User login
Implementing a Telehealth Shared Counseling and Decision-Making Visit for Lung Cancer Screening in a Veterans Affairs Medical Center
Lung cancer is the second most frequently diagnosed cancer among US veterans and the leading cause of cancer death.1 Clinical trials have shown that annual screening of high-risk persons with low-dose computed tomography (LDCT) can reduce the risk of dying of lung cancer.2 In 2011, the National Lung Screening Trial (NLST) reported that over a 3-year period, annual LDCT screening reduced the risk of dying of lung cancer by 20% compared with chest radiograph screening.3 Lung cancer screening (LCS), however, was associated with harms, including false-positive results, complications from invasive diagnostic procedures, incidental findings, overdiagnosis, and radiation exposure.
The US Preventive Services Task Force (USPSTF) began recommending annual screening of high-risk persons after publication of the NLST results.4 The Veterans Health Administration (VHA) recommended implementing LCS in 2017.5 Guidelines, however, have consistently highlighted the complexity of the decision and the importance of engaging patients in thorough discussions about the potential benefits and harms of screening (shared decision making [SDM]). The Centers for Medicare and Medicaid Services (CMS) has issued coverage determinations mandating that eligible patients undergo a counseling visit that uses a decision aid to support SDM for LCS and addresses tobacco use.6,7 However, primary care practitioners (PCPs) face many challenges in delivering SDM, including a lack of awareness of clinical trial results and screening guidelines, competing clinical demands, being untrained in SDM, and not having educational resources.8 Patients in rural locations face travel burdens in attending counseling visits.9
We conducted a pilot study to address concerns with delivering SDM for LCS to veterans. We implemented a centralized screening model in which veterans were referred by clinicians to a trained decision coach who conducted telephone visits to discuss the initial LCS decision, addressed tobacco cessation, and placed LDCT orders. We evaluated the outcomes of this telemedicine visit by using decision quality metrics and tracking LCS uptake, referrals for tobacco cessation, and clinical outcomes. The University of Iowa Institutional Review Board considered this study to be a quality improvement project and waived informed consent and HIPAA (Health Insurance Portability and Accountability Act) authorization requirements.
Implementation
We implemented the LCS program at the Iowa City Veterans Affairs Health Care System (ICVAHCS), which has both resident and staff clinicians, and 2 community-based outpatient clinics (Coralville, Cedar Rapids) with staff clinicians. The pilot study, conducted from November 2020 through July 2022, was led by a multidisciplinary team that included a nurse, primary care physician, pulmonologist, and radiologist. The team conducted online presentations to educate PCPs about the epidemiology of lung cancer, results of screening trials, LCS guidelines, the rationale for a centralized model of SDM, and the ICVAHCS screening protocols.
Screening Referrals
When the study began in 2020, we used the 2015 USPSTF criteria for annual LCS: individuals aged 55 to 80 years with a 30 pack-year smoking history and current tobacco user or who had quit within 15 years.4 We lowered the starting age to 50 years and the pack-year requirement to 20 after the USPSTF issued updated guidelines in 2021.10 Clinicians were notified about potentially eligible patients through the US Department of Veterans Affairs (VA) Computerized Personal Record System (CPRS) reminders or by the nurse program coordinator (NPC) who reviewed health records of patients with upcoming appointments. If the clinician determined that screening was appropriate, they ordered an LCS consult. The NPC called the veteran to confirm eligibility, mailed a decision aid, and scheduled a telephone visit to conduct SDM. We used the VA decision aid developed for the LCS demonstration project conducted at 8 academic VA medical centers between 2013 and 2017.11
Shared Decision-Making Telephone Visit
The NPC adapted a telephone script developed for a Cancer Prevention and Research Institute of Texas–funded project conducted by 2 coauthors (RJV and LML).12 The NPC asked about receipt/review of the decision aid, described the screening process, and addressed benefits and potential harms of screening. The NPC also offered smoking cessation interventions for veterans who were currently smoking, including referrals to the VA patient aligned care team clinical pharmacist for management of tobacco cessation or to the national VA Quit Line. The encounter ended by assessing the veteran’s understanding of screening issues and eliciting the veteran’s preferences for LDCT and willingness to adhere with the LCS program.
LDCT Imaging
The NPC placed LDCT orders for veterans interested in screening and alerted the referring clinician to sign the order. Veterans who agreed to be screened were placed in an LCS dashboard developed by the Veterans Integrated Services Network (VISN) 23 LCS program that was used as a patient management tool. The dashboard allowed the NPC to track patients, ensuring that veterans were being scheduled for and completing initial and follow-up testing. Radiologists used the Lung-RADS (Lung Imaging Reporting and Data System) to categorize LDCT results (1, normal; 2, benign nodule; 3, probably benign nodule; 4, suspicious nodule).13 Veterans with Lung-RADS 1 or 2 results were scheduled for an annual LDCT (if they remained eligible). Veterans with Lung-RADS 3 results were scheduled for a 6-month follow-up CT. The screening program sent electronic consults to pulmonary for veterans with Lung-RADS 4 to determine whether they should undergo additional imaging or be evaluated in the pulmonary clinic.
Evaluating Shared Decision Making
We audio taped and transcribed randomly selected SDM encounters to assess fidelity with the 2016 CMS required discussion elements for counseling about lung cancer, including the benefit of reducing lung cancer mortality; the potential for harms from false alarms, incidental findings, overdiagnosis, and radiation exposure; the need for annual screening; the importance of smoking cessation; and the possibility of undergoing follow-up testing and diagnostic procedures. An investigator coded the transcripts to assess for the presence of each required element and scored the encounter from 0 to 7.
We also surveyed veterans completing SDM, using a convenience sampling strategy to evaluate knowledge, the quality of the SDM process, and decisional conflict. Initially, we sent mailed surveys to subjects to be completed 1 week after the SDM visit. To increase the response rate, we subsequently called patients to complete the surveys by telephone 1 week after the SDM visit.
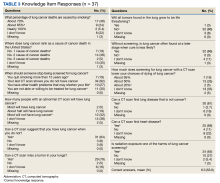
We used the validated LCS-12 knowledge measure to assess awareness of lung cancer risks, screening eligibility, and the benefits and harms of screening.14 We evaluated the quality of the SDM visit by using the 3-item CollaboRATE scale (Table 1).15
The NPC also took field notes during interviews to help identify additional SDM issues. After each call, the NPC noted her impressions of the veteran’s engagement with SDM and understanding of the screening issues.
Clinical Outcomes
We used the screening dashboard and CPRS to track clinical outcomes, including screening uptake, referrals for tobacco cessation, appropriate (screening or diagnostic) follow-up testing, and cancer diagnoses. We used descriptive statistics to characterize demographic data and survey responses.
Initial Findings
We conducted 105 SDM telephone visits from November 2020 through July 2022 (Table 2).
We surveyed 47 of the veterans completing SDM visits (45%) and received 37 completed surveys (79%). All respondents were male, mean age 61.9 years, 89% White, 38% married/partnered, 70% rural, 65% currently smoking, with a mean 44.8 pack-years smoking history. On average, veterans answered 6.3 (53%) of knowledge questions correctly (Table 3).
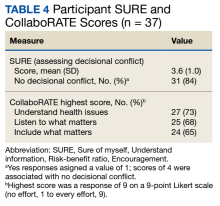
Only 1 respondent (3%) correctly answered the multiple-choice question about indications for stopping screening. Two (5%) correctly answered the question on the magnitude of benefit, most overestimated or did not know. Similarly, 23 (62%) overestimated or did not know the predictive value of an abnormal scan. About two-thirds of veterans underestimated or did not know the attributable risk of lung cancer from tobacco, and about four-fifths did not know the mortality rank of lung cancer. Among the 37 respondents, 31 (84%) indicated not having any decisional conflict as defined by a score of 4 on the SURE scale.
Implementing SDM
The NPC’s field notes indicated that many veterans did not perceive any need to discuss the screening decision and believed that their PCP had referred them just for screening. However, they reported having cursory discussions with their PCP, being told that only their history of heavy tobacco use meant they should be screened. For veterans who had not read the decision aid, the NPC attempted to summarize benefits and harms. However, the discussions were often inadequate because the veterans were not interested in receiving information, particularly numerical data, or indicated that they had limited time for the call.
Seventy-two (69%) of the veterans who met with the NPC were currently smoking. Tobacco cessation counseling was offered to 66; 29 were referred to the VA Quit Line, 10 were referred to the tobacco cessation pharmacist, and the NPC contacted the PCPs for 9 patients who wanted prescriptions for nicotine replacement therapy.
After the SDM visit, 91 veterans (87%) agreed to screening. By the end of the study period, 73 veterans (80%) completed testing. Most veterans had Lung-RADS 1 or 2 results, 11 (1%) had a Lung-RADS 3, and 7 (10%) had a Lung-RADS 4. All 9 veterans with Lung-RADS 3 results and at least 6 months of follow-up underwent repeat imaging within 4 to 13 months (median, 7). All veterans with a Lung-RADS 4 result were referred to pulmonary. One patient was diagnosed with an early-stage non–small cell lung cancer.
We identified several problems with LDCT coding. Radiologists did not consistently use Lung-RADS when interpreting screening LDCTs; some used the Fleischner lung nodule criteria.18 We also found discordant readings for abnormal LDCTs, where the assigned Lung-RADS score was not consistent with the nodule description in the radiology report.
Discussion
Efforts to implement LCS with a telemedicine SDM intervention were mixed. An NPC-led SDM phone call was successfully incorporated into the clinical workflow. Most veterans identified as being eligible for screening participated in the counseling visit and underwent screening. However, they were often reluctant to engage in SDM, feeling that their clinician had already recommended screening and that there was no need for further discussion. Unfortunately, many veterans had not received or reviewed the decision aid and were not interested in receiving information about benefits and harms. Because we relied on telephone calls, we could not share visual information in real time.
Overall, the surveys indicated that most veterans were very satisfied with the quality of the discussion and reported feeling no decisional conflict. However, based on the NPC’s field notes and audio recordings, we believe that the responses may have reflected earlier discussions with the PCP that reportedly emphasized only the veteran’s eligibility for screening. The fidelity assessments indicated that the NPC consistently addressed the harms and benefits of screening.
Nonetheless, the performance on knowledge measures was uneven. Veterans were generally aware of harms, including false alarms, overdiagnosis, radiation exposure, and incidental findings. They did not, however, appreciate when screening should stop. They also underestimated the risks of developing lung cancer and the portion of that risk attributable to tobacco use, and overestimated the benefits of screening. These results suggest that the veterans, at least those who completed the surveys, may not be making well-informed decisions.
Our findings echo those of other VA investigators in finding knowledge deficits among screened veterans, including being unaware that LDCT was for LCS, believing that screening could prevent cancer, receiving little information about screening harms, and feeling that negative tests meant they were among the “lucky ones” who would avoid harm from continued smoking.19,20
The VA is currently implementing centralized screening models with the Lung Precision Oncology Program and the VA partnership to increase access to lung screening (VA-PALS).5 The centralized model, which readily supports the tracking, monitoring, and reporting needs of a screening program, also has advantages in delivering SDM because counselors have been trained in SDM, are more familiar with LCS evidence and processes, can better incorporate decision tools, and do not face the same time constraints as clinicians.21 However, studies have shown that most patients have already decided to be screened when they show up for the SDM visit.22 In contrast, about one-third of patients in primary care settings who receive decision support chose not to be screened.23,24 We found that 13% of our patients decided against screening after a telephone discussion, suggesting that a virtually conducted SDM visit can meaningfully support decision making. Telemedicine also may reduce health inequities in centralized models arising from patients having limited access to screening centers.
Our results suggest that PCPs referring patients to a centralized program, even for virtual visits, should frame the decision to initiate LCS as SDM, where an informed patient is being supported in making a decision consistent with their values and preferences. Furthermore, engaging patients in SDM should not be construed as endorsing screening. When centralized support is less available, individual clinics may need to provide SDM, perhaps using a nonclinician decision coach if clinicians lack the time to lead the discussions. Decision coaches have been effectively used to increase patients’ knowledge about the benefits and harms of screening.12 Regardless of the program model, PCPs will also be responsible for determining whether patients are healthy enough to undergo invasive diagnostic testing and treatment and ensuring that tobacco use is addressed.
SDM delivered in any setting will be enhanced by ensuring that patients are provided with decision aids before a counseling visit. This will help them better understand the benefits and harms of screening and the need to elicit values. The discussion can then focus on areas of concern or questions raised by reviewing the decision aid. The clinician and patient could also use a decision aid during either a face-to-face or video clinical encounter to facilitate SDM. A Cochrane review has shown that using decision aids for people facing screening decisions increases knowledge, reduces decisional conflict, and effectively elicits values and preferences.25 Providing high-quality decision support is a patient-centered approach that respects a patient’s autonomy and may promote health equity and improve adherence.
We recognized the importance of having a multidisciplinary team, involving primary care, radiology, pulmonary, and nursing, with a shared understanding of the screening processes. These are essential features for a high-quality screening program where eligible veterans are readily identified and receive prompt and appropriate follow-up. Radiologists need to use Lung-RADS categories consistently and appropriately when reading LDCTs. This may require ongoing educational efforts, particularly given the new CMS guidelines accepting nonsubspecialist chest readers.7 Additionally, fellows and board-eligible residents may interpret images in academic settings and at VA facilities. The program needs to work closely with the pulmonary service to ensure that Lung-RADS 4 patients are promptly assessed. Radiologists and pulmonologists should calibrate the application of Lung-RADS categories to pulmonary nodules through jointly participating in meetings to review selected cases.
Challenges and Limitations
We faced some notable implementation challenges. The COVID-19 pandemic was extremely disruptive to LCS as it was to all health care. In addition, screening workflow processes were hampered by a lack of clinical reminders, which ideally would trigger for clinicians based on the tobacco history. The absence of this reminder meant that numerous patients were found to be ineligible for screening. We have a long-standing lung nodule clinic, and clinicians were confused about whether to order a surveillance imaging for an incidental nodule or a screening LDCT.
