User login
Is there a new role for metformin in the management of gestational diabetes?
Dunne F, Newman C, Alvarez-Iglesia A, et al. Early metformin in gestational diabetes: a randomized clinical trial. JAMA. 2023;330:1547-1556. doi:10.1001/jama .2023.19869
EXPERT COMMENTARY
Gestational diabetes mellitus occurs in 4% to 7% of pregnancies, and the prevalence is likely to continue to increase given the rising rates of hypertension, obesity, advanced maternal age, and other medical comorbidities in pregnant persons in the United States.1,2 Uncontrolled hyperglycemia in pregnancy is associated swith many adverse perinatal outcomes, including stillbirth, macrosomia, admission to the neonatal intensive care unit (NICU), development of hypertensive disorders, and cesarean deliveries. Hence, it is important to investigate and identify the optimal management of gestational diabetes.
Metformin, an oral biguanide, although studied for gestational diabetes treatment in phase 3 randomized clinical open-label trials, often is avoided in patients who are pregnant (with the exception of patients who have needle aversions, are financially unable to use insulin, or are unable to administer insulin safely).1,2 Metformin is a highly effective first-line agent in the management of both prediabetes and type 2 diabetes, which begs us to question if there is a role for it in the management of gestational diabetes.
Details about the study
The study by Dunne and colleagues was a randomized controlled trial (RCT) conducted in a 1:1 parallel fashion at two institutions in Ireland from 2017–2022. The primary outcome assessed if treatment with metformin would reduce fasting blood glucose levels and the initiation of insulin among women diagnosed with gestational diabetes. A total of 510 participants enrolled in the study, with 268 receiving metformin (up to a maximum dose of 2,500 mg) at diagnosis and 267 receiving an identical placebo. Blood sugar levels were monitored 7 times a day, and medication adherence was assessed every 4 weeks.
Results. At 32 or 38 weeks’ gestation, 56.8% of patients in the metformin arm, and 63.7% of patients in the placebo arm required insulin or had fasting blood glucose levels above 5.1 mmol/L (91.8mg/dL), which was a statistically insignificant difference (P = .13). Although there was similarly no difference in the total amount of insulin used in each study group, the percentage of patients who required insulin initiation was decreased in the metformin arm (38.4% vs 51.1%; P = .004).
Study strengths and weaknesses
The authors conducted a well-designed double-blinded RCT—in both rural and tertiary care settings. Additionally, the study had an impressive 90% patient adherence rate for home blood glucose monitoring 7 times per day. The study arms were balanced for body mass index, as obesity is a known contributor to the development of gestational diabetes and response to insulin.
This study findings’ generalizability is limited across subpopulations given the lack of ethnic and racial diversity—the study population was 80% White. Additionally, utilization of the World Health Organization guidelines for diagnosing gestational diabetes, although adopted by most associations across the world, limits its application to areas of the world that use the National Diabetes Data Group or the Carpenter-Coustan diagnosis guidelines.3,4 Furthermore, the diagnosis of gestational diabetes, which was based on 1 elevated value of a 2-hour glucose tolerance test, has limited scientific support, has not been proven to improve obstetric outcomes, and may increase health care costs when compared with the 2-step method.5 The criteria for insulin initiation in the trial was based on having 2 elevated measures of blood glucose during home glucose monitoring, a criteria that is much stricter than what is used in other countries or clinical practice. The trial authors concluded that use of metformin had a statistically significant reduction in neonates weighing > 4,000 g and > 90th% of weight, but they did not assess study group differences in neonatal skin fold thickness or anthropometric measurements, as reported in the Metformin in Gestational Diabetes trials.6 ●
The study findings by Dunne and colleagues reinforce the current standard practice for the management of gestational diabetes: prescribe medical nutrition therapy and exercise followed by insulin initiation in the setting of persistently elevated blood glucose levels. Knowing that metformin crosses the placenta, future studies should also address the long-term metabolic and health outcomes of fetuses exposed to metformin.
NKECHINYELUM OGU, MD; CHARLOTTE NIZNIK, APRN; MICHELLE A. KOMINIAREK, MD, MS
- Rowan JA, Hague WM, Gao W, et al. Metformin versus insulin for the treatment of gestational diabetes. N Engl J Med. 2008;358:2003-2015. doi: 10.1056/NEJMoa0707193
- American College of Obstetricians and Gynecologists. Gestational diabetes mellitus: Practice Bulletin No. 180. Obstet Gynecol. 2017;130:e17-31. doi: 10.1097/AOG.0000000000002159
- Classification and diagnosis of diabetes mellitus and other categories of glucose intolerance. National Diabetes Data Group. Diabetes. 1979;28:1039-1057. doi: 10.2337 /diab.28.12.1039
- Carpenter MW, Coustan DR. Criteria for screening tests for gestational diabetes. Am J Obstet Gynecol. 1982;144:768-773. doi: 10.1016/0002-9378(82)90349-0
- Vandorsten JP, Dodson WC, Espeland MA, et al. NIH consensus development conference: diagnosing gestational diabetes mellitus. NIH Consens State Sci Statements. 2013;29:1-31.
- Rowan JA, Rush EC, Obolonkin V, et al. Metformin in gestational diabetes: the offspring follow-up (MiG TOFU) body composition at 2 years of age. Diabetes Care. 2011;34:2279-2284. https://doi.org/10.2337/dc11-0660
Dunne F, Newman C, Alvarez-Iglesia A, et al. Early metformin in gestational diabetes: a randomized clinical trial. JAMA. 2023;330:1547-1556. doi:10.1001/jama .2023.19869
EXPERT COMMENTARY
Gestational diabetes mellitus occurs in 4% to 7% of pregnancies, and the prevalence is likely to continue to increase given the rising rates of hypertension, obesity, advanced maternal age, and other medical comorbidities in pregnant persons in the United States.1,2 Uncontrolled hyperglycemia in pregnancy is associated swith many adverse perinatal outcomes, including stillbirth, macrosomia, admission to the neonatal intensive care unit (NICU), development of hypertensive disorders, and cesarean deliveries. Hence, it is important to investigate and identify the optimal management of gestational diabetes.
Metformin, an oral biguanide, although studied for gestational diabetes treatment in phase 3 randomized clinical open-label trials, often is avoided in patients who are pregnant (with the exception of patients who have needle aversions, are financially unable to use insulin, or are unable to administer insulin safely).1,2 Metformin is a highly effective first-line agent in the management of both prediabetes and type 2 diabetes, which begs us to question if there is a role for it in the management of gestational diabetes.
Details about the study
The study by Dunne and colleagues was a randomized controlled trial (RCT) conducted in a 1:1 parallel fashion at two institutions in Ireland from 2017–2022. The primary outcome assessed if treatment with metformin would reduce fasting blood glucose levels and the initiation of insulin among women diagnosed with gestational diabetes. A total of 510 participants enrolled in the study, with 268 receiving metformin (up to a maximum dose of 2,500 mg) at diagnosis and 267 receiving an identical placebo. Blood sugar levels were monitored 7 times a day, and medication adherence was assessed every 4 weeks.
Results. At 32 or 38 weeks’ gestation, 56.8% of patients in the metformin arm, and 63.7% of patients in the placebo arm required insulin or had fasting blood glucose levels above 5.1 mmol/L (91.8mg/dL), which was a statistically insignificant difference (P = .13). Although there was similarly no difference in the total amount of insulin used in each study group, the percentage of patients who required insulin initiation was decreased in the metformin arm (38.4% vs 51.1%; P = .004).
Study strengths and weaknesses
The authors conducted a well-designed double-blinded RCT—in both rural and tertiary care settings. Additionally, the study had an impressive 90% patient adherence rate for home blood glucose monitoring 7 times per day. The study arms were balanced for body mass index, as obesity is a known contributor to the development of gestational diabetes and response to insulin.
This study findings’ generalizability is limited across subpopulations given the lack of ethnic and racial diversity—the study population was 80% White. Additionally, utilization of the World Health Organization guidelines for diagnosing gestational diabetes, although adopted by most associations across the world, limits its application to areas of the world that use the National Diabetes Data Group or the Carpenter-Coustan diagnosis guidelines.3,4 Furthermore, the diagnosis of gestational diabetes, which was based on 1 elevated value of a 2-hour glucose tolerance test, has limited scientific support, has not been proven to improve obstetric outcomes, and may increase health care costs when compared with the 2-step method.5 The criteria for insulin initiation in the trial was based on having 2 elevated measures of blood glucose during home glucose monitoring, a criteria that is much stricter than what is used in other countries or clinical practice. The trial authors concluded that use of metformin had a statistically significant reduction in neonates weighing > 4,000 g and > 90th% of weight, but they did not assess study group differences in neonatal skin fold thickness or anthropometric measurements, as reported in the Metformin in Gestational Diabetes trials.6 ●
The study findings by Dunne and colleagues reinforce the current standard practice for the management of gestational diabetes: prescribe medical nutrition therapy and exercise followed by insulin initiation in the setting of persistently elevated blood glucose levels. Knowing that metformin crosses the placenta, future studies should also address the long-term metabolic and health outcomes of fetuses exposed to metformin.
NKECHINYELUM OGU, MD; CHARLOTTE NIZNIK, APRN; MICHELLE A. KOMINIAREK, MD, MS
Dunne F, Newman C, Alvarez-Iglesia A, et al. Early metformin in gestational diabetes: a randomized clinical trial. JAMA. 2023;330:1547-1556. doi:10.1001/jama .2023.19869
EXPERT COMMENTARY
Gestational diabetes mellitus occurs in 4% to 7% of pregnancies, and the prevalence is likely to continue to increase given the rising rates of hypertension, obesity, advanced maternal age, and other medical comorbidities in pregnant persons in the United States.1,2 Uncontrolled hyperglycemia in pregnancy is associated swith many adverse perinatal outcomes, including stillbirth, macrosomia, admission to the neonatal intensive care unit (NICU), development of hypertensive disorders, and cesarean deliveries. Hence, it is important to investigate and identify the optimal management of gestational diabetes.
Metformin, an oral biguanide, although studied for gestational diabetes treatment in phase 3 randomized clinical open-label trials, often is avoided in patients who are pregnant (with the exception of patients who have needle aversions, are financially unable to use insulin, or are unable to administer insulin safely).1,2 Metformin is a highly effective first-line agent in the management of both prediabetes and type 2 diabetes, which begs us to question if there is a role for it in the management of gestational diabetes.
Details about the study
The study by Dunne and colleagues was a randomized controlled trial (RCT) conducted in a 1:1 parallel fashion at two institutions in Ireland from 2017–2022. The primary outcome assessed if treatment with metformin would reduce fasting blood glucose levels and the initiation of insulin among women diagnosed with gestational diabetes. A total of 510 participants enrolled in the study, with 268 receiving metformin (up to a maximum dose of 2,500 mg) at diagnosis and 267 receiving an identical placebo. Blood sugar levels were monitored 7 times a day, and medication adherence was assessed every 4 weeks.
Results. At 32 or 38 weeks’ gestation, 56.8% of patients in the metformin arm, and 63.7% of patients in the placebo arm required insulin or had fasting blood glucose levels above 5.1 mmol/L (91.8mg/dL), which was a statistically insignificant difference (P = .13). Although there was similarly no difference in the total amount of insulin used in each study group, the percentage of patients who required insulin initiation was decreased in the metformin arm (38.4% vs 51.1%; P = .004).
