User login
Cardiac sympathetic denervation preceding motor signs in Parkinson disease*
In Parkinson disease (PD), by the time the movement disorder develops, most of the nigrostriatal dopamine terminals have been lost. Identification of biomarkers of PD should improve early diagnosis and spur development of effective treatments.
Braak has proposed a pathogenetic sequence beginning outside the brain, with invasion of peripheral, vulnerable autonomic neurons, followed by alpha-synucleinopathy in lower brainstem nuclei and then by alpha-synucleinopathy in the midbrain substantia nigra and then finally in the cerebral cortex.3,4 Consistent with early involvement of peripheral autonomic or lower brainstem centers, several studies of de novo PD have reported evidence of cardiac noradrenergic denervation5,8,14,22 or of decreased baroreflex-cardiovagal function.1,2,6,14,18
Whether these abnormalities can actually precede symptomatic PD has been unknown. Here we report the case of a patient who had cardiac noradrenergic denervation, detected by 6-[18F]fluorodopamine positron emission tomography, and decreased baroreflex-cardiovagal gain, detected by abnormal beat-to-beat blood pressure and heart rate responses to the Valsalva maneuver, 4 years before the clinical onset of PD.
CASE REPORT
A 56-year-old man was referred for possible pheochromocytoma, based on episodic hypertensive episodes and symptoms suggesting excessive catecholamine effects.
He had no serious health problems until about 1998, when he began to experience malaise and exercise intolerance and episodes of hypertension or hypotension, palpitations, and chest tightness. He also had a long history of constipation and dyspepsia, a tendency to urinary retention, and complained of a sense of fullness in the left neck. The patient’s career was in marketing and business development, until he quit work due to his symptoms. His mother had died of PD. Cardiac catheterization showed normal coronary arteries. Gastrointestinal endoscopy was unrevealing. Biochemical testing showed elevated plasma levels and urinary excretion of epinephrine. Thyroid function was normal.
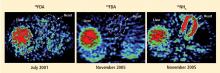
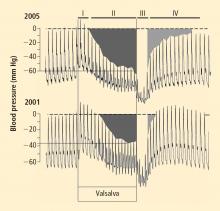
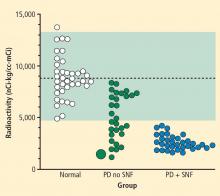
Over several months in 2005 the patient noted progressive slowing of movement and inability to relax the arms, small handwriting, decreased facial expression, and decreased voice volume. The patient returned to the NIH in November 2005, to participate in a protocol on pseudopheochromocytoma, the evaluation again including 6-[18F]fluorodopamine positron emission tomographic scanning and beat-to-beat blood pressure and heart rate associated with the Valsalva maneuver. 6-[18F]fluorodopamine PET again revealed severely decreased 6-[18F]fluorodopaminederived radioactivity throughout the left ventricular myocardium (Figure 1). In the interventricular septum, radioactivity at the midpoint of the scanning frame between 5 and 10 minutes after initiation of injection of 6-[18F]fluorodopamine was 1,286 nCi-kg/cc-mCi, more than 2 standard deviations below the normal mean and one of the lowest values we have recorded so far (Figure 3). Blood pressure decreased progressively in Phase II of the Valsalva maneuver, to a greater extent than in 2001, there was no overshoot of pressure after release of the maneuver, and the return of pressure toward baseline was prolonged, findings pointing to failure of sympathetically mediated reflexive vasoconstriction.12,23 Baroreflex-cardiovagal gain was also lower than in 2001 (1.2 msec/mm Hg from the results in Phase II, 2.6 msec/mm Hg from those in Phase IV), both because the range of heart was smaller and the extent of change in systolic pressure larger in 2005 than in 2001.
Neurological consultation in November 2005 noted stooped posture and axial instability, cogwheel rigidity in all four extremities, paucity of spontaneous movements, masked face with infrequent blinking, and monotone voice, but with normal speed of gait and no resting tremor. The patient was diagnosed with mild PD.
DISCUSSION
In this patient, results of 6-[18F]fluorodopamine PET scanning indicated cardiac sympathetic denervation 4 years before the clinical onset of PD. Considering that in PD loss of cardiac noradrenergic innervation progresses slowly over years,13 and that the patient already had evidence for markedly decreased cardiac noradrenergic innervation at the time of initial evaluation, loss of cardiac sympathetic nerves probably preceded the movement disorder by many more than the 4 years between initial testing and the onset of PD.
