User login
Unrelated Death After Colorectal Cancer Screening: Implications for Improving Colonoscopy Referrals
Colorectal cancer (CRC) ranks among the most common causes of cancer and cancer-related death in the US. The US Multi-Society Task Force (USMSTF) on Colorectal Cancer thus strongly endorsed using several available screening options.1 The published guidelines largely rely on age to define the target population (Table 1). For average-risk individuals, national and Veterans Health Administration (VHA) guidelines currently recommend CRC screening in individuals aged between 50 and 75 years with a life expectancy of > 5 years.1
Although case-control studies also point to a potential benefit in persons aged > 75 years,2,3 the USMSTF cited less convincing evidence and suggested an individualized approach that should consider relative cancer risk and comorbidity burden. Such an approach is supported by modeling studies, which suggest reduced benefit and increased risk of screening with increasing age. The reduced benefit also is significantly affected by comorbidity and relative cancer risk.4 The VHA has successfully implemented CRC screening, capturing the majority of eligible patients based on age criteria. A recent survey showed that more than three-quarters of veterans between age 50 and 75 years had undergone some screening test for CRC as part of routine preventive care. Colonoscopy clearly emerged as the dominant modality chosen for CRC screening and accounted for nearly 84% of these screening tests.5 Consistent with these data, a case-control study confirmed that the widespread implementation of colonoscopy as CRC screening method reduced cancer-related mortality in veterans for cases of left but not right-sided colon cancer.6
With calls to expand the age range of CRC screening beyond aged 75 years, we decided to assess survival rates of a cohort of veterans who underwent a screening or surveillance colonoscopy between 2008 and 2014.7 The goals were to characterize the portion of the cohort that had died, the time between a screening colonoscopy and death, the portion of deaths that were aged ≥ 80 years, and the causes of the deaths. In addition, we focused on a subgroup of the cohort, defined by death within 2 years after the index colonoscopy, to identify predictors of early death that were independent of age.
Methods
We queried the endoscopy reporting system (EndoWorks; Olympus America, Center Valley, PA) for all colonoscopies performed by 2 of 14 physicians at the George Wahlen VA Medical Center (GWVAMC) in Salt Lake City, Utah, who performed endoscopic procedures between January 1, 2008 and December 1, 2014. These physicians had focused their clinical practice exclusively on elective outpatient colonoscopies and accounted for 37.4% of the examinations at GWVAMC during the study period. All colonoscopy requests were triaged and assigned based on availability of open and appropriate procedure time slots without direct physician-specific referral, thus reducing the chance of skewing results. The reports were filtered through a text search to focus on examinations that listed screening or surveillance as indication. The central patient electronic health record was then reviewed to extract basic demographic data, survival status (as of August 1, 2018), and survival time in years after the index or subsequent colonoscopy. For deceased veterans, the age at the time of death, cause of death, and comorbidities were queried.
This study compared cases and control across the study. Cases were persons who clearly died early (defined as > 2 years following the index examination). They were matched with controls who lived for ≥ 5 years after their colonoscopy. These periods were selected because the USMSTF recommended that CRC screening or surveillance colonoscopy should be discontinued in persons with a life expectancy of < 5 years, and most study patients underwent their index procedure ≥ 5 years before August 2018. Cases and controls underwent a colonoscopy in the same year and were matched for age, sex, and presence of underlying inflammatory bowel disease (IBD). For cases and controls, we identified the ordering health care provider specialty, (ie, primary care, gastroenterology, or other).
In addition, we reviewed the encounter linked to the order and abstracted relevant comorbidities listed at that time, noted the use of anticoagulants, opioid analgesics, and benzodiazepines. The comorbidity burden was quantified using the Charlson Comorbidity Index.8 In addition, we denoted the presence of psychiatric problems (eg, anxiety, depression, bipolar disease, psychosis, substance abuse), the diagnosis of atrial fibrillation (AF) or other cardiac arrhythmias, and whether the patient had previously been treated for a malignancy that was in apparent clinical remission. Finally, we searched for routine laboratory tests at the time of this visit or, when not obtained, within 6 months of the encounter, and abstracted serum creatinine, hemoglobin (Hgb), platelet number, serum protein, and albumin. In clinical practice, cutoff values of test results are often more helpful in decision making. We, therefore, dichotomized results for Hgb (cutoff: 10 g/dL), creatinine (cutoff: 2 mg/dL), and albumin (cutoff: 3.2 mg/dL).
Descriptive and analytical statistics were obtained with Stata Version 14.1 (College Station, TX). Unless indicated otherwise, continuous data are shown as mean with 95% CIs. For dichotomous data, we used percentages with their 95% CIs. Analytic statistics were performed with the t test for continuous variables and the 2-tailed test for proportions. A P < .05 was considered a significant difference. To determine independent predictors of early death, we performed a logistic regression analysis with results being expressed as odds ratio with 95% CIs. Survival status was chosen as a dependent variable, and we entered variables that significantly correlated with survival in the bivariate analysis as independent variables.
The study was designed and conducted as a quality improvement project to assess colonoscopy performance and outcomes with the Salt Lake City Specialty Care Center of Innovation (COI), one of 5 regional COIs with an operational mission to improve health care access, utilization, and quality. Our work related to colonoscopy and access within the COI region, including Salt Lake City, has been reviewed and acknowledged by the GWVAMC Institutional Review Board as quality improvement. Andrew Gawron has an operational appointment in the GWVAMC COI, which is part of a US Department of Veterans Affairs (VA) central office initiative established in 2015. The COIs are charged with identifying best practices within the VA and applying those practices throughout the COI region. This local project to identify practice patterns and outcomes locally was sponsored by the GWVAMC COI with a focus to generate information to improve colonoscopy referral quality in patients at Salt Lake City and inform regional and national efforts in this domain.
Results
During the study period, 4,879 veterans (96.9% male) underwent at least 1 colonoscopy for screening or surveillance by 1 of the 2 providers. A total of 306 persons (6.3%) were aged > 80 years. The indication for surveillance colonoscopies included IBD in 78 (1.6%) veterans 2 of whom were women. The mean (SD) follow-up period between the index colonoscopy and study closure or death was 7.4 years (1.7). During the study time, 1,439 persons underwent a repeat examination for surveillance. The percentage of veterans with at least 1 additional colonoscopy after the index test was significantly higher in patients with known IBD compared with those without IBD (78.2% vs 28.7%; P < .01).
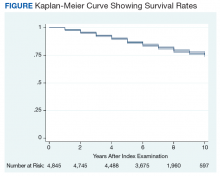
Between the index colonoscopy and August 2018, 974 patients (20.0%) died (Figure). The mean (SD) time between the colonoscopy and recorded year of death was 4.4 years (4.1). The fraction of women in the cohort that died (n = 18) was lower compared with 132 for the group of persons still alive (1.8% vs 3.4%; P < .05). The fraction of veterans with IBD who died by August 2018 did not differ from that of patients with IBD in the cohort of individuals who survived (19.2% vs 20.0%; P = .87). The cohort of veterans who died before study closure included 107 persons who were aged > 80 years at the time of their index colonoscopy, which is significantly more than in the cohort of persons still alive (11.0% vs 5.1%; P < .01).
Cause of Death
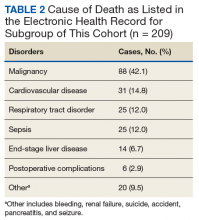
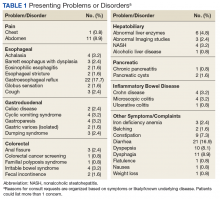
In 209 of the 974 (21.5%) veteran deaths a cause was recorded. Malignancies accounted for 88 of the deaths (42.1%), and CRCs were responsible for 14 (6.7%) deaths (Table 2). In 8 of these patients, the cancer had been identified at an advanced stage, not allowing for curative therapy. One patient had been asked to return for a repeat test as residual fecal matter did not allow proper visualization. He died 1 year later due to complications of sepsis after colonic perforation caused by a proximal colon cancer. Five patients underwent surgery with curative intent but suffered recurrences. In addition to malignancies, advanced diseases, such as cardiovascular, bronchopulmonary illnesses, and infections, were other commonly listed causes of death.
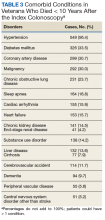
We also abstracted comorbidities that were known at the time of death or the most recent encounter within the VHA system. Hypertension was most commonly listed (549) followed by a current or prior diagnosis of malignancies (355) and diabetes mellitus (DM) (Table 3). Prostate cancer was the most commonly diagnosed malignancy (80), 17 of whom had a second malignancy. CRC accounted for 54 of the malignancies, 1 of which developed in a patient with long-standing ulcerative colitis, 2 were a manifestation of a known hereditary cancer syndrome (Lynch syndrome), and the remaining 51 cases were various cancers without known predisposition. The diagnosis of CRC was made during the study period in 29 veterans. In the remaining 25 patients, the colonoscopy was performed as a surveillance examination after previous surgery for CRC.
Potential Predictors of Early Death
To better define potential predictors of early death, we focused on the 258 persons (5.3%) who died within 2 years after the index procedure and paired them with matched controls. One patient underwent a colonoscopy for surveillance of previously treated cancer and was excluded due to very advanced age, as no matched control could be identified. The mean (SD) age of this male-predominant cohort was 68.2 (9.6) vs 67.9 (9.4) years for cases and controls, respectively. At the time of referral for the test, 29 persons (11.3%) were aged > 80 years, which is significantly more than seen for the overall cohort with 306 (6.3%; P < .001). While primary care providers accounted for most referrals in cases (85.2%) and controls (93.0%), the fraction of veterans referred by gastroenterologists or other specialty care providers was significantly higher in the case group compared with that in the controls (14.8% vs 7.0%; P < .05).
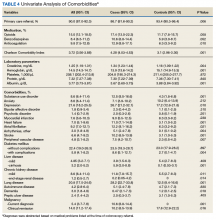
In our age-matched analysis, we examined other potential factors that could influence survival. The burden of comorbid conditions summarized in the Charlson Comorbidity Index significantly correlated with survival status (Table 4). As this composite index does not include psychiatric conditions, we separately examined the impact of anxiety, depression, bipolar disease, psychotic disorders, and substance abuse. The diagnoses of depression and substance use disorders (SUDs) were associated with higher rates of early death. Considering concerns about SUDs, we also assessed the association between prescription for opioids or benzodiazepines and survival status, which showed a marginal correlation. Anticoagulant use, a likely surrogate for cardiovascular disorders, were more commonly listed in the cases than they were in the controls.
Looking at specific comorbid conditions, significant problems affecting key organ systems from heart to lung, liver, kidneys, or brain (dementia) were all predictors of poor outcome. Similarly, DM with secondary complication correlated with early death after the index procedure. In contrast, a history of prior myocardial infarction, prior cancer treatment without evidence of persistent or recurrent disease, or prior peptic ulcer disease did not differ between cases and controls. Focusing on routine blood tests, we noted marginal, but statistically different results for Hgb, serum creatinine, and albumin in cases compared with controls.
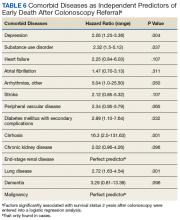
Next we performed a logistic regression to identify independent predictors of survival status. The referring provider specialty, Charlson Comorbidity Index, the diagnosis of a SUD, current benzodiazepine use, and significant anemia or hypoalbuminemia independently predicted death within 2 years of the index examination (Table 5). Considering the composite nature of the Charlson Comorbidity Index, we separately examined the relative importance of different comorbid conditions using a logistic regression analysis. Consistent with the univariate analyses, a known malignancy; severe liver, lung, or kidney disease; and DM with secondary complications were associated with poor outcome. Only arrhythmias other than AF were independent marginal predictors of early death, whereas other variables related to cardiac performance did not reach the level of significance (Table 6). As was true for our analysis examining the composite comorbidity index, the diagnosis of a SUD remained significant as a predictor of death within 2 years of the index colonoscopy.
Discussion
This retrospective analysis followed patients for a mean time of 7 years after a colonoscopy for CRC screening or polyp surveillance. We noted a high rate of all-cause mortality, with 20% of the cohort dying within the period studied. Malignancies, cardiovascular diseases, and advanced lung diseases were most commonly listed causes of death. As expected, CRC was among the 3 most common malignancies and was the cause of death in 6.7% of the group with sufficiently detailed information. While these results fall within the expected range for the mortality related to CRC,9 the results do not allow us to assess the impact of screening, which has been shown to decrease cancer-related mortality in veterans.6 This was limited because the sample size was too small to assess the impact of screening and the cause of death was ascertained for a small percentage of the sample.
Although our findings are limited to a subset of patients seen in a single center, they suggest the importance of appropriate eligibility criteria for screening tests, as also defined in national guidelines.1 As a key anchoring point that describes the target population, age contributed to the rate of relatively early death after the index procedure. Consistent with previously published data, we saw a significant impact of comorbid diseases.10,11 However, our findings go beyond prior reports and show the important impact of psychiatric disease burden, most important the role of SUDs. The predictive value of a summary score, such as the Charlson Comorbidity Index, supports the idea of a cumulative impact, with an increasing disease burden decreasing life expectancy.10-14 It is important to consider the ongoing impact of such coexisting illnesses. Our analysis shows, the mere history of prior problems did not independently predict survival status in our cohort.
Although age is the key anchoring point that defines the target population for CRC screening programs, the benefit of earlier cancer detection or, in the context of colonoscopy with polypectomy, cancer prevention comes with a delay. Thus, cancer risk, procedural risk, and life expectancy should all be weighed when discussing and deciding on the appropriateness of CRC screening. When we disregard inherited cancer syndromes, CRC is clearly a disease of the second half of life with the incidence increasing with age.15 However, other disease burdens rise, which may affects the risk of screening and treatment should cancer be found.
