User login
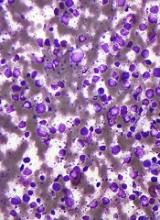
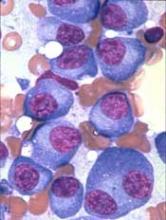
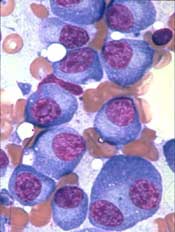
Study of DBA provides new insight into hematopoiesis
In studying Diamond-Blackfan anemia (DBA), researchers have found evidence to suggest that ribosome levels play a key role in hematopoiesis.
The researchers found that a reduction in ribosomes is responsible for the disruption in red blood cell production observed in patients with DBA.
This reduction in ribosomes impairs the translation of certain messenger RNAs (mRNAs), which inhibits erythroid lineage commitment in hematopoietic stem and progenitor cells (HSCPs).
The researchers said this work has provided insight into how lineage commitment functions normally and in the setting of DBA.
Vijay Sankaran, MD, PhD, of Boston Children’s Hospital in Massachusetts, and his colleagues described these findings in Cell.
The team noted that most cases of DBA are caused by heterozygous loss-of-function mutations in ribosomal protein (RP) genes, which result in RP haploinsufficiency.
Past research revealed DBA-causing mutations in the transcription factor GATA1. Subsequent work showed that RP haploinsufficiency results in reduced translation of GATA1 mRNA, and increasing GATA1 protein levels can reduce the erythroid defects in DBA cells.
However, the mechanisms underlying these phenomena were unclear.
In the current study, the researchers found that DBA-associated perturbations reduced ribosome levels but did not affect the composition of ribosomes.
“There has been controversy over whether a ribosomal protein mutation changes the composition of the ribosomes or the quantity of the ribosomes,” Dr Sankaran said. “We know now that it is the latter.”
The researchers said the reduction of ribosome levels was largely promoted through reduced translation of RP mRNAs.
The team identified 525 transcripts with translation efficiencies that were downregulated by RP haploinsufficiency. They said a subset of these transcripts, including GATA1, have proven essential for hematopoietic cell growth and are upregulated during early erythropoiesis.
The researchers confirmed their prior findings that translation of GATA1 mRNA is decreased by about 2-fold in differentiating HSPCs with RP haploinsufficiency.
The team also found that GATA1 has “unique” 5’ untranslated region features that confer sensitivity to ribosome levels.
Taken together, these findings suggest the presence of GATA1 proteins in HSPCs helps prime them for erythroid lineage commitment. Without enough ribosomes to produce enough GATA1 proteins, the HSPCs never receive the signal to become red blood cells.
“This raises the question of whether we can design a gene therapy to overcome the GATA1 deficiency,” Dr Sankaran said. “We now have a tremendous interest in this approach and believe it can be done.”
In studying Diamond-Blackfan anemia (DBA), researchers have found evidence to suggest that ribosome levels play a key role in hematopoiesis.
The researchers found that a reduction in ribosomes is responsible for the disruption in red blood cell production observed in patients with DBA.
This reduction in ribosomes impairs the translation of certain messenger RNAs (mRNAs), which inhibits erythroid lineage commitment in hematopoietic stem and progenitor cells (HSCPs).
The researchers said this work has provided insight into how lineage commitment functions normally and in the setting of DBA.
Vijay Sankaran, MD, PhD, of Boston Children’s Hospital in Massachusetts, and his colleagues described these findings in Cell.
The team noted that most cases of DBA are caused by heterozygous loss-of-function mutations in ribosomal protein (RP) genes, which result in RP haploinsufficiency.
Past research revealed DBA-causing mutations in the transcription factor GATA1. Subsequent work showed that RP haploinsufficiency results in reduced translation of GATA1 mRNA, and increasing GATA1 protein levels can reduce the erythroid defects in DBA cells.
However, the mechanisms underlying these phenomena were unclear.
In the current study, the researchers found that DBA-associated perturbations reduced ribosome levels but did not affect the composition of ribosomes.
“There has been controversy over whether a ribosomal protein mutation changes the composition of the ribosomes or the quantity of the ribosomes,” Dr Sankaran said. “We know now that it is the latter.”
The researchers said the reduction of ribosome levels was largely promoted through reduced translation of RP mRNAs.
The team identified 525 transcripts with translation efficiencies that were downregulated by RP haploinsufficiency. They said a subset of these transcripts, including GATA1, have proven essential for hematopoietic cell growth and are upregulated during early erythropoiesis.
The researchers confirmed their prior findings that translation of GATA1 mRNA is decreased by about 2-fold in differentiating HSPCs with RP haploinsufficiency.
The team also found that GATA1 has “unique” 5’ untranslated region features that confer sensitivity to ribosome levels.
Taken together, these findings suggest the presence of GATA1 proteins in HSPCs helps prime them for erythroid lineage commitment. Without enough ribosomes to produce enough GATA1 proteins, the HSPCs never receive the signal to become red blood cells.
“This raises the question of whether we can design a gene therapy to overcome the GATA1 deficiency,” Dr Sankaran said. “We now have a tremendous interest in this approach and believe it can be done.”
In studying Diamond-Blackfan anemia (DBA), researchers have found evidence to suggest that ribosome levels play a key role in hematopoiesis.
The researchers found that a reduction in ribosomes is responsible for the disruption in red blood cell production observed in patients with DBA.
This reduction in ribosomes impairs the translation of certain messenger RNAs (mRNAs), which inhibits erythroid lineage commitment in hematopoietic stem and progenitor cells (HSCPs).
The researchers said this work has provided insight into how lineage commitment functions normally and in the setting of DBA.
Vijay Sankaran, MD, PhD, of Boston Children’s Hospital in Massachusetts, and his colleagues described these findings in Cell.
The team noted that most cases of DBA are caused by heterozygous loss-of-function mutations in ribosomal protein (RP) genes, which result in RP haploinsufficiency.
Past research revealed DBA-causing mutations in the transcription factor GATA1. Subsequent work showed that RP haploinsufficiency results in reduced translation of GATA1 mRNA, and increasing GATA1 protein levels can reduce the erythroid defects in DBA cells.
However, the mechanisms underlying these phenomena were unclear.
In the current study, the researchers found that DBA-associated perturbations reduced ribosome levels but did not affect the composition of ribosomes.
“There has been controversy over whether a ribosomal protein mutation changes the composition of the ribosomes or the quantity of the ribosomes,” Dr Sankaran said. “We know now that it is the latter.”
The researchers said the reduction of ribosome levels was largely promoted through reduced translation of RP mRNAs.
The team identified 525 transcripts with translation efficiencies that were downregulated by RP haploinsufficiency. They said a subset of these transcripts, including GATA1, have proven essential for hematopoietic cell growth and are upregulated during early erythropoiesis.
The researchers confirmed their prior findings that translation of GATA1 mRNA is decreased by about 2-fold in differentiating HSPCs with RP haploinsufficiency.
The team also found that GATA1 has “unique” 5’ untranslated region features that confer sensitivity to ribosome levels.
Taken together, these findings suggest the presence of GATA1 proteins in HSPCs helps prime them for erythroid lineage commitment. Without enough ribosomes to produce enough GATA1 proteins, the HSPCs never receive the signal to become red blood cells.
“This raises the question of whether we can design a gene therapy to overcome the GATA1 deficiency,” Dr Sankaran said. “We now have a tremendous interest in this approach and believe it can be done.”
Research reveals new subtypes of DLBCL
New research helps explain why some patients with diffuse large B-cell lymphoma (DLBCL) respond well to immunochemotherapy and others do not.
Researchers analyzed samples from nearly 600 DLBCL patients and identified 4 new genetic subtypes of the disease.
Patients with 2 of these subtypes had overall survival (OS) rates that were roughly twice as high as OS rates in patients with the other 2 subtypes.
Louis M. Staudt, MD, PhD, of the National Cancer Institute in Bethesda, Maryland, and his colleagues described these findings in NEJM.
The researchers noted that the current subtypes of DLBCL—germinal center B-cell-like (GCB) and activated B-cell-like (ABC) DLBCL—are associated with OS after treatment with R-CHOP (rituximab, cyclophosphamide, doxorubicin, vincristine, and prednisone).
Patients with ABC DLBCL have a much lower OS rate, on average, than patients with GCB DLBCL. However, even in the GCB subgroup, many patients relapse after treatment.
“The first question we wanted to tackle was whether there were other molecular features of the tumors that could help us explain why some people were well-served by chemotherapy,” Dr Staudt said.
“And the second, related question was, if we could understand who was not responding well to treatment, could we understand the genetics of these tumors to suggest new potential therapies beyond chemotherapy? The answer to both questions was ‘yes.’”
Dr Staudt and his colleagues analyzed tumor samples from 574 patients with DLBCL, performing exome and transcriptome sequencing, array-based DNA copy-number analysis, and targeted amplicon resequencing of 372 genes to find recurrent aberrations.
The team also developed an algorithm to discover genetic subtypes based on the co-occurrence of genetic alterations.
In this way, they identified 4 genetic subtypes:
- MCD, which was named based on the co-occurrence of MYD88L265P and CD79B mutations
- BN2, whose name was based on the presence of BCL6 fusions and NOTCH2 mutations
- N1, named for NOTCH1 mutations
- EZB, named for EZH2 mutations and BCL2 translocations.
The researchers said aberrations in multiple genes distinguished each of these subtypes from other DLBCLs, and the subtypes differed phenotypically.
Patients with BN2 or EZB subtypes had much higher OS rates after receiving R-CHOP than patients with MCD or N1 subtypes. The predicted 5-year OS rates were 26% for MCD patients, 36% for N1 patients, 65% for BN2 patients, and 68% for EZB patients.
The researchers said they found evidence to suggest that MCD and BN2 DLBCLs rely on chronic active B-cell receptor signaling that is amenable to therapeutic inhibition.
The team also noted that some of the subtypes they identified can be found in both ABC and GCB DLBCLs. For example, a patient could have ABC DLBCL, which is associated with a lower OS rate after R-CHOP, but also have the BN2 genetic subtype that responds well to R-CHOP.
“This shows we’ve gone beyond where we were,” Dr Staudt said. “Before, even with our most advanced molecular diagnosis, we would have said all ABC tumors are the ‘bad’ type, and they need to be treated aggressively.”
“Now, we can implement this kind of classification and say that, even if a patient has the ‘bad’ ABC type, they have the ‘good’ genetic type, BN2. So there’s a much better chance of chemotherapy curing the disease.”
