User login
Oral cancer survival lower with positive margins, public insurance
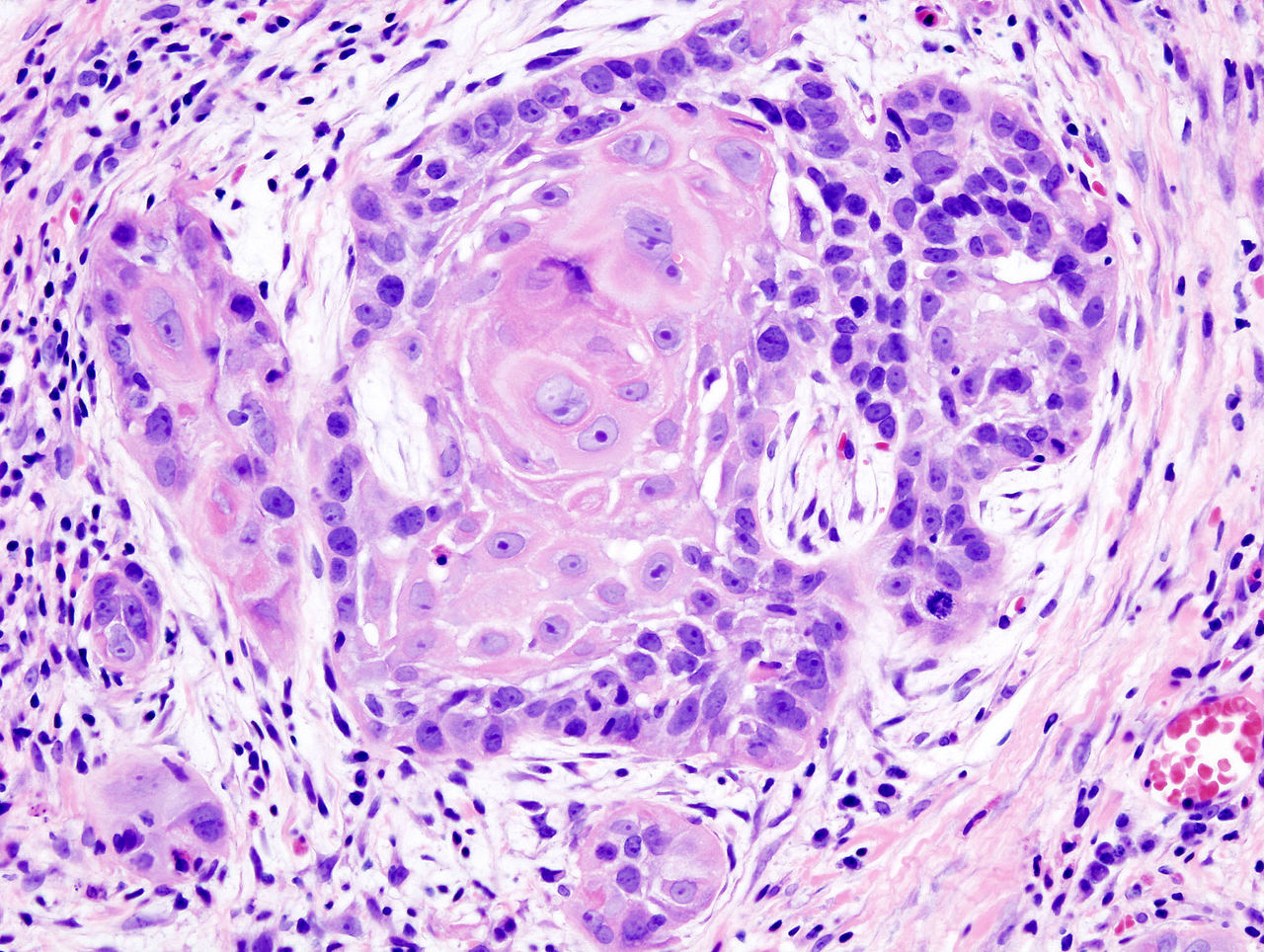
In patients who underwent surgical treatment for stage I or II oral cavity squamous cell cancer, positive tumor margin, the use of radiation or chemotherapy, treatment in a nonacademic facility, and having public health insurance were significantly associated with lower 5-year survival rates, according to a retrospective analysis published online in the JAMA Otolaryngology–Head & Neck Surgery.
The findings suggest that some factors associated with lower 5-year survival rates “may be targets for quality improvement efforts,” wrote Alexander L. Luryi of Yale University, New Haven, Conn., and colleagues.
Seventy percent of 6,830 patients who underwent surgery for stage I or II oral cavity squamous cell cancer (OCSCC) from 2003 to 2006 survived 5 years, according to information from the National Cancer Data Base.
Multivariate analysis showed higher survival rates were significantly associated with neck dissection (hazard ratio, 0.85; P = .003). Lower survival rates were significantly associated with radiation therapy (HR, 1.31; P < .001), chemotherapy (HR, 1.34; P = .03), nonprivate insurance (HR Medicaid, 1.96; HR Medicare, 1.45; P < .001), and nonacademic treatment facility (HR, 1.13; P = .03).
Care at academic centers compared with nonacademic centers was associated with improved survival, possibly due to health care provider expertise, the study authors noted (JAMA Otolaryngol. Head Neck Surg. 2015 May 14 [doi:10.1001/jamaoto.2015.0719]).
Survival rates were lower in patients treated at nonacademic cancer centers, but multivariate analysis showed no association between facility-based case volume and survival. Patients insured through Medicaid and Medicare had significantly lower 5-year survival rates (P < .001 for both). That finding may be the result of inconsistent treatment and follow-up, the investigators said, or worse baseline health among that patient population.
Controversy exists over the relationship between positive margins and outcomes, and the implications for aggressiveness of surgery. The study found positive margins were significantly associated with poorer outcomes, the researchers noted, which supports the use of aggressive surgery in early OCSCC to achieve negative margins.
Radiation and chemotherapy were linked to worse outcomes, and those therapies were possibly indicators of less aggressive resection in localized disease. The analysis could not adjust for potential confounding effects of perineural and lymphovascular invasion, because the information was not recorded in the National Cancer Data Base.
The study indicated a positive impact by neck dissection on survival. Patients with occult neck disease who underwent neck dissection likely would have been restaged to stage III or higher and removed from the early stage sample, the authors explained, which would account for higher survival rates for those remaining. Prospective trials are needed to determine the role of elective neck dissection in early OCSCC, the researchers added.
The William U. Gardner Memorial Research Fund at Yale University supported the study. Dr. Luryi and coauthors reported having no disclosures.
In patients who underwent surgical treatment for stage I or II oral cavity squamous cell cancer, positive tumor margin, the use of radiation or chemotherapy, treatment in a nonacademic facility, and having public health insurance were significantly associated with lower 5-year survival rates, according to a retrospective analysis published online in the JAMA Otolaryngology–Head & Neck Surgery.
The findings suggest that some factors associated with lower 5-year survival rates “may be targets for quality improvement efforts,” wrote Alexander L. Luryi of Yale University, New Haven, Conn., and colleagues.
Seventy percent of 6,830 patients who underwent surgery for stage I or II oral cavity squamous cell cancer (OCSCC) from 2003 to 2006 survived 5 years, according to information from the National Cancer Data Base.
Multivariate analysis showed higher survival rates were significantly associated with neck dissection (hazard ratio, 0.85; P = .003). Lower survival rates were significantly associated with radiation therapy (HR, 1.31; P < .001), chemotherapy (HR, 1.34; P = .03), nonprivate insurance (HR Medicaid, 1.96; HR Medicare, 1.45; P < .001), and nonacademic treatment facility (HR, 1.13; P = .03).
Care at academic centers compared with nonacademic centers was associated with improved survival, possibly due to health care provider expertise, the study authors noted (JAMA Otolaryngol. Head Neck Surg. 2015 May 14 [doi:10.1001/jamaoto.2015.0719]).
Survival rates were lower in patients treated at nonacademic cancer centers, but multivariate analysis showed no association between facility-based case volume and survival. Patients insured through Medicaid and Medicare had significantly lower 5-year survival rates (P < .001 for both). That finding may be the result of inconsistent treatment and follow-up, the investigators said, or worse baseline health among that patient population.
Controversy exists over the relationship between positive margins and outcomes, and the implications for aggressiveness of surgery. The study found positive margins were significantly associated with poorer outcomes, the researchers noted, which supports the use of aggressive surgery in early OCSCC to achieve negative margins.
Radiation and chemotherapy were linked to worse outcomes, and those therapies were possibly indicators of less aggressive resection in localized disease. The analysis could not adjust for potential confounding effects of perineural and lymphovascular invasion, because the information was not recorded in the National Cancer Data Base.
The study indicated a positive impact by neck dissection on survival. Patients with occult neck disease who underwent neck dissection likely would have been restaged to stage III or higher and removed from the early stage sample, the authors explained, which would account for higher survival rates for those remaining. Prospective trials are needed to determine the role of elective neck dissection in early OCSCC, the researchers added.
The William U. Gardner Memorial Research Fund at Yale University supported the study. Dr. Luryi and coauthors reported having no disclosures.
In patients who underwent surgical treatment for stage I or II oral cavity squamous cell cancer, positive tumor margin, the use of radiation or chemotherapy, treatment in a nonacademic facility, and having public health insurance were significantly associated with lower 5-year survival rates, according to a retrospective analysis published online in the JAMA Otolaryngology–Head & Neck Surgery.
The findings suggest that some factors associated with lower 5-year survival rates “may be targets for quality improvement efforts,” wrote Alexander L. Luryi of Yale University, New Haven, Conn., and colleagues.
Seventy percent of 6,830 patients who underwent surgery for stage I or II oral cavity squamous cell cancer (OCSCC) from 2003 to 2006 survived 5 years, according to information from the National Cancer Data Base.
Multivariate analysis showed higher survival rates were significantly associated with neck dissection (hazard ratio, 0.85; P = .003). Lower survival rates were significantly associated with radiation therapy (HR, 1.31; P < .001), chemotherapy (HR, 1.34; P = .03), nonprivate insurance (HR Medicaid, 1.96; HR Medicare, 1.45; P < .001), and nonacademic treatment facility (HR, 1.13; P = .03).
Care at academic centers compared with nonacademic centers was associated with improved survival, possibly due to health care provider expertise, the study authors noted (JAMA Otolaryngol. Head Neck Surg. 2015 May 14 [doi:10.1001/jamaoto.2015.0719]).
Survival rates were lower in patients treated at nonacademic cancer centers, but multivariate analysis showed no association between facility-based case volume and survival. Patients insured through Medicaid and Medicare had significantly lower 5-year survival rates (P < .001 for both). That finding may be the result of inconsistent treatment and follow-up, the investigators said, or worse baseline health among that patient population.
Controversy exists over the relationship between positive margins and outcomes, and the implications for aggressiveness of surgery. The study found positive margins were significantly associated with poorer outcomes, the researchers noted, which supports the use of aggressive surgery in early OCSCC to achieve negative margins.
Radiation and chemotherapy were linked to worse outcomes, and those therapies were possibly indicators of less aggressive resection in localized disease. The analysis could not adjust for potential confounding effects of perineural and lymphovascular invasion, because the information was not recorded in the National Cancer Data Base.
The study indicated a positive impact by neck dissection on survival. Patients with occult neck disease who underwent neck dissection likely would have been restaged to stage III or higher and removed from the early stage sample, the authors explained, which would account for higher survival rates for those remaining. Prospective trials are needed to determine the role of elective neck dissection in early OCSCC, the researchers added.
The William U. Gardner Memorial Research Fund at Yale University supported the study. Dr. Luryi and coauthors reported having no disclosures.
FROM JAMA OTOLARYNGOLOGY–HEAD & NECK SURGERY
Key clinical point: Treatment factors such as neck dissection, tumor margins, type of insurance, and health care facility impact 5-year survival after surgery for early stage oral cavity cancer.
Major finding: Radiation, chemotherapy, positive tumor margin, nonacademic facility, and nonprivate insurance were significantly associated with lower 5-year survival.
Data source: Retrospective study of 6,830 patients in National Cancer Data Base who underwent surgery to treat stage I or II oral cavity squamous cell cancer.
Disclosures: The William U. Gardner Memorial Research Fund at Yale University supported the study. Dr. Luryi and coauthors reported having no disclosures.
Similar 5-year outcomes from accelerated partial-breast irradiation, whole-breast irradiation
Treatment with accelerated partial-breast irradiation (APBI) versus conventional whole-breast irradiation for women with early-stage breast cancer resulted in similar tumor recurrence rates and overall survival, but APBI was associated with less toxicity, according to a report published in the European Journal of Cancer.
“There was no evidence of significant differences regarding the true incidence of recurrence nor new-onset ipsilateral tumors. [Overall survival] did not differ between the two treatment groups, with the same number of deaths related to” breast cancer, wrote Dr. Lorenzo Livi, a radiation oncologist at the University of Florence, Italy, and his colleagues (Eur. J. Cancer 2015;51:451-63).
Six of the 520 patients, three in each study arm, had ipsilateral breast tumor recurrence. No significant differences were observed in contralateral breast cancer occurrence, distant metastases, or overall survival. The low rate of events at the median 5-year follow up “underlines the importance of longer follow up in addition to an appropriate selection of patient candidates for APBI,” they wrote, urging caution in interpreting the results because longer follow-up is required.
Most of the patient cohort had tumor grade G1-2 (89%), positive estrogen-receptor status (95%), negative nodal status (86%), and were human epidermal growth factor receptor 2 negative (96%).
The APBI group had significantly fewer adverse events than the whole-breast irradiation (WBI) group (P < .0001). Erythema was the most frequently observed event in both arms of the study, at 20% for APBI and 66.5% for WBI. The most represented late skin adverse event was grade 1-2 fibrosis (11% in WBI, 4.5% in APBI). No grade-3 toxicity was recorded in either study arm.
Potential advantages of APBI include shorter treatment time, lower costs, and improved cosmesis, compared with convention treatment. Cosmetic results for both groups in the study were rated excellent/good in greater than 90% of patients, with APBI having slightly better outcomes (P = .045).
The randomized, phase III trial was conducted at the radiation-oncology department of the University of Florence (Italy) between 2005 and 2013 and compared APBI using intensity-modulated radiotherapy (IMRT) with conventional, tangential-field WBI. For the WBI arm, a total dose of 50 Gy was given in 25 fractions, followed by a radiation boost of 10 Gy in five fractions. For the APBI group, a dose of 30 Gy in five fractions at 6 Gy/fraction was given (treatment time, 2 weeks), which is equivalent to 54 Gy in a standard 2-Gy fractionation.
Compared with conformal techniques, IMRT produces optimal dosimeter results while allowing easier, less time-consuming treatment delivery. Additionally, APBI appears to be more cost effective than conventional WBI radiation therapy. The trial “demonstrated excellent results in terms of safety, with a very low rate of local recurrences. APBI using the IMRT technique with the administration of 30 Gy in five non-consecutive fractions should be part of the multidisciplinary discussion to offer a tailored treatment for the patient,” Dr. Livi and his colleagues wrote.
The investigators did not declare any outside funding or conflicts of interest.
Treatment with accelerated partial-breast irradiation (APBI) versus conventional whole-breast irradiation for women with early-stage breast cancer resulted in similar tumor recurrence rates and overall survival, but APBI was associated with less toxicity, according to a report published in the European Journal of Cancer.
“There was no evidence of significant differences regarding the true incidence of recurrence nor new-onset ipsilateral tumors. [Overall survival] did not differ between the two treatment groups, with the same number of deaths related to” breast cancer, wrote Dr. Lorenzo Livi, a radiation oncologist at the University of Florence, Italy, and his colleagues (Eur. J. Cancer 2015;51:451-63).
Six of the 520 patients, three in each study arm, had ipsilateral breast tumor recurrence. No significant differences were observed in contralateral breast cancer occurrence, distant metastases, or overall survival. The low rate of events at the median 5-year follow up “underlines the importance of longer follow up in addition to an appropriate selection of patient candidates for APBI,” they wrote, urging caution in interpreting the results because longer follow-up is required.
Most of the patient cohort had tumor grade G1-2 (89%), positive estrogen-receptor status (95%), negative nodal status (86%), and were human epidermal growth factor receptor 2 negative (96%).
The APBI group had significantly fewer adverse events than the whole-breast irradiation (WBI) group (P < .0001). Erythema was the most frequently observed event in both arms of the study, at 20% for APBI and 66.5% for WBI. The most represented late skin adverse event was grade 1-2 fibrosis (11% in WBI, 4.5% in APBI). No grade-3 toxicity was recorded in either study arm.
Potential advantages of APBI include shorter treatment time, lower costs, and improved cosmesis, compared with convention treatment. Cosmetic results for both groups in the study were rated excellent/good in greater than 90% of patients, with APBI having slightly better outcomes (P = .045).
The randomized, phase III trial was conducted at the radiation-oncology department of the University of Florence (Italy) between 2005 and 2013 and compared APBI using intensity-modulated radiotherapy (IMRT) with conventional, tangential-field WBI. For the WBI arm, a total dose of 50 Gy was given in 25 fractions, followed by a radiation boost of 10 Gy in five fractions. For the APBI group, a dose of 30 Gy in five fractions at 6 Gy/fraction was given (treatment time, 2 weeks), which is equivalent to 54 Gy in a standard 2-Gy fractionation.
Compared with conformal techniques, IMRT produces optimal dosimeter results while allowing easier, less time-consuming treatment delivery. Additionally, APBI appears to be more cost effective than conventional WBI radiation therapy. The trial “demonstrated excellent results in terms of safety, with a very low rate of local recurrences. APBI using the IMRT technique with the administration of 30 Gy in five non-consecutive fractions should be part of the multidisciplinary discussion to offer a tailored treatment for the patient,” Dr. Livi and his colleagues wrote.
The investigators did not declare any outside funding or conflicts of interest.
Treatment with accelerated partial-breast irradiation (APBI) versus conventional whole-breast irradiation for women with early-stage breast cancer resulted in similar tumor recurrence rates and overall survival, but APBI was associated with less toxicity, according to a report published in the European Journal of Cancer.
“There was no evidence of significant differences regarding the true incidence of recurrence nor new-onset ipsilateral tumors. [Overall survival] did not differ between the two treatment groups, with the same number of deaths related to” breast cancer, wrote Dr. Lorenzo Livi, a radiation oncologist at the University of Florence, Italy, and his colleagues (Eur. J. Cancer 2015;51:451-63).
Six of the 520 patients, three in each study arm, had ipsilateral breast tumor recurrence. No significant differences were observed in contralateral breast cancer occurrence, distant metastases, or overall survival. The low rate of events at the median 5-year follow up “underlines the importance of longer follow up in addition to an appropriate selection of patient candidates for APBI,” they wrote, urging caution in interpreting the results because longer follow-up is required.
Most of the patient cohort had tumor grade G1-2 (89%), positive estrogen-receptor status (95%), negative nodal status (86%), and were human epidermal growth factor receptor 2 negative (96%).
The APBI group had significantly fewer adverse events than the whole-breast irradiation (WBI) group (P < .0001). Erythema was the most frequently observed event in both arms of the study, at 20% for APBI and 66.5% for WBI. The most represented late skin adverse event was grade 1-2 fibrosis (11% in WBI, 4.5% in APBI). No grade-3 toxicity was recorded in either study arm.
Potential advantages of APBI include shorter treatment time, lower costs, and improved cosmesis, compared with convention treatment. Cosmetic results for both groups in the study were rated excellent/good in greater than 90% of patients, with APBI having slightly better outcomes (P = .045).
The randomized, phase III trial was conducted at the radiation-oncology department of the University of Florence (Italy) between 2005 and 2013 and compared APBI using intensity-modulated radiotherapy (IMRT) with conventional, tangential-field WBI. For the WBI arm, a total dose of 50 Gy was given in 25 fractions, followed by a radiation boost of 10 Gy in five fractions. For the APBI group, a dose of 30 Gy in five fractions at 6 Gy/fraction was given (treatment time, 2 weeks), which is equivalent to 54 Gy in a standard 2-Gy fractionation.
Compared with conformal techniques, IMRT produces optimal dosimeter results while allowing easier, less time-consuming treatment delivery. Additionally, APBI appears to be more cost effective than conventional WBI radiation therapy. The trial “demonstrated excellent results in terms of safety, with a very low rate of local recurrences. APBI using the IMRT technique with the administration of 30 Gy in five non-consecutive fractions should be part of the multidisciplinary discussion to offer a tailored treatment for the patient,” Dr. Livi and his colleagues wrote.
The investigators did not declare any outside funding or conflicts of interest.
FROM THE EUROPEAN JOURNAL OF CANCER
Key clinical point: Patients with early-stage breast cancer had similar outcomes after 5 years whether they received APBI or WBI .
Major finding: 6 of 520 patients, three in each study arm, had ipsilateral breast tumor recurrence.
Data source: From 2005 to 2013, the single-center phase III study randomized 520 patients to receive APBI or WBI; clinicians, investigators, and patients were aware of arm assignments.
Disclosures: The investigators did not declare any outside funding or conflicts of interest.
Omega-3 fatty acids similar to placebo for aromatase inhibitor–induced musculoskeletal pain
Aromatase inhibitor-induced arthralgia substantially improved in women who received omega-3 fatty acid capsules, as well as in those who took placebo, according to a study published online May 4 in the Journal of Clinical Oncology.
Brief Pain Inventory (BPI) worst pain scores were significantly lower than were baseline scores after treatment with O3-FAs and placebo. On the 10-point scale, the median scores reported by women taking O3-FAs were lower by 1.69, 1.74, and 2.23 at 6, 12, and 24 weeks, respectively (P < .001). For those taking placebo, the scores also were significantly lower: 1.36, 1.50, and 1.81 points at 6, 12, and 24 weeks respectively (P < .001). Differences between the groups were not significant, reported Dr. Dawn Hershman of the Herbert Irving Comprehensive Cancer Center, Columbia University, New York, and associates (J. Clin. Onc. 2015 May 4 [doi: 10.1200/JCO.2014.59.5595]).
“The improvement in symptoms in both the treatment and placebo groups was unexpected. The magnitude of the expected placebo effect reported in the literature can vary from 6% to 59% and can be higher in symptom-management studies. We found an effect >50%,” Dr. Hershman and associates wrote.
The large placebo effect may have resulted from a number of factors, including the natural history of arthralgia (which can improve over time), the soy/corn oil ingredients in the placebo capsule, or O3-FA contamination in the placebo arm due to supplementation by patients.
There are no proven therapies for AI-associated arthralgia, and its mechanism is unclear. Evidence suggests that inflammation may play a role. Given that studies have shown O3-FAs may benefit symptoms of rheumatoid arthritis, this multicenter, placebo controlled trial evaluated whether O3-FAs reduce pain and stiffness in 249 women undergoing adjuvant AI therapy for early-stage breast cancer.
At week 12, patients who received O3-FAs had significantly decreased serum triglyceride levels (–22.1 mg/dL, P < .001) and increased HDL (2.9 mg/dL, P < .007). Triglyceride and HDL levels for the placebo arm did not significantly change, which suggests that O3-FA contamination in the placebo arm was not a factor in the high placebo effect. Other serum measures (cholesterol, CRP, and LDL) did not significantly change for either group, Dr. Hershman and associates said.
The placebo effect is related to patient expectations and perceptions of treatment. It is heightened with a supportive patient-provider relationship and typically diminished in blinded clinical trials where patients do not know if they are receiving the active drug. However, in clinical trials that have evaluated symptom management, substantial placebo effects were more common. This observation points to the importance of placebo-controlled, randomized trials vs. uncontrolled trials for symptom management therapies.
The larger-than-expected placebo response in the Hershman et. al. study may have resulted from several factors, including O3-FA supplementation causing placebo contamination, ingredients within the placebo capsule, the natural history of AIMSS, and patient selection.
Because O3-FAs are readily available as supplements, placebo contamination should be considered. The authors examined a surrogate marker for exposure, longitudinal change in triglycerides, and found only the active arm had decreased triglyceride levels. This observation suggests that participants in the placebo arm were not significantly exposed to O3-FAs.
The ingredients of the placebo capsule, soybean oil or other components, may have contributed to symptom improvement. If AIMSS is related to estrogen deprivation, estrogenic compounds in the placebo may have affected symptoms.
Previous studies indicate that many patients do not spontaneously improve over time, as would be the case if the natural history of AIMSS accounted for the high placebo effect.
Regarding the patients enrolled in the study, there is no way to definitively determine if their pain and stiffness was caused by AIs; distinguishing between AIMSS and worsening of osteoarthritis in this patient population with high baseline joint pain is difficult. It is possible that patients with preexisting musculoskeletal pain not related to AIs participated in this study, particularly since there is a low perceived toxicity of the drug.
Patients enrolled in this trial may have experienced symptom improvement solely due to the expectation of benefit by participation in the trial. A similar placebo response was reported in a controlled trial of modafinil for fatigue.
The results of this well-conducted SWOG trial clearly demonstrate that acknowledging and controlling for placebo effects is crucial to high-quality research in the field of symptom management.
Dr. N. Lynn Henry is assistant professor in the division of hematology/oncology, department of internal medicine, at the University of Michigan, Ann Arbor. Dr. Jennifer Griggs is professor of medicine in the division of hematology/oncology and director of the breast cancer survivorship program at the University of Michigan Comprehensive Cancer Center, Ann Arbor. Dr. Henry reported receiving research funding from Sanofi-Aventis, BioMarin, and Celldex. These comments were taken from an editorial accompanying the report by Hershman et al. (J. Clin. Onc. 2015 May 4 [doi:10.1200/JCO.2015.61.1004]).
