User login
Considering Probiotics? What You Must Know First
Probiotics—live microorganisms that are consumed as supplements or food for purported health benefits—are a popular OTC remedy for various gastrointestinal (GI) ailments and other conditions, but the evidence supporting their use is mixed. Probiotics interact with the normal flora of the human body. They are believed to act by multiple mechanisms to deliver beneficial effects, including providing a protective barrier, altering intestinal pH to favor the growth of nonpathogenic bacteria, enhancing the host’s immunologic response, producing antimicrobial substances, and directly competing with pathogenic bacteria for receptors in the GI tract1 (see “The normal human intestinal flora,”).
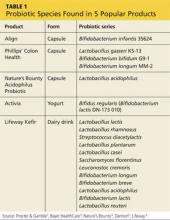
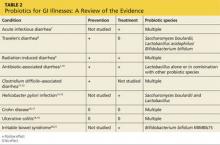
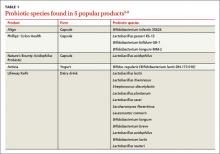
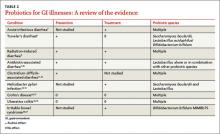
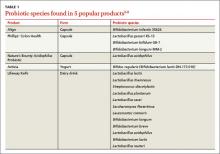
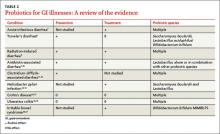
In the United States, Lactobacillus and Bifidobacterium are the probiotic genera that are most commonly used. (For a list of the specific probiotic species found in five popular products, see Table 1.2-6) The review that follows examines the evidence for using probiotics for select GI ailments, including several types of diarrheal illness, inflammatory bowel disease (Crohn disease and ulcerative colitis), and irritable bowel syndrome (IBS). These findings are summarized in Table 2.1,7-21
Continue for probiotics may help with some types of diarrhea >>
PROBIOTICS MAY HELP WITH SOME TYPES OF DIARRHEA
Acute infectious diarrhea. Viruses, bacteria, and parasites cause acute infectious diarrhea, and probiotics are thought to act against these pathogens by competing for available nutrients and pattern-recognition receptors in the GI endothelium, acidifying the local environment, and increasing immune responses within the GI tract. In a meta-analysis of 63 studies (N = 8,014) that used multiple strains and dosages of probiotics, investigators found probiotics shortened the duration of acute infectious diarrhea by approximately 24 h.7 Probiotics also reduced both the risk for diarrhea lasting longer than four days (relative risk [RR], 0.41) and stool frequency on day 2 of illness (mean difference of 0.80 stools).
Traveler’s diarrhea. The incidence of traveler’s diarrhea is > 50% for travel to high-risk areas such as the Middle East, North Africa, Latin America, and Southeast Asia, and 5% to 10% when traveling to areas such as North America, Northern Europe, the United Kingdom, Australia, and New Zealand.8 Traveler’s diarrhea may be caused by ingesting food and liquids contaminated with fecal material. Symptoms include diarrhea, cramps, and nausea that, if untreated, typically last from two to six days but can last for as long as a month.8
In a meta-analysis of 12 studies (N = 5,171) that evaluated various probiotic strains, researchers found probiotics effectively prevented traveler’s diarrhea in US and European travelers who visited a variety of vacation spots (pooled RR, 0.85).8 No serious adverse events were reported.
Radiation-induced diarrhea. Radiation treatments to the abdomen and pelvis can damage the lower GI tract and cause diarrhea. The pooled results from a meta-analysis that included six studies (N = 1,449) significantly favored the use of probiotics over placebo for decreasing the incidence of radiation-induced diarrhea (odds ratio [OR], 0.44).9 Probiotic use also was associated with decreased loperamide use (OR, 0.29) and decreased incidence of watery stools (OR, 0.36), but these outcomes did not reach statistical significance.
