User login
History, exam, and labs: Is one enough to diagnose acute adult appendicitis?
No, none of the 3—history, exam, or labs— is sufficiently accurate to diagnose acute appendicitis (strength of recommendation [SOR]: A, based on meta-analysis of high-quality studies). When combined, the following tests are helpful: an elevated C-reactive protein (CRP), elevated total white blood cell (WBC) count, elevated percentage of polymorphonuclear leukocyte (PMN) cells (left shift), and the presence of guarding or rebound on physical examination. The combination of any 2 of these tests yields a very high positive likelihood ratio (LR+), but the absence of these does not exclude appendicitis (SOR: A, based on meta-analysis of high-quality studies).
2 inexpensive tests can lower costs in low-probability presentations
Fereshteh Gerayli, MD
East Tennessee State University, Johnson City
Unlike physicians in other parts of the world, us physicians rely heavily on imaging studies to diagnose acute appendicitis. This has decreased the rate of negative appendectomies by 15% to 20%. However, the liberal and indiscriminate use of imaging studies increases medical costs while diminishing physicians’ clinical diagnostic skills.
The systematic review our authors cited demonstrated a high likelihood ratio for the presence of appendicitis by combining 2 inexpensive tests. Adding a thorough history and physical exam and a clinical scoring system can further enhance our clinical diagnosis. Considering the cost and the wide range of diagnostic accuracy of imaging studies (which depend on the experience of the reader), it is reasonable to skip CT scan in low probability presentations.
Evidence summary
Radiographic imaging to rule out appendicitis has become more commonplace, but it comes with an increased financial cost and additional delay in surgical intervention. Knowing the accuracy of common diagnostic tests may reduce the need for confirmatory imaging studies that increase both cost and time to surgery.
High levels of 2 or more inflammatory values are helpful
A meta-analysis of patients hospitalized for suspected acute appendicitis analyzed 28 different diagnostic variables in 24 studies.1 Variables included WBC, granulocyte count, PMN proportion, CRP level, and body temperature; histopathology was the gold standard. In no circumstance did an isolated elevation of any 1 factor result in a significant LR+. In addition, the absence of any 1 variable failed to yield a LR– <0.01 (low enough to exclude appendicitis).
Clinicians inherently combine multiple variables when evaluating patients, and when evaluating patients with abdominal pain, this technique can result in identification of adequate likelihood ratios (TABLE).1 In general, when 2 or more of the aforementioned inflammatory variables are present, the diagnosis of acute appendicitis is likely. When all markers of inflammation are normal, though acute appendicitis is less likely, the power is insufficient to exclude it as a possible diagnosis.
The value of CRP in the evaluation of suspected appendicitis was confirmed in a retrospective evaluation of 566 patients who underwent appendectomies.2 The sensitivity and specificity of the test improved depending on the duration of symptoms for both appendicitis and ruptured appendicitis. For appendicitis, CRP levels >1.4, 4.0, and 10.5 on Days 1, 2, and 3 had sensitivities/specificities of 0.38/0.81, 0.63/0.78, and 0.72/0.83, respectively. For ruptured appendicitis, levels of 3.3, 8.5, and 12.0 on Days 1, 2, and 3 had sensitivities/specificities of 0.77/0.89, 0.70/0.95, and 0.90/0.96, respectively.
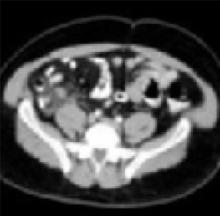
Enlarged appendix with inflammatory changes to mesenteric fatIn a series of 439 patients with symptoms suggestive of acute appendicitis, those with confirmed appendicitis (n=101) had a mean WBC count of 14.8 K/μL (95% CI, 13.9–15.8) and a mean neutrophil percentage of 82 (95% CI, 80–84).1 In contrast, those without appendicitis (n=338) had a mean WBC count of 9.2 K/μL (95% CI, 9.0–9.4) and a mean neutrophil percentage of 68 (95% CI, 66–70).
TABLE
How much do the inflammatory markers tell us? A look at likelihood ratios for appendicitis
| COMBINATION OF TESTS | LIKELIHOOD RATIOS | |
|---|---|---|
| POSITIVE (>10=STRONG EVIDENCE FOR DIAGNOSIS) | NEGATIVE (<0.1=EVIDENCE AGAINST DIAGNOSIS) | |
| WBC >10.0 × 109/L CRP >8 mg/L | 23.32 (95% CI, 6.87–84.79) | 0.03 (95% CI, 0.00–0.14) |
| WBC >10.0 × 109/L PMN cells >70% CRP >12 mg/L | 20.85 (95% CI, 5.47–80.27) | 0.03 (95% CI, 0.01–0.16) |
| Guarding/rebound tenderness WBC >10.0×109 | 11.34 (95% CI, 6.65–19.56) | 0.14 (95% CI, 0.08–0.24) |
| WBC, white blood cell count; CRP, C-reactive protein; PMN, polymorphonuclear leukocyte; CI, confidence interval. | ||
| Source: Andersson, Br J Surg 2004.1 | ||
Recommendations from others
A review of medical and professional associations revealed no official guidelines regarding the evaluation of suspected acute appendicitis. Surgical textbooks confirm that the diagnosis of acute appendicitis is made primarily by history and examination, with help from laboratory and radiographic studies.3
1. Andersson RE. Meta-analysis of the clinical and laboratory diagnosis of appendicitis. Br J Surg 2004;91:28-37.
2. Birkhahn R, Briggs M, Datillo PA, Van Deusen SK, Gaeta TJ. Classifying patient suspected of appendicitis with regard to likelihood. Am J Surgery 2006;191:497-502.
3. Townsend CM, Sabiston DC. Sabiston Textbook of Surgery. 17th ed. Philadelphia, Pa: Saunders, 2004:1381–1395.
No, none of the 3—history, exam, or labs— is sufficiently accurate to diagnose acute appendicitis (strength of recommendation [SOR]: A, based on meta-analysis of high-quality studies). When combined, the following tests are helpful: an elevated C-reactive protein (CRP), elevated total white blood cell (WBC) count, elevated percentage of polymorphonuclear leukocyte (PMN) cells (left shift), and the presence of guarding or rebound on physical examination. The combination of any 2 of these tests yields a very high positive likelihood ratio (LR+), but the absence of these does not exclude appendicitis (SOR: A, based on meta-analysis of high-quality studies).
2 inexpensive tests can lower costs in low-probability presentations
Fereshteh Gerayli, MD
East Tennessee State University, Johnson City
Unlike physicians in other parts of the world, us physicians rely heavily on imaging studies to diagnose acute appendicitis. This has decreased the rate of negative appendectomies by 15% to 20%. However, the liberal and indiscriminate use of imaging studies increases medical costs while diminishing physicians’ clinical diagnostic skills.
The systematic review our authors cited demonstrated a high likelihood ratio for the presence of appendicitis by combining 2 inexpensive tests. Adding a thorough history and physical exam and a clinical scoring system can further enhance our clinical diagnosis. Considering the cost and the wide range of diagnostic accuracy of imaging studies (which depend on the experience of the reader), it is reasonable to skip CT scan in low probability presentations.
Evidence summary
Radiographic imaging to rule out appendicitis has become more commonplace, but it comes with an increased financial cost and additional delay in surgical intervention. Knowing the accuracy of common diagnostic tests may reduce the need for confirmatory imaging studies that increase both cost and time to surgery.
High levels of 2 or more inflammatory values are helpful
A meta-analysis of patients hospitalized for suspected acute appendicitis analyzed 28 different diagnostic variables in 24 studies.1 Variables included WBC, granulocyte count, PMN proportion, CRP level, and body temperature; histopathology was the gold standard. In no circumstance did an isolated elevation of any 1 factor result in a significant LR+. In addition, the absence of any 1 variable failed to yield a LR– <0.01 (low enough to exclude appendicitis).
Clinicians inherently combine multiple variables when evaluating patients, and when evaluating patients with abdominal pain, this technique can result in identification of adequate likelihood ratios (TABLE).1 In general, when 2 or more of the aforementioned inflammatory variables are present, the diagnosis of acute appendicitis is likely. When all markers of inflammation are normal, though acute appendicitis is less likely, the power is insufficient to exclude it as a possible diagnosis.
The value of CRP in the evaluation of suspected appendicitis was confirmed in a retrospective evaluation of 566 patients who underwent appendectomies.2 The sensitivity and specificity of the test improved depending on the duration of symptoms for both appendicitis and ruptured appendicitis. For appendicitis, CRP levels >1.4, 4.0, and 10.5 on Days 1, 2, and 3 had sensitivities/specificities of 0.38/0.81, 0.63/0.78, and 0.72/0.83, respectively. For ruptured appendicitis, levels of 3.3, 8.5, and 12.0 on Days 1, 2, and 3 had sensitivities/specificities of 0.77/0.89, 0.70/0.95, and 0.90/0.96, respectively.
Enlarged appendix with inflammatory changes to mesenteric fatIn a series of 439 patients with symptoms suggestive of acute appendicitis, those with confirmed appendicitis (n=101) had a mean WBC count of 14.8 K/μL (95% CI, 13.9–15.8) and a mean neutrophil percentage of 82 (95% CI, 80–84).1 In contrast, those without appendicitis (n=338) had a mean WBC count of 9.2 K/μL (95% CI, 9.0–9.4) and a mean neutrophil percentage of 68 (95% CI, 66–70).
TABLE
How much do the inflammatory markers tell us? A look at likelihood ratios for appendicitis
| COMBINATION OF TESTS | LIKELIHOOD RATIOS | |
|---|---|---|
| POSITIVE (>10=STRONG EVIDENCE FOR DIAGNOSIS) | NEGATIVE (<0.1=EVIDENCE AGAINST DIAGNOSIS) | |
| WBC >10.0 × 109/L CRP >8 mg/L | 23.32 (95% CI, 6.87–84.79) | 0.03 (95% CI, 0.00–0.14) |
| WBC >10.0 × 109/L PMN cells >70% CRP >12 mg/L | 20.85 (95% CI, 5.47–80.27) | 0.03 (95% CI, 0.01–0.16) |
| Guarding/rebound tenderness WBC >10.0×109 | 11.34 (95% CI, 6.65–19.56) | 0.14 (95% CI, 0.08–0.24) |
| WBC, white blood cell count; CRP, C-reactive protein; PMN, polymorphonuclear leukocyte; CI, confidence interval. | ||
| Source: Andersson, Br J Surg 2004.1 | ||
Recommendations from others
A review of medical and professional associations revealed no official guidelines regarding the evaluation of suspected acute appendicitis. Surgical textbooks confirm that the diagnosis of acute appendicitis is made primarily by history and examination, with help from laboratory and radiographic studies.3
No, none of the 3—history, exam, or labs— is sufficiently accurate to diagnose acute appendicitis (strength of recommendation [SOR]: A, based on meta-analysis of high-quality studies). When combined, the following tests are helpful: an elevated C-reactive protein (CRP), elevated total white blood cell (WBC) count, elevated percentage of polymorphonuclear leukocyte (PMN) cells (left shift), and the presence of guarding or rebound on physical examination. The combination of any 2 of these tests yields a very high positive likelihood ratio (LR+), but the absence of these does not exclude appendicitis (SOR: A, based on meta-analysis of high-quality studies).
2 inexpensive tests can lower costs in low-probability presentations
Fereshteh Gerayli, MD
East Tennessee State University, Johnson City
Unlike physicians in other parts of the world, us physicians rely heavily on imaging studies to diagnose acute appendicitis. This has decreased the rate of negative appendectomies by 15% to 20%. However, the liberal and indiscriminate use of imaging studies increases medical costs while diminishing physicians’ clinical diagnostic skills.
The systematic review our authors cited demonstrated a high likelihood ratio for the presence of appendicitis by combining 2 inexpensive tests. Adding a thorough history and physical exam and a clinical scoring system can further enhance our clinical diagnosis. Considering the cost and the wide range of diagnostic accuracy of imaging studies (which depend on the experience of the reader), it is reasonable to skip CT scan in low probability presentations.
Evidence summary
Radiographic imaging to rule out appendicitis has become more commonplace, but it comes with an increased financial cost and additional delay in surgical intervention. Knowing the accuracy of common diagnostic tests may reduce the need for confirmatory imaging studies that increase both cost and time to surgery.
High levels of 2 or more inflammatory values are helpful
A meta-analysis of patients hospitalized for suspected acute appendicitis analyzed 28 different diagnostic variables in 24 studies.1 Variables included WBC, granulocyte count, PMN proportion, CRP level, and body temperature; histopathology was the gold standard. In no circumstance did an isolated elevation of any 1 factor result in a significant LR+. In addition, the absence of any 1 variable failed to yield a LR– <0.01 (low enough to exclude appendicitis).
Clinicians inherently combine multiple variables when evaluating patients, and when evaluating patients with abdominal pain, this technique can result in identification of adequate likelihood ratios (TABLE).1 In general, when 2 or more of the aforementioned inflammatory variables are present, the diagnosis of acute appendicitis is likely. When all markers of inflammation are normal, though acute appendicitis is less likely, the power is insufficient to exclude it as a possible diagnosis.
The value of CRP in the evaluation of suspected appendicitis was confirmed in a retrospective evaluation of 566 patients who underwent appendectomies.2 The sensitivity and specificity of the test improved depending on the duration of symptoms for both appendicitis and ruptured appendicitis. For appendicitis, CRP levels >1.4, 4.0, and 10.5 on Days 1, 2, and 3 had sensitivities/specificities of 0.38/0.81, 0.63/0.78, and 0.72/0.83, respectively. For ruptured appendicitis, levels of 3.3, 8.5, and 12.0 on Days 1, 2, and 3 had sensitivities/specificities of 0.77/0.89, 0.70/0.95, and 0.90/0.96, respectively.
Enlarged appendix with inflammatory changes to mesenteric fatIn a series of 439 patients with symptoms suggestive of acute appendicitis, those with confirmed appendicitis (n=101) had a mean WBC count of 14.8 K/μL (95% CI, 13.9–15.8) and a mean neutrophil percentage of 82 (95% CI, 80–84).1 In contrast, those without appendicitis (n=338) had a mean WBC count of 9.2 K/μL (95% CI, 9.0–9.4) and a mean neutrophil percentage of 68 (95% CI, 66–70).
TABLE
How much do the inflammatory markers tell us? A look at likelihood ratios for appendicitis
| COMBINATION OF TESTS | LIKELIHOOD RATIOS | |
|---|---|---|
| POSITIVE (>10=STRONG EVIDENCE FOR DIAGNOSIS) | NEGATIVE (<0.1=EVIDENCE AGAINST DIAGNOSIS) | |
| WBC >10.0 × 109/L CRP >8 mg/L | 23.32 (95% CI, 6.87–84.79) | 0.03 (95% CI, 0.00–0.14) |
| WBC >10.0 × 109/L PMN cells >70% CRP >12 mg/L | 20.85 (95% CI, 5.47–80.27) | 0.03 (95% CI, 0.01–0.16) |
| Guarding/rebound tenderness WBC >10.0×109 | 11.34 (95% CI, 6.65–19.56) | 0.14 (95% CI, 0.08–0.24) |
| WBC, white blood cell count; CRP, C-reactive protein; PMN, polymorphonuclear leukocyte; CI, confidence interval. | ||
| Source: Andersson, Br J Surg 2004.1 | ||
Recommendations from others
A review of medical and professional associations revealed no official guidelines regarding the evaluation of suspected acute appendicitis. Surgical textbooks confirm that the diagnosis of acute appendicitis is made primarily by history and examination, with help from laboratory and radiographic studies.3
1. Andersson RE. Meta-analysis of the clinical and laboratory diagnosis of appendicitis. Br J Surg 2004;91:28-37.
2. Birkhahn R, Briggs M, Datillo PA, Van Deusen SK, Gaeta TJ. Classifying patient suspected of appendicitis with regard to likelihood. Am J Surgery 2006;191:497-502.
3. Townsend CM, Sabiston DC. Sabiston Textbook of Surgery. 17th ed. Philadelphia, Pa: Saunders, 2004:1381–1395.
1. Andersson RE. Meta-analysis of the clinical and laboratory diagnosis of appendicitis. Br J Surg 2004;91:28-37.
2. Birkhahn R, Briggs M, Datillo PA, Van Deusen SK, Gaeta TJ. Classifying patient suspected of appendicitis with regard to likelihood. Am J Surgery 2006;191:497-502.
3. Townsend CM, Sabiston DC. Sabiston Textbook of Surgery. 17th ed. Philadelphia, Pa: Saunders, 2004:1381–1395.
Evidence-based answers from the Family Physicians Inquiries Network
Can you differentiate bacterial from viral pediatric infections based on the CBC?
No—the complete blood count (CBC) alone does not have adequate sensitivity or specificity to tell bacterial from viral infections (strength of recommendation [SOR]: B, cohort studies). When used in conjunction with other clinical parameters in validated decision-making algorithms, the CBC can help detect serious bacterial infections in pediatric patients with fever (SOR: B, cohort studies).
There’s no substitute for history, physical exam, and good judgment
John D. Hallgren, MD
Uniformed Services University of the Health Sciences, RAF Menwith Hill, United Kingdom
Viral vs bacterial—often these are surrogate terms for minor vs serious illness. This review is a great lesson in likelihood ratios. Based on the low likelihood ratio, a CBC alone does not shift our suspicion greatly for serious bacterial infections in intermediate-risk patients; however, if you combine it with a clinical decision rule, it can greatly help decision-making, as evidenced by negative predictive values of 99% and above.
In contrast, we don’t need the CBC to tell us that an adult with the sniffles has a rhino/corona/whatevervirus, nor do we need it to tell us that a febrile, lethargic child with a petechial rash has a life-threatening bacteremia. If you enjoy the muck and the mess of primary care as much as I do, this inquiry should provide you with the validation that there’s no substitute for the history, physical exam, and judgment of a good clinician.
Evidence summary
For acutely febrile patients, the presence of an elevated white blood cell (WBC) count with elevated band forms has dogmatically been thought of as a marker for bacterial infection.1 Current literature, however, does not support this.2
Neisseria meningitides
A retrospective study of 5353 infants ages 3 to 89 days presenting to the emergency department for evaluation of fever showed that 3 of 4 infants ultimately diagnosed with bacterial meningitis would have been missed if the WBC count alone were used to predict which infants need a lumbar puncture.3 A prospective study of 2492 children ages 3 to 24 months presenting to the emergency department with acute fever and an absolute WBC count >15,000/mm3 revealed that neither a polymorphonuclear count of >10,000/mm3 (>66% segmented forms) nor a band count of >500/mm3 was associated with an increased likelihood of occult bacterial infection.4 Other studies show that the WBC alone is poorly discriminatory for identifying either bacteremia or meningitis.5,6
To improve the diagnostic utility of the CBC, other studies have examined individual components of the white blood cell differential count (TABLE 1). In particular, the use of the absolute neutrophil count (ANC) has been proposed as a superior marker of serious bacterial infection.7 A review of 6579 outpatients aged 3 to 36 months presenting to the emergency department with temperatures of 39°C or higher showed an ANC of >10,000/mm3 as more predictive of occult pneumococcal bacteremia than an elevated WBC count (>15,000/mm3) alone.8 Another retrospective review of more than 10,000 patients aged 3 to 36 months presenting to the emergency department used logistic regression to identify predictors of bacteremia. In this study, ANC (>9500/mm3) and WBC (>14,300/mm3) were of equal sensitivity (75%) and specificity (75%) in identifying serious bacterial infection.9 Finally, the band count alone does not accurately predict serious bacterial infection.10
In summary, the CBC cannot be used in isolation to differentiate bacterial from viral illness. The CBC can, however, augment clinical data from the history and physical examination to predict the likelihood of serious bacterial illness. As a result, numerous diagnostic criteria, each incorporating elements of the CBC, have been developed in an attempt to accurately differentiate bacterial from viral illness in acutely febrile patients, most typically children (TABLE 2). These criteria differ by age of the patient, clinical testing recommendations, indications for antibiotic therapy, as well as WBC cutoffs.
