User login
The shrinking woman
A 45-year-old woman who has been undergoing hemodialysis for 20 years presents with diffuse bone pain, pruritic skin, muscle weakness, and disability. About 8 years ago, she was diagnosed with uremic hyperparathyroidism and underwent two parathyroidectomy procedures and eight sessions of percutaneous alcohol ablation of the parathyroid gland.
A technetium-99m sestamibi radionuclide scan shows bilateral parathyroid hyperplasia with no ectopic parathyroid adenomas. Although a surgeon she consulted 1 month ago declined to perform another parathyroidectomy for technical reasons, another surgeon agreed to do it at this time. Parathyroidectomy was successfully performed, after which the parathyroid hormone level decreased drastically, to 85 pg/mL.
To our surprise, her total body length increased by 6 to 7 cm after surgery, with partial straightening of the back and legs noted about 4 to 5 weeks after surgery. Furthermore, she was able to walk a short distance just a few weeks after surgery. Unfortunately, she died from sepsis the next year.
SEVERE UREMIC HYPERPARATHYROIDISM: A CLINICAL DILEMMA
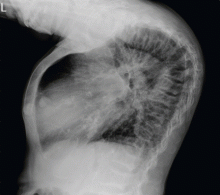
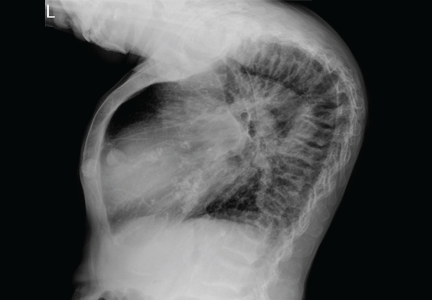
The clinical appearance of reduced body length and diffuse bony deformity leading to “shrinking” as a consequence of prolonged severe uremic hyperparathyroidism has only rarely been reported.1 However, it is not uncommon for surgeons to decide against parathyroidectomy because of concerns of extensive subcutaneous fibrosis and recurrent laryngeal nerve damage associated with previous operations. The result is that the patient’s uremic hyperparathyroidism goes untreated, increasing the risk of long-term complications, as in this patient.
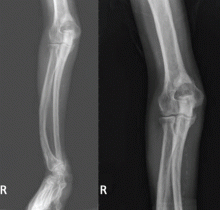
TYPICAL RADIOGRAPHIC FEATURES
Why the body length increases after parathyroidectomy is not yet known, but plausible mechanisms are the effects of a recovery of muscle strength and the vigorous bony remineralization that strengthens weight-bearing bones after resolution of uremic hyperparathyroidism.
THE DANGERS OF DELAYED TREATMENT
Delaying parathyroidectomy may induce prolonged and severe uremic hyperparathyroidism, as in this patient. Nevertheless, despite the delay, surgery was able to partially ameliorate the symptoms of hyperparathyroidism and improve the extreme bone deformity. However, the patient’s informed consent, a detailed preoperative evaluation, and exclusion of ectopic parathyroid adenomas are imperative before surgical treatment.
- Horensten ML, Boner G, Rosenfeld JB. The shrinking man. A manifestation of severe renal osteodystrophy. JAMA 1980; 244:267–268.
- Jevtic V. Imaging of renal osteodystrophy. Eur J Radiol 2003; 46:85–95.
- Ferreira MA. Diagnosis of renal osteodystrophy: when and how to use biochemical markers and non-invasive methods; when bone biopsy is needed. Nephrol Dial Transplant 2000; 15(suppl 5):8–14.
A 45-year-old woman who has been undergoing hemodialysis for 20 years presents with diffuse bone pain, pruritic skin, muscle weakness, and disability. About 8 years ago, she was diagnosed with uremic hyperparathyroidism and underwent two parathyroidectomy procedures and eight sessions of percutaneous alcohol ablation of the parathyroid gland.
A technetium-99m sestamibi radionuclide scan shows bilateral parathyroid hyperplasia with no ectopic parathyroid adenomas. Although a surgeon she consulted 1 month ago declined to perform another parathyroidectomy for technical reasons, another surgeon agreed to do it at this time. Parathyroidectomy was successfully performed, after which the parathyroid hormone level decreased drastically, to 85 pg/mL.
