User login
Tumor Volume: An Adjunct Prognostic Factor in Cutaneous Melanoma
Melanoma continues to be a devastating disease unless diagnosed and treated early. According to the National Cancer Institute, there will be more than 76,000 new cases of invasive melanoma and nearly 10,000 melanoma-related deaths in 2014 in the United States.1 If diagnosed early, more than 93% of melanoma patients can expect to be cured, but later diagnosis of thicker melanoma is associated with a worse prognosis. Surgery remains the mainstay of therapy for cutaneous melanoma, including wide excision and sentinel lymph node (SLN) biopsy for staging of the regional nodal basins in appropriate patients. Although novel targeted therapies and immunotherapies have been associated with improved survival in metastatic melanoma, detection of cutaneous melanoma in its early phases remains the best chance for cure.
Tumor thickness, or Breslow depth, is the most important histologic determinant of prognosis in melanoma patients and is measured vertically in millimeters from the top of the granular layer (or base of superficial ulceration) to the deepest point of the tumor involvement. Increased tumor thickness confers a higher metastatic potential and poorer prognosis.2 Other histologic prognostic factors that have been incorporated into the American Joint Committee on Cancer melanoma staging system include the presence or absence of ulceration and mitotic index (measured per square millimeter), particularly for T1 melanomas (<1 mm thick), though Breslow depth greater than 0.75 mm appears to be the most reliable predictor of SLN metastasis in thin (T1) melanomas (≤1 mm).3
Tumor volume assessment may be a helpful adjunct to Breslow depth as a prognostic indicator for melanoma, particularly for predicting SLN metastasis.4 This retrospective study was designed to assess the improvement in the accuracy of Breslow depth as a prognostic factor by utilizing tumor volume combined with mitotic index, presence or absence of ulceration, and inflammatory host reaction (eg, tumor-infiltrating lymphocytes).
Methods
The study was approved by the Stanford University (Stanford, California) institutional review board. A retrospective review of invasive primary melanomas recorded in Stanford University’s pathology/dermatopathology database from January 2007 through December 2010 was conducted. Because cases included both Stanford Health Care (formerly Stanford Hospital & Clinics) and outside pathology consultations, clinical assessment of patient outcome was not possible for all cases and thus was not performed.
Assessment
Information extracted from the pathology reports included Breslow depth; estimated surface area of the primary tumor (measured by the longest vertical and horizontal dimensions recorded by the clinician prior to diagnostic biopsy and reported on the biopsy requisition form [>90% of cases] or reported by the pathologist on gross measurement of the pigmented lesion in formalin [<10% of cases]); mitotic index (measured per square millimeter); presence or absence of ulceration; and inflammatory host reaction (as noted by tumor-infiltrating response). Our method of estimating the tumor volume (lesion surface area • Breslow depth) did not take into account border irregularities in the primary tumor. This method also was limited because prebiopsy clinical measurement could differ from gross pathologic measurement of the tumor due to shrinkage of the latter ex vivo and following formalin fixation. However, when both measurements were documented, the pathological measurement was only slightly less than the clinical measurement. Metastases were defined as those in lymph nodes (microscopic or macroscopic), skin, or in distant organs, as identified through review of subsequent pathology reports.
Statistical Analysis
Statistical analyses were conducted using SAS version 9.3. Test statistics were preset at a significance level of α=.05. Using metastasis status as the outcome, univariate regression models were first fitted to assess the predictive ability of each prognostic indicator. In univariate analyses, continuous prognostic indicators (Breslow depth, tumor volume, and surface area) were included in the model while seeking the best functional form by means of fractional polynomials modeling.5,6 Predictive ability of prognostic indicators was determined by the area under the receiver operating characteristic curve (AUC).7 Using best functional form for Breslow depth, all other prognostic indicators were added to the model to assess their individual contributions to improve the predictive ability for tumor metastasis. The functional forms used for tumor volume and surface area were those determined in the univariate analysis. Multivariable models were compared aiming for an improvement of the best Breslow model indices: Schwarz criterion, Hosmer-Lemeshow goodness-of-fit test, generalized R2, and AUC.5 The added contribution of clinical predictors to the model for Breslow depth was judged by the significance of the coefficient for the added clinical predictor, the significance of the change in AUC, and the change in the model indices listed above. A check on overdispersion was carried out on the final model selected.
