User login
The effect of centralizing breast cancer care in an urban public hospital
When cancer care is centralized in a comprehensive fashion, the quality of care and the outcomes improve.1,2 Unfortunately, because of the medical insurance structure in New York City, most patients of lower socioeconomic status do not receive their cancer care in such dedicated cancer centers. In New York City, the majority of the underserved vulnerable populations – that is, those without health insurance – receive their care from the public hospital system known as NYC Health and Hospitals. Cancer care in this system is not centralized and may result in fragmented implementation of various modalities of treatment. In addition, because there is no centralized care, needs such as early screening and prevention programs are often not addressed. This problem was evident in Queens in 2000 and before when many patients with late-stage cancers were presenting for cancer care. Queens, which is one of the 5 boroughs of New York City, has more than 2.3 million residents. It has 2 public hospitals, Elmhurst Hospital Center and Queens Hospital Center (QHC). In 2001, the plan was devised for the establishment of a cancer center at QHC, mainly because of the high rate of late-stage cancers that were being seen at presentation and recognition of the need for more comprehensive care. In 2002, the Queens Cancer Center (QCC) began to see patients. QCC is a single facility that provides medical, surgical, radiation, gynecologic, and urologic oncology all in one area of the QHC.
This study is an investigation of the possible impact on care for breast cancer patients of low socioeconomic status who were treated at a comprehensive cancer center, with specific consideration of the change or improvement in treatment modalities and outcomes. Data on treatment modalities and outcomes of cancer patients who were treated at the QHC during 2000, before the QCC was set up, were compared with data of patients treated during 2008 (2008 was selected because we have 5-year survival data for those patients). The public hospital system treats all patients regardless of their ability to pay, so the majority of patients in the system are of lower socioeconomic status. In addition, 92% of the patients seen QHC are from a minority population. These are the populations that tend to have a worse prognosis and often are not given optimal treatment.3 The payer mix of patients in the public hospital system is different than that of private hospitals. Most of the patients present at the hospital with no insurance and if they are diagnosed with cancer they may be converted to emergency Medicaid. About 10% of patients will not be converted because of their document status.
Patients and methods
We used the Queens Hospital Tumor Registry to identify the patients who had been diagnosed with and treated for breast cancer in 2000 and 2008. The electronic medical records were reviewed, and in the case of the 2000-year patients, the written charts were also reviewed. The study was approved by the Mount Sinai institutional review board. It was not necessary to obtain patient consent because it was a retrospective study.
Only patients diagnosed with stage 0, I, II, or III breast cancer who received their treatment at QHC were included in the study. Patients who were seen in consultation at QHC but not treated there were excluded. Statistics were done using the 2x2 chi-squared SPSS analysis; a P value of .05 was considered significant. The survival data was analyzed using SAS.
Results
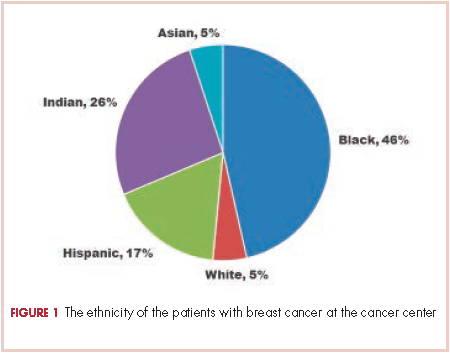
There were 24 evaluable patients in 2000 and 78 evaluable patients in 2008 who had stage 0, I, II, or III primary breast cancer and were treated at QHC. The average age of the patients in 2000 was 53.5 years and 54.7 years in 2008. The mean age for both groups was 55 years. The patients were ethnically diverse in both groups with 46% black, 17% Hispanic, 25% ethnic Asian Indian, and 6% white (Figure 1).
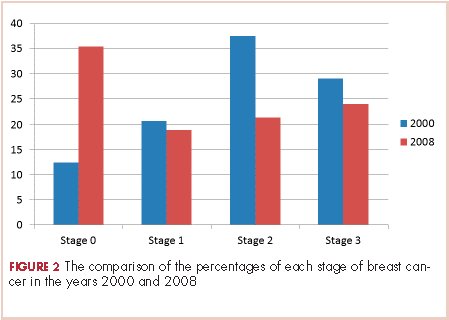
The payer mix in 2000 was 9 patients (37.5%) self-pay, 7 (29%) Medicaid, and 8 (33%) Medicare. In 2008, 11 patients (14%) were self-pay, 46 (59%) Medicaid, 11 (14%) Medicare, and 10 (13%) were private insurance. In 2000, there were 3 (12%) patients with stage 0 disease, 5 (21%) with stage I; 9 (37.5%) with stage II, and 7 (29%) with stage III. In 2008 there were 28 (36%) patients with stage 0 disease, 15 (19%) with stage I, 17 (22%) with stage II, and 18 (23%) with stage III (Figure 2).
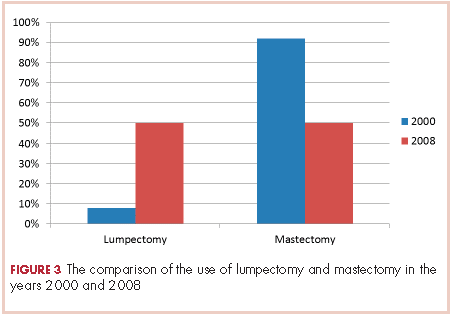
None of those values are statistically different. In 2000, 2 of the 24 patients had lumpectomies (partial mastectomy) and the rest had mastectomies. In 2008, 39 (50%) patients had mastectomy and 39 (50%) had lumpectomies (Figure 3). This was a statistically significant difference.
Radiation was given to both patients with lumpectomy in the 2000 group. In the 2008 group, all patients with lumpectomies were evaluated for radiation, and 6 of them did not receive radiation for the following reasons: 3 had very small foci of ductal carcinoma in situ (DCIS) and were treated with hormone therapy and no radiation; 1 patient had a lumpectomy for stage 1 cancer and also did not get radiation therapy because of a low oncotype and very small lesion; 2 patients were older than 70 years and had DCIS and were treated with tamoxifen alone as per NCCN Guidelines for women in that age group. The rest of the patients with lumpectomies received postoperative radiation.
