User login
Magnesium Sulfate for Fetal Neuroprotection in Preterm Birth
Introduction: The Many Lanes of Research on Magnesium Sulfate
The research that improves human health in the most expedient and most impactful ways is multitiered, with basic or fundamental research, translational research, interventional studies, and retrospective research often occurring simultaneously. There should be no “single lane” of research and one type of research does not preclude the other.
Too often, we fall short in one of these lanes. While we have achieved many moonshots in obstetrics and maternal-fetal medicine, we have tended not to place a high priority on basic research, which can provide a strong understanding of the biology of major diseases and conditions affecting women and their offspring. When conducted with proper commitment and funding, such research can lead to biologically directed therapy.
Within our specialty, research on how we can effectively prevent preterm birth, prematurity, and preeclampsia has taken a long road, with various types of therapies being tried, but none being overwhelmingly effective — with an ongoing need for more basic or fundamental research. Nevertheless, we can benefit and gain great insights from retrospective and interventional studies associated with clinical therapies used to treat premature labor and preeclampsia when these therapies have an unanticipated and important secondary benefit.
This month our Master Class is focused on the neuroprotection of prematurity. Magnesium sulfate is a valuable tool for the treatment of both premature labor and preeclampsia, and more recently, also for neuroprotection of the fetus. Interestingly, this use stemmed from researchers looking retrospectively at outcomes in women who received the compound for other reasons. It took many years for researchers to prove its neuroprotective value through interventional trials, while researchers simultaneously strove to understand on a basic biologic level how magnesium sulfate works to prevent outcomes such as cerebral palsy.
Basic research underway today continues to improve our understanding of its precise mechanisms of action. Combined with other tiers of research — including more interventional studies and more translational research — we can improve its utility for the neuroprotection of prematurity. Alternatively, ongoing research may lead to different, even more effective treatments.
Our guest author is Irina Burd, MD, PhD, Sylvan Freiman, MD Endowed Professor and Chair of the department of obstetrics, gynecology, and reproductive sciences at the University of Maryland School of Medicine.* Dr. Burd is also a physician-scientist. She recounts the important story of magnesium sulfate and what is currently known about its biologic plausibility in neuroprotection — including through her own studies – as well as what may be coming in the future.
E. Albert Reece, MD, PhD, MBA, a maternal-fetal medicine specialist, is dean emeritus of the University of Maryland School of Medicine, former university executive vice president; currently the endowed professor and director of the Center for Advanced Research Training and Innovation (CARTI), and senior scientist in the Center for Birth Defects Research. Dr. Reece reported no relevant disclosures. He is the medical editor of this column. Contact him at obnews@mdedge.com.
Magnesium Sulfate for Fetal Neuroprotection in Preterm Birth
Without a doubt, magnesium sulfate (MgSO4) given before anticipated preterm birth reduces the risk of cerebral palsy. It is a valuable tool for fetal neuroprotection at a time when there are no proven alternatives. Yet without the persistent research that occurred over more than 20 years, it may not have won the endorsement of the American College of Obstetrics and Gynecologists in 2010 and worked its way into routine practice.
Its history is worthy of reflection. It took years of observational trials (not all of which showed neuroprotective effects), six randomized controlled trials (none of which met their primary endpoint), three meta-analyses, and a Cochrane Database Systematic Review to arrive at the conclusion that antenatal magnesium sulfate therapy given to women at risk of preterm birth has definitive neuroprotective benefit.
This history also holds lessons for our specialty given the dearth of drugs approved for use in pregnancy and the recent withdrawal from the market of Makena — one of only nine drugs to ever be approved by the Food and Drug Administration for use in pregnancy — after a second trial showed lack of benefit in preventing recurrent preterm birth. The story of MgSO4 tells us it’s acceptable to have major stumbling blocks: At one point, MgSO4 was considered to be not only not helpful, but harmful, causing neonatal death. Further research disproved this initial finding.
Moreover, the MgSO4 story is one that remains unfinished, as my laboratory and other researchers work to better understand its biologic plausibility and to discover additional neuroprotective agents for anticipated preterm birth that may further reduce the risk of cerebral palsy. This leading cause of chronic childhood disability is estimated by the United Cerebral Palsy Foundation to affect approximately 800,000 people in the United States.
Origins and Biologic Plausibility
The MgSO4 story is rooted in the late seventeenth century discovery by physician Nehemiah Grew that the compound was the key component of the then-famous medicinal spring waters in Epsom, England.1 MgSO4 was first used for eclampsia in 1906,2 and was first reported in the American literature for eclampsia in 1925.3 In 1959, its effect as a tocolytic agent was reported.4
More than 30 years later, in 1995, an observational study coauthored by Karin B. Nelson, MD, and Judith K. Grether, PhD of the National Institutes of Health, showed a reduced risk of cerebral palsy in very-low-birth-weight infants (VLBW).5 The report marked a turning point in research interest on neuroprotection for anticipated preterm birth.
The precise molecular mechanisms of action of MgSO4 for neuroprotection are still not well understood. However, research findings from the University of Maryland and other institutions have provided biologic plausibility for its use to prevent cerebral palsy. Our current thinking is that it involves the prevention of periventricular white matter injury and/or the prevention of oxidative stress and a neuronal injury mechanism called excitotoxicity.