The findings in this case fit with previous reports of cardiac noradrenergic denervation in de novo PD and with the concept of a peripheral-to-central and caudal-to-rostral pathogenetic sequence. Orimo and co-workers have noted loss of noradrenergic terminal innervation of the myocardium before loss of cell bodies in sympathetic ganglia in PD.16
Our patient also had evidence for decreased baroreflex-cardiovagal function 4 years before the movement disorder. The baroreflex is a homeostatic arc, and abnormalities of afferent neurotransmission, central integration by brainstem centers, or vagal efferent pre-ganglionic or post-ganglionic fibers could result in the same clinical laboratory finding of low baroreflex-cardiovagal gain. In particular, the extent to which baroreflex-cardiovagal failure in PD reflects a brainstem lesion, as opposed to an afferent lesion or loss of parasympathetic cholinergic efferents, remains unknown. The results in our patient are consistent with the view that baroreflex-cardiovagal function worsens over years before the onset of PD.
Chronic constipation, which also preceded parkinsonism in our case, would be consistent with early dysregulation of gastrointestinal autonomic function. Accumulations of alpha-synuclein in enteric neurons and in the dorsal motor nucleus of the vagus nerve, the central neural site of origin of parasympathetic innervation of much of the gastrointestinal tract, has been reported to be an early pathological finding.3 As noted above, however, the occurrence of central neural pathology would not exclude a concurrent afferent or efferent lesion, and studies have found Lewy bodies in the myenteric plexus of both the esophagus and colon,9 as well as loss of enteric dopaminergic neurons in PD with chronic constipation.19
Evidence for abnormalities of the sympathetic norad renergic and parasympathetic cholinergic components of the autonomic nervous system in our patient occurred without evidence for compromised adrenomedullary function. On the contrary, the patient had augmented plasma epinephrine responses to glucagon injection, both upon initial evaluation and at follow-up. The patient therefore did not appear to have diffuse loss of catecholaminergic cells. Although studies have noted decreased adrenomedullary catecholamine concentrations in patients with severe PD,7,20,21 plasma levels of epinephrine and its O-methylated metabolite, metanephrine, have been reported to be normal.10
Combined cardiac sympathetic denervation (with attendant denervation supersensitivity), baroreflex-cardiovagal hypofunction, and adrenomedullary hyper-responsiveness might explain the symptoms and signs of cardiovascular instability, such as episodic hypertensive paroxysms, tachycardia, palpitations, and chest pain despite normal coronary arteries, that led to clinical suspicion of pheochromocytoma in this patient.
The results in this case lead us to propose that cardiac sympathetic denervation and decreased baroreflex-cardiovagal gain may be biomarkers of early autonomic involvement in PD. Studies in progress about autonomic function in relatives of patients with familial PD should help test this hypothesis.
- Bonuccelli U, Lucetti C, Del Dotto P, et al. Orthostatic hypotension in de novo Parkinson disease. Arch Neurol 2003; 60:1400–1404.
- Bouhaddi M, Vuillier F, Fortrat JO, et al. Impaired cardiovascular autonomic control in newly and long-term-treated patients with Parkinson’s disease: involvement of L-dopa therapy. Auton Neurosci 2004; 116:30–38.
- Braak H, de Vos RA, Bohl J, Del Tredici K. Gastric alpha-synuclein immunoreactive inclusions in Meissner’s and Auerbach’s plexuses in cases staged for Parkinson’s disease-related brain pathology. Neurosci Lett 2006; 396:67–72.
- Braak H, Rub U, Gai WP, Del Tredici K. Idiopathic Parkinson’s disease: possible routes by which vulnerable neuronal types may be subject to neuroinvasion by an unknown pathogen. J Neural Transm 2003; 110:517–536.
- Braune S. The role of cardiac metaiodobenzylguanidine uptake in the differential diagnosis of parkinsonian syndromes. Clin Auton Res 2001; 11:351–355.
- Camerlingo M, Aillon C, Bottacchi E, et al. Parasympathetic assessment in Parkinson’s disease. Adv Neurol 1987; 45:267–269.
- Carmichael SW, Wilson RJ, Brimijoin WS, et al. Decreased catecholamines in the adrenal medulla of patients with parkinsonism. N Engl J Med 1988; 318:254.
