User login
Avulsion of the Anterior Lateral Meniscal Root Secondary to Tibial Eminence Fracture
ABSTRACT
The lateral tibial eminence shares a close relationship with the anterior root of the lateral meniscus. Limited studies have reported traumatic injury to the anterior meniscal roots in the setting of tibial eminence fractures, and reported rates of occurrence of concomitant meniscal and chondral injuries vary widely. The purpose of this article is to describe the case of a 28-year-old woman who had a complete avulsion of the anterolateral meniscal root caused by a tibial eminence fracture with resultant malunion and root displacement. The anterolateral meniscal root was anatomically repaired following arthroscopic resection of the malunited fragment.
The lateral tibial eminence is intimately associated with the root attachment of the anterior horn of the lateral meniscus.1-3 Previous studies have demonstrated both the close proximity of the anterior cruciate ligament (ACL) insertion to the meniscal roots and the potential for disruption in surgical interventions, such as tibial tunnel drilling in ACL reconstruction or placement of intramedullary tibial nails.4-6 The meniscal roots play a crucial role in force distribution, and disruption of these structures has been shown to significantly increase joint contact forces. Despite the deleterious effects of this injury, limited studies have reported on traumatic injury to the meniscal roots in the setting of tibial eminence fractures.
Reported rates of occurrence of concomitant meniscal and chondral injuries occurring with tibial eminence fractures vary widely, ranging from <5% to 40%.7,8 Although fractures to the tibial eminence are more common in children, an association between these injuries and concomitant soft tissue injuries, including meniscal, chondral, and collateral ligament injuries, in the adult population has been reported.7 Monto and Cameron-Donaldson8 used magnetic resonance imaging (MRI) to evaluate tibial eminence fractures in adults and found that 23% of study subjects had associated medial meniscus tears and 18% had lateral meniscus tears. In a similar study, Ishibashi and colleagues9 found that 25% of tibial eminence fractures were associated with lateral meniscus tears and 16% with medial meniscus tears.
These studies demonstrate the potential for meniscus injuries during tibial eminence fractures. However, the authors are unaware of any reports of complete tearing of the anterior horn of the lateral meniscus in association with this injury. This is an important injury to recognize and identify intraoperatively because an injury of this nature could potentially compromise the mechanical loading patterns and health of the articular cartilage of the lateral compartment of the knee. The purpose of this article is to describe a complete avulsion of the anterolateral meniscal root due to a tibial eminence fracture with resultant malunion and displacement of the root in a nonanatomical position. The patient provided written informed consent for print and electronic publication of this case report.
Continue to: A 28-year-old active woman...
CASE
A 28-year-old active woman presented to our clinic 22 months after sustaining a right knee tibial eminence fracture that was initially treated with extension immobilization, which resulted in a fibrous malunion. She subsequently sustained a second injury resulting in displacement of the malunion fracture fragment, and was treated at another institution 10 months prior to presentation at our clinic with arthroscopic reduction and internal fixation with a cannulated screw and washer of the tibial eminence fracture. This was followed by hardware removal 6 months prior to her office visit at our clinic. At presentation, she reported worsening right knee pain, mechanical symptoms, and loss of both flexion and extension compared with her uninjured knee. Conservative management, including activity modification, extensive physical therapy, and anti-inflammatory medication following her most recent procedure, had not resulted in improvement of her symptoms.
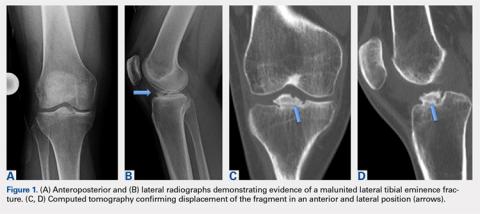
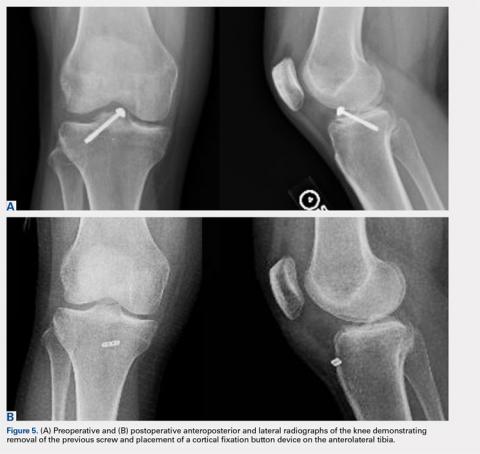
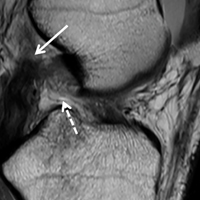
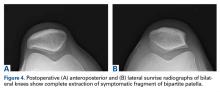
Physical examination revealed significantly reduced knee flexion and extension (+15°-120° on the affected side compared with 5° of hyperextension to 130° flexion of the contralateral knee). Ligamentous examination demonstrated no laxity with varus or valgus stress at 0° to 30° of flexion, negative posterior drawer, and a Grade 2 Lachman and positive pivot shift. She also exhibited pain with attempted right knee terminal extension. Radiographs and computed tomography scans were obtained and reviewed. They revealed a malunited tibial eminence fracture (Figures 1A-1D).
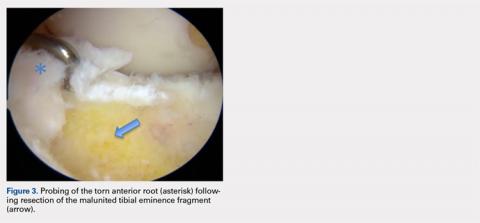
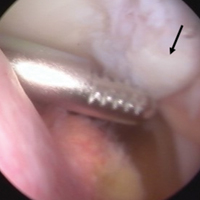
Arthroscopic assessment of the right knee demonstrated the large osseous fragment located in the anterolateral aspect of the joint with the displaced anterior horn of the lateral meniscus attached as well as significant anterior impingement limiting knee extension. Probing of the anterolateral meniscal root in the lateral compartment showed abundant surrounding scar tissue with an abnormal attachment, representing a chronic root avulsion. A mechanical shaver was used to débride the scar tissue and expose the malunited fragment, followed by complete osseous fragment excision with a high-speed burr (Figure 3).
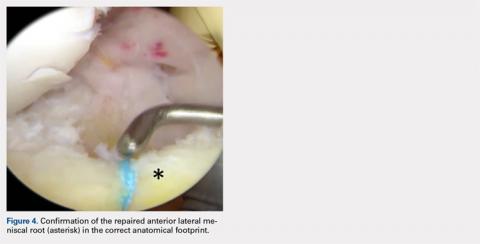
A soft tissue anterolateral meniscal root repair was performed by creating a 2-cm to 3-cm incision on the anterolateral tibia, just distal to the medial aspect of the Gerdy tubercle. To best restore the footprint of the repair and increase the potential for biologic healing, 2 transtibial tunnels were created at the location of the root attachment. An ACL aiming device with a cannulated sleeve was used to drill 2 bony tunnels approximately 5 mm apart, exiting at the anatomic root footprint. The drill pins were removed, leaving the 2 cannulas in place for later suture passage. A suture-passing device was used to pass 2 separate sutures through the detached meniscal root.
Continue to: Postoperatively, the patient was placed...
Postoperatively, the patient was placed on a non-weight-bearing protocol for her operative lower extremity for 6 weeks. A brace locked in extension was used for the same period of time (being removed only for physical therapy exercises). Enoxaparin was used for the first 2 weeks for deep vein thrombosis prophylaxis, followed by aspirin for an additional 4 weeks. Physical therapy was started on postoperative day 1 to begin working on early passive ROM exercises. Knee flexion was limited to 0° to 90° of flexion for the first 2 weeks and then progressed as tolerated.
DISCUSSION
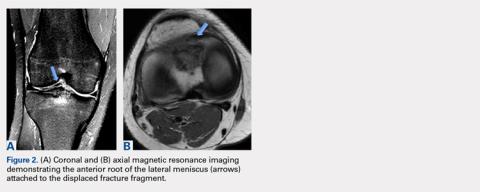
This article describes a rare case of a patient with lateral meniscal anterior root avulsion in the setting of a tibial eminence fracture with subsequent malunion and root displacement. In a case such as this, delineation of the true extent of the injury is difficult because the anterior meniscal root can be torn, displaced, and nonanatomically scarred to surrounding soft tissues, making MRI interpretation challenging. Clinically, patients can present with a wide range of symptoms, including pain, mechanical symptoms, instability, and loss of knee motion.10
The anterior root of the lateral meniscus has been reported to be attached anterior to the lateral tibial eminence and adjacent to the insertion of the ACL. Fibrous connections extending from the anterior horn of the lateral meniscus attachment to the lateral tibial eminence are constant.11 Furumatsu and colleagues12 demonstrated the existence of dense fibers linking the anterior root of the lateral meniscus with the lateral aspect of the ACL tibial insertion. Acknowledging the close relationship of these structures is key to comprehending the importance of evaluating the anterior horn of the lateral meniscus in cases of tibial eminence fractures at the initial time of injury. Failure to diagnose this pathology can lead to poor clinical outcomes and early degenerative changes of the knee.
Tibial intercondylar eminence avulsion fractures are most likely to occur in children and adolescents, and are equivalent to an ACL tear in adults.13 When tibial eminence fractures occur in an older cohort, they are often combined with lesions of the menisci, capsule, or collateral ligaments.14 The initial injury in our patient demonstrated concomitant anterior root injury that progressed with time to nonanatomical healing of the root, leading to altered biomechanics. Surgical techniques available for meniscal root repair are broadly divided into transosseous suture repairs and suture anchor repairs.10 The transtibial pullout technique using 2 transtibial bone tunnels as described in this report is the senior author’s (RFL) preference because it provides a strong construct with minimal displacement of the repaired meniscus.15-17
This article describes a complete avulsion of the anterolateral meniscal root caused by a tibial eminence fracture with resultant malunion and displacement of the root in a nonanatomic position. Anterior meniscal root tears have been reported to result in altered biomechanics and force transmission across the knee, and therefore, anatomic repair of the anterior root is indicated.
1. James EW, LaPrade CM, Ellman MB, Wijdicks CA, Engebretsen L, LaPrade RF. Radiographic identification of the anterior and posterior root attachments of the medial and lateral menisci. Am J Sports Med. 2014;42(11):2707-2714. doi:10.1177/0363546514545863.
2. LaPrade CM, Foad A, Smith SD, et al. Biomechanical consequences of a nonanatomic posterior medial meniscal root repair. Am J Sports Med. 2015;43(4):912-920. doi:10.1177/0363546514566191.
3. LaPrade CM, James EW, Cram TR, Feagin JA, Engebretsen L, LaPrade RF. Meniscal root tears: a classification system based on tear morphology. Am J Sports Med. 2015;43(2):363-369. doi:10.1177/0363546514559684.
4. Ellman MB, James EW, LaPrade CM, LaPrade RF. Anterior meniscus root avulsion following intramedullary nailing for a tibial shaft fracture. Knee Surg Sports Traumatol Arthrosc. 2015;23(4):1188-1191. doi:10.1007/s00167-014-2941-5.
5. Padalecki JR, Jansson KS, Smith SD, et al. Biomechanical consequences of a complete radial tear adjacent to the medial meniscus posterior root attachment site: in situ pull-out repair restores derangement of joint mechanics. Am J Sports Med. 2014;42(3):699-707. doi:10.1177/0363546513499314.
6. LaPrade CM, Jisa KA, Cram TR, LaPrade RF. Posterior lateral meniscal root tear due to a malpositioned double-bundle anterior cruciate ligament reconstruction tibial tunnel. Knee Surg Sports Traumatol Arthrosc. 2015;23(12):3670-3673. doi:10.1007/s00167-014-3273-1.
7. Mitchell JJ, Sjostrom R, Mansour AA, et al. Incidence of meniscal injury and chondral pathology in anterior tibial spine fractures of children. J Pediatr Orthop. 2015;35(2):130-135. doi:10.1097/BPO.0000000000000249.
8. Monto RR, Cameron-Donaldson ML. Magnetic resonance imaging in the evaluation of tibial eminence fractures in adults. J Knee Surg. 2006;19(3):187-190.
9. Ishibashi Y, Tsuda E, Sasaki T, Toh S. Magnetic resonance imaging AIDS in detecting concomitant injuries in patients with tibial spine fractures. Clin Orthop Relat Res. 2005;(434):207-212.
10. Bhatia S, LaPrade CM, Ellman MB, LaPrade RF. Meniscal root tears significance, diagnosis, and treatment. Am J Sports Med. 2014;42(12):3016-3030. doi:10.1177/0363546514524162.
11. Ziegler CG, Pietrini SD, Westerhaus BD, et al. Arthroscopically pertinent landmarks for tunnel positioning in single-bundle and double-bundle anterior cruciate ligament reconstructions. Am J Sports Med. 2011;39(4):743-752. doi:10.1177/0363546510387511.
12. Furumatsu T, Kodama Y, Maehara A, et al. The anterior cruciate ligament-lateral meniscus complex: a histological study. Connect Tissue Res. 2016;57(2):91-98. doi:10.3109/03008207.2015.1081899.
13. Lubowitz JH, Grauer JD. Arthroscopic treatment of anterior cruciate ligament avulsion. Clin Orthop Rel Res. 1993;(294):242-246.
14. Falstie-Jensen S, Sondergard Petersen PE. Incarceration of the meniscus in fractures of the intercondylar eminence of the tibia in children. Injury. 1984;15(4):236-238.
15. LaPrade CM, LaPrade MD, Turnbull TL, Wijdicks CA, LaPrade RF. Biomechanical evaluation of the transtibial pull-out technique for posterior medial meniscal root repairs using 1 and 2 transtibial bone tunnels. Am J Sports Med. 2015;43(4):899-904. doi:10.1177/0363546514563278.
16. Menge TJ, Chahla J, Dean CS, Mitchell JJ, Moatshe G, LaPrade RF. Anterior meniscal root repair using a transtibial double-tunnel pullout technique. Arthrosc Tech. 2016;5(3):e679-e684. doi:10.1016/j.eats.2016.02.026.
17. Menge TJ, Dean CS, Chahla J, Mitchell JJ, LaPrade RF. Anterior horn meniscal repair using an outside-in suture technique. Arthrosc Tech. 2016;5(5):e1111-e1116. doi:10.1016/j.eats.2016.06.005.
ABSTRACT
The lateral tibial eminence shares a close relationship with the anterior root of the lateral meniscus. Limited studies have reported traumatic injury to the anterior meniscal roots in the setting of tibial eminence fractures, and reported rates of occurrence of concomitant meniscal and chondral injuries vary widely. The purpose of this article is to describe the case of a 28-year-old woman who had a complete avulsion of the anterolateral meniscal root caused by a tibial eminence fracture with resultant malunion and root displacement. The anterolateral meniscal root was anatomically repaired following arthroscopic resection of the malunited fragment.
The lateral tibial eminence is intimately associated with the root attachment of the anterior horn of the lateral meniscus.1-3 Previous studies have demonstrated both the close proximity of the anterior cruciate ligament (ACL) insertion to the meniscal roots and the potential for disruption in surgical interventions, such as tibial tunnel drilling in ACL reconstruction or placement of intramedullary tibial nails.4-6 The meniscal roots play a crucial role in force distribution, and disruption of these structures has been shown to significantly increase joint contact forces. Despite the deleterious effects of this injury, limited studies have reported on traumatic injury to the meniscal roots in the setting of tibial eminence fractures.
Reported rates of occurrence of concomitant meniscal and chondral injuries occurring with tibial eminence fractures vary widely, ranging from <5% to 40%.7,8 Although fractures to the tibial eminence are more common in children, an association between these injuries and concomitant soft tissue injuries, including meniscal, chondral, and collateral ligament injuries, in the adult population has been reported.7 Monto and Cameron-Donaldson8 used magnetic resonance imaging (MRI) to evaluate tibial eminence fractures in adults and found that 23% of study subjects had associated medial meniscus tears and 18% had lateral meniscus tears. In a similar study, Ishibashi and colleagues9 found that 25% of tibial eminence fractures were associated with lateral meniscus tears and 16% with medial meniscus tears.
These studies demonstrate the potential for meniscus injuries during tibial eminence fractures. However, the authors are unaware of any reports of complete tearing of the anterior horn of the lateral meniscus in association with this injury. This is an important injury to recognize and identify intraoperatively because an injury of this nature could potentially compromise the mechanical loading patterns and health of the articular cartilage of the lateral compartment of the knee. The purpose of this article is to describe a complete avulsion of the anterolateral meniscal root due to a tibial eminence fracture with resultant malunion and displacement of the root in a nonanatomical position. The patient provided written informed consent for print and electronic publication of this case report.
Continue to: A 28-year-old active woman...