Antibiotic-associated diarrhea. Antibiotic use has long been associated with the development of diarrheal illness, sometimes due to the acceleration of GI motility (eg, erythromycin) or by causing osmotic diarrhea by decreasing GI bacteria that assist in carbohydrate breakdown.11 A meta-analysis that evaluated 63 randomized controlled trials (RCTs) (N = 11,811) showed that probiotics are effective for treating and preventing antibiotic-associated diarrhea (AAD).1 There was a statistically significant reduction in AAD among patients who received probiotics (RR, 0.58; number needed to treat [NNT], 13). Most of the studies in this meta-analysis used a Lactobacillus probiotic alone or in combination with another probiotic. Researchers did not analyze whether the efficacy varied by patient population, probiotic used, causative antibiotic, or duration of treatment.1 Another meta-analysis of 34 studies (N = 4,138) also found probiotic therapy can prevent AAD.10 The pooled RR for AAD was 0.53 for patients treated with probiotics compared to placebo, with an NNT of 8. The effects remained significant when results were grouped by probiotic species, patient age, and duration of antibiotic treatment. Among a subgroup of patients in this meta-analysis who were being treated for Helicobacter pylori, the pooled RR of AAD was 0.37 and the NNT was 5.10 However, the 2013 PLACIDE trial (N = 17,420) found no significant decrease in AAD rates in hospitalized patients older than 65 being treated with antibiotics who received probiotics (RR, 1.04).22
Clostridium difficile–associated diarrhea. As we know, antibiotics can disrupt the normal GI flora and permit overgrowth of Clostridium difficile, which can result in C difficile–associated diarrhea (CDAD).12 This can occur with oral, parenteral, and even topical antibiotics.11 Researchers have investigated whether probiotics can prevent this opportunistic C difficile overgrowth.
A 2012 meta-analysis of 20 trials (N = 38,180) found probiotic prophylaxis prevented CDAD in both inpatients and outpatients while not increasing the incidence of significant adverse effects.12 Probiotics decreased the incidence of CDAD by 66% (pooled RR, 0.34).12 Adverse events occurred in 9.3% of patients taking probiotics, compared with 12.6% of controls (RR, 0.82).12
Conversely, a 2008 review of four studies (N = 336) concluded there is insufficient evidence for using probiotics to treat CDAD, either as monotherapy or adjunct therapy.11 One trial in this meta-analysis (N = 124) found patients who received the probiotic Saccharomyces boulardii in addition to antibiotic therapy were significantly less likely to experience CDAD recurrence than those who received placebo (RR, 0.59).11 However, this benefit was not found in the other trials in this meta-analysis.11
The PLACIDE trial found probiotics did not prevent CDAD in hospitalized patients older than 65; 0.8% of patients who received probiotics developed CDAD, compared to 1.2% in the placebo group (RR, 0.71).22
Helicobacter pylori infection. The triple-therapy regimen of a proton pump inhibitor plus the antibiotics clarithromycin and amoxicillin is the recommended treatment for H pylori infection.13 Associated adverse effects include diarrhea and decreased eradication rates, in part due to antibiotic resistance. Certain Lactobacillus species have been shown to inhibit or kill H pylori in vitro,13 and evidence from several meta-analyses suggests probiotics should be an adjunct therapy for the treatment of H pylori.
In a meta-analysis of 10 RCTs (N = 963), fermented milk-based probiotics improved H pylori eradication rates by 5% to 15%.14 In another meta-analysis that evaluated five RCTs (N = 1,307), S boulardii significantly increased the H pylori eradication rate when used as an adjunct to triple therapy (RR, 1.13) and reduced the rate of treatment related adverse effects (RR, 0.46).13 In a third meta-analysis of 10 trials (N = 1,469), Lactobacillus supplementation increased H pylori eradication rates (OR, 2.1) while decreasing the overall incidence of adverse effects (OR, 0.3).15
Next: For inflammatory bowel disease, probiotics are unlikely to help >>
FOR INFLAMMATORY BOWEL DISEASE, PROBIOTICS ARE UNLIKELY TO HELP
Current therapies for Crohn disease and ulcerative colitis, such as corticosteroids and other immunosuppressive agents, are effective but have significant adverse events.18 Researchers explored whether probiotics might help treat these diseases by improving immune response, the balance of microbes in the GI tract, and the intestinal barrier.18
Crohn disease. In a meta-analysis that was able to identify only one small RCT (N = 11), 80% of patients receiving probiotic treatment went into remission, compared to 83% in the placebo group (OR, 0.80).16 Researchers concluded there was insufficient evidence for the use of probiotics for inducing remission in Crohn disease.
Another meta-analysis of seven small studies (N = 160) found no significant evidence supporting probiotic use for maintaining remission in Crohn disease compared with aminosalicylates or azathioprine.17 One small study in this review found there was a benefit to combining S boulardii with a reduced level of standard maintenance therapy when compared to standard therapy alone, but this difference was not statistically significant.17
Ulcerative colitis. A systematic review of four RCTs (N = 244) that compared conventional treatment alone to conventional treatment plus probiotics for remission or clinical improvement in patients with active ulcerative colitis found no significant differences between groups.18 Another meta-analysis of four studies (N = 587) found that compared to placebo or treatment with mesalazine, probiotics had no benefit for maintaining remission in ulcerative colitis.19 The rate of relapse was 40.1% in the probiotics group, compared to 34.1% in the mesalazine group. The number of adverse effects was similar in both groups.
Continue for most evidence suggests probiotics are useful for IBS >>
MOST EVIDENCE SUGGESTS PROBIOTICS ARE USEFUL FOR IBS
In RCTs, probiotic supplements—but not yogurt containing probiotics—reduced IBS symptoms. Research suggests that imbalances in GI flora, along with subsequent dysfunction in intestinal barriers and translocation of intestinal flora, may play a role in symptoms associated with IBS, such as abdominal pain, bloating, and diarrhea/constipation.20 There are few effective therapeutic options for patients with IBS.
In a systematic review of 19 RCTs (N = 1,650), probiotics were significantly more effective than placebo for patients with IBS, with an NNT of 4.21 This review did not evaluate the difference between various probiotic species and strains.
In an RCT (N = 122), the probiotic strain Bifidobacterium bifidum MIMBb75 was found to be safe and beneficial for treating IBS symptoms and improving patients’ quality of life.20 On a 7-point scale of global assessment of IBS symptoms, the score was reduced by 0.88 points in the group that received B bifidum MIMBb75 and 0.16 points in the placebo group (P < .0001). Almost half (47%) of the patients who received B bifidum MIMBb75 reported adequate relief, compared to 11% in the placebo group (P < .0001).
An RCT (N = 179) that compared yogurt containing probiotics to nonprobiotic yogurt found that the former had no benefits for treating IBS symptoms.23 After four weeks, 57% of patients who ate the probiotic yogurt reported adequate relief, compared to 53% of those who ate nonprobiotic yogurt (P = .71). After eight weeks, those numbers were 47% and 68%, respectively.23
REFERENCES
1. Hempel S, Newberry SJ, Maher AR, et al. Probiotics for the prevention and treatment of antibiotic-associated diarrhea: a systematic review and meta-analysis. JAMA. 2012;307:1959-1969.
2. Procter & Gamble. Align product information. www.aligngi.com/information-on-Align-probiotic-supplement. Accessed May 19, 2015.
3. Bayer HealthCare. Phillip’s Colon Health product information. http://phillipspro.com/en/home/product-information/index.php. Accessed May 19, 2015.
4. Nature’s Bounty. Nature’s Bounty Acidophilus Probiotic product label. http://images.vitaminimages.com/cdn/sd/pdf/L002610-NB.PDF. Accessed May 19, 2015.
5. Dannon. Activia. http://activia.us.com/probiotic-yogurt/activia. Accessed May 19, 2015.
6. Lifeway. Lifeway Kefir frequently asked questions. http://lifewaykefir.com/faq/. Accessed May 19, 2015.
7. Allen SJ, Martinez EG, Gregorio GV, et al. Probiotics for treating acute infectious diarrhoea. Cochrane Database Syst Rev. 2010;(11):CD003048.
8. McFarland LV. Meta-analysis of probiotics for the prevention of traveler’s diarrhea. Travel Med Infect Dis. 2007;5:97-105.
9. Hamad A, Fragkos KC, Forbes A. A systemic review and meta-analysis of probiotics for the management of radiation induced bowel disease. Clin Nutr. 2013;32:353-360.
10. Videlock EJ, Cremonini F. Meