User login
Treating type 2 diabetes: Targeting the causative factors
- Self-monitoring of blood glucose is an integral component of diabetes therapy and should always be included in the management plan (SOR:C).
- Medical nutrition therapy should be individualized, preferably by a registered dietitian familiar with diabetes (SOR:B).
- A regular physical activity program is recommended for all patients with diabetes who are capable of participating (SOR:B).
- When a monotherapy fails, combine drugs with different mechanisms of action to achieve an additive effect (SOR:A).
- The combination of sulfonylurea and metformin has proven effective in many studies. One showed that initial treatment with glyburide/metformin improved glycemic control better than either glyburide or metformin monotherapy (SOR: A).
Glycemic control in diabetes begins with a patient’s adherence to several nonpharmacologic measures. Without such a commitment, success in controlling the disease will be difficult to achieve, and otherwise appropriate drug therapy will be hindered.
Most antidiabetic agents comparably reduce glycosylated hemoglobin (A1c) levels. However, a particular agent may be preferred depending on a patient’s characteristics. And some circumstances call for combination therapy. This article reviews the advantages and disadvantages of the many pharmacologic treatments for glucose control and hyperglycemia in type 2 diabetes.
Benefits Of Diabetes Control
The benefits of diabetes control are detailed in this issue of THE JOURNAL OF FAMILY PRACTICE (“Strategies to reduce complications in type 2 diabetes,” pages 366–374). For every percentage-point reduction in hemoglobin A1c, it is possible to achieve a 22% to 35% reduction in microvascular complications.1,2 Cardiovascular disease can be reduced in patients with diabetes by treating hypertension3,4 and hyperlipidemia, prescribing aspirin therapy, using angiotensin-converting enzyme (ACE) inhibitors, and with smoking cessation.5,6
Targets For Glycemic Control
The American Diabetes Association’s (ADA) recommended targets for glycemic control are a preprandial blood glucose level of 80–120 mg/dL, a bedtime blood glucose level of 100–140 mg/dL, and a hemoglobin A1c level of <7% (with a level of >8% requiring additional measures). Hemoglobin A1c is the best determinant of glycemic exposure, and its mean value is a nationally recognized indicator of how well diabetes is being managed.7 The American College of Endocrinology has adopted a more aggressive approach by designating an A1c level of 6.5% as both a target and action level.8
Self-monitoring of blood glucose
Self-monitoring of blood glucose (SMBG) is an integral component of diabetes therapy (strength of recommendation [SOR]: C) and should always be included in the management plan (SOR: C). The optimal frequency and timing of SMBG for type 2 diabetes is not known, but they should be sufficient to facilitate reaching glucose goals. The A1c test should be performed at least semi-annually for patients with stable glycemic control, and quarterly for patients not meeting glycemic goals or those who are changing therapy. A1c levels and mean plasma glucose levels can be approximately correlated (Table 1).7
TABLE 1
Correlation between hemoglobin A1clevels and mean plasma glucose levels
| Hemoglobin A1c(%) | Mean plasma glucose (mg/dL) |
|---|---|
| 6 | 135 |
| 7 | 170 |
| 8 | 205 |
| 9 | 240 |
| 10 | 275 |
| 11 | 310 |
| 12 | 345 |
Nonpharmacologic Therapy
Nonpharmacologic measures remain the cornerstone of managing type 2 diabetes. Hyperglycemia adversely and reversibly affects both insulin resistance and insulin secretion. Improvement in glycemic control can occur through dietary modification and regular exercise.
A recent meta-analysis of randomized controlled trials of diabetes patient education observed a net decrease in HbA1c of 0.32% in intervention groups vs control.9 Interventions that included a face-to-face delivery, cognitive reframing teaching method, and exercise content were more likely to improve glycemic control.
Education
Lifestyle changes involving diet, exercise, and usually weight loss are key to effective management of diabetes. If patients are to change their behavior, they must be given detailed training.6 Self-management also necessitates that patients engage in problem solving. This requires that each aspect of the management plan is understood and agreed upon by the patient and providers, and that the goals and treatment plan are individualized and reasonable.
Diet: recommend soluble fiber, reduce calories
Medical nutrition therapy should be individualized and preferably provided by a registered dietitian familiar with diabetes (SOR: B). The goals of nutrition therapy, according to the ADA, are to attain recommended body weight and prevent or reverse obesity. The means of achieving these goals are nutrition assessment and modification of nutrient intake and lifestyle through healthy food choices and physical activity.7
A high intake of dietary fiber (particularly the soluble type) above the level recommended by the ADA improves glycemic control, decreases hyperinsulinemia, and lowers plasma lipid concentrations.10
Hypocaloric diets cause glucose plasma levels to fall, in some cases to a normal level with a weight loss of even 5 to 10 pounds.7,11 Hypoglycemic medications are of course most effective in nonobese persons. But effectiveness is also improved if weight that is gained can be limited. Despite the clear benefit of weight loss, only a few patients are able to attain and maintain substantial weight loss. Maintenance of a reduced or elevated body weight is associated with compensatory changes in energy expenditure, which oppose the maintenance of body weight that is different from the usual weight.13 Part of the individualization of therapy is respect of personal and cultural preferences, lifestyles, and financial considerations.
Physical activity: a little goes a long way
A regular physical activity program is recommended for all patients with diabetes who are capable of participating (SOR: B).7 It improves blood glucose control, reduces cardiovascular risk factors, aids weight loss, and enhances well being.7 A recently published prospective cohort study showed that walking at least 2 hours a week was associated with a 39% lower all-cause mortality (hazard rate ratio [HRR], 0.61; 95% CI, 0.48–0.78) and a 34% lower cardiovascular mortality (HRR, 0.66; 95% CI, 0.45–0.96) across a diverse spectrum of adults with diabetes. The NNT (to prevent 1 death per year) is 61 for patients who walk at least 2 hours/week.14
In prescribing a physical activity plan for a patient, consider cardiovascular disease risk factors or complications to minimize the risk of untoward events. Micro- and macrovascular disease are of course prevalent among persons with diabetes, often resulting in functional limitations that make exercise more difficult.
Other priorities
Other recommended components of care include daily aspirin use, foot care exams, tobacco cessation, pneumococcal and influenza vaccinations, and an annual dilated retinal exam.
Pharmacologic Therapy
The coexisting defects in type 2 diabetes mellitus are as follows:
- resistance to insulin action in muscle
- defective pancreatic insulin secretion
- unrestrained hepatic glucose production, aggravated by increased lipolysis in adipose tissue.
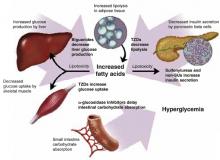
Drug therapy is aimed at each of these defects, and also at reducing carbohydrate absorption in the small intestine (Figure 1). As far as antihyperglycemic effect is concerned, no one category of antidiabetic agent is favored over another.15 Except for nateglinide and α-glucosidase inhibitors (AGIs), each of the drug categories leads to a similar reduction in A1c.16 However, patient characteristics may lead to selection of a particular agent. Table 2 summarizes oral treatment options, their relative advantages and costs.
FIGURE 1
Drug therapies for coexisting defects in type 2 diabetes
TABLE 2
Pharmacologic treatments for type 2 diabetes: monotherapies
| Target population | Advantages | Disadvantages | Dosing | Cost* |
|---|---|---|---|---|
| Sulfonylureas | ||||
| Recent type 2 DM diagnosis | Rapid FPG reduction | Weight gain | Glyburide: 1.25–20 mg once or twice daily (micronized, 0.75–12 mg once or twice daily) | $22.80 (5 mg, #120) |
| Type 2 DM <5 years duration | Low cost | Increased risk of hypoglycemia | Glipizide: 2.5–40 mg once or twice daily (extended-release, 2.5–20 mg once daily) | $14.66 (10 mg, #120) |
| Glimepiride: 1–8 mg once daily | $51.98 (10 mg, #60) | |||
| $57.98 (4 mg, #60) | ||||
| Non-sulfonylurea secretagogues (meglitinides) | ||||
| Recent type 2 DM diagnosis | Reduced risk of hypoclycemia | Higher cost | Nateglinide: 60–120 mg 3 times daily | $85.99 (120mg, #90) |
| Elevated PPG | Short-acting | Frequent dosing | Repaglinide: 0.5–4 mg 3 or 4 times daily | $218.06 (2 mg, #240) |
| Meal-adjusted dosing | ||||
| Biguanides | ||||
| Overweight/obese | No weight gain | GI side effects | Metformin: 500–1000 mg 2 or 3 times daily | $77.99 (850 mg, #90) |
| Insulin resistant | Reduced risk of hypoglycemia | High cost | Metformin XR: 1000–2000 mg once or twice daily | $89.98 (500 mg, #120) |
| Rare lactic acidosis | ||||
| TZDs | ||||
| Insulin resistant | Reduced amount of insulin | High cost | Rosiglitazone: 4–8 mg once or twice daily | $135.99 (8 mg, #30) |
| Overweight/obese | Reduced risk of hypoglycemia | Weight gain | Pioglitazone: 15–45 mg once daily | $153.99 (45 mg, #30) |
| Slow onset of action | ||||
| Liver toxicity | ||||
| AGIs | ||||
| Elevated PPG | Reduced risk of hypoglycemia | High cost | Acarbose: 50–100 mg 3 times daily | $67.99 (100 mg, #90) |
| Contraindications to other agents | Non-systemic action | GI side effects | Miglitol: 50–100 mg 3 times daily | $66.99 (100 mg, #90) |
| *Drug costs for 30 days’ supply of maximum daily dosage. From www.drugstore.com, December 2003. | ||||
| DM, diabetes mellitus; TZD, thiazolidinediones; AGT, a-glucosidase inhibitors; FPG, fasting plasma glucose; PPG, postprandial glucose; GI, gastrointestinal | ||||
Sulfonylureas
Sulfonylureas directly increase insulin secretion by binding to the sulfonylurea receptor on pancreatic beta cells; they provide a relatively quick onset of action. First-generation sulfonylureas (eg, tolbutamide, chlorpropamide) and second-generation sulfonylureas (eg, glyburide, glipizide, glimepiride) are equivalent in their maximum hypoglycemic effect.17
Second-generation agents are used more commonly than first-generation. They all contain the sulfonylurea moiety, but different chemical substitutions in the basic molecule change pharmacokinetics, resulting in different durations of action.17 Second-generation agents are probably safer than first-generation drugs, being less likely to cause hyponatremia, disulfiram-like reactions, or prolonged hypoglycemia.18
At maximal doses, sulfonylureas lower A1c levels by 1–2 percentage points and fasting plasma glucose concentrations by 60–70 mg/dL;15 however, the glucose lowering effect typically plateaus after half the maximal recommended dose is reached. Sulfonylureas have no consistent effect on dyslipidemia. In UK Prospective Diabetes Study (UKPDS) 33, though improved glycemic control with sulfonylureas (or insulin) led to a 25% reduction in microvascular endpoints (mostly less retinal photocoagulation) (P<.01), sulfonylureas (or insulin) did not significantly reduce death or all-cause mortality compared with diet treatment.2
Adverse effects. The primary adverse effects of sulfonylureas are weight gain and hypoglycemia. In UKPDS 33, weight gain at 10 years was 2.6 kg (99% confidence interval [CI], 1.6–3.6) with chlorpropamide and 1.7 kg (99% CI, 0.7–2.7) with glyburide, compared with patients receiving diet therapy (each P<.001).2 In the same study, the rate of major hypoglycemic episodes (third-party help or medical intervention necessary) while on therapy was 0.4%/year for chlorpropamide and 0.6%/year for glyburide, compared with 0.1%/year for diet.
Glyburide and chlorpropamide have active metabolites with renal elimination, and they should therefore be used with caution in patients with renal insufficiency. In 1971, the University Group Diabetes Project (UGDP) observed a twofold increase in the rate of cardiovascular death among patients receiving tolbutamide compared with those receiving insulin or placebo.18 This led to a decades long debate on the validity of this conclusion.19 More recently, UKPDS 33 did not demonstrate any increased cardiovascular mortality among patients receiving glyburide or chlorpropamide, and has largely negated this earlier concern.2
Cost. Sulfonylureas are the least expensive oral agents used to treat type 2 diabetes.
TABLE 3
Pharmacological treatments for type 2 diabetes: combination therapies
| Sulfonylureas | Meglitinides | Biguanides | TZDs | AGIs | |
|---|---|---|---|---|---|
| Double combination therapy option* | ✓ | ✓ | |||
| Double combination therapy option† | ✓ | ✓ | |||
| Double combination therapy option | ✓ | ✓ | |||
| Double combination therapy option | ✓ | ✓ | |||
| Double combination therapy option | ✓ | ✓ | |||
| Double combination therapy option | ✓ | ✓ | |||
| Triple combination therapy option | ✓ | ✓ | ✓ | ||
| Triple combination therapy option | ✓ | ✓ | ✓ | ||
| If therapeutic goals are not met using the above combinations; switch to insulin with or without oral agent. | |||||
| *Available as Glucovance (metformin/glyburide) or as Metaglip (metformin/glipizide) | |||||
| † Available as Avandamet (rosiglitazone/metformin) | |||||
Non-sulfonylurea secretagogues
Like sulfonylureas, the non-sulfonylurea secretagogues (non-SU), repaglinide and nateglinide, stimulate beta cells to increase insulin secretion. However, the non-SU agents mediate their action through a different, adjacent site on the “sulfonylurea receptor.” Comparatively, the non-SU agents have a faster onset of action (20 minutes), shorter half-life (about 1.0–1.5 hours), and greater effects on postprandial glucose excursions than do sulfonylureas.20 In contrast to the sulfonylureas, the extent of insulin release with non-SU agents is glucose dependent, and therefore they may have less risk of hypoglycemia several hours after meals.15
A group of metabolic abnormalities that increase cardiovascular risk has been recognized since 1988 and has been given many names—Syndrome X, insulin resistance syndrome, dysmetabolic syndrome, The Deadly Quartet.73 The National Cholesterol Education Program Adult Treatment Panel III recently recodified this syndrome as shown below. The principles for diet and exercise discussed in this article also apply to the goals of reducing obesity and physical inactivity in the metabolic syndrome, and preliminary data suggest a reduction in the risk for type 2 diabetes (NNT per year=27; P=.000174) and for cardiovascular disease.75
| Risk factor | Defining level |
|---|---|
| Abdominal obesity | Waist circumference |
| Men | >102 cm (>40 in) |
| Women | >88 cm (>35 in) |
| Triglycerides | ≥150 mg/dL |
| HDL cholesterol | |
| Men | <40 mg/dL |
| Women | <50 mg/dL |
| Blood pressure | ≥130/85 mm Hg |
| Fasting glucose | ≥110 mg/dL |
Repaglinide lowers the A1c level by 1.7–1.9 percentage points, similar in efficacy to sulfonylureas. Nateglinide appears somewhat less efficacious and lowers A1c by 0.6–1.0 percentage points.15 Nateglinide was significantly less effective than glyburide at lowering A1c levels and the fasting plasma glucose in one 24-week study. Non-SUs added to sulfonylureas produce no additional benefit in glycemic control. The effect of non-SUs on microvascular or macrovascular endpoints is unknown.
Adverse effects. Hypoglycemia is the primary adverse effect of non-SUs. Confirmed hypoglycemia (plasma glucose <60 mg/dL) was observed in 2.4% of patients taking nateglinide compared with 0.4% of those receiving placebo. Mild or moderate hypoglycemia occurred in 16% of repaglinide patients, 20% of glyburide patients, and 19% of glipizide patients in one-year comparative studies. Further comparative studies are needed to determine if non-SUs produce significantly less hypoglycemia and weight gain than sulfonylureas.
Cost. Non-SUs must be dosed 3 times daily at the start of meals. One relative disadvantage is their increased cost compared with sulfonylureas.
Biguanides
The only biguanide marketed in the US is metformin. Its primary action is to inhibit hepatic glucose production and, to a much lesser extent, enhance insulin sensitivity in peripheral tissues.21 Metformin does not stimulate insulin secretion and does not cause hypoglycemia when used as monotherapy, but it can potentiate hypoglycemia in combination with insulin or insulin secretagogues.
Metformin is similar in efficacy to the sulfonylureas. It lowers A1c by 1.5–2.0 percentage points and fasting plasma glucose by 60–80 mg/dL. Its antihyperglycemic efficacy is independent of patient age, duration of diabetes, or BMI.22
In the UKPDS 34 study, a subgroup of obese patients was randomized to receive intensive control (group 1, metformin; group 2, a sulfonylurea or insulin) or conventional diet therapy (group 3). Despite a similar reduction in the A1c level between the 2 intensive-treatment groups, patients treated with metformin had a 32% reduction for any diabetes-related endpoint (95% CI, 13–47; P=.002), 43% fewer diabetes-related deaths (95% CI, 9–63; P=.017), and a 36% reduction in all cause mortality, compared with the diet therapy group (95% CI, 9–55; P=.011).23
Metformin also showed significant benefit when compared with patients receiving sulfonylurea or insulin (group 2). The absolute risk of any diabetes endpoint was 29.8 vs. 40.1 (events per 1000 patient-years; P=.0034), all-cause mortality (13.5 vs 18.9; P=.021), and stroke (3.3 vs 6.2; P=.032), respectively, for metformin vs sulfonylurea or insulin (group 2). Thus, metformin is the only oral hypoglycemic agent proven to reduce macrovascular risk in overweight patients with type 2 diabetes. For perspective, in overweight patients, metformin significantly reduced all-cause mortality (NNT per year=141; 95% CI, 115–183; P=.011), and any diabetes-related outcome (NNT per year=74; 95% CI, 63–90; P=.0023), compared with diet alone.23,24
Metformin induces weight loss (2–3 kg), preferentially involving adipose tissue in obese patients with type 2 diabetes over 4 to 6 months.22,25 In UKPDS 34, weight gain was similar among those treated with metformin and diet (approximately 2 kg); weight gain over 10 years was less with metformin, however, than with sulfonylurea (approximately 4 kg) or insulin (approximately 6 kg).23 Metformin also significantly improved levels of total cholesterol, low-density lipoprotein (LDL) cholesterol, and triglycerides when compared with glyburide or placebo.22
Risk of lactic acidosis. Lactic acidosis associated with metformin is a rare but serious adverse event, with an estimated prevalence of 3 cases per 100,000.26 The product labeling notes most of these cases have occurred among patients with significant renal insufficiency, including both intrinsic renal disease and renal hypoperfusion. Absolute contraindications include renal disease (serum creatinine ≥1.5 mg/dL [males] and ≥1.4 mg/dL [females]), congestive heart failure requiring pharmacological treatment, and acute or chronic metabolic acidosis. It should also be discontinued at the time of radiologic studies using intravascular iodinated contrast materials.
Additional “precautionary conditions” include age ≥80 years (unless measurement of creatinine clearance demonstrates that renal function is not reduced), hepatic disease, cationic drug use, conditions associated with hypoxia (eg, chronic obstructive pulmonary disease [COPD], acute myocardial infarction, dehydration, sepsis), excessive alcohol intake, and surgery, until patient’s oral intake is resumed.
Is the risk overstated? Despite these extensive precautions, published studies show that metformin is commonly prescribed to patients with absolute contraindications.27,28 One recent study observed that 11.2% of Medicare beneficiaries hospitalized with congestive heart failure and concomitant diabetes were treated with metformin.28 In the absence of advanced renal dysfunction, metformin rarely accumulates in the body,29 and accumulation of metformin is rarely reported as a cause of lactic acidosis.30,31 Rather, tissue hypoxia acts as a trigger in most cases. Metformin should therefore be discontinued whenever tissue hypoxia is suspected.31
A recent systematic review and meta analysis found no evidence that metformin was associated with an increased risk of lactic acidosis if the drug was prescribed under study conditions, taking into account contraindications.32 Refinement and clarification of the risk for lactic acidosis in these various populations is needed, to ensure optimal patient safety and to further assess this highly effective medication.
Common adverse effects associated with metformin are diarrhea and nausea, which can be minimized by administering the drug with meals and slowly titrating the dose, or perhaps by using the extended-release formulation.
Thiazolidinediones
Thiazolidinediones (TZDs) include rosiglitazone and pioglitazone. These agents, like metformin, do not increase insulin secretion but depend on the presence of insulin for their activity. TZDs are agonists at peroxisome-proliferator-activated receptor gamma (PPAR-γ) receptors in peripheral tissues such as skeletal muscle, where they increase glucose uptake.15 Thus, their predominant effect is to decrease insulin resistance.
TZDs have similar antihyperglycemic efficacy as sulfonylureas or metformin. They decrease A1c levels by 0.6–1.9 percentage points and lower fasting plasma glucose levels by 50–80 mg/dL.15 They have a slower onset of action compared with other hypoglycemic drugs, and intervals of 3 to 4 weeks should be allowed between doses before increasing the dosage. TZDs also have favorable effects on lipid levels: HDL concentrations increase and triglyceride concentrations decrease with their use.33 It is not known whether they decrease macrovascular or microvascular complications, although such studies are underway.
Adverse effects. TZDs are typically well tolerated, though weight gain of 1–3 kg, edema (4%–5%) and anemia (1%–2%) can occur. Weight gain and edema are more pronounced when TZDs are used in combination with insulin. Anemia is likely due to increased plasma volume rather than any significant hematological effect.
Due to adverse events related to volume expansion, TZDs are not recommended for patients with New York Heart Association class III or IV heart failure. A recent consensus statement from the American Heart Association and the ADA stresses that before administering TZD treatment, the physician should explore the possible presence of cardiac disease, use of other drugs that cause fluid retention, and the pathogenesis of any existing edema or dyspnea.34
Although troglitazone was removed from the market due to its association with hepatocellular injury, pioglitazone and rosiglitazone are not as convincingly associated with liver injury.15 In preapproval clinical studies, less than 0.5% of patients treated with rosiglitzone and pioglitazone had elevations in alanine transaminase (ALT) >3 times the upper limit of normal.
The incidence of hepatitis or acute liver failure from troglitazone was compared with rosiglitazone, pioglitazone, metformin, and glyburide, by analysis of spontaneously reported adverse events to the Food and Drug Administration (FDA) MEDWATCH database during the first 15 months of marketing of each drug.35,36 The incidence of hepatitis per million prescriptions was 21.5, 14.7, 9.4, 2.9, and 4.1, respectively, while the incidence of acute liver failure per 100,000 prescriptions was 4.6, 0.9, 0.8, 0.2, and 0. It appears that postmarketing data support preclinical studies, in that the incidence of acute liver failure is an order of magnitude higher for troglitazone vs. other TZDs.35 However, the FDA recommends avoiding their use in patients with baseline ALT levels >2.5 times the upper limit of normal. The FDA recently reduced the recommended frequency for ALT monitoring for pioglitazone (and is currently considering the same for rosiglitazone). Serum ALT is recommended prior to initiation and then periodically thereafter.
Cost. TZDs are expensive relative to other hypoglycemic agents.
α-glucosidase inhibitors
The α-glucosidase inhibitors (AGIs), acarbose and miglitol, act through competitive, reversible inhibition of membrane-bound intestinal α-glucosidase, which hydrolyzes complex carbohydrates to glucose and other monosaccharides. This inhibition delays glucose absorption and decreases postprandial hyperglycemia.37 Thus, they have a nonsystemic mechanism of action.
These agents cause a modest reduction in the A1c level (0.5–1.0 percentage points) and are thus less effective than sulfonylureas, metformin, or TZDs. They do not reduce fasting plasma glucose levels, but reduce postprandial hyperglycemia by 50 mg/dL.38 No long-term studies have evaluated whether AGIs reduce diabetes-related macrovascular or microvascular outcomes.
Adverse effects. While AGIs are virtually free of serious toxicities, patient tolerability can be a problem due to adverse gastrointestinal effects. In indirect comparisons from placebo-controlled trials, patients treated with miglitol and acarbose commonly reported abdominal pain (11.7%, 19%), diarrhea (28.7%, 31%), and flatulence (41.5%, 74%), respectively. Systemic accumulation of AGIs has been shown to increase in proportion to the degree of renal insufficiency, and their use is not recommended for patients with serum creatinine >2.0 mg/dL. However, whether such patients are at greater risk of any toxicity is unknown. Acarbose at doses above 100 mg 3 times daily has been associated with elevated serum transaminase levels; however, this risk appears negligible at standard doses.
Insulin
Insulin is the oldest therapy for diabetes, and it has no upper dose limit.39 It increases insulin levels and can reduce A1c levels by 1.5 to 2.5 percentage points. Though half of diabetes patients need insulin eventually for optimal control, historically it has been introduced late in the disease process unless patients have severe hyperglycemia (fasting blood sugar >350 mg/dL) or ketonuria.38 However, it is effective in gaining initial control, decreasing gluconeogenesis and increasing glucose uptake. Disadvantages are weight gain, hypoglycemia, and patient reluctance to give injections.
When insulin is indicated. Patients who exhibit persistent hyperglycemia despite oral hypoglycemic therapy may stop the oral drug(s) and begin insulin. By combining insulin with oral therapy, lower insulin doses may be used to achieve desired control vs using insulin alone.40 For some patients a basal supplement of insulin may be sufficient and can be given as a single dose at bedtime, without an oral hypoglycemic drug.41
Insulin regimens. Various insulin regimens are available: very rapid acting (lispro and aspart), rapid acting (regular), intermediate acting (isophane insulin [NPH] and lente) and very long acting (ultralente and glargine). Glargine insulin (Lantus) has more predictable absorption than NPH, lente, and ultralente. Lantus, compared with NPH, has been associated with less nocturnal and postprandial hypoglycemia.38,42,43 This is consistent with the peakless and longer duration of glargine compared with NPH.44 A recent randomized controlled trial demonstrated that morning insulin glargine lowered A1c levels more than a bedtime dose of NPH (–1.24 vs –0.84; 95% CI, 0.23%–0.58%) or a bedtime dose of glargine (–1.24 vs –0.96%; 95% CI, 0.11%–0.46%).45 Glargine’s only relative disadvantage is increased cost.
Combination products. Combination insulin options are 70 NPH/30 regular, 50 NPH/50 regular, and 75 lispro protamine/25 lispro. Many combinations of insulin regimens have been used successfully. The typical range of insulin needed for monotherapy is 0.4–1 U/kg/d. Once-daily injection of intermediate acting or long acting insulins at bedtime or before breakfast, once-daily or twice-daily combinations of intermediate and rapid acting insulins, and more complex regimens have been used to good effect.
