User login
What’s Eating You? Ixodes Tick and Related Diseases, Part 3: Coinfection and Tick-Bite Prevention
Tick-borne diseases are increasing in prevalence, likely due to climate change in combination with human movement into tick habitats.1-3 The Ixodes genus of hard ticks is a common vector for the transmission of pathogenic viruses, bacteria, parasites, and toxins. Among these, Lyme disease, which is caused by Borrelia burgdorferi, is the most prevalent, followed by babesiosis and human granulocytic anaplasmosis (HGA), respectively.4 In Europe, tick-borne encephalitis is commonly encountered. More recently identified diseases transmitted by Ixodes ticks include Powassan virus and Borrelia miyamotoi infection; however, these diseases are less frequently encountered than other tick-borne diseases.5,6
As tick-borne diseases become more prevalent, the likelihood of coinfection with more than one Ixodes-transmitted pathogen is increasing.7 Therefore, it is important for physicians who practice in endemic areas to be aware of the possibility of coinfection, which can alter clinical presentation, disease severity, and treatment response in tick-borne diseases. Additionally, public education on tick-bite prevention and prompt tick removal is necessary to combat the rising prevalence of these diseases.
Coinfection
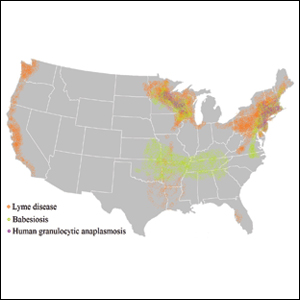
Risk of coinfection with more than one tick-borne disease is contingent on the geographic distribution of the tick species as well as the particular pathogen’s prevalence within reservoir hosts in a given area (Figure). Most coinfections occur with B. burgdorferi and an additional pathogen, usually Anaplasma phagocytophilum (which causes human granulocytic anaplasmosis [HGA]) or Babesia microti (which causes babesiosis). In Europe, coinfection with tick-borne encephalitis virus may occur. There is limited evidence of human coinfection with B miyamotoi or Powassan virus, as isolated infection with either of these pathogens is rare.
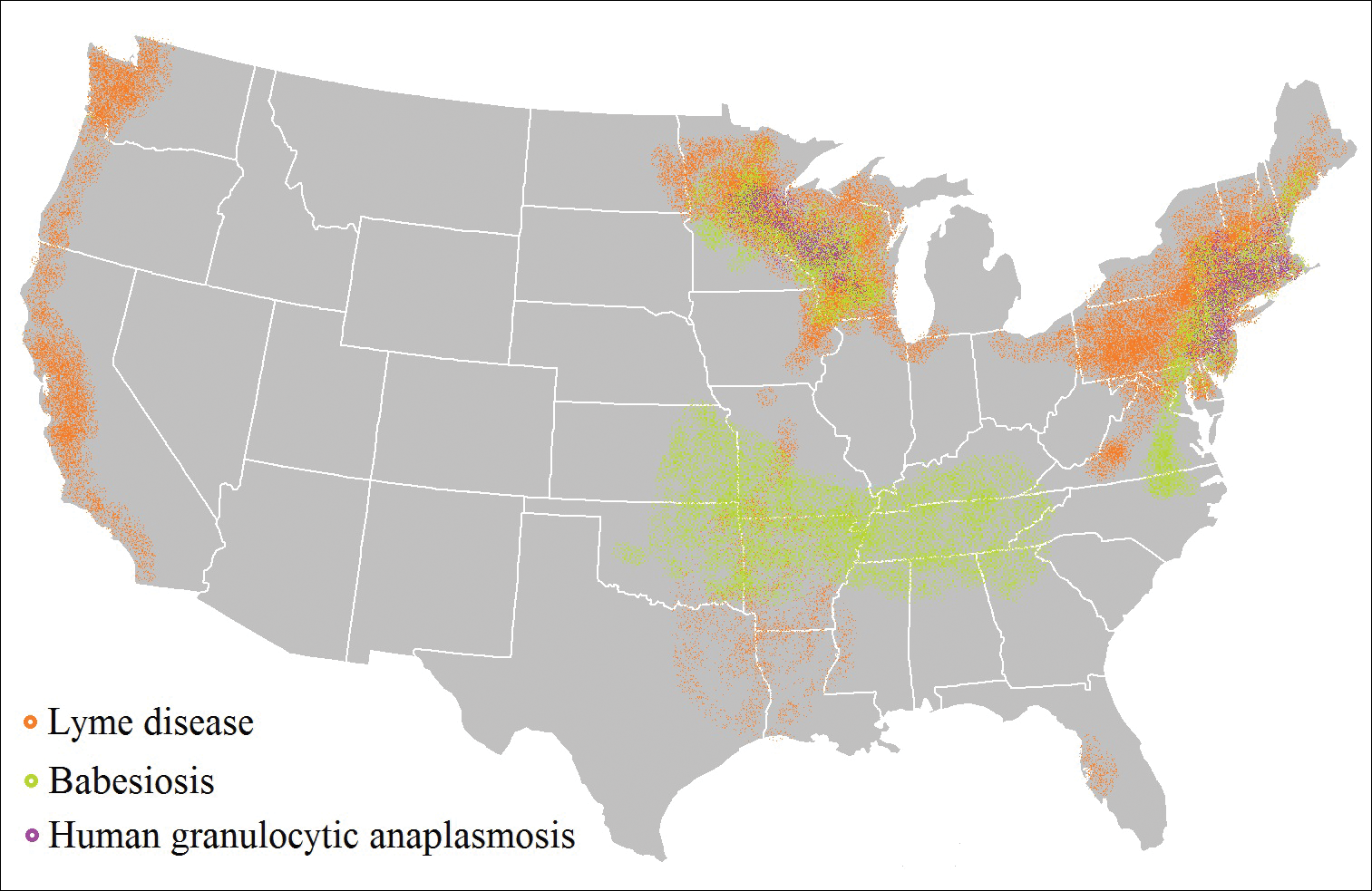
In patients with Lyme disease, as many as 35% may have concurrent babesiosis, and as many as 12% may have concurrent HGA in endemic areas (eg, northeast and northern central United States).7-9 Concurrent HGA and babesiosis in the absence of Lyme disease also has been documented.7-9 Coinfection generally increases the diversity of presenting symptoms, often obscuring the primary diagnosis. In addition, these patients may have more severe and prolonged illness.8,10,11
In endemic areas, coinfection with B burgdorferi and an additional pathogen should be suspected if a patient presents with typical symptoms of early Lyme disease, especially erythema migrans, along with (1) combination of fever, chills, and headache; (2) prolonged viral-like illness, particularly 48 hours after appropriate antibiotic treatment; and (3) unexplained blood dyscrasia.7,11,12 When a patient presents with erythema migrans, it is unnecessary to test for HGA, as treatment of Lyme disease with doxycycline also is adequate for treating HGA; however, if systemic symptoms persist despite treatment, testing for babesiosis and other tick-borne illnesses should be considered, as babesiosis requires treatment with atovaquone plus azithromycin or clindamycin plus quinine.13
A complete blood count and peripheral blood smear can aid in the diagnosis of coinfection. The complete blood count may reveal leukopenia, anemia, or thrombocytopenia associated with HGA or babesiosis. The peripheral blood smear can reveal inclusions of intra-erythrocytic ring forms and tetrads (the “Maltese cross” appearance) in babesiosis and intragranulocytic morulae in HGA.12 The most sensitive diagnostic tests for tick-borne diseases are organism-specific IgM and IgG serology for Lyme disease, babesiosis, and HGA and polymerase chain reaction for babesiosis and HGA.7
Prevention Strategies
The most effective means of controlling tick-borne disease is avoiding tick bites altogether. One method is to avoid spending time in high-risk areas that may be infested with ticks, particularly low-lying brush, where ticks are likely to hide.14 For individuals traveling in environments with a high risk of tick exposure, behavioral methods of avoidance are indicated, including wearing long pants and a shirt with long sleeves, tucking the shirt into the pants, and wearing closed-toe shoes. Wearing light-colored clothing may aid in tick identification and prompt removal prior to attachment. Permethrin-impregnated clothing has been proven to decrease the likelihood of tick bites in adults working outdoors.15-17
Topical repellents also play a role in the prevention of tick-borne diseases. The most effective and safe synthetic repellents are N,N-diethyl-meta-toluamide (DEET); picaridin; p-menthane-3,8-diol; and insect repellent 3535 (IR3535)(ethyl butylacetylaminopropionate).16-19 Plant-based repellents also are available, but their efficacy is strongly influenced by the surrounding environment (eg, temperature, humidity, organic matter).20-22 Individuals also may be exposed to ticks following contact with domesticated animals and pets.23,24 Tick prevention in pets with the use of ectoparasiticides should be directed by a qualified veterinarian.25
Tick Removal
Following a bite, the tick should be removed promptly to avoid transmission of pathogens. Numerous commercial and in-home methods of tick removal are available, but not all are equally effective. Detachment techniques include removal with a card or commercially available radiofrequency device, lassoing, or freezing.26,27 However, the most effective method is simple removal with tweezers. The tick should be grasped close to the skin surface and pulled upward with an even pressure. Commercially available tick-removal devices have not been shown to produce better outcomes than removal of the tick with tweezers.28
Conclusion
When patients do not respond to therapy for presumed tick-borne infection, the diagnosis should be reconsidered. One important consideration is coinfection with a second organism. Prompt identification and removal of ticks can prevent disease transmission.
- McMichael C, Barnett J, McMichael AJ. An ill wind? climate change, migration, and health. Environ Health Perspect. 2012;120:646-654.
- Ostfeld RS, Brunner JL. Climate change and Ixodes tick-borne diseases of humans. Philos Trans R Soc Lond B Biol Sci. 2015;370:20140051.
- Ogden NH, Bigras-Poulin M, O’Callaghan CJ, et al. Vector seasonality, host infection dynamics and fitness of pathogens transmitted by the tick Ixodes scapularis. Parasitology. 2007;134(pt 2):209-227.
- Tickborne diseases of the United States. Centers for Disease Control and Prevention website. http://www.cdc.gov/ticks/diseases/index.html. Updated July 25, 2017. Accessed April 10, 2018.
- Hinten SR, Beckett GA, Gensheimer KF, et al. Increased recognition of Powassan encephalitis in the United States, 1999-2005. Vector Borne Zoonotic Dis. 2008;8:733-740.
- Platonov AE, Karan LS, Kolyasnikova NM, et al. Humans infected with relapsing fever spirochete Borrelia miyamotoi, Russia. Emerg Infect Dis. 2011;17:1816-1823.
- Krause PJ, McKay K, Thompson CA, et al; Deer-Associated Infection Study Group. Disease-specific diagnosis of coinfecting tickborne zoonoses: babesiosis, human granulocytic ehrlichiosis, and Lyme disease. Clin Infect Dis. 2002;34:1184-1191.
- Krause PJ, Telford SR 3rd, Spielman A, et al. Concurrent Lyme disease and babesiosis. evidence for increased severity and duration of illness. JAMA. 1996;275:1657-1660.
- Belongia EA, Reed KD, Mitchell PD, et al. Clinical and epidemiological features of early Lyme disease and human granulocytic ehrlichiosis in Wisconsin. Clin Infect Dis. 1999;29:1472-1477.
- Sweeny CJ, Ghassemi M, Agger WA, et al. Coinfection with Babesia microti and Borrelia burgdorferi in a western Wisconsin resident. Mayo Clin Proc.1998;73:338-341.
- Nadelman RB, Horowitz HW, Hsieh TC, et al. Simultaneous human granulocytic ehrlichiosis and Lyme borreliosis. N Engl J Med. 1997;337:27-30.
- Wormser GP, Dattwyler RJ, Shapiro ED, et al. The clinical assessment, treatment, and prevention of Lyme disease, human granulocytic anaplasmosis, and babesiosis: clinical practice guidelines by the Infectious Diseases Society of America. Clin Infect Dis. 2006;43:1089-1134.
- Swanson SJ, Neitzel D, Reed DK, et al. Coinfections acquired from Ixodes ticks. Clin Microbiol Rev. 2006;19:708-727.
- Hayes EB, Piesman J. How can we prevent Lyme disease? N Engl J Med. 2003;348:2424-2430.
- Vaughn MF, Funkhouser SW, Lin FC, et al. Long-lasting permethrin impregnated uniforms: a randomized-controlled trial for tick bite prevention. Am J Prev Med. 2014;46:473-480.
- Miller NJ, Rainone EE, Dyer MC, et al. Tick bite protection with permethrin-treated summer-weight clothing. J Med Entomol. 2011;48:327-333.
- Richards SL, Balanay JAG, Harris JW. Effectiveness of permethrin-treated clothing to prevent tick exposure in foresters in the central Appalachian region of the USA. Int J Environ Health Res. 2015;25:453-462.
- Pages F, Dautel H, Duvallet G, et al. Tick repellents for human use: prevention of tick bites and tick-borne diseases. Vector Borne Zoonotic Dis. 2014;14:85-93.
- Büchel K, Bendin J, Gharbi A, et al. Repellent efficacy of DEET, icaridin, and EBAAP against Ixodes ricinus and Ixodes scapularis nymphs (Acari, Ixodidae). Ticks Tick Borne Dis. 2015;6:494-498.
- Schwantes U, Dautel H, Jung G. Prevention of infectious tick-borne diseases in humans: comparative studies of the repellency of different dodecanoic acid-formulations against Ixodes ricinus ticks (Acari: Ixodidae). Parasit Vectors. 2008;8:1-8.
- Bissinger BW, Apperson CS, Sonenshine DE, et al. Efficacy of the new repellent BioUD against three species of ixodid ticks. Exp Appl Acarol. 2009;48:239-250.
- Feaster JE, Scialdone MA, Todd RG, et al. Dihydronepetalactones deter feeding activity by mosquitoes, stable flies, and deer ticks. J Med Entomol. 2009;46:832-840.
- Jennett AL, Smith FD, Wall R. Tick infestation risk for dogs in a peri-urban park. Parasit Vectors. 2013;6:358.
- Rand PW, Smith RP Jr, Lacombe EH. Canine seroprevalence and the distribution of Ixodes dammini in an area of emerging Lyme disease. Am J Public Health. 1991;81:1331-1334.
- Baneth G, Bourdeau P, Bourdoiseau G, et al; CVBD World Forum. Vector-borne diseases—constant challenge for practicing veterinarians: recommendations from the CVBD World Forum. Parasit Vectors. 2012;5:55.
- Akin Belli A, Dervis E, Kar S, et al. Revisiting detachment techniques in human-biting ticks. J Am Acad Dermatol. 2016;75:393-397.
- Ashique KT, Kaliyadan F. Radiofrequency device for tick removal. J Am Acad Dermatol. 2015;72:155-156.
- Due C, Fox W, Medlock JM, et al. Tick bite prevention and tick removal. BMJ. 2013;347:f7123.
Tick-borne diseases are increasing in prevalence, likely due to climate change in combination with human movement into tick habitats.1-3 The Ixodes genus of hard ticks is a common vector for the transmission of pathogenic viruses, bacteria, parasites, and toxins. Among these, Lyme disease, which is caused by Borrelia burgdorferi, is the most prevalent, followed by babesiosis and human granulocytic anaplasmosis (HGA), respectively.4 In Europe, tick-borne encephalitis is commonly encountered. More recently identified diseases transmitted by Ixodes ticks include Powassan virus and Borrelia miyamotoi infection; however, these diseases are less frequently encountered than other tick-borne diseases.5,6
As tick-borne diseases become more prevalent, the likelihood of coinfection with more than one Ixodes-transmitted pathogen is increasing.7 Therefore, it is important for physicians who practice in endemic areas to be aware of the possibility of coinfection, which can alter clinical presentation, disease severity, and treatment response in tick-borne diseases. Additionally, public education on tick-bite prevention and prompt tick removal is necessary to combat the rising prevalence of these diseases.
Coinfection
Risk of coinfection with more than one tick-borne disease is contingent on the geographic distribution of the tick species as well as the particular pathogen’s prevalence within reservoir hosts in a given area (Figure). Most coinfections occur with B. burgdorferi and an additional pathogen, usually Anaplasma phagocytophilum (which causes human granulocytic anaplasmosis [HGA]) or Babesia microti (which causes babesiosis). In Europe, coinfection with tick-borne encephalitis virus may occur. There is limited evidence of human coinfection with B miyamotoi or Powassan virus, as isolated infection with either of these pathogens is rare.

