User login
GO approved to treat AML in Europe
The European Commission has authorized use of gemtuzumab ozogamicin (GO, Mylotarg™) as a treatment for patients with acute myeloid leukemia (AML).
GO is now approved for use in combination with daunorubicin and cytarabine to treat patients age 15 and older who have previously untreated, de novo, CD33-positive AML, not including acute promyelocytic leukemia.
GO is an antibody-drug conjugate composed of the cytotoxic agent calicheamicin attached to a monoclonal antibody targeting CD33, an antigen expressed on the surface of myeloblasts in up to 90% of AML patients.
When GO binds to the CD33 antigen on the cell surface, it is absorbed into the cell, and calicheamicin is released, causing cell death.
Previous rejection
The European Commission’s approval of GO follows a positive opinion from the European Medicines Agency’s Committee for Medicinal Products for Human Use (CHMP). In February, the CHMP recommended that GO receive marketing authorization for the aforementioned indication.
However, the CHMP previously issued a negative opinion of GO (first in 2007, confirmed in 2008), saying the drug should not receive marketing authorization.
The proposed indication for GO at that time was as re-induction treatment in adults with CD33-positive AML in first relapse who were not candidates for other intensive re-induction chemotherapy regimens and were either older than 60 or had a duration of first remission lasting less than 12 months.
The CHMP said there was insufficient evidence to establish the effectiveness of GO in AML, and the drug’s benefits did not outweigh its risks.
Phase 3 trial
The current marketing authorization application for GO is supported by data from an investigator-led, phase 3, randomized trial known as ALFA-0701. Updated results from this trial are available in the US prescribing information for GO.
ALFA-0701 included 271 patients with newly diagnosed, de novo AML who were 50 to 70 years of age.
Patients were randomized (1:1) to receive induction consisting of daunorubicin (60 mg/m2 on days 1 to 3) and cytarabine (200 mg/m2 on days 1 to 7) with (n=135) or without (n=136) GO at 3 mg/m2 (up to a maximum of 1 vial) on days 1, 4, and 7. Patients who did not achieve a response after first induction could receive a second induction with daunorubicin and cytarabine alone.
Patients with a response received consolidation therapy with 2 courses of treatment including daunorubicin (60 mg/m2 on day 1 of first consolidation course; 60 mg/m2 on days 1 and 2 of second consolidation course) and cytarabine (1 g/m2 every 12 hours on days 1 to 4) with or without GO at 3 mg/m2 (up to a maximum of 1 vial) on day 1 according to their initial randomization.
Patients who achieved remission were also eligible for allogeneic transplant. An interval of at least 2 months between the last dose of GO and transplant was recommended.
Baseline characteristics were largely well balanced between the treatment arms, but there was a higher percentage of males in the GO arm than the control arm—55% and 44%, respectively.
The study’s primary endpoint was event-free survival. The median event-free survival was 17.3 months in the GO arm and 9.5 months in the control arm (hazard ratio=0.56; 95% CI: 0.42-0.76; P<0.001).
There was no significant difference in overall survival between the treatment arms. (Updated overall survival data have not been released).
All patients in this trial developed severe neutropenia, thrombocytopenia, and anemia. However, the incidence of prolonged, grade 3–4 thrombocytopenia in the absence of active leukemia was higher in the GO arm.
Treatment-emergent adverse events (AEs) considered most important for understanding the safety profile of GO were hemorrhage, veno-occlusive liver disease (VOD), and severe infections.
Treatment discontinuation due to any AE occurred in 31% of patients in the GO arm and 7% of those in the control arm. The most frequent AEs leading to discontinuation for patients on GO were thrombocytopenia (15%), VOD (3%), and septic shock (2%).
Fatal AEs occurred in 8 patients (6%) in the GO arm and 3 (2%) in the control arm. In the GO arm, 3 patients died of VOD, 4 died of hemorrhage-related events, and 1 died of a suspected cardiac cause. All 3 fatal AEs in the control arm were sepsis.
The European Commission has authorized use of gemtuzumab ozogamicin (GO, Mylotarg™) as a treatment for patients with acute myeloid leukemia (AML).
GO is now approved for use in combination with daunorubicin and cytarabine to treat patients age 15 and older who have previously untreated, de novo, CD33-positive AML, not including acute promyelocytic leukemia.
GO is an antibody-drug conjugate composed of the cytotoxic agent calicheamicin attached to a monoclonal antibody targeting CD33, an antigen expressed on the surface of myeloblasts in up to 90% of AML patients.
When GO binds to the CD33 antigen on the cell surface, it is absorbed into the cell, and calicheamicin is released, causing cell death.
Previous rejection
The European Commission’s approval of GO follows a positive opinion from the European Medicines Agency’s Committee for Medicinal Products for Human Use (CHMP). In February, the CHMP recommended that GO receive marketing authorization for the aforementioned indication.
However, the CHMP previously issued a negative opinion of GO (first in 2007, confirmed in 2008), saying the drug should not receive marketing authorization.
The proposed indication for GO at that time was as re-induction treatment in adults with CD33-positive AML in first relapse who were not candidates for other intensive re-induction chemotherapy regimens and were either older than 60 or had a duration of first remission lasting less than 12 months.
The CHMP said there was insufficient evidence to establish the effectiveness of GO in AML, and the drug’s benefits did not outweigh its risks.
Phase 3 trial
The current marketing authorization application for GO is supported by data from an investigator-led, phase 3, randomized trial known as ALFA-0701. Updated results from this trial are available in the US prescribing information for GO.
ALFA-0701 included 271 patients with newly diagnosed, de novo AML who were 50 to 70 years of age.
Patients were randomized (1:1) to receive induction consisting of daunorubicin (60 mg/m2 on days 1 to 3) and cytarabine (200 mg/m2 on days 1 to 7) with (n=135) or without (n=136) GO at 3 mg/m2 (up to a maximum of 1 vial) on days 1, 4, and 7. Patients who did not achieve a response after first induction could receive a second induction with daunorubicin and cytarabine alone.
Patients with a response received consolidation therapy with 2 courses of treatment including daunorubicin (60 mg/m2 on day 1 of first consolidation course; 60 mg/m2 on days 1 and 2 of second consolidation course) and cytarabine (1 g/m2 every 12 hours on days 1 to 4) with or without GO at 3 mg/m2 (up to a maximum of 1 vial) on day 1 according to their initial randomization.
Patients who achieved remission were also eligible for allogeneic transplant. An interval of at least 2 months between the last dose of GO and transplant was recommended.
Baseline characteristics were largely well balanced between the treatment arms, but there was a higher percentage of males in the GO arm than the control arm—55% and 44%, respectively.
The study’s primary endpoint was event-free survival. The median event-free survival was 17.3 months in the GO arm and 9.5 months in the control arm (hazard ratio=0.56; 95% CI: 0.42-0.76; P<0.001).
There was no significant difference in overall survival between the treatment arms. (Updated overall survival data have not been released).
All patients in this trial developed severe neutropenia, thrombocytopenia, and anemia. However, the incidence of prolonged, grade 3–4 thrombocytopenia in the absence of active leukemia was higher in the GO arm.
Treatment-emergent adverse events (AEs) considered most important for understanding the safety profile of GO were hemorrhage, veno-occlusive liver disease (VOD), and severe infections.
Treatment discontinuation due to any AE occurred in 31% of patients in the GO arm and 7% of those in the control arm. The most frequent AEs leading to discontinuation for patients on GO were thrombocytopenia (15%), VOD (3%), and septic shock (2%).
Fatal AEs occurred in 8 patients (6%) in the GO arm and 3 (2%) in the control arm. In the GO arm, 3 patients died of VOD, 4 died of hemorrhage-related events, and 1 died of a suspected cardiac cause. All 3 fatal AEs in the control arm were sepsis.
The European Commission has authorized use of gemtuzumab ozogamicin (GO, Mylotarg™) as a treatment for patients with acute myeloid leukemia (AML).
GO is now approved for use in combination with daunorubicin and cytarabine to treat patients age 15 and older who have previously untreated, de novo, CD33-positive AML, not including acute promyelocytic leukemia.
GO is an antibody-drug conjugate composed of the cytotoxic agent calicheamicin attached to a monoclonal antibody targeting CD33, an antigen expressed on the surface of myeloblasts in up to 90% of AML patients.
When GO binds to the CD33 antigen on the cell surface, it is absorbed into the cell, and calicheamicin is released, causing cell death.
Previous rejection
The European Commission’s approval of GO follows a positive opinion from the European Medicines Agency’s Committee for Medicinal Products for Human Use (CHMP). In February, the CHMP recommended that GO receive marketing authorization for the aforementioned indication.
However, the CHMP previously issued a negative opinion of GO (first in 2007, confirmed in 2008), saying the drug should not receive marketing authorization.
The proposed indication for GO at that time was as re-induction treatment in adults with CD33-positive AML in first relapse who were not candidates for other intensive re-induction chemotherapy regimens and were either older than 60 or had a duration of first remission lasting less than 12 months.
The CHMP said there was insufficient evidence to establish the effectiveness of GO in AML, and the drug’s benefits did not outweigh its risks.
Phase 3 trial
The current marketing authorization application for GO is supported by data from an investigator-led, phase 3, randomized trial known as ALFA-0701. Updated results from this trial are available in the US prescribing information for GO.
ALFA-0701 included 271 patients with newly diagnosed, de novo AML who were 50 to 70 years of age.
Patients were randomized (1:1) to receive induction consisting of daunorubicin (60 mg/m2 on days 1 to 3) and cytarabine (200 mg/m2 on days 1 to 7) with (n=135) or without (n=136) GO at 3 mg/m2 (up to a maximum of 1 vial) on days 1, 4, and 7. Patients who did not achieve a response after first induction could receive a second induction with daunorubicin and cytarabine alone.
Patients with a response received consolidation therapy with 2 courses of treatment including daunorubicin (60 mg/m2 on day 1 of first consolidation course; 60 mg/m2 on days 1 and 2 of second consolidation course) and cytarabine (1 g/m2 every 12 hours on days 1 to 4) with or without GO at 3 mg/m2 (up to a maximum of 1 vial) on day 1 according to their initial randomization.
Patients who achieved remission were also eligible for allogeneic transplant. An interval of at least 2 months between the last dose of GO and transplant was recommended.
Baseline characteristics were largely well balanced between the treatment arms, but there was a higher percentage of males in the GO arm than the control arm—55% and 44%, respectively.
The study’s primary endpoint was event-free survival. The median event-free survival was 17.3 months in the GO arm and 9.5 months in the control arm (hazard ratio=0.56; 95% CI: 0.42-0.76; P<0.001).
There was no significant difference in overall survival between the treatment arms. (Updated overall survival data have not been released).
All patients in this trial developed severe neutropenia, thrombocytopenia, and anemia. However, the incidence of prolonged, grade 3–4 thrombocytopenia in the absence of active leukemia was higher in the GO arm.
Treatment-emergent adverse events (AEs) considered most important for understanding the safety profile of GO were hemorrhage, veno-occlusive liver disease (VOD), and severe infections.
Treatment discontinuation due to any AE occurred in 31% of patients in the GO arm and 7% of those in the control arm. The most frequent AEs leading to discontinuation for patients on GO were thrombocytopenia (15%), VOD (3%), and septic shock (2%).
Fatal AEs occurred in 8 patients (6%) in the GO arm and 3 (2%) in the control arm. In the GO arm, 3 patients died of VOD, 4 died of hemorrhage-related events, and 1 died of a suspected cardiac cause. All 3 fatal AEs in the control arm were sepsis.
Exercise linked to risk of death in cancer patients
CHICAGO—Researchers have identified a link between habitual physical activity (PA) and mortality among cancer patients.
Engaging in regular PA, both pre- and post-diagnosis, was associated with a significantly lower risk of death for the entire population studied and for patients with 8 specific types of cancer.
However, the association was not significant for patients with other cancer types, including hematologic malignancies.
