User login
HIV management in pregnancy
Human immunodeficiency virus (HIV) is a single-stranded enveloped RNA retrovirus that was first described in the 1980s and is known for its severity of systemic immune dysregulation and associated opportunistic infections. It is transmitted through contact with blood or bodily fluids, and it can be transmitted vertically, most often at the time of delivery. Since the advent of antiretroviral therapy, the average life expectancy and natural course of HIV infection has improved notably.1
In 2019, just over 1 million adults and adolescents in the United States were living with the diagnosis of HIV.2 In the same year, the rate of new HIV diagnoses in the United States had stabilized at a rate of 13.2 new cases per 100,000 individuals.2 Among this cohort, individuals identifying as females at birth accounted for 19% of the total population living with HIV.2 Sexual contact was the most common route of transmission, followed by injection drug use—77% and 20%, respectively.2
It is important to note that the incidence and prevalence of HIV does not reflect the individuals who unknowingly are living with the disease. The disease burden associated with HIV infection and the availability of effective treatment modalities has led to the recommendation that all individuals undergo HIV screening at least once in their lifetime.3 Early identification of HIV infection is important to optimize the health of all individuals and future generations.
The interplay between high-risk sexual practices and the risk for HIV exposure and unintended pregnancy places the ObGyn at the forefront of HIV prevention and identification. Early diagnosis and standardized treatment with antiretroviral therapies have led to both a dramatic improvement in adult disease burden and a dramatic decrease in perinatal transmission.4,5 In 2019, perinatal transmission accounted for less than 1% of HIV transmission in the United States.2 This is a decrease of greater than 54% from 2014, which, again, emphasizes the role of the ObGyn in HIV management.6
Preconception care: Gynecologic screening, diagnosis, and management
The Centers for Disease Control and Prevention (CDC) recommends that an individual undergo HIV screening at least once in their lifetime.3 HIV screening algorithms have changed over the last 20 years to reduce the number of false-positive and/or false-negative results obtained through HIV antibody testing alone.7 HIV-1/2 antibody/antigen immunoassay is recommended as the initial screening test. If reactive, this should be followed by an HIV p24-specific antigen test. Reactivity for both the HIV-1/2 immunoassay and the HIV p24-specific antigen test confirms the diagnosis of HIV infection. However, if HIV p24-specific antigen testing is indeterminate or an acute HIV infection is suspected, an HIV nucleic acid test (NAT) should be performed.7,8
Upon a positive diagnosis, a multidisciplinary team approach is recommended to address the mental, social, and physical care of the patient. Team members should include an adult medicine clinician, an infectious disease clinician, an ObGyn, social services staff, and behavioral health support to achieve the goal of obtaining and maintaining the patient’s optimal health status.
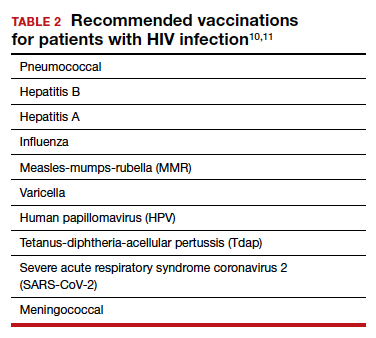
TABLE 1 lists the recommended initial laboratory assessments that should follow a new diagnosis of HIV infection. Based on the laboratory results, the indicated vaccinations, antibiotic prophylaxis for opportunistic infections, and optimal combined antiretroviral therapy (cART) can be determined.9 The vaccinations listed in TABLE 2 should be up to date.10,11 Additionally, cervical cancer screening with cytology and human papillomavirus (HPV) testing and treatment should be performed in accordance with the 2019 American Society for Cervical Cancer Prevention (ASCCP) guidelines.12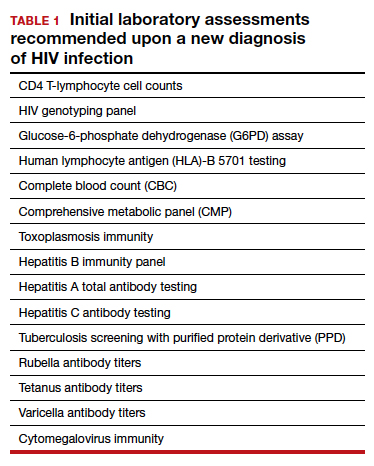
Promptly initiating cART is of utmost importance; this decreases the rate of HIV transmission via sexual contact and decreases the rate of perinatal transmission.5,13 Results of the initial laboratory assessment, hepatitis B status, and desire for pregnancy/contraception should be considered when initiating cART.3,14,15
It is imperative to discuss sharing the positive diagnostic results with the patient’s partner. The CDC provides guidance for these discussions,16 which should address the use of preexposure prophylaxis (PrEP) if partner screening establishes partner serodiscordance (that is, HIV positivity in one partner and HIV negativity in the other partner). PrEP is a single pill approved by the US Food and Drug Administration (FDA) that combines tenofovir 300 mg and emtricitabine 200 mg daily17 and has been recommended since 2012.18-20 PrEP also should be considered in sexually active individuals who have higher-risk behaviors within an area with high HIV prevalence.18-21 Despite the CDC’s strong recommendations for PrEP use, lack of insurance coverage and high cost are barriers to universal use. The National Alliance of State and Territorial AIDS Directors (NASTAD) provides a list of patient and copayment assistance programs that can be found at the NASTAD website: https://nastad.org/prepcost-resources/prep-assitance-programs.
Continue to: Preconception considerations...
Preconception considerations
In individuals with known HIV infection, preconception consultation with an ObGyn or maternal-fetal medicine (MFM) specialist should be recommended prior to conception.22 Preconception recommendations include addressing optimization of maternal medical comorbidities, addressing routine health screening and vaccinations, performing sexually transmitted infection screening, and optimizing HIV disease status.3,22,23
With the assistance of adult medicine and infectious disease clinicians, a cART regimen that is sufficient to reliably maintain viral suppression (that is, viral load < 50 copies/mL on 2 separate occasions at least 3 months apart) and is safe for use in pregnancy should be established.3 In serodiscordant couples, recommended mechanisms to prevent HIV transmission during conception include sustained viral suppression in the HIV-positive partner, PrEP use in the HIV-negative partner, and timing of unprotected intercourse during peak fertility only.3
Antepartum care
The initial prenatal visit
Women who have no prior screening for HIV or prior negative HIV results should undergo HIV screening at the first prenatal visit.3 Screening should be performed in accordance with the “opt out method.”6 Using this method, a woman without a known diagnosis of HIV infection is told that she will undergo HIV screening as a component of routine prenatal care unless she decides that she does not want this test performed.6,24,25 At the time of screening, all pregnant women should be provided with comprehensive information regarding HIV screening, HIV screening results, and the implications of HIV infection on pregnancy.26
In the pregnant patient with confirmed HIV infection, all preconception considerations should be addressed. If not already in place, referrals to appropriate providers (infectious disease specialist, ObGyn, MFM specialist) and ancillary support staff (social services, behavioral health support) should be arranged. All efforts should be implemented to optimize additional medical comorbidities. TABLE 3 lists additional prenatal testing requirements.22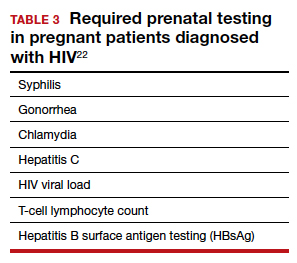
Antiretroviral therapy should be assessed for safety and efficacy in pregnancy and should comply with the CDC recommendations for cART in pregnancy.3 Patients with a T-lymphocyte cell count of less than 200 cells/mm3 and/or a viral load greater than 50 copies/mL despite adherent cART use should be referred to an infectious disease specialist to determine the need for alternative cART and/or the need for chemoprophylaxis against opportunistic infections.23
First and second trimester
Antiretroviral adherence and barriers to adherence should be addressed at every prenatal visit. If the patient is started on antiretroviral therapy in pregnancy or is switched to an alternative cART regimen, viral load assessment should be performed 2 to 4 weeks after the start or change in cART and then repeated monthly until undetectable levels are achieved.3,26 If an undetectable viral load cannot be obtained, cART adherence should be thoroughly evaluated, and the patient should be referred to an infectious disease or HIV treatment specialist.26
If the initial prenatal testing indicates an undetectable viral load, repeat viral load assessment can be performed every 3 months throughout the pregnancy.3 If initial prenatal testing indicates an undetectable HIV viral load and the T-lymphocyte count is greater than 200 cells/mm3, repeat viral load testing can be performed every 6 months to ensure stability.3
Early screening for gestational diabetes should be performed in patients receiving protease inhibitors because these agents may interfere with carbohydrate tolerance.22,26
Continue to: Third trimester...
Third trimester
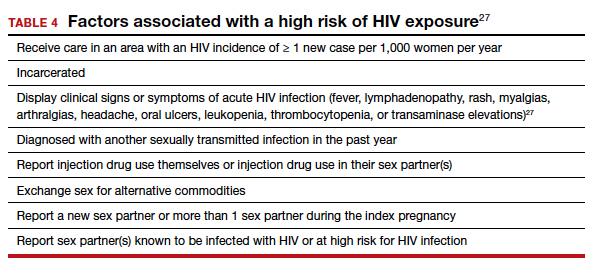
Women with negative HIV screening at the initial prenatal evaluation should undergo repeat HIV screening in the third trimester if they are at high risk for HIV exposure.25 Factors that determine high-risk status are listed in TABLE 4.27 Sexually transmitted infection screening should be repeated in the third trimester.26
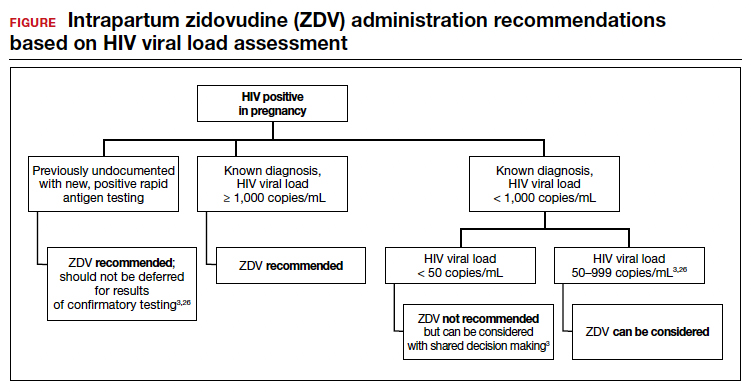
Repeat assessment of the viral load should be completed between 34 and 36 weeks’ gestation or sooner if additional indications for early term or late preterm delivery arise.3 Viral load assessments aid in determining delivery timing and route and the need for zidovudine (ZDV) treatment (FIGURE).
Studies that were performed prior to standardized cART use found higher rates of perinatal transmission associated with vaginal delivery when compared with cesarean delivery (CD).28-30 However, these studies did not account for measures of viral load within their study populations.28-30
In more recent studies performed in the era of standardized cART and viral load monitoring, CD does not provide protection from perinatal transmission when the maternal viral load is less than 1,000 copies/mL at the time of delivery.31 Similarly, delivery prior to 40 weeks’ gestation does not confer protection from perinatal transmission.32
Alternatively, if the maternal viral load is 1,000 copies/mL or greater, CD should be considered to reduce the risk of perinatal transmission. A scheduled, elective CD at 38 weeks’ gestation is recommended in those with a maternal viral load of 1,000 copies/mL or greater and no medical indication for earlier delivery in order to decrease the likelihood of labor onset and/or rupture of membranes prior to delivery.3,33
Intrapartum care
Rapid antigen testing (with follow-up confirmatory testing as indicated) is recommended in patients presenting to labor and delivery with no prior documentation of HIV status.3,8,26
Despite a significant decrease in perinatal transmission rates over the last 30 years, a large proportion of perinatal transmission cases are thought to result from intrapartum fetal exposure. While the mechanism of transmission is not known, a correlation between maternal viral load and risk for perinatal transmission has been shown. A maternal viral load of less than 1,000 copies/mL has been associated with a perinatal transmission risk of less than 2%.34,35 A maternal viral load between 50 and 999 copies/mL has been associated with a perinatal transmission rate of 1% to 2% compared with less than 1% for a maternal viral load of less than 50 copies/mL or undetectable measures.5,36,37
These differences in perinatal transmission rates have prompted the recommendation for administration of ZDV for a minimum of 3 hours prior to delivery in mothers with a viral load of 1,000 copies/mL or greater.4,38 The recommended ZDV dosing is: a 1-hour intravenous loading dose of 2 mg/kg followed by continuous infusion of 1 mg/kg per hour until delivery.39,40 Patients who opt for vaginal delivery despite nonsuppressed viral loads (≥1,000 copies/mL) after thorough perinatal counseling should receive ZDV at the start of labor through delivery.3 All patients should be continued on cART throughout their intrapartum and postpartum course.
The duration of membrane rupture and the use of invasive fetal monitoring (that is, fetal scalp electrodes) have been assessed as mechanisms of perinatal transmission. Although they were performed prior to the standardized use of cART, several studies demonstrated that increased perinatal transmission rates were associated with invasive fetal monitoring.34,41,42 While limited data have refuted this finding in women with suppressed viral loads (< 50 copies/mL), the American College of Obstetricians and Gynecologists recommends avoiding the use of invasive fetal monitoring in labor.26
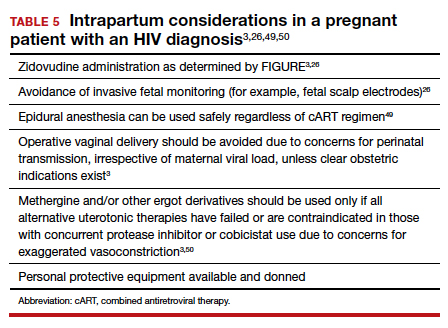
Pre-cART studies demonstrated increased rates of perinatal transmission with longer durations of membrane rupture prior to delivery.43,44 More recent studies have reevaluated this association and determined that the increased perinatal transmission rates are more likely associated with higher maternal viral loads at the time of delivery rather than duration of membrane rupture.45-47 No clear evidence describes when or if CD after the onset of labor or rupture of membranes provides protection from perinatal HIV transmission in pregnant women with HIV receiving no antiretroviral drugs or only ZDV during labor.43,48 CD can be considered for patients in whom scheduled, pre-labor CD was planned who present in labor or with rupture of membranes prior to scheduled CD.26 These, and additional intrapartum considerations, are listed in TABLE 5.49,50
Appropriate personal protective equipment should be available and donned for all providers present throughout intrapartum management and at delivery.23,26 Should any provider injury occur, immediate cleansing of the injury site should be performed, followed by referral to proper workplace supervisors for additional laboratory testing and antiretroviral prophylaxis.
Continue to: Postpartum care...
Postpartum care
Postpartum contraception should be offered and provided in accordance with patient request. Regardless of other birth control methods, strict condom use should be advised. PrEP should be discussed and offered for all partners of serodiscordant couples.
Upon outpatient follow-up, assessment and provision of routine health maintenance should be performed. Any abnormal cervical pathology encountered during prenatal care should be managed in accordance with ASCCP guidelines.12 Follow-up care should be established with adult medicine, infectious disease, and ObGyn clinicians.26
Neonatal considerations
Neonates born to mothers with positive or unknown HIV status should undergo expedited HIV testing.51,52 Consultation should be conducted with pediatric or neonatology colleagues to determine the antiretroviral regimen and duration of therapy based on presumed HIV status of the neonate. Ideally, antiretroviral therapy should be initiated within 6 hours of delivery.3,53
Formula feeding should be implemented as maternal HIV infection is one of the few contraindications to breastfeeding.54,55 The risk of late breast milk transmission, defined as postnatal transmission that occurs after 1 month of age, may vary based on maternal viral load, but it has been reported as high as 8.9 transmissions per 100 person-years of breastfeeding.56
Resources available
Care of the pregnant patient with HIV and the reduction of perinatal transmission both depend on early diagnosis of HIV and effective treatment with cART. Such patients benefit from a team-based care model that includes the ObGyn and/or MFM specialist, infectious disease clinician, pediatrician, and social worker. As guidelines evolve for care of these patients, a reference checklist, such as the examples provided at the Society for Maternal-Fetal Medicine website (smfm.org) or at HIV.gov, provide an outline for:
- management before, during, and after pregnancy
- suggestions for management teams of interest to successfully carry out the checklist requirements
- proposals for measurements of quality performance with the use of checklists in the management of HIV in pregnancy.
In addition, assistance with clinical decision making for patients with HIV in pregnancy can be obtained via telephone consultation with the National Clinician Consultation Center–Perinatal HIV/AIDS (888-448-8765), which is available 24 hours a day, 7 days a week. ●
- Samji H, Cescon A, Hogg RS, et al; North American AIDS Cohort Collaboration on Research and Design (NA-ACCORD) of IeDEA. Closing the gap: increases in life expectancy among treated HIV-positive individuals in the United States and Canada. PLoS One. 2013;8:e81355. doi: 10.1371/journal. pone.0081355.
- Centers for Disease Control and Prevention. May 1, 2021. HIV Surveillance Report, 2019, vol. 32: Diagnosis of HIV infection in the United States and dependent areas, 2019. Accessed February 15, 2022. http://www.cdc.gov/hiv/library/reports /hiv-surveillance.html
- Panel on Antiretroviral Therapy and Medical Management of Children Living with HIV. Guidelines for the use of antiretroviral agents in pediatric HIV infection. https: //clinicalinfo.hiv.gov/en/guidelines/pediatric-arv. Accessed February 15, 2022.
- Connor EM, Sperling RS, Gelber R, et al. Reduction of maternal-infant transmission of human immunodeficiency virus type 1 with zidovudine treatment. Pediatric AIDS Clinical Trials Group Protocol 076 Study Group. N Engl J Med. 1994;331:1173-1180.
- Townsend CL, Byrne L, Cortina-Borja M, et al. Earlier initiation of ART and further decline in mother-to-child HIV transmission rates, 2000-2011. AIDS. 2014;28:1049-1057.
- Centers for Disease Control and Prevention. January 26, 2022. HIV and pregnant women, infants, and children. Accessed February 15, 2022. https://www.cdc.gov/hiv/group/gender /pregnantwomen/index.html
- Centers for Disease Control and Prevention. 2018 Quick reference guide: Recommended laboratory HIV testing algorithm for serum or plasma specimens. National Center for HIV/AIDS, Viral Hepatitis, and TB Prevention (US); Division of HIV/AIDS Prevention; Association of Public Health Laboratories. Updated January 2018. https://stacks. cdc.gov/view/cdc/50872
- Centers for Disease Control and Prevention, Association of Public Health Laboratories. June 27, 2014. Laboratory testing for the diagnosis of HIV infection: updated recommendations. Accessed February 15, 2022. http://stacks.cdc.gov/view /cdc/23447
- Guidelines for the prevention and treatment of opportunistic infections in adults and adolescents with HIV. Updated April 12, 2022. Accessed July 6, 2022. https://clinicalinfo.hiv .gov/en/guidelines/adult-and-adolescent-opportunistic -infection/whats-new-guidelines
- Rubin LG, Levin MJ, Ljungman P, et al. 2013 IDSA clinical practice guideline for vaccination of the immunocompromised host. Clin Infect Dis. 2014;58: e44–e100. doi: 10.1093/cid/cit684.
- Centers for Disease Control and Prevention. ACIP: Guidance for vaccine recommendations for pregnant and breastfeeding women. Accessed July 5, 2022. https://www.cdc.gov /vaccines/hcp/acip-recs/rec-vac-preg.html?CDC_AA _refVal=https%3A%2F%2Fwww.cdc.gov%2Fvaccines%2Facip %2Fcommittee%2Fguidance%2Frec-vac-preg.html
- Perkins RB, Guido RS, Castle PE, et al; for the 2019 ASCCP Risk-Based Management Consensus Guidelines Committee. 2019 ASCCP risk-based management consensus guidelines for abnormal cervical cancer screening tests and cancer precursors. J Low Genit Tract Dis. 2020;24:102-131. doi: 10.1097/LGT.0000000000000525. Erratum in: J Low Genit Tract Dis. 2020;24:427.
- Cohen MS, Chen YQ, McCauley M, et al; HPTN 052 Study Team. Prevention of HIV-1 infection with early antiretroviral therapy. N Engl J Med. 2011;365:493-505.
- Drug interactions between antiretroviral agents and hormonal contraceptives. Accessed July 6, 2022. https://clinicalinfo .hiv.gov/en/table/table-3-drug-interactions-between -antiretroviral-agents-and-hormonal-contraceptives
- Panel on Treatment of HIV During Pregnancy and Prevention of Perinatal Transmission. Recommendations for use of antiretroviral drugs in pregnancy and interventions to reduce perinatal HIV transmission in the United States. Accessed July 7, 2022. https://clinicalinfo.hiv.gov/en/guidelines/perinatal /whats-new-guidelines
- Centers for Disease Control and Prevention. Recommendations for partner services programs for HIV infection, syphilis, gonorrhea, and chlamydial infection. MMWR Recomm Rep. 2008;57(RR-9):1–83.
- Gilead Sciences, Inc. Truvada (emtricitabine 200 mg/ tenofovir disoproxil fumarate 300 mg tablets). Accessed July 6, 2022. https://truvada.com
- Centers for Disease Control and Prevention. Interim guidance for clinicians considering the use of preexposure prophylaxis for the prevention of HIV infection in heterosexually active adults. MMWR Morb Mortal Wkly Rep. 2012;61:586-589.
- Baeten JM, Donnell D, Ndase P, et al; Partners PrEP Study Team. Antiretroviral prophylaxis for HIV prevention in heterosexual men and women. N Engl J Med. 2012;367: 399-410.
- Celum C, Baeten JM. Antiretroviral-based HIV-1 prevention: antiretroviral treatment and pre-exposure prophylaxis. Antivir Ther. 2012;17:1483-1493.
- Thigpen MC, Kebaabetswe PM, Paxton LA, et al; TDF2 Study Group. Antiretroviral preexposure prophylaxis for heterosexual HIV transmission in Botswana. N Engl J Med. 2012;367:423-434.
- Society for Maternal-Fetal Medicine. Special statement: updated checklists for pregnancy management in persons with HIV. Accessed July 5, 2022. https://www.smfm.org /publications/334-smfm-special-statement-updated -checklists-for-pregnancy-management-in-persons-with-hiv
- American College of Obstetricians and Gynecologists. ACOG committee opinion no. 752. Prenatal and perinatal human immunodeficiency virus testing. Obstet Gynecol. 2018;132:e138-e142.
- Human immunodeficiency virus screening. Joint statement of the American Academy of Pediatrics and the American College of Obstetricians and Gynecologists. Pediatrics. 1999;104(1 pt 1):128.
- Branson BM, Handsfield HH, Lampe MA, et al; Centers for Disease Control and Prevention. Revised recommendations for HIV testing of adults, adolescents, and pregnant women in health care settings. MMWR Recomm Rep. 2006; 55(RR-14):1-17.
- American College of Obstetricians and Gynecologists. ACOG committee opinion no. 751. Labor and delivery management of women with human immunodeficiency virus infection. Obstet Gynecol. 2018;132:e131-e137.
- Centers for Disease Control and Prevention. Factors increasing the risk of acquiring or transmitting HIV. November 12, 2019. Accessed July 29, 2022. https://www.cdc .gov/hiv/risk/estimates/riskfactors.html
- Mandelbrot L, Le Chenadec J, Berrebi A, et al. Perinatal HIV1 transmission: interaction between zidovudine prophylaxis and mode of delivery in the French Perinatal Cohort. JAMA. 1998;280:55-60.
- European Mode of Delivery Collaboration. Elective caesarean-section versus vaginal delivery in prevention of vertical HIV-1 transmission: a randomised clinical trial. Lancet. 1999;353:1035-1039.
- International Perinatal HIV Group; Andiman W, Bryson Y, de Martino M, et al. The mode of delivery and the risk of vertical transmission of human immunodeficiency virus type 1—a meta-analysis of 15 prospective cohort studies. N Engl J Med. 1999;340:977-987.
- Briand N, Jasseron C, Sibiude J, et al. Cesarean section for HIV-infected women in the combination antiretroviral therapies era, 2000–2010. Am J Obstet Gynecol. 2013;209: 335.e1-335.e12.
- Scott RK, Chakhtoura N, Burke MM, et al. Delivery after 40 weeks of gestation in pregnant women with well-controlled human immunodeficiency virus. Obstet Gynecol. 2017;130:502-510.
- American College of Obstetricians and Gynecologists. Committee opinion no. 560. Medically indicated late-preterm and early-term deliveries. Obstet Gynecol. 2013;121:908-910.
- Mofenson LM, Lambert JS, Stiehm ER, et al. Risk factors for perinatal transmission of human immunodeficiency virus type 1 in women treated with zidovudine. Pediatric AIDS Clinical Trials Group Study 185 Team. N Engl J Med. 1999;341:385-393.
- Garcia PM, Kalish LA, Pitt J, et al. Maternal levels of plasma human immunodeficiency virus type 1 RNA and the risk of perinatal transmission. Women and Infants Transmission Study Group. N Engl J Med. 1999;341:394-402.
- Briand N, Warszawski J, Mandelbrot L, et al; ANRS-EPF CO1CO11 Study Group. Is intrapartum intravenous zidovudine for prevention of mother-to-child HIV-1 transmission still useful in the combination antiretroviral therapy era? Clin Infect Dis. 2013;57:903-914.
- Myer L, Phillips TK, McIntyre JA, et al. HIV viraemia and mother-to-child transmission risk after antiretroviral therapy initiation in pregnancy in Cape Town, South Africa. HIV Med. 2017;18:80-88.
- Rodman JH, Flynn PM, Robbins B, et al. Systemic pharmacokinetics and cellular pharmacology of zidovudine in human immunodeficiency virus type 1-infected women and newborn infants. J Infect Dis. 1999;180:1844-1850.
- Wade NA, Birkhead GS, Warren BL, et al. Abbreviated regimens of zidovudine prophylaxis and perinatal transmission of the human immunodeficiency virus. N Engl J Med. 1998;339:1409-1414.
- Nielsen-Saines K, Watts HD, Veloso VS, et al; NICHD HPTN 040/PACTG 1043 Protocol Team. Three postpartum antiretroviral regimens to prevent intrapartum HIV infection. N Engl J Med. 2012;366:2368-2379.
- Mandelbrot L, Mayaux MJ, Bongain A, et al. Obstetric factors and mother-to-child transmission of human immunodeficiency virus type 1: the French perinatal cohorts. SEROGEST French Pediatric HIV Infection Study Group. Am J Obstet Gynecol. 1996;175(3 pt 1):661-667.
- Shapiro DE, Sperling RS, Mandelbrot L, et al. Risk factors for perinatal human immunodeficiency virus transmission in patients receiving zidovudine prophylaxis. Pediatric AIDS Clinical Trials Group protocol 076 Study Group. Obstet Gynecol. 1999;94:897-908.
- International Perinatal HIV Group. Duration of ruptured membranes and vertical transmission of HIV-1: a meta-analysis from 15 prospective cohort studies. AIDS. 2001;15:357-368.
- Nielsen TF, Hokegard KH. Postoperative cesarean section morbidity: a prospective study. Am J Obstet Gynecol. 1983;146:911-916.
- Mark S, Murphy KE, Read S, et al. HIV mother-to-child transmission, mode of delivery, and duration of rupture of membranes: experience in the current era. Infect Dis Obstet Gynecol. 2012;2012:267969.
- Cotter AM, Brookfield KF, Duthely LM, et al. Duration of membrane rupture and risk of perinatal transmission of HIV1 in the era of combination antiretroviral therapy. Am J Obstet Gynecol. 2012;207:482.e1-482.e5.
- Peters H, Byrne L, De Ruiter A, et al. Duration of ruptured membranes and mother-to-child HIV transmission: a prospective population-based surveillance study. BJOG. 2016;123:975-981.
- Jamieson DJ, Read JS, Kourtis AP, et al. Cesarean delivery for HIV-infected women: recommendations and controversies. Am J Obstet Gynecol. 2007;197(3 suppl):S96-S100.
- Cambic CR, Avram MJ, Gupta DK, et al. Effect of ritonavir-induced cytochrome P450 3A4 inhibition on plasma fentanyl concentrations during patient-controlled epidural labor analgesia: a pharmacokinetic simulation. Int J Obstet Anesth. 2014;23:45-51.
- Navarro J, Curran A, Burgos J, et al. Acute leg ischaemia in an HIV-infected patient receiving antiretroviral treatment. Antivir Ther. 2017;22:89-90.
- American Academy of Pediatrics, American College of Obstetricians and Gynecologists. Guidelines for Perinatal Care. 8th ed. American Academy of Pediatrics, American College of Obstetricians and Gynecologists; 2017.
- Siberry GK, Abzug MJ, Nachman S, et al; Panel on Opportunistic Infections in HIV-Exposed and HIV-Infected Children. Guidelines for the prevention and treatment of opportunistic infections in HIV-exposed and HIV-infected children: recommendations from the National Institutes of Health, Centers for Disease Control and Prevention, the HIV Medicine Association of the Infectious Diseases Society of America, the Pediatric Infectious Diseases Society, and the American Academy of Pediatrics. Pediatr Infect Dis J. 32(suppl 2[0 2]):i–KK4.
- Panel on Antiretroviral Therapy and Medical Management of Children Living with HIV. Guidelines for the use of antiretroviral agents in pediatric HIV infection. Accessed February 15, 2022. https://clinicalinfo.hiv.gov/en/guidelines /pediatric-arv
- Committee on Health Care for Underserved Women, American College of Obstetricians and Gynecologists. ACOG committee opinion no. 361. Breastfeeding: maternal and infant aspects. Obstet Gynecol. 2007;109(2 pt 1):479-480.
- Committee on Pediatric AIDS; Mofenson LM, Flynn PM, Aldrovandi GM, et al. Infant feeding and transmission of human immunodeficiency virus in the United States. Pediatrics. 2013;131:391-396.
- Breastfeeding and HIV International Transmission Study Group; Coutsoudis A, Dabis F, Fawzi W, et al. Late postnatal transmission of HIV-1 in breast-fed children: an individual patient data meta-analysis. J Infect Dis. 2004;189:2154-2166.
Human immunodeficiency virus (HIV) is a single-stranded enveloped RNA retrovirus that was first described in the 1980s and is known for its severity of systemic immune dysregulation and associated opportunistic infections. It is transmitted through contact with blood or bodily fluids, and it can be transmitted vertically, most often at the time of delivery. Since the advent of antiretroviral therapy, the average life expectancy and natural course of HIV infection has improved notably.1
In 2019, just over 1 million adults and adolescents in the United States were living with the diagnosis of HIV.2 In the same year, the rate of new HIV diagnoses in the United States had stabilized at a rate of 13.2 new cases per 100,000 individuals.2 Among this cohort, individuals identifying as females at birth accounted for 19% of the total population living with HIV.2 Sexual contact was the most common route of transmission, followed by injection drug use—77% and 20%, respectively.2
It is important to note that the incidence and prevalence of HIV does not reflect the individuals who unknowingly are living with the disease. The disease burden associated with HIV infection and the availability of effective treatment modalities has led to the recommendation that all individuals undergo HIV screening at least once in their lifetime.3 Early identification of HIV infection is important to optimize the health of all individuals and future generations.
The interplay between high-risk sexual practices and the risk for HIV exposure and unintended pregnancy places the ObGyn at the forefront of HIV prevention and identification. Early diagnosis and standardized treatment with antiretroviral therapies have led to both a dramatic improvement in adult disease burden and a dramatic decrease in perinatal transmission.4,5 In 2019, perinatal transmission accounted for less than 1% of HIV transmission in the United States.2 This is a decrease of greater than 54% from 2014, which, again, emphasizes the role of the ObGyn in HIV management.6
Preconception care: Gynecologic screening, diagnosis, and management
The Centers for Disease Control and Prevention (CDC) recommends that an individual undergo HIV screening at least once in their lifetime.3 HIV screening algorithms have changed over the last 20 years to reduce the number of false-positive and/or false-negative results obtained through HIV antibody testing alone.7 HIV-1/2 antibody/antigen immunoassay is recommended as the initial screening test. If reactive, this should be followed by an HIV p24-specific antigen test. Reactivity for both the HIV-1/2 immunoassay and the HIV p24-specific antigen test confirms the diagnosis of HIV infection. However, if HIV p24-specific antigen testing is indeterminate or an acute HIV infection is suspected, an HIV nucleic acid test (NAT) should be performed.7,8
Upon a positive diagnosis, a multidisciplinary team approach is recommended to address the mental, social, and physical care of the patient. Team members should include an adult medicine clinician, an infectious disease clinician, an ObGyn, social services staff, and behavioral health support to achieve the goal of obtaining and maintaining the patient’s optimal health status.
TABLE 1 lists the recommended initial laboratory assessments that should follow a new diagnosis of HIV infection. Based on the laboratory results, the indicated vaccinations, antibiotic prophylaxis for opportunistic infections, and optimal combined antiretroviral therapy (cART) can be determined.9 The vaccinations listed in TABLE 2 should be up to date.10,11 Additionally, cervical cancer screening with cytology and human papillomavirus (HPV) testing and treatment should be performed in accordance with the 2019 American Society for Cervical Cancer Prevention (ASCCP) guidelines.12

Promptly initiating cART is of utmost importance; this decreases the rate of HIV transmission via sexual contact and decreases the rate of perinatal transmission.5,13 Results of the initial laboratory assessment, hepatitis B status, and desire for pregnancy/contraception should be considered when initiating cART.3,14,15
It is imperative to discuss sharing the positive diagnostic results with the patient’s partner. The CDC provides guidance for these discussions,16 which should address the use of preexposure prophylaxis (PrEP) if partner screening establishes partner serodiscordance (that is, HIV positivity in one partner and HIV negativity in the other partner). PrEP is a single pill approved by the US Food and Drug Administration (FDA) that combines tenofovir 300 mg and emtricitabine 200 mg daily17 and has been recommended since 2012.18-20 PrEP also should be considered in sexually active individuals who have higher-risk behaviors within an area with high HIV prevalence.18-21 Despite the CDC’s strong recommendations for PrEP use, lack of insurance coverage and high cost are barriers to universal use. The National Alliance of State and Territorial AIDS Directors (NASTAD) provides a list of patient and copayment assistance programs that can be found at the NASTAD website: https://nastad.org/prepcost-resources/prep-assitance-programs.
Continue to: Preconception considerations...
Preconception considerations
In individuals with known HIV infection, preconception consultation with an ObGyn or maternal-fetal medicine (MFM) specialist should be recommended prior to conception.22 Preconception recommendations include addressing optimization of maternal medical comorbidities, addressing routine health screening and vaccinations, performing sexually transmitted infection screening, and optimizing HIV disease status.3,22,23
With the assistance of adult medicine and infectious disease clinicians, a cART regimen that is sufficient to reliably maintain viral suppression (that is, viral load < 50 copies/mL on 2 separate occasions at least 3 months apart) and is safe for use in pregnancy should be established.3 In serodiscordant couples, recommended mechanisms to prevent HIV transmission during conception include sustained viral suppression in the HIV-positive partner, PrEP use in the HIV-negative partner, and timing of unprotected intercourse during peak fertility only.3
Antepartum care
The initial prenatal visit
Women who have no prior screening for HIV or prior negative HIV results should undergo HIV screening at the first prenatal visit.3 Screening should be performed in accordance with the “opt out method.”6 Using this method, a woman without a known diagnosis of HIV infection is told that she will undergo HIV screening as a component of routine prenatal care unless she decides that she does not want this test performed.6,24,25 At the time of screening, all pregnant women should be provided with comprehensive information regarding HIV screening, HIV screening results, and the implications of HIV infection on pregnancy.26
In the pregnant patient with confirmed HIV infection, all preconception considerations should be addressed. If not already in place, referrals to appropriate providers (infectious disease specialist, ObGyn, MFM specialist) and ancillary support staff (social services, behavioral health support) should be arranged. All efforts should be implemented to optimize additional medical comorbidities. TABLE 3 lists additional prenatal testing requirements.22
Antiretroviral therapy should be assessed for safety and efficacy in pregnancy and should comply with the CDC recommendations for cART in pregnancy.3 Patients with a T-lymphocyte cell count of less than 200 cells/mm3 and/or a viral load greater than 50 copies/mL despite adherent cART use should be referred to an infectious disease specialist to determine the need for alternative cART and/or the need for chemoprophylaxis against opportunistic infections.23
First and second trimester
Antiretroviral adherence and barriers to adherence should be addressed at every prenatal visit. If the patient is started on antiretroviral therapy in pregnancy or is switched to an alternative cART regimen, viral load assessment should be performed 2 to 4 weeks after the start or change in cART and then repeated monthly until undetectable levels are achieved.3,26 If an undetectable viral load cannot be obtained, cART adherence should be thoroughly evaluated, and the patient should be referred to an infectious disease or HIV treatment specialist.26
If the initial prenatal testing indicates an undetectable viral load, repeat viral load assessment can be performed every 3 months throughout the pregnancy.3 If initial prenatal testing indicates an undetectable HIV viral load and the T-lymphocyte count is greater than 200 cells/mm3, repeat viral load testing can be performed every 6 months to ensure stability.3
Early screening for gestational diabetes should be performed in patients receiving protease inhibitors because these agents may interfere with carbohydrate tolerance.22,26
Continue to: Third trimester...
Third trimester
Women with negative HIV screening at the initial prenatal evaluation should undergo repeat HIV screening in the third trimester if they are at high risk for HIV exposure.25 Factors that determine high-risk status are listed in TABLE 4.27 Sexually transmitted infection screening should be repeated in the third trimester.26

Repeat assessment of the viral load should be completed between 34 and 36 weeks’ gestation or sooner if additional indications for early term or late preterm delivery arise.3 Viral load assessments aid in determining delivery timing and route and the need for zidovudine (ZDV) treatment (FIGURE).

Studies that were performed prior to standardized cART use found higher rates of perinatal transmission associated with vaginal delivery when compared with cesarean delivery (CD).28-30 However, these studies did not account for measures of viral load within their study populations.28-30
In more recent studies performed in the era of standardized cART and viral load monitoring, CD does not provide protection from perinatal transmission when the maternal viral load is less than 1,000 copies/mL at the time of delivery.31 Similarly, delivery prior to 40 weeks’ gestation does not confer protection from perinatal transmission.32
Alternatively, if the maternal viral load is 1,000 copies/mL or greater, CD should be considered to reduce the risk of perinatal transmission. A scheduled, elective CD at 38 weeks’ gestation is recommended in those with a maternal viral load of 1,000 copies/mL or greater and no medical indication for earlier delivery in order to decrease the likelihood of labor onset and/or rupture of membranes prior to delivery.3,33
Intrapartum care
Rapid antigen testing (with follow-up confirmatory testing as indicated) is recommended in patients presenting to labor and delivery with no prior documentation of HIV status.3,8,26
Despite a significant decrease in perinatal transmission rates over the last 30 years, a large proportion of perinatal transmission cases are thought to result from intrapartum fetal exposure. While the mechanism of transmission is not known, a correlation between maternal viral load and risk for perinatal transmission has been shown. A maternal viral load of less than 1,000 copies/mL has been associated with a perinatal transmission risk of less than 2%.34,35 A maternal viral load between 50 and 999 copies/mL has been associated with a perinatal transmission rate of 1% to 2% compared with less than 1% for a maternal viral load of less than 50 copies/mL or undetectable measures.5,36,37
These differences in perinatal transmission rates have prompted the recommendation for administration of ZDV for a minimum of 3 hours prior to delivery in mothers with a viral load of 1,000 copies/mL or greater.4,38 The recommended ZDV dosing is: a 1-hour intravenous loading dose of 2 mg/kg followed by continuous infusion of 1 mg/kg per hour until delivery.39,40 Patients who opt for vaginal delivery despite nonsuppressed viral loads (≥1,000 copies/mL) after thorough perinatal counseling should receive ZDV at the start of labor through delivery.3 All patients should be continued on cART throughout their intrapartum and postpartum course.
The duration of membrane rupture and the use of invasive fetal monitoring (that is, fetal scalp electrodes) have been assessed as mechanisms of perinatal transmission. Although they were performed prior to the standardized use of cART, several studies demonstrated that increased perinatal transmission rates were associated with invasive fetal monitoring.34,41,42 While limited data have refuted this finding in women with suppressed viral loads (< 50 copies/mL), the American College of Obstetricians and Gynecologists recommends avoiding the use of invasive fetal monitoring in labor.26
Pre-cART studies demonstrated increased rates of perinatal transmission with longer durations of membrane rupture prior to delivery.43,44 More recent studies have reevaluated this association and determined that the increased perinatal transmission rates are more likely associated with higher maternal viral loads at the time of delivery rather than duration of membrane rupture.45-47 No clear evidence describes when or if CD after the onset of labor or rupture of membranes provides protection from perinatal HIV transmission in pregnant women with HIV receiving no antiretroviral drugs or only ZDV during labor.43,48 CD can be considered for patients in whom scheduled, pre-labor CD was planned who present in labor or with rupture of membranes prior to scheduled CD.26 These, and additional intrapartum considerations, are listed in TABLE 5.49,50
Appropriate personal protective equipment should be available and donned for all providers present throughout intrapartum management and at delivery.23,26 Should any provider injury occur, immediate cleansing of the injury site should be performed, followed by referral to proper workplace supervisors for additional laboratory testing and antiretroviral prophylaxis.

Continue to: Postpartum care...
Postpartum care
Postpartum contraception should be offered and provided in accordance with patient request. Regardless of other birth control methods, strict condom use should be advised. PrEP should be discussed and offered for all partners of serodiscordant couples.
Upon outpatient follow-up, assessment and provision of routine health maintenance should be performed. Any abnormal cervical pathology encountered during prenatal care should be managed in accordance with ASCCP guidelines.12 Follow-up care should be established with adult medicine, infectious disease, and ObGyn clinicians.26
Neonatal considerations
Neonates born to mothers with positive or unknown HIV status should undergo expedited HIV testing.51,52 Consultation should be conducted with pediatric or neonatology colleagues to determine the antiretroviral regimen and duration of therapy based on presumed HIV status of the neonate. Ideally, antiretroviral therapy should be initiated within 6 hours of delivery.3,53
Formula feeding should be implemented as maternal HIV infection is one of the few contraindications to breastfeeding.54,55 The risk of late breast milk transmission, defined as postnatal transmission that occurs after 1 month of age, may vary based on maternal viral load, but it has been reported as high as 8.9 transmissions per 100 person-years of breastfeeding.56
Resources available
Care of the pregnant patient with HIV and the reduction of perinatal transmission both depend on early diagnosis of HIV and effective treatment with cART. Such patients benefit from a team-based care model that includes the ObGyn and/or MFM specialist, infectious disease clinician, pediatrician, and social worker. As guidelines evolve for care of these patients, a reference checklist, such as the examples provided at the Society for Maternal-Fetal Medicine website (smfm.org) or at HIV.gov, provide an outline for:
- management before, during, and after pregnancy
- suggestions for management teams of interest to successfully carry out the checklist requirements
- proposals for measurements of quality performance with the use of checklists in the management of HIV in pregnancy.
In addition, assistance with clinical decision making for patients with HIV in pregnancy can be obtained via telephone consultation with the National Clinician Consultation Center–Perinatal HIV/AIDS (888-448-8765), which is available 24 hours a day, 7 days a week. ●
Human immunodeficiency virus (HIV) is a single-stranded enveloped RNA retrovirus that was first described in the 1980s and is known for its severity of systemic immune dysregulation and associated opportunistic infections. It is transmitted through contact with blood or bodily fluids, and it can be transmitted vertically, most often at the time of delivery. Since the advent of antiretroviral therapy, the average life expectancy and natural course of HIV infection has improved notably.1
In 2019, just over 1 million adults and adolescents in the United States were living with the diagnosis of HIV.2 In the same year, the rate of new HIV diagnoses in the United States had stabilized at a rate of 13.2 new cases per 100,000 individuals.2 Among this cohort, individuals identifying as females at birth accounted for 19% of the total population living with HIV.2 Sexual contact was the most common route of transmission, followed by injection drug use—77% and 20%, respectively.2
It is important to note that the incidence and prevalence of HIV does not reflect the individuals who unknowingly are living with the disease. The disease burden associated with HIV infection and the availability of effective treatment modalities has led to the recommendation that all individuals undergo HIV screening at least once in their lifetime.3 Early identification of HIV infection is important to optimize the health of all individuals and future generations.
The interplay between high-risk sexual practices and the risk for HIV exposure and unintended pregnancy places the ObGyn at the forefront of HIV prevention and identification. Early diagnosis and standardized treatment with antiretroviral therapies have led to both a dramatic improvement in adult disease burden and a dramatic decrease in perinatal transmission.4,5 In 2019, perinatal transmission accounted for less than 1% of HIV transmission in the United States.2 This is a decrease of greater than 54% from 2014, which, again, emphasizes the role of the ObGyn in HIV management.6
Preconception care: Gynecologic screening, diagnosis, and management
The Centers for Disease Control and Prevention (CDC) recommends that an individual undergo HIV screening at least once in their lifetime.3 HIV screening algorithms have changed over the last 20 years to reduce the number of false-positive and/or false-negative results obtained through HIV antibody testing alone.7 HIV-1/2 antibody/antigen immunoassay is recommended as the initial screening test. If reactive, this should be followed by an HIV p24-specific antigen test. Reactivity for both the HIV-1/2 immunoassay and the HIV p24-specific antigen test confirms the diagnosis of HIV infection. However, if HIV p24-specific antigen testing is indeterminate or an acute HIV infection is suspected, an HIV nucleic acid test (NAT) should be performed.7,8
Upon a positive diagnosis, a multidisciplinary team approach is recommended to address the mental, social, and physical care of the patient. Team members should include an adult medicine clinician, an infectious disease clinician, an ObGyn, social services staff, and behavioral health support to achieve the goal of obtaining and maintaining the patient’s optimal health status.
TABLE 1 lists the recommended initial laboratory assessments that should follow a new diagnosis of HIV infection. Based on the laboratory results, the indicated vaccinations, antibiotic prophylaxis for opportunistic infections, and optimal combined antiretroviral therapy (cART) can be determined.9 The vaccinations listed in TABLE 2 should be up to date.10,11 Additionally, cervical cancer screening with cytology and human papillomavirus (HPV) testing and treatment should be performed in accordance with the 2019 American Society for Cervical Cancer Prevention (ASCCP) guidelines.12

Promptly initiating cART is of utmost importance; this decreases the rate of HIV transmission via sexual contact and decreases the rate of perinatal transmission.5,13 Results of the initial laboratory assessment, hepatitis B status, and desire for pregnancy/contraception should be considered when initiating cART.3,14,15
It is imperative to discuss sharing the positive diagnostic results with the patient’s partner. The CDC provides guidance for these discussions,16 which should address the use of preexposure prophylaxis (PrEP) if partner screening establishes partner serodiscordance (that is, HIV positivity in one partner and HIV negativity in the other partner). PrEP is a single pill approved by the US Food and Drug Administration (FDA) that combines tenofovir 300 mg and emtricitabine 200 mg daily17 and has been recommended since 2012.18-20 PrEP also should be considered in sexually active individuals who have higher-risk behaviors within an area with high HIV prevalence.18-21 Despite the CDC’s strong recommendations for PrEP use, lack of insurance coverage and high cost are barriers to universal use. The National Alliance of State and Territorial AIDS Directors (NASTAD) provides a list of patient and copayment assistance programs that can be found at the NASTAD website: https://nastad.org/prepcost-resources/prep-assitance-programs.
Continue to: Preconception considerations...