Hormone and HER2 (human epidermal growth factor receptor 2) status was obtained on all patients. For the 2000 patients, 71% had 1 hormone receptor–positive (estrogen receptor [ER] or progesterone receptor [PR]), 21% were triple negative (ER-PR and HER2-neu), and 42% had HER2-neu–positive tumors. For the 2008, patients 65% were positive for 1 hormone receptor (ER or PR), 28% were triple negative (ER-PR and HER2-neu), and 7% had HER2-neu-positive tumors.
All patients were offered chemotherapy and hormone therapy if appropriate, as per NCCN guidelines. If a patient’s tumor was found to be HER2-positive, then the chemotherapy regimen would include the use of trastuzumab in both groups.
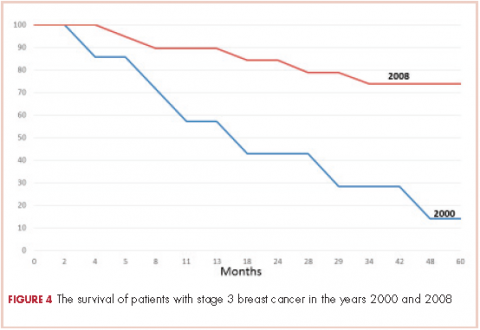
The 5-year survival for the 2008 stage III patients was 73.7%, compared with 14.2% for the 2000 stage III patients. The only deaths in the 2008 group were in patients with stage III disease. In the 2000 group, 4 of the 5 patients with stage III cancer died, and 33% of patients with stage I or II either died or were lost to follow-up before 5 years. This survival difference is significant by a chi-square and Wilcoxon analysis, with a P value of .01.
In 2000, 86% of patients with cancer were termed self-pay, that is, they had no insurance and they were not converted to emergency Medicaid. In 2008, 16% of patients were self-pay, and the rest were converted to Medicaid. In 2000, fewer than 2% of patients had commercial insurance, compared with 9% in 2008.
Discussion
There have been numerous studies reporting on disparities in the treatment of patients with breast cancer based on race or socioeconomic status.4-18 Many studies have shown inferior survival for black women with breast cancer, but it is not entirely clear whether these differences are the result of the quality of medical care received or biologic differences.14,19 A moderately large study from a metropolitan medical center in Detroit showed no difference in survival in their patients based on race when all of the patients received equal treatments.15 A meta-analysis of survival in black and white breast cancer patients showed that the black women had significantly poorer outcomes.19
Findings from a recent study showed that patients of lower socioeconomic status are more likely to undergo mastectomy than breast conserving therapy.20 The study, which identified 727,927 patients with early-stage breast cancer during 1998-2011, found that the rate of breast conservation increased from 54% to 59% during that time period and that there were significant barriers to women receiving breast-conserving therapy based on their type of insurance and having a lower socioeconomic status.20
The treatment of breast cancer is best delivered in a multimodality setting, but many inner-city public hospitals do not have such a facility for their patients. QHC is the only public hospital in New York City that has established a comprehensive cancer center. The patient population of QHC is overwhelmingly of minority origin (only 5% of patients are white). In addition, it is a safety net hospital, so no patient is turned away because they cannot pay, and most patients are of lower socioeconomic status and do not have insurance. The purpose of the cancer center was to provide a single site at which our patients could receive all their treatment. It was to ensure that our patients had easy access to care and treatment during all phases of their disease trajectory and did not “fall through the cracks” of the system. Those goals were addressed by having all of the center’s physicians in one place. Physicians involved in care included medical, surgical, and radiation oncologists, a gynecologic oncologist, a genitourinary oncologist, and a thoracic surgery oncologist. The support groups organized for the cancer patients included 3 oncology social workers, an oncology navigator, a nutritionist, a pastoral care supporter, and an oncology psychologist, all located in the same area. All of the clerical and financial aspects of care were also placed within the center. This made the experience as seamless as possible for both the patients and the treating physicians. A “survivors clinic” was established so the cancer patients could be seen by integrated primary care providers to address all noncancer-related health issues such as hypertension, diabetes, or heart disease. Finally, a robust clinical oncology research team was established in the same location. The research included several protocols for new drug treatments for breast cancer from pharmaceutical companies as well as the multi-institutional oncology groups.
Part of the mission of the cancer center was to reach out into the community of Queens to provide education about early detection, cancer prevention, and other public health issues such as tobacco cessation. We established a close working relationship with the Queens Public Library System to connect with their users and dispense information about cancer care and early detection. The Queens Library system is the largest in the United States, and everyone who lives in Queens has easy access to one of its 63 branch libraries. We arranged several lectures about breast cancer awareness in some of the branch libraries. We also procured a mobile mammogram unit for free screening events at the lectures, especially in neighborhoods with a large number residents who were of lower socioeconomic status.
To study the possible effect of these changes on our patients with breast cancer, we compared 2 groups of patients. One group was from the year 2000, a year before the cancer center was opened. The other was from the year 2008, the last year we could get real 5-year survival statistics. We explored how establishing the cancer center might have changed the patients’ stage at diagnosis, care, treatment modalities such as type of surgery, and outcomes. It is difficult to compare these 2 groups because of differences in the patients’ cancers, such as their receptor status, as well as differences in treatment options between the two time periods. However, we had no other way to compare the data to see if there were any trends.
There was a migration to earlier-stage cancer at diagnosis during the 6-year period after the cancer center was opened. It is likely that the educational sessions that were done in the community contributed to this migration. We also saw an increase in the number of mammograms done, from 6,300 in 2000 to 8,800 in 2008. This increase in screening also could account for more patients being identified with earlier-stage disease and might be attributable to the community education through the outreach programs.
As a quality control method, the cancer center has been evaluated by the Commission on Cancer every 3 years. At the 2013 evaluation, we received the Gold Commendation – the highest possible recognition for having 8 out of 8 commendations – and a 3-year accreditation.