Using our understanding of disease development, researchers have introduced the concept of time to benefit or lag time to help decisions about screening strategies. The period defines the likely time for a precursor or early form of cancer potentially detected by screening to manifest as a clinically relevant lesion. This lag time becomes an especially important consideration in screening of older and/or chronically ill adults with life expectancies that may be close to or even less than the time to benefit.16 Modeling studies suggest that 1,000 flexible sigmoidoscopy screenings are needed to prevent 1 cancer that would manifest about 10 years after the index examination.17,18 The mean life expectancy of a healthy person aged 75 years exceeds 10 years but drops with comorbidity burden. Consistent with these considerations, an analysis of Medicare claims data concluded that individuals with ≥ 3 significant comorbidities do not derive any benefit from screening colonoscopy.14 Looking at the impact of comorbidities, mathematical models concluded that colorectal cancer screening should not be continued in persons with moderate or severe comorbid conditions aged 66 years and 72 years, respectively.19 In contrast, modeling results suggest a benefit of continued screening up to and even above the age of 80 years if persons have an increased cancer risk and if there are no confounding comorbidities.4
Life expectancy and time to benefit describe probabilities. Although such probabilities are relevant in public policy decision, providers and patients may struggle with probabilistic thinking when faced with decisions that involve probabilities of individual health care vs population health care. Both are concerned about the seemingly gloomy or pessimistic undertone of discussing life expectancy and the inherent uncertainty of prognostic tools.20,21 Prior research indicates that this reluctance translates into clinical practice. When faced with vignettes, most clinicians would offer CRC screening to healthy persons aged 80 years with rates falling when the description included a significant comorbid burden; however, more than 40% would still consider screening in octogenarians with poor health.22
Consistent with these responses to theoretical scenarios, CRC screening of veterans dropped with age but was still continued in persons with significant comorbidity.23 Large studies of the veteran population suggest that about 10% of veterans aged > 70 years have chronic medical problems that limit their life expectancy to < 5 years; nonetheless, more than 40% of this cohort underwent colonoscopies for CRC screening.24,25 Interestingly, more illness burden and more clinical encounters translated into more screening examinations in older sick veterans compared with that of the cohort of healthier older persons, suggesting an impact of clinical reminders and the key role of age as the main anchoring variable.23
Ongoing screening despite limited or even no benefit is not unique to CRC. Using validated tools, Pollock and colleagues showed comparable screening rates for breast and prostate cancer when they examined cohorts at either high or low risk of early mortality.26 Similar results have been reported in veterans with about one-third of elderly males with poor life expectancy still undergoing prostate cancer screening.27 Interestingly, inappropriate screening is more common in nonacademic centers and influenced by provider characteristics: nurse practitioner, physician assistants, older attending physicians and male physicians were more likely to order such tests.27,28
Limitations
In this study, we examined a cohort of veterans enrolled in CRC screening within a single institution and obtained survival data for a mean follow-up of > 7 years. We also restricted our study to patients undergoing examinations that explicitly listed screening as indication or polyp surveillance for the test. However, inclusion was based on the indication listed in the report, which may differ from the intent of the ordering provider. Reporting systems often come with default settings, which may skew data. Comorbidities for the entire cohort of veterans who died within the time frame of the study were extracted from the chart without controlling for time-dependent changes, which may more appropriately describe the comorbidity burden at the time of the test. Using a case-control design, we addressed this potential caveat and included only illnesses recorded in the encounter linked to the colonoscopy order. Despite these limitations, our results highlight the importance to more effectively define and target appropriate candidates for CRC screening.
Conclusion
This study shows that age is a simple but not sufficiently accurate criterion to define potential candidates for CRC screening. As automated reminders often prompt discussions about and referral to screening examinations, we should develop algorithms that estimate the individual cancer risk and/or integrate an automatically calculated comorbidity index with these alerts or insert such a tool into order-sets. In addition, providers and patients need to be educated about the rationale and need for a more comprehensive approach to CRC screening that considers anticipated life expectancy. On an individual and health system level, our goal should be to reduce overall mortality rather than only cancer-specific death rates.
1. Rex DK, Boland CR, Dominitz JA, et al. Colorectal cancer screening: recommendations for physicians and patients from the U.S. Multi-Society Task Force on Colorectal Cancer. Gastroenterology. 2017;153(1):307-323.
2. Kahi CJ, Myers LJ, Slaven JE, et al. Lower endoscopy reduces colorectal cancer incidence in older individuals. Gastroenterology. 2014;146(3):718-725.e3.
3. Wang YR, Cangemi JR, Loftus EV Jr, Picco MF. Decreased risk of colorectal cancer after colonoscopy in patients 76-85 years old in the United States. Digestion. 2016;93(2):132-138.
4. van Hees F, Saini SD, Lansdorp-Vogelaar I, et al. Personalizing colonoscopy screening for elderly individuals based on screening history, cancer risk, and comorbidity status could increase cost effectiveness. Gastroenterology. 2015;149(6):1425-1437.
5. May FP, Yano EM, Provenzale D, Steers NW, Washington DL. The association between primary source of healthcare coverage and colorectal cancer screening among US veterans. Dig Dis Sci. 2017;62(8):1923-1932.
6. Kahi CJ, Pohl H, Myers LJ, Mobarek D, Robertson DJ, Imperiale TF. Colonoscopy and colorectal cancer mortality in the Veterans Affairs Health Care System: a case-control study. Ann Intern Med. 2018;168(7):481-488.
7. Holt PR, Kozuch P, Mewar S. Colon cancer and the elderly: from screening to treatment in management of GI disease in the elderly. Best Pract Res Clin Gastroenterol. 2009;23(6):889-907.
8. Charlson M, Szatrowski TP, Peterson J, Gold J. Validation of a combined comorbidity index. J Clin Epidemiol. 1994;47(11):1245-1251.
9. Mandel JS, Bond JH, Church TR, et al. Reducing mortality from colorectal cancer by screening for fecal occult blood. Minnesota Colon Cancer Control Study. N Engl J Med. 1993;328(19):1365-1371.
10. Lee TA, Shields AE, Vogeli C, et al. Mortality rate in veterans with multiple chronic conditions. J Gen Intern Med. 2007;22(suppl 3):403-407.
11. Nguyen-Nielsen M, Norgaard M, Jacobsen JB, et al. Comorbidity and survival of Danish prostate cancer patients from 2000-2011: a population-based cohort study. Clin Epidemiol. 2013;5(suppl 1):47-55.
12. Jang SH, Chea JW, Lee KB. Charlson comorbidity index using administrative database in incident PD patients. Clin Nephrol. 2010;73(3):204-209.
13. Fried L, Bernardini J, Piraino B. Charlson comorbidity index as a predictor of outcomes in incident peritoneal dialysis patients. Am J Kidney Dis. 2001;37(2):337-342.
14. Gross CP, Soulos PR, Ross JS, et al. Assessing the impact of screening colonoscopy on mortality in the medicare population. J Gen Intern Med. 2011;26(12):1441-1449.
15. Chouhan V, Mansoor E, Parasa S, Cooper GS. Rates of prevalent colorectal cancer occurrence in persons 75 years of age and older: a population-based national study. Dig Dis Sci. 2018;63(7):1929-1936.
16. Lee SJ, Kim CM. Individualizing prevention for older adults. J Am Geriatr Soc. 2018;66(2):229-234.
17. Tang V, Boscardin WJ, Stijacic-Cenzer I, et al. Time to benefit for colorectal cancer screening: survival meta-analysis of flexible sigmoidoscopy trials. BMJ. 2015;350:h1662.
18. Lee SJ, Boscardin WJ, Stijacic-Cenzer I, et al. Time lag to benefit after screening for breast and colorectal cancer: meta-analysis of survival data from the United States, Sweden, United Kingdom, and Denmark. BMJ. 2013;346:e8441.
19. Lansdorp-Vogelaar I, Gulati R, Mariotto AB, et al. Personalizing age of cancer screening cessation based on comorbid conditions: model estimates of harms and benefits. Ann Intern Med. 2014;161(2):104-112.
20. Schoenborn NL, Bowman TL II, Cayea D, Pollack CE, Feeser S, Boyd C. Primary care practitioners’ views on incorporating long-term prognosis in the care of older adults. JAMA Intern Med. 2016;176(5):671-678.
21. Schoenborn NL, Lee K, Pollack CE, et al. Older adults’ views and communication preferences about cancer screening cessation. JAMA Intern Med. 2017;177(8):1121-1128.
22. Lewis CL, Esserman D, DeLeon C, Pignone MP, Pathman DE, Golin C. Physician decision making for colorectal cancer screening in the elderly. J Gen Intern Med. 2013;28(9):1202-1217.
23. Saini SD, Vijan S, Schoenfeld P, Powell AA, Moser S, Kerr EA. Role of quality measurement in inappropriate use of screening for colorectal cancer: retrospective cohort study. BMJ. 2014;348:g1247.
24. Walter LC, Lindquist K, Nugent S, et al. Impact of age and comorbidity on colorectal cancer screening among older veterans. Ann Intern Med. 2009;150(7):465-473.
25. Powell AA, Saini SD, Breitenstein MK, et al. Rates and correlates of potentially inappropriate colorectal cancer screening in the Veterans Health Administration. J Gen Intern Med. 2015;30(6):732-741.
26. Pollack CE, Blackford AL, Schoenborn NL, Boyd CM, Peairs KS, DuGoff EH. Comparing prognostic tools for cancer screening: considerations for clinical practice and performance assessment. J Am Geriatr Soc. 2016;64(5):1032-1038.
27. So C, Kirby KA, Mehta K, et al. Medical center characteristics associated with PSA screening in elderly veterans with limited life expectancy. J Gen Intern Med. 2012;27(6):653-660.
28. Tang VL, Shi Y, Fung K, et al. Clinician factors associated with prostate-specific antigen screening in older veterans with limited life expectancy. JAMA Intern Med. 2016;176(5):654-661.
Colorectal cancer (CRC) ranks among the most common causes of cancer and cancer-related death in the US. The US Multi-Society Task Force (USMSTF) on Colorectal Cancer thus strongly endorsed using several available screening options.1 The published guidelines largely rely on age to define the target population (Table 1). For average-risk individuals, national and Veterans Health Administration (VHA) guidelines currently recommend CRC screening in individuals aged between 50 and 75 years with a life expectancy of > 5 years.1
Although case-control studies also point to a potential benefit in persons aged > 75 years,2,3 the USMSTF cited less convincing evidence and suggested an individualized approach that should consider relative cancer risk and comorbidity burden. Such an approach is supported by modeling studies, which suggest reduced benefit and increased risk of screening with increasing age. The reduced benefit also is significantly affected by comorbidity and relative cancer risk.4 The VHA has successfully implemented CRC screening, capturing the majority of eligible patients based on age criteria. A recent survey showed that more than three-quarters of veterans between age 50 and 75 years had undergone some screening test for CRC as part of routine preventive care. Colonoscopy clearly emerged as the dominant modality chosen for CRC screening and accounted for nearly 84% of these screening tests.5 Consistent with these data, a case-control study confirmed that the widespread implementation of colonoscopy as CRC screening method reduced cancer-related mortality in veterans for cases of left but not right-sided colon cancer.6
With calls to expand the age range of CRC screening beyond aged 75 years, we decided to assess survival rates of a cohort of veterans who underwent a screening or surveillance colonoscopy between 2008 and 2014.7 The goals were to characterize the portion of the cohort that had died, the time between a screening colonoscopy and death, the portion of deaths that were aged ≥ 80 years, and the causes of the deaths. In addition, we focused on a subgroup of the cohort, defined by death within 2 years after the index colonoscopy, to identify predictors of early death that were independent of age.
Methods
We queried the endoscopy reporting system (EndoWorks; Olympus America, Center Valley, PA) for all colonoscopies performed by 2 of 14 physicians at the George Wahlen VA Medical Center (GWVAMC) in Salt Lake City, Utah, who performed endoscopic procedures between January 1, 2008 and December 1, 2014. These physicians had focused their clinical practice exclusively on elective outpatient colonoscopies and accounted for 37.4% of the examinations at GWVAMC during the study period. All colonoscopy requests were triaged and assigned based on availability of open and appropriate procedure time slots without direct physician-specific referral, thus reducing the chance of skewing results. The reports were filtered through a text search to focus on examinations that listed screening or surveillance as indication. The central patient electronic health record was then reviewed to extract basic demographic data, survival status (as of August 1, 2018), and survival time in years after the index or subsequent colonoscopy. For deceased veterans, the age at the time of death, cause of death, and comorbidities were queried.
This study compared cases and control across the study. Cases were persons who clearly died early (defined as > 2 years following the index examination). They were matched with controls who lived for ≥ 5 years after their colonoscopy. These periods were selected because the USMSTF recommended that CRC screening or surveillance colonoscopy should be discontinued in persons with a life expectancy of < 5 years, and most study patients underwent their index procedure ≥ 5 years before August 2018. Cases and controls underwent a colonoscopy in the same year and were matched for age, sex, and presence of underlying inflammatory bowel disease (IBD). For cases and controls, we identified the ordering health care provider specialty, (ie, primary care, gastroenterology, or other).
In addition, we reviewed the encounter linked to the order and abstracted relevant comorbidities listed at that time, noted the use of anticoagulants, opioid analgesics, and benzodiazepines. The comorbidity burden was quantified using the Charlson Comorbidity Index.8 In addition, we denoted the presence of psychiatric problems (eg, anxiety, depression, bipolar disease, psychosis, substance abuse), the diagnosis of atrial fibrillation (AF) or other cardiac arrhythmias, and whether the patient had previously been treated for a malignancy that was in apparent clinical remission. Finally, we searched for routine laboratory tests at the time of this visit or, when not obtained, within 6 months of the encounter, and abstracted serum creatinine, hemoglobin (Hgb), platelet number, serum protein, and albumin. In clinical practice, cutoff values of test results are often more helpful in decision making. We, therefore, dichotomized results for Hgb (cutoff: 10 g/dL), creatinine (cutoff: 2 mg/dL), and albumin (cutoff: 3.2 mg/dL).
Descriptive and analytical statistics were obtained with Stata Version 14.1 (College Station, TX). Unless indicated otherwise, continuous data are shown as mean with 95% CIs. For dichotomous data, we used percentages with their 95% CIs. Analytic statistics were performed with the t test for continuous variables and the 2-tailed test for proportions. A P < .05 was considered a significant difference. To determine independent predictors of early death, we performed a logistic regression analysis with results being expressed as odds ratio with 95% CIs. Survival status was chosen as a dependent variable, and we entered variables that significantly correlated with survival in the bivariate analysis as independent variables.
The study was designed and conducted as a quality improvement project to assess colonoscopy performance and outcomes with the Salt Lake City Specialty Care Center of Innovation (COI), one of 5 regional COIs with an operational mission to improve health care access, utilization, and quality. Our work related to colonoscopy and access within the COI region, including Salt Lake City, has been reviewed and acknowledged by the GWVAMC Institutional Review Board as quality improvement. Andrew Gawron has an operational appointment in the GWVAMC COI, which is part of a US Department of Veterans Affairs (VA) central office initiative established in 2015. The COIs are charged with identifying best practices within the VA and applying those practices throughout the COI region. This local project to identify practice patterns and outcomes locally was sponsored by the GWVAMC COI with a focus to generate information to improve colonoscopy referral quality in patients at Salt Lake City and inform regional and national efforts in this domain.