The radiology service was able to update order sets in CPRS to help guide clinicians in distinguishing indications and prerequisites for enrolling in LCS. This helped reduce the number of inappropriate orders and crossover orders between the VISN nodule tracking program and the LCS program.
Our results were preliminary and based on a small sample. We did not survey all veterans who underwent SDM, though the response rate was 79% and patient characteristics were similar to the larger cohort. Our results were potentially subject to selection bias, which could inflate the positive responses about decision quality and decisional conflict. However, the knowledge deficits are likely to be valid and suggest a need to better inform eligible veterans about the benefits and harms of screening. We did not have sufficient follow-up time to determine whether veterans were adherent to annual screenings. We showed that almost all those with abnormal imaging results completed diagnostic evaluations and/or were evaluated by pulmonary. As the program matures, we will be able to track outcomes related to cancer diagnoses and treatment.
Conclusions
A centralized LCS program was able to deliver SDM and enroll veterans in a screening program. While veterans were confident in their decision to screen and felt that they participated in decision making, knowledge testing indicated important deficits. Furthermore, we observed that many veterans did not meaningfully engage in SDM. Clinicians will need to frame the decision as patient centered at the time of referral, highlight the role of the NPC and importance of SDM, and be able to provide adequate decision support. The SDM visits can be enhanced by ensuring that veterans are able to review decision aids. Telemedicine is an acceptable and effective approach for supporting screening discussions, particularly for rural veterans.26
Acknowledgments
The authors thank the following individuals for their contributions to the study: John Paul Hornbeck, program support specialist; Kelly Miell, PhD; Bradley Mecham, PhD; Christopher C. Richards, MA; Bailey Noble, NP; Rebecca Barnhart, program analyst.
1. Zullig LL, Jackson GL, Dorn RA, et al. Cancer incidence among patients of the U.S. Veterans Affairs Health Care System. Mil Med. 2012;177(6):693-701. doi:10.7205/milmed-d-11-00434
2. Hoffman RM, Atallah RP, Struble RD, Badgett RG. Lung cancer screening with low-dose CT: a meta-analysis. J Gen Intern Med. 2020;35(10):3015-3025. doi:10.1007/s11606-020-05951-7
3. National Lung Screening Trial Research Team, Aberle DR, Adams AM, et al. Reduced lung-cancer mortality with low-dose computed tomographic screening. N Engl J Med. 2011;365(5):395-409. doi:10.1056/NEJMoa1102873
4. Moyer VA, US Preventive Services Task Force. Screening for lung cancer: U.S. Preventive Services Task Force recommendation statement. Ann Intern Med. 2014;160(5):330-338. doi:10.7326/M13-2771
5. Maurice NM, Tanner NT. Lung cancer screening at the VA: past, present and future. Semin Oncol. 2022;S0093-7754(22)00041-0. doi:10.1053/j.seminoncol.2022.06.001
6. Centers for Medicare & Medicaid Services. Screening for lung cancer with low dose computed tomography (LDCT) (CAG-00439N). Published 2015. Accessed July 10, 2023. http://www.cms.gov/medicare-coverage-database/details/nca-decision-memo.aspx?NCAId=274
7. Centers for Medicare & Medicaid Services. Screening for lung cancer with low dose computed tomography (LDCT) (CAG-00439R). Published 2022. Accessed July 10, 2023. https://www.cms.gov/medicare-coverage-database/view/ncacal-decision-memo.aspx?proposed=N&ncaid=304
8. National Academies of Sciences, Engineering, and Medicine; Health and Medicine Division; Board on Health Care Services; National Cancer Policy Forum. Implementation of Lung Cancer Screening: Proceedings of a Workshop. The National Academies Press; November 17, 2016. doi:10.172216/23680
9. Bernstein E, Bade BC, Akgün KM, Rose MG, Cain HC. Barriers and facilitators to lung cancer screening and follow-up. Semin Oncol. 2022;S0093-7754(22)00058-6. doi:10.1053/j.seminoncol.2022.07.004
10. US Preventive Services Task Force, Krist AH, Davidson KW, et al. Screening for lung cancer: US Preventive Services Task Force recommendation statement. JAMA. 2021;325(10):962-970. doi:10.1001/jama.2021.1117
11. Kinsinger LS, Atkins D, Provenzale D, Anderson C, Petzel R. Implementation of a new screening recommendation in health care: the Veterans Health Administration’s approach to lung cancer screening. Ann Intern Med. 2014;161(8):597-598. doi:10.7326/M14-1070
12. Lowenstein LM, Godoy MCB, Erasmus JJ, et al. Implementing decision coaching for lung cancer screening in the low-dose computed tomography setting. JCO Oncol Pract. 2020;16(8):e703-e725. doi:10.1200/JOP.19.00453
13. American College of Radiology Committee on Lung-RADS. Lung-RADS assessment categories 2022. Published November 2022. Accessed July 3, 2023. https://www.acr.org/-/media/ACR/Files/RADS/Lung-RADS/Lung-RADS-2022.pdf
14. Lowenstein LM, Richards VF, Leal VB, et al. A brief measure of smokers’ knowledge of lung cancer screening with low-dose computed tomography. Prev Med Rep. 2016;4:351-356. doi:10.1016/j.pmedr.2016.07.008
15. Elwyn G, Barr PJ, Grande SW, Thompson R, Walsh T, Ozanne EM. Developing CollaboRATE: a fast and frugal patient-reported measure of shared decision making in clinical encounters. Patient Educ Couns. 2013;93(1):102-107. doi:10.1016/j.pec.2013.05.009
16. Barr PJ, Thompson R, Walsh T, Grande SW, Ozanne EM, Elwyn G. The psychometric properties of CollaboRATE: a fast and frugal patient-reported measure of the shared decision-making process. J Med Internet Res. 2014;16(1):e2. doi:10.2196/jmir.3085
17. Légaré F, Kearing S, Clay K, et al. Are you SURE?: Assessing patient decisional conflict with a 4-item screening test. Can Fam Physician. 2010;56(8):e308-e314.
18. MacMahon H, Naidich DP, Goo JM, et al. Guidelines for management of incidental pulmonary nodules detected on CT images: from the Fleischner Society 2017. Radiology. 2017;284(1):228-243. doi:10.1148/radiol.2017161659
19. Wiener RS, Koppelman E, Bolton R, et al. Patient and clinician perspectives on shared decision-making in early adopting lung cancer screening programs: a qualitative study. J Gen Intern Med. 2018;33(7):1035-1042. doi:10.1007/s11606-018-4350-9
20. Zeliadt SB, Heffner JL, Sayre G, et al. Attitudes and perceptions about smoking cessation in the context of lung cancer screening. JAMA Intern Med. 2015;175(9):1530-1537. doi:10.1001/jamainternmed.2015.3558
21. Mazzone PJ, White CS, Kazerooni EA, Smith RA, Thomson CC. Proposed quality metrics for lung cancer screening programs: a National Lung Cancer Roundtable Project. Chest. 2021;160(1):368-378. doi:10.1016/j.chest.2021.01.063
22. Mazzone PJ, Tenenbaum A, Seeley M, et al. Impact of a lung cancer screening counseling and shared decision-making visit. Chest. 2017;151(3):572-578. doi:10.1016/j.chest.2016.10.027
23. Reuland DS, Cubillos L, Brenner AT, Harris RP, Minish B, Pignone MP. A pre-post study testing a lung cancer screening decision aid in primary care. BMC Med Inform Decis Mak. 2018;18(1):5. doi:10.1186/s12911-018-0582-1
24. Dharod A, Bellinger C, Foley K, Case LD, Miller D. The reach and feasibility of an interactive lung cancer screening decision aid delivered by patient portal. Appl Clin Inform. 2019;10(1):19-27. doi:10.1055/s-0038-1676807
25. Stacey D, Légaré F, Lewis K, et al. Decision aids for people facing health treatment or screening decisions. Cochrane Database Syst Rev. 2017;4:CD001431. doi:10.1002/14651858.CD001431.pub5
26. Tanner NT, Banas E, Yeager D, Dai L, Hughes Halbert C, Silvestri GA. In-person and telephonic shared decision-making visits for people considering lung cancer screening: an assessment of decision quality. Chest. 2019;155(1):236-238. doi:10.1016/j.chest.2018.07.046
Lung cancer is the second most frequently diagnosed cancer among US veterans and the leading cause of cancer death.1 Clinical trials have shown that annual screening of high-risk persons with low-dose computed tomography (LDCT) can reduce the risk of dying of lung cancer.2 In 2011, the National Lung Screening Trial (NLST) reported that over a 3-year period, annual LDCT screening reduced the risk of dying of lung cancer by 20% compared with chest radiograph screening.3 Lung cancer screening (LCS), however, was associated with harms, including false-positive results, complications from invasive diagnostic procedures, incidental findings, overdiagnosis, and radiation exposure.
The US Preventive Services Task Force (USPSTF) began recommending annual screening of high-risk persons after publication of the NLST results.4 The Veterans Health Administration (VHA) recommended implementing LCS in 2017.5 Guidelines, however, have consistently highlighted the complexity of the decision and the importance of engaging patients in thorough discussions about the potential benefits and harms of screening (shared decision making [SDM]). The Centers for Medicare and Medicaid Services (CMS) has issued coverage determinations mandating that eligible patients undergo a counseling visit that uses a decision aid to support SDM for LCS and addresses tobacco use.6,7 However, primary care practitioners (PCPs) face many challenges in delivering SDM, including a lack of awareness of clinical trial results and screening guidelines, competing clinical demands, being untrained in SDM, and not having educational resources.8 Patients in rural locations face travel burdens in attending counseling visits.9
We conducted a pilot study to address concerns with delivering SDM for LCS to veterans. We implemented a centralized screening model in which veterans were referred by clinicians to a trained decision coach who conducted telephone visits to discuss the initial LCS decision, addressed tobacco cessation, and placed LDCT orders. We evaluated the outcomes of this telemedicine visit by using decision quality metrics and tracking LCS uptake, referrals for tobacco cessation, and clinical outcomes. The University of Iowa Institutional Review Board considered this study to be a quality improvement project and waived informed consent and HIPAA (Health Insurance Portability and Accountability Act) authorization requirements.
Implementation
We implemented the LCS program at the Iowa City Veterans Affairs Health Care System (ICVAHCS), which has both resident and staff clinicians, and 2 community-based outpatient clinics (Coralville, Cedar Rapids) with staff clinicians. The pilot study, conducted from November 2020 through July 2022, was led by a multidisciplinary team that included a nurse, primary care physician, pulmonologist, and radiologist. The team conducted online presentations to educate PCPs about the epidemiology of lung cancer, results of screening trials, LCS guidelines, the rationale for a centralized model of SDM, and the ICVAHCS screening protocols.
Screening Referrals
When the study began in 2020, we used the 2015 USPSTF criteria for annual LCS: individuals aged 55 to 80 years with a 30 pack-year smoking history and current tobacco user or who had quit within 15 years.4 We lowered the starting age to 50 years and the pack-year requirement to 20 after the USPSTF issued updated guidelines in 2021.10 Clinicians were notified about potentially eligible patients through the US Department of Veterans Affairs (VA) Computerized Personal Record System (CPRS) reminders or by the nurse program coordinator (NPC) who reviewed health records of patients with upcoming appointments. If the clinician determined that screening was appropriate, they ordered an LCS consult. The NPC called the veteran to confirm eligibility, mailed a decision aid, and scheduled a telephone visit to conduct SDM. We used the VA decision aid developed for the LCS demonstration project conducted at 8 academic VA medical centers between 2013 and 2017.11
Shared Decision-Making Telephone Visit
The NPC adapted a telephone script developed for a Cancer Prevention and Research Institute of Texas–funded project conducted by 2 coauthors (RJV and LML).12 The NPC asked about receipt/review of the decision aid, described the screening process, and addressed benefits and potential harms of screening. The NPC also offered smoking cessation interventions for veterans who were currently smoking, including referrals to the VA patient aligned care team clinical pharmacist for management of tobacco cessation or to the national VA Quit Line. The encounter ended by assessing the veteran’s understanding of screening issues and eliciting the veteran’s preferences for LDCT and willingness to adhere with the LCS program.
LDCT Imaging
The NPC placed LDCT orders for veterans interested in screening and alerted the referring clinician to sign the order. Veterans who agreed to be screened were placed in an LCS dashboard developed by the Veterans Integrated Services Network (VISN) 23 LCS program that was used as a patient management tool. The dashboard allowed the NPC to track patients, ensuring that veterans were being scheduled for and completing initial and follow-up testing. Radiologists used the Lung-RADS (Lung Imaging Reporting and Data System) to categorize LDCT results (1, normal; 2, benign nodule; 3, probably benign nodule; 4, suspicious nodule).13 Veterans with Lung-RADS 1 or 2 results were scheduled for an annual LDCT (if they remained eligible). Veterans with Lung-RADS 3 results were scheduled for a 6-month follow-up CT. The screening program sent electronic consults to pulmonary for veterans with Lung-RADS 4 to determine whether they should undergo additional imaging or be evaluated in the pulmonary clinic.
Evaluating Shared Decision Making
We audio taped and transcribed randomly selected SDM encounters to assess fidelity with the 2016 CMS required discussion elements for counseling about lung cancer, including the benefit of reducing lung cancer mortality; the potential for harms from false alarms, incidental findings, overdiagnosis, and radiation exposure; the need for annual screening; the importance of smoking cessation; and the possibility of undergoing follow-up testing and diagnostic procedures. An investigator coded the transcripts to assess for the presence of each required element and scored the encounter from 0 to 7.
We also surveyed veterans completing SDM, using a convenience sampling strategy to evaluate knowledge, the quality of the SDM process, and decisional conflict. Initially, we sent mailed surveys to subjects to be completed 1 week after the SDM visit. To increase the response rate, we subsequently called patients to complete the surveys by telephone 1 week after the SDM visit.