Data from this study will be shared through the National Cancer Institute’s Genomic Data Commons to make it available for future research.
Dr Staudt said he and his colleagues hope their new molecular classification will be used in clinical trials so that DLBCL treatment can move toward more targeted therapies.
“The goal is to find the right drug for the right person at the right time,” Dr Staudt said. “And we feel this genetic understanding of diffuse lymphoma is a step forward in precision therapy.”
New research helps explain why some patients with diffuse large B-cell lymphoma (DLBCL) respond well to immunochemotherapy and others do not.
Researchers analyzed samples from nearly 600 DLBCL patients and identified 4 new genetic subtypes of the disease.
Patients with 2 of these subtypes had overall survival (OS) rates that were roughly twice as high as OS rates in patients with the other 2 subtypes.
Louis M. Staudt, MD, PhD, of the National Cancer Institute in Bethesda, Maryland, and his colleagues described these findings in NEJM.
The researchers noted that the current subtypes of DLBCL—germinal center B-cell-like (GCB) and activated B-cell-like (ABC) DLBCL—are associated with OS after treatment with R-CHOP (rituximab, cyclophosphamide, doxorubicin, vincristine, and prednisone).
Patients with ABC DLBCL have a much lower OS rate, on average, than patients with GCB DLBCL. However, even in the GCB subgroup, many patients relapse after treatment.
“The first question we wanted to tackle was whether there were other molecular features of the tumors that could help us explain why some people were well-served by chemotherapy,” Dr Staudt said.
“And the second, related question was, if we could understand who was not responding well to treatment, could we understand the genetics of these tumors to suggest new potential therapies beyond chemotherapy? The answer to both questions was ‘yes.’”
Dr Staudt and his colleagues analyzed tumor samples from 574 patients with DLBCL, performing exome and transcriptome sequencing, array-based DNA copy-number analysis, and targeted amplicon resequencing of 372 genes to find recurrent aberrations.
The team also developed an algorithm to discover genetic subtypes based on the co-occurrence of genetic alterations.
In this way, they identified 4 genetic subtypes:
- MCD, which was named based on the co-occurrence of MYD88L265P and CD79B mutations
- BN2, whose name was based on the presence of BCL6 fusions and NOTCH2 mutations
- N1, named for NOTCH1 mutations
- EZB, named for EZH2 mutations and BCL2 translocations.
The researchers said aberrations in multiple genes distinguished each of these subtypes from other DLBCLs, and the subtypes differed phenotypically.
Patients with BN2 or EZB subtypes had much higher OS rates after receiving R-CHOP than patients with MCD or N1 subtypes. The predicted 5-year OS rates were 26% for MCD patients, 36% for N1 patients, 65% for BN2 patients, and 68% for EZB patients.
The researchers said they found evidence to suggest that MCD and BN2 DLBCLs rely on chronic active B-cell receptor signaling that is amenable to therapeutic inhibition.
The team also noted that some of the subtypes they identified can be found in both ABC and GCB DLBCLs. For example, a patient could have ABC DLBCL, which is associated with a lower OS rate after R-CHOP, but also have the BN2 genetic subtype that responds well to R-CHOP.
“This shows we’ve gone beyond where we were,” Dr Staudt said. “Before, even with our most advanced molecular diagnosis, we would have said all ABC tumors are the ‘bad’ type, and they need to be treated aggressively.”
“Now, we can implement this kind of classification and say that, even if a patient has the ‘bad’ ABC type, they have the ‘good’ genetic type, BN2. So there’s a much better chance of chemotherapy curing the disease.”
Data from this study will be shared through the National Cancer Institute’s Genomic Data Commons to make it available for future research.
Dr Staudt said he and his colleagues hope their new molecular classification will be used in clinical trials so that DLBCL treatment can move toward more targeted therapies.
“The goal is to find the right drug for the right person at the right time,” Dr Staudt said. “And we feel this genetic understanding of diffuse lymphoma is a step forward in precision therapy.”
New research helps explain why some patients with diffuse large B-cell lymphoma (DLBCL) respond well to immunochemotherapy and others do not.
Researchers analyzed samples from nearly 600 DLBCL patients and identified 4 new genetic subtypes of the disease.
Patients with 2 of these subtypes had overall survival (OS) rates that were roughly twice as high as OS rates in patients with the other 2 subtypes.
Louis M. Staudt, MD, PhD, of the National Cancer Institute in Bethesda, Maryland, and his colleagues described these findings in NEJM.
The researchers noted that the current subtypes of DLBCL—germinal center B-cell-like (GCB) and activated B-cell-like (ABC) DLBCL—are associated with OS after treatment with R-CHOP (rituximab, cyclophosphamide, doxorubicin, vincristine, and prednisone).
Patients with ABC DLBCL have a much lower OS rate, on average, than patients with GCB DLBCL. However, even in the GCB subgroup, many patients relapse after treatment.
“The first question we wanted to tackle was whether there were other molecular features of the tumors that could help us explain why some people were well-served by chemotherapy,” Dr Staudt said.
“And the second, related question was, if we could understand who was not responding well to treatment, could we understand the genetics of these tumors to suggest new potential therapies beyond chemotherapy? The answer to both questions was ‘yes.’”
Dr Staudt and his colleagues analyzed tumor samples from 574 patients with DLBCL, performing exome and transcriptome sequencing, array-based DNA copy-number analysis, and targeted amplicon resequencing of 372 genes to find recurrent aberrations.
The team also developed an algorithm to discover genetic subtypes based on the co-occurrence of genetic alterations.
In this way, they identified 4 genetic subtypes:
- MCD, which was named based on the co-occurrence of MYD88L265P and CD79B mutations
- BN2, whose name was based on the presence of BCL6 fusions and NOTCH2 mutations
- N1, named for NOTCH1 mutations
- EZB, named for EZH2 mutations and BCL2 translocations.
The researchers said aberrations in multiple genes distinguished each of these subtypes from other DLBCLs, and the subtypes differed phenotypically.
Patients with BN2 or EZB subtypes had much higher OS rates after receiving R-CHOP than patients with MCD or N1 subtypes. The predicted 5-year OS rates were 26% for MCD patients, 36% for N1 patients, 65% for BN2 patients, and 68% for EZB patients.
The researchers said they found evidence to suggest that MCD and BN2 DLBCLs rely on chronic active B-cell receptor signaling that is amenable to therapeutic inhibition.
The team also noted that some of the subtypes they identified can be found in both ABC and GCB DLBCLs. For example, a patient could have ABC DLBCL, which is associated with a lower OS rate after R-CHOP, but also have the BN2 genetic subtype that responds well to R-CHOP.
“This shows we’ve gone beyond where we were,” Dr Staudt said. “Before, even with our most advanced molecular diagnosis, we would have said all ABC tumors are the ‘bad’ type, and they need to be treated aggressively.”
“Now, we can implement this kind of classification and say that, even if a patient has the ‘bad’ ABC type, they have the ‘good’ genetic type, BN2. So there’s a much better chance of chemotherapy curing the disease.”
Data from this study will be shared through the National Cancer Institute’s Genomic Data Commons to make it available for future research.
Dr Staudt said he and his colleagues hope their new molecular classification will be used in clinical trials so that DLBCL treatment can move toward more targeted therapies.
“The goal is to find the right drug for the right person at the right time,” Dr Staudt said. “And we feel this genetic understanding of diffuse lymphoma is a step forward in precision therapy.”
Drug shows promise for treating AML, MDS
Preclinical results support clinical testing of an experimental agent in acute myeloid leukemia (AML) and myelodysplastic syndromes (MDS), according to researchers.
The agent, ALRN-6924, was shown to combat AML and MDS by restoring activity of the tumor-suppressing protein p53.
ALRN-6924 exhibited antileukemic activity in AML cells and mouse models of the disease, as well as in a patient with MDS and excess leukemic blasts who received the drug on a compassionate-use basis.
These results, published in Science Translational Medicine, have led to a phase 1 trial of ALRN-6924 in patients with AML or MDS.
ALRN-6924 was developed by Aileron Therapeutics Inc., to target p53 by inhibiting 2 naturally occurring proteins, MDMX and MDM2. Overexpression of these proteins inactivates p53, allowing cancer cells to multiply unchecked.
In the current study, researchers set out to confirm ALRN-6924’s mechanism of action and determine the efficacy of the drug in AML/MDS. This work was supported, in part, by grants from Aileron Therapeutics Inc., and the National Institutes of Health.
The researchers did find that ALRN-6924 targets both MDMX and MDM2, blocking their interaction with p53 in AML cells.
The team said ALRN-6924 inhibited proliferation by inducing cell-cycle arrest and apoptosis in AML cell lines and AML patient cells, including leukemic stem cell-enriched populations.
“This is important because AML is driven by stem cells, and, if you don’t target stem cells, the disease will come back very quickly,” said study author Ulrich Steidl, MD, PhD, of Albert Einstein College of Medicine in Bronx, New York.
The researchers also found that ALRN-6924 greatly increased survival in a mouse model of AML. The median survival was 34 days in vehicle-treated control mice, 83 days in mice that received ALRN-6924 at 20 mg/kg twice a week, and 151 days in mice that received ALRN-6924 at 20 mg/kg three times a week.
“This is a very striking response,” Dr Steidl said. “Most experimental drugs for leukemia achieve an increase in survival of only a few days in these preclinical models. Even more importantly, ALRN-6924 effectively cured about 40% of the treated mice, meaning they were disease-free more than 1 year after treatment, essentially a lifetime for a mouse.”
Finally, the researchers assessed the effects of ALRN-6924 in a patient who had high-risk MDS with excess leukemic blasts.
The team found the p53 protein “was rapidly induced” in CD34+ leukemic blasts but not in healthy lymphocytes. And ALRN-6924 reduced the number of malignant cells circulating in the blood.
“This test was not designed to assess the efficacy of the drug in humans,” Dr Steidl noted. “That has to be done in a proper clinical trial. Our goal was to determine whether it can hit the desired target in human cells in a clinical setting, which it did in this individual.”
ALRN-6924 is a stapled alpha-helical peptide, a class of drugs whose helical structure is stabilized using hydrocarbon “staples.” The stapling prevents the peptides from being degraded by enzymes before reaching their intended target. ALRN-6924 is the first stapled peptide therapeutic to be tested in patients.
In the phase 1 trial (NCT02909972), researchers are testing ALRN-6924 in patients with relapsed/refractory AML or advanced MDS.
