User login
PU-H71 receives orphan drug designation for myelofibrosis
The US Food and Drug Administration (FDA) has granted orphan drug designation to PU-H71 to treat patients with myelofibrosis.
The drug specifically targets the epichaperome, a network of high-molecular-weight complexes found in multiple diseases, including cancer and neurologic disorders. These complexes enhance cellular survival, irrespective of tissue of origin or genetic background.
According to research published in Nature Reviews Cancer, Pu-H71 interferes with the epichaperome function in diseases and does not affect normal cells.
PU-H71 is being evaluated in a phase 1b trial in myelofibrosis and advanced metastatic breast cancer.
“In myelofibrosis, the epichaperome plays a central role in optimizing the JAK-STAT pathway,” said Srdan Verstovsek, MD, PhD, “allowing JAK2 to form dimers that evade inhibition with a JAK2 inhibitor such as ruxolitinib.”
“By inhibiting epichaperome function and breaking this mechanism, we believe PU-H71 can increase anti-cancer activity of JAK2 inhibitors,” he said. Dr Verstovsek, of the MD Anderson Cancer Center in Houston, Texas, is lead clinical research advisor for the phase 1b myelofibrosis study.
Phase 1b Study (NCT01393509)
This is a multicenter study designed to assess the safety, tolerability, pharmacokinetic and preliminary efficacy of PU-H71 in patients taking concomitant ruxolitinib.
The safety expansion phase of the trial is open for accrual only to patients with myeloproliferative neoplasms (MPNs).
These patients must have been on ruxolitinib for at least 3 months, be on a stable dose for at least 1 month prior to enrollment and be taking at least 5 mg twice daily.
Patients must have persistent disease manifestations, despite ruxolitinib therapy. These include persistent splenomegaly, abnormal blood counts, persistent constitutional symptoms, residual fibrosis in bone marrow (2+ or greater), or measurable allele burden as evidenced by clonal JAK2 or MPL mutation.
Samus Therapeutics, the developer of PU-H71, announced, simultaneously with the orphan drug designation, the dosing of the first patient in the phase 1b myelofibrosis study.
“Targeting the epichaperome offers an exciting new avenue for treating myelofibrosis and related diseases,” Dr Verstovsek said.
“I look forward to seeing how the combination of these therapies can affect outcomes in patients for whom this resistance is associated with poor prognoses.”
The US Food and Drug Administration (FDA) has granted orphan drug designation to PU-H71 to treat patients with myelofibrosis.
The drug specifically targets the epichaperome, a network of high-molecular-weight complexes found in multiple diseases, including cancer and neurologic disorders. These complexes enhance cellular survival, irrespective of tissue of origin or genetic background.
According to research published in Nature Reviews Cancer, Pu-H71 interferes with the epichaperome function in diseases and does not affect normal cells.
PU-H71 is being evaluated in a phase 1b trial in myelofibrosis and advanced metastatic breast cancer.
“In myelofibrosis, the epichaperome plays a central role in optimizing the JAK-STAT pathway,” said Srdan Verstovsek, MD, PhD, “allowing JAK2 to form dimers that evade inhibition with a JAK2 inhibitor such as ruxolitinib.”
“By inhibiting epichaperome function and breaking this mechanism, we believe PU-H71 can increase anti-cancer activity of JAK2 inhibitors,” he said. Dr Verstovsek, of the MD Anderson Cancer Center in Houston, Texas, is lead clinical research advisor for the phase 1b myelofibrosis study.
Phase 1b Study (NCT01393509)
This is a multicenter study designed to assess the safety, tolerability, pharmacokinetic and preliminary efficacy of PU-H71 in patients taking concomitant ruxolitinib.
The safety expansion phase of the trial is open for accrual only to patients with myeloproliferative neoplasms (MPNs).
These patients must have been on ruxolitinib for at least 3 months, be on a stable dose for at least 1 month prior to enrollment and be taking at least 5 mg twice daily.
Patients must have persistent disease manifestations, despite ruxolitinib therapy. These include persistent splenomegaly, abnormal blood counts, persistent constitutional symptoms, residual fibrosis in bone marrow (2+ or greater), or measurable allele burden as evidenced by clonal JAK2 or MPL mutation.
Samus Therapeutics, the developer of PU-H71, announced, simultaneously with the orphan drug designation, the dosing of the first patient in the phase 1b myelofibrosis study.
“Targeting the epichaperome offers an exciting new avenue for treating myelofibrosis and related diseases,” Dr Verstovsek said.
“I look forward to seeing how the combination of these therapies can affect outcomes in patients for whom this resistance is associated with poor prognoses.”
The US Food and Drug Administration (FDA) has granted orphan drug designation to PU-H71 to treat patients with myelofibrosis.
The drug specifically targets the epichaperome, a network of high-molecular-weight complexes found in multiple diseases, including cancer and neurologic disorders. These complexes enhance cellular survival, irrespective of tissue of origin or genetic background.
According to research published in Nature Reviews Cancer, Pu-H71 interferes with the epichaperome function in diseases and does not affect normal cells.
PU-H71 is being evaluated in a phase 1b trial in myelofibrosis and advanced metastatic breast cancer.
“In myelofibrosis, the epichaperome plays a central role in optimizing the JAK-STAT pathway,” said Srdan Verstovsek, MD, PhD, “allowing JAK2 to form dimers that evade inhibition with a JAK2 inhibitor such as ruxolitinib.”
“By inhibiting epichaperome function and breaking this mechanism, we believe PU-H71 can increase anti-cancer activity of JAK2 inhibitors,” he said. Dr Verstovsek, of the MD Anderson Cancer Center in Houston, Texas, is lead clinical research advisor for the phase 1b myelofibrosis study.
Phase 1b Study (NCT01393509)
This is a multicenter study designed to assess the safety, tolerability, pharmacokinetic and preliminary efficacy of PU-H71 in patients taking concomitant ruxolitinib.
The safety expansion phase of the trial is open for accrual only to patients with myeloproliferative neoplasms (MPNs).
These patients must have been on ruxolitinib for at least 3 months, be on a stable dose for at least 1 month prior to enrollment and be taking at least 5 mg twice daily.
Patients must have persistent disease manifestations, despite ruxolitinib therapy. These include persistent splenomegaly, abnormal blood counts, persistent constitutional symptoms, residual fibrosis in bone marrow (2+ or greater), or measurable allele burden as evidenced by clonal JAK2 or MPL mutation.
Samus Therapeutics, the developer of PU-H71, announced, simultaneously with the orphan drug designation, the dosing of the first patient in the phase 1b myelofibrosis study.
“Targeting the epichaperome offers an exciting new avenue for treating myelofibrosis and related diseases,” Dr Verstovsek said.
“I look forward to seeing how the combination of these therapies can affect outcomes in patients for whom this resistance is associated with poor prognoses.”
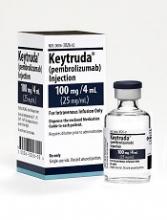
FDA grants pembrolizumab accelerated approval for PMBCL
The US Food and Drug Administration (FDA) granted accelerated approval to the anti-PD-1 therapy pembrolizumab (Keytruda) for the treatment of adult and pediatric patients with refractory primary mediastinal large B-cell lymphoma (PMBCL).
The indication also includes patients who have relapsed after 2 or more prior lines of therapy.
Pembrolizumab had received priority review for PMBCL late last year and also has orphan drug designation and breakthrough therapy designation for this indication.
The FDA based its approval on data from the KEYNOTE-170 (NCT02576990 ) trial.
Investigators enrolled 53 patients onto the multicenter, open-label, single-arm trial. Patients received pembrolizumab 200 mg intravenously every 3 weeks until unacceptable toxicity or documented disease progression.
Patients whose disease did not progress received the drug for up to 24 months.
Patient characteristics
Patients were a median age of 33 years (range, 20 – 61), 43% were male, 92% white, 43% had an ECOG performance status of 0, and 57% had an ECOG performance status of 1.
Almost half (49%) had relapsed disease, and 36% had primary refractory disease.
About a quarter (26%) had undergone prior autologous hematopoietic stem cell transplant, and 32% had prior radiation therapy.
All patients had received prior rituximab.
Results
At a median follow-up of 9.7 months, the overall response rate was 45% (24 responders), including 11% complete responses and 34% partial responses.
The median duration of response was not reached during the follow-up period and ranged from a median 1.1 to 19.2 months.
Median time to first objective response was 2.8 months (range, 2.1 – 8.5). Accordingly, investigators do not recommend pembrolizumab for PMBCL patients who require urgent cytoreductive therapy.
Safety
The most common adverse events occurring in 10% or more of patients were musculoskeletal pain (30%), upper respiratory tract infection (28%), pyrexia (28%), fatigue (23%), cough (26%), dyspnea (21%), diarrhea (13%), abdominal pain (13%), nausea (11%), arrhythmia (11%), and headache (11%).
Eight percent of patients discontinued treatment, and 15% interrupted treatment due to adverse reactions.
Adverse events requiring systemic corticosteroid therapy occurred in 25% of patients.
Serious adverse events occurred in 26% and included arrhythmia (4 %), cardiac tamponade (2%), myocardial infarction (2%), pericardial effusion (2%), and pericarditis (2%).
Six (11%) patients died within 30 days of start of treatment.
The recommended pembrolizumab dose for treatment of adults with PMBCL is 200 mg every 3 weeks. The recommended dose in pediatric patients is 2 mg/kg (up to a maximum of 200 mg) every 3 weeks.