We used the validated LCS-12 knowledge measure to assess awareness of lung cancer risks, screening eligibility, and the benefits and harms of screening.14 We evaluated the quality of the SDM visit by using the 3-item CollaboRATE scale (Table 1).15
The NPC also took field notes during interviews to help identify additional SDM issues. After each call, the NPC noted her impressions of the veteran’s engagement with SDM and understanding of the screening issues.
Clinical Outcomes
We used the screening dashboard and CPRS to track clinical outcomes, including screening uptake, referrals for tobacco cessation, appropriate (screening or diagnostic) follow-up testing, and cancer diagnoses. We used descriptive statistics to characterize demographic data and survey responses.
Initial Findings
We conducted 105 SDM telephone visits from November 2020 through July 2022 (Table 2).
We surveyed 47 of the veterans completing SDM visits (45%) and received 37 completed surveys (79%). All respondents were male, mean age 61.9 years, 89% White, 38% married/partnered, 70% rural, 65% currently smoking, with a mean 44.8 pack-years smoking history. On average, veterans answered 6.3 (53%) of knowledge questions correctly (Table 3).
Only 1 respondent (3%) correctly answered the multiple-choice question about indications for stopping screening. Two (5%) correctly answered the question on the magnitude of benefit, most overestimated or did not know. Similarly, 23 (62%) overestimated or did not know the predictive value of an abnormal scan. About two-thirds of veterans underestimated or did not know the attributable risk of lung cancer from tobacco, and about four-fifths did not know the mortality rank of lung cancer. Among the 37 respondents, 31 (84%) indicated not having any decisional conflict as defined by a score of 4 on the SURE scale.
Implementing SDM
The NPC’s field notes indicated that many veterans did not perceive any need to discuss the screening decision and believed that their PCP had referred them just for screening. However, they reported having cursory discussions with their PCP, being told that only their history of heavy tobacco use meant they should be screened. For veterans who had not read the decision aid, the NPC attempted to summarize benefits and harms. However, the discussions were often inadequate because the veterans were not interested in receiving information, particularly numerical data, or indicated that they had limited time for the call.
Seventy-two (69%) of the veterans who met with the NPC were currently smoking. Tobacco cessation counseling was offered to 66; 29 were referred to the VA Quit Line, 10 were referred to the tobacco cessation pharmacist, and the NPC contacted the PCPs for 9 patients who wanted prescriptions for nicotine replacement therapy.
After the SDM visit, 91 veterans (87%) agreed to screening. By the end of the study period, 73 veterans (80%) completed testing. Most veterans had Lung-RADS 1 or 2 results, 11 (1%) had a Lung-RADS 3, and 7 (10%) had a Lung-RADS 4. All 9 veterans with Lung-RADS 3 results and at least 6 months of follow-up underwent repeat imaging within 4 to 13 months (median, 7). All veterans with a Lung-RADS 4 result were referred to pulmonary. One patient was diagnosed with an early-stage non–small cell lung cancer.
We identified several problems with LDCT coding. Radiologists did not consistently use Lung-RADS when interpreting screening LDCTs; some used the Fleischner lung nodule criteria.18 We also found discordant readings for abnormal LDCTs, where the assigned Lung-RADS score was not consistent with the nodule description in the radiology report.
Discussion
Efforts to implement LCS with a telemedicine SDM intervention were mixed. An NPC-led SDM phone call was successfully incorporated into the clinical workflow. Most veterans identified as being eligible for screening participated in the counseling visit and underwent screening. However, they were often reluctant to engage in SDM, feeling that their clinician had already recommended screening and that there was no need for further discussion. Unfortunately, many veterans had not received or reviewed the decision aid and were not interested in receiving information about benefits and harms. Because we relied on telephone calls, we could not share visual information in real time.
Overall, the surveys indicated that most veterans were very satisfied with the quality of the discussion and reported feeling no decisional conflict. However, based on the NPC’s field notes and audio recordings, we believe that the responses may have reflected earlier discussions with the PCP that reportedly emphasized only the veteran’s eligibility for screening. The fidelity assessments indicated that the NPC consistently addressed the harms and benefits of screening.
Nonetheless, the performance on knowledge measures was uneven. Veterans were generally aware of harms, including false alarms, overdiagnosis, radiation exposure, and incidental findings. They did not, however, appreciate when screening should stop. They also underestimated the risks of developing lung cancer and the portion of that risk attributable to tobacco use, and overestimated the benefits of screening. These results suggest that the veterans, at least those who completed the surveys, may not be making well-informed decisions.
Our findings echo those of other VA investigators in finding knowledge deficits among screened veterans, including being unaware that LDCT was for LCS, believing that screening could prevent cancer, receiving little information about screening harms, and feeling that negative tests meant they were among the “lucky ones” who would avoid harm from continued smoking.19,20
The VA is currently implementing centralized screening models with the Lung Precision Oncology Program and the VA partnership to increase access to lung screening (VA-PALS).5 The centralized model, which readily supports the tracking, monitoring, and reporting needs of a screening program, also has advantages in delivering SDM because counselors have been trained in SDM, are more familiar with LCS evidence and processes, can better incorporate decision tools, and do not face the same time constraints as clinicians.21 However, studies have shown that most patients have already decided to be screened when they show up for the SDM visit.22 In contrast, about one-third of patients in primary care settings who receive decision support chose not to be screened.23,24 We found that 13% of our patients decided against screening after a telephone discussion, suggesting that a virtually conducted SDM visit can meaningfully support decision making. Telemedicine also may reduce health inequities in centralized models arising from patients having limited access to screening centers.
Our results suggest that PCPs referring patients to a centralized program, even for virtual visits, should frame the decision to initiate LCS as SDM, where an informed patient is being supported in making a decision consistent with their values and preferences. Furthermore, engaging patients in SDM should not be construed as endorsing screening. When centralized support is less available, individual clinics may need to provide SDM, perhaps using a nonclinician decision coach if clinicians lack the time to lead the discussions. Decision coaches have been effectively used to increase patients’ knowledge about the benefits and harms of screening.12 Regardless of the program model, PCPs will also be responsible for determining whether patients are healthy enough to undergo invasive diagnostic testing and treatment and ensuring that tobacco use is addressed.
SDM delivered in any setting will be enhanced by ensuring that patients are provided with decision aids before a counseling visit. This will help them better understand the benefits and harms of screening and the need to elicit values. The discussion can then focus on areas of concern or questions raised by reviewing the decision aid. The clinician and patient could also use a decision aid during either a face-to-face or video clinical encounter to facilitate SDM. A Cochrane review has shown that using decision aids for people facing screening decisions increases knowledge, reduces decisional conflict, and effectively elicits values and preferences.25 Providing high-quality decision support is a patient-centered approach that respects a patient’s autonomy and may promote health equity and improve adherence.
We recognized the importance of having a multidisciplinary team, involving primary care, radiology, pulmonary, and nursing, with a shared understanding of the screening processes. These are essential features for a high-quality screening program where eligible veterans are readily identified and receive prompt and appropriate follow-up. Radiologists need to use Lung-RADS categories consistently and appropriately when reading LDCTs. This may require ongoing educational efforts, particularly given the new CMS guidelines accepting nonsubspecialist chest readers.7 Additionally, fellows and board-eligible residents may interpret images in academic settings and at VA facilities. The program needs to work closely with the pulmonary service to ensure that Lung-RADS 4 patients are promptly assessed. Radiologists and pulmonologists should calibrate the application of Lung-RADS categories to pulmonary nodules through jointly participating in meetings to review selected cases.
Challenges and Limitations
We faced some notable implementation challenges. The COVID-19 pandemic was extremely disruptive to LCS as it was to all health care. In addition, screening workflow processes were hampered by a lack of clinical reminders, which ideally would trigger for clinicians based on the tobacco history. The absence of this reminder meant that numerous patients were found to be ineligible for screening. We have a long-standing lung nodule clinic, and clinicians were confused about whether to order a surveillance imaging for an incidental nodule or a screening LDCT.
The radiology service was able to update order sets in CPRS to help guide clinicians in distinguishing indications and prerequisites for enrolling in LCS. This helped reduce the number of inappropriate orders and crossover orders between the VISN nodule tracking program and the LCS program.
Our results were preliminary and based on a small sample. We did not survey all veterans who underwent SDM, though the response rate was 79% and patient characteristics were similar to the larger cohort. Our results were potentially subject to selection bias, which could inflate the positive responses about decision quality and decisional conflict. However, the knowledge deficits are likely to be valid and suggest a need to better inform eligible veterans about the benefits and harms of screening. We did not have sufficient follow-up time to determine whether veterans were adherent to annual screenings. We showed that almost all those with abnormal imaging results completed diagnostic evaluations and/or were evaluated by pulmonary. As the program matures, we will be able to track outcomes related to cancer diagnoses and treatment.
Conclusions
A centralized LCS program was able to deliver SDM and enroll veterans in a screening program. While veterans were confident in their decision to screen and felt that they participated in decision making, knowledge testing indicated important deficits. Furthermore, we observed that many veterans did not meaningfully engage in SDM. Clinicians will need to frame the decision as patient centered at the time of referral, highlight the role of the NPC and importance of SDM, and be able to provide adequate decision support. The SDM visits can be enhanced by ensuring that veterans are able to review decision aids. Telemedicine is an acceptable and effective approach for supporting screening discussions, particularly for rural veterans.26
Acknowledgments
The authors thank the following individuals for their contributions to the study: John Paul Hornbeck, program support specialist; Kelly Miell, PhD; Bradley Mecham, PhD; Christopher C. Richards, MA; Bailey Noble, NP; Rebecca Barnhart, program analyst.
Lung cancer is the second most frequently diagnosed cancer among US veterans and the leading cause of cancer death.1 Clinical trials have shown that annual screening of high-risk persons with low-dose computed tomography (LDCT) can reduce the risk of dying of lung cancer.2 In 2011, the National Lung Screening Trial (NLST) reported that over a 3-year period, annual LDCT screening reduced the risk of dying of lung cancer by 20% compared with chest radiograph screening.3 Lung cancer screening (LCS), however, was associated with harms, including false-positive results, complications from invasive diagnostic procedures, incidental findings, overdiagnosis, and radiation exposure.
The US Preventive Services Task Force (USPSTF) began recommending annual screening of high-risk persons after publication of the NLST results.4 The Veterans Health Administration (VHA) recommended implementing LCS in 2017.5 Guidelines, however, have consistently highlighted the complexity of the decision and the importance of engaging patients in thorough discussions about the potential benefits and harms of screening (shared decision making [SDM]). The Centers for Medicare and Medicaid Services (CMS) has issued coverage determinations mandating that eligible patients undergo a counseling visit that uses a decision aid to support SDM for LCS and addresses tobacco use.6,7 However, primary care practitioners (PCPs) face many challenges in delivering SDM, including a lack of awareness of clinical trial results and screening guidelines, competing clinical demands, being untrained in SDM, and not having educational resources.8 Patients in rural locations face travel burdens in attending counseling visits.9
We conducted a pilot study to address concerns with delivering SDM for LCS to veterans. We implemented a centralized screening model in which veterans were referred by clinicians to a trained decision coach who conducted telephone visits to discuss the initial LCS decision, addressed tobacco cessation, and placed LDCT orders. We evaluated the outcomes of this telemedicine visit by using decision quality metrics and tracking LCS uptake, referrals for tobacco cessation, and clinical outcomes. The University of Iowa Institutional Review Board considered this study to be a quality improvement project and waived informed consent and HIPAA (Health Insurance Portability and Accountability Act) authorization requirements.
Implementation
We implemented the LCS program at the Iowa City Veterans Affairs Health Care System (ICVAHCS), which has both resident and staff clinicians, and 2 community-based outpatient clinics (Coralville, Cedar Rapids) with staff clinicians. The pilot study, conducted from November 2020 through July 2022, was led by a multidisciplinary team that included a nurse, primary care physician, pulmonologist, and radiologist. The team conducted online presentations to educate PCPs about the epidemiology of lung cancer, results of screening trials, LCS guidelines, the rationale for a centralized model of SDM, and the ICVAHCS screening protocols.
Screening Referrals
When the study began in 2020, we used the 2015 USPSTF criteria for annual LCS: individuals aged 55 to 80 years with a 30 pack-year smoking history and current tobacco user or who had quit within 15 years.4 We lowered the starting age to 50 years and the pack-year requirement to 20 after the USPSTF issued updated guidelines in 2021.10 Clinicians were notified about potentially eligible patients through the US Department of Veterans Affairs (VA) Computerized Personal Record System (CPRS) reminders or by the nurse program coordinator (NPC) who reviewed health records of patients with upcoming appointments. If the clinician determined that screening was appropriate, they ordered an LCS consult. The NPC called the veteran to confirm eligibility, mailed a decision aid, and scheduled a telephone visit to conduct SDM. We used the VA decision aid developed for the LCS demonstration project conducted at 8 academic VA medical centers between 2013 and 2017.11
Shared Decision-Making Telephone Visit
The NPC adapted a telephone script developed for a Cancer Prevention and Research Institute of Texas–funded project conducted by 2 coauthors (RJV and LML).12 The NPC asked about receipt/review of the decision aid, described the screening process, and addressed benefits and potential harms of screening. The NPC also offered smoking cessation interventions for veterans who were currently smoking, including referrals to the VA patient aligned care team clinical pharmacist for management of tobacco cessation or to the national VA Quit Line. The encounter ended by assessing the veteran’s understanding of screening issues and eliciting the veteran’s preferences for LDCT and willingness to adhere with the LCS program.