To our surprise, her total body length increased by 6 to 7 cm after surgery, with partial straightening of the back and legs noted about 4 to 5 weeks after surgery. Furthermore, she was able to walk a short distance just a few weeks after surgery. Unfortunately, she died from sepsis the next year.
SEVERE UREMIC HYPERPARATHYROIDISM: A CLINICAL DILEMMA
The clinical appearance of reduced body length and diffuse bony deformity leading to “shrinking” as a consequence of prolonged severe uremic hyperparathyroidism has only rarely been reported.1 However, it is not uncommon for surgeons to decide against parathyroidectomy because of concerns of extensive subcutaneous fibrosis and recurrent laryngeal nerve damage associated with previous operations. The result is that the patient’s uremic hyperparathyroidism goes untreated, increasing the risk of long-term complications, as in this patient.
TYPICAL RADIOGRAPHIC FEATURES
Why the body length increases after parathyroidectomy is not yet known, but plausible mechanisms are the effects of a recovery of muscle strength and the vigorous bony remineralization that strengthens weight-bearing bones after resolution of uremic hyperparathyroidism.
THE DANGERS OF DELAYED TREATMENT
Delaying parathyroidectomy may induce prolonged and severe uremic hyperparathyroidism, as in this patient. Nevertheless, despite the delay, surgery was able to partially ameliorate the symptoms of hyperparathyroidism and improve the extreme bone deformity. However, the patient’s informed consent, a detailed preoperative evaluation, and exclusion of ectopic parathyroid adenomas are imperative before surgical treatment.
A 45-year-old woman who has been undergoing hemodialysis for 20 years presents with diffuse bone pain, pruritic skin, muscle weakness, and disability. About 8 years ago, she was diagnosed with uremic hyperparathyroidism and underwent two parathyroidectomy procedures and eight sessions of percutaneous alcohol ablation of the parathyroid gland.
A technetium-99m sestamibi radionuclide scan shows bilateral parathyroid hyperplasia with no ectopic parathyroid adenomas. Although a surgeon she consulted 1 month ago declined to perform another parathyroidectomy for technical reasons, another surgeon agreed to do it at this time. Parathyroidectomy was successfully performed, after which the parathyroid hormone level decreased drastically, to 85 pg/mL.
To our surprise, her total body length increased by 6 to 7 cm after surgery, with partial straightening of the back and legs noted about 4 to 5 weeks after surgery. Furthermore, she was able to walk a short distance just a few weeks after surgery. Unfortunately, she died from sepsis the next year.
SEVERE UREMIC HYPERPARATHYROIDISM: A CLINICAL DILEMMA
The clinical appearance of reduced body length and diffuse bony deformity leading to “shrinking” as a consequence of prolonged severe uremic hyperparathyroidism has only rarely been reported.1 However, it is not uncommon for surgeons to decide against parathyroidectomy because of concerns of extensive subcutaneous fibrosis and recurrent laryngeal nerve damage associated with previous operations. The result is that the patient’s uremic hyperparathyroidism goes untreated, increasing the risk of long-term complications, as in this patient.
TYPICAL RADIOGRAPHIC FEATURES
Why the body length increases after parathyroidectomy is not yet known, but plausible mechanisms are the effects of a recovery of muscle strength and the vigorous bony remineralization that strengthens weight-bearing bones after resolution of uremic hyperparathyroidism.
THE DANGERS OF DELAYED TREATMENT
Delaying parathyroidectomy may induce prolonged and severe uremic hyperparathyroidism, as in this patient. Nevertheless, despite the delay, surgery was able to partially ameliorate the symptoms of hyperparathyroidism and improve the extreme bone deformity. However, the patient’s informed consent, a detailed preoperative evaluation, and exclusion of ectopic parathyroid adenomas are imperative before surgical treatment.
- Horensten ML, Boner G, Rosenfeld JB. The shrinking man. A manifestation of severe renal osteodystrophy. JAMA 1980; 244:267–268.
- Jevtic V. Imaging of renal osteodystrophy. Eur J Radiol 2003; 46:85–95.