Preclinical results support clinical testing of an experimental agent in acute myeloid leukemia (AML) and myelodysplastic syndromes (MDS), according to researchers.
The agent, ALRN-6924, was shown to combat AML and MDS by restoring activity of the tumor-suppressing protein p53.
ALRN-6924 exhibited antileukemic activity in AML cells and mouse models of the disease, as well as in a patient with MDS and excess leukemic blasts who received the drug on a compassionate-use basis.
These results, published in Science Translational Medicine, have led to a phase 1 trial of ALRN-6924 in patients with AML or MDS.
ALRN-6924 was developed by Aileron Therapeutics Inc., to target p53 by inhibiting 2 naturally occurring proteins, MDMX and MDM2. Overexpression of these proteins inactivates p53, allowing cancer cells to multiply unchecked.
In the current study, researchers set out to confirm ALRN-6924’s mechanism of action and determine the efficacy of the drug in AML/MDS. This work was supported, in part, by grants from Aileron Therapeutics Inc., and the National Institutes of Health.
The researchers did find that ALRN-6924 targets both MDMX and MDM2, blocking their interaction with p53 in AML cells.
The team said ALRN-6924 inhibited proliferation by inducing cell-cycle arrest and apoptosis in AML cell lines and AML patient cells, including leukemic stem cell-enriched populations.
“This is important because AML is driven by stem cells, and, if you don’t target stem cells, the disease will come back very quickly,” said study author Ulrich Steidl, MD, PhD, of Albert Einstein College of Medicine in Bronx, New York.
The researchers also found that ALRN-6924 greatly increased survival in a mouse model of AML. The median survival was 34 days in vehicle-treated control mice, 83 days in mice that received ALRN-6924 at 20 mg/kg twice a week, and 151 days in mice that received ALRN-6924 at 20 mg/kg three times a week.
“This is a very striking response,” Dr Steidl said. “Most experimental drugs for leukemia achieve an increase in survival of only a few days in these preclinical models. Even more importantly, ALRN-6924 effectively cured about 40% of the treated mice, meaning they were disease-free more than 1 year after treatment, essentially a lifetime for a mouse.”
Finally, the researchers assessed the effects of ALRN-6924 in a patient who had high-risk MDS with excess leukemic blasts.
The team found the p53 protein “was rapidly induced” in CD34+ leukemic blasts but not in healthy lymphocytes. And ALRN-6924 reduced the number of malignant cells circulating in the blood.
“This test was not designed to assess the efficacy of the drug in humans,” Dr Steidl noted. “That has to be done in a proper clinical trial. Our goal was to determine whether it can hit the desired target in human cells in a clinical setting, which it did in this individual.”
ALRN-6924 is a stapled alpha-helical peptide, a class of drugs whose helical structure is stabilized using hydrocarbon “staples.” The stapling prevents the peptides from being degraded by enzymes before reaching their intended target. ALRN-6924 is the first stapled peptide therapeutic to be tested in patients.
In the phase 1 trial (NCT02909972), researchers are testing ALRN-6924 in patients with relapsed/refractory AML or advanced MDS.
Preclinical results support clinical testing of an experimental agent in acute myeloid leukemia (AML) and myelodysplastic syndromes (MDS), according to researchers.
The agent, ALRN-6924, was shown to combat AML and MDS by restoring activity of the tumor-suppressing protein p53.
ALRN-6924 exhibited antileukemic activity in AML cells and mouse models of the disease, as well as in a patient with MDS and excess leukemic blasts who received the drug on a compassionate-use basis.
These results, published in Science Translational Medicine, have led to a phase 1 trial of ALRN-6924 in patients with AML or MDS.
ALRN-6924 was developed by Aileron Therapeutics Inc., to target p53 by inhibiting 2 naturally occurring proteins, MDMX and MDM2. Overexpression of these proteins inactivates p53, allowing cancer cells to multiply unchecked.
In the current study, researchers set out to confirm ALRN-6924’s mechanism of action and determine the efficacy of the drug in AML/MDS. This work was supported, in part, by grants from Aileron Therapeutics Inc., and the National Institutes of Health.
The researchers did find that ALRN-6924 targets both MDMX and MDM2, blocking their interaction with p53 in AML cells.
The team said ALRN-6924 inhibited proliferation by inducing cell-cycle arrest and apoptosis in AML cell lines and AML patient cells, including leukemic stem cell-enriched populations.
“This is important because AML is driven by stem cells, and, if you don’t target stem cells, the disease will come back very quickly,” said study author Ulrich Steidl, MD, PhD, of Albert Einstein College of Medicine in Bronx, New York.
The researchers also found that ALRN-6924 greatly increased survival in a mouse model of AML. The median survival was 34 days in vehicle-treated control mice, 83 days in mice that received ALRN-6924 at 20 mg/kg twice a week, and 151 days in mice that received ALRN-6924 at 20 mg/kg three times a week.
“This is a very striking response,” Dr Steidl said. “Most experimental drugs for leukemia achieve an increase in survival of only a few days in these preclinical models. Even more importantly, ALRN-6924 effectively cured about 40% of the treated mice, meaning they were disease-free more than 1 year after treatment, essentially a lifetime for a mouse.”
Finally, the researchers assessed the effects of ALRN-6924 in a patient who had high-risk MDS with excess leukemic blasts.
The team found the p53 protein “was rapidly induced” in CD34+ leukemic blasts but not in healthy lymphocytes. And ALRN-6924 reduced the number of malignant cells circulating in the blood.
“This test was not designed to assess the efficacy of the drug in humans,” Dr Steidl noted. “That has to be done in a proper clinical trial. Our goal was to determine whether it can hit the desired target in human cells in a clinical setting, which it did in this individual.”
ALRN-6924 is a stapled alpha-helical peptide, a class of drugs whose helical structure is stabilized using hydrocarbon “staples.” The stapling prevents the peptides from being degraded by enzymes before reaching their intended target. ALRN-6924 is the first stapled peptide therapeutic to be tested in patients.
In the phase 1 trial (NCT02909972), researchers are testing ALRN-6924 in patients with relapsed/refractory AML or advanced MDS.
Group identifies novel genes involved in MM development
Researchers have identified novel genes involved in the development of multiple myeloma (MM), according to a paper published in Leukemia.
The team’s analyses revealed regions of coding and non-coding DNA that appear to drive MM development.
The researchers analyzed whole-exome sequencing data from 804 MM patients and whole-genome sequencing data from 765 MM patients.
This revealed 16 novel genes that were disrupted in coding regions of DNA and 15 novel genes disrupted in non-coding regions.
There were 5 genes disrupted by structural variants in coding regions—CD96, PRDM1, FBXW7, MAP3K14, and CCND2.
There were also 11 genes disrupted by single nucleotide variants (SNVs) and indels in coding regions—BAX, C8orf86, FAM154B, FTL, HIST1H4H, LEMD2, PABPC1, RPN1, RPS3A, SGPP1, and TBC1D29.
Among the novel genes disrupted by mutations in non-coding regions was NBPF1, a promoter disrupted by SNVs. The researchers noted that NBPF1 is directly regulated by NF-κB, and the NF-κB pathway is recurrently affected in MM.
The team also identified 7 cis-regulatory elements (CREs) disrupted by SNVs—CALCB, COBLL1, HOXB3, ST6GAL1, PAX5, ATP13A2, and TPRG1.
The researchers said the SNVs in PAX5 and HOXB3 reduced gene expression, suggesting PAX5 and HOXB3 function as tumor suppressors in MM. On the other hand, the SNVs in ST6GAL1 increased gene expression, which may contribute to the aberrant immunoglobulin-G glycosylation seen in MM.
Finally, there were 7 CREs disrupted by copy number variations—MYC, PLD4, KDM3B, SP110, RAB36, PACS2, and TEX22.
The researchers noted that, with the exception of MYC, these genes reside close to regions of common structural variation, so their relevance in MM is not clear.
The team also said it’s well known that MYC is upregulated in MM through gene amplification or translocation, but this research shows that MYC can be dysregulated by alternative mechanisms.
“We need smarter, kinder treatments for myeloma that are more tailored to each person’s cancer,” said study author Richard Houlston, MD, PhD, of The Institute of Cancer Research in London, UK.
“Exhaustive genetic research like this is helping us to make that possible. Our findings should now open up new avenues for discovering treatments that target the genes driving myeloma.”
Researchers have identified novel genes involved in the development of multiple myeloma (MM), according to a paper published in Leukemia.
The team’s analyses revealed regions of coding and non-coding DNA that appear to drive MM development.
The researchers analyzed whole-exome sequencing data from 804 MM patients and whole-genome sequencing data from 765 MM patients.
This revealed 16 novel genes that were disrupted in coding regions of DNA and 15 novel genes disrupted in non-coding regions.
There were 5 genes disrupted by structural variants in coding regions—CD96, PRDM1, FBXW7, MAP3K14, and CCND2.
There were also 11 genes disrupted by single nucleotide variants (SNVs) and indels in coding regions—BAX, C8orf86, FAM154B, FTL, HIST1H4H, LEMD2, PABPC1, RPN1, RPS3A, SGPP1, and TBC1D29.
Among the novel genes disrupted by mutations in non-coding regions was NBPF1, a promoter disrupted by SNVs. The researchers noted that NBPF1 is directly regulated by NF-κB, and the NF-κB pathway is recurrently affected in MM.
The team also identified 7 cis-regulatory elements (CREs) disrupted by SNVs—CALCB, COBLL1, HOXB3, ST6GAL1, PAX5, ATP13A2, and TPRG1.
The researchers said the SNVs in PAX5 and HOXB3 reduced gene expression, suggesting PAX5 and HOXB3 function as tumor suppressors in MM. On the other hand, the SNVs in ST6GAL1 increased gene expression, which may contribute to the aberrant immunoglobulin-G glycosylation seen in MM.
Finally, there were 7 CREs disrupted by copy number variations—MYC, PLD4, KDM3B, SP110, RAB36, PACS2, and TEX22.
The researchers noted that, with the exception of MYC, these genes reside close to regions of common structural variation, so their relevance in MM is not clear.
The team also said it’s well known that MYC is upregulated in MM through gene amplification or translocation, but this research shows that MYC can be dysregulated by alternative mechanisms.
“We need smarter, kinder treatments for myeloma that are more tailored to each person’s cancer,” said study author Richard Houlston, MD, PhD, of The Institute of Cancer Research in London, UK.