LDCT Imaging
The NPC placed LDCT orders for veterans interested in screening and alerted the referring clinician to sign the order. Veterans who agreed to be screened were placed in an LCS dashboard developed by the Veterans Integrated Services Network (VISN) 23 LCS program that was used as a patient management tool. The dashboard allowed the NPC to track patients, ensuring that veterans were being scheduled for and completing initial and follow-up testing. Radiologists used the Lung-RADS (Lung Imaging Reporting and Data System) to categorize LDCT results (1, normal; 2, benign nodule; 3, probably benign nodule; 4, suspicious nodule).13 Veterans with Lung-RADS 1 or 2 results were scheduled for an annual LDCT (if they remained eligible). Veterans with Lung-RADS 3 results were scheduled for a 6-month follow-up CT. The screening program sent electronic consults to pulmonary for veterans with Lung-RADS 4 to determine whether they should undergo additional imaging or be evaluated in the pulmonary clinic.
Evaluating Shared Decision Making
We audio taped and transcribed randomly selected SDM encounters to assess fidelity with the 2016 CMS required discussion elements for counseling about lung cancer, including the benefit of reducing lung cancer mortality; the potential for harms from false alarms, incidental findings, overdiagnosis, and radiation exposure; the need for annual screening; the importance of smoking cessation; and the possibility of undergoing follow-up testing and diagnostic procedures. An investigator coded the transcripts to assess for the presence of each required element and scored the encounter from 0 to 7.
We also surveyed veterans completing SDM, using a convenience sampling strategy to evaluate knowledge, the quality of the SDM process, and decisional conflict. Initially, we sent mailed surveys to subjects to be completed 1 week after the SDM visit. To increase the response rate, we subsequently called patients to complete the surveys by telephone 1 week after the SDM visit.
We used the validated LCS-12 knowledge measure to assess awareness of lung cancer risks, screening eligibility, and the benefits and harms of screening.14 We evaluated the quality of the SDM visit by using the 3-item CollaboRATE scale (Table 1).15
The NPC also took field notes during interviews to help identify additional SDM issues. After each call, the NPC noted her impressions of the veteran’s engagement with SDM and understanding of the screening issues.
Clinical Outcomes
We used the screening dashboard and CPRS to track clinical outcomes, including screening uptake, referrals for tobacco cessation, appropriate (screening or diagnostic) follow-up testing, and cancer diagnoses. We used descriptive statistics to characterize demographic data and survey responses.
Initial Findings
We conducted 105 SDM telephone visits from November 2020 through July 2022 (Table 2).
We surveyed 47 of the veterans completing SDM visits (45%) and received 37 completed surveys (79%). All respondents were male, mean age 61.9 years, 89% White, 38% married/partnered, 70% rural, 65% currently smoking, with a mean 44.8 pack-years smoking history. On average, veterans answered 6.3 (53%) of knowledge questions correctly (Table 3).
Only 1 respondent (3%) correctly answered the multiple-choice question about indications for stopping screening. Two (5%) correctly answered the question on the magnitude of benefit, most overestimated or did not know. Similarly, 23 (62%) overestimated or did not know the predictive value of an abnormal scan. About two-thirds of veterans underestimated or did not know the attributable risk of lung cancer from tobacco, and about four-fifths did not know the mortality rank of lung cancer. Among the 37 respondents, 31 (84%) indicated not having any decisional conflict as defined by a score of 4 on the SURE scale.
Implementing SDM
The NPC’s field notes indicated that many veterans did not perceive any need to discuss the screening decision and believed that their PCP had referred them just for screening. However, they reported having cursory discussions with their PCP, being told that only their history of heavy tobacco use meant they should be screened. For veterans who had not read the decision aid, the NPC attempted to summarize benefits and harms. However, the discussions were often inadequate because the veterans were not interested in receiving information, particularly numerical data, or indicated that they had limited time for the call.
Seventy-two (69%) of the veterans who met with the NPC were currently smoking. Tobacco cessation counseling was offered to 66; 29 were referred to the VA Quit Line, 10 were referred to the tobacco cessation pharmacist, and the NPC contacted the PCPs for 9 patients who wanted prescriptions for nicotine replacement therapy.
After the SDM visit, 91 veterans (87%) agreed to screening. By the end of the study period, 73 veterans (80%) completed testing. Most veterans had Lung-RADS 1 or 2 results, 11 (1%) had a Lung-RADS 3, and 7 (10%) had a Lung-RADS 4. All 9 veterans with Lung-RADS 3 results and at least 6 months of follow-up underwent repeat imaging within 4 to 13 months (median, 7). All veterans with a Lung-RADS 4 result were referred to pulmonary. One patient was diagnosed with an early-stage non–small cell lung cancer.
We identified several problems with LDCT coding. Radiologists did not consistently use Lung-RADS when interpreting screening LDCTs; some used the Fleischner lung nodule criteria.18 We also found discordant readings for abnormal LDCTs, where the assigned Lung-RADS score was not consistent with the nodule description in the radiology report.
Discussion
Efforts to implement LCS with a telemedicine SDM intervention were mixed. An NPC-led SDM phone call was successfully incorporated into the clinical workflow. Most veterans identified as being eligible for screening participated in the counseling visit and underwent screening. However, they were often reluctant to engage in SDM, feeling that their clinician had already recommended screening and that there was no need for further discussion. Unfortunately, many veterans had not received or reviewed the decision aid and were not interested in receiving information about benefits and harms. Because we relied on telephone calls, we could not share visual information in real time.
Overall, the surveys indicated that most veterans were very satisfied with the quality of the discussion and reported feeling no decisional conflict. However, based on the NPC’s field notes and audio recordings, we believe that the responses may have reflected earlier discussions with the PCP that reportedly emphasized only the veteran’s eligibility for screening. The fidelity assessments indicated that the NPC consistently addressed the harms and benefits of screening.
Nonetheless, the performance on knowledge measures was uneven. Veterans were generally aware of harms, including false alarms, overdiagnosis, radiation exposure, and incidental findings. They did not, however, appreciate when screening should stop. They also underestimated the risks of developing lung cancer and the portion of that risk attributable to tobacco use, and overestimated the benefits of screening. These results suggest that the veterans, at least those who completed the surveys, may not be making well-informed decisions.
Our findings echo those of other VA investigators in finding knowledge deficits among screened veterans, including being unaware that LDCT was for LCS, believing that screening could prevent cancer, receiving little information about screening harms, and feeling that negative tests meant they were among the “lucky ones” who would avoid harm from continued smoking.19,20
The VA is currently implementing centralized screening models with the Lung Precision Oncology Program and the VA partnership to increase access to lung screening (VA-PALS).5 The centralized model, which readily supports the tracking, monitoring, and reporting needs of a screening program, also has advantages in delivering SDM because counselors have been trained in SDM, are more familiar with LCS evidence and processes, can better incorporate decision tools, and do not face the same time constraints as clinicians.21 However, studies have shown that most patients have already decided to be screened when they show up for the SDM visit.22 In contrast, about one-third of patients in primary care settings who receive decision support chose not to be screened.23,24 We found that 13% of our patients decided against screening after a telephone discussion, suggesting that a virtually conducted SDM visit can meaningfully support decision making. Telemedicine also may reduce health inequities in centralized models arising from patients having limited access to screening centers.
Our results suggest that PCPs referring patients to a centralized program, even for virtual visits, should frame the decision to initiate LCS as SDM, where an informed patient is being supported in making a decision consistent with their values and preferences. Furthermore, engaging patients in SDM should not be construed as endorsing screening. When centralized support is less available, individual clinics may need to provide SDM, perhaps using a nonclinician decision coach if clinicians lack the time to lead the discussions. Decision coaches have been effectively used to increase patients’ knowledge about the benefits and harms of screening.12 Regardless of the program model, PCPs will also be responsible for determining whether patients are healthy enough to undergo invasive diagnostic testing and treatment and ensuring that tobacco use is addressed.
SDM delivered in any setting will be enhanced by ensuring that patients are provided with decision aids before a counseling visit. This will help them better understand the benefits and harms of screening and the need to elicit values. The discussion can then focus on areas of concern or questions raised by reviewing the decision aid. The clinician and patient could also use a decision aid during either a face-to-face or video clinical encounter to facilitate SDM. A Cochrane review has shown that using decision aids for people facing screening decisions increases knowledge, reduces decisional conflict, and effectively elicits values and preferences.25 Providing high-quality decision support is a patient-centered approach that respects a patient’s autonomy and may promote health equity and improve adherence.
We recognized the importance of having a multidisciplinary team, involving primary care, radiology, pulmonary, and nursing, with a shared understanding of the screening processes. These are essential features for a high-quality screening program where eligible veterans are readily identified and receive prompt and appropriate follow-up. Radiologists need to use Lung-RADS categories consistently and appropriately when reading LDCTs. This may require ongoing educational efforts, particularly given the new CMS guidelines accepting nonsubspecialist chest readers.7 Additionally, fellows and board-eligible residents may interpret images in academic settings and at VA facilities. The program needs to work closely with the pulmonary service to ensure that Lung-RADS 4 patients are promptly assessed. Radiologists and pulmonologists should calibrate the application of Lung-RADS categories to pulmonary nodules through jointly participating in meetings to review selected cases.
Challenges and Limitations
We faced some notable implementation challenges. The COVID-19 pandemic was extremely disruptive to LCS as it was to all health care. In addition, screening workflow processes were hampered by a lack of clinical reminders, which ideally would trigger for clinicians based on the tobacco history. The absence of this reminder meant that numerous patients were found to be ineligible for screening. We have a long-standing lung nodule clinic, and clinicians were confused about whether to order a surveillance imaging for an incidental nodule or a screening LDCT.
The radiology service was able to update order sets in CPRS to help guide clinicians in distinguishing indications and prerequisites for enrolling in LCS. This helped reduce the number of inappropriate orders and crossover orders between the VISN nodule tracking program and the LCS program.
Our results were preliminary and based on a small sample. We did not survey all veterans who underwent SDM, though the response rate was 79% and patient characteristics were similar to the larger cohort. Our results were potentially subject to selection bias, which could inflate the positive responses about decision quality and decisional conflict. However, the knowledge deficits are likely to be valid and suggest a need to better inform eligible veterans about the benefits and harms of screening. We did not have sufficient follow-up time to determine whether veterans were adherent to annual screenings. We showed that almost all those with abnormal imaging results completed diagnostic evaluations and/or were evaluated by pulmonary. As the program matures, we will be able to track outcomes related to cancer diagnoses and treatment.
Conclusions
A centralized LCS program was able to deliver SDM and enroll veterans in a screening program. While veterans were confident in their decision to screen and felt that they participated in decision making, knowledge testing indicated important deficits. Furthermore, we observed that many veterans did not meaningfully engage in SDM. Clinicians will need to frame the decision as patient centered at the time of referral, highlight the role of the NPC and importance of SDM, and be able to provide adequate decision support. The SDM visits can be enhanced by ensuring that veterans are able to review decision aids. Telemedicine is an acceptable and effective approach for supporting screening discussions, particularly for rural veterans.26
Acknowledgments
The authors thank the following individuals for their contributions to the study: John Paul Hornbeck, program support specialist; Kelly Miell, PhD; Bradley Mecham, PhD; Christopher C. Richards, MA; Bailey Noble, NP; Rebecca Barnhart, program analyst.
1. Zullig LL, Jackson GL, Dorn RA, et al. Cancer incidence among patients of the U.S. Veterans Affairs Health Care System. Mil Med. 2012;177(6):693-701. doi:10.7205/milmed-d-11-00434
2. Hoffman RM, Atallah RP, Struble RD, Badgett RG. Lung cancer screening with low-dose CT: a meta-analysis. J Gen Intern Med. 2020;35(10):3015-3025. doi:10.1007/s11606-020-05951-7
3. National Lung Screening Trial Research Team, Aberle DR, Adams AM, et al. Reduced lung-cancer mortality with low-dose computed tomographic screening. N Engl J Med. 2011;365(5):395-409. doi:10.1056/NEJMoa1102873
4. Moyer VA, US Preventive Services Task Force. Screening for lung cancer: U.S. Preventive Services Task Force recommendation statement. Ann Intern Med. 2014;160(5):330-338. doi:10.7326/M13-2771
5. Maurice NM, Tanner NT. Lung cancer screening at the VA: past, present and future. Semin Oncol. 2022;S0093-7754(22)00041-0. doi:10.1053/j.seminoncol.2022.06.001
6. Centers for Medicare & Medicaid Services. Screening for lung cancer with low dose computed tomography (LDCT) (CAG-00439N). Published 2015. Accessed July 10, 2023. http://www.cms.gov/medicare-coverage-database/details/nca-decision-memo.aspx?NCAId=274
7. Centers for Medicare & Medicaid Services. Screening for lung cancer with low dose computed tomography (LDCT) (CAG-00439R). Published 2022. Accessed July 10, 2023. https://www.cms.gov/medicare-coverage-database/view/ncacal-decision-memo.aspx?proposed=N&ncaid=304
8. National Academies of Sciences, Engineering, and Medicine; Health and Medicine Division; Board on Health Care Services; National Cancer Policy Forum. Implementation of Lung Cancer Screening: Proceedings of a Workshop. The National Academies Press; November 17, 2016. doi:10.172216/23680
9. Bernstein E, Bade BC, Akgün KM, Rose MG, Cain HC. Barriers and facilitators to lung cancer screening and follow-up. Semin Oncol. 2022;S0093-7754(22)00058-6. doi:10.1053/j.seminoncol.2022.07.004
10. US Preventive Services Task Force, Krist AH, Davidson KW, et al. Screening for lung cancer: US Preventive Services Task Force recommendation statement. JAMA. 2021;325(10):962-970. doi:10.1001/jama.2021.1117
11. Kinsinger LS, Atkins D, Provenzale D, Anderson C, Petzel R. Implementation of a new screening recommendation in health care: the Veterans Health Administration’s approach to lung cancer screening. Ann Intern Med. 2014;161(8):597-598. doi:10.7326/M14-1070
12. Lowenstein LM, Godoy MCB, Erasmus JJ, et al. Implementing decision coaching for lung cancer screening in the low-dose computed tomography setting. JCO Oncol Pract. 2020;16(8):e703-e725. doi:10.1200/JOP.19.00453
13. American College of Radiology Committee on Lung-RADS. Lung-RADS assessment categories 2022. Published November 2022. Accessed July 3, 2023. https://www.acr.org/-/media/ACR/Files/RADS/Lung-RADS/Lung-RADS-2022.pdf
14. Lowenstein LM, Richards VF, Leal VB, et al. A brief measure of smokers’ knowledge of lung cancer screening with low-dose computed tomography. Prev Med Rep. 2016;4:351-356. doi:10.1016/j.pmedr.2016.07.008
15. Elwyn G, Barr PJ, Grande SW, Thompson R, Walsh T, Ozanne EM. Developing CollaboRATE: a fast and frugal patient-reported measure of shared decision making in clinical encounters. Patient Educ Couns. 2013;93(1):102-107. doi:10.1016/j.pec.2013.05.009
16. Barr PJ, Thompson R, Walsh T, Grande SW, Ozanne EM, Elwyn G. The psychometric properties of CollaboRATE: a fast and frugal patient-reported measure of the shared decision-making process. J Med Internet Res. 2014;16(1):e2. doi:10.2196/jmir.3085
17. Légaré F, Kearing S, Clay K, et al. Are you SURE?: Assessing patient decisional conflict with a 4-item screening test. Can Fam Physician. 2010;56(8):e308-e314.