There was a notable increase in the use of lumpectomy over mastectomy after the establishment of the cancer center, possibly due to the addition of 2 surgical oncologists to the cancer center’s care team. The integration of multimodiality care for each patient may also have increased the use of breast-conserving surgery.
There was a significant increase from 2000 to 2008 in the survival of patients treated for stage III breast cancer. New drugs and new patterns of adjuvant care might have been partly responsible for that change. The establishment of the comprehensive cancer center with access to new protocols ensured that patients received state-of-the-art cancer treatment. Moreover, the facility addressed all aspects of patient care throughout the disease trajectory by including designated social workers, psychologists, a nutritionist, pastoral care, and patient and survivor support groups to ensure that patients would keep coming to the center for their therapy, with no delays and very little loss to follow-up.
Most patients without insurance were able to acquire emergency Medicaid through the cancer center. This was done by having 2 financial counselors who met with every patient and who could facilitate access to Medicaid as needed. As a result of that, the percentage of patients with no coverage went from 86% in 2000 to 16% in 2008. Before this system was set up, patients who were designated self-pay would pay a fee as low as $15 for each visit and received thousands of dollars’ worth of care. Thus, by forming a cancer center and facilitating patient access to Medicaid, we were able to save money for this public institution because of the gain in revenue from Medicaid.
Our findings suggest that the development of comprehensive cancer centers within inner-city health systems can ensure better treatment for patients of lower socioeconomic status. We present evidence that this may result in increased survival, more sophisticated surgical options, and better patient quality of life. Moreover, this can be achieved while effectively increasing revenue for the public hospitals. Correcting the inequality of access to care and better therapeutic options by setting up comprehensive cancer centers could contribute to improved parity of outcomes for underserved populations.
The author acknowledges the statistical help of Brian Altonen, MPH.
1. Kesson EM, Allardice GM, George WD, Morrison DS. Effects of multidisciplinary team working on breast cancer survival: retrospective, comparative, interventional cohort study of 13,722 women. BMJ. 2012;344:e2718.
2. Vrijens F, Stordeur S, Beirens K, Devriese S, Van Eycken E, Vlayen J. Effect of hospital volume on processes of care and 5-year survival after breast cancer: a population-based study on 25000 women. Breast. 2012;21(3):261-266.
3. Bradley CJ, Given CW, Roberts C. Race, socioeconomic status and breast cancer treatment and survival. J Natl Cancer Inst. 2002;94(7):490-496.
4. Wheeler SB, Hayes-Reeder KE, Carey LA. Disparities in breast cancer treatment and outcomes: biological, social, and health system determinants and opportunities for research. Oncologist. 2013;18:986-993.
5. Ward E, Jemal A, Cokkinides V, et al. Cancer disparities by race/ethnicity and socioeconomic status. CA Cancer J Clin. 2004;54:78-93.
6. Chen F, Puig M, Yermilov I, et al. Using breast cancer quality indicators in a vulnerable population. Cancer. 2011;117:3311-3321.
7. Banerjee M, George J, Yee C, Hryniuk W, Schwartz K. Disentangling the effects of race on breast cancer treatment. Cancer. 2007;110:2169-2177.
8. Freedman RA, He Y, Winer EP, Keating NL. Trends in racial and age disparities in definitive local therapy of early-stage breast cancer. J Clin Oncol. 2009;27:713-719.
9. Bickell NA, Shastri K, Fei K, et al. A tracking and feedback registry to reduce racial disparities in breast cancer care. J Natl Cancer Inst. 2008;100:1717-1723.
19. Bickell NA, Wang JJ, Oluwole S, et al. Missed opportunities: Racial disparities in adjuvant breast cancer treatment. J Clin Oncol. 2006;24:1357-1362.
11. Harper S, Lynch J, Meersman SC, Breen N, Davis WW, Reichman MC. Trends in area-socioeconomic and race-ethnic disparities in breast cancer incidence, stage at diagnosis, screening, mortality, and survival among women ages 50 years and over (1987-2005). Cancer Epidemiol Biomarkers Prev. 2009;18:121-131.
12. Ward E, Halpern M, Schrag N, et al. Association of insurance with cancer care utilization and outcomes. CA Cancer J Clin. 2008;58:9-31.
13. Naik AM, Joseph K, Harris M, Davis C, Shapiro R, Hiotis KL. Indigent breast cancer patients among all racial and ethnic groups present with more advanced disease compared with nationally reported date. Am J Surg. 2003;186:400-403.
14. Hersman DL, Unger JM, Barlow WE, et al. Treatment quality and outcomes of African American versus white breast cancer patients: retrospective analysis of southwest oncology studies S8814/S8897. J Clin Oncol. 2009;27: 2157-2162.
15
16. Brawley OW. Disaggregating the effects of race and poverty on breast cancer outcomes. J Natl Cancer Inst. 2002;94:471-473.
17. Baquet CR, Commiskey P. Socioeconomic factors and breast carcinoma in multicultural women. Cancer. 2000;88:1256-1264.
18. Cross C, Harris J, Recht A. Race, socioeconomic status, and breast carcinoma in the US. Cancer. 2002;95:1988-1999.
19. Newman LA, Griffith KA, Jatoi I, Simon MS, Crowe JP, Colditz GA. Meta-analysis of survival in African American and white American patients with breast cancer: Ethnicity compared with socioeconomic status. J Clin Oncol. 2006;24:1342-1349.
20. Lautner M, Lin H, Shen Y, et al. Disparities in the use of breast-conserving therapy among patients with early-stage breast cancer. JAMA. 2015;150:778-786.