Results
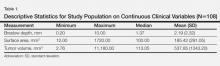
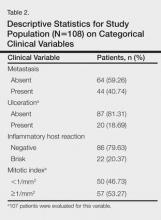
There were 108 eligible cases in the 4-year time period in which tumor volume assessment could be determined based on the pathology report in conjunction with Breslow depth, mitotic index, presence or absence of ulceration, and tumor infiltrating response. Breslow depth ranged from 0.20 to 10.00 mm, with a median depth of 1.37 mm. Surface area ranged from 12.00 to 1720.00 mm2 (median, 100.00 mm2). Tumor volume was calculated by multiplying Breslow depth by surface area and ranged from 2.76 to 11,180.00 mm3 (median, 113.05 mm3)(Table 1). Ulceration was present in 18.69% of the tumors, 20.37% exhibited a brisk inflammatory host reaction, and 53.27% had a mitotic index of 1/mm2 or more. Tumor metastasis was noted in 40.74% (44/108) of patients (Table 2), all of whom had a primary melanoma with a Breslow depth greater than 1 mm. Only one T1 melanoma had a tumor volume greater than 250 mm3. Metastasis in patients with T2 (1- to 2-mm thick) and T3 (2- to 4-mm thick) melanoma was associated with a tumor volume greater than 250 mm3 in 16 of 26 patients (61.54%), and all 18 patients with T4 melanomas (>4-mm thick) had tumor volume greater than 250 mm3.
Univariate analysis demonstrated that Breslow depth was the best prognostic indicator of metastasis (AUC=0.946) but that tumor volume (as a continuous variable) was nearly equally predictive (AUC=0.940)(Table 3). Tumor volume alone (categorized as <250 mm3 vs >250 mm3) had lower prognostic value (AUC=0.855). Mitotic index, presence or absence of ulceration, inflammatory host reaction, and surface area also had lower prognostic values, though all were significant factors (P values ranging from <.0001 to .0077)(Table 3).
Importantly, the addition of surface area, mitotic index, presence or absence of ulceration, and inflammatory host reaction to the model to Breslow depth did not improve predictive ability for metastasis, and AUC values did not increase significantly after adding these factors (Table 4). In particular, the change in AUC for adding surface area to the model with Breslow depth was 0.023 (P=.1095). Models in Table 4 were checked for interaction of these 2 predictors, and the interaction term for thickness and surface area was not statistically significant (P=.0932)(data not shown).
Comment
Decades after the concept of measuring tumor thickness in cutaneous melanomas was proposed by Dr. Alexander Breslow, it remains the most reliable predictor of prognosis in melanoma patients.2 Our study demonstrated that tumor volume may be contributory to thickness, despite our relatively imprecise assessment of tumor volume based on clinical or pathological reporting of primary tumor area. Because more than 90% of our tumor volume measurements were based on clinician reports of the lesion size before diagnostic biopsy rather than gross measurement of the tumor by the pathologist after biopsy, we believe that measurement and assessment of tumor volume could be readily incorporated into the clinical practice setting. Although we could not demonstrate a correlation between SLN positivity and tumor volume in T1 melanomas because none of the T1 tumors exhibited microscopic nodal metastasis, assessment of tumor volume may assist the clinician in patient management, using a 250-mm3 cutoff point. Gross tumor measurement is important to allow for accurate assessment of volume and would preferably be recorded by the clinician prior to biopsy with notation of clinical lesion size on the pathology requisition form, as is recommended in the American Academy of Dermatology’s melanoma practice guidelines.8
A prior assessment of 123 patients with invasive primary melanomas demonstrated that greater tumor volume (>250 mm3) was associated with metastasis across all tumor thicknesses.4 In T1 melanoma, no patients with a tumor volume less than 250 mm3 demonstrated SLN metastasis,4 suggesting that volume assessment may aid in consideration of staging with SLN biopsy in conjunction with tumor thickness and other established prognostic factors for SLN positivity in thin melanomas (eg, high mitotic index [particularly in tumors >0.75-mm thick]), histologic ulceration, and/or lymphovascular invasion).2,8
It should be noted, however, that lentigo maligna melanoma, which often is predominantly in situ with only focal papillary dermal invasion, may have an erroneously high tumor volume due to its larger total surface area. However, tumor volume would not be expected to correlate with tumor metastasis given the thin invasive component. The current study was limited by not accounting for melanoma subtype in the overall analysis.