“Exhaustive genetic research like this is helping us to make that possible. Our findings should now open up new avenues for discovering treatments that target the genes driving myeloma.”
Researchers have identified novel genes involved in the development of multiple myeloma (MM), according to a paper published in Leukemia.
The team’s analyses revealed regions of coding and non-coding DNA that appear to drive MM development.
The researchers analyzed whole-exome sequencing data from 804 MM patients and whole-genome sequencing data from 765 MM patients.
This revealed 16 novel genes that were disrupted in coding regions of DNA and 15 novel genes disrupted in non-coding regions.
There were 5 genes disrupted by structural variants in coding regions—CD96, PRDM1, FBXW7, MAP3K14, and CCND2.
There were also 11 genes disrupted by single nucleotide variants (SNVs) and indels in coding regions—BAX, C8orf86, FAM154B, FTL, HIST1H4H, LEMD2, PABPC1, RPN1, RPS3A, SGPP1, and TBC1D29.
Among the novel genes disrupted by mutations in non-coding regions was NBPF1, a promoter disrupted by SNVs. The researchers noted that NBPF1 is directly regulated by NF-κB, and the NF-κB pathway is recurrently affected in MM.
The team also identified 7 cis-regulatory elements (CREs) disrupted by SNVs—CALCB, COBLL1, HOXB3, ST6GAL1, PAX5, ATP13A2, and TPRG1.
The researchers said the SNVs in PAX5 and HOXB3 reduced gene expression, suggesting PAX5 and HOXB3 function as tumor suppressors in MM. On the other hand, the SNVs in ST6GAL1 increased gene expression, which may contribute to the aberrant immunoglobulin-G glycosylation seen in MM.
Finally, there were 7 CREs disrupted by copy number variations—MYC, PLD4, KDM3B, SP110, RAB36, PACS2, and TEX22.
The researchers noted that, with the exception of MYC, these genes reside close to regions of common structural variation, so their relevance in MM is not clear.
The team also said it’s well known that MYC is upregulated in MM through gene amplification or translocation, but this research shows that MYC can be dysregulated by alternative mechanisms.
“We need smarter, kinder treatments for myeloma that are more tailored to each person’s cancer,” said study author Richard Houlston, MD, PhD, of The Institute of Cancer Research in London, UK.
“Exhaustive genetic research like this is helping us to make that possible. Our findings should now open up new avenues for discovering treatments that target the genes driving myeloma.”
Generic antiemetic now available in US
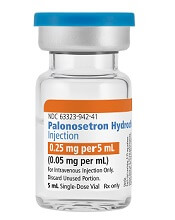
Palonosetron Hydrochloride Injection, a generic alternative to Aloxi®, is now available in the US.
Fresenius Kabi’s Palonosetron Hydrochloride Injection is a 5-HT3 serotonin receptor that is approved for the prevention of nausea and vomiting in certain adults.
Palonosetron Hydrochloride Injection is available in a single-dose vial (0.25 mg per 5 mL).
In the US, Palonosetron Hydrochloride Injection is approved for the prevention of acute and delayed nausea and vomiting associated with initial and repeat courses of moderately emetogenic cancer chemotherapy.
The drug is also approved for the prevention of acute nausea and vomiting associated with initial and repeat courses of highly emetogenic cancer chemotherapy.
And Palonosetron Hydrochloride Injection is approved for the prevention of post-operative nausea and vomiting for up to 24 hours after surgery. Efficacy beyond 24 hours has not been demonstrated.
The full prescribing information for Palonosetron Hydrochloride Injection can be found on the Fresenius Kabi website.
Palonosetron Hydrochloride Injection, a generic alternative to Aloxi®, is now available in the US.
Fresenius Kabi’s Palonosetron Hydrochloride Injection is a 5-HT3 serotonin receptor that is approved for the prevention of nausea and vomiting in certain adults.
Palonosetron Hydrochloride Injection is available in a single-dose vial (0.25 mg per 5 mL).
In the US, Palonosetron Hydrochloride Injection is approved for the prevention of acute and delayed nausea and vomiting associated with initial and repeat courses of moderately emetogenic cancer chemotherapy.
The drug is also approved for the prevention of acute nausea and vomiting associated with initial and repeat courses of highly emetogenic cancer chemotherapy.
And Palonosetron Hydrochloride Injection is approved for the prevention of post-operative nausea and vomiting for up to 24 hours after surgery. Efficacy beyond 24 hours has not been demonstrated.
The full prescribing information for Palonosetron Hydrochloride Injection can be found on the Fresenius Kabi website.
Palonosetron Hydrochloride Injection, a generic alternative to Aloxi®, is now available in the US.
Fresenius Kabi’s Palonosetron Hydrochloride Injection is a 5-HT3 serotonin receptor that is approved for the prevention of nausea and vomiting in certain adults.
Palonosetron Hydrochloride Injection is available in a single-dose vial (0.25 mg per 5 mL).
In the US, Palonosetron Hydrochloride Injection is approved for the prevention of acute and delayed nausea and vomiting associated with initial and repeat courses of moderately emetogenic cancer chemotherapy.
The drug is also approved for the prevention of acute nausea and vomiting associated with initial and repeat courses of highly emetogenic cancer chemotherapy.
And Palonosetron Hydrochloride Injection is approved for the prevention of post-operative nausea and vomiting for up to 24 hours after surgery. Efficacy beyond 24 hours has not been demonstrated.
The full prescribing information for Palonosetron Hydrochloride Injection can be found on the Fresenius Kabi website.
Gene variants linked to survival after HSCT
New research has revealed a link between rare gene variants and survival after hematopoietic stem cell transplant (HSCT).
Researchers performed exome sequencing in nearly 2500 HSCT recipients and their matched, unrelated donors.
The sequencing revealed several gene variants—in both donors and recipients—that were significantly associated with overall survival (OS), transplant-related mortality (TRM), and disease-related mortality (DRM) after HSCT.
Qianqian Zhu, PhD, of Roswell Park Comprehensive Cancer Center in Buffalo, New York, and her colleagues described these findings in Blood.
The team performed exome sequencing—using the Illumina HumanExome BeadChip—in patients who participated in the DISCOVeRY-BMT study.
This included 2473 HSCT recipients who had acute myeloid leukemia, acute lymphoblastic leukemia, or myelodysplastic syndromes. It also included 2221 donors who were a 10/10 human leukocyte antigen match for each recipient.
The researchers looked at genetic variants in donors and recipients and assessed the variants’ associations with OS, TRM, and DRM.
Variants in recipients
Analyses revealed an increased risk of TRM when there was a mismatch between donors and recipients for a variant in TEX38—rs200092801. The increased risk was even more pronounced when either the recipient or the donor was female.
Among the recipients mismatched with their donors at rs200092801, every female recipient and every recipient with a female donor died from TRM. In comparison, 44% of the male recipients with male donors died from TRM.
The researchers said the rs200092801 variant may prompt the production of a mutant peptide that can be presented by MHC-I molecules to immune cells to trigger downstream immune response and TRM.
Dr Zhu and her colleagues also identified variants that appeared to have a positive impact on TRM and OS.
Recipients who had any of 6 variants in the gene OR51D1 had a decreased risk of TRM and improved OS.
The variants (rs138224979, rs148606808, rs141786655, rs61745314, rs200394876, and rs149135276) were not associated with DRM, so the researchers concluded that the improvement in OS was driven by protection against TRM.
Donor variants linked to OS
Donors had variants in 4 genes—ALPP, EMID1, SLC44A5, and LRP1—that were associated with OS but not TRM or DRM.
The 3 variants identified in ALPP (rs144454460, rs140078460, and rs142493383) were associated with improved OS.
And the 2 variants in SLC44A5 (rs143004355 and rs149696907) were associated with worse OS.
There were 2 variants in EMID1. One was associated with improved OS (rs34772704), and the other was associated with decreased OS (rs139996840).
And there were 27 variants in LRP1. Some had a positive association with OS, and others had a negative association.
Donor variants linked to TRM and DRM
Six variants in the HHAT gene were associated with TRM. Five of the variants appeared to have a protective effect against TRM (rs145455128, rs146916002, rs61744143, rs149597734, and rs145943928). For the other variant (rs141591165), the apparent effect was inconsistent between patient cohorts.
There were 3 variants in LYZL4 associated with DRM. Two were associated with an increased risk of DRM (rs147770623 and rs76947105), and 1 appeared to have a protective effect (rs181886204).
Six variants in NT5E appeared to have a protective effect against DRM (rs200250022, rs200369370, rs41271617, rs200648774, rs144719925, and rs145505137).
The researchers said the variants in NT5E probably reduce the enzyme activity of the gene. This supports preclinical findings showing that targeted blockade of NT5E can slow tumor growth.
“We have just started to uncover the biological relevance of these new and unexpected genes to a patient’s survival after [HSCT],” Dr Zhu said.
“Our findings shed light on new areas that were not considered before, but we need to further replicate and test our findings. We’re hoping that additional studies of this type will continue to discover novel genes leading to improved outcomes for patients.”
New research has revealed a link between rare gene variants and survival after hematopoietic stem cell transplant (HSCT).
Researchers performed exome sequencing in nearly 2500 HSCT recipients and their matched, unrelated donors.
The sequencing revealed several gene variants—in both donors and recipients—that were significantly associated with overall survival (OS), transplant-related mortality (TRM), and disease-related mortality (DRM) after HSCT.
Qianqian Zhu, PhD, of Roswell Park Comprehensive Cancer Center in Buffalo, New York, and her colleagues described these findings in Blood.
The team performed exome sequencing—using the Illumina HumanExome BeadChip—in patients who participated in the DISCOVeRY-BMT study.
This included 2473 HSCT recipients who had acute myeloid leukemia, acute lymphoblastic leukemia, or myelodysplastic syndromes. It also included 2221 donors who were a 10/10 human leukocyte antigen match for each recipient.
The researchers looked at genetic variants in donors and recipients and assessed the variants’ associations with OS, TRM, and DRM.
Variants in recipients
Analyses revealed an increased risk of TRM when there was a mismatch between donors and recipients for a variant in TEX38—rs200092801. The increased risk was even more pronounced when either the recipient or the donor was female.
Among the recipients mismatched with their donors at rs200092801, every female recipient and every recipient with a female donor died from TRM. In comparison, 44% of the male recipients with male donors died from TRM.
The researchers said the rs200092801 variant may prompt the production of a mutant peptide that can be presented by MHC-I molecules to immune cells to trigger downstream immune response and TRM.