When cancer care is centralized in a comprehensive fashion, the quality of care and the outcomes improve.1,2 Unfortunately, because of the medical insurance structure in New York City, most patients of lower socioeconomic status do not receive their cancer care in such dedicated cancer centers. In New York City, the majority of the underserved vulnerable populations – that is, those without health insurance – receive their care from the public hospital system known as NYC Health and Hospitals. Cancer care in this system is not centralized and may result in fragmented implementation of various modalities of treatment. In addition, because there is no centralized care, needs such as early screening and prevention programs are often not addressed. This problem was evident in Queens in 2000 and before when many patients with late-stage cancers were presenting for cancer care. Queens, which is one of the 5 boroughs of New York City, has more than 2.3 million residents. It has 2 public hospitals, Elmhurst Hospital Center and Queens Hospital Center (QHC). In 2001, the plan was devised for the establishment of a cancer center at QHC, mainly because of the high rate of late-stage cancers that were being seen at presentation and recognition of the need for more comprehensive care. In 2002, the Queens Cancer Center (QCC) began to see patients. QCC is a single facility that provides medical, surgical, radiation, gynecologic, and urologic oncology all in one area of the QHC.
This study is an investigation of the possible impact on care for breast cancer patients of low socioeconomic status who were treated at a comprehensive cancer center, with specific consideration of the change or improvement in treatment modalities and outcomes. Data on treatment modalities and outcomes of cancer patients who were treated at the QHC during 2000, before the QCC was set up, were compared with data of patients treated during 2008 (2008 was selected because we have 5-year survival data for those patients). The public hospital system treats all patients regardless of their ability to pay, so the majority of patients in the system are of lower socioeconomic status. In addition, 92% of the patients seen QHC are from a minority population. These are the populations that tend to have a worse prognosis and often are not given optimal treatment.3 The payer mix of patients in the public hospital system is different than that of private hospitals. Most of the patients present at the hospital with no insurance and if they are diagnosed with cancer they may be converted to emergency Medicaid. About 10% of patients will not be converted because of their document status.
Patients and methods
We used the Queens Hospital Tumor Registry to identify the patients who had been diagnosed with and treated for breast cancer in 2000 and 2008. The electronic medical records were reviewed, and in the case of the 2000-year patients, the written charts were also reviewed. The study was approved by the Mount Sinai institutional review board. It was not necessary to obtain patient consent because it was a retrospective study.
Only patients diagnosed with stage 0, I, II, or III breast cancer who received their treatment at QHC were included in the study. Patients who were seen in consultation at QHC but not treated there were excluded. Statistics were done using the 2x2 chi-squared SPSS analysis; a P value of .05 was considered significant. The survival data was analyzed using SAS.
Results
There were 24 evaluable patients in 2000 and 78 evaluable patients in 2008 who had stage 0, I, II, or III primary breast cancer and were treated at QHC. The average age of the patients in 2000 was 53.5 years and 54.7 years in 2008. The mean age for both groups was 55 years. The patients were ethnically diverse in both groups with 46% black, 17% Hispanic, 25% ethnic Asian Indian, and 6% white (Figure 1).
The payer mix in 2000 was 9 patients (37.5%) self-pay, 7 (29%) Medicaid, and 8 (33%) Medicare. In 2008, 11 patients (14%) were self-pay, 46 (59%) Medicaid, 11 (14%) Medicare, and 10 (13%) were private insurance. In 2000, there were 3 (12%) patients with stage 0 disease, 5 (21%) with stage I; 9 (37.5%) with stage II, and 7 (29%) with stage III. In 2008 there were 28 (36%) patients with stage 0 disease, 15 (19%) with stage I, 17 (22%) with stage II, and 18 (23%) with stage III (Figure 2).
None of those values are statistically different. In 2000, 2 of the 24 patients had lumpectomies (partial mastectomy) and the rest had mastectomies. In 2008, 39 (50%) patients had mastectomy and 39 (50%) had lumpectomies (Figure 3). This was a statistically significant difference.
Radiation was given to both patients with lumpectomy in the 2000 group. In the 2008 group, all patients with lumpectomies were evaluated for radiation, and 6 of them did not receive radiation for the following reasons: 3 had very small foci of ductal carcinoma in situ (DCIS) and were treated with hormone therapy and no radiation; 1 patient had a lumpectomy for stage 1 cancer and also did not get radiation therapy because of a low oncotype and very small lesion; 2 patients were older than 70 years and had DCIS and were treated with tamoxifen alone as per NCCN Guidelines for women in that age group. The rest of the patients with lumpectomies received postoperative radiation.
Hormone and HER2 (human epidermal growth factor receptor 2) status was obtained on all patients. For the 2000 patients, 71% had 1 hormone receptor–positive (estrogen receptor [ER] or progesterone receptor [PR]), 21% were triple negative (ER-PR and HER2-neu), and 42% had HER2-neu–positive tumors. For the 2008, patients 65% were positive for 1 hormone receptor (ER or PR), 28% were triple negative (ER-PR and HER2-neu), and 7% had HER2-neu-positive tumors.
All patients were offered chemotherapy and hormone therapy if appropriate, as per NCCN guidelines. If a patient’s tumor was found to be HER2-positive, then the chemotherapy regimen would include the use of trastuzumab in both groups.
The 5-year survival for the 2008 stage III patients was 73.7%, compared with 14.2% for the 2000 stage III patients. The only deaths in the 2008 group were in patients with stage III disease. In the 2000 group, 4 of the 5 patients with stage III cancer died, and 33% of patients with stage I or II either died or were lost to follow-up before 5 years. This survival difference is significant by a chi-square and Wilcoxon analysis, with a P value of .01.
In 2000, 86% of patients with cancer were termed self-pay, that is, they had no insurance and they were not converted to emergency Medicaid. In 2008, 16% of patients were self-pay, and the rest were converted to Medicaid. In 2000, fewer than 2% of patients had commercial insurance, compared with 9% in 2008.
