User login
Seven Effective Strategies of Highly Successful Resuscitations (or A New Look at an Ancient Practice)
Despite more intensive guidelines and advances in resuscitation research, the survival rate for victims of cardiopulmonary arrest remains virtually unchanged from forty years ago when modern cardiopulmonary resuscitation (CPR) was first described (1). Perhaps in part because the guidelines for ACLS set forth by the American Heart Association (AHA) and the International Liaison Committee on Resuscitation (ILCOR) have become so complex―and continue to increase in breadth and scope into topics well beyond cardiopulmonary arrest with each revision―the critical aspects of resuscitation have become diluted by unnecessarily difficult algorithms. Critical skill sets―such as proper performance of CPR and rapid defibrillation―have become dwarfed by less critical aspects of acute resuscitation. Remarkably common errors usurp the dual fundamental goals of ACLS: neurological preservation and prevention of early death. This review will address the historical context of resuscitation and then will focus on seven of the most essential, evidence-based strategies for improving outcomes in ACLS.
History of Resuscitation
The modern resuscitation era began in 1960 when Kouwenhoven, Jude, and Knickerbocker published a pair of landmark papers on the use of closed chest compressions (CCC) as a means to resuscitate patients in cardiopulmonary arrest (2,3). Interestingly, the culmination of their three and a half decades of work was initially motivated and sponsored by an electric company seeking to reduce the death rate of its linemen from ventricular fibrillation. While innovative, their technique built on millennia of creative, and sometimes bizarre ancient practices geared at reversing death. Given the lack of in-depth knowledge of anatomy and physiology combined with a rich overlap between shamanism and medicine, it is perhaps stunning to realize that the oldest recorded reasonably physiologic approach to resuscitation stems from over 3500 years ago. Egyptian hieroglyphs show the story of the healing goddess, Isis, reviving her husband Osiris using mouth-to-mouth ventilation (4). Still other Egyptian texts advocated hanging drowned victims upside down, compressing and releasing the thorax with the goal to ventilate and revive the patient (5). Hebrew midwives were documented as having performed mouth-to-mouth on deceased newborns as early as 1300 BCE (6). And even the Bible tells of the prophet Elishah’s successful resuscitation of a deceased child through artificial respiration:
...And he went up, and lay upon the child, and put his mouth upon his mouth, and his eyes upon his eyes, and his hands upon his hands; and he stretched himself upon the child; and the flesh of the child waxed warm (7).
One of the forefathers of modern medicine, the Greek physician Galen, was the first doctor to use an artificial ventilation strategy in 177, filling dead animals’ lungs with air from a bellows (8).
In 1628, physician William Harvey, the first to accurately describe circulation, used his newfound knowledge to successfully stop ventricular fibrillation in a pigeon using open heart massage (9 ). John Hunter created a bellows that could deliver positive and negative pressure ventilation, which he used to resuscitate dogs in 1755 (10). The Dutch Humane Society immediately tapped Hunter’s knowledge to help reduce the death rate of drowning victims (11). The resultant 1767 publication was the first ever to advocate the use of “artificial respiration”:
…the operator closed the patient’s nostrils, applied his mouth to the patient’s mouth, inflated the lungs and expanded the chest and belly, and produced expiration by compressing the abdomen with his free hand (12).
Despite giving ventilation equal measure with another popular technique at the time called fumigation―the use of tobacco smoke to fill the colon of drowned victims via a rectal tube―scientists rapidly began to use true physiologic practices to advance resuscitation.
Within eight years, Priestly would discover the element oxygen and Squires of London would record the first-ever use of electricity in resuscitation:
…he tried the effects of electricity. Twenty minutes elapsed before he could apply the shock [to the 3 year old child who had fallen out of a 1st story window], which he gave to various parts of the body in vain; but upon transmitting a few shocks through the thorax, he perceived a small pulsation; in a few minutes the child began to breathe….her health was restored (4).
Resuscitation became a legitimate science during the 19th century with literature replete of experimental successes in laboratory animal resuscitations. Techniques included using closed and open heart massage, manual ventilation using specialized medical bellows, and then finally, in 1899, documented cessation of ventricular fibrillation by electricity. Jean Louis Prevost and Frederic Batelli reported that they had defibrillated a dog successfully with the use of two electrodes―one on the head and one in the rectum―with high voltage AC current (13).
As the 20th century dawned, George Washington Crile―the cofounder of the Cleveland Clinic and considered by many as the most innovative researcher in the field of resuscitation―described successful closed chest cardiac massage in man and the first use of saline and epinephrine infusion in cardiac arrest in 1903. after the Russians Gurvich and Yuniev had demonstrated the superiority of DC current to AC current in defibrillation in 1939, Beck (1947) and Zoll (1956) published their successes in humans of open and closed chest defibrillation respectively.13 Even though the advent of the modern defibrillator loomed imminently, the practicality of widespread dissemination of the cumbersome equipment needed to provide these shocks was not yet manifest. Thus, Kouwenhoven and colleagues created a technique of closed chest cardiac massage that could keep patients alive long enough to receive definitive treatment, and modern CPR was born (14).
Epidemiology of Cardiopulmonary Arrest
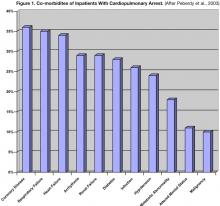
In the United States an estimated 375,000 to 750,000 hospitalized patients suffer a cardiopulmonary arrest (CA) requiring advanced cardiac life support (ACLS) annually (15). The incidence of CA is estimated to be as high as 1–2% of all patients admitted to academic hospitals with a prevalence of 58 to 71 people per 100,000 nationally (16,17). The demographics of over 14,000 patients resuscitated for a CA are summarized in Table 1; patient comorbidities are listed in Figure 1.18 The typical CA patient is a white male in his seventh decade of life with a history of cardiac, pulmonary, or renal disease suffering from a pre-arrest arrhythmia or respiratory problem. Over 86% of patients are either on continuous cardiac monitoring (telemetry) or have a witnessed CA.
When primary respiratory arrests are excluded (such as from opiate overdose or post-anesthesia), only approximately 1 in 7 patients will survive an in-hospital resuscitation to discharge (1,19). Survivors’ initial rhythms are typically either pulseless ventricular tachycardia (VT) (35%) or ventricular fibrillation (VF) (34%), but fully 20% of survivors have initial “rhythms” of asystole or pulseless electrical activity (PEA)―which comprise virtually two–thirds of all arrests―suggesting a meager benefit to resuscitation of this subgroup of patients. Almost one third of survivors who lived independently pre-arrest are unable to be discharged home and between 14-23% of survivors―whose pre-arrest neurological function was normal―develop moderate to severe cognitive deficits after resuscitation (18,20). Fewer than 2% of survivors suffer coma or a persistent vegetative state. Neither gender nor advanced age appears to be a negative predictor of survival (1,21).
Time is Life Lost
The goals of resuscitation are two-fold: preservation of neurological function and prevention of early death. Expedient resuscitation maximizes the likelihood of positive outcomes, but too often, precious time is life lost spent performing unnecessary diagnostic maneuvers or unimportant interventions that have little impact on prognosis. The overarching target in in-hospital adult resuscitation is minimizing the time it takes for patients to receive defibrillation. All other activities are only a mean to this end. The remainder of this review will focus on seven highly effective strategies for successful resuscitations.
Primary Survey
Guidelines continue to stress the importance of airway, breathing, and circulation as the basic tenets of initial response to a CA. While this approach has merit in out-of-hospital arrests, it is an anathema in hospitalized patients. Valuable time is often lost trying to ascertain the presence or absence of respirations or pulse. Earle et al. designed a very creative way to gauge the operating characteristics of the carotid pulse check. Providers were asked to assess randomized patients who were to undergo open-heart surgery; some of the patients were already on cardiopulmonary bypass (“true negatives” with no spontaneous pulse) and the remainder were not (“true positives” with a pulse). With a median time of over 30 seconds, care providers could only accurately determine pulselessness 65% of the time (90% sensitivity and 55% specificity).22 Given that this study occurred in a very controlled environment without the drama of a real CA, it is likely that these data would even be worse in the chaos of resuscitation.
First Effective Stratagem: If an unresponsive adult inpatient clinically appears to be suffering a cardiopulmonary arrest, treatment (activation of a “code team” and application of CPR) should be initiated immediately without performing a pulse check.
CPR Physiology and Impact
CPR is a critical bridge to defibrillation but is not an end unto itself. The physiology that occurs during CPR is remarkably complex, and our current understanding is incomplete. Kouwenhoven posited that chest compressions result in a functional equivalent to open cardiac massage (2). In this “cardiac pump model,” the physiology is similar to a surgeon’s hands squeezing the non-beating heart: artificial systole from the down stroke of a compression compresses the heart against the spinal column forcing blood from the ventricles and forcing closure of the mitral and tricuspid valves. During artificial diastole, reversing pressure gradients result in closure of the aortic and pulmonic valves resulting in bi-ventricular filling of blood and perfusion of the coronary arteries.
An alternative model, the “thoracic pump model,” looks at the entire thoracic cavity as a pump with functional “valves” at the thoracic inlet preventing back-flow from the intrathoracic veins into the extrathoracic veins (23). The intrathoracic pressure rapidly increases during artificial systole leading to antegrade flow of blood from vessels under relatively higher pressure (the aorta and the pulmonary vasculature) to blood vessels under relatively lower pressure (the carotid arteries). The elevated intrathoracic pressure collapses the comparably weak vena cava, and, combined with tricuspid valve closure, prevents simultaneous retrograde venous flow. With three of the cardiac valves open during artificial systole, the heart is relegated to the role of a passive conduit for blood rather than providing any meaningful pumping action. During artificial diastole, intrathoracic pressure drops to near zero resulting in transient back flow of blood from the carotid arteries toward the heart. This induces aortic valve closure and generates only meager coronary artery perfusion.
