User login
Low back pain in youth: Recognizing red flags
Low back pain in not uncommon in children and adolescents.1-3 Although the prevalence of low back pain in children < 7 years is low, it increases with age, with studies reporting lifetime prevalence at age 12 years between 16% and 18% and rates as high as 66% by 16 years of age.4,5 Although children and adolescents usually have pain that is transient and benign without a defined cause, structural causes of low back pain should be considered in school-aged children with pain that persists for > 3 to 6 weeks. 4 The most common structural causes of adolescent low back pain are reviewed here.
Etiology: A mixed bag
Back pain in school-aged children is most commonly due to muscular strain, overuse, or poor posture. The pain is often transient in nature and responds to rest and postural education.4,6 A herniated disc is an uncommon finding in younger school-aged children, but incidence increases slightly among older adolescents, particularly those who are active in collision sports and/or weight-lifting.7,8 Pain caused by a herniated disc often radiates along the distribution of the sciatic nerve and worsens during lumbar flexion.
Spondylolysis and spondylolisthesis are important causes of back pain in children. Spondylolysis is defined as a defect or abnormality of the pars interarticularis and surrounding lamina and pedicle. Spondylolisthesis, which is less common, is defined as the translation or “slippage” of one vertebral segment in relation to the next caudal segment. These conditions commonly occur as a result of repetitive stress.
In a prospective study of adolescents < 19 years with low back pain for > 2 weeks, the prevalence of spondylolysis was 39.7%.9 Adolescent athletes with symptomatic low back pain are more likely to have spondylolysis than nonathletes (32% vs 2%, respectively).2,10 Pain is often made worse by extension of the spine. Spondylolysis and spondylolisthesis can be congenital or acquired, and both can be asymptomatic. Children and teens who are athletes are at higher risk for symptomatic spondylolysis and spondylolisthesis.10-12 This is especially true for those involved in gymnastics, dance, football, and/or volleyball, where a repetitive load is placed onto an extended spine.
Idiopathic scoliosis is an abnormal lateral curvature of the spine that usually develops during adolescence and worsens with growth. Historically, painful scoliosis was considered rare, but more recently researchers determined that children with scoliosis have a higher rate of pain compared to their peers.13,14 School-aged children with scoliosis were found to be at 2 times the risk of low back pain compared to those without scoliosis.13 It is important to identify scoliosis in adolescents so that progression can be monitored.
Screening for scoliosis in primary care is somewhat controversial. The US Preventive Services Task Force (USPSTF) finds insufficient evidence for screening asymptomatic adolescents for scoliosis.15 This recommendation is based on the fact that there is little evidence on the effect of screening on long-term outcomes. Screening may also lead to unnecessary radiation. Conversely, a position statement released by the Scoliosis Research Society, the Pediatric Orthopedic Society of North America, the American Association of Orthopedic Surgeons, and the American Academy of Pediatrics recommends scoliosis screening during routine pediatric office visits.16 Screening for girls is recommended at ages 10 and 12 years, and for boys, once between ages 13 and 14 years. The statement highlights evidence showing that focused screening by appropriate personnel has value in detecting a clinically significant curve (> 20°).
Scheuermann disease is a rare cause of back pain in children that usually develops during adolescence and results in increasing thoracic kyphosis. An autosomal dominant mutation plays a role in this disease of the growth cartilage endplate; repetitive strain on the growth cartilage is also a contributing factor.17,18 An atypical variant manifests with kyphosis in the thoracolumbar region.17
Continue to: Other causes of low back pain
Other causes of low back pain—including inflammatory arthritis, infection (eg, discitis), and tumor—are rare in children but must always be considered, especially in the setting of persistent symptoms.4,19-21 More on the features of these conditions is listed in TABLE 1.1-7,13-15,17-30
History: Focus on onset, timing, and duration of symptoms
As with adults, obtaining a history that includes the onset, timing, and duration of symptoms is key in the evaluation of low back pain in children, as is obtaining a history of the patient’s activities; sports that repetitively load the lumbar spine in an extended position increase the risk of injury.10
Specific risk factors for low back pain in children and adolescents are poorly understood.4,9,31 Pain can be associated with trauma, or it can have a more progressive or insidious onset. Generally, pain that is present for up to 6 weeks and is intermittent or improving has a self-limited course. Pain that persists beyond 3 to 6 weeks or is worsening is more likely to have an anatomical cause that needs further evaluation.2,3,10,21
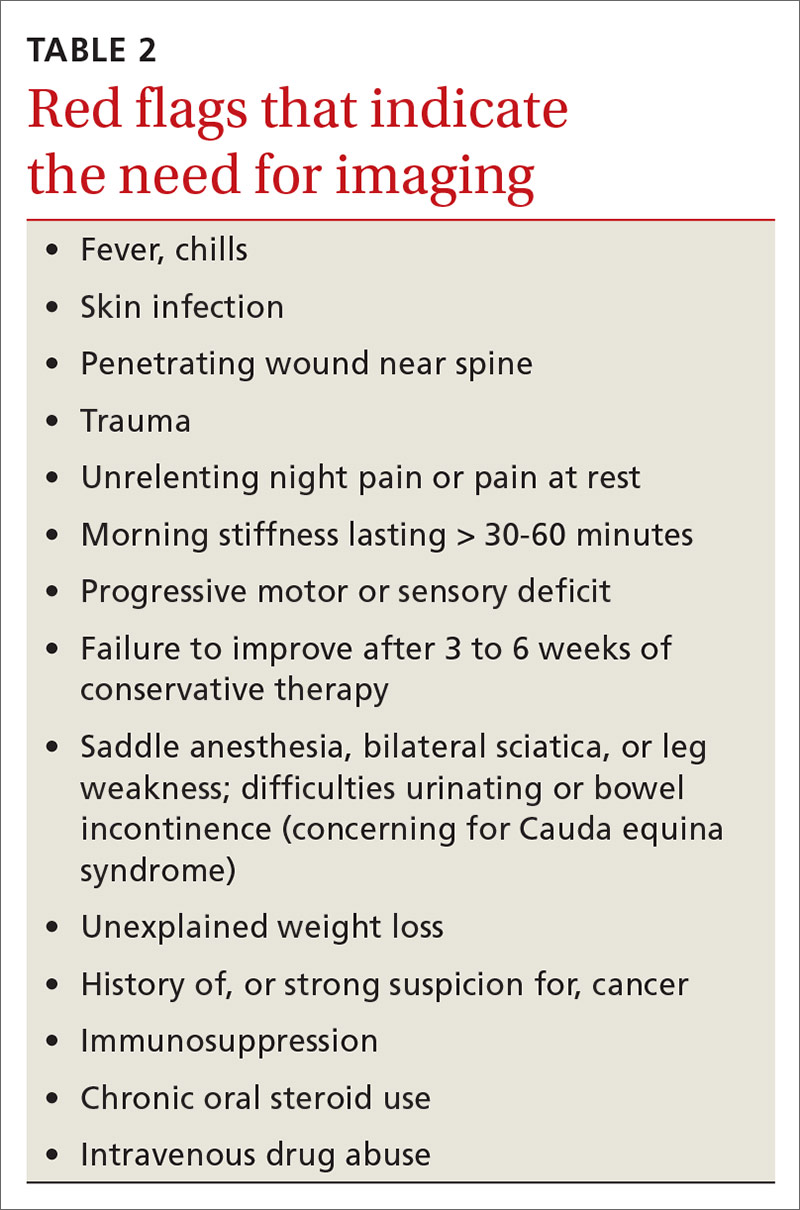
Identifying exacerbating and alleviating factors can provide useful information. Pain that is worse with lumbar flexion is more likely to come from muscular strain or disc pathology. Pain with extension is more likely due to a structural cause such as spondylolysis/spondylolisthesis, scoliosis, or Scheuermann disease.2,4,10,17,18,21 See TABLE 2 for red flag symptoms that indicate the need for imaging and further work-up.
The physical exam: Visualize, assess range of motion, and reproduce pain
The physical examination of any patient with low back pain should include direct visualization and inspection of the back, spine, and pelvis; palpation of the spine and paraspinal regions; assessment of lumbar range of motion and of the lumbar nerve roots, including tests of sensation, strength, and deep tendon reflexes; and an evaluation of the patient’s posture, which can provide clues to underlying causes of pain.
Continue to: Increased thoracic kyphosis...
Increased thoracic kyphosis that is not reversible is concerning for Scheuermann disease.9,17,18 A significant elevation in one shoulder or side of the pelvis can be indicative of scoliosis. Increased lumbar lordosis may predispose a patient to spondylolysis.
In patients with spondylolysis, lumbar extension will usually reproduce pain, which is often unilateral. Hyperextension in a single-leg stance, commonly known as the Stork test, is positive for unilateral spondylolysis when it reproduces pain on the ipsilateral side. The sensitivity of the Stork test for unilateral spondylolysis is approximately 50%.32 (For more information on the Stork test, see www.physio-pedia.com/Stork_test.)
Pain reproduced with lumbar flexion is less concerning for bony pathology and is most often related to soft-tissue strain. Lumbar flexion with concomitant radicular pain is associated with disc pathology.8 Pain with a straight-leg raise is also associated with disk pathology, especially if raising the contralateral leg increases pain.8
Using a scoliometer. Evaluate the flexed spine for the presence of asymmetry, which can indicate scoliosis.33 If asymmetry is present, use a scoliometer to determine the degree of asymmetry. Zero to 5° is considered clinically insignificant; monitor and reevaluate these patients at subsequent visits.34,35 Ten degrees or more of asymmetry with a scoliometer should prompt you to order radiographs.35,36 A smartphone-based scoliometer for iPhones was evaluated in 1 study and was shown to have reasonable reliability and validity for clinical use.37
Deformity of the lower extremities. Because low back pain may be caused by biomechanical or structural deformity of the lower extremities, examine the flexibility of the hip flexors, gluteal musculature, hamstrings, and the iliotibial band.38 In addition, evaluate for leg-length discrepancy and lower-extremity malalignment, such as femoral anteversion, tibial torsion, or pes planus.
Continue to: Imaging
Imaging: Know when it’s needed
Although imaging of the lumbar spine is often unnecessary in the presence of acute low back pain in children, always consider imaging in the setting of bony tenderness, pain that wakes a patient from sleep, and in the setting of other red flag symptoms (see TABLE 2). Low back pain in children that is reproducible with lumbar extension is concerning for spondylolysis or spondylolisthesis. If the pain with extension persists beyond 3 to 6 weeks, order imaging starting with radiographs.2,39
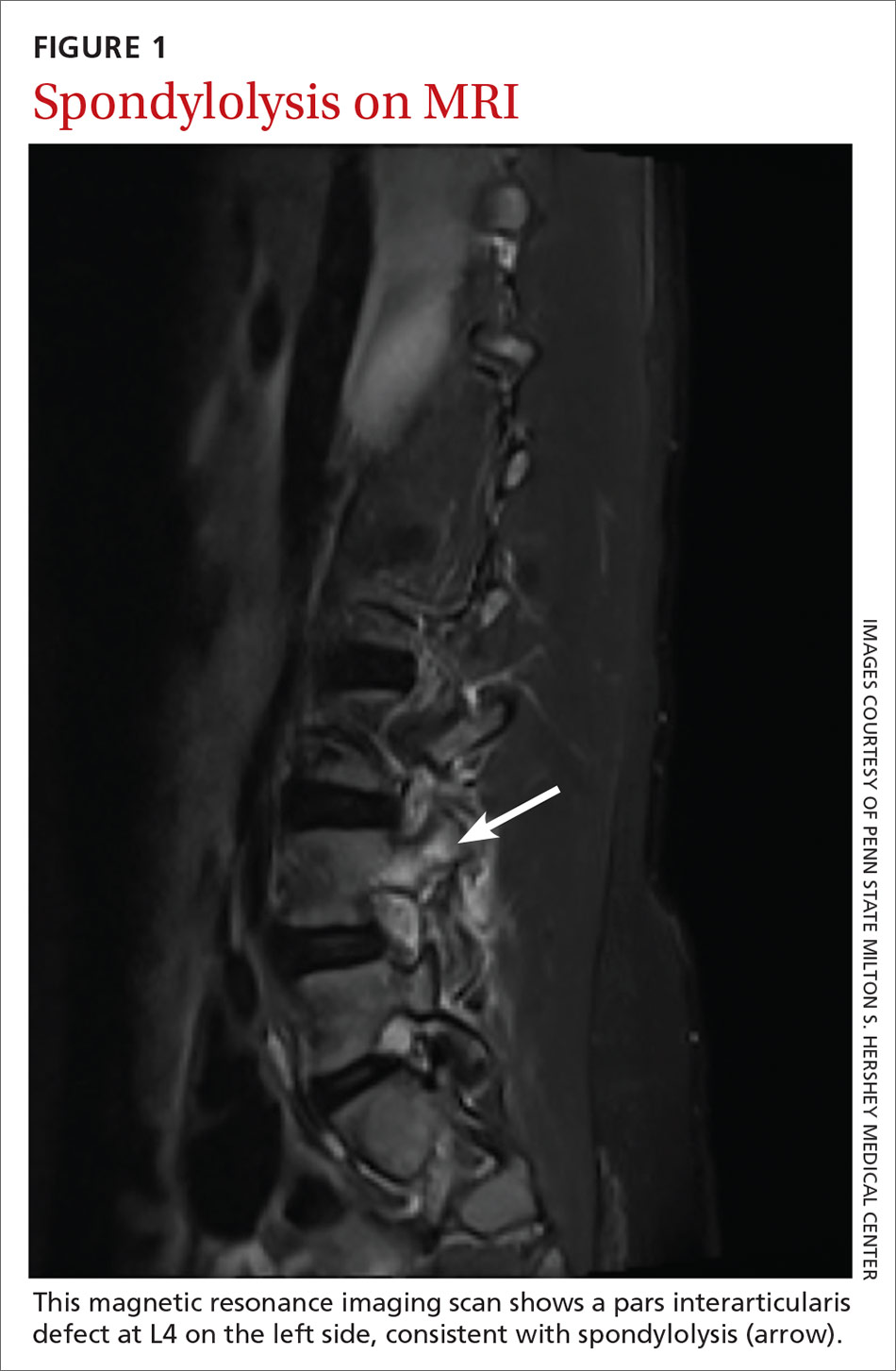
Traditionally, 4 views of the spine—anteroposterior (AP), lateral, and oblique (one right and one left)—were obtained, but recent evidence indicates that 2 views (AP and lateral) have similar sensitivity and specificity to 4 views with significantly reduced radiation exposure.2,39 Because the sensitivity of plain films is relatively low, consider more advanced imaging if spondylolysis or spondylolisthesis is strongly suspected. Recent studies indicate that magnetic resonance imaging (MRI) may be as effective as computed tomography (CT) or bone scan and has the advantage of lower radiation (FIGURE 1).2,22
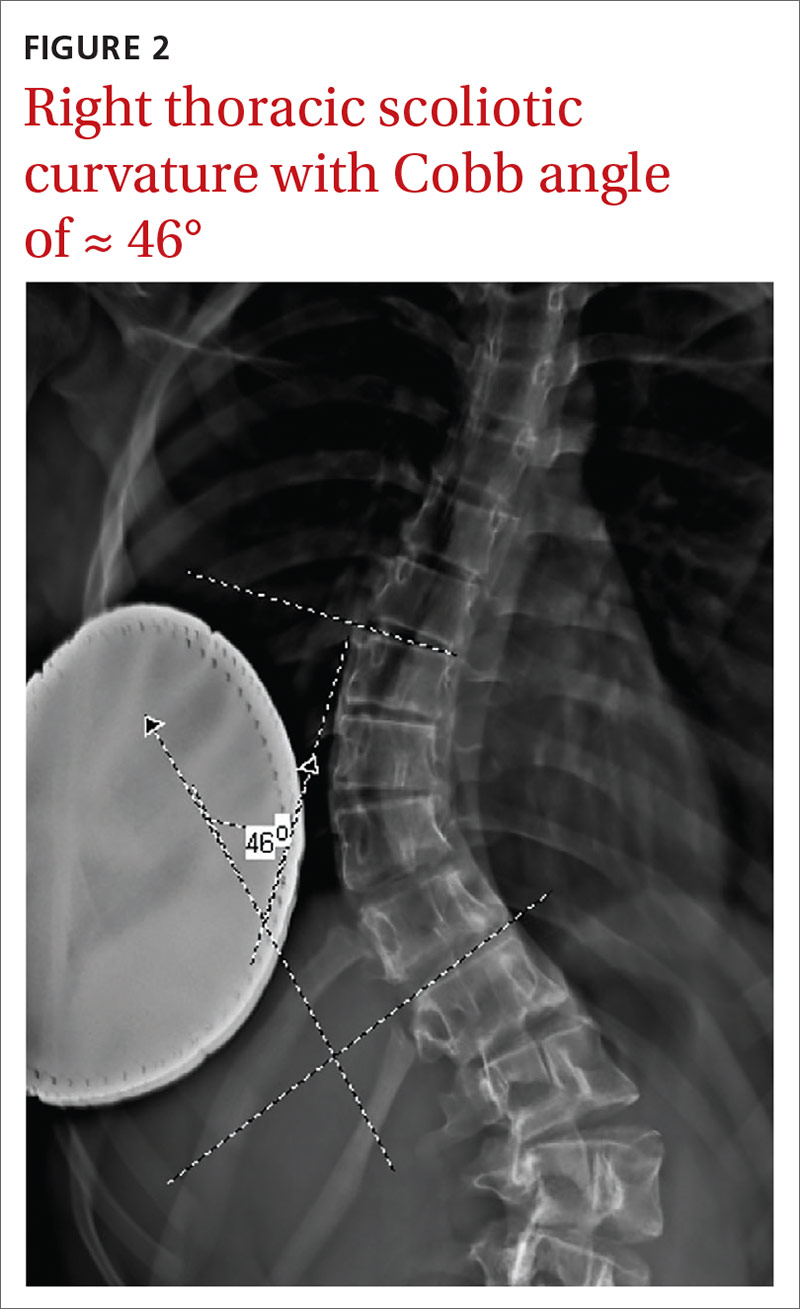
Similarly, order radiographs if there is > 10° of asymmetry noted on physical exam using a scoliometer.15,23 Calculate the Cobb angle to determine the severity of scoliosis. Refer patients with angles ≥ 20° to a pediatric orthopedist for monitoring of progression and consideration of bracing (FIGURE 2).23,34 For patients with curvatures between 10° and 19°, repeat imaging every 6 to 12 months. Because scoliosis is a risk factor for spondylolysis, evaluate radiographs in the setting of painful scoliosis for the presence of a spondylolysis.34,35
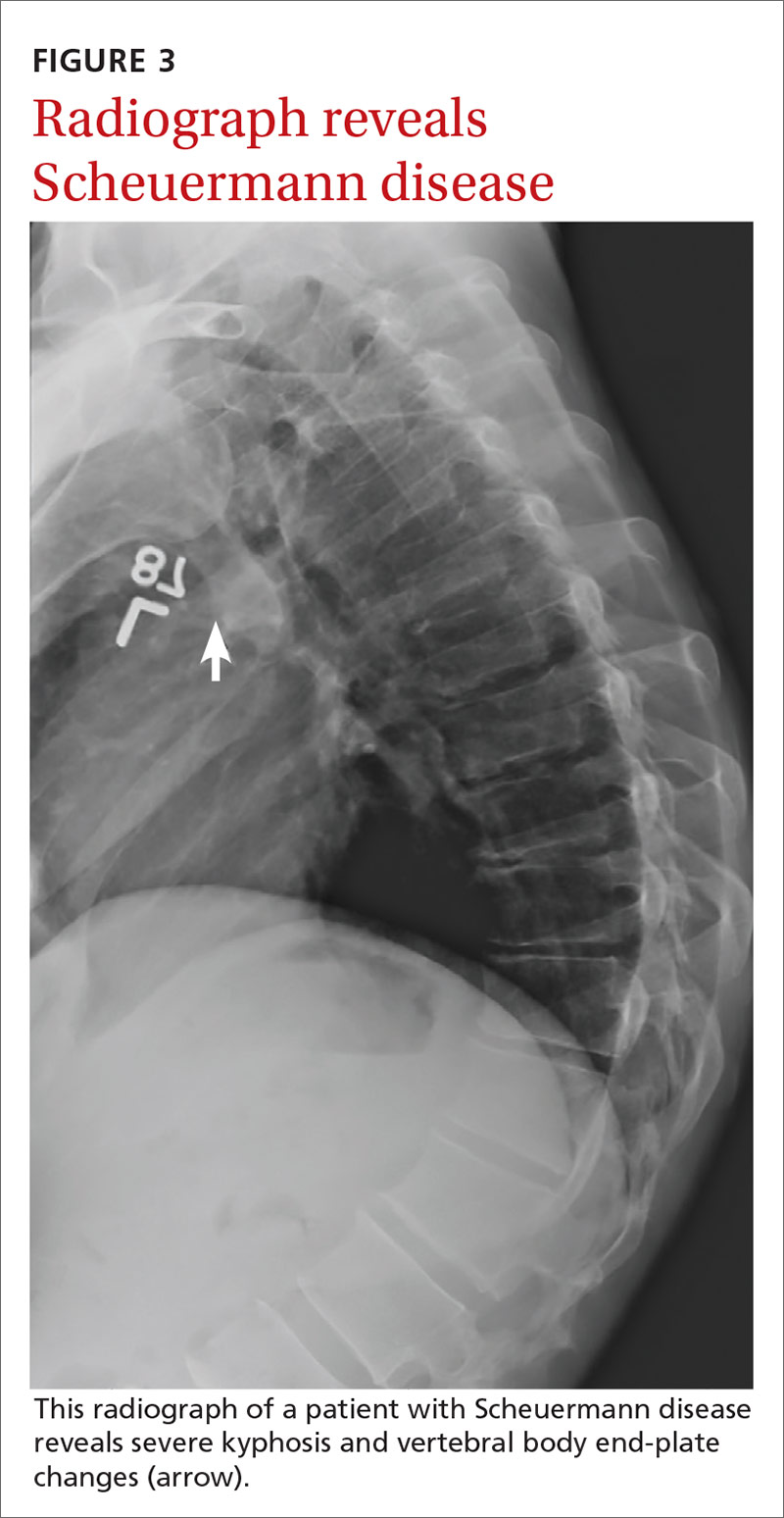
If excessive kyphosis is noted on exam, order radiographs to evaluate for Scheuermann disease. Classic imaging findings include Schmorl nodes, vertebral endplate changes, and anterior wedging (FIGURE 3).17,18
In the absence of the above concerns, defer imaging of the lumbar spine until after adequate rest and rehabilitation have been attempted.
