User login
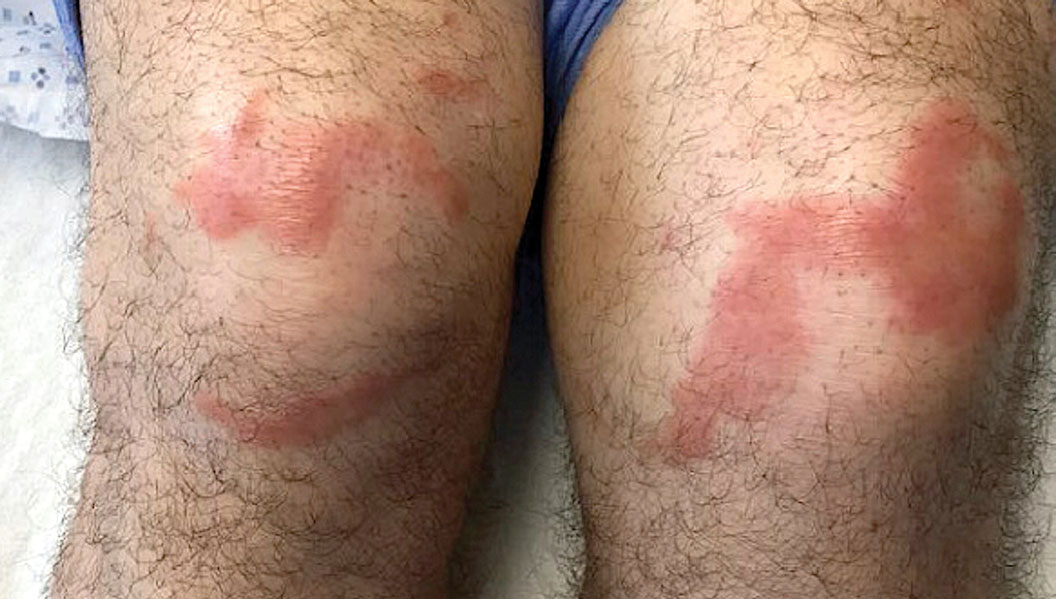
Multiple Annular Erythematous Plaques
The Diagnosis: Mid-Borderline Multibacillary Leprosy
The biopsies showed a granulomatous dermatitis involving the dermis and subcutaneous adipose tissue (Figure, A). Fite staining also revealed numerous acid-fast bacilli (AFB) throughout the dermis (Figure, B); however, polymerase chain reaction (PCR) for Mycobacterium tuberculosis was negative, and concomitant AFB tissue culture showed no growth after 8 weeks of incubation from the left wrist biopsy (Table). Interestingly, a left inguinal lymph node biopsy performed 6 months prior to presentation that helped to establish the diagnosis of follicular lymphoma also revealed nonnecrotizing granulomas and the presence of rare AFB; this formalin-fixed specimen subsequently tested negative for M tuberculosis and nontuberculous mycobacteria (NTM) by broad-range PCR. Due to a high index of suspicion, another unpreserved skin biopsy of the right knee was sent for NTM testing with PCR. Primers to 16S ribosomal RNA and the beta subunit of RNA polymerase, rpoB, gene detected Mycobacterium leprae DNA, leading to the diagnosis of mid-borderline (or borderline-borderline) multibacillary leprosy. Our patient subsequently reported subtle hypoesthesia of the plaques on the knees. He recalled eating undercooked armadillo meat in the southern United States more than 30 years prior to admission. In addition, he had a history of being incarcerated in the northeastern United States. This case was reported to the National Hansen’s Disease Program, and our patient was started on a 2-year course of daily clarithromycin, daily minocycline, and once-monthly moxifloxacin. His family also was evaluated and did not have any skin lesions concerning for leprosy.