Subsequent work by Paradis et al. shows that, in essence, both “pump” models have equal validity, and one or the other physiology dominates in any given patient (24). Regardless of which type of physiology occurs during resuscitation, neither provides physiologically sufficient circulation to maintain organ viability for long. When performed ideally, chest compressions during actual resuscitations in humans yield systolic blood pressures of only 60–80mmHg; and blood flow of less than one third the normal cardiac output, less than 10–15% of normal cerebral blood flow, and less than 1–5% of normal coronary artery blood flow (25).
Such subphysiologic circulation leaves little latitude for improper technique. Yet, care providers rarely perform chest compressions properly, erring towards too shallow a compression depth 62.6% of the time and too slow a compression rate 71.9% of the time on actual resuscitations―errors that increase in frequency the longer it has been since the caregiver was trained (26,27). Observational data on the quality of CPR suggest that these are not just esoteric technical deviations, but that compared with those in whom CPR is correctly performed, 14-day survival was almost 75% lower in those on whom CPR was incorrectly performed (16% vs. 4%)(28).
Furthermore, routine interruptions in chest compressions― such as for positive pressure ventilations in non-intubated patients―likely further hinder survival rather than contribute meaningfully to outcome. For example, medical students performing traditional CPR took an average of 14 seconds to administer two mouth-to-mouth ventilations after each group of 15 compressions. This effectively reduced the number of compressions to a mere 43 per minute, or less than half the guideline-mandated 100 per minute, thus theoretically reducing circulation to the heart and brain by a similar percentage (29). Yu et al demonstrated that swine receiving more than 80 compressions per minute during CPR had a 100% survival at 24 hours compared with a dismal 10% survival in animals that had less than 80 compressions per minute (30). Kern et al found statistically significantly higher coronary artery perfusion pressures and markedly higher neurological normal 24 hour survival in swine receiving continuous chest compressions compared with controls receiving traditional CPR (31).
Increasingly data such as these do raise the question of what―if any―benefit rescue breathing has in adult resuscitation. Human data show that a strategy of continuous chest compressions―without rescue breathing―is equally efficacious to traditional CPR in terms of outcome (32). Two physiologic theories prevail: 1) the mechanics of chest wall compression may be sufficient to provide a limited minute ventilation independent of supplemental ventilation (author’s speculation); and 2) the improved oxygenation that occurs in those receiving artificial ventilation is offset by the deleterious impact on hemodynamics that occur when chest compressions are interrupted for ventilation (34).
Second Effective Stratagem: Until an adult inpatient can be defibrillated, the focus of resuscitation should be on proper continuous chest compression depth and rate, not on ventilation.
Ventilations are Harmful
Cardiopulmonary collapse has immediate consequences on cellular physiology. Both due to a lack of expiration of carbon dioxide and the development of lactatemia due to the shift to anaerobic metabolism, blood pH drops precipitously. As the pH shifts out of the physiologic range, drugs begin to perform in unexpected ways or fail altogether; ion trapping occurs; and many electrolytes begin to shift in or out of cells affecting their serum concentration. Diminished or absent cardiac output accelerates these derangements in a relentless positive feedback loop. Logic, therefore, dictates that anything that can improve oxygenation and ventilation would be helpful at slowing or reversing this pathophysiology. Surprisingly, however, clinicians’ lack of knowledge of both equipment and technique often promotes, rather than mitigates, the physiologic derangements.
The bag-valve-mask (BVM) is one of the least understood resuscitation devices. For example, clinicians occasionally place a BVM over spontaneously breathing patients who are exceptionally ill with the goal of augmenting patients’ meager ventilations. However, the BVM is constructed only for positive pressure ventilation and thus, unless the clinician squeezes the bag in perfect coordination with the patient’s ventilatory effort, the BVM will paradoxically smother the patient.
The bag portion of the BVM is designed for a one-handed squeeze to deliver a tidal volume that is roughly 750cc; this volume is in keeping with guideline recommendations of positive pressure ventilation volumes of roughly 10cc/kg/ventilation (29). Yet, clinicians commonly use two hands to compress the BVM maximally during resuscitation (theoretically delivering upwards of double the recommended volume).
In addition to excessive volume, clinicians also deliver ventilations too rapidly. Abella et al. showed that during human resuscitations, ventilation rate exceeded the recommended goal of 20 ventilations/minute 60.9% of the time (27). Theoretically, high minute ventilations lead to an increased incidence of gastric insufflation, regurgitation, and post-resuscitation aspiration.
Though no studies have ever been performed to understand why clinicians hyperventilate patients during resuscitation, it is interesting to speculate that clinicians are not only trying to raise blood oxygen levels rapidly, but also to reverse the profound metabolic and respiratory acidosis that occur during CA. While seemingly mechanistically sound, the logic that supraphysiologic minute ventilations will profoundly change blood pH without other physiologic costs is specious at best. Aufderheide et al. Demonstrated that hyperventilation during resuscitation in swine resulted in increased intrathoracic pressures, markedly reduced coronary artery perfusion pressures, and resultant proportional reductions in survival rates as hyperventilation increased (35). Clinically these findings are known as auto-PEEP, a known complication of artificial ventilation that results in systemic arterial hypotension. Thus the paradox: aggressive attempts to overcorrect systemic acidoses via higher minute ventilations leads to worsening systemic blood pressures and thus worsening lactic acidosis. I believe that iatrogenic hypotension is one of the most common problems to complicate an otherwise successful resuscitation and that more research is urgently needed on this issue.
Third Effective Stratagem: Bag-valve-mask ventilations should be performed with precision; when used, the bag should be compressed with only one hand and delivered no faster than one breath every 3–5 seconds.
Fourth Effective Stratagem: In cases of pulseless electrical activity (PEA) or post-resuscitation hypotension, auto-PEEP should be considered foremost as a proximal cause.
Fifth Effective Stratagem: Ventilations in resuscitation should be viewed primarily as a means to oxygenate the patient rather than as a means to compensate from a systemic acidemia.
Shocking Revelations
Even though patients in ventricular fibrillation (VF) and pulseless ventricular tachycardia (PVT) together only comprise about one–third of all inpatient cardiac arrests, they account for almost 70% of survivors of CA. Survival in these arrhythmias is predicated solely on rapid defibrillation: the simultaneous depolarization of a critical mass of myocytes by an electrical current with resultant resumption of normal cardiac conduction (36). Perhaps the best attestation to the importance of rapid defibrillation actually comes from the survival of patients with cardiac arrest at casinos: security cameras provided exact documentation of time of collapse and defibrillation. Survival to hospital discharge was cut in half when the time-from-collapse-to-defibrillation was greater than 3 minutes (74% vs. 49%)(37).
Sixth Effective Stratagem: Since patients in VF/PVT are the most likely to survive CA―and that rate is directly related to immediacy of defibrillation―all patients should be presumptively treated as if they are in VF/PVT and should be defibrillated within 3 minutes of collapse unless there are data to support another arrhythmia or cause of collapse.
Much of the electrical current delivered during a defibrillation attempt is either dissipated as heat or is conducted around the thorax without penetrating the myocardium; Lerman and Deale have shown that the amount of current reaching the heart may be as little as 4% during a defibrillation attempt (38). From Ohm’s Law, the current that reaches the heart is directly proportional to the voltage across the chest and inversely related to the resistance of the supervening tissues. Interestingly, increasing the voltage of a shock only increases the amount of heat produced without a commensurate increase in current delivered. Therefore, techniques that reduce thoracic resistance yield the highest current delivery: the use of manual defibrillator paddles (as opposed to self adhesive defibrillation pads), the amount of pressure applied to the paddles (>25 pounds), the correct placement (underneath, rather than on top of breast tissue), the use of a conduction material (gel or pads), and the rapid delivery of a stacked shock (resistance transiently decreases after a counter-shock) all improve current delivery and thus may improve defibrillation outcome (36).
Physician leadership during resuscitations is critical for maximizing likelihood of patient survival. Perhaps due to physicians’ lack of familiarity with defibrillators, the mechanics of defibrillation are often deferred unnecessarily to nursing staff. Unfortunately, this may have profoundly negative effects on the resuscitation. Since many hospitals reduce educational expenses by foregoing training nurses in ACLS unless they work in intensive care units, nurses on a code may have even less comfort in using defibrillators than physicians. One study showed nearly a 72.5% decrease in patient survival when the nurse who arrived first at the resuscitation was untrained in ACLS (37.5% vs. 10.3%); a difference that probably is related to delayed defibrillation though the study could not establish direct causality (39).
A separate study showed that 85% of patients on cardiac monitored wards were defibrillated in the target time of 3 minutes from collapse, while only 28% of patients on unmonitored wards were defibrillated within the target time; this led to an adjusted odds ratio for survival-to-discharge in patients on monitored versus non-monitored wards of 1.45 (95% CI, 0.95–2.20)(40). While at first these data seem to indicate faster responses on monitored wards, these data were specific to time-from-collapse-to-defibrillation and therefore should be location-independent once the need for resuscitation was identified. Instead, I interpret these data as showing the lower comfort nurses on non-monitored wards have with rapid defibrillation. Rather than serving as an indictment against nurses, these data in aggregate underscore the vital role physician responders have to ensure rapid defibrillation of patients in cardiac arrest.
Seventh Effective Stratagem: Physicians should aggressively pursue defibrillation as early as possible during resuscitation especially on non-monitored wards where nurses are less likely to be ACLS trained and less likely to be familiar with defibrillator operation.
Conclusions
Resuscitation, the act of bringing back life from imminent death, is one of the most sensationalized practices in medicine and dates almost to the beginning of recorded history. In the past two decades, increasingly evidence-based guidelines have tried to provide a guide to help practitioners treat patients effectively in the minutes after cardiopulmonary collapse. Unfortunately, with each new iteration of the guidelines, it has become increasingly difficult to determine which strategies offer the highest yield or the most import. As ILCOR meets in 2005 to revise the guidelines once again, hopefully the most important strategies will be given their proper highlight, providing clinicians with increased comfort and confidence with cardiopulmonary resuscitation.