Continue to: Treatment typically involves restor physical therapy
Treatment typically involves restor physical therapy
Most cases of low back pain in children and adolescents are benign and self-limited. Many children with low back pain can be treated with relative rest from the offending activity. For children with more persistent pain, physical therapy (PT) is often indicated. Similar to that for adults, there is little evidence for specific PT programs to help children with low back pain. Rehabilitation should be individualized based on the condition being treated.
Medications. There have been no high-quality studies on the benefit of medications to treat low back pain in children. Studies have shown nonsteroidal anti-inflammatory drugs (NSAIDs) have value in adults, and they are likely safe for use in children,40 but the risk of opiate abuse is significantly increased in adolescents who have been prescribed opiate pain medication prior to 12th grade.41
Lumbar disc herniation. Although still relatively rare, lumbar disc herniation is more common in older children and adolescents than in younger children and is treated similarly to that in adults.8 Range-of-motion exercise to restore lumbar motion is often first-line treatment. Research has shown that exercises that strengthen the abdominal or “core” musculature help prevent the return of low back pain.24,25
In the case of spondylolysis or spondylolisthesis, rest from activity is generally required for a minimum of 4 to 6 weeks. Rehabilitation in the form of range of motion, especially into the lumbar extension, and spinal stabilization exercises are effective for both reducing pain and restoring range-of-motion and strength.42 Have patients avoid heavy backpacks, which can reproduce pain. Children often benefit from leaving a second set of schoolbooks at home. For most patients with spondylolysis, conservative treatment with rehabilitation is equal to or better than surgical intervention in returning the patient to his/her pre-injury activity level.26,43,44 When returning athletes to their sport, aggressive PT, defined as rest for < 10 weeks prior to initiating PT, is superior to delaying PT beyond 10 weeks of rest.27
Idiopathic scoliosis. Much of the literature on the treatment of scoliosis is focused on limiting progression of the scoliotic curvature. Researchers thought that more severe curves were associated with more severe pain, but a recent systematic review showed that back pain can occur in patients with even small curvatures.28 Treatment for patients with smaller degrees of curvature is similar to that for mechanical low back pain. PT may have a role in the treatment of scoliosis, but there is little evidence in the literature of its effectiveness.
Continue to: A Cochrane review showed...
A Cochrane review showed that PT and exercise-based treatments had no effect on back pain or disability in patients with scoliosis.29 And outpatient PT alone, in the absence of bracing, does not arrest progression of the scoliotic curvature.35 One trial did demonstrate that an intensive inpatient treatment program of 4 to 6 weeks for patients with curvature of at least 40° reduced progression of curvature compared to an untreated control group at 1 year.34 The outcomes of functional mobility and pain were not measured. Follow-up data on curve progression beyond 1 year are not available. Unfortunately, intensive inpatient treatment is not readily available or cost-effective for most patients with scoliosis.
Scheuermann disease. The mainstay of treatment for mild Scheuermann disease is advising the patient to avoid repetitive loading of the spine. Patients should avoid sports such as competitive weight-lifting, gymnastics, and football. Lower impact athletics are encouraged. Refer patients with pain to PT to address posture and core stabilization. Patients with severe kyphosis may require surgery.17,18
Bracing: Rarely helpful for low back pain
The use of lumbar braces or corsets is rarely helpful for low back pain in children. Bracing in the setting of spondylolysis is controversial.One study indicated that bracing in combination with activity restriction and lumbar extension exercise is superior to activity restriction and lumbar flexion exercises alone.43 But a meta-analysis did not demonstrate a significant difference in recovery when bracing was added.44 Bracing may help to reduce pain initially in patients with spondylolysis who have pain at rest. Bracing is not recommended for patients with pain that abates with activity modification.
Scoliosis and Scheuermann kyphosis. Treatment of adolescent idiopathic scoliosis usually consists of observation and periodic reevaluation. Bracing is a mainstay of the nonsurgical management of scoliosis and is appropriate for curves of 20° to 40°; studies have reported successful control of curve progression in > 70% of patients.36 According to 1 study, the number of cases of scoliosis needed to treat with bracing to prevent 1 surgery is 3.30 Surgery is often indicated for patients with curvatures > 40°, although this is also debated.33
Bracing is used rarely for Scheuermann kyphosis but may be helpful in more severe or painful cases.17
CORRESPONDENCE
Shawn F. Phillips, MD, MSPT, 500 University Drive H154, Hershey, PA, 17033; sphillips6@pennstatehealth.psu.edu.
1. MacDonald J, Stuart E, Rodenberg R. Musculoskeletal low back pain in school-aged children: a review. JAMA Pediatr. 2017;171:280-287.
2. Tofte JN CarlLee TL, Holte AJ, et al. Imaging pediatric spondylolysis: a systematic review. Spine. 2017;42:777-782.
3. Sakai T, Sairyo K, Suzue N, et al. Incidence and etiology of lumbar spondylolysis: review of the literature. J Orthop Sci. 2010;15:281-288.
4. Calvo-Muñoz I, Gómez-Conesa A, Sánchez-Meca J. Prevalence of low back pain in children and adolescents: a meta-analysis. BMC Pediatrics. 2013;13:14.
5. Bernstein RM, Cozen H. Evaluation of back pain in children and adolescents. Am Fam Physician. 2007;76:1669-1676.
6. Taxter AJ, Chauvin NA, Weiss PF. Diagnosis and treatment of low back pain in the pediatric population. Phys Sportsmed. 2014;42:94-104.
7. Haus BM, Micheli LJ. Back pain in the pediatric and adolescent athlete. Clin Sports Med. 2012;31:423-440.
8. Lavelle WF, Bianco A, Mason R, et al. Pediatric disk herniation. J Am Acad Orthop Surg. 2011;19:649-656.
9. Taimela S, Kujala UM, Salminen JJ, et al. The prevalence of low back pain among children and adolescents: a nationwide, cohort-based questionnaire survey in Finland. Spine. 1997;22:1132-1136.
10. Schroeder GD, LaBella CR, Mendoza M, et al. The role of intense athletic activity on structural lumbar abnormalities in adolescent patients with symptomatic low back pain. Eur Spine J. 2016;25:2842-2848.
11. Waicus KM, Smith BW. Back injuries in the pediatric athlete. Curr Sports Med Rep. 2002;1:52-58.
12. Daniels JM, Pontius G, El-Amin S, et al. Evaluation of low back pain in athletes. Sports Health. 2011;3:336-345.
13. Sato T, Hirano T, Ito T, et al. Back pain in adolescents with idiopathic scoliosis: epidemiological study for 43,630 pupils in Niigata City, Japan. Eur Spine J. 2011;20:274-279.
14. Smorgick Y, Mirovsky Y, Baker KC, et al. Predictors of back pain in adolescent idiopathic scoliosis surgical candidates. J Pediatr Orthop. 2013;33:289-292.
15. US Preventive Services Task Force. Screening for Adolescent Idiopathic Scoliosis. US Preventive Services Task Force Recommendation Statement. JAMA. 2018;319:165-172.
16. Hresko MT, Talwalkar VR, Schwend RM. Position statement–Screening for the early detection of idiopathic scoliosis in adolescents. SRS/POSNA/AAOS/AAP Position Statement. 2015. www.srs.org/about-srs/news-and-announcements/position-statement---screening-for-the-early-detection-for-idiopathic-scoliosis-in-adolescents. Accessed September 30, 2020.
17. Palazzo C, Sailhan F, Revel M. Scheuermann’s disease: an update. Joint Bone Spine. 2014;81:209-214.
18. Ali RM, Green DW, Patel TC. Scheuermann’s kyphosis. Curr Opin Pediatr. 1999;11:70-75.
19. de Moraes Barros Fucs PM, Meves R, Yamada HH, et al. Spinal infections in children: a review. Int Orthop. 2012;36:387-395.
20. Joaquim AF, Ghizoni E, Valadares MG, et al. Spinal tumors in children. Revista da Associação Médica Brasileira. 2017;63:459-465.
21. Weiss PF, Colbert RA. Juvenile spondyloarthritis: a distinct form of juvenile arthritis. Pediatr Clin North Am. 2018;65:675-690.
22. Rush JK, Astur N, Scott S, et al. Use of magnetic resonance imaging in the evaluation of spondylolysis. J Pediatr Orthop. 2015;35:271-275.
23. Janicki JA, Alman B. Scoliosis: review of diagnosis and treatment. Pediatr Child Health. 2007;12:771-776.
24. O’Sullivan PB, Phyty GD, Twomey LT, et al. Evaluation of specific stabilizing exercise in the treatment of chronic low back pain with radiologic diagnosis of spondylolysis or spondylolisthesis. Spine.1997;22:2959-2967.
25. Inani SB, Selkar SP. Effect of core stabilization exercises versus conventional exercises on pain and functional status in patients with non-specific low back pain: a randomized clinical trial. J Back Musculoskelet Rehabil. 2013;26:37-43.
26. Garet M, Reiman MP, Mathers J, et al. Nonoperative treatment in lumbar spondylolysis and spondylolisthesis: a systematic review. Sports Health. 2013;5:225-232.
27. Selhorst M, Fischer A, Graft K, et al. Timing of physical therapy referral in adolescent athletes with acute spondylolysis: a retrospective chart review. Clin J Sport Med. 2017;27:296-301.
28. Théroux J, Stomski N, Hodgetts CJ, et al. Prevalence of low back pain in adolescents with idiopathic scoliosis: a systematic review. Chiropr Man Ther. 2017;25:10.
29. Romano M, Minozzi S, Zaina F, et al. Exercises for adolescent idiopathic scoliosis: a Cochrane systematic review. Spine (Phila Pa 1976). 2013;38:E883-E893.
30. Sanders JO, Newton PO, Browne RH, et al. Bracing for idiopathic scoliosis: how many patients require treatment to prevent one surgery? J Bone Joint Surg Am. 2014;96:649-653.
31. Hill JJ, Keating JL. Risk factors for the first episode of low back pain in children are infrequently validated across samples and conditions: a systematic review. J Physiother. 2010;56:237-244.
32. Grødahl LHJ, Fawcett L, Nazareth M, et al. Diagnostic utility of patient history and physical examination data to detect spondylolysis and spondylolisthesis in athletes with low back pain: a systematic review. Man Ther. 2016;24:7-17.
33. Asher MA, Burton DC. Adolescent idiopathic scoliosis: natural history and long term treatment effects. Scoliosis. 2006;1:2.
34. Weiss HR, Weiss G, Petermann F. Incidence of curvature progression in idiopathic scoliosis patients treated with scoliosis inpatient rehabilitation (SIR): an age- and sex-matched controlled study. Pediatr Rehabil. 2003;6:23-30.
35. Gomez JA, Hresko MT, Glotzbecker MP. Nonsurgical management of adolescent idiopathic scoliosis. J Am Acad Orthop Surg. 2016;24:555-564.
36. Weinstein SL, Dolan LA, Wright JG, et al. Effects of bracing in adolescents with idiopathic scoliosis. N Engl J Med. 2013;369:1512-1521.
37. Balg F, Juteau M, Theoret C, et al. Validity and reliability of the iPhone to measure rib hump in scoliosis. J Pediatr Orthop. 2014;34:774-779.
38. Auerbach JD, Ahn J, Zgonis MH, et al. Streamlining the evaluation of low back pain in children. Clin Orthop Relatl Res. 2008;466:1971-1977.
39. Beck NA, Miller R, Baldwin K, et al. Do oblique views add value in the diagnosis of spondylolysis in adolescents? J Bone Joint Surg Am. 2013;95:e65.
40. Roelofs PD, Deyo RA, Koes BW, et al. Nonsteroidal anti-inflammatory drugs for low back pain: an updated Cochrane review. Spine (Phila Pa 1976). 2008;33:1766-1774.
41. Miech R, Johnston L, O’Malley PM, et al. Prescription opioids in adolescence and future opioid misuse. Pediatrics. 2015;136:e1169-e1177.
42. Hu S, Tribus C, Diab M, et al. Spondylolysis and spondylolisthesis. J Bone Joint Surg. 2008;90:655-671.
43. Panteliadis P, Nagra NS, Edwards KL, et al. Athletic population with spondylolysis: review of outcomes following surgical repair or conservative management. Global Spine J. 2016;6:615-625.
44. Klein G, Mehlman CT, McCarty M. Nonoperative treatment of spondylolysis and grade I spondylolisthesis in children and young adults: a meta-analysis of observational studies. J Pediatr Orthop. 2009;29:146-156.
Low back pain in not uncommon in children and adolescents.1-3 Although the prevalence of low back pain in children < 7 years is low, it increases with age, with studies reporting lifetime prevalence at age 12 years between 16% and 18% and rates as high as 66% by 16 years of age.4,5 Although children and adolescents usually have pain that is transient and benign without a defined cause, structural causes of low back pain should be considered in school-aged children with pain that persists for > 3 to 6 weeks. 4 The most common structural causes of adolescent low back pain are reviewed here.
Etiology: A mixed bag
Back pain in school-aged children is most commonly due to muscular strain, overuse, or poor posture. The pain is often transient in nature and responds to rest and postural education.4,6 A herniated disc is an uncommon finding in younger school-aged children, but incidence increases slightly among older adolescents, particularly those who are active in collision sports and/or weight-lifting.7,8 Pain caused by a herniated disc often radiates along the distribution of the sciatic nerve and worsens during lumbar flexion.
Spondylolysis and spondylolisthesis are important causes of back pain in children. Spondylolysis is defined as a defect or abnormality of the pars interarticularis and surrounding lamina and pedicle. Spondylolisthesis, which is less common, is defined as the translation or “slippage” of one vertebral segment in relation to the next caudal segment. These conditions commonly occur as a result of repetitive stress.
In a prospective study of adolescents < 19 years with low back pain for > 2 weeks, the prevalence of spondylolysis was 39.7%.9 Adolescent athletes with symptomatic low back pain are more likely to have spondylolysis than nonathletes (32% vs 2%, respectively).2,10 Pain is often made worse by extension of the spine. Spondylolysis and spondylolisthesis can be congenital or acquired, and both can be asymptomatic. Children and teens who are athletes are at higher risk for symptomatic spondylolysis and spondylolisthesis.10-12 This is especially true for those involved in gymnastics, dance, football, and/or volleyball, where a repetitive load is placed onto an extended spine.
Idiopathic scoliosis is an abnormal lateral curvature of the spine that usually develops during adolescence and worsens with growth. Historically, painful scoliosis was considered rare, but more recently researchers determined that children with scoliosis have a higher rate of pain compared to their peers.13,14 School-aged children with scoliosis were found to be at 2 times the risk of low back pain compared to those without scoliosis.13 It is important to identify scoliosis in adolescents so that progression can be monitored.
