User login
Large-Diameter Femoral Heads in Total Hip Arthroplasty: An Evidence-Based Review
A common cause for total hip arthroplasty (THA) revision is joint instability.1,2 The reported incidence of dislocation in primary THA ranges from 0.4% to 5.8%,3-5 but this rate increases after revision surgery.1,3-8 Use of large-diameter femoral heads has been proposed to decrease the risks for instability and to improve impingement-free range of motion (ROM).
The biomechanical rationale for using large-diameter femoral heads is that they must travel farther before subluxation or dislocation occurs (jump distance). Despite these benefits, there were initial concerns that catastrophic failure and high levels of volumetric wear would occur if these heads were used with conventional polyethylene liners. These concerns led to the development of alternative bearing surfaces, particularly metal-on-metal bearings, which offered theoretical benefits of large-diameter articulations that improved stability while purportedly being highly wear-resistant.9-11 However, concerns about adverse local soft-tissue reactions and high blood concentrations of metal ions tempered the initial enthusiasm for metal bearings.12-16 Fortunately, highly cross-linked polyethylene and fourth-generation ceramic bearing surfaces, with improved toughness and better wear properties, may allow use of large-diameter heads without the need for metal-on-metal bearings.17,18
In this article, we review the concepts and principles behind use of large-diameter ceramic or cobalt-chromium femoral heads on polyethylene-bearing surfaces in THA with particular attention to biomechanics, early concerns about polyethylene wear and rim fractures, recent improvements in material properties of polyethylene and ceramic bearings, dislocation rates, and clinical and functional outcomes.
Definitions
For this review, we define large-diameter femoral heads as having diameters of 36 mm or more and conventional or small-diameter femoral heads as having diameters between 22 and 32 mm.
Biomechanics
Head–Neck Ratio, Impingement-Free ROM, and Jump Distance
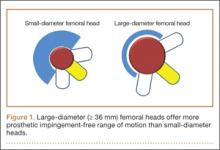
Several implant design principles have been proposed to reduce the risks for impingement and dislocation. Of these, large femoral head diameters have been extensively studied.19,20 It is well known that impingement of the femoral neck on the cup edge promotes edge loading and higher wear rates. In addition, impingement increases the tendency of the head to sublux from the acetabulum. One strategy for avoiding this component-to-component impingement is to increase the head–neck ratio (HNR), the ratio of the femoral head to the neck diameter. Biomechanically, increased HNRs lead to delayed contact between the femoral neck and the acetabular liner.21,22 Therefore, with large femoral heads, which have large HNRs, impingement occurs later and at larger ROMs—compared with small-diameter femoral heads, which have lower HNRs and are more prone to early impingement and subluxation (Figure 1).23-26
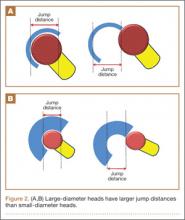
In a cadaveric study of 6 hips, Bartz and colleagues23 reported a significantly higher preimpingement ROM when the prosthetic head size increased from 22 mm to 28 mm (P < .05). They found a change from prosthetic to osseous impingement when the head size increased from 22 mm to 32 mm. Similar results were observed in a computer simulation model by Cinotti and colleagues,27 who demonstrated that increasing the femoral head size from 28 mm to 38 mm resulted in a 5° improvement in ROM. However, the largest gains were observed when the heads with the smallest diameters were upsized; ROM improved only marginally when femoral head size was further increased from 32 mm to 38 mm. The primary reason for the lack of expected improvement in ROM with head sizes of more than 32 mm is often bone-on-bone impingement. Burroughs and colleagues28 demonstrated that the 38-mm and 44-mm heads virtually eliminated component-to-component impingement except in extremes of external rotation. However, there were no differences in ROM between 38-mm and 44-mm heads because of osseous impingement. In addition, large heads are less likely to sublux or dislocate, as they need to travel farther before reaching the edge of the acetabular cup before dislocation. This is known as the jump distance, and it corresponds to the depth of the acetabular shell, which in turn equates with the radius of the femoral head (Figures 2A, 2B). For this reason, the larger the femoral head diameter, the farther the jump distance and, correspondingly, the lower the risk for dislocation.29
Elevated liners historically were used to increase the jump distance for dislocation.30 These liners, however, can increase impingement at the extremes of motion.31 Some of these problems can be avoided with use of larger heads, which have increased jump distances without additional risks for impingement. Moreover, large heads create a suction effect that provides passive resistance to dislocation.32 With head diameters beyond 38 mm, impingement-free ROM often plateaus. However, the jump distance required for dislocations to occur continues to increase as femoral head diameters increase in size. Thus, patients may experience fewer motion benefits but continue to benefit from overall stability with femoral head sizes increasing beyond 38 mm.
Current evidence suggests there may be substantial benefits toward improved stability from increasing head diameters from 22 mm to 38 mm because of the increase in jump distances and improvements in prosthetic impingement-free ROM. However, there may be little gain in ROM from increasing the head diameters beyond these dimensions because of the potential risks of bony impingement. Nevertheless, there may be some additional benefits toward stability from improvement in jump distances with incremental head sizes
beyond 38 mm.29,33,34
Finite Element Analysis Studies
Finite element analysis of large-diameter heads in THA has shown that, at optimal cup inclination (45°), most stresses occur on the articular surface of the liner. However, these stresses remain well below the yield strength of the polyethylene liners.29 With increasing abduction angles, the stress concentration increases substantially because of the decreased contact surface area. At these angles, the point of maximum contact moves toward the rim of the polyethylene liner, which can lead to rim fractures or failure of locking mechanisms.29,35,36
Early Concerns With Large-Diameter Femoral Heads: Wear, Liner Failure, and Fracture of Ceramic Components
Use of small-diameter femoral heads started with the first report by Charnley37 of “low frictional torque arthroplasty.” Charnley initially considered a 41.5-mm femoral head, but he thought it would increase risks for acetabular loosening from high frictional torque generated by the large head, and he switched to a small-diameter (22.5 mm) design. One of the tradeoffs with smaller diameter heads was decreased jump height in addition to increased linear wear.
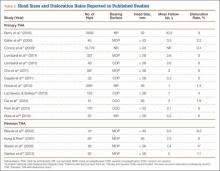
Large femoral heads used with cemented polyethylene acetabular components historically have been associated with increased rates of volumetric wear but low rates of linear wear, which potentially may increase the risk for osteolysis.38-40 However, newer highly cross-linked polyethylene liners have shown improved in vitro and in vivo volumetric wear characteristics and potentially lower linear wear rates compared with earlier designs (Table 1).28,41-43
Another concern about earlier generations of large femoral heads was the risk for catastrophic liner failure on conventional polyethylene. This was originally reported by Berry and colleagues,47 who described wear-through and failure in patients with thin (< 5 mm) acetabular cups. However, these concerns have been largely addressed by the development of highly cross-linked polyethylene, which has improved wear characteristics and fatigue resistance.48
Recent Improvements in Material Properties of Polyethylene and Ceramic Bearings
The development of highly cross-linked polyethylene and fourth-generation ceramics has renewed interest in large-diameter bearings in THA. These bearing surfaces improve wear, enhance material properties, and have superior oxidation resistance.42,48-53
We now briefly describe the methods used to improve the material properties of polyethylene and ceramics. Studies have shown that increasing the radiation dose (up to 200 kGy) increases cross-linking and causes an inverse exponential decrease in polyethylene wear.28,41,48-51 However, increasing radiation doses also increases production of free radicals, which diminish the material strength of these polyethylenes. The current generation of highly cross-linked polyethylene liners is produced through a variety of manufacturing strategies to improve cross-linking and reduce wear. These strategies include differential radiation doses (50-100 kGy), techniques (electron beam, radiation), and thermal treatments (melting, annealing). Moreover, to enhance the material properties and reduce the incidence of rim cracking and delamination, authors have proposed using vitamin E supplementation to minimize the amount of subsurface oxidation that occurs as an inevitable consequence of free radical formation during fabrication.54,55 A terminal sterilization process (eg, gas plasma, ethylene oxide, or gamma sterilization in nitrogen) is needed to make commercial, highly cross-linked polyethylene.52,53
Fourth-generation ceramics manufactured with nano-sized yttria-stabilized tetragonal zirconia particles in a stable alumina matrix have more fracture toughness and improved wear characteristics.54,55 In addition, oxide additives (eg, chromium oxide, strontium oxide) improve hardness and dissipate energy by deflecting cracks to prevent their propagation.56 Moreover, the smaller grain sizes of fourth-generation ceramic bearings compared with third-generation designs (0.8 µm vs 1-5 µm) cause less disruption of the fluid film layer, which ultimately results in improved wear performance.57
Multiple studies have found reduced wear rates with metal and ceramic large heads coupled with highly cross-linked polyethylene-bearings (Table 2).17,41,50,58 Bragdon and colleagues,58 using radiostereometric analysis in 25 patients, found no significant differences in mean head penetration rates between 36-mm and 28-mm cobalt-chromium (Co-Cr) heads articulating with highly cross-linked polyethylene cups at a mean follow-up of 3 years (0.035 mm/y vs 0.046 mm/y; P = .11). Geller and colleagues,64 in their study of 42 patients with large-diameter (> 32 mm) Co-Cr femoral heads, found low mean (SD) linear wear rates of 0.06 (0.41) mm/y at a mean follow-up of 3 years. D’Antonio and colleagues,65 in a multicenter study, reported low average linear wear (0.015 mm/y) and volumetric wear (12.1 mm3/y) over 5 years using sequentially annealed cross-linked polyethylene. In vitro reports suggest that large-diameter ceramic heads may have lower wear properties than Co-Cr heads do. Galvin and colleagues,66 in an in vitro hip simulator study, found that large-diameter ceramic heads on highly cross-linked ultrahigh-molecular-weight polyethylene had 40% reductions in steady-state wear rates compared with Co-Cr heads on highly cross-linked bearings (4.7 vs 8.1 mm3/million cycles; P < 0.01).
Dislocation Rates
Several patient, surgeon, and implant factors affect the rate of dislocations after THA. Multiple implant options utilize the biomechanical advantage that large-diameter heads have in improving stability. Various alternatives include use of constrained tripolar heads, dual-mobility bearings, and conventional large-diameter heads with standard liners.67-69
Large-Diameter Heads
Despite the biomechanical advantages of large-diameter metal-on-polyethylene bearings, prior studies have questioned use of these bearings because of risks for increased wear and rim failures. However, the improved wear properties of highly cross-linked polyethylene, elaborated earlier, have led to a reappraisal of this option (Table 2).4,70 Howie and colleagues,71 in a randomized control trial of 644 patients, also found significantly lower rates of dislocation after primary THA with 36-mm heads compared with 28-mm heads (1.3% vs 5.4%; P = .012); in addition, fewer dislocations occurred with 36-mm heads than with 28-mm heads (4.9% vs 12.2%; P = .27) in a series of 44 patients in revision settings. Similarly, in a study conducted with 39,271 Medicare patients between 1998 and 2007, Malkani and colleagues72 found a decrease in the dislocation rate, from 4.21% to 2.14%, with use of large-diameter femoral heads. These results have been confirmed by several other authors.34,66,73,74 Similar results were observed in 65,992 patients in the Australian National Joint Replacement Registry by Conroy and colleagues,75 who reported a significant decrease in the risk for dislocation with large heads (≥ 30 mm) compared with 22-mm heads (relative risk, 1.0 vs 3.1; P ≤ .001).
Few studies have analyzed the role of large-diameter femoral heads in the presence of compromised soft tissues around the hip. Kung and Ries,76 evaluating the influence of large-diameter heads in the presence and absence of a deficient abductor mechanism, demonstrated statistically significant reductions in rates of dislocation after 230 revision THAs when the abductor mechanism was intact with use of 36-mm heads compared with 28-mm heads (12.7% vs 0%; P = .015). With abductor deficiency, though, the positive effect of large heads in reducing dislocation rates was substantially reduced and was similar to that of small heads (P = .74).76
Large heads considerably improve overall stability and lower dislocation rates in THA. With the development of newer ceramics and highly cross-linked polyethylenes, the wear rates reported in multiple studies appear to be less concerning.
Constrained Tripolar Heads
Tripolar heads have been proposed as treatment options for improving stability in patients with chronic and recurrent instability after THA. The tripolar implant consists of a metal head that snap-fits into a polyethylene liner with a polished Co-Cr backing. This bipolar head articulates with a polyethylene bearing that is press-fitted onto a metal acetabular shell and constrained by a metal ring snapped to the outer polyethylene bearing. The bipolar component behaves as a large-diameter femoral head, and the metal ring provides additional restraint, further improving stability.
Williams and colleagues77 performed a systematic review and reported on the outcomes of constrained tripolar liners in 1199 hips at a mean follow-up of 4 years (range, 2-10 years). The mean dislocation rate was 10%, and the mean rate of revision surgery unrelated to instability was 4%. In a study of 43 hips at a mean follow-up of 4 years (range, 2-9 years), Zywiel and colleagues78 reported on the clinical and radiographic outcomes of tripolar constrained liners. Their study group had a mean Harris Hip Score (HHS) of 82 points (range, 38-100 points) and overall survival of 91%, with no evidence of radiographic loosening during follow-up. Despite the improvements in stability with constrained tripolar liners, some authors have reported multiple mechanisms of failure with these devices.79-81 In a study of 43 failed constrained tripolar liners with a mean time to failure of about 2 years, Guyen and colleagues79 identified 5 different failure modes (types 1-5) involving all 4 interfaces in these components.