Additional indications for pembrolizumab include melanoma, non-small cell lung cancer, head and neck squamous cell cancer, classical Hodgkin lymphoma, urothelial carcinoma, microsatellite instability-high cancer, gastric cancer, and cervical cancer.
The full prescribing information is available on the FDA website.
Pembrolizumab (Keytruda) is a product of Merck & Co, Inc.
The US Food and Drug Administration (FDA) granted accelerated approval to the anti-PD-1 therapy pembrolizumab (Keytruda) for the treatment of adult and pediatric patients with refractory primary mediastinal large B-cell lymphoma (PMBCL).
The indication also includes patients who have relapsed after 2 or more prior lines of therapy.
Pembrolizumab had received priority review for PMBCL late last year and also has orphan drug designation and breakthrough therapy designation for this indication.
The FDA based its approval on data from the KEYNOTE-170 (NCT02576990 ) trial.
Investigators enrolled 53 patients onto the multicenter, open-label, single-arm trial. Patients received pembrolizumab 200 mg intravenously every 3 weeks until unacceptable toxicity or documented disease progression.
Patients whose disease did not progress received the drug for up to 24 months.
Patient characteristics
Patients were a median age of 33 years (range, 20 – 61), 43% were male, 92% white, 43% had an ECOG performance status of 0, and 57% had an ECOG performance status of 1.
Almost half (49%) had relapsed disease, and 36% had primary refractory disease.
About a quarter (26%) had undergone prior autologous hematopoietic stem cell transplant, and 32% had prior radiation therapy.
All patients had received prior rituximab.
Results
At a median follow-up of 9.7 months, the overall response rate was 45% (24 responders), including 11% complete responses and 34% partial responses.
The median duration of response was not reached during the follow-up period and ranged from a median 1.1 to 19.2 months.
Median time to first objective response was 2.8 months (range, 2.1 – 8.5). Accordingly, investigators do not recommend pembrolizumab for PMBCL patients who require urgent cytoreductive therapy.
Safety
The most common adverse events occurring in 10% or more of patients were musculoskeletal pain (30%), upper respiratory tract infection (28%), pyrexia (28%), fatigue (23%), cough (26%), dyspnea (21%), diarrhea (13%), abdominal pain (13%), nausea (11%), arrhythmia (11%), and headache (11%).
Eight percent of patients discontinued treatment, and 15% interrupted treatment due to adverse reactions.
Adverse events requiring systemic corticosteroid therapy occurred in 25% of patients.
Serious adverse events occurred in 26% and included arrhythmia (4 %), cardiac tamponade (2%), myocardial infarction (2%), pericardial effusion (2%), and pericarditis (2%).
Six (11%) patients died within 30 days of start of treatment.
The recommended pembrolizumab dose for treatment of adults with PMBCL is 200 mg every 3 weeks. The recommended dose in pediatric patients is 2 mg/kg (up to a maximum of 200 mg) every 3 weeks.
Additional indications for pembrolizumab include melanoma, non-small cell lung cancer, head and neck squamous cell cancer, classical Hodgkin lymphoma, urothelial carcinoma, microsatellite instability-high cancer, gastric cancer, and cervical cancer.
The full prescribing information is available on the FDA website.
Pembrolizumab (Keytruda) is a product of Merck & Co, Inc.
The US Food and Drug Administration (FDA) granted accelerated approval to the anti-PD-1 therapy pembrolizumab (Keytruda) for the treatment of adult and pediatric patients with refractory primary mediastinal large B-cell lymphoma (PMBCL).
The indication also includes patients who have relapsed after 2 or more prior lines of therapy.
Pembrolizumab had received priority review for PMBCL late last year and also has orphan drug designation and breakthrough therapy designation for this indication.
The FDA based its approval on data from the KEYNOTE-170 (NCT02576990 ) trial.
Investigators enrolled 53 patients onto the multicenter, open-label, single-arm trial. Patients received pembrolizumab 200 mg intravenously every 3 weeks until unacceptable toxicity or documented disease progression.
Patients whose disease did not progress received the drug for up to 24 months.
Patient characteristics
Patients were a median age of 33 years (range, 20 – 61), 43% were male, 92% white, 43% had an ECOG performance status of 0, and 57% had an ECOG performance status of 1.
Almost half (49%) had relapsed disease, and 36% had primary refractory disease.
About a quarter (26%) had undergone prior autologous hematopoietic stem cell transplant, and 32% had prior radiation therapy.
All patients had received prior rituximab.
Results
At a median follow-up of 9.7 months, the overall response rate was 45% (24 responders), including 11% complete responses and 34% partial responses.
The median duration of response was not reached during the follow-up period and ranged from a median 1.1 to 19.2 months.
Median time to first objective response was 2.8 months (range, 2.1 – 8.5). Accordingly, investigators do not recommend pembrolizumab for PMBCL patients who require urgent cytoreductive therapy.
Safety
The most common adverse events occurring in 10% or more of patients were musculoskeletal pain (30%), upper respiratory tract infection (28%), pyrexia (28%), fatigue (23%), cough (26%), dyspnea (21%), diarrhea (13%), abdominal pain (13%), nausea (11%), arrhythmia (11%), and headache (11%).
Eight percent of patients discontinued treatment, and 15% interrupted treatment due to adverse reactions.
Adverse events requiring systemic corticosteroid therapy occurred in 25% of patients.
Serious adverse events occurred in 26% and included arrhythmia (4 %), cardiac tamponade (2%), myocardial infarction (2%), pericardial effusion (2%), and pericarditis (2%).
Six (11%) patients died within 30 days of start of treatment.
The recommended pembrolizumab dose for treatment of adults with PMBCL is 200 mg every 3 weeks. The recommended dose in pediatric patients is 2 mg/kg (up to a maximum of 200 mg) every 3 weeks.
Additional indications for pembrolizumab include melanoma, non-small cell lung cancer, head and neck squamous cell cancer, classical Hodgkin lymphoma, urothelial carcinoma, microsatellite instability-high cancer, gastric cancer, and cervical cancer.
The full prescribing information is available on the FDA website.
Pembrolizumab (Keytruda) is a product of Merck & Co, Inc.
Mircera approved for anemia in pediatric patients with CKD
Mircera®, methoxy polyethylene glycol-epoetin beta, was approved by the US Food and Drug Administration (FDA) to treat anemia in pediatric patients who have chronic kidney disease (CKD).
The drug is indicated for patients ages 5 to 17 years on hemodialysis who are switching from another erythropoiesis-stimulating agent (ESA) after their hemoglobin levels have stabilized.
The FDA also approved the agent to treat adult patients with CKD-associated anemia.
However, the drug is not approved to treat anemia caused by cancer chemotherapy.
The FDA based its approval on data from an open-label, multiple-dose, multicenter, dose-finding trial (NCT00717366).
Investigators enrolled 64 pediatric patients with CKD on hemodialysis. The patients had to have stable hemoglobin levels while receiving another ESA, such as epoetin alfa/beta or darbepoetin alfa.
Patients received Mircera intravenously once every 4 weeks for 20 weeks. Investigators adjusted the dosages, if necessary, after the first administration to maintain target hemoglobin levels.
Efficacy was based on the patients’ ability to maintain target hemoglobin levels and also on data extrapolated from trials of Mircera in adults with CKD.
Patients who received Mircera had a mean change in hemoglobin concentration from baseline of -0.15g/dL and 75% maintained hemoglobin values within ± 1g/dL of baseline.
Eighty-one percent maintained hemoglobin values within 10–12g/dL during the evaluation period.
The safety findings in pediatric patients were consistent with those previously reported in adults.
The most common adverse reactions occurring in 10% or more patients, as indicated in the prescribing information, are hypertension, diarrhea, and nasopharyngitis.
The drug carries a black box warning for increased risk of death, myocardial infarction, stroke, venous thromboembolism, thrombosis of vascular access, and tumor progression of recurrence.
Mircera is an erythropoietin receptor activator with greater activity in vivo as well as increased half-life, compared to erythropoietin.
Mircera is manufactured by Vifor (International) Inc.
Mircera®, methoxy polyethylene glycol-epoetin beta, was approved by the US Food and Drug Administration (FDA) to treat anemia in pediatric patients who have chronic kidney disease (CKD).
The drug is indicated for patients ages 5 to 17 years on hemodialysis who are switching from another erythropoiesis-stimulating agent (ESA) after their hemoglobin levels have stabilized.
The FDA also approved the agent to treat adult patients with CKD-associated anemia.
However, the drug is not approved to treat anemia caused by cancer chemotherapy.
The FDA based its approval on data from an open-label, multiple-dose, multicenter, dose-finding trial (NCT00717366).
Investigators enrolled 64 pediatric patients with CKD on hemodialysis. The patients had to have stable hemoglobin levels while receiving another ESA, such as epoetin alfa/beta or darbepoetin alfa.
Patients received Mircera intravenously once every 4 weeks for 20 weeks. Investigators adjusted the dosages, if necessary, after the first administration to maintain target hemoglobin levels.
Efficacy was based on the patients’ ability to maintain target hemoglobin levels and also on data extrapolated from trials of Mircera in adults with CKD.
Patients who received Mircera had a mean change in hemoglobin concentration from baseline of -0.15g/dL and 75% maintained hemoglobin values within ± 1g/dL of baseline.
Eighty-one percent maintained hemoglobin values within 10–12g/dL during the evaluation period.
The safety findings in pediatric patients were consistent with those previously reported in adults.
The most common adverse reactions occurring in 10% or more patients, as indicated in the prescribing information, are hypertension, diarrhea, and nasopharyngitis.
The drug carries a black box warning for increased risk of death, myocardial infarction, stroke, venous thromboembolism, thrombosis of vascular access, and tumor progression of recurrence.