Preconception considerations
In individuals with known HIV infection, preconception consultation with an ObGyn or maternal-fetal medicine (MFM) specialist should be recommended prior to conception.22 Preconception recommendations include addressing optimization of maternal medical comorbidities, addressing routine health screening and vaccinations, performing sexually transmitted infection screening, and optimizing HIV disease status.3,22,23
With the assistance of adult medicine and infectious disease clinicians, a cART regimen that is sufficient to reliably maintain viral suppression (that is, viral load < 50 copies/mL on 2 separate occasions at least 3 months apart) and is safe for use in pregnancy should be established.3 In serodiscordant couples, recommended mechanisms to prevent HIV transmission during conception include sustained viral suppression in the HIV-positive partner, PrEP use in the HIV-negative partner, and timing of unprotected intercourse during peak fertility only.3
Antepartum care
The initial prenatal visit
Women who have no prior screening for HIV or prior negative HIV results should undergo HIV screening at the first prenatal visit.3 Screening should be performed in accordance with the “opt out method.”6 Using this method, a woman without a known diagnosis of HIV infection is told that she will undergo HIV screening as a component of routine prenatal care unless she decides that she does not want this test performed.6,24,25 At the time of screening, all pregnant women should be provided with comprehensive information regarding HIV screening, HIV screening results, and the implications of HIV infection on pregnancy.26
In the pregnant patient with confirmed HIV infection, all preconception considerations should be addressed. If not already in place, referrals to appropriate providers (infectious disease specialist, ObGyn, MFM specialist) and ancillary support staff (social services, behavioral health support) should be arranged. All efforts should be implemented to optimize additional medical comorbidities. TABLE 3 lists additional prenatal testing requirements.22
Antiretroviral therapy should be assessed for safety and efficacy in pregnancy and should comply with the CDC recommendations for cART in pregnancy.3 Patients with a T-lymphocyte cell count of less than 200 cells/mm3 and/or a viral load greater than 50 copies/mL despite adherent cART use should be referred to an infectious disease specialist to determine the need for alternative cART and/or the need for chemoprophylaxis against opportunistic infections.23
First and second trimester
Antiretroviral adherence and barriers to adherence should be addressed at every prenatal visit. If the patient is started on antiretroviral therapy in pregnancy or is switched to an alternative cART regimen, viral load assessment should be performed 2 to 4 weeks after the start or change in cART and then repeated monthly until undetectable levels are achieved.3,26 If an undetectable viral load cannot be obtained, cART adherence should be thoroughly evaluated, and the patient should be referred to an infectious disease or HIV treatment specialist.26
If the initial prenatal testing indicates an undetectable viral load, repeat viral load assessment can be performed every 3 months throughout the pregnancy.3 If initial prenatal testing indicates an undetectable HIV viral load and the T-lymphocyte count is greater than 200 cells/mm3, repeat viral load testing can be performed every 6 months to ensure stability.3
Early screening for gestational diabetes should be performed in patients receiving protease inhibitors because these agents may interfere with carbohydrate tolerance.22,26
Continue to: Third trimester...
Third trimester
Women with negative HIV screening at the initial prenatal evaluation should undergo repeat HIV screening in the third trimester if they are at high risk for HIV exposure.25 Factors that determine high-risk status are listed in TABLE 4.27 Sexually transmitted infection screening should be repeated in the third trimester.26

Repeat assessment of the viral load should be completed between 34 and 36 weeks’ gestation or sooner if additional indications for early term or late preterm delivery arise.3 Viral load assessments aid in determining delivery timing and route and the need for zidovudine (ZDV) treatment (FIGURE).

Studies that were performed prior to standardized cART use found higher rates of perinatal transmission associated with vaginal delivery when compared with cesarean delivery (CD).28-30 However, these studies did not account for measures of viral load within their study populations.28-30
In more recent studies performed in the era of standardized cART and viral load monitoring, CD does not provide protection from perinatal transmission when the maternal viral load is less than 1,000 copies/mL at the time of delivery.31 Similarly, delivery prior to 40 weeks’ gestation does not confer protection from perinatal transmission.32
Alternatively, if the maternal viral load is 1,000 copies/mL or greater, CD should be considered to reduce the risk of perinatal transmission. A scheduled, elective CD at 38 weeks’ gestation is recommended in those with a maternal viral load of 1,000 copies/mL or greater and no medical indication for earlier delivery in order to decrease the likelihood of labor onset and/or rupture of membranes prior to delivery.3,33
Intrapartum care
Rapid antigen testing (with follow-up confirmatory testing as indicated) is recommended in patients presenting to labor and delivery with no prior documentation of HIV status.3,8,26
Despite a significant decrease in perinatal transmission rates over the last 30 years, a large proportion of perinatal transmission cases are thought to result from intrapartum fetal exposure. While the mechanism of transmission is not known, a correlation between maternal viral load and risk for perinatal transmission has been shown. A maternal viral load of less than 1,000 copies/mL has been associated with a perinatal transmission risk of less than 2%.34,35 A maternal viral load between 50 and 999 copies/mL has been associated with a perinatal transmission rate of 1% to 2% compared with less than 1% for a maternal viral load of less than 50 copies/mL or undetectable measures.5,36,37
These differences in perinatal transmission rates have prompted the recommendation for administration of ZDV for a minimum of 3 hours prior to delivery in mothers with a viral load of 1,000 copies/mL or greater.4,38 The recommended ZDV dosing is: a 1-hour intravenous loading dose of 2 mg/kg followed by continuous infusion of 1 mg/kg per hour until delivery.39,40 Patients who opt for vaginal delivery despite nonsuppressed viral loads (≥1,000 copies/mL) after thorough perinatal counseling should receive ZDV at the start of labor through delivery.3 All patients should be continued on cART throughout their intrapartum and postpartum course.
The duration of membrane rupture and the use of invasive fetal monitoring (that is, fetal scalp electrodes) have been assessed as mechanisms of perinatal transmission. Although they were performed prior to the standardized use of cART, several studies demonstrated that increased perinatal transmission rates were associated with invasive fetal monitoring.34,41,42 While limited data have refuted this finding in women with suppressed viral loads (< 50 copies/mL), the American College of Obstetricians and Gynecologists recommends avoiding the use of invasive fetal monitoring in labor.26
Pre-cART studies demonstrated increased rates of perinatal transmission with longer durations of membrane rupture prior to delivery.43,44 More recent studies have reevaluated this association and determined that the increased perinatal transmission rates are more likely associated with higher maternal viral loads at the time of delivery rather than duration of membrane rupture.45-47 No clear evidence describes when or if CD after the onset of labor or rupture of membranes provides protection from perinatal HIV transmission in pregnant women with HIV receiving no antiretroviral drugs or only ZDV during labor.43,48 CD can be considered for patients in whom scheduled, pre-labor CD was planned who present in labor or with rupture of membranes prior to scheduled CD.26 These, and additional intrapartum considerations, are listed in TABLE 5.49,50
Appropriate personal protective equipment should be available and donned for all providers present throughout intrapartum management and at delivery.23,26 Should any provider injury occur, immediate cleansing of the injury site should be performed, followed by referral to proper workplace supervisors for additional laboratory testing and antiretroviral prophylaxis.

Continue to: Postpartum care...
Postpartum care
Postpartum contraception should be offered and provided in accordance with patient request. Regardless of other birth control methods, strict condom use should be advised. PrEP should be discussed and offered for all partners of serodiscordant couples.
Upon outpatient follow-up, assessment and provision of routine health maintenance should be performed. Any abnormal cervical pathology encountered during prenatal care should be managed in accordance with ASCCP guidelines.12 Follow-up care should be established with adult medicine, infectious disease, and ObGyn clinicians.26
Neonatal considerations
Neonates born to mothers with positive or unknown HIV status should undergo expedited HIV testing.51,52 Consultation should be conducted with pediatric or neonatology colleagues to determine the antiretroviral regimen and duration of therapy based on presumed HIV status of the neonate. Ideally, antiretroviral therapy should be initiated within 6 hours of delivery.3,53
Formula feeding should be implemented as maternal HIV infection is one of the few contraindications to breastfeeding.54,55 The risk of late breast milk transmission, defined as postnatal transmission that occurs after 1 month of age, may vary based on maternal viral load, but it has been reported as high as 8.9 transmissions per 100 person-years of breastfeeding.56
Resources available
Care of the pregnant patient with HIV and the reduction of perinatal transmission both depend on early diagnosis of HIV and effective treatment with cART. Such patients benefit from a team-based care model that includes the ObGyn and/or MFM specialist, infectious disease clinician, pediatrician, and social worker. As guidelines evolve for care of these patients, a reference checklist, such as the examples provided at the Society for Maternal-Fetal Medicine website (smfm.org) or at HIV.gov, provide an outline for:
- management before, during, and after pregnancy
- suggestions for management teams of interest to successfully carry out the checklist requirements
- proposals for measurements of quality performance with the use of checklists in the management of HIV in pregnancy.
In addition, assistance with clinical decision making for patients with HIV in pregnancy can be obtained via telephone consultation with the National Clinician Consultation Center–Perinatal HIV/AIDS (888-448-8765), which is available 24 hours a day, 7 days a week. ●
- Samji H, Cescon A, Hogg RS, et al; North American AIDS Cohort Collaboration on Research and Design (NA-ACCORD) of IeDEA. Closing the gap: increases in life expectancy among treated HIV-positive individuals in the United States and Canada. PLoS One. 2013;8:e81355. doi: 10.1371/journal. pone.0081355.
- Centers for Disease Control and Prevention. May 1, 2021. HIV Surveillance Report, 2019, vol. 32: Diagnosis of HIV infection in the United States and dependent areas, 2019. Accessed February 15, 2022. http://www.cdc.gov/hiv/library/reports /hiv-surveillance.html
- Panel on Antiretroviral Therapy and Medical Management of Children Living with HIV. Guidelines for the use of antiretroviral agents in pediatric HIV infection. https: //clinicalinfo.hiv.gov/en/guidelines/pediatric-arv. Accessed February 15, 2022.
- Connor EM, Sperling RS, Gelber R, et al. Reduction of maternal-infant transmission of human immunodeficiency virus type 1 with zidovudine treatment. Pediatric AIDS Clinical Trials Group Protocol 076 Study Group. N Engl J Med. 1994;331:1173-1180.
- Townsend CL, Byrne L, Cortina-Borja M, et al. Earlier initiation of ART and further decline in mother-to-child HIV transmission rates, 2000-2011. AIDS. 2014;28:1049-1057.
- Centers for Disease Control and Prevention. January 26, 2022. HIV and pregnant women, infants, and children. Accessed February 15, 2022. https://www.cdc.gov/hiv/group/gender /pregnantwomen/index.html
- Centers for Disease Control and Prevention. 2018 Quick reference guide: Recommended laboratory HIV testing algorithm for serum or plasma specimens. National Center for HIV/AIDS, Viral Hepatitis, and TB Prevention (US); Division of HIV/AIDS Prevention; Association of Public Health Laboratories. Updated January 2018. https://stacks. cdc.gov/view/cdc/50872
- Centers for Disease Control and Prevention, Association of Public Health Laboratories. June 27, 2014. Laboratory testing for the diagnosis of HIV infection: updated recommendations. Accessed February 15, 2022. http://stacks.cdc.gov/view /cdc/23447
- Guidelines for the prevention and treatment of opportunistic infections in adults and adolescents with HIV. Updated April 12, 2022. Accessed July 6, 2022. https://clinicalinfo.hiv .gov/en/guidelines/adult-and-adolescent-opportunistic -infection/whats-new-guidelines
- Rubin LG, Levin MJ, Ljungman P, et al. 2013 IDSA clinical practice guideline for vaccination of the immunocompromised host. Clin Infect Dis. 2014;58: e44–e100. doi: 10.1093/cid/cit684.
- Centers for Disease Control and Prevention. ACIP: Guidance for vaccine recommendations for pregnant and breastfeeding women. Accessed July 5, 2022. https://www.cdc.gov /vaccines/hcp/acip-recs/rec-vac-preg.html?CDC_AA _refVal=https%3A%2F%2Fwww.cdc.gov%2Fvaccines%2Facip %2Fcommittee%2Fguidance%2Frec-vac-preg.html
- Perkins RB, Guido RS, Castle PE, et al; for the 2019 ASCCP Risk-Based Management Consensus Guidelines Committee. 2019 ASCCP risk-based management consensus guidelines for abnormal cervical cancer screening tests and cancer precursors. J Low Genit Tract Dis. 2020;24:102-131. doi: 10.1097/LGT.0000000000000525. Erratum in: J Low Genit Tract Dis. 2020;24:427.
- Cohen MS, Chen YQ, McCauley M, et al; HPTN 052 Study Team. Prevention of HIV-1 infection with early antiretroviral therapy. N Engl J Med. 2011;365:493-505.
- Drug interactions between antiretroviral agents and hormonal contraceptives. Accessed July 6, 2022. https://clinicalinfo .hiv.gov/en/table/table-3-drug-interactions-between -antiretroviral-agents-and-hormonal-contraceptives
- Panel on Treatment of HIV During Pregnancy and Prevention of Perinatal Transmission. Recommendations for use of antiretroviral drugs in pregnancy and interventions to reduce perinatal HIV transmission in the United States. Accessed July 7, 2022. https://clinicalinfo.hiv.gov/en/guidelines/perinatal /whats-new-guidelines
- Centers for Disease Control and Prevention. Recommendations for partner services programs for HIV infection, syphilis, gonorrhea, and chlamydial infection. MMWR Recomm Rep. 2008;57(RR-9):1–83.
- Gilead Sciences, Inc. Truvada (emtricitabine 200 mg/ tenofovir disoproxil fumarate 300 mg tablets). Accessed July 6, 2022. https://truvada.com
- Centers for Disease Control and Prevention. Interim guidance for clinicians considering the use of preexposure prophylaxis for the prevention of HIV infection in heterosexually active adults. MMWR Morb Mortal Wkly Rep. 2012;61:586-589.
- Baeten JM, Donnell D, Ndase P, et al; Partners PrEP Study Team. Antiretroviral prophylaxis for HIV prevention in heterosexual men and women. N Engl J Med. 2012;367: 399-410.
- Celum C, Baeten JM. Antiretroviral-based HIV-1 prevention: antiretroviral treatment and pre-exposure prophylaxis. Antivir Ther. 2012;17:1483-1493.
- Thigpen MC, Kebaabetswe PM, Paxton LA, et al; TDF2 Study Group. Antiretroviral preexposure prophylaxis for heterosexual HIV transmission in Botswana. N Engl J Med. 2012;367:423-434.
- Society for Maternal-Fetal Medicine. Special statement: updated checklists for pregnancy management in persons with HIV. Accessed July 5, 2022. https://www.smfm.org /publications/334-smfm-special-statement-updated -checklists-for-pregnancy-management-in-persons-with-hiv
- American College of Obstetricians and Gynecologists. ACOG committee opinion no. 752. Prenatal and perinatal human immunodeficiency virus testing. Obstet Gynecol. 2018;132:e138-e142.
- Human immunodeficiency virus screening. Joint statement of the American Academy of Pediatrics and the American College of Obstetricians and Gynecologists. Pediatrics. 1999;104(1 pt 1):128.
- Branson BM, Handsfield HH, Lampe MA, et al; Centers for Disease Control and Prevention. Revised recommendations for HIV testing of adults, adolescents, and pregnant women in health care settings. MMWR Recomm Rep. 2006; 55(RR-14):1-17.
- American College of Obstetricians and Gynecologists. ACOG committee opinion no. 751. Labor and delivery management of women with human immunodeficiency virus infection. Obstet Gynecol. 2018;132:e131-e137.
- Centers for Disease Control and Prevention. Factors increasing the risk of acquiring or transmitting HIV. November 12, 2019. Accessed July 29, 2022. https://www.cdc .gov/hiv/risk/estimates/riskfactors.html
- Mandelbrot L, Le Chenadec J, Berrebi A, et al. Perinatal HIV1 transmission: interaction between zidovudine prophylaxis and mode of delivery in the French Perinatal Cohort. JAMA. 1998;280:55-60.