A practical estimation of tumor volume based on clinical measurement of tumor size (ie, surface area of the suspicious lesion prior to biopsy) in combination with the pathologist’s assessment of Breslow depth may be a helpful adjunct to predicting likelihood of development of metastasis. We suggest that the concept of tumor volume should be subjected to more rigorous investigation with standardized clinical/prebiopsy measurement of the lesion; correlation with known histologic prognostic factors, SLN positivity, and/or development of additional nodal or visceral metastasis; and most importantly long-term patient outcome in terms of survival. Our preliminary data suggest the value of this enterprise.
1. American Cancer Society. Cancer Facts & Figures 2014. Atlanta, GA: American Cancer Society; 2014.
2. Balch CM, Gershenwald JE, Soong SJ, et al. Final version of 2009 AJCC melanoma staging and classification. J Clin Oncol. 2009;27:6199-6206.
3. Coit DG, Andtbacka R, Anker CJ, et al. Melanoma, version 2.2013: featured updates to the NCCN guidelines. J Natl Compr Canc Netw. 2013;11:395-407.
4. Walton RG, Velasco C. Volume as a prognostic indicator in cutaneous malignant melanoma. Practical Dermatol. September 2010:26-28.
5. Hosmer DW, Lemeshow S. Applied Logistic Regression. 2nd ed. Hoboken, NJ: John Wiley & Sons, Inc; 2000.
6. Royston P, Sauerbrei W. Multivariable Model-Building: A Pragmatic Approach to Regression Analysis Based on Fractional Polynomials for Modelling Continuous Variables. Chichester, England: John Wiley & Sons; 2008.
7. Pepe MS. The Statistical Evaluation of Medical Tests for Classification and Prediction. Vol 28. Oxford, England: Oxford University Press; 2004.
8. Bichakjian CK, Halpern AC, Johnson TM, et al; American Academy of Dermatology. Guidelines of care for the management of primary cutaneous melanoma. American Academy of Dermatology. J Am Acad Dermatol. 2011;65:1032-1047.
Melanoma continues to be a devastating disease unless diagnosed and treated early. According to the National Cancer Institute, there will be more than 76,000 new cases of invasive melanoma and nearly 10,000 melanoma-related deaths in 2014 in the United States.1 If diagnosed early, more than 93% of melanoma patients can expect to be cured, but later diagnosis of thicker melanoma is associated with a worse prognosis. Surgery remains the mainstay of therapy for cutaneous melanoma, including wide excision and sentinel lymph node (SLN) biopsy for staging of the regional nodal basins in appropriate patients. Although novel targeted therapies and immunotherapies have been associated with improved survival in metastatic melanoma, detection of cutaneous melanoma in its early phases remains the best chance for cure.
Tumor thickness, or Breslow depth, is the most important histologic determinant of prognosis in melanoma patients and is measured vertically in millimeters from the top of the granular layer (or base of superficial ulceration) to the deepest point of the tumor involvement. Increased tumor thickness confers a higher metastatic potential and poorer prognosis.2 Other histologic prognostic factors that have been incorporated into the American Joint Committee on Cancer melanoma staging system include the presence or absence of ulceration and mitotic index (measured per square millimeter), particularly for T1 melanomas (<1 mm thick), though Breslow depth greater than 0.75 mm appears to be the most reliable predictor of SLN metastasis in thin (T1) melanomas (≤1 mm).3
Tumor volume assessment may be a helpful adjunct to Breslow depth as a prognostic indicator for melanoma, particularly for predicting SLN metastasis.4 This retrospective study was designed to assess the improvement in the accuracy of Breslow depth as a prognostic factor by utilizing tumor volume combined with mitotic index, presence or absence of ulceration, and inflammatory host reaction (eg, tumor-infiltrating lymphocytes).