Mircera is an erythropoietin receptor activator with greater activity in vivo as well as increased half-life, compared to erythropoietin.
Mircera is manufactured by Vifor (International) Inc.
Mircera®, methoxy polyethylene glycol-epoetin beta, was approved by the US Food and Drug Administration (FDA) to treat anemia in pediatric patients who have chronic kidney disease (CKD).
The drug is indicated for patients ages 5 to 17 years on hemodialysis who are switching from another erythropoiesis-stimulating agent (ESA) after their hemoglobin levels have stabilized.
The FDA also approved the agent to treat adult patients with CKD-associated anemia.
However, the drug is not approved to treat anemia caused by cancer chemotherapy.
The FDA based its approval on data from an open-label, multiple-dose, multicenter, dose-finding trial (NCT00717366).
Investigators enrolled 64 pediatric patients with CKD on hemodialysis. The patients had to have stable hemoglobin levels while receiving another ESA, such as epoetin alfa/beta or darbepoetin alfa.
Patients received Mircera intravenously once every 4 weeks for 20 weeks. Investigators adjusted the dosages, if necessary, after the first administration to maintain target hemoglobin levels.
Efficacy was based on the patients’ ability to maintain target hemoglobin levels and also on data extrapolated from trials of Mircera in adults with CKD.
Patients who received Mircera had a mean change in hemoglobin concentration from baseline of -0.15g/dL and 75% maintained hemoglobin values within ± 1g/dL of baseline.
Eighty-one percent maintained hemoglobin values within 10–12g/dL during the evaluation period.
The safety findings in pediatric patients were consistent with those previously reported in adults.
The most common adverse reactions occurring in 10% or more patients, as indicated in the prescribing information, are hypertension, diarrhea, and nasopharyngitis.
The drug carries a black box warning for increased risk of death, myocardial infarction, stroke, venous thromboembolism, thrombosis of vascular access, and tumor progression of recurrence.
Mircera is an erythropoietin receptor activator with greater activity in vivo as well as increased half-life, compared to erythropoietin.
Mircera is manufactured by Vifor (International) Inc.
FDA approves venetoclax for CLL/SLL with or without del 17p
The US Food and Drug Administration (FDA) has approved venetoclax tablets (Venclexta ®) in combination with rituximab to treat patients with chronic lymphocytic leukemia (CLL) or small lymphocytic lymphoma (SLL) who have received 1 prior therapy.
The combination is approved for patients with or without deletion of 17p (del 17p).
The FDA based its approval on the phase 3 MURANO trial, in which venetoclax in combination with rituximab (VEN+R) significantly improved progression-free survival (PFS) in relapsed or refractory CLL patients compared to the chemoimmunotherapy regimen of bendamustine plus rituximab(B+R).
This approval, according to the drug’s developers, makes venetoclax plus rituximab the first oral-based, chemotherapy-free combination with a fixed treatment duration for CLL.
The FDA has also converted venetoclax's accelerated approval to a full approval. The drug was previously granted accelerated approval as a single agent for the treatment of people with CLL with 17p deletion.
Venetoclax is being developed by AbbVie and Roche and jointly commercialized by AbbVie and Genentech in the US and by AbbVie outside the US.
Phase 3 MURANO trial (NCT02005471)
The multicenter, open-label trial randomized 389 patients to VEN+R (194 patients) or B+R (195 patients). Median age of the patients was 65 years (range, 22 – 85).
Patients in the VEN+R arm completed a 5-week ramp-up of venetoclax followed by venetoclax 400 mg once daily for 24 months measured from the rituximab start date.
Tumor lysis syndrome (TLS), caused by a rapid reduction in tumor volume, is an identified risk with venetoclax treatment. The dose ramp-up was intended to mitigate this risk.
Rituximab was initiated after venetoclax ramp-up and given for 6 cycles (375 mg/m2 intravenously on cycle 1 day 1 and 500 mg/m2 intravenously on day 1 of cycles 2-6, with a 28-day cycle length).
Patients in the B+R arm received 6 cycles of B+R (bendamustine 70 mg/m2 on days 1 and 2 of each 28-day cycle and rituximab at the above described dose and schedule).
Efficacy was based on PFS as assessed by an independent review committee.
After a median follow-up of 23 months, the median PFS was not reached in the VEN+R arm and was 18.1 months in the B+R arm (P<0.0001).
The overall response rate was 92% for patients treated with VEN+R compared to 72% for those treated with B+R.
Safety
The most common adverse events (AEs) in the VEN+R arms that occurred in 20% or more patients were neutropenia (65%), diarrhea (40%), upper respiratory tract infection (39%), fatigue (22%), cough (22%), and nausea (21%).
Grade 3 or 4 neutropenia developed in 64% of patients, and grade 4 neutropenia in 31%.
Serious adverse events (SAEs) developed in 46% of patients and serious infections in 21%, consisting most frequently of pneumonia (9%).
The incidence of TLS was 3%, occurring in 6 of 194 patients.
In the VEN+R arm, discontinuations due to any AEs occurred in 16% of patients, dose reductions in 15%, and dose interruptions in 71%.
Neutropenia led to dose interruptions in 46% of patients and discontinuations in 3%. Thrombocytopenia led to discontinuations in 3% of patients.
Fatal AEs that occurred in the absence of disease progression and within 30 days of the last VEN+R treatment and/or 90 days of the last rituximab infusion were reported in 2% (4/194) of patients.
In the B+R arm, AEs led to treatment discontinuations in 10% of patients, dose reductions in 15%, and dose interruptions in 40 %.
Investigators previously reported data from the phase 3 MURANO study as a late-breaking abstract at the 2017 ASH Annual Meeting and published the findings in NEJM.
John Seymour, MBBS, PhD, lead investigator of the MURANO study, said in the corporate release, the approval "validates the results seen in the phase 3 trial, including the significant improvement in progression-free survival over a standard of care comparator arm."
"Progression-free survival is considered a gold standard for demonstrating clinical benefit in oncology," he added.
Full prescribing information for venetoclax is available here.
The US Food and Drug Administration (FDA) has approved venetoclax tablets (Venclexta ®) in combination with rituximab to treat patients with chronic lymphocytic leukemia (CLL) or small lymphocytic lymphoma (SLL) who have received 1 prior therapy.
The combination is approved for patients with or without deletion of 17p (del 17p).
The FDA based its approval on the phase 3 MURANO trial, in which venetoclax in combination with rituximab (VEN+R) significantly improved progression-free survival (PFS) in relapsed or refractory CLL patients compared to the chemoimmunotherapy regimen of bendamustine plus rituximab(B+R).
This approval, according to the drug’s developers, makes venetoclax plus rituximab the first oral-based, chemotherapy-free combination with a fixed treatment duration for CLL.
The FDA has also converted venetoclax's accelerated approval to a full approval. The drug was previously granted accelerated approval as a single agent for the treatment of people with CLL with 17p deletion.
Venetoclax is being developed by AbbVie and Roche and jointly commercialized by AbbVie and Genentech in the US and by AbbVie outside the US.
Phase 3 MURANO trial (NCT02005471)
The multicenter, open-label trial randomized 389 patients to VEN+R (194 patients) or B+R (195 patients). Median age of the patients was 65 years (range, 22 – 85).
Patients in the VEN+R arm completed a 5-week ramp-up of venetoclax followed by venetoclax 400 mg once daily for 24 months measured from the rituximab start date.
Tumor lysis syndrome (TLS), caused by a rapid reduction in tumor volume, is an identified risk with venetoclax treatment. The dose ramp-up was intended to mitigate this risk.
Rituximab was initiated after venetoclax ramp-up and given for 6 cycles (375 mg/m2 intravenously on cycle 1 day 1 and 500 mg/m2 intravenously on day 1 of cycles 2-6, with a 28-day cycle length).
Patients in the B+R arm received 6 cycles of B+R (bendamustine 70 mg/m2 on days 1 and 2 of each 28-day cycle and rituximab at the above described dose and schedule).
Efficacy was based on PFS as assessed by an independent review committee.
After a median follow-up of 23 months, the median PFS was not reached in the VEN+R arm and was 18.1 months in the B+R arm (P<0.0001).
The overall response rate was 92% for patients treated with VEN+R compared to 72% for those treated with B+R.
Safety
The most common adverse events (AEs) in the VEN+R arms that occurred in 20% or more patients were neutropenia (65%), diarrhea (40%), upper respiratory tract infection (39%), fatigue (22%), cough (22%), and nausea (21%).
Grade 3 or 4 neutropenia developed in 64% of patients, and grade 4 neutropenia in 31%.
Serious adverse events (SAEs) developed in 46% of patients and serious infections in 21%, consisting most frequently of pneumonia (9%).
The incidence of TLS was 3%, occurring in 6 of 194 patients.
In the VEN+R arm, discontinuations due to any AEs occurred in 16% of patients, dose reductions in 15%, and dose interruptions in 71%.
Neutropenia led to dose interruptions in 46% of patients and discontinuations in 3%. Thrombocytopenia led to discontinuations in 3% of patients.
Fatal AEs that occurred in the absence of disease progression and within 30 days of the last VEN+R treatment and/or 90 days of the last rituximab infusion were reported in 2% (4/194) of patients.
In the B+R arm, AEs led to treatment discontinuations in 10% of patients, dose reductions in 15%, and dose interruptions in 40 %.
Investigators previously reported data from the phase 3 MURANO study as a late-breaking abstract at the 2017 ASH Annual Meeting and published the findings in NEJM.