Results
During the study period, 4,879 veterans (96.9% male) underwent at least 1 colonoscopy for screening or surveillance by 1 of the 2 providers. A total of 306 persons (6.3%) were aged > 80 years. The indication for surveillance colonoscopies included IBD in 78 (1.6%) veterans 2 of whom were women. The mean (SD) follow-up period between the index colonoscopy and study closure or death was 7.4 years (1.7). During the study time, 1,439 persons underwent a repeat examination for surveillance. The percentage of veterans with at least 1 additional colonoscopy after the index test was significantly higher in patients with known IBD compared with those without IBD (78.2% vs 28.7%; P < .01).
Between the index colonoscopy and August 2018, 974 patients (20.0%) died (Figure). The mean (SD) time between the colonoscopy and recorded year of death was 4.4 years (4.1). The fraction of women in the cohort that died (n = 18) was lower compared with 132 for the group of persons still alive (1.8% vs 3.4%; P < .05). The fraction of veterans with IBD who died by August 2018 did not differ from that of patients with IBD in the cohort of individuals who survived (19.2% vs 20.0%; P = .87). The cohort of veterans who died before study closure included 107 persons who were aged > 80 years at the time of their index colonoscopy, which is significantly more than in the cohort of persons still alive (11.0% vs 5.1%; P < .01).
Cause of Death
In 209 of the 974 (21.5%) veteran deaths a cause was recorded. Malignancies accounted for 88 of the deaths (42.1%), and CRCs were responsible for 14 (6.7%) deaths (Table 2). In 8 of these patients, the cancer had been identified at an advanced stage, not allowing for curative therapy. One patient had been asked to return for a repeat test as residual fecal matter did not allow proper visualization. He died 1 year later due to complications of sepsis after colonic perforation caused by a proximal colon cancer. Five patients underwent surgery with curative intent but suffered recurrences. In addition to malignancies, advanced diseases, such as cardiovascular, bronchopulmonary illnesses, and infections, were other commonly listed causes of death.
We also abstracted comorbidities that were known at the time of death or the most recent encounter within the VHA system. Hypertension was most commonly listed (549) followed by a current or prior diagnosis of malignancies (355) and diabetes mellitus (DM) (Table 3). Prostate cancer was the most commonly diagnosed malignancy (80), 17 of whom had a second malignancy. CRC accounted for 54 of the malignancies, 1 of which developed in a patient with long-standing ulcerative colitis, 2 were a manifestation of a known hereditary cancer syndrome (Lynch syndrome), and the remaining 51 cases were various cancers without known predisposition. The diagnosis of CRC was made during the study period in 29 veterans. In the remaining 25 patients, the colonoscopy was performed as a surveillance examination after previous surgery for CRC.
Potential Predictors of Early Death
To better define potential predictors of early death, we focused on the 258 persons (5.3%) who died within 2 years after the index procedure and paired them with matched controls. One patient underwent a colonoscopy for surveillance of previously treated cancer and was excluded due to very advanced age, as no matched control could be identified. The mean (SD) age of this male-predominant cohort was 68.2 (9.6) vs 67.9 (9.4) years for cases and controls, respectively. At the time of referral for the test, 29 persons (11.3%) were aged > 80 years, which is significantly more than seen for the overall cohort with 306 (6.3%; P < .001). While primary care providers accounted for most referrals in cases (85.2%) and controls (93.0%), the fraction of veterans referred by gastroenterologists or other specialty care providers was significantly higher in the case group compared with that in the controls (14.8% vs 7.0%; P < .05).
In our age-matched analysis, we examined other potential factors that could influence survival. The burden of comorbid conditions summarized in the Charlson Comorbidity Index significantly correlated with survival status (Table 4). As this composite index does not include psychiatric conditions, we separately examined the impact of anxiety, depression, bipolar disease, psychotic disorders, and substance abuse. The diagnoses of depression and substance use disorders (SUDs) were associated with higher rates of early death. Considering concerns about SUDs, we also assessed the association between prescription for opioids or benzodiazepines and survival status, which showed a marginal correlation. Anticoagulant use, a likely surrogate for cardiovascular disorders, were more commonly listed in the cases than they were in the controls.
Looking at specific comorbid conditions, significant problems affecting key organ systems from heart to lung, liver, kidneys, or brain (dementia) were all predictors of poor outcome. Similarly, DM with secondary complication correlated with early death after the index procedure. In contrast, a history of prior myocardial infarction, prior cancer treatment without evidence of persistent or recurrent disease, or prior peptic ulcer disease did not differ between cases and controls. Focusing on routine blood tests, we noted marginal, but statistically different results for Hgb, serum creatinine, and albumin in cases compared with controls.
Next we performed a logistic regression to identify independent predictors of survival status. The referring provider specialty, Charlson Comorbidity Index, the diagnosis of a SUD, current benzodiazepine use, and significant anemia or hypoalbuminemia independently predicted death within 2 years of the index examination (Table 5). Considering the composite nature of the Charlson Comorbidity Index, we separately examined the relative importance of different comorbid conditions using a logistic regression analysis. Consistent with the univariate analyses, a known malignancy; severe liver, lung, or kidney disease; and DM with secondary complications were associated with poor outcome. Only arrhythmias other than AF were independent marginal predictors of early death, whereas other variables related to cardiac performance did not reach the level of significance (Table 6). As was true for our analysis examining the composite comorbidity index, the diagnosis of a SUD remained significant as a predictor of death within 2 years of the index colonoscopy.
Discussion
This retrospective analysis followed patients for a mean time of 7 years after a colonoscopy for CRC screening or polyp surveillance. We noted a high rate of all-cause mortality, with 20% of the cohort dying within the period studied. Malignancies, cardiovascular diseases, and advanced lung diseases were most commonly listed causes of death. As expected, CRC was among the 3 most common malignancies and was the cause of death in 6.7% of the group with sufficiently detailed information. While these results fall within the expected range for the mortality related to CRC,9 the results do not allow us to assess the impact of screening, which has been shown to decrease cancer-related mortality in veterans.6 This was limited because the sample size was too small to assess the impact of screening and the cause of death was ascertained for a small percentage of the sample.
Although our findings are limited to a subset of patients seen in a single center, they suggest the importance of appropriate eligibility criteria for screening tests, as also defined in national guidelines.1 As a key anchoring point that describes the target population, age contributed to the rate of relatively early death after the index procedure. Consistent with previously published data, we saw a significant impact of comorbid diseases.10,11 However, our findings go beyond prior reports and show the important impact of psychiatric disease burden, most important the role of SUDs. The predictive value of a summary score, such as the Charlson Comorbidity Index, supports the idea of a cumulative impact, with an increasing disease burden decreasing life expectancy.10-14 It is important to consider the ongoing impact of such coexisting illnesses. Our analysis shows, the mere history of prior problems did not independently predict survival status in our cohort.
Although age is the key anchoring point that defines the target population for CRC screening programs, the benefit of earlier cancer detection or, in the context of colonoscopy with polypectomy, cancer prevention comes with a delay. Thus, cancer risk, procedural risk, and life expectancy should all be weighed when discussing and deciding on the appropriateness of CRC screening. When we disregard inherited cancer syndromes, CRC is clearly a disease of the second half of life with the incidence increasing with age.15 However, other disease burdens rise, which may affects the risk of screening and treatment should cancer be found.
Using our understanding of disease development, researchers have introduced the concept of time to benefit or lag time to help decisions about screening strategies. The period defines the likely time for a precursor or early form of cancer potentially detected by screening to manifest as a clinically relevant lesion. This lag time becomes an especially important consideration in screening of older and/or chronically ill adults with life expectancies that may be close to or even less than the time to benefit.16 Modeling studies suggest that 1,000 flexible sigmoidoscopy screenings are needed to prevent 1 cancer that would manifest about 10 years after the index examination.17,18 The mean life expectancy of a healthy person aged 75 years exceeds 10 years but drops with comorbidity burden. Consistent with these considerations, an analysis of Medicare claims data concluded that individuals with ≥ 3 significant comorbidities do not derive any benefit from screening colonoscopy.14 Looking at the impact of comorbidities, mathematical models concluded that colorectal cancer screening should not be continued in persons with moderate or severe comorbid conditions aged 66 years and 72 years, respectively.19 In contrast, modeling results suggest a benefit of continued screening up to and even above the age of 80 years if persons have an increased cancer risk and if there are no confounding comorbidities.4
Life expectancy and time to benefit describe probabilities. Although such probabilities are relevant in public policy decision, providers and patients may struggle with probabilistic thinking when faced with decisions that involve probabilities of individual health care vs population health care. Both are concerned about the seemingly gloomy or pessimistic undertone of discussing life expectancy and the inherent uncertainty of prognostic tools.20,21 Prior research indicates that this reluctance translates into clinical practice. When faced with vignettes, most clinicians would offer CRC screening to healthy persons aged 80 years with rates falling when the description included a significant comorbid burden; however, more than 40% would still consider screening in octogenarians with poor health.22
Consistent with these responses to theoretical scenarios, CRC screening of veterans dropped with age but was still continued in persons with significant comorbidity.23 Large studies of the veteran population suggest that about 10% of veterans aged > 70 years have chronic medical problems that limit their life expectancy to < 5 years; nonetheless, more than 40% of this cohort underwent colonoscopies for CRC screening.24,25 Interestingly, more illness burden and more clinical encounters translated into more screening examinations in older sick veterans compared with that of the cohort of healthier older persons, suggesting an impact of clinical reminders and the key role of age as the main anchoring variable.23
Ongoing screening despite limited or even no benefit is not unique to CRC. Using validated tools, Pollock and colleagues showed comparable screening rates for breast and prostate cancer when they examined cohorts at either high or low risk of early mortality.26 Similar results have been reported in veterans with about one-third of elderly males with poor life expectancy still undergoing prostate cancer screening.27 Interestingly, inappropriate screening is more common in nonacademic centers and influenced by provider characteristics: nurse practitioner, physician assistants, older attending physicians and male physicians were more likely to order such tests.27,28
Limitations
In this study, we examined a cohort of veterans enrolled in CRC screening within a single institution and obtained survival data for a mean follow-up of > 7 years. We also restricted our study to patients undergoing examinations that explicitly listed screening as indication or polyp surveillance for the test. However, inclusion was based on the indication listed in the report, which may differ from the intent of the ordering provider. Reporting systems often come with default settings, which may skew data. Comorbidities for the entire cohort of veterans who died within the time frame of the study were extracted from the chart without controlling for time-dependent changes, which may more appropriately describe the comorbidity burden at the time of the test. Using a case-control design, we addressed this potential caveat and included only illnesses recorded in the encounter linked to the colonoscopy order. Despite these limitations, our results highlight the importance to more effectively define and target appropriate candidates for CRC screening.
Conclusion
This study shows that age is a simple but not sufficiently accurate criterion to define potential candidates for CRC screening. As automated reminders often prompt discussions about and referral to screening examinations, we should develop algorithms that estimate the individual cancer risk and/or integrate an automatically calculated comorbidity index with these alerts or insert such a tool into order-sets. In addition, providers and patients need to be educated about the rationale and need for a more comprehensive approach to CRC screening that considers anticipated life expectancy. On an individual and health system level, our goal should be to reduce overall mortality rather than only cancer-specific death rates.
Colorectal cancer (CRC) ranks among the most common causes of cancer and cancer-related death in the US. The US Multi-Society Task Force (USMSTF) on Colorectal Cancer thus strongly endorsed using several available screening options.1 The published guidelines largely rely on age to define the target population (Table 1). For average-risk individuals, national and Veterans Health Administration (VHA) guidelines currently recommend CRC screening in individuals aged between 50 and 75 years with a life expectancy of > 5 years.1
Although case-control studies also point to a potential benefit in persons aged > 75 years,2,3 the USMSTF cited less convincing evidence and suggested an individualized approach that should consider relative cancer risk and comorbidity burden. Such an approach is supported by modeling studies, which suggest reduced benefit and increased risk of screening with increasing age. The reduced benefit also is significantly affected by comorbidity and relative cancer risk.4 The VHA has successfully implemented CRC screening, capturing the majority of eligible patients based on age criteria. A recent survey showed that more than three-quarters of veterans between age 50 and 75 years had undergone some screening test for CRC as part of routine preventive care. Colonoscopy clearly emerged as the dominant modality chosen for CRC screening and accounted for nearly 84% of these screening tests.5 Consistent with these data, a case-control study confirmed that the widespread implementation of colonoscopy as CRC screening method reduced cancer-related mortality in veterans for cases of left but not right-sided colon cancer.6
With calls to expand the age range of CRC screening beyond aged 75 years, we decided to assess survival rates of a cohort of veterans who underwent a screening or surveillance colonoscopy between 2008 and 2014.7 The goals were to characterize the portion of the cohort that had died, the time between a screening colonoscopy and death, the portion of deaths that were aged ≥ 80 years, and the causes of the deaths. In addition, we focused on a subgroup of the cohort, defined by death within 2 years after the index colonoscopy, to identify predictors of early death that were independent of age.
Methods
We queried the endoscopy reporting system (EndoWorks; Olympus America, Center Valley, PA) for all colonoscopies performed by 2 of 14 physicians at the George Wahlen VA Medical Center (GWVAMC) in Salt Lake City, Utah, who performed endoscopic procedures between January 1, 2008 and December 1, 2014. These physicians had focused their clinical practice exclusively on elective outpatient colonoscopies and accounted for 37.4% of the examinations at GWVAMC during the study period. All colonoscopy requests were triaged and assigned based on availability of open and appropriate procedure time slots without direct physician-specific referral, thus reducing the chance of skewing results. The reports were filtered through a text search to focus on examinations that listed screening or surveillance as indication. The central patient electronic health record was then reviewed to extract basic demographic data, survival status (as of August 1, 2018), and survival time in years after the index or subsequent colonoscopy. For deceased veterans, the age at the time of death, cause of death, and comorbidities were queried.
This study compared cases and control across the study. Cases were persons who clearly died early (defined as > 2 years following the index examination). They were matched with controls who lived for ≥ 5 years after their colonoscopy. These periods were selected because the USMSTF recommended that CRC screening or surveillance colonoscopy should be discontinued in persons with a life expectancy of < 5 years, and most study patients underwent their index procedure ≥ 5 years before August 2018. Cases and controls underwent a colonoscopy in the same year and were matched for age, sex, and presence of underlying inflammatory bowel disease (IBD). For cases and controls, we identified the ordering health care provider specialty, (ie, primary care, gastroenterology, or other).