In patients with Lyme disease, as many as 35% may have concurrent babesiosis, and as many as 12% may have concurrent HGA in endemic areas (eg, northeast and northern central United States).7-9 Concurrent HGA and babesiosis in the absence of Lyme disease also has been documented.7-9 Coinfection generally increases the diversity of presenting symptoms, often obscuring the primary diagnosis. In addition, these patients may have more severe and prolonged illness.8,10,11
In endemic areas, coinfection with B burgdorferi and an additional pathogen should be suspected if a patient presents with typical symptoms of early Lyme disease, especially erythema migrans, along with (1) combination of fever, chills, and headache; (2) prolonged viral-like illness, particularly 48 hours after appropriate antibiotic treatment; and (3) unexplained blood dyscrasia.7,11,12 When a patient presents with erythema migrans, it is unnecessary to test for HGA, as treatment of Lyme disease with doxycycline also is adequate for treating HGA; however, if systemic symptoms persist despite treatment, testing for babesiosis and other tick-borne illnesses should be considered, as babesiosis requires treatment with atovaquone plus azithromycin or clindamycin plus quinine.13
A complete blood count and peripheral blood smear can aid in the diagnosis of coinfection. The complete blood count may reveal leukopenia, anemia, or thrombocytopenia associated with HGA or babesiosis. The peripheral blood smear can reveal inclusions of intra-erythrocytic ring forms and tetrads (the “Maltese cross” appearance) in babesiosis and intragranulocytic morulae in HGA.12 The most sensitive diagnostic tests for tick-borne diseases are organism-specific IgM and IgG serology for Lyme disease, babesiosis, and HGA and polymerase chain reaction for babesiosis and HGA.7
Prevention Strategies
The most effective means of controlling tick-borne disease is avoiding tick bites altogether. One method is to avoid spending time in high-risk areas that may be infested with ticks, particularly low-lying brush, where ticks are likely to hide.14 For individuals traveling in environments with a high risk of tick exposure, behavioral methods of avoidance are indicated, including wearing long pants and a shirt with long sleeves, tucking the shirt into the pants, and wearing closed-toe shoes. Wearing light-colored clothing may aid in tick identification and prompt removal prior to attachment. Permethrin-impregnated clothing has been proven to decrease the likelihood of tick bites in adults working outdoors.15-17
Topical repellents also play a role in the prevention of tick-borne diseases. The most effective and safe synthetic repellents are N,N-diethyl-meta-toluamide (DEET); picaridin; p-menthane-3,8-diol; and insect repellent 3535 (IR3535)(ethyl butylacetylaminopropionate).16-19 Plant-based repellents also are available, but their efficacy is strongly influenced by the surrounding environment (eg, temperature, humidity, organic matter).20-22 Individuals also may be exposed to ticks following contact with domesticated animals and pets.23,24 Tick prevention in pets with the use of ectoparasiticides should be directed by a qualified veterinarian.25
Tick Removal
Following a bite, the tick should be removed promptly to avoid transmission of pathogens. Numerous commercial and in-home methods of tick removal are available, but not all are equally effective. Detachment techniques include removal with a card or commercially available radiofrequency device, lassoing, or freezing.26,27 However, the most effective method is simple removal with tweezers. The tick should be grasped close to the skin surface and pulled upward with an even pressure. Commercially available tick-removal devices have not been shown to produce better outcomes than removal of the tick with tweezers.28
Conclusion
When patients do not respond to therapy for presumed tick-borne infection, the diagnosis should be reconsidered. One important consideration is coinfection with a second organism. Prompt identification and removal of ticks can prevent disease transmission.
Tick-borne diseases are increasing in prevalence, likely due to climate change in combination with human movement into tick habitats.1-3 The Ixodes genus of hard ticks is a common vector for the transmission of pathogenic viruses, bacteria, parasites, and toxins. Among these, Lyme disease, which is caused by Borrelia burgdorferi, is the most prevalent, followed by babesiosis and human granulocytic anaplasmosis (HGA), respectively.4 In Europe, tick-borne encephalitis is commonly encountered. More recently identified diseases transmitted by Ixodes ticks include Powassan virus and Borrelia miyamotoi infection; however, these diseases are less frequently encountered than other tick-borne diseases.5,6
As tick-borne diseases become more prevalent, the likelihood of coinfection with more than one Ixodes-transmitted pathogen is increasing.7 Therefore, it is important for physicians who practice in endemic areas to be aware of the possibility of coinfection, which can alter clinical presentation, disease severity, and treatment response in tick-borne diseases. Additionally, public education on tick-bite prevention and prompt tick removal is necessary to combat the rising prevalence of these diseases.
Coinfection
Risk of coinfection with more than one tick-borne disease is contingent on the geographic distribution of the tick species as well as the particular pathogen’s prevalence within reservoir hosts in a given area (Figure). Most coinfections occur with B. burgdorferi and an additional pathogen, usually Anaplasma phagocytophilum (which causes human granulocytic anaplasmosis [HGA]) or Babesia microti (which causes babesiosis). In Europe, coinfection with tick-borne encephalitis virus may occur. There is limited evidence of human coinfection with B miyamotoi or Powassan virus, as isolated infection with either of these pathogens is rare.

In patients with Lyme disease, as many as 35% may have concurrent babesiosis, and as many as 12% may have concurrent HGA in endemic areas (eg, northeast and northern central United States).7-9 Concurrent HGA and babesiosis in the absence of Lyme disease also has been documented.7-9 Coinfection generally increases the diversity of presenting symptoms, often obscuring the primary diagnosis. In addition, these patients may have more severe and prolonged illness.8,10,11
In endemic areas, coinfection with B burgdorferi and an additional pathogen should be suspected if a patient presents with typical symptoms of early Lyme disease, especially erythema migrans, along with (1) combination of fever, chills, and headache; (2) prolonged viral-like illness, particularly 48 hours after appropriate antibiotic treatment; and (3) unexplained blood dyscrasia.7,11,12 When a patient presents with erythema migrans, it is unnecessary to test for HGA, as treatment of Lyme disease with doxycycline also is adequate for treating HGA; however, if systemic symptoms persist despite treatment, testing for babesiosis and other tick-borne illnesses should be considered, as babesiosis requires treatment with atovaquone plus azithromycin or clindamycin plus quinine.13
A complete blood count and peripheral blood smear can aid in the diagnosis of coinfection. The complete blood count may reveal leukopenia, anemia, or thrombocytopenia associated with HGA or babesiosis. The peripheral blood smear can reveal inclusions of intra-erythrocytic ring forms and tetrads (the “Maltese cross” appearance) in babesiosis and intragranulocytic morulae in HGA.12 The most sensitive diagnostic tests for tick-borne diseases are organism-specific IgM and IgG serology for Lyme disease, babesiosis, and HGA and polymerase chain reaction for babesiosis and HGA.7
Prevention Strategies
The most effective means of controlling tick-borne disease is avoiding tick bites altogether. One method is to avoid spending time in high-risk areas that may be infested with ticks, particularly low-lying brush, where ticks are likely to hide.14 For individuals traveling in environments with a high risk of tick exposure, behavioral methods of avoidance are indicated, including wearing long pants and a shirt with long sleeves, tucking the shirt into the pants, and wearing closed-toe shoes. Wearing light-colored clothing may aid in tick identification and prompt removal prior to attachment. Permethrin-impregnated clothing has been proven to decrease the likelihood of tick bites in adults working outdoors.15-17
Topical repellents also play a role in the prevention of tick-borne diseases. The most effective and safe synthetic repellents are N,N-diethyl-meta-toluamide (DEET); picaridin; p-menthane-3,8-diol; and insect repellent 3535 (IR3535)(ethyl butylacetylaminopropionate).16-19 Plant-based repellents also are available, but their efficacy is strongly influenced by the surrounding environment (eg, temperature, humidity, organic matter).20-22 Individuals also may be exposed to ticks following contact with domesticated animals and pets.23,24 Tick prevention in pets with the use of ectoparasiticides should be directed by a qualified veterinarian.25
Tick Removal
Following a bite, the tick should be removed promptly to avoid transmission of pathogens. Numerous commercial and in-home methods of tick removal are available, but not all are equally effective. Detachment techniques include removal with a card or commercially available radiofrequency device, lassoing, or freezing.26,27 However, the most effective method is simple removal with tweezers. The tick should be grasped close to the skin surface and pulled upward with an even pressure. Commercially available tick-removal devices have not been shown to produce better outcomes than removal of the tick with tweezers.28
Conclusion
When patients do not respond to therapy for presumed tick-borne infection, the diagnosis should be reconsidered. One important consideration is coinfection with a second organism. Prompt identification and removal of ticks can prevent disease transmission.
- McMichael C, Barnett J, McMichael AJ. An ill wind? climate change, migration, and health. Environ Health Perspect. 2012;120:646-654.
- Ostfeld RS, Brunner JL. Climate change and Ixodes tick-borne diseases of humans. Philos Trans R Soc Lond B Biol Sci. 2015;370:20140051.
- Ogden NH, Bigras-Poulin M, O’Callaghan CJ, et al. Vector seasonality, host infection dynamics and fitness of pathogens transmitted by the tick Ixodes scapularis. Parasitology. 2007;134(pt 2):209-227.
- Tickborne diseases of the United States. Centers for Disease Control and Prevention website. http://www.cdc.gov/ticks/diseases/index.html. Updated July 25, 2017. Accessed April 10, 2018.
- Hinten SR, Beckett GA, Gensheimer KF, et al. Increased recognition of Powassan encephalitis in the United States, 1999-2005. Vector Borne Zoonotic Dis. 2008;8:733-740.
- Platonov AE, Karan LS, Kolyasnikova NM, et al. Humans infected with relapsing fever spirochete Borrelia miyamotoi, Russia. Emerg Infect Dis. 2011;17:1816-1823.
- Krause PJ, McKay K, Thompson CA, et al; Deer-Associated Infection Study Group. Disease-specific diagnosis of coinfecting tickborne zoonoses: babesiosis, human granulocytic ehrlichiosis, and Lyme disease. Clin Infect Dis. 2002;34:1184-1191.
- Krause PJ, Telford SR 3rd, Spielman A, et al. Concurrent Lyme disease and babesiosis. evidence for increased severity and duration of illness. JAMA. 1996;275:1657-1660.
- Belongia EA, Reed KD, Mitchell PD, et al. Clinical and epidemiological features of early Lyme disease and human granulocytic ehrlichiosis in Wisconsin. Clin Infect Dis. 1999;29:1472-1477.
- Sweeny CJ, Ghassemi M, Agger WA, et al. Coinfection with Babesia microti and Borrelia burgdorferi in a western Wisconsin resident. Mayo Clin Proc.1998;73:338-341.
- Nadelman RB, Horowitz HW, Hsieh TC, et al. Simultaneous human granulocytic ehrlichiosis and Lyme borreliosis. N Engl J Med. 1997;337:27-30.
- Wormser GP, Dattwyler RJ, Shapiro ED, et al. The clinical assessment, treatment, and prevention of Lyme disease, human granulocytic anaplasmosis, and babesiosis: clinical practice guidelines by the Infectious Diseases Society of America. Clin Infect Dis. 2006;43:1089-1134.
- Swanson SJ, Neitzel D, Reed DK, et al. Coinfections acquired from Ixodes ticks. Clin Microbiol Rev. 2006;19:708-727.
- Hayes EB, Piesman J. How can we prevent Lyme disease? N Engl J Med. 2003;348:2424-2430.
- Vaughn MF, Funkhouser SW, Lin FC, et al. Long-lasting permethrin impregnated uniforms: a randomized-controlled trial for tick bite prevention. Am J Prev Med. 2014;46:473-480.
- Miller NJ, Rainone EE, Dyer MC, et al. Tick bite protection with permethrin-treated summer-weight clothing. J Med Entomol. 2011;48:327-333.
- Richards SL, Balanay JAG, Harris JW. Effectiveness of permethrin-treated clothing to prevent tick exposure in foresters in the central Appalachian region of the USA. Int J Environ Health Res. 2015;25:453-462.
- Pages F, Dautel H, Duvallet G, et al. Tick repellents for human use: prevention of tick bites and tick-borne diseases. Vector Borne Zoonotic Dis. 2014;14:85-93.
- Büchel K, Bendin J, Gharbi A, et al. Repellent efficacy of DEET, icaridin, and EBAAP against Ixodes ricinus and Ixodes scapularis nymphs (Acari, Ixodidae). Ticks Tick Borne Dis. 2015;6:494-498.
- Schwantes U, Dautel H, Jung G. Prevention of infectious tick-borne diseases in humans: comparative studies of the repellency of different dodecanoic acid-formulations against Ixodes ricinus ticks (Acari: Ixodidae). Parasit Vectors. 2008;8:1-8.
- Bissinger BW, Apperson CS, Sonenshine DE, et al. Efficacy of the new repellent BioUD against three species of ixodid ticks. Exp Appl Acarol. 2009;48:239-250.
- Feaster JE, Scialdone MA, Todd RG, et al. Dihydronepetalactones deter feeding activity by mosquitoes, stable flies, and deer ticks. J Med Entomol. 2009;46:832-840.
- Jennett AL, Smith FD, Wall R. Tick infestation risk for dogs in a peri-urban park. Parasit Vectors. 2013;6:358.
- Rand PW, Smith RP Jr, Lacombe EH. Canine seroprevalence and the distribution of Ixodes dammini in an area of emerging Lyme disease. Am J Public Health. 1991;81:1331-1334.
- Baneth G, Bourdeau P, Bourdoiseau G, et al; CVBD World Forum. Vector-borne diseases—constant challenge for practicing veterinarians: recommendations from the CVBD World Forum. Parasit Vectors. 2012;5:55.
- Akin Belli A, Dervis E, Kar S, et al. Revisiting detachment techniques in human-biting ticks. J Am Acad Dermatol. 2016;75:393-397.
- Ashique KT, Kaliyadan F. Radiofrequency device for tick removal. J Am Acad Dermatol. 2015;72:155-156.
- Due C, Fox W, Medlock JM, et al. Tick bite prevention and tick removal. BMJ. 2013;347:f7123.
- McMichael C, Barnett J, McMichael AJ. An ill wind? climate change, migration, and health. Environ Health Perspect. 2012;120:646-654.
- Ostfeld RS, Brunner JL. Climate change and Ixodes tick-borne diseases of humans. Philos Trans R Soc Lond B Biol Sci. 2015;370:20140051.
- Ogden NH, Bigras-Poulin M, O’Callaghan CJ, et al. Vector seasonality, host infection dynamics and fitness of pathogens transmitted by the tick Ixodes scapularis. Parasitology. 2007;134(pt 2):209-227.
- Tickborne diseases of the United States. Centers for Disease Control and Prevention website. http://www.cdc.gov/ticks/diseases/index.html. Updated July 25, 2017. Accessed April 10, 2018.
- Hinten SR, Beckett GA, Gensheimer KF, et al. Increased recognition of Powassan encephalitis in the United States, 1999-2005. Vector Borne Zoonotic Dis. 2008;8:733-740.
- Platonov AE, Karan LS, Kolyasnikova NM, et al. Humans infected with relapsing fever spirochete Borrelia miyamotoi, Russia. Emerg Infect Dis. 2011;17:1816-1823.
- Krause PJ, McKay K, Thompson CA, et al; Deer-Associated Infection Study Group. Disease-specific diagnosis of coinfecting tickborne zoonoses: babesiosis, human granulocytic ehrlichiosis, and Lyme disease. Clin Infect Dis. 2002;34:1184-1191.
- Krause PJ, Telford SR 3rd, Spielman A, et al. Concurrent Lyme disease and babesiosis. evidence for increased severity and duration of illness. JAMA. 1996;275:1657-1660.
- Belongia EA, Reed KD, Mitchell PD, et al. Clinical and epidemiological features of early Lyme disease and human granulocytic ehrlichiosis in Wisconsin. Clin Infect Dis. 1999;29:1472-1477.
- Sweeny CJ, Ghassemi M, Agger WA, et al. Coinfection with Babesia microti and Borrelia burgdorferi in a western Wisconsin resident. Mayo Clin Proc.1998;73:338-341.
- Nadelman RB, Horowitz HW, Hsieh TC, et al. Simultaneous human granulocytic ehrlichiosis and Lyme borreliosis. N Engl J Med. 1997;337:27-30.
- Wormser GP, Dattwyler RJ, Shapiro ED, et al. The clinical assessment, treatment, and prevention of Lyme disease, human granulocytic anaplasmosis, and babesiosis: clinical practice guidelines by the Infectious Diseases Society of America. Clin Infect Dis. 2006;43:1089-1134.
- Swanson SJ, Neitzel D, Reed DK, et al. Coinfections acquired from Ixodes ticks. Clin Microbiol Rev. 2006;19:708-727.
- Hayes EB, Piesman J. How can we prevent Lyme disease? N Engl J Med. 2003;348:2424-2430.
- Vaughn MF, Funkhouser SW, Lin FC, et al. Long-lasting permethrin impregnated uniforms: a randomized-controlled trial for tick bite prevention. Am J Prev Med. 2014;46:473-480.
- Miller NJ, Rainone EE, Dyer MC, et al. Tick bite protection with permethrin-treated summer-weight clothing. J Med Entomol. 2011;48:327-333.
- Richards SL, Balanay JAG, Harris JW. Effectiveness of permethrin-treated clothing to prevent tick exposure in foresters in the central Appalachian region of the USA. Int J Environ Health Res. 2015;25:453-462.
- Pages F, Dautel H, Duvallet G, et al. Tick repellents for human use: prevention of tick bites and tick-borne diseases. Vector Borne Zoonotic Dis. 2014;14:85-93.
- Büchel K, Bendin J, Gharbi A, et al. Repellent efficacy of DEET, icaridin, and EBAAP against Ixodes ricinus and Ixodes scapularis nymphs (Acari, Ixodidae). Ticks Tick Borne Dis. 2015;6:494-498.
- Schwantes U, Dautel H, Jung G. Prevention of infectious tick-borne diseases in humans: comparative studies of the repellency of different dodecanoic acid-formulations against Ixodes ricinus ticks (Acari: Ixodidae). Parasit Vectors. 2008;8:1-8.
- Bissinger BW, Apperson CS, Sonenshine DE, et al. Efficacy of the new repellent BioUD against three species of ixodid ticks. Exp Appl Acarol. 2009;48:239-250.
- Feaster JE, Scialdone MA, Todd RG, et al. Dihydronepetalactones deter feeding activity by mosquitoes, stable flies, and deer ticks. J Med Entomol. 2009;46:832-840.
- Jennett AL, Smith FD, Wall R. Tick infestation risk for dogs in a peri-urban park. Parasit Vectors. 2013;6:358.
- Rand PW, Smith RP Jr, Lacombe EH. Canine seroprevalence and the distribution of Ixodes dammini in an area of emerging Lyme disease. Am J Public Health. 1991;81:1331-1334.
- Baneth G, Bourdeau P, Bourdoiseau G, et al; CVBD World Forum. Vector-borne diseases—constant challenge for practicing veterinarians: recommendations from the CVBD World Forum. Parasit Vectors. 2012;5:55.
- Akin Belli A, Dervis E, Kar S, et al. Revisiting detachment techniques in human-biting ticks. J Am Acad Dermatol. 2016;75:393-397.
- Ashique KT, Kaliyadan F. Radiofrequency device for tick removal. J Am Acad Dermatol. 2015;72:155-156.
- Due C, Fox W, Medlock JM, et al. Tick bite prevention and tick removal. BMJ. 2013;347:f7123.
Practice Points
- As tick-borne diseases become more prevalent, the likelihood of coinfection with more than one Ixodes-transmitted pathogen is increasing, particularly in endemic areas.
- Coinfection generally increases the diversity of presenting symptoms, obscuring the primary diagnosis. The disease course also may be prolonged and more severe.
- Prevention of tick attachment and prompt tick removal are critical to combating the rising prevalence of tick-borne diseases.
What’s Eating You? Ixodes Tick and Related Diseases, Part 2: Diagnosis and Treatment of Regional Tick-borne Diseases
The Ixodes tick is prevalent in temperate climates worldwide. During a blood meal, pathogens may be transmitted from the tick to its host. Borrelia burgdorferi, a spirochete responsible for Lyme disease, is the most prevalent pathogen transmitted by Ixodes ticks.1 Borrelia mayonii recently was identified as an additional cause of Lyme disease in the United States.2
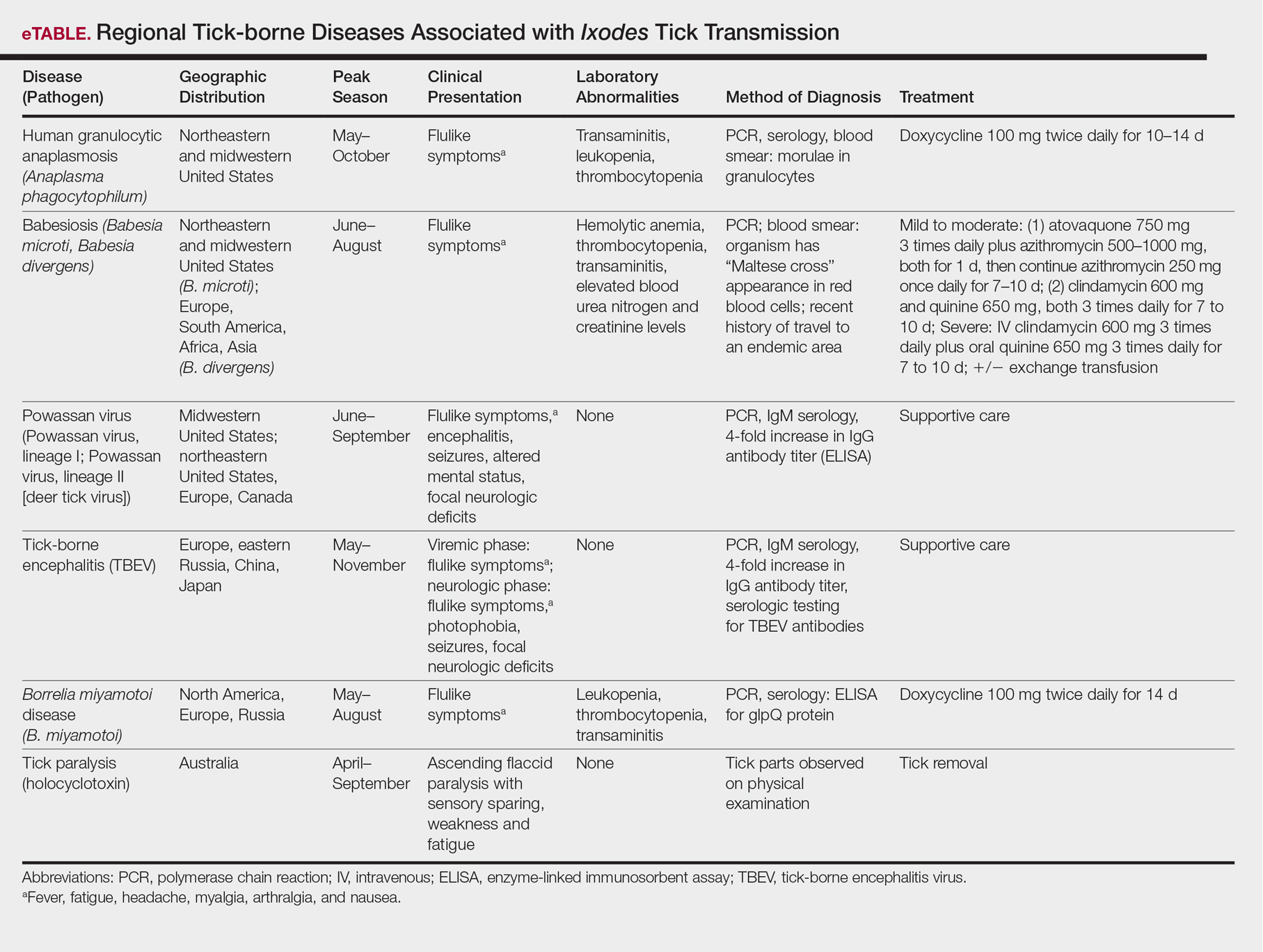
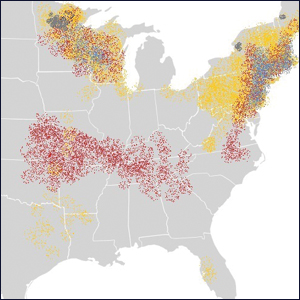
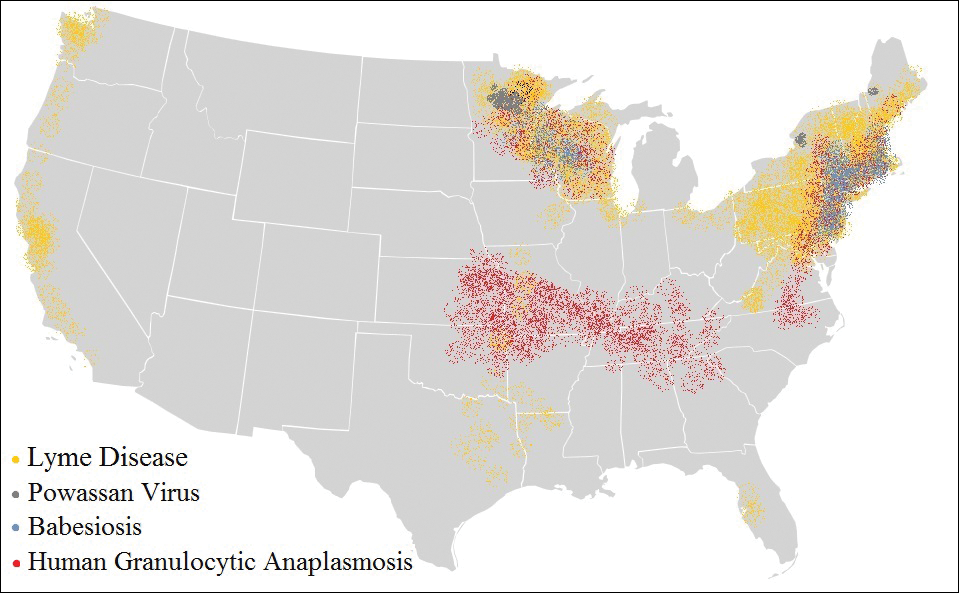
The Ixodes tick also is associated with several less common pathogens, including Babesia microti and the tick-borne encephalitis virus, which have been recognized as Ixodes-associated pathogens for many years.3,4 Other pathogens have been identified, including Anaplasma phagocytophilum, recognized in the 1990s as the cause of human granulocytic anaplasmosis, as well as the Powassan virus and Borrelia miyamotoi.5-7 Additionally, tick paralysis has been associated with toxins in the saliva of various species of several genera of ticks, including some Ixodes species.8 Due to an overlap in geographic distribution (Figure) and disease presentations (eTable), it is important that physicians be familiar with these regional pathogens transmitted by Ixodes ticks.
Human Granulocytic Anaplasmosis
Formerly known as human granulocytic ehrlichiosis, human granulocytic anaplasmosis is caused by A phagocytophilum and is transmitted by Ixodes scapularis, Ixodes pacificus, and Ixodes persulcatus. The incidence of human granulocytic anaplasmosis in the United States increased 12-fold from 2001 to 2011.9
Presenting symptoms generally are nonspecific, including fever, night sweats, headache, myalgias, and arthralgias, often resulting in misdiagnosis as a viral infection. Laboratory abnormalities include mild transaminitis, leukopenia, and thrombocytopenia.9,10 Although most infections resolve spontaneously, 3% of patients develop serious complications. The mortality rate is 0.6%.11
A diagnosis of human granulocytic anaplasmosis should be suspected in patients with a viral-like illness and exposure to ticks in an endemic area. The diagnosis can be confirmed by polymerase chain reaction (PCR), acute- and convalescent-phase serologic testing, or direct fluorescent antibody screening. Characteristic morulae may be present in granulocytes.12 Treatment typically includes doxycycline, which also covers B burgdorferi coinfection. When a diagnosis of human granulocytic anaplasmosis is suspected, treatment should never be delayed to await laboratory confirmation. If no clinical improvement is seen within 48 hours, alternate diagnoses or coinfection with B microti should be considered.10
Babesiosis
The protozoan B microti causes babesiosis in the United States, with Babesia divergens being more common in Europe.13 Reported cases of babesiosis in New York increased as much as 20-fold from 2001 to 2008.14 Transmission primarily is from the Ixodes tick but rarely can occur from blood transfusion.15 Tick attachment for at least 36 hours is required for transmission.13
The clinical presentation of babesiosis ranges from asymptomatic to fatal. Symptoms generally are nonspecific, resembling a viral infection and including headache, nausea, diarrhea, arthralgia, and myalgia. Laboratory evaluation may reveal hemolytic anemia, thrombocytopenia, transaminitis, and elevated blood urea nitrogen and creatinine levels.16 Rash is not typical. Resolution of symptoms generally occurs within 2 weeks of presentation, although anemia may persist for months.13 Severe disease is more common among elderly and immunocompromised patients. Complications include respiratory failure, renal failure, congestive heart failure, and disseminated intravascular coagulation. The mortality rate in the United States is approximately 10%.10,16
A diagnosis of babesiosis is made based on the presence of flulike symptoms, laboratory results, and history of recent travel to an endemic area. A thin blood smear allows identification of the organism in erythrocytes as ring forms or tetrads (a “Maltese cross” appearance).17 Polymerase chain reaction is more sensitive than a blood smear, especially in early disease.18 Indirect fluorescent antibody testing is species-specific but cannot verify active infection.10
Treatment of babesiosis is indicated for symptomatic patients with active infection. Positive serology alone is not an indication for treatment. Asymptomatic patients with positive serology should have diagnostic testing repeated in 3 months with subsequent treatment if parasitemia persists. Mild disease is treated with atovaquone plus azithromycin or clindamycin plus quinine. Severe babesiosis is treated with quinine and intravenous clindamycin and may require exchange transfusion.10 Coinfection with B burgdorferi should be considered in patients with flulike symptoms and erythema migrans or treatment failure. Coinfection is diagnosed by Lyme serology plus PCR for B microti. This is an important consideration because treatment of babesiosis does not eradicate B burgdorferi infection.19
Powassan Virus
Powassan virus is a flavivirus that causes encephalitis. It is transmitted by Ixodes cookei (Powassan virus, lineage I) in the Great Lakes region and by I scapularis (Powassan virus, lineage II, or deer tick virus) in the northeastern United States. Transmission can occur within 15 minutes of tick attachment.6,20,21
Patients typically present with fever, headache, altered mental status, seizures, and focal neurologic deficits. Gastrointestinal symptoms and rash also have been reported.21 The diagnosis is made based on clinical presentation and laboratory testing with PCR or enzyme-linked immunosorbent assay (ELISA). Cross-reactivity on ELISA exists, necessitating confirmation with a neutralizing antibody or PCR. Treatment is supportive. Corticosteroids and intravenous immunoglobulin have been proposed as treatment modalities, but evidence of their efficacy is limited.22
Tick-borne Encephalitis
Tick-borne encephalitis is caused by the flavivirus tick-borne encephalitis virus in Europe and Asia. The tick-borne encephalitis virus is transmitted by Ixodes ricinus in Europe and by Ixodes persulcatus in eastern Russia, China, and Japan. It also has been associated with consumption of unpasteurized milk.23,24
Tick-borne encephalitis presents in a biphasic pattern. The initial viremic phase can persist for as long as 8 days with headache, nausea, myalgia, and fever. One-third of patients then enter an asymptomatic phase, followed by virus penetration into the central nervous system. The neurologic phase produces continued headache and fever with photophobia, focal neurologic deficits, seizures, respiratory depression, or coma. Neurologic sequelae persist in 10% to 20% of patients.25,26
In the viremic stage, diagnosis is made with PCR or culture. During the latent phase or neurologic phase, serologic testing for tick-borne encephalitis virus antibodies is indicated. Neutralizing antibody evaluation may be necessary due to cross-reactivity among flaviviruses.27 Treatment is supportive. An inactivated vaccine is available for high-risk populations.28
Borrelia miyamotoi Disease
Borrelia miyamotoi is a symbiont of the Ixodes tick formerly believed to have no pathogenic significance; however, B miyamotoi was isolated in febrile patients in Russia in 20117 and was identified as a pathogen in both North America29 and Europe in 2013.30 Disease presentation includes nonspecific symptoms of fever, fatigue, headache, arthralgia, myalgia, and nausea. Rash is uncommon. Laboratory abnormalities include leukopenia, thrombocytopenia, and transaminitis.31,32 Meningoencephalitis may occur in immunocompromised patients.29,30
The diagnosis of B miyamotoi disease is confirmed by PCR or serology. An ELISA that is positive for B burgdorferi IgM but negative with confirmatory immunoblot suggests B miyamotoi disease. Seroconversion using a glpQ protein ELISA also can be assessed.31 If ELISA is positive, Lyme disease can be excluded because B burgdorferi does not possess g1pQ. Treatment is with doxycycline.32
Tick Paralysis
Tick paralysis is an intoxication with holocyclotoxin from the saliva of gravid hard ticks. In the United States, intoxication is associated with ticks of various species of Amblyomma, Dermacentor, and Ixodes in the Northwest, Southeast, and Northeast. In Australia, intoxication is associated with Ixodes.33 Patients present with weakness and fatigue, progressing to ascending flaccid paralysis with sensory sparing. The treatment is tick removal.8,33
Conclusion
Arthropods carry many regional pathogens. Physicians outside of those regions should seek a travel history and be alert for imported disease.
- Steere AC, Grodzicki RL, Kornblatt AN, et al. The spirochetal etiology of Lyme disease. N Engl J Med. 1983;308:733-740.
- Dolan MC, Hojgaard A, Hoxmeier JC, et al. Vector competence of the blacklegged tick, Ixodes scapularis, for the recently recognized Lyme borreliosis spirochete Candidatus Borrelia mayonii. Ticks Tick Borne Dis. 2016;7:665-669.
- Rudzinska MA, Spielman A, Riek RF, et al. Intraerythrocytic ‘gametocytes’ of Babesia microti and their maturation in ticks. Can J Zool. 1979;57:424-434.
- Casals J, Olitsky PK. Enduring immunity following vaccination of mice with formalin-inactivated virus of Russian spring-summer (Far Eastern, tick-borne) encephalitis; correlation with serum-neutralizing and complement-fixing antibodies. J Exp Med. 1945;82:431-443.
- Magnarelli LA, Stafford KC III, Mather TN, et al. Hemocytic rickettsia-like organisms in ticks: serologic reactivity with antisera to Ehrlichiae and detection of DNA of agent of human granulocytic ehrlichiosis by PCR. J Clin Microbiol. 1995;33:2710-2714.
- McLean DM, Donohue WL. Powassan virus: isolation of virus from a fatal case of encephalitis. Can Med Assoc J. 1959;80:708-711.
- Platonov AE, Karan LS, Kolyasnikova NM, et al. Humans infected with relapsing fever spirochete Borrelia miyamotoi, Russia. Emerg Infect Dis. 2011;17:1816-1823.
- Diaz JH. A 60-year meta-analysis of tick paralysis in the United States: a predictable, preventable, and often misdiagnosed poisoning. J Med Toxicol. 2010;6:15-21.
- Bakken J, Dumler JS. Human granulocytic anaplasmosis. Infect Dis Clin North Am. 2015;29:341-355.
- Chapman AS, Bakken JS, Folk SM, et al; Tickborne Rickettsial Diseases Working Group; CDC. Diagnosis and management of tickborne rickettsial diseases: Rocky Mountain spotted fever, ehrlichioses, and anaplasmosis—United States: a practical guide for physicians and other health-care and public health professionals. MMWR Recomm Rep. 2006;55(RR-4):1-27.
- Dahlgren FS, Mandel EJ, Krebs JW, et al. Increasing incidence of Ehrlichia chaffeensis and Anaplasma phagocytophilum in the United States, 2000-2007. Am J Trop Med Hyg. 2011;85:124-130.
- Aguero-Rosenfeld ME. Diagnosis of human granulocytic ehrlichiosis: state of the art. Vector Borne Zoonotic Dis. 2002;2:233-239.
- Vannier EG, Diuk-Wasser MA, Ben Mamoun C, et al. Babesiosis. Infect Dis Clin North Am. 2015;29:357-370.
- Joseph JT, Roy SS, Shams N, et al. Babesiosis in Lower Hudson Valley, New York, USA. Emerg Infect Dis. 2011;17:843-847.
- McQuiston JH, Childs JE, Chamberland ME, et al. Transmission of tickborne agents by blood transfusions: a review of known and potential risks in the United States. Transfusion. 2000;40:274-284.
- Hatcher JC, Greenberg PD, Antique J, et al. Severe babesiosis in Long Island: review of 34 cases and their complications. Clin Infect Dis. 2001;32:1117-1125.
- Healy GR, Ruebush TK. Morphology of Babesia microti in human blood smears. Am J Clin Pathol. 1980;73:107-109.
- Kowalski TJ, Jobe DA, Dolan EC, et al. The emergence of clinically relevant babesiosis in southwestern Wisconsin. WMJ. 2015;114:152-157.
- Krause PJ, Telford SR III, Spielman A, et al. Concurrent Lyme disease and babesiosis. evidence for increased severity and duration of illness. JAMA. 1996;275:1657-1660.
- Centers for Disease Control and Prevention. Statistics & maps. http://www.cdc.gov/powassan/statistics.html. Updated February 14, 2017. Accessed December 11, 2017.
- Piantadosi A, Rubin DB, McQuillen DP, et al. Emerging cases of Powassan virus encephalitis in New England: clinical presentation, imaging, and review of the literature. Clin Infect Dis. 2016;62:707-713.
- El Khoury MY, Camargo JF, White JL, et al. Potential role of deer tick virus in Powassan encephalitis cases in Lyme disease-endemic areas of New York, U.S.A. Emerg Infect Dis. 2013;19:1926-1933.
- World Health Organization (WHO). Vaccines against tick-borne encephalitis: WHO position paper. Wkly Epidemiol Rec. 2011;86:241-256.
- Centers for Disease Control and Prevention (CDC). Tick-borne encephalitis among U.S. travelers to Europe and Asia—2000-2009. JAMA. 2010;303:2132-2135.
- Valarcher JF, Hägglund S, Juremalm M, et al. Tick-borne encephalitits. Rev Sci Tech. 2015;34:453-466.
- Schultze D, Dollenmaier G, Rohner A, et al. Benefit of detecting tick-borne encephalitis viremia in the first phase of illness. J Clin Virol. 2007;38:172-175.
- Holzmann H. Diagnosis of tick-borne encephalitis. Vaccine. 2003;21(suppl 1):S36-S40.
- Zavadska D, Anca I, André F, et al. Recommendations for tick-borne encephalitis vaccination from the Central European Vaccination Awareness Group. Hum Vaccin Immunother. 2013;9:362-374.
- Gugliotta JL, Goethert HK, Berardi VP, et al. Meningoencephalitis from Borrelia miyamotoi in an immunocompromised patient. N Engl J Med. 2013;368:240-245.
- Hovius JW, de Wever B, Sohne M, et al. A case of meningoencephalitis by the relapsing fever spirochaete Borrelia miyamotoi in Europe. Lancet. 2013;382:658.
- Molloy PJ, Telford SR III, Chowdri HR, et al. Borrelia miyamotoi disease in the northeastern United States: a case series. Ann Intern Med. 2015;163:91-98.
- Telford SR 3rd, Goethert HK, Molloy PJ, et al. Borrelia miyamotoi disease: neither Lyme disease nor relapsing fever. Clin Lab Med. 2015;35:867-882.
- Diaz JH. A comparative meta-analysis of tick paralysis in the United States and Australia. Clin Toxicol (Phila). 2015;53:874-883.
The Ixodes tick is prevalent in temperate climates worldwide. During a blood meal, pathogens may be transmitted from the tick to its host. Borrelia burgdorferi, a spirochete responsible for Lyme disease, is the most prevalent pathogen transmitted by Ixodes ticks.1 Borrelia mayonii recently was identified as an additional cause of Lyme disease in the United States.2
The Ixodes tick also is associated with several less common pathogens, including Babesia microti and the tick-borne encephalitis virus, which have been recognized as Ixodes-associated pathogens for many years.3,4 Other pathogens have been identified, including Anaplasma phagocytophilum, recognized in the 1990s as the cause of human granulocytic anaplasmosis, as well as the Powassan virus and Borrelia miyamotoi.5-7 Additionally, tick paralysis has been associated with toxins in the saliva of various species of several genera of ticks, including some Ixodes species.8 Due to an overlap in geographic distribution (Figure) and disease presentations (eTable), it is important that physicians be familiar with these regional pathogens transmitted by Ixodes ticks.

Human Granulocytic Anaplasmosis
Formerly known as human granulocytic ehrlichiosis, human granulocytic anaplasmosis is caused by A phagocytophilum and is transmitted by Ixodes scapularis, Ixodes pacificus, and Ixodes persulcatus. The incidence of human granulocytic anaplasmosis in the United States increased 12-fold from 2001 to 2011.9
Presenting symptoms generally are nonspecific, including fever, night sweats, headache, myalgias, and arthralgias, often resulting in misdiagnosis as a viral infection. Laboratory abnormalities include mild transaminitis, leukopenia, and thrombocytopenia.9,10 Although most infections resolve spontaneously, 3% of patients develop serious complications. The mortality rate is 0.6%.11
A diagnosis of human granulocytic anaplasmosis should be suspected in patients with a viral-like illness and exposure to ticks in an endemic area. The diagnosis can be confirmed by polymerase chain reaction (PCR), acute- and convalescent-phase serologic testing, or direct fluorescent antibody screening. Characteristic morulae may be present in granulocytes.12 Treatment typically includes doxycycline, which also covers B burgdorferi coinfection. When a diagnosis of human granulocytic anaplasmosis is suspected, treatment should never be delayed to await laboratory confirmation. If no clinical improvement is seen within 48 hours, alternate diagnoses or coinfection with B microti should be considered.10
Babesiosis
The protozoan B microti causes babesiosis in the United States, with Babesia divergens being more common in Europe.13 Reported cases of babesiosis in New York increased as much as 20-fold from 2001 to 2008.14 Transmission primarily is from the Ixodes tick but rarely can occur from blood transfusion.15 Tick attachment for at least 36 hours is required for transmission.13
The clinical presentation of babesiosis ranges from asymptomatic to fatal. Symptoms generally are nonspecific, resembling a viral infection and including headache, nausea, diarrhea, arthralgia, and myalgia. Laboratory evaluation may reveal hemolytic anemia, thrombocytopenia, transaminitis, and elevated blood urea nitrogen and creatinine levels.16 Rash is not typical. Resolution of symptoms generally occurs within 2 weeks of presentation, although anemia may persist for months.13 Severe disease is more common among elderly and immunocompromised patients. Complications include respiratory failure, renal failure, congestive heart failure, and disseminated intravascular coagulation. The mortality rate in the United States is approximately 10%.10,16
A diagnosis of babesiosis is made based on the presence of flulike symptoms, laboratory results, and history of recent travel to an endemic area. A thin blood smear allows identification of the organism in erythrocytes as ring forms or tetrads (a “Maltese cross” appearance).17 Polymerase chain reaction is more sensitive than a blood smear, especially in early disease.18 Indirect fluorescent antibody testing is species-specific but cannot verify active infection.10
Treatment of babesiosis is indicated for symptomatic patients with active infection. Positive serology alone is not an indication for treatment. Asymptomatic patients with positive serology should have diagnostic testing repeated in 3 months with subsequent treatment if parasitemia persists. Mild disease is treated with atovaquone plus azithromycin or clindamycin plus quinine. Severe babesiosis is treated with quinine and intravenous clindamycin and may require exchange transfusion.10 Coinfection with B burgdorferi should be considered in patients with flulike symptoms and erythema migrans or treatment failure. Coinfection is diagnosed by Lyme serology plus PCR for B microti. This is an important consideration because treatment of babesiosis does not eradicate B burgdorferi infection.19
Powassan Virus
Powassan virus is a flavivirus that causes encephalitis. It is transmitted by Ixodes cookei (Powassan virus, lineage I) in the Great Lakes region and by I scapularis (Powassan virus, lineage II, or deer tick virus) in the northeastern United States. Transmission can occur within 15 minutes of tick attachment.6,20,21
Patients typically present with fever, headache, altered mental status, seizures, and focal neurologic deficits. Gastrointestinal symptoms and rash also have been reported.21 The diagnosis is made based on clinical presentation and laboratory testing with PCR or enzyme-linked immunosorbent assay (ELISA). Cross-reactivity on ELISA exists, necessitating confirmation with a neutralizing antibody or PCR. Treatment is supportive. Corticosteroids and intravenous immunoglobulin have been proposed as treatment modalities, but evidence of their efficacy is limited.22
Tick-borne Encephalitis
Tick-borne encephalitis is caused by the flavivirus tick-borne encephalitis virus in Europe and Asia. The tick-borne encephalitis virus is transmitted by Ixodes ricinus in Europe and by Ixodes persulcatus in eastern Russia, China, and Japan. It also has been associated with consumption of unpasteurized milk.23,24
Tick-borne encephalitis presents in a biphasic pattern. The initial viremic phase can persist for as long as 8 days with headache, nausea, myalgia, and fever. One-third of patients then enter an asymptomatic phase, followed by virus penetration into the central nervous system. The neurologic phase produces continued headache and fever with photophobia, focal neurologic deficits, seizures, respiratory depression, or coma. Neurologic sequelae persist in 10% to 20% of patients.25,26
In the viremic stage, diagnosis is made with PCR or culture. During the latent phase or neurologic phase, serologic testing for tick-borne encephalitis virus antibodies is indicated. Neutralizing antibody evaluation may be necessary due to cross-reactivity among flaviviruses.27 Treatment is supportive. An inactivated vaccine is available for high-risk populations.28
Borrelia miyamotoi Disease
Borrelia miyamotoi is a symbiont of the Ixodes tick formerly believed to have no pathogenic significance; however, B miyamotoi was isolated in febrile patients in Russia in 20117 and was identified as a pathogen in both North America29 and Europe in 2013.30 Disease presentation includes nonspecific symptoms of fever, fatigue, headache, arthralgia, myalgia, and nausea. Rash is uncommon. Laboratory abnormalities include leukopenia, thrombocytopenia, and transaminitis.31,32 Meningoencephalitis may occur in immunocompromised patients.29,30
The diagnosis of B miyamotoi disease is confirmed by PCR or serology. An ELISA that is positive for B burgdorferi IgM but negative with confirmatory immunoblot suggests B miyamotoi disease. Seroconversion using a glpQ protein ELISA also can be assessed.31 If ELISA is positive, Lyme disease can be excluded because B burgdorferi does not possess g1pQ. Treatment is with doxycycline.32
Tick Paralysis
Tick paralysis is an intoxication with holocyclotoxin from the saliva of gravid hard ticks. In the United States, intoxication is associated with ticks of various species of Amblyomma, Dermacentor, and Ixodes in the Northwest, Southeast, and Northeast. In Australia, intoxication is associated with Ixodes.33 Patients present with weakness and fatigue, progressing to ascending flaccid paralysis with sensory sparing. The treatment is tick removal.8,33
Conclusion
Arthropods carry many regional pathogens. Physicians outside of those regions should seek a travel history and be alert for imported disease.
The Ixodes tick is prevalent in temperate climates worldwide. During a blood meal, pathogens may be transmitted from the tick to its host. Borrelia burgdorferi, a spirochete responsible for Lyme disease, is the most prevalent pathogen transmitted by Ixodes ticks.1 Borrelia mayonii recently was identified as an additional cause of Lyme disease in the United States.2
The Ixodes tick also is associated with several less common pathogens, including Babesia microti and the tick-borne encephalitis virus, which have been recognized as Ixodes-associated pathogens for many years.3,4 Other pathogens have been identified, including Anaplasma phagocytophilum, recognized in the 1990s as the cause of human granulocytic anaplasmosis, as well as the Powassan virus and Borrelia miyamotoi.5-7 Additionally, tick paralysis has been associated with toxins in the saliva of various species of several genera of ticks, including some Ixodes species.8 Due to an overlap in geographic distribution (Figure) and disease presentations (eTable), it is important that physicians be familiar with these regional pathogens transmitted by Ixodes ticks.