Bibliography
- Brindley PG, Markland DM, Mayers I, Kutsogiannis DJ. Predictors of survival following inhospital adult cardiopulmonary resuscitation. CMAJ. 2002;167:343-8.
- Kouwenhoven WB, Jude JR, Knickerbocker GG. Closed chest cardiac massage. JAMA. 1960;173:1064-7.
- Jude JR, Kouwenhoven WB, Knickerbocker GG. Cardiac arrest; report of application of external cardiac massage on 118 patients. JAMA. 1961;178:1063-71.
- Varon J, Sternback GL. Cardiopulmonary resuscitation: lessons from the past. J Emerg Med. 1991;9:5037.
- Liss HP. A history of resuscitation. Ann Emerg Med.1986;15: 65-72.
- Thangam S, Weil MH, Rackow EC. Cardiopulmonary resuscitation: a historical review. Acute Care. 1986;12:63-94.
- Kings II, 4:34-35 (KJV).
- Baker AB. Artificial respiration: the history of an idea. Med Hist. 1971;15:336-46.
- Stephenson HE. Cardiac Arrest and Resuscitation. St Louis: CV Mosby; 1969.
- Lee RV. Cardiopulmonary resuscitation in the eighteenth century: a historical perspective on present practice. J Hist Med. 1972;27:418-33.
- Varon J, Marik PE, Fromm RE. Cardiopulmonary resuscitation: a review for clinicians. Resuscitation. 1998;36: 133-45.
- Keith A. Three Hunterian lectures on the various mechanisms underlying the various methods of artificial respiration. Lancet. 1909;1:895-9.
- DeBard ML. The history of cardiopulmonary resuscitation. Ann Emerg Med. 1980;9:273-5.
- Acosta P, Varion J, Sternbach GL, BaskeQ P. Resuscitation great. Kouwenhoven, Jude and Knickerbocker: the introduction of defibrillation and external chest compressions into modern resuscitation. Resuscitation. 2005;64:139-43.
- US Congress, Office of Technology Assessment. Life-sustaining technologies and the elderly. Washington, DC: US Government Printing Office, 1987; 11, Publication OTA-BA-306.
- DeBard ML. Cardiopulmonary resuscitation: analysis of six years’ experience and review of the literature. Ann Emerg Med. 1981;10:408-11.
- Cassel CK, et al. Cardiopulmonary resuscitation in the elderly. Office of Technology Assessment, U.S. Congress, Washington, DC, November, 1985.
- Peberdy MA, Kaye W, Ornato JP, et al. Cardiopulmonary resuscitation of adults in the hospital: a report of 14 720 cardiac arrests from the National Registry of Cardiopulmonary Resuscitation. Resuscitation. 2003;58297-308.
- Valentin A, Karnik R, Donath P, Winkler WB, Slany J. Outcome of cardiopulmonary resuscitation in hospitalized patients. Resuscitation. 1995;30:217-21.
- Robinson GR, Hess D. Postdischarge survival and functional status following in-hospital cardiopulmonary resuscitation. Chest. 1994;105:991-6.
- Murphy DI, Murray AM, Robinson BE. Outcomes of cardiopulmonary resuscitation in the elderly. Ann Int Med. 1989;111:199-205.
- Eberle B, Dick WF, Schneider T, Wisser G, Doetsch S, Tzanova I. Checking the carotid pulse check: diagnostic accuracy of first responders in patients with and without a pulse. Resuscitation. 1996;33:107-16.
- Rudikoff MT, Maughan WL, Effron M, Freund P, Weisfeldt ML. Mechanisms of blood flow during cardiopulmonary resuscitation. Circulation. 1980;61:345-52.
- Paradis NA, Martin GB, Goetting MG, et al. Simultaneous aortic, jugular bulb, and right atrial pressures during cardiopulmonary resuscitation in humans: insight into mechanisms. Circulation. 1989;80:361-8.
- Sanders AB, Ogle M, Ewy GA. Coronary perfusion pressure during cardiopulmonary resuscitation. Am J Emerg Med. 1985;3:11-4.
- Heidenreich JW, Higdon TA, Kern KB, et al. Single-rescuer cardiopulmonary resuscitation: “two quick breaths”―an oxymoron. Resuscitation. 2004;62:283-89.
- Abella BS, Alvarado JP, Myklebust H, et al. Quality of cardiopulmonary resuscitation during in-hospital cardiac arrest. JAMA. 2005;293:305-10.
- Van Hoeyweghen RJ, Bossaert LL, Mullie A, et al. (Belgian Cerebral Resuscitation Study Group.) Quality and efficiency of bystander CPR. Resuscitation. 1993;26:47-52.
- American Heart Association in collaboration with the International Liaison Committee on Resuscitation (ILCOR). International Guidelines 2000 for Cardiopulmonary Resuscitation and Emergency Cardiovascular Care―a consensus on science. Resuscitation. 2000.46:1-448.
- Yu T, Weil MH, Tang W, et al. Adverse outcomes of interrupted precordial compression during automated defibrillation. Circulation. 2002;106:368-72.
- Kern KB, Hilwig RW, Berg RA, Sanders AB, Ewy GA. Importance of continuous chest compressions during cardiopulmonary resuscitation: improved outcome during a simulated single lay-rescuer scenario. Circulation. 2002;105:645-649.
- Hallstrom A, Cobb L, Johnson E, Copass M. Cardiopulmonary resuscitation by chest compression alone or with to-mouth ventilation. N Engl J Med. ;342: 154-653.
- Berg RA, Sanders AB, Kern KB, et al. Adverse hemodynamic effects of interrupting chest compressions for rescue breathing during cardiopulmonary resuscitation for ventricular fibrillation cardiac arrest. Circulation. 2001;104:2465-70.
- Aufderheide TP, Sigurdsson G, Pirallo RG, et al. Hyperventilation-induced hypotension during cardiopulmonary resuscitation. Circulation. 2004;109:1960-5.
- Dalzell GWN. Determinants of successful defibrillation. Heart. ;80:405-7.
- Valenzuela TD, Roe DJ, Nichol G, Clark LL, Spaite DW,Hardman RG. Outcomes of rapid defibrillation by security ad er cardiac arrest in casinos. N Engl J Med. ;343:120-69.
- Lerman BB, Deale C. Relation between transcardiac and thransthoracic current during defibrillation in humans. Circ Res. 1990;67:1420-6.
- Dane FC, RussellLindgren KS, Parish DC, Durham MD, Brown TD. Inhospital resuscitation: association between ACLS training and survival to discharge. Resuscitation. 2000;47:83-7.
- Herlitz J, Bang A, Aune S, Ekstrom L, Lundstrom G, Holmberg S. Characteristics and outcome among patients suffering inhospital cardiac arrest in monitored and nonmonitored areas. Resuscitation. ;48:125-35.
Despite more intensive guidelines and advances in resuscitation research, the survival rate for victims of cardiopulmonary arrest remains virtually unchanged from forty years ago when modern cardiopulmonary resuscitation (CPR) was first described (1). Perhaps in part because the guidelines for ACLS set forth by the American Heart Association (AHA) and the International Liaison Committee on Resuscitation (ILCOR) have become so complex―and continue to increase in breadth and scope into topics well beyond cardiopulmonary arrest with each revision―the critical aspects of resuscitation have become diluted by unnecessarily difficult algorithms. Critical skill sets―such as proper performance of CPR and rapid defibrillation―have become dwarfed by less critical aspects of acute resuscitation. Remarkably common errors usurp the dual fundamental goals of ACLS: neurological preservation and prevention of early death. This review will address the historical context of resuscitation and then will focus on seven of the most essential, evidence-based strategies for improving outcomes in ACLS.
History of Resuscitation
The modern resuscitation era began in 1960 when Kouwenhoven, Jude, and Knickerbocker published a pair of landmark papers on the use of closed chest compressions (CCC) as a means to resuscitate patients in cardiopulmonary arrest (2,3). Interestingly, the culmination of their three and a half decades of work was initially motivated and sponsored by an electric company seeking to reduce the death rate of its linemen from ventricular fibrillation. While innovative, their technique built on millennia of creative, and sometimes bizarre ancient practices geared at reversing death. Given the lack of in-depth knowledge of anatomy and physiology combined with a rich overlap between shamanism and medicine, it is perhaps stunning to realize that the oldest recorded reasonably physiologic approach to resuscitation stems from over 3500 years ago. Egyptian hieroglyphs show the story of the healing goddess, Isis, reviving her husband Osiris using mouth-to-mouth ventilation (4). Still other Egyptian texts advocated hanging drowned victims upside down, compressing and releasing the thorax with the goal to ventilate and revive the patient (5). Hebrew midwives were documented as having performed mouth-to-mouth on deceased newborns as early as 1300 BCE (6). And even the Bible tells of the prophet Elishah’s successful resuscitation of a deceased child through artificial respiration:
...And he went up, and lay upon the child, and put his mouth upon his mouth, and his eyes upon his eyes, and his hands upon his hands; and he stretched himself upon the child; and the flesh of the child waxed warm (7).
One of the forefathers of modern medicine, the Greek physician Galen, was the first doctor to use an artificial ventilation strategy in 177, filling dead animals’ lungs with air from a bellows (8).
In 1628, physician William Harvey, the first to accurately describe circulation, used his newfound knowledge to successfully stop ventricular fibrillation in a pigeon using open heart massage (9 ). John Hunter created a bellows that could deliver positive and negative pressure ventilation, which he used to resuscitate dogs in 1755 (10). The Dutch Humane Society immediately tapped Hunter’s knowledge to help reduce the death rate of drowning victims (11). The resultant 1767 publication was the first ever to advocate the use of “artificial respiration”:
…the operator closed the patient’s nostrils, applied his mouth to the patient’s mouth, inflated the lungs and expanded the chest and belly, and produced expiration by compressing the abdomen with his free hand (12).
Despite giving ventilation equal measure with another popular technique at the time called fumigation―the use of tobacco smoke to fill the colon of drowned victims via a rectal tube―scientists rapidly began to use true physiologic practices to advance resuscitation.
Within eight years, Priestly would discover the element oxygen and Squires of London would record the first-ever use of electricity in resuscitation:
…he tried the effects of electricity. Twenty minutes elapsed before he could apply the shock [to the 3 year old child who had fallen out of a 1st story window], which he gave to various parts of the body in vain; but upon transmitting a few shocks through the thorax, he perceived a small pulsation; in a few minutes the child began to breathe….her health was restored (4).
Resuscitation became a legitimate science during the 19th century with literature replete of experimental successes in laboratory animal resuscitations. Techniques included using closed and open heart massage, manual ventilation using specialized medical bellows, and then finally, in 1899, documented cessation of ventricular fibrillation by electricity. Jean Louis Prevost and Frederic Batelli reported that they had defibrillated a dog successfully with the use of two electrodes―one on the head and one in the rectum―with high voltage AC current (13).
As the 20th century dawned, George Washington Crile―the cofounder of the Cleveland Clinic and considered by many as the most innovative researcher in the field of resuscitation―described successful closed chest cardiac massage in man and the first use of saline and epinephrine infusion in cardiac arrest in 1903. after the Russians Gurvich and Yuniev had demonstrated the superiority of DC current to AC current in defibrillation in 1939, Beck (1947) and Zoll (1956) published their successes in humans of open and closed chest defibrillation respectively.13 Even though the advent of the modern defibrillator loomed imminently, the practicality of widespread dissemination of the cumbersome equipment needed to provide these shocks was not yet manifest. Thus, Kouwenhoven and colleagues created a technique of closed chest cardiac massage that could keep patients alive long enough to receive definitive treatment, and modern CPR was born (14).
Epidemiology of Cardiopulmonary Arrest
In the United States an estimated 375,000 to 750,000 hospitalized patients suffer a cardiopulmonary arrest (CA) requiring advanced cardiac life support (ACLS) annually (15). The incidence of CA is estimated to be as high as 1–2% of all patients admitted to academic hospitals with a prevalence of 58 to 71 people per 100,000 nationally (16,17). The demographics of over 14,000 patients resuscitated for a CA are summarized in Table 1; patient comorbidities are listed in Figure 1.18 The typical CA patient is a white male in his seventh decade of life with a history of cardiac, pulmonary, or renal disease suffering from a pre-arrest arrhythmia or respiratory problem. Over 86% of patients are either on continuous cardiac monitoring (telemetry) or have a witnessed CA.
When primary respiratory arrests are excluded (such as from opiate overdose or post-anesthesia), only approximately 1 in 7 patients will survive an in-hospital resuscitation to discharge (1,19). Survivors’ initial rhythms are typically either pulseless ventricular tachycardia (VT) (35%) or ventricular fibrillation (VF) (34%), but fully 20% of survivors have initial “rhythms” of asystole or pulseless electrical activity (PEA)―which comprise virtually two–thirds of all arrests―suggesting a meager benefit to resuscitation of this subgroup of patients. Almost one third of survivors who lived independently pre-arrest are unable to be discharged home and between 14-23% of survivors―whose pre-arrest neurological function was normal―develop moderate to severe cognitive deficits after resuscitation (18,20). Fewer than 2% of survivors suffer coma or a persistent vegetative state. Neither gender nor advanced age appears to be a negative predictor of survival (1,21).
Time is Life Lost
The goals of resuscitation are two-fold: preservation of neurological function and prevention of early death. Expedient resuscitation maximizes the likelihood of positive outcomes, but too often, precious time is life lost spent performing unnecessary diagnostic maneuvers or unimportant interventions that have little impact on prognosis. The overarching target in in-hospital adult resuscitation is minimizing the time it takes for patients to receive defibrillation. All other activities are only a mean to this end. The remainder of this review will focus on seven highly effective strategies for successful resuscitations.
Primary Survey
Guidelines continue to stress the importance of airway, breathing, and circulation as the basic tenets of initial response to a CA. While this approach has merit in out-of-hospital arrests, it is an anathema in hospitalized patients. Valuable time is often lost trying to ascertain the presence or absence of respirations or pulse. Earle et al. designed a very creative way to gauge the operating characteristics of the carotid pulse check. Providers were asked to assess randomized patients who were to undergo open-heart surgery; some of the patients were already on cardiopulmonary bypass (“true negatives” with no spontaneous pulse) and the remainder were not (“true positives” with a pulse). With a median time of over 30 seconds, care providers could only accurately determine pulselessness 65% of the time (90% sensitivity and 55% specificity).22 Given that this study occurred in a very controlled environment without the drama of a real CA, it is likely that these data would even be worse in the chaos of resuscitation.
First Effective Stratagem: If an unresponsive adult inpatient clinically appears to be suffering a cardiopulmonary arrest, treatment (activation of a “code team” and application of CPR) should be initiated immediately without performing a pulse check.
CPR Physiology and Impact
CPR is a critical bridge to defibrillation but is not an end unto itself. The physiology that occurs during CPR is remarkably complex, and our current understanding is incomplete. Kouwenhoven posited that chest compressions result in a functional equivalent to open cardiac massage (2). In this “cardiac pump model,” the physiology is similar to a surgeon’s hands squeezing the non-beating heart: artificial systole from the down stroke of a compression compresses the heart against the spinal column forcing blood from the ventricles and forcing closure of the mitral and tricuspid valves. During artificial diastole, reversing pressure gradients result in closure of the aortic and pulmonic valves resulting in bi-ventricular filling of blood and perfusion of the coronary arteries.
An alternative model, the “thoracic pump model,” looks at the entire thoracic cavity as a pump with functional “valves” at the thoracic inlet preventing back-flow from the intrathoracic veins into the extrathoracic veins (23). The intrathoracic pressure rapidly increases during artificial systole leading to antegrade flow of blood from vessels under relatively higher pressure (the aorta and the pulmonary vasculature) to blood vessels under relatively lower pressure (the carotid arteries). The elevated intrathoracic pressure collapses the comparably weak vena cava, and, combined with tricuspid valve closure, prevents simultaneous retrograde venous flow. With three of the cardiac valves open during artificial systole, the heart is relegated to the role of a passive conduit for blood rather than providing any meaningful pumping action. During artificial diastole, intrathoracic pressure drops to near zero resulting in transient back flow of blood from the carotid arteries toward the heart. This induces aortic valve closure and generates only meager coronary artery perfusion.
Subsequent work by Paradis et al. shows that, in essence, both “pump” models have equal validity, and one or the other physiology dominates in any given patient (24). Regardless of which type of physiology occurs during resuscitation, neither provides physiologically sufficient circulation to maintain organ viability for long. When performed ideally, chest compressions during actual resuscitations in humans yield systolic blood pressures of only 60–80mmHg; and blood flow of less than one third the normal cardiac output, less than 10–15% of normal cerebral blood flow, and less than 1–5% of normal coronary artery blood flow (25).
Such subphysiologic circulation leaves little latitude for improper technique. Yet, care providers rarely perform chest compressions properly, erring towards too shallow a compression depth 62.6% of the time and too slow a compression rate 71.9% of the time on actual resuscitations―errors that increase in frequency the longer it has been since the caregiver was trained (26,27). Observational data on the quality of CPR suggest that these are not just esoteric technical deviations, but that compared with those in whom CPR is correctly performed, 14-day survival was almost 75% lower in those on whom CPR was incorrectly performed (16% vs. 4%)(28).
Furthermore, routine interruptions in chest compressions― such as for positive pressure ventilations in non-intubated patients―likely further hinder survival rather than contribute meaningfully to outcome. For example, medical students performing traditional CPR took an average of 14 seconds to administer two mouth-to-mouth ventilations after each group of 15 compressions. This effectively reduced the number of compressions to a mere 43 per minute, or less than half the guideline-mandated 100 per minute, thus theoretically reducing circulation to the heart and brain by a similar percentage (29). Yu et al demonstrated that swine receiving more than 80 compressions per minute during CPR had a 100% survival at 24 hours compared with a dismal 10% survival in animals that had less than 80 compressions per minute (30). Kern et al found statistically significantly higher coronary artery perfusion pressures and markedly higher neurological normal 24 hour survival in swine receiving continuous chest compressions compared with controls receiving traditional CPR (31).
Increasingly data such as these do raise the question of what―if any―benefit rescue breathing has in adult resuscitation. Human data show that a strategy of continuous chest compressions―without rescue breathing―is equally efficacious to traditional CPR in terms of outcome (32). Two physiologic theories prevail: 1) the mechanics of chest wall compression may be sufficient to provide a limited minute ventilation independent of supplemental ventilation (author’s speculation); and 2) the improved oxygenation that occurs in those receiving artificial ventilation is offset by the deleterious impact on hemodynamics that occur when chest compressions are interrupted for ventilation (34).
Second Effective Stratagem: Until an adult inpatient can be defibrillated, the focus of resuscitation should be on proper continuous chest compression depth and rate, not on ventilation.
Ventilations are Harmful
Cardiopulmonary collapse has immediate consequences on cellular physiology. Both due to a lack of expiration of carbon dioxide and the development of lactatemia due to the shift to anaerobic metabolism, blood pH drops precipitously. As the pH shifts out of the physiologic range, drugs begin to perform in unexpected ways or fail altogether; ion trapping occurs; and many electrolytes begin to shift in or out of cells affecting their serum concentration. Diminished or absent cardiac output accelerates these derangements in a relentless positive feedback loop. Logic, therefore, dictates that anything that can improve oxygenation and ventilation would be helpful at slowing or reversing this pathophysiology. Surprisingly, however, clinicians’ lack of knowledge of both equipment and technique often promotes, rather than mitigates, the physiologic derangements.