18. MacMahon H, Naidich DP, Goo JM, et al. Guidelines for management of incidental pulmonary nodules detected on CT images: from the Fleischner Society 2017. Radiology. 2017;284(1):228-243. doi:10.1148/radiol.2017161659
19. Wiener RS, Koppelman E, Bolton R, et al. Patient and clinician perspectives on shared decision-making in early adopting lung cancer screening programs: a qualitative study. J Gen Intern Med. 2018;33(7):1035-1042. doi:10.1007/s11606-018-4350-9
20. Zeliadt SB, Heffner JL, Sayre G, et al. Attitudes and perceptions about smoking cessation in the context of lung cancer screening. JAMA Intern Med. 2015;175(9):1530-1537. doi:10.1001/jamainternmed.2015.3558
21. Mazzone PJ, White CS, Kazerooni EA, Smith RA, Thomson CC. Proposed quality metrics for lung cancer screening programs: a National Lung Cancer Roundtable Project. Chest. 2021;160(1):368-378. doi:10.1016/j.chest.2021.01.063
22. Mazzone PJ, Tenenbaum A, Seeley M, et al. Impact of a lung cancer screening counseling and shared decision-making visit. Chest. 2017;151(3):572-578. doi:10.1016/j.chest.2016.10.027
23. Reuland DS, Cubillos L, Brenner AT, Harris RP, Minish B, Pignone MP. A pre-post study testing a lung cancer screening decision aid in primary care. BMC Med Inform Decis Mak. 2018;18(1):5. doi:10.1186/s12911-018-0582-1
24. Dharod A, Bellinger C, Foley K, Case LD, Miller D. The reach and feasibility of an interactive lung cancer screening decision aid delivered by patient portal. Appl Clin Inform. 2019;10(1):19-27. doi:10.1055/s-0038-1676807
25. Stacey D, Légaré F, Lewis K, et al. Decision aids for people facing health treatment or screening decisions. Cochrane Database Syst Rev. 2017;4:CD001431. doi:10.1002/14651858.CD001431.pub5
26. Tanner NT, Banas E, Yeager D, Dai L, Hughes Halbert C, Silvestri GA. In-person and telephonic shared decision-making visits for people considering lung cancer screening: an assessment of decision quality. Chest. 2019;155(1):236-238. doi:10.1016/j.chest.2018.07.046
1. Zullig LL, Jackson GL, Dorn RA, et al. Cancer incidence among patients of the U.S. Veterans Affairs Health Care System. Mil Med. 2012;177(6):693-701. doi:10.7205/milmed-d-11-00434
2. Hoffman RM, Atallah RP, Struble RD, Badgett RG. Lung cancer screening with low-dose CT: a meta-analysis. J Gen Intern Med. 2020;35(10):3015-3025. doi:10.1007/s11606-020-05951-7
3. National Lung Screening Trial Research Team, Aberle DR, Adams AM, et al. Reduced lung-cancer mortality with low-dose computed tomographic screening. N Engl J Med. 2011;365(5):395-409. doi:10.1056/NEJMoa1102873
4. Moyer VA, US Preventive Services Task Force. Screening for lung cancer: U.S. Preventive Services Task Force recommendation statement. Ann Intern Med. 2014;160(5):330-338. doi:10.7326/M13-2771
5. Maurice NM, Tanner NT. Lung cancer screening at the VA: past, present and future. Semin Oncol. 2022;S0093-7754(22)00041-0. doi:10.1053/j.seminoncol.2022.06.001
6. Centers for Medicare & Medicaid Services. Screening for lung cancer with low dose computed tomography (LDCT) (CAG-00439N). Published 2015. Accessed July 10, 2023. http://www.cms.gov/medicare-coverage-database/details/nca-decision-memo.aspx?NCAId=274
7. Centers for Medicare & Medicaid Services. Screening for lung cancer with low dose computed tomography (LDCT) (CAG-00439R). Published 2022. Accessed July 10, 2023. https://www.cms.gov/medicare-coverage-database/view/ncacal-decision-memo.aspx?proposed=N&ncaid=304
8. National Academies of Sciences, Engineering, and Medicine; Health and Medicine Division; Board on Health Care Services; National Cancer Policy Forum. Implementation of Lung Cancer Screening: Proceedings of a Workshop. The National Academies Press; November 17, 2016. doi:10.172216/23680
9. Bernstein E, Bade BC, Akgün KM, Rose MG, Cain HC. Barriers and facilitators to lung cancer screening and follow-up. Semin Oncol. 2022;S0093-7754(22)00058-6. doi:10.1053/j.seminoncol.2022.07.004
10. US Preventive Services Task Force, Krist AH, Davidson KW, et al. Screening for lung cancer: US Preventive Services Task Force recommendation statement. JAMA. 2021;325(10):962-970. doi:10.1001/jama.2021.1117
11. Kinsinger LS, Atkins D, Provenzale D, Anderson C, Petzel R. Implementation of a new screening recommendation in health care: the Veterans Health Administration’s approach to lung cancer screening. Ann Intern Med. 2014;161(8):597-598. doi:10.7326/M14-1070
12. Lowenstein LM, Godoy MCB, Erasmus JJ, et al. Implementing decision coaching for lung cancer screening in the low-dose computed tomography setting. JCO Oncol Pract. 2020;16(8):e703-e725. doi:10.1200/JOP.19.00453
13. American College of Radiology Committee on Lung-RADS. Lung-RADS assessment categories 2022. Published November 2022. Accessed July 3, 2023. https://www.acr.org/-/media/ACR/Files/RADS/Lung-RADS/Lung-RADS-2022.pdf
14. Lowenstein LM, Richards VF, Leal VB, et al. A brief measure of smokers’ knowledge of lung cancer screening with low-dose computed tomography. Prev Med Rep. 2016;4:351-356. doi:10.1016/j.pmedr.2016.07.008
15. Elwyn G, Barr PJ, Grande SW, Thompson R, Walsh T, Ozanne EM. Developing CollaboRATE: a fast and frugal patient-reported measure of shared decision making in clinical encounters. Patient Educ Couns. 2013;93(1):102-107. doi:10.1016/j.pec.2013.05.009
16. Barr PJ, Thompson R, Walsh T, Grande SW, Ozanne EM, Elwyn G. The psychometric properties of CollaboRATE: a fast and frugal patient-reported measure of the shared decision-making process. J Med Internet Res. 2014;16(1):e2. doi:10.2196/jmir.3085
17. Légaré F, Kearing S, Clay K, et al. Are you SURE?: Assessing patient decisional conflict with a 4-item screening test. Can Fam Physician. 2010;56(8):e308-e314.
18. MacMahon H, Naidich DP, Goo JM, et al. Guidelines for management of incidental pulmonary nodules detected on CT images: from the Fleischner Society 2017. Radiology. 2017;284(1):228-243. doi:10.1148/radiol.2017161659
19. Wiener RS, Koppelman E, Bolton R, et al. Patient and clinician perspectives on shared decision-making in early adopting lung cancer screening programs: a qualitative study. J Gen Intern Med. 2018;33(7):1035-1042. doi:10.1007/s11606-018-4350-9
20. Zeliadt SB, Heffner JL, Sayre G, et al. Attitudes and perceptions about smoking cessation in the context of lung cancer screening. JAMA Intern Med. 2015;175(9):1530-1537. doi:10.1001/jamainternmed.2015.3558
21. Mazzone PJ, White CS, Kazerooni EA, Smith RA, Thomson CC. Proposed quality metrics for lung cancer screening programs: a National Lung Cancer Roundtable Project. Chest. 2021;160(1):368-378. doi:10.1016/j.chest.2021.01.063
22. Mazzone PJ, Tenenbaum A, Seeley M, et al. Impact of a lung cancer screening counseling and shared decision-making visit. Chest. 2017;151(3):572-578. doi:10.1016/j.chest.2016.10.027
23. Reuland DS, Cubillos L, Brenner AT, Harris RP, Minish B, Pignone MP. A pre-post study testing a lung cancer screening decision aid in primary care. BMC Med Inform Decis Mak. 2018;18(1):5. doi:10.1186/s12911-018-0582-1
24. Dharod A, Bellinger C, Foley K, Case LD, Miller D. The reach and feasibility of an interactive lung cancer screening decision aid delivered by patient portal. Appl Clin Inform. 2019;10(1):19-27. doi:10.1055/s-0038-1676807
25. Stacey D, Légaré F, Lewis K, et al. Decision aids for people facing health treatment or screening decisions. Cochrane Database Syst Rev. 2017;4:CD001431. doi:10.1002/14651858.CD001431.pub5
26. Tanner NT, Banas E, Yeager D, Dai L, Hughes Halbert C, Silvestri GA. In-person and telephonic shared decision-making visits for people considering lung cancer screening: an assessment of decision quality. Chest. 2019;155(1):236-238. doi:10.1016/j.chest.2018.07.046
Applying a Text-Search Algorithm to Radiology Reports Can Find More Patients With Pulmonary Nodules Than Radiology Coding Alone (FULL)
Rapid advances in imaging technology have led to better spatial resolution with lower radiation doses to patients. These advances have helped to increase the use of diagnostic chest imaging, particularly in emergency departments and oncology centers, and in screening for coronary artery disease. As a result, there has been an explosion of incidental findings on chest imaging—including indeterminate lung nodules.1,2
Lung nodules are rounded and well-circumscribed lung opacities (≤ 3 cm in diameter) that may present as solitary or multiple lesions in usually asymptomatic patients. Most lung nodules are benign, the result of an infectious or inflammatory process. Nodules that are ≤ 8 mm in diameter, unless they show increase in size over time, often can be safely followed with imaging surveillance. In contrast, lung nodules > 8 mm could represent an early-stage lung cancer, especially among patients with high-risk for developing lung cancer (ie, those with advanced age, heavy tobacco abuse, or emphysema) and should be further assessed with close imaging surveillance, either chest computed tomography (CT) alone or positron-emission tomography (PET)/CT, or tissue biopsy, based on the underlying likelihood of malignancy.
Patients who receive an early-stage lung cancer diagnosis can be offered curative treatments leading to improved 5-year survival rates.3,4 Consequently, health care systems need to be able to identify these nodules accurately, in order to categorize and manage them accordingly to the Fleischner radiographic and American College of Chest Physicians clinical guidelines.5,6 Unfortunately, many hospitals struggle to identify patients with incidental lung nodules found during diagnostic chest and abdominal imaging, due in part to poor adherence to Fleischner guidelines among radiologists for categorizing pulmonary nodules.7,8
The Veterans Health Administration (VHA) system is interested in effectively detecting patients with incidental lung nodules. Veterans have a higher risk of developing lung cancer when compared with the entire US population, mainly due to a higher incidence of tobacco use.6 The prevalence of lung nodules among veterans with significant risk factors for lung cancer is about 60% nationwide, and up to 85% in the Midwest, due to the high prevalence of histoplasmosis.7 However, only a small percentage of these nodules represent an early stage primary lung cancer.
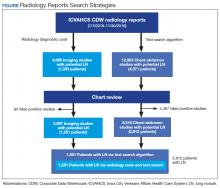
Several Veterans Integrated Service Networks (VISNs) in the VHA use a radiology diagnostic code to systematically identify imaging studies with presence of lung nodules. In VISN 23, which includes Minnesota, North Dakota, South Dakota, Iowa, and portions of neighboring states, the code used to identify these radiology studies is 44. However, there is high variability in the reporting and coding of imaging studies among radiologists, which could lead to misclassifying patients with lung nodules.8
Some studies suggest that using an automated text search algorithm within radiology reports can be a highly effective strategy to identify patients with lung nodules.9,10 In this study, we compared the diagnostic performance of a newly developed text search algorithm applied to radiology reports with the current standard practice of using a radiology diagnostic code for identifying patients with lung nodules at the Iowa City US Department of Veterans Affairs (VA) Health Care System (ICVAHCS) hospital in Iowa.
Methods
Since 2014, The ICVAHCS has used a radiology diagnostic code to identify any imaging studies with lung nodules. The radiologist enters “44” at the end of the reading process using the Nuance Powerscribe 360 radiation reporting system. The code is uploaded into the VHA Corporate Data Warehouse (CDW), and it is located within the radiology exam domain. This strategy was created and implemented by the Minneapolis VA Health Care System in Minnesota for all the VA hospitals in VISN 23. A lung nodule registry nurse was provided with a list of radiology studies flagged with this radiology diagnostic code every 2 weeks. A chart review was then performed for all these studies to determine the presence of a lung nodule. When detected, the ordering health care provider was alerted and given recommendations for managing the nodule.