Encouraging outcomes have been reported at midterm follow-up with tripolar constrained liners. However, concerns about failure at the interfaces suggest that use of these components should be restricted to patients with deficient abductor mechanisms or neuromuscular compromise, low-demand elderly patients, and salvage cases of recurrent dislocations.79
Dual-Mobility Bearings
For more than 20 years, different dual-mobility bearings have been used for difficult acetabular reconstructive scenarios and prevention of instability.82,83 Dual-mobility cups provide constructs that snap-fit a small-diameter femoral head within a large polyethylene insert that articulates with a fixed metal shell. This effectively increases the functional head diameter.
Various authors have reported excellent survivorship rates (92%-99%) and low dislocation rates for these bearings at 5- to 10-year follow-up.82,84-90 Philippot and colleagues,86 in a recent study of 438 hips with dual-mobility cups, reported excellent survivorship (96%) and no early or late instability within a 15-year follow-up. Bouchet and colleagues69 compared dual-mobility bearings (105 hips) with conventional metal-on-polythene bearings (108 hips) and found significantly (P < .05) lower dislocation rates for the dual-mobility implants at a minimum 1-year follow-up. The French Society of Orthopaedics and Traumatology performed a multicenter analysis of 3473 hips with dual-mobility cups implanted in France between January 1998 and December 2003.87 During a mean follow-up of 7 years (range, 5-11 years), there were 15 dislocations (0.43%), 14 of which occurred early, within 3 months of implantation (0.4%). Aseptic implant survivorship was 95% at 10-year follow-up.
Use of these bearings has recently increased in the United States. Short-term and midterm follow-up data show low rates of dislocation and wear. Long-term data are to come.
Clinical and Functional Outcomes of Large-Diameter Femoral Heads
There is a paucity of long-term outcomes data on use of large-diameter heads with highly cross-linked polyethylene bearings. Short-term and midterm clinical results appear to be excellent, with low rates of wear, osteolysis, and aseptic loosening.28,41,73,89-92
Plate and colleagues91 compared the effects of large-diameter (≥ 36 mm) and small-diameter (26 mm, 28 mm) metal heads on highly cross-linked polyethylene bearings. At a mean follow-up of 5 years (range, 4-8.4 years), the large-head cohort had a mean HHS of 90 points (range, 50-100 points) and no dislocations or radiographic evidence of stem or cup loosening. Similarly, Meftah and colleagues93 reported 100% stem survivorship and excellent clinical outcomes—a mean Western Ontario and McMaster Universities Arthritis Index (WOMAC) score of 30 points—for 72 hips with use of large ceramic heads (≥ 32 mm) on highly cross-linked polyethylene at a mean follow-up of 3 years. Gagala and colleagues94 reported excellent clinical and radiographic outcomes in 50 hips (18 ceramic on ceramic, 32 ceramic on polyethylene; 36-mm heads) at a mean follow-up of 3.5 years. Mean HHS was 94 points, and there was no evidence of liner fractures, aseptic loosening, or osteolysis.
In summary, large-diameter femoral heads in THA have become increasingly popular because of improvements in the material properties and wear characteristics of highly cross-linked polyethylene and fourth-generation ceramics. Despite the potential advantages of large heads in preventing dislocations, the basic surgical tenets of placing the acetabular component in appropriate alignment remain firmly established. Implants with functionally large heads (eg, dual-mobility bearings, constrained tripolar liners) may play an important role in patients at high risk for dislocation—particularly elderly patients with poor neuromuscular muscle coordination or deficient abductors, trauma patients, and patients with prior dislocations. Short-term and midterm results are excellent; rates of wear, aseptic loosening, and osteolysis are low. However, long-term outcomes data are needed to support widespread use of large heads in younger and more active patients.
1. Dorr LD, Wolf AW, Chandler R, Conaty JP. Classification and treatment of dislocations of total hip arthroplasty. Clin Orthop. 1983;(173):151-158.
2. Dorr LD, Wan Z. Causes of and treatment protocol for instability of total hip replacement. Clin Orthop. 1998;(355):144-151.
3. Turner RS. Postoperative total hip prosthetic femoral head dislocations. Incidence, etiologic factors, and management. Clin Orthop. 1994;(301):196-204.
4. Woo RY, Morrey BF. Dislocations after total hip arthroplasty. J Bone Joint Surg Am. 1982;64(9):1295-1306.
5. Etienne A, Cupic Z, Charnley J. Postoperative dislocation after Charnley low-friction arthroplasty. Clin Orthop. 1978;(132):19-23.
6. Fackler CD, Poss R. Dislocation in total hip arthroplasties. Clin Orthop. 1980;(151):169-178.
7. Joshi A, Lee CM, Markovic L, Vlatis G, Murphy JC. Prognosis of dislocation after total hip arthroplasty. J Arthroplasty. 1998;13(1):17-21.
8. Lindberg HO, Carlsson AS, Gentz CF, Pettersson H. Recurrent and non-recurrent dislocation following total hip arthroplasty. Acta Orthop Scand. 1982;53(6):947-952.
9. Eswaramoorthy V, Moonot P, Kalairajah Y, Biant LC, Field RE. The Metasul metal-on-metal articulation in primary total hip replacement: clinical and radiological results at ten years. J Bone Joint Surg Br. 2008;90(10):1278-1283.
10. Grubl A, Marker M, Brodner W, et al. Long-term follow-up of metal-on-metal total hip replacement. J Orthop Res. 2007;25(7):841-848.
11. Leslie I, Williams S, Brown C, et al. Effect of bearing size on the long-term wear, wear debris, and ion levels of large diameter metal-on-metal hip replacements—an in vitro study. J Biomed Mater Res B Appl Biomater. 2008;87(1):163-172.
12. Verhaar JA. The hard lesson of metal-on-metal hip implants [in Dutch]. Ned Tijdschr Geneeskd. 2012;156(42):A5564.
13. Fabi D, Levine B, Paprosky W, et al. Metal-on-metal total hip arthroplasty: causes and high incidence of early failure. Orthopedics. 2012;35(7):e1009-e1016.
14. Heneghan C, Langton D, Thompson M. Ongoing problems with metal-on-metal hip implants. BMJ. 2012;344:e1349.
15. Lee RK, Nevelos J, Vigdorchik J, Markel DC. Bearing surfaces for hip arthroplasty—is metal-on-metal a passing fancy? Surg Technol Int. 2012;22:243-249.
16. Voleti PB, Baldwin KD, Lee GC. Metal-on-metal vs conventional total hip arthroplasty: a systematic review and meta-analysis of randomized controlled trials. J Arthroplasty. 2012;27(10):1844-1849.
17. Urban JA, Garvin KL, Boese CK, et al. Ceramic-on-polyethylene bearing surfaces in total hip arthroplasty. Seventeen to twenty-one-year results.
J Bone Joint Surg Am. 2001;83(11):1688-1694.
18. Callaghan JJ, Liu SS. Ceramic on crosslinked polyethylene in total hip replacement: any better than metal on crosslinked polyethylene? Iowa Orthop J. 2009;29:1-4.
19. Barrack RL. Dislocation after total hip arthroplasty: implant design and orientation. J Am Acad Orthop Surg. 2003;11(2):89-99.
20. Krushell RJ, Burke DW, Harris WH. Elevated-rim acetabular components. Effect on range of motion and stability in total hip arthroplasty. J Arthroplasty. 1991;6(suppl):S53-S58.
21. Morrey BF. Instability after total hip arthroplasty. Orthop Clin North Am. 1992;23(2):237-248.
22. Morrey BF. Dislocation. In: Morrey BF, ed. Joint Replacement Arthroplasty. New York, NY: Churchill Livingstone; 1991:851-865.
23. Bartz RL, Nobel PC, Kadakia NR, Tullos HS. The effect of femoral component head size on posterior dislocation of the artificial hip joint. J Bone Joint Surg Am. 2000;82(9):1300-1307.
24. Nicholas RM, Orr JF, Mollan RA, Calderwood JW, Nixon JR, Watson P. Dislocation of total hip replacements. A comparative study of standard, long posterior wall and augmented acetabular components. J Bone Joint Surg Br. 1990;72(3):418-422.
25. McCollum DE, Gray WJ. Dislocation after total hip arthroplasty. Causes and prevention. Clin Orthop. 1990;(261):159-170.
26. Herrlin K, Selvik G, Pettersson H, Kesek P, Onnerfalt R, Ohlin A. Position, orientation and component interaction in dislocation of the total hip prosthesis. Acta Radiol. 1988;29(4):441-444.
27. Cinotti G, Lucioli N, Malagoli A, Calderoli C, Cassese F. Do large femoral heads reduce the risks of impingement in total hip arthroplasty with optimal and non-optimal cup positioning? Int Orthop. 2011;35(3):317-323.
28. Burroughs BR, Rubash HE, Harris WH. Femoral head sizes larger than 32 mm against highly cross-linked polyethylene. Clin Orthop. 2002;(405):150-157.
29. Crowninshield RD, Maloney WJ, Wentz DH, Humphrey SM, Blanchard CR. Biomechanics of large femoral heads: what they do and don‘t do. Clin Orthop. 2004;(429):102-107.
30. Charnley J. Low Friction Arthroplasty of the Hip: Theory and Practice. New York, NY: Springer; 1979.
31. Yamaguchi M, Akisue T, Bauer TW, Hashimoto Y. The spatial location of impingement in total hip arthroplasty. J Arthroplasty. 2000;15(3):305-313.
32. Peters CL, McPherson E, Jackson JD, Erickson JA. Reduction in early dislocation rate with large-diameter femoral heads in primary total hip arthroplasty. J Arthroplasty. 2007;22(6 suppl 2):140-144.
33. Masonis JL, Bourne RB. Surgical approach, abductor function, and total hip arthroplasty dislocation. Clin Orthop. 2002;(405):46-53.
34. Beaule PE, Schmalzried TP, Udomkiat P, Amstutz HC. Jumbo femoral head for the treatment of recurrent dislocation following total hip replacement. J Bone Joint Surg Am. 2002;84(2):256-263.
35. Oral E, Malhi AS, Muratoglu OK. Mechanisms of decrease in fatigue crack propagation resistance in irradiated and melted UHMWPE. Biomaterials. 2006;27(6):917-925.
36. Baker DA, Bellare A, Pruitt L. The effects of degree of crosslinking on the fatigue crack initiation and propagation resistance of orthopedic-grade polyethylene. J Biomed Mater Res A. 2003;66(1):146-154.
37. Charnley J. Total hip replacement by low-friction arthroplasty. Clin Orthop. 1970;(72):7-21.
38. Kabo JM, Gebhard JS, Loren G, Amstutz HC. In vivo wear of polyethylene acetabular components. J Bone Joint Surg Br. 1993;75(2):254-258.
39. Livermore J, Ilstrup D, Morrey B. Effect of femoral head size on wear of the polyethylene acetabular component. J Bone Joint Surg Am. 1990;72(4):518-528.
40. Ma SM, Kabo JM, Amstutz HC. Frictional torque in surface and conventional hip replacement. J Bone Joint Surg Am. 1983;65(3):366-370.
41. Muratoglu OK, Bragdon CR, O‘Connor D, et al. Larger diameter femoral heads used in conjunction with a highly cross-linked ultra-high molecular weight polyethylene: a new concept. J Arthroplasty. 2001;16(8 suppl 1):24-30.
42. Thomas GER, Simpson DJ, Mehmood S, et al. The seven-year wear of highly cross-linked polyethylene in total hip arthroplasty: a double-blind, randomized controlled trial using radiostereometric analysis. J Bone Joint Surg Am. 2011;93(8):716-722.
43. Mutimer J, Devane PA, Adams K, Horne JG. Highly crosslinked polyethylene reduces wear in total hip arthroplasty at 5 years. Clin Orthop. 2010;468(12):3228-3233.
44. Bragdon CR, Doerner M, Martell J, Jarrett B, Palm H, Malchau H. The 2012 John Charnley Award: clinical multicenter studies of the wear performance of highly crosslinked remelted polyethylene in THA. Clin Orthop. 2013;471(2):393-402.
45. Lachiewicz PF, Heckman DS, Soileau ES, Mangla J, Martell JM. Femoral head size and wear of highly cross-linked polyethylene at 5 to 8 years. Clin Orthop. 2009;467(12):3290-3296.
46. Sychterz CJ, Engh CA Jr, Young AM, Hopper RH Jr, Engh CA. Comparison of in vivo wear between polyethylene liners articulating with ceramic and cobalt-chrome femoral heads. J Bone Joint Surg Br. 2000;82(7):948-951.
47. Berry DJ, Barnes CL, Scott RD, Cabanela ME, Poss R. Catastrophic failure of the polyethylene liner of uncemented acetabular components.
J Bone Joint Surg Br. 1994;76(4):575-578.
48. McKellop H, Shen FW, Lu B, Campbell P, Salovey R. Development of an extremely wear-resistant ultra high molecular weight polyethylene for total hip replacements. J Orthop Res. 1999;17(2):157-167.
49. Wang A, Essner A, Polineni VK, Stark C, Dumbleton JH. Lubrication and wear of ultra-high molecular weight polyethylene in total joint replacements. Tribol Int. 1998;31(1-3):17-33.
50. Estok DM 2nd, Burroughs BR, Muratoglu OK, Harris WH. Comparison of hip simulator wear of 2 different highly cross-linked ultra high molecular weight polyethylene acetabular components using both 32- and 38-mm femoral heads. J Arthroplasty. 2007;22(4):581-589.
51. Muratoglu OK, Bragdon CR, O‘Connor DO, et al. Unified wear model for highly crosslinked ultra-high molecular weight polyethylenes (UHMWPE). Biomaterials. 1999;20(16):1463-1470.
52. Harris WH, Muratoglu OK. A review of current cross-linked polyethylenes used in total joint arthroplasty. Clin Orthop. 2005;(430):46-52.
53. Muratoglu OK, Bragdon CR, O‘Connor DO, Jasty M, Harris WH. A novel method of cross-linking ultra-high-molecular-weight polyethylene to improve wear, reduce oxidation, and retain mechanical properties. Recipient of the 1999 HAP Paul Award. J Arthroplasty. 2001;16(2):149-160.
54. Bal BS, Garino J, Ries M, Rahaman MN. A review of ceramic bearing materials in total joint arthroplasty. Hip Int. 2007;17(1):21-30.
55. Traina F, De Fine M, Di Martino A, Faldini C. Fracture of ceramic bearing surfaces following total hip replacement: a systematic review. Biomed Res Int. 2013;2013:157247.
56. Cai YZ, Yan SG. Development of ceramic-on-ceramic implants for total hip arthroplasty. Orthop Surg. 2010;2(3):175-181.
57. Stewart TD, Tipper JL, Insley G, Streicher RM, Ingham E, Fisher J. Long-term wear of ceramic matrix composite materials for hip prostheses under severe swing phase microseparation. J Biomed Mater Res B Appl Biomater. 2003;66(2):567-573.
58. Bragdon CR, Greene ME, Freiberg AA, Harris WH, Malchau H. Radiostereometric analysis comparison of wear of highly cross-linked polyethylene against 36- vs 28-mm femoral heads. J Arthroplasty. 2007;22(6 suppl 2):125-129.
59. Lombardi AV Jr, Skeels MD, Berend KR, Adams JB, Franchi OJ. Do large heads enhance stability and restore native anatomy in primary total hip arthroplasty? Clin Orthop. 2011;469(6):1547-1553.
60. Lachiewicz PF, Soileau ES. Low early and late dislocation rates with 36- and 40-mm heads in patients at high risk for dislocation. Clin Orthop. 2013;471(2):439-443.
61. Cai P, Hu Y, Xie J. Large-diameter Delta ceramic-on-ceramic versus common-sized ceramic-on-polyethylene bearings in THA. Orthopedics. 2012;35(9):e1307-e1313.
62. Park KS, Yoon TR, Hwang SY, Lee KB. Modified minimally invasive two-incision total hip arthroplasty using large diameter femoral head. Indian
J Orthop. 2012;46(1):29-35.
63. Garbuz DS, Masri BA, Duncan CP, et al. The Frank Stinchfield Award: dislocation in revision THA: do large heads (36 and 40 mm) result in reduced dislocation rates in a randomized clinical trial? Clin Orthop. 2012;470(2):351-356.
64. Geller JA, Malchau H, Bragdon C, Greene M, Harris WH, Freiberg AA. Large diameter femoral heads on highly cross-linked polyethylene: minimum 3-year results. Clin Orthop. 2006;(447):53-59.
65. D’Antonio JA, Capello WN, Ramakrishnan R. Second-generation annealed highly cross-linked polyethylene exhibits low wear. Clin Orthop. 2012;470(6):1696-1704.
66. Galvin AL, Jennings LM, Tipper JL, Ingham E, Fisher J. Wear and creep of highly crosslinked polyethylene against cobalt chrome and ceramic femoral heads. Proc Inst Mech Eng H. 2010;224(10):1175-1183.
67. Skeels MD, Berend KR, Lombardi AV Jr. The dislocator, early and late: the role of large heads. Orthopedics. 2009;32(9).
68. Plate JF, Seyler TM, Stroh DA, Issa K, Akbar M, Mont MA. Risk of dislocation using large- vs. small-diameter femoral heads in total hip arthroplasty. BMC Res Notes. 2012;5(1):553.
69. Bouchet R, Mercier N, Saragaglia D. Posterior approach and dislocation rate: a 213 total hip replacements case–control study comparing the dual mobility cup with a conventional 28-mm metal head/polyethylene prosthesis. Orthop Traumatol Surg Res. 2011;97(1):2-7.
70. Ali Khan MA, Brakenbury PH, Reynolds IS. Dislocation following total hip replacement. J Bone Joint Surg Br. 1981;63(2):214-218.
71. Howie DW, Holubowycz OT, Middleton R. Large femoral heads decrease the incidence of dislocation after total hip arthroplasty: a randomized controlled trial. J Bone Joint Surg Am. 2012;94(12):1095-1102.
72. Malkani AL, Ong KL, Lau E, Kurtz SM, Justice BJ, Manley MT. Early- and late-term dislocation risk after primary hip arthroplasty in the Medicare population. J Arthroplasty. 2010;25(6 suppl):21-25.
73. Berry DJ, von Knoch M, Schleck CD, Harmsen WS. Effect of femoral head diameter and operative approach on risk of dislocation after primary total hip arthroplasty. J Bone Joint Surg Am. 2005;87(11):2456-2463.
74. Cho MR, Lee HS, Lee SW, Choi CH, Kim SK, Ko SB. Results after total hip arthroplasty with a large head and bipolar arthroplasty in patients with displaced femoral neck fractures. J Arthroplasty. 2011;26(6):893-896.
75. Conroy JL, Whitehouse SL, Graves SE, Pratt NL, Ryan P, Crawford RW. Risk factors for revision for early dislocation in total hip arthroplasty.
J Arthroplasty. 2008;23(6):867-872.
76. Kung PL, Ries MD. Effect of femoral head size and abductors on dislocation after revision THA. Clin Orthop. 2007;(465):170-174.
77. Williams JT Jr, Ragland PS, Clarke S. Constrained components for the unstable hip following total hip arthroplasty: a literature review. Int Orthop. 2007;31(3):273-277.
78. Zywiel MG, Mustafa LH, Bonutti PM, Mont MA. Are abductor muscle quality and previous revision surgery predictors of constrained liner failure in hip arthroplasty? Int Orthop. 2011;35(6):797-802.
79. Guyen O, Lewallen DG, Cabanela ME. Modes of failure of Osteonics constrained tripolar implants: a retrospective analysis of forty-three failed implants. J Bone Joint Surg Am. 2008;90(7):1553-1560.
80. Banks LN, McElwain JP. An unusual mode of failure of a tripolar constrained acetabular liner: a case report. Arch Orthop Trauma Surg. 2010;130(4):503-505.
81. Robertson WJ, Mattern CJ, Hur J, Su EP, Pellicci PM. Failure mechanisms and closed reduction of a constrained tripolar acetabular liner.
J Arthroplasty. 2009;24(2):322.e5-e11.
82. Aubriot JH, Lesimple P, Leclercq S. Study of Bousquet‘s non-cemented acetabular implant in 100 hybrid total hip prostheses (Charnley type cemented femoral component). Average 5-year follow-up [in French]. Acta Orthop Belg. 1993;59(suppl 1):267-271.
83. Farizon F, de Lavison R, Azoulai JJ, Bousquet G. Results with a cementless alumina-coated cup with dual mobility. A twelve-year follow-up study. Int Orthop. 1998;22(4):219-224.
84. Mertl P, Combes A, Leiber-Wackenheim F, Fessy MH, Girard J, Migaud H. Recurrence of dislocation following total hip arthroplasty revision using dual mobility cups was rare in 180 hips followed over 7 years. HSS J. 2012;8(3):251-256.
85. Langlais FL, Ropars M, Gaucher F, Musset T, Chaix O. Dual mobility cemented cups have low dislocation rates in THA revisions. Clin Orthop. 2008;466(2):389-395.
86. Philippot R, Farizon F, Camilleri JP, et al. Survival of dual mobility socket with a mean 17 years follow-up [in French]. Rev Chir Orthop Reparatrice Appar Mot. 2008;94(1):43-48.
87. Adam P, Philippe R, Ehlinger M, et al. Dual mobility cups hip arthroplasty as a treatment for displaced fracture of the femoral neck in the elderly.
A prospective, systematic, multicenter study with specific focus on postoperative dislocation. Orthop Traumatol Surg Res. 2012;98(3):296-300.
88. Fessy MH. La double mobilité [Dual mobility]. Revue de Chirurgie Orthopédique et Traumatologique. 2010;96(7):891-898.
89. Mont MA, Issa K, Naziri Q, Harwin SF, Delanois RE, Johnson AJ. The use of dual-mobility bearings in difficult hip arthroplasty reconstructive cases. Surg Technol Int. 2011;21:234-240.
90. Sayeed SA, Mont MA, Costa CR, et al. Early outcomes of sequentially cross-linked thin polyethylene liners with large diameter femoral heads in total hip arthroplasty. Bull NYU Hosp Jt Dis. 2011;69(suppl 1):S90-S94.
91. Plate JF, Seyler TM, Stroh DA, Issa K, Akbar M, Mont MA. Risk of dislocation using large- vs. small-diameter femoral heads in total hip arthroplasty. BMC Res Notes. 2012;5(1):553.
92. Sato T, Nakashima Y, Akiyama M, et al. Wear resistant performance of highly cross-linked and annealed ultra-high molecular weight polyethylene against ceramic heads in total hip arthroplasty. J Orthop Res. 2012;30(12):2031-2037.
93. Meftah M, Ebrahimpour PB, He C, Ranawat AS, Ranawat CS. Preliminary clinical and radiographic results of large ceramic heads on highly cross-linked polyethylene. Orthopedics. 2011;34(6):133.
94. Gagala J, Mazurkiewicz T, Dajewski Z. Large diameter femoral heads in primary alumina/alumina and XSPE/alumina total hip arthroplasty.
A follow-up study of 50 hips after average 40 months and review of literature [in Polish]. Chir Narzadow Ruchu Ortop Pol. 2011;76(1):14-20.
A common cause for total hip arthroplasty (THA) revision is joint instability.1,2 The reported incidence of dislocation in primary THA ranges from 0.4% to 5.8%,3-5 but this rate increases after revision surgery.1,3-8 Use of large-diameter femoral heads has been proposed to decrease the risks for instability and to improve impingement-free range of motion (ROM).
The biomechanical rationale for using large-diameter femoral heads is that they must travel farther before subluxation or dislocation occurs (jump distance). Despite these benefits, there were initial concerns that catastrophic failure and high levels of volumetric wear would occur if these heads were used with conventional polyethylene liners. These concerns led to the development of alternative bearing surfaces, particularly metal-on-metal bearings, which offered theoretical benefits of large-diameter articulations that improved stability while purportedly being highly wear-resistant.9-11 However, concerns about adverse local soft-tissue reactions and high blood concentrations of metal ions tempered the initial enthusiasm for metal bearings.12-16 Fortunately, highly cross-linked polyethylene and fourth-generation ceramic bearing surfaces, with improved toughness and better wear properties, may allow use of large-diameter heads without the need for metal-on-metal bearings.17,18
In this article, we review the concepts and principles behind use of large-diameter ceramic or cobalt-chromium femoral heads on polyethylene-bearing surfaces in THA with particular attention to biomechanics, early concerns about polyethylene wear and rim fractures, recent improvements in material properties of polyethylene and ceramic bearings, dislocation rates, and clinical and functional outcomes.
Definitions
For this review, we define large-diameter femoral heads as having diameters of 36 mm or more and conventional or small-diameter femoral heads as having diameters between 22 and 32 mm.
Biomechanics
Head–Neck Ratio, Impingement-Free ROM, and Jump Distance
Several implant design principles have been proposed to reduce the risks for impingement and dislocation. Of these, large femoral head diameters have been extensively studied.19,20 It is well known that impingement of the femoral neck on the cup edge promotes edge loading and higher wear rates. In addition, impingement increases the tendency of the head to sublux from the acetabulum. One strategy for avoiding this component-to-component impingement is to increase the head–neck ratio (HNR), the ratio of the femoral head to the neck diameter. Biomechanically, increased HNRs lead to delayed contact between the femoral neck and the acetabular liner.21,22 Therefore, with large femoral heads, which have large HNRs, impingement occurs later and at larger ROMs—compared with small-diameter femoral heads, which have lower HNRs and are more prone to early impingement and subluxation (Figure 1).23-26
In a cadaveric study of 6 hips, Bartz and colleagues23 reported a significantly higher preimpingement ROM when the prosthetic head size increased from 22 mm to 28 mm (P < .05). They found a change from prosthetic to osseous impingement when the head size increased from 22 mm to 32 mm. Similar results were observed in a computer simulation model by Cinotti and colleagues,27 who demonstrated that increasing the femoral head size from 28 mm to 38 mm resulted in a 5° improvement in ROM. However, the largest gains were observed when the heads with the smallest diameters were upsized; ROM improved only marginally when femoral head size was further increased from 32 mm to 38 mm. The primary reason for the lack of expected improvement in ROM with head sizes of more than 32 mm is often bone-on-bone impingement. Burroughs and colleagues28 demonstrated that the 38-mm and 44-mm heads virtually eliminated component-to-component impingement except in extremes of external rotation. However, there were no differences in ROM between 38-mm and 44-mm heads because of osseous impingement. In addition, large heads are less likely to sublux or dislocate, as they need to travel farther before reaching the edge of the acetabular cup before dislocation. This is known as the jump distance, and it corresponds to the depth of the acetabular shell, which in turn equates with the radius of the femoral head (Figures 2A, 2B). For this reason, the larger the femoral head diameter, the farther the jump distance and, correspondingly, the lower the risk for dislocation.29
Elevated liners historically were used to increase the jump distance for dislocation.30 These liners, however, can increase impingement at the extremes of motion.31 Some of these problems can be avoided with use of larger heads, which have increased jump distances without additional risks for impingement. Moreover, large heads create a suction effect that provides passive resistance to dislocation.32 With head diameters beyond 38 mm, impingement-free ROM often plateaus. However, the jump distance required for dislocations to occur continues to increase as femoral head diameters increase in size. Thus, patients may experience fewer motion benefits but continue to benefit from overall stability with femoral head sizes increasing beyond 38 mm.
Current evidence suggests there may be substantial benefits toward improved stability from increasing head diameters from 22 mm to 38 mm because of the increase in jump distances and improvements in prosthetic impingement-free ROM. However, there may be little gain in ROM from increasing the head diameters beyond these dimensions because of the potential risks of bony impingement. Nevertheless, there may be some additional benefits toward stability from improvement in jump distances with incremental head sizes
beyond 38 mm.29,33,34
Finite Element Analysis Studies
Finite element analysis of large-diameter heads in THA has shown that, at optimal cup inclination (45°), most stresses occur on the articular surface of the liner. However, these stresses remain well below the yield strength of the polyethylene liners.29 With increasing abduction angles, the stress concentration increases substantially because of the decreased contact surface area. At these angles, the point of maximum contact moves toward the rim of the polyethylene liner, which can lead to rim fractures or failure of locking mechanisms.29,35,36
Early Concerns With Large-Diameter Femoral Heads: Wear, Liner Failure, and Fracture of Ceramic Components
Use of small-diameter femoral heads started with the first report by Charnley37 of “low frictional torque arthroplasty.” Charnley initially considered a 41.5-mm femoral head, but he thought it would increase risks for acetabular loosening from high frictional torque generated by the large head, and he switched to a small-diameter (22.5 mm) design. One of the tradeoffs with smaller diameter heads was decreased jump height in addition to increased linear wear.
Large femoral heads used with cemented polyethylene acetabular components historically have been associated with increased rates of volumetric wear but low rates of linear wear, which potentially may increase the risk for osteolysis.38-40 However, newer highly cross-linked polyethylene liners have shown improved in vitro and in vivo volumetric wear characteristics and potentially lower linear wear rates compared with earlier designs (Table 1).28,41-43
Another concern about earlier generations of large femoral heads was the risk for catastrophic liner failure on conventional polyethylene. This was originally reported by Berry and colleagues,47 who described wear-through and failure in patients with thin (< 5 mm) acetabular cups. However, these concerns have been largely addressed by the development of highly cross-linked polyethylene, which has improved wear characteristics and fatigue resistance.48
Recent Improvements in Material Properties of Polyethylene and Ceramic Bearings
The development of highly cross-linked polyethylene and fourth-generation ceramics has renewed interest in large-diameter bearings in THA. These bearing surfaces improve wear, enhance material properties, and have superior oxidation resistance.42,48-53
We now briefly describe the methods used to improve the material properties of polyethylene and ceramics. Studies have shown that increasing the radiation dose (up to 200 kGy) increases cross-linking and causes an inverse exponential decrease in polyethylene wear.28,41,48-51 However, increasing radiation doses also increases production of free radicals, which diminish the material strength of these polyethylenes. The current generation of highly cross-linked polyethylene liners is produced through a variety of manufacturing strategies to improve cross-linking and reduce wear. These strategies include differential radiation doses (50-100 kGy), techniques (electron beam, radiation), and thermal treatments (melting, annealing). Moreover, to enhance the material properties and reduce the incidence of rim cracking and delamination, authors have proposed using vitamin E supplementation to minimize the amount of subsurface oxidation that occurs as an inevitable consequence of free radical formation during fabrication.54,55 A terminal sterilization process (eg, gas plasma, ethylene oxide, or gamma sterilization in nitrogen) is needed to make commercial, highly cross-linked polyethylene.52,53
Fourth-generation ceramics manufactured with nano-sized yttria-stabilized tetragonal zirconia particles in a stable alumina matrix have more fracture toughness and improved wear characteristics.54,55 In addition, oxide additives (eg, chromium oxide, strontium oxide) improve hardness and dissipate energy by deflecting cracks to prevent their propagation.56 Moreover, the smaller grain sizes of fourth-generation ceramic bearings compared with third-generation designs (0.8 µm vs 1-5 µm) cause less disruption of the fluid film layer, which ultimately results in improved wear performance.57
Multiple studies have found reduced wear rates with metal and ceramic large heads coupled with highly cross-linked polyethylene-bearings (Table 2).17,41,50,58 Bragdon and colleagues,58 using radiostereometric analysis in 25 patients, found no significant differences in mean head penetration rates between 36-mm and 28-mm cobalt-chromium (Co-Cr) heads articulating with highly cross-linked polyethylene cups at a mean follow-up of 3 years (0.035 mm/y vs 0.046 mm/y; P = .11). Geller and colleagues,64 in their study of 42 patients with large-diameter (> 32 mm) Co-Cr femoral heads, found low mean (SD) linear wear rates of 0.06 (0.41) mm/y at a mean follow-up of 3 years. D’Antonio and colleagues,65 in a multicenter study, reported low average linear wear (0.015 mm/y) and volumetric wear (12.1 mm3/y) over 5 years using sequentially annealed cross-linked polyethylene. In vitro reports suggest that large-diameter ceramic heads may have lower wear properties than Co-Cr heads do. Galvin and colleagues,66 in an in vitro hip simulator study, found that large-diameter ceramic heads on highly cross-linked ultrahigh-molecular-weight polyethylene had 40% reductions in steady-state wear rates compared with Co-Cr heads on highly cross-linked bearings (4.7 vs 8.1 mm3/million cycles; P < 0.01).
Dislocation Rates
Several patient, surgeon, and implant factors affect the rate of dislocations after THA. Multiple implant options utilize the biomechanical advantage that large-diameter heads have in improving stability. Various alternatives include use of constrained tripolar heads, dual-mobility bearings, and conventional large-diameter heads with standard liners.67-69
Large-Diameter Heads
Despite the biomechanical advantages of large-diameter metal-on-polyethylene bearings, prior studies have questioned use of these bearings because of risks for increased wear and rim failures. However, the improved wear properties of highly cross-linked polyethylene, elaborated earlier, have led to a reappraisal of this option (Table 2).4,70 Howie and colleagues,71 in a randomized control trial of 644 patients, also found significantly lower rates of dislocation after primary THA with 36-mm heads compared with 28-mm heads (1.3% vs 5.4%; P = .012); in addition, fewer dislocations occurred with 36-mm heads than with 28-mm heads (4.9% vs 12.2%; P = .27) in a series of 44 patients in revision settings. Similarly, in a study conducted with 39,271 Medicare patients between 1998 and 2007, Malkani and colleagues72 found a decrease in the dislocation rate, from 4.21% to 2.14%, with use of large-diameter femoral heads. These results have been confirmed by several other authors.34,66,73,74 Similar results were observed in 65,992 patients in the Australian National Joint Replacement Registry by Conroy and colleagues,75 who reported a significant decrease in the risk for dislocation with large heads (≥ 30 mm) compared with 22-mm heads (relative risk, 1.0 vs 3.1; P ≤ .001).
Few studies have analyzed the role of large-diameter femoral heads in the presence of compromised soft tissues around the hip. Kung and Ries,76 evaluating the influence of large-diameter heads in the presence and absence of a deficient abductor mechanism, demonstrated statistically significant reductions in rates of dislocation after 230 revision THAs when the abductor mechanism was intact with use of 36-mm heads compared with 28-mm heads (12.7% vs 0%; P = .015). With abductor deficiency, though, the positive effect of large heads in reducing dislocation rates was substantially reduced and was similar to that of small heads (P = .74).76
Large heads considerably improve overall stability and lower dislocation rates in THA. With the development of newer ceramics and highly cross-linked polyethylenes, the wear rates reported in multiple studies appear to be less concerning.
Constrained Tripolar Heads
Tripolar heads have been proposed as treatment options for improving stability in patients with chronic and recurrent instability after THA. The tripolar implant consists of a metal head that snap-fits into a polyethylene liner with a polished Co-Cr backing. This bipolar head articulates with a polyethylene bearing that is press-fitted onto a metal acetabular shell and constrained by a metal ring snapped to the outer polyethylene bearing. The bipolar component behaves as a large-diameter femoral head, and the metal ring provides additional restraint, further improving stability.
Williams and colleagues77 performed a systematic review and reported on the outcomes of constrained tripolar liners in 1199 hips at a mean follow-up of 4 years (range, 2-10 years). The mean dislocation rate was 10%, and the mean rate of revision surgery unrelated to instability was 4%. In a study of 43 hips at a mean follow-up of 4 years (range, 2-9 years), Zywiel and colleagues78 reported on the clinical and radiographic outcomes of tripolar constrained liners. Their study group had a mean Harris Hip Score (HHS) of 82 points (range, 38-100 points) and overall survival of 91%, with no evidence of radiographic loosening during follow-up. Despite the improvements in stability with constrained tripolar liners, some authors have reported multiple mechanisms of failure with these devices.79-81 In a study of 43 failed constrained tripolar liners with a mean time to failure of about 2 years, Guyen and colleagues79 identified 5 different failure modes (types 1-5) involving all 4 interfaces in these components.
Encouraging outcomes have been reported at midterm follow-up with tripolar constrained liners. However, concerns about failure at the interfaces suggest that use of these components should be restricted to patients with deficient abductor mechanisms or neuromuscular compromise, low-demand elderly patients, and salvage cases of recurrent dislocations.79
Dual-Mobility Bearings
For more than 20 years, different dual-mobility bearings have been used for difficult acetabular reconstructive scenarios and prevention of instability.82,83 Dual-mobility cups provide constructs that snap-fit a small-diameter femoral head within a large polyethylene insert that articulates with a fixed metal shell. This effectively increases the functional head diameter.
Various authors have reported excellent survivorship rates (92%-99%) and low dislocation rates for these bearings at 5- to 10-year follow-up.82,84-90 Philippot and colleagues,86 in a recent study of 438 hips with dual-mobility cups, reported excellent survivorship (96%) and no early or late instability within a 15-year follow-up. Bouchet and colleagues69 compared dual-mobility bearings (105 hips) with conventional metal-on-polythene bearings (108 hips) and found significantly (P < .05) lower dislocation rates for the dual-mobility implants at a minimum 1-year follow-up. The French Society of Orthopaedics and Traumatology performed a multicenter analysis of 3473 hips with dual-mobility cups implanted in France between January 1998 and December 2003.87 During a mean follow-up of 7 years (range, 5-11 years), there were 15 dislocations (0.43%), 14 of which occurred early, within 3 months of implantation (0.4%). Aseptic implant survivorship was 95% at 10-year follow-up.
Use of these bearings has recently increased in the United States. Short-term and midterm follow-up data show low rates of dislocation and wear. Long-term data are to come.
Clinical and Functional Outcomes of Large-Diameter Femoral Heads
There is a paucity of long-term outcomes data on use of large-diameter heads with highly cross-linked polyethylene bearings. Short-term and midterm clinical results appear to be excellent, with low rates of wear, osteolysis, and aseptic loosening.28,41,73,89-92
Plate and colleagues91 compared the effects of large-diameter (≥ 36 mm) and small-diameter (26 mm, 28 mm) metal heads on highly cross-linked polyethylene bearings. At a mean follow-up of 5 years (range, 4-8.4 years), the large-head cohort had a mean HHS of 90 points (range, 50-100 points) and no dislocations or radiographic evidence of stem or cup loosening. Similarly, Meftah and colleagues93 reported 100% stem survivorship and excellent clinical outcomes—a mean Western Ontario and McMaster Universities Arthritis Index (WOMAC) score of 30 points—for 72 hips with use of large ceramic heads (≥ 32 mm) on highly cross-linked polyethylene at a mean follow-up of 3 years. Gagala and colleagues94 reported excellent clinical and radiographic outcomes in 50 hips (18 ceramic on ceramic, 32 ceramic on polyethylene; 36-mm heads) at a mean follow-up of 3.5 years. Mean HHS was 94 points, and there was no evidence of liner fractures, aseptic loosening, or osteolysis.
In summary, large-diameter femoral heads in THA have become increasingly popular because of improvements in the material properties and wear characteristics of highly cross-linked polyethylene and fourth-generation ceramics. Despite the potential advantages of large heads in preventing dislocations, the basic surgical tenets of placing the acetabular component in appropriate alignment remain firmly established. Implants with functionally large heads (eg, dual-mobility bearings, constrained tripolar liners) may play an important role in patients at high risk for dislocation—particularly elderly patients with poor neuromuscular muscle coordination or deficient abductors, trauma patients, and patients with prior dislocations. Short-term and midterm results are excellent; rates of wear, aseptic loosening, and osteolysis are low. However, long-term outcomes data are needed to support widespread use of large heads in younger and more active patients.
A common cause for total hip arthroplasty (THA) revision is joint instability.1,2 The reported incidence of dislocation in primary THA ranges from 0.4% to 5.8%,3-5 but this rate increases after revision surgery.1,3-8 Use of large-diameter femoral heads has been proposed to decrease the risks for instability and to improve impingement-free range of motion (ROM).
The biomechanical rationale for using large-diameter femoral heads is that they must travel farther before subluxation or dislocation occurs (jump distance). Despite these benefits, there were initial concerns that catastrophic failure and high levels of volumetric wear would occur if these heads were used with conventional polyethylene liners. These concerns led to the development of alternative bearing surfaces, particularly metal-on-metal bearings, which offered theoretical benefits of large-diameter articulations that improved stability while purportedly being highly wear-resistant.9-11 However, concerns about adverse local soft-tissue reactions and high blood concentrations of metal ions tempered the initial enthusiasm for metal bearings.12-16 Fortunately, highly cross-linked polyethylene and fourth-generation ceramic bearing surfaces, with improved toughness and better wear properties, may allow use of large-diameter heads without the need for metal-on-metal bearings.17,18
In this article, we review the concepts and principles behind use of large-diameter ceramic or cobalt-chromium femoral heads on polyethylene-bearing surfaces in THA with particular attention to biomechanics, early concerns about polyethylene wear and rim fractures, recent improvements in material properties of polyethylene and ceramic bearings, dislocation rates, and clinical and functional outcomes.
