User login
Chagas disease: An unusual and dangerous infection for both mother and baby
CASE Pregnant woman with a suspected parasitic infection
A 20-year-old, previously healthy, primigravid woman at 24 weeks’ gestation immigrated from Bolivia to the United States 3 days ago. On the morning of her international flight, she awoke to discover a small insect bite just below her left eye. She sought medical evaluation because her eyelid is now significantly swollen, and she has a headache, anorexia, fatigue, and a fever of 38.4° C. The examining physician ordered a polymerase chain reaction (PCR) test for Trypanosoma cruzi, and the test is positive.
- How should this patient be treated during, and after, her delivery?
- Does this infection pose a risk to the newborn baby?
- What type of surveillance and treatment is indicated for the baby?
Chagas disease is common in South America, Central America, and Mexico and is well known to physicians in those countries. Clinicians who practice in the United States are much less familiar with the condition, but it is becoming increasingly common as a result of international travel within the Americas.
In this article, we review the interesting microbiology and epidemiology of Chagas disease, focus on its clinical manifestations, and discuss the most useful diagnostic tests for the illness. We conclude with a summary of preventive and treatment measures, with particular emphasis on managing the disease in pregnancy.
How Chagas disease is transmitted and who is at risk
Chagas disease was named in honor of a Brazilian physician, Carlos Chagas, who first described the condition in 1909. The disease is endemic in South America, Central America, and Mexico, and, recently, its prevalence has increased in the southern United States. Approximately 300,000 people in the United States are infected.1,2
The illness is caused by the parasite Trypanosoma cruzi, and it is also known as American trypanosomiasis. The parasite is spread primarily by the bite of triatomine insects (“kissing bugs”). Approximately 60% of these insects are infected with the parasite. The insects live and thrive in the interspaces of mud walls (adobe homes) and thatched roofs. At night, the insects leave their darkened spaces and feed on the exposed skin of sleeping persons. They are particularly likely to bite the moist skin surfaces near the eye and mouth, and, as they do, they defecate and excrete the parasite into the blood vessels beneath the skin. Within the blood, the trypomastigotes invade various host cells. Inside the host cells, the organism transforms into an amastigote, which is the replicative form of the parasite. After several rounds of replication, the amastigote transforms back into a trypomastigote, bursts from the cell, and goes on to infect other host cells.1
In addition to transmission by the insect vector, the parasite also can be transmitted by blood transfusion and organ donation. When contaminated blood is transfused, the risk of transmission is approximately 10% to 25% for each unit. Following implementation of effective screening programs by blood banks in Central America, South America, Mexico, and the United States, the risk of transmission from undetected infection is now approximately 1:200,000 per unit.
When a transplant procedure with an infected heart is performed, the risk of transmission is 75% to 100%. For liver transplants, the frequency of transmission is 0% to 29%; for kidney transplants, the risk of transmission is 0% to 19%.
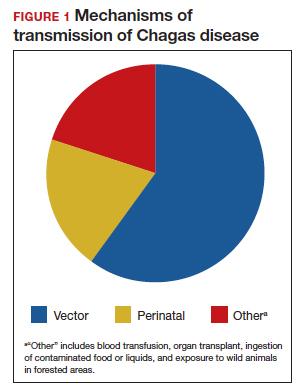
Consumption of contaminated food or drink, particularly nonpasteurized items sold by street vendors, is also an important mechanism of transmission. In addition, transmission can occur as a result of laboratory exposure and by exposure to wild animals (racoons, opossums, marmosets, bats, armadillos) in forested areas. Finally, perinatal transmission now accounts for about 22% of infections. As effective vector control programs have been introduced in endemic areas, the proportion of cases caused by the insect vector has steadily decreased1-3 (FIGURE 1).
Continue to: Clinical manifestations of Chagas disease...
Clinical manifestations of Chagas disease
Chagas disease occurs in 2 stages, acute and chronic.1,2,4 In patients who are infected via an insect vector, the acute stage typically begins 1 to 2 weeks after the insect bite. This phase of the illness usually lasts 4 to 8 weeks and almost always resolves without treatment.
Some infected patients will be completely free of symptoms. Others will have manifestations such as:
- fever
- malaise
- headache
- hepatosplenomegaly
- lymphadenopathy
- swollen nodule at the site of infection
—Romaña’s sign, when the lesion is on the eyelid
—Chagoma, when the lesion is elsewhere on the skin.
Fortunately, less than 5% of patients will have severe illness, manifested by myocarditis, pericarditis, encephalitis, or meningitis.
People infected by ingestion of the parasite in food or drink often become more severely ill within 3 weeks. Their clinical manifestations include fever, vomiting, dyspnea, cough, chest pain, abdominal pain, and myalgias. Individuals infected through organ transplant or blood transfusion present more like those infected by the insect vector, but their illness may not develop until several weeks to 5 months after exposure.
In the absence of effective treatment, approximately 40% of patients with acute infection will develop chronic infection, often several decades later. The most common, and most ominous, feature of chronic illness is cardiac disease, experienced by about 30% of patients. Cardiac disease may be manifested as a serious arrhythmia, chest pain, congestive heart failure, or thromboembolism.
The other organ system that is likely to be adversely affected in patients with chronic disease is the gastrointestinal (GI) system, and approximately 10% of chronically infected patients experience this complication. Patients may develop a dilated esophagus, which leads to odynophagia and dysphagia. Diminished motility in other areas of the GI tract also may result in chronic constipation and even bowel obstruction. Chronically infected patients who are immunosuppressed due to HIV infection may become gravely ill as a result of encephalitis and brain abscesses. Cardiac and GI dysfunction is due to the parasite’s massive destruction of nerve endings.
Continue to: Making the diagnosis...
Making the diagnosis
The diagnosis of Chagas disease begins with screening patients who have epidemiologic risk factors that place them at high risk for contracting the infection and at significantly increased risk for morbidity and mortality as a result of either the acute infection or the later chronic stage of infection. A thorough history is vital in the evaluation because the acute illness can have such vague clinical manifestations, and many patients remain asymptomatic until signs of chronic infection appear.
Risk factors that warrant screening include being born in a country endemic for Chagas disease, living in an endemic country for more than 6 months, living with someone who has a confirmed diagnosis, residing in a house made of natural materials (mud walls, thatched roof) in an endemic area, and a history of discovering the triatomine bug in the household.