CASE
A 28-year-old active woman presented to our clinic 22 months after sustaining a right knee tibial eminence fracture that was initially treated with extension immobilization, which resulted in a fibrous malunion. She subsequently sustained a second injury resulting in displacement of the malunion fracture fragment, and was treated at another institution 10 months prior to presentation at our clinic with arthroscopic reduction and internal fixation with a cannulated screw and washer of the tibial eminence fracture. This was followed by hardware removal 6 months prior to her office visit at our clinic. At presentation, she reported worsening right knee pain, mechanical symptoms, and loss of both flexion and extension compared with her uninjured knee. Conservative management, including activity modification, extensive physical therapy, and anti-inflammatory medication following her most recent procedure, had not resulted in improvement of her symptoms.
Physical examination revealed significantly reduced knee flexion and extension (+15°-120° on the affected side compared with 5° of hyperextension to 130° flexion of the contralateral knee). Ligamentous examination demonstrated no laxity with varus or valgus stress at 0° to 30° of flexion, negative posterior drawer, and a Grade 2 Lachman and positive pivot shift. She also exhibited pain with attempted right knee terminal extension. Radiographs and computed tomography scans were obtained and reviewed. They revealed a malunited tibial eminence fracture (Figures 1A-1D).
Arthroscopic assessment of the right knee demonstrated the large osseous fragment located in the anterolateral aspect of the joint with the displaced anterior horn of the lateral meniscus attached as well as significant anterior impingement limiting knee extension. Probing of the anterolateral meniscal root in the lateral compartment showed abundant surrounding scar tissue with an abnormal attachment, representing a chronic root avulsion. A mechanical shaver was used to débride the scar tissue and expose the malunited fragment, followed by complete osseous fragment excision with a high-speed burr (Figure 3).
A soft tissue anterolateral meniscal root repair was performed by creating a 2-cm to 3-cm incision on the anterolateral tibia, just distal to the medial aspect of the Gerdy tubercle. To best restore the footprint of the repair and increase the potential for biologic healing, 2 transtibial tunnels were created at the location of the root attachment. An ACL aiming device with a cannulated sleeve was used to drill 2 bony tunnels approximately 5 mm apart, exiting at the anatomic root footprint. The drill pins were removed, leaving the 2 cannulas in place for later suture passage. A suture-passing device was used to pass 2 separate sutures through the detached meniscal root.
Continue to: Postoperatively, the patient was placed...
Postoperatively, the patient was placed on a non-weight-bearing protocol for her operative lower extremity for 6 weeks. A brace locked in extension was used for the same period of time (being removed only for physical therapy exercises). Enoxaparin was used for the first 2 weeks for deep vein thrombosis prophylaxis, followed by aspirin for an additional 4 weeks. Physical therapy was started on postoperative day 1 to begin working on early passive ROM exercises. Knee flexion was limited to 0° to 90° of flexion for the first 2 weeks and then progressed as tolerated.
DISCUSSION
This article describes a rare case of a patient with lateral meniscal anterior root avulsion in the setting of a tibial eminence fracture with subsequent malunion and root displacement. In a case such as this, delineation of the true extent of the injury is difficult because the anterior meniscal root can be torn, displaced, and nonanatomically scarred to surrounding soft tissues, making MRI interpretation challenging. Clinically, patients can present with a wide range of symptoms, including pain, mechanical symptoms, instability, and loss of knee motion.10
The anterior root of the lateral meniscus has been reported to be attached anterior to the lateral tibial eminence and adjacent to the insertion of the ACL. Fibrous connections extending from the anterior horn of the lateral meniscus attachment to the lateral tibial eminence are constant.11 Furumatsu and colleagues12 demonstrated the existence of dense fibers linking the anterior root of the lateral meniscus with the lateral aspect of the ACL tibial insertion. Acknowledging the close relationship of these structures is key to comprehending the importance of evaluating the anterior horn of the lateral meniscus in cases of tibial eminence fractures at the initial time of injury. Failure to diagnose this pathology can lead to poor clinical outcomes and early degenerative changes of the knee.
Tibial intercondylar eminence avulsion fractures are most likely to occur in children and adolescents, and are equivalent to an ACL tear in adults.13 When tibial eminence fractures occur in an older cohort, they are often combined with lesions of the menisci, capsule, or collateral ligaments.14 The initial injury in our patient demonstrated concomitant anterior root injury that progressed with time to nonanatomical healing of the root, leading to altered biomechanics. Surgical techniques available for meniscal root repair are broadly divided into transosseous suture repairs and suture anchor repairs.10 The transtibial pullout technique using 2 transtibial bone tunnels as described in this report is the senior author’s (RFL) preference because it provides a strong construct with minimal displacement of the repaired meniscus.15-17
This article describes a complete avulsion of the anterolateral meniscal root caused by a tibial eminence fracture with resultant malunion and displacement of the root in a nonanatomic position. Anterior meniscal root tears have been reported to result in altered biomechanics and force transmission across the knee, and therefore, anatomic repair of the anterior root is indicated.
ABSTRACT
The lateral tibial eminence shares a close relationship with the anterior root of the lateral meniscus. Limited studies have reported traumatic injury to the anterior meniscal roots in the setting of tibial eminence fractures, and reported rates of occurrence of concomitant meniscal and chondral injuries vary widely. The purpose of this article is to describe the case of a 28-year-old woman who had a complete avulsion of the anterolateral meniscal root caused by a tibial eminence fracture with resultant malunion and root displacement. The anterolateral meniscal root was anatomically repaired following arthroscopic resection of the malunited fragment.
The lateral tibial eminence is intimately associated with the root attachment of the anterior horn of the lateral meniscus.1-3 Previous studies have demonstrated both the close proximity of the anterior cruciate ligament (ACL) insertion to the meniscal roots and the potential for disruption in surgical interventions, such as tibial tunnel drilling in ACL reconstruction or placement of intramedullary tibial nails.4-6 The meniscal roots play a crucial role in force distribution, and disruption of these structures has been shown to significantly increase joint contact forces. Despite the deleterious effects of this injury, limited studies have reported on traumatic injury to the meniscal roots in the setting of tibial eminence fractures.
Reported rates of occurrence of concomitant meniscal and chondral injuries occurring with tibial eminence fractures vary widely, ranging from <5% to 40%.7,8 Although fractures to the tibial eminence are more common in children, an association between these injuries and concomitant soft tissue injuries, including meniscal, chondral, and collateral ligament injuries, in the adult population has been reported.7 Monto and Cameron-Donaldson8 used magnetic resonance imaging (MRI) to evaluate tibial eminence fractures in adults and found that 23% of study subjects had associated medial meniscus tears and 18% had lateral meniscus tears. In a similar study, Ishibashi and colleagues9 found that 25% of tibial eminence fractures were associated with lateral meniscus tears and 16% with medial meniscus tears.
These studies demonstrate the potential for meniscus injuries during tibial eminence fractures. However, the authors are unaware of any reports of complete tearing of the anterior horn of the lateral meniscus in association with this injury. This is an important injury to recognize and identify intraoperatively because an injury of this nature could potentially compromise the mechanical loading patterns and health of the articular cartilage of the lateral compartment of the knee. The purpose of this article is to describe a complete avulsion of the anterolateral meniscal root due to a tibial eminence fracture with resultant malunion and displacement of the root in a nonanatomical position. The patient provided written informed consent for print and electronic publication of this case report.
Continue to: A 28-year-old active woman...
CASE
A 28-year-old active woman presented to our clinic 22 months after sustaining a right knee tibial eminence fracture that was initially treated with extension immobilization, which resulted in a fibrous malunion. She subsequently sustained a second injury resulting in displacement of the malunion fracture fragment, and was treated at another institution 10 months prior to presentation at our clinic with arthroscopic reduction and internal fixation with a cannulated screw and washer of the tibial eminence fracture. This was followed by hardware removal 6 months prior to her office visit at our clinic. At presentation, she reported worsening right knee pain, mechanical symptoms, and loss of both flexion and extension compared with her uninjured knee. Conservative management, including activity modification, extensive physical therapy, and anti-inflammatory medication following her most recent procedure, had not resulted in improvement of her symptoms.
Physical examination revealed significantly reduced knee flexion and extension (+15°-120° on the affected side compared with 5° of hyperextension to 130° flexion of the contralateral knee). Ligamentous examination demonstrated no laxity with varus or valgus stress at 0° to 30° of flexion, negative posterior drawer, and a Grade 2 Lachman and positive pivot shift. She also exhibited pain with attempted right knee terminal extension. Radiographs and computed tomography scans were obtained and reviewed. They revealed a malunited tibial eminence fracture (Figures 1A-1D).
Arthroscopic assessment of the right knee demonstrated the large osseous fragment located in the anterolateral aspect of the joint with the displaced anterior horn of the lateral meniscus attached as well as significant anterior impingement limiting knee extension. Probing of the anterolateral meniscal root in the lateral compartment showed abundant surrounding scar tissue with an abnormal attachment, representing a chronic root avulsion. A mechanical shaver was used to débride the scar tissue and expose the malunited fragment, followed by complete osseous fragment excision with a high-speed burr (Figure 3).
A soft tissue anterolateral meniscal root repair was performed by creating a 2-cm to 3-cm incision on the anterolateral tibia, just distal to the medial aspect of the Gerdy tubercle. To best restore the footprint of the repair and increase the potential for biologic healing, 2 transtibial tunnels were created at the location of the root attachment. An ACL aiming device with a cannulated sleeve was used to drill 2 bony tunnels approximately 5 mm apart, exiting at the anatomic root footprint. The drill pins were removed, leaving the 2 cannulas in place for later suture passage. A suture-passing device was used to pass 2 separate sutures through the detached meniscal root.
Continue to: Postoperatively, the patient was placed...
Postoperatively, the patient was placed on a non-weight-bearing protocol for her operative lower extremity for 6 weeks. A brace locked in extension was used for the same period of time (being removed only for physical therapy exercises). Enoxaparin was used for the first 2 weeks for deep vein thrombosis prophylaxis, followed by aspirin for an additional 4 weeks. Physical therapy was started on postoperative day 1 to begin working on early passive ROM exercises. Knee flexion was limited to 0° to 90° of flexion for the first 2 weeks and then progressed as tolerated.
DISCUSSION
This article describes a rare case of a patient with lateral meniscal anterior root avulsion in the setting of a tibial eminence fracture with subsequent malunion and root displacement. In a case such as this, delineation of the true extent of the injury is difficult because the anterior meniscal root can be torn, displaced, and nonanatomically scarred to surrounding soft tissues, making MRI interpretation challenging. Clinically, patients can present with a wide range of symptoms, including pain, mechanical symptoms, instability, and loss of knee motion.10
The anterior root of the lateral meniscus has been reported to be attached anterior to the lateral tibial eminence and adjacent to the insertion of the ACL. Fibrous connections extending from the anterior horn of the lateral meniscus attachment to the lateral tibial eminence are constant.11 Furumatsu and colleagues12 demonstrated the existence of dense fibers linking the anterior root of the lateral meniscus with the lateral aspect of the ACL tibial insertion. Acknowledging the close relationship of these structures is key to comprehending the importance of evaluating the anterior horn of the lateral meniscus in cases of tibial eminence fractures at the initial time of injury. Failure to diagnose this pathology can lead to poor clinical outcomes and early degenerative changes of the knee.
Tibial intercondylar eminence avulsion fractures are most likely to occur in children and adolescents, and are equivalent to an ACL tear in adults.13 When tibial eminence fractures occur in an older cohort, they are often combined with lesions of the menisci, capsule, or collateral ligaments.14 The initial injury in our patient demonstrated concomitant anterior root injury that progressed with time to nonanatomical healing of the root, leading to altered biomechanics. Surgical techniques available for meniscal root repair are broadly divided into transosseous suture repairs and suture anchor repairs.10 The transtibial pullout technique using 2 transtibial bone tunnels as described in this report is the senior author’s (RFL) preference because it provides a strong construct with minimal displacement of the repaired meniscus.15-17
This article describes a complete avulsion of the anterolateral meniscal root caused by a tibial eminence fracture with resultant malunion and displacement of the root in a nonanatomic position. Anterior meniscal root tears have been reported to result in altered biomechanics and force transmission across the knee, and therefore, anatomic repair of the anterior root is indicated.
1. James EW, LaPrade CM, Ellman MB, Wijdicks CA, Engebretsen L, LaPrade RF. Radiographic identification of the anterior and posterior root attachments of the medial and lateral menisci. Am J Sports Med. 2014;42(11):2707-2714. doi:10.1177/0363546514545863.
2. LaPrade CM, Foad A, Smith SD, et al. Biomechanical consequences of a nonanatomic posterior medial meniscal root repair. Am J Sports Med. 2015;43(4):912-920. doi:10.1177/0363546514566191.
3. LaPrade CM, James EW, Cram TR, Feagin JA, Engebretsen L, LaPrade RF. Meniscal root tears: a classification system based on tear morphology. Am J Sports Med. 2015;43(2):363-369. doi:10.1177/0363546514559684.
4. Ellman MB, James EW, LaPrade CM, LaPrade RF. Anterior meniscus root avulsion following intramedullary nailing for a tibial shaft fracture. Knee Surg Sports Traumatol Arthrosc. 2015;23(4):1188-1191. doi:10.1007/s00167-014-2941-5.
5. Padalecki JR, Jansson KS, Smith SD, et al. Biomechanical consequences of a complete radial tear adjacent to the medial meniscus posterior root attachment site: in situ pull-out repair restores derangement of joint mechanics. Am J Sports Med. 2014;42(3):699-707. doi:10.1177/0363546513499314.
6. LaPrade CM, Jisa KA, Cram TR, LaPrade RF. Posterior lateral meniscal root tear due to a malpositioned double-bundle anterior cruciate ligament reconstruction tibial tunnel. Knee Surg Sports Traumatol Arthrosc. 2015;23(12):3670-3673. doi:10.1007/s00167-014-3273-1.
7. Mitchell JJ, Sjostrom R, Mansour AA, et al. Incidence of meniscal injury and chondral pathology in anterior tibial spine fractures of children. J Pediatr Orthop. 2015;35(2):130-135. doi:10.1097/BPO.0000000000000249.
8. Monto RR, Cameron-Donaldson ML. Magnetic resonance imaging in the evaluation of tibial eminence fractures in adults. J Knee Surg. 2006;19(3):187-190.
9. Ishibashi Y, Tsuda E, Sasaki T, Toh S. Magnetic resonance imaging AIDS in detecting concomitant injuries in patients with tibial spine fractures. Clin Orthop Relat Res. 2005;(434):207-212.
10. Bhatia S, LaPrade CM, Ellman MB, LaPrade RF. Meniscal root tears significance, diagnosis, and treatment. Am J Sports Med. 2014;42(12):3016-3030. doi:10.1177/0363546514524162.
11. Ziegler CG, Pietrini SD, Westerhaus BD, et al. Arthroscopically pertinent landmarks for tunnel positioning in single-bundle and double-bundle anterior cruciate ligament reconstructions. Am J Sports Med. 2011;39(4):743-752. doi:10.1177/0363546510387511.
12. Furumatsu T, Kodama Y, Maehara A, et al. The anterior cruciate ligament-lateral meniscus complex: a histological study. Connect Tissue Res. 2016;57(2):91-98. doi:10.3109/03008207.2015.1081899.
13. Lubowitz JH, Grauer JD. Arthroscopic treatment of anterior cruciate ligament avulsion. Clin Orthop Rel Res. 1993;(294):242-246.
14. Falstie-Jensen S, Sondergard Petersen PE. Incarceration of the meniscus in fractures of the intercondylar eminence of the tibia in children. Injury. 1984;15(4):236-238.
15. LaPrade CM, LaPrade MD, Turnbull TL, Wijdicks CA, LaPrade RF. Biomechanical evaluation of the transtibial pull-out technique for posterior medial meniscal root repairs using 1 and 2 transtibial bone tunnels. Am J Sports Med. 2015;43(4):899-904. doi:10.1177/0363546514563278.
16. Menge TJ, Chahla J, Dean CS, Mitchell JJ, Moatshe G, LaPrade RF. Anterior meniscal root repair using a transtibial double-tunnel pullout technique. Arthrosc Tech. 2016;5(3):e679-e684. doi:10.1016/j.eats.2016.02.026.
17. Menge TJ, Dean CS, Chahla J, Mitchell JJ, LaPrade RF. Anterior horn meniscal repair using an outside-in suture technique. Arthrosc Tech. 2016;5(5):e1111-e1116. doi:10.1016/j.eats.2016.06.005.
1. James EW, LaPrade CM, Ellman MB, Wijdicks CA, Engebretsen L, LaPrade RF. Radiographic identification of the anterior and posterior root attachments of the medial and lateral menisci. Am J Sports Med. 2014;42(11):2707-2714. doi:10.1177/0363546514545863.
2. LaPrade CM, Foad A, Smith SD, et al. Biomechanical consequences of a nonanatomic posterior medial meniscal root repair. Am J Sports Med. 2015;43(4):912-920. doi:10.1177/0363546514566191.
3. LaPrade CM, James EW, Cram TR, Feagin JA, Engebretsen L, LaPrade RF. Meniscal root tears: a classification system based on tear morphology. Am J Sports Med. 2015;43(2):363-369. doi:10.1177/0363546514559684.
4. Ellman MB, James EW, LaPrade CM, LaPrade RF. Anterior meniscus root avulsion following intramedullary nailing for a tibial shaft fracture. Knee Surg Sports Traumatol Arthrosc. 2015;23(4):1188-1191. doi:10.1007/s00167-014-2941-5.