Discussion
There have been numerous studies reporting on disparities in the treatment of patients with breast cancer based on race or socioeconomic status.4-18 Many studies have shown inferior survival for black women with breast cancer, but it is not entirely clear whether these differences are the result of the quality of medical care received or biologic differences.14,19 A moderately large study from a metropolitan medical center in Detroit showed no difference in survival in their patients based on race when all of the patients received equal treatments.15 A meta-analysis of survival in black and white breast cancer patients showed that the black women had significantly poorer outcomes.19
Findings from a recent study showed that patients of lower socioeconomic status are more likely to undergo mastectomy than breast conserving therapy.20 The study, which identified 727,927 patients with early-stage breast cancer during 1998-2011, found that the rate of breast conservation increased from 54% to 59% during that time period and that there were significant barriers to women receiving breast-conserving therapy based on their type of insurance and having a lower socioeconomic status.20
The treatment of breast cancer is best delivered in a multimodality setting, but many inner-city public hospitals do not have such a facility for their patients. QHC is the only public hospital in New York City that has established a comprehensive cancer center. The patient population of QHC is overwhelmingly of minority origin (only 5% of patients are white). In addition, it is a safety net hospital, so no patient is turned away because they cannot pay, and most patients are of lower socioeconomic status and do not have insurance. The purpose of the cancer center was to provide a single site at which our patients could receive all their treatment. It was to ensure that our patients had easy access to care and treatment during all phases of their disease trajectory and did not “fall through the cracks” of the system. Those goals were addressed by having all of the center’s physicians in one place. Physicians involved in care included medical, surgical, and radiation oncologists, a gynecologic oncologist, a genitourinary oncologist, and a thoracic surgery oncologist. The support groups organized for the cancer patients included 3 oncology social workers, an oncology navigator, a nutritionist, a pastoral care supporter, and an oncology psychologist, all located in the same area. All of the clerical and financial aspects of care were also placed within the center. This made the experience as seamless as possible for both the patients and the treating physicians. A “survivors clinic” was established so the cancer patients could be seen by integrated primary care providers to address all noncancer-related health issues such as hypertension, diabetes, or heart disease. Finally, a robust clinical oncology research team was established in the same location. The research included several protocols for new drug treatments for breast cancer from pharmaceutical companies as well as the multi-institutional oncology groups.
Part of the mission of the cancer center was to reach out into the community of Queens to provide education about early detection, cancer prevention, and other public health issues such as tobacco cessation. We established a close working relationship with the Queens Public Library System to connect with their users and dispense information about cancer care and early detection. The Queens Library system is the largest in the United States, and everyone who lives in Queens has easy access to one of its 63 branch libraries. We arranged several lectures about breast cancer awareness in some of the branch libraries. We also procured a mobile mammogram unit for free screening events at the lectures, especially in neighborhoods with a large number residents who were of lower socioeconomic status.
To study the possible effect of these changes on our patients with breast cancer, we compared 2 groups of patients. One group was from the year 2000, a year before the cancer center was opened. The other was from the year 2008, the last year we could get real 5-year survival statistics. We explored how establishing the cancer center might have changed the patients’ stage at diagnosis, care, treatment modalities such as type of surgery, and outcomes. It is difficult to compare these 2 groups because of differences in the patients’ cancers, such as their receptor status, as well as differences in treatment options between the two time periods. However, we had no other way to compare the data to see if there were any trends.
There was a migration to earlier-stage cancer at diagnosis during the 6-year period after the cancer center was opened. It is likely that the educational sessions that were done in the community contributed to this migration. We also saw an increase in the number of mammograms done, from 6,300 in 2000 to 8,800 in 2008. This increase in screening also could account for more patients being identified with earlier-stage disease and might be attributable to the community education through the outreach programs.
As a quality control method, the cancer center has been evaluated by the Commission on Cancer every 3 years. At the 2013 evaluation, we received the Gold Commendation – the highest possible recognition for having 8 out of 8 commendations – and a 3-year accreditation.
There was a notable increase in the use of lumpectomy over mastectomy after the establishment of the cancer center, possibly due to the addition of 2 surgical oncologists to the cancer center’s care team. The integration of multimodiality care for each patient may also have increased the use of breast-conserving surgery.
There was a significant increase from 2000 to 2008 in the survival of patients treated for stage III breast cancer. New drugs and new patterns of adjuvant care might have been partly responsible for that change. The establishment of the comprehensive cancer center with access to new protocols ensured that patients received state-of-the-art cancer treatment. Moreover, the facility addressed all aspects of patient care throughout the disease trajectory by including designated social workers, psychologists, a nutritionist, pastoral care, and patient and survivor support groups to ensure that patients would keep coming to the center for their therapy, with no delays and very little loss to follow-up.
Most patients without insurance were able to acquire emergency Medicaid through the cancer center. This was done by having 2 financial counselors who met with every patient and who could facilitate access to Medicaid as needed. As a result of that, the percentage of patients with no coverage went from 86% in 2000 to 16% in 2008. Before this system was set up, patients who were designated self-pay would pay a fee as low as $15 for each visit and received thousands of dollars’ worth of care. Thus, by forming a cancer center and facilitating patient access to Medicaid, we were able to save money for this public institution because of the gain in revenue from Medicaid.
Our findings suggest that the development of comprehensive cancer centers within inner-city health systems can ensure better treatment for patients of lower socioeconomic status. We present evidence that this may result in increased survival, more sophisticated surgical options, and better patient quality of life. Moreover, this can be achieved while effectively increasing revenue for the public hospitals. Correcting the inequality of access to care and better therapeutic options by setting up comprehensive cancer centers could contribute to improved parity of outcomes for underserved populations.
The author acknowledges the statistical help of Brian Altonen, MPH.
When cancer care is centralized in a comprehensive fashion, the quality of care and the outcomes improve.1,2 Unfortunately, because of the medical insurance structure in New York City, most patients of lower socioeconomic status do not receive their cancer care in such dedicated cancer centers. In New York City, the majority of the underserved vulnerable populations – that is, those without health insurance – receive their care from the public hospital system known as NYC Health and Hospitals. Cancer care in this system is not centralized and may result in fragmented implementation of various modalities of treatment. In addition, because there is no centralized care, needs such as early screening and prevention programs are often not addressed. This problem was evident in Queens in 2000 and before when many patients with late-stage cancers were presenting for cancer care. Queens, which is one of the 5 boroughs of New York City, has more than 2.3 million residents. It has 2 public hospitals, Elmhurst Hospital Center and Queens Hospital Center (QHC). In 2001, the plan was devised for the establishment of a cancer center at QHC, mainly because of the high rate of late-stage cancers that were being seen at presentation and recognition of the need for more comprehensive care. In 2002, the Queens Cancer Center (QCC) began to see patients. QCC is a single facility that provides medical, surgical, radiation, gynecologic, and urologic oncology all in one area of the QHC.