Periventricular white matter injury involving injury to preoligodendrocytes before 32 weeks’ gestation is the most prevalent injury seen in cerebral palsy; preoligodendrocytes are precursors of myelinating oligodendrocytes, which constitute a major glial population in the white matter. Our research in a mouse model demonstrated that the intrauterine inflammation frequently associated with preterm birth can lead to neuronal injury as well as white matter damage, and that MgSO4 may ameliorate both.6,7
Excitotoxicity results from excessive stimulation of N-methyl-D-aspartate (NMDA) glutamatergic receptors on preoligodendrocytes and a rush of calcium through the voltage-gated channels. This calcium influx leads to the production of nitric oxide, oxidative stress, and subsequent mitochondrial damage and cell death. As a bivalent ion, MgSO4 sits in the voltage-gated channels of the NMDA receptors and reduces glutamatergic signaling, thus serving as a calcium antagonist and modulating calcium influx (See Figure).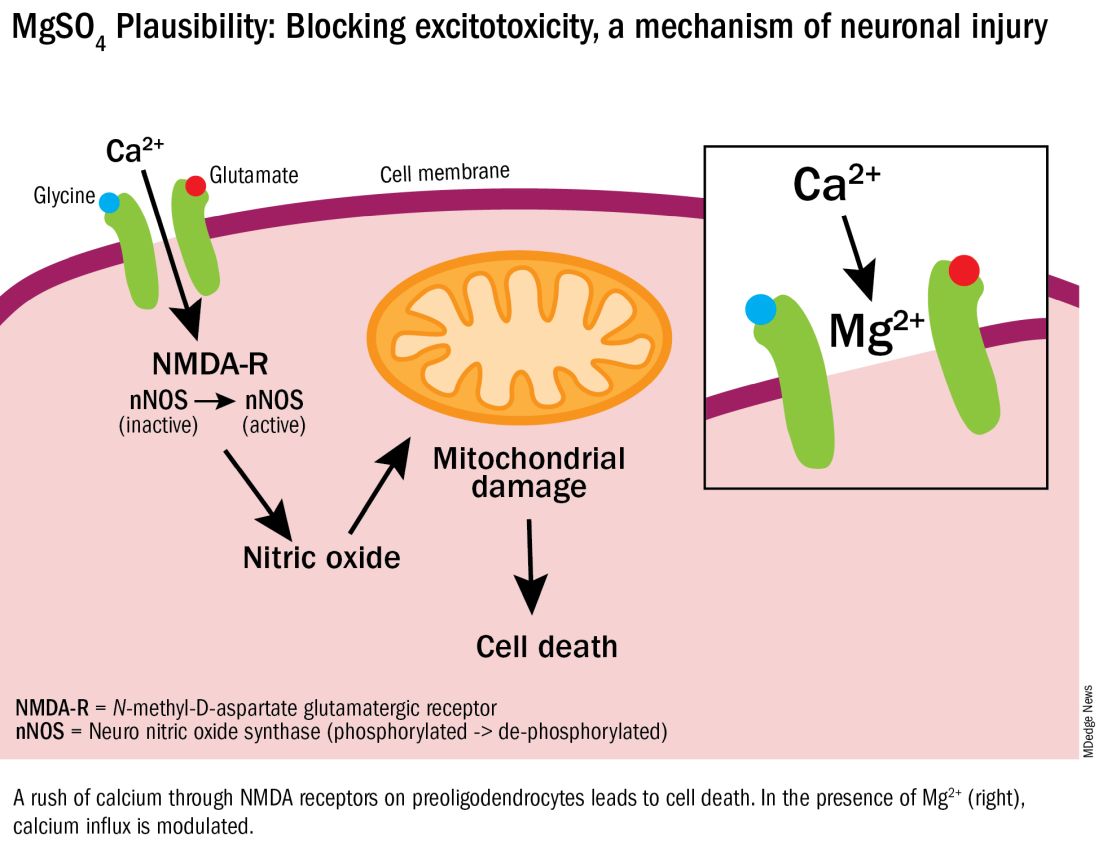
In vitro research in our laboratory has also shown that MgSO4 may dampen inflammatory reactions driven by intrauterine infections, which, like preterm birth, increase the risk of cerebral palsy and adverse neurodevelopmental outcomes.8 MgSO4 appears to do so by blocking the voltage-gated P2X7 receptor in umbilical vein endothelial cells, thus blocking endothelial secretion of the proinflammatory cytokine interleukin (IL)–1beta. Much more research is needed to determine whether MgSO4 could help prevent cerebral palsy through this mechanism.
The Long Route of Research
The 1995 Nelson-Grether study compared VLBW (< 1500 g) infants who survived and developed moderate/severe cerebral palsy within 3 years to randomly selected VLBW controls with respect to whether their mothers had received MgSO4 to prevent seizures in preeclampsia or as a tocolytic agent.5 In a population of more than 155,000 children born between 1983 and 1985, in utero exposure to MgSO4 was reported in 7.1% of 42 VLBW infants with cerebral palsy and 36% of 75 VLBW controls (odds ratio [OR], 0.14; 95% CI, 0.05-0.51). In women without preeclampsia the OR increased to 0.25.
This motivating study had been preceded by several observational studies showing that infants born to women with preeclampsia who received MgSO4 had significantly lower risks of developing intraventricular hemorrhage (IVH) and germinal matrix hemorrhage (GMH). In one of these studies, published in 1992, Karl C. Kuban, MD, and coauthors reported that “maternal receipt of magnesium sulfate was associated with diminished risk of GMH-IVH even in those babies born to mothers who apparently did not have preeclampsia.”9
In the several years following the 1995 Nelson-Grether study, several other case-control/observational studies were reported, with conflicting conclusions, and investigators around the world began designing and conducting needed randomized controlled trials.