- Ferreira MA. Diagnosis of renal osteodystrophy: when and how to use biochemical markers and non-invasive methods; when bone biopsy is needed. Nephrol Dial Transplant 2000; 15(suppl 5):8–14.
- Horensten ML, Boner G, Rosenfeld JB. The shrinking man. A manifestation of severe renal osteodystrophy. JAMA 1980; 244:267–268.
- Jevtic V. Imaging of renal osteodystrophy. Eur J Radiol 2003; 46:85–95.
- Ferreira MA. Diagnosis of renal osteodystrophy: when and how to use biochemical markers and non-invasive methods; when bone biopsy is needed. Nephrol Dial Transplant 2000; 15(suppl 5):8–14.
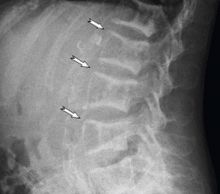
Recurrent pyelonephritis as a sign of ‘sponge kidney’
KEY FEATURES
Medullary sponge kidney causes extensive cystic dilation of medullary collecting tubules.1 It is usually an incidental finding in patients undergoing intravenous urography as part of the evaluation for infection, hematuria, or kidney stones.
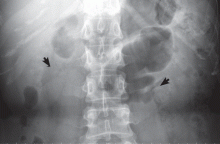
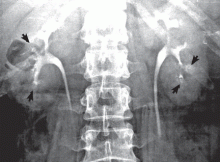
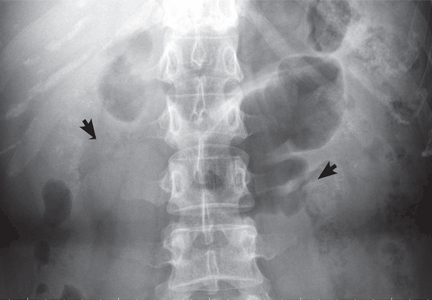
The classic urographic appearance is linear striations with small brushes or “bouquets of flowers,” which represent the collection of contrast material in small papillary cysts.
Medullary sponge kidney has long been considered a congenital disorder, but the genetic defect has not yet been identified, and the pathogenesis is not yet known. It has only rarely been reported in children.2
It is usually asymptomatic, but complications may occur, including nephrocalcinosis or lithiasis, urinary tract infection, renal tubular acidosis, and impaired urine concentrating ability.
RISK OF LITHIASIS
Gambaro et al3 estimated that medullary sponge kidney is found in up to 20% of patients with urolithiasis, and that more than 70% of patients with medullary sponge kidney develop stones.
Cystic dilation of medullary collecting tubules (an anatomic abnormality) inevitably causes urinary stasis. This, combined with hypercalciuria and reduced excretion of urinary citrate and magnesium (metabolic abnormalities), contributes to lithiasis.4 Lithiasis in medullary sponge kidney is a well-known cause of urinary tract infection, and it tends to facilitate infective stone formation after episodes of urinary tract infection.5
The unique anatomic derangement of medullary sponge kidney contributes to the recurrence of pyelonephritis, in addition to the conventional risk factors—frequent sexual intercourse, avoidance of voiding because it is inconvenient, incomplete bladder emptying, ureteropelvic junction obstruction, ectopic ureter, and impaired immunity.6
DIAGNOSIS AND TREATMENT
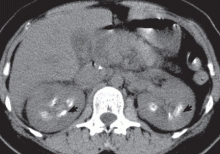
Intravenous urography is the gold standard for the diagnosis of medullary sponge kidney; computed tomography and ultrasonography are generally limited in their ability to clearly show the tubular ectasia.7
Treatment includes antibiotics for acute pyelonephritis and thiazide diuretics and potassium citrate to prevent stone formation and renal tubular acidosis. Due to its silent course, medullary sponge kidney should be considered not only as a cause of nephrocalcinosis and nephrolithiasis, but also as a distinct entity complicating recurrent pyelonephritis.
- Gambaro G, Feltrin GP, Lupo A, Bonfante L, D'Angelo A, Antonello A. Medullary sponge kidney (Lenarduzzi-Cacchi-Ricci disease): a Padua Medical School discovery in the 1930s. Kidney Int 2006; 69:663–670.