In addition, we reviewed the encounter linked to the order and abstracted relevant comorbidities listed at that time, noted the use of anticoagulants, opioid analgesics, and benzodiazepines. The comorbidity burden was quantified using the Charlson Comorbidity Index.8 In addition, we denoted the presence of psychiatric problems (eg, anxiety, depression, bipolar disease, psychosis, substance abuse), the diagnosis of atrial fibrillation (AF) or other cardiac arrhythmias, and whether the patient had previously been treated for a malignancy that was in apparent clinical remission. Finally, we searched for routine laboratory tests at the time of this visit or, when not obtained, within 6 months of the encounter, and abstracted serum creatinine, hemoglobin (Hgb), platelet number, serum protein, and albumin. In clinical practice, cutoff values of test results are often more helpful in decision making. We, therefore, dichotomized results for Hgb (cutoff: 10 g/dL), creatinine (cutoff: 2 mg/dL), and albumin (cutoff: 3.2 mg/dL).
Descriptive and analytical statistics were obtained with Stata Version 14.1 (College Station, TX). Unless indicated otherwise, continuous data are shown as mean with 95% CIs. For dichotomous data, we used percentages with their 95% CIs. Analytic statistics were performed with the t test for continuous variables and the 2-tailed test for proportions. A P < .05 was considered a significant difference. To determine independent predictors of early death, we performed a logistic regression analysis with results being expressed as odds ratio with 95% CIs. Survival status was chosen as a dependent variable, and we entered variables that significantly correlated with survival in the bivariate analysis as independent variables.
The study was designed and conducted as a quality improvement project to assess colonoscopy performance and outcomes with the Salt Lake City Specialty Care Center of Innovation (COI), one of 5 regional COIs with an operational mission to improve health care access, utilization, and quality. Our work related to colonoscopy and access within the COI region, including Salt Lake City, has been reviewed and acknowledged by the GWVAMC Institutional Review Board as quality improvement. Andrew Gawron has an operational appointment in the GWVAMC COI, which is part of a US Department of Veterans Affairs (VA) central office initiative established in 2015. The COIs are charged with identifying best practices within the VA and applying those practices throughout the COI region. This local project to identify practice patterns and outcomes locally was sponsored by the GWVAMC COI with a focus to generate information to improve colonoscopy referral quality in patients at Salt Lake City and inform regional and national efforts in this domain.
Results
During the study period, 4,879 veterans (96.9% male) underwent at least 1 colonoscopy for screening or surveillance by 1 of the 2 providers. A total of 306 persons (6.3%) were aged > 80 years. The indication for surveillance colonoscopies included IBD in 78 (1.6%) veterans 2 of whom were women. The mean (SD) follow-up period between the index colonoscopy and study closure or death was 7.4 years (1.7). During the study time, 1,439 persons underwent a repeat examination for surveillance. The percentage of veterans with at least 1 additional colonoscopy after the index test was significantly higher in patients with known IBD compared with those without IBD (78.2% vs 28.7%; P < .01).
Between the index colonoscopy and August 2018, 974 patients (20.0%) died (Figure). The mean (SD) time between the colonoscopy and recorded year of death was 4.4 years (4.1). The fraction of women in the cohort that died (n = 18) was lower compared with 132 for the group of persons still alive (1.8% vs 3.4%; P < .05). The fraction of veterans with IBD who died by August 2018 did not differ from that of patients with IBD in the cohort of individuals who survived (19.2% vs 20.0%; P = .87). The cohort of veterans who died before study closure included 107 persons who were aged > 80 years at the time of their index colonoscopy, which is significantly more than in the cohort of persons still alive (11.0% vs 5.1%; P < .01).
Cause of Death
In 209 of the 974 (21.5%) veteran deaths a cause was recorded. Malignancies accounted for 88 of the deaths (42.1%), and CRCs were responsible for 14 (6.7%) deaths (Table 2). In 8 of these patients, the cancer had been identified at an advanced stage, not allowing for curative therapy. One patient had been asked to return for a repeat test as residual fecal matter did not allow proper visualization. He died 1 year later due to complications of sepsis after colonic perforation caused by a proximal colon cancer. Five patients underwent surgery with curative intent but suffered recurrences. In addition to malignancies, advanced diseases, such as cardiovascular, bronchopulmonary illnesses, and infections, were other commonly listed causes of death.
We also abstracted comorbidities that were known at the time of death or the most recent encounter within the VHA system. Hypertension was most commonly listed (549) followed by a current or prior diagnosis of malignancies (355) and diabetes mellitus (DM) (Table 3). Prostate cancer was the most commonly diagnosed malignancy (80), 17 of whom had a second malignancy. CRC accounted for 54 of the malignancies, 1 of which developed in a patient with long-standing ulcerative colitis, 2 were a manifestation of a known hereditary cancer syndrome (Lynch syndrome), and the remaining 51 cases were various cancers without known predisposition. The diagnosis of CRC was made during the study period in 29 veterans. In the remaining 25 patients, the colonoscopy was performed as a surveillance examination after previous surgery for CRC.
Potential Predictors of Early Death
To better define potential predictors of early death, we focused on the 258 persons (5.3%) who died within 2 years after the index procedure and paired them with matched controls. One patient underwent a colonoscopy for surveillance of previously treated cancer and was excluded due to very advanced age, as no matched control could be identified. The mean (SD) age of this male-predominant cohort was 68.2 (9.6) vs 67.9 (9.4) years for cases and controls, respectively. At the time of referral for the test, 29 persons (11.3%) were aged > 80 years, which is significantly more than seen for the overall cohort with 306 (6.3%; P < .001). While primary care providers accounted for most referrals in cases (85.2%) and controls (93.0%), the fraction of veterans referred by gastroenterologists or other specialty care providers was significantly higher in the case group compared with that in the controls (14.8% vs 7.0%; P < .05).
In our age-matched analysis, we examined other potential factors that could influence survival. The burden of comorbid conditions summarized in the Charlson Comorbidity Index significantly correlated with survival status (Table 4). As this composite index does not include psychiatric conditions, we separately examined the impact of anxiety, depression, bipolar disease, psychotic disorders, and substance abuse. The diagnoses of depression and substance use disorders (SUDs) were associated with higher rates of early death. Considering concerns about SUDs, we also assessed the association between prescription for opioids or benzodiazepines and survival status, which showed a marginal correlation. Anticoagulant use, a likely surrogate for cardiovascular disorders, were more commonly listed in the cases than they were in the controls.
Looking at specific comorbid conditions, significant problems affecting key organ systems from heart to lung, liver, kidneys, or brain (dementia) were all predictors of poor outcome. Similarly, DM with secondary complication correlated with early death after the index procedure. In contrast, a history of prior myocardial infarction, prior cancer treatment without evidence of persistent or recurrent disease, or prior peptic ulcer disease did not differ between cases and controls. Focusing on routine blood tests, we noted marginal, but statistically different results for Hgb, serum creatinine, and albumin in cases compared with controls.
Next we performed a logistic regression to identify independent predictors of survival status. The referring provider specialty, Charlson Comorbidity Index, the diagnosis of a SUD, current benzodiazepine use, and significant anemia or hypoalbuminemia independently predicted death within 2 years of the index examination (Table 5). Considering the composite nature of the Charlson Comorbidity Index, we separately examined the relative importance of different comorbid conditions using a logistic regression analysis. Consistent with the univariate analyses, a known malignancy; severe liver, lung, or kidney disease; and DM with secondary complications were associated with poor outcome. Only arrhythmias other than AF were independent marginal predictors of early death, whereas other variables related to cardiac performance did not reach the level of significance (Table 6). As was true for our analysis examining the composite comorbidity index, the diagnosis of a SUD remained significant as a predictor of death within 2 years of the index colonoscopy.
Discussion
This retrospective analysis followed patients for a mean time of 7 years after a colonoscopy for CRC screening or polyp surveillance. We noted a high rate of all-cause mortality, with 20% of the cohort dying within the period studied. Malignancies, cardiovascular diseases, and advanced lung diseases were most commonly listed causes of death. As expected, CRC was among the 3 most common malignancies and was the cause of death in 6.7% of the group with sufficiently detailed information. While these results fall within the expected range for the mortality related to CRC,9 the results do not allow us to assess the impact of screening, which has been shown to decrease cancer-related mortality in veterans.6 This was limited because the sample size was too small to assess the impact of screening and the cause of death was ascertained for a small percentage of the sample.
Although our findings are limited to a subset of patients seen in a single center, they suggest the importance of appropriate eligibility criteria for screening tests, as also defined in national guidelines.1 As a key anchoring point that describes the target population, age contributed to the rate of relatively early death after the index procedure. Consistent with previously published data, we saw a significant impact of comorbid diseases.10,11 However, our findings go beyond prior reports and show the important impact of psychiatric disease burden, most important the role of SUDs. The predictive value of a summary score, such as the Charlson Comorbidity Index, supports the idea of a cumulative impact, with an increasing disease burden decreasing life expectancy.10-14 It is important to consider the ongoing impact of such coexisting illnesses. Our analysis shows, the mere history of prior problems did not independently predict survival status in our cohort.
Although age is the key anchoring point that defines the target population for CRC screening programs, the benefit of earlier cancer detection or, in the context of colonoscopy with polypectomy, cancer prevention comes with a delay. Thus, cancer risk, procedural risk, and life expectancy should all be weighed when discussing and deciding on the appropriateness of CRC screening. When we disregard inherited cancer syndromes, CRC is clearly a disease of the second half of life with the incidence increasing with age.15 However, other disease burdens rise, which may affects the risk of screening and treatment should cancer be found.
Using our understanding of disease development, researchers have introduced the concept of time to benefit or lag time to help decisions about screening strategies. The period defines the likely time for a precursor or early form of cancer potentially detected by screening to manifest as a clinically relevant lesion. This lag time becomes an especially important consideration in screening of older and/or chronically ill adults with life expectancies that may be close to or even less than the time to benefit.16 Modeling studies suggest that 1,000 flexible sigmoidoscopy screenings are needed to prevent 1 cancer that would manifest about 10 years after the index examination.17,18 The mean life expectancy of a healthy person aged 75 years exceeds 10 years but drops with comorbidity burden. Consistent with these considerations, an analysis of Medicare claims data concluded that individuals with ≥ 3 significant comorbidities do not derive any benefit from screening colonoscopy.14 Looking at the impact of comorbidities, mathematical models concluded that colorectal cancer screening should not be continued in persons with moderate or severe comorbid conditions aged 66 years and 72 years, respectively.19 In contrast, modeling results suggest a benefit of continued screening up to and even above the age of 80 years if persons have an increased cancer risk and if there are no confounding comorbidities.4
Life expectancy and time to benefit describe probabilities. Although such probabilities are relevant in public policy decision, providers and patients may struggle with probabilistic thinking when faced with decisions that involve probabilities of individual health care vs population health care. Both are concerned about the seemingly gloomy or pessimistic undertone of discussing life expectancy and the inherent uncertainty of prognostic tools.20,21 Prior research indicates that this reluctance translates into clinical practice. When faced with vignettes, most clinicians would offer CRC screening to healthy persons aged 80 years with rates falling when the description included a significant comorbid burden; however, more than 40% would still consider screening in octogenarians with poor health.22
Consistent with these responses to theoretical scenarios, CRC screening of veterans dropped with age but was still continued in persons with significant comorbidity.23 Large studies of the veteran population suggest that about 10% of veterans aged > 70 years have chronic medical problems that limit their life expectancy to < 5 years; nonetheless, more than 40% of this cohort underwent colonoscopies for CRC screening.24,25 Interestingly, more illness burden and more clinical encounters translated into more screening examinations in older sick veterans compared with that of the cohort of healthier older persons, suggesting an impact of clinical reminders and the key role of age as the main anchoring variable.23
Ongoing screening despite limited or even no benefit is not unique to CRC. Using validated tools, Pollock and colleagues showed comparable screening rates for breast and prostate cancer when they examined cohorts at either high or low risk of early mortality.26 Similar results have been reported in veterans with about one-third of elderly males with poor life expectancy still undergoing prostate cancer screening.27 Interestingly, inappropriate screening is more common in nonacademic centers and influenced by provider characteristics: nurse practitioner, physician assistants, older attending physicians and male physicians were more likely to order such tests.27,28
Limitations
In this study, we examined a cohort of veterans enrolled in CRC screening within a single institution and obtained survival data for a mean follow-up of > 7 years. We also restricted our study to patients undergoing examinations that explicitly listed screening as indication or polyp surveillance for the test. However, inclusion was based on the indication listed in the report, which may differ from the intent of the ordering provider. Reporting systems often come with default settings, which may skew data. Comorbidities for the entire cohort of veterans who died within the time frame of the study were extracted from the chart without controlling for time-dependent changes, which may more appropriately describe the comorbidity burden at the time of the test. Using a case-control design, we addressed this potential caveat and included only illnesses recorded in the encounter linked to the colonoscopy order. Despite these limitations, our results highlight the importance to more effectively define and target appropriate candidates for CRC screening.
Conclusion
This study shows that age is a simple but not sufficiently accurate criterion to define potential candidates for CRC screening. As automated reminders often prompt discussions about and referral to screening examinations, we should develop algorithms that estimate the individual cancer risk and/or integrate an automatically calculated comorbidity index with these alerts or insert such a tool into order-sets. In addition, providers and patients need to be educated about the rationale and need for a more comprehensive approach to CRC screening that considers anticipated life expectancy. On an individual and health system level, our goal should be to reduce overall mortality rather than only cancer-specific death rates.
1. Rex DK, Boland CR, Dominitz JA, et al. Colorectal cancer screening: recommendations for physicians and patients from the U.S. Multi-Society Task Force on Colorectal Cancer. Gastroenterology. 2017;153(1):307-323.
2. Kahi CJ, Myers LJ, Slaven JE, et al. Lower endoscopy reduces colorectal cancer incidence in older individuals. Gastroenterology. 2014;146(3):718-725.e3.
3. Wang YR, Cangemi JR, Loftus EV Jr, Picco MF. Decreased risk of colorectal cancer after colonoscopy in patients 76-85 years old in the United States. Digestion. 2016;93(2):132-138.
4. van Hees F, Saini SD, Lansdorp-Vogelaar I, et al. Personalizing colonoscopy screening for elderly individuals based on screening history, cancer risk, and comorbidity status could increase cost effectiveness. Gastroenterology. 2015;149(6):1425-1437.
5. May FP, Yano EM, Provenzale D, Steers NW, Washington DL. The association between primary source of healthcare coverage and colorectal cancer screening among US veterans. Dig Dis Sci. 2017;62(8):1923-1932.
