User login
Contraceptive care best practices
While the unintended pregnancy rate for women ages 15 to 44 years decreased by 18% between 2008 and 2011, almost half of pregnancies in the United States remain unintended.1 On a more positive note, however, women who use birth control consistently and correctly account for only 5% of unintended pregnancies.2 As family physicians (FPs), we can support and facilitate our female patients’ efforts to consistently use highly effective forms of contraception. The 5 initiatives detailed here can help toward that end.
1. Routinely screen patients for their reproductive intentions
All women of reproductive age should be screened routinely for their pregnancy intentions. The American College of Obstetricians and Gynecologists (ACOG) encourages clinicians to ask women about pregnancy intendedness and encourages patients to develop a reproductive life plan, or a set of personal goals about whether or when to have children.3 The Centers for Disease Control and Prevention (CDC) has also developed a reproductive life plan tool for health professionals to encourage women and men to reflect upon their plans.4 So just as we regularly screen and document cigarette use and blood pressure (BP), so too, should we routinely screen women for their reproductive goals.
Ask women this one question. The Oregon Foundation for Reproductive Health launched the One Key Question Initiative, which proposes that the care team ask women ages 18 to 50: “Would you like to become pregnant in the next year?”5 A common workflow includes the medical assistant asking women about pregnancy intentions and providing a preconception and/or contraceptive handout, if appropriate. The physician provides additional counseling as needed. Pilot studies of One Key Question indicate that 30% to 40% of women screened needed follow-up counseling, suggesting the need for clinicians to be proactive in asking about reproductive plans. (Additional information on the Initiative is available on the Foundation’s Web site at http://www.orfrh.org/.)
This approach assumes women feel in control of their reproduction; however, this may not be the reality for many, especially low-income women.6 Additionally, women commonly cite planning a pregnancy as appropriate only when they are in an ideal relationship and when they are living in a financially stable environment—conditions that some women may never achieve.
Another caveat is that women may not have explicit pregnancy intentions, in which case, this particular approach may not be effective. A study of low-income women found only 60% intended to use the method prescribed after contraception counseling, with 37% of those stopping because of adverse effects, 23% saying they wanted another method, and 17% citing method complexity.7
Reproductive coercion from male partners, ranging from pressure to become pregnant to method sabotage, is also common in low-income women.8 Regular conversations that prioritize a woman’s values and experience are needed to promote reproductive autonomy.
2. Decouple provision of contraception from unnecessary exams
Pelvic exams and pap smears should not be required prior to offering patients hormonal contraception, according to the Choosing Wisely campaign of the American Board of Internal Medicine and ACOG.9,10 Hormonal contraception may instead be provided safely based on a medical history and BP assessment. Adolescents, minority groups, obese women, and victims of sexual trauma, in particular may avoid asking about birth control because of anxiety and fear of pain from these exams.11 The American College of Physicians recommends against speculum and bimanual exams in asymptomatic, non-pregnant, adult women.12 Pap smears and sexually transmitted infection (STI) testing should be performed at their normally scheduled intervals as recommended by the US Preventive Services Task Force (USPSTF) and not be tied to contraceptive provision.13
Assess pregnancy status using criteria,rather than a pregnancy text
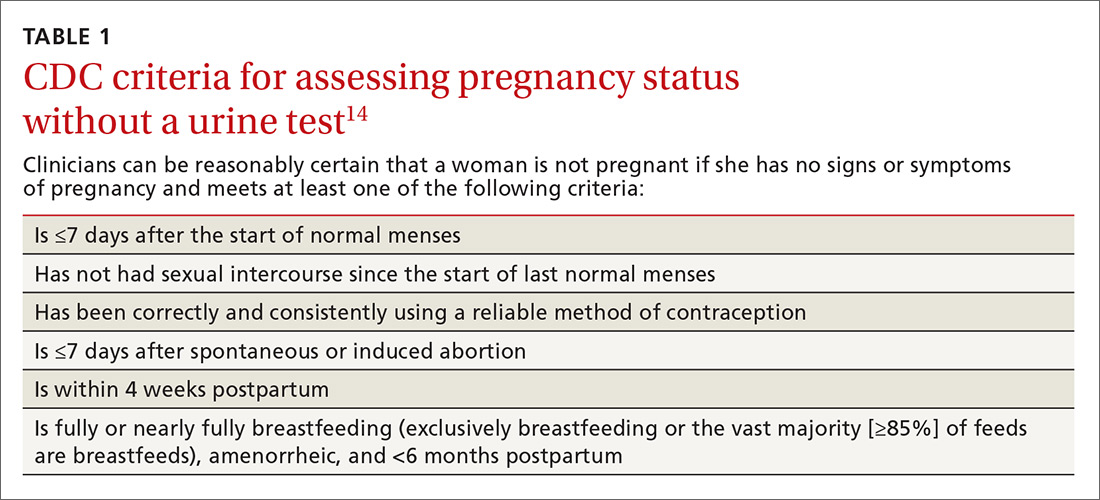
Use the CDC’s criteria to assess pregnancy status rather than relying on a urine pregnancy test prior to providing contraception. Once you are reasonably sure that a woman is not pregnant (TABLE 114), contraception may be started. Some physicians have traditionally requested that a woman delay starting contraception until the next menses to ensure that she is not already pregnant. However, given the evidence that hormonal contraception does not cause birth defects, such a delay is not warranted and puts the woman at risk of an unintended pregnancy during the gap.15
Furthermore, there is an approximate 2-week window in which a woman could have a negative urine pregnancy test despite being pregnant, so the test alone is not completely reliable. In addition, obese women may experience irregular cycles, further complicating the traditional approach.16
Another largely unnecessary step … The US Selected Practice Recommendations (US SPR) from the CDC notes that additional STI screening prior to an intrauterine device (IUD) insertion is unnecessary for most women if appropriate screening guidelines have been previously followed.14 For those who have not been screened according to guidelines, the CDC recommends same-day screening and IUD insertion. You can then treat an STI without removing the IUD. Women with purulent cervicitis or a current chlamydial or gonorrheal infection should delay IUD insertion until after treatment.
3. Expand long-acting reversible contraception counseling and access
Offer long-acting reversible contraception (LARC), such as IUDs and implants, as first-line options for most women. ACOG endorses LARC as the most effective reversible method for most women, including those who have not given birth and adolescents.17 Unfortunately, a 2012 study found that family physicians were less likely than OB-GYNs to have enough time for contraceptive counseling and fewer than half felt competent inserting IUDs.18 While 79% of OB-GYNs routinely discussed IUDs with their patients, only 47% of family physicians did. In 2014, the American Academy of Pediatrics (AAP) endorsed a LARC-first tiered counseling approach for adolescents.19
A test of LARC-first counseling
The Contraceptive CHOICE project, a St. Louis, Missouri-based initiative, was launched to reduce unintended pregnancies in women ages 14 to 45 years by offering LARC-first counseling and free contraception of their choice.20 This project involved more than 9000 women at high risk for unintended pregnancy. Same-day LARC insertion was available. Seventy-five percent of women chose a LARC method and they reported greater continuation at 12 and 24 months, when compared to women who did not choose a LARC method. LARC users also reported higher satisfaction at one year. Provision of contraception through the project contributed to a reduction in repeat abortions as well as decreased rates of teenage pregnancy, birth, and abortion. Three years after the start of the project, IUDs had continuation rates of nearly 70%, implants of 56%, and non-LARC methods of 31%.21
When counseling women, it’s important to remember that effectiveness may not be the only criterium a woman uses when choosing a method. A 2010 study found that for 91% of women at high risk for unintended pregnancy, no single method possessed all the features they deemed “extremely important.”22 Clinicians should take a patient-centered approach to find birth control that fits each patient’s priorities.
Clinicians need proper training in LARC methods
Only 20% of FPs regularly insert IUDs, and 11% offer contraceptive implants, according to estimates from physicians recertifying with the American Board of Family Medicine in 2014.23 Access to training during residency is a key component to increasing these rates. FPs who practice obstetrics should be trained in postpartum LARC insertion and offer this option prior to hospital discharge as well as during the postpartum office visit.
Performing LARC insertions on the same day as counseling is ideal, and clinics should strive to reduce barriers to same-day procedures. Time constraints may be addressed by shifting tasks among the medical team. In the CHOICE project, contraceptive counselors—half of whom had no clinical experience—were trained to provide tiered counseling to participants. By working with a cross-trained health care team and offering prepared resources, clinicians can save time and improve access.
Physicians may want to incorporate the free online resources Bedsider.org or Stayteen.org to help women learn about contraceptive methods.24 The user-friendly Web sites, operated by the National Campaign to Prevent Teen and Unplanned Pregnancy, describe various forms of contraception and offer text and email reminders. Incorporating Bedsider into the counseling workflow and discussing the various reminder tools available may improve patients’ knowledge and enhance their compliance.
Additional barriers for practices may include high upfront costs associated with stocking devices. Practices that may be unable to sustain the costs surrounding enhanced contraception counseling and provision can collaborate with family planning clinics that are able to offer same-day services. A study of clinics in California found that Title X clinics were more likely to provide on-site LARC services than non-Title X public and private providers.25
4. Follow CDC guidelines for initiating and continuing contraception
Follow the US SPR for guidance on initiating and continuing contraceptive methods.14 The CDC’s Medical Eligibility Criteria for Contraceptive Use is another vital resource, providing recommendations for contraceptive methods to patients who have specific medical conditions or characteristics.26
Utilize the “quick start” method for hormonal contraception, where birth control is started on the same day as its prescription regardless of timing of the menstrual cycle. If you can’t be reasonably certain that a woman is not pregnant based on the criteria listed in TABLE 1,14 conduct a pregnancy test (while recognizing the aforementioned 2-week window of limitations) and counsel the patient to use back-up protection for the first 7 days along with repeating a pregnancy test in 2 weeks’ time.
The quick start method may lead to higher adherence than delayed initiation.27 Differences in continuation rates between women who use the quick start method and those who follow the delayed approach may disappear over time.28
Prescribe and provide a year’s supply of oral contraceptive pills (OCPs) as recommended by the CDC US SPR.14 It is important to note that pharmacists are usually restricted by insurance companies to only fill a one or 3 month’s supply.
In January 2016, Oregon began requiring private and state health insurance providers to reimburse for a year’s supply of prescription contraception; in January 2017, insurers in Washington, DC, were also required to offer women a year’s supply of prescription contraception.29,30 Several other states have followed suit. The California Health Benefits Review Program estimates a savings of $42.8 million a year from fewer office visits and 15,000 fewer unintended pregnancies if their state enacts a similar policy.31
Pharmacist initiatives are worth watching. In January 2016, Oregon pharmacists with additional training were allowed to prescribe OCs and hormonal patches to women 18 years and older.32 In April 2016, a similar law went into effect in California, but without a minimum age requirement and with the additional coverage of vaginal rings and Depo-Provera (depo) injections.33 Pharmacists in both states must review a health questionnaire completed by the woman and can refer to a physician as necessary.
The CDC recommends that clinicians extend the allowed window for repeat depo injections to 15 weeks.14 Common institutional protocol is to give repeat injections every 11 to 13 weeks. If past that window, protocol often dictates the woman abstain from unprotected sex for 2 weeks and then return for a negative pregnancy test (or await menses) before the next injection. However, the CDC notes that depo is effective for longer than the 13-week period.14 No additional birth control or pregnancy testing is needed and the woman can receive the next depo shot if she is up to 15 weeks from the previous shot.
One study found no additional pregnancy risks for those who were up to 4 weeks “late” for their next shot, suggesting there is potential for an even larger grace period.34 The World Health Organization advises allowing a repeat injection up to 4 weeks late.35 We encourage institutions to change their policies to comply with the CDC’s 15-week window.
Another initiative is over-the-counter (OTC) access to OCs, which the American Academy of Family Physicians (AAFP) and ACOG support.36,37 ACOG notes that “no drug or intervention is completely without risk of harm” and that the risk of venous thromboembolism for OC users is lower than the risk of pregnancy.37 Women can successfully self-screen for contraindications using a checklist. Concerns about women potentially being less adherent or less likely to choose LARCs are not reasons to preclude access to other methods. The AAFP supports insurance coverage of OCs, regardless of prescription status.36
5. Routinely counsel about, and advance-prescribe, emergency contraception pills
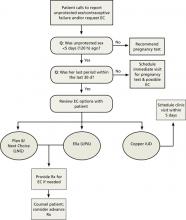
Physicians should counsel and advance-prescribe emergency contraception pills (ECPs) to women, including adolescents, using less reliable contraception, as recommended by ACOG, AAP, and the CDC.14,37,38 It’s also important to provide information on the copper IUD as the most effective method of emergency contraception, with nearly 100% efficacy if placed within 5 days.39 An easy-to-read patient hand-out in English and Spanish on EC options can be found at http://beyondthepill.ucsf.edu/tools-materials.
Only 3% of respondents participating in the 2006-2010 National Survey of Family Growth received counseling about emergency contraception in the past year.40 ECPs are most effective when used within 24 hours but have some efficacy up to 5 days.37 Due to the Affordable Care Act, most insurance plans will cover ECPs if purchased with a prescription, but coverage varies by state.41 Ulipristal acetate (UPA) ECP is only available with a prescription. Advance prescriptions can alleviate financial burdens on women when they need to access ECPs quickly.
Women should wait at least 5 days before resuming or starting hormonal contraception after taking UPA-based ECP, as it may reduce the ovulation-delaying effect of the ECP.14 For IUDs, implants, and depo, which require a visit to a health care provider, physicians evaluating earlier provision should consider the risks of reduced efficacy against the many barriers to access.
UPA-based ECPs (such as ella) may be more effective for overweight and obese women than levonorgestrel-based ECPs (such as Plan B and Next Choice).14 Consider advance-prescribing UPA ECPs to women with a body mass index (BMI) >25 kg/m2.42 Such considerations are important as the prevalence of obesity in women between 2013 and 2014 was 40.4%.43
In May 2016, the FDA noted that while current data are insufficient regarding whether the effectiveness of levonorgestrel ECPs is reduced in overweight or obese women, there are no safety concerns regarding their use in this population.44 Therefore, a woman with a BMI >25 kg/m2 should use UPA ECPs if available; but if not, she can still use levonorgestrel ECPs. One study, however, has found that UPA ECPs are only as effective as a placebo when BMI is ≥35 kg/m2, at which point a copper IUD may be the only effective form of emergency contraception.45
Transitioning from customary practices to best practices
Following these practical steps, FPs can improve contraceptive care for women. However, to make a significant impact, clinicians must be willing to change customary practices that are based on tradition, routines, or outdated protocols in favor of those based on current evidence.
One good place to start the transition to best practices is to familiarize yourself with the 2016 US Medical Eligibility Criteria for Contraceptive Use26 and Selected Practice Recommendations for Contraceptive Use.14 TABLES 214,26,46,47 and 3 offer additional resources that can enhance contraceptive counseling and further promote access to contraceptive care.
The contraceptive coverage guarantee under the Affordable Care Act has allowed many women to make contraceptive choices based on personal needs and preferences rather than cost. The new contraceptive coverage exemptions issued under the Trump administration will bring cost back as the driving decision factor for women whose employers choose not to provide contraceptive coverage. Providers should be aware of the typical costs associated with the various contraceptive options offered in their practice and community.
CORRESPONDENCE
Jessica Dalby, MD, Department of Family Medicine and Community Health, University of Wisconsin School of Medicine and Public Health, 1102 South Park St, Suite 100, Madison, WI 53715; Jessica.Dalby@fammed.wisc.edu.
1. Finer LB, Zolna MR. Declines in unintended pregnancy in the United States, 2008–2011. N Engl J Med. 2016; 374:843-852.
2. Sonfield A, Hasstedt K, Gold RB. Moving Forward: Family Planning in the Era of Health Reform. New York: Guttmacher Institute. 2014. Available at: https://www.guttmacher.org/report/moving-forward-family-planning-era-health-reform. Accessed October 5, 2017.