John Seymour, MBBS, PhD, lead investigator of the MURANO study, said in the corporate release, the approval "validates the results seen in the phase 3 trial, including the significant improvement in progression-free survival over a standard of care comparator arm."
"Progression-free survival is considered a gold standard for demonstrating clinical benefit in oncology," he added.
Full prescribing information for venetoclax is available here.
The US Food and Drug Administration (FDA) has approved venetoclax tablets (Venclexta ®) in combination with rituximab to treat patients with chronic lymphocytic leukemia (CLL) or small lymphocytic lymphoma (SLL) who have received 1 prior therapy.
The combination is approved for patients with or without deletion of 17p (del 17p).
The FDA based its approval on the phase 3 MURANO trial, in which venetoclax in combination with rituximab (VEN+R) significantly improved progression-free survival (PFS) in relapsed or refractory CLL patients compared to the chemoimmunotherapy regimen of bendamustine plus rituximab(B+R).
This approval, according to the drug’s developers, makes venetoclax plus rituximab the first oral-based, chemotherapy-free combination with a fixed treatment duration for CLL.
The FDA has also converted venetoclax's accelerated approval to a full approval. The drug was previously granted accelerated approval as a single agent for the treatment of people with CLL with 17p deletion.
Venetoclax is being developed by AbbVie and Roche and jointly commercialized by AbbVie and Genentech in the US and by AbbVie outside the US.
Phase 3 MURANO trial (NCT02005471)
The multicenter, open-label trial randomized 389 patients to VEN+R (194 patients) or B+R (195 patients). Median age of the patients was 65 years (range, 22 – 85).
Patients in the VEN+R arm completed a 5-week ramp-up of venetoclax followed by venetoclax 400 mg once daily for 24 months measured from the rituximab start date.
Tumor lysis syndrome (TLS), caused by a rapid reduction in tumor volume, is an identified risk with venetoclax treatment. The dose ramp-up was intended to mitigate this risk.
Rituximab was initiated after venetoclax ramp-up and given for 6 cycles (375 mg/m2 intravenously on cycle 1 day 1 and 500 mg/m2 intravenously on day 1 of cycles 2-6, with a 28-day cycle length).
Patients in the B+R arm received 6 cycles of B+R (bendamustine 70 mg/m2 on days 1 and 2 of each 28-day cycle and rituximab at the above described dose and schedule).
Efficacy was based on PFS as assessed by an independent review committee.
After a median follow-up of 23 months, the median PFS was not reached in the VEN+R arm and was 18.1 months in the B+R arm (P<0.0001).
The overall response rate was 92% for patients treated with VEN+R compared to 72% for those treated with B+R.
Safety
The most common adverse events (AEs) in the VEN+R arms that occurred in 20% or more patients were neutropenia (65%), diarrhea (40%), upper respiratory tract infection (39%), fatigue (22%), cough (22%), and nausea (21%).
Grade 3 or 4 neutropenia developed in 64% of patients, and grade 4 neutropenia in 31%.
Serious adverse events (SAEs) developed in 46% of patients and serious infections in 21%, consisting most frequently of pneumonia (9%).
The incidence of TLS was 3%, occurring in 6 of 194 patients.
In the VEN+R arm, discontinuations due to any AEs occurred in 16% of patients, dose reductions in 15%, and dose interruptions in 71%.
Neutropenia led to dose interruptions in 46% of patients and discontinuations in 3%. Thrombocytopenia led to discontinuations in 3% of patients.
Fatal AEs that occurred in the absence of disease progression and within 30 days of the last VEN+R treatment and/or 90 days of the last rituximab infusion were reported in 2% (4/194) of patients.
In the B+R arm, AEs led to treatment discontinuations in 10% of patients, dose reductions in 15%, and dose interruptions in 40 %.
Investigators previously reported data from the phase 3 MURANO study as a late-breaking abstract at the 2017 ASH Annual Meeting and published the findings in NEJM.
John Seymour, MBBS, PhD, lead investigator of the MURANO study, said in the corporate release, the approval "validates the results seen in the phase 3 trial, including the significant improvement in progression-free survival over a standard of care comparator arm."
"Progression-free survival is considered a gold standard for demonstrating clinical benefit in oncology," he added.
Full prescribing information for venetoclax is available here.
Dasatinib outcomes similar to imatinib in pediatric Ph+ ALL
Dasatinib used during induction and consolidation in the Children’s Oncology Group (COG) AALL0622 trial provided early response rates for children with Ph-positive (Ph+) acute lymphoblastic leukemia (ALL), according to investigators.
But the early response rates did not improve event-free survival (EFS) compared to the use of consolidation imatinib in the AALL0031 study.
Incidence of cranial relapse was more than doubled in AALL0622 compared to AALL0031.
The investigators believe the incidence of cranial relapse may explain the results of AALL0622.
“We cannot yet conclude that the current dasatinib plus chemotherapy combination is better than imatinib plus chemotherapy,” the authors stated.
AALL0622 was designed to be an improvement on AALL0031, which demonstrated that adding the tyrosine kinase inhibitor (TKI) imatinib to intensive chemotherapy in the consolidation phase significantly improved survival for children with Ph+ ALL.
In AALL0622 dasatinib was given early in induction (day 15) and then in consolidation with the hope that patients could achieve early remission.
Another departure from AALL0031 was that cranial irradiation was not provided for control of central nervous system (CNS) metastasis. Because dasatinib accumulates in the CNS, which is a ‘sanctuary site’ for leukemia, it was presumed that patients could benefit from a TKI yet be spared from cranial irradiation.
As expected, adding dasatinib mid-induction provided a complete remission rate of 98% at the end of induction (day 29), which was better than the 89% seen in AALL0031.
In addition, more patients in AALL0622 showed minimal residual disease (MRD) <0.01% at the end of induction: 59% vs 25% in AALL0031 (P <0.001). At the end of consolidation, corresponding rates were 89% vs 71% for AALL0031.
For the primary outcome, 3-year EFS was 84.6% for patients in AALL0622 in standard-risk patients. Five-year OS and EFS rates were 86% and 60%, respectively.
In patients with overt brain metastasis (CNS3 status), 5-year CNS relapse was 15% for patients in the AALL0622 study vs 6.6% for patients in the AALL031 study.
However, 5-year OS rates were similar in the two groups of patients: 86% for AALL0622 vs 81% for AALL0031.
HSCT
AALL0622 allowed the use of hematopoietic stem cell transplantation (HSCT) in high-risk patients as well as in standard-risk patients with a sibling donor.
Five-year OS and EFS for standard-risk patients (19% underwent HSCT at first remission) and high-risk patients (91% underwent HSCT in first remission) were similar.
Children who did not undergo HSCT had a similar 5-year OS of 88%, which suggested that children with Ph+ ALL should not undergo transplantation at first remission.
Samples from a subset of patients was analyzed for IKZF1 mutations and correlated with outcomes.
Five-year OS was 80% in those harboring the mutation versus 100% who had the wild-type gene (P=0.04); 4-year EFS was also significantly lower—10% vs 82% (P=0.04).
Screening for IKZF1 may be used to identify high-risk patients suitable for HSCT and/or alternate treatment, the authors note.
The investigators reported their findings in The Journal of Clinical Oncology.
Dasatinib used during induction and consolidation in the Children’s Oncology Group (COG) AALL0622 trial provided early response rates for children with Ph-positive (Ph+) acute lymphoblastic leukemia (ALL), according to investigators.
But the early response rates did not improve event-free survival (EFS) compared to the use of consolidation imatinib in the AALL0031 study.
Incidence of cranial relapse was more than doubled in AALL0622 compared to AALL0031.
The investigators believe the incidence of cranial relapse may explain the results of AALL0622.
“We cannot yet conclude that the current dasatinib plus chemotherapy combination is better than imatinib plus chemotherapy,” the authors stated.
AALL0622 was designed to be an improvement on AALL0031, which demonstrated that adding the tyrosine kinase inhibitor (TKI) imatinib to intensive chemotherapy in the consolidation phase significantly improved survival for children with Ph+ ALL.
In AALL0622 dasatinib was given early in induction (day 15) and then in consolidation with the hope that patients could achieve early remission.
Another departure from AALL0031 was that cranial irradiation was not provided for control of central nervous system (CNS) metastasis. Because dasatinib accumulates in the CNS, which is a ‘sanctuary site’ for leukemia, it was presumed that patients could benefit from a TKI yet be spared from cranial irradiation.
As expected, adding dasatinib mid-induction provided a complete remission rate of 98% at the end of induction (day 29), which was better than the 89% seen in AALL0031.
In addition, more patients in AALL0622 showed minimal residual disease (MRD) <0.01% at the end of induction: 59% vs 25% in AALL0031 (P <0.001). At the end of consolidation, corresponding rates were 89% vs 71% for AALL0031.
For the primary outcome, 3-year EFS was 84.6% for patients in AALL0622 in standard-risk patients. Five-year OS and EFS rates were 86% and 60%, respectively.
In patients with overt brain metastasis (CNS3 status), 5-year CNS relapse was 15% for patients in the AALL0622 study vs 6.6% for patients in the AALL031 study.
However, 5-year OS rates were similar in the two groups of patients: 86% for AALL0622 vs 81% for AALL0031.
HSCT
AALL0622 allowed the use of hematopoietic stem cell transplantation (HSCT) in high-risk patients as well as in standard-risk patients with a sibling donor.
Five-year OS and EFS for standard-risk patients (19% underwent HSCT at first remission) and high-risk patients (91% underwent HSCT in first remission) were similar.
Children who did not undergo HSCT had a similar 5-year OS of 88%, which suggested that children with Ph+ ALL should not undergo transplantation at first remission.