We initially searched for the radiology studies with a presumptive lung nodule using the radiology code 44 within the CDW. Separately, we applied the text search strategy only to radiology reports from chest and abdomen studies (ie, X-rays, CT, magnetic resonance imaging [MRI], and PET) that contained any of the keyword phrases. The text search strategy was modeled based on a natural language processing (NLP) algorithm developed by the Puget Sound VA Healthcare System in Seattle, Washington to identify lung nodules on radiology reports.9 Our algorithm included a series of text searches using Microsoft SQL. After several simulations using a random group of radiology reports, we chose the keywords: “lung AND nodul”; “pulm AND nodul”; “pulm AND mass”; “lung AND mass”; and “ground glass”. We selected only chest and abdomen studies because on several simulations using a random group of radiology reports, the vast majority of lung nodules were identified on chest and abdomen imaging studies. Also, it would not have been feasible to chart review the approximately 30,000 total radiology reports that were generated during the study period.
From January 1, 2016 through November 30, 2016, we applied both search strategies independently: radiology diagnostic code for lung nodules to all imaging studies, and text search to all radiology reports of chest and abdomen imaging studies in the CDW (Figure). We also collected demographic (eg, age, sex, race, rurality) and clinical (eg, medical comorbidities, tobacco use) information that were uploaded to the database automatically from CDW using International Statistical Classification of Diseases, Tenth Edition and demographic codes. The VHA uses the Rural-Urban Commuting Areas (RUCA) system to define rurality, which takes into account population density and how closely a community is linked socioeconomically to larger urban centers.11 The protocol was reviewed and approved by the institutional review board of ICVAHCS and the University of Iowa.
The presence of a lung nodule was established by having the lung nodule registry nurse manually review the charts of every patient with a radiology report identified by either code 44 or the text search algorithm. The goal was to ensure that our text search strategy identified all reports with a code 44 to be compliant with VISN expectations. Cases in which a lung nodule was described in the radiology report were considered true positives, and those without a lung nodule description were considered false positives.
We compared the sociodemographic and clinical characteristics of patients with lung nodules between those identified with both code 44 and the text search and those identified with the text search alone. We used χ2 tests for categorical variables (eg, age, gender, RUCA, chronic obstructive pulmonary disease (COPD), smoking status) and t tests for continuous variables (eg, Charlson comorbidity score). A P value ≤ .05 was considered statistically significant. To assess the yield of each search strategy, we determined the number of patients with lung nodules detected by the text search and the radiology diagnostic code. We also calculated the positive predictive value (PPV) and 95% CI of each search strategy.
Results
We identified 12,983 radiology studies that required manual review during the study period. We confirmed that 8,516 imaging studies had lung nodules, representing 2,912 patients. Subjects with lung nodules were predominantly male (96%), aged between 60 and 79 years (71%), and lived in a rural area (72%). More than 50% of these patients had COPD and over a third were current smokers (Table 1). The text search algorithm identified all of the patients identified by the radiology diagnostic code (n = 1,251). It also identified an additional 1,661 patients with lung nodules that otherwise would have been missed by the radiology code. Compared with those identified only by the text search, those identified by both the radiology coding and text search were older, had lower Charlson comorbidity scores, and were more likely to be a current smoker.
The text search algorithm identified more than twice as many patients with potential lung nodules compared with the radiology diagnostic code (4,071 vs 1,363) (Table 2). However, the text search algorithm was associated with a much higher number of false positives than was the diagnostic code (1,159 vs 112) and a lower PPV (72% [95% CI, 70.6-73.4] vs 92% [95% CI, 90.6-93.4], respectively). The text search algorithm identified 130 patients with lung nodules of moderate to high risk for malignancy (> 8 mm diameter) that were not identified by the radiology code. When the PPV of each search strategy was calculated based on imaging studies with nodules (most patients had > 1 imaging study), the results remained similar (98% for radiology code and 66% for text search). A larger proportion of the lung nodules detected by code 44 vs the text search algorithm were from CT chest studies.
Discussion
In a population of predominantly older male veterans with significant risk factors for lung cancer and high incidence of incidental lung nodules, applying a text search algorithm on radiology reports identified a substantial number of patients with lung nodules, including some with nodules > 8 mm, that were missed by the radiologist-generated code.9,10 Improving the yield of detection for lung nodules in a population with high risk for lung cancer would increase the likelihood of detecting patients with potentially curable early-stage lung cancers, decreasing lung cancer mortality.
The reasons for the high number of patients with lung nodules missed by the radiology code are unclear. Potential explanations may include the lack of standardization of imaging reports by the radiologists (ie, only 21% of chest CTs used a standardized template describing a lung nodule in our study), a problem well recognized both within and outside VHA.8,12
The text search algorithm identified more patients with lung nodules but had a higher rate of false positives when compared with the diagnostic code. The high rate of false positives resulted in more charts to review and an increased workload for the lung nodule registry team. The challenges presented by an increased workload should be balanced against the potential harms of missing nodules that develop into advanced cancer.
Text Search Adjustments
Refining the text search criteria algorithm and the chart review process may decrease the rate of false positives significantly without affecting detection of lung nodules. In subsequent simulations, we found that by adding an exclusion criteria to text search algorithm to remove reports with specific keywords we could substantially reduce the number of false positive reports without affecting the detection rate of the lung nodules. These exclusion criteria would exclude any reports that: (1) contain “nodul” within the next 8 words after mentioning “no”; (2) contain “clear” within the next 8 words after mentioning “lung” in the text (eg, “lungs appear to be clear”); (3) contain “clear” within the next 4 words after mentioning “otherwise” in the text (eg, “otherwise appear to be clear”). Based on our study results, we further refined the text search strategy by limiting the search to only chest imaging studies. When we applied the revised algorithm to a random sample of imaging reports, we found all the code 44 radiology reports were still captured, but we were able to reduce the number of radiology reports needing review by about 80%.
Although classification approaches are being refined to improve radiology performance in multiple categories of nodules, this study suggests that alternative approaches based on text algorithms can improve the capture of pulmonary nodules that require surveillance. These algorithms also can be used to augment radiologist reporting systems. This represents an investment in resources to build a team that should include a bioinformatics specialist, lung nodule registry personnel (review charts of the detected imaging studies with lung nodules, populating the lung nodule database, and determining and tracking the need of imaging follow up), a lung nodule clinic nurse coordinator, and a dedicated lung nodule clinic pulmonologist.
Radiology departments could employ this text search approach to identify missed nodules and use an audit and feedback system to train radiologists to code lung nodules consistently at the time of the initial reading to avoid delays in identifying patients with nodules. Alternatively, the more widespread use of a standardized CT chest radiology reports using Fleischner or the American College of Radiology Lung Imaging Reporting and Data System (Lung RADS) templates might improve the detection of patients with lung nodules.5,13,14
The VHA system should have an effective strategy for identifying incidental lung nodules during routine radiology examinations. Relying only on radiologists to identify and code pulmonary nodules can lead to missing a significant number of patients with lung nodules and some patients with early stage lung cancer who could receive curative therapy.12,14-16 The use of a standardized algorithm, like a text search strategy, might decrease the risk of variation in the execution and result in a more sensitive detection of patients with lung nodules. The text search strategy might be easily implemented and shared with other hospitals both within and outside the VHA.
Limitations
This study was performed in a single VHA hospital and the findings may not be generalizable to other settings of care. Second, our study design is susceptible to work-up bias because the results of a diagnostic test (eg, chest or abdomen imaging) affected whether the chart review was used to verify the test result. It was not feasible to review the patient records of all radiology studies done at the facility during the study period, consequently complete 2 × 2 tables could not be created to calculate sensitivity, specificity, and negative predictive value.
Conclusion
A text search algorithm of radiology reports increased the detection of patients with lung nodules when compared with radiology diagnostic coding alone. However, the improved detection was associated with a higher rate of false positives, which requires manually reviewing a larger number of patient’s chart reports. Future research and quality improvement should focus on standardizing the radiology reporting process and improving the efficiency and reliability of follow up and tracking of incidental lung nodules.
Acknowledgments
The work reported here was supported by a grant from the Office of Rural Health (N32-FY16Q1-S1-P01577), US Department of Veterans Affairs, Veterans Health Administration. We also had the support from the Veterans Rural Health Resource Center-Iowa City, and the Health Services Research and Development (HSR&D) Service through the Comprehensive Access and Delivery Research and Evaluation (CADRE) Center (REA 09-220).
1. Jacobs PC, Mali WP, Grobbee DE, van der Graaf Y. Prevalence of incidental findings in computed tomographic screening of the chest: a systematic review. Journal of computer assisted tomography. 2008;32(2):214-221.
2. Frank L, Quint LE. Chest CT incidentalomas: thyroid lesions, enlarged mediastinal lymph nodes, and lung nodules. Cancer Imaging. 2012;12(1):41-48.
3. National Institutes of Health, National Cancer Institute, Surveillance, Epidemiology, and End Results Program. Cancer stat facts: lung and bronchus cancer. https://seer.cancer.gov/statfacts/html/lungb.html. Accessed April 8, 2020.
4. Alberg AJ, Brock MV, Ford JG, Samet JM, Spivack SD. Epidemiology of lung cancer: Diagnosis and management of lung cancer, 3rd ed: American College of Chest Physicians evidence-based clinical practice guidelines. Chest. 2013;143(5 Suppl):e1S-e29S.
5. MacMahon H, Naidich DP, Goo JM, et al. Guidelines for Management of Incidental Pulmonary Nodules Detected on CT Images: From the Fleischner Society 2017. Radiology. 2017;284(1):228-243.
6. Zullig LL, Jackson GL, Dorn RA, et al. Cancer incidence among patients of the U.S. Veterans Affairs Health Care System. Mil Med. 2012;177(6):693-701.
7. Kinsinger LS, Anderson C, Kim J, et al. Implementation of lung cancer screening in the Veterans Health Administration. JAMA Intern Med. 2017;177(3):399-406.
8. Iqbal MN, Stott E, Huml AM, et al. What’s in a name? Factors associated with documentation and evaluation of incidental pulmonary nodules. Ann Am Thorac Soc. 2016;13(10):1704-1711.
9. Farjah F, Halgrim S, Buist DS, et al. An automated method for identifying individuals with a lung nodule can be feasibly implemented across health systems. Egems (Wash DC). 2016;4(1):1254.
10. Danforth KN, Early MI, Ngan S, Kosco AE, Zheng C, Gould MK. Automated identification of patients with pulmonary nodules in an integrated health system using administrative health plan data, radiology reports, and natural language processing. J Thorac Oncol. 2012;7(8):1257-1262.
11. US Department of Veterans Affairs, Office of Rural Health. https://www.ruralhealth.va.gov/aboutus/ruralvets.asp. Updated January 28, 2020. Accessed April 8, 2020.
12. Blagev DP, Lloyd JF, Conner K, et al. Follow-up of incidental pulmonary nodules and the radiology report. J Am Coll Radiol. 2016;13(2 suppl):R18-R24.
13. Eisenberg RL, Fleischner S. Ways to improve radiologists’ adherence to Fleischner Society guidelines for management of pulmonary nodules. J Am Coll Radiol. 2013;10(6):439-441.
14. Aberle DR. Implementing lung cancer screening: the US experience. Clin Radiol. 2017;72(5):401-406.
15. Gould MK, Donington J, Lynch WR, et al. Evaluation of individuals with pulmonary nodules: when is it lung cancer? Diagnosis and management of lung cancer, 3rd ed: American College of Chest Physicians evidence-based clinical practice guidelines. Chest. 2013;143(5 Suppl):e93S-e120S.
16. Callister ME, Baldwin DR. How should pulmonary nodules be optimally investigated and managed? Lung Cancer. 2016;91:48-55.
Rapid advances in imaging technology have led to better spatial resolution with lower radiation doses to patients. These advances have helped to increase the use of diagnostic chest imaging, particularly in emergency departments and oncology centers, and in screening for coronary artery disease. As a result, there has been an explosion of incidental findings on chest imaging—including indeterminate lung nodules.1,2
Lung nodules are rounded and well-circumscribed lung opacities (≤ 3 cm in diameter) that may present as solitary or multiple lesions in usually asymptomatic patients. Most lung nodules are benign, the result of an infectious or inflammatory process. Nodules that are ≤ 8 mm in diameter, unless they show increase in size over time, often can be safely followed with imaging surveillance. In contrast, lung nodules > 8 mm could represent an early-stage lung cancer, especially among patients with high-risk for developing lung cancer (ie, those with advanced age, heavy tobacco abuse, or emphysema) and should be further assessed with close imaging surveillance, either chest computed tomography (CT) alone or positron-emission tomography (PET)/CT, or tissue biopsy, based on the underlying likelihood of malignancy.
Patients who receive an early-stage lung cancer diagnosis can be offered curative treatments leading to improved 5-year survival rates.3,4 Consequently, health care systems need to be able to identify these nodules accurately, in order to categorize and manage them accordingly to the Fleischner radiographic and American College of Chest Physicians clinical guidelines.5,6 Unfortunately, many hospitals struggle to identify patients with incidental lung nodules found during diagnostic chest and abdominal imaging, due in part to poor adherence to Fleischner guidelines among radiologists for categorizing pulmonary nodules.7,8
The Veterans Health Administration (VHA) system is interested in effectively detecting patients with incidental lung nodules. Veterans have a higher risk of developing lung cancer when compared with the entire US population, mainly due to a higher incidence of tobacco use.6 The prevalence of lung nodules among veterans with significant risk factors for lung cancer is about 60% nationwide, and up to 85% in the Midwest, due to the high prevalence of histoplasmosis.7 However, only a small percentage of these nodules represent an early stage primary lung cancer.
Several Veterans Integrated Service Networks (VISNs) in the VHA use a radiology diagnostic code to systematically identify imaging studies with presence of lung nodules. In VISN 23, which includes Minnesota, North Dakota, South Dakota, Iowa, and portions of neighboring states, the code used to identify these radiology studies is 44. However, there is high variability in the reporting and coding of imaging studies among radiologists, which could lead to misclassifying patients with lung nodules.8
Some studies suggest that using an automated text search algorithm within radiology reports can be a highly effective strategy to identify patients with lung nodules.9,10 In this study, we compared the diagnostic performance of a newly developed text search algorithm applied to radiology reports with the current standard practice of using a radiology diagnostic code for identifying patients with lung nodules at the Iowa City US Department of Veterans Affairs (VA) Health Care System (ICVAHCS) hospital in Iowa.