Definitions
For this review, we define large-diameter femoral heads as having diameters of 36 mm or more and conventional or small-diameter femoral heads as having diameters between 22 and 32 mm.
Biomechanics
Head–Neck Ratio, Impingement-Free ROM, and Jump Distance
Several implant design principles have been proposed to reduce the risks for impingement and dislocation. Of these, large femoral head diameters have been extensively studied.19,20 It is well known that impingement of the femoral neck on the cup edge promotes edge loading and higher wear rates. In addition, impingement increases the tendency of the head to sublux from the acetabulum. One strategy for avoiding this component-to-component impingement is to increase the head–neck ratio (HNR), the ratio of the femoral head to the neck diameter. Biomechanically, increased HNRs lead to delayed contact between the femoral neck and the acetabular liner.21,22 Therefore, with large femoral heads, which have large HNRs, impingement occurs later and at larger ROMs—compared with small-diameter femoral heads, which have lower HNRs and are more prone to early impingement and subluxation (Figure 1).23-26
In a cadaveric study of 6 hips, Bartz and colleagues23 reported a significantly higher preimpingement ROM when the prosthetic head size increased from 22 mm to 28 mm (P < .05). They found a change from prosthetic to osseous impingement when the head size increased from 22 mm to 32 mm. Similar results were observed in a computer simulation model by Cinotti and colleagues,27 who demonstrated that increasing the femoral head size from 28 mm to 38 mm resulted in a 5° improvement in ROM. However, the largest gains were observed when the heads with the smallest diameters were upsized; ROM improved only marginally when femoral head size was further increased from 32 mm to 38 mm. The primary reason for the lack of expected improvement in ROM with head sizes of more than 32 mm is often bone-on-bone impingement. Burroughs and colleagues28 demonstrated that the 38-mm and 44-mm heads virtually eliminated component-to-component impingement except in extremes of external rotation. However, there were no differences in ROM between 38-mm and 44-mm heads because of osseous impingement. In addition, large heads are less likely to sublux or dislocate, as they need to travel farther before reaching the edge of the acetabular cup before dislocation. This is known as the jump distance, and it corresponds to the depth of the acetabular shell, which in turn equates with the radius of the femoral head (Figures 2A, 2B). For this reason, the larger the femoral head diameter, the farther the jump distance and, correspondingly, the lower the risk for dislocation.29
Elevated liners historically were used to increase the jump distance for dislocation.30 These liners, however, can increase impingement at the extremes of motion.31 Some of these problems can be avoided with use of larger heads, which have increased jump distances without additional risks for impingement. Moreover, large heads create a suction effect that provides passive resistance to dislocation.32 With head diameters beyond 38 mm, impingement-free ROM often plateaus. However, the jump distance required for dislocations to occur continues to increase as femoral head diameters increase in size. Thus, patients may experience fewer motion benefits but continue to benefit from overall stability with femoral head sizes increasing beyond 38 mm.
Current evidence suggests there may be substantial benefits toward improved stability from increasing head diameters from 22 mm to 38 mm because of the increase in jump distances and improvements in prosthetic impingement-free ROM. However, there may be little gain in ROM from increasing the head diameters beyond these dimensions because of the potential risks of bony impingement. Nevertheless, there may be some additional benefits toward stability from improvement in jump distances with incremental head sizes
beyond 38 mm.29,33,34
Finite Element Analysis Studies
Finite element analysis of large-diameter heads in THA has shown that, at optimal cup inclination (45°), most stresses occur on the articular surface of the liner. However, these stresses remain well below the yield strength of the polyethylene liners.29 With increasing abduction angles, the stress concentration increases substantially because of the decreased contact surface area. At these angles, the point of maximum contact moves toward the rim of the polyethylene liner, which can lead to rim fractures or failure of locking mechanisms.29,35,36
Early Concerns With Large-Diameter Femoral Heads: Wear, Liner Failure, and Fracture of Ceramic Components
Use of small-diameter femoral heads started with the first report by Charnley37 of “low frictional torque arthroplasty.” Charnley initially considered a 41.5-mm femoral head, but he thought it would increase risks for acetabular loosening from high frictional torque generated by the large head, and he switched to a small-diameter (22.5 mm) design. One of the tradeoffs with smaller diameter heads was decreased jump height in addition to increased linear wear.
Large femoral heads used with cemented polyethylene acetabular components historically have been associated with increased rates of volumetric wear but low rates of linear wear, which potentially may increase the risk for osteolysis.38-40 However, newer highly cross-linked polyethylene liners have shown improved in vitro and in vivo volumetric wear characteristics and potentially lower linear wear rates compared with earlier designs (Table 1).28,41-43
Another concern about earlier generations of large femoral heads was the risk for catastrophic liner failure on conventional polyethylene. This was originally reported by Berry and colleagues,47 who described wear-through and failure in patients with thin (< 5 mm) acetabular cups. However, these concerns have been largely addressed by the development of highly cross-linked polyethylene, which has improved wear characteristics and fatigue resistance.48
Recent Improvements in Material Properties of Polyethylene and Ceramic Bearings
The development of highly cross-linked polyethylene and fourth-generation ceramics has renewed interest in large-diameter bearings in THA. These bearing surfaces improve wear, enhance material properties, and have superior oxidation resistance.42,48-53
We now briefly describe the methods used to improve the material properties of polyethylene and ceramics. Studies have shown that increasing the radiation dose (up to 200 kGy) increases cross-linking and causes an inverse exponential decrease in polyethylene wear.28,41,48-51 However, increasing radiation doses also increases production of free radicals, which diminish the material strength of these polyethylenes. The current generation of highly cross-linked polyethylene liners is produced through a variety of manufacturing strategies to improve cross-linking and reduce wear. These strategies include differential radiation doses (50-100 kGy), techniques (electron beam, radiation), and thermal treatments (melting, annealing). Moreover, to enhance the material properties and reduce the incidence of rim cracking and delamination, authors have proposed using vitamin E supplementation to minimize the amount of subsurface oxidation that occurs as an inevitable consequence of free radical formation during fabrication.54,55 A terminal sterilization process (eg, gas plasma, ethylene oxide, or gamma sterilization in nitrogen) is needed to make commercial, highly cross-linked polyethylene.52,53
Fourth-generation ceramics manufactured with nano-sized yttria-stabilized tetragonal zirconia particles in a stable alumina matrix have more fracture toughness and improved wear characteristics.54,55 In addition, oxide additives (eg, chromium oxide, strontium oxide) improve hardness and dissipate energy by deflecting cracks to prevent their propagation.56 Moreover, the smaller grain sizes of fourth-generation ceramic bearings compared with third-generation designs (0.8 µm vs 1-5 µm) cause less disruption of the fluid film layer, which ultimately results in improved wear performance.57
Multiple studies have found reduced wear rates with metal and ceramic large heads coupled with highly cross-linked polyethylene-bearings (Table 2).17,41,50,58 Bragdon and colleagues,58 using radiostereometric analysis in 25 patients, found no significant differences in mean head penetration rates between 36-mm and 28-mm cobalt-chromium (Co-Cr) heads articulating with highly cross-linked polyethylene cups at a mean follow-up of 3 years (0.035 mm/y vs 0.046 mm/y; P = .11). Geller and colleagues,64 in their study of 42 patients with large-diameter (> 32 mm) Co-Cr femoral heads, found low mean (SD) linear wear rates of 0.06 (0.41) mm/y at a mean follow-up of 3 years. D’Antonio and colleagues,65 in a multicenter study, reported low average linear wear (0.015 mm/y) and volumetric wear (12.1 mm3/y) over 5 years using sequentially annealed cross-linked polyethylene. In vitro reports suggest that large-diameter ceramic heads may have lower wear properties than Co-Cr heads do. Galvin and colleagues,66 in an in vitro hip simulator study, found that large-diameter ceramic heads on highly cross-linked ultrahigh-molecular-weight polyethylene had 40% reductions in steady-state wear rates compared with Co-Cr heads on highly cross-linked bearings (4.7 vs 8.1 mm3/million cycles; P < 0.01).
Dislocation Rates
Several patient, surgeon, and implant factors affect the rate of dislocations after THA. Multiple implant options utilize the biomechanical advantage that large-diameter heads have in improving stability. Various alternatives include use of constrained tripolar heads, dual-mobility bearings, and conventional large-diameter heads with standard liners.67-69
Large-Diameter Heads
Despite the biomechanical advantages of large-diameter metal-on-polyethylene bearings, prior studies have questioned use of these bearings because of risks for increased wear and rim failures. However, the improved wear properties of highly cross-linked polyethylene, elaborated earlier, have led to a reappraisal of this option (Table 2).4,70 Howie and colleagues,71 in a randomized control trial of 644 patients, also found significantly lower rates of dislocation after primary THA with 36-mm heads compared with 28-mm heads (1.3% vs 5.4%; P = .012); in addition, fewer dislocations occurred with 36-mm heads than with 28-mm heads (4.9% vs 12.2%; P = .27) in a series of 44 patients in revision settings. Similarly, in a study conducted with 39,271 Medicare patients between 1998 and 2007, Malkani and colleagues72 found a decrease in the dislocation rate, from 4.21% to 2.14%, with use of large-diameter femoral heads. These results have been confirmed by several other authors.34,66,73,74 Similar results were observed in 65,992 patients in the Australian National Joint Replacement Registry by Conroy and colleagues,75 who reported a significant decrease in the risk for dislocation with large heads (≥ 30 mm) compared with 22-mm heads (relative risk, 1.0 vs 3.1; P ≤ .001).
Few studies have analyzed the role of large-diameter femoral heads in the presence of compromised soft tissues around the hip. Kung and Ries,76 evaluating the influence of large-diameter heads in the presence and absence of a deficient abductor mechanism, demonstrated statistically significant reductions in rates of dislocation after 230 revision THAs when the abductor mechanism was intact with use of 36-mm heads compared with 28-mm heads (12.7% vs 0%; P = .015). With abductor deficiency, though, the positive effect of large heads in reducing dislocation rates was substantially reduced and was similar to that of small heads (P = .74).76
Large heads considerably improve overall stability and lower dislocation rates in THA. With the development of newer ceramics and highly cross-linked polyethylenes, the wear rates reported in multiple studies appear to be less concerning.
Constrained Tripolar Heads
Tripolar heads have been proposed as treatment options for improving stability in patients with chronic and recurrent instability after THA. The tripolar implant consists of a metal head that snap-fits into a polyethylene liner with a polished Co-Cr backing. This bipolar head articulates with a polyethylene bearing that is press-fitted onto a metal acetabular shell and constrained by a metal ring snapped to the outer polyethylene bearing. The bipolar component behaves as a large-diameter femoral head, and the metal ring provides additional restraint, further improving stability.
Williams and colleagues77 performed a systematic review and reported on the outcomes of constrained tripolar liners in 1199 hips at a mean follow-up of 4 years (range, 2-10 years). The mean dislocation rate was 10%, and the mean rate of revision surgery unrelated to instability was 4%. In a study of 43 hips at a mean follow-up of 4 years (range, 2-9 years), Zywiel and colleagues78 reported on the clinical and radiographic outcomes of tripolar constrained liners. Their study group had a mean Harris Hip Score (HHS) of 82 points (range, 38-100 points) and overall survival of 91%, with no evidence of radiographic loosening during follow-up. Despite the improvements in stability with constrained tripolar liners, some authors have reported multiple mechanisms of failure with these devices.79-81 In a study of 43 failed constrained tripolar liners with a mean time to failure of about 2 years, Guyen and colleagues79 identified 5 different failure modes (types 1-5) involving all 4 interfaces in these components.
Encouraging outcomes have been reported at midterm follow-up with tripolar constrained liners. However, concerns about failure at the interfaces suggest that use of these components should be restricted to patients with deficient abductor mechanisms or neuromuscular compromise, low-demand elderly patients, and salvage cases of recurrent dislocations.79
Dual-Mobility Bearings
For more than 20 years, different dual-mobility bearings have been used for difficult acetabular reconstructive scenarios and prevention of instability.82,83 Dual-mobility cups provide constructs that snap-fit a small-diameter femoral head within a large polyethylene insert that articulates with a fixed metal shell. This effectively increases the functional head diameter.
Various authors have reported excellent survivorship rates (92%-99%) and low dislocation rates for these bearings at 5- to 10-year follow-up.82,84-90 Philippot and colleagues,86 in a recent study of 438 hips with dual-mobility cups, reported excellent survivorship (96%) and no early or late instability within a 15-year follow-up. Bouchet and colleagues69 compared dual-mobility bearings (105 hips) with conventional metal-on-polythene bearings (108 hips) and found significantly (P < .05) lower dislocation rates for the dual-mobility implants at a minimum 1-year follow-up. The French Society of Orthopaedics and Traumatology performed a multicenter analysis of 3473 hips with dual-mobility cups implanted in France between January 1998 and December 2003.87 During a mean follow-up of 7 years (range, 5-11 years), there were 15 dislocations (0.43%), 14 of which occurred early, within 3 months of implantation (0.4%). Aseptic implant survivorship was 95% at 10-year follow-up.
Use of these bearings has recently increased in the United States. Short-term and midterm follow-up data show low rates of dislocation and wear. Long-term data are to come.
