User login
Outcomes and Aseptic Survivorship of Revision Total Knee Arthroplasty
Over the past 3 decades, total knee arthroplasty (TKA) has been considered a safe and effective treatment for end-stage knee arthritis.1 However, as the population, the incidence of obesity, and life expectancy continue to increase, the number of TKAs will rise as well.2,3 It is expected that over the next 16 years, the number of TKAs performed annually will exceed 3 million in the United States alone.4 This projection represents an over 600% increase from 2005 figures.5 Given the demographic shift expected over the next 2 decades, patients are anticipated to undergo these procedures at younger ages compared with previous generations, such that those age 65 years or younger will account for more than 55% of primary TKAs.6 More important, given this exponential growth in primary TKAs, there will be a concordant rise in revision procedures. It is expected that, the annual number has roughly doubled from that recorded for 2005.4
Compared with primary TKAs, however, revision TKAs have had less promising results, with survivorship as low as 60% over shorter periods.7,8 In addition, recent studies have found an even higher degree of dissatisfaction and functional limitations among revision TKA patients than among primary TKA patients, 15% to 30% of whom are unhappy with their procedures.9-11 These shortcomings of revision TKAs are thought to result from several factors, including poor bone quality, insufficient bone stock, ligamentous instability, soft-tissue incompetence, infection, malalignment, problems with extensor mechanisms, and substantial pain of uncertain etiology.
Despite there being several complex factors that can lead to worse outcomes with revision TKAs, surgeons are expected to produce results equivalent to those of primary TKAs. It is therefore imperative to delineate the objective and subjective outcomes of revision techniques to identify areas in need of improvement. In this article, we provide a concise overview of revision TKA outcomes in order to stimulate manufacturers, surgeons, and hospitals to improve on implant designs, surgical techniques, and care guidelines for revision TKA. We review the evidence on 5 points: aseptic survivorship, functional outcomes, patient satisfaction, quality of life (QOL), and economic impact. In addition, we compare available outcome data for revision and primary TKAs.
1. Aseptic survivorship
Fehring and colleagues12 in 2001 and Sharkey and colleagues13 in 2002 evaluated mechanisms of failure for revision TKA and reported many failures resulted from infection or were associated with the implant, and occurred within 2 years after the primary procedure. More recently, Dy and colleagues14 found the most common reason for revision was aseptic loosening, followed by infection. The present review focuses on aseptic femoral and tibial revision.
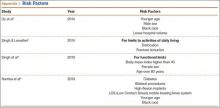
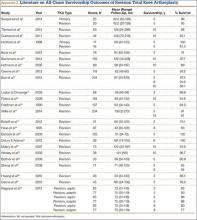
The failure rate for revision TKA is substantially higher than for primary TKA with the same type of prosthesis because of the complexity of the revision procedure, the increasing constraint of the implant design, and the higher degree of bone loss. (Appendix 1 lists risk factors for revision surgery. Appendix 2 is a complete list of survivorship outcomes of revision TKA.)
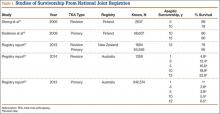
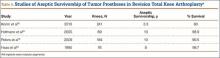
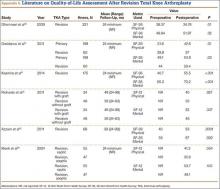
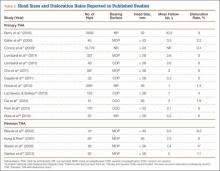
Sheng and colleagues15 in 2006 and Koskinen and colleagues16 in 2008 analyzed Finnish Arthroplasty Register data to determine failure rates for revision and primary TKA. Sheng and colleagues15 examined survivorship of 2637 revision TKAs (performed between 1990 and 2002) for all-cause endpoints after first revision procedure. Survivorship rates were 89% (5 years) and 79% (10 years), while Koskinen and colleagues16 noted all-cause survival rates of 80% at 15 years. More recently, in 2013, the New Zealand Orthopaedic Association17 analyzed New Zealand Joint Registry data for revision and re-revision rates (rates of revision per 100 component years) for 64,556 primary TKAs performed between 1999 and 2012. During the period studied, 1684 revisions were performed, reflecting a 2.6% revision rate, a 0.50% rate of revision per 100 component years, and a 13-year Kaplan-Meier survivorship of 94.5%. The most common reasons for revision were pain, deep infection, and tibial component loosening (Table 1).
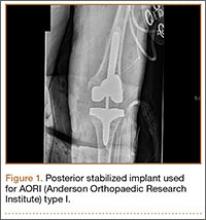
Posterior stabilized implants
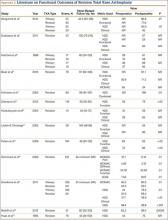
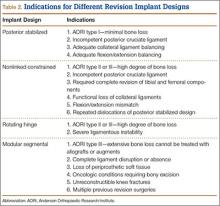
Laskin and Ohnsorge18 retrospectively reviewed the cases of 58 patients who underwent unilateral revision TKA (with a posterior stabilized implant), of which 42% were for coronal instability and 44% for a loose tibial component. At minimum 4-year follow-up, 52 of the 58 patients had anteroposterior instability of less than 5 mm. In addition, 5 years after surgery, aseptic survivorship was 96%. Meijer and colleagues19 conducted a retrospective comparative study of 69 revision TKAs (65 patients) in which 9 knees received a primary implant and 60 received a revision implant with stems and augmentation (60 = 37 posterior stabilized, 20 constrained, 3 rotating hinge). Survival rates for the primary implants were 100% (1 year), 73% (2 years), and 44% (5 years), and survival rates for the revision implants were significantly better: 95% (1 year), 92% (2 years), and 92% (5 years) (hazard ratio, 5.87; P = .008). The authors therefore indicated that it was unclear whether using a primary implant should still be an option in revision TKA and, if it is used, whether it should be limited to less complex situations in which bone loss and ligament damage are minimal (Table 2).
Constrained and semiconstrained implants
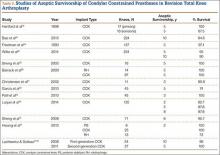
In a study of 234 knees (209 patients) with soft-tissue deficiency, Wilke and colleagues20 evaluated the long-term survivorship of revision TKA with use of a semiconstrained modular fixed-bearing implant system. Overall Kaplan-Meier survival rates were 91% (5 years) and 81% (10 years) at a mean follow-up of 9 years. When aseptic revision was evaluated, however, the survival rates increased to 95% (5 years) and 90% (10 years). The authors noted that male sex was the only variable that significantly increased the risk for re-revision (hazard ratio, 2.07; P = .02), which they attributed to potentially higher activity levels. In 2006 and 2011, Lachiewicz and Soileau21,22 evaluated the survival of first- and second-generation constrained condylar prostheses in primary TKA cases with severe valgus deformities, incompetent collateral ligaments, or severe flexion contractures. Of the 54 knees (44 patients) with first-generation prostheses, 42 (34 patients) had a mean follow-up of 9 years (range, 5-16 years). Ten-year survival with failure, defined as component revision for loosening, was 96%. The 27 TKAs using second-generation prostheses had a mean follow-up of about 5 years (range, 2-12 years). At final follow-up, there were no revisions for loosening or patellar problems, but 6 knees (22%) required lateral retinacular release of the patella (Table 3).
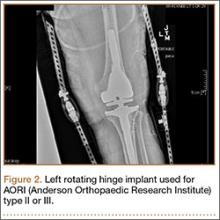
Rotating hinge implants
Neumann and colleagues23 evaluated the clinical and radiographic outcomes of 24 rotating hinge prostheses used for aseptic loosening with substantial bone loss and collateral ligament instability. At a mean follow-up of 56 months (range, 3-5 years), there was no evidence of loosening of any implants, and nonprogressive radiolucent lines were found in only 2 tibial components. Kowalczewski and colleagues24 evaluated the clinical and radiologic outcomes of 12 primary TKAs using a rotating hinge knee prosthesis at a minimum follow-up of 10 years. By most recent follow-up, no implants had been revised for loosening, and only 3 had nonprogressive radiolucent lines (Table 4).
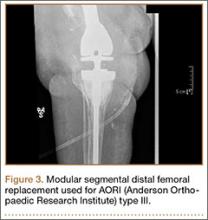
Endoprostheses (modular segmental implants)
In a systematic review of 9 studies, Korim and colleagues25 evaluated 241 endoprostheses used for limb salvage under nononcologic conditions. Mean follow-up was about 3 years (range, 1-5 years). The devices were used to treat various conditions, including periprosthetic fracture, bone loss with aseptic loosening, and ligament insufficiency. The overall reoperation rate was 17% (41/241 cases). Mechanical failures were less frequent (6%-19%) (Table 5).
2. Functional outcomes
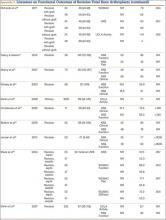
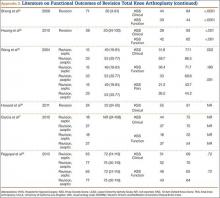
The goal in both primary and revision TKA is to restore the function and mobility of the knee and to alleviate pain. Whereas primary TKAs are realistically predictable and reproducible in their outcomes, revision TKAs are vastly more complicated, which can result in worse postoperative outcomes and function. In addition, revision TKAs may require extensive surgical exposure, which causes more tissue and muscle damage, prolonging rehabilitation. (Appendix 3 is a complete list of studies of functional outcomes of revision TKA.)
This discrepancy in functional outcomes between primary and revision TKA begins as early as the postoperative inpatient rehabilitation period. Using the functional independence measurement (FIM), which estimates performance of activities of daily living, mobility, and cognition, Vincent and colleagues26 evaluated the functional improvement produced by revision versus primary TKA during inpatient rehabilitation. They compared 424 consecutive primary TKAs with 138 revision TKAs. For both groups, FIM scores increased significantly (P = .015) between admission and discharge. On discharge, however, FIM scores were significantly (P = .01) higher for the primary group than the revision group (29 and 27 points, respectively). Furthermore, in the evaluation of mechanisms of failure, patients who had revision TKA for mechanical or pain-related problems did markedly better than those who had revision TKA for infection.
Compared with primary knee implants, revision implants require increasing constraint. We assume increasing constraint affects knee biomechanics, leading to worsening functional outcomes. In a study of 60 revision TKAs (57 patients) using posterior stabilized, condylar constrained, or rotating hinge prostheses, Vasso and colleagues27 examined functional outcomes at a median follow-up of 9 years (range, 4-12 years). At most recent follow-up, mean International Knee Society (IKS) Knee and Function scores were 81 (range, 48-97) and 79 (range, 56-92), mean Hospital for Special Surgery (HSS) score was 84 (range, 62-98), and mean range of motion (ROM) was 121° (range, 98°-132°) (P < .001). Although there were no significant differences in IKS and HSS scores between prosthesis types, ROM was significantly (P < .01) wider in the posterior stabilized group than in the condylar constrained and rotating hinge groups (127° vs 112° and 108°), suggesting increasing constraint resulted in decreased ROM. Several studies have found increasing constraint might lead to reduced function.28-30
However, Hwang and colleagues31 evaluated functional outcomes in 36 revision TKAs and noted that the cemented posterior stabilized (n = 8), condylar constrained (n = 25), and rotating hinge (n = 13) prostheses used did not differ in their mean Knee Society scores (78, 81, and 83, respectively).
There remains a marked disparity in patient limitations seen after revision versus primary TKA. Given the positive results being obtained with newer implants, studies might suggest recent generations of prostheses have allowed designs to be comparable. As design development continues, we may come closer to achieving outcomes comparable to those of primary TKA.
3. Patient satisfaction
Several recent reports have shown that 10% to 25% of patients who underwent primary TKA were dissatisfied with their surgery30,32; other studies have found patient satisfaction often correlating to function and pain.33-35 Given the worse outcomes for revision TKA (outlined in the preceding section), the substantial pain accompanying a second, more complex procedure, and the extensive rehabilitation expected, we suspect patients who undergo revision TKA are even less satisfied with their surgery than their primary counterparts are. (See Appendix 4 for a complete list of studies of patient satisfaction after revision TKA.)
Barrack and colleagues32 evaluated a consecutive series of 238 patients followed up for at least 1 year after revision TKA. Patients were asked to rate their degree of satisfaction with both their primary procedure and the revision and to indicate their expectations regarding their revision prosthesis. Mean satisfaction score was 7.4 (maximum = 10), with 13% of patients dissatisfied, 18% somewhat satisfied, and 69% satisfied. Seventy-four percent of patients expected their revision prosthesis to last longer than the primary prosthesis.
Greidanus and colleagues36 evaluated patient satisfaction in 60 revision TKA cases and 199 primary TKA cases at 2-year follow-up. The primary TKA group had significantly (P < .01) higher satisfaction scores in a comparison with the revision TKA group: Global (86 vs 73), Pain Relief (88 vs 70), Function (83 vs 67), and Recreation (77 vs 62). These findings support the satisfaction rates reported by Dahm and colleagues33,34: 91% for primary TKA patients and 77% for revision TKA patients.
4. Quality of life
Procedure complexity leads to reduced survivorship, function, and mobility, longer rehabilitation, and decreased QOL for revision TKA patients relative to primary TKA patients.37 (See Appendix 5 for a complete list of studies of QOL outcomes of revision TKA.)
Greidanus and colleagues36 evaluated joint-specific QOL (using the 12-item Oxford Knee Score; OKS) and generic QOL (using the 12-Item Short Form Health Survey; SF-12) in 60 revision TKA cases and 199 primary TKA cases at a mean follow-up of 2 years. (The OKS survey is used to evaluate patient perspectives on TKA outcomes,38 and the multipurpose SF-12 questionnaire is used to assess mental and physical function and general health-related QOL.39) Compared with the revision TKA group, the primary TKA group had significantly higher OKS after surgery (78 vs 68; P = .01) as well as significantly higher SF-12 scores: Global (84 vs 72; P = .01), Mental (54 vs 50; P = .03), and Physical (43 vs 37; P = .01). Similarly, Ghomrawi and colleagues40 evaluated patterns of improvement in 308 patients (318 knees) who had revision TKA. At 24-month follow-up, mean SF-36 Physical and Mental scores were 35 and 52, respectively.
Deehan and colleagues41 used the Nottingham Health Profile (NHP) to compare 94 patients’ health-related QOL scores before revision TKA with their scores 3 months, 1 year, and 5 years after revision. NHP Pain subscale scores were significantly lower 3 and 12 months after surgery than before surgery, but this difference was no longer seen at the 5-year follow-up. There was no significant improvement in scores on the other 5 NHP subscales (Sleep, Energy, Emotion, Mobility, Social Isolation) at any time points.
As shown in the literature, patients’ QOL outcomes improve after revision TKA, but these gains are not at the level of patients who undergo primary TKA.36,41 Given that revision surgery is more extensive, and that perhaps revision patients have poorer muscle function, they usually do not return to the level they attained after their index procedure.
5. Economic impact
Consistent with the outcomes already described, the economic impact of revision TKAs is excess expenditures and costs to patients and health care institutions.42 The sources of this impact are higher implant costs, extra operative trays and times, longer hospital stays, more rehabilitation, and increased medication use.43 Revision TKA costs range from $49,000 to more than $100,000—a tremendous increase over primary TKA costs ($25,000-$30,000).43-45 Furthermore, the annual economic burden associated with revision TKA, now $2.7 billion, is expected to exceed $13 billion by 2030.46 In the United States, about $23.2 billion will be spent on 926,527 primary TKAs in 2015; significantly, the costs associated with revising just 10% of these cases account for almost 50% of the total cost of the primary procedures.46
In a retrospective cost-identification multicenter cohort study, Bozic and colleagues47 found that both-component and single-component revisions, compared with primary procedures, were associated with significantly increased operative time (~265 and 221 minutes vs 200 minutes), use of allograft bone (23% and 14% vs 1%), length of stay (5.4 and 5.7 days vs 5.0 days), and percentage of patients discharged to extended-care facilities (26% and 26% vs 25%) (P < .0001). Hospital costs for both- and single-component revisions were 138% and 114% higher than costs for primary procedures (P < .0001). More recently, Kallala and colleagues44 analyzed UK National Health Service data and compared the costs of revision for infection with revision for other causes (pain, instability, aseptic loosening, fracture). Mean length of stay associated with revision for infection (21.5 days) was more than double that associated with revision for aseptic loosening (9.5 days; P < .0001), and mean cost of revision for septic causes (£30,011) was more than 3 times that of revision for other causes (£9655; P < .0001). The authors concluded that the higher costs of revision knee surgery have a considerable economic impact, especially in infection cases.
With more extensive procedures, long-stem or more constrained prostheses are often needed to obtain adequate fixation and stability. The resulting increased, substantial economic burden is felt by patients and the health care system. Given that health care reimbursements are declining, hospitals that perform revision TKAs can sustain marked financial losses. Some centers are asking whether it is cost-effective to continue to perform these types of procedures. We must find new ways to provide revision procedures using less costly implants and tools so that centers will continue to make these procedures available to patients.
Conclusion
Given the exponential growth in primary TKAs, there will be a concordant increase in revision TKAs in the decades to come. This review provides a concise overview of revision TKA outcomes. Given the low level of evidence regarding revision TKAs, we need further higher quality studies of their prostheses and outcomes. Specifically, we need systematic reviews and meta-analyses to provide higher quality evidence regarding outcomes of using individual prosthetic designs.
1. Cram P, Lu X, Kates SL, Singh JA, Li Y, Wolf BR. Total knee arthroplasty volume, utilization, and outcomes among Medicare beneficiaries, 1991–2010. JAMA. 2012;308(12):1227-1236.
2. Crowninshield RD, Rosenberg AG, Sporer SM. Changing demographics of patients with total joint replacement. Clin Orthop Relat Res. 2006;443:266-272.
3. Ravi B, Croxford R, Reichmann WM, Losina E, Katz JN, Hawker GA. The changing demographics of total joint arthroplasty recipients in the United States and Ontario from 2001 to 2007. Best Pract Res Clin Rheumatol. 2012;26(5):637-647.
4. Kurtz S, Ong K, Lau E, Mowat F, Halpern M. Projections of primary and revision hip and knee arthroplasty in the United States from 2005 to 2030. J Bone Joint Surg Am. 2007;89(4):780-785.
5. Kurtz SM, Ong KL, Schmier J, Zhao K, Mowat F, Lau E. Primary and revision arthroplasty surgery caseloads in the United States from 1990 to 2004. J Arthroplasty. 2009;24(2):195-203.
6. Kurtz SM, Lau E, Ong K, Zhao K, Kelly M, Bozic KJ. Future young patient demand for primary and revision joint replacement: national projections from 2010 to 2030. Clin Orthop Relat Res. 2009;467(10):2606-2612.
7. Bryan RS, Rand JA. Revision total knee arthroplasty. Clin Orthop Relat Res. 1982;170:116-122.
8. Rand JA, Bryan RS. Revision after total knee arthroplasty. Orthop Clin North Am. 1982;13(1):201-212.
9. Bozic KJ, Kurtz SM, Lau E, et al. The epidemiology of revision total knee arthroplasty in the United States. Clin Orthop Relat Res. 2010;468(1):45-51.
10. Parvizi J, Nunley RM, Berend KR, et al. High level of residual symptoms in young patients after total knee arthroplasty. Clin Orthop Relat Res. 2014;472(1):133-137.
11. Ali A, Sundberg M, Robertsson O, et al. Dissatisfied patients after total knee arthroplasty: a registry study involving 114 patients with 8-13 years of followup. Acta Orthop. 2014;85(3):229-233.
12. Fehring TK, Odum S, Griffin WL, Mason JB, Nadaud M. Early failures in total knee arthroplasty. Clin Orthop Relat Res. 2001;392:315-318.
13. Sharkey PF, Hozack WJ, Rothman RH, Shastri S, Jacoby SM. Insall Award paper. Why are total knee arthroplasties failing today? Clin Orthop Relat Res. 2002;404:7-13.
14. Dy CJ, Marx RG, Bozic KJ, Pan TJ, Padgett DE, Lyman S. Risk factors for revision within 10 years of total knee arthroplasty. Clin Orthop Relat Res. 2014;472(4):1198-1207.
15. Sheng PY, Konttinen L, Lehto M, et al. Revision total knee arthroplasty: 1990 through 2002. A review of the Finnish Arthroplasty Registry. J Bone Joint Surg Am. 2006;88(7):1425-1430.
16. Koskinen E, Eskelinen A, Paavolainen P, Pulkkinen P, Remes V. Comparison of survival and cost-effectiveness between unicondylar arthroplasty and total knee arthroplasty in patients with primary osteoarthritis: a follow-up study of 50,493 knee replacements from the Finnish Arthroplasty Register. Acta Orthop. 2008;79(4):499-507.
17. New Zealand Orthopaedic Association. The New Zealand Joint Registry Fourteen Year Report (January 1999 to December 2012). http://www.nzoa.org.nz/system/files/NJR%2014%20Year%20Report.pdf. Published November 2013. Accessed December 16, 2015.
18. Laskin RS, Ohnsorge J. The use of standard posterior stabilized implants in revision total knee arthroplasty. Clin Orthop Relat Res. 2005;(440):122-125.
19. Meijer MF, Reininga IH, Boerboom AL, Stevens M, Bulstra SK. Poorer survival after a primary implant during revision total knee arthroplasty. Int Orthop. 2013;37(3):415-419.
20. Wilke BK, Wagner ER, Trousdale RT. Long-term survival of semi-constrained total knee arthroplasty for revision surgery. J Arthroplasty. 2014;29(5):1005-1008.
21. Lachiewicz PF, Soileau ES. Ten-year survival and clinical results of constrained components in primary total knee arthroplasty. J Arthroplasty. 2006;21(6):803-808.
22. Lachiewicz PF, Soileau ES. Results of a second-generation constrained condylar prosthesis in primary total knee arthroplasty. J Arthroplasty. 2011;26(8):1228-1231.
23. Neumann DR, Hofstaedter T, Dorn U. Follow-up of a modular rotating hinge knee system in salvage revision total knee arthroplasty. J Arthroplasty. 2012;27(5):814-819.
24. Kowalczewski J, Marczak D, Synder M, Sibinski M. Primary rotating-hinge total knee arthroplasty: good outcomes at mid-term follow-up. J Arthroplasty. 2014;29(6):1202-1206.
25. Korim MT, Esler CN, Reddy VR, Ashford RU. A systematic review of endoprosthetic replacement for non-tumour indications around the knee joint. Knee. 2013;20(6):367-375.
26. Vincent KR, Vincent HK, Lee LW, Alfano AP. Inpatient rehabilitation outcomes in primary and revision total knee arthroplasty patients. Clin Orthop Relat Res. 2006;(446):201-207.
27. Vasso M, Beaufils P, Schiavone Panni A. Constraint choice in revision knee arthroplasty. Int Orthop. 2013;37(7):1279-1284.
28. Baier C, Luring C, Schaumburger J, et al. Assessing patient-oriented results after revision total knee arthroplasty. J Orthop Sci. 2013;18(6):955-961.
29. Hartford JM, Goodman SB, Schurman DJ, Knoblick G. Complex primary and revision total knee arthroplasty using the condylar constrained prosthesis: an average 5-year follow-up. J Arthroplasty. 1998;13(4):380-387.
30. Haidukewych GJ, Jacofsky DJ, Pagnano MW, Trousdale RT. Functional results after revision of well-fixed components for stiffness after primary total knee arthroplasty. J Arthroplasty. 2005;20(2):133-138.
31. Hwang SC, Kong JY, Nam DC, et al. Revision total knee arthroplasty with a cemented posterior stabilized, condylar constrained or fully constrained prosthesis: a minimum 2-year follow-up analysis. Clin Orthop Surg. 2010;2(2):112-120.
32. Barrack RL, McClure JT, Burak CF, Clohisy JC, Parvizi J, Sharkey P. Revision total knee arthroplasty: the patient’s perspective. Clin Orthop Relat Res. 2007;464:146-150.
33. Dahm DL, Barnes SA, Harrington JR, Berry DJ. Patient reported activity after revision total knee arthroplasty. J Arthroplasty. 2007;22(6 suppl 2):106-110.
34. Dahm DL, Barnes SA, Harrington JR, Sayeed SA, Berry DJ. Patient-reported activity level after total knee arthroplasty. J Arthroplasty. 2008;23(3):401-407.
35. Richards CJ, Garbuz DS, Pugh L, Masri BA. Revision total knee arthroplasty: clinical outcome comparison with and without the use of femoral head structural allograft. J Arthroplasty. 2011;26(8):1299-1304.
36. Greidanus NV, Peterson RC, Masri BA, Garbuz DS. Quality of life outcomes in revision versus primary total knee arthroplasty. J Arthroplasty. 2011;26(4):615-620.
37. Ethgen O, Bruyere O, Richy F, Dardennes C, Reginster JY. Health-related quality of life in total hip and total knee arthroplasty. A qualitative and systematic review of the literature. J Bone Joint Surg Am. 2004;86(5):963-974.
38. Murray DW, Fitzpatrick R, Rogers K, et al. The use of the Oxford hip and knee scores. J Bone Joint Surg Br. 2007;89(8):1010-1014.
39. Ware J Jr, Kosinski M, Keller SD. A 12-Item Short-Form Health Survey: construction of scales and preliminary tests of reliability and validity. Med Care. 1996;34(3):220-233.
40. Ghomrawi HM, Kane RL, Eberly LE, Bershadsky B, Saleh KJ; North American Knee Arthroplasty Revision Study Group. Patterns of functional improvement after revision knee arthroplasty. J Bone Joint Surg Am. 2009;91(12):2838-2845.
41. Deehan DJ, Murray JD, Birdsall PD, Pinder IM. Quality of life after knee revision arthroplasty. Acta Orthop. 2006;77(5):761-766.
42. Kapadia BH, McElroy MJ, Issa K, Johnson AJ, Bozic KJ, Mont MA. The economic impact of periprosthetic infections following total knee arthroplasty at a specialized tertiary-care center. J Arthroplasty. 2014;29(5):929-932.
43. Bhandari M, Smith J, Miller LE, Block JE. Clinical and economic burden of revision knee arthroplasty. Clin Med Insights Arthritis Musculoskelet Disord. 2012;5:89-94.
44. Kallala RF, Vanhegan IS, Ibrahim MS, Sarmah S, Haddad FS. Financial analysis of revision knee surgery based on NHS tariffs and hospital costs: does it pay to provide a revision service? Bone Joint J Br. 2015;97(2):197-201.
45. Ong KL, Mowat FS, Chan N, Lau E, Halpern MT, Kurtz SM. Economic burden of revision hip and knee arthroplasty in Medicare enrollees. Clin Orthop Relat Res. 2006;446:22-28.
46. Kurtz SM, Ong KL, Lau E, Bozic KJ. Impact of the economic downturn on total joint replacement demand in the United States: updated projections to 2021. J Bone Joint Surg Am. 2014;96(8):624-630.
47. Bozic KJ, Durbhakula S, Berry DJ, et al. Differences in patient and procedure characteristics and hospital resource use in primary and revision total joint arthroplasty: a multicenter study. J Arthroplasty. 2005;20(7 suppl 3):17-25.
48. Lee KJ, Moon JY, Song EK, Lim HA, Seon JK. Minimum Two-year Results of Revision Total Knee Arthroplasty Following Infectious or Non-infectious Causes. Knee Surg Relat Res. 2012;24(4):227-234.
49. Bae DK, Song SJ, Heo DB, Lee SH, Song WJ. Long-term survival rate of implants and modes of failure after revision total knee arthroplasty by a single surgeon. J Arthroplasty. 2013;28(7):1130-1134.
50. Sheng PY, Jämsen E, Lehto MU, Konttinen YT, Pajamäki J, Halonen P. Revision total knee arthroplasty with the Total Condylar III system in inflammatory arthritis. J Bone Joint Surg Br. 2005;87(9):1222-1224.
51. Lachiewicz PF, Soileau ES. Ten-year survival and clinical results of constrained components in primary total knee arthroplasty. J Arthroplasty. 2006;21(6):803-808.
52. Haas SB, Insall JN, Montgomery W 3rd, Windsor RE. Revision total knee arthroplasty with use of modular components with stems inserted without cement. J Bone Joint Surg Am. 1995;77(11):1700-1707.
53. Mabry TM, Vessely MB, Schleck CD, Harmsen WS, Berry DJ. Revision total knee arthroplasty with modular cemented stems: long-term follow-up. J Arthroplasty. 2007;22(6 Suppl 2):100-105.
54. Gudnason A, Milbrink J, Hailer NP. Implant survival and outcome after rotating-hinge total knee revision arthroplasty: a minimum 6-year follow-up. Arch Orthop Trauma Surg. 2011;131(11):1601-1607.
55. Hofmann AA, Goldberg T, Tanner AM, Kurtin SM. Treatment of infected total knee arthroplasty using an articulating spacer: 2- to 12-year experience. Clin Orthop Relat Res. 2005;430:125-131.
56. Greene JW, Reynolds SM, Stimac JD, Malkani AL, Massini MA. Midterm results of hybrid cement technique in revision total knee arthroplasty. J Arthroplasty. 2013;28(4):570-574.
57. Dalury DF, Adams MJ. Minimum 6-year follow-up of revision total knee arthroplasty without patella reimplantation. Journal Arthroplasty. 2012;27(8 Suppl):91-94.
58. Whaley AL, Trousdale RT, Rand JA, Hanssen AD. Cemented long-stem revision total knee arthroplasty. J Arthroplasty. 2003;18(5):592-599.
59. Friedman RJ, Hirst P, Poss R, Kelley K, Sledge CB. Results of revision total knee arthroplasty performed for aseptic loosening. Clinical Orthop Relat Res. 1990;255:235-241.
60. Barrack RL, Rorabeck C, Partington P, Sawhney J, Engh G. The results of retaining a well-fixed patellar component in revision total knee arthroplasty. J Arthroplasty. 2000;15(4):413-417.
61. Christensen CP, Crawford JJ, Olin MD, Vail TP. Revision of the stiff total knee arthroplasty. J Arthroplasty. 2002;17(4):409-415.
62. Garcia RM, Hardy BT, Kraay MJ, Goldberg VM. Revision total knee arthroplasty for aseptic and septic causes in patients with rheumatoid arthritis. Clin Orthop Relat Res. 2010;468(1):82-89.
63. Patil N, Lee K, Huddleston JI, Harris AH, Goodman SB. Aseptic versus septic revision total knee arthroplasty: patient satisfaction, outcome and quality of life improvement. Knee. 2010;17(3):200-203.
64. Luque R, Rizo B, Urda A, et al. Predictive factors for failure after total knee replacement revision. Int Orthop. 2014;38(2):429-435.
65. Bistolfi A, Massazza G, Rosso F, Crova M. Rotating-hinge total knee for revision total knee arthroplasty. Orthopedics. 2012;35(3):e325-e330.
66. Bottner F, Laskin R, Windsor RE, Haas SB. Hybrid component fixation in revision total knee arthroplasty. Clin Orthop Relat Res. 2006;446:127-131.
67. Jensen CL, Winther N, Schroder HM, Petersen MM. Outcome of revision total knee arthroplasty with the use of trabecular metal cone for reconstruction of severe bone loss at the proximal tibia. Knee. 2014;21(6):1233-1237.
68. Howard JL, Kudera J, Lewallen DG, Hanssen AD. Early results of the use of tantalum femoral cones for revision total knee arthroplasty. J Bone Joint Surg Am. 2011;93(5):478-484.
69. Yang JH, Yoon JR, Oh CH, Kim TS. Hybrid component fixation in total knee arthroplasty: minimum of 10-year follow-up study. J Arthroplasty. 2012;27(6):1111-1118.
70. Peters CL, Erickson JA, Gililland JM. Clinical and radiographic results of 184 consecutive revision total knee arthroplasties placed with modular cementless stems. J Arthroplasty. 2009;24(6 Suppl):48-53.
71. Registry AOANJR. Hip and Knee Arthroplasty. Annual Report 2014. 2014.
72. Registry AOANJR. Hip and Knee Arthroplasty. Annual Report 2013. 2013.
Over the past 3 decades, total knee arthroplasty (TKA) has been considered a safe and effective treatment for end-stage knee arthritis.1 However, as the population, the incidence of obesity, and life expectancy continue to increase, the number of TKAs will rise as well.2,3 It is expected that over the next 16 years, the number of TKAs performed annually will exceed 3 million in the United States alone.4 This projection represents an over 600% increase from 2005 figures.5 Given the demographic shift expected over the next 2 decades, patients are anticipated to undergo these procedures at younger ages compared with previous generations, such that those age 65 years or younger will account for more than 55% of primary TKAs.6 More important, given this exponential growth in primary TKAs, there will be a concordant rise in revision procedures. It is expected that, the annual number has roughly doubled from that recorded for 2005.4
Compared with primary TKAs, however, revision TKAs have had less promising results, with survivorship as low as 60% over shorter periods.7,8 In addition, recent studies have found an even higher degree of dissatisfaction and functional limitations among revision TKA patients than among primary TKA patients, 15% to 30% of whom are unhappy with their procedures.9-11 These shortcomings of revision TKAs are thought to result from several factors, including poor bone quality, insufficient bone stock, ligamentous instability, soft-tissue incompetence, infection, malalignment, problems with extensor mechanisms, and substantial pain of uncertain etiology.
Despite there being several complex factors that can lead to worse outcomes with revision TKAs, surgeons are expected to produce results equivalent to those of primary TKAs. It is therefore imperative to delineate the objective and subjective outcomes of revision techniques to identify areas in need of improvement. In this article, we provide a concise overview of revision TKA outcomes in order to stimulate manufacturers, surgeons, and hospitals to improve on implant designs, surgical techniques, and care guidelines for revision TKA. We review the evidence on 5 points: aseptic survivorship, functional outcomes, patient satisfaction, quality of life (QOL), and economic impact. In addition, we compare available outcome data for revision and primary TKAs.
1. Aseptic survivorship
Fehring and colleagues12 in 2001 and Sharkey and colleagues13 in 2002 evaluated mechanisms of failure for revision TKA and reported many failures resulted from infection or were associated with the implant, and occurred within 2 years after the primary procedure. More recently, Dy and colleagues14 found the most common reason for revision was aseptic loosening, followed by infection. The present review focuses on aseptic femoral and tibial revision.
The failure rate for revision TKA is substantially higher than for primary TKA with the same type of prosthesis because of the complexity of the revision procedure, the increasing constraint of the implant design, and the higher degree of bone loss. (Appendix 1 lists risk factors for revision surgery. Appendix 2 is a complete list of survivorship outcomes of revision TKA.)
Sheng and colleagues15 in 2006 and Koskinen and colleagues16 in 2008 analyzed Finnish Arthroplasty Register data to determine failure rates for revision and primary TKA. Sheng and colleagues15 examined survivorship of 2637 revision TKAs (performed between 1990 and 2002) for all-cause endpoints after first revision procedure. Survivorship rates were 89% (5 years) and 79% (10 years), while Koskinen and colleagues16 noted all-cause survival rates of 80% at 15 years. More recently, in 2013, the New Zealand Orthopaedic Association17 analyzed New Zealand Joint Registry data for revision and re-revision rates (rates of revision per 100 component years) for 64,556 primary TKAs performed between 1999 and 2012. During the period studied, 1684 revisions were performed, reflecting a 2.6% revision rate, a 0.50% rate of revision per 100 component years, and a 13-year Kaplan-Meier survivorship of 94.5%. The most common reasons for revision were pain, deep infection, and tibial component loosening (Table 1).