5. Padalecki JR, Jansson KS, Smith SD, et al. Biomechanical consequences of a complete radial tear adjacent to the medial meniscus posterior root attachment site: in situ pull-out repair restores derangement of joint mechanics. Am J Sports Med. 2014;42(3):699-707. doi:10.1177/0363546513499314.
6. LaPrade CM, Jisa KA, Cram TR, LaPrade RF. Posterior lateral meniscal root tear due to a malpositioned double-bundle anterior cruciate ligament reconstruction tibial tunnel. Knee Surg Sports Traumatol Arthrosc. 2015;23(12):3670-3673. doi:10.1007/s00167-014-3273-1.
7. Mitchell JJ, Sjostrom R, Mansour AA, et al. Incidence of meniscal injury and chondral pathology in anterior tibial spine fractures of children. J Pediatr Orthop. 2015;35(2):130-135. doi:10.1097/BPO.0000000000000249.
8. Monto RR, Cameron-Donaldson ML. Magnetic resonance imaging in the evaluation of tibial eminence fractures in adults. J Knee Surg. 2006;19(3):187-190.
9. Ishibashi Y, Tsuda E, Sasaki T, Toh S. Magnetic resonance imaging AIDS in detecting concomitant injuries in patients with tibial spine fractures. Clin Orthop Relat Res. 2005;(434):207-212.
10. Bhatia S, LaPrade CM, Ellman MB, LaPrade RF. Meniscal root tears significance, diagnosis, and treatment. Am J Sports Med. 2014;42(12):3016-3030. doi:10.1177/0363546514524162.
11. Ziegler CG, Pietrini SD, Westerhaus BD, et al. Arthroscopically pertinent landmarks for tunnel positioning in single-bundle and double-bundle anterior cruciate ligament reconstructions. Am J Sports Med. 2011;39(4):743-752. doi:10.1177/0363546510387511.
12. Furumatsu T, Kodama Y, Maehara A, et al. The anterior cruciate ligament-lateral meniscus complex: a histological study. Connect Tissue Res. 2016;57(2):91-98. doi:10.3109/03008207.2015.1081899.
13. Lubowitz JH, Grauer JD. Arthroscopic treatment of anterior cruciate ligament avulsion. Clin Orthop Rel Res. 1993;(294):242-246.
14. Falstie-Jensen S, Sondergard Petersen PE. Incarceration of the meniscus in fractures of the intercondylar eminence of the tibia in children. Injury. 1984;15(4):236-238.
15. LaPrade CM, LaPrade MD, Turnbull TL, Wijdicks CA, LaPrade RF. Biomechanical evaluation of the transtibial pull-out technique for posterior medial meniscal root repairs using 1 and 2 transtibial bone tunnels. Am J Sports Med. 2015;43(4):899-904. doi:10.1177/0363546514563278.
16. Menge TJ, Chahla J, Dean CS, Mitchell JJ, Moatshe G, LaPrade RF. Anterior meniscal root repair using a transtibial double-tunnel pullout technique. Arthrosc Tech. 2016;5(3):e679-e684. doi:10.1016/j.eats.2016.02.026.
17. Menge TJ, Dean CS, Chahla J, Mitchell JJ, LaPrade RF. Anterior horn meniscal repair using an outside-in suture technique. Arthrosc Tech. 2016;5(5):e1111-e1116. doi:10.1016/j.eats.2016.06.005.
TAKE-HOME POINTS
- Root tears of all meniscal attachments have been described. A comprehensive anatomic understanding of the meniscal roots is of utmost importance to suspect root lesions.
- A detailed physical examination along with imaging methods should be performed to make the correct diagnosis. In cases of evident injuries, such as a tibial spine fracture, additional soft tissue pathology should also be assessed.
- It is important to restore all torn root attachments to restore joint loading and contact areas. An anatomical root repair is needed to yield optimal results.
- Progressive rehabilitation with early ROM starting on postoperative day 1 can help avoid loss of knee motion and arthrofibrosis.
Paraskiing Crash and Knee Dislocation With Multiligament Reconstruction and Iliotibial Band Repair
Take-Home Points
- Reconstruction of a torn ITB is important in restoration of native anatomy and function given its properties in anterolateral stabilization and resistance to varus stress and internal tibial rotation.
- Restoration of posterolateral instability primarily involves reconstructing the FCL, PLT, and popliteofibular ligament.
- For combined PLC injuries, concurrent reconstruction of the cruciate ligaments in one stage is highly recommended.
- Post-surgery, a 6-week non-weight-bearing, limited flexion rehab protocol utilizing a dynamic PCL brace, such as the PCL Rebound brace, is recommended to prevent posterior tibial sag.
- Arthrofibrosis and decreased ROM can be seen following a violent knee injury which requires extensive multiligament reconstruction surgeries, occasionally requiring a secondary surgery for further restoration of knee motion.
Tibiofemoral knee dislocations are uncommon injuries that have devastating complications and potentially result in complex surgeries.1 Knee dislocations (KDs) can be classified with the Schenck system.2 KD-I is a multiligament injury involving the anterior cruciate ligament (ACL) or the posterior cruciate ligament (PCL), and the scale increases in severity/number of ligaments involved, with KD-V being a multiligament injury with periarticular fracture.2
In this article, we report the case of a complex multiligament knee reconstruction performed with a midsubstance iliotibial band (ITB) repair. The patient provided written informed consent for print and electronic publication of this case report.
Case Report
A 27-year-old man presented 12 days after a paraskiing crash in which he collided with a tree at 45 mph and fell 40 feet before hitting snow. Physical examination revealed a large hemarthrosis of the left lower extremity and ecchymosis about the posterolateral aspect of the knee and popliteal fossa. Range of motion (ROM) was limited from 5° of hyperextension to 90° of flexion. Additional motion was deferred secondary to pain. Varus stress testing at 0° and 30° of knee flexion demonstrated significant side-to-side differences. The Lachman test, posterior drawer test, and posterolateral drawer test were all 3+. The dial test was 3 to 4+ compared with the contralateral knee. Valgus stress testing at 0° and 30° of flexion did not reveal any side-to-side laxity. The calf was nontender, and all compartments were soft. The patient reported no neurovascular symptoms and had no neuromotor deficits other than mild common peroneal nerve dysesthesias.
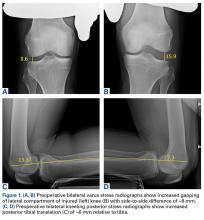
Varus stress radiographs showed increased side-to-side gapping (8 mm) of the lateral compartment of the injured knee. Kneeling posterior stress radiographs, limited because of the patient’s inability to apply full stress on the injured knee secondary to pain, showed a difference of 6 mm in increased posterior translation on the uninjured leg (Figures 1A-1D).
First Surgery
1. PLC Approach. A lateral hockey-stick skin incision was made along the ITB and extended distally between the fibular head and the Gerdy tubercle. The subcutaneous tissue was then dissected, and a posteriorly based flap was developed for preservation of vascular support to the superficial tissues. The ITB and the lateral capsule had completely torn off of the femur, allowing exposure directly into the joint. The long and short heads of the biceps femoris were exposed, with about 50% of the biceps attachment torn. The FCL was torn midsubstance, and the PLT had no remnant attachment left on the femur.
2. ITB and Lateral Capsule Tag Stitched. The torn ends of the ITB were dissected and tag stitches placed in each end. Tag stitches were also placed in the lateral capsule in preparation for a direct repair.
3. Neurolysis. The common peroneal nerve was found encased in a significant amount of scar tissue, and extensive neurolysis was required. Slow, methodical dissection was performed under the partially torn long head of the biceps femoris and was continued through the scar tissue and adhesions. Distally, 5 mm to 7 mm of the peroneus longus fascia was incised as part of the neurolysis in order to prevent nerve irritation or foot drop caused by postoperative swelling.
4. PLC Tunnels. The margin between the lateral gastrocnemius tendon and the soleus muscle was identified by blunt dissection that allowed palpation of the posteromedial aspect of the fibular styloid and the popliteus musculotendinous junction. The underlying biceps bursa was incised in order to locate the midportion of the FCL remnant, which typically is tag-stitched with No. 2 FiberWire to help identify the femoral attachment (this was not done because of the complete tear at the midsubstance of the FCL).
Subperiosteal dissection of the lateral aspect of the fibular head was performed anterior to posterior and distally extended to the champagne-glass drop-off of the fibular head. Continuing the dissection distally beyond this point can endanger the common peroneal nerve. A small sulcus can be palpated where the distal FCL inserts on the fibular head. Posteriorly, a small elevator was used to dissect the soleus muscle off of the posteromedial aspect of the fibular head, where the fibular tunnel would later be created.
A Chandler retractor was placed posterior to the fibular head to protect the neurovascular bundle. With the aid of a collateral ligament aiming device, a guide pin was drilled from the lateral aspect of the fibular head (FCL attachment) to the posteromedial downslope of the fibular styloid (popliteofibular ligament attachment). The entry point of the guide pin was immediately above the champagne- glass drop-off, at the distal insertion site of the FCL, which was described as being 28.4 mm from the styloid tip and 8.2 mm posterior to the anterior margin of the fibular head.3 Care should be taken not to ream the tunnel too proximal, as doing so increases the risk of iatrogenic fracture. A 7-mm reamer was then used to drill the fibular tunnel. To facilitate later passage of the graft, a passing suture was placed through the tunnel, leaving the loop anterolateral.
Next, the starting point for the tibial tunnel was located on the flat spot of the anterolateral tibia distal and medial to the Gerdy tubercle, just lateral to the tibial tubercle. The tibial popliteal sulcus was identified by palpation of the posterolateral tibial plateau to localize the site of the popliteus musculotendinous junction, which is the ideal location of the posterior aperture of the tibial tunnel. This point is 1 cm proximal and 1 cm medial to the posteromedial exit of the fibular tunnel. A Chandler retractor was placed anterior to the lateral gastrocnemius to protect the neurovascular bundle. In the locations described earlier, a cruciate aiming device was used to place a guide pin anterior to posterior. A 9-mm tunnel was overreamed and a passing suture placed, leaving the loop posterior to facilitate graft passage.
The femoral insertions of the FCL and the PLT were then identified. ITB splitting was not necessary, given the complete midsubstance tear of this structure. The FCL attachment was identified 1.4 mm proximal and 3.1 mm posterior to the lateral epicondyle.3 Sharp dissection was performed in this location, proximal to distal, exposing the lateral epicondyle and the small sulcus at the FCL attachment site. A collateral ligament reconstruction aiming sleeve was used to drill a guide pin over the FCL femoral attachment site and out the medial aspect of the distal thigh, about 5 cm proximal and anterior to the adductor tubercle.
The femoral attachment of the PLT was reported located 18.5 mm anterior to the FCL insertion, in the anterior fifth of the popliteal sulcus.3 Although arthrotomy is usually required in order to access the PLT attachment, it was not necessary in this case, given the lateral capsule tear. A guide pin was inserted at the PLT attachment site, parallel to the FCL pin. After proper placement was verified, a 9-mm reamer was used to drill the FCL and PLT tunnels to a depth of 25 mm (socket), and a passing suture was placed into each tunnel to facilitate graft passage.
5. ACL Graft Harvest. The central third of the ipsilateral patellar tendon was harvested for use in the ACL reconstruction. Included were a 10-mm × 20-mm bone plug from the patella and a 10-mm × 25-mm bone plug from the tibial tubercle. The patella defect was then bone-grafted, and the patellar tendon closed side-to-side.
6. Graft Preparation. For the PLC, we used a split Achilles tendon allograft that had two 9-mm × 25-mm bone plugs proximally and were tubularized distally. For the PCL, we used an anterolateral bundle (ALB), which consisted of an Achilles tendon allograft that had an 11-mm × 25-mm bone plug proximally and was tubularized distally, and a posteromedial bundle (PMB), which consisted of a tibialis anterior allograft that was tubularized at both ends. For the ACL, we used a bone–patellar tendon–bone autograft 10 mm in diameter with a 20-mm femoral bone plug and a 25-mm tibial bone plug distally.
7. Arthroscopy. We created standard anterolateral and anteromedial parapatellar portals and performed arthroscopy, including lysis of adhesions. Cartilage and menisci were lesion-free.
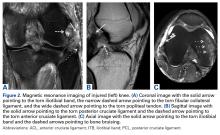
8. PCL Femoral Tunnels. The ALB attachment was identified and outlined with a coagulator between the trochlear point and the medial arch point, adjacent to the edge of the articular cartilage. Similarly, the PMB attachment was marked about 8 mm or 9 mm posterior to the edge of the articular cartilage of the medial femoral condyle and slightly posterior to the ALB tunnel.4
In the anterolateral tunnel, an acorn reamer 11 mm in diameter was used to score the entry point of the ALB femoral tunnel. An eyelet pin was then drilled through the reamer anteromedially out the knee. Then a closed socket tunnel was reamed over the eyelet pin to a depth of 25 mm. A passing suture was pulled through the tunnel in preparation for graft passage.
With use of the same technique, a 7-mm reamer was placed against the outline of the PMB attachment site, and an eyelet pin was drilled through this reamer and out the anteromedial aspect of the knee. Again, a 25-mm deep closed socket was reamed. A bone bridge distance of 2 mm was maintained between the 2 femoral PCL bundle tunnels.
9. ACL Femoral Tunnel. The femoral ACL attachment was identified and outlined. An over-the-top guide was used to determine proper placement of the 10-mm low-profile reamer. A guide pin was drilled through the center of the reamer. The reamer was used to create a 25-mm deep closed socket tunnel, and a passing stitch was placed.
10. PCL Tibial Tunnel. With use of a 70° arthroscope for visualization, a posteromedial arthroscopic portal was created, and a shaver and a coagulator were used to identify the tibial PCL attachment, located distally along the PCL facet, until the proximal aspect of the popliteus muscle fibers were visualized. A guide pin was drilled starting at the anteromedial aspect of the tibia, about 6 cm distal to the joint line and centered between the anterior tibial crest and the medial tibial border. The pin exited posteriorly at the center of the PCL tibial attachment along the PCL bundle ridge, which was reported located between the ALB and the PMB on the tibia.5 Pin placement was verified with intraoperative lateral and anteroposterior radiographs. On the lateral radiograph, the pin should be about 6 mm or 7 mm proximal to the champagne-glass drop-off at the PCL facet on the posterior aspect of the tibia. On the anteroposterior radiograph, the pin should be 1 mm to 2 mm distal to the joint line and at the medial aspect of the lateral tibial eminence. A large curette was passed through the posteromedial arthroscopic portal both to retract the posterior tissues away from the reamer and to protect against guide-pin protrusion The guide pin was then overreamed with a 12-mm acorn reamer.
A large smoother was passed proximally up the tibial tunnel and then pulled out the anteromedial portal with a grasper. The smoother was gently cycled to smooth the intra-articular tibial tunnel aperture to remove any bony spicules that could interfere with graft passage. The smoother was then pulled back into the joint, passed out the anterolateral arthroscopic portal, and secured with a small clamp.4
11. ACL Tibial Tunnel. The ACL tibial attachment site was identified and cleaned of soft tissue. A guide pin was placed and then overreamed with a 10-mm acorn reamer.
12. PCL Femoral Fixation. The PMB graft was passed into its tunnel and secured with a 7-mm × 23-mm titanium screw. Next, the ALB was secured to the femur with a 7-mm × 20-mm titanium screw. The smoother was used to pull both grafts down through the tibial tunnel.
13. ACL Femoral Fixation. A 7-mm × 20-mm titanium screw was then used to fix the ACL autograft inside the femur. Traction was applied to the 3 cruciate grafts. There was no sign of impingement.
14. PLC Femoral Fixation. The FCL and the popliteus bone plugs were passed into their respective femoral sockets and secured with 7-mm × 20-mm titanium screws.
15. Lateral Capsule Femoral Anchors. Two suture anchors were placed into the femur, and the sutures were passed through the femoral portion of the lateral capsule for later repair.
16. PCL Tibial Fixation. Both grafts were fixed with a fully threaded bicortical 6.5-mm × 40-mm cannulated cancellous screw and an 18-mm spiked washer. The ALB was fixed first, with the knee flexed to 90°, traction on the graft, and the tibia in neutral rotation. Restoration of the normal tibiofemoral step-off was verified. The PMB was then fixed with the knee in full extension. A posterior drawer test was performed to verify restoration of stability.
17. PLC Fibula Fixation. The PLT graft was passed down the popliteal hiatus, and the FCL graft was passed under the remnant of the biceps bursa on the fibular head and then through the fibular head, anterolateral to posteromedial. The FCL graft was fixed in the fibular tunnel with the knee in 20° of flexion, a slight valgus reduction force, the tibia in neutral rotation, and traction on the graft. A 7-mm × 23-mm bioabsorbable screw was used.
18. Lateral Capsular Repair. The lateral capsule was directly repaired with the previously placed sutures. The sutures were tied with the knee in 20° of flexion.