Dr Zhu and her colleagues also identified variants that appeared to have a positive impact on TRM and OS.
Recipients who had any of 6 variants in the gene OR51D1 had a decreased risk of TRM and improved OS.
The variants (rs138224979, rs148606808, rs141786655, rs61745314, rs200394876, and rs149135276) were not associated with DRM, so the researchers concluded that the improvement in OS was driven by protection against TRM.
Donor variants linked to OS
Donors had variants in 4 genes—ALPP, EMID1, SLC44A5, and LRP1—that were associated with OS but not TRM or DRM.
The 3 variants identified in ALPP (rs144454460, rs140078460, and rs142493383) were associated with improved OS.
And the 2 variants in SLC44A5 (rs143004355 and rs149696907) were associated with worse OS.
There were 2 variants in EMID1. One was associated with improved OS (rs34772704), and the other was associated with decreased OS (rs139996840).
And there were 27 variants in LRP1. Some had a positive association with OS, and others had a negative association.
Donor variants linked to TRM and DRM
Six variants in the HHAT gene were associated with TRM. Five of the variants appeared to have a protective effect against TRM (rs145455128, rs146916002, rs61744143, rs149597734, and rs145943928). For the other variant (rs141591165), the apparent effect was inconsistent between patient cohorts.
There were 3 variants in LYZL4 associated with DRM. Two were associated with an increased risk of DRM (rs147770623 and rs76947105), and 1 appeared to have a protective effect (rs181886204).
Six variants in NT5E appeared to have a protective effect against DRM (rs200250022, rs200369370, rs41271617, rs200648774, rs144719925, and rs145505137).
The researchers said the variants in NT5E probably reduce the enzyme activity of the gene. This supports preclinical findings showing that targeted blockade of NT5E can slow tumor growth.
“We have just started to uncover the biological relevance of these new and unexpected genes to a patient’s survival after [HSCT],” Dr Zhu said.
“Our findings shed light on new areas that were not considered before, but we need to further replicate and test our findings. We’re hoping that additional studies of this type will continue to discover novel genes leading to improved outcomes for patients.”
New research has revealed a link between rare gene variants and survival after hematopoietic stem cell transplant (HSCT).
Researchers performed exome sequencing in nearly 2500 HSCT recipients and their matched, unrelated donors.
The sequencing revealed several gene variants—in both donors and recipients—that were significantly associated with overall survival (OS), transplant-related mortality (TRM), and disease-related mortality (DRM) after HSCT.
Qianqian Zhu, PhD, of Roswell Park Comprehensive Cancer Center in Buffalo, New York, and her colleagues described these findings in Blood.
The team performed exome sequencing—using the Illumina HumanExome BeadChip—in patients who participated in the DISCOVeRY-BMT study.
This included 2473 HSCT recipients who had acute myeloid leukemia, acute lymphoblastic leukemia, or myelodysplastic syndromes. It also included 2221 donors who were a 10/10 human leukocyte antigen match for each recipient.
The researchers looked at genetic variants in donors and recipients and assessed the variants’ associations with OS, TRM, and DRM.
Variants in recipients
Analyses revealed an increased risk of TRM when there was a mismatch between donors and recipients for a variant in TEX38—rs200092801. The increased risk was even more pronounced when either the recipient or the donor was female.
Among the recipients mismatched with their donors at rs200092801, every female recipient and every recipient with a female donor died from TRM. In comparison, 44% of the male recipients with male donors died from TRM.
The researchers said the rs200092801 variant may prompt the production of a mutant peptide that can be presented by MHC-I molecules to immune cells to trigger downstream immune response and TRM.
Dr Zhu and her colleagues also identified variants that appeared to have a positive impact on TRM and OS.
Recipients who had any of 6 variants in the gene OR51D1 had a decreased risk of TRM and improved OS.
The variants (rs138224979, rs148606808, rs141786655, rs61745314, rs200394876, and rs149135276) were not associated with DRM, so the researchers concluded that the improvement in OS was driven by protection against TRM.
Donor variants linked to OS
Donors had variants in 4 genes—ALPP, EMID1, SLC44A5, and LRP1—that were associated with OS but not TRM or DRM.
The 3 variants identified in ALPP (rs144454460, rs140078460, and rs142493383) were associated with improved OS.
And the 2 variants in SLC44A5 (rs143004355 and rs149696907) were associated with worse OS.
There were 2 variants in EMID1. One was associated with improved OS (rs34772704), and the other was associated with decreased OS (rs139996840).
And there were 27 variants in LRP1. Some had a positive association with OS, and others had a negative association.
Donor variants linked to TRM and DRM
Six variants in the HHAT gene were associated with TRM. Five of the variants appeared to have a protective effect against TRM (rs145455128, rs146916002, rs61744143, rs149597734, and rs145943928). For the other variant (rs141591165), the apparent effect was inconsistent between patient cohorts.
There were 3 variants in LYZL4 associated with DRM. Two were associated with an increased risk of DRM (rs147770623 and rs76947105), and 1 appeared to have a protective effect (rs181886204).
Six variants in NT5E appeared to have a protective effect against DRM (rs200250022, rs200369370, rs41271617, rs200648774, rs144719925, and rs145505137).
The researchers said the variants in NT5E probably reduce the enzyme activity of the gene. This supports preclinical findings showing that targeted blockade of NT5E can slow tumor growth.
“We have just started to uncover the biological relevance of these new and unexpected genes to a patient’s survival after [HSCT],” Dr Zhu said.
“Our findings shed light on new areas that were not considered before, but we need to further replicate and test our findings. We’re hoping that additional studies of this type will continue to discover novel genes leading to improved outcomes for patients.”
Tazemetostat exhibits antitumor activity in phase 1 trial
The EZH2 inhibitor tazemetostat demonstrated a “favorable safety profile and antitumor activity” in a phase 1 study, according to researchers.
The drug produced responses in 8 of 21 patients with relapsed/refractory B-cell non-Hodgkin lymphoma (NHL), including 3 complete responses (CRs).
The maximum tolerated dose of tazemetostat was not reached, and there were no fatal adverse events (AEs) related to treatment.
Grade 3/4 treatment-related AEs included thrombocytopenia, neutropenia, hepatocellular injury, and hypertension.
Antoine Italiano, MD, PhD, of Institut Bergonié in Bordeaux, France, and his colleagues reported these results in The Lancet Oncology. The trial was sponsored by Epizyme and Eisai.
The study enrolled 64 patients—43 with solid tumors and 21 with B-cell NHL. The following characteristics and dosing information pertain only to the patients with NHL.
Thirteen patients had diffuse large B-cell lymphoma (DLBCL), 7 had follicular lymphoma (FL), and 1 had marginal zone lymphoma (MZL).
The patients’ median age was 62 (range, 53-70), and 71% were male. They had an ECOG performance status of 0 (62%) or 1 (38%).
Most patients had received at least 3 prior therapies—38% had 3, 14% had 4, and 33% had 5 or more prior therapies. Forty-eight percent had prior hematopoietic stem cell transplant.
The patients received escalating doses of tazemetostat twice daily—100 mg (n=1), 200 mg (n=2), 400 mg (n=1), 800 mg (n=8), and 1600 mg (n=4).
The remaining 5 patients were enrolled in a substudy to evaluate food effect. These patients received a single 200 mg dose on day -8 and day -1, with or without food, followed by 400 mg twice daily starting on day 1. Specific results on the food effects were not included in the paper.
Safety
In the entire study cohort, there was 1 dose-limiting toxicity—grade 4 thrombocytopenia—at the 1600 mg dose. The maximum tolerated dose of tazemetostat was not reached, but the researchers decided upon 800 mg twice daily as the recommended phase 2 dose.
Overall, 77% (n=49) of patients had treatment-related AEs. Grade 3/4 treatment-related AEs included thrombocytopenia (4%, n=2), neutropenia (4%, n=2), hepatocellular injury (2%, n=1), and hypertension (2%, n=1).
Serious treatment-related AEs were neutropenia in 1 patient (800 mg group) and anemia and thrombocytopenia in another patient (1600 mg group).
Seven patients (11%) had fatal AEs, but none were considered treatment-related. They included general physical health deterioration (1 at 200 mg, 1 at 1600 mg, and 2 at 400 mg), respiratory distress (2 at 400 mg), and septic shock (1 at 1600 mg).
Efficacy
Eight of the 21 NHL patients responded to treatment. Three patients had a CR—1 with DLBCL and 2 with FL. Of the 5 partial responders, 3 had DLBCL, 1 had FL, and 1 had MZL.
The median time to first response was 3.5 months, and the median duration of response was 12.4 months.
The 3 complete responders remained on tazemetostat beyond 27.6 months (FL patient), 28.8 months (FL patient), and 33.6 months (DLBCL patient).
Two of the 43 patients with solid tumors responded to tazemetostat—1 with a CR and 1 with a partial response.
The complete responder had an INI1-negative malignant rhabdoid tumor, and the partial responder had a SMARCA4-negative malignant rhabdoid tumor of the ovary.
“Today’s publication in The Lancet Oncology reports the safety and tolerability endpoints for tazemetostat in this study, which enabled further evaluation of EZH2 inhibition in INI1- and SMARCA4-negative solid tumors and NHL,” Dr Italiano said. “I’m also encouraged by the preliminary antitumor activity observed in this study.”
The EZH2 inhibitor tazemetostat demonstrated a “favorable safety profile and antitumor activity” in a phase 1 study, according to researchers.
The drug produced responses in 8 of 21 patients with relapsed/refractory B-cell non-Hodgkin lymphoma (NHL), including 3 complete responses (CRs).
The maximum tolerated dose of tazemetostat was not reached, and there were no fatal adverse events (AEs) related to treatment.
Grade 3/4 treatment-related AEs included thrombocytopenia, neutropenia, hepatocellular injury, and hypertension.
Antoine Italiano, MD, PhD, of Institut Bergonié in Bordeaux, France, and his colleagues reported these results in The Lancet Oncology. The trial was sponsored by Epizyme and Eisai.
The study enrolled 64 patients—43 with solid tumors and 21 with B-cell NHL. The following characteristics and dosing information pertain only to the patients with NHL.
Thirteen patients had diffuse large B-cell lymphoma (DLBCL), 7 had follicular lymphoma (FL), and 1 had marginal zone lymphoma (MZL).
The patients’ median age was 62 (range, 53-70), and 71% were male. They had an ECOG performance status of 0 (62%) or 1 (38%).