TABLE 1
WBC markers: How good are they at predicting serious bacterial infection?9,18,19
| VARIABLE | CUTOFF | SENSITIVITY | SPECIFICITY | LR (95% CI) |
|---|---|---|---|---|
| White blood cell count | 15,000/mm3 | 64%–82% | 67%–75% | 1.9–2.7 (1.1–3.8) |
| Absolute neutrophil count | 10,000/mm3 | 64%–76% | 76%–81% | 3.0–3.3 (1.6–6.2) |
| LR, likelihood ratio; CI, confidence interval. | ||||
TABLE 2
Clinical criteria for predicting serious bacterial infection in febrile children
| CRITERION | ROCHESTER CRITERIA11 | BOSTON CRITERIA12 | PHILADELPHIA CRITERIA13 |
|---|---|---|---|
| Predictive value | 98.9% PV–in ruling out serious bacterial infection | 95% PV+ to identify serious bacterial infection | 100% PV–in ruling out serious bacterial infection |
| Age | <60 days | 1–3 mos Present to emergency dept. with fever ≥38.0°C | 29–56 days Present with fever ≥38.2°C |
| Appearance | Well-appearing Previously healthy No evidence of infection (skin, bone, joint, soft tissue or ear) | Healthy appearing No ear, soft tissue, joint or bone infection on exam | Well-appearing |
| White blood cell count | WBC 5–15,000/mm3 Bands ≤1,500/mm3 | Peripheral WBC ≤20,000/mm3 | WBC ≤15,000/mm3 Band-to-neutrophil ratio of ≤0.2 |
| Urinalysis | ≤10 WBC/hpf of centrifuged urine | Urinalysis ≤10 WBC/hpf | Urinalysis ≤10 WBC/hpf |
| Other tests | If diarrhea, ≤5 WBC/hpf of stool smear | CSF WBC ≤10/hpf | CSF WBC ≤8/hpf with negative gram stain If watery diarrhea, few or no WBC/hpf on stool smear |
| WBC, white blood cell count; hpf, high-powered field; CSF, cerebrospinal fluid; PV, predictive value | |||
Recommendations from others
The American College of Emergency Physicians recommends considering antibiotic therapy for previously healthy, well-appearing children ages 3 to 36 months who present with a fever without a clinical source and a WBC count >15,000/mm3.3,14
The University of Cincinnati Evidence-Based Clinical Practice Guidelines for fever of uncertain source in children ages 2 to 36 months recommends obtaining a CBC for any child who is ill-appearing or at high risk for bacteremia (determined by the clinicians’ judgment). A WBC of ≥15,000/mm3 or ANC >10,000/mm3 provide support for antibiotic therapy.15 The 1993 American Academy of Pediatrics guidelines for fever ≥39°C without a source in children ages 3 months to 3 years recommends a CBC; if the WBC count ≥15,000/mm3, they recommend a blood culture and treatment with antibiotics pending culture results.3,16
It is important to note that in the age of Haemophilus influenza and Streptococcus pneumonia vaccination, the rate of occult bacteremia in febrile children presenting without a source has fallen from 3% to 10% to 1% or less.17 A lower prevalence reduces the utility of routine CBC or blood culture in the evaluation of immunized, febrile children. Parameters such as procalcitonin, interleukin-6, interleukin-8, interleukin-1 receptor antagonist and C-reactive protein show future promise as biochemical markers for identifying serious bacterial infections.18
1. Wile MJ, Homer LD, Gaehler S, Phillips S, Millan J. Manual differential cell counts help predict bacterial infection. A multivariate analysis. Am J Clin Pathol 2001;115:644-649.
2. Seebach JD, Morant R, Ruegg R, Seifert B, Fehr J. The diagnostic value of the neutrophil left shift in predicting inflammatory and infectious disease. Am J Clin Pathol 1997;107:582-591.
3. Bonsu BK, Harper MB. Utility of the peripheral blood white blood cell count for identifying sick young infants who need lumbar puncture. Ann Emerg Med 2003;41:206-214.
4. Kramer MS, Tange SM, Mills EL, Ciampi A, Bernstein ML, Drummond KN. Role of the complete blood count in detecting occult focal bacterial infection in the young febrile child. J Clin Epidemiol 1993;46:349-357.
5. Brown L, Shaw T, Wittlake WA. Does leucocytosis identify bacterial infections in febrile neonates presenting to the emergency department? Emerg Med J 2005;22:256-259.
6. Garges HP, Moody MA, Cotten CM, et al. Neonatal meningitis: what is the correlation among cerebrospinal fluid cultures, blood cultures, and cerebrospinal fluid parameters? Pediatrics 2006;117:1094-1100.
7. Gombos MM, Bienkowski RS, Gochman RF, Billett HH. The absolute neutrophil count: is it the best indicator for occult bacteremia in infants? Am J Clin Pathol 1998;109:221-225.
8. Kuppermann N, Fleisher GR, Jaffe DM. Predictors of occult pneumococcal bacteremia in young febrile children. Ann Emerg Med 1998;31:679-687.
9. Isaacman DJ, Shults J, Gross TK, Davis PH, Harper M. Predictors of bacteremia in febrile children 3 to 36 months of age. Pediatrics 2000;106:977-982.
10. Cornbleet PJ. Clinical utility of the band count. Clin Lab Med 2002;22:101-136.
11. Dagan R, Powell KR, Hall CB, Menegus MA. Identification of infants unlikely to have serious bacterial infection although hospitalized for suspected sepsis. J Pediatr 1985;107:855-860.
12. Baskin MN, O’Rourke EJ, Fleisher GR. Outpatient treatment of febrile infants 28 to 89 days of age with intramuscular administration of ceftriaxone. J Pediatr 1992;120:22-27.
13. Baker MD, Bell LM, Avner JR. The efficacy of routine outpatient management without antibiotics of fever in selected infants. Pediatrics 1999;103:627-631.
14. American College of emergency Physicians. Clinical policy for children younger than three years presenting to the emergency department with fever. Ann Emerg Med 2003;42:530-545.
15. Cincinnati Children’s Hospital Medical Center. Evidence-based clinical practice guideline for fever of uncertain source in children in 2 to 36 months of age. Cincinnati, Ohio: Cincinnati Children’s Hospital Medical Center; 2003.
16. Baraff LJ, Bass JW, Fleisher GR, et al. Practice guideline for the management of infants and children 0 to 36 months of age with fever without source. Ann Emerg Med 1993;22:1198-1210.
17. Stoll ML, Rubin LG. Incidence of occult bacteremia among highly febrile young children in the era of the pneumococcal conjugate vaccine. Arch Pediatr Adolesc Med 2004;158:671-675.
18. Pulliam PN, Attia MW, Cronan KM. C-reactive protein in febrile children 1 to 36 months of age with clinically undetectable serious bacterial infection. Pediatrics 2001;108:1275-1279.
19. Pratt A, Attia MW. Duration of fever and markers of serious bacterial infection in young febrile children. Pediatr Int 2007;49:31-35.
No—the complete blood count (CBC) alone does not have adequate sensitivity or specificity to tell bacterial from viral infections (strength of recommendation [SOR]: B, cohort studies). When used in conjunction with other clinical parameters in validated decision-making algorithms, the CBC can help detect serious bacterial infections in pediatric patients with fever (SOR: B, cohort studies).
There’s no substitute for history, physical exam, and good judgment
John D. Hallgren, MD
Uniformed Services University of the Health Sciences, RAF Menwith Hill, United Kingdom
Viral vs bacterial—often these are surrogate terms for minor vs serious illness. This review is a great lesson in likelihood ratios. Based on the low likelihood ratio, a CBC alone does not shift our suspicion greatly for serious bacterial infections in intermediate-risk patients; however, if you combine it with a clinical decision rule, it can greatly help decision-making, as evidenced by negative predictive values of 99% and above.
In contrast, we don’t need the CBC to tell us that an adult with the sniffles has a rhino/corona/whatevervirus, nor do we need it to tell us that a febrile, lethargic child with a petechial rash has a life-threatening bacteremia. If you enjoy the muck and the mess of primary care as much as I do, this inquiry should provide you with the validation that there’s no substitute for the history, physical exam, and judgment of a good clinician.
Evidence summary
For acutely febrile patients, the presence of an elevated white blood cell (WBC) count with elevated band forms has dogmatically been thought of as a marker for bacterial infection.1 Current literature, however, does not support this.2
Neisseria meningitides
A retrospective study of 5353 infants ages 3 to 89 days presenting to the emergency department for evaluation of fever showed that 3 of 4 infants ultimately diagnosed with bacterial meningitis would have been missed if the WBC count alone were used to predict which infants need a lumbar puncture.3 A prospective study of 2492 children ages 3 to 24 months presenting to the emergency department with acute fever and an absolute WBC count >15,000/mm3 revealed that neither a polymorphonuclear count of >10,000/mm3 (>66% segmented forms) nor a band count of >500/mm3 was associated with an increased likelihood of occult bacterial infection.4 Other studies show that the WBC alone is poorly discriminatory for identifying either bacteremia or meningitis.5,6
To improve the diagnostic utility of the CBC, other studies have examined individual components of the white blood cell differential count (TABLE 1). In particular, the use of the absolute neutrophil count (ANC) has been proposed as a superior marker of serious bacterial infection.7 A review of 6579 outpatients aged 3 to 36 months presenting to the emergency department with temperatures of 39°C or higher showed an ANC of >10,000/mm3 as more predictive of occult pneumococcal bacteremia than an elevated WBC count (>15,000/mm3) alone.8 Another retrospective review of more than 10,000 patients aged 3 to 36 months presenting to the emergency department used logistic regression to identify predictors of bacteremia. In this study, ANC (>9500/mm3) and WBC (>14,300/mm3) were of equal sensitivity (75%) and specificity (75%) in identifying serious bacterial infection.9 Finally, the band count alone does not accurately predict serious bacterial infection.10
In summary, the CBC cannot be used in isolation to differentiate bacterial from viral illness. The CBC can, however, augment clinical data from the history and physical examination to predict the likelihood of serious bacterial illness. As a result, numerous diagnostic criteria, each incorporating elements of the CBC, have been developed in an attempt to accurately differentiate bacterial from viral illness in acutely febrile patients, most typically children (TABLE 2). These criteria differ by age of the patient, clinical testing recommendations, indications for antibiotic therapy, as well as WBC cutoffs.
TABLE 1
WBC markers: How good are they at predicting serious bacterial infection?9,18,19
| VARIABLE | CUTOFF | SENSITIVITY | SPECIFICITY | LR (95% CI) |
|---|---|---|---|---|
| White blood cell count | 15,000/mm3 | 64%–82% | 67%–75% | 1.9–2.7 (1.1–3.8) |
| Absolute neutrophil count | 10,000/mm3 | 64%–76% | 76%–81% | 3.0–3.3 (1.6–6.2) |
| LR, likelihood ratio; CI, confidence interval. | ||||
TABLE 2
Clinical criteria for predicting serious bacterial infection in febrile children
| CRITERION | ROCHESTER CRITERIA11 | BOSTON CRITERIA12 | PHILADELPHIA CRITERIA13 |
|---|---|---|---|
| Predictive value | 98.9% PV–in ruling out serious bacterial infection | 95% PV+ to identify serious bacterial infection | 100% PV–in ruling out serious bacterial infection |
| Age | <60 days | 1–3 mos Present to emergency dept. with fever ≥38.0°C | 29–56 days Present with fever ≥38.2°C |
| Appearance | Well-appearing Previously healthy No evidence of infection (skin, bone, joint, soft tissue or ear) | Healthy appearing No ear, soft tissue, joint or bone infection on exam | Well-appearing |
| White blood cell count | WBC 5–15,000/mm3 Bands ≤1,500/mm3 | Peripheral WBC ≤20,000/mm3 | WBC ≤15,000/mm3 Band-to-neutrophil ratio of ≤0.2 |
| Urinalysis | ≤10 WBC/hpf of centrifuged urine | Urinalysis ≤10 WBC/hpf | Urinalysis ≤10 WBC/hpf |
| Other tests | If diarrhea, ≤5 WBC/hpf of stool smear | CSF WBC ≤10/hpf | CSF WBC ≤8/hpf with negative gram stain If watery diarrhea, few or no WBC/hpf on stool smear |
| WBC, white blood cell count; hpf, high-powered field; CSF, cerebrospinal fluid; PV, predictive value | |||
Recommendations from others
The American College of Emergency Physicians recommends considering antibiotic therapy for previously healthy, well-appearing children ages 3 to 36 months who present with a fever without a clinical source and a WBC count >15,000/mm3.3,14
The University of Cincinnati Evidence-Based Clinical Practice Guidelines for fever of uncertain source in children ages 2 to 36 months recommends obtaining a CBC for any child who is ill-appearing or at high risk for bacteremia (determined by the clinicians’ judgment). A WBC of ≥15,000/mm3 or ANC >10,000/mm3 provide support for antibiotic therapy.15 The 1993 American Academy of Pediatrics guidelines for fever ≥39°C without a source in children ages 3 months to 3 years recommends a CBC; if the WBC count ≥15,000/mm3, they recommend a blood culture and treatment with antibiotics pending culture results.3,16
It is important to note that in the age of Haemophilus influenza and Streptococcus pneumonia vaccination, the rate of occult bacteremia in febrile children presenting without a source has fallen from 3% to 10% to 1% or less.17 A lower prevalence reduces the utility of routine CBC or blood culture in the evaluation of immunized, febrile children. Parameters such as procalcitonin, interleukin-6, interleukin-8, interleukin-1 receptor antagonist and C-reactive protein show future promise as biochemical markers for identifying serious bacterial infections.18
No—the complete blood count (CBC) alone does not have adequate sensitivity or specificity to tell bacterial from viral infections (strength of recommendation [SOR]: B, cohort studies). When used in conjunction with other clinical parameters in validated decision-making algorithms, the CBC can help detect serious bacterial infections in pediatric patients with fever (SOR: B, cohort studies).
There’s no substitute for history, physical exam, and good judgment
John D. Hallgren, MD
Uniformed Services University of the Health Sciences, RAF Menwith Hill, United Kingdom
Viral vs bacterial—often these are surrogate terms for minor vs serious illness. This review is a great lesson in likelihood ratios. Based on the low likelihood ratio, a CBC alone does not shift our suspicion greatly for serious bacterial infections in intermediate-risk patients; however, if you combine it with a clinical decision rule, it can greatly help decision-making, as evidenced by negative predictive values of 99% and above.
In contrast, we don’t need the CBC to tell us that an adult with the sniffles has a rhino/corona/whatevervirus, nor do we need it to tell us that a febrile, lethargic child with a petechial rash has a life-threatening bacteremia. If you enjoy the muck and the mess of primary care as much as I do, this inquiry should provide you with the validation that there’s no substitute for the history, physical exam, and judgment of a good clinician.
Evidence summary
For acutely febrile patients, the presence of an elevated white blood cell (WBC) count with elevated band forms has dogmatically been thought of as a marker for bacterial infection.1 Current literature, however, does not support this.2
Neisseria meningitides
A retrospective study of 5353 infants ages 3 to 89 days presenting to the emergency department for evaluation of fever showed that 3 of 4 infants ultimately diagnosed with bacterial meningitis would have been missed if the WBC count alone were used to predict which infants need a lumbar puncture.3 A prospective study of 2492 children ages 3 to 24 months presenting to the emergency department with acute fever and an absolute WBC count >15,000/mm3 revealed that neither a polymorphonuclear count of >10,000/mm3 (>66% segmented forms) nor a band count of >500/mm3 was associated with an increased likelihood of occult bacterial infection.4 Other studies show that the WBC alone is poorly discriminatory for identifying either bacteremia or meningitis.5,6
To improve the diagnostic utility of the CBC, other studies have examined individual components of the white blood cell differential count (TABLE 1). In particular, the use of the absolute neutrophil count (ANC) has been proposed as a superior marker of serious bacterial infection.7 A review of 6579 outpatients aged 3 to 36 months presenting to the emergency department with temperatures of 39°C or higher showed an ANC of >10,000/mm3 as more predictive of occult pneumococcal bacteremia than an elevated WBC count (>15,000/mm3) alone.8 Another retrospective review of more than 10,000 patients aged 3 to 36 months presenting to the emergency department used logistic regression to identify predictors of bacteremia. In this study, ANC (>9500/mm3) and WBC (>14,300/mm3) were of equal sensitivity (75%) and specificity (75%) in identifying serious bacterial infection.9 Finally, the band count alone does not accurately predict serious bacterial infection.10
In summary, the CBC cannot be used in isolation to differentiate bacterial from viral illness. The CBC can, however, augment clinical data from the history and physical examination to predict the likelihood of serious bacterial illness. As a result, numerous diagnostic criteria, each incorporating elements of the CBC, have been developed in an attempt to accurately differentiate bacterial from viral illness in acutely febrile patients, most typically children (TABLE 2). These criteria differ by age of the patient, clinical testing recommendations, indications for antibiotic therapy, as well as WBC cutoffs.
TABLE 1
WBC markers: How good are they at predicting serious bacterial infection?9,18,19
| VARIABLE | CUTOFF | SENSITIVITY | SPECIFICITY | LR (95% CI) |
|---|---|---|---|---|
| White blood cell count | 15,000/mm3 | 64%–82% | 67%–75% | 1.9–2.7 (1.1–3.8) |
| Absolute neutrophil count | 10,000/mm3 | 64%–76% | 76%–81% | 3.0–3.3 (1.6–6.2) |
| LR, likelihood ratio; CI, confidence interval. | ||||
TABLE 2
Clinical criteria for predicting serious bacterial infection in febrile children
| CRITERION | ROCHESTER CRITERIA11 | BOSTON CRITERIA12 | PHILADELPHIA CRITERIA13 |
|---|---|---|---|
| Predictive value | 98.9% PV–in ruling out serious bacterial infection | 95% PV+ to identify serious bacterial infection | 100% PV–in ruling out serious bacterial infection |
| Age | <60 days | 1–3 mos Present to emergency dept. with fever ≥38.0°C | 29–56 days Present with fever ≥38.2°C |
| Appearance | Well-appearing Previously healthy No evidence of infection (skin, bone, joint, soft tissue or ear) | Healthy appearing No ear, soft tissue, joint or bone infection on exam | Well-appearing |
| White blood cell count | WBC 5–15,000/mm3 Bands ≤1,500/mm3 | Peripheral WBC ≤20,000/mm3 | WBC ≤15,000/mm3 Band-to-neutrophil ratio of ≤0.2 |
| Urinalysis | ≤10 WBC/hpf of centrifuged urine | Urinalysis ≤10 WBC/hpf | Urinalysis ≤10 WBC/hpf |
| Other tests | If diarrhea, ≤5 WBC/hpf of stool smear | CSF WBC ≤10/hpf | CSF WBC ≤8/hpf with negative gram stain If watery diarrhea, few or no WBC/hpf on stool smear |
| WBC, white blood cell count; hpf, high-powered field; CSF, cerebrospinal fluid; PV, predictive value | |||
Recommendations from others
The American College of Emergency Physicians recommends considering antibiotic therapy for previously healthy, well-appearing children ages 3 to 36 months who present with a fever without a clinical source and a WBC count >15,000/mm3.3,14
The University of Cincinnati Evidence-Based Clinical Practice Guidelines for fever of uncertain source in children ages 2 to 36 months recommends obtaining a CBC for any child who is ill-appearing or at high risk for bacteremia (determined by the clinicians’ judgment). A WBC of ≥15,000/mm3 or ANC >10,000/mm3 provide support for antibiotic therapy.15 The 1993 American Academy of Pediatrics guidelines for fever ≥39°C without a source in children ages 3 months to 3 years recommends a CBC; if the WBC count ≥15,000/mm3, they recommend a blood culture and treatment with antibiotics pending culture results.3,16
It is important to note that in the age of Haemophilus influenza and Streptococcus pneumonia vaccination, the rate of occult bacteremia in febrile children presenting without a source has fallen from 3% to 10% to 1% or less.17 A lower prevalence reduces the utility of routine CBC or blood culture in the evaluation of immunized, febrile children. Parameters such as procalcitonin, interleukin-6, interleukin-8, interleukin-1 receptor antagonist and C-reactive protein show future promise as biochemical markers for identifying serious bacterial infections.18
1. Wile MJ, Homer LD, Gaehler S, Phillips S, Millan J. Manual differential cell counts help predict bacterial infection. A multivariate analysis. Am J Clin Pathol 2001;115:644-649.
2. Seebach JD, Morant R, Ruegg R, Seifert B, Fehr J. The diagnostic value of the neutrophil left shift in predicting inflammatory and infectious disease. Am J Clin Pathol 1997;107:582-591.
3. Bonsu BK, Harper MB. Utility of the peripheral blood white blood cell count for identifying sick young infants who need lumbar puncture. Ann Emerg Med 2003;41:206-214.
4. Kramer MS, Tange SM, Mills EL, Ciampi A, Bernstein ML, Drummond KN. Role of the complete blood count in detecting occult focal bacterial infection in the young febrile child. J Clin Epidemiol 1993;46:349-357.
5. Brown L, Shaw T, Wittlake WA. Does leucocytosis identify bacterial infections in febrile neonates presenting to the emergency department? Emerg Med J 2005;22:256-259.
6. Garges HP, Moody MA, Cotten CM, et al. Neonatal meningitis: what is the correlation among cerebrospinal fluid cultures, blood cultures, and cerebrospinal fluid parameters? Pediatrics 2006;117:1094-1100.
7. Gombos MM, Bienkowski RS, Gochman RF, Billett HH. The absolute neutrophil count: is it the best indicator for occult bacteremia in infants? Am J Clin Pathol 1998;109:221-225.
8. Kuppermann N, Fleisher GR, Jaffe DM. Predictors of occult pneumococcal bacteremia in young febrile children. Ann Emerg Med 1998;31:679-687.
9. Isaacman DJ, Shults J, Gross TK, Davis PH, Harper M. Predictors of bacteremia in febrile children 3 to 36 months of age. Pediatrics 2000;106:977-982.
10. Cornbleet PJ. Clinical utility of the band count. Clin Lab Med 2002;22:101-136.
11. Dagan R, Powell KR, Hall CB, Menegus MA. Identification of infants unlikely to have serious bacterial infection although hospitalized for suspected sepsis. J Pediatr 1985;107:855-860.
12. Baskin MN, O’Rourke EJ, Fleisher GR. Outpatient treatment of febrile infants 28 to 89 days of age with intramuscular administration of ceftriaxone. J Pediatr 1992;120:22-27.
13. Baker MD, Bell LM, Avner JR. The efficacy of routine outpatient management without antibiotics of fever in selected infants. Pediatrics 1999;103:627-631.
14. American College of emergency Physicians. Clinical policy for children younger than three years presenting to the emergency department with fever. Ann Emerg Med 2003;42:530-545.
15. Cincinnati Children’s Hospital Medical Center. Evidence-based clinical practice guideline for fever of uncertain source in children in 2 to 36 months of age. Cincinnati, Ohio: Cincinnati Children’s Hospital Medical Center; 2003.
16. Baraff LJ, Bass JW, Fleisher GR, et al. Practice guideline for the management of infants and children 0 to 36 months of age with fever without source. Ann Emerg Med 1993;22:1198-1210.
17. Stoll ML, Rubin LG. Incidence of occult bacteremia among highly febrile young children in the era of the pneumococcal conjugate vaccine. Arch Pediatr Adolesc Med 2004;158:671-675.
18. Pulliam PN, Attia MW, Cronan KM. C-reactive protein in febrile children 1 to 36 months of age with clinically undetectable serious bacterial infection. Pediatrics 2001;108:1275-1279.
19. Pratt A, Attia MW. Duration of fever and markers of serious bacterial infection in young febrile children. Pediatr Int 2007;49:31-35.
1. Wile MJ, Homer LD, Gaehler S, Phillips S, Millan J. Manual differential cell counts help predict bacterial infection. A multivariate analysis. Am J Clin Pathol 2001;115:644-649.
2. Seebach JD, Morant R, Ruegg R, Seifert B, Fehr J. The diagnostic value of the neutrophil left shift in predicting inflammatory and infectious disease. Am J Clin Pathol 1997;107:582-591.
3. Bonsu BK, Harper MB. Utility of the peripheral blood white blood cell count for identifying sick young infants who need lumbar puncture. Ann Emerg Med 2003;41:206-214.
4. Kramer MS, Tange SM, Mills EL, Ciampi A, Bernstein ML, Drummond KN. Role of the complete blood count in detecting occult focal bacterial infection in the young febrile child. J Clin Epidemiol 1993;46:349-357.
5. Brown L, Shaw T, Wittlake WA. Does leucocytosis identify bacterial infections in febrile neonates presenting to the emergency department? Emerg Med J 2005;22:256-259.
6. Garges HP, Moody MA, Cotten CM, et al. Neonatal meningitis: what is the correlation among cerebrospinal fluid cultures, blood cultures, and cerebrospinal fluid parameters? Pediatrics 2006;117:1094-1100.
7. Gombos MM, Bienkowski RS, Gochman RF, Billett HH. The absolute neutrophil count: is it the best indicator for occult bacteremia in infants? Am J Clin Pathol 1998;109:221-225.
8. Kuppermann N, Fleisher GR, Jaffe DM. Predictors of occult pneumococcal bacteremia in young febrile children. Ann Emerg Med 1998;31:679-687.
9. Isaacman DJ, Shults J, Gross TK, Davis PH, Harper M. Predictors of bacteremia in febrile children 3 to 36 months of age. Pediatrics 2000;106:977-982.
10. Cornbleet PJ. Clinical utility of the band count. Clin Lab Med 2002;22:101-136.
11. Dagan R, Powell KR, Hall CB, Menegus MA. Identification of infants unlikely to have serious bacterial infection although hospitalized for suspected sepsis. J Pediatr 1985;107:855-860.
12. Baskin MN, O’Rourke EJ, Fleisher GR. Outpatient treatment of febrile infants 28 to 89 days of age with intramuscular administration of ceftriaxone. J Pediatr 1992;120:22-27.
13. Baker MD, Bell LM, Avner JR. The efficacy of routine outpatient management without antibiotics of fever in selected infants. Pediatrics 1999;103:627-631.
14. American College of emergency Physicians. Clinical policy for children younger than three years presenting to the emergency department with fever. Ann Emerg Med 2003;42:530-545.
15. Cincinnati Children’s Hospital Medical Center. Evidence-based clinical practice guideline for fever of uncertain source in children in 2 to 36 months of age. Cincinnati, Ohio: Cincinnati Children’s Hospital Medical Center; 2003.
16. Baraff LJ, Bass JW, Fleisher GR, et al. Practice guideline for the management of infants and children 0 to 36 months of age with fever without source. Ann Emerg Med 1993;22:1198-1210.
17. Stoll ML, Rubin LG. Incidence of occult bacteremia among highly febrile young children in the era of the pneumococcal conjugate vaccine. Arch Pediatr Adolesc Med 2004;158:671-675.
18. Pulliam PN, Attia MW, Cronan KM. C-reactive protein in febrile children 1 to 36 months of age with clinically undetectable serious bacterial infection. Pediatrics 2001;108:1275-1279.
19. Pratt A, Attia MW. Duration of fever and markers of serious bacterial infection in young febrile children. Pediatr Int 2007;49:31-35.
Evidence-based answers from the Family Physicians Inquiries Network
Are there big differences among beta-blockers in treating essential hypertension?
Yes, a number of beta-blockers are effective in lowering blood pressure (strength of recommendation [SOR]: A, multiple, consistent randomized controlled trials [RCTs]). Cardioselective beta-blockers do not alter lung function studies for patients with chronic obstructive pulmonary disease (COPD) or reversible airway disease (SOR: A, meta-analysis of RCTs).
Propranolol and timolol have greater risks of causing fatigue as a side effect (SOR: A, meta-analysis of RCTs). Recent meta-analyses have stirred debate on the effectiveness of the agents in preventing adverse outcomes. The level of evidence has reached the point where the practice of using beta-blockers as monotherapy should be questioned (SOR: C, expert opinion).
Beta-blocker debate may be irrelevant when these drugs are taken with other antihypertensives
Joseph Saseen, PharmD, FCCP, BCPS
University of Colorado Health Sciences Center
Definitive evidence has demonstrated reduced risk of cardiovascular events with beta-blockers as a primary antihypertensive agent for patients with concurrent coronary heart disease. However, using a beta-blocker as a primary antihypertensive for patients without such compelling indications is now considered controversial. In 2006, the UK’s National Institute for Health and Clinical Excellence published a clinical guideline for hypertension1 in which beta-blockers are no longer preferred as a routine initial therapy for hypertension and are reserved as alternative agents after diuretics, angiotensin-converting enzyme (ACE) inhibitors, angiotensin receptor blockers (ARBs), and calcium channel blockers.
This recommendation was based on results from meta-analyses that suggest beta-blockers, especially atenolol, may not be as cardioprotective as other antihypertensives. This has been confirmed by a 2007 Cochrane analysis.2 Despite a half-life of only 6 to 7 hours, atenolol is nearly always dosed once daily, while carvedilol and metoprolol have half-lives of 6 to 10 and 3 to 7 hours, respectively, and are dosed at least twice daily. It is possible that the controversy with beta-blockers arises because atenolol should really be a twice-daily drug.
In clinical practice, most patients with hypertension need more than one agent to attain goal blood pressure values. The debate over whether one beta-blocker is better or worse may be clinically irrelevant when beta-blockers are used in combination with another antihypertensive.
Evidence summary
Numerous trials have shown that beta-blockers lower blood pressure for patients with hypertension. No head-to-head trials of beta-blockers have been conducted that reveal differences in terms of patient-oriented outcomes, such as all-cause mortality, in the treatment of hypertension.
No effect on lung function, but fatigue is a factor
A Cochrane review on the cardioselective beta-blockers atenolol (Tenormin), bisoprolol (Zebeta), and metoprolol (Lopressor) found that single-dose and multiple-treatment studies showed no decline in lung function among patients with mild to moderate reversible airway disease or chronic obstructive pulmonary disease.3,4 The analysis was not able to identify any differential effect of these beta-blockers with or without intrinsic sympathomimetic activity for patients with lung disease.
That said, beta-blockers do have side effects. One meta-analysis found no difference in the development of depression with beta-blocker therapy; however, first-generation beta-blockers (propranolol and timolol) had higher rates of fatigue than did the later beta-blockers.5 They reported that the risk of fatigue was only 18 per 1000 patients (95% confidence interval [CI], 5–30) and the risk for sexual dysfunction was 5 per 1000 patients (95% CI, 2–8) for all beta-blockers as a class. Importantly, they also stratified side-effect findings on the basis of lipophilic vs nonlipophilic and found no difference in side effect frequency.
Adverse outcomes data give reason to pause
Two recent meta-analyses6,7 on beta-blockers have called into question the effectiveness of these agents in preventing adverse outcomes in treating hypertension.
The first meta-analysis6 reviewed 4 studies that compared atenolol with placebo or no treatment, and 5 that compared atenolol with other antihypertensive drugs. They found no outcome differences between atenolol and placebo in the 4 studies, comprising 6825 patients, followed for a mean of 4.6 years. There was no difference in all-cause mortality (relative risk [RR]=1.01; 95% CI, 0.89–1.15), cardiovascular mortality (RR=0.99; 95% CI, 0.83–1.18), or myocardial infarction (RR=0.99; 95% CI, 0.83–1.19). The risk of stroke appeared to be lower in the atenolol than in the placebo group (RR=0.85; 95% CI, 0.72–1.01). When atenolol was compared with other antihypertensives, there were no major differences in blood pressure lowering between the treatment arms.
The authors found a significantly higher mortality (RR=1.13; 95% CI, 1.02–1.25) with atenolol treatment than with other active treatment, in 5 studies comprising 17,671 patients who were followed up for a mean of 4.6 years. Stroke was also more frequent with atenolol in comparison with other agents.
The second meta-analysis7 covered 13 randomized controlled trials (n=105,951) comparing treatment with beta-blockers with other antihypertensive drugs. Seven studies (n=27,433) were included in a comparison of beta-blockers and placebo or no treatment. The relative risk of stroke was 16% higher for beta-blockers (95% CI, 4%–30%) than for other drugs. No difference was seen for myocardial infarction. When the effect of beta-blockers was compared with that of placebo or no treatment, the relative risk of stroke was reduced by 19% for all beta-blockers (95% CI, 7%–29%). There was no difference for myocardial infarction or mortality.
An age divide appears with adverse events
A subsequent meta-analysis found that beta-blocker therapy in younger patients (less than 60 years of age) is associated with a significant reduction in cardiovascular morbidity and mortality.8 Researchers used data from 145,811 participants in 21 hypertension trials, beta-blockers reduced major cardiovascular outcomes in younger patients (risk ratio=0.86; 95% CI, 0.74–0.99) but not in older patients (risk ratio=0.89; 95% CI, 0.75–1.05).
In active comparator trials, beta-blockers demonstrated similar reductions in morbidity and mortality to other antihypertensive agents in younger patients (risk ratio=0.97; 95% CI, 0.88–1.07) but not in older patients (risk ratio=1.06; 95% CI, 1.01–1.10), with the excess risk being particularly marked for strokes (risk ratio=1.18; 95% CI, 1.07–1.30). The primary outcome researchers evaluated was a composite of stroke, myocardial infarction, and death.
Calcium channel blockers beat beta-blockers in recent review
Finally, a more recent systematic review found beta blockers to be inferior to calcium channel blockers and renin-angiotensin system inhibitors (ACE inhibitors or ARBs) for major endpoints of all-cause mortality, coronary heart disease, stroke, total cardiovascular events, and cardiovascular mortality.9 This review found beta-blockers had similar outcomes as diuretics but were less well tolerated than diuretics (RR=1.80; 95% CI, 1.33–2.42) or renin-angiotensin system inhibitors (RR=1.41; 1.29–1.54).
Thirteen trials with 91,561 participants, meeting inclusion criteria, compared beta-blockers with placebo (4 trials; n=23,613), diuretics (5 trials; n=18,241), calcium-channel blockers (4 trials; n=44,825), and renin-angiotensin system inhibitors (3 trials; n=10,828). Compared with placebo, beta-blockers reduced the risk of stroke (RR=0.80; 95% CI, 0.66–0.96) with a marginal fall in total cardiovascular events (RR=0.88; 95% CI, 0.79–0.97), but did not affect all-cause mortality (RR=0.99, 0.88–1.11), coronary heart disease (RR=0.93, 0.81–1.07), or cardiovascular mortality (RR=0.93, 0.80–1.09). The effect on stroke was less than that of calcium-channel blockers (RR=1.24, 1.11–1.40) and renin-angiotensin system inhibitors (RR=1.30, 1.11–1.53). The effect on total cardiovascular events was less than that of calcium-channel blockers (RR=1.18, 1.08–1.29).
Recommendations from others
The Joint National Committee on Hypertension (JNC-7) states that excellent clinical trial data demonstrate that lowering blood pressure with beta-blockers (and several other drug classes) will reduce the complications of hypertension.10
The European Society of Cardiology recommends beta-blockers as the first choice for antihypertensive therapy, alone or in combination, for patients with previous myocardial infarction, ischemic heart disease, arrhythmias or heart failure, asymptomatic left ventricular dysfunction, diabetes, or high risk of coronary disease, based on the efficacy of these drugs in these patient populations.11
1. Hypertension: Management of hypertension in adults in primary care. London: Royal College of Physicians; June 2006. Available at www.nice.org.uk/CG034. Accessed on March 7, 2007.
2. Wiysonge C, Bradley H, Mayosi B, et al. Beta-blockers for hypertension. Cochrane Database Syst Rev 2007;(1):CD002003.-
3. Salpeter S, Ormiston T, Salpeter E, Wood-Baker R. Cardioselective beta-blockers for COPD. Cochrane Database Syst Rev 2005;(4):CD003566.-
4. Salpeter S, Ormiston T, Salpeter E. Cardioselective beta-blockers for reversible airway disease. Cochrane Database Syst Rev 2002;(4):CD002992.-
5. Ko DT, Hebert PR, Coffey CS, Sedrakyan A, Curtis JP, Krumholz HM. Beta-blocker therapy and symptoms of depression, fatigue, and sexual dysfunction. JAMA 2002;288:351-357.
6. Carlberg B, Samuelsson O, Lindholm LH. Atenolol in hypertension: is it a wise choice? Lancet 2004;364:1684-1689.
7. Lindholm LH, Carlberg B, Samuelsson O. Should beta blockers remain first choice in the treatment of primary hypertension? A meta-analysis. Lancet 2005;366:1545-1553.
8. Khan N, McAlister FA. Re-examining the efficacy of beta-blockers for the treatment of hypertension: a meta-analysis. CMAJ 2006;174:1737-1742.
9. Bradley HA, Wiysonge CS, Volmink JA, et al. How strong is the evidence for use of beta-blockers as first-line therapy for hypertension? Systematic review and meta-analysis. J Hypertens 2006;24:2131-2141.
10. Chobanian AV, Bakris GL, Black HR, et al. Seventh report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure. Hypertension 2003;42:1206-1252.
11. Lopez-Sendon J, Swedberg K, McMurray J, et al. Expert consensus document on beta-adrenergic receptor blockers. Eur Heart J 2004;25:1341-1362.
Yes, a number of beta-blockers are effective in lowering blood pressure (strength of recommendation [SOR]: A, multiple, consistent randomized controlled trials [RCTs]). Cardioselective beta-blockers do not alter lung function studies for patients with chronic obstructive pulmonary disease (COPD) or reversible airway disease (SOR: A, meta-analysis of RCTs).
Propranolol and timolol have greater risks of causing fatigue as a side effect (SOR: A, meta-analysis of RCTs). Recent meta-analyses have stirred debate on the effectiveness of the agents in preventing adverse outcomes. The level of evidence has reached the point where the practice of using beta-blockers as monotherapy should be questioned (SOR: C, expert opinion).
Beta-blocker debate may be irrelevant when these drugs are taken with other antihypertensives
Joseph Saseen, PharmD, FCCP, BCPS
University of Colorado Health Sciences Center
Definitive evidence has demonstrated reduced risk of cardiovascular events with beta-blockers as a primary antihypertensive agent for patients with concurrent coronary heart disease. However, using a beta-blocker as a primary antihypertensive for patients without such compelling indications is now considered controversial. In 2006, the UK’s National Institute for Health and Clinical Excellence published a clinical guideline for hypertension1 in which beta-blockers are no longer preferred as a routine initial therapy for hypertension and are reserved as alternative agents after diuretics, angiotensin-converting enzyme (ACE) inhibitors, angiotensin receptor blockers (ARBs), and calcium channel blockers.
This recommendation was based on results from meta-analyses that suggest beta-blockers, especially atenolol, may not be as cardioprotective as other antihypertensives. This has been confirmed by a 2007 Cochrane analysis.2 Despite a half-life of only 6 to 7 hours, atenolol is nearly always dosed once daily, while carvedilol and metoprolol have half-lives of 6 to 10 and 3 to 7 hours, respectively, and are dosed at least twice daily. It is possible that the controversy with beta-blockers arises because atenolol should really be a twice-daily drug.
In clinical practice, most patients with hypertension need more than one agent to attain goal blood pressure values. The debate over whether one beta-blocker is better or worse may be clinically irrelevant when beta-blockers are used in combination with another antihypertensive.
Evidence summary
Numerous trials have shown that beta-blockers lower blood pressure for patients with hypertension. No head-to-head trials of beta-blockers have been conducted that reveal differences in terms of patient-oriented outcomes, such as all-cause mortality, in the treatment of hypertension.
No effect on lung function, but fatigue is a factor
A Cochrane review on the cardioselective beta-blockers atenolol (Tenormin), bisoprolol (Zebeta), and metoprolol (Lopressor) found that single-dose and multiple-treatment studies showed no decline in lung function among patients with mild to moderate reversible airway disease or chronic obstructive pulmonary disease.3,4 The analysis was not able to identify any differential effect of these beta-blockers with or without intrinsic sympathomimetic activity for patients with lung disease.
That said, beta-blockers do have side effects. One meta-analysis found no difference in the development of depression with beta-blocker therapy; however, first-generation beta-blockers (propranolol and timolol) had higher rates of fatigue than did the later beta-blockers.5 They reported that the risk of fatigue was only 18 per 1000 patients (95% confidence interval [CI], 5–30) and the risk for sexual dysfunction was 5 per 1000 patients (95% CI, 2–8) for all beta-blockers as a class. Importantly, they also stratified side-effect findings on the basis of lipophilic vs nonlipophilic and found no difference in side effect frequency.
Adverse outcomes data give reason to pause
Two recent meta-analyses6,7 on beta-blockers have called into question the effectiveness of these agents in preventing adverse outcomes in treating hypertension.
The first meta-analysis6 reviewed 4 studies that compared atenolol with placebo or no treatment, and 5 that compared atenolol with other antihypertensive drugs. They found no outcome differences between atenolol and placebo in the 4 studies, comprising 6825 patients, followed for a mean of 4.6 years. There was no difference in all-cause mortality (relative risk [RR]=1.01; 95% CI, 0.89–1.15), cardiovascular mortality (RR=0.99; 95% CI, 0.83–1.18), or myocardial infarction (RR=0.99; 95% CI, 0.83–1.19). The risk of stroke appeared to be lower in the atenolol than in the placebo group (RR=0.85; 95% CI, 0.72–1.01). When atenolol was compared with other antihypertensives, there were no major differences in blood pressure lowering between the treatment arms.
The authors found a significantly higher mortality (RR=1.13; 95% CI, 1.02–1.25) with atenolol treatment than with other active treatment, in 5 studies comprising 17,671 patients who were followed up for a mean of 4.6 years. Stroke was also more frequent with atenolol in comparison with other agents.
The second meta-analysis7 covered 13 randomized controlled trials (n=105,951) comparing treatment with beta-blockers with other antihypertensive drugs. Seven studies (n=27,433) were included in a comparison of beta-blockers and placebo or no treatment. The relative risk of stroke was 16% higher for beta-blockers (95% CI, 4%–30%) than for other drugs. No difference was seen for myocardial infarction. When the effect of beta-blockers was compared with that of placebo or no treatment, the relative risk of stroke was reduced by 19% for all beta-blockers (95% CI, 7%–29%). There was no difference for myocardial infarction or mortality.
An age divide appears with adverse events
A subsequent meta-analysis found that beta-blocker therapy in younger patients (less than 60 years of age) is associated with a significant reduction in cardiovascular morbidity and mortality.8 Researchers used data from 145,811 participants in 21 hypertension trials, beta-blockers reduced major cardiovascular outcomes in younger patients (risk ratio=0.86; 95% CI, 0.74–0.99) but not in older patients (risk ratio=0.89; 95% CI, 0.75–1.05).
In active comparator trials, beta-blockers demonstrated similar reductions in morbidity and mortality to other antihypertensive agents in younger patients (risk ratio=0.97; 95% CI, 0.88–1.07) but not in older patients (risk ratio=1.06; 95% CI, 1.01–1.10), with the excess risk being particularly marked for strokes (risk ratio=1.18; 95% CI, 1.07–1.30). The primary outcome researchers evaluated was a composite of stroke, myocardial infarction, and death.