Rikki Cannioto, PhD, of Roswell Park Comprehensive Cancer Center in Buffalo, New York, and her colleagues presented these findings at the AACR Annual Meeting 2018 (abstract 5254*).
The researchers examined the association between habitual PA and outcomes in 5807 cancer patients enrolled in the Data Bank and BioRepository at Roswell Park between 2003 and 2016.
The population was 54.8% female and 93% white. The average age at diagnosis was 60.6 years.
The researchers looked at patterns of PA over time, from the decade before the cancer was diagnosed and continuing for up to 1 year after diagnosis.
Patients who engaged in regular, moderate- to vigorous-intensity PA (such as walking, running, aerobics, or other cardiovascular exercise) both before and after their diagnosis were considered habitually active, whereas those who did not exercise regularly were considered habitually inactive.
Overall, 52% of patients reported habitual activity, and 19% reported habitual inactivity. Twenty-three percent of patients said their activity level decreased after diagnosis, and 6% said their activity level increased.
Patients were followed through January 31, 2018. The median time to follow-up was 53 months, and 33.7% of patients (n=1956) died during the follow-up period.
Results
The researchers found that patients who were active before and after diagnosis were 40% more likely to survive than those who were habitually inactive (P<0.001). Habitually inactive patients had a 66% increased risk of mortality compared to active patients.
The habitually active patients had a 37-month mean survival advantage over the inactive patients.
In addition, patients whose activity level increased after diagnosis had a 25% lower risk of death than patients who remained inactive after diagnosis.
The researchers observed a significant (P<0.05) association between habitual PA and decreased mortality in patients with breast, colon, prostate, bladder, endometrial, ovarian, esophageal, and skin cancers.
However, the association between PA and mortality was not significant for patients with hematologic malignancies (P=0.59) or kidney, liver, lung, pancreas, stomach, or “other” cancers.
The researchers said the associations between habitual PA and decreased mortality remained consistent regardless of a patient’s sex, tumor stage, smoking status, or body mass index.
“[W]hen it comes to exercise, something is better than nothing, but regular, weekly exercise seems to really make a difference,” Dr Cannioto said.
“In fact, patients who were physically active 3 or 4 days a week experienced an even greater benefit than those who exercised daily, and patients who had only 1 or 2 days of regular activity per week did nearly as well. This is particularly encouraging, as cancer patients and survivors can be overwhelmed by current physical activity recommendations.”
*Information in the abstract differs from the presentation.
CHICAGO—Researchers have identified a link between habitual physical activity (PA) and mortality among cancer patients.
Engaging in regular PA, both pre- and post-diagnosis, was associated with a significantly lower risk of death for the entire population studied and for patients with 8 specific types of cancer.
However, the association was not significant for patients with other cancer types, including hematologic malignancies.
Rikki Cannioto, PhD, of Roswell Park Comprehensive Cancer Center in Buffalo, New York, and her colleagues presented these findings at the AACR Annual Meeting 2018 (abstract 5254*).
The researchers examined the association between habitual PA and outcomes in 5807 cancer patients enrolled in the Data Bank and BioRepository at Roswell Park between 2003 and 2016.
The population was 54.8% female and 93% white. The average age at diagnosis was 60.6 years.
The researchers looked at patterns of PA over time, from the decade before the cancer was diagnosed and continuing for up to 1 year after diagnosis.
Patients who engaged in regular, moderate- to vigorous-intensity PA (such as walking, running, aerobics, or other cardiovascular exercise) both before and after their diagnosis were considered habitually active, whereas those who did not exercise regularly were considered habitually inactive.
Overall, 52% of patients reported habitual activity, and 19% reported habitual inactivity. Twenty-three percent of patients said their activity level decreased after diagnosis, and 6% said their activity level increased.
Patients were followed through January 31, 2018. The median time to follow-up was 53 months, and 33.7% of patients (n=1956) died during the follow-up period.
Results
The researchers found that patients who were active before and after diagnosis were 40% more likely to survive than those who were habitually inactive (P<0.001). Habitually inactive patients had a 66% increased risk of mortality compared to active patients.
The habitually active patients had a 37-month mean survival advantage over the inactive patients.
In addition, patients whose activity level increased after diagnosis had a 25% lower risk of death than patients who remained inactive after diagnosis.
The researchers observed a significant (P<0.05) association between habitual PA and decreased mortality in patients with breast, colon, prostate, bladder, endometrial, ovarian, esophageal, and skin cancers.
However, the association between PA and mortality was not significant for patients with hematologic malignancies (P=0.59) or kidney, liver, lung, pancreas, stomach, or “other” cancers.
The researchers said the associations between habitual PA and decreased mortality remained consistent regardless of a patient’s sex, tumor stage, smoking status, or body mass index.
“[W]hen it comes to exercise, something is better than nothing, but regular, weekly exercise seems to really make a difference,” Dr Cannioto said.
“In fact, patients who were physically active 3 or 4 days a week experienced an even greater benefit than those who exercised daily, and patients who had only 1 or 2 days of regular activity per week did nearly as well. This is particularly encouraging, as cancer patients and survivors can be overwhelmed by current physical activity recommendations.”
*Information in the abstract differs from the presentation.
CHICAGO—Researchers have identified a link between habitual physical activity (PA) and mortality among cancer patients.
Engaging in regular PA, both pre- and post-diagnosis, was associated with a significantly lower risk of death for the entire population studied and for patients with 8 specific types of cancer.
However, the association was not significant for patients with other cancer types, including hematologic malignancies.
Rikki Cannioto, PhD, of Roswell Park Comprehensive Cancer Center in Buffalo, New York, and her colleagues presented these findings at the AACR Annual Meeting 2018 (abstract 5254*).
The researchers examined the association between habitual PA and outcomes in 5807 cancer patients enrolled in the Data Bank and BioRepository at Roswell Park between 2003 and 2016.
The population was 54.8% female and 93% white. The average age at diagnosis was 60.6 years.
The researchers looked at patterns of PA over time, from the decade before the cancer was diagnosed and continuing for up to 1 year after diagnosis.
Patients who engaged in regular, moderate- to vigorous-intensity PA (such as walking, running, aerobics, or other cardiovascular exercise) both before and after their diagnosis were considered habitually active, whereas those who did not exercise regularly were considered habitually inactive.
Overall, 52% of patients reported habitual activity, and 19% reported habitual inactivity. Twenty-three percent of patients said their activity level decreased after diagnosis, and 6% said their activity level increased.
Patients were followed through January 31, 2018. The median time to follow-up was 53 months, and 33.7% of patients (n=1956) died during the follow-up period.
Results
The researchers found that patients who were active before and after diagnosis were 40% more likely to survive than those who were habitually inactive (P<0.001). Habitually inactive patients had a 66% increased risk of mortality compared to active patients.
The habitually active patients had a 37-month mean survival advantage over the inactive patients.
In addition, patients whose activity level increased after diagnosis had a 25% lower risk of death than patients who remained inactive after diagnosis.
The researchers observed a significant (P<0.05) association between habitual PA and decreased mortality in patients with breast, colon, prostate, bladder, endometrial, ovarian, esophageal, and skin cancers.
However, the association between PA and mortality was not significant for patients with hematologic malignancies (P=0.59) or kidney, liver, lung, pancreas, stomach, or “other” cancers.
The researchers said the associations between habitual PA and decreased mortality remained consistent regardless of a patient’s sex, tumor stage, smoking status, or body mass index.
“[W]hen it comes to exercise, something is better than nothing, but regular, weekly exercise seems to really make a difference,” Dr Cannioto said.
“In fact, patients who were physically active 3 or 4 days a week experienced an even greater benefit than those who exercised daily, and patients who had only 1 or 2 days of regular activity per week did nearly as well. This is particularly encouraging, as cancer patients and survivors can be overwhelmed by current physical activity recommendations.”
*Information in the abstract differs from the presentation.
Company stops development of drug for AL amyloidosis
Prothena Corporation plc is discontinuing development of NEOD001, an investigational antibody intended for the treatment of AL amyloidosis.
The company’s decision was based on results from the phase 2b PRONTO study and a futility analysis of the phase 3 VITAL study.
NEOD001 did not meet the primary or secondary endpoints of the PRONTO study, so Prothena asked an independent data monitoring committee to review a futility analysis of the ongoing VITAL study.
The committee recommended discontinuation of the VITAL study, so Prothena decided to discontinue all development of NEOD001, including the VITAL study and open-label extension studies.
“We are deeply disappointed by this outcome, particularly for patients suffering from this devastating disease,” said Gene Kinney, PhD, president and chief executive officer of Prothena.
“We are surprised by the results from these 2 placebo-controlled studies and will continue to analyze the resulting data to share insights with our collaborators in the scientific, medical, and advocacy communities.”
Phase 3 VITAL study
The VITAL study was a multicenter, randomized, double-blind, placebo-controlled study of NEOD001 in treatment-naïve patients with AL amyloidosis and cardiac dysfunction.
The study enrolled 260 patients who were randomized on a 1:1 basis to receive 24 mg/kg of NEOD001 or placebo via intravenous infusion every 28 days. Patients in both arms received concurrent standard of care therapy.
The composite primary endpoint was event-based, with all-cause mortality or cardiac hospitalizations counted as events.
The futility analysis, based on 103 adjudicated events of the 156 events specified to complete the study, was not statistically significant. The hazard ratio was 0.84 favoring NEOD001 versus the control arm.
Phase 2b PRONTO study
The PRONTO study was a multicenter, randomized, double-blind, placebo-controlled study of NEOD001 in previously treated patients with AL amyloidosis and persistent cardiac dysfunction.
The study enrolled 129 patients who were randomized on a 1:1 basis to receive 24 mg/kg of NEOD001 (n=66) or placebo (n=63) via intravenous infusion every 28 days.
There was no significant difference between the treatment arms for any of the study’s endpoints.
The primary endpoint was cardiac best response, as assessed by N-terminal pro B-type natriuretic peptide (NT-proBNP) through 12 months of treatment. This endpoint was achieved by 39.4% of patients in the NEOD001 arm and 47.6% in the placebo arm.
The NT-proBNP rate of change (slope) through 12 months of treatment was 9.80 in the NEOD001 arm and 81.42 in the placebo arm.
The mean change in Short-form 36 Physical Component Summary Score after 12 months of treatment was 0.19 and 0.97, respectively.
The median change in 6-Minute Walk Test distance after 12 months of treatment was 19.25 m and 8.00 m, respectively.
And the mean change in Neuropathy Impairment Score of the Lower Limb after 12 months of treatment was -1.20 and -0.60, respectively.
The rate of renal best response (as assessed by proteinuria and estimated glomerular filtration rate) through 12 months of treatment was 53.8% in the NEOD001 arm and 33.3% in the placebo arm.
The rate of all-cause mortality was 4.5% (n=3) and 9.5% (n=6), respectively.
Prothena said NEOD001 was generally safe and well tolerated in this trial.
Prothena Corporation plc is discontinuing development of NEOD001, an investigational antibody intended for the treatment of AL amyloidosis.
The company’s decision was based on results from the phase 2b PRONTO study and a futility analysis of the phase 3 VITAL study.
NEOD001 did not meet the primary or secondary endpoints of the PRONTO study, so Prothena asked an independent data monitoring committee to review a futility analysis of the ongoing VITAL study.
The committee recommended discontinuation of the VITAL study, so Prothena decided to discontinue all development of NEOD001, including the VITAL study and open-label extension studies.
“We are deeply disappointed by this outcome, particularly for patients suffering from this devastating disease,” said Gene Kinney, PhD, president and chief executive officer of Prothena.
“We are surprised by the results from these 2 placebo-controlled studies and will continue to analyze the resulting data to share insights with our collaborators in the scientific, medical, and advocacy communities.”
Phase 3 VITAL study
The VITAL study was a multicenter, randomized, double-blind, placebo-controlled study of NEOD001 in treatment-naïve patients with AL amyloidosis and cardiac dysfunction.