Study strengths and weaknesses
The authors conducted a well-designed double-blinded RCT—in both rural and tertiary care settings. Additionally, the study had an impressive 90% patient adherence rate for home blood glucose monitoring 7 times per day. The study arms were balanced for body mass index, as obesity is a known contributor to the development of gestational diabetes and response to insulin.
This study findings’ generalizability is limited across subpopulations given the lack of ethnic and racial diversity—the study population was 80% White. Additionally, utilization of the World Health Organization guidelines for diagnosing gestational diabetes, although adopted by most associations across the world, limits its application to areas of the world that use the National Diabetes Data Group or the Carpenter-Coustan diagnosis guidelines.3,4 Furthermore, the diagnosis of gestational diabetes, which was based on 1 elevated value of a 2-hour glucose tolerance test, has limited scientific support, has not been proven to improve obstetric outcomes, and may increase health care costs when compared with the 2-step method.5 The criteria for insulin initiation in the trial was based on having 2 elevated measures of blood glucose during home glucose monitoring, a criteria that is much stricter than what is used in other countries or clinical practice. The trial authors concluded that use of metformin had a statistically significant reduction in neonates weighing > 4,000 g and > 90th% of weight, but they did not assess study group differences in neonatal skin fold thickness or anthropometric measurements, as reported in the Metformin in Gestational Diabetes trials.6 ●
The study findings by Dunne and colleagues reinforce the current standard practice for the management of gestational diabetes: prescribe medical nutrition therapy and exercise followed by insulin initiation in the setting of persistently elevated blood glucose levels. Knowing that metformin crosses the placenta, future studies should also address the long-term metabolic and health outcomes of fetuses exposed to metformin.
NKECHINYELUM OGU, MD; CHARLOTTE NIZNIK, APRN; MICHELLE A. KOMINIAREK, MD, MS
- Rowan JA, Hague WM, Gao W, et al. Metformin versus insulin for the treatment of gestational diabetes. N Engl J Med. 2008;358:2003-2015. doi: 10.1056/NEJMoa0707193
- American College of Obstetricians and Gynecologists. Gestational diabetes mellitus: Practice Bulletin No. 180. Obstet Gynecol. 2017;130:e17-31. doi: 10.1097/AOG.0000000000002159
- Classification and diagnosis of diabetes mellitus and other categories of glucose intolerance. National Diabetes Data Group. Diabetes. 1979;28:1039-1057. doi: 10.2337 /diab.28.12.1039
- Carpenter MW, Coustan DR. Criteria for screening tests for gestational diabetes. Am J Obstet Gynecol. 1982;144:768-773. doi: 10.1016/0002-9378(82)90349-0
- Vandorsten JP, Dodson WC, Espeland MA, et al. NIH consensus development conference: diagnosing gestational diabetes mellitus. NIH Consens State Sci Statements. 2013;29:1-31.
- Rowan JA, Rush EC, Obolonkin V, et al. Metformin in gestational diabetes: the offspring follow-up (MiG TOFU) body composition at 2 years of age. Diabetes Care. 2011;34:2279-2284. https://doi.org/10.2337/dc11-0660
- Rowan JA, Hague WM, Gao W, et al. Metformin versus insulin for the treatment of gestational diabetes. N Engl J Med. 2008;358:2003-2015. doi: 10.1056/NEJMoa0707193
- American College of Obstetricians and Gynecologists. Gestational diabetes mellitus: Practice Bulletin No. 180. Obstet Gynecol. 2017;130:e17-31. doi: 10.1097/AOG.0000000000002159
- Classification and diagnosis of diabetes mellitus and other categories of glucose intolerance. National Diabetes Data Group. Diabetes. 1979;28:1039-1057. doi: 10.2337 /diab.28.12.1039
- Carpenter MW, Coustan DR. Criteria for screening tests for gestational diabetes. Am J Obstet Gynecol. 1982;144:768-773. doi: 10.1016/0002-9378(82)90349-0
- Vandorsten JP, Dodson WC, Espeland MA, et al. NIH consensus development conference: diagnosing gestational diabetes mellitus. NIH Consens State Sci Statements. 2013;29:1-31.
- Rowan JA, Rush EC, Obolonkin V, et al. Metformin in gestational diabetes: the offspring follow-up (MiG TOFU) body composition at 2 years of age. Diabetes Care. 2011;34:2279-2284. https://doi.org/10.2337/dc11-0660
To what extent do growth abnormalities increase the risk of stillbirth near term in pregnancies complicated by diabetes?
McElwee ER, Oliver EA, McFarling K, et al. Risk of stillbirth in pregnancies complicated by diabetes, stratified by fetal growth. Obstet Gynecol. 2023;141:801-809. doi:10.1097/AOG.0000000000005102.
EXPERT COMMENTARY
Stillbirth is defined as intrauterine demise at or beyond 20 weeks’ gestation. Pregestational DM and GDM significantly increase the risk of stillbirth. Both fetal growth restriction and macrosomia are common complications of pregnancies affected by diabetes, and they further increase the risk of stillbirth. While maternal variables such as glycemic control and medication requirement are currently used to assess the risks of expectant management and inform delivery timing, abnormal fetal growth is not.
Investigators sought to evaluate the stillbirth rates per week of expectant management during the late third trimester stratified by birth weight (as a surrogate for fetal growth) in pregnancies complicated by PG-DM or GDM.
Details of the study
McElwee and colleagues used the US National Vital Statistics System to identify nonanomalous singleton pregnancies complicated by PG-DM or GDM from 2014 to 2017.1 Pregnancies were stratified by birth weight and categorized as being LGA (birth weight > 90th percentile for gestational age), SGA (birth weight < 10th percentile for gestational age), or AGA. Stillbirths were identified from 34 0/7 through 39 6/7 weeks of gestation, and conditional stillbirth rates per 10,000 pregnancies were calculated for each week of gestation.
Results. Among 834,631 pregnancies complicated by PG-DM (13.1%) or GDM (86.9%), there were 3,033 stillbirths, of which 61% were in pregnancies with PG-DM. Stillbirth rates increased with advancing gestational age for both PG-DM and GDM regardless of birth weight. In pregnancies with PG-DM, fetuses that were LGA or SGA had a higher relative risk of stillbirth compared with their AGA counterparts at each gestational age. This stillbirth risk was highest in pregnancies with PG-DM that were LGA. At 39 weeks, the stillbirth rate in this population was 96.9/10,000 ongoing pregnancies and was 5 times higher than pregnancies with PG-DM that were AGA. When the GDM-related AGA group was selected as the referent (as the lowest-risk comparison group), pregnancies with PG-DM that were LGA had a 21-times higher relative risk of stillbirth at 37 and 38 weeks of gestation.
Study strengths and limitations
Decisions on the optimal timing of delivery seek to strike a balance between the increased neonatal morbidity with delivery before 39 weeks’ gestation and the increased risk of stillbirth with expectant management. In pregnancies complicated by diabetes, current guidelines from the American College of Obstetricians and Gynecologists recommend consideration of maternal variables, such as medication requirement, glycemic control, and vascular sequelae, to inform decisions on delivery timing, as these factors have been postulated to influence the risk of stillbirth with pregnancy prolongation.2 These recommendations are based largely on expert opinion and retrospective data.
The question of how fetal growth abnormalities factor into this complicated decision making is also an area of low-quality evidence despite studies that demonstrate that both SGA and LGA fetuses in pregnancies complicated by diabetes are at increased risk of stillbirth.3
The large population-based study design by McElwee and colleagues allowed the investigators to examine a rare event (stillbirth) with multiple stratification levels and sufficient statistical power and to contribute to this literature.
Significant limitations, however, must be considered before generalizing these results. The data were restricted to variables available on birth and death certificates, and more granular information—such as the type of DM, level of glycemic control, frequency of antenatal testing, and stillbirth work-up—could not be assessed. Ultrasonographic estimations of fetal weight also were not included. Birth weight data were used as a proxy, although we know that these variables do not always correlate well given the limited accuracy of ultrasonography in assessing projected birth weight, particularly later in pregnancy. The authors also did not control for highly prevalent variables (for example, hypertension, obesity) that are likely associated with abnormal fetal growth and stillbirth in these populations. ●
The present study demonstrates that both SGA and LGA are significant risk factors for stillbirth in pregnancies with either PG-DM or GDM in the late preterm and early term periods, and this risk should be considered when making decisions on appropriate timing of delivery. The conditional stillbirth rate was highest in pregnancies with PG-DM with LGA fetuses, and this risk increased with each week of expectant management. This population may benefit the most from critical assessment of the risk of stillbirth with ongoing pregnancy. Notably, the quality of evidence is not sufficient to universally alter delivery timing guidelines in this population. We recommend individual assessment of each clinical scenario when making these decisions.
NIGEL MADDEN, MD; MICHELLE A. KOMINIAREK, MD, MS
- McElwee ER, Oliver EA, McFarling K, et al. Risk of stillbirth in pregnancies complicated by diabetes, stratified by fetal growth. Obstet Gynecol. 2023;141:801-809. doi:10.1097 /AOG.0000000000005102
- ACOG Committee Opinion No. 764. Medically indicated late-preterm and early-term deliveries. Obstet Gynecol. 2019;133:e151-e155. doi:10.1097/AOG.0000000000003083
- Starikov R, Dudley D, Reddy UM. Stillbirth in the pregnancy complicated by diabetes. Curr Diab Rep. 2015;15:11. doi:10.1007/s11892-015-0580-y
McElwee ER, Oliver EA, McFarling K, et al. Risk of stillbirth in pregnancies complicated by diabetes, stratified by fetal growth. Obstet Gynecol. 2023;141:801-809. doi:10.1097/AOG.0000000000005102.
EXPERT COMMENTARY
Stillbirth is defined as intrauterine demise at or beyond 20 weeks’ gestation. Pregestational DM and GDM significantly increase the risk of stillbirth. Both fetal growth restriction and macrosomia are common complications of pregnancies affected by diabetes, and they further increase the risk of stillbirth. While maternal variables such as glycemic control and medication requirement are currently used to assess the risks of expectant management and inform delivery timing, abnormal fetal growth is not.
Investigators sought to evaluate the stillbirth rates per week of expectant management during the late third trimester stratified by birth weight (as a surrogate for fetal growth) in pregnancies complicated by PG-DM or GDM.
Details of the study
McElwee and colleagues used the US National Vital Statistics System to identify nonanomalous singleton pregnancies complicated by PG-DM or GDM from 2014 to 2017.1 Pregnancies were stratified by birth weight and categorized as being LGA (birth weight > 90th percentile for gestational age), SGA (birth weight < 10th percentile for gestational age), or AGA. Stillbirths were identified from 34 0/7 through 39 6/7 weeks of gestation, and conditional stillbirth rates per 10,000 pregnancies were calculated for each week of gestation.
Results. Among 834,631 pregnancies complicated by PG-DM (13.1%) or GDM (86.9%), there were 3,033 stillbirths, of which 61% were in pregnancies with PG-DM. Stillbirth rates increased with advancing gestational age for both PG-DM and GDM regardless of birth weight. In pregnancies with PG-DM, fetuses that were LGA or SGA had a higher relative risk of stillbirth compared with their AGA counterparts at each gestational age. This stillbirth risk was highest in pregnancies with PG-DM that were LGA. At 39 weeks, the stillbirth rate in this population was 96.9/10,000 ongoing pregnancies and was 5 times higher than pregnancies with PG-DM that were AGA. When the GDM-related AGA group was selected as the referent (as the lowest-risk comparison group), pregnancies with PG-DM that were LGA had a 21-times higher relative risk of stillbirth at 37 and 38 weeks of gestation.