Posterior stabilized implants
Laskin and Ohnsorge18 retrospectively reviewed the cases of 58 patients who underwent unilateral revision TKA (with a posterior stabilized implant), of which 42% were for coronal instability and 44% for a loose tibial component. At minimum 4-year follow-up, 52 of the 58 patients had anteroposterior instability of less than 5 mm. In addition, 5 years after surgery, aseptic survivorship was 96%. Meijer and colleagues19 conducted a retrospective comparative study of 69 revision TKAs (65 patients) in which 9 knees received a primary implant and 60 received a revision implant with stems and augmentation (60 = 37 posterior stabilized, 20 constrained, 3 rotating hinge). Survival rates for the primary implants were 100% (1 year), 73% (2 years), and 44% (5 years), and survival rates for the revision implants were significantly better: 95% (1 year), 92% (2 years), and 92% (5 years) (hazard ratio, 5.87; P = .008). The authors therefore indicated that it was unclear whether using a primary implant should still be an option in revision TKA and, if it is used, whether it should be limited to less complex situations in which bone loss and ligament damage are minimal (Table 2).
Constrained and semiconstrained implants
In a study of 234 knees (209 patients) with soft-tissue deficiency, Wilke and colleagues20 evaluated the long-term survivorship of revision TKA with use of a semiconstrained modular fixed-bearing implant system. Overall Kaplan-Meier survival rates were 91% (5 years) and 81% (10 years) at a mean follow-up of 9 years. When aseptic revision was evaluated, however, the survival rates increased to 95% (5 years) and 90% (10 years). The authors noted that male sex was the only variable that significantly increased the risk for re-revision (hazard ratio, 2.07; P = .02), which they attributed to potentially higher activity levels. In 2006 and 2011, Lachiewicz and Soileau21,22 evaluated the survival of first- and second-generation constrained condylar prostheses in primary TKA cases with severe valgus deformities, incompetent collateral ligaments, or severe flexion contractures. Of the 54 knees (44 patients) with first-generation prostheses, 42 (34 patients) had a mean follow-up of 9 years (range, 5-16 years). Ten-year survival with failure, defined as component revision for loosening, was 96%. The 27 TKAs using second-generation prostheses had a mean follow-up of about 5 years (range, 2-12 years). At final follow-up, there were no revisions for loosening or patellar problems, but 6 knees (22%) required lateral retinacular release of the patella (Table 3).
Rotating hinge implants
Neumann and colleagues23 evaluated the clinical and radiographic outcomes of 24 rotating hinge prostheses used for aseptic loosening with substantial bone loss and collateral ligament instability. At a mean follow-up of 56 months (range, 3-5 years), there was no evidence of loosening of any implants, and nonprogressive radiolucent lines were found in only 2 tibial components. Kowalczewski and colleagues24 evaluated the clinical and radiologic outcomes of 12 primary TKAs using a rotating hinge knee prosthesis at a minimum follow-up of 10 years. By most recent follow-up, no implants had been revised for loosening, and only 3 had nonprogressive radiolucent lines (Table 4).
Endoprostheses (modular segmental implants)
In a systematic review of 9 studies, Korim and colleagues25 evaluated 241 endoprostheses used for limb salvage under nononcologic conditions. Mean follow-up was about 3 years (range, 1-5 years). The devices were used to treat various conditions, including periprosthetic fracture, bone loss with aseptic loosening, and ligament insufficiency. The overall reoperation rate was 17% (41/241 cases). Mechanical failures were less frequent (6%-19%) (Table 5).
2. Functional outcomes
The goal in both primary and revision TKA is to restore the function and mobility of the knee and to alleviate pain. Whereas primary TKAs are realistically predictable and reproducible in their outcomes, revision TKAs are vastly more complicated, which can result in worse postoperative outcomes and function. In addition, revision TKAs may require extensive surgical exposure, which causes more tissue and muscle damage, prolonging rehabilitation. (Appendix 3 is a complete list of studies of functional outcomes of revision TKA.)
This discrepancy in functional outcomes between primary and revision TKA begins as early as the postoperative inpatient rehabilitation period. Using the functional independence measurement (FIM), which estimates performance of activities of daily living, mobility, and cognition, Vincent and colleagues26 evaluated the functional improvement produced by revision versus primary TKA during inpatient rehabilitation. They compared 424 consecutive primary TKAs with 138 revision TKAs. For both groups, FIM scores increased significantly (P = .015) between admission and discharge. On discharge, however, FIM scores were significantly (P = .01) higher for the primary group than the revision group (29 and 27 points, respectively). Furthermore, in the evaluation of mechanisms of failure, patients who had revision TKA for mechanical or pain-related problems did markedly better than those who had revision TKA for infection.
Compared with primary knee implants, revision implants require increasing constraint. We assume increasing constraint affects knee biomechanics, leading to worsening functional outcomes. In a study of 60 revision TKAs (57 patients) using posterior stabilized, condylar constrained, or rotating hinge prostheses, Vasso and colleagues27 examined functional outcomes at a median follow-up of 9 years (range, 4-12 years). At most recent follow-up, mean International Knee Society (IKS) Knee and Function scores were 81 (range, 48-97) and 79 (range, 56-92), mean Hospital for Special Surgery (HSS) score was 84 (range, 62-98), and mean range of motion (ROM) was 121° (range, 98°-132°) (P < .001). Although there were no significant differences in IKS and HSS scores between prosthesis types, ROM was significantly (P < .01) wider in the posterior stabilized group than in the condylar constrained and rotating hinge groups (127° vs 112° and 108°), suggesting increasing constraint resulted in decreased ROM. Several studies have found increasing constraint might lead to reduced function.28-30
However, Hwang and colleagues31 evaluated functional outcomes in 36 revision TKAs and noted that the cemented posterior stabilized (n = 8), condylar constrained (n = 25), and rotating hinge (n = 13) prostheses used did not differ in their mean Knee Society scores (78, 81, and 83, respectively).
There remains a marked disparity in patient limitations seen after revision versus primary TKA. Given the positive results being obtained with newer implants, studies might suggest recent generations of prostheses have allowed designs to be comparable. As design development continues, we may come closer to achieving outcomes comparable to those of primary TKA.
3. Patient satisfaction
Several recent reports have shown that 10% to 25% of patients who underwent primary TKA were dissatisfied with their surgery30,32; other studies have found patient satisfaction often correlating to function and pain.33-35 Given the worse outcomes for revision TKA (outlined in the preceding section), the substantial pain accompanying a second, more complex procedure, and the extensive rehabilitation expected, we suspect patients who undergo revision TKA are even less satisfied with their surgery than their primary counterparts are. (See Appendix 4 for a complete list of studies of patient satisfaction after revision TKA.)
Barrack and colleagues32 evaluated a consecutive series of 238 patients followed up for at least 1 year after revision TKA. Patients were asked to rate their degree of satisfaction with both their primary procedure and the revision and to indicate their expectations regarding their revision prosthesis. Mean satisfaction score was 7.4 (maximum = 10), with 13% of patients dissatisfied, 18% somewhat satisfied, and 69% satisfied. Seventy-four percent of patients expected their revision prosthesis to last longer than the primary prosthesis.
Greidanus and colleagues36 evaluated patient satisfaction in 60 revision TKA cases and 199 primary TKA cases at 2-year follow-up. The primary TKA group had significantly (P < .01) higher satisfaction scores in a comparison with the revision TKA group: Global (86 vs 73), Pain Relief (88 vs 70), Function (83 vs 67), and Recreation (77 vs 62). These findings support the satisfaction rates reported by Dahm and colleagues33,34: 91% for primary TKA patients and 77% for revision TKA patients.
4. Quality of life
Procedure complexity leads to reduced survivorship, function, and mobility, longer rehabilitation, and decreased QOL for revision TKA patients relative to primary TKA patients.37 (See Appendix 5 for a complete list of studies of QOL outcomes of revision TKA.)
Greidanus and colleagues36 evaluated joint-specific QOL (using the 12-item Oxford Knee Score; OKS) and generic QOL (using the 12-Item Short Form Health Survey; SF-12) in 60 revision TKA cases and 199 primary TKA cases at a mean follow-up of 2 years. (The OKS survey is used to evaluate patient perspectives on TKA outcomes,38 and the multipurpose SF-12 questionnaire is used to assess mental and physical function and general health-related QOL.39) Compared with the revision TKA group, the primary TKA group had significantly higher OKS after surgery (78 vs 68; P = .01) as well as significantly higher SF-12 scores: Global (84 vs 72; P = .01), Mental (54 vs 50; P = .03), and Physical (43 vs 37; P = .01). Similarly, Ghomrawi and colleagues40 evaluated patterns of improvement in 308 patients (318 knees) who had revision TKA. At 24-month follow-up, mean SF-36 Physical and Mental scores were 35 and 52, respectively.
Deehan and colleagues41 used the Nottingham Health Profile (NHP) to compare 94 patients’ health-related QOL scores before revision TKA with their scores 3 months, 1 year, and 5 years after revision. NHP Pain subscale scores were significantly lower 3 and 12 months after surgery than before surgery, but this difference was no longer seen at the 5-year follow-up. There was no significant improvement in scores on the other 5 NHP subscales (Sleep, Energy, Emotion, Mobility, Social Isolation) at any time points.
As shown in the literature, patients’ QOL outcomes improve after revision TKA, but these gains are not at the level of patients who undergo primary TKA.36,41 Given that revision surgery is more extensive, and that perhaps revision patients have poorer muscle function, they usually do not return to the level they attained after their index procedure.
5. Economic impact
Consistent with the outcomes already described, the economic impact of revision TKAs is excess expenditures and costs to patients and health care institutions.42 The sources of this impact are higher implant costs, extra operative trays and times, longer hospital stays, more rehabilitation, and increased medication use.43 Revision TKA costs range from $49,000 to more than $100,000—a tremendous increase over primary TKA costs ($25,000-$30,000).43-45 Furthermore, the annual economic burden associated with revision TKA, now $2.7 billion, is expected to exceed $13 billion by 2030.46 In the United States, about $23.2 billion will be spent on 926,527 primary TKAs in 2015; significantly, the costs associated with revising just 10% of these cases account for almost 50% of the total cost of the primary procedures.46
In a retrospective cost-identification multicenter cohort study, Bozic and colleagues47 found that both-component and single-component revisions, compared with primary procedures, were associated with significantly increased operative time (~265 and 221 minutes vs 200 minutes), use of allograft bone (23% and 14% vs 1%), length of stay (5.4 and 5.7 days vs 5.0 days), and percentage of patients discharged to extended-care facilities (26% and 26% vs 25%) (P < .0001). Hospital costs for both- and single-component revisions were 138% and 114% higher than costs for primary procedures (P < .0001). More recently, Kallala and colleagues44 analyzed UK National Health Service data and compared the costs of revision for infection with revision for other causes (pain, instability, aseptic loosening, fracture). Mean length of stay associated with revision for infection (21.5 days) was more than double that associated with revision for aseptic loosening (9.5 days; P < .0001), and mean cost of revision for septic causes (£30,011) was more than 3 times that of revision for other causes (£9655; P < .0001). The authors concluded that the higher costs of revision knee surgery have a considerable economic impact, especially in infection cases.
With more extensive procedures, long-stem or more constrained prostheses are often needed to obtain adequate fixation and stability. The resulting increased, substantial economic burden is felt by patients and the health care system. Given that health care reimbursements are declining, hospitals that perform revision TKAs can sustain marked financial losses. Some centers are asking whether it is cost-effective to continue to perform these types of procedures. We must find new ways to provide revision procedures using less costly implants and tools so that centers will continue to make these procedures available to patients.
Conclusion
Given the exponential growth in primary TKAs, there will be a concordant increase in revision TKAs in the decades to come. This review provides a concise overview of revision TKA outcomes. Given the low level of evidence regarding revision TKAs, we need further higher quality studies of their prostheses and outcomes. Specifically, we need systematic reviews and meta-analyses to provide higher quality evidence regarding outcomes of using individual prosthetic designs.
Over the past 3 decades, total knee arthroplasty (TKA) has been considered a safe and effective treatment for end-stage knee arthritis.1 However, as the population, the incidence of obesity, and life expectancy continue to increase, the number of TKAs will rise as well.2,3 It is expected that over the next 16 years, the number of TKAs performed annually will exceed 3 million in the United States alone.4 This projection represents an over 600% increase from 2005 figures.5 Given the demographic shift expected over the next 2 decades, patients are anticipated to undergo these procedures at younger ages compared with previous generations, such that those age 65 years or younger will account for more than 55% of primary TKAs.6 More important, given this exponential growth in primary TKAs, there will be a concordant rise in revision procedures. It is expected that, the annual number has roughly doubled from that recorded for 2005.4
Compared with primary TKAs, however, revision TKAs have had less promising results, with survivorship as low as 60% over shorter periods.7,8 In addition, recent studies have found an even higher degree of dissatisfaction and functional limitations among revision TKA patients than among primary TKA patients, 15% to 30% of whom are unhappy with their procedures.9-11 These shortcomings of revision TKAs are thought to result from several factors, including poor bone quality, insufficient bone stock, ligamentous instability, soft-tissue incompetence, infection, malalignment, problems with extensor mechanisms, and substantial pain of uncertain etiology.
Despite there being several complex factors that can lead to worse outcomes with revision TKAs, surgeons are expected to produce results equivalent to those of primary TKAs. It is therefore imperative to delineate the objective and subjective outcomes of revision techniques to identify areas in need of improvement. In this article, we provide a concise overview of revision TKA outcomes in order to stimulate manufacturers, surgeons, and hospitals to improve on implant designs, surgical techniques, and care guidelines for revision TKA. We review the evidence on 5 points: aseptic survivorship, functional outcomes, patient satisfaction, quality of life (QOL), and economic impact. In addition, we compare available outcome data for revision and primary TKAs.
1. Aseptic survivorship
Fehring and colleagues12 in 2001 and Sharkey and colleagues13 in 2002 evaluated mechanisms of failure for revision TKA and reported many failures resulted from infection or were associated with the implant, and occurred within 2 years after the primary procedure. More recently, Dy and colleagues14 found the most common reason for revision was aseptic loosening, followed by infection. The present review focuses on aseptic femoral and tibial revision.
The failure rate for revision TKA is substantially higher than for primary TKA with the same type of prosthesis because of the complexity of the revision procedure, the increasing constraint of the implant design, and the higher degree of bone loss. (Appendix 1 lists risk factors for revision surgery. Appendix 2 is a complete list of survivorship outcomes of revision TKA.)
Sheng and colleagues15 in 2006 and Koskinen and colleagues16 in 2008 analyzed Finnish Arthroplasty Register data to determine failure rates for revision and primary TKA. Sheng and colleagues15 examined survivorship of 2637 revision TKAs (performed between 1990 and 2002) for all-cause endpoints after first revision procedure. Survivorship rates were 89% (5 years) and 79% (10 years), while Koskinen and colleagues16 noted all-cause survival rates of 80% at 15 years. More recently, in 2013, the New Zealand Orthopaedic Association17 analyzed New Zealand Joint Registry data for revision and re-revision rates (rates of revision per 100 component years) for 64,556 primary TKAs performed between 1999 and 2012. During the period studied, 1684 revisions were performed, reflecting a 2.6% revision rate, a 0.50% rate of revision per 100 component years, and a 13-year Kaplan-Meier survivorship of 94.5%. The most common reasons for revision were pain, deep infection, and tibial component loosening (Table 1).
Posterior stabilized implants
Laskin and Ohnsorge18 retrospectively reviewed the cases of 58 patients who underwent unilateral revision TKA (with a posterior stabilized implant), of which 42% were for coronal instability and 44% for a loose tibial component. At minimum 4-year follow-up, 52 of the 58 patients had anteroposterior instability of less than 5 mm. In addition, 5 years after surgery, aseptic survivorship was 96%. Meijer and colleagues19 conducted a retrospective comparative study of 69 revision TKAs (65 patients) in which 9 knees received a primary implant and 60 received a revision implant with stems and augmentation (60 = 37 posterior stabilized, 20 constrained, 3 rotating hinge). Survival rates for the primary implants were 100% (1 year), 73% (2 years), and 44% (5 years), and survival rates for the revision implants were significantly better: 95% (1 year), 92% (2 years), and 92% (5 years) (hazard ratio, 5.87; P = .008). The authors therefore indicated that it was unclear whether using a primary implant should still be an option in revision TKA and, if it is used, whether it should be limited to less complex situations in which bone loss and ligament damage are minimal (Table 2).
Constrained and semiconstrained implants
In a study of 234 knees (209 patients) with soft-tissue deficiency, Wilke and colleagues20 evaluated the long-term survivorship of revision TKA with use of a semiconstrained modular fixed-bearing implant system. Overall Kaplan-Meier survival rates were 91% (5 years) and 81% (10 years) at a mean follow-up of 9 years. When aseptic revision was evaluated, however, the survival rates increased to 95% (5 years) and 90% (10 years). The authors noted that male sex was the only variable that significantly increased the risk for re-revision (hazard ratio, 2.07; P = .02), which they attributed to potentially higher activity levels. In 2006 and 2011, Lachiewicz and Soileau21,22 evaluated the survival of first- and second-generation constrained condylar prostheses in primary TKA cases with severe valgus deformities, incompetent collateral ligaments, or severe flexion contractures. Of the 54 knees (44 patients) with first-generation prostheses, 42 (34 patients) had a mean follow-up of 9 years (range, 5-16 years). Ten-year survival with failure, defined as component revision for loosening, was 96%. The 27 TKAs using second-generation prostheses had a mean follow-up of about 5 years (range, 2-12 years). At final follow-up, there were no revisions for loosening or patellar problems, but 6 knees (22%) required lateral retinacular release of the patella (Table 3).
Rotating hinge implants
Neumann and colleagues23 evaluated the clinical and radiographic outcomes of 24 rotating hinge prostheses used for aseptic loosening with substantial bone loss and collateral ligament instability. At a mean follow-up of 56 months (range, 3-5 years), there was no evidence of loosening of any implants, and nonprogressive radiolucent lines were found in only 2 tibial components. Kowalczewski and colleagues24 evaluated the clinical and radiologic outcomes of 12 primary TKAs using a rotating hinge knee prosthesis at a minimum follow-up of 10 years. By most recent follow-up, no implants had been revised for loosening, and only 3 had nonprogressive radiolucent lines (Table 4).
Endoprostheses (modular segmental implants)
In a systematic review of 9 studies, Korim and colleagues25 evaluated 241 endoprostheses used for limb salvage under nononcologic conditions. Mean follow-up was about 3 years (range, 1-5 years). The devices were used to treat various conditions, including periprosthetic fracture, bone loss with aseptic loosening, and ligament insufficiency. The overall reoperation rate was 17% (41/241 cases). Mechanical failures were less frequent (6%-19%) (Table 5).
2. Functional outcomes
The goal in both primary and revision TKA is to restore the function and mobility of the knee and to alleviate pain. Whereas primary TKAs are realistically predictable and reproducible in their outcomes, revision TKAs are vastly more complicated, which can result in worse postoperative outcomes and function. In addition, revision TKAs may require extensive surgical exposure, which causes more tissue and muscle damage, prolonging rehabilitation. (Appendix 3 is a complete list of studies of functional outcomes of revision TKA.)
This discrepancy in functional outcomes between primary and revision TKA begins as early as the postoperative inpatient rehabilitation period. Using the functional independence measurement (FIM), which estimates performance of activities of daily living, mobility, and cognition, Vincent and colleagues26 evaluated the functional improvement produced by revision versus primary TKA during inpatient rehabilitation. They compared 424 consecutive primary TKAs with 138 revision TKAs. For both groups, FIM scores increased significantly (P = .015) between admission and discharge. On discharge, however, FIM scores were significantly (P = .01) higher for the primary group than the revision group (29 and 27 points, respectively). Furthermore, in the evaluation of mechanisms of failure, patients who had revision TKA for mechanical or pain-related problems did markedly better than those who had revision TKA for infection.
Compared with primary knee implants, revision implants require increasing constraint. We assume increasing constraint affects knee biomechanics, leading to worsening functional outcomes. In a study of 60 revision TKAs (57 patients) using posterior stabilized, condylar constrained, or rotating hinge prostheses, Vasso and colleagues27 examined functional outcomes at a median follow-up of 9 years (range, 4-12 years). At most recent follow-up, mean International Knee Society (IKS) Knee and Function scores were 81 (range, 48-97) and 79 (range, 56-92), mean Hospital for Special Surgery (HSS) score was 84 (range, 62-98), and mean range of motion (ROM) was 121° (range, 98°-132°) (P < .001). Although there were no significant differences in IKS and HSS scores between prosthesis types, ROM was significantly (P < .01) wider in the posterior stabilized group than in the condylar constrained and rotating hinge groups (127° vs 112° and 108°), suggesting increasing constraint resulted in decreased ROM. Several studies have found increasing constraint might lead to reduced function.28-30
However, Hwang and colleagues31 evaluated functional outcomes in 36 revision TKAs and noted that the cemented posterior stabilized (n = 8), condylar constrained (n = 25), and rotating hinge (n = 13) prostheses used did not differ in their mean Knee Society scores (78, 81, and 83, respectively).
There remains a marked disparity in patient limitations seen after revision versus primary TKA. Given the positive results being obtained with newer implants, studies might suggest recent generations of prostheses have allowed designs to be comparable. As design development continues, we may come closer to achieving outcomes comparable to those of primary TKA.
3. Patient satisfaction
Several recent reports have shown that 10% to 25% of patients who underwent primary TKA were dissatisfied with their surgery30,32; other studies have found patient satisfaction often correlating to function and pain.33-35 Given the worse outcomes for revision TKA (outlined in the preceding section), the substantial pain accompanying a second, more complex procedure, and the extensive rehabilitation expected, we suspect patients who undergo revision TKA are even less satisfied with their surgery than their primary counterparts are. (See Appendix 4 for a complete list of studies of patient satisfaction after revision TKA.)
Barrack and colleagues32 evaluated a consecutive series of 238 patients followed up for at least 1 year after revision TKA. Patients were asked to rate their degree of satisfaction with both their primary procedure and the revision and to indicate their expectations regarding their revision prosthesis. Mean satisfaction score was 7.4 (maximum = 10), with 13% of patients dissatisfied, 18% somewhat satisfied, and 69% satisfied. Seventy-four percent of patients expected their revision prosthesis to last longer than the primary prosthesis.
Greidanus and colleagues36 evaluated patient satisfaction in 60 revision TKA cases and 199 primary TKA cases at 2-year follow-up. The primary TKA group had significantly (P < .01) higher satisfaction scores in a comparison with the revision TKA group: Global (86 vs 73), Pain Relief (88 vs 70), Function (83 vs 67), and Recreation (77 vs 62). These findings support the satisfaction rates reported by Dahm and colleagues33,34: 91% for primary TKA patients and 77% for revision TKA patients.
4. Quality of life
Procedure complexity leads to reduced survivorship, function, and mobility, longer rehabilitation, and decreased QOL for revision TKA patients relative to primary TKA patients.37 (See Appendix 5 for a complete list of studies of QOL outcomes of revision TKA.)
Greidanus and colleagues36 evaluated joint-specific QOL (using the 12-item Oxford Knee Score; OKS) and generic QOL (using the 12-Item Short Form Health Survey; SF-12) in 60 revision TKA cases and 199 primary TKA cases at a mean follow-up of 2 years. (The OKS survey is used to evaluate patient perspectives on TKA outcomes,38 and the multipurpose SF-12 questionnaire is used to assess mental and physical function and general health-related QOL.39) Compared with the revision TKA group, the primary TKA group had significantly higher OKS after surgery (78 vs 68; P = .01) as well as significantly higher SF-12 scores: Global (84 vs 72; P = .01), Mental (54 vs 50; P = .03), and Physical (43 vs 37; P = .01). Similarly, Ghomrawi and colleagues40 evaluated patterns of improvement in 308 patients (318 knees) who had revision TKA. At 24-month follow-up, mean SF-36 Physical and Mental scores were 35 and 52, respectively.
Deehan and colleagues41 used the Nottingham Health Profile (NHP) to compare 94 patients’ health-related QOL scores before revision TKA with their scores 3 months, 1 year, and 5 years after revision. NHP Pain subscale scores were significantly lower 3 and 12 months after surgery than before surgery, but this difference was no longer seen at the 5-year follow-up. There was no significant improvement in scores on the other 5 NHP subscales (Sleep, Energy, Emotion, Mobility, Social Isolation) at any time points.
As shown in the literature, patients’ QOL outcomes improve after revision TKA, but these gains are not at the level of patients who undergo primary TKA.36,41 Given that revision surgery is more extensive, and that perhaps revision patients have poorer muscle function, they usually do not return to the level they attained after their index procedure.
5. Economic impact
Consistent with the outcomes already described, the economic impact of revision TKAs is excess expenditures and costs to patients and health care institutions.42 The sources of this impact are higher implant costs, extra operative trays and times, longer hospital stays, more rehabilitation, and increased medication use.43 Revision TKA costs range from $49,000 to more than $100,000—a tremendous increase over primary TKA costs ($25,000-$30,000).43-45 Furthermore, the annual economic burden associated with revision TKA, now $2.7 billion, is expected to exceed $13 billion by 2030.46 In the United States, about $23.2 billion will be spent on 926,527 primary TKAs in 2015; significantly, the costs associated with revising just 10% of these cases account for almost 50% of the total cost of the primary procedures.46
In a retrospective cost-identification multicenter cohort study, Bozic and colleagues47 found that both-component and single-component revisions, compared with primary procedures, were associated with significantly increased operative time (~265 and 221 minutes vs 200 minutes), use of allograft bone (23% and 14% vs 1%), length of stay (5.4 and 5.7 days vs 5.0 days), and percentage of patients discharged to extended-care facilities (26% and 26% vs 25%) (P < .0001). Hospital costs for both- and single-component revisions were 138% and 114% higher than costs for primary procedures (P < .0001). More recently, Kallala and colleagues44 analyzed UK National Health Service data and compared the costs of revision for infection with revision for other causes (pain, instability, aseptic loosening, fracture). Mean length of stay associated with revision for infection (21.5 days) was more than double that associated with revision for aseptic loosening (9.5 days; P < .0001), and mean cost of revision for septic causes (£30,011) was more than 3 times that of revision for other causes (£9655; P < .0001). The authors concluded that the higher costs of revision knee surgery have a considerable economic impact, especially in infection cases.
With more extensive procedures, long-stem or more constrained prostheses are often needed to obtain adequate fixation and stability. The resulting increased, substantial economic burden is felt by patients and the health care system. Given that health care reimbursements are declining, hospitals that perform revision TKAs can sustain marked financial losses. Some centers are asking whether it is cost-effective to continue to perform these types of procedures. We must find new ways to provide revision procedures using less costly implants and tools so that centers will continue to make these procedures available to patients.
Conclusion
Given the exponential growth in primary TKAs, there will be a concordant increase in revision TKAs in the decades to come. This review provides a concise overview of revision TKA outcomes. Given the low level of evidence regarding revision TKAs, we need further higher quality studies of their prostheses and outcomes. Specifically, we need systematic reviews and meta-analyses to provide higher quality evidence regarding outcomes of using individual prosthetic designs.
1. Cram P, Lu X, Kates SL, Singh JA, Li Y, Wolf BR. Total knee arthroplasty volume, utilization, and outcomes among Medicare beneficiaries, 1991–2010. JAMA. 2012;308(12):1227-1236.
2. Crowninshield RD, Rosenberg AG, Sporer SM. Changing demographics of patients with total joint replacement. Clin Orthop Relat Res. 2006;443:266-272.
3. Ravi B, Croxford R, Reichmann WM, Losina E, Katz JN, Hawker GA. The changing demographics of total joint arthroplasty recipients in the United States and Ontario from 2001 to 2007. Best Pract Res Clin Rheumatol. 2012;26(5):637-647.
4. Kurtz S, Ong K, Lau E, Mowat F, Halpern M. Projections of primary and revision hip and knee arthroplasty in the United States from 2005 to 2030. J Bone Joint Surg Am. 2007;89(4):780-785.
5. Kurtz SM, Ong KL, Schmier J, Zhao K, Mowat F, Lau E. Primary and revision arthroplasty surgery caseloads in the United States from 1990 to 2004. J Arthroplasty. 2009;24(2):195-203.
6. Kurtz SM, Lau E, Ong K, Zhao K, Kelly M, Bozic KJ. Future young patient demand for primary and revision joint replacement: national projections from 2010 to 2030. Clin Orthop Relat Res. 2009;467(10):2606-2612.
7. Bryan RS, Rand JA. Revision total knee arthroplasty. Clin Orthop Relat Res. 1982;170:116-122.
8. Rand JA, Bryan RS. Revision after total knee arthroplasty. Orthop Clin North Am. 1982;13(1):201-212.
9. Bozic KJ, Kurtz SM, Lau E, et al. The epidemiology of revision total knee arthroplasty in the United States. Clin Orthop Relat Res. 2010;468(1):45-51.
10. Parvizi J, Nunley RM, Berend KR, et al. High level of residual symptoms in young patients after total knee arthroplasty. Clin Orthop Relat Res. 2014;472(1):133-137.
11. Ali A, Sundberg M, Robertsson O, et al. Dissatisfied patients after total knee arthroplasty: a registry study involving 114 patients with 8-13 years of followup. Acta Orthop. 2014;85(3):229-233.
12. Fehring TK, Odum S, Griffin WL, Mason JB, Nadaud M. Early failures in total knee arthroplasty. Clin Orthop Relat Res. 2001;392:315-318.
13. Sharkey PF, Hozack WJ, Rothman RH, Shastri S, Jacoby SM. Insall Award paper. Why are total knee arthroplasties failing today? Clin Orthop Relat Res. 2002;404:7-13.
14. Dy CJ, Marx RG, Bozic KJ, Pan TJ, Padgett DE, Lyman S. Risk factors for revision within 10 years of total knee arthroplasty. Clin Orthop Relat Res. 2014;472(4):1198-1207.
15. Sheng PY, Konttinen L, Lehto M, et al. Revision total knee arthroplasty: 1990 through 2002. A review of the Finnish Arthroplasty Registry. J Bone Joint Surg Am. 2006;88(7):1425-1430.
16. Koskinen E, Eskelinen A, Paavolainen P, Pulkkinen P, Remes V. Comparison of survival and cost-effectiveness between unicondylar arthroplasty and total knee arthroplasty in patients with primary osteoarthritis: a follow-up study of 50,493 knee replacements from the Finnish Arthroplasty Register. Acta Orthop. 2008;79(4):499-507.
17. New Zealand Orthopaedic Association. The New Zealand Joint Registry Fourteen Year Report (January 1999 to December 2012). http://www.nzoa.org.nz/system/files/NJR%2014%20Year%20Report.pdf. Published November 2013. Accessed December 16, 2015.
18. Laskin RS, Ohnsorge J. The use of standard posterior stabilized implants in revision total knee arthroplasty. Clin Orthop Relat Res. 2005;(440):122-125.
19. Meijer MF, Reininga IH, Boerboom AL, Stevens M, Bulstra SK. Poorer survival after a primary implant during revision total knee arthroplasty. Int Orthop. 2013;37(3):415-419.
20. Wilke BK, Wagner ER, Trousdale RT. Long-term survival of semi-constrained total knee arthroplasty for revision surgery. J Arthroplasty. 2014;29(5):1005-1008.
21. Lachiewicz PF, Soileau ES. Ten-year survival and clinical results of constrained components in primary total knee arthroplasty. J Arthroplasty. 2006;21(6):803-808.
22. Lachiewicz PF, Soileau ES. Results of a second-generation constrained condylar prosthesis in primary total knee arthroplasty. J Arthroplasty. 2011;26(8):1228-1231.
23. Neumann DR, Hofstaedter T, Dorn U. Follow-up of a modular rotating hinge knee system in salvage revision total knee arthroplasty. J Arthroplasty. 2012;27(5):814-819.
24. Kowalczewski J, Marczak D, Synder M, Sibinski M. Primary rotating-hinge total knee arthroplasty: good outcomes at mid-term follow-up. J Arthroplasty. 2014;29(6):1202-1206.
25. Korim MT, Esler CN, Reddy VR, Ashford RU. A systematic review of endoprosthetic replacement for non-tumour indications around the knee joint. Knee. 2013;20(6):367-375.
26. Vincent KR, Vincent HK, Lee LW, Alfano AP. Inpatient rehabilitation outcomes in primary and revision total knee arthroplasty patients. Clin Orthop Relat Res. 2006;(446):201-207.
27. Vasso M, Beaufils P, Schiavone Panni A. Constraint choice in revision knee arthroplasty. Int Orthop. 2013;37(7):1279-1284.
28. Baier C, Luring C, Schaumburger J, et al. Assessing patient-oriented results after revision total knee arthroplasty. J Orthop Sci. 2013;18(6):955-961.
29. Hartford JM, Goodman SB, Schurman DJ, Knoblick G. Complex primary and revision total knee arthroplasty using the condylar constrained prosthesis: an average 5-year follow-up. J Arthroplasty. 1998;13(4):380-387.
30. Haidukewych GJ, Jacofsky DJ, Pagnano MW, Trousdale RT. Functional results after revision of well-fixed components for stiffness after primary total knee arthroplasty. J Arthroplasty. 2005;20(2):133-138.
31. Hwang SC, Kong JY, Nam DC, et al. Revision total knee arthroplasty with a cemented posterior stabilized, condylar constrained or fully constrained prosthesis: a minimum 2-year follow-up analysis. Clin Orthop Surg. 2010;2(2):112-120.
32. Barrack RL, McClure JT, Burak CF, Clohisy JC, Parvizi J, Sharkey P. Revision total knee arthroplasty: the patient’s perspective. Clin Orthop Relat Res. 2007;464:146-150.
33. Dahm DL, Barnes SA, Harrington JR, Berry DJ. Patient reported activity after revision total knee arthroplasty. J Arthroplasty. 2007;22(6 suppl 2):106-110.
34. Dahm DL, Barnes SA, Harrington JR, Sayeed SA, Berry DJ. Patient-reported activity level after total knee arthroplasty. J Arthroplasty. 2008;23(3):401-407.
35. Richards CJ, Garbuz DS, Pugh L, Masri BA. Revision total knee arthroplasty: clinical outcome comparison with and without the use of femoral head structural allograft. J Arthroplasty. 2011;26(8):1299-1304.
36. Greidanus NV, Peterson RC, Masri BA, Garbuz DS. Quality of life outcomes in revision versus primary total knee arthroplasty. J Arthroplasty. 2011;26(4):615-620.
37. Ethgen O, Bruyere O, Richy F, Dardennes C, Reginster JY. Health-related quality of life in total hip and total knee arthroplasty. A qualitative and systematic review of the literature. J Bone Joint Surg Am. 2004;86(5):963-974.
38. Murray DW, Fitzpatrick R, Rogers K, et al. The use of the Oxford hip and knee scores. J Bone Joint Surg Br. 2007;89(8):1010-1014.
39. Ware J Jr, Kosinski M, Keller SD. A 12-Item Short-Form Health Survey: construction of scales and preliminary tests of reliability and validity. Med Care. 1996;34(3):220-233.
40. Ghomrawi HM, Kane RL, Eberly LE, Bershadsky B, Saleh KJ; North American Knee Arthroplasty Revision Study Group. Patterns of functional improvement after revision knee arthroplasty. J Bone Joint Surg Am. 2009;91(12):2838-2845.
41. Deehan DJ, Murray JD, Birdsall PD, Pinder IM. Quality of life after knee revision arthroplasty. Acta Orthop. 2006;77(5):761-766.
42. Kapadia BH, McElroy MJ, Issa K, Johnson AJ, Bozic KJ, Mont MA. The economic impact of periprosthetic infections following total knee arthroplasty at a specialized tertiary-care center. J Arthroplasty. 2014;29(5):929-932.
43. Bhandari M, Smith J, Miller LE, Block JE. Clinical and economic burden of revision knee arthroplasty. Clin Med Insights Arthritis Musculoskelet Disord. 2012;5:89-94.