- European Mode of Delivery Collaboration. Elective caesarean-section versus vaginal delivery in prevention of vertical HIV-1 transmission: a randomised clinical trial. Lancet. 1999;353:1035-1039.
- International Perinatal HIV Group; Andiman W, Bryson Y, de Martino M, et al. The mode of delivery and the risk of vertical transmission of human immunodeficiency virus type 1—a meta-analysis of 15 prospective cohort studies. N Engl J Med. 1999;340:977-987.
- Briand N, Jasseron C, Sibiude J, et al. Cesarean section for HIV-infected women in the combination antiretroviral therapies era, 2000–2010. Am J Obstet Gynecol. 2013;209: 335.e1-335.e12.
- Scott RK, Chakhtoura N, Burke MM, et al. Delivery after 40 weeks of gestation in pregnant women with well-controlled human immunodeficiency virus. Obstet Gynecol. 2017;130:502-510.
- American College of Obstetricians and Gynecologists. Committee opinion no. 560. Medically indicated late-preterm and early-term deliveries. Obstet Gynecol. 2013;121:908-910.
- Mofenson LM, Lambert JS, Stiehm ER, et al. Risk factors for perinatal transmission of human immunodeficiency virus type 1 in women treated with zidovudine. Pediatric AIDS Clinical Trials Group Study 185 Team. N Engl J Med. 1999;341:385-393.
- Garcia PM, Kalish LA, Pitt J, et al. Maternal levels of plasma human immunodeficiency virus type 1 RNA and the risk of perinatal transmission. Women and Infants Transmission Study Group. N Engl J Med. 1999;341:394-402.
- Briand N, Warszawski J, Mandelbrot L, et al; ANRS-EPF CO1CO11 Study Group. Is intrapartum intravenous zidovudine for prevention of mother-to-child HIV-1 transmission still useful in the combination antiretroviral therapy era? Clin Infect Dis. 2013;57:903-914.
- Myer L, Phillips TK, McIntyre JA, et al. HIV viraemia and mother-to-child transmission risk after antiretroviral therapy initiation in pregnancy in Cape Town, South Africa. HIV Med. 2017;18:80-88.
- Rodman JH, Flynn PM, Robbins B, et al. Systemic pharmacokinetics and cellular pharmacology of zidovudine in human immunodeficiency virus type 1-infected women and newborn infants. J Infect Dis. 1999;180:1844-1850.
- Wade NA, Birkhead GS, Warren BL, et al. Abbreviated regimens of zidovudine prophylaxis and perinatal transmission of the human immunodeficiency virus. N Engl J Med. 1998;339:1409-1414.
- Nielsen-Saines K, Watts HD, Veloso VS, et al; NICHD HPTN 040/PACTG 1043 Protocol Team. Three postpartum antiretroviral regimens to prevent intrapartum HIV infection. N Engl J Med. 2012;366:2368-2379.
- Mandelbrot L, Mayaux MJ, Bongain A, et al. Obstetric factors and mother-to-child transmission of human immunodeficiency virus type 1: the French perinatal cohorts. SEROGEST French Pediatric HIV Infection Study Group. Am J Obstet Gynecol. 1996;175(3 pt 1):661-667.
- Shapiro DE, Sperling RS, Mandelbrot L, et al. Risk factors for perinatal human immunodeficiency virus transmission in patients receiving zidovudine prophylaxis. Pediatric AIDS Clinical Trials Group protocol 076 Study Group. Obstet Gynecol. 1999;94:897-908.
- International Perinatal HIV Group. Duration of ruptured membranes and vertical transmission of HIV-1: a meta-analysis from 15 prospective cohort studies. AIDS. 2001;15:357-368.
- Nielsen TF, Hokegard KH. Postoperative cesarean section morbidity: a prospective study. Am J Obstet Gynecol. 1983;146:911-916.
- Mark S, Murphy KE, Read S, et al. HIV mother-to-child transmission, mode of delivery, and duration of rupture of membranes: experience in the current era. Infect Dis Obstet Gynecol. 2012;2012:267969.
- Cotter AM, Brookfield KF, Duthely LM, et al. Duration of membrane rupture and risk of perinatal transmission of HIV1 in the era of combination antiretroviral therapy. Am J Obstet Gynecol. 2012;207:482.e1-482.e5.
- Peters H, Byrne L, De Ruiter A, et al. Duration of ruptured membranes and mother-to-child HIV transmission: a prospective population-based surveillance study. BJOG. 2016;123:975-981.
- Jamieson DJ, Read JS, Kourtis AP, et al. Cesarean delivery for HIV-infected women: recommendations and controversies. Am J Obstet Gynecol. 2007;197(3 suppl):S96-S100.
- Cambic CR, Avram MJ, Gupta DK, et al. Effect of ritonavir-induced cytochrome P450 3A4 inhibition on plasma fentanyl concentrations during patient-controlled epidural labor analgesia: a pharmacokinetic simulation. Int J Obstet Anesth. 2014;23:45-51.
- Navarro J, Curran A, Burgos J, et al. Acute leg ischaemia in an HIV-infected patient receiving antiretroviral treatment. Antivir Ther. 2017;22:89-90.
- American Academy of Pediatrics, American College of Obstetricians and Gynecologists. Guidelines for Perinatal Care. 8th ed. American Academy of Pediatrics, American College of Obstetricians and Gynecologists; 2017.
- Siberry GK, Abzug MJ, Nachman S, et al; Panel on Opportunistic Infections in HIV-Exposed and HIV-Infected Children. Guidelines for the prevention and treatment of opportunistic infections in HIV-exposed and HIV-infected children: recommendations from the National Institutes of Health, Centers for Disease Control and Prevention, the HIV Medicine Association of the Infectious Diseases Society of America, the Pediatric Infectious Diseases Society, and the American Academy of Pediatrics. Pediatr Infect Dis J. 32(suppl 2[0 2]):i–KK4.
- Panel on Antiretroviral Therapy and Medical Management of Children Living with HIV. Guidelines for the use of antiretroviral agents in pediatric HIV infection. Accessed February 15, 2022. https://clinicalinfo.hiv.gov/en/guidelines /pediatric-arv
- Committee on Health Care for Underserved Women, American College of Obstetricians and Gynecologists. ACOG committee opinion no. 361. Breastfeeding: maternal and infant aspects. Obstet Gynecol. 2007;109(2 pt 1):479-480.
- Committee on Pediatric AIDS; Mofenson LM, Flynn PM, Aldrovandi GM, et al. Infant feeding and transmission of human immunodeficiency virus in the United States. Pediatrics. 2013;131:391-396.
- Breastfeeding and HIV International Transmission Study Group; Coutsoudis A, Dabis F, Fawzi W, et al. Late postnatal transmission of HIV-1 in breast-fed children: an individual patient data meta-analysis. J Infect Dis. 2004;189:2154-2166.
- Samji H, Cescon A, Hogg RS, et al; North American AIDS Cohort Collaboration on Research and Design (NA-ACCORD) of IeDEA. Closing the gap: increases in life expectancy among treated HIV-positive individuals in the United States and Canada. PLoS One. 2013;8:e81355. doi: 10.1371/journal. pone.0081355.
- Centers for Disease Control and Prevention. May 1, 2021. HIV Surveillance Report, 2019, vol. 32: Diagnosis of HIV infection in the United States and dependent areas, 2019. Accessed February 15, 2022. http://www.cdc.gov/hiv/library/reports /hiv-surveillance.html
- Panel on Antiretroviral Therapy and Medical Management of Children Living with HIV. Guidelines for the use of antiretroviral agents in pediatric HIV infection. https: //clinicalinfo.hiv.gov/en/guidelines/pediatric-arv. Accessed February 15, 2022.
- Connor EM, Sperling RS, Gelber R, et al. Reduction of maternal-infant transmission of human immunodeficiency virus type 1 with zidovudine treatment. Pediatric AIDS Clinical Trials Group Protocol 076 Study Group. N Engl J Med. 1994;331:1173-1180.
- Townsend CL, Byrne L, Cortina-Borja M, et al. Earlier initiation of ART and further decline in mother-to-child HIV transmission rates, 2000-2011. AIDS. 2014;28:1049-1057.
- Centers for Disease Control and Prevention. January 26, 2022. HIV and pregnant women, infants, and children. Accessed February 15, 2022. https://www.cdc.gov/hiv/group/gender /pregnantwomen/index.html
- Centers for Disease Control and Prevention. 2018 Quick reference guide: Recommended laboratory HIV testing algorithm for serum or plasma specimens. National Center for HIV/AIDS, Viral Hepatitis, and TB Prevention (US); Division of HIV/AIDS Prevention; Association of Public Health Laboratories. Updated January 2018. https://stacks. cdc.gov/view/cdc/50872
- Centers for Disease Control and Prevention, Association of Public Health Laboratories. June 27, 2014. Laboratory testing for the diagnosis of HIV infection: updated recommendations. Accessed February 15, 2022. http://stacks.cdc.gov/view /cdc/23447
- Guidelines for the prevention and treatment of opportunistic infections in adults and adolescents with HIV. Updated April 12, 2022. Accessed July 6, 2022. https://clinicalinfo.hiv .gov/en/guidelines/adult-and-adolescent-opportunistic -infection/whats-new-guidelines
- Rubin LG, Levin MJ, Ljungman P, et al. 2013 IDSA clinical practice guideline for vaccination of the immunocompromised host. Clin Infect Dis. 2014;58: e44–e100. doi: 10.1093/cid/cit684.
- Centers for Disease Control and Prevention. ACIP: Guidance for vaccine recommendations for pregnant and breastfeeding women. Accessed July 5, 2022. https://www.cdc.gov /vaccines/hcp/acip-recs/rec-vac-preg.html?CDC_AA _refVal=https%3A%2F%2Fwww.cdc.gov%2Fvaccines%2Facip %2Fcommittee%2Fguidance%2Frec-vac-preg.html
- Perkins RB, Guido RS, Castle PE, et al; for the 2019 ASCCP Risk-Based Management Consensus Guidelines Committee. 2019 ASCCP risk-based management consensus guidelines for abnormal cervical cancer screening tests and cancer precursors. J Low Genit Tract Dis. 2020;24:102-131. doi: 10.1097/LGT.0000000000000525. Erratum in: J Low Genit Tract Dis. 2020;24:427.
- Cohen MS, Chen YQ, McCauley M, et al; HPTN 052 Study Team. Prevention of HIV-1 infection with early antiretroviral therapy. N Engl J Med. 2011;365:493-505.
- Drug interactions between antiretroviral agents and hormonal contraceptives. Accessed July 6, 2022. https://clinicalinfo .hiv.gov/en/table/table-3-drug-interactions-between -antiretroviral-agents-and-hormonal-contraceptives
- Panel on Treatment of HIV During Pregnancy and Prevention of Perinatal Transmission. Recommendations for use of antiretroviral drugs in pregnancy and interventions to reduce perinatal HIV transmission in the United States. Accessed July 7, 2022. https://clinicalinfo.hiv.gov/en/guidelines/perinatal /whats-new-guidelines
- Centers for Disease Control and Prevention. Recommendations for partner services programs for HIV infection, syphilis, gonorrhea, and chlamydial infection. MMWR Recomm Rep. 2008;57(RR-9):1–83.
- Gilead Sciences, Inc. Truvada (emtricitabine 200 mg/ tenofovir disoproxil fumarate 300 mg tablets). Accessed July 6, 2022. https://truvada.com
- Centers for Disease Control and Prevention. Interim guidance for clinicians considering the use of preexposure prophylaxis for the prevention of HIV infection in heterosexually active adults. MMWR Morb Mortal Wkly Rep. 2012;61:586-589.
- Baeten JM, Donnell D, Ndase P, et al; Partners PrEP Study Team. Antiretroviral prophylaxis for HIV prevention in heterosexual men and women. N Engl J Med. 2012;367: 399-410.
- Celum C, Baeten JM. Antiretroviral-based HIV-1 prevention: antiretroviral treatment and pre-exposure prophylaxis. Antivir Ther. 2012;17:1483-1493.
- Thigpen MC, Kebaabetswe PM, Paxton LA, et al; TDF2 Study Group. Antiretroviral preexposure prophylaxis for heterosexual HIV transmission in Botswana. N Engl J Med. 2012;367:423-434.
- Society for Maternal-Fetal Medicine. Special statement: updated checklists for pregnancy management in persons with HIV. Accessed July 5, 2022. https://www.smfm.org /publications/334-smfm-special-statement-updated -checklists-for-pregnancy-management-in-persons-with-hiv
- American College of Obstetricians and Gynecologists. ACOG committee opinion no. 752. Prenatal and perinatal human immunodeficiency virus testing. Obstet Gynecol. 2018;132:e138-e142.
- Human immunodeficiency virus screening. Joint statement of the American Academy of Pediatrics and the American College of Obstetricians and Gynecologists. Pediatrics. 1999;104(1 pt 1):128.
- Branson BM, Handsfield HH, Lampe MA, et al; Centers for Disease Control and Prevention. Revised recommendations for HIV testing of adults, adolescents, and pregnant women in health care settings. MMWR Recomm Rep. 2006; 55(RR-14):1-17.
- American College of Obstetricians and Gynecologists. ACOG committee opinion no. 751. Labor and delivery management of women with human immunodeficiency virus infection. Obstet Gynecol. 2018;132:e131-e137.
- Centers for Disease Control and Prevention. Factors increasing the risk of acquiring or transmitting HIV. November 12, 2019. Accessed July 29, 2022. https://www.cdc .gov/hiv/risk/estimates/riskfactors.html
- Mandelbrot L, Le Chenadec J, Berrebi A, et al. Perinatal HIV1 transmission: interaction between zidovudine prophylaxis and mode of delivery in the French Perinatal Cohort. JAMA. 1998;280:55-60.
- European Mode of Delivery Collaboration. Elective caesarean-section versus vaginal delivery in prevention of vertical HIV-1 transmission: a randomised clinical trial. Lancet. 1999;353:1035-1039.
- International Perinatal HIV Group; Andiman W, Bryson Y, de Martino M, et al. The mode of delivery and the risk of vertical transmission of human immunodeficiency virus type 1—a meta-analysis of 15 prospective cohort studies. N Engl J Med. 1999;340:977-987.
- Briand N, Jasseron C, Sibiude J, et al. Cesarean section for HIV-infected women in the combination antiretroviral therapies era, 2000–2010. Am J Obstet Gynecol. 2013;209: 335.e1-335.e12.
- Scott RK, Chakhtoura N, Burke MM, et al. Delivery after 40 weeks of gestation in pregnant women with well-controlled human immunodeficiency virus. Obstet Gynecol. 2017;130:502-510.
- American College of Obstetricians and Gynecologists. Committee opinion no. 560. Medically indicated late-preterm and early-term deliveries. Obstet Gynecol. 2013;121:908-910.
- Mofenson LM, Lambert JS, Stiehm ER, et al. Risk factors for perinatal transmission of human immunodeficiency virus type 1 in women treated with zidovudine. Pediatric AIDS Clinical Trials Group Study 185 Team. N Engl J Med. 1999;341:385-393.
- Garcia PM, Kalish LA, Pitt J, et al. Maternal levels of plasma human immunodeficiency virus type 1 RNA and the risk of perinatal transmission. Women and Infants Transmission Study Group. N Engl J Med. 1999;341:394-402.
- Briand N, Warszawski J, Mandelbrot L, et al; ANRS-EPF CO1CO11 Study Group. Is intrapartum intravenous zidovudine for prevention of mother-to-child HIV-1 transmission still useful in the combination antiretroviral therapy era? Clin Infect Dis. 2013;57:903-914.
- Myer L, Phillips TK, McIntyre JA, et al. HIV viraemia and mother-to-child transmission risk after antiretroviral therapy initiation in pregnancy in Cape Town, South Africa. HIV Med. 2017;18:80-88.
- Rodman JH, Flynn PM, Robbins B, et al. Systemic pharmacokinetics and cellular pharmacology of zidovudine in human immunodeficiency virus type 1-infected women and newborn infants. J Infect Dis. 1999;180:1844-1850.