Screening for scoliosis in primary care is somewhat controversial. The US Preventive Services Task Force (USPSTF) finds insufficient evidence for screening asymptomatic adolescents for scoliosis.15 This recommendation is based on the fact that there is little evidence on the effect of screening on long-term outcomes. Screening may also lead to unnecessary radiation. Conversely, a position statement released by the Scoliosis Research Society, the Pediatric Orthopedic Society of North America, the American Association of Orthopedic Surgeons, and the American Academy of Pediatrics recommends scoliosis screening during routine pediatric office visits.16 Screening for girls is recommended at ages 10 and 12 years, and for boys, once between ages 13 and 14 years. The statement highlights evidence showing that focused screening by appropriate personnel has value in detecting a clinically significant curve (> 20°).
Scheuermann disease is a rare cause of back pain in children that usually develops during adolescence and results in increasing thoracic kyphosis. An autosomal dominant mutation plays a role in this disease of the growth cartilage endplate; repetitive strain on the growth cartilage is also a contributing factor.17,18 An atypical variant manifests with kyphosis in the thoracolumbar region.17
Continue to: Other causes of low back pain
Other causes of low back pain—including inflammatory arthritis, infection (eg, discitis), and tumor—are rare in children but must always be considered, especially in the setting of persistent symptoms.4,19-21 More on the features of these conditions is listed in TABLE 1.1-7,13-15,17-30
History: Focus on onset, timing, and duration of symptoms
As with adults, obtaining a history that includes the onset, timing, and duration of symptoms is key in the evaluation of low back pain in children, as is obtaining a history of the patient’s activities; sports that repetitively load the lumbar spine in an extended position increase the risk of injury.10
Specific risk factors for low back pain in children and adolescents are poorly understood.4,9,31 Pain can be associated with trauma, or it can have a more progressive or insidious onset. Generally, pain that is present for up to 6 weeks and is intermittent or improving has a self-limited course. Pain that persists beyond 3 to 6 weeks or is worsening is more likely to have an anatomical cause that needs further evaluation.2,3,10,21
Identifying exacerbating and alleviating factors can provide useful information. Pain that is worse with lumbar flexion is more likely to come from muscular strain or disc pathology. Pain with extension is more likely due to a structural cause such as spondylolysis/spondylolisthesis, scoliosis, or Scheuermann disease.2,4,10,17,18,21 See TABLE 2 for red flag symptoms that indicate the need for imaging and further work-up.
The physical exam: Visualize, assess range of motion, and reproduce pain
The physical examination of any patient with low back pain should include direct visualization and inspection of the back, spine, and pelvis; palpation of the spine and paraspinal regions; assessment of lumbar range of motion and of the lumbar nerve roots, including tests of sensation, strength, and deep tendon reflexes; and an evaluation of the patient’s posture, which can provide clues to underlying causes of pain.
Continue to: Increased thoracic kyphosis...
Increased thoracic kyphosis that is not reversible is concerning for Scheuermann disease.9,17,18 A significant elevation in one shoulder or side of the pelvis can be indicative of scoliosis. Increased lumbar lordosis may predispose a patient to spondylolysis.
In patients with spondylolysis, lumbar extension will usually reproduce pain, which is often unilateral. Hyperextension in a single-leg stance, commonly known as the Stork test, is positive for unilateral spondylolysis when it reproduces pain on the ipsilateral side. The sensitivity of the Stork test for unilateral spondylolysis is approximately 50%.32 (For more information on the Stork test, see www.physio-pedia.com/Stork_test.)
Pain reproduced with lumbar flexion is less concerning for bony pathology and is most often related to soft-tissue strain. Lumbar flexion with concomitant radicular pain is associated with disc pathology.8 Pain with a straight-leg raise is also associated with disk pathology, especially if raising the contralateral leg increases pain.8
Using a scoliometer. Evaluate the flexed spine for the presence of asymmetry, which can indicate scoliosis.33 If asymmetry is present, use a scoliometer to determine the degree of asymmetry. Zero to 5° is considered clinically insignificant; monitor and reevaluate these patients at subsequent visits.34,35 Ten degrees or more of asymmetry with a scoliometer should prompt you to order radiographs.35,36 A smartphone-based scoliometer for iPhones was evaluated in 1 study and was shown to have reasonable reliability and validity for clinical use.37
Deformity of the lower extremities. Because low back pain may be caused by biomechanical or structural deformity of the lower extremities, examine the flexibility of the hip flexors, gluteal musculature, hamstrings, and the iliotibial band.38 In addition, evaluate for leg-length discrepancy and lower-extremity malalignment, such as femoral anteversion, tibial torsion, or pes planus.
Continue to: Imaging
Imaging: Know when it’s needed
Although imaging of the lumbar spine is often unnecessary in the presence of acute low back pain in children, always consider imaging in the setting of bony tenderness, pain that wakes a patient from sleep, and in the setting of other red flag symptoms (see TABLE 2). Low back pain in children that is reproducible with lumbar extension is concerning for spondylolysis or spondylolisthesis. If the pain with extension persists beyond 3 to 6 weeks, order imaging starting with radiographs.2,39
Traditionally, 4 views of the spine—anteroposterior (AP), lateral, and oblique (one right and one left)—were obtained, but recent evidence indicates that 2 views (AP and lateral) have similar sensitivity and specificity to 4 views with significantly reduced radiation exposure.2,39 Because the sensitivity of plain films is relatively low, consider more advanced imaging if spondylolysis or spondylolisthesis is strongly suspected. Recent studies indicate that magnetic resonance imaging (MRI) may be as effective as computed tomography (CT) or bone scan and has the advantage of lower radiation (FIGURE 1).2,22
Similarly, order radiographs if there is > 10° of asymmetry noted on physical exam using a scoliometer.15,23 Calculate the Cobb angle to determine the severity of scoliosis. Refer patients with angles ≥ 20° to a pediatric orthopedist for monitoring of progression and consideration of bracing (FIGURE 2).23,34 For patients with curvatures between 10° and 19°, repeat imaging every 6 to 12 months. Because scoliosis is a risk factor for spondylolysis, evaluate radiographs in the setting of painful scoliosis for the presence of a spondylolysis.34,35
If excessive kyphosis is noted on exam, order radiographs to evaluate for Scheuermann disease. Classic imaging findings include Schmorl nodes, vertebral endplate changes, and anterior wedging (FIGURE 3).17,18
In the absence of the above concerns, defer imaging of the lumbar spine until after adequate rest and rehabilitation have been attempted.
Continue to: Treatment typically involves restor physical therapy
Treatment typically involves restor physical therapy
Most cases of low back pain in children and adolescents are benign and self-limited. Many children with low back pain can be treated with relative rest from the offending activity. For children with more persistent pain, physical therapy (PT) is often indicated. Similar to that for adults, there is little evidence for specific PT programs to help children with low back pain. Rehabilitation should be individualized based on the condition being treated.
Medications. There have been no high-quality studies on the benefit of medications to treat low back pain in children. Studies have shown nonsteroidal anti-inflammatory drugs (NSAIDs) have value in adults, and they are likely safe for use in children,40 but the risk of opiate abuse is significantly increased in adolescents who have been prescribed opiate pain medication prior to 12th grade.41
Lumbar disc herniation. Although still relatively rare, lumbar disc herniation is more common in older children and adolescents than in younger children and is treated similarly to that in adults.8 Range-of-motion exercise to restore lumbar motion is often first-line treatment. Research has shown that exercises that strengthen the abdominal or “core” musculature help prevent the return of low back pain.24,25
In the case of spondylolysis or spondylolisthesis, rest from activity is generally required for a minimum of 4 to 6 weeks. Rehabilitation in the form of range of motion, especially into the lumbar extension, and spinal stabilization exercises are effective for both reducing pain and restoring range-of-motion and strength.42 Have patients avoid heavy backpacks, which can reproduce pain. Children often benefit from leaving a second set of schoolbooks at home. For most patients with spondylolysis, conservative treatment with rehabilitation is equal to or better than surgical intervention in returning the patient to his/her pre-injury activity level.26,43,44 When returning athletes to their sport, aggressive PT, defined as rest for < 10 weeks prior to initiating PT, is superior to delaying PT beyond 10 weeks of rest.27
Idiopathic scoliosis. Much of the literature on the treatment of scoliosis is focused on limiting progression of the scoliotic curvature. Researchers thought that more severe curves were associated with more severe pain, but a recent systematic review showed that back pain can occur in patients with even small curvatures.28 Treatment for patients with smaller degrees of curvature is similar to that for mechanical low back pain. PT may have a role in the treatment of scoliosis, but there is little evidence in the literature of its effectiveness.
Continue to: A Cochrane review showed...
A Cochrane review showed that PT and exercise-based treatments had no effect on back pain or disability in patients with scoliosis.29 And outpatient PT alone, in the absence of bracing, does not arrest progression of the scoliotic curvature.35 One trial did demonstrate that an intensive inpatient treatment program of 4 to 6 weeks for patients with curvature of at least 40° reduced progression of curvature compared to an untreated control group at 1 year.34 The outcomes of functional mobility and pain were not measured. Follow-up data on curve progression beyond 1 year are not available. Unfortunately, intensive inpatient treatment is not readily available or cost-effective for most patients with scoliosis.
Scheuermann disease. The mainstay of treatment for mild Scheuermann disease is advising the patient to avoid repetitive loading of the spine. Patients should avoid sports such as competitive weight-lifting, gymnastics, and football. Lower impact athletics are encouraged. Refer patients with pain to PT to address posture and core stabilization. Patients with severe kyphosis may require surgery.17,18
Bracing: Rarely helpful for low back pain
The use of lumbar braces or corsets is rarely helpful for low back pain in children. Bracing in the setting of spondylolysis is controversial.One study indicated that bracing in combination with activity restriction and lumbar extension exercise is superior to activity restriction and lumbar flexion exercises alone.43 But a meta-analysis did not demonstrate a significant difference in recovery when bracing was added.44 Bracing may help to reduce pain initially in patients with spondylolysis who have pain at rest. Bracing is not recommended for patients with pain that abates with activity modification.
Scoliosis and Scheuermann kyphosis. Treatment of adolescent idiopathic scoliosis usually consists of observation and periodic reevaluation. Bracing is a mainstay of the nonsurgical management of scoliosis and is appropriate for curves of 20° to 40°; studies have reported successful control of curve progression in > 70% of patients.36 According to 1 study, the number of cases of scoliosis needed to treat with bracing to prevent 1 surgery is 3.30 Surgery is often indicated for patients with curvatures > 40°, although this is also debated.33
Bracing is used rarely for Scheuermann kyphosis but may be helpful in more severe or painful cases.17
CORRESPONDENCE
Shawn F. Phillips, MD, MSPT, 500 University Drive H154, Hershey, PA, 17033; sphillips6@pennstatehealth.psu.edu.
Low back pain in not uncommon in children and adolescents.1-3 Although the prevalence of low back pain in children < 7 years is low, it increases with age, with studies reporting lifetime prevalence at age 12 years between 16% and 18% and rates as high as 66% by 16 years of age.4,5 Although children and adolescents usually have pain that is transient and benign without a defined cause, structural causes of low back pain should be considered in school-aged children with pain that persists for > 3 to 6 weeks. 4 The most common structural causes of adolescent low back pain are reviewed here.
Etiology: A mixed bag
Back pain in school-aged children is most commonly due to muscular strain, overuse, or poor posture. The pain is often transient in nature and responds to rest and postural education.4,6 A herniated disc is an uncommon finding in younger school-aged children, but incidence increases slightly among older adolescents, particularly those who are active in collision sports and/or weight-lifting.7,8 Pain caused by a herniated disc often radiates along the distribution of the sciatic nerve and worsens during lumbar flexion.
Spondylolysis and spondylolisthesis are important causes of back pain in children. Spondylolysis is defined as a defect or abnormality of the pars interarticularis and surrounding lamina and pedicle. Spondylolisthesis, which is less common, is defined as the translation or “slippage” of one vertebral segment in relation to the next caudal segment. These conditions commonly occur as a result of repetitive stress.
In a prospective study of adolescents < 19 years with low back pain for > 2 weeks, the prevalence of spondylolysis was 39.7%.9 Adolescent athletes with symptomatic low back pain are more likely to have spondylolysis than nonathletes (32% vs 2%, respectively).2,10 Pain is often made worse by extension of the spine. Spondylolysis and spondylolisthesis can be congenital or acquired, and both can be asymptomatic. Children and teens who are athletes are at higher risk for symptomatic spondylolysis and spondylolisthesis.10-12 This is especially true for those involved in gymnastics, dance, football, and/or volleyball, where a repetitive load is placed onto an extended spine.
Idiopathic scoliosis is an abnormal lateral curvature of the spine that usually develops during adolescence and worsens with growth. Historically, painful scoliosis was considered rare, but more recently researchers determined that children with scoliosis have a higher rate of pain compared to their peers.13,14 School-aged children with scoliosis were found to be at 2 times the risk of low back pain compared to those without scoliosis.13 It is important to identify scoliosis in adolescents so that progression can be monitored.
Screening for scoliosis in primary care is somewhat controversial. The US Preventive Services Task Force (USPSTF) finds insufficient evidence for screening asymptomatic adolescents for scoliosis.15 This recommendation is based on the fact that there is little evidence on the effect of screening on long-term outcomes. Screening may also lead to unnecessary radiation. Conversely, a position statement released by the Scoliosis Research Society, the Pediatric Orthopedic Society of North America, the American Association of Orthopedic Surgeons, and the American Academy of Pediatrics recommends scoliosis screening during routine pediatric office visits.16 Screening for girls is recommended at ages 10 and 12 years, and for boys, once between ages 13 and 14 years. The statement highlights evidence showing that focused screening by appropriate personnel has value in detecting a clinically significant curve (> 20°).
Scheuermann disease is a rare cause of back pain in children that usually develops during adolescence and results in increasing thoracic kyphosis. An autosomal dominant mutation plays a role in this disease of the growth cartilage endplate; repetitive strain on the growth cartilage is also a contributing factor.17,18 An atypical variant manifests with kyphosis in the thoracolumbar region.17
Continue to: Other causes of low back pain
Other causes of low back pain—including inflammatory arthritis, infection (eg, discitis), and tumor—are rare in children but must always be considered, especially in the setting of persistent symptoms.4,19-21 More on the features of these conditions is listed in TABLE 1.1-7,13-15,17-30
History: Focus on onset, timing, and duration of symptoms
As with adults, obtaining a history that includes the onset, timing, and duration of symptoms is key in the evaluation of low back pain in children, as is obtaining a history of the patient’s activities; sports that repetitively load the lumbar spine in an extended position increase the risk of injury.10
Specific risk factors for low back pain in children and adolescents are poorly understood.4,9,31 Pain can be associated with trauma, or it can have a more progressive or insidious onset. Generally, pain that is present for up to 6 weeks and is intermittent or improving has a self-limited course. Pain that persists beyond 3 to 6 weeks or is worsening is more likely to have an anatomical cause that needs further evaluation.2,3,10,21
Identifying exacerbating and alleviating factors can provide useful information. Pain that is worse with lumbar flexion is more likely to come from muscular strain or disc pathology. Pain with extension is more likely due to a structural cause such as spondylolysis/spondylolisthesis, scoliosis, or Scheuermann disease.2,4,10,17,18,21 See TABLE 2 for red flag symptoms that indicate the need for imaging and further work-up.
The physical exam: Visualize, assess range of motion, and reproduce pain
The physical examination of any patient with low back pain should include direct visualization and inspection of the back, spine, and pelvis; palpation of the spine and paraspinal regions; assessment of lumbar range of motion and of the lumbar nerve roots, including tests of sensation, strength, and deep tendon reflexes; and an evaluation of the patient’s posture, which can provide clues to underlying causes of pain.
Continue to: Increased thoracic kyphosis...
Increased thoracic kyphosis that is not reversible is concerning for Scheuermann disease.9,17,18 A significant elevation in one shoulder or side of the pelvis can be indicative of scoliosis. Increased lumbar lordosis may predispose a patient to spondylolysis.
In patients with spondylolysis, lumbar extension will usually reproduce pain, which is often unilateral. Hyperextension in a single-leg stance, commonly known as the Stork test, is positive for unilateral spondylolysis when it reproduces pain on the ipsilateral side. The sensitivity of the Stork test for unilateral spondylolysis is approximately 50%.32 (For more information on the Stork test, see www.physio-pedia.com/Stork_test.)
Pain reproduced with lumbar flexion is less concerning for bony pathology and is most often related to soft-tissue strain. Lumbar flexion with concomitant radicular pain is associated with disc pathology.8 Pain with a straight-leg raise is also associated with disk pathology, especially if raising the contralateral leg increases pain.8
Using a scoliometer. Evaluate the flexed spine for the presence of asymmetry, which can indicate scoliosis.33 If asymmetry is present, use a scoliometer to determine the degree of asymmetry. Zero to 5° is considered clinically insignificant; monitor and reevaluate these patients at subsequent visits.34,35 Ten degrees or more of asymmetry with a scoliometer should prompt you to order radiographs.35,36 A smartphone-based scoliometer for iPhones was evaluated in 1 study and was shown to have reasonable reliability and validity for clinical use.37
Deformity of the lower extremities. Because low back pain may be caused by biomechanical or structural deformity of the lower extremities, examine the flexibility of the hip flexors, gluteal musculature, hamstrings, and the iliotibial band.38 In addition, evaluate for leg-length discrepancy and lower-extremity malalignment, such as femoral anteversion, tibial torsion, or pes planus.
Continue to: Imaging
Imaging: Know when it’s needed
Although imaging of the lumbar spine is often unnecessary in the presence of acute low back pain in children, always consider imaging in the setting of bony tenderness, pain that wakes a patient from sleep, and in the setting of other red flag symptoms (see TABLE 2). Low back pain in children that is reproducible with lumbar extension is concerning for spondylolysis or spondylolisthesis. If the pain with extension persists beyond 3 to 6 weeks, order imaging starting with radiographs.2,39
Traditionally, 4 views of the spine—anteroposterior (AP), lateral, and oblique (one right and one left)—were obtained, but recent evidence indicates that 2 views (AP and lateral) have similar sensitivity and specificity to 4 views with significantly reduced radiation exposure.2,39 Because the sensitivity of plain films is relatively low, consider more advanced imaging if spondylolysis or spondylolisthesis is strongly suspected. Recent studies indicate that magnetic resonance imaging (MRI) may be as effective as computed tomography (CT) or bone scan and has the advantage of lower radiation (FIGURE 1).2,22
Similarly, order radiographs if there is > 10° of asymmetry noted on physical exam using a scoliometer.15,23 Calculate the Cobb angle to determine the severity of scoliosis. Refer patients with angles ≥ 20° to a pediatric orthopedist for monitoring of progression and consideration of bracing (FIGURE 2).23,34 For patients with curvatures between 10° and 19°, repeat imaging every 6 to 12 months. Because scoliosis is a risk factor for spondylolysis, evaluate radiographs in the setting of painful scoliosis for the presence of a spondylolysis.34,35
If excessive kyphosis is noted on exam, order radiographs to evaluate for Scheuermann disease. Classic imaging findings include Schmorl nodes, vertebral endplate changes, and anterior wedging (FIGURE 3).17,18
In the absence of the above concerns, defer imaging of the lumbar spine until after adequate rest and rehabilitation have been attempted.