44. Kallala RF, Vanhegan IS, Ibrahim MS, Sarmah S, Haddad FS. Financial analysis of revision knee surgery based on NHS tariffs and hospital costs: does it pay to provide a revision service? Bone Joint J Br. 2015;97(2):197-201.
45. Ong KL, Mowat FS, Chan N, Lau E, Halpern MT, Kurtz SM. Economic burden of revision hip and knee arthroplasty in Medicare enrollees. Clin Orthop Relat Res. 2006;446:22-28.
46. Kurtz SM, Ong KL, Lau E, Bozic KJ. Impact of the economic downturn on total joint replacement demand in the United States: updated projections to 2021. J Bone Joint Surg Am. 2014;96(8):624-630.
47. Bozic KJ, Durbhakula S, Berry DJ, et al. Differences in patient and procedure characteristics and hospital resource use in primary and revision total joint arthroplasty: a multicenter study. J Arthroplasty. 2005;20(7 suppl 3):17-25.
48. Lee KJ, Moon JY, Song EK, Lim HA, Seon JK. Minimum Two-year Results of Revision Total Knee Arthroplasty Following Infectious or Non-infectious Causes. Knee Surg Relat Res. 2012;24(4):227-234.
49. Bae DK, Song SJ, Heo DB, Lee SH, Song WJ. Long-term survival rate of implants and modes of failure after revision total knee arthroplasty by a single surgeon. J Arthroplasty. 2013;28(7):1130-1134.
50. Sheng PY, Jämsen E, Lehto MU, Konttinen YT, Pajamäki J, Halonen P. Revision total knee arthroplasty with the Total Condylar III system in inflammatory arthritis. J Bone Joint Surg Br. 2005;87(9):1222-1224.
51. Lachiewicz PF, Soileau ES. Ten-year survival and clinical results of constrained components in primary total knee arthroplasty. J Arthroplasty. 2006;21(6):803-808.
52. Haas SB, Insall JN, Montgomery W 3rd, Windsor RE. Revision total knee arthroplasty with use of modular components with stems inserted without cement. J Bone Joint Surg Am. 1995;77(11):1700-1707.
53. Mabry TM, Vessely MB, Schleck CD, Harmsen WS, Berry DJ. Revision total knee arthroplasty with modular cemented stems: long-term follow-up. J Arthroplasty. 2007;22(6 Suppl 2):100-105.
54. Gudnason A, Milbrink J, Hailer NP. Implant survival and outcome after rotating-hinge total knee revision arthroplasty: a minimum 6-year follow-up. Arch Orthop Trauma Surg. 2011;131(11):1601-1607.
55. Hofmann AA, Goldberg T, Tanner AM, Kurtin SM. Treatment of infected total knee arthroplasty using an articulating spacer: 2- to 12-year experience. Clin Orthop Relat Res. 2005;430:125-131.
56. Greene JW, Reynolds SM, Stimac JD, Malkani AL, Massini MA. Midterm results of hybrid cement technique in revision total knee arthroplasty. J Arthroplasty. 2013;28(4):570-574.
57. Dalury DF, Adams MJ. Minimum 6-year follow-up of revision total knee arthroplasty without patella reimplantation. Journal Arthroplasty. 2012;27(8 Suppl):91-94.
58. Whaley AL, Trousdale RT, Rand JA, Hanssen AD. Cemented long-stem revision total knee arthroplasty. J Arthroplasty. 2003;18(5):592-599.
59. Friedman RJ, Hirst P, Poss R, Kelley K, Sledge CB. Results of revision total knee arthroplasty performed for aseptic loosening. Clinical Orthop Relat Res. 1990;255:235-241.
60. Barrack RL, Rorabeck C, Partington P, Sawhney J, Engh G. The results of retaining a well-fixed patellar component in revision total knee arthroplasty. J Arthroplasty. 2000;15(4):413-417.
61. Christensen CP, Crawford JJ, Olin MD, Vail TP. Revision of the stiff total knee arthroplasty. J Arthroplasty. 2002;17(4):409-415.
62. Garcia RM, Hardy BT, Kraay MJ, Goldberg VM. Revision total knee arthroplasty for aseptic and septic causes in patients with rheumatoid arthritis. Clin Orthop Relat Res. 2010;468(1):82-89.
63. Patil N, Lee K, Huddleston JI, Harris AH, Goodman SB. Aseptic versus septic revision total knee arthroplasty: patient satisfaction, outcome and quality of life improvement. Knee. 2010;17(3):200-203.
64. Luque R, Rizo B, Urda A, et al. Predictive factors for failure after total knee replacement revision. Int Orthop. 2014;38(2):429-435.
65. Bistolfi A, Massazza G, Rosso F, Crova M. Rotating-hinge total knee for revision total knee arthroplasty. Orthopedics. 2012;35(3):e325-e330.
66. Bottner F, Laskin R, Windsor RE, Haas SB. Hybrid component fixation in revision total knee arthroplasty. Clin Orthop Relat Res. 2006;446:127-131.
67. Jensen CL, Winther N, Schroder HM, Petersen MM. Outcome of revision total knee arthroplasty with the use of trabecular metal cone for reconstruction of severe bone loss at the proximal tibia. Knee. 2014;21(6):1233-1237.
68. Howard JL, Kudera J, Lewallen DG, Hanssen AD. Early results of the use of tantalum femoral cones for revision total knee arthroplasty. J Bone Joint Surg Am. 2011;93(5):478-484.
69. Yang JH, Yoon JR, Oh CH, Kim TS. Hybrid component fixation in total knee arthroplasty: minimum of 10-year follow-up study. J Arthroplasty. 2012;27(6):1111-1118.
70. Peters CL, Erickson JA, Gililland JM. Clinical and radiographic results of 184 consecutive revision total knee arthroplasties placed with modular cementless stems. J Arthroplasty. 2009;24(6 Suppl):48-53.
71. Registry AOANJR. Hip and Knee Arthroplasty. Annual Report 2014. 2014.
72. Registry AOANJR. Hip and Knee Arthroplasty. Annual Report 2013. 2013.
1. Cram P, Lu X, Kates SL, Singh JA, Li Y, Wolf BR. Total knee arthroplasty volume, utilization, and outcomes among Medicare beneficiaries, 1991–2010. JAMA. 2012;308(12):1227-1236.
2. Crowninshield RD, Rosenberg AG, Sporer SM. Changing demographics of patients with total joint replacement. Clin Orthop Relat Res. 2006;443:266-272.
3. Ravi B, Croxford R, Reichmann WM, Losina E, Katz JN, Hawker GA. The changing demographics of total joint arthroplasty recipients in the United States and Ontario from 2001 to 2007. Best Pract Res Clin Rheumatol. 2012;26(5):637-647.
4. Kurtz S, Ong K, Lau E, Mowat F, Halpern M. Projections of primary and revision hip and knee arthroplasty in the United States from 2005 to 2030. J Bone Joint Surg Am. 2007;89(4):780-785.
5. Kurtz SM, Ong KL, Schmier J, Zhao K, Mowat F, Lau E. Primary and revision arthroplasty surgery caseloads in the United States from 1990 to 2004. J Arthroplasty. 2009;24(2):195-203.
6. Kurtz SM, Lau E, Ong K, Zhao K, Kelly M, Bozic KJ. Future young patient demand for primary and revision joint replacement: national projections from 2010 to 2030. Clin Orthop Relat Res. 2009;467(10):2606-2612.
7. Bryan RS, Rand JA. Revision total knee arthroplasty. Clin Orthop Relat Res. 1982;170:116-122.
8. Rand JA, Bryan RS. Revision after total knee arthroplasty. Orthop Clin North Am. 1982;13(1):201-212.
9. Bozic KJ, Kurtz SM, Lau E, et al. The epidemiology of revision total knee arthroplasty in the United States. Clin Orthop Relat Res. 2010;468(1):45-51.
10. Parvizi J, Nunley RM, Berend KR, et al. High level of residual symptoms in young patients after total knee arthroplasty. Clin Orthop Relat Res. 2014;472(1):133-137.
11. Ali A, Sundberg M, Robertsson O, et al. Dissatisfied patients after total knee arthroplasty: a registry study involving 114 patients with 8-13 years of followup. Acta Orthop. 2014;85(3):229-233.
12. Fehring TK, Odum S, Griffin WL, Mason JB, Nadaud M. Early failures in total knee arthroplasty. Clin Orthop Relat Res. 2001;392:315-318.
13. Sharkey PF, Hozack WJ, Rothman RH, Shastri S, Jacoby SM. Insall Award paper. Why are total knee arthroplasties failing today? Clin Orthop Relat Res. 2002;404:7-13.
14. Dy CJ, Marx RG, Bozic KJ, Pan TJ, Padgett DE, Lyman S. Risk factors for revision within 10 years of total knee arthroplasty. Clin Orthop Relat Res. 2014;472(4):1198-1207.
15. Sheng PY, Konttinen L, Lehto M, et al. Revision total knee arthroplasty: 1990 through 2002. A review of the Finnish Arthroplasty Registry. J Bone Joint Surg Am. 2006;88(7):1425-1430.
16. Koskinen E, Eskelinen A, Paavolainen P, Pulkkinen P, Remes V. Comparison of survival and cost-effectiveness between unicondylar arthroplasty and total knee arthroplasty in patients with primary osteoarthritis: a follow-up study of 50,493 knee replacements from the Finnish Arthroplasty Register. Acta Orthop. 2008;79(4):499-507.
17. New Zealand Orthopaedic Association. The New Zealand Joint Registry Fourteen Year Report (January 1999 to December 2012). http://www.nzoa.org.nz/system/files/NJR%2014%20Year%20Report.pdf. Published November 2013. Accessed December 16, 2015.
18. Laskin RS, Ohnsorge J. The use of standard posterior stabilized implants in revision total knee arthroplasty. Clin Orthop Relat Res. 2005;(440):122-125.
19. Meijer MF, Reininga IH, Boerboom AL, Stevens M, Bulstra SK. Poorer survival after a primary implant during revision total knee arthroplasty. Int Orthop. 2013;37(3):415-419.
20. Wilke BK, Wagner ER, Trousdale RT. Long-term survival of semi-constrained total knee arthroplasty for revision surgery. J Arthroplasty. 2014;29(5):1005-1008.
21. Lachiewicz PF, Soileau ES. Ten-year survival and clinical results of constrained components in primary total knee arthroplasty. J Arthroplasty. 2006;21(6):803-808.
22. Lachiewicz PF, Soileau ES. Results of a second-generation constrained condylar prosthesis in primary total knee arthroplasty. J Arthroplasty. 2011;26(8):1228-1231.
23. Neumann DR, Hofstaedter T, Dorn U. Follow-up of a modular rotating hinge knee system in salvage revision total knee arthroplasty. J Arthroplasty. 2012;27(5):814-819.
24. Kowalczewski J, Marczak D, Synder M, Sibinski M. Primary rotating-hinge total knee arthroplasty: good outcomes at mid-term follow-up. J Arthroplasty. 2014;29(6):1202-1206.
25. Korim MT, Esler CN, Reddy VR, Ashford RU. A systematic review of endoprosthetic replacement for non-tumour indications around the knee joint. Knee. 2013;20(6):367-375.
26. Vincent KR, Vincent HK, Lee LW, Alfano AP. Inpatient rehabilitation outcomes in primary and revision total knee arthroplasty patients. Clin Orthop Relat Res. 2006;(446):201-207.
27. Vasso M, Beaufils P, Schiavone Panni A. Constraint choice in revision knee arthroplasty. Int Orthop. 2013;37(7):1279-1284.
28. Baier C, Luring C, Schaumburger J, et al. Assessing patient-oriented results after revision total knee arthroplasty. J Orthop Sci. 2013;18(6):955-961.
29. Hartford JM, Goodman SB, Schurman DJ, Knoblick G. Complex primary and revision total knee arthroplasty using the condylar constrained prosthesis: an average 5-year follow-up. J Arthroplasty. 1998;13(4):380-387.
30. Haidukewych GJ, Jacofsky DJ, Pagnano MW, Trousdale RT. Functional results after revision of well-fixed components for stiffness after primary total knee arthroplasty. J Arthroplasty. 2005;20(2):133-138.
31. Hwang SC, Kong JY, Nam DC, et al. Revision total knee arthroplasty with a cemented posterior stabilized, condylar constrained or fully constrained prosthesis: a minimum 2-year follow-up analysis. Clin Orthop Surg. 2010;2(2):112-120.
32. Barrack RL, McClure JT, Burak CF, Clohisy JC, Parvizi J, Sharkey P. Revision total knee arthroplasty: the patient’s perspective. Clin Orthop Relat Res. 2007;464:146-150.
33. Dahm DL, Barnes SA, Harrington JR, Berry DJ. Patient reported activity after revision total knee arthroplasty. J Arthroplasty. 2007;22(6 suppl 2):106-110.
34. Dahm DL, Barnes SA, Harrington JR, Sayeed SA, Berry DJ. Patient-reported activity level after total knee arthroplasty. J Arthroplasty. 2008;23(3):401-407.
35. Richards CJ, Garbuz DS, Pugh L, Masri BA. Revision total knee arthroplasty: clinical outcome comparison with and without the use of femoral head structural allograft. J Arthroplasty. 2011;26(8):1299-1304.
36. Greidanus NV, Peterson RC, Masri BA, Garbuz DS. Quality of life outcomes in revision versus primary total knee arthroplasty. J Arthroplasty. 2011;26(4):615-620.
37. Ethgen O, Bruyere O, Richy F, Dardennes C, Reginster JY. Health-related quality of life in total hip and total knee arthroplasty. A qualitative and systematic review of the literature. J Bone Joint Surg Am. 2004;86(5):963-974.
38. Murray DW, Fitzpatrick R, Rogers K, et al. The use of the Oxford hip and knee scores. J Bone Joint Surg Br. 2007;89(8):1010-1014.
39. Ware J Jr, Kosinski M, Keller SD. A 12-Item Short-Form Health Survey: construction of scales and preliminary tests of reliability and validity. Med Care. 1996;34(3):220-233.
40. Ghomrawi HM, Kane RL, Eberly LE, Bershadsky B, Saleh KJ; North American Knee Arthroplasty Revision Study Group. Patterns of functional improvement after revision knee arthroplasty. J Bone Joint Surg Am. 2009;91(12):2838-2845.
41. Deehan DJ, Murray JD, Birdsall PD, Pinder IM. Quality of life after knee revision arthroplasty. Acta Orthop. 2006;77(5):761-766.
42. Kapadia BH, McElroy MJ, Issa K, Johnson AJ, Bozic KJ, Mont MA. The economic impact of periprosthetic infections following total knee arthroplasty at a specialized tertiary-care center. J Arthroplasty. 2014;29(5):929-932.
43. Bhandari M, Smith J, Miller LE, Block JE. Clinical and economic burden of revision knee arthroplasty. Clin Med Insights Arthritis Musculoskelet Disord. 2012;5:89-94.
44. Kallala RF, Vanhegan IS, Ibrahim MS, Sarmah S, Haddad FS. Financial analysis of revision knee surgery based on NHS tariffs and hospital costs: does it pay to provide a revision service? Bone Joint J Br. 2015;97(2):197-201.
45. Ong KL, Mowat FS, Chan N, Lau E, Halpern MT, Kurtz SM. Economic burden of revision hip and knee arthroplasty in Medicare enrollees. Clin Orthop Relat Res. 2006;446:22-28.
46. Kurtz SM, Ong KL, Lau E, Bozic KJ. Impact of the economic downturn on total joint replacement demand in the United States: updated projections to 2021. J Bone Joint Surg Am. 2014;96(8):624-630.
47. Bozic KJ, Durbhakula S, Berry DJ, et al. Differences in patient and procedure characteristics and hospital resource use in primary and revision total joint arthroplasty: a multicenter study. J Arthroplasty. 2005;20(7 suppl 3):17-25.
48. Lee KJ, Moon JY, Song EK, Lim HA, Seon JK. Minimum Two-year Results of Revision Total Knee Arthroplasty Following Infectious or Non-infectious Causes. Knee Surg Relat Res. 2012;24(4):227-234.
49. Bae DK, Song SJ, Heo DB, Lee SH, Song WJ. Long-term survival rate of implants and modes of failure after revision total knee arthroplasty by a single surgeon. J Arthroplasty. 2013;28(7):1130-1134.
50. Sheng PY, Jämsen E, Lehto MU, Konttinen YT, Pajamäki J, Halonen P. Revision total knee arthroplasty with the Total Condylar III system in inflammatory arthritis. J Bone Joint Surg Br. 2005;87(9):1222-1224.
51. Lachiewicz PF, Soileau ES. Ten-year survival and clinical results of constrained components in primary total knee arthroplasty. J Arthroplasty. 2006;21(6):803-808.
52. Haas SB, Insall JN, Montgomery W 3rd, Windsor RE. Revision total knee arthroplasty with use of modular components with stems inserted without cement. J Bone Joint Surg Am. 1995;77(11):1700-1707.
53. Mabry TM, Vessely MB, Schleck CD, Harmsen WS, Berry DJ. Revision total knee arthroplasty with modular cemented stems: long-term follow-up. J Arthroplasty. 2007;22(6 Suppl 2):100-105.
54. Gudnason A, Milbrink J, Hailer NP. Implant survival and outcome after rotating-hinge total knee revision arthroplasty: a minimum 6-year follow-up. Arch Orthop Trauma Surg. 2011;131(11):1601-1607.
55. Hofmann AA, Goldberg T, Tanner AM, Kurtin SM. Treatment of infected total knee arthroplasty using an articulating spacer: 2- to 12-year experience. Clin Orthop Relat Res. 2005;430:125-131.
56. Greene JW, Reynolds SM, Stimac JD, Malkani AL, Massini MA. Midterm results of hybrid cement technique in revision total knee arthroplasty. J Arthroplasty. 2013;28(4):570-574.
57. Dalury DF, Adams MJ. Minimum 6-year follow-up of revision total knee arthroplasty without patella reimplantation. Journal Arthroplasty. 2012;27(8 Suppl):91-94.
58. Whaley AL, Trousdale RT, Rand JA, Hanssen AD. Cemented long-stem revision total knee arthroplasty. J Arthroplasty. 2003;18(5):592-599.
59. Friedman RJ, Hirst P, Poss R, Kelley K, Sledge CB. Results of revision total knee arthroplasty performed for aseptic loosening. Clinical Orthop Relat Res. 1990;255:235-241.
60. Barrack RL, Rorabeck C, Partington P, Sawhney J, Engh G. The results of retaining a well-fixed patellar component in revision total knee arthroplasty. J Arthroplasty. 2000;15(4):413-417.
61. Christensen CP, Crawford JJ, Olin MD, Vail TP. Revision of the stiff total knee arthroplasty. J Arthroplasty. 2002;17(4):409-415.
62. Garcia RM, Hardy BT, Kraay MJ, Goldberg VM. Revision total knee arthroplasty for aseptic and septic causes in patients with rheumatoid arthritis. Clin Orthop Relat Res. 2010;468(1):82-89.
63. Patil N, Lee K, Huddleston JI, Harris AH, Goodman SB. Aseptic versus septic revision total knee arthroplasty: patient satisfaction, outcome and quality of life improvement. Knee. 2010;17(3):200-203.
64. Luque R, Rizo B, Urda A, et al. Predictive factors for failure after total knee replacement revision. Int Orthop. 2014;38(2):429-435.
65. Bistolfi A, Massazza G, Rosso F, Crova M. Rotating-hinge total knee for revision total knee arthroplasty. Orthopedics. 2012;35(3):e325-e330.
66. Bottner F, Laskin R, Windsor RE, Haas SB. Hybrid component fixation in revision total knee arthroplasty. Clin Orthop Relat Res. 2006;446:127-131.
67. Jensen CL, Winther N, Schroder HM, Petersen MM. Outcome of revision total knee arthroplasty with the use of trabecular metal cone for reconstruction of severe bone loss at the proximal tibia. Knee. 2014;21(6):1233-1237.
68. Howard JL, Kudera J, Lewallen DG, Hanssen AD. Early results of the use of tantalum femoral cones for revision total knee arthroplasty. J Bone Joint Surg Am. 2011;93(5):478-484.
69. Yang JH, Yoon JR, Oh CH, Kim TS. Hybrid component fixation in total knee arthroplasty: minimum of 10-year follow-up study. J Arthroplasty. 2012;27(6):1111-1118.
70. Peters CL, Erickson JA, Gililland JM. Clinical and radiographic results of 184 consecutive revision total knee arthroplasties placed with modular cementless stems. J Arthroplasty. 2009;24(6 Suppl):48-53.
71. Registry AOANJR. Hip and Knee Arthroplasty. Annual Report 2014. 2014.
72. Registry AOANJR. Hip and Knee Arthroplasty. Annual Report 2013. 2013.
Implant Designs in Revision Total Knee Arthroplasty
Before 1990, a considerable number of revisions were performed, largely for implant-associated failures, in the first few years after index primary knee arthroplasties.1,2 Since then, surgeons, manufacturers, and hospitals have collaborated to improve implant designs, techniques, and care guidelines.3,4 Despite the substantial improvements in designs, which led to implant longevity of more than 15 years in many cases, these devices still have limited life spans. Large studies have estimated that the risk for revision required after primary knee arthroplasty ranges from as low as 5% at 15 years to up to 9% at 10 years.4,5
The surgical goals of revision total knee arthroplasty (TKA) are to obtain stable fixation of the prosthesis to host bone, to obtain a stable range of motion compatible with the patient’s activities of daily living, and to achieve these goals while using the smallest amount of prosthetic augments and constraint so that the soft tissues may share in load transfer.6 As prosthetic constraint increases, the soft tissues participate less in load sharing, and increasing stresses are put on the implant–bone interface, which further increases the risk for early implant loosening.7 Hence, as characteristics of a revision implant become more constrained, there is often a higher rate of aseptic loosening expected.8
Controversy remains regarding the ideal implant type for revision TKA. To ensure the success of revision surgery and to reduce the risks for postoperative dissatisfaction, complications, and re-revision, orthopedists must understand the types of revision implant designs available, particularly as each has its own indications and potential complications.
In this article, we review the classification systems used for revision TKA as well as the types of prosthetic designs that can be used: posterior stabilized, nonlinked constrained, rotating hinge, and modular segmental.
1. Classification of bone loss and soft-tissue integrity
To further understand revision TKA, we must consider the complexity level of these cases, particularly by evaluating degree of bone loss and soft-tissue deficiency. The most accepted way to assess bone loss both before and during surgery is to use the AORI (Anderson Orthopaedic Research Institute) classification system.9 Bone loss can be classified into 3 types: I, in which metaphyseal bone is intact and small bone defects do not compromise component stability; II, in which metaphyseal bone is damaged and cancellous bone loss requires cement fill, augments, or bone graft; and III, in which metaphyseal bone is deficient, and lost bone comprises a major portion of condyle or plateau and occasionally requires bone grafts or custom implants (Table 1). These patterns of bone loss are occasionally associated with detachment of the collateral ligament or patellar tendon.
In addition to understanding bone loss in revision TKA, surgeons must be aware of soft-tissue deficiencies (eg, collateral ligaments, extensor mechanism), which also influence type and amount of prosthesis constraint. Specifically, constraint choice depends on amount of bone loss and on the condition of stabilizing tissues, such as the collateral ligaments. Under conditions of minimal bone loss and intact peripheral ligaments, a less constrained device, such as a primary posterior stabilized system, can be considered. When ligaments are present but insufficient, a semiconstrained device is recommended. In the presence of medial collateral ligament attenuation or complete medial or lateral collateral ligament dysfunction, a fully constrained prosthesis is required.8 Therefore, amount of bone loss or soft-tissue deficiency often dictates which prosthesis to use.
For radiographic classification, the Knee Society roentgenographic evaluation and scoring system10 has been implemented to allow for uniform reporting of radiographic results and to ensure adequate preoperative planning and postoperative assessment of component alignment. This system incorporates the evaluation of alignment in the coronal, sagittal, and patellofemoral planes and assesses radiolucency using zones dividing the implant–bone interface into segments to allow for easier classification of areas of lucency. More recently, a modified version of the Knee Society system was constructed.11 This modification simplifies zone classifications and accommodates more complex revision knee designs and stem extensions.
2. Posterior stabilized designs
Cruciate-retaining prostheses are seldom applicable in the revision TKA setting because of frequent damage to the posterior cruciate ligament, except in the case of simple polyethylene exchanges or, potentially, revisions of failed unicompartmental TKAs. Thus, posterior stabilized designs are the first-line choice for revision TKA (Figure 1). These prostheses are indicated only when the posterior cruciate ligament is incompetent and in the setting of adequate flexion and extension and medial and lateral collateral ligament balancing.
However, studies have shown that posterior stabilized TKAs have a limited role in revision TKAs, as the amount of ligamentous and bony damage is often underestimated in these patients, and use of a primary implant in a revision setting often requires additional augments, all of which may have contributed to the high failure rate. Thus, this design should be used only when the patient has adequate bone stock (AORI type I) and collateral ligament tension. This situation further emphasizes the importance of performing intraoperative testing for ligamentous balance and bone deficit evaluation in order to determine the most appropriate implant (Table 2).
3. Nonlinked constrained designs
Nonlinked constrained (condylar constrained) designs are the devices most commonly used for revision TKAs (>50% of revision knees). These prostheses provide increased articular constraint, which is required in patients with persistent instability, despite appropriate soft-tissue balancing. Increased articular constraint allows for more knee stability by providing progressive varus-valgus, coronal, and rotational stability with the aid of taller and wider tibial posts.12 Specifically, these implants incorporate a tibial post that fits closely between the femoral condyles, allowing for less motion compared with a standard posterior stabilized design.12
In addition, these designs may be used with augments, stems, and allografts when bone loss is more substantial. In particular, stem extensions allow for load distribution to the diaphyseal regions of the tibia and femur and thereby aid in reducing the increased stress at the bone–implant interface, which is a common concern with these implants. However, these extensions cost more, require intramedullary invasion, and are associated with higher rates of leg and thigh pain.12
These prostheses are often implicated in cases involving a high degree of bone loss (eg, AORI type II or III). They are ideally used in cases in which complete revision of both tibial and femoral components is needed and are indicated in cases of incompetent posterior cruciate ligament, partial functional loss of medial or lateral collateral ligaments, or flexion-extension mismatch.13 Furthermore, use of a constrained prosthesis is recommended in the setting of varus or valgus instability, or repeated dislocations of a posterior stabilized design (Table 2).
Ten-year survivorship ranges from 85% to 96%, but this is substantially lower than the 95% to 96% for condylar constrained prostheses used in primary TKAs.14-17 Moreover, the large discrepancy between survivorship of primary TKA and revision TKA with a constrained prosthesis further affirms that the complexity of revision surgery, rather than the prosthesis used, may have more deleterious effects on outcomes. However, surgeons must be aware that increased constraint leads to increased stress on the prosthetic interfaces with associated aseptic loosening and early failure, and this continues to be a legitimate concern.
4. Rotating hinge designs
Many patients who undergo revision TKA can be managed with a posterior stabilizing or nonlinked constrained design. However, in patients who present with severe ligamentous instability and bone loss (AORI type II or III), a rotating hinge prostheses, or highly constrained device, is often recommended (Figure 2).18 By using a rotating mobile-bearing platform, this prosthesis permits axial rotation through a metal-reinforced polyethylene-post articulation in the tibial tray. In addition, it involves use of modular diaphyseal-engaging stems and diaphyseal sleeves, which allow for the bypass of bony defects and areas of bone loss (Table 2).
However, the rigid biomechanics of hinged prostheses is associated with increased risk for aseptic loosening (aseptic 10-year survival, 60%-80%), imparted by the transfer of stresses across the bone. The higher risk for early loosening, osteolysis, and excessive wear—caused by the highly restricted biomechanics of early generations of fixed hinged designs—has led to the development of new devices with mobile mechanics. Prosthetic designs have been improved with an added rotational axis to reduce torsional stress, a patellar resurfacing option, and better stem fixation and patellofemoral kinematics. Overall, these are aimed to improve rates of instability and aseptic loosening, with promising results demonstrated in the literature.
5. Modular segmental arthroplasty designs
Segmental arthroplasty prostheses, which typically are end-of-the-line revision TKA options, are applicable only in cases of extensive bone loss (more than can be treated with allografts or augments; AORI type 3), complete ligamentous disruption/absence, loss of periprosthetic soft tissue, and multiple previous revision procedures (Figure 3). Despite the limited indications for these prostheses, they yield quick return to function without graft nonunion or resorption, and they augment ingrowth/ongrowth. Furthermore, the next surgical option could be fusion or amputation. When failures were specifically evaluated for aseptic loosening across 4 studies, the survival rate ranged from 83% to 99.5%, with the most frequent complication being infection (up to 33% in one series).6,19-21
The major roles for segmental arthroplasty prostheses in primary TKAs are in the setting of oncologic conditions that require bony excision, or unreconstuctable fractures about the knee. Used after ancillary metastatic disease, these prostheses demonstrate positive results, according to several reports.22,23 In the setting of revision TKA, however, these prostheses should be used only when other surgical options are unfeasible, given the high risk for infection and the re-revision rates. Currently, revision TKAs with tumor prostheses have a high failure rate (up to 50%) because of the extensive surgery and the lack of bony and soft-tissue support (Table 2).
Conclusion
Orthopedists performing revision TKAs must consider bone stock and remaining ligament stability. In particular, they should choose implants for least constraint and adequate knee stability, as these are essential in minimizing the stresses on the implant–bone interface. Ultimately, functional outcomes, survivorship, and postoperative satisfaction determine the success of these designs. However, predictors of outcomes of revision surgery are often multifactorial, and surgeons must also consider procedure complexity and patient-specific characteristics.
1. Fehring TK, Odum S, Griffin WL, Mason JB, Nadaud M. Early failures in total knee arthroplasty. Clin Orthop Relat Res. 2001;392:315-318.
2. Sharkey PF, Hozack WJ, Rothman RH, Shastri S, Jacoby SM. Insall Award paper. Why are total knee arthroplasties failing today? Clin Orthop Relat Res. 2002;404:7-13.
3. Schroer WC, Berend KR, Lombardi AV, et al. Why are total knees failing today? Etiology of total knee revision in 2010 and 2011. J Arthroplasty. 2013;28(8 suppl):116-119.
4. Kim TK. CORR Insights(®): risk factors for revision within 10 years of total knee arthroplasty. Clin Orthop Relat Res. 2014;472(4):1208-1209.
5. Sheng PY, Jämsen E, Lehto MU, Konttinen YT, Pajamäki J, Halonen P. Revision total knee arthroplasty with the Total Condylar III system in inflammatory arthritis. J Bone Joint Surg Br. 2005;87(9):1222-1224.
6. Haas SB, Insall JN, Montgomery W 3rd, Windsor RE. Revision total knee arthroplasty with use of modular components with stems inserted without cement. J Bone Joint Surg Am. 1995;77(11):1700-1707.
7. Dennis DA. A stepwise approach to revision total knee arthroplasty. J Arthroplasty. 2007;22(4 suppl 1):32-38.
8. Vasso M, Beaufils P, Schiavone Panni A. Constraint choice in revision knee arthroplasty. Int Orthop. 2013;37(7):1279-1284.
9. Engh GA, Ammeen DJ. Bone loss with revision total knee arthroplasty: defect classification and alternatives for reconstruction. Instr Course Lect. 1999;48:167-175.
10. Ewald FC. The Knee Society total knee arthroplasty roentgenographic evaluation and scoring system. Clin Orthop Relat Res. 1989;248:9-12.
11. Meneghini RM, Mont MA, Backstein DB, Bourne RB, Dennis DA, Scuderi GR. Development of a modern Knee Society radiographic evaluation system and methodology for total knee arthroplasty. J Arthroplasty. 2015;30(12):2311-2314.
12. Nam D, Umunna BP, Cross MB, Reinhardt KR, Duggal S, Cornell CN. Clinical results and failure mechanisms of a nonmodular constrained knee without stem extensions. HSS J. 2012;8(2):96-102.
13. Lombardi AV Jr, Berend KR. The role of implant constraint in revision TKA: striking the balance. Orthopedics. 2006;29(9):847-849.
14. Lachiewicz PF, Soileau ES. Results of a second-generation constrained condylar prosthesis in primary total knee arthroplasty. J Arthroplasty. 2011;26(8):1228-1231.
15. Bae DK, Song SJ, Heo DB, Lee SH, Song WJ. Long-term survival rate of implants and modes of failure after revision total knee arthroplasty by a single surgeon. J Arthroplasty. 2013;28(7):1130-1134.
16. Wilke BK, Wagner ER, Trousdale RT. Long-term survival of semi-constrained total knee arthroplasty for revision surgery. J Arthroplasty. 2014;29(5):1005-1008.
17. Lachiewicz PF, Soileau ES. Ten-year survival and clinical results of constrained components in primary total knee arthroplasty. J Arthroplasty. 2006;21(6):803-808.
18. Jones RE. Total knee arthroplasty with modular rotating-platform hinge. Orthopedics. 2006;29(9 suppl):S80-S82.
19. Korim MT, Esler CN, Reddy VR, Ashford RU. A systematic review of endoprosthetic replacement for non-tumour indications around the knee joint. The Knee. 2013;20:367-375.
20. Hofmann AA, Goldberg T, Tanner AM, Kurtin SM. Treatment of infected total knee arthroplasty using an articulating spacer: 2- to 12-year experience. Clin Orthop Relat Res. 2005;(430):125-131.
21. Peters CL, Erickson J, Kloepper RG, Mohr RA. Revision total knee arthroplasty with modular components inserted with metaphyseal cement and stems without cement. J Arthroplasty. 2005;20:302-308.
22. Pala E, Trovarelli G, Calabro T, Angelini A, Abati CN, Ruggieri P. Survival of modern knee tumor megaprostheses: failures, functional results, and a comparative statistical analysis. Clinical Orthop Relat Res. 2015;473:891-899.
23. Angelini A, Henderson E, Trovarelli G, Ruggieri P. Is there a role for knee arthrodesis with modular endoprostheses for tumor and revision of failed endoprostheses? Clin Orthop Relat Res. 2013;471(10):3326-3335.
Before 1990, a considerable number of revisions were performed, largely for implant-associated failures, in the first few years after index primary knee arthroplasties.1,2 Since then, surgeons, manufacturers, and hospitals have collaborated to improve implant designs, techniques, and care guidelines.3,4 Despite the substantial improvements in designs, which led to implant longevity of more than 15 years in many cases, these devices still have limited life spans. Large studies have estimated that the risk for revision required after primary knee arthroplasty ranges from as low as 5% at 15 years to up to 9% at 10 years.4,5
The surgical goals of revision total knee arthroplasty (TKA) are to obtain stable fixation of the prosthesis to host bone, to obtain a stable range of motion compatible with the patient’s activities of daily living, and to achieve these goals while using the smallest amount of prosthetic augments and constraint so that the soft tissues may share in load transfer.6 As prosthetic constraint increases, the soft tissues participate less in load sharing, and increasing stresses are put on the implant–bone interface, which further increases the risk for early implant loosening.7 Hence, as characteristics of a revision implant become more constrained, there is often a higher rate of aseptic loosening expected.8
Controversy remains regarding the ideal implant type for revision TKA. To ensure the success of revision surgery and to reduce the risks for postoperative dissatisfaction, complications, and re-revision, orthopedists must understand the types of revision implant designs available, particularly as each has its own indications and potential complications.
In this article, we review the classification systems used for revision TKA as well as the types of prosthetic designs that can be used: posterior stabilized, nonlinked constrained, rotating hinge, and modular segmental.