Screening options include serology, microscopy, and PCR testing. Screening with a single, highly sensitive immunoglobulin G (IgG) serologic test is recommended for nonendemic clinical or community settings. In patients who were born in or who lived in an endemic area for more than 6 months, special consideration should be given to screening women of reproductive age, patients of all ages who were born to a mother with a confirmed diagnosis, individuals who were exposed to a triatomine insect, and people who are immunocompromised.5
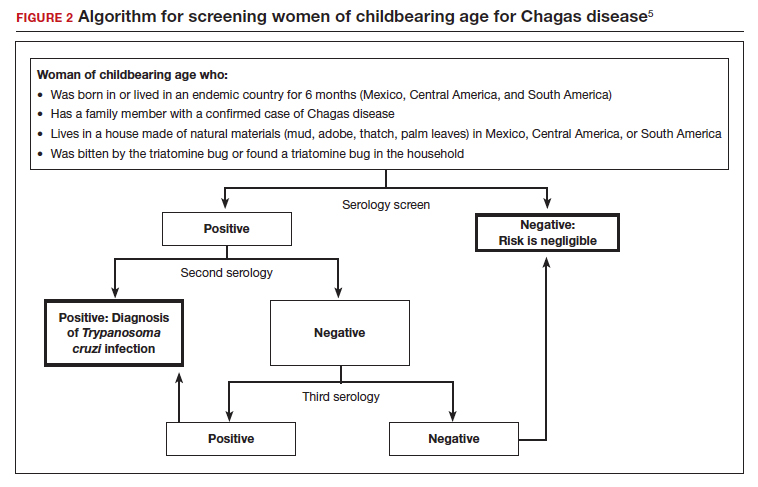
A positive serologic test should be confirmed with a second assay based on a different antigen. Currently, 4 IgG tests have US Food and Drug Administration (FDA) approval for diagnosis. If a patient has 2 positive serologic tests, the diagnosis is confirmed, regardless of clinical presentation. Discordant results warrant a third test to differentiate between positive and negative results (FIGURE 2).5 All patients with a confirmed diagnosis should have an electrocardiogram, echocardiogram, and abdominal computed tomography (CT) scan to assess for cardiac or GI abnormalities.
Neonates and infants of mothers with suspected or confirmed infection merit special attention. These children may demonstrate hepatomegaly, splenomegaly, anemia, thrombocytopenia, pneumonitis, heart failure, cardiac arrhythmias, or meningoencephalitis. Newborns delivered to infected mothers will invariably have positive tests for IgG antibody because of transplacental transfer of maternal antibody. Therefore, they should be evaluated by PCR or by direct microscopic examination of the blood for trypomastigotes. In neonates with a negative initial result, repeat testing should be performed by PCR at 4 to 6 weeks of age. Even if the second screening test is negative, the infant should be retested at 9 to 12 months. At this point, maternal IgG no longer should be circulating in the infant’s blood. Three negative tests should effectively rule out T cruzi infection (FIGURE 3).5-7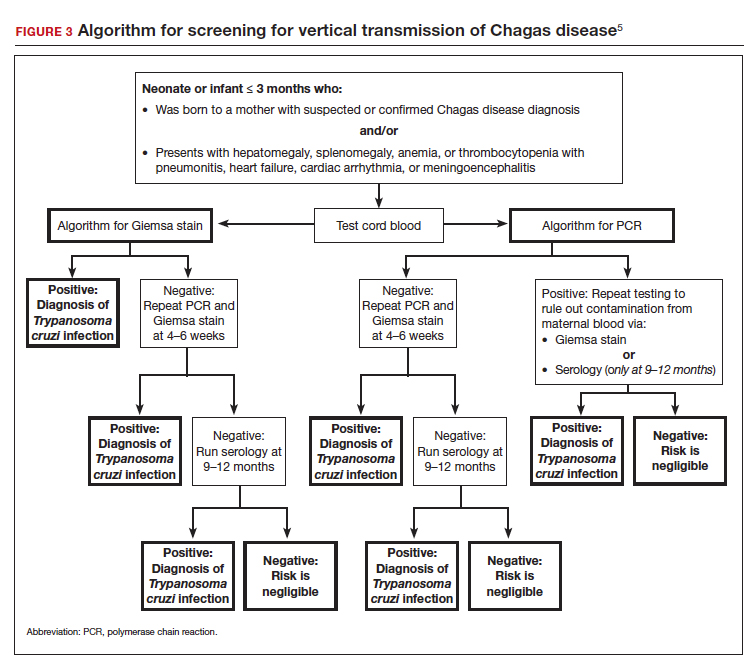
Organ recipients merit special consideration because, in these individuals, the late stages of Chagas disease may be fatal. In these patients, the preferred diagnostic test is PCR. For transplant patients, monitoring should occur every week for 2 months, bimonthly for the third month, and monthly for 6 months after transplantation. Routine monitoring is not recommended in patients with HIV infection who show no clinical signs of Chagas disease and who are not from endemic areas.
Treatment options
No vaccine or hyperimmune globulin can be used to treat Chagas disease. At this time, 2 antiparasitic drugs are available to treat the condition. One is benznidazole, which inhibits DNA, RNA, and protein synthesis within the microorganism. The medication is given in a dose of 5 to 8 mg/kg per day, divided into 2 doses, for 60 days. Benznidazole is FDA approved for the treatment of individuals older than age 2. It has been used off-label in children younger than 2 years of age. The drug is commercially available at http://www.benznidazoletablets.com.
Benznidazole causes multiple minor side effects and several very serious adverse effects. The serious adverse effects include acute generalized exanthematous pustulosis, toxic epidermal necrolysis, peripheral neuropathy, marrow suppression, and hepatotoxicity. Benznidazole has been teratogenic and carcinogenic in animal studies and should not be used in pregnancy.1,3,6
The second drug is nifurtimox. This drug is FDA approved for the treatment of Chagas disease in adults and for newborns and young children. It is commercially available for pharmacies to purchase from several drug wholesalers. Nifurtimox produces reactive oxygen species and toxic intermediates that induce DNA damage and cause cell death of the microorganism. The appropriate oral dose is 8 to 10 mg/kg per day, divided into 3 to 4 equal doses. The duration of treatment is 60 to 90 days, depending on the patient’s response. Like benznidazole, nifurtimox also is highly toxic. Severe adverse effects include a hypersensitivity reaction, anaphylaxis, angioedema, syncope, seizures, and psychosis. Nifurtimox also is teratogenic and is contraindicated in pregnancy.1,3,6
Clinicians who have questions about the use of either of these medications should contact the Centers for Disease Control and Prevention, Division of Parasitic Diseases public inquiries telephone line at (404) 718-4745.