Samples from a subset of patients was analyzed for IKZF1 mutations and correlated with outcomes.
Five-year OS was 80% in those harboring the mutation versus 100% who had the wild-type gene (P=0.04); 4-year EFS was also significantly lower—10% vs 82% (P=0.04).
Screening for IKZF1 may be used to identify high-risk patients suitable for HSCT and/or alternate treatment, the authors note.
The investigators reported their findings in The Journal of Clinical Oncology.
Dasatinib used during induction and consolidation in the Children’s Oncology Group (COG) AALL0622 trial provided early response rates for children with Ph-positive (Ph+) acute lymphoblastic leukemia (ALL), according to investigators.
But the early response rates did not improve event-free survival (EFS) compared to the use of consolidation imatinib in the AALL0031 study.
Incidence of cranial relapse was more than doubled in AALL0622 compared to AALL0031.
The investigators believe the incidence of cranial relapse may explain the results of AALL0622.
“We cannot yet conclude that the current dasatinib plus chemotherapy combination is better than imatinib plus chemotherapy,” the authors stated.
AALL0622 was designed to be an improvement on AALL0031, which demonstrated that adding the tyrosine kinase inhibitor (TKI) imatinib to intensive chemotherapy in the consolidation phase significantly improved survival for children with Ph+ ALL.
In AALL0622 dasatinib was given early in induction (day 15) and then in consolidation with the hope that patients could achieve early remission.
Another departure from AALL0031 was that cranial irradiation was not provided for control of central nervous system (CNS) metastasis. Because dasatinib accumulates in the CNS, which is a ‘sanctuary site’ for leukemia, it was presumed that patients could benefit from a TKI yet be spared from cranial irradiation.
As expected, adding dasatinib mid-induction provided a complete remission rate of 98% at the end of induction (day 29), which was better than the 89% seen in AALL0031.
In addition, more patients in AALL0622 showed minimal residual disease (MRD) <0.01% at the end of induction: 59% vs 25% in AALL0031 (P <0.001). At the end of consolidation, corresponding rates were 89% vs 71% for AALL0031.
For the primary outcome, 3-year EFS was 84.6% for patients in AALL0622 in standard-risk patients. Five-year OS and EFS rates were 86% and 60%, respectively.
In patients with overt brain metastasis (CNS3 status), 5-year CNS relapse was 15% for patients in the AALL0622 study vs 6.6% for patients in the AALL031 study.
However, 5-year OS rates were similar in the two groups of patients: 86% for AALL0622 vs 81% for AALL0031.
HSCT
AALL0622 allowed the use of hematopoietic stem cell transplantation (HSCT) in high-risk patients as well as in standard-risk patients with a sibling donor.
Five-year OS and EFS for standard-risk patients (19% underwent HSCT at first remission) and high-risk patients (91% underwent HSCT in first remission) were similar.
Children who did not undergo HSCT had a similar 5-year OS of 88%, which suggested that children with Ph+ ALL should not undergo transplantation at first remission.
Samples from a subset of patients was analyzed for IKZF1 mutations and correlated with outcomes.
Five-year OS was 80% in those harboring the mutation versus 100% who had the wild-type gene (P=0.04); 4-year EFS was also significantly lower—10% vs 82% (P=0.04).
Screening for IKZF1 may be used to identify high-risk patients suitable for HSCT and/or alternate treatment, the authors note.
The investigators reported their findings in The Journal of Clinical Oncology.
Emicizumab granted priority review for hemophilia A without inhibitors
The US Food and Drug Administration (FDA) has granted priority review for emicizumab (Hemlibra®) for adults and children with hemophilia A without factor VIII inhibitors.
Earlier this year, the agency awarded emicizumab breakthrough therapy designation for the same population.
Emicizumab is a bispecific factor IXa- and factor X-directed antibody approved by the FDA for routine prophylaxis to prevent or reduce the frequency of bleeding episodes in adults and children who have hemophilia A with factor VIII inhibitors.
The FDA based its decision to grant emicizumab priority review on the phase 3 HAVEN 3 study, results of which were presented recently at the World Federation of Hemophilia congress.
In HAVEN 3, emicizumab demonstrated a 68% reduction (P<0.0001) in treated bleeds based on an intra-patient comparison in patients who were previously enrolled in a prospective non-interventional study.
According to Genentech, co-developer of the drug, this makes emicizumab the first medicine to show superior efficacy to prior treatment with factor VIII prophylaxis, the current standard of care for people with hemophilia A without factor VIII inhibitors.
About HAVEN 3
The randomized, multicenter, open-label trial evaluated prophylaxis versus no prophylaxis in patients without factor VIII inhibitors.
The study included 152 patients 12 years or older who were previously treated with factor VIII therapy on-demand or as prophylaxis.
Patients previously treated with on-demand factor VIII were randomized in a 2:2:1 fashion to 1 of 3 treatment groups:
- Arm A received emicizumab prophylaxis at 3 mg/kg/wk for 4 weeks, followed by 1.5 mg/kg/wk until the end of study.
- Arm B received emicizumab prophylaxis at 3 mg/kg/wk for 4 weeks, followed by 3 mg/kg/2wks for at least 24 weeks.
- Arm C received no prophylaxis
Patients previously treated prophylactically with factor VIII were enrolled in Arm D and received emicizumab prophylaxis at 3 mg/kg/wk for 4 weeks, followed by 1.5 mg/kg/wk until the end of study.
The protocol permitted episodic treatment of breakthrough bleeds with factor VIII therapy.
Patients in the prophylaxis groups achieved a 96% (P<0.0001) and 97% (P<0.0001) reduction in treated bleeds, respectively, compared to those who received no prophylaxis.
Additionally, 55.6% of patients treated weekly and 60% treated every 2 weeks had no treated bleeds. In contrast, 0% in the prophylaxis group achieved zero treated bleeds.
Investigators observed no unexpected or serious adverse events (AEs), no thrombotic events, and no cases of thrombotic microangiopathy.
The most common AEs occurring in 5% or more of patients were injection site reactions, arthralgia, nasopharyngitis, headache, upper respiratory tract infection, and influenza.
The FDA is expected to make a decision regarding approval by October 4.
The US Food and Drug Administration (FDA) has granted priority review for emicizumab (Hemlibra®) for adults and children with hemophilia A without factor VIII inhibitors.
Earlier this year, the agency awarded emicizumab breakthrough therapy designation for the same population.
Emicizumab is a bispecific factor IXa- and factor X-directed antibody approved by the FDA for routine prophylaxis to prevent or reduce the frequency of bleeding episodes in adults and children who have hemophilia A with factor VIII inhibitors.
The FDA based its decision to grant emicizumab priority review on the phase 3 HAVEN 3 study, results of which were presented recently at the World Federation of Hemophilia congress.
In HAVEN 3, emicizumab demonstrated a 68% reduction (P<0.0001) in treated bleeds based on an intra-patient comparison in patients who were previously enrolled in a prospective non-interventional study.
According to Genentech, co-developer of the drug, this makes emicizumab the first medicine to show superior efficacy to prior treatment with factor VIII prophylaxis, the current standard of care for people with hemophilia A without factor VIII inhibitors.
About HAVEN 3
The randomized, multicenter, open-label trial evaluated prophylaxis versus no prophylaxis in patients without factor VIII inhibitors.
The study included 152 patients 12 years or older who were previously treated with factor VIII therapy on-demand or as prophylaxis.
Patients previously treated with on-demand factor VIII were randomized in a 2:2:1 fashion to 1 of 3 treatment groups:
- Arm A received emicizumab prophylaxis at 3 mg/kg/wk for 4 weeks, followed by 1.5 mg/kg/wk until the end of study.
- Arm B received emicizumab prophylaxis at 3 mg/kg/wk for 4 weeks, followed by 3 mg/kg/2wks for at least 24 weeks.
- Arm C received no prophylaxis
Patients previously treated prophylactically with factor VIII were enrolled in Arm D and received emicizumab prophylaxis at 3 mg/kg/wk for 4 weeks, followed by 1.5 mg/kg/wk until the end of study.
The protocol permitted episodic treatment of breakthrough bleeds with factor VIII therapy.
Patients in the prophylaxis groups achieved a 96% (P<0.0001) and 97% (P<0.0001) reduction in treated bleeds, respectively, compared to those who received no prophylaxis.
Additionally, 55.6% of patients treated weekly and 60% treated every 2 weeks had no treated bleeds. In contrast, 0% in the prophylaxis group achieved zero treated bleeds.
Investigators observed no unexpected or serious adverse events (AEs), no thrombotic events, and no cases of thrombotic microangiopathy.
The most common AEs occurring in 5% or more of patients were injection site reactions, arthralgia, nasopharyngitis, headache, upper respiratory tract infection, and influenza.
The FDA is expected to make a decision regarding approval by October 4.
The US Food and Drug Administration (FDA) has granted priority review for emicizumab (Hemlibra®) for adults and children with hemophilia A without factor VIII inhibitors.
Earlier this year, the agency awarded emicizumab breakthrough therapy designation for the same population.
Emicizumab is a bispecific factor IXa- and factor X-directed antibody approved by the FDA for routine prophylaxis to prevent or reduce the frequency of bleeding episodes in adults and children who have hemophilia A with factor VIII inhibitors.
The FDA based its decision to grant emicizumab priority review on the phase 3 HAVEN 3 study, results of which were presented recently at the World Federation of Hemophilia congress.
In HAVEN 3, emicizumab demonstrated a 68% reduction (P<0.0001) in treated bleeds based on an intra-patient comparison in patients who were previously enrolled in a prospective non-interventional study.