19. PLC Tibial Fixation. The grafts were passed together, posterior to anterior, through the tibial tunnel. The knee was cycled several times through complete flexion/extension ROM. A 9-mm × 23-mm bioabsorbable screw was then used to fix the grafts to the tibia. During this fixation, the knee was kept in 60° of flexion and neutral rotation while traction was being applied to the distal end of both grafts.
20. ACL Tibial Fixation. A 9-mm × 20-mm titanium screw was used to fix the ACL graft with the knee in full extension. The graft was then viewed intra-articularly to confirm there was no impingement. The Lachman, posterior drawer, posterolateral drawer, dial, and varus stress tests were performed to ensure restoration of stability.
21. ITB Repair. A portion of the remaining Achilles tendon allograft was used to perform ITB reconstruction (reconstitution of the gaped portion of the ITB). Orthocord (DePuy Synthes) and Vicryl (Ethicon) sutures were used for this reconstruction. Knee stability was deemed restored, and the incisions were closed in standard layered fashion.
First Surgery: Postoperative Management
The patient remained non-weight-bearing the first 6 weeks after surgery, with prone knee flexion limited (0°-90°) the first 2 weeks. In addition, a PCL Jack brace (Albrecht) was placed 1 week after surgery and was to be worn at all times to decrease stress on the PCL grafts.
As ROM was not progressing as expected, the patient was instructed to use a continuous passive motion (CPM) machine 2 hours 3 times a day. About 4 weeks after surgery, with ROM still not progressing, the frequency of use of this machine was increased.
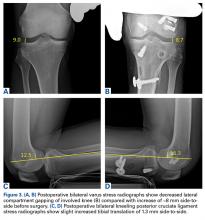
Despite continued physical therapy, use of the CPM machine, and pain management, ROM was limited (11°-90° of flexion) 5.5 months after left knee multiligament reconstruction. However, stress radiographs showed excellent stability. Varus stress radiographs showed a side-to-side difference of 0.3 mm less on the left (injured) knee, and kneeling PCL stress radiographs showed a side-to-side difference of 1.3 mm more on the left knee (Figures 3A-3D).
Second Surgery and Postoperative Management
As gentle manipulation under anesthesia was unsuccessful, the patient underwent knee arthroscopy, including 4-compartment lysis of adhesions, arthroscopically assisted posteromedial capsular release, and post-débridement manipulation under anesthesia. During manipulation, full extension and knee flexion up to 135° were achieved. ACL, PCL, and popliteus grafts were visualized and confirmed to be intact.
After this second surgery, the patient was to resume physical therapy and begin weight- bearing as tolerated. Active ROM was prioritized in an attempt to reach full ROM. In addition, a CPM machine was to be used from 0° to 135° of knee flexion 4 hours 3 times a day for 6 weeks.
Two weeks after surgery, the patient had continued pain, and extracapsular swelling in the left knee. However, ROM (0°-115° of flexion) was improved relative to before surgery (11°-90° of flexion), though it remained below the range on the contralateral side. Of note, the patient reported having a flexion contracture (~10°) in the immediate postoperative period. He had woken up with it after sleeping with the CPM machine the night before. The contracture delayed his physical therapy for several hours and resulted in a redesign of his therapy protocol to emphasize full, active knee extension and patellar mobilization, as well as discontinuation of use of the CPM machine. Corticosteroids were initiated to help with the extracapsular swelling, and the new therapy regimen brought adequate progress in ROM. Four months after the second surgery, the patient had full extension and 135° of flexion and was transitioned into wearing the PCL Rebound brace.
Discussion
This case was unique because of the midsubstance ITB tear and simultaneous multiligament injury caused by a KD-IIIL, a KD involving the ACL, the PCL, and the PLC with the medial side intact. There is limited research on ITB repair generally, with or without KD involvement. In a retrospective review of acute knee trauma cases, ITB pathologies were seen on 45% of reviewed MRI scans, and only 3% of the injuries were grade III; in addition, only 9 (5%) of the 200 cases involved both ITB and multiligament (ACL, PCL) knee injuries.6
After our patient’s ACL, PCL, and PLC were reconstructed, a fan piece of the Achilles tendon allograft from the PLC reconstruction was used to repair the ITB. The graft was used to reconstitute the torn gapped portion of the band in multiple locations, and this repair helped restore stability. The literature has reported numerous surgical uses for a portion of the ITB but few studies on repairing this anatomical structure. Preservation of the ITB is important to restoration of native anatomy and function. The ITB helps with anterolateral stabilization of the knee and with resistance of varus stress and internal tibial rotation.
The PLC reconstruction used in this case has been biomechanically validated as restoring the knee to near native stability through anatomical reconstruction of the PLC’s 3 main static stabilizers: the FCL, the PLT, and the popliteofibular ligament.7-9 First described in 2004,7 this anatomical PLC reconstruction technique has improved subjective and objective patient outcomes.10,11 For combined PLC injuries (eg, our patient’s injuries), Geeslin and LaPrade10 recommended concurrent reconstruction of the cruciate ligaments. In addition to the PLC reconstruction, the anatomical double-bundle PCL reconstruction used in this case has demonstrated significant improvements in subjective and objective outcome scores and objective knee stability.12
Although the stability and anatomy of this patient’s injured knee were reestablished, his development of arthrofibrosis is important. Many have discussed the commonality of arthrofibrosis or decreased ROM after extensive multiligament reconstruction surgeries.13,14 One study involving surgical management and outcomes of multiligament knee injuries found that, in more than half of its cases, restoration of full ROM required at least one operation after the initial one.13 Therefore, it is not unusual that our patient required a second operation for decreased ROM.
Conclusion
After surgery, excellent stabilization was achieved. Although the patient had setbacks related to pain and decreased ROM, his second surgery and continued physical therapy likely will help him return to his preoperative recreational activity levels.
1. Delos D, Warren RF, Marx RG. Multiligament knee injuries and their treatment. Oper Tech Sports Med. 2010;18(4):219-226.
2. Hobby B, Treme G, Wascher DC, Schenck RC. How I manage knee dislocations. Oper Tech Sports Med. 2010;18(4):227-234.
3. LaPrade RF, Ly TV, Wentorf FA, Engebretsen L. The posterolateral attachments of the knee: a qualitative and quantitative morphologic analysis of the fibular collateral ligament, popliteus tendon, popliteofibular ligament, and lateral gastrocnemius tendon. Am J Sports Med. 2003;31(6):854-860.
4. Chahla J, Nitri M, Civitarese D, Dean CS, Moulton SG, LaPrade RF. Anatomic double-bundle posterior cruciate ligament reconstruction. Arthrosc Tech. 2016;5(1):e149-e156.
5. Anderson CJ, Ziegler CG, Wijdicks CA, Engebretsen L, LaPrade RF. Arthroscopically pertinent anatomy of the anterolateral and posteromedial bundles of the posterior cruciate ligament. J Bone Joint Surg Am. 2012;94(21):1936-1945.
6. Mansour R, Yoong P, McKean D, Teh JL. The iliotibial band in acute knee trauma: patterns of injury on MR imaging. Skeletal Radiol. 2014;43(10):1369-1375.
7. LaPrade RF, Johansen S, Wentorf FA, Engebretsen L, Esterberg JL, Tso A. An analysis of an anatomical posterolateral knee reconstruction: an in vitro biomechanical study and development of a surgical technique. Am J Sports Med. 2004;32(6):1405-1414.
8. McCarthy M, Camarda L, Wijdicks CA, Johansen S, Engebretsen L, LaPrade RF. Anatomic posterolateral knee reconstructions require a popliteofibular ligament reconstruction through a tibial tunnel. Am J Sports Med. 2010;38(8):1674-1681.
9. LaPrade RF, Wozniczka JK, Stellmaker MP, Wijdicks CA. Analysis of the static function of the popliteus tendon and evaluation of an anatomic reconstruction: the “fifth ligament” of the knee. Am J Sports Med. 2010;38(3):543-549.
10. Geeslin AG, LaPrade RF. Outcomes of treatment of acute grade-III isolated and combined posterolateral knee injuries: a prospective case series and surgical technique. J Bone Joint Surg Am. 2011;93(18):1672-1683.
11. LaPrade RF, Johansen S, Agel J, Risberg MA, Moksnes H, Engebretsen L. Outcomes of an anatomic posterolateral knee reconstruction. J Bone Joint Surg Am. 2010;92(1):16-22.
12. Spiridonov SI, Slinkard NJ, LaPrade RF. Isolated and combined grade-III posterior cruciate ligament tears treated with double-bundle reconstruction with use of endoscopically placed femoral tunnels and grafts: operative technique and clinical outcomes. J Bone Joint Surg Am. 2011;93(19):1773-1780.
13. Noyes FR, Barber-Westin SD. Reconstruction of the anterior and posterior cruciate ligaments after knee dislocation. Use of early protected postoperative motion to decrease arthrofibrosis. Am J Sports Med. 1997;25(6):769-778.
14. Yenchak AJ, Wilk KE, Arrigo CA, Simpson CD, Andrews JR. Criteria-based management of an acute multistructure knee injury in a professional football player: a case report. J Orthop Sports Phys Ther. 2011;41(9):675-686.
Take-Home Points
- Reconstruction of a torn ITB is important in restoration of native anatomy and function given its properties in anterolateral stabilization and resistance to varus stress and internal tibial rotation.
- Restoration of posterolateral instability primarily involves reconstructing the FCL, PLT, and popliteofibular ligament.
- For combined PLC injuries, concurrent reconstruction of the cruciate ligaments in one stage is highly recommended.
- Post-surgery, a 6-week non-weight-bearing, limited flexion rehab protocol utilizing a dynamic PCL brace, such as the PCL Rebound brace, is recommended to prevent posterior tibial sag.
- Arthrofibrosis and decreased ROM can be seen following a violent knee injury which requires extensive multiligament reconstruction surgeries, occasionally requiring a secondary surgery for further restoration of knee motion.
Tibiofemoral knee dislocations are uncommon injuries that have devastating complications and potentially result in complex surgeries.1 Knee dislocations (KDs) can be classified with the Schenck system.2 KD-I is a multiligament injury involving the anterior cruciate ligament (ACL) or the posterior cruciate ligament (PCL), and the scale increases in severity/number of ligaments involved, with KD-V being a multiligament injury with periarticular fracture.2
In this article, we report the case of a complex multiligament knee reconstruction performed with a midsubstance iliotibial band (ITB) repair. The patient provided written informed consent for print and electronic publication of this case report.
Case Report
A 27-year-old man presented 12 days after a paraskiing crash in which he collided with a tree at 45 mph and fell 40 feet before hitting snow. Physical examination revealed a large hemarthrosis of the left lower extremity and ecchymosis about the posterolateral aspect of the knee and popliteal fossa. Range of motion (ROM) was limited from 5° of hyperextension to 90° of flexion. Additional motion was deferred secondary to pain. Varus stress testing at 0° and 30° of knee flexion demonstrated significant side-to-side differences. The Lachman test, posterior drawer test, and posterolateral drawer test were all 3+. The dial test was 3 to 4+ compared with the contralateral knee. Valgus stress testing at 0° and 30° of flexion did not reveal any side-to-side laxity. The calf was nontender, and all compartments were soft. The patient reported no neurovascular symptoms and had no neuromotor deficits other than mild common peroneal nerve dysesthesias.
Varus stress radiographs showed increased side-to-side gapping (8 mm) of the lateral compartment of the injured knee. Kneeling posterior stress radiographs, limited because of the patient’s inability to apply full stress on the injured knee secondary to pain, showed a difference of 6 mm in increased posterior translation on the uninjured leg (Figures 1A-1D).
First Surgery
1. PLC Approach. A lateral hockey-stick skin incision was made along the ITB and extended distally between the fibular head and the Gerdy tubercle. The subcutaneous tissue was then dissected, and a posteriorly based flap was developed for preservation of vascular support to the superficial tissues. The ITB and the lateral capsule had completely torn off of the femur, allowing exposure directly into the joint. The long and short heads of the biceps femoris were exposed, with about 50% of the biceps attachment torn. The FCL was torn midsubstance, and the PLT had no remnant attachment left on the femur.
2. ITB and Lateral Capsule Tag Stitched. The torn ends of the ITB were dissected and tag stitches placed in each end. Tag stitches were also placed in the lateral capsule in preparation for a direct repair.
3. Neurolysis. The common peroneal nerve was found encased in a significant amount of scar tissue, and extensive neurolysis was required. Slow, methodical dissection was performed under the partially torn long head of the biceps femoris and was continued through the scar tissue and adhesions. Distally, 5 mm to 7 mm of the peroneus longus fascia was incised as part of the neurolysis in order to prevent nerve irritation or foot drop caused by postoperative swelling.
4. PLC Tunnels. The margin between the lateral gastrocnemius tendon and the soleus muscle was identified by blunt dissection that allowed palpation of the posteromedial aspect of the fibular styloid and the popliteus musculotendinous junction. The underlying biceps bursa was incised in order to locate the midportion of the FCL remnant, which typically is tag-stitched with No. 2 FiberWire to help identify the femoral attachment (this was not done because of the complete tear at the midsubstance of the FCL).
Subperiosteal dissection of the lateral aspect of the fibular head was performed anterior to posterior and distally extended to the champagne-glass drop-off of the fibular head. Continuing the dissection distally beyond this point can endanger the common peroneal nerve. A small sulcus can be palpated where the distal FCL inserts on the fibular head. Posteriorly, a small elevator was used to dissect the soleus muscle off of the posteromedial aspect of the fibular head, where the fibular tunnel would later be created.
A Chandler retractor was placed posterior to the fibular head to protect the neurovascular bundle. With the aid of a collateral ligament aiming device, a guide pin was drilled from the lateral aspect of the fibular head (FCL attachment) to the posteromedial downslope of the fibular styloid (popliteofibular ligament attachment). The entry point of the guide pin was immediately above the champagne- glass drop-off, at the distal insertion site of the FCL, which was described as being 28.4 mm from the styloid tip and 8.2 mm posterior to the anterior margin of the fibular head.3 Care should be taken not to ream the tunnel too proximal, as doing so increases the risk of iatrogenic fracture. A 7-mm reamer was then used to drill the fibular tunnel. To facilitate later passage of the graft, a passing suture was placed through the tunnel, leaving the loop anterolateral.
Next, the starting point for the tibial tunnel was located on the flat spot of the anterolateral tibia distal and medial to the Gerdy tubercle, just lateral to the tibial tubercle. The tibial popliteal sulcus was identified by palpation of the posterolateral tibial plateau to localize the site of the popliteus musculotendinous junction, which is the ideal location of the posterior aperture of the tibial tunnel. This point is 1 cm proximal and 1 cm medial to the posteromedial exit of the fibular tunnel. A Chandler retractor was placed anterior to the lateral gastrocnemius to protect the neurovascular bundle. In the locations described earlier, a cruciate aiming device was used to place a guide pin anterior to posterior. A 9-mm tunnel was overreamed and a passing suture placed, leaving the loop posterior to facilitate graft passage.
The femoral insertions of the FCL and the PLT were then identified. ITB splitting was not necessary, given the complete midsubstance tear of this structure. The FCL attachment was identified 1.4 mm proximal and 3.1 mm posterior to the lateral epicondyle.3 Sharp dissection was performed in this location, proximal to distal, exposing the lateral epicondyle and the small sulcus at the FCL attachment site. A collateral ligament reconstruction aiming sleeve was used to drill a guide pin over the FCL femoral attachment site and out the medial aspect of the distal thigh, about 5 cm proximal and anterior to the adductor tubercle.
The femoral attachment of the PLT was reported located 18.5 mm anterior to the FCL insertion, in the anterior fifth of the popliteal sulcus.3 Although arthrotomy is usually required in order to access the PLT attachment, it was not necessary in this case, given the lateral capsule tear. A guide pin was inserted at the PLT attachment site, parallel to the FCL pin. After proper placement was verified, a 9-mm reamer was used to drill the FCL and PLT tunnels to a depth of 25 mm (socket), and a passing suture was placed into each tunnel to facilitate graft passage.
5. ACL Graft Harvest. The central third of the ipsilateral patellar tendon was harvested for use in the ACL reconstruction. Included were a 10-mm × 20-mm bone plug from the patella and a 10-mm × 25-mm bone plug from the tibial tubercle. The patella defect was then bone-grafted, and the patellar tendon closed side-to-side.
6. Graft Preparation. For the PLC, we used a split Achilles tendon allograft that had two 9-mm × 25-mm bone plugs proximally and were tubularized distally. For the PCL, we used an anterolateral bundle (ALB), which consisted of an Achilles tendon allograft that had an 11-mm × 25-mm bone plug proximally and was tubularized distally, and a posteromedial bundle (PMB), which consisted of a tibialis anterior allograft that was tubularized at both ends. For the ACL, we used a bone–patellar tendon–bone autograft 10 mm in diameter with a 20-mm femoral bone plug and a 25-mm tibial bone plug distally.
7. Arthroscopy. We created standard anterolateral and anteromedial parapatellar portals and performed arthroscopy, including lysis of adhesions. Cartilage and menisci were lesion-free.