Study strengths and limitations
Decisions on the optimal timing of delivery seek to strike a balance between the increased neonatal morbidity with delivery before 39 weeks’ gestation and the increased risk of stillbirth with expectant management. In pregnancies complicated by diabetes, current guidelines from the American College of Obstetricians and Gynecologists recommend consideration of maternal variables, such as medication requirement, glycemic control, and vascular sequelae, to inform decisions on delivery timing, as these factors have been postulated to influence the risk of stillbirth with pregnancy prolongation.2 These recommendations are based largely on expert opinion and retrospective data.
The question of how fetal growth abnormalities factor into this complicated decision making is also an area of low-quality evidence despite studies that demonstrate that both SGA and LGA fetuses in pregnancies complicated by diabetes are at increased risk of stillbirth.3
The large population-based study design by McElwee and colleagues allowed the investigators to examine a rare event (stillbirth) with multiple stratification levels and sufficient statistical power and to contribute to this literature.
Significant limitations, however, must be considered before generalizing these results. The data were restricted to variables available on birth and death certificates, and more granular information—such as the type of DM, level of glycemic control, frequency of antenatal testing, and stillbirth work-up—could not be assessed. Ultrasonographic estimations of fetal weight also were not included. Birth weight data were used as a proxy, although we know that these variables do not always correlate well given the limited accuracy of ultrasonography in assessing projected birth weight, particularly later in pregnancy. The authors also did not control for highly prevalent variables (for example, hypertension, obesity) that are likely associated with abnormal fetal growth and stillbirth in these populations. ●
The present study demonstrates that both SGA and LGA are significant risk factors for stillbirth in pregnancies with either PG-DM or GDM in the late preterm and early term periods, and this risk should be considered when making decisions on appropriate timing of delivery. The conditional stillbirth rate was highest in pregnancies with PG-DM with LGA fetuses, and this risk increased with each week of expectant management. This population may benefit the most from critical assessment of the risk of stillbirth with ongoing pregnancy. Notably, the quality of evidence is not sufficient to universally alter delivery timing guidelines in this population. We recommend individual assessment of each clinical scenario when making these decisions.
NIGEL MADDEN, MD; MICHELLE A. KOMINIAREK, MD, MS
McElwee ER, Oliver EA, McFarling K, et al. Risk of stillbirth in pregnancies complicated by diabetes, stratified by fetal growth. Obstet Gynecol. 2023;141:801-809. doi:10.1097/AOG.0000000000005102.
EXPERT COMMENTARY
Stillbirth is defined as intrauterine demise at or beyond 20 weeks’ gestation. Pregestational DM and GDM significantly increase the risk of stillbirth. Both fetal growth restriction and macrosomia are common complications of pregnancies affected by diabetes, and they further increase the risk of stillbirth. While maternal variables such as glycemic control and medication requirement are currently used to assess the risks of expectant management and inform delivery timing, abnormal fetal growth is not.
Investigators sought to evaluate the stillbirth rates per week of expectant management during the late third trimester stratified by birth weight (as a surrogate for fetal growth) in pregnancies complicated by PG-DM or GDM.
Details of the study
McElwee and colleagues used the US National Vital Statistics System to identify nonanomalous singleton pregnancies complicated by PG-DM or GDM from 2014 to 2017.1 Pregnancies were stratified by birth weight and categorized as being LGA (birth weight > 90th percentile for gestational age), SGA (birth weight < 10th percentile for gestational age), or AGA. Stillbirths were identified from 34 0/7 through 39 6/7 weeks of gestation, and conditional stillbirth rates per 10,000 pregnancies were calculated for each week of gestation.
Results. Among 834,631 pregnancies complicated by PG-DM (13.1%) or GDM (86.9%), there were 3,033 stillbirths, of which 61% were in pregnancies with PG-DM. Stillbirth rates increased with advancing gestational age for both PG-DM and GDM regardless of birth weight. In pregnancies with PG-DM, fetuses that were LGA or SGA had a higher relative risk of stillbirth compared with their AGA counterparts at each gestational age. This stillbirth risk was highest in pregnancies with PG-DM that were LGA. At 39 weeks, the stillbirth rate in this population was 96.9/10,000 ongoing pregnancies and was 5 times higher than pregnancies with PG-DM that were AGA. When the GDM-related AGA group was selected as the referent (as the lowest-risk comparison group), pregnancies with PG-DM that were LGA had a 21-times higher relative risk of stillbirth at 37 and 38 weeks of gestation.
Study strengths and limitations
Decisions on the optimal timing of delivery seek to strike a balance between the increased neonatal morbidity with delivery before 39 weeks’ gestation and the increased risk of stillbirth with expectant management. In pregnancies complicated by diabetes, current guidelines from the American College of Obstetricians and Gynecologists recommend consideration of maternal variables, such as medication requirement, glycemic control, and vascular sequelae, to inform decisions on delivery timing, as these factors have been postulated to influence the risk of stillbirth with pregnancy prolongation.2 These recommendations are based largely on expert opinion and retrospective data.
The question of how fetal growth abnormalities factor into this complicated decision making is also an area of low-quality evidence despite studies that demonstrate that both SGA and LGA fetuses in pregnancies complicated by diabetes are at increased risk of stillbirth.3
The large population-based study design by McElwee and colleagues allowed the investigators to examine a rare event (stillbirth) with multiple stratification levels and sufficient statistical power and to contribute to this literature.
Significant limitations, however, must be considered before generalizing these results. The data were restricted to variables available on birth and death certificates, and more granular information—such as the type of DM, level of glycemic control, frequency of antenatal testing, and stillbirth work-up—could not be assessed. Ultrasonographic estimations of fetal weight also were not included. Birth weight data were used as a proxy, although we know that these variables do not always correlate well given the limited accuracy of ultrasonography in assessing projected birth weight, particularly later in pregnancy. The authors also did not control for highly prevalent variables (for example, hypertension, obesity) that are likely associated with abnormal fetal growth and stillbirth in these populations. ●
The present study demonstrates that both SGA and LGA are significant risk factors for stillbirth in pregnancies with either PG-DM or GDM in the late preterm and early term periods, and this risk should be considered when making decisions on appropriate timing of delivery. The conditional stillbirth rate was highest in pregnancies with PG-DM with LGA fetuses, and this risk increased with each week of expectant management. This population may benefit the most from critical assessment of the risk of stillbirth with ongoing pregnancy. Notably, the quality of evidence is not sufficient to universally alter delivery timing guidelines in this population. We recommend individual assessment of each clinical scenario when making these decisions.
NIGEL MADDEN, MD; MICHELLE A. KOMINIAREK, MD, MS
- McElwee ER, Oliver EA, McFarling K, et al. Risk of stillbirth in pregnancies complicated by diabetes, stratified by fetal growth. Obstet Gynecol. 2023;141:801-809. doi:10.1097 /AOG.0000000000005102
- ACOG Committee Opinion No. 764. Medically indicated late-preterm and early-term deliveries. Obstet Gynecol. 2019;133:e151-e155. doi:10.1097/AOG.0000000000003083
- Starikov R, Dudley D, Reddy UM. Stillbirth in the pregnancy complicated by diabetes. Curr Diab Rep. 2015;15:11. doi:10.1007/s11892-015-0580-y
- McElwee ER, Oliver EA, McFarling K, et al. Risk of stillbirth in pregnancies complicated by diabetes, stratified by fetal growth. Obstet Gynecol. 2023;141:801-809. doi:10.1097 /AOG.0000000000005102
- ACOG Committee Opinion No. 764. Medically indicated late-preterm and early-term deliveries. Obstet Gynecol. 2019;133:e151-e155. doi:10.1097/AOG.0000000000003083
- Starikov R, Dudley D, Reddy UM. Stillbirth in the pregnancy complicated by diabetes. Curr Diab Rep. 2015;15:11. doi:10.1007/s11892-015-0580-y
Treating PPH: A novel vacuum-induced hemorrhage control device
Postpartum hemorrhage (PPH) continues to be a leading cause of maternal morbidity and mortality both worldwide and in the United States.1-3 A PPH is defined as the cumulative blood loss of 1,000 mL or more, or blood loss accompanied by signs or symptoms of hypovolemia, within 24 hours following the birth process (including intrapartum loss).4
Approximately 70% to 80% of hemorrhages are due to abnormal uterine tone.5 Bimanual massage and medical management, the primary treatments for uterine atony, attempt to restore the normal uterine tone that compresses the vessels in the placental implantation site and limits bleeding. For women in whom the primary treatments are not effective, only uterine compression sutures in a laparotomy can achieve physiologic contracture of the uterus. The second-line treatment option, intrauterine tamponade, places pressure over the placental implantation site while distending the uterus.
In October 2020, the US Food and Drug Administration (FDA) granted clearance to a novel device that offers an alternative treatment option. The Jada System (Alydia Health), an intrauterine vacuum-induced hemorrhage control device, is placed in the uterus and uses wall suction to induce physiologic contraction of the uterus to control bleeding.6
In this article, within the context of a case vignette, we discuss the recent study on the Jada System and how this device can be used in the management of PPH.6
CASE Woman with PPH history fears repeat hemorrhage
Ms. B. is a 25-year-old woman (G2P1) who presents for prenatal care at 10 weeks’ gestation. Her medical history is significant for asthma and PPH after her first delivery. When you review her prior delivery records, you learn that she had a protracted labor and delivered a healthy 10 lb 8 oz baby boy after 3 hours of pushing. After delivery, she received postpartum intravenous oxytocin followed by intramuscular uterotonics when her bleeding was heavy during her laceration repair. Her estimated blood loss at delivery was 600 mL. The team was called back to her bedside for the continued bleeding. Uterine atony was diagnosed. Although she received additional uterotonics, the bleeding continued. An intrauterine tamponade balloon was placed, and the bleeding ultimately was controlled. The total estimated blood loss (EBL) was 2.5 L, and the patient then was transfused with 2 U of packed red blood cells.
Currently, Ms. B. is very worried about having another hemorrhage as the bleeding terrified her and her partner, disrupted breastfeeding initiation while the tamponade was in place, and made her anxious about having another baby.
What steps would you take to prepare for a potential PPH in this patient?
Risk factors
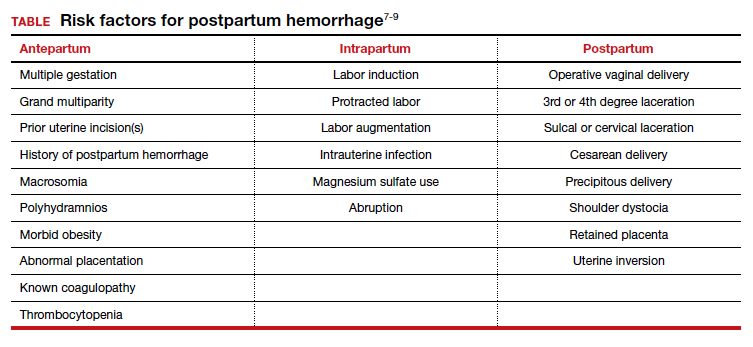
While PPH often is unpredictable, many risk factors have been identified (TABLE).7-9 Some risk factors are present during the antepartum period while others arise during labor. In some cases, obstetric clinicians may be able to intervene during prenatal care, such as by giving iron supplementation to address anemia. Other factors, however, are not modifiable, including multiparity, polyhydramnios, and multiple gestations. On presentation to the labor unit, new risk factors may arise, such as magnesium sulfate use, chorioamnionitis, protracted labor, or the need for general anesthesia. In addition, the presence of a fibroid uterus or a uterine inversion can impede effective uterine contractions.5
Various tools are available for assessing these risk factors on admission, during labor, and after delivery, such as the AWHONN postpartum hemorrhage risk assessment table and the CMQCC obstetric hemorrhage toolkit.10,11
Continue to: CASE continued Patient’s history reveals risk factors...