The placebo effect is related to patient expectations and perceptions of treatment. It is heightened with a supportive patient-provider relationship and typically diminished in blinded clinical trials where patients do not know if they are receiving the active drug. However, in clinical trials that have evaluated symptom management, substantial placebo effects were more common. This observation points to the importance of placebo-controlled, randomized trials vs. uncontrolled trials for symptom management therapies.
The larger-than-expected placebo response in the Hershman et. al. study may have resulted from several factors, including O3-FA supplementation causing placebo contamination, ingredients within the placebo capsule, the natural history of AIMSS, and patient selection.
Because O3-FAs are readily available as supplements, placebo contamination should be considered. The authors examined a surrogate marker for exposure, longitudinal change in triglycerides, and found only the active arm had decreased triglyceride levels. This observation suggests that participants in the placebo arm were not significantly exposed to O3-FAs.
The ingredients of the placebo capsule, soybean oil or other components, may have contributed to symptom improvement. If AIMSS is related to estrogen deprivation, estrogenic compounds in the placebo may have affected symptoms.
Previous studies indicate that many patients do not spontaneously improve over time, as would be the case if the natural history of AIMSS accounted for the high placebo effect.
Regarding the patients enrolled in the study, there is no way to definitively determine if their pain and stiffness was caused by AIs; distinguishing between AIMSS and worsening of osteoarthritis in this patient population with high baseline joint pain is difficult. It is possible that patients with preexisting musculoskeletal pain not related to AIs participated in this study, particularly since there is a low perceived toxicity of the drug.
Patients enrolled in this trial may have experienced symptom improvement solely due to the expectation of benefit by participation in the trial. A similar placebo response was reported in a controlled trial of modafinil for fatigue.
The results of this well-conducted SWOG trial clearly demonstrate that acknowledging and controlling for placebo effects is crucial to high-quality research in the field of symptom management.
Dr. N. Lynn Henry is assistant professor in the division of hematology/oncology, department of internal medicine, at the University of Michigan, Ann Arbor. Dr. Jennifer Griggs is professor of medicine in the division of hematology/oncology and director of the breast cancer survivorship program at the University of Michigan Comprehensive Cancer Center, Ann Arbor. Dr. Henry reported receiving research funding from Sanofi-Aventis, BioMarin, and Celldex. These comments were taken from an editorial accompanying the report by Hershman et al. (J. Clin. Onc. 2015 May 4 [doi:10.1200/JCO.2015.61.1004]).
The placebo effect is related to patient expectations and perceptions of treatment. It is heightened with a supportive patient-provider relationship and typically diminished in blinded clinical trials where patients do not know if they are receiving the active drug. However, in clinical trials that have evaluated symptom management, substantial placebo effects were more common. This observation points to the importance of placebo-controlled, randomized trials vs. uncontrolled trials for symptom management therapies.
The larger-than-expected placebo response in the Hershman et. al. study may have resulted from several factors, including O3-FA supplementation causing placebo contamination, ingredients within the placebo capsule, the natural history of AIMSS, and patient selection.
Because O3-FAs are readily available as supplements, placebo contamination should be considered. The authors examined a surrogate marker for exposure, longitudinal change in triglycerides, and found only the active arm had decreased triglyceride levels. This observation suggests that participants in the placebo arm were not significantly exposed to O3-FAs.
The ingredients of the placebo capsule, soybean oil or other components, may have contributed to symptom improvement. If AIMSS is related to estrogen deprivation, estrogenic compounds in the placebo may have affected symptoms.
Previous studies indicate that many patients do not spontaneously improve over time, as would be the case if the natural history of AIMSS accounted for the high placebo effect.
Regarding the patients enrolled in the study, there is no way to definitively determine if their pain and stiffness was caused by AIs; distinguishing between AIMSS and worsening of osteoarthritis in this patient population with high baseline joint pain is difficult. It is possible that patients with preexisting musculoskeletal pain not related to AIs participated in this study, particularly since there is a low perceived toxicity of the drug.
Patients enrolled in this trial may have experienced symptom improvement solely due to the expectation of benefit by participation in the trial. A similar placebo response was reported in a controlled trial of modafinil for fatigue.
The results of this well-conducted SWOG trial clearly demonstrate that acknowledging and controlling for placebo effects is crucial to high-quality research in the field of symptom management.
Dr. N. Lynn Henry is assistant professor in the division of hematology/oncology, department of internal medicine, at the University of Michigan, Ann Arbor. Dr. Jennifer Griggs is professor of medicine in the division of hematology/oncology and director of the breast cancer survivorship program at the University of Michigan Comprehensive Cancer Center, Ann Arbor. Dr. Henry reported receiving research funding from Sanofi-Aventis, BioMarin, and Celldex. These comments were taken from an editorial accompanying the report by Hershman et al. (J. Clin. Onc. 2015 May 4 [doi:10.1200/JCO.2015.61.1004]).
Aromatase inhibitor-induced arthralgia substantially improved in women who received omega-3 fatty acid capsules, as well as in those who took placebo, according to a study published online May 4 in the Journal of Clinical Oncology.
Brief Pain Inventory (BPI) worst pain scores were significantly lower than were baseline scores after treatment with O3-FAs and placebo. On the 10-point scale, the median scores reported by women taking O3-FAs were lower by 1.69, 1.74, and 2.23 at 6, 12, and 24 weeks, respectively (P < .001). For those taking placebo, the scores also were significantly lower: 1.36, 1.50, and 1.81 points at 6, 12, and 24 weeks respectively (P < .001). Differences between the groups were not significant, reported Dr. Dawn Hershman of the Herbert Irving Comprehensive Cancer Center, Columbia University, New York, and associates (J. Clin. Onc. 2015 May 4 [doi: 10.1200/JCO.2014.59.5595]).
“The improvement in symptoms in both the treatment and placebo groups was unexpected. The magnitude of the expected placebo effect reported in the literature can vary from 6% to 59% and can be higher in symptom-management studies. We found an effect >50%,” Dr. Hershman and associates wrote.
The large placebo effect may have resulted from a number of factors, including the natural history of arthralgia (which can improve over time), the soy/corn oil ingredients in the placebo capsule, or O3-FA contamination in the placebo arm due to supplementation by patients.
There are no proven therapies for AI-associated arthralgia, and its mechanism is unclear. Evidence suggests that inflammation may play a role. Given that studies have shown O3-FAs may benefit symptoms of rheumatoid arthritis, this multicenter, placebo controlled trial evaluated whether O3-FAs reduce pain and stiffness in 249 women undergoing adjuvant AI therapy for early-stage breast cancer.
At week 12, patients who received O3-FAs had significantly decreased serum triglyceride levels (–22.1 mg/dL, P < .001) and increased HDL (2.9 mg/dL, P < .007). Triglyceride and HDL levels for the placebo arm did not significantly change, which suggests that O3-FA contamination in the placebo arm was not a factor in the high placebo effect. Other serum measures (cholesterol, CRP, and LDL) did not significantly change for either group, Dr. Hershman and associates said.
Aromatase inhibitor-induced arthralgia substantially improved in women who received omega-3 fatty acid capsules, as well as in those who took placebo, according to a study published online May 4 in the Journal of Clinical Oncology.
Brief Pain Inventory (BPI) worst pain scores were significantly lower than were baseline scores after treatment with O3-FAs and placebo. On the 10-point scale, the median scores reported by women taking O3-FAs were lower by 1.69, 1.74, and 2.23 at 6, 12, and 24 weeks, respectively (P < .001). For those taking placebo, the scores also were significantly lower: 1.36, 1.50, and 1.81 points at 6, 12, and 24 weeks respectively (P < .001). Differences between the groups were not significant, reported Dr. Dawn Hershman of the Herbert Irving Comprehensive Cancer Center, Columbia University, New York, and associates (J. Clin. Onc. 2015 May 4 [doi: 10.1200/JCO.2014.59.5595]).
“The improvement in symptoms in both the treatment and placebo groups was unexpected. The magnitude of the expected placebo effect reported in the literature can vary from 6% to 59% and can be higher in symptom-management studies. We found an effect >50%,” Dr. Hershman and associates wrote.
The large placebo effect may have resulted from a number of factors, including the natural history of arthralgia (which can improve over time), the soy/corn oil ingredients in the placebo capsule, or O3-FA contamination in the placebo arm due to supplementation by patients.
There are no proven therapies for AI-associated arthralgia, and its mechanism is unclear. Evidence suggests that inflammation may play a role. Given that studies have shown O3-FAs may benefit symptoms of rheumatoid arthritis, this multicenter, placebo controlled trial evaluated whether O3-FAs reduce pain and stiffness in 249 women undergoing adjuvant AI therapy for early-stage breast cancer.
At week 12, patients who received O3-FAs had significantly decreased serum triglyceride levels (–22.1 mg/dL, P < .001) and increased HDL (2.9 mg/dL, P < .007). Triglyceride and HDL levels for the placebo arm did not significantly change, which suggests that O3-FA contamination in the placebo arm was not a factor in the high placebo effect. Other serum measures (cholesterol, CRP, and LDL) did not significantly change for either group, Dr. Hershman and associates said.
Key clinical point: Patients who received omega-3 fatty acid (O3-FA) capsules, as well as those who received placebo, had significant, sustained improvement in aromatase inhibitor-induced musculoskeletal pain.
Major finding: Mean observed Brief Pain Inventory worst pain scores for the O3-FA arm and the placebo arm were about 50% lower (1.74 and 1.49 points lower, respectively) than baseline scores after 12 weeks.
Data source: A multicenter, placebo controlled trial evaluating the effect of O3-FAs in 249 women with a history of breast cancer who had muscle pain and stiffness subsequent to the initiation of AI treatment.
Disclosures: Dr. Hershman reported having no disclosures. His coauthors reported ties to several industry sources.
Lung adenocarcinoma subtype predicts benefit from adjuvant chemotherapy
Analysis of a large cohort of patient tumor samples from four international adjuvant chemotherapy trials confirms the prognostic value of the latest, soon-to-be-published, World Health Organization lung adenocarcinoma classifications, investigators reported online April 27 in the Journal of Clinical Oncology.
More than half of all patients with early-stage non–small cell lung cancer will have recurrence after primary surgery and “identifying prognostic factors beyond stage is crucial to select patients who need adjuvant therapies … The results of our study represent the first markers, to our knowledge, from the LACE-Bio [the Lung Adjuvant Cisplatin Evaluation Biomarker] project that suggest a significant predictive value for survival benefit from ACT [adjuvant chemotherapy] in patients with early-stage lung adenocarcinoma,” Dr. Ming-Sound Tsao with the Princess Margaret Cancer Centre, University of Toronto, and his colleagues wrote (J. Clin. Oncol. 2015 Apr. 27 [doi:10.1200/JCO.2014.58.8335]).
A new lung adenocarcinoma classification system based on the predominant histological pattern observed in resected tumors, including lepidic (LEP), papillary (PAP), acinar (ACN), micropapillary (MIP), and predominantly solid (SOL) subgroups, forms the basis for the fourth edition of the WHO classification to be published in 2015. The LACE-Bio collaborative group evaluated biomarkers using data from a large cohort of patients participating in four ACT trials: the IALT (International Adjuvant Lung Cancer Trial), ANITA (Adjuvant Navelbine International Trialist Association), JBR-10, and CALGB (Cancer and Leukemia Group B; now Alliance for Clinical Trials in Oncology).
The current study evaluated the prognostic value of the classification regarding survival from adjuvant chemotherapy using hematoxylin and eosin (HE)-stained slides from a subset of these patients. Because of the low numbers of representative samples in some groups, the five subtypes were collapsed into three groups: LEP, ACN/PAP, and MIP/SOL.
Patients with invasive lung adenocarcinoma with MIP and SOL patterns had poorer disease-free survival (DFS) and specific disease-free survival (SDFS) compared with the ACN/PAP subtypes. Furthermore, in early-stage adenocarcinoma, MIP/SOL-predominant histology predicted benefit from ACT in DFS and SDFS.
Multivariate analysis showed a marginally significant chemotherapy benefit in OS for MIP/SOL (hazard ratio, 0.71; 95% confidence interval, 0.51-0.99; P = .04) but not for ACN/PAP. There was a significant ACT benefit within the MIP/SOL group for DFS and SDFS (P < .001 for both), but not within the ACN/PAP group.
At a median follow-up of 5.6 years, among 575 patients, there were 269 (47%) events for OS, 320 (56%) for DFS, and 292 (51%) for SDFS. The primary endpoint was OS, and secondary endpoints were DFS, defined as time from random assignment to first event (recurrence or death) and specific DFS defined as time to first cancer-related event. The prognostic impact of adenocarcinoma subtype on ACT benefit was determined by comparison with 293 patients in the observation arm who did not receive ACT.
Several previous studies demonstrated that patients with lung adenocarcinoma with predominantly MIP and SOL patterns had the worst outcomes. The association of the MIP growth pattern and poor outcomes in breast cancer is well known. The SOL pattern describes poorly differentiated carcinomas, and higher rates of this subtype have been observed in studies that included patients with higher stage disease.
“We have shown the first evidence to our knowledge that MIP/SOL–predominant histology is a promising predictive marker for benefit from ACT in DFS and SDFS in patients with early-stage lung adenocarcinoma; however, more analyses are needed to confirm these findings,” they wrote.
Analysis of a large cohort of patient tumor samples from four international adjuvant chemotherapy trials confirms the prognostic value of the latest, soon-to-be-published, World Health Organization lung adenocarcinoma classifications, investigators reported online April 27 in the Journal of Clinical Oncology.
More than half of all patients with early-stage non–small cell lung cancer will have recurrence after primary surgery and “identifying prognostic factors beyond stage is crucial to select patients who need adjuvant therapies … The results of our study represent the first markers, to our knowledge, from the LACE-Bio [the Lung Adjuvant Cisplatin Evaluation Biomarker] project that suggest a significant predictive value for survival benefit from ACT [adjuvant chemotherapy] in patients with early-stage lung adenocarcinoma,” Dr. Ming-Sound Tsao with the Princess Margaret Cancer Centre, University of Toronto, and his colleagues wrote (J. Clin. Oncol. 2015 Apr. 27 [doi:10.1200/JCO.2014.58.8335]).
A new lung adenocarcinoma classification system based on the predominant histological pattern observed in resected tumors, including lepidic (LEP), papillary (PAP), acinar (ACN), micropapillary (MIP), and predominantly solid (SOL) subgroups, forms the basis for the fourth edition of the WHO classification to be published in 2015. The LACE-Bio collaborative group evaluated biomarkers using data from a large cohort of patients participating in four ACT trials: the IALT (International Adjuvant Lung Cancer Trial), ANITA (Adjuvant Navelbine International Trialist Association), JBR-10, and CALGB (Cancer and Leukemia Group B; now Alliance for Clinical Trials in Oncology).
The current study evaluated the prognostic value of the classification regarding survival from adjuvant chemotherapy using hematoxylin and eosin (HE)-stained slides from a subset of these patients. Because of the low numbers of representative samples in some groups, the five subtypes were collapsed into three groups: LEP, ACN/PAP, and MIP/SOL.
Patients with invasive lung adenocarcinoma with MIP and SOL patterns had poorer disease-free survival (DFS) and specific disease-free survival (SDFS) compared with the ACN/PAP subtypes. Furthermore, in early-stage adenocarcinoma, MIP/SOL-predominant histology predicted benefit from ACT in DFS and SDFS.
Multivariate analysis showed a marginally significant chemotherapy benefit in OS for MIP/SOL (hazard ratio, 0.71; 95% confidence interval, 0.51-0.99; P = .04) but not for ACN/PAP. There was a significant ACT benefit within the MIP/SOL group for DFS and SDFS (P < .001 for both), but not within the ACN/PAP group.
At a median follow-up of 5.6 years, among 575 patients, there were 269 (47%) events for OS, 320 (56%) for DFS, and 292 (51%) for SDFS. The primary endpoint was OS, and secondary endpoints were DFS, defined as time from random assignment to first event (recurrence or death) and specific DFS defined as time to first cancer-related event. The prognostic impact of adenocarcinoma subtype on ACT benefit was determined by comparison with 293 patients in the observation arm who did not receive ACT.
Several previous studies demonstrated that patients with lung adenocarcinoma with predominantly MIP and SOL patterns had the worst outcomes. The association of the MIP growth pattern and poor outcomes in breast cancer is well known. The SOL pattern describes poorly differentiated carcinomas, and higher rates of this subtype have been observed in studies that included patients with higher stage disease.
“We have shown the first evidence to our knowledge that MIP/SOL–predominant histology is a promising predictive marker for benefit from ACT in DFS and SDFS in patients with early-stage lung adenocarcinoma; however, more analyses are needed to confirm these findings,” they wrote.
Analysis of a large cohort of patient tumor samples from four international adjuvant chemotherapy trials confirms the prognostic value of the latest, soon-to-be-published, World Health Organization lung adenocarcinoma classifications, investigators reported online April 27 in the Journal of Clinical Oncology.
More than half of all patients with early-stage non–small cell lung cancer will have recurrence after primary surgery and “identifying prognostic factors beyond stage is crucial to select patients who need adjuvant therapies … The results of our study represent the first markers, to our knowledge, from the LACE-Bio [the Lung Adjuvant Cisplatin Evaluation Biomarker] project that suggest a significant predictive value for survival benefit from ACT [adjuvant chemotherapy] in patients with early-stage lung adenocarcinoma,” Dr. Ming-Sound Tsao with the Princess Margaret Cancer Centre, University of Toronto, and his colleagues wrote (J. Clin. Oncol. 2015 Apr. 27 [doi:10.1200/JCO.2014.58.8335]).
A new lung adenocarcinoma classification system based on the predominant histological pattern observed in resected tumors, including lepidic (LEP), papillary (PAP), acinar (ACN), micropapillary (MIP), and predominantly solid (SOL) subgroups, forms the basis for the fourth edition of the WHO classification to be published in 2015. The LACE-Bio collaborative group evaluated biomarkers using data from a large cohort of patients participating in four ACT trials: the IALT (International Adjuvant Lung Cancer Trial), ANITA (Adjuvant Navelbine International Trialist Association), JBR-10, and CALGB (Cancer and Leukemia Group B; now Alliance for Clinical Trials in Oncology).
The current study evaluated the prognostic value of the classification regarding survival from adjuvant chemotherapy using hematoxylin and eosin (HE)-stained slides from a subset of these patients. Because of the low numbers of representative samples in some groups, the five subtypes were collapsed into three groups: LEP, ACN/PAP, and MIP/SOL.
Patients with invasive lung adenocarcinoma with MIP and SOL patterns had poorer disease-free survival (DFS) and specific disease-free survival (SDFS) compared with the ACN/PAP subtypes. Furthermore, in early-stage adenocarcinoma, MIP/SOL-predominant histology predicted benefit from ACT in DFS and SDFS.
Multivariate analysis showed a marginally significant chemotherapy benefit in OS for MIP/SOL (hazard ratio, 0.71; 95% confidence interval, 0.51-0.99; P = .04) but not for ACN/PAP. There was a significant ACT benefit within the MIP/SOL group for DFS and SDFS (P < .001 for both), but not within the ACN/PAP group.
At a median follow-up of 5.6 years, among 575 patients, there were 269 (47%) events for OS, 320 (56%) for DFS, and 292 (51%) for SDFS. The primary endpoint was OS, and secondary endpoints were DFS, defined as time from random assignment to first event (recurrence or death) and specific DFS defined as time to first cancer-related event. The prognostic impact of adenocarcinoma subtype on ACT benefit was determined by comparison with 293 patients in the observation arm who did not receive ACT.
Several previous studies demonstrated that patients with lung adenocarcinoma with predominantly MIP and SOL patterns had the worst outcomes. The association of the MIP growth pattern and poor outcomes in breast cancer is well known. The SOL pattern describes poorly differentiated carcinomas, and higher rates of this subtype have been observed in studies that included patients with higher stage disease.
“We have shown the first evidence to our knowledge that MIP/SOL–predominant histology is a promising predictive marker for benefit from ACT in DFS and SDFS in patients with early-stage lung adenocarcinoma; however, more analyses are needed to confirm these findings,” they wrote.
Key clinical point: Invasive adenocarcinomas with micropapillary (MIP) and predominantly solid (SOL) patterns had greater benefit from adjuvant chemotherapy than acinar (ACN) and papillary (PAP) subtypes.
Major finding: Grouped by subtype, patients in the MIP/SOL group had poorer disease-free survival (DFS) and specific disease-free survival (SDFS) than the ACN/PAP group (P < .01). The MIP/SOL group derived significant benefit in DFS (P < .001) and SDFS (P < .001), but not OS, from adjuvant chemotherapy.
Data source: Histology data was analyzed from 575 patients with lung adenocarcinomas who participated in adjuvant chemotherapy clinical trials and were part of the Lung Adjuvant Cisplatin Evaluation Biomarker (LACE-Bio) collaborative group.
Disclosures: Dr. Tsao reported having no disclosures. Many of his coauthors reported ties to several industry sources.
Children with advanced cancer need greater palliative care
Children over 2 years of age with advanced cancer experience high physical and psychological symptom distress, resulting mostly from pain, fatigue, drowsiness, and irritability, according to a study published online April 27 in the Journal of Clinical Oncology.
In the Pediatric Quality of Life and Evaluation of Symptoms Technology (PediQUEST) Study, children often reported suffering from pain that is known to be treatable, wrote Dr. Joanne Wolfe, director of pediatric palliative care at Boston Children’s Hospital and division chief of pediatric palliative care service in the department of psychosocial oncology and palliative care at Dana-Farber Cancer Institute, Boston (J. Clin. Oncol. 2015 April 27 [doi: 10.1200/JCO.2014.59.1222]).
“The study makes it evident that there is considerable room for improvement in easing distress in children with advanced cancer. Children experienced substantial suffering from pain throughout the course of the illness and especially at the end of life,” they wrote.
Among 104 children enrolled in the study with 920 completed symptom assessments over a 9-month follow-up, pain was most frequently reported (48%), followed by fatigue (46%), drowsiness (39%), and nausea (35%). Emotional distress was prevalent as well: irritability (37%), sleep disturbance (29%), nervousness (25%), sadness (24%), and worrying (24%). High distress was indicated for most scores.
During the last 12 weeks of life, prevalence of symptoms and levels of high distress worsened. The prevalence of pain increased to 62%; 58% with high distress. Fatigue rose to 49%, and drowsiness to 50%.
To establish patient reported outcomes (PROs) for children, PediQUEST collects child-reported symptoms (or parent-reported when necessary) and HRQoL data via computer and generates feedback reports and email alerts.
Higher symptom scores were associated with several factors, including being female, having a brain tumor, having disease progression within the previous 10 days, and receiving moderate- to high-intensity cancer treatment within the previous 10 days.
“Implications from these findings are not necessarily to decrease the amount or intensity of cancer-directed therapy offered but rather to increase efforts to ameliorate symptom distress,” the investigators wrote.
Children over 2 years of age with advanced cancer experience high physical and psychological symptom distress, resulting mostly from pain, fatigue, drowsiness, and irritability, according to a study published online April 27 in the Journal of Clinical Oncology.
In the Pediatric Quality of Life and Evaluation of Symptoms Technology (PediQUEST) Study, children often reported suffering from pain that is known to be treatable, wrote Dr. Joanne Wolfe, director of pediatric palliative care at Boston Children’s Hospital and division chief of pediatric palliative care service in the department of psychosocial oncology and palliative care at Dana-Farber Cancer Institute, Boston (J. Clin. Oncol. 2015 April 27 [doi: 10.1200/JCO.2014.59.1222]).
“The study makes it evident that there is considerable room for improvement in easing distress in children with advanced cancer. Children experienced substantial suffering from pain throughout the course of the illness and especially at the end of life,” they wrote.
Among 104 children enrolled in the study with 920 completed symptom assessments over a 9-month follow-up, pain was most frequently reported (48%), followed by fatigue (46%), drowsiness (39%), and nausea (35%). Emotional distress was prevalent as well: irritability (37%), sleep disturbance (29%), nervousness (25%), sadness (24%), and worrying (24%). High distress was indicated for most scores.
During the last 12 weeks of life, prevalence of symptoms and levels of high distress worsened. The prevalence of pain increased to 62%; 58% with high distress. Fatigue rose to 49%, and drowsiness to 50%.
To establish patient reported outcomes (PROs) for children, PediQUEST collects child-reported symptoms (or parent-reported when necessary) and HRQoL data via computer and generates feedback reports and email alerts.
Higher symptom scores were associated with several factors, including being female, having a brain tumor, having disease progression within the previous 10 days, and receiving moderate- to high-intensity cancer treatment within the previous 10 days.
“Implications from these findings are not necessarily to decrease the amount or intensity of cancer-directed therapy offered but rather to increase efforts to ameliorate symptom distress,” the investigators wrote.
Children over 2 years of age with advanced cancer experience high physical and psychological symptom distress, resulting mostly from pain, fatigue, drowsiness, and irritability, according to a study published online April 27 in the Journal of Clinical Oncology.
In the Pediatric Quality of Life and Evaluation of Symptoms Technology (PediQUEST) Study, children often reported suffering from pain that is known to be treatable, wrote Dr. Joanne Wolfe, director of pediatric palliative care at Boston Children’s Hospital and division chief of pediatric palliative care service in the department of psychosocial oncology and palliative care at Dana-Farber Cancer Institute, Boston (J. Clin. Oncol. 2015 April 27 [doi: 10.1200/JCO.2014.59.1222]).
“The study makes it evident that there is considerable room for improvement in easing distress in children with advanced cancer. Children experienced substantial suffering from pain throughout the course of the illness and especially at the end of life,” they wrote.
Among 104 children enrolled in the study with 920 completed symptom assessments over a 9-month follow-up, pain was most frequently reported (48%), followed by fatigue (46%), drowsiness (39%), and nausea (35%). Emotional distress was prevalent as well: irritability (37%), sleep disturbance (29%), nervousness (25%), sadness (24%), and worrying (24%). High distress was indicated for most scores.
During the last 12 weeks of life, prevalence of symptoms and levels of high distress worsened. The prevalence of pain increased to 62%; 58% with high distress. Fatigue rose to 49%, and drowsiness to 50%.
To establish patient reported outcomes (PROs) for children, PediQUEST collects child-reported symptoms (or parent-reported when necessary) and HRQoL data via computer and generates feedback reports and email alerts.
Higher symptom scores were associated with several factors, including being female, having a brain tumor, having disease progression within the previous 10 days, and receiving moderate- to high-intensity cancer treatment within the previous 10 days.
“Implications from these findings are not necessarily to decrease the amount or intensity of cancer-directed therapy offered but rather to increase efforts to ameliorate symptom distress,” the investigators wrote.
Key clinical point: According to their own reports, children with advanced cancer experience high suffering, mostly due to cancer-directed therapy.
Major finding: During the last 12 weeks of life, pain was prevalent in 62% of patients; pain with high distress in 58%.
Data source: For 104 patients who were enrolled in the PediQUEST RCT, symptoms were recorded via PediQUEST Memorial Symptom Assessment Scale (920 total assessments) and clinical data was obtained from medical records.
Disclosures: Dr. Wolfe and coauthors reported having no disclosures.
Immunotherapy-related toxicities are varied
Toxicities associated with immunotherapies cover a wide range from capillary leakage with cytokine therapies, to damaging cross reactivities of cell therapies, to autoinflammatory reactions observed with immune checkpoint inhibitors, according to a review published online April 27 in the Journal of Clinical Oncology.
Most toxicities involve hyperactivated T-cell responses directed against healthy tissue. In general, cytokine therapy generates diffuse, nonspecific T-cell reactivity, while vaccines, adoptive cell therapy, and checkpoint protein inhibitors generate specific T-cell responses directed to normal tissue that can cause organ damage. Dr. Jeffrey S. Weber, director of the Donald A. Adam Comprehensive Melanoma Research Center at the Moffitt Cancer Center and Research Institute, Tampa, and his colleagues summarized the toxicities specific to immunotherapies and emphasized the importance of oncology practitioners understanding the spectrum of adverse events and treatment options available (J. Clin. Oncol. 2015 Apr. 27 [doi:10.1200/JCO.2014.60.0379]).
Minimal toxicity has been observed with cancer vaccines, possibly because the tumor-associated targets are overexpressed in cancer cells but are found at low or undetectable levels in normal cells. Human epidermal growth factor receptor 2 (HER2), p53, and survivin are the most common vaccine targets. Sipuleucel-T, the one currently approved vaccine, has a favorable toxicity profile, with transient chills, fatigue, and fever the most common adverse events (AEs).
By contrast, cytokines are associated with frequent and severe AEs, which has dampened enthusiasm for the treatment. The FDA approved recombinant human interferon-alpha (IFN) for the treatment of hairy cell leukemia and high-risk melanoma, and interleukin-2 was approved for advanced renal cell carcinoma and melanoma.
IFN treatment results in constitutional symptoms of fever and fatigue in more than 80% of recipients, as well as headache and myalgia. Nonsteroidal anti-inflammatory drugs are helpful. Up to one-third of patients have diarrhea and two thirds have nausea and anorexia. Neuropsychiatric issues are observed, such as confusion in about 10% of patients, depression in up to 45%, and psychosis in less than 1%. Prophylactic antidepressants may reduce the risk of depression in those with a history of depression, but careful monitoring of all patients is suggested.
Thrombocytopenia and leukopenia are observed in up to 10% of patients. Hyperthyroidism or hypothyroidism occurs in 10%-15% of patients. Sarcoid is rare, but may be confused with disease progression in patients with melanoma or lymphoma. IFN may cause autoimmune events, but some investigators noted that this may be associated with improved treatment outcomes.
IL-2 treatment leads to increased vascular permeability and fluid retention, including pleural effusions and pulmonary edema, hypotension, and prerenal azotemia. Thrombocytopenia, anemia, coagulopathy, or neutrophil chemotaxis impairment leading to catheter infections may occur. Neurotoxicity associated with IL-2 can be subtle, such as lethargy and irritability, or severe as in florid psychosis.
Mediators of IL-2 toxicity include nitric oxide, IL-1, tumor necrosis factor–alpha, and IFN-gamma, but inhibitors of these toxic factors have been unsuccessful.
Adoptive T-cell therapies have effectively treated patients with certain metastatic cancers, including melanoma, metastatic cervical cancer, and B-cell malignancies. Preparative chemotherapy for lymphodepletion leaves patients at risk for sepsis and bleeding before hematopoietic recovery, which is the dominant cause of a 1%-2% rate of mortality.
Cytokine release syndrome resembles sepsis and is also seen with high-dose IL-2. With supportive care, even severe renal failure, coma, and respiratory failure are completely reversed usually. IL-6 was identified as a mediator in one case involving B-cell malignancy, and an IL-6 receptor-blocking antibody showed apparent benefit.
Autoimmunity induced by the T cells may occur, the consequences of which depend on the level and distribution of the normal tissue expression of the target.
Standard interventions for life-threatening adverse responses to T-cell therapy include high-dose corticosteroids and alemtuzumab (anti-CD52 antibody) to suppress lymphocytes, a treatment which may circumvent antitumor effects as well.
Since 2011, checkpoint inhibitors ipilimumab (anti-CTLA-4), pembrolizumab, and nivolumab (both anti-PD-1) have been approved by the Food and Drug Administration and are commonly used for patients with melanoma and other cancers. Autoimmune reactions are common, and all patients are recommended to have thyroid function studies, complete blood counts, and liver function and metabolic panels at each treatment and regular intervals for 6 months following treatment.
Ipilimumab toxicities are dose related, while toxicities associated with PD-1 blockade do not appear to be dose related. The most common drug related AEs of any grade were fatigue, pruritus, and rash.
Toxicities associated with PD-1 antibodies vary depending on the histology treated. For severe colitis caused by ipilimumab or PD-1 antibodies, high doses of corticosteroids are required, and infliximab administered in patients whose colitis fails to resolve within three days.
The onset of immune-related AEs follows a predictable pattern, with skin-related toxicities occurring first, followed by colitis, then hepatitis and endocrinopathies observed between weeks 12 and 24.
“The key to successful management of checkpoint protein antibody toxicities is early diagnosis, high suspicion, excellent patient-provider communication, and rapid and aggressive use of corticosteroids and other immune suppressants for immune-related AEs,” Dr. Weber and his associates wrote.
Dr. Weber and his associates reported receiving research funding from or having consulting or advisory roles with several industry sources.
Toxicities associated with immunotherapies cover a wide range from capillary leakage with cytokine therapies, to damaging cross reactivities of cell therapies, to autoinflammatory reactions observed with immune checkpoint inhibitors, according to a review published online April 27 in the Journal of Clinical Oncology.
Most toxicities involve hyperactivated T-cell responses directed against healthy tissue. In general, cytokine therapy generates diffuse, nonspecific T-cell reactivity, while vaccines, adoptive cell therapy, and checkpoint protein inhibitors generate specific T-cell responses directed to normal tissue that can cause organ damage. Dr. Jeffrey S. Weber, director of the Donald A. Adam Comprehensive Melanoma Research Center at the Moffitt Cancer Center and Research Institute, Tampa, and his colleagues summarized the toxicities specific to immunotherapies and emphasized the importance of oncology practitioners understanding the spectrum of adverse events and treatment options available (J. Clin. Oncol. 2015 Apr. 27 [doi:10.1200/JCO.2014.60.0379]).
Minimal toxicity has been observed with cancer vaccines, possibly because the tumor-associated targets are overexpressed in cancer cells but are found at low or undetectable levels in normal cells. Human epidermal growth factor receptor 2 (HER2), p53, and survivin are the most common vaccine targets. Sipuleucel-T, the one currently approved vaccine, has a favorable toxicity profile, with transient chills, fatigue, and fever the most common adverse events (AEs).
By contrast, cytokines are associated with frequent and severe AEs, which has dampened enthusiasm for the treatment. The FDA approved recombinant human interferon-alpha (IFN) for the treatment of hairy cell leukemia and high-risk melanoma, and interleukin-2 was approved for advanced renal cell carcinoma and melanoma.
IFN treatment results in constitutional symptoms of fever and fatigue in more than 80% of recipients, as well as headache and myalgia. Nonsteroidal anti-inflammatory drugs are helpful. Up to one-third of patients have diarrhea and two thirds have nausea and anorexia. Neuropsychiatric issues are observed, such as confusion in about 10% of patients, depression in up to 45%, and psychosis in less than 1%. Prophylactic antidepressants may reduce the risk of depression in those with a history of depression, but careful monitoring of all patients is suggested.
Thrombocytopenia and leukopenia are observed in up to 10% of patients. Hyperthyroidism or hypothyroidism occurs in 10%-15% of patients. Sarcoid is rare, but may be confused with disease progression in patients with melanoma or lymphoma. IFN may cause autoimmune events, but some investigators noted that this may be associated with improved treatment outcomes.
IL-2 treatment leads to increased vascular permeability and fluid retention, including pleural effusions and pulmonary edema, hypotension, and prerenal azotemia. Thrombocytopenia, anemia, coagulopathy, or neutrophil chemotaxis impairment leading to catheter infections may occur. Neurotoxicity associated with IL-2 can be subtle, such as lethargy and irritability, or severe as in florid psychosis.
Mediators of IL-2 toxicity include nitric oxide, IL-1, tumor necrosis factor–alpha, and IFN-gamma, but inhibitors of these toxic factors have been unsuccessful.
Adoptive T-cell therapies have effectively treated patients with certain metastatic cancers, including melanoma, metastatic cervical cancer, and B-cell malignancies. Preparative chemotherapy for lymphodepletion leaves patients at risk for sepsis and bleeding before hematopoietic recovery, which is the dominant cause of a 1%-2% rate of mortality.
Cytokine release syndrome resembles sepsis and is also seen with high-dose IL-2. With supportive care, even severe renal failure, coma, and respiratory failure are completely reversed usually. IL-6 was identified as a mediator in one case involving B-cell malignancy, and an IL-6 receptor-blocking antibody showed apparent benefit.
Autoimmunity induced by the T cells may occur, the consequences of which depend on the level and distribution of the normal tissue expression of the target.
Standard interventions for life-threatening adverse responses to T-cell therapy include high-dose corticosteroids and alemtuzumab (anti-CD52 antibody) to suppress lymphocytes, a treatment which may circumvent antitumor effects as well.
Since 2011, checkpoint inhibitors ipilimumab (anti-CTLA-4), pembrolizumab, and nivolumab (both anti-PD-1) have been approved by the Food and Drug Administration and are commonly used for patients with melanoma and other cancers. Autoimmune reactions are common, and all patients are recommended to have thyroid function studies, complete blood counts, and liver function and metabolic panels at each treatment and regular intervals for 6 months following treatment.
Ipilimumab toxicities are dose related, while toxicities associated with PD-1 blockade do not appear to be dose related. The most common drug related AEs of any grade were fatigue, pruritus, and rash.
Toxicities associated with PD-1 antibodies vary depending on the histology treated. For severe colitis caused by ipilimumab or PD-1 antibodies, high doses of corticosteroids are required, and infliximab administered in patients whose colitis fails to resolve within three days.
The onset of immune-related AEs follows a predictable pattern, with skin-related toxicities occurring first, followed by colitis, then hepatitis and endocrinopathies observed between weeks 12 and 24.
“The key to successful management of checkpoint protein antibody toxicities is early diagnosis, high suspicion, excellent patient-provider communication, and rapid and aggressive use of corticosteroids and other immune suppressants for immune-related AEs,” Dr. Weber and his associates wrote.
Dr. Weber and his associates reported receiving research funding from or having consulting or advisory roles with several industry sources.
Toxicities associated with immunotherapies cover a wide range from capillary leakage with cytokine therapies, to damaging cross reactivities of cell therapies, to autoinflammatory reactions observed with immune checkpoint inhibitors, according to a review published online April 27 in the Journal of Clinical Oncology.
Most toxicities involve hyperactivated T-cell responses directed against healthy tissue. In general, cytokine therapy generates diffuse, nonspecific T-cell reactivity, while vaccines, adoptive cell therapy, and checkpoint protein inhibitors generate specific T-cell responses directed to normal tissue that can cause organ damage. Dr. Jeffrey S. Weber, director of the Donald A. Adam Comprehensive Melanoma Research Center at the Moffitt Cancer Center and Research Institute, Tampa, and his colleagues summarized the toxicities specific to immunotherapies and emphasized the importance of oncology practitioners understanding the spectrum of adverse events and treatment options available (J. Clin. Oncol. 2015 Apr. 27 [doi:10.1200/JCO.2014.60.0379]).
Minimal toxicity has been observed with cancer vaccines, possibly because the tumor-associated targets are overexpressed in cancer cells but are found at low or undetectable levels in normal cells. Human epidermal growth factor receptor 2 (HER2), p53, and survivin are the most common vaccine targets. Sipuleucel-T, the one currently approved vaccine, has a favorable toxicity profile, with transient chills, fatigue, and fever the most common adverse events (AEs).
By contrast, cytokines are associated with frequent and severe AEs, which has dampened enthusiasm for the treatment. The FDA approved recombinant human interferon-alpha (IFN) for the treatment of hairy cell leukemia and high-risk melanoma, and interleukin-2 was approved for advanced renal cell carcinoma and melanoma.
IFN treatment results in constitutional symptoms of fever and fatigue in more than 80% of recipients, as well as headache and myalgia. Nonsteroidal anti-inflammatory drugs are helpful. Up to one-third of patients have diarrhea and two thirds have nausea and anorexia. Neuropsychiatric issues are observed, such as confusion in about 10% of patients, depression in up to 45%, and psychosis in less than 1%. Prophylactic antidepressants may reduce the risk of depression in those with a history of depression, but careful monitoring of all patients is suggested.
Thrombocytopenia and leukopenia are observed in up to 10% of patients. Hyperthyroidism or hypothyroidism occurs in 10%-15% of patients. Sarcoid is rare, but may be confused with disease progression in patients with melanoma or lymphoma. IFN may cause autoimmune events, but some investigators noted that this may be associated with improved treatment outcomes.
IL-2 treatment leads to increased vascular permeability and fluid retention, including pleural effusions and pulmonary edema, hypotension, and prerenal azotemia. Thrombocytopenia, anemia, coagulopathy, or neutrophil chemotaxis impairment leading to catheter infections may occur. Neurotoxicity associated with IL-2 can be subtle, such as lethargy and irritability, or severe as in florid psychosis.
Mediators of IL-2 toxicity include nitric oxide, IL-1, tumor necrosis factor–alpha, and IFN-gamma, but inhibitors of these toxic factors have been unsuccessful.
Adoptive T-cell therapies have effectively treated patients with certain metastatic cancers, including melanoma, metastatic cervical cancer, and B-cell malignancies. Preparative chemotherapy for lymphodepletion leaves patients at risk for sepsis and bleeding before hematopoietic recovery, which is the dominant cause of a 1%-2% rate of mortality.
Cytokine release syndrome resembles sepsis and is also seen with high-dose IL-2. With supportive care, even severe renal failure, coma, and respiratory failure are completely reversed usually. IL-6 was identified as a mediator in one case involving B-cell malignancy, and an IL-6 receptor-blocking antibody showed apparent benefit.
Autoimmunity induced by the T cells may occur, the consequences of which depend on the level and distribution of the normal tissue expression of the target.
Standard interventions for life-threatening adverse responses to T-cell therapy include high-dose corticosteroids and alemtuzumab (anti-CD52 antibody) to suppress lymphocytes, a treatment which may circumvent antitumor effects as well.
Since 2011, checkpoint inhibitors ipilimumab (anti-CTLA-4), pembrolizumab, and nivolumab (both anti-PD-1) have been approved by the Food and Drug Administration and are commonly used for patients with melanoma and other cancers. Autoimmune reactions are common, and all patients are recommended to have thyroid function studies, complete blood counts, and liver function and metabolic panels at each treatment and regular intervals for 6 months following treatment.
Ipilimumab toxicities are dose related, while toxicities associated with PD-1 blockade do not appear to be dose related. The most common drug related AEs of any grade were fatigue, pruritus, and rash.
Toxicities associated with PD-1 antibodies vary depending on the histology treated. For severe colitis caused by ipilimumab or PD-1 antibodies, high doses of corticosteroids are required, and infliximab administered in patients whose colitis fails to resolve within three days.
The onset of immune-related AEs follows a predictable pattern, with skin-related toxicities occurring first, followed by colitis, then hepatitis and endocrinopathies observed between weeks 12 and 24.
“The key to successful management of checkpoint protein antibody toxicities is early diagnosis, high suspicion, excellent patient-provider communication, and rapid and aggressive use of corticosteroids and other immune suppressants for immune-related AEs,” Dr. Weber and his associates wrote.
Dr. Weber and his associates reported receiving research funding from or having consulting or advisory roles with several industry sources.
Exercise pumps up chemotherapy completion rates for breast cancer patients
Compared with usual care, a moderate- to high-intensity exercise intervention had beneficial effects on chemotherapy completion rates, symptom burden, and return-to-work rates among women with breast cancer who were undergoing adjuvant chemotherapy, according to a study published online April 27 in the Journal of Clinical Oncology.
For the multicenter Physical Exercise During Adjuvant Chemotherapy Effectiveness Study (PACES), 230 women (mean age 51 years) with breast cancer who were undergoing adjuvant chemotherapy were randomized to participate in a high- to moderate-intensity exercise program supervised by physical therapists (n = 76), a low-intensity home-based program (n = 77), or the usual care group (n = 77).
Dose adjustments in the chemotherapy regimen were less frequent in the moderate- to high-intensity exercise group (12%) than in the usual care or low-intensity groups (both 34%, P = .002). Patients in the exercise interventions were more likely to return to work by the 6-month follow up than those with usual care, wrote Ms. Hanna van Waart, a doctoral candidate at the Netherlands Cancer Institute, Amsterdam, and colleagues (J. Clin. Oncol. 2015 Apr. 27 [doi:10.1200/JCO.2014.59.1081]).
“This not only has financial implications, but also carries meaning in terms of quality of life and a sense of return to normalcy,” they wrote.
At completion of chemotherapy, patients from both activity groups reported significantly better physical functioning, less nausea and vomiting, and less pain than did those in the usual care group.
Compared with usual care, a moderate- to high-intensity exercise intervention had beneficial effects on chemotherapy completion rates, symptom burden, and return-to-work rates among women with breast cancer who were undergoing adjuvant chemotherapy, according to a study published online April 27 in the Journal of Clinical Oncology.
For the multicenter Physical Exercise During Adjuvant Chemotherapy Effectiveness Study (PACES), 230 women (mean age 51 years) with breast cancer who were undergoing adjuvant chemotherapy were randomized to participate in a high- to moderate-intensity exercise program supervised by physical therapists (n = 76), a low-intensity home-based program (n = 77), or the usual care group (n = 77).
Dose adjustments in the chemotherapy regimen were less frequent in the moderate- to high-intensity exercise group (12%) than in the usual care or low-intensity groups (both 34%, P = .002). Patients in the exercise interventions were more likely to return to work by the 6-month follow up than those with usual care, wrote Ms. Hanna van Waart, a doctoral candidate at the Netherlands Cancer Institute, Amsterdam, and colleagues (J. Clin. Oncol. 2015 Apr. 27 [doi:10.1200/JCO.2014.59.1081]).
“This not only has financial implications, but also carries meaning in terms of quality of life and a sense of return to normalcy,” they wrote.
At completion of chemotherapy, patients from both activity groups reported significantly better physical functioning, less nausea and vomiting, and less pain than did those in the usual care group.
Compared with usual care, a moderate- to high-intensity exercise intervention had beneficial effects on chemotherapy completion rates, symptom burden, and return-to-work rates among women with breast cancer who were undergoing adjuvant chemotherapy, according to a study published online April 27 in the Journal of Clinical Oncology.
For the multicenter Physical Exercise During Adjuvant Chemotherapy Effectiveness Study (PACES), 230 women (mean age 51 years) with breast cancer who were undergoing adjuvant chemotherapy were randomized to participate in a high- to moderate-intensity exercise program supervised by physical therapists (n = 76), a low-intensity home-based program (n = 77), or the usual care group (n = 77).
Dose adjustments in the chemotherapy regimen were less frequent in the moderate- to high-intensity exercise group (12%) than in the usual care or low-intensity groups (both 34%, P = .002). Patients in the exercise interventions were more likely to return to work by the 6-month follow up than those with usual care, wrote Ms. Hanna van Waart, a doctoral candidate at the Netherlands Cancer Institute, Amsterdam, and colleagues (J. Clin. Oncol. 2015 Apr. 27 [doi:10.1200/JCO.2014.59.1081]).
“This not only has financial implications, but also carries meaning in terms of quality of life and a sense of return to normalcy,” they wrote.
At completion of chemotherapy, patients from both activity groups reported significantly better physical functioning, less nausea and vomiting, and less pain than did those in the usual care group.
Key clinical point: Moderate- to high-intensity exercise during adjuvant chemotherapy improves completion rates. Low-intensity physical activity resulted in less pronounced benefits.
Major finding: Dose adjustments in the chemotherapy regimen were less frequent in the moderate- to high-intensity exercise group (12%) than in the usual care or low-intensity groups (both 34%, P = .002).
Data source: PACES, a controlled multicenter study that randomized 230 patients with breast cancer to participate in high- to moderate-intensity (n = 76) or low intensity (n = 77) exercise or usual care (n = 77) while undergoing adjuvant chemotherapy.
Disclosures: Dr. van Waart reported having no disclosures. Two of her coauthors reported ties to several industry sources.
Oophorectomy improves survival after breast cancer in BRCA1 carriers
Women with breast cancer and BRCA1 mutations had a lower risk of mortality if they underwent oophorectomy, and the benefit was apparent beyond age 50, investigators reported online April 23 in JAMA Oncology.
In the entire cohort that included women with BRCA1 and BRCA2 mutations, breast cancer–related mortality was reduced 56%, and all-cause mortality (including 9 deaths due to ovarian cancer) was reduced 65% with oophorectomy (adjusted HR, 0.35; 95% CI, 0.22-0.56; P < .001).
Dr. Kelly Metcalfe of the Women’s College Research Institute, Toronto, and her colleagues provided evidence in support of BRCA1 testing in women with early-stage breast cancer at the time of diagnosis.
“The data presented here suggest that oophorectomy should be discussed with the patient [with a BRCA1 mutation] shortly after diagnosis. We recommend that the operation be performed in the first year of treatment to maximize the benefit,” the investigators wrote (JAMA Oncol. 2015 Apr. 23 [doi:10.1001/jamaoncol.2015.0658]).
The historical cohort study evaluated 676 women with BRCA1 or BRCA2 mutations and early-stage breast cancer diagnosed between 1975 and 2008. In order to examine the impact of subsequent oophorectomy on mortality risk due to breast cancer, a requirement for inclusion was that both ovaries be intact at the time of diagnosis. The subsequent oophorectomy group included 345 women.
Among the women who underwent oophorectomy, women with a BRCA1 mutation had a significantly reduced risk of breast cancer–related death, but the association was not significant for those with BRCA2 mutations. However, the number of BRCA2 carriers was much smaller than the number of BRCA1 carriers, and the 95% CI range was wide (0.23-1.43). A larger sample is required to determine the strength of an association.
Regardless of BRCA1/2 mutation status, in patients with estrogen receptor–negative breast cancer, oophorectomy had a protective effect (HR, 0.07; 95% CI, 0.01-0.51; P = .009).
Among 14 women over age 50 with estrogen receptor–negative cancer who did not undergo oophorectomy, 3 (21%) died; of the 15 who underwent the surgery, none died. Based on this finding, and a previous study indicating a protective effect of oophorectomy on the risk of primary breast cancer in women over 50, the researchers concluded that “the postmenopausal ovary remains active in terms of androgen production and that this affects cancer risk and progression, either directly or through aromatization to estrogen.”
Women with breast cancer and BRCA1 mutations had a lower risk of mortality if they underwent oophorectomy, and the benefit was apparent beyond age 50, investigators reported online April 23 in JAMA Oncology.
In the entire cohort that included women with BRCA1 and BRCA2 mutations, breast cancer–related mortality was reduced 56%, and all-cause mortality (including 9 deaths due to ovarian cancer) was reduced 65% with oophorectomy (adjusted HR, 0.35; 95% CI, 0.22-0.56; P < .001).
Dr. Kelly Metcalfe of the Women’s College Research Institute, Toronto, and her colleagues provided evidence in support of BRCA1 testing in women with early-stage breast cancer at the time of diagnosis.
“The data presented here suggest that oophorectomy should be discussed with the patient [with a BRCA1 mutation] shortly after diagnosis. We recommend that the operation be performed in the first year of treatment to maximize the benefit,” the investigators wrote (JAMA Oncol. 2015 Apr. 23 [doi:10.1001/jamaoncol.2015.0658]).
The historical cohort study evaluated 676 women with BRCA1 or BRCA2 mutations and early-stage breast cancer diagnosed between 1975 and 2008. In order to examine the impact of subsequent oophorectomy on mortality risk due to breast cancer, a requirement for inclusion was that both ovaries be intact at the time of diagnosis. The subsequent oophorectomy group included 345 women.
Among the women who underwent oophorectomy, women with a BRCA1 mutation had a significantly reduced risk of breast cancer–related death, but the association was not significant for those with BRCA2 mutations. However, the number of BRCA2 carriers was much smaller than the number of BRCA1 carriers, and the 95% CI range was wide (0.23-1.43). A larger sample is required to determine the strength of an association.