The study enrolled 260 patients who were randomized on a 1:1 basis to receive 24 mg/kg of NEOD001 or placebo via intravenous infusion every 28 days. Patients in both arms received concurrent standard of care therapy.
The composite primary endpoint was event-based, with all-cause mortality or cardiac hospitalizations counted as events.
The futility analysis, based on 103 adjudicated events of the 156 events specified to complete the study, was not statistically significant. The hazard ratio was 0.84 favoring NEOD001 versus the control arm.
Phase 2b PRONTO study
The PRONTO study was a multicenter, randomized, double-blind, placebo-controlled study of NEOD001 in previously treated patients with AL amyloidosis and persistent cardiac dysfunction.
The study enrolled 129 patients who were randomized on a 1:1 basis to receive 24 mg/kg of NEOD001 (n=66) or placebo (n=63) via intravenous infusion every 28 days.
There was no significant difference between the treatment arms for any of the study’s endpoints.
The primary endpoint was cardiac best response, as assessed by N-terminal pro B-type natriuretic peptide (NT-proBNP) through 12 months of treatment. This endpoint was achieved by 39.4% of patients in the NEOD001 arm and 47.6% in the placebo arm.
The NT-proBNP rate of change (slope) through 12 months of treatment was 9.80 in the NEOD001 arm and 81.42 in the placebo arm.
The mean change in Short-form 36 Physical Component Summary Score after 12 months of treatment was 0.19 and 0.97, respectively.
The median change in 6-Minute Walk Test distance after 12 months of treatment was 19.25 m and 8.00 m, respectively.
And the mean change in Neuropathy Impairment Score of the Lower Limb after 12 months of treatment was -1.20 and -0.60, respectively.
The rate of renal best response (as assessed by proteinuria and estimated glomerular filtration rate) through 12 months of treatment was 53.8% in the NEOD001 arm and 33.3% in the placebo arm.
The rate of all-cause mortality was 4.5% (n=3) and 9.5% (n=6), respectively.
Prothena said NEOD001 was generally safe and well tolerated in this trial.
Prothena Corporation plc is discontinuing development of NEOD001, an investigational antibody intended for the treatment of AL amyloidosis.
The company’s decision was based on results from the phase 2b PRONTO study and a futility analysis of the phase 3 VITAL study.
NEOD001 did not meet the primary or secondary endpoints of the PRONTO study, so Prothena asked an independent data monitoring committee to review a futility analysis of the ongoing VITAL study.
The committee recommended discontinuation of the VITAL study, so Prothena decided to discontinue all development of NEOD001, including the VITAL study and open-label extension studies.
“We are deeply disappointed by this outcome, particularly for patients suffering from this devastating disease,” said Gene Kinney, PhD, president and chief executive officer of Prothena.
“We are surprised by the results from these 2 placebo-controlled studies and will continue to analyze the resulting data to share insights with our collaborators in the scientific, medical, and advocacy communities.”
Phase 3 VITAL study
The VITAL study was a multicenter, randomized, double-blind, placebo-controlled study of NEOD001 in treatment-naïve patients with AL amyloidosis and cardiac dysfunction.
The study enrolled 260 patients who were randomized on a 1:1 basis to receive 24 mg/kg of NEOD001 or placebo via intravenous infusion every 28 days. Patients in both arms received concurrent standard of care therapy.
The composite primary endpoint was event-based, with all-cause mortality or cardiac hospitalizations counted as events.
The futility analysis, based on 103 adjudicated events of the 156 events specified to complete the study, was not statistically significant. The hazard ratio was 0.84 favoring NEOD001 versus the control arm.
Phase 2b PRONTO study
The PRONTO study was a multicenter, randomized, double-blind, placebo-controlled study of NEOD001 in previously treated patients with AL amyloidosis and persistent cardiac dysfunction.
The study enrolled 129 patients who were randomized on a 1:1 basis to receive 24 mg/kg of NEOD001 (n=66) or placebo (n=63) via intravenous infusion every 28 days.
There was no significant difference between the treatment arms for any of the study’s endpoints.
The primary endpoint was cardiac best response, as assessed by N-terminal pro B-type natriuretic peptide (NT-proBNP) through 12 months of treatment. This endpoint was achieved by 39.4% of patients in the NEOD001 arm and 47.6% in the placebo arm.
The NT-proBNP rate of change (slope) through 12 months of treatment was 9.80 in the NEOD001 arm and 81.42 in the placebo arm.
The mean change in Short-form 36 Physical Component Summary Score after 12 months of treatment was 0.19 and 0.97, respectively.
The median change in 6-Minute Walk Test distance after 12 months of treatment was 19.25 m and 8.00 m, respectively.
And the mean change in Neuropathy Impairment Score of the Lower Limb after 12 months of treatment was -1.20 and -0.60, respectively.
The rate of renal best response (as assessed by proteinuria and estimated glomerular filtration rate) through 12 months of treatment was 53.8% in the NEOD001 arm and 33.3% in the placebo arm.
The rate of all-cause mortality was 4.5% (n=3) and 9.5% (n=6), respectively.
Prothena said NEOD001 was generally safe and well tolerated in this trial.
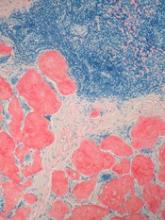
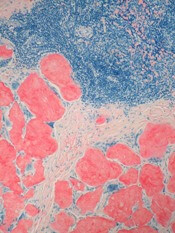
Art education benefits blood cancer patients
New research suggests a bedside visual art intervention (BVAI) can reduce pain and anxiety in inpatients with hematologic malignancies, including those undergoing transplant.
The BVAI involved an educator teaching patients art technique one-on-one for approximately 30 minutes.
After a single session, patients had significant improvements in positive mood and pain scores, as well as decreases in negative mood and anxiety.
Alexandra P. Wolanskyj, MD, of Mayo Clinic in Rochester, Minnesota, and her colleagues reported these results in the European Journal of Cancer Care.
The study included 21 patients, 19 of them female. Their median age was 53.5 (range, 19-75). Six patients were undergoing hematopoietic stem cell transplant.
The patients had multiple myeloma (n=5), acute myeloid leukemia (n=5), non-Hodgkin lymphoma (n=3), Hodgkin lymphoma (n=2), acute lymphoblastic leukemia (n=1), chronic lymphocytic leukemia (n=1), amyloidosis (n=1), Gardner-Diamond syndrome (n=1), myelodysplastic syndrome (n=1), and Waldenstrom’s macroglobulinemia (n=1).
Nearly half of patients had relapsed disease (47.6%), 23.8% had active and new disease, 19.0% had active disease with primary resistance on chemotherapy, and 9.5% of patients were in remission.
Intervention
The researchers recruited an educator from a community art center to teach art at the patients’ bedsides. Sessions were intended to be about 30 minutes. However, patients could stop at any time or continue beyond 30 minutes.
Patients and their families could make art or just observe. Materials used included watercolors, oil pastels, colored pencils, and clay (all non-toxic and odorless). The materials were left with patients so they could continue to use them after the sessions.
Results
The researchers assessed patients’ pain, anxiety, and mood at baseline and after the patients had a session with the art educator.
After the BVAI, patients had a significant decrease in pain, according to the Visual Analog Scale (VAS). The 14 patients who reported any pain at baseline had a mean reduction in VAS score of 1.5, or a 35.1% reduction in pain (P=0.017).
Patients had a 21.6% reduction in anxiety after the BVAI. Among the 20 patients who completed this assessment, there was a mean 9.2-point decrease in State-Trait Anxiety Inventory (STAI) score (P=0.001).
In addition, patients had a significant increase in positive mood and a significant decrease in negative mood after the BVAI. Mood was assessed in 20 patients using the Positive and Negative Affect Schedule (PANAS) scale.
Positive mood increased 14.6% (P=0.003), and negative mood decreased 18.0% (P=0.015) after the BVAI. Patients’ mean PANAS scores increased 4.6 points for positive mood and decreased 3.3 points for negative mood.
All 21 patients completed a questionnaire on the BVAI. All but 1 patient (95%) said the intervention was positive overall, and 85% of patients (n=18) said they would be interested in participating in future art-based interventions.
The researchers said these results suggest experiences provided by artists in the community may be an adjunct to conventional treatments in patients with cancer-related mood symptoms and pain.
New research suggests a bedside visual art intervention (BVAI) can reduce pain and anxiety in inpatients with hematologic malignancies, including those undergoing transplant.
The BVAI involved an educator teaching patients art technique one-on-one for approximately 30 minutes.
After a single session, patients had significant improvements in positive mood and pain scores, as well as decreases in negative mood and anxiety.
Alexandra P. Wolanskyj, MD, of Mayo Clinic in Rochester, Minnesota, and her colleagues reported these results in the European Journal of Cancer Care.
The study included 21 patients, 19 of them female. Their median age was 53.5 (range, 19-75). Six patients were undergoing hematopoietic stem cell transplant.
The patients had multiple myeloma (n=5), acute myeloid leukemia (n=5), non-Hodgkin lymphoma (n=3), Hodgkin lymphoma (n=2), acute lymphoblastic leukemia (n=1), chronic lymphocytic leukemia (n=1), amyloidosis (n=1), Gardner-Diamond syndrome (n=1), myelodysplastic syndrome (n=1), and Waldenstrom’s macroglobulinemia (n=1).
Nearly half of patients had relapsed disease (47.6%), 23.8% had active and new disease, 19.0% had active disease with primary resistance on chemotherapy, and 9.5% of patients were in remission.
Intervention
The researchers recruited an educator from a community art center to teach art at the patients’ bedsides. Sessions were intended to be about 30 minutes. However, patients could stop at any time or continue beyond 30 minutes.
Patients and their families could make art or just observe. Materials used included watercolors, oil pastels, colored pencils, and clay (all non-toxic and odorless). The materials were left with patients so they could continue to use them after the sessions.
Results
The researchers assessed patients’ pain, anxiety, and mood at baseline and after the patients had a session with the art educator.
After the BVAI, patients had a significant decrease in pain, according to the Visual Analog Scale (VAS). The 14 patients who reported any pain at baseline had a mean reduction in VAS score of 1.5, or a 35.1% reduction in pain (P=0.017).
Patients had a 21.6% reduction in anxiety after the BVAI. Among the 20 patients who completed this assessment, there was a mean 9.2-point decrease in State-Trait Anxiety Inventory (STAI) score (P=0.001).
In addition, patients had a significant increase in positive mood and a significant decrease in negative mood after the BVAI. Mood was assessed in 20 patients using the Positive and Negative Affect Schedule (PANAS) scale.
Positive mood increased 14.6% (P=0.003), and negative mood decreased 18.0% (P=0.015) after the BVAI. Patients’ mean PANAS scores increased 4.6 points for positive mood and decreased 3.3 points for negative mood.
All 21 patients completed a questionnaire on the BVAI. All but 1 patient (95%) said the intervention was positive overall, and 85% of patients (n=18) said they would be interested in participating in future art-based interventions.
The researchers said these results suggest experiences provided by artists in the community may be an adjunct to conventional treatments in patients with cancer-related mood symptoms and pain.
New research suggests a bedside visual art intervention (BVAI) can reduce pain and anxiety in inpatients with hematologic malignancies, including those undergoing transplant.
The BVAI involved an educator teaching patients art technique one-on-one for approximately 30 minutes.
After a single session, patients had significant improvements in positive mood and pain scores, as well as decreases in negative mood and anxiety.
Alexandra P. Wolanskyj, MD, of Mayo Clinic in Rochester, Minnesota, and her colleagues reported these results in the European Journal of Cancer Care.
The study included 21 patients, 19 of them female. Their median age was 53.5 (range, 19-75). Six patients were undergoing hematopoietic stem cell transplant.
The patients had multiple myeloma (n=5), acute myeloid leukemia (n=5), non-Hodgkin lymphoma (n=3), Hodgkin lymphoma (n=2), acute lymphoblastic leukemia (n=1), chronic lymphocytic leukemia (n=1), amyloidosis (n=1), Gardner-Diamond syndrome (n=1), myelodysplastic syndrome (n=1), and Waldenstrom’s macroglobulinemia (n=1).