- Wade NA, Birkhead GS, Warren BL, et al. Abbreviated regimens of zidovudine prophylaxis and perinatal transmission of the human immunodeficiency virus. N Engl J Med. 1998;339:1409-1414.
- Nielsen-Saines K, Watts HD, Veloso VS, et al; NICHD HPTN 040/PACTG 1043 Protocol Team. Three postpartum antiretroviral regimens to prevent intrapartum HIV infection. N Engl J Med. 2012;366:2368-2379.
- Mandelbrot L, Mayaux MJ, Bongain A, et al. Obstetric factors and mother-to-child transmission of human immunodeficiency virus type 1: the French perinatal cohorts. SEROGEST French Pediatric HIV Infection Study Group. Am J Obstet Gynecol. 1996;175(3 pt 1):661-667.
- Shapiro DE, Sperling RS, Mandelbrot L, et al. Risk factors for perinatal human immunodeficiency virus transmission in patients receiving zidovudine prophylaxis. Pediatric AIDS Clinical Trials Group protocol 076 Study Group. Obstet Gynecol. 1999;94:897-908.
- International Perinatal HIV Group. Duration of ruptured membranes and vertical transmission of HIV-1: a meta-analysis from 15 prospective cohort studies. AIDS. 2001;15:357-368.
- Nielsen TF, Hokegard KH. Postoperative cesarean section morbidity: a prospective study. Am J Obstet Gynecol. 1983;146:911-916.
- Mark S, Murphy KE, Read S, et al. HIV mother-to-child transmission, mode of delivery, and duration of rupture of membranes: experience in the current era. Infect Dis Obstet Gynecol. 2012;2012:267969.
- Cotter AM, Brookfield KF, Duthely LM, et al. Duration of membrane rupture and risk of perinatal transmission of HIV1 in the era of combination antiretroviral therapy. Am J Obstet Gynecol. 2012;207:482.e1-482.e5.
- Peters H, Byrne L, De Ruiter A, et al. Duration of ruptured membranes and mother-to-child HIV transmission: a prospective population-based surveillance study. BJOG. 2016;123:975-981.
- Jamieson DJ, Read JS, Kourtis AP, et al. Cesarean delivery for HIV-infected women: recommendations and controversies. Am J Obstet Gynecol. 2007;197(3 suppl):S96-S100.
- Cambic CR, Avram MJ, Gupta DK, et al. Effect of ritonavir-induced cytochrome P450 3A4 inhibition on plasma fentanyl concentrations during patient-controlled epidural labor analgesia: a pharmacokinetic simulation. Int J Obstet Anesth. 2014;23:45-51.
- Navarro J, Curran A, Burgos J, et al. Acute leg ischaemia in an HIV-infected patient receiving antiretroviral treatment. Antivir Ther. 2017;22:89-90.
- American Academy of Pediatrics, American College of Obstetricians and Gynecologists. Guidelines for Perinatal Care. 8th ed. American Academy of Pediatrics, American College of Obstetricians and Gynecologists; 2017.
- Siberry GK, Abzug MJ, Nachman S, et al; Panel on Opportunistic Infections in HIV-Exposed and HIV-Infected Children. Guidelines for the prevention and treatment of opportunistic infections in HIV-exposed and HIV-infected children: recommendations from the National Institutes of Health, Centers for Disease Control and Prevention, the HIV Medicine Association of the Infectious Diseases Society of America, the Pediatric Infectious Diseases Society, and the American Academy of Pediatrics. Pediatr Infect Dis J. 32(suppl 2[0 2]):i–KK4.
- Panel on Antiretroviral Therapy and Medical Management of Children Living with HIV. Guidelines for the use of antiretroviral agents in pediatric HIV infection. Accessed February 15, 2022. https://clinicalinfo.hiv.gov/en/guidelines /pediatric-arv
- Committee on Health Care for Underserved Women, American College of Obstetricians and Gynecologists. ACOG committee opinion no. 361. Breastfeeding: maternal and infant aspects. Obstet Gynecol. 2007;109(2 pt 1):479-480.
- Committee on Pediatric AIDS; Mofenson LM, Flynn PM, Aldrovandi GM, et al. Infant feeding and transmission of human immunodeficiency virus in the United States. Pediatrics. 2013;131:391-396.
- Breastfeeding and HIV International Transmission Study Group; Coutsoudis A, Dabis F, Fawzi W, et al. Late postnatal transmission of HIV-1 in breast-fed children: an individual patient data meta-analysis. J Infect Dis. 2004;189:2154-2166.
Treating PPH: A novel vacuum-induced hemorrhage control device
Postpartum hemorrhage (PPH) continues to be a leading cause of maternal morbidity and mortality both worldwide and in the United States.1-3 A PPH is defined as the cumulative blood loss of 1,000 mL or more, or blood loss accompanied by signs or symptoms of hypovolemia, within 24 hours following the birth process (including intrapartum loss).4
Approximately 70% to 80% of hemorrhages are due to abnormal uterine tone.5 Bimanual massage and medical management, the primary treatments for uterine atony, attempt to restore the normal uterine tone that compresses the vessels in the placental implantation site and limits bleeding. For women in whom the primary treatments are not effective, only uterine compression sutures in a laparotomy can achieve physiologic contracture of the uterus. The second-line treatment option, intrauterine tamponade, places pressure over the placental implantation site while distending the uterus.
In October 2020, the US Food and Drug Administration (FDA) granted clearance to a novel device that offers an alternative treatment option. The Jada System (Alydia Health), an intrauterine vacuum-induced hemorrhage control device, is placed in the uterus and uses wall suction to induce physiologic contraction of the uterus to control bleeding.6
In this article, within the context of a case vignette, we discuss the recent study on the Jada System and how this device can be used in the management of PPH.6
CASE Woman with PPH history fears repeat hemorrhage
Ms. B. is a 25-year-old woman (G2P1) who presents for prenatal care at 10 weeks’ gestation. Her medical history is significant for asthma and PPH after her first delivery. When you review her prior delivery records, you learn that she had a protracted labor and delivered a healthy 10 lb 8 oz baby boy after 3 hours of pushing. After delivery, she received postpartum intravenous oxytocin followed by intramuscular uterotonics when her bleeding was heavy during her laceration repair. Her estimated blood loss at delivery was 600 mL. The team was called back to her bedside for the continued bleeding. Uterine atony was diagnosed. Although she received additional uterotonics, the bleeding continued. An intrauterine tamponade balloon was placed, and the bleeding ultimately was controlled. The total estimated blood loss (EBL) was 2.5 L, and the patient then was transfused with 2 U of packed red blood cells.
Currently, Ms. B. is very worried about having another hemorrhage as the bleeding terrified her and her partner, disrupted breastfeeding initiation while the tamponade was in place, and made her anxious about having another baby.
What steps would you take to prepare for a potential PPH in this patient?
Risk factors
While PPH often is unpredictable, many risk factors have been identified (TABLE).7-9 Some risk factors are present during the antepartum period while others arise during labor. In some cases, obstetric clinicians may be able to intervene during prenatal care, such as by giving iron supplementation to address anemia. Other factors, however, are not modifiable, including multiparity, polyhydramnios, and multiple gestations. On presentation to the labor unit, new risk factors may arise, such as magnesium sulfate use, chorioamnionitis, protracted labor, or the need for general anesthesia. In addition, the presence of a fibroid uterus or a uterine inversion can impede effective uterine contractions.5
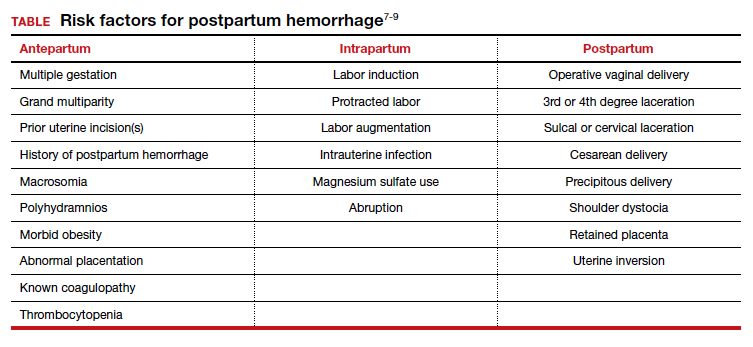
Various tools are available for assessing these risk factors on admission, during labor, and after delivery, such as the AWHONN postpartum hemorrhage risk assessment table and the CMQCC obstetric hemorrhage toolkit.10,11
Continue to: CASE continued Patient’s history reveals risk factors...
CASE continued Patient’s history reveals risk factors
You review with Ms. B. that she had several risk factors present during labor. She had a large baby and a protracted labor. Knowing her history in this pregnancy will allow the clinical team to be prepared for a potential recurrent hemorrhage and to respond proactively to bleeding.
Consider the management options
The initial treatment for PPH includes bimanual massage, oxytocin, and other uterotonics (methylergonovine, 15-methyl prostaglandin F2α, and misoprostol). While various algorithms are available on the order of treatment, a single agent has not been shown superior to others.12 The antifibrinolytic medication tranexamic acid also was shown to reduce the risk of death from obstetric hemorrhage in the international WOMAN trial.13
While these agents often are used simultaneously to achieve hemostasis, their systemic effects are associated with contraindications. Specifically, F2α prostaglandins cannot be used in patients with asthma or active hepatic, pulmonary, or cardiac disease. Ergot derivatives cannot be used in patients with hypertension, pre-eclampsia, or cardiovascular disease. Given the rising rate of medical comorbidities during pregnancy, such contraindications limit the treatment options for many patients.
In cases in which medical management is not sufficient or is contraindicated for controlling hemorrhage, second-line treatment includes the use of tamponade techniques, such as intrauterine packing or balloons. The tamponade applies pressure directly to the placental implantation site for 12 to 24 hours, which allows time for the uterus to contract and return to normal tone. While this method may seem counterintuitive to achieving uterine tone, studies suggest a success rate between 75% and 86% with balloon tamponade.12
Third-line treatment options are increasingly invasive but should be used to prevent further maternal morbidity and mortality. These include uterine artery embolization and surgery. Uterine artery embolization is an option for a stable patient at a center with available interventional radiology services. If embolization is either not successful or not available, an exploratory laparotomy should be performed. Uterine compression sutures can be placed along with vascular ligation sutures of the uterine arteries (O’Leary sutures) and the hypogastric arteries. If all other methods have failed, a hysterectomy is the definitive treatment for hemorrhage.
CASE continued Patient desires an alternative to tamponade if needed
Following your visit, Ms. B. has an ultrasound scan that shows a dichorionic diamniotic twin pregnancy. She also has a microcytic anemia. After you discuss iron supplementation with the patient, she asks if there are any other options should medical management fail in the event of a recurrent hemorrhage. While intrauterine tamponade balloon did treat her hemorrhage, she was not happy with the length of time it had to remain in place, the discomfort while it was used, and the disruption to her planned recovery. You inform her of a new treatment option available for PPH, a vacuum-induced hemorrhage control device that was recently FDA cleared.
Continue to: New device controls bleeding fast...
New device controls bleeding fast
In 2020, D’Alton and colleagues reported on their multicenter, prospective single-arm treatment study on the effectiveness and safety of an intrauterine vacuum-induced hemorrhage control device.6 This device, the Jada System, uses low-level vacuum to induce uterine contraction to control bleeding from uterine atony. The prospective study, which followed a 2016 feasibility study, enrolled more than 100 women at 12 centers across the United States.6,14 Women were eligible to participate if they delivered at a gestational age of 34 weeks or later and had an EBL between 500 and 1,000 mL after a vaginal delivery or an EBL between 1,000 and 1,500 mL after a cesarean delivery.
Treatment with the vacuum device was successful in 94% (100/106, 95% confidence interval, 88%–98%) of women, and definitive control of abnormal bleeding was achieved in a median of 3 minutes (interquartile range [IQR], 2.0–5.0) after connection to the vacuum device.6
CASE continued Patient has questions
Your patient expresses interest in this device, but she wants to understand how it works. Would it require transfer to another unit or prolonged monitoring?
How the device works
Compared with intrauterine tamponade balloon devices, which apply pressure by distending the uterus, the Jada System applies low-level intrauterine vacuum to facilitate the physiologic forces of uterine contractions to constrict myometrial blood vessels and achieve hemostasis.6 The device is made of medical-grade silicone. Its distal end, which is placed in the uterus, is an elliptical loop. The loop’s inner surface contains 20 vacuum pores protected by a shield that facilitate creation of a vacuum within the uterine cavity. The loop is soft and smooth to limit the chance of tissue damage during insertion, treatment, and removal of the device. The device’s proximal end has a vacuum connector. The vacuum source is hospital-grade wall suction, but a portable vacuum source also can be used (FIGURE 1).
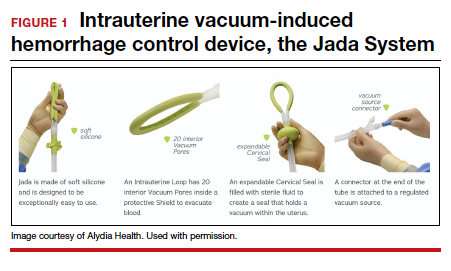
Prior to placing the device, a manual sweep of the uterine cavity is performed. If needed, ultrasonography can be used with the manual sweep to ensure that there is no retained placental tissue or clot. The loop of the Jada System is then inserted in the uterine cavity, and the circular cervical seal, just outside the external cervical os, is filled with sterile water.
Low-level vacuum (80 ± 10 mm Hg) is applied so that pooled blood is evacuated from the uterus as it collapses (FIGURE 2). The volume of any ongoing bleeding is measured in the suction tubing while the uterine response to treatment can be palpated. Once there is no bleeding without any need for further treatment, the device should remain in the uterus for at least 1 hour. The suction is then turned off, and bleeding is monitored for 30 minutes. If bleeding remains controlled, the device can be removed.
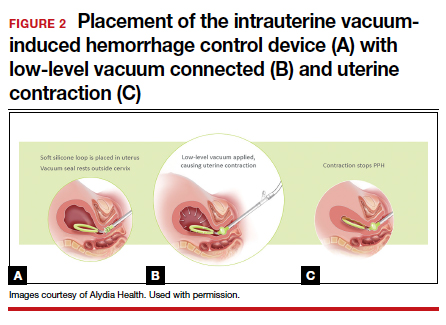
CASE continued The question of complications
Ms. B. is concerned about safety and asks about potential complications with the device’s use.
Safety findings
In the prospective study and FDA review, the device was deemed safe. There were 8 possibly related adverse events (endometritis, laceration disruption, and vaginal infection), which all resolved without serious clinical sequelae. Forty women (38%) received a blood transfusion, but only 5 required 4 U or more of red blood cells.6
Continue to: CASE continued What do other physicians think?...
CASE continued What do other physicians think?
Your patient is curious about the time it takes for the device to work and whether other clinicians like using this new device for hemorrhage treatment.
Duration of treatment
The times to achieve uterine collapse and control of hemorrhage are both relatively short. In the prospective study, the initial collapse of the uterus took a median of 1 minute (IQR, 1–2 min) from the time of vacuum connection.6 Bleeding was controlled in less than 5 minutes in 82% of women, with an overall median time of 3 minutes (IQR, 2–5 min). The median duration of vacuum treatment was 144.0 minutes (IQR, 85.8–295.8 min), which includes the required minimum of 60 minutes for vacuum treatment time and 30 minutes of observation without the vacuum connected but with the device still in place.6
When polled, the majority of clinicians—98%—reported that the intrauterine vacuum-induced hemorrhage control device was easy to use, and 97% would recommend its use for future patients.6
Further, recognizing the device’s potential, the Cleveland Clinic cited it as one of the top 10 health care innovations for 2021 for offering a low-tech and minimally invasive tool for obstetric clinicians.15
CASE continued Final questions
Ms. B. thanks you for the information and asks, should she know anything else about the device?
Vacuum device vs other treatments
The study by D’Alton and colleagues was a single-arm treatment trial that did not directly compare the effectiveness of the device with that of other PPH treatment options, such as balloon tamponade.6 At this point, we know that clinicians can safely and quickly use the device to treat uterine atony, but we do not know if it is superior to other treatments for PPH.