The bag-valve-mask (BVM) is one of the least understood resuscitation devices. For example, clinicians occasionally place a BVM over spontaneously breathing patients who are exceptionally ill with the goal of augmenting patients’ meager ventilations. However, the BVM is constructed only for positive pressure ventilation and thus, unless the clinician squeezes the bag in perfect coordination with the patient’s ventilatory effort, the BVM will paradoxically smother the patient.
The bag portion of the BVM is designed for a one-handed squeeze to deliver a tidal volume that is roughly 750cc; this volume is in keeping with guideline recommendations of positive pressure ventilation volumes of roughly 10cc/kg/ventilation (29). Yet, clinicians commonly use two hands to compress the BVM maximally during resuscitation (theoretically delivering upwards of double the recommended volume).
In addition to excessive volume, clinicians also deliver ventilations too rapidly. Abella et al. showed that during human resuscitations, ventilation rate exceeded the recommended goal of 20 ventilations/minute 60.9% of the time (27). Theoretically, high minute ventilations lead to an increased incidence of gastric insufflation, regurgitation, and post-resuscitation aspiration.
Though no studies have ever been performed to understand why clinicians hyperventilate patients during resuscitation, it is interesting to speculate that clinicians are not only trying to raise blood oxygen levels rapidly, but also to reverse the profound metabolic and respiratory acidosis that occur during CA. While seemingly mechanistically sound, the logic that supraphysiologic minute ventilations will profoundly change blood pH without other physiologic costs is specious at best. Aufderheide et al. Demonstrated that hyperventilation during resuscitation in swine resulted in increased intrathoracic pressures, markedly reduced coronary artery perfusion pressures, and resultant proportional reductions in survival rates as hyperventilation increased (35). Clinically these findings are known as auto-PEEP, a known complication of artificial ventilation that results in systemic arterial hypotension. Thus the paradox: aggressive attempts to overcorrect systemic acidoses via higher minute ventilations leads to worsening systemic blood pressures and thus worsening lactic acidosis. I believe that iatrogenic hypotension is one of the most common problems to complicate an otherwise successful resuscitation and that more research is urgently needed on this issue.
Third Effective Stratagem: Bag-valve-mask ventilations should be performed with precision; when used, the bag should be compressed with only one hand and delivered no faster than one breath every 3–5 seconds.
Fourth Effective Stratagem: In cases of pulseless electrical activity (PEA) or post-resuscitation hypotension, auto-PEEP should be considered foremost as a proximal cause.
Fifth Effective Stratagem: Ventilations in resuscitation should be viewed primarily as a means to oxygenate the patient rather than as a means to compensate from a systemic acidemia.
Shocking Revelations
Even though patients in ventricular fibrillation (VF) and pulseless ventricular tachycardia (PVT) together only comprise about one–third of all inpatient cardiac arrests, they account for almost 70% of survivors of CA. Survival in these arrhythmias is predicated solely on rapid defibrillation: the simultaneous depolarization of a critical mass of myocytes by an electrical current with resultant resumption of normal cardiac conduction (36). Perhaps the best attestation to the importance of rapid defibrillation actually comes from the survival of patients with cardiac arrest at casinos: security cameras provided exact documentation of time of collapse and defibrillation. Survival to hospital discharge was cut in half when the time-from-collapse-to-defibrillation was greater than 3 minutes (74% vs. 49%)(37).
Sixth Effective Stratagem: Since patients in VF/PVT are the most likely to survive CA―and that rate is directly related to immediacy of defibrillation―all patients should be presumptively treated as if they are in VF/PVT and should be defibrillated within 3 minutes of collapse unless there are data to support another arrhythmia or cause of collapse.
Much of the electrical current delivered during a defibrillation attempt is either dissipated as heat or is conducted around the thorax without penetrating the myocardium; Lerman and Deale have shown that the amount of current reaching the heart may be as little as 4% during a defibrillation attempt (38). From Ohm’s Law, the current that reaches the heart is directly proportional to the voltage across the chest and inversely related to the resistance of the supervening tissues. Interestingly, increasing the voltage of a shock only increases the amount of heat produced without a commensurate increase in current delivered. Therefore, techniques that reduce thoracic resistance yield the highest current delivery: the use of manual defibrillator paddles (as opposed to self adhesive defibrillation pads), the amount of pressure applied to the paddles (>25 pounds), the correct placement (underneath, rather than on top of breast tissue), the use of a conduction material (gel or pads), and the rapid delivery of a stacked shock (resistance transiently decreases after a counter-shock) all improve current delivery and thus may improve defibrillation outcome (36).
Physician leadership during resuscitations is critical for maximizing likelihood of patient survival. Perhaps due to physicians’ lack of familiarity with defibrillators, the mechanics of defibrillation are often deferred unnecessarily to nursing staff. Unfortunately, this may have profoundly negative effects on the resuscitation. Since many hospitals reduce educational expenses by foregoing training nurses in ACLS unless they work in intensive care units, nurses on a code may have even less comfort in using defibrillators than physicians. One study showed nearly a 72.5% decrease in patient survival when the nurse who arrived first at the resuscitation was untrained in ACLS (37.5% vs. 10.3%); a difference that probably is related to delayed defibrillation though the study could not establish direct causality (39).
A separate study showed that 85% of patients on cardiac monitored wards were defibrillated in the target time of 3 minutes from collapse, while only 28% of patients on unmonitored wards were defibrillated within the target time; this led to an adjusted odds ratio for survival-to-discharge in patients on monitored versus non-monitored wards of 1.45 (95% CI, 0.95–2.20)(40). While at first these data seem to indicate faster responses on monitored wards, these data were specific to time-from-collapse-to-defibrillation and therefore should be location-independent once the need for resuscitation was identified. Instead, I interpret these data as showing the lower comfort nurses on non-monitored wards have with rapid defibrillation. Rather than serving as an indictment against nurses, these data in aggregate underscore the vital role physician responders have to ensure rapid defibrillation of patients in cardiac arrest.
Seventh Effective Stratagem: Physicians should aggressively pursue defibrillation as early as possible during resuscitation especially on non-monitored wards where nurses are less likely to be ACLS trained and less likely to be familiar with defibrillator operation.
Conclusions
Resuscitation, the act of bringing back life from imminent death, is one of the most sensationalized practices in medicine and dates almost to the beginning of recorded history. In the past two decades, increasingly evidence-based guidelines have tried to provide a guide to help practitioners treat patients effectively in the minutes after cardiopulmonary collapse. Unfortunately, with each new iteration of the guidelines, it has become increasingly difficult to determine which strategies offer the highest yield or the most import. As ILCOR meets in 2005 to revise the guidelines once again, hopefully the most important strategies will be given their proper highlight, providing clinicians with increased comfort and confidence with cardiopulmonary resuscitation.
Bibliography
- Brindley PG, Markland DM, Mayers I, Kutsogiannis DJ. Predictors of survival following inhospital adult cardiopulmonary resuscitation. CMAJ. 2002;167:343-8.
- Kouwenhoven WB, Jude JR, Knickerbocker GG. Closed chest cardiac massage. JAMA. 1960;173:1064-7.
- Jude JR, Kouwenhoven WB, Knickerbocker GG. Cardiac arrest; report of application of external cardiac massage on 118 patients. JAMA. 1961;178:1063-71.
- Varon J, Sternback GL. Cardiopulmonary resuscitation: lessons from the past. J Emerg Med. 1991;9:5037.
- Liss HP. A history of resuscitation. Ann Emerg Med.1986;15: 65-72.
- Thangam S, Weil MH, Rackow EC. Cardiopulmonary resuscitation: a historical review. Acute Care. 1986;12:63-94.
- Kings II, 4:34-35 (KJV).
- Baker AB. Artificial respiration: the history of an idea. Med Hist. 1971;15:336-46.
- Stephenson HE. Cardiac Arrest and Resuscitation. St Louis: CV Mosby; 1969.
- Lee RV. Cardiopulmonary resuscitation in the eighteenth century: a historical perspective on present practice. J Hist Med. 1972;27:418-33.
- Varon J, Marik PE, Fromm RE. Cardiopulmonary resuscitation: a review for clinicians. Resuscitation. 1998;36: 133-45.
- Keith A. Three Hunterian lectures on the various mechanisms underlying the various methods of artificial respiration. Lancet. 1909;1:895-9.
- DeBard ML. The history of cardiopulmonary resuscitation. Ann Emerg Med. 1980;9:273-5.
- Acosta P, Varion J, Sternbach GL, BaskeQ P. Resuscitation great. Kouwenhoven, Jude and Knickerbocker: the introduction of defibrillation and external chest compressions into modern resuscitation. Resuscitation. 2005;64:139-43.
- US Congress, Office of Technology Assessment. Life-sustaining technologies and the elderly. Washington, DC: US Government Printing Office, 1987; 11, Publication OTA-BA-306.
- DeBard ML. Cardiopulmonary resuscitation: analysis of six years’ experience and review of the literature. Ann Emerg Med. 1981;10:408-11.
- Cassel CK, et al. Cardiopulmonary resuscitation in the elderly. Office of Technology Assessment, U.S. Congress, Washington, DC, November, 1985.
- Peberdy MA, Kaye W, Ornato JP, et al. Cardiopulmonary resuscitation of adults in the hospital: a report of 14 720 cardiac arrests from the National Registry of Cardiopulmonary Resuscitation. Resuscitation. 2003;58297-308.
- Valentin A, Karnik R, Donath P, Winkler WB, Slany J. Outcome of cardiopulmonary resuscitation in hospitalized patients. Resuscitation. 1995;30:217-21.
- Robinson GR, Hess D. Postdischarge survival and functional status following in-hospital cardiopulmonary resuscitation. Chest. 1994;105:991-6.
- Murphy DI, Murray AM, Robinson BE. Outcomes of cardiopulmonary resuscitation in the elderly. Ann Int Med. 1989;111:199-205.
- Eberle B, Dick WF, Schneider T, Wisser G, Doetsch S, Tzanova I. Checking the carotid pulse check: diagnostic accuracy of first responders in patients with and without a pulse. Resuscitation. 1996;33:107-16.