Potential for cure. When either benznidazole or nifurtimox is administered early in the course of a patient’s acute infection, the chance for complete cure is excellent. The same is true for early treatment of the infected neonate. When treatment is delayed, or if it cannot be completed because of intolerable adverse effects, the prognosis for complete cure is diminished.
In adults who have chronic disease, antiparasitic treatment is unlikely to be effective. In such a situation, secondary treatment is directed toward correction of heart failure, control of cardiac rhythm disturbances, and control of GI motility disorders. For both cardiac and GI conditions, medication and surgery may be indicated. Antiparasitic treatment is more effective in children with chronic disease but it is still not uniformly effective.1,3,5,6
Preventing infection
Vector control is the key to preventing infection in areas where Chagas disease is endemic. One important, but often financially unaffordable, measure is construction of homes with building materials that do not support the growth of the triatomine insects that transmit the disease. A second critical preventive measure is the spraying of mud and thatched homes and surrounding areas with long-lasting insecticides. Pyrethroids are the preferred agents today. Alternative agents include fenitrothion and bendiocarb.1
Other important preventive measures include:
- screening the blood supply for T cruzi and eliminating units contaminated with the parasite
- screening for the parasite in organs targeted for transplant
- screening infected women of reproductive age in endemic areas and treating those who are positive before they become pregnant; this measure may be almost 95% effective in preventing congenital infection
- using mosquito netting when housing is insecure and air conditioning is not available
- in endemic areas, avoiding unpasteurized fruit drinks and unwashed fruits and vegetables.
Unique considerations in pregnancy
Chagas disease does not cause specific anatomic birth defects. However, infected women are more likely to experience spontaneous abortion, preterm premature rupture of membranes, preterm labor, and fetal growth restriction. Overall, the risk of perinatal transmission is approximately 5%, but it may be higher in women who have a very high parasite load. Infected neonates who remain untreated are at risk for developing the serious sequelae of chronic infection. At least half of neonates who are infected will initially be asymptomatic. Therefore, screening of at-risk neonates is essential in order to implement effective treatment.3,6
As noted earlier, the usual drugs used for treating Chagas disease should not be used in pregnancy. Nevertheless, it is still important to screen certain individuals for infection and, subsequently, target them and their neonates for treatment immediately following delivery. The following pregnant patients should be screened5,6:
- women with clinical manifestations that suggest acute or chronic infection
- women from areas of the world in which Chagas disease is endemic, namely, from the southern United States to northern Chile and Argentina. Although the disease is endemic in 21 countries, the countries with the highest prevalence are Bolivia, Argentina, and Paraguay.
- newborns delivered to mothers who have been identified as infected.
As mentioned, several tests are available for screening: PCR, antibody assays, and examination of peripheral blood smears. At least 2 test results should be positive to confirm the diagnosis of infection. Neonates should be followed for 9 to 12 months after delivery to determine if perinatal transmission has occurred. Treatment with antiparasitic drugs is indicated for all infected children.5
CASE Continue surveillance during pregnancy, treat after delivery
This patient should not be treated during pregnancy because the 2 major antiparasitic drugs are teratogenic. Antenatally, she should be followed for evidence of preterm labor and fetal growth restriction. She also should have an electrocardiogram and echocardiogram to evaluate for cardiac disease. Immediately after delivery, the patient should be treated with benznidazole for 60 days. Breastfeeding is acceptable. Her neonate should be screened for infection for up to 9 months, following the algorithm outlined earlier (FIGURE 3), and treated if the surveillance tests are positive. ●
- Chagas disease is caused by the parasite Trypanosoma cruzi, which is spread by the bite of the triatomine insect (the “kissing bug”).
- The condition is widespread among impoverished populations in South America, Central America, and Mexico, but it is rare in the United States except in individuals who immigrated here from endemic areas.
- Chagas disease evolves through 2 phases: acute and chronic. Manifestations of acute infection include fever, malaise, headache, hepatosplenomegaly, lymphadenopathy, and swelling at the site of the insect bite. The chronic phase is manifested by serious cardiac and gastrointestinal dysfunction.
- The diagnosis can be established by identifying the organism in a blood smear and by detecting antibody or antigen in the blood.
- The 2 drugs of choice for treatment of Chagas disease are benznidazole and nifurtimox. These drugs are teratogenic and are contraindicated in pregnancy.
- Women at risk for infection should be screened prior to, or during, pregnancy. Infants of infected mothers should be screened for infection for up to 9 to 12 months after delivery and treated if they test positive. Treatment of the infant is almost 100% effective in preventing chronic illness.
- Bern C. Chagas disease: epidemiology, screening, and prevention. UpToDate. Updated April 8, 2022. Accessed October 6, 2022. https://www.uptodate.com/contents /chagas-disease-epidemiology-screening-and-prevention
- Chagas disease. Cleveland Clinic. Reviewed October 8, 2021. Accessed October 6, 2022. https://my.clevelandclinic.org /health/diseases/21876-chagas-disease
- Howard EJ, Xiong X, Carlier Y, et al. Frequency of the congenital transmission of Trypanosoma cruzi: a systematic review and meta-analysis. BJOG. 2014;121:22-33.
- Chagas disease. Mayo Clinic. November 12, 2020. Accessed October 6, 2022. https://www.mayoclinic.org/diseases -conditions/chagas-disease/symptoms-causes/syc-20356212
- Forsyth CJ, Manne-Goehler J, Bern C, et al. Recommendations for screening and diagnosis of Chagas disease in the United States. J Infect Dis. 2022;225:1601-1610.
- Torrico F, Alonso-Vega C, Suarez E. et al. Maternal Trypanosoma cruzi infection, pregnancy outcome, morbidity, and mortality of congenitally infected and non-infected newborns in Bolivia. Am J Trop Med Hyg. 2004;70:201-209.