Nearly half of patients had relapsed disease (47.6%), 23.8% had active and new disease, 19.0% had active disease with primary resistance on chemotherapy, and 9.5% of patients were in remission.
Intervention
The researchers recruited an educator from a community art center to teach art at the patients’ bedsides. Sessions were intended to be about 30 minutes. However, patients could stop at any time or continue beyond 30 minutes.
Patients and their families could make art or just observe. Materials used included watercolors, oil pastels, colored pencils, and clay (all non-toxic and odorless). The materials were left with patients so they could continue to use them after the sessions.
Results
The researchers assessed patients’ pain, anxiety, and mood at baseline and after the patients had a session with the art educator.
After the BVAI, patients had a significant decrease in pain, according to the Visual Analog Scale (VAS). The 14 patients who reported any pain at baseline had a mean reduction in VAS score of 1.5, or a 35.1% reduction in pain (P=0.017).
Patients had a 21.6% reduction in anxiety after the BVAI. Among the 20 patients who completed this assessment, there was a mean 9.2-point decrease in State-Trait Anxiety Inventory (STAI) score (P=0.001).
In addition, patients had a significant increase in positive mood and a significant decrease in negative mood after the BVAI. Mood was assessed in 20 patients using the Positive and Negative Affect Schedule (PANAS) scale.
Positive mood increased 14.6% (P=0.003), and negative mood decreased 18.0% (P=0.015) after the BVAI. Patients’ mean PANAS scores increased 4.6 points for positive mood and decreased 3.3 points for negative mood.
All 21 patients completed a questionnaire on the BVAI. All but 1 patient (95%) said the intervention was positive overall, and 85% of patients (n=18) said they would be interested in participating in future art-based interventions.
The researchers said these results suggest experiences provided by artists in the community may be an adjunct to conventional treatments in patients with cancer-related mood symptoms and pain.
Therapy shows early promise in phase 1 MM trial
CHICAGO—Early phase 1 results suggest a chimeric antigen receptor (CAR) T-cell therapy can induce tumor regression in heavily pretreated patients with multiple myeloma (MM).
The therapy, P-BCMA-101, has only been tested in 3 patients in the lowest dose cohort.
However, signs of efficacy have been seen in all 3 patients, including a lasting partial response in 1 patient.
There have been no dose-limiting toxicities, and none of the patients have developed cytokine release syndrome (CRS).
“The results from the first cohort of the phase 1 P-BCMA-101 study have surpassed historical benchmarks of safety and efficacy in multiple myeloma at this dose level and give us confidence to move ahead into additional dose cohorts,” said Eric Ostertag, MD, PhD, chief executive officer of Poseida Therapeutics, Inc.
Dr Ostertag and his colleagues presented these results at the AACR Annual Meeting 2018 (abstract CT130). The trial is sponsored by Poseida Therapeutics, Inc.
The trial is enrolling patients with relapsed/refractory MM who have received a proteasome inhibitor and immunomodulatory agent.
Conditioning consists of standard cyclophosphamide (300 mg/m2) and fludarabine (30 mg/m2) on days -5 to -3. Patients then receive a single dose of P-BCMA-101 on day 0.
The trial has a 3+3 dose-escalation design, with up to 6 dose levels. The first dose level is 0.75 x 106 P-BCMA-101 cells/kg.
The 3 patients who have received this dose had 6 to 9 prior therapies.
Patient 1
The first patient was a 54-year-old female with lambda light chain MM. She had t(11;14), del13q14, and 11q23(x3).
Before she received P-BCMA-101, the patient had an increase in free light chains (FLCs) to 3290 mg/L, which caused renal failure. She was treated with cyclophosphamide/prednisone and plasmapheresis bridging therapy before proceeding to P-BCMA-101.
The patient achieved a partial response 2 weeks after receiving P-BCMA-101, and this has persisted through week 12. She had a maximal reduction in urine M-protein of 92% and a reduction in plasma FLCs of 79%.
The patient did not experience any adverse events (AEs) considered related to P-BCMA-101.
Patient 2
The second patient was a 50-year-old female with oligosecretory kappa light chain MM and plasmacytomas. She had del17p (TP53) and 11q13(x3).
Due to enlarging plasmacytomas, the patient was treated with DT-PACE (dexamethasone plus thalidomide with cisplatin, doxorubicin, cyclophosphamide, and etoposide) before receiving P-BCMA-101.
Her bone plasmacytomas resolved to below background within 4 to 8 weeks of P-BCMA-101 administration, but 1 new non-bone lesion appeared months later.
AEs considered at least possibly related to treatment in this patient included grade 2-4 neutropenia as well as grade 3-4 thrombocytopenia.
Patient 3
The third patient was a 65-year-old female with lambda light chain MM and del13q14.
Her urine M-protein and FLCs briefly dipped and rose after she received bridging therapy with lenalidomide and dexamethasone, but they decreased at 4 weeks after P-BCMA-101 administration, which corresponded with P-BCMA-101 expansion in the peripheral blood.
AEs considered at least possibly related to treatment included easy bruising (grade 1), fatigue (grade 2), febrile neutropenia (grade 3), hypogammaglobinemia (grade 2), neutropenia (grade 2-3), and thrombocytopenia (grade 3-4).
“The lack of cytokine release syndrome in any of the 3 patients, in spite of marked efficacy, is unprecedented at this dose, which we believe is attributable to multiple differentiated aspects of our technology, resulting in a highly purified CAR T product with a high percentage of cells with a T stem cell memory phenotype,” Dr Ostertag said.
In addition to a lack of CRS, there were no dose-limiting toxicities. Therefore, the dose has been escalated to 2 x 106 P-BCMA-101+ CAR T cells/kg for the next patient cohort. The first patient has been treated at this dose level, with no CRS yet reported.
About P-BCMA-101
P-BCMA-101 employs a B-cell maturation antigen-specific Centyrin™ fused to a second-generation CAR scaffold (a CARTyrin) rather than a single-chain variable fragment (scFv).
Centyrins have similar binding affinities as scFvs but are said to be potentially less immunogenic than scFvs, more stable at the cell surface, and resistant to antigen/ligand-independent tonic signaling.
P-BCMA-101 is engineered using PiggyBac™, a transposon-based system requiring only mRNA and plasmid DNA (no virus).
The increased cargo capacity of PiggyBac allows for the incorporation of a safety switch and a selectable gene. The safety switch can be activated to enable depletion in case AEs occur. And the selectable gene allows for enrichment of CAR+ cells.
CHICAGO—Early phase 1 results suggest a chimeric antigen receptor (CAR) T-cell therapy can induce tumor regression in heavily pretreated patients with multiple myeloma (MM).
The therapy, P-BCMA-101, has only been tested in 3 patients in the lowest dose cohort.
However, signs of efficacy have been seen in all 3 patients, including a lasting partial response in 1 patient.
There have been no dose-limiting toxicities, and none of the patients have developed cytokine release syndrome (CRS).
“The results from the first cohort of the phase 1 P-BCMA-101 study have surpassed historical benchmarks of safety and efficacy in multiple myeloma at this dose level and give us confidence to move ahead into additional dose cohorts,” said Eric Ostertag, MD, PhD, chief executive officer of Poseida Therapeutics, Inc.
Dr Ostertag and his colleagues presented these results at the AACR Annual Meeting 2018 (abstract CT130). The trial is sponsored by Poseida Therapeutics, Inc.
The trial is enrolling patients with relapsed/refractory MM who have received a proteasome inhibitor and immunomodulatory agent.
Conditioning consists of standard cyclophosphamide (300 mg/m2) and fludarabine (30 mg/m2) on days -5 to -3. Patients then receive a single dose of P-BCMA-101 on day 0.
The trial has a 3+3 dose-escalation design, with up to 6 dose levels. The first dose level is 0.75 x 106 P-BCMA-101 cells/kg.
The 3 patients who have received this dose had 6 to 9 prior therapies.
Patient 1
The first patient was a 54-year-old female with lambda light chain MM. She had t(11;14), del13q14, and 11q23(x3).
Before she received P-BCMA-101, the patient had an increase in free light chains (FLCs) to 3290 mg/L, which caused renal failure. She was treated with cyclophosphamide/prednisone and plasmapheresis bridging therapy before proceeding to P-BCMA-101.
The patient achieved a partial response 2 weeks after receiving P-BCMA-101, and this has persisted through week 12. She had a maximal reduction in urine M-protein of 92% and a reduction in plasma FLCs of 79%.
The patient did not experience any adverse events (AEs) considered related to P-BCMA-101.
Patient 2
The second patient was a 50-year-old female with oligosecretory kappa light chain MM and plasmacytomas. She had del17p (TP53) and 11q13(x3).
Due to enlarging plasmacytomas, the patient was treated with DT-PACE (dexamethasone plus thalidomide with cisplatin, doxorubicin, cyclophosphamide, and etoposide) before receiving P-BCMA-101.
Her bone plasmacytomas resolved to below background within 4 to 8 weeks of P-BCMA-101 administration, but 1 new non-bone lesion appeared months later.
AEs considered at least possibly related to treatment in this patient included grade 2-4 neutropenia as well as grade 3-4 thrombocytopenia.
Patient 3
The third patient was a 65-year-old female with lambda light chain MM and del13q14.
Her urine M-protein and FLCs briefly dipped and rose after she received bridging therapy with lenalidomide and dexamethasone, but they decreased at 4 weeks after P-BCMA-101 administration, which corresponded with P-BCMA-101 expansion in the peripheral blood.
AEs considered at least possibly related to treatment included easy bruising (grade 1), fatigue (grade 2), febrile neutropenia (grade 3), hypogammaglobinemia (grade 2), neutropenia (grade 2-3), and thrombocytopenia (grade 3-4).
“The lack of cytokine release syndrome in any of the 3 patients, in spite of marked efficacy, is unprecedented at this dose, which we believe is attributable to multiple differentiated aspects of our technology, resulting in a highly purified CAR T product with a high percentage of cells with a T stem cell memory phenotype,” Dr Ostertag said.
In addition to a lack of CRS, there were no dose-limiting toxicities. Therefore, the dose has been escalated to 2 x 106 P-BCMA-101+ CAR T cells/kg for the next patient cohort. The first patient has been treated at this dose level, with no CRS yet reported.
About P-BCMA-101
P-BCMA-101 employs a B-cell maturation antigen-specific Centyrin™ fused to a second-generation CAR scaffold (a CARTyrin) rather than a single-chain variable fragment (scFv).
Centyrins have similar binding affinities as scFvs but are said to be potentially less immunogenic than scFvs, more stable at the cell surface, and resistant to antigen/ligand-independent tonic signaling.
P-BCMA-101 is engineered using PiggyBac™, a transposon-based system requiring only mRNA and plasmid DNA (no virus).
The increased cargo capacity of PiggyBac allows for the incorporation of a safety switch and a selectable gene. The safety switch can be activated to enable depletion in case AEs occur. And the selectable gene allows for enrichment of CAR+ cells.
CHICAGO—Early phase 1 results suggest a chimeric antigen receptor (CAR) T-cell therapy can induce tumor regression in heavily pretreated patients with multiple myeloma (MM).
The therapy, P-BCMA-101, has only been tested in 3 patients in the lowest dose cohort.
However, signs of efficacy have been seen in all 3 patients, including a lasting partial response in 1 patient.
There have been no dose-limiting toxicities, and none of the patients have developed cytokine release syndrome (CRS).
“The results from the first cohort of the phase 1 P-BCMA-101 study have surpassed historical benchmarks of safety and efficacy in multiple myeloma at this dose level and give us confidence to move ahead into additional dose cohorts,” said Eric Ostertag, MD, PhD, chief executive officer of Poseida Therapeutics, Inc.
Dr Ostertag and his colleagues presented these results at the AACR Annual Meeting 2018 (abstract CT130). The trial is sponsored by Poseida Therapeutics, Inc.