Human Granulocytic Anaplasmosis
Formerly known as human granulocytic ehrlichiosis, human granulocytic anaplasmosis is caused by A phagocytophilum and is transmitted by Ixodes scapularis, Ixodes pacificus, and Ixodes persulcatus. The incidence of human granulocytic anaplasmosis in the United States increased 12-fold from 2001 to 2011.9
Presenting symptoms generally are nonspecific, including fever, night sweats, headache, myalgias, and arthralgias, often resulting in misdiagnosis as a viral infection. Laboratory abnormalities include mild transaminitis, leukopenia, and thrombocytopenia.9,10 Although most infections resolve spontaneously, 3% of patients develop serious complications. The mortality rate is 0.6%.11
A diagnosis of human granulocytic anaplasmosis should be suspected in patients with a viral-like illness and exposure to ticks in an endemic area. The diagnosis can be confirmed by polymerase chain reaction (PCR), acute- and convalescent-phase serologic testing, or direct fluorescent antibody screening. Characteristic morulae may be present in granulocytes.12 Treatment typically includes doxycycline, which also covers B burgdorferi coinfection. When a diagnosis of human granulocytic anaplasmosis is suspected, treatment should never be delayed to await laboratory confirmation. If no clinical improvement is seen within 48 hours, alternate diagnoses or coinfection with B microti should be considered.10
Babesiosis
The protozoan B microti causes babesiosis in the United States, with Babesia divergens being more common in Europe.13 Reported cases of babesiosis in New York increased as much as 20-fold from 2001 to 2008.14 Transmission primarily is from the Ixodes tick but rarely can occur from blood transfusion.15 Tick attachment for at least 36 hours is required for transmission.13
The clinical presentation of babesiosis ranges from asymptomatic to fatal. Symptoms generally are nonspecific, resembling a viral infection and including headache, nausea, diarrhea, arthralgia, and myalgia. Laboratory evaluation may reveal hemolytic anemia, thrombocytopenia, transaminitis, and elevated blood urea nitrogen and creatinine levels.16 Rash is not typical. Resolution of symptoms generally occurs within 2 weeks of presentation, although anemia may persist for months.13 Severe disease is more common among elderly and immunocompromised patients. Complications include respiratory failure, renal failure, congestive heart failure, and disseminated intravascular coagulation. The mortality rate in the United States is approximately 10%.10,16
A diagnosis of babesiosis is made based on the presence of flulike symptoms, laboratory results, and history of recent travel to an endemic area. A thin blood smear allows identification of the organism in erythrocytes as ring forms or tetrads (a “Maltese cross” appearance).17 Polymerase chain reaction is more sensitive than a blood smear, especially in early disease.18 Indirect fluorescent antibody testing is species-specific but cannot verify active infection.10
Treatment of babesiosis is indicated for symptomatic patients with active infection. Positive serology alone is not an indication for treatment. Asymptomatic patients with positive serology should have diagnostic testing repeated in 3 months with subsequent treatment if parasitemia persists. Mild disease is treated with atovaquone plus azithromycin or clindamycin plus quinine. Severe babesiosis is treated with quinine and intravenous clindamycin and may require exchange transfusion.10 Coinfection with B burgdorferi should be considered in patients with flulike symptoms and erythema migrans or treatment failure. Coinfection is diagnosed by Lyme serology plus PCR for B microti. This is an important consideration because treatment of babesiosis does not eradicate B burgdorferi infection.19
Powassan Virus
Powassan virus is a flavivirus that causes encephalitis. It is transmitted by Ixodes cookei (Powassan virus, lineage I) in the Great Lakes region and by I scapularis (Powassan virus, lineage II, or deer tick virus) in the northeastern United States. Transmission can occur within 15 minutes of tick attachment.6,20,21
Patients typically present with fever, headache, altered mental status, seizures, and focal neurologic deficits. Gastrointestinal symptoms and rash also have been reported.21 The diagnosis is made based on clinical presentation and laboratory testing with PCR or enzyme-linked immunosorbent assay (ELISA). Cross-reactivity on ELISA exists, necessitating confirmation with a neutralizing antibody or PCR. Treatment is supportive. Corticosteroids and intravenous immunoglobulin have been proposed as treatment modalities, but evidence of their efficacy is limited.22
Tick-borne Encephalitis
Tick-borne encephalitis is caused by the flavivirus tick-borne encephalitis virus in Europe and Asia. The tick-borne encephalitis virus is transmitted by Ixodes ricinus in Europe and by Ixodes persulcatus in eastern Russia, China, and Japan. It also has been associated with consumption of unpasteurized milk.23,24
Tick-borne encephalitis presents in a biphasic pattern. The initial viremic phase can persist for as long as 8 days with headache, nausea, myalgia, and fever. One-third of patients then enter an asymptomatic phase, followed by virus penetration into the central nervous system. The neurologic phase produces continued headache and fever with photophobia, focal neurologic deficits, seizures, respiratory depression, or coma. Neurologic sequelae persist in 10% to 20% of patients.25,26
In the viremic stage, diagnosis is made with PCR or culture. During the latent phase or neurologic phase, serologic testing for tick-borne encephalitis virus antibodies is indicated. Neutralizing antibody evaluation may be necessary due to cross-reactivity among flaviviruses.27 Treatment is supportive. An inactivated vaccine is available for high-risk populations.28
Borrelia miyamotoi Disease
Borrelia miyamotoi is a symbiont of the Ixodes tick formerly believed to have no pathogenic significance; however, B miyamotoi was isolated in febrile patients in Russia in 20117 and was identified as a pathogen in both North America29 and Europe in 2013.30 Disease presentation includes nonspecific symptoms of fever, fatigue, headache, arthralgia, myalgia, and nausea. Rash is uncommon. Laboratory abnormalities include leukopenia, thrombocytopenia, and transaminitis.31,32 Meningoencephalitis may occur in immunocompromised patients.29,30
The diagnosis of B miyamotoi disease is confirmed by PCR or serology. An ELISA that is positive for B burgdorferi IgM but negative with confirmatory immunoblot suggests B miyamotoi disease. Seroconversion using a glpQ protein ELISA also can be assessed.31 If ELISA is positive, Lyme disease can be excluded because B burgdorferi does not possess g1pQ. Treatment is with doxycycline.32
Tick Paralysis
Tick paralysis is an intoxication with holocyclotoxin from the saliva of gravid hard ticks. In the United States, intoxication is associated with ticks of various species of Amblyomma, Dermacentor, and Ixodes in the Northwest, Southeast, and Northeast. In Australia, intoxication is associated with Ixodes.33 Patients present with weakness and fatigue, progressing to ascending flaccid paralysis with sensory sparing. The treatment is tick removal.8,33
Conclusion
Arthropods carry many regional pathogens. Physicians outside of those regions should seek a travel history and be alert for imported disease.
- Steere AC, Grodzicki RL, Kornblatt AN, et al. The spirochetal etiology of Lyme disease. N Engl J Med. 1983;308:733-740.
- Dolan MC, Hojgaard A, Hoxmeier JC, et al. Vector competence of the blacklegged tick, Ixodes scapularis, for the recently recognized Lyme borreliosis spirochete Candidatus Borrelia mayonii. Ticks Tick Borne Dis. 2016;7:665-669.
- Rudzinska MA, Spielman A, Riek RF, et al. Intraerythrocytic ‘gametocytes’ of Babesia microti and their maturation in ticks. Can J Zool. 1979;57:424-434.
- Casals J, Olitsky PK. Enduring immunity following vaccination of mice with formalin-inactivated virus of Russian spring-summer (Far Eastern, tick-borne) encephalitis; correlation with serum-neutralizing and complement-fixing antibodies. J Exp Med. 1945;82:431-443.
- Magnarelli LA, Stafford KC III, Mather TN, et al. Hemocytic rickettsia-like organisms in ticks: serologic reactivity with antisera to Ehrlichiae and detection of DNA of agent of human granulocytic ehrlichiosis by PCR. J Clin Microbiol. 1995;33:2710-2714.
- McLean DM, Donohue WL. Powassan virus: isolation of virus from a fatal case of encephalitis. Can Med Assoc J. 1959;80:708-711.
- Platonov AE, Karan LS, Kolyasnikova NM, et al. Humans infected with relapsing fever spirochete Borrelia miyamotoi, Russia. Emerg Infect Dis. 2011;17:1816-1823.
- Diaz JH. A 60-year meta-analysis of tick paralysis in the United States: a predictable, preventable, and often misdiagnosed poisoning. J Med Toxicol. 2010;6:15-21.
- Bakken J, Dumler JS. Human granulocytic anaplasmosis. Infect Dis Clin North Am. 2015;29:341-355.
- Chapman AS, Bakken JS, Folk SM, et al; Tickborne Rickettsial Diseases Working Group; CDC. Diagnosis and management of tickborne rickettsial diseases: Rocky Mountain spotted fever, ehrlichioses, and anaplasmosis—United States: a practical guide for physicians and other health-care and public health professionals. MMWR Recomm Rep. 2006;55(RR-4):1-27.
- Dahlgren FS, Mandel EJ, Krebs JW, et al. Increasing incidence of Ehrlichia chaffeensis and Anaplasma phagocytophilum in the United States, 2000-2007. Am J Trop Med Hyg. 2011;85:124-130.
- Aguero-Rosenfeld ME. Diagnosis of human granulocytic ehrlichiosis: state of the art. Vector Borne Zoonotic Dis. 2002;2:233-239.
- Vannier EG, Diuk-Wasser MA, Ben Mamoun C, et al. Babesiosis. Infect Dis Clin North Am. 2015;29:357-370.
- Joseph JT, Roy SS, Shams N, et al. Babesiosis in Lower Hudson Valley, New York, USA. Emerg Infect Dis. 2011;17:843-847.
- McQuiston JH, Childs JE, Chamberland ME, et al. Transmission of tickborne agents by blood transfusions: a review of known and potential risks in the United States. Transfusion. 2000;40:274-284.
- Hatcher JC, Greenberg PD, Antique J, et al. Severe babesiosis in Long Island: review of 34 cases and their complications. Clin Infect Dis. 2001;32:1117-1125.
- Healy GR, Ruebush TK. Morphology of Babesia microti in human blood smears. Am J Clin Pathol. 1980;73:107-109.
- Kowalski TJ, Jobe DA, Dolan EC, et al. The emergence of clinically relevant babesiosis in southwestern Wisconsin. WMJ. 2015;114:152-157.
- Krause PJ, Telford SR III, Spielman A, et al. Concurrent Lyme disease and babesiosis. evidence for increased severity and duration of illness. JAMA. 1996;275:1657-1660.
- Centers for Disease Control and Prevention. Statistics & maps. http://www.cdc.gov/powassan/statistics.html. Updated February 14, 2017. Accessed December 11, 2017.
- Piantadosi A, Rubin DB, McQuillen DP, et al. Emerging cases of Powassan virus encephalitis in New England: clinical presentation, imaging, and review of the literature. Clin Infect Dis. 2016;62:707-713.
- El Khoury MY, Camargo JF, White JL, et al. Potential role of deer tick virus in Powassan encephalitis cases in Lyme disease-endemic areas of New York, U.S.A. Emerg Infect Dis. 2013;19:1926-1933.
- World Health Organization (WHO). Vaccines against tick-borne encephalitis: WHO position paper. Wkly Epidemiol Rec. 2011;86:241-256.
- Centers for Disease Control and Prevention (CDC). Tick-borne encephalitis among U.S. travelers to Europe and Asia—2000-2009. JAMA. 2010;303:2132-2135.
- Valarcher JF, Hägglund S, Juremalm M, et al. Tick-borne encephalitits. Rev Sci Tech. 2015;34:453-466.
- Schultze D, Dollenmaier G, Rohner A, et al. Benefit of detecting tick-borne encephalitis viremia in the first phase of illness. J Clin Virol. 2007;38:172-175.
- Holzmann H. Diagnosis of tick-borne encephalitis. Vaccine. 2003;21(suppl 1):S36-S40.
- Zavadska D, Anca I, André F, et al. Recommendations for tick-borne encephalitis vaccination from the Central European Vaccination Awareness Group. Hum Vaccin Immunother. 2013;9:362-374.
- Gugliotta JL, Goethert HK, Berardi VP, et al. Meningoencephalitis from Borrelia miyamotoi in an immunocompromised patient. N Engl J Med. 2013;368:240-245.
- Hovius JW, de Wever B, Sohne M, et al. A case of meningoencephalitis by the relapsing fever spirochaete Borrelia miyamotoi in Europe. Lancet. 2013;382:658.
- Molloy PJ, Telford SR III, Chowdri HR, et al. Borrelia miyamotoi disease in the northeastern United States: a case series. Ann Intern Med. 2015;163:91-98.
- Telford SR 3rd, Goethert HK, Molloy PJ, et al. Borrelia miyamotoi disease: neither Lyme disease nor relapsing fever. Clin Lab Med. 2015;35:867-882.
- Diaz JH. A comparative meta-analysis of tick paralysis in the United States and Australia. Clin Toxicol (Phila). 2015;53:874-883.
- Steere AC, Grodzicki RL, Kornblatt AN, et al. The spirochetal etiology of Lyme disease. N Engl J Med. 1983;308:733-740.
- Dolan MC, Hojgaard A, Hoxmeier JC, et al. Vector competence of the blacklegged tick, Ixodes scapularis, for the recently recognized Lyme borreliosis spirochete Candidatus Borrelia mayonii. Ticks Tick Borne Dis. 2016;7:665-669.
- Rudzinska MA, Spielman A, Riek RF, et al. Intraerythrocytic ‘gametocytes’ of Babesia microti and their maturation in ticks. Can J Zool. 1979;57:424-434.
- Casals J, Olitsky PK. Enduring immunity following vaccination of mice with formalin-inactivated virus of Russian spring-summer (Far Eastern, tick-borne) encephalitis; correlation with serum-neutralizing and complement-fixing antibodies. J Exp Med. 1945;82:431-443.
- Magnarelli LA, Stafford KC III, Mather TN, et al. Hemocytic rickettsia-like organisms in ticks: serologic reactivity with antisera to Ehrlichiae and detection of DNA of agent of human granulocytic ehrlichiosis by PCR. J Clin Microbiol. 1995;33:2710-2714.
- McLean DM, Donohue WL. Powassan virus: isolation of virus from a fatal case of encephalitis. Can Med Assoc J. 1959;80:708-711.
- Platonov AE, Karan LS, Kolyasnikova NM, et al. Humans infected with relapsing fever spirochete Borrelia miyamotoi, Russia. Emerg Infect Dis. 2011;17:1816-1823.
- Diaz JH. A 60-year meta-analysis of tick paralysis in the United States: a predictable, preventable, and often misdiagnosed poisoning. J Med Toxicol. 2010;6:15-21.
- Bakken J, Dumler JS. Human granulocytic anaplasmosis. Infect Dis Clin North Am. 2015;29:341-355.
- Chapman AS, Bakken JS, Folk SM, et al; Tickborne Rickettsial Diseases Working Group; CDC. Diagnosis and management of tickborne rickettsial diseases: Rocky Mountain spotted fever, ehrlichioses, and anaplasmosis—United States: a practical guide for physicians and other health-care and public health professionals. MMWR Recomm Rep. 2006;55(RR-4):1-27.
- Dahlgren FS, Mandel EJ, Krebs JW, et al. Increasing incidence of Ehrlichia chaffeensis and Anaplasma phagocytophilum in the United States, 2000-2007. Am J Trop Med Hyg. 2011;85:124-130.
- Aguero-Rosenfeld ME. Diagnosis of human granulocytic ehrlichiosis: state of the art. Vector Borne Zoonotic Dis. 2002;2:233-239.
- Vannier EG, Diuk-Wasser MA, Ben Mamoun C, et al. Babesiosis. Infect Dis Clin North Am. 2015;29:357-370.
- Joseph JT, Roy SS, Shams N, et al. Babesiosis in Lower Hudson Valley, New York, USA. Emerg Infect Dis. 2011;17:843-847.
- McQuiston JH, Childs JE, Chamberland ME, et al. Transmission of tickborne agents by blood transfusions: a review of known and potential risks in the United States. Transfusion. 2000;40:274-284.
- Hatcher JC, Greenberg PD, Antique J, et al. Severe babesiosis in Long Island: review of 34 cases and their complications. Clin Infect Dis. 2001;32:1117-1125.
- Healy GR, Ruebush TK. Morphology of Babesia microti in human blood smears. Am J Clin Pathol. 1980;73:107-109.
- Kowalski TJ, Jobe DA, Dolan EC, et al. The emergence of clinically relevant babesiosis in southwestern Wisconsin. WMJ. 2015;114:152-157.
- Krause PJ, Telford SR III, Spielman A, et al. Concurrent Lyme disease and babesiosis. evidence for increased severity and duration of illness. JAMA. 1996;275:1657-1660.
- Centers for Disease Control and Prevention. Statistics & maps. http://www.cdc.gov/powassan/statistics.html. Updated February 14, 2017. Accessed December 11, 2017.
- Piantadosi A, Rubin DB, McQuillen DP, et al. Emerging cases of Powassan virus encephalitis in New England: clinical presentation, imaging, and review of the literature. Clin Infect Dis. 2016;62:707-713.
- El Khoury MY, Camargo JF, White JL, et al. Potential role of deer tick virus in Powassan encephalitis cases in Lyme disease-endemic areas of New York, U.S.A. Emerg Infect Dis. 2013;19:1926-1933.
- World Health Organization (WHO). Vaccines against tick-borne encephalitis: WHO position paper. Wkly Epidemiol Rec. 2011;86:241-256.
- Centers for Disease Control and Prevention (CDC). Tick-borne encephalitis among U.S. travelers to Europe and Asia—2000-2009. JAMA. 2010;303:2132-2135.
- Valarcher JF, Hägglund S, Juremalm M, et al. Tick-borne encephalitits. Rev Sci Tech. 2015;34:453-466.
- Schultze D, Dollenmaier G, Rohner A, et al. Benefit of detecting tick-borne encephalitis viremia in the first phase of illness. J Clin Virol. 2007;38:172-175.
- Holzmann H. Diagnosis of tick-borne encephalitis. Vaccine. 2003;21(suppl 1):S36-S40.
- Zavadska D, Anca I, André F, et al. Recommendations for tick-borne encephalitis vaccination from the Central European Vaccination Awareness Group. Hum Vaccin Immunother. 2013;9:362-374.
- Gugliotta JL, Goethert HK, Berardi VP, et al. Meningoencephalitis from Borrelia miyamotoi in an immunocompromised patient. N Engl J Med. 2013;368:240-245.
- Hovius JW, de Wever B, Sohne M, et al. A case of meningoencephalitis by the relapsing fever spirochaete Borrelia miyamotoi in Europe. Lancet. 2013;382:658.
- Molloy PJ, Telford SR III, Chowdri HR, et al. Borrelia miyamotoi disease in the northeastern United States: a case series. Ann Intern Med. 2015;163:91-98.
- Telford SR 3rd, Goethert HK, Molloy PJ, et al. Borrelia miyamotoi disease: neither Lyme disease nor relapsing fever. Clin Lab Med. 2015;35:867-882.
- Diaz JH. A comparative meta-analysis of tick paralysis in the United States and Australia. Clin Toxicol (Phila). 2015;53:874-883.
Practice Points
- Apart from the more familiar Borrelia burgdorferi, several less common pathogens associated with diseases transmitted by Ixodes ticks include Anaplasma phagocytophilum, Babesia microti, Borrelia miyamotoi, the Powassan virus, and the tick-borne encephalitis virus.