This study is an investigation of the possible impact on care for breast cancer patients of low socioeconomic status who were treated at a comprehensive cancer center, with specific consideration of the change or improvement in treatment modalities and outcomes. Data on treatment modalities and outcomes of cancer patients who were treated at the QHC during 2000, before the QCC was set up, were compared with data of patients treated during 2008 (2008 was selected because we have 5-year survival data for those patients). The public hospital system treats all patients regardless of their ability to pay, so the majority of patients in the system are of lower socioeconomic status. In addition, 92% of the patients seen QHC are from a minority population. These are the populations that tend to have a worse prognosis and often are not given optimal treatment.3 The payer mix of patients in the public hospital system is different than that of private hospitals. Most of the patients present at the hospital with no insurance and if they are diagnosed with cancer they may be converted to emergency Medicaid. About 10% of patients will not be converted because of their document status.
Patients and methods
We used the Queens Hospital Tumor Registry to identify the patients who had been diagnosed with and treated for breast cancer in 2000 and 2008. The electronic medical records were reviewed, and in the case of the 2000-year patients, the written charts were also reviewed. The study was approved by the Mount Sinai institutional review board. It was not necessary to obtain patient consent because it was a retrospective study.
Only patients diagnosed with stage 0, I, II, or III breast cancer who received their treatment at QHC were included in the study. Patients who were seen in consultation at QHC but not treated there were excluded. Statistics were done using the 2x2 chi-squared SPSS analysis; a P value of .05 was considered significant. The survival data was analyzed using SAS.
Results
There were 24 evaluable patients in 2000 and 78 evaluable patients in 2008 who had stage 0, I, II, or III primary breast cancer and were treated at QHC. The average age of the patients in 2000 was 53.5 years and 54.7 years in 2008. The mean age for both groups was 55 years. The patients were ethnically diverse in both groups with 46% black, 17% Hispanic, 25% ethnic Asian Indian, and 6% white (Figure 1).
The payer mix in 2000 was 9 patients (37.5%) self-pay, 7 (29%) Medicaid, and 8 (33%) Medicare. In 2008, 11 patients (14%) were self-pay, 46 (59%) Medicaid, 11 (14%) Medicare, and 10 (13%) were private insurance. In 2000, there were 3 (12%) patients with stage 0 disease, 5 (21%) with stage I; 9 (37.5%) with stage II, and 7 (29%) with stage III. In 2008 there were 28 (36%) patients with stage 0 disease, 15 (19%) with stage I, 17 (22%) with stage II, and 18 (23%) with stage III (Figure 2).
None of those values are statistically different. In 2000, 2 of the 24 patients had lumpectomies (partial mastectomy) and the rest had mastectomies. In 2008, 39 (50%) patients had mastectomy and 39 (50%) had lumpectomies (Figure 3). This was a statistically significant difference.
Radiation was given to both patients with lumpectomy in the 2000 group. In the 2008 group, all patients with lumpectomies were evaluated for radiation, and 6 of them did not receive radiation for the following reasons: 3 had very small foci of ductal carcinoma in situ (DCIS) and were treated with hormone therapy and no radiation; 1 patient had a lumpectomy for stage 1 cancer and also did not get radiation therapy because of a low oncotype and very small lesion; 2 patients were older than 70 years and had DCIS and were treated with tamoxifen alone as per NCCN Guidelines for women in that age group. The rest of the patients with lumpectomies received postoperative radiation.
Hormone and HER2 (human epidermal growth factor receptor 2) status was obtained on all patients. For the 2000 patients, 71% had 1 hormone receptor–positive (estrogen receptor [ER] or progesterone receptor [PR]), 21% were triple negative (ER-PR and HER2-neu), and 42% had HER2-neu–positive tumors. For the 2008, patients 65% were positive for 1 hormone receptor (ER or PR), 28% were triple negative (ER-PR and HER2-neu), and 7% had HER2-neu-positive tumors.
All patients were offered chemotherapy and hormone therapy if appropriate, as per NCCN guidelines. If a patient’s tumor was found to be HER2-positive, then the chemotherapy regimen would include the use of trastuzumab in both groups.
The 5-year survival for the 2008 stage III patients was 73.7%, compared with 14.2% for the 2000 stage III patients. The only deaths in the 2008 group were in patients with stage III disease. In the 2000 group, 4 of the 5 patients with stage III cancer died, and 33% of patients with stage I or II either died or were lost to follow-up before 5 years. This survival difference is significant by a chi-square and Wilcoxon analysis, with a P value of .01.
In 2000, 86% of patients with cancer were termed self-pay, that is, they had no insurance and they were not converted to emergency Medicaid. In 2008, 16% of patients were self-pay, and the rest were converted to Medicaid. In 2000, fewer than 2% of patients had commercial insurance, compared with 9% in 2008.
Discussion
There have been numerous studies reporting on disparities in the treatment of patients with breast cancer based on race or socioeconomic status.4-18 Many studies have shown inferior survival for black women with breast cancer, but it is not entirely clear whether these differences are the result of the quality of medical care received or biologic differences.14,19 A moderately large study from a metropolitan medical center in Detroit showed no difference in survival in their patients based on race when all of the patients received equal treatments.15 A meta-analysis of survival in black and white breast cancer patients showed that the black women had significantly poorer outcomes.19
Findings from a recent study showed that patients of lower socioeconomic status are more likely to undergo mastectomy than breast conserving therapy.20 The study, which identified 727,927 patients with early-stage breast cancer during 1998-2011, found that the rate of breast conservation increased from 54% to 59% during that time period and that there were significant barriers to women receiving breast-conserving therapy based on their type of insurance and having a lower socioeconomic status.20
The treatment of breast cancer is best delivered in a multimodality setting, but many inner-city public hospitals do not have such a facility for their patients. QHC is the only public hospital in New York City that has established a comprehensive cancer center. The patient population of QHC is overwhelmingly of minority origin (only 5% of patients are white). In addition, it is a safety net hospital, so no patient is turned away because they cannot pay, and most patients are of lower socioeconomic status and do not have insurance. The purpose of the cancer center was to provide a single site at which our patients could receive all their treatment. It was to ensure that our patients had easy access to care and treatment during all phases of their disease trajectory and did not “fall through the cracks” of the system. Those goals were addressed by having all of the center’s physicians in one place. Physicians involved in care included medical, surgical, and radiation oncologists, a gynecologic oncologist, a genitourinary oncologist, and a thoracic surgery oncologist. The support groups organized for the cancer patients included 3 oncology social workers, an oncology navigator, a nutritionist, a pastoral care supporter, and an oncology psychologist, all located in the same area. All of the clerical and financial aspects of care were also placed within the center. This made the experience as seamless as possible for both the patients and the treating physicians. A “survivors clinic” was established so the cancer patients could be seen by integrated primary care providers to address all noncancer-related health issues such as hypertension, diabetes, or heart disease. Finally, a robust clinical oncology research team was established in the same location. The research included several protocols for new drug treatments for breast cancer from pharmaceutical companies as well as the multi-institutional oncology groups.