- Rudikoff MT, Maughan WL, Effron M, Freund P, Weisfeldt ML. Mechanisms of blood flow during cardiopulmonary resuscitation. Circulation. 1980;61:345-52.
- Paradis NA, Martin GB, Goetting MG, et al. Simultaneous aortic, jugular bulb, and right atrial pressures during cardiopulmonary resuscitation in humans: insight into mechanisms. Circulation. 1989;80:361-8.
- Sanders AB, Ogle M, Ewy GA. Coronary perfusion pressure during cardiopulmonary resuscitation. Am J Emerg Med. 1985;3:11-4.
- Heidenreich JW, Higdon TA, Kern KB, et al. Single-rescuer cardiopulmonary resuscitation: “two quick breaths”―an oxymoron. Resuscitation. 2004;62:283-89.
- Abella BS, Alvarado JP, Myklebust H, et al. Quality of cardiopulmonary resuscitation during in-hospital cardiac arrest. JAMA. 2005;293:305-10.
- Van Hoeyweghen RJ, Bossaert LL, Mullie A, et al. (Belgian Cerebral Resuscitation Study Group.) Quality and efficiency of bystander CPR. Resuscitation. 1993;26:47-52.
- American Heart Association in collaboration with the International Liaison Committee on Resuscitation (ILCOR). International Guidelines 2000 for Cardiopulmonary Resuscitation and Emergency Cardiovascular Care―a consensus on science. Resuscitation. 2000.46:1-448.
- Yu T, Weil MH, Tang W, et al. Adverse outcomes of interrupted precordial compression during automated defibrillation. Circulation. 2002;106:368-72.
- Kern KB, Hilwig RW, Berg RA, Sanders AB, Ewy GA. Importance of continuous chest compressions during cardiopulmonary resuscitation: improved outcome during a simulated single lay-rescuer scenario. Circulation. 2002;105:645-649.
- Hallstrom A, Cobb L, Johnson E, Copass M. Cardiopulmonary resuscitation by chest compression alone or with to-mouth ventilation. N Engl J Med. ;342: 154-653.
- Berg RA, Sanders AB, Kern KB, et al. Adverse hemodynamic effects of interrupting chest compressions for rescue breathing during cardiopulmonary resuscitation for ventricular fibrillation cardiac arrest. Circulation. 2001;104:2465-70.
- Aufderheide TP, Sigurdsson G, Pirallo RG, et al. Hyperventilation-induced hypotension during cardiopulmonary resuscitation. Circulation. 2004;109:1960-5.
- Dalzell GWN. Determinants of successful defibrillation. Heart. ;80:405-7.
- Valenzuela TD, Roe DJ, Nichol G, Clark LL, Spaite DW,Hardman RG. Outcomes of rapid defibrillation by security ad er cardiac arrest in casinos. N Engl J Med. ;343:120-69.
- Lerman BB, Deale C. Relation between transcardiac and thransthoracic current during defibrillation in humans. Circ Res. 1990;67:1420-6.
- Dane FC, RussellLindgren KS, Parish DC, Durham MD, Brown TD. Inhospital resuscitation: association between ACLS training and survival to discharge. Resuscitation. 2000;47:83-7.
- Herlitz J, Bang A, Aune S, Ekstrom L, Lundstrom G, Holmberg S. Characteristics and outcome among patients suffering inhospital cardiac arrest in monitored and nonmonitored areas. Resuscitation. ;48:125-35.
Despite more intensive guidelines and advances in resuscitation research, the survival rate for victims of cardiopulmonary arrest remains virtually unchanged from forty years ago when modern cardiopulmonary resuscitation (CPR) was first described (1). Perhaps in part because the guidelines for ACLS set forth by the American Heart Association (AHA) and the International Liaison Committee on Resuscitation (ILCOR) have become so complex―and continue to increase in breadth and scope into topics well beyond cardiopulmonary arrest with each revision―the critical aspects of resuscitation have become diluted by unnecessarily difficult algorithms. Critical skill sets―such as proper performance of CPR and rapid defibrillation―have become dwarfed by less critical aspects of acute resuscitation. Remarkably common errors usurp the dual fundamental goals of ACLS: neurological preservation and prevention of early death. This review will address the historical context of resuscitation and then will focus on seven of the most essential, evidence-based strategies for improving outcomes in ACLS.
History of Resuscitation
The modern resuscitation era began in 1960 when Kouwenhoven, Jude, and Knickerbocker published a pair of landmark papers on the use of closed chest compressions (CCC) as a means to resuscitate patients in cardiopulmonary arrest (2,3). Interestingly, the culmination of their three and a half decades of work was initially motivated and sponsored by an electric company seeking to reduce the death rate of its linemen from ventricular fibrillation. While innovative, their technique built on millennia of creative, and sometimes bizarre ancient practices geared at reversing death. Given the lack of in-depth knowledge of anatomy and physiology combined with a rich overlap between shamanism and medicine, it is perhaps stunning to realize that the oldest recorded reasonably physiologic approach to resuscitation stems from over 3500 years ago. Egyptian hieroglyphs show the story of the healing goddess, Isis, reviving her husband Osiris using mouth-to-mouth ventilation (4). Still other Egyptian texts advocated hanging drowned victims upside down, compressing and releasing the thorax with the goal to ventilate and revive the patient (5). Hebrew midwives were documented as having performed mouth-to-mouth on deceased newborns as early as 1300 BCE (6). And even the Bible tells of the prophet Elishah’s successful resuscitation of a deceased child through artificial respiration:
...And he went up, and lay upon the child, and put his mouth upon his mouth, and his eyes upon his eyes, and his hands upon his hands; and he stretched himself upon the child; and the flesh of the child waxed warm (7).
One of the forefathers of modern medicine, the Greek physician Galen, was the first doctor to use an artificial ventilation strategy in 177, filling dead animals’ lungs with air from a bellows (8).
In 1628, physician William Harvey, the first to accurately describe circulation, used his newfound knowledge to successfully stop ventricular fibrillation in a pigeon using open heart massage (9 ). John Hunter created a bellows that could deliver positive and negative pressure ventilation, which he used to resuscitate dogs in 1755 (10). The Dutch Humane Society immediately tapped Hunter’s knowledge to help reduce the death rate of drowning victims (11). The resultant 1767 publication was the first ever to advocate the use of “artificial respiration”:
…the operator closed the patient’s nostrils, applied his mouth to the patient’s mouth, inflated the lungs and expanded the chest and belly, and produced expiration by compressing the abdomen with his free hand (12).
Despite giving ventilation equal measure with another popular technique at the time called fumigation―the use of tobacco smoke to fill the colon of drowned victims via a rectal tube―scientists rapidly began to use true physiologic practices to advance resuscitation.
Within eight years, Priestly would discover the element oxygen and Squires of London would record the first-ever use of electricity in resuscitation:
…he tried the effects of electricity. Twenty minutes elapsed before he could apply the shock [to the 3 year old child who had fallen out of a 1st story window], which he gave to various parts of the body in vain; but upon transmitting a few shocks through the thorax, he perceived a small pulsation; in a few minutes the child began to breathe….her health was restored (4).
Resuscitation became a legitimate science during the 19th century with literature replete of experimental successes in laboratory animal resuscitations. Techniques included using closed and open heart massage, manual ventilation using specialized medical bellows, and then finally, in 1899, documented cessation of ventricular fibrillation by electricity. Jean Louis Prevost and Frederic Batelli reported that they had defibrillated a dog successfully with the use of two electrodes―one on the head and one in the rectum―with high voltage AC current (13).
As the 20th century dawned, George Washington Crile―the cofounder of the Cleveland Clinic and considered by many as the most innovative researcher in the field of resuscitation―described successful closed chest cardiac massage in man and the first use of saline and epinephrine infusion in cardiac arrest in 1903. after the Russians Gurvich and Yuniev had demonstrated the superiority of DC current to AC current in defibrillation in 1939, Beck (1947) and Zoll (1956) published their successes in humans of open and closed chest defibrillation respectively.13 Even though the advent of the modern defibrillator loomed imminently, the practicality of widespread dissemination of the cumbersome equipment needed to provide these shocks was not yet manifest. Thus, Kouwenhoven and colleagues created a technique of closed chest cardiac massage that could keep patients alive long enough to receive definitive treatment, and modern CPR was born (14).
Epidemiology of Cardiopulmonary Arrest
In the United States an estimated 375,000 to 750,000 hospitalized patients suffer a cardiopulmonary arrest (CA) requiring advanced cardiac life support (ACLS) annually (15). The incidence of CA is estimated to be as high as 1–2% of all patients admitted to academic hospitals with a prevalence of 58 to 71 people per 100,000 nationally (16,17). The demographics of over 14,000 patients resuscitated for a CA are summarized in Table 1; patient comorbidities are listed in Figure 1.18 The typical CA patient is a white male in his seventh decade of life with a history of cardiac, pulmonary, or renal disease suffering from a pre-arrest arrhythmia or respiratory problem. Over 86% of patients are either on continuous cardiac monitoring (telemetry) or have a witnessed CA.
When primary respiratory arrests are excluded (such as from opiate overdose or post-anesthesia), only approximately 1 in 7 patients will survive an in-hospital resuscitation to discharge (1,19). Survivors’ initial rhythms are typically either pulseless ventricular tachycardia (VT) (35%) or ventricular fibrillation (VF) (34%), but fully 20% of survivors have initial “rhythms” of asystole or pulseless electrical activity (PEA)―which comprise virtually two–thirds of all arrests―suggesting a meager benefit to resuscitation of this subgroup of patients. Almost one third of survivors who lived independently pre-arrest are unable to be discharged home and between 14-23% of survivors―whose pre-arrest neurological function was normal―develop moderate to severe cognitive deficits after resuscitation (18,20). Fewer than 2% of survivors suffer coma or a persistent vegetative state. Neither gender nor advanced age appears to be a negative predictor of survival (1,21).