1. Classification of bone loss and soft-tissue integrity
To further understand revision TKA, we must consider the complexity level of these cases, particularly by evaluating degree of bone loss and soft-tissue deficiency. The most accepted way to assess bone loss both before and during surgery is to use the AORI (Anderson Orthopaedic Research Institute) classification system.9 Bone loss can be classified into 3 types: I, in which metaphyseal bone is intact and small bone defects do not compromise component stability; II, in which metaphyseal bone is damaged and cancellous bone loss requires cement fill, augments, or bone graft; and III, in which metaphyseal bone is deficient, and lost bone comprises a major portion of condyle or plateau and occasionally requires bone grafts or custom implants (Table 1). These patterns of bone loss are occasionally associated with detachment of the collateral ligament or patellar tendon.
In addition to understanding bone loss in revision TKA, surgeons must be aware of soft-tissue deficiencies (eg, collateral ligaments, extensor mechanism), which also influence type and amount of prosthesis constraint. Specifically, constraint choice depends on amount of bone loss and on the condition of stabilizing tissues, such as the collateral ligaments. Under conditions of minimal bone loss and intact peripheral ligaments, a less constrained device, such as a primary posterior stabilized system, can be considered. When ligaments are present but insufficient, a semiconstrained device is recommended. In the presence of medial collateral ligament attenuation or complete medial or lateral collateral ligament dysfunction, a fully constrained prosthesis is required.8 Therefore, amount of bone loss or soft-tissue deficiency often dictates which prosthesis to use.
For radiographic classification, the Knee Society roentgenographic evaluation and scoring system10 has been implemented to allow for uniform reporting of radiographic results and to ensure adequate preoperative planning and postoperative assessment of component alignment. This system incorporates the evaluation of alignment in the coronal, sagittal, and patellofemoral planes and assesses radiolucency using zones dividing the implant–bone interface into segments to allow for easier classification of areas of lucency. More recently, a modified version of the Knee Society system was constructed.11 This modification simplifies zone classifications and accommodates more complex revision knee designs and stem extensions.
2. Posterior stabilized designs
Cruciate-retaining prostheses are seldom applicable in the revision TKA setting because of frequent damage to the posterior cruciate ligament, except in the case of simple polyethylene exchanges or, potentially, revisions of failed unicompartmental TKAs. Thus, posterior stabilized designs are the first-line choice for revision TKA (Figure 1). These prostheses are indicated only when the posterior cruciate ligament is incompetent and in the setting of adequate flexion and extension and medial and lateral collateral ligament balancing.
However, studies have shown that posterior stabilized TKAs have a limited role in revision TKAs, as the amount of ligamentous and bony damage is often underestimated in these patients, and use of a primary implant in a revision setting often requires additional augments, all of which may have contributed to the high failure rate. Thus, this design should be used only when the patient has adequate bone stock (AORI type I) and collateral ligament tension. This situation further emphasizes the importance of performing intraoperative testing for ligamentous balance and bone deficit evaluation in order to determine the most appropriate implant (Table 2).
3. Nonlinked constrained designs
Nonlinked constrained (condylar constrained) designs are the devices most commonly used for revision TKAs (>50% of revision knees). These prostheses provide increased articular constraint, which is required in patients with persistent instability, despite appropriate soft-tissue balancing. Increased articular constraint allows for more knee stability by providing progressive varus-valgus, coronal, and rotational stability with the aid of taller and wider tibial posts.12 Specifically, these implants incorporate a tibial post that fits closely between the femoral condyles, allowing for less motion compared with a standard posterior stabilized design.12
In addition, these designs may be used with augments, stems, and allografts when bone loss is more substantial. In particular, stem extensions allow for load distribution to the diaphyseal regions of the tibia and femur and thereby aid in reducing the increased stress at the bone–implant interface, which is a common concern with these implants. However, these extensions cost more, require intramedullary invasion, and are associated with higher rates of leg and thigh pain.12
These prostheses are often implicated in cases involving a high degree of bone loss (eg, AORI type II or III). They are ideally used in cases in which complete revision of both tibial and femoral components is needed and are indicated in cases of incompetent posterior cruciate ligament, partial functional loss of medial or lateral collateral ligaments, or flexion-extension mismatch.13 Furthermore, use of a constrained prosthesis is recommended in the setting of varus or valgus instability, or repeated dislocations of a posterior stabilized design (Table 2).
Ten-year survivorship ranges from 85% to 96%, but this is substantially lower than the 95% to 96% for condylar constrained prostheses used in primary TKAs.14-17 Moreover, the large discrepancy between survivorship of primary TKA and revision TKA with a constrained prosthesis further affirms that the complexity of revision surgery, rather than the prosthesis used, may have more deleterious effects on outcomes. However, surgeons must be aware that increased constraint leads to increased stress on the prosthetic interfaces with associated aseptic loosening and early failure, and this continues to be a legitimate concern.
4. Rotating hinge designs
Many patients who undergo revision TKA can be managed with a posterior stabilizing or nonlinked constrained design. However, in patients who present with severe ligamentous instability and bone loss (AORI type II or III), a rotating hinge prostheses, or highly constrained device, is often recommended (Figure 2).18 By using a rotating mobile-bearing platform, this prosthesis permits axial rotation through a metal-reinforced polyethylene-post articulation in the tibial tray. In addition, it involves use of modular diaphyseal-engaging stems and diaphyseal sleeves, which allow for the bypass of bony defects and areas of bone loss (Table 2).
However, the rigid biomechanics of hinged prostheses is associated with increased risk for aseptic loosening (aseptic 10-year survival, 60%-80%), imparted by the transfer of stresses across the bone. The higher risk for early loosening, osteolysis, and excessive wear—caused by the highly restricted biomechanics of early generations of fixed hinged designs—has led to the development of new devices with mobile mechanics. Prosthetic designs have been improved with an added rotational axis to reduce torsional stress, a patellar resurfacing option, and better stem fixation and patellofemoral kinematics. Overall, these are aimed to improve rates of instability and aseptic loosening, with promising results demonstrated in the literature.
5. Modular segmental arthroplasty designs
Segmental arthroplasty prostheses, which typically are end-of-the-line revision TKA options, are applicable only in cases of extensive bone loss (more than can be treated with allografts or augments; AORI type 3), complete ligamentous disruption/absence, loss of periprosthetic soft tissue, and multiple previous revision procedures (Figure 3). Despite the limited indications for these prostheses, they yield quick return to function without graft nonunion or resorption, and they augment ingrowth/ongrowth. Furthermore, the next surgical option could be fusion or amputation. When failures were specifically evaluated for aseptic loosening across 4 studies, the survival rate ranged from 83% to 99.5%, with the most frequent complication being infection (up to 33% in one series).6,19-21
The major roles for segmental arthroplasty prostheses in primary TKAs are in the setting of oncologic conditions that require bony excision, or unreconstuctable fractures about the knee. Used after ancillary metastatic disease, these prostheses demonstrate positive results, according to several reports.22,23 In the setting of revision TKA, however, these prostheses should be used only when other surgical options are unfeasible, given the high risk for infection and the re-revision rates. Currently, revision TKAs with tumor prostheses have a high failure rate (up to 50%) because of the extensive surgery and the lack of bony and soft-tissue support (Table 2).
Conclusion
Orthopedists performing revision TKAs must consider bone stock and remaining ligament stability. In particular, they should choose implants for least constraint and adequate knee stability, as these are essential in minimizing the stresses on the implant–bone interface. Ultimately, functional outcomes, survivorship, and postoperative satisfaction determine the success of these designs. However, predictors of outcomes of revision surgery are often multifactorial, and surgeons must also consider procedure complexity and patient-specific characteristics.
Before 1990, a considerable number of revisions were performed, largely for implant-associated failures, in the first few years after index primary knee arthroplasties.1,2 Since then, surgeons, manufacturers, and hospitals have collaborated to improve implant designs, techniques, and care guidelines.3,4 Despite the substantial improvements in designs, which led to implant longevity of more than 15 years in many cases, these devices still have limited life spans. Large studies have estimated that the risk for revision required after primary knee arthroplasty ranges from as low as 5% at 15 years to up to 9% at 10 years.4,5
The surgical goals of revision total knee arthroplasty (TKA) are to obtain stable fixation of the prosthesis to host bone, to obtain a stable range of motion compatible with the patient’s activities of daily living, and to achieve these goals while using the smallest amount of prosthetic augments and constraint so that the soft tissues may share in load transfer.6 As prosthetic constraint increases, the soft tissues participate less in load sharing, and increasing stresses are put on the implant–bone interface, which further increases the risk for early implant loosening.7 Hence, as characteristics of a revision implant become more constrained, there is often a higher rate of aseptic loosening expected.8
Controversy remains regarding the ideal implant type for revision TKA. To ensure the success of revision surgery and to reduce the risks for postoperative dissatisfaction, complications, and re-revision, orthopedists must understand the types of revision implant designs available, particularly as each has its own indications and potential complications.
In this article, we review the classification systems used for revision TKA as well as the types of prosthetic designs that can be used: posterior stabilized, nonlinked constrained, rotating hinge, and modular segmental.
1. Classification of bone loss and soft-tissue integrity
To further understand revision TKA, we must consider the complexity level of these cases, particularly by evaluating degree of bone loss and soft-tissue deficiency. The most accepted way to assess bone loss both before and during surgery is to use the AORI (Anderson Orthopaedic Research Institute) classification system.9 Bone loss can be classified into 3 types: I, in which metaphyseal bone is intact and small bone defects do not compromise component stability; II, in which metaphyseal bone is damaged and cancellous bone loss requires cement fill, augments, or bone graft; and III, in which metaphyseal bone is deficient, and lost bone comprises a major portion of condyle or plateau and occasionally requires bone grafts or custom implants (Table 1). These patterns of bone loss are occasionally associated with detachment of the collateral ligament or patellar tendon.
In addition to understanding bone loss in revision TKA, surgeons must be aware of soft-tissue deficiencies (eg, collateral ligaments, extensor mechanism), which also influence type and amount of prosthesis constraint. Specifically, constraint choice depends on amount of bone loss and on the condition of stabilizing tissues, such as the collateral ligaments. Under conditions of minimal bone loss and intact peripheral ligaments, a less constrained device, such as a primary posterior stabilized system, can be considered. When ligaments are present but insufficient, a semiconstrained device is recommended. In the presence of medial collateral ligament attenuation or complete medial or lateral collateral ligament dysfunction, a fully constrained prosthesis is required.8 Therefore, amount of bone loss or soft-tissue deficiency often dictates which prosthesis to use.
For radiographic classification, the Knee Society roentgenographic evaluation and scoring system10 has been implemented to allow for uniform reporting of radiographic results and to ensure adequate preoperative planning and postoperative assessment of component alignment. This system incorporates the evaluation of alignment in the coronal, sagittal, and patellofemoral planes and assesses radiolucency using zones dividing the implant–bone interface into segments to allow for easier classification of areas of lucency. More recently, a modified version of the Knee Society system was constructed.11 This modification simplifies zone classifications and accommodates more complex revision knee designs and stem extensions.
2. Posterior stabilized designs
Cruciate-retaining prostheses are seldom applicable in the revision TKA setting because of frequent damage to the posterior cruciate ligament, except in the case of simple polyethylene exchanges or, potentially, revisions of failed unicompartmental TKAs. Thus, posterior stabilized designs are the first-line choice for revision TKA (Figure 1). These prostheses are indicated only when the posterior cruciate ligament is incompetent and in the setting of adequate flexion and extension and medial and lateral collateral ligament balancing.
However, studies have shown that posterior stabilized TKAs have a limited role in revision TKAs, as the amount of ligamentous and bony damage is often underestimated in these patients, and use of a primary implant in a revision setting often requires additional augments, all of which may have contributed to the high failure rate. Thus, this design should be used only when the patient has adequate bone stock (AORI type I) and collateral ligament tension. This situation further emphasizes the importance of performing intraoperative testing for ligamentous balance and bone deficit evaluation in order to determine the most appropriate implant (Table 2).
3. Nonlinked constrained designs
Nonlinked constrained (condylar constrained) designs are the devices most commonly used for revision TKAs (>50% of revision knees). These prostheses provide increased articular constraint, which is required in patients with persistent instability, despite appropriate soft-tissue balancing. Increased articular constraint allows for more knee stability by providing progressive varus-valgus, coronal, and rotational stability with the aid of taller and wider tibial posts.12 Specifically, these implants incorporate a tibial post that fits closely between the femoral condyles, allowing for less motion compared with a standard posterior stabilized design.12
In addition, these designs may be used with augments, stems, and allografts when bone loss is more substantial. In particular, stem extensions allow for load distribution to the diaphyseal regions of the tibia and femur and thereby aid in reducing the increased stress at the bone–implant interface, which is a common concern with these implants. However, these extensions cost more, require intramedullary invasion, and are associated with higher rates of leg and thigh pain.12
These prostheses are often implicated in cases involving a high degree of bone loss (eg, AORI type II or III). They are ideally used in cases in which complete revision of both tibial and femoral components is needed and are indicated in cases of incompetent posterior cruciate ligament, partial functional loss of medial or lateral collateral ligaments, or flexion-extension mismatch.13 Furthermore, use of a constrained prosthesis is recommended in the setting of varus or valgus instability, or repeated dislocations of a posterior stabilized design (Table 2).
Ten-year survivorship ranges from 85% to 96%, but this is substantially lower than the 95% to 96% for condylar constrained prostheses used in primary TKAs.14-17 Moreover, the large discrepancy between survivorship of primary TKA and revision TKA with a constrained prosthesis further affirms that the complexity of revision surgery, rather than the prosthesis used, may have more deleterious effects on outcomes. However, surgeons must be aware that increased constraint leads to increased stress on the prosthetic interfaces with associated aseptic loosening and early failure, and this continues to be a legitimate concern.
4. Rotating hinge designs
Many patients who undergo revision TKA can be managed with a posterior stabilizing or nonlinked constrained design. However, in patients who present with severe ligamentous instability and bone loss (AORI type II or III), a rotating hinge prostheses, or highly constrained device, is often recommended (Figure 2).18 By using a rotating mobile-bearing platform, this prosthesis permits axial rotation through a metal-reinforced polyethylene-post articulation in the tibial tray. In addition, it involves use of modular diaphyseal-engaging stems and diaphyseal sleeves, which allow for the bypass of bony defects and areas of bone loss (Table 2).
However, the rigid biomechanics of hinged prostheses is associated with increased risk for aseptic loosening (aseptic 10-year survival, 60%-80%), imparted by the transfer of stresses across the bone. The higher risk for early loosening, osteolysis, and excessive wear—caused by the highly restricted biomechanics of early generations of fixed hinged designs—has led to the development of new devices with mobile mechanics. Prosthetic designs have been improved with an added rotational axis to reduce torsional stress, a patellar resurfacing option, and better stem fixation and patellofemoral kinematics. Overall, these are aimed to improve rates of instability and aseptic loosening, with promising results demonstrated in the literature.
5. Modular segmental arthroplasty designs
Segmental arthroplasty prostheses, which typically are end-of-the-line revision TKA options, are applicable only in cases of extensive bone loss (more than can be treated with allografts or augments; AORI type 3), complete ligamentous disruption/absence, loss of periprosthetic soft tissue, and multiple previous revision procedures (Figure 3). Despite the limited indications for these prostheses, they yield quick return to function without graft nonunion or resorption, and they augment ingrowth/ongrowth. Furthermore, the next surgical option could be fusion or amputation. When failures were specifically evaluated for aseptic loosening across 4 studies, the survival rate ranged from 83% to 99.5%, with the most frequent complication being infection (up to 33% in one series).6,19-21
The major roles for segmental arthroplasty prostheses in primary TKAs are in the setting of oncologic conditions that require bony excision, or unreconstuctable fractures about the knee. Used after ancillary metastatic disease, these prostheses demonstrate positive results, according to several reports.22,23 In the setting of revision TKA, however, these prostheses should be used only when other surgical options are unfeasible, given the high risk for infection and the re-revision rates. Currently, revision TKAs with tumor prostheses have a high failure rate (up to 50%) because of the extensive surgery and the lack of bony and soft-tissue support (Table 2).
Conclusion
Orthopedists performing revision TKAs must consider bone stock and remaining ligament stability. In particular, they should choose implants for least constraint and adequate knee stability, as these are essential in minimizing the stresses on the implant–bone interface. Ultimately, functional outcomes, survivorship, and postoperative satisfaction determine the success of these designs. However, predictors of outcomes of revision surgery are often multifactorial, and surgeons must also consider procedure complexity and patient-specific characteristics.
1. Fehring TK, Odum S, Griffin WL, Mason JB, Nadaud M. Early failures in total knee arthroplasty. Clin Orthop Relat Res. 2001;392:315-318.
2. Sharkey PF, Hozack WJ, Rothman RH, Shastri S, Jacoby SM. Insall Award paper. Why are total knee arthroplasties failing today? Clin Orthop Relat Res. 2002;404:7-13.
3. Schroer WC, Berend KR, Lombardi AV, et al. Why are total knees failing today? Etiology of total knee revision in 2010 and 2011. J Arthroplasty. 2013;28(8 suppl):116-119.
4. Kim TK. CORR Insights(®): risk factors for revision within 10 years of total knee arthroplasty. Clin Orthop Relat Res. 2014;472(4):1208-1209.
5. Sheng PY, Jämsen E, Lehto MU, Konttinen YT, Pajamäki J, Halonen P. Revision total knee arthroplasty with the Total Condylar III system in inflammatory arthritis. J Bone Joint Surg Br. 2005;87(9):1222-1224.
6. Haas SB, Insall JN, Montgomery W 3rd, Windsor RE. Revision total knee arthroplasty with use of modular components with stems inserted without cement. J Bone Joint Surg Am. 1995;77(11):1700-1707.
7. Dennis DA. A stepwise approach to revision total knee arthroplasty. J Arthroplasty. 2007;22(4 suppl 1):32-38.
8. Vasso M, Beaufils P, Schiavone Panni A. Constraint choice in revision knee arthroplasty. Int Orthop. 2013;37(7):1279-1284.
9. Engh GA, Ammeen DJ. Bone loss with revision total knee arthroplasty: defect classification and alternatives for reconstruction. Instr Course Lect. 1999;48:167-175.
10. Ewald FC. The Knee Society total knee arthroplasty roentgenographic evaluation and scoring system. Clin Orthop Relat Res. 1989;248:9-12.
11. Meneghini RM, Mont MA, Backstein DB, Bourne RB, Dennis DA, Scuderi GR. Development of a modern Knee Society radiographic evaluation system and methodology for total knee arthroplasty. J Arthroplasty. 2015;30(12):2311-2314.
12. Nam D, Umunna BP, Cross MB, Reinhardt KR, Duggal S, Cornell CN. Clinical results and failure mechanisms of a nonmodular constrained knee without stem extensions. HSS J. 2012;8(2):96-102.
13. Lombardi AV Jr, Berend KR. The role of implant constraint in revision TKA: striking the balance. Orthopedics. 2006;29(9):847-849.
14. Lachiewicz PF, Soileau ES. Results of a second-generation constrained condylar prosthesis in primary total knee arthroplasty. J Arthroplasty. 2011;26(8):1228-1231.
15. Bae DK, Song SJ, Heo DB, Lee SH, Song WJ. Long-term survival rate of implants and modes of failure after revision total knee arthroplasty by a single surgeon. J Arthroplasty. 2013;28(7):1130-1134.
16. Wilke BK, Wagner ER, Trousdale RT. Long-term survival of semi-constrained total knee arthroplasty for revision surgery. J Arthroplasty. 2014;29(5):1005-1008.
17. Lachiewicz PF, Soileau ES. Ten-year survival and clinical results of constrained components in primary total knee arthroplasty. J Arthroplasty. 2006;21(6):803-808.
18. Jones RE. Total knee arthroplasty with modular rotating-platform hinge. Orthopedics. 2006;29(9 suppl):S80-S82.
19. Korim MT, Esler CN, Reddy VR, Ashford RU. A systematic review of endoprosthetic replacement for non-tumour indications around the knee joint. The Knee. 2013;20:367-375.
20. Hofmann AA, Goldberg T, Tanner AM, Kurtin SM. Treatment of infected total knee arthroplasty using an articulating spacer: 2- to 12-year experience. Clin Orthop Relat Res. 2005;(430):125-131.
21. Peters CL, Erickson J, Kloepper RG, Mohr RA. Revision total knee arthroplasty with modular components inserted with metaphyseal cement and stems without cement. J Arthroplasty. 2005;20:302-308.
22. Pala E, Trovarelli G, Calabro T, Angelini A, Abati CN, Ruggieri P. Survival of modern knee tumor megaprostheses: failures, functional results, and a comparative statistical analysis. Clinical Orthop Relat Res. 2015;473:891-899.
23. Angelini A, Henderson E, Trovarelli G, Ruggieri P. Is there a role for knee arthrodesis with modular endoprostheses for tumor and revision of failed endoprostheses? Clin Orthop Relat Res. 2013;471(10):3326-3335.
1. Fehring TK, Odum S, Griffin WL, Mason JB, Nadaud M. Early failures in total knee arthroplasty. Clin Orthop Relat Res. 2001;392:315-318.
2. Sharkey PF, Hozack WJ, Rothman RH, Shastri S, Jacoby SM. Insall Award paper. Why are total knee arthroplasties failing today? Clin Orthop Relat Res. 2002;404:7-13.
3. Schroer WC, Berend KR, Lombardi AV, et al. Why are total knees failing today? Etiology of total knee revision in 2010 and 2011. J Arthroplasty. 2013;28(8 suppl):116-119.
4. Kim TK. CORR Insights(®): risk factors for revision within 10 years of total knee arthroplasty. Clin Orthop Relat Res. 2014;472(4):1208-1209.
5. Sheng PY, Jämsen E, Lehto MU, Konttinen YT, Pajamäki J, Halonen P. Revision total knee arthroplasty with the Total Condylar III system in inflammatory arthritis. J Bone Joint Surg Br. 2005;87(9):1222-1224.
6. Haas SB, Insall JN, Montgomery W 3rd, Windsor RE. Revision total knee arthroplasty with use of modular components with stems inserted without cement. J Bone Joint Surg Am. 1995;77(11):1700-1707.
7. Dennis DA. A stepwise approach to revision total knee arthroplasty. J Arthroplasty. 2007;22(4 suppl 1):32-38.
8. Vasso M, Beaufils P, Schiavone Panni A. Constraint choice in revision knee arthroplasty. Int Orthop. 2013;37(7):1279-1284.
9. Engh GA, Ammeen DJ. Bone loss with revision total knee arthroplasty: defect classification and alternatives for reconstruction. Instr Course Lect. 1999;48:167-175.
10. Ewald FC. The Knee Society total knee arthroplasty roentgenographic evaluation and scoring system. Clin Orthop Relat Res. 1989;248:9-12.
11. Meneghini RM, Mont MA, Backstein DB, Bourne RB, Dennis DA, Scuderi GR. Development of a modern Knee Society radiographic evaluation system and methodology for total knee arthroplasty. J Arthroplasty. 2015;30(12):2311-2314.
12. Nam D, Umunna BP, Cross MB, Reinhardt KR, Duggal S, Cornell CN. Clinical results and failure mechanisms of a nonmodular constrained knee without stem extensions. HSS J. 2012;8(2):96-102.
13. Lombardi AV Jr, Berend KR. The role of implant constraint in revision TKA: striking the balance. Orthopedics. 2006;29(9):847-849.
14. Lachiewicz PF, Soileau ES. Results of a second-generation constrained condylar prosthesis in primary total knee arthroplasty. J Arthroplasty. 2011;26(8):1228-1231.
15. Bae DK, Song SJ, Heo DB, Lee SH, Song WJ. Long-term survival rate of implants and modes of failure after revision total knee arthroplasty by a single surgeon. J Arthroplasty. 2013;28(7):1130-1134.
16. Wilke BK, Wagner ER, Trousdale RT. Long-term survival of semi-constrained total knee arthroplasty for revision surgery. J Arthroplasty. 2014;29(5):1005-1008.
17. Lachiewicz PF, Soileau ES. Ten-year survival and clinical results of constrained components in primary total knee arthroplasty. J Arthroplasty. 2006;21(6):803-808.
18. Jones RE. Total knee arthroplasty with modular rotating-platform hinge. Orthopedics. 2006;29(9 suppl):S80-S82.
19. Korim MT, Esler CN, Reddy VR, Ashford RU. A systematic review of endoprosthetic replacement for non-tumour indications around the knee joint. The Knee. 2013;20:367-375.
20. Hofmann AA, Goldberg T, Tanner AM, Kurtin SM. Treatment of infected total knee arthroplasty using an articulating spacer: 2- to 12-year experience. Clin Orthop Relat Res. 2005;(430):125-131.
21. Peters CL, Erickson J, Kloepper RG, Mohr RA. Revision total knee arthroplasty with modular components inserted with metaphyseal cement and stems without cement. J Arthroplasty. 2005;20:302-308.
22. Pala E, Trovarelli G, Calabro T, Angelini A, Abati CN, Ruggieri P. Survival of modern knee tumor megaprostheses: failures, functional results, and a comparative statistical analysis. Clinical Orthop Relat Res. 2015;473:891-899.
23. Angelini A, Henderson E, Trovarelli G, Ruggieri P. Is there a role for knee arthrodesis with modular endoprostheses for tumor and revision of failed endoprostheses? Clin Orthop Relat Res. 2013;471(10):3326-3335.
The Value of National and Hospital Registries
Following Dr. Sarmiento’s commentary, “Orthopedic Registries: Second Thoughts,” we agree that it is important and appropriate to question the value of any new additions to the orthopedic field, and registries are no exception. We thank Dr. Sarmiento for his comments on the viability of registries and the need for continued critical evaluation. Before joint registries, however, we had to rely on small-cohort analyses to assess outcomes and complications. Now, national and hospital registries, specifically joint registries, may be an invaluable source of information for orthopedic surgeons, patients, health care administrators, regulators, and implant suppliers.1,2
Contrary to Dr. Sarmiento’s belief that registry data results are likely to have been reported in the literature, it is difficult to refute the value of recent years’ registry data in helping surgeons shape their practice. For example, according to Lewallen and Etkin,3 the National Joint Registry of England and Wales information has provided orthopedic surgeons with crucial findings regarding the outcomes of metal-on-metal hip arthroplasties. Using the England and Wales registry data from more than 400,000 primary total hip arthroplasties, Smith and colleagues4 noted that metal-on-metal stemmed articulations led to poor implant survival, particularly in young women with large-diameter heads, and indicated these articulations should not be used. Australian registry data on metal-on-metal devices and reports of failure rates up to 11%5 led one manufacturer to recall its implants.6 In addition, the Norwegian Arthroplasty Register evaluated survival rates and reasons for revision for 7 types of cemented primary total knee arthroplasty (TKA) between 1994 and 2009.7 Data on more than 17,000 primary TKAs allowed Plate and colleagues8 to confidently determine that aseptic loosening was related to certain TKA designs. Using registry information, they identified patients at risk for dislocation in total hip arthroplasty and concluded that large-diameter femoral head articulations could reduce dislocation rates.
Obtaining such large cohorts of patients in individual studies is not only difficult but highly unlikely. Unlike registry data, these studies are often impractical in evaluating factors of low incidence, such as revision rates, as it is often difficult to find significant differences in small populations.9 Furthermore, these controlled trials homogenize patients—using exclusion and inclusion criteria to eliminate potential confounders—and thus poorly represent the heterogeneity of a typical hospital’s patient population.10 Although the literature may indeed have alluded to such complications, only a database as extensive as a registry can allow us to fully comprehend the outcomes of particular implants and devices.
Dr. Sarmiento points to the AO Swiss Fracture Registry as being of little benefit and raises the concern that the American Joint Replacement Registry (AJRR) may follow with the same results. However, realizing a registry’s benefits may take time and the gradual accumulation of data. Supporting this, Hübschle and colleagues11 recently used AO Swiss Fracture Registry data to validate use of balloon kyphoplasty for vertebral compression fractures and concluded that the technique is safe and effective in reducing pain—thus possibly providing the federal office with the evidence needed for reimbursement for this intervention. Therefore, this registry is now providing useful information.
We can never truly know the veracity of participating surgeons, but it is naïve to assume that this issue arises only vis-à-vis registries. If we were to debate the ethical and professional standards of colleagues in our field, such questions could extend to all studies performed, even peer-reviewed studies. Therefore, we do not think this is reason to exclude the patient data and outcomes found in registries. We must emphasize that ultimately registry data are often most useful in highlighting trends and determining triggers for further study rather than in arriving at conclusions.1 In particular, registry data may be used in cohort studies that evaluate the risk factors for and incidence of certain outcomes. Focused higher-level interventional studies can then follow the trends observed.1 However, registry data are also valuable on their own, when higher-level, randomized controlled trials may be impractical or unethical.12
Dr. Sarmiento refers to corrupt relationships between companies and orthopedists as “representing a widespread loss of professionalism in our ranks.” Despite a US Justice Department investigation into these relationships, only a few doctors were found to have had inappropriate relationships.13 In addition, the investigation and prosecution of companies led to an agreement requiring federal monitoring and new corporate compliance procedures, which should ensure stricter adherence to regulations.14 We do not believe this should undermine the value of registries and the work that has been contributed by thousands of surgeons hoping to improve the field of orthopedics. In addition, concerns about the influence of well-known individuals may be better directed at individual institution–based research, particularly as these specific authors also often have conflicts of interest that may skew the presentation of results. The strength of registry data is in providing collective data and large samples from a multitude of surgeons rather than from just high-volume surgeons, and therefore registry data provide a better overall picture of patients and their procedures.15 Furthermore, trends observed in national registries in countries such as New Zealand16 may aid in effectively reducing the revision rate, possibly up to 10%.17 If a US national joint registry is marginally as effective, then we may see considerable savings for our health care services.17,18
We wholeheartedly agree that a yearly review of registries may be constructive. Dr. Sarmiento suggests an annual publication summarizing peer-reviewed articles and the opportunity for orthopedists to decide for themselves what treatments to choose based on reports from independent investigators. Although this sounds feasible, it would be difficult to decide which articles should be selected as pertinent for this type of publication. Any selection would be biased, and not all studies with high-level evidence are necessarily important or relevant. Therefore, selecting what is most appropriate to cite is not without its difficulties. We appreciate that there are problems in standardizing data reporting among registries. However, to improve interregistry collaboration, the US Food and Drug Administration is sponsoring the International Consortium of Orthopaedic Registries (ICOR) to facilitate data presentation.19 ICOR aims to increase cooperation, standardize analyses, and improve reporting, which will only strengthen the data available to us. Such efforts will ultimately enhance coordination and international collaboration among registries.15 In addition, incorporating patient-reported outcomes into our national registry will aid in quantifying arthroplasty outcomes from the patient’s perspective and will continue to improve total joint arthroplasties.20
Overall, this debate is useful and highly relevant in highlighting potential issues with registries. Although registries are not without their flaws, like all aspects of orthopedics they are ever evolving, and they must be continually modified and improved. However, disregard for the potential value of AJRR, which has benefits for orthopedists and patients alike, is premature. Once again, we thank Dr. Sarmiento for starting this discussion, which will allow us to continue to evaluate and improve our registries.
1. Konan S, Haddad FS. Joint registries: a Ptolemaic model of data interpretation? Bone Joint J Br. 2013;95(12):1585-1586.
2. Banerjee S, Cafri G, Isaacs AJ, et al. A distributed health data network analysis of survival outcomes: the International Consortium of Orthopaedic Registries perspective. J Bone Joint Surg Am. 2014;96(suppl 1):7-11.
3. Lewallen DG, Etkin CD. The need for a national total joint registry. Orthop Nurs. 2013;32(1):4-5.
4. Smith AJ, Dieppe P, Vernon K, Porter M, Blom AW; National Joint Registry of England and Wales. Failure rates of stemmed metal-on-metal hip replacements: analysis of data from the National Joint Registry of England and Wales. Lancet. 2012;379(9822):1199-1204.
5. de Steiger RN, Hang JR, Miller LN, Graves SE, Davidson DC. Five-year results of the ASR XL Acetabular System and the ASR Hip Resurfacing System: an analysis from the Australian Orthopaedic Association National Joint Replacement Registry. J Bone Joint Surg Am. 2011;93(24):2287-2293.
6. Hug KT, Watters TS, Vail TP, Bolognesi MP. The withdrawn ASR™ THA and hip resurfacing systems: how have our patients fared over 1 to 6 years? Clin Orthop. 2013;471(2):430-438.
7. Gøthesen O, Espehaug B, Havelin L, et al. Survival rates and causes of revision in cemented primary total knee replacement: a report from the Norwegian Arthroplasty Register 1994–2009. Bone Joint J Br. 2013;95(5):636-642.
8. Plate JF, Seyler TM, Stroh DA, Issa K, Akbar M, Mont MA. Risk of dislocation using large- vs. small-diameter femoral heads in total hip arthroplasty. BMC Res Notes. 2012;5:553.
9. Daruwalla ZJ, Wong KL, Pillay KR, Leong KM, Murphy DP. Does ageing Singapore need an electronic database of hip fracture patients? The value and role of a national joint registry and an electronic database of intertrochanteric and femoral neck fractures. Singapore Med J. 2014;55(5):287-288.
10. Rasmussen JV, Olsen BS, Fevang BT, et al. A review of national shoulder and elbow joint replacement registries. J Shoulder Elbow Surg. 2012;21(10):1328-1335.
11. Hübschle L, Borgström F, Olafsson G, et al. Real-life results of balloon kyphoplasty for vertebral compression fractures from the SWISSspine registry. Spine J. 2014;14(9):2063-2077.
12. Ahn H, Court-Brown CM, McQueen MM, Schemitsch EH. The use of hospital registries in orthopaedic surgery. J Bone Joint Surg Am. 2009;91(suppl 3):68-72.
13. Youngstrom N. Swept up in major medical device case, physician pays $650,000 to settle kickback charges. AIS Health Business Daily. May 3, 2010.
14. Five companies in hip and knee replacement industry avoid prosecution by agreeing to compliance rules and monitoring [press release]. US Department of Justice website. http://www.justice.gov/usao/nj/Press/files/pdffiles/Older/hips0927.rel.pdf. Published September 27, 2007. Accessed February 19, 2015.
15. Namba RS, Inacio MC, Paxton EW, Robertsson O, Graves SE. The role of registry data in the evaluation of mobile-bearing total knee arthroplasty. J Bone Joint Surg Am. 2011;93(suppl 3):48-50.
16. Insull PJ, Cobbett H, Frampton CM, Munro JT. The use of a lipped acetabular liner decreases the rate of revision for instability after total hip replacement: a study using data from the New Zealand Joint Registry. Bone Joint J Br. 2014;96(7):884-888.
17. Rankin EA. AJRR: becoming a national US joint registry. Orthopedics. 2013;36(3):175-176.
18. American Joint Replacement Registry website. https://teamwork.aaos.org/ajrr/SitePages/About%20Us.aspx. Accessed February 19, 2015.
19. International Consortium of Orthopaedic Registries website. http://www.icor-initiative.org. Accessed February 19, 2015.
20. Franklin PD, Harrold L, Ayers DC. Incorporating patient-reported outcomes in total joint arthroplasty registries: challenges and opportunities. Clin Orthop. 2013;471(11):3482-3488.
Following Dr. Sarmiento’s commentary, “Orthopedic Registries: Second Thoughts,” we agree that it is important and appropriate to question the value of any new additions to the orthopedic field, and registries are no exception. We thank Dr. Sarmiento for his comments on the viability of registries and the need for continued critical evaluation. Before joint registries, however, we had to rely on small-cohort analyses to assess outcomes and complications. Now, national and hospital registries, specifically joint registries, may be an invaluable source of information for orthopedic surgeons, patients, health care administrators, regulators, and implant suppliers.1,2
Contrary to Dr. Sarmiento’s belief that registry data results are likely to have been reported in the literature, it is difficult to refute the value of recent years’ registry data in helping surgeons shape their practice. For example, according to Lewallen and Etkin,3 the National Joint Registry of England and Wales information has provided orthopedic surgeons with crucial findings regarding the outcomes of metal-on-metal hip arthroplasties. Using the England and Wales registry data from more than 400,000 primary total hip arthroplasties, Smith and colleagues4 noted that metal-on-metal stemmed articulations led to poor implant survival, particularly in young women with large-diameter heads, and indicated these articulations should not be used. Australian registry data on metal-on-metal devices and reports of failure rates up to 11%5 led one manufacturer to recall its implants.6 In addition, the Norwegian Arthroplasty Register evaluated survival rates and reasons for revision for 7 types of cemented primary total knee arthroplasty (TKA) between 1994 and 2009.7 Data on more than 17,000 primary TKAs allowed Plate and colleagues8 to confidently determine that aseptic loosening was related to certain TKA designs. Using registry information, they identified patients at risk for dislocation in total hip arthroplasty and concluded that large-diameter femoral head articulations could reduce dislocation rates.
Obtaining such large cohorts of patients in individual studies is not only difficult but highly unlikely. Unlike registry data, these studies are often impractical in evaluating factors of low incidence, such as revision rates, as it is often difficult to find significant differences in small populations.9 Furthermore, these controlled trials homogenize patients—using exclusion and inclusion criteria to eliminate potential confounders—and thus poorly represent the heterogeneity of a typical hospital’s patient population.10 Although the literature may indeed have alluded to such complications, only a database as extensive as a registry can allow us to fully comprehend the outcomes of particular implants and devices.
Dr. Sarmiento points to the AO Swiss Fracture Registry as being of little benefit and raises the concern that the American Joint Replacement Registry (AJRR) may follow with the same results. However, realizing a registry’s benefits may take time and the gradual accumulation of data. Supporting this, Hübschle and colleagues11 recently used AO Swiss Fracture Registry data to validate use of balloon kyphoplasty for vertebral compression fractures and concluded that the technique is safe and effective in reducing pain—thus possibly providing the federal office with the evidence needed for reimbursement for this intervention. Therefore, this registry is now providing useful information.
We can never truly know the veracity of participating surgeons, but it is naïve to assume that this issue arises only vis-à-vis registries. If we were to debate the ethical and professional standards of colleagues in our field, such questions could extend to all studies performed, even peer-reviewed studies. Therefore, we do not think this is reason to exclude the patient data and outcomes found in registries. We must emphasize that ultimately registry data are often most useful in highlighting trends and determining triggers for further study rather than in arriving at conclusions.1 In particular, registry data may be used in cohort studies that evaluate the risk factors for and incidence of certain outcomes. Focused higher-level interventional studies can then follow the trends observed.1 However, registry data are also valuable on their own, when higher-level, randomized controlled trials may be impractical or unethical.12
Dr. Sarmiento refers to corrupt relationships between companies and orthopedists as “representing a widespread loss of professionalism in our ranks.” Despite a US Justice Department investigation into these relationships, only a few doctors were found to have had inappropriate relationships.13 In addition, the investigation and prosecution of companies led to an agreement requiring federal monitoring and new corporate compliance procedures, which should ensure stricter adherence to regulations.14 We do not believe this should undermine the value of registries and the work that has been contributed by thousands of surgeons hoping to improve the field of orthopedics. In addition, concerns about the influence of well-known individuals may be better directed at individual institution–based research, particularly as these specific authors also often have conflicts of interest that may skew the presentation of results. The strength of registry data is in providing collective data and large samples from a multitude of surgeons rather than from just high-volume surgeons, and therefore registry data provide a better overall picture of patients and their procedures.15 Furthermore, trends observed in national registries in countries such as New Zealand16 may aid in effectively reducing the revision rate, possibly up to 10%.17 If a US national joint registry is marginally as effective, then we may see considerable savings for our health care services.17,18
We wholeheartedly agree that a yearly review of registries may be constructive. Dr. Sarmiento suggests an annual publication summarizing peer-reviewed articles and the opportunity for orthopedists to decide for themselves what treatments to choose based on reports from independent investigators. Although this sounds feasible, it would be difficult to decide which articles should be selected as pertinent for this type of publication. Any selection would be biased, and not all studies with high-level evidence are necessarily important or relevant. Therefore, selecting what is most appropriate to cite is not without its difficulties. We appreciate that there are problems in standardizing data reporting among registries. However, to improve interregistry collaboration, the US Food and Drug Administration is sponsoring the International Consortium of Orthopaedic Registries (ICOR) to facilitate data presentation.19 ICOR aims to increase cooperation, standardize analyses, and improve reporting, which will only strengthen the data available to us. Such efforts will ultimately enhance coordination and international collaboration among registries.15 In addition, incorporating patient-reported outcomes into our national registry will aid in quantifying arthroplasty outcomes from the patient’s perspective and will continue to improve total joint arthroplasties.20
Overall, this debate is useful and highly relevant in highlighting potential issues with registries. Although registries are not without their flaws, like all aspects of orthopedics they are ever evolving, and they must be continually modified and improved. However, disregard for the potential value of AJRR, which has benefits for orthopedists and patients alike, is premature. Once again, we thank Dr. Sarmiento for starting this discussion, which will allow us to continue to evaluate and improve our registries.
Following Dr. Sarmiento’s commentary, “Orthopedic Registries: Second Thoughts,” we agree that it is important and appropriate to question the value of any new additions to the orthopedic field, and registries are no exception. We thank Dr. Sarmiento for his comments on the viability of registries and the need for continued critical evaluation. Before joint registries, however, we had to rely on small-cohort analyses to assess outcomes and complications. Now, national and hospital registries, specifically joint registries, may be an invaluable source of information for orthopedic surgeons, patients, health care administrators, regulators, and implant suppliers.1,2
Contrary to Dr. Sarmiento’s belief that registry data results are likely to have been reported in the literature, it is difficult to refute the value of recent years’ registry data in helping surgeons shape their practice. For example, according to Lewallen and Etkin,3 the National Joint Registry of England and Wales information has provided orthopedic surgeons with crucial findings regarding the outcomes of metal-on-metal hip arthroplasties. Using the England and Wales registry data from more than 400,000 primary total hip arthroplasties, Smith and colleagues4 noted that metal-on-metal stemmed articulations led to poor implant survival, particularly in young women with large-diameter heads, and indicated these articulations should not be used. Australian registry data on metal-on-metal devices and reports of failure rates up to 11%5 led one manufacturer to recall its implants.6 In addition, the Norwegian Arthroplasty Register evaluated survival rates and reasons for revision for 7 types of cemented primary total knee arthroplasty (TKA) between 1994 and 2009.7 Data on more than 17,000 primary TKAs allowed Plate and colleagues8 to confidently determine that aseptic loosening was related to certain TKA designs. Using registry information, they identified patients at risk for dislocation in total hip arthroplasty and concluded that large-diameter femoral head articulations could reduce dislocation rates.
Obtaining such large cohorts of patients in individual studies is not only difficult but highly unlikely. Unlike registry data, these studies are often impractical in evaluating factors of low incidence, such as revision rates, as it is often difficult to find significant differences in small populations.9 Furthermore, these controlled trials homogenize patients—using exclusion and inclusion criteria to eliminate potential confounders—and thus poorly represent the heterogeneity of a typical hospital’s patient population.10 Although the literature may indeed have alluded to such complications, only a database as extensive as a registry can allow us to fully comprehend the outcomes of particular implants and devices.
Dr. Sarmiento points to the AO Swiss Fracture Registry as being of little benefit and raises the concern that the American Joint Replacement Registry (AJRR) may follow with the same results. However, realizing a registry’s benefits may take time and the gradual accumulation of data. Supporting this, Hübschle and colleagues11 recently used AO Swiss Fracture Registry data to validate use of balloon kyphoplasty for vertebral compression fractures and concluded that the technique is safe and effective in reducing pain—thus possibly providing the federal office with the evidence needed for reimbursement for this intervention. Therefore, this registry is now providing useful information.
We can never truly know the veracity of participating surgeons, but it is naïve to assume that this issue arises only vis-à-vis registries. If we were to debate the ethical and professional standards of colleagues in our field, such questions could extend to all studies performed, even peer-reviewed studies. Therefore, we do not think this is reason to exclude the patient data and outcomes found in registries. We must emphasize that ultimately registry data are often most useful in highlighting trends and determining triggers for further study rather than in arriving at conclusions.1 In particular, registry data may be used in cohort studies that evaluate the risk factors for and incidence of certain outcomes. Focused higher-level interventional studies can then follow the trends observed.1 However, registry data are also valuable on their own, when higher-level, randomized controlled trials may be impractical or unethical.12
Dr. Sarmiento refers to corrupt relationships between companies and orthopedists as “representing a widespread loss of professionalism in our ranks.” Despite a US Justice Department investigation into these relationships, only a few doctors were found to have had inappropriate relationships.13 In addition, the investigation and prosecution of companies led to an agreement requiring federal monitoring and new corporate compliance procedures, which should ensure stricter adherence to regulations.14 We do not believe this should undermine the value of registries and the work that has been contributed by thousands of surgeons hoping to improve the field of orthopedics. In addition, concerns about the influence of well-known individuals may be better directed at individual institution–based research, particularly as these specific authors also often have conflicts of interest that may skew the presentation of results. The strength of registry data is in providing collective data and large samples from a multitude of surgeons rather than from just high-volume surgeons, and therefore registry data provide a better overall picture of patients and their procedures.15 Furthermore, trends observed in national registries in countries such as New Zealand16 may aid in effectively reducing the revision rate, possibly up to 10%.17 If a US national joint registry is marginally as effective, then we may see considerable savings for our health care services.17,18
We wholeheartedly agree that a yearly review of registries may be constructive. Dr. Sarmiento suggests an annual publication summarizing peer-reviewed articles and the opportunity for orthopedists to decide for themselves what treatments to choose based on reports from independent investigators. Although this sounds feasible, it would be difficult to decide which articles should be selected as pertinent for this type of publication. Any selection would be biased, and not all studies with high-level evidence are necessarily important or relevant. Therefore, selecting what is most appropriate to cite is not without its difficulties. We appreciate that there are problems in standardizing data reporting among registries. However, to improve interregistry collaboration, the US Food and Drug Administration is sponsoring the International Consortium of Orthopaedic Registries (ICOR) to facilitate data presentation.19 ICOR aims to increase cooperation, standardize analyses, and improve reporting, which will only strengthen the data available to us. Such efforts will ultimately enhance coordination and international collaboration among registries.15 In addition, incorporating patient-reported outcomes into our national registry will aid in quantifying arthroplasty outcomes from the patient’s perspective and will continue to improve total joint arthroplasties.20
Overall, this debate is useful and highly relevant in highlighting potential issues with registries. Although registries are not without their flaws, like all aspects of orthopedics they are ever evolving, and they must be continually modified and improved. However, disregard for the potential value of AJRR, which has benefits for orthopedists and patients alike, is premature. Once again, we thank Dr. Sarmiento for starting this discussion, which will allow us to continue to evaluate and improve our registries.
1. Konan S, Haddad FS. Joint registries: a Ptolemaic model of data interpretation? Bone Joint J Br. 2013;95(12):1585-1586.
2. Banerjee S, Cafri G, Isaacs AJ, et al. A distributed health data network analysis of survival outcomes: the International Consortium of Orthopaedic Registries perspective. J Bone Joint Surg Am. 2014;96(suppl 1):7-11.
3. Lewallen DG, Etkin CD. The need for a national total joint registry. Orthop Nurs. 2013;32(1):4-5.
4. Smith AJ, Dieppe P, Vernon K, Porter M, Blom AW; National Joint Registry of England and Wales. Failure rates of stemmed metal-on-metal hip replacements: analysis of data from the National Joint Registry of England and Wales. Lancet. 2012;379(9822):1199-1204.
5. de Steiger RN, Hang JR, Miller LN, Graves SE, Davidson DC. Five-year results of the ASR XL Acetabular System and the ASR Hip Resurfacing System: an analysis from the Australian Orthopaedic Association National Joint Replacement Registry. J Bone Joint Surg Am. 2011;93(24):2287-2293.
6. Hug KT, Watters TS, Vail TP, Bolognesi MP. The withdrawn ASR™ THA and hip resurfacing systems: how have our patients fared over 1 to 6 years? Clin Orthop. 2013;471(2):430-438.
7. Gøthesen O, Espehaug B, Havelin L, et al. Survival rates and causes of revision in cemented primary total knee replacement: a report from the Norwegian Arthroplasty Register 1994–2009. Bone Joint J Br. 2013;95(5):636-642.
8. Plate JF, Seyler TM, Stroh DA, Issa K, Akbar M, Mont MA. Risk of dislocation using large- vs. small-diameter femoral heads in total hip arthroplasty. BMC Res Notes. 2012;5:553.
9. Daruwalla ZJ, Wong KL, Pillay KR, Leong KM, Murphy DP. Does ageing Singapore need an electronic database of hip fracture patients? The value and role of a national joint registry and an electronic database of intertrochanteric and femoral neck fractures. Singapore Med J. 2014;55(5):287-288.
10. Rasmussen JV, Olsen BS, Fevang BT, et al. A review of national shoulder and elbow joint replacement registries. J Shoulder Elbow Surg. 2012;21(10):1328-1335.
11. Hübschle L, Borgström F, Olafsson G, et al. Real-life results of balloon kyphoplasty for vertebral compression fractures from the SWISSspine registry. Spine J. 2014;14(9):2063-2077.
12. Ahn H, Court-Brown CM, McQueen MM, Schemitsch EH. The use of hospital registries in orthopaedic surgery. J Bone Joint Surg Am. 2009;91(suppl 3):68-72.
13. Youngstrom N. Swept up in major medical device case, physician pays $650,000 to settle kickback charges. AIS Health Business Daily. May 3, 2010.
14. Five companies in hip and knee replacement industry avoid prosecution by agreeing to compliance rules and monitoring [press release]. US Department of Justice website. http://www.justice.gov/usao/nj/Press/files/pdffiles/Older/hips0927.rel.pdf. Published September 27, 2007. Accessed February 19, 2015.
15. Namba RS, Inacio MC, Paxton EW, Robertsson O, Graves SE. The role of registry data in the evaluation of mobile-bearing total knee arthroplasty. J Bone Joint Surg Am. 2011;93(suppl 3):48-50.
16. Insull PJ, Cobbett H, Frampton CM, Munro JT. The use of a lipped acetabular liner decreases the rate of revision for instability after total hip replacement: a study using data from the New Zealand Joint Registry. Bone Joint J Br. 2014;96(7):884-888.
17. Rankin EA. AJRR: becoming a national US joint registry. Orthopedics. 2013;36(3):175-176.
18. American Joint Replacement Registry website. https://teamwork.aaos.org/ajrr/SitePages/About%20Us.aspx. Accessed February 19, 2015.
19. International Consortium of Orthopaedic Registries website. http://www.icor-initiative.org. Accessed February 19, 2015.
20. Franklin PD, Harrold L, Ayers DC. Incorporating patient-reported outcomes in total joint arthroplasty registries: challenges and opportunities. Clin Orthop. 2013;471(11):3482-3488.
1. Konan S, Haddad FS. Joint registries: a Ptolemaic model of data interpretation? Bone Joint J Br. 2013;95(12):1585-1586.
2. Banerjee S, Cafri G, Isaacs AJ, et al. A distributed health data network analysis of survival outcomes: the International Consortium of Orthopaedic Registries perspective. J Bone Joint Surg Am. 2014;96(suppl 1):7-11.
3. Lewallen DG, Etkin CD. The need for a national total joint registry. Orthop Nurs. 2013;32(1):4-5.
4. Smith AJ, Dieppe P, Vernon K, Porter M, Blom AW; National Joint Registry of England and Wales. Failure rates of stemmed metal-on-metal hip replacements: analysis of data from the National Joint Registry of England and Wales. Lancet. 2012;379(9822):1199-1204.
5. de Steiger RN, Hang JR, Miller LN, Graves SE, Davidson DC. Five-year results of the ASR XL Acetabular System and the ASR Hip Resurfacing System: an analysis from the Australian Orthopaedic Association National Joint Replacement Registry. J Bone Joint Surg Am. 2011;93(24):2287-2293.
6. Hug KT, Watters TS, Vail TP, Bolognesi MP. The withdrawn ASR™ THA and hip resurfacing systems: how have our patients fared over 1 to 6 years? Clin Orthop. 2013;471(2):430-438.
7. Gøthesen O, Espehaug B, Havelin L, et al. Survival rates and causes of revision in cemented primary total knee replacement: a report from the Norwegian Arthroplasty Register 1994–2009. Bone Joint J Br. 2013;95(5):636-642.
8. Plate JF, Seyler TM, Stroh DA, Issa K, Akbar M, Mont MA. Risk of dislocation using large- vs. small-diameter femoral heads in total hip arthroplasty. BMC Res Notes. 2012;5:553.
9. Daruwalla ZJ, Wong KL, Pillay KR, Leong KM, Murphy DP. Does ageing Singapore need an electronic database of hip fracture patients? The value and role of a national joint registry and an electronic database of intertrochanteric and femoral neck fractures. Singapore Med J. 2014;55(5):287-288.
10. Rasmussen JV, Olsen BS, Fevang BT, et al. A review of national shoulder and elbow joint replacement registries. J Shoulder Elbow Surg. 2012;21(10):1328-1335.
11. Hübschle L, Borgström F, Olafsson G, et al. Real-life results of balloon kyphoplasty for vertebral compression fractures from the SWISSspine registry. Spine J. 2014;14(9):2063-2077.
12. Ahn H, Court-Brown CM, McQueen MM, Schemitsch EH. The use of hospital registries in orthopaedic surgery. J Bone Joint Surg Am. 2009;91(suppl 3):68-72.
13. Youngstrom N. Swept up in major medical device case, physician pays $650,000 to settle kickback charges. AIS Health Business Daily. May 3, 2010.
14. Five companies in hip and knee replacement industry avoid prosecution by agreeing to compliance rules and monitoring [press release]. US Department of Justice website. http://www.justice.gov/usao/nj/Press/files/pdffiles/Older/hips0927.rel.pdf. Published September 27, 2007. Accessed February 19, 2015.
15. Namba RS, Inacio MC, Paxton EW, Robertsson O, Graves SE. The role of registry data in the evaluation of mobile-bearing total knee arthroplasty. J Bone Joint Surg Am. 2011;93(suppl 3):48-50.
16. Insull PJ, Cobbett H, Frampton CM, Munro JT. The use of a lipped acetabular liner decreases the rate of revision for instability after total hip replacement: a study using data from the New Zealand Joint Registry. Bone Joint J Br. 2014;96(7):884-888.
17. Rankin EA. AJRR: becoming a national US joint registry. Orthopedics. 2013;36(3):175-176.
18. American Joint Replacement Registry website. https://teamwork.aaos.org/ajrr/SitePages/About%20Us.aspx. Accessed February 19, 2015.
19. International Consortium of Orthopaedic Registries website. http://www.icor-initiative.org. Accessed February 19, 2015.
20. Franklin PD, Harrold L, Ayers DC. Incorporating patient-reported outcomes in total joint arthroplasty registries: challenges and opportunities. Clin Orthop. 2013;471(11):3482-3488.
Large-Diameter Femoral Heads in Total Hip Arthroplasty: An Evidence-Based Review
A common cause for total hip arthroplasty (THA) revision is joint instability.1,2 The reported incidence of dislocation in primary THA ranges from 0.4% to 5.8%,3-5 but this rate increases after revision surgery.1,3-8 Use of large-diameter femoral heads has been proposed to decrease the risks for instability and to improve impingement-free range of motion (ROM).
The biomechanical rationale for using large-diameter femoral heads is that they must travel farther before subluxation or dislocation occurs (jump distance). Despite these benefits, there were initial concerns that catastrophic failure and high levels of volumetric wear would occur if these heads were used with conventional polyethylene liners. These concerns led to the development of alternative bearing surfaces, particularly metal-on-metal bearings, which offered theoretical benefits of large-diameter articulations that improved stability while purportedly being highly wear-resistant.9-11 However, concerns about adverse local soft-tissue reactions and high blood concentrations of metal ions tempered the initial enthusiasm for metal bearings.12-16 Fortunately, highly cross-linked polyethylene and fourth-generation ceramic bearing surfaces, with improved toughness and better wear properties, may allow use of large-diameter heads without the need for metal-on-metal bearings.17,18
In this article, we review the concepts and principles behind use of large-diameter ceramic or cobalt-chromium femoral heads on polyethylene-bearing surfaces in THA with particular attention to biomechanics, early concerns about polyethylene wear and rim fractures, recent improvements in material properties of polyethylene and ceramic bearings, dislocation rates, and clinical and functional outcomes.
Definitions
For this review, we define large-diameter femoral heads as having diameters of 36 mm or more and conventional or small-diameter femoral heads as having diameters between 22 and 32 mm.
Biomechanics
Head–Neck Ratio, Impingement-Free ROM, and Jump Distance
Several implant design principles have been proposed to reduce the risks for impingement and dislocation. Of these, large femoral head diameters have been extensively studied.19,20 It is well known that impingement of the femoral neck on the cup edge promotes edge loading and higher wear rates. In addition, impingement increases the tendency of the head to sublux from the acetabulum. One strategy for avoiding this component-to-component impingement is to increase the head–neck ratio (HNR), the ratio of the femoral head to the neck diameter. Biomechanically, increased HNRs lead to delayed contact between the femoral neck and the acetabular liner.21,22 Therefore, with large femoral heads, which have large HNRs, impingement occurs later and at larger ROMs—compared with small-diameter femoral heads, which have lower HNRs and are more prone to early impingement and subluxation (Figure 1).23-26
In a cadaveric study of 6 hips, Bartz and colleagues23 reported a significantly higher preimpingement ROM when the prosthetic head size increased from 22 mm to 28 mm (P < .05). They found a change from prosthetic to osseous impingement when the head size increased from 22 mm to 32 mm. Similar results were observed in a computer simulation model by Cinotti and colleagues,27 who demonstrated that increasing the femoral head size from 28 mm to 38 mm resulted in a 5° improvement in ROM. However, the largest gains were observed when the heads with the smallest diameters were upsized; ROM improved only marginally when femoral head size was further increased from 32 mm to 38 mm. The primary reason for the lack of expected improvement in ROM with head sizes of more than 32 mm is often bone-on-bone impingement. Burroughs and colleagues28 demonstrated that the 38-mm and 44-mm heads virtually eliminated component-to-component impingement except in extremes of external rotation. However, there were no differences in ROM between 38-mm and 44-mm heads because of osseous impingement. In addition, large heads are less likely to sublux or dislocate, as they need to travel farther before reaching the edge of the acetabular cup before dislocation. This is known as the jump distance, and it corresponds to the depth of the acetabular shell, which in turn equates with the radius of the femoral head (Figures 2A, 2B). For this reason, the larger the femoral head diameter, the farther the jump distance and, correspondingly, the lower the risk for dislocation.29
Elevated liners historically were used to increase the jump distance for dislocation.30 These liners, however, can increase impingement at the extremes of motion.31 Some of these problems can be avoided with use of larger heads, which have increased jump distances without additional risks for impingement. Moreover, large heads create a suction effect that provides passive resistance to dislocation.32 With head diameters beyond 38 mm, impingement-free ROM often plateaus. However, the jump distance required for dislocations to occur continues to increase as femoral head diameters increase in size. Thus, patients may experience fewer motion benefits but continue to benefit from overall stability with femoral head sizes increasing beyond 38 mm.
Current evidence suggests there may be substantial benefits toward improved stability from increasing head diameters from 22 mm to 38 mm because of the increase in jump distances and improvements in prosthetic impingement-free ROM. However, there may be little gain in ROM from increasing the head diameters beyond these dimensions because of the potential risks of bony impingement. Nevertheless, there may be some additional benefits toward stability from improvement in jump distances with incremental head sizes
beyond 38 mm.29,33,34
Finite Element Analysis Studies
Finite element analysis of large-diameter heads in THA has shown that, at optimal cup inclination (45°), most stresses occur on the articular surface of the liner. However, these stresses remain well below the yield strength of the polyethylene liners.29 With increasing abduction angles, the stress concentration increases substantially because of the decreased contact surface area. At these angles, the point of maximum contact moves toward the rim of the polyethylene liner, which can lead to rim fractures or failure of locking mechanisms.29,35,36
Early Concerns With Large-Diameter Femoral Heads: Wear, Liner Failure, and Fracture of Ceramic Components
Use of small-diameter femoral heads started with the first report by Charnley37 of “low frictional torque arthroplasty.” Charnley initially considered a 41.5-mm femoral head, but he thought it would increase risks for acetabular loosening from high frictional torque generated by the large head, and he switched to a small-diameter (22.5 mm) design. One of the tradeoffs with smaller diameter heads was decreased jump height in addition to increased linear wear.
Large femoral heads used with cemented polyethylene acetabular components historically have been associated with increased rates of volumetric wear but low rates of linear wear, which potentially may increase the risk for osteolysis.38-40 However, newer highly cross-linked polyethylene liners have shown improved in vitro and in vivo volumetric wear characteristics and potentially lower linear wear rates compared with earlier designs (Table 1).28,41-43
Another concern about earlier generations of large femoral heads was the risk for catastrophic liner failure on conventional polyethylene. This was originally reported by Berry and colleagues,47 who described wear-through and failure in patients with thin (< 5 mm) acetabular cups. However, these concerns have been largely addressed by the development of highly cross-linked polyethylene, which has improved wear characteristics and fatigue resistance.48
Recent Improvements in Material Properties of Polyethylene and Ceramic Bearings
The development of highly cross-linked polyethylene and fourth-generation ceramics has renewed interest in large-diameter bearings in THA. These bearing surfaces improve wear, enhance material properties, and have superior oxidation resistance.42,48-53
We now briefly describe the methods used to improve the material properties of polyethylene and ceramics. Studies have shown that increasing the radiation dose (up to 200 kGy) increases cross-linking and causes an inverse exponential decrease in polyethylene wear.28,41,48-51 However, increasing radiation doses also increases production of free radicals, which diminish the material strength of these polyethylenes. The current generation of highly cross-linked polyethylene liners is produced through a variety of manufacturing strategies to improve cross-linking and reduce wear. These strategies include differential radiation doses (50-100 kGy), techniques (electron beam, radiation), and thermal treatments (melting, annealing). Moreover, to enhance the material properties and reduce the incidence of rim cracking and delamination, authors have proposed using vitamin E supplementation to minimize the amount of subsurface oxidation that occurs as an inevitable consequence of free radical formation during fabrication.54,55 A terminal sterilization process (eg, gas plasma, ethylene oxide, or gamma sterilization in nitrogen) is needed to make commercial, highly cross-linked polyethylene.52,53
Fourth-generation ceramics manufactured with nano-sized yttria-stabilized tetragonal zirconia particles in a stable alumina matrix have more fracture toughness and improved wear characteristics.54,55 In addition, oxide additives (eg, chromium oxide, strontium oxide) improve hardness and dissipate energy by deflecting cracks to prevent their propagation.56 Moreover, the smaller grain sizes of fourth-generation ceramic bearings compared with third-generation designs (0.8 µm vs 1-5 µm) cause less disruption of the fluid film layer, which ultimately results in improved wear performance.57
Multiple studies have found reduced wear rates with metal and ceramic large heads coupled with highly cross-linked polyethylene-bearings (Table 2).17,41,50,58 Bragdon and colleagues,58 using radiostereometric analysis in 25 patients, found no significant differences in mean head penetration rates between 36-mm and 28-mm cobalt-chromium (Co-Cr) heads articulating with highly cross-linked polyethylene cups at a mean follow-up of 3 years (0.035 mm/y vs 0.046 mm/y; P = .11). Geller and colleagues,64 in their study of 42 patients with large-diameter (> 32 mm) Co-Cr femoral heads, found low mean (SD) linear wear rates of 0.06 (0.41) mm/y at a mean follow-up of 3 years. D’Antonio and colleagues,65 in a multicenter study, reported low average linear wear (0.015 mm/y) and volumetric wear (12.1 mm3/y) over 5 years using sequentially annealed cross-linked polyethylene. In vitro reports suggest that large-diameter ceramic heads may have lower wear properties than Co-Cr heads do. Galvin and colleagues,66 in an in vitro hip simulator study, found that large-diameter ceramic heads on highly cross-linked ultrahigh-molecular-weight polyethylene had 40% reductions in steady-state wear rates compared with Co-Cr heads on highly cross-linked bearings (4.7 vs 8.1 mm3/million cycles; P < 0.01).
Dislocation Rates
Several patient, surgeon, and implant factors affect the rate of dislocations after THA. Multiple implant options utilize the biomechanical advantage that large-diameter heads have in improving stability. Various alternatives include use of constrained tripolar heads, dual-mobility bearings, and conventional large-diameter heads with standard liners.67-69
Large-Diameter Heads
Despite the biomechanical advantages of large-diameter metal-on-polyethylene bearings, prior studies have questioned use of these bearings because of risks for increased wear and rim failures. However, the improved wear properties of highly cross-linked polyethylene, elaborated earlier, have led to a reappraisal of this option (Table 2).4,70 Howie and colleagues,71 in a randomized control trial of 644 patients, also found significantly lower rates of dislocation after primary THA with 36-mm heads compared with 28-mm heads (1.3% vs 5.4%; P = .012); in addition, fewer dislocations occurred with 36-mm heads than with 28-mm heads (4.9% vs 12.2%; P = .27) in a series of 44 patients in revision settings. Similarly, in a study conducted with 39,271 Medicare patients between 1998 and 2007, Malkani and colleagues72 found a decrease in the dislocation rate, from 4.21% to 2.14%, with use of large-diameter femoral heads. These results have been confirmed by several other authors.34,66,73,74 Similar results were observed in 65,992 patients in the Australian National Joint Replacement Registry by Conroy and colleagues,75 who reported a significant decrease in the risk for dislocation with large heads (≥ 30 mm) compared with 22-mm heads (relative risk, 1.0 vs 3.1; P ≤ .001).
Few studies have analyzed the role of large-diameter femoral heads in the presence of compromised soft tissues around the hip. Kung and Ries,76 evaluating the influence of large-diameter heads in the presence and absence of a deficient abductor mechanism, demonstrated statistically significant reductions in rates of dislocation after 230 revision THAs when the abductor mechanism was intact with use of 36-mm heads compared with 28-mm heads (12.7% vs 0%; P = .015). With abductor deficiency, though, the positive effect of large heads in reducing dislocation rates was substantially reduced and was similar to that of small heads (P = .74).76
Large heads considerably improve overall stability and lower dislocation rates in THA. With the development of newer ceramics and highly cross-linked polyethylenes, the wear rates reported in multiple studies appear to be less concerning.
Constrained Tripolar Heads
Tripolar heads have been proposed as treatment options for improving stability in patients with chronic and recurrent instability after THA. The tripolar implant consists of a metal head that snap-fits into a polyethylene liner with a polished Co-Cr backing. This bipolar head articulates with a polyethylene bearing that is press-fitted onto a metal acetabular shell and constrained by a metal ring snapped to the outer polyethylene bearing. The bipolar component behaves as a large-diameter femoral head, and the metal ring provides additional restraint, further improving stability.
Williams and colleagues77 performed a systematic review and reported on the outcomes of constrained tripolar liners in 1199 hips at a mean follow-up of 4 years (range, 2-10 years). The mean dislocation rate was 10%, and the mean rate of revision surgery unrelated to instability was 4%. In a study of 43 hips at a mean follow-up of 4 years (range, 2-9 years), Zywiel and colleagues78 reported on the clinical and radiographic outcomes of tripolar constrained liners. Their study group had a mean Harris Hip Score (HHS) of 82 points (range, 38-100 points) and overall survival of 91%, with no evidence of radiographic loosening during follow-up. Despite the improvements in stability with constrained tripolar liners, some authors have reported multiple mechanisms of failure with these devices.79-81 In a study of 43 failed constrained tripolar liners with a mean time to failure of about 2 years, Guyen and colleagues79 identified 5 different failure modes (types 1-5) involving all 4 interfaces in these components.
Encouraging outcomes have been reported at midterm follow-up with tripolar constrained liners. However, concerns about failure at the interfaces suggest that use of these components should be restricted to patients with deficient abductor mechanisms or neuromuscular compromise, low-demand elderly patients, and salvage cases of recurrent dislocations.79
Dual-Mobility Bearings
For more than 20 years, different dual-mobility bearings have been used for difficult acetabular reconstructive scenarios and prevention of instability.82,83 Dual-mobility cups provide constructs that snap-fit a small-diameter femoral head within a large polyethylene insert that articulates with a fixed metal shell. This effectively increases the functional head diameter.
Various authors have reported excellent survivorship rates (92%-99%) and low dislocation rates for these bearings at 5- to 10-year follow-up.82,84-90 Philippot and colleagues,86 in a recent study of 438 hips with dual-mobility cups, reported excellent survivorship (96%) and no early or late instability within a 15-year follow-up. Bouchet and colleagues69 compared dual-mobility bearings (105 hips) with conventional metal-on-polythene bearings (108 hips) and found significantly (P < .05) lower dislocation rates for the dual-mobility implants at a minimum 1-year follow-up. The French Society of Orthopaedics and Traumatology performed a multicenter analysis of 3473 hips with dual-mobility cups implanted in France between January 1998 and December 2003.87 During a mean follow-up of 7 years (range, 5-11 years), there were 15 dislocations (0.43%), 14 of which occurred early, within 3 months of implantation (0.4%). Aseptic implant survivorship was 95% at 10-year follow-up.
Use of these bearings has recently increased in the United States. Short-term and midterm follow-up data show low rates of dislocation and wear. Long-term data are to come.
Clinical and Functional Outcomes of Large-Diameter Femoral Heads
There is a paucity of long-term outcomes data on use of large-diameter heads with highly cross-linked polyethylene bearings. Short-term and midterm clinical results appear to be excellent, with low rates of wear, osteolysis, and aseptic loosening.28,41,73,89-92
Plate and colleagues91 compared the effects of large-diameter (≥ 36 mm) and small-diameter (26 mm, 28 mm) metal heads on highly cross-linked polyethylene bearings. At a mean follow-up of 5 years (range, 4-8.4 years), the large-head cohort had a mean HHS of 90 points (range, 50-100 points) and no dislocations or radiographic evidence of stem or cup loosening. Similarly, Meftah and colleagues93 reported 100% stem survivorship and excellent clinical outcomes—a mean Western Ontario and McMaster Universities Arthritis Index (WOMAC) score of 30 points—for 72 hips with use of large ceramic heads (≥ 32 mm) on highly cross-linked polyethylene at a mean follow-up of 3 years. Gagala and colleagues94 reported excellent clinical and radiographic outcomes in 50 hips (18 ceramic on ceramic, 32 ceramic on polyethylene; 36-mm heads) at a mean follow-up of 3.5 years. Mean HHS was 94 points, and there was no evidence of liner fractures, aseptic loosening, or osteolysis.
In summary, large-diameter femoral heads in THA have become increasingly popular because of improvements in the material properties and wear characteristics of highly cross-linked polyethylene and fourth-generation ceramics. Despite the potential advantages of large heads in preventing dislocations, the basic surgical tenets of placing the acetabular component in appropriate alignment remain firmly established. Implants with functionally large heads (eg, dual-mobility bearings, constrained tripolar liners) may play an important role in patients at high risk for dislocation—particularly elderly patients with poor neuromuscular muscle coordination or deficient abductors, trauma patients, and patients with prior dislocations. Short-term and midterm results are excellent; rates of wear, aseptic loosening, and osteolysis are low. However, long-term outcomes data are needed to support widespread use of large heads in younger and more active patients.
1. Dorr LD, Wolf AW, Chandler R, Conaty JP. Classification and treatment of dislocations of total hip arthroplasty. Clin Orthop. 1983;(173):151-158.
2. Dorr LD, Wan Z. Causes of and treatment protocol for instability of total hip replacement. Clin Orthop. 1998;(355):144-151.
3. Turner RS. Postoperative total hip prosthetic femoral head dislocations. Incidence, etiologic factors, and management. Clin Orthop. 1994;(301):196-204.
4. Woo RY, Morrey BF. Dislocations after total hip arthroplasty. J Bone Joint Surg Am. 1982;64(9):1295-1306.
5. Etienne A, Cupic Z, Charnley J. Postoperative dislocation after Charnley low-friction arthroplasty. Clin Orthop. 1978;(132):19-23.
6. Fackler CD, Poss R. Dislocation in total hip arthroplasties. Clin Orthop. 1980;(151):169-178.
7. Joshi A, Lee CM, Markovic L, Vlatis G, Murphy JC. Prognosis of dislocation after total hip arthroplasty. J Arthroplasty. 1998;13(1):17-21.
8. Lindberg HO, Carlsson AS, Gentz CF, Pettersson H. Recurrent and non-recurrent dislocation following total hip arthroplasty. Acta Orthop Scand. 1982;53(6):947-952.
9. Eswaramoorthy V, Moonot P, Kalairajah Y, Biant LC, Field RE. The Metasul metal-on-metal articulation in primary total hip replacement: clinical and radiological results at ten years. J Bone Joint Surg Br. 2008;90(10):1278-1283.
10. Grubl A, Marker M, Brodner W, et al. Long-term follow-up of metal-on-metal total hip replacement. J Orthop Res. 2007;25(7):841-848.
11. Leslie I, Williams S, Brown C, et al. Effect of bearing size on the long-term wear, wear debris, and ion levels of large diameter metal-on-metal hip replacements—an in vitro study. J Biomed Mater Res B Appl Biomater. 2008;87(1):163-172.
12. Verhaar JA. The hard lesson of metal-on-metal hip implants [in Dutch]. Ned Tijdschr Geneeskd. 2012;156(42):A5564.
13. Fabi D, Levine B, Paprosky W, et al. Metal-on-metal total hip arthroplasty: causes and high incidence of early failure. Orthopedics. 2012;35(7):e1009-e1016.
14. Heneghan C, Langton D, Thompson M. Ongoing problems with metal-on-metal hip implants. BMJ. 2012;344:e1349.
15. Lee RK, Nevelos J, Vigdorchik J, Markel DC. Bearing surfaces for hip arthroplasty—is metal-on-metal a passing fancy? Surg Technol Int. 2012;22:243-249.
16. Voleti PB, Baldwin KD, Lee GC. Metal-on-metal vs conventional total hip arthroplasty: a systematic review and meta-analysis of randomized controlled trials. J Arthroplasty. 2012;27(10):1844-1849.
17. Urban JA, Garvin KL, Boese CK, et al. Ceramic-on-polyethylene bearing surfaces in total hip arthroplasty. Seventeen to twenty-one-year results.
J Bone Joint Surg Am. 2001;83(11):1688-1694.
18. Callaghan JJ, Liu SS. Ceramic on crosslinked polyethylene in total hip replacement: any better than metal on crosslinked polyethylene? Iowa Orthop J. 2009;29:1-4.
19. Barrack RL. Dislocation after total hip arthroplasty: implant design and orientation. J Am Acad Orthop Surg. 2003;11(2):89-99.
20. Krushell RJ, Burke DW, Harris WH. Elevated-rim acetabular components. Effect on range of motion and stability in total hip arthroplasty. J Arthroplasty. 1991;6(suppl):S53-S58.
21. Morrey BF. Instability after total hip arthroplasty. Orthop Clin North Am. 1992;23(2):237-248.
22. Morrey BF. Dislocation. In: Morrey BF, ed. Joint Replacement Arthroplasty. New York, NY: Churchill Livingstone; 1991:851-865.
23. Bartz RL, Nobel PC, Kadakia NR, Tullos HS. The effect of femoral component head size on posterior dislocation of the artificial hip joint. J Bone Joint Surg Am. 2000;82(9):1300-1307.
24. Nicholas RM, Orr JF, Mollan RA, Calderwood JW, Nixon JR, Watson P. Dislocation of total hip replacements. A comparative study of standard, long posterior wall and augmented acetabular components. J Bone Joint Surg Br. 1990;72(3):418-422.
25. McCollum DE, Gray WJ. Dislocation after total hip arthroplasty. Causes and prevention. Clin Orthop. 1990;(261):159-170.
26. Herrlin K, Selvik G, Pettersson H, Kesek P, Onnerfalt R, Ohlin A. Position, orientation and component interaction in dislocation of the total hip prosthesis. Acta Radiol. 1988;29(4):441-444.
27. Cinotti G, Lucioli N, Malagoli A, Calderoli C, Cassese F. Do large femoral heads reduce the risks of impingement in total hip arthroplasty with optimal and non-optimal cup positioning? Int Orthop. 2011;35(3):317-323.
28. Burroughs BR, Rubash HE, Harris WH. Femoral head sizes larger than 32 mm against highly cross-linked polyethylene. Clin Orthop. 2002;(405):150-157.
29. Crowninshield RD, Maloney WJ, Wentz DH, Humphrey SM, Blanchard CR. Biomechanics of large femoral heads: what they do and don‘t do. Clin Orthop. 2004;(429):102-107.
30. Charnley J. Low Friction Arthroplasty of the Hip: Theory and Practice. New York, NY: Springer; 1979.
31. Yamaguchi M, Akisue T, Bauer TW, Hashimoto Y. The spatial location of impingement in total hip arthroplasty. J Arthroplasty. 2000;15(3):305-313.
32. Peters CL, McPherson E, Jackson JD, Erickson JA. Reduction in early dislocation rate with large-diameter femoral heads in primary total hip arthroplasty. J Arthroplasty. 2007;22(6 suppl 2):140-144.
33. Masonis JL, Bourne RB. Surgical approach, abductor function, and total hip arthroplasty dislocation. Clin Orthop. 2002;(405):46-53.
34. Beaule PE, Schmalzried TP, Udomkiat P, Amstutz HC. Jumbo femoral head for the treatment of recurrent dislocation following total hip replacement. J Bone Joint Surg Am. 2002;84(2):256-263.
35. Oral E, Malhi AS, Muratoglu OK. Mechanisms of decrease in fatigue crack propagation resistance in irradiated and melted UHMWPE. Biomaterials. 2006;27(6):917-925.
36. Baker DA, Bellare A, Pruitt L. The effects of degree of crosslinking on the fatigue crack initiation and propagation resistance of orthopedic-grade polyethylene. J Biomed Mater Res A. 2003;66(1):146-154.
37. Charnley J. Total hip replacement by low-friction arthroplasty. Clin Orthop. 1970;(72):7-21.
38. Kabo JM, Gebhard JS, Loren G, Amstutz HC. In vivo wear of polyethylene acetabular components. J Bone Joint Surg Br. 1993;75(2):254-258.
39. Livermore J, Ilstrup D, Morrey B. Effect of femoral head size on wear of the polyethylene acetabular component. J Bone Joint Surg Am. 1990;72(4):518-528.
40. Ma SM, Kabo JM, Amstutz HC. Frictional torque in surface and conventional hip replacement. J Bone Joint Surg Am. 1983;65(3):366-370.
41. Muratoglu OK, Bragdon CR, O‘Connor D, et al. Larger diameter femoral heads used in conjunction with a highly cross-linked ultra-high molecular weight polyethylene: a new concept. J Arthroplasty. 2001;16(8 suppl 1):24-30.
42. Thomas GER, Simpson DJ, Mehmood S, et al. The seven-year wear of highly cross-linked polyethylene in total hip arthroplasty: a double-blind, randomized controlled trial using radiostereometric analysis. J Bone Joint Surg Am. 2011;93(8):716-722.
43. Mutimer J, Devane PA, Adams K, Horne JG. Highly crosslinked polyethylene reduces wear in total hip arthroplasty at 5 years. Clin Orthop. 2010;468(12):3228-3233.
44. Bragdon CR, Doerner M, Martell J, Jarrett B, Palm H, Malchau H. The 2012 John Charnley Award: clinical multicenter studies of the wear performance of highly crosslinked remelted polyethylene in THA. Clin Orthop. 2013;471(2):393-402.
45. Lachiewicz PF, Heckman DS, Soileau ES, Mangla J, Martell JM. Femoral head size and wear of highly cross-linked polyethylene at 5 to 8 years. Clin Orthop. 2009;467(12):3290-3296.
46. Sychterz CJ, Engh CA Jr, Young AM, Hopper RH Jr, Engh CA. Comparison of in vivo wear between polyethylene liners articulating with ceramic and cobalt-chrome femoral heads. J Bone Joint Surg Br. 2000;82(7):948-951.
47. Berry DJ, Barnes CL, Scott RD, Cabanela ME, Poss R. Catastrophic failure of the polyethylene liner of uncemented acetabular components.
J Bone Joint Surg Br. 1994;76(4):575-578.
48. McKellop H, Shen FW, Lu B, Campbell P, Salovey R. Development of an extremely wear-resistant ultra high molecular weight polyethylene for total hip replacements. J Orthop Res. 1999;17(2):157-167.
49. Wang A, Essner A, Polineni VK, Stark C, Dumbleton JH. Lubrication and wear of ultra-high molecular weight polyethylene in total joint replacements. Tribol Int. 1998;31(1-3):17-33.
50. Estok DM 2nd, Burroughs BR, Muratoglu OK, Harris WH. Comparison of hip simulator wear of 2 different highly cross-linked ultra high molecular weight polyethylene acetabular components using both 32- and 38-mm femoral heads. J Arthroplasty. 2007;22(4):581-589.
51. Muratoglu OK, Bragdon CR, O‘Connor DO, et al. Unified wear model for highly crosslinked ultra-high molecular weight polyethylenes (UHMWPE). Biomaterials. 1999;20(16):1463-1470.
52. Harris WH, Muratoglu OK. A review of current cross-linked polyethylenes used in total joint arthroplasty. Clin Orthop. 2005;(430):46-52.
53. Muratoglu OK, Bragdon CR, O‘Connor DO, Jasty M, Harris WH. A novel method of cross-linking ultra-high-molecular-weight polyethylene to improve wear, reduce oxidation, and retain mechanical properties. Recipient of the 1999 HAP Paul Award. J Arthroplasty. 2001;16(2):149-160.
54. Bal BS, Garino J, Ries M, Rahaman MN. A review of ceramic bearing materials in total joint arthroplasty. Hip Int. 2007;17(1):21-30.
55. Traina F, De Fine M, Di Martino A, Faldini C. Fracture of ceramic bearing surfaces following total hip replacement: a systematic review. Biomed Res Int. 2013;2013:157247.
56. Cai YZ, Yan SG. Development of ceramic-on-ceramic implants for total hip arthroplasty. Orthop Surg. 2010;2(3):175-181.
57. Stewart TD, Tipper JL, Insley G, Streicher RM, Ingham E, Fisher J. Long-term wear of ceramic matrix composite materials for hip prostheses under severe swing phase microseparation. J Biomed Mater Res B Appl Biomater. 2003;66(2):567-573.
58. Bragdon CR, Greene ME, Freiberg AA, Harris WH, Malchau H. Radiostereometric analysis comparison of wear of highly cross-linked polyethylene against 36- vs 28-mm femoral heads. J Arthroplasty. 2007;22(6 suppl 2):125-129.
59. Lombardi AV Jr, Skeels MD, Berend KR, Adams JB, Franchi OJ. Do large heads enhance stability and restore native anatomy in primary total hip arthroplasty? Clin Orthop. 2011;469(6):1547-1553.
60. Lachiewicz PF, Soileau ES. Low early and late dislocation rates with 36- and 40-mm heads in patients at high risk for dislocation. Clin Orthop. 2013;471(2):439-443.
61. Cai P, Hu Y, Xie J. Large-diameter Delta ceramic-on-ceramic versus common-sized ceramic-on-polyethylene bearings in THA. Orthopedics. 2012;35(9):e1307-e1313.
62. Park KS, Yoon TR, Hwang SY, Lee KB. Modified minimally invasive two-incision total hip arthroplasty using large diameter femoral head. Indian
J Orthop. 2012;46(1):29-35.
63. Garbuz DS, Masri BA, Duncan CP, et al. The Frank Stinchfield Award: dislocation in revision THA: do large heads (36 and 40 mm) result in reduced dislocation rates in a randomized clinical trial? Clin Orthop. 2012;470(2):351-356.
64. Geller JA, Malchau H, Bragdon C, Greene M, Harris WH, Freiberg AA. Large diameter femoral heads on highly cross-linked polyethylene: minimum 3-year results. Clin Orthop. 2006;(447):53-59.
65. D’Antonio JA, Capello WN, Ramakrishnan R. Second-generation annealed highly cross-linked polyethylene exhibits low wear. Clin Orthop. 2012;470(6):1696-1704.
66. Galvin AL, Jennings LM, Tipper JL, Ingham E, Fisher J. Wear and creep of highly crosslinked polyethylene against cobalt chrome and ceramic femoral heads. Proc Inst Mech Eng H. 2010;224(10):1175-1183.
67. Skeels MD, Berend KR, Lombardi AV Jr. The dislocator, early and late: the role of large heads. Orthopedics. 2009;32(9).
68. Plate JF, Seyler TM, Stroh DA, Issa K, Akbar M, Mont MA. Risk of dislocation using large- vs. small-diameter femoral heads in total hip arthroplasty. BMC Res Notes. 2012;5(1):553.
69. Bouchet R, Mercier N, Saragaglia D. Posterior approach and dislocation rate: a 213 total hip replacements case–control study comparing the dual mobility cup with a conventional 28-mm metal head/polyethylene prosthesis. Orthop Traumatol Surg Res. 2011;97(1):2-7.
70. Ali Khan MA, Brakenbury PH, Reynolds IS. Dislocation following total hip replacement. J Bone Joint Surg Br. 1981;63(2):214-218.
71. Howie DW, Holubowycz OT, Middleton R. Large femoral heads decrease the incidence of dislocation after total hip arthroplasty: a randomized controlled trial. J Bone Joint Surg Am. 2012;94(12):1095-1102.
72. Malkani AL, Ong KL, Lau E, Kurtz SM, Justice BJ, Manley MT. Early- and late-term dislocation risk after primary hip arthroplasty in the Medicare population. J Arthroplasty. 2010;25(6 suppl):21-25.
73. Berry DJ, von Knoch M, Schleck CD, Harmsen WS. Effect of femoral head diameter and operative approach on risk of dislocation after primary total hip arthroplasty. J Bone Joint Surg Am. 2005;87(11):2456-2463.
74. Cho MR, Lee HS, Lee SW, Choi CH, Kim SK, Ko SB. Results after total hip arthroplasty with a large head and bipolar arthroplasty in patients with displaced femoral neck fractures. J Arthroplasty. 2011;26(6):893-896.
75. Conroy JL, Whitehouse SL, Graves SE, Pratt NL, Ryan P, Crawford RW. Risk factors for revision for early dislocation in total hip arthroplasty.
J Arthroplasty. 2008;23(6):867-872.
76. Kung PL, Ries MD. Effect of femoral head size and abductors on dislocation after revision THA. Clin Orthop. 2007;(465):170-174.
77. Williams JT Jr, Ragland PS, Clarke S. Constrained components for the unstable hip following total hip arthroplasty: a literature review. Int Orthop. 2007;31(3):273-277.
78. Zywiel MG, Mustafa LH, Bonutti PM, Mont MA. Are abductor muscle quality and previous revision surgery predictors of constrained liner failure in hip arthroplasty? Int Orthop. 2011;35(6):797-802.
79. Guyen O, Lewallen DG, Cabanela ME. Modes of failure of Osteonics constrained tripolar implants: a retrospective analysis of forty-three failed implants. J Bone Joint Surg Am. 2008;90(7):1553-1560.
80. Banks LN, McElwain JP. An unusual mode of failure of a tripolar constrained acetabular liner: a case report. Arch Orthop Trauma Surg. 2010;130(4):503-505.
81. Robertson WJ, Mattern CJ, Hur J, Su EP, Pellicci PM. Failure mechanisms and closed reduction of a constrained tripolar acetabular liner.
J Arthroplasty. 2009;24(2):322.e5-e11.
82. Aubriot JH, Lesimple P, Leclercq S. Study of Bousquet‘s non-cemented acetabular implant in 100 hybrid total hip prostheses (Charnley type cemented femoral component). Average 5-year follow-up [in French]. Acta Orthop Belg. 1993;59(suppl 1):267-271.
83. Farizon F, de Lavison R, Azoulai JJ, Bousquet G. Results with a cementless alumina-coated cup with dual mobility. A twelve-year follow-up study. Int Orthop. 1998;22(4):219-224.
84. Mertl P, Combes A, Leiber-Wackenheim F, Fessy MH, Girard J, Migaud H. Recurrence of dislocation following total hip arthroplasty revision using dual mobility cups was rare in 180 hips followed over 7 years. HSS J. 2012;8(3):251-256.
85. Langlais FL, Ropars M, Gaucher F, Musset T, Chaix O. Dual mobility cemented cups have low dislocation rates in THA revisions. Clin Orthop. 2008;466(2):389-395.
86. Philippot R, Farizon F, Camilleri JP, et al. Survival of dual mobility socket with a mean 17 years follow-up [in French]. Rev Chir Orthop Reparatrice Appar Mot. 2008;94(1):43-48.
87. Adam P, Philippe R, Ehlinger M, et al. Dual mobility cups hip arthroplasty as a treatment for displaced fracture of the femoral neck in the elderly.
A prospective, systematic, multicenter study with specific focus on postoperative dislocation. Orthop Traumatol Surg Res. 2012;98(3):296-300.
88. Fessy MH. La double mobilité [Dual mobility]. Revue de Chirurgie Orthopédique et Traumatologique. 2010;96(7):891-898.
89. Mont MA, Issa K, Naziri Q, Harwin SF, Delanois RE, Johnson AJ. The use of dual-mobility bearings in difficult hip arthroplasty reconstructive cases. Surg Technol Int. 2011;21:234-240.
90. Sayeed SA, Mont MA, Costa CR, et al. Early outcomes of sequentially cross-linked thin polyethylene liners with large diameter femoral heads in total hip arthroplasty. Bull NYU Hosp Jt Dis. 2011;69(suppl 1):S90-S94.
91. Plate JF, Seyler TM, Stroh DA, Issa K, Akbar M, Mont MA. Risk of dislocation using large- vs. small-diameter femoral heads in total hip arthroplasty. BMC Res Notes. 2012;5(1):553.
92. Sato T, Nakashima Y, Akiyama M, et al. Wear resistant performance of highly cross-linked and annealed ultra-high molecular weight polyethylene against ceramic heads in total hip arthroplasty. J Orthop Res. 2012;30(12):2031-2037.
93. Meftah M, Ebrahimpour PB, He C, Ranawat AS, Ranawat CS. Preliminary clinical and radiographic results of large ceramic heads on highly cross-linked polyethylene. Orthopedics. 2011;34(6):133.
94. Gagala J, Mazurkiewicz T, Dajewski Z. Large diameter femoral heads in primary alumina/alumina and XSPE/alumina total hip arthroplasty.
A follow-up study of 50 hips after average 40 months and review of literature [in Polish]. Chir Narzadow Ruchu Ortop Pol. 2011;76(1):14-20.
A common cause for total hip arthroplasty (THA) revision is joint instability.1,2 The reported incidence of dislocation in primary THA ranges from 0.4% to 5.8%,3-5 but this rate increases after revision surgery.1,3-8 Use of large-diameter femoral heads has been proposed to decrease the risks for instability and to improve impingement-free range of motion (ROM).
The biomechanical rationale for using large-diameter femoral heads is that they must travel farther before subluxation or dislocation occurs (jump distance). Despite these benefits, there were initial concerns that catastrophic failure and high levels of volumetric wear would occur if these heads were used with conventional polyethylene liners. These concerns led to the development of alternative bearing surfaces, particularly metal-on-metal bearings, which offered theoretical benefits of large-diameter articulations that improved stability while purportedly being highly wear-resistant.9-11 However, concerns about adverse local soft-tissue reactions and high blood concentrations of metal ions tempered the initial enthusiasm for metal bearings.12-16 Fortunately, highly cross-linked polyethylene and fourth-generation ceramic bearing surfaces, with improved toughness and better wear properties, may allow use of large-diameter heads without the need for metal-on-metal bearings.17,18
In this article, we review the concepts and principles behind use of large-diameter ceramic or cobalt-chromium femoral heads on polyethylene-bearing surfaces in THA with particular attention to biomechanics, early concerns about polyethylene wear and rim fractures, recent improvements in material properties of polyethylene and ceramic bearings, dislocation rates, and clinical and functional outcomes.
Definitions
For this review, we define large-diameter femoral heads as having diameters of 36 mm or more and conventional or small-diameter femoral heads as having diameters between 22 and 32 mm.
Biomechanics
Head–Neck Ratio, Impingement-Free ROM, and Jump Distance
Several implant design principles have been proposed to reduce the risks for impingement and dislocation. Of these, large femoral head diameters have been extensively studied.19,20 It is well known that impingement of the femoral neck on the cup edge promotes edge loading and higher wear rates. In addition, impingement increases the tendency of the head to sublux from the acetabulum. One strategy for avoiding this component-to-component impingement is to increase the head–neck ratio (HNR), the ratio of the femoral head to the neck diameter. Biomechanically, increased HNRs lead to delayed contact between the femoral neck and the acetabular liner.21,22 Therefore, with large femoral heads, which have large HNRs, impingement occurs later and at larger ROMs—compared with small-diameter femoral heads, which have lower HNRs and are more prone to early impingement and subluxation (Figure 1).23-26
In a cadaveric study of 6 hips, Bartz and colleagues23 reported a significantly higher preimpingement ROM when the prosthetic head size increased from 22 mm to 28 mm (P < .05). They found a change from prosthetic to osseous impingement when the head size increased from 22 mm to 32 mm. Similar results were observed in a computer simulation model by Cinotti and colleagues,27 who demonstrated that increasing the femoral head size from 28 mm to 38 mm resulted in a 5° improvement in ROM. However, the largest gains were observed when the heads with the smallest diameters were upsized; ROM improved only marginally when femoral head size was further increased from 32 mm to 38 mm. The primary reason for the lack of expected improvement in ROM with head sizes of more than 32 mm is often bone-on-bone impingement. Burroughs and colleagues28 demonstrated that the 38-mm and 44-mm heads virtually eliminated component-to-component impingement except in extremes of external rotation. However, there were no differences in ROM between 38-mm and 44-mm heads because of osseous impingement. In addition, large heads are less likely to sublux or dislocate, as they need to travel farther before reaching the edge of the acetabular cup before dislocation. This is known as the jump distance, and it corresponds to the depth of the acetabular shell, which in turn equates with the radius of the femoral head (Figures 2A, 2B). For this reason, the larger the femoral head diameter, the farther the jump distance and, correspondingly, the lower the risk for dislocation.29
Elevated liners historically were used to increase the jump distance for dislocation.30 These liners, however, can increase impingement at the extremes of motion.31 Some of these problems can be avoided with use of larger heads, which have increased jump distances without additional risks for impingement. Moreover, large heads create a suction effect that provides passive resistance to dislocation.32 With head diameters beyond 38 mm, impingement-free ROM often plateaus. However, the jump distance required for dislocations to occur continues to increase as femoral head diameters increase in size. Thus, patients may experience fewer motion benefits but continue to benefit from overall stability with femoral head sizes increasing beyond 38 mm.
Current evidence suggests there may be substantial benefits toward improved stability from increasing head diameters from 22 mm to 38 mm because of the increase in jump distances and improvements in prosthetic impingement-free ROM. However, there may be little gain in ROM from increasing the head diameters beyond these dimensions because of the potential risks of bony impingement. Nevertheless, there may be some additional benefits toward stability from improvement in jump distances with incremental head sizes
beyond 38 mm.29,33,34
Finite Element Analysis Studies
Finite element analysis of large-diameter heads in THA has shown that, at optimal cup inclination (45°), most stresses occur on the articular surface of the liner. However, these stresses remain well below the yield strength of the polyethylene liners.29 With increasing abduction angles, the stress concentration increases substantially because of the decreased contact surface area. At these angles, the point of maximum contact moves toward the rim of the polyethylene liner, which can lead to rim fractures or failure of locking mechanisms.29,35,36
Early Concerns With Large-Diameter Femoral Heads: Wear, Liner Failure, and Fracture of Ceramic Components
Use of small-diameter femoral heads started with the first report by Charnley37 of “low frictional torque arthroplasty.” Charnley initially considered a 41.5-mm femoral head, but he thought it would increase risks for acetabular loosening from high frictional torque generated by the large head, and he switched to a small-diameter (22.5 mm) design. One of the tradeoffs with smaller diameter heads was decreased jump height in addition to increased linear wear.
Large femoral heads used with cemented polyethylene acetabular components historically have been associated with increased rates of volumetric wear but low rates of linear wear, which potentially may increase the risk for osteolysis.38-40 However, newer highly cross-linked polyethylene liners have shown improved in vitro and in vivo volumetric wear characteristics and potentially lower linear wear rates compared with earlier designs (Table 1).28,41-43
Another concern about earlier generations of large femoral heads was the risk for catastrophic liner failure on conventional polyethylene. This was originally reported by Berry and colleagues,47 who described wear-through and failure in patients with thin (< 5 mm) acetabular cups. However, these concerns have been largely addressed by the development of highly cross-linked polyethylene, which has improved wear characteristics and fatigue resistance.48
Recent Improvements in Material Properties of Polyethylene and Ceramic Bearings
The development of highly cross-linked polyethylene and fourth-generation ceramics has renewed interest in large-diameter bearings in THA. These bearing surfaces improve wear, enhance material properties, and have superior oxidation resistance.42,48-53
We now briefly describe the methods used to improve the material properties of polyethylene and ceramics. Studies have shown that increasing the radiation dose (up to 200 kGy) increases cross-linking and causes an inverse exponential decrease in polyethylene wear.28,41,48-51 However, increasing radiation doses also increases production of free radicals, which diminish the material strength of these polyethylenes. The current generation of highly cross-linked polyethylene liners is produced through a variety of manufacturing strategies to improve cross-linking and reduce wear. These strategies include differential radiation doses (50-100 kGy), techniques (electron beam, radiation), and thermal treatments (melting, annealing). Moreover, to enhance the material properties and reduce the incidence of rim cracking and delamination, authors have proposed using vitamin E supplementation to minimize the amount of subsurface oxidation that occurs as an inevitable consequence of free radical formation during fabrication.54,55 A terminal sterilization process (eg, gas plasma, ethylene oxide, or gamma sterilization in nitrogen) is needed to make commercial, highly cross-linked polyethylene.52,53
Fourth-generation ceramics manufactured with nano-sized yttria-stabilized tetragonal zirconia particles in a stable alumina matrix have more fracture toughness and improved wear characteristics.54,55 In addition, oxide additives (eg, chromium oxide, strontium oxide) improve hardness and dissipate energy by deflecting cracks to prevent their propagation.56 Moreover, the smaller grain sizes of fourth-generation ceramic bearings compared with third-generation designs (0.8 µm vs 1-5 µm) cause less disruption of the fluid film layer, which ultimately results in improved wear performance.57
Multiple studies have found reduced wear rates with metal and ceramic large heads coupled with highly cross-linked polyethylene-bearings (Table 2).17,41,50,58 Bragdon and colleagues,58 using radiostereometric analysis in 25 patients, found no significant differences in mean head penetration rates between 36-mm and 28-mm cobalt-chromium (Co-Cr) heads articulating with highly cross-linked polyethylene cups at a mean follow-up of 3 years (0.035 mm/y vs 0.046 mm/y; P = .11). Geller and colleagues,64 in their study of 42 patients with large-diameter (> 32 mm) Co-Cr femoral heads, found low mean (SD) linear wear rates of 0.06 (0.41) mm/y at a mean follow-up of 3 years. D’Antonio and colleagues,65 in a multicenter study, reported low average linear wear (0.015 mm/y) and volumetric wear (12.1 mm3/y) over 5 years using sequentially annealed cross-linked polyethylene. In vitro reports suggest that large-diameter ceramic heads may have lower wear properties than Co-Cr heads do. Galvin and colleagues,66 in an in vitro hip simulator study, found that large-diameter ceramic heads on highly cross-linked ultrahigh-molecular-weight polyethylene had 40% reductions in steady-state wear rates compared with Co-Cr heads on highly cross-linked bearings (4.7 vs 8.1 mm3/million cycles; P < 0.01).
Dislocation Rates
Several patient, surgeon, and implant factors affect the rate of dislocations after THA. Multiple implant options utilize the biomechanical advantage that large-diameter heads have in improving stability. Various alternatives include use of constrained tripolar heads, dual-mobility bearings, and conventional large-diameter heads with standard liners.67-69
Large-Diameter Heads
Despite the biomechanical advantages of large-diameter metal-on-polyethylene bearings, prior studies have questioned use of these bearings because of risks for increased wear and rim failures. However, the improved wear properties of highly cross-linked polyethylene, elaborated earlier, have led to a reappraisal of this option (Table 2).4,70 Howie and colleagues,71 in a randomized control trial of 644 patients, also found significantly lower rates of dislocation after primary THA with 36-mm heads compared with 28-mm heads (1.3% vs 5.4%; P = .012); in addition, fewer dislocations occurred with 36-mm heads than with 28-mm heads (4.9% vs 12.2%; P = .27) in a series of 44 patients in revision settings. Similarly, in a study conducted with 39,271 Medicare patients between 1998 and 2007, Malkani and colleagues72 found a decrease in the dislocation rate, from 4.21% to 2.14%, with use of large-diameter femoral heads. These results have been confirmed by several other authors.34,66,73,74 Similar results were observed in 65,992 patients in the Australian National Joint Replacement Registry by Conroy and colleagues,75 who reported a significant decrease in the risk for dislocation with large heads (≥ 30 mm) compared with 22-mm heads (relative risk, 1.0 vs 3.1; P ≤ .001).
Few studies have analyzed the role of large-diameter femoral heads in the presence of compromised soft tissues around the hip. Kung and Ries,76 evaluating the influence of large-diameter heads in the presence and absence of a deficient abductor mechanism, demonstrated statistically significant reductions in rates of dislocation after 230 revision THAs when the abductor mechanism was intact with use of 36-mm heads compared with 28-mm heads (12.7% vs 0%; P = .015). With abductor deficiency, though, the positive effect of large heads in reducing dislocation rates was substantially reduced and was similar to that of small heads (P = .74).76
Large heads considerably improve overall stability and lower dislocation rates in THA. With the development of newer ceramics and highly cross-linked polyethylenes, the wear rates reported in multiple studies appear to be less concerning.
Constrained Tripolar Heads
Tripolar heads have been proposed as treatment options for improving stability in patients with chronic and recurrent instability after THA. The tripolar implant consists of a metal head that snap-fits into a polyethylene liner with a polished Co-Cr backing. This bipolar head articulates with a polyethylene bearing that is press-fitted onto a metal acetabular shell and constrained by a metal ring snapped to the outer polyethylene bearing. The bipolar component behaves as a large-diameter femoral head, and the metal ring provides additional restraint, further improving stability.
Williams and colleagues77 performed a systematic review and reported on the outcomes of constrained tripolar liners in 1199 hips at a mean follow-up of 4 years (range, 2-10 years). The mean dislocation rate was 10%, and the mean rate of revision surgery unrelated to instability was 4%. In a study of 43 hips at a mean follow-up of 4 years (range, 2-9 years), Zywiel and colleagues78 reported on the clinical and radiographic outcomes of tripolar constrained liners. Their study group had a mean Harris Hip Score (HHS) of 82 points (range, 38-100 points) and overall survival of 91%, with no evidence of radiographic loosening during follow-up. Despite the improvements in stability with constrained tripolar liners, some authors have reported multiple mechanisms of failure with these devices.79-81 In a study of 43 failed constrained tripolar liners with a mean time to failure of about 2 years, Guyen and colleagues79 identified 5 different failure modes (types 1-5) involving all 4 interfaces in these components.
Encouraging outcomes have been reported at midterm follow-up with tripolar constrained liners. However, concerns about failure at the interfaces suggest that use of these components should be restricted to patients with deficient abductor mechanisms or neuromuscular compromise, low-demand elderly patients, and salvage cases of recurrent dislocations.79
Dual-Mobility Bearings
For more than 20 years, different dual-mobility bearings have been used for difficult acetabular reconstructive scenarios and prevention of instability.82,83 Dual-mobility cups provide constructs that snap-fit a small-diameter femoral head within a large polyethylene insert that articulates with a fixed metal shell. This effectively increases the functional head diameter.
Various authors have reported excellent survivorship rates (92%-99%) and low dislocation rates for these bearings at 5- to 10-year follow-up.82,84-90 Philippot and colleagues,86 in a recent study of 438 hips with dual-mobility cups, reported excellent survivorship (96%) and no early or late instability within a 15-year follow-up. Bouchet and colleagues69 compared dual-mobility bearings (105 hips) with conventional metal-on-polythene bearings (108 hips) and found significantly (P < .05) lower dislocation rates for the dual-mobility implants at a minimum 1-year follow-up. The French Society of Orthopaedics and Traumatology performed a multicenter analysis of 3473 hips with dual-mobility cups implanted in France between January 1998 and December 2003.87 During a mean follow-up of 7 years (range, 5-11 years), there were 15 dislocations (0.43%), 14 of which occurred early, within 3 months of implantation (0.4%). Aseptic implant survivorship was 95% at 10-year follow-up.
Use of these bearings has recently increased in the United States. Short-term and midterm follow-up data show low rates of dislocation and wear. Long-term data are to come.
Clinical and Functional Outcomes of Large-Diameter Femoral Heads
There is a paucity of long-term outcomes data on use of large-diameter heads with highly cross-linked polyethylene bearings. Short-term and midterm clinical results appear to be excellent, with low rates of wear, osteolysis, and aseptic loosening.28,41,73,89-92
Plate and colleagues91 compared the effects of large-diameter (≥ 36 mm) and small-diameter (26 mm, 28 mm) metal heads on highly cross-linked polyethylene bearings. At a mean follow-up of 5 years (range, 4-8.4 years), the large-head cohort had a mean HHS of 90 points (range, 50-100 points) and no dislocations or radiographic evidence of stem or cup loosening. Similarly, Meftah and colleagues93 reported 100% stem survivorship and excellent clinical outcomes—a mean Western Ontario and McMaster Universities Arthritis Index (WOMAC) score of 30 points—for 72 hips with use of large ceramic heads (≥ 32 mm) on highly cross-linked polyethylene at a mean follow-up of 3 years. Gagala and colleagues94 reported excellent clinical and radiographic outcomes in 50 hips (18 ceramic on ceramic, 32 ceramic on polyethylene; 36-mm heads) at a mean follow-up of 3.5 years. Mean HHS was 94 points, and there was no evidence of liner fractures, aseptic loosening, or osteolysis.
In summary, large-diameter femoral heads in THA have become increasingly popular because of improvements in the material properties and wear characteristics of highly cross-linked polyethylene and fourth-generation ceramics. Despite the potential advantages of large heads in preventing dislocations, the basic surgical tenets of placing the acetabular component in appropriate alignment remain firmly established. Implants with functionally large heads (eg, dual-mobility bearings, constrained tripolar liners) may play an important role in patients at high risk for dislocation—particularly elderly patients with poor neuromuscular muscle coordination or deficient abductors, trauma patients, and patients with prior dislocations. Short-term and midterm results are excellent; rates of wear, aseptic loosening, and osteolysis are low. However, long-term outcomes data are needed to support widespread use of large heads in younger and more active patients.