Continue to: Treatment typically involves restor physical therapy
Treatment typically involves restor physical therapy
Most cases of low back pain in children and adolescents are benign and self-limited. Many children with low back pain can be treated with relative rest from the offending activity. For children with more persistent pain, physical therapy (PT) is often indicated. Similar to that for adults, there is little evidence for specific PT programs to help children with low back pain. Rehabilitation should be individualized based on the condition being treated.
Medications. There have been no high-quality studies on the benefit of medications to treat low back pain in children. Studies have shown nonsteroidal anti-inflammatory drugs (NSAIDs) have value in adults, and they are likely safe for use in children,40 but the risk of opiate abuse is significantly increased in adolescents who have been prescribed opiate pain medication prior to 12th grade.41
Lumbar disc herniation. Although still relatively rare, lumbar disc herniation is more common in older children and adolescents than in younger children and is treated similarly to that in adults.8 Range-of-motion exercise to restore lumbar motion is often first-line treatment. Research has shown that exercises that strengthen the abdominal or “core” musculature help prevent the return of low back pain.24,25
In the case of spondylolysis or spondylolisthesis, rest from activity is generally required for a minimum of 4 to 6 weeks. Rehabilitation in the form of range of motion, especially into the lumbar extension, and spinal stabilization exercises are effective for both reducing pain and restoring range-of-motion and strength.42 Have patients avoid heavy backpacks, which can reproduce pain. Children often benefit from leaving a second set of schoolbooks at home. For most patients with spondylolysis, conservative treatment with rehabilitation is equal to or better than surgical intervention in returning the patient to his/her pre-injury activity level.26,43,44 When returning athletes to their sport, aggressive PT, defined as rest for < 10 weeks prior to initiating PT, is superior to delaying PT beyond 10 weeks of rest.27
Idiopathic scoliosis. Much of the literature on the treatment of scoliosis is focused on limiting progression of the scoliotic curvature. Researchers thought that more severe curves were associated with more severe pain, but a recent systematic review showed that back pain can occur in patients with even small curvatures.28 Treatment for patients with smaller degrees of curvature is similar to that for mechanical low back pain. PT may have a role in the treatment of scoliosis, but there is little evidence in the literature of its effectiveness.
Continue to: A Cochrane review showed...
A Cochrane review showed that PT and exercise-based treatments had no effect on back pain or disability in patients with scoliosis.29 And outpatient PT alone, in the absence of bracing, does not arrest progression of the scoliotic curvature.35 One trial did demonstrate that an intensive inpatient treatment program of 4 to 6 weeks for patients with curvature of at least 40° reduced progression of curvature compared to an untreated control group at 1 year.34 The outcomes of functional mobility and pain were not measured. Follow-up data on curve progression beyond 1 year are not available. Unfortunately, intensive inpatient treatment is not readily available or cost-effective for most patients with scoliosis.
Scheuermann disease. The mainstay of treatment for mild Scheuermann disease is advising the patient to avoid repetitive loading of the spine. Patients should avoid sports such as competitive weight-lifting, gymnastics, and football. Lower impact athletics are encouraged. Refer patients with pain to PT to address posture and core stabilization. Patients with severe kyphosis may require surgery.17,18
Bracing: Rarely helpful for low back pain
The use of lumbar braces or corsets is rarely helpful for low back pain in children. Bracing in the setting of spondylolysis is controversial.One study indicated that bracing in combination with activity restriction and lumbar extension exercise is superior to activity restriction and lumbar flexion exercises alone.43 But a meta-analysis did not demonstrate a significant difference in recovery when bracing was added.44 Bracing may help to reduce pain initially in patients with spondylolysis who have pain at rest. Bracing is not recommended for patients with pain that abates with activity modification.
Scoliosis and Scheuermann kyphosis. Treatment of adolescent idiopathic scoliosis usually consists of observation and periodic reevaluation. Bracing is a mainstay of the nonsurgical management of scoliosis and is appropriate for curves of 20° to 40°; studies have reported successful control of curve progression in > 70% of patients.36 According to 1 study, the number of cases of scoliosis needed to treat with bracing to prevent 1 surgery is 3.30 Surgery is often indicated for patients with curvatures > 40°, although this is also debated.33
Bracing is used rarely for Scheuermann kyphosis but may be helpful in more severe or painful cases.17
CORRESPONDENCE
Shawn F. Phillips, MD, MSPT, 500 University Drive H154, Hershey, PA, 17033; sphillips6@pennstatehealth.psu.edu.
1. MacDonald J, Stuart E, Rodenberg R. Musculoskeletal low back pain in school-aged children: a review. JAMA Pediatr. 2017;171:280-287.
2. Tofte JN CarlLee TL, Holte AJ, et al. Imaging pediatric spondylolysis: a systematic review. Spine. 2017;42:777-782.
3. Sakai T, Sairyo K, Suzue N, et al. Incidence and etiology of lumbar spondylolysis: review of the literature. J Orthop Sci. 2010;15:281-288.
4. Calvo-Muñoz I, Gómez-Conesa A, Sánchez-Meca J. Prevalence of low back pain in children and adolescents: a meta-analysis. BMC Pediatrics. 2013;13:14.
5. Bernstein RM, Cozen H. Evaluation of back pain in children and adolescents. Am Fam Physician. 2007;76:1669-1676.
6. Taxter AJ, Chauvin NA, Weiss PF. Diagnosis and treatment of low back pain in the pediatric population. Phys Sportsmed. 2014;42:94-104.
7. Haus BM, Micheli LJ. Back pain in the pediatric and adolescent athlete. Clin Sports Med. 2012;31:423-440.
8. Lavelle WF, Bianco A, Mason R, et al. Pediatric disk herniation. J Am Acad Orthop Surg. 2011;19:649-656.
9. Taimela S, Kujala UM, Salminen JJ, et al. The prevalence of low back pain among children and adolescents: a nationwide, cohort-based questionnaire survey in Finland. Spine. 1997;22:1132-1136.
10. Schroeder GD, LaBella CR, Mendoza M, et al. The role of intense athletic activity on structural lumbar abnormalities in adolescent patients with symptomatic low back pain. Eur Spine J. 2016;25:2842-2848.
11. Waicus KM, Smith BW. Back injuries in the pediatric athlete. Curr Sports Med Rep. 2002;1:52-58.
12. Daniels JM, Pontius G, El-Amin S, et al. Evaluation of low back pain in athletes. Sports Health. 2011;3:336-345.
13. Sato T, Hirano T, Ito T, et al. Back pain in adolescents with idiopathic scoliosis: epidemiological study for 43,630 pupils in Niigata City, Japan. Eur Spine J. 2011;20:274-279.
14. Smorgick Y, Mirovsky Y, Baker KC, et al. Predictors of back pain in adolescent idiopathic scoliosis surgical candidates. J Pediatr Orthop. 2013;33:289-292.
15. US Preventive Services Task Force. Screening for Adolescent Idiopathic Scoliosis. US Preventive Services Task Force Recommendation Statement. JAMA. 2018;319:165-172.
16. Hresko MT, Talwalkar VR, Schwend RM. Position statement–Screening for the early detection of idiopathic scoliosis in adolescents. SRS/POSNA/AAOS/AAP Position Statement. 2015. www.srs.org/about-srs/news-and-announcements/position-statement---screening-for-the-early-detection-for-idiopathic-scoliosis-in-adolescents. Accessed September 30, 2020.
17. Palazzo C, Sailhan F, Revel M. Scheuermann’s disease: an update. Joint Bone Spine. 2014;81:209-214.
18. Ali RM, Green DW, Patel TC. Scheuermann’s kyphosis. Curr Opin Pediatr. 1999;11:70-75.
19. de Moraes Barros Fucs PM, Meves R, Yamada HH, et al. Spinal infections in children: a review. Int Orthop. 2012;36:387-395.
20. Joaquim AF, Ghizoni E, Valadares MG, et al. Spinal tumors in children. Revista da Associação Médica Brasileira. 2017;63:459-465.
21. Weiss PF, Colbert RA. Juvenile spondyloarthritis: a distinct form of juvenile arthritis. Pediatr Clin North Am. 2018;65:675-690.
22. Rush JK, Astur N, Scott S, et al. Use of magnetic resonance imaging in the evaluation of spondylolysis. J Pediatr Orthop. 2015;35:271-275.
23. Janicki JA, Alman B. Scoliosis: review of diagnosis and treatment. Pediatr Child Health. 2007;12:771-776.
24. O’Sullivan PB, Phyty GD, Twomey LT, et al. Evaluation of specific stabilizing exercise in the treatment of chronic low back pain with radiologic diagnosis of spondylolysis or spondylolisthesis. Spine.1997;22:2959-2967.
25. Inani SB, Selkar SP. Effect of core stabilization exercises versus conventional exercises on pain and functional status in patients with non-specific low back pain: a randomized clinical trial. J Back Musculoskelet Rehabil. 2013;26:37-43.
26. Garet M, Reiman MP, Mathers J, et al. Nonoperative treatment in lumbar spondylolysis and spondylolisthesis: a systematic review. Sports Health. 2013;5:225-232.
27. Selhorst M, Fischer A, Graft K, et al. Timing of physical therapy referral in adolescent athletes with acute spondylolysis: a retrospective chart review. Clin J Sport Med. 2017;27:296-301.
28. Théroux J, Stomski N, Hodgetts CJ, et al. Prevalence of low back pain in adolescents with idiopathic scoliosis: a systematic review. Chiropr Man Ther. 2017;25:10.
29. Romano M, Minozzi S, Zaina F, et al. Exercises for adolescent idiopathic scoliosis: a Cochrane systematic review. Spine (Phila Pa 1976). 2013;38:E883-E893.
30. Sanders JO, Newton PO, Browne RH, et al. Bracing for idiopathic scoliosis: how many patients require treatment to prevent one surgery? J Bone Joint Surg Am. 2014;96:649-653.
31. Hill JJ, Keating JL. Risk factors for the first episode of low back pain in children are infrequently validated across samples and conditions: a systematic review. J Physiother. 2010;56:237-244.
32. Grødahl LHJ, Fawcett L, Nazareth M, et al. Diagnostic utility of patient history and physical examination data to detect spondylolysis and spondylolisthesis in athletes with low back pain: a systematic review. Man Ther. 2016;24:7-17.
33. Asher MA, Burton DC. Adolescent idiopathic scoliosis: natural history and long term treatment effects. Scoliosis. 2006;1:2.
34. Weiss HR, Weiss G, Petermann F. Incidence of curvature progression in idiopathic scoliosis patients treated with scoliosis inpatient rehabilitation (SIR): an age- and sex-matched controlled study. Pediatr Rehabil. 2003;6:23-30.
35. Gomez JA, Hresko MT, Glotzbecker MP. Nonsurgical management of adolescent idiopathic scoliosis. J Am Acad Orthop Surg. 2016;24:555-564.
36. Weinstein SL, Dolan LA, Wright JG, et al. Effects of bracing in adolescents with idiopathic scoliosis. N Engl J Med. 2013;369:1512-1521.
37. Balg F, Juteau M, Theoret C, et al. Validity and reliability of the iPhone to measure rib hump in scoliosis. J Pediatr Orthop. 2014;34:774-779.
38. Auerbach JD, Ahn J, Zgonis MH, et al. Streamlining the evaluation of low back pain in children. Clin Orthop Relatl Res. 2008;466:1971-1977.
39. Beck NA, Miller R, Baldwin K, et al. Do oblique views add value in the diagnosis of spondylolysis in adolescents? J Bone Joint Surg Am. 2013;95:e65.
40. Roelofs PD, Deyo RA, Koes BW, et al. Nonsteroidal anti-inflammatory drugs for low back pain: an updated Cochrane review. Spine (Phila Pa 1976). 2008;33:1766-1774.
41. Miech R, Johnston L, O’Malley PM, et al. Prescription opioids in adolescence and future opioid misuse. Pediatrics. 2015;136:e1169-e1177.
42. Hu S, Tribus C, Diab M, et al. Spondylolysis and spondylolisthesis. J Bone Joint Surg. 2008;90:655-671.
43. Panteliadis P, Nagra NS, Edwards KL, et al. Athletic population with spondylolysis: review of outcomes following surgical repair or conservative management. Global Spine J. 2016;6:615-625.
44. Klein G, Mehlman CT, McCarty M. Nonoperative treatment of spondylolysis and grade I spondylolisthesis in children and young adults: a meta-analysis of observational studies. J Pediatr Orthop. 2009;29:146-156.
1. MacDonald J, Stuart E, Rodenberg R. Musculoskeletal low back pain in school-aged children: a review. JAMA Pediatr. 2017;171:280-287.
2. Tofte JN CarlLee TL, Holte AJ, et al. Imaging pediatric spondylolysis: a systematic review. Spine. 2017;42:777-782.
3. Sakai T, Sairyo K, Suzue N, et al. Incidence and etiology of lumbar spondylolysis: review of the literature. J Orthop Sci. 2010;15:281-288.
4. Calvo-Muñoz I, Gómez-Conesa A, Sánchez-Meca J. Prevalence of low back pain in children and adolescents: a meta-analysis. BMC Pediatrics. 2013;13:14.
5. Bernstein RM, Cozen H. Evaluation of back pain in children and adolescents. Am Fam Physician. 2007;76:1669-1676.
6. Taxter AJ, Chauvin NA, Weiss PF. Diagnosis and treatment of low back pain in the pediatric population. Phys Sportsmed. 2014;42:94-104.
7. Haus BM, Micheli LJ. Back pain in the pediatric and adolescent athlete. Clin Sports Med. 2012;31:423-440.
8. Lavelle WF, Bianco A, Mason R, et al. Pediatric disk herniation. J Am Acad Orthop Surg. 2011;19:649-656.
9. Taimela S, Kujala UM, Salminen JJ, et al. The prevalence of low back pain among children and adolescents: a nationwide, cohort-based questionnaire survey in Finland. Spine. 1997;22:1132-1136.
10. Schroeder GD, LaBella CR, Mendoza M, et al. The role of intense athletic activity on structural lumbar abnormalities in adolescent patients with symptomatic low back pain. Eur Spine J. 2016;25:2842-2848.
11. Waicus KM, Smith BW. Back injuries in the pediatric athlete. Curr Sports Med Rep. 2002;1:52-58.
12. Daniels JM, Pontius G, El-Amin S, et al. Evaluation of low back pain in athletes. Sports Health. 2011;3:336-345.
13. Sato T, Hirano T, Ito T, et al. Back pain in adolescents with idiopathic scoliosis: epidemiological study for 43,630 pupils in Niigata City, Japan. Eur Spine J. 2011;20:274-279.
14. Smorgick Y, Mirovsky Y, Baker KC, et al. Predictors of back pain in adolescent idiopathic scoliosis surgical candidates. J Pediatr Orthop. 2013;33:289-292.
15. US Preventive Services Task Force. Screening for Adolescent Idiopathic Scoliosis. US Preventive Services Task Force Recommendation Statement. JAMA. 2018;319:165-172.
16. Hresko MT, Talwalkar VR, Schwend RM. Position statement–Screening for the early detection of idiopathic scoliosis in adolescents. SRS/POSNA/AAOS/AAP Position Statement. 2015. www.srs.org/about-srs/news-and-announcements/position-statement---screening-for-the-early-detection-for-idiopathic-scoliosis-in-adolescents. Accessed September 30, 2020.
17. Palazzo C, Sailhan F, Revel M. Scheuermann’s disease: an update. Joint Bone Spine. 2014;81:209-214.
18. Ali RM, Green DW, Patel TC. Scheuermann’s kyphosis. Curr Opin Pediatr. 1999;11:70-75.
19. de Moraes Barros Fucs PM, Meves R, Yamada HH, et al. Spinal infections in children: a review. Int Orthop. 2012;36:387-395.
20. Joaquim AF, Ghizoni E, Valadares MG, et al. Spinal tumors in children. Revista da Associação Médica Brasileira. 2017;63:459-465.
21. Weiss PF, Colbert RA. Juvenile spondyloarthritis: a distinct form of juvenile arthritis. Pediatr Clin North Am. 2018;65:675-690.
22. Rush JK, Astur N, Scott S, et al. Use of magnetic resonance imaging in the evaluation of spondylolysis. J Pediatr Orthop. 2015;35:271-275.
23. Janicki JA, Alman B. Scoliosis: review of diagnosis and treatment. Pediatr Child Health. 2007;12:771-776.
24. O’Sullivan PB, Phyty GD, Twomey LT, et al. Evaluation of specific stabilizing exercise in the treatment of chronic low back pain with radiologic diagnosis of spondylolysis or spondylolisthesis. Spine.1997;22:2959-2967.
25. Inani SB, Selkar SP. Effect of core stabilization exercises versus conventional exercises on pain and functional status in patients with non-specific low back pain: a randomized clinical trial. J Back Musculoskelet Rehabil. 2013;26:37-43.
26. Garet M, Reiman MP, Mathers J, et al. Nonoperative treatment in lumbar spondylolysis and spondylolisthesis: a systematic review. Sports Health. 2013;5:225-232.
27. Selhorst M, Fischer A, Graft K, et al. Timing of physical therapy referral in adolescent athletes with acute spondylolysis: a retrospective chart review. Clin J Sport Med. 2017;27:296-301.
28. Théroux J, Stomski N, Hodgetts CJ, et al. Prevalence of low back pain in adolescents with idiopathic scoliosis: a systematic review. Chiropr Man Ther. 2017;25:10.
29. Romano M, Minozzi S, Zaina F, et al. Exercises for adolescent idiopathic scoliosis: a Cochrane systematic review. Spine (Phila Pa 1976). 2013;38:E883-E893.
30. Sanders JO, Newton PO, Browne RH, et al. Bracing for idiopathic scoliosis: how many patients require treatment to prevent one surgery? J Bone Joint Surg Am. 2014;96:649-653.
31. Hill JJ, Keating JL. Risk factors for the first episode of low back pain in children are infrequently validated across samples and conditions: a systematic review. J Physiother. 2010;56:237-244.