CASE continued Patient’s history reveals risk factors
You review with Ms. B. that she had several risk factors present during labor. She had a large baby and a protracted labor. Knowing her history in this pregnancy will allow the clinical team to be prepared for a potential recurrent hemorrhage and to respond proactively to bleeding.
Consider the management options
The initial treatment for PPH includes bimanual massage, oxytocin, and other uterotonics (methylergonovine, 15-methyl prostaglandin F2α, and misoprostol). While various algorithms are available on the order of treatment, a single agent has not been shown superior to others.12 The antifibrinolytic medication tranexamic acid also was shown to reduce the risk of death from obstetric hemorrhage in the international WOMAN trial.13
While these agents often are used simultaneously to achieve hemostasis, their systemic effects are associated with contraindications. Specifically, F2α prostaglandins cannot be used in patients with asthma or active hepatic, pulmonary, or cardiac disease. Ergot derivatives cannot be used in patients with hypertension, pre-eclampsia, or cardiovascular disease. Given the rising rate of medical comorbidities during pregnancy, such contraindications limit the treatment options for many patients.
In cases in which medical management is not sufficient or is contraindicated for controlling hemorrhage, second-line treatment includes the use of tamponade techniques, such as intrauterine packing or balloons. The tamponade applies pressure directly to the placental implantation site for 12 to 24 hours, which allows time for the uterus to contract and return to normal tone. While this method may seem counterintuitive to achieving uterine tone, studies suggest a success rate between 75% and 86% with balloon tamponade.12
Third-line treatment options are increasingly invasive but should be used to prevent further maternal morbidity and mortality. These include uterine artery embolization and surgery. Uterine artery embolization is an option for a stable patient at a center with available interventional radiology services. If embolization is either not successful or not available, an exploratory laparotomy should be performed. Uterine compression sutures can be placed along with vascular ligation sutures of the uterine arteries (O’Leary sutures) and the hypogastric arteries. If all other methods have failed, a hysterectomy is the definitive treatment for hemorrhage.
CASE continued Patient desires an alternative to tamponade if needed
Following your visit, Ms. B. has an ultrasound scan that shows a dichorionic diamniotic twin pregnancy. She also has a microcytic anemia. After you discuss iron supplementation with the patient, she asks if there are any other options should medical management fail in the event of a recurrent hemorrhage. While intrauterine tamponade balloon did treat her hemorrhage, she was not happy with the length of time it had to remain in place, the discomfort while it was used, and the disruption to her planned recovery. You inform her of a new treatment option available for PPH, a vacuum-induced hemorrhage control device that was recently FDA cleared.
Continue to: New device controls bleeding fast...
New device controls bleeding fast
In 2020, D’Alton and colleagues reported on their multicenter, prospective single-arm treatment study on the effectiveness and safety of an intrauterine vacuum-induced hemorrhage control device.6 This device, the Jada System, uses low-level vacuum to induce uterine contraction to control bleeding from uterine atony. The prospective study, which followed a 2016 feasibility study, enrolled more than 100 women at 12 centers across the United States.6,14 Women were eligible to participate if they delivered at a gestational age of 34 weeks or later and had an EBL between 500 and 1,000 mL after a vaginal delivery or an EBL between 1,000 and 1,500 mL after a cesarean delivery.
Treatment with the vacuum device was successful in 94% (100/106, 95% confidence interval, 88%–98%) of women, and definitive control of abnormal bleeding was achieved in a median of 3 minutes (interquartile range [IQR], 2.0–5.0) after connection to the vacuum device.6
CASE continued Patient has questions
Your patient expresses interest in this device, but she wants to understand how it works. Would it require transfer to another unit or prolonged monitoring?
How the device works
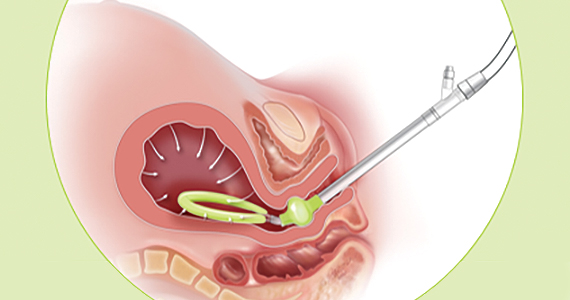
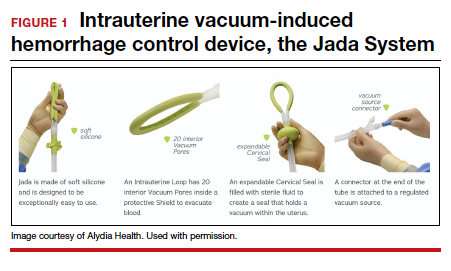
Compared with intrauterine tamponade balloon devices, which apply pressure by distending the uterus, the Jada System applies low-level intrauterine vacuum to facilitate the physiologic forces of uterine contractions to constrict myometrial blood vessels and achieve hemostasis.6 The device is made of medical-grade silicone. Its distal end, which is placed in the uterus, is an elliptical loop. The loop’s inner surface contains 20 vacuum pores protected by a shield that facilitate creation of a vacuum within the uterine cavity. The loop is soft and smooth to limit the chance of tissue damage during insertion, treatment, and removal of the device. The device’s proximal end has a vacuum connector. The vacuum source is hospital-grade wall suction, but a portable vacuum source also can be used (FIGURE 1).
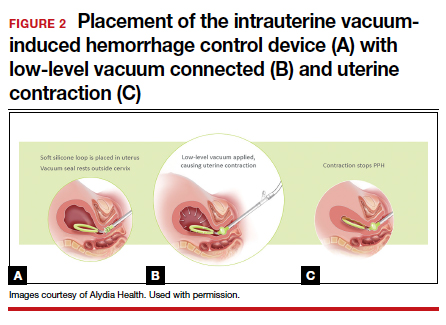
Prior to placing the device, a manual sweep of the uterine cavity is performed. If needed, ultrasonography can be used with the manual sweep to ensure that there is no retained placental tissue or clot. The loop of the Jada System is then inserted in the uterine cavity, and the circular cervical seal, just outside the external cervical os, is filled with sterile water.
Low-level vacuum (80 ± 10 mm Hg) is applied so that pooled blood is evacuated from the uterus as it collapses (FIGURE 2). The volume of any ongoing bleeding is measured in the suction tubing while the uterine response to treatment can be palpated. Once there is no bleeding without any need for further treatment, the device should remain in the uterus for at least 1 hour. The suction is then turned off, and bleeding is monitored for 30 minutes. If bleeding remains controlled, the device can be removed.
CASE continued The question of complications
Ms. B. is concerned about safety and asks about potential complications with the device’s use.
Safety findings
In the prospective study and FDA review, the device was deemed safe. There were 8 possibly related adverse events (endometritis, laceration disruption, and vaginal infection), which all resolved without serious clinical sequelae. Forty women (38%) received a blood transfusion, but only 5 required 4 U or more of red blood cells.6
Continue to: CASE continued What do other physicians think?...
CASE continued What do other physicians think?
Your patient is curious about the time it takes for the device to work and whether other clinicians like using this new device for hemorrhage treatment.
Duration of treatment
The times to achieve uterine collapse and control of hemorrhage are both relatively short. In the prospective study, the initial collapse of the uterus took a median of 1 minute (IQR, 1–2 min) from the time of vacuum connection.6 Bleeding was controlled in less than 5 minutes in 82% of women, with an overall median time of 3 minutes (IQR, 2–5 min). The median duration of vacuum treatment was 144.0 minutes (IQR, 85.8–295.8 min), which includes the required minimum of 60 minutes for vacuum treatment time and 30 minutes of observation without the vacuum connected but with the device still in place.6
When polled, the majority of clinicians—98%—reported that the intrauterine vacuum-induced hemorrhage control device was easy to use, and 97% would recommend its use for future patients.6
Further, recognizing the device’s potential, the Cleveland Clinic cited it as one of the top 10 health care innovations for 2021 for offering a low-tech and minimally invasive tool for obstetric clinicians.15
CASE continued Final questions
Ms. B. thanks you for the information and asks, should she know anything else about the device?
Vacuum device vs other treatments
The study by D’Alton and colleagues was a single-arm treatment trial that did not directly compare the effectiveness of the device with that of other PPH treatment options, such as balloon tamponade.6 At this point, we know that clinicians can safely and quickly use the device to treat uterine atony, but we do not know if it is superior to other treatments for PPH.
Key takeaways
Postpartum hemorrhage is a leading cause of maternal morbidity and mortality. When first-line uterotonics fail, obstetric clinicians previously had only balloon tamponade or invasive procedures to treat patients. The novel intrauterine vacuum-induced hemorrhage control device takes a new approach that simulates the physiologic process of uterine contractions. The device can rapidly and effectively control abnormal postpartum uterine bleeding. More studies are needed, however, to compare the device’s effectiveness with that of other PPH treatments and to consider its use in women with more severe degrees of postpartum hemorrhage as well as its cost-effectiveness. ●
- Say L, Chou D, Gemmill A, et al. Global causes of maternal death: a WHO systematic analysis. Lancet Glob Health. 2014;2:e323-e333.
- Callaghan WM, Creanga AA, Kuklina EV. Severe maternal morbidity among delivery and postpartum hospitalizations in the United States. Obstet Gynecol. 2012;120:1029-1036.
- Centers for Disease Control and Prevention. Severe maternal morbidity in the United States. http://www .cdc.gov/reproductivehealth/maternalinfanthealth /severematernalmorbidity.html. Accessed November 6, 2020.
- Menard MK, Main EK, Currigan SM. Executive summary of the reVITALize initiative: standardizing obstetric data definitions. Obstet Gynecol. 2014;124:150-153.
- American College of Obstetricians and Gynecologists Committee on Practice Bulletins–Obstetrics. Practice bulletin no. 183: postpartum hemorrhage. Obstet Gynecol. 2017;130:e168-e186.
- D’Alton ME, Rood KM, Smid MC, et al. Intrauterine vacuum-induced hemorrhage-control device for rapid treatment of postpartum hemorrhage. Obstet Gynecol. 2020;136:882-891.
- Mavrides E, Allard S, Chandraharan E, et al; on behalf of the Royal College of Obstetricians and Gynaecologists. Prevention and management of postpartum hemorrhage. BJOG. 2016;124:e106-e149.
- Lyndon A, Lagrew D, Shields L, et al. Improving health care response to obstetric hemorrhage, version 2.0 (California Maternal Quality Care Collaborative Toolkit to Transform Maternity Care). Developed under contract #11-10006 with the California Department of Public Health; Maternal, Child and Adolescent Health Division; Published by the California Maternal Quality Care Collaborative, March 17, 2015.
- Main EK, Goffman D, Scavone BM, et al; National Partnership for Maternal Safety; Council on Patient Safety in Women’s Health Care. National Partnership for Maternal Safety: consensus bundle on obstetric hemorrhage. Obstet Gynecol. 2015;126:155-162.