- Messenger LA, Bern C. Congenital Chagas disease: current diagnostics, limitations and future perspectives. Curr Opin Infect Dis. 2018;31:415-421.
CASE Pregnant woman with a suspected parasitic infection
A 20-year-old, previously healthy, primigravid woman at 24 weeks’ gestation immigrated from Bolivia to the United States 3 days ago. On the morning of her international flight, she awoke to discover a small insect bite just below her left eye. She sought medical evaluation because her eyelid is now significantly swollen, and she has a headache, anorexia, fatigue, and a fever of 38.4° C. The examining physician ordered a polymerase chain reaction (PCR) test for Trypanosoma cruzi, and the test is positive.
- How should this patient be treated during, and after, her delivery?
- Does this infection pose a risk to the newborn baby?
- What type of surveillance and treatment is indicated for the baby?
Chagas disease is common in South America, Central America, and Mexico and is well known to physicians in those countries. Clinicians who practice in the United States are much less familiar with the condition, but it is becoming increasingly common as a result of international travel within the Americas.
In this article, we review the interesting microbiology and epidemiology of Chagas disease, focus on its clinical manifestations, and discuss the most useful diagnostic tests for the illness. We conclude with a summary of preventive and treatment measures, with particular emphasis on managing the disease in pregnancy.
How Chagas disease is transmitted and who is at risk
Chagas disease was named in honor of a Brazilian physician, Carlos Chagas, who first described the condition in 1909. The disease is endemic in South America, Central America, and Mexico, and, recently, its prevalence has increased in the southern United States. Approximately 300,000 people in the United States are infected.1,2
The illness is caused by the parasite Trypanosoma cruzi, and it is also known as American trypanosomiasis. The parasite is spread primarily by the bite of triatomine insects (“kissing bugs”). Approximately 60% of these insects are infected with the parasite. The insects live and thrive in the interspaces of mud walls (adobe homes) and thatched roofs. At night, the insects leave their darkened spaces and feed on the exposed skin of sleeping persons. They are particularly likely to bite the moist skin surfaces near the eye and mouth, and, as they do, they defecate and excrete the parasite into the blood vessels beneath the skin. Within the blood, the trypomastigotes invade various host cells. Inside the host cells, the organism transforms into an amastigote, which is the replicative form of the parasite. After several rounds of replication, the amastigote transforms back into a trypomastigote, bursts from the cell, and goes on to infect other host cells.1
In addition to transmission by the insect vector, the parasite also can be transmitted by blood transfusion and organ donation. When contaminated blood is transfused, the risk of transmission is approximately 10% to 25% for each unit. Following implementation of effective screening programs by blood banks in Central America, South America, Mexico, and the United States, the risk of transmission from undetected infection is now approximately 1:200,000 per unit.
When a transplant procedure with an infected heart is performed, the risk of transmission is 75% to 100%. For liver transplants, the frequency of transmission is 0% to 29%; for kidney transplants, the risk of transmission is 0% to 19%.
Consumption of contaminated food or drink, particularly nonpasteurized items sold by street vendors, is also an important mechanism of transmission. In addition, transmission can occur as a result of laboratory exposure and by exposure to wild animals (racoons, opossums, marmosets, bats, armadillos) in forested areas. Finally, perinatal transmission now accounts for about 22% of infections. As effective vector control programs have been introduced in endemic areas, the proportion of cases caused by the insect vector has steadily decreased1-3 (FIGURE 1).
Continue to: Clinical manifestations of Chagas disease...
Clinical manifestations of Chagas disease
Chagas disease occurs in 2 stages, acute and chronic.1,2,4 In patients who are infected via an insect vector, the acute stage typically begins 1 to 2 weeks after the insect bite. This phase of the illness usually lasts 4 to 8 weeks and almost always resolves without treatment.
Some infected patients will be completely free of symptoms. Others will have manifestations such as:
- fever
- malaise
- headache
- hepatosplenomegaly
- lymphadenopathy
- swollen nodule at the site of infection
—Romaña’s sign, when the lesion is on the eyelid
—Chagoma, when the lesion is elsewhere on the skin.
Fortunately, less than 5% of patients will have severe illness, manifested by myocarditis, pericarditis, encephalitis, or meningitis.
People infected by ingestion of the parasite in food or drink often become more severely ill within 3 weeks. Their clinical manifestations include fever, vomiting, dyspnea, cough, chest pain, abdominal pain, and myalgias. Individuals infected through organ transplant or blood transfusion present more like those infected by the insect vector, but their illness may not develop until several weeks to 5 months after exposure.
In the absence of effective treatment, approximately 40% of patients with acute infection will develop chronic infection, often several decades later. The most common, and most ominous, feature of chronic illness is cardiac disease, experienced by about 30% of patients. Cardiac disease may be manifested as a serious arrhythmia, chest pain, congestive heart failure, or thromboembolism.
The other organ system that is likely to be adversely affected in patients with chronic disease is the gastrointestinal (GI) system, and approximately 10% of chronically infected patients experience this complication. Patients may develop a dilated esophagus, which leads to odynophagia and dysphagia. Diminished motility in other areas of the GI tract also may result in chronic constipation and even bowel obstruction. Chronically infected patients who are immunosuppressed due to HIV infection may become gravely ill as a result of encephalitis and brain abscesses. Cardiac and GI dysfunction is due to the parasite’s massive destruction of nerve endings.
Continue to: Making the diagnosis...
Making the diagnosis
The diagnosis of Chagas disease begins with screening patients who have epidemiologic risk factors that place them at high risk for contracting the infection and at significantly increased risk for morbidity and mortality as a result of either the acute infection or the later chronic stage of infection. A thorough history is vital in the evaluation because the acute illness can have such vague clinical manifestations, and many patients remain asymptomatic until signs of chronic infection appear.
Risk factors that warrant screening include being born in a country endemic for Chagas disease, living in an endemic country for more than 6 months, living with someone who has a confirmed diagnosis, residing in a house made of natural materials (mud walls, thatched roof) in an endemic area, and a history of discovering the triatomine bug in the household.