Methods
The study was approved by the Stanford University (Stanford, California) institutional review board. A retrospective review of invasive primary melanomas recorded in Stanford University’s pathology/dermatopathology database from January 2007 through December 2010 was conducted. Because cases included both Stanford Health Care (formerly Stanford Hospital & Clinics) and outside pathology consultations, clinical assessment of patient outcome was not possible for all cases and thus was not performed.
Assessment
Information extracted from the pathology reports included Breslow depth; estimated surface area of the primary tumor (measured by the longest vertical and horizontal dimensions recorded by the clinician prior to diagnostic biopsy and reported on the biopsy requisition form [>90% of cases] or reported by the pathologist on gross measurement of the pigmented lesion in formalin [<10% of cases]); mitotic index (measured per square millimeter); presence or absence of ulceration; and inflammatory host reaction (as noted by tumor-infiltrating response). Our method of estimating the tumor volume (lesion surface area • Breslow depth) did not take into account border irregularities in the primary tumor. This method also was limited because prebiopsy clinical measurement could differ from gross pathologic measurement of the tumor due to shrinkage of the latter ex vivo and following formalin fixation. However, when both measurements were documented, the pathological measurement was only slightly less than the clinical measurement. Metastases were defined as those in lymph nodes (microscopic or macroscopic), skin, or in distant organs, as identified through review of subsequent pathology reports.
Statistical Analysis
Statistical analyses were conducted using SAS version 9.3. Test statistics were preset at a significance level of α=.05. Using metastasis status as the outcome, univariate regression models were first fitted to assess the predictive ability of each prognostic indicator. In univariate analyses, continuous prognostic indicators (Breslow depth, tumor volume, and surface area) were included in the model while seeking the best functional form by means of fractional polynomials modeling.5,6 Predictive ability of prognostic indicators was determined by the area under the receiver operating characteristic curve (AUC).7 Using best functional form for Breslow depth, all other prognostic indicators were added to the model to assess their individual contributions to improve the predictive ability for tumor metastasis. The functional forms used for tumor volume and surface area were those determined in the univariate analysis. Multivariable models were compared aiming for an improvement of the best Breslow model indices: Schwarz criterion, Hosmer-Lemeshow goodness-of-fit test, generalized R2, and AUC.5 The added contribution of clinical predictors to the model for Breslow depth was judged by the significance of the coefficient for the added clinical predictor, the significance of the change in AUC, and the change in the model indices listed above. A check on overdispersion was carried out on the final model selected.
Results
There were 108 eligible cases in the 4-year time period in which tumor volume assessment could be determined based on the pathology report in conjunction with Breslow depth, mitotic index, presence or absence of ulceration, and tumor infiltrating response. Breslow depth ranged from 0.20 to 10.00 mm, with a median depth of 1.37 mm. Surface area ranged from 12.00 to 1720.00 mm2 (median, 100.00 mm2). Tumor volume was calculated by multiplying Breslow depth by surface area and ranged from 2.76 to 11,180.00 mm3 (median, 113.05 mm3)(Table 1). Ulceration was present in 18.69% of the tumors, 20.37% exhibited a brisk inflammatory host reaction, and 53.27% had a mitotic index of 1/mm2 or more. Tumor metastasis was noted in 40.74% (44/108) of patients (Table 2), all of whom had a primary melanoma with a Breslow depth greater than 1 mm. Only one T1 melanoma had a tumor volume greater than 250 mm3. Metastasis in patients with T2 (1- to 2-mm thick) and T3 (2- to 4-mm thick) melanoma was associated with a tumor volume greater than 250 mm3 in 16 of 26 patients (61.54%), and all 18 patients with T4 melanomas (>4-mm thick) had tumor volume greater than 250 mm3.