Regardless of BRCA1/2 mutation status, in patients with estrogen receptor–negative breast cancer, oophorectomy had a protective effect (HR, 0.07; 95% CI, 0.01-0.51; P = .009).
Among 14 women over age 50 with estrogen receptor–negative cancer who did not undergo oophorectomy, 3 (21%) died; of the 15 who underwent the surgery, none died. Based on this finding, and a previous study indicating a protective effect of oophorectomy on the risk of primary breast cancer in women over 50, the researchers concluded that “the postmenopausal ovary remains active in terms of androgen production and that this affects cancer risk and progression, either directly or through aromatization to estrogen.”
Women with breast cancer and BRCA1 mutations had a lower risk of mortality if they underwent oophorectomy, and the benefit was apparent beyond age 50, investigators reported online April 23 in JAMA Oncology.
In the entire cohort that included women with BRCA1 and BRCA2 mutations, breast cancer–related mortality was reduced 56%, and all-cause mortality (including 9 deaths due to ovarian cancer) was reduced 65% with oophorectomy (adjusted HR, 0.35; 95% CI, 0.22-0.56; P < .001).
Dr. Kelly Metcalfe of the Women’s College Research Institute, Toronto, and her colleagues provided evidence in support of BRCA1 testing in women with early-stage breast cancer at the time of diagnosis.
“The data presented here suggest that oophorectomy should be discussed with the patient [with a BRCA1 mutation] shortly after diagnosis. We recommend that the operation be performed in the first year of treatment to maximize the benefit,” the investigators wrote (JAMA Oncol. 2015 Apr. 23 [doi:10.1001/jamaoncol.2015.0658]).
The historical cohort study evaluated 676 women with BRCA1 or BRCA2 mutations and early-stage breast cancer diagnosed between 1975 and 2008. In order to examine the impact of subsequent oophorectomy on mortality risk due to breast cancer, a requirement for inclusion was that both ovaries be intact at the time of diagnosis. The subsequent oophorectomy group included 345 women.
Among the women who underwent oophorectomy, women with a BRCA1 mutation had a significantly reduced risk of breast cancer–related death, but the association was not significant for those with BRCA2 mutations. However, the number of BRCA2 carriers was much smaller than the number of BRCA1 carriers, and the 95% CI range was wide (0.23-1.43). A larger sample is required to determine the strength of an association.
Regardless of BRCA1/2 mutation status, in patients with estrogen receptor–negative breast cancer, oophorectomy had a protective effect (HR, 0.07; 95% CI, 0.01-0.51; P = .009).
Among 14 women over age 50 with estrogen receptor–negative cancer who did not undergo oophorectomy, 3 (21%) died; of the 15 who underwent the surgery, none died. Based on this finding, and a previous study indicating a protective effect of oophorectomy on the risk of primary breast cancer in women over 50, the researchers concluded that “the postmenopausal ovary remains active in terms of androgen production and that this affects cancer risk and progression, either directly or through aromatization to estrogen.”
FROM JAMA ONCOLOGY
Key clinical point: Oophorectomy significantly improved the prognosis of women with breast cancer and a BRCA1 mutation.
Major finding: The adjusted hazard ratio for breast cancer mortality in women with a BRCA1 mutation who had breast cancer and a subsequent oophorectomy was 0.38 (95% CI, 0.19-0.077; P = .007).
Data source: The retrospective cohort study evaluated 676 women with BRCA1 or BRCA2 mutations and breast cancer diagnosed from 1975 to 2008.
Disclosures: Dr. Kelly Metcalfe reported having no disclosures. The research was funded by the Canadian Breast Cancer Foundation.
TKI benefit greater with certain EGFR mutations in NSCLC tumors
Risk of non–small cell lung cancer progression is reduced by 63% for patients with epidermal growth factor receptor mutations who take tyrosine kinase inhibitors over chemotherapy, and the benefit is 50% greater for those with exon 19 deletions compared with exon 21 leucine to arginine substitutions, according to a meta-analysis published online April 20 in the Journal of Clinical Oncology.
More than 90% of epidermal growth factor receptor (EGFR) mutations in tumors of non–small cell lung cancer (NSCLC) patients are so-called common mutations of exon 19 deletion or exon 21 L858R substitution, and while both mutations are recognized as predicting benefit with EGFR TKIs, this meta-analysis of seven studies of NSCLC patients with the mutations (56% exon 19 deletions and 44% L858R), showed 50% greater relative benefit in progression free survival (PFS) for patients with exon 19 deletions relative to L858R (HR, 0.24; 95% CI, 0.20-0.29 vs. 0.48; 0.39-0.58).
“These findings have important implications for clinical trial design and interpretation, economic analysis, and future drug development for EGFR-mutated, advanced NSCLC,” wrote Dr. Chee Khoon Lee of the National Health and Medical Research Council Clinical Trials Centre, University of Sydney, Australia, and associates (J. Clin. Oncol. 2015 April 20 [doi: 10.1200/JCO.2014.58.1736]).
The researchers also found a 36% greater PFS benefit for never-smokers compared with current or former smokers. The pooled HR for PFS among the 70% of patients who never smoked was 0.32 (0.27-0.37) compared with 0.50 (0.40-0.63) for the 30% who currently or formerly smoked (Pinteraction = .002).
Women had a 27% greater PFS benefit than did men, a difference previously thought due to the higher EGFR mutation rate in women or due to lower smoking rates in women than men. All patients in the current study had EGFR mutations, and the sex-based difference in PFS benefit was still apparent. Multivariate analysis showed also that the benefit was independent of smoking status.
Regarding associations between EGFR mutation type and clinical characteristics, no significant correlations were observed between mutation type and age, performance status, sex, histology, or smoking.
In patients treated with TKIs, exon 19 deletions were associated with longer PFS than L858R (median PFS 11.8 vs. 10.0 months; P = .006). By contrast, among those randomly assigned to chemotherapy, L858R mutations were associated with longer PFS (6.1 vs. 5.1 months; P = .003).
The report was limited by the absence of overall survival data, which the researchers said they plan to include in an upcoming analysis when the data become available.
Risk of non–small cell lung cancer progression is reduced by 63% for patients with epidermal growth factor receptor mutations who take tyrosine kinase inhibitors over chemotherapy, and the benefit is 50% greater for those with exon 19 deletions compared with exon 21 leucine to arginine substitutions, according to a meta-analysis published online April 20 in the Journal of Clinical Oncology.
More than 90% of epidermal growth factor receptor (EGFR) mutations in tumors of non–small cell lung cancer (NSCLC) patients are so-called common mutations of exon 19 deletion or exon 21 L858R substitution, and while both mutations are recognized as predicting benefit with EGFR TKIs, this meta-analysis of seven studies of NSCLC patients with the mutations (56% exon 19 deletions and 44% L858R), showed 50% greater relative benefit in progression free survival (PFS) for patients with exon 19 deletions relative to L858R (HR, 0.24; 95% CI, 0.20-0.29 vs. 0.48; 0.39-0.58).
“These findings have important implications for clinical trial design and interpretation, economic analysis, and future drug development for EGFR-mutated, advanced NSCLC,” wrote Dr. Chee Khoon Lee of the National Health and Medical Research Council Clinical Trials Centre, University of Sydney, Australia, and associates (J. Clin. Oncol. 2015 April 20 [doi: 10.1200/JCO.2014.58.1736]).
The researchers also found a 36% greater PFS benefit for never-smokers compared with current or former smokers. The pooled HR for PFS among the 70% of patients who never smoked was 0.32 (0.27-0.37) compared with 0.50 (0.40-0.63) for the 30% who currently or formerly smoked (Pinteraction = .002).
Women had a 27% greater PFS benefit than did men, a difference previously thought due to the higher EGFR mutation rate in women or due to lower smoking rates in women than men. All patients in the current study had EGFR mutations, and the sex-based difference in PFS benefit was still apparent. Multivariate analysis showed also that the benefit was independent of smoking status.
Regarding associations between EGFR mutation type and clinical characteristics, no significant correlations were observed between mutation type and age, performance status, sex, histology, or smoking.
In patients treated with TKIs, exon 19 deletions were associated with longer PFS than L858R (median PFS 11.8 vs. 10.0 months; P = .006). By contrast, among those randomly assigned to chemotherapy, L858R mutations were associated with longer PFS (6.1 vs. 5.1 months; P = .003).
The report was limited by the absence of overall survival data, which the researchers said they plan to include in an upcoming analysis when the data become available.
Risk of non–small cell lung cancer progression is reduced by 63% for patients with epidermal growth factor receptor mutations who take tyrosine kinase inhibitors over chemotherapy, and the benefit is 50% greater for those with exon 19 deletions compared with exon 21 leucine to arginine substitutions, according to a meta-analysis published online April 20 in the Journal of Clinical Oncology.
More than 90% of epidermal growth factor receptor (EGFR) mutations in tumors of non–small cell lung cancer (NSCLC) patients are so-called common mutations of exon 19 deletion or exon 21 L858R substitution, and while both mutations are recognized as predicting benefit with EGFR TKIs, this meta-analysis of seven studies of NSCLC patients with the mutations (56% exon 19 deletions and 44% L858R), showed 50% greater relative benefit in progression free survival (PFS) for patients with exon 19 deletions relative to L858R (HR, 0.24; 95% CI, 0.20-0.29 vs. 0.48; 0.39-0.58).
“These findings have important implications for clinical trial design and interpretation, economic analysis, and future drug development for EGFR-mutated, advanced NSCLC,” wrote Dr. Chee Khoon Lee of the National Health and Medical Research Council Clinical Trials Centre, University of Sydney, Australia, and associates (J. Clin. Oncol. 2015 April 20 [doi: 10.1200/JCO.2014.58.1736]).
The researchers also found a 36% greater PFS benefit for never-smokers compared with current or former smokers. The pooled HR for PFS among the 70% of patients who never smoked was 0.32 (0.27-0.37) compared with 0.50 (0.40-0.63) for the 30% who currently or formerly smoked (Pinteraction = .002).
Women had a 27% greater PFS benefit than did men, a difference previously thought due to the higher EGFR mutation rate in women or due to lower smoking rates in women than men. All patients in the current study had EGFR mutations, and the sex-based difference in PFS benefit was still apparent. Multivariate analysis showed also that the benefit was independent of smoking status.
Regarding associations between EGFR mutation type and clinical characteristics, no significant correlations were observed between mutation type and age, performance status, sex, histology, or smoking.
In patients treated with TKIs, exon 19 deletions were associated with longer PFS than L858R (median PFS 11.8 vs. 10.0 months; P = .006). By contrast, among those randomly assigned to chemotherapy, L858R mutations were associated with longer PFS (6.1 vs. 5.1 months; P = .003).
The report was limited by the absence of overall survival data, which the researchers said they plan to include in an upcoming analysis when the data become available.
FROM JOURNAL OF CLINICAL ONCOLOGY
Key clinical point: Risk of non–small cell lung cancer (NSCLC) progression is reduced by 63% for patients with epidermal growth factor receptor (EGFR) mutations who take tyrosine kinase inhibitors over chemotherapy, and the reduction is 50% greater for exon 19 deletions compared with exon 21 leucine to arginine substitutions.
Major finding: Patients with exon 19 deletions had prolonged PFS compared with exon 21 L858R substitutions: hazard ratios were 0.24 (95% CI, 0.20-0.29) and 0.48 (0.39-0.58), respectively (Pinteraction < .001).
Data source: A meta-analysis of seven trials that compared EGFR tyrosine kinase inhibitors with chemotherapy in a total of 1,649 patients with NSCLC and EGFR mutations.
Disclosures: Dr. Chee Khoon Lee reported receiving funding from GlaxoSmithKline and Boehringer Ingelheim. Many of his coauthors reported ties to several industry sources.
Alzheimer’s drug improves cognitive function after RT for brain tumors
Adult brain tumor survivors taking donepezil, a drug approved for the treatment of Alzheimer’s disease, showed significant improvements in the cognitive functions of memory, motor speed, and dexterity, compared with those taking a placebo. However, improvements in the primary outcome of composite cognitive function were similar for the two arms, investigators reported.
The study results were published online April 20 in the Journal of Clinical Oncology.
Patients with greater pretreatment deficits saw greater improvements in cognitive functioning with donepezil treatment, reported Stephen Rapp, Ph.D., professor of psychiatry and behavioral medicine at Wake Forest School of Medicine, Winston-Salem, N.C., and associates.
“This suggests that treatment with a daily dose of donepezil can provide benefit to some adult long-term brain tumor survivors after PBI or WBI [partial- or whole-brain irradiation], particularly those with greater pretreatment cognitive impairment,” they wrote (J. Clin. Oncol. 2015 Apr. 20 [doi: 10.1200/JCO.2014.58.4508]).
The phase III trial enrolled 198 primary or metastatic brain tumor survivors who underwent fractionated PBI or WBI at least 6 months previously. Patients received either donepezil at 5 mg daily for 6 weeks, followed by 10 mg daily for 18 weeks if well tolerated, or placebo for 24 weeks. Composite cognitive scores improved for both arms and did not differ significantly. Donepezil treatment resulted in significantly greater improvements in memory (recognition, P = .027; discrimination, P = .007) and motor speed and dexterity (P = .016).