Time is Life Lost
The goals of resuscitation are two-fold: preservation of neurological function and prevention of early death. Expedient resuscitation maximizes the likelihood of positive outcomes, but too often, precious time is life lost spent performing unnecessary diagnostic maneuvers or unimportant interventions that have little impact on prognosis. The overarching target in in-hospital adult resuscitation is minimizing the time it takes for patients to receive defibrillation. All other activities are only a mean to this end. The remainder of this review will focus on seven highly effective strategies for successful resuscitations.
Primary Survey
Guidelines continue to stress the importance of airway, breathing, and circulation as the basic tenets of initial response to a CA. While this approach has merit in out-of-hospital arrests, it is an anathema in hospitalized patients. Valuable time is often lost trying to ascertain the presence or absence of respirations or pulse. Earle et al. designed a very creative way to gauge the operating characteristics of the carotid pulse check. Providers were asked to assess randomized patients who were to undergo open-heart surgery; some of the patients were already on cardiopulmonary bypass (“true negatives” with no spontaneous pulse) and the remainder were not (“true positives” with a pulse). With a median time of over 30 seconds, care providers could only accurately determine pulselessness 65% of the time (90% sensitivity and 55% specificity).22 Given that this study occurred in a very controlled environment without the drama of a real CA, it is likely that these data would even be worse in the chaos of resuscitation.
First Effective Stratagem: If an unresponsive adult inpatient clinically appears to be suffering a cardiopulmonary arrest, treatment (activation of a “code team” and application of CPR) should be initiated immediately without performing a pulse check.
CPR Physiology and Impact
CPR is a critical bridge to defibrillation but is not an end unto itself. The physiology that occurs during CPR is remarkably complex, and our current understanding is incomplete. Kouwenhoven posited that chest compressions result in a functional equivalent to open cardiac massage (2). In this “cardiac pump model,” the physiology is similar to a surgeon’s hands squeezing the non-beating heart: artificial systole from the down stroke of a compression compresses the heart against the spinal column forcing blood from the ventricles and forcing closure of the mitral and tricuspid valves. During artificial diastole, reversing pressure gradients result in closure of the aortic and pulmonic valves resulting in bi-ventricular filling of blood and perfusion of the coronary arteries.
An alternative model, the “thoracic pump model,” looks at the entire thoracic cavity as a pump with functional “valves” at the thoracic inlet preventing back-flow from the intrathoracic veins into the extrathoracic veins (23). The intrathoracic pressure rapidly increases during artificial systole leading to antegrade flow of blood from vessels under relatively higher pressure (the aorta and the pulmonary vasculature) to blood vessels under relatively lower pressure (the carotid arteries). The elevated intrathoracic pressure collapses the comparably weak vena cava, and, combined with tricuspid valve closure, prevents simultaneous retrograde venous flow. With three of the cardiac valves open during artificial systole, the heart is relegated to the role of a passive conduit for blood rather than providing any meaningful pumping action. During artificial diastole, intrathoracic pressure drops to near zero resulting in transient back flow of blood from the carotid arteries toward the heart. This induces aortic valve closure and generates only meager coronary artery perfusion.
Subsequent work by Paradis et al. shows that, in essence, both “pump” models have equal validity, and one or the other physiology dominates in any given patient (24). Regardless of which type of physiology occurs during resuscitation, neither provides physiologically sufficient circulation to maintain organ viability for long. When performed ideally, chest compressions during actual resuscitations in humans yield systolic blood pressures of only 60–80mmHg; and blood flow of less than one third the normal cardiac output, less than 10–15% of normal cerebral blood flow, and less than 1–5% of normal coronary artery blood flow (25).
Such subphysiologic circulation leaves little latitude for improper technique. Yet, care providers rarely perform chest compressions properly, erring towards too shallow a compression depth 62.6% of the time and too slow a compression rate 71.9% of the time on actual resuscitations―errors that increase in frequency the longer it has been since the caregiver was trained (26,27). Observational data on the quality of CPR suggest that these are not just esoteric technical deviations, but that compared with those in whom CPR is correctly performed, 14-day survival was almost 75% lower in those on whom CPR was incorrectly performed (16% vs. 4%)(28).
Furthermore, routine interruptions in chest compressions― such as for positive pressure ventilations in non-intubated patients―likely further hinder survival rather than contribute meaningfully to outcome. For example, medical students performing traditional CPR took an average of 14 seconds to administer two mouth-to-mouth ventilations after each group of 15 compressions. This effectively reduced the number of compressions to a mere 43 per minute, or less than half the guideline-mandated 100 per minute, thus theoretically reducing circulation to the heart and brain by a similar percentage (29). Yu et al demonstrated that swine receiving more than 80 compressions per minute during CPR had a 100% survival at 24 hours compared with a dismal 10% survival in animals that had less than 80 compressions per minute (30). Kern et al found statistically significantly higher coronary artery perfusion pressures and markedly higher neurological normal 24 hour survival in swine receiving continuous chest compressions compared with controls receiving traditional CPR (31).
Increasingly data such as these do raise the question of what―if any―benefit rescue breathing has in adult resuscitation. Human data show that a strategy of continuous chest compressions―without rescue breathing―is equally efficacious to traditional CPR in terms of outcome (32). Two physiologic theories prevail: 1) the mechanics of chest wall compression may be sufficient to provide a limited minute ventilation independent of supplemental ventilation (author’s speculation); and 2) the improved oxygenation that occurs in those receiving artificial ventilation is offset by the deleterious impact on hemodynamics that occur when chest compressions are interrupted for ventilation (34).
Second Effective Stratagem: Until an adult inpatient can be defibrillated, the focus of resuscitation should be on proper continuous chest compression depth and rate, not on ventilation.
Ventilations are Harmful
Cardiopulmonary collapse has immediate consequences on cellular physiology. Both due to a lack of expiration of carbon dioxide and the development of lactatemia due to the shift to anaerobic metabolism, blood pH drops precipitously. As the pH shifts out of the physiologic range, drugs begin to perform in unexpected ways or fail altogether; ion trapping occurs; and many electrolytes begin to shift in or out of cells affecting their serum concentration. Diminished or absent cardiac output accelerates these derangements in a relentless positive feedback loop. Logic, therefore, dictates that anything that can improve oxygenation and ventilation would be helpful at slowing or reversing this pathophysiology. Surprisingly, however, clinicians’ lack of knowledge of both equipment and technique often promotes, rather than mitigates, the physiologic derangements.
The bag-valve-mask (BVM) is one of the least understood resuscitation devices. For example, clinicians occasionally place a BVM over spontaneously breathing patients who are exceptionally ill with the goal of augmenting patients’ meager ventilations. However, the BVM is constructed only for positive pressure ventilation and thus, unless the clinician squeezes the bag in perfect coordination with the patient’s ventilatory effort, the BVM will paradoxically smother the patient.
The bag portion of the BVM is designed for a one-handed squeeze to deliver a tidal volume that is roughly 750cc; this volume is in keeping with guideline recommendations of positive pressure ventilation volumes of roughly 10cc/kg/ventilation (29). Yet, clinicians commonly use two hands to compress the BVM maximally during resuscitation (theoretically delivering upwards of double the recommended volume).
In addition to excessive volume, clinicians also deliver ventilations too rapidly. Abella et al. showed that during human resuscitations, ventilation rate exceeded the recommended goal of 20 ventilations/minute 60.9% of the time (27). Theoretically, high minute ventilations lead to an increased incidence of gastric insufflation, regurgitation, and post-resuscitation aspiration.
Though no studies have ever been performed to understand why clinicians hyperventilate patients during resuscitation, it is interesting to speculate that clinicians are not only trying to raise blood oxygen levels rapidly, but also to reverse the profound metabolic and respiratory acidosis that occur during CA. While seemingly mechanistically sound, the logic that supraphysiologic minute ventilations will profoundly change blood pH without other physiologic costs is specious at best. Aufderheide et al. Demonstrated that hyperventilation during resuscitation in swine resulted in increased intrathoracic pressures, markedly reduced coronary artery perfusion pressures, and resultant proportional reductions in survival rates as hyperventilation increased (35). Clinically these findings are known as auto-PEEP, a known complication of artificial ventilation that results in systemic arterial hypotension. Thus the paradox: aggressive attempts to overcorrect systemic acidoses via higher minute ventilations leads to worsening systemic blood pressures and thus worsening lactic acidosis. I believe that iatrogenic hypotension is one of the most common problems to complicate an otherwise successful resuscitation and that more research is urgently needed on this issue.
Third Effective Stratagem: Bag-valve-mask ventilations should be performed with precision; when used, the bag should be compressed with only one hand and delivered no faster than one breath every 3–5 seconds.
Fourth Effective Stratagem: In cases of pulseless electrical activity (PEA) or post-resuscitation hypotension, auto-PEEP should be considered foremost as a proximal cause.
Fifth Effective Stratagem: Ventilations in resuscitation should be viewed primarily as a means to oxygenate the patient rather than as a means to compensate from a systemic acidemia.
Shocking Revelations
Even though patients in ventricular fibrillation (VF) and pulseless ventricular tachycardia (PVT) together only comprise about one–third of all inpatient cardiac arrests, they account for almost 70% of survivors of CA. Survival in these arrhythmias is predicated solely on rapid defibrillation: the simultaneous depolarization of a critical mass of myocytes by an electrical current with resultant resumption of normal cardiac conduction (36). Perhaps the best attestation to the importance of rapid defibrillation actually comes from the survival of patients with cardiac arrest at casinos: security cameras provided exact documentation of time of collapse and defibrillation. Survival to hospital discharge was cut in half when the time-from-collapse-to-defibrillation was greater than 3 minutes (74% vs. 49%)(37).
Sixth Effective Stratagem: Since patients in VF/PVT are the most likely to survive CA―and that rate is directly related to immediacy of defibrillation―all patients should be presumptively treated as if they are in VF/PVT and should be defibrillated within 3 minutes of collapse unless there are data to support another arrhythmia or cause of collapse.