Univariate analysis demonstrated that Breslow depth was the best prognostic indicator of metastasis (AUC=0.946) but that tumor volume (as a continuous variable) was nearly equally predictive (AUC=0.940)(Table 3). Tumor volume alone (categorized as <250 mm3 vs >250 mm3) had lower prognostic value (AUC=0.855). Mitotic index, presence or absence of ulceration, inflammatory host reaction, and surface area also had lower prognostic values, though all were significant factors (P values ranging from <.0001 to .0077)(Table 3).
Importantly, the addition of surface area, mitotic index, presence or absence of ulceration, and inflammatory host reaction to the model to Breslow depth did not improve predictive ability for metastasis, and AUC values did not increase significantly after adding these factors (Table 4). In particular, the change in AUC for adding surface area to the model with Breslow depth was 0.023 (P=.1095). Models in Table 4 were checked for interaction of these 2 predictors, and the interaction term for thickness and surface area was not statistically significant (P=.0932)(data not shown).
Comment
Decades after the concept of measuring tumor thickness in cutaneous melanomas was proposed by Dr. Alexander Breslow, it remains the most reliable predictor of prognosis in melanoma patients.2 Our study demonstrated that tumor volume may be contributory to thickness, despite our relatively imprecise assessment of tumor volume based on clinical or pathological reporting of primary tumor area. Because more than 90% of our tumor volume measurements were based on clinician reports of the lesion size before diagnostic biopsy rather than gross measurement of the tumor by the pathologist after biopsy, we believe that measurement and assessment of tumor volume could be readily incorporated into the clinical practice setting. Although we could not demonstrate a correlation between SLN positivity and tumor volume in T1 melanomas because none of the T1 tumors exhibited microscopic nodal metastasis, assessment of tumor volume may assist the clinician in patient management, using a 250-mm3 cutoff point. Gross tumor measurement is important to allow for accurate assessment of volume and would preferably be recorded by the clinician prior to biopsy with notation of clinical lesion size on the pathology requisition form, as is recommended in the American Academy of Dermatology’s melanoma practice guidelines.8
A prior assessment of 123 patients with invasive primary melanomas demonstrated that greater tumor volume (>250 mm3) was associated with metastasis across all tumor thicknesses.4 In T1 melanoma, no patients with a tumor volume less than 250 mm3 demonstrated SLN metastasis,4 suggesting that volume assessment may aid in consideration of staging with SLN biopsy in conjunction with tumor thickness and other established prognostic factors for SLN positivity in thin melanomas (eg, high mitotic index [particularly in tumors >0.75-mm thick]), histologic ulceration, and/or lymphovascular invasion).2,8
It should be noted, however, that lentigo maligna melanoma, which often is predominantly in situ with only focal papillary dermal invasion, may have an erroneously high tumor volume due to its larger total surface area. However, tumor volume would not be expected to correlate with tumor metastasis given the thin invasive component. The current study was limited by not accounting for melanoma subtype in the overall analysis.
A practical estimation of tumor volume based on clinical measurement of tumor size (ie, surface area of the suspicious lesion prior to biopsy) in combination with the pathologist’s assessment of Breslow depth may be a helpful adjunct to predicting likelihood of development of metastasis. We suggest that the concept of tumor volume should be subjected to more rigorous investigation with standardized clinical/prebiopsy measurement of the lesion; correlation with known histologic prognostic factors, SLN positivity, and/or development of additional nodal or visceral metastasis; and most importantly long-term patient outcome in terms of survival. Our preliminary data suggest the value of this enterprise.