- AWHONN Postpartum Hemorrhage Project. Postpartum hemorrhage (PPH) risk assessment table 1.0. https:// mygnosis.com/Content/Chunks/3504/assets/pdfs/PPH _Risk_Assessment_Table-7-17-15.pdf. Accessed November 15, 2020.
- Bingham D, Melsop K, Main E. CMQCC obstetric hemorrhage toolkit: hospital level implementation guide. 2010. California Maternal Quality Care Collaborative (CMQCC). Palo Alto, CA: Stanford University. https://www.cmqcc.org/resource/1489 /download. Accessed November 15, 2020.
- Likis FE, Sathe NA, Morgans AK, et al. Management of postpartum hemorrhage. Comparative effectiveness review no. 151. AHRQ publication no. 15-EHC013-EF. Rockville, MD: Agency for Healthcare Research and Quality; 2015.
- WOMAN Trial Collaborators. Effect of early tranexamic acid administration on mortality, hysterectomy, and other morbidities in women with post-partum haemorrhage (WOMAN): an international, randomised, double-blind, placebo-controlled trial. Lancet. 2017;389:2105–2116.
- Purwosunu Y, Sarkoen W, Arulkumaran S, et al. Control of postpartum hemorrhage using vacuum-induced uterine tamponade. Obstet Gynecol. 2016;128:33-36.
- Cleveland Clinic Innovations. Cleveland Clinic unveils top 10 medical innovations for 2021. October 6, 2020. https:// innovations.clevelandclinic.org/Programs/Top-10-Medical -Innovations/Top-10-for-2021. Accessed November 6, 2020.
Postpartum hemorrhage (PPH) continues to be a leading cause of maternal morbidity and mortality both worldwide and in the United States.1-3 A PPH is defined as the cumulative blood loss of 1,000 mL or more, or blood loss accompanied by signs or symptoms of hypovolemia, within 24 hours following the birth process (including intrapartum loss).4
Approximately 70% to 80% of hemorrhages are due to abnormal uterine tone.5 Bimanual massage and medical management, the primary treatments for uterine atony, attempt to restore the normal uterine tone that compresses the vessels in the placental implantation site and limits bleeding. For women in whom the primary treatments are not effective, only uterine compression sutures in a laparotomy can achieve physiologic contracture of the uterus. The second-line treatment option, intrauterine tamponade, places pressure over the placental implantation site while distending the uterus.
In October 2020, the US Food and Drug Administration (FDA) granted clearance to a novel device that offers an alternative treatment option. The Jada System (Alydia Health), an intrauterine vacuum-induced hemorrhage control device, is placed in the uterus and uses wall suction to induce physiologic contraction of the uterus to control bleeding.6
In this article, within the context of a case vignette, we discuss the recent study on the Jada System and how this device can be used in the management of PPH.6
CASE Woman with PPH history fears repeat hemorrhage
Ms. B. is a 25-year-old woman (G2P1) who presents for prenatal care at 10 weeks’ gestation. Her medical history is significant for asthma and PPH after her first delivery. When you review her prior delivery records, you learn that she had a protracted labor and delivered a healthy 10 lb 8 oz baby boy after 3 hours of pushing. After delivery, she received postpartum intravenous oxytocin followed by intramuscular uterotonics when her bleeding was heavy during her laceration repair. Her estimated blood loss at delivery was 600 mL. The team was called back to her bedside for the continued bleeding. Uterine atony was diagnosed. Although she received additional uterotonics, the bleeding continued. An intrauterine tamponade balloon was placed, and the bleeding ultimately was controlled. The total estimated blood loss (EBL) was 2.5 L, and the patient then was transfused with 2 U of packed red blood cells.
Currently, Ms. B. is very worried about having another hemorrhage as the bleeding terrified her and her partner, disrupted breastfeeding initiation while the tamponade was in place, and made her anxious about having another baby.
What steps would you take to prepare for a potential PPH in this patient?
Risk factors
While PPH often is unpredictable, many risk factors have been identified (TABLE).7-9 Some risk factors are present during the antepartum period while others arise during labor. In some cases, obstetric clinicians may be able to intervene during prenatal care, such as by giving iron supplementation to address anemia. Other factors, however, are not modifiable, including multiparity, polyhydramnios, and multiple gestations. On presentation to the labor unit, new risk factors may arise, such as magnesium sulfate use, chorioamnionitis, protracted labor, or the need for general anesthesia. In addition, the presence of a fibroid uterus or a uterine inversion can impede effective uterine contractions.5

Various tools are available for assessing these risk factors on admission, during labor, and after delivery, such as the AWHONN postpartum hemorrhage risk assessment table and the CMQCC obstetric hemorrhage toolkit.10,11
Continue to: CASE continued Patient’s history reveals risk factors...
CASE continued Patient’s history reveals risk factors
You review with Ms. B. that she had several risk factors present during labor. She had a large baby and a protracted labor. Knowing her history in this pregnancy will allow the clinical team to be prepared for a potential recurrent hemorrhage and to respond proactively to bleeding.
Consider the management options
The initial treatment for PPH includes bimanual massage, oxytocin, and other uterotonics (methylergonovine, 15-methyl prostaglandin F2α, and misoprostol). While various algorithms are available on the order of treatment, a single agent has not been shown superior to others.12 The antifibrinolytic medication tranexamic acid also was shown to reduce the risk of death from obstetric hemorrhage in the international WOMAN trial.13
While these agents often are used simultaneously to achieve hemostasis, their systemic effects are associated with contraindications. Specifically, F2α prostaglandins cannot be used in patients with asthma or active hepatic, pulmonary, or cardiac disease. Ergot derivatives cannot be used in patients with hypertension, pre-eclampsia, or cardiovascular disease. Given the rising rate of medical comorbidities during pregnancy, such contraindications limit the treatment options for many patients.
In cases in which medical management is not sufficient or is contraindicated for controlling hemorrhage, second-line treatment includes the use of tamponade techniques, such as intrauterine packing or balloons. The tamponade applies pressure directly to the placental implantation site for 12 to 24 hours, which allows time for the uterus to contract and return to normal tone. While this method may seem counterintuitive to achieving uterine tone, studies suggest a success rate between 75% and 86% with balloon tamponade.12
Third-line treatment options are increasingly invasive but should be used to prevent further maternal morbidity and mortality. These include uterine artery embolization and surgery. Uterine artery embolization is an option for a stable patient at a center with available interventional radiology services. If embolization is either not successful or not available, an exploratory laparotomy should be performed. Uterine compression sutures can be placed along with vascular ligation sutures of the uterine arteries (O’Leary sutures) and the hypogastric arteries. If all other methods have failed, a hysterectomy is the definitive treatment for hemorrhage.
CASE continued Patient desires an alternative to tamponade if needed
Following your visit, Ms. B. has an ultrasound scan that shows a dichorionic diamniotic twin pregnancy. She also has a microcytic anemia. After you discuss iron supplementation with the patient, she asks if there are any other options should medical management fail in the event of a recurrent hemorrhage. While intrauterine tamponade balloon did treat her hemorrhage, she was not happy with the length of time it had to remain in place, the discomfort while it was used, and the disruption to her planned recovery. You inform her of a new treatment option available for PPH, a vacuum-induced hemorrhage control device that was recently FDA cleared.
Continue to: New device controls bleeding fast...
New device controls bleeding fast
In 2020, D’Alton and colleagues reported on their multicenter, prospective single-arm treatment study on the effectiveness and safety of an intrauterine vacuum-induced hemorrhage control device.6 This device, the Jada System, uses low-level vacuum to induce uterine contraction to control bleeding from uterine atony. The prospective study, which followed a 2016 feasibility study, enrolled more than 100 women at 12 centers across the United States.6,14 Women were eligible to participate if they delivered at a gestational age of 34 weeks or later and had an EBL between 500 and 1,000 mL after a vaginal delivery or an EBL between 1,000 and 1,500 mL after a cesarean delivery.
Treatment with the vacuum device was successful in 94% (100/106, 95% confidence interval, 88%–98%) of women, and definitive control of abnormal bleeding was achieved in a median of 3 minutes (interquartile range [IQR], 2.0–5.0) after connection to the vacuum device.6
CASE continued Patient has questions
Your patient expresses interest in this device, but she wants to understand how it works. Would it require transfer to another unit or prolonged monitoring?
How the device works
Compared with intrauterine tamponade balloon devices, which apply pressure by distending the uterus, the Jada System applies low-level intrauterine vacuum to facilitate the physiologic forces of uterine contractions to constrict myometrial blood vessels and achieve hemostasis.6 The device is made of medical-grade silicone. Its distal end, which is placed in the uterus, is an elliptical loop. The loop’s inner surface contains 20 vacuum pores protected by a shield that facilitate creation of a vacuum within the uterine cavity. The loop is soft and smooth to limit the chance of tissue damage during insertion, treatment, and removal of the device. The device’s proximal end has a vacuum connector. The vacuum source is hospital-grade wall suction, but a portable vacuum source also can be used (FIGURE 1).

Prior to placing the device, a manual sweep of the uterine cavity is performed. If needed, ultrasonography can be used with the manual sweep to ensure that there is no retained placental tissue or clot. The loop of the Jada System is then inserted in the uterine cavity, and the circular cervical seal, just outside the external cervical os, is filled with sterile water.
Low-level vacuum (80 ± 10 mm Hg) is applied so that pooled blood is evacuated from the uterus as it collapses (FIGURE 2). The volume of any ongoing bleeding is measured in the suction tubing while the uterine response to treatment can be palpated. Once there is no bleeding without any need for further treatment, the device should remain in the uterus for at least 1 hour. The suction is then turned off, and bleeding is monitored for 30 minutes. If bleeding remains controlled, the device can be removed.

CASE continued The question of complications
Ms. B. is concerned about safety and asks about potential complications with the device’s use.
Safety findings
In the prospective study and FDA review, the device was deemed safe. There were 8 possibly related adverse events (endometritis, laceration disruption, and vaginal infection), which all resolved without serious clinical sequelae. Forty women (38%) received a blood transfusion, but only 5 required 4 U or more of red blood cells.6
Continue to: CASE continued What do other physicians think?...
CASE continued What do other physicians think?
Your patient is curious about the time it takes for the device to work and whether other clinicians like using this new device for hemorrhage treatment.
Duration of treatment
The times to achieve uterine collapse and control of hemorrhage are both relatively short. In the prospective study, the initial collapse of the uterus took a median of 1 minute (IQR, 1–2 min) from the time of vacuum connection.6 Bleeding was controlled in less than 5 minutes in 82% of women, with an overall median time of 3 minutes (IQR, 2–5 min). The median duration of vacuum treatment was 144.0 minutes (IQR, 85.8–295.8 min), which includes the required minimum of 60 minutes for vacuum treatment time and 30 minutes of observation without the vacuum connected but with the device still in place.6
When polled, the majority of clinicians—98%—reported that the intrauterine vacuum-induced hemorrhage control device was easy to use, and 97% would recommend its use for future patients.6
Further, recognizing the device’s potential, the Cleveland Clinic cited it as one of the top 10 health care innovations for 2021 for offering a low-tech and minimally invasive tool for obstetric clinicians.15
CASE continued Final questions
Ms. B. thanks you for the information and asks, should she know anything else about the device?