Most patients had received at least 3 prior therapies—38% had 3, 14% had 4, and 33% had 5 or more prior therapies. Forty-eight percent had prior hematopoietic stem cell transplant.
The patients received escalating doses of tazemetostat twice daily—100 mg (n=1), 200 mg (n=2), 400 mg (n=1), 800 mg (n=8), and 1600 mg (n=4).
The remaining 5 patients were enrolled in a substudy to evaluate food effect. These patients received a single 200 mg dose on day -8 and day -1, with or without food, followed by 400 mg twice daily starting on day 1. Specific results on the food effects were not included in the paper.
Safety
In the entire study cohort, there was 1 dose-limiting toxicity—grade 4 thrombocytopenia—at the 1600 mg dose. The maximum tolerated dose of tazemetostat was not reached, but the researchers decided upon 800 mg twice daily as the recommended phase 2 dose.
Overall, 77% (n=49) of patients had treatment-related AEs. Grade 3/4 treatment-related AEs included thrombocytopenia (4%, n=2), neutropenia (4%, n=2), hepatocellular injury (2%, n=1), and hypertension (2%, n=1).
Serious treatment-related AEs were neutropenia in 1 patient (800 mg group) and anemia and thrombocytopenia in another patient (1600 mg group).
Seven patients (11%) had fatal AEs, but none were considered treatment-related. They included general physical health deterioration (1 at 200 mg, 1 at 1600 mg, and 2 at 400 mg), respiratory distress (2 at 400 mg), and septic shock (1 at 1600 mg).
Efficacy
Eight of the 21 NHL patients responded to treatment. Three patients had a CR—1 with DLBCL and 2 with FL. Of the 5 partial responders, 3 had DLBCL, 1 had FL, and 1 had MZL.
The median time to first response was 3.5 months, and the median duration of response was 12.4 months.
The 3 complete responders remained on tazemetostat beyond 27.6 months (FL patient), 28.8 months (FL patient), and 33.6 months (DLBCL patient).
Two of the 43 patients with solid tumors responded to tazemetostat—1 with a CR and 1 with a partial response.
The complete responder had an INI1-negative malignant rhabdoid tumor, and the partial responder had a SMARCA4-negative malignant rhabdoid tumor of the ovary.
“Today’s publication in The Lancet Oncology reports the safety and tolerability endpoints for tazemetostat in this study, which enabled further evaluation of EZH2 inhibition in INI1- and SMARCA4-negative solid tumors and NHL,” Dr Italiano said. “I’m also encouraged by the preliminary antitumor activity observed in this study.”
The EZH2 inhibitor tazemetostat demonstrated a “favorable safety profile and antitumor activity” in a phase 1 study, according to researchers.
The drug produced responses in 8 of 21 patients with relapsed/refractory B-cell non-Hodgkin lymphoma (NHL), including 3 complete responses (CRs).
The maximum tolerated dose of tazemetostat was not reached, and there were no fatal adverse events (AEs) related to treatment.
Grade 3/4 treatment-related AEs included thrombocytopenia, neutropenia, hepatocellular injury, and hypertension.
Antoine Italiano, MD, PhD, of Institut Bergonié in Bordeaux, France, and his colleagues reported these results in The Lancet Oncology. The trial was sponsored by Epizyme and Eisai.
The study enrolled 64 patients—43 with solid tumors and 21 with B-cell NHL. The following characteristics and dosing information pertain only to the patients with NHL.
Thirteen patients had diffuse large B-cell lymphoma (DLBCL), 7 had follicular lymphoma (FL), and 1 had marginal zone lymphoma (MZL).
The patients’ median age was 62 (range, 53-70), and 71% were male. They had an ECOG performance status of 0 (62%) or 1 (38%).
Most patients had received at least 3 prior therapies—38% had 3, 14% had 4, and 33% had 5 or more prior therapies. Forty-eight percent had prior hematopoietic stem cell transplant.
The patients received escalating doses of tazemetostat twice daily—100 mg (n=1), 200 mg (n=2), 400 mg (n=1), 800 mg (n=8), and 1600 mg (n=4).
The remaining 5 patients were enrolled in a substudy to evaluate food effect. These patients received a single 200 mg dose on day -8 and day -1, with or without food, followed by 400 mg twice daily starting on day 1. Specific results on the food effects were not included in the paper.
Safety
In the entire study cohort, there was 1 dose-limiting toxicity—grade 4 thrombocytopenia—at the 1600 mg dose. The maximum tolerated dose of tazemetostat was not reached, but the researchers decided upon 800 mg twice daily as the recommended phase 2 dose.
Overall, 77% (n=49) of patients had treatment-related AEs. Grade 3/4 treatment-related AEs included thrombocytopenia (4%, n=2), neutropenia (4%, n=2), hepatocellular injury (2%, n=1), and hypertension (2%, n=1).
Serious treatment-related AEs were neutropenia in 1 patient (800 mg group) and anemia and thrombocytopenia in another patient (1600 mg group).
Seven patients (11%) had fatal AEs, but none were considered treatment-related. They included general physical health deterioration (1 at 200 mg, 1 at 1600 mg, and 2 at 400 mg), respiratory distress (2 at 400 mg), and septic shock (1 at 1600 mg).
Efficacy
Eight of the 21 NHL patients responded to treatment. Three patients had a CR—1 with DLBCL and 2 with FL. Of the 5 partial responders, 3 had DLBCL, 1 had FL, and 1 had MZL.
The median time to first response was 3.5 months, and the median duration of response was 12.4 months.
The 3 complete responders remained on tazemetostat beyond 27.6 months (FL patient), 28.8 months (FL patient), and 33.6 months (DLBCL patient).
Two of the 43 patients with solid tumors responded to tazemetostat—1 with a CR and 1 with a partial response.
The complete responder had an INI1-negative malignant rhabdoid tumor, and the partial responder had a SMARCA4-negative malignant rhabdoid tumor of the ovary.
“Today’s publication in The Lancet Oncology reports the safety and tolerability endpoints for tazemetostat in this study, which enabled further evaluation of EZH2 inhibition in INI1- and SMARCA4-negative solid tumors and NHL,” Dr Italiano said. “I’m also encouraged by the preliminary antitumor activity observed in this study.”
Selinexor receives fast track designation for MM
The US Food and Drug Administration (FDA) has granted fast track designation to selinexor for the treatment of patients with penta-refractory multiple myeloma (MM).
The patients must have received at least 3 prior lines of therapy that included an alkylating agent, a glucocorticoid, bortezomib, carfilzomib, lenalidomide, pomalidomide, and daratumumab.
In addition, the patients must have disease that is refractory to at least 1 proteasome inhibitor, at least 1 immunomodulatory agent, glucocorticoids, daratumumab, and the patients’ most recent therapy.
“The designation of fast track for selinexor represents important recognition by the FDA of the potential of this anticancer agent to address the significant unmet need in the treatment of patients with penta-refractory myeloma that has continued to progress despite available therapies,” said Sharon Shacham, PhD, founder, president, and chief scientific officer of Karyopharm Therapeutics Inc., the company developing selinexor.
The FDA’s fast track drug development program is designed to expedite clinical development and submission of new drug applications for medicines with the potential to treat serious or life-threatening conditions and address unmet medical needs.
Fast track designation facilitates frequent interactions with the FDA review team, including meetings to discuss all aspects of development to support a drug’s approval, and also provides the opportunity to submit sections of a new drug application on a rolling basis as data become available.
About selinexor
Selinexor (formerly KPT-330) is a first-in-class, oral selective inhibitor of nuclear export compound.
Selinexor functions by binding with and inhibiting the nuclear export protein XPO1 (also called CRM1), leading to the accumulation of tumor suppressor proteins in the cell nucleus. This reinitiates and amplifies their tumor suppressor function and is believed to lead to apoptosis in cancer cells while largely sparing normal cells.
Selinexor is currently being evaluated in several clinical trials across multiple cancer indications.
In the phase 2 STORM trial, researchers are testing selinexor in combination with low-dose dexamethasone for patients with penta-refractory MM. Karyopharm Therapeutics plans to report top-line data from this study at the end of this month.
Trials of selinexor were placed on partial clinical hold in mid-March last year due to a lack of information about serious adverse events. However, the hold was lifted for trials of patients with hematologic malignancies at the end of that same month.
The US Food and Drug Administration (FDA) has granted fast track designation to selinexor for the treatment of patients with penta-refractory multiple myeloma (MM).
The patients must have received at least 3 prior lines of therapy that included an alkylating agent, a glucocorticoid, bortezomib, carfilzomib, lenalidomide, pomalidomide, and daratumumab.
In addition, the patients must have disease that is refractory to at least 1 proteasome inhibitor, at least 1 immunomodulatory agent, glucocorticoids, daratumumab, and the patients’ most recent therapy.
“The designation of fast track for selinexor represents important recognition by the FDA of the potential of this anticancer agent to address the significant unmet need in the treatment of patients with penta-refractory myeloma that has continued to progress despite available therapies,” said Sharon Shacham, PhD, founder, president, and chief scientific officer of Karyopharm Therapeutics Inc., the company developing selinexor.
The FDA’s fast track drug development program is designed to expedite clinical development and submission of new drug applications for medicines with the potential to treat serious or life-threatening conditions and address unmet medical needs.
Fast track designation facilitates frequent interactions with the FDA review team, including meetings to discuss all aspects of development to support a drug’s approval, and also provides the opportunity to submit sections of a new drug application on a rolling basis as data become available.
About selinexor
Selinexor (formerly KPT-330) is a first-in-class, oral selective inhibitor of nuclear export compound.
Selinexor functions by binding with and inhibiting the nuclear export protein XPO1 (also called CRM1), leading to the accumulation of tumor suppressor proteins in the cell nucleus. This reinitiates and amplifies their tumor suppressor function and is believed to lead to apoptosis in cancer cells while largely sparing normal cells.
Selinexor is currently being evaluated in several clinical trials across multiple cancer indications.
In the phase 2 STORM trial, researchers are testing selinexor in combination with low-dose dexamethasone for patients with penta-refractory MM. Karyopharm Therapeutics plans to report top-line data from this study at the end of this month.
Trials of selinexor were placed on partial clinical hold in mid-March last year due to a lack of information about serious adverse events. However, the hold was lifted for trials of patients with hematologic malignancies at the end of that same month.
The US Food and Drug Administration (FDA) has granted fast track designation to selinexor for the treatment of patients with penta-refractory multiple myeloma (MM).