The trial is enrolling patients with relapsed/refractory MM who have received a proteasome inhibitor and immunomodulatory agent.
Conditioning consists of standard cyclophosphamide (300 mg/m2) and fludarabine (30 mg/m2) on days -5 to -3. Patients then receive a single dose of P-BCMA-101 on day 0.
The trial has a 3+3 dose-escalation design, with up to 6 dose levels. The first dose level is 0.75 x 106 P-BCMA-101 cells/kg.
The 3 patients who have received this dose had 6 to 9 prior therapies.
Patient 1
The first patient was a 54-year-old female with lambda light chain MM. She had t(11;14), del13q14, and 11q23(x3).
Before she received P-BCMA-101, the patient had an increase in free light chains (FLCs) to 3290 mg/L, which caused renal failure. She was treated with cyclophosphamide/prednisone and plasmapheresis bridging therapy before proceeding to P-BCMA-101.
The patient achieved a partial response 2 weeks after receiving P-BCMA-101, and this has persisted through week 12. She had a maximal reduction in urine M-protein of 92% and a reduction in plasma FLCs of 79%.
The patient did not experience any adverse events (AEs) considered related to P-BCMA-101.
Patient 2
The second patient was a 50-year-old female with oligosecretory kappa light chain MM and plasmacytomas. She had del17p (TP53) and 11q13(x3).
Due to enlarging plasmacytomas, the patient was treated with DT-PACE (dexamethasone plus thalidomide with cisplatin, doxorubicin, cyclophosphamide, and etoposide) before receiving P-BCMA-101.
Her bone plasmacytomas resolved to below background within 4 to 8 weeks of P-BCMA-101 administration, but 1 new non-bone lesion appeared months later.
AEs considered at least possibly related to treatment in this patient included grade 2-4 neutropenia as well as grade 3-4 thrombocytopenia.
Patient 3
The third patient was a 65-year-old female with lambda light chain MM and del13q14.
Her urine M-protein and FLCs briefly dipped and rose after she received bridging therapy with lenalidomide and dexamethasone, but they decreased at 4 weeks after P-BCMA-101 administration, which corresponded with P-BCMA-101 expansion in the peripheral blood.
AEs considered at least possibly related to treatment included easy bruising (grade 1), fatigue (grade 2), febrile neutropenia (grade 3), hypogammaglobinemia (grade 2), neutropenia (grade 2-3), and thrombocytopenia (grade 3-4).
“The lack of cytokine release syndrome in any of the 3 patients, in spite of marked efficacy, is unprecedented at this dose, which we believe is attributable to multiple differentiated aspects of our technology, resulting in a highly purified CAR T product with a high percentage of cells with a T stem cell memory phenotype,” Dr Ostertag said.
In addition to a lack of CRS, there were no dose-limiting toxicities. Therefore, the dose has been escalated to 2 x 106 P-BCMA-101+ CAR T cells/kg for the next patient cohort. The first patient has been treated at this dose level, with no CRS yet reported.
About P-BCMA-101
P-BCMA-101 employs a B-cell maturation antigen-specific Centyrin™ fused to a second-generation CAR scaffold (a CARTyrin) rather than a single-chain variable fragment (scFv).
Centyrins have similar binding affinities as scFvs but are said to be potentially less immunogenic than scFvs, more stable at the cell surface, and resistant to antigen/ligand-independent tonic signaling.
P-BCMA-101 is engineered using PiggyBac™, a transposon-based system requiring only mRNA and plasmid DNA (no virus).
The increased cargo capacity of PiggyBac allows for the incorporation of a safety switch and a selectable gene. The safety switch can be activated to enable depletion in case AEs occur. And the selectable gene allows for enrichment of CAR+ cells.
BET inhibitor has lasting effects in AML, MM
CHICAGO—A BET inhibitor can have potent and long-lasting effects against leukemia and multiple myeloma (MM), according to researchers.
The inhibitor, TG-1601 (or CK-103), exhibited cytotoxicity in MM and leukemia cell lines but did not affect the growth of normal cell lines.
TG-1601 also reduced tumor volume in mouse models of MM and acute myeloid leukemia (AML), and drug holidays had little impact on this activity.
Furthermore, researchers observed enduring MYC inhibition in mice treated with TG-1601.
This research was presented at the AACR Annual Meeting 2018 (abstract 5790).
The work was conducted by researchers from TG Therapeutics and Checkpoint Therapeutics—the companies developing TG-1601—as well as Jubilant Biosys.
In vitro activity
Researchers assessed the cytotoxic activity of TG-1601 in leukemia, MM, and normal cell lines by incubating the cells with increasing concentrations of the drug for 72 hours.
The results suggested TG-1601 inhibits MM and leukemia cell growth, as all EC50 values were below 100 nM.
In the leukemia cell lines, EC50 values were 35 nM (Jurkat), 31 nM (HEL92.1.7), 24 nM (CCRF-CEM and MV4-11), and 18 nM (OCI-AML3).
In the MM cell lines, EC50 values were 85 nM (RPMI8226), 32 nM (KMS28PE), 24 nM (KMS28BM), 21 nM (MOLP8), and 15 nM (MM1s).
In the normal cell lines (Beas2B and WT9-12), cell growth wasn’t inhibited more than 50% with TG-1601 at 10 μM.
In vivo activity
For their MM model, researchers used mice inoculated with MM1 cells. The mice received TG-1601 at 10 mg/kg twice a day.
At day 17 after treatment initiation, there was a 70% reduction in tumor volume. During a week-long drug holiday, tumors did not grow back as fast in TG-1601-treated mice as they did in vehicle control mice.
For their AML model, researchers used mice inoculated with MV4-11 cells. The mice received TG-1601 as a single dose of 20 mg/kg/day—continuously or with 2, 3, or 4 days off per week—or at 10 mg/kg twice a day.
At day 15, 100% of mice that received the drug at 10 mg/kg twice a day were tumor-free. Mice that received the single 20 mg/kg dose had a 94% reduction in tumor volume.
The reduction in tumor volume was 91% in mice with the 2-day drug holiday, 78% in those with the 3-day holiday, and 82% in those with the 4-day holiday.
The researchers also found that TG-1601 had synergistic antitumor activity with an anti-PD-1 antibody in a mouse model of melanoma.
Pharmacodynamic activity
In the MV4-11 cell line, TG-1601 induced “rapid” downregulation of MYC and BCL2 and an increase of p21 mRNA, according to the researchers.
The team also assessed MYC expression in mice with MV4-11 tumors. They said MYC levels rapidly decreased in the tumors and were undetectable at 3 hours after a single dose of TG-1601.
The researchers noted that, at 24 hours after dosing, TG-1601 was cleared from the tumor. However, MYC levels remained below 40% their initial level.
The team said this suggests a long-lasting effect of TG-1601 that may be attributed to its enhanced binding affinity.
“These data demonstrate [TG-1601’s] potential to be a novel BET inhibitor that potently inhibits MYC expression,” said James F. Oliviero, president and chief executive officer of Checkpoint Therapeutics.
“We believe the preclinical data presented today provides encouraging evidence to support the development of [TG-1601] as an anticancer agent, alone and in combination with our anti-PD-L1 antibody, and look forward to the advancement of [TG-1601] into a first-in-human phase 1 trial expected to commence later this year.”
CHICAGO—A BET inhibitor can have potent and long-lasting effects against leukemia and multiple myeloma (MM), according to researchers.
The inhibitor, TG-1601 (or CK-103), exhibited cytotoxicity in MM and leukemia cell lines but did not affect the growth of normal cell lines.
TG-1601 also reduced tumor volume in mouse models of MM and acute myeloid leukemia (AML), and drug holidays had little impact on this activity.
Furthermore, researchers observed enduring MYC inhibition in mice treated with TG-1601.
This research was presented at the AACR Annual Meeting 2018 (abstract 5790).
The work was conducted by researchers from TG Therapeutics and Checkpoint Therapeutics—the companies developing TG-1601—as well as Jubilant Biosys.
In vitro activity
Researchers assessed the cytotoxic activity of TG-1601 in leukemia, MM, and normal cell lines by incubating the cells with increasing concentrations of the drug for 72 hours.
The results suggested TG-1601 inhibits MM and leukemia cell growth, as all EC50 values were below 100 nM.
In the leukemia cell lines, EC50 values were 35 nM (Jurkat), 31 nM (HEL92.1.7), 24 nM (CCRF-CEM and MV4-11), and 18 nM (OCI-AML3).
In the MM cell lines, EC50 values were 85 nM (RPMI8226), 32 nM (KMS28PE), 24 nM (KMS28BM), 21 nM (MOLP8), and 15 nM (MM1s).
In the normal cell lines (Beas2B and WT9-12), cell growth wasn’t inhibited more than 50% with TG-1601 at 10 μM.
In vivo activity
For their MM model, researchers used mice inoculated with MM1 cells. The mice received TG-1601 at 10 mg/kg twice a day.
At day 17 after treatment initiation, there was a 70% reduction in tumor volume. During a week-long drug holiday, tumors did not grow back as fast in TG-1601-treated mice as they did in vehicle control mice.
For their AML model, researchers used mice inoculated with MV4-11 cells. The mice received TG-1601 as a single dose of 20 mg/kg/day—continuously or with 2, 3, or 4 days off per week—or at 10 mg/kg twice a day.
At day 15, 100% of mice that received the drug at 10 mg/kg twice a day were tumor-free. Mice that received the single 20 mg/kg dose had a 94% reduction in tumor volume.
The reduction in tumor volume was 91% in mice with the 2-day drug holiday, 78% in those with the 3-day holiday, and 82% in those with the 4-day holiday.
The researchers also found that TG-1601 had synergistic antitumor activity with an anti-PD-1 antibody in a mouse model of melanoma.
Pharmacodynamic activity
In the MV4-11 cell line, TG-1601 induced “rapid” downregulation of MYC and BCL2 and an increase of p21 mRNA, according to the researchers.
The team also assessed MYC expression in mice with MV4-11 tumors. They said MYC levels rapidly decreased in the tumors and were undetectable at 3 hours after a single dose of TG-1601.
The researchers noted that, at 24 hours after dosing, TG-1601 was cleared from the tumor. However, MYC levels remained below 40% their initial level.
The team said this suggests a long-lasting effect of TG-1601 that may be attributed to its enhanced binding affinity.
“These data demonstrate [TG-1601’s] potential to be a novel BET inhibitor that potently inhibits MYC expression,” said James F. Oliviero, president and chief executive officer of Checkpoint Therapeutics.
“We believe the preclinical data presented today provides encouraging evidence to support the development of [TG-1601] as an anticancer agent, alone and in combination with our anti-PD-L1 antibody, and look forward to the advancement of [TG-1601] into a first-in-human phase 1 trial expected to commence later this year.”
CHICAGO—A BET inhibitor can have potent and long-lasting effects against leukemia and multiple myeloma (MM), according to researchers.
The inhibitor, TG-1601 (or CK-103), exhibited cytotoxicity in MM and leukemia cell lines but did not affect the growth of normal cell lines.
TG-1601 also reduced tumor volume in mouse models of MM and acute myeloid leukemia (AML), and drug holidays had little impact on this activity.
Furthermore, researchers observed enduring MYC inhibition in mice treated with TG-1601.
This research was presented at the AACR Annual Meeting 2018 (abstract 5790).
The work was conducted by researchers from TG Therapeutics and Checkpoint Therapeutics—the companies developing TG-1601—as well as Jubilant Biosys.
In vitro activity
Researchers assessed the cytotoxic activity of TG-1601 in leukemia, MM, and normal cell lines by incubating the cells with increasing concentrations of the drug for 72 hours.
The results suggested TG-1601 inhibits MM and leukemia cell growth, as all EC50 values were below 100 nM.
In the leukemia cell lines, EC50 values were 35 nM (Jurkat), 31 nM (HEL92.1.7), 24 nM (CCRF-CEM and MV4-11), and 18 nM (OCI-AML3).