Vacuum device vs other treatments
The study by D’Alton and colleagues was a single-arm treatment trial that did not directly compare the effectiveness of the device with that of other PPH treatment options, such as balloon tamponade.6 At this point, we know that clinicians can safely and quickly use the device to treat uterine atony, but we do not know if it is superior to other treatments for PPH.
Key takeaways
Postpartum hemorrhage is a leading cause of maternal morbidity and mortality. When first-line uterotonics fail, obstetric clinicians previously had only balloon tamponade or invasive procedures to treat patients. The novel intrauterine vacuum-induced hemorrhage control device takes a new approach that simulates the physiologic process of uterine contractions. The device can rapidly and effectively control abnormal postpartum uterine bleeding. More studies are needed, however, to compare the device’s effectiveness with that of other PPH treatments and to consider its use in women with more severe degrees of postpartum hemorrhage as well as its cost-effectiveness. ●
Postpartum hemorrhage (PPH) continues to be a leading cause of maternal morbidity and mortality both worldwide and in the United States.1-3 A PPH is defined as the cumulative blood loss of 1,000 mL or more, or blood loss accompanied by signs or symptoms of hypovolemia, within 24 hours following the birth process (including intrapartum loss).4
Approximately 70% to 80% of hemorrhages are due to abnormal uterine tone.5 Bimanual massage and medical management, the primary treatments for uterine atony, attempt to restore the normal uterine tone that compresses the vessels in the placental implantation site and limits bleeding. For women in whom the primary treatments are not effective, only uterine compression sutures in a laparotomy can achieve physiologic contracture of the uterus. The second-line treatment option, intrauterine tamponade, places pressure over the placental implantation site while distending the uterus.
In October 2020, the US Food and Drug Administration (FDA) granted clearance to a novel device that offers an alternative treatment option. The Jada System (Alydia Health), an intrauterine vacuum-induced hemorrhage control device, is placed in the uterus and uses wall suction to induce physiologic contraction of the uterus to control bleeding.6
In this article, within the context of a case vignette, we discuss the recent study on the Jada System and how this device can be used in the management of PPH.6
CASE Woman with PPH history fears repeat hemorrhage
Ms. B. is a 25-year-old woman (G2P1) who presents for prenatal care at 10 weeks’ gestation. Her medical history is significant for asthma and PPH after her first delivery. When you review her prior delivery records, you learn that she had a protracted labor and delivered a healthy 10 lb 8 oz baby boy after 3 hours of pushing. After delivery, she received postpartum intravenous oxytocin followed by intramuscular uterotonics when her bleeding was heavy during her laceration repair. Her estimated blood loss at delivery was 600 mL. The team was called back to her bedside for the continued bleeding. Uterine atony was diagnosed. Although she received additional uterotonics, the bleeding continued. An intrauterine tamponade balloon was placed, and the bleeding ultimately was controlled. The total estimated blood loss (EBL) was 2.5 L, and the patient then was transfused with 2 U of packed red blood cells.
Currently, Ms. B. is very worried about having another hemorrhage as the bleeding terrified her and her partner, disrupted breastfeeding initiation while the tamponade was in place, and made her anxious about having another baby.
What steps would you take to prepare for a potential PPH in this patient?
Risk factors
While PPH often is unpredictable, many risk factors have been identified (TABLE).7-9 Some risk factors are present during the antepartum period while others arise during labor. In some cases, obstetric clinicians may be able to intervene during prenatal care, such as by giving iron supplementation to address anemia. Other factors, however, are not modifiable, including multiparity, polyhydramnios, and multiple gestations. On presentation to the labor unit, new risk factors may arise, such as magnesium sulfate use, chorioamnionitis, protracted labor, or the need for general anesthesia. In addition, the presence of a fibroid uterus or a uterine inversion can impede effective uterine contractions.5

Various tools are available for assessing these risk factors on admission, during labor, and after delivery, such as the AWHONN postpartum hemorrhage risk assessment table and the CMQCC obstetric hemorrhage toolkit.10,11
Continue to: CASE continued Patient’s history reveals risk factors...
CASE continued Patient’s history reveals risk factors
You review with Ms. B. that she had several risk factors present during labor. She had a large baby and a protracted labor. Knowing her history in this pregnancy will allow the clinical team to be prepared for a potential recurrent hemorrhage and to respond proactively to bleeding.
Consider the management options
The initial treatment for PPH includes bimanual massage, oxytocin, and other uterotonics (methylergonovine, 15-methyl prostaglandin F2α, and misoprostol). While various algorithms are available on the order of treatment, a single agent has not been shown superior to others.12 The antifibrinolytic medication tranexamic acid also was shown to reduce the risk of death from obstetric hemorrhage in the international WOMAN trial.13
While these agents often are used simultaneously to achieve hemostasis, their systemic effects are associated with contraindications. Specifically, F2α prostaglandins cannot be used in patients with asthma or active hepatic, pulmonary, or cardiac disease. Ergot derivatives cannot be used in patients with hypertension, pre-eclampsia, or cardiovascular disease. Given the rising rate of medical comorbidities during pregnancy, such contraindications limit the treatment options for many patients.
In cases in which medical management is not sufficient or is contraindicated for controlling hemorrhage, second-line treatment includes the use of tamponade techniques, such as intrauterine packing or balloons. The tamponade applies pressure directly to the placental implantation site for 12 to 24 hours, which allows time for the uterus to contract and return to normal tone. While this method may seem counterintuitive to achieving uterine tone, studies suggest a success rate between 75% and 86% with balloon tamponade.12
Third-line treatment options are increasingly invasive but should be used to prevent further maternal morbidity and mortality. These include uterine artery embolization and surgery. Uterine artery embolization is an option for a stable patient at a center with available interventional radiology services. If embolization is either not successful or not available, an exploratory laparotomy should be performed. Uterine compression sutures can be placed along with vascular ligation sutures of the uterine arteries (O’Leary sutures) and the hypogastric arteries. If all other methods have failed, a hysterectomy is the definitive treatment for hemorrhage.
CASE continued Patient desires an alternative to tamponade if needed
Following your visit, Ms. B. has an ultrasound scan that shows a dichorionic diamniotic twin pregnancy. She also has a microcytic anemia. After you discuss iron supplementation with the patient, she asks if there are any other options should medical management fail in the event of a recurrent hemorrhage. While intrauterine tamponade balloon did treat her hemorrhage, she was not happy with the length of time it had to remain in place, the discomfort while it was used, and the disruption to her planned recovery. You inform her of a new treatment option available for PPH, a vacuum-induced hemorrhage control device that was recently FDA cleared.
Continue to: New device controls bleeding fast...
New device controls bleeding fast
In 2020, D’Alton and colleagues reported on their multicenter, prospective single-arm treatment study on the effectiveness and safety of an intrauterine vacuum-induced hemorrhage control device.6 This device, the Jada System, uses low-level vacuum to induce uterine contraction to control bleeding from uterine atony. The prospective study, which followed a 2016 feasibility study, enrolled more than 100 women at 12 centers across the United States.6,14 Women were eligible to participate if they delivered at a gestational age of 34 weeks or later and had an EBL between 500 and 1,000 mL after a vaginal delivery or an EBL between 1,000 and 1,500 mL after a cesarean delivery.
Treatment with the vacuum device was successful in 94% (100/106, 95% confidence interval, 88%–98%) of women, and definitive control of abnormal bleeding was achieved in a median of 3 minutes (interquartile range [IQR], 2.0–5.0) after connection to the vacuum device.6
CASE continued Patient has questions
Your patient expresses interest in this device, but she wants to understand how it works. Would it require transfer to another unit or prolonged monitoring?
How the device works
Compared with intrauterine tamponade balloon devices, which apply pressure by distending the uterus, the Jada System applies low-level intrauterine vacuum to facilitate the physiologic forces of uterine contractions to constrict myometrial blood vessels and achieve hemostasis.6 The device is made of medical-grade silicone. Its distal end, which is placed in the uterus, is an elliptical loop. The loop’s inner surface contains 20 vacuum pores protected by a shield that facilitate creation of a vacuum within the uterine cavity. The loop is soft and smooth to limit the chance of tissue damage during insertion, treatment, and removal of the device. The device’s proximal end has a vacuum connector. The vacuum source is hospital-grade wall suction, but a portable vacuum source also can be used (FIGURE 1).

Prior to placing the device, a manual sweep of the uterine cavity is performed. If needed, ultrasonography can be used with the manual sweep to ensure that there is no retained placental tissue or clot. The loop of the Jada System is then inserted in the uterine cavity, and the circular cervical seal, just outside the external cervical os, is filled with sterile water.
Low-level vacuum (80 ± 10 mm Hg) is applied so that pooled blood is evacuated from the uterus as it collapses (FIGURE 2). The volume of any ongoing bleeding is measured in the suction tubing while the uterine response to treatment can be palpated. Once there is no bleeding without any need for further treatment, the device should remain in the uterus for at least 1 hour. The suction is then turned off, and bleeding is monitored for 30 minutes. If bleeding remains controlled, the device can be removed.

CASE continued The question of complications
Ms. B. is concerned about safety and asks about potential complications with the device’s use.
Safety findings
In the prospective study and FDA review, the device was deemed safe. There were 8 possibly related adverse events (endometritis, laceration disruption, and vaginal infection), which all resolved without serious clinical sequelae. Forty women (38%) received a blood transfusion, but only 5 required 4 U or more of red blood cells.6
Continue to: CASE continued What do other physicians think?...
CASE continued What do other physicians think?
Your patient is curious about the time it takes for the device to work and whether other clinicians like using this new device for hemorrhage treatment.
Duration of treatment
The times to achieve uterine collapse and control of hemorrhage are both relatively short. In the prospective study, the initial collapse of the uterus took a median of 1 minute (IQR, 1–2 min) from the time of vacuum connection.6 Bleeding was controlled in less than 5 minutes in 82% of women, with an overall median time of 3 minutes (IQR, 2–5 min). The median duration of vacuum treatment was 144.0 minutes (IQR, 85.8–295.8 min), which includes the required minimum of 60 minutes for vacuum treatment time and 30 minutes of observation without the vacuum connected but with the device still in place.6
When polled, the majority of clinicians—98%—reported that the intrauterine vacuum-induced hemorrhage control device was easy to use, and 97% would recommend its use for future patients.6
Further, recognizing the device’s potential, the Cleveland Clinic cited it as one of the top 10 health care innovations for 2021 for offering a low-tech and minimally invasive tool for obstetric clinicians.15
CASE continued Final questions
Ms. B. thanks you for the information and asks, should she know anything else about the device?
Vacuum device vs other treatments
The study by D’Alton and colleagues was a single-arm treatment trial that did not directly compare the effectiveness of the device with that of other PPH treatment options, such as balloon tamponade.6 At this point, we know that clinicians can safely and quickly use the device to treat uterine atony, but we do not know if it is superior to other treatments for PPH.
Key takeaways
Postpartum hemorrhage is a leading cause of maternal morbidity and mortality. When first-line uterotonics fail, obstetric clinicians previously had only balloon tamponade or invasive procedures to treat patients. The novel intrauterine vacuum-induced hemorrhage control device takes a new approach that simulates the physiologic process of uterine contractions. The device can rapidly and effectively control abnormal postpartum uterine bleeding. More studies are needed, however, to compare the device’s effectiveness with that of other PPH treatments and to consider its use in women with more severe degrees of postpartum hemorrhage as well as its cost-effectiveness. ●
- Say L, Chou D, Gemmill A, et al. Global causes of maternal death: a WHO systematic analysis. Lancet Glob Health. 2014;2:e323-e333.