Clinical and Functional Outcomes of Large-Diameter Femoral Heads
There is a paucity of long-term outcomes data on use of large-diameter heads with highly cross-linked polyethylene bearings. Short-term and midterm clinical results appear to be excellent, with low rates of wear, osteolysis, and aseptic loosening.28,41,73,89-92
Plate and colleagues91 compared the effects of large-diameter (≥ 36 mm) and small-diameter (26 mm, 28 mm) metal heads on highly cross-linked polyethylene bearings. At a mean follow-up of 5 years (range, 4-8.4 years), the large-head cohort had a mean HHS of 90 points (range, 50-100 points) and no dislocations or radiographic evidence of stem or cup loosening. Similarly, Meftah and colleagues93 reported 100% stem survivorship and excellent clinical outcomes—a mean Western Ontario and McMaster Universities Arthritis Index (WOMAC) score of 30 points—for 72 hips with use of large ceramic heads (≥ 32 mm) on highly cross-linked polyethylene at a mean follow-up of 3 years. Gagala and colleagues94 reported excellent clinical and radiographic outcomes in 50 hips (18 ceramic on ceramic, 32 ceramic on polyethylene; 36-mm heads) at a mean follow-up of 3.5 years. Mean HHS was 94 points, and there was no evidence of liner fractures, aseptic loosening, or osteolysis.
In summary, large-diameter femoral heads in THA have become increasingly popular because of improvements in the material properties and wear characteristics of highly cross-linked polyethylene and fourth-generation ceramics. Despite the potential advantages of large heads in preventing dislocations, the basic surgical tenets of placing the acetabular component in appropriate alignment remain firmly established. Implants with functionally large heads (eg, dual-mobility bearings, constrained tripolar liners) may play an important role in patients at high risk for dislocation—particularly elderly patients with poor neuromuscular muscle coordination or deficient abductors, trauma patients, and patients with prior dislocations. Short-term and midterm results are excellent; rates of wear, aseptic loosening, and osteolysis are low. However, long-term outcomes data are needed to support widespread use of large heads in younger and more active patients.
1. Dorr LD, Wolf AW, Chandler R, Conaty JP. Classification and treatment of dislocations of total hip arthroplasty. Clin Orthop. 1983;(173):151-158.
2. Dorr LD, Wan Z. Causes of and treatment protocol for instability of total hip replacement. Clin Orthop. 1998;(355):144-151.
3. Turner RS. Postoperative total hip prosthetic femoral head dislocations. Incidence, etiologic factors, and management. Clin Orthop. 1994;(301):196-204.
4. Woo RY, Morrey BF. Dislocations after total hip arthroplasty. J Bone Joint Surg Am. 1982;64(9):1295-1306.
5. Etienne A, Cupic Z, Charnley J. Postoperative dislocation after Charnley low-friction arthroplasty. Clin Orthop. 1978;(132):19-23.
6. Fackler CD, Poss R. Dislocation in total hip arthroplasties. Clin Orthop. 1980;(151):169-178.
7. Joshi A, Lee CM, Markovic L, Vlatis G, Murphy JC. Prognosis of dislocation after total hip arthroplasty. J Arthroplasty. 1998;13(1):17-21.
8. Lindberg HO, Carlsson AS, Gentz CF, Pettersson H. Recurrent and non-recurrent dislocation following total hip arthroplasty. Acta Orthop Scand. 1982;53(6):947-952.
9. Eswaramoorthy V, Moonot P, Kalairajah Y, Biant LC, Field RE. The Metasul metal-on-metal articulation in primary total hip replacement: clinical and radiological results at ten years. J Bone Joint Surg Br. 2008;90(10):1278-1283.
10. Grubl A, Marker M, Brodner W, et al. Long-term follow-up of metal-on-metal total hip replacement. J Orthop Res. 2007;25(7):841-848.
11. Leslie I, Williams S, Brown C, et al. Effect of bearing size on the long-term wear, wear debris, and ion levels of large diameter metal-on-metal hip replacements—an in vitro study. J Biomed Mater Res B Appl Biomater. 2008;87(1):163-172.
12. Verhaar JA. The hard lesson of metal-on-metal hip implants [in Dutch]. Ned Tijdschr Geneeskd. 2012;156(42):A5564.
13. Fabi D, Levine B, Paprosky W, et al. Metal-on-metal total hip arthroplasty: causes and high incidence of early failure. Orthopedics. 2012;35(7):e1009-e1016.
14. Heneghan C, Langton D, Thompson M. Ongoing problems with metal-on-metal hip implants. BMJ. 2012;344:e1349.
15. Lee RK, Nevelos J, Vigdorchik J, Markel DC. Bearing surfaces for hip arthroplasty—is metal-on-metal a passing fancy? Surg Technol Int. 2012;22:243-249.
16. Voleti PB, Baldwin KD, Lee GC. Metal-on-metal vs conventional total hip arthroplasty: a systematic review and meta-analysis of randomized controlled trials. J Arthroplasty. 2012;27(10):1844-1849.
17. Urban JA, Garvin KL, Boese CK, et al. Ceramic-on-polyethylene bearing surfaces in total hip arthroplasty. Seventeen to twenty-one-year results.
J Bone Joint Surg Am. 2001;83(11):1688-1694.
18. Callaghan JJ, Liu SS. Ceramic on crosslinked polyethylene in total hip replacement: any better than metal on crosslinked polyethylene? Iowa Orthop J. 2009;29:1-4.
19. Barrack RL. Dislocation after total hip arthroplasty: implant design and orientation. J Am Acad Orthop Surg. 2003;11(2):89-99.
20. Krushell RJ, Burke DW, Harris WH. Elevated-rim acetabular components. Effect on range of motion and stability in total hip arthroplasty. J Arthroplasty. 1991;6(suppl):S53-S58.
21. Morrey BF. Instability after total hip arthroplasty. Orthop Clin North Am. 1992;23(2):237-248.
22. Morrey BF. Dislocation. In: Morrey BF, ed. Joint Replacement Arthroplasty. New York, NY: Churchill Livingstone; 1991:851-865.
23. Bartz RL, Nobel PC, Kadakia NR, Tullos HS. The effect of femoral component head size on posterior dislocation of the artificial hip joint. J Bone Joint Surg Am. 2000;82(9):1300-1307.
24. Nicholas RM, Orr JF, Mollan RA, Calderwood JW, Nixon JR, Watson P. Dislocation of total hip replacements. A comparative study of standard, long posterior wall and augmented acetabular components. J Bone Joint Surg Br. 1990;72(3):418-422.
25. McCollum DE, Gray WJ. Dislocation after total hip arthroplasty. Causes and prevention. Clin Orthop. 1990;(261):159-170.
26. Herrlin K, Selvik G, Pettersson H, Kesek P, Onnerfalt R, Ohlin A. Position, orientation and component interaction in dislocation of the total hip prosthesis. Acta Radiol. 1988;29(4):441-444.
27. Cinotti G, Lucioli N, Malagoli A, Calderoli C, Cassese F. Do large femoral heads reduce the risks of impingement in total hip arthroplasty with optimal and non-optimal cup positioning? Int Orthop. 2011;35(3):317-323.
28. Burroughs BR, Rubash HE, Harris WH. Femoral head sizes larger than 32 mm against highly cross-linked polyethylene. Clin Orthop. 2002;(405):150-157.
29. Crowninshield RD, Maloney WJ, Wentz DH, Humphrey SM, Blanchard CR. Biomechanics of large femoral heads: what they do and don‘t do. Clin Orthop. 2004;(429):102-107.
30. Charnley J. Low Friction Arthroplasty of the Hip: Theory and Practice. New York, NY: Springer; 1979.
31. Yamaguchi M, Akisue T, Bauer TW, Hashimoto Y. The spatial location of impingement in total hip arthroplasty. J Arthroplasty. 2000;15(3):305-313.
32. Peters CL, McPherson E, Jackson JD, Erickson JA. Reduction in early dislocation rate with large-diameter femoral heads in primary total hip arthroplasty. J Arthroplasty. 2007;22(6 suppl 2):140-144.
33. Masonis JL, Bourne RB. Surgical approach, abductor function, and total hip arthroplasty dislocation. Clin Orthop. 2002;(405):46-53.
34. Beaule PE, Schmalzried TP, Udomkiat P, Amstutz HC. Jumbo femoral head for the treatment of recurrent dislocation following total hip replacement. J Bone Joint Surg Am. 2002;84(2):256-263.
35. Oral E, Malhi AS, Muratoglu OK. Mechanisms of decrease in fatigue crack propagation resistance in irradiated and melted UHMWPE. Biomaterials. 2006;27(6):917-925.
36. Baker DA, Bellare A, Pruitt L. The effects of degree of crosslinking on the fatigue crack initiation and propagation resistance of orthopedic-grade polyethylene. J Biomed Mater Res A. 2003;66(1):146-154.
37. Charnley J. Total hip replacement by low-friction arthroplasty. Clin Orthop. 1970;(72):7-21.
38. Kabo JM, Gebhard JS, Loren G, Amstutz HC. In vivo wear of polyethylene acetabular components. J Bone Joint Surg Br. 1993;75(2):254-258.
39. Livermore J, Ilstrup D, Morrey B. Effect of femoral head size on wear of the polyethylene acetabular component. J Bone Joint Surg Am. 1990;72(4):518-528.
40. Ma SM, Kabo JM, Amstutz HC. Frictional torque in surface and conventional hip replacement. J Bone Joint Surg Am. 1983;65(3):366-370.
41. Muratoglu OK, Bragdon CR, O‘Connor D, et al. Larger diameter femoral heads used in conjunction with a highly cross-linked ultra-high molecular weight polyethylene: a new concept. J Arthroplasty. 2001;16(8 suppl 1):24-30.
42. Thomas GER, Simpson DJ, Mehmood S, et al. The seven-year wear of highly cross-linked polyethylene in total hip arthroplasty: a double-blind, randomized controlled trial using radiostereometric analysis. J Bone Joint Surg Am. 2011;93(8):716-722.
43. Mutimer J, Devane PA, Adams K, Horne JG. Highly crosslinked polyethylene reduces wear in total hip arthroplasty at 5 years. Clin Orthop. 2010;468(12):3228-3233.
44. Bragdon CR, Doerner M, Martell J, Jarrett B, Palm H, Malchau H. The 2012 John Charnley Award: clinical multicenter studies of the wear performance of highly crosslinked remelted polyethylene in THA. Clin Orthop. 2013;471(2):393-402.
45. Lachiewicz PF, Heckman DS, Soileau ES, Mangla J, Martell JM. Femoral head size and wear of highly cross-linked polyethylene at 5 to 8 years. Clin Orthop. 2009;467(12):3290-3296.
46. Sychterz CJ, Engh CA Jr, Young AM, Hopper RH Jr, Engh CA. Comparison of in vivo wear between polyethylene liners articulating with ceramic and cobalt-chrome femoral heads. J Bone Joint Surg Br. 2000;82(7):948-951.
47. Berry DJ, Barnes CL, Scott RD, Cabanela ME, Poss R. Catastrophic failure of the polyethylene liner of uncemented acetabular components.
J Bone Joint Surg Br. 1994;76(4):575-578.
48. McKellop H, Shen FW, Lu B, Campbell P, Salovey R. Development of an extremely wear-resistant ultra high molecular weight polyethylene for total hip replacements. J Orthop Res. 1999;17(2):157-167.
49. Wang A, Essner A, Polineni VK, Stark C, Dumbleton JH. Lubrication and wear of ultra-high molecular weight polyethylene in total joint replacements. Tribol Int. 1998;31(1-3):17-33.
50. Estok DM 2nd, Burroughs BR, Muratoglu OK, Harris WH. Comparison of hip simulator wear of 2 different highly cross-linked ultra high molecular weight polyethylene acetabular components using both 32- and 38-mm femoral heads. J Arthroplasty. 2007;22(4):581-589.
51. Muratoglu OK, Bragdon CR, O‘Connor DO, et al. Unified wear model for highly crosslinked ultra-high molecular weight polyethylenes (UHMWPE). Biomaterials. 1999;20(16):1463-1470.
52. Harris WH, Muratoglu OK. A review of current cross-linked polyethylenes used in total joint arthroplasty. Clin Orthop. 2005;(430):46-52.
53. Muratoglu OK, Bragdon CR, O‘Connor DO, Jasty M, Harris WH. A novel method of cross-linking ultra-high-molecular-weight polyethylene to improve wear, reduce oxidation, and retain mechanical properties. Recipient of the 1999 HAP Paul Award. J Arthroplasty. 2001;16(2):149-160.
54. Bal BS, Garino J, Ries M, Rahaman MN. A review of ceramic bearing materials in total joint arthroplasty. Hip Int. 2007;17(1):21-30.
55. Traina F, De Fine M, Di Martino A, Faldini C. Fracture of ceramic bearing surfaces following total hip replacement: a systematic review. Biomed Res Int. 2013;2013:157247.
56. Cai YZ, Yan SG. Development of ceramic-on-ceramic implants for total hip arthroplasty. Orthop Surg. 2010;2(3):175-181.
57. Stewart TD, Tipper JL, Insley G, Streicher RM, Ingham E, Fisher J. Long-term wear of ceramic matrix composite materials for hip prostheses under severe swing phase microseparation. J Biomed Mater Res B Appl Biomater. 2003;66(2):567-573.
58. Bragdon CR, Greene ME, Freiberg AA, Harris WH, Malchau H. Radiostereometric analysis comparison of wear of highly cross-linked polyethylene against 36- vs 28-mm femoral heads. J Arthroplasty. 2007;22(6 suppl 2):125-129.
59. Lombardi AV Jr, Skeels MD, Berend KR, Adams JB, Franchi OJ. Do large heads enhance stability and restore native anatomy in primary total hip arthroplasty? Clin Orthop. 2011;469(6):1547-1553.
60. Lachiewicz PF, Soileau ES. Low early and late dislocation rates with 36- and 40-mm heads in patients at high risk for dislocation. Clin Orthop. 2013;471(2):439-443.