Using prandial insulin at each meal with separate basal insulin adds flexibility to meal times and doses administered.43 With multiple-dose intensive insulin therapy, a basal dose suppresses hepatic glucose output and the bolus doses enhance postprandial glucose uptake. This intensive insulin treatment reduces mortality among critically ill patients in surgical intensive care units and for those with acute myocardial infarction.46,47 An algorithm for using progressive therapy in diabetes mellitus is shown in Figure 2.48
FIGURE 2
ADA recommendations for the treatment of type 2 diabetes
Combination Therapy
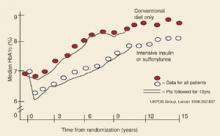
Over time glycemic control becomes more difficult, even with maximum monotherapy for patients with healthy lifestyles. It was shown in UKPDS 49 that monotherapy with sulfonylurea, metformin, or insulin eventually fails in most cases—by 3 years after diagnosis, about 50% of patients need more than monotherapy; 75% by 9 years.49 In UKPDS 33, the median A1c level increased steadily over 10 years with both conventional therapy and intensive therapy (Figure 3).2
Several options are available when monotherapy fails. Based on expert opinion, the principle is to combine drugs with different mechanisms of action to achieve an additive effect for glycemic control. Combination products may simplify the treatment regimen and improve adherence. In many instances, they may also cost less.50
Successful combinations. The combination of sulfonylurea and metformin has proven effective in many studies.22,51,52 One study showed that initial treatment with glyburide/metformin improved glycemic control better than either glyburide or metformin monotherapy (SOR: A).53,54 The addition of the non-SU secretagogues repaglinide and nateglinide to metformin significantly improved glycemic control, with repaglinide showing superiority over nateglinide.55 A TZD added to a sulfonylurea has also significantly improved A1c and fasting blood sugar results.56 Patients whose diabetes was inadequately controlled with diet alone or diet plus a sulfonylurea showed improvement with the addition of the AGI miglitol, compared with addition of placebo.57 The AGI acarbose has shown to be an effective addition to diet, metformin, sulfonylurea, and insulin.58 A TZD added to metformin has also been shown to improve glycemic control.59 A non-SU added to patients inadequately controlled with a TZD has also been effective.60
The early addition of insulin when maximal sulfonylurea therapy is inadequate has been effective.61-63 When introducing insulin, a nighttime regimen of NPH or glargine, 10 units at bedtime, is an appropriate dose (SOR: C). This is easier and less costly than often assumed, and helps improve glycemic control.64 Most patients require combination therapy as their disease progresses.39
FIGURE 3
Glycemic control in type 2 DM
Improving Outcomes
Cumulative survey data reveal a wide gap between guideline recommendations and the care patients receive.65 One study showed that physicians initiated treatment changes only after the A1c level had reached 9.0% or higher instead of the 8.0% level recommended by ADA.66 How can the quality of management be improved?
In private practices and institutions, many interventions have been shown to improve outcomes in diabetes mellitus. Education measures work, and they include chart audits, reminder cards, pharmacist collaboration, flow sheets, and nursing initiatives.67,68 Effective disease-management programs have also used clinical guidelines, outcomes reporting, coverage of glucose meters and strips, and the support of clinical leadership.69
Computerized systems that track patients and recommended laboratory tests have improved screening rates and glycemic and blood pressure control.70 Monitoring patients’ readiness to change has allowed targeted education to improve A1c levels.71 Continuity of care has also improved the quality of disease control by increasing adherence to recommended tests and exams.72
Acknowlegments
The authors thank Marie Hamer, RN, for her continuous diabetes quality improvement efforts and Jean Camarata for her editorial and reference acquisition assistance.
Corresponding author
John E. Sutherland, MD, Northeast Iowa Family Practice Residency Program, University of Iowa College of Medicine, 2055 Kimball Avenue, Waterloo, Iowa 50702. E-mail: jsutherl@neimef.org.
1. Vinik AI, Vinik E. Prevention of the complications of diabetes. Am J Manag Care. 2003;9 suppl:S63-S80.
2. UK Prospective Diabetes Study (UKPDS) Group. Intensive blood-glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33). Lancet. 1998;352:837-853.
3. Arauz-Pacheco C, Parrott MA, Raskin P. American Diabetes Association Treatment of hypertension in adults with diabetes. Diabetes Care. 2004;27 (suppl):S65-67.
4. UK Prospective Diabetes Study Group. Tight blood pressure control and risk of macrovascular and microvascular complications in type 2 diabetes: UKPDS 38. BMJ. 1998;317:703-713.
5. Beckman JA, Creager MA, Libby P. Diabetes and atherosclerosis: epidemiology, pathophysiology, and management. JAMA. 2002;287:2570-2581.
6. Kendall DM, Bergenstal RM. Comprehensive management of patients with type 2 diabetes: establishing priorities of care. Am J Manag Care. 2001;7 (suppl):S327-S343.
7. American Diabetes Association. Standards of medical care in Diabetes. Diabetes Care. 2004;27(suppl):15-35.
8. Peterson KA. Diabetes management in the primary care setting: summary. Am J Med. 2002;113(suppl 6A):36S-40S.
9. Ellis SE, Speroff T, Dittus RS, Brown A, Pichert JW, Elasy TA. Diabetes patient education: A meta-analysis and meta-regression. Patient Educ Couns. 2004;52:97-105.
10. Chandalia M, Garg A, Lutjohann D, et al. Beneficial effects of high dietary fiber intake in patients with type 2 diabetes mellitus. N Engl J Med. 2000;342:1392-1398.
11. Hadden DR, Montgomery DAD, Skelly RJ, et al. Maturity onset diabetes mellitus: response to intensive dietary management. Br Med J. 1975;3:276-278.
12. Niskenen LK, Uusitupa MI, Surlund H, et al. Five-year follow-up study on plasma insulin levels in newly diagnosed NIDDM patients and nondiabetic subjects. Diabetes Care. 1009;13:41-48.
13. Leibel R, Rosenbaum M, Hirsch J. Changes in energy expenditure resulting from altered body weight. N Engl J Med. 1995;332:621-628.
14. Gregg EW, Gerzoff RB, Caspersen CJ, et al. Relationship of walking to mortality among US adults with diabetes. Arch Intern Med. 2003;163:1440-1447.
15. Inzucchi SE. Oral antihyperglycemic therapy for type 2 diabetes: scientific review. JAMA. 2002;287:360-372.
16. Holmboe ES. Oral antihyperglycemic therapy for type 2 diabetes: clinical apparatus. JAMA. 2002;287:373-376.
17. Rang HP, Dale MM, Ritter JM, Moore PK. The endocrine pancreas and the control of blood glucose. In: Pharmacology. 5th ed. London: Churchill-Living-stone/Elsevier Science; 2003;380-394.
18. Davis SN, Granner DK. Insulin, oral hypoglycemic agents, and the pharmacology of the endocrine pancreas. In: Hardman JG, Limbird LE, Gilman AG, eds. Goodman & Gilman’s The Pharmacological Basis of Therapeutics. 10th ed. New York, NY: McGraw-Hill; 2001;1679-1714.
19. Goldner MG, Knatterud GL, Prout TE. Effects of hypoglycemic agents on vascular complications in patients with adult-onset diabetes. 3. Clinical implications of UGDP results. JAMA. 1971;218:1400-1410.
20. Hollander P, Schwartz SL, Gatlin MR, et al. Importance of early insulin secretion: comparison of nateglinide and glyburide in previously diet-treated patients with type 2 diabetes. Diabetes Care. 2001;24:983-988.
21. Inzucchi SE, Maggs DG, Spollett GR, et al. Efficacy and metabolic effects of metformin and troglitazone in type II diabetes mellitus. N Engl J Med. 1998;338:867-872.
22. DeFronzo RA, Goodman AM. Efficacy of metformin in patients with non-insulin-dependent diabetes mellitus. The Multicenter Metformin Study Group. N Engl J Med. 1995;333:541-549.
23. UKPDS Group Effect of intensive blood-glucose control with metformin on complications in overweight patients with type 2 diabetes (UKPDS 34). Lancet. 1998;352:854-865.
24. Shaughnessy AF, Slawson DC. What happened to the valid POEMs? A survey of review articles on the treatment of type 2 diabetes. BMJ. 2003;327:266.-
25. Stumvoll M, Nurjhan N, Perriello G, Dailey G, Gerich JE. Metabolic effects of metformin in non insulin-dependent diabetes mellitus. N Engl J Med. 1995;333:550-554.
26. Brown JB, Pedula MS, Barzilay J, et al. Lactic acidosis rates in type 2 diabetes. Diabetes Care. 1998;21:1659-1663.
27. Calabrese AT, Coley KC, DaPos SV, Swanson D, Rao RH. Evaluation of prescribing practices: risk of lactic acidosis with metformin therapy. Arch Intern Med. 2002;162:434-437.
28. Masoudi FA, Wang Y, Inzucchi SE, et al. Metformin and thiazolidinedione use in Medicare patients with heart failure. JAMA. 2003;290:81-85.
29. Scheen AJ. Clinical pharmacokinetics of metformin. Clin Pharmacokinet. 1996;30:359-371.
30. Lalau JD, Lacroix C, De Cagny B, Fournier A. Metformin-associated lactic acidosis in diabetic patients with acute renal failure. A critical analysis of its pathogenesis and prognosis. Nephrol Dial Transplant. 1994;9 (suppl 4):126-129.
31. Jones GC, Macklin JP, Alexander WD. Contraindications to the use of metformin. BMJ. 2003;3:131-132.
32. Salpeter SR, Greyber E, Pasternak GA, Salpeter EE. Risk of fatal and nonfatal lactic acidosis with metformin use in type 2 diabetes mellitus: systematic review and metaanalysis. Arch Intern Med. 2003;63:2594-2602.
33. Aronoff S, Rosenblatt S, Braithwaite S, Egan JW, Mathiesen AL, Schnieder RL. Pioglitazone hydrochloride monotherapy improves glycemic control in the treatment of patients with type 2 diabetes: a 6 month randomized placebo-controlled dose-response study. The Pioglitazone 001 Study Group. Diabetes Care. 2000;23:1605-1611.
34. Nesto RW, Bell D, Bonow RO, et al. Thiazolidinedione use, fluid retention, and congestive heart failure: a consensus statement from the American Heart Association and American Diabetes Association. October 7, 2003. Circulation. 2003;108:2941-2948.
35. Tolman KG, Chandramouli J. Hepatotoxicity of the thiazolidinediones. Clin Liver Dis. 2003;7:369-379.
36. Zawadzki JK, Green L, Graham BJ. Thioglitazone-associated 15-month post-marketing hepatotoxicity. Poster abstract. FDA Science Forum. Available at: vm.cfsan.fda.gov/~frf/forum02/a187ab4.htm. Accessed on February 25, 2004.;
37. Lebowitz HE. a-Glucosidase inhibitors as agents in the treatment of diabetes. Diabetes Rev. 1998;6:132-145.
38. Chan JL, Abrahamson MJ. Pharmacological management of type 2 diabetes mellitus: rationale for rational use of insulin. Mayo Clin Proc. 2003;78:459-467.
39. Nathan DM. Initial management of glycemia in type 2 diabetes mellitus. N Engl J Med. 2002;347:1342-1349.
40. Pugh JA, Wagner ML, Sawyer J, Ramirez G, Tuley M, Friedberg SJ. Is combination sulfonylurea and insulin therapy useful in NIDDM patients? A metaanalysis. Diabetes Care. 1992;15:953-959.
41. Cusi K, Cunningham GR, Comstock JP. Safety and efficacy of normalizing fasting glucose with bedtime NPH insulin alone in NIDDM. Diabetes Care. 1995;18:843-851.
42. White JR, Davis SN, Cooppan R, et al. Clarifying the role of insulin in type 2 diabetes management. Clinical Diabetes. 2003;1:14-21.
43. DeWitt DE, Hirsch IR. Outpatient insulin therapy in type 1 and type 2 diabetes mellitus: scientific review. JAMA. 2003;289:2254-2264.
44. - Yki, Jarvinen H, Dressler A, Ziemen M. HOE 901/3002 Study Group Less nocturnal hypoglycemia and better post-dinner glucose control with bedtime insulin glargine compared with bedtime NPH insulin during insulin combination therapy in type 2 diabetes. Diabetes Care. 2000;23:1130-1136.
45. Fritsche A, Schweitzer MA, Haring HU. 4001 Study Group Glimepiride combined with morning insulin glargine, bedtime neutral protamine hagedorn insulin, or bedtime insulin glargine in patients with type 2 diabetes. A randomized, controlled trial. Ann Intern Med. 2003;138:952-959.
46. van den Berghe G, Wouters P, Weekers F, et al. Intensive insulin therapy in critically ill patients. N Engl J Med. 2001;345:1359-1367.
47. Malmberg K, Norhammar A, Wedel H, Ryden L. Glycometabolic state at admission: important risk marker of mortality in conventionally treated patients with diabetes mellitus and acute myocardial infarction: long-term results from the Diabetes and Insulin-Glucose Infusion in Acute Myocardial Infarction (DIGAMI) study. Circulation. 1999;99:2626-2632.
48. Zimmerman BR. Therapy for type 2 diabetes mellitus. In: Medical Management of Type 2 Diabetes. 4th ed. Alexandria, Va: American Diabetes Association; 1998.;
49. Turner RC, Cull CA, Frighi V, Holman RR. Glycemic control with diet, sulfonylurea, metformin, or insulin in patients with type 2 diabetes mellitus. Progressive requirement for multiple therapies (UKPDS 49). JAMA. 1999;281:2005-2012.
50. Leichter SB, Thomas S. combination medications in diabetes care: an opportunity that merits more attention. Clin Diabetes. 2003;21:175-178.
51. Hermann LS, Schersten B, Bitzen P, et al. Therapeutic comparison of metformin and sulfonylurea, alone and in various combinations. A double-blind controlled study. Diabetes Care. 1994;17:1100-1109.
52. Jeppesen J, Zhou M, Chen Y, Reaven G. Effect of metformin on postprandial lipemia in patients with fairly to poorly controlled NIDDM. Diabetes Care. 1994;17:1093-1099.
53. Garber AJ, Larsen J, Schneider SH, et al. Simultaneous glyburide/metformin therapy is superior to component monotherapy as an initial pharmacological treatment for type 2 diabetes. Diabetes Obes Metab. 2002;4:201-208.
54. Riddle M. Combining sulfonylureas and other oral agents. Am J Med. 2000;108(suppl 6A):15S-22S.
55. Raskin P, Klaff L, McGill J, et al. Efficacy and safety of combination therapy: repaglinide plus metformin versus nateglinide plus metformin. Diabetes Care. 2003;26:2063-2068.
56. Kipnes MS, Krosnick A, Rendell MS, Egan JW, Mathisen AL, Schneider RL. Pioglitazone hydrochloride in combination with sulfonylurea therapy improves glycemic control in patients with type 2 diabetes mellitus: a randomized, placebo-controlled study. Am J Med. 2001;111:10-17.
57. Johnston PS, Feig PU, Coniff RF, Krol A, Davidson JA, Haffner SM. Long-term titrated-dose a-glucosidase inhibition in non-insulin-requiring Hispanic NIDDM patients. Diabetes Care. 1998;21:409-415.
58. Chiasson J, Josse RG, Hunt JA, et al. The efficacy of acarbose in the treatment of patients with non-insulin-dependent diabetes mellitus. A multicenter controlled clinical trial. Ann Intern Med. 1994;121:928-935.
59. Fonseca V, Rosenstock J, Patwardhan R, Salzman A. Effect of metformin and rosiglitazone combination therapy in patients with type 2 diabetes mellitus: a randomized controlled trial. JAMA. 2000;283:1695-1702.
60. Fonseca V, Grunberger G, Gupta S, Shen S, Foley JE. Addition of nateglinide to rosiglitazone monotherapy suppresses mealtime hyperglycemia and improves overall glycemic control. Diabetes Care. 2003;26:1685-1690.
61. Wright A, Burden ACF, Paisey RB, Cull CA, Holman RR; UKPDS. Sulfonylurea inadequacy: efficacy of addition of insulin over 6 years in patients with type 2 diabetes in the U.K. Prospective Diabetes Study (UKPDS 57). Diabetes Care. 2002;25:330-336.
62. Garber AJ. Benefits of combination therapy of insulin and oral hypoglycemic agents. Arch Intern Med. 2003;163:1781-1782.
63. Westphal SA, Palumbo PJ. Insulin and oral hypoglycemic agents should not be used in combination in the treatment of type 2 diabetes. Arch Intern Med. 2003;163:1783-1785.
64. DeWitt DE, Dugdale DC. Using new insulin strategies in the outpatient treatment of diabetes: clinical applications. JAMA. 2003;289:2265-2269.
65. Saaddine JB, Engelgau MM, Beckles GL, Gregg EW, Thompson TJ, Narayan KM. A diabetes report card for the United States: quality of care in the 1990s. Ann Intern Med. 2002;136:565-574.
66. Brown JB, Nichols GA. Slow response to loss of glycemic control in type 2 diabetes mellitus. Am J Manag Care. 2003;9:213-217.
67. Sutherland JE, Hoehns JD, O’Donnell B, Wiblin RT. Diabetes management quality improvement in a family practice residency program. J Am Board Fam Pract. 2001;14:243-251.
68. De Grauw W, van Gerwen W, van de Lisdonk EH, van den Hoogen HJ, van den Bosch WJ, van Weel C. Outcomes of audit-enhanced monitoring of patients with type 2 diabetes. J Fam Pract. 2002;51:459-464.
69. Sidorov J, Gabbay R, Harris R, et al. Disease management for diabetes mellitus: impact on hemoglobin A1c. Am J Manag Care. 2000;6:1217-1226.
70. Domurat ES. Diabetes managed care and clinical outcomes: the Harbor City, California Kaiser Permanente diabetes care system. Am J Manag Care. 1999;5:1299-1307.
71. Peterson K, Hughes M. Readiness to change and clinical success in a diabetes educational program. J Am Board Fam Pract. 2002;15:266-270.
72. Parchman ML, Burge SK. Continuity and quality of care in type 2 diabetes: a Residency Research Network at South Texas study. J Fam Pract. 2002;51:619-624.
73. Fagan TC, Deedwania PC. The cardiovascular dysmetabolic syndrome. Am J Med. 1998;105(suppl):77S-82S.
74. Tuomilehto J, Lindstrom J, Eriksson JG, et al. Prevention of type 2 diabetes mellitus by changes in lifestyle among subjects with impaired glucose tolerance. N Engl J Med. 2001;344:1343-1350.
75. Meigs JB. The metabolic syndrome. BMJ. 2003;327:61-62.
- Self-monitoring of blood glucose is an integral component of diabetes therapy and should always be included in the management plan (SOR:C).
- Medical nutrition therapy should be individualized, preferably by a registered dietitian familiar with diabetes (SOR:B).
- A regular physical activity program is recommended for all patients with diabetes who are capable of participating (SOR:B).
- When a monotherapy fails, combine drugs with different mechanisms of action to achieve an additive effect (SOR:A).
- The combination of sulfonylurea and metformin has proven effective in many studies. One showed that initial treatment with glyburide/metformin improved glycemic control better than either glyburide or metformin monotherapy (SOR: A).
Glycemic control in diabetes begins with a patient’s adherence to several nonpharmacologic measures. Without such a commitment, success in controlling the disease will be difficult to achieve, and otherwise appropriate drug therapy will be hindered.
Most antidiabetic agents comparably reduce glycosylated hemoglobin (A1c) levels. However, a particular agent may be preferred depending on a patient’s characteristics. And some circumstances call for combination therapy. This article reviews the advantages and disadvantages of the many pharmacologic treatments for glucose control and hyperglycemia in type 2 diabetes.
Benefits Of Diabetes Control
The benefits of diabetes control are detailed in this issue of THE JOURNAL OF FAMILY PRACTICE (“Strategies to reduce complications in type 2 diabetes,” pages 366–374). For every percentage-point reduction in hemoglobin A1c, it is possible to achieve a 22% to 35% reduction in microvascular complications.1,2 Cardiovascular disease can be reduced in patients with diabetes by treating hypertension3,4 and hyperlipidemia, prescribing aspirin therapy, using angiotensin-converting enzyme (ACE) inhibitors, and with smoking cessation.5,6
Targets For Glycemic Control
The American Diabetes Association’s (ADA) recommended targets for glycemic control are a preprandial blood glucose level of 80–120 mg/dL, a bedtime blood glucose level of 100–140 mg/dL, and a hemoglobin A1c level of <7% (with a level of >8% requiring additional measures). Hemoglobin A1c is the best determinant of glycemic exposure, and its mean value is a nationally recognized indicator of how well diabetes is being managed.7 The American College of Endocrinology has adopted a more aggressive approach by designating an A1c level of 6.5% as both a target and action level.8
Self-monitoring of blood glucose
Self-monitoring of blood glucose (SMBG) is an integral component of diabetes therapy (strength of recommendation [SOR]: C) and should always be included in the management plan (SOR: C). The optimal frequency and timing of SMBG for type 2 diabetes is not known, but they should be sufficient to facilitate reaching glucose goals. The A1c test should be performed at least semi-annually for patients with stable glycemic control, and quarterly for patients not meeting glycemic goals or those who are changing therapy. A1c levels and mean plasma glucose levels can be approximately correlated (Table 1).7
TABLE 1
Correlation between hemoglobin A1clevels and mean plasma glucose levels
| Hemoglobin A1c(%) | Mean plasma glucose (mg/dL) |
|---|---|
| 6 | 135 |
| 7 | 170 |
| 8 | 205 |
| 9 | 240 |
| 10 | 275 |
| 11 | 310 |
| 12 | 345 |
Nonpharmacologic Therapy
Nonpharmacologic measures remain the cornerstone of managing type 2 diabetes. Hyperglycemia adversely and reversibly affects both insulin resistance and insulin secretion. Improvement in glycemic control can occur through dietary modification and regular exercise.
A recent meta-analysis of randomized controlled trials of diabetes patient education observed a net decrease in HbA1c of 0.32% in intervention groups vs control.9 Interventions that included a face-to-face delivery, cognitive reframing teaching method, and exercise content were more likely to improve glycemic control.
Education
Lifestyle changes involving diet, exercise, and usually weight loss are key to effective management of diabetes. If patients are to change their behavior, they must be given detailed training.6 Self-management also necessitates that patients engage in problem solving. This requires that each aspect of the management plan is understood and agreed upon by the patient and providers, and that the goals and treatment plan are individualized and reasonable.
Diet: recommend soluble fiber, reduce calories
Medical nutrition therapy should be individualized and preferably provided by a registered dietitian familiar with diabetes (SOR: B). The goals of nutrition therapy, according to the ADA, are to attain recommended body weight and prevent or reverse obesity. The means of achieving these goals are nutrition assessment and modification of nutrient intake and lifestyle through healthy food choices and physical activity.7
A high intake of dietary fiber (particularly the soluble type) above the level recommended by the ADA improves glycemic control, decreases hyperinsulinemia, and lowers plasma lipid concentrations.10
Hypocaloric diets cause glucose plasma levels to fall, in some cases to a normal level with a weight loss of even 5 to 10 pounds.7,11 Hypoglycemic medications are of course most effective in nonobese persons. But effectiveness is also improved if weight that is gained can be limited. Despite the clear benefit of weight loss, only a few patients are able to attain and maintain substantial weight loss. Maintenance of a reduced or elevated body weight is associated with compensatory changes in energy expenditure, which oppose the maintenance of body weight that is different from the usual weight.13 Part of the individualization of therapy is respect of personal and cultural preferences, lifestyles, and financial considerations.
Physical activity: a little goes a long way
A regular physical activity program is recommended for all patients with diabetes who are capable of participating (SOR: B).7 It improves blood glucose control, reduces cardiovascular risk factors, aids weight loss, and enhances well being.7 A recently published prospective cohort study showed that walking at least 2 hours a week was associated with a 39% lower all-cause mortality (hazard rate ratio [HRR], 0.61; 95% CI, 0.48–0.78) and a 34% lower cardiovascular mortality (HRR, 0.66; 95% CI, 0.45–0.96) across a diverse spectrum of adults with diabetes. The NNT (to prevent 1 death per year) is 61 for patients who walk at least 2 hours/week.14
In prescribing a physical activity plan for a patient, consider cardiovascular disease risk factors or complications to minimize the risk of untoward events. Micro- and macrovascular disease are of course prevalent among persons with diabetes, often resulting in functional limitations that make exercise more difficult.
Other priorities
Other recommended components of care include daily aspirin use, foot care exams, tobacco cessation, pneumococcal and influenza vaccinations, and an annual dilated retinal exam.
Pharmacologic Therapy
The coexisting defects in type 2 diabetes mellitus are as follows:
- resistance to insulin action in muscle
- defective pancreatic insulin secretion
- unrestrained hepatic glucose production, aggravated by increased lipolysis in adipose tissue.
Drug therapy is aimed at each of these defects, and also at reducing carbohydrate absorption in the small intestine (Figure 1). As far as antihyperglycemic effect is concerned, no one category of antidiabetic agent is favored over another.15 Except for nateglinide and α-glucosidase inhibitors (AGIs), each of the drug categories leads to a similar reduction in A1c.16 However, patient characteristics may lead to selection of a particular agent. Table 2 summarizes oral treatment options, their relative advantages and costs.
FIGURE 1
Drug therapies for coexisting defects in type 2 diabetes
TABLE 2
Pharmacologic treatments for type 2 diabetes: monotherapies
| Target population | Advantages | Disadvantages | Dosing | Cost* |
|---|---|---|---|---|
| Sulfonylureas | ||||
| Recent type 2 DM diagnosis | Rapid FPG reduction | Weight gain | Glyburide: 1.25–20 mg once or twice daily (micronized, 0.75–12 mg once or twice daily) | $22.80 (5 mg, #120) |
| Type 2 DM <5 years duration | Low cost | Increased risk of hypoglycemia | Glipizide: 2.5–40 mg once or twice daily (extended-release, 2.5–20 mg once daily) | $14.66 (10 mg, #120) |
| Glimepiride: 1–8 mg once daily | $51.98 (10 mg, #60) | |||
| $57.98 (4 mg, #60) | ||||
| Non-sulfonylurea secretagogues (meglitinides) | ||||
| Recent type 2 DM diagnosis | Reduced risk of hypoclycemia | Higher cost | Nateglinide: 60–120 mg 3 times daily | $85.99 (120mg, #90) |
| Elevated PPG | Short-acting | Frequent dosing | Repaglinide: 0.5–4 mg 3 or 4 times daily | $218.06 (2 mg, #240) |
| Meal-adjusted dosing | ||||
| Biguanides | ||||
| Overweight/obese | No weight gain | GI side effects | Metformin: 500–1000 mg 2 or 3 times daily | $77.99 (850 mg, #90) |
| Insulin resistant | Reduced risk of hypoglycemia | High cost | Metformin XR: 1000–2000 mg once or twice daily | $89.98 (500 mg, #120) |
| Rare lactic acidosis | ||||
| TZDs | ||||
| Insulin resistant | Reduced amount of insulin | High cost | Rosiglitazone: 4–8 mg once or twice daily | $135.99 (8 mg, #30) |
| Overweight/obese | Reduced risk of hypoglycemia | Weight gain | Pioglitazone: 15–45 mg once daily | $153.99 (45 mg, #30) |
| Slow onset of action | ||||
| Liver toxicity | ||||
| AGIs | ||||
| Elevated PPG | Reduced risk of hypoglycemia | High cost | Acarbose: 50–100 mg 3 times daily | $67.99 (100 mg, #90) |
| Contraindications to other agents | Non-systemic action | GI side effects | Miglitol: 50–100 mg 3 times daily | $66.99 (100 mg, #90) |
| *Drug costs for 30 days’ supply of maximum daily dosage. From www.drugstore.com, December 2003. | ||||
| DM, diabetes mellitus; TZD, thiazolidinediones; AGT, a-glucosidase inhibitors; FPG, fasting plasma glucose; PPG, postprandial glucose; GI, gastrointestinal | ||||
Sulfonylureas
Sulfonylureas directly increase insulin secretion by binding to the sulfonylurea receptor on pancreatic beta cells; they provide a relatively quick onset of action. First-generation sulfonylureas (eg, tolbutamide, chlorpropamide) and second-generation sulfonylureas (eg, glyburide, glipizide, glimepiride) are equivalent in their maximum hypoglycemic effect.17
Second-generation agents are used more commonly than first-generation. They all contain the sulfonylurea moiety, but different chemical substitutions in the basic molecule change pharmacokinetics, resulting in different durations of action.17 Second-generation agents are probably safer than first-generation drugs, being less likely to cause hyponatremia, disulfiram-like reactions, or prolonged hypoglycemia.18
At maximal doses, sulfonylureas lower A1c levels by 1–2 percentage points and fasting plasma glucose concentrations by 60–70 mg/dL;15 however, the glucose lowering effect typically plateaus after half the maximal recommended dose is reached. Sulfonylureas have no consistent effect on dyslipidemia. In UK Prospective Diabetes Study (UKPDS) 33, though improved glycemic control with sulfonylureas (or insulin) led to a 25% reduction in microvascular endpoints (mostly less retinal photocoagulation) (P<.01), sulfonylureas (or insulin) did not significantly reduce death or all-cause mortality compared with diet treatment.2
Adverse effects. The primary adverse effects of sulfonylureas are weight gain and hypoglycemia. In UKPDS 33, weight gain at 10 years was 2.6 kg (99% confidence interval [CI], 1.6–3.6) with chlorpropamide and 1.7 kg (99% CI, 0.7–2.7) with glyburide, compared with patients receiving diet therapy (each P<.001).2 In the same study, the rate of major hypoglycemic episodes (third-party help or medical intervention necessary) while on therapy was 0.4%/year for chlorpropamide and 0.6%/year for glyburide, compared with 0.1%/year for diet.
Glyburide and chlorpropamide have active metabolites with renal elimination, and they should therefore be used with caution in patients with renal insufficiency. In 1971, the University Group Diabetes Project (UGDP) observed a twofold increase in the rate of cardiovascular death among patients receiving tolbutamide compared with those receiving insulin or placebo.18 This led to a decades long debate on the validity of this conclusion.19 More recently, UKPDS 33 did not demonstrate any increased cardiovascular mortality among patients receiving glyburide or chlorpropamide, and has largely negated this earlier concern.2
Cost. Sulfonylureas are the least expensive oral agents used to treat type 2 diabetes.
TABLE 3
Pharmacological treatments for type 2 diabetes: combination therapies
| Sulfonylureas | Meglitinides | Biguanides | TZDs | AGIs | |
|---|---|---|---|---|---|
| Double combination therapy option* | ✓ | ✓ | |||
| Double combination therapy option† | ✓ | ✓ | |||
| Double combination therapy option | ✓ | ✓ | |||
| Double combination therapy option | ✓ | ✓ | |||
| Double combination therapy option | ✓ | ✓ | |||
| Double combination therapy option | ✓ | ✓ | |||
| Triple combination therapy option | ✓ | ✓ | ✓ | ||
| Triple combination therapy option | ✓ | ✓ | ✓ | ||
| If therapeutic goals are not met using the above combinations; switch to insulin with or without oral agent. | |||||
| *Available as Glucovance (metformin/glyburide) or as Metaglip (metformin/glipizide) | |||||
| † Available as Avandamet (rosiglitazone/metformin) | |||||
Non-sulfonylurea secretagogues
Like sulfonylureas, the non-sulfonylurea secretagogues (non-SU), repaglinide and nateglinide, stimulate beta cells to increase insulin secretion. However, the non-SU agents mediate their action through a different, adjacent site on the “sulfonylurea receptor.” Comparatively, the non-SU agents have a faster onset of action (20 minutes), shorter half-life (about 1.0–1.5 hours), and greater effects on postprandial glucose excursions than do sulfonylureas.20 In contrast to the sulfonylureas, the extent of insulin release with non-SU agents is glucose dependent, and therefore they may have less risk of hypoglycemia several hours after meals.15
A group of metabolic abnormalities that increase cardiovascular risk has been recognized since 1988 and has been given many names—Syndrome X, insulin resistance syndrome, dysmetabolic syndrome, The Deadly Quartet.73 The National Cholesterol Education Program Adult Treatment Panel III recently recodified this syndrome as shown below. The principles for diet and exercise discussed in this article also apply to the goals of reducing obesity and physical inactivity in the metabolic syndrome, and preliminary data suggest a reduction in the risk for type 2 diabetes (NNT per year=27; P=.000174) and for cardiovascular disease.75
| Risk factor | Defining level |
|---|---|
| Abdominal obesity | Waist circumference |
| Men | >102 cm (>40 in) |
| Women | >88 cm (>35 in) |
| Triglycerides | ≥150 mg/dL |
| HDL cholesterol | |
| Men | <40 mg/dL |
| Women | <50 mg/dL |
| Blood pressure | ≥130/85 mm Hg |
| Fasting glucose | ≥110 mg/dL |
Repaglinide lowers the A1c level by 1.7–1.9 percentage points, similar in efficacy to sulfonylureas. Nateglinide appears somewhat less efficacious and lowers A1c by 0.6–1.0 percentage points.15 Nateglinide was significantly less effective than glyburide at lowering A1c levels and the fasting plasma glucose in one 24-week study. Non-SUs added to sulfonylureas produce no additional benefit in glycemic control. The effect of non-SUs on microvascular or macrovascular endpoints is unknown.
Adverse effects. Hypoglycemia is the primary adverse effect of non-SUs. Confirmed hypoglycemia (plasma glucose <60 mg/dL) was observed in 2.4% of patients taking nateglinide compared with 0.4% of those receiving placebo. Mild or moderate hypoglycemia occurred in 16% of repaglinide patients, 20% of glyburide patients, and 19% of glipizide patients in one-year comparative studies. Further comparative studies are needed to determine if non-SUs produce significantly less hypoglycemia and weight gain than sulfonylureas.
Cost. Non-SUs must be dosed 3 times daily at the start of meals. One relative disadvantage is their increased cost compared with sulfonylureas.
Biguanides
The only biguanide marketed in the US is metformin. Its primary action is to inhibit hepatic glucose production and, to a much lesser extent, enhance insulin sensitivity in peripheral tissues.21 Metformin does not stimulate insulin secretion and does not cause hypoglycemia when used as monotherapy, but it can potentiate hypoglycemia in combination with insulin or insulin secretagogues.
Metformin is similar in efficacy to the sulfonylureas. It lowers A1c by 1.5–2.0 percentage points and fasting plasma glucose by 60–80 mg/dL. Its antihyperglycemic efficacy is independent of patient age, duration of diabetes, or BMI.22
In the UKPDS 34 study, a subgroup of obese patients was randomized to receive intensive control (group 1, metformin; group 2, a sulfonylurea or insulin) or conventional diet therapy (group 3). Despite a similar reduction in the A1c level between the 2 intensive-treatment groups, patients treated with metformin had a 32% reduction for any diabetes-related endpoint (95% CI, 13–47; P=.002), 43% fewer diabetes-related deaths (95% CI, 9–63; P=.017), and a 36% reduction in all cause mortality, compared with the diet therapy group (95% CI, 9–55; P=.011).23
Metformin also showed significant benefit when compared with patients receiving sulfonylurea or insulin (group 2). The absolute risk of any diabetes endpoint was 29.8 vs. 40.1 (events per 1000 patient-years; P=.0034), all-cause mortality (13.5 vs 18.9; P=.021), and stroke (3.3 vs 6.2; P=.032), respectively, for metformin vs sulfonylurea or insulin (group 2). Thus, metformin is the only oral hypoglycemic agent proven to reduce macrovascular risk in overweight patients with type 2 diabetes. For perspective, in overweight patients, metformin significantly reduced all-cause mortality (NNT per year=141; 95% CI, 115–183; P=.011), and any diabetes-related outcome (NNT per year=74; 95% CI, 63–90; P=.0023), compared with diet alone.23,24
Metformin induces weight loss (2–3 kg), preferentially involving adipose tissue in obese patients with type 2 diabetes over 4 to 6 months.22,25 In UKPDS 34, weight gain was similar among those treated with metformin and diet (approximately 2 kg); weight gain over 10 years was less with metformin, however, than with sulfonylurea (approximately 4 kg) or insulin (approximately 6 kg).23 Metformin also significantly improved levels of total cholesterol, low-density lipoprotein (LDL) cholesterol, and triglycerides when compared with glyburide or placebo.22
Risk of lactic acidosis. Lactic acidosis associated with metformin is a rare but serious adverse event, with an estimated prevalence of 3 cases per 100,000.26 The product labeling notes most of these cases have occurred among patients with significant renal insufficiency, including both intrinsic renal disease and renal hypoperfusion. Absolute contraindications include renal disease (serum creatinine ≥1.5 mg/dL [males] and ≥1.4 mg/dL [females]), congestive heart failure requiring pharmacological treatment, and acute or chronic metabolic acidosis. It should also be discontinued at the time of radiologic studies using intravascular iodinated contrast materials.
Additional “precautionary conditions” include age ≥80 years (unless measurement of creatinine clearance demonstrates that renal function is not reduced), hepatic disease, cationic drug use, conditions associated with hypoxia (eg, chronic obstructive pulmonary disease [COPD], acute myocardial infarction, dehydration, sepsis), excessive alcohol intake, and surgery, until patient’s oral intake is resumed.
Is the risk overstated? Despite these extensive precautions, published studies show that metformin is commonly prescribed to patients with absolute contraindications.27,28 One recent study observed that 11.2% of Medicare beneficiaries hospitalized with congestive heart failure and concomitant diabetes were treated with metformin.28 In the absence of advanced renal dysfunction, metformin rarely accumulates in the body,29 and accumulation of metformin is rarely reported as a cause of lactic acidosis.30,31 Rather, tissue hypoxia acts as a trigger in most cases. Metformin should therefore be discontinued whenever tissue hypoxia is suspected.31
A recent systematic review and meta analysis found no evidence that metformin was associated with an increased risk of lactic acidosis if the drug was prescribed under study conditions, taking into account contraindications.32 Refinement and clarification of the risk for lactic acidosis in these various populations is needed, to ensure optimal patient safety and to further assess this highly effective medication.
Common adverse effects associated with metformin are diarrhea and nausea, which can be minimized by administering the drug with meals and slowly titrating the dose, or perhaps by using the extended-release formulation.
Thiazolidinediones
Thiazolidinediones (TZDs) include rosiglitazone and pioglitazone. These agents, like metformin, do not increase insulin secretion but depend on the presence of insulin for their activity. TZDs are agonists at peroxisome-proliferator-activated receptor gamma (PPAR-γ) receptors in peripheral tissues such as skeletal muscle, where they increase glucose uptake.15 Thus, their predominant effect is to decrease insulin resistance.
TZDs have similar antihyperglycemic efficacy as sulfonylureas or metformin. They decrease A1c levels by 0.6–1.9 percentage points and lower fasting plasma glucose levels by 50–80 mg/dL.15 They have a slower onset of action compared with other hypoglycemic drugs, and intervals of 3 to 4 weeks should be allowed between doses before increasing the dosage. TZDs also have favorable effects on lipid levels: HDL concentrations increase and triglyceride concentrations decrease with their use.33 It is not known whether they decrease macrovascular or microvascular complications, although such studies are underway.
Adverse effects. TZDs are typically well tolerated, though weight gain of 1–3 kg, edema (4%–5%) and anemia (1%–2%) can occur. Weight gain and edema are more pronounced when TZDs are used in combination with insulin. Anemia is likely due to increased plasma volume rather than any significant hematological effect.
Due to adverse events related to volume expansion, TZDs are not recommended for patients with New York Heart Association class III or IV heart failure. A recent consensus statement from the American Heart Association and the ADA stresses that before administering TZD treatment, the physician should explore the possible presence of cardiac disease, use of other drugs that cause fluid retention, and the pathogenesis of any existing edema or dyspnea.34
Although troglitazone was removed from the market due to its association with hepatocellular injury, pioglitazone and rosiglitazone are not as convincingly associated with liver injury.15 In preapproval clinical studies, less than 0.5% of patients treated with rosiglitzone and pioglitazone had elevations in alanine transaminase (ALT) >3 times the upper limit of normal.
The incidence of hepatitis or acute liver failure from troglitazone was compared with rosiglitazone, pioglitazone, metformin, and glyburide, by analysis of spontaneously reported adverse events to the Food and Drug Administration (FDA) MEDWATCH database during the first 15 months of marketing of each drug.35,36 The incidence of hepatitis per million prescriptions was 21.5, 14.7, 9.4, 2.9, and 4.1, respectively, while the incidence of acute liver failure per 100,000 prescriptions was 4.6, 0.9, 0.8, 0.2, and 0. It appears that postmarketing data support preclinical studies, in that the incidence of acute liver failure is an order of magnitude higher for troglitazone vs. other TZDs.35 However, the FDA recommends avoiding their use in patients with baseline ALT levels >2.5 times the upper limit of normal. The FDA recently reduced the recommended frequency for ALT monitoring for pioglitazone (and is currently considering the same for rosiglitazone). Serum ALT is recommended prior to initiation and then periodically thereafter.
Cost. TZDs are expensive relative to other hypoglycemic agents.
α-glucosidase inhibitors
The α-glucosidase inhibitors (AGIs), acarbose and miglitol, act through competitive, reversible inhibition of membrane-bound intestinal α-glucosidase, which hydrolyzes complex carbohydrates to glucose and other monosaccharides. This inhibition delays glucose absorption and decreases postprandial hyperglycemia.37 Thus, they have a nonsystemic mechanism of action.
These agents cause a modest reduction in the A1c level (0.5–1.0 percentage points) and are thus less effective than sulfonylureas, metformin, or TZDs. They do not reduce fasting plasma glucose levels, but reduce postprandial hyperglycemia by 50 mg/dL.38 No long-term studies have evaluated whether AGIs reduce diabetes-related macrovascular or microvascular outcomes.
Adverse effects. While AGIs are virtually free of serious toxicities, patient tolerability can be a problem due to adverse gastrointestinal effects. In indirect comparisons from placebo-controlled trials, patients treated with miglitol and acarbose commonly reported abdominal pain (11.7%, 19%), diarrhea (28.7%, 31%), and flatulence (41.5%, 74%), respectively. Systemic accumulation of AGIs has been shown to increase in proportion to the degree of renal insufficiency, and their use is not recommended for patients with serum creatinine >2.0 mg/dL. However, whether such patients are at greater risk of any toxicity is unknown. Acarbose at doses above 100 mg 3 times daily has been associated with elevated serum transaminase levels; however, this risk appears negligible at standard doses.
Insulin
Insulin is the oldest therapy for diabetes, and it has no upper dose limit.39 It increases insulin levels and can reduce A1c levels by 1.5 to 2.5 percentage points. Though half of diabetes patients need insulin eventually for optimal control, historically it has been introduced late in the disease process unless patients have severe hyperglycemia (fasting blood sugar >350 mg/dL) or ketonuria.38 However, it is effective in gaining initial control, decreasing gluconeogenesis and increasing glucose uptake. Disadvantages are weight gain, hypoglycemia, and patient reluctance to give injections.
When insulin is indicated. Patients who exhibit persistent hyperglycemia despite oral hypoglycemic therapy may stop the oral drug(s) and begin insulin. By combining insulin with oral therapy, lower insulin doses may be used to achieve desired control vs using insulin alone.40 For some patients a basal supplement of insulin may be sufficient and can be given as a single dose at bedtime, without an oral hypoglycemic drug.41
Insulin regimens. Various insulin regimens are available: very rapid acting (lispro and aspart), rapid acting (regular), intermediate acting (isophane insulin [NPH] and lente) and very long acting (ultralente and glargine). Glargine insulin (Lantus) has more predictable absorption than NPH, lente, and ultralente. Lantus, compared with NPH, has been associated with less nocturnal and postprandial hypoglycemia.38,42,43 This is consistent with the peakless and longer duration of glargine compared with NPH.44 A recent randomized controlled trial demonstrated that morning insulin glargine lowered A1c levels more than a bedtime dose of NPH (–1.24 vs –0.84; 95% CI, 0.23%–0.58%) or a bedtime dose of glargine (–1.24 vs –0.96%; 95% CI, 0.11%–0.46%).45 Glargine’s only relative disadvantage is increased cost.
Combination products. Combination insulin options are 70 NPH/30 regular, 50 NPH/50 regular, and 75 lispro protamine/25 lispro. Many combinations of insulin regimens have been used successfully. The typical range of insulin needed for monotherapy is 0.4–1 U/kg/d. Once-daily injection of intermediate acting or long acting insulins at bedtime or before breakfast, once-daily or twice-daily combinations of intermediate and rapid acting insulins, and more complex regimens have been used to good effect.
Using prandial insulin at each meal with separate basal insulin adds flexibility to meal times and doses administered.43 With multiple-dose intensive insulin therapy, a basal dose suppresses hepatic glucose output and the bolus doses enhance postprandial glucose uptake. This intensive insulin treatment reduces mortality among critically ill patients in surgical intensive care units and for those with acute myocardial infarction.46,47 An algorithm for using progressive therapy in diabetes mellitus is shown in Figure 2.48
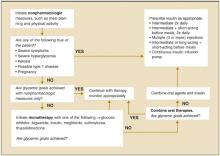
FIGURE 2
ADA recommendations for the treatment of type 2 diabetes
Combination Therapy
Over time glycemic control becomes more difficult, even with maximum monotherapy for patients with healthy lifestyles. It was shown in UKPDS 49 that monotherapy with sulfonylurea, metformin, or insulin eventually fails in most cases—by 3 years after diagnosis, about 50% of patients need more than monotherapy; 75% by 9 years.49 In UKPDS 33, the median A1c level increased steadily over 10 years with both conventional therapy and intensive therapy (Figure 3).2
Several options are available when monotherapy fails. Based on expert opinion, the principle is to combine drugs with different mechanisms of action to achieve an additive effect for glycemic control. Combination products may simplify the treatment regimen and improve adherence. In many instances, they may also cost less.50
Successful combinations. The combination of sulfonylurea and metformin has proven effective in many studies.22,51,52 One study showed that initial treatment with glyburide/metformin improved glycemic control better than either glyburide or metformin monotherapy (SOR: A).53,54 The addition of the non-SU secretagogues repaglinide and nateglinide to metformin significantly improved glycemic control, with repaglinide showing superiority over nateglinide.55 A TZD added to a sulfonylurea has also significantly improved A1c and fasting blood sugar results.56 Patients whose diabetes was inadequately controlled with diet alone or diet plus a sulfonylurea showed improvement with the addition of the AGI miglitol, compared with addition of placebo.57 The AGI acarbose has shown to be an effective addition to diet, metformin, sulfonylurea, and insulin.58 A TZD added to metformin has also been shown to improve glycemic control.59 A non-SU added to patients inadequately controlled with a TZD has also been effective.60
The early addition of insulin when maximal sulfonylurea therapy is inadequate has been effective.61-63 When introducing insulin, a nighttime regimen of NPH or glargine, 10 units at bedtime, is an appropriate dose (SOR: C). This is easier and less costly than often assumed, and helps improve glycemic control.64 Most patients require combination therapy as their disease progresses.39
FIGURE 3
Glycemic control in type 2 DM
Improving Outcomes
Cumulative survey data reveal a wide gap between guideline recommendations and the care patients receive.65 One study showed that physicians initiated treatment changes only after the A1c level had reached 9.0% or higher instead of the 8.0% level recommended by ADA.66 How can the quality of management be improved?
In private practices and institutions, many interventions have been shown to improve outcomes in diabetes mellitus. Education measures work, and they include chart audits, reminder cards, pharmacist collaboration, flow sheets, and nursing initiatives.67,68 Effective disease-management programs have also used clinical guidelines, outcomes reporting, coverage of glucose meters and strips, and the support of clinical leadership.69
Computerized systems that track patients and recommended laboratory tests have improved screening rates and glycemic and blood pressure control.70 Monitoring patients’ readiness to change has allowed targeted education to improve A1c levels.71 Continuity of care has also improved the quality of disease control by increasing adherence to recommended tests and exams.72
Acknowlegments
The authors thank Marie Hamer, RN, for her continuous diabetes quality improvement efforts and Jean Camarata for her editorial and reference acquisition assistance.
Corresponding author
John E. Sutherland, MD, Northeast Iowa Family Practice Residency Program, University of Iowa College of Medicine, 2055 Kimball Avenue, Waterloo, Iowa 50702. E-mail: jsutherl@neimef.org.
- Self-monitoring of blood glucose is an integral component of diabetes therapy and should always be included in the management plan (SOR:C).
- Medical nutrition therapy should be individualized, preferably by a registered dietitian familiar with diabetes (SOR:B).
- A regular physical activity program is recommended for all patients with diabetes who are capable of participating (SOR:B).
- When a monotherapy fails, combine drugs with different mechanisms of action to achieve an additive effect (SOR:A).
- The combination of sulfonylurea and metformin has proven effective in many studies. One showed that initial treatment with glyburide/metformin improved glycemic control better than either glyburide or metformin monotherapy (SOR: A).
Glycemic control in diabetes begins with a patient’s adherence to several nonpharmacologic measures. Without such a commitment, success in controlling the disease will be difficult to achieve, and otherwise appropriate drug therapy will be hindered.
Most antidiabetic agents comparably reduce glycosylated hemoglobin (A1c) levels. However, a particular agent may be preferred depending on a patient’s characteristics. And some circumstances call for combination therapy. This article reviews the advantages and disadvantages of the many pharmacologic treatments for glucose control and hyperglycemia in type 2 diabetes.
Benefits Of Diabetes Control
The benefits of diabetes control are detailed in this issue of THE JOURNAL OF FAMILY PRACTICE (“Strategies to reduce complications in type 2 diabetes,” pages 366–374). For every percentage-point reduction in hemoglobin A1c, it is possible to achieve a 22% to 35% reduction in microvascular complications.1,2 Cardiovascular disease can be reduced in patients with diabetes by treating hypertension3,4 and hyperlipidemia, prescribing aspirin therapy, using angiotensin-converting enzyme (ACE) inhibitors, and with smoking cessation.5,6
Targets For Glycemic Control
The American Diabetes Association’s (ADA) recommended targets for glycemic control are a preprandial blood glucose level of 80–120 mg/dL, a bedtime blood glucose level of 100–140 mg/dL, and a hemoglobin A1c level of <7% (with a level of >8% requiring additional measures). Hemoglobin A1c is the best determinant of glycemic exposure, and its mean value is a nationally recognized indicator of how well diabetes is being managed.7 The American College of Endocrinology has adopted a more aggressive approach by designating an A1c level of 6.5% as both a target and action level.8
Self-monitoring of blood glucose
Self-monitoring of blood glucose (SMBG) is an integral component of diabetes therapy (strength of recommendation [SOR]: C) and should always be included in the management plan (SOR: C). The optimal frequency and timing of SMBG for type 2 diabetes is not known, but they should be sufficient to facilitate reaching glucose goals. The A1c test should be performed at least semi-annually for patients with stable glycemic control, and quarterly for patients not meeting glycemic goals or those who are changing therapy. A1c levels and mean plasma glucose levels can be approximately correlated (Table 1).7
TABLE 1
Correlation between hemoglobin A1clevels and mean plasma glucose levels
| Hemoglobin A1c(%) | Mean plasma glucose (mg/dL) |
|---|---|
| 6 | 135 |
| 7 | 170 |
| 8 | 205 |
| 9 | 240 |
| 10 | 275 |
| 11 | 310 |
| 12 | 345 |
Nonpharmacologic Therapy
Nonpharmacologic measures remain the cornerstone of managing type 2 diabetes. Hyperglycemia adversely and reversibly affects both insulin resistance and insulin secretion. Improvement in glycemic control can occur through dietary modification and regular exercise.
A recent meta-analysis of randomized controlled trials of diabetes patient education observed a net decrease in HbA1c of 0.32% in intervention groups vs control.9 Interventions that included a face-to-face delivery, cognitive reframing teaching method, and exercise content were more likely to improve glycemic control.
Education
Lifestyle changes involving diet, exercise, and usually weight loss are key to effective management of diabetes. If patients are to change their behavior, they must be given detailed training.6 Self-management also necessitates that patients engage in problem solving. This requires that each aspect of the management plan is understood and agreed upon by the patient and providers, and that the goals and treatment plan are individualized and reasonable.
Diet: recommend soluble fiber, reduce calories
Medical nutrition therapy should be individualized and preferably provided by a registered dietitian familiar with diabetes (SOR: B). The goals of nutrition therapy, according to the ADA, are to attain recommended body weight and prevent or reverse obesity. The means of achieving these goals are nutrition assessment and modification of nutrient intake and lifestyle through healthy food choices and physical activity.7
A high intake of dietary fiber (particularly the soluble type) above the level recommended by the ADA improves glycemic control, decreases hyperinsulinemia, and lowers plasma lipid concentrations.10
Hypocaloric diets cause glucose plasma levels to fall, in some cases to a normal level with a weight loss of even 5 to 10 pounds.7,11 Hypoglycemic medications are of course most effective in nonobese persons. But effectiveness is also improved if weight that is gained can be limited. Despite the clear benefit of weight loss, only a few patients are able to attain and maintain substantial weight loss. Maintenance of a reduced or elevated body weight is associated with compensatory changes in energy expenditure, which oppose the maintenance of body weight that is different from the usual weight.13 Part of the individualization of therapy is respect of personal and cultural preferences, lifestyles, and financial considerations.
Physical activity: a little goes a long way
A regular physical activity program is recommended for all patients with diabetes who are capable of participating (SOR: B).7 It improves blood glucose control, reduces cardiovascular risk factors, aids weight loss, and enhances well being.7 A recently published prospective cohort study showed that walking at least 2 hours a week was associated with a 39% lower all-cause mortality (hazard rate ratio [HRR], 0.61; 95% CI, 0.48–0.78) and a 34% lower cardiovascular mortality (HRR, 0.66; 95% CI, 0.45–0.96) across a diverse spectrum of adults with diabetes. The NNT (to prevent 1 death per year) is 61 for patients who walk at least 2 hours/week.14
In prescribing a physical activity plan for a patient, consider cardiovascular disease risk factors or complications to minimize the risk of untoward events. Micro- and macrovascular disease are of course prevalent among persons with diabetes, often resulting in functional limitations that make exercise more difficult.
Other priorities
Other recommended components of care include daily aspirin use, foot care exams, tobacco cessation, pneumococcal and influenza vaccinations, and an annual dilated retinal exam.
Pharmacologic Therapy
The coexisting defects in type 2 diabetes mellitus are as follows:
- resistance to insulin action in muscle
- defective pancreatic insulin secretion
- unrestrained hepatic glucose production, aggravated by increased lipolysis in adipose tissue.
Drug therapy is aimed at each of these defects, and also at reducing carbohydrate absorption in the small intestine (Figure 1). As far as antihyperglycemic effect is concerned, no one category of antidiabetic agent is favored over another.15 Except for nateglinide and α-glucosidase inhibitors (AGIs), each of the drug categories leads to a similar reduction in A1c.16 However, patient characteristics may lead to selection of a particular agent. Table 2 summarizes oral treatment options, their relative advantages and costs.
FIGURE 1
Drug therapies for coexisting defects in type 2 diabetes
TABLE 2
Pharmacologic treatments for type 2 diabetes: monotherapies
| Target population | Advantages | Disadvantages | Dosing | Cost* |
|---|---|---|---|---|
| Sulfonylureas | ||||
| Recent type 2 DM diagnosis | Rapid FPG reduction | Weight gain | Glyburide: 1.25–20 mg once or twice daily (micronized, 0.75–12 mg once or twice daily) | $22.80 (5 mg, #120) |
| Type 2 DM <5 years duration | Low cost | Increased risk of hypoglycemia | Glipizide: 2.5–40 mg once or twice daily (extended-release, 2.5–20 mg once daily) | $14.66 (10 mg, #120) |
| Glimepiride: 1–8 mg once daily | $51.98 (10 mg, #60) | |||
| $57.98 (4 mg, #60) | ||||
| Non-sulfonylurea secretagogues (meglitinides) | ||||
| Recent type 2 DM diagnosis | Reduced risk of hypoclycemia | Higher cost | Nateglinide: 60–120 mg 3 times daily | $85.99 (120mg, #90) |
| Elevated PPG | Short-acting | Frequent dosing | Repaglinide: 0.5–4 mg 3 or 4 times daily | $218.06 (2 mg, #240) |
| Meal-adjusted dosing | ||||
| Biguanides | ||||
| Overweight/obese | No weight gain | GI side effects | Metformin: 500–1000 mg 2 or 3 times daily | $77.99 (850 mg, #90) |
| Insulin resistant | Reduced risk of hypoglycemia | High cost | Metformin XR: 1000–2000 mg once or twice daily | $89.98 (500 mg, #120) |
| Rare lactic acidosis | ||||
| TZDs | ||||
| Insulin resistant | Reduced amount of insulin | High cost | Rosiglitazone: 4–8 mg once or twice daily | $135.99 (8 mg, #30) |
| Overweight/obese | Reduced risk of hypoglycemia | Weight gain | Pioglitazone: 15–45 mg once daily | $153.99 (45 mg, #30) |
| Slow onset of action | ||||
| Liver toxicity | ||||
| AGIs | ||||
| Elevated PPG | Reduced risk of hypoglycemia | High cost | Acarbose: 50–100 mg 3 times daily | $67.99 (100 mg, #90) |
| Contraindications to other agents | Non-systemic action | GI side effects | Miglitol: 50–100 mg 3 times daily | $66.99 (100 mg, #90) |
| *Drug costs for 30 days’ supply of maximum daily dosage. From www.drugstore.com, December 2003. | ||||
| DM, diabetes mellitus; TZD, thiazolidinediones; AGT, a-glucosidase inhibitors; FPG, fasting plasma glucose; PPG, postprandial glucose; GI, gastrointestinal | ||||
Sulfonylureas
Sulfonylureas directly increase insulin secretion by binding to the sulfonylurea receptor on pancreatic beta cells; they provide a relatively quick onset of action. First-generation sulfonylureas (eg, tolbutamide, chlorpropamide) and second-generation sulfonylureas (eg, glyburide, glipizide, glimepiride) are equivalent in their maximum hypoglycemic effect.17
Second-generation agents are used more commonly than first-generation. They all contain the sulfonylurea moiety, but different chemical substitutions in the basic molecule change pharmacokinetics, resulting in different durations of action.17 Second-generation agents are probably safer than first-generation drugs, being less likely to cause hyponatremia, disulfiram-like reactions, or prolonged hypoglycemia.18
At maximal doses, sulfonylureas lower A1c levels by 1–2 percentage points and fasting plasma glucose concentrations by 60–70 mg/dL;15 however, the glucose lowering effect typically plateaus after half the maximal recommended dose is reached. Sulfonylureas have no consistent effect on dyslipidemia. In UK Prospective Diabetes Study (UKPDS) 33, though improved glycemic control with sulfonylureas (or insulin) led to a 25% reduction in microvascular endpoints (mostly less retinal photocoagulation) (P<.01), sulfonylureas (or insulin) did not significantly reduce death or all-cause mortality compared with diet treatment.2
Adverse effects. The primary adverse effects of sulfonylureas are weight gain and hypoglycemia. In UKPDS 33, weight gain at 10 years was 2.6 kg (99% confidence interval [CI], 1.6–3.6) with chlorpropamide and 1.7 kg (99% CI, 0.7–2.7) with glyburide, compared with patients receiving diet therapy (each P<.001).2 In the same study, the rate of major hypoglycemic episodes (third-party help or medical intervention necessary) while on therapy was 0.4%/year for chlorpropamide and 0.6%/year for glyburide, compared with 0.1%/year for diet.
Glyburide and chlorpropamide have active metabolites with renal elimination, and they should therefore be used with caution in patients with renal insufficiency. In 1971, the University Group Diabetes Project (UGDP) observed a twofold increase in the rate of cardiovascular death among patients receiving tolbutamide compared with those receiving insulin or placebo.18 This led to a decades long debate on the validity of this conclusion.19 More recently, UKPDS 33 did not demonstrate any increased cardiovascular mortality among patients receiving glyburide or chlorpropamide, and has largely negated this earlier concern.2
Cost. Sulfonylureas are the least expensive oral agents used to treat type 2 diabetes.
TABLE 3
Pharmacological treatments for type 2 diabetes: combination therapies
| Sulfonylureas | Meglitinides | Biguanides | TZDs | AGIs | |
|---|---|---|---|---|---|
| Double combination therapy option* | ✓ | ✓ | |||
| Double combination therapy option† | ✓ | ✓ | |||
| Double combination therapy option | ✓ | ✓ | |||
| Double combination therapy option | ✓ | ✓ | |||
| Double combination therapy option | ✓ | ✓ | |||
| Double combination therapy option | ✓ | ✓ | |||
| Triple combination therapy option | ✓ | ✓ | ✓ | ||
| Triple combination therapy option | ✓ | ✓ | ✓ | ||
| If therapeutic goals are not met using the above combinations; switch to insulin with or without oral agent. | |||||
| *Available as Glucovance (metformin/glyburide) or as Metaglip (metformin/glipizide) | |||||
| † Available as Avandamet (rosiglitazone/metformin) | |||||
Non-sulfonylurea secretagogues
Like sulfonylureas, the non-sulfonylurea secretagogues (non-SU), repaglinide and nateglinide, stimulate beta cells to increase insulin secretion. However, the non-SU agents mediate their action through a different, adjacent site on the “sulfonylurea receptor.” Comparatively, the non-SU agents have a faster onset of action (20 minutes), shorter half-life (about 1.0–1.5 hours), and greater effects on postprandial glucose excursions than do sulfonylureas.20 In contrast to the sulfonylureas, the extent of insulin release with non-SU agents is glucose dependent, and therefore they may have less risk of hypoglycemia several hours after meals.15
A group of metabolic abnormalities that increase cardiovascular risk has been recognized since 1988 and has been given many names—Syndrome X, insulin resistance syndrome, dysmetabolic syndrome, The Deadly Quartet.73 The National Cholesterol Education Program Adult Treatment Panel III recently recodified this syndrome as shown below. The principles for diet and exercise discussed in this article also apply to the goals of reducing obesity and physical inactivity in the metabolic syndrome, and preliminary data suggest a reduction in the risk for type 2 diabetes (NNT per year=27; P=.000174) and for cardiovascular disease.75
| Risk factor | Defining level |
|---|---|
| Abdominal obesity | Waist circumference |
| Men | >102 cm (>40 in) |
| Women | >88 cm (>35 in) |
| Triglycerides | ≥150 mg/dL |
| HDL cholesterol | |
| Men | <40 mg/dL |
| Women | <50 mg/dL |
| Blood pressure | ≥130/85 mm Hg |
| Fasting glucose | ≥110 mg/dL |
Repaglinide lowers the A1c level by 1.7–1.9 percentage points, similar in efficacy to sulfonylureas. Nateglinide appears somewhat less efficacious and lowers A1c by 0.6–1.0 percentage points.15 Nateglinide was significantly less effective than glyburide at lowering A1c levels and the fasting plasma glucose in one 24-week study. Non-SUs added to sulfonylureas produce no additional benefit in glycemic control. The effect of non-SUs on microvascular or macrovascular endpoints is unknown.
Adverse effects. Hypoglycemia is the primary adverse effect of non-SUs. Confirmed hypoglycemia (plasma glucose <60 mg/dL) was observed in 2.4% of patients taking nateglinide compared with 0.4% of those receiving placebo. Mild or moderate hypoglycemia occurred in 16% of repaglinide patients, 20% of glyburide patients, and 19% of glipizide patients in one-year comparative studies. Further comparative studies are needed to determine if non-SUs produce significantly less hypoglycemia and weight gain than sulfonylureas.
Cost. Non-SUs must be dosed 3 times daily at the start of meals. One relative disadvantage is their increased cost compared with sulfonylureas.
Biguanides
The only biguanide marketed in the US is metformin. Its primary action is to inhibit hepatic glucose production and, to a much lesser extent, enhance insulin sensitivity in peripheral tissues.21 Metformin does not stimulate insulin secretion and does not cause hypoglycemia when used as monotherapy, but it can potentiate hypoglycemia in combination with insulin or insulin secretagogues.
Metformin is similar in efficacy to the sulfonylureas. It lowers A1c by 1.5–2.0 percentage points and fasting plasma glucose by 60–80 mg/dL. Its antihyperglycemic efficacy is independent of patient age, duration of diabetes, or BMI.22
In the UKPDS 34 study, a subgroup of obese patients was randomized to receive intensive control (group 1, metformin; group 2, a sulfonylurea or insulin) or conventional diet therapy (group 3). Despite a similar reduction in the A1c level between the 2 intensive-treatment groups, patients treated with metformin had a 32% reduction for any diabetes-related endpoint (95% CI, 13–47; P=.002), 43% fewer diabetes-related deaths (95% CI, 9–63; P=.017), and a 36% reduction in all cause mortality, compared with the diet therapy group (95% CI, 9–55; P=.011).23
Metformin also showed significant benefit when compared with patients receiving sulfonylurea or insulin (group 2). The absolute risk of any diabetes endpoint was 29.8 vs. 40.1 (events per 1000 patient-years; P=.0034), all-cause mortality (13.5 vs 18.9; P=.021), and stroke (3.3 vs 6.2; P=.032), respectively, for metformin vs sulfonylurea or insulin (group 2). Thus, metformin is the only oral hypoglycemic agent proven to reduce macrovascular risk in overweight patients with type 2 diabetes. For perspective, in overweight patients, metformin significantly reduced all-cause mortality (NNT per year=141; 95% CI, 115–183; P=.011), and any diabetes-related outcome (NNT per year=74; 95% CI, 63–90; P=.0023), compared with diet alone.23,24
Metformin induces weight loss (2–3 kg), preferentially involving adipose tissue in obese patients with type 2 diabetes over 4 to 6 months.22,25 In UKPDS 34, weight gain was similar among those treated with metformin and diet (approximately 2 kg); weight gain over 10 years was less with metformin, however, than with sulfonylurea (approximately 4 kg) or insulin (approximately 6 kg).23 Metformin also significantly improved levels of total cholesterol, low-density lipoprotein (LDL) cholesterol, and triglycerides when compared with glyburide or placebo.22
Risk of lactic acidosis. Lactic acidosis associated with metformin is a rare but serious adverse event, with an estimated prevalence of 3 cases per 100,000.26 The product labeling notes most of these cases have occurred among patients with significant renal insufficiency, including both intrinsic renal disease and renal hypoperfusion. Absolute contraindications include renal disease (serum creatinine ≥1.5 mg/dL [males] and ≥1.4 mg/dL [females]), congestive heart failure requiring pharmacological treatment, and acute or chronic metabolic acidosis. It should also be discontinued at the time of radiologic studies using intravascular iodinated contrast materials.
Additional “precautionary conditions” include age ≥80 years (unless measurement of creatinine clearance demonstrates that renal function is not reduced), hepatic disease, cationic drug use, conditions associated with hypoxia (eg, chronic obstructive pulmonary disease [COPD], acute myocardial infarction, dehydration, sepsis), excessive alcohol intake, and surgery, until patient’s oral intake is resumed.
Is the risk overstated? Despite these extensive precautions, published studies show that metformin is commonly prescribed to patients with absolute contraindications.27,28 One recent study observed that 11.2% of Medicare beneficiaries hospitalized with congestive heart failure and concomitant diabetes were treated with metformin.28 In the absence of advanced renal dysfunction, metformin rarely accumulates in the body,29 and accumulation of metformin is rarely reported as a cause of lactic acidosis.30,31 Rather, tissue hypoxia acts as a trigger in most cases. Metformin should therefore be discontinued whenever tissue hypoxia is suspected.31
A recent systematic review and meta analysis found no evidence that metformin was associated with an increased risk of lactic acidosis if the drug was prescribed under study conditions, taking into account contraindications.32 Refinement and clarification of the risk for lactic acidosis in these various populations is needed, to ensure optimal patient safety and to further assess this highly effective medication.
Common adverse effects associated with metformin are diarrhea and nausea, which can be minimized by administering the drug with meals and slowly titrating the dose, or perhaps by using the extended-release formulation.
Thiazolidinediones
Thiazolidinediones (TZDs) include rosiglitazone and pioglitazone. These agents, like metformin, do not increase insulin secretion but depend on the presence of insulin for their activity. TZDs are agonists at peroxisome-proliferator-activated receptor gamma (PPAR-γ) receptors in peripheral tissues such as skeletal muscle, where they increase glucose uptake.15 Thus, their predominant effect is to decrease insulin resistance.
TZDs have similar antihyperglycemic efficacy as sulfonylureas or metformin. They decrease A1c levels by 0.6–1.9 percentage points and lower fasting plasma glucose levels by 50–80 mg/dL.15 They have a slower onset of action compared with other hypoglycemic drugs, and intervals of 3 to 4 weeks should be allowed between doses before increasing the dosage. TZDs also have favorable effects on lipid levels: HDL concentrations increase and triglyceride concentrations decrease with their use.33 It is not known whether they decrease macrovascular or microvascular complications, although such studies are underway.
Adverse effects. TZDs are typically well tolerated, though weight gain of 1–3 kg, edema (4%–5%) and anemia (1%–2%) can occur. Weight gain and edema are more pronounced when TZDs are used in combination with insulin. Anemia is likely due to increased plasma volume rather than any significant hematological effect.
Due to adverse events related to volume expansion, TZDs are not recommended for patients with New York Heart Association class III or IV heart failure. A recent consensus statement from the American Heart Association and the ADA stresses that before administering TZD treatment, the physician should explore the possible presence of cardiac disease, use of other drugs that cause fluid retention, and the pathogenesis of any existing edema or dyspnea.34
Although troglitazone was removed from the market due to its association with hepatocellular injury, pioglitazone and rosiglitazone are not as convincingly associated with liver injury.15 In preapproval clinical studies, less than 0.5% of patients treated with rosiglitzone and pioglitazone had elevations in alanine transaminase (ALT) >3 times the upper limit of normal.
The incidence of hepatitis or acute liver failure from troglitazone was compared with rosiglitazone, pioglitazone, metformin, and glyburide, by analysis of spontaneously reported adverse events to the Food and Drug Administration (FDA) MEDWATCH database during the first 15 months of marketing of each drug.35,36 The incidence of hepatitis per million prescriptions was 21.5, 14.7, 9.4, 2.9, and 4.1, respectively, while the incidence of acute liver failure per 100,000 prescriptions was 4.6, 0.9, 0.8, 0.2, and 0. It appears that postmarketing data support preclinical studies, in that the incidence of acute liver failure is an order of magnitude higher for troglitazone vs. other TZDs.35 However, the FDA recommends avoiding their use in patients with baseline ALT levels >2.5 times the upper limit of normal. The FDA recently reduced the recommended frequency for ALT monitoring for pioglitazone (and is currently considering the same for rosiglitazone). Serum ALT is recommended prior to initiation and then periodically thereafter.
Cost. TZDs are expensive relative to other hypoglycemic agents.
α-glucosidase inhibitors
The α-glucosidase inhibitors (AGIs), acarbose and miglitol, act through competitive, reversible inhibition of membrane-bound intestinal α-glucosidase, which hydrolyzes complex carbohydrates to glucose and other monosaccharides. This inhibition delays glucose absorption and decreases postprandial hyperglycemia.37 Thus, they have a nonsystemic mechanism of action.
These agents cause a modest reduction in the A1c level (0.5–1.0 percentage points) and are thus less effective than sulfonylureas, metformin, or TZDs. They do not reduce fasting plasma glucose levels, but reduce postprandial hyperglycemia by 50 mg/dL.38 No long-term studies have evaluated whether AGIs reduce diabetes-related macrovascular or microvascular outcomes.
Adverse effects. While AGIs are virtually free of serious toxicities, patient tolerability can be a problem due to adverse gastrointestinal effects. In indirect comparisons from placebo-controlled trials, patients treated with miglitol and acarbose commonly reported abdominal pain (11.7%, 19%), diarrhea (28.7%, 31%), and flatulence (41.5%, 74%), respectively. Systemic accumulation of AGIs has been shown to increase in proportion to the degree of renal insufficiency, and their use is not recommended for patients with serum creatinine >2.0 mg/dL. However, whether such patients are at greater risk of any toxicity is unknown. Acarbose at doses above 100 mg 3 times daily has been associated with elevated serum transaminase levels; however, this risk appears negligible at standard doses.
Insulin
Insulin is the oldest therapy for diabetes, and it has no upper dose limit.39 It increases insulin levels and can reduce A1c levels by 1.5 to 2.5 percentage points. Though half of diabetes patients need insulin eventually for optimal control, historically it has been introduced late in the disease process unless patients have severe hyperglycemia (fasting blood sugar >350 mg/dL) or ketonuria.38 However, it is effective in gaining initial control, decreasing gluconeogenesis and increasing glucose uptake. Disadvantages are weight gain, hypoglycemia, and patient reluctance to give injections.
When insulin is indicated. Patients who exhibit persistent hyperglycemia despite oral hypoglycemic therapy may stop the oral drug(s) and begin insulin. By combining insulin with oral therapy, lower insulin doses may be used to achieve desired control vs using insulin alone.40 For some patients a basal supplement of insulin may be sufficient and can be given as a single dose at bedtime, without an oral hypoglycemic drug.41
Insulin regimens. Various insulin regimens are available: very rapid acting (lispro and aspart), rapid acting (regular), intermediate acting (isophane insulin [NPH] and lente) and very long acting (ultralente and glargine). Glargine insulin (Lantus) has more predictable absorption than NPH, lente, and ultralente. Lantus, compared with NPH, has been associated with less nocturnal and postprandial hypoglycemia.38,42,43 This is consistent with the peakless and longer duration of glargine compared with NPH.44 A recent randomized controlled trial demonstrated that morning insulin glargine lowered A1c levels more than a bedtime dose of NPH (–1.24 vs –0.84; 95% CI, 0.23%–0.58%) or a bedtime dose of glargine (–1.24 vs –0.96%; 95% CI, 0.11%–0.46%).45 Glargine’s only relative disadvantage is increased cost.
Combination products. Combination insulin options are 70 NPH/30 regular, 50 NPH/50 regular, and 75 lispro protamine/25 lispro. Many combinations of insulin regimens have been used successfully. The typical range of insulin needed for monotherapy is 0.4–1 U/kg/d. Once-daily injection of intermediate acting or long acting insulins at bedtime or before breakfast, once-daily or twice-daily combinations of intermediate and rapid acting insulins, and more complex regimens have been used to good effect.
Using prandial insulin at each meal with separate basal insulin adds flexibility to meal times and doses administered.43 With multiple-dose intensive insulin therapy, a basal dose suppresses hepatic glucose output and the bolus doses enhance postprandial glucose uptake. This intensive insulin treatment reduces mortality among critically ill patients in surgical intensive care units and for those with acute myocardial infarction.46,47 An algorithm for using progressive therapy in diabetes mellitus is shown in Figure 2.48
FIGURE 2
ADA recommendations for the treatment of type 2 diabetes
Combination Therapy
Over time glycemic control becomes more difficult, even with maximum monotherapy for patients with healthy lifestyles. It was shown in UKPDS 49 that monotherapy with sulfonylurea, metformin, or insulin eventually fails in most cases—by 3 years after diagnosis, about 50% of patients need more than monotherapy; 75% by 9 years.49 In UKPDS 33, the median A1c level increased steadily over 10 years with both conventional therapy and intensive therapy (Figure 3).2
Several options are available when monotherapy fails. Based on expert opinion, the principle is to combine drugs with different mechanisms of action to achieve an additive effect for glycemic control. Combination products may simplify the treatment regimen and improve adherence. In many instances, they may also cost less.50
Successful combinations. The combination of sulfonylurea and metformin has proven effective in many studies.22,51,52 One study showed that initial treatment with glyburide/metformin improved glycemic control better than either glyburide or metformin monotherapy (SOR: A).53,54 The addition of the non-SU secretagogues repaglinide and nateglinide to metformin significantly improved glycemic control, with repaglinide showing superiority over nateglinide.55 A TZD added to a sulfonylurea has also significantly improved A1c and fasting blood sugar results.56 Patients whose diabetes was inadequately controlled with diet alone or diet plus a sulfonylurea showed improvement with the addition of the AGI miglitol, compared with addition of placebo.57 The AGI acarbose has shown to be an effective addition to diet, metformin, sulfonylurea, and insulin.58 A TZD added to metformin has also been shown to improve glycemic control.59 A non-SU added to patients inadequately controlled with a TZD has also been effective.60
The early addition of insulin when maximal sulfonylurea therapy is inadequate has been effective.61-63 When introducing insulin, a nighttime regimen of NPH or glargine, 10 units at bedtime, is an appropriate dose (SOR: C). This is easier and less costly than often assumed, and helps improve glycemic control.64 Most patients require combination therapy as their disease progresses.39
FIGURE 3
Glycemic control in type 2 DM
Improving Outcomes
Cumulative survey data reveal a wide gap between guideline recommendations and the care patients receive.65 One study showed that physicians initiated treatment changes only after the A1c level had reached 9.0% or higher instead of the 8.0% level recommended by ADA.66 How can the quality of management be improved?
In private practices and institutions, many interventions have been shown to improve outcomes in diabetes mellitus. Education measures work, and they include chart audits, reminder cards, pharmacist collaboration, flow sheets, and nursing initiatives.67,68 Effective disease-management programs have also used clinical guidelines, outcomes reporting, coverage of glucose meters and strips, and the support of clinical leadership.69
Computerized systems that track patients and recommended laboratory tests have improved screening rates and glycemic and blood pressure control.70 Monitoring patients’ readiness to change has allowed targeted education to improve A1c levels.71 Continuity of care has also improved the quality of disease control by increasing adherence to recommended tests and exams.72
Acknowlegments
The authors thank Marie Hamer, RN, for her continuous diabetes quality improvement efforts and Jean Camarata for her editorial and reference acquisition assistance.
Corresponding author
John E. Sutherland, MD, Northeast Iowa Family Practice Residency Program, University of Iowa College of Medicine, 2055 Kimball Avenue, Waterloo, Iowa 50702. E-mail: jsutherl@neimef.org.
1. Vinik AI, Vinik E. Prevention of the complications of diabetes. Am J Manag Care. 2003;9 suppl:S63-S80.
2. UK Prospective Diabetes Study (UKPDS) Group. Intensive blood-glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33). Lancet. 1998;352:837-853.
3. Arauz-Pacheco C, Parrott MA, Raskin P. American Diabetes Association Treatment of hypertension in adults with diabetes. Diabetes Care. 2004;27 (suppl):S65-67.
4. UK Prospective Diabetes Study Group. Tight blood pressure control and risk of macrovascular and microvascular complications in type 2 diabetes: UKPDS 38. BMJ. 1998;317:703-713.
5. Beckman JA, Creager MA, Libby P. Diabetes and atherosclerosis: epidemiology, pathophysiology, and management. JAMA. 2002;287:2570-2581.
6. Kendall DM, Bergenstal RM. Comprehensive management of patients with type 2 diabetes: establishing priorities of care. Am J Manag Care. 2001;7 (suppl):S327-S343.
7. American Diabetes Association. Standards of medical care in Diabetes. Diabetes Care. 2004;27(suppl):15-35.
8. Peterson KA. Diabetes management in the primary care setting: summary. Am J Med. 2002;113(suppl 6A):36S-40S.
9. Ellis SE, Speroff T, Dittus RS, Brown A, Pichert JW, Elasy TA. Diabetes patient education: A meta-analysis and meta-regression. Patient Educ Couns. 2004;52:97-105.
10. Chandalia M, Garg A, Lutjohann D, et al. Beneficial effects of high dietary fiber intake in patients with type 2 diabetes mellitus. N Engl J Med. 2000;342:1392-1398.
11. Hadden DR, Montgomery DAD, Skelly RJ, et al. Maturity onset diabetes mellitus: response to intensive dietary management. Br Med J. 1975;3:276-278.
12. Niskenen LK, Uusitupa MI, Surlund H, et al. Five-year follow-up study on plasma insulin levels in newly diagnosed NIDDM patients and nondiabetic subjects. Diabetes Care. 1009;13:41-48.
13. Leibel R, Rosenbaum M, Hirsch J. Changes in energy expenditure resulting from altered body weight. N Engl J Med. 1995;332:621-628.
14. Gregg EW, Gerzoff RB, Caspersen CJ, et al. Relationship of walking to mortality among US adults with diabetes. Arch Intern Med. 2003;163:1440-1447.
15. Inzucchi SE. Oral antihyperglycemic therapy for type 2 diabetes: scientific review. JAMA. 2002;287:360-372.
16. Holmboe ES. Oral antihyperglycemic therapy for type 2 diabetes: clinical apparatus. JAMA. 2002;287:373-376.
17. Rang HP, Dale MM, Ritter JM, Moore PK. The endocrine pancreas and the control of blood glucose. In: Pharmacology. 5th ed. London: Churchill-Living-stone/Elsevier Science; 2003;380-394.
18. Davis SN, Granner DK. Insulin, oral hypoglycemic agents, and the pharmacology of the endocrine pancreas. In: Hardman JG, Limbird LE, Gilman AG, eds. Goodman & Gilman’s The Pharmacological Basis of Therapeutics. 10th ed. New York, NY: McGraw-Hill; 2001;1679-1714.
19. Goldner MG, Knatterud GL, Prout TE. Effects of hypoglycemic agents on vascular complications in patients with adult-onset diabetes. 3. Clinical implications of UGDP results. JAMA. 1971;218:1400-1410.
20. Hollander P, Schwartz SL, Gatlin MR, et al. Importance of early insulin secretion: comparison of nateglinide and glyburide in previously diet-treated patients with type 2 diabetes. Diabetes Care. 2001;24:983-988.
21. Inzucchi SE, Maggs DG, Spollett GR, et al. Efficacy and metabolic effects of metformin and troglitazone in type II diabetes mellitus. N Engl J Med. 1998;338:867-872.
22. DeFronzo RA, Goodman AM. Efficacy of metformin in patients with non-insulin-dependent diabetes mellitus. The Multicenter Metformin Study Group. N Engl J Med. 1995;333:541-549.
23. UKPDS Group Effect of intensive blood-glucose control with metformin on complications in overweight patients with type 2 diabetes (UKPDS 34). Lancet. 1998;352:854-865.
24. Shaughnessy AF, Slawson DC. What happened to the valid POEMs? A survey of review articles on the treatment of type 2 diabetes. BMJ. 2003;327:266.-
25. Stumvoll M, Nurjhan N, Perriello G, Dailey G, Gerich JE. Metabolic effects of metformin in non insulin-dependent diabetes mellitus. N Engl J Med. 1995;333:550-554.
26. Brown JB, Pedula MS, Barzilay J, et al. Lactic acidosis rates in type 2 diabetes. Diabetes Care. 1998;21:1659-1663.
27. Calabrese AT, Coley KC, DaPos SV, Swanson D, Rao RH. Evaluation of prescribing practices: risk of lactic acidosis with metformin therapy. Arch Intern Med. 2002;162:434-437.
28. Masoudi FA, Wang Y, Inzucchi SE, et al. Metformin and thiazolidinedione use in Medicare patients with heart failure. JAMA. 2003;290:81-85.
29. Scheen AJ. Clinical pharmacokinetics of metformin. Clin Pharmacokinet. 1996;30:359-371.
30. Lalau JD, Lacroix C, De Cagny B, Fournier A. Metformin-associated lactic acidosis in diabetic patients with acute renal failure. A critical analysis of its pathogenesis and prognosis. Nephrol Dial Transplant. 1994;9 (suppl 4):126-129.
31. Jones GC, Macklin JP, Alexander WD. Contraindications to the use of metformin. BMJ. 2003;3:131-132.
32. Salpeter SR, Greyber E, Pasternak GA, Salpeter EE. Risk of fatal and nonfatal lactic acidosis with metformin use in type 2 diabetes mellitus: systematic review and metaanalysis. Arch Intern Med. 2003;63:2594-2602.
33. Aronoff S, Rosenblatt S, Braithwaite S, Egan JW, Mathiesen AL, Schnieder RL. Pioglitazone hydrochloride monotherapy improves glycemic control in the treatment of patients with type 2 diabetes: a 6 month randomized placebo-controlled dose-response study. The Pioglitazone 001 Study Group. Diabetes Care. 2000;23:1605-1611.
34. Nesto RW, Bell D, Bonow RO, et al. Thiazolidinedione use, fluid retention, and congestive heart failure: a consensus statement from the American Heart Association and American Diabetes Association. October 7, 2003. Circulation. 2003;108:2941-2948.
35. Tolman KG, Chandramouli J. Hepatotoxicity of the thiazolidinediones. Clin Liver Dis. 2003;7:369-379.
36. Zawadzki JK, Green L, Graham BJ. Thioglitazone-associated 15-month post-marketing hepatotoxicity. Poster abstract. FDA Science Forum. Available at: vm.cfsan.fda.gov/~frf/forum02/a187ab4.htm. Accessed on February 25, 2004.;
37. Lebowitz HE. a-Glucosidase inhibitors as agents in the treatment of diabetes. Diabetes Rev. 1998;6:132-145.
38. Chan JL, Abrahamson MJ. Pharmacological management of type 2 diabetes mellitus: rationale for rational use of insulin. Mayo Clin Proc. 2003;78:459-467.
39. Nathan DM. Initial management of glycemia in type 2 diabetes mellitus. N Engl J Med. 2002;347:1342-1349.
40. Pugh JA, Wagner ML, Sawyer J, Ramirez G, Tuley M, Friedberg SJ. Is combination sulfonylurea and insulin therapy useful in NIDDM patients? A metaanalysis. Diabetes Care. 1992;15:953-959.
41. Cusi K, Cunningham GR, Comstock JP. Safety and efficacy of normalizing fasting glucose with bedtime NPH insulin alone in NIDDM. Diabetes Care. 1995;18:843-851.
42. White JR, Davis SN, Cooppan R, et al. Clarifying the role of insulin in type 2 diabetes management. Clinical Diabetes. 2003;1:14-21.
43. DeWitt DE, Hirsch IR. Outpatient insulin therapy in type 1 and type 2 diabetes mellitus: scientific review. JAMA. 2003;289:2254-2264.
44. - Yki, Jarvinen H, Dressler A, Ziemen M. HOE 901/3002 Study Group Less nocturnal hypoglycemia and better post-dinner glucose control with bedtime insulin glargine compared with bedtime NPH insulin during insulin combination therapy in type 2 diabetes. Diabetes Care. 2000;23:1130-1136.
45. Fritsche A, Schweitzer MA, Haring HU. 4001 Study Group Glimepiride combined with morning insulin glargine, bedtime neutral protamine hagedorn insulin, or bedtime insulin glargine in patients with type 2 diabetes. A randomized, controlled trial. Ann Intern Med. 2003;138:952-959.
46. van den Berghe G, Wouters P, Weekers F, et al. Intensive insulin therapy in critically ill patients. N Engl J Med. 2001;345:1359-1367.
47. Malmberg K, Norhammar A, Wedel H, Ryden L. Glycometabolic state at admission: important risk marker of mortality in conventionally treated patients with diabetes mellitus and acute myocardial infarction: long-term results from the Diabetes and Insulin-Glucose Infusion in Acute Myocardial Infarction (DIGAMI) study. Circulation. 1999;99:2626-2632.
48. Zimmerman BR. Therapy for type 2 diabetes mellitus. In: Medical Management of Type 2 Diabetes. 4th ed. Alexandria, Va: American Diabetes Association; 1998.;
49. Turner RC, Cull CA, Frighi V, Holman RR. Glycemic control with diet, sulfonylurea, metformin, or insulin in patients with type 2 diabetes mellitus. Progressive requirement for multiple therapies (UKPDS 49). JAMA. 1999;281:2005-2012.
50. Leichter SB, Thomas S. combination medications in diabetes care: an opportunity that merits more attention. Clin Diabetes. 2003;21:175-178.
51. Hermann LS, Schersten B, Bitzen P, et al. Therapeutic comparison of metformin and sulfonylurea, alone and in various combinations. A double-blind controlled study. Diabetes Care. 1994;17:1100-1109.
52. Jeppesen J, Zhou M, Chen Y, Reaven G. Effect of metformin on postprandial lipemia in patients with fairly to poorly controlled NIDDM. Diabetes Care. 1994;17:1093-1099.
53. Garber AJ, Larsen J, Schneider SH, et al. Simultaneous glyburide/metformin therapy is superior to component monotherapy as an initial pharmacological treatment for type 2 diabetes. Diabetes Obes Metab. 2002;4:201-208.
54. Riddle M. Combining sulfonylureas and other oral agents. Am J Med. 2000;108(suppl 6A):15S-22S.
55. Raskin P, Klaff L, McGill J, et al. Efficacy and safety of combination therapy: repaglinide plus metformin versus nateglinide plus metformin. Diabetes Care. 2003;26:2063-2068.
56. Kipnes MS, Krosnick A, Rendell MS, Egan JW, Mathisen AL, Schneider RL. Pioglitazone hydrochloride in combination with sulfonylurea therapy improves glycemic control in patients with type 2 diabetes mellitus: a randomized, placebo-controlled study. Am J Med. 2001;111:10-17.
57. Johnston PS, Feig PU, Coniff RF, Krol A, Davidson JA, Haffner SM. Long-term titrated-dose a-glucosidase inhibition in non-insulin-requiring Hispanic NIDDM patients. Diabetes Care. 1998;21:409-415.
58. Chiasson J, Josse RG, Hunt JA, et al. The efficacy of acarbose in the treatment of patients with non-insulin-dependent diabetes mellitus. A multicenter controlled clinical trial. Ann Intern Med. 1994;121:928-935.
59. Fonseca V, Rosenstock J, Patwardhan R, Salzman A. Effect of metformin and rosiglitazone combination therapy in patients with type 2 diabetes mellitus: a randomized controlled trial. JAMA. 2000;283:1695-1702.
60. Fonseca V, Grunberger G, Gupta S, Shen S, Foley JE. Addition of nateglinide to rosiglitazone monotherapy suppresses mealtime hyperglycemia and improves overall glycemic control. Diabetes Care. 2003;26:1685-1690.
61. Wright A, Burden ACF, Paisey RB, Cull CA, Holman RR; UKPDS. Sulfonylurea inadequacy: efficacy of addition of insulin over 6 years in patients with type 2 diabetes in the U.K. Prospective Diabetes Study (UKPDS 57). Diabetes Care. 2002;25:330-336.
62. Garber AJ. Benefits of combination therapy of insulin and oral hypoglycemic agents. Arch Intern Med. 2003;163:1781-1782.
63. Westphal SA, Palumbo PJ. Insulin and oral hypoglycemic agents should not be used in combination in the treatment of type 2 diabetes. Arch Intern Med. 2003;163:1783-1785.
64. DeWitt DE, Dugdale DC. Using new insulin strategies in the outpatient treatment of diabetes: clinical applications. JAMA. 2003;289:2265-2269.
65. Saaddine JB, Engelgau MM, Beckles GL, Gregg EW, Thompson TJ, Narayan KM. A diabetes report card for the United States: quality of care in the 1990s. Ann Intern Med. 2002;136:565-574.
66. Brown JB, Nichols GA. Slow response to loss of glycemic control in type 2 diabetes mellitus. Am J Manag Care. 2003;9:213-217.
67. Sutherland JE, Hoehns JD, O’Donnell B, Wiblin RT. Diabetes management quality improvement in a family practice residency program. J Am Board Fam Pract. 2001;14:243-251.
68. De Grauw W, van Gerwen W, van de Lisdonk EH, van den Hoogen HJ, van den Bosch WJ, van Weel C. Outcomes of audit-enhanced monitoring of patients with type 2 diabetes. J Fam Pract. 2002;51:459-464.
69. Sidorov J, Gabbay R, Harris R, et al. Disease management for diabetes mellitus: impact on hemoglobin A1c. Am J Manag Care. 2000;6:1217-1226.
70. Domurat ES. Diabetes managed care and clinical outcomes: the Harbor City, California Kaiser Permanente diabetes care system. Am J Manag Care. 1999;5:1299-1307.
71. Peterson K, Hughes M. Readiness to change and clinical success in a diabetes educational program. J Am Board Fam Pract. 2002;15:266-270.
72. Parchman ML, Burge SK. Continuity and quality of care in type 2 diabetes: a Residency Research Network at South Texas study. J Fam Pract. 2002;51:619-624.
73. Fagan TC, Deedwania PC. The cardiovascular dysmetabolic syndrome. Am J Med. 1998;105(suppl):77S-82S.
74. Tuomilehto J, Lindstrom J, Eriksson JG, et al. Prevention of type 2 diabetes mellitus by changes in lifestyle among subjects with impaired glucose tolerance. N Engl J Med. 2001;344:1343-1350.
75. Meigs JB. The metabolic syndrome. BMJ. 2003;327:61-62.
1. Vinik AI, Vinik E. Prevention of the complications of diabetes. Am J Manag Care. 2003;9 suppl:S63-S80.
2. UK Prospective Diabetes Study (UKPDS) Group. Intensive blood-glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33). Lancet. 1998;352:837-853.
3. Arauz-Pacheco C, Parrott MA, Raskin P. American Diabetes Association Treatment of hypertension in adults with diabetes. Diabetes Care. 2004;27 (suppl):S65-67.
4. UK Prospective Diabetes Study Group. Tight blood pressure control and risk of macrovascular and microvascular complications in type 2 diabetes: UKPDS 38. BMJ. 1998;317:703-713.
5. Beckman JA, Creager MA, Libby P. Diabetes and atherosclerosis: epidemiology, pathophysiology, and management. JAMA. 2002;287:2570-2581.
6. Kendall DM, Bergenstal RM. Comprehensive management of patients with type 2 diabetes: establishing priorities of care. Am J Manag Care. 2001;7 (suppl):S327-S343.
7. American Diabetes Association. Standards of medical care in Diabetes. Diabetes Care. 2004;27(suppl):15-35.
8. Peterson KA. Diabetes management in the primary care setting: summary. Am J Med. 2002;113(suppl 6A):36S-40S.
9. Ellis SE, Speroff T, Dittus RS, Brown A, Pichert JW, Elasy TA. Diabetes patient education: A meta-analysis and meta-regression. Patient Educ Couns. 2004;52:97-105.
10. Chandalia M, Garg A, Lutjohann D, et al. Beneficial effects of high dietary fiber intake in patients with type 2 diabetes mellitus. N Engl J Med. 2000;342:1392-1398.
11. Hadden DR, Montgomery DAD, Skelly RJ, et al. Maturity onset diabetes mellitus: response to intensive dietary management. Br Med J. 1975;3:276-278.
12. Niskenen LK, Uusitupa MI, Surlund H, et al. Five-year follow-up study on plasma insulin levels in newly diagnosed NIDDM patients and nondiabetic subjects. Diabetes Care. 1009;13:41-48.
13. Leibel R, Rosenbaum M, Hirsch J. Changes in energy expenditure resulting from altered body weight. N Engl J Med. 1995;332:621-628.
14. Gregg EW, Gerzoff RB, Caspersen CJ, et al. Relationship of walking to mortality among US adults with diabetes. Arch Intern Med. 2003;163:1440-1447.
15. Inzucchi SE. Oral antihyperglycemic therapy for type 2 diabetes: scientific review. JAMA. 2002;287:360-372.
16. Holmboe ES. Oral antihyperglycemic therapy for type 2 diabetes: clinical apparatus. JAMA. 2002;287:373-376.
17. Rang HP, Dale MM, Ritter JM, Moore PK. The endocrine pancreas and the control of blood glucose. In: Pharmacology. 5th ed. London: Churchill-Living-stone/Elsevier Science; 2003;380-394.
18. Davis SN, Granner DK. Insulin, oral hypoglycemic agents, and the pharmacology of the endocrine pancreas. In: Hardman JG, Limbird LE, Gilman AG, eds. Goodman & Gilman’s The Pharmacological Basis of Therapeutics. 10th ed. New York, NY: McGraw-Hill; 2001;1679-1714.
19. Goldner MG, Knatterud GL, Prout TE. Effects of hypoglycemic agents on vascular complications in patients with adult-onset diabetes. 3. Clinical implications of UGDP results. JAMA. 1971;218:1400-1410.
20. Hollander P, Schwartz SL, Gatlin MR, et al. Importance of early insulin secretion: comparison of nateglinide and glyburide in previously diet-treated patients with type 2 diabetes. Diabetes Care. 2001;24:983-988.
21. Inzucchi SE, Maggs DG, Spollett GR, et al. Efficacy and metabolic effects of metformin and troglitazone in type II diabetes mellitus. N Engl J Med. 1998;338:867-872.
22. DeFronzo RA, Goodman AM. Efficacy of metformin in patients with non-insulin-dependent diabetes mellitus. The Multicenter Metformin Study Group. N Engl J Med. 1995;333:541-549.
23. UKPDS Group Effect of intensive blood-glucose control with metformin on complications in overweight patients with type 2 diabetes (UKPDS 34). Lancet. 1998;352:854-865.
24. Shaughnessy AF, Slawson DC. What happened to the valid POEMs? A survey of review articles on the treatment of type 2 diabetes. BMJ. 2003;327:266.-
25. Stumvoll M, Nurjhan N, Perriello G, Dailey G, Gerich JE. Metabolic effects of metformin in non insulin-dependent diabetes mellitus. N Engl J Med. 1995;333:550-554.
26. Brown JB, Pedula MS, Barzilay J, et al. Lactic acidosis rates in type 2 diabetes. Diabetes Care. 1998;21:1659-1663.
27. Calabrese AT, Coley KC, DaPos SV, Swanson D, Rao RH. Evaluation of prescribing practices: risk of lactic acidosis with metformin therapy. Arch Intern Med. 2002;162:434-437.
28. Masoudi FA, Wang Y, Inzucchi SE, et al. Metformin and thiazolidinedione use in Medicare patients with heart failure. JAMA. 2003;290:81-85.
29. Scheen AJ. Clinical pharmacokinetics of metformin. Clin Pharmacokinet. 1996;30:359-371.
30. Lalau JD, Lacroix C, De Cagny B, Fournier A. Metformin-associated lactic acidosis in diabetic patients with acute renal failure. A critical analysis of its pathogenesis and prognosis. Nephrol Dial Transplant. 1994;9 (suppl 4):126-129.
31. Jones GC, Macklin JP, Alexander WD. Contraindications to the use of metformin. BMJ. 2003;3:131-132.
32. Salpeter SR, Greyber E, Pasternak GA, Salpeter EE. Risk of fatal and nonfatal lactic acidosis with metformin use in type 2 diabetes mellitus: systematic review and metaanalysis. Arch Intern Med. 2003;63:2594-2602.
33. Aronoff S, Rosenblatt S, Braithwaite S, Egan JW, Mathiesen AL, Schnieder RL. Pioglitazone hydrochloride monotherapy improves glycemic control in the treatment of patients with type 2 diabetes: a 6 month randomized placebo-controlled dose-response study. The Pioglitazone 001 Study Group. Diabetes Care. 2000;23:1605-1611.
34. Nesto RW, Bell D, Bonow RO, et al. Thiazolidinedione use, fluid retention, and congestive heart failure: a consensus statement from the American Heart Association and American Diabetes Association. October 7, 2003. Circulation. 2003;108:2941-2948.
35. Tolman KG, Chandramouli J. Hepatotoxicity of the thiazolidinediones. Clin Liver Dis. 2003;7:369-379.
36. Zawadzki JK, Green L, Graham BJ. Thioglitazone-associated 15-month post-marketing hepatotoxicity. Poster abstract. FDA Science Forum. Available at: vm.cfsan.fda.gov/~frf/forum02/a187ab4.htm. Accessed on February 25, 2004.;
37. Lebowitz HE. a-Glucosidase inhibitors as agents in the treatment of diabetes. Diabetes Rev. 1998;6:132-145.
38. Chan JL, Abrahamson MJ. Pharmacological management of type 2 diabetes mellitus: rationale for rational use of insulin. Mayo Clin Proc. 2003;78:459-467.
39. Nathan DM. Initial management of glycemia in type 2 diabetes mellitus. N Engl J Med. 2002;347:1342-1349.
40. Pugh JA, Wagner ML, Sawyer J, Ramirez G, Tuley M, Friedberg SJ. Is combination sulfonylurea and insulin therapy useful in NIDDM patients? A metaanalysis. Diabetes Care. 1992;15:953-959.
41. Cusi K, Cunningham GR, Comstock JP. Safety and efficacy of normalizing fasting glucose with bedtime NPH insulin alone in NIDDM. Diabetes Care. 1995;18:843-851.
42. White JR, Davis SN, Cooppan R, et al. Clarifying the role of insulin in type 2 diabetes management. Clinical Diabetes. 2003;1:14-21.
43. DeWitt DE, Hirsch IR. Outpatient insulin therapy in type 1 and type 2 diabetes mellitus: scientific review. JAMA. 2003;289:2254-2264.
44. - Yki, Jarvinen H, Dressler A, Ziemen M. HOE 901/3002 Study Group Less nocturnal hypoglycemia and better post-dinner glucose control with bedtime insulin glargine compared with bedtime NPH insulin during insulin combination therapy in type 2 diabetes. Diabetes Care. 2000;23:1130-1136.
45. Fritsche A, Schweitzer MA, Haring HU. 4001 Study Group Glimepiride combined with morning insulin glargine, bedtime neutral protamine hagedorn insulin, or bedtime insulin glargine in patients with type 2 diabetes. A randomized, controlled trial. Ann Intern Med. 2003;138:952-959.
46. van den Berghe G, Wouters P, Weekers F, et al. Intensive insulin therapy in critically ill patients. N Engl J Med. 2001;345:1359-1367.
47. Malmberg K, Norhammar A, Wedel H, Ryden L. Glycometabolic state at admission: important risk marker of mortality in conventionally treated patients with diabetes mellitus and acute myocardial infarction: long-term results from the Diabetes and Insulin-Glucose Infusion in Acute Myocardial Infarction (DIGAMI) study. Circulation. 1999;99:2626-2632.
48. Zimmerman BR. Therapy for type 2 diabetes mellitus. In: Medical Management of Type 2 Diabetes. 4th ed. Alexandria, Va: American Diabetes Association; 1998.;
49. Turner RC, Cull CA, Frighi V, Holman RR. Glycemic control with diet, sulfonylurea, metformin, or insulin in patients with type 2 diabetes mellitus. Progressive requirement for multiple therapies (UKPDS 49). JAMA. 1999;281:2005-2012.
50. Leichter SB, Thomas S. combination medications in diabetes care: an opportunity that merits more attention. Clin Diabetes. 2003;21:175-178.
51. Hermann LS, Schersten B, Bitzen P, et al. Therapeutic comparison of metformin and sulfonylurea, alone and in various combinations. A double-blind controlled study. Diabetes Care. 1994;17:1100-1109.
52. Jeppesen J, Zhou M, Chen Y, Reaven G. Effect of metformin on postprandial lipemia in patients with fairly to poorly controlled NIDDM. Diabetes Care. 1994;17:1093-1099.
53. Garber AJ, Larsen J, Schneider SH, et al. Simultaneous glyburide/metformin therapy is superior to component monotherapy as an initial pharmacological treatment for type 2 diabetes. Diabetes Obes Metab. 2002;4:201-208.
54. Riddle M. Combining sulfonylureas and other oral agents. Am J Med. 2000;108(suppl 6A):15S-22S.
55. Raskin P, Klaff L, McGill J, et al. Efficacy and safety of combination therapy: repaglinide plus metformin versus nateglinide plus metformin. Diabetes Care. 2003;26:2063-2068.
56. Kipnes MS, Krosnick A, Rendell MS, Egan JW, Mathisen AL, Schneider RL. Pioglitazone hydrochloride in combination with sulfonylurea therapy improves glycemic control in patients with type 2 diabetes mellitus: a randomized, placebo-controlled study. Am J Med. 2001;111:10-17.
57. Johnston PS, Feig PU, Coniff RF, Krol A, Davidson JA, Haffner SM. Long-term titrated-dose a-glucosidase inhibition in non-insulin-requiring Hispanic NIDDM patients. Diabetes Care. 1998;21:409-415.
58. Chiasson J, Josse RG, Hunt JA, et al. The efficacy of acarbose in the treatment of patients with non-insulin-dependent diabetes mellitus. A multicenter controlled clinical trial. Ann Intern Med. 1994;121:928-935.
59. Fonseca V, Rosenstock J, Patwardhan R, Salzman A. Effect of metformin and rosiglitazone combination therapy in patients with type 2 diabetes mellitus: a randomized controlled trial. JAMA. 2000;283:1695-1702.
60. Fonseca V, Grunberger G, Gupta S, Shen S, Foley JE. Addition of nateglinide to rosiglitazone monotherapy suppresses mealtime hyperglycemia and improves overall glycemic control. Diabetes Care. 2003;26:1685-1690.
61. Wright A, Burden ACF, Paisey RB, Cull CA, Holman RR; UKPDS. Sulfonylurea inadequacy: efficacy of addition of insulin over 6 years in patients with type 2 diabetes in the U.K. Prospective Diabetes Study (UKPDS 57). Diabetes Care. 2002;25:330-336.
62. Garber AJ. Benefits of combination therapy of insulin and oral hypoglycemic agents. Arch Intern Med. 2003;163:1781-1782.
63. Westphal SA, Palumbo PJ. Insulin and oral hypoglycemic agents should not be used in combination in the treatment of type 2 diabetes. Arch Intern Med. 2003;163:1783-1785.
64. DeWitt DE, Dugdale DC. Using new insulin strategies in the outpatient treatment of diabetes: clinical applications. JAMA. 2003;289:2265-2269.
65. Saaddine JB, Engelgau MM, Beckles GL, Gregg EW, Thompson TJ, Narayan KM. A diabetes report card for the United States: quality of care in the 1990s. Ann Intern Med. 2002;136:565-574.
66. Brown JB, Nichols GA. Slow response to loss of glycemic control in type 2 diabetes mellitus. Am J Manag Care. 2003;9:213-217.
67. Sutherland JE, Hoehns JD, O’Donnell B, Wiblin RT. Diabetes management quality improvement in a family practice residency program. J Am Board Fam Pract. 2001;14:243-251.
68. De Grauw W, van Gerwen W, van de Lisdonk EH, van den Hoogen HJ, van den Bosch WJ, van Weel C. Outcomes of audit-enhanced monitoring of patients with type 2 diabetes. J Fam Pract. 2002;51:459-464.
69. Sidorov J, Gabbay R, Harris R, et al. Disease management for diabetes mellitus: impact on hemoglobin A1c. Am J Manag Care. 2000;6:1217-1226.
70. Domurat ES. Diabetes managed care and clinical outcomes: the Harbor City, California Kaiser Permanente diabetes care system. Am J Manag Care. 1999;5:1299-1307.
71. Peterson K, Hughes M. Readiness to change and clinical success in a diabetes educational program. J Am Board Fam Pract. 2002;15:266-270.
72. Parchman ML, Burge SK. Continuity and quality of care in type 2 diabetes: a Residency Research Network at South Texas study. J Fam Pract. 2002;51:619-624.
73. Fagan TC, Deedwania PC. The cardiovascular dysmetabolic syndrome. Am J Med. 1998;105(suppl):77S-82S.
74. Tuomilehto J, Lindstrom J, Eriksson JG, et al. Prevention of type 2 diabetes mellitus by changes in lifestyle among subjects with impaired glucose tolerance. N Engl J Med. 2001;344:1343-1350.
75. Meigs JB. The metabolic syndrome. BMJ. 2003;327:61-62.
Achieving the best outcome in treatment of depression
- Combined treatment with psychotherapy or psychiatric consult and drug therapy has shown better response in several studies than either therapy alone (A).
- Although not proven by clinical trials, selecting a medication by matching its side-effect profile to patient characteristics is supported by case reports and likely enhances compliance.
- Patients who do not improve with initial therapy often benefit from being switched to another class of antidepressants (A), or having a drug from another class added to their therapy (B).
You are more likely to see depression in your practice than any other disorder except hypertension.1 Given the prevalence of depression* and the variability of its clinical symptoms and comorbidities, how do you determine the optimal therapy for a given patient?
A sobering thought: nearly half of all patients stop taking their antidepressant prescription medication within the first month of treatment.1 We discuss the critical factors you can address to help patients stick with treatment and achieve the best outcome.
Therapeutic Options
Pharmacotherapy
Antidepressants are thought to exert their therapeutic and adverse effects through 3 chemical monamine neurotransmission systems; by increasing levels of norepinephrine, serotonin, or dopamine in the synapse; and by resultant secondary changes in presynaptic and postsynaptic receptor physiology.3,8,9 Newer medications—such as selective serotonin reuptake inhibitors (SSRIs)—have simpler dose schedules, different (and for some patients more favorable) adverse effect profiles, and less likelihood of causing death from overdose compared with older tricyclic antidepressants (TCAs) and monamine oxidase inhibitors (MAOIs).
Patients are less likely to discontinue treatment with SSRIs than with TCAs (odds ratio=1.21; 95% confidence interval [CI], 1.12–1.30).10
However, there are no clinically significant differences in effectiveness between SSRIs and TCAs (strength of recommendation [SOR]: A).11 Importantly, although practice patterns in the use of antidepressants have changed, some reasons for the preference of newer effective agents have not been substantiated. For instance, we do not know whether the patient population taking newer agents has a lower rate of suicide, despite the difference in fatality risk mentioned earlier.
Combined pharmacotherapy and psychiatric consultation
Combining pharmacotherapy and psychotherapy can be more effective than either modality alone. In one study, 73% of patients with chronic depression treated with combination therapy showed a reduction of 50% or more on the Hamilton Rating Scale for Depression (HRSD), compared with just 48% in the nefazodone-only and psychotherapy-only groups (SOR: A). Among those who completed the study, the rates of response were 85%, 55%, and 52%, respectively (although the results considered compliant patients only, which biases the results in favor of treatment).2
Among elderly depressed patients who received home care, 58% of those who underwent intervention by a psychogeriatric team recovered, compared with just 25% in the control group (SOR: A).12 The intervention group received a multidisciplinary team evaluation and an individualized management plan, which could include any combination of physical, psychological, or social interventions. The control group received usual care from their general practitioner.
Studies of combination therapy have yielded mixed results, but guidelines from the psychiatric literature based on clinical experience advocate concomitant psychotherapy and medication (SOR: A).13 For patients with persistent symptoms after 6 to 8 weeks of taking antidepressant medication, concomitant psychotherapy improved compliance, satisfaction, and outcomes when compared with usual care.14
At any one time, at least 3% of the US population suffers from chronic depression.2 More than 17% of the population have had a major depressive episode in their lifetime, and more than 10% have experienced an episode within the past 12 months.3 The incidence and prevalence of depression in women are approximately twice that seen in men.4
Major depression is the fourth leading cause of worldwide disease burden.5
Natural history and prognosis
An untreated episode of depression usually lasts 6 months or longer. About half of persons experiencing major depression will have a second episode; a second episode increases the risk for a third episode to 80%.1,3 Patients diagnosed with depression average 5 depressive episodes in their life and may have recurrences every 4 to 6 years. Episodes usually become longer and more frequent with advancing age. In about 20% to 35% of cases, only partial remission occurs and functioning remains impaired.1
Fifteen percent of severely depressed patients commit suicide. The 2 most powerful predictors of suicide are a history of major depression or schizophrenia and a history of addictive disorders.6
Outpatient treatment of depression has increased markedly in the United States, with greater involvement on the part of physicians, greater use of psychotropic medications, expanding availability of third-party payment, and less use of psychotherapy.7
The concomitant therapy group participated in a multifaceted program including education, psychiatric referral, pharmacy utilization records, and primary physician feedback. The usual care group received standard antidepressants and follow-up visits from their family physician, with optional referral to a mental health provider.
Psychotherapy has also been shown to decrease the risk of relapse once symptoms have remitted.15 Primary care physicians can also incorporate counseling as adjunctive therapy.
Herbal and nutritional products
St. John’s wort. St. John’s wort (Hypericum perforatum L.) has been used as an herbal medication for more than 2000 years. Its efficacy in the treatment of depression has been studied extensively. Some studies demonstrated that these extracts are more effective than placebo for the short-term treatment of mild and moderate depression.16,17,18 Two randomized controlled trials demonstrated minimal efficacy of St. John’s wort in moderately severe major depression.19,20 The National Institutes of Health is sponsoring a placebo-controlled, double-blinded trial comparing St. John’s wort with SSRIs.21
Omega-3 fatty acids. Chronic deficiencies of essential fatty acids may adversely affect central nervous system function. In a small, 4-week double-blind study, outpatients receiving antidepressant therapy who were also given eicosapentaenoic acid exhibited improvement in core depressive symptoms (eg, worthlessness, guilt, insomnia) compared with the antidepressantplus-placebo group. Larger, long-term prospective trials are needed to confirm an antidepressant effect with omega-3 fatty acids.22
S-adenosyl-L-methionine. S-adenosyl-L-methionine is possibly effective for short-term treatment of major depression. Data for other herbal or nutritional remedies are negligible.23
Exercise
Physical activity may play an important role in relieving depression. One randomized controlled trial showed that an aerobic exercise program, sertraline therapy, or a combination of both were equally effective in the treatment of depression, although there was a more rapid initial response with sertraline.24
A systematic review and meta-analysis concluded that exercise may reduce depression symptoms short term, but much of the evidence is of poor quality.25 Well-controlled studies are needed to clarify the role of exercise in the treatment of depression. However, exercise is promising enough to consider implementation in clinical practice at this time.
Treatment strategy
Guidelines for medicating patients and setting expectations
Start antidepressant therapy promptly when depression is diagnosed. Maintain the initial dosage for at least 3 to 4 weeks before increasing it. A trial of 6 to 8 weeks at maximum dosage (or the maximum tolerated dosage) is necessary to confirm treatment success or failure.26,27,28
An improvement in symptoms will usually not be noted until after 2 to 6 weeks of therapy. Depending on depression severity, schedule weekly or monthly visits for patients during the initial treatment phase. The response rate to initial treatment is only 50% to 60%, but more than 80% of depressed patients will respond to at least 1 medication.1
Response to placebo is highly variable. It is often substantial and has increased in recent years. In an analysis of 75 trials between 1981 and 2001, the mean proportion of patients in the placebo group who responded (50% improvement on the HRSD) was 29.7%, compared with 50.1% in the active medication group.29 The placebo effect may reflect some combination of patient expectations, the natural history of depression with possible spontaneous remission, and limitations of study methods.
Antidepressant therapy is effective compared with placebo for depression secondary to medical illness (number needed to treat [NNT], 4.2; 95% CI, 3.2–6.4), with minimal treatment dropout (number needed to harm, 9.8; 95% CI, 5.4–42.9).30 Although many patients settle for partial improvement of their symptoms, the treatment goal should be complete remission.
Factors in drug selection
Selecting an antidepressant can be challenging: more than 24 drugs are on the market, each working through 1 or more of 7 pharmacologic mechanisms. Theoretically, choosing a drug is made easier by matching patient symptoms to likely medication side effects or by knowing that the patient or a family member responded favorably to a particular antidepressant in the past.
This intuitive model has not been proven superior to any other model of selecting antidepres-sants, but it is clinically sound, pharmacodynamically appealing, and supported by case reports. Its strength may lie in enhancing patient adherence during the critical initial phase of treatment.
A recent randomized, prospective comparison of the SSRIs paroxetine, fluoxetine, and sertraline showed similar effectiveness and tolerability (SOR: A).31 This suggests that efforts to individualize therapy based on comorbidities or likely side effects may not be as useful when choosing from among analogous SSRIs.
Nevertheless, choosing a drug that is effective, convenient, and well tolerated will improve the likelihood of achieving and maintaining a full remission. The data on adverse effects of antidepressants are widely available and well understood. Also consider cost (Table).
Preferences based on characteristics. For a patient whose depression is not complicated by other clinical conditions, the initial choice of antidepressant would usually be an SSRI. But nefazodone, mirtazapine, bupropion, or low-dose venlafaxine may be equally appropriate.
For a patient whose depression has other specific components, use your knowledge of drugs’ common side effects to fit the patient’s clinical profile.
- If there is generalized anxiety, agitation, and insomnia, both nefazodone8 and mirtazapine32 are excellent choices. Trazodone at low doses is often used as a sedative with nonsedating antidepressants.8
- If weight gain is desired, mirtazapine is indicated.32
- If tobacco cessation is a secondary goal, bupropion is preferred.31
- Those suffering from hypersomnia, retarded depression, cognitive slowing, and pseudodementia would benefit from bupropion or venlafaxine.9
- For more severely depressed patients, venlafaxine may be advantageous due to its dual serotonergic and noradrenergic activity at moderate to high doses.34,35,36 Mirtazapine and TCAs are also useful in severe depression, as well as for coexisting chronic pain syndromes.8 For refractory or atypical depression in motivated and compliant patients, MAOIs my be useful.8
When to avoid specific drugs.
- Patients with hypersomnia and motor retardation should avoid nefazodone and mirtazapine.8,32
- With obesity, mirtazapine and TCAs are least preferred.8,32
- If sexual dysfunction preceded depression, avoid giving SSRIs and venlafaxine.3
- Those experiencing agitation and insomnia should avoid bupropion and venlafaxine.3
- Seizure disorder is a contraindication to bupropion.3
- Hypertension is a relative contraindication to venlafaxine.3
- Liver disease is a contraindication to nefazodone.37
- Preexisting heart disease and increased suicide risk are both relative contraindications to TCAs.8
TABLE
Comparative dosages and costs of antidepressant drugs
| Agents | Initial target dose | Maximum effective dose | Monthly cost of initial target dose* |
|---|---|---|---|
| Selective serotonin reuptake inhibitors | |||
| Citalopram (Celexa) | 20 mg qd | 60 mg qd | $61.58 |
| Escitalopram (Lexapro) | 10 mg qd | 20 mg qd | $65.28 |
| Fluoxetine (Prozac) | 20 mg qd | 80 mg qd | $81.78 |
| Fluoxetine (generic) | 20 mg qd | 80 mg qd | $61.80 |
| Fluoxetine (Prozac Weekly) | 90 mg qwk | $71.04 | |
| Fluvoxamine (Luvox) | 50 mg qd | 150 mg bid | $59.70 |
| Paroxetine (Paxil) | 20 mg qd | 60 mg qd | $70.98 |
| Paroxetine (Paxil CR) | 25 mg qd | 75 mg qd | $75.86 |
| Sertraline (Zoloft) | 50 mg qd | 200 mg qd | $65.24 |
| Tricyclic antidepressants | |||
| Amitriptyline (Elavil, Endep, Vanatrip) | 100 mg qhs | 300 mg qhs | $ 7.99 |
| Desipramine (Norpramin) | 100 mg qhs | 200 mg qhs | $18.54 |
| Doxepin (Adapin, Sinequan) | 100 mg qhs | 300 mg qhs | $8.12 |
| Imipramine (Tofranil) | 100 mg qhs | 300 mg qhs | $31.96 |
| Nortriptyline (Aventil, Pamelor) | 75 mg qhs | 150 mg qhs | $ 8.71 |
| Others | |||
| Bupropion (Wellbutrin) | 100 mg tid | 150 mg tid | $92.33 |
| Bupropion (generic) | 100 mg tid | 150 mg tid | $64.62 |
| Bupropion (Wellbutrin SR) | 150 mg bid | 200 mg bid | $87.09 |
| Mirtazapine (Remeron) | 30 mg qhs | 45 mg qhs | $80.79 |
| Nefazodone (Serzone) | 100 mg bid | 300 mg bid | $74.94 |
| Trazodone (Desyrel) | 100 mg bid | 300 mg bid | $15.98 |
| Venlafaxine (Effexor) | 37.5 mg bid | 150 mg tid | $74.39 |
| Venlafaxine (Effexor XL) | 75 mg qd | 225 mg qd | $66.25 |
| Lithium (Eskalith, Lithobid, Lithonate, Lithotabs) | 300 mg bid | 600 mg bid | $13.70 |
| *Costs from www.drugstore.com, November 2002. | |||
Helping nonresponders
Patients whose symptoms do not improve with therapy could be switched to a different monotherapy or to multiple drugs. Drug choices for treatment-refractory and nonresponding patients have evolved more by anecdote than by systematic study.9
Switch drugs. The benefit of switching patients to another category of antidepressant was recently demonstrated in a study where nearly half of patients who did not respond to an initial antidepressant, whether SSRI or TCA, responded when switched to the alternate agent (SOR: A).38 It is also beneficial to switch medications within a category (SOR: B).27,39,40
Add a drug. Adding a second antidepressant from a category with a different mechanism of action often enhances clinical efficacy. This has been demonstrated in combining an SSRI with a TCA (SOR: B).41 Though response rates are very similar for various antidepressants, complete remission and rates of response in severely depressed patients may be higher in dual-action antidepressants (SOR: A).34,35,36
Add lithium. A great deal of evidence supports the use of lithium augmentation (SOR: A).42,43 This agent should be used more in primary care and not only by psychiatrists. A recent meta-analysis of double-blind, placebo-controlled studies of lithium (given at a dosage of at least 800 mg/d or at a level high enough to achieve a serum drug concentration of 0.5 mEq/L for at least 2 weeks) found a summary pooled odds ratio of response to lithium of 3.31 (95% CI, 1.46–7.53) with a NNT of 3.7.44 Other studies have been less clear on the optimal dose or blood level, so a starting dose of 300 mg twice daily with a serum drug concentration of 0.4 mEq/L has been recommended.
If renal function is normal, the concentration of lithium can be checked 5 days after a patient has received a stable dosage, at least 8 hours fol-lowing the last dose. Lithium may cause thyroid abnormalities; monitoring should include a measurement of thyroid-stimulating hormone, repeated at 6 months and 1 year.
Other augmentation options. Augmentation of antidepressants with buspirone has been proven useful in major depression (SOR: B).45 Thyroid supplementation may also increase the effectiveness of antidepressant therapy using triiodothyronine (T3), at doses not to exceed 50 mcg per day (SOR: B).46,47 Electroconvulsive therapy (ECT) has a high rate of therapeutic success, including speed and safety, but it is not administered as first-line treatment by psychiatrists except in severe cases (SOR: A).48,49 Augmentation with antipsychotic or anticonvulsants is another strategy that shows some benefit for select patients.50
Texas medication algorithm project
The process of drug selection just described can avoid treatment-threatening side effects, enable patient adherence to treatment, and maximize the potential for therapeutic response. However, the model can become disorienting for the clinician and the patient if 1 or 2 initial selections for treatment do not succeed. A useful synergy may be achieved by adapting the intuitive model to an algorithmic model—the Texas Medication Algorithm Project (TMAP). TMAP is an evolving model that reflects ongoing clinical research in the treatment of depression.27
Developed in 1995 from a review of existing antidepressant research and several consensus conferences, the TMAP (continually updated with new research findings) has developed algorithms for treatment of schizophrenia and bipolar disorder in addition to major depression. At each stage in the depression algorithm, treatment plans similar in efficacy and safety are grouped together, and the clinician is given a limited number of options. The later stages in the algorithm are more complex, admittedly with a greater potential for medical complications (Figure).51
The algorithm represents a tentative foundation for a sequenced medication plan. Research pertaining to the selection of antidepressant medicationis underway, sponsored by the National Institute of Mental Health. Unlike most antide-pressant trials, this study includes subjects with significant concomitant medical illnesses.
FIGURE
Treatment of chronic major depression*
When to refer
Patients requiring referral to a psychiatrist include those with suicidal ideation or severe depression, aggressive ideation, bipolar disorder, atypical depression, psychotic depression, substance abuse, or treatment resistance.52 Referral to a licensed counselor should be offered to most patients with depression, with or without psychiatric involvement, though many factors (eg, patient motivation, capacity for insight, patient perceptions of therapist) will affect follow-through and outcome.
Maintenance therapy
Once full remission has been achieved, 6 to 12 months of continued pharmacotherapy at the same dose is recommended, as it decreases the risk of relapse by 70%.5,21,26 More than half of patients will have a recurrence of depression in their lifetime, and they should be advised about this risk.1
A second episode of major depression confers an 80% chance of additional recurrences, and patients should therefore be maintained on medication for 1 to 2 years.
A third episode requires indefinite maintenance treatment because of a 90% recurrence rate.3,26
Follow-up visits after remission can be tapered gradually to once every 3 months. Discontinuation of therapy should be done gradually to minimize withdrawal reactions; it also necessitates follow-up visits or phone calls.
* For a review of screening for depression, see Nease DE, Malouin JM. Depression screening: A practical strategy. J Fam Pract 2003; 52(2):118–126.
1. Whooley MA, Simon GE. Managing depression in medical outpatients. N Engl J Med 2000;343:1942-1950.
2. Keller MB, McCullough JP, Klein DN, et al. A comparison of nefazodone, the cognitive behavioral-analysis system for psychotherapy, and their combination for the treatment of chronic depression. N Engl J Med 2000;342:1462-1470.
3. Cohen L. Rational drug use in the treatment of depression. Pharmacotherapy 1997;17:45-61.
4. Bhatia SC, Bhatia SK. Depression in women: diagnostic and treatment considerations. Am Fam Physician 1999;60:225-240.
5. Glass RM. Treating depression as a recurrent or chronic disease. JAMA 1999;281:83.-
6. Harwitz D, Ravizza L. Psychiatric emergencies; suicide and depression. Emergency Medical Clinics of North America 2000;18:263-271.
7. Olfson M, Marcus S, Druss B, et al. National trends in the out-patient treatment of depression. JAMA 2002;287:203-209.
8. Stahl SM. Selecting an antidepressant by using mechanism of action to enhance efficacy and avoid side effects. J Clin Psychiatry 1998;59(supp1 18):23-29.
9. Stahl SM. Depression and bipolar disorders. In: Stahl SM. Essential Psychopharmacology, Neuroscientific Basis and Clinical Applications. Cambridge: Cambridge University Press, 1996;135-295.
10. Barbui C, Hotopf M, Freemantle N, et al. Treatment discontinuation with selective serotonin reuptake inhibitors (SSRIs) versus tricyclic antidepressants (TCAs) (Cochrane Review). In: The Cochrane Library, 1, 2002. Oxford; Update Software.
11. Geddes JR, Freemantle N, Mason J, et al. Selective serotonin reuptake inhibitors (SSRIs) for depression (Cochrane Review). In: The Cochrane Library, 1, 2002. Oxford: Update Software.
12. Banerjee S, Shamash K, Macdonald AJD, Mann AH. Randomized controlled trial of effect of intervention by psychogeriatric team on depression in frail elderly people at home. BMJ 1996;313:1058-1061.
13. Reynolds III CF, Frank E, Perel JM, et al. Nortriptyline and interpersonal psychotherapy as maintenance therapies for recurrent major depression. JAMA 1999;281:39-45.
14. Katon W, Von Korff M, Lin E, et al. Stepped collaborative care for primary care patients with persistent symptoms of depression: a randomized trial. Arch Gen Psychiatry 1999;56:1109-1115.
15. Fava GA, Rafanelli C, Grandi S, Grandi S, Conti S, Belluardo P. Prevention of recurrent depression with cognitive behavioral therapy: preliminary findings. Arch Gen Psychiatry 1998;55:816-820.
16. Linde K, Mulrow CD. St John’s wort for depression (Cochrane Review). In: The Cochrane Library, 1, 2002. Oxford; Update Software.
17. Williams Jr JW, Mulrow CD, Chiquette E, et al. A systematic review of newer pharmacotherapies for depression in adults: evidence report summary. Ann Intern Med 2000;132:743-756.
18. Lecrubier Y, Clerc G, Didi R, Kieser M. Efficacy of St. John’s wort extract WS 5570 in major depression: a double-blind, placebo-controlled trial. Am J Psychiatry 2002;159:1361-1366.
19. Shelton RC, Keller MB, Gelenberg A, et al. Effectiveness of St. John’s wort in major depression, a randomized controlled trial. JAMA 2001;285:1978-1986.
20. Hypericum Depression Trial Study Group. Effect of Hypericum perforatum (St. John’s wort) in major depressive disorder, a randomized controlled trial. JAMA 2002;287:1807-1814.
21. Evidence Report/Technology Assessment. Number 7, Treatment of depression-newer pharmacotherapies (AHCPR Publication No. 99-E014). Rockville, Md: US Department of Health and Human Services; 1999.
22. Nemets B, Stahl Z, Belmaker RH. Addition of Omega-3 fatty acid to maintenance medication treatment for recurrent unipolar depressive disorder. Am J Psychiatry 2002;159:477-479.
23. Jellin JM, Gregory P, Balz F, et al. Pharmacist’s Letter/Prescriber’s Letter. Natural Medicines Comprehensive Database. 3rd ed. Stockton, Calif: Therapeutic Research Faculty; 2000;925-928.
24. Blumenthal JA, Babyak MA, Moore KA, Craighead WE, et al. Effects of exercise training on older patients with major depression. Arch Intern Med 1999;159:2349-2356.
25. Lawler DA, Hopker SW. The effectiveness of exercise as an intervention in the management of depression: systematic review and meta-regression analysis of randomized controlled trials. Br Med J 2001;322:763-767.
26. American Psychiatric Association. Practice guideline for the treatment of patients with major depressive disorder in adults. Am J Psychiatry 2000;157(4 suppl):1-45.
27. Crismon ML, Trivedi M, Pigott TA, et al. The Texas Medication Algorithm Project: Report of the Texas Consensus Conference. Panel on medication treatment of major depressive disorder. J Clin Psychiatry 1999;60:142-156.
28. Quitkin FM, Rabkin JG, Ross D, McGrath PJ. Duration of antidepressant drug treatment: what is an adequate trial? Arch Gen Psychiatry 1984;41:238-245.
29. Walsh BT, Seidman SN, Sysko R, Gould M. Placebo response in studies of major depression, variable, substantial, and growing. JAMA 2002;287:1840-1847.
30. Gill D, Hatcher S. Antidepressants for depression in medical illness (Cochrane Review). In: The Cochrane Library,. 1, 2002. Oxford; Update Software.
31. Kroenke K, West SL, Swindle R, et al. Similar effectiveness of paroxetine, fluoxetine, and sertraline in primary care: A randomized trial. JAMA 2001;286:2947-2955.
32. Hartmann PM. Mirtazapine: a newer antidepressant. Am Fam Physician 1999;59:159-161.
33. Hurt RD, Sachs DPL, Glover ED, Offord KP, et al. A comparison of sustained-release bupropion and placebo for smoking cessation. N Engl J Med 1997;337:1195-1202.
34. Clerc GE, Ruimy P, Verdeau-Pailles J, et al. A double-blind comparison of venlafaxine and fluoxetine in patients hospitalized for major depression and melancholia. Int Clin Psychopharmacology 1994;9:139-143.
35. Thase ME, Entsuah AR, Rudolph RL. Remission rates during treatment with venlafaxine or selective serotonin reuptake inhibitors. Br J of Psychiatry 2001;178:234-241.
36. Schweitzer E, Feighner J, Mandos L, Rickles K. Comparison of venlafaxine and imipramine in the acute treatment of major depression in outpatients. J Clin Psychiatry 1994;55:104-108.
37. Product information. Serzone(c) (nefazodone). Princeton, NJ Bristol Myers Squibb Co., 2002 (January).
38. Thase ME, Rush AJ, Howland RH, Kornstein SG, Kocsis JH, Gelenberg AJ, et al. Double-blind switch study of imipramine or sertraline treatment of antidepressant-resistant chronic depression. Arch Gen Psychiatry 2002;59:233-239.
39. Ballenger JC, Davidson JR, Lecrubier Y, Nutt DJ. A proposed algorithm for improved recognition and treatment of depression/anxiety spectrum in primary care. J Clin Psychiatry 2001;3:44-52.
40. Thase ME, Feighner JP, Lydiard RB. Citalopram treatment of fluoxetine nonresponders. J Clin Psychiatry 2001;62:683-687.
41. Nelson JC, Mazure CM, Bowers MB, Jatlow PI. A preliminary, open study of the combination of fluoxetine and desipramine for rapid treatment of major depression. Arch Gen Psychiatry 1991;48:303-307.
42. Stein G, Bernardt M. Lithium augmentation therapy in tri-cyclic-resistant depression: a controlled trial using lithium in low and normal doses. Br J Psychiatry 1993;162:634-640.
43. Joffe RT, Singer W, Levitt AJ, et al. A placebo-controlled comparison of lithium and triiodothyronine augmentation of tricyclic antidepressants in unipolar refractory depression. Arch Gen Psychiatry 1993;50:387-393.
44. Bauer M, Dopfmer S. Lithium augmentation in treatment-resistant depression: meta-analysis of placebo-controlled studies. J Clin Psychopharmacol 1999;19:427-434.
45. Harvey KV, Balon R. Augmentation with buspirone: a review. Ann Clin Psychiatry 1995;2:143-147.
46. Thase ME, Rush AJ. Treatment-resistant depression. In Bloom FE, Kupfer DJ. Psychopharmacology: The Fourth Generation of Progress. New York, NY: Karen Press; 1995;1081-1097.
47. Prange AJ Jr, Loosen PT, Wilson IC, Lipton MA. The therapeutic use of hormone of the thyroid axis in depression. In: Post R, Ballenger J. Neurobiology of mood disorders.. Vol 1. Baltimore, Md: Williams & Wilkins; 1980;311-322.
48. Gagne GG, Furman MJ, Carpenter LL, Price LH. Efficacy of continuation ECT and antidepressant drugs compared to long-term antidepressants alone in depressed patients. Am J Psychiatry 2000;157:1960-1965.
49. American Psychiatric Association.The practice of electroconvulsive therapy: recommendations for treatment, training, and privileging. Washington, DC: American Psychiatric Association, 1990.
50. Shelton RC, Tollefson GD, Tohen M, et al. A novel augmentation strategy for treating resistant major depression. Am J Psychiatry 2001;158:131-134.