- Callaghan WM, Creanga AA, Kuklina EV. Severe maternal morbidity among delivery and postpartum hospitalizations in the United States. Obstet Gynecol. 2012;120:1029-1036.
- Centers for Disease Control and Prevention. Severe maternal morbidity in the United States. http://www .cdc.gov/reproductivehealth/maternalinfanthealth /severematernalmorbidity.html. Accessed November 6, 2020.
- Menard MK, Main EK, Currigan SM. Executive summary of the reVITALize initiative: standardizing obstetric data definitions. Obstet Gynecol. 2014;124:150-153.
- American College of Obstetricians and Gynecologists Committee on Practice Bulletins–Obstetrics. Practice bulletin no. 183: postpartum hemorrhage. Obstet Gynecol. 2017;130:e168-e186.
- D’Alton ME, Rood KM, Smid MC, et al. Intrauterine vacuum-induced hemorrhage-control device for rapid treatment of postpartum hemorrhage. Obstet Gynecol. 2020;136:882-891.
- Mavrides E, Allard S, Chandraharan E, et al; on behalf of the Royal College of Obstetricians and Gynaecologists. Prevention and management of postpartum hemorrhage. BJOG. 2016;124:e106-e149.
- Lyndon A, Lagrew D, Shields L, et al. Improving health care response to obstetric hemorrhage, version 2.0 (California Maternal Quality Care Collaborative Toolkit to Transform Maternity Care). Developed under contract #11-10006 with the California Department of Public Health; Maternal, Child and Adolescent Health Division; Published by the California Maternal Quality Care Collaborative, March 17, 2015.
- Main EK, Goffman D, Scavone BM, et al; National Partnership for Maternal Safety; Council on Patient Safety in Women’s Health Care. National Partnership for Maternal Safety: consensus bundle on obstetric hemorrhage. Obstet Gynecol. 2015;126:155-162.
- AWHONN Postpartum Hemorrhage Project. Postpartum hemorrhage (PPH) risk assessment table 1.0. https:// mygnosis.com/Content/Chunks/3504/assets/pdfs/PPH _Risk_Assessment_Table-7-17-15.pdf. Accessed November 15, 2020.
- Bingham D, Melsop K, Main E. CMQCC obstetric hemorrhage toolkit: hospital level implementation guide. 2010. California Maternal Quality Care Collaborative (CMQCC). Palo Alto, CA: Stanford University. https://www.cmqcc.org/resource/1489 /download. Accessed November 15, 2020.
- Likis FE, Sathe NA, Morgans AK, et al. Management of postpartum hemorrhage. Comparative effectiveness review no. 151. AHRQ publication no. 15-EHC013-EF. Rockville, MD: Agency for Healthcare Research and Quality; 2015.
- WOMAN Trial Collaborators. Effect of early tranexamic acid administration on mortality, hysterectomy, and other morbidities in women with post-partum haemorrhage (WOMAN): an international, randomised, double-blind, placebo-controlled trial. Lancet. 2017;389:2105–2116.
- Purwosunu Y, Sarkoen W, Arulkumaran S, et al. Control of postpartum hemorrhage using vacuum-induced uterine tamponade. Obstet Gynecol. 2016;128:33-36.
- Cleveland Clinic Innovations. Cleveland Clinic unveils top 10 medical innovations for 2021. October 6, 2020. https:// innovations.clevelandclinic.org/Programs/Top-10-Medical -Innovations/Top-10-for-2021. Accessed November 6, 2020.
- Say L, Chou D, Gemmill A, et al. Global causes of maternal death: a WHO systematic analysis. Lancet Glob Health. 2014;2:e323-e333.
- Callaghan WM, Creanga AA, Kuklina EV. Severe maternal morbidity among delivery and postpartum hospitalizations in the United States. Obstet Gynecol. 2012;120:1029-1036.
- Centers for Disease Control and Prevention. Severe maternal morbidity in the United States. http://www .cdc.gov/reproductivehealth/maternalinfanthealth /severematernalmorbidity.html. Accessed November 6, 2020.
- Menard MK, Main EK, Currigan SM. Executive summary of the reVITALize initiative: standardizing obstetric data definitions. Obstet Gynecol. 2014;124:150-153.
- American College of Obstetricians and Gynecologists Committee on Practice Bulletins–Obstetrics. Practice bulletin no. 183: postpartum hemorrhage. Obstet Gynecol. 2017;130:e168-e186.
- D’Alton ME, Rood KM, Smid MC, et al. Intrauterine vacuum-induced hemorrhage-control device for rapid treatment of postpartum hemorrhage. Obstet Gynecol. 2020;136:882-891.
- Mavrides E, Allard S, Chandraharan E, et al; on behalf of the Royal College of Obstetricians and Gynaecologists. Prevention and management of postpartum hemorrhage. BJOG. 2016;124:e106-e149.
- Lyndon A, Lagrew D, Shields L, et al. Improving health care response to obstetric hemorrhage, version 2.0 (California Maternal Quality Care Collaborative Toolkit to Transform Maternity Care). Developed under contract #11-10006 with the California Department of Public Health; Maternal, Child and Adolescent Health Division; Published by the California Maternal Quality Care Collaborative, March 17, 2015.
- Main EK, Goffman D, Scavone BM, et al; National Partnership for Maternal Safety; Council on Patient Safety in Women’s Health Care. National Partnership for Maternal Safety: consensus bundle on obstetric hemorrhage. Obstet Gynecol. 2015;126:155-162.
- AWHONN Postpartum Hemorrhage Project. Postpartum hemorrhage (PPH) risk assessment table 1.0. https:// mygnosis.com/Content/Chunks/3504/assets/pdfs/PPH _Risk_Assessment_Table-7-17-15.pdf. Accessed November 15, 2020.
- Bingham D, Melsop K, Main E. CMQCC obstetric hemorrhage toolkit: hospital level implementation guide. 2010. California Maternal Quality Care Collaborative (CMQCC). Palo Alto, CA: Stanford University. https://www.cmqcc.org/resource/1489 /download. Accessed November 15, 2020.
- Likis FE, Sathe NA, Morgans AK, et al. Management of postpartum hemorrhage. Comparative effectiveness review no. 151. AHRQ publication no. 15-EHC013-EF. Rockville, MD: Agency for Healthcare Research and Quality; 2015.
- WOMAN Trial Collaborators. Effect of early tranexamic acid administration on mortality, hysterectomy, and other morbidities in women with post-partum haemorrhage (WOMAN): an international, randomised, double-blind, placebo-controlled trial. Lancet. 2017;389:2105–2116.
- Purwosunu Y, Sarkoen W, Arulkumaran S, et al. Control of postpartum hemorrhage using vacuum-induced uterine tamponade. Obstet Gynecol. 2016;128:33-36.
- Cleveland Clinic Innovations. Cleveland Clinic unveils top 10 medical innovations for 2021. October 6, 2020. https:// innovations.clevelandclinic.org/Programs/Top-10-Medical -Innovations/Top-10-for-2021. Accessed November 6, 2020.
Daily calorie intake requirements during pregnancy: Does one size fit all?
Most J, St Amant M, Hsia DS, et al. Evidence-based recommendations for energy intake in pregnant women with obesity. J Clin Invest. 2019;129:4682-4690.
EXPERT COMMENTARY
In 2009, the Institute of Medicine, now known as the National Academy of Medicine, updated its gestational weight gain guideline. This guideline’s major difference, compared with the 1990 guideline, is a specific weight gain range for women with obesity: 5 to 9 kg, or 11 to 20 lb.1 This weight gain range was chosen in part because it allows for a minimum weight gain that supports the growth and development of tissues (fetus, placenta, breast, uterus) and fluids (blood volume, intracellular and extracellular fluid), also known as the “fat-free” mass.
Many studies have since shown not only associations between lower-than-guideline-recommended weight gain and improved pregnancy outcomes (for example, reductions in preeclampsia and cesarean deliveries), but also increases in low birth weight for infants of women with obesity.2,3 Although the weight gain guideline differs based on a woman’s prepregnancy body mass index, the energy requirements, or how many additional calories a woman should consume daily, are the same for all, regardless of weight prior to pregnancy: an increase by 340 to 452 kcal/day in the second and third trimesters.1
Recently, Most and colleagues challenged this recommendation for energy requirements with results from their prospective observational study of 54 women with obesity during pregnancy.4 They aimed to evaluate energy intake with the energy intake-balance method (doubly labeled water and whole-room indirect calorimetry and body composition) according to tests done at 13 to 16 weeks’ gestation and 35 to 37 weeks’ gestation and according to the current National Academy of Medicine gestational weight gain guideline (inadequate, recommended, or excessive weight gain groups).4
Details of the study
Women who participated in this study were recruited from the Pennington Biomedical Research Center in Louisiana and were mostly multiparas (57%); about half had a college degree or higher (52%) and 41% were African American. The investigators found that gestational weight gain in their participants was similar to that found in other large epidemiologic studies in that 67% of women had excessive gestational weight gain.5
Findings. For women who gained the recommended amount of weight (n = 8), mean (SD) daily energy intake was 2,698 (99) kcal/day and energy expenditure was 2,824 (105) kcal/day. Therefore, to meet the recommended amount of weight gain, these women had a negative energy balance (-125 [52] kcal/day). Women with inadequate weight gain
(n = 10) also had a negative energy balance (-262 [32] kcal/day), but the difference was not significantly different compared with that in the recommended gestational weight gain group (P = .08). By contrast, women with excessive gestational weight gain (n = 36) had a mean (SD) positive energy balance of 186 (29) kcal/day.
Fat-free mass and fat mass weight gains. The body weight gains of the fat-free and fat mass compartments also were compared with linear mixed effect models among the 3 weight gain groups. There were no differences in the amount of fat-free mass gained among the 3 weight gain groups (P>.05), but women with excessive gestational weight gain had significantly higher increases in fat mass compared with the other 2 weight gain groups (P<.001).
Pregnancy outcomes. Although there were no differences in cesarean deliveries or birth weight among the 3 weight gain groups, the study was not powered to detect these differences.
Continue to: Study strengths and limitations...
Study strengths and limitations
It is important to note that this study by Most and colleagues was not a health behavior intervention for gestational weight gain. Women who participated in the study did not receive specific directions or advice on diet or physical activity. Furthermore, the study used the current gestational weight gain guideline as a reference to determine energy intake. As such, findings from this study alone cannot be used to adapt the current gestational weight gain guideline for women with obesity.
The study methods were rigorous in terms of the energy intake measurements, but a larger and more diverse sample size is needed to confirm the study findings.
Most and colleagues’ data suggest that maintaining energy balance can support obligatory growth and development of women and their fetuses during pregnancy (fat-free mass). In doing so, women with obesity meet the current gestational weight gain guideline. It is hoped that this important research will be used in future studies, with larger sample sizes, to evaluate energy requirements during pregnancy, especially in women with different classes of obesity. Ultimately, these new recommendations for energy requirements should be combined with studies of health behavior interventions for gestational weight gain.
The study by Most and colleagues supports the concept that energy requirements need to be individualized for women to meet the recommended amount of gestational weight gain. If women meet their gestational weight gain goals, they have the potential to improve their health and the health of their offspring.
MICHELLE A. KOMINIAREK, MD, MS
- Institute of Medicine and National Research Council Committee to Reexamine IOM Pregnacy Weight Guidelines. Rasmussen KM, Yaktine AL, eds. Weight Gain During Pregnancy: Reexamining the Guidelines. Washington, DC: National Academies Press; 2009.