3. Committee on Health Care for Underserved Women. Reproductive Life Planning to Reduce Unintended Pregnancy: American College of Obstetricians and Gynecologists. 2016. Available at: https://www.acog.org/Resources-And-Publications/Committee-Opinions/Committee-on-Health-Care-for-Underserved-Women/Reproductive-Life-Planning-to-Reduce-Unintended-Pregnancy. Accessed October 5, 2017.
4. Centers for Disease Control and Prevention. Reproductive Life Plan Tool for Health Care Providers. 2016. Available at: http://www.cdc.gov/preconception/rlptool.html. Accessed August 31, 2016.
5. Oregon Health Authority. Effective Contraceptive Use among Women at Risk of Unintended Pregnancy Guidance Document. 2014. Available at: http://www.oregon.gov/oha/HPA/ANALYTICS/CCOData/Effective%20Contraceptive%20Use%20Guidance%20Document.pdf. Accessed October 5, 2017.
6. Borrero S, Nikolajski C, Steinberg JR, et al. “It just happens”: a qualitative study exploring low-income women’s perspectives on pregnancy intention and planning. Contraception. 2015;91:150-156.
7. Yee LM, Farner KC, King E, et al. What do women want? Experiences of low-income women with postpartum contraception and contraceptive counseling. J Pregnancy Child Health. 2015;2.
8. Kalichman SC, Williams EA, Cherry C, et al. Sexual coercion, domestic violence, and negotiating condom use among low-income African American women. J Womens Health. 1998;7:371-378.
9. ABIM Foundation. Pelvic Exams, Pap Tests and Oral Contraceptives. 2016. Available at: http://www.choosingwisely.org/patient-resources/pelvic-exams-pap-tests-and-oral-contraceptives/. Accessed May 31, 2016.
10. Committee on Health Care for Underserved Women. Access to Contraception: American College of Obstetricians and Gynecologists. 2015. Number 615. Available at: https://www.acog.org/-/media/Committee-Opinions/Committee-on-Health-Care-for-Underserved-Women/co615.pdf?dmc=1&ts=201710. Accessed October 5, 2017.
11. Bates CK, Carroll N, Potter J. The challenging pelvic examination. J Gen Intern Med. 2011;26:651-657.
12. Qaseem A, Humphrey LL, Harris R, et al. Screening pelvic examination in adult women: a clinical practice guideline from the American College of Physicians. Ann Intern Med. 2014;161:67-72.
13. U.S. Preventive Services Task Force. Cervical Cancer: Screening. 2012. Available at: http://www.uspreventiveservicestaskforce.org/Page/Document/UpdateSummaryFinal/cervical-cancer-screening. Accessed May 25, 2016.
14. Curtis KM, Jatlaoui TC, Tepper NK, et al. U.S. selected practice recommendations for contraceptive use, 2016. MMWR Morb Mortal Wkly Rep. 2016;65:1-66.
15. Lesnewski R, Prine L. Initiating hormonal contraception. Am Fam Physician. 2006;74:105-112.
16. Jacobsen BK, Knutsen SF, Oda K, et al. Obesity at age 20 and the risk of miscarriages, irregular periods and reported problems of becoming pregnant: the Adventist Health Study-2. Eur J Epidemiol. 2012; 27:923-931.
17. Committee on Gynecologic Practice. Increasing Access to Contraceptive Implants and Intrauterine Devices to Reduce Unintended Pregnancy: American College of Obstetricians and Gynecologists. 2015. Number 642. Available at: https://www.acog.org/Resources-And-Publications/Committee-Opinions/Committee-on-Gynecologic-Practice/Increasing-Access-to-Contraceptive-Implants-and-Intrauterine-Devices-to-Reduce-Unintended-Pregnancy. Accessed October 5, 2017.
18. Harper CC, Henderson JT, Raine TR, et al. Evidence-based IUD practice: family physicians and obstetrician-gynecologists. Fam Med. 2012;44:637-645.
19. American Academy of Pediatrics, Committee on Adolescence. Policy statement: Contraception for Adolescents. 2014. Available at: http://pediatrics.aappublications.org/content/pediatrics/early/2014/09/24/peds.2014-2299.full.pdf. Accessed October 5, 2017.
20. Birgisson NE, Zhao Q, Secura GM, et al. Preventing unintended pregnancy: The Contraceptive CHOICE Project in review. J Womens Health (Larchmt). 2015;24:349-353.
21. Diedrich JT, Zhao Q, Madden T, et al. Three-year continuation of reversible contraception. Am J Obstet Gynecol. 2015;213:662.e1-e8.
22. Lessard LN, Karasek D, Ma S, et al. Contraceptive features preferred by women at high risk of unintended pregnancy. Perspect Sex Reprod Health. 2012;44:194-200.
23. Nisen MB, Peterson LE, Cochrane A, et al. US family physicians’ intrauterine and implantable contraception provision: results from a national survey. Contraception. 2016;93:432-437.
24. National Campaign to Prevent Teen and Unplanned Pregnancy. Bedsider. Available at: https://bedsider.org/. Accessed June 14, 2016.
25. Park HY, Rodriguez MI, Hulett D, et al. Long-acting reversible contraception method use among Title X providers and non-Title X providers in California. Contraception. 2012;86:557-561.
26. Curtis KM, Tepper NK, Jatlaoui TC, et al. U.S. medical eligibility criteria for contraceptive use, 2016. MMWR Morb Mortal Wkly Rep. 2016;65:1-103.
27. Westhoff C, Kerns J, Morroni C, et al. Quick start: novel oral contraceptive initiation method. Contraception. 2002;66:141-145.
28. Brahmi D, Curtis KM. When can a woman start combined hormonal contraceptives (CHCs)? A systematic review. Contraception. 2013;87:524-538.
29. Lachman S. Oregon To Require Insurers To Cover A Year’s Supply Of Birth Control. Huffington Post. June 11, 2015. Available at: https://www.huffingtonpost.com/2015/06/11/oregon-birth-control-_n_7564712.html. Accessed October 16, 2017.
30. Andrews M. D.C. Women To Get Access To Full Year’s Worth Of Contraceptives. Kaiser Health News. September 25, 2015. Available at: https://khn.org/news/d-c-women-to-get-access-to-full-years-worth-of-contraceptives/. Accessed October 16, 2017.
31. Analysis of California Senate Bill (SB) 999 Contraceptives: Annual Supply: A Report to the 2015-2016 California State Legislature: California Health Benefits Review Program. 2016. Available at: http://chbrp.ucop.edu/index.php?action=read&bill_id=195&doc_type=1000. Accessed October 5, 2017.
32. Frazier A. Pharmacist-prescribed birth control in effect Jan 1. KOIN News. December 30, 2015. Available at: http://koin.com/2015/12/30/pharmacist-provided-birth-control-in-effect-jan-1/. Accessed October 5, 2017.
33. Karlamangla S. Birth control pills without prescriptions, coming soon to California under new law. Los Angeles Times. February 14, 2016. Available at: http://www.latimes.com/health/la-me-birth-control-pharmacies-20160214-story.html. Accessed October 16, 2017.
34. Steiner MJ, Kwok C, Stanback J, et al. Injectable contraception: what should the longest interval be for reinjections? Contraception. 2008;77:410-414.
35. World Health Organization. Family Planning: A Global Handbook for Providers. 2011. Available at: http://apps.who.int/iris/bitstream/10665/44028/1/9780978856373_eng.pdf. Accessed October 5, 2017.
36. American Academy of Family Physicians. Over-the-Counter Oral Contraceptives. 2014; Available at: http://www.aafp.org/about/policies/all/otc-oral-contraceptives.html. Accessed June 2, 2016.
37. Committee on Gynecologic Practice. Over-the-Counter Access to Oral Contraceptives: American College of Obstetricians and Gynecologists. 2012. Number 544. Available at: https://www.acog.org/Resources-And-Publications/Committee-Opinions/Committee-on-Gynecologic-Practice/Over-the-Counter-Access-to-Oral-Contraceptives. Accessed October 5, 2017.
38. Committee on Adolescence. Emergency contraception. Pediatrics. 2012;130:1174-1182.
39. Cleland K, Zhu H, Goldstuck N, et al. The efficacy of intrauterine devices for emergency contraception: a systematic review of 35 years of experience. Hum Reprod. 2012;27:1994-2000.
40. Martinez G, Chandra A, Febo-Vazquez I, et al. Use of Family Planning and Related Medical Services Among Women Aged 15–44 in the United States: National Survey of Family Growth, 2006–2010: National Center for Health Statistics, Centers for Disease Control and Prevention. 2013. Available at: https://www.cdc.gov/nchs/data/nhsr/nhsr068.pdf. Accessed October 5, 2017.
41. Guttmacher Institute. Insurance Coverage of Contraceptives: Guttmacher Institute;2017. Available at: https://www.guttmacher.org/state-policy/explore/insurance-coverage-contraceptives Accessed October 7, 2017.
42. Glasier A, Cameron ST, Blithe D, et al. Can we identify women at risk of pregnancy despite using emergency contraception? Data from randomized trials of ulipristal acetate and levonorgestrel. Contraception. 2011;84:363-367.
43. Flegal KM, Kruszon-Moran D, Carroll MD, et al. Trends in obesity among adults in the United States, 2005 to 2014. JAMA. 2016;315:2284-2291.
44. US Food & Drug Administration. Postmarket Drug Safety Information for Patients and Providers - Plan B (0.75mg levonorgestrel) and Plan B One-Step (1.5 mg levonorgestrel) Tablets Information. 2016; Available at: http://www.fda.gov/Drugs/DrugSafety/PostmarketDrugSafetyInformationforPatientsandProviders/ucm109775.htm. Accessed May 25, 2016.
45. Simmons KB, Edelman AB. Contraception and sexual health in obese women. Best Pract Res Clin Obstet Gynaecol. 2015;29:466-478.
46. Centers for Disease Control and Prevention. Providing quality family planning services: recommendations of CDC and the U.S. Office of Population Affairs. MMWR Recomm Rep. 2014;63:1-29.
47. LARC FIRST. Available at: http://www.larcfirst.com/index.html. Accessed May 2016.
While the unintended pregnancy rate for women ages 15 to 44 years decreased by 18% between 2008 and 2011, almost half of pregnancies in the United States remain unintended.1 On a more positive note, however, women who use birth control consistently and correctly account for only 5% of unintended pregnancies.2 As family physicians (FPs), we can support and facilitate our female patients’ efforts to consistently use highly effective forms of contraception. The 5 initiatives detailed here can help toward that end.
1. Routinely screen patients for their reproductive intentions
All women of reproductive age should be screened routinely for their pregnancy intentions. The American College of Obstetricians and Gynecologists (ACOG) encourages clinicians to ask women about pregnancy intendedness and encourages patients to develop a reproductive life plan, or a set of personal goals about whether or when to have children.3 The Centers for Disease Control and Prevention (CDC) has also developed a reproductive life plan tool for health professionals to encourage women and men to reflect upon their plans.4 So just as we regularly screen and document cigarette use and blood pressure (BP), so too, should we routinely screen women for their reproductive goals.
Ask women this one question. The Oregon Foundation for Reproductive Health launched the One Key Question Initiative, which proposes that the care team ask women ages 18 to 50: “Would you like to become pregnant in the next year?”5 A common workflow includes the medical assistant asking women about pregnancy intentions and providing a preconception and/or contraceptive handout, if appropriate. The physician provides additional counseling as needed. Pilot studies of One Key Question indicate that 30% to 40% of women screened needed follow-up counseling, suggesting the need for clinicians to be proactive in asking about reproductive plans. (Additional information on the Initiative is available on the Foundation’s Web site at http://www.orfrh.org/.)
This approach assumes women feel in control of their reproduction; however, this may not be the reality for many, especially low-income women.6 Additionally, women commonly cite planning a pregnancy as appropriate only when they are in an ideal relationship and when they are living in a financially stable environment—conditions that some women may never achieve.
Another caveat is that women may not have explicit pregnancy intentions, in which case, this particular approach may not be effective. A study of low-income women found only 60% intended to use the method prescribed after contraception counseling, with 37% of those stopping because of adverse effects, 23% saying they wanted another method, and 17% citing method complexity.7
Reproductive coercion from male partners, ranging from pressure to become pregnant to method sabotage, is also common in low-income women.8 Regular conversations that prioritize a woman’s values and experience are needed to promote reproductive autonomy.
2. Decouple provision of contraception from unnecessary exams
Pelvic exams and pap smears should not be required prior to offering patients hormonal contraception, according to the Choosing Wisely campaign of the American Board of Internal Medicine and ACOG.9,10 Hormonal contraception may instead be provided safely based on a medical history and BP assessment. Adolescents, minority groups, obese women, and victims of sexual trauma, in particular may avoid asking about birth control because of anxiety and fear of pain from these exams.11 The American College of Physicians recommends against speculum and bimanual exams in asymptomatic, non-pregnant, adult women.12 Pap smears and sexually transmitted infection (STI) testing should be performed at their normally scheduled intervals as recommended by the US Preventive Services Task Force (USPSTF) and not be tied to contraceptive provision.13
Assess pregnancy status using criteria,rather than a pregnancy text
Use the CDC’s criteria to assess pregnancy status rather than relying on a urine pregnancy test prior to providing contraception. Once you are reasonably sure that a woman is not pregnant (TABLE 114), contraception may be started. Some physicians have traditionally requested that a woman delay starting contraception until the next menses to ensure that she is not already pregnant. However, given the evidence that hormonal contraception does not cause birth defects, such a delay is not warranted and puts the woman at risk of an unintended pregnancy during the gap.15
Furthermore, there is an approximate 2-week window in which a woman could have a negative urine pregnancy test despite being pregnant, so the test alone is not completely reliable. In addition, obese women may experience irregular cycles, further complicating the traditional approach.16
Another largely unnecessary step … The US Selected Practice Recommendations (US SPR) from the CDC notes that additional STI screening prior to an intrauterine device (IUD) insertion is unnecessary for most women if appropriate screening guidelines have been previously followed.14 For those who have not been screened according to guidelines, the CDC recommends same-day screening and IUD insertion. You can then treat an STI without removing the IUD. Women with purulent cervicitis or a current chlamydial or gonorrheal infection should delay IUD insertion until after treatment.
3. Expand long-acting reversible contraception counseling and access
Offer long-acting reversible contraception (LARC), such as IUDs and implants, as first-line options for most women. ACOG endorses LARC as the most effective reversible method for most women, including those who have not given birth and adolescents.17 Unfortunately, a 2012 study found that family physicians were less likely than OB-GYNs to have enough time for contraceptive counseling and fewer than half felt competent inserting IUDs.18 While 79% of OB-GYNs routinely discussed IUDs with their patients, only 47% of family physicians did. In 2014, the American Academy of Pediatrics (AAP) endorsed a LARC-first tiered counseling approach for adolescents.19
A test of LARC-first counseling
The Contraceptive CHOICE project, a St. Louis, Missouri-based initiative, was launched to reduce unintended pregnancies in women ages 14 to 45 years by offering LARC-first counseling and free contraception of their choice.20 This project involved more than 9000 women at high risk for unintended pregnancy. Same-day LARC insertion was available. Seventy-five percent of women chose a LARC method and they reported greater continuation at 12 and 24 months, when compared to women who did not choose a LARC method. LARC users also reported higher satisfaction at one year. Provision of contraception through the project contributed to a reduction in repeat abortions as well as decreased rates of teenage pregnancy, birth, and abortion. Three years after the start of the project, IUDs had continuation rates of nearly 70%, implants of 56%, and non-LARC methods of 31%.21
When counseling women, it’s important to remember that effectiveness may not be the only criterium a woman uses when choosing a method. A 2010 study found that for 91% of women at high risk for unintended pregnancy, no single method possessed all the features they deemed “extremely important.”22 Clinicians should take a patient-centered approach to find birth control that fits each patient’s priorities.
Clinicians need proper training in LARC methods
Only 20% of FPs regularly insert IUDs, and 11% offer contraceptive implants, according to estimates from physicians recertifying with the American Board of Family Medicine in 2014.23 Access to training during residency is a key component to increasing these rates. FPs who practice obstetrics should be trained in postpartum LARC insertion and offer this option prior to hospital discharge as well as during the postpartum office visit.
Performing LARC insertions on the same day as counseling is ideal, and clinics should strive to reduce barriers to same-day procedures. Time constraints may be addressed by shifting tasks among the medical team. In the CHOICE project, contraceptive counselors—half of whom had no clinical experience—were trained to provide tiered counseling to participants. By working with a cross-trained health care team and offering prepared resources, clinicians can save time and improve access.
Physicians may want to incorporate the free online resources Bedsider.org or Stayteen.org to help women learn about contraceptive methods.24 The user-friendly Web sites, operated by the National Campaign to Prevent Teen and Unplanned Pregnancy, describe various forms of contraception and offer text and email reminders. Incorporating Bedsider into the counseling workflow and discussing the various reminder tools available may improve patients’ knowledge and enhance their compliance.
Additional barriers for practices may include high upfront costs associated with stocking devices. Practices that may be unable to sustain the costs surrounding enhanced contraception counseling and provision can collaborate with family planning clinics that are able to offer same-day services. A study of clinics in California found that Title X clinics were more likely to provide on-site LARC services than non-Title X public and private providers.25
4. Follow CDC guidelines for initiating and continuing contraception
Follow the US SPR for guidance on initiating and continuing contraceptive methods.14 The CDC’s Medical Eligibility Criteria for Contraceptive Use is another vital resource, providing recommendations for contraceptive methods to patients who have specific medical conditions or characteristics.26
Utilize the “quick start” method for hormonal contraception, where birth control is started on the same day as its prescription regardless of timing of the menstrual cycle. If you can’t be reasonably certain that a woman is not pregnant based on the criteria listed in TABLE 1,14 conduct a pregnancy test (while recognizing the aforementioned 2-week window of limitations) and counsel the patient to use back-up protection for the first 7 days along with repeating a pregnancy test in 2 weeks’ time.
The quick start method may lead to higher adherence than delayed initiation.27 Differences in continuation rates between women who use the quick start method and those who follow the delayed approach may disappear over time.28
Prescribe and provide a year’s supply of oral contraceptive pills (OCPs) as recommended by the CDC US SPR.14 It is important to note that pharmacists are usually restricted by insurance companies to only fill a one or 3 month’s supply.
In January 2016, Oregon began requiring private and state health insurance providers to reimburse for a year’s supply of prescription contraception; in January 2017, insurers in Washington, DC, were also required to offer women a year’s supply of prescription contraception.29,30 Several other states have followed suit. The California Health Benefits Review Program estimates a savings of $42.8 million a year from fewer office visits and 15,000 fewer unintended pregnancies if their state enacts a similar policy.31
Pharmacist initiatives are worth watching. In January 2016, Oregon pharmacists with additional training were allowed to prescribe OCs and hormonal patches to women 18 years and older.32 In April 2016, a similar law went into effect in California, but without a minimum age requirement and with the additional coverage of vaginal rings and Depo-Provera (depo) injections.33 Pharmacists in both states must review a health questionnaire completed by the woman and can refer to a physician as necessary.
The CDC recommends that clinicians extend the allowed window for repeat depo injections to 15 weeks.14 Common institutional protocol is to give repeat injections every 11 to 13 weeks. If past that window, protocol often dictates the woman abstain from unprotected sex for 2 weeks and then return for a negative pregnancy test (or await menses) before the next injection. However, the CDC notes that depo is effective for longer than the 13-week period.14 No additional birth control or pregnancy testing is needed and the woman can receive the next depo shot if she is up to 15 weeks from the previous shot.
One study found no additional pregnancy risks for those who were up to 4 weeks “late” for their next shot, suggesting there is potential for an even larger grace period.34 The World Health Organization advises allowing a repeat injection up to 4 weeks late.35 We encourage institutions to change their policies to comply with the CDC’s 15-week window.
Another initiative is over-the-counter (OTC) access to OCs, which the American Academy of Family Physicians (AAFP) and ACOG support.36,37 ACOG notes that “no drug or intervention is completely without risk of harm” and that the risk of venous thromboembolism for OC users is lower than the risk of pregnancy.37 Women can successfully self-screen for contraindications using a checklist. Concerns about women potentially being less adherent or less likely to choose LARCs are not reasons to preclude access to other methods. The AAFP supports insurance coverage of OCs, regardless of prescription status.36
5. Routinely counsel about, and advance-prescribe, emergency contraception pills
Physicians should counsel and advance-prescribe emergency contraception pills (ECPs) to women, including adolescents, using less reliable contraception, as recommended by ACOG, AAP, and the CDC.14,37,38 It’s also important to provide information on the copper IUD as the most effective method of emergency contraception, with nearly 100% efficacy if placed within 5 days.39 An easy-to-read patient hand-out in English and Spanish on EC options can be found at http://beyondthepill.ucsf.edu/tools-materials.
Only 3% of respondents participating in the 2006-2010 National Survey of Family Growth received counseling about emergency contraception in the past year.40 ECPs are most effective when used within 24 hours but have some efficacy up to 5 days.37 Due to the Affordable Care Act, most insurance plans will cover ECPs if purchased with a prescription, but coverage varies by state.41 Ulipristal acetate (UPA) ECP is only available with a prescription. Advance prescriptions can alleviate financial burdens on women when they need to access ECPs quickly.
Women should wait at least 5 days before resuming or starting hormonal contraception after taking UPA-based ECP, as it may reduce the ovulation-delaying effect of the ECP.14 For IUDs, implants, and depo, which require a visit to a health care provider, physicians evaluating earlier provision should consider the risks of reduced efficacy against the many barriers to access.
UPA-based ECPs (such as ella) may be more effective for overweight and obese women than levonorgestrel-based ECPs (such as Plan B and Next Choice).14 Consider advance-prescribing UPA ECPs to women with a body mass index (BMI) >25 kg/m2.42 Such considerations are important as the prevalence of obesity in women between 2013 and 2014 was 40.4%.43
In May 2016, the FDA noted that while current data are insufficient regarding whether the effectiveness of levonorgestrel ECPs is reduced in overweight or obese women, there are no safety concerns regarding their use in this population.44 Therefore, a woman with a BMI >25 kg/m2 should use UPA ECPs if available; but if not, she can still use levonorgestrel ECPs. One study, however, has found that UPA ECPs are only as effective as a placebo when BMI is ≥35 kg/m2, at which point a copper IUD may be the only effective form of emergency contraception.45
Transitioning from customary practices to best practices
Following these practical steps, FPs can improve contraceptive care for women. However, to make a significant impact, clinicians must be willing to change customary practices that are based on tradition, routines, or outdated protocols in favor of those based on current evidence.
One good place to start the transition to best practices is to familiarize yourself with the 2016 US Medical Eligibility Criteria for Contraceptive Use26 and Selected Practice Recommendations for Contraceptive Use.14 TABLES 214,26,46,47 and 3 offer additional resources that can enhance contraceptive counseling and further promote access to contraceptive care.
The contraceptive coverage guarantee under the Affordable Care Act has allowed many women to make contraceptive choices based on personal needs and preferences rather than cost. The new contraceptive coverage exemptions issued under the Trump administration will bring cost back as the driving decision factor for women whose employers choose not to provide contraceptive coverage. Providers should be aware of the typical costs associated with the various contraceptive options offered in their practice and community.
CORRESPONDENCE
Jessica Dalby, MD, Department of Family Medicine and Community Health, University of Wisconsin School of Medicine and Public Health, 1102 South Park St, Suite 100, Madison, WI 53715; Jessica.Dalby@fammed.wisc.edu.
While the unintended pregnancy rate for women ages 15 to 44 years decreased by 18% between 2008 and 2011, almost half of pregnancies in the United States remain unintended.1 On a more positive note, however, women who use birth control consistently and correctly account for only 5% of unintended pregnancies.2 As family physicians (FPs), we can support and facilitate our female patients’ efforts to consistently use highly effective forms of contraception. The 5 initiatives detailed here can help toward that end.
1. Routinely screen patients for their reproductive intentions
All women of reproductive age should be screened routinely for their pregnancy intentions. The American College of Obstetricians and Gynecologists (ACOG) encourages clinicians to ask women about pregnancy intendedness and encourages patients to develop a reproductive life plan, or a set of personal goals about whether or when to have children.3 The Centers for Disease Control and Prevention (CDC) has also developed a reproductive life plan tool for health professionals to encourage women and men to reflect upon their plans.4 So just as we regularly screen and document cigarette use and blood pressure (BP), so too, should we routinely screen women for their reproductive goals.
Ask women this one question. The Oregon Foundation for Reproductive Health launched the One Key Question Initiative, which proposes that the care team ask women ages 18 to 50: “Would you like to become pregnant in the next year?”5 A common workflow includes the medical assistant asking women about pregnancy intentions and providing a preconception and/or contraceptive handout, if appropriate. The physician provides additional counseling as needed. Pilot studies of One Key Question indicate that 30% to 40% of women screened needed follow-up counseling, suggesting the need for clinicians to be proactive in asking about reproductive plans. (Additional information on the Initiative is available on the Foundation’s Web site at http://www.orfrh.org/.)
This approach assumes women feel in control of their reproduction; however, this may not be the reality for many, especially low-income women.6 Additionally, women commonly cite planning a pregnancy as appropriate only when they are in an ideal relationship and when they are living in a financially stable environment—conditions that some women may never achieve.
Another caveat is that women may not have explicit pregnancy intentions, in which case, this particular approach may not be effective. A study of low-income women found only 60% intended to use the method prescribed after contraception counseling, with 37% of those stopping because of adverse effects, 23% saying they wanted another method, and 17% citing method complexity.7
Reproductive coercion from male partners, ranging from pressure to become pregnant to method sabotage, is also common in low-income women.8 Regular conversations that prioritize a woman’s values and experience are needed to promote reproductive autonomy.
2. Decouple provision of contraception from unnecessary exams
Pelvic exams and pap smears should not be required prior to offering patients hormonal contraception, according to the Choosing Wisely campaign of the American Board of Internal Medicine and ACOG.9,10 Hormonal contraception may instead be provided safely based on a medical history and BP assessment. Adolescents, minority groups, obese women, and victims of sexual trauma, in particular may avoid asking about birth control because of anxiety and fear of pain from these exams.11 The American College of Physicians recommends against speculum and bimanual exams in asymptomatic, non-pregnant, adult women.12 Pap smears and sexually transmitted infection (STI) testing should be performed at their normally scheduled intervals as recommended by the US Preventive Services Task Force (USPSTF) and not be tied to contraceptive provision.13
Assess pregnancy status using criteria,rather than a pregnancy text
Use the CDC’s criteria to assess pregnancy status rather than relying on a urine pregnancy test prior to providing contraception. Once you are reasonably sure that a woman is not pregnant (TABLE 114), contraception may be started. Some physicians have traditionally requested that a woman delay starting contraception until the next menses to ensure that she is not already pregnant. However, given the evidence that hormonal contraception does not cause birth defects, such a delay is not warranted and puts the woman at risk of an unintended pregnancy during the gap.15
Furthermore, there is an approximate 2-week window in which a woman could have a negative urine pregnancy test despite being pregnant, so the test alone is not completely reliable. In addition, obese women may experience irregular cycles, further complicating the traditional approach.16
Another largely unnecessary step … The US Selected Practice Recommendations (US SPR) from the CDC notes that additional STI screening prior to an intrauterine device (IUD) insertion is unnecessary for most women if appropriate screening guidelines have been previously followed.14 For those who have not been screened according to guidelines, the CDC recommends same-day screening and IUD insertion. You can then treat an STI without removing the IUD. Women with purulent cervicitis or a current chlamydial or gonorrheal infection should delay IUD insertion until after treatment.
3. Expand long-acting reversible contraception counseling and access
Offer long-acting reversible contraception (LARC), such as IUDs and implants, as first-line options for most women. ACOG endorses LARC as the most effective reversible method for most women, including those who have not given birth and adolescents.17 Unfortunately, a 2012 study found that family physicians were less likely than OB-GYNs to have enough time for contraceptive counseling and fewer than half felt competent inserting IUDs.18 While 79% of OB-GYNs routinely discussed IUDs with their patients, only 47% of family physicians did. In 2014, the American Academy of Pediatrics (AAP) endorsed a LARC-first tiered counseling approach for adolescents.19
A test of LARC-first counseling
The Contraceptive CHOICE project, a St. Louis, Missouri-based initiative, was launched to reduce unintended pregnancies in women ages 14 to 45 years by offering LARC-first counseling and free contraception of their choice.20 This project involved more than 9000 women at high risk for unintended pregnancy. Same-day LARC insertion was available. Seventy-five percent of women chose a LARC method and they reported greater continuation at 12 and 24 months, when compared to women who did not choose a LARC method. LARC users also reported higher satisfaction at one year. Provision of contraception through the project contributed to a reduction in repeat abortions as well as decreased rates of teenage pregnancy, birth, and abortion. Three years after the start of the project, IUDs had continuation rates of nearly 70%, implants of 56%, and non-LARC methods of 31%.21
When counseling women, it’s important to remember that effectiveness may not be the only criterium a woman uses when choosing a method. A 2010 study found that for 91% of women at high risk for unintended pregnancy, no single method possessed all the features they deemed “extremely important.”22 Clinicians should take a patient-centered approach to find birth control that fits each patient’s priorities.
Clinicians need proper training in LARC methods
Only 20% of FPs regularly insert IUDs, and 11% offer contraceptive implants, according to estimates from physicians recertifying with the American Board of Family Medicine in 2014.23 Access to training during residency is a key component to increasing these rates. FPs who practice obstetrics should be trained in postpartum LARC insertion and offer this option prior to hospital discharge as well as during the postpartum office visit.
Performing LARC insertions on the same day as counseling is ideal, and clinics should strive to reduce barriers to same-day procedures. Time constraints may be addressed by shifting tasks among the medical team. In the CHOICE project, contraceptive counselors—half of whom had no clinical experience—were trained to provide tiered counseling to participants. By working with a cross-trained health care team and offering prepared resources, clinicians can save time and improve access.
Physicians may want to incorporate the free online resources Bedsider.org or Stayteen.org to help women learn about contraceptive methods.24 The user-friendly Web sites, operated by the National Campaign to Prevent Teen and Unplanned Pregnancy, describe various forms of contraception and offer text and email reminders. Incorporating Bedsider into the counseling workflow and discussing the various reminder tools available may improve patients’ knowledge and enhance their compliance.
Additional barriers for practices may include high upfront costs associated with stocking devices. Practices that may be unable to sustain the costs surrounding enhanced contraception counseling and provision can collaborate with family planning clinics that are able to offer same-day services. A study of clinics in California found that Title X clinics were more likely to provide on-site LARC services than non-Title X public and private providers.25
4. Follow CDC guidelines for initiating and continuing contraception
Follow the US SPR for guidance on initiating and continuing contraceptive methods.14 The CDC’s Medical Eligibility Criteria for Contraceptive Use is another vital resource, providing recommendations for contraceptive methods to patients who have specific medical conditions or characteristics.26
Utilize the “quick start” method for hormonal contraception, where birth control is started on the same day as its prescription regardless of timing of the menstrual cycle. If you can’t be reasonably certain that a woman is not pregnant based on the criteria listed in TABLE 1,14 conduct a pregnancy test (while recognizing the aforementioned 2-week window of limitations) and counsel the patient to use back-up protection for the first 7 days along with repeating a pregnancy test in 2 weeks’ time.
The quick start method may lead to higher adherence than delayed initiation.27 Differences in continuation rates between women who use the quick start method and those who follow the delayed approach may disappear over time.28
Prescribe and provide a year’s supply of oral contraceptive pills (OCPs) as recommended by the CDC US SPR.14 It is important to note that pharmacists are usually restricted by insurance companies to only fill a one or 3 month’s supply.
In January 2016, Oregon began requiring private and state health insurance providers to reimburse for a year’s supply of prescription contraception; in January 2017, insurers in Washington, DC, were also required to offer women a year’s supply of prescription contraception.29,30 Several other states have followed suit. The California Health Benefits Review Program estimates a savings of $42.8 million a year from fewer office visits and 15,000 fewer unintended pregnancies if their state enacts a similar policy.31
Pharmacist initiatives are worth watching. In January 2016, Oregon pharmacists with additional training were allowed to prescribe OCs and hormonal patches to women 18 years and older.32 In April 2016, a similar law went into effect in California, but without a minimum age requirement and with the additional coverage of vaginal rings and Depo-Provera (depo) injections.33 Pharmacists in both states must review a health questionnaire completed by the woman and can refer to a physician as necessary.
The CDC recommends that clinicians extend the allowed window for repeat depo injections to 15 weeks.14 Common institutional protocol is to give repeat injections every 11 to 13 weeks. If past that window, protocol often dictates the woman abstain from unprotected sex for 2 weeks and then return for a negative pregnancy test (or await menses) before the next injection. However, the CDC notes that depo is effective for longer than the 13-week period.14 No additional birth control or pregnancy testing is needed and the woman can receive the next depo shot if she is up to 15 weeks from the previous shot.
One study found no additional pregnancy risks for those who were up to 4 weeks “late” for their next shot, suggesting there is potential for an even larger grace period.34 The World Health Organization advises allowing a repeat injection up to 4 weeks late.35 We encourage institutions to change their policies to comply with the CDC’s 15-week window.
Another initiative is over-the-counter (OTC) access to OCs, which the American Academy of Family Physicians (AAFP) and ACOG support.36,37 ACOG notes that “no drug or intervention is completely without risk of harm” and that the risk of venous thromboembolism for OC users is lower than the risk of pregnancy.37 Women can successfully self-screen for contraindications using a checklist. Concerns about women potentially being less adherent or less likely to choose LARCs are not reasons to preclude access to other methods. The AAFP supports insurance coverage of OCs, regardless of prescription status.36
5. Routinely counsel about, and advance-prescribe, emergency contraception pills
Physicians should counsel and advance-prescribe emergency contraception pills (ECPs) to women, including adolescents, using less reliable contraception, as recommended by ACOG, AAP, and the CDC.14,37,38 It’s also important to provide information on the copper IUD as the most effective method of emergency contraception, with nearly 100% efficacy if placed within 5 days.39 An easy-to-read patient hand-out in English and Spanish on EC options can be found at http://beyondthepill.ucsf.edu/tools-materials.
Only 3% of respondents participating in the 2006-2010 National Survey of Family Growth received counseling about emergency contraception in the past year.40 ECPs are most effective when used within 24 hours but have some efficacy up to 5 days.37 Due to the Affordable Care Act, most insurance plans will cover ECPs if purchased with a prescription, but coverage varies by state.41 Ulipristal acetate (UPA) ECP is only available with a prescription. Advance prescriptions can alleviate financial burdens on women when they need to access ECPs quickly.
Women should wait at least 5 days before resuming or starting hormonal contraception after taking UPA-based ECP, as it may reduce the ovulation-delaying effect of the ECP.14 For IUDs, implants, and depo, which require a visit to a health care provider, physicians evaluating earlier provision should consider the risks of reduced efficacy against the many barriers to access.
UPA-based ECPs (such as ella) may be more effective for overweight and obese women than levonorgestrel-based ECPs (such as Plan B and Next Choice).14 Consider advance-prescribing UPA ECPs to women with a body mass index (BMI) >25 kg/m2.42 Such considerations are important as the prevalence of obesity in women between 2013 and 2014 was 40.4%.43
In May 2016, the FDA noted that while current data are insufficient regarding whether the effectiveness of levonorgestrel ECPs is reduced in overweight or obese women, there are no safety concerns regarding their use in this population.44 Therefore, a woman with a BMI >25 kg/m2 should use UPA ECPs if available; but if not, she can still use levonorgestrel ECPs. One study, however, has found that UPA ECPs are only as effective as a placebo when BMI is ≥35 kg/m2, at which point a copper IUD may be the only effective form of emergency contraception.45
Transitioning from customary practices to best practices
Following these practical steps, FPs can improve contraceptive care for women. However, to make a significant impact, clinicians must be willing to change customary practices that are based on tradition, routines, or outdated protocols in favor of those based on current evidence.
One good place to start the transition to best practices is to familiarize yourself with the 2016 US Medical Eligibility Criteria for Contraceptive Use26 and Selected Practice Recommendations for Contraceptive Use.14 TABLES 214,26,46,47 and 3 offer additional resources that can enhance contraceptive counseling and further promote access to contraceptive care.
The contraceptive coverage guarantee under the Affordable Care Act has allowed many women to make contraceptive choices based on personal needs and preferences rather than cost. The new contraceptive coverage exemptions issued under the Trump administration will bring cost back as the driving decision factor for women whose employers choose not to provide contraceptive coverage. Providers should be aware of the typical costs associated with the various contraceptive options offered in their practice and community.
CORRESPONDENCE
Jessica Dalby, MD, Department of Family Medicine and Community Health, University of Wisconsin School of Medicine and Public Health, 1102 South Park St, Suite 100, Madison, WI 53715; Jessica.Dalby@fammed.wisc.edu.
1. Finer LB, Zolna MR. Declines in unintended pregnancy in the United States, 2008–2011. N Engl J Med. 2016; 374:843-852.
2. Sonfield A, Hasstedt K, Gold RB. Moving Forward: Family Planning in the Era of Health Reform. New York: Guttmacher Institute. 2014. Available at: https://www.guttmacher.org/report/moving-forward-family-planning-era-health-reform. Accessed October 5, 2017.
3. Committee on Health Care for Underserved Women. Reproductive Life Planning to Reduce Unintended Pregnancy: American College of Obstetricians and Gynecologists. 2016. Available at: https://www.acog.org/Resources-And-Publications/Committee-Opinions/Committee-on-Health-Care-for-Underserved-Women/Reproductive-Life-Planning-to-Reduce-Unintended-Pregnancy. Accessed October 5, 2017.
4. Centers for Disease Control and Prevention. Reproductive Life Plan Tool for Health Care Providers. 2016. Available at: http://www.cdc.gov/preconception/rlptool.html. Accessed August 31, 2016.
5. Oregon Health Authority. Effective Contraceptive Use among Women at Risk of Unintended Pregnancy Guidance Document. 2014. Available at: http://www.oregon.gov/oha/HPA/ANALYTICS/CCOData/Effective%20Contraceptive%20Use%20Guidance%20Document.pdf. Accessed October 5, 2017.
6. Borrero S, Nikolajski C, Steinberg JR, et al. “It just happens”: a qualitative study exploring low-income women’s perspectives on pregnancy intention and planning. Contraception. 2015;91:150-156.
7. Yee LM, Farner KC, King E, et al. What do women want? Experiences of low-income women with postpartum contraception and contraceptive counseling. J Pregnancy Child Health. 2015;2.
8. Kalichman SC, Williams EA, Cherry C, et al. Sexual coercion, domestic violence, and negotiating condom use among low-income African American women. J Womens Health. 1998;7:371-378.
9. ABIM Foundation. Pelvic Exams, Pap Tests and Oral Contraceptives. 2016. Available at: http://www.choosingwisely.org/patient-resources/pelvic-exams-pap-tests-and-oral-contraceptives/. Accessed May 31, 2016.
10. Committee on Health Care for Underserved Women. Access to Contraception: American College of Obstetricians and Gynecologists. 2015. Number 615. Available at: https://www.acog.org/-/media/Committee-Opinions/Committee-on-Health-Care-for-Underserved-Women/co615.pdf?dmc=1&ts=201710. Accessed October 5, 2017.
11. Bates CK, Carroll N, Potter J. The challenging pelvic examination. J Gen Intern Med. 2011;26:651-657.
12. Qaseem A, Humphrey LL, Harris R, et al. Screening pelvic examination in adult women: a clinical practice guideline from the American College of Physicians. Ann Intern Med. 2014;161:67-72.
13. U.S. Preventive Services Task Force. Cervical Cancer: Screening. 2012. Available at: http://www.uspreventiveservicestaskforce.org/Page/Document/UpdateSummaryFinal/cervical-cancer-screening. Accessed May 25, 2016.
14. Curtis KM, Jatlaoui TC, Tepper NK, et al. U.S. selected practice recommendations for contraceptive use, 2016. MMWR Morb Mortal Wkly Rep. 2016;65:1-66.
15. Lesnewski R, Prine L. Initiating hormonal contraception. Am Fam Physician. 2006;74:105-112.
16. Jacobsen BK, Knutsen SF, Oda K, et al. Obesity at age 20 and the risk of miscarriages, irregular periods and reported problems of becoming pregnant: the Adventist Health Study-2. Eur J Epidemiol. 2012; 27:923-931.
17. Committee on Gynecologic Practice. Increasing Access to Contraceptive Implants and Intrauterine Devices to Reduce Unintended Pregnancy: American College of Obstetricians and Gynecologists. 2015. Number 642. Available at: https://www.acog.org/Resources-And-Publications/Committee-Opinions/Committee-on-Gynecologic-Practice/Increasing-Access-to-Contraceptive-Implants-and-Intrauterine-Devices-to-Reduce-Unintended-Pregnancy. Accessed October 5, 2017.
18. Harper CC, Henderson JT, Raine TR, et al. Evidence-based IUD practice: family physicians and obstetrician-gynecologists. Fam Med. 2012;44:637-645.
19. American Academy of Pediatrics, Committee on Adolescence. Policy statement: Contraception for Adolescents. 2014. Available at: http://pediatrics.aappublications.org/content/pediatrics/early/2014/09/24/peds.2014-2299.full.pdf. Accessed October 5, 2017.
20. Birgisson NE, Zhao Q, Secura GM, et al. Preventing unintended pregnancy: The Contraceptive CHOICE Project in review. J Womens Health (Larchmt). 2015;24:349-353.
21. Diedrich JT, Zhao Q, Madden T, et al. Three-year continuation of reversible contraception. Am J Obstet Gynecol. 2015;213:662.e1-e8.
22. Lessard LN, Karasek D, Ma S, et al. Contraceptive features preferred by women at high risk of unintended pregnancy. Perspect Sex Reprod Health. 2012;44:194-200.
23. Nisen MB, Peterson LE, Cochrane A, et al. US family physicians’ intrauterine and implantable contraception provision: results from a national survey. Contraception. 2016;93:432-437.
24. National Campaign to Prevent Teen and Unplanned Pregnancy. Bedsider. Available at: https://bedsider.org/. Accessed June 14, 2016.
25. Park HY, Rodriguez MI, Hulett D, et al. Long-acting reversible contraception method use among Title X providers and non-Title X providers in California. Contraception. 2012;86:557-561.
26. Curtis KM, Tepper NK, Jatlaoui TC, et al. U.S. medical eligibility criteria for contraceptive use, 2016. MMWR Morb Mortal Wkly Rep. 2016;65:1-103.
27. Westhoff C, Kerns J, Morroni C, et al. Quick start: novel oral contraceptive initiation method. Contraception. 2002;66:141-145.
28. Brahmi D, Curtis KM. When can a woman start combined hormonal contraceptives (CHCs)? A systematic review. Contraception. 2013;87:524-538.
29. Lachman S. Oregon To Require Insurers To Cover A Year’s Supply Of Birth Control. Huffington Post. June 11, 2015. Available at: https://www.huffingtonpost.com/2015/06/11/oregon-birth-control-_n_7564712.html. Accessed October 16, 2017.
30. Andrews M. D.C. Women To Get Access To Full Year’s Worth Of Contraceptives. Kaiser Health News. September 25, 2015. Available at: https://khn.org/news/d-c-women-to-get-access-to-full-years-worth-of-contraceptives/. Accessed October 16, 2017.
31. Analysis of California Senate Bill (SB) 999 Contraceptives: Annual Supply: A Report to the 2015-2016 California State Legislature: California Health Benefits Review Program. 2016. Available at: http://chbrp.ucop.edu/index.php?action=read&bill_id=195&doc_type=1000. Accessed October 5, 2017.
32. Frazier A. Pharmacist-prescribed birth control in effect Jan 1. KOIN News. December 30, 2015. Available at: http://koin.com/2015/12/30/pharmacist-provided-birth-control-in-effect-jan-1/. Accessed October 5, 2017.
33. Karlamangla S. Birth control pills without prescriptions, coming soon to California under new law. Los Angeles Times. February 14, 2016. Available at: http://www.latimes.com/health/la-me-birth-control-pharmacies-20160214-story.html. Accessed October 16, 2017.
34. Steiner MJ, Kwok C, Stanback J, et al. Injectable contraception: what should the longest interval be for reinjections? Contraception. 2008;77:410-414.
35. World Health Organization. Family Planning: A Global Handbook for Providers. 2011. Available at: http://apps.who.int/iris/bitstream/10665/44028/1/9780978856373_eng.pdf. Accessed October 5, 2017.
36. American Academy of Family Physicians. Over-the-Counter Oral Contraceptives. 2014; Available at: http://www.aafp.org/about/policies/all/otc-oral-contraceptives.html. Accessed June 2, 2016.
37. Committee on Gynecologic Practice. Over-the-Counter Access to Oral Contraceptives: American College of Obstetricians and Gynecologists. 2012. Number 544. Available at: https://www.acog.org/Resources-And-Publications/Committee-Opinions/Committee-on-Gynecologic-Practice/Over-the-Counter-Access-to-Oral-Contraceptives. Accessed October 5, 2017.
38. Committee on Adolescence. Emergency contraception. Pediatrics. 2012;130:1174-1182.
39. Cleland K, Zhu H, Goldstuck N, et al. The efficacy of intrauterine devices for emergency contraception: a systematic review of 35 years of experience. Hum Reprod. 2012;27:1994-2000.
40. Martinez G, Chandra A, Febo-Vazquez I, et al. Use of Family Planning and Related Medical Services Among Women Aged 15–44 in the United States: National Survey of Family Growth, 2006–2010: National Center for Health Statistics, Centers for Disease Control and Prevention. 2013. Available at: https://www.cdc.gov/nchs/data/nhsr/nhsr068.pdf. Accessed October 5, 2017.
41. Guttmacher Institute. Insurance Coverage of Contraceptives: Guttmacher Institute;2017. Available at: https://www.guttmacher.org/state-policy/explore/insurance-coverage-contraceptives Accessed October 7, 2017.
42. Glasier A, Cameron ST, Blithe D, et al. Can we identify women at risk of pregnancy despite using emergency contraception? Data from randomized trials of ulipristal acetate and levonorgestrel. Contraception. 2011;84:363-367.
43. Flegal KM, Kruszon-Moran D, Carroll MD, et al. Trends in obesity among adults in the United States, 2005 to 2014. JAMA. 2016;315:2284-2291.
44. US Food & Drug Administration. Postmarket Drug Safety Information for Patients and Providers - Plan B (0.75mg levonorgestrel) and Plan B One-Step (1.5 mg levonorgestrel) Tablets Information. 2016; Available at: http://www.fda.gov/Drugs/DrugSafety/PostmarketDrugSafetyInformationforPatientsandProviders/ucm109775.htm. Accessed May 25, 2016.
45. Simmons KB, Edelman AB. Contraception and sexual health in obese women. Best Pract Res Clin Obstet Gynaecol. 2015;29:466-478.
46. Centers for Disease Control and Prevention. Providing quality family planning services: recommendations of CDC and the U.S. Office of Population Affairs. MMWR Recomm Rep. 2014;63:1-29.
47. LARC FIRST. Available at: http://www.larcfirst.com/index.html. Accessed May 2016.
1. Finer LB, Zolna MR. Declines in unintended pregnancy in the United States, 2008–2011. N Engl J Med. 2016; 374:843-852.
2. Sonfield A, Hasstedt K, Gold RB. Moving Forward: Family Planning in the Era of Health Reform. New York: Guttmacher Institute. 2014. Available at: https://www.guttmacher.org/report/moving-forward-family-planning-era-health-reform. Accessed October 5, 2017.
3. Committee on Health Care for Underserved Women. Reproductive Life Planning to Reduce Unintended Pregnancy: American College of Obstetricians and Gynecologists. 2016. Available at: https://www.acog.org/Resources-And-Publications/Committee-Opinions/Committee-on-Health-Care-for-Underserved-Women/Reproductive-Life-Planning-to-Reduce-Unintended-Pregnancy. Accessed October 5, 2017.
4. Centers for Disease Control and Prevention. Reproductive Life Plan Tool for Health Care Providers. 2016. Available at: http://www.cdc.gov/preconception/rlptool.html. Accessed August 31, 2016.
5. Oregon Health Authority. Effective Contraceptive Use among Women at Risk of Unintended Pregnancy Guidance Document. 2014. Available at: http://www.oregon.gov/oha/HPA/ANALYTICS/CCOData/Effective%20Contraceptive%20Use%20Guidance%20Document.pdf. Accessed October 5, 2017.
6. Borrero S, Nikolajski C, Steinberg JR, et al. “It just happens”: a qualitative study exploring low-income women’s perspectives on pregnancy intention and planning. Contraception. 2015;91:150-156.
7. Yee LM, Farner KC, King E, et al. What do women want? Experiences of low-income women with postpartum contraception and contraceptive counseling. J Pregnancy Child Health. 2015;2.
8. Kalichman SC, Williams EA, Cherry C, et al. Sexual coercion, domestic violence, and negotiating condom use among low-income African American women. J Womens Health. 1998;7:371-378.
9. ABIM Foundation. Pelvic Exams, Pap Tests and Oral Contraceptives. 2016. Available at: http://www.choosingwisely.org/patient-resources/pelvic-exams-pap-tests-and-oral-contraceptives/. Accessed May 31, 2016.
10. Committee on Health Care for Underserved Women. Access to Contraception: American College of Obstetricians and Gynecologists. 2015. Number 615. Available at: https://www.acog.org/-/media/Committee-Opinions/Committee-on-Health-Care-for-Underserved-Women/co615.pdf?dmc=1&ts=201710. Accessed October 5, 2017.
11. Bates CK, Carroll N, Potter J. The challenging pelvic examination. J Gen Intern Med. 2011;26:651-657.
12. Qaseem A, Humphrey LL, Harris R, et al. Screening pelvic examination in adult women: a clinical practice guideline from the American College of Physicians. Ann Intern Med. 2014;161:67-72.
13. U.S. Preventive Services Task Force. Cervical Cancer: Screening. 2012. Available at: http://www.uspreventiveservicestaskforce.org/Page/Document/UpdateSummaryFinal/cervical-cancer-screening. Accessed May 25, 2016.
14. Curtis KM, Jatlaoui TC, Tepper NK, et al. U.S. selected practice recommendations for contraceptive use, 2016. MMWR Morb Mortal Wkly Rep. 2016;65:1-66.
15. Lesnewski R, Prine L. Initiating hormonal contraception. Am Fam Physician. 2006;74:105-112.
16. Jacobsen BK, Knutsen SF, Oda K, et al. Obesity at age 20 and the risk of miscarriages, irregular periods and reported problems of becoming pregnant: the Adventist Health Study-2. Eur J Epidemiol. 2012; 27:923-931.
17. Committee on Gynecologic Practice. Increasing Access to Contraceptive Implants and Intrauterine Devices to Reduce Unintended Pregnancy: American College of Obstetricians and Gynecologists. 2015. Number 642. Available at: https://www.acog.org/Resources-And-Publications/Committee-Opinions/Committee-on-Gynecologic-Practice/Increasing-Access-to-Contraceptive-Implants-and-Intrauterine-Devices-to-Reduce-Unintended-Pregnancy. Accessed October 5, 2017.
18. Harper CC, Henderson JT, Raine TR, et al. Evidence-based IUD practice: family physicians and obstetrician-gynecologists. Fam Med. 2012;44:637-645.
19. American Academy of Pediatrics, Committee on Adolescence. Policy statement: Contraception for Adolescents. 2014. Available at: http://pediatrics.aappublications.org/content/pediatrics/early/2014/09/24/peds.2014-2299.full.pdf. Accessed October 5, 2017.
20. Birgisson NE, Zhao Q, Secura GM, et al. Preventing unintended pregnancy: The Contraceptive CHOICE Project in review. J Womens Health (Larchmt). 2015;24:349-353.
21. Diedrich JT, Zhao Q, Madden T, et al. Three-year continuation of reversible contraception. Am J Obstet Gynecol. 2015;213:662.e1-e8.
22. Lessard LN, Karasek D, Ma S, et al. Contraceptive features preferred by women at high risk of unintended pregnancy. Perspect Sex Reprod Health. 2012;44:194-200.
23. Nisen MB, Peterson LE, Cochrane A, et al. US family physicians’ intrauterine and implantable contraception provision: results from a national survey. Contraception. 2016;93:432-437.
24. National Campaign to Prevent Teen and Unplanned Pregnancy. Bedsider. Available at: https://bedsider.org/. Accessed June 14, 2016.
25. Park HY, Rodriguez MI, Hulett D, et al. Long-acting reversible contraception method use among Title X providers and non-Title X providers in California. Contraception. 2012;86:557-561.
26. Curtis KM, Tepper NK, Jatlaoui TC, et al. U.S. medical eligibility criteria for contraceptive use, 2016. MMWR Morb Mortal Wkly Rep. 2016;65:1-103.
27. Westhoff C, Kerns J, Morroni C, et al. Quick start: novel oral contraceptive initiation method. Contraception. 2002;66:141-145.
28. Brahmi D, Curtis KM. When can a woman start combined hormonal contraceptives (CHCs)? A systematic review. Contraception. 2013;87:524-538.
29. Lachman S. Oregon To Require Insurers To Cover A Year’s Supply Of Birth Control. Huffington Post. June 11, 2015. Available at: https://www.huffingtonpost.com/2015/06/11/oregon-birth-control-_n_7564712.html. Accessed October 16, 2017.
30. Andrews M. D.C. Women To Get Access To Full Year’s Worth Of Contraceptives. Kaiser Health News. September 25, 2015. Available at: https://khn.org/news/d-c-women-to-get-access-to-full-years-worth-of-contraceptives/. Accessed October 16, 2017.
31. Analysis of California Senate Bill (SB) 999 Contraceptives: Annual Supply: A Report to the 2015-2016 California State Legislature: California Health Benefits Review Program. 2016. Available at: http://chbrp.ucop.edu/index.php?action=read&bill_id=195&doc_type=1000. Accessed October 5, 2017.
32. Frazier A. Pharmacist-prescribed birth control in effect Jan 1. KOIN News. December 30, 2015. Available at: http://koin.com/2015/12/30/pharmacist-provided-birth-control-in-effect-jan-1/. Accessed October 5, 2017.
33. Karlamangla S. Birth control pills without prescriptions, coming soon to California under new law. Los Angeles Times. February 14, 2016. Available at: http://www.latimes.com/health/la-me-birth-control-pharmacies-20160214-story.html. Accessed October 16, 2017.
34. Steiner MJ, Kwok C, Stanback J, et al. Injectable contraception: what should the longest interval be for reinjections? Contraception. 2008;77:410-414.
35. World Health Organization. Family Planning: A Global Handbook for Providers. 2011. Available at: http://apps.who.int/iris/bitstream/10665/44028/1/9780978856373_eng.pdf. Accessed October 5, 2017.
36. American Academy of Family Physicians. Over-the-Counter Oral Contraceptives. 2014; Available at: http://www.aafp.org/about/policies/all/otc-oral-contraceptives.html. Accessed June 2, 2016.
37. Committee on Gynecologic Practice. Over-the-Counter Access to Oral Contraceptives: American College of Obstetricians and Gynecologists. 2012. Number 544. Available at: https://www.acog.org/Resources-And-Publications/Committee-Opinions/Committee-on-Gynecologic-Practice/Over-the-Counter-Access-to-Oral-Contraceptives. Accessed October 5, 2017.
38. Committee on Adolescence. Emergency contraception. Pediatrics. 2012;130:1174-1182.
39. Cleland K, Zhu H, Goldstuck N, et al. The efficacy of intrauterine devices for emergency contraception: a systematic review of 35 years of experience. Hum Reprod. 2012;27:1994-2000.
40. Martinez G, Chandra A, Febo-Vazquez I, et al. Use of Family Planning and Related Medical Services Among Women Aged 15–44 in the United States: National Survey of Family Growth, 2006–2010: National Center for Health Statistics, Centers for Disease Control and Prevention. 2013. Available at: https://www.cdc.gov/nchs/data/nhsr/nhsr068.pdf. Accessed October 5, 2017.
41. Guttmacher Institute. Insurance Coverage of Contraceptives: Guttmacher Institute;2017. Available at: https://www.guttmacher.org/state-policy/explore/insurance-coverage-contraceptives Accessed October 7, 2017.
42. Glasier A, Cameron ST, Blithe D, et al. Can we identify women at risk of pregnancy despite using emergency contraception? Data from randomized trials of ulipristal acetate and levonorgestrel. Contraception. 2011;84:363-367.
43. Flegal KM, Kruszon-Moran D, Carroll MD, et al. Trends in obesity among adults in the United States, 2005 to 2014. JAMA. 2016;315:2284-2291.
44. US Food & Drug Administration. Postmarket Drug Safety Information for Patients and Providers - Plan B (0.75mg levonorgestrel) and Plan B One-Step (1.5 mg levonorgestrel) Tablets Information. 2016; Available at: http://www.fda.gov/Drugs/DrugSafety/PostmarketDrugSafetyInformationforPatientsandProviders/ucm109775.htm. Accessed May 25, 2016.
45. Simmons KB, Edelman AB. Contraception and sexual health in obese women. Best Pract Res Clin Obstet Gynaecol. 2015;29:466-478.
46. Centers for Disease Control and Prevention. Providing quality family planning services: recommendations of CDC and the U.S. Office of Population Affairs. MMWR Recomm Rep. 2014;63:1-29.
47. LARC FIRST. Available at: http://www.larcfirst.com/index.html. Accessed May 2016.
Emergency contraception: An underutilized resource
• Offer emergency contraception (EC) to any woman who reports contraceptive failure or unprotected intercourse within the last 5 days; no clinical exam is necessary. B
• Prescribe a progestin-only EC or ulipristal acetate, both of which are more effective and have fewer adverse effects than an estrogen-progestin combination. A
• Consider giving sexually active teens <17 years an advance prescription for EC, as it is not available over the counter to this age group. C
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
The average American woman will spend more than 30 years of her life trying to prevent pregnancy—not always successfully. Each year, half of the approximately 6 million pregnancies in the United States are unintended.1 Emergency contraception (EC) gives a woman a second chance to prevent pregnancy after a contraceptive failure or unprotected sex. But all too often, it isn’t offered and she doesn’t request it.
Lack of knowledge about EC continues to be a barrier to its use. Some women have heard about the “morning after pill,” but may not know that EC can be effective for up to 5 days after intercourse—or even that it’s available in this country.2 Others are unaware that it is possible to prevent pregnancy after intercourse,2 and mistakenly believe that EC drugs are abortifacients. In fact, they work primarily by interfering with ovulation and have not been found to prevent implantation or to disrupt an existing pregnancy.3-5
Providers also contribute to the limited use of EC, often because they’re unfamiliar with the options or uncomfortable discussing them with patients, particularly sexually active teens.2
This update can help you clear up misconceptions about EC with your patients. It also provides evidence-based information about the various types of EC, a review of issues affecting accessibility, and a telephone triage protocol to guide your response to women seeking postcoital contraception.
EC today: Plan B and beyond
Hormonal EC was first studied in the 1920s, when researchers found that estrogenic ovarian extracts interfered with pregnancy in animals. The first regimen was a high-dose estrogen-only formulation. In 1974, a combined estrogen-progestin replaced it. Known as the Yuzpe method for the physician who discovered it,6 this regimen used a widely available brand of combined estrogen-progestin oral contraceptive pills. The standard dose consisted of 100 mcg ethinyl estradiol (EE) and 0.5 mg levonorgestrel (LNG) taken 12 hours apart.2,7
Although the Yuzpe method is still in use, progestin-only EC—Plan B as well as generic (Next Choice) and single-dose (Plan B One-Step) LNG formulations—has become the standard of care because it has greater efficacy and fewer adverse effects.2 There are 2 additional options: the copper intrauterine device (IUD), which is highly effective both as EC and as a long-term contraceptive,6 and ulipristal acetate (UPA), which received US Food and Drug Administration (FDA) approval in 2010. This second-generation antiprogestin, sold under the brand name Ella, is well tolerated and highly effective.8
EC efficacy: What the evidence shows
EC is most likely to work when used within 24 hours, but remains effective—albeit to varying degrees—for up to 120 hours (TABLE).2,5,8,9 Thus, which EC is best for a particular patient depends, in part, on timing.
TABLE
Emergency contraception: Comparing methods*2,5,8,9
| EC method | Dose and timing | Benefits | Adverse effects/ drawbacks |
|---|---|---|---|
| Estrogen-progestin OCs | 100 mcg EE and 0.5 mg LNG, taken 12 h apart First dose within 72 h | Easily accessible and widely available; patient may use OCs she already has at home | Higher rates of adverse effects, including nausea, vomiting, headache; less effective than other methods |
| Progestin-only (Plan B, Next Choice, others) | 1.5 mg LNG within 72 h (available in divided doses or in a single tablet; 2 tablets may be taken as a single dose) | Available OTC for patients ≥17 y; more effective and fewer adverse effects than estrogen-progestin Convenience of single dose | Prescription required for patients <17 y Approved for use within 72 h; effectiveness diminishes thereafter |
| UPA (Ella) | 30 mg UPA, taken ≤120 h† | More effective than LNG; fewer adverse effects than estrogen-progestin Efficacy remains high ≤5 days Convenience of single dose | Prescription required; not available at all pharmacies Not studied in breastfeeding‡ |
| Copper IUD | Insert ≤120 h | Extremely effective Provides immediate, long-term contraception | Insertion requires staff training; higher cost than oral EC |
| EC, emergency contraception; EE, ethinyl estradiol; IUD, intrauterine device; LNG, levonorgestrel; OCs, oral contraceptives; OTC, over the counter; UPA, ulipristal acetate. *Low doses of mifepristone (<25-50 mg)—approved as an abortifacient in much larger doses—may also be used as EC. †Dosage should be repeated if vomiting occurs within 3 hours. ‡Advise patients to avoid breastfeeding for 36 hours | |||
Copper IUDs have the highest success rate: Studies have found the copper IUD to be >99% effective in preventing pregnancy when inserted within 5 days of unprotected intercourse.9,10 The copper ions it contains have a toxic effect on sperm, and impair the potential for fertilization; the device may also make the endometrium inhospitable to implantation.9,10
A just-published systematic review of 42 studies in 6 countries over a period of more than 30 years yielded similar results: Among more than 7000 women who had the IUDs inserted after unprotected intercourse, the pregnancy rate was 0.09%.11
But an IUD is appropriate only for women who want long-term contraception and would otherwise qualify for IUD insertion. By comparison, hormonal EC is not as effective and generally works best when used within a shorter time frame.
Progestin alone vs estrogen-progestin combo. To compare hormonal contraception, many researchers use a “prevented fraction”—an estimated percentage of pregnancies averted by treatment. A large World Health Organization-sponsored study found that the efficacy of progestin-only EC is superior to that of the estrogen-progestin combination, with prevented fractions of 85% and 57%, respectively. The progestin-only EC was also associated with significantly fewer adverse effects.12
In more recent studies, the prevented fraction for progestin-only EC has been found to range from 60% to 94%, while a meta-analysis of studies assessing estrogen-progestin EC r evealed a prevented fraction of ≥74%.2
Although there is evidence suggesting that progestin-only EC may work for up to 5 days,13,14 it has FDA approval only for use within 72 hours of intercourse.13 A time-sensitive analysis showed that when it was used within 12 hours of intercourse, the pregnancy rate was 0.5%. The rate increased steadily to 4.1% when the progestin-based EC was taken 61 to 72 hours after intercourse, and rose by an additional 50% after an additional 12-hour delay.15
Hormonal EC is only effective before ovulation occurs. Once luteinizing hormone (LH) starts to rise, it is ineffective. However, the likelihood of pregnancy drops precipitously after ovulation, and there is no risk of pregnancy in the luteal phase, with or without EC.
One pill or 2? Both Plan B and the generic Next Choice are sold as 2-dose regimens, with one 0.75-mg tablet taken within 72 hours and the second taken 12 hours later. Plan B One-Step, which consists of a single 1.5-mg tablet, is clinically equivalent to the 2-dose formula,16 but is more convenient and may improve adherence. Notably, though, one large randomized controlled trial (RCT) in China found that the 2-pill regimen was significantly more effective in preventing pregnancy in women who had further acts of unprotected intercourse after treatment.17
UPA has a 5-day window. UPA has FDA approval for use within 120 hours of unprotected intercourse and has been found to be more effective than progestin-only EC, especially when used on Day 4 or 5 (72-120 hours).8 Adverse effects are mild to moderate, similar to those of LNG, and may include headache, abdominal pain, nausea, dysmenorrhea, fatigue, and dizziness.8
The medication binds to progesterone receptors, acting as an antagonist as well as a partial agonist. The mechanism of action depends on the phase of the woman’s cycle. Taken during the midfollicular phase, UPA inhibits follicle development.18 When used in the advanced follicular phase, just prior to ovulation, it delays LH peak and postpones ovulation.19
In one small study in which women were randomized to either UPA or placebo, researchers found that the drug delayed ovulation for ≥5 days in about 60% of those who took it; in comparison, ovulation occurred by Day 5 in every woman in the placebo group.19
How accessible is EC?
EC has a tumultuous history in the United States,20 and accessibility depends on a variety of factors—age among them.
Plan B, for instance, is subject to a 2-tier system. It was approved in 1999 as a prescription-only product and has been available over the counter (OTC) to women 17 years and older since 2009. Younger women can get it only by prescription.21
Nonetheless, Plan B made the news again last year, when US Health and Human Services Secretary Kathleen Sebelius overruled an FDA decision to give teens younger than 17 OTC access.22 Thus, the age restriction remains in place, although there is no medical evidence to support it.23 Other forms of EC, including UPA, are available to all women only by prescription.
Accessibility of EC also may vary from one part of the country to another. Some states have enacted laws with conscience clauses that allow pharmacists to refuse to dispense EC. Others have worked to increase access by authorizing pharmacists to initiate and dispense EC on their own, provided they work in collaboration with a doctor or other licensed prescriber. As of 2011, 9 states—Alaska, California, Hawaii, Maine, Massachusetts, New Hampshire, New Mexico, Vermont, and Washington—had such agreements in place.24
Cost is another potential barrier. The cost of oral EC varies from about $10 to $70, plus the cost of a doctor visit for a teen who needs a prescription. Obtaining the copper IUD without insurance coverage would cost hundreds of dollars, to cover the price of insertion as well as the device.5
Increasing access: What you can do
In view of the barriers that adolescents face in obtaining EC, the American College of Obstetricians and Gynecologists and the American Academy of Pediatrics, among other organizations, recommend that physicians give advance prescriptions to teens under the age of 17. 2,25
But how likely are they to actually buy the medication and use it on an emergency basis?
A 2007 Cochrane review found that giving women advance prescriptions for EC did not reduce pregnancy or abortion rates.26 Other studies have found that EC use is highest among women with the lowest risk of pregnancy—those who are already using contraception and are less likely to have unprotected intercourse. Those at the highest risk for unintended pregnancy were found to be less likely to use EC after every episode of unprotected intercourse.23,26 One RCT demonstrated that rates of pregnancy and sexually transmitted infection were not significantly increased by advance provision of EC, leading the researchers to conclude that it was therefore unreasonable to restrict access.27
While it is prudent to make women aware that EC is available should they need it, the focus should be on the fact that consistent use of a reliable form of contraception—an IUD or hormonal contraception, in particular—gives them the best chance of preventing an unwanted pregnancy.
What to do when that call comes in
When a woman calls to report a contraceptive failure or tells you she has had unprotected intercourse, start by finding out how recently it occurred. Subsequent questions and actions that can be used by triage nurses or physicians on call are detailed in the easy-to-use EC telephone triage protocol (FIGURE)28 on page 395. Whether you prescribe oral EC or schedule an appointment to insert a copper IUD within the next few days, there are a number of key points to keep in mind.
Initiate EC as soon as possible, but make it available to any woman who requests it for up to 5 days after unprotected intercourse.
Advise patients that oral EC is safe for most women—even those with contraindications to oral contraceptives. No physical examination is necessary, and there’s usually no need for a pregnancy test.2 The one exception: A woman who has not had a period in the past 30 days should be given a pregnancy test before taking UPA.2
Offer EC at any time in the cycle. Although EC works primarily in the preovulatory phase, it should be offered regardless of the phase of the patient’s menstrual cycle. That’s because of the possibility of late ovulation, as well as the difficulty in accurately determining the phase of a woman’s cycle based on a history alone.
Make EC available to any woman who has been sexually abused. At many emergency departments, EC is not routinely offered to women who come in after being raped, although it clearly should be.29
FIGURE
Telephone triage protocol for emergency contraception
EC, emergency contraception; IUD, intrauterine device; LNG, levonorgestrel; Rx, prescription; UPA, ulipristal acetate.
Adapted from: Reproductive Health Access Project. http://www.reproductiveaccess.org/contraception/tel_triage_ec.htm.28
Patient counseling about EC
Advise patients for whom you prescribe oral EC that the medication delays ovulation, which means they could be at risk for pregnancy later in the cycle. Stress the need to use an alternative means of contraception (a barrier method is recommended for women taking UPA) until their next menses and to come in for a pregnancy test if their period is more than a week late.
Point out, too, that EC can be used more than once within the same cycle, if necessary. That said, even a single request for EC should result in a discussion of effective, longer-term contraception, including the possibility of an IUD.
CORRESPONDENCE Sarina Schrager, MD, MS, University of Wisconsin School of Public Health, Department of Family Medicine, 1100 Delaplaine Court, Madison, WI 53715; sbschrag@wisc.edu
1. Guttmacher Institute. National Reproductive Health profile. Available at: http://www.guttmacher.org/datacenter/profiles/print/US.jsp. Accessed November 3, 2011.
2. American College of Obstetricians and Gynecologists ACOG practice bulletin No. 112. Emergency contraception. Obstet Gynecol. 2010;115:1100–1109.
3. Belluck P. No abortion role seen for morning-after pill. New York Times. June 6, 2012; A1.
4. Trussell J, Raymond E. Emergency contraception: a last chance to prevent unintended pregnancy. Princeton, NJ: Office of Population Research at Princeton University; June 2012. Available at: http://ec.princeton.edu/questions/ec-review.pdf. Accessed June 20, 2012.
5. Planned Parenthood. Morning-after pill (emergency contraception). Available at: http://www.plannedparenthood.org/health-topics/emergency-contraception-morning-after-pill-4363.asp. Accessed June 7, 2012.
6. Ellertson C. History and efficacy of emergency contraception: beyond Coca-Cola. Fam Plann Perspect. 1996;28:44-48.
7. Yuzpe AA, Thurlow HJ, Ramzy I, et al. Post coital contraception—a pilot study. J Reprod Med. 1974;13:53-58.
8. Glasier AF, Cameron ST, Fine PM, et al. Ulipristal acetate versus levonorgestrel for emergency contraception: a randomised non-inferiority trial and meta-analysis. Lancet. 2010;375:555-562.
9. Belden P, Harper CC, Speidel J. The copper IUD for emergency contraception, a neglected option. Contraception. 2012;85:338-339.
10. Wu S, Godfrey EM, Wojdyla D, et al. Copper T380A intrauterine device for emergency contraception: a prospective, multicentre, cohort clinical trial. Br J Obstet Gynaecol. 2010;117:1205-1210.
11. Cleland K, Zhu H, Goldstuck N, et al. The efficacy of intrauterine devices for emergency contraception: a systematic review of 35 years of experience. Hum Reprod. 2012 May 8 [Epub ahead of print].
12. World Health Organization’s Task Force on postovulatory methods of fertility regulation. Randomised controlled trial of levonorgestrel versus the Yuzpe regimen of combined oral contraceptives for emergency contraception. Lancet. 1998;352:428-433.
13. US Food and Drug Administration. Plan B: questions and answers. Updated December 14, 2006. Available at: http://www.fda.gov/Drugs/DrugSafety/PostmarketDrugSafetyInformationforPatientsandProviders/ucm109783.htm. Accessed June 7, 2012.
14. Trussell J, Rodriguez G, Ellertson C. A meta-analysis of efficacy for the Yuzpe method (estrogen-progestin). Contraception. 1999;59:147-151.
15. Piaggio G, von Hertzen H, Grimes DA, et al. Task Force on Postovulatory Methods of Fertility. Timing of emergency contraception with levonorgestrel or the Yuzpe regimen. Lancet. 1999;353:721.
16. Cheng L, Gülmezoglu AM, Piaggio GGP, et al. Interventions for emergency contraception. Cochrane Database Syst Rev. 2008;(2):CD001324.
17. Ngai SW, Fan S, Li S, et al. A randomized trial to compare 24 h versus 12 h double dose regimen of levonorgestrel for emergency contraception. Hum Reprod. 2005;20:307-311.
18. Stratton P, Hartog B, Hajizadeh N, et al. A single mid-follicular dose of CDB-2914, a new antiprogestin, inhibits folliculogenesis and endometrial differentiation in normally cycling women. Hum Reprod. 2000;5:1092-1099.
19. Brache V, Cochon L, Jesam C, et al. Immediate pre-ovulatory administration of 30 mg ulipristal acetate significantly delays follicular rupture. Hum Reprod. 2010;25:2256-2263.
20. Kliff S. Plan B’s complicated history. Available at: http://www.thedailybeast.com/newsweek/2009/08/24/plan-b-s-complicated-history.html/. Accessed June 7, 2012.
21. US Food and Drug Administration Updated FDA action on Plan B (levonorgestrel) tablets. April 22, 2009. Available at: http://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/2009/ucm149568.htm. Accessed June 7, 2012.
22. US Department of Health and Human Services. A statement by U.S. Department of Health and Human Services Secretary Kathleen Sebelius. December 7, 2011. Available at: http://www.hhs.gov/news/press/2011pres/12/20111207a.html. Accessed June 7, 2012.
23. Duffy K, Gold M. Adolescents and emergency contraception: update 2011. Curr Opin Obstet Gynecol. 2011;23:328-333.
24. National Conference of State Legislatures. Emergency contraception state laws. Updated July 2011. Available at: http://www.ncsl.org/issues-research/health/emergency-contraception-state-laws.aspx. Accessed May 31, 2012.
25. Cash S. New AAP policy advises on emergency contraception use. AAP News. 2005;26:1.
26. Polis CB, Grimes DA, Schaffer K, et al. Advance provision of EC for pregnancy prevention. Cochrane Database Syst Rev. 2010;(2):CD005497.
27. Raine T, Harper CC, Rocca CH, et al. Direct access to emergency contraception through pharmacies and effect on unintended pregnancy and STIs: a randomized controlled trial. JAMA. 2005;293:54-62.
28. Reproductive Health Access Project. Telephone triage protocol for emergency contraception. Available at: http://www.reproductiveaccess.org/contraception/tel_triage_ec.htm. Accessed May 31, 2012.
29. American College of Obstetricians and Gynecologists. Sexual assault. Committee Opinion No. 499. Obstet Gynecol. 2011;118:296-399.
• Offer emergency contraception (EC) to any woman who reports contraceptive failure or unprotected intercourse within the last 5 days; no clinical exam is necessary. B
• Prescribe a progestin-only EC or ulipristal acetate, both of which are more effective and have fewer adverse effects than an estrogen-progestin combination. A
• Consider giving sexually active teens <17 years an advance prescription for EC, as it is not available over the counter to this age group. C
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
The average American woman will spend more than 30 years of her life trying to prevent pregnancy—not always successfully. Each year, half of the approximately 6 million pregnancies in the United States are unintended.1 Emergency contraception (EC) gives a woman a second chance to prevent pregnancy after a contraceptive failure or unprotected sex. But all too often, it isn’t offered and she doesn’t request it.
Lack of knowledge about EC continues to be a barrier to its use. Some women have heard about the “morning after pill,” but may not know that EC can be effective for up to 5 days after intercourse—or even that it’s available in this country.2 Others are unaware that it is possible to prevent pregnancy after intercourse,2 and mistakenly believe that EC drugs are abortifacients. In fact, they work primarily by interfering with ovulation and have not been found to prevent implantation or to disrupt an existing pregnancy.3-5
Providers also contribute to the limited use of EC, often because they’re unfamiliar with the options or uncomfortable discussing them with patients, particularly sexually active teens.2
This update can help you clear up misconceptions about EC with your patients. It also provides evidence-based information about the various types of EC, a review of issues affecting accessibility, and a telephone triage protocol to guide your response to women seeking postcoital contraception.
EC today: Plan B and beyond
Hormonal EC was first studied in the 1920s, when researchers found that estrogenic ovarian extracts interfered with pregnancy in animals. The first regimen was a high-dose estrogen-only formulation. In 1974, a combined estrogen-progestin replaced it. Known as the Yuzpe method for the physician who discovered it,6 this regimen used a widely available brand of combined estrogen-progestin oral contraceptive pills. The standard dose consisted of 100 mcg ethinyl estradiol (EE) and 0.5 mg levonorgestrel (LNG) taken 12 hours apart.2,7
Although the Yuzpe method is still in use, progestin-only EC—Plan B as well as generic (Next Choice) and single-dose (Plan B One-Step) LNG formulations—has become the standard of care because it has greater efficacy and fewer adverse effects.2 There are 2 additional options: the copper intrauterine device (IUD), which is highly effective both as EC and as a long-term contraceptive,6 and ulipristal acetate (UPA), which received US Food and Drug Administration (FDA) approval in 2010. This second-generation antiprogestin, sold under the brand name Ella, is well tolerated and highly effective.8
EC efficacy: What the evidence shows
EC is most likely to work when used within 24 hours, but remains effective—albeit to varying degrees—for up to 120 hours (TABLE).2,5,8,9 Thus, which EC is best for a particular patient depends, in part, on timing.