Calcium channel blockers beat beta-blockers in recent review
Finally, a more recent systematic review found beta blockers to be inferior to calcium channel blockers and renin-angiotensin system inhibitors (ACE inhibitors or ARBs) for major endpoints of all-cause mortality, coronary heart disease, stroke, total cardiovascular events, and cardiovascular mortality.9 This review found beta-blockers had similar outcomes as diuretics but were less well tolerated than diuretics (RR=1.80; 95% CI, 1.33–2.42) or renin-angiotensin system inhibitors (RR=1.41; 1.29–1.54).
Thirteen trials with 91,561 participants, meeting inclusion criteria, compared beta-blockers with placebo (4 trials; n=23,613), diuretics (5 trials; n=18,241), calcium-channel blockers (4 trials; n=44,825), and renin-angiotensin system inhibitors (3 trials; n=10,828). Compared with placebo, beta-blockers reduced the risk of stroke (RR=0.80; 95% CI, 0.66–0.96) with a marginal fall in total cardiovascular events (RR=0.88; 95% CI, 0.79–0.97), but did not affect all-cause mortality (RR=0.99, 0.88–1.11), coronary heart disease (RR=0.93, 0.81–1.07), or cardiovascular mortality (RR=0.93, 0.80–1.09). The effect on stroke was less than that of calcium-channel blockers (RR=1.24, 1.11–1.40) and renin-angiotensin system inhibitors (RR=1.30, 1.11–1.53). The effect on total cardiovascular events was less than that of calcium-channel blockers (RR=1.18, 1.08–1.29).
Recommendations from others
The Joint National Committee on Hypertension (JNC-7) states that excellent clinical trial data demonstrate that lowering blood pressure with beta-blockers (and several other drug classes) will reduce the complications of hypertension.10
The European Society of Cardiology recommends beta-blockers as the first choice for antihypertensive therapy, alone or in combination, for patients with previous myocardial infarction, ischemic heart disease, arrhythmias or heart failure, asymptomatic left ventricular dysfunction, diabetes, or high risk of coronary disease, based on the efficacy of these drugs in these patient populations.11
Yes, a number of beta-blockers are effective in lowering blood pressure (strength of recommendation [SOR]: A, multiple, consistent randomized controlled trials [RCTs]). Cardioselective beta-blockers do not alter lung function studies for patients with chronic obstructive pulmonary disease (COPD) or reversible airway disease (SOR: A, meta-analysis of RCTs).
Propranolol and timolol have greater risks of causing fatigue as a side effect (SOR: A, meta-analysis of RCTs). Recent meta-analyses have stirred debate on the effectiveness of the agents in preventing adverse outcomes. The level of evidence has reached the point where the practice of using beta-blockers as monotherapy should be questioned (SOR: C, expert opinion).
Beta-blocker debate may be irrelevant when these drugs are taken with other antihypertensives
Joseph Saseen, PharmD, FCCP, BCPS
University of Colorado Health Sciences Center
Definitive evidence has demonstrated reduced risk of cardiovascular events with beta-blockers as a primary antihypertensive agent for patients with concurrent coronary heart disease. However, using a beta-blocker as a primary antihypertensive for patients without such compelling indications is now considered controversial. In 2006, the UK’s National Institute for Health and Clinical Excellence published a clinical guideline for hypertension1 in which beta-blockers are no longer preferred as a routine initial therapy for hypertension and are reserved as alternative agents after diuretics, angiotensin-converting enzyme (ACE) inhibitors, angiotensin receptor blockers (ARBs), and calcium channel blockers.
This recommendation was based on results from meta-analyses that suggest beta-blockers, especially atenolol, may not be as cardioprotective as other antihypertensives. This has been confirmed by a 2007 Cochrane analysis.2 Despite a half-life of only 6 to 7 hours, atenolol is nearly always dosed once daily, while carvedilol and metoprolol have half-lives of 6 to 10 and 3 to 7 hours, respectively, and are dosed at least twice daily. It is possible that the controversy with beta-blockers arises because atenolol should really be a twice-daily drug.
In clinical practice, most patients with hypertension need more than one agent to attain goal blood pressure values. The debate over whether one beta-blocker is better or worse may be clinically irrelevant when beta-blockers are used in combination with another antihypertensive.
Evidence summary
Numerous trials have shown that beta-blockers lower blood pressure for patients with hypertension. No head-to-head trials of beta-blockers have been conducted that reveal differences in terms of patient-oriented outcomes, such as all-cause mortality, in the treatment of hypertension.
No effect on lung function, but fatigue is a factor
A Cochrane review on the cardioselective beta-blockers atenolol (Tenormin), bisoprolol (Zebeta), and metoprolol (Lopressor) found that single-dose and multiple-treatment studies showed no decline in lung function among patients with mild to moderate reversible airway disease or chronic obstructive pulmonary disease.3,4 The analysis was not able to identify any differential effect of these beta-blockers with or without intrinsic sympathomimetic activity for patients with lung disease.
That said, beta-blockers do have side effects. One meta-analysis found no difference in the development of depression with beta-blocker therapy; however, first-generation beta-blockers (propranolol and timolol) had higher rates of fatigue than did the later beta-blockers.5 They reported that the risk of fatigue was only 18 per 1000 patients (95% confidence interval [CI], 5–30) and the risk for sexual dysfunction was 5 per 1000 patients (95% CI, 2–8) for all beta-blockers as a class. Importantly, they also stratified side-effect findings on the basis of lipophilic vs nonlipophilic and found no difference in side effect frequency.
Adverse outcomes data give reason to pause
Two recent meta-analyses6,7 on beta-blockers have called into question the effectiveness of these agents in preventing adverse outcomes in treating hypertension.
The first meta-analysis6 reviewed 4 studies that compared atenolol with placebo or no treatment, and 5 that compared atenolol with other antihypertensive drugs. They found no outcome differences between atenolol and placebo in the 4 studies, comprising 6825 patients, followed for a mean of 4.6 years. There was no difference in all-cause mortality (relative risk [RR]=1.01; 95% CI, 0.89–1.15), cardiovascular mortality (RR=0.99; 95% CI, 0.83–1.18), or myocardial infarction (RR=0.99; 95% CI, 0.83–1.19). The risk of stroke appeared to be lower in the atenolol than in the placebo group (RR=0.85; 95% CI, 0.72–1.01). When atenolol was compared with other antihypertensives, there were no major differences in blood pressure lowering between the treatment arms.
The authors found a significantly higher mortality (RR=1.13; 95% CI, 1.02–1.25) with atenolol treatment than with other active treatment, in 5 studies comprising 17,671 patients who were followed up for a mean of 4.6 years. Stroke was also more frequent with atenolol in comparison with other agents.
The second meta-analysis7 covered 13 randomized controlled trials (n=105,951) comparing treatment with beta-blockers with other antihypertensive drugs. Seven studies (n=27,433) were included in a comparison of beta-blockers and placebo or no treatment. The relative risk of stroke was 16% higher for beta-blockers (95% CI, 4%–30%) than for other drugs. No difference was seen for myocardial infarction. When the effect of beta-blockers was compared with that of placebo or no treatment, the relative risk of stroke was reduced by 19% for all beta-blockers (95% CI, 7%–29%). There was no difference for myocardial infarction or mortality.
An age divide appears with adverse events
A subsequent meta-analysis found that beta-blocker therapy in younger patients (less than 60 years of age) is associated with a significant reduction in cardiovascular morbidity and mortality.8 Researchers used data from 145,811 participants in 21 hypertension trials, beta-blockers reduced major cardiovascular outcomes in younger patients (risk ratio=0.86; 95% CI, 0.74–0.99) but not in older patients (risk ratio=0.89; 95% CI, 0.75–1.05).
In active comparator trials, beta-blockers demonstrated similar reductions in morbidity and mortality to other antihypertensive agents in younger patients (risk ratio=0.97; 95% CI, 0.88–1.07) but not in older patients (risk ratio=1.06; 95% CI, 1.01–1.10), with the excess risk being particularly marked for strokes (risk ratio=1.18; 95% CI, 1.07–1.30). The primary outcome researchers evaluated was a composite of stroke, myocardial infarction, and death.
Calcium channel blockers beat beta-blockers in recent review
Finally, a more recent systematic review found beta blockers to be inferior to calcium channel blockers and renin-angiotensin system inhibitors (ACE inhibitors or ARBs) for major endpoints of all-cause mortality, coronary heart disease, stroke, total cardiovascular events, and cardiovascular mortality.9 This review found beta-blockers had similar outcomes as diuretics but were less well tolerated than diuretics (RR=1.80; 95% CI, 1.33–2.42) or renin-angiotensin system inhibitors (RR=1.41; 1.29–1.54).
Thirteen trials with 91,561 participants, meeting inclusion criteria, compared beta-blockers with placebo (4 trials; n=23,613), diuretics (5 trials; n=18,241), calcium-channel blockers (4 trials; n=44,825), and renin-angiotensin system inhibitors (3 trials; n=10,828). Compared with placebo, beta-blockers reduced the risk of stroke (RR=0.80; 95% CI, 0.66–0.96) with a marginal fall in total cardiovascular events (RR=0.88; 95% CI, 0.79–0.97), but did not affect all-cause mortality (RR=0.99, 0.88–1.11), coronary heart disease (RR=0.93, 0.81–1.07), or cardiovascular mortality (RR=0.93, 0.80–1.09). The effect on stroke was less than that of calcium-channel blockers (RR=1.24, 1.11–1.40) and renin-angiotensin system inhibitors (RR=1.30, 1.11–1.53). The effect on total cardiovascular events was less than that of calcium-channel blockers (RR=1.18, 1.08–1.29).
Recommendations from others
The Joint National Committee on Hypertension (JNC-7) states that excellent clinical trial data demonstrate that lowering blood pressure with beta-blockers (and several other drug classes) will reduce the complications of hypertension.10
The European Society of Cardiology recommends beta-blockers as the first choice for antihypertensive therapy, alone or in combination, for patients with previous myocardial infarction, ischemic heart disease, arrhythmias or heart failure, asymptomatic left ventricular dysfunction, diabetes, or high risk of coronary disease, based on the efficacy of these drugs in these patient populations.11
1. Hypertension: Management of hypertension in adults in primary care. London: Royal College of Physicians; June 2006. Available at www.nice.org.uk/CG034. Accessed on March 7, 2007.
2. Wiysonge C, Bradley H, Mayosi B, et al. Beta-blockers for hypertension. Cochrane Database Syst Rev 2007;(1):CD002003.-
3. Salpeter S, Ormiston T, Salpeter E, Wood-Baker R. Cardioselective beta-blockers for COPD. Cochrane Database Syst Rev 2005;(4):CD003566.-
4. Salpeter S, Ormiston T, Salpeter E. Cardioselective beta-blockers for reversible airway disease. Cochrane Database Syst Rev 2002;(4):CD002992.-
5. Ko DT, Hebert PR, Coffey CS, Sedrakyan A, Curtis JP, Krumholz HM. Beta-blocker therapy and symptoms of depression, fatigue, and sexual dysfunction. JAMA 2002;288:351-357.
6. Carlberg B, Samuelsson O, Lindholm LH. Atenolol in hypertension: is it a wise choice? Lancet 2004;364:1684-1689.
7. Lindholm LH, Carlberg B, Samuelsson O. Should beta blockers remain first choice in the treatment of primary hypertension? A meta-analysis. Lancet 2005;366:1545-1553.
8. Khan N, McAlister FA. Re-examining the efficacy of beta-blockers for the treatment of hypertension: a meta-analysis. CMAJ 2006;174:1737-1742.
9. Bradley HA, Wiysonge CS, Volmink JA, et al. How strong is the evidence for use of beta-blockers as first-line therapy for hypertension? Systematic review and meta-analysis. J Hypertens 2006;24:2131-2141.
10. Chobanian AV, Bakris GL, Black HR, et al. Seventh report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure. Hypertension 2003;42:1206-1252.
11. Lopez-Sendon J, Swedberg K, McMurray J, et al. Expert consensus document on beta-adrenergic receptor blockers. Eur Heart J 2004;25:1341-1362.
1. Hypertension: Management of hypertension in adults in primary care. London: Royal College of Physicians; June 2006. Available at www.nice.org.uk/CG034. Accessed on March 7, 2007.
2. Wiysonge C, Bradley H, Mayosi B, et al. Beta-blockers for hypertension. Cochrane Database Syst Rev 2007;(1):CD002003.-
3. Salpeter S, Ormiston T, Salpeter E, Wood-Baker R. Cardioselective beta-blockers for COPD. Cochrane Database Syst Rev 2005;(4):CD003566.-
4. Salpeter S, Ormiston T, Salpeter E. Cardioselective beta-blockers for reversible airway disease. Cochrane Database Syst Rev 2002;(4):CD002992.-
5. Ko DT, Hebert PR, Coffey CS, Sedrakyan A, Curtis JP, Krumholz HM. Beta-blocker therapy and symptoms of depression, fatigue, and sexual dysfunction. JAMA 2002;288:351-357.
6. Carlberg B, Samuelsson O, Lindholm LH. Atenolol in hypertension: is it a wise choice? Lancet 2004;364:1684-1689.
7. Lindholm LH, Carlberg B, Samuelsson O. Should beta blockers remain first choice in the treatment of primary hypertension? A meta-analysis. Lancet 2005;366:1545-1553.
8. Khan N, McAlister FA. Re-examining the efficacy of beta-blockers for the treatment of hypertension: a meta-analysis. CMAJ 2006;174:1737-1742.
9. Bradley HA, Wiysonge CS, Volmink JA, et al. How strong is the evidence for use of beta-blockers as first-line therapy for hypertension? Systematic review and meta-analysis. J Hypertens 2006;24:2131-2141.
10. Chobanian AV, Bakris GL, Black HR, et al. Seventh report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure. Hypertension 2003;42:1206-1252.
11. Lopez-Sendon J, Swedberg K, McMurray J, et al. Expert consensus document on beta-adrenergic receptor blockers. Eur Heart J 2004;25:1341-1362.
Evidence-based answers from the Family Physicians Inquiries Network
What other STI testing should we do for a patient with chlamydia?
Testing for gonorrhea is recommended for a patient with genital chlamydia; also test for gonorrhea and chlamydia in their sexual partner because of the high prevalence of coinfection, particularly among younger patients (strength of recommendation [SOR]: C, based on expert opinion and observational studies). Testing for syphilis and HIV is also recommended for the patient and their partner (SOR: C, based on expert opinion).
David B. , MD, FAAFP
Tacoma Family Medicine, Tacoma, Wash
Counsel younger adults and teens regarding the risks for STIs by taking an adequate sexual history Many outpatient labs offer combined upfront testing of chlamydia and gonorrhea—the information here supports that approach. More important, the recommendation to “screen” all patients seeking treatment for sexually transmitted infections (STIs) for HIV carries an essential underlying message: the need to talk with and counsel our patients, especially younger adults and teens, regarding their risks for STIs by taking an adequate sexual history.
The Centers for Disease Control and Prevention (CDC) suggests one organized approach using “The Five Ps”: Partners (gender, number), Prevention of pregnancy, Protection from STIs, Practices (type of sex and condom use), Past history of STIs. This important conversation not only guides decisions about completing testing for other STIs but provides an avenue for potentially life-saving conversations.
Evidence Summary
A pilot study evaluated the yield of testing patients for other STIs among patients with genital chlamydia diagnosed during opportunistic screening. The study screened patients of both sexes in primary health care settings, as well as men attending a genitourinary medicine clinic. All patients testing positive in the community were advised to attend the genitourinary medicine clinic for STI screening, partner notification, and testing of patients and their contacts. More than 90% of the patients testing positive for chlamydia attended the genitourinary medicine clinic for management (total numbers seen in the clinic; women n=1245 [957 screened in the community] and men n=490 [280 screened in the community]).
At the clinic, further workup included evaluation and testing for chlamydia, gonorrhea, trichomonas, and bacterial vaginosis. Of the patients whose initial screening was in the genitourinary medicine clinic, 28% had an additional STI. Of the patients initially screened in the community setting, 4% had another STI. Partner testing showed that 55% of male partners of female patients had an STI and 76% of female partners of male patients had one or more STI.1
The high prevalence of coinfection of chlamydia and gonorrhea has been shown in several studies. One cross-sectional study of new clients to a hospital-based STI clinic with gonorrhea, chlamydia, or both infections found 39% of 1239 women and 24% of 1141 heterosexual men with gonorrhea also had chlamydia. Thirteen percent of females and 19% of heterosexual males with chlamydia also had gonorrhea. More than half of the women and a third of the men aged 15 to 19 had both gonorrhea and chlamydia. Patients with both STIs tended to be younger than those with one.2 A study of the prevalence rate of chlamydia, gonorrhea, and their coinfection in an adolescent population (women n=131,915 and men n=71,074) of juvenile detention centers between 1997 and 2002 found that 18% of women and 13% of males with chlamydia were coinfected with gonorrhea.3 In non–STI clinic settings, gonorrhea has been found in 9% of men4,5 and 6% of women5 with chlamydia.
Recommendations from others
The American Academy of Family Physicians (AAFP) strongly recommends testing for chlamydia in all sexually active women aged 25 years or younger and those at increased risk. The AAFP recommends screening all sexually active women for gonorrhea if they are at increased risk for infection; strongly recommends screening persons at increased risk for syphilis infection; and strongly recommends screening for HIV for persons seeking treatment for STIs.6
The CDC guidelines recommend evaluation, testing and treatment of partners of persons with chlamydia. As well as testing for other STIs, the guidelines suggest Pap smear screening for women who have not been adequately screened, as they often are at high risk for later cervical cancer.7 All patients seeking treatment for STDs, including all patients attending STD clinics, should be screened routinely for HIV during each visit for a new complaint, regardless of whether the patient is known or suspected to have specific behavior risks for HIV infection.8
The Institute for Clinical Systems Improvement recommends screening for chlamydia and gonorrhea for all sexually active women aged 25 years and younger and other asymptomatic women at risk for infection. Routine screening for HIV is also recommended to all persons at high risk, including those seeking treatment for any STI.9
1. Harindra V, Tobin JM, Underhill G. Opportunistic chlamydia screening; should positive patients be screened for co-infections? Int J STD AIDS 2002;13:821-825
2. Creighton S, Tenant-Flowers M, Taylor CB, Miller R, Low N. Co-infection with gonorrhoea and chlamydia: how much is there and what does it mean? Int J STD AIDS 2003;14:109-113
3. Kahn RH, Mosure DJ, Blank S, et al for the jail STD Prevalence Monitoring Project. Chlamydia trachomatis and Neisseria gonorrhoeae Prevalence and Coinfection in adolescents entering selected US juvenile Detention Centers, 1997-2002. Sex Transm Dis 2005;32:255-259.
4. Gaydos CA, Kent CK, Rietmeijer CA, et al. Prevalence of Neisseria Gonorrheae among men screened for Chlamydia Trachomatis in four US cities, 1999-2003 Sex Transm D 2006;33:314-319.
5. KMiller WC, Ford CA, Morris M, et al. Prevalence of chlamydial and gonococcal infections among young adults in the united states. JAMA 2004;291:2229-2236
6. American Academy of Family Physicians (AAFP). Summary of the recommendations for clinical preventive services. Revision 6.0. Leawood, Kan: AAFP; August 2005.
7. Workowski KA, Berman SM. Centers for Disease Control and Prevention. Sexually transmitted diseases treatment guidelines, 2006. MMWR Recomm Rep 2006;55(RR-11):1-94.
8. Branson BM, Handsfield HH, Lampe MA, et al. Revised recommendations for HIV testing of adults, adolescents, and pregnant women in health-care settings. MMWR Recomm Rep 2006;55(RR-14):1-17.
9. Institute for Clinical systems Improvement Preventive services for adults. Bloomington, Minn: Institute for Clinical systems Improvement; 2004. Available online at www guideline.gov. Accessed on December 13, 2006.
Testing for gonorrhea is recommended for a patient with genital chlamydia; also test for gonorrhea and chlamydia in their sexual partner because of the high prevalence of coinfection, particularly among younger patients (strength of recommendation [SOR]: C, based on expert opinion and observational studies). Testing for syphilis and HIV is also recommended for the patient and their partner (SOR: C, based on expert opinion).
David B. , MD, FAAFP
Tacoma Family Medicine, Tacoma, Wash
Counsel younger adults and teens regarding the risks for STIs by taking an adequate sexual history Many outpatient labs offer combined upfront testing of chlamydia and gonorrhea—the information here supports that approach. More important, the recommendation to “screen” all patients seeking treatment for sexually transmitted infections (STIs) for HIV carries an essential underlying message: the need to talk with and counsel our patients, especially younger adults and teens, regarding their risks for STIs by taking an adequate sexual history.
The Centers for Disease Control and Prevention (CDC) suggests one organized approach using “The Five Ps”: Partners (gender, number), Prevention of pregnancy, Protection from STIs, Practices (type of sex and condom use), Past history of STIs. This important conversation not only guides decisions about completing testing for other STIs but provides an avenue for potentially life-saving conversations.