Screening options include serology, microscopy, and PCR testing. Screening with a single, highly sensitive immunoglobulin G (IgG) serologic test is recommended for nonendemic clinical or community settings. In patients who were born in or who lived in an endemic area for more than 6 months, special consideration should be given to screening women of reproductive age, patients of all ages who were born to a mother with a confirmed diagnosis, individuals who were exposed to a triatomine insect, and people who are immunocompromised.5
A positive serologic test should be confirmed with a second assay based on a different antigen. Currently, 4 IgG tests have US Food and Drug Administration (FDA) approval for diagnosis. If a patient has 2 positive serologic tests, the diagnosis is confirmed, regardless of clinical presentation. Discordant results warrant a third test to differentiate between positive and negative results (FIGURE 2).5 All patients with a confirmed diagnosis should have an electrocardiogram, echocardiogram, and abdominal computed tomography (CT) scan to assess for cardiac or GI abnormalities.

Neonates and infants of mothers with suspected or confirmed infection merit special attention. These children may demonstrate hepatomegaly, splenomegaly, anemia, thrombocytopenia, pneumonitis, heart failure, cardiac arrhythmias, or meningoencephalitis. Newborns delivered to infected mothers will invariably have positive tests for IgG antibody because of transplacental transfer of maternal antibody. Therefore, they should be evaluated by PCR or by direct microscopic examination of the blood for trypomastigotes. In neonates with a negative initial result, repeat testing should be performed by PCR at 4 to 6 weeks of age. Even if the second screening test is negative, the infant should be retested at 9 to 12 months. At this point, maternal IgG no longer should be circulating in the infant’s blood. Three negative tests should effectively rule out T cruzi infection (FIGURE 3).5-7
Organ recipients merit special consideration because, in these individuals, the late stages of Chagas disease may be fatal. In these patients, the preferred diagnostic test is PCR. For transplant patients, monitoring should occur every week for 2 months, bimonthly for the third month, and monthly for 6 months after transplantation. Routine monitoring is not recommended in patients with HIV infection who show no clinical signs of Chagas disease and who are not from endemic areas.
Treatment options
No vaccine or hyperimmune globulin can be used to treat Chagas disease. At this time, 2 antiparasitic drugs are available to treat the condition. One is benznidazole, which inhibits DNA, RNA, and protein synthesis within the microorganism. The medication is given in a dose of 5 to 8 mg/kg per day, divided into 2 doses, for 60 days. Benznidazole is FDA approved for the treatment of individuals older than age 2. It has been used off-label in children younger than 2 years of age. The drug is commercially available at http://www.benznidazoletablets.com.
Benznidazole causes multiple minor side effects and several very serious adverse effects. The serious adverse effects include acute generalized exanthematous pustulosis, toxic epidermal necrolysis, peripheral neuropathy, marrow suppression, and hepatotoxicity. Benznidazole has been teratogenic and carcinogenic in animal studies and should not be used in pregnancy.1,3,6
The second drug is nifurtimox. This drug is FDA approved for the treatment of Chagas disease in adults and for newborns and young children. It is commercially available for pharmacies to purchase from several drug wholesalers. Nifurtimox produces reactive oxygen species and toxic intermediates that induce DNA damage and cause cell death of the microorganism. The appropriate oral dose is 8 to 10 mg/kg per day, divided into 3 to 4 equal doses. The duration of treatment is 60 to 90 days, depending on the patient’s response. Like benznidazole, nifurtimox also is highly toxic. Severe adverse effects include a hypersensitivity reaction, anaphylaxis, angioedema, syncope, seizures, and psychosis. Nifurtimox also is teratogenic and is contraindicated in pregnancy.1,3,6
Clinicians who have questions about the use of either of these medications should contact the Centers for Disease Control and Prevention, Division of Parasitic Diseases public inquiries telephone line at (404) 718-4745.
Potential for cure. When either benznidazole or nifurtimox is administered early in the course of a patient’s acute infection, the chance for complete cure is excellent. The same is true for early treatment of the infected neonate. When treatment is delayed, or if it cannot be completed because of intolerable adverse effects, the prognosis for complete cure is diminished.
In adults who have chronic disease, antiparasitic treatment is unlikely to be effective. In such a situation, secondary treatment is directed toward correction of heart failure, control of cardiac rhythm disturbances, and control of GI motility disorders. For both cardiac and GI conditions, medication and surgery may be indicated. Antiparasitic treatment is more effective in children with chronic disease but it is still not uniformly effective.1,3,5,6
Preventing infection
Vector control is the key to preventing infection in areas where Chagas disease is endemic. One important, but often financially unaffordable, measure is construction of homes with building materials that do not support the growth of the triatomine insects that transmit the disease. A second critical preventive measure is the spraying of mud and thatched homes and surrounding areas with long-lasting insecticides. Pyrethroids are the preferred agents today. Alternative agents include fenitrothion and bendiocarb.1
Other important preventive measures include:
- screening the blood supply for T cruzi and eliminating units contaminated with the parasite
- screening for the parasite in organs targeted for transplant
- screening infected women of reproductive age in endemic areas and treating those who are positive before they become pregnant; this measure may be almost 95% effective in preventing congenital infection
- using mosquito netting when housing is insecure and air conditioning is not available
- in endemic areas, avoiding unpasteurized fruit drinks and unwashed fruits and vegetables.
Unique considerations in pregnancy
Chagas disease does not cause specific anatomic birth defects. However, infected women are more likely to experience spontaneous abortion, preterm premature rupture of membranes, preterm labor, and fetal growth restriction. Overall, the risk of perinatal transmission is approximately 5%, but it may be higher in women who have a very high parasite load. Infected neonates who remain untreated are at risk for developing the serious sequelae of chronic infection. At least half of neonates who are infected will initially be asymptomatic. Therefore, screening of at-risk neonates is essential in order to implement effective treatment.3,6
As noted earlier, the usual drugs used for treating Chagas disease should not be used in pregnancy. Nevertheless, it is still important to screen certain individuals for infection and, subsequently, target them and their neonates for treatment immediately following delivery. The following pregnant patients should be screened5,6:
- women with clinical manifestations that suggest acute or chronic infection
- women from areas of the world in which Chagas disease is endemic, namely, from the southern United States to northern Chile and Argentina. Although the disease is endemic in 21 countries, the countries with the highest prevalence are Bolivia, Argentina, and Paraguay.
- newborns delivered to mothers who have been identified as infected.