6. Kahi CJ, Pohl H, Myers LJ, Mobarek D, Robertson DJ, Imperiale TF. Colonoscopy and colorectal cancer mortality in the Veterans Affairs Health Care System: a case-control study. Ann Intern Med. 2018;168(7):481-488.
7. Holt PR, Kozuch P, Mewar S. Colon cancer and the elderly: from screening to treatment in management of GI disease in the elderly. Best Pract Res Clin Gastroenterol. 2009;23(6):889-907.
8. Charlson M, Szatrowski TP, Peterson J, Gold J. Validation of a combined comorbidity index. J Clin Epidemiol. 1994;47(11):1245-1251.
9. Mandel JS, Bond JH, Church TR, et al. Reducing mortality from colorectal cancer by screening for fecal occult blood. Minnesota Colon Cancer Control Study. N Engl J Med. 1993;328(19):1365-1371.
10. Lee TA, Shields AE, Vogeli C, et al. Mortality rate in veterans with multiple chronic conditions. J Gen Intern Med. 2007;22(suppl 3):403-407.
11. Nguyen-Nielsen M, Norgaard M, Jacobsen JB, et al. Comorbidity and survival of Danish prostate cancer patients from 2000-2011: a population-based cohort study. Clin Epidemiol. 2013;5(suppl 1):47-55.
12. Jang SH, Chea JW, Lee KB. Charlson comorbidity index using administrative database in incident PD patients. Clin Nephrol. 2010;73(3):204-209.
13. Fried L, Bernardini J, Piraino B. Charlson comorbidity index as a predictor of outcomes in incident peritoneal dialysis patients. Am J Kidney Dis. 2001;37(2):337-342.
14. Gross CP, Soulos PR, Ross JS, et al. Assessing the impact of screening colonoscopy on mortality in the medicare population. J Gen Intern Med. 2011;26(12):1441-1449.
15. Chouhan V, Mansoor E, Parasa S, Cooper GS. Rates of prevalent colorectal cancer occurrence in persons 75 years of age and older: a population-based national study. Dig Dis Sci. 2018;63(7):1929-1936.
16. Lee SJ, Kim CM. Individualizing prevention for older adults. J Am Geriatr Soc. 2018;66(2):229-234.
17. Tang V, Boscardin WJ, Stijacic-Cenzer I, et al. Time to benefit for colorectal cancer screening: survival meta-analysis of flexible sigmoidoscopy trials. BMJ. 2015;350:h1662.
18. Lee SJ, Boscardin WJ, Stijacic-Cenzer I, et al. Time lag to benefit after screening for breast and colorectal cancer: meta-analysis of survival data from the United States, Sweden, United Kingdom, and Denmark. BMJ. 2013;346:e8441.
19. Lansdorp-Vogelaar I, Gulati R, Mariotto AB, et al. Personalizing age of cancer screening cessation based on comorbid conditions: model estimates of harms and benefits. Ann Intern Med. 2014;161(2):104-112.
20. Schoenborn NL, Bowman TL II, Cayea D, Pollack CE, Feeser S, Boyd C. Primary care practitioners’ views on incorporating long-term prognosis in the care of older adults. JAMA Intern Med. 2016;176(5):671-678.
21. Schoenborn NL, Lee K, Pollack CE, et al. Older adults’ views and communication preferences about cancer screening cessation. JAMA Intern Med. 2017;177(8):1121-1128.
22. Lewis CL, Esserman D, DeLeon C, Pignone MP, Pathman DE, Golin C. Physician decision making for colorectal cancer screening in the elderly. J Gen Intern Med. 2013;28(9):1202-1217.
23. Saini SD, Vijan S, Schoenfeld P, Powell AA, Moser S, Kerr EA. Role of quality measurement in inappropriate use of screening for colorectal cancer: retrospective cohort study. BMJ. 2014;348:g1247.
24. Walter LC, Lindquist K, Nugent S, et al. Impact of age and comorbidity on colorectal cancer screening among older veterans. Ann Intern Med. 2009;150(7):465-473.
25. Powell AA, Saini SD, Breitenstein MK, et al. Rates and correlates of potentially inappropriate colorectal cancer screening in the Veterans Health Administration. J Gen Intern Med. 2015;30(6):732-741.
26. Pollack CE, Blackford AL, Schoenborn NL, Boyd CM, Peairs KS, DuGoff EH. Comparing prognostic tools for cancer screening: considerations for clinical practice and performance assessment. J Am Geriatr Soc. 2016;64(5):1032-1038.
27. So C, Kirby KA, Mehta K, et al. Medical center characteristics associated with PSA screening in elderly veterans with limited life expectancy. J Gen Intern Med. 2012;27(6):653-660.
28. Tang VL, Shi Y, Fung K, et al. Clinician factors associated with prostate-specific antigen screening in older veterans with limited life expectancy. JAMA Intern Med. 2016;176(5):654-661.
1. Rex DK, Boland CR, Dominitz JA, et al. Colorectal cancer screening: recommendations for physicians and patients from the U.S. Multi-Society Task Force on Colorectal Cancer. Gastroenterology. 2017;153(1):307-323.
2. Kahi CJ, Myers LJ, Slaven JE, et al. Lower endoscopy reduces colorectal cancer incidence in older individuals. Gastroenterology. 2014;146(3):718-725.e3.
3. Wang YR, Cangemi JR, Loftus EV Jr, Picco MF. Decreased risk of colorectal cancer after colonoscopy in patients 76-85 years old in the United States. Digestion. 2016;93(2):132-138.
4. van Hees F, Saini SD, Lansdorp-Vogelaar I, et al. Personalizing colonoscopy screening for elderly individuals based on screening history, cancer risk, and comorbidity status could increase cost effectiveness. Gastroenterology. 2015;149(6):1425-1437.
5. May FP, Yano EM, Provenzale D, Steers NW, Washington DL. The association between primary source of healthcare coverage and colorectal cancer screening among US veterans. Dig Dis Sci. 2017;62(8):1923-1932.
6. Kahi CJ, Pohl H, Myers LJ, Mobarek D, Robertson DJ, Imperiale TF. Colonoscopy and colorectal cancer mortality in the Veterans Affairs Health Care System: a case-control study. Ann Intern Med. 2018;168(7):481-488.
7. Holt PR, Kozuch P, Mewar S. Colon cancer and the elderly: from screening to treatment in management of GI disease in the elderly. Best Pract Res Clin Gastroenterol. 2009;23(6):889-907.
8. Charlson M, Szatrowski TP, Peterson J, Gold J. Validation of a combined comorbidity index. J Clin Epidemiol. 1994;47(11):1245-1251.
9. Mandel JS, Bond JH, Church TR, et al. Reducing mortality from colorectal cancer by screening for fecal occult blood. Minnesota Colon Cancer Control Study. N Engl J Med. 1993;328(19):1365-1371.
10. Lee TA, Shields AE, Vogeli C, et al. Mortality rate in veterans with multiple chronic conditions. J Gen Intern Med. 2007;22(suppl 3):403-407.
11. Nguyen-Nielsen M, Norgaard M, Jacobsen JB, et al. Comorbidity and survival of Danish prostate cancer patients from 2000-2011: a population-based cohort study. Clin Epidemiol. 2013;5(suppl 1):47-55.
12. Jang SH, Chea JW, Lee KB. Charlson comorbidity index using administrative database in incident PD patients. Clin Nephrol. 2010;73(3):204-209.
13. Fried L, Bernardini J, Piraino B. Charlson comorbidity index as a predictor of outcomes in incident peritoneal dialysis patients. Am J Kidney Dis. 2001;37(2):337-342.
14. Gross CP, Soulos PR, Ross JS, et al. Assessing the impact of screening colonoscopy on mortality in the medicare population. J Gen Intern Med. 2011;26(12):1441-1449.
15. Chouhan V, Mansoor E, Parasa S, Cooper GS. Rates of prevalent colorectal cancer occurrence in persons 75 years of age and older: a population-based national study. Dig Dis Sci. 2018;63(7):1929-1936.
16. Lee SJ, Kim CM. Individualizing prevention for older adults. J Am Geriatr Soc. 2018;66(2):229-234.
17. Tang V, Boscardin WJ, Stijacic-Cenzer I, et al. Time to benefit for colorectal cancer screening: survival meta-analysis of flexible sigmoidoscopy trials. BMJ. 2015;350:h1662.
18. Lee SJ, Boscardin WJ, Stijacic-Cenzer I, et al. Time lag to benefit after screening for breast and colorectal cancer: meta-analysis of survival data from the United States, Sweden, United Kingdom, and Denmark. BMJ. 2013;346:e8441.
19. Lansdorp-Vogelaar I, Gulati R, Mariotto AB, et al. Personalizing age of cancer screening cessation based on comorbid conditions: model estimates of harms and benefits. Ann Intern Med. 2014;161(2):104-112.
20. Schoenborn NL, Bowman TL II, Cayea D, Pollack CE, Feeser S, Boyd C. Primary care practitioners’ views on incorporating long-term prognosis in the care of older adults. JAMA Intern Med. 2016;176(5):671-678.
21. Schoenborn NL, Lee K, Pollack CE, et al. Older adults’ views and communication preferences about cancer screening cessation. JAMA Intern Med. 2017;177(8):1121-1128.
22. Lewis CL, Esserman D, DeLeon C, Pignone MP, Pathman DE, Golin C. Physician decision making for colorectal cancer screening in the elderly. J Gen Intern Med. 2013;28(9):1202-1217.
23. Saini SD, Vijan S, Schoenfeld P, Powell AA, Moser S, Kerr EA. Role of quality measurement in inappropriate use of screening for colorectal cancer: retrospective cohort study. BMJ. 2014;348:g1247.
24. Walter LC, Lindquist K, Nugent S, et al. Impact of age and comorbidity on colorectal cancer screening among older veterans. Ann Intern Med. 2009;150(7):465-473.
25. Powell AA, Saini SD, Breitenstein MK, et al. Rates and correlates of potentially inappropriate colorectal cancer screening in the Veterans Health Administration. J Gen Intern Med. 2015;30(6):732-741.
26. Pollack CE, Blackford AL, Schoenborn NL, Boyd CM, Peairs KS, DuGoff EH. Comparing prognostic tools for cancer screening: considerations for clinical practice and performance assessment. J Am Geriatr Soc. 2016;64(5):1032-1038.
27. So C, Kirby KA, Mehta K, et al. Medical center characteristics associated with PSA screening in elderly veterans with limited life expectancy. J Gen Intern Med. 2012;27(6):653-660.
28. Tang VL, Shi Y, Fung K, et al. Clinician factors associated with prostate-specific antigen screening in older veterans with limited life expectancy. JAMA Intern Med. 2016;176(5):654-661.
Expanding the Scope of Telemedicine in Gastroenterology
Access to specialized services has been a consistently complex problem for many integrated health care systems, including the Veterans Health Administration (VHA). About two-thirds of veterans experience significant barriers when trying to obtain medical care.1 While these problems partly mirror difficulties that nonveterans face as well, there is a unique obligation toward those who put life and health at risk during their military service.2
To better meet demands, the VHA expanded personnel and clinic infrastructure with more providers and a network of community-based outpatient clinics (CBOC) that created more openings for clinic visits.3 Yet regional variability remains a significant problem for primary and even more so for specialty medical services.
Recent data show that more than one-fifth of all veterans live in areas with low population density and shortages of health care providers.4 The data point at a special problem in this context because these veterans often face long travel times to centers offering specialty services. The introduction of electronic consults functions as an alternative venue to obtain expert input but amounts to only 2% of total consult volume.5 A more interactive approach with face-to-face teleconferencing, case discussions, and special training led by expert clinicians has further improved access in such underserved areas and played a key role in the success of the VHA hepatitis C treatment initiative.6
Despite its clearly proven role and success, these e-consults come with some conceptual shortcomings. A key caveat is the lack of direct patient involvement. Obtaining information from the source rather than relying on symptoms documented by a third person can be essential in approaching medical problems. Experts may be able to tease out the often essential details of a history when making a diagnosis. A direct contact adds an additional, perhaps less tangible, component to the interaction that relies on verbal and nonverbal components of personal interactions and plays an important role in treatment success. Prior studies strongly link credibility of and trust in a provider as well as the related treatment success to such aspects of communication.7,8
Gastroenterology Telemedicine Services
The George E. Wahlen VA Medical Center in Salt Lake City, Utah, draws from a large catchment area that extends from the southern border of Utah to the neighboring states of Idaho, Wyoming, Nevada, and Montana. Large stretches of this territory are remote with population densities well below 5 persons per square mile. The authors therefore devised a specialty outreach program relying on telemedicine for patients with gastrointestinal and liver diseases and present the initial experience with the implementation of this program.
Phase 1: Finding the Champions
Prior studies clearly emphasized that most successful telemedicine clinics relied on key persons (“champions”) promoting the idea and carrying the additional logistic and time issues required to start and maintain the new program.9,10 Thus we created a small team that defined and refined goals, identified target groups, and worked out the logistics. Based on prior experiences, we focused initially on veterans with more chronic and likely functional disorders, such as diarrhea, constipation, dyspepsia, or nausea. The team also planned to accept patients with chronic liver disorders or abnormal test results that required further clarification. By consensus, the group excluded acute problems and bleeding as well as disorders with pain as primary manifestations. The underlying assumption was that a direct physical examination was less critical in most of these cases.
Phase 2: Outreach
Clinic managers and medical directors of the affiliated CBOC were informed of the planned telemedicine clinic. Also, we identified local champions who could function as point persons and assist in the organization of visits. One member of the team personally visited key sites to discuss needs and opportunities with CBOC personnel during a routine staff meeting. The goal was to introduce the program, the key personnel, to explain criteria for appropriate candidates that may benefit from telemedicine consults, and to agree on a referral pathway. Finally, we emphasized that the consultant would always defer to the referring provider or patient and honor their requests.
Phase 3: Identifying Appropriate Patients
The team planned for and has since used 4 different pathways to identify possible candidates for telemedicine visits. The consult triaging process with telemedicine is an option that is brought up with patients if their travel to the facility exceeds 100 miles. Similarly, the team reviews procedural requests to optimize diagnostic yields and limit patient burden. For example, if endoscopic testing is requested to address chronic abdominal pain or other concerns that had already prompted a similar request with negative results, then the team will ask for feedback and recommend a telemedicine consultation prior to performing the procedure. Telemedicine also is offered for follow-up encounters to veterans seen in the facility for clinical or procedural evaluations if they live ≥ 40 miles away. The 2 other pathways are requests from referring providers or patients that specifically ask for telemedicine visits.