According to Genentech, co-developer of the drug, this makes emicizumab the first medicine to show superior efficacy to prior treatment with factor VIII prophylaxis, the current standard of care for people with hemophilia A without factor VIII inhibitors.
About HAVEN 3
The randomized, multicenter, open-label trial evaluated prophylaxis versus no prophylaxis in patients without factor VIII inhibitors.
The study included 152 patients 12 years or older who were previously treated with factor VIII therapy on-demand or as prophylaxis.
Patients previously treated with on-demand factor VIII were randomized in a 2:2:1 fashion to 1 of 3 treatment groups:
- Arm A received emicizumab prophylaxis at 3 mg/kg/wk for 4 weeks, followed by 1.5 mg/kg/wk until the end of study.
- Arm B received emicizumab prophylaxis at 3 mg/kg/wk for 4 weeks, followed by 3 mg/kg/2wks for at least 24 weeks.
- Arm C received no prophylaxis
Patients previously treated prophylactically with factor VIII were enrolled in Arm D and received emicizumab prophylaxis at 3 mg/kg/wk for 4 weeks, followed by 1.5 mg/kg/wk until the end of study.
The protocol permitted episodic treatment of breakthrough bleeds with factor VIII therapy.
Patients in the prophylaxis groups achieved a 96% (P<0.0001) and 97% (P<0.0001) reduction in treated bleeds, respectively, compared to those who received no prophylaxis.
Additionally, 55.6% of patients treated weekly and 60% treated every 2 weeks had no treated bleeds. In contrast, 0% in the prophylaxis group achieved zero treated bleeds.
Investigators observed no unexpected or serious adverse events (AEs), no thrombotic events, and no cases of thrombotic microangiopathy.
The most common AEs occurring in 5% or more of patients were injection site reactions, arthralgia, nasopharyngitis, headache, upper respiratory tract infection, and influenza.
The FDA is expected to make a decision regarding approval by October 4.
CAR T-cell technology making headway in MM
Chimeric antigen receptor (CAR) T-cell technology has been successfully used in the treatment of hematologic malignancies such as leukemias and lymphomas. Now, this technology has come to the forefront in multiple myeloma (MM).
Researchers at the National Cancer Institute, led by James N. Kochenderfer, MD, generated CAR T cells expressing the B-cell maturation antigen (BCMA), which is uniquely found on MM cells.
In the first in humans study, 24 patients received CAR-BCMA T cells at doses ranging between 0.3 and 3 x 106 CAR+ T cells/kg. Sixteen patients were treated at the highest dose. These patients had a median of 9.5 lines of prior therapy and 63% were refractory to their last treatment.
Thirteen patients had responses that were either partial or better and the overall response rate was 81%. Median event-free survival was 31 weeks. At the time of the report, 6 patients still have ongoing responses.
Patient cases
The report highlights a case study of a patient who had a large abdominal mass that resolved on computed tomography imaging, with λ light chains decreasing dramatically and becoming undetectable after CAR T-cell infusion. Recovery of normal plasma cells was noticeable with λ chain increases, but the ratio of κ to λ ratio remained normal 6 months after CAR T-cell infusion.
Using immunohistochemistry staining of CS138 before and 2 months after CAR-BMCA infusion, the researchers found effective depletion in bone marrow plasma cells in patients who were evaluated.
In patients who responded to treatment, decline in serum BCMA was also observed. However, in a patient who later progressed, BCMA increases were seen, leading the researchers to suggest that BCMA may be a tumor marker for MM.
The researchers noted that peak levels of CAR T cells occurred between 7 and 14 days after infusion and highest levels were seen in patients who showed antimyeloma responses.
Toxicity
CAR T-cell technology is also associated with accompanying toxicities.
At the highest dose, cytokine release syndrome (CRS) was a substantial toxicity especially in 2 patients who had a significant MM burden: in one case 80% of bone marrow cells were MM plasma cells and in the other the MM burden was 90%.
Six of the 16 patients required vasopressor support for hypotension and 1 patient required mechanical ventilation. The researchers noted that CRS of grade 3/4 was seen in patients who had a higher MM plasma cell burden.
The researchers indicated there is room for improvement. “The importance of persistence of CAR T cells in treating MM requires additional study,” they stated.
The sometimes low or absence of BCMA expression on MM cells may prompt the search for other antigens. “Treatment outcomes varied substantially between patients, and much room for improvement remains in improving the durability of antimyeloma responses and in reducing toxicity,” they concluded.
The researchers reported their findings in the Journal of Clinical Oncology.
Chimeric antigen receptor (CAR) T-cell technology has been successfully used in the treatment of hematologic malignancies such as leukemias and lymphomas. Now, this technology has come to the forefront in multiple myeloma (MM).
Researchers at the National Cancer Institute, led by James N. Kochenderfer, MD, generated CAR T cells expressing the B-cell maturation antigen (BCMA), which is uniquely found on MM cells.
In the first in humans study, 24 patients received CAR-BCMA T cells at doses ranging between 0.3 and 3 x 106 CAR+ T cells/kg. Sixteen patients were treated at the highest dose. These patients had a median of 9.5 lines of prior therapy and 63% were refractory to their last treatment.
Thirteen patients had responses that were either partial or better and the overall response rate was 81%. Median event-free survival was 31 weeks. At the time of the report, 6 patients still have ongoing responses.
Patient cases
The report highlights a case study of a patient who had a large abdominal mass that resolved on computed tomography imaging, with λ light chains decreasing dramatically and becoming undetectable after CAR T-cell infusion. Recovery of normal plasma cells was noticeable with λ chain increases, but the ratio of κ to λ ratio remained normal 6 months after CAR T-cell infusion.
Using immunohistochemistry staining of CS138 before and 2 months after CAR-BMCA infusion, the researchers found effective depletion in bone marrow plasma cells in patients who were evaluated.
In patients who responded to treatment, decline in serum BCMA was also observed. However, in a patient who later progressed, BCMA increases were seen, leading the researchers to suggest that BCMA may be a tumor marker for MM.
The researchers noted that peak levels of CAR T cells occurred between 7 and 14 days after infusion and highest levels were seen in patients who showed antimyeloma responses.
Toxicity
CAR T-cell technology is also associated with accompanying toxicities.
At the highest dose, cytokine release syndrome (CRS) was a substantial toxicity especially in 2 patients who had a significant MM burden: in one case 80% of bone marrow cells were MM plasma cells and in the other the MM burden was 90%.
Six of the 16 patients required vasopressor support for hypotension and 1 patient required mechanical ventilation. The researchers noted that CRS of grade 3/4 was seen in patients who had a higher MM plasma cell burden.
The researchers indicated there is room for improvement. “The importance of persistence of CAR T cells in treating MM requires additional study,” they stated.
The sometimes low or absence of BCMA expression on MM cells may prompt the search for other antigens. “Treatment outcomes varied substantially between patients, and much room for improvement remains in improving the durability of antimyeloma responses and in reducing toxicity,” they concluded.
The researchers reported their findings in the Journal of Clinical Oncology.
Chimeric antigen receptor (CAR) T-cell technology has been successfully used in the treatment of hematologic malignancies such as leukemias and lymphomas. Now, this technology has come to the forefront in multiple myeloma (MM).
Researchers at the National Cancer Institute, led by James N. Kochenderfer, MD, generated CAR T cells expressing the B-cell maturation antigen (BCMA), which is uniquely found on MM cells.
In the first in humans study, 24 patients received CAR-BCMA T cells at doses ranging between 0.3 and 3 x 106 CAR+ T cells/kg. Sixteen patients were treated at the highest dose. These patients had a median of 9.5 lines of prior therapy and 63% were refractory to their last treatment.
Thirteen patients had responses that were either partial or better and the overall response rate was 81%. Median event-free survival was 31 weeks. At the time of the report, 6 patients still have ongoing responses.
Patient cases
The report highlights a case study of a patient who had a large abdominal mass that resolved on computed tomography imaging, with λ light chains decreasing dramatically and becoming undetectable after CAR T-cell infusion. Recovery of normal plasma cells was noticeable with λ chain increases, but the ratio of κ to λ ratio remained normal 6 months after CAR T-cell infusion.
Using immunohistochemistry staining of CS138 before and 2 months after CAR-BMCA infusion, the researchers found effective depletion in bone marrow plasma cells in patients who were evaluated.
In patients who responded to treatment, decline in serum BCMA was also observed. However, in a patient who later progressed, BCMA increases were seen, leading the researchers to suggest that BCMA may be a tumor marker for MM.
The researchers noted that peak levels of CAR T cells occurred between 7 and 14 days after infusion and highest levels were seen in patients who showed antimyeloma responses.
Toxicity
CAR T-cell technology is also associated with accompanying toxicities.
At the highest dose, cytokine release syndrome (CRS) was a substantial toxicity especially in 2 patients who had a significant MM burden: in one case 80% of bone marrow cells were MM plasma cells and in the other the MM burden was 90%.
Six of the 16 patients required vasopressor support for hypotension and 1 patient required mechanical ventilation. The researchers noted that CRS of grade 3/4 was seen in patients who had a higher MM plasma cell burden.
The researchers indicated there is room for improvement. “The importance of persistence of CAR T cells in treating MM requires additional study,” they stated.
The sometimes low or absence of BCMA expression on MM cells may prompt the search for other antigens. “Treatment outcomes varied substantially between patients, and much room for improvement remains in improving the durability of antimyeloma responses and in reducing toxicity,” they concluded.
The researchers reported their findings in the Journal of Clinical Oncology.
FDA approves first biosimilar pegfilgrastim
The US Food and Drug Association (FDA) has approved pegfilgrastim-jmdb (Fulphila™) as the first biosimilar to Neulasta®.
The agents reduce the risk of infection or the duration of febrile neutropenia in patients treated with immunosuppressive chemotherapy for non-myeloid hematologic malignancies.
The FDA approved Fulphila based on evidence that included extensive structural and functional characterization, animal study data, human pharmacokinetic and pharmacodynamic data, clinical immunogenicity data, and other clinical safety and effectiveness data.
The evidence demonstrated that Fulphila is biosimilar to Amgen’s Neulasta. The FDA, in its announcement, noted that Fulphila has been approved as a biosimilar and not as an interchangeable product.
A biosimilar is a biological product approved based on data showing it is highly similar to a biological product already approved by the FDA, termed the reference product.
A biosimilar has no clinically meaningful differences from the reference product in terms of safety, purity, and effectiveness.
Common side effects of Fulphila include bone pain and pain in extremities.
The FDA cautions that patients with a history of serious allergic reaction to human granulocyte colony-stimulating factors, such as pegfilgrastim or filgrastim products, should not take Fulphila.
Serious side effects from Fulphila include:
- rupture of the spleen
- acute respiratory distress syndrome
- serious allergic reactions including anaphylaxis
- glomerulonephritis
- leukocytosis
- capillary leak syndrome
- potential for tumor growth
Fatal sickle cell crises have also occurred with Fulphila use.
Fulphila is not indicated for the mobilization of peripheral blood progenitor cells for hematopoietic stem cell transplantation.
The FDA is planning to release a comprehensive new plan to advance policy efforts that promote biosimilar product development, according to FDA Commissioner Scott Gotlieb, MD.
“We want to make sure that the pathway for developing biosimilar versions of approved biologics is efficient and effective, so that patients benefit from competition to existing biologics once lawful intellectual property has lapsed on these products,” he said in the announcement.
The FDA granted approval of Fulphila to Mylan GmbH. Mylan is co-developing Fulphila with Biocon.
Last fall, the agency had issued a complete response letter saying it could not approve the proposed biosimilar pending an update to the application.
The complete response letter did not raise any questions on the biosimilarity of Fulphila (investigational drug product MYL-1401H), pharmacokinetic/pharmacodynamic data, clinical data, or immunogenicity, however.
Mylan anticipates launching Fulphila in the coming weeks.
The US Food and Drug Association (FDA) has approved pegfilgrastim-jmdb (Fulphila™) as the first biosimilar to Neulasta®.
The agents reduce the risk of infection or the duration of febrile neutropenia in patients treated with immunosuppressive chemotherapy for non-myeloid hematologic malignancies.
The FDA approved Fulphila based on evidence that included extensive structural and functional characterization, animal study data, human pharmacokinetic and pharmacodynamic data, clinical immunogenicity data, and other clinical safety and effectiveness data.
The evidence demonstrated that Fulphila is biosimilar to Amgen’s Neulasta. The FDA, in its announcement, noted that Fulphila has been approved as a biosimilar and not as an interchangeable product.
A biosimilar is a biological product approved based on data showing it is highly similar to a biological product already approved by the FDA, termed the reference product.
A biosimilar has no clinically meaningful differences from the reference product in terms of safety, purity, and effectiveness.
Common side effects of Fulphila include bone pain and pain in extremities.
The FDA cautions that patients with a history of serious allergic reaction to human granulocyte colony-stimulating factors, such as pegfilgrastim or filgrastim products, should not take Fulphila.
Serious side effects from Fulphila include:
- rupture of the spleen
- acute respiratory distress syndrome
- serious allergic reactions including anaphylaxis
- glomerulonephritis
- leukocytosis
- capillary leak syndrome
- potential for tumor growth
Fatal sickle cell crises have also occurred with Fulphila use.
Fulphila is not indicated for the mobilization of peripheral blood progenitor cells for hematopoietic stem cell transplantation.
The FDA is planning to release a comprehensive new plan to advance policy efforts that promote biosimilar product development, according to FDA Commissioner Scott Gotlieb, MD.
“We want to make sure that the pathway for developing biosimilar versions of approved biologics is efficient and effective, so that patients benefit from competition to existing biologics once lawful intellectual property has lapsed on these products,” he said in the announcement.
The FDA granted approval of Fulphila to Mylan GmbH. Mylan is co-developing Fulphila with Biocon.
Last fall, the agency had issued a complete response letter saying it could not approve the proposed biosimilar pending an update to the application.
The complete response letter did not raise any questions on the biosimilarity of Fulphila (investigational drug product MYL-1401H), pharmacokinetic/pharmacodynamic data, clinical data, or immunogenicity, however.
Mylan anticipates launching Fulphila in the coming weeks.
The US Food and Drug Association (FDA) has approved pegfilgrastim-jmdb (Fulphila™) as the first biosimilar to Neulasta®.
The agents reduce the risk of infection or the duration of febrile neutropenia in patients treated with immunosuppressive chemotherapy for non-myeloid hematologic malignancies.
The FDA approved Fulphila based on evidence that included extensive structural and functional characterization, animal study data, human pharmacokinetic and pharmacodynamic data, clinical immunogenicity data, and other clinical safety and effectiveness data.
The evidence demonstrated that Fulphila is biosimilar to Amgen’s Neulasta. The FDA, in its announcement, noted that Fulphila has been approved as a biosimilar and not as an interchangeable product.
A biosimilar is a biological product approved based on data showing it is highly similar to a biological product already approved by the FDA, termed the reference product.
A biosimilar has no clinically meaningful differences from the reference product in terms of safety, purity, and effectiveness.
Common side effects of Fulphila include bone pain and pain in extremities.
The FDA cautions that patients with a history of serious allergic reaction to human granulocyte colony-stimulating factors, such as pegfilgrastim or filgrastim products, should not take Fulphila.
Serious side effects from Fulphila include:
- rupture of the spleen
- acute respiratory distress syndrome
- serious allergic reactions including anaphylaxis
- glomerulonephritis
- leukocytosis
- capillary leak syndrome
- potential for tumor growth
Fatal sickle cell crises have also occurred with Fulphila use.
Fulphila is not indicated for the mobilization of peripheral blood progenitor cells for hematopoietic stem cell transplantation.
The FDA is planning to release a comprehensive new plan to advance policy efforts that promote biosimilar product development, according to FDA Commissioner Scott Gotlieb, MD.
“We want to make sure that the pathway for developing biosimilar versions of approved biologics is efficient and effective, so that patients benefit from competition to existing biologics once lawful intellectual property has lapsed on these products,” he said in the announcement.
The FDA granted approval of Fulphila to Mylan GmbH. Mylan is co-developing Fulphila with Biocon.
Last fall, the agency had issued a complete response letter saying it could not approve the proposed biosimilar pending an update to the application.
The complete response letter did not raise any questions on the biosimilarity of Fulphila (investigational drug product MYL-1401H), pharmacokinetic/pharmacodynamic data, clinical data, or immunogenicity, however.
Mylan anticipates launching Fulphila in the coming weeks.
Over 1100 new meds, vaccines being developed to treat cancer
Currently, 1,120 new medicines and vaccines are being developed to treat cancer, according to a new report of the Pharmaceutical Research and Manufacturers of America (PhRMA).
And all of them, the organization states, are in clinical trials or awaiting review by the US Food and Drug Administration (FDA).
Leading the way are treatments for solid tumors, with 397 in development. Treatments for blood cancers are not far behind, with nearly 340 medicines in development: 137 for leukemias, 135 for lymphomas, and 62 for multiple myeloma.
Immuno-oncology and personalized medicine have a hand in this increase.
In the last year, according to PhRMA’s "Medicines in Development for Cancer 2018 Report," 47 new immune-oncology treatments have been added to the development pipeline, including CAR-T therapies and checkpoint inhibitors.
This brings the total to 295 immuno-oncology medicines and vaccines in the development pipeline this year.
The report also states that about 85% of these medicines in the oncology pipeline are first-in-class.
And PhRMA attributes the approximately 73% of survival gains in cancer to the new medicines.
Despite the bright picture, PhRMA acknowledges the financial burden and medical care challenges patients encounter.
It addresses them in a new chart pack, "Cancer Medicines: Value in Context," which puts cancer costs in perspective and offers solutions for improving the current system in the United States.
The association reports the top medical financial concerns of patients to be diagnostic tests or scans (53%), prescription medicines (43%), physician office visits (39%), outpatient treatments-including radiation (37%), and surgery (36%).
Spending on cancer medicines represents about 1% of overall healthcare spending, according to the organization, with cancer medications accounting for $49.8 billion of the $3.49 trillion healthcare spending in the United States.
Cancer medicines represent about 20% of spending on cancer, PhrMA notes, and some insurance plans place treatments for certain high-cost conditions on the highest drug formulary cost-sharing tier.
And patients with the highest copay were 5 times more likely to abandon treatment than the lowest copay group, PhRMA points out.
“No patient should struggle to afford their needed treatments,” PhRMA stated in a release, “and it is important that we address patient access challenges.”
Currently, 1,120 new medicines and vaccines are being developed to treat cancer, according to a new report of the Pharmaceutical Research and Manufacturers of America (PhRMA).
And all of them, the organization states, are in clinical trials or awaiting review by the US Food and Drug Administration (FDA).
Leading the way are treatments for solid tumors, with 397 in development. Treatments for blood cancers are not far behind, with nearly 340 medicines in development: 137 for leukemias, 135 for lymphomas, and 62 for multiple myeloma.