As mentioned, several tests are available for screening: PCR, antibody assays, and examination of peripheral blood smears. At least 2 test results should be positive to confirm the diagnosis of infection. Neonates should be followed for 9 to 12 months after delivery to determine if perinatal transmission has occurred. Treatment with antiparasitic drugs is indicated for all infected children.5
CASE Continue surveillance during pregnancy, treat after delivery
This patient should not be treated during pregnancy because the 2 major antiparasitic drugs are teratogenic. Antenatally, she should be followed for evidence of preterm labor and fetal growth restriction. She also should have an electrocardiogram and echocardiogram to evaluate for cardiac disease. Immediately after delivery, the patient should be treated with benznidazole for 60 days. Breastfeeding is acceptable. Her neonate should be screened for infection for up to 9 months, following the algorithm outlined earlier (FIGURE 3), and treated if the surveillance tests are positive. ●
- Chagas disease is caused by the parasite Trypanosoma cruzi, which is spread by the bite of the triatomine insect (the “kissing bug”).
- The condition is widespread among impoverished populations in South America, Central America, and Mexico, but it is rare in the United States except in individuals who immigrated here from endemic areas.
- Chagas disease evolves through 2 phases: acute and chronic. Manifestations of acute infection include fever, malaise, headache, hepatosplenomegaly, lymphadenopathy, and swelling at the site of the insect bite. The chronic phase is manifested by serious cardiac and gastrointestinal dysfunction.
- The diagnosis can be established by identifying the organism in a blood smear and by detecting antibody or antigen in the blood.
- The 2 drugs of choice for treatment of Chagas disease are benznidazole and nifurtimox. These drugs are teratogenic and are contraindicated in pregnancy.
- Women at risk for infection should be screened prior to, or during, pregnancy. Infants of infected mothers should be screened for infection for up to 9 to 12 months after delivery and treated if they test positive. Treatment of the infant is almost 100% effective in preventing chronic illness.
CASE Pregnant woman with a suspected parasitic infection
A 20-year-old, previously healthy, primigravid woman at 24 weeks’ gestation immigrated from Bolivia to the United States 3 days ago. On the morning of her international flight, she awoke to discover a small insect bite just below her left eye. She sought medical evaluation because her eyelid is now significantly swollen, and she has a headache, anorexia, fatigue, and a fever of 38.4° C. The examining physician ordered a polymerase chain reaction (PCR) test for Trypanosoma cruzi, and the test is positive.
- How should this patient be treated during, and after, her delivery?
- Does this infection pose a risk to the newborn baby?
- What type of surveillance and treatment is indicated for the baby?
Chagas disease is common in South America, Central America, and Mexico and is well known to physicians in those countries. Clinicians who practice in the United States are much less familiar with the condition, but it is becoming increasingly common as a result of international travel within the Americas.
In this article, we review the interesting microbiology and epidemiology of Chagas disease, focus on its clinical manifestations, and discuss the most useful diagnostic tests for the illness. We conclude with a summary of preventive and treatment measures, with particular emphasis on managing the disease in pregnancy.
How Chagas disease is transmitted and who is at risk
Chagas disease was named in honor of a Brazilian physician, Carlos Chagas, who first described the condition in 1909. The disease is endemic in South America, Central America, and Mexico, and, recently, its prevalence has increased in the southern United States. Approximately 300,000 people in the United States are infected.1,2
The illness is caused by the parasite Trypanosoma cruzi, and it is also known as American trypanosomiasis. The parasite is spread primarily by the bite of triatomine insects (“kissing bugs”). Approximately 60% of these insects are infected with the parasite. The insects live and thrive in the interspaces of mud walls (adobe homes) and thatched roofs. At night, the insects leave their darkened spaces and feed on the exposed skin of sleeping persons. They are particularly likely to bite the moist skin surfaces near the eye and mouth, and, as they do, they defecate and excrete the parasite into the blood vessels beneath the skin. Within the blood, the trypomastigotes invade various host cells. Inside the host cells, the organism transforms into an amastigote, which is the replicative form of the parasite. After several rounds of replication, the amastigote transforms back into a trypomastigote, bursts from the cell, and goes on to infect other host cells.1
In addition to transmission by the insect vector, the parasite also can be transmitted by blood transfusion and organ donation. When contaminated blood is transfused, the risk of transmission is approximately 10% to 25% for each unit. Following implementation of effective screening programs by blood banks in Central America, South America, Mexico, and the United States, the risk of transmission from undetected infection is now approximately 1:200,000 per unit.
When a transplant procedure with an infected heart is performed, the risk of transmission is 75% to 100%. For liver transplants, the frequency of transmission is 0% to 29%; for kidney transplants, the risk of transmission is 0% to 19%.
Consumption of contaminated food or drink, particularly nonpasteurized items sold by street vendors, is also an important mechanism of transmission. In addition, transmission can occur as a result of laboratory exposure and by exposure to wild animals (racoons, opossums, marmosets, bats, armadillos) in forested areas. Finally, perinatal transmission now accounts for about 22% of infections. As effective vector control programs have been introduced in endemic areas, the proportion of cases caused by the insect vector has steadily decreased1-3 (FIGURE 1).
Continue to: Clinical manifestations of Chagas disease...
Clinical manifestations of Chagas disease
Chagas disease occurs in 2 stages, acute and chronic.1,2,4 In patients who are infected via an insect vector, the acute stage typically begins 1 to 2 weeks after the insect bite. This phase of the illness usually lasts 4 to 8 weeks and almost always resolves without treatment.
Some infected patients will be completely free of symptoms. Others will have manifestations such as:
- fever
- malaise
- headache
- hepatosplenomegaly
- lymphadenopathy
- swollen nodule at the site of infection
—Romaña’s sign, when the lesion is on the eyelid
—Chagoma, when the lesion is elsewhere on the skin.
Fortunately, less than 5% of patients will have severe illness, manifested by myocarditis, pericarditis, encephalitis, or meningitis.
People infected by ingestion of the parasite in food or drink often become more severely ill within 3 weeks. Their clinical manifestations include fever, vomiting, dyspnea, cough, chest pain, abdominal pain, and myalgias. Individuals infected through organ transplant or blood transfusion present more like those infected by the insect vector, but their illness may not develop until several weeks to 5 months after exposure.