Phase 4: Implementation
Since rolling out the program in November 2016, video visits have been used for more than 150 clinic encounters. Within the first 12 months, 124 patients were seen at least once using telemedicine links. Of 144 visits, 54 (38%) were follow-up visits; the rest constituted initial consultations. Focusing on initial encounters only, veterans specifically asked for a telemedicine visit in 16 cases (17.8%). One-third of these referrals was specifically marked as a telemedicine visit by the primary care provider. In the remaining cases, the triaging personnel brought up the possibility of a telemedicine interaction and requested feedback from the referring provider.
Veterans resided in many different areas within and outside of the facility’s immediate referral area (Figure).
Abnormal bowel patterns, gastroesophageal reflux, and dyspepsia accounted for most concerns (Table 1).
Beyond obtaining contextual data and information about the specific clinical manifestations, the rationale for these encounters was a detailed discussion of the problem and treatment options available. Ablative therapy in Barrett esophagus best exemplifies the potential relevance of such an encounter: Although conceptually appealing to decrease cancer risk, the approach requires a significant commitment typically involving repeated sessions of radiofrequency ablation followed by intense endoscopic surveillance. With travel distances of several hundred miles in these cases, these encounters provide relevant information to patients and the opportunity to make informed decisions without the burden and cost of a long trip.
A shift in telemedicine encounters will likely occur that will increasingly rely on access from home computers or handheld devices. However, the initial phase of this program relied on connections through a CBOC. Coordination between 2 sites adds a level of complexity to ensure availability of space and videoconferencing equipment. To limit the logistic burden and improve cost-effectiveness, the authors did not expect or request the presence of the primary or another independent provider. Instead, the team communicated with a locally designated point person who coordinated the remote encounters and assisted in implementing some of the suggested next steps. Prior site visits and communications with referring providers had established channels of communication to define concerns or highlight findings. The same channels also allowed the team to direct its attention to specific aspects of the physical examination to support or rule out a presumptive diagnosis.
If additional testing was suggested, Telemedicine Services generally ordered the appropriate assessments unless veterans requested relying on local resources better known to personnel at the remote site. The most common diagnostic steps recommended were laboratory tests (n = 21; 14.6%), endoscopic procedures (n = 18; 12.5%), and radiologic studies (n = 17; 11.8%) (Table 2).
Most of the treatment changes focused on medication and dietary management, followed by lifestyle modifications and behavioral or psychological interventions. Some treatments, such as ablation of dysplastic epithelium in patients with Barrett esophagus or pneumatic dilation of achalasia required traveling to the George E. Wahlen VAMC. Nonetheless, the number of trips were limited as the team could assess appropriateness, explain approaches, and evaluate symptomatic outcomes with the initial or subsequent remote encounters. Most of the follow-up involved the primary care providers (n = 62; 43.1%), while repeat remote encounters were suggested in 31 visits (21.5%) and an in-person clinic follow-up in 7 cases (n = 4.9%). In the remaining cases, veterans were asked to contact the team directly or through their primary care provider if additional input was needed.
Discussion
The initial implementation of a specialty telemedicine clinic taught us several lessons that will not only guide this program expansion, but also may be relevant for others introducing telemedicine into their specialty clinics. At first glance, videoconferencing with patients resembles more conventional clinic encounters. However, it adds another angle as many steps from scheduling a visit to implementing recommendations rely on different members at the remote site. Thus, the success of such a program depends on establishing a true partnership with the teams at the various satellite sites. It also requires ongoing feedback from all team members and fine-tuning to effectively integrate it into the routine operations of both sites.
Feedback about the program has been very positive with comments often asking for an expansion beyond gastroenterology. Concerns largely were limited to scheduling problems that may become less relevant if the new telehealth initiative moves forward and enables health care providers to directly connect with computers or handheld devices at the patient’s home. Prior studies demonstrated that most individuals have access to such technology and accept it as a viable or even attractive option for medical encounters.11,12
For some, remaining in the comfort of their own home is not only more convenient, but also adds a sense of security, further adding to its appeal.13 As suggested by the economist Richard Thaler, simple nudges may be required to increase use and perhaps utility of telemedicine or e-consults.14 At this stage, it is the active choice of the referral or triaging provider to consider telemedicine as an option. To facilitate deviation from the established routine, we plan to revise the consult requests by using a drop-down menu option that brings up e-consult, telemedicine, or clinic visit as alternatives and requires an active choice rather than defaulting to conventional face-to-face visits.
Despite an overall successful launch of the specialty telemedicine clinic, several conceptual questions require additional in-depth assessments. While video visits indeed include the literal face time that characterizes normal clinic visits, does this translate into the “face value” that may contribute to treatment success? If detailed information about physical findings is needed, remote encounters require a third person at the distant site to complete this step, which may not only be a logistic burden, but also could influence the perceived utility and affect outcomes.
Previously published studies have demonstrated the effectiveness of video-based interactions and allow providers to address these points to some degree. Remote encounters have established roles in mental healthcare that is less dependent on physical findings.15 Distance monitoring of devices or biomarkers, such as blood sugar levels or blood pressure, are becoming routine and often are combined with corrective interventions.16-18
Recently completed trials showed satisfaction did not differ from conventional clinic encounters when telemedicine encounters were used to manage chronic headaches or provide postoperative follow-up after urologic surgery.19,20 For gastroenterology, telemedicine outreach after hospitalizations not only improved care, but also lowered rates of testing after discharge.21 In patients with inflammatory bowel disease, a group that was not targeted during this initial phase, proactive and close follow-up with remote technology can decrease the need for hospitalization.22
These data are consistent with encouraging feedback received. Nonetheless, it is important to assess whether this approach is superior to established and cheaper alternatives, most notably simple telephone interactions. Video-linkage obviously allows nonverbal elements of communication, which play an important role in patient preference and satisfaction, treatment implementation, and impact.7,8,23-25 Providers described patients as more focused and engaged compared with telephone interactions and valued the ability to incorporate body language in their assessment.26
Telemedicine clinics offered by specialty providers may not improve access as defined by wait times only, which would require adding more clinical time and personnel. However, it can lower barriers to care imposed by long distances between rural areas and facilities with specialized expertise. Even if a remote encounter concludes with the recommendation to visit the clinic for more detailed testing or treatment, explaining the need for such steps and involving the patient in the decision-making process may affect adherence.
Conclusion
Although the experiences of the team at George E. Wahlen VA Medical Center support the use of telemedicine in specialty clinics, the next phase of the project needs to address the utility of this approach and define the perceived value and potential problems of telemedicine. Obtaining this insight will require complex data sets with feedback from patients and referring and consulting providers. As trade-offs will likely vary between different diseases or symptoms, such studies will provide a better definition of clinical scenarios best suited for remote encounters. In addition, they may provide approximate values for distance or efforts that may make the cost of a direct clinic visit worth it, thereby defining boundary-condition.
1. Elnitsky CA, Andresen EM, Clark ME, McGarity S, Hall CG, Kerns RD. Access to the US Department of Veterans Affairs health system: self-reported barriers to care among returnees of Operations Enduring Freedom and Iraqi Freedom. BMC Health Serv Res. 2013;13:498.
2. Woolhandler S, Himmelstein DU, Distajo R, et al. America’s neglected veterans: 1.7 million who served have no health coverage. Int J Health Serv. 2005;35(2):313-323.
3. Rosenheck R. Primary care satellite clinics and improved access to general and mental health services. Health Serv Res. 2000;35:777-790.
4. Doyle JM, Streeter RA. Veterans’ location in health professional shortage areas: implications for access to care and workforce supply. Health Serv Res. 2017;52(suppl 1):459-480.
5. Kirsh S, Carey E, Aron DC, et al. Impact of a national specialty e-consultation implementation project on access. Am J Manag Care. 2015;21(12):e648-e654.
6. Belperio PS, Chartier M, Ross DB, Alaigh P, Shulkin D. Curing hepatitis C virus infection: best practices from the U.S. Department of Veterans Affairs. Ann Intern Med. 2017;167(7):499-504.
7. Kaptchuk TJ, Kelley JM, Conboy LA, et al. Components of placebo effect: randomised controlled trial in patients with irritable bowel syndrome. BMJ. 2008;333(7651):999-1003.
8. Weinland SR, Morris CB, Dalton C, et al. Cognitive factors affect treatment response to medical and psychological treatments in functional bowel disorders. Am J Gastroenterol. 2010;105(6):1397-1406.
9. Wade V, Eliott J. The role of the champion in telehealth service development: a qualitative analysis. J Telemed Telecare. 2012;18(8):490-492.
10. Postema TR, Peeters JM, Friele RD. Key factors influencing the implementation success of a home telecare application. Int J Med Inform. 2012;81(6):415-423.
11. Tahir D. Trump and VA unveil telehealth initiative. https://www.politico.com/tipsheets/morning-ehealth/2017/08/04/trump-and-va-unveil-telehealth-initiative-221706. Published August 4, 2017. Accessed July 11, 2018.
12. Gardner MR, Jenkins SM, O’Neil DA, Gardner MR, Jenkins SM, O’Neil DA. Perceptions of video-based appointments from the patient’s home: a patient survey. Telemed J E Health. 2015;21(4):281-285.
13. Powell RE, Henstenburg JM, Cooper G, Hollander JE, Rising KL. Patient perceptions of telehealth primary care video visits. Ann Fam Med. 2017;15(3):225-229.
14. Benartzi S, Beshears J, Milkman KL, et al. Should governments invest more in nudging? Psychol Sci. 2017;28(8):1041-1055.
15. Turgoose D, Ashwick R, Murphy D. Systematic review of lessons learned from delivering tele-therapy to veterans with post-traumatic stress disorder. J Telemed Telecare. 2017:1357633x17730443.
16. Dalouk K, Gandhi N, Jessel P, et al. Outcomes of telemedicine video-conferencing clinic versus in-person clinic follow-up for implantable cardioverter-defibrillator recipients. Circ Arrhythm Electrophysiol. 2017;10(9) pii: e005217.
17. Warren R, Carlisle K, Mihala G, Scuffham PA. Effects of telemonitoring on glycaemic control and healthcare costs in type 2 diabetes: a randomised controlled trial. J Telemed Telecare. 2017:1357633x17723943.
18. Tucker KL, Sheppard JP, Stevens R, et al. Self-monitoring of blood pressure in hypertension: a systematic review and individual patient data meta-analysis. PLoS Med. 2017;14(9):e1002389.
19. Müller KI, Alstadhaug KB, Bekkelund SI. Headache patients’ satisfaction with telemedicine: a 12-month follow-up randomized non-inferiority trial. Eur J Neurol. 2017;24(6):807-815.
20. Viers BR, Lightner DJ, Rivera ME, et al. Efficiency, satisfaction, and costs for remote video visits following radical prostatectomy: a randomized controlled trial. Eur Urol. 2015;68:729-735.
21. Wallace P, Barber J, Clayton W, et al. Virtual outreach: a randomised controlled trial and economic evaluation of joint teleconferenced medical consultations. Health Technol Assess. 2004;8(50):1-106, iii-iv.
22. de Jong MJ, van der Meulen-de Jong AE, Romberg-Camps MJ, et al. Telemedicine for management of inflammatory bowel disease (myIBDcoach): a pragmatic, multicentre, randomised controlled trial. Lancet. 2017;390(10098):959-968.
23. Czerniak E, Biegon A, Ziv A, et al. Manipulating the placebo response in experimental pain by altering doctor’s performance style. Front Psychol. 2016;7:874.
24. Moffet HH, Parker MM, Sarkar U, et al. Adherence to laboratory test requests by patients with diabetes: the Diabetes Study of Northern California (DISTANCE). Am J Manag Care. 2011;17(5):339-344.
25. Richter KP, Shireman TI, Ellerbeck EF, et al. Comparative and cost effectiveness of telemedicine versus telephone counseling for smoking cessation. J Med Internet Res. 2015;17(5):e113.
26. Voils CI, Venne VL, Weidenbacher H, Sperber N, Datta S. Comparison of telephone and televideo modes for delivery of genetic counseling: a randomized trial. J Genet Couns. 2018;27(2):339-348.
Access to specialized services has been a consistently complex problem for many integrated health care systems, including the Veterans Health Administration (VHA). About two-thirds of veterans experience significant barriers when trying to obtain medical care.1 While these problems partly mirror difficulties that nonveterans face as well, there is a unique obligation toward those who put life and health at risk during their military service.2
To better meet demands, the VHA expanded personnel and clinic infrastructure with more providers and a network of community-based outpatient clinics (CBOC) that created more openings for clinic visits.3 Yet regional variability remains a significant problem for primary and even more so for specialty medical services.
Recent data show that more than one-fifth of all veterans live in areas with low population density and shortages of health care providers.4 The data point at a special problem in this context because these veterans often face long travel times to centers offering specialty services. The introduction of electronic consults functions as an alternative venue to obtain expert input but amounts to only 2% of total consult volume.5 A more interactive approach with face-to-face teleconferencing, case discussions, and special training led by expert clinicians has further improved access in such underserved areas and played a key role in the success of the VHA hepatitis C treatment initiative.6
Despite its clearly proven role and success, these e-consults come with some conceptual shortcomings. A key caveat is the lack of direct patient involvement. Obtaining information from the source rather than relying on symptoms documented by a third person can be essential in approaching medical problems. Experts may be able to tease out the often essential details of a history when making a diagnosis. A direct contact adds an additional, perhaps less tangible, component to the interaction that relies on verbal and nonverbal components of personal interactions and plays an important role in treatment success. Prior studies strongly link credibility of and trust in a provider as well as the related treatment success to such aspects of communication.7,8
Gastroenterology Telemedicine Services
The George E. Wahlen VA Medical Center in Salt Lake City, Utah, draws from a large catchment area that extends from the southern border of Utah to the neighboring states of Idaho, Wyoming, Nevada, and Montana. Large stretches of this territory are remote with population densities well below 5 persons per square mile. The authors therefore devised a specialty outreach program relying on telemedicine for patients with gastrointestinal and liver diseases and present the initial experience with the implementation of this program.
Phase 1: Finding the Champions
Prior studies clearly emphasized that most successful telemedicine clinics relied on key persons (“champions”) promoting the idea and carrying the additional logistic and time issues required to start and maintain the new program.9,10 Thus we created a small team that defined and refined goals, identified target groups, and worked out the logistics. Based on prior experiences, we focused initially on veterans with more chronic and likely functional disorders, such as diarrhea, constipation, dyspepsia, or nausea. The team also planned to accept patients with chronic liver disorders or abnormal test results that required further clarification. By consensus, the group excluded acute problems and bleeding as well as disorders with pain as primary manifestations. The underlying assumption was that a direct physical examination was less critical in most of these cases.