- Overlap in both the geographic distribution and the clinical presentations of these uncommon pathogens underscores the importance of being familiar with their capacity for causing illness and effective treatment.
- Intoxication with the saliva of some Ixodes species can cause an ascending flaccid tick paralysis.
What’s Eating You? Ixodes Tick and Related Diseases, Part 1: Life Cycle, Local Reactions, and Lyme Disease
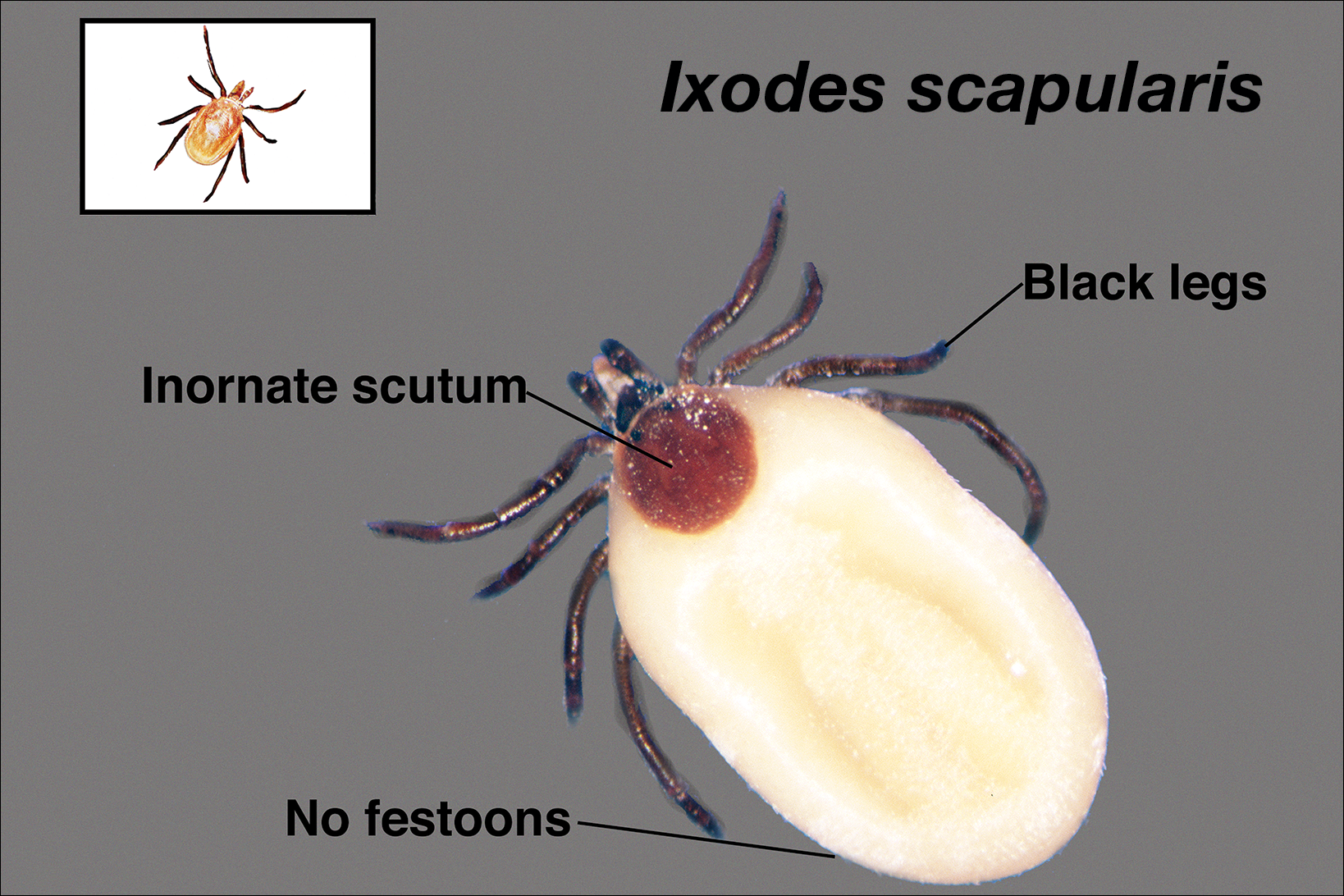
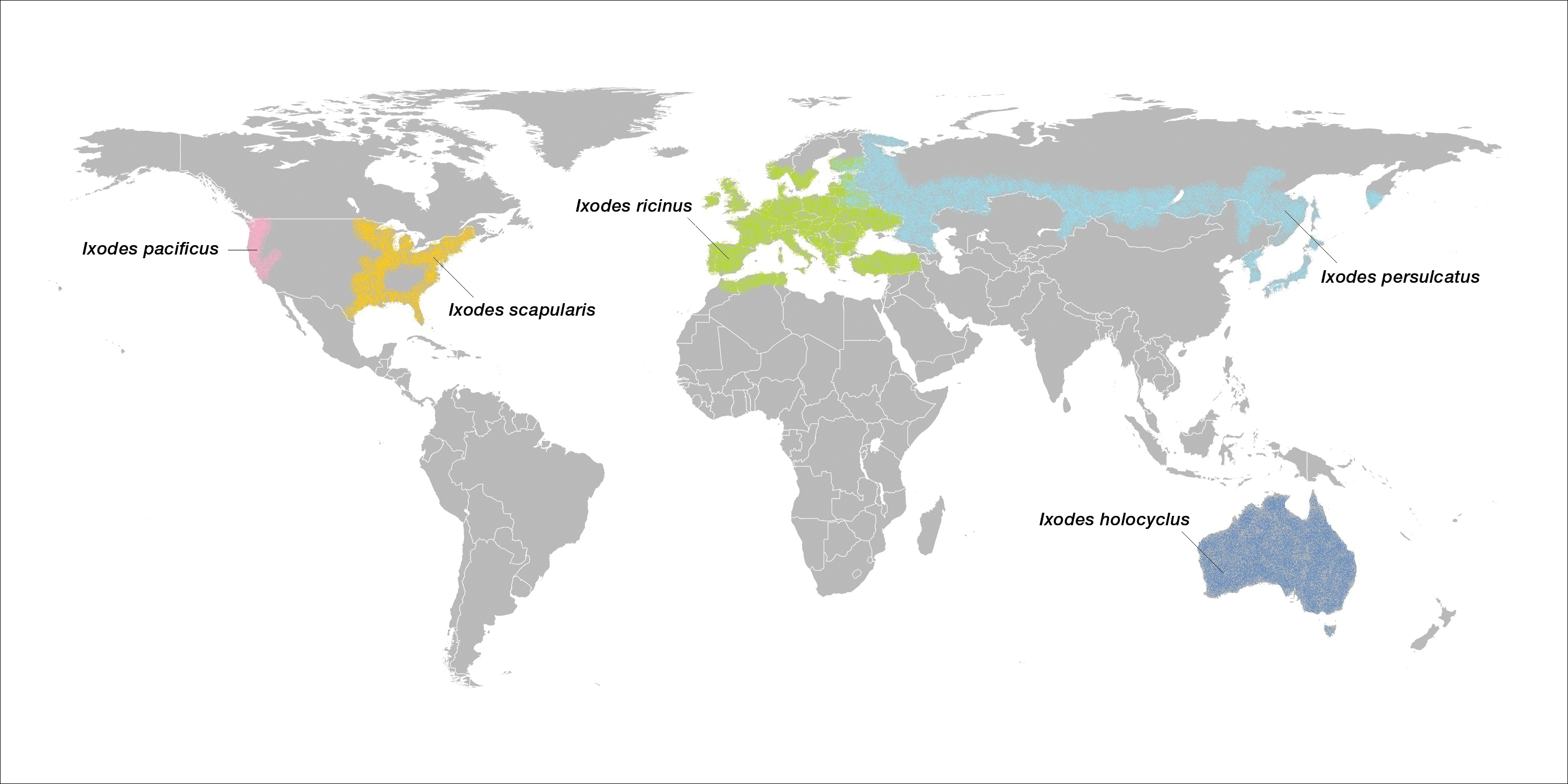
Ticks are ectoparasitic hemophages that feed on mammals, reptiles, and birds. The Ixodidae family comprises the hard ticks. A hard dorsal plate, scutum, and capitulum that extends outward from the body are features that distinguish the hard tick. 1Ixodes is the largest genus of hard ticks, with more than 250 species localized in temperate climates.2 It has an inornate scutum and lacks festoons (Figure 1).1 The Ixodes ricinus species complex accounts for most species relevant to the spread of human disease (Figure 2), with Ixodes scapularis in the northeastern, north midwestern, and southern United States; Ixodes pacificus in western United States; I ricinus in Europe and North Africa; and Ixodes persulcatus in Russia and Asia. Ixodes holocyclus is endemic to Australia.3,4
Life Cycle
Ixodes species progress through 4 life stages—egg, larvae, nymph, and adult—during their 3-host life cycle. Lifespan is 2 to 6 years, varying with environmental factors. A blood meal is required between each stage. Female ticks have a small scutum, allowing the abdomen to engorge during meals (Figure 3).

Larvae hatch in the early summer and remain dormant until the spring, emerging as a nymph. Following a blood meal, the nymph molts and reemerges as an adult in autumn. During autumn and winter, the female lays as many as 2000 eggs that emerge in early summer.5 Nymphs are small and easily undetected for the duration required for pathogen transmission, making nymphs the stage most likely to transmit disease.6
The majority of tick-borne diseases present from May to July, corresponding to nymph activity. Fewer cases present in the autumn and early spring because the adult female feeds during cooler months.7
Larvae have 6 legs and are about the size of a sesame seed when engorged. Nymphs are slightly larger with 8 legs. Adults are largest and have 8 legs. Following a blood meal, the tick becomes engorged, increasing in size and lightening in color (Figure 3).1
Ticks are found in low-lying shrubs and tall grass as well as on the forest floor. They search for a host by detecting CO2, warmth, the smell of sweat, and the color white, prompting attachment.8 Habitats hospitable to Ixodes have expanded in the wake of climate, environmental, and socioeconomic changes, potentially contributing to the increasing incidence and expansion of zoonoses associated with this vector.9,10
Local Reactions
A tick bite may induce local hypersensitivity, leading to a red papule or plaque at the bite site, followed by swelling, warmth, and erythema. A cellular immune reaction induces induration and pruritus. Hard ticks are less likely than soft ticks to cause a serious local reaction.11,12
A variety of clinical and histologic features are observed following an arthropod bite. Histologically, acute tick bites show a neutrophilic infiltrate with fibrin deposition. Chronic reactions demonstrate a wedge-shaped, mixed infiltrate with prominent endothelial swelling. Eosinophilic cellulitis, or Wells syndrome, reveals tissue eosinophilia and flame figures.13 Tick mouthparts may be identified in the tissue. B-cell hyperplasia is seen in Borrelia lymphocytoma and is more common in Europe, presenting as erythematous to plum–colored nodules on the ear and areola.14
Lyme Disease
Disease manifestations vary by location. Lyme disease is associated with Borrelia burgdorferi and the recently identified Borrelia mayonii in the United States15; in Europe and Asia, acrodermatitis chronica atrophicans is associated with Borrelia afzelii and neuroborreliosis, with Borrelia garinii. Lyme disease is the most common tick-borne illness in the United States.16 The I ricinus species complex is the most common vector harboring Borrelia species.17 At least 36 hours of tick adherence is required for disease transmission.18 The incubation period is 3 to 20 days (median, 12 days).19
Clinical Findings
Erythema migrans is the most characteristic sign, seen in 80% of cases of Lyme disease. The typical rash is a centrifugally spreading, erythematous, annular patch with central clearing at the site of the tick bite.20 Atypical rashes include vesicular, indurated, ulcerated, and follicular variants.21 Histopathology commonly shows a superficial and deep perivascular lymphocytic infiltrate with plasma cells, histiocytes, and eosinophils.22 Typically, the rash resolves in 3 to 5 weeks.18
Early disseminated Lyme disease can present with any of the following findings: multiple erythema migrans; neurologic involvement, including cranial nerve palsy and meningitis; and Lyme carditis, which may result in atrioventricular block.23,24 Late findings include arthritis, encephalopathy, and polyneuropathy. A late cutaneous manifestation, acrodermatitis chronica atrophicans, is rare in the United States but occurs in as many as 10% of Lyme disease cases in Europe. An initial inflammatory response manifests as blue-red erythema and edema of the extensor surfaces of the extremities, commonly on the dorsal hands, feet, elbows, and knees. Firm fibrotic nodules may develop later over the olecranon and patella.23,24
The term chronic Lyme disease has been used to describe the persistence of symptoms after treatment; however, large clinical trials have not detected a difference in symptom frequency between patients with a history of Lyme disease and matched controls.25,26 Many patients with chronic Lyme disease may instead have posttreatment Lyme disease syndrome, described as nonspecific symptoms including fatigue, arthralgia, and decreased mental acuity following treatment of confirmed Lyme disease. Symptoms generally improve within 1 year.27
Laboratory Testing
The gold standard for laboratory diagnosis of Lyme disease is 2-tiered serologic testing. First, an enzyme immunoassay or immunofluorescence assay is used to screen for antibodies. A Western blot follows if the result of the screen is positive or equivocal. Western blot testing for IgM and IgG is used when illness duration is less than 4 weeks; after 4 weeks, a Western blot for IgG alone is sufficient.27,28 The 2-tiered test has 99% specificity. Sensitivity increases with duration of disease (29%–40% with erythema migrans; 42%–87% in early disseminated disease; 97%–100% in late disease).29,30 A false-positive result can occur in the presence of infectious mononucleosis, an autoimmune disorder, and syphilis. If serologic testing is negative and suspicion remains high, testing should be repeated in 2 to 4 weeks.31 When a patient in a Lyme-endemic area presents with typical erythema migrans, serologic testing is unnecessary prior to treatment.32
Management
Treatment of Lyme disease centers on antibiotic therapy (Table). First-line treatment of early disseminated disease is doxycycline for 14 days (range, 10–21 days).27 In pregnant women, children younger than 8 years, and tetracycline-allergic patients, amoxicillin or cefuroxime axetil for 14 days (range, 14–21 days) may be used.33 For erythema migrans without complications, doxycycline for 10 days is effective. Complications that require hospitalization are treated with intravenous ceftriaxone.27 Re-treatment in patients with posttreatment Lyme disease syndrome is not recommended.34 Prophylaxis with a single dose of doxycycline 200 mg may be indicated when all of the following conditions are met: (1) the patient is in an area where more than 20% of Ixodes ticks are infected with B burgdorferi, (2) the attached tick is I scapularis, (3) the tick has been attached for more than 36 hours, and (4) treatment is begun within 72 hours of tick removal.27
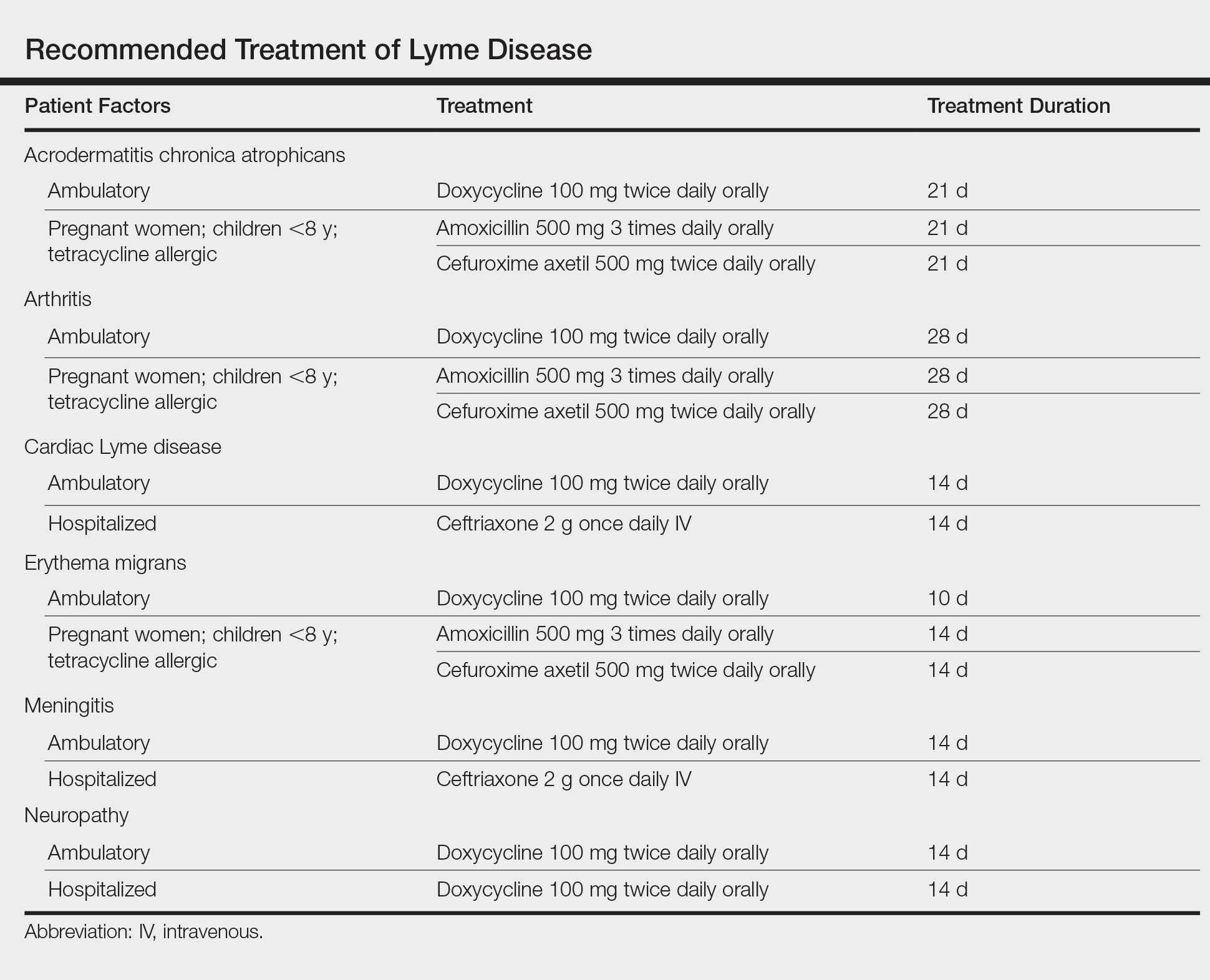
- Anderson JF, Magnarelli LA. Biology of ticks. Infect Dis Clin North Am. 2008;22:195-215.