TABLE
Emergency contraception: Comparing methods*2,5,8,9
| EC method | Dose and timing | Benefits | Adverse effects/ drawbacks |
|---|---|---|---|
| Estrogen-progestin OCs | 100 mcg EE and 0.5 mg LNG, taken 12 h apart First dose within 72 h | Easily accessible and widely available; patient may use OCs she already has at home | Higher rates of adverse effects, including nausea, vomiting, headache; less effective than other methods |
| Progestin-only (Plan B, Next Choice, others) | 1.5 mg LNG within 72 h (available in divided doses or in a single tablet; 2 tablets may be taken as a single dose) | Available OTC for patients ≥17 y; more effective and fewer adverse effects than estrogen-progestin Convenience of single dose | Prescription required for patients <17 y Approved for use within 72 h; effectiveness diminishes thereafter |
| UPA (Ella) | 30 mg UPA, taken ≤120 h† | More effective than LNG; fewer adverse effects than estrogen-progestin Efficacy remains high ≤5 days Convenience of single dose | Prescription required; not available at all pharmacies Not studied in breastfeeding‡ |
| Copper IUD | Insert ≤120 h | Extremely effective Provides immediate, long-term contraception | Insertion requires staff training; higher cost than oral EC |
| EC, emergency contraception; EE, ethinyl estradiol; IUD, intrauterine device; LNG, levonorgestrel; OCs, oral contraceptives; OTC, over the counter; UPA, ulipristal acetate. *Low doses of mifepristone (<25-50 mg)—approved as an abortifacient in much larger doses—may also be used as EC. †Dosage should be repeated if vomiting occurs within 3 hours. ‡Advise patients to avoid breastfeeding for 36 hours | |||
Copper IUDs have the highest success rate: Studies have found the copper IUD to be >99% effective in preventing pregnancy when inserted within 5 days of unprotected intercourse.9,10 The copper ions it contains have a toxic effect on sperm, and impair the potential for fertilization; the device may also make the endometrium inhospitable to implantation.9,10
A just-published systematic review of 42 studies in 6 countries over a period of more than 30 years yielded similar results: Among more than 7000 women who had the IUDs inserted after unprotected intercourse, the pregnancy rate was 0.09%.11
But an IUD is appropriate only for women who want long-term contraception and would otherwise qualify for IUD insertion. By comparison, hormonal EC is not as effective and generally works best when used within a shorter time frame.
Progestin alone vs estrogen-progestin combo. To compare hormonal contraception, many researchers use a “prevented fraction”—an estimated percentage of pregnancies averted by treatment. A large World Health Organization-sponsored study found that the efficacy of progestin-only EC is superior to that of the estrogen-progestin combination, with prevented fractions of 85% and 57%, respectively. The progestin-only EC was also associated with significantly fewer adverse effects.12
In more recent studies, the prevented fraction for progestin-only EC has been found to range from 60% to 94%, while a meta-analysis of studies assessing estrogen-progestin EC r evealed a prevented fraction of ≥74%.2
Although there is evidence suggesting that progestin-only EC may work for up to 5 days,13,14 it has FDA approval only for use within 72 hours of intercourse.13 A time-sensitive analysis showed that when it was used within 12 hours of intercourse, the pregnancy rate was 0.5%. The rate increased steadily to 4.1% when the progestin-based EC was taken 61 to 72 hours after intercourse, and rose by an additional 50% after an additional 12-hour delay.15
Hormonal EC is only effective before ovulation occurs. Once luteinizing hormone (LH) starts to rise, it is ineffective. However, the likelihood of pregnancy drops precipitously after ovulation, and there is no risk of pregnancy in the luteal phase, with or without EC.
One pill or 2? Both Plan B and the generic Next Choice are sold as 2-dose regimens, with one 0.75-mg tablet taken within 72 hours and the second taken 12 hours later. Plan B One-Step, which consists of a single 1.5-mg tablet, is clinically equivalent to the 2-dose formula,16 but is more convenient and may improve adherence. Notably, though, one large randomized controlled trial (RCT) in China found that the 2-pill regimen was significantly more effective in preventing pregnancy in women who had further acts of unprotected intercourse after treatment.17
UPA has a 5-day window. UPA has FDA approval for use within 120 hours of unprotected intercourse and has been found to be more effective than progestin-only EC, especially when used on Day 4 or 5 (72-120 hours).8 Adverse effects are mild to moderate, similar to those of LNG, and may include headache, abdominal pain, nausea, dysmenorrhea, fatigue, and dizziness.8
The medication binds to progesterone receptors, acting as an antagonist as well as a partial agonist. The mechanism of action depends on the phase of the woman’s cycle. Taken during the midfollicular phase, UPA inhibits follicle development.18 When used in the advanced follicular phase, just prior to ovulation, it delays LH peak and postpones ovulation.19
In one small study in which women were randomized to either UPA or placebo, researchers found that the drug delayed ovulation for ≥5 days in about 60% of those who took it; in comparison, ovulation occurred by Day 5 in every woman in the placebo group.19
How accessible is EC?
EC has a tumultuous history in the United States,20 and accessibility depends on a variety of factors—age among them.
Plan B, for instance, is subject to a 2-tier system. It was approved in 1999 as a prescription-only product and has been available over the counter (OTC) to women 17 years and older since 2009. Younger women can get it only by prescription.21
Nonetheless, Plan B made the news again last year, when US Health and Human Services Secretary Kathleen Sebelius overruled an FDA decision to give teens younger than 17 OTC access.22 Thus, the age restriction remains in place, although there is no medical evidence to support it.23 Other forms of EC, including UPA, are available to all women only by prescription.
Accessibility of EC also may vary from one part of the country to another. Some states have enacted laws with conscience clauses that allow pharmacists to refuse to dispense EC. Others have worked to increase access by authorizing pharmacists to initiate and dispense EC on their own, provided they work in collaboration with a doctor or other licensed prescriber. As of 2011, 9 states—Alaska, California, Hawaii, Maine, Massachusetts, New Hampshire, New Mexico, Vermont, and Washington—had such agreements in place.24
Cost is another potential barrier. The cost of oral EC varies from about $10 to $70, plus the cost of a doctor visit for a teen who needs a prescription. Obtaining the copper IUD without insurance coverage would cost hundreds of dollars, to cover the price of insertion as well as the device.5
Increasing access: What you can do
In view of the barriers that adolescents face in obtaining EC, the American College of Obstetricians and Gynecologists and the American Academy of Pediatrics, among other organizations, recommend that physicians give advance prescriptions to teens under the age of 17. 2,25
But how likely are they to actually buy the medication and use it on an emergency basis?
A 2007 Cochrane review found that giving women advance prescriptions for EC did not reduce pregnancy or abortion rates.26 Other studies have found that EC use is highest among women with the lowest risk of pregnancy—those who are already using contraception and are less likely to have unprotected intercourse. Those at the highest risk for unintended pregnancy were found to be less likely to use EC after every episode of unprotected intercourse.23,26 One RCT demonstrated that rates of pregnancy and sexually transmitted infection were not significantly increased by advance provision of EC, leading the researchers to conclude that it was therefore unreasonable to restrict access.27
While it is prudent to make women aware that EC is available should they need it, the focus should be on the fact that consistent use of a reliable form of contraception—an IUD or hormonal contraception, in particular—gives them the best chance of preventing an unwanted pregnancy.
What to do when that call comes in
When a woman calls to report a contraceptive failure or tells you she has had unprotected intercourse, start by finding out how recently it occurred. Subsequent questions and actions that can be used by triage nurses or physicians on call are detailed in the easy-to-use EC telephone triage protocol (FIGURE)28 on page 395. Whether you prescribe oral EC or schedule an appointment to insert a copper IUD within the next few days, there are a number of key points to keep in mind.
Initiate EC as soon as possible, but make it available to any woman who requests it for up to 5 days after unprotected intercourse.
Advise patients that oral EC is safe for most women—even those with contraindications to oral contraceptives. No physical examination is necessary, and there’s usually no need for a pregnancy test.2 The one exception: A woman who has not had a period in the past 30 days should be given a pregnancy test before taking UPA.2
Offer EC at any time in the cycle. Although EC works primarily in the preovulatory phase, it should be offered regardless of the phase of the patient’s menstrual cycle. That’s because of the possibility of late ovulation, as well as the difficulty in accurately determining the phase of a woman’s cycle based on a history alone.
Make EC available to any woman who has been sexually abused. At many emergency departments, EC is not routinely offered to women who come in after being raped, although it clearly should be.29
FIGURE
Telephone triage protocol for emergency contraception
EC, emergency contraception; IUD, intrauterine device; LNG, levonorgestrel; Rx, prescription; UPA, ulipristal acetate.
Adapted from: Reproductive Health Access Project. http://www.reproductiveaccess.org/contraception/tel_triage_ec.htm.28
Patient counseling about EC
Advise patients for whom you prescribe oral EC that the medication delays ovulation, which means they could be at risk for pregnancy later in the cycle. Stress the need to use an alternative means of contraception (a barrier method is recommended for women taking UPA) until their next menses and to come in for a pregnancy test if their period is more than a week late.
Point out, too, that EC can be used more than once within the same cycle, if necessary. That said, even a single request for EC should result in a discussion of effective, longer-term contraception, including the possibility of an IUD.
CORRESPONDENCE Sarina Schrager, MD, MS, University of Wisconsin School of Public Health, Department of Family Medicine, 1100 Delaplaine Court, Madison, WI 53715; sbschrag@wisc.edu
• Offer emergency contraception (EC) to any woman who reports contraceptive failure or unprotected intercourse within the last 5 days; no clinical exam is necessary. B
• Prescribe a progestin-only EC or ulipristal acetate, both of which are more effective and have fewer adverse effects than an estrogen-progestin combination. A
• Consider giving sexually active teens <17 years an advance prescription for EC, as it is not available over the counter to this age group. C
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
The average American woman will spend more than 30 years of her life trying to prevent pregnancy—not always successfully. Each year, half of the approximately 6 million pregnancies in the United States are unintended.1 Emergency contraception (EC) gives a woman a second chance to prevent pregnancy after a contraceptive failure or unprotected sex. But all too often, it isn’t offered and she doesn’t request it.
Lack of knowledge about EC continues to be a barrier to its use. Some women have heard about the “morning after pill,” but may not know that EC can be effective for up to 5 days after intercourse—or even that it’s available in this country.2 Others are unaware that it is possible to prevent pregnancy after intercourse,2 and mistakenly believe that EC drugs are abortifacients. In fact, they work primarily by interfering with ovulation and have not been found to prevent implantation or to disrupt an existing pregnancy.3-5
Providers also contribute to the limited use of EC, often because they’re unfamiliar with the options or uncomfortable discussing them with patients, particularly sexually active teens.2
This update can help you clear up misconceptions about EC with your patients. It also provides evidence-based information about the various types of EC, a review of issues affecting accessibility, and a telephone triage protocol to guide your response to women seeking postcoital contraception.
EC today: Plan B and beyond
Hormonal EC was first studied in the 1920s, when researchers found that estrogenic ovarian extracts interfered with pregnancy in animals. The first regimen was a high-dose estrogen-only formulation. In 1974, a combined estrogen-progestin replaced it. Known as the Yuzpe method for the physician who discovered it,6 this regimen used a widely available brand of combined estrogen-progestin oral contraceptive pills. The standard dose consisted of 100 mcg ethinyl estradiol (EE) and 0.5 mg levonorgestrel (LNG) taken 12 hours apart.2,7
Although the Yuzpe method is still in use, progestin-only EC—Plan B as well as generic (Next Choice) and single-dose (Plan B One-Step) LNG formulations—has become the standard of care because it has greater efficacy and fewer adverse effects.2 There are 2 additional options: the copper intrauterine device (IUD), which is highly effective both as EC and as a long-term contraceptive,6 and ulipristal acetate (UPA), which received US Food and Drug Administration (FDA) approval in 2010. This second-generation antiprogestin, sold under the brand name Ella, is well tolerated and highly effective.8
EC efficacy: What the evidence shows
EC is most likely to work when used within 24 hours, but remains effective—albeit to varying degrees—for up to 120 hours (TABLE).2,5,8,9 Thus, which EC is best for a particular patient depends, in part, on timing.
TABLE
Emergency contraception: Comparing methods*2,5,8,9
| EC method | Dose and timing | Benefits | Adverse effects/ drawbacks |
|---|---|---|---|
| Estrogen-progestin OCs | 100 mcg EE and 0.5 mg LNG, taken 12 h apart First dose within 72 h | Easily accessible and widely available; patient may use OCs she already has at home | Higher rates of adverse effects, including nausea, vomiting, headache; less effective than other methods |
| Progestin-only (Plan B, Next Choice, others) | 1.5 mg LNG within 72 h (available in divided doses or in a single tablet; 2 tablets may be taken as a single dose) | Available OTC for patients ≥17 y; more effective and fewer adverse effects than estrogen-progestin Convenience of single dose | Prescription required for patients <17 y Approved for use within 72 h; effectiveness diminishes thereafter |
| UPA (Ella) | 30 mg UPA, taken ≤120 h† | More effective than LNG; fewer adverse effects than estrogen-progestin Efficacy remains high ≤5 days Convenience of single dose | Prescription required; not available at all pharmacies Not studied in breastfeeding‡ |
| Copper IUD | Insert ≤120 h | Extremely effective Provides immediate, long-term contraception | Insertion requires staff training; higher cost than oral EC |
| EC, emergency contraception; EE, ethinyl estradiol; IUD, intrauterine device; LNG, levonorgestrel; OCs, oral contraceptives; OTC, over the counter; UPA, ulipristal acetate. *Low doses of mifepristone (<25-50 mg)—approved as an abortifacient in much larger doses—may also be used as EC. †Dosage should be repeated if vomiting occurs within 3 hours. ‡Advise patients to avoid breastfeeding for 36 hours | |||
Copper IUDs have the highest success rate: Studies have found the copper IUD to be >99% effective in preventing pregnancy when inserted within 5 days of unprotected intercourse.9,10 The copper ions it contains have a toxic effect on sperm, and impair the potential for fertilization; the device may also make the endometrium inhospitable to implantation.9,10
A just-published systematic review of 42 studies in 6 countries over a period of more than 30 years yielded similar results: Among more than 7000 women who had the IUDs inserted after unprotected intercourse, the pregnancy rate was 0.09%.11
But an IUD is appropriate only for women who want long-term contraception and would otherwise qualify for IUD insertion. By comparison, hormonal EC is not as effective and generally works best when used within a shorter time frame.
Progestin alone vs estrogen-progestin combo. To compare hormonal contraception, many researchers use a “prevented fraction”—an estimated percentage of pregnancies averted by treatment. A large World Health Organization-sponsored study found that the efficacy of progestin-only EC is superior to that of the estrogen-progestin combination, with prevented fractions of 85% and 57%, respectively. The progestin-only EC was also associated with significantly fewer adverse effects.12
In more recent studies, the prevented fraction for progestin-only EC has been found to range from 60% to 94%, while a meta-analysis of studies assessing estrogen-progestin EC r evealed a prevented fraction of ≥74%.2
Although there is evidence suggesting that progestin-only EC may work for up to 5 days,13,14 it has FDA approval only for use within 72 hours of intercourse.13 A time-sensitive analysis showed that when it was used within 12 hours of intercourse, the pregnancy rate was 0.5%. The rate increased steadily to 4.1% when the progestin-based EC was taken 61 to 72 hours after intercourse, and rose by an additional 50% after an additional 12-hour delay.15
Hormonal EC is only effective before ovulation occurs. Once luteinizing hormone (LH) starts to rise, it is ineffective. However, the likelihood of pregnancy drops precipitously after ovulation, and there is no risk of pregnancy in the luteal phase, with or without EC.
One pill or 2? Both Plan B and the generic Next Choice are sold as 2-dose regimens, with one 0.75-mg tablet taken within 72 hours and the second taken 12 hours later. Plan B One-Step, which consists of a single 1.5-mg tablet, is clinically equivalent to the 2-dose formula,16 but is more convenient and may improve adherence. Notably, though, one large randomized controlled trial (RCT) in China found that the 2-pill regimen was significantly more effective in preventing pregnancy in women who had further acts of unprotected intercourse after treatment.17
UPA has a 5-day window. UPA has FDA approval for use within 120 hours of unprotected intercourse and has been found to be more effective than progestin-only EC, especially when used on Day 4 or 5 (72-120 hours).8 Adverse effects are mild to moderate, similar to those of LNG, and may include headache, abdominal pain, nausea, dysmenorrhea, fatigue, and dizziness.8
The medication binds to progesterone receptors, acting as an antagonist as well as a partial agonist. The mechanism of action depends on the phase of the woman’s cycle. Taken during the midfollicular phase, UPA inhibits follicle development.18 When used in the advanced follicular phase, just prior to ovulation, it delays LH peak and postpones ovulation.19
In one small study in which women were randomized to either UPA or placebo, researchers found that the drug delayed ovulation for ≥5 days in about 60% of those who took it; in comparison, ovulation occurred by Day 5 in every woman in the placebo group.19
How accessible is EC?