51. Trivedi MH, Kleiber BA. Algorithm for the treatment of chronic depression. J Clin Psychiatry 2001;62(suppl 6):22-29.
52. Montana CB. Recognition and treatment of depression in a primary care setting. J Clin Psychiatry 1994;55(suppl 1):18-34.
- Combined treatment with psychotherapy or psychiatric consult and drug therapy has shown better response in several studies than either therapy alone (A).
- Although not proven by clinical trials, selecting a medication by matching its side-effect profile to patient characteristics is supported by case reports and likely enhances compliance.
- Patients who do not improve with initial therapy often benefit from being switched to another class of antidepressants (A), or having a drug from another class added to their therapy (B).
You are more likely to see depression in your practice than any other disorder except hypertension.1 Given the prevalence of depression* and the variability of its clinical symptoms and comorbidities, how do you determine the optimal therapy for a given patient?
A sobering thought: nearly half of all patients stop taking their antidepressant prescription medication within the first month of treatment.1 We discuss the critical factors you can address to help patients stick with treatment and achieve the best outcome.
Therapeutic Options
Pharmacotherapy
Antidepressants are thought to exert their therapeutic and adverse effects through 3 chemical monamine neurotransmission systems; by increasing levels of norepinephrine, serotonin, or dopamine in the synapse; and by resultant secondary changes in presynaptic and postsynaptic receptor physiology.3,8,9 Newer medications—such as selective serotonin reuptake inhibitors (SSRIs)—have simpler dose schedules, different (and for some patients more favorable) adverse effect profiles, and less likelihood of causing death from overdose compared with older tricyclic antidepressants (TCAs) and monamine oxidase inhibitors (MAOIs).
Patients are less likely to discontinue treatment with SSRIs than with TCAs (odds ratio=1.21; 95% confidence interval [CI], 1.12–1.30).10
However, there are no clinically significant differences in effectiveness between SSRIs and TCAs (strength of recommendation [SOR]: A).11 Importantly, although practice patterns in the use of antidepressants have changed, some reasons for the preference of newer effective agents have not been substantiated. For instance, we do not know whether the patient population taking newer agents has a lower rate of suicide, despite the difference in fatality risk mentioned earlier.
Combined pharmacotherapy and psychiatric consultation
Combining pharmacotherapy and psychotherapy can be more effective than either modality alone. In one study, 73% of patients with chronic depression treated with combination therapy showed a reduction of 50% or more on the Hamilton Rating Scale for Depression (HRSD), compared with just 48% in the nefazodone-only and psychotherapy-only groups (SOR: A). Among those who completed the study, the rates of response were 85%, 55%, and 52%, respectively (although the results considered compliant patients only, which biases the results in favor of treatment).2
Among elderly depressed patients who received home care, 58% of those who underwent intervention by a psychogeriatric team recovered, compared with just 25% in the control group (SOR: A).12 The intervention group received a multidisciplinary team evaluation and an individualized management plan, which could include any combination of physical, psychological, or social interventions. The control group received usual care from their general practitioner.
Studies of combination therapy have yielded mixed results, but guidelines from the psychiatric literature based on clinical experience advocate concomitant psychotherapy and medication (SOR: A).13 For patients with persistent symptoms after 6 to 8 weeks of taking antidepressant medication, concomitant psychotherapy improved compliance, satisfaction, and outcomes when compared with usual care.14
At any one time, at least 3% of the US population suffers from chronic depression.2 More than 17% of the population have had a major depressive episode in their lifetime, and more than 10% have experienced an episode within the past 12 months.3 The incidence and prevalence of depression in women are approximately twice that seen in men.4
Major depression is the fourth leading cause of worldwide disease burden.5
Natural history and prognosis
An untreated episode of depression usually lasts 6 months or longer. About half of persons experiencing major depression will have a second episode; a second episode increases the risk for a third episode to 80%.1,3 Patients diagnosed with depression average 5 depressive episodes in their life and may have recurrences every 4 to 6 years. Episodes usually become longer and more frequent with advancing age. In about 20% to 35% of cases, only partial remission occurs and functioning remains impaired.1
Fifteen percent of severely depressed patients commit suicide. The 2 most powerful predictors of suicide are a history of major depression or schizophrenia and a history of addictive disorders.6
Outpatient treatment of depression has increased markedly in the United States, with greater involvement on the part of physicians, greater use of psychotropic medications, expanding availability of third-party payment, and less use of psychotherapy.7
The concomitant therapy group participated in a multifaceted program including education, psychiatric referral, pharmacy utilization records, and primary physician feedback. The usual care group received standard antidepressants and follow-up visits from their family physician, with optional referral to a mental health provider.
Psychotherapy has also been shown to decrease the risk of relapse once symptoms have remitted.15 Primary care physicians can also incorporate counseling as adjunctive therapy.
Herbal and nutritional products
St. John’s wort. St. John’s wort (Hypericum perforatum L.) has been used as an herbal medication for more than 2000 years. Its efficacy in the treatment of depression has been studied extensively. Some studies demonstrated that these extracts are more effective than placebo for the short-term treatment of mild and moderate depression.16,17,18 Two randomized controlled trials demonstrated minimal efficacy of St. John’s wort in moderately severe major depression.19,20 The National Institutes of Health is sponsoring a placebo-controlled, double-blinded trial comparing St. John’s wort with SSRIs.21
Omega-3 fatty acids. Chronic deficiencies of essential fatty acids may adversely affect central nervous system function. In a small, 4-week double-blind study, outpatients receiving antidepressant therapy who were also given eicosapentaenoic acid exhibited improvement in core depressive symptoms (eg, worthlessness, guilt, insomnia) compared with the antidepressantplus-placebo group. Larger, long-term prospective trials are needed to confirm an antidepressant effect with omega-3 fatty acids.22
S-adenosyl-L-methionine. S-adenosyl-L-methionine is possibly effective for short-term treatment of major depression. Data for other herbal or nutritional remedies are negligible.23
Exercise
Physical activity may play an important role in relieving depression. One randomized controlled trial showed that an aerobic exercise program, sertraline therapy, or a combination of both were equally effective in the treatment of depression, although there was a more rapid initial response with sertraline.24
A systematic review and meta-analysis concluded that exercise may reduce depression symptoms short term, but much of the evidence is of poor quality.25 Well-controlled studies are needed to clarify the role of exercise in the treatment of depression. However, exercise is promising enough to consider implementation in clinical practice at this time.
Treatment strategy
Guidelines for medicating patients and setting expectations
Start antidepressant therapy promptly when depression is diagnosed. Maintain the initial dosage for at least 3 to 4 weeks before increasing it. A trial of 6 to 8 weeks at maximum dosage (or the maximum tolerated dosage) is necessary to confirm treatment success or failure.26,27,28
An improvement in symptoms will usually not be noted until after 2 to 6 weeks of therapy. Depending on depression severity, schedule weekly or monthly visits for patients during the initial treatment phase. The response rate to initial treatment is only 50% to 60%, but more than 80% of depressed patients will respond to at least 1 medication.1
Response to placebo is highly variable. It is often substantial and has increased in recent years. In an analysis of 75 trials between 1981 and 2001, the mean proportion of patients in the placebo group who responded (50% improvement on the HRSD) was 29.7%, compared with 50.1% in the active medication group.29 The placebo effect may reflect some combination of patient expectations, the natural history of depression with possible spontaneous remission, and limitations of study methods.
Antidepressant therapy is effective compared with placebo for depression secondary to medical illness (number needed to treat [NNT], 4.2; 95% CI, 3.2–6.4), with minimal treatment dropout (number needed to harm, 9.8; 95% CI, 5.4–42.9).30 Although many patients settle for partial improvement of their symptoms, the treatment goal should be complete remission.
Factors in drug selection
Selecting an antidepressant can be challenging: more than 24 drugs are on the market, each working through 1 or more of 7 pharmacologic mechanisms. Theoretically, choosing a drug is made easier by matching patient symptoms to likely medication side effects or by knowing that the patient or a family member responded favorably to a particular antidepressant in the past.
This intuitive model has not been proven superior to any other model of selecting antidepres-sants, but it is clinically sound, pharmacodynamically appealing, and supported by case reports. Its strength may lie in enhancing patient adherence during the critical initial phase of treatment.
A recent randomized, prospective comparison of the SSRIs paroxetine, fluoxetine, and sertraline showed similar effectiveness and tolerability (SOR: A).31 This suggests that efforts to individualize therapy based on comorbidities or likely side effects may not be as useful when choosing from among analogous SSRIs.
Nevertheless, choosing a drug that is effective, convenient, and well tolerated will improve the likelihood of achieving and maintaining a full remission. The data on adverse effects of antidepressants are widely available and well understood. Also consider cost (Table).
Preferences based on characteristics. For a patient whose depression is not complicated by other clinical conditions, the initial choice of antidepressant would usually be an SSRI. But nefazodone, mirtazapine, bupropion, or low-dose venlafaxine may be equally appropriate.
For a patient whose depression has other specific components, use your knowledge of drugs’ common side effects to fit the patient’s clinical profile.
- If there is generalized anxiety, agitation, and insomnia, both nefazodone8 and mirtazapine32 are excellent choices. Trazodone at low doses is often used as a sedative with nonsedating antidepressants.8
- If weight gain is desired, mirtazapine is indicated.32
- If tobacco cessation is a secondary goal, bupropion is preferred.31
- Those suffering from hypersomnia, retarded depression, cognitive slowing, and pseudodementia would benefit from bupropion or venlafaxine.9
- For more severely depressed patients, venlafaxine may be advantageous due to its dual serotonergic and noradrenergic activity at moderate to high doses.34,35,36 Mirtazapine and TCAs are also useful in severe depression, as well as for coexisting chronic pain syndromes.8 For refractory or atypical depression in motivated and compliant patients, MAOIs my be useful.8
When to avoid specific drugs.
- Patients with hypersomnia and motor retardation should avoid nefazodone and mirtazapine.8,32
- With obesity, mirtazapine and TCAs are least preferred.8,32
- If sexual dysfunction preceded depression, avoid giving SSRIs and venlafaxine.3
- Those experiencing agitation and insomnia should avoid bupropion and venlafaxine.3
- Seizure disorder is a contraindication to bupropion.3
- Hypertension is a relative contraindication to venlafaxine.3
- Liver disease is a contraindication to nefazodone.37
- Preexisting heart disease and increased suicide risk are both relative contraindications to TCAs.8
TABLE
Comparative dosages and costs of antidepressant drugs
| Agents | Initial target dose | Maximum effective dose | Monthly cost of initial target dose* |
|---|---|---|---|
| Selective serotonin reuptake inhibitors | |||
| Citalopram (Celexa) | 20 mg qd | 60 mg qd | $61.58 |
| Escitalopram (Lexapro) | 10 mg qd | 20 mg qd | $65.28 |
| Fluoxetine (Prozac) | 20 mg qd | 80 mg qd | $81.78 |
| Fluoxetine (generic) | 20 mg qd | 80 mg qd | $61.80 |
| Fluoxetine (Prozac Weekly) | 90 mg qwk | $71.04 | |
| Fluvoxamine (Luvox) | 50 mg qd | 150 mg bid | $59.70 |
| Paroxetine (Paxil) | 20 mg qd | 60 mg qd | $70.98 |
| Paroxetine (Paxil CR) | 25 mg qd | 75 mg qd | $75.86 |
| Sertraline (Zoloft) | 50 mg qd | 200 mg qd | $65.24 |
| Tricyclic antidepressants | |||
| Amitriptyline (Elavil, Endep, Vanatrip) | 100 mg qhs | 300 mg qhs | $ 7.99 |
| Desipramine (Norpramin) | 100 mg qhs | 200 mg qhs | $18.54 |
| Doxepin (Adapin, Sinequan) | 100 mg qhs | 300 mg qhs | $8.12 |
| Imipramine (Tofranil) | 100 mg qhs | 300 mg qhs | $31.96 |
| Nortriptyline (Aventil, Pamelor) | 75 mg qhs | 150 mg qhs | $ 8.71 |
| Others | |||
| Bupropion (Wellbutrin) | 100 mg tid | 150 mg tid | $92.33 |
| Bupropion (generic) | 100 mg tid | 150 mg tid | $64.62 |
| Bupropion (Wellbutrin SR) | 150 mg bid | 200 mg bid | $87.09 |
| Mirtazapine (Remeron) | 30 mg qhs | 45 mg qhs | $80.79 |
| Nefazodone (Serzone) | 100 mg bid | 300 mg bid | $74.94 |
| Trazodone (Desyrel) | 100 mg bid | 300 mg bid | $15.98 |
| Venlafaxine (Effexor) | 37.5 mg bid | 150 mg tid | $74.39 |
| Venlafaxine (Effexor XL) | 75 mg qd | 225 mg qd | $66.25 |
| Lithium (Eskalith, Lithobid, Lithonate, Lithotabs) | 300 mg bid | 600 mg bid | $13.70 |
| *Costs from www.drugstore.com, November 2002. | |||
Helping nonresponders
Patients whose symptoms do not improve with therapy could be switched to a different monotherapy or to multiple drugs. Drug choices for treatment-refractory and nonresponding patients have evolved more by anecdote than by systematic study.9
Switch drugs. The benefit of switching patients to another category of antidepressant was recently demonstrated in a study where nearly half of patients who did not respond to an initial antidepressant, whether SSRI or TCA, responded when switched to the alternate agent (SOR: A).38 It is also beneficial to switch medications within a category (SOR: B).27,39,40
Add a drug. Adding a second antidepressant from a category with a different mechanism of action often enhances clinical efficacy. This has been demonstrated in combining an SSRI with a TCA (SOR: B).41 Though response rates are very similar for various antidepressants, complete remission and rates of response in severely depressed patients may be higher in dual-action antidepressants (SOR: A).34,35,36
Add lithium. A great deal of evidence supports the use of lithium augmentation (SOR: A).42,43 This agent should be used more in primary care and not only by psychiatrists. A recent meta-analysis of double-blind, placebo-controlled studies of lithium (given at a dosage of at least 800 mg/d or at a level high enough to achieve a serum drug concentration of 0.5 mEq/L for at least 2 weeks) found a summary pooled odds ratio of response to lithium of 3.31 (95% CI, 1.46–7.53) with a NNT of 3.7.44 Other studies have been less clear on the optimal dose or blood level, so a starting dose of 300 mg twice daily with a serum drug concentration of 0.4 mEq/L has been recommended.
If renal function is normal, the concentration of lithium can be checked 5 days after a patient has received a stable dosage, at least 8 hours fol-lowing the last dose. Lithium may cause thyroid abnormalities; monitoring should include a measurement of thyroid-stimulating hormone, repeated at 6 months and 1 year.
Other augmentation options. Augmentation of antidepressants with buspirone has been proven useful in major depression (SOR: B).45 Thyroid supplementation may also increase the effectiveness of antidepressant therapy using triiodothyronine (T3), at doses not to exceed 50 mcg per day (SOR: B).46,47 Electroconvulsive therapy (ECT) has a high rate of therapeutic success, including speed and safety, but it is not administered as first-line treatment by psychiatrists except in severe cases (SOR: A).48,49 Augmentation with antipsychotic or anticonvulsants is another strategy that shows some benefit for select patients.50
Texas medication algorithm project
The process of drug selection just described can avoid treatment-threatening side effects, enable patient adherence to treatment, and maximize the potential for therapeutic response. However, the model can become disorienting for the clinician and the patient if 1 or 2 initial selections for treatment do not succeed. A useful synergy may be achieved by adapting the intuitive model to an algorithmic model—the Texas Medication Algorithm Project (TMAP). TMAP is an evolving model that reflects ongoing clinical research in the treatment of depression.27
Developed in 1995 from a review of existing antidepressant research and several consensus conferences, the TMAP (continually updated with new research findings) has developed algorithms for treatment of schizophrenia and bipolar disorder in addition to major depression. At each stage in the depression algorithm, treatment plans similar in efficacy and safety are grouped together, and the clinician is given a limited number of options. The later stages in the algorithm are more complex, admittedly with a greater potential for medical complications (Figure).51
The algorithm represents a tentative foundation for a sequenced medication plan. Research pertaining to the selection of antidepressant medicationis underway, sponsored by the National Institute of Mental Health. Unlike most antide-pressant trials, this study includes subjects with significant concomitant medical illnesses.
FIGURE
Treatment of chronic major depression*
When to refer
Patients requiring referral to a psychiatrist include those with suicidal ideation or severe depression, aggressive ideation, bipolar disorder, atypical depression, psychotic depression, substance abuse, or treatment resistance.52 Referral to a licensed counselor should be offered to most patients with depression, with or without psychiatric involvement, though many factors (eg, patient motivation, capacity for insight, patient perceptions of therapist) will affect follow-through and outcome.
Maintenance therapy
Once full remission has been achieved, 6 to 12 months of continued pharmacotherapy at the same dose is recommended, as it decreases the risk of relapse by 70%.5,21,26 More than half of patients will have a recurrence of depression in their lifetime, and they should be advised about this risk.1
A second episode of major depression confers an 80% chance of additional recurrences, and patients should therefore be maintained on medication for 1 to 2 years.
A third episode requires indefinite maintenance treatment because of a 90% recurrence rate.3,26
Follow-up visits after remission can be tapered gradually to once every 3 months. Discontinuation of therapy should be done gradually to minimize withdrawal reactions; it also necessitates follow-up visits or phone calls.
* For a review of screening for depression, see Nease DE, Malouin JM. Depression screening: A practical strategy. J Fam Pract 2003; 52(2):118–126.
- Combined treatment with psychotherapy or psychiatric consult and drug therapy has shown better response in several studies than either therapy alone (A).
- Although not proven by clinical trials, selecting a medication by matching its side-effect profile to patient characteristics is supported by case reports and likely enhances compliance.
- Patients who do not improve with initial therapy often benefit from being switched to another class of antidepressants (A), or having a drug from another class added to their therapy (B).
You are more likely to see depression in your practice than any other disorder except hypertension.1 Given the prevalence of depression* and the variability of its clinical symptoms and comorbidities, how do you determine the optimal therapy for a given patient?
A sobering thought: nearly half of all patients stop taking their antidepressant prescription medication within the first month of treatment.1 We discuss the critical factors you can address to help patients stick with treatment and achieve the best outcome.
Therapeutic Options
Pharmacotherapy
Antidepressants are thought to exert their therapeutic and adverse effects through 3 chemical monamine neurotransmission systems; by increasing levels of norepinephrine, serotonin, or dopamine in the synapse; and by resultant secondary changes in presynaptic and postsynaptic receptor physiology.3,8,9 Newer medications—such as selective serotonin reuptake inhibitors (SSRIs)—have simpler dose schedules, different (and for some patients more favorable) adverse effect profiles, and less likelihood of causing death from overdose compared with older tricyclic antidepressants (TCAs) and monamine oxidase inhibitors (MAOIs).
Patients are less likely to discontinue treatment with SSRIs than with TCAs (odds ratio=1.21; 95% confidence interval [CI], 1.12–1.30).10
However, there are no clinically significant differences in effectiveness between SSRIs and TCAs (strength of recommendation [SOR]: A).11 Importantly, although practice patterns in the use of antidepressants have changed, some reasons for the preference of newer effective agents have not been substantiated. For instance, we do not know whether the patient population taking newer agents has a lower rate of suicide, despite the difference in fatality risk mentioned earlier.
Combined pharmacotherapy and psychiatric consultation
Combining pharmacotherapy and psychotherapy can be more effective than either modality alone. In one study, 73% of patients with chronic depression treated with combination therapy showed a reduction of 50% or more on the Hamilton Rating Scale for Depression (HRSD), compared with just 48% in the nefazodone-only and psychotherapy-only groups (SOR: A). Among those who completed the study, the rates of response were 85%, 55%, and 52%, respectively (although the results considered compliant patients only, which biases the results in favor of treatment).2
Among elderly depressed patients who received home care, 58% of those who underwent intervention by a psychogeriatric team recovered, compared with just 25% in the control group (SOR: A).12 The intervention group received a multidisciplinary team evaluation and an individualized management plan, which could include any combination of physical, psychological, or social interventions. The control group received usual care from their general practitioner.
Studies of combination therapy have yielded mixed results, but guidelines from the psychiatric literature based on clinical experience advocate concomitant psychotherapy and medication (SOR: A).13 For patients with persistent symptoms after 6 to 8 weeks of taking antidepressant medication, concomitant psychotherapy improved compliance, satisfaction, and outcomes when compared with usual care.14
At any one time, at least 3% of the US population suffers from chronic depression.2 More than 17% of the population have had a major depressive episode in their lifetime, and more than 10% have experienced an episode within the past 12 months.3 The incidence and prevalence of depression in women are approximately twice that seen in men.4
Major depression is the fourth leading cause of worldwide disease burden.5
Natural history and prognosis
An untreated episode of depression usually lasts 6 months or longer. About half of persons experiencing major depression will have a second episode; a second episode increases the risk for a third episode to 80%.1,3 Patients diagnosed with depression average 5 depressive episodes in their life and may have recurrences every 4 to 6 years. Episodes usually become longer and more frequent with advancing age. In about 20% to 35% of cases, only partial remission occurs and functioning remains impaired.1
Fifteen percent of severely depressed patients commit suicide. The 2 most powerful predictors of suicide are a history of major depression or schizophrenia and a history of addictive disorders.6
Outpatient treatment of depression has increased markedly in the United States, with greater involvement on the part of physicians, greater use of psychotropic medications, expanding availability of third-party payment, and less use of psychotherapy.7
The concomitant therapy group participated in a multifaceted program including education, psychiatric referral, pharmacy utilization records, and primary physician feedback. The usual care group received standard antidepressants and follow-up visits from their family physician, with optional referral to a mental health provider.
Psychotherapy has also been shown to decrease the risk of relapse once symptoms have remitted.15 Primary care physicians can also incorporate counseling as adjunctive therapy.
Herbal and nutritional products
St. John’s wort. St. John’s wort (Hypericum perforatum L.) has been used as an herbal medication for more than 2000 years. Its efficacy in the treatment of depression has been studied extensively. Some studies demonstrated that these extracts are more effective than placebo for the short-term treatment of mild and moderate depression.16,17,18 Two randomized controlled trials demonstrated minimal efficacy of St. John’s wort in moderately severe major depression.19,20 The National Institutes of Health is sponsoring a placebo-controlled, double-blinded trial comparing St. John’s wort with SSRIs.21
Omega-3 fatty acids. Chronic deficiencies of essential fatty acids may adversely affect central nervous system function. In a small, 4-week double-blind study, outpatients receiving antidepressant therapy who were also given eicosapentaenoic acid exhibited improvement in core depressive symptoms (eg, worthlessness, guilt, insomnia) compared with the antidepressantplus-placebo group. Larger, long-term prospective trials are needed to confirm an antidepressant effect with omega-3 fatty acids.22
S-adenosyl-L-methionine. S-adenosyl-L-methionine is possibly effective for short-term treatment of major depression. Data for other herbal or nutritional remedies are negligible.23
Exercise
Physical activity may play an important role in relieving depression. One randomized controlled trial showed that an aerobic exercise program, sertraline therapy, or a combination of both were equally effective in the treatment of depression, although there was a more rapid initial response with sertraline.24
A systematic review and meta-analysis concluded that exercise may reduce depression symptoms short term, but much of the evidence is of poor quality.25 Well-controlled studies are needed to clarify the role of exercise in the treatment of depression. However, exercise is promising enough to consider implementation in clinical practice at this time.
Treatment strategy
Guidelines for medicating patients and setting expectations
Start antidepressant therapy promptly when depression is diagnosed. Maintain the initial dosage for at least 3 to 4 weeks before increasing it. A trial of 6 to 8 weeks at maximum dosage (or the maximum tolerated dosage) is necessary to confirm treatment success or failure.26,27,28
An improvement in symptoms will usually not be noted until after 2 to 6 weeks of therapy. Depending on depression severity, schedule weekly or monthly visits for patients during the initial treatment phase. The response rate to initial treatment is only 50% to 60%, but more than 80% of depressed patients will respond to at least 1 medication.1
Response to placebo is highly variable. It is often substantial and has increased in recent years. In an analysis of 75 trials between 1981 and 2001, the mean proportion of patients in the placebo group who responded (50% improvement on the HRSD) was 29.7%, compared with 50.1% in the active medication group.29 The placebo effect may reflect some combination of patient expectations, the natural history of depression with possible spontaneous remission, and limitations of study methods.
Antidepressant therapy is effective compared with placebo for depression secondary to medical illness (number needed to treat [NNT], 4.2; 95% CI, 3.2–6.4), with minimal treatment dropout (number needed to harm, 9.8; 95% CI, 5.4–42.9).30 Although many patients settle for partial improvement of their symptoms, the treatment goal should be complete remission.
Factors in drug selection
Selecting an antidepressant can be challenging: more than 24 drugs are on the market, each working through 1 or more of 7 pharmacologic mechanisms. Theoretically, choosing a drug is made easier by matching patient symptoms to likely medication side effects or by knowing that the patient or a family member responded favorably to a particular antidepressant in the past.
This intuitive model has not been proven superior to any other model of selecting antidepres-sants, but it is clinically sound, pharmacodynamically appealing, and supported by case reports. Its strength may lie in enhancing patient adherence during the critical initial phase of treatment.
A recent randomized, prospective comparison of the SSRIs paroxetine, fluoxetine, and sertraline showed similar effectiveness and tolerability (SOR: A).31 This suggests that efforts to individualize therapy based on comorbidities or likely side effects may not be as useful when choosing from among analogous SSRIs.
Nevertheless, choosing a drug that is effective, convenient, and well tolerated will improve the likelihood of achieving and maintaining a full remission. The data on adverse effects of antidepressants are widely available and well understood. Also consider cost (Table).
Preferences based on characteristics. For a patient whose depression is not complicated by other clinical conditions, the initial choice of antidepressant would usually be an SSRI. But nefazodone, mirtazapine, bupropion, or low-dose venlafaxine may be equally appropriate.
For a patient whose depression has other specific components, use your knowledge of drugs’ common side effects to fit the patient’s clinical profile.
- If there is generalized anxiety, agitation, and insomnia, both nefazodone8 and mirtazapine32 are excellent choices. Trazodone at low doses is often used as a sedative with nonsedating antidepressants.8
- If weight gain is desired, mirtazapine is indicated.32
- If tobacco cessation is a secondary goal, bupropion is preferred.31
- Those suffering from hypersomnia, retarded depression, cognitive slowing, and pseudodementia would benefit from bupropion or venlafaxine.9
- For more severely depressed patients, venlafaxine may be advantageous due to its dual serotonergic and noradrenergic activity at moderate to high doses.34,35,36 Mirtazapine and TCAs are also useful in severe depression, as well as for coexisting chronic pain syndromes.8 For refractory or atypical depression in motivated and compliant patients, MAOIs my be useful.8
When to avoid specific drugs.