In the MM cell lines, EC50 values were 85 nM (RPMI8226), 32 nM (KMS28PE), 24 nM (KMS28BM), 21 nM (MOLP8), and 15 nM (MM1s).
In the normal cell lines (Beas2B and WT9-12), cell growth wasn’t inhibited more than 50% with TG-1601 at 10 μM.
In vivo activity
For their MM model, researchers used mice inoculated with MM1 cells. The mice received TG-1601 at 10 mg/kg twice a day.
At day 17 after treatment initiation, there was a 70% reduction in tumor volume. During a week-long drug holiday, tumors did not grow back as fast in TG-1601-treated mice as they did in vehicle control mice.
For their AML model, researchers used mice inoculated with MV4-11 cells. The mice received TG-1601 as a single dose of 20 mg/kg/day—continuously or with 2, 3, or 4 days off per week—or at 10 mg/kg twice a day.
At day 15, 100% of mice that received the drug at 10 mg/kg twice a day were tumor-free. Mice that received the single 20 mg/kg dose had a 94% reduction in tumor volume.
The reduction in tumor volume was 91% in mice with the 2-day drug holiday, 78% in those with the 3-day holiday, and 82% in those with the 4-day holiday.
The researchers also found that TG-1601 had synergistic antitumor activity with an anti-PD-1 antibody in a mouse model of melanoma.
Pharmacodynamic activity
In the MV4-11 cell line, TG-1601 induced “rapid” downregulation of MYC and BCL2 and an increase of p21 mRNA, according to the researchers.
The team also assessed MYC expression in mice with MV4-11 tumors. They said MYC levels rapidly decreased in the tumors and were undetectable at 3 hours after a single dose of TG-1601.
The researchers noted that, at 24 hours after dosing, TG-1601 was cleared from the tumor. However, MYC levels remained below 40% their initial level.
The team said this suggests a long-lasting effect of TG-1601 that may be attributed to its enhanced binding affinity.
“These data demonstrate [TG-1601’s] potential to be a novel BET inhibitor that potently inhibits MYC expression,” said James F. Oliviero, president and chief executive officer of Checkpoint Therapeutics.
“We believe the preclinical data presented today provides encouraging evidence to support the development of [TG-1601] as an anticancer agent, alone and in combination with our anti-PD-L1 antibody, and look forward to the advancement of [TG-1601] into a first-in-human phase 1 trial expected to commence later this year.”
Study reveals gene variants that predispose kids to ALL
Germline variants in IKZF1 can predispose carriers to acute lymphoblastic leukemia (ALL), according to a study published in Cancer Cell.
The research began with the discovery of an IKZF1 variant in 3 generations of a German family affected by pediatric ALL.
Researchers then analyzed data from nearly 5000 children with ALL and identified 27 additional germline variants in IKZF1.
These variants were present in 0.9% of the patients analyzed, and most of the patients with the variants had B-cell ALL.
“This finding adds to the growing body of evidence that, while germline variations still account for a small percentage of pediatric ALL cases overall, more children than previously recognized inherit a predisposition to develop ALL,” said Charles Mullighan, MBBS, MD, of St. Jude Children’s Research Hospital in Memphis, Tennessee.
In the Cancer Cell paper, Dr Mullighan and his colleagues report the discovery of a germline deletion variant in IKZF1 (c.del556 or D186fs), which was present in 3 generations of a family.
Two of the 6 family members with this variant had developed B-ALL as children and died. The remaining 4 subjects are apparently healthy, despite having reduced numbers of B cells.
To build upon this discovery, the researchers performed targeted sequencing of IKZF1 in 4963 children with ALL.
This revealed 27 additional IKZF1 variants in 43 patients, most of whom had B-ALL. (One patient had T-cell ALL, and, for 8 patients, their subtype was unknown.)
The researchers noted that the variants were distributed across the gene.
“The pattern of IKZF1 variants was surprising because many of the variants were in regions of the gene that are rarely mutated in leukemic cells,” said study author Jun J. Yang, PhD, of St. Jude. “These regions of the gene have not been well characterized.”
The researchers also found that 22 of the 28 IKZF1 variants adversely affect gene function, while the remaining 6 variants appear to be benign.
The team said the deleterious variants impair DNA binding and regulation of transcriptional targets, induce aberrant leukemic cell adhesion, and reduce ALL cells’ sensitivity to treatment with dasatinib and dexamethasone.
The researchers identified the most deleterious variants as 5 that are located outside of the zinc-finger domains (M31V, M347V, R423C, A434G, and L449F), 2 variants affecting the N-terminal DNA-binding domain (R162P and H163Y), 2 truncating nonsense variants (M306* and C394*), and the frameshift variant discovered in the German family (D186fs).
“This [research] will expand the number of genes to consider when screening for predisposition to leukemia, particularly B-ALL,” said study author Kim Nichols, of St. Jude.
“And while not everyone carrying a germline IKZF1 variant will develop leukemia, these results will help us educate families about the potential risk of leukemia.”
Germline variants in IKZF1 can predispose carriers to acute lymphoblastic leukemia (ALL), according to a study published in Cancer Cell.
The research began with the discovery of an IKZF1 variant in 3 generations of a German family affected by pediatric ALL.
Researchers then analyzed data from nearly 5000 children with ALL and identified 27 additional germline variants in IKZF1.
These variants were present in 0.9% of the patients analyzed, and most of the patients with the variants had B-cell ALL.
“This finding adds to the growing body of evidence that, while germline variations still account for a small percentage of pediatric ALL cases overall, more children than previously recognized inherit a predisposition to develop ALL,” said Charles Mullighan, MBBS, MD, of St. Jude Children’s Research Hospital in Memphis, Tennessee.
In the Cancer Cell paper, Dr Mullighan and his colleagues report the discovery of a germline deletion variant in IKZF1 (c.del556 or D186fs), which was present in 3 generations of a family.
Two of the 6 family members with this variant had developed B-ALL as children and died. The remaining 4 subjects are apparently healthy, despite having reduced numbers of B cells.
To build upon this discovery, the researchers performed targeted sequencing of IKZF1 in 4963 children with ALL.
This revealed 27 additional IKZF1 variants in 43 patients, most of whom had B-ALL. (One patient had T-cell ALL, and, for 8 patients, their subtype was unknown.)
The researchers noted that the variants were distributed across the gene.
“The pattern of IKZF1 variants was surprising because many of the variants were in regions of the gene that are rarely mutated in leukemic cells,” said study author Jun J. Yang, PhD, of St. Jude. “These regions of the gene have not been well characterized.”
The researchers also found that 22 of the 28 IKZF1 variants adversely affect gene function, while the remaining 6 variants appear to be benign.
The team said the deleterious variants impair DNA binding and regulation of transcriptional targets, induce aberrant leukemic cell adhesion, and reduce ALL cells’ sensitivity to treatment with dasatinib and dexamethasone.
The researchers identified the most deleterious variants as 5 that are located outside of the zinc-finger domains (M31V, M347V, R423C, A434G, and L449F), 2 variants affecting the N-terminal DNA-binding domain (R162P and H163Y), 2 truncating nonsense variants (M306* and C394*), and the frameshift variant discovered in the German family (D186fs).
“This [research] will expand the number of genes to consider when screening for predisposition to leukemia, particularly B-ALL,” said study author Kim Nichols, of St. Jude.
“And while not everyone carrying a germline IKZF1 variant will develop leukemia, these results will help us educate families about the potential risk of leukemia.”
Germline variants in IKZF1 can predispose carriers to acute lymphoblastic leukemia (ALL), according to a study published in Cancer Cell.
The research began with the discovery of an IKZF1 variant in 3 generations of a German family affected by pediatric ALL.
Researchers then analyzed data from nearly 5000 children with ALL and identified 27 additional germline variants in IKZF1.
These variants were present in 0.9% of the patients analyzed, and most of the patients with the variants had B-cell ALL.
“This finding adds to the growing body of evidence that, while germline variations still account for a small percentage of pediatric ALL cases overall, more children than previously recognized inherit a predisposition to develop ALL,” said Charles Mullighan, MBBS, MD, of St. Jude Children’s Research Hospital in Memphis, Tennessee.
In the Cancer Cell paper, Dr Mullighan and his colleagues report the discovery of a germline deletion variant in IKZF1 (c.del556 or D186fs), which was present in 3 generations of a family.
Two of the 6 family members with this variant had developed B-ALL as children and died. The remaining 4 subjects are apparently healthy, despite having reduced numbers of B cells.
To build upon this discovery, the researchers performed targeted sequencing of IKZF1 in 4963 children with ALL.
This revealed 27 additional IKZF1 variants in 43 patients, most of whom had B-ALL. (One patient had T-cell ALL, and, for 8 patients, their subtype was unknown.)
The researchers noted that the variants were distributed across the gene.
“The pattern of IKZF1 variants was surprising because many of the variants were in regions of the gene that are rarely mutated in leukemic cells,” said study author Jun J. Yang, PhD, of St. Jude. “These regions of the gene have not been well characterized.”
The researchers also found that 22 of the 28 IKZF1 variants adversely affect gene function, while the remaining 6 variants appear to be benign.
The team said the deleterious variants impair DNA binding and regulation of transcriptional targets, induce aberrant leukemic cell adhesion, and reduce ALL cells’ sensitivity to treatment with dasatinib and dexamethasone.
The researchers identified the most deleterious variants as 5 that are located outside of the zinc-finger domains (M31V, M347V, R423C, A434G, and L449F), 2 variants affecting the N-terminal DNA-binding domain (R162P and H163Y), 2 truncating nonsense variants (M306* and C394*), and the frameshift variant discovered in the German family (D186fs).
“This [research] will expand the number of genes to consider when screening for predisposition to leukemia, particularly B-ALL,” said study author Kim Nichols, of St. Jude.
“And while not everyone carrying a germline IKZF1 variant will develop leukemia, these results will help us educate families about the potential risk of leukemia.”
Inhibitor outperforms rivals in leukemia, lymphoma
CHICAGO—Preclinical research suggests the pan-FLT3/pan-BTK inhibitor CG’806 is more effective than other kinase inhibitors in fighting certain hematologic malignancies.
In one study, CG’806 proved more potent than comparator drugs in primary samples of acute myeloid leukemia (AML) and chronic lymphocytic leukemia (CLL).
In another study, CG’806 demonstrated greater cytotoxicity than comparators in a range of malignant B cell lines.
Data from both studies were presented at the AACR Annual Meeting 2018 (abstracts 791 and 794).
The research was supported by Aptose Biosciences, Inc., the company developing CG’806.
CG’806 is a small molecule that inhibits wild-type (WT) FLT3, as well as FLT3 housing the ITD mutation or with point mutations in the tyrosine kinase domain (TKD, including D835G, D835Y, D835H) or in the gatekeeper region (F691L). CG’806 also inhibits BTK-WT and BTK-C481S.
Stephen E. Kurtz, PhD, of Oregon Health & Science University in Portland, and his colleagues presented results with CG’806 in primary patient samples.
The team found that CG’806 demonstrated greater potency against AML samples relative to other FLT3 inhibitors.
Median IC50 values in 188 AML patient samples were 0.0765 µM for CG’806, 0.125 µM for gilteritinib, 0.199 µM for quizartinib, 0.551 µM for dovitinib, 2.25 µM for midostaurin, 2.93 µM for sorafenib, and 5.01 µM for crenolanib.
The researchers said CG’806 sensitivity was enhanced in FLT3-ITD and FLT3-TKD positive cases.
In CLL patient samples, CG’806 exhibited greater potency and a greater range of activity than the BTK inhibitor ibrutinib. Across 95 CLL samples, the median IC50 values were 0.114 µM for CG’806 and 4.09 µM for ibrutinib.
The researchers said this greater potency of CG’806 may be due to the activity of CG’806 on CSF1R, which has been identified as a therapeutic target in CLL.