- Druschky A, Hilz MJ, Platsch G, et al. Differentiation of Parkinson’s disease and multiple system atrophy in early disease stages by means of I-123-MIBG-SPECT. J Neurol Sci 2000; 175:3–12.
- Edwards LL, Quigley EM, Pfeiffer RF. Gastrointestinal dysfunction in Parkinson’s disease: frequency and pathophysiology. Neurology 1992; 42:726–732.
- Goldstein DS, Holmes C, Sharabi Y, Brentzel S, Eisenhofer G. Plasma levels of catechols and metanephrines in neurogenic orthostatic hypotension. Neurology 2003; 60:1327–1332.
- Goldstein DS, Horwitz D, Keiser HR. Comparison of techniques for measuring baroreflex sensitivity in man. Circulation 1982; 66:432–439.
- Goldstein DS, Tack C. Noninvasive detection of sympathetic neurocirculatory failure. Clin Auton Res 2000; 10:285–291.
- Li ST, Dendi R, Holmes C, Goldstein DS. Progressive loss of cardiac sympathetic innervation in Parkinson’s disease. Ann Neurol 2002; 52:220–223.
- Oka H, Mochio S, Onouchi K, Morita M, Yoshioka M, Inoue K. Cardiovascular dysautonomia in de novo Parkinson’s disease. J Neurol Sci 2006; 241:59–65.
- Oka H, Mochio S, Yoshioka M, Morita M, Onouchi K, Inoue K. Cardiovascular dysautonomia in Parkinson’s disease and multiple system atrophy. Acta Neurol Scand 2006; 113:221–227.
- Orimo S, Amino T, Itoh Y, et al. Cardiac sympathetic denervation precedes neuronal loss in the sympathetic ganglia in Lewy body disease. Acta Neuropathol (Berl) 2005; 109:583–588.
- Pacak K, Eisenhofer G, Carrasquillo JA, Chen CC, Li ST, Goldstein DS. 6-[18F]fluorodopamine positron emission tomographic (PET) scanning for diagnostic localization of pheochromocytoma. Hypertension 2001; 38:6–8.
- Quadri R, Comino I, Scarzella L, et al. Autonomic nervous function in de novo parkinsonian patients in basal condition and after acute levodopa administration. Funct Neurol 2000; 15:81–86.
- Singaram C, Ashraf W, Gaumnitz EA, et al. Dopaminergic defect of enteric nervous system in Parkinson’s disease patients with chronic constipation. Lancet 1995; 346:861–864.
- Stoddard SL, Ahlskog JE, Kelly PJ, et al. Decreased adrenal medullary catecholamines in adrenal transplanted parkinsonian patients compared to nephrectomy patients. Exp Neurol 1989; 104:218–222.
- Stoddard SL, Tyce GM, Ahlskog JE, Zinsmeister AR, Carmichael SW. Decreased catecholamine content in parkinsonian adrenal medullae. Exp Neurol 1989; 104:22–27.
- Takatsu H, Nishida H, Matsuo H, et al. Cardiac sympathetic denervation from the early stage of Parkinson’s disease: clinical and experimental studies with radiolabeled MIBG. J Nucl Med 2000; 41:71–77.
- Vogel ER, Sandroni P, Low PA. Blood pressure recovery from Valsalva maneuver in patients with autonomic failure. Neurology 2005; 65:1533–1537.
In Parkinson disease (PD), by the time the movement disorder develops, most of the nigrostriatal dopamine terminals have been lost. Identification of biomarkers of PD should improve early diagnosis and spur development of effective treatments.
Braak has proposed a pathogenetic sequence beginning outside the brain, with invasion of peripheral, vulnerable autonomic neurons, followed by alpha-synucleinopathy in lower brainstem nuclei and then by alpha-synucleinopathy in the midbrain substantia nigra and then finally in the cerebral cortex.3,4 Consistent with early involvement of peripheral autonomic or lower brainstem centers, several studies of de novo PD have reported evidence of cardiac noradrenergic denervation5,8,14,22 or of decreased baroreflex-cardiovagal function.1,2,6,14,18
Whether these abnormalities can actually precede symptomatic PD has been unknown. Here we report the case of a patient who had cardiac noradrenergic denervation, detected by 6-[18F]fluorodopamine positron emission tomography, and decreased baroreflex-cardiovagal gain, detected by abnormal beat-to-beat blood pressure and heart rate responses to the Valsalva maneuver, 4 years before the clinical onset of PD.
CASE REPORT
A 56-year-old man was referred for possible pheochromocytoma, based on episodic hypertensive episodes and symptoms suggesting excessive catecholamine effects.
He had no serious health problems until about 1998, when he began to experience malaise and exercise intolerance and episodes of hypertension or hypotension, palpitations, and chest tightness. He also had a long history of constipation and dyspepsia, a tendency to urinary retention, and complained of a sense of fullness in the left neck. The patient’s career was in marketing and business development, until he quit work due to his symptoms. His mother had died of PD. Cardiac catheterization showed normal coronary arteries. Gastrointestinal endoscopy was unrevealing. Biochemical testing showed elevated plasma levels and urinary excretion of epinephrine. Thyroid function was normal.
Over several months in 2005 the patient noted progressive slowing of movement and inability to relax the arms, small handwriting, decreased facial expression, and decreased voice volume. The patient returned to the NIH in November 2005, to participate in a protocol on pseudopheochromocytoma, the evaluation again including 6-[18F]fluorodopamine positron emission tomographic scanning and beat-to-beat blood pressure and heart rate associated with the Valsalva maneuver. 6-[18F]fluorodopamine PET again revealed severely decreased 6-[18F]fluorodopaminederived radioactivity throughout the left ventricular myocardium (Figure 1). In the interventricular septum, radioactivity at the midpoint of the scanning frame between 5 and 10 minutes after initiation of injection of 6-[18F]fluorodopamine was 1,286 nCi-kg/cc-mCi, more than 2 standard deviations below the normal mean and one of the lowest values we have recorded so far (Figure 3). Blood pressure decreased progressively in Phase II of the Valsalva maneuver, to a greater extent than in 2001, there was no overshoot of pressure after release of the maneuver, and the return of pressure toward baseline was prolonged, findings pointing to failure of sympathetically mediated reflexive vasoconstriction.12,23 Baroreflex-cardiovagal gain was also lower than in 2001 (1.2 msec/mm Hg from the results in Phase II, 2.6 msec/mm Hg from those in Phase IV), both because the range of heart was smaller and the extent of change in systolic pressure larger in 2005 than in 2001.
Neurological consultation in November 2005 noted stooped posture and axial instability, cogwheel rigidity in all four extremities, paucity of spontaneous movements, masked face with infrequent blinking, and monotone voice, but with normal speed of gait and no resting tremor. The patient was diagnosed with mild PD.
DISCUSSION
In this patient, results of 6-[18F]fluorodopamine PET scanning indicated cardiac sympathetic denervation 4 years before the clinical onset of PD. Considering that in PD loss of cardiac noradrenergic innervation progresses slowly over years,13 and that the patient already had evidence for markedly decreased cardiac noradrenergic innervation at the time of initial evaluation, loss of cardiac sympathetic nerves probably preceded the movement disorder by many more than the 4 years between initial testing and the onset of PD.
The findings in this case fit with previous reports of cardiac noradrenergic denervation in de novo PD and with the concept of a peripheral-to-central and caudal-to-rostral pathogenetic sequence. Orimo and co-workers have noted loss of noradrenergic terminal innervation of the myocardium before loss of cell bodies in sympathetic ganglia in PD.16
Our patient also had evidence for decreased baroreflex-cardiovagal function 4 years before the movement disorder. The baroreflex is a homeostatic arc, and abnormalities of afferent neurotransmission, central integration by brainstem centers, or vagal efferent pre-ganglionic or post-ganglionic fibers could result in the same clinical laboratory finding of low baroreflex-cardiovagal gain. In particular, the extent to which baroreflex-cardiovagal failure in PD reflects a brainstem lesion, as opposed to an afferent lesion or loss of parasympathetic cholinergic efferents, remains unknown. The results in our patient are consistent with the view that baroreflex-cardiovagal function worsens over years before the onset of PD.