Methods
Since 2014, The ICVAHCS has used a radiology diagnostic code to identify any imaging studies with lung nodules. The radiologist enters “44” at the end of the reading process using the Nuance Powerscribe 360 radiation reporting system. The code is uploaded into the VHA Corporate Data Warehouse (CDW), and it is located within the radiology exam domain. This strategy was created and implemented by the Minneapolis VA Health Care System in Minnesota for all the VA hospitals in VISN 23. A lung nodule registry nurse was provided with a list of radiology studies flagged with this radiology diagnostic code every 2 weeks. A chart review was then performed for all these studies to determine the presence of a lung nodule. When detected, the ordering health care provider was alerted and given recommendations for managing the nodule.
We initially searched for the radiology studies with a presumptive lung nodule using the radiology code 44 within the CDW. Separately, we applied the text search strategy only to radiology reports from chest and abdomen studies (ie, X-rays, CT, magnetic resonance imaging [MRI], and PET) that contained any of the keyword phrases. The text search strategy was modeled based on a natural language processing (NLP) algorithm developed by the Puget Sound VA Healthcare System in Seattle, Washington to identify lung nodules on radiology reports.9 Our algorithm included a series of text searches using Microsoft SQL. After several simulations using a random group of radiology reports, we chose the keywords: “lung AND nodul”; “pulm AND nodul”; “pulm AND mass”; “lung AND mass”; and “ground glass”. We selected only chest and abdomen studies because on several simulations using a random group of radiology reports, the vast majority of lung nodules were identified on chest and abdomen imaging studies. Also, it would not have been feasible to chart review the approximately 30,000 total radiology reports that were generated during the study period.
From January 1, 2016 through November 30, 2016, we applied both search strategies independently: radiology diagnostic code for lung nodules to all imaging studies, and text search to all radiology reports of chest and abdomen imaging studies in the CDW (Figure). We also collected demographic (eg, age, sex, race, rurality) and clinical (eg, medical comorbidities, tobacco use) information that were uploaded to the database automatically from CDW using International Statistical Classification of Diseases, Tenth Edition and demographic codes. The VHA uses the Rural-Urban Commuting Areas (RUCA) system to define rurality, which takes into account population density and how closely a community is linked socioeconomically to larger urban centers.11 The protocol was reviewed and approved by the institutional review board of ICVAHCS and the University of Iowa.
The presence of a lung nodule was established by having the lung nodule registry nurse manually review the charts of every patient with a radiology report identified by either code 44 or the text search algorithm. The goal was to ensure that our text search strategy identified all reports with a code 44 to be compliant with VISN expectations. Cases in which a lung nodule was described in the radiology report were considered true positives, and those without a lung nodule description were considered false positives.
We compared the sociodemographic and clinical characteristics of patients with lung nodules between those identified with both code 44 and the text search and those identified with the text search alone. We used χ2 tests for categorical variables (eg, age, gender, RUCA, chronic obstructive pulmonary disease (COPD), smoking status) and t tests for continuous variables (eg, Charlson comorbidity score). A P value ≤ .05 was considered statistically significant. To assess the yield of each search strategy, we determined the number of patients with lung nodules detected by the text search and the radiology diagnostic code. We also calculated the positive predictive value (PPV) and 95% CI of each search strategy.
Results
We identified 12,983 radiology studies that required manual review during the study period. We confirmed that 8,516 imaging studies had lung nodules, representing 2,912 patients. Subjects with lung nodules were predominantly male (96%), aged between 60 and 79 years (71%), and lived in a rural area (72%). More than 50% of these patients had COPD and over a third were current smokers (Table 1). The text search algorithm identified all of the patients identified by the radiology diagnostic code (n = 1,251). It also identified an additional 1,661 patients with lung nodules that otherwise would have been missed by the radiology code. Compared with those identified only by the text search, those identified by both the radiology coding and text search were older, had lower Charlson comorbidity scores, and were more likely to be a current smoker.
The text search algorithm identified more than twice as many patients with potential lung nodules compared with the radiology diagnostic code (4,071 vs 1,363) (Table 2). However, the text search algorithm was associated with a much higher number of false positives than was the diagnostic code (1,159 vs 112) and a lower PPV (72% [95% CI, 70.6-73.4] vs 92% [95% CI, 90.6-93.4], respectively). The text search algorithm identified 130 patients with lung nodules of moderate to high risk for malignancy (> 8 mm diameter) that were not identified by the radiology code. When the PPV of each search strategy was calculated based on imaging studies with nodules (most patients had > 1 imaging study), the results remained similar (98% for radiology code and 66% for text search). A larger proportion of the lung nodules detected by code 44 vs the text search algorithm were from CT chest studies.
Discussion
In a population of predominantly older male veterans with significant risk factors for lung cancer and high incidence of incidental lung nodules, applying a text search algorithm on radiology reports identified a substantial number of patients with lung nodules, including some with nodules > 8 mm, that were missed by the radiologist-generated code.9,10 Improving the yield of detection for lung nodules in a population with high risk for lung cancer would increase the likelihood of detecting patients with potentially curable early-stage lung cancers, decreasing lung cancer mortality.
The reasons for the high number of patients with lung nodules missed by the radiology code are unclear. Potential explanations may include the lack of standardization of imaging reports by the radiologists (ie, only 21% of chest CTs used a standardized template describing a lung nodule in our study), a problem well recognized both within and outside VHA.8,12
The text search algorithm identified more patients with lung nodules but had a higher rate of false positives when compared with the diagnostic code. The high rate of false positives resulted in more charts to review and an increased workload for the lung nodule registry team. The challenges presented by an increased workload should be balanced against the potential harms of missing nodules that develop into advanced cancer.
Text Search Adjustments
Refining the text search criteria algorithm and the chart review process may decrease the rate of false positives significantly without affecting detection of lung nodules. In subsequent simulations, we found that by adding an exclusion criteria to text search algorithm to remove reports with specific keywords we could substantially reduce the number of false positive reports without affecting the detection rate of the lung nodules. These exclusion criteria would exclude any reports that: (1) contain “nodul” within the next 8 words after mentioning “no”; (2) contain “clear” within the next 8 words after mentioning “lung” in the text (eg, “lungs appear to be clear”); (3) contain “clear” within the next 4 words after mentioning “otherwise” in the text (eg, “otherwise appear to be clear”). Based on our study results, we further refined the text search strategy by limiting the search to only chest imaging studies. When we applied the revised algorithm to a random sample of imaging reports, we found all the code 44 radiology reports were still captured, but we were able to reduce the number of radiology reports needing review by about 80%.
Although classification approaches are being refined to improve radiology performance in multiple categories of nodules, this study suggests that alternative approaches based on text algorithms can improve the capture of pulmonary nodules that require surveillance. These algorithms also can be used to augment radiologist reporting systems. This represents an investment in resources to build a team that should include a bioinformatics specialist, lung nodule registry personnel (review charts of the detected imaging studies with lung nodules, populating the lung nodule database, and determining and tracking the need of imaging follow up), a lung nodule clinic nurse coordinator, and a dedicated lung nodule clinic pulmonologist.
Radiology departments could employ this text search approach to identify missed nodules and use an audit and feedback system to train radiologists to code lung nodules consistently at the time of the initial reading to avoid delays in identifying patients with nodules. Alternatively, the more widespread use of a standardized CT chest radiology reports using Fleischner or the American College of Radiology Lung Imaging Reporting and Data System (Lung RADS) templates might improve the detection of patients with lung nodules.5,13,14
The VHA system should have an effective strategy for identifying incidental lung nodules during routine radiology examinations. Relying only on radiologists to identify and code pulmonary nodules can lead to missing a significant number of patients with lung nodules and some patients with early stage lung cancer who could receive curative therapy.12,14-16 The use of a standardized algorithm, like a text search strategy, might decrease the risk of variation in the execution and result in a more sensitive detection of patients with lung nodules. The text search strategy might be easily implemented and shared with other hospitals both within and outside the VHA.
Limitations
This study was performed in a single VHA hospital and the findings may not be generalizable to other settings of care. Second, our study design is susceptible to work-up bias because the results of a diagnostic test (eg, chest or abdomen imaging) affected whether the chart review was used to verify the test result. It was not feasible to review the patient records of all radiology studies done at the facility during the study period, consequently complete 2 × 2 tables could not be created to calculate sensitivity, specificity, and negative predictive value.
Conclusion
A text search algorithm of radiology reports increased the detection of patients with lung nodules when compared with radiology diagnostic coding alone. However, the improved detection was associated with a higher rate of false positives, which requires manually reviewing a larger number of patient’s chart reports. Future research and quality improvement should focus on standardizing the radiology reporting process and improving the efficiency and reliability of follow up and tracking of incidental lung nodules.
Acknowledgments
The work reported here was supported by a grant from the Office of Rural Health (N32-FY16Q1-S1-P01577), US Department of Veterans Affairs, Veterans Health Administration. We also had the support from the Veterans Rural Health Resource Center-Iowa City, and the Health Services Research and Development (HSR&D) Service through the Comprehensive Access and Delivery Research and Evaluation (CADRE) Center (REA 09-220).
Rapid advances in imaging technology have led to better spatial resolution with lower radiation doses to patients. These advances have helped to increase the use of diagnostic chest imaging, particularly in emergency departments and oncology centers, and in screening for coronary artery disease. As a result, there has been an explosion of incidental findings on chest imaging—including indeterminate lung nodules.1,2
Lung nodules are rounded and well-circumscribed lung opacities (≤ 3 cm in diameter) that may present as solitary or multiple lesions in usually asymptomatic patients. Most lung nodules are benign, the result of an infectious or inflammatory process. Nodules that are ≤ 8 mm in diameter, unless they show increase in size over time, often can be safely followed with imaging surveillance. In contrast, lung nodules > 8 mm could represent an early-stage lung cancer, especially among patients with high-risk for developing lung cancer (ie, those with advanced age, heavy tobacco abuse, or emphysema) and should be further assessed with close imaging surveillance, either chest computed tomography (CT) alone or positron-emission tomography (PET)/CT, or tissue biopsy, based on the underlying likelihood of malignancy.
Patients who receive an early-stage lung cancer diagnosis can be offered curative treatments leading to improved 5-year survival rates.3,4 Consequently, health care systems need to be able to identify these nodules accurately, in order to categorize and manage them accordingly to the Fleischner radiographic and American College of Chest Physicians clinical guidelines.5,6 Unfortunately, many hospitals struggle to identify patients with incidental lung nodules found during diagnostic chest and abdominal imaging, due in part to poor adherence to Fleischner guidelines among radiologists for categorizing pulmonary nodules.7,8
The Veterans Health Administration (VHA) system is interested in effectively detecting patients with incidental lung nodules. Veterans have a higher risk of developing lung cancer when compared with the entire US population, mainly due to a higher incidence of tobacco use.6 The prevalence of lung nodules among veterans with significant risk factors for lung cancer is about 60% nationwide, and up to 85% in the Midwest, due to the high prevalence of histoplasmosis.7 However, only a small percentage of these nodules represent an early stage primary lung cancer.
Several Veterans Integrated Service Networks (VISNs) in the VHA use a radiology diagnostic code to systematically identify imaging studies with presence of lung nodules. In VISN 23, which includes Minnesota, North Dakota, South Dakota, Iowa, and portions of neighboring states, the code used to identify these radiology studies is 44. However, there is high variability in the reporting and coding of imaging studies among radiologists, which could lead to misclassifying patients with lung nodules.8
Some studies suggest that using an automated text search algorithm within radiology reports can be a highly effective strategy to identify patients with lung nodules.9,10 In this study, we compared the diagnostic performance of a newly developed text search algorithm applied to radiology reports with the current standard practice of using a radiology diagnostic code for identifying patients with lung nodules at the Iowa City US Department of Veterans Affairs (VA) Health Care System (ICVAHCS) hospital in Iowa.
Methods
Since 2014, The ICVAHCS has used a radiology diagnostic code to identify any imaging studies with lung nodules. The radiologist enters “44” at the end of the reading process using the Nuance Powerscribe 360 radiation reporting system. The code is uploaded into the VHA Corporate Data Warehouse (CDW), and it is located within the radiology exam domain. This strategy was created and implemented by the Minneapolis VA Health Care System in Minnesota for all the VA hospitals in VISN 23. A lung nodule registry nurse was provided with a list of radiology studies flagged with this radiology diagnostic code every 2 weeks. A chart review was then performed for all these studies to determine the presence of a lung nodule. When detected, the ordering health care provider was alerted and given recommendations for managing the nodule.
We initially searched for the radiology studies with a presumptive lung nodule using the radiology code 44 within the CDW. Separately, we applied the text search strategy only to radiology reports from chest and abdomen studies (ie, X-rays, CT, magnetic resonance imaging [MRI], and PET) that contained any of the keyword phrases. The text search strategy was modeled based on a natural language processing (NLP) algorithm developed by the Puget Sound VA Healthcare System in Seattle, Washington to identify lung nodules on radiology reports.9 Our algorithm included a series of text searches using Microsoft SQL. After several simulations using a random group of radiology reports, we chose the keywords: “lung AND nodul”; “pulm AND nodul”; “pulm AND mass”; “lung AND mass”; and “ground glass”. We selected only chest and abdomen studies because on several simulations using a random group of radiology reports, the vast majority of lung nodules were identified on chest and abdomen imaging studies. Also, it would not have been feasible to chart review the approximately 30,000 total radiology reports that were generated during the study period.