The six published randomized controlled trials looking at MgSO4 and neuroprotection varied in their inclusion and exclusion criteria, their recruitment and enrollment style, the gestational ages for MgSO4 administration, loading and maintenance doses, how cerebral palsy or neuroprotection was assessed, and other factors (See Table for RCT characteristics and main outcomes).10-14 One of the trials aimed primarily at evaluating the efficacy of MgSO4 for preventing preeclampsia.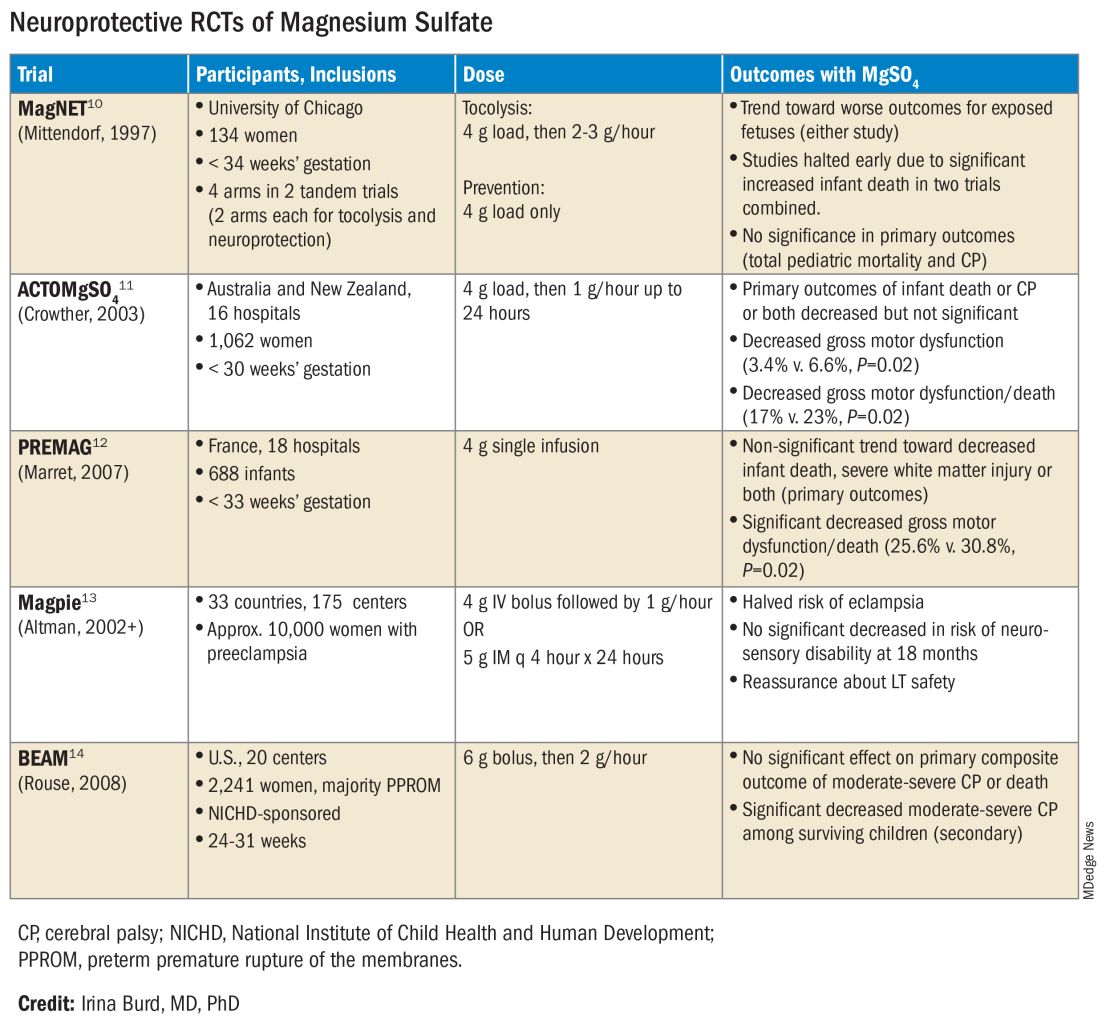
Again, none of the randomized controlled trials demonstrated statistical significance for their primary outcomes or concluded that there was a significant neuroprotective effect for cerebral palsy. Rather, most suggested benefit through secondary analyses. Moreover, as mentioned earlier, research that proceeded after the first published randomized controlled trial — the Magnesium and Neurologic Endpoints (MAGnet) trial — was suspended early when an interim analysis showed a significantly increased risk of mortality in MgSO4-exposed fetuses. All told, it wasn’t until researchers obtained unpublished data and conducted meta-analyses and systematic reviews that a significant effect of MgSO4 on cerebral palsy could be seen.
The three systematic reviews and the Cochrane review, each of which used slightly different methodologies, were published in rapid succession in 2009. One review calculated a relative risk of cerebral palsy of 0.71 (95% CI, 0.55-0.91) — and a relative risk for the combined outcome of death and cerebral palsy at 0.85 (95% CI, 0.74-0.98) — when women at risk of preterm birth were given MgSO4.15 The number needed to treat (NNT) to prevent one case of cerebral palsy was 63, investigators determined, and the NNT to prevent one case of cerebral palsy or infant death was 44.
Another review estimated the NNT for prevention of one case of cerebral palsy at 52 when MgSO4 is given at less than 34 weeks’ gestation, and similarly concluded that MgSO4 is associated with a significantly “reduced risk of moderate/severe CP and substantial gross motor dysfunction without any statistically significant effect on the risk of total pediatric mortality.”16
A third review, from the National Institute of Child Health and Human Development (NICHD) Maternal-Fetal Medicine Units Network (MFMU), estimated an NNT of 46 to prevent one case of cerebral palsy in infants exposed to MgSO4 before 30 weeks, and an NNT of 56 when exposure occurs before 32-34 weeks.17
The Cochrane Review, meanwhile, reported a relative reduction in the risk of cerebral palsy of 0.68 (95% CI, 0.54-0.87) when antenatal MgSO4 is given at less than 37 weeks’ gestation, as well as a significant reduction in the rate of substantial gross motor dysfunction (RR, 0.61; 95% CI, 0.44-0.85).18 The NNT to avoid one case of cerebral palsy, researchers reported, was 63.
Moving Forward
The NNTs calculated in these reviews — ranging from 44 to 63 — are convincing, and are comparable with evidence-based medicine data for prevention of other common diseases.19 For instance, the NNT for a life saved when aspirin is given immediately after a heart attack is 42. Statins given for 5 years in people with known heart disease have an NNT of 83 to save one life, an NNT of 39 to prevent one nonfatal heart attack, and an NNT of 125 to prevent one stroke. For oral anticoagulants used in nonvalvular atrial fibrillation for primary stroke prevention, the NNTs to prevent one stroke, and one death, are 22 and 42, respectively.19
In its 2010 Committee Opinion on Magnesium Sulfate Before Anticipated Preterm Birth for Neuroprotection (reaffirmed in 2020), the American College of Obstetricians and Gynecologists left it to institutions to develop their own guidelines “regarding inclusion criteria, treatment regimens, concurrent tocolysis, and monitoring in accordance with one of the larger trials.”20
Not surprisingly, most if not all hospitals have chosen a higher dose of MgSO4 administered up to 31 weeks’ gestation in keeping with the protocols employed in the NICHD-sponsored BEAM trial (See Table).
The hope moving forward is to expand treatment options for neuroprotection in cases of imminent preterm birth. Researchers have been assessing the ability of melatonin to provide neuroprotection in cases of growth restriction and neonatal asphyxia. Melatonin has anti-inflammatory and antioxidant properties and is known to mediate neuronal generation and synaptic plasticity.21
N-acetyl-L-cysteine is another potential neuroprotective agent. It acts as an antioxidant, a precursor to glutathione, and a modulator of the glutamate system and has been studied as a neuroprotective agent in cases of maternal chorioamnionitis.21 Both melatonin and N-acetyl-L-cysteine are regarded as safe in pregnancy, but much more clinical study is needed to prove their neuroprotective potential when given shortly before birth or earlier.
Dr. Burd is the Sylvan Freiman, MD Endowed Professor and Chair of the department of obstetrics, gynecology, and reproductive sciences at the University of Maryland School of Medicine, Baltimore. She has no conflicts of interest.
References
1. Clio Med. 1984;19(1-2):1-21.
2. Medicinsk Rev. (Bergen) 1906;32:264-272.
3. Am J Obstet Gynecol. 1996;174(4):1390-1391.
4. Am J Obstet Gynecol. 1959;78(1):27-32.
5. Pediatrics. 1995;95(2):263-269.
6. Am J Obstet Gynecol. 2009;201(3):279.e1-279.e8.
7. Am J Obstet Gynecol. 2010;202(3):292.e1-292.e9.
8. Pediatr Res. 2020;87(3):463-471.
9. J Child Neurol. 1992;7(1):70-76.
10. Lancet. 1997;350:1517-1518.
11. JAMA. 2003;290:2669-2676.
12. BJOG. 2007;114(3):310-318.
13. Lancet. 2002;359(9321):1877-1890.
14. N Engl J Med. 2008;359:895-905.
15. Obstet Gynecol. 2009;113(6):1327-1333.
16. Am J Obstet Gynecol. 2009;200(6):595-609.
17. Obstet Gynecol 2009;114:354-364.
18. Cochrane Database Syst Rev. 2009 Jan 21:(1):CD004661.
19. www.thennt.com.
20. Obstet Gynecol. 2010;115:669-671.
21. Front Synaptic Neurosci. 2012;13:680899.
*This story was corrected on June 10, 2024.
Introduction: The Many Lanes of Research on Magnesium Sulfate
The research that improves human health in the most expedient and most impactful ways is multitiered, with basic or fundamental research, translational research, interventional studies, and retrospective research often occurring simultaneously. There should be no “single lane” of research and one type of research does not preclude the other.
Too often, we fall short in one of these lanes. While we have achieved many moonshots in obstetrics and maternal-fetal medicine, we have tended not to place a high priority on basic research, which can provide a strong understanding of the biology of major diseases and conditions affecting women and their offspring. When conducted with proper commitment and funding, such research can lead to biologically directed therapy.
Within our specialty, research on how we can effectively prevent preterm birth, prematurity, and preeclampsia has taken a long road, with various types of therapies being tried, but none being overwhelmingly effective — with an ongoing need for more basic or fundamental research. Nevertheless, we can benefit and gain great insights from retrospective and interventional studies associated with clinical therapies used to treat premature labor and preeclampsia when these therapies have an unanticipated and important secondary benefit.
This month our Master Class is focused on the neuroprotection of prematurity. Magnesium sulfate is a valuable tool for the treatment of both premature labor and preeclampsia, and more recently, also for neuroprotection of the fetus. Interestingly, this use stemmed from researchers looking retrospectively at outcomes in women who received the compound for other reasons. It took many years for researchers to prove its neuroprotective value through interventional trials, while researchers simultaneously strove to understand on a basic biologic level how magnesium sulfate works to prevent outcomes such as cerebral palsy.
Basic research underway today continues to improve our understanding of its precise mechanisms of action. Combined with other tiers of research — including more interventional studies and more translational research — we can improve its utility for the neuroprotection of prematurity. Alternatively, ongoing research may lead to different, even more effective treatments.
Our guest author is Irina Burd, MD, PhD, Sylvan Freiman, MD Endowed Professor and Chair of the department of obstetrics, gynecology, and reproductive sciences at the University of Maryland School of Medicine.* Dr. Burd is also a physician-scientist. She recounts the important story of magnesium sulfate and what is currently known about its biologic plausibility in neuroprotection — including through her own studies – as well as what may be coming in the future.
E. Albert Reece, MD, PhD, MBA, a maternal-fetal medicine specialist, is dean emeritus of the University of Maryland School of Medicine, former university executive vice president; currently the endowed professor and director of the Center for Advanced Research Training and Innovation (CARTI), and senior scientist in the Center for Birth Defects Research. Dr. Reece reported no relevant disclosures. He is the medical editor of this column. Contact him at obnews@mdedge.com.
Magnesium Sulfate for Fetal Neuroprotection in Preterm Birth
Without a doubt, magnesium sulfate (MgSO4) given before anticipated preterm birth reduces the risk of cerebral palsy. It is a valuable tool for fetal neuroprotection at a time when there are no proven alternatives. Yet without the persistent research that occurred over more than 20 years, it may not have won the endorsement of the American College of Obstetrics and Gynecologists in 2010 and worked its way into routine practice.
Its history is worthy of reflection. It took years of observational trials (not all of which showed neuroprotective effects), six randomized controlled trials (none of which met their primary endpoint), three meta-analyses, and a Cochrane Database Systematic Review to arrive at the conclusion that antenatal magnesium sulfate therapy given to women at risk of preterm birth has definitive neuroprotective benefit.
This history also holds lessons for our specialty given the dearth of drugs approved for use in pregnancy and the recent withdrawal from the market of Makena — one of only nine drugs to ever be approved by the Food and Drug Administration for use in pregnancy — after a second trial showed lack of benefit in preventing recurrent preterm birth. The story of MgSO4 tells us it’s acceptable to have major stumbling blocks: At one point, MgSO4 was considered to be not only not helpful, but harmful, causing neonatal death. Further research disproved this initial finding.
Moreover, the MgSO4 story is one that remains unfinished, as my laboratory and other researchers work to better understand its biologic plausibility and to discover additional neuroprotective agents for anticipated preterm birth that may further reduce the risk of cerebral palsy. This leading cause of chronic childhood disability is estimated by the United Cerebral Palsy Foundation to affect approximately 800,000 people in the United States.
Origins and Biologic Plausibility
The MgSO4 story is rooted in the late seventeenth century discovery by physician Nehemiah Grew that the compound was the key component of the then-famous medicinal spring waters in Epsom, England.1 MgSO4 was first used for eclampsia in 1906,2 and was first reported in the American literature for eclampsia in 1925.3 In 1959, its effect as a tocolytic agent was reported.4
More than 30 years later, in 1995, an observational study coauthored by Karin B. Nelson, MD, and Judith K. Grether, PhD of the National Institutes of Health, showed a reduced risk of cerebral palsy in very-low-birth-weight infants (VLBW).5 The report marked a turning point in research interest on neuroprotection for anticipated preterm birth.
The precise molecular mechanisms of action of MgSO4 for neuroprotection are still not well understood. However, research findings from the University of Maryland and other institutions have provided biologic plausibility for its use to prevent cerebral palsy. Our current thinking is that it involves the prevention of periventricular white matter injury and/or the prevention of oxidative stress and a neuronal injury mechanism called excitotoxicity.
Periventricular white matter injury involving injury to preoligodendrocytes before 32 weeks’ gestation is the most prevalent injury seen in cerebral palsy; preoligodendrocytes are precursors of myelinating oligodendrocytes, which constitute a major glial population in the white matter. Our research in a mouse model demonstrated that the intrauterine inflammation frequently associated with preterm birth can lead to neuronal injury as well as white matter damage, and that MgSO4 may ameliorate both.6,7
Excitotoxicity results from excessive stimulation of N-methyl-D-aspartate (NMDA) glutamatergic receptors on preoligodendrocytes and a rush of calcium through the voltage-gated channels. This calcium influx leads to the production of nitric oxide, oxidative stress, and subsequent mitochondrial damage and cell death. As a bivalent ion, MgSO4 sits in the voltage-gated channels of the NMDA receptors and reduces glutamatergic signaling, thus serving as a calcium antagonist and modulating calcium influx (See Figure).
In vitro research in our laboratory has also shown that MgSO4 may dampen inflammatory reactions driven by intrauterine infections, which, like preterm birth, increase the risk of cerebral palsy and adverse neurodevelopmental outcomes.8 MgSO4 appears to do so by blocking the voltage-gated P2X7 receptor in umbilical vein endothelial cells, thus blocking endothelial secretion of the proinflammatory cytokine interleukin (IL)–1beta. Much more research is needed to determine whether MgSO4 could help prevent cerebral palsy through this mechanism.
The Long Route of Research
The 1995 Nelson-Grether study compared VLBW (< 1500 g) infants who survived and developed moderate/severe cerebral palsy within 3 years to randomly selected VLBW controls with respect to whether their mothers had received MgSO4 to prevent seizures in preeclampsia or as a tocolytic agent.5 In a population of more than 155,000 children born between 1983 and 1985, in utero exposure to MgSO4 was reported in 7.1% of 42 VLBW infants with cerebral palsy and 36% of 75 VLBW controls (odds ratio [OR], 0.14; 95% CI, 0.05-0.51). In women without preeclampsia the OR increased to 0.25.
This motivating study had been preceded by several observational studies showing that infants born to women with preeclampsia who received MgSO4 had significantly lower risks of developing intraventricular hemorrhage (IVH) and germinal matrix hemorrhage (GMH). In one of these studies, published in 1992, Karl C. Kuban, MD, and coauthors reported that “maternal receipt of magnesium sulfate was associated with diminished risk of GMH-IVH even in those babies born to mothers who apparently did not have preeclampsia.”9
In the several years following the 1995 Nelson-Grether study, several other case-control/observational studies were reported, with conflicting conclusions, and investigators around the world began designing and conducting needed randomized controlled trials.
The six published randomized controlled trials looking at MgSO4 and neuroprotection varied in their inclusion and exclusion criteria, their recruitment and enrollment style, the gestational ages for MgSO4 administration, loading and maintenance doses, how cerebral palsy or neuroprotection was assessed, and other factors (See Table for RCT characteristics and main outcomes).10-14 One of the trials aimed primarily at evaluating the efficacy of MgSO4 for preventing preeclampsia.
Again, none of the randomized controlled trials demonstrated statistical significance for their primary outcomes or concluded that there was a significant neuroprotective effect for cerebral palsy. Rather, most suggested benefit through secondary analyses. Moreover, as mentioned earlier, research that proceeded after the first published randomized controlled trial — the Magnesium and Neurologic Endpoints (MAGnet) trial — was suspended early when an interim analysis showed a significantly increased risk of mortality in MgSO4-exposed fetuses. All told, it wasn’t until researchers obtained unpublished data and conducted meta-analyses and systematic reviews that a significant effect of MgSO4 on cerebral palsy could be seen.
The three systematic reviews and the Cochrane review, each of which used slightly different methodologies, were published in rapid succession in 2009. One review calculated a relative risk of cerebral palsy of 0.71 (95% CI, 0.55-0.91) — and a relative risk for the combined outcome of death and cerebral palsy at 0.85 (95% CI, 0.74-0.98) — when women at risk of preterm birth were given MgSO4.15 The number needed to treat (NNT) to prevent one case of cerebral palsy was 63, investigators determined, and the NNT to prevent one case of cerebral palsy or infant death was 44.
Another review estimated the NNT for prevention of one case of cerebral palsy at 52 when MgSO4 is given at less than 34 weeks’ gestation, and similarly concluded that MgSO4 is associated with a significantly “reduced risk of moderate/severe CP and substantial gross motor dysfunction without any statistically significant effect on the risk of total pediatric mortality.”16
A third review, from the National Institute of Child Health and Human Development (NICHD) Maternal-Fetal Medicine Units Network (MFMU), estimated an NNT of 46 to prevent one case of cerebral palsy in infants exposed to MgSO4 before 30 weeks, and an NNT of 56 when exposure occurs before 32-34 weeks.17
The Cochrane Review, meanwhile, reported a relative reduction in the risk of cerebral palsy of 0.68 (95% CI, 0.54-0.87) when antenatal MgSO4 is given at less than 37 weeks’ gestation, as well as a significant reduction in the rate of substantial gross motor dysfunction (RR, 0.61; 95% CI, 0.44-0.85).18 The NNT to avoid one case of cerebral palsy, researchers reported, was 63.
Moving Forward
The NNTs calculated in these reviews — ranging from 44 to 63 — are convincing, and are comparable with evidence-based medicine data for prevention of other common diseases.19 For instance, the NNT for a life saved when aspirin is given immediately after a heart attack is 42. Statins given for 5 years in people with known heart disease have an NNT of 83 to save one life, an NNT of 39 to prevent one nonfatal heart attack, and an NNT of 125 to prevent one stroke. For oral anticoagulants used in nonvalvular atrial fibrillation for primary stroke prevention, the NNTs to prevent one stroke, and one death, are 22 and 42, respectively.19
In its 2010 Committee Opinion on Magnesium Sulfate Before Anticipated Preterm Birth for Neuroprotection (reaffirmed in 2020), the American College of Obstetricians and Gynecologists left it to institutions to develop their own guidelines “regarding inclusion criteria, treatment regimens, concurrent tocolysis, and monitoring in accordance with one of the larger trials.”20
Not surprisingly, most if not all hospitals have chosen a higher dose of MgSO4 administered up to 31 weeks’ gestation in keeping with the protocols employed in the NICHD-sponsored BEAM trial (See Table).
The hope moving forward is to expand treatment options for neuroprotection in cases of imminent preterm birth. Researchers have been assessing the ability of melatonin to provide neuroprotection in cases of growth restriction and neonatal asphyxia. Melatonin has anti-inflammatory and antioxidant properties and is known to mediate neuronal generation and synaptic plasticity.21
N-acetyl-L-cysteine is another potential neuroprotective agent. It acts as an antioxidant, a precursor to glutathione, and a modulator of the glutamate system and has been studied as a neuroprotective agent in cases of maternal chorioamnionitis.21 Both melatonin and N-acetyl-L-cysteine are regarded as safe in pregnancy, but much more clinical study is needed to prove their neuroprotective potential when given shortly before birth or earlier.
Dr. Burd is the Sylvan Freiman, MD Endowed Professor and Chair of the department of obstetrics, gynecology, and reproductive sciences at the University of Maryland School of Medicine, Baltimore. She has no conflicts of interest.
References
1. Clio Med. 1984;19(1-2):1-21.
2. Medicinsk Rev. (Bergen) 1906;32:264-272.
3. Am J Obstet Gynecol. 1996;174(4):1390-1391.
4. Am J Obstet Gynecol. 1959;78(1):27-32.
5. Pediatrics. 1995;95(2):263-269.
6. Am J Obstet Gynecol. 2009;201(3):279.e1-279.e8.
7. Am J Obstet Gynecol. 2010;202(3):292.e1-292.e9.
8. Pediatr Res. 2020;87(3):463-471.
9. J Child Neurol. 1992;7(1):70-76.
10. Lancet. 1997;350:1517-1518.
11. JAMA. 2003;290:2669-2676.
12. BJOG. 2007;114(3):310-318.
13. Lancet. 2002;359(9321):1877-1890.
14. N Engl J Med. 2008;359:895-905.
15. Obstet Gynecol. 2009;113(6):1327-1333.
16. Am J Obstet Gynecol. 2009;200(6):595-609.
17. Obstet Gynecol 2009;114:354-364.
18. Cochrane Database Syst Rev. 2009 Jan 21:(1):CD004661.
19. www.thennt.com.
20. Obstet Gynecol. 2010;115:669-671.
21. Front Synaptic Neurosci. 2012;13:680899.
*This story was corrected on June 10, 2024.
Introduction: The Many Lanes of Research on Magnesium Sulfate
The research that improves human health in the most expedient and most impactful ways is multitiered, with basic or fundamental research, translational research, interventional studies, and retrospective research often occurring simultaneously. There should be no “single lane” of research and one type of research does not preclude the other.
Too often, we fall short in one of these lanes. While we have achieved many moonshots in obstetrics and maternal-fetal medicine, we have tended not to place a high priority on basic research, which can provide a strong understanding of the biology of major diseases and conditions affecting women and their offspring. When conducted with proper commitment and funding, such research can lead to biologically directed therapy.
Within our specialty, research on how we can effectively prevent preterm birth, prematurity, and preeclampsia has taken a long road, with various types of therapies being tried, but none being overwhelmingly effective — with an ongoing need for more basic or fundamental research. Nevertheless, we can benefit and gain great insights from retrospective and interventional studies associated with clinical therapies used to treat premature labor and preeclampsia when these therapies have an unanticipated and important secondary benefit.
This month our Master Class is focused on the neuroprotection of prematurity. Magnesium sulfate is a valuable tool for the treatment of both premature labor and preeclampsia, and more recently, also for neuroprotection of the fetus. Interestingly, this use stemmed from researchers looking retrospectively at outcomes in women who received the compound for other reasons. It took many years for researchers to prove its neuroprotective value through interventional trials, while researchers simultaneously strove to understand on a basic biologic level how magnesium sulfate works to prevent outcomes such as cerebral palsy.
Basic research underway today continues to improve our understanding of its precise mechanisms of action. Combined with other tiers of research — including more interventional studies and more translational research — we can improve its utility for the neuroprotection of prematurity. Alternatively, ongoing research may lead to different, even more effective treatments.