32. Grødahl LHJ, Fawcett L, Nazareth M, et al. Diagnostic utility of patient history and physical examination data to detect spondylolysis and spondylolisthesis in athletes with low back pain: a systematic review. Man Ther. 2016;24:7-17.
33. Asher MA, Burton DC. Adolescent idiopathic scoliosis: natural history and long term treatment effects. Scoliosis. 2006;1:2.
34. Weiss HR, Weiss G, Petermann F. Incidence of curvature progression in idiopathic scoliosis patients treated with scoliosis inpatient rehabilitation (SIR): an age- and sex-matched controlled study. Pediatr Rehabil. 2003;6:23-30.
35. Gomez JA, Hresko MT, Glotzbecker MP. Nonsurgical management of adolescent idiopathic scoliosis. J Am Acad Orthop Surg. 2016;24:555-564.
36. Weinstein SL, Dolan LA, Wright JG, et al. Effects of bracing in adolescents with idiopathic scoliosis. N Engl J Med. 2013;369:1512-1521.
37. Balg F, Juteau M, Theoret C, et al. Validity and reliability of the iPhone to measure rib hump in scoliosis. J Pediatr Orthop. 2014;34:774-779.
38. Auerbach JD, Ahn J, Zgonis MH, et al. Streamlining the evaluation of low back pain in children. Clin Orthop Relatl Res. 2008;466:1971-1977.
39. Beck NA, Miller R, Baldwin K, et al. Do oblique views add value in the diagnosis of spondylolysis in adolescents? J Bone Joint Surg Am. 2013;95:e65.
40. Roelofs PD, Deyo RA, Koes BW, et al. Nonsteroidal anti-inflammatory drugs for low back pain: an updated Cochrane review. Spine (Phila Pa 1976). 2008;33:1766-1774.
41. Miech R, Johnston L, O’Malley PM, et al. Prescription opioids in adolescence and future opioid misuse. Pediatrics. 2015;136:e1169-e1177.
42. Hu S, Tribus C, Diab M, et al. Spondylolysis and spondylolisthesis. J Bone Joint Surg. 2008;90:655-671.
43. Panteliadis P, Nagra NS, Edwards KL, et al. Athletic population with spondylolysis: review of outcomes following surgical repair or conservative management. Global Spine J. 2016;6:615-625.
44. Klein G, Mehlman CT, McCarty M. Nonoperative treatment of spondylolysis and grade I spondylolisthesis in children and young adults: a meta-analysis of observational studies. J Pediatr Orthop. 2009;29:146-156.
PRACTICE RECOMMENDATIONS
› Be aware that low back pain is rare in children < 7 years but increases in incidence as children near adolescence. A
› Consider imaging in the setting of bony tenderness, pain that awakens the patient from sleep, or in the presence of other “red flag” symptoms. A
› Consider spondylolysis and spondylolisthesis in adolescent athletes with low back pain lasting longer than 3 to 6 weeks. A
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
Getting injured runners back on track
• Advise patients with metatarsalgia to use metatarsal pads, consider orthotics, use contrast baths, and avoid high heels and pointy-toed shoes. C
• Recommend that runners with stress fractures of the foot have at least 4 weeks of rest before a gradual return to activity. C
• Consider short-term physical therapy for patients with plantar fasciitis to enable them to learn proper stretching and strengthening techniques. C
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
CASE Jim F, 40 years old and overweight (BMI=28 kg/m2), has come to see you because of foot pain that began shortly after he took up running. Jim tells you that turning 40 was “an eye opener” that prompted him to “get healthy.” He says that while he was a competitive athlete in high school, he never ran regularly—until he embarked on a running program 3 months ago.
Jim denies acute injury, bruising, swelling, redness, fever, or chills, but states that the pain, which he describes as dull and achy, is gradually getting worse. It hurts the most when he stands for long periods of time. He says that he occasionally takes ibuprofen for the foot pain, but has not tried icing or stretching. When you ask him what kind of sneakers he wears during his runs, Jim reports that his running shoes—purchased at a discount store—are about 5 years old.
Participation in running has grown by more than 40% in the United States in the past decade.1 As a result, patients like Jim are bound to have their share of aches, pains, and injuries that prompt them to visit their family physician. And that’s where this review can help. This rundown of the most common foot pain diagnoses, as well as the at-a-glance summaries of the differential diagnosis (TABLE 1)2-5 and treatment options (TABLE 2),3,6-25 can help you quickly get patients the relief they need to return to running.
TABLE 1
Differential diagnosis for runners’ foot pain2-5
| Symptom | Differential diagnosis |
|---|---|
| Foot pain |
|
| Heel pain |
|
| *Represents a more common diagnosis. | |
TABLE 2
Diagnosing and treating common runners’ injuries
| Diagnosis | History | Physical exam | Interventions |
|---|---|---|---|
| Metatarsalgia | Plantar foot pain, insidious onset; occasional swelling, bruising, or deformity | Tenderness of MT heads; possible edema or hyperkeratosis; negative tuning fork test | Footwear: cushioning, wide toe box, MT pads; consider orthotics. Contrast baths; NSAIDs6-9 |
| Stress fracture | Pain, insidious onset, increasing in intensity and duration | Localized TTP; possible swelling or bruising; positive tuning fork test; X-rays/MRI may be helpful | Boot for minimum of 3-4 weeks, followed by PT for foot/ankle ROM, strength, proprioception Ice, acetaminophen (NSAIDs controversial)10-12 Progressive return to running* |
| Plantar fasciitis | Plantar foot/heel pain, worse with first steps in AM and after prolonged weight-bearing | TTP at medial calcaneal tubercle | Relative rest, NSAIDs, PT for HEP, Graston technique, taping; possible night splinting13-15 Consider ESWT, corticosteroid injection for refractory cases16-18 |
| MAT | Posterior heel/Achilles pain in midportion; insidious onset, increasing in intensity, worse with activity | Tenderness midportion Achilles; possible tendon thickening; warmth, crepitus, nodules | Relative rest; PT for eccentric exercises; heel lift, with or without orthotics19-22 Consider PRP, prolotherapy, ESWT, or ultrasound in refractory cases†23,24 Surgical intervention rarely indicated3 |
| IAT | Posterior heel/Achilles pain in insertion of Achilles; insidious onset, increasing in intensity; swelling possible; worse with activity | Tenderness with or without swelling; deformity at Achilles insertion | Relative rest; footwear modification (heel lift, possibly with orthotics); PT for eccentric exercises, though less valuable than for MAT†25 |
| *Starting with cross-training exercise, progressing to running on a treadmill, then to running outdoors. †Corticosteroid injection contraindicated. ESWT, extracorporeal shock wave therapy; HEP, home exercise program; IAT, insertional Achilles tendinopathy; MAT, midportion Achilles tendinopathy; MRI, magnetic resonance imaging; MT, metatarsal; NSAIDs, nonsteroidal anti-inflammatory drugs; PRP, plasma-rich protein; PT, physical therapy; ROM, range of motion; TTP, tenderness to palpation. | |||
Metatarsalgia: Pain on the plantar surface
Typically associated with a recent increase in activity or change in footwear, metatarsalgia is defined by pain on the plantar surface of the forefoot in the area of the metatarsal heads. The second, third, and fourth metatarsals are the most common offenders, and the pain may or may not be accompanied by swelling, bruising, or deformity.
Mechanical irregularities in the foot are thought to contribute to the development of metatarsalgia, which is typically inflammatory in nature. Physical exam often reveals tenderness at the affected metatarsal heads, with or without pain in the corresponding metatarsophalangeal joint, and occasionally, with overlying edema or hyperkeratosis.
Tuning fork test. Commonly used but weakly supported, this diagnostic test is performed by applying a vibrating tuning fork to a site of possible fracture. If the maneuver produces focal pain, the test is positive and may be helpful in ruling in metatarsal stress fractures.26
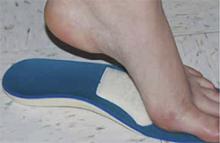
Treatment: Change shoes, consider NSAIDs. Treatment for metatarsalgia begins conservatively, with a change in footwear. High heels or pointy-toed shoes should be avoided, and metatarsal pads (FIGURE 1) can be placed inside the shoes to help off-load the metatarsal head.6 The pads come prefabricated or can be custom made, and are typically placed by physical therapists to ensure proper placement. Orthotics should also be considered, as they can help normalize abnormal foot mechanics that may contribute to metatarsalgia.7,8 (See “A word about runners’ footwear”.9,27-31)
Metatarsalgia is believed to be an inflammatory process, and NSAIDs may be helpful. Contrast baths—alternately submerging the affected foot in a basin of hot (but not scalding) water for 1 to 2 minutes, then immersing it in cold water for 30 to 60 seconds and repeating the process for about 20 minutes once or twice daily—may be helpful. Magnetic insoles are not recommended, as they have been found to be no better than sham insoles.32 Rarely, surgical repair of underlying mechanical abnormalities is indicated for treatment of refractory metatarsalgia.
CASE On examination, Jim F has no swelling, but some hyperkeratosis overlying the second and third metatarsal heads. He has tenderness to palpation at these heads as well as the corresponding metatarsophalangeal joints, and a negative tuning fork test.
You advise Jim that he has metatarsalgia, educate him about the pathophysiology of this condition, and give him a prescription for a nonsteroidal anti-inflammatory drug. You suggest he use contrast baths—and explain how this is done—once or twice a day and refer him to physical therapy for proper placement of metatarsal pads in his shoes, and schedule an appointment for a 6-week follow-up.
Return to running. There is no firm recommendation regarding abstaining from running with metatarsalgia. Advise patients to use pain as a guide in determining the intensity and duration of activity.
FIGURE 1
Treatment for metatarsalgia is conservative
In addition to changing to more comfortable footwear, patients with metatarsalgia can place metatarsal pads like the one shown here in their shoes to ease the metatarsal load.
The proper footwear for runners is subject to considerable debate, with arguments supported by contradictory evidence. What is known, however, is that running shoes should:
- be a comfortable fit with cushioning chosen to accommodate arch type
- be replaced after running 300 to 500 miles or every 12 months, whichever comes first27,28
- be purchased from a sporting goods or running store, rather than at a discount retailer. That’s because the shoes sold at discount stores are often older, and breakdown of the protective cushioning is more likely to have occurred prior to purchase.28
The most expensive shoe is not automatically the best choice for the runner, however. Some studies have found no benefit in foot strike pressures with expensive cushioned running shoes compared with low- or medium-cost brands.29 Shoes should be selected based on comfort, although the patient’s arch type should also be considered when selecting running footwear.30
Barefoot running shoes, designed to simulate barefoot running, are also an option. As with cushioned running shoes, evidence regarding barefoot running is contradictory. Some studies suggest that running mechanics are improved with barefoot running or barefoot running shoes; others have had unfavorable or inconsistent results, indicating a need for further research.9,31
Stress fracture: Tenderness and pain of insidious onset
Stress fractures of the foot (SFF)—overuse injuries also known as fatigue fractures—are common in recreational runners. They are thought to result from microtraumas, which alone are not sufficient to break bone but together overwhelm the bone’s natural ability to remodel and recover over time. SFF are characterized by tenderness and pain of insidious onset, and typically occur when more than one training variable (eg, frequency, duration, and intensity) is changed simultaneously. SFF can also result from a change in exercise mechanics, such as foot strike.
Stress fractures can occur in any bone in the foot, but are most common in the metatarsal bones, specifically the mid or distal portion of the second or third metatarsal, or the tarsal navicular.2,33 On examination, the patient will have tenderness to palpation, often well localized. A positive tuning fork test (see page 647) is highly suggestive of a stress fracture.
In female runners, stress fractures may be associated with the female athlete triad—osteoporosis or osteopenia, disordered eating (specifically caloric deficiency and low BMI), and amenorrhea. In addition to the major long-term health problems that may result from even one component of the triad, SFF may be a short-term consequence.34
Although SFF is a clinical diagnosis, x-rays—including 3-view plain films of the foot, with the area of concern clearly noted on the order—are recommended. Magnetic resonance imaging may be used for secondary imaging if doubt about the source of the pain remains.35
Of note: Occasionally, a metatarsal stress fracture progresses to a frank fracture, specifically of the metaphyseal-diaphyseal junction of the fifth metatarsal—known as a Jones fracture. This type of fracture has a high rate of malunion or nonunion.36 If there is any suspicion of a fracture in this area, consider a referral to a sports medicine specialist or orthopedic surgeon.
Treatment: Icing, analgesics, and a boot. Standard treatment for SFF includes icing for 15 to 20 minutes up to 3 times a day for a minimum of 72 hours after injury, but may be continued throughout the healing period. Analgesics such as acetaminophen and a walking boot for 3 to 4 weeks, with follow-up at 3 weeks, should also be implemented. Recent evidence suggests that NSAIDs may hinder the bone healing process, and their use in treating SFF is controversial.10-12
Weaning from the boot can begin when the patient is pain free with the boot on, usually by 3 to 4 weeks. Patients often progress quickly from wearing the boot at all times to wearing it only outside of the house, to not wearing it at all. Advise patients who need to walk long distances for a good portion of the day to keep the boot nearby and to put it on if the pain returns.
Once weaning from the boot begins, physical therapy (PT) should be considered to help the patient regain foot and ankle range of motion (ROM), proprioception, and strength. Once he or she learns the exercises, rehabilitation can be accomplished with a home exercise program. Foot deformities, such as pes planus or pes cavus, may indicate a need for orthotics. A well-structured athletic shoe may help to prevent future injury.7,8
Return to running. Once adequate ROM and strength in the foot and ankle are recovered, the patient can begin to resume activity, starting with a low-impact cross-training exercise, such as a stationary bike or elliptical, for a week or 2. A patient who remains pain free can progress from cross-training to running on a treadmill for another week or 2, then gradually switch to outdoor running.
Plantar fasciitis: Heel pain with an insidious onset
Plantar fasciitis is one of the most common causes of heel pain in athletes (primarily runners) and nonathletes alike. Plantar fasciitis may be associated with acute trauma, but is more commonly insidious in onset. The diagnosis is clinical and rarely requires imaging.
Pain associated with plantar fasciitis may be described as sharp and stabbing or dull and aching. It is on the plantar surface of the heel, sometimes radiating to the arch, and may localize to the insertion of the plantar fascia on the medial calcaneal tubercle (FIGURE 2). The pain is typically most severe with the first few steps in the morning or after other periods of prolonged rest. It usually improves after a few steps, but may return later in the day. Plantar fasciitis does not cause paresthesias or other neurologic symptoms, so their presence is suggestive of a different diagnosis, such as nerve entrapment, compartment syndrome, or tarsal tunnel syndrome.3,5
Treatment: It’s multifactorial. NSAIDs are commonly used. Relative rest is recommended, but cross training may be considered to maintain fitness.37 Short-term PT is also recommended to teach the patient proper stretching and strengthening techniques in the form of a home exercise plan. Modalities such as iontophoresis (a system of transdermal delivery of medication with the use of electrical currents), Graston (a form of instrument-assisted soft tissue mobilization), and taping may be incorporated into PT, as well.13
Night splinting may also be used to keep the foot in a dorsiflexed position. A splint can be purchased without a prescription and prevents the plantar fascia from shortening overnight by providing a continuous passive stretch, thus reducing pain with first steps.14
Orthotics may also help to reduce symptom severity and duration, and studies have found no difference in outcomes with prefabricated vs custom-made devices.15 Another treatment to consider, particularly for recalcitrant cases of plantar fasciitis, is extracorporeal shock wave therapy, which has been studied for more than a decade with conflicting results.16 Corticosteroid injection may also be used for treatment-refractory plantar fasciitis, but caution is required, as the injection may increase the risk of rupture of the plantar fascia.17,18
Return to running. There are no set guidelines for when an athlete with plantar fasciitis can return to running. Typically, after 2 to 4 weeks of relative rest and other treatments, the runner can begin to transition from cross-training to treadmill running.
FIGURE 2
Severe pain with first steps of the day
The pain of plantar fasciitis—often most severe first thing in the morning—may localize to the insertion of the plantar fascia on the medial calcaneal tubercle, as shown above.
Achilles tendinopathy: An overuse injury
Achilles tendinopathy (AT) is typically an overuse injury incurred by athletes, although it is sometimes seen in patients who are sedentary and overweight. With a prevalence among runners of approximately 11%, AT is sometimes called the “runners’ disease.”4
Tendinopathy is a more accurate description than tendonitis, as histologic studies of affected Achilles tendons suggest that AT is a degenerative, rather than an inflammatory, condition.38 A diagnosis of AT can be further classified as midportion or insertional.
Midportion Achilles tendinopathy (MAT), characterized by pain that occurs in the body of the Achilles tendon and worsens with activity, is often a clinical diagnosis. Physical findings suggestive of MAT are tenderness to palpation of the midportion of the Achilles tendon, with thickening of the tendon, warmth, crepitus, or palpable nodules in the tendon body. Onset is insidious and is commonly associated with an increase in activity.
Treatment: Orthotics or a heel lift. Like that of plantar fasciitis, treatment of midportion Achilles tendinopathy is primarily conservative. The use of orthotics, or a heel lift, is one of the most cost-effective interventions, and they are widely used, despite limited evidence of efficacy.39 Custom orthotics are costly, and patients often benefit from trying prefabricated orthotics first to determine whether they will help.
Eccentric exercises. One of the most studied interventions for MAT is eccentric exercise training. Studies of eccentric exercises have been very favorable, and the exercises can be taught during routine PT sessions.19-22 Modalities such as ultrasound therapy and extracorporeal shock wave therapy (ESWT) have also been studied. But because results have been inconsistent, they are generally reserved for treatment-refractory cases.23
In patients with no contraindications, NSAIDs may be a good choice for pain management with relatively favorable results in the literature.24 Corticosteroid injections should not be used, as they have been directly linked to rupture of the Achilles tendon.23
Other interventions, such as plasma-rich protein injections and prolotherapy—a technique in which an irritant is injected into the tendon in an attempt to create an inflammatory reaction, thus increasing local blood flow and healing—are being studied for the treatment of AT, but are not routinely used or covered by insurance for this purpose. Surgical intervention may be considered for patients whose symptoms last for more than 3 to 6 months despite conservative treatment.
Insertional Achilles tendinopathy (IAT) can be clinically differentiated from MAT by the location of symptoms and tenderness to palpation at the insertion site of the Achilles into the calcaneous. Like MAT, IAT is exacerbated by activity. Other conditions that may contribute to, or be mistaken for, IAT are a Haglund deformity and retrocalcaneal bursitis.