“The clinical benefit of current FLT3 inhibitors in AML is transient, as resistance develops after several months of treatment,” Dr Kurtz noted. “Similarly, ibrutinib . . . is limited by acquired resistance as well as refractory disease and tolerance challenges. As a pan-FLT3/pan-BTK inhibitor . . ., CG’806 offers important potential to address these limitations.”
Hongying Zhang, MD, PhD, of Aptose Biosciences, and her colleagues presented results with CG’806 in malignant B-cell and AML cell lines.
The researchers found that CG’806 inhibited FLT3-ITD signaling and induced apoptosis more effectively than quizartinib in FLT3-ITD AML cells (MV4-11). The team noted that CG’806 caused G0/G1 cell-cycle arrest in the cells.
CG’806 also exhibited greater cytotoxic activity than quizartinib in FLT3-WT AML cell lines (KG-1 and NOMO-1).
In addition, CG’806 was more potent than quizartinib, gilteritinib, and crenolanib in Ba/F3 cells transfected with FLT3-WT, ITD, D835Y, and ITD-F691. CG’806 was more potent than quizartinib and crenolanib—but not gilteritinib—in Ba/F3 cells transfected with FLT3-ITD-D835Y.
The researchers said they found that CG’806 inhibits BTK, AURK, and downstream signals in FLT3-WT AML cells.
The team also found that CG’806 decreased BTK phosphorylation in all tested cell lines of B-cell malignancies. This included acute lymphoblastic leukemia, mantle cell lymphoma, Burkitt lymphoma, diffuse large B-cell lymphoma, and follicular lymphoma cell lines.
Across all cell lines, CG’806 killed malignant B cells more effectively than ibrutinib. And CG’806 was “equally potent” against WT and C481S-mutant BTK, according to the researchers.
The team also said CG’806 inhibited AURK and induced polyploidy in B-cell malignancies.
“[C]G’806 has demonstrated the ability to kill a broad range of AML and B-cell malignancies through inhibition of multiple oncogenic pathways,” said William G. Rice, PhD, chairman and chief executive officer of Aptose.
“These studies are critical for understanding how to develop and position CG’806 as we prepare for clinical development in these challenging hematologic malignancies.”
CHICAGO—Preclinical research suggests the pan-FLT3/pan-BTK inhibitor CG’806 is more effective than other kinase inhibitors in fighting certain hematologic malignancies.
In one study, CG’806 proved more potent than comparator drugs in primary samples of acute myeloid leukemia (AML) and chronic lymphocytic leukemia (CLL).
In another study, CG’806 demonstrated greater cytotoxicity than comparators in a range of malignant B cell lines.
Data from both studies were presented at the AACR Annual Meeting 2018 (abstracts 791 and 794).
The research was supported by Aptose Biosciences, Inc., the company developing CG’806.
CG’806 is a small molecule that inhibits wild-type (WT) FLT3, as well as FLT3 housing the ITD mutation or with point mutations in the tyrosine kinase domain (TKD, including D835G, D835Y, D835H) or in the gatekeeper region (F691L). CG’806 also inhibits BTK-WT and BTK-C481S.
Stephen E. Kurtz, PhD, of Oregon Health & Science University in Portland, and his colleagues presented results with CG’806 in primary patient samples.
The team found that CG’806 demonstrated greater potency against AML samples relative to other FLT3 inhibitors.
Median IC50 values in 188 AML patient samples were 0.0765 µM for CG’806, 0.125 µM for gilteritinib, 0.199 µM for quizartinib, 0.551 µM for dovitinib, 2.25 µM for midostaurin, 2.93 µM for sorafenib, and 5.01 µM for crenolanib.
The researchers said CG’806 sensitivity was enhanced in FLT3-ITD and FLT3-TKD positive cases.
In CLL patient samples, CG’806 exhibited greater potency and a greater range of activity than the BTK inhibitor ibrutinib. Across 95 CLL samples, the median IC50 values were 0.114 µM for CG’806 and 4.09 µM for ibrutinib.
The researchers said this greater potency of CG’806 may be due to the activity of CG’806 on CSF1R, which has been identified as a therapeutic target in CLL.
“The clinical benefit of current FLT3 inhibitors in AML is transient, as resistance develops after several months of treatment,” Dr Kurtz noted. “Similarly, ibrutinib . . . is limited by acquired resistance as well as refractory disease and tolerance challenges. As a pan-FLT3/pan-BTK inhibitor . . ., CG’806 offers important potential to address these limitations.”
Hongying Zhang, MD, PhD, of Aptose Biosciences, and her colleagues presented results with CG’806 in malignant B-cell and AML cell lines.
The researchers found that CG’806 inhibited FLT3-ITD signaling and induced apoptosis more effectively than quizartinib in FLT3-ITD AML cells (MV4-11). The team noted that CG’806 caused G0/G1 cell-cycle arrest in the cells.
CG’806 also exhibited greater cytotoxic activity than quizartinib in FLT3-WT AML cell lines (KG-1 and NOMO-1).
In addition, CG’806 was more potent than quizartinib, gilteritinib, and crenolanib in Ba/F3 cells transfected with FLT3-WT, ITD, D835Y, and ITD-F691. CG’806 was more potent than quizartinib and crenolanib—but not gilteritinib—in Ba/F3 cells transfected with FLT3-ITD-D835Y.
The researchers said they found that CG’806 inhibits BTK, AURK, and downstream signals in FLT3-WT AML cells.
The team also found that CG’806 decreased BTK phosphorylation in all tested cell lines of B-cell malignancies. This included acute lymphoblastic leukemia, mantle cell lymphoma, Burkitt lymphoma, diffuse large B-cell lymphoma, and follicular lymphoma cell lines.
Across all cell lines, CG’806 killed malignant B cells more effectively than ibrutinib. And CG’806 was “equally potent” against WT and C481S-mutant BTK, according to the researchers.
The team also said CG’806 inhibited AURK and induced polyploidy in B-cell malignancies.
“[C]G’806 has demonstrated the ability to kill a broad range of AML and B-cell malignancies through inhibition of multiple oncogenic pathways,” said William G. Rice, PhD, chairman and chief executive officer of Aptose.
“These studies are critical for understanding how to develop and position CG’806 as we prepare for clinical development in these challenging hematologic malignancies.”
CHICAGO—Preclinical research suggests the pan-FLT3/pan-BTK inhibitor CG’806 is more effective than other kinase inhibitors in fighting certain hematologic malignancies.
In one study, CG’806 proved more potent than comparator drugs in primary samples of acute myeloid leukemia (AML) and chronic lymphocytic leukemia (CLL).
In another study, CG’806 demonstrated greater cytotoxicity than comparators in a range of malignant B cell lines.
Data from both studies were presented at the AACR Annual Meeting 2018 (abstracts 791 and 794).
The research was supported by Aptose Biosciences, Inc., the company developing CG’806.
CG’806 is a small molecule that inhibits wild-type (WT) FLT3, as well as FLT3 housing the ITD mutation or with point mutations in the tyrosine kinase domain (TKD, including D835G, D835Y, D835H) or in the gatekeeper region (F691L). CG’806 also inhibits BTK-WT and BTK-C481S.
Stephen E. Kurtz, PhD, of Oregon Health & Science University in Portland, and his colleagues presented results with CG’806 in primary patient samples.
The team found that CG’806 demonstrated greater potency against AML samples relative to other FLT3 inhibitors.
Median IC50 values in 188 AML patient samples were 0.0765 µM for CG’806, 0.125 µM for gilteritinib, 0.199 µM for quizartinib, 0.551 µM for dovitinib, 2.25 µM for midostaurin, 2.93 µM for sorafenib, and 5.01 µM for crenolanib.
The researchers said CG’806 sensitivity was enhanced in FLT3-ITD and FLT3-TKD positive cases.
In CLL patient samples, CG’806 exhibited greater potency and a greater range of activity than the BTK inhibitor ibrutinib. Across 95 CLL samples, the median IC50 values were 0.114 µM for CG’806 and 4.09 µM for ibrutinib.
The researchers said this greater potency of CG’806 may be due to the activity of CG’806 on CSF1R, which has been identified as a therapeutic target in CLL.
“The clinical benefit of current FLT3 inhibitors in AML is transient, as resistance develops after several months of treatment,” Dr Kurtz noted. “Similarly, ibrutinib . . . is limited by acquired resistance as well as refractory disease and tolerance challenges. As a pan-FLT3/pan-BTK inhibitor . . ., CG’806 offers important potential to address these limitations.”
Hongying Zhang, MD, PhD, of Aptose Biosciences, and her colleagues presented results with CG’806 in malignant B-cell and AML cell lines.
The researchers found that CG’806 inhibited FLT3-ITD signaling and induced apoptosis more effectively than quizartinib in FLT3-ITD AML cells (MV4-11). The team noted that CG’806 caused G0/G1 cell-cycle arrest in the cells.
CG’806 also exhibited greater cytotoxic activity than quizartinib in FLT3-WT AML cell lines (KG-1 and NOMO-1).
In addition, CG’806 was more potent than quizartinib, gilteritinib, and crenolanib in Ba/F3 cells transfected with FLT3-WT, ITD, D835Y, and ITD-F691. CG’806 was more potent than quizartinib and crenolanib—but not gilteritinib—in Ba/F3 cells transfected with FLT3-ITD-D835Y.
The researchers said they found that CG’806 inhibits BTK, AURK, and downstream signals in FLT3-WT AML cells.
The team also found that CG’806 decreased BTK phosphorylation in all tested cell lines of B-cell malignancies. This included acute lymphoblastic leukemia, mantle cell lymphoma, Burkitt lymphoma, diffuse large B-cell lymphoma, and follicular lymphoma cell lines.
Across all cell lines, CG’806 killed malignant B cells more effectively than ibrutinib. And CG’806 was “equally potent” against WT and C481S-mutant BTK, according to the researchers.
The team also said CG’806 inhibited AURK and induced polyploidy in B-cell malignancies.
“[C]G’806 has demonstrated the ability to kill a broad range of AML and B-cell malignancies through inhibition of multiple oncogenic pathways,” said William G. Rice, PhD, chairman and chief executive officer of Aptose.
“These studies are critical for understanding how to develop and position CG’806 as we prepare for clinical development in these challenging hematologic malignancies.”
Team uses iPSCs to create ‘universal’ CAR T cells
CHICAGO—Researchers have used induced pluripotent stem cells (iPSCs) to create a “universal” chimeric antigen receptor (CAR) T-cell therapy known as FT819.
The team says FT819 has the potential to be mass-produced, stored, and made readily available for cancer patients.
In in vitro experiments, FT819 demonstrated activity against leukemia and lymphoma.
These results were presented at the AACR Annual Meeting 2018 (abstract LB-108).
The research was conducted by employees of Fate Therapeutics, Inc., the company developing FT819, as well as Memorial Sloan-Kettering Cancer Center.
About FT819
FT819 is produced from a master iPSC line generated using T cells from healthy donors.
“A master iPSC line has unlimited capacity to self-renew and can be banked and renewably used,” said Bob Valamehr, PhD, vice-president of cancer immunotherapy at Fate Therapeutics, Inc.
“We started with cells from a healthy donor rather than the patient, created a master cell line, and used the master cell line to produce large quantities of ‘universal’ CAR19 T cells that are not patient-restricted. These first-of-kind CAR19 T cells, called FT819, can be packaged, stored, and made readily available for treatment of a large number of patients.”
FT819 has 2 targeting receptors—a CAR targeting CD19-positive tumor cells and a CD16 Fc receptor that can engage other therapies (such as tumor antigen-targeting monoclonal antibodies) to overcome antigen escape.
The master iPSC line used for the production of FT819 is engineered in a one-time event to insert a CD19 CAR into the T-cell receptor α constant (TRAC) locus. This is done to eliminate T-cell receptor expression and reduce the likelihood of graft-versus-host disease.
Previous research showed that targeting a CAR to the TRAC locus results in uniform CAR expression and enhances T-cell potency. In fact, TRAC-CAR T cells outperformed conventionally generated CAR T cells by preventing T-cell exhaustion in a mouse model of acute lymphoblastic leukemia.