- Kapadia MZ, Park CK, Beyene J, et al. Weight loss instead of weight gain within the guidelines in obese women during pregnancy: a systematic review and metaanalyses of maternal and infant outcomes. PLoS One. 2015;10:e0132650.
- Kapadia MZ, Park CK, Beyene J, et al. Can we safely recommend gestational weight gain below the 2009 guidelines in obese women? A systematic review and metaanalysis. Obes Rev. 2015;16:189-206.
- Most J, St Amant M, Hsia DS, et al. Evidence-based recommendations for energy intake in pregnant women with obesity. J Clin Invest. 2019;129:4682-4690.
- Deputy NP, Sharma AJ, Kim SY, et al. Prevalence and characteristics associated with gestational weight gain adequacy. Obstet Gynecol. 2015;125:773-781.
Most J, St Amant M, Hsia DS, et al. Evidence-based recommendations for energy intake in pregnant women with obesity. J Clin Invest. 2019;129:4682-4690.
EXPERT COMMENTARY
In 2009, the Institute of Medicine, now known as the National Academy of Medicine, updated its gestational weight gain guideline. This guideline’s major difference, compared with the 1990 guideline, is a specific weight gain range for women with obesity: 5 to 9 kg, or 11 to 20 lb.1 This weight gain range was chosen in part because it allows for a minimum weight gain that supports the growth and development of tissues (fetus, placenta, breast, uterus) and fluids (blood volume, intracellular and extracellular fluid), also known as the “fat-free” mass.
Many studies have since shown not only associations between lower-than-guideline-recommended weight gain and improved pregnancy outcomes (for example, reductions in preeclampsia and cesarean deliveries), but also increases in low birth weight for infants of women with obesity.2,3 Although the weight gain guideline differs based on a woman’s prepregnancy body mass index, the energy requirements, or how many additional calories a woman should consume daily, are the same for all, regardless of weight prior to pregnancy: an increase by 340 to 452 kcal/day in the second and third trimesters.1
Recently, Most and colleagues challenged this recommendation for energy requirements with results from their prospective observational study of 54 women with obesity during pregnancy.4 They aimed to evaluate energy intake with the energy intake-balance method (doubly labeled water and whole-room indirect calorimetry and body composition) according to tests done at 13 to 16 weeks’ gestation and 35 to 37 weeks’ gestation and according to the current National Academy of Medicine gestational weight gain guideline (inadequate, recommended, or excessive weight gain groups).4
Details of the study
Women who participated in this study were recruited from the Pennington Biomedical Research Center in Louisiana and were mostly multiparas (57%); about half had a college degree or higher (52%) and 41% were African American. The investigators found that gestational weight gain in their participants was similar to that found in other large epidemiologic studies in that 67% of women had excessive gestational weight gain.5
Findings. For women who gained the recommended amount of weight (n = 8), mean (SD) daily energy intake was 2,698 (99) kcal/day and energy expenditure was 2,824 (105) kcal/day. Therefore, to meet the recommended amount of weight gain, these women had a negative energy balance (-125 [52] kcal/day). Women with inadequate weight gain
(n = 10) also had a negative energy balance (-262 [32] kcal/day), but the difference was not significantly different compared with that in the recommended gestational weight gain group (P = .08). By contrast, women with excessive gestational weight gain (n = 36) had a mean (SD) positive energy balance of 186 (29) kcal/day.
Fat-free mass and fat mass weight gains. The body weight gains of the fat-free and fat mass compartments also were compared with linear mixed effect models among the 3 weight gain groups. There were no differences in the amount of fat-free mass gained among the 3 weight gain groups (P>.05), but women with excessive gestational weight gain had significantly higher increases in fat mass compared with the other 2 weight gain groups (P<.001).
Pregnancy outcomes. Although there were no differences in cesarean deliveries or birth weight among the 3 weight gain groups, the study was not powered to detect these differences.
Continue to: Study strengths and limitations...
Study strengths and limitations
It is important to note that this study by Most and colleagues was not a health behavior intervention for gestational weight gain. Women who participated in the study did not receive specific directions or advice on diet or physical activity. Furthermore, the study used the current gestational weight gain guideline as a reference to determine energy intake. As such, findings from this study alone cannot be used to adapt the current gestational weight gain guideline for women with obesity.
The study methods were rigorous in terms of the energy intake measurements, but a larger and more diverse sample size is needed to confirm the study findings.
Most and colleagues’ data suggest that maintaining energy balance can support obligatory growth and development of women and their fetuses during pregnancy (fat-free mass). In doing so, women with obesity meet the current gestational weight gain guideline. It is hoped that this important research will be used in future studies, with larger sample sizes, to evaluate energy requirements during pregnancy, especially in women with different classes of obesity. Ultimately, these new recommendations for energy requirements should be combined with studies of health behavior interventions for gestational weight gain.
The study by Most and colleagues supports the concept that energy requirements need to be individualized for women to meet the recommended amount of gestational weight gain. If women meet their gestational weight gain goals, they have the potential to improve their health and the health of their offspring.
MICHELLE A. KOMINIAREK, MD, MS
Most J, St Amant M, Hsia DS, et al. Evidence-based recommendations for energy intake in pregnant women with obesity. J Clin Invest. 2019;129:4682-4690.
EXPERT COMMENTARY
In 2009, the Institute of Medicine, now known as the National Academy of Medicine, updated its gestational weight gain guideline. This guideline’s major difference, compared with the 1990 guideline, is a specific weight gain range for women with obesity: 5 to 9 kg, or 11 to 20 lb.1 This weight gain range was chosen in part because it allows for a minimum weight gain that supports the growth and development of tissues (fetus, placenta, breast, uterus) and fluids (blood volume, intracellular and extracellular fluid), also known as the “fat-free” mass.
Many studies have since shown not only associations between lower-than-guideline-recommended weight gain and improved pregnancy outcomes (for example, reductions in preeclampsia and cesarean deliveries), but also increases in low birth weight for infants of women with obesity.2,3 Although the weight gain guideline differs based on a woman’s prepregnancy body mass index, the energy requirements, or how many additional calories a woman should consume daily, are the same for all, regardless of weight prior to pregnancy: an increase by 340 to 452 kcal/day in the second and third trimesters.1
Recently, Most and colleagues challenged this recommendation for energy requirements with results from their prospective observational study of 54 women with obesity during pregnancy.4 They aimed to evaluate energy intake with the energy intake-balance method (doubly labeled water and whole-room indirect calorimetry and body composition) according to tests done at 13 to 16 weeks’ gestation and 35 to 37 weeks’ gestation and according to the current National Academy of Medicine gestational weight gain guideline (inadequate, recommended, or excessive weight gain groups).4
Details of the study
Women who participated in this study were recruited from the Pennington Biomedical Research Center in Louisiana and were mostly multiparas (57%); about half had a college degree or higher (52%) and 41% were African American. The investigators found that gestational weight gain in their participants was similar to that found in other large epidemiologic studies in that 67% of women had excessive gestational weight gain.5
Findings. For women who gained the recommended amount of weight (n = 8), mean (SD) daily energy intake was 2,698 (99) kcal/day and energy expenditure was 2,824 (105) kcal/day. Therefore, to meet the recommended amount of weight gain, these women had a negative energy balance (-125 [52] kcal/day). Women with inadequate weight gain
(n = 10) also had a negative energy balance (-262 [32] kcal/day), but the difference was not significantly different compared with that in the recommended gestational weight gain group (P = .08). By contrast, women with excessive gestational weight gain (n = 36) had a mean (SD) positive energy balance of 186 (29) kcal/day.
Fat-free mass and fat mass weight gains. The body weight gains of the fat-free and fat mass compartments also were compared with linear mixed effect models among the 3 weight gain groups. There were no differences in the amount of fat-free mass gained among the 3 weight gain groups (P>.05), but women with excessive gestational weight gain had significantly higher increases in fat mass compared with the other 2 weight gain groups (P<.001).
Pregnancy outcomes. Although there were no differences in cesarean deliveries or birth weight among the 3 weight gain groups, the study was not powered to detect these differences.
Continue to: Study strengths and limitations...
Study strengths and limitations
It is important to note that this study by Most and colleagues was not a health behavior intervention for gestational weight gain. Women who participated in the study did not receive specific directions or advice on diet or physical activity. Furthermore, the study used the current gestational weight gain guideline as a reference to determine energy intake. As such, findings from this study alone cannot be used to adapt the current gestational weight gain guideline for women with obesity.
The study methods were rigorous in terms of the energy intake measurements, but a larger and more diverse sample size is needed to confirm the study findings.
Most and colleagues’ data suggest that maintaining energy balance can support obligatory growth and development of women and their fetuses during pregnancy (fat-free mass). In doing so, women with obesity meet the current gestational weight gain guideline. It is hoped that this important research will be used in future studies, with larger sample sizes, to evaluate energy requirements during pregnancy, especially in women with different classes of obesity. Ultimately, these new recommendations for energy requirements should be combined with studies of health behavior interventions for gestational weight gain.
The study by Most and colleagues supports the concept that energy requirements need to be individualized for women to meet the recommended amount of gestational weight gain. If women meet their gestational weight gain goals, they have the potential to improve their health and the health of their offspring.
MICHELLE A. KOMINIAREK, MD, MS
- Institute of Medicine and National Research Council Committee to Reexamine IOM Pregnacy Weight Guidelines. Rasmussen KM, Yaktine AL, eds. Weight Gain During Pregnancy: Reexamining the Guidelines. Washington, DC: National Academies Press; 2009.
- Kapadia MZ, Park CK, Beyene J, et al. Weight loss instead of weight gain within the guidelines in obese women during pregnancy: a systematic review and metaanalyses of maternal and infant outcomes. PLoS One. 2015;10:e0132650.
- Kapadia MZ, Park CK, Beyene J, et al. Can we safely recommend gestational weight gain below the 2009 guidelines in obese women? A systematic review and metaanalysis. Obes Rev. 2015;16:189-206.
- Most J, St Amant M, Hsia DS, et al. Evidence-based recommendations for energy intake in pregnant women with obesity. J Clin Invest. 2019;129:4682-4690.
- Deputy NP, Sharma AJ, Kim SY, et al. Prevalence and characteristics associated with gestational weight gain adequacy. Obstet Gynecol. 2015;125:773-781.
- Institute of Medicine and National Research Council Committee to Reexamine IOM Pregnacy Weight Guidelines. Rasmussen KM, Yaktine AL, eds. Weight Gain During Pregnancy: Reexamining the Guidelines. Washington, DC: National Academies Press; 2009.
- Kapadia MZ, Park CK, Beyene J, et al. Weight loss instead of weight gain within the guidelines in obese women during pregnancy: a systematic review and metaanalyses of maternal and infant outcomes. PLoS One. 2015;10:e0132650.
- Kapadia MZ, Park CK, Beyene J, et al. Can we safely recommend gestational weight gain below the 2009 guidelines in obese women? A systematic review and metaanalysis. Obes Rev. 2015;16:189-206.
- Most J, St Amant M, Hsia DS, et al. Evidence-based recommendations for energy intake in pregnant women with obesity. J Clin Invest. 2019;129:4682-4690.
- Deputy NP, Sharma AJ, Kim SY, et al. Prevalence and characteristics associated with gestational weight gain adequacy. Obstet Gynecol. 2015;125:773-781.