- Kasap B, Soylu A, Oren O, Turkmen M, Kavukcu S. Medullary sponge kidney associated with distal renal tubular acidosis in a 5-year-old girl. Eur J Pediatr 2006; 165:648–651.
- Gambaro G, Fabris A, Puliatta D, Lupo A. Lithiasis in cystic kidney disease and malformations of the urinary tract. Urol Res 2006; 34:102–107.
- O'Neill M, Breslau NA, Pak CY. Metabolic evaluation of nephrolithiasis in patients with medullary sponge kidney. JAMA 1981; 245:1233–1236.
- Miano R, Germani S, Vespasiani G. Stones and urinary tract infections. Urol Int 2007; 79(suppl 1):32–36.
- Scholes D, Hooton TM, Roberts PL, Gupta K, Stapleton AE, Stamm WE. Risk factors associated with acute pyelonephritis in healthy women. Ann Intern Med 2005; 142:20–27.
- Maw AM, Megibow AJ, Grasso M, Goldfarb DS. Diagnosis of medullary sponge kidney by computed tomographic urography. Am J Kidney Dis 2007; 50:146–150.
KEY FEATURES
Medullary sponge kidney causes extensive cystic dilation of medullary collecting tubules.1 It is usually an incidental finding in patients undergoing intravenous urography as part of the evaluation for infection, hematuria, or kidney stones.
The classic urographic appearance is linear striations with small brushes or “bouquets of flowers,” which represent the collection of contrast material in small papillary cysts.
Medullary sponge kidney has long been considered a congenital disorder, but the genetic defect has not yet been identified, and the pathogenesis is not yet known. It has only rarely been reported in children.2
It is usually asymptomatic, but complications may occur, including nephrocalcinosis or lithiasis, urinary tract infection, renal tubular acidosis, and impaired urine concentrating ability.
RISK OF LITHIASIS
Gambaro et al3 estimated that medullary sponge kidney is found in up to 20% of patients with urolithiasis, and that more than 70% of patients with medullary sponge kidney develop stones.
Cystic dilation of medullary collecting tubules (an anatomic abnormality) inevitably causes urinary stasis. This, combined with hypercalciuria and reduced excretion of urinary citrate and magnesium (metabolic abnormalities), contributes to lithiasis.4 Lithiasis in medullary sponge kidney is a well-known cause of urinary tract infection, and it tends to facilitate infective stone formation after episodes of urinary tract infection.5
The unique anatomic derangement of medullary sponge kidney contributes to the recurrence of pyelonephritis, in addition to the conventional risk factors—frequent sexual intercourse, avoidance of voiding because it is inconvenient, incomplete bladder emptying, ureteropelvic junction obstruction, ectopic ureter, and impaired immunity.6
DIAGNOSIS AND TREATMENT
Intravenous urography is the gold standard for the diagnosis of medullary sponge kidney; computed tomography and ultrasonography are generally limited in their ability to clearly show the tubular ectasia.7
Treatment includes antibiotics for acute pyelonephritis and thiazide diuretics and potassium citrate to prevent stone formation and renal tubular acidosis. Due to its silent course, medullary sponge kidney should be considered not only as a cause of nephrocalcinosis and nephrolithiasis, but also as a distinct entity complicating recurrent pyelonephritis.
KEY FEATURES
Medullary sponge kidney causes extensive cystic dilation of medullary collecting tubules.1 It is usually an incidental finding in patients undergoing intravenous urography as part of the evaluation for infection, hematuria, or kidney stones.
The classic urographic appearance is linear striations with small brushes or “bouquets of flowers,” which represent the collection of contrast material in small papillary cysts.
Medullary sponge kidney has long been considered a congenital disorder, but the genetic defect has not yet been identified, and the pathogenesis is not yet known. It has only rarely been reported in children.2
It is usually asymptomatic, but complications may occur, including nephrocalcinosis or lithiasis, urinary tract infection, renal tubular acidosis, and impaired urine concentrating ability.
RISK OF LITHIASIS
Gambaro et al3 estimated that medullary sponge kidney is found in up to 20% of patients with urolithiasis, and that more than 70% of patients with medullary sponge kidney develop stones.