Donepezil was generally well tolerated, except for diarrhea in 25% of the active arm vs. 9% in the placebo arm (P = .005). The study retention rate was 74% at 24 weeks for both groups.
Although enrolled patients had a high level of cognitive impairment relative to noncancer controls, with 91% having at least one test score at least 1.5 standard deviations below the normal comparison group, scores across most measures varied widely from significantly lower to higher than the comparison group. This heterogeneity may underlie the less than significant improvement observed with the study treatment. Patients with greater cognitive deficits saw greater benefits.
“This indicates that brain tumors and their treatments, including cranial irradiation, are associated with clinically significant cognitive impairment among some but not all patients. In future studies, demonstrable cognitive impairment should be an inclusion criterion for enrollment,” Dr. Rapp and associates wrote.
The study by Rapp et al. suggests that for brain tumor survivors experiencing cognitive difficulties, intervention with donepezil, a drug approved for use in Alzheimer’s disease (AD), may be helpful. Although average improvements were small, the trial indicates clear benefit for some patients, especially those most impaired. The study followed patients taking the drug for 6 months, but patients who responded to treatment likely will continue with lifelong therapy, based on experience with donepezil in AD. After cessation of the agent, neurocognitive function of treated AD patients declined to the level of untreated patients.
The results of the current study justify administering the drug to affected patients and monitoring for effects. In the absence of evidence of clinical benefit, the data do not support continuing treatment. Donepezil use in AD is continued for some patients even without signs of improvement, on the basis of slowing expected decline. However, cognitive declines due to tumor and treatment injury do not progress over time, and donepezil use in this population is supported only with evidence of benefit.
Optimal dosing for cancer patients requires further study, but studies with AD patients showed no clear benefit of dose escalation that outweighed GI adverse effects.
Maintaining maximal cognitive functioning in patients who often begin treatment with brain injury due to the tumor and unrelated illnesses, requires first the prevention of further damage. Strategies include functional image-guided surgery, limiting daily radiation fraction size, improved image-guided radiotherapy target definition, highly conformal radiotherapy administration techniques, and highly focused stereotactic radiosurgery in place of whole-brain radiotherapy for many patients with brain metastasis. Research on improvements in imaging of tumor and functional brain to better guide surgery and radiation is worthwhile.
Neurocognitive rehabilitation is recommended for patients with cognitive deficits that persist after therapy had ended. A recent randomized study showed clear benefit of rehabilitation for attention, verbal memory, and mental fatigue.
By taking steps to prevent injury, rehabilitate patients with deficits, and administer drug therapies while monitoring for benefit, improvements to cognitive function in brain tumor survivors may begin to increase. To best employ these strategies, a validated, easy-to-use instrument that measures mild to moderate impairment is needed for routine oncology practice.
Dr. Lawrence Kleinberg is associate professor of radiation oncology and molecular radiation sciences at Johns Hopkins Sidney Kimmel Comprehensive Cancer Center, Baltimore. These comments were excerpted from the editorial accompanying the report by Dr. Rapp et al. (J. Clin. Oncol. 2015 April 20 [doi: 10.1200/JCO.2014.60.2805]).
The study by Rapp et al. suggests that for brain tumor survivors experiencing cognitive difficulties, intervention with donepezil, a drug approved for use in Alzheimer’s disease (AD), may be helpful. Although average improvements were small, the trial indicates clear benefit for some patients, especially those most impaired. The study followed patients taking the drug for 6 months, but patients who responded to treatment likely will continue with lifelong therapy, based on experience with donepezil in AD. After cessation of the agent, neurocognitive function of treated AD patients declined to the level of untreated patients.
The results of the current study justify administering the drug to affected patients and monitoring for effects. In the absence of evidence of clinical benefit, the data do not support continuing treatment. Donepezil use in AD is continued for some patients even without signs of improvement, on the basis of slowing expected decline. However, cognitive declines due to tumor and treatment injury do not progress over time, and donepezil use in this population is supported only with evidence of benefit.
Optimal dosing for cancer patients requires further study, but studies with AD patients showed no clear benefit of dose escalation that outweighed GI adverse effects.
Maintaining maximal cognitive functioning in patients who often begin treatment with brain injury due to the tumor and unrelated illnesses, requires first the prevention of further damage. Strategies include functional image-guided surgery, limiting daily radiation fraction size, improved image-guided radiotherapy target definition, highly conformal radiotherapy administration techniques, and highly focused stereotactic radiosurgery in place of whole-brain radiotherapy for many patients with brain metastasis. Research on improvements in imaging of tumor and functional brain to better guide surgery and radiation is worthwhile.
Neurocognitive rehabilitation is recommended for patients with cognitive deficits that persist after therapy had ended. A recent randomized study showed clear benefit of rehabilitation for attention, verbal memory, and mental fatigue.
By taking steps to prevent injury, rehabilitate patients with deficits, and administer drug therapies while monitoring for benefit, improvements to cognitive function in brain tumor survivors may begin to increase. To best employ these strategies, a validated, easy-to-use instrument that measures mild to moderate impairment is needed for routine oncology practice.
Dr. Lawrence Kleinberg is associate professor of radiation oncology and molecular radiation sciences at Johns Hopkins Sidney Kimmel Comprehensive Cancer Center, Baltimore. These comments were excerpted from the editorial accompanying the report by Dr. Rapp et al. (J. Clin. Oncol. 2015 April 20 [doi: 10.1200/JCO.2014.60.2805]).
The study by Rapp et al. suggests that for brain tumor survivors experiencing cognitive difficulties, intervention with donepezil, a drug approved for use in Alzheimer’s disease (AD), may be helpful. Although average improvements were small, the trial indicates clear benefit for some patients, especially those most impaired. The study followed patients taking the drug for 6 months, but patients who responded to treatment likely will continue with lifelong therapy, based on experience with donepezil in AD. After cessation of the agent, neurocognitive function of treated AD patients declined to the level of untreated patients.
The results of the current study justify administering the drug to affected patients and monitoring for effects. In the absence of evidence of clinical benefit, the data do not support continuing treatment. Donepezil use in AD is continued for some patients even without signs of improvement, on the basis of slowing expected decline. However, cognitive declines due to tumor and treatment injury do not progress over time, and donepezil use in this population is supported only with evidence of benefit.
Optimal dosing for cancer patients requires further study, but studies with AD patients showed no clear benefit of dose escalation that outweighed GI adverse effects.
Maintaining maximal cognitive functioning in patients who often begin treatment with brain injury due to the tumor and unrelated illnesses, requires first the prevention of further damage. Strategies include functional image-guided surgery, limiting daily radiation fraction size, improved image-guided radiotherapy target definition, highly conformal radiotherapy administration techniques, and highly focused stereotactic radiosurgery in place of whole-brain radiotherapy for many patients with brain metastasis. Research on improvements in imaging of tumor and functional brain to better guide surgery and radiation is worthwhile.
Neurocognitive rehabilitation is recommended for patients with cognitive deficits that persist after therapy had ended. A recent randomized study showed clear benefit of rehabilitation for attention, verbal memory, and mental fatigue.
By taking steps to prevent injury, rehabilitate patients with deficits, and administer drug therapies while monitoring for benefit, improvements to cognitive function in brain tumor survivors may begin to increase. To best employ these strategies, a validated, easy-to-use instrument that measures mild to moderate impairment is needed for routine oncology practice.
Dr. Lawrence Kleinberg is associate professor of radiation oncology and molecular radiation sciences at Johns Hopkins Sidney Kimmel Comprehensive Cancer Center, Baltimore. These comments were excerpted from the editorial accompanying the report by Dr. Rapp et al. (J. Clin. Oncol. 2015 April 20 [doi: 10.1200/JCO.2014.60.2805]).
Adult brain tumor survivors taking donepezil, a drug approved for the treatment of Alzheimer’s disease, showed significant improvements in the cognitive functions of memory, motor speed, and dexterity, compared with those taking a placebo. However, improvements in the primary outcome of composite cognitive function were similar for the two arms, investigators reported.
The study results were published online April 20 in the Journal of Clinical Oncology.
Patients with greater pretreatment deficits saw greater improvements in cognitive functioning with donepezil treatment, reported Stephen Rapp, Ph.D., professor of psychiatry and behavioral medicine at Wake Forest School of Medicine, Winston-Salem, N.C., and associates.
“This suggests that treatment with a daily dose of donepezil can provide benefit to some adult long-term brain tumor survivors after PBI or WBI [partial- or whole-brain irradiation], particularly those with greater pretreatment cognitive impairment,” they wrote (J. Clin. Oncol. 2015 Apr. 20 [doi: 10.1200/JCO.2014.58.4508]).
The phase III trial enrolled 198 primary or metastatic brain tumor survivors who underwent fractionated PBI or WBI at least 6 months previously. Patients received either donepezil at 5 mg daily for 6 weeks, followed by 10 mg daily for 18 weeks if well tolerated, or placebo for 24 weeks. Composite cognitive scores improved for both arms and did not differ significantly. Donepezil treatment resulted in significantly greater improvements in memory (recognition, P = .027; discrimination, P = .007) and motor speed and dexterity (P = .016).
Donepezil was generally well tolerated, except for diarrhea in 25% of the active arm vs. 9% in the placebo arm (P = .005). The study retention rate was 74% at 24 weeks for both groups.
Although enrolled patients had a high level of cognitive impairment relative to noncancer controls, with 91% having at least one test score at least 1.5 standard deviations below the normal comparison group, scores across most measures varied widely from significantly lower to higher than the comparison group. This heterogeneity may underlie the less than significant improvement observed with the study treatment. Patients with greater cognitive deficits saw greater benefits.
“This indicates that brain tumors and their treatments, including cranial irradiation, are associated with clinically significant cognitive impairment among some but not all patients. In future studies, demonstrable cognitive impairment should be an inclusion criterion for enrollment,” Dr. Rapp and associates wrote.
Adult brain tumor survivors taking donepezil, a drug approved for the treatment of Alzheimer’s disease, showed significant improvements in the cognitive functions of memory, motor speed, and dexterity, compared with those taking a placebo. However, improvements in the primary outcome of composite cognitive function were similar for the two arms, investigators reported.
The study results were published online April 20 in the Journal of Clinical Oncology.
Patients with greater pretreatment deficits saw greater improvements in cognitive functioning with donepezil treatment, reported Stephen Rapp, Ph.D., professor of psychiatry and behavioral medicine at Wake Forest School of Medicine, Winston-Salem, N.C., and associates.
“This suggests that treatment with a daily dose of donepezil can provide benefit to some adult long-term brain tumor survivors after PBI or WBI [partial- or whole-brain irradiation], particularly those with greater pretreatment cognitive impairment,” they wrote (J. Clin. Oncol. 2015 Apr. 20 [doi: 10.1200/JCO.2014.58.4508]).
The phase III trial enrolled 198 primary or metastatic brain tumor survivors who underwent fractionated PBI or WBI at least 6 months previously. Patients received either donepezil at 5 mg daily for 6 weeks, followed by 10 mg daily for 18 weeks if well tolerated, or placebo for 24 weeks. Composite cognitive scores improved for both arms and did not differ significantly. Donepezil treatment resulted in significantly greater improvements in memory (recognition, P = .027; discrimination, P = .007) and motor speed and dexterity (P = .016).
Donepezil was generally well tolerated, except for diarrhea in 25% of the active arm vs. 9% in the placebo arm (P = .005). The study retention rate was 74% at 24 weeks for both groups.
Although enrolled patients had a high level of cognitive impairment relative to noncancer controls, with 91% having at least one test score at least 1.5 standard deviations below the normal comparison group, scores across most measures varied widely from significantly lower to higher than the comparison group. This heterogeneity may underlie the less than significant improvement observed with the study treatment. Patients with greater cognitive deficits saw greater benefits.
“This indicates that brain tumors and their treatments, including cranial irradiation, are associated with clinically significant cognitive impairment among some but not all patients. In future studies, demonstrable cognitive impairment should be an inclusion criterion for enrollment,” Dr. Rapp and associates wrote.
FROM THE JOURNAL OF CLINICAL ONCOLOGY
Key clinical point: Among adult brain tumor survivors who underwent partial- or whole-brain irradiation, treatment with donepezil compared with placebo was associated with significant improvements in memory and motor speed and dexterity, but composite cognitive scores were similar.
Major finding: After 24 weeks of treatment, patients taking donepezil (vs. placebo) had significantly more improvement in memory (recognition, P = .027; discrimination, P = .007) and motor speed and dexterity (P = .016).
Data source: A double-blind phase III trial that randomized 198 patients who had undergone irradiation at least 6 months previously to receive donepezil (5 mg for 6 weeks followed by 10 mg for 18 weeks) or placebo (24 weeks).
Disclosures: Dr. Rapp reported having no financial disclosures.