Our guest author is Irina Burd, MD, PhD, Sylvan Freiman, MD Endowed Professor and Chair of the department of obstetrics, gynecology, and reproductive sciences at the University of Maryland School of Medicine.* Dr. Burd is also a physician-scientist. She recounts the important story of magnesium sulfate and what is currently known about its biologic plausibility in neuroprotection — including through her own studies – as well as what may be coming in the future.
E. Albert Reece, MD, PhD, MBA, a maternal-fetal medicine specialist, is dean emeritus of the University of Maryland School of Medicine, former university executive vice president; currently the endowed professor and director of the Center for Advanced Research Training and Innovation (CARTI), and senior scientist in the Center for Birth Defects Research. Dr. Reece reported no relevant disclosures. He is the medical editor of this column. Contact him at obnews@mdedge.com.
Magnesium Sulfate for Fetal Neuroprotection in Preterm Birth
Without a doubt, magnesium sulfate (MgSO4) given before anticipated preterm birth reduces the risk of cerebral palsy. It is a valuable tool for fetal neuroprotection at a time when there are no proven alternatives. Yet without the persistent research that occurred over more than 20 years, it may not have won the endorsement of the American College of Obstetrics and Gynecologists in 2010 and worked its way into routine practice.
Its history is worthy of reflection. It took years of observational trials (not all of which showed neuroprotective effects), six randomized controlled trials (none of which met their primary endpoint), three meta-analyses, and a Cochrane Database Systematic Review to arrive at the conclusion that antenatal magnesium sulfate therapy given to women at risk of preterm birth has definitive neuroprotective benefit.
This history also holds lessons for our specialty given the dearth of drugs approved for use in pregnancy and the recent withdrawal from the market of Makena — one of only nine drugs to ever be approved by the Food and Drug Administration for use in pregnancy — after a second trial showed lack of benefit in preventing recurrent preterm birth. The story of MgSO4 tells us it’s acceptable to have major stumbling blocks: At one point, MgSO4 was considered to be not only not helpful, but harmful, causing neonatal death. Further research disproved this initial finding.
Moreover, the MgSO4 story is one that remains unfinished, as my laboratory and other researchers work to better understand its biologic plausibility and to discover additional neuroprotective agents for anticipated preterm birth that may further reduce the risk of cerebral palsy. This leading cause of chronic childhood disability is estimated by the United Cerebral Palsy Foundation to affect approximately 800,000 people in the United States.
Origins and Biologic Plausibility
The MgSO4 story is rooted in the late seventeenth century discovery by physician Nehemiah Grew that the compound was the key component of the then-famous medicinal spring waters in Epsom, England.1 MgSO4 was first used for eclampsia in 1906,2 and was first reported in the American literature for eclampsia in 1925.3 In 1959, its effect as a tocolytic agent was reported.4
More than 30 years later, in 1995, an observational study coauthored by Karin B. Nelson, MD, and Judith K. Grether, PhD of the National Institutes of Health, showed a reduced risk of cerebral palsy in very-low-birth-weight infants (VLBW).5 The report marked a turning point in research interest on neuroprotection for anticipated preterm birth.
The precise molecular mechanisms of action of MgSO4 for neuroprotection are still not well understood. However, research findings from the University of Maryland and other institutions have provided biologic plausibility for its use to prevent cerebral palsy. Our current thinking is that it involves the prevention of periventricular white matter injury and/or the prevention of oxidative stress and a neuronal injury mechanism called excitotoxicity.
Periventricular white matter injury involving injury to preoligodendrocytes before 32 weeks’ gestation is the most prevalent injury seen in cerebral palsy; preoligodendrocytes are precursors of myelinating oligodendrocytes, which constitute a major glial population in the white matter. Our research in a mouse model demonstrated that the intrauterine inflammation frequently associated with preterm birth can lead to neuronal injury as well as white matter damage, and that MgSO4 may ameliorate both.6,7
Excitotoxicity results from excessive stimulation of N-methyl-D-aspartate (NMDA) glutamatergic receptors on preoligodendrocytes and a rush of calcium through the voltage-gated channels. This calcium influx leads to the production of nitric oxide, oxidative stress, and subsequent mitochondrial damage and cell death. As a bivalent ion, MgSO4 sits in the voltage-gated channels of the NMDA receptors and reduces glutamatergic signaling, thus serving as a calcium antagonist and modulating calcium influx (See Figure).
In vitro research in our laboratory has also shown that MgSO4 may dampen inflammatory reactions driven by intrauterine infections, which, like preterm birth, increase the risk of cerebral palsy and adverse neurodevelopmental outcomes.8 MgSO4 appears to do so by blocking the voltage-gated P2X7 receptor in umbilical vein endothelial cells, thus blocking endothelial secretion of the proinflammatory cytokine interleukin (IL)–1beta. Much more research is needed to determine whether MgSO4 could help prevent cerebral palsy through this mechanism.
The Long Route of Research
The 1995 Nelson-Grether study compared VLBW (< 1500 g) infants who survived and developed moderate/severe cerebral palsy within 3 years to randomly selected VLBW controls with respect to whether their mothers had received MgSO4 to prevent seizures in preeclampsia or as a tocolytic agent.5 In a population of more than 155,000 children born between 1983 and 1985, in utero exposure to MgSO4 was reported in 7.1% of 42 VLBW infants with cerebral palsy and 36% of 75 VLBW controls (odds ratio [OR], 0.14; 95% CI, 0.05-0.51). In women without preeclampsia the OR increased to 0.25.
This motivating study had been preceded by several observational studies showing that infants born to women with preeclampsia who received MgSO4 had significantly lower risks of developing intraventricular hemorrhage (IVH) and germinal matrix hemorrhage (GMH). In one of these studies, published in 1992, Karl C. Kuban, MD, and coauthors reported that “maternal receipt of magnesium sulfate was associated with diminished risk of GMH-IVH even in those babies born to mothers who apparently did not have preeclampsia.”9
In the several years following the 1995 Nelson-Grether study, several other case-control/observational studies were reported, with conflicting conclusions, and investigators around the world began designing and conducting needed randomized controlled trials.