8. PCL Femoral Tunnels. The ALB attachment was identified and outlined with a coagulator between the trochlear point and the medial arch point, adjacent to the edge of the articular cartilage. Similarly, the PMB attachment was marked about 8 mm or 9 mm posterior to the edge of the articular cartilage of the medial femoral condyle and slightly posterior to the ALB tunnel.4
In the anterolateral tunnel, an acorn reamer 11 mm in diameter was used to score the entry point of the ALB femoral tunnel. An eyelet pin was then drilled through the reamer anteromedially out the knee. Then a closed socket tunnel was reamed over the eyelet pin to a depth of 25 mm. A passing suture was pulled through the tunnel in preparation for graft passage.
With use of the same technique, a 7-mm reamer was placed against the outline of the PMB attachment site, and an eyelet pin was drilled through this reamer and out the anteromedial aspect of the knee. Again, a 25-mm deep closed socket was reamed. A bone bridge distance of 2 mm was maintained between the 2 femoral PCL bundle tunnels.
9. ACL Femoral Tunnel. The femoral ACL attachment was identified and outlined. An over-the-top guide was used to determine proper placement of the 10-mm low-profile reamer. A guide pin was drilled through the center of the reamer. The reamer was used to create a 25-mm deep closed socket tunnel, and a passing stitch was placed.
10. PCL Tibial Tunnel. With use of a 70° arthroscope for visualization, a posteromedial arthroscopic portal was created, and a shaver and a coagulator were used to identify the tibial PCL attachment, located distally along the PCL facet, until the proximal aspect of the popliteus muscle fibers were visualized. A guide pin was drilled starting at the anteromedial aspect of the tibia, about 6 cm distal to the joint line and centered between the anterior tibial crest and the medial tibial border. The pin exited posteriorly at the center of the PCL tibial attachment along the PCL bundle ridge, which was reported located between the ALB and the PMB on the tibia.5 Pin placement was verified with intraoperative lateral and anteroposterior radiographs. On the lateral radiograph, the pin should be about 6 mm or 7 mm proximal to the champagne-glass drop-off at the PCL facet on the posterior aspect of the tibia. On the anteroposterior radiograph, the pin should be 1 mm to 2 mm distal to the joint line and at the medial aspect of the lateral tibial eminence. A large curette was passed through the posteromedial arthroscopic portal both to retract the posterior tissues away from the reamer and to protect against guide-pin protrusion The guide pin was then overreamed with a 12-mm acorn reamer.
A large smoother was passed proximally up the tibial tunnel and then pulled out the anteromedial portal with a grasper. The smoother was gently cycled to smooth the intra-articular tibial tunnel aperture to remove any bony spicules that could interfere with graft passage. The smoother was then pulled back into the joint, passed out the anterolateral arthroscopic portal, and secured with a small clamp.4
11. ACL Tibial Tunnel. The ACL tibial attachment site was identified and cleaned of soft tissue. A guide pin was placed and then overreamed with a 10-mm acorn reamer.
12. PCL Femoral Fixation. The PMB graft was passed into its tunnel and secured with a 7-mm × 23-mm titanium screw. Next, the ALB was secured to the femur with a 7-mm × 20-mm titanium screw. The smoother was used to pull both grafts down through the tibial tunnel.
13. ACL Femoral Fixation. A 7-mm × 20-mm titanium screw was then used to fix the ACL autograft inside the femur. Traction was applied to the 3 cruciate grafts. There was no sign of impingement.
14. PLC Femoral Fixation. The FCL and the popliteus bone plugs were passed into their respective femoral sockets and secured with 7-mm × 20-mm titanium screws.
15. Lateral Capsule Femoral Anchors. Two suture anchors were placed into the femur, and the sutures were passed through the femoral portion of the lateral capsule for later repair.
16. PCL Tibial Fixation. Both grafts were fixed with a fully threaded bicortical 6.5-mm × 40-mm cannulated cancellous screw and an 18-mm spiked washer. The ALB was fixed first, with the knee flexed to 90°, traction on the graft, and the tibia in neutral rotation. Restoration of the normal tibiofemoral step-off was verified. The PMB was then fixed with the knee in full extension. A posterior drawer test was performed to verify restoration of stability.
17. PLC Fibula Fixation. The PLT graft was passed down the popliteal hiatus, and the FCL graft was passed under the remnant of the biceps bursa on the fibular head and then through the fibular head, anterolateral to posteromedial. The FCL graft was fixed in the fibular tunnel with the knee in 20° of flexion, a slight valgus reduction force, the tibia in neutral rotation, and traction on the graft. A 7-mm × 23-mm bioabsorbable screw was used.
18. Lateral Capsular Repair. The lateral capsule was directly repaired with the previously placed sutures. The sutures were tied with the knee in 20° of flexion.
19. PLC Tibial Fixation. The grafts were passed together, posterior to anterior, through the tibial tunnel. The knee was cycled several times through complete flexion/extension ROM. A 9-mm × 23-mm bioabsorbable screw was then used to fix the grafts to the tibia. During this fixation, the knee was kept in 60° of flexion and neutral rotation while traction was being applied to the distal end of both grafts.
20. ACL Tibial Fixation. A 9-mm × 20-mm titanium screw was used to fix the ACL graft with the knee in full extension. The graft was then viewed intra-articularly to confirm there was no impingement. The Lachman, posterior drawer, posterolateral drawer, dial, and varus stress tests were performed to ensure restoration of stability.
21. ITB Repair. A portion of the remaining Achilles tendon allograft was used to perform ITB reconstruction (reconstitution of the gaped portion of the ITB). Orthocord (DePuy Synthes) and Vicryl (Ethicon) sutures were used for this reconstruction. Knee stability was deemed restored, and the incisions were closed in standard layered fashion.
First Surgery: Postoperative Management
The patient remained non-weight-bearing the first 6 weeks after surgery, with prone knee flexion limited (0°-90°) the first 2 weeks. In addition, a PCL Jack brace (Albrecht) was placed 1 week after surgery and was to be worn at all times to decrease stress on the PCL grafts.
As ROM was not progressing as expected, the patient was instructed to use a continuous passive motion (CPM) machine 2 hours 3 times a day. About 4 weeks after surgery, with ROM still not progressing, the frequency of use of this machine was increased.
Despite continued physical therapy, use of the CPM machine, and pain management, ROM was limited (11°-90° of flexion) 5.5 months after left knee multiligament reconstruction. However, stress radiographs showed excellent stability. Varus stress radiographs showed a side-to-side difference of 0.3 mm less on the left (injured) knee, and kneeling PCL stress radiographs showed a side-to-side difference of 1.3 mm more on the left knee (Figures 3A-3D).
Second Surgery and Postoperative Management
As gentle manipulation under anesthesia was unsuccessful, the patient underwent knee arthroscopy, including 4-compartment lysis of adhesions, arthroscopically assisted posteromedial capsular release, and post-débridement manipulation under anesthesia. During manipulation, full extension and knee flexion up to 135° were achieved. ACL, PCL, and popliteus grafts were visualized and confirmed to be intact.
After this second surgery, the patient was to resume physical therapy and begin weight- bearing as tolerated. Active ROM was prioritized in an attempt to reach full ROM. In addition, a CPM machine was to be used from 0° to 135° of knee flexion 4 hours 3 times a day for 6 weeks.
Two weeks after surgery, the patient had continued pain, and extracapsular swelling in the left knee. However, ROM (0°-115° of flexion) was improved relative to before surgery (11°-90° of flexion), though it remained below the range on the contralateral side. Of note, the patient reported having a flexion contracture (~10°) in the immediate postoperative period. He had woken up with it after sleeping with the CPM machine the night before. The contracture delayed his physical therapy for several hours and resulted in a redesign of his therapy protocol to emphasize full, active knee extension and patellar mobilization, as well as discontinuation of use of the CPM machine. Corticosteroids were initiated to help with the extracapsular swelling, and the new therapy regimen brought adequate progress in ROM. Four months after the second surgery, the patient had full extension and 135° of flexion and was transitioned into wearing the PCL Rebound brace.
Discussion
This case was unique because of the midsubstance ITB tear and simultaneous multiligament injury caused by a KD-IIIL, a KD involving the ACL, the PCL, and the PLC with the medial side intact. There is limited research on ITB repair generally, with or without KD involvement. In a retrospective review of acute knee trauma cases, ITB pathologies were seen on 45% of reviewed MRI scans, and only 3% of the injuries were grade III; in addition, only 9 (5%) of the 200 cases involved both ITB and multiligament (ACL, PCL) knee injuries.6
After our patient’s ACL, PCL, and PLC were reconstructed, a fan piece of the Achilles tendon allograft from the PLC reconstruction was used to repair the ITB. The graft was used to reconstitute the torn gapped portion of the band in multiple locations, and this repair helped restore stability. The literature has reported numerous surgical uses for a portion of the ITB but few studies on repairing this anatomical structure. Preservation of the ITB is important to restoration of native anatomy and function. The ITB helps with anterolateral stabilization of the knee and with resistance of varus stress and internal tibial rotation.
The PLC reconstruction used in this case has been biomechanically validated as restoring the knee to near native stability through anatomical reconstruction of the PLC’s 3 main static stabilizers: the FCL, the PLT, and the popliteofibular ligament.7-9 First described in 2004,7 this anatomical PLC reconstruction technique has improved subjective and objective patient outcomes.10,11 For combined PLC injuries (eg, our patient’s injuries), Geeslin and LaPrade10 recommended concurrent reconstruction of the cruciate ligaments. In addition to the PLC reconstruction, the anatomical double-bundle PCL reconstruction used in this case has demonstrated significant improvements in subjective and objective outcome scores and objective knee stability.12
Although the stability and anatomy of this patient’s injured knee were reestablished, his development of arthrofibrosis is important. Many have discussed the commonality of arthrofibrosis or decreased ROM after extensive multiligament reconstruction surgeries.13,14 One study involving surgical management and outcomes of multiligament knee injuries found that, in more than half of its cases, restoration of full ROM required at least one operation after the initial one.13 Therefore, it is not unusual that our patient required a second operation for decreased ROM.
Conclusion
After surgery, excellent stabilization was achieved. Although the patient had setbacks related to pain and decreased ROM, his second surgery and continued physical therapy likely will help him return to his preoperative recreational activity levels.
Take-Home Points
- Reconstruction of a torn ITB is important in restoration of native anatomy and function given its properties in anterolateral stabilization and resistance to varus stress and internal tibial rotation.
- Restoration of posterolateral instability primarily involves reconstructing the FCL, PLT, and popliteofibular ligament.
- For combined PLC injuries, concurrent reconstruction of the cruciate ligaments in one stage is highly recommended.
- Post-surgery, a 6-week non-weight-bearing, limited flexion rehab protocol utilizing a dynamic PCL brace, such as the PCL Rebound brace, is recommended to prevent posterior tibial sag.
- Arthrofibrosis and decreased ROM can be seen following a violent knee injury which requires extensive multiligament reconstruction surgeries, occasionally requiring a secondary surgery for further restoration of knee motion.
Tibiofemoral knee dislocations are uncommon injuries that have devastating complications and potentially result in complex surgeries.1 Knee dislocations (KDs) can be classified with the Schenck system.2 KD-I is a multiligament injury involving the anterior cruciate ligament (ACL) or the posterior cruciate ligament (PCL), and the scale increases in severity/number of ligaments involved, with KD-V being a multiligament injury with periarticular fracture.2
In this article, we report the case of a complex multiligament knee reconstruction performed with a midsubstance iliotibial band (ITB) repair. The patient provided written informed consent for print and electronic publication of this case report.
Case Report
A 27-year-old man presented 12 days after a paraskiing crash in which he collided with a tree at 45 mph and fell 40 feet before hitting snow. Physical examination revealed a large hemarthrosis of the left lower extremity and ecchymosis about the posterolateral aspect of the knee and popliteal fossa. Range of motion (ROM) was limited from 5° of hyperextension to 90° of flexion. Additional motion was deferred secondary to pain. Varus stress testing at 0° and 30° of knee flexion demonstrated significant side-to-side differences. The Lachman test, posterior drawer test, and posterolateral drawer test were all 3+. The dial test was 3 to 4+ compared with the contralateral knee. Valgus stress testing at 0° and 30° of flexion did not reveal any side-to-side laxity. The calf was nontender, and all compartments were soft. The patient reported no neurovascular symptoms and had no neuromotor deficits other than mild common peroneal nerve dysesthesias.
Varus stress radiographs showed increased side-to-side gapping (8 mm) of the lateral compartment of the injured knee. Kneeling posterior stress radiographs, limited because of the patient’s inability to apply full stress on the injured knee secondary to pain, showed a difference of 6 mm in increased posterior translation on the uninjured leg (Figures 1A-1D).
First Surgery
1. PLC Approach. A lateral hockey-stick skin incision was made along the ITB and extended distally between the fibular head and the Gerdy tubercle. The subcutaneous tissue was then dissected, and a posteriorly based flap was developed for preservation of vascular support to the superficial tissues. The ITB and the lateral capsule had completely torn off of the femur, allowing exposure directly into the joint. The long and short heads of the biceps femoris were exposed, with about 50% of the biceps attachment torn. The FCL was torn midsubstance, and the PLT had no remnant attachment left on the femur.
2. ITB and Lateral Capsule Tag Stitched. The torn ends of the ITB were dissected and tag stitches placed in each end. Tag stitches were also placed in the lateral capsule in preparation for a direct repair.
3. Neurolysis. The common peroneal nerve was found encased in a significant amount of scar tissue, and extensive neurolysis was required. Slow, methodical dissection was performed under the partially torn long head of the biceps femoris and was continued through the scar tissue and adhesions. Distally, 5 mm to 7 mm of the peroneus longus fascia was incised as part of the neurolysis in order to prevent nerve irritation or foot drop caused by postoperative swelling.
4. PLC Tunnels. The margin between the lateral gastrocnemius tendon and the soleus muscle was identified by blunt dissection that allowed palpation of the posteromedial aspect of the fibular styloid and the popliteus musculotendinous junction. The underlying biceps bursa was incised in order to locate the midportion of the FCL remnant, which typically is tag-stitched with No. 2 FiberWire to help identify the femoral attachment (this was not done because of the complete tear at the midsubstance of the FCL).
Subperiosteal dissection of the lateral aspect of the fibular head was performed anterior to posterior and distally extended to the champagne-glass drop-off of the fibular head. Continuing the dissection distally beyond this point can endanger the common peroneal nerve. A small sulcus can be palpated where the distal FCL inserts on the fibular head. Posteriorly, a small elevator was used to dissect the soleus muscle off of the posteromedial aspect of the fibular head, where the fibular tunnel would later be created.
A Chandler retractor was placed posterior to the fibular head to protect the neurovascular bundle. With the aid of a collateral ligament aiming device, a guide pin was drilled from the lateral aspect of the fibular head (FCL attachment) to the posteromedial downslope of the fibular styloid (popliteofibular ligament attachment). The entry point of the guide pin was immediately above the champagne- glass drop-off, at the distal insertion site of the FCL, which was described as being 28.4 mm from the styloid tip and 8.2 mm posterior to the anterior margin of the fibular head.3 Care should be taken not to ream the tunnel too proximal, as doing so increases the risk of iatrogenic fracture. A 7-mm reamer was then used to drill the fibular tunnel. To facilitate later passage of the graft, a passing suture was placed through the tunnel, leaving the loop anterolateral.
Next, the starting point for the tibial tunnel was located on the flat spot of the anterolateral tibia distal and medial to the Gerdy tubercle, just lateral to the tibial tubercle. The tibial popliteal sulcus was identified by palpation of the posterolateral tibial plateau to localize the site of the popliteus musculotendinous junction, which is the ideal location of the posterior aperture of the tibial tunnel. This point is 1 cm proximal and 1 cm medial to the posteromedial exit of the fibular tunnel. A Chandler retractor was placed anterior to the lateral gastrocnemius to protect the neurovascular bundle. In the locations described earlier, a cruciate aiming device was used to place a guide pin anterior to posterior. A 9-mm tunnel was overreamed and a passing suture placed, leaving the loop posterior to facilitate graft passage.
The femoral insertions of the FCL and the PLT were then identified. ITB splitting was not necessary, given the complete midsubstance tear of this structure. The FCL attachment was identified 1.4 mm proximal and 3.1 mm posterior to the lateral epicondyle.3 Sharp dissection was performed in this location, proximal to distal, exposing the lateral epicondyle and the small sulcus at the FCL attachment site. A collateral ligament reconstruction aiming sleeve was used to drill a guide pin over the FCL femoral attachment site and out the medial aspect of the distal thigh, about 5 cm proximal and anterior to the adductor tubercle.
The femoral attachment of the PLT was reported located 18.5 mm anterior to the FCL insertion, in the anterior fifth of the popliteal sulcus.3 Although arthrotomy is usually required in order to access the PLT attachment, it was not necessary in this case, given the lateral capsule tear. A guide pin was inserted at the PLT attachment site, parallel to the FCL pin. After proper placement was verified, a 9-mm reamer was used to drill the FCL and PLT tunnels to a depth of 25 mm (socket), and a passing suture was placed into each tunnel to facilitate graft passage.