EC has a tumultuous history in the United States,20 and accessibility depends on a variety of factors—age among them.
Plan B, for instance, is subject to a 2-tier system. It was approved in 1999 as a prescription-only product and has been available over the counter (OTC) to women 17 years and older since 2009. Younger women can get it only by prescription.21
Nonetheless, Plan B made the news again last year, when US Health and Human Services Secretary Kathleen Sebelius overruled an FDA decision to give teens younger than 17 OTC access.22 Thus, the age restriction remains in place, although there is no medical evidence to support it.23 Other forms of EC, including UPA, are available to all women only by prescription.
Accessibility of EC also may vary from one part of the country to another. Some states have enacted laws with conscience clauses that allow pharmacists to refuse to dispense EC. Others have worked to increase access by authorizing pharmacists to initiate and dispense EC on their own, provided they work in collaboration with a doctor or other licensed prescriber. As of 2011, 9 states—Alaska, California, Hawaii, Maine, Massachusetts, New Hampshire, New Mexico, Vermont, and Washington—had such agreements in place.24
Cost is another potential barrier. The cost of oral EC varies from about $10 to $70, plus the cost of a doctor visit for a teen who needs a prescription. Obtaining the copper IUD without insurance coverage would cost hundreds of dollars, to cover the price of insertion as well as the device.5
Increasing access: What you can do
In view of the barriers that adolescents face in obtaining EC, the American College of Obstetricians and Gynecologists and the American Academy of Pediatrics, among other organizations, recommend that physicians give advance prescriptions to teens under the age of 17. 2,25
But how likely are they to actually buy the medication and use it on an emergency basis?
A 2007 Cochrane review found that giving women advance prescriptions for EC did not reduce pregnancy or abortion rates.26 Other studies have found that EC use is highest among women with the lowest risk of pregnancy—those who are already using contraception and are less likely to have unprotected intercourse. Those at the highest risk for unintended pregnancy were found to be less likely to use EC after every episode of unprotected intercourse.23,26 One RCT demonstrated that rates of pregnancy and sexually transmitted infection were not significantly increased by advance provision of EC, leading the researchers to conclude that it was therefore unreasonable to restrict access.27
While it is prudent to make women aware that EC is available should they need it, the focus should be on the fact that consistent use of a reliable form of contraception—an IUD or hormonal contraception, in particular—gives them the best chance of preventing an unwanted pregnancy.
What to do when that call comes in
When a woman calls to report a contraceptive failure or tells you she has had unprotected intercourse, start by finding out how recently it occurred. Subsequent questions and actions that can be used by triage nurses or physicians on call are detailed in the easy-to-use EC telephone triage protocol (FIGURE)28 on page 395. Whether you prescribe oral EC or schedule an appointment to insert a copper IUD within the next few days, there are a number of key points to keep in mind.
Initiate EC as soon as possible, but make it available to any woman who requests it for up to 5 days after unprotected intercourse.
Advise patients that oral EC is safe for most women—even those with contraindications to oral contraceptives. No physical examination is necessary, and there’s usually no need for a pregnancy test.2 The one exception: A woman who has not had a period in the past 30 days should be given a pregnancy test before taking UPA.2
Offer EC at any time in the cycle. Although EC works primarily in the preovulatory phase, it should be offered regardless of the phase of the patient’s menstrual cycle. That’s because of the possibility of late ovulation, as well as the difficulty in accurately determining the phase of a woman’s cycle based on a history alone.
Make EC available to any woman who has been sexually abused. At many emergency departments, EC is not routinely offered to women who come in after being raped, although it clearly should be.29
FIGURE
Telephone triage protocol for emergency contraception
EC, emergency contraception; IUD, intrauterine device; LNG, levonorgestrel; Rx, prescription; UPA, ulipristal acetate.
Adapted from: Reproductive Health Access Project. http://www.reproductiveaccess.org/contraception/tel_triage_ec.htm.28
Patient counseling about EC
Advise patients for whom you prescribe oral EC that the medication delays ovulation, which means they could be at risk for pregnancy later in the cycle. Stress the need to use an alternative means of contraception (a barrier method is recommended for women taking UPA) until their next menses and to come in for a pregnancy test if their period is more than a week late.
Point out, too, that EC can be used more than once within the same cycle, if necessary. That said, even a single request for EC should result in a discussion of effective, longer-term contraception, including the possibility of an IUD.
CORRESPONDENCE Sarina Schrager, MD, MS, University of Wisconsin School of Public Health, Department of Family Medicine, 1100 Delaplaine Court, Madison, WI 53715; sbschrag@wisc.edu
1. Guttmacher Institute. National Reproductive Health profile. Available at: http://www.guttmacher.org/datacenter/profiles/print/US.jsp. Accessed November 3, 2011.
2. American College of Obstetricians and Gynecologists ACOG practice bulletin No. 112. Emergency contraception. Obstet Gynecol. 2010;115:1100–1109.
3. Belluck P. No abortion role seen for morning-after pill. New York Times. June 6, 2012; A1.
4. Trussell J, Raymond E. Emergency contraception: a last chance to prevent unintended pregnancy. Princeton, NJ: Office of Population Research at Princeton University; June 2012. Available at: http://ec.princeton.edu/questions/ec-review.pdf. Accessed June 20, 2012.
5. Planned Parenthood. Morning-after pill (emergency contraception). Available at: http://www.plannedparenthood.org/health-topics/emergency-contraception-morning-after-pill-4363.asp. Accessed June 7, 2012.
6. Ellertson C. History and efficacy of emergency contraception: beyond Coca-Cola. Fam Plann Perspect. 1996;28:44-48.
7. Yuzpe AA, Thurlow HJ, Ramzy I, et al. Post coital contraception—a pilot study. J Reprod Med. 1974;13:53-58.
8. Glasier AF, Cameron ST, Fine PM, et al. Ulipristal acetate versus levonorgestrel for emergency contraception: a randomised non-inferiority trial and meta-analysis. Lancet. 2010;375:555-562.
9. Belden P, Harper CC, Speidel J. The copper IUD for emergency contraception, a neglected option. Contraception. 2012;85:338-339.
10. Wu S, Godfrey EM, Wojdyla D, et al. Copper T380A intrauterine device for emergency contraception: a prospective, multicentre, cohort clinical trial. Br J Obstet Gynaecol. 2010;117:1205-1210.
11. Cleland K, Zhu H, Goldstuck N, et al. The efficacy of intrauterine devices for emergency contraception: a systematic review of 35 years of experience. Hum Reprod. 2012 May 8 [Epub ahead of print].
12. World Health Organization’s Task Force on postovulatory methods of fertility regulation. Randomised controlled trial of levonorgestrel versus the Yuzpe regimen of combined oral contraceptives for emergency contraception. Lancet. 1998;352:428-433.
13. US Food and Drug Administration. Plan B: questions and answers. Updated December 14, 2006. Available at: http://www.fda.gov/Drugs/DrugSafety/PostmarketDrugSafetyInformationforPatientsandProviders/ucm109783.htm. Accessed June 7, 2012.
14. Trussell J, Rodriguez G, Ellertson C. A meta-analysis of efficacy for the Yuzpe method (estrogen-progestin). Contraception. 1999;59:147-151.
15. Piaggio G, von Hertzen H, Grimes DA, et al. Task Force on Postovulatory Methods of Fertility. Timing of emergency contraception with levonorgestrel or the Yuzpe regimen. Lancet. 1999;353:721.
16. Cheng L, Gülmezoglu AM, Piaggio GGP, et al. Interventions for emergency contraception. Cochrane Database Syst Rev. 2008;(2):CD001324.
17. Ngai SW, Fan S, Li S, et al. A randomized trial to compare 24 h versus 12 h double dose regimen of levonorgestrel for emergency contraception. Hum Reprod. 2005;20:307-311.
18. Stratton P, Hartog B, Hajizadeh N, et al. A single mid-follicular dose of CDB-2914, a new antiprogestin, inhibits folliculogenesis and endometrial differentiation in normally cycling women. Hum Reprod. 2000;5:1092-1099.
19. Brache V, Cochon L, Jesam C, et al. Immediate pre-ovulatory administration of 30 mg ulipristal acetate significantly delays follicular rupture. Hum Reprod. 2010;25:2256-2263.
20. Kliff S. Plan B’s complicated history. Available at: http://www.thedailybeast.com/newsweek/2009/08/24/plan-b-s-complicated-history.html/. Accessed June 7, 2012.
21. US Food and Drug Administration Updated FDA action on Plan B (levonorgestrel) tablets. April 22, 2009. Available at: http://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/2009/ucm149568.htm. Accessed June 7, 2012.
22. US Department of Health and Human Services. A statement by U.S. Department of Health and Human Services Secretary Kathleen Sebelius. December 7, 2011. Available at: http://www.hhs.gov/news/press/2011pres/12/20111207a.html. Accessed June 7, 2012.
23. Duffy K, Gold M. Adolescents and emergency contraception: update 2011. Curr Opin Obstet Gynecol. 2011;23:328-333.
24. National Conference of State Legislatures. Emergency contraception state laws. Updated July 2011. Available at: http://www.ncsl.org/issues-research/health/emergency-contraception-state-laws.aspx. Accessed May 31, 2012.
25. Cash S. New AAP policy advises on emergency contraception use. AAP News. 2005;26:1.
26. Polis CB, Grimes DA, Schaffer K, et al. Advance provision of EC for pregnancy prevention. Cochrane Database Syst Rev. 2010;(2):CD005497.
27. Raine T, Harper CC, Rocca CH, et al. Direct access to emergency contraception through pharmacies and effect on unintended pregnancy and STIs: a randomized controlled trial. JAMA. 2005;293:54-62.
28. Reproductive Health Access Project. Telephone triage protocol for emergency contraception. Available at: http://www.reproductiveaccess.org/contraception/tel_triage_ec.htm. Accessed May 31, 2012.
29. American College of Obstetricians and Gynecologists. Sexual assault. Committee Opinion No. 499. Obstet Gynecol. 2011;118:296-399.
1. Guttmacher Institute. National Reproductive Health profile. Available at: http://www.guttmacher.org/datacenter/profiles/print/US.jsp. Accessed November 3, 2011.
2. American College of Obstetricians and Gynecologists ACOG practice bulletin No. 112. Emergency contraception. Obstet Gynecol. 2010;115:1100–1109.
3. Belluck P. No abortion role seen for morning-after pill. New York Times. June 6, 2012; A1.
4. Trussell J, Raymond E. Emergency contraception: a last chance to prevent unintended pregnancy. Princeton, NJ: Office of Population Research at Princeton University; June 2012. Available at: http://ec.princeton.edu/questions/ec-review.pdf. Accessed June 20, 2012.
5. Planned Parenthood. Morning-after pill (emergency contraception). Available at: http://www.plannedparenthood.org/health-topics/emergency-contraception-morning-after-pill-4363.asp. Accessed June 7, 2012.
6. Ellertson C. History and efficacy of emergency contraception: beyond Coca-Cola. Fam Plann Perspect. 1996;28:44-48.
7. Yuzpe AA, Thurlow HJ, Ramzy I, et al. Post coital contraception—a pilot study. J Reprod Med. 1974;13:53-58.
8. Glasier AF, Cameron ST, Fine PM, et al. Ulipristal acetate versus levonorgestrel for emergency contraception: a randomised non-inferiority trial and meta-analysis. Lancet. 2010;375:555-562.
9. Belden P, Harper CC, Speidel J. The copper IUD for emergency contraception, a neglected option. Contraception. 2012;85:338-339.
10. Wu S, Godfrey EM, Wojdyla D, et al. Copper T380A intrauterine device for emergency contraception: a prospective, multicentre, cohort clinical trial. Br J Obstet Gynaecol. 2010;117:1205-1210.
11. Cleland K, Zhu H, Goldstuck N, et al. The efficacy of intrauterine devices for emergency contraception: a systematic review of 35 years of experience. Hum Reprod. 2012 May 8 [Epub ahead of print].
12. World Health Organization’s Task Force on postovulatory methods of fertility regulation. Randomised controlled trial of levonorgestrel versus the Yuzpe regimen of combined oral contraceptives for emergency contraception. Lancet. 1998;352:428-433.
13. US Food and Drug Administration. Plan B: questions and answers. Updated December 14, 2006. Available at: http://www.fda.gov/Drugs/DrugSafety/PostmarketDrugSafetyInformationforPatientsandProviders/ucm109783.htm. Accessed June 7, 2012.
14. Trussell J, Rodriguez G, Ellertson C. A meta-analysis of efficacy for the Yuzpe method (estrogen-progestin). Contraception. 1999;59:147-151.
15. Piaggio G, von Hertzen H, Grimes DA, et al. Task Force on Postovulatory Methods of Fertility. Timing of emergency contraception with levonorgestrel or the Yuzpe regimen. Lancet. 1999;353:721.
16. Cheng L, Gülmezoglu AM, Piaggio GGP, et al. Interventions for emergency contraception. Cochrane Database Syst Rev. 2008;(2):CD001324.
17. Ngai SW, Fan S, Li S, et al. A randomized trial to compare 24 h versus 12 h double dose regimen of levonorgestrel for emergency contraception. Hum Reprod. 2005;20:307-311.
18. Stratton P, Hartog B, Hajizadeh N, et al. A single mid-follicular dose of CDB-2914, a new antiprogestin, inhibits folliculogenesis and endometrial differentiation in normally cycling women. Hum Reprod. 2000;5:1092-1099.
19. Brache V, Cochon L, Jesam C, et al. Immediate pre-ovulatory administration of 30 mg ulipristal acetate significantly delays follicular rupture. Hum Reprod. 2010;25:2256-2263.
20. Kliff S. Plan B’s complicated history. Available at: http://www.thedailybeast.com/newsweek/2009/08/24/plan-b-s-complicated-history.html/. Accessed June 7, 2012.
21. US Food and Drug Administration Updated FDA action on Plan B (levonorgestrel) tablets. April 22, 2009. Available at: http://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/2009/ucm149568.htm. Accessed June 7, 2012.
22. US Department of Health and Human Services. A statement by U.S. Department of Health and Human Services Secretary Kathleen Sebelius. December 7, 2011. Available at: http://www.hhs.gov/news/press/2011pres/12/20111207a.html. Accessed June 7, 2012.
23. Duffy K, Gold M. Adolescents and emergency contraception: update 2011. Curr Opin Obstet Gynecol. 2011;23:328-333.
24. National Conference of State Legislatures. Emergency contraception state laws. Updated July 2011. Available at: http://www.ncsl.org/issues-research/health/emergency-contraception-state-laws.aspx. Accessed May 31, 2012.
25. Cash S. New AAP policy advises on emergency contraception use. AAP News. 2005;26:1.
26. Polis CB, Grimes DA, Schaffer K, et al. Advance provision of EC for pregnancy prevention. Cochrane Database Syst Rev. 2010;(2):CD005497.
27. Raine T, Harper CC, Rocca CH, et al. Direct access to emergency contraception through pharmacies and effect on unintended pregnancy and STIs: a randomized controlled trial. JAMA. 2005;293:54-62.
28. Reproductive Health Access Project. Telephone triage protocol for emergency contraception. Available at: http://www.reproductiveaccess.org/contraception/tel_triage_ec.htm. Accessed May 31, 2012.
29. American College of Obstetricians and Gynecologists. Sexual assault. Committee Opinion No. 499. Obstet Gynecol. 2011;118:296-399.