61. Cai P, Hu Y, Xie J. Large-diameter Delta ceramic-on-ceramic versus common-sized ceramic-on-polyethylene bearings in THA. Orthopedics. 2012;35(9):e1307-e1313.
62. Park KS, Yoon TR, Hwang SY, Lee KB. Modified minimally invasive two-incision total hip arthroplasty using large diameter femoral head. Indian
J Orthop. 2012;46(1):29-35.
63. Garbuz DS, Masri BA, Duncan CP, et al. The Frank Stinchfield Award: dislocation in revision THA: do large heads (36 and 40 mm) result in reduced dislocation rates in a randomized clinical trial? Clin Orthop. 2012;470(2):351-356.
64. Geller JA, Malchau H, Bragdon C, Greene M, Harris WH, Freiberg AA. Large diameter femoral heads on highly cross-linked polyethylene: minimum 3-year results. Clin Orthop. 2006;(447):53-59.
65. D’Antonio JA, Capello WN, Ramakrishnan R. Second-generation annealed highly cross-linked polyethylene exhibits low wear. Clin Orthop. 2012;470(6):1696-1704.
66. Galvin AL, Jennings LM, Tipper JL, Ingham E, Fisher J. Wear and creep of highly crosslinked polyethylene against cobalt chrome and ceramic femoral heads. Proc Inst Mech Eng H. 2010;224(10):1175-1183.
67. Skeels MD, Berend KR, Lombardi AV Jr. The dislocator, early and late: the role of large heads. Orthopedics. 2009;32(9).
68. Plate JF, Seyler TM, Stroh DA, Issa K, Akbar M, Mont MA. Risk of dislocation using large- vs. small-diameter femoral heads in total hip arthroplasty. BMC Res Notes. 2012;5(1):553.
69. Bouchet R, Mercier N, Saragaglia D. Posterior approach and dislocation rate: a 213 total hip replacements case–control study comparing the dual mobility cup with a conventional 28-mm metal head/polyethylene prosthesis. Orthop Traumatol Surg Res. 2011;97(1):2-7.
70. Ali Khan MA, Brakenbury PH, Reynolds IS. Dislocation following total hip replacement. J Bone Joint Surg Br. 1981;63(2):214-218.
71. Howie DW, Holubowycz OT, Middleton R. Large femoral heads decrease the incidence of dislocation after total hip arthroplasty: a randomized controlled trial. J Bone Joint Surg Am. 2012;94(12):1095-1102.
72. Malkani AL, Ong KL, Lau E, Kurtz SM, Justice BJ, Manley MT. Early- and late-term dislocation risk after primary hip arthroplasty in the Medicare population. J Arthroplasty. 2010;25(6 suppl):21-25.
73. Berry DJ, von Knoch M, Schleck CD, Harmsen WS. Effect of femoral head diameter and operative approach on risk of dislocation after primary total hip arthroplasty. J Bone Joint Surg Am. 2005;87(11):2456-2463.
74. Cho MR, Lee HS, Lee SW, Choi CH, Kim SK, Ko SB. Results after total hip arthroplasty with a large head and bipolar arthroplasty in patients with displaced femoral neck fractures. J Arthroplasty. 2011;26(6):893-896.
75. Conroy JL, Whitehouse SL, Graves SE, Pratt NL, Ryan P, Crawford RW. Risk factors for revision for early dislocation in total hip arthroplasty.
J Arthroplasty. 2008;23(6):867-872.
76. Kung PL, Ries MD. Effect of femoral head size and abductors on dislocation after revision THA. Clin Orthop. 2007;(465):170-174.
77. Williams JT Jr, Ragland PS, Clarke S. Constrained components for the unstable hip following total hip arthroplasty: a literature review. Int Orthop. 2007;31(3):273-277.
78. Zywiel MG, Mustafa LH, Bonutti PM, Mont MA. Are abductor muscle quality and previous revision surgery predictors of constrained liner failure in hip arthroplasty? Int Orthop. 2011;35(6):797-802.
79. Guyen O, Lewallen DG, Cabanela ME. Modes of failure of Osteonics constrained tripolar implants: a retrospective analysis of forty-three failed implants. J Bone Joint Surg Am. 2008;90(7):1553-1560.
80. Banks LN, McElwain JP. An unusual mode of failure of a tripolar constrained acetabular liner: a case report. Arch Orthop Trauma Surg. 2010;130(4):503-505.
81. Robertson WJ, Mattern CJ, Hur J, Su EP, Pellicci PM. Failure mechanisms and closed reduction of a constrained tripolar acetabular liner.
J Arthroplasty. 2009;24(2):322.e5-e11.
82. Aubriot JH, Lesimple P, Leclercq S. Study of Bousquet‘s non-cemented acetabular implant in 100 hybrid total hip prostheses (Charnley type cemented femoral component). Average 5-year follow-up [in French]. Acta Orthop Belg. 1993;59(suppl 1):267-271.
83. Farizon F, de Lavison R, Azoulai JJ, Bousquet G. Results with a cementless alumina-coated cup with dual mobility. A twelve-year follow-up study. Int Orthop. 1998;22(4):219-224.
84. Mertl P, Combes A, Leiber-Wackenheim F, Fessy MH, Girard J, Migaud H. Recurrence of dislocation following total hip arthroplasty revision using dual mobility cups was rare in 180 hips followed over 7 years. HSS J. 2012;8(3):251-256.
85. Langlais FL, Ropars M, Gaucher F, Musset T, Chaix O. Dual mobility cemented cups have low dislocation rates in THA revisions. Clin Orthop. 2008;466(2):389-395.
86. Philippot R, Farizon F, Camilleri JP, et al. Survival of dual mobility socket with a mean 17 years follow-up [in French]. Rev Chir Orthop Reparatrice Appar Mot. 2008;94(1):43-48.
87. Adam P, Philippe R, Ehlinger M, et al. Dual mobility cups hip arthroplasty as a treatment for displaced fracture of the femoral neck in the elderly.
A prospective, systematic, multicenter study with specific focus on postoperative dislocation. Orthop Traumatol Surg Res. 2012;98(3):296-300.
88. Fessy MH. La double mobilité [Dual mobility]. Revue de Chirurgie Orthopédique et Traumatologique. 2010;96(7):891-898.
89. Mont MA, Issa K, Naziri Q, Harwin SF, Delanois RE, Johnson AJ. The use of dual-mobility bearings in difficult hip arthroplasty reconstructive cases. Surg Technol Int. 2011;21:234-240.
90. Sayeed SA, Mont MA, Costa CR, et al. Early outcomes of sequentially cross-linked thin polyethylene liners with large diameter femoral heads in total hip arthroplasty. Bull NYU Hosp Jt Dis. 2011;69(suppl 1):S90-S94.
91. Plate JF, Seyler TM, Stroh DA, Issa K, Akbar M, Mont MA. Risk of dislocation using large- vs. small-diameter femoral heads in total hip arthroplasty. BMC Res Notes. 2012;5(1):553.
92. Sato T, Nakashima Y, Akiyama M, et al. Wear resistant performance of highly cross-linked and annealed ultra-high molecular weight polyethylene against ceramic heads in total hip arthroplasty. J Orthop Res. 2012;30(12):2031-2037.
93. Meftah M, Ebrahimpour PB, He C, Ranawat AS, Ranawat CS. Preliminary clinical and radiographic results of large ceramic heads on highly cross-linked polyethylene. Orthopedics. 2011;34(6):133.
94. Gagala J, Mazurkiewicz T, Dajewski Z. Large diameter femoral heads in primary alumina/alumina and XSPE/alumina total hip arthroplasty.
A follow-up study of 50 hips after average 40 months and review of literature [in Polish]. Chir Narzadow Ruchu Ortop Pol. 2011;76(1):14-20.
1. Dorr LD, Wolf AW, Chandler R, Conaty JP. Classification and treatment of dislocations of total hip arthroplasty. Clin Orthop. 1983;(173):151-158.
2. Dorr LD, Wan Z. Causes of and treatment protocol for instability of total hip replacement. Clin Orthop. 1998;(355):144-151.
3. Turner RS. Postoperative total hip prosthetic femoral head dislocations. Incidence, etiologic factors, and management. Clin Orthop. 1994;(301):196-204.
4. Woo RY, Morrey BF. Dislocations after total hip arthroplasty. J Bone Joint Surg Am. 1982;64(9):1295-1306.
5. Etienne A, Cupic Z, Charnley J. Postoperative dislocation after Charnley low-friction arthroplasty. Clin Orthop. 1978;(132):19-23.
6. Fackler CD, Poss R. Dislocation in total hip arthroplasties. Clin Orthop. 1980;(151):169-178.
7. Joshi A, Lee CM, Markovic L, Vlatis G, Murphy JC. Prognosis of dislocation after total hip arthroplasty. J Arthroplasty. 1998;13(1):17-21.
8. Lindberg HO, Carlsson AS, Gentz CF, Pettersson H. Recurrent and non-recurrent dislocation following total hip arthroplasty. Acta Orthop Scand. 1982;53(6):947-952.
9. Eswaramoorthy V, Moonot P, Kalairajah Y, Biant LC, Field RE. The Metasul metal-on-metal articulation in primary total hip replacement: clinical and radiological results at ten years. J Bone Joint Surg Br. 2008;90(10):1278-1283.
10. Grubl A, Marker M, Brodner W, et al. Long-term follow-up of metal-on-metal total hip replacement. J Orthop Res. 2007;25(7):841-848.
11. Leslie I, Williams S, Brown C, et al. Effect of bearing size on the long-term wear, wear debris, and ion levels of large diameter metal-on-metal hip replacements—an in vitro study. J Biomed Mater Res B Appl Biomater. 2008;87(1):163-172.
12. Verhaar JA. The hard lesson of metal-on-metal hip implants [in Dutch]. Ned Tijdschr Geneeskd. 2012;156(42):A5564.
13. Fabi D, Levine B, Paprosky W, et al. Metal-on-metal total hip arthroplasty: causes and high incidence of early failure. Orthopedics. 2012;35(7):e1009-e1016.
14. Heneghan C, Langton D, Thompson M. Ongoing problems with metal-on-metal hip implants. BMJ. 2012;344:e1349.
15. Lee RK, Nevelos J, Vigdorchik J, Markel DC. Bearing surfaces for hip arthroplasty—is metal-on-metal a passing fancy? Surg Technol Int. 2012;22:243-249.
16. Voleti PB, Baldwin KD, Lee GC. Metal-on-metal vs conventional total hip arthroplasty: a systematic review and meta-analysis of randomized controlled trials. J Arthroplasty. 2012;27(10):1844-1849.
17. Urban JA, Garvin KL, Boese CK, et al. Ceramic-on-polyethylene bearing surfaces in total hip arthroplasty. Seventeen to twenty-one-year results.
J Bone Joint Surg Am. 2001;83(11):1688-1694.
18. Callaghan JJ, Liu SS. Ceramic on crosslinked polyethylene in total hip replacement: any better than metal on crosslinked polyethylene? Iowa Orthop J. 2009;29:1-4.
19. Barrack RL. Dislocation after total hip arthroplasty: implant design and orientation. J Am Acad Orthop Surg. 2003;11(2):89-99.
20. Krushell RJ, Burke DW, Harris WH. Elevated-rim acetabular components. Effect on range of motion and stability in total hip arthroplasty. J Arthroplasty. 1991;6(suppl):S53-S58.
21. Morrey BF. Instability after total hip arthroplasty. Orthop Clin North Am. 1992;23(2):237-248.
22. Morrey BF. Dislocation. In: Morrey BF, ed. Joint Replacement Arthroplasty. New York, NY: Churchill Livingstone; 1991:851-865.
23. Bartz RL, Nobel PC, Kadakia NR, Tullos HS. The effect of femoral component head size on posterior dislocation of the artificial hip joint. J Bone Joint Surg Am. 2000;82(9):1300-1307.
24. Nicholas RM, Orr JF, Mollan RA, Calderwood JW, Nixon JR, Watson P. Dislocation of total hip replacements. A comparative study of standard, long posterior wall and augmented acetabular components. J Bone Joint Surg Br. 1990;72(3):418-422.
25. McCollum DE, Gray WJ. Dislocation after total hip arthroplasty. Causes and prevention. Clin Orthop. 1990;(261):159-170.
26. Herrlin K, Selvik G, Pettersson H, Kesek P, Onnerfalt R, Ohlin A. Position, orientation and component interaction in dislocation of the total hip prosthesis. Acta Radiol. 1988;29(4):441-444.
27. Cinotti G, Lucioli N, Malagoli A, Calderoli C, Cassese F. Do large femoral heads reduce the risks of impingement in total hip arthroplasty with optimal and non-optimal cup positioning? Int Orthop. 2011;35(3):317-323.
28. Burroughs BR, Rubash HE, Harris WH. Femoral head sizes larger than 32 mm against highly cross-linked polyethylene. Clin Orthop. 2002;(405):150-157.
29. Crowninshield RD, Maloney WJ, Wentz DH, Humphrey SM, Blanchard CR. Biomechanics of large femoral heads: what they do and don‘t do. Clin Orthop. 2004;(429):102-107.
30. Charnley J. Low Friction Arthroplasty of the Hip: Theory and Practice. New York, NY: Springer; 1979.
31. Yamaguchi M, Akisue T, Bauer TW, Hashimoto Y. The spatial location of impingement in total hip arthroplasty. J Arthroplasty. 2000;15(3):305-313.
32. Peters CL, McPherson E, Jackson JD, Erickson JA. Reduction in early dislocation rate with large-diameter femoral heads in primary total hip arthroplasty. J Arthroplasty. 2007;22(6 suppl 2):140-144.