Treatment: Footwear modification. Treatment of IAT, like that of MAT, is primarily conservative. Orthotics or heel lifts are commonly used. However, there is greater emphasis on footwear modification due to the mechanical irritation and resultant posterior heel swelling often associated with IAT. While eccentric exercises play a role in IAT treatment, the benefits are limited.25
As with MAT, corticosteroid injections are contraindicated due to the risk of tendon rupture. Modalities such as ultrasound, ESWT, plasma-rich protein, and prolotherapy lack sufficient evidence to be widely recommended.
For refractory cases of IAT, surgical intervention often relieves the pain.
Return to running. After an initial rest of 2 to 4 weeks, patients may return to running while completing therapy. It’s not necessary to wait until the patient is completely pain free, but pain should be used to guide decisions about intensity and duration of activity.
CASE When Jim returns 6 weeks later, he reports that he took 3 weeks off from running because of the pain. Initially, he used contrast baths daily, Jim says, but now he uses them only when he is symptomatic, and he discontinued the NSAID a few weeks ago. Jim tells you he went to the local running store for a new pair of running shoes and that he is now able to run at his previous pace while remaining relatively pain free.
CORRESPONDENCE Jessica Favero Butts, MD, One American Square, Suite 185, Indianapolis, IN 46282; Jbutts2@iuhealth.org
1. Sporting Goods Manufacturers Association (SGMA) 2010 Sports & Fitness Participation Report. Silver Spring, Md: SGMA; 2011.
2. Tuan K, Wu S, Sennett B. Stress fractures in athletes: risk fractures, diagnosis, and management. Orthopedics. 2004;27:583-593.
3. Wapner KL, Parekh SG. Heel pain. In: DeLee J, Drez D, Miller M, eds. DeLee and Drez’s Orthopaedic Sports Medicine. 3rd ed. Philadelphia, Pa: Saunders; 2010:2030–2056.
4. Lysholm J, Wiklander J. Injuries in runners. Am J Sports Med. 1987;15:168-171.
5. Guyton G, Gomez L, Mann R. Entrapment neuropathies of the foot. In: DeLee J, Drez D, Miller M, eds. DeLee and Drez’s Orthopaedic Sports Medicine. 3rd ed. Philadelphia, Pa: Saunders; 2010:2057–2063.
6. Kang JH, Chen MD, Chen SC, et al. Correlations between subjective treatment responses and plantar pressure parameters of metatarsal pad treatment in metatarsalgia patients: a prospective study. BMC Musculoskelet Disord. 2006;7:95.-
7. MacLean CL, van Emmerik R, Hamill J. Influence of custom foot orthotic intervention on lower extremity intralimb coupling during a 30-minute run. J Appl Biomech. 2010;26:390-399.
8. MacLean CL, Davis IS, Hamill J. Short- and long-term influences of a custom foot orthotic intervention on lower extremity dynamics. Clin J Sport Med. 2008;18:338-343.
9. Bishop M, Fiolkowski P, Conrad B, et al. Athletic footwear, leg stiffness, and running kinematics. J Athl Train. 2006;41:387-392.
10. Burd TA, Hughes MS, Anglen JO. Heterotopic ossification prophylaxis with indomethacin increases the risk of long-bone nonunion. J Bone Joint Surg Br. 2003;85:700-705.
11. Butcher CK, Marsh DR. Nonsteroidal anti-inflammatory drugs delay tibial fracture union. Injury. 1996;27:375.-
12. Yates JE, Shah SH. Do NSAIDS impede fracture healing? J Fam Pract. 2011;60:41-42.
13. Hyland M, Webber-Gaffney A, Cohen L. Randomized controlled trial of calcaneal taping, sham taping, and plantar fascia stretching for the short-term management of plantar heel pain. J Orthop Sports Phys Ther. 2006;36:364-371.
14. Powell M, Post WR, Keener J, et al. Effective treatment of chronic plantar fasciitis with dorsiflexion night splints: a crossover prospective randomized outcome study. Foot Ankle Int. 1998;19:10-18.
15. Baldassin V, Gomes CR, Beraldo PS. Effectiveness of prefabricated and customized foot orthoses made from low-cost foam for noncomplicated plantar fasciitis: a randomized controlled trial. Arch Phys Med Rehabil. 2009;90:701-706.
16. Rompe JD, Furia J, Weil L, et al. Shock wave therapy for chronic plantar fasciopathy. Br Med Bull. 2007;81-82:183-208.
17. Kleinman M, Gross AF. Achilles tendon rupture following steroid injection. Report of three cases. J Bone Joint Surg Am. 1983;65:1345-1347.
18. Hamilton B, Remedios D, Loosemore M, et al. Achilles tendon rupture in an elite athlete following multiple injection therapies. J Sci Med Sport. 2008;11:566-568.
19. Wasielewski NJ, Kotsko KM. Does eccentric exercise reduce pain and improve strength in physically active adults with symptomatic lower extremity tendinosis? A systematic review. J Athl Train. 2007;42:409-421.
20. Kingma JJ, de Knikker R, Wittink HM, et al. Eccentric overload training in patients with chronic Achilles tendinopathy: a systematic review. Br J Sports Med. 2007;41:e3.-
21. Norregaard J, Larsen CC, Bieler T, et al. Eccentric exercise in treatment of Achilles tendinopathy. Scand J Med Sci Sports. 2007;17:133-138.
22. Roos EM, Engstrom M, Lagerquist A, et al. Clinical improvement after 6 weeks of eccentric exercise in patients with mid-portion Achilles tendinopathy – a randomized trial with 1-year follow-up. Scand J Med Sci Sports. 2004;14:286-295.
23. Magnusse RA, Dunn WR, Thompson AB. Nonoperative treatment of midportion Achilles tendinopathy: a systematic review. Clin J Sports Med. 2009;19:54-64.
24. McShane JM, Ostick B, McCabe F. Noninsertional Achilles tendinopathy: pathology and management. Curr Sports Med Rep. 2007;6:288-292.
25. Fahlstrom M, Jonsson P, Lorentzon R, et al. Chronic Achilles tendon pain treated with eccentric calf-muscle training. Knee Surg Sports Traumatol Arthrosc. 2003;11:327-333.
26. Lesho EP. Can tuning forks replace bone scans for identification of tibial stress fractures? Mil Med. 1997;162:802-803.
27. Clinghan R, Arnold GP, Drew TS, et al. Do you get value for money when you buy an expensive pair of running shoes? Br J Sports Med. 2008;42:189-193.
28. Butler RJ, Davis IS, Hamill J. Interaction of arch type and footwear on running mechanics. Am J Sports Med. 2006;34:1998-2005.
29. Divert C, Mornieux G, Freychat P, et al. Barefoot-shot running differences: shoe or mass effect? Int J Sports Med. 2008;29:512-518.
30. Taunton JE, Ryan MB, Clement DB, et al. A prospective study of running injuries: the Vancouver Sun Run “In Training” clinics. Br J Sports Med. 2003;37:239-244.
31. Verdejo R, Mills NJ. Heel-shoe interactions and the durability of EVA foam running-shoe midsoles. J Biomech. 2004;37:1379-1386.
32. Winemiller MH, Billow RG, Laskowski ER, et al. Effect of magnetic vs sham-magnetic insoles on nonspecific foot pain in the workplace: a randomized, double-blind, placebo-controlled trial. Mayo Clin Proc. 2005;80:1138-1145.
33. Logan K. Stress fractures in the adolescent athlete. Pediatr Ann. 2007;36:738-745.
34. Thein-Nissenbaum JM, Carr KE. Female athlete triad syndrome in the high school athlete. Phys Ther Sport. 2011;12:108-116.
35. Umans H. Imaging sports medicine injuries of the foot and toes. Clin Sports Med. 2006;25:763-780.
36. Vorlat P, Achtergael W, Haentjens P. Predictors of outcome of non-displaced fractures of the base of the fifth metatarsal. Int Orthop. 2007;31:5-10.
37. Dyck D, Boyajian-O’Neill L. Plantar fasciitis. Clin J Sports Med. 2004;14:305-309.
38. Alfredson H, Thorsen K, Lorentzon R. In situ microdialysis in tendon tissue: high levels of glutamate, but not prostaglandin E2 in chronic Achilles tendon pain. Knee Surg Sports Traumatol Arthrosc. 1999;7:378-381.
39. Seligman DA, Dawson DR. Customized heel pads and soft orthotics to treat heel pain and plantar fasciitis. Arch Phys Med Rehab. 2003;84:1564-1567.
• Advise patients with metatarsalgia to use metatarsal pads, consider orthotics, use contrast baths, and avoid high heels and pointy-toed shoes. C
• Recommend that runners with stress fractures of the foot have at least 4 weeks of rest before a gradual return to activity. C
• Consider short-term physical therapy for patients with plantar fasciitis to enable them to learn proper stretching and strengthening techniques. C
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
CASE Jim F, 40 years old and overweight (BMI=28 kg/m2), has come to see you because of foot pain that began shortly after he took up running. Jim tells you that turning 40 was “an eye opener” that prompted him to “get healthy.” He says that while he was a competitive athlete in high school, he never ran regularly—until he embarked on a running program 3 months ago.
Jim denies acute injury, bruising, swelling, redness, fever, or chills, but states that the pain, which he describes as dull and achy, is gradually getting worse. It hurts the most when he stands for long periods of time. He says that he occasionally takes ibuprofen for the foot pain, but has not tried icing or stretching. When you ask him what kind of sneakers he wears during his runs, Jim reports that his running shoes—purchased at a discount store—are about 5 years old.
Participation in running has grown by more than 40% in the United States in the past decade.1 As a result, patients like Jim are bound to have their share of aches, pains, and injuries that prompt them to visit their family physician. And that’s where this review can help. This rundown of the most common foot pain diagnoses, as well as the at-a-glance summaries of the differential diagnosis (TABLE 1)2-5 and treatment options (TABLE 2),3,6-25 can help you quickly get patients the relief they need to return to running.
TABLE 1
Differential diagnosis for runners’ foot pain2-5
| Symptom | Differential diagnosis |
|---|---|
| Foot pain |
|
| Heel pain |
|
| *Represents a more common diagnosis. | |
TABLE 2
Diagnosing and treating common runners’ injuries
| Diagnosis | History | Physical exam | Interventions |
|---|---|---|---|
| Metatarsalgia | Plantar foot pain, insidious onset; occasional swelling, bruising, or deformity | Tenderness of MT heads; possible edema or hyperkeratosis; negative tuning fork test | Footwear: cushioning, wide toe box, MT pads; consider orthotics. Contrast baths; NSAIDs6-9 |
| Stress fracture | Pain, insidious onset, increasing in intensity and duration | Localized TTP; possible swelling or bruising; positive tuning fork test; X-rays/MRI may be helpful | Boot for minimum of 3-4 weeks, followed by PT for foot/ankle ROM, strength, proprioception Ice, acetaminophen (NSAIDs controversial)10-12 Progressive return to running* |
| Plantar fasciitis | Plantar foot/heel pain, worse with first steps in AM and after prolonged weight-bearing | TTP at medial calcaneal tubercle | Relative rest, NSAIDs, PT for HEP, Graston technique, taping; possible night splinting13-15 Consider ESWT, corticosteroid injection for refractory cases16-18 |
| MAT | Posterior heel/Achilles pain in midportion; insidious onset, increasing in intensity, worse with activity | Tenderness midportion Achilles; possible tendon thickening; warmth, crepitus, nodules | Relative rest; PT for eccentric exercises; heel lift, with or without orthotics19-22 Consider PRP, prolotherapy, ESWT, or ultrasound in refractory cases†23,24 Surgical intervention rarely indicated3 |
| IAT | Posterior heel/Achilles pain in insertion of Achilles; insidious onset, increasing in intensity; swelling possible; worse with activity | Tenderness with or without swelling; deformity at Achilles insertion | Relative rest; footwear modification (heel lift, possibly with orthotics); PT for eccentric exercises, though less valuable than for MAT†25 |
| *Starting with cross-training exercise, progressing to running on a treadmill, then to running outdoors. †Corticosteroid injection contraindicated. ESWT, extracorporeal shock wave therapy; HEP, home exercise program; IAT, insertional Achilles tendinopathy; MAT, midportion Achilles tendinopathy; MRI, magnetic resonance imaging; MT, metatarsal; NSAIDs, nonsteroidal anti-inflammatory drugs; PRP, plasma-rich protein; PT, physical therapy; ROM, range of motion; TTP, tenderness to palpation. | |||
Metatarsalgia: Pain on the plantar surface
Typically associated with a recent increase in activity or change in footwear, metatarsalgia is defined by pain on the plantar surface of the forefoot in the area of the metatarsal heads. The second, third, and fourth metatarsals are the most common offenders, and the pain may or may not be accompanied by swelling, bruising, or deformity.
Mechanical irregularities in the foot are thought to contribute to the development of metatarsalgia, which is typically inflammatory in nature. Physical exam often reveals tenderness at the affected metatarsal heads, with or without pain in the corresponding metatarsophalangeal joint, and occasionally, with overlying edema or hyperkeratosis.
Tuning fork test. Commonly used but weakly supported, this diagnostic test is performed by applying a vibrating tuning fork to a site of possible fracture. If the maneuver produces focal pain, the test is positive and may be helpful in ruling in metatarsal stress fractures.26
Treatment: Change shoes, consider NSAIDs. Treatment for metatarsalgia begins conservatively, with a change in footwear. High heels or pointy-toed shoes should be avoided, and metatarsal pads (FIGURE 1) can be placed inside the shoes to help off-load the metatarsal head.6 The pads come prefabricated or can be custom made, and are typically placed by physical therapists to ensure proper placement. Orthotics should also be considered, as they can help normalize abnormal foot mechanics that may contribute to metatarsalgia.7,8 (See “A word about runners’ footwear”.9,27-31)
Metatarsalgia is believed to be an inflammatory process, and NSAIDs may be helpful. Contrast baths—alternately submerging the affected foot in a basin of hot (but not scalding) water for 1 to 2 minutes, then immersing it in cold water for 30 to 60 seconds and repeating the process for about 20 minutes once or twice daily—may be helpful. Magnetic insoles are not recommended, as they have been found to be no better than sham insoles.32 Rarely, surgical repair of underlying mechanical abnormalities is indicated for treatment of refractory metatarsalgia.
CASE On examination, Jim F has no swelling, but some hyperkeratosis overlying the second and third metatarsal heads. He has tenderness to palpation at these heads as well as the corresponding metatarsophalangeal joints, and a negative tuning fork test.
You advise Jim that he has metatarsalgia, educate him about the pathophysiology of this condition, and give him a prescription for a nonsteroidal anti-inflammatory drug. You suggest he use contrast baths—and explain how this is done—once or twice a day and refer him to physical therapy for proper placement of metatarsal pads in his shoes, and schedule an appointment for a 6-week follow-up.
Return to running. There is no firm recommendation regarding abstaining from running with metatarsalgia. Advise patients to use pain as a guide in determining the intensity and duration of activity.
FIGURE 1
Treatment for metatarsalgia is conservative
In addition to changing to more comfortable footwear, patients with metatarsalgia can place metatarsal pads like the one shown here in their shoes to ease the metatarsal load.
The proper footwear for runners is subject to considerable debate, with arguments supported by contradictory evidence. What is known, however, is that running shoes should:
- be a comfortable fit with cushioning chosen to accommodate arch type
- be replaced after running 300 to 500 miles or every 12 months, whichever comes first27,28
- be purchased from a sporting goods or running store, rather than at a discount retailer. That’s because the shoes sold at discount stores are often older, and breakdown of the protective cushioning is more likely to have occurred prior to purchase.28
The most expensive shoe is not automatically the best choice for the runner, however. Some studies have found no benefit in foot strike pressures with expensive cushioned running shoes compared with low- or medium-cost brands.29 Shoes should be selected based on comfort, although the patient’s arch type should also be considered when selecting running footwear.30
Barefoot running shoes, designed to simulate barefoot running, are also an option. As with cushioned running shoes, evidence regarding barefoot running is contradictory. Some studies suggest that running mechanics are improved with barefoot running or barefoot running shoes; others have had unfavorable or inconsistent results, indicating a need for further research.9,31
Stress fracture: Tenderness and pain of insidious onset
Stress fractures of the foot (SFF)—overuse injuries also known as fatigue fractures—are common in recreational runners. They are thought to result from microtraumas, which alone are not sufficient to break bone but together overwhelm the bone’s natural ability to remodel and recover over time. SFF are characterized by tenderness and pain of insidious onset, and typically occur when more than one training variable (eg, frequency, duration, and intensity) is changed simultaneously. SFF can also result from a change in exercise mechanics, such as foot strike.
Stress fractures can occur in any bone in the foot, but are most common in the metatarsal bones, specifically the mid or distal portion of the second or third metatarsal, or the tarsal navicular.2,33 On examination, the patient will have tenderness to palpation, often well localized. A positive tuning fork test (see page 647) is highly suggestive of a stress fracture.
In female runners, stress fractures may be associated with the female athlete triad—osteoporosis or osteopenia, disordered eating (specifically caloric deficiency and low BMI), and amenorrhea. In addition to the major long-term health problems that may result from even one component of the triad, SFF may be a short-term consequence.34
Although SFF is a clinical diagnosis, x-rays—including 3-view plain films of the foot, with the area of concern clearly noted on the order—are recommended. Magnetic resonance imaging may be used for secondary imaging if doubt about the source of the pain remains.35
Of note: Occasionally, a metatarsal stress fracture progresses to a frank fracture, specifically of the metaphyseal-diaphyseal junction of the fifth metatarsal—known as a Jones fracture. This type of fracture has a high rate of malunion or nonunion.36 If there is any suspicion of a fracture in this area, consider a referral to a sports medicine specialist or orthopedic surgeon.
Treatment: Icing, analgesics, and a boot. Standard treatment for SFF includes icing for 15 to 20 minutes up to 3 times a day for a minimum of 72 hours after injury, but may be continued throughout the healing period. Analgesics such as acetaminophen and a walking boot for 3 to 4 weeks, with follow-up at 3 weeks, should also be implemented. Recent evidence suggests that NSAIDs may hinder the bone healing process, and their use in treating SFF is controversial.10-12
Weaning from the boot can begin when the patient is pain free with the boot on, usually by 3 to 4 weeks. Patients often progress quickly from wearing the boot at all times to wearing it only outside of the house, to not wearing it at all. Advise patients who need to walk long distances for a good portion of the day to keep the boot nearby and to put it on if the pain returns.
Once weaning from the boot begins, physical therapy (PT) should be considered to help the patient regain foot and ankle range of motion (ROM), proprioception, and strength. Once he or she learns the exercises, rehabilitation can be accomplished with a home exercise program. Foot deformities, such as pes planus or pes cavus, may indicate a need for orthotics. A well-structured athletic shoe may help to prevent future injury.7,8
Return to running. Once adequate ROM and strength in the foot and ankle are recovered, the patient can begin to resume activity, starting with a low-impact cross-training exercise, such as a stationary bike or elliptical, for a week or 2. A patient who remains pain free can progress from cross-training to running on a treadmill for another week or 2, then gradually switch to outdoor running.