Immuno-oncology and personalized medicine have a hand in this increase.
In the last year, according to PhRMA’s "Medicines in Development for Cancer 2018 Report," 47 new immune-oncology treatments have been added to the development pipeline, including CAR-T therapies and checkpoint inhibitors.
This brings the total to 295 immuno-oncology medicines and vaccines in the development pipeline this year.
The report also states that about 85% of these medicines in the oncology pipeline are first-in-class.
And PhRMA attributes the approximately 73% of survival gains in cancer to the new medicines.
Despite the bright picture, PhRMA acknowledges the financial burden and medical care challenges patients encounter.
It addresses them in a new chart pack, "Cancer Medicines: Value in Context," which puts cancer costs in perspective and offers solutions for improving the current system in the United States.
The association reports the top medical financial concerns of patients to be diagnostic tests or scans (53%), prescription medicines (43%), physician office visits (39%), outpatient treatments-including radiation (37%), and surgery (36%).
Spending on cancer medicines represents about 1% of overall healthcare spending, according to the organization, with cancer medications accounting for $49.8 billion of the $3.49 trillion healthcare spending in the United States.
Cancer medicines represent about 20% of spending on cancer, PhrMA notes, and some insurance plans place treatments for certain high-cost conditions on the highest drug formulary cost-sharing tier.
And patients with the highest copay were 5 times more likely to abandon treatment than the lowest copay group, PhRMA points out.
“No patient should struggle to afford their needed treatments,” PhRMA stated in a release, “and it is important that we address patient access challenges.”
Currently, 1,120 new medicines and vaccines are being developed to treat cancer, according to a new report of the Pharmaceutical Research and Manufacturers of America (PhRMA).
And all of them, the organization states, are in clinical trials or awaiting review by the US Food and Drug Administration (FDA).
Leading the way are treatments for solid tumors, with 397 in development. Treatments for blood cancers are not far behind, with nearly 340 medicines in development: 137 for leukemias, 135 for lymphomas, and 62 for multiple myeloma.
Immuno-oncology and personalized medicine have a hand in this increase.
In the last year, according to PhRMA’s "Medicines in Development for Cancer 2018 Report," 47 new immune-oncology treatments have been added to the development pipeline, including CAR-T therapies and checkpoint inhibitors.
This brings the total to 295 immuno-oncology medicines and vaccines in the development pipeline this year.
The report also states that about 85% of these medicines in the oncology pipeline are first-in-class.
And PhRMA attributes the approximately 73% of survival gains in cancer to the new medicines.
Despite the bright picture, PhRMA acknowledges the financial burden and medical care challenges patients encounter.
It addresses them in a new chart pack, "Cancer Medicines: Value in Context," which puts cancer costs in perspective and offers solutions for improving the current system in the United States.
The association reports the top medical financial concerns of patients to be diagnostic tests or scans (53%), prescription medicines (43%), physician office visits (39%), outpatient treatments-including radiation (37%), and surgery (36%).
Spending on cancer medicines represents about 1% of overall healthcare spending, according to the organization, with cancer medications accounting for $49.8 billion of the $3.49 trillion healthcare spending in the United States.
Cancer medicines represent about 20% of spending on cancer, PhrMA notes, and some insurance plans place treatments for certain high-cost conditions on the highest drug formulary cost-sharing tier.
And patients with the highest copay were 5 times more likely to abandon treatment than the lowest copay group, PhRMA points out.
“No patient should struggle to afford their needed treatments,” PhRMA stated in a release, “and it is important that we address patient access challenges.”
Eltrombopag receives priority review designation for SAA
Eltrombopag (Promacta®) in combination with standard immunosuppressive therapy (IST) has received priority review designation from the US Food and Drug Administration (FDA) for first-line treatment of severe aplastic anemia (SAA).
The drug is already approved for SAA in the refractory setting for patients who have had an insufficient response to IST.
And it is approved for adults and children with chronic immune thrombocytopenia (ITP) who are refractory to other treatments and for patients with chronic hepatitis C virus infection who are thrombocytopenic.
Eltrombopag, an oral thrombopoietin receptor agonist, had received breakthrough therapy designation from the FDA earlier this year for use in combination with IST as first-line treatment of SAA.
The priority review designation for the agent as a first-line treatment for SAA is supported by data from a phase 1/2 trial published in NEJM in April 2017 and subsequent data on file with Novartis.
The FDA intends to take action on a priority review application within 6 months of receiving it, rather than the standard 10 months.
The agency grants priority review to applications for products that may provide significant improvements in the treatment, diagnosis, or prevention of serious conditions.
Trial data
Ninety-two patients with previously untreated SAA were enrolled on the trial and received IST and eltrombopag in 3 different cohorts.
Cohorts varied by start day of eltrombopag and duration of eltrombopag therapy. Patients in cohort 1 received eltrombopag from day 14 to 6 months. Patients in cohort 2 received the drug from day 14 to 3 months. And patients in cohort 3 received eltrombopag from day 1 to 6 months.
The overall response rate (ORR) at 6 months was 80% (cohort 1), 87% (cohort 2), and 94% (cohort 3).
The complete response rate at 6 months was 33%, 26%, and 58% in the 3 cohorts, respectively.
At a median follow-up of 2 years, the overall survival rate was 97%.
In the corporate announcement of the priority review designation, Novartis reported an ORR of 85% at 6 months.
Adverse events included transient elevations in liver enzyme levels (7 patients) and 2 severe adverse events—grades 2 and 3 cutaneous eruption—related to eltrombopag that resulted in patients stopping the drug.
Eltrombopag is marketed as Revolade in countries outside the US.
Eltrombopag (Promacta®) in combination with standard immunosuppressive therapy (IST) has received priority review designation from the US Food and Drug Administration (FDA) for first-line treatment of severe aplastic anemia (SAA).
The drug is already approved for SAA in the refractory setting for patients who have had an insufficient response to IST.
And it is approved for adults and children with chronic immune thrombocytopenia (ITP) who are refractory to other treatments and for patients with chronic hepatitis C virus infection who are thrombocytopenic.
Eltrombopag, an oral thrombopoietin receptor agonist, had received breakthrough therapy designation from the FDA earlier this year for use in combination with IST as first-line treatment of SAA.
The priority review designation for the agent as a first-line treatment for SAA is supported by data from a phase 1/2 trial published in NEJM in April 2017 and subsequent data on file with Novartis.
The FDA intends to take action on a priority review application within 6 months of receiving it, rather than the standard 10 months.
The agency grants priority review to applications for products that may provide significant improvements in the treatment, diagnosis, or prevention of serious conditions.
Trial data
Ninety-two patients with previously untreated SAA were enrolled on the trial and received IST and eltrombopag in 3 different cohorts.
Cohorts varied by start day of eltrombopag and duration of eltrombopag therapy. Patients in cohort 1 received eltrombopag from day 14 to 6 months. Patients in cohort 2 received the drug from day 14 to 3 months. And patients in cohort 3 received eltrombopag from day 1 to 6 months.
The overall response rate (ORR) at 6 months was 80% (cohort 1), 87% (cohort 2), and 94% (cohort 3).
The complete response rate at 6 months was 33%, 26%, and 58% in the 3 cohorts, respectively.
At a median follow-up of 2 years, the overall survival rate was 97%.
In the corporate announcement of the priority review designation, Novartis reported an ORR of 85% at 6 months.
Adverse events included transient elevations in liver enzyme levels (7 patients) and 2 severe adverse events—grades 2 and 3 cutaneous eruption—related to eltrombopag that resulted in patients stopping the drug.
Eltrombopag is marketed as Revolade in countries outside the US.
Eltrombopag (Promacta®) in combination with standard immunosuppressive therapy (IST) has received priority review designation from the US Food and Drug Administration (FDA) for first-line treatment of severe aplastic anemia (SAA).
The drug is already approved for SAA in the refractory setting for patients who have had an insufficient response to IST.
And it is approved for adults and children with chronic immune thrombocytopenia (ITP) who are refractory to other treatments and for patients with chronic hepatitis C virus infection who are thrombocytopenic.
Eltrombopag, an oral thrombopoietin receptor agonist, had received breakthrough therapy designation from the FDA earlier this year for use in combination with IST as first-line treatment of SAA.
The priority review designation for the agent as a first-line treatment for SAA is supported by data from a phase 1/2 trial published in NEJM in April 2017 and subsequent data on file with Novartis.
The FDA intends to take action on a priority review application within 6 months of receiving it, rather than the standard 10 months.
The agency grants priority review to applications for products that may provide significant improvements in the treatment, diagnosis, or prevention of serious conditions.
Trial data
Ninety-two patients with previously untreated SAA were enrolled on the trial and received IST and eltrombopag in 3 different cohorts.
Cohorts varied by start day of eltrombopag and duration of eltrombopag therapy. Patients in cohort 1 received eltrombopag from day 14 to 6 months. Patients in cohort 2 received the drug from day 14 to 3 months. And patients in cohort 3 received eltrombopag from day 1 to 6 months.
The overall response rate (ORR) at 6 months was 80% (cohort 1), 87% (cohort 2), and 94% (cohort 3).
The complete response rate at 6 months was 33%, 26%, and 58% in the 3 cohorts, respectively.
At a median follow-up of 2 years, the overall survival rate was 97%.
In the corporate announcement of the priority review designation, Novartis reported an ORR of 85% at 6 months.
Adverse events included transient elevations in liver enzyme levels (7 patients) and 2 severe adverse events—grades 2 and 3 cutaneous eruption—related to eltrombopag that resulted in patients stopping the drug.
Eltrombopag is marketed as Revolade in countries outside the US.