The patients must have received at least 3 prior lines of therapy that included an alkylating agent, a glucocorticoid, bortezomib, carfilzomib, lenalidomide, pomalidomide, and daratumumab.
In addition, the patients must have disease that is refractory to at least 1 proteasome inhibitor, at least 1 immunomodulatory agent, glucocorticoids, daratumumab, and the patients’ most recent therapy.
“The designation of fast track for selinexor represents important recognition by the FDA of the potential of this anticancer agent to address the significant unmet need in the treatment of patients with penta-refractory myeloma that has continued to progress despite available therapies,” said Sharon Shacham, PhD, founder, president, and chief scientific officer of Karyopharm Therapeutics Inc., the company developing selinexor.
The FDA’s fast track drug development program is designed to expedite clinical development and submission of new drug applications for medicines with the potential to treat serious or life-threatening conditions and address unmet medical needs.
Fast track designation facilitates frequent interactions with the FDA review team, including meetings to discuss all aspects of development to support a drug’s approval, and also provides the opportunity to submit sections of a new drug application on a rolling basis as data become available.
About selinexor
Selinexor (formerly KPT-330) is a first-in-class, oral selective inhibitor of nuclear export compound.
Selinexor functions by binding with and inhibiting the nuclear export protein XPO1 (also called CRM1), leading to the accumulation of tumor suppressor proteins in the cell nucleus. This reinitiates and amplifies their tumor suppressor function and is believed to lead to apoptosis in cancer cells while largely sparing normal cells.
Selinexor is currently being evaluated in several clinical trials across multiple cancer indications.
In the phase 2 STORM trial, researchers are testing selinexor in combination with low-dose dexamethasone for patients with penta-refractory MM. Karyopharm Therapeutics plans to report top-line data from this study at the end of this month.
Trials of selinexor were placed on partial clinical hold in mid-March last year due to a lack of information about serious adverse events. However, the hold was lifted for trials of patients with hematologic malignancies at the end of that same month.
Health Canada approves product for adult ALL
Health Canada has approved inotuzumab ozogamicin (Besponsa™) as monotherapy for adults with relapsed or refractory, CD22-positive, B-cell precursor acute lymphoblastic leukemia (ALL).
Inotuzumab ozogamicin is the first and only CD22-directed antibody-drug conjugate approved for this indication.
The product consists of a monoclonal antibody targeting CD22 and a cytotoxic agent known as calicheamicin.
Health Canada’s approval of inotuzumab ozogamicin is based on results from the phase 3 INO-VATE trial, which were published in NEJM in June 2016.
The trial enrolled 326 adults with relapsed or refractory B-cell precursor ALL.
Patients received inotuzumab ozogamicin or 1 of 3 chemotherapy regimens—high-dose cytarabine; cytarabine plus mitoxantrone; or fludarabine, cytarabine, and granulocyte colony-stimulating factor.
The rate of complete remission, including incomplete hematologic recovery, was 80.7% in the inotuzumab arm and 29.4% in the chemotherapy arm (P<0.001). The median duration of remission was 4.6 months and 3.1 months, respectively (P=0.03).
Forty-one percent of patients treated with inotuzumab and 11% of those who received chemotherapy proceeded to stem cell transplant directly after treatment (P<0.001).
The median progression-free survival was 5.0 months in the inotuzumab arm and 1.8 months in the chemotherapy arm (P<0.001).
The median overall survival was 7.7 months and 6.7 months, respectively (P=0.04). This did not meet the prespecified boundary of significance (P=0.0208).
Liver-related adverse events were more common in the inotuzumab arm than the chemotherapy arm. The most frequent of these were increased aspartate aminotransferase level (20% vs 10%), hyperbilirubinemia (15% vs 10%), and increased alanine aminotransferase level (14% vs 11%).
Veno-occlusive liver disease occurred in 11% of patients in the inotuzumab arm and 1% in the chemotherapy arm.
There were 17 deaths during treatment in the inotuzumab arm and 11 in the chemotherapy arm. Four deaths were considered related to inotuzumab, and 2 were deemed related to chemotherapy.
Health Canada has approved inotuzumab ozogamicin (Besponsa™) as monotherapy for adults with relapsed or refractory, CD22-positive, B-cell precursor acute lymphoblastic leukemia (ALL).
Inotuzumab ozogamicin is the first and only CD22-directed antibody-drug conjugate approved for this indication.
The product consists of a monoclonal antibody targeting CD22 and a cytotoxic agent known as calicheamicin.
Health Canada’s approval of inotuzumab ozogamicin is based on results from the phase 3 INO-VATE trial, which were published in NEJM in June 2016.
The trial enrolled 326 adults with relapsed or refractory B-cell precursor ALL.
Patients received inotuzumab ozogamicin or 1 of 3 chemotherapy regimens—high-dose cytarabine; cytarabine plus mitoxantrone; or fludarabine, cytarabine, and granulocyte colony-stimulating factor.
The rate of complete remission, including incomplete hematologic recovery, was 80.7% in the inotuzumab arm and 29.4% in the chemotherapy arm (P<0.001). The median duration of remission was 4.6 months and 3.1 months, respectively (P=0.03).
Forty-one percent of patients treated with inotuzumab and 11% of those who received chemotherapy proceeded to stem cell transplant directly after treatment (P<0.001).
The median progression-free survival was 5.0 months in the inotuzumab arm and 1.8 months in the chemotherapy arm (P<0.001).
The median overall survival was 7.7 months and 6.7 months, respectively (P=0.04). This did not meet the prespecified boundary of significance (P=0.0208).
Liver-related adverse events were more common in the inotuzumab arm than the chemotherapy arm. The most frequent of these were increased aspartate aminotransferase level (20% vs 10%), hyperbilirubinemia (15% vs 10%), and increased alanine aminotransferase level (14% vs 11%).
Veno-occlusive liver disease occurred in 11% of patients in the inotuzumab arm and 1% in the chemotherapy arm.
There were 17 deaths during treatment in the inotuzumab arm and 11 in the chemotherapy arm. Four deaths were considered related to inotuzumab, and 2 were deemed related to chemotherapy.
Health Canada has approved inotuzumab ozogamicin (Besponsa™) as monotherapy for adults with relapsed or refractory, CD22-positive, B-cell precursor acute lymphoblastic leukemia (ALL).
Inotuzumab ozogamicin is the first and only CD22-directed antibody-drug conjugate approved for this indication.
The product consists of a monoclonal antibody targeting CD22 and a cytotoxic agent known as calicheamicin.
Health Canada’s approval of inotuzumab ozogamicin is based on results from the phase 3 INO-VATE trial, which were published in NEJM in June 2016.
The trial enrolled 326 adults with relapsed or refractory B-cell precursor ALL.
Patients received inotuzumab ozogamicin or 1 of 3 chemotherapy regimens—high-dose cytarabine; cytarabine plus mitoxantrone; or fludarabine, cytarabine, and granulocyte colony-stimulating factor.
The rate of complete remission, including incomplete hematologic recovery, was 80.7% in the inotuzumab arm and 29.4% in the chemotherapy arm (P<0.001). The median duration of remission was 4.6 months and 3.1 months, respectively (P=0.03).
Forty-one percent of patients treated with inotuzumab and 11% of those who received chemotherapy proceeded to stem cell transplant directly after treatment (P<0.001).
The median progression-free survival was 5.0 months in the inotuzumab arm and 1.8 months in the chemotherapy arm (P<0.001).
The median overall survival was 7.7 months and 6.7 months, respectively (P=0.04). This did not meet the prespecified boundary of significance (P=0.0208).
Liver-related adverse events were more common in the inotuzumab arm than the chemotherapy arm. The most frequent of these were increased aspartate aminotransferase level (20% vs 10%), hyperbilirubinemia (15% vs 10%), and increased alanine aminotransferase level (14% vs 11%).
Veno-occlusive liver disease occurred in 11% of patients in the inotuzumab arm and 1% in the chemotherapy arm.
There were 17 deaths during treatment in the inotuzumab arm and 11 in the chemotherapy arm. Four deaths were considered related to inotuzumab, and 2 were deemed related to chemotherapy.
Combo improves outcomes in MCL
A 2-drug combination can improve outcomes in patients with mantle cell lymphoma (MCL), according to researchers.
In a phase 2 trial of MCL patients, the BTK inhibitor ibrutinib and the BCL2 inhibitor venetoclax produced an overall response rate of 71% and a complete response (CR) rate of 62%.
“This was in patients who we expected to have a poor outcome on conventional therapy, and in which treatment with either ibrutinib or venetoclax alone was expected to see only 21% of patients show a complete response,” said Constantine Tam, MBBS, MD, of the Peter MacCallum Cancer Centre in Melbourne, Victoria, Australia.
The most common adverse events (AEs) in patients receiving venetoclax and ibrutinib were diarrhea (83%), fatigue (75%), and nausea/vomiting (71%). Fourteen patients (58%) had serious AEs, including 2 with tumor lysis syndrome.
Dr Tam and his colleagues reported these results in NEJM.
The study included 24 patients—23 with relapsed/refractory MCL and 1 with previously untreated MCL. They had a median age of 68 (range, 47-81).
Most patients had high-risk (75%) or intermediate-risk (21%) disease, according to the MCL International Prognostic Index. Half of patients (including the previously untreated patient) had a TP53 aberration, and 25% had an NF-κB pathway mutation.
The relapsed/refractory patients had a median of 2 prior therapies (range, 1-6), and 48% were refractory to their most recent therapy.
Patients received ibrutinib monotherapy at 560 mg daily for 4 weeks. Then, patients began receiving venetoclax as well, in increasing doses, up to 400 mg per day. Patients received treatment until progression or unacceptable toxicity.
Efficacy
The study’s primary endpoint was CR at week 16, as assessed without PET. This was to allow the researchers to compare CR results in this trial to CR results in the PCYC-1104-CA study, a phase 2 trial of ibrutinib monotherapy in MCL.
According to CT, the CR rate was 42% in patients who received venetoclax and ibrutinib. This is significantly higher than the 9% CR rate observed in the patients treated with ibrutinib alone (P<0.001).
However, according to PET/CT, the 16-week CR rate was 62% in patients who received venetoclax and ibrutinib, and the overall response rate was 71%.
Overall, the rate of minimal residual disease (MRD) negativity was 67% (n=16) in the bone marrow according to flow cytometry and 38% (n=9) in the blood according to allele-specific oligonucleotide-polymerase chain reaction (ASO-PCR). However, not all patients were evaluable for MRD.