A common cause for total hip arthroplasty (THA) revision is joint instability.1,2 The reported incidence of dislocation in primary THA ranges from 0.4% to 5.8%,3-5 but this rate increases after revision surgery.1,3-8 Use of large-diameter femoral heads has been proposed to decrease the risks for instability and to improve impingement-free range of motion (ROM).
The biomechanical rationale for using large-diameter femoral heads is that they must travel farther before subluxation or dislocation occurs (jump distance). Despite these benefits, there were initial concerns that catastrophic failure and high levels of volumetric wear would occur if these heads were used with conventional polyethylene liners. These concerns led to the development of alternative bearing surfaces, particularly metal-on-metal bearings, which offered theoretical benefits of large-diameter articulations that improved stability while purportedly being highly wear-resistant.9-11 However, concerns about adverse local soft-tissue reactions and high blood concentrations of metal ions tempered the initial enthusiasm for metal bearings.12-16 Fortunately, highly cross-linked polyethylene and fourth-generation ceramic bearing surfaces, with improved toughness and better wear properties, may allow use of large-diameter heads without the need for metal-on-metal bearings.17,18
In this article, we review the concepts and principles behind use of large-diameter ceramic or cobalt-chromium femoral heads on polyethylene-bearing surfaces in THA with particular attention to biomechanics, early concerns about polyethylene wear and rim fractures, recent improvements in material properties of polyethylene and ceramic bearings, dislocation rates, and clinical and functional outcomes.
Definitions
For this review, we define large-diameter femoral heads as having diameters of 36 mm or more and conventional or small-diameter femoral heads as having diameters between 22 and 32 mm.
Biomechanics
Head–Neck Ratio, Impingement-Free ROM, and Jump Distance
Several implant design principles have been proposed to reduce the risks for impingement and dislocation. Of these, large femoral head diameters have been extensively studied.19,20 It is well known that impingement of the femoral neck on the cup edge promotes edge loading and higher wear rates. In addition, impingement increases the tendency of the head to sublux from the acetabulum. One strategy for avoiding this component-to-component impingement is to increase the head–neck ratio (HNR), the ratio of the femoral head to the neck diameter. Biomechanically, increased HNRs lead to delayed contact between the femoral neck and the acetabular liner.21,22 Therefore, with large femoral heads, which have large HNRs, impingement occurs later and at larger ROMs—compared with small-diameter femoral heads, which have lower HNRs and are more prone to early impingement and subluxation (Figure 1).23-26
In a cadaveric study of 6 hips, Bartz and colleagues23 reported a significantly higher preimpingement ROM when the prosthetic head size increased from 22 mm to 28 mm (P < .05). They found a change from prosthetic to osseous impingement when the head size increased from 22 mm to 32 mm. Similar results were observed in a computer simulation model by Cinotti and colleagues,27 who demonstrated that increasing the femoral head size from 28 mm to 38 mm resulted in a 5° improvement in ROM. However, the largest gains were observed when the heads with the smallest diameters were upsized; ROM improved only marginally when femoral head size was further increased from 32 mm to 38 mm. The primary reason for the lack of expected improvement in ROM with head sizes of more than 32 mm is often bone-on-bone impingement. Burroughs and colleagues28 demonstrated that the 38-mm and 44-mm heads virtually eliminated component-to-component impingement except in extremes of external rotation. However, there were no differences in ROM between 38-mm and 44-mm heads because of osseous impingement. In addition, large heads are less likely to sublux or dislocate, as they need to travel farther before reaching the edge of the acetabular cup before dislocation. This is known as the jump distance, and it corresponds to the depth of the acetabular shell, which in turn equates with the radius of the femoral head (Figures 2A, 2B). For this reason, the larger the femoral head diameter, the farther the jump distance and, correspondingly, the lower the risk for dislocation.29
Elevated liners historically were used to increase the jump distance for dislocation.30 These liners, however, can increase impingement at the extremes of motion.31 Some of these problems can be avoided with use of larger heads, which have increased jump distances without additional risks for impingement. Moreover, large heads create a suction effect that provides passive resistance to dislocation.32 With head diameters beyond 38 mm, impingement-free ROM often plateaus. However, the jump distance required for dislocations to occur continues to increase as femoral head diameters increase in size. Thus, patients may experience fewer motion benefits but continue to benefit from overall stability with femoral head sizes increasing beyond 38 mm.
Current evidence suggests there may be substantial benefits toward improved stability from increasing head diameters from 22 mm to 38 mm because of the increase in jump distances and improvements in prosthetic impingement-free ROM. However, there may be little gain in ROM from increasing the head diameters beyond these dimensions because of the potential risks of bony impingement. Nevertheless, there may be some additional benefits toward stability from improvement in jump distances with incremental head sizes
beyond 38 mm.29,33,34
Finite Element Analysis Studies
Finite element analysis of large-diameter heads in THA has shown that, at optimal cup inclination (45°), most stresses occur on the articular surface of the liner. However, these stresses remain well below the yield strength of the polyethylene liners.29 With increasing abduction angles, the stress concentration increases substantially because of the decreased contact surface area. At these angles, the point of maximum contact moves toward the rim of the polyethylene liner, which can lead to rim fractures or failure of locking mechanisms.29,35,36
Early Concerns With Large-Diameter Femoral Heads: Wear, Liner Failure, and Fracture of Ceramic Components
Use of small-diameter femoral heads started with the first report by Charnley37 of “low frictional torque arthroplasty.” Charnley initially considered a 41.5-mm femoral head, but he thought it would increase risks for acetabular loosening from high frictional torque generated by the large head, and he switched to a small-diameter (22.5 mm) design. One of the tradeoffs with smaller diameter heads was decreased jump height in addition to increased linear wear.
Large femoral heads used with cemented polyethylene acetabular components historically have been associated with increased rates of volumetric wear but low rates of linear wear, which potentially may increase the risk for osteolysis.38-40 However, newer highly cross-linked polyethylene liners have shown improved in vitro and in vivo volumetric wear characteristics and potentially lower linear wear rates compared with earlier designs (Table 1).28,41-43
Another concern about earlier generations of large femoral heads was the risk for catastrophic liner failure on conventional polyethylene. This was originally reported by Berry and colleagues,47 who described wear-through and failure in patients with thin (< 5 mm) acetabular cups. However, these concerns have been largely addressed by the development of highly cross-linked polyethylene, which has improved wear characteristics and fatigue resistance.48
Recent Improvements in Material Properties of Polyethylene and Ceramic Bearings
The development of highly cross-linked polyethylene and fourth-generation ceramics has renewed interest in large-diameter bearings in THA. These bearing surfaces improve wear, enhance material properties, and have superior oxidation resistance.42,48-53
We now briefly describe the methods used to improve the material properties of polyethylene and ceramics. Studies have shown that increasing the radiation dose (up to 200 kGy) increases cross-linking and causes an inverse exponential decrease in polyethylene wear.28,41,48-51 However, increasing radiation doses also increases production of free radicals, which diminish the material strength of these polyethylenes. The current generation of highly cross-linked polyethylene liners is produced through a variety of manufacturing strategies to improve cross-linking and reduce wear. These strategies include differential radiation doses (50-100 kGy), techniques (electron beam, radiation), and thermal treatments (melting, annealing). Moreover, to enhance the material properties and reduce the incidence of rim cracking and delamination, authors have proposed using vitamin E supplementation to minimize the amount of subsurface oxidation that occurs as an inevitable consequence of free radical formation during fabrication.54,55 A terminal sterilization process (eg, gas plasma, ethylene oxide, or gamma sterilization in nitrogen) is needed to make commercial, highly cross-linked polyethylene.52,53
Fourth-generation ceramics manufactured with nano-sized yttria-stabilized tetragonal zirconia particles in a stable alumina matrix have more fracture toughness and improved wear characteristics.54,55 In addition, oxide additives (eg, chromium oxide, strontium oxide) improve hardness and dissipate energy by deflecting cracks to prevent their propagation.56 Moreover, the smaller grain sizes of fourth-generation ceramic bearings compared with third-generation designs (0.8 µm vs 1-5 µm) cause less disruption of the fluid film layer, which ultimately results in improved wear performance.57
Multiple studies have found reduced wear rates with metal and ceramic large heads coupled with highly cross-linked polyethylene-bearings (Table 2).17,41,50,58 Bragdon and colleagues,58 using radiostereometric analysis in 25 patients, found no significant differences in mean head penetration rates between 36-mm and 28-mm cobalt-chromium (Co-Cr) heads articulating with highly cross-linked polyethylene cups at a mean follow-up of 3 years (0.035 mm/y vs 0.046 mm/y; P = .11). Geller and colleagues,64 in their study of 42 patients with large-diameter (> 32 mm) Co-Cr femoral heads, found low mean (SD) linear wear rates of 0.06 (0.41) mm/y at a mean follow-up of 3 years. D’Antonio and colleagues,65 in a multicenter study, reported low average linear wear (0.015 mm/y) and volumetric wear (12.1 mm3/y) over 5 years using sequentially annealed cross-linked polyethylene. In vitro reports suggest that large-diameter ceramic heads may have lower wear properties than Co-Cr heads do. Galvin and colleagues,66 in an in vitro hip simulator study, found that large-diameter ceramic heads on highly cross-linked ultrahigh-molecular-weight polyethylene had 40% reductions in steady-state wear rates compared with Co-Cr heads on highly cross-linked bearings (4.7 vs 8.1 mm3/million cycles; P < 0.01).
Dislocation Rates
Several patient, surgeon, and implant factors affect the rate of dislocations after THA. Multiple implant options utilize the biomechanical advantage that large-diameter heads have in improving stability. Various alternatives include use of constrained tripolar heads, dual-mobility bearings, and conventional large-diameter heads with standard liners.67-69
Large-Diameter Heads
Despite the biomechanical advantages of large-diameter metal-on-polyethylene bearings, prior studies have questioned use of these bearings because of risks for increased wear and rim failures. However, the improved wear properties of highly cross-linked polyethylene, elaborated earlier, have led to a reappraisal of this option (Table 2).4,70 Howie and colleagues,71 in a randomized control trial of 644 patients, also found significantly lower rates of dislocation after primary THA with 36-mm heads compared with 28-mm heads (1.3% vs 5.4%; P = .012); in addition, fewer dislocations occurred with 36-mm heads than with 28-mm heads (4.9% vs 12.2%; P = .27) in a series of 44 patients in revision settings. Similarly, in a study conducted with 39,271 Medicare patients between 1998 and 2007, Malkani and colleagues72 found a decrease in the dislocation rate, from 4.21% to 2.14%, with use of large-diameter femoral heads. These results have been confirmed by several other authors.34,66,73,74 Similar results were observed in 65,992 patients in the Australian National Joint Replacement Registry by Conroy and colleagues,75 who reported a significant decrease in the risk for dislocation with large heads (≥ 30 mm) compared with 22-mm heads (relative risk, 1.0 vs 3.1; P ≤ .001).
Few studies have analyzed the role of large-diameter femoral heads in the presence of compromised soft tissues around the hip. Kung and Ries,76 evaluating the influence of large-diameter heads in the presence and absence of a deficient abductor mechanism, demonstrated statistically significant reductions in rates of dislocation after 230 revision THAs when the abductor mechanism was intact with use of 36-mm heads compared with 28-mm heads (12.7% vs 0%; P = .015). With abductor deficiency, though, the positive effect of large heads in reducing dislocation rates was substantially reduced and was similar to that of small heads (P = .74).76
Large heads considerably improve overall stability and lower dislocation rates in THA. With the development of newer ceramics and highly cross-linked polyethylenes, the wear rates reported in multiple studies appear to be less concerning.
Constrained Tripolar Heads
Tripolar heads have been proposed as treatment options for improving stability in patients with chronic and recurrent instability after THA. The tripolar implant consists of a metal head that snap-fits into a polyethylene liner with a polished Co-Cr backing. This bipolar head articulates with a polyethylene bearing that is press-fitted onto a metal acetabular shell and constrained by a metal ring snapped to the outer polyethylene bearing. The bipolar component behaves as a large-diameter femoral head, and the metal ring provides additional restraint, further improving stability.
Williams and colleagues77 performed a systematic review and reported on the outcomes of constrained tripolar liners in 1199 hips at a mean follow-up of 4 years (range, 2-10 years). The mean dislocation rate was 10%, and the mean rate of revision surgery unrelated to instability was 4%. In a study of 43 hips at a mean follow-up of 4 years (range, 2-9 years), Zywiel and colleagues78 reported on the clinical and radiographic outcomes of tripolar constrained liners. Their study group had a mean Harris Hip Score (HHS) of 82 points (range, 38-100 points) and overall survival of 91%, with no evidence of radiographic loosening during follow-up. Despite the improvements in stability with constrained tripolar liners, some authors have reported multiple mechanisms of failure with these devices.79-81 In a study of 43 failed constrained tripolar liners with a mean time to failure of about 2 years, Guyen and colleagues79 identified 5 different failure modes (types 1-5) involving all 4 interfaces in these components.
Encouraging outcomes have been reported at midterm follow-up with tripolar constrained liners. However, concerns about failure at the interfaces suggest that use of these components should be restricted to patients with deficient abductor mechanisms or neuromuscular compromise, low-demand elderly patients, and salvage cases of recurrent dislocations.79
Dual-Mobility Bearings
For more than 20 years, different dual-mobility bearings have been used for difficult acetabular reconstructive scenarios and prevention of instability.82,83 Dual-mobility cups provide constructs that snap-fit a small-diameter femoral head within a large polyethylene insert that articulates with a fixed metal shell. This effectively increases the functional head diameter.
Various authors have reported excellent survivorship rates (92%-99%) and low dislocation rates for these bearings at 5- to 10-year follow-up.82,84-90 Philippot and colleagues,86 in a recent study of 438 hips with dual-mobility cups, reported excellent survivorship (96%) and no early or late instability within a 15-year follow-up. Bouchet and colleagues69 compared dual-mobility bearings (105 hips) with conventional metal-on-polythene bearings (108 hips) and found significantly (P < .05) lower dislocation rates for the dual-mobility implants at a minimum 1-year follow-up. The French Society of Orthopaedics and Traumatology performed a multicenter analysis of 3473 hips with dual-mobility cups implanted in France between January 1998 and December 2003.87 During a mean follow-up of 7 years (range, 5-11 years), there were 15 dislocations (0.43%), 14 of which occurred early, within 3 months of implantation (0.4%). Aseptic implant survivorship was 95% at 10-year follow-up.
Use of these bearings has recently increased in the United States. Short-term and midterm follow-up data show low rates of dislocation and wear. Long-term data are to come.
Clinical and Functional Outcomes of Large-Diameter Femoral Heads
There is a paucity of long-term outcomes data on use of large-diameter heads with highly cross-linked polyethylene bearings. Short-term and midterm clinical results appear to be excellent, with low rates of wear, osteolysis, and aseptic loosening.28,41,73,89-92
Plate and colleagues91 compared the effects of large-diameter (≥ 36 mm) and small-diameter (26 mm, 28 mm) metal heads on highly cross-linked polyethylene bearings. At a mean follow-up of 5 years (range, 4-8.4 years), the large-head cohort had a mean HHS of 90 points (range, 50-100 points) and no dislocations or radiographic evidence of stem or cup loosening. Similarly, Meftah and colleagues93 reported 100% stem survivorship and excellent clinical outcomes—a mean Western Ontario and McMaster Universities Arthritis Index (WOMAC) score of 30 points—for 72 hips with use of large ceramic heads (≥ 32 mm) on highly cross-linked polyethylene at a mean follow-up of 3 years. Gagala and colleagues94 reported excellent clinical and radiographic outcomes in 50 hips (18 ceramic on ceramic, 32 ceramic on polyethylene; 36-mm heads) at a mean follow-up of 3.5 years. Mean HHS was 94 points, and there was no evidence of liner fractures, aseptic loosening, or osteolysis.
In summary, large-diameter femoral heads in THA have become increasingly popular because of improvements in the material properties and wear characteristics of highly cross-linked polyethylene and fourth-generation ceramics. Despite the potential advantages of large heads in preventing dislocations, the basic surgical tenets of placing the acetabular component in appropriate alignment remain firmly established. Implants with functionally large heads (eg, dual-mobility bearings, constrained tripolar liners) may play an important role in patients at high risk for dislocation—particularly elderly patients with poor neuromuscular muscle coordination or deficient abductors, trauma patients, and patients with prior dislocations. Short-term and midterm results are excellent; rates of wear, aseptic loosening, and osteolysis are low. However, long-term outcomes data are needed to support widespread use of large heads in younger and more active patients.
1. Dorr LD, Wolf AW, Chandler R, Conaty JP. Classification and treatment of dislocations of total hip arthroplasty. Clin Orthop. 1983;(173):151-158.
2. Dorr LD, Wan Z. Causes of and treatment protocol for instability of total hip replacement. Clin Orthop. 1998;(355):144-151.
3. Turner RS. Postoperative total hip prosthetic femoral head dislocations. Incidence, etiologic factors, and management. Clin Orthop. 1994;(301):196-204.
4. Woo RY, Morrey BF. Dislocations after total hip arthroplasty. J Bone Joint Surg Am. 1982;64(9):1295-1306.
5. Etienne A, Cupic Z, Charnley J. Postoperative dislocation after Charnley low-friction arthroplasty. Clin Orthop. 1978;(132):19-23.
6. Fackler CD, Poss R. Dislocation in total hip arthroplasties. Clin Orthop. 1980;(151):169-178.
7. Joshi A, Lee CM, Markovic L, Vlatis G, Murphy JC. Prognosis of dislocation after total hip arthroplasty. J Arthroplasty. 1998;13(1):17-21.
8. Lindberg HO, Carlsson AS, Gentz CF, Pettersson H. Recurrent and non-recurrent dislocation following total hip arthroplasty. Acta Orthop Scand. 1982;53(6):947-952.
9. Eswaramoorthy V, Moonot P, Kalairajah Y, Biant LC, Field RE. The Metasul metal-on-metal articulation in primary total hip replacement: clinical and radiological results at ten years. J Bone Joint Surg Br. 2008;90(10):1278-1283.
10. Grubl A, Marker M, Brodner W, et al. Long-term follow-up of metal-on-metal total hip replacement. J Orthop Res. 2007;25(7):841-848.
11. Leslie I, Williams S, Brown C, et al. Effect of bearing size on the long-term wear, wear debris, and ion levels of large diameter metal-on-metal hip replacements—an in vitro study. J Biomed Mater Res B Appl Biomater. 2008;87(1):163-172.
12. Verhaar JA. The hard lesson of metal-on-metal hip implants [in Dutch]. Ned Tijdschr Geneeskd. 2012;156(42):A5564.
13. Fabi D, Levine B, Paprosky W, et al. Metal-on-metal total hip arthroplasty: causes and high incidence of early failure. Orthopedics. 2012;35(7):e1009-e1016.
14. Heneghan C, Langton D, Thompson M. Ongoing problems with metal-on-metal hip implants. BMJ. 2012;344:e1349.
15. Lee RK, Nevelos J, Vigdorchik J, Markel DC. Bearing surfaces for hip arthroplasty—is metal-on-metal a passing fancy? Surg Technol Int. 2012;22:243-249.
16. Voleti PB, Baldwin KD, Lee GC. Metal-on-metal vs conventional total hip arthroplasty: a systematic review and meta-analysis of randomized controlled trials. J Arthroplasty. 2012;27(10):1844-1849.
17. Urban JA, Garvin KL, Boese CK, et al. Ceramic-on-polyethylene bearing surfaces in total hip arthroplasty. Seventeen to twenty-one-year results.
J Bone Joint Surg Am. 2001;83(11):1688-1694.
18. Callaghan JJ, Liu SS. Ceramic on crosslinked polyethylene in total hip replacement: any better than metal on crosslinked polyethylene? Iowa Orthop J. 2009;29:1-4.
19. Barrack RL. Dislocation after total hip arthroplasty: implant design and orientation. J Am Acad Orthop Surg. 2003;11(2):89-99.
20. Krushell RJ, Burke DW, Harris WH. Elevated-rim acetabular components. Effect on range of motion and stability in total hip arthroplasty. J Arthroplasty. 1991;6(suppl):S53-S58.
21. Morrey BF. Instability after total hip arthroplasty. Orthop Clin North Am. 1992;23(2):237-248.
22. Morrey BF. Dislocation. In: Morrey BF, ed. Joint Replacement Arthroplasty. New York, NY: Churchill Livingstone; 1991:851-865.
23. Bartz RL, Nobel PC, Kadakia NR, Tullos HS. The effect of femoral component head size on posterior dislocation of the artificial hip joint. J Bone Joint Surg Am. 2000;82(9):1300-1307.
24. Nicholas RM, Orr JF, Mollan RA, Calderwood JW, Nixon JR, Watson P. Dislocation of total hip replacements. A comparative study of standard, long posterior wall and augmented acetabular components. J Bone Joint Surg Br. 1990;72(3):418-422.
25. McCollum DE, Gray WJ. Dislocation after total hip arthroplasty. Causes and prevention. Clin Orthop. 1990;(261):159-170.
26. Herrlin K, Selvik G, Pettersson H, Kesek P, Onnerfalt R, Ohlin A. Position, orientation and component interaction in dislocation of the total hip prosthesis. Acta Radiol. 1988;29(4):441-444.
27. Cinotti G, Lucioli N, Malagoli A, Calderoli C, Cassese F. Do large femoral heads reduce the risks of impingement in total hip arthroplasty with optimal and non-optimal cup positioning? Int Orthop. 2011;35(3):317-323.
28. Burroughs BR, Rubash HE, Harris WH. Femoral head sizes larger than 32 mm against highly cross-linked polyethylene. Clin Orthop. 2002;(405):150-157.
29. Crowninshield RD, Maloney WJ, Wentz DH, Humphrey SM, Blanchard CR. Biomechanics of large femoral heads: what they do and don‘t do. Clin Orthop. 2004;(429):102-107.
30. Charnley J. Low Friction Arthroplasty of the Hip: Theory and Practice. New York, NY: Springer; 1979.
31. Yamaguchi M, Akisue T, Bauer TW, Hashimoto Y. The spatial location of impingement in total hip arthroplasty. J Arthroplasty. 2000;15(3):305-313.
32. Peters CL, McPherson E, Jackson JD, Erickson JA. Reduction in early dislocation rate with large-diameter femoral heads in primary total hip arthroplasty. J Arthroplasty. 2007;22(6 suppl 2):140-144.
33. Masonis JL, Bourne RB. Surgical approach, abductor function, and total hip arthroplasty dislocation. Clin Orthop. 2002;(405):46-53.
34. Beaule PE, Schmalzried TP, Udomkiat P, Amstutz HC. Jumbo femoral head for the treatment of recurrent dislocation following total hip replacement. J Bone Joint Surg Am. 2002;84(2):256-263.
35. Oral E, Malhi AS, Muratoglu OK. Mechanisms of decrease in fatigue crack propagation resistance in irradiated and melted UHMWPE. Biomaterials. 2006;27(6):917-925.
36. Baker DA, Bellare A, Pruitt L. The effects of degree of crosslinking on the fatigue crack initiation and propagation resistance of orthopedic-grade polyethylene. J Biomed Mater Res A. 2003;66(1):146-154.
37. Charnley J. Total hip replacement by low-friction arthroplasty. Clin Orthop. 1970;(72):7-21.
38. Kabo JM, Gebhard JS, Loren G, Amstutz HC. In vivo wear of polyethylene acetabular components. J Bone Joint Surg Br. 1993;75(2):254-258.
39. Livermore J, Ilstrup D, Morrey B. Effect of femoral head size on wear of the polyethylene acetabular component. J Bone Joint Surg Am. 1990;72(4):518-528.
40. Ma SM, Kabo JM, Amstutz HC. Frictional torque in surface and conventional hip replacement. J Bone Joint Surg Am. 1983;65(3):366-370.
41. Muratoglu OK, Bragdon CR, O‘Connor D, et al. Larger diameter femoral heads used in conjunction with a highly cross-linked ultra-high molecular weight polyethylene: a new concept. J Arthroplasty. 2001;16(8 suppl 1):24-30.
42. Thomas GER, Simpson DJ, Mehmood S, et al. The seven-year wear of highly cross-linked polyethylene in total hip arthroplasty: a double-blind, randomized controlled trial using radiostereometric analysis. J Bone Joint Surg Am. 2011;93(8):716-722.
43. Mutimer J, Devane PA, Adams K, Horne JG. Highly crosslinked polyethylene reduces wear in total hip arthroplasty at 5 years. Clin Orthop. 2010;468(12):3228-3233.
44. Bragdon CR, Doerner M, Martell J, Jarrett B, Palm H, Malchau H. The 2012 John Charnley Award: clinical multicenter studies of the wear performance of highly crosslinked remelted polyethylene in THA. Clin Orthop. 2013;471(2):393-402.
45. Lachiewicz PF, Heckman DS, Soileau ES, Mangla J, Martell JM. Femoral head size and wear of highly cross-linked polyethylene at 5 to 8 years. Clin Orthop. 2009;467(12):3290-3296.
46. Sychterz CJ, Engh CA Jr, Young AM, Hopper RH Jr, Engh CA. Comparison of in vivo wear between polyethylene liners articulating with ceramic and cobalt-chrome femoral heads. J Bone Joint Surg Br. 2000;82(7):948-951.
47. Berry DJ, Barnes CL, Scott RD, Cabanela ME, Poss R. Catastrophic failure of the polyethylene liner of uncemented acetabular components.
J Bone Joint Surg Br. 1994;76(4):575-578.
48. McKellop H, Shen FW, Lu B, Campbell P, Salovey R. Development of an extremely wear-resistant ultra high molecular weight polyethylene for total hip replacements. J Orthop Res. 1999;17(2):157-167.
49. Wang A, Essner A, Polineni VK, Stark C, Dumbleton JH. Lubrication and wear of ultra-high molecular weight polyethylene in total joint replacements. Tribol Int. 1998;31(1-3):17-33.
50. Estok DM 2nd, Burroughs BR, Muratoglu OK, Harris WH. Comparison of hip simulator wear of 2 different highly cross-linked ultra high molecular weight polyethylene acetabular components using both 32- and 38-mm femoral heads. J Arthroplasty. 2007;22(4):581-589.
51. Muratoglu OK, Bragdon CR, O‘Connor DO, et al. Unified wear model for highly crosslinked ultra-high molecular weight polyethylenes (UHMWPE). Biomaterials. 1999;20(16):1463-1470.
52. Harris WH, Muratoglu OK. A review of current cross-linked polyethylenes used in total joint arthroplasty. Clin Orthop. 2005;(430):46-52.
53. Muratoglu OK, Bragdon CR, O‘Connor DO, Jasty M, Harris WH. A novel method of cross-linking ultra-high-molecular-weight polyethylene to improve wear, reduce oxidation, and retain mechanical properties. Recipient of the 1999 HAP Paul Award. J Arthroplasty. 2001;16(2):149-160.
54. Bal BS, Garino J, Ries M, Rahaman MN. A review of ceramic bearing materials in total joint arthroplasty. Hip Int. 2007;17(1):21-30.
55. Traina F, De Fine M, Di Martino A, Faldini C. Fracture of ceramic bearing surfaces following total hip replacement: a systematic review. Biomed Res Int. 2013;2013:157247.
56. Cai YZ, Yan SG. Development of ceramic-on-ceramic implants for total hip arthroplasty. Orthop Surg. 2010;2(3):175-181.
57. Stewart TD, Tipper JL, Insley G, Streicher RM, Ingham E, Fisher J. Long-term wear of ceramic matrix composite materials for hip prostheses under severe swing phase microseparation. J Biomed Mater Res B Appl Biomater. 2003;66(2):567-573.
58. Bragdon CR, Greene ME, Freiberg AA, Harris WH, Malchau H. Radiostereometric analysis comparison of wear of highly cross-linked polyethylene against 36- vs 28-mm femoral heads. J Arthroplasty. 2007;22(6 suppl 2):125-129.
59. Lombardi AV Jr, Skeels MD, Berend KR, Adams JB, Franchi OJ. Do large heads enhance stability and restore native anatomy in primary total hip arthroplasty? Clin Orthop. 2011;469(6):1547-1553.
60. Lachiewicz PF, Soileau ES. Low early and late dislocation rates with 36- and 40-mm heads in patients at high risk for dislocation. Clin Orthop. 2013;471(2):439-443.
61. Cai P, Hu Y, Xie J. Large-diameter Delta ceramic-on-ceramic versus common-sized ceramic-on-polyethylene bearings in THA. Orthopedics. 2012;35(9):e1307-e1313.
62. Park KS, Yoon TR, Hwang SY, Lee KB. Modified minimally invasive two-incision total hip arthroplasty using large diameter femoral head. Indian
J Orthop. 2012;46(1):29-35.
63. Garbuz DS, Masri BA, Duncan CP, et al. The Frank Stinchfield Award: dislocation in revision THA: do large heads (36 and 40 mm) result in reduced dislocation rates in a randomized clinical trial? Clin Orthop. 2012;470(2):351-356.
64. Geller JA, Malchau H, Bragdon C, Greene M, Harris WH, Freiberg AA. Large diameter femoral heads on highly cross-linked polyethylene: minimum 3-year results. Clin Orthop. 2006;(447):53-59.
65. D’Antonio JA, Capello WN, Ramakrishnan R. Second-generation annealed highly cross-linked polyethylene exhibits low wear. Clin Orthop. 2012;470(6):1696-1704.
66. Galvin AL, Jennings LM, Tipper JL, Ingham E, Fisher J. Wear and creep of highly crosslinked polyethylene against cobalt chrome and ceramic femoral heads. Proc Inst Mech Eng H. 2010;224(10):1175-1183.
67. Skeels MD, Berend KR, Lombardi AV Jr. The dislocator, early and late: the role of large heads. Orthopedics. 2009;32(9).
68. Plate JF, Seyler TM, Stroh DA, Issa K, Akbar M, Mont MA. Risk of dislocation using large- vs. small-diameter femoral heads in total hip arthroplasty. BMC Res Notes. 2012;5(1):553.
69. Bouchet R, Mercier N, Saragaglia D. Posterior approach and dislocation rate: a 213 total hip replacements case–control study comparing the dual mobility cup with a conventional 28-mm metal head/polyethylene prosthesis. Orthop Traumatol Surg Res. 2011;97(1):2-7.
70. Ali Khan MA, Brakenbury PH, Reynolds IS. Dislocation following total hip replacement. J Bone Joint Surg Br. 1981;63(2):214-218.
71. Howie DW, Holubowycz OT, Middleton R. Large femoral heads decrease the incidence of dislocation after total hip arthroplasty: a randomized controlled trial. J Bone Joint Surg Am. 2012;94(12):1095-1102.
72. Malkani AL, Ong KL, Lau E, Kurtz SM, Justice BJ, Manley MT. Early- and late-term dislocation risk after primary hip arthroplasty in the Medicare population. J Arthroplasty. 2010;25(6 suppl):21-25.
73. Berry DJ, von Knoch M, Schleck CD, Harmsen WS. Effect of femoral head diameter and operative approach on risk of dislocation after primary total hip arthroplasty. J Bone Joint Surg Am. 2005;87(11):2456-2463.
74. Cho MR, Lee HS, Lee SW, Choi CH, Kim SK, Ko SB. Results after total hip arthroplasty with a large head and bipolar arthroplasty in patients with displaced femoral neck fractures. J Arthroplasty. 2011;26(6):893-896.
75. Conroy JL, Whitehouse SL, Graves SE, Pratt NL, Ryan P, Crawford RW. Risk factors for revision for early dislocation in total hip arthroplasty.
J Arthroplasty. 2008;23(6):867-872.
76. Kung PL, Ries MD. Effect of femoral head size and abductors on dislocation after revision THA. Clin Orthop. 2007;(465):170-174.
77. Williams JT Jr, Ragland PS, Clarke S. Constrained components for the unstable hip following total hip arthroplasty: a literature review. Int Orthop. 2007;31(3):273-277.
78. Zywiel MG, Mustafa LH, Bonutti PM, Mont MA. Are abductor muscle quality and previous revision surgery predictors of constrained liner failure in hip arthroplasty? Int Orthop. 2011;35(6):797-802.
79. Guyen O, Lewallen DG, Cabanela ME. Modes of failure of Osteonics constrained tripolar implants: a retrospective analysis of forty-three failed implants. J Bone Joint Surg Am. 2008;90(7):1553-1560.
80. Banks LN, McElwain JP. An unusual mode of failure of a tripolar constrained acetabular liner: a case report. Arch Orthop Trauma Surg. 2010;130(4):503-505.
81. Robertson WJ, Mattern CJ, Hur J, Su EP, Pellicci PM. Failure mechanisms and closed reduction of a constrained tripolar acetabular liner.
J Arthroplasty. 2009;24(2):322.e5-e11.
82. Aubriot JH, Lesimple P, Leclercq S. Study of Bousquet‘s non-cemented acetabular implant in 100 hybrid total hip prostheses (Charnley type cemented femoral component). Average 5-year follow-up [in French]. Acta Orthop Belg. 1993;59(suppl 1):267-271.
83. Farizon F, de Lavison R, Azoulai JJ, Bousquet G. Results with a cementless alumina-coated cup with dual mobility. A twelve-year follow-up study. Int Orthop. 1998;22(4):219-224.
84. Mertl P, Combes A, Leiber-Wackenheim F, Fessy MH, Girard J, Migaud H. Recurrence of dislocation following total hip arthroplasty revision using dual mobility cups was rare in 180 hips followed over 7 years. HSS J. 2012;8(3):251-256.
85. Langlais FL, Ropars M, Gaucher F, Musset T, Chaix O. Dual mobility cemented cups have low dislocation rates in THA revisions. Clin Orthop. 2008;466(2):389-395.
86. Philippot R, Farizon F, Camilleri JP, et al. Survival of dual mobility socket with a mean 17 years follow-up [in French]. Rev Chir Orthop Reparatrice Appar Mot. 2008;94(1):43-48.
87. Adam P, Philippe R, Ehlinger M, et al. Dual mobility cups hip arthroplasty as a treatment for displaced fracture of the femoral neck in the elderly.
A prospective, systematic, multicenter study with specific focus on postoperative dislocation. Orthop Traumatol Surg Res. 2012;98(3):296-300.
88. Fessy MH. La double mobilité [Dual mobility]. Revue de Chirurgie Orthopédique et Traumatologique. 2010;96(7):891-898.
89. Mont MA, Issa K, Naziri Q, Harwin SF, Delanois RE, Johnson AJ. The use of dual-mobility bearings in difficult hip arthroplasty reconstructive cases. Surg Technol Int. 2011;21:234-240.
90. Sayeed SA, Mont MA, Costa CR, et al. Early outcomes of sequentially cross-linked thin polyethylene liners with large diameter femoral heads in total hip arthroplasty. Bull NYU Hosp Jt Dis. 2011;69(suppl 1):S90-S94.
91. Plate JF, Seyler TM, Stroh DA, Issa K, Akbar M, Mont MA. Risk of dislocation using large- vs. small-diameter femoral heads in total hip arthroplasty. BMC Res Notes. 2012;5(1):553.
92. Sato T, Nakashima Y, Akiyama M, et al. Wear resistant performance of highly cross-linked and annealed ultra-high molecular weight polyethylene against ceramic heads in total hip arthroplasty. J Orthop Res. 2012;30(12):2031-2037.
93. Meftah M, Ebrahimpour PB, He C, Ranawat AS, Ranawat CS. Preliminary clinical and radiographic results of large ceramic heads on highly cross-linked polyethylene. Orthopedics. 2011;34(6):133.
94. Gagala J, Mazurkiewicz T, Dajewski Z. Large diameter femoral heads in primary alumina/alumina and XSPE/alumina total hip arthroplasty.