In the absence of effective treatment, approximately 40% of patients with acute infection will develop chronic infection, often several decades later. The most common, and most ominous, feature of chronic illness is cardiac disease, experienced by about 30% of patients. Cardiac disease may be manifested as a serious arrhythmia, chest pain, congestive heart failure, or thromboembolism.
The other organ system that is likely to be adversely affected in patients with chronic disease is the gastrointestinal (GI) system, and approximately 10% of chronically infected patients experience this complication. Patients may develop a dilated esophagus, which leads to odynophagia and dysphagia. Diminished motility in other areas of the GI tract also may result in chronic constipation and even bowel obstruction. Chronically infected patients who are immunosuppressed due to HIV infection may become gravely ill as a result of encephalitis and brain abscesses. Cardiac and GI dysfunction is due to the parasite’s massive destruction of nerve endings.
Continue to: Making the diagnosis...
Making the diagnosis
The diagnosis of Chagas disease begins with screening patients who have epidemiologic risk factors that place them at high risk for contracting the infection and at significantly increased risk for morbidity and mortality as a result of either the acute infection or the later chronic stage of infection. A thorough history is vital in the evaluation because the acute illness can have such vague clinical manifestations, and many patients remain asymptomatic until signs of chronic infection appear.
Risk factors that warrant screening include being born in a country endemic for Chagas disease, living in an endemic country for more than 6 months, living with someone who has a confirmed diagnosis, residing in a house made of natural materials (mud walls, thatched roof) in an endemic area, and a history of discovering the triatomine bug in the household.
Screening options include serology, microscopy, and PCR testing. Screening with a single, highly sensitive immunoglobulin G (IgG) serologic test is recommended for nonendemic clinical or community settings. In patients who were born in or who lived in an endemic area for more than 6 months, special consideration should be given to screening women of reproductive age, patients of all ages who were born to a mother with a confirmed diagnosis, individuals who were exposed to a triatomine insect, and people who are immunocompromised.5
A positive serologic test should be confirmed with a second assay based on a different antigen. Currently, 4 IgG tests have US Food and Drug Administration (FDA) approval for diagnosis. If a patient has 2 positive serologic tests, the diagnosis is confirmed, regardless of clinical presentation. Discordant results warrant a third test to differentiate between positive and negative results (FIGURE 2).5 All patients with a confirmed diagnosis should have an electrocardiogram, echocardiogram, and abdominal computed tomography (CT) scan to assess for cardiac or GI abnormalities.

Neonates and infants of mothers with suspected or confirmed infection merit special attention. These children may demonstrate hepatomegaly, splenomegaly, anemia, thrombocytopenia, pneumonitis, heart failure, cardiac arrhythmias, or meningoencephalitis. Newborns delivered to infected mothers will invariably have positive tests for IgG antibody because of transplacental transfer of maternal antibody. Therefore, they should be evaluated by PCR or by direct microscopic examination of the blood for trypomastigotes. In neonates with a negative initial result, repeat testing should be performed by PCR at 4 to 6 weeks of age. Even if the second screening test is negative, the infant should be retested at 9 to 12 months. At this point, maternal IgG no longer should be circulating in the infant’s blood. Three negative tests should effectively rule out T cruzi infection (FIGURE 3).5-7
Organ recipients merit special consideration because, in these individuals, the late stages of Chagas disease may be fatal. In these patients, the preferred diagnostic test is PCR. For transplant patients, monitoring should occur every week for 2 months, bimonthly for the third month, and monthly for 6 months after transplantation. Routine monitoring is not recommended in patients with HIV infection who show no clinical signs of Chagas disease and who are not from endemic areas.
Treatment options
No vaccine or hyperimmune globulin can be used to treat Chagas disease. At this time, 2 antiparasitic drugs are available to treat the condition. One is benznidazole, which inhibits DNA, RNA, and protein synthesis within the microorganism. The medication is given in a dose of 5 to 8 mg/kg per day, divided into 2 doses, for 60 days. Benznidazole is FDA approved for the treatment of individuals older than age 2. It has been used off-label in children younger than 2 years of age. The drug is commercially available at http://www.benznidazoletablets.com.
Benznidazole causes multiple minor side effects and several very serious adverse effects. The serious adverse effects include acute generalized exanthematous pustulosis, toxic epidermal necrolysis, peripheral neuropathy, marrow suppression, and hepatotoxicity. Benznidazole has been teratogenic and carcinogenic in animal studies and should not be used in pregnancy.1,3,6
The second drug is nifurtimox. This drug is FDA approved for the treatment of Chagas disease in adults and for newborns and young children. It is commercially available for pharmacies to purchase from several drug wholesalers. Nifurtimox produces reactive oxygen species and toxic intermediates that induce DNA damage and cause cell death of the microorganism. The appropriate oral dose is 8 to 10 mg/kg per day, divided into 3 to 4 equal doses. The duration of treatment is 60 to 90 days, depending on the patient’s response. Like benznidazole, nifurtimox also is highly toxic. Severe adverse effects include a hypersensitivity reaction, anaphylaxis, angioedema, syncope, seizures, and psychosis. Nifurtimox also is teratogenic and is contraindicated in pregnancy.1,3,6
Clinicians who have questions about the use of either of these medications should contact the Centers for Disease Control and Prevention, Division of Parasitic Diseases public inquiries telephone line at (404) 718-4745.
Potential for cure. When either benznidazole or nifurtimox is administered early in the course of a patient’s acute infection, the chance for complete cure is excellent. The same is true for early treatment of the infected neonate. When treatment is delayed, or if it cannot be completed because of intolerable adverse effects, the prognosis for complete cure is diminished.
In adults who have chronic disease, antiparasitic treatment is unlikely to be effective. In such a situation, secondary treatment is directed toward correction of heart failure, control of cardiac rhythm disturbances, and control of GI motility disorders. For both cardiac and GI conditions, medication and surgery may be indicated. Antiparasitic treatment is more effective in children with chronic disease but it is still not uniformly effective.1,3,5,6
Preventing infection
Vector control is the key to preventing infection in areas where Chagas disease is endemic. One important, but often financially unaffordable, measure is construction of homes with building materials that do not support the growth of the triatomine insects that transmit the disease. A second critical preventive measure is the spraying of mud and thatched homes and surrounding areas with long-lasting insecticides. Pyrethroids are the preferred agents today. Alternative agents include fenitrothion and bendiocarb.1
Other important preventive measures include:
- screening the blood supply for T cruzi and eliminating units contaminated with the parasite
- screening for the parasite in organs targeted for transplant
- screening infected women of reproductive age in endemic areas and treating those who are positive before they become pregnant; this measure may be almost 95% effective in preventing congenital infection
- using mosquito netting when housing is insecure and air conditioning is not available
- in endemic areas, avoiding unpasteurized fruit drinks and unwashed fruits and vegetables.