Melanoma continues to be a devastating disease unless diagnosed and treated early. According to the National Cancer Institute, there will be more than 76,000 new cases of invasive melanoma and nearly 10,000 melanoma-related deaths in 2014 in the United States.1 If diagnosed early, more than 93% of melanoma patients can expect to be cured, but later diagnosis of thicker melanoma is associated with a worse prognosis. Surgery remains the mainstay of therapy for cutaneous melanoma, including wide excision and sentinel lymph node (SLN) biopsy for staging of the regional nodal basins in appropriate patients. Although novel targeted therapies and immunotherapies have been associated with improved survival in metastatic melanoma, detection of cutaneous melanoma in its early phases remains the best chance for cure.
Tumor thickness, or Breslow depth, is the most important histologic determinant of prognosis in melanoma patients and is measured vertically in millimeters from the top of the granular layer (or base of superficial ulceration) to the deepest point of the tumor involvement. Increased tumor thickness confers a higher metastatic potential and poorer prognosis.2 Other histologic prognostic factors that have been incorporated into the American Joint Committee on Cancer melanoma staging system include the presence or absence of ulceration and mitotic index (measured per square millimeter), particularly for T1 melanomas (<1 mm thick), though Breslow depth greater than 0.75 mm appears to be the most reliable predictor of SLN metastasis in thin (T1) melanomas (≤1 mm).3
Tumor volume assessment may be a helpful adjunct to Breslow depth as a prognostic indicator for melanoma, particularly for predicting SLN metastasis.4 This retrospective study was designed to assess the improvement in the accuracy of Breslow depth as a prognostic factor by utilizing tumor volume combined with mitotic index, presence or absence of ulceration, and inflammatory host reaction (eg, tumor-infiltrating lymphocytes).
Methods
The study was approved by the Stanford University (Stanford, California) institutional review board. A retrospective review of invasive primary melanomas recorded in Stanford University’s pathology/dermatopathology database from January 2007 through December 2010 was conducted. Because cases included both Stanford Health Care (formerly Stanford Hospital & Clinics) and outside pathology consultations, clinical assessment of patient outcome was not possible for all cases and thus was not performed.
Assessment
Information extracted from the pathology reports included Breslow depth; estimated surface area of the primary tumor (measured by the longest vertical and horizontal dimensions recorded by the clinician prior to diagnostic biopsy and reported on the biopsy requisition form [>90% of cases] or reported by the pathologist on gross measurement of the pigmented lesion in formalin [<10% of cases]); mitotic index (measured per square millimeter); presence or absence of ulceration; and inflammatory host reaction (as noted by tumor-infiltrating response). Our method of estimating the tumor volume (lesion surface area • Breslow depth) did not take into account border irregularities in the primary tumor. This method also was limited because prebiopsy clinical measurement could differ from gross pathologic measurement of the tumor due to shrinkage of the latter ex vivo and following formalin fixation. However, when both measurements were documented, the pathological measurement was only slightly less than the clinical measurement. Metastases were defined as those in lymph nodes (microscopic or macroscopic), skin, or in distant organs, as identified through review of subsequent pathology reports.
Statistical Analysis
Statistical analyses were conducted using SAS version 9.3. Test statistics were preset at a significance level of α=.05. Using metastasis status as the outcome, univariate regression models were first fitted to assess the predictive ability of each prognostic indicator. In univariate analyses, continuous prognostic indicators (Breslow depth, tumor volume, and surface area) were included in the model while seeking the best functional form by means of fractional polynomials modeling.5,6 Predictive ability of prognostic indicators was determined by the area under the receiver operating characteristic curve (AUC).7 Using best functional form for Breslow depth, all other prognostic indicators were added to the model to assess their individual contributions to improve the predictive ability for tumor metastasis. The functional forms used for tumor volume and surface area were those determined in the univariate analysis. Multivariable models were compared aiming for an improvement of the best Breslow model indices: Schwarz criterion, Hosmer-Lemeshow goodness-of-fit test, generalized R2, and AUC.5 The added contribution of clinical predictors to the model for Breslow depth was judged by the significance of the coefficient for the added clinical predictor, the significance of the change in AUC, and the change in the model indices listed above. A check on overdispersion was carried out on the final model selected.