Leprosy is a major global health concern, transmitted via breaks in the skin, respiratory secretions, and contact with armadillos. It continues to be endemic in India, Brazil, and Indonesia.1 In the United States where leprosy is nonendemic, 159 new cases were detected in 2020; the most notable risk factors in the United States are armadillo exposure and travel history.2,3Mycobacterium leprae are intracellular bacilli that preferentially infect macrophages and Schwann cells, resulting in erythematous or hypopigmented skin lesions that often are anesthetic. Mycobacterium leprae has the longest doubling time of all bacteria with unknown in vitro growth requirements and a typical in vivo incubation period of 2 to 10 years.4 Therefore, in vitro cultures will yield no growth, as seen in our case. In our patient, Fite stain showed acid-fast organisms in multiple tissue specimens, but AFB cultures demonstrated no growth after 8 weeks of incubation. Although clinicopathologic correlation is most important, PCR analysis can help to assist in the diagnosis of leprosy. Unpreserved tissue should be used when possible, as the fixation process may adversely affect the analytic sensitivity of subsequent PCR-based assays.5 In our case, NTM were not detected by PCR in the inguinal lymph node specimen despite demonstrating rare AFB staining. This result likely was multifactorial, including the effect of formalin fixation and paraffin embedding as well as concomitant low biomass.

Leprosy is known as a great imitator, and clinical manifestations (both neurologic and cutaneous) depend on host immune response to the mycobacteria. Although tuberculoid leprosy (associated with T helper type 1 immune response) is distinguished by few asymmetric, well-demarcated, and often hypopigmented plaques, lepromatous leprosy (associated with T helper type 2 response) is characterized by numerous symmetric and poorly defined lesions. Borderline leprosy, as seen in our patient, is the most common type of leprosy and shows features of both tuberculoid and lepromatous leprosy.4 It also may be particularly difficult to diagnose.6,7 Borderline-borderline leprosy involves lesions that mostly are of the lepromatous type and symmetric but also may include raised plaques, as in tuberculoid leprosy.4 Plaques in an annular configuration with central clearing, as seen in our patient, are considered suggestive.8 Histopathology of borderline-borderline leprosy lesions shows subepidermal clear zones, and granulomas are more diffuse than in tuberculoid leprosy.4
Given the noncaseating granulomatous dermatitis seen on histopathology and the relatively higher incidence of sarcoidosis in our region of practice, our initial differential included sarcoidosis and other granulomatous disorders such as granuloma annulare. Interestingly, sarcoidosis has been misdiagnosed as leprosy on multiple occasions in countries where leprosy is endemic.9,10 Localized cutaneous leishmaniasis typically presents with infiltrated plaques and nodules that may ulcerate; diffuse and disseminated as well as mucocutaneous presentations may occur depending on the species and severity of infection. Parasitized macrophages containing amastigotes may be seen in the dermis highlighted by CD1a immunostaining. Mycosis fungoides presents as papulosquamous patches or plaques, often favoring sunprotected sites; the hypopigmented variant may mimic the central clearing seen in leprosy.
The diagnosis of leprosy can be challenging due to varying clinical presentation; indolent growth of the causative organism; and indeterminate nature of stains, including the Fite stain. Although leprosy is an uncommon diagnosis, this case underscores the need to keep it in the differential of granulomatous dermatoses in the appropriate clinical setting, particularly in patients with risk factors for exposure.8
- Blok DJ, De Vlas SJ, Richardus JH. Global elimination of leprosy by 2020: are we on track? Parasit Vectors. 2015;8:548. doi:10.1186/s13071-015-1143-4
- National Hansen’s disease (leprosy) program caring and curing since 1894. Health Resources and Services Administration website. Published April 13, 2017. Accessed November 17, 2022. https://www.hrsa.gov/hansens-disease/index.html
- Aslam S, Peraza J, Mekaiel A, et al. Major risk factors for leprosy in a non-endemic area of the United States: a case series. IDCases. 2019;17:E00557. doi:10.1016/j.idcr.2019.e00557
- Kundakci N, Erdem C. Leprosy: a great imitator. Clin Dermatol. 2019;37:200-212. doi:10.1016/j.clindermatol.2019.01.002
- Marchetti G, Gori A, Catozzi L, et al. Evaluation of PCR in detection of Mycobacterium tuberculosis from formalin-fixed, paraffin-embedded tissues: comparison of four amplification assays. J Clin Microbiol. 1998;36:1512-1517.
- Pawar M, Zawar V. Mid-borderline leprosy masquerading as an overlap syndrome. Rheumatology (Oxford). 2018;57:1686-1688. doi:10.1093 /rheumatology/key125
- Day W, Prodanovic E. Borderline lepromatous leprosy masking as tinea versicolor. Int J Dermatol. 2019;58:E125-E126. doi:10.1111/ijd.14439
- Lastória JC, de Abreu MAMM. Leprosy: review of the epidemiological, clinical, and etiopathogenic aspects: part 1. An Bras Dermatol. 2014;89:205-218. doi:10.1590/abd1806-4841.20142450
- Kaushik A, Vinay K, Narang T, et al. Ichthyosiform sarcoidosis: a mimic of leprosy? Clin Exp Dermatol. 2019;44:677-680. doi:10.1111/ced.13863
- Chowdhary KN, Rao R, Priya P, et al. Cutaneous sarcoidosis misdiagnosed as leprosy. report of two cases and review of literature. Indian J Lepr. 2016;88:177-183.
The Diagnosis: Mid-Borderline Multibacillary Leprosy
The biopsies showed a granulomatous dermatitis involving the dermis and subcutaneous adipose tissue (Figure, A). Fite staining also revealed numerous acid-fast bacilli (AFB) throughout the dermis (Figure, B); however, polymerase chain reaction (PCR) for Mycobacterium tuberculosis was negative, and concomitant AFB tissue culture showed no growth after 8 weeks of incubation from the left wrist biopsy (Table). Interestingly, a left inguinal lymph node biopsy performed 6 months prior to presentation that helped to establish the diagnosis of follicular lymphoma also revealed nonnecrotizing granulomas and the presence of rare AFB; this formalin-fixed specimen subsequently tested negative for M tuberculosis and nontuberculous mycobacteria (NTM) by broad-range PCR. Due to a high index of suspicion, another unpreserved skin biopsy of the right knee was sent for NTM testing with PCR. Primers to 16S ribosomal RNA and the beta subunit of RNA polymerase, rpoB, gene detected Mycobacterium leprae DNA, leading to the diagnosis of mid-borderline (or borderline-borderline) multibacillary leprosy. Our patient subsequently reported subtle hypoesthesia of the plaques on the knees. He recalled eating undercooked armadillo meat in the southern United States more than 30 years prior to admission. In addition, he had a history of being incarcerated in the northeastern United States. This case was reported to the National Hansen’s Disease Program, and our patient was started on a 2-year course of daily clarithromycin, daily minocycline, and once-monthly moxifloxacin. His family also was evaluated and did not have any skin lesions concerning for leprosy.

Leprosy is a major global health concern, transmitted via breaks in the skin, respiratory secretions, and contact with armadillos. It continues to be endemic in India, Brazil, and Indonesia.1 In the United States where leprosy is nonendemic, 159 new cases were detected in 2020; the most notable risk factors in the United States are armadillo exposure and travel history.2,3Mycobacterium leprae are intracellular bacilli that preferentially infect macrophages and Schwann cells, resulting in erythematous or hypopigmented skin lesions that often are anesthetic. Mycobacterium leprae has the longest doubling time of all bacteria with unknown in vitro growth requirements and a typical in vivo incubation period of 2 to 10 years.4 Therefore, in vitro cultures will yield no growth, as seen in our case. In our patient, Fite stain showed acid-fast organisms in multiple tissue specimens, but AFB cultures demonstrated no growth after 8 weeks of incubation. Although clinicopathologic correlation is most important, PCR analysis can help to assist in the diagnosis of leprosy. Unpreserved tissue should be used when possible, as the fixation process may adversely affect the analytic sensitivity of subsequent PCR-based assays.5 In our case, NTM were not detected by PCR in the inguinal lymph node specimen despite demonstrating rare AFB staining. This result likely was multifactorial, including the effect of formalin fixation and paraffin embedding as well as concomitant low biomass.

Leprosy is known as a great imitator, and clinical manifestations (both neurologic and cutaneous) depend on host immune response to the mycobacteria. Although tuberculoid leprosy (associated with T helper type 1 immune response) is distinguished by few asymmetric, well-demarcated, and often hypopigmented plaques, lepromatous leprosy (associated with T helper type 2 response) is characterized by numerous symmetric and poorly defined lesions. Borderline leprosy, as seen in our patient, is the most common type of leprosy and shows features of both tuberculoid and lepromatous leprosy.4 It also may be particularly difficult to diagnose.6,7 Borderline-borderline leprosy involves lesions that mostly are of the lepromatous type and symmetric but also may include raised plaques, as in tuberculoid leprosy.4 Plaques in an annular configuration with central clearing, as seen in our patient, are considered suggestive.8 Histopathology of borderline-borderline leprosy lesions shows subepidermal clear zones, and granulomas are more diffuse than in tuberculoid leprosy.4
Given the noncaseating granulomatous dermatitis seen on histopathology and the relatively higher incidence of sarcoidosis in our region of practice, our initial differential included sarcoidosis and other granulomatous disorders such as granuloma annulare. Interestingly, sarcoidosis has been misdiagnosed as leprosy on multiple occasions in countries where leprosy is endemic.9,10 Localized cutaneous leishmaniasis typically presents with infiltrated plaques and nodules that may ulcerate; diffuse and disseminated as well as mucocutaneous presentations may occur depending on the species and severity of infection. Parasitized macrophages containing amastigotes may be seen in the dermis highlighted by CD1a immunostaining. Mycosis fungoides presents as papulosquamous patches or plaques, often favoring sunprotected sites; the hypopigmented variant may mimic the central clearing seen in leprosy.
The diagnosis of leprosy can be challenging due to varying clinical presentation; indolent growth of the causative organism; and indeterminate nature of stains, including the Fite stain. Although leprosy is an uncommon diagnosis, this case underscores the need to keep it in the differential of granulomatous dermatoses in the appropriate clinical setting, particularly in patients with risk factors for exposure.8
The Diagnosis: Mid-Borderline Multibacillary Leprosy
The biopsies showed a granulomatous dermatitis involving the dermis and subcutaneous adipose tissue (Figure, A). Fite staining also revealed numerous acid-fast bacilli (AFB) throughout the dermis (Figure, B); however, polymerase chain reaction (PCR) for Mycobacterium tuberculosis was negative, and concomitant AFB tissue culture showed no growth after 8 weeks of incubation from the left wrist biopsy (Table). Interestingly, a left inguinal lymph node biopsy performed 6 months prior to presentation that helped to establish the diagnosis of follicular lymphoma also revealed nonnecrotizing granulomas and the presence of rare AFB; this formalin-fixed specimen subsequently tested negative for M tuberculosis and nontuberculous mycobacteria (NTM) by broad-range PCR. Due to a high index of suspicion, another unpreserved skin biopsy of the right knee was sent for NTM testing with PCR. Primers to 16S ribosomal RNA and the beta subunit of RNA polymerase, rpoB, gene detected Mycobacterium leprae DNA, leading to the diagnosis of mid-borderline (or borderline-borderline) multibacillary leprosy. Our patient subsequently reported subtle hypoesthesia of the plaques on the knees. He recalled eating undercooked armadillo meat in the southern United States more than 30 years prior to admission. In addition, he had a history of being incarcerated in the northeastern United States. This case was reported to the National Hansen’s Disease Program, and our patient was started on a 2-year course of daily clarithromycin, daily minocycline, and once-monthly moxifloxacin. His family also was evaluated and did not have any skin lesions concerning for leprosy.

Leprosy is a major global health concern, transmitted via breaks in the skin, respiratory secretions, and contact with armadillos. It continues to be endemic in India, Brazil, and Indonesia.1 In the United States where leprosy is nonendemic, 159 new cases were detected in 2020; the most notable risk factors in the United States are armadillo exposure and travel history.2,3Mycobacterium leprae are intracellular bacilli that preferentially infect macrophages and Schwann cells, resulting in erythematous or hypopigmented skin lesions that often are anesthetic. Mycobacterium leprae has the longest doubling time of all bacteria with unknown in vitro growth requirements and a typical in vivo incubation period of 2 to 10 years.4 Therefore, in vitro cultures will yield no growth, as seen in our case. In our patient, Fite stain showed acid-fast organisms in multiple tissue specimens, but AFB cultures demonstrated no growth after 8 weeks of incubation. Although clinicopathologic correlation is most important, PCR analysis can help to assist in the diagnosis of leprosy. Unpreserved tissue should be used when possible, as the fixation process may adversely affect the analytic sensitivity of subsequent PCR-based assays.5 In our case, NTM were not detected by PCR in the inguinal lymph node specimen despite demonstrating rare AFB staining. This result likely was multifactorial, including the effect of formalin fixation and paraffin embedding as well as concomitant low biomass.

Leprosy is known as a great imitator, and clinical manifestations (both neurologic and cutaneous) depend on host immune response to the mycobacteria. Although tuberculoid leprosy (associated with T helper type 1 immune response) is distinguished by few asymmetric, well-demarcated, and often hypopigmented plaques, lepromatous leprosy (associated with T helper type 2 response) is characterized by numerous symmetric and poorly defined lesions. Borderline leprosy, as seen in our patient, is the most common type of leprosy and shows features of both tuberculoid and lepromatous leprosy.4 It also may be particularly difficult to diagnose.6,7 Borderline-borderline leprosy involves lesions that mostly are of the lepromatous type and symmetric but also may include raised plaques, as in tuberculoid leprosy.4 Plaques in an annular configuration with central clearing, as seen in our patient, are considered suggestive.8 Histopathology of borderline-borderline leprosy lesions shows subepidermal clear zones, and granulomas are more diffuse than in tuberculoid leprosy.4
Given the noncaseating granulomatous dermatitis seen on histopathology and the relatively higher incidence of sarcoidosis in our region of practice, our initial differential included sarcoidosis and other granulomatous disorders such as granuloma annulare. Interestingly, sarcoidosis has been misdiagnosed as leprosy on multiple occasions in countries where leprosy is endemic.9,10 Localized cutaneous leishmaniasis typically presents with infiltrated plaques and nodules that may ulcerate; diffuse and disseminated as well as mucocutaneous presentations may occur depending on the species and severity of infection. Parasitized macrophages containing amastigotes may be seen in the dermis highlighted by CD1a immunostaining. Mycosis fungoides presents as papulosquamous patches or plaques, often favoring sunprotected sites; the hypopigmented variant may mimic the central clearing seen in leprosy.
The diagnosis of leprosy can be challenging due to varying clinical presentation; indolent growth of the causative organism; and indeterminate nature of stains, including the Fite stain. Although leprosy is an uncommon diagnosis, this case underscores the need to keep it in the differential of granulomatous dermatoses in the appropriate clinical setting, particularly in patients with risk factors for exposure.8
- Blok DJ, De Vlas SJ, Richardus JH. Global elimination of leprosy by 2020: are we on track? Parasit Vectors. 2015;8:548. doi:10.1186/s13071-015-1143-4
- National Hansen’s disease (leprosy) program caring and curing since 1894. Health Resources and Services Administration website. Published April 13, 2017. Accessed November 17, 2022. https://www.hrsa.gov/hansens-disease/index.html
- Aslam S, Peraza J, Mekaiel A, et al. Major risk factors for leprosy in a non-endemic area of the United States: a case series. IDCases. 2019;17:E00557. doi:10.1016/j.idcr.2019.e00557
- Kundakci N, Erdem C. Leprosy: a great imitator. Clin Dermatol. 2019;37:200-212. doi:10.1016/j.clindermatol.2019.01.002
- Marchetti G, Gori A, Catozzi L, et al. Evaluation of PCR in detection of Mycobacterium tuberculosis from formalin-fixed, paraffin-embedded tissues: comparison of four amplification assays. J Clin Microbiol. 1998;36:1512-1517.