In vitro experiments
With the current work, the researchers found that FT819 displayed an efficient cytotoxic T-cell response when challenged with CD19-positive tumor cells. FT819 produced cytokines (IFN-gamma, TNF-alpha, and IL-2) and mediators of cell death (CD107a/b, perforin, and granzyme B).
FT819 was also target-specific, attacking only CD19-positive tumor cells and sparing CD19-negative tumor cells in experiments with Raji (Burkitt lymphoma) and Nalm-6 (B-cell acute lymphoblastic leukemia) cell lines.
The researchers said they observed consistent antigen-specific cytotoxicity against Nalm-6 cells with FT819 but variability in antigen-specific cytotoxicity with conventional CAR T cells.
In addition, when combined with rituximab, FT819 elicited antibody-dependent cell-mediated cytotoxicity against CD19-negative, CD20-positive tumor cells.
“Through the development of FT819, we believe there is significant opportunity to lower the cost of CAR T-cell manufacture, enhance the quality of the product, and create a readily available supply of a more efficacious product to reach more patients in need,” Dr Valamehr said.
CHICAGO—Researchers have used induced pluripotent stem cells (iPSCs) to create a “universal” chimeric antigen receptor (CAR) T-cell therapy known as FT819.
The team says FT819 has the potential to be mass-produced, stored, and made readily available for cancer patients.
In in vitro experiments, FT819 demonstrated activity against leukemia and lymphoma.
These results were presented at the AACR Annual Meeting 2018 (abstract LB-108).
The research was conducted by employees of Fate Therapeutics, Inc., the company developing FT819, as well as Memorial Sloan-Kettering Cancer Center.
About FT819
FT819 is produced from a master iPSC line generated using T cells from healthy donors.
“A master iPSC line has unlimited capacity to self-renew and can be banked and renewably used,” said Bob Valamehr, PhD, vice-president of cancer immunotherapy at Fate Therapeutics, Inc.
“We started with cells from a healthy donor rather than the patient, created a master cell line, and used the master cell line to produce large quantities of ‘universal’ CAR19 T cells that are not patient-restricted. These first-of-kind CAR19 T cells, called FT819, can be packaged, stored, and made readily available for treatment of a large number of patients.”
FT819 has 2 targeting receptors—a CAR targeting CD19-positive tumor cells and a CD16 Fc receptor that can engage other therapies (such as tumor antigen-targeting monoclonal antibodies) to overcome antigen escape.
The master iPSC line used for the production of FT819 is engineered in a one-time event to insert a CD19 CAR into the T-cell receptor α constant (TRAC) locus. This is done to eliminate T-cell receptor expression and reduce the likelihood of graft-versus-host disease.
Previous research showed that targeting a CAR to the TRAC locus results in uniform CAR expression and enhances T-cell potency. In fact, TRAC-CAR T cells outperformed conventionally generated CAR T cells by preventing T-cell exhaustion in a mouse model of acute lymphoblastic leukemia.
In vitro experiments
With the current work, the researchers found that FT819 displayed an efficient cytotoxic T-cell response when challenged with CD19-positive tumor cells. FT819 produced cytokines (IFN-gamma, TNF-alpha, and IL-2) and mediators of cell death (CD107a/b, perforin, and granzyme B).
FT819 was also target-specific, attacking only CD19-positive tumor cells and sparing CD19-negative tumor cells in experiments with Raji (Burkitt lymphoma) and Nalm-6 (B-cell acute lymphoblastic leukemia) cell lines.
The researchers said they observed consistent antigen-specific cytotoxicity against Nalm-6 cells with FT819 but variability in antigen-specific cytotoxicity with conventional CAR T cells.
In addition, when combined with rituximab, FT819 elicited antibody-dependent cell-mediated cytotoxicity against CD19-negative, CD20-positive tumor cells.
“Through the development of FT819, we believe there is significant opportunity to lower the cost of CAR T-cell manufacture, enhance the quality of the product, and create a readily available supply of a more efficacious product to reach more patients in need,” Dr Valamehr said.
CHICAGO—Researchers have used induced pluripotent stem cells (iPSCs) to create a “universal” chimeric antigen receptor (CAR) T-cell therapy known as FT819.
The team says FT819 has the potential to be mass-produced, stored, and made readily available for cancer patients.
In in vitro experiments, FT819 demonstrated activity against leukemia and lymphoma.
These results were presented at the AACR Annual Meeting 2018 (abstract LB-108).
The research was conducted by employees of Fate Therapeutics, Inc., the company developing FT819, as well as Memorial Sloan-Kettering Cancer Center.
About FT819
FT819 is produced from a master iPSC line generated using T cells from healthy donors.
“A master iPSC line has unlimited capacity to self-renew and can be banked and renewably used,” said Bob Valamehr, PhD, vice-president of cancer immunotherapy at Fate Therapeutics, Inc.
“We started with cells from a healthy donor rather than the patient, created a master cell line, and used the master cell line to produce large quantities of ‘universal’ CAR19 T cells that are not patient-restricted. These first-of-kind CAR19 T cells, called FT819, can be packaged, stored, and made readily available for treatment of a large number of patients.”
FT819 has 2 targeting receptors—a CAR targeting CD19-positive tumor cells and a CD16 Fc receptor that can engage other therapies (such as tumor antigen-targeting monoclonal antibodies) to overcome antigen escape.
The master iPSC line used for the production of FT819 is engineered in a one-time event to insert a CD19 CAR into the T-cell receptor α constant (TRAC) locus. This is done to eliminate T-cell receptor expression and reduce the likelihood of graft-versus-host disease.
Previous research showed that targeting a CAR to the TRAC locus results in uniform CAR expression and enhances T-cell potency. In fact, TRAC-CAR T cells outperformed conventionally generated CAR T cells by preventing T-cell exhaustion in a mouse model of acute lymphoblastic leukemia.
In vitro experiments
With the current work, the researchers found that FT819 displayed an efficient cytotoxic T-cell response when challenged with CD19-positive tumor cells. FT819 produced cytokines (IFN-gamma, TNF-alpha, and IL-2) and mediators of cell death (CD107a/b, perforin, and granzyme B).
FT819 was also target-specific, attacking only CD19-positive tumor cells and sparing CD19-negative tumor cells in experiments with Raji (Burkitt lymphoma) and Nalm-6 (B-cell acute lymphoblastic leukemia) cell lines.
The researchers said they observed consistent antigen-specific cytotoxicity against Nalm-6 cells with FT819 but variability in antigen-specific cytotoxicity with conventional CAR T cells.
In addition, when combined with rituximab, FT819 elicited antibody-dependent cell-mediated cytotoxicity against CD19-negative, CD20-positive tumor cells.
“Through the development of FT819, we believe there is significant opportunity to lower the cost of CAR T-cell manufacture, enhance the quality of the product, and create a readily available supply of a more efficacious product to reach more patients in need,” Dr Valamehr said.
Groups launch open portal for cancer models
The European Bioinformatics Institute (EMBL-EBI) and The Jackson Laboratory (JAX) have developed an open cancer research portal for patient-derived xenograft (PDX) models.
The portal, known as PDX Finder, catalogues PDX models from numerous global repositories.
At present, PDX Finder contains more than 1900 models for a range of cancers, including hematologic malignancies.
Researchers can search for PDX models and submit their own on the PDX Finder website: http://www.pdxfinder.org.
“PDX models are increasingly recognized as clinically relevant because they retain the patient tumor characteristics and imitate a specific patient’s response to drugs more accurately than other models,” said Nathalie Conte, PDX Finder project lead at EMBL-EBI.
“However, until now, there was no open central catalogue for PDX models. PDX Finder enables cancer researchers to search through a wider variety of models more quickly, saving valuable time and enabling collaborations.”
PDX Finder builds on JAX’s PDX resource, which was developed in partnership with more than 20 medical centers in the US, and EMBL-EBI’s membership of EurOPDX. This ensures the scope and reach of PDX Finder is wider than any other individual resource available, according to JAX and EMBL-EBI.
“Both EMBL-EBI and JAX received independent funding from the National Cancer Institute [NCI] for projects that included implementation of online catalogues for PDX models,” noted Carol Bult, scientific director of the JAX PDX resource.
“Both groups had the goal to help basic and clinical cancer researchers find relevant models fast. With approval from the NCI, we instead collaborated to build a single, unified portal with international scope.”
The launch of PDX Finder comes just months after EMBL-EBI and JAX published the PDX minimal information standard, which sets standards for basic information needed to describe essential properties of a PDX model.
The standard ensures that every model in PDX Finder is carefully described to help researchers choose models that are most relevant to their work.
The European Bioinformatics Institute (EMBL-EBI) and The Jackson Laboratory (JAX) have developed an open cancer research portal for patient-derived xenograft (PDX) models.
The portal, known as PDX Finder, catalogues PDX models from numerous global repositories.
At present, PDX Finder contains more than 1900 models for a range of cancers, including hematologic malignancies.
Researchers can search for PDX models and submit their own on the PDX Finder website: http://www.pdxfinder.org.
“PDX models are increasingly recognized as clinically relevant because they retain the patient tumor characteristics and imitate a specific patient’s response to drugs more accurately than other models,” said Nathalie Conte, PDX Finder project lead at EMBL-EBI.
“However, until now, there was no open central catalogue for PDX models. PDX Finder enables cancer researchers to search through a wider variety of models more quickly, saving valuable time and enabling collaborations.”
PDX Finder builds on JAX’s PDX resource, which was developed in partnership with more than 20 medical centers in the US, and EMBL-EBI’s membership of EurOPDX. This ensures the scope and reach of PDX Finder is wider than any other individual resource available, according to JAX and EMBL-EBI.
“Both EMBL-EBI and JAX received independent funding from the National Cancer Institute [NCI] for projects that included implementation of online catalogues for PDX models,” noted Carol Bult, scientific director of the JAX PDX resource.
“Both groups had the goal to help basic and clinical cancer researchers find relevant models fast. With approval from the NCI, we instead collaborated to build a single, unified portal with international scope.”
The launch of PDX Finder comes just months after EMBL-EBI and JAX published the PDX minimal information standard, which sets standards for basic information needed to describe essential properties of a PDX model.
The standard ensures that every model in PDX Finder is carefully described to help researchers choose models that are most relevant to their work.
The European Bioinformatics Institute (EMBL-EBI) and The Jackson Laboratory (JAX) have developed an open cancer research portal for patient-derived xenograft (PDX) models.
The portal, known as PDX Finder, catalogues PDX models from numerous global repositories.
At present, PDX Finder contains more than 1900 models for a range of cancers, including hematologic malignancies.
Researchers can search for PDX models and submit their own on the PDX Finder website: http://www.pdxfinder.org.
“PDX models are increasingly recognized as clinically relevant because they retain the patient tumor characteristics and imitate a specific patient’s response to drugs more accurately than other models,” said Nathalie Conte, PDX Finder project lead at EMBL-EBI.
“However, until now, there was no open central catalogue for PDX models. PDX Finder enables cancer researchers to search through a wider variety of models more quickly, saving valuable time and enabling collaborations.”
PDX Finder builds on JAX’s PDX resource, which was developed in partnership with more than 20 medical centers in the US, and EMBL-EBI’s membership of EurOPDX. This ensures the scope and reach of PDX Finder is wider than any other individual resource available, according to JAX and EMBL-EBI.
“Both EMBL-EBI and JAX received independent funding from the National Cancer Institute [NCI] for projects that included implementation of online catalogues for PDX models,” noted Carol Bult, scientific director of the JAX PDX resource.
“Both groups had the goal to help basic and clinical cancer researchers find relevant models fast. With approval from the NCI, we instead collaborated to build a single, unified portal with international scope.”
The launch of PDX Finder comes just months after EMBL-EBI and JAX published the PDX minimal information standard, which sets standards for basic information needed to describe essential properties of a PDX model.
The standard ensures that every model in PDX Finder is carefully described to help researchers choose models that are most relevant to their work.