Results
There were 108 eligible cases in the 4-year time period in which tumor volume assessment could be determined based on the pathology report in conjunction with Breslow depth, mitotic index, presence or absence of ulceration, and tumor infiltrating response. Breslow depth ranged from 0.20 to 10.00 mm, with a median depth of 1.37 mm. Surface area ranged from 12.00 to 1720.00 mm2 (median, 100.00 mm2). Tumor volume was calculated by multiplying Breslow depth by surface area and ranged from 2.76 to 11,180.00 mm3 (median, 113.05 mm3)(Table 1). Ulceration was present in 18.69% of the tumors, 20.37% exhibited a brisk inflammatory host reaction, and 53.27% had a mitotic index of 1/mm2 or more. Tumor metastasis was noted in 40.74% (44/108) of patients (Table 2), all of whom had a primary melanoma with a Breslow depth greater than 1 mm. Only one T1 melanoma had a tumor volume greater than 250 mm3. Metastasis in patients with T2 (1- to 2-mm thick) and T3 (2- to 4-mm thick) melanoma was associated with a tumor volume greater than 250 mm3 in 16 of 26 patients (61.54%), and all 18 patients with T4 melanomas (>4-mm thick) had tumor volume greater than 250 mm3.
Univariate analysis demonstrated that Breslow depth was the best prognostic indicator of metastasis (AUC=0.946) but that tumor volume (as a continuous variable) was nearly equally predictive (AUC=0.940)(Table 3). Tumor volume alone (categorized as <250 mm3 vs >250 mm3) had lower prognostic value (AUC=0.855). Mitotic index, presence or absence of ulceration, inflammatory host reaction, and surface area also had lower prognostic values, though all were significant factors (P values ranging from <.0001 to .0077)(Table 3).
Importantly, the addition of surface area, mitotic index, presence or absence of ulceration, and inflammatory host reaction to the model to Breslow depth did not improve predictive ability for metastasis, and AUC values did not increase significantly after adding these factors (Table 4). In particular, the change in AUC for adding surface area to the model with Breslow depth was 0.023 (P=.1095). Models in Table 4 were checked for interaction of these 2 predictors, and the interaction term for thickness and surface area was not statistically significant (P=.0932)(data not shown).
Comment
Decades after the concept of measuring tumor thickness in cutaneous melanomas was proposed by Dr. Alexander Breslow, it remains the most reliable predictor of prognosis in melanoma patients.2 Our study demonstrated that tumor volume may be contributory to thickness, despite our relatively imprecise assessment of tumor volume based on clinical or pathological reporting of primary tumor area. Because more than 90% of our tumor volume measurements were based on clinician reports of the lesion size before diagnostic biopsy rather than gross measurement of the tumor by the pathologist after biopsy, we believe that measurement and assessment of tumor volume could be readily incorporated into the clinical practice setting. Although we could not demonstrate a correlation between SLN positivity and tumor volume in T1 melanomas because none of the T1 tumors exhibited microscopic nodal metastasis, assessment of tumor volume may assist the clinician in patient management, using a 250-mm3 cutoff point. Gross tumor measurement is important to allow for accurate assessment of volume and would preferably be recorded by the clinician prior to biopsy with notation of clinical lesion size on the pathology requisition form, as is recommended in the American Academy of Dermatology’s melanoma practice guidelines.8
A prior assessment of 123 patients with invasive primary melanomas demonstrated that greater tumor volume (>250 mm3) was associated with metastasis across all tumor thicknesses.4 In T1 melanoma, no patients with a tumor volume less than 250 mm3 demonstrated SLN metastasis,4 suggesting that volume assessment may aid in consideration of staging with SLN biopsy in conjunction with tumor thickness and other established prognostic factors for SLN positivity in thin melanomas (eg, high mitotic index [particularly in tumors >0.75-mm thick]), histologic ulceration, and/or lymphovascular invasion).2,8
It should be noted, however, that lentigo maligna melanoma, which often is predominantly in situ with only focal papillary dermal invasion, may have an erroneously high tumor volume due to its larger total surface area. However, tumor volume would not be expected to correlate with tumor metastasis given the thin invasive component. The current study was limited by not accounting for melanoma subtype in the overall analysis.
