User login
Using the ankle-brachial index to diagnose peripheral artery disease and assess cardiovascular risk
In this article, we seek to convince you to measure the ankle-brachial index for any patient you suspect may have peripheral artery disease. This would include patients who are elderly, who smoke, or who have diabetes, regardless of whether or not they have symptoms. The ankle-brachial index is a simple test that involves taking the blood pressure in all four limbs using a hand-held Doppler device and then dividing the leg systolic pressure by the arm systolic pressure.
This simple test is both sensitive and specific for peripheral artery disease. It also gives a good assessment of cardiovascular risk. The downside: you or a member of your staff spends a few minutes doing it. Also, for patients without leg symptoms or abnormal findings on physical examination, you may not be paid for doing it.
Despite these limitations, the ankle-brachial index is a powerful clinical tool that deserves to be performed more often in primary care.
PERIPHERAL ARTERY DISEASE IS COMMON AND SERIOUS
Peripheral artery disease is important to detect, as it is common, it has serious consequences, and effective treatments are available. However, many patients with the disease do not have typical symptoms.
Peripheral artery disease affects up to 29% of people over age 70, depending on the population sampled.1,2 Its classic symptom is intermittent claudication, ie, leg pain with walking that improves with rest. However, most patients do not have intermittent claudication; they have atypical leg symptoms or no symptoms at all.2,3 While the risk factors for peripheral artery disease are similar to those for coronary artery disease, the factors most strongly associated with peripheral artery disease are older age, tobacco smoking, and diabetes mellitus.4 Blacks are twice as likely to have it compared with whites, even after adjusting for other cardiovascular risk factors.5
Untreated peripheral artery disease may have serious consequences, such as amputation, impaired functional capacity, poor quality of life, and depression.3,6,7 In addition, it is a strong marker of atherosclerotic burden and cardiovascular risk and has been recognized as a coronary risk equivalent. Patients with peripheral artery disease are at higher risk of death, myocardial infarction, stroke, and hospitalization, with event rates as high as 21% per year.8
Fortunately, simple therapies have been shown to prevent adverse cardiovascular events in peripheral artery disease, including antiplatelet drugs, statins, and angiotensin-converting enzyme inhibitors.9–11
THE ANKLE-BRACHIAL INDEX IS SENSITIVE AND SPECIFIC
The evaluation for possible peripheral artery disease should begin with the medical history, a cardiovascular review of systems, and a focused physical examination in which one should:
- Measure the blood pressure in both arms to assess for occult subclavian stenosis
- Auscultate for bruits over the carotid, abdominal, and femoral arteries
- Palpate the pulses in the lower extremities and the abdominal aorta
- Inspect the bare legs and feet for thinning of the skin, hair loss, thickening of the nails (which are nonspecific signs), and ulceration.
However, the physical examination has limited sensitivity and specificity for diagnosing peripheral artery disease. In general, the most reliable finding is the absence of a palpable posterior tibial artery pulse, which has been reported to have a specificity of 71% and a sensitivity of 91% for peripheral artery disease.12
The ankle-brachial index is the first-line test for both screening for peripheral artery disease and for diagnosing it. It is inexpensive and noninvasive to obtain and has a high sensitivity (79% to 95%) and specificity (95% to 96%) compared with angiography as the gold standard.13–18 It can be measured easily in the office, and every practitioner who cares for patients at risk of cardiovascular disease can be trained to measure it competently.
HOW TO MEASURE THE ANKLE-BRACHIAL INDEX
The ankle-brachial index is the ratio of the systolic pressure in the ankle to the systolic pressure in the arm. In healthy people, this ratio is typically greater than 1.0 or 1.1.
You can measure the ankle-brachial index in the office with a blood pressure cuff, sphygmomanometer, and handheld Doppler device. Alternatively, it can be measured in a noninvasive vascular laboratory as part of a more detailed examination that allows for assessment of blood pressures and waveforms (Doppler or pulse-volume recordings) at multiple segments along the limb. These more detailed vascular studies are generally reserved for patients with confirmed peripheral artery disease to locate the level and extent of blockage or for patients in whom lower-extremity revascularization is contemplated.
The use of a stethoscope to measure blood pressures for the ankle-brachial index has been studied in a few small series,19,20 but is thought to be less accurate than Doppler, especially in the setting of significant arterial occlusive disease. Because of this, it is recommended and assumed that a Doppler device be used to measure all blood pressures for the ankle-brachial index.
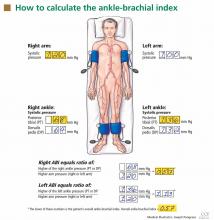
After the patient has been resting quietly for 5 to 10 minutes in the supine position, the systolic blood pressure is measured in both arms and in both ankles in the dorsalis pedis and posterior tibial arteries (Figure 1). The blood pressure cuff is placed about 1 inch above the antecubital fossa for the brachial pressure and about 2 inches above the medial malleolus for the ankle pressures. A clear arterial pulse signal should be heard using the Doppler probe before inflating the blood pressure cuff. The cuff is then inflated to at least 20 mm Hg above the point where the arterial Doppler sounds disappear and then slowly deflated until the Doppler sounds reappear. The blood pressure at which the Doppler signal of the arterial pulse reappears is the systolic pressure for that vessel.
The ankle-brachial index is calculated by dividing the higher of the two ankle systolic blood pressures in each leg by the higher of the two brachial systolic blood pressures. The higher of the two brachial pressures is used as the denominator to account for the possibility of subclavian artery stenosis, which can decrease the blood pressure in the upper extremity. The ankle-brachial index is calculated for each leg, and the lower value is the patient’s overall ankle-brachial index. An abnormal value in either leg indicates peripheral artery disease.
While other ways of calculating the ankle-brachial index have been proposed, such as averaging the two pressures at each ankle or reporting the lower of the two ankle pressures, these methods are not standard for use in clinical practice.
Similarly, the use of oscillometric blood pressure devices has been proposed, which would eliminate the need for a Doppler device and personnel trained in its use. However, results of validation studies of oscillometric measurement of the ankle-brachial index have been inconsistent, likely because the devices were designed for measuring blood pressure in nonobstructed arms, not the legs, and especially not diseased legs.21–25 In general, oscillometric devices tend to overestimate ankle pressure, giving a falsely high ankle-brachial index in patients with moderate to severe peripheral artery disease.21 Their utility in screening for peripheral artery disease has not been evaluated in broad, population-based studies. Efforts to develop and validate new oscillometric devices for diagnosing peripheral artery disease are ongoing.
INTERPRETING THE ANKLE-BRACHIAL INDEX
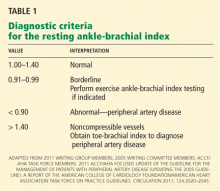
Diagnostic criteria for the ankle-brachial index were standardized in 2011 (Table 1).26 Most healthy adults have a value greater than 1.0. A value of less than 0.91 is consistent with significant peripheral artery disease, and a value lower than 0.40 at rest generally indicates severe disease. A value between 0.91 and 0.99 is borderline abnormal and does not rule out peripheral artery disease. A value greater than 1.40 reflects noncompressibility of the leg arteries and is not diagnostic (see below).
The ankle-brachial index after exercise. In patients strongly suspected of having peripheral artery disease but who have a normal ankle-brachial index at rest, and especially if the resting value is borderline (ie, 0.91–0.99), the measurement should be repeated after exercise, the better to detect “mild” peripheral artery disease.15 With exercise, increased flow across a fixed stenosis leads to a significant fall in ankle pressure and a lower ankle-brachial index. In one study,27 the ankle-brachial index fell below 0.9 after exercise in 31% of outpatients with symptoms who had initially tested normal.
The exercise is optimally done on a motorized treadmill set at an incline. A number of exercise protocols are in use; at our institution, we use a fixed workload protocol. The ankle-brachial index and ankle pulse-volume recordings are recorded on both sides at rest, after which the patient generally walks for 5 minutes at a 12% grade at 2.0 mph or until symptoms force the patient to stop. The advantage of treadmill testing is the ability to assess functional capacity by measuring the time to the onset of pain and the total walking time.
Alternatively, active pedal plantar flexion maneuvers (heel raises) or corridor walking to the point at which limiting symptoms occur can be done if a treadmill is not available, though this is not the favored approach and does not qualify as formal exercise testing for reimbursement purposes. The patient is asked to do heel raises as high and as fast as possible for 30 seconds or until limiting pain symptoms occur. Results with this maneuver have been shown to correlate well with those of treadmill exercise testing.28
Immediately after any exercise maneuver, arm and ankle pressures are remeasured and bilateral ankle-brachial indices are recalculated. A fall in ankle pressure or the ankle-brachial index after exercise (generally, a fall of more than 20%) supports the diagnosis of peripheral artery disease. If the patient develops leg symptoms during exercise while his or her ankle-brachial index falls significantly, this also supports the vasculogenic nature of the leg symptoms.
An ankle-brachial index greater than 1.40 means that the pedal arteries are stiff and cannot be compressed by the blood pressure cuff. This is considered abnormal, though not necessarily diagnostic of peripheral artery disease. Noncompressible leg arteries are common among patients with long-standing diabetes mellitus or end-stage renal disease, and also can be found in obese patients.
Because toe arteries are usually compressible even when the pedal arteries are not, a toe-brachial index can be obtained to confirm the diagnosis of peripheral artery disease in these cases. This is calculated by measuring the blood pressure in the great toe using a small digital blood pressure cuff and a Doppler probe or a plethysmographic flow sensor. The toe-brachial index is calculated by dividing the toe blood pressure by the higher of the two brachial artery pressures; a value of 0.7 or less generally indicates peripheral artery disease.
WHAT SHOULD BE DONE WITH AN ABNORMAL RESULT?
An abnormal ankle-brachial index establishes the diagnosis of peripheral artery disease, and in many cases no additional diagnostic testing is necessary.
Care of patients with peripheral artery disease has three elements:
- Cardiovascular risk factor assessment and reduction to prevent myocardial infarction, stroke, and death
- Assessment and treatment of leg symptoms to improve function and quality of life
- Foot care to prevent ulcers and amputation.
Risk factor reduction. Because they have a markedly greater risk of cardiovascular disease and death, all patients with peripheral artery disease should undergo aggressive cardiovascular risk factor modification,26,29 including:
- Antiplatelet therapy in the form of aspirin 75–325 mg daily or clopidogrel 75 mg daily as an alternative to aspirin
- Counseling and therapy for immediate smoking cessation if the patient smokes
- Treatment of hypertension to Seventh Joint National Committee goals30
- Treatment of lipids to Adult Treatment Panel III goals31 (generally to a goal low-density lipoprotein cholesterol of less than 100 mg/dL, and less than 70 mg/dL if possible)
- Treatment of diabetes to a goal hemoglobin A1c of less than 7% (in the absence of contraindications).32
Exercise and anticlaudication medication. Patients with an abnormal ankle-brachial index and intermittent claudication may benefit from a supervised exercise program, a trial of drug therapy for claudication, or both. All patients with peripheral artery disease, regardless of symptoms, should be advised to incorporate aerobic exercise (ideally, walking) into their daily routine.
Cilostazol (Pletal), a phosphodiesterase inhibitor, has been given a class IA recommendation in the American College of Cardiology/American Heart Association guidelines for the treatment of intermittent claudication. The dose is generally 100 mg by mouth twice daily.29
Revascularization. Patients with an abnormal ankle-brachial index and lifestyle-limiting claudication that has failed to improve with medical therapy or a course of supervised exercise training should be referred to a vascular specialist for evaluation for revascularization (endovascular therapy or surgical bypass). 29 Endovascular therapy is particularly attractive for patients with claudication and evidence of aortoiliac disease (suspected in patients with gluteal or thigh claudication, diminution of the femoral pulse, or a bruit over the femoral artery on examination and confirmed by noninvasive vascular laboratory testing).
Patients who have ischemic pain at rest, gangrene, or a nonhealing lower-extremity wound that has been present for at least 2 weeks should be referred for revascularization on an urgent basis, given the risk of impending limb loss associated with critical limb ischemia.
A detailed review of the medical, endovascular, and surgical management of peripheral artery disease can be found in a supplement to the Cleveland Clinic Journal of Medicine published in 200633 and in comprehensive multi-society guidelines.26,29
THE ANKLE-BRACHIAL INDEX AS A MARKER OF RISK
Low values: Peripheral artery disease
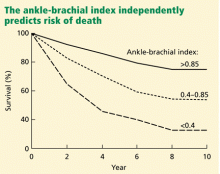
Peripheral artery disease, as diagnosed by a low ankle-brachial index, confers an excess risk of death from all causes in a graded fashion: ie, the more severe the disease, the lower the survival rate (Figure 2).34 Because peripheral artery disease is a sign of systemic atherosclerosis and one-third to one-half of patients with peripheral artery disease have evidence of cerebrovascular or coronary artery disease,35–37 peripheral artery disease also confers a higher risk of cardiovascular death.
The Edinburgh Artery Study,38 a prospective cohort study of 1,592 randomly selected patients age 55 to 74 years, demonstrated the relationship between a low ankle-brachial index and an increased risk of cardiovascular death. Over 5 years of follow-up, compared with patients with a normal ankle-brachial index, the relative risk of cardiovascular death in symptomatic patients with a value of 0.9 or lower was 2.67 (95% confidence interval [CI] 1.34–5.29). The relative risk in patients with asymptomatic disease was between 1.74 (95% CI 1.09–2.76) and 2.08 (95% CI 1.13–3.83), depending on the level of ankle-brachial index decrement and ankle blood pressure response to hyperemia.