Phase 2: Outreach
Clinic managers and medical directors of the affiliated CBOC were informed of the planned telemedicine clinic. Also, we identified local champions who could function as point persons and assist in the organization of visits. One member of the team personally visited key sites to discuss needs and opportunities with CBOC personnel during a routine staff meeting. The goal was to introduce the program, the key personnel, to explain criteria for appropriate candidates that may benefit from telemedicine consults, and to agree on a referral pathway. Finally, we emphasized that the consultant would always defer to the referring provider or patient and honor their requests.
Phase 3: Identifying Appropriate Patients
The team planned for and has since used 4 different pathways to identify possible candidates for telemedicine visits. The consult triaging process with telemedicine is an option that is brought up with patients if their travel to the facility exceeds 100 miles. Similarly, the team reviews procedural requests to optimize diagnostic yields and limit patient burden. For example, if endoscopic testing is requested to address chronic abdominal pain or other concerns that had already prompted a similar request with negative results, then the team will ask for feedback and recommend a telemedicine consultation prior to performing the procedure. Telemedicine also is offered for follow-up encounters to veterans seen in the facility for clinical or procedural evaluations if they live ≥ 40 miles away. The 2 other pathways are requests from referring providers or patients that specifically ask for telemedicine visits.
Phase 4: Implementation
Since rolling out the program in November 2016, video visits have been used for more than 150 clinic encounters. Within the first 12 months, 124 patients were seen at least once using telemedicine links. Of 144 visits, 54 (38%) were follow-up visits; the rest constituted initial consultations. Focusing on initial encounters only, veterans specifically asked for a telemedicine visit in 16 cases (17.8%). One-third of these referrals was specifically marked as a telemedicine visit by the primary care provider. In the remaining cases, the triaging personnel brought up the possibility of a telemedicine interaction and requested feedback from the referring provider.
Veterans resided in many different areas within and outside of the facility’s immediate referral area (Figure).
Abnormal bowel patterns, gastroesophageal reflux, and dyspepsia accounted for most concerns (Table 1).
Beyond obtaining contextual data and information about the specific clinical manifestations, the rationale for these encounters was a detailed discussion of the problem and treatment options available. Ablative therapy in Barrett esophagus best exemplifies the potential relevance of such an encounter: Although conceptually appealing to decrease cancer risk, the approach requires a significant commitment typically involving repeated sessions of radiofrequency ablation followed by intense endoscopic surveillance. With travel distances of several hundred miles in these cases, these encounters provide relevant information to patients and the opportunity to make informed decisions without the burden and cost of a long trip.
A shift in telemedicine encounters will likely occur that will increasingly rely on access from home computers or handheld devices. However, the initial phase of this program relied on connections through a CBOC. Coordination between 2 sites adds a level of complexity to ensure availability of space and videoconferencing equipment. To limit the logistic burden and improve cost-effectiveness, the authors did not expect or request the presence of the primary or another independent provider. Instead, the team communicated with a locally designated point person who coordinated the remote encounters and assisted in implementing some of the suggested next steps. Prior site visits and communications with referring providers had established channels of communication to define concerns or highlight findings. The same channels also allowed the team to direct its attention to specific aspects of the physical examination to support or rule out a presumptive diagnosis.
If additional testing was suggested, Telemedicine Services generally ordered the appropriate assessments unless veterans requested relying on local resources better known to personnel at the remote site. The most common diagnostic steps recommended were laboratory tests (n = 21; 14.6%), endoscopic procedures (n = 18; 12.5%), and radiologic studies (n = 17; 11.8%) (Table 2).
Most of the treatment changes focused on medication and dietary management, followed by lifestyle modifications and behavioral or psychological interventions. Some treatments, such as ablation of dysplastic epithelium in patients with Barrett esophagus or pneumatic dilation of achalasia required traveling to the George E. Wahlen VAMC. Nonetheless, the number of trips were limited as the team could assess appropriateness, explain approaches, and evaluate symptomatic outcomes with the initial or subsequent remote encounters. Most of the follow-up involved the primary care providers (n = 62; 43.1%), while repeat remote encounters were suggested in 31 visits (21.5%) and an in-person clinic follow-up in 7 cases (n = 4.9%). In the remaining cases, veterans were asked to contact the team directly or through their primary care provider if additional input was needed.
Discussion
The initial implementation of a specialty telemedicine clinic taught us several lessons that will not only guide this program expansion, but also may be relevant for others introducing telemedicine into their specialty clinics. At first glance, videoconferencing with patients resembles more conventional clinic encounters. However, it adds another angle as many steps from scheduling a visit to implementing recommendations rely on different members at the remote site. Thus, the success of such a program depends on establishing a true partnership with the teams at the various satellite sites. It also requires ongoing feedback from all team members and fine-tuning to effectively integrate it into the routine operations of both sites.
Feedback about the program has been very positive with comments often asking for an expansion beyond gastroenterology. Concerns largely were limited to scheduling problems that may become less relevant if the new telehealth initiative moves forward and enables health care providers to directly connect with computers or handheld devices at the patient’s home. Prior studies demonstrated that most individuals have access to such technology and accept it as a viable or even attractive option for medical encounters.11,12
For some, remaining in the comfort of their own home is not only more convenient, but also adds a sense of security, further adding to its appeal.13 As suggested by the economist Richard Thaler, simple nudges may be required to increase use and perhaps utility of telemedicine or e-consults.14 At this stage, it is the active choice of the referral or triaging provider to consider telemedicine as an option. To facilitate deviation from the established routine, we plan to revise the consult requests by using a drop-down menu option that brings up e-consult, telemedicine, or clinic visit as alternatives and requires an active choice rather than defaulting to conventional face-to-face visits.
Despite an overall successful launch of the specialty telemedicine clinic, several conceptual questions require additional in-depth assessments. While video visits indeed include the literal face time that characterizes normal clinic visits, does this translate into the “face value” that may contribute to treatment success? If detailed information about physical findings is needed, remote encounters require a third person at the distant site to complete this step, which may not only be a logistic burden, but also could influence the perceived utility and affect outcomes.
Previously published studies have demonstrated the effectiveness of video-based interactions and allow providers to address these points to some degree. Remote encounters have established roles in mental healthcare that is less dependent on physical findings.15 Distance monitoring of devices or biomarkers, such as blood sugar levels or blood pressure, are becoming routine and often are combined with corrective interventions.16-18
Recently completed trials showed satisfaction did not differ from conventional clinic encounters when telemedicine encounters were used to manage chronic headaches or provide postoperative follow-up after urologic surgery.19,20 For gastroenterology, telemedicine outreach after hospitalizations not only improved care, but also lowered rates of testing after discharge.21 In patients with inflammatory bowel disease, a group that was not targeted during this initial phase, proactive and close follow-up with remote technology can decrease the need for hospitalization.22
These data are consistent with encouraging feedback received. Nonetheless, it is important to assess whether this approach is superior to established and cheaper alternatives, most notably simple telephone interactions. Video-linkage obviously allows nonverbal elements of communication, which play an important role in patient preference and satisfaction, treatment implementation, and impact.7,8,23-25 Providers described patients as more focused and engaged compared with telephone interactions and valued the ability to incorporate body language in their assessment.26
Telemedicine clinics offered by specialty providers may not improve access as defined by wait times only, which would require adding more clinical time and personnel. However, it can lower barriers to care imposed by long distances between rural areas and facilities with specialized expertise. Even if a remote encounter concludes with the recommendation to visit the clinic for more detailed testing or treatment, explaining the need for such steps and involving the patient in the decision-making process may affect adherence.
Conclusion
Although the experiences of the team at George E. Wahlen VA Medical Center support the use of telemedicine in specialty clinics, the next phase of the project needs to address the utility of this approach and define the perceived value and potential problems of telemedicine. Obtaining this insight will require complex data sets with feedback from patients and referring and consulting providers. As trade-offs will likely vary between different diseases or symptoms, such studies will provide a better definition of clinical scenarios best suited for remote encounters. In addition, they may provide approximate values for distance or efforts that may make the cost of a direct clinic visit worth it, thereby defining boundary-condition.
Access to specialized services has been a consistently complex problem for many integrated health care systems, including the Veterans Health Administration (VHA). About two-thirds of veterans experience significant barriers when trying to obtain medical care.1 While these problems partly mirror difficulties that nonveterans face as well, there is a unique obligation toward those who put life and health at risk during their military service.2
To better meet demands, the VHA expanded personnel and clinic infrastructure with more providers and a network of community-based outpatient clinics (CBOC) that created more openings for clinic visits.3 Yet regional variability remains a significant problem for primary and even more so for specialty medical services.
Recent data show that more than one-fifth of all veterans live in areas with low population density and shortages of health care providers.4 The data point at a special problem in this context because these veterans often face long travel times to centers offering specialty services. The introduction of electronic consults functions as an alternative venue to obtain expert input but amounts to only 2% of total consult volume.5 A more interactive approach with face-to-face teleconferencing, case discussions, and special training led by expert clinicians has further improved access in such underserved areas and played a key role in the success of the VHA hepatitis C treatment initiative.6
Despite its clearly proven role and success, these e-consults come with some conceptual shortcomings. A key caveat is the lack of direct patient involvement. Obtaining information from the source rather than relying on symptoms documented by a third person can be essential in approaching medical problems. Experts may be able to tease out the often essential details of a history when making a diagnosis. A direct contact adds an additional, perhaps less tangible, component to the interaction that relies on verbal and nonverbal components of personal interactions and plays an important role in treatment success. Prior studies strongly link credibility of and trust in a provider as well as the related treatment success to such aspects of communication.7,8
Gastroenterology Telemedicine Services
The George E. Wahlen VA Medical Center in Salt Lake City, Utah, draws from a large catchment area that extends from the southern border of Utah to the neighboring states of Idaho, Wyoming, Nevada, and Montana. Large stretches of this territory are remote with population densities well below 5 persons per square mile. The authors therefore devised a specialty outreach program relying on telemedicine for patients with gastrointestinal and liver diseases and present the initial experience with the implementation of this program.
Phase 1: Finding the Champions
Prior studies clearly emphasized that most successful telemedicine clinics relied on key persons (“champions”) promoting the idea and carrying the additional logistic and time issues required to start and maintain the new program.9,10 Thus we created a small team that defined and refined goals, identified target groups, and worked out the logistics. Based on prior experiences, we focused initially on veterans with more chronic and likely functional disorders, such as diarrhea, constipation, dyspepsia, or nausea. The team also planned to accept patients with chronic liver disorders or abnormal test results that required further clarification. By consensus, the group excluded acute problems and bleeding as well as disorders with pain as primary manifestations. The underlying assumption was that a direct physical examination was less critical in most of these cases.
Phase 2: Outreach
Clinic managers and medical directors of the affiliated CBOC were informed of the planned telemedicine clinic. Also, we identified local champions who could function as point persons and assist in the organization of visits. One member of the team personally visited key sites to discuss needs and opportunities with CBOC personnel during a routine staff meeting. The goal was to introduce the program, the key personnel, to explain criteria for appropriate candidates that may benefit from telemedicine consults, and to agree on a referral pathway. Finally, we emphasized that the consultant would always defer to the referring provider or patient and honor their requests.
Phase 3: Identifying Appropriate Patients
The team planned for and has since used 4 different pathways to identify possible candidates for telemedicine visits. The consult triaging process with telemedicine is an option that is brought up with patients if their travel to the facility exceeds 100 miles. Similarly, the team reviews procedural requests to optimize diagnostic yields and limit patient burden. For example, if endoscopic testing is requested to address chronic abdominal pain or other concerns that had already prompted a similar request with negative results, then the team will ask for feedback and recommend a telemedicine consultation prior to performing the procedure. Telemedicine also is offered for follow-up encounters to veterans seen in the facility for clinical or procedural evaluations if they live ≥ 40 miles away. The 2 other pathways are requests from referring providers or patients that specifically ask for telemedicine visits.
Phase 4: Implementation
Since rolling out the program in November 2016, video visits have been used for more than 150 clinic encounters. Within the first 12 months, 124 patients were seen at least once using telemedicine links. Of 144 visits, 54 (38%) were follow-up visits; the rest constituted initial consultations. Focusing on initial encounters only, veterans specifically asked for a telemedicine visit in 16 cases (17.8%). One-third of these referrals was specifically marked as a telemedicine visit by the primary care provider. In the remaining cases, the triaging personnel brought up the possibility of a telemedicine interaction and requested feedback from the referring provider.
Veterans resided in many different areas within and outside of the facility’s immediate referral area (Figure).
Abnormal bowel patterns, gastroesophageal reflux, and dyspepsia accounted for most concerns (Table 1).
Beyond obtaining contextual data and information about the specific clinical manifestations, the rationale for these encounters was a detailed discussion of the problem and treatment options available. Ablative therapy in Barrett esophagus best exemplifies the potential relevance of such an encounter: Although conceptually appealing to decrease cancer risk, the approach requires a significant commitment typically involving repeated sessions of radiofrequency ablation followed by intense endoscopic surveillance. With travel distances of several hundred miles in these cases, these encounters provide relevant information to patients and the opportunity to make informed decisions without the burden and cost of a long trip.
A shift in telemedicine encounters will likely occur that will increasingly rely on access from home computers or handheld devices. However, the initial phase of this program relied on connections through a CBOC. Coordination between 2 sites adds a level of complexity to ensure availability of space and videoconferencing equipment. To limit the logistic burden and improve cost-effectiveness, the authors did not expect or request the presence of the primary or another independent provider. Instead, the team communicated with a locally designated point person who coordinated the remote encounters and assisted in implementing some of the suggested next steps. Prior site visits and communications with referring providers had established channels of communication to define concerns or highlight findings. The same channels also allowed the team to direct its attention to specific aspects of the physical examination to support or rule out a presumptive diagnosis.
If additional testing was suggested, Telemedicine Services generally ordered the appropriate assessments unless veterans requested relying on local resources better known to personnel at the remote site. The most common diagnostic steps recommended were laboratory tests (n = 21; 14.6%), endoscopic procedures (n = 18; 12.5%), and radiologic studies (n = 17; 11.8%) (Table 2).
Most of the treatment changes focused on medication and dietary management, followed by lifestyle modifications and behavioral or psychological interventions. Some treatments, such as ablation of dysplastic epithelium in patients with Barrett esophagus or pneumatic dilation of achalasia required traveling to the George E. Wahlen VAMC. Nonetheless, the number of trips were limited as the team could assess appropriateness, explain approaches, and evaluate symptomatic outcomes with the initial or subsequent remote encounters. Most of the follow-up involved the primary care providers (n = 62; 43.1%), while repeat remote encounters were suggested in 31 visits (21.5%) and an in-person clinic follow-up in 7 cases (n = 4.9%). In the remaining cases, veterans were asked to contact the team directly or through their primary care provider if additional input was needed.