Evidence Summary
A pilot study evaluated the yield of testing patients for other STIs among patients with genital chlamydia diagnosed during opportunistic screening. The study screened patients of both sexes in primary health care settings, as well as men attending a genitourinary medicine clinic. All patients testing positive in the community were advised to attend the genitourinary medicine clinic for STI screening, partner notification, and testing of patients and their contacts. More than 90% of the patients testing positive for chlamydia attended the genitourinary medicine clinic for management (total numbers seen in the clinic; women n=1245 [957 screened in the community] and men n=490 [280 screened in the community]).
At the clinic, further workup included evaluation and testing for chlamydia, gonorrhea, trichomonas, and bacterial vaginosis. Of the patients whose initial screening was in the genitourinary medicine clinic, 28% had an additional STI. Of the patients initially screened in the community setting, 4% had another STI. Partner testing showed that 55% of male partners of female patients had an STI and 76% of female partners of male patients had one or more STI.1
The high prevalence of coinfection of chlamydia and gonorrhea has been shown in several studies. One cross-sectional study of new clients to a hospital-based STI clinic with gonorrhea, chlamydia, or both infections found 39% of 1239 women and 24% of 1141 heterosexual men with gonorrhea also had chlamydia. Thirteen percent of females and 19% of heterosexual males with chlamydia also had gonorrhea. More than half of the women and a third of the men aged 15 to 19 had both gonorrhea and chlamydia. Patients with both STIs tended to be younger than those with one.2 A study of the prevalence rate of chlamydia, gonorrhea, and their coinfection in an adolescent population (women n=131,915 and men n=71,074) of juvenile detention centers between 1997 and 2002 found that 18% of women and 13% of males with chlamydia were coinfected with gonorrhea.3 In non–STI clinic settings, gonorrhea has been found in 9% of men4,5 and 6% of women5 with chlamydia.
Recommendations from others
The American Academy of Family Physicians (AAFP) strongly recommends testing for chlamydia in all sexually active women aged 25 years or younger and those at increased risk. The AAFP recommends screening all sexually active women for gonorrhea if they are at increased risk for infection; strongly recommends screening persons at increased risk for syphilis infection; and strongly recommends screening for HIV for persons seeking treatment for STIs.6
The CDC guidelines recommend evaluation, testing and treatment of partners of persons with chlamydia. As well as testing for other STIs, the guidelines suggest Pap smear screening for women who have not been adequately screened, as they often are at high risk for later cervical cancer.7 All patients seeking treatment for STDs, including all patients attending STD clinics, should be screened routinely for HIV during each visit for a new complaint, regardless of whether the patient is known or suspected to have specific behavior risks for HIV infection.8
The Institute for Clinical Systems Improvement recommends screening for chlamydia and gonorrhea for all sexually active women aged 25 years and younger and other asymptomatic women at risk for infection. Routine screening for HIV is also recommended to all persons at high risk, including those seeking treatment for any STI.9
Testing for gonorrhea is recommended for a patient with genital chlamydia; also test for gonorrhea and chlamydia in their sexual partner because of the high prevalence of coinfection, particularly among younger patients (strength of recommendation [SOR]: C, based on expert opinion and observational studies). Testing for syphilis and HIV is also recommended for the patient and their partner (SOR: C, based on expert opinion).
David B. , MD, FAAFP
Tacoma Family Medicine, Tacoma, Wash
Counsel younger adults and teens regarding the risks for STIs by taking an adequate sexual history Many outpatient labs offer combined upfront testing of chlamydia and gonorrhea—the information here supports that approach. More important, the recommendation to “screen” all patients seeking treatment for sexually transmitted infections (STIs) for HIV carries an essential underlying message: the need to talk with and counsel our patients, especially younger adults and teens, regarding their risks for STIs by taking an adequate sexual history.
The Centers for Disease Control and Prevention (CDC) suggests one organized approach using “The Five Ps”: Partners (gender, number), Prevention of pregnancy, Protection from STIs, Practices (type of sex and condom use), Past history of STIs. This important conversation not only guides decisions about completing testing for other STIs but provides an avenue for potentially life-saving conversations.
Evidence Summary
A pilot study evaluated the yield of testing patients for other STIs among patients with genital chlamydia diagnosed during opportunistic screening. The study screened patients of both sexes in primary health care settings, as well as men attending a genitourinary medicine clinic. All patients testing positive in the community were advised to attend the genitourinary medicine clinic for STI screening, partner notification, and testing of patients and their contacts. More than 90% of the patients testing positive for chlamydia attended the genitourinary medicine clinic for management (total numbers seen in the clinic; women n=1245 [957 screened in the community] and men n=490 [280 screened in the community]).
At the clinic, further workup included evaluation and testing for chlamydia, gonorrhea, trichomonas, and bacterial vaginosis. Of the patients whose initial screening was in the genitourinary medicine clinic, 28% had an additional STI. Of the patients initially screened in the community setting, 4% had another STI. Partner testing showed that 55% of male partners of female patients had an STI and 76% of female partners of male patients had one or more STI.1
The high prevalence of coinfection of chlamydia and gonorrhea has been shown in several studies. One cross-sectional study of new clients to a hospital-based STI clinic with gonorrhea, chlamydia, or both infections found 39% of 1239 women and 24% of 1141 heterosexual men with gonorrhea also had chlamydia. Thirteen percent of females and 19% of heterosexual males with chlamydia also had gonorrhea. More than half of the women and a third of the men aged 15 to 19 had both gonorrhea and chlamydia. Patients with both STIs tended to be younger than those with one.2 A study of the prevalence rate of chlamydia, gonorrhea, and their coinfection in an adolescent population (women n=131,915 and men n=71,074) of juvenile detention centers between 1997 and 2002 found that 18% of women and 13% of males with chlamydia were coinfected with gonorrhea.3 In non–STI clinic settings, gonorrhea has been found in 9% of men4,5 and 6% of women5 with chlamydia.
Recommendations from others
The American Academy of Family Physicians (AAFP) strongly recommends testing for chlamydia in all sexually active women aged 25 years or younger and those at increased risk. The AAFP recommends screening all sexually active women for gonorrhea if they are at increased risk for infection; strongly recommends screening persons at increased risk for syphilis infection; and strongly recommends screening for HIV for persons seeking treatment for STIs.6
The CDC guidelines recommend evaluation, testing and treatment of partners of persons with chlamydia. As well as testing for other STIs, the guidelines suggest Pap smear screening for women who have not been adequately screened, as they often are at high risk for later cervical cancer.7 All patients seeking treatment for STDs, including all patients attending STD clinics, should be screened routinely for HIV during each visit for a new complaint, regardless of whether the patient is known or suspected to have specific behavior risks for HIV infection.8
The Institute for Clinical Systems Improvement recommends screening for chlamydia and gonorrhea for all sexually active women aged 25 years and younger and other asymptomatic women at risk for infection. Routine screening for HIV is also recommended to all persons at high risk, including those seeking treatment for any STI.9
1. Harindra V, Tobin JM, Underhill G. Opportunistic chlamydia screening; should positive patients be screened for co-infections? Int J STD AIDS 2002;13:821-825
2. Creighton S, Tenant-Flowers M, Taylor CB, Miller R, Low N. Co-infection with gonorrhoea and chlamydia: how much is there and what does it mean? Int J STD AIDS 2003;14:109-113
3. Kahn RH, Mosure DJ, Blank S, et al for the jail STD Prevalence Monitoring Project. Chlamydia trachomatis and Neisseria gonorrhoeae Prevalence and Coinfection in adolescents entering selected US juvenile Detention Centers, 1997-2002. Sex Transm Dis 2005;32:255-259.
4. Gaydos CA, Kent CK, Rietmeijer CA, et al. Prevalence of Neisseria Gonorrheae among men screened for Chlamydia Trachomatis in four US cities, 1999-2003 Sex Transm D 2006;33:314-319.
5. KMiller WC, Ford CA, Morris M, et al. Prevalence of chlamydial and gonococcal infections among young adults in the united states. JAMA 2004;291:2229-2236
6. American Academy of Family Physicians (AAFP). Summary of the recommendations for clinical preventive services. Revision 6.0. Leawood, Kan: AAFP; August 2005.
7. Workowski KA, Berman SM. Centers for Disease Control and Prevention. Sexually transmitted diseases treatment guidelines, 2006. MMWR Recomm Rep 2006;55(RR-11):1-94.
8. Branson BM, Handsfield HH, Lampe MA, et al. Revised recommendations for HIV testing of adults, adolescents, and pregnant women in health-care settings. MMWR Recomm Rep 2006;55(RR-14):1-17.
9. Institute for Clinical systems Improvement Preventive services for adults. Bloomington, Minn: Institute for Clinical systems Improvement; 2004. Available online at www guideline.gov. Accessed on December 13, 2006.
1. Harindra V, Tobin JM, Underhill G. Opportunistic chlamydia screening; should positive patients be screened for co-infections? Int J STD AIDS 2002;13:821-825
2. Creighton S, Tenant-Flowers M, Taylor CB, Miller R, Low N. Co-infection with gonorrhoea and chlamydia: how much is there and what does it mean? Int J STD AIDS 2003;14:109-113
3. Kahn RH, Mosure DJ, Blank S, et al for the jail STD Prevalence Monitoring Project. Chlamydia trachomatis and Neisseria gonorrhoeae Prevalence and Coinfection in adolescents entering selected US juvenile Detention Centers, 1997-2002. Sex Transm Dis 2005;32:255-259.
4. Gaydos CA, Kent CK, Rietmeijer CA, et al. Prevalence of Neisseria Gonorrheae among men screened for Chlamydia Trachomatis in four US cities, 1999-2003 Sex Transm D 2006;33:314-319.
5. KMiller WC, Ford CA, Morris M, et al. Prevalence of chlamydial and gonococcal infections among young adults in the united states. JAMA 2004;291:2229-2236
6. American Academy of Family Physicians (AAFP). Summary of the recommendations for clinical preventive services. Revision 6.0. Leawood, Kan: AAFP; August 2005.
7. Workowski KA, Berman SM. Centers for Disease Control and Prevention. Sexually transmitted diseases treatment guidelines, 2006. MMWR Recomm Rep 2006;55(RR-11):1-94.
8. Branson BM, Handsfield HH, Lampe MA, et al. Revised recommendations for HIV testing of adults, adolescents, and pregnant women in health-care settings. MMWR Recomm Rep 2006;55(RR-14):1-17.
9. Institute for Clinical systems Improvement Preventive services for adults. Bloomington, Minn: Institute for Clinical systems Improvement; 2004. Available online at www guideline.gov. Accessed on December 13, 2006.
Evidence-based answers from the Family Physicians Inquiries Network
What is the role of combination therapy (insulin plus oral medication) in type 2 diabetes?
Combination therapy using insulin plus metformin (Glucophage), a sulfonylurea, or both produces glycemic control comparable with using insulin alone, but there is less weight gain when metformin is used (strength of recommendation [SOR]: B, based on systematic review of randomized controlled trials [RCTs] with some heterogeneity). Combination therapy using insulin and pioglitazone (Actos) reduces glycosylated hemoglobin (HbA1c) more than either insulin alone or adding pioglitazone to a sulfonylurea, but results in more weight gain (SOR: A, based on RCT). Using insulin glargine (Lantus) in combination therapy produces fewer nocturnal hypoglycemic events than using neutral protamine Hagedorn (NPH) insulin, while producing equivalent HbA1c reduction (SOR: B, based on RCT).
When the HbA1c is high (above 9.0% to 9.5%) on 1 or 2 oral agents, beginning combination therapy is more effective than adding another oral agent (SOR: B, based on subpopulation analysis in RCTs).
Educate patients from the time of diagnosis that insulin is not a failure
Vincent Lo, MD
San Joaquin General Hospital, French Camp, Calif
Combination therapy for patients with type 2 diabetes is a safe and effective stepping stone between oral therapy and insulin therapy. Unfortunately, significant barriers remain to getting insulin started when oral agents alone are insufficient. Patients often do not understand the common need for insulin therapy as type 2 diabetes advances, and some physicians continue to use the threat of insulin as a punitive incentive to promote patient compliance. It is little wonder that many patients perceive a physician’s eventual recommendation for insulin therapy as a personal failure. Patients are also concerned about the discomfort, inconvenience, and risk of insulin injections. Physicians should focus on educating their patients from the time of diagnosis that insulin is not a failure, but just another tool that will help them achieve their blood sugar goals.
Evidence summary
A systematic review evaluated beginning combination therapy (adding insulin to oral medication) compared with switching to insulin alone in patients with type 2 diabetes mellitus with inadequate glycemic control on oral medication.1 Twenty RCTs studied a total of 1811 patients; glycemic control was the primary outcome measure. Oral medication comprised either sulfonylureas (75%), metformin (4%), or both (21%). Individual studies used different insulin dosing schedules and statistical measures. However, overall, combination therapy provided glucose control comparable with insulin alone. In only 1 small, low-quality study did insulin plus metformin reduce HbA1c more than other combination therapy regimens or insulin alone. Ten studies reported a trend toward less weight gain with combination therapy that included metformin. Fourteen studies found the same incidence of hypoglycemic episodes in combination therapy and insulin alone.
Three later RCTs of overweight patients with inadequate control on oral agents (HbA1c >7% on a sulfonylurea, metformin, or both) also compared beginning combination therapy with switching to insulin alone (with 70/30 or NPH insulin twice daily). In one study with 64 patients followed for 12 months, HbA1c fell by 0.14% less (nonsignificant) in the combination therapy group (bedtime NPH plus sulfonylurea and metformin) than in the insulin alone group (70/30 twice daily).2 The combination therapy group gained significantly less weight than the insulin-alone group (1.3 kg vs 4.2 kg; P=.01).
In the second study of 261 patients, the combination therapy group (glimepiride [Amaryl] plus bedtime NPH) had a significantly higher HbA1c after 9 months than 2 groups using insulin alone (twice daily 70/30, and twice daily NPH insulin) (8.9% vs 8.3% and 8.4%).3 Mean weight gain was similar in all 3 groups but only a minority of patients reached a target HbA1c of 6.5%. In the final study of only 16 patients, HbA1c after 6 months improved significantly and equally in both groups (baseline: 8.3%, combination therapy final: 6.8%; insulin alone final: 7.0%). However, the combination therapy group gained significantly less weight.4
An open-label RCT with 341 patients who were inadequately controlled on metformin compared beginning combination therapy (biphasic insulin aspart 30/70 [Novolog Mix 70/30] and metformin) with switching to insulin alone (biphasic insulin aspart 30/70).5 A third group added a second oral medication (sulfonylurea and metformin). After 16 weeks, patients taking combination therapy had a significantly lower HbA1c than those on insulin alone (treatment difference 0.39%, P=.007).
Overall, combination therapy and 2 oral medications reduced HbA1c by the same amount, but combination therapy reduced HbA1c more in a subpopulation of patients with HbA1c >9.0% at baseline (treatment difference 0.46%, P=.027). The group on insulin alone weighed significantly more (4.6 kg, P<.001) at the end of the trial than the group taking 2 oral medications.
An open-label RCT of 756 patients with inadequate glycemic control (HbA1c >7.5%, mean 8.6%) on either 1 or 2 oral agents (70% taking both metformin and a sulfonylurea) compared combination therapy using bedtime insulin glargine with combination therapy using morning NPH.6 Each group titrated insulin doses to achieve a target fasting glucose ≤100. By 24 weeks, both groups had equivalently reduced HbA1c (mean HbA1c=6.96% with glargine, and 6.97% with NPH; P=NS), but fewer patients experienced nocturnal hypoglycemia with glargine than with NPH (33.2% vs 26.7%, P<.05).
Another open-label RCT evaluated 281 patients with at least 3 months of inadequate glycemic control (HbA1c=7.4%–14.7%) on a sulfonylurea.7 Patients were randomized to a) switching to a combination of biphasic insulin aspart 30/70 plus pioglitazone, b) adding pioglitazone to the sulfonylurea, or c) switching to insulin alone (biphasic insulin aspart 30/70). After 18 weeks, insulin plus pioglitazone reduced HbA1c significantly more than either glyburide plus pioglitazone (P=.005) or insulin alone (P=.005). However, the insulin plus pioglitazone group had the most weight gain (mean 4 kg, similar to other pioglitazone trials). There were no major hypoglycemic events.
Another open-label RCT evaluated 217 patients inadequately controlled (HbA1c=7.5%–11%) on a 2-drug oral regimen (metformin and a sulfonylurea, each drug dosed at ≥50% of the recommended maximum), randomized to add either insulin glargine or rosiglitazone (Avandia).8 Both groups reduced HbA1c equivalently after 24 weeks (–1.7% for glargine vs –1.5% for rosiglitazone). However, in patients with a baseline HbA1c >9.5%, adding insulin glargine reduced HbA1c significantly more than rosiglitazone.
Recommendations from others
A comparative analysis of guidelines on diabetes from 13 different countries (including the US) found general agreement in the recommendation to add a second oral agent to maximum doses of an initial agent in patients with poor glycemic control.9 However, no consensus was reached on the value or indications of combination therapy with oral agents and insulin.
The European Diabetes Policy Group recommends adding a second oral agent when the maximum dose of a single agent is reached, and using triple therapy when targets are not reached on maximum tolerated doses of 2 agents. Continued therapy with oral agents is advised when initiating insulin.10
1. Goudswaard AN, Furlong NJ, Rutten GE, Stolk RP, Valk GD. Insulin monotherapy versus combinations of insulin with oral hypoglycaemic agents in patients with type 2 diabetes mellitus. ochrane Database Syst Rev 2004;(4):CD003418.-
2. Goudswaard AN, Stolk RP, Zuithoff P, de Valk HW, Rutten GE. Starting insulin in type 2 diabetes: continue oral hypoglycemic agents? A randomized trial in primary care. J Fam Pract 2004;53:393-399.
3. Stehouwer MHA, DeVries JH, Lumeij JA, et al. Combined bedtime insulin-daytime sulphonylurea regimen compared with two different daily insulin regimens in type 2 diabetes: effects on HbA1c and hypoglycemia rate-a randomized trial . Diabetes Metab Res Rev 2003;19:148-152.
4. Olsson PO, Lindstrom T. Combination-therapy with bedtime NPH insulin and sulphonylureas gives similar glycaemic control but lower weight gain than insulin twice daily in patients with type 2 diabetes. Diabetes Metab 2002;28(4 Pt 1):272-277.
5. Kvapil M, Swatko A, Hilberg C, Shestakova M. Biphasic insulin aspart 30 plus metformin: an effective combination in type 2 diabetes. Diabetes Obes Metab 2006;8:39-48.
6. Riddle MC, Rosenstock J, Gerich J. The Insulin Glargine 4002 Study Investigators. The treat-to-target trial: randomized addition of glargine or human NPH insulin to oral therapy of type 2 diabetic patients. Diabetes Care 2003;26:3080-3086.
7. Raz I, Stranks S, Filipczak R, et al. Efficacy and safety of biphasic insulin aspart 30 combined with pioglitazone in type 2 diabetes poorly controlled on glibenclamide (glyburide) monotherapy or combination therapy: an 18 week, randomized, open-label study. Clin Ther 2005;27:1432-1443.
8. Rosenstock J, Sugimoto D, Strange P, Stewart JA, SoltesRak E, Dailey G. Triple therapy in type 2 diabetes: insulin glargine or rosiglitazone added to combination therapy of sulfonylurea plus metformin in insulin-naïve patients. Diabetes Care 2006;29:554-559.
9. Burgers JS, Grol R, Klazinga NS, et al. Inside guidelines: comparative analysis of recommendations and evidence in diabetes guidelines from 13 countries. Diabetes Care 2002;25:1933-1939.
10. European Diabetes Policy Group 1999. A desktop guide to Type 2 diabetes mellitus. Diabet Med 1999;16:716-730.
Combination therapy using insulin plus metformin (Glucophage), a sulfonylurea, or both produces glycemic control comparable with using insulin alone, but there is less weight gain when metformin is used (strength of recommendation [SOR]: B, based on systematic review of randomized controlled trials [RCTs] with some heterogeneity). Combination therapy using insulin and pioglitazone (Actos) reduces glycosylated hemoglobin (HbA1c) more than either insulin alone or adding pioglitazone to a sulfonylurea, but results in more weight gain (SOR: A, based on RCT). Using insulin glargine (Lantus) in combination therapy produces fewer nocturnal hypoglycemic events than using neutral protamine Hagedorn (NPH) insulin, while producing equivalent HbA1c reduction (SOR: B, based on RCT).
When the HbA1c is high (above 9.0% to 9.5%) on 1 or 2 oral agents, beginning combination therapy is more effective than adding another oral agent (SOR: B, based on subpopulation analysis in RCTs).
Educate patients from the time of diagnosis that insulin is not a failure
Vincent Lo, MD
San Joaquin General Hospital, French Camp, Calif
Combination therapy for patients with type 2 diabetes is a safe and effective stepping stone between oral therapy and insulin therapy. Unfortunately, significant barriers remain to getting insulin started when oral agents alone are insufficient. Patients often do not understand the common need for insulin therapy as type 2 diabetes advances, and some physicians continue to use the threat of insulin as a punitive incentive to promote patient compliance. It is little wonder that many patients perceive a physician’s eventual recommendation for insulin therapy as a personal failure. Patients are also concerned about the discomfort, inconvenience, and risk of insulin injections. Physicians should focus on educating their patients from the time of diagnosis that insulin is not a failure, but just another tool that will help them achieve their blood sugar goals.