5. ACL Graft Harvest. The central third of the ipsilateral patellar tendon was harvested for use in the ACL reconstruction. Included were a 10-mm × 20-mm bone plug from the patella and a 10-mm × 25-mm bone plug from the tibial tubercle. The patella defect was then bone-grafted, and the patellar tendon closed side-to-side.
6. Graft Preparation. For the PLC, we used a split Achilles tendon allograft that had two 9-mm × 25-mm bone plugs proximally and were tubularized distally. For the PCL, we used an anterolateral bundle (ALB), which consisted of an Achilles tendon allograft that had an 11-mm × 25-mm bone plug proximally and was tubularized distally, and a posteromedial bundle (PMB), which consisted of a tibialis anterior allograft that was tubularized at both ends. For the ACL, we used a bone–patellar tendon–bone autograft 10 mm in diameter with a 20-mm femoral bone plug and a 25-mm tibial bone plug distally.
7. Arthroscopy. We created standard anterolateral and anteromedial parapatellar portals and performed arthroscopy, including lysis of adhesions. Cartilage and menisci were lesion-free.
8. PCL Femoral Tunnels. The ALB attachment was identified and outlined with a coagulator between the trochlear point and the medial arch point, adjacent to the edge of the articular cartilage. Similarly, the PMB attachment was marked about 8 mm or 9 mm posterior to the edge of the articular cartilage of the medial femoral condyle and slightly posterior to the ALB tunnel.4
In the anterolateral tunnel, an acorn reamer 11 mm in diameter was used to score the entry point of the ALB femoral tunnel. An eyelet pin was then drilled through the reamer anteromedially out the knee. Then a closed socket tunnel was reamed over the eyelet pin to a depth of 25 mm. A passing suture was pulled through the tunnel in preparation for graft passage.
With use of the same technique, a 7-mm reamer was placed against the outline of the PMB attachment site, and an eyelet pin was drilled through this reamer and out the anteromedial aspect of the knee. Again, a 25-mm deep closed socket was reamed. A bone bridge distance of 2 mm was maintained between the 2 femoral PCL bundle tunnels.
9. ACL Femoral Tunnel. The femoral ACL attachment was identified and outlined. An over-the-top guide was used to determine proper placement of the 10-mm low-profile reamer. A guide pin was drilled through the center of the reamer. The reamer was used to create a 25-mm deep closed socket tunnel, and a passing stitch was placed.
10. PCL Tibial Tunnel. With use of a 70° arthroscope for visualization, a posteromedial arthroscopic portal was created, and a shaver and a coagulator were used to identify the tibial PCL attachment, located distally along the PCL facet, until the proximal aspect of the popliteus muscle fibers were visualized. A guide pin was drilled starting at the anteromedial aspect of the tibia, about 6 cm distal to the joint line and centered between the anterior tibial crest and the medial tibial border. The pin exited posteriorly at the center of the PCL tibial attachment along the PCL bundle ridge, which was reported located between the ALB and the PMB on the tibia.5 Pin placement was verified with intraoperative lateral and anteroposterior radiographs. On the lateral radiograph, the pin should be about 6 mm or 7 mm proximal to the champagne-glass drop-off at the PCL facet on the posterior aspect of the tibia. On the anteroposterior radiograph, the pin should be 1 mm to 2 mm distal to the joint line and at the medial aspect of the lateral tibial eminence. A large curette was passed through the posteromedial arthroscopic portal both to retract the posterior tissues away from the reamer and to protect against guide-pin protrusion The guide pin was then overreamed with a 12-mm acorn reamer.
A large smoother was passed proximally up the tibial tunnel and then pulled out the anteromedial portal with a grasper. The smoother was gently cycled to smooth the intra-articular tibial tunnel aperture to remove any bony spicules that could interfere with graft passage. The smoother was then pulled back into the joint, passed out the anterolateral arthroscopic portal, and secured with a small clamp.4
11. ACL Tibial Tunnel. The ACL tibial attachment site was identified and cleaned of soft tissue. A guide pin was placed and then overreamed with a 10-mm acorn reamer.
12. PCL Femoral Fixation. The PMB graft was passed into its tunnel and secured with a 7-mm × 23-mm titanium screw. Next, the ALB was secured to the femur with a 7-mm × 20-mm titanium screw. The smoother was used to pull both grafts down through the tibial tunnel.
13. ACL Femoral Fixation. A 7-mm × 20-mm titanium screw was then used to fix the ACL autograft inside the femur. Traction was applied to the 3 cruciate grafts. There was no sign of impingement.
14. PLC Femoral Fixation. The FCL and the popliteus bone plugs were passed into their respective femoral sockets and secured with 7-mm × 20-mm titanium screws.
15. Lateral Capsule Femoral Anchors. Two suture anchors were placed into the femur, and the sutures were passed through the femoral portion of the lateral capsule for later repair.
16. PCL Tibial Fixation. Both grafts were fixed with a fully threaded bicortical 6.5-mm × 40-mm cannulated cancellous screw and an 18-mm spiked washer. The ALB was fixed first, with the knee flexed to 90°, traction on the graft, and the tibia in neutral rotation. Restoration of the normal tibiofemoral step-off was verified. The PMB was then fixed with the knee in full extension. A posterior drawer test was performed to verify restoration of stability.
17. PLC Fibula Fixation. The PLT graft was passed down the popliteal hiatus, and the FCL graft was passed under the remnant of the biceps bursa on the fibular head and then through the fibular head, anterolateral to posteromedial. The FCL graft was fixed in the fibular tunnel with the knee in 20° of flexion, a slight valgus reduction force, the tibia in neutral rotation, and traction on the graft. A 7-mm × 23-mm bioabsorbable screw was used.
18. Lateral Capsular Repair. The lateral capsule was directly repaired with the previously placed sutures. The sutures were tied with the knee in 20° of flexion.
19. PLC Tibial Fixation. The grafts were passed together, posterior to anterior, through the tibial tunnel. The knee was cycled several times through complete flexion/extension ROM. A 9-mm × 23-mm bioabsorbable screw was then used to fix the grafts to the tibia. During this fixation, the knee was kept in 60° of flexion and neutral rotation while traction was being applied to the distal end of both grafts.
20. ACL Tibial Fixation. A 9-mm × 20-mm titanium screw was used to fix the ACL graft with the knee in full extension. The graft was then viewed intra-articularly to confirm there was no impingement. The Lachman, posterior drawer, posterolateral drawer, dial, and varus stress tests were performed to ensure restoration of stability.
21. ITB Repair. A portion of the remaining Achilles tendon allograft was used to perform ITB reconstruction (reconstitution of the gaped portion of the ITB). Orthocord (DePuy Synthes) and Vicryl (Ethicon) sutures were used for this reconstruction. Knee stability was deemed restored, and the incisions were closed in standard layered fashion.
First Surgery: Postoperative Management
The patient remained non-weight-bearing the first 6 weeks after surgery, with prone knee flexion limited (0°-90°) the first 2 weeks. In addition, a PCL Jack brace (Albrecht) was placed 1 week after surgery and was to be worn at all times to decrease stress on the PCL grafts.
As ROM was not progressing as expected, the patient was instructed to use a continuous passive motion (CPM) machine 2 hours 3 times a day. About 4 weeks after surgery, with ROM still not progressing, the frequency of use of this machine was increased.
Despite continued physical therapy, use of the CPM machine, and pain management, ROM was limited (11°-90° of flexion) 5.5 months after left knee multiligament reconstruction. However, stress radiographs showed excellent stability. Varus stress radiographs showed a side-to-side difference of 0.3 mm less on the left (injured) knee, and kneeling PCL stress radiographs showed a side-to-side difference of 1.3 mm more on the left knee (Figures 3A-3D).
Second Surgery and Postoperative Management
As gentle manipulation under anesthesia was unsuccessful, the patient underwent knee arthroscopy, including 4-compartment lysis of adhesions, arthroscopically assisted posteromedial capsular release, and post-débridement manipulation under anesthesia. During manipulation, full extension and knee flexion up to 135° were achieved. ACL, PCL, and popliteus grafts were visualized and confirmed to be intact.
After this second surgery, the patient was to resume physical therapy and begin weight- bearing as tolerated. Active ROM was prioritized in an attempt to reach full ROM. In addition, a CPM machine was to be used from 0° to 135° of knee flexion 4 hours 3 times a day for 6 weeks.
Two weeks after surgery, the patient had continued pain, and extracapsular swelling in the left knee. However, ROM (0°-115° of flexion) was improved relative to before surgery (11°-90° of flexion), though it remained below the range on the contralateral side. Of note, the patient reported having a flexion contracture (~10°) in the immediate postoperative period. He had woken up with it after sleeping with the CPM machine the night before. The contracture delayed his physical therapy for several hours and resulted in a redesign of his therapy protocol to emphasize full, active knee extension and patellar mobilization, as well as discontinuation of use of the CPM machine. Corticosteroids were initiated to help with the extracapsular swelling, and the new therapy regimen brought adequate progress in ROM. Four months after the second surgery, the patient had full extension and 135° of flexion and was transitioned into wearing the PCL Rebound brace.
Discussion
This case was unique because of the midsubstance ITB tear and simultaneous multiligament injury caused by a KD-IIIL, a KD involving the ACL, the PCL, and the PLC with the medial side intact. There is limited research on ITB repair generally, with or without KD involvement. In a retrospective review of acute knee trauma cases, ITB pathologies were seen on 45% of reviewed MRI scans, and only 3% of the injuries were grade III; in addition, only 9 (5%) of the 200 cases involved both ITB and multiligament (ACL, PCL) knee injuries.6
After our patient’s ACL, PCL, and PLC were reconstructed, a fan piece of the Achilles tendon allograft from the PLC reconstruction was used to repair the ITB. The graft was used to reconstitute the torn gapped portion of the band in multiple locations, and this repair helped restore stability. The literature has reported numerous surgical uses for a portion of the ITB but few studies on repairing this anatomical structure. Preservation of the ITB is important to restoration of native anatomy and function. The ITB helps with anterolateral stabilization of the knee and with resistance of varus stress and internal tibial rotation.
The PLC reconstruction used in this case has been biomechanically validated as restoring the knee to near native stability through anatomical reconstruction of the PLC’s 3 main static stabilizers: the FCL, the PLT, and the popliteofibular ligament.7-9 First described in 2004,7 this anatomical PLC reconstruction technique has improved subjective and objective patient outcomes.10,11 For combined PLC injuries (eg, our patient’s injuries), Geeslin and LaPrade10 recommended concurrent reconstruction of the cruciate ligaments. In addition to the PLC reconstruction, the anatomical double-bundle PCL reconstruction used in this case has demonstrated significant improvements in subjective and objective outcome scores and objective knee stability.12
Although the stability and anatomy of this patient’s injured knee were reestablished, his development of arthrofibrosis is important. Many have discussed the commonality of arthrofibrosis or decreased ROM after extensive multiligament reconstruction surgeries.13,14 One study involving surgical management and outcomes of multiligament knee injuries found that, in more than half of its cases, restoration of full ROM required at least one operation after the initial one.13 Therefore, it is not unusual that our patient required a second operation for decreased ROM.
Conclusion
After surgery, excellent stabilization was achieved. Although the patient had setbacks related to pain and decreased ROM, his second surgery and continued physical therapy likely will help him return to his preoperative recreational activity levels.
1. Delos D, Warren RF, Marx RG. Multiligament knee injuries and their treatment. Oper Tech Sports Med. 2010;18(4):219-226.
2. Hobby B, Treme G, Wascher DC, Schenck RC. How I manage knee dislocations. Oper Tech Sports Med. 2010;18(4):227-234.
3. LaPrade RF, Ly TV, Wentorf FA, Engebretsen L. The posterolateral attachments of the knee: a qualitative and quantitative morphologic analysis of the fibular collateral ligament, popliteus tendon, popliteofibular ligament, and lateral gastrocnemius tendon. Am J Sports Med. 2003;31(6):854-860.
4. Chahla J, Nitri M, Civitarese D, Dean CS, Moulton SG, LaPrade RF. Anatomic double-bundle posterior cruciate ligament reconstruction. Arthrosc Tech. 2016;5(1):e149-e156.
5. Anderson CJ, Ziegler CG, Wijdicks CA, Engebretsen L, LaPrade RF. Arthroscopically pertinent anatomy of the anterolateral and posteromedial bundles of the posterior cruciate ligament. J Bone Joint Surg Am. 2012;94(21):1936-1945.
6. Mansour R, Yoong P, McKean D, Teh JL. The iliotibial band in acute knee trauma: patterns of injury on MR imaging. Skeletal Radiol. 2014;43(10):1369-1375.
7. LaPrade RF, Johansen S, Wentorf FA, Engebretsen L, Esterberg JL, Tso A. An analysis of an anatomical posterolateral knee reconstruction: an in vitro biomechanical study and development of a surgical technique. Am J Sports Med. 2004;32(6):1405-1414.
8. McCarthy M, Camarda L, Wijdicks CA, Johansen S, Engebretsen L, LaPrade RF. Anatomic posterolateral knee reconstructions require a popliteofibular ligament reconstruction through a tibial tunnel. Am J Sports Med. 2010;38(8):1674-1681.
9. LaPrade RF, Wozniczka JK, Stellmaker MP, Wijdicks CA. Analysis of the static function of the popliteus tendon and evaluation of an anatomic reconstruction: the “fifth ligament” of the knee. Am J Sports Med. 2010;38(3):543-549.
10. Geeslin AG, LaPrade RF. Outcomes of treatment of acute grade-III isolated and combined posterolateral knee injuries: a prospective case series and surgical technique. J Bone Joint Surg Am. 2011;93(18):1672-1683.
11. LaPrade RF, Johansen S, Agel J, Risberg MA, Moksnes H, Engebretsen L. Outcomes of an anatomic posterolateral knee reconstruction. J Bone Joint Surg Am. 2010;92(1):16-22.
12. Spiridonov SI, Slinkard NJ, LaPrade RF. Isolated and combined grade-III posterior cruciate ligament tears treated with double-bundle reconstruction with use of endoscopically placed femoral tunnels and grafts: operative technique and clinical outcomes. J Bone Joint Surg Am. 2011;93(19):1773-1780.
13. Noyes FR, Barber-Westin SD. Reconstruction of the anterior and posterior cruciate ligaments after knee dislocation. Use of early protected postoperative motion to decrease arthrofibrosis. Am J Sports Med. 1997;25(6):769-778.
14. Yenchak AJ, Wilk KE, Arrigo CA, Simpson CD, Andrews JR. Criteria-based management of an acute multistructure knee injury in a professional football player: a case report. J Orthop Sports Phys Ther. 2011;41(9):675-686.
1. Delos D, Warren RF, Marx RG. Multiligament knee injuries and their treatment. Oper Tech Sports Med. 2010;18(4):219-226.
2. Hobby B, Treme G, Wascher DC, Schenck RC. How I manage knee dislocations. Oper Tech Sports Med. 2010;18(4):227-234.
3. LaPrade RF, Ly TV, Wentorf FA, Engebretsen L. The posterolateral attachments of the knee: a qualitative and quantitative morphologic analysis of the fibular collateral ligament, popliteus tendon, popliteofibular ligament, and lateral gastrocnemius tendon. Am J Sports Med. 2003;31(6):854-860.
4. Chahla J, Nitri M, Civitarese D, Dean CS, Moulton SG, LaPrade RF. Anatomic double-bundle posterior cruciate ligament reconstruction. Arthrosc Tech. 2016;5(1):e149-e156.
5. Anderson CJ, Ziegler CG, Wijdicks CA, Engebretsen L, LaPrade RF. Arthroscopically pertinent anatomy of the anterolateral and posteromedial bundles of the posterior cruciate ligament. J Bone Joint Surg Am. 2012;94(21):1936-1945.
6. Mansour R, Yoong P, McKean D, Teh JL. The iliotibial band in acute knee trauma: patterns of injury on MR imaging. Skeletal Radiol. 2014;43(10):1369-1375.
7. LaPrade RF, Johansen S, Wentorf FA, Engebretsen L, Esterberg JL, Tso A. An analysis of an anatomical posterolateral knee reconstruction: an in vitro biomechanical study and development of a surgical technique. Am J Sports Med. 2004;32(6):1405-1414.
8. McCarthy M, Camarda L, Wijdicks CA, Johansen S, Engebretsen L, LaPrade RF. Anatomic posterolateral knee reconstructions require a popliteofibular ligament reconstruction through a tibial tunnel. Am J Sports Med. 2010;38(8):1674-1681.
9. LaPrade RF, Wozniczka JK, Stellmaker MP, Wijdicks CA. Analysis of the static function of the popliteus tendon and evaluation of an anatomic reconstruction: the “fifth ligament” of the knee. Am J Sports Med. 2010;38(3):543-549.
10. Geeslin AG, LaPrade RF. Outcomes of treatment of acute grade-III isolated and combined posterolateral knee injuries: a prospective case series and surgical technique. J Bone Joint Surg Am. 2011;93(18):1672-1683.
11. LaPrade RF, Johansen S, Agel J, Risberg MA, Moksnes H, Engebretsen L. Outcomes of an anatomic posterolateral knee reconstruction. J Bone Joint Surg Am. 2010;92(1):16-22.