Discussion
The initial implementation of a specialty telemedicine clinic taught us several lessons that will not only guide this program expansion, but also may be relevant for others introducing telemedicine into their specialty clinics. At first glance, videoconferencing with patients resembles more conventional clinic encounters. However, it adds another angle as many steps from scheduling a visit to implementing recommendations rely on different members at the remote site. Thus, the success of such a program depends on establishing a true partnership with the teams at the various satellite sites. It also requires ongoing feedback from all team members and fine-tuning to effectively integrate it into the routine operations of both sites.
Feedback about the program has been very positive with comments often asking for an expansion beyond gastroenterology. Concerns largely were limited to scheduling problems that may become less relevant if the new telehealth initiative moves forward and enables health care providers to directly connect with computers or handheld devices at the patient’s home. Prior studies demonstrated that most individuals have access to such technology and accept it as a viable or even attractive option for medical encounters.11,12
For some, remaining in the comfort of their own home is not only more convenient, but also adds a sense of security, further adding to its appeal.13 As suggested by the economist Richard Thaler, simple nudges may be required to increase use and perhaps utility of telemedicine or e-consults.14 At this stage, it is the active choice of the referral or triaging provider to consider telemedicine as an option. To facilitate deviation from the established routine, we plan to revise the consult requests by using a drop-down menu option that brings up e-consult, telemedicine, or clinic visit as alternatives and requires an active choice rather than defaulting to conventional face-to-face visits.
Despite an overall successful launch of the specialty telemedicine clinic, several conceptual questions require additional in-depth assessments. While video visits indeed include the literal face time that characterizes normal clinic visits, does this translate into the “face value” that may contribute to treatment success? If detailed information about physical findings is needed, remote encounters require a third person at the distant site to complete this step, which may not only be a logistic burden, but also could influence the perceived utility and affect outcomes.
Previously published studies have demonstrated the effectiveness of video-based interactions and allow providers to address these points to some degree. Remote encounters have established roles in mental healthcare that is less dependent on physical findings.15 Distance monitoring of devices or biomarkers, such as blood sugar levels or blood pressure, are becoming routine and often are combined with corrective interventions.16-18
Recently completed trials showed satisfaction did not differ from conventional clinic encounters when telemedicine encounters were used to manage chronic headaches or provide postoperative follow-up after urologic surgery.19,20 For gastroenterology, telemedicine outreach after hospitalizations not only improved care, but also lowered rates of testing after discharge.21 In patients with inflammatory bowel disease, a group that was not targeted during this initial phase, proactive and close follow-up with remote technology can decrease the need for hospitalization.22
These data are consistent with encouraging feedback received. Nonetheless, it is important to assess whether this approach is superior to established and cheaper alternatives, most notably simple telephone interactions. Video-linkage obviously allows nonverbal elements of communication, which play an important role in patient preference and satisfaction, treatment implementation, and impact.7,8,23-25 Providers described patients as more focused and engaged compared with telephone interactions and valued the ability to incorporate body language in their assessment.26
Telemedicine clinics offered by specialty providers may not improve access as defined by wait times only, which would require adding more clinical time and personnel. However, it can lower barriers to care imposed by long distances between rural areas and facilities with specialized expertise. Even if a remote encounter concludes with the recommendation to visit the clinic for more detailed testing or treatment, explaining the need for such steps and involving the patient in the decision-making process may affect adherence.
Conclusion
Although the experiences of the team at George E. Wahlen VA Medical Center support the use of telemedicine in specialty clinics, the next phase of the project needs to address the utility of this approach and define the perceived value and potential problems of telemedicine. Obtaining this insight will require complex data sets with feedback from patients and referring and consulting providers. As trade-offs will likely vary between different diseases or symptoms, such studies will provide a better definition of clinical scenarios best suited for remote encounters. In addition, they may provide approximate values for distance or efforts that may make the cost of a direct clinic visit worth it, thereby defining boundary-condition.
1. Elnitsky CA, Andresen EM, Clark ME, McGarity S, Hall CG, Kerns RD. Access to the US Department of Veterans Affairs health system: self-reported barriers to care among returnees of Operations Enduring Freedom and Iraqi Freedom. BMC Health Serv Res. 2013;13:498.
2. Woolhandler S, Himmelstein DU, Distajo R, et al. America’s neglected veterans: 1.7 million who served have no health coverage. Int J Health Serv. 2005;35(2):313-323.
3. Rosenheck R. Primary care satellite clinics and improved access to general and mental health services. Health Serv Res. 2000;35:777-790.
4. Doyle JM, Streeter RA. Veterans’ location in health professional shortage areas: implications for access to care and workforce supply. Health Serv Res. 2017;52(suppl 1):459-480.
5. Kirsh S, Carey E, Aron DC, et al. Impact of a national specialty e-consultation implementation project on access. Am J Manag Care. 2015;21(12):e648-e654.
6. Belperio PS, Chartier M, Ross DB, Alaigh P, Shulkin D. Curing hepatitis C virus infection: best practices from the U.S. Department of Veterans Affairs. Ann Intern Med. 2017;167(7):499-504.
7. Kaptchuk TJ, Kelley JM, Conboy LA, et al. Components of placebo effect: randomised controlled trial in patients with irritable bowel syndrome. BMJ. 2008;333(7651):999-1003.
8. Weinland SR, Morris CB, Dalton C, et al. Cognitive factors affect treatment response to medical and psychological treatments in functional bowel disorders. Am J Gastroenterol. 2010;105(6):1397-1406.
9. Wade V, Eliott J. The role of the champion in telehealth service development: a qualitative analysis. J Telemed Telecare. 2012;18(8):490-492.
10. Postema TR, Peeters JM, Friele RD. Key factors influencing the implementation success of a home telecare application. Int J Med Inform. 2012;81(6):415-423.
11. Tahir D. Trump and VA unveil telehealth initiative. https://www.politico.com/tipsheets/morning-ehealth/2017/08/04/trump-and-va-unveil-telehealth-initiative-221706. Published August 4, 2017. Accessed July 11, 2018.
12. Gardner MR, Jenkins SM, O’Neil DA, Gardner MR, Jenkins SM, O’Neil DA. Perceptions of video-based appointments from the patient’s home: a patient survey. Telemed J E Health. 2015;21(4):281-285.
13. Powell RE, Henstenburg JM, Cooper G, Hollander JE, Rising KL. Patient perceptions of telehealth primary care video visits. Ann Fam Med. 2017;15(3):225-229.
14. Benartzi S, Beshears J, Milkman KL, et al. Should governments invest more in nudging? Psychol Sci. 2017;28(8):1041-1055.
15. Turgoose D, Ashwick R, Murphy D. Systematic review of lessons learned from delivering tele-therapy to veterans with post-traumatic stress disorder. J Telemed Telecare. 2017:1357633x17730443.
16. Dalouk K, Gandhi N, Jessel P, et al. Outcomes of telemedicine video-conferencing clinic versus in-person clinic follow-up for implantable cardioverter-defibrillator recipients. Circ Arrhythm Electrophysiol. 2017;10(9) pii: e005217.
17. Warren R, Carlisle K, Mihala G, Scuffham PA. Effects of telemonitoring on glycaemic control and healthcare costs in type 2 diabetes: a randomised controlled trial. J Telemed Telecare. 2017:1357633x17723943.
18. Tucker KL, Sheppard JP, Stevens R, et al. Self-monitoring of blood pressure in hypertension: a systematic review and individual patient data meta-analysis. PLoS Med. 2017;14(9):e1002389.
19. Müller KI, Alstadhaug KB, Bekkelund SI. Headache patients’ satisfaction with telemedicine: a 12-month follow-up randomized non-inferiority trial. Eur J Neurol. 2017;24(6):807-815.
20. Viers BR, Lightner DJ, Rivera ME, et al. Efficiency, satisfaction, and costs for remote video visits following radical prostatectomy: a randomized controlled trial. Eur Urol. 2015;68:729-735.
21. Wallace P, Barber J, Clayton W, et al. Virtual outreach: a randomised controlled trial and economic evaluation of joint teleconferenced medical consultations. Health Technol Assess. 2004;8(50):1-106, iii-iv.
22. de Jong MJ, van der Meulen-de Jong AE, Romberg-Camps MJ, et al. Telemedicine for management of inflammatory bowel disease (myIBDcoach): a pragmatic, multicentre, randomised controlled trial. Lancet. 2017;390(10098):959-968.
23. Czerniak E, Biegon A, Ziv A, et al. Manipulating the placebo response in experimental pain by altering doctor’s performance style. Front Psychol. 2016;7:874.
24. Moffet HH, Parker MM, Sarkar U, et al. Adherence to laboratory test requests by patients with diabetes: the Diabetes Study of Northern California (DISTANCE). Am J Manag Care. 2011;17(5):339-344.
25. Richter KP, Shireman TI, Ellerbeck EF, et al. Comparative and cost effectiveness of telemedicine versus telephone counseling for smoking cessation. J Med Internet Res. 2015;17(5):e113.
26. Voils CI, Venne VL, Weidenbacher H, Sperber N, Datta S. Comparison of telephone and televideo modes for delivery of genetic counseling: a randomized trial. J Genet Couns. 2018;27(2):339-348.
1. Elnitsky CA, Andresen EM, Clark ME, McGarity S, Hall CG, Kerns RD. Access to the US Department of Veterans Affairs health system: self-reported barriers to care among returnees of Operations Enduring Freedom and Iraqi Freedom. BMC Health Serv Res. 2013;13:498.
2. Woolhandler S, Himmelstein DU, Distajo R, et al. America’s neglected veterans: 1.7 million who served have no health coverage. Int J Health Serv. 2005;35(2):313-323.
3. Rosenheck R. Primary care satellite clinics and improved access to general and mental health services. Health Serv Res. 2000;35:777-790.
4. Doyle JM, Streeter RA. Veterans’ location in health professional shortage areas: implications for access to care and workforce supply. Health Serv Res. 2017;52(suppl 1):459-480.
5. Kirsh S, Carey E, Aron DC, et al. Impact of a national specialty e-consultation implementation project on access. Am J Manag Care. 2015;21(12):e648-e654.
6. Belperio PS, Chartier M, Ross DB, Alaigh P, Shulkin D. Curing hepatitis C virus infection: best practices from the U.S. Department of Veterans Affairs. Ann Intern Med. 2017;167(7):499-504.
7. Kaptchuk TJ, Kelley JM, Conboy LA, et al. Components of placebo effect: randomised controlled trial in patients with irritable bowel syndrome. BMJ. 2008;333(7651):999-1003.
8. Weinland SR, Morris CB, Dalton C, et al. Cognitive factors affect treatment response to medical and psychological treatments in functional bowel disorders. Am J Gastroenterol. 2010;105(6):1397-1406.
9. Wade V, Eliott J. The role of the champion in telehealth service development: a qualitative analysis. J Telemed Telecare. 2012;18(8):490-492.
10. Postema TR, Peeters JM, Friele RD. Key factors influencing the implementation success of a home telecare application. Int J Med Inform. 2012;81(6):415-423.
11. Tahir D. Trump and VA unveil telehealth initiative. https://www.politico.com/tipsheets/morning-ehealth/2017/08/04/trump-and-va-unveil-telehealth-initiative-221706. Published August 4, 2017. Accessed July 11, 2018.
12. Gardner MR, Jenkins SM, O’Neil DA, Gardner MR, Jenkins SM, O’Neil DA. Perceptions of video-based appointments from the patient’s home: a patient survey. Telemed J E Health. 2015;21(4):281-285.
13. Powell RE, Henstenburg JM, Cooper G, Hollander JE, Rising KL. Patient perceptions of telehealth primary care video visits. Ann Fam Med. 2017;15(3):225-229.
14. Benartzi S, Beshears J, Milkman KL, et al. Should governments invest more in nudging? Psychol Sci. 2017;28(8):1041-1055.
15. Turgoose D, Ashwick R, Murphy D. Systematic review of lessons learned from delivering tele-therapy to veterans with post-traumatic stress disorder. J Telemed Telecare. 2017:1357633x17730443.
16. Dalouk K, Gandhi N, Jessel P, et al. Outcomes of telemedicine video-conferencing clinic versus in-person clinic follow-up for implantable cardioverter-defibrillator recipients. Circ Arrhythm Electrophysiol. 2017;10(9) pii: e005217.
17. Warren R, Carlisle K, Mihala G, Scuffham PA. Effects of telemonitoring on glycaemic control and healthcare costs in type 2 diabetes: a randomised controlled trial. J Telemed Telecare. 2017:1357633x17723943.
18. Tucker KL, Sheppard JP, Stevens R, et al. Self-monitoring of blood pressure in hypertension: a systematic review and individual patient data meta-analysis. PLoS Med. 2017;14(9):e1002389.
19. Müller KI, Alstadhaug KB, Bekkelund SI. Headache patients’ satisfaction with telemedicine: a 12-month follow-up randomized non-inferiority trial. Eur J Neurol. 2017;24(6):807-815.
20. Viers BR, Lightner DJ, Rivera ME, et al. Efficiency, satisfaction, and costs for remote video visits following radical prostatectomy: a randomized controlled trial. Eur Urol. 2015;68:729-735.
21. Wallace P, Barber J, Clayton W, et al. Virtual outreach: a randomised controlled trial and economic evaluation of joint teleconferenced medical consultations. Health Technol Assess. 2004;8(50):1-106, iii-iv.
22. de Jong MJ, van der Meulen-de Jong AE, Romberg-Camps MJ, et al. Telemedicine for management of inflammatory bowel disease (myIBDcoach): a pragmatic, multicentre, randomised controlled trial. Lancet. 2017;390(10098):959-968.
23. Czerniak E, Biegon A, Ziv A, et al. Manipulating the placebo response in experimental pain by altering doctor’s performance style. Front Psychol. 2016;7:874.
24. Moffet HH, Parker MM, Sarkar U, et al. Adherence to laboratory test requests by patients with diabetes: the Diabetes Study of Northern California (DISTANCE). Am J Manag Care. 2011;17(5):339-344.
25. Richter KP, Shireman TI, Ellerbeck EF, et al. Comparative and cost effectiveness of telemedicine versus telephone counseling for smoking cessation. J Med Internet Res. 2015;17(5):e113.
26. Voils CI, Venne VL, Weidenbacher H, Sperber N, Datta S. Comparison of telephone and televideo modes for delivery of genetic counseling: a randomized trial. J Genet Couns. 2018;27(2):339-348.