A follow-up study of 50 hips after average 40 months and review of literature [in Polish]. Chir Narzadow Ruchu Ortop Pol. 2011;76(1):14-20.
1. Dorr LD, Wolf AW, Chandler R, Conaty JP. Classification and treatment of dislocations of total hip arthroplasty. Clin Orthop. 1983;(173):151-158.
2. Dorr LD, Wan Z. Causes of and treatment protocol for instability of total hip replacement. Clin Orthop. 1998;(355):144-151.
3. Turner RS. Postoperative total hip prosthetic femoral head dislocations. Incidence, etiologic factors, and management. Clin Orthop. 1994;(301):196-204.
4. Woo RY, Morrey BF. Dislocations after total hip arthroplasty. J Bone Joint Surg Am. 1982;64(9):1295-1306.
5. Etienne A, Cupic Z, Charnley J. Postoperative dislocation after Charnley low-friction arthroplasty. Clin Orthop. 1978;(132):19-23.
6. Fackler CD, Poss R. Dislocation in total hip arthroplasties. Clin Orthop. 1980;(151):169-178.
7. Joshi A, Lee CM, Markovic L, Vlatis G, Murphy JC. Prognosis of dislocation after total hip arthroplasty. J Arthroplasty. 1998;13(1):17-21.
8. Lindberg HO, Carlsson AS, Gentz CF, Pettersson H. Recurrent and non-recurrent dislocation following total hip arthroplasty. Acta Orthop Scand. 1982;53(6):947-952.
9. Eswaramoorthy V, Moonot P, Kalairajah Y, Biant LC, Field RE. The Metasul metal-on-metal articulation in primary total hip replacement: clinical and radiological results at ten years. J Bone Joint Surg Br. 2008;90(10):1278-1283.
10. Grubl A, Marker M, Brodner W, et al. Long-term follow-up of metal-on-metal total hip replacement. J Orthop Res. 2007;25(7):841-848.
11. Leslie I, Williams S, Brown C, et al. Effect of bearing size on the long-term wear, wear debris, and ion levels of large diameter metal-on-metal hip replacements—an in vitro study. J Biomed Mater Res B Appl Biomater. 2008;87(1):163-172.
12. Verhaar JA. The hard lesson of metal-on-metal hip implants [in Dutch]. Ned Tijdschr Geneeskd. 2012;156(42):A5564.
13. Fabi D, Levine B, Paprosky W, et al. Metal-on-metal total hip arthroplasty: causes and high incidence of early failure. Orthopedics. 2012;35(7):e1009-e1016.
14. Heneghan C, Langton D, Thompson M. Ongoing problems with metal-on-metal hip implants. BMJ. 2012;344:e1349.
15. Lee RK, Nevelos J, Vigdorchik J, Markel DC. Bearing surfaces for hip arthroplasty—is metal-on-metal a passing fancy? Surg Technol Int. 2012;22:243-249.
16. Voleti PB, Baldwin KD, Lee GC. Metal-on-metal vs conventional total hip arthroplasty: a systematic review and meta-analysis of randomized controlled trials. J Arthroplasty. 2012;27(10):1844-1849.
17. Urban JA, Garvin KL, Boese CK, et al. Ceramic-on-polyethylene bearing surfaces in total hip arthroplasty. Seventeen to twenty-one-year results.
J Bone Joint Surg Am. 2001;83(11):1688-1694.
18. Callaghan JJ, Liu SS. Ceramic on crosslinked polyethylene in total hip replacement: any better than metal on crosslinked polyethylene? Iowa Orthop J. 2009;29:1-4.
19. Barrack RL. Dislocation after total hip arthroplasty: implant design and orientation. J Am Acad Orthop Surg. 2003;11(2):89-99.
20. Krushell RJ, Burke DW, Harris WH. Elevated-rim acetabular components. Effect on range of motion and stability in total hip arthroplasty. J Arthroplasty. 1991;6(suppl):S53-S58.
21. Morrey BF. Instability after total hip arthroplasty. Orthop Clin North Am. 1992;23(2):237-248.
22. Morrey BF. Dislocation. In: Morrey BF, ed. Joint Replacement Arthroplasty. New York, NY: Churchill Livingstone; 1991:851-865.
23. Bartz RL, Nobel PC, Kadakia NR, Tullos HS. The effect of femoral component head size on posterior dislocation of the artificial hip joint. J Bone Joint Surg Am. 2000;82(9):1300-1307.
24. Nicholas RM, Orr JF, Mollan RA, Calderwood JW, Nixon JR, Watson P. Dislocation of total hip replacements. A comparative study of standard, long posterior wall and augmented acetabular components. J Bone Joint Surg Br. 1990;72(3):418-422.
25. McCollum DE, Gray WJ. Dislocation after total hip arthroplasty. Causes and prevention. Clin Orthop. 1990;(261):159-170.
26. Herrlin K, Selvik G, Pettersson H, Kesek P, Onnerfalt R, Ohlin A. Position, orientation and component interaction in dislocation of the total hip prosthesis. Acta Radiol. 1988;29(4):441-444.
27. Cinotti G, Lucioli N, Malagoli A, Calderoli C, Cassese F. Do large femoral heads reduce the risks of impingement in total hip arthroplasty with optimal and non-optimal cup positioning? Int Orthop. 2011;35(3):317-323.
28. Burroughs BR, Rubash HE, Harris WH. Femoral head sizes larger than 32 mm against highly cross-linked polyethylene. Clin Orthop. 2002;(405):150-157.
29. Crowninshield RD, Maloney WJ, Wentz DH, Humphrey SM, Blanchard CR. Biomechanics of large femoral heads: what they do and don‘t do. Clin Orthop. 2004;(429):102-107.
30. Charnley J. Low Friction Arthroplasty of the Hip: Theory and Practice. New York, NY: Springer; 1979.
31. Yamaguchi M, Akisue T, Bauer TW, Hashimoto Y. The spatial location of impingement in total hip arthroplasty. J Arthroplasty. 2000;15(3):305-313.
32. Peters CL, McPherson E, Jackson JD, Erickson JA. Reduction in early dislocation rate with large-diameter femoral heads in primary total hip arthroplasty. J Arthroplasty. 2007;22(6 suppl 2):140-144.
33. Masonis JL, Bourne RB. Surgical approach, abductor function, and total hip arthroplasty dislocation. Clin Orthop. 2002;(405):46-53.
34. Beaule PE, Schmalzried TP, Udomkiat P, Amstutz HC. Jumbo femoral head for the treatment of recurrent dislocation following total hip replacement. J Bone Joint Surg Am. 2002;84(2):256-263.
35. Oral E, Malhi AS, Muratoglu OK. Mechanisms of decrease in fatigue crack propagation resistance in irradiated and melted UHMWPE. Biomaterials. 2006;27(6):917-925.
36. Baker DA, Bellare A, Pruitt L. The effects of degree of crosslinking on the fatigue crack initiation and propagation resistance of orthopedic-grade polyethylene. J Biomed Mater Res A. 2003;66(1):146-154.
37. Charnley J. Total hip replacement by low-friction arthroplasty. Clin Orthop. 1970;(72):7-21.
38. Kabo JM, Gebhard JS, Loren G, Amstutz HC. In vivo wear of polyethylene acetabular components. J Bone Joint Surg Br. 1993;75(2):254-258.
39. Livermore J, Ilstrup D, Morrey B. Effect of femoral head size on wear of the polyethylene acetabular component. J Bone Joint Surg Am. 1990;72(4):518-528.
40. Ma SM, Kabo JM, Amstutz HC. Frictional torque in surface and conventional hip replacement. J Bone Joint Surg Am. 1983;65(3):366-370.
41. Muratoglu OK, Bragdon CR, O‘Connor D, et al. Larger diameter femoral heads used in conjunction with a highly cross-linked ultra-high molecular weight polyethylene: a new concept. J Arthroplasty. 2001;16(8 suppl 1):24-30.
42. Thomas GER, Simpson DJ, Mehmood S, et al. The seven-year wear of highly cross-linked polyethylene in total hip arthroplasty: a double-blind, randomized controlled trial using radiostereometric analysis. J Bone Joint Surg Am. 2011;93(8):716-722.
43. Mutimer J, Devane PA, Adams K, Horne JG. Highly crosslinked polyethylene reduces wear in total hip arthroplasty at 5 years. Clin Orthop. 2010;468(12):3228-3233.
44. Bragdon CR, Doerner M, Martell J, Jarrett B, Palm H, Malchau H. The 2012 John Charnley Award: clinical multicenter studies of the wear performance of highly crosslinked remelted polyethylene in THA. Clin Orthop. 2013;471(2):393-402.
45. Lachiewicz PF, Heckman DS, Soileau ES, Mangla J, Martell JM. Femoral head size and wear of highly cross-linked polyethylene at 5 to 8 years. Clin Orthop. 2009;467(12):3290-3296.
46. Sychterz CJ, Engh CA Jr, Young AM, Hopper RH Jr, Engh CA. Comparison of in vivo wear between polyethylene liners articulating with ceramic and cobalt-chrome femoral heads. J Bone Joint Surg Br. 2000;82(7):948-951.
47. Berry DJ, Barnes CL, Scott RD, Cabanela ME, Poss R. Catastrophic failure of the polyethylene liner of uncemented acetabular components.
J Bone Joint Surg Br. 1994;76(4):575-578.
48. McKellop H, Shen FW, Lu B, Campbell P, Salovey R. Development of an extremely wear-resistant ultra high molecular weight polyethylene for total hip replacements. J Orthop Res. 1999;17(2):157-167.
49. Wang A, Essner A, Polineni VK, Stark C, Dumbleton JH. Lubrication and wear of ultra-high molecular weight polyethylene in total joint replacements. Tribol Int. 1998;31(1-3):17-33.
50. Estok DM 2nd, Burroughs BR, Muratoglu OK, Harris WH. Comparison of hip simulator wear of 2 different highly cross-linked ultra high molecular weight polyethylene acetabular components using both 32- and 38-mm femoral heads. J Arthroplasty. 2007;22(4):581-589.
51. Muratoglu OK, Bragdon CR, O‘Connor DO, et al. Unified wear model for highly crosslinked ultra-high molecular weight polyethylenes (UHMWPE). Biomaterials. 1999;20(16):1463-1470.
52. Harris WH, Muratoglu OK. A review of current cross-linked polyethylenes used in total joint arthroplasty. Clin Orthop. 2005;(430):46-52.
53. Muratoglu OK, Bragdon CR, O‘Connor DO, Jasty M, Harris WH. A novel method of cross-linking ultra-high-molecular-weight polyethylene to improve wear, reduce oxidation, and retain mechanical properties. Recipient of the 1999 HAP Paul Award. J Arthroplasty. 2001;16(2):149-160.
54. Bal BS, Garino J, Ries M, Rahaman MN. A review of ceramic bearing materials in total joint arthroplasty. Hip Int. 2007;17(1):21-30.
55. Traina F, De Fine M, Di Martino A, Faldini C. Fracture of ceramic bearing surfaces following total hip replacement: a systematic review. Biomed Res Int. 2013;2013:157247.
56. Cai YZ, Yan SG. Development of ceramic-on-ceramic implants for total hip arthroplasty. Orthop Surg. 2010;2(3):175-181.
57. Stewart TD, Tipper JL, Insley G, Streicher RM, Ingham E, Fisher J. Long-term wear of ceramic matrix composite materials for hip prostheses under severe swing phase microseparation. J Biomed Mater Res B Appl Biomater. 2003;66(2):567-573.
58. Bragdon CR, Greene ME, Freiberg AA, Harris WH, Malchau H. Radiostereometric analysis comparison of wear of highly cross-linked polyethylene against 36- vs 28-mm femoral heads. J Arthroplasty. 2007;22(6 suppl 2):125-129.
59. Lombardi AV Jr, Skeels MD, Berend KR, Adams JB, Franchi OJ. Do large heads enhance stability and restore native anatomy in primary total hip arthroplasty? Clin Orthop. 2011;469(6):1547-1553.
60. Lachiewicz PF, Soileau ES. Low early and late dislocation rates with 36- and 40-mm heads in patients at high risk for dislocation. Clin Orthop. 2013;471(2):439-443.
61. Cai P, Hu Y, Xie J. Large-diameter Delta ceramic-on-ceramic versus common-sized ceramic-on-polyethylene bearings in THA. Orthopedics. 2012;35(9):e1307-e1313.
62. Park KS, Yoon TR, Hwang SY, Lee KB. Modified minimally invasive two-incision total hip arthroplasty using large diameter femoral head. Indian
J Orthop. 2012;46(1):29-35.
63. Garbuz DS, Masri BA, Duncan CP, et al. The Frank Stinchfield Award: dislocation in revision THA: do large heads (36 and 40 mm) result in reduced dislocation rates in a randomized clinical trial? Clin Orthop. 2012;470(2):351-356.
64. Geller JA, Malchau H, Bragdon C, Greene M, Harris WH, Freiberg AA. Large diameter femoral heads on highly cross-linked polyethylene: minimum 3-year results. Clin Orthop. 2006;(447):53-59.
65. D’Antonio JA, Capello WN, Ramakrishnan R. Second-generation annealed highly cross-linked polyethylene exhibits low wear. Clin Orthop. 2012;470(6):1696-1704.
66. Galvin AL, Jennings LM, Tipper JL, Ingham E, Fisher J. Wear and creep of highly crosslinked polyethylene against cobalt chrome and ceramic femoral heads. Proc Inst Mech Eng H. 2010;224(10):1175-1183.
67. Skeels MD, Berend KR, Lombardi AV Jr. The dislocator, early and late: the role of large heads. Orthopedics. 2009;32(9).
68. Plate JF, Seyler TM, Stroh DA, Issa K, Akbar M, Mont MA. Risk of dislocation using large- vs. small-diameter femoral heads in total hip arthroplasty. BMC Res Notes. 2012;5(1):553.
69. Bouchet R, Mercier N, Saragaglia D. Posterior approach and dislocation rate: a 213 total hip replacements case–control study comparing the dual mobility cup with a conventional 28-mm metal head/polyethylene prosthesis. Orthop Traumatol Surg Res. 2011;97(1):2-7.
70. Ali Khan MA, Brakenbury PH, Reynolds IS. Dislocation following total hip replacement. J Bone Joint Surg Br. 1981;63(2):214-218.
71. Howie DW, Holubowycz OT, Middleton R. Large femoral heads decrease the incidence of dislocation after total hip arthroplasty: a randomized controlled trial. J Bone Joint Surg Am. 2012;94(12):1095-1102.
72. Malkani AL, Ong KL, Lau E, Kurtz SM, Justice BJ, Manley MT. Early- and late-term dislocation risk after primary hip arthroplasty in the Medicare population. J Arthroplasty. 2010;25(6 suppl):21-25.
73. Berry DJ, von Knoch M, Schleck CD, Harmsen WS. Effect of femoral head diameter and operative approach on risk of dislocation after primary total hip arthroplasty. J Bone Joint Surg Am. 2005;87(11):2456-2463.
74. Cho MR, Lee HS, Lee SW, Choi CH, Kim SK, Ko SB. Results after total hip arthroplasty with a large head and bipolar arthroplasty in patients with displaced femoral neck fractures. J Arthroplasty. 2011;26(6):893-896.
75. Conroy JL, Whitehouse SL, Graves SE, Pratt NL, Ryan P, Crawford RW. Risk factors for revision for early dislocation in total hip arthroplasty.
J Arthroplasty. 2008;23(6):867-872.
76. Kung PL, Ries MD. Effect of femoral head size and abductors on dislocation after revision THA. Clin Orthop. 2007;(465):170-174.
77. Williams JT Jr, Ragland PS, Clarke S. Constrained components for the unstable hip following total hip arthroplasty: a literature review. Int Orthop. 2007;31(3):273-277.
78. Zywiel MG, Mustafa LH, Bonutti PM, Mont MA. Are abductor muscle quality and previous revision surgery predictors of constrained liner failure in hip arthroplasty? Int Orthop. 2011;35(6):797-802.
79. Guyen O, Lewallen DG, Cabanela ME. Modes of failure of Osteonics constrained tripolar implants: a retrospective analysis of forty-three failed implants. J Bone Joint Surg Am. 2008;90(7):1553-1560.
80. Banks LN, McElwain JP. An unusual mode of failure of a tripolar constrained acetabular liner: a case report. Arch Orthop Trauma Surg. 2010;130(4):503-505.
81. Robertson WJ, Mattern CJ, Hur J, Su EP, Pellicci PM. Failure mechanisms and closed reduction of a constrained tripolar acetabular liner.
J Arthroplasty. 2009;24(2):322.e5-e11.
82. Aubriot JH, Lesimple P, Leclercq S. Study of Bousquet‘s non-cemented acetabular implant in 100 hybrid total hip prostheses (Charnley type cemented femoral component). Average 5-year follow-up [in French]. Acta Orthop Belg. 1993;59(suppl 1):267-271.
83. Farizon F, de Lavison R, Azoulai JJ, Bousquet G. Results with a cementless alumina-coated cup with dual mobility. A twelve-year follow-up study. Int Orthop. 1998;22(4):219-224.
84. Mertl P, Combes A, Leiber-Wackenheim F, Fessy MH, Girard J, Migaud H. Recurrence of dislocation following total hip arthroplasty revision using dual mobility cups was rare in 180 hips followed over 7 years. HSS J. 2012;8(3):251-256.
85. Langlais FL, Ropars M, Gaucher F, Musset T, Chaix O. Dual mobility cemented cups have low dislocation rates in THA revisions. Clin Orthop. 2008;466(2):389-395.
86. Philippot R, Farizon F, Camilleri JP, et al. Survival of dual mobility socket with a mean 17 years follow-up [in French]. Rev Chir Orthop Reparatrice Appar Mot. 2008;94(1):43-48.
87. Adam P, Philippe R, Ehlinger M, et al. Dual mobility cups hip arthroplasty as a treatment for displaced fracture of the femoral neck in the elderly.
A prospective, systematic, multicenter study with specific focus on postoperative dislocation. Orthop Traumatol Surg Res. 2012;98(3):296-300.
88. Fessy MH. La double mobilité [Dual mobility]. Revue de Chirurgie Orthopédique et Traumatologique. 2010;96(7):891-898.
89. Mont MA, Issa K, Naziri Q, Harwin SF, Delanois RE, Johnson AJ. The use of dual-mobility bearings in difficult hip arthroplasty reconstructive cases. Surg Technol Int. 2011;21:234-240.
90. Sayeed SA, Mont MA, Costa CR, et al. Early outcomes of sequentially cross-linked thin polyethylene liners with large diameter femoral heads in total hip arthroplasty. Bull NYU Hosp Jt Dis. 2011;69(suppl 1):S90-S94.
91. Plate JF, Seyler TM, Stroh DA, Issa K, Akbar M, Mont MA. Risk of dislocation using large- vs. small-diameter femoral heads in total hip arthroplasty. BMC Res Notes. 2012;5(1):553.
92. Sato T, Nakashima Y, Akiyama M, et al. Wear resistant performance of highly cross-linked and annealed ultra-high molecular weight polyethylene against ceramic heads in total hip arthroplasty. J Orthop Res. 2012;30(12):2031-2037.
93. Meftah M, Ebrahimpour PB, He C, Ranawat AS, Ranawat CS. Preliminary clinical and radiographic results of large ceramic heads on highly cross-linked polyethylene. Orthopedics. 2011;34(6):133.
94. Gagala J, Mazurkiewicz T, Dajewski Z. Large diameter femoral heads in primary alumina/alumina and XSPE/alumina total hip arthroplasty.
A follow-up study of 50 hips after average 40 months and review of literature [in Polish]. Chir Narzadow Ruchu Ortop Pol. 2011;76(1):14-20.
Effect of Acetabular Cup Abduction Angle on Wear of Ultrahigh-Molecular-Weight Polyethylene in Hip Simulator Testing
Navigation in Total Knee Arthroplasty: Truth, Myths, and Controversies
The overall success of total knee arthroplasty (TKA) depends on proper implant choice, meticulous surgical technique, appropriate patient selection, and
effective postoperative rehabilitation. Inappropriate technique leads to suboptimal placement of implants in coronal, sagittal, or axial planes.1-3 This results in eccentric prosthetic loading, which may contribute to accelerated polyethylene wear, early component loosening, higher rates of revision surgery, and unsatisfactory clinical outcomes. The need to optimize component positioning during TKA stimulated the development of computer-assisted navigation in TKA in the late 1990’s. Proponents of this technology believe that it helps to reduce outliers, improves coronal, sagittal, and rotational alignment, and optimizes flexion and extension gap-balancing. This is believed to result in improved implant survival and better functional outcomes. However, despite these postulated advantages, less than 5% of surgeons in the United States currently use navigation during TKA perhaps due to concerns of costs, increased operating time, learn- ing curve issues, and lack of improvement in functional outcomes at mid-term follow-up.
Navigated TKA, due to its accuracy and low margins of error, has the potential to reduce component malalignment to within 1o to 2o of neutral mechanical axis.4 However, others have reported that alignment of the femoral and tibial components achieved with computer navigation is not different than TKA using conventional techniques.5-12 This lack of improvement reported in these studies may be due to a number of potential sources of errors, which can be either surgeon- or device-related. These errors may pre-dispose to discrepancies between alignments calculated by the computer and the actual position of the implants. Apart from software- and hardware-related calibration issues, the majority of inaccuracies, which are often surgeon-related, result from registration of anatomical landmarks, pin array movements after registration, incorrect bone cuts despite accurate jig placement, and incorrect placement of final components during cementation. Of these surgeon-related factors, variability in the identification of the anatomical landmarks appears to be critical and occurs due to anatomical variations or from inaccurate recognition of intraoperative bony landmarks. A recent study found that registration of the distal femoral epicondyles was more likely to be inaccurate than other anatomical landmarks, as it was found that a small change of 2 mm in the sagittal plane can lead to a 1o change in the femoral component rotation.13
Nevertheless, the general consensus from recent high- level evidence (Level I and II) suggests that navigated TKA leads to improved coronal-alignment outcomes and reduced numbers of outliers.14-18 In a recent systematic review of 27 randomized controlled trials of 2541 patients, Hetaimish and colleagues19 compared the alignment outcomes of navigated with conventional TKA. The authors found that the navigated cohort had a significantly lower risk of producing a mechanical axis deviation of greater than 3o, compared with conventional TKA (relative risk [RR] = 0.37; P<.001). The femoral and tibial, coronal and sagittal malalignment (>3o) were also found to be significantly lower with navigated TKA, compared with conventional techniques. However, no substantial differences were found in the rotation alignment of the femoral component between the 2 comparison cohorts (navigated group, 18.8%; conventional group, 14.5%).
Advocates of navigation believe that improved component alignment would lead to better functional outcomes and lower revision rates.20,21 However, at short- to mid-term follow-up, most studies have failed to show any substantial benefits in terms of functional outcomes, revision rates, patient satisfaction, or patient-perceived quality-of-life, when comparing computer-assisted navigation to conventional techniques.11,22-25 Recent systematic reviews by Zamora et al24 and Burnett et al25 found no significant differences in the functional outcomes between navigated and conventional TKA (P>.05). This lack of the expected improvement in functional outcomes reported in various studies with navigation could be due to variability in registration of anatomical landmarks leading to errors in the rotational axis, or a lack of complete understanding of the interplay of alignment, ligament balance, in vivo joint loading and kinematics. In a report from the Mayo Clinic,26 the authors believed that there may be little practical value in relying on a mechanical alignment of ±3o from neutral as an isolated variable in predicting the longevity of modern TKA. In addition, they suggested that factors apart from mechanical alignment may have a more profound impact on implant durability.
Several studies27-31 that compared the joint line changes or ligament balance between navigated and conventional TKA, report no substantial differences in the maintenance of the joint line, quality of life, and functional outcomes. Despite claims of decreased blood loss, length-of stay, cardiac complications, and lower risks of fat embolism with computer-assisted navigation by some authors, other reports have failed to demonstrate any substantial advantages, therefore, it is controversial if any clear benefit exists.6,32-34 It is postulated that the high initial institutional costs of navigation can even out in the long run if the goals of improved survivorship and functional outcomes are achieved.35 However, as mid-term follow-up studies have failed to show a survival or functional benefit, the purported costs savings from computer navigation may not be accurate. Navigated TKA has been reported to increase operative time by about 15 to 20 minutes, compared with conventional TKA. Although, this increases operative time, it has not been reported to increase the risk of deep prosthetic joint infections.
Navigation provides some benefits in terms of radiological alignment. However, the clinical advantages are yet to be defined. Currently, there are many unanswered ques- tions concerning alignment in TKA, such as having a more individual approach based on the patients’ own anatomic variations including considerations about the presence of constitutional varus in patients. Navigation may have a role when TKA is performed for complex deformities, fractures, or in the presence of retained implants that prevent the use of conventional guides. Nevertheless, one should always keep in mind cost considerations. This has been true with any technological advancement we have had in the past and will be of concern in the future as well, especially with rising healthcare costs. When analyzing costs with naviga- tion, one must take in to account not only the overall costs of technology, but also the added costs of training, increased operating room times, and disposables when performing these procedures. Although we are advocates of change and are excited about this technology, the cost-benefit ratio for computer navigated TKA needs to be reconciled.
The overall success of total knee arthroplasty (TKA) depends on proper implant choice, meticulous surgical technique, appropriate patient selection, and
effective postoperative rehabilitation. Inappropriate technique leads to suboptimal placement of implants in coronal, sagittal, or axial planes.1-3 This results in eccentric prosthetic loading, which may contribute to accelerated polyethylene wear, early component loosening, higher rates of revision surgery, and unsatisfactory clinical outcomes. The need to optimize component positioning during TKA stimulated the development of computer-assisted navigation in TKA in the late 1990’s. Proponents of this technology believe that it helps to reduce outliers, improves coronal, sagittal, and rotational alignment, and optimizes flexion and extension gap-balancing. This is believed to result in improved implant survival and better functional outcomes. However, despite these postulated advantages, less than 5% of surgeons in the United States currently use navigation during TKA perhaps due to concerns of costs, increased operating time, learn- ing curve issues, and lack of improvement in functional outcomes at mid-term follow-up.
Navigated TKA, due to its accuracy and low margins of error, has the potential to reduce component malalignment to within 1o to 2o of neutral mechanical axis.4 However, others have reported that alignment of the femoral and tibial components achieved with computer navigation is not different than TKA using conventional techniques.5-12 This lack of improvement reported in these studies may be due to a number of potential sources of errors, which can be either surgeon- or device-related. These errors may pre-dispose to discrepancies between alignments calculated by the computer and the actual position of the implants. Apart from software- and hardware-related calibration issues, the majority of inaccuracies, which are often surgeon-related, result from registration of anatomical landmarks, pin array movements after registration, incorrect bone cuts despite accurate jig placement, and incorrect placement of final components during cementation. Of these surgeon-related factors, variability in the identification of the anatomical landmarks appears to be critical and occurs due to anatomical variations or from inaccurate recognition of intraoperative bony landmarks. A recent study found that registration of the distal femoral epicondyles was more likely to be inaccurate than other anatomical landmarks, as it was found that a small change of 2 mm in the sagittal plane can lead to a 1o change in the femoral component rotation.13
Nevertheless, the general consensus from recent high- level evidence (Level I and II) suggests that navigated TKA leads to improved coronal-alignment outcomes and reduced numbers of outliers.14-18 In a recent systematic review of 27 randomized controlled trials of 2541 patients, Hetaimish and colleagues19 compared the alignment outcomes of navigated with conventional TKA. The authors found that the navigated cohort had a significantly lower risk of producing a mechanical axis deviation of greater than 3o, compared with conventional TKA (relative risk [RR] = 0.37; P<.001). The femoral and tibial, coronal and sagittal malalignment (>3o) were also found to be significantly lower with navigated TKA, compared with conventional techniques. However, no substantial differences were found in the rotation alignment of the femoral component between the 2 comparison cohorts (navigated group, 18.8%; conventional group, 14.5%).
Advocates of navigation believe that improved component alignment would lead to better functional outcomes and lower revision rates.20,21 However, at short- to mid-term follow-up, most studies have failed to show any substantial benefits in terms of functional outcomes, revision rates, patient satisfaction, or patient-perceived quality-of-life, when comparing computer-assisted navigation to conventional techniques.11,22-25 Recent systematic reviews by Zamora et al24 and Burnett et al25 found no significant differences in the functional outcomes between navigated and conventional TKA (P>.05). This lack of the expected improvement in functional outcomes reported in various studies with navigation could be due to variability in registration of anatomical landmarks leading to errors in the rotational axis, or a lack of complete understanding of the interplay of alignment, ligament balance, in vivo joint loading and kinematics. In a report from the Mayo Clinic,26 the authors believed that there may be little practical value in relying on a mechanical alignment of ±3o from neutral as an isolated variable in predicting the longevity of modern TKA. In addition, they suggested that factors apart from mechanical alignment may have a more profound impact on implant durability.
Several studies27-31 that compared the joint line changes or ligament balance between navigated and conventional TKA, report no substantial differences in the maintenance of the joint line, quality of life, and functional outcomes. Despite claims of decreased blood loss, length-of stay, cardiac complications, and lower risks of fat embolism with computer-assisted navigation by some authors, other reports have failed to demonstrate any substantial advantages, therefore, it is controversial if any clear benefit exists.6,32-34 It is postulated that the high initial institutional costs of navigation can even out in the long run if the goals of improved survivorship and functional outcomes are achieved.35 However, as mid-term follow-up studies have failed to show a survival or functional benefit, the purported costs savings from computer navigation may not be accurate. Navigated TKA has been reported to increase operative time by about 15 to 20 minutes, compared with conventional TKA. Although, this increases operative time, it has not been reported to increase the risk of deep prosthetic joint infections.
Navigation provides some benefits in terms of radiological alignment. However, the clinical advantages are yet to be defined. Currently, there are many unanswered ques- tions concerning alignment in TKA, such as having a more individual approach based on the patients’ own anatomic variations including considerations about the presence of constitutional varus in patients. Navigation may have a role when TKA is performed for complex deformities, fractures, or in the presence of retained implants that prevent the use of conventional guides. Nevertheless, one should always keep in mind cost considerations. This has been true with any technological advancement we have had in the past and will be of concern in the future as well, especially with rising healthcare costs. When analyzing costs with naviga- tion, one must take in to account not only the overall costs of technology, but also the added costs of training, increased operating room times, and disposables when performing these procedures. Although we are advocates of change and are excited about this technology, the cost-benefit ratio for computer navigated TKA needs to be reconciled.
The overall success of total knee arthroplasty (TKA) depends on proper implant choice, meticulous surgical technique, appropriate patient selection, and
effective postoperative rehabilitation. Inappropriate technique leads to suboptimal placement of implants in coronal, sagittal, or axial planes.1-3 This results in eccentric prosthetic loading, which may contribute to accelerated polyethylene wear, early component loosening, higher rates of revision surgery, and unsatisfactory clinical outcomes. The need to optimize component positioning during TKA stimulated the development of computer-assisted navigation in TKA in the late 1990’s. Proponents of this technology believe that it helps to reduce outliers, improves coronal, sagittal, and rotational alignment, and optimizes flexion and extension gap-balancing. This is believed to result in improved implant survival and better functional outcomes. However, despite these postulated advantages, less than 5% of surgeons in the United States currently use navigation during TKA perhaps due to concerns of costs, increased operating time, learn- ing curve issues, and lack of improvement in functional outcomes at mid-term follow-up.
Navigated TKA, due to its accuracy and low margins of error, has the potential to reduce component malalignment to within 1o to 2o of neutral mechanical axis.4 However, others have reported that alignment of the femoral and tibial components achieved with computer navigation is not different than TKA using conventional techniques.5-12 This lack of improvement reported in these studies may be due to a number of potential sources of errors, which can be either surgeon- or device-related. These errors may pre-dispose to discrepancies between alignments calculated by the computer and the actual position of the implants. Apart from software- and hardware-related calibration issues, the majority of inaccuracies, which are often surgeon-related, result from registration of anatomical landmarks, pin array movements after registration, incorrect bone cuts despite accurate jig placement, and incorrect placement of final components during cementation. Of these surgeon-related factors, variability in the identification of the anatomical landmarks appears to be critical and occurs due to anatomical variations or from inaccurate recognition of intraoperative bony landmarks. A recent study found that registration of the distal femoral epicondyles was more likely to be inaccurate than other anatomical landmarks, as it was found that a small change of 2 mm in the sagittal plane can lead to a 1o change in the femoral component rotation.13
Nevertheless, the general consensus from recent high- level evidence (Level I and II) suggests that navigated TKA leads to improved coronal-alignment outcomes and reduced numbers of outliers.14-18 In a recent systematic review of 27 randomized controlled trials of 2541 patients, Hetaimish and colleagues19 compared the alignment outcomes of navigated with conventional TKA. The authors found that the navigated cohort had a significantly lower risk of producing a mechanical axis deviation of greater than 3o, compared with conventional TKA (relative risk [RR] = 0.37; P<.001). The femoral and tibial, coronal and sagittal malalignment (>3o) were also found to be significantly lower with navigated TKA, compared with conventional techniques. However, no substantial differences were found in the rotation alignment of the femoral component between the 2 comparison cohorts (navigated group, 18.8%; conventional group, 14.5%).
Advocates of navigation believe that improved component alignment would lead to better functional outcomes and lower revision rates.20,21 However, at short- to mid-term follow-up, most studies have failed to show any substantial benefits in terms of functional outcomes, revision rates, patient satisfaction, or patient-perceived quality-of-life, when comparing computer-assisted navigation to conventional techniques.11,22-25 Recent systematic reviews by Zamora et al24 and Burnett et al25 found no significant differences in the functional outcomes between navigated and conventional TKA (P>.05). This lack of the expected improvement in functional outcomes reported in various studies with navigation could be due to variability in registration of anatomical landmarks leading to errors in the rotational axis, or a lack of complete understanding of the interplay of alignment, ligament balance, in vivo joint loading and kinematics. In a report from the Mayo Clinic,26 the authors believed that there may be little practical value in relying on a mechanical alignment of ±3o from neutral as an isolated variable in predicting the longevity of modern TKA. In addition, they suggested that factors apart from mechanical alignment may have a more profound impact on implant durability.
Several studies27-31 that compared the joint line changes or ligament balance between navigated and conventional TKA, report no substantial differences in the maintenance of the joint line, quality of life, and functional outcomes. Despite claims of decreased blood loss, length-of stay, cardiac complications, and lower risks of fat embolism with computer-assisted navigation by some authors, other reports have failed to demonstrate any substantial advantages, therefore, it is controversial if any clear benefit exists.6,32-34 It is postulated that the high initial institutional costs of navigation can even out in the long run if the goals of improved survivorship and functional outcomes are achieved.35 However, as mid-term follow-up studies have failed to show a survival or functional benefit, the purported costs savings from computer navigation may not be accurate. Navigated TKA has been reported to increase operative time by about 15 to 20 minutes, compared with conventional TKA. Although, this increases operative time, it has not been reported to increase the risk of deep prosthetic joint infections.
Navigation provides some benefits in terms of radiological alignment. However, the clinical advantages are yet to be defined. Currently, there are many unanswered ques- tions concerning alignment in TKA, such as having a more individual approach based on the patients’ own anatomic variations including considerations about the presence of constitutional varus in patients. Navigation may have a role when TKA is performed for complex deformities, fractures, or in the presence of retained implants that prevent the use of conventional guides. Nevertheless, one should always keep in mind cost considerations. This has been true with any technological advancement we have had in the past and will be of concern in the future as well, especially with rising healthcare costs. When analyzing costs with naviga- tion, one must take in to account not only the overall costs of technology, but also the added costs of training, increased operating room times, and disposables when performing these procedures. Although we are advocates of change and are excited about this technology, the cost-benefit ratio for computer navigated TKA needs to be reconciled.