Plantar fasciitis: Heel pain with an insidious onset
Plantar fasciitis is one of the most common causes of heel pain in athletes (primarily runners) and nonathletes alike. Plantar fasciitis may be associated with acute trauma, but is more commonly insidious in onset. The diagnosis is clinical and rarely requires imaging.
Pain associated with plantar fasciitis may be described as sharp and stabbing or dull and aching. It is on the plantar surface of the heel, sometimes radiating to the arch, and may localize to the insertion of the plantar fascia on the medial calcaneal tubercle (FIGURE 2). The pain is typically most severe with the first few steps in the morning or after other periods of prolonged rest. It usually improves after a few steps, but may return later in the day. Plantar fasciitis does not cause paresthesias or other neurologic symptoms, so their presence is suggestive of a different diagnosis, such as nerve entrapment, compartment syndrome, or tarsal tunnel syndrome.3,5
Treatment: It’s multifactorial. NSAIDs are commonly used. Relative rest is recommended, but cross training may be considered to maintain fitness.37 Short-term PT is also recommended to teach the patient proper stretching and strengthening techniques in the form of a home exercise plan. Modalities such as iontophoresis (a system of transdermal delivery of medication with the use of electrical currents), Graston (a form of instrument-assisted soft tissue mobilization), and taping may be incorporated into PT, as well.13
Night splinting may also be used to keep the foot in a dorsiflexed position. A splint can be purchased without a prescription and prevents the plantar fascia from shortening overnight by providing a continuous passive stretch, thus reducing pain with first steps.14
Orthotics may also help to reduce symptom severity and duration, and studies have found no difference in outcomes with prefabricated vs custom-made devices.15 Another treatment to consider, particularly for recalcitrant cases of plantar fasciitis, is extracorporeal shock wave therapy, which has been studied for more than a decade with conflicting results.16 Corticosteroid injection may also be used for treatment-refractory plantar fasciitis, but caution is required, as the injection may increase the risk of rupture of the plantar fascia.17,18
Return to running. There are no set guidelines for when an athlete with plantar fasciitis can return to running. Typically, after 2 to 4 weeks of relative rest and other treatments, the runner can begin to transition from cross-training to treadmill running.
FIGURE 2
Severe pain with first steps of the day
The pain of plantar fasciitis—often most severe first thing in the morning—may localize to the insertion of the plantar fascia on the medial calcaneal tubercle, as shown above.
Achilles tendinopathy: An overuse injury
Achilles tendinopathy (AT) is typically an overuse injury incurred by athletes, although it is sometimes seen in patients who are sedentary and overweight. With a prevalence among runners of approximately 11%, AT is sometimes called the “runners’ disease.”4
Tendinopathy is a more accurate description than tendonitis, as histologic studies of affected Achilles tendons suggest that AT is a degenerative, rather than an inflammatory, condition.38 A diagnosis of AT can be further classified as midportion or insertional.
Midportion Achilles tendinopathy (MAT), characterized by pain that occurs in the body of the Achilles tendon and worsens with activity, is often a clinical diagnosis. Physical findings suggestive of MAT are tenderness to palpation of the midportion of the Achilles tendon, with thickening of the tendon, warmth, crepitus, or palpable nodules in the tendon body. Onset is insidious and is commonly associated with an increase in activity.
Treatment: Orthotics or a heel lift. Like that of plantar fasciitis, treatment of midportion Achilles tendinopathy is primarily conservative. The use of orthotics, or a heel lift, is one of the most cost-effective interventions, and they are widely used, despite limited evidence of efficacy.39 Custom orthotics are costly, and patients often benefit from trying prefabricated orthotics first to determine whether they will help.
Eccentric exercises. One of the most studied interventions for MAT is eccentric exercise training. Studies of eccentric exercises have been very favorable, and the exercises can be taught during routine PT sessions.19-22 Modalities such as ultrasound therapy and extracorporeal shock wave therapy (ESWT) have also been studied. But because results have been inconsistent, they are generally reserved for treatment-refractory cases.23
In patients with no contraindications, NSAIDs may be a good choice for pain management with relatively favorable results in the literature.24 Corticosteroid injections should not be used, as they have been directly linked to rupture of the Achilles tendon.23
Other interventions, such as plasma-rich protein injections and prolotherapy—a technique in which an irritant is injected into the tendon in an attempt to create an inflammatory reaction, thus increasing local blood flow and healing—are being studied for the treatment of AT, but are not routinely used or covered by insurance for this purpose. Surgical intervention may be considered for patients whose symptoms last for more than 3 to 6 months despite conservative treatment.
Insertional Achilles tendinopathy (IAT) can be clinically differentiated from MAT by the location of symptoms and tenderness to palpation at the insertion site of the Achilles into the calcaneous. Like MAT, IAT is exacerbated by activity. Other conditions that may contribute to, or be mistaken for, IAT are a Haglund deformity and retrocalcaneal bursitis.
Treatment: Footwear modification. Treatment of IAT, like that of MAT, is primarily conservative. Orthotics or heel lifts are commonly used. However, there is greater emphasis on footwear modification due to the mechanical irritation and resultant posterior heel swelling often associated with IAT. While eccentric exercises play a role in IAT treatment, the benefits are limited.25
As with MAT, corticosteroid injections are contraindicated due to the risk of tendon rupture. Modalities such as ultrasound, ESWT, plasma-rich protein, and prolotherapy lack sufficient evidence to be widely recommended.
For refractory cases of IAT, surgical intervention often relieves the pain.
Return to running. After an initial rest of 2 to 4 weeks, patients may return to running while completing therapy. It’s not necessary to wait until the patient is completely pain free, but pain should be used to guide decisions about intensity and duration of activity.
CASE When Jim returns 6 weeks later, he reports that he took 3 weeks off from running because of the pain. Initially, he used contrast baths daily, Jim says, but now he uses them only when he is symptomatic, and he discontinued the NSAID a few weeks ago. Jim tells you he went to the local running store for a new pair of running shoes and that he is now able to run at his previous pace while remaining relatively pain free.
CORRESPONDENCE Jessica Favero Butts, MD, One American Square, Suite 185, Indianapolis, IN 46282; Jbutts2@iuhealth.org
• Advise patients with metatarsalgia to use metatarsal pads, consider orthotics, use contrast baths, and avoid high heels and pointy-toed shoes. C
• Recommend that runners with stress fractures of the foot have at least 4 weeks of rest before a gradual return to activity. C
• Consider short-term physical therapy for patients with plantar fasciitis to enable them to learn proper stretching and strengthening techniques. C
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
CASE Jim F, 40 years old and overweight (BMI=28 kg/m2), has come to see you because of foot pain that began shortly after he took up running. Jim tells you that turning 40 was “an eye opener” that prompted him to “get healthy.” He says that while he was a competitive athlete in high school, he never ran regularly—until he embarked on a running program 3 months ago.
Jim denies acute injury, bruising, swelling, redness, fever, or chills, but states that the pain, which he describes as dull and achy, is gradually getting worse. It hurts the most when he stands for long periods of time. He says that he occasionally takes ibuprofen for the foot pain, but has not tried icing or stretching. When you ask him what kind of sneakers he wears during his runs, Jim reports that his running shoes—purchased at a discount store—are about 5 years old.
Participation in running has grown by more than 40% in the United States in the past decade.1 As a result, patients like Jim are bound to have their share of aches, pains, and injuries that prompt them to visit their family physician. And that’s where this review can help. This rundown of the most common foot pain diagnoses, as well as the at-a-glance summaries of the differential diagnosis (TABLE 1)2-5 and treatment options (TABLE 2),3,6-25 can help you quickly get patients the relief they need to return to running.
TABLE 1
Differential diagnosis for runners’ foot pain2-5
| Symptom | Differential diagnosis |
|---|---|
| Foot pain |
|
| Heel pain |
|
| *Represents a more common diagnosis. | |
TABLE 2
Diagnosing and treating common runners’ injuries
| Diagnosis | History | Physical exam | Interventions |
|---|---|---|---|
| Metatarsalgia | Plantar foot pain, insidious onset; occasional swelling, bruising, or deformity | Tenderness of MT heads; possible edema or hyperkeratosis; negative tuning fork test | Footwear: cushioning, wide toe box, MT pads; consider orthotics. Contrast baths; NSAIDs6-9 |
| Stress fracture | Pain, insidious onset, increasing in intensity and duration | Localized TTP; possible swelling or bruising; positive tuning fork test; X-rays/MRI may be helpful | Boot for minimum of 3-4 weeks, followed by PT for foot/ankle ROM, strength, proprioception Ice, acetaminophen (NSAIDs controversial)10-12 Progressive return to running* |
| Plantar fasciitis | Plantar foot/heel pain, worse with first steps in AM and after prolonged weight-bearing | TTP at medial calcaneal tubercle | Relative rest, NSAIDs, PT for HEP, Graston technique, taping; possible night splinting13-15 Consider ESWT, corticosteroid injection for refractory cases16-18 |
| MAT | Posterior heel/Achilles pain in midportion; insidious onset, increasing in intensity, worse with activity | Tenderness midportion Achilles; possible tendon thickening; warmth, crepitus, nodules | Relative rest; PT for eccentric exercises; heel lift, with or without orthotics19-22 Consider PRP, prolotherapy, ESWT, or ultrasound in refractory cases†23,24 Surgical intervention rarely indicated3 |
| IAT | Posterior heel/Achilles pain in insertion of Achilles; insidious onset, increasing in intensity; swelling possible; worse with activity | Tenderness with or without swelling; deformity at Achilles insertion | Relative rest; footwear modification (heel lift, possibly with orthotics); PT for eccentric exercises, though less valuable than for MAT†25 |
| *Starting with cross-training exercise, progressing to running on a treadmill, then to running outdoors. †Corticosteroid injection contraindicated. ESWT, extracorporeal shock wave therapy; HEP, home exercise program; IAT, insertional Achilles tendinopathy; MAT, midportion Achilles tendinopathy; MRI, magnetic resonance imaging; MT, metatarsal; NSAIDs, nonsteroidal anti-inflammatory drugs; PRP, plasma-rich protein; PT, physical therapy; ROM, range of motion; TTP, tenderness to palpation. | |||
Metatarsalgia: Pain on the plantar surface
Typically associated with a recent increase in activity or change in footwear, metatarsalgia is defined by pain on the plantar surface of the forefoot in the area of the metatarsal heads. The second, third, and fourth metatarsals are the most common offenders, and the pain may or may not be accompanied by swelling, bruising, or deformity.
Mechanical irregularities in the foot are thought to contribute to the development of metatarsalgia, which is typically inflammatory in nature. Physical exam often reveals tenderness at the affected metatarsal heads, with or without pain in the corresponding metatarsophalangeal joint, and occasionally, with overlying edema or hyperkeratosis.
Tuning fork test. Commonly used but weakly supported, this diagnostic test is performed by applying a vibrating tuning fork to a site of possible fracture. If the maneuver produces focal pain, the test is positive and may be helpful in ruling in metatarsal stress fractures.26
Treatment: Change shoes, consider NSAIDs. Treatment for metatarsalgia begins conservatively, with a change in footwear. High heels or pointy-toed shoes should be avoided, and metatarsal pads (FIGURE 1) can be placed inside the shoes to help off-load the metatarsal head.6 The pads come prefabricated or can be custom made, and are typically placed by physical therapists to ensure proper placement. Orthotics should also be considered, as they can help normalize abnormal foot mechanics that may contribute to metatarsalgia.7,8 (See “A word about runners’ footwear”.9,27-31)
Metatarsalgia is believed to be an inflammatory process, and NSAIDs may be helpful. Contrast baths—alternately submerging the affected foot in a basin of hot (but not scalding) water for 1 to 2 minutes, then immersing it in cold water for 30 to 60 seconds and repeating the process for about 20 minutes once or twice daily—may be helpful. Magnetic insoles are not recommended, as they have been found to be no better than sham insoles.32 Rarely, surgical repair of underlying mechanical abnormalities is indicated for treatment of refractory metatarsalgia.
CASE On examination, Jim F has no swelling, but some hyperkeratosis overlying the second and third metatarsal heads. He has tenderness to palpation at these heads as well as the corresponding metatarsophalangeal joints, and a negative tuning fork test.
You advise Jim that he has metatarsalgia, educate him about the pathophysiology of this condition, and give him a prescription for a nonsteroidal anti-inflammatory drug. You suggest he use contrast baths—and explain how this is done—once or twice a day and refer him to physical therapy for proper placement of metatarsal pads in his shoes, and schedule an appointment for a 6-week follow-up.
Return to running. There is no firm recommendation regarding abstaining from running with metatarsalgia. Advise patients to use pain as a guide in determining the intensity and duration of activity.
FIGURE 1
Treatment for metatarsalgia is conservative
In addition to changing to more comfortable footwear, patients with metatarsalgia can place metatarsal pads like the one shown here in their shoes to ease the metatarsal load.
The proper footwear for runners is subject to considerable debate, with arguments supported by contradictory evidence. What is known, however, is that running shoes should:
- be a comfortable fit with cushioning chosen to accommodate arch type
- be replaced after running 300 to 500 miles or every 12 months, whichever comes first27,28
- be purchased from a sporting goods or running store, rather than at a discount retailer. That’s because the shoes sold at discount stores are often older, and breakdown of the protective cushioning is more likely to have occurred prior to purchase.28
The most expensive shoe is not automatically the best choice for the runner, however. Some studies have found no benefit in foot strike pressures with expensive cushioned running shoes compared with low- or medium-cost brands.29 Shoes should be selected based on comfort, although the patient’s arch type should also be considered when selecting running footwear.30
Barefoot running shoes, designed to simulate barefoot running, are also an option. As with cushioned running shoes, evidence regarding barefoot running is contradictory. Some studies suggest that running mechanics are improved with barefoot running or barefoot running shoes; others have had unfavorable or inconsistent results, indicating a need for further research.9,31
Stress fracture: Tenderness and pain of insidious onset
Stress fractures of the foot (SFF)—overuse injuries also known as fatigue fractures—are common in recreational runners. They are thought to result from microtraumas, which alone are not sufficient to break bone but together overwhelm the bone’s natural ability to remodel and recover over time. SFF are characterized by tenderness and pain of insidious onset, and typically occur when more than one training variable (eg, frequency, duration, and intensity) is changed simultaneously. SFF can also result from a change in exercise mechanics, such as foot strike.
Stress fractures can occur in any bone in the foot, but are most common in the metatarsal bones, specifically the mid or distal portion of the second or third metatarsal, or the tarsal navicular.2,33 On examination, the patient will have tenderness to palpation, often well localized. A positive tuning fork test (see page 647) is highly suggestive of a stress fracture.
In female runners, stress fractures may be associated with the female athlete triad—osteoporosis or osteopenia, disordered eating (specifically caloric deficiency and low BMI), and amenorrhea. In addition to the major long-term health problems that may result from even one component of the triad, SFF may be a short-term consequence.34
Although SFF is a clinical diagnosis, x-rays—including 3-view plain films of the foot, with the area of concern clearly noted on the order—are recommended. Magnetic resonance imaging may be used for secondary imaging if doubt about the source of the pain remains.35
Of note: Occasionally, a metatarsal stress fracture progresses to a frank fracture, specifically of the metaphyseal-diaphyseal junction of the fifth metatarsal—known as a Jones fracture. This type of fracture has a high rate of malunion or nonunion.36 If there is any suspicion of a fracture in this area, consider a referral to a sports medicine specialist or orthopedic surgeon.
Treatment: Icing, analgesics, and a boot. Standard treatment for SFF includes icing for 15 to 20 minutes up to 3 times a day for a minimum of 72 hours after injury, but may be continued throughout the healing period. Analgesics such as acetaminophen and a walking boot for 3 to 4 weeks, with follow-up at 3 weeks, should also be implemented. Recent evidence suggests that NSAIDs may hinder the bone healing process, and their use in treating SFF is controversial.10-12
Weaning from the boot can begin when the patient is pain free with the boot on, usually by 3 to 4 weeks. Patients often progress quickly from wearing the boot at all times to wearing it only outside of the house, to not wearing it at all. Advise patients who need to walk long distances for a good portion of the day to keep the boot nearby and to put it on if the pain returns.
Once weaning from the boot begins, physical therapy (PT) should be considered to help the patient regain foot and ankle range of motion (ROM), proprioception, and strength. Once he or she learns the exercises, rehabilitation can be accomplished with a home exercise program. Foot deformities, such as pes planus or pes cavus, may indicate a need for orthotics. A well-structured athletic shoe may help to prevent future injury.7,8
Return to running. Once adequate ROM and strength in the foot and ankle are recovered, the patient can begin to resume activity, starting with a low-impact cross-training exercise, such as a stationary bike or elliptical, for a week or 2. A patient who remains pain free can progress from cross-training to running on a treadmill for another week or 2, then gradually switch to outdoor running.
Plantar fasciitis: Heel pain with an insidious onset
Plantar fasciitis is one of the most common causes of heel pain in athletes (primarily runners) and nonathletes alike. Plantar fasciitis may be associated with acute trauma, but is more commonly insidious in onset. The diagnosis is clinical and rarely requires imaging.
Pain associated with plantar fasciitis may be described as sharp and stabbing or dull and aching. It is on the plantar surface of the heel, sometimes radiating to the arch, and may localize to the insertion of the plantar fascia on the medial calcaneal tubercle (FIGURE 2). The pain is typically most severe with the first few steps in the morning or after other periods of prolonged rest. It usually improves after a few steps, but may return later in the day. Plantar fasciitis does not cause paresthesias or other neurologic symptoms, so their presence is suggestive of a different diagnosis, such as nerve entrapment, compartment syndrome, or tarsal tunnel syndrome.3,5
Treatment: It’s multifactorial. NSAIDs are commonly used. Relative rest is recommended, but cross training may be considered to maintain fitness.37 Short-term PT is also recommended to teach the patient proper stretching and strengthening techniques in the form of a home exercise plan. Modalities such as iontophoresis (a system of transdermal delivery of medication with the use of electrical currents), Graston (a form of instrument-assisted soft tissue mobilization), and taping may be incorporated into PT, as well.13
Night splinting may also be used to keep the foot in a dorsiflexed position. A splint can be purchased without a prescription and prevents the plantar fascia from shortening overnight by providing a continuous passive stretch, thus reducing pain with first steps.14
Orthotics may also help to reduce symptom severity and duration, and studies have found no difference in outcomes with prefabricated vs custom-made devices.15 Another treatment to consider, particularly for recalcitrant cases of plantar fasciitis, is extracorporeal shock wave therapy, which has been studied for more than a decade with conflicting results.16 Corticosteroid injection may also be used for treatment-refractory plantar fasciitis, but caution is required, as the injection may increase the risk of rupture of the plantar fascia.17,18
Return to running. There are no set guidelines for when an athlete with plantar fasciitis can return to running. Typically, after 2 to 4 weeks of relative rest and other treatments, the runner can begin to transition from cross-training to treadmill running.