Evidence summary
A systematic review evaluated beginning combination therapy (adding insulin to oral medication) compared with switching to insulin alone in patients with type 2 diabetes mellitus with inadequate glycemic control on oral medication.1 Twenty RCTs studied a total of 1811 patients; glycemic control was the primary outcome measure. Oral medication comprised either sulfonylureas (75%), metformin (4%), or both (21%). Individual studies used different insulin dosing schedules and statistical measures. However, overall, combination therapy provided glucose control comparable with insulin alone. In only 1 small, low-quality study did insulin plus metformin reduce HbA1c more than other combination therapy regimens or insulin alone. Ten studies reported a trend toward less weight gain with combination therapy that included metformin. Fourteen studies found the same incidence of hypoglycemic episodes in combination therapy and insulin alone.
Three later RCTs of overweight patients with inadequate control on oral agents (HbA1c >7% on a sulfonylurea, metformin, or both) also compared beginning combination therapy with switching to insulin alone (with 70/30 or NPH insulin twice daily). In one study with 64 patients followed for 12 months, HbA1c fell by 0.14% less (nonsignificant) in the combination therapy group (bedtime NPH plus sulfonylurea and metformin) than in the insulin alone group (70/30 twice daily).2 The combination therapy group gained significantly less weight than the insulin-alone group (1.3 kg vs 4.2 kg; P=.01).
In the second study of 261 patients, the combination therapy group (glimepiride [Amaryl] plus bedtime NPH) had a significantly higher HbA1c after 9 months than 2 groups using insulin alone (twice daily 70/30, and twice daily NPH insulin) (8.9% vs 8.3% and 8.4%).3 Mean weight gain was similar in all 3 groups but only a minority of patients reached a target HbA1c of 6.5%. In the final study of only 16 patients, HbA1c after 6 months improved significantly and equally in both groups (baseline: 8.3%, combination therapy final: 6.8%; insulin alone final: 7.0%). However, the combination therapy group gained significantly less weight.4
An open-label RCT with 341 patients who were inadequately controlled on metformin compared beginning combination therapy (biphasic insulin aspart 30/70 [Novolog Mix 70/30] and metformin) with switching to insulin alone (biphasic insulin aspart 30/70).5 A third group added a second oral medication (sulfonylurea and metformin). After 16 weeks, patients taking combination therapy had a significantly lower HbA1c than those on insulin alone (treatment difference 0.39%, P=.007).
Overall, combination therapy and 2 oral medications reduced HbA1c by the same amount, but combination therapy reduced HbA1c more in a subpopulation of patients with HbA1c >9.0% at baseline (treatment difference 0.46%, P=.027). The group on insulin alone weighed significantly more (4.6 kg, P<.001) at the end of the trial than the group taking 2 oral medications.
An open-label RCT of 756 patients with inadequate glycemic control (HbA1c >7.5%, mean 8.6%) on either 1 or 2 oral agents (70% taking both metformin and a sulfonylurea) compared combination therapy using bedtime insulin glargine with combination therapy using morning NPH.6 Each group titrated insulin doses to achieve a target fasting glucose ≤100. By 24 weeks, both groups had equivalently reduced HbA1c (mean HbA1c=6.96% with glargine, and 6.97% with NPH; P=NS), but fewer patients experienced nocturnal hypoglycemia with glargine than with NPH (33.2% vs 26.7%, P<.05).
Another open-label RCT evaluated 281 patients with at least 3 months of inadequate glycemic control (HbA1c=7.4%–14.7%) on a sulfonylurea.7 Patients were randomized to a) switching to a combination of biphasic insulin aspart 30/70 plus pioglitazone, b) adding pioglitazone to the sulfonylurea, or c) switching to insulin alone (biphasic insulin aspart 30/70). After 18 weeks, insulin plus pioglitazone reduced HbA1c significantly more than either glyburide plus pioglitazone (P=.005) or insulin alone (P=.005). However, the insulin plus pioglitazone group had the most weight gain (mean 4 kg, similar to other pioglitazone trials). There were no major hypoglycemic events.
Another open-label RCT evaluated 217 patients inadequately controlled (HbA1c=7.5%–11%) on a 2-drug oral regimen (metformin and a sulfonylurea, each drug dosed at ≥50% of the recommended maximum), randomized to add either insulin glargine or rosiglitazone (Avandia).8 Both groups reduced HbA1c equivalently after 24 weeks (–1.7% for glargine vs –1.5% for rosiglitazone). However, in patients with a baseline HbA1c >9.5%, adding insulin glargine reduced HbA1c significantly more than rosiglitazone.
Recommendations from others
A comparative analysis of guidelines on diabetes from 13 different countries (including the US) found general agreement in the recommendation to add a second oral agent to maximum doses of an initial agent in patients with poor glycemic control.9 However, no consensus was reached on the value or indications of combination therapy with oral agents and insulin.
The European Diabetes Policy Group recommends adding a second oral agent when the maximum dose of a single agent is reached, and using triple therapy when targets are not reached on maximum tolerated doses of 2 agents. Continued therapy with oral agents is advised when initiating insulin.10
Combination therapy using insulin plus metformin (Glucophage), a sulfonylurea, or both produces glycemic control comparable with using insulin alone, but there is less weight gain when metformin is used (strength of recommendation [SOR]: B, based on systematic review of randomized controlled trials [RCTs] with some heterogeneity). Combination therapy using insulin and pioglitazone (Actos) reduces glycosylated hemoglobin (HbA1c) more than either insulin alone or adding pioglitazone to a sulfonylurea, but results in more weight gain (SOR: A, based on RCT). Using insulin glargine (Lantus) in combination therapy produces fewer nocturnal hypoglycemic events than using neutral protamine Hagedorn (NPH) insulin, while producing equivalent HbA1c reduction (SOR: B, based on RCT).
When the HbA1c is high (above 9.0% to 9.5%) on 1 or 2 oral agents, beginning combination therapy is more effective than adding another oral agent (SOR: B, based on subpopulation analysis in RCTs).
Educate patients from the time of diagnosis that insulin is not a failure
Vincent Lo, MD
San Joaquin General Hospital, French Camp, Calif
Combination therapy for patients with type 2 diabetes is a safe and effective stepping stone between oral therapy and insulin therapy. Unfortunately, significant barriers remain to getting insulin started when oral agents alone are insufficient. Patients often do not understand the common need for insulin therapy as type 2 diabetes advances, and some physicians continue to use the threat of insulin as a punitive incentive to promote patient compliance. It is little wonder that many patients perceive a physician’s eventual recommendation for insulin therapy as a personal failure. Patients are also concerned about the discomfort, inconvenience, and risk of insulin injections. Physicians should focus on educating their patients from the time of diagnosis that insulin is not a failure, but just another tool that will help them achieve their blood sugar goals.
Evidence summary
A systematic review evaluated beginning combination therapy (adding insulin to oral medication) compared with switching to insulin alone in patients with type 2 diabetes mellitus with inadequate glycemic control on oral medication.1 Twenty RCTs studied a total of 1811 patients; glycemic control was the primary outcome measure. Oral medication comprised either sulfonylureas (75%), metformin (4%), or both (21%). Individual studies used different insulin dosing schedules and statistical measures. However, overall, combination therapy provided glucose control comparable with insulin alone. In only 1 small, low-quality study did insulin plus metformin reduce HbA1c more than other combination therapy regimens or insulin alone. Ten studies reported a trend toward less weight gain with combination therapy that included metformin. Fourteen studies found the same incidence of hypoglycemic episodes in combination therapy and insulin alone.
Three later RCTs of overweight patients with inadequate control on oral agents (HbA1c >7% on a sulfonylurea, metformin, or both) also compared beginning combination therapy with switching to insulin alone (with 70/30 or NPH insulin twice daily). In one study with 64 patients followed for 12 months, HbA1c fell by 0.14% less (nonsignificant) in the combination therapy group (bedtime NPH plus sulfonylurea and metformin) than in the insulin alone group (70/30 twice daily).2 The combination therapy group gained significantly less weight than the insulin-alone group (1.3 kg vs 4.2 kg; P=.01).
In the second study of 261 patients, the combination therapy group (glimepiride [Amaryl] plus bedtime NPH) had a significantly higher HbA1c after 9 months than 2 groups using insulin alone (twice daily 70/30, and twice daily NPH insulin) (8.9% vs 8.3% and 8.4%).3 Mean weight gain was similar in all 3 groups but only a minority of patients reached a target HbA1c of 6.5%. In the final study of only 16 patients, HbA1c after 6 months improved significantly and equally in both groups (baseline: 8.3%, combination therapy final: 6.8%; insulin alone final: 7.0%). However, the combination therapy group gained significantly less weight.4
An open-label RCT with 341 patients who were inadequately controlled on metformin compared beginning combination therapy (biphasic insulin aspart 30/70 [Novolog Mix 70/30] and metformin) with switching to insulin alone (biphasic insulin aspart 30/70).5 A third group added a second oral medication (sulfonylurea and metformin). After 16 weeks, patients taking combination therapy had a significantly lower HbA1c than those on insulin alone (treatment difference 0.39%, P=.007).
Overall, combination therapy and 2 oral medications reduced HbA1c by the same amount, but combination therapy reduced HbA1c more in a subpopulation of patients with HbA1c >9.0% at baseline (treatment difference 0.46%, P=.027). The group on insulin alone weighed significantly more (4.6 kg, P<.001) at the end of the trial than the group taking 2 oral medications.
An open-label RCT of 756 patients with inadequate glycemic control (HbA1c >7.5%, mean 8.6%) on either 1 or 2 oral agents (70% taking both metformin and a sulfonylurea) compared combination therapy using bedtime insulin glargine with combination therapy using morning NPH.6 Each group titrated insulin doses to achieve a target fasting glucose ≤100. By 24 weeks, both groups had equivalently reduced HbA1c (mean HbA1c=6.96% with glargine, and 6.97% with NPH; P=NS), but fewer patients experienced nocturnal hypoglycemia with glargine than with NPH (33.2% vs 26.7%, P<.05).
Another open-label RCT evaluated 281 patients with at least 3 months of inadequate glycemic control (HbA1c=7.4%–14.7%) on a sulfonylurea.7 Patients were randomized to a) switching to a combination of biphasic insulin aspart 30/70 plus pioglitazone, b) adding pioglitazone to the sulfonylurea, or c) switching to insulin alone (biphasic insulin aspart 30/70). After 18 weeks, insulin plus pioglitazone reduced HbA1c significantly more than either glyburide plus pioglitazone (P=.005) or insulin alone (P=.005). However, the insulin plus pioglitazone group had the most weight gain (mean 4 kg, similar to other pioglitazone trials). There were no major hypoglycemic events.
Another open-label RCT evaluated 217 patients inadequately controlled (HbA1c=7.5%–11%) on a 2-drug oral regimen (metformin and a sulfonylurea, each drug dosed at ≥50% of the recommended maximum), randomized to add either insulin glargine or rosiglitazone (Avandia).8 Both groups reduced HbA1c equivalently after 24 weeks (–1.7% for glargine vs –1.5% for rosiglitazone). However, in patients with a baseline HbA1c >9.5%, adding insulin glargine reduced HbA1c significantly more than rosiglitazone.
Recommendations from others
A comparative analysis of guidelines on diabetes from 13 different countries (including the US) found general agreement in the recommendation to add a second oral agent to maximum doses of an initial agent in patients with poor glycemic control.9 However, no consensus was reached on the value or indications of combination therapy with oral agents and insulin.
The European Diabetes Policy Group recommends adding a second oral agent when the maximum dose of a single agent is reached, and using triple therapy when targets are not reached on maximum tolerated doses of 2 agents. Continued therapy with oral agents is advised when initiating insulin.10
1. Goudswaard AN, Furlong NJ, Rutten GE, Stolk RP, Valk GD. Insulin monotherapy versus combinations of insulin with oral hypoglycaemic agents in patients with type 2 diabetes mellitus. ochrane Database Syst Rev 2004;(4):CD003418.-
2. Goudswaard AN, Stolk RP, Zuithoff P, de Valk HW, Rutten GE. Starting insulin in type 2 diabetes: continue oral hypoglycemic agents? A randomized trial in primary care. J Fam Pract 2004;53:393-399.
3. Stehouwer MHA, DeVries JH, Lumeij JA, et al. Combined bedtime insulin-daytime sulphonylurea regimen compared with two different daily insulin regimens in type 2 diabetes: effects on HbA1c and hypoglycemia rate-a randomized trial . Diabetes Metab Res Rev 2003;19:148-152.
4. Olsson PO, Lindstrom T. Combination-therapy with bedtime NPH insulin and sulphonylureas gives similar glycaemic control but lower weight gain than insulin twice daily in patients with type 2 diabetes. Diabetes Metab 2002;28(4 Pt 1):272-277.
5. Kvapil M, Swatko A, Hilberg C, Shestakova M. Biphasic insulin aspart 30 plus metformin: an effective combination in type 2 diabetes. Diabetes Obes Metab 2006;8:39-48.
6. Riddle MC, Rosenstock J, Gerich J. The Insulin Glargine 4002 Study Investigators. The treat-to-target trial: randomized addition of glargine or human NPH insulin to oral therapy of type 2 diabetic patients. Diabetes Care 2003;26:3080-3086.
7. Raz I, Stranks S, Filipczak R, et al. Efficacy and safety of biphasic insulin aspart 30 combined with pioglitazone in type 2 diabetes poorly controlled on glibenclamide (glyburide) monotherapy or combination therapy: an 18 week, randomized, open-label study. Clin Ther 2005;27:1432-1443.
8. Rosenstock J, Sugimoto D, Strange P, Stewart JA, SoltesRak E, Dailey G. Triple therapy in type 2 diabetes: insulin glargine or rosiglitazone added to combination therapy of sulfonylurea plus metformin in insulin-naïve patients. Diabetes Care 2006;29:554-559.
9. Burgers JS, Grol R, Klazinga NS, et al. Inside guidelines: comparative analysis of recommendations and evidence in diabetes guidelines from 13 countries. Diabetes Care 2002;25:1933-1939.
10. European Diabetes Policy Group 1999. A desktop guide to Type 2 diabetes mellitus. Diabet Med 1999;16:716-730.
1. Goudswaard AN, Furlong NJ, Rutten GE, Stolk RP, Valk GD. Insulin monotherapy versus combinations of insulin with oral hypoglycaemic agents in patients with type 2 diabetes mellitus. ochrane Database Syst Rev 2004;(4):CD003418.-
2. Goudswaard AN, Stolk RP, Zuithoff P, de Valk HW, Rutten GE. Starting insulin in type 2 diabetes: continue oral hypoglycemic agents? A randomized trial in primary care. J Fam Pract 2004;53:393-399.
3. Stehouwer MHA, DeVries JH, Lumeij JA, et al. Combined bedtime insulin-daytime sulphonylurea regimen compared with two different daily insulin regimens in type 2 diabetes: effects on HbA1c and hypoglycemia rate-a randomized trial . Diabetes Metab Res Rev 2003;19:148-152.
4. Olsson PO, Lindstrom T. Combination-therapy with bedtime NPH insulin and sulphonylureas gives similar glycaemic control but lower weight gain than insulin twice daily in patients with type 2 diabetes. Diabetes Metab 2002;28(4 Pt 1):272-277.
5. Kvapil M, Swatko A, Hilberg C, Shestakova M. Biphasic insulin aspart 30 plus metformin: an effective combination in type 2 diabetes. Diabetes Obes Metab 2006;8:39-48.
6. Riddle MC, Rosenstock J, Gerich J. The Insulin Glargine 4002 Study Investigators. The treat-to-target trial: randomized addition of glargine or human NPH insulin to oral therapy of type 2 diabetic patients. Diabetes Care 2003;26:3080-3086.
7. Raz I, Stranks S, Filipczak R, et al. Efficacy and safety of biphasic insulin aspart 30 combined with pioglitazone in type 2 diabetes poorly controlled on glibenclamide (glyburide) monotherapy or combination therapy: an 18 week, randomized, open-label study. Clin Ther 2005;27:1432-1443.
8. Rosenstock J, Sugimoto D, Strange P, Stewart JA, SoltesRak E, Dailey G. Triple therapy in type 2 diabetes: insulin glargine or rosiglitazone added to combination therapy of sulfonylurea plus metformin in insulin-naïve patients. Diabetes Care 2006;29:554-559.
9. Burgers JS, Grol R, Klazinga NS, et al. Inside guidelines: comparative analysis of recommendations and evidence in diabetes guidelines from 13 countries. Diabetes Care 2002;25:1933-1939.
10. European Diabetes Policy Group 1999. A desktop guide to Type 2 diabetes mellitus. Diabet Med 1999;16:716-730.
Evidence-based answers from the Family Physicians Inquiries Network
Should patients receive 23-valent pneumococcal vaccination more than once?
No patient-oriented evidence supports pneumococcal revaccination of any patient (high-risk or otherwise). Antibody levels may be augmented by revaccination; however, the clinical efficacy of revaccination, even among high-risk patients, is unknown. Revaccination is recommended by the Advisory Committee on Immunization Practices (ACIP) in certain circumstances. (strength of recommendation [SOR]: C, expert opinion based on physiology/bench research). Revaccination once appears to be safe, especially if provided 5 years or more after primary vaccination (SOR: B, based upon consistent results of cohort studies and nonrandomized prospective trials).
Until research proves otherwise, keep revaccinating high-risk patients
Richard Kim, MD
Glendale, Ariz
It is unfortunate that we lack any clinical data for or against pneumococcal revaccination. As a result, we may disagree on the correct revaccination schedule. Several questions arise from this uncertainty. First, do increasing antibody levels increase or prolong immunity? Second, how do we weigh the estimated numbers needed to treat (NNT) versus the numbers needed to harm (NNH)? And third, considering the prevalence and severity of the disease, should we err on the side of immunizing more rather than less? Since booster shots seem to be safe (implying a high NNH), the ACIP guideline is probably a good compromise between increasing antibody titers (theoretical benefit), and lack of efficacy data. Until we have further information to the contrary, I’m going to continue to revaccinate high-risk patients.
Evidence summary
Streptococcus pneumoniae is the most common cause of community-acquired pneumonia and the second most common cause of bacterial meningitis in the United States.1 An estimated 40,000 people die annually in the US from pneumococcal infections.1 Even with antibiotic treatment and intensive care unit support, the mortality of patients with pneumococcal bacteremia approaches 25% to 30%.1
Because of the importance of this pathogen, it has been the focus of several trials to demonstrate the efficacy of the primary vaccination. To date, 7 meta-analyses have been completed to assess the efficacy of pneumococcal vaccine in adults, with varying results.2-7 Most recently, a Cochrane Review (updated in 2005)8 concluded that “the combined results from randomized studies fail to show that the polysaccharide vaccine is effective in preventing either pneumonia or death.” However, they did recognize that the nonrandomized studies have consistently shown that the polysaccharide vaccine is effective in reducing the more specific outcome of invasive pneumococcal disease (bacteremia and meningitis).
Multiple studies have used measurement of antibody levels to assess the response of patients to the vaccine and for justification of the need for revaccination. However, measurement of antibody levels to pneumococcal serotypes is difficult, inexact, and is only a surrogate marker for the immune status of a patient, which also relies on the overall function of their immune system. Although pneumococcal vaccine is most highly recommended in patients with chronic disease or immunodeficiency, these patients have a poorer initial response rate and a faster decline in antibody levels than younger, immunocompetent recipients of the vaccine.1,9
A study of pneumococcal strains cultured from hospitalized patients demonstrated a duration of protection against pneumococcal infection that was much longer than that predicted by the shorter duration of antibody levels. The vaccine’s ability to reduce infection (due to serotypes included in the vaccine) lasted for at least 9 years and overall efficacy for preventing infection caused by the serotypes included in the vaccine was 57%.1
Revaccination is safe; particularly when performed more than 5 years after the initial vaccination. Injection site reactions are more common and more severe in revaccinated persons (rising from 3% to 15% in the immunocompetent patient).10 Revaccination, however, does not result in increased rates of hospitalization, and few severe reactions have been reported.11
No randomized or prospective trials regarding the clinical efficacy of revaccination have been completed. However, when reviewing the studies of antibody response, several summary conclusions can be made. Among those who were nonresponders to the initial vaccination, revaccination (even repeated revaccination) is not effective in stimulating any significant antibody response.12-14 Among those who responded to the primary vaccination, revaccination can stimulate a second antibody response—albeit to lower levels and with less duration than after the initial vaccination.13,15-17 Among those who do respond to revaccination, antibody levels can rapidly decline to undetectable levels in a matter of months, and they may or may not retain protection against disease over time.14,15 It appears that revaccination recommendations have been based on the safety of the vaccination, concern for patients at risk and reduced antibody levels, rather than on proven clinical utility.