12. Spiridonov SI, Slinkard NJ, LaPrade RF. Isolated and combined grade-III posterior cruciate ligament tears treated with double-bundle reconstruction with use of endoscopically placed femoral tunnels and grafts: operative technique and clinical outcomes. J Bone Joint Surg Am. 2011;93(19):1773-1780.
13. Noyes FR, Barber-Westin SD. Reconstruction of the anterior and posterior cruciate ligaments after knee dislocation. Use of early protected postoperative motion to decrease arthrofibrosis. Am J Sports Med. 1997;25(6):769-778.
14. Yenchak AJ, Wilk KE, Arrigo CA, Simpson CD, Andrews JR. Criteria-based management of an acute multistructure knee injury in a professional football player: a case report. J Orthop Sports Phys Ther. 2011;41(9):675-686.
Arthroscopic Excision of Bipartite Patella With Preservation of Lateral Retinaculum in an Adolescent Ice Hockey Player
Take-Home Points
- Bipartite patella is an asymptomatic anatomical variant.
- Occasionally, some adolescent athletes can present with AKP, resulting in decreased participation and performance.
- Bipartite patella is classified in type I, inferior pole; type II, lateral margin; and type III, superior lateral pole, depending on where the accessory patellar fragment is.
- Nonoperative treatment is advocated first. If symptoms persist surgical treatment should be attempted.
In 2% to 3% of the general population, the finding of bipartite patella on knee radiographs is often incidental.1,2 During development, the patella normally originates in a primary ossification center. Occasionally, secondary ossification centers emerge around the margins of the primary center and typically join that center. In some cases, the secondary2 center remains separated, leading to patella partita and an accessory patellar fragment.3,4
The bipartite patella is connected to the primary patella by fibrocartilage. The fibrous attachment may become irritated or separated as a result of trauma, overuse, or strenuous activity.1,5-7 Saupe classification of bipartite patella is based on accessory patellar fragment location: type I, inferior pole; type II, lateral margin; and type III, superior lateral pole.8 When an individual with a bipartite patella becomes symptomatic, anterior knee pain (AKP) is the most common complaint—it has been described in adolescent athletes in numerous sports.7,9-11For most patients, first-line treatment is nonoperative management. A typical regimen includes reduced activity, use of nonsteroidal anti-inflammatory drugs, physical therapy, and isometric quadriceps-strengthening exercises.1,12 Other nonoperative approaches described in the literature are immobilization,5,10 steroid and anesthetic injection, and ultrasound therapy.13 If symptoms do not improve, surgical treatment should be considered. Surgical treatment options include open excision of fragment,3,9,12 arthroscopic excision of fragment,7,14,15 tension band wiring,5,16 open reduction and internal fixation,17 open or arthroscopic vastus lateralis release,18-20 and lateral retinacular release.21 However, the optimal surgical option remains controversial.
In this case report, we present a modification of an arthroscopic surgical technique for excising a symptomatic bipartite patella and report midterm clinical outcomes. The patient provided written informed consent for print and electronic publication of this report.
Case Report
A 16-year-old elite male ice hockey player presented to clinic with a 2-week history of left AKP. He could not recall a specific injury that triggered the symptoms. Radiographs were obtained at an outside institution, and knee patellar fracture was diagnosed. The patient, placed in a straight-leg immobilizer, later presented to a referral clinic for a second opinion and further evaluation. Physical examination revealed significant tenderness to palpation of the lateral aspect of the patella. Range of motion was symmetric and fully intact. Patellar mobility was excellent. However, the patient could not perform a straight-leg raise because of the pain.
We obtained anteroposterior and lateral radiographs (Figures 1A, 1B), which showed evidence of a Saupe type III bipartite patella with separation at the superolateral pole.
Two years later, the patient returned with left AKP, again localized to the lateral aspect of the patella, over the bipartite fragment. The pain was significant with compression. Given the patient’s history, arthroscopic excision of the bipartite patella was recommended. After discussing all treatment options, the patient elected to proceed with the surgery.
Surgical Technique
The patient was positioned supine on the operating table. Medial and lateral parapatellar arthroscopic portals were created. Menisci, cruciate ligaments, and tibiofemoral articular cartilage were arthroscopically visualized and determined to be normal. The bipartite patella was easily visualized, and notably loose when probed. Grade 2 chondromalacia was present diffusely throughout the bipartite patella and on the far lateral aspect of the patella, at the fragment interface.
Attention was then turned to arthroscopic removal of the accessory patellar fragment (Figures 3A, 3B).
Postoperative Rehabilitation
Rehabilitation focused on protection of the healing patella and accelerated rehabilitation for early return to play. Range-of-motion exercises and stationary bicycling were initiated on postoperative day 1. Weight-bearing was allowed as tolerated. Quadriceps sets, straight-leg raises, and ankle pumps were performed 5 times daily for 6 weeks. Six weeks after surgery, the patient was cleared, and he returned to full on-ice activities.
Outcomes
This study was approved by an Institutional Review Board. Preoperative and postoperative outcomes were obtained and stored in a data registry. The patient’s Lysholm score22 improved from 71 before surgery to 100 at 31-month follow-up. In addition, his subjective International Knee Documentation Committee score23 improved from 65.5 before surgery to 72.4 after surgery. At follow-up, patient satisfaction with outcome was 10/10. In addition, the patient had returned to playing hockey at a higher national level without functional limitation.
Discussion
The most important finding in this case is that arthroscopic excision of a bipartite patella with preservation of the lateral retinaculum in an elite adolescent hockey player resulted in improved subjective clinical outcomes scores and early return to competition. Arthroscopic excision was favored over open excision in this patient because of potential quicker recovery,14 less pain, and expedited return to competition. In addition, previous arthroscopic techniques were modified to shorten postoperative rehabilitation. The modified technique included preservation of the lateral retinaculum and total arthroscopic excision of the accessory bipartite patella fragment.
Although results of open techniques have been favorable,3,8,9 these procedures are far more invasive than arthroscopic techniques and may result in loss of quadriceps strength and prolonged rehabilitation.18 Weckström and colleagues12 followed 25 male military recruits for a minimum of 10 years after open excision of symptomatic bipartite patella. Mean Kujala score was 95 (range, 75-100), and median visual analog scale score for knee pain was 1.0 (range, 0.0-6.0). In a study by Bourne and Bianco,3 13 of 16 patients who were followed for an average of 7 years experienced complete pain relief with an average recovery time of 2 months.
Other studies have described the arthroscopic excision technique for symptomatic bipartite patella,7,14,15 but outcomes are underreported, especially for follow-ups longer than 2 years. Felli and colleagues7 described a case of arthroscopic excision and lateral release in a 23-year-old female professional volleyball player; at 1-year follow-up, the patient was symptom-free and back to full athletic participation. Azarbod and colleagues14 also reported on a patient who was symptom-free, 6 weeks after arthroscopic excision of bipartite patella. Carney and colleagues15 indicated that successful excision of bipartite patella was evident on 6-month radiographic follow-up. Our 31-month follow-up is the longest of any study on arthroscopic excision of bipartite patella. Clinical outcomes were excellent both in our patient’s case and in the earlier studies.
Our patient was a high-level hockey player who wanted to return to competition as quickly as possible. Conservative management, including physical therapy, initially resolved his symptoms and allowed him to resume on-ice activities after 6 weeks. In time, however, his symptoms returned and began limiting his on-ice performance. Arthroscopic removal of the bipartite patella accessory fragment allowed him to return to full on-ice activities after 6 weeks. His case provides evidence that arthroscopic management of bipartite patella with preservation of the vastus lateralis and lateral retinaculum may be an excellent treatment option for patients who want to return to athletics as quickly as possible.
Our technique of arthroscopic excision with preservation of lateral retinaculum is an excellent treatment option for symptomatic bipartite patella. This option, combined with an aggressive rehabilitation protocol, allows for pain relief and expedited return to competition.
Am J Orthop. 2017;46(3):135-138. Copyright Frontline Medical Communications Inc. 2017. All rights reserved.
1. Atesok K, Doral MN, Lowe J, Finsterbush A. Symptomatic bipartite patella: treatment alternatives. J Am Acad Orthop Surg. 2008;16(8):455-461.
2. Insall J. Current concepts review: patellar pain. J Bone Joint Surg Am. 1982;64(1):147-152.
3. Bourne MH, Bianco AJ Jr. Bipartite patella in the adolescent: results of surgical excision. J Pediatr Orthop. 1990;10(1):69-73.
4. Oohashi Y, Koshino T, Oohashi Y. Clinical features and classification of bipartite or tripartite patella. Knee Surg Sports Traumatol Arthrosc. 2010;18(11):1465-1469.
5. Okuno H, Sugita T, Kawamata T, Ohnuma M, Yamada N, Yoshizumi Y. Traumatic separation of a type I bipartite patella: a report of four knees. Clin Orthop Relat Res. 2004;(420):257-260.
6. Yoo JH, Kim EH, Ryu HK. Arthroscopic removal of separated bipartite patella causing snapping knee syndrome. Orthopedics. 2008;31(7):717.
7. Felli L, Fiore M, Biglieni L. Arthroscopic treatment of symptomatic bipartite patella. Knee Surg Sports Traumatol Arthrosc. 2011;19(3):398-399.
8. Green WT Jr. Painful bipartite patellae. A report of three cases. Clin Orthop Relat Res. 1975;(110):197-200.
9. Ishikawa H, Sakurai A, Hirata S, et al. Painful bipartite patella in young athletes. The diagnostic value of skyline views taken in squatting position and the results of surgical excision. Clin Orthop Relat Res. 1994;(305):223-228.
10. Stocker RL, van Laer L. Injury of a bipartite patella in a young upcoming sportsman. Arch Orthop Trauma Surg. 2011;131(1):75-78.
11. Wong CK. Bipartite patella in a young athlete. J Orthop Sports Phys Ther. 2009;39(7):560.
12. Weckström M, Parviainen M, Pihlajamäki HK. Excision of painful bipartite patella: good long-term outcome in young adults. Clin Orthop Relat Res. 2008;466(11):2848-2855.
13. Kumahashi N, Uchio Y, Iwasa J, Kawasaki K, Adachi N, Ochi M. Bone union of painful bipartite patella after treatment with low-intensity pulsed ultrasound: report of two cases. Knee. 2008;15(1):50-53.
14. Azarbod P, Agar G, Patel V. Arthroscopic excision of a painful bipartite patella fragment. Arthroscopy. 2005;21(8):1006.
15. Carney J, Thompson D, O’Daniel J, Cassidy J. Arthroscopic excision of a painful bipartite patella fragment. Am J Orthop. 2010;39(1):40-43.
16. Tauber M, Matis N, Resch H. Traumatic separation of an uncommon bipartite patella type: a case report. Knee Surg Sports Traumatol Arthrosc. 2007;15(1):83-87.
17. Werner S, Durkan M, Jones J, Quilici S, Crawford D. Symptomatic bipartite patella: three subtypes, three representative cases. J Knee Surg. 2013;26(suppl 1):S72-S76.
18. Adachi N, Ochi M, Yamaguchi H, Uchio Y, Kuriwaka M. Vastus lateralis release for painful bipartite patella. Arthroscopy. 2002;18(4):404-411.
19. Maeno S, Hashimoto D, Otani T, Masumoto K, Hui C. The “coiling-up procedure”: a novel technique for extra-articular arthroscopy. Arthroscopy. 2010;26(11):1551-1555.
20. Ogata K. Painful bipartite patella. A new approach to operative treatment. J Bone Joint Surg Am. 1994;76(4):573-578.
21. Mori Y, Okumo H, Iketani H, Kuroki Y. Efficacy of lateral retinacular release for painful bipartite patella. Am J Sports Med. 1995;23(1):13-18.
22. Lysholm J, Gillquist J. Evaluation of knee ligament surgery results with special emphasis on use of a scoring scale. Am J Sports Med. 1982;10(3):150-154
23. Grevnerts HT, Terwee CB, Kvist J. The measurement properties of the IKDC-subjective knee form. Knee Surg Sports Traumatol Arthrosc. 2015;23(12):3698-3706.
Take-Home Points
- Bipartite patella is an asymptomatic anatomical variant.
- Occasionally, some adolescent athletes can present with AKP, resulting in decreased participation and performance.
- Bipartite patella is classified in type I, inferior pole; type II, lateral margin; and type III, superior lateral pole, depending on where the accessory patellar fragment is.
- Nonoperative treatment is advocated first. If symptoms persist surgical treatment should be attempted.
In 2% to 3% of the general population, the finding of bipartite patella on knee radiographs is often incidental.1,2 During development, the patella normally originates in a primary ossification center. Occasionally, secondary ossification centers emerge around the margins of the primary center and typically join that center. In some cases, the secondary2 center remains separated, leading to patella partita and an accessory patellar fragment.3,4
The bipartite patella is connected to the primary patella by fibrocartilage. The fibrous attachment may become irritated or separated as a result of trauma, overuse, or strenuous activity.1,5-7 Saupe classification of bipartite patella is based on accessory patellar fragment location: type I, inferior pole; type II, lateral margin; and type III, superior lateral pole.8 When an individual with a bipartite patella becomes symptomatic, anterior knee pain (AKP) is the most common complaint—it has been described in adolescent athletes in numerous sports.7,9-11For most patients, first-line treatment is nonoperative management. A typical regimen includes reduced activity, use of nonsteroidal anti-inflammatory drugs, physical therapy, and isometric quadriceps-strengthening exercises.1,12 Other nonoperative approaches described in the literature are immobilization,5,10 steroid and anesthetic injection, and ultrasound therapy.13 If symptoms do not improve, surgical treatment should be considered. Surgical treatment options include open excision of fragment,3,9,12 arthroscopic excision of fragment,7,14,15 tension band wiring,5,16 open reduction and internal fixation,17 open or arthroscopic vastus lateralis release,18-20 and lateral retinacular release.21 However, the optimal surgical option remains controversial.
In this case report, we present a modification of an arthroscopic surgical technique for excising a symptomatic bipartite patella and report midterm clinical outcomes. The patient provided written informed consent for print and electronic publication of this report.
Case Report
A 16-year-old elite male ice hockey player presented to clinic with a 2-week history of left AKP. He could not recall a specific injury that triggered the symptoms. Radiographs were obtained at an outside institution, and knee patellar fracture was diagnosed. The patient, placed in a straight-leg immobilizer, later presented to a referral clinic for a second opinion and further evaluation. Physical examination revealed significant tenderness to palpation of the lateral aspect of the patella. Range of motion was symmetric and fully intact. Patellar mobility was excellent. However, the patient could not perform a straight-leg raise because of the pain.
We obtained anteroposterior and lateral radiographs (Figures 1A, 1B), which showed evidence of a Saupe type III bipartite patella with separation at the superolateral pole.
Two years later, the patient returned with left AKP, again localized to the lateral aspect of the patella, over the bipartite fragment. The pain was significant with compression. Given the patient’s history, arthroscopic excision of the bipartite patella was recommended. After discussing all treatment options, the patient elected to proceed with the surgery.
Surgical Technique
The patient was positioned supine on the operating table. Medial and lateral parapatellar arthroscopic portals were created. Menisci, cruciate ligaments, and tibiofemoral articular cartilage were arthroscopically visualized and determined to be normal. The bipartite patella was easily visualized, and notably loose when probed. Grade 2 chondromalacia was present diffusely throughout the bipartite patella and on the far lateral aspect of the patella, at the fragment interface.
Attention was then turned to arthroscopic removal of the accessory patellar fragment (Figures 3A, 3B).
Postoperative Rehabilitation
Rehabilitation focused on protection of the healing patella and accelerated rehabilitation for early return to play. Range-of-motion exercises and stationary bicycling were initiated on postoperative day 1. Weight-bearing was allowed as tolerated. Quadriceps sets, straight-leg raises, and ankle pumps were performed 5 times daily for 6 weeks. Six weeks after surgery, the patient was cleared, and he returned to full on-ice activities.
Outcomes
This study was approved by an Institutional Review Board. Preoperative and postoperative outcomes were obtained and stored in a data registry. The patient’s Lysholm score22 improved from 71 before surgery to 100 at 31-month follow-up. In addition, his subjective International Knee Documentation Committee score23 improved from 65.5 before surgery to 72.4 after surgery. At follow-up, patient satisfaction with outcome was 10/10. In addition, the patient had returned to playing hockey at a higher national level without functional limitation.
Discussion
The most important finding in this case is that arthroscopic excision of a bipartite patella with preservation of the lateral retinaculum in an elite adolescent hockey player resulted in improved subjective clinical outcomes scores and early return to competition. Arthroscopic excision was favored over open excision in this patient because of potential quicker recovery,14 less pain, and expedited return to competition. In addition, previous arthroscopic techniques were modified to shorten postoperative rehabilitation. The modified technique included preservation of the lateral retinaculum and total arthroscopic excision of the accessory bipartite patella fragment.