Among evaluable patients with a CR, 93% (14/15) were MRD negative according to flow cytometry, and 82% (9/11) were negative according to ASO-PCR.
The median progression-free survival was not reached at a median follow-up of 15.9 months. The estimated progression-free survival was 75% at 12 months and 57% at 18 months.
The rate of overall survival was 79% at 12 months and 74% at 18 months.
“These very promising results have triggered additional and larger studies to better understand the synergistic benefits of the venetoclax-ibrutinib treatment combination in MCL patients,” Dr Tam said.
Safety
The most common AEs were diarrhea (83%); fatigue (75%); nausea/vomiting (71%); bleeding, bruising, or postoperative hemorrhage (54%); musculoskeletal or connective-tissue pain (50%); cough or dyspnea (46%); soft-tissue infection (42%); upper respiratory tract infection (42%); neutropenia (33%); and lower respiratory tract infection (33%).
Grade 3/4 AEs included neutropenia (33%), thrombocytopenia (17%), anemia (12%), diarrhea (12%), tumor lysis syndrome (8%), atrial fibrillation (8%), lower respiratory tract infection (8%), soft-tissue infection (8%), cough or dyspnea (4%), musculoskeletal or connective-tissue pain (4%), and bleeding, bruising, or postoperative hemorrhage (4%).
Serious AEs included diarrhea (12%), tumor lysis syndrome (8%), atrial fibrillation (8%), pyrexia (8%), pleural effusion (8%), cardiac failure (4%), and soft-tissue infection (4%).
The patients who developed tumor lysis syndrome were among the first 15 patients who started venetoclax at a dose of 50 mg per day. Because of these cases, the study protocol was amended to lower the starting dose of venetoclax to 20 mg daily. After that, there were no additional cases of tumor lysis syndrome.
There were 6 deaths during the study. Four were attributed to disease progression, 1 to malignant otitis externa, and 1 to cardiac failure in a patient in CR.
A 2-drug combination can improve outcomes in patients with mantle cell lymphoma (MCL), according to researchers.
In a phase 2 trial of MCL patients, the BTK inhibitor ibrutinib and the BCL2 inhibitor venetoclax produced an overall response rate of 71% and a complete response (CR) rate of 62%.
“This was in patients who we expected to have a poor outcome on conventional therapy, and in which treatment with either ibrutinib or venetoclax alone was expected to see only 21% of patients show a complete response,” said Constantine Tam, MBBS, MD, of the Peter MacCallum Cancer Centre in Melbourne, Victoria, Australia.
The most common adverse events (AEs) in patients receiving venetoclax and ibrutinib were diarrhea (83%), fatigue (75%), and nausea/vomiting (71%). Fourteen patients (58%) had serious AEs, including 2 with tumor lysis syndrome.
Dr Tam and his colleagues reported these results in NEJM.
The study included 24 patients—23 with relapsed/refractory MCL and 1 with previously untreated MCL. They had a median age of 68 (range, 47-81).
Most patients had high-risk (75%) or intermediate-risk (21%) disease, according to the MCL International Prognostic Index. Half of patients (including the previously untreated patient) had a TP53 aberration, and 25% had an NF-κB pathway mutation.
The relapsed/refractory patients had a median of 2 prior therapies (range, 1-6), and 48% were refractory to their most recent therapy.
Patients received ibrutinib monotherapy at 560 mg daily for 4 weeks. Then, patients began receiving venetoclax as well, in increasing doses, up to 400 mg per day. Patients received treatment until progression or unacceptable toxicity.
Efficacy
The study’s primary endpoint was CR at week 16, as assessed without PET. This was to allow the researchers to compare CR results in this trial to CR results in the PCYC-1104-CA study, a phase 2 trial of ibrutinib monotherapy in MCL.
According to CT, the CR rate was 42% in patients who received venetoclax and ibrutinib. This is significantly higher than the 9% CR rate observed in the patients treated with ibrutinib alone (P<0.001).
However, according to PET/CT, the 16-week CR rate was 62% in patients who received venetoclax and ibrutinib, and the overall response rate was 71%.
Overall, the rate of minimal residual disease (MRD) negativity was 67% (n=16) in the bone marrow according to flow cytometry and 38% (n=9) in the blood according to allele-specific oligonucleotide-polymerase chain reaction (ASO-PCR). However, not all patients were evaluable for MRD.
Among evaluable patients with a CR, 93% (14/15) were MRD negative according to flow cytometry, and 82% (9/11) were negative according to ASO-PCR.
The median progression-free survival was not reached at a median follow-up of 15.9 months. The estimated progression-free survival was 75% at 12 months and 57% at 18 months.
The rate of overall survival was 79% at 12 months and 74% at 18 months.
“These very promising results have triggered additional and larger studies to better understand the synergistic benefits of the venetoclax-ibrutinib treatment combination in MCL patients,” Dr Tam said.
Safety
The most common AEs were diarrhea (83%); fatigue (75%); nausea/vomiting (71%); bleeding, bruising, or postoperative hemorrhage (54%); musculoskeletal or connective-tissue pain (50%); cough or dyspnea (46%); soft-tissue infection (42%); upper respiratory tract infection (42%); neutropenia (33%); and lower respiratory tract infection (33%).
Grade 3/4 AEs included neutropenia (33%), thrombocytopenia (17%), anemia (12%), diarrhea (12%), tumor lysis syndrome (8%), atrial fibrillation (8%), lower respiratory tract infection (8%), soft-tissue infection (8%), cough or dyspnea (4%), musculoskeletal or connective-tissue pain (4%), and bleeding, bruising, or postoperative hemorrhage (4%).
Serious AEs included diarrhea (12%), tumor lysis syndrome (8%), atrial fibrillation (8%), pyrexia (8%), pleural effusion (8%), cardiac failure (4%), and soft-tissue infection (4%).
The patients who developed tumor lysis syndrome were among the first 15 patients who started venetoclax at a dose of 50 mg per day. Because of these cases, the study protocol was amended to lower the starting dose of venetoclax to 20 mg daily. After that, there were no additional cases of tumor lysis syndrome.
There were 6 deaths during the study. Four were attributed to disease progression, 1 to malignant otitis externa, and 1 to cardiac failure in a patient in CR.
A 2-drug combination can improve outcomes in patients with mantle cell lymphoma (MCL), according to researchers.
In a phase 2 trial of MCL patients, the BTK inhibitor ibrutinib and the BCL2 inhibitor venetoclax produced an overall response rate of 71% and a complete response (CR) rate of 62%.
“This was in patients who we expected to have a poor outcome on conventional therapy, and in which treatment with either ibrutinib or venetoclax alone was expected to see only 21% of patients show a complete response,” said Constantine Tam, MBBS, MD, of the Peter MacCallum Cancer Centre in Melbourne, Victoria, Australia.
The most common adverse events (AEs) in patients receiving venetoclax and ibrutinib were diarrhea (83%), fatigue (75%), and nausea/vomiting (71%). Fourteen patients (58%) had serious AEs, including 2 with tumor lysis syndrome.
Dr Tam and his colleagues reported these results in NEJM.
The study included 24 patients—23 with relapsed/refractory MCL and 1 with previously untreated MCL. They had a median age of 68 (range, 47-81).
Most patients had high-risk (75%) or intermediate-risk (21%) disease, according to the MCL International Prognostic Index. Half of patients (including the previously untreated patient) had a TP53 aberration, and 25% had an NF-κB pathway mutation.
The relapsed/refractory patients had a median of 2 prior therapies (range, 1-6), and 48% were refractory to their most recent therapy.
Patients received ibrutinib monotherapy at 560 mg daily for 4 weeks. Then, patients began receiving venetoclax as well, in increasing doses, up to 400 mg per day. Patients received treatment until progression or unacceptable toxicity.
Efficacy
The study’s primary endpoint was CR at week 16, as assessed without PET. This was to allow the researchers to compare CR results in this trial to CR results in the PCYC-1104-CA study, a phase 2 trial of ibrutinib monotherapy in MCL.
According to CT, the CR rate was 42% in patients who received venetoclax and ibrutinib. This is significantly higher than the 9% CR rate observed in the patients treated with ibrutinib alone (P<0.001).
However, according to PET/CT, the 16-week CR rate was 62% in patients who received venetoclax and ibrutinib, and the overall response rate was 71%.
Overall, the rate of minimal residual disease (MRD) negativity was 67% (n=16) in the bone marrow according to flow cytometry and 38% (n=9) in the blood according to allele-specific oligonucleotide-polymerase chain reaction (ASO-PCR). However, not all patients were evaluable for MRD.
Among evaluable patients with a CR, 93% (14/15) were MRD negative according to flow cytometry, and 82% (9/11) were negative according to ASO-PCR.
The median progression-free survival was not reached at a median follow-up of 15.9 months. The estimated progression-free survival was 75% at 12 months and 57% at 18 months.
The rate of overall survival was 79% at 12 months and 74% at 18 months.
“These very promising results have triggered additional and larger studies to better understand the synergistic benefits of the venetoclax-ibrutinib treatment combination in MCL patients,” Dr Tam said.
Safety
The most common AEs were diarrhea (83%); fatigue (75%); nausea/vomiting (71%); bleeding, bruising, or postoperative hemorrhage (54%); musculoskeletal or connective-tissue pain (50%); cough or dyspnea (46%); soft-tissue infection (42%); upper respiratory tract infection (42%); neutropenia (33%); and lower respiratory tract infection (33%).
Grade 3/4 AEs included neutropenia (33%), thrombocytopenia (17%), anemia (12%), diarrhea (12%), tumor lysis syndrome (8%), atrial fibrillation (8%), lower respiratory tract infection (8%), soft-tissue infection (8%), cough or dyspnea (4%), musculoskeletal or connective-tissue pain (4%), and bleeding, bruising, or postoperative hemorrhage (4%).
Serious AEs included diarrhea (12%), tumor lysis syndrome (8%), atrial fibrillation (8%), pyrexia (8%), pleural effusion (8%), cardiac failure (4%), and soft-tissue infection (4%).
The patients who developed tumor lysis syndrome were among the first 15 patients who started venetoclax at a dose of 50 mg per day. Because of these cases, the study protocol was amended to lower the starting dose of venetoclax to 20 mg daily. After that, there were no additional cases of tumor lysis syndrome.
There were 6 deaths during the study. Four were attributed to disease progression, 1 to malignant otitis externa, and 1 to cardiac failure in a patient in CR.