- Pawar M, Zawar V. Mid-borderline leprosy masquerading as an overlap syndrome. Rheumatology (Oxford). 2018;57:1686-1688. doi:10.1093 /rheumatology/key125
- Day W, Prodanovic E. Borderline lepromatous leprosy masking as tinea versicolor. Int J Dermatol. 2019;58:E125-E126. doi:10.1111/ijd.14439
- Lastória JC, de Abreu MAMM. Leprosy: review of the epidemiological, clinical, and etiopathogenic aspects: part 1. An Bras Dermatol. 2014;89:205-218. doi:10.1590/abd1806-4841.20142450
- Kaushik A, Vinay K, Narang T, et al. Ichthyosiform sarcoidosis: a mimic of leprosy? Clin Exp Dermatol. 2019;44:677-680. doi:10.1111/ced.13863
- Chowdhary KN, Rao R, Priya P, et al. Cutaneous sarcoidosis misdiagnosed as leprosy. report of two cases and review of literature. Indian J Lepr. 2016;88:177-183.
- Blok DJ, De Vlas SJ, Richardus JH. Global elimination of leprosy by 2020: are we on track? Parasit Vectors. 2015;8:548. doi:10.1186/s13071-015-1143-4
- National Hansen’s disease (leprosy) program caring and curing since 1894. Health Resources and Services Administration website. Published April 13, 2017. Accessed November 17, 2022. https://www.hrsa.gov/hansens-disease/index.html
- Aslam S, Peraza J, Mekaiel A, et al. Major risk factors for leprosy in a non-endemic area of the United States: a case series. IDCases. 2019;17:E00557. doi:10.1016/j.idcr.2019.e00557
- Kundakci N, Erdem C. Leprosy: a great imitator. Clin Dermatol. 2019;37:200-212. doi:10.1016/j.clindermatol.2019.01.002
- Marchetti G, Gori A, Catozzi L, et al. Evaluation of PCR in detection of Mycobacterium tuberculosis from formalin-fixed, paraffin-embedded tissues: comparison of four amplification assays. J Clin Microbiol. 1998;36:1512-1517.
- Pawar M, Zawar V. Mid-borderline leprosy masquerading as an overlap syndrome. Rheumatology (Oxford). 2018;57:1686-1688. doi:10.1093 /rheumatology/key125
- Day W, Prodanovic E. Borderline lepromatous leprosy masking as tinea versicolor. Int J Dermatol. 2019;58:E125-E126. doi:10.1111/ijd.14439
- Lastória JC, de Abreu MAMM. Leprosy: review of the epidemiological, clinical, and etiopathogenic aspects: part 1. An Bras Dermatol. 2014;89:205-218. doi:10.1590/abd1806-4841.20142450
- Kaushik A, Vinay K, Narang T, et al. Ichthyosiform sarcoidosis: a mimic of leprosy? Clin Exp Dermatol. 2019;44:677-680. doi:10.1111/ced.13863
- Chowdhary KN, Rao R, Priya P, et al. Cutaneous sarcoidosis misdiagnosed as leprosy. report of two cases and review of literature. Indian J Lepr. 2016;88:177-183.
A 59-year-old man was admitted to the medical ward with multiple annular erythematous plaques and polyarthralgia of several months’ duration. His medical history included low-grade stage IIA follicular lymphoma diagnosed 6 months prior to presentation, substance abuse with opiates and cocaine, coronary artery disease, ascending aortic aneurysm, and chronic lower back pain. Physical examination revealed multiple red to red-brown papules and plaques, some in an annular configuration, that were distributed on the cheeks, left wrist, knees, dorsal feet, and soles. Bilateral inguinal lymphadenopathy also was noted. Serological testing for HIV, hepatitis B and C viruses, Treponema pallidum, Borrelia burgdorferi, and tuberculosis assay were negative. Arthrocentesis of the left wrist 1 week prior to admission noted 5333 nucleated cells/μL (reference range, <3000 cells/μL) and no crystals; culture of the fluid was sterile. Skin biopsies of plaques on the left wrist, left dorsal foot, and right knee were obtained for histopathologic analysis.