(Reactive hyperemia is an alternative to exercise testing. It is performed by inflating a blood pressure cuff at the thigh above the systolic pressure for 3 to 5 minutes or until the patient can no longer tolerate the inflation. Blood pressures at the ankle are remeasured after cuff release.)
Several other epidemiologic studies have established the association between low ankle-brachial index and the risk of cardiovascular death.
Heald et al39 performed a meta-analysis of 44,590 patients in 11 epidemiologic studies and found that, after adjustment for age, sex, conventional cardiovascular risk factors, and prevalent cardiovascular disease, an ankle-brachial index lower than 0.9 conferred a higher risk of:
- All-cause mortality (pooled risk ratio [RR] 1.60, 95% CI 1.32–1.95)
- Cardiovascular mortality (pooled RR 1.96, 95% CI 1.46–2.64)
- Coronary heart disease (pooled RR 1.45, 95% CI 1.08–1.93)
- Stroke (pooled RR 1.35, 95% CI 1.10–1.65).
Fowkes et al,40 in a meta-analysis of 16 population cohort studies including 48,294 patients over 480,325 person-years of follow-up, found that a low ankle-brachial index predicted cardiovascular events and death even after adjusting for the Framingham risk score, Hazard ratios for cardiovascular death were:
- 2.92 (95% CI 2.31–3.70) in men
- 2.97 (95% CI 2.02–4.35) in women.
Hazard ratios for death from any cause were:
- 2.34 (95% CI 1.97–2.78) in men
- 2.35 (95% CI 1.76–3.13) in women.
Adding the ankle-brachial index to the Framingham risk score resulted in reclassification of risk category in approximately 19% of men and 36% of women.40
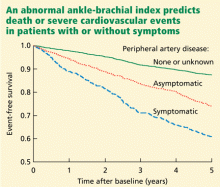
The German Epidemiological Trial on Ankle Brachial Index (getABI) screened 6,880 patients 65 years of age and found an abnormal ankle-brachial index in 20.9% of them.41 In more than 5 years of follow-up, a value of less than 0.90 was associated with a higher rate of cardiovascular events and death from any cause in patients with both symptomatic and asymptomatic peripheral artery disease (Figure 3).41
In addition, the lower the ankle-brachial index, the greater the rate of death or severe cardiovascular events. An index between 0.7 and 0.9 was associated with a statistically significant twofold increase (adjusted hazard ratio 2.03), and a value lower than 0.5 was associated with a nearly fivefold increase (hazard ratio 4.65) in the risk of events compared with the group of patients with normal values.41
Abnormal results after exercise
Exercise testing may increase the sensitivity of the ankle-brachial index to detect peripheral artery disease in patients with normal resting values and especially in patients with borderline values. As such, abnormal exercise values have also been associated with an increased risk of death due to any cause and of cardiovascular death.
In a prospective cohort study of 3,209 patients with suspected or known peripheral artery disease referred to a vascular surgery clinic in the Netherlands, patients with lower postexercise values had a higher rate of overall and cardiac death (hazard ratio per 10% lower value 1.16 [95% CI 1.13–1.18] and 1.10 [95% CI 1.09–1.13], respectively).42
Sheikh et al43 reported similar findings in patients with normal resting ankle-brachial indices at Cleveland Clinic. In this study, an abnormal postexercise ankle-brachial index (defined as < 0.85) was associated with a hazard ratio of 1.67 for all-cause mortality compared with a normal postexercise value among individuals with no history of cardiovascular events.
High values: Noncompressible vessels
While the relationship between low values and increased mortality and cardiovascular risk is well accepted, there have been conflicting reports regarding high values (> 1.4) and adverse outcomes.44,45
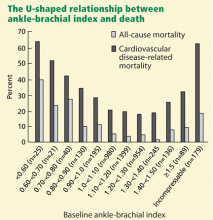
The Strong Heart Study44 was a population-based study in 4,393 Native Americans followed for more than 8 years for the rate of all-cause and cardiovascular mortality. Most (n = 3,773) of the cohort had a normal ankle-brachial index (≥ 0.9 and ≤ 1.4); 4.9% (n = 216) had a low value (< 0.9); and 9.2% (n = 404) had a high value (> 1.4 or noncompressible). Relative risk ratios for all-cause mortality were 1.69 (95% CI 1.34–2.14) for low values and 1.77 (95% CI 1.48–2.13) for high values compared with those with normal values. Low and high ankle-brachial indices also conferred a risk of cardiovascular death, with relative risk ratios of 2.52 (95% CI 1.74–3.64) and 2.09 (95% CI 1.49–2.94), respectively. There was a U-shaped relationship between the ankle-brachial index and the mortality rate (Figure 4).44
The Atherosclerosis Risk in Communities (ARIC) study45 had different findings. In 14,777 participants followed for a mean of 12.2 years, the cardiovascular disease event rates of patients whose ankle-brachial index-was categorized as high (> 1.3, > 1.4, or > 1.5) were similar to those of patients with a normal value (between 0.9 and 1.3).
Differences in event rates between the two studies may be due to a higher prevalence of values greater than 1.4 in the Strong Heart Study cohort as well as to a higher prevalence of concomitant risk factors (diabetes, older age, hypertension, lipid abnormality) in the high ankle-brachial index group in the Strong Heart Study compared with the ARIC study.
DIFFERING RECOMMENDATIONS
The ankle-brachial index can be used to screen for asymptomatic peripheral artery disease. It can also be used to confirm the diagnosis in patients with symptoms such as intermittent claudication, ischemic pain at rest, or lower extremity ulcers or in patients with signs such as abnormal pulses, bruits, or lower-extremity skin changes. It is also used to reassess the severity of known peripheral artery disease and as a part of a routine surveillance program to assess the patency of bypass grafts and endovascular stents after revascularization procedures.
The complication of peripheral artery disease that patients dread the most is limb loss, but of greater clinical consequence are the alarming rates of cardiovascular events and death in these patients. Epidemiologic studies have shown that fewer than 5% of patients age 55 or older with claudication or asymptomatic peripheral artery disease experience major amputation over a 5-year follow-up period, but 20% of these patients will have a stroke or myocardial infarction and 15% to 30% will die. Of those who die, 75% die of a coronary or cerebrovascular cause.36 Because of the markedly increased risk of death or cardiovascular morbidity in patients with peripheral artery disease, many have advocated screening patients at high risk using the ankle-brachial index. However, there have been conflicting recommendations from national societies and agencies.29,46–48
The United States Preventive Services Task Force (USPSTF) updated its 1996 recommendations on screening for peripheral artery disease in 2005 and recommended against routinely screening for it, giving the practice a “D” recommendation (not recommended). Specifically, it stated that it found “fair evidence that screening asymptomatic adults with the ankle brachial index could lead to some small degree of harm, including false-positive results and unnecessary work-ups,”46 and concluded that “for asymptomatic adults, harms of routine screening for [peripheral artery disease] exceed benefits.”46
This negative recommendation was intensely debated among vascular specialty groups, and a rebuttal was published in 2006.49 The major area of contention was the task force’s assumption that decreased disease-specific morbidity (especially limb loss) is the most important outcome to be prevented by screening for asymptomatic peripheral artery disease, rather than adverse cardiovascular events. The USPSTF has announced plans for an update on screening for peripheral artery disease, anticipated for 2013.50
The American College of Cardiology/American Heart Association task force in 2005 recommended that a history of walking impairment, intermittent claudication, ischemic rest pain, or nonhealing wounds be solicited as part of a standard review of systems for adults age 70 and older or adults age 50 and older who have risk factors for atherosclerosis (class IC recommendation—based only on a consensus opinion of experts, case studies, or standard of care).29 In contrast to the USPSTF recommendations, the joint guidelines further recommended that patients with asymptomatic lower-extremity peripheral artery disease be identified by physical examination, ankle-brachial index, or both, to prevent myocardial infarction, stroke, or death (class IC).29 Patients at risk for lower-extremity peripheral artery disease for whom ankle-brachial index measurement is recommended include those with exertional leg symptoms, those with nonhealing ulcers, those age 70 and older, and those age 50 and older who have a history of moking or diabetes.
The American Diabetes Association and the second Inter-Society Consensus for the Management of Peripheral Arterial Disease (TASC II) issued similar recommendations.48
In 2011, the American College of Cardiology/American Heart Association task force issued a focused update to its 2005 guidelines, broadening the recommendation for testing to include patients age 65 and older on the basis of the getABI study, as well as maintaining the recommendation for testing for those age 50 and older with a history of smoking or diabetes (class IB recommendation).26,41
The task force’s Guideline for the Assessment of Cardiovascular Risk in Asymptomatic Adults says that measuring the ankle-brachial index is reasonable for cardiovascular risk assessment in asymptomatic adults at intermediate risk (class IIA—conflicting evidence or divergence of opinion, from multiple randomized clinical trials).51 Also recommended as risk stratification tools for this patient population are measurement of carotid intima-media thickness and measurement of coronary artery calcium (both class IIA recommendations).
Unlike these tests, however, the ankle-brachial index does not require highly trained technical and medical personnel to perform and interpret. In addition, there is no risk of radiation exposure as is the case in coronary calcium measurement. It is a simpler, lower-cost, and more widely available tool for cardiovascular risk assessment.
LIMITATIONS TO ANKLE-BRACHIAL SCREENING IN PRACTICE
Although this test is relatively simple and noninvasive, it is not widely performed in primary care and cardiovascular medicine. In a study by Mohler and colleagues,52 the most common barriers to its use among primary care providers were the time required to perform it, lack of reimbursement for it, and limited staff availability. Currently, third-party payers do not generally reimburse for an ankle-brachial index examination performed to screen a patient who is asymptomatic but at risk for peripheral artery disease. Unfortunately, this has limited the widespread adoption of a program to detect peripheral artery disease in patients at risk.
Despite these limitations, the ankle-brachial index is an invaluable tool to both screen for peripheral artery disease in the appropriate at-risk patient populations and to diagnose it in patients who present with lower extremity symptoms. There are few diagnostic tests available today that provide such a high degree of diagnostic accuracy with as much prognostic information as the ankle-brachial index and with such little expense and risk to the patient.
- Selvin E, Erlinger TP. Prevalence of and risk factors for peripheral arterial disease in the United States: results from the National Health and Nutrition Examination Survey, 1999–2000. Circulation 2004; 110:738–743.
- Hirsch AT, Criqui MH, Treat-Jacobson D, et al. Peripheral arterial disease detection, awareness, and treatment in primary care. JAMA 2001; 286:1317–1324.
- McDermott MM, Greenland P, Liu K, et al. Leg symptoms in peripheral arterial disease: associated clinical characteristics and functional impairment. JAMA 2001; 286:1599–1606.
- McDermott MM. The magnitude of the problem of peripheral arterial disease: epidemiology and clinical significance. Cleve Clin J Med 2006; 73(suppl 4):S2–S7.
- Criqui MH, Vargas V, Denenberg JO, et al. Ethnicity and peripheral arterial disease: the San Diego Population Study. Circulation 2005; 112:2703–2707.
- Regensteiner JG, Hiatt WR, Coll JR, et al The impact of peripheral arterial disease on health-related quality of life in the Peripheral Arterial Disease Awareness, Risk, and Treatment: New Resources for Survival (PARTNERS) Program. Vasc Med 2008; 13:15–24.
- Arseven A, Guralnik JM, O’Brien E, Liu K, McDermott MM. Peripheral arterial disease and depressed mood in older men and women. Vasc Med 2001; 6:229–234.
- Steg PG, Bhatt DL, Wilson PW, et al; REACH Registry Investigators. One-year cardiovascular event rates in outpatients with atherothrombosis. JAMA 2007; 297:1197–1206.
- Gornik HL, Creager MA. Contemporary management of peripheral arterial disease: I. cardiovascular risk-factor modification. Cleve Clin J Med 2006; 73(suppl 4):S30–S37.
- Antithrombotic Trialists’ Collaboration. Collaborative meta-analysis of randomised trials of antiplatelet therapy for prevention of death, myocardial infarction, and stroke in high risk patients. BMJ 2002; 324:71–86.
- Heart Protection Study Collaborative Group. Randomized trial of the effects of cholesterol-lowering with simvastatin on peripheral vascular and other major vascular outcomes in 20,536 people with peripheral arterial disease and other high-risk conditions. J Vasc Surg 2007; 45:645–654.
- Criqui MH, Fronek A, Klauber MR, Barrett-Connor E, Gabriel S. The sensitivity, specificity, and predictive value of traditional clinical evaluation of peripheral arterial disease: results from noninvasive testing in a defined population. Circulation 1985; 71:516–522.
- Carter SA. Clinical measurement of systolic pressures in limbs with arterial occlusive disease. JAMA 1969; 207:1869–1874.
- Ouriel K, Zarins CK. Doppler ankle pressure: an evaluation of three methods of expression. Arch Surg 1982; 117:1297–1300.
- Yao ST, Hobbs JT, Irvine WT. Ankle systolic pressure measurements in arterial disease affecting the lower extremities. Br J Surg 1969; 56:676–679.
- Carter SA. Indirect systolic pressures and pulse waves in arterial occlusive diseases of the lower extremities. Circulation 1968; 37:624–637.
- Belch JJ, Topol EJ, Agnelli G, et al; Prevention of Atherothrombotic Disease Network. Critical issues in peripheral arterial disease detection and management: a call to action. Arch Intern Med 2003; 163:884–892.
- Lijmer JG, Hunink MG, van den Dungen JJ, Loonstra J, Smit AJ. ROC analysis of noninvasive tests for peripheral arterial disease. Ultrasound Med Biol 1996; 22:391–398.
- Carmo GA, Mandil A, Nascimento BR, et al. Can we measure the ankle-brachial index using only a stethoscope? A pilot study. Fam Pract 2009; 26:22–26.
- Chesbro SB, Asongwed ET, Brown J, John EB. Reliability of Doppler and stethoscope methods of determining systolic blood pressures: considerations for calculating an ankle-brachial index. J Natl Med Assoc 2011; 103:863–869.
- Jönsson B, Lindberg LG, Skau T, Thulesius O. Is oscillometric ankle pressure reliable in leg vascular disease? Clin Physiol 2001; 21:155–163.
- Ramanathan A, Conaghan PJ, Jenkinson AD, Bishop CR. Comparison of ankle-brachial pressure index measurements using an automated oscillometric device with the standard Doppler ultrasound technique. ANZ J Surg 2003; 73:105–108.
- Beckman JA, Higgins CO, Gerhard-Herman M. Automated oscillometric determination of the ankle-brachial index provides accuracy necessary for office practice. Hypertension 2006; 47:35–38.
- Mehlsen J, Wiinberg N, Bruce C. Oscillometric blood pressure measurement: a simple method in screening for peripheral arterial disease. Clin Physiol Funct Imaging 2008; 28:426–429.
- Aboyans V, Lacroix P, Doucet S, Preux PM, Criqui MH, Laskar M. Diagnosis of peripheral arterial disease in general practice: can the ankle-brachial index be measured either by pulse palpation or an automatic blood pressure device? Int J Clin Pract 2008; 62:1001–1007.
- 2011 Writing Group Members. 2011 ACCF/AHA Focused Update of the Guideline for the Management of patients with peripheral artery disease (updating the 2005 guideline): a report of the American College of Cardiology Foundation/American Heart Association Task Force on practice guidelines. Circulation 2011; 124:2020–2045.
- Stein R, Hriljac I, Halperin JL, Gustavson SM, Teodorescu V, Olin JW. Limitation of the resting ankle-brachial index in symptomatic patients with peripheral arterial disease. Vasc Med 2006; 11:29–33.
- Amirhamzeh MM, Chant HJ, Rees JL, Hands LJ, Powell RJ, Campbell WB. A comparative study of treadmill tests and heel raising exercise for peripheral arterial disease. Eur J Vasc Endovasc Surg 1997; 13:301–305.
- Hirsch AT, Haskal ZJ, Hertzer NR, et al; American Association for Vascular Surgery; Society for Vascular Surgery; Society for Cardiovascular Angiography and Interventions; Society for Vascular Medicine and Biology; Society of Interventional Radiology; ACC/AHA Task Force on Practice Guidelines Writing Committee to Develop Guidelines for the Management of Patients With Peripheral Arterial Disease; American Association of Cardiovascular and Pulmonary Rehabilitation; National Heart, Lung, and Blood Institute; Society for Vascular Nursing; TransAtlantic Inter-Society Consensus; Vascular Disease Foundation. ACC/AHA 2005 practice guidelines for the management of patients with peripheral arterial disease (lower extremity, renal, mesenteric, and abdominal aortic): a collaborative report from the American Association for Vascular Surgery/Society for Vascular Surgery, Society for Cardiovascular Angiography and Interventions, Society for Vascular Medicine and Biology, Society of Interventional Radiology, and the ACC/AHA Task Force on Practice Guidelines (Writing Committee to Develop Guidelines for the Management of Patients With Peripheral Arterial Disease): endorsed by the American Association of Cardiovascular and Pulmonary Rehabilitation; National Heart, Lung, and Blood Institute; Society for Vascular Nursing; TransAtlantic Inter-Society Consensus; and Vascular Disease Foundation. Circulation 2006; 113:e463–e654.
- Chobanian AV, Bakris GL, Black HR, et al; Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure; National Heart, Lung, and Blood Institute; National High Blood Pressure Education Program Coordinating Committee. Seventh report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure. Hypertension 2003; 42:1206–1252.
- National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III). Third Report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III) final report. Circulation 2002; 106:3143–3421.
- Skyler JS, Bergenstal R, Bonow RO, et al; American Diabetes Association; American College of Cardiology Foundation; American Heart Association. Intensive glycemic control and the prevention of cardiovascular events: implications of the ACCORD, ADVANCE, and VA Diabetes Trials: a position statement of the American Diabetes Association and a Scientific Statement of the American College of Cardiology Foundation and the American Heart Association. J Am Coll Cardiol 2009; 53:298–304.
- Almahameed A, Bartholomew JR, editors. Peripheral arterial disease: recognition and contemporary management. Cleve Clin J Med 2006; 73(suppl 4):S1–S51.
- McKenna M, Wolfson S, Kuller L. The ratio of ankle and arm arterial pressure as an independent predictor of mortality. Atherosclerosis 1991; 87:119–128.
- Valentine RJ, Grayburn PA, Eichhorn EJ, Myers SI, Clagett GP. Coronary artery disease is highly prevalent among patients with premature peripheral vascular disease. J Vasc Surg 1994; 19:668–674.
- Weitz JI, Byrne J, Clagett GP, et al. Diagnosis and treatment of chronic arterial insufficiency of the lower extremities: a critical review. Circulation 1996; 94:3026–3049.
- Hertzer NR, Beven EG, Young JR, et al. Coronary artery disease in peripheral vascular patients. A classification of 1000 coronary angiograms and results of surgical management. Ann Surg 1984; 199:223–233.
- Leng GC, Lee AJ, Fowkes FG, et al. Incidence, natural history and cardiovascular events in symptomatic and asymptomatic peripheral arterial disease in the general population. Int J Epidemiol 1996; 25:1172–1181.
- Heald CL, Fowkes FG, Murray GD, Price JF; Ankle Brachial Index Collaboration. Risk of mortality and cardiovascular disease associated with the ankle-brachial index: Systematic review. Atherosclerosis 2006; 189:61–69.
- Ankle Brachial Index; Fowkes FG, Murray GD, Butcher I, et al. Collaboration Ankle brachial index combined with Framingham Risk Score to predict cardiovascular events and mortality: a meta-analysis. JAMA 2008; 300:197–208.
- Diehm C, Allenberg JR, Pittrow D, et al; German Epidemiological Trial on Ankle Brachial Index Study Group. Mortality and vascular morbidity in older adults with asymptomatic versus symptomatic peripheral artery disease. Circulation 2009; 120:2053–2061.
- Feringa HH, Bax JJ, van Waning VH, et al. The long-term prognostic value of the resting and postexercise ankle-brachial index. Arch Intern Med 2006; 166:529–535.
- Sheikh MA, Bhatt DL, Li J, Lin S, Bartholomew JR. Usefulness of postexercise ankle-brachial index to predict all-cause mortality. Am J Cardiol 2011; 107:778–782.
- Resnick HE, Lindsay RS, McDermott MM, et al. Relationship of high and low ankle brachial index to all-cause and cardiovascular disease mortality: the Strong Heart Study. Circulation 2004; 109:733–739.
- Wattanakit K, Folsom AR, Duprez DA, Weatherley BD, Hirsch AT. Clinical significance of a high ankle-brachial index: insights from the Atherosclerosis Risk in Communities (ARIC) Study. Atherosclerosis 2007; 190:459–464.
- US Preventive Services Task Force. Screening for peripheral arterial disease: Recommendation statement. http://www.ahrq.gov/clinic/uspstf05/pad/padrs.pdf. Accessed July 25, 2012.
- Mayfield JA, Reiber GE, Sanders LJ, Janisse D, Pogach LM; American Diabetes Association. Preventive foot care in diabetes. Diabetes Care 2004; 27(suppl 1):S63–S64.
- Norgren L, Hiatt WR, Dormandy JA, Nehler MR, Harris KA, Fowkes FG; TASC II Working Group. Inter-Society Consensus for the Management of Peripheral Arterial Disease (TASC II). J Vasc Surg 2007; 45(suppl S):S5–S67.
- Beckman JA, Jaff MR, Creager MA. The United States preventive services task force recommendation statement on screening for peripheral arterial disease: more harm than benefit? Circulation 2006; 114:861–866.
- US Preventive Services Task Force. Screening for peripheral artery disease draft research plan. http://www.uspreventiveservicestaskforce.org/uspstf12/pad/padslides.htm. Accessed July 26, 2012.
- Greenland P, Alpert JS, Beller GA, et al; American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. 2010 ACCF/AHA guideline for assessment of cardiovascular risk in asymptomatic adults: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. Circulation 2010; 122:e584–e636.
- Mohler ER, Treat-Jacobson D, Reilly MP, et al. Utility and barriers to performance of the ankle-brachial index in primary care practice. Vasc Med 2004; 9:253–260.
In this article, we seek to convince you to measure the ankle-brachial index for any patient you suspect may have peripheral artery disease. This would include patients who are elderly, who smoke, or who have diabetes, regardless of whether or not they have symptoms. The ankle-brachial index is a simple test that involves taking the blood pressure in all four limbs using a hand-held Doppler device and then dividing the leg systolic pressure by the arm systolic pressure.
This simple test is both sensitive and specific for peripheral artery disease. It also gives a good assessment of cardiovascular risk. The downside: you or a member of your staff spends a few minutes doing it. Also, for patients without leg symptoms or abnormal findings on physical examination, you may not be paid for doing it.
Despite these limitations, the ankle-brachial index is a powerful clinical tool that deserves to be performed more often in primary care.
PERIPHERAL ARTERY DISEASE IS COMMON AND SERIOUS
Peripheral artery disease is important to detect, as it is common, it has serious consequences, and effective treatments are available. However, many patients with the disease do not have typical symptoms.
Peripheral artery disease affects up to 29% of people over age 70, depending on the population sampled.1,2 Its classic symptom is intermittent claudication, ie, leg pain with walking that improves with rest. However, most patients do not have intermittent claudication; they have atypical leg symptoms or no symptoms at all.2,3 While the risk factors for peripheral artery disease are similar to those for coronary artery disease, the factors most strongly associated with peripheral artery disease are older age, tobacco smoking, and diabetes mellitus.4 Blacks are twice as likely to have it compared with whites, even after adjusting for other cardiovascular risk factors.5
Untreated peripheral artery disease may have serious consequences, such as amputation, impaired functional capacity, poor quality of life, and depression.3,6,7 In addition, it is a strong marker of atherosclerotic burden and cardiovascular risk and has been recognized as a coronary risk equivalent. Patients with peripheral artery disease are at higher risk of death, myocardial infarction, stroke, and hospitalization, with event rates as high as 21% per year.8
Fortunately, simple therapies have been shown to prevent adverse cardiovascular events in peripheral artery disease, including antiplatelet drugs, statins, and angiotensin-converting enzyme inhibitors.9–11
THE ANKLE-BRACHIAL INDEX IS SENSITIVE AND SPECIFIC
The evaluation for possible peripheral artery disease should begin with the medical history, a cardiovascular review of systems, and a focused physical examination in which one should:
- Measure the blood pressure in both arms to assess for occult subclavian stenosis
- Auscultate for bruits over the carotid, abdominal, and femoral arteries
- Palpate the pulses in the lower extremities and the abdominal aorta
- Inspect the bare legs and feet for thinning of the skin, hair loss, thickening of the nails (which are nonspecific signs), and ulceration.
However, the physical examination has limited sensitivity and specificity for diagnosing peripheral artery disease. In general, the most reliable finding is the absence of a palpable posterior tibial artery pulse, which has been reported to have a specificity of 71% and a sensitivity of 91% for peripheral artery disease.12
The ankle-brachial index is the first-line test for both screening for peripheral artery disease and for diagnosing it. It is inexpensive and noninvasive to obtain and has a high sensitivity (79% to 95%) and specificity (95% to 96%) compared with angiography as the gold standard.13–18 It can be measured easily in the office, and every practitioner who cares for patients at risk of cardiovascular disease can be trained to measure it competently.
HOW TO MEASURE THE ANKLE-BRACHIAL INDEX
The ankle-brachial index is the ratio of the systolic pressure in the ankle to the systolic pressure in the arm. In healthy people, this ratio is typically greater than 1.0 or 1.1.
You can measure the ankle-brachial index in the office with a blood pressure cuff, sphygmomanometer, and handheld Doppler device. Alternatively, it can be measured in a noninvasive vascular laboratory as part of a more detailed examination that allows for assessment of blood pressures and waveforms (Doppler or pulse-volume recordings) at multiple segments along the limb. These more detailed vascular studies are generally reserved for patients with confirmed peripheral artery disease to locate the level and extent of blockage or for patients in whom lower-extremity revascularization is contemplated.
The use of a stethoscope to measure blood pressures for the ankle-brachial index has been studied in a few small series,19,20 but is thought to be less accurate than Doppler, especially in the setting of significant arterial occlusive disease. Because of this, it is recommended and assumed that a Doppler device be used to measure all blood pressures for the ankle-brachial index.
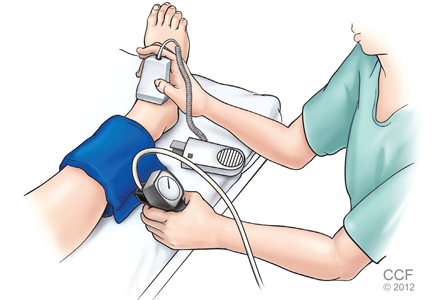
After the patient has been resting quietly for 5 to 10 minutes in the supine position, the systolic blood pressure is measured in both arms and in both ankles in the dorsalis pedis and posterior tibial arteries (Figure 1). The blood pressure cuff is placed about 1 inch above the antecubital fossa for the brachial pressure and about 2 inches above the medial malleolus for the ankle pressures. A clear arterial pulse signal should be heard using the Doppler probe before inflating the blood pressure cuff. The cuff is then inflated to at least 20 mm Hg above the point where the arterial Doppler sounds disappear and then slowly deflated until the Doppler sounds reappear. The blood pressure at which the Doppler signal of the arterial pulse reappears is the systolic pressure for that vessel.
The ankle-brachial index is calculated by dividing the higher of the two ankle systolic blood pressures in each leg by the higher of the two brachial systolic blood pressures. The higher of the two brachial pressures is used as the denominator to account for the possibility of subclavian artery stenosis, which can decrease the blood pressure in the upper extremity. The ankle-brachial index is calculated for each leg, and the lower value is the patient’s overall ankle-brachial index. An abnormal value in either leg indicates peripheral artery disease.
While other ways of calculating the ankle-brachial index have been proposed, such as averaging the two pressures at each ankle or reporting the lower of the two ankle pressures, these methods are not standard for use in clinical practice.
Similarly, the use of oscillometric blood pressure devices has been proposed, which would eliminate the need for a Doppler device and personnel trained in its use. However, results of validation studies of oscillometric measurement of the ankle-brachial index have been inconsistent, likely because the devices were designed for measuring blood pressure in nonobstructed arms, not the legs, and especially not diseased legs.21–25 In general, oscillometric devices tend to overestimate ankle pressure, giving a falsely high ankle-brachial index in patients with moderate to severe peripheral artery disease.21 Their utility in screening for peripheral artery disease has not been evaluated in broad, population-based studies. Efforts to develop and validate new oscillometric devices for diagnosing peripheral artery disease are ongoing.
INTERPRETING THE ANKLE-BRACHIAL INDEX
Diagnostic criteria for the ankle-brachial index were standardized in 2011 (Table 1).26 Most healthy adults have a value greater than 1.0. A value of less than 0.91 is consistent with significant peripheral artery disease, and a value lower than 0.40 at rest generally indicates severe disease. A value between 0.91 and 0.99 is borderline abnormal and does not rule out peripheral artery disease. A value greater than 1.40 reflects noncompressibility of the leg arteries and is not diagnostic (see below).
The ankle-brachial index after exercise. In patients strongly suspected of having peripheral artery disease but who have a normal ankle-brachial index at rest, and especially if the resting value is borderline (ie, 0.91–0.99), the measurement should be repeated after exercise, the better to detect “mild” peripheral artery disease.15 With exercise, increased flow across a fixed stenosis leads to a significant fall in ankle pressure and a lower ankle-brachial index. In one study,27 the ankle-brachial index fell below 0.9 after exercise in 31% of outpatients with symptoms who had initially tested normal.
The exercise is optimally done on a motorized treadmill set at an incline. A number of exercise protocols are in use; at our institution, we use a fixed workload protocol. The ankle-brachial index and ankle pulse-volume recordings are recorded on both sides at rest, after which the patient generally walks for 5 minutes at a 12% grade at 2.0 mph or until symptoms force the patient to stop. The advantage of treadmill testing is the ability to assess functional capacity by measuring the time to the onset of pain and the total walking time.
Alternatively, active pedal plantar flexion maneuvers (heel raises) or corridor walking to the point at which limiting symptoms occur can be done if a treadmill is not available, though this is not the favored approach and does not qualify as formal exercise testing for reimbursement purposes. The patient is asked to do heel raises as high and as fast as possible for 30 seconds or until limiting pain symptoms occur. Results with this maneuver have been shown to correlate well with those of treadmill exercise testing.28
Immediately after any exercise maneuver, arm and ankle pressures are remeasured and bilateral ankle-brachial indices are recalculated. A fall in ankle pressure or the ankle-brachial index after exercise (generally, a fall of more than 20%) supports the diagnosis of peripheral artery disease. If the patient develops leg symptoms during exercise while his or her ankle-brachial index falls significantly, this also supports the vasculogenic nature of the leg symptoms.
An ankle-brachial index greater than 1.40 means that the pedal arteries are stiff and cannot be compressed by the blood pressure cuff. This is considered abnormal, though not necessarily diagnostic of peripheral artery disease. Noncompressible leg arteries are common among patients with long-standing diabetes mellitus or end-stage renal disease, and also can be found in obese patients.
Because toe arteries are usually compressible even when the pedal arteries are not, a toe-brachial index can be obtained to confirm the diagnosis of peripheral artery disease in these cases. This is calculated by measuring the blood pressure in the great toe using a small digital blood pressure cuff and a Doppler probe or a plethysmographic flow sensor. The toe-brachial index is calculated by dividing the toe blood pressure by the higher of the two brachial artery pressures; a value of 0.7 or less generally indicates peripheral artery disease.
WHAT SHOULD BE DONE WITH AN ABNORMAL RESULT?
An abnormal ankle-brachial index establishes the diagnosis of peripheral artery disease, and in many cases no additional diagnostic testing is necessary.
Care of patients with peripheral artery disease has three elements:
- Cardiovascular risk factor assessment and reduction to prevent myocardial infarction, stroke, and death
- Assessment and treatment of leg symptoms to improve function and quality of life
- Foot care to prevent ulcers and amputation.
Risk factor reduction. Because they have a markedly greater risk of cardiovascular disease and death, all patients with peripheral artery disease should undergo aggressive cardiovascular risk factor modification,26,29 including:
- Antiplatelet therapy in the form of aspirin 75–325 mg daily or clopidogrel 75 mg daily as an alternative to aspirin
- Counseling and therapy for immediate smoking cessation if the patient smokes
- Treatment of hypertension to Seventh Joint National Committee goals30
- Treatment of lipids to Adult Treatment Panel III goals31 (generally to a goal low-density lipoprotein cholesterol of less than 100 mg/dL, and less than 70 mg/dL if possible)
- Treatment of diabetes to a goal hemoglobin A1c of less than 7% (in the absence of contraindications).32
Exercise and anticlaudication medication. Patients with an abnormal ankle-brachial index and intermittent claudication may benefit from a supervised exercise program, a trial of drug therapy for claudication, or both. All patients with peripheral artery disease, regardless of symptoms, should be advised to incorporate aerobic exercise (ideally, walking) into their daily routine.
Cilostazol (Pletal), a phosphodiesterase inhibitor, has been given a class IA recommendation in the American College of Cardiology/American Heart Association guidelines for the treatment of intermittent claudication. The dose is generally 100 mg by mouth twice daily.29
Revascularization. Patients with an abnormal ankle-brachial index and lifestyle-limiting claudication that has failed to improve with medical therapy or a course of supervised exercise training should be referred to a vascular specialist for evaluation for revascularization (endovascular therapy or surgical bypass). 29 Endovascular therapy is particularly attractive for patients with claudication and evidence of aortoiliac disease (suspected in patients with gluteal or thigh claudication, diminution of the femoral pulse, or a bruit over the femoral artery on examination and confirmed by noninvasive vascular laboratory testing).
Patients who have ischemic pain at rest, gangrene, or a nonhealing lower-extremity wound that has been present for at least 2 weeks should be referred for revascularization on an urgent basis, given the risk of impending limb loss associated with critical limb ischemia.
A detailed review of the medical, endovascular, and surgical management of peripheral artery disease can be found in a supplement to the Cleveland Clinic Journal of Medicine published in 200633 and in comprehensive multi-society guidelines.26,29
THE ANKLE-BRACHIAL INDEX AS A MARKER OF RISK
Low values: Peripheral artery disease
Peripheral artery disease, as diagnosed by a low ankle-brachial index, confers an excess risk of death from all causes in a graded fashion: ie, the more severe the disease, the lower the survival rate (Figure 2).34 Because peripheral artery disease is a sign of systemic atherosclerosis and one-third to one-half of patients with peripheral artery disease have evidence of cerebrovascular or coronary artery disease,35–37 peripheral artery disease also confers a higher risk of cardiovascular death.
The Edinburgh Artery Study,38 a prospective cohort study of 1,592 randomly selected patients age 55 to 74 years, demonstrated the relationship between a low ankle-brachial index and an increased risk of cardiovascular death. Over 5 years of follow-up, compared with patients with a normal ankle-brachial index, the relative risk of cardiovascular death in symptomatic patients with a value of 0.9 or lower was 2.67 (95% confidence interval [CI] 1.34–5.29). The relative risk in patients with asymptomatic disease was between 1.74 (95% CI 1.09–2.76) and 2.08 (95% CI 1.13–3.83), depending on the level of ankle-brachial index decrement and ankle blood pressure response to hyperemia.
(Reactive hyperemia is an alternative to exercise testing. It is performed by inflating a blood pressure cuff at the thigh above the systolic pressure for 3 to 5 minutes or until the patient can no longer tolerate the inflation. Blood pressures at the ankle are remeasured after cuff release.)
Several other epidemiologic studies have established the association between low ankle-brachial index and the risk of cardiovascular death.
Heald et al39 performed a meta-analysis of 44,590 patients in 11 epidemiologic studies and found that, after adjustment for age, sex, conventional cardiovascular risk factors, and prevalent cardiovascular disease, an ankle-brachial index lower than 0.9 conferred a higher risk of:
- All-cause mortality (pooled risk ratio [RR] 1.60, 95% CI 1.32–1.95)
- Cardiovascular mortality (pooled RR 1.96, 95% CI 1.46–2.64)
- Coronary heart disease (pooled RR 1.45, 95% CI 1.08–1.93)
- Stroke (pooled RR 1.35, 95% CI 1.10–1.65).
Fowkes et al,40 in a meta-analysis of 16 population cohort studies including 48,294 patients over 480,325 person-years of follow-up, found that a low ankle-brachial index predicted cardiovascular events and death even after adjusting for the Framingham risk score, Hazard ratios for cardiovascular death were:
- 2.92 (95% CI 2.31–3.70) in men
- 2.97 (95% CI 2.02–4.35) in women.
Hazard ratios for death from any cause were:
- 2.34 (95% CI 1.97–2.78) in men
- 2.35 (95% CI 1.76–3.13) in women.
Adding the ankle-brachial index to the Framingham risk score resulted in reclassification of risk category in approximately 19% of men and 36% of women.40
The German Epidemiological Trial on Ankle Brachial Index (getABI) screened 6,880 patients 65 years of age and found an abnormal ankle-brachial index in 20.9% of them.41 In more than 5 years of follow-up, a value of less than 0.90 was associated with a higher rate of cardiovascular events and death from any cause in patients with both symptomatic and asymptomatic peripheral artery disease (Figure 3).41
In addition, the lower the ankle-brachial index, the greater the rate of death or severe cardiovascular events. An index between 0.7 and 0.9 was associated with a statistically significant twofold increase (adjusted hazard ratio 2.03), and a value lower than 0.5 was associated with a nearly fivefold increase (hazard ratio 4.65) in the risk of events compared with the group of patients with normal values.41
Abnormal results after exercise
Exercise testing may increase the sensitivity of the ankle-brachial index to detect peripheral artery disease in patients with normal resting values and especially in patients with borderline values. As such, abnormal exercise values have also been associated with an increased risk of death due to any cause and of cardiovascular death.
In a prospective cohort study of 3,209 patients with suspected or known peripheral artery disease referred to a vascular surgery clinic in the Netherlands, patients with lower postexercise values had a higher rate of overall and cardiac death (hazard ratio per 10% lower value 1.16 [95% CI 1.13–1.18] and 1.10 [95% CI 1.09–1.13], respectively).42
Sheikh et al43 reported similar findings in patients with normal resting ankle-brachial indices at Cleveland Clinic. In this study, an abnormal postexercise ankle-brachial index (defined as < 0.85) was associated with a hazard ratio of 1.67 for all-cause mortality compared with a normal postexercise value among individuals with no history of cardiovascular events.
High values: Noncompressible vessels
While the relationship between low values and increased mortality and cardiovascular risk is well accepted, there have been conflicting reports regarding high values (> 1.4) and adverse outcomes.44,45
The Strong Heart Study44 was a population-based study in 4,393 Native Americans followed for more than 8 years for the rate of all-cause and cardiovascular mortality. Most (n = 3,773) of the cohort had a normal ankle-brachial index (≥ 0.9 and ≤ 1.4); 4.9% (n = 216) had a low value (< 0.9); and 9.2% (n = 404) had a high value (> 1.4 or noncompressible). Relative risk ratios for all-cause mortality were 1.69 (95% CI 1.34–2.14) for low values and 1.77 (95% CI 1.48–2.13) for high values compared with those with normal values. Low and high ankle-brachial indices also conferred a risk of cardiovascular death, with relative risk ratios of 2.52 (95% CI 1.74–3.64) and 2.09 (95% CI 1.49–2.94), respectively. There was a U-shaped relationship between the ankle-brachial index and the mortality rate (Figure 4).44
The Atherosclerosis Risk in Communities (ARIC) study45 had different findings. In 14,777 participants followed for a mean of 12.2 years, the cardiovascular disease event rates of patients whose ankle-brachial index-was categorized as high (> 1.3, > 1.4, or > 1.5) were similar to those of patients with a normal value (between 0.9 and 1.3).
Differences in event rates between the two studies may be due to a higher prevalence of values greater than 1.4 in the Strong Heart Study cohort as well as to a higher prevalence of concomitant risk factors (diabetes, older age, hypertension, lipid abnormality) in the high ankle-brachial index group in the Strong Heart Study compared with the ARIC study.
DIFFERING RECOMMENDATIONS
The ankle-brachial index can be used to screen for asymptomatic peripheral artery disease. It can also be used to confirm the diagnosis in patients with symptoms such as intermittent claudication, ischemic pain at rest, or lower extremity ulcers or in patients with signs such as abnormal pulses, bruits, or lower-extremity skin changes. It is also used to reassess the severity of known peripheral artery disease and as a part of a routine surveillance program to assess the patency of bypass grafts and endovascular stents after revascularization procedures.
The complication of peripheral artery disease that patients dread the most is limb loss, but of greater clinical consequence are the alarming rates of cardiovascular events and death in these patients. Epidemiologic studies have shown that fewer than 5% of patients age 55 or older with claudication or asymptomatic peripheral artery disease experience major amputation over a 5-year follow-up period, but 20% of these patients will have a stroke or myocardial infarction and 15% to 30% will die. Of those who die, 75% die of a coronary or cerebrovascular cause.36 Because of the markedly increased risk of death or cardiovascular morbidity in patients with peripheral artery disease, many have advocated screening patients at high risk using the ankle-brachial index. However, there have been conflicting recommendations from national societies and agencies.29,46–48
The United States Preventive Services Task Force (USPSTF) updated its 1996 recommendations on screening for peripheral artery disease in 2005 and recommended against routinely screening for it, giving the practice a “D” recommendation (not recommended). Specifically, it stated that it found “fair evidence that screening asymptomatic adults with the ankle brachial index could lead to some small degree of harm, including false-positive results and unnecessary work-ups,”46 and concluded that “for asymptomatic adults, harms of routine screening for [peripheral artery disease] exceed benefits.”46
This negative recommendation was intensely debated among vascular specialty groups, and a rebuttal was published in 2006.49 The major area of contention was the task force’s assumption that decreased disease-specific morbidity (especially limb loss) is the most important outcome to be prevented by screening for asymptomatic peripheral artery disease, rather than adverse cardiovascular events. The USPSTF has announced plans for an update on screening for peripheral artery disease, anticipated for 2013.50
The American College of Cardiology/American Heart Association task force in 2005 recommended that a history of walking impairment, intermittent claudication, ischemic rest pain, or nonhealing wounds be solicited as part of a standard review of systems for adults age 70 and older or adults age 50 and older who have risk factors for atherosclerosis (class IC recommendation—based only on a consensus opinion of experts, case studies, or standard of care).29 In contrast to the USPSTF recommendations, the joint guidelines further recommended that patients with asymptomatic lower-extremity peripheral artery disease be identified by physical examination, ankle-brachial index, or both, to prevent myocardial infarction, stroke, or death (class IC).29 Patients at risk for lower-extremity peripheral artery disease for whom ankle-brachial index measurement is recommended include those with exertional leg symptoms, those with nonhealing ulcers, those age 70 and older, and those age 50 and older who have a history of moking or diabetes.
The American Diabetes Association and the second Inter-Society Consensus for the Management of Peripheral Arterial Disease (TASC II) issued similar recommendations.48
In 2011, the American College of Cardiology/American Heart Association task force issued a focused update to its 2005 guidelines, broadening the recommendation for testing to include patients age 65 and older on the basis of the getABI study, as well as maintaining the recommendation for testing for those age 50 and older with a history of smoking or diabetes (class IB recommendation).26,41
The task force’s Guideline for the Assessment of Cardiovascular Risk in Asymptomatic Adults says that measuring the ankle-brachial index is reasonable for cardiovascular risk assessment in asymptomatic adults at intermediate risk (class IIA—conflicting evidence or divergence of opinion, from multiple randomized clinical trials).51 Also recommended as risk stratification tools for this patient population are measurement of carotid intima-media thickness and measurement of coronary artery calcium (both class IIA recommendations).
Unlike these tests, however, the ankle-brachial index does not require highly trained technical and medical personnel to perform and interpret. In addition, there is no risk of radiation exposure as is the case in coronary calcium measurement. It is a simpler, lower-cost, and more widely available tool for cardiovascular risk assessment.
LIMITATIONS TO ANKLE-BRACHIAL SCREENING IN PRACTICE
Although this test is relatively simple and noninvasive, it is not widely performed in primary care and cardiovascular medicine. In a study by Mohler and colleagues,52 the most common barriers to its use among primary care providers were the time required to perform it, lack of reimbursement for it, and limited staff availability. Currently, third-party payers do not generally reimburse for an ankle-brachial index examination performed to screen a patient who is asymptomatic but at risk for peripheral artery disease. Unfortunately, this has limited the widespread adoption of a program to detect peripheral artery disease in patients at risk.
Despite these limitations, the ankle-brachial index is an invaluable tool to both screen for peripheral artery disease in the appropriate at-risk patient populations and to diagnose it in patients who present with lower extremity symptoms. There are few diagnostic tests available today that provide such a high degree of diagnostic accuracy with as much prognostic information as the ankle-brachial index and with such little expense and risk to the patient.
In this article, we seek to convince you to measure the ankle-brachial index for any patient you suspect may have peripheral artery disease. This would include patients who are elderly, who smoke, or who have diabetes, regardless of whether or not they have symptoms. The ankle-brachial index is a simple test that involves taking the blood pressure in all four limbs using a hand-held Doppler device and then dividing the leg systolic pressure by the arm systolic pressure.
This simple test is both sensitive and specific for peripheral artery disease. It also gives a good assessment of cardiovascular risk. The downside: you or a member of your staff spends a few minutes doing it. Also, for patients without leg symptoms or abnormal findings on physical examination, you may not be paid for doing it.
Despite these limitations, the ankle-brachial index is a powerful clinical tool that deserves to be performed more often in primary care.
PERIPHERAL ARTERY DISEASE IS COMMON AND SERIOUS
Peripheral artery disease is important to detect, as it is common, it has serious consequences, and effective treatments are available. However, many patients with the disease do not have typical symptoms.
Peripheral artery disease affects up to 29% of people over age 70, depending on the population sampled.1,2 Its classic symptom is intermittent claudication, ie, leg pain with walking that improves with rest. However, most patients do not have intermittent claudication; they have atypical leg symptoms or no symptoms at all.2,3 While the risk factors for peripheral artery disease are similar to those for coronary artery disease, the factors most strongly associated with peripheral artery disease are older age, tobacco smoking, and diabetes mellitus.4 Blacks are twice as likely to have it compared with whites, even after adjusting for other cardiovascular risk factors.5
Untreated peripheral artery disease may have serious consequences, such as amputation, impaired functional capacity, poor quality of life, and depression.3,6,7 In addition, it is a strong marker of atherosclerotic burden and cardiovascular risk and has been recognized as a coronary risk equivalent. Patients with peripheral artery disease are at higher risk of death, myocardial infarction, stroke, and hospitalization, with event rates as high as 21% per year.8
Fortunately, simple therapies have been shown to prevent adverse cardiovascular events in peripheral artery disease, including antiplatelet drugs, statins, and angiotensin-converting enzyme inhibitors.9–11
THE ANKLE-BRACHIAL INDEX IS SENSITIVE AND SPECIFIC
The evaluation for possible peripheral artery disease should begin with the medical history, a cardiovascular review of systems, and a focused physical examination in which one should:
- Measure the blood pressure in both arms to assess for occult subclavian stenosis
- Auscultate for bruits over the carotid, abdominal, and femoral arteries
- Palpate the pulses in the lower extremities and the abdominal aorta
- Inspect the bare legs and feet for thinning of the skin, hair loss, thickening of the nails (which are nonspecific signs), and ulceration.
However, the physical examination has limited sensitivity and specificity for diagnosing peripheral artery disease. In general, the most reliable finding is the absence of a palpable posterior tibial artery pulse, which has been reported to have a specificity of 71% and a sensitivity of 91% for peripheral artery disease.12
The ankle-brachial index is the first-line test for both screening for peripheral artery disease and for diagnosing it. It is inexpensive and noninvasive to obtain and has a high sensitivity (79% to 95%) and specificity (95% to 96%) compared with angiography as the gold standard.13–18 It can be measured easily in the office, and every practitioner who cares for patients at risk of cardiovascular disease can be trained to measure it competently.
HOW TO MEASURE THE ANKLE-BRACHIAL INDEX
The ankle-brachial index is the ratio of the systolic pressure in the ankle to the systolic pressure in the arm. In healthy people, this ratio is typically greater than 1.0 or 1.1.
You can measure the ankle-brachial index in the office with a blood pressure cuff, sphygmomanometer, and handheld Doppler device. Alternatively, it can be measured in a noninvasive vascular laboratory as part of a more detailed examination that allows for assessment of blood pressures and waveforms (Doppler or pulse-volume recordings) at multiple segments along the limb. These more detailed vascular studies are generally reserved for patients with confirmed peripheral artery disease to locate the level and extent of blockage or for patients in whom lower-extremity revascularization is contemplated.
The use of a stethoscope to measure blood pressures for the ankle-brachial index has been studied in a few small series,19,20 but is thought to be less accurate than Doppler, especially in the setting of significant arterial occlusive disease. Because of this, it is recommended and assumed that a Doppler device be used to measure all blood pressures for the ankle-brachial index.
After the patient has been resting quietly for 5 to 10 minutes in the supine position, the systolic blood pressure is measured in both arms and in both ankles in the dorsalis pedis and posterior tibial arteries (Figure 1). The blood pressure cuff is placed about 1 inch above the antecubital fossa for the brachial pressure and about 2 inches above the medial malleolus for the ankle pressures. A clear arterial pulse signal should be heard using the Doppler probe before inflating the blood pressure cuff. The cuff is then inflated to at least 20 mm Hg above the point where the arterial Doppler sounds disappear and then slowly deflated until the Doppler sounds reappear. The blood pressure at which the Doppler signal of the arterial pulse reappears is the systolic pressure for that vessel.
The ankle-brachial index is calculated by dividing the higher of the two ankle systolic blood pressures in each leg by the higher of the two brachial systolic blood pressures. The higher of the two brachial pressures is used as the denominator to account for the possibility of subclavian artery stenosis, which can decrease the blood pressure in the upper extremity. The ankle-brachial index is calculated for each leg, and the lower value is the patient’s overall ankle-brachial index. An abnormal value in either leg indicates peripheral artery disease.
While other ways of calculating the ankle-brachial index have been proposed, such as averaging the two pressures at each ankle or reporting the lower of the two ankle pressures, these methods are not standard for use in clinical practice.
Similarly, the use of oscillometric blood pressure devices has been proposed, which would eliminate the need for a Doppler device and personnel trained in its use. However, results of validation studies of oscillometric measurement of the ankle-brachial index have been inconsistent, likely because the devices were designed for measuring blood pressure in nonobstructed arms, not the legs, and especially not diseased legs.21–25 In general, oscillometric devices tend to overestimate ankle pressure, giving a falsely high ankle-brachial index in patients with moderate to severe peripheral artery disease.21 Their utility in screening for peripheral artery disease has not been evaluated in broad, population-based studies. Efforts to develop and validate new oscillometric devices for diagnosing peripheral artery disease are ongoing.
INTERPRETING THE ANKLE-BRACHIAL INDEX
Diagnostic criteria for the ankle-brachial index were standardized in 2011 (Table 1).26 Most healthy adults have a value greater than 1.0. A value of less than 0.91 is consistent with significant peripheral artery disease, and a value lower than 0.40 at rest generally indicates severe disease. A value between 0.91 and 0.99 is borderline abnormal and does not rule out peripheral artery disease. A value greater than 1.40 reflects noncompressibility of the leg arteries and is not diagnostic (see below).
The ankle-brachial index after exercise. In patients strongly suspected of having peripheral artery disease but who have a normal ankle-brachial index at rest, and especially if the resting value is borderline (ie, 0.91–0.99), the measurement should be repeated after exercise, the better to detect “mild” peripheral artery disease.15 With exercise, increased flow across a fixed stenosis leads to a significant fall in ankle pressure and a lower ankle-brachial index. In one study,27 the ankle-brachial index fell below 0.9 after exercise in 31% of outpatients with symptoms who had initially tested normal.
The exercise is optimally done on a motorized treadmill set at an incline. A number of exercise protocols are in use; at our institution, we use a fixed workload protocol. The ankle-brachial index and ankle pulse-volume recordings are recorded on both sides at rest, after which the patient generally walks for 5 minutes at a 12% grade at 2.0 mph or until symptoms force the patient to stop. The advantage of treadmill testing is the ability to assess functional capacity by measuring the time to the onset of pain and the total walking time.