Cystic dilation of medullary collecting tubules (an anatomic abnormality) inevitably causes urinary stasis. This, combined with hypercalciuria and reduced excretion of urinary citrate and magnesium (metabolic abnormalities), contributes to lithiasis.4 Lithiasis in medullary sponge kidney is a well-known cause of urinary tract infection, and it tends to facilitate infective stone formation after episodes of urinary tract infection.5
The unique anatomic derangement of medullary sponge kidney contributes to the recurrence of pyelonephritis, in addition to the conventional risk factors—frequent sexual intercourse, avoidance of voiding because it is inconvenient, incomplete bladder emptying, ureteropelvic junction obstruction, ectopic ureter, and impaired immunity.6
DIAGNOSIS AND TREATMENT
Intravenous urography is the gold standard for the diagnosis of medullary sponge kidney; computed tomography and ultrasonography are generally limited in their ability to clearly show the tubular ectasia.7
Treatment includes antibiotics for acute pyelonephritis and thiazide diuretics and potassium citrate to prevent stone formation and renal tubular acidosis. Due to its silent course, medullary sponge kidney should be considered not only as a cause of nephrocalcinosis and nephrolithiasis, but also as a distinct entity complicating recurrent pyelonephritis.
- Gambaro G, Feltrin GP, Lupo A, Bonfante L, D'Angelo A, Antonello A. Medullary sponge kidney (Lenarduzzi-Cacchi-Ricci disease): a Padua Medical School discovery in the 1930s. Kidney Int 2006; 69:663–670.
- Kasap B, Soylu A, Oren O, Turkmen M, Kavukcu S. Medullary sponge kidney associated with distal renal tubular acidosis in a 5-year-old girl. Eur J Pediatr 2006; 165:648–651.
- Gambaro G, Fabris A, Puliatta D, Lupo A. Lithiasis in cystic kidney disease and malformations of the urinary tract. Urol Res 2006; 34:102–107.
- O'Neill M, Breslau NA, Pak CY. Metabolic evaluation of nephrolithiasis in patients with medullary sponge kidney. JAMA 1981; 245:1233–1236.
- Miano R, Germani S, Vespasiani G. Stones and urinary tract infections. Urol Int 2007; 79(suppl 1):32–36.
- Scholes D, Hooton TM, Roberts PL, Gupta K, Stapleton AE, Stamm WE. Risk factors associated with acute pyelonephritis in healthy women. Ann Intern Med 2005; 142:20–27.
- Maw AM, Megibow AJ, Grasso M, Goldfarb DS. Diagnosis of medullary sponge kidney by computed tomographic urography. Am J Kidney Dis 2007; 50:146–150.
- Gambaro G, Feltrin GP, Lupo A, Bonfante L, D'Angelo A, Antonello A. Medullary sponge kidney (Lenarduzzi-Cacchi-Ricci disease): a Padua Medical School discovery in the 1930s. Kidney Int 2006; 69:663–670.
- Kasap B, Soylu A, Oren O, Turkmen M, Kavukcu S. Medullary sponge kidney associated with distal renal tubular acidosis in a 5-year-old girl. Eur J Pediatr 2006; 165:648–651.
- Gambaro G, Fabris A, Puliatta D, Lupo A. Lithiasis in cystic kidney disease and malformations of the urinary tract. Urol Res 2006; 34:102–107.
- O'Neill M, Breslau NA, Pak CY. Metabolic evaluation of nephrolithiasis in patients with medullary sponge kidney. JAMA 1981; 245:1233–1236.
- Miano R, Germani S, Vespasiani G. Stones and urinary tract infections. Urol Int 2007; 79(suppl 1):32–36.
- Scholes D, Hooton TM, Roberts PL, Gupta K, Stapleton AE, Stamm WE. Risk factors associated with acute pyelonephritis in healthy women. Ann Intern Med 2005; 142:20–27.
- Maw AM, Megibow AJ, Grasso M, Goldfarb DS. Diagnosis of medullary sponge kidney by computed tomographic urography. Am J Kidney Dis 2007; 50:146–150.