- Jongejan F, Uilenberg G. The global importance of ticks. Parasitology. 2004;129(suppl):S3-S14.
- Xu G, Fang QQ, Keirans JE, et al. Molecular phylogenetic analyses indicate that the Ixodes ricinus complex is a paraphyletic group. J Parasitol. 2003;89:452-457.
- Swanson SJ, Neitzel D, Reed DK, et al. Coinfections acquired from Ixodes ticks. Clin Microbiol Rev. 2006;19:708-727.
- Mathison BA, Pritt BS. Laboratory identification of arthropod ectoparasites. Clin Microbol Rev. 2014;27:48-67.
- Falco RC, Fish D, Piesman J. Duration of tick bites in a Lyme disease-endemic area. Am J Epidemiol. 1996;143:187-192.
- Centers for Disease Control and Prevention. Lyme disease graphs. http://www.cdc.gov/lyme/stats/graphs.html. Updated November 21, 2016. Accessed November 21, 2017.
- Randolph SE. The impact of tick ecology on pathogen transmission dynamics. In: Bowman AS, Nuttall PA, eds. Ticks: Biology, Disease and Control. Cambridge, UK: Cambridge University Press; 2008:40-72.
- Ostfeld RS, Brunner JL. Climate change and Ixodes tick-borne diseases of humans. Philos Trans R Soc Lond B Biol Sci. 2015;370. pii:20140051. doi:10.1098/rstb.2014.0051.
- Medlock JM, Hansford KM, Bormane A, et al. Driving forces for changes in geographical distribution of Ixodes ricinus ticks in Europe. Parasit Vectors. 2013;6:1.
- McGinley-Smith DE, Tsao SS. Dermatoses from ticks. J Am Acad Dermatol. 2003;49:393-396.
- Middleton DB. Tick-borne infections. What starts as a tiny bite may have a serious outcome. Postgrad Med. 1994;95:131-139.
- Melski JW. Wells’ syndrome, insect bites, and eosinophils. Dermatol Clin. 2015;8:287-293.
- Castelli E, Caputo V, Morello V, et al. Local reactions to tick bites. Am J Dermatopathol. 2008;30:241-248.
- Pritt BS, Mead PS, Johnson DK, et al. Identification of a novel pathogenic Borrelia species causing Lyme borreliosis with unusually high spirochaetaemia: a descriptive study. Lancet Infect Dis. 2016;16:556-564.
- Orloski KA, Hayes EB, Campbell GL, et al. Surveillance for Lyme disease—United States, 1992-1998. MMWR CDC Surveill Summ. 2000;49:1-11.
- Gray JS. The ecology of ticks transmitting Lyme borreliosis. Exp Appl Acarol. 1998;22:249-258.
- Piesman J, Mather TN, Sinsky RJ, et al. Duration of tick attachment and Borrelia burgdorferi transmission. J Clin Microbiol. 1987;25:557-558.
- Richardson M, Elliman D, Maguire H, et al. Evidence base of incubation periods, periods of infectiousness and exclusion policies for the control of communicable diseases in schools and preschools. Pediatr Infect Dis J. 2001;20:380-391.
- Myers SA, Sexton DJ. Dermatologic manifestations of arthropod-borne diseases. Infect Dis Clin North Am. 1994;8:689-712.
- Ducroux E, Debarbieux S, Boibieux A, et al. Follicular borreliosis: an atypical presentation of erythema chronicum migrans. Dermatology. 2009;219:84-85.
- Miraflor AP, Seidel GD, Perry AE, et al. The many masks of cutaneous Lyme disease. J Cutan Pathol. 2016:43:32-40.
- Lenormand C, Jaulhac B, Debarbieux S, et al. Expanding the clinicopathological spectrum of late cutaneous Lyme borreliosis (acrodermatitis chronica atrophicans): a prospective study of 20 culture and/or polymerase chain reaction (PCR) documented cases. J Am Acad Dermatol. 2016;74:685-692.
- Zajkowska J, Czupryna P, Pancewicz SA, et al. Acrodermatitis chronica atrophicans. Lancet Infect Dis. 2011;11:800.
- Seltzer EG, Gerber MA, Cartter ML, et al. Long-term outcomes of persons with Lyme disease. JAMA. 2000;283:609-616.
- Shadick NA, Phillips CB, Sangha O, et al. Musculoskeletal and neurologic outcomes in patients with previously treated Lyme disease. Ann Intern Med. 1999;131:919-926.
- Wormser GP, Dattwyler RJ, Shapiro ED, et al. The clinical assessment, treatment, and prevention of Lyme disease, human granulocytic anaplasmosis, and babesiosis: clinical practice guidelines by the Infectious Diseases Society of America. Clin Infect Dis. 2006;43:1089-1134.
- Schriefer ME. Lyme disease diagnosis: serology. Clin Lab Med. 2015;35:797-814.
- Wormser GP, Nowakowski J, Nadelman RB, et al. Impact of clinical variables on Borrelia burgdorferi-specific antibody seropositivity in acute-phase sera from patients in North America with culture-confirmed early Lyme disease. Clin Vaccine Immunol. 2008;15:1519-1522.
- Leeflang MM, Ang CW, Berkhout J, et al. The diagnostic accuracy of serological tests for Lyme borreliosis in Europe: a systematic review and meta-analysis. BMC Infect Dis. 2016;16:140.
- Sanchez E, Vannier E, Wormser GP, et al. Diagnosis, treatment, and prevention of Lyme disease, human granulocytic anaplasmosis, and babesiosis: a review. JAMA. 2016;315:1767-1777.
- Lantos PM, Brinkerhoff RJ, Wormser GP, et al. Empiric antibiotic treatment of erythema migrans-like skin lesions as a function of geography: a clinical and cost effectiveness modeling study. Vector Borne Zoonotic Dis. 2013;13:877-883.
- Smith GN, Gemmill I, Moore KM. Management of tick bites and Lyme disease during pregnancy. J Obstet Gynaecol Can. 2012;34:1087-1091.
- Berende A, ter Hofstede HJ, Vos FJ, et al. Randomized trial of longer-term therapy for symptoms attributed to Lyme disease. N Engl J Med. 2016;374:1209-1220.
Ticks are ectoparasitic hemophages that feed on mammals, reptiles, and birds. The Ixodidae family comprises the hard ticks. A hard dorsal plate, scutum, and capitulum that extends outward from the body are features that distinguish the hard tick. 1Ixodes is the largest genus of hard ticks, with more than 250 species localized in temperate climates.2 It has an inornate scutum and lacks festoons (Figure 1).1 The Ixodes ricinus species complex accounts for most species relevant to the spread of human disease (Figure 2), with Ixodes scapularis in the northeastern, north midwestern, and southern United States; Ixodes pacificus in western United States; I ricinus in Europe and North Africa; and Ixodes persulcatus in Russia and Asia. Ixodes holocyclus is endemic to Australia.3,4


Life Cycle
Ixodes species progress through 4 life stages—egg, larvae, nymph, and adult—during their 3-host life cycle. Lifespan is 2 to 6 years, varying with environmental factors. A blood meal is required between each stage. Female ticks have a small scutum, allowing the abdomen to engorge during meals (Figure 3).

Larvae hatch in the early summer and remain dormant until the spring, emerging as a nymph. Following a blood meal, the nymph molts and reemerges as an adult in autumn. During autumn and winter, the female lays as many as 2000 eggs that emerge in early summer.5 Nymphs are small and easily undetected for the duration required for pathogen transmission, making nymphs the stage most likely to transmit disease.6
The majority of tick-borne diseases present from May to July, corresponding to nymph activity. Fewer cases present in the autumn and early spring because the adult female feeds during cooler months.7
Larvae have 6 legs and are about the size of a sesame seed when engorged. Nymphs are slightly larger with 8 legs. Adults are largest and have 8 legs. Following a blood meal, the tick becomes engorged, increasing in size and lightening in color (Figure 3).1
Ticks are found in low-lying shrubs and tall grass as well as on the forest floor. They search for a host by detecting CO2, warmth, the smell of sweat, and the color white, prompting attachment.8 Habitats hospitable to Ixodes have expanded in the wake of climate, environmental, and socioeconomic changes, potentially contributing to the increasing incidence and expansion of zoonoses associated with this vector.9,10
Local Reactions
A tick bite may induce local hypersensitivity, leading to a red papule or plaque at the bite site, followed by swelling, warmth, and erythema. A cellular immune reaction induces induration and pruritus. Hard ticks are less likely than soft ticks to cause a serious local reaction.11,12
A variety of clinical and histologic features are observed following an arthropod bite. Histologically, acute tick bites show a neutrophilic infiltrate with fibrin deposition. Chronic reactions demonstrate a wedge-shaped, mixed infiltrate with prominent endothelial swelling. Eosinophilic cellulitis, or Wells syndrome, reveals tissue eosinophilia and flame figures.13 Tick mouthparts may be identified in the tissue. B-cell hyperplasia is seen in Borrelia lymphocytoma and is more common in Europe, presenting as erythematous to plum–colored nodules on the ear and areola.14
Lyme Disease
Disease manifestations vary by location. Lyme disease is associated with Borrelia burgdorferi and the recently identified Borrelia mayonii in the United States15; in Europe and Asia, acrodermatitis chronica atrophicans is associated with Borrelia afzelii and neuroborreliosis, with Borrelia garinii. Lyme disease is the most common tick-borne illness in the United States.16 The I ricinus species complex is the most common vector harboring Borrelia species.17 At least 36 hours of tick adherence is required for disease transmission.18 The incubation period is 3 to 20 days (median, 12 days).19
Clinical Findings
Erythema migrans is the most characteristic sign, seen in 80% of cases of Lyme disease. The typical rash is a centrifugally spreading, erythematous, annular patch with central clearing at the site of the tick bite.20 Atypical rashes include vesicular, indurated, ulcerated, and follicular variants.21 Histopathology commonly shows a superficial and deep perivascular lymphocytic infiltrate with plasma cells, histiocytes, and eosinophils.22 Typically, the rash resolves in 3 to 5 weeks.18
Early disseminated Lyme disease can present with any of the following findings: multiple erythema migrans; neurologic involvement, including cranial nerve palsy and meningitis; and Lyme carditis, which may result in atrioventricular block.23,24 Late findings include arthritis, encephalopathy, and polyneuropathy. A late cutaneous manifestation, acrodermatitis chronica atrophicans, is rare in the United States but occurs in as many as 10% of Lyme disease cases in Europe. An initial inflammatory response manifests as blue-red erythema and edema of the extensor surfaces of the extremities, commonly on the dorsal hands, feet, elbows, and knees. Firm fibrotic nodules may develop later over the olecranon and patella.23,24
The term chronic Lyme disease has been used to describe the persistence of symptoms after treatment; however, large clinical trials have not detected a difference in symptom frequency between patients with a history of Lyme disease and matched controls.25,26 Many patients with chronic Lyme disease may instead have posttreatment Lyme disease syndrome, described as nonspecific symptoms including fatigue, arthralgia, and decreased mental acuity following treatment of confirmed Lyme disease. Symptoms generally improve within 1 year.27
Laboratory Testing
The gold standard for laboratory diagnosis of Lyme disease is 2-tiered serologic testing. First, an enzyme immunoassay or immunofluorescence assay is used to screen for antibodies. A Western blot follows if the result of the screen is positive or equivocal. Western blot testing for IgM and IgG is used when illness duration is less than 4 weeks; after 4 weeks, a Western blot for IgG alone is sufficient.27,28 The 2-tiered test has 99% specificity. Sensitivity increases with duration of disease (29%–40% with erythema migrans; 42%–87% in early disseminated disease; 97%–100% in late disease).29,30 A false-positive result can occur in the presence of infectious mononucleosis, an autoimmune disorder, and syphilis. If serologic testing is negative and suspicion remains high, testing should be repeated in 2 to 4 weeks.31 When a patient in a Lyme-endemic area presents with typical erythema migrans, serologic testing is unnecessary prior to treatment.32
Management
Treatment of Lyme disease centers on antibiotic therapy (Table). First-line treatment of early disseminated disease is doxycycline for 14 days (range, 10–21 days).27 In pregnant women, children younger than 8 years, and tetracycline-allergic patients, amoxicillin or cefuroxime axetil for 14 days (range, 14–21 days) may be used.33 For erythema migrans without complications, doxycycline for 10 days is effective. Complications that require hospitalization are treated with intravenous ceftriaxone.27 Re-treatment in patients with posttreatment Lyme disease syndrome is not recommended.34 Prophylaxis with a single dose of doxycycline 200 mg may be indicated when all of the following conditions are met: (1) the patient is in an area where more than 20% of Ixodes ticks are infected with B burgdorferi, (2) the attached tick is I scapularis, (3) the tick has been attached for more than 36 hours, and (4) treatment is begun within 72 hours of tick removal.27

Ticks are ectoparasitic hemophages that feed on mammals, reptiles, and birds. The Ixodidae family comprises the hard ticks. A hard dorsal plate, scutum, and capitulum that extends outward from the body are features that distinguish the hard tick. 1Ixodes is the largest genus of hard ticks, with more than 250 species localized in temperate climates.2 It has an inornate scutum and lacks festoons (Figure 1).1 The Ixodes ricinus species complex accounts for most species relevant to the spread of human disease (Figure 2), with Ixodes scapularis in the northeastern, north midwestern, and southern United States; Ixodes pacificus in western United States; I ricinus in Europe and North Africa; and Ixodes persulcatus in Russia and Asia. Ixodes holocyclus is endemic to Australia.3,4


Life Cycle
Ixodes species progress through 4 life stages—egg, larvae, nymph, and adult—during their 3-host life cycle. Lifespan is 2 to 6 years, varying with environmental factors. A blood meal is required between each stage. Female ticks have a small scutum, allowing the abdomen to engorge during meals (Figure 3).