The six published randomized controlled trials looking at MgSO4 and neuroprotection varied in their inclusion and exclusion criteria, their recruitment and enrollment style, the gestational ages for MgSO4 administration, loading and maintenance doses, how cerebral palsy or neuroprotection was assessed, and other factors (See Table for RCT characteristics and main outcomes).10-14 One of the trials aimed primarily at evaluating the efficacy of MgSO4 for preventing preeclampsia.
Again, none of the randomized controlled trials demonstrated statistical significance for their primary outcomes or concluded that there was a significant neuroprotective effect for cerebral palsy. Rather, most suggested benefit through secondary analyses. Moreover, as mentioned earlier, research that proceeded after the first published randomized controlled trial — the Magnesium and Neurologic Endpoints (MAGnet) trial — was suspended early when an interim analysis showed a significantly increased risk of mortality in MgSO4-exposed fetuses. All told, it wasn’t until researchers obtained unpublished data and conducted meta-analyses and systematic reviews that a significant effect of MgSO4 on cerebral palsy could be seen.
The three systematic reviews and the Cochrane review, each of which used slightly different methodologies, were published in rapid succession in 2009. One review calculated a relative risk of cerebral palsy of 0.71 (95% CI, 0.55-0.91) — and a relative risk for the combined outcome of death and cerebral palsy at 0.85 (95% CI, 0.74-0.98) — when women at risk of preterm birth were given MgSO4.15 The number needed to treat (NNT) to prevent one case of cerebral palsy was 63, investigators determined, and the NNT to prevent one case of cerebral palsy or infant death was 44.
Another review estimated the NNT for prevention of one case of cerebral palsy at 52 when MgSO4 is given at less than 34 weeks’ gestation, and similarly concluded that MgSO4 is associated with a significantly “reduced risk of moderate/severe CP and substantial gross motor dysfunction without any statistically significant effect on the risk of total pediatric mortality.”16
A third review, from the National Institute of Child Health and Human Development (NICHD) Maternal-Fetal Medicine Units Network (MFMU), estimated an NNT of 46 to prevent one case of cerebral palsy in infants exposed to MgSO4 before 30 weeks, and an NNT of 56 when exposure occurs before 32-34 weeks.17
The Cochrane Review, meanwhile, reported a relative reduction in the risk of cerebral palsy of 0.68 (95% CI, 0.54-0.87) when antenatal MgSO4 is given at less than 37 weeks’ gestation, as well as a significant reduction in the rate of substantial gross motor dysfunction (RR, 0.61; 95% CI, 0.44-0.85).18 The NNT to avoid one case of cerebral palsy, researchers reported, was 63.
Moving Forward
The NNTs calculated in these reviews — ranging from 44 to 63 — are convincing, and are comparable with evidence-based medicine data for prevention of other common diseases.19 For instance, the NNT for a life saved when aspirin is given immediately after a heart attack is 42. Statins given for 5 years in people with known heart disease have an NNT of 83 to save one life, an NNT of 39 to prevent one nonfatal heart attack, and an NNT of 125 to prevent one stroke. For oral anticoagulants used in nonvalvular atrial fibrillation for primary stroke prevention, the NNTs to prevent one stroke, and one death, are 22 and 42, respectively.19
In its 2010 Committee Opinion on Magnesium Sulfate Before Anticipated Preterm Birth for Neuroprotection (reaffirmed in 2020), the American College of Obstetricians and Gynecologists left it to institutions to develop their own guidelines “regarding inclusion criteria, treatment regimens, concurrent tocolysis, and monitoring in accordance with one of the larger trials.”20
Not surprisingly, most if not all hospitals have chosen a higher dose of MgSO4 administered up to 31 weeks’ gestation in keeping with the protocols employed in the NICHD-sponsored BEAM trial (See Table).
The hope moving forward is to expand treatment options for neuroprotection in cases of imminent preterm birth. Researchers have been assessing the ability of melatonin to provide neuroprotection in cases of growth restriction and neonatal asphyxia. Melatonin has anti-inflammatory and antioxidant properties and is known to mediate neuronal generation and synaptic plasticity.21
N-acetyl-L-cysteine is another potential neuroprotective agent. It acts as an antioxidant, a precursor to glutathione, and a modulator of the glutamate system and has been studied as a neuroprotective agent in cases of maternal chorioamnionitis.21 Both melatonin and N-acetyl-L-cysteine are regarded as safe in pregnancy, but much more clinical study is needed to prove their neuroprotective potential when given shortly before birth or earlier.
Dr. Burd is the Sylvan Freiman, MD Endowed Professor and Chair of the department of obstetrics, gynecology, and reproductive sciences at the University of Maryland School of Medicine, Baltimore. She has no conflicts of interest.
References
1. Clio Med. 1984;19(1-2):1-21.
2. Medicinsk Rev. (Bergen) 1906;32:264-272.
3. Am J Obstet Gynecol. 1996;174(4):1390-1391.
4. Am J Obstet Gynecol. 1959;78(1):27-32.
5. Pediatrics. 1995;95(2):263-269.
6. Am J Obstet Gynecol. 2009;201(3):279.e1-279.e8.
7. Am J Obstet Gynecol. 2010;202(3):292.e1-292.e9.
8. Pediatr Res. 2020;87(3):463-471.
9. J Child Neurol. 1992;7(1):70-76.
10. Lancet. 1997;350:1517-1518.
11. JAMA. 2003;290:2669-2676.
12. BJOG. 2007;114(3):310-318.
13. Lancet. 2002;359(9321):1877-1890.
14. N Engl J Med. 2008;359:895-905.
15. Obstet Gynecol. 2009;113(6):1327-1333.
16. Am J Obstet Gynecol. 2009;200(6):595-609.
17. Obstet Gynecol 2009;114:354-364.
18. Cochrane Database Syst Rev. 2009 Jan 21:(1):CD004661.
19. www.thennt.com.
20. Obstet Gynecol. 2010;115:669-671.
21. Front Synaptic Neurosci. 2012;13:680899.
*This story was corrected on June 10, 2024.