FIGURE 2
Severe pain with first steps of the day
The pain of plantar fasciitis—often most severe first thing in the morning—may localize to the insertion of the plantar fascia on the medial calcaneal tubercle, as shown above.
Achilles tendinopathy: An overuse injury
Achilles tendinopathy (AT) is typically an overuse injury incurred by athletes, although it is sometimes seen in patients who are sedentary and overweight. With a prevalence among runners of approximately 11%, AT is sometimes called the “runners’ disease.”4
Tendinopathy is a more accurate description than tendonitis, as histologic studies of affected Achilles tendons suggest that AT is a degenerative, rather than an inflammatory, condition.38 A diagnosis of AT can be further classified as midportion or insertional.
Midportion Achilles tendinopathy (MAT), characterized by pain that occurs in the body of the Achilles tendon and worsens with activity, is often a clinical diagnosis. Physical findings suggestive of MAT are tenderness to palpation of the midportion of the Achilles tendon, with thickening of the tendon, warmth, crepitus, or palpable nodules in the tendon body. Onset is insidious and is commonly associated with an increase in activity.
Treatment: Orthotics or a heel lift. Like that of plantar fasciitis, treatment of midportion Achilles tendinopathy is primarily conservative. The use of orthotics, or a heel lift, is one of the most cost-effective interventions, and they are widely used, despite limited evidence of efficacy.39 Custom orthotics are costly, and patients often benefit from trying prefabricated orthotics first to determine whether they will help.
Eccentric exercises. One of the most studied interventions for MAT is eccentric exercise training. Studies of eccentric exercises have been very favorable, and the exercises can be taught during routine PT sessions.19-22 Modalities such as ultrasound therapy and extracorporeal shock wave therapy (ESWT) have also been studied. But because results have been inconsistent, they are generally reserved for treatment-refractory cases.23
In patients with no contraindications, NSAIDs may be a good choice for pain management with relatively favorable results in the literature.24 Corticosteroid injections should not be used, as they have been directly linked to rupture of the Achilles tendon.23
Other interventions, such as plasma-rich protein injections and prolotherapy—a technique in which an irritant is injected into the tendon in an attempt to create an inflammatory reaction, thus increasing local blood flow and healing—are being studied for the treatment of AT, but are not routinely used or covered by insurance for this purpose. Surgical intervention may be considered for patients whose symptoms last for more than 3 to 6 months despite conservative treatment.
Insertional Achilles tendinopathy (IAT) can be clinically differentiated from MAT by the location of symptoms and tenderness to palpation at the insertion site of the Achilles into the calcaneous. Like MAT, IAT is exacerbated by activity. Other conditions that may contribute to, or be mistaken for, IAT are a Haglund deformity and retrocalcaneal bursitis.
Treatment: Footwear modification. Treatment of IAT, like that of MAT, is primarily conservative. Orthotics or heel lifts are commonly used. However, there is greater emphasis on footwear modification due to the mechanical irritation and resultant posterior heel swelling often associated with IAT. While eccentric exercises play a role in IAT treatment, the benefits are limited.25
As with MAT, corticosteroid injections are contraindicated due to the risk of tendon rupture. Modalities such as ultrasound, ESWT, plasma-rich protein, and prolotherapy lack sufficient evidence to be widely recommended.
For refractory cases of IAT, surgical intervention often relieves the pain.
Return to running. After an initial rest of 2 to 4 weeks, patients may return to running while completing therapy. It’s not necessary to wait until the patient is completely pain free, but pain should be used to guide decisions about intensity and duration of activity.
CASE When Jim returns 6 weeks later, he reports that he took 3 weeks off from running because of the pain. Initially, he used contrast baths daily, Jim says, but now he uses them only when he is symptomatic, and he discontinued the NSAID a few weeks ago. Jim tells you he went to the local running store for a new pair of running shoes and that he is now able to run at his previous pace while remaining relatively pain free.
CORRESPONDENCE Jessica Favero Butts, MD, One American Square, Suite 185, Indianapolis, IN 46282; Jbutts2@iuhealth.org
1. Sporting Goods Manufacturers Association (SGMA) 2010 Sports & Fitness Participation Report. Silver Spring, Md: SGMA; 2011.
2. Tuan K, Wu S, Sennett B. Stress fractures in athletes: risk fractures, diagnosis, and management. Orthopedics. 2004;27:583-593.
3. Wapner KL, Parekh SG. Heel pain. In: DeLee J, Drez D, Miller M, eds. DeLee and Drez’s Orthopaedic Sports Medicine. 3rd ed. Philadelphia, Pa: Saunders; 2010:2030–2056.
4. Lysholm J, Wiklander J. Injuries in runners. Am J Sports Med. 1987;15:168-171.
5. Guyton G, Gomez L, Mann R. Entrapment neuropathies of the foot. In: DeLee J, Drez D, Miller M, eds. DeLee and Drez’s Orthopaedic Sports Medicine. 3rd ed. Philadelphia, Pa: Saunders; 2010:2057–2063.
6. Kang JH, Chen MD, Chen SC, et al. Correlations between subjective treatment responses and plantar pressure parameters of metatarsal pad treatment in metatarsalgia patients: a prospective study. BMC Musculoskelet Disord. 2006;7:95.-
7. MacLean CL, van Emmerik R, Hamill J. Influence of custom foot orthotic intervention on lower extremity intralimb coupling during a 30-minute run. J Appl Biomech. 2010;26:390-399.
8. MacLean CL, Davis IS, Hamill J. Short- and long-term influences of a custom foot orthotic intervention on lower extremity dynamics. Clin J Sport Med. 2008;18:338-343.
9. Bishop M, Fiolkowski P, Conrad B, et al. Athletic footwear, leg stiffness, and running kinematics. J Athl Train. 2006;41:387-392.
10. Burd TA, Hughes MS, Anglen JO. Heterotopic ossification prophylaxis with indomethacin increases the risk of long-bone nonunion. J Bone Joint Surg Br. 2003;85:700-705.
11. Butcher CK, Marsh DR. Nonsteroidal anti-inflammatory drugs delay tibial fracture union. Injury. 1996;27:375.-
12. Yates JE, Shah SH. Do NSAIDS impede fracture healing? J Fam Pract. 2011;60:41-42.
13. Hyland M, Webber-Gaffney A, Cohen L. Randomized controlled trial of calcaneal taping, sham taping, and plantar fascia stretching for the short-term management of plantar heel pain. J Orthop Sports Phys Ther. 2006;36:364-371.
14. Powell M, Post WR, Keener J, et al. Effective treatment of chronic plantar fasciitis with dorsiflexion night splints: a crossover prospective randomized outcome study. Foot Ankle Int. 1998;19:10-18.
15. Baldassin V, Gomes CR, Beraldo PS. Effectiveness of prefabricated and customized foot orthoses made from low-cost foam for noncomplicated plantar fasciitis: a randomized controlled trial. Arch Phys Med Rehabil. 2009;90:701-706.
16. Rompe JD, Furia J, Weil L, et al. Shock wave therapy for chronic plantar fasciopathy. Br Med Bull. 2007;81-82:183-208.
17. Kleinman M, Gross AF. Achilles tendon rupture following steroid injection. Report of three cases. J Bone Joint Surg Am. 1983;65:1345-1347.
18. Hamilton B, Remedios D, Loosemore M, et al. Achilles tendon rupture in an elite athlete following multiple injection therapies. J Sci Med Sport. 2008;11:566-568.
19. Wasielewski NJ, Kotsko KM. Does eccentric exercise reduce pain and improve strength in physically active adults with symptomatic lower extremity tendinosis? A systematic review. J Athl Train. 2007;42:409-421.
20. Kingma JJ, de Knikker R, Wittink HM, et al. Eccentric overload training in patients with chronic Achilles tendinopathy: a systematic review. Br J Sports Med. 2007;41:e3.-
21. Norregaard J, Larsen CC, Bieler T, et al. Eccentric exercise in treatment of Achilles tendinopathy. Scand J Med Sci Sports. 2007;17:133-138.
22. Roos EM, Engstrom M, Lagerquist A, et al. Clinical improvement after 6 weeks of eccentric exercise in patients with mid-portion Achilles tendinopathy – a randomized trial with 1-year follow-up. Scand J Med Sci Sports. 2004;14:286-295.
23. Magnusse RA, Dunn WR, Thompson AB. Nonoperative treatment of midportion Achilles tendinopathy: a systematic review. Clin J Sports Med. 2009;19:54-64.
24. McShane JM, Ostick B, McCabe F. Noninsertional Achilles tendinopathy: pathology and management. Curr Sports Med Rep. 2007;6:288-292.
25. Fahlstrom M, Jonsson P, Lorentzon R, et al. Chronic Achilles tendon pain treated with eccentric calf-muscle training. Knee Surg Sports Traumatol Arthrosc. 2003;11:327-333.
26. Lesho EP. Can tuning forks replace bone scans for identification of tibial stress fractures? Mil Med. 1997;162:802-803.
27. Clinghan R, Arnold GP, Drew TS, et al. Do you get value for money when you buy an expensive pair of running shoes? Br J Sports Med. 2008;42:189-193.
28. Butler RJ, Davis IS, Hamill J. Interaction of arch type and footwear on running mechanics. Am J Sports Med. 2006;34:1998-2005.
29. Divert C, Mornieux G, Freychat P, et al. Barefoot-shot running differences: shoe or mass effect? Int J Sports Med. 2008;29:512-518.
30. Taunton JE, Ryan MB, Clement DB, et al. A prospective study of running injuries: the Vancouver Sun Run “In Training” clinics. Br J Sports Med. 2003;37:239-244.
31. Verdejo R, Mills NJ. Heel-shoe interactions and the durability of EVA foam running-shoe midsoles. J Biomech. 2004;37:1379-1386.
32. Winemiller MH, Billow RG, Laskowski ER, et al. Effect of magnetic vs sham-magnetic insoles on nonspecific foot pain in the workplace: a randomized, double-blind, placebo-controlled trial. Mayo Clin Proc. 2005;80:1138-1145.
33. Logan K. Stress fractures in the adolescent athlete. Pediatr Ann. 2007;36:738-745.
34. Thein-Nissenbaum JM, Carr KE. Female athlete triad syndrome in the high school athlete. Phys Ther Sport. 2011;12:108-116.
35. Umans H. Imaging sports medicine injuries of the foot and toes. Clin Sports Med. 2006;25:763-780.
36. Vorlat P, Achtergael W, Haentjens P. Predictors of outcome of non-displaced fractures of the base of the fifth metatarsal. Int Orthop. 2007;31:5-10.
37. Dyck D, Boyajian-O’Neill L. Plantar fasciitis. Clin J Sports Med. 2004;14:305-309.
38. Alfredson H, Thorsen K, Lorentzon R. In situ microdialysis in tendon tissue: high levels of glutamate, but not prostaglandin E2 in chronic Achilles tendon pain. Knee Surg Sports Traumatol Arthrosc. 1999;7:378-381.
39. Seligman DA, Dawson DR. Customized heel pads and soft orthotics to treat heel pain and plantar fasciitis. Arch Phys Med Rehab. 2003;84:1564-1567.
1. Sporting Goods Manufacturers Association (SGMA) 2010 Sports & Fitness Participation Report. Silver Spring, Md: SGMA; 2011.
2. Tuan K, Wu S, Sennett B. Stress fractures in athletes: risk fractures, diagnosis, and management. Orthopedics. 2004;27:583-593.
3. Wapner KL, Parekh SG. Heel pain. In: DeLee J, Drez D, Miller M, eds. DeLee and Drez’s Orthopaedic Sports Medicine. 3rd ed. Philadelphia, Pa: Saunders; 2010:2030–2056.
4. Lysholm J, Wiklander J. Injuries in runners. Am J Sports Med. 1987;15:168-171.
5. Guyton G, Gomez L, Mann R. Entrapment neuropathies of the foot. In: DeLee J, Drez D, Miller M, eds. DeLee and Drez’s Orthopaedic Sports Medicine. 3rd ed. Philadelphia, Pa: Saunders; 2010:2057–2063.
6. Kang JH, Chen MD, Chen SC, et al. Correlations between subjective treatment responses and plantar pressure parameters of metatarsal pad treatment in metatarsalgia patients: a prospective study. BMC Musculoskelet Disord. 2006;7:95.-
7. MacLean CL, van Emmerik R, Hamill J. Influence of custom foot orthotic intervention on lower extremity intralimb coupling during a 30-minute run. J Appl Biomech. 2010;26:390-399.
8. MacLean CL, Davis IS, Hamill J. Short- and long-term influences of a custom foot orthotic intervention on lower extremity dynamics. Clin J Sport Med. 2008;18:338-343.
9. Bishop M, Fiolkowski P, Conrad B, et al. Athletic footwear, leg stiffness, and running kinematics. J Athl Train. 2006;41:387-392.
10. Burd TA, Hughes MS, Anglen JO. Heterotopic ossification prophylaxis with indomethacin increases the risk of long-bone nonunion. J Bone Joint Surg Br. 2003;85:700-705.
11. Butcher CK, Marsh DR. Nonsteroidal anti-inflammatory drugs delay tibial fracture union. Injury. 1996;27:375.-
12. Yates JE, Shah SH. Do NSAIDS impede fracture healing? J Fam Pract. 2011;60:41-42.
13. Hyland M, Webber-Gaffney A, Cohen L. Randomized controlled trial of calcaneal taping, sham taping, and plantar fascia stretching for the short-term management of plantar heel pain. J Orthop Sports Phys Ther. 2006;36:364-371.
14. Powell M, Post WR, Keener J, et al. Effective treatment of chronic plantar fasciitis with dorsiflexion night splints: a crossover prospective randomized outcome study. Foot Ankle Int. 1998;19:10-18.
15. Baldassin V, Gomes CR, Beraldo PS. Effectiveness of prefabricated and customized foot orthoses made from low-cost foam for noncomplicated plantar fasciitis: a randomized controlled trial. Arch Phys Med Rehabil. 2009;90:701-706.
16. Rompe JD, Furia J, Weil L, et al. Shock wave therapy for chronic plantar fasciopathy. Br Med Bull. 2007;81-82:183-208.
17. Kleinman M, Gross AF. Achilles tendon rupture following steroid injection. Report of three cases. J Bone Joint Surg Am. 1983;65:1345-1347.
18. Hamilton B, Remedios D, Loosemore M, et al. Achilles tendon rupture in an elite athlete following multiple injection therapies. J Sci Med Sport. 2008;11:566-568.
19. Wasielewski NJ, Kotsko KM. Does eccentric exercise reduce pain and improve strength in physically active adults with symptomatic lower extremity tendinosis? A systematic review. J Athl Train. 2007;42:409-421.
20. Kingma JJ, de Knikker R, Wittink HM, et al. Eccentric overload training in patients with chronic Achilles tendinopathy: a systematic review. Br J Sports Med. 2007;41:e3.-
21. Norregaard J, Larsen CC, Bieler T, et al. Eccentric exercise in treatment of Achilles tendinopathy. Scand J Med Sci Sports. 2007;17:133-138.
22. Roos EM, Engstrom M, Lagerquist A, et al. Clinical improvement after 6 weeks of eccentric exercise in patients with mid-portion Achilles tendinopathy – a randomized trial with 1-year follow-up. Scand J Med Sci Sports. 2004;14:286-295.
23. Magnusse RA, Dunn WR, Thompson AB. Nonoperative treatment of midportion Achilles tendinopathy: a systematic review. Clin J Sports Med. 2009;19:54-64.
24. McShane JM, Ostick B, McCabe F. Noninsertional Achilles tendinopathy: pathology and management. Curr Sports Med Rep. 2007;6:288-292.
25. Fahlstrom M, Jonsson P, Lorentzon R, et al. Chronic Achilles tendon pain treated with eccentric calf-muscle training. Knee Surg Sports Traumatol Arthrosc. 2003;11:327-333.
26. Lesho EP. Can tuning forks replace bone scans for identification of tibial stress fractures? Mil Med. 1997;162:802-803.
27. Clinghan R, Arnold GP, Drew TS, et al. Do you get value for money when you buy an expensive pair of running shoes? Br J Sports Med. 2008;42:189-193.
28. Butler RJ, Davis IS, Hamill J. Interaction of arch type and footwear on running mechanics. Am J Sports Med. 2006;34:1998-2005.
29. Divert C, Mornieux G, Freychat P, et al. Barefoot-shot running differences: shoe or mass effect? Int J Sports Med. 2008;29:512-518.
30. Taunton JE, Ryan MB, Clement DB, et al. A prospective study of running injuries: the Vancouver Sun Run “In Training” clinics. Br J Sports Med. 2003;37:239-244.
31. Verdejo R, Mills NJ. Heel-shoe interactions and the durability of EVA foam running-shoe midsoles. J Biomech. 2004;37:1379-1386.
32. Winemiller MH, Billow RG, Laskowski ER, et al. Effect of magnetic vs sham-magnetic insoles on nonspecific foot pain in the workplace: a randomized, double-blind, placebo-controlled trial. Mayo Clin Proc. 2005;80:1138-1145.
33. Logan K. Stress fractures in the adolescent athlete. Pediatr Ann. 2007;36:738-745.
34. Thein-Nissenbaum JM, Carr KE. Female athlete triad syndrome in the high school athlete. Phys Ther Sport. 2011;12:108-116.
35. Umans H. Imaging sports medicine injuries of the foot and toes. Clin Sports Med. 2006;25:763-780.
36. Vorlat P, Achtergael W, Haentjens P. Predictors of outcome of non-displaced fractures of the base of the fifth metatarsal. Int Orthop. 2007;31:5-10.
37. Dyck D, Boyajian-O’Neill L. Plantar fasciitis. Clin J Sports Med. 2004;14:305-309.
38. Alfredson H, Thorsen K, Lorentzon R. In situ microdialysis in tendon tissue: high levels of glutamate, but not prostaglandin E2 in chronic Achilles tendon pain. Knee Surg Sports Traumatol Arthrosc. 1999;7:378-381.
39. Seligman DA, Dawson DR. Customized heel pads and soft orthotics to treat heel pain and plantar fasciitis. Arch Phys Med Rehab. 2003;84:1564-1567.