Unique considerations in pregnancy
Chagas disease does not cause specific anatomic birth defects. However, infected women are more likely to experience spontaneous abortion, preterm premature rupture of membranes, preterm labor, and fetal growth restriction. Overall, the risk of perinatal transmission is approximately 5%, but it may be higher in women who have a very high parasite load. Infected neonates who remain untreated are at risk for developing the serious sequelae of chronic infection. At least half of neonates who are infected will initially be asymptomatic. Therefore, screening of at-risk neonates is essential in order to implement effective treatment.3,6
As noted earlier, the usual drugs used for treating Chagas disease should not be used in pregnancy. Nevertheless, it is still important to screen certain individuals for infection and, subsequently, target them and their neonates for treatment immediately following delivery. The following pregnant patients should be screened5,6:
- women with clinical manifestations that suggest acute or chronic infection
- women from areas of the world in which Chagas disease is endemic, namely, from the southern United States to northern Chile and Argentina. Although the disease is endemic in 21 countries, the countries with the highest prevalence are Bolivia, Argentina, and Paraguay.
- newborns delivered to mothers who have been identified as infected.
As mentioned, several tests are available for screening: PCR, antibody assays, and examination of peripheral blood smears. At least 2 test results should be positive to confirm the diagnosis of infection. Neonates should be followed for 9 to 12 months after delivery to determine if perinatal transmission has occurred. Treatment with antiparasitic drugs is indicated for all infected children.5
CASE Continue surveillance during pregnancy, treat after delivery
This patient should not be treated during pregnancy because the 2 major antiparasitic drugs are teratogenic. Antenatally, she should be followed for evidence of preterm labor and fetal growth restriction. She also should have an electrocardiogram and echocardiogram to evaluate for cardiac disease. Immediately after delivery, the patient should be treated with benznidazole for 60 days. Breastfeeding is acceptable. Her neonate should be screened for infection for up to 9 months, following the algorithm outlined earlier (FIGURE 3), and treated if the surveillance tests are positive. ●
- Chagas disease is caused by the parasite Trypanosoma cruzi, which is spread by the bite of the triatomine insect (the “kissing bug”).
- The condition is widespread among impoverished populations in South America, Central America, and Mexico, but it is rare in the United States except in individuals who immigrated here from endemic areas.
- Chagas disease evolves through 2 phases: acute and chronic. Manifestations of acute infection include fever, malaise, headache, hepatosplenomegaly, lymphadenopathy, and swelling at the site of the insect bite. The chronic phase is manifested by serious cardiac and gastrointestinal dysfunction.
- The diagnosis can be established by identifying the organism in a blood smear and by detecting antibody or antigen in the blood.
- The 2 drugs of choice for treatment of Chagas disease are benznidazole and nifurtimox. These drugs are teratogenic and are contraindicated in pregnancy.
- Women at risk for infection should be screened prior to, or during, pregnancy. Infants of infected mothers should be screened for infection for up to 9 to 12 months after delivery and treated if they test positive. Treatment of the infant is almost 100% effective in preventing chronic illness.
- Bern C. Chagas disease: epidemiology, screening, and prevention. UpToDate. Updated April 8, 2022. Accessed October 6, 2022. https://www.uptodate.com/contents /chagas-disease-epidemiology-screening-and-prevention
- Chagas disease. Cleveland Clinic. Reviewed October 8, 2021. Accessed October 6, 2022. https://my.clevelandclinic.org /health/diseases/21876-chagas-disease
- Howard EJ, Xiong X, Carlier Y, et al. Frequency of the congenital transmission of Trypanosoma cruzi: a systematic review and meta-analysis. BJOG. 2014;121:22-33.
- Chagas disease. Mayo Clinic. November 12, 2020. Accessed October 6, 2022. https://www.mayoclinic.org/diseases -conditions/chagas-disease/symptoms-causes/syc-20356212
- Forsyth CJ, Manne-Goehler J, Bern C, et al. Recommendations for screening and diagnosis of Chagas disease in the United States. J Infect Dis. 2022;225:1601-1610.
- Torrico F, Alonso-Vega C, Suarez E. et al. Maternal Trypanosoma cruzi infection, pregnancy outcome, morbidity, and mortality of congenitally infected and non-infected newborns in Bolivia. Am J Trop Med Hyg. 2004;70:201-209.
- Messenger LA, Bern C. Congenital Chagas disease: current diagnostics, limitations and future perspectives. Curr Opin Infect Dis. 2018;31:415-421.
- Bern C. Chagas disease: epidemiology, screening, and prevention. UpToDate. Updated April 8, 2022. Accessed October 6, 2022. https://www.uptodate.com/contents /chagas-disease-epidemiology-screening-and-prevention
- Chagas disease. Cleveland Clinic. Reviewed October 8, 2021. Accessed October 6, 2022. https://my.clevelandclinic.org /health/diseases/21876-chagas-disease
- Howard EJ, Xiong X, Carlier Y, et al. Frequency of the congenital transmission of Trypanosoma cruzi: a systematic review and meta-analysis. BJOG. 2014;121:22-33.
- Chagas disease. Mayo Clinic. November 12, 2020. Accessed October 6, 2022. https://www.mayoclinic.org/diseases -conditions/chagas-disease/symptoms-causes/syc-20356212
- Forsyth CJ, Manne-Goehler J, Bern C, et al. Recommendations for screening and diagnosis of Chagas disease in the United States. J Infect Dis. 2022;225:1601-1610.
- Torrico F, Alonso-Vega C, Suarez E. et al. Maternal Trypanosoma cruzi infection, pregnancy outcome, morbidity, and mortality of congenitally infected and non-infected newborns in Bolivia. Am J Trop Med Hyg. 2004;70:201-209.
- Messenger LA, Bern C. Congenital Chagas disease: current diagnostics, limitations and future perspectives. Curr Opin Infect Dis. 2018;31:415-421.