Although results of open techniques have been favorable,3,8,9 these procedures are far more invasive than arthroscopic techniques and may result in loss of quadriceps strength and prolonged rehabilitation.18 Weckström and colleagues12 followed 25 male military recruits for a minimum of 10 years after open excision of symptomatic bipartite patella. Mean Kujala score was 95 (range, 75-100), and median visual analog scale score for knee pain was 1.0 (range, 0.0-6.0). In a study by Bourne and Bianco,3 13 of 16 patients who were followed for an average of 7 years experienced complete pain relief with an average recovery time of 2 months.
Other studies have described the arthroscopic excision technique for symptomatic bipartite patella,7,14,15 but outcomes are underreported, especially for follow-ups longer than 2 years. Felli and colleagues7 described a case of arthroscopic excision and lateral release in a 23-year-old female professional volleyball player; at 1-year follow-up, the patient was symptom-free and back to full athletic participation. Azarbod and colleagues14 also reported on a patient who was symptom-free, 6 weeks after arthroscopic excision of bipartite patella. Carney and colleagues15 indicated that successful excision of bipartite patella was evident on 6-month radiographic follow-up. Our 31-month follow-up is the longest of any study on arthroscopic excision of bipartite patella. Clinical outcomes were excellent both in our patient’s case and in the earlier studies.
Our patient was a high-level hockey player who wanted to return to competition as quickly as possible. Conservative management, including physical therapy, initially resolved his symptoms and allowed him to resume on-ice activities after 6 weeks. In time, however, his symptoms returned and began limiting his on-ice performance. Arthroscopic removal of the bipartite patella accessory fragment allowed him to return to full on-ice activities after 6 weeks. His case provides evidence that arthroscopic management of bipartite patella with preservation of the vastus lateralis and lateral retinaculum may be an excellent treatment option for patients who want to return to athletics as quickly as possible.
Our technique of arthroscopic excision with preservation of lateral retinaculum is an excellent treatment option for symptomatic bipartite patella. This option, combined with an aggressive rehabilitation protocol, allows for pain relief and expedited return to competition.
Am J Orthop. 2017;46(3):135-138. Copyright Frontline Medical Communications Inc. 2017. All rights reserved.
Take-Home Points
- Bipartite patella is an asymptomatic anatomical variant.
- Occasionally, some adolescent athletes can present with AKP, resulting in decreased participation and performance.
- Bipartite patella is classified in type I, inferior pole; type II, lateral margin; and type III, superior lateral pole, depending on where the accessory patellar fragment is.
- Nonoperative treatment is advocated first. If symptoms persist surgical treatment should be attempted.
In 2% to 3% of the general population, the finding of bipartite patella on knee radiographs is often incidental.1,2 During development, the patella normally originates in a primary ossification center. Occasionally, secondary ossification centers emerge around the margins of the primary center and typically join that center. In some cases, the secondary2 center remains separated, leading to patella partita and an accessory patellar fragment.3,4
The bipartite patella is connected to the primary patella by fibrocartilage. The fibrous attachment may become irritated or separated as a result of trauma, overuse, or strenuous activity.1,5-7 Saupe classification of bipartite patella is based on accessory patellar fragment location: type I, inferior pole; type II, lateral margin; and type III, superior lateral pole.8 When an individual with a bipartite patella becomes symptomatic, anterior knee pain (AKP) is the most common complaint—it has been described in adolescent athletes in numerous sports.7,9-11For most patients, first-line treatment is nonoperative management. A typical regimen includes reduced activity, use of nonsteroidal anti-inflammatory drugs, physical therapy, and isometric quadriceps-strengthening exercises.1,12 Other nonoperative approaches described in the literature are immobilization,5,10 steroid and anesthetic injection, and ultrasound therapy.13 If symptoms do not improve, surgical treatment should be considered. Surgical treatment options include open excision of fragment,3,9,12 arthroscopic excision of fragment,7,14,15 tension band wiring,5,16 open reduction and internal fixation,17 open or arthroscopic vastus lateralis release,18-20 and lateral retinacular release.21 However, the optimal surgical option remains controversial.
In this case report, we present a modification of an arthroscopic surgical technique for excising a symptomatic bipartite patella and report midterm clinical outcomes. The patient provided written informed consent for print and electronic publication of this report.
Case Report
A 16-year-old elite male ice hockey player presented to clinic with a 2-week history of left AKP. He could not recall a specific injury that triggered the symptoms. Radiographs were obtained at an outside institution, and knee patellar fracture was diagnosed. The patient, placed in a straight-leg immobilizer, later presented to a referral clinic for a second opinion and further evaluation. Physical examination revealed significant tenderness to palpation of the lateral aspect of the patella. Range of motion was symmetric and fully intact. Patellar mobility was excellent. However, the patient could not perform a straight-leg raise because of the pain.
We obtained anteroposterior and lateral radiographs (Figures 1A, 1B), which showed evidence of a Saupe type III bipartite patella with separation at the superolateral pole.
Two years later, the patient returned with left AKP, again localized to the lateral aspect of the patella, over the bipartite fragment. The pain was significant with compression. Given the patient’s history, arthroscopic excision of the bipartite patella was recommended. After discussing all treatment options, the patient elected to proceed with the surgery.
Surgical Technique
The patient was positioned supine on the operating table. Medial and lateral parapatellar arthroscopic portals were created. Menisci, cruciate ligaments, and tibiofemoral articular cartilage were arthroscopically visualized and determined to be normal. The bipartite patella was easily visualized, and notably loose when probed. Grade 2 chondromalacia was present diffusely throughout the bipartite patella and on the far lateral aspect of the patella, at the fragment interface.
Attention was then turned to arthroscopic removal of the accessory patellar fragment (Figures 3A, 3B).
Postoperative Rehabilitation
Rehabilitation focused on protection of the healing patella and accelerated rehabilitation for early return to play. Range-of-motion exercises and stationary bicycling were initiated on postoperative day 1. Weight-bearing was allowed as tolerated. Quadriceps sets, straight-leg raises, and ankle pumps were performed 5 times daily for 6 weeks. Six weeks after surgery, the patient was cleared, and he returned to full on-ice activities.
Outcomes
This study was approved by an Institutional Review Board. Preoperative and postoperative outcomes were obtained and stored in a data registry. The patient’s Lysholm score22 improved from 71 before surgery to 100 at 31-month follow-up. In addition, his subjective International Knee Documentation Committee score23 improved from 65.5 before surgery to 72.4 after surgery. At follow-up, patient satisfaction with outcome was 10/10. In addition, the patient had returned to playing hockey at a higher national level without functional limitation.
Discussion
The most important finding in this case is that arthroscopic excision of a bipartite patella with preservation of the lateral retinaculum in an elite adolescent hockey player resulted in improved subjective clinical outcomes scores and early return to competition. Arthroscopic excision was favored over open excision in this patient because of potential quicker recovery,14 less pain, and expedited return to competition. In addition, previous arthroscopic techniques were modified to shorten postoperative rehabilitation. The modified technique included preservation of the lateral retinaculum and total arthroscopic excision of the accessory bipartite patella fragment.
Although results of open techniques have been favorable,3,8,9 these procedures are far more invasive than arthroscopic techniques and may result in loss of quadriceps strength and prolonged rehabilitation.18 Weckström and colleagues12 followed 25 male military recruits for a minimum of 10 years after open excision of symptomatic bipartite patella. Mean Kujala score was 95 (range, 75-100), and median visual analog scale score for knee pain was 1.0 (range, 0.0-6.0). In a study by Bourne and Bianco,3 13 of 16 patients who were followed for an average of 7 years experienced complete pain relief with an average recovery time of 2 months.
Other studies have described the arthroscopic excision technique for symptomatic bipartite patella,7,14,15 but outcomes are underreported, especially for follow-ups longer than 2 years. Felli and colleagues7 described a case of arthroscopic excision and lateral release in a 23-year-old female professional volleyball player; at 1-year follow-up, the patient was symptom-free and back to full athletic participation. Azarbod and colleagues14 also reported on a patient who was symptom-free, 6 weeks after arthroscopic excision of bipartite patella. Carney and colleagues15 indicated that successful excision of bipartite patella was evident on 6-month radiographic follow-up. Our 31-month follow-up is the longest of any study on arthroscopic excision of bipartite patella. Clinical outcomes were excellent both in our patient’s case and in the earlier studies.
Our patient was a high-level hockey player who wanted to return to competition as quickly as possible. Conservative management, including physical therapy, initially resolved his symptoms and allowed him to resume on-ice activities after 6 weeks. In time, however, his symptoms returned and began limiting his on-ice performance. Arthroscopic removal of the bipartite patella accessory fragment allowed him to return to full on-ice activities after 6 weeks. His case provides evidence that arthroscopic management of bipartite patella with preservation of the vastus lateralis and lateral retinaculum may be an excellent treatment option for patients who want to return to athletics as quickly as possible.
Our technique of arthroscopic excision with preservation of lateral retinaculum is an excellent treatment option for symptomatic bipartite patella. This option, combined with an aggressive rehabilitation protocol, allows for pain relief and expedited return to competition.
Am J Orthop. 2017;46(3):135-138. Copyright Frontline Medical Communications Inc. 2017. All rights reserved.
1. Atesok K, Doral MN, Lowe J, Finsterbush A. Symptomatic bipartite patella: treatment alternatives. J Am Acad Orthop Surg. 2008;16(8):455-461.
2. Insall J. Current concepts review: patellar pain. J Bone Joint Surg Am. 1982;64(1):147-152.
3. Bourne MH, Bianco AJ Jr. Bipartite patella in the adolescent: results of surgical excision. J Pediatr Orthop. 1990;10(1):69-73.
4. Oohashi Y, Koshino T, Oohashi Y. Clinical features and classification of bipartite or tripartite patella. Knee Surg Sports Traumatol Arthrosc. 2010;18(11):1465-1469.
5. Okuno H, Sugita T, Kawamata T, Ohnuma M, Yamada N, Yoshizumi Y. Traumatic separation of a type I bipartite patella: a report of four knees. Clin Orthop Relat Res. 2004;(420):257-260.
6. Yoo JH, Kim EH, Ryu HK. Arthroscopic removal of separated bipartite patella causing snapping knee syndrome. Orthopedics. 2008;31(7):717.
7. Felli L, Fiore M, Biglieni L. Arthroscopic treatment of symptomatic bipartite patella. Knee Surg Sports Traumatol Arthrosc. 2011;19(3):398-399.
8. Green WT Jr. Painful bipartite patellae. A report of three cases. Clin Orthop Relat Res. 1975;(110):197-200.
9. Ishikawa H, Sakurai A, Hirata S, et al. Painful bipartite patella in young athletes. The diagnostic value of skyline views taken in squatting position and the results of surgical excision. Clin Orthop Relat Res. 1994;(305):223-228.
10. Stocker RL, van Laer L. Injury of a bipartite patella in a young upcoming sportsman. Arch Orthop Trauma Surg. 2011;131(1):75-78.
11. Wong CK. Bipartite patella in a young athlete. J Orthop Sports Phys Ther. 2009;39(7):560.
12. Weckström M, Parviainen M, Pihlajamäki HK. Excision of painful bipartite patella: good long-term outcome in young adults. Clin Orthop Relat Res. 2008;466(11):2848-2855.
13. Kumahashi N, Uchio Y, Iwasa J, Kawasaki K, Adachi N, Ochi M. Bone union of painful bipartite patella after treatment with low-intensity pulsed ultrasound: report of two cases. Knee. 2008;15(1):50-53.
14. Azarbod P, Agar G, Patel V. Arthroscopic excision of a painful bipartite patella fragment. Arthroscopy. 2005;21(8):1006.
15. Carney J, Thompson D, O’Daniel J, Cassidy J. Arthroscopic excision of a painful bipartite patella fragment. Am J Orthop. 2010;39(1):40-43.
16. Tauber M, Matis N, Resch H. Traumatic separation of an uncommon bipartite patella type: a case report. Knee Surg Sports Traumatol Arthrosc. 2007;15(1):83-87.
17. Werner S, Durkan M, Jones J, Quilici S, Crawford D. Symptomatic bipartite patella: three subtypes, three representative cases. J Knee Surg. 2013;26(suppl 1):S72-S76.
18. Adachi N, Ochi M, Yamaguchi H, Uchio Y, Kuriwaka M. Vastus lateralis release for painful bipartite patella. Arthroscopy. 2002;18(4):404-411.
19. Maeno S, Hashimoto D, Otani T, Masumoto K, Hui C. The “coiling-up procedure”: a novel technique for extra-articular arthroscopy. Arthroscopy. 2010;26(11):1551-1555.
20. Ogata K. Painful bipartite patella. A new approach to operative treatment. J Bone Joint Surg Am. 1994;76(4):573-578.
21. Mori Y, Okumo H, Iketani H, Kuroki Y. Efficacy of lateral retinacular release for painful bipartite patella. Am J Sports Med. 1995;23(1):13-18.
22. Lysholm J, Gillquist J. Evaluation of knee ligament surgery results with special emphasis on use of a scoring scale. Am J Sports Med. 1982;10(3):150-154
23. Grevnerts HT, Terwee CB, Kvist J. The measurement properties of the IKDC-subjective knee form. Knee Surg Sports Traumatol Arthrosc. 2015;23(12):3698-3706.
1. Atesok K, Doral MN, Lowe J, Finsterbush A. Symptomatic bipartite patella: treatment alternatives. J Am Acad Orthop Surg. 2008;16(8):455-461.
2. Insall J. Current concepts review: patellar pain. J Bone Joint Surg Am. 1982;64(1):147-152.
3. Bourne MH, Bianco AJ Jr. Bipartite patella in the adolescent: results of surgical excision. J Pediatr Orthop. 1990;10(1):69-73.
4. Oohashi Y, Koshino T, Oohashi Y. Clinical features and classification of bipartite or tripartite patella. Knee Surg Sports Traumatol Arthrosc. 2010;18(11):1465-1469.
5. Okuno H, Sugita T, Kawamata T, Ohnuma M, Yamada N, Yoshizumi Y. Traumatic separation of a type I bipartite patella: a report of four knees. Clin Orthop Relat Res. 2004;(420):257-260.
6. Yoo JH, Kim EH, Ryu HK. Arthroscopic removal of separated bipartite patella causing snapping knee syndrome. Orthopedics. 2008;31(7):717.
7. Felli L, Fiore M, Biglieni L. Arthroscopic treatment of symptomatic bipartite patella. Knee Surg Sports Traumatol Arthrosc. 2011;19(3):398-399.
8. Green WT Jr. Painful bipartite patellae. A report of three cases. Clin Orthop Relat Res. 1975;(110):197-200.
9. Ishikawa H, Sakurai A, Hirata S, et al. Painful bipartite patella in young athletes. The diagnostic value of skyline views taken in squatting position and the results of surgical excision. Clin Orthop Relat Res. 1994;(305):223-228.
10. Stocker RL, van Laer L. Injury of a bipartite patella in a young upcoming sportsman. Arch Orthop Trauma Surg. 2011;131(1):75-78.
11. Wong CK. Bipartite patella in a young athlete. J Orthop Sports Phys Ther. 2009;39(7):560.
12. Weckström M, Parviainen M, Pihlajamäki HK. Excision of painful bipartite patella: good long-term outcome in young adults. Clin Orthop Relat Res. 2008;466(11):2848-2855.
13. Kumahashi N, Uchio Y, Iwasa J, Kawasaki K, Adachi N, Ochi M. Bone union of painful bipartite patella after treatment with low-intensity pulsed ultrasound: report of two cases. Knee. 2008;15(1):50-53.
14. Azarbod P, Agar G, Patel V. Arthroscopic excision of a painful bipartite patella fragment. Arthroscopy. 2005;21(8):1006.
15. Carney J, Thompson D, O’Daniel J, Cassidy J. Arthroscopic excision of a painful bipartite patella fragment. Am J Orthop. 2010;39(1):40-43.
16. Tauber M, Matis N, Resch H. Traumatic separation of an uncommon bipartite patella type: a case report. Knee Surg Sports Traumatol Arthrosc. 2007;15(1):83-87.
17. Werner S, Durkan M, Jones J, Quilici S, Crawford D. Symptomatic bipartite patella: three subtypes, three representative cases. J Knee Surg. 2013;26(suppl 1):S72-S76.
18. Adachi N, Ochi M, Yamaguchi H, Uchio Y, Kuriwaka M. Vastus lateralis release for painful bipartite patella. Arthroscopy. 2002;18(4):404-411.
19. Maeno S, Hashimoto D, Otani T, Masumoto K, Hui C. The “coiling-up procedure”: a novel technique for extra-articular arthroscopy. Arthroscopy. 2010;26(11):1551-1555.
20. Ogata K. Painful bipartite patella. A new approach to operative treatment. J Bone Joint Surg Am. 1994;76(4):573-578.
21. Mori Y, Okumo H, Iketani H, Kuroki Y. Efficacy of lateral retinacular release for painful bipartite patella. Am J Sports Med. 1995;23(1):13-18.
22. Lysholm J, Gillquist J. Evaluation of knee ligament surgery results with special emphasis on use of a scoring scale. Am J Sports Med. 1982;10(3):150-154
23. Grevnerts HT, Terwee CB, Kvist J. The measurement properties of the IKDC-subjective knee form. Knee Surg Sports Traumatol Arthrosc. 2015;23(12):3698-3706.