33. Masonis JL, Bourne RB. Surgical approach, abductor function, and total hip arthroplasty dislocation. Clin Orthop. 2002;(405):46-53.
34. Beaule PE, Schmalzried TP, Udomkiat P, Amstutz HC. Jumbo femoral head for the treatment of recurrent dislocation following total hip replacement. J Bone Joint Surg Am. 2002;84(2):256-263.
35. Oral E, Malhi AS, Muratoglu OK. Mechanisms of decrease in fatigue crack propagation resistance in irradiated and melted UHMWPE. Biomaterials. 2006;27(6):917-925.
36. Baker DA, Bellare A, Pruitt L. The effects of degree of crosslinking on the fatigue crack initiation and propagation resistance of orthopedic-grade polyethylene. J Biomed Mater Res A. 2003;66(1):146-154.
37. Charnley J. Total hip replacement by low-friction arthroplasty. Clin Orthop. 1970;(72):7-21.
38. Kabo JM, Gebhard JS, Loren G, Amstutz HC. In vivo wear of polyethylene acetabular components. J Bone Joint Surg Br. 1993;75(2):254-258.
39. Livermore J, Ilstrup D, Morrey B. Effect of femoral head size on wear of the polyethylene acetabular component. J Bone Joint Surg Am. 1990;72(4):518-528.
40. Ma SM, Kabo JM, Amstutz HC. Frictional torque in surface and conventional hip replacement. J Bone Joint Surg Am. 1983;65(3):366-370.
41. Muratoglu OK, Bragdon CR, O‘Connor D, et al. Larger diameter femoral heads used in conjunction with a highly cross-linked ultra-high molecular weight polyethylene: a new concept. J Arthroplasty. 2001;16(8 suppl 1):24-30.
42. Thomas GER, Simpson DJ, Mehmood S, et al. The seven-year wear of highly cross-linked polyethylene in total hip arthroplasty: a double-blind, randomized controlled trial using radiostereometric analysis. J Bone Joint Surg Am. 2011;93(8):716-722.
43. Mutimer J, Devane PA, Adams K, Horne JG. Highly crosslinked polyethylene reduces wear in total hip arthroplasty at 5 years. Clin Orthop. 2010;468(12):3228-3233.
44. Bragdon CR, Doerner M, Martell J, Jarrett B, Palm H, Malchau H. The 2012 John Charnley Award: clinical multicenter studies of the wear performance of highly crosslinked remelted polyethylene in THA. Clin Orthop. 2013;471(2):393-402.
45. Lachiewicz PF, Heckman DS, Soileau ES, Mangla J, Martell JM. Femoral head size and wear of highly cross-linked polyethylene at 5 to 8 years. Clin Orthop. 2009;467(12):3290-3296.
46. Sychterz CJ, Engh CA Jr, Young AM, Hopper RH Jr, Engh CA. Comparison of in vivo wear between polyethylene liners articulating with ceramic and cobalt-chrome femoral heads. J Bone Joint Surg Br. 2000;82(7):948-951.
47. Berry DJ, Barnes CL, Scott RD, Cabanela ME, Poss R. Catastrophic failure of the polyethylene liner of uncemented acetabular components.
J Bone Joint Surg Br. 1994;76(4):575-578.
48. McKellop H, Shen FW, Lu B, Campbell P, Salovey R. Development of an extremely wear-resistant ultra high molecular weight polyethylene for total hip replacements. J Orthop Res. 1999;17(2):157-167.
49. Wang A, Essner A, Polineni VK, Stark C, Dumbleton JH. Lubrication and wear of ultra-high molecular weight polyethylene in total joint replacements. Tribol Int. 1998;31(1-3):17-33.
50. Estok DM 2nd, Burroughs BR, Muratoglu OK, Harris WH. Comparison of hip simulator wear of 2 different highly cross-linked ultra high molecular weight polyethylene acetabular components using both 32- and 38-mm femoral heads. J Arthroplasty. 2007;22(4):581-589.
51. Muratoglu OK, Bragdon CR, O‘Connor DO, et al. Unified wear model for highly crosslinked ultra-high molecular weight polyethylenes (UHMWPE). Biomaterials. 1999;20(16):1463-1470.
52. Harris WH, Muratoglu OK. A review of current cross-linked polyethylenes used in total joint arthroplasty. Clin Orthop. 2005;(430):46-52.
53. Muratoglu OK, Bragdon CR, O‘Connor DO, Jasty M, Harris WH. A novel method of cross-linking ultra-high-molecular-weight polyethylene to improve wear, reduce oxidation, and retain mechanical properties. Recipient of the 1999 HAP Paul Award. J Arthroplasty. 2001;16(2):149-160.
54. Bal BS, Garino J, Ries M, Rahaman MN. A review of ceramic bearing materials in total joint arthroplasty. Hip Int. 2007;17(1):21-30.
55. Traina F, De Fine M, Di Martino A, Faldini C. Fracture of ceramic bearing surfaces following total hip replacement: a systematic review. Biomed Res Int. 2013;2013:157247.
56. Cai YZ, Yan SG. Development of ceramic-on-ceramic implants for total hip arthroplasty. Orthop Surg. 2010;2(3):175-181.
57. Stewart TD, Tipper JL, Insley G, Streicher RM, Ingham E, Fisher J. Long-term wear of ceramic matrix composite materials for hip prostheses under severe swing phase microseparation. J Biomed Mater Res B Appl Biomater. 2003;66(2):567-573.
58. Bragdon CR, Greene ME, Freiberg AA, Harris WH, Malchau H. Radiostereometric analysis comparison of wear of highly cross-linked polyethylene against 36- vs 28-mm femoral heads. J Arthroplasty. 2007;22(6 suppl 2):125-129.
59. Lombardi AV Jr, Skeels MD, Berend KR, Adams JB, Franchi OJ. Do large heads enhance stability and restore native anatomy in primary total hip arthroplasty? Clin Orthop. 2011;469(6):1547-1553.
60. Lachiewicz PF, Soileau ES. Low early and late dislocation rates with 36- and 40-mm heads in patients at high risk for dislocation. Clin Orthop. 2013;471(2):439-443.
61. Cai P, Hu Y, Xie J. Large-diameter Delta ceramic-on-ceramic versus common-sized ceramic-on-polyethylene bearings in THA. Orthopedics. 2012;35(9):e1307-e1313.
62. Park KS, Yoon TR, Hwang SY, Lee KB. Modified minimally invasive two-incision total hip arthroplasty using large diameter femoral head. Indian
J Orthop. 2012;46(1):29-35.
63. Garbuz DS, Masri BA, Duncan CP, et al. The Frank Stinchfield Award: dislocation in revision THA: do large heads (36 and 40 mm) result in reduced dislocation rates in a randomized clinical trial? Clin Orthop. 2012;470(2):351-356.
64. Geller JA, Malchau H, Bragdon C, Greene M, Harris WH, Freiberg AA. Large diameter femoral heads on highly cross-linked polyethylene: minimum 3-year results. Clin Orthop. 2006;(447):53-59.
65. D’Antonio JA, Capello WN, Ramakrishnan R. Second-generation annealed highly cross-linked polyethylene exhibits low wear. Clin Orthop. 2012;470(6):1696-1704.
66. Galvin AL, Jennings LM, Tipper JL, Ingham E, Fisher J. Wear and creep of highly crosslinked polyethylene against cobalt chrome and ceramic femoral heads. Proc Inst Mech Eng H. 2010;224(10):1175-1183.
67. Skeels MD, Berend KR, Lombardi AV Jr. The dislocator, early and late: the role of large heads. Orthopedics. 2009;32(9).
68. Plate JF, Seyler TM, Stroh DA, Issa K, Akbar M, Mont MA. Risk of dislocation using large- vs. small-diameter femoral heads in total hip arthroplasty. BMC Res Notes. 2012;5(1):553.
69. Bouchet R, Mercier N, Saragaglia D. Posterior approach and dislocation rate: a 213 total hip replacements case–control study comparing the dual mobility cup with a conventional 28-mm metal head/polyethylene prosthesis. Orthop Traumatol Surg Res. 2011;97(1):2-7.
70. Ali Khan MA, Brakenbury PH, Reynolds IS. Dislocation following total hip replacement. J Bone Joint Surg Br. 1981;63(2):214-218.
71. Howie DW, Holubowycz OT, Middleton R. Large femoral heads decrease the incidence of dislocation after total hip arthroplasty: a randomized controlled trial. J Bone Joint Surg Am. 2012;94(12):1095-1102.
72. Malkani AL, Ong KL, Lau E, Kurtz SM, Justice BJ, Manley MT. Early- and late-term dislocation risk after primary hip arthroplasty in the Medicare population. J Arthroplasty. 2010;25(6 suppl):21-25.
73. Berry DJ, von Knoch M, Schleck CD, Harmsen WS. Effect of femoral head diameter and operative approach on risk of dislocation after primary total hip arthroplasty. J Bone Joint Surg Am. 2005;87(11):2456-2463.
74. Cho MR, Lee HS, Lee SW, Choi CH, Kim SK, Ko SB. Results after total hip arthroplasty with a large head and bipolar arthroplasty in patients with displaced femoral neck fractures. J Arthroplasty. 2011;26(6):893-896.
75. Conroy JL, Whitehouse SL, Graves SE, Pratt NL, Ryan P, Crawford RW. Risk factors for revision for early dislocation in total hip arthroplasty.
J Arthroplasty. 2008;23(6):867-872.
76. Kung PL, Ries MD. Effect of femoral head size and abductors on dislocation after revision THA. Clin Orthop. 2007;(465):170-174.
77. Williams JT Jr, Ragland PS, Clarke S. Constrained components for the unstable hip following total hip arthroplasty: a literature review. Int Orthop. 2007;31(3):273-277.
78. Zywiel MG, Mustafa LH, Bonutti PM, Mont MA. Are abductor muscle quality and previous revision surgery predictors of constrained liner failure in hip arthroplasty? Int Orthop. 2011;35(6):797-802.
79. Guyen O, Lewallen DG, Cabanela ME. Modes of failure of Osteonics constrained tripolar implants: a retrospective analysis of forty-three failed implants. J Bone Joint Surg Am. 2008;90(7):1553-1560.
80. Banks LN, McElwain JP. An unusual mode of failure of a tripolar constrained acetabular liner: a case report. Arch Orthop Trauma Surg. 2010;130(4):503-505.
81. Robertson WJ, Mattern CJ, Hur J, Su EP, Pellicci PM. Failure mechanisms and closed reduction of a constrained tripolar acetabular liner.
J Arthroplasty. 2009;24(2):322.e5-e11.
82. Aubriot JH, Lesimple P, Leclercq S. Study of Bousquet‘s non-cemented acetabular implant in 100 hybrid total hip prostheses (Charnley type cemented femoral component). Average 5-year follow-up [in French]. Acta Orthop Belg. 1993;59(suppl 1):267-271.
83. Farizon F, de Lavison R, Azoulai JJ, Bousquet G. Results with a cementless alumina-coated cup with dual mobility. A twelve-year follow-up study. Int Orthop. 1998;22(4):219-224.
84. Mertl P, Combes A, Leiber-Wackenheim F, Fessy MH, Girard J, Migaud H. Recurrence of dislocation following total hip arthroplasty revision using dual mobility cups was rare in 180 hips followed over 7 years. HSS J. 2012;8(3):251-256.
85. Langlais FL, Ropars M, Gaucher F, Musset T, Chaix O. Dual mobility cemented cups have low dislocation rates in THA revisions. Clin Orthop. 2008;466(2):389-395.
86. Philippot R, Farizon F, Camilleri JP, et al. Survival of dual mobility socket with a mean 17 years follow-up [in French]. Rev Chir Orthop Reparatrice Appar Mot. 2008;94(1):43-48.
87. Adam P, Philippe R, Ehlinger M, et al. Dual mobility cups hip arthroplasty as a treatment for displaced fracture of the femoral neck in the elderly.
A prospective, systematic, multicenter study with specific focus on postoperative dislocation. Orthop Traumatol Surg Res. 2012;98(3):296-300.
88. Fessy MH. La double mobilité [Dual mobility]. Revue de Chirurgie Orthopédique et Traumatologique. 2010;96(7):891-898.
89. Mont MA, Issa K, Naziri Q, Harwin SF, Delanois RE, Johnson AJ. The use of dual-mobility bearings in difficult hip arthroplasty reconstructive cases. Surg Technol Int. 2011;21:234-240.
90. Sayeed SA, Mont MA, Costa CR, et al. Early outcomes of sequentially cross-linked thin polyethylene liners with large diameter femoral heads in total hip arthroplasty. Bull NYU Hosp Jt Dis. 2011;69(suppl 1):S90-S94.
91. Plate JF, Seyler TM, Stroh DA, Issa K, Akbar M, Mont MA. Risk of dislocation using large- vs. small-diameter femoral heads in total hip arthroplasty. BMC Res Notes. 2012;5(1):553.
92. Sato T, Nakashima Y, Akiyama M, et al. Wear resistant performance of highly cross-linked and annealed ultra-high molecular weight polyethylene against ceramic heads in total hip arthroplasty. J Orthop Res. 2012;30(12):2031-2037.
93. Meftah M, Ebrahimpour PB, He C, Ranawat AS, Ranawat CS. Preliminary clinical and radiographic results of large ceramic heads on highly cross-linked polyethylene. Orthopedics. 2011;34(6):133.
94. Gagala J, Mazurkiewicz T, Dajewski Z. Large diameter femoral heads in primary alumina/alumina and XSPE/alumina total hip arthroplasty.
A follow-up study of 50 hips after average 40 months and review of literature [in Polish]. Chir Narzadow Ruchu Ortop Pol. 2011;76(1):14-20.