- Patients with hypersomnia and motor retardation should avoid nefazodone and mirtazapine.8,32
- With obesity, mirtazapine and TCAs are least preferred.8,32
- If sexual dysfunction preceded depression, avoid giving SSRIs and venlafaxine.3
- Those experiencing agitation and insomnia should avoid bupropion and venlafaxine.3
- Seizure disorder is a contraindication to bupropion.3
- Hypertension is a relative contraindication to venlafaxine.3
- Liver disease is a contraindication to nefazodone.37
- Preexisting heart disease and increased suicide risk are both relative contraindications to TCAs.8
TABLE
Comparative dosages and costs of antidepressant drugs
| Agents | Initial target dose | Maximum effective dose | Monthly cost of initial target dose* |
|---|---|---|---|
| Selective serotonin reuptake inhibitors | |||
| Citalopram (Celexa) | 20 mg qd | 60 mg qd | $61.58 |
| Escitalopram (Lexapro) | 10 mg qd | 20 mg qd | $65.28 |
| Fluoxetine (Prozac) | 20 mg qd | 80 mg qd | $81.78 |
| Fluoxetine (generic) | 20 mg qd | 80 mg qd | $61.80 |
| Fluoxetine (Prozac Weekly) | 90 mg qwk | $71.04 | |
| Fluvoxamine (Luvox) | 50 mg qd | 150 mg bid | $59.70 |
| Paroxetine (Paxil) | 20 mg qd | 60 mg qd | $70.98 |
| Paroxetine (Paxil CR) | 25 mg qd | 75 mg qd | $75.86 |
| Sertraline (Zoloft) | 50 mg qd | 200 mg qd | $65.24 |
| Tricyclic antidepressants | |||
| Amitriptyline (Elavil, Endep, Vanatrip) | 100 mg qhs | 300 mg qhs | $ 7.99 |
| Desipramine (Norpramin) | 100 mg qhs | 200 mg qhs | $18.54 |
| Doxepin (Adapin, Sinequan) | 100 mg qhs | 300 mg qhs | $8.12 |
| Imipramine (Tofranil) | 100 mg qhs | 300 mg qhs | $31.96 |
| Nortriptyline (Aventil, Pamelor) | 75 mg qhs | 150 mg qhs | $ 8.71 |
| Others | |||
| Bupropion (Wellbutrin) | 100 mg tid | 150 mg tid | $92.33 |
| Bupropion (generic) | 100 mg tid | 150 mg tid | $64.62 |
| Bupropion (Wellbutrin SR) | 150 mg bid | 200 mg bid | $87.09 |
| Mirtazapine (Remeron) | 30 mg qhs | 45 mg qhs | $80.79 |
| Nefazodone (Serzone) | 100 mg bid | 300 mg bid | $74.94 |
| Trazodone (Desyrel) | 100 mg bid | 300 mg bid | $15.98 |
| Venlafaxine (Effexor) | 37.5 mg bid | 150 mg tid | $74.39 |
| Venlafaxine (Effexor XL) | 75 mg qd | 225 mg qd | $66.25 |
| Lithium (Eskalith, Lithobid, Lithonate, Lithotabs) | 300 mg bid | 600 mg bid | $13.70 |
| *Costs from www.drugstore.com, November 2002. | |||
Helping nonresponders
Patients whose symptoms do not improve with therapy could be switched to a different monotherapy or to multiple drugs. Drug choices for treatment-refractory and nonresponding patients have evolved more by anecdote than by systematic study.9
Switch drugs. The benefit of switching patients to another category of antidepressant was recently demonstrated in a study where nearly half of patients who did not respond to an initial antidepressant, whether SSRI or TCA, responded when switched to the alternate agent (SOR: A).38 It is also beneficial to switch medications within a category (SOR: B).27,39,40
Add a drug. Adding a second antidepressant from a category with a different mechanism of action often enhances clinical efficacy. This has been demonstrated in combining an SSRI with a TCA (SOR: B).41 Though response rates are very similar for various antidepressants, complete remission and rates of response in severely depressed patients may be higher in dual-action antidepressants (SOR: A).34,35,36
Add lithium. A great deal of evidence supports the use of lithium augmentation (SOR: A).42,43 This agent should be used more in primary care and not only by psychiatrists. A recent meta-analysis of double-blind, placebo-controlled studies of lithium (given at a dosage of at least 800 mg/d or at a level high enough to achieve a serum drug concentration of 0.5 mEq/L for at least 2 weeks) found a summary pooled odds ratio of response to lithium of 3.31 (95% CI, 1.46–7.53) with a NNT of 3.7.44 Other studies have been less clear on the optimal dose or blood level, so a starting dose of 300 mg twice daily with a serum drug concentration of 0.4 mEq/L has been recommended.
If renal function is normal, the concentration of lithium can be checked 5 days after a patient has received a stable dosage, at least 8 hours fol-lowing the last dose. Lithium may cause thyroid abnormalities; monitoring should include a measurement of thyroid-stimulating hormone, repeated at 6 months and 1 year.
Other augmentation options. Augmentation of antidepressants with buspirone has been proven useful in major depression (SOR: B).45 Thyroid supplementation may also increase the effectiveness of antidepressant therapy using triiodothyronine (T3), at doses not to exceed 50 mcg per day (SOR: B).46,47 Electroconvulsive therapy (ECT) has a high rate of therapeutic success, including speed and safety, but it is not administered as first-line treatment by psychiatrists except in severe cases (SOR: A).48,49 Augmentation with antipsychotic or anticonvulsants is another strategy that shows some benefit for select patients.50
Texas medication algorithm project
The process of drug selection just described can avoid treatment-threatening side effects, enable patient adherence to treatment, and maximize the potential for therapeutic response. However, the model can become disorienting for the clinician and the patient if 1 or 2 initial selections for treatment do not succeed. A useful synergy may be achieved by adapting the intuitive model to an algorithmic model—the Texas Medication Algorithm Project (TMAP). TMAP is an evolving model that reflects ongoing clinical research in the treatment of depression.27
Developed in 1995 from a review of existing antidepressant research and several consensus conferences, the TMAP (continually updated with new research findings) has developed algorithms for treatment of schizophrenia and bipolar disorder in addition to major depression. At each stage in the depression algorithm, treatment plans similar in efficacy and safety are grouped together, and the clinician is given a limited number of options. The later stages in the algorithm are more complex, admittedly with a greater potential for medical complications (Figure).51
The algorithm represents a tentative foundation for a sequenced medication plan. Research pertaining to the selection of antidepressant medicationis underway, sponsored by the National Institute of Mental Health. Unlike most antide-pressant trials, this study includes subjects with significant concomitant medical illnesses.
FIGURE
Treatment of chronic major depression*
When to refer
Patients requiring referral to a psychiatrist include those with suicidal ideation or severe depression, aggressive ideation, bipolar disorder, atypical depression, psychotic depression, substance abuse, or treatment resistance.52 Referral to a licensed counselor should be offered to most patients with depression, with or without psychiatric involvement, though many factors (eg, patient motivation, capacity for insight, patient perceptions of therapist) will affect follow-through and outcome.
Maintenance therapy
Once full remission has been achieved, 6 to 12 months of continued pharmacotherapy at the same dose is recommended, as it decreases the risk of relapse by 70%.5,21,26 More than half of patients will have a recurrence of depression in their lifetime, and they should be advised about this risk.1
A second episode of major depression confers an 80% chance of additional recurrences, and patients should therefore be maintained on medication for 1 to 2 years.
A third episode requires indefinite maintenance treatment because of a 90% recurrence rate.3,26
Follow-up visits after remission can be tapered gradually to once every 3 months. Discontinuation of therapy should be done gradually to minimize withdrawal reactions; it also necessitates follow-up visits or phone calls.
* For a review of screening for depression, see Nease DE, Malouin JM. Depression screening: A practical strategy. J Fam Pract 2003; 52(2):118–126.
1. Whooley MA, Simon GE. Managing depression in medical outpatients. N Engl J Med 2000;343:1942-1950.
2. Keller MB, McCullough JP, Klein DN, et al. A comparison of nefazodone, the cognitive behavioral-analysis system for psychotherapy, and their combination for the treatment of chronic depression. N Engl J Med 2000;342:1462-1470.
3. Cohen L. Rational drug use in the treatment of depression. Pharmacotherapy 1997;17:45-61.
4. Bhatia SC, Bhatia SK. Depression in women: diagnostic and treatment considerations. Am Fam Physician 1999;60:225-240.
5. Glass RM. Treating depression as a recurrent or chronic disease. JAMA 1999;281:83.-
6. Harwitz D, Ravizza L. Psychiatric emergencies; suicide and depression. Emergency Medical Clinics of North America 2000;18:263-271.
7. Olfson M, Marcus S, Druss B, et al. National trends in the out-patient treatment of depression. JAMA 2002;287:203-209.
8. Stahl SM. Selecting an antidepressant by using mechanism of action to enhance efficacy and avoid side effects. J Clin Psychiatry 1998;59(supp1 18):23-29.
9. Stahl SM. Depression and bipolar disorders. In: Stahl SM. Essential Psychopharmacology, Neuroscientific Basis and Clinical Applications. Cambridge: Cambridge University Press, 1996;135-295.
10. Barbui C, Hotopf M, Freemantle N, et al. Treatment discontinuation with selective serotonin reuptake inhibitors (SSRIs) versus tricyclic antidepressants (TCAs) (Cochrane Review). In: The Cochrane Library, 1, 2002. Oxford; Update Software.
11. Geddes JR, Freemantle N, Mason J, et al. Selective serotonin reuptake inhibitors (SSRIs) for depression (Cochrane Review). In: The Cochrane Library, 1, 2002. Oxford: Update Software.
12. Banerjee S, Shamash K, Macdonald AJD, Mann AH. Randomized controlled trial of effect of intervention by psychogeriatric team on depression in frail elderly people at home. BMJ 1996;313:1058-1061.
13. Reynolds III CF, Frank E, Perel JM, et al. Nortriptyline and interpersonal psychotherapy as maintenance therapies for recurrent major depression. JAMA 1999;281:39-45.
14. Katon W, Von Korff M, Lin E, et al. Stepped collaborative care for primary care patients with persistent symptoms of depression: a randomized trial. Arch Gen Psychiatry 1999;56:1109-1115.
15. Fava GA, Rafanelli C, Grandi S, Grandi S, Conti S, Belluardo P. Prevention of recurrent depression with cognitive behavioral therapy: preliminary findings. Arch Gen Psychiatry 1998;55:816-820.
16. Linde K, Mulrow CD. St John’s wort for depression (Cochrane Review). In: The Cochrane Library, 1, 2002. Oxford; Update Software.
17. Williams Jr JW, Mulrow CD, Chiquette E, et al. A systematic review of newer pharmacotherapies for depression in adults: evidence report summary. Ann Intern Med 2000;132:743-756.
18. Lecrubier Y, Clerc G, Didi R, Kieser M. Efficacy of St. John’s wort extract WS 5570 in major depression: a double-blind, placebo-controlled trial. Am J Psychiatry 2002;159:1361-1366.
19. Shelton RC, Keller MB, Gelenberg A, et al. Effectiveness of St. John’s wort in major depression, a randomized controlled trial. JAMA 2001;285:1978-1986.
20. Hypericum Depression Trial Study Group. Effect of Hypericum perforatum (St. John’s wort) in major depressive disorder, a randomized controlled trial. JAMA 2002;287:1807-1814.
21. Evidence Report/Technology Assessment. Number 7, Treatment of depression-newer pharmacotherapies (AHCPR Publication No. 99-E014). Rockville, Md: US Department of Health and Human Services; 1999.
22. Nemets B, Stahl Z, Belmaker RH. Addition of Omega-3 fatty acid to maintenance medication treatment for recurrent unipolar depressive disorder. Am J Psychiatry 2002;159:477-479.
23. Jellin JM, Gregory P, Balz F, et al. Pharmacist’s Letter/Prescriber’s Letter. Natural Medicines Comprehensive Database. 3rd ed. Stockton, Calif: Therapeutic Research Faculty; 2000;925-928.
24. Blumenthal JA, Babyak MA, Moore KA, Craighead WE, et al. Effects of exercise training on older patients with major depression. Arch Intern Med 1999;159:2349-2356.
25. Lawler DA, Hopker SW. The effectiveness of exercise as an intervention in the management of depression: systematic review and meta-regression analysis of randomized controlled trials. Br Med J 2001;322:763-767.
26. American Psychiatric Association. Practice guideline for the treatment of patients with major depressive disorder in adults. Am J Psychiatry 2000;157(4 suppl):1-45.
27. Crismon ML, Trivedi M, Pigott TA, et al. The Texas Medication Algorithm Project: Report of the Texas Consensus Conference. Panel on medication treatment of major depressive disorder. J Clin Psychiatry 1999;60:142-156.
28. Quitkin FM, Rabkin JG, Ross D, McGrath PJ. Duration of antidepressant drug treatment: what is an adequate trial? Arch Gen Psychiatry 1984;41:238-245.
29. Walsh BT, Seidman SN, Sysko R, Gould M. Placebo response in studies of major depression, variable, substantial, and growing. JAMA 2002;287:1840-1847.
30. Gill D, Hatcher S. Antidepressants for depression in medical illness (Cochrane Review). In: The Cochrane Library,. 1, 2002. Oxford; Update Software.
31. Kroenke K, West SL, Swindle R, et al. Similar effectiveness of paroxetine, fluoxetine, and sertraline in primary care: A randomized trial. JAMA 2001;286:2947-2955.
32. Hartmann PM. Mirtazapine: a newer antidepressant. Am Fam Physician 1999;59:159-161.
33. Hurt RD, Sachs DPL, Glover ED, Offord KP, et al. A comparison of sustained-release bupropion and placebo for smoking cessation. N Engl J Med 1997;337:1195-1202.
34. Clerc GE, Ruimy P, Verdeau-Pailles J, et al. A double-blind comparison of venlafaxine and fluoxetine in patients hospitalized for major depression and melancholia. Int Clin Psychopharmacology 1994;9:139-143.
35. Thase ME, Entsuah AR, Rudolph RL. Remission rates during treatment with venlafaxine or selective serotonin reuptake inhibitors. Br J of Psychiatry 2001;178:234-241.
36. Schweitzer E, Feighner J, Mandos L, Rickles K. Comparison of venlafaxine and imipramine in the acute treatment of major depression in outpatients. J Clin Psychiatry 1994;55:104-108.
37. Product information. Serzone(c) (nefazodone). Princeton, NJ Bristol Myers Squibb Co., 2002 (January).
38. Thase ME, Rush AJ, Howland RH, Kornstein SG, Kocsis JH, Gelenberg AJ, et al. Double-blind switch study of imipramine or sertraline treatment of antidepressant-resistant chronic depression. Arch Gen Psychiatry 2002;59:233-239.
39. Ballenger JC, Davidson JR, Lecrubier Y, Nutt DJ. A proposed algorithm for improved recognition and treatment of depression/anxiety spectrum in primary care. J Clin Psychiatry 2001;3:44-52.
40. Thase ME, Feighner JP, Lydiard RB. Citalopram treatment of fluoxetine nonresponders. J Clin Psychiatry 2001;62:683-687.
41. Nelson JC, Mazure CM, Bowers MB, Jatlow PI. A preliminary, open study of the combination of fluoxetine and desipramine for rapid treatment of major depression. Arch Gen Psychiatry 1991;48:303-307.
42. Stein G, Bernardt M. Lithium augmentation therapy in tri-cyclic-resistant depression: a controlled trial using lithium in low and normal doses. Br J Psychiatry 1993;162:634-640.
43. Joffe RT, Singer W, Levitt AJ, et al. A placebo-controlled comparison of lithium and triiodothyronine augmentation of tricyclic antidepressants in unipolar refractory depression. Arch Gen Psychiatry 1993;50:387-393.
44. Bauer M, Dopfmer S. Lithium augmentation in treatment-resistant depression: meta-analysis of placebo-controlled studies. J Clin Psychopharmacol 1999;19:427-434.
45. Harvey KV, Balon R. Augmentation with buspirone: a review. Ann Clin Psychiatry 1995;2:143-147.
46. Thase ME, Rush AJ. Treatment-resistant depression. In Bloom FE, Kupfer DJ. Psychopharmacology: The Fourth Generation of Progress. New York, NY: Karen Press; 1995;1081-1097.
47. Prange AJ Jr, Loosen PT, Wilson IC, Lipton MA. The therapeutic use of hormone of the thyroid axis in depression. In: Post R, Ballenger J. Neurobiology of mood disorders.. Vol 1. Baltimore, Md: Williams & Wilkins; 1980;311-322.
48. Gagne GG, Furman MJ, Carpenter LL, Price LH. Efficacy of continuation ECT and antidepressant drugs compared to long-term antidepressants alone in depressed patients. Am J Psychiatry 2000;157:1960-1965.
49. American Psychiatric Association.The practice of electroconvulsive therapy: recommendations for treatment, training, and privileging. Washington, DC: American Psychiatric Association, 1990.
50. Shelton RC, Tollefson GD, Tohen M, et al. A novel augmentation strategy for treating resistant major depression. Am J Psychiatry 2001;158:131-134.
51. Trivedi MH, Kleiber BA. Algorithm for the treatment of chronic depression. J Clin Psychiatry 2001;62(suppl 6):22-29.
52. Montana CB. Recognition and treatment of depression in a primary care setting. J Clin Psychiatry 1994;55(suppl 1):18-34.
1. Whooley MA, Simon GE. Managing depression in medical outpatients. N Engl J Med 2000;343:1942-1950.
2. Keller MB, McCullough JP, Klein DN, et al. A comparison of nefazodone, the cognitive behavioral-analysis system for psychotherapy, and their combination for the treatment of chronic depression. N Engl J Med 2000;342:1462-1470.
3. Cohen L. Rational drug use in the treatment of depression. Pharmacotherapy 1997;17:45-61.
4. Bhatia SC, Bhatia SK. Depression in women: diagnostic and treatment considerations. Am Fam Physician 1999;60:225-240.
5. Glass RM. Treating depression as a recurrent or chronic disease. JAMA 1999;281:83.-
6. Harwitz D, Ravizza L. Psychiatric emergencies; suicide and depression. Emergency Medical Clinics of North America 2000;18:263-271.
7. Olfson M, Marcus S, Druss B, et al. National trends in the out-patient treatment of depression. JAMA 2002;287:203-209.
8. Stahl SM. Selecting an antidepressant by using mechanism of action to enhance efficacy and avoid side effects. J Clin Psychiatry 1998;59(supp1 18):23-29.
9. Stahl SM. Depression and bipolar disorders. In: Stahl SM. Essential Psychopharmacology, Neuroscientific Basis and Clinical Applications. Cambridge: Cambridge University Press, 1996;135-295.
10. Barbui C, Hotopf M, Freemantle N, et al. Treatment discontinuation with selective serotonin reuptake inhibitors (SSRIs) versus tricyclic antidepressants (TCAs) (Cochrane Review). In: The Cochrane Library, 1, 2002. Oxford; Update Software.
11. Geddes JR, Freemantle N, Mason J, et al. Selective serotonin reuptake inhibitors (SSRIs) for depression (Cochrane Review). In: The Cochrane Library, 1, 2002. Oxford: Update Software.
12. Banerjee S, Shamash K, Macdonald AJD, Mann AH. Randomized controlled trial of effect of intervention by psychogeriatric team on depression in frail elderly people at home. BMJ 1996;313:1058-1061.
13. Reynolds III CF, Frank E, Perel JM, et al. Nortriptyline and interpersonal psychotherapy as maintenance therapies for recurrent major depression. JAMA 1999;281:39-45.
14. Katon W, Von Korff M, Lin E, et al. Stepped collaborative care for primary care patients with persistent symptoms of depression: a randomized trial. Arch Gen Psychiatry 1999;56:1109-1115.
15. Fava GA, Rafanelli C, Grandi S, Grandi S, Conti S, Belluardo P. Prevention of recurrent depression with cognitive behavioral therapy: preliminary findings. Arch Gen Psychiatry 1998;55:816-820.
16. Linde K, Mulrow CD. St John’s wort for depression (Cochrane Review). In: The Cochrane Library, 1, 2002. Oxford; Update Software.
17. Williams Jr JW, Mulrow CD, Chiquette E, et al. A systematic review of newer pharmacotherapies for depression in adults: evidence report summary. Ann Intern Med 2000;132:743-756.
18. Lecrubier Y, Clerc G, Didi R, Kieser M. Efficacy of St. John’s wort extract WS 5570 in major depression: a double-blind, placebo-controlled trial. Am J Psychiatry 2002;159:1361-1366.
19. Shelton RC, Keller MB, Gelenberg A, et al. Effectiveness of St. John’s wort in major depression, a randomized controlled trial. JAMA 2001;285:1978-1986.
20. Hypericum Depression Trial Study Group. Effect of Hypericum perforatum (St. John’s wort) in major depressive disorder, a randomized controlled trial. JAMA 2002;287:1807-1814.
21. Evidence Report/Technology Assessment. Number 7, Treatment of depression-newer pharmacotherapies (AHCPR Publication No. 99-E014). Rockville, Md: US Department of Health and Human Services; 1999.
22. Nemets B, Stahl Z, Belmaker RH. Addition of Omega-3 fatty acid to maintenance medication treatment for recurrent unipolar depressive disorder. Am J Psychiatry 2002;159:477-479.
23. Jellin JM, Gregory P, Balz F, et al. Pharmacist’s Letter/Prescriber’s Letter. Natural Medicines Comprehensive Database. 3rd ed. Stockton, Calif: Therapeutic Research Faculty; 2000;925-928.
24. Blumenthal JA, Babyak MA, Moore KA, Craighead WE, et al. Effects of exercise training on older patients with major depression. Arch Intern Med 1999;159:2349-2356.
25. Lawler DA, Hopker SW. The effectiveness of exercise as an intervention in the management of depression: systematic review and meta-regression analysis of randomized controlled trials. Br Med J 2001;322:763-767.
26. American Psychiatric Association. Practice guideline for the treatment of patients with major depressive disorder in adults. Am J Psychiatry 2000;157(4 suppl):1-45.
27. Crismon ML, Trivedi M, Pigott TA, et al. The Texas Medication Algorithm Project: Report of the Texas Consensus Conference. Panel on medication treatment of major depressive disorder. J Clin Psychiatry 1999;60:142-156.
28. Quitkin FM, Rabkin JG, Ross D, McGrath PJ. Duration of antidepressant drug treatment: what is an adequate trial? Arch Gen Psychiatry 1984;41:238-245.
29. Walsh BT, Seidman SN, Sysko R, Gould M. Placebo response in studies of major depression, variable, substantial, and growing. JAMA 2002;287:1840-1847.
30. Gill D, Hatcher S. Antidepressants for depression in medical illness (Cochrane Review). In: The Cochrane Library,. 1, 2002. Oxford; Update Software.
31. Kroenke K, West SL, Swindle R, et al. Similar effectiveness of paroxetine, fluoxetine, and sertraline in primary care: A randomized trial. JAMA 2001;286:2947-2955.
32. Hartmann PM. Mirtazapine: a newer antidepressant. Am Fam Physician 1999;59:159-161.
33. Hurt RD, Sachs DPL, Glover ED, Offord KP, et al. A comparison of sustained-release bupropion and placebo for smoking cessation. N Engl J Med 1997;337:1195-1202.
34. Clerc GE, Ruimy P, Verdeau-Pailles J, et al. A double-blind comparison of venlafaxine and fluoxetine in patients hospitalized for major depression and melancholia. Int Clin Psychopharmacology 1994;9:139-143.
35. Thase ME, Entsuah AR, Rudolph RL. Remission rates during treatment with venlafaxine or selective serotonin reuptake inhibitors. Br J of Psychiatry 2001;178:234-241.
36. Schweitzer E, Feighner J, Mandos L, Rickles K. Comparison of venlafaxine and imipramine in the acute treatment of major depression in outpatients. J Clin Psychiatry 1994;55:104-108.
37. Product information. Serzone(c) (nefazodone). Princeton, NJ Bristol Myers Squibb Co., 2002 (January).
38. Thase ME, Rush AJ, Howland RH, Kornstein SG, Kocsis JH, Gelenberg AJ, et al. Double-blind switch study of imipramine or sertraline treatment of antidepressant-resistant chronic depression. Arch Gen Psychiatry 2002;59:233-239.
39. Ballenger JC, Davidson JR, Lecrubier Y, Nutt DJ. A proposed algorithm for improved recognition and treatment of depression/anxiety spectrum in primary care. J Clin Psychiatry 2001;3:44-52.
40. Thase ME, Feighner JP, Lydiard RB. Citalopram treatment of fluoxetine nonresponders. J Clin Psychiatry 2001;62:683-687.
41. Nelson JC, Mazure CM, Bowers MB, Jatlow PI. A preliminary, open study of the combination of fluoxetine and desipramine for rapid treatment of major depression. Arch Gen Psychiatry 1991;48:303-307.
42. Stein G, Bernardt M. Lithium augmentation therapy in tri-cyclic-resistant depression: a controlled trial using lithium in low and normal doses. Br J Psychiatry 1993;162:634-640.
43. Joffe RT, Singer W, Levitt AJ, et al. A placebo-controlled comparison of lithium and triiodothyronine augmentation of tricyclic antidepressants in unipolar refractory depression. Arch Gen Psychiatry 1993;50:387-393.
44. Bauer M, Dopfmer S. Lithium augmentation in treatment-resistant depression: meta-analysis of placebo-controlled studies. J Clin Psychopharmacol 1999;19:427-434.
45. Harvey KV, Balon R. Augmentation with buspirone: a review. Ann Clin Psychiatry 1995;2:143-147.
46. Thase ME, Rush AJ. Treatment-resistant depression. In Bloom FE, Kupfer DJ. Psychopharmacology: The Fourth Generation of Progress. New York, NY: Karen Press; 1995;1081-1097.
47. Prange AJ Jr, Loosen PT, Wilson IC, Lipton MA. The therapeutic use of hormone of the thyroid axis in depression. In: Post R, Ballenger J. Neurobiology of mood disorders.. Vol 1. Baltimore, Md: Williams & Wilkins; 1980;311-322.
48. Gagne GG, Furman MJ, Carpenter LL, Price LH. Efficacy of continuation ECT and antidepressant drugs compared to long-term antidepressants alone in depressed patients. Am J Psychiatry 2000;157:1960-1965.
49. American Psychiatric Association.The practice of electroconvulsive therapy: recommendations for treatment, training, and privileging. Washington, DC: American Psychiatric Association, 1990.
50. Shelton RC, Tollefson GD, Tohen M, et al. A novel augmentation strategy for treating resistant major depression. Am J Psychiatry 2001;158:131-134.
51. Trivedi MH, Kleiber BA. Algorithm for the treatment of chronic depression. J Clin Psychiatry 2001;62(suppl 6):22-29.
52. Montana CB. Recognition and treatment of depression in a primary care setting. J Clin Psychiatry 1994;55(suppl 1):18-34.