Larvae hatch in the early summer and remain dormant until the spring, emerging as a nymph. Following a blood meal, the nymph molts and reemerges as an adult in autumn. During autumn and winter, the female lays as many as 2000 eggs that emerge in early summer.5 Nymphs are small and easily undetected for the duration required for pathogen transmission, making nymphs the stage most likely to transmit disease.6
The majority of tick-borne diseases present from May to July, corresponding to nymph activity. Fewer cases present in the autumn and early spring because the adult female feeds during cooler months.7
Larvae have 6 legs and are about the size of a sesame seed when engorged. Nymphs are slightly larger with 8 legs. Adults are largest and have 8 legs. Following a blood meal, the tick becomes engorged, increasing in size and lightening in color (Figure 3).1
Ticks are found in low-lying shrubs and tall grass as well as on the forest floor. They search for a host by detecting CO2, warmth, the smell of sweat, and the color white, prompting attachment.8 Habitats hospitable to Ixodes have expanded in the wake of climate, environmental, and socioeconomic changes, potentially contributing to the increasing incidence and expansion of zoonoses associated with this vector.9,10
Local Reactions
A tick bite may induce local hypersensitivity, leading to a red papule or plaque at the bite site, followed by swelling, warmth, and erythema. A cellular immune reaction induces induration and pruritus. Hard ticks are less likely than soft ticks to cause a serious local reaction.11,12
A variety of clinical and histologic features are observed following an arthropod bite. Histologically, acute tick bites show a neutrophilic infiltrate with fibrin deposition. Chronic reactions demonstrate a wedge-shaped, mixed infiltrate with prominent endothelial swelling. Eosinophilic cellulitis, or Wells syndrome, reveals tissue eosinophilia and flame figures.13 Tick mouthparts may be identified in the tissue. B-cell hyperplasia is seen in Borrelia lymphocytoma and is more common in Europe, presenting as erythematous to plum–colored nodules on the ear and areola.14
Lyme Disease
Disease manifestations vary by location. Lyme disease is associated with Borrelia burgdorferi and the recently identified Borrelia mayonii in the United States15; in Europe and Asia, acrodermatitis chronica atrophicans is associated with Borrelia afzelii and neuroborreliosis, with Borrelia garinii. Lyme disease is the most common tick-borne illness in the United States.16 The I ricinus species complex is the most common vector harboring Borrelia species.17 At least 36 hours of tick adherence is required for disease transmission.18 The incubation period is 3 to 20 days (median, 12 days).19
Clinical Findings
Erythema migrans is the most characteristic sign, seen in 80% of cases of Lyme disease. The typical rash is a centrifugally spreading, erythematous, annular patch with central clearing at the site of the tick bite.20 Atypical rashes include vesicular, indurated, ulcerated, and follicular variants.21 Histopathology commonly shows a superficial and deep perivascular lymphocytic infiltrate with plasma cells, histiocytes, and eosinophils.22 Typically, the rash resolves in 3 to 5 weeks.18
Early disseminated Lyme disease can present with any of the following findings: multiple erythema migrans; neurologic involvement, including cranial nerve palsy and meningitis; and Lyme carditis, which may result in atrioventricular block.23,24 Late findings include arthritis, encephalopathy, and polyneuropathy. A late cutaneous manifestation, acrodermatitis chronica atrophicans, is rare in the United States but occurs in as many as 10% of Lyme disease cases in Europe. An initial inflammatory response manifests as blue-red erythema and edema of the extensor surfaces of the extremities, commonly on the dorsal hands, feet, elbows, and knees. Firm fibrotic nodules may develop later over the olecranon and patella.23,24
The term chronic Lyme disease has been used to describe the persistence of symptoms after treatment; however, large clinical trials have not detected a difference in symptom frequency between patients with a history of Lyme disease and matched controls.25,26 Many patients with chronic Lyme disease may instead have posttreatment Lyme disease syndrome, described as nonspecific symptoms including fatigue, arthralgia, and decreased mental acuity following treatment of confirmed Lyme disease. Symptoms generally improve within 1 year.27
Laboratory Testing
The gold standard for laboratory diagnosis of Lyme disease is 2-tiered serologic testing. First, an enzyme immunoassay or immunofluorescence assay is used to screen for antibodies. A Western blot follows if the result of the screen is positive or equivocal. Western blot testing for IgM and IgG is used when illness duration is less than 4 weeks; after 4 weeks, a Western blot for IgG alone is sufficient.27,28 The 2-tiered test has 99% specificity. Sensitivity increases with duration of disease (29%–40% with erythema migrans; 42%–87% in early disseminated disease; 97%–100% in late disease).29,30 A false-positive result can occur in the presence of infectious mononucleosis, an autoimmune disorder, and syphilis. If serologic testing is negative and suspicion remains high, testing should be repeated in 2 to 4 weeks.31 When a patient in a Lyme-endemic area presents with typical erythema migrans, serologic testing is unnecessary prior to treatment.32
Management
Treatment of Lyme disease centers on antibiotic therapy (Table). First-line treatment of early disseminated disease is doxycycline for 14 days (range, 10–21 days).27 In pregnant women, children younger than 8 years, and tetracycline-allergic patients, amoxicillin or cefuroxime axetil for 14 days (range, 14–21 days) may be used.33 For erythema migrans without complications, doxycycline for 10 days is effective. Complications that require hospitalization are treated with intravenous ceftriaxone.27 Re-treatment in patients with posttreatment Lyme disease syndrome is not recommended.34 Prophylaxis with a single dose of doxycycline 200 mg may be indicated when all of the following conditions are met: (1) the patient is in an area where more than 20% of Ixodes ticks are infected with B burgdorferi, (2) the attached tick is I scapularis, (3) the tick has been attached for more than 36 hours, and (4) treatment is begun within 72 hours of tick removal.27

- Anderson JF, Magnarelli LA. Biology of ticks. Infect Dis Clin North Am. 2008;22:195-215.
- Jongejan F, Uilenberg G. The global importance of ticks. Parasitology. 2004;129(suppl):S3-S14.
- Xu G, Fang QQ, Keirans JE, et al. Molecular phylogenetic analyses indicate that the Ixodes ricinus complex is a paraphyletic group. J Parasitol. 2003;89:452-457.
- Swanson SJ, Neitzel D, Reed DK, et al. Coinfections acquired from Ixodes ticks. Clin Microbiol Rev. 2006;19:708-727.
- Mathison BA, Pritt BS. Laboratory identification of arthropod ectoparasites. Clin Microbol Rev. 2014;27:48-67.
- Falco RC, Fish D, Piesman J. Duration of tick bites in a Lyme disease-endemic area. Am J Epidemiol. 1996;143:187-192.
- Centers for Disease Control and Prevention. Lyme disease graphs. http://www.cdc.gov/lyme/stats/graphs.html. Updated November 21, 2016. Accessed November 21, 2017.
- Randolph SE. The impact of tick ecology on pathogen transmission dynamics. In: Bowman AS, Nuttall PA, eds. Ticks: Biology, Disease and Control. Cambridge, UK: Cambridge University Press; 2008:40-72.
- Ostfeld RS, Brunner JL. Climate change and Ixodes tick-borne diseases of humans. Philos Trans R Soc Lond B Biol Sci. 2015;370. pii:20140051. doi:10.1098/rstb.2014.0051.
- Medlock JM, Hansford KM, Bormane A, et al. Driving forces for changes in geographical distribution of Ixodes ricinus ticks in Europe. Parasit Vectors. 2013;6:1.
- McGinley-Smith DE, Tsao SS. Dermatoses from ticks. J Am Acad Dermatol. 2003;49:393-396.
- Middleton DB. Tick-borne infections. What starts as a tiny bite may have a serious outcome. Postgrad Med. 1994;95:131-139.
- Melski JW. Wells’ syndrome, insect bites, and eosinophils. Dermatol Clin. 2015;8:287-293.
- Castelli E, Caputo V, Morello V, et al. Local reactions to tick bites. Am J Dermatopathol. 2008;30:241-248.
- Pritt BS, Mead PS, Johnson DK, et al. Identification of a novel pathogenic Borrelia species causing Lyme borreliosis with unusually high spirochaetaemia: a descriptive study. Lancet Infect Dis. 2016;16:556-564.
- Orloski KA, Hayes EB, Campbell GL, et al. Surveillance for Lyme disease—United States, 1992-1998. MMWR CDC Surveill Summ. 2000;49:1-11.
- Gray JS. The ecology of ticks transmitting Lyme borreliosis. Exp Appl Acarol. 1998;22:249-258.
- Piesman J, Mather TN, Sinsky RJ, et al. Duration of tick attachment and Borrelia burgdorferi transmission. J Clin Microbiol. 1987;25:557-558.
- Richardson M, Elliman D, Maguire H, et al. Evidence base of incubation periods, periods of infectiousness and exclusion policies for the control of communicable diseases in schools and preschools. Pediatr Infect Dis J. 2001;20:380-391.
- Myers SA, Sexton DJ. Dermatologic manifestations of arthropod-borne diseases. Infect Dis Clin North Am. 1994;8:689-712.
- Ducroux E, Debarbieux S, Boibieux A, et al. Follicular borreliosis: an atypical presentation of erythema chronicum migrans. Dermatology. 2009;219:84-85.
- Miraflor AP, Seidel GD, Perry AE, et al. The many masks of cutaneous Lyme disease. J Cutan Pathol. 2016:43:32-40.
- Lenormand C, Jaulhac B, Debarbieux S, et al. Expanding the clinicopathological spectrum of late cutaneous Lyme borreliosis (acrodermatitis chronica atrophicans): a prospective study of 20 culture and/or polymerase chain reaction (PCR) documented cases. J Am Acad Dermatol. 2016;74:685-692.
- Zajkowska J, Czupryna P, Pancewicz SA, et al. Acrodermatitis chronica atrophicans. Lancet Infect Dis. 2011;11:800.
- Seltzer EG, Gerber MA, Cartter ML, et al. Long-term outcomes of persons with Lyme disease. JAMA. 2000;283:609-616.
- Shadick NA, Phillips CB, Sangha O, et al. Musculoskeletal and neurologic outcomes in patients with previously treated Lyme disease. Ann Intern Med. 1999;131:919-926.
- Wormser GP, Dattwyler RJ, Shapiro ED, et al. The clinical assessment, treatment, and prevention of Lyme disease, human granulocytic anaplasmosis, and babesiosis: clinical practice guidelines by the Infectious Diseases Society of America. Clin Infect Dis. 2006;43:1089-1134.
- Schriefer ME. Lyme disease diagnosis: serology. Clin Lab Med. 2015;35:797-814.
- Wormser GP, Nowakowski J, Nadelman RB, et al. Impact of clinical variables on Borrelia burgdorferi-specific antibody seropositivity in acute-phase sera from patients in North America with culture-confirmed early Lyme disease. Clin Vaccine Immunol. 2008;15:1519-1522.
- Leeflang MM, Ang CW, Berkhout J, et al. The diagnostic accuracy of serological tests for Lyme borreliosis in Europe: a systematic review and meta-analysis. BMC Infect Dis. 2016;16:140.
- Sanchez E, Vannier E, Wormser GP, et al. Diagnosis, treatment, and prevention of Lyme disease, human granulocytic anaplasmosis, and babesiosis: a review. JAMA. 2016;315:1767-1777.
- Lantos PM, Brinkerhoff RJ, Wormser GP, et al. Empiric antibiotic treatment of erythema migrans-like skin lesions as a function of geography: a clinical and cost effectiveness modeling study. Vector Borne Zoonotic Dis. 2013;13:877-883.
- Smith GN, Gemmill I, Moore KM. Management of tick bites and Lyme disease during pregnancy. J Obstet Gynaecol Can. 2012;34:1087-1091.
- Berende A, ter Hofstede HJ, Vos FJ, et al. Randomized trial of longer-term therapy for symptoms attributed to Lyme disease. N Engl J Med. 2016;374:1209-1220.
- Anderson JF, Magnarelli LA. Biology of ticks. Infect Dis Clin North Am. 2008;22:195-215.
- Jongejan F, Uilenberg G. The global importance of ticks. Parasitology. 2004;129(suppl):S3-S14.
- Xu G, Fang QQ, Keirans JE, et al. Molecular phylogenetic analyses indicate that the Ixodes ricinus complex is a paraphyletic group. J Parasitol. 2003;89:452-457.
- Swanson SJ, Neitzel D, Reed DK, et al. Coinfections acquired from Ixodes ticks. Clin Microbiol Rev. 2006;19:708-727.
- Mathison BA, Pritt BS. Laboratory identification of arthropod ectoparasites. Clin Microbol Rev. 2014;27:48-67.
- Falco RC, Fish D, Piesman J. Duration of tick bites in a Lyme disease-endemic area. Am J Epidemiol. 1996;143:187-192.
- Centers for Disease Control and Prevention. Lyme disease graphs. http://www.cdc.gov/lyme/stats/graphs.html. Updated November 21, 2016. Accessed November 21, 2017.
- Randolph SE. The impact of tick ecology on pathogen transmission dynamics. In: Bowman AS, Nuttall PA, eds. Ticks: Biology, Disease and Control. Cambridge, UK: Cambridge University Press; 2008:40-72.
- Ostfeld RS, Brunner JL. Climate change and Ixodes tick-borne diseases of humans. Philos Trans R Soc Lond B Biol Sci. 2015;370. pii:20140051. doi:10.1098/rstb.2014.0051.
- Medlock JM, Hansford KM, Bormane A, et al. Driving forces for changes in geographical distribution of Ixodes ricinus ticks in Europe. Parasit Vectors. 2013;6:1.
- McGinley-Smith DE, Tsao SS. Dermatoses from ticks. J Am Acad Dermatol. 2003;49:393-396.
- Middleton DB. Tick-borne infections. What starts as a tiny bite may have a serious outcome. Postgrad Med. 1994;95:131-139.
- Melski JW. Wells’ syndrome, insect bites, and eosinophils. Dermatol Clin. 2015;8:287-293.
- Castelli E, Caputo V, Morello V, et al. Local reactions to tick bites. Am J Dermatopathol. 2008;30:241-248.
- Pritt BS, Mead PS, Johnson DK, et al. Identification of a novel pathogenic Borrelia species causing Lyme borreliosis with unusually high spirochaetaemia: a descriptive study. Lancet Infect Dis. 2016;16:556-564.
- Orloski KA, Hayes EB, Campbell GL, et al. Surveillance for Lyme disease—United States, 1992-1998. MMWR CDC Surveill Summ. 2000;49:1-11.
- Gray JS. The ecology of ticks transmitting Lyme borreliosis. Exp Appl Acarol. 1998;22:249-258.
- Piesman J, Mather TN, Sinsky RJ, et al. Duration of tick attachment and Borrelia burgdorferi transmission. J Clin Microbiol. 1987;25:557-558.
- Richardson M, Elliman D, Maguire H, et al. Evidence base of incubation periods, periods of infectiousness and exclusion policies for the control of communicable diseases in schools and preschools. Pediatr Infect Dis J. 2001;20:380-391.
- Myers SA, Sexton DJ. Dermatologic manifestations of arthropod-borne diseases. Infect Dis Clin North Am. 1994;8:689-712.
- Ducroux E, Debarbieux S, Boibieux A, et al. Follicular borreliosis: an atypical presentation of erythema chronicum migrans. Dermatology. 2009;219:84-85.
- Miraflor AP, Seidel GD, Perry AE, et al. The many masks of cutaneous Lyme disease. J Cutan Pathol. 2016:43:32-40.
- Lenormand C, Jaulhac B, Debarbieux S, et al. Expanding the clinicopathological spectrum of late cutaneous Lyme borreliosis (acrodermatitis chronica atrophicans): a prospective study of 20 culture and/or polymerase chain reaction (PCR) documented cases. J Am Acad Dermatol. 2016;74:685-692.
- Zajkowska J, Czupryna P, Pancewicz SA, et al. Acrodermatitis chronica atrophicans. Lancet Infect Dis. 2011;11:800.
- Seltzer EG, Gerber MA, Cartter ML, et al. Long-term outcomes of persons with Lyme disease. JAMA. 2000;283:609-616.
- Shadick NA, Phillips CB, Sangha O, et al. Musculoskeletal and neurologic outcomes in patients with previously treated Lyme disease. Ann Intern Med. 1999;131:919-926.
- Wormser GP, Dattwyler RJ, Shapiro ED, et al. The clinical assessment, treatment, and prevention of Lyme disease, human granulocytic anaplasmosis, and babesiosis: clinical practice guidelines by the Infectious Diseases Society of America. Clin Infect Dis. 2006;43:1089-1134.
- Schriefer ME. Lyme disease diagnosis: serology. Clin Lab Med. 2015;35:797-814.
- Wormser GP, Nowakowski J, Nadelman RB, et al. Impact of clinical variables on Borrelia burgdorferi-specific antibody seropositivity in acute-phase sera from patients in North America with culture-confirmed early Lyme disease. Clin Vaccine Immunol. 2008;15:1519-1522.
- Leeflang MM, Ang CW, Berkhout J, et al. The diagnostic accuracy of serological tests for Lyme borreliosis in Europe: a systematic review and meta-analysis. BMC Infect Dis. 2016;16:140.
- Sanchez E, Vannier E, Wormser GP, et al. Diagnosis, treatment, and prevention of Lyme disease, human granulocytic anaplasmosis, and babesiosis: a review. JAMA. 2016;315:1767-1777.
- Lantos PM, Brinkerhoff RJ, Wormser GP, et al. Empiric antibiotic treatment of erythema migrans-like skin lesions as a function of geography: a clinical and cost effectiveness modeling study. Vector Borne Zoonotic Dis. 2013;13:877-883.
- Smith GN, Gemmill I, Moore KM. Management of tick bites and Lyme disease during pregnancy. J Obstet Gynaecol Can. 2012;34:1087-1091.
- Berende A, ter Hofstede HJ, Vos FJ, et al. Randomized trial of longer-term therapy for symptoms attributed to Lyme disease. N Engl J Med. 2016;374:1209-1220.
Practice Points
- Lyme disease is transmitted by Ixodes ticks in the northeastern, midwestern, and far western United States.
- Most tick-borne illnesses, including Lyme disease, respond to treatment with doxycycline.
- Babesiosis, a malarialike illness, can be transmitted concurrently with Lyme disease.