A practical estimation of tumor volume based on clinical measurement of tumor size (ie, surface area of the suspicious lesion prior to biopsy) in combination with the pathologist’s assessment of Breslow depth may be a helpful adjunct to predicting likelihood of development of metastasis. We suggest that the concept of tumor volume should be subjected to more rigorous investigation with standardized clinical/prebiopsy measurement of the lesion; correlation with known histologic prognostic factors, SLN positivity, and/or development of additional nodal or visceral metastasis; and most importantly long-term patient outcome in terms of survival. Our preliminary data suggest the value of this enterprise.
1. American Cancer Society. Cancer Facts & Figures 2014. Atlanta, GA: American Cancer Society; 2014.
2. Balch CM, Gershenwald JE, Soong SJ, et al. Final version of 2009 AJCC melanoma staging and classification. J Clin Oncol. 2009;27:6199-6206.
3. Coit DG, Andtbacka R, Anker CJ, et al. Melanoma, version 2.2013: featured updates to the NCCN guidelines. J Natl Compr Canc Netw. 2013;11:395-407.
4. Walton RG, Velasco C. Volume as a prognostic indicator in cutaneous malignant melanoma. Practical Dermatol. September 2010:26-28.
5. Hosmer DW, Lemeshow S. Applied Logistic Regression. 2nd ed. Hoboken, NJ: John Wiley & Sons, Inc; 2000.
6. Royston P, Sauerbrei W. Multivariable Model-Building: A Pragmatic Approach to Regression Analysis Based on Fractional Polynomials for Modelling Continuous Variables. Chichester, England: John Wiley & Sons; 2008.
7. Pepe MS. The Statistical Evaluation of Medical Tests for Classification and Prediction. Vol 28. Oxford, England: Oxford University Press; 2004.
8. Bichakjian CK, Halpern AC, Johnson TM, et al; American Academy of Dermatology. Guidelines of care for the management of primary cutaneous melanoma. American Academy of Dermatology. J Am Acad Dermatol. 2011;65:1032-1047.
1. American Cancer Society. Cancer Facts & Figures 2014. Atlanta, GA: American Cancer Society; 2014.
2. Balch CM, Gershenwald JE, Soong SJ, et al. Final version of 2009 AJCC melanoma staging and classification. J Clin Oncol. 2009;27:6199-6206.
3. Coit DG, Andtbacka R, Anker CJ, et al. Melanoma, version 2.2013: featured updates to the NCCN guidelines. J Natl Compr Canc Netw. 2013;11:395-407.
4. Walton RG, Velasco C. Volume as a prognostic indicator in cutaneous malignant melanoma. Practical Dermatol. September 2010:26-28.
5. Hosmer DW, Lemeshow S. Applied Logistic Regression. 2nd ed. Hoboken, NJ: John Wiley & Sons, Inc; 2000.
6. Royston P, Sauerbrei W. Multivariable Model-Building: A Pragmatic Approach to Regression Analysis Based on Fractional Polynomials for Modelling Continuous Variables. Chichester, England: John Wiley & Sons; 2008.
7. Pepe MS. The Statistical Evaluation of Medical Tests for Classification and Prediction. Vol 28. Oxford, England: Oxford University Press; 2004.
8. Bichakjian CK, Halpern AC, Johnson TM, et al; American Academy of Dermatology. Guidelines of care for the management of primary cutaneous melanoma. American Academy of Dermatology. J Am Acad Dermatol. 2011;65:1032-1047.
Practice Points
- Measurement of melanoma tumor volume using clinical area (length • width of the lesion before diagnostic biopsy) multiplied by Breslow depth may provide additional prognostic information.
- Further study is needed to validate the use of tumor volume as an adjunct to established histopathologic prognostic factors in cutaneous melanoma.