Recommendations from others
The Advisory Committee on Immunization Practices (ACIP) advises that the vaccine be used in “persons with diseases and other conditions predisposing to the development of bacteremic pneumococcal pneumonia” and that “revaccination should not be done at intervals less than five years.” They state "the value of vaccination on the basis of advanced age is not clear at this time.” (Further details may be found at: www.aafp.org/PreBuilt/agecharts_adultimmunization.pdf.)
The American Academy of Family Physicians, American College of Physicians, the American College of Obstetricians and Gynecologists and the American Diabetes Association follow the ACIP recommendations.
1. Butler JC, Breiman RF, Campbell JF, Lipman HB, Broome CV, Facklam RR. Pneumococcal polysaccharide vaccine efficacy: An Evaluation of current recommendations. JAMA 1993;270:1826-1831.
2. Fine MJ, Smith MA, Carson CA, et al. Efficacy of pneumococcal vaccination in adults: A meta-analysis of randomized controlled trials. Arch Intern Med 1994;154:2666-2677.
3. Hutchinson BG, Oxman AD, Shannon HS, Lloyd S, Altmayer CA, Thomas K. Clinical effectiveness of pneumococcal vaccine. Meta analysis. Can Fam Physician 1999;45:2381-2393.
4. Cornu C, Yzebe D, Leophonte P, Gaillat J, Boissel JP, Cucherat M. Efficacy of pneumococcal polysaccharide vaccine in immunocompetent adults: a meta-analysis of randomized trials. Vaccine 2001;19:4780-4790.
5. Moore RA, Wiffen PJ, Lipsky BA. Are the pneumococcal polysaccharide vaccines effective? Meta-analysis of the prospective trials. BMC Fam Pract 2000;1:1.-
6. Puig-Barbera J, Belenguer Varea A, Goterris Pinto M, Brines Benlliure MJ. Pneumococcal vaccine effectiveness in the elderly. Systematic review and meta-analysis. Aten Primaria 2002;30:269-281.
7. Watson I, Wilson BJ, Waugh N. Pneumococcal polysaccharide vaccine: a systematic review of clinical effectiveness in adults. Vaccine 2002;20:2166-2173.
8. Dear KB, Holden J, Andrews R, Tatham D. Vaccines for preventing pneumococcal infection in adults. Cochrane Database Syst Rev 2003;(4):CD000422.-
9. Whitney CG, Schaffner W, Butler JC. Rethinking recommendations for use of pneumococcal vaccines in adults. Clin Infect Dis 2001;33:662-675.
10. Jackson LA, Benson P, Snellar VP, et al. Safety of revaccination with pneumococcal polysaccharide vaccine. JAMA 1999;281:243-248.
11. Snow R, Babish JD, McBean AM. Is there any connection between a second pneumonia shot and hospitalization among Medicare beneficiaries? Public Health Rep 1995;110:720-725.
12. Petrasch S, Kuhnemund O, Reinacher A, et al. Antibody responses of splenectomized patients with non-Hodgkin’s lymphoma to immunization with polyvalent pneumococcal vaccines. Clin Diagn Lab Immunol 1997;4:635-638.
13. Rodriguez-Barradas MC, Groover JE, Lacke CE, et al. IgG antibody to pneumococcal capsular polysaccharide in human immunodeficiency virus-infected subjects: persistence of antibody in responders, revaccination in nonresponders, and relationship of immunoglobulin allotype to response. J Infect Dis 1996;173:1347-1353.
14. Cherif H, Landgren O, Konradsen HB, Kalin M, Bjorkholm M. Poor antibody response to pneumococcal polysaccharide vaccination suggests increased susceptibility to pneumococcal infection in splenectomized patients with hematological diseases. Vaccine. 2006;24(1):75-81.
15. Lackner TE, Hamilton RG, Hill JJ, Davey C, Guay DR. Pneumococcal polysaccharide revaccination: immunoglobulin g seroconversion, persistence, and safety in frail, chronically ill older subjects. J Am Geriatr Soc 2003;51:240-245.
16. Landgren O, Bjorkholm M, Konradsen HB, et al. A prospective study on antibody response to repeated vaccinations with pneumococcal capsular polysaccharide in splenectomized individuals with special reference to Hodgkin’s lymphoma. J Intern Med 2004;255:664-673.
17. Torling J, Hedlund J, Konradsen HB, Ortqvist A. Revaccination with the 23-valent pneumococcal polysaccharide vaccine in middle-aged and elderly persons previously treated for pneumonia. Vaccine 2003;22:96-103.
No patient-oriented evidence supports pneumococcal revaccination of any patient (high-risk or otherwise). Antibody levels may be augmented by revaccination; however, the clinical efficacy of revaccination, even among high-risk patients, is unknown. Revaccination is recommended by the Advisory Committee on Immunization Practices (ACIP) in certain circumstances. (strength of recommendation [SOR]: C, expert opinion based on physiology/bench research). Revaccination once appears to be safe, especially if provided 5 years or more after primary vaccination (SOR: B, based upon consistent results of cohort studies and nonrandomized prospective trials).
Until research proves otherwise, keep revaccinating high-risk patients
Richard Kim, MD
Glendale, Ariz
It is unfortunate that we lack any clinical data for or against pneumococcal revaccination. As a result, we may disagree on the correct revaccination schedule. Several questions arise from this uncertainty. First, do increasing antibody levels increase or prolong immunity? Second, how do we weigh the estimated numbers needed to treat (NNT) versus the numbers needed to harm (NNH)? And third, considering the prevalence and severity of the disease, should we err on the side of immunizing more rather than less? Since booster shots seem to be safe (implying a high NNH), the ACIP guideline is probably a good compromise between increasing antibody titers (theoretical benefit), and lack of efficacy data. Until we have further information to the contrary, I’m going to continue to revaccinate high-risk patients.
Evidence summary
Streptococcus pneumoniae is the most common cause of community-acquired pneumonia and the second most common cause of bacterial meningitis in the United States.1 An estimated 40,000 people die annually in the US from pneumococcal infections.1 Even with antibiotic treatment and intensive care unit support, the mortality of patients with pneumococcal bacteremia approaches 25% to 30%.1
Because of the importance of this pathogen, it has been the focus of several trials to demonstrate the efficacy of the primary vaccination. To date, 7 meta-analyses have been completed to assess the efficacy of pneumococcal vaccine in adults, with varying results.2-7 Most recently, a Cochrane Review (updated in 2005)8 concluded that “the combined results from randomized studies fail to show that the polysaccharide vaccine is effective in preventing either pneumonia or death.” However, they did recognize that the nonrandomized studies have consistently shown that the polysaccharide vaccine is effective in reducing the more specific outcome of invasive pneumococcal disease (bacteremia and meningitis).
Multiple studies have used measurement of antibody levels to assess the response of patients to the vaccine and for justification of the need for revaccination. However, measurement of antibody levels to pneumococcal serotypes is difficult, inexact, and is only a surrogate marker for the immune status of a patient, which also relies on the overall function of their immune system. Although pneumococcal vaccine is most highly recommended in patients with chronic disease or immunodeficiency, these patients have a poorer initial response rate and a faster decline in antibody levels than younger, immunocompetent recipients of the vaccine.1,9
A study of pneumococcal strains cultured from hospitalized patients demonstrated a duration of protection against pneumococcal infection that was much longer than that predicted by the shorter duration of antibody levels. The vaccine’s ability to reduce infection (due to serotypes included in the vaccine) lasted for at least 9 years and overall efficacy for preventing infection caused by the serotypes included in the vaccine was 57%.1
Revaccination is safe; particularly when performed more than 5 years after the initial vaccination. Injection site reactions are more common and more severe in revaccinated persons (rising from 3% to 15% in the immunocompetent patient).10 Revaccination, however, does not result in increased rates of hospitalization, and few severe reactions have been reported.11
No randomized or prospective trials regarding the clinical efficacy of revaccination have been completed. However, when reviewing the studies of antibody response, several summary conclusions can be made. Among those who were nonresponders to the initial vaccination, revaccination (even repeated revaccination) is not effective in stimulating any significant antibody response.12-14 Among those who responded to the primary vaccination, revaccination can stimulate a second antibody response—albeit to lower levels and with less duration than after the initial vaccination.13,15-17 Among those who do respond to revaccination, antibody levels can rapidly decline to undetectable levels in a matter of months, and they may or may not retain protection against disease over time.14,15 It appears that revaccination recommendations have been based on the safety of the vaccination, concern for patients at risk and reduced antibody levels, rather than on proven clinical utility.
Recommendations from others
The Advisory Committee on Immunization Practices (ACIP) advises that the vaccine be used in “persons with diseases and other conditions predisposing to the development of bacteremic pneumococcal pneumonia” and that “revaccination should not be done at intervals less than five years.” They state "the value of vaccination on the basis of advanced age is not clear at this time.” (Further details may be found at: www.aafp.org/PreBuilt/agecharts_adultimmunization.pdf.)
The American Academy of Family Physicians, American College of Physicians, the American College of Obstetricians and Gynecologists and the American Diabetes Association follow the ACIP recommendations.
No patient-oriented evidence supports pneumococcal revaccination of any patient (high-risk or otherwise). Antibody levels may be augmented by revaccination; however, the clinical efficacy of revaccination, even among high-risk patients, is unknown. Revaccination is recommended by the Advisory Committee on Immunization Practices (ACIP) in certain circumstances. (strength of recommendation [SOR]: C, expert opinion based on physiology/bench research). Revaccination once appears to be safe, especially if provided 5 years or more after primary vaccination (SOR: B, based upon consistent results of cohort studies and nonrandomized prospective trials).
Until research proves otherwise, keep revaccinating high-risk patients
Richard Kim, MD
Glendale, Ariz
It is unfortunate that we lack any clinical data for or against pneumococcal revaccination. As a result, we may disagree on the correct revaccination schedule. Several questions arise from this uncertainty. First, do increasing antibody levels increase or prolong immunity? Second, how do we weigh the estimated numbers needed to treat (NNT) versus the numbers needed to harm (NNH)? And third, considering the prevalence and severity of the disease, should we err on the side of immunizing more rather than less? Since booster shots seem to be safe (implying a high NNH), the ACIP guideline is probably a good compromise between increasing antibody titers (theoretical benefit), and lack of efficacy data. Until we have further information to the contrary, I’m going to continue to revaccinate high-risk patients.
Evidence summary
Streptococcus pneumoniae is the most common cause of community-acquired pneumonia and the second most common cause of bacterial meningitis in the United States.1 An estimated 40,000 people die annually in the US from pneumococcal infections.1 Even with antibiotic treatment and intensive care unit support, the mortality of patients with pneumococcal bacteremia approaches 25% to 30%.1
Because of the importance of this pathogen, it has been the focus of several trials to demonstrate the efficacy of the primary vaccination. To date, 7 meta-analyses have been completed to assess the efficacy of pneumococcal vaccine in adults, with varying results.2-7 Most recently, a Cochrane Review (updated in 2005)8 concluded that “the combined results from randomized studies fail to show that the polysaccharide vaccine is effective in preventing either pneumonia or death.” However, they did recognize that the nonrandomized studies have consistently shown that the polysaccharide vaccine is effective in reducing the more specific outcome of invasive pneumococcal disease (bacteremia and meningitis).
Multiple studies have used measurement of antibody levels to assess the response of patients to the vaccine and for justification of the need for revaccination. However, measurement of antibody levels to pneumococcal serotypes is difficult, inexact, and is only a surrogate marker for the immune status of a patient, which also relies on the overall function of their immune system. Although pneumococcal vaccine is most highly recommended in patients with chronic disease or immunodeficiency, these patients have a poorer initial response rate and a faster decline in antibody levels than younger, immunocompetent recipients of the vaccine.1,9
A study of pneumococcal strains cultured from hospitalized patients demonstrated a duration of protection against pneumococcal infection that was much longer than that predicted by the shorter duration of antibody levels. The vaccine’s ability to reduce infection (due to serotypes included in the vaccine) lasted for at least 9 years and overall efficacy for preventing infection caused by the serotypes included in the vaccine was 57%.1
Revaccination is safe; particularly when performed more than 5 years after the initial vaccination. Injection site reactions are more common and more severe in revaccinated persons (rising from 3% to 15% in the immunocompetent patient).10 Revaccination, however, does not result in increased rates of hospitalization, and few severe reactions have been reported.11
No randomized or prospective trials regarding the clinical efficacy of revaccination have been completed. However, when reviewing the studies of antibody response, several summary conclusions can be made. Among those who were nonresponders to the initial vaccination, revaccination (even repeated revaccination) is not effective in stimulating any significant antibody response.12-14 Among those who responded to the primary vaccination, revaccination can stimulate a second antibody response—albeit to lower levels and with less duration than after the initial vaccination.13,15-17 Among those who do respond to revaccination, antibody levels can rapidly decline to undetectable levels in a matter of months, and they may or may not retain protection against disease over time.14,15 It appears that revaccination recommendations have been based on the safety of the vaccination, concern for patients at risk and reduced antibody levels, rather than on proven clinical utility.
Recommendations from others
The Advisory Committee on Immunization Practices (ACIP) advises that the vaccine be used in “persons with diseases and other conditions predisposing to the development of bacteremic pneumococcal pneumonia” and that “revaccination should not be done at intervals less than five years.” They state "the value of vaccination on the basis of advanced age is not clear at this time.” (Further details may be found at: www.aafp.org/PreBuilt/agecharts_adultimmunization.pdf.)
The American Academy of Family Physicians, American College of Physicians, the American College of Obstetricians and Gynecologists and the American Diabetes Association follow the ACIP recommendations.
1. Butler JC, Breiman RF, Campbell JF, Lipman HB, Broome CV, Facklam RR. Pneumococcal polysaccharide vaccine efficacy: An Evaluation of current recommendations. JAMA 1993;270:1826-1831.
2. Fine MJ, Smith MA, Carson CA, et al. Efficacy of pneumococcal vaccination in adults: A meta-analysis of randomized controlled trials. Arch Intern Med 1994;154:2666-2677.
3. Hutchinson BG, Oxman AD, Shannon HS, Lloyd S, Altmayer CA, Thomas K. Clinical effectiveness of pneumococcal vaccine. Meta analysis. Can Fam Physician 1999;45:2381-2393.
4. Cornu C, Yzebe D, Leophonte P, Gaillat J, Boissel JP, Cucherat M. Efficacy of pneumococcal polysaccharide vaccine in immunocompetent adults: a meta-analysis of randomized trials. Vaccine 2001;19:4780-4790.
5. Moore RA, Wiffen PJ, Lipsky BA. Are the pneumococcal polysaccharide vaccines effective? Meta-analysis of the prospective trials. BMC Fam Pract 2000;1:1.-
6. Puig-Barbera J, Belenguer Varea A, Goterris Pinto M, Brines Benlliure MJ. Pneumococcal vaccine effectiveness in the elderly. Systematic review and meta-analysis. Aten Primaria 2002;30:269-281.
7. Watson I, Wilson BJ, Waugh N. Pneumococcal polysaccharide vaccine: a systematic review of clinical effectiveness in adults. Vaccine 2002;20:2166-2173.
8. Dear KB, Holden J, Andrews R, Tatham D. Vaccines for preventing pneumococcal infection in adults. Cochrane Database Syst Rev 2003;(4):CD000422.-
9. Whitney CG, Schaffner W, Butler JC. Rethinking recommendations for use of pneumococcal vaccines in adults. Clin Infect Dis 2001;33:662-675.
10. Jackson LA, Benson P, Snellar VP, et al. Safety of revaccination with pneumococcal polysaccharide vaccine. JAMA 1999;281:243-248.
11. Snow R, Babish JD, McBean AM. Is there any connection between a second pneumonia shot and hospitalization among Medicare beneficiaries? Public Health Rep 1995;110:720-725.
12. Petrasch S, Kuhnemund O, Reinacher A, et al. Antibody responses of splenectomized patients with non-Hodgkin’s lymphoma to immunization with polyvalent pneumococcal vaccines. Clin Diagn Lab Immunol 1997;4:635-638.
13. Rodriguez-Barradas MC, Groover JE, Lacke CE, et al. IgG antibody to pneumococcal capsular polysaccharide in human immunodeficiency virus-infected subjects: persistence of antibody in responders, revaccination in nonresponders, and relationship of immunoglobulin allotype to response. J Infect Dis 1996;173:1347-1353.
14. Cherif H, Landgren O, Konradsen HB, Kalin M, Bjorkholm M. Poor antibody response to pneumococcal polysaccharide vaccination suggests increased susceptibility to pneumococcal infection in splenectomized patients with hematological diseases. Vaccine. 2006;24(1):75-81.
15. Lackner TE, Hamilton RG, Hill JJ, Davey C, Guay DR. Pneumococcal polysaccharide revaccination: immunoglobulin g seroconversion, persistence, and safety in frail, chronically ill older subjects. J Am Geriatr Soc 2003;51:240-245.
16. Landgren O, Bjorkholm M, Konradsen HB, et al. A prospective study on antibody response to repeated vaccinations with pneumococcal capsular polysaccharide in splenectomized individuals with special reference to Hodgkin’s lymphoma. J Intern Med 2004;255:664-673.
17. Torling J, Hedlund J, Konradsen HB, Ortqvist A. Revaccination with the 23-valent pneumococcal polysaccharide vaccine in middle-aged and elderly persons previously treated for pneumonia. Vaccine 2003;22:96-103.
1. Butler JC, Breiman RF, Campbell JF, Lipman HB, Broome CV, Facklam RR. Pneumococcal polysaccharide vaccine efficacy: An Evaluation of current recommendations. JAMA 1993;270:1826-1831.
2. Fine MJ, Smith MA, Carson CA, et al. Efficacy of pneumococcal vaccination in adults: A meta-analysis of randomized controlled trials. Arch Intern Med 1994;154:2666-2677.
3. Hutchinson BG, Oxman AD, Shannon HS, Lloyd S, Altmayer CA, Thomas K. Clinical effectiveness of pneumococcal vaccine. Meta analysis. Can Fam Physician 1999;45:2381-2393.
4. Cornu C, Yzebe D, Leophonte P, Gaillat J, Boissel JP, Cucherat M. Efficacy of pneumococcal polysaccharide vaccine in immunocompetent adults: a meta-analysis of randomized trials. Vaccine 2001;19:4780-4790.
5. Moore RA, Wiffen PJ, Lipsky BA. Are the pneumococcal polysaccharide vaccines effective? Meta-analysis of the prospective trials. BMC Fam Pract 2000;1:1.-
6. Puig-Barbera J, Belenguer Varea A, Goterris Pinto M, Brines Benlliure MJ. Pneumococcal vaccine effectiveness in the elderly. Systematic review and meta-analysis. Aten Primaria 2002;30:269-281.
7. Watson I, Wilson BJ, Waugh N. Pneumococcal polysaccharide vaccine: a systematic review of clinical effectiveness in adults. Vaccine 2002;20:2166-2173.
8. Dear KB, Holden J, Andrews R, Tatham D. Vaccines for preventing pneumococcal infection in adults. Cochrane Database Syst Rev 2003;(4):CD000422.-
9. Whitney CG, Schaffner W, Butler JC. Rethinking recommendations for use of pneumococcal vaccines in adults. Clin Infect Dis 2001;33:662-675.
10. Jackson LA, Benson P, Snellar VP, et al. Safety of revaccination with pneumococcal polysaccharide vaccine. JAMA 1999;281:243-248.
11. Snow R, Babish JD, McBean AM. Is there any connection between a second pneumonia shot and hospitalization among Medicare beneficiaries? Public Health Rep 1995;110:720-725.
12. Petrasch S, Kuhnemund O, Reinacher A, et al. Antibody responses of splenectomized patients with non-Hodgkin’s lymphoma to immunization with polyvalent pneumococcal vaccines. Clin Diagn Lab Immunol 1997;4:635-638.
13. Rodriguez-Barradas MC, Groover JE, Lacke CE, et al. IgG antibody to pneumococcal capsular polysaccharide in human immunodeficiency virus-infected subjects: persistence of antibody in responders, revaccination in nonresponders, and relationship of immunoglobulin allotype to response. J Infect Dis 1996;173:1347-1353.
14. Cherif H, Landgren O, Konradsen HB, Kalin M, Bjorkholm M. Poor antibody response to pneumococcal polysaccharide vaccination suggests increased susceptibility to pneumococcal infection in splenectomized patients with hematological diseases. Vaccine. 2006;24(1):75-81.
15. Lackner TE, Hamilton RG, Hill JJ, Davey C, Guay DR. Pneumococcal polysaccharide revaccination: immunoglobulin g seroconversion, persistence, and safety in frail, chronically ill older subjects. J Am Geriatr Soc 2003;51:240-245.
16. Landgren O, Bjorkholm M, Konradsen HB, et al. A prospective study on antibody response to repeated vaccinations with pneumococcal capsular polysaccharide in splenectomized individuals with special reference to Hodgkin’s lymphoma. J Intern Med 2004;255:664-673.
17. Torling J, Hedlund J, Konradsen HB, Ortqvist A. Revaccination with the 23-valent pneumococcal polysaccharide vaccine in middle-aged and elderly persons previously treated for pneumonia. Vaccine 2003;22:96-103.
Evidence-based answers from the Family Physicians Inquiries Network
Does stopping a statin increase the short-term risk of a cardiovascular event?