From January 1, 2016 through November 30, 2016, we applied both search strategies independently: radiology diagnostic code for lung nodules to all imaging studies, and text search to all radiology reports of chest and abdomen imaging studies in the CDW (Figure). We also collected demographic (eg, age, sex, race, rurality) and clinical (eg, medical comorbidities, tobacco use) information that were uploaded to the database automatically from CDW using International Statistical Classification of Diseases, Tenth Edition and demographic codes. The VHA uses the Rural-Urban Commuting Areas (RUCA) system to define rurality, which takes into account population density and how closely a community is linked socioeconomically to larger urban centers.11 The protocol was reviewed and approved by the institutional review board of ICVAHCS and the University of Iowa.
The presence of a lung nodule was established by having the lung nodule registry nurse manually review the charts of every patient with a radiology report identified by either code 44 or the text search algorithm. The goal was to ensure that our text search strategy identified all reports with a code 44 to be compliant with VISN expectations. Cases in which a lung nodule was described in the radiology report were considered true positives, and those without a lung nodule description were considered false positives.
We compared the sociodemographic and clinical characteristics of patients with lung nodules between those identified with both code 44 and the text search and those identified with the text search alone. We used χ2 tests for categorical variables (eg, age, gender, RUCA, chronic obstructive pulmonary disease (COPD), smoking status) and t tests for continuous variables (eg, Charlson comorbidity score). A P value ≤ .05 was considered statistically significant. To assess the yield of each search strategy, we determined the number of patients with lung nodules detected by the text search and the radiology diagnostic code. We also calculated the positive predictive value (PPV) and 95% CI of each search strategy.
Results
We identified 12,983 radiology studies that required manual review during the study period. We confirmed that 8,516 imaging studies had lung nodules, representing 2,912 patients. Subjects with lung nodules were predominantly male (96%), aged between 60 and 79 years (71%), and lived in a rural area (72%). More than 50% of these patients had COPD and over a third were current smokers (Table 1). The text search algorithm identified all of the patients identified by the radiology diagnostic code (n = 1,251). It also identified an additional 1,661 patients with lung nodules that otherwise would have been missed by the radiology code. Compared with those identified only by the text search, those identified by both the radiology coding and text search were older, had lower Charlson comorbidity scores, and were more likely to be a current smoker.
The text search algorithm identified more than twice as many patients with potential lung nodules compared with the radiology diagnostic code (4,071 vs 1,363) (Table 2). However, the text search algorithm was associated with a much higher number of false positives than was the diagnostic code (1,159 vs 112) and a lower PPV (72% [95% CI, 70.6-73.4] vs 92% [95% CI, 90.6-93.4], respectively). The text search algorithm identified 130 patients with lung nodules of moderate to high risk for malignancy (> 8 mm diameter) that were not identified by the radiology code. When the PPV of each search strategy was calculated based on imaging studies with nodules (most patients had > 1 imaging study), the results remained similar (98% for radiology code and 66% for text search). A larger proportion of the lung nodules detected by code 44 vs the text search algorithm were from CT chest studies.
Discussion
In a population of predominantly older male veterans with significant risk factors for lung cancer and high incidence of incidental lung nodules, applying a text search algorithm on radiology reports identified a substantial number of patients with lung nodules, including some with nodules > 8 mm, that were missed by the radiologist-generated code.9,10 Improving the yield of detection for lung nodules in a population with high risk for lung cancer would increase the likelihood of detecting patients with potentially curable early-stage lung cancers, decreasing lung cancer mortality.
The reasons for the high number of patients with lung nodules missed by the radiology code are unclear. Potential explanations may include the lack of standardization of imaging reports by the radiologists (ie, only 21% of chest CTs used a standardized template describing a lung nodule in our study), a problem well recognized both within and outside VHA.8,12
The text search algorithm identified more patients with lung nodules but had a higher rate of false positives when compared with the diagnostic code. The high rate of false positives resulted in more charts to review and an increased workload for the lung nodule registry team. The challenges presented by an increased workload should be balanced against the potential harms of missing nodules that develop into advanced cancer.
Text Search Adjustments
Refining the text search criteria algorithm and the chart review process may decrease the rate of false positives significantly without affecting detection of lung nodules. In subsequent simulations, we found that by adding an exclusion criteria to text search algorithm to remove reports with specific keywords we could substantially reduce the number of false positive reports without affecting the detection rate of the lung nodules. These exclusion criteria would exclude any reports that: (1) contain “nodul” within the next 8 words after mentioning “no”; (2) contain “clear” within the next 8 words after mentioning “lung” in the text (eg, “lungs appear to be clear”); (3) contain “clear” within the next 4 words after mentioning “otherwise” in the text (eg, “otherwise appear to be clear”). Based on our study results, we further refined the text search strategy by limiting the search to only chest imaging studies. When we applied the revised algorithm to a random sample of imaging reports, we found all the code 44 radiology reports were still captured, but we were able to reduce the number of radiology reports needing review by about 80%.
Although classification approaches are being refined to improve radiology performance in multiple categories of nodules, this study suggests that alternative approaches based on text algorithms can improve the capture of pulmonary nodules that require surveillance. These algorithms also can be used to augment radiologist reporting systems. This represents an investment in resources to build a team that should include a bioinformatics specialist, lung nodule registry personnel (review charts of the detected imaging studies with lung nodules, populating the lung nodule database, and determining and tracking the need of imaging follow up), a lung nodule clinic nurse coordinator, and a dedicated lung nodule clinic pulmonologist.
Radiology departments could employ this text search approach to identify missed nodules and use an audit and feedback system to train radiologists to code lung nodules consistently at the time of the initial reading to avoid delays in identifying patients with nodules. Alternatively, the more widespread use of a standardized CT chest radiology reports using Fleischner or the American College of Radiology Lung Imaging Reporting and Data System (Lung RADS) templates might improve the detection of patients with lung nodules.5,13,14
The VHA system should have an effective strategy for identifying incidental lung nodules during routine radiology examinations. Relying only on radiologists to identify and code pulmonary nodules can lead to missing a significant number of patients with lung nodules and some patients with early stage lung cancer who could receive curative therapy.12,14-16 The use of a standardized algorithm, like a text search strategy, might decrease the risk of variation in the execution and result in a more sensitive detection of patients with lung nodules. The text search strategy might be easily implemented and shared with other hospitals both within and outside the VHA.
Limitations
This study was performed in a single VHA hospital and the findings may not be generalizable to other settings of care. Second, our study design is susceptible to work-up bias because the results of a diagnostic test (eg, chest or abdomen imaging) affected whether the chart review was used to verify the test result. It was not feasible to review the patient records of all radiology studies done at the facility during the study period, consequently complete 2 × 2 tables could not be created to calculate sensitivity, specificity, and negative predictive value.
Conclusion
A text search algorithm of radiology reports increased the detection of patients with lung nodules when compared with radiology diagnostic coding alone. However, the improved detection was associated with a higher rate of false positives, which requires manually reviewing a larger number of patient’s chart reports. Future research and quality improvement should focus on standardizing the radiology reporting process and improving the efficiency and reliability of follow up and tracking of incidental lung nodules.
Acknowledgments
The work reported here was supported by a grant from the Office of Rural Health (N32-FY16Q1-S1-P01577), US Department of Veterans Affairs, Veterans Health Administration. We also had the support from the Veterans Rural Health Resource Center-Iowa City, and the Health Services Research and Development (HSR&D) Service through the Comprehensive Access and Delivery Research and Evaluation (CADRE) Center (REA 09-220).
1. Jacobs PC, Mali WP, Grobbee DE, van der Graaf Y. Prevalence of incidental findings in computed tomographic screening of the chest: a systematic review. Journal of computer assisted tomography. 2008;32(2):214-221.
2. Frank L, Quint LE. Chest CT incidentalomas: thyroid lesions, enlarged mediastinal lymph nodes, and lung nodules. Cancer Imaging. 2012;12(1):41-48.
3. National Institutes of Health, National Cancer Institute, Surveillance, Epidemiology, and End Results Program. Cancer stat facts: lung and bronchus cancer. https://seer.cancer.gov/statfacts/html/lungb.html. Accessed April 8, 2020.
4. Alberg AJ, Brock MV, Ford JG, Samet JM, Spivack SD. Epidemiology of lung cancer: Diagnosis and management of lung cancer, 3rd ed: American College of Chest Physicians evidence-based clinical practice guidelines. Chest. 2013;143(5 Suppl):e1S-e29S.
5. MacMahon H, Naidich DP, Goo JM, et al. Guidelines for Management of Incidental Pulmonary Nodules Detected on CT Images: From the Fleischner Society 2017. Radiology. 2017;284(1):228-243.
6. Zullig LL, Jackson GL, Dorn RA, et al. Cancer incidence among patients of the U.S. Veterans Affairs Health Care System. Mil Med. 2012;177(6):693-701.
7. Kinsinger LS, Anderson C, Kim J, et al. Implementation of lung cancer screening in the Veterans Health Administration. JAMA Intern Med. 2017;177(3):399-406.
8. Iqbal MN, Stott E, Huml AM, et al. What’s in a name? Factors associated with documentation and evaluation of incidental pulmonary nodules. Ann Am Thorac Soc. 2016;13(10):1704-1711.
9. Farjah F, Halgrim S, Buist DS, et al. An automated method for identifying individuals with a lung nodule can be feasibly implemented across health systems. Egems (Wash DC). 2016;4(1):1254.
10. Danforth KN, Early MI, Ngan S, Kosco AE, Zheng C, Gould MK. Automated identification of patients with pulmonary nodules in an integrated health system using administrative health plan data, radiology reports, and natural language processing. J Thorac Oncol. 2012;7(8):1257-1262.
11. US Department of Veterans Affairs, Office of Rural Health. https://www.ruralhealth.va.gov/aboutus/ruralvets.asp. Updated January 28, 2020. Accessed April 8, 2020.
12. Blagev DP, Lloyd JF, Conner K, et al. Follow-up of incidental pulmonary nodules and the radiology report. J Am Coll Radiol. 2016;13(2 suppl):R18-R24.
13. Eisenberg RL, Fleischner S. Ways to improve radiologists’ adherence to Fleischner Society guidelines for management of pulmonary nodules. J Am Coll Radiol. 2013;10(6):439-441.
14. Aberle DR. Implementing lung cancer screening: the US experience. Clin Radiol. 2017;72(5):401-406.
15. Gould MK, Donington J, Lynch WR, et al. Evaluation of individuals with pulmonary nodules: when is it lung cancer? Diagnosis and management of lung cancer, 3rd ed: American College of Chest Physicians evidence-based clinical practice guidelines. Chest. 2013;143(5 Suppl):e93S-e120S.
16. Callister ME, Baldwin DR. How should pulmonary nodules be optimally investigated and managed? Lung Cancer. 2016;91:48-55.
1. Jacobs PC, Mali WP, Grobbee DE, van der Graaf Y. Prevalence of incidental findings in computed tomographic screening of the chest: a systematic review. Journal of computer assisted tomography. 2008;32(2):214-221.
2. Frank L, Quint LE. Chest CT incidentalomas: thyroid lesions, enlarged mediastinal lymph nodes, and lung nodules. Cancer Imaging. 2012;12(1):41-48.
3. National Institutes of Health, National Cancer Institute, Surveillance, Epidemiology, and End Results Program. Cancer stat facts: lung and bronchus cancer. https://seer.cancer.gov/statfacts/html/lungb.html. Accessed April 8, 2020.
4. Alberg AJ, Brock MV, Ford JG, Samet JM, Spivack SD. Epidemiology of lung cancer: Diagnosis and management of lung cancer, 3rd ed: American College of Chest Physicians evidence-based clinical practice guidelines. Chest. 2013;143(5 Suppl):e1S-e29S.
5. MacMahon H, Naidich DP, Goo JM, et al. Guidelines for Management of Incidental Pulmonary Nodules Detected on CT Images: From the Fleischner Society 2017. Radiology. 2017;284(1):228-243.
6. Zullig LL, Jackson GL, Dorn RA, et al. Cancer incidence among patients of the U.S. Veterans Affairs Health Care System. Mil Med. 2012;177(6):693-701.
7. Kinsinger LS, Anderson C, Kim J, et al. Implementation of lung cancer screening in the Veterans Health Administration. JAMA Intern Med. 2017;177(3):399-406.
8. Iqbal MN, Stott E, Huml AM, et al. What’s in a name? Factors associated with documentation and evaluation of incidental pulmonary nodules. Ann Am Thorac Soc. 2016;13(10):1704-1711.
9. Farjah F, Halgrim S, Buist DS, et al. An automated method for identifying individuals with a lung nodule can be feasibly implemented across health systems. Egems (Wash DC). 2016;4(1):1254.
10. Danforth KN, Early MI, Ngan S, Kosco AE, Zheng C, Gould MK. Automated identification of patients with pulmonary nodules in an integrated health system using administrative health plan data, radiology reports, and natural language processing. J Thorac Oncol. 2012;7(8):1257-1262.
11. US Department of Veterans Affairs, Office of Rural Health. https://www.ruralhealth.va.gov/aboutus/ruralvets.asp. Updated January 28, 2020. Accessed April 8, 2020.
12. Blagev DP, Lloyd JF, Conner K, et al. Follow-up of incidental pulmonary nodules and the radiology report. J Am Coll Radiol. 2016;13(2 suppl):R18-R24.
13. Eisenberg RL, Fleischner S. Ways to improve radiologists’ adherence to Fleischner Society guidelines for management of pulmonary nodules. J Am Coll Radiol. 2013;10(6):439-441.
14. Aberle DR. Implementing lung cancer screening: the US experience. Clin Radiol. 2017;72(5):401-406.
15. Gould MK, Donington J, Lynch WR, et al. Evaluation of individuals with pulmonary nodules: when is it lung cancer? Diagnosis and management of lung cancer, 3rd ed: American College of Chest Physicians evidence-based clinical practice guidelines. Chest. 2013;143(5 Suppl):e93S-e120S.
16. Callister ME, Baldwin DR. How should pulmonary nodules be optimally investigated and managed? Lung Cancer. 2016;91:48-55.