Much of the electrical current delivered during a defibrillation attempt is either dissipated as heat or is conducted around the thorax without penetrating the myocardium; Lerman and Deale have shown that the amount of current reaching the heart may be as little as 4% during a defibrillation attempt (38). From Ohm’s Law, the current that reaches the heart is directly proportional to the voltage across the chest and inversely related to the resistance of the supervening tissues. Interestingly, increasing the voltage of a shock only increases the amount of heat produced without a commensurate increase in current delivered. Therefore, techniques that reduce thoracic resistance yield the highest current delivery: the use of manual defibrillator paddles (as opposed to self adhesive defibrillation pads), the amount of pressure applied to the paddles (>25 pounds), the correct placement (underneath, rather than on top of breast tissue), the use of a conduction material (gel or pads), and the rapid delivery of a stacked shock (resistance transiently decreases after a counter-shock) all improve current delivery and thus may improve defibrillation outcome (36).
Physician leadership during resuscitations is critical for maximizing likelihood of patient survival. Perhaps due to physicians’ lack of familiarity with defibrillators, the mechanics of defibrillation are often deferred unnecessarily to nursing staff. Unfortunately, this may have profoundly negative effects on the resuscitation. Since many hospitals reduce educational expenses by foregoing training nurses in ACLS unless they work in intensive care units, nurses on a code may have even less comfort in using defibrillators than physicians. One study showed nearly a 72.5% decrease in patient survival when the nurse who arrived first at the resuscitation was untrained in ACLS (37.5% vs. 10.3%); a difference that probably is related to delayed defibrillation though the study could not establish direct causality (39).
A separate study showed that 85% of patients on cardiac monitored wards were defibrillated in the target time of 3 minutes from collapse, while only 28% of patients on unmonitored wards were defibrillated within the target time; this led to an adjusted odds ratio for survival-to-discharge in patients on monitored versus non-monitored wards of 1.45 (95% CI, 0.95–2.20)(40). While at first these data seem to indicate faster responses on monitored wards, these data were specific to time-from-collapse-to-defibrillation and therefore should be location-independent once the need for resuscitation was identified. Instead, I interpret these data as showing the lower comfort nurses on non-monitored wards have with rapid defibrillation. Rather than serving as an indictment against nurses, these data in aggregate underscore the vital role physician responders have to ensure rapid defibrillation of patients in cardiac arrest.
Seventh Effective Stratagem: Physicians should aggressively pursue defibrillation as early as possible during resuscitation especially on non-monitored wards where nurses are less likely to be ACLS trained and less likely to be familiar with defibrillator operation.
Conclusions
Resuscitation, the act of bringing back life from imminent death, is one of the most sensationalized practices in medicine and dates almost to the beginning of recorded history. In the past two decades, increasingly evidence-based guidelines have tried to provide a guide to help practitioners treat patients effectively in the minutes after cardiopulmonary collapse. Unfortunately, with each new iteration of the guidelines, it has become increasingly difficult to determine which strategies offer the highest yield or the most import. As ILCOR meets in 2005 to revise the guidelines once again, hopefully the most important strategies will be given their proper highlight, providing clinicians with increased comfort and confidence with cardiopulmonary resuscitation.
Bibliography
- Brindley PG, Markland DM, Mayers I, Kutsogiannis DJ. Predictors of survival following inhospital adult cardiopulmonary resuscitation. CMAJ. 2002;167:343-8.
- Kouwenhoven WB, Jude JR, Knickerbocker GG. Closed chest cardiac massage. JAMA. 1960;173:1064-7.
- Jude JR, Kouwenhoven WB, Knickerbocker GG. Cardiac arrest; report of application of external cardiac massage on 118 patients. JAMA. 1961;178:1063-71.
- Varon J, Sternback GL. Cardiopulmonary resuscitation: lessons from the past. J Emerg Med. 1991;9:5037.
- Liss HP. A history of resuscitation. Ann Emerg Med.1986;15: 65-72.
- Thangam S, Weil MH, Rackow EC. Cardiopulmonary resuscitation: a historical review. Acute Care. 1986;12:63-94.
- Kings II, 4:34-35 (KJV).
- Baker AB. Artificial respiration: the history of an idea. Med Hist. 1971;15:336-46.
- Stephenson HE. Cardiac Arrest and Resuscitation. St Louis: CV Mosby; 1969.
- Lee RV. Cardiopulmonary resuscitation in the eighteenth century: a historical perspective on present practice. J Hist Med. 1972;27:418-33.
- Varon J, Marik PE, Fromm RE. Cardiopulmonary resuscitation: a review for clinicians. Resuscitation. 1998;36: 133-45.
- Keith A. Three Hunterian lectures on the various mechanisms underlying the various methods of artificial respiration. Lancet. 1909;1:895-9.
- DeBard ML. The history of cardiopulmonary resuscitation. Ann Emerg Med. 1980;9:273-5.
- Acosta P, Varion J, Sternbach GL, BaskeQ P. Resuscitation great. Kouwenhoven, Jude and Knickerbocker: the introduction of defibrillation and external chest compressions into modern resuscitation. Resuscitation. 2005;64:139-43.
- US Congress, Office of Technology Assessment. Life-sustaining technologies and the elderly. Washington, DC: US Government Printing Office, 1987; 11, Publication OTA-BA-306.
- DeBard ML. Cardiopulmonary resuscitation: analysis of six years’ experience and review of the literature. Ann Emerg Med. 1981;10:408-11.
- Cassel CK, et al. Cardiopulmonary resuscitation in the elderly. Office of Technology Assessment, U.S. Congress, Washington, DC, November, 1985.
- Peberdy MA, Kaye W, Ornato JP, et al. Cardiopulmonary resuscitation of adults in the hospital: a report of 14 720 cardiac arrests from the National Registry of Cardiopulmonary Resuscitation. Resuscitation. 2003;58297-308.
- Valentin A, Karnik R, Donath P, Winkler WB, Slany J. Outcome of cardiopulmonary resuscitation in hospitalized patients. Resuscitation. 1995;30:217-21.
- Robinson GR, Hess D. Postdischarge survival and functional status following in-hospital cardiopulmonary resuscitation. Chest. 1994;105:991-6.
- Murphy DI, Murray AM, Robinson BE. Outcomes of cardiopulmonary resuscitation in the elderly. Ann Int Med. 1989;111:199-205.
- Eberle B, Dick WF, Schneider T, Wisser G, Doetsch S, Tzanova I. Checking the carotid pulse check: diagnostic accuracy of first responders in patients with and without a pulse. Resuscitation. 1996;33:107-16.
- Rudikoff MT, Maughan WL, Effron M, Freund P, Weisfeldt ML. Mechanisms of blood flow during cardiopulmonary resuscitation. Circulation. 1980;61:345-52.
- Paradis NA, Martin GB, Goetting MG, et al. Simultaneous aortic, jugular bulb, and right atrial pressures during cardiopulmonary resuscitation in humans: insight into mechanisms. Circulation. 1989;80:361-8.
- Sanders AB, Ogle M, Ewy GA. Coronary perfusion pressure during cardiopulmonary resuscitation. Am J Emerg Med. 1985;3:11-4.
- Heidenreich JW, Higdon TA, Kern KB, et al. Single-rescuer cardiopulmonary resuscitation: “two quick breaths”―an oxymoron. Resuscitation. 2004;62:283-89.
- Abella BS, Alvarado JP, Myklebust H, et al. Quality of cardiopulmonary resuscitation during in-hospital cardiac arrest. JAMA. 2005;293:305-10.
- Van Hoeyweghen RJ, Bossaert LL, Mullie A, et al. (Belgian Cerebral Resuscitation Study Group.) Quality and efficiency of bystander CPR. Resuscitation. 1993;26:47-52.
- American Heart Association in collaboration with the International Liaison Committee on Resuscitation (ILCOR). International Guidelines 2000 for Cardiopulmonary Resuscitation and Emergency Cardiovascular Care―a consensus on science. Resuscitation. 2000.46:1-448.
- Yu T, Weil MH, Tang W, et al. Adverse outcomes of interrupted precordial compression during automated defibrillation. Circulation. 2002;106:368-72.
- Kern KB, Hilwig RW, Berg RA, Sanders AB, Ewy GA. Importance of continuous chest compressions during cardiopulmonary resuscitation: improved outcome during a simulated single lay-rescuer scenario. Circulation. 2002;105:645-649.
- Hallstrom A, Cobb L, Johnson E, Copass M. Cardiopulmonary resuscitation by chest compression alone or with to-mouth ventilation. N Engl J Med. ;342: 154-653.
- Berg RA, Sanders AB, Kern KB, et al. Adverse hemodynamic effects of interrupting chest compressions for rescue breathing during cardiopulmonary resuscitation for ventricular fibrillation cardiac arrest. Circulation. 2001;104:2465-70.
- Aufderheide TP, Sigurdsson G, Pirallo RG, et al. Hyperventilation-induced hypotension during cardiopulmonary resuscitation. Circulation. 2004;109:1960-5.
- Dalzell GWN. Determinants of successful defibrillation. Heart. ;80:405-7.
- Valenzuela TD, Roe DJ, Nichol G, Clark LL, Spaite DW,Hardman RG. Outcomes of rapid defibrillation by security ad er cardiac arrest in casinos. N Engl J Med. ;343:120-69.
- Lerman BB, Deale C. Relation between transcardiac and thransthoracic current during defibrillation in humans. Circ Res. 1990;67:1420-6.
- Dane FC, RussellLindgren KS, Parish DC, Durham MD, Brown TD. Inhospital resuscitation: association between ACLS training and survival to discharge. Resuscitation. 2000;47:83-7.
- Herlitz J, Bang A, Aune S, Ekstrom L, Lundstrom G, Holmberg S. Characteristics and outcome among patients suffering inhospital cardiac arrest in monitored and nonmonitored areas. Resuscitation. ;48:125-35.