Chronic constipation, which also preceded parkinsonism in our case, would be consistent with early dysregulation of gastrointestinal autonomic function. Accumulations of alpha-synuclein in enteric neurons and in the dorsal motor nucleus of the vagus nerve, the central neural site of origin of parasympathetic innervation of much of the gastrointestinal tract, has been reported to be an early pathological finding.3 As noted above, however, the occurrence of central neural pathology would not exclude a concurrent afferent or efferent lesion, and studies have found Lewy bodies in the myenteric plexus of both the esophagus and colon,9 as well as loss of enteric dopaminergic neurons in PD with chronic constipation.19
Evidence for abnormalities of the sympathetic norad renergic and parasympathetic cholinergic components of the autonomic nervous system in our patient occurred without evidence for compromised adrenomedullary function. On the contrary, the patient had augmented plasma epinephrine responses to glucagon injection, both upon initial evaluation and at follow-up. The patient therefore did not appear to have diffuse loss of catecholaminergic cells. Although studies have noted decreased adrenomedullary catecholamine concentrations in patients with severe PD,7,20,21 plasma levels of epinephrine and its O-methylated metabolite, metanephrine, have been reported to be normal.10
Combined cardiac sympathetic denervation (with attendant denervation supersensitivity), baroreflex-cardiovagal hypofunction, and adrenomedullary hyper-responsiveness might explain the symptoms and signs of cardiovascular instability, such as episodic hypertensive paroxysms, tachycardia, palpitations, and chest pain despite normal coronary arteries, that led to clinical suspicion of pheochromocytoma in this patient.
The results in this case lead us to propose that cardiac sympathetic denervation and decreased baroreflex-cardiovagal gain may be biomarkers of early autonomic involvement in PD. Studies in progress about autonomic function in relatives of patients with familial PD should help test this hypothesis.
In Parkinson disease (PD), by the time the movement disorder develops, most of the nigrostriatal dopamine terminals have been lost. Identification of biomarkers of PD should improve early diagnosis and spur development of effective treatments.
Braak has proposed a pathogenetic sequence beginning outside the brain, with invasion of peripheral, vulnerable autonomic neurons, followed by alpha-synucleinopathy in lower brainstem nuclei and then by alpha-synucleinopathy in the midbrain substantia nigra and then finally in the cerebral cortex.3,4 Consistent with early involvement of peripheral autonomic or lower brainstem centers, several studies of de novo PD have reported evidence of cardiac noradrenergic denervation5,8,14,22 or of decreased baroreflex-cardiovagal function.1,2,6,14,18
Whether these abnormalities can actually precede symptomatic PD has been unknown. Here we report the case of a patient who had cardiac noradrenergic denervation, detected by 6-[18F]fluorodopamine positron emission tomography, and decreased baroreflex-cardiovagal gain, detected by abnormal beat-to-beat blood pressure and heart rate responses to the Valsalva maneuver, 4 years before the clinical onset of PD.
CASE REPORT
A 56-year-old man was referred for possible pheochromocytoma, based on episodic hypertensive episodes and symptoms suggesting excessive catecholamine effects.
He had no serious health problems until about 1998, when he began to experience malaise and exercise intolerance and episodes of hypertension or hypotension, palpitations, and chest tightness. He also had a long history of constipation and dyspepsia, a tendency to urinary retention, and complained of a sense of fullness in the left neck. The patient’s career was in marketing and business development, until he quit work due to his symptoms. His mother had died of PD. Cardiac catheterization showed normal coronary arteries. Gastrointestinal endoscopy was unrevealing. Biochemical testing showed elevated plasma levels and urinary excretion of epinephrine. Thyroid function was normal.
Over several months in 2005 the patient noted progressive slowing of movement and inability to relax the arms, small handwriting, decreased facial expression, and decreased voice volume. The patient returned to the NIH in November 2005, to participate in a protocol on pseudopheochromocytoma, the evaluation again including 6-[18F]fluorodopamine positron emission tomographic scanning and beat-to-beat blood pressure and heart rate associated with the Valsalva maneuver. 6-[18F]fluorodopamine PET again revealed severely decreased 6-[18F]fluorodopaminederived radioactivity throughout the left ventricular myocardium (Figure 1). In the interventricular septum, radioactivity at the midpoint of the scanning frame between 5 and 10 minutes after initiation of injection of 6-[18F]fluorodopamine was 1,286 nCi-kg/cc-mCi, more than 2 standard deviations below the normal mean and one of the lowest values we have recorded so far (Figure 3). Blood pressure decreased progressively in Phase II of the Valsalva maneuver, to a greater extent than in 2001, there was no overshoot of pressure after release of the maneuver, and the return of pressure toward baseline was prolonged, findings pointing to failure of sympathetically mediated reflexive vasoconstriction.12,23 Baroreflex-cardiovagal gain was also lower than in 2001 (1.2 msec/mm Hg from the results in Phase II, 2.6 msec/mm Hg from those in Phase IV), both because the range of heart was smaller and the extent of change in systolic pressure larger in 2005 than in 2001.
Neurological consultation in November 2005 noted stooped posture and axial instability, cogwheel rigidity in all four extremities, paucity of spontaneous movements, masked face with infrequent blinking, and monotone voice, but with normal speed of gait and no resting tremor. The patient was diagnosed with mild PD.
DISCUSSION
In this patient, results of 6-[18F]fluorodopamine PET scanning indicated cardiac sympathetic denervation 4 years before the clinical onset of PD. Considering that in PD loss of cardiac noradrenergic innervation progresses slowly over years,13 and that the patient already had evidence for markedly decreased cardiac noradrenergic innervation at the time of initial evaluation, loss of cardiac sympathetic nerves probably preceded the movement disorder by many more than the 4 years between initial testing and the onset of PD.
The findings in this case fit with previous reports of cardiac noradrenergic denervation in de novo PD and with the concept of a peripheral-to-central and caudal-to-rostral pathogenetic sequence. Orimo and co-workers have noted loss of noradrenergic terminal innervation of the myocardium before loss of cell bodies in sympathetic ganglia in PD.16
Our patient also had evidence for decreased baroreflex-cardiovagal function 4 years before the movement disorder. The baroreflex is a homeostatic arc, and abnormalities of afferent neurotransmission, central integration by brainstem centers, or vagal efferent pre-ganglionic or post-ganglionic fibers could result in the same clinical laboratory finding of low baroreflex-cardiovagal gain. In particular, the extent to which baroreflex-cardiovagal failure in PD reflects a brainstem lesion, as opposed to an afferent lesion or loss of parasympathetic cholinergic efferents, remains unknown. The results in our patient are consistent with the view that baroreflex-cardiovagal function worsens over years before the onset of PD.
Chronic constipation, which also preceded parkinsonism in our case, would be consistent with early dysregulation of gastrointestinal autonomic function. Accumulations of alpha-synuclein in enteric neurons and in the dorsal motor nucleus of the vagus nerve, the central neural site of origin of parasympathetic innervation of much of the gastrointestinal tract, has been reported to be an early pathological finding.3 As noted above, however, the occurrence of central neural pathology would not exclude a concurrent afferent or efferent lesion, and studies have found Lewy bodies in the myenteric plexus of both the esophagus and colon,9 as well as loss of enteric dopaminergic neurons in PD with chronic constipation.19
Evidence for abnormalities of the sympathetic norad renergic and parasympathetic cholinergic components of the autonomic nervous system in our patient occurred without evidence for compromised adrenomedullary function. On the contrary, the patient had augmented plasma epinephrine responses to glucagon injection, both upon initial evaluation and at follow-up. The patient therefore did not appear to have diffuse loss of catecholaminergic cells. Although studies have noted decreased adrenomedullary catecholamine concentrations in patients with severe PD,7,20,21 plasma levels of epinephrine and its O-methylated metabolite, metanephrine, have been reported to be normal.10
Combined cardiac sympathetic denervation (with attendant denervation supersensitivity), baroreflex-cardiovagal hypofunction, and adrenomedullary hyper-responsiveness might explain the symptoms and signs of cardiovascular instability, such as episodic hypertensive paroxysms, tachycardia, palpitations, and chest pain despite normal coronary arteries, that led to clinical suspicion of pheochromocytoma in this patient.
The results in this case lead us to propose that cardiac sympathetic denervation and decreased baroreflex-cardiovagal gain may be biomarkers of early autonomic involvement in PD. Studies in progress about autonomic function in relatives of patients with familial PD should help test this hypothesis.
- Bonuccelli U, Lucetti C, Del Dotto P, et al. Orthostatic hypotension in de novo Parkinson disease. Arch Neurol 2003; 60:1400–1404.
- Bouhaddi M, Vuillier F, Fortrat JO, et al. Impaired cardiovascular autonomic control in newly and long-term-treated patients with Parkinson’s disease: involvement of L-dopa therapy. Auton Neurosci 2004; 116:30–38.
- Braak H, de Vos RA, Bohl J, Del Tredici K. Gastric alpha-synuclein immunoreactive inclusions in Meissner’s and Auerbach’s plexuses in cases staged for Parkinson’s disease-related brain pathology. Neurosci Lett 2006; 396:67–72.
- Braak H, Rub U, Gai WP, Del Tredici K. Idiopathic Parkinson’s disease: possible routes by which vulnerable neuronal types may be subject to neuroinvasion by an unknown pathogen. J Neural Transm 2003; 110:517–536.
- Braune S. The role of cardiac metaiodobenzylguanidine uptake in the differential diagnosis of parkinsonian syndromes. Clin Auton Res 2001; 11:351–355.
- Camerlingo M, Aillon C, Bottacchi E, et al. Parasympathetic assessment in Parkinson’s disease. Adv Neurol 1987; 45:267–269.
- Carmichael SW, Wilson RJ, Brimijoin WS, et al. Decreased catecholamines in the adrenal medulla of patients with parkinsonism. N Engl J Med 1988; 318:254.
- Druschky A, Hilz MJ, Platsch G, et al. Differentiation of Parkinson’s disease and multiple system atrophy in early disease stages by means of I-123-MIBG-SPECT. J Neurol Sci 2000; 175:3–12.
- Edwards LL, Quigley EM, Pfeiffer RF. Gastrointestinal dysfunction in Parkinson’s disease: frequency and pathophysiology. Neurology 1992; 42:726–732.
- Goldstein DS, Holmes C, Sharabi Y, Brentzel S, Eisenhofer G. Plasma levels of catechols and metanephrines in neurogenic orthostatic hypotension. Neurology 2003; 60:1327–1332.
- Goldstein DS, Horwitz D, Keiser HR. Comparison of techniques for measuring baroreflex sensitivity in man. Circulation 1982; 66:432–439.
- Goldstein DS, Tack C. Noninvasive detection of sympathetic neurocirculatory failure. Clin Auton Res 2000; 10:285–291.
- Li ST, Dendi R, Holmes C, Goldstein DS. Progressive loss of cardiac sympathetic innervation in Parkinson’s disease. Ann Neurol 2002; 52:220–223.
- Oka H, Mochio S, Onouchi K, Morita M, Yoshioka M, Inoue K. Cardiovascular dysautonomia in de novo Parkinson’s disease. J Neurol Sci 2006; 241:59–65.
- Oka H, Mochio S, Yoshioka M, Morita M, Onouchi K, Inoue K. Cardiovascular dysautonomia in Parkinson’s disease and multiple system atrophy. Acta Neurol Scand 2006; 113:221–227.
- Orimo S, Amino T, Itoh Y, et al. Cardiac sympathetic denervation precedes neuronal loss in the sympathetic ganglia in Lewy body disease. Acta Neuropathol (Berl) 2005; 109:583–588.
- Pacak K, Eisenhofer G, Carrasquillo JA, Chen CC, Li ST, Goldstein DS. 6-[18F]fluorodopamine positron emission tomographic (PET) scanning for diagnostic localization of pheochromocytoma. Hypertension 2001; 38:6–8.
- Quadri R, Comino I, Scarzella L, et al. Autonomic nervous function in de novo parkinsonian patients in basal condition and after acute levodopa administration. Funct Neurol 2000; 15:81–86.
- Singaram C, Ashraf W, Gaumnitz EA, et al. Dopaminergic defect of enteric nervous system in Parkinson’s disease patients with chronic constipation. Lancet 1995; 346:861–864.
- Stoddard SL, Ahlskog JE, Kelly PJ, et al. Decreased adrenal medullary catecholamines in adrenal transplanted parkinsonian patients compared to nephrectomy patients. Exp Neurol 1989; 104:218–222.
- Stoddard SL, Tyce GM, Ahlskog JE, Zinsmeister AR, Carmichael SW. Decreased catecholamine content in parkinsonian adrenal medullae. Exp Neurol 1989; 104:22–27.
- Takatsu H, Nishida H, Matsuo H, et al. Cardiac sympathetic denervation from the early stage of Parkinson’s disease: clinical and experimental studies with radiolabeled MIBG. J Nucl Med 2000; 41:71–77.
- Vogel ER, Sandroni P, Low PA. Blood pressure recovery from Valsalva maneuver in patients with autonomic failure. Neurology 2005; 65:1533–1537.
- Bonuccelli U, Lucetti C, Del Dotto P, et al. Orthostatic hypotension in de novo Parkinson disease. Arch Neurol 2003; 60:1400–1404.
- Bouhaddi M, Vuillier F, Fortrat JO, et al. Impaired cardiovascular autonomic control in newly and long-term-treated patients with Parkinson’s disease: involvement of L-dopa therapy. Auton Neurosci 2004; 116:30–38.
- Braak H, de Vos RA, Bohl J, Del Tredici K. Gastric alpha-synuclein immunoreactive inclusions in Meissner’s and Auerbach’s plexuses in cases staged for Parkinson’s disease-related brain pathology. Neurosci Lett 2006; 396:67–72.
- Braak H, Rub U, Gai WP, Del Tredici K. Idiopathic Parkinson’s disease: possible routes by which vulnerable neuronal types may be subject to neuroinvasion by an unknown pathogen. J Neural Transm 2003; 110:517–536.
- Braune S. The role of cardiac metaiodobenzylguanidine uptake in the differential diagnosis of parkinsonian syndromes. Clin Auton Res 2001; 11:351–355.
- Camerlingo M, Aillon C, Bottacchi E, et al. Parasympathetic assessment in Parkinson’s disease. Adv Neurol 1987; 45:267–269.
- Carmichael SW, Wilson RJ, Brimijoin WS, et al. Decreased catecholamines in the adrenal medulla of patients with parkinsonism. N Engl J Med 1988; 318:254.
- Druschky A, Hilz MJ, Platsch G, et al. Differentiation of Parkinson’s disease and multiple system atrophy in early disease stages by means of I-123-MIBG-SPECT. J Neurol Sci 2000; 175:3–12.
- Edwards LL, Quigley EM, Pfeiffer RF. Gastrointestinal dysfunction in Parkinson’s disease: frequency and pathophysiology. Neurology 1992; 42:726–732.
- Goldstein DS, Holmes C, Sharabi Y, Brentzel S, Eisenhofer G. Plasma levels of catechols and metanephrines in neurogenic orthostatic hypotension. Neurology 2003; 60:1327–1332.
- Goldstein DS, Horwitz D, Keiser HR. Comparison of techniques for measuring baroreflex sensitivity in man. Circulation 1982; 66:432–439.
- Goldstein DS, Tack C. Noninvasive detection of sympathetic neurocirculatory failure. Clin Auton Res 2000; 10:285–291.
- Li ST, Dendi R, Holmes C, Goldstein DS. Progressive loss of cardiac sympathetic innervation in Parkinson’s disease. Ann Neurol 2002; 52:220–223.
- Oka H, Mochio S, Onouchi K, Morita M, Yoshioka M, Inoue K. Cardiovascular dysautonomia in de novo Parkinson’s disease. J Neurol Sci 2006; 241:59–65.
- Oka H, Mochio S, Yoshioka M, Morita M, Onouchi K, Inoue K. Cardiovascular dysautonomia in Parkinson’s disease and multiple system atrophy. Acta Neurol Scand 2006; 113:221–227.
- Orimo S, Amino T, Itoh Y, et al. Cardiac sympathetic denervation precedes neuronal loss in the sympathetic ganglia in Lewy body disease. Acta Neuropathol (Berl) 2005; 109:583–588.
- Pacak K, Eisenhofer G, Carrasquillo JA, Chen CC, Li ST, Goldstein DS. 6-[18F]fluorodopamine positron emission tomographic (PET) scanning for diagnostic localization of pheochromocytoma. Hypertension 2001; 38:6–8.
- Quadri R, Comino I, Scarzella L, et al. Autonomic nervous function in de novo parkinsonian patients in basal condition and after acute levodopa administration. Funct Neurol 2000; 15:81–86.
- Singaram C, Ashraf W, Gaumnitz EA, et al. Dopaminergic defect of enteric nervous system in Parkinson’s disease patients with chronic constipation. Lancet 1995; 346:861–864.
- Stoddard SL, Ahlskog JE, Kelly PJ, et al. Decreased adrenal medullary catecholamines in adrenal transplanted parkinsonian patients compared to nephrectomy patients. Exp Neurol 1989; 104:218–222.
- Stoddard SL, Tyce GM, Ahlskog JE, Zinsmeister AR, Carmichael SW. Decreased catecholamine content in parkinsonian adrenal medullae. Exp Neurol 1989; 104:22–27.
- Takatsu H, Nishida H, Matsuo H, et al. Cardiac sympathetic denervation from the early stage of Parkinson’s disease: clinical and experimental studies with radiolabeled MIBG. J Nucl Med 2000; 41:71–77.
- Vogel ER, Sandroni P, Low PA. Blood pressure recovery from Valsalva maneuver in patients with autonomic failure. Neurology 2005; 65:1533–1537.