Alternatively, active pedal plantar flexion maneuvers (heel raises) or corridor walking to the point at which limiting symptoms occur can be done if a treadmill is not available, though this is not the favored approach and does not qualify as formal exercise testing for reimbursement purposes. The patient is asked to do heel raises as high and as fast as possible for 30 seconds or until limiting pain symptoms occur. Results with this maneuver have been shown to correlate well with those of treadmill exercise testing.28
Immediately after any exercise maneuver, arm and ankle pressures are remeasured and bilateral ankle-brachial indices are recalculated. A fall in ankle pressure or the ankle-brachial index after exercise (generally, a fall of more than 20%) supports the diagnosis of peripheral artery disease. If the patient develops leg symptoms during exercise while his or her ankle-brachial index falls significantly, this also supports the vasculogenic nature of the leg symptoms.
An ankle-brachial index greater than 1.40 means that the pedal arteries are stiff and cannot be compressed by the blood pressure cuff. This is considered abnormal, though not necessarily diagnostic of peripheral artery disease. Noncompressible leg arteries are common among patients with long-standing diabetes mellitus or end-stage renal disease, and also can be found in obese patients.
Because toe arteries are usually compressible even when the pedal arteries are not, a toe-brachial index can be obtained to confirm the diagnosis of peripheral artery disease in these cases. This is calculated by measuring the blood pressure in the great toe using a small digital blood pressure cuff and a Doppler probe or a plethysmographic flow sensor. The toe-brachial index is calculated by dividing the toe blood pressure by the higher of the two brachial artery pressures; a value of 0.7 or less generally indicates peripheral artery disease.
WHAT SHOULD BE DONE WITH AN ABNORMAL RESULT?
An abnormal ankle-brachial index establishes the diagnosis of peripheral artery disease, and in many cases no additional diagnostic testing is necessary.
Care of patients with peripheral artery disease has three elements:
- Cardiovascular risk factor assessment and reduction to prevent myocardial infarction, stroke, and death
- Assessment and treatment of leg symptoms to improve function and quality of life
- Foot care to prevent ulcers and amputation.
Risk factor reduction. Because they have a markedly greater risk of cardiovascular disease and death, all patients with peripheral artery disease should undergo aggressive cardiovascular risk factor modification,26,29 including:
- Antiplatelet therapy in the form of aspirin 75–325 mg daily or clopidogrel 75 mg daily as an alternative to aspirin
- Counseling and therapy for immediate smoking cessation if the patient smokes
- Treatment of hypertension to Seventh Joint National Committee goals30
- Treatment of lipids to Adult Treatment Panel III goals31 (generally to a goal low-density lipoprotein cholesterol of less than 100 mg/dL, and less than 70 mg/dL if possible)
- Treatment of diabetes to a goal hemoglobin A1c of less than 7% (in the absence of contraindications).32
Exercise and anticlaudication medication. Patients with an abnormal ankle-brachial index and intermittent claudication may benefit from a supervised exercise program, a trial of drug therapy for claudication, or both. All patients with peripheral artery disease, regardless of symptoms, should be advised to incorporate aerobic exercise (ideally, walking) into their daily routine.
Cilostazol (Pletal), a phosphodiesterase inhibitor, has been given a class IA recommendation in the American College of Cardiology/American Heart Association guidelines for the treatment of intermittent claudication. The dose is generally 100 mg by mouth twice daily.29
Revascularization. Patients with an abnormal ankle-brachial index and lifestyle-limiting claudication that has failed to improve with medical therapy or a course of supervised exercise training should be referred to a vascular specialist for evaluation for revascularization (endovascular therapy or surgical bypass). 29 Endovascular therapy is particularly attractive for patients with claudication and evidence of aortoiliac disease (suspected in patients with gluteal or thigh claudication, diminution of the femoral pulse, or a bruit over the femoral artery on examination and confirmed by noninvasive vascular laboratory testing).
Patients who have ischemic pain at rest, gangrene, or a nonhealing lower-extremity wound that has been present for at least 2 weeks should be referred for revascularization on an urgent basis, given the risk of impending limb loss associated with critical limb ischemia.
A detailed review of the medical, endovascular, and surgical management of peripheral artery disease can be found in a supplement to the Cleveland Clinic Journal of Medicine published in 200633 and in comprehensive multi-society guidelines.26,29
THE ANKLE-BRACHIAL INDEX AS A MARKER OF RISK
Low values: Peripheral artery disease
Peripheral artery disease, as diagnosed by a low ankle-brachial index, confers an excess risk of death from all causes in a graded fashion: ie, the more severe the disease, the lower the survival rate (Figure 2).34 Because peripheral artery disease is a sign of systemic atherosclerosis and one-third to one-half of patients with peripheral artery disease have evidence of cerebrovascular or coronary artery disease,35–37 peripheral artery disease also confers a higher risk of cardiovascular death.
The Edinburgh Artery Study,38 a prospective cohort study of 1,592 randomly selected patients age 55 to 74 years, demonstrated the relationship between a low ankle-brachial index and an increased risk of cardiovascular death. Over 5 years of follow-up, compared with patients with a normal ankle-brachial index, the relative risk of cardiovascular death in symptomatic patients with a value of 0.9 or lower was 2.67 (95% confidence interval [CI] 1.34–5.29). The relative risk in patients with asymptomatic disease was between 1.74 (95% CI 1.09–2.76) and 2.08 (95% CI 1.13–3.83), depending on the level of ankle-brachial index decrement and ankle blood pressure response to hyperemia.
(Reactive hyperemia is an alternative to exercise testing. It is performed by inflating a blood pressure cuff at the thigh above the systolic pressure for 3 to 5 minutes or until the patient can no longer tolerate the inflation. Blood pressures at the ankle are remeasured after cuff release.)
Several other epidemiologic studies have established the association between low ankle-brachial index and the risk of cardiovascular death.
Heald et al39 performed a meta-analysis of 44,590 patients in 11 epidemiologic studies and found that, after adjustment for age, sex, conventional cardiovascular risk factors, and prevalent cardiovascular disease, an ankle-brachial index lower than 0.9 conferred a higher risk of:
- All-cause mortality (pooled risk ratio [RR] 1.60, 95% CI 1.32–1.95)
- Cardiovascular mortality (pooled RR 1.96, 95% CI 1.46–2.64)
- Coronary heart disease (pooled RR 1.45, 95% CI 1.08–1.93)
- Stroke (pooled RR 1.35, 95% CI 1.10–1.65).
Fowkes et al,40 in a meta-analysis of 16 population cohort studies including 48,294 patients over 480,325 person-years of follow-up, found that a low ankle-brachial index predicted cardiovascular events and death even after adjusting for the Framingham risk score, Hazard ratios for cardiovascular death were:
- 2.92 (95% CI 2.31–3.70) in men
- 2.97 (95% CI 2.02–4.35) in women.
Hazard ratios for death from any cause were:
- 2.34 (95% CI 1.97–2.78) in men
- 2.35 (95% CI 1.76–3.13) in women.
Adding the ankle-brachial index to the Framingham risk score resulted in reclassification of risk category in approximately 19% of men and 36% of women.40
The German Epidemiological Trial on Ankle Brachial Index (getABI) screened 6,880 patients 65 years of age and found an abnormal ankle-brachial index in 20.9% of them.41 In more than 5 years of follow-up, a value of less than 0.90 was associated with a higher rate of cardiovascular events and death from any cause in patients with both symptomatic and asymptomatic peripheral artery disease (Figure 3).41
In addition, the lower the ankle-brachial index, the greater the rate of death or severe cardiovascular events. An index between 0.7 and 0.9 was associated with a statistically significant twofold increase (adjusted hazard ratio 2.03), and a value lower than 0.5 was associated with a nearly fivefold increase (hazard ratio 4.65) in the risk of events compared with the group of patients with normal values.41
Abnormal results after exercise
Exercise testing may increase the sensitivity of the ankle-brachial index to detect peripheral artery disease in patients with normal resting values and especially in patients with borderline values. As such, abnormal exercise values have also been associated with an increased risk of death due to any cause and of cardiovascular death.
In a prospective cohort study of 3,209 patients with suspected or known peripheral artery disease referred to a vascular surgery clinic in the Netherlands, patients with lower postexercise values had a higher rate of overall and cardiac death (hazard ratio per 10% lower value 1.16 [95% CI 1.13–1.18] and 1.10 [95% CI 1.09–1.13], respectively).42
Sheikh et al43 reported similar findings in patients with normal resting ankle-brachial indices at Cleveland Clinic. In this study, an abnormal postexercise ankle-brachial index (defined as < 0.85) was associated with a hazard ratio of 1.67 for all-cause mortality compared with a normal postexercise value among individuals with no history of cardiovascular events.
High values: Noncompressible vessels
While the relationship between low values and increased mortality and cardiovascular risk is well accepted, there have been conflicting reports regarding high values (> 1.4) and adverse outcomes.44,45
The Strong Heart Study44 was a population-based study in 4,393 Native Americans followed for more than 8 years for the rate of all-cause and cardiovascular mortality. Most (n = 3,773) of the cohort had a normal ankle-brachial index (≥ 0.9 and ≤ 1.4); 4.9% (n = 216) had a low value (< 0.9); and 9.2% (n = 404) had a high value (> 1.4 or noncompressible). Relative risk ratios for all-cause mortality were 1.69 (95% CI 1.34–2.14) for low values and 1.77 (95% CI 1.48–2.13) for high values compared with those with normal values. Low and high ankle-brachial indices also conferred a risk of cardiovascular death, with relative risk ratios of 2.52 (95% CI 1.74–3.64) and 2.09 (95% CI 1.49–2.94), respectively. There was a U-shaped relationship between the ankle-brachial index and the mortality rate (Figure 4).44
The Atherosclerosis Risk in Communities (ARIC) study45 had different findings. In 14,777 participants followed for a mean of 12.2 years, the cardiovascular disease event rates of patients whose ankle-brachial index-was categorized as high (> 1.3, > 1.4, or > 1.5) were similar to those of patients with a normal value (between 0.9 and 1.3).
Differences in event rates between the two studies may be due to a higher prevalence of values greater than 1.4 in the Strong Heart Study cohort as well as to a higher prevalence of concomitant risk factors (diabetes, older age, hypertension, lipid abnormality) in the high ankle-brachial index group in the Strong Heart Study compared with the ARIC study.
DIFFERING RECOMMENDATIONS
The ankle-brachial index can be used to screen for asymptomatic peripheral artery disease. It can also be used to confirm the diagnosis in patients with symptoms such as intermittent claudication, ischemic pain at rest, or lower extremity ulcers or in patients with signs such as abnormal pulses, bruits, or lower-extremity skin changes. It is also used to reassess the severity of known peripheral artery disease and as a part of a routine surveillance program to assess the patency of bypass grafts and endovascular stents after revascularization procedures.
The complication of peripheral artery disease that patients dread the most is limb loss, but of greater clinical consequence are the alarming rates of cardiovascular events and death in these patients. Epidemiologic studies have shown that fewer than 5% of patients age 55 or older with claudication or asymptomatic peripheral artery disease experience major amputation over a 5-year follow-up period, but 20% of these patients will have a stroke or myocardial infarction and 15% to 30% will die. Of those who die, 75% die of a coronary or cerebrovascular cause.36 Because of the markedly increased risk of death or cardiovascular morbidity in patients with peripheral artery disease, many have advocated screening patients at high risk using the ankle-brachial index. However, there have been conflicting recommendations from national societies and agencies.29,46–48
The United States Preventive Services Task Force (USPSTF) updated its 1996 recommendations on screening for peripheral artery disease in 2005 and recommended against routinely screening for it, giving the practice a “D” recommendation (not recommended). Specifically, it stated that it found “fair evidence that screening asymptomatic adults with the ankle brachial index could lead to some small degree of harm, including false-positive results and unnecessary work-ups,”46 and concluded that “for asymptomatic adults, harms of routine screening for [peripheral artery disease] exceed benefits.”46
This negative recommendation was intensely debated among vascular specialty groups, and a rebuttal was published in 2006.49 The major area of contention was the task force’s assumption that decreased disease-specific morbidity (especially limb loss) is the most important outcome to be prevented by screening for asymptomatic peripheral artery disease, rather than adverse cardiovascular events. The USPSTF has announced plans for an update on screening for peripheral artery disease, anticipated for 2013.50
The American College of Cardiology/American Heart Association task force in 2005 recommended that a history of walking impairment, intermittent claudication, ischemic rest pain, or nonhealing wounds be solicited as part of a standard review of systems for adults age 70 and older or adults age 50 and older who have risk factors for atherosclerosis (class IC recommendation—based only on a consensus opinion of experts, case studies, or standard of care).29 In contrast to the USPSTF recommendations, the joint guidelines further recommended that patients with asymptomatic lower-extremity peripheral artery disease be identified by physical examination, ankle-brachial index, or both, to prevent myocardial infarction, stroke, or death (class IC).29 Patients at risk for lower-extremity peripheral artery disease for whom ankle-brachial index measurement is recommended include those with exertional leg symptoms, those with nonhealing ulcers, those age 70 and older, and those age 50 and older who have a history of moking or diabetes.
The American Diabetes Association and the second Inter-Society Consensus for the Management of Peripheral Arterial Disease (TASC II) issued similar recommendations.48
In 2011, the American College of Cardiology/American Heart Association task force issued a focused update to its 2005 guidelines, broadening the recommendation for testing to include patients age 65 and older on the basis of the getABI study, as well as maintaining the recommendation for testing for those age 50 and older with a history of smoking or diabetes (class IB recommendation).26,41
The task force’s Guideline for the Assessment of Cardiovascular Risk in Asymptomatic Adults says that measuring the ankle-brachial index is reasonable for cardiovascular risk assessment in asymptomatic adults at intermediate risk (class IIA—conflicting evidence or divergence of opinion, from multiple randomized clinical trials).51 Also recommended as risk stratification tools for this patient population are measurement of carotid intima-media thickness and measurement of coronary artery calcium (both class IIA recommendations).
Unlike these tests, however, the ankle-brachial index does not require highly trained technical and medical personnel to perform and interpret. In addition, there is no risk of radiation exposure as is the case in coronary calcium measurement. It is a simpler, lower-cost, and more widely available tool for cardiovascular risk assessment.
LIMITATIONS TO ANKLE-BRACHIAL SCREENING IN PRACTICE
Although this test is relatively simple and noninvasive, it is not widely performed in primary care and cardiovascular medicine. In a study by Mohler and colleagues,52 the most common barriers to its use among primary care providers were the time required to perform it, lack of reimbursement for it, and limited staff availability. Currently, third-party payers do not generally reimburse for an ankle-brachial index examination performed to screen a patient who is asymptomatic but at risk for peripheral artery disease. Unfortunately, this has limited the widespread adoption of a program to detect peripheral artery disease in patients at risk.
Despite these limitations, the ankle-brachial index is an invaluable tool to both screen for peripheral artery disease in the appropriate at-risk patient populations and to diagnose it in patients who present with lower extremity symptoms. There are few diagnostic tests available today that provide such a high degree of diagnostic accuracy with as much prognostic information as the ankle-brachial index and with such little expense and risk to the patient.
- Selvin E, Erlinger TP. Prevalence of and risk factors for peripheral arterial disease in the United States: results from the National Health and Nutrition Examination Survey, 1999–2000. Circulation 2004; 110:738–743.
- Hirsch AT, Criqui MH, Treat-Jacobson D, et al. Peripheral arterial disease detection, awareness, and treatment in primary care. JAMA 2001; 286:1317–1324.
- McDermott MM, Greenland P, Liu K, et al. Leg symptoms in peripheral arterial disease: associated clinical characteristics and functional impairment. JAMA 2001; 286:1599–1606.
- McDermott MM. The magnitude of the problem of peripheral arterial disease: epidemiology and clinical significance. Cleve Clin J Med 2006; 73(suppl 4):S2–S7.
- Criqui MH, Vargas V, Denenberg JO, et al. Ethnicity and peripheral arterial disease: the San Diego Population Study. Circulation 2005; 112:2703–2707.
- Regensteiner JG, Hiatt WR, Coll JR, et al The impact of peripheral arterial disease on health-related quality of life in the Peripheral Arterial Disease Awareness, Risk, and Treatment: New Resources for Survival (PARTNERS) Program. Vasc Med 2008; 13:15–24.
- Arseven A, Guralnik JM, O’Brien E, Liu K, McDermott MM. Peripheral arterial disease and depressed mood in older men and women. Vasc Med 2001; 6:229–234.
- Steg PG, Bhatt DL, Wilson PW, et al; REACH Registry Investigators. One-year cardiovascular event rates in outpatients with atherothrombosis. JAMA 2007; 297:1197–1206.
- Gornik HL, Creager MA. Contemporary management of peripheral arterial disease: I. cardiovascular risk-factor modification. Cleve Clin J Med 2006; 73(suppl 4):S30–S37.
- Antithrombotic Trialists’ Collaboration. Collaborative meta-analysis of randomised trials of antiplatelet therapy for prevention of death, myocardial infarction, and stroke in high risk patients. BMJ 2002; 324:71–86.
- Heart Protection Study Collaborative Group. Randomized trial of the effects of cholesterol-lowering with simvastatin on peripheral vascular and other major vascular outcomes in 20,536 people with peripheral arterial disease and other high-risk conditions. J Vasc Surg 2007; 45:645–654.
- Criqui MH, Fronek A, Klauber MR, Barrett-Connor E, Gabriel S. The sensitivity, specificity, and predictive value of traditional clinical evaluation of peripheral arterial disease: results from noninvasive testing in a defined population. Circulation 1985; 71:516–522.
- Carter SA. Clinical measurement of systolic pressures in limbs with arterial occlusive disease. JAMA 1969; 207:1869–1874.
- Ouriel K, Zarins CK. Doppler ankle pressure: an evaluation of three methods of expression. Arch Surg 1982; 117:1297–1300.
- Yao ST, Hobbs JT, Irvine WT. Ankle systolic pressure measurements in arterial disease affecting the lower extremities. Br J Surg 1969; 56:676–679.
- Carter SA. Indirect systolic pressures and pulse waves in arterial occlusive diseases of the lower extremities. Circulation 1968; 37:624–637.
- Belch JJ, Topol EJ, Agnelli G, et al; Prevention of Atherothrombotic Disease Network. Critical issues in peripheral arterial disease detection and management: a call to action. Arch Intern Med 2003; 163:884–892.
- Lijmer JG, Hunink MG, van den Dungen JJ, Loonstra J, Smit AJ. ROC analysis of noninvasive tests for peripheral arterial disease. Ultrasound Med Biol 1996; 22:391–398.
- Carmo GA, Mandil A, Nascimento BR, et al. Can we measure the ankle-brachial index using only a stethoscope? A pilot study. Fam Pract 2009; 26:22–26.
- Chesbro SB, Asongwed ET, Brown J, John EB. Reliability of Doppler and stethoscope methods of determining systolic blood pressures: considerations for calculating an ankle-brachial index. J Natl Med Assoc 2011; 103:863–869.
- Jönsson B, Lindberg LG, Skau T, Thulesius O. Is oscillometric ankle pressure reliable in leg vascular disease? Clin Physiol 2001; 21:155–163.
- Ramanathan A, Conaghan PJ, Jenkinson AD, Bishop CR. Comparison of ankle-brachial pressure index measurements using an automated oscillometric device with the standard Doppler ultrasound technique. ANZ J Surg 2003; 73:105–108.
- Beckman JA, Higgins CO, Gerhard-Herman M. Automated oscillometric determination of the ankle-brachial index provides accuracy necessary for office practice. Hypertension 2006; 47:35–38.
- Mehlsen J, Wiinberg N, Bruce C. Oscillometric blood pressure measurement: a simple method in screening for peripheral arterial disease. Clin Physiol Funct Imaging 2008; 28:426–429.
- Aboyans V, Lacroix P, Doucet S, Preux PM, Criqui MH, Laskar M. Diagnosis of peripheral arterial disease in general practice: can the ankle-brachial index be measured either by pulse palpation or an automatic blood pressure device? Int J Clin Pract 2008; 62:1001–1007.
- 2011 Writing Group Members. 2011 ACCF/AHA Focused Update of the Guideline for the Management of patients with peripheral artery disease (updating the 2005 guideline): a report of the American College of Cardiology Foundation/American Heart Association Task Force on practice guidelines. Circulation 2011; 124:2020–2045.
- Stein R, Hriljac I, Halperin JL, Gustavson SM, Teodorescu V, Olin JW. Limitation of the resting ankle-brachial index in symptomatic patients with peripheral arterial disease. Vasc Med 2006; 11:29–33.
- Amirhamzeh MM, Chant HJ, Rees JL, Hands LJ, Powell RJ, Campbell WB. A comparative study of treadmill tests and heel raising exercise for peripheral arterial disease. Eur J Vasc Endovasc Surg 1997; 13:301–305.
- Hirsch AT, Haskal ZJ, Hertzer NR, et al; American Association for Vascular Surgery; Society for Vascular Surgery; Society for Cardiovascular Angiography and Interventions; Society for Vascular Medicine and Biology; Society of Interventional Radiology; ACC/AHA Task Force on Practice Guidelines Writing Committee to Develop Guidelines for the Management of Patients With Peripheral Arterial Disease; American Association of Cardiovascular and Pulmonary Rehabilitation; National Heart, Lung, and Blood Institute; Society for Vascular Nursing; TransAtlantic Inter-Society Consensus; Vascular Disease Foundation. ACC/AHA 2005 practice guidelines for the management of patients with peripheral arterial disease (lower extremity, renal, mesenteric, and abdominal aortic): a collaborative report from the American Association for Vascular Surgery/Society for Vascular Surgery, Society for Cardiovascular Angiography and Interventions, Society for Vascular Medicine and Biology, Society of Interventional Radiology, and the ACC/AHA Task Force on Practice Guidelines (Writing Committee to Develop Guidelines for the Management of Patients With Peripheral Arterial Disease): endorsed by the American Association of Cardiovascular and Pulmonary Rehabilitation; National Heart, Lung, and Blood Institute; Society for Vascular Nursing; TransAtlantic Inter-Society Consensus; and Vascular Disease Foundation. Circulation 2006; 113:e463–e654.
- Chobanian AV, Bakris GL, Black HR, et al; Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure; National Heart, Lung, and Blood Institute; National High Blood Pressure Education Program Coordinating Committee. Seventh report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure. Hypertension 2003; 42:1206–1252.
- National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III). Third Report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III) final report. Circulation 2002; 106:3143–3421.
- Skyler JS, Bergenstal R, Bonow RO, et al; American Diabetes Association; American College of Cardiology Foundation; American Heart Association. Intensive glycemic control and the prevention of cardiovascular events: implications of the ACCORD, ADVANCE, and VA Diabetes Trials: a position statement of the American Diabetes Association and a Scientific Statement of the American College of Cardiology Foundation and the American Heart Association. J Am Coll Cardiol 2009; 53:298–304.
- Almahameed A, Bartholomew JR, editors. Peripheral arterial disease: recognition and contemporary management. Cleve Clin J Med 2006; 73(suppl 4):S1–S51.
- McKenna M, Wolfson S, Kuller L. The ratio of ankle and arm arterial pressure as an independent predictor of mortality. Atherosclerosis 1991; 87:119–128.
- Valentine RJ, Grayburn PA, Eichhorn EJ, Myers SI, Clagett GP. Coronary artery disease is highly prevalent among patients with premature peripheral vascular disease. J Vasc Surg 1994; 19:668–674.
- Weitz JI, Byrne J, Clagett GP, et al. Diagnosis and treatment of chronic arterial insufficiency of the lower extremities: a critical review. Circulation 1996; 94:3026–3049.
- Hertzer NR, Beven EG, Young JR, et al. Coronary artery disease in peripheral vascular patients. A classification of 1000 coronary angiograms and results of surgical management. Ann Surg 1984; 199:223–233.
- Leng GC, Lee AJ, Fowkes FG, et al. Incidence, natural history and cardiovascular events in symptomatic and asymptomatic peripheral arterial disease in the general population. Int J Epidemiol 1996; 25:1172–1181.
- Heald CL, Fowkes FG, Murray GD, Price JF; Ankle Brachial Index Collaboration. Risk of mortality and cardiovascular disease associated with the ankle-brachial index: Systematic review. Atherosclerosis 2006; 189:61–69.
- Ankle Brachial Index; Fowkes FG, Murray GD, Butcher I, et al. Collaboration Ankle brachial index combined with Framingham Risk Score to predict cardiovascular events and mortality: a meta-analysis. JAMA 2008; 300:197–208.
- Diehm C, Allenberg JR, Pittrow D, et al; German Epidemiological Trial on Ankle Brachial Index Study Group. Mortality and vascular morbidity in older adults with asymptomatic versus symptomatic peripheral artery disease. Circulation 2009; 120:2053–2061.
- Feringa HH, Bax JJ, van Waning VH, et al. The long-term prognostic value of the resting and postexercise ankle-brachial index. Arch Intern Med 2006; 166:529–535.
- Sheikh MA, Bhatt DL, Li J, Lin S, Bartholomew JR. Usefulness of postexercise ankle-brachial index to predict all-cause mortality. Am J Cardiol 2011; 107:778–782.
- Resnick HE, Lindsay RS, McDermott MM, et al. Relationship of high and low ankle brachial index to all-cause and cardiovascular disease mortality: the Strong Heart Study. Circulation 2004; 109:733–739.
- Wattanakit K, Folsom AR, Duprez DA, Weatherley BD, Hirsch AT. Clinical significance of a high ankle-brachial index: insights from the Atherosclerosis Risk in Communities (ARIC) Study. Atherosclerosis 2007; 190:459–464.
- US Preventive Services Task Force. Screening for peripheral arterial disease: Recommendation statement. http://www.ahrq.gov/clinic/uspstf05/pad/padrs.pdf. Accessed July 25, 2012.
- Mayfield JA, Reiber GE, Sanders LJ, Janisse D, Pogach LM; American Diabetes Association. Preventive foot care in diabetes. Diabetes Care 2004; 27(suppl 1):S63–S64.
- Norgren L, Hiatt WR, Dormandy JA, Nehler MR, Harris KA, Fowkes FG; TASC II Working Group. Inter-Society Consensus for the Management of Peripheral Arterial Disease (TASC II). J Vasc Surg 2007; 45(suppl S):S5–S67.
- Beckman JA, Jaff MR, Creager MA. The United States preventive services task force recommendation statement on screening for peripheral arterial disease: more harm than benefit? Circulation 2006; 114:861–866.
- US Preventive Services Task Force. Screening for peripheral artery disease draft research plan. http://www.uspreventiveservicestaskforce.org/uspstf12/pad/padslides.htm. Accessed July 26, 2012.
- Greenland P, Alpert JS, Beller GA, et al; American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. 2010 ACCF/AHA guideline for assessment of cardiovascular risk in asymptomatic adults: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. Circulation 2010; 122:e584–e636.
- Mohler ER, Treat-Jacobson D, Reilly MP, et al. Utility and barriers to performance of the ankle-brachial index in primary care practice. Vasc Med 2004; 9:253–260.
- Selvin E, Erlinger TP. Prevalence of and risk factors for peripheral arterial disease in the United States: results from the National Health and Nutrition Examination Survey, 1999–2000. Circulation 2004; 110:738–743.
- Hirsch AT, Criqui MH, Treat-Jacobson D, et al. Peripheral arterial disease detection, awareness, and treatment in primary care. JAMA 2001; 286:1317–1324.
- McDermott MM, Greenland P, Liu K, et al. Leg symptoms in peripheral arterial disease: associated clinical characteristics and functional impairment. JAMA 2001; 286:1599–1606.
- McDermott MM. The magnitude of the problem of peripheral arterial disease: epidemiology and clinical significance. Cleve Clin J Med 2006; 73(suppl 4):S2–S7.
- Criqui MH, Vargas V, Denenberg JO, et al. Ethnicity and peripheral arterial disease: the San Diego Population Study. Circulation 2005; 112:2703–2707.
- Regensteiner JG, Hiatt WR, Coll JR, et al The impact of peripheral arterial disease on health-related quality of life in the Peripheral Arterial Disease Awareness, Risk, and Treatment: New Resources for Survival (PARTNERS) Program. Vasc Med 2008; 13:15–24.
- Arseven A, Guralnik JM, O’Brien E, Liu K, McDermott MM. Peripheral arterial disease and depressed mood in older men and women. Vasc Med 2001; 6:229–234.
- Steg PG, Bhatt DL, Wilson PW, et al; REACH Registry Investigators. One-year cardiovascular event rates in outpatients with atherothrombosis. JAMA 2007; 297:1197–1206.
- Gornik HL, Creager MA. Contemporary management of peripheral arterial disease: I. cardiovascular risk-factor modification. Cleve Clin J Med 2006; 73(suppl 4):S30–S37.
- Antithrombotic Trialists’ Collaboration. Collaborative meta-analysis of randomised trials of antiplatelet therapy for prevention of death, myocardial infarction, and stroke in high risk patients. BMJ 2002; 324:71–86.
- Heart Protection Study Collaborative Group. Randomized trial of the effects of cholesterol-lowering with simvastatin on peripheral vascular and other major vascular outcomes in 20,536 people with peripheral arterial disease and other high-risk conditions. J Vasc Surg 2007; 45:645–654.
- Criqui MH, Fronek A, Klauber MR, Barrett-Connor E, Gabriel S. The sensitivity, specificity, and predictive value of traditional clinical evaluation of peripheral arterial disease: results from noninvasive testing in a defined population. Circulation 1985; 71:516–522.
- Carter SA. Clinical measurement of systolic pressures in limbs with arterial occlusive disease. JAMA 1969; 207:1869–1874.
- Ouriel K, Zarins CK. Doppler ankle pressure: an evaluation of three methods of expression. Arch Surg 1982; 117:1297–1300.
- Yao ST, Hobbs JT, Irvine WT. Ankle systolic pressure measurements in arterial disease affecting the lower extremities. Br J Surg 1969; 56:676–679.
- Carter SA. Indirect systolic pressures and pulse waves in arterial occlusive diseases of the lower extremities. Circulation 1968; 37:624–637.
- Belch JJ, Topol EJ, Agnelli G, et al; Prevention of Atherothrombotic Disease Network. Critical issues in peripheral arterial disease detection and management: a call to action. Arch Intern Med 2003; 163:884–892.
- Lijmer JG, Hunink MG, van den Dungen JJ, Loonstra J, Smit AJ. ROC analysis of noninvasive tests for peripheral arterial disease. Ultrasound Med Biol 1996; 22:391–398.
- Carmo GA, Mandil A, Nascimento BR, et al. Can we measure the ankle-brachial index using only a stethoscope? A pilot study. Fam Pract 2009; 26:22–26.
- Chesbro SB, Asongwed ET, Brown J, John EB. Reliability of Doppler and stethoscope methods of determining systolic blood pressures: considerations for calculating an ankle-brachial index. J Natl Med Assoc 2011; 103:863–869.
- Jönsson B, Lindberg LG, Skau T, Thulesius O. Is oscillometric ankle pressure reliable in leg vascular disease? Clin Physiol 2001; 21:155–163.
- Ramanathan A, Conaghan PJ, Jenkinson AD, Bishop CR. Comparison of ankle-brachial pressure index measurements using an automated oscillometric device with the standard Doppler ultrasound technique. ANZ J Surg 2003; 73:105–108.
- Beckman JA, Higgins CO, Gerhard-Herman M. Automated oscillometric determination of the ankle-brachial index provides accuracy necessary for office practice. Hypertension 2006; 47:35–38.
- Mehlsen J, Wiinberg N, Bruce C. Oscillometric blood pressure measurement: a simple method in screening for peripheral arterial disease. Clin Physiol Funct Imaging 2008; 28:426–429.
- Aboyans V, Lacroix P, Doucet S, Preux PM, Criqui MH, Laskar M. Diagnosis of peripheral arterial disease in general practice: can the ankle-brachial index be measured either by pulse palpation or an automatic blood pressure device? Int J Clin Pract 2008; 62:1001–1007.
- 2011 Writing Group Members. 2011 ACCF/AHA Focused Update of the Guideline for the Management of patients with peripheral artery disease (updating the 2005 guideline): a report of the American College of Cardiology Foundation/American Heart Association Task Force on practice guidelines. Circulation 2011; 124:2020–2045.
- Stein R, Hriljac I, Halperin JL, Gustavson SM, Teodorescu V, Olin JW. Limitation of the resting ankle-brachial index in symptomatic patients with peripheral arterial disease. Vasc Med 2006; 11:29–33.
- Amirhamzeh MM, Chant HJ, Rees JL, Hands LJ, Powell RJ, Campbell WB. A comparative study of treadmill tests and heel raising exercise for peripheral arterial disease. Eur J Vasc Endovasc Surg 1997; 13:301–305.
- Hirsch AT, Haskal ZJ, Hertzer NR, et al; American Association for Vascular Surgery; Society for Vascular Surgery; Society for Cardiovascular Angiography and Interventions; Society for Vascular Medicine and Biology; Society of Interventional Radiology; ACC/AHA Task Force on Practice Guidelines Writing Committee to Develop Guidelines for the Management of Patients With Peripheral Arterial Disease; American Association of Cardiovascular and Pulmonary Rehabilitation; National Heart, Lung, and Blood Institute; Society for Vascular Nursing; TransAtlantic Inter-Society Consensus; Vascular Disease Foundation. ACC/AHA 2005 practice guidelines for the management of patients with peripheral arterial disease (lower extremity, renal, mesenteric, and abdominal aortic): a collaborative report from the American Association for Vascular Surgery/Society for Vascular Surgery, Society for Cardiovascular Angiography and Interventions, Society for Vascular Medicine and Biology, Society of Interventional Radiology, and the ACC/AHA Task Force on Practice Guidelines (Writing Committee to Develop Guidelines for the Management of Patients With Peripheral Arterial Disease): endorsed by the American Association of Cardiovascular and Pulmonary Rehabilitation; National Heart, Lung, and Blood Institute; Society for Vascular Nursing; TransAtlantic Inter-Society Consensus; and Vascular Disease Foundation. Circulation 2006; 113:e463–e654.
- Chobanian AV, Bakris GL, Black HR, et al; Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure; National Heart, Lung, and Blood Institute; National High Blood Pressure Education Program Coordinating Committee. Seventh report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure. Hypertension 2003; 42:1206–1252.
- National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III). Third Report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III) final report. Circulation 2002; 106:3143–3421.
- Skyler JS, Bergenstal R, Bonow RO, et al; American Diabetes Association; American College of Cardiology Foundation; American Heart Association. Intensive glycemic control and the prevention of cardiovascular events: implications of the ACCORD, ADVANCE, and VA Diabetes Trials: a position statement of the American Diabetes Association and a Scientific Statement of the American College of Cardiology Foundation and the American Heart Association. J Am Coll Cardiol 2009; 53:298–304.
- Almahameed A, Bartholomew JR, editors. Peripheral arterial disease: recognition and contemporary management. Cleve Clin J Med 2006; 73(suppl 4):S1–S51.
- McKenna M, Wolfson S, Kuller L. The ratio of ankle and arm arterial pressure as an independent predictor of mortality. Atherosclerosis 1991; 87:119–128.
- Valentine RJ, Grayburn PA, Eichhorn EJ, Myers SI, Clagett GP. Coronary artery disease is highly prevalent among patients with premature peripheral vascular disease. J Vasc Surg 1994; 19:668–674.
- Weitz JI, Byrne J, Clagett GP, et al. Diagnosis and treatment of chronic arterial insufficiency of the lower extremities: a critical review. Circulation 1996; 94:3026–3049.
- Hertzer NR, Beven EG, Young JR, et al. Coronary artery disease in peripheral vascular patients. A classification of 1000 coronary angiograms and results of surgical management. Ann Surg 1984; 199:223–233.
- Leng GC, Lee AJ, Fowkes FG, et al. Incidence, natural history and cardiovascular events in symptomatic and asymptomatic peripheral arterial disease in the general population. Int J Epidemiol 1996; 25:1172–1181.
- Heald CL, Fowkes FG, Murray GD, Price JF; Ankle Brachial Index Collaboration. Risk of mortality and cardiovascular disease associated with the ankle-brachial index: Systematic review. Atherosclerosis 2006; 189:61–69.
- Ankle Brachial Index; Fowkes FG, Murray GD, Butcher I, et al. Collaboration Ankle brachial index combined with Framingham Risk Score to predict cardiovascular events and mortality: a meta-analysis. JAMA 2008; 300:197–208.
- Diehm C, Allenberg JR, Pittrow D, et al; German Epidemiological Trial on Ankle Brachial Index Study Group. Mortality and vascular morbidity in older adults with asymptomatic versus symptomatic peripheral artery disease. Circulation 2009; 120:2053–2061.
- Feringa HH, Bax JJ, van Waning VH, et al. The long-term prognostic value of the resting and postexercise ankle-brachial index. Arch Intern Med 2006; 166:529–535.
- Sheikh MA, Bhatt DL, Li J, Lin S, Bartholomew JR. Usefulness of postexercise ankle-brachial index to predict all-cause mortality. Am J Cardiol 2011; 107:778–782.
- Resnick HE, Lindsay RS, McDermott MM, et al. Relationship of high and low ankle brachial index to all-cause and cardiovascular disease mortality: the Strong Heart Study. Circulation 2004; 109:733–739.
- Wattanakit K, Folsom AR, Duprez DA, Weatherley BD, Hirsch AT. Clinical significance of a high ankle-brachial index: insights from the Atherosclerosis Risk in Communities (ARIC) Study. Atherosclerosis 2007; 190:459–464.
- US Preventive Services Task Force. Screening for peripheral arterial disease: Recommendation statement. http://www.ahrq.gov/clinic/uspstf05/pad/padrs.pdf. Accessed July 25, 2012.
- Mayfield JA, Reiber GE, Sanders LJ, Janisse D, Pogach LM; American Diabetes Association. Preventive foot care in diabetes. Diabetes Care 2004; 27(suppl 1):S63–S64.
- Norgren L, Hiatt WR, Dormandy JA, Nehler MR, Harris KA, Fowkes FG; TASC II Working Group. Inter-Society Consensus for the Management of Peripheral Arterial Disease (TASC II). J Vasc Surg 2007; 45(suppl S):S5–S67.
- Beckman JA, Jaff MR, Creager MA. The United States preventive services task force recommendation statement on screening for peripheral arterial disease: more harm than benefit? Circulation 2006; 114:861–866.
- US Preventive Services Task Force. Screening for peripheral artery disease draft research plan. http://www.uspreventiveservicestaskforce.org/uspstf12/pad/padslides.htm. Accessed July 26, 2012.
- Greenland P, Alpert JS, Beller GA, et al; American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. 2010 ACCF/AHA guideline for assessment of cardiovascular risk in asymptomatic adults: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. Circulation 2010; 122:e584–e636.
- Mohler ER, Treat-Jacobson D, Reilly MP, et al. Utility and barriers to performance of the ankle-brachial index in primary care practice. Vasc Med 2004; 9:253–260.
KEY POINTS
- The ankle-brachial index is the systolic pressure in the ankle (either the dorsalis pedis or the posterior tibial artery, whichever has the higher pressure) divided by the systolic pressure in the arm (either the left or right, whichever is higher). The lower of the two values obtained (left and right) is the patient’s overall ankle-brachial index.
- Most healthy adults have a value greater than 1.0. A value of less than 0.91 indicates significant peripheral artery disease, and a value lower than 0.40 at rest generally indicates severe disease. Values higher than 1.4 indicate stiffened, noncompressible arteries.
- Measuring the ankle-brachial index after exercise can uncover peripheral artery disease in patients with a normal resting ankle-brachial index.