Part of the mission of the cancer center was to reach out into the community of Queens to provide education about early detection, cancer prevention, and other public health issues such as tobacco cessation. We established a close working relationship with the Queens Public Library System to connect with their users and dispense information about cancer care and early detection. The Queens Library system is the largest in the United States, and everyone who lives in Queens has easy access to one of its 63 branch libraries. We arranged several lectures about breast cancer awareness in some of the branch libraries. We also procured a mobile mammogram unit for free screening events at the lectures, especially in neighborhoods with a large number residents who were of lower socioeconomic status.
To study the possible effect of these changes on our patients with breast cancer, we compared 2 groups of patients. One group was from the year 2000, a year before the cancer center was opened. The other was from the year 2008, the last year we could get real 5-year survival statistics. We explored how establishing the cancer center might have changed the patients’ stage at diagnosis, care, treatment modalities such as type of surgery, and outcomes. It is difficult to compare these 2 groups because of differences in the patients’ cancers, such as their receptor status, as well as differences in treatment options between the two time periods. However, we had no other way to compare the data to see if there were any trends.
There was a migration to earlier-stage cancer at diagnosis during the 6-year period after the cancer center was opened. It is likely that the educational sessions that were done in the community contributed to this migration. We also saw an increase in the number of mammograms done, from 6,300 in 2000 to 8,800 in 2008. This increase in screening also could account for more patients being identified with earlier-stage disease and might be attributable to the community education through the outreach programs.
As a quality control method, the cancer center has been evaluated by the Commission on Cancer every 3 years. At the 2013 evaluation, we received the Gold Commendation – the highest possible recognition for having 8 out of 8 commendations – and a 3-year accreditation.
There was a notable increase in the use of lumpectomy over mastectomy after the establishment of the cancer center, possibly due to the addition of 2 surgical oncologists to the cancer center’s care team. The integration of multimodiality care for each patient may also have increased the use of breast-conserving surgery.
There was a significant increase from 2000 to 2008 in the survival of patients treated for stage III breast cancer. New drugs and new patterns of adjuvant care might have been partly responsible for that change. The establishment of the comprehensive cancer center with access to new protocols ensured that patients received state-of-the-art cancer treatment. Moreover, the facility addressed all aspects of patient care throughout the disease trajectory by including designated social workers, psychologists, a nutritionist, pastoral care, and patient and survivor support groups to ensure that patients would keep coming to the center for their therapy, with no delays and very little loss to follow-up.
Most patients without insurance were able to acquire emergency Medicaid through the cancer center. This was done by having 2 financial counselors who met with every patient and who could facilitate access to Medicaid as needed. As a result of that, the percentage of patients with no coverage went from 86% in 2000 to 16% in 2008. Before this system was set up, patients who were designated self-pay would pay a fee as low as $15 for each visit and received thousands of dollars’ worth of care. Thus, by forming a cancer center and facilitating patient access to Medicaid, we were able to save money for this public institution because of the gain in revenue from Medicaid.
Our findings suggest that the development of comprehensive cancer centers within inner-city health systems can ensure better treatment for patients of lower socioeconomic status. We present evidence that this may result in increased survival, more sophisticated surgical options, and better patient quality of life. Moreover, this can be achieved while effectively increasing revenue for the public hospitals. Correcting the inequality of access to care and better therapeutic options by setting up comprehensive cancer centers could contribute to improved parity of outcomes for underserved populations.
The author acknowledges the statistical help of Brian Altonen, MPH.
1. Kesson EM, Allardice GM, George WD, Morrison DS. Effects of multidisciplinary team working on breast cancer survival: retrospective, comparative, interventional cohort study of 13,722 women. BMJ. 2012;344:e2718.
2. Vrijens F, Stordeur S, Beirens K, Devriese S, Van Eycken E, Vlayen J. Effect of hospital volume on processes of care and 5-year survival after breast cancer: a population-based study on 25000 women. Breast. 2012;21(3):261-266.
3. Bradley CJ, Given CW, Roberts C. Race, socioeconomic status and breast cancer treatment and survival. J Natl Cancer Inst. 2002;94(7):490-496.
4. Wheeler SB, Hayes-Reeder KE, Carey LA. Disparities in breast cancer treatment and outcomes: biological, social, and health system determinants and opportunities for research. Oncologist. 2013;18:986-993.
5. Ward E, Jemal A, Cokkinides V, et al. Cancer disparities by race/ethnicity and socioeconomic status. CA Cancer J Clin. 2004;54:78-93.
6. Chen F, Puig M, Yermilov I, et al. Using breast cancer quality indicators in a vulnerable population. Cancer. 2011;117:3311-3321.
7. Banerjee M, George J, Yee C, Hryniuk W, Schwartz K. Disentangling the effects of race on breast cancer treatment. Cancer. 2007;110:2169-2177.
8. Freedman RA, He Y, Winer EP, Keating NL. Trends in racial and age disparities in definitive local therapy of early-stage breast cancer. J Clin Oncol. 2009;27:713-719.