When hydroxymethyl glutaryl coenzyme A (HMG CoA)inhibitors (statins) are stopped by asymptomatic patients, there appears to be no increased risk of cardiovascular events (strength of recommendation [SOR]: B,). However, for patients who have recently experienced a cardiovascular event, discontinuation of statins increases the risk of further events and death (SOR: B,).
Rely on low-tech skills, like shared decision-making, to improve adherence
Vincent Lo, MD
San Joaquin Family Medicine Residency, San Joaquin General Hospital, French Camp, Calif
One might hope that all patients taking statins would have excellent compliance, given these drugs’ well-established benefit. Unfortunately, long-term adherence remains suboptimal, and patients are going to stop their statins.1 A Canadian study2 found that patients aged >65 years, with and without recent acute coronary syndrome (ACS), had low rates of adherence to statins 2 years after initiation of therapy (40.1% for ACS, 36.1% for chronic coronary artery disease, and 25.4% for primary prevention). A recent Cochrane review3 found small improved adherence despite attempted intervention (range improvement: −3% to 25%). They concluded no intervention aimed at improving adherence to lipid-lowing drugs can be recommended over another, given the limited effects.
Clinicians are left to rely on low-tech skills, such as focusing on the patient’s perspective and shared decision-making, to improve their patients’ adherence. This focus is especially important for those who had a recent cardiovascular event. Nevertheless, it is reassuring that this review did not find significantly increased harm after abrupt stopping of statins among stable patients without recent ACS.
Evidence summary
The benefits of statin therapy appear to extend beyond the realm of their cholesterol-lowering properties.4 These benefits, such as reduction in post—myocardial infarction (MI) deaths and reinfarctions, are seen quickly after initiation of therapy. Other drugs, such as aspirin and beta-blockers, have also been shown to improve early outcomes when started after cardiovascular events, although waiting until the patient is hemodynamically stable to initiate beta-blockade reduces the risk of cardiogenic shock.5 If standard agents are either not started or withdrawn after a cardiovascular event, patients are at increased risk of harm.6,7
Physiological research. Studies of patients with stroke and those with only risk factors for cardiovascular disease show that platelet activity is increased when statins are discontinued.8,9 Additionally, tissue plasminogen activator levels are decreased after discontinuation of statins, resulting in a relatively hypercoagulable state.10 Animal studies of stroke in mice showed mice whose statin was abruptly stopped had more damage from stroke than those whose statin was continued.11
High-risk cardiovascular patients. Preliminary human data suggested that stopping statins increased risk of recurrent events for patients who recently had a primary cardiovascular event. One retrospective case-control study evaluated 4870 patients who had statin therapy withdrawn on admission to the hospital for non-ST segment elevation MI (NSTEMI). Patients who had their statins withheld had increased rates of heart failure, arrhythmia, shock, and death (hazard ratio=2.32; 95% confidence interval [CI], 2.02–2.67).12 A post-hoc analysis of data from the PRISM trial found that among the 86 patients who were admitted for chest pain and had their statin withdrawn, a higher rate of death and nonfatal MI was observed, compared with the 379 patients whose statins were continued (hazard ratio=2.93; 95% CI, 1.64–6.27). This effect was seen in the first week and was independent of cholesterol levels and measures of severity of illness.13
Low-risk cardiovascular patients. A post-hoc analysis of the washout period of a prospective study of 9473 asymptomatic outpatients who were previously taking statins showed that for these lower-risk patients, similar rates of cardiovascular events could be expected during withdrawal (any statin) or initiation of atorvastatin therapy. The monthly event rate during the discontinuation phase was 0.20% and during initiation was 0.26% (P=NS).14
Recommendations from others
Currently, no expert panels or specialty bodies make recommendations regarding how or when to discontinue statins. The Institute for Clinical Systems Improvement recommends that all patients with chronic stable coronary artery disease should be considered for statin use regardless of their lipid levels; however, no mention of discontinuing statins is made.15
1. Benner JS, Glynn RJ, Neumann PJ, Mogun H, Weinsten MC, Avorn J. Long-term persistence in use of statin therapy in elderly patients. JAMA 2002;288:455-461.
2. Jackevicius CA, Mamdani M, Tu JV. Adherence with statin therapy in elderly patients with and without acute coronary syndromes. JAMA 2002;288:462-467.
3. Schedlbauer A, Schroeder K, Peters TJ, Fahey T. Interventions to improve adherence to lipid lowering medication. Cochrane Database Syst Rev 2004;(4)CD004371.
4. Thompson PL, Meredeth I, Amerena J, et al. Effect of pravastatin compared with placebo initiated within 24 hours of onset of acute myocardial infarction or unstable angina: the Pravastatin in Acute Coronary Treatment (PACT) trial. Am Heart J 2004;148:e2.
5. Chen ZM, Pan HC, Chen YP, et al. Early intravenous then oral metoprolol in 45,852 patients with acute myocardial infarction: randomised placebo-controlled trial. Lancet 2005;366:1622-1632.
6. Olsson G, Oden A, Johansson L, Sjogren A, Rehnqvist N. Prognosis after withdrawal of chronic postinfarction metoprolol treatment: a 2-7 year follow-up. Eur Heart J 1988;9:365-372.
7. ISIS-2 (Second International Study of Infarct Survival) Collaborative Group. Randomised trial of intravenous streptokinase, oral aspirin, both, or neither among 17,187 cases of suspected acute myocardial infarction: ISIS-2. Lancet 1988; 2(8607):349–360.
8. Puccetti L, Pasqui AL, Pastorelli M, et al. Platelet hyperactivity after statin treatment discontinuation. Thromb Haemost 2003;90:476-482.
9. Cha JK, Jeong MH, Kim JW. Statin reduces the platelet P-selectin expression in atherosclerotic ischemic stroke. J Thrombosis Thrombolysis 2004;18:39-42.
10. Lai WT, Lee KT, Chu CS, et al. Influence of withdrawal of statin treatment on proinflammatory response and fibrinolytic activity in humans: an effect independent on cholesterol elevation. Int J Cardiol 2005;98:459-464.
11. Gertz K, Laufs U, Lindauer U, et al. Withdrawal of statin treatment abrogates stroke protection in mice. Stroke 2003;34:551-557.
12. Spencer FA, Fonarow GC, Frederick PD, et al. Early withdrawal of statin therapy in patients with non-ST-segment elevation myocardial infarction: national registry of myocardial infarction. Arch Intern Med 2004;164:2162-2168.
13. Heeschen C, Hamm CW, Laufs U, et al. Withdrawal of statins increases event rates in patients with acute coronary syndromes. Circulation 2002;105:1446-1452.
14. McGowan MP. and the Treating to New Target (TNT) Study Group. There is no evidence for an increase in acute coronary syndromes after short-term abrupt discontinuation of statins in stable cardiac patients. Circulation 2004;110:2333-2335.
15. Stable coronary artery disease. Institute for Clinical Systems Improvement. Bloomington, Minn: Institute for Clinical Systems Improvement (ICSI); July 1994. Revised April 2005. Available at: www.icsi.org/knowledge/detail.asp?catID=29&itemID=192. Accessed on May 17, 2006.
When hydroxymethyl glutaryl coenzyme A (HMG CoA)inhibitors (statins) are stopped by asymptomatic patients, there appears to be no increased risk of cardiovascular events (strength of recommendation [SOR]: B,). However, for patients who have recently experienced a cardiovascular event, discontinuation of statins increases the risk of further events and death (SOR: B,).
Rely on low-tech skills, like shared decision-making, to improve adherence
Vincent Lo, MD
San Joaquin Family Medicine Residency, San Joaquin General Hospital, French Camp, Calif
One might hope that all patients taking statins would have excellent compliance, given these drugs’ well-established benefit. Unfortunately, long-term adherence remains suboptimal, and patients are going to stop their statins.1 A Canadian study2 found that patients aged >65 years, with and without recent acute coronary syndrome (ACS), had low rates of adherence to statins 2 years after initiation of therapy (40.1% for ACS, 36.1% for chronic coronary artery disease, and 25.4% for primary prevention). A recent Cochrane review3 found small improved adherence despite attempted intervention (range improvement: −3% to 25%). They concluded no intervention aimed at improving adherence to lipid-lowing drugs can be recommended over another, given the limited effects.
Clinicians are left to rely on low-tech skills, such as focusing on the patient’s perspective and shared decision-making, to improve their patients’ adherence. This focus is especially important for those who had a recent cardiovascular event. Nevertheless, it is reassuring that this review did not find significantly increased harm after abrupt stopping of statins among stable patients without recent ACS.
Evidence summary
The benefits of statin therapy appear to extend beyond the realm of their cholesterol-lowering properties.4 These benefits, such as reduction in post—myocardial infarction (MI) deaths and reinfarctions, are seen quickly after initiation of therapy. Other drugs, such as aspirin and beta-blockers, have also been shown to improve early outcomes when started after cardiovascular events, although waiting until the patient is hemodynamically stable to initiate beta-blockade reduces the risk of cardiogenic shock.5 If standard agents are either not started or withdrawn after a cardiovascular event, patients are at increased risk of harm.6,7
Physiological research. Studies of patients with stroke and those with only risk factors for cardiovascular disease show that platelet activity is increased when statins are discontinued.8,9 Additionally, tissue plasminogen activator levels are decreased after discontinuation of statins, resulting in a relatively hypercoagulable state.10 Animal studies of stroke in mice showed mice whose statin was abruptly stopped had more damage from stroke than those whose statin was continued.11
High-risk cardiovascular patients. Preliminary human data suggested that stopping statins increased risk of recurrent events for patients who recently had a primary cardiovascular event. One retrospective case-control study evaluated 4870 patients who had statin therapy withdrawn on admission to the hospital for non-ST segment elevation MI (NSTEMI). Patients who had their statins withheld had increased rates of heart failure, arrhythmia, shock, and death (hazard ratio=2.32; 95% confidence interval [CI], 2.02–2.67).12 A post-hoc analysis of data from the PRISM trial found that among the 86 patients who were admitted for chest pain and had their statin withdrawn, a higher rate of death and nonfatal MI was observed, compared with the 379 patients whose statins were continued (hazard ratio=2.93; 95% CI, 1.64–6.27). This effect was seen in the first week and was independent of cholesterol levels and measures of severity of illness.13
Low-risk cardiovascular patients. A post-hoc analysis of the washout period of a prospective study of 9473 asymptomatic outpatients who were previously taking statins showed that for these lower-risk patients, similar rates of cardiovascular events could be expected during withdrawal (any statin) or initiation of atorvastatin therapy. The monthly event rate during the discontinuation phase was 0.20% and during initiation was 0.26% (P=NS).14
Recommendations from others
Currently, no expert panels or specialty bodies make recommendations regarding how or when to discontinue statins. The Institute for Clinical Systems Improvement recommends that all patients with chronic stable coronary artery disease should be considered for statin use regardless of their lipid levels; however, no mention of discontinuing statins is made.15
When hydroxymethyl glutaryl coenzyme A (HMG CoA)inhibitors (statins) are stopped by asymptomatic patients, there appears to be no increased risk of cardiovascular events (strength of recommendation [SOR]: B,). However, for patients who have recently experienced a cardiovascular event, discontinuation of statins increases the risk of further events and death (SOR: B,).
Rely on low-tech skills, like shared decision-making, to improve adherence
Vincent Lo, MD
San Joaquin Family Medicine Residency, San Joaquin General Hospital, French Camp, Calif
One might hope that all patients taking statins would have excellent compliance, given these drugs’ well-established benefit. Unfortunately, long-term adherence remains suboptimal, and patients are going to stop their statins.1 A Canadian study2 found that patients aged >65 years, with and without recent acute coronary syndrome (ACS), had low rates of adherence to statins 2 years after initiation of therapy (40.1% for ACS, 36.1% for chronic coronary artery disease, and 25.4% for primary prevention). A recent Cochrane review3 found small improved adherence despite attempted intervention (range improvement: −3% to 25%). They concluded no intervention aimed at improving adherence to lipid-lowing drugs can be recommended over another, given the limited effects.
Clinicians are left to rely on low-tech skills, such as focusing on the patient’s perspective and shared decision-making, to improve their patients’ adherence. This focus is especially important for those who had a recent cardiovascular event. Nevertheless, it is reassuring that this review did not find significantly increased harm after abrupt stopping of statins among stable patients without recent ACS.
Evidence summary
The benefits of statin therapy appear to extend beyond the realm of their cholesterol-lowering properties.4 These benefits, such as reduction in post—myocardial infarction (MI) deaths and reinfarctions, are seen quickly after initiation of therapy. Other drugs, such as aspirin and beta-blockers, have also been shown to improve early outcomes when started after cardiovascular events, although waiting until the patient is hemodynamically stable to initiate beta-blockade reduces the risk of cardiogenic shock.5 If standard agents are either not started or withdrawn after a cardiovascular event, patients are at increased risk of harm.6,7
Physiological research. Studies of patients with stroke and those with only risk factors for cardiovascular disease show that platelet activity is increased when statins are discontinued.8,9 Additionally, tissue plasminogen activator levels are decreased after discontinuation of statins, resulting in a relatively hypercoagulable state.10 Animal studies of stroke in mice showed mice whose statin was abruptly stopped had more damage from stroke than those whose statin was continued.11
High-risk cardiovascular patients. Preliminary human data suggested that stopping statins increased risk of recurrent events for patients who recently had a primary cardiovascular event. One retrospective case-control study evaluated 4870 patients who had statin therapy withdrawn on admission to the hospital for non-ST segment elevation MI (NSTEMI). Patients who had their statins withheld had increased rates of heart failure, arrhythmia, shock, and death (hazard ratio=2.32; 95% confidence interval [CI], 2.02–2.67).12 A post-hoc analysis of data from the PRISM trial found that among the 86 patients who were admitted for chest pain and had their statin withdrawn, a higher rate of death and nonfatal MI was observed, compared with the 379 patients whose statins were continued (hazard ratio=2.93; 95% CI, 1.64–6.27). This effect was seen in the first week and was independent of cholesterol levels and measures of severity of illness.13
Low-risk cardiovascular patients. A post-hoc analysis of the washout period of a prospective study of 9473 asymptomatic outpatients who were previously taking statins showed that for these lower-risk patients, similar rates of cardiovascular events could be expected during withdrawal (any statin) or initiation of atorvastatin therapy. The monthly event rate during the discontinuation phase was 0.20% and during initiation was 0.26% (P=NS).14
Recommendations from others
Currently, no expert panels or specialty bodies make recommendations regarding how or when to discontinue statins. The Institute for Clinical Systems Improvement recommends that all patients with chronic stable coronary artery disease should be considered for statin use regardless of their lipid levels; however, no mention of discontinuing statins is made.15
1. Benner JS, Glynn RJ, Neumann PJ, Mogun H, Weinsten MC, Avorn J. Long-term persistence in use of statin therapy in elderly patients. JAMA 2002;288:455-461.
2. Jackevicius CA, Mamdani M, Tu JV. Adherence with statin therapy in elderly patients with and without acute coronary syndromes. JAMA 2002;288:462-467.
3. Schedlbauer A, Schroeder K, Peters TJ, Fahey T. Interventions to improve adherence to lipid lowering medication. Cochrane Database Syst Rev 2004;(4)CD004371.
4. Thompson PL, Meredeth I, Amerena J, et al. Effect of pravastatin compared with placebo initiated within 24 hours of onset of acute myocardial infarction or unstable angina: the Pravastatin in Acute Coronary Treatment (PACT) trial. Am Heart J 2004;148:e2.
5. Chen ZM, Pan HC, Chen YP, et al. Early intravenous then oral metoprolol in 45,852 patients with acute myocardial infarction: randomised placebo-controlled trial. Lancet 2005;366:1622-1632.
6. Olsson G, Oden A, Johansson L, Sjogren A, Rehnqvist N. Prognosis after withdrawal of chronic postinfarction metoprolol treatment: a 2-7 year follow-up. Eur Heart J 1988;9:365-372.
7. ISIS-2 (Second International Study of Infarct Survival) Collaborative Group. Randomised trial of intravenous streptokinase, oral aspirin, both, or neither among 17,187 cases of suspected acute myocardial infarction: ISIS-2. Lancet 1988; 2(8607):349–360.
8. Puccetti L, Pasqui AL, Pastorelli M, et al. Platelet hyperactivity after statin treatment discontinuation. Thromb Haemost 2003;90:476-482.
9. Cha JK, Jeong MH, Kim JW. Statin reduces the platelet P-selectin expression in atherosclerotic ischemic stroke. J Thrombosis Thrombolysis 2004;18:39-42.
10. Lai WT, Lee KT, Chu CS, et al. Influence of withdrawal of statin treatment on proinflammatory response and fibrinolytic activity in humans: an effect independent on cholesterol elevation. Int J Cardiol 2005;98:459-464.
11. Gertz K, Laufs U, Lindauer U, et al. Withdrawal of statin treatment abrogates stroke protection in mice. Stroke 2003;34:551-557.
12. Spencer FA, Fonarow GC, Frederick PD, et al. Early withdrawal of statin therapy in patients with non-ST-segment elevation myocardial infarction: national registry of myocardial infarction. Arch Intern Med 2004;164:2162-2168.
13. Heeschen C, Hamm CW, Laufs U, et al. Withdrawal of statins increases event rates in patients with acute coronary syndromes. Circulation 2002;105:1446-1452.
14. McGowan MP. and the Treating to New Target (TNT) Study Group. There is no evidence for an increase in acute coronary syndromes after short-term abrupt discontinuation of statins in stable cardiac patients. Circulation 2004;110:2333-2335.
15. Stable coronary artery disease. Institute for Clinical Systems Improvement. Bloomington, Minn: Institute for Clinical Systems Improvement (ICSI); July 1994. Revised April 2005. Available at: www.icsi.org/knowledge/detail.asp?catID=29&itemID=192. Accessed on May 17, 2006.
1. Benner JS, Glynn RJ, Neumann PJ, Mogun H, Weinsten MC, Avorn J. Long-term persistence in use of statin therapy in elderly patients. JAMA 2002;288:455-461.
2. Jackevicius CA, Mamdani M, Tu JV. Adherence with statin therapy in elderly patients with and without acute coronary syndromes. JAMA 2002;288:462-467.
3. Schedlbauer A, Schroeder K, Peters TJ, Fahey T. Interventions to improve adherence to lipid lowering medication. Cochrane Database Syst Rev 2004;(4)CD004371.
4. Thompson PL, Meredeth I, Amerena J, et al. Effect of pravastatin compared with placebo initiated within 24 hours of onset of acute myocardial infarction or unstable angina: the Pravastatin in Acute Coronary Treatment (PACT) trial. Am Heart J 2004;148:e2.
5. Chen ZM, Pan HC, Chen YP, et al. Early intravenous then oral metoprolol in 45,852 patients with acute myocardial infarction: randomised placebo-controlled trial. Lancet 2005;366:1622-1632.
6. Olsson G, Oden A, Johansson L, Sjogren A, Rehnqvist N. Prognosis after withdrawal of chronic postinfarction metoprolol treatment: a 2-7 year follow-up. Eur Heart J 1988;9:365-372.
7. ISIS-2 (Second International Study of Infarct Survival) Collaborative Group. Randomised trial of intravenous streptokinase, oral aspirin, both, or neither among 17,187 cases of suspected acute myocardial infarction: ISIS-2. Lancet 1988; 2(8607):349–360.
8. Puccetti L, Pasqui AL, Pastorelli M, et al. Platelet hyperactivity after statin treatment discontinuation. Thromb Haemost 2003;90:476-482.
9. Cha JK, Jeong MH, Kim JW. Statin reduces the platelet P-selectin expression in atherosclerotic ischemic stroke. J Thrombosis Thrombolysis 2004;18:39-42.
10. Lai WT, Lee KT, Chu CS, et al. Influence of withdrawal of statin treatment on proinflammatory response and fibrinolytic activity in humans: an effect independent on cholesterol elevation. Int J Cardiol 2005;98:459-464.
11. Gertz K, Laufs U, Lindauer U, et al. Withdrawal of statin treatment abrogates stroke protection in mice. Stroke 2003;34:551-557.
12. Spencer FA, Fonarow GC, Frederick PD, et al. Early withdrawal of statin therapy in patients with non-ST-segment elevation myocardial infarction: national registry of myocardial infarction. Arch Intern Med 2004;164:2162-2168.
13. Heeschen C, Hamm CW, Laufs U, et al. Withdrawal of statins increases event rates in patients with acute coronary syndromes. Circulation 2002;105:1446-1452.
14. McGowan MP. and the Treating to New Target (TNT) Study Group. There is no evidence for an increase in acute coronary syndromes after short-term abrupt discontinuation of statins in stable cardiac patients. Circulation 2004;110:2333-2335.
15. Stable coronary artery disease. Institute for Clinical Systems Improvement. Bloomington, Minn: Institute for Clinical Systems Improvement (ICSI); July 1994. Revised April 2005. Available at: www.icsi.org/knowledge/detail.asp?catID=29&itemID=192. Accessed on May 17, 2006.
Evidence-based answers from the Family Physicians Inquiries Network