Key takeaways
Postpartum hemorrhage is a leading cause of maternal morbidity and mortality. When first-line uterotonics fail, obstetric clinicians previously had only balloon tamponade or invasive procedures to treat patients. The novel intrauterine vacuum-induced hemorrhage control device takes a new approach that simulates the physiologic process of uterine contractions. The device can rapidly and effectively control abnormal postpartum uterine bleeding. More studies are needed, however, to compare the device’s effectiveness with that of other PPH treatments and to consider its use in women with more severe degrees of postpartum hemorrhage as well as its cost-effectiveness. ●
- Say L, Chou D, Gemmill A, et al. Global causes of maternal death: a WHO systematic analysis. Lancet Glob Health. 2014;2:e323-e333.
- Callaghan WM, Creanga AA, Kuklina EV. Severe maternal morbidity among delivery and postpartum hospitalizations in the United States. Obstet Gynecol. 2012;120:1029-1036.
- Centers for Disease Control and Prevention. Severe maternal morbidity in the United States. http://www .cdc.gov/reproductivehealth/maternalinfanthealth /severematernalmorbidity.html. Accessed November 6, 2020.
- Menard MK, Main EK, Currigan SM. Executive summary of the reVITALize initiative: standardizing obstetric data definitions. Obstet Gynecol. 2014;124:150-153.
- American College of Obstetricians and Gynecologists Committee on Practice Bulletins–Obstetrics. Practice bulletin no. 183: postpartum hemorrhage. Obstet Gynecol. 2017;130:e168-e186.
- D’Alton ME, Rood KM, Smid MC, et al. Intrauterine vacuum-induced hemorrhage-control device for rapid treatment of postpartum hemorrhage. Obstet Gynecol. 2020;136:882-891.
- Mavrides E, Allard S, Chandraharan E, et al; on behalf of the Royal College of Obstetricians and Gynaecologists. Prevention and management of postpartum hemorrhage. BJOG. 2016;124:e106-e149.
- Lyndon A, Lagrew D, Shields L, et al. Improving health care response to obstetric hemorrhage, version 2.0 (California Maternal Quality Care Collaborative Toolkit to Transform Maternity Care). Developed under contract #11-10006 with the California Department of Public Health; Maternal, Child and Adolescent Health Division; Published by the California Maternal Quality Care Collaborative, March 17, 2015.
- Main EK, Goffman D, Scavone BM, et al; National Partnership for Maternal Safety; Council on Patient Safety in Women’s Health Care. National Partnership for Maternal Safety: consensus bundle on obstetric hemorrhage. Obstet Gynecol. 2015;126:155-162.
- AWHONN Postpartum Hemorrhage Project. Postpartum hemorrhage (PPH) risk assessment table 1.0. https:// mygnosis.com/Content/Chunks/3504/assets/pdfs/PPH _Risk_Assessment_Table-7-17-15.pdf. Accessed November 15, 2020.
- Bingham D, Melsop K, Main E. CMQCC obstetric hemorrhage toolkit: hospital level implementation guide. 2010. California Maternal Quality Care Collaborative (CMQCC). Palo Alto, CA: Stanford University. https://www.cmqcc.org/resource/1489 /download. Accessed November 15, 2020.
- Likis FE, Sathe NA, Morgans AK, et al. Management of postpartum hemorrhage. Comparative effectiveness review no. 151. AHRQ publication no. 15-EHC013-EF. Rockville, MD: Agency for Healthcare Research and Quality; 2015.
- WOMAN Trial Collaborators. Effect of early tranexamic acid administration on mortality, hysterectomy, and other morbidities in women with post-partum haemorrhage (WOMAN): an international, randomised, double-blind, placebo-controlled trial. Lancet. 2017;389:2105–2116.
- Purwosunu Y, Sarkoen W, Arulkumaran S, et al. Control of postpartum hemorrhage using vacuum-induced uterine tamponade. Obstet Gynecol. 2016;128:33-36.
- Cleveland Clinic Innovations. Cleveland Clinic unveils top 10 medical innovations for 2021. October 6, 2020. https:// innovations.clevelandclinic.org/Programs/Top-10-Medical -Innovations/Top-10-for-2021. Accessed November 6, 2020.
Postpartum hemorrhage (PPH) continues to be a leading cause of maternal morbidity and mortality both worldwide and in the United States.1-3 A PPH is defined as the cumulative blood loss of 1,000 mL or more, or blood loss accompanied by signs or symptoms of hypovolemia, within 24 hours following the birth process (including intrapartum loss).4
Approximately 70% to 80% of hemorrhages are due to abnormal uterine tone.5 Bimanual massage and medical management, the primary treatments for uterine atony, attempt to restore the normal uterine tone that compresses the vessels in the placental implantation site and limits bleeding. For women in whom the primary treatments are not effective, only uterine compression sutures in a laparotomy can achieve physiologic contracture of the uterus. The second-line treatment option, intrauterine tamponade, places pressure over the placental implantation site while distending the uterus.
In October 2020, the US Food and Drug Administration (FDA) granted clearance to a novel device that offers an alternative treatment option. The Jada System (Alydia Health), an intrauterine vacuum-induced hemorrhage control device, is placed in the uterus and uses wall suction to induce physiologic contraction of the uterus to control bleeding.6
In this article, within the context of a case vignette, we discuss the recent study on the Jada System and how this device can be used in the management of PPH.6
CASE Woman with PPH history fears repeat hemorrhage
Ms. B. is a 25-year-old woman (G2P1) who presents for prenatal care at 10 weeks’ gestation. Her medical history is significant for asthma and PPH after her first delivery. When you review her prior delivery records, you learn that she had a protracted labor and delivered a healthy 10 lb 8 oz baby boy after 3 hours of pushing. After delivery, she received postpartum intravenous oxytocin followed by intramuscular uterotonics when her bleeding was heavy during her laceration repair. Her estimated blood loss at delivery was 600 mL. The team was called back to her bedside for the continued bleeding. Uterine atony was diagnosed. Although she received additional uterotonics, the bleeding continued. An intrauterine tamponade balloon was placed, and the bleeding ultimately was controlled. The total estimated blood loss (EBL) was 2.5 L, and the patient then was transfused with 2 U of packed red blood cells.
Currently, Ms. B. is very worried about having another hemorrhage as the bleeding terrified her and her partner, disrupted breastfeeding initiation while the tamponade was in place, and made her anxious about having another baby.
What steps would you take to prepare for a potential PPH in this patient?
Risk factors
While PPH often is unpredictable, many risk factors have been identified (TABLE).7-9 Some risk factors are present during the antepartum period while others arise during labor. In some cases, obstetric clinicians may be able to intervene during prenatal care, such as by giving iron supplementation to address anemia. Other factors, however, are not modifiable, including multiparity, polyhydramnios, and multiple gestations. On presentation to the labor unit, new risk factors may arise, such as magnesium sulfate use, chorioamnionitis, protracted labor, or the need for general anesthesia. In addition, the presence of a fibroid uterus or a uterine inversion can impede effective uterine contractions.5

Various tools are available for assessing these risk factors on admission, during labor, and after delivery, such as the AWHONN postpartum hemorrhage risk assessment table and the CMQCC obstetric hemorrhage toolkit.10,11
Continue to: CASE continued Patient’s history reveals risk factors...
CASE continued Patient’s history reveals risk factors
You review with Ms. B. that she had several risk factors present during labor. She had a large baby and a protracted labor. Knowing her history in this pregnancy will allow the clinical team to be prepared for a potential recurrent hemorrhage and to respond proactively to bleeding.
Consider the management options
The initial treatment for PPH includes bimanual massage, oxytocin, and other uterotonics (methylergonovine, 15-methyl prostaglandin F2α, and misoprostol). While various algorithms are available on the order of treatment, a single agent has not been shown superior to others.12 The antifibrinolytic medication tranexamic acid also was shown to reduce the risk of death from obstetric hemorrhage in the international WOMAN trial.13
While these agents often are used simultaneously to achieve hemostasis, their systemic effects are associated with contraindications. Specifically, F2α prostaglandins cannot be used in patients with asthma or active hepatic, pulmonary, or cardiac disease. Ergot derivatives cannot be used in patients with hypertension, pre-eclampsia, or cardiovascular disease. Given the rising rate of medical comorbidities during pregnancy, such contraindications limit the treatment options for many patients.
In cases in which medical management is not sufficient or is contraindicated for controlling hemorrhage, second-line treatment includes the use of tamponade techniques, such as intrauterine packing or balloons. The tamponade applies pressure directly to the placental implantation site for 12 to 24 hours, which allows time for the uterus to contract and return to normal tone. While this method may seem counterintuitive to achieving uterine tone, studies suggest a success rate between 75% and 86% with balloon tamponade.12
Third-line treatment options are increasingly invasive but should be used to prevent further maternal morbidity and mortality. These include uterine artery embolization and surgery. Uterine artery embolization is an option for a stable patient at a center with available interventional radiology services. If embolization is either not successful or not available, an exploratory laparotomy should be performed. Uterine compression sutures can be placed along with vascular ligation sutures of the uterine arteries (O’Leary sutures) and the hypogastric arteries. If all other methods have failed, a hysterectomy is the definitive treatment for hemorrhage.
CASE continued Patient desires an alternative to tamponade if needed
Following your visit, Ms. B. has an ultrasound scan that shows a dichorionic diamniotic twin pregnancy. She also has a microcytic anemia. After you discuss iron supplementation with the patient, she asks if there are any other options should medical management fail in the event of a recurrent hemorrhage. While intrauterine tamponade balloon did treat her hemorrhage, she was not happy with the length of time it had to remain in place, the discomfort while it was used, and the disruption to her planned recovery. You inform her of a new treatment option available for PPH, a vacuum-induced hemorrhage control device that was recently FDA cleared.
Continue to: New device controls bleeding fast...
New device controls bleeding fast
In 2020, D’Alton and colleagues reported on their multicenter, prospective single-arm treatment study on the effectiveness and safety of an intrauterine vacuum-induced hemorrhage control device.6 This device, the Jada System, uses low-level vacuum to induce uterine contraction to control bleeding from uterine atony. The prospective study, which followed a 2016 feasibility study, enrolled more than 100 women at 12 centers across the United States.6,14 Women were eligible to participate if they delivered at a gestational age of 34 weeks or later and had an EBL between 500 and 1,000 mL after a vaginal delivery or an EBL between 1,000 and 1,500 mL after a cesarean delivery.
Treatment with the vacuum device was successful in 94% (100/106, 95% confidence interval, 88%–98%) of women, and definitive control of abnormal bleeding was achieved in a median of 3 minutes (interquartile range [IQR], 2.0–5.0) after connection to the vacuum device.6
CASE continued Patient has questions
Your patient expresses interest in this device, but she wants to understand how it works. Would it require transfer to another unit or prolonged monitoring?
How the device works
Compared with intrauterine tamponade balloon devices, which apply pressure by distending the uterus, the Jada System applies low-level intrauterine vacuum to facilitate the physiologic forces of uterine contractions to constrict myometrial blood vessels and achieve hemostasis.6 The device is made of medical-grade silicone. Its distal end, which is placed in the uterus, is an elliptical loop. The loop’s inner surface contains 20 vacuum pores protected by a shield that facilitate creation of a vacuum within the uterine cavity. The loop is soft and smooth to limit the chance of tissue damage during insertion, treatment, and removal of the device. The device’s proximal end has a vacuum connector. The vacuum source is hospital-grade wall suction, but a portable vacuum source also can be used (FIGURE 1).

Prior to placing the device, a manual sweep of the uterine cavity is performed. If needed, ultrasonography can be used with the manual sweep to ensure that there is no retained placental tissue or clot. The loop of the Jada System is then inserted in the uterine cavity, and the circular cervical seal, just outside the external cervical os, is filled with sterile water.
Low-level vacuum (80 ± 10 mm Hg) is applied so that pooled blood is evacuated from the uterus as it collapses (FIGURE 2). The volume of any ongoing bleeding is measured in the suction tubing while the uterine response to treatment can be palpated. Once there is no bleeding without any need for further treatment, the device should remain in the uterus for at least 1 hour. The suction is then turned off, and bleeding is monitored for 30 minutes. If bleeding remains controlled, the device can be removed.

CASE continued The question of complications
Ms. B. is concerned about safety and asks about potential complications with the device’s use.
Safety findings
In the prospective study and FDA review, the device was deemed safe. There were 8 possibly related adverse events (endometritis, laceration disruption, and vaginal infection), which all resolved without serious clinical sequelae. Forty women (38%) received a blood transfusion, but only 5 required 4 U or more of red blood cells.6
Continue to: CASE continued What do other physicians think?...
CASE continued What do other physicians think?
Your patient is curious about the time it takes for the device to work and whether other clinicians like using this new device for hemorrhage treatment.
Duration of treatment
The times to achieve uterine collapse and control of hemorrhage are both relatively short. In the prospective study, the initial collapse of the uterus took a median of 1 minute (IQR, 1–2 min) from the time of vacuum connection.6 Bleeding was controlled in less than 5 minutes in 82% of women, with an overall median time of 3 minutes (IQR, 2–5 min). The median duration of vacuum treatment was 144.0 minutes (IQR, 85.8–295.8 min), which includes the required minimum of 60 minutes for vacuum treatment time and 30 minutes of observation without the vacuum connected but with the device still in place.6
When polled, the majority of clinicians—98%—reported that the intrauterine vacuum-induced hemorrhage control device was easy to use, and 97% would recommend its use for future patients.6
Further, recognizing the device’s potential, the Cleveland Clinic cited it as one of the top 10 health care innovations for 2021 for offering a low-tech and minimally invasive tool for obstetric clinicians.15
CASE continued Final questions
Ms. B. thanks you for the information and asks, should she know anything else about the device?
Vacuum device vs other treatments
The study by D’Alton and colleagues was a single-arm treatment trial that did not directly compare the effectiveness of the device with that of other PPH treatment options, such as balloon tamponade.6 At this point, we know that clinicians can safely and quickly use the device to treat uterine atony, but we do not know if it is superior to other treatments for PPH.
Key takeaways
Postpartum hemorrhage is a leading cause of maternal morbidity and mortality. When first-line uterotonics fail, obstetric clinicians previously had only balloon tamponade or invasive procedures to treat patients. The novel intrauterine vacuum-induced hemorrhage control device takes a new approach that simulates the physiologic process of uterine contractions. The device can rapidly and effectively control abnormal postpartum uterine bleeding. More studies are needed, however, to compare the device’s effectiveness with that of other PPH treatments and to consider its use in women with more severe degrees of postpartum hemorrhage as well as its cost-effectiveness. ●
Postpartum hemorrhage (PPH) continues to be a leading cause of maternal morbidity and mortality both worldwide and in the United States.1-3 A PPH is defined as the cumulative blood loss of 1,000 mL or more, or blood loss accompanied by signs or symptoms of hypovolemia, within 24 hours following the birth process (including intrapartum loss).4
Approximately 70% to 80% of hemorrhages are due to abnormal uterine tone.5 Bimanual massage and medical management, the primary treatments for uterine atony, attempt to restore the normal uterine tone that compresses the vessels in the placental implantation site and limits bleeding. For women in whom the primary treatments are not effective, only uterine compression sutures in a laparotomy can achieve physiologic contracture of the uterus. The second-line treatment option, intrauterine tamponade, places pressure over the placental implantation site while distending the uterus.
In October 2020, the US Food and Drug Administration (FDA) granted clearance to a novel device that offers an alternative treatment option. The Jada System (Alydia Health), an intrauterine vacuum-induced hemorrhage control device, is placed in the uterus and uses wall suction to induce physiologic contraction of the uterus to control bleeding.6
In this article, within the context of a case vignette, we discuss the recent study on the Jada System and how this device can be used in the management of PPH.6
CASE Woman with PPH history fears repeat hemorrhage
Ms. B. is a 25-year-old woman (G2P1) who presents for prenatal care at 10 weeks’ gestation. Her medical history is significant for asthma and PPH after her first delivery. When you review her prior delivery records, you learn that she had a protracted labor and delivered a healthy 10 lb 8 oz baby boy after 3 hours of pushing. After delivery, she received postpartum intravenous oxytocin followed by intramuscular uterotonics when her bleeding was heavy during her laceration repair. Her estimated blood loss at delivery was 600 mL. The team was called back to her bedside for the continued bleeding. Uterine atony was diagnosed. Although she received additional uterotonics, the bleeding continued. An intrauterine tamponade balloon was placed, and the bleeding ultimately was controlled. The total estimated blood loss (EBL) was 2.5 L, and the patient then was transfused with 2 U of packed red blood cells.