9. Bickell NA, Shastri K, Fei K, et al. A tracking and feedback registry to reduce racial disparities in breast cancer care. J Natl Cancer Inst. 2008;100:1717-1723.
19. Bickell NA, Wang JJ, Oluwole S, et al. Missed opportunities: Racial disparities in adjuvant breast cancer treatment. J Clin Oncol. 2006;24:1357-1362.
11. Harper S, Lynch J, Meersman SC, Breen N, Davis WW, Reichman MC. Trends in area-socioeconomic and race-ethnic disparities in breast cancer incidence, stage at diagnosis, screening, mortality, and survival among women ages 50 years and over (1987-2005). Cancer Epidemiol Biomarkers Prev. 2009;18:121-131.
12. Ward E, Halpern M, Schrag N, et al. Association of insurance with cancer care utilization and outcomes. CA Cancer J Clin. 2008;58:9-31.
13. Naik AM, Joseph K, Harris M, Davis C, Shapiro R, Hiotis KL. Indigent breast cancer patients among all racial and ethnic groups present with more advanced disease compared with nationally reported date. Am J Surg. 2003;186:400-403.
14. Hersman DL, Unger JM, Barlow WE, et al. Treatment quality and outcomes of African American versus white breast cancer patients: retrospective analysis of southwest oncology studies S8814/S8897. J Clin Oncol. 2009;27: 2157-2162.
15
16. Brawley OW. Disaggregating the effects of race and poverty on breast cancer outcomes. J Natl Cancer Inst. 2002;94:471-473.
17. Baquet CR, Commiskey P. Socioeconomic factors and breast carcinoma in multicultural women. Cancer. 2000;88:1256-1264.
18. Cross C, Harris J, Recht A. Race, socioeconomic status, and breast carcinoma in the US. Cancer. 2002;95:1988-1999.
19. Newman LA, Griffith KA, Jatoi I, Simon MS, Crowe JP, Colditz GA. Meta-analysis of survival in African American and white American patients with breast cancer: Ethnicity compared with socioeconomic status. J Clin Oncol. 2006;24:1342-1349.
20. Lautner M, Lin H, Shen Y, et al. Disparities in the use of breast-conserving therapy among patients with early-stage breast cancer. JAMA. 2015;150:778-786.
1. Kesson EM, Allardice GM, George WD, Morrison DS. Effects of multidisciplinary team working on breast cancer survival: retrospective, comparative, interventional cohort study of 13,722 women. BMJ. 2012;344:e2718.
2. Vrijens F, Stordeur S, Beirens K, Devriese S, Van Eycken E, Vlayen J. Effect of hospital volume on processes of care and 5-year survival after breast cancer: a population-based study on 25000 women. Breast. 2012;21(3):261-266.
3. Bradley CJ, Given CW, Roberts C. Race, socioeconomic status and breast cancer treatment and survival. J Natl Cancer Inst. 2002;94(7):490-496.
4. Wheeler SB, Hayes-Reeder KE, Carey LA. Disparities in breast cancer treatment and outcomes: biological, social, and health system determinants and opportunities for research. Oncologist. 2013;18:986-993.
5. Ward E, Jemal A, Cokkinides V, et al. Cancer disparities by race/ethnicity and socioeconomic status. CA Cancer J Clin. 2004;54:78-93.
6. Chen F, Puig M, Yermilov I, et al. Using breast cancer quality indicators in a vulnerable population. Cancer. 2011;117:3311-3321.
7. Banerjee M, George J, Yee C, Hryniuk W, Schwartz K. Disentangling the effects of race on breast cancer treatment. Cancer. 2007;110:2169-2177.
8. Freedman RA, He Y, Winer EP, Keating NL. Trends in racial and age disparities in definitive local therapy of early-stage breast cancer. J Clin Oncol. 2009;27:713-719.
9. Bickell NA, Shastri K, Fei K, et al. A tracking and feedback registry to reduce racial disparities in breast cancer care. J Natl Cancer Inst. 2008;100:1717-1723.
19. Bickell NA, Wang JJ, Oluwole S, et al. Missed opportunities: Racial disparities in adjuvant breast cancer treatment. J Clin Oncol. 2006;24:1357-1362.
11. Harper S, Lynch J, Meersman SC, Breen N, Davis WW, Reichman MC. Trends in area-socioeconomic and race-ethnic disparities in breast cancer incidence, stage at diagnosis, screening, mortality, and survival among women ages 50 years and over (1987-2005). Cancer Epidemiol Biomarkers Prev. 2009;18:121-131.
12. Ward E, Halpern M, Schrag N, et al. Association of insurance with cancer care utilization and outcomes. CA Cancer J Clin. 2008;58:9-31.
13. Naik AM, Joseph K, Harris M, Davis C, Shapiro R, Hiotis KL. Indigent breast cancer patients among all racial and ethnic groups present with more advanced disease compared with nationally reported date. Am J Surg. 2003;186:400-403.
14. Hersman DL, Unger JM, Barlow WE, et al. Treatment quality and outcomes of African American versus white breast cancer patients: retrospective analysis of southwest oncology studies S8814/S8897. J Clin Oncol. 2009;27: 2157-2162.
15
16. Brawley OW. Disaggregating the effects of race and poverty on breast cancer outcomes. J Natl Cancer Inst. 2002;94:471-473.
17. Baquet CR, Commiskey P. Socioeconomic factors and breast carcinoma in multicultural women. Cancer. 2000;88:1256-1264.
18. Cross C, Harris J, Recht A. Race, socioeconomic status, and breast carcinoma in the US. Cancer. 2002;95:1988-1999.
19. Newman LA, Griffith KA, Jatoi I, Simon MS, Crowe JP, Colditz GA. Meta-analysis of survival in African American and white American patients with breast cancer: Ethnicity compared with socioeconomic status. J Clin Oncol. 2006;24:1342-1349.
20. Lautner M, Lin H, Shen Y, et al. Disparities in the use of breast-conserving therapy among patients with early-stage breast cancer. JAMA. 2015;150:778-786.