Currently, Ms. B. is very worried about having another hemorrhage as the bleeding terrified her and her partner, disrupted breastfeeding initiation while the tamponade was in place, and made her anxious about having another baby.
What steps would you take to prepare for a potential PPH in this patient?
Risk factors
While PPH often is unpredictable, many risk factors have been identified (TABLE).7-9 Some risk factors are present during the antepartum period while others arise during labor. In some cases, obstetric clinicians may be able to intervene during prenatal care, such as by giving iron supplementation to address anemia. Other factors, however, are not modifiable, including multiparity, polyhydramnios, and multiple gestations. On presentation to the labor unit, new risk factors may arise, such as magnesium sulfate use, chorioamnionitis, protracted labor, or the need for general anesthesia. In addition, the presence of a fibroid uterus or a uterine inversion can impede effective uterine contractions.5

Various tools are available for assessing these risk factors on admission, during labor, and after delivery, such as the AWHONN postpartum hemorrhage risk assessment table and the CMQCC obstetric hemorrhage toolkit.10,11
Continue to: CASE continued Patient’s history reveals risk factors...
CASE continued Patient’s history reveals risk factors
You review with Ms. B. that she had several risk factors present during labor. She had a large baby and a protracted labor. Knowing her history in this pregnancy will allow the clinical team to be prepared for a potential recurrent hemorrhage and to respond proactively to bleeding.
Consider the management options
The initial treatment for PPH includes bimanual massage, oxytocin, and other uterotonics (methylergonovine, 15-methyl prostaglandin F2α, and misoprostol). While various algorithms are available on the order of treatment, a single agent has not been shown superior to others.12 The antifibrinolytic medication tranexamic acid also was shown to reduce the risk of death from obstetric hemorrhage in the international WOMAN trial.13
While these agents often are used simultaneously to achieve hemostasis, their systemic effects are associated with contraindications. Specifically, F2α prostaglandins cannot be used in patients with asthma or active hepatic, pulmonary, or cardiac disease. Ergot derivatives cannot be used in patients with hypertension, pre-eclampsia, or cardiovascular disease. Given the rising rate of medical comorbidities during pregnancy, such contraindications limit the treatment options for many patients.
In cases in which medical management is not sufficient or is contraindicated for controlling hemorrhage, second-line treatment includes the use of tamponade techniques, such as intrauterine packing or balloons. The tamponade applies pressure directly to the placental implantation site for 12 to 24 hours, which allows time for the uterus to contract and return to normal tone. While this method may seem counterintuitive to achieving uterine tone, studies suggest a success rate between 75% and 86% with balloon tamponade.12
Third-line treatment options are increasingly invasive but should be used to prevent further maternal morbidity and mortality. These include uterine artery embolization and surgery. Uterine artery embolization is an option for a stable patient at a center with available interventional radiology services. If embolization is either not successful or not available, an exploratory laparotomy should be performed. Uterine compression sutures can be placed along with vascular ligation sutures of the uterine arteries (O’Leary sutures) and the hypogastric arteries. If all other methods have failed, a hysterectomy is the definitive treatment for hemorrhage.
CASE continued Patient desires an alternative to tamponade if needed
Following your visit, Ms. B. has an ultrasound scan that shows a dichorionic diamniotic twin pregnancy. She also has a microcytic anemia. After you discuss iron supplementation with the patient, she asks if there are any other options should medical management fail in the event of a recurrent hemorrhage. While intrauterine tamponade balloon did treat her hemorrhage, she was not happy with the length of time it had to remain in place, the discomfort while it was used, and the disruption to her planned recovery. You inform her of a new treatment option available for PPH, a vacuum-induced hemorrhage control device that was recently FDA cleared.
Continue to: New device controls bleeding fast...
New device controls bleeding fast
In 2020, D’Alton and colleagues reported on their multicenter, prospective single-arm treatment study on the effectiveness and safety of an intrauterine vacuum-induced hemorrhage control device.6 This device, the Jada System, uses low-level vacuum to induce uterine contraction to control bleeding from uterine atony. The prospective study, which followed a 2016 feasibility study, enrolled more than 100 women at 12 centers across the United States.6,14 Women were eligible to participate if they delivered at a gestational age of 34 weeks or later and had an EBL between 500 and 1,000 mL after a vaginal delivery or an EBL between 1,000 and 1,500 mL after a cesarean delivery.
Treatment with the vacuum device was successful in 94% (100/106, 95% confidence interval, 88%–98%) of women, and definitive control of abnormal bleeding was achieved in a median of 3 minutes (interquartile range [IQR], 2.0–5.0) after connection to the vacuum device.6
CASE continued Patient has questions
Your patient expresses interest in this device, but she wants to understand how it works. Would it require transfer to another unit or prolonged monitoring?
How the device works
Compared with intrauterine tamponade balloon devices, which apply pressure by distending the uterus, the Jada System applies low-level intrauterine vacuum to facilitate the physiologic forces of uterine contractions to constrict myometrial blood vessels and achieve hemostasis.6 The device is made of medical-grade silicone. Its distal end, which is placed in the uterus, is an elliptical loop. The loop’s inner surface contains 20 vacuum pores protected by a shield that facilitate creation of a vacuum within the uterine cavity. The loop is soft and smooth to limit the chance of tissue damage during insertion, treatment, and removal of the device. The device’s proximal end has a vacuum connector. The vacuum source is hospital-grade wall suction, but a portable vacuum source also can be used (FIGURE 1).

Prior to placing the device, a manual sweep of the uterine cavity is performed. If needed, ultrasonography can be used with the manual sweep to ensure that there is no retained placental tissue or clot. The loop of the Jada System is then inserted in the uterine cavity, and the circular cervical seal, just outside the external cervical os, is filled with sterile water.
Low-level vacuum (80 ± 10 mm Hg) is applied so that pooled blood is evacuated from the uterus as it collapses (FIGURE 2). The volume of any ongoing bleeding is measured in the suction tubing while the uterine response to treatment can be palpated. Once there is no bleeding without any need for further treatment, the device should remain in the uterus for at least 1 hour. The suction is then turned off, and bleeding is monitored for 30 minutes. If bleeding remains controlled, the device can be removed.

CASE continued The question of complications
Ms. B. is concerned about safety and asks about potential complications with the device’s use.
Safety findings
In the prospective study and FDA review, the device was deemed safe. There were 8 possibly related adverse events (endometritis, laceration disruption, and vaginal infection), which all resolved without serious clinical sequelae. Forty women (38%) received a blood transfusion, but only 5 required 4 U or more of red blood cells.6
Continue to: CASE continued What do other physicians think?...
CASE continued What do other physicians think?
Your patient is curious about the time it takes for the device to work and whether other clinicians like using this new device for hemorrhage treatment.
Duration of treatment
The times to achieve uterine collapse and control of hemorrhage are both relatively short. In the prospective study, the initial collapse of the uterus took a median of 1 minute (IQR, 1–2 min) from the time of vacuum connection.6 Bleeding was controlled in less than 5 minutes in 82% of women, with an overall median time of 3 minutes (IQR, 2–5 min). The median duration of vacuum treatment was 144.0 minutes (IQR, 85.8–295.8 min), which includes the required minimum of 60 minutes for vacuum treatment time and 30 minutes of observation without the vacuum connected but with the device still in place.6
When polled, the majority of clinicians—98%—reported that the intrauterine vacuum-induced hemorrhage control device was easy to use, and 97% would recommend its use for future patients.6
Further, recognizing the device’s potential, the Cleveland Clinic cited it as one of the top 10 health care innovations for 2021 for offering a low-tech and minimally invasive tool for obstetric clinicians.15
CASE continued Final questions
Ms. B. thanks you for the information and asks, should she know anything else about the device?
Vacuum device vs other treatments
The study by D’Alton and colleagues was a single-arm treatment trial that did not directly compare the effectiveness of the device with that of other PPH treatment options, such as balloon tamponade.6 At this point, we know that clinicians can safely and quickly use the device to treat uterine atony, but we do not know if it is superior to other treatments for PPH.
Key takeaways
Postpartum hemorrhage is a leading cause of maternal morbidity and mortality. When first-line uterotonics fail, obstetric clinicians previously had only balloon tamponade or invasive procedures to treat patients. The novel intrauterine vacuum-induced hemorrhage control device takes a new approach that simulates the physiologic process of uterine contractions. The device can rapidly and effectively control abnormal postpartum uterine bleeding. More studies are needed, however, to compare the device’s effectiveness with that of other PPH treatments and to consider its use in women with more severe degrees of postpartum hemorrhage as well as its cost-effectiveness. ●
- Say L, Chou D, Gemmill A, et al. Global causes of maternal death: a WHO systematic analysis. Lancet Glob Health. 2014;2:e323-e333.
- Callaghan WM, Creanga AA, Kuklina EV. Severe maternal morbidity among delivery and postpartum hospitalizations in the United States. Obstet Gynecol. 2012;120:1029-1036.
- Centers for Disease Control and Prevention. Severe maternal morbidity in the United States. http://www .cdc.gov/reproductivehealth/maternalinfanthealth /severematernalmorbidity.html. Accessed November 6, 2020.
- Menard MK, Main EK, Currigan SM. Executive summary of the reVITALize initiative: standardizing obstetric data definitions. Obstet Gynecol. 2014;124:150-153.
- American College of Obstetricians and Gynecologists Committee on Practice Bulletins–Obstetrics. Practice bulletin no. 183: postpartum hemorrhage. Obstet Gynecol. 2017;130:e168-e186.
- D’Alton ME, Rood KM, Smid MC, et al. Intrauterine vacuum-induced hemorrhage-control device for rapid treatment of postpartum hemorrhage. Obstet Gynecol. 2020;136:882-891.
- Mavrides E, Allard S, Chandraharan E, et al; on behalf of the Royal College of Obstetricians and Gynaecologists. Prevention and management of postpartum hemorrhage. BJOG. 2016;124:e106-e149.
- Lyndon A, Lagrew D, Shields L, et al. Improving health care response to obstetric hemorrhage, version 2.0 (California Maternal Quality Care Collaborative Toolkit to Transform Maternity Care). Developed under contract #11-10006 with the California Department of Public Health; Maternal, Child and Adolescent Health Division; Published by the California Maternal Quality Care Collaborative, March 17, 2015.
- Main EK, Goffman D, Scavone BM, et al; National Partnership for Maternal Safety; Council on Patient Safety in Women’s Health Care. National Partnership for Maternal Safety: consensus bundle on obstetric hemorrhage. Obstet Gynecol. 2015;126:155-162.
- AWHONN Postpartum Hemorrhage Project. Postpartum hemorrhage (PPH) risk assessment table 1.0. https:// mygnosis.com/Content/Chunks/3504/assets/pdfs/PPH _Risk_Assessment_Table-7-17-15.pdf. Accessed November 15, 2020.
- Bingham D, Melsop K, Main E. CMQCC obstetric hemorrhage toolkit: hospital level implementation guide. 2010. California Maternal Quality Care Collaborative (CMQCC). Palo Alto, CA: Stanford University. https://www.cmqcc.org/resource/1489 /download. Accessed November 15, 2020.
- Likis FE, Sathe NA, Morgans AK, et al. Management of postpartum hemorrhage. Comparative effectiveness review no. 151. AHRQ publication no. 15-EHC013-EF. Rockville, MD: Agency for Healthcare Research and Quality; 2015.
- WOMAN Trial Collaborators. Effect of early tranexamic acid administration on mortality, hysterectomy, and other morbidities in women with post-partum haemorrhage (WOMAN): an international, randomised, double-blind, placebo-controlled trial. Lancet. 2017;389:2105–2116.
- Purwosunu Y, Sarkoen W, Arulkumaran S, et al. Control of postpartum hemorrhage using vacuum-induced uterine tamponade. Obstet Gynecol. 2016;128:33-36.
- Cleveland Clinic Innovations. Cleveland Clinic unveils top 10 medical innovations for 2021. October 6, 2020. https:// innovations.clevelandclinic.org/Programs/Top-10-Medical -Innovations/Top-10-for-2021. Accessed November 6, 2020.
- Say L, Chou D, Gemmill A, et al. Global causes of maternal death: a WHO systematic analysis. Lancet Glob Health. 2014;2:e323-e333.
- Callaghan WM, Creanga AA, Kuklina EV. Severe maternal morbidity among delivery and postpartum hospitalizations in the United States. Obstet Gynecol. 2012;120:1029-1036.
- Centers for Disease Control and Prevention. Severe maternal morbidity in the United States. http://www .cdc.gov/reproductivehealth/maternalinfanthealth /severematernalmorbidity.html. Accessed November 6, 2020.
- Menard MK, Main EK, Currigan SM. Executive summary of the reVITALize initiative: standardizing obstetric data definitions. Obstet Gynecol. 2014;124:150-153.
- American College of Obstetricians and Gynecologists Committee on Practice Bulletins–Obstetrics. Practice bulletin no. 183: postpartum hemorrhage. Obstet Gynecol. 2017;130:e168-e186.
- D’Alton ME, Rood KM, Smid MC, et al. Intrauterine vacuum-induced hemorrhage-control device for rapid treatment of postpartum hemorrhage. Obstet Gynecol. 2020;136:882-891.
- Mavrides E, Allard S, Chandraharan E, et al; on behalf of the Royal College of Obstetricians and Gynaecologists. Prevention and management of postpartum hemorrhage. BJOG. 2016;124:e106-e149.
- Lyndon A, Lagrew D, Shields L, et al. Improving health care response to obstetric hemorrhage, version 2.0 (California Maternal Quality Care Collaborative Toolkit to Transform Maternity Care). Developed under contract #11-10006 with the California Department of Public Health; Maternal, Child and Adolescent Health Division; Published by the California Maternal Quality Care Collaborative, March 17, 2015.
- Main EK, Goffman D, Scavone BM, et al; National Partnership for Maternal Safety; Council on Patient Safety in Women’s Health Care. National Partnership for Maternal Safety: consensus bundle on obstetric hemorrhage. Obstet Gynecol. 2015;126:155-162.
- AWHONN Postpartum Hemorrhage Project. Postpartum hemorrhage (PPH) risk assessment table 1.0. https:// mygnosis.com/Content/Chunks/3504/assets/pdfs/PPH _Risk_Assessment_Table-7-17-15.pdf. Accessed November 15, 2020.
- Bingham D, Melsop K, Main E. CMQCC obstetric hemorrhage toolkit: hospital level implementation guide. 2010. California Maternal Quality Care Collaborative (CMQCC). Palo Alto, CA: Stanford University. https://www.cmqcc.org/resource/1489 /download. Accessed November 15, 2020.
- Likis FE, Sathe NA, Morgans AK, et al. Management of postpartum hemorrhage. Comparative effectiveness review no. 151. AHRQ publication no. 15-EHC013-EF. Rockville, MD: Agency for Healthcare Research and Quality; 2015.
- WOMAN Trial Collaborators. Effect of early tranexamic acid administration on mortality, hysterectomy, and other morbidities in women with post-partum haemorrhage (WOMAN): an international, randomised, double-blind, placebo-controlled trial. Lancet. 2017;389:2105–2116.
- Purwosunu Y, Sarkoen W, Arulkumaran S, et al. Control of postpartum hemorrhage using vacuum-induced uterine tamponade. Obstet Gynecol. 2016;128:33-36.
- Cleveland Clinic Innovations. Cleveland Clinic unveils top 10 medical innovations for 2021. October 6, 2020. https:// innovations.clevelandclinic.org/Programs/Top-10-Medical -Innovations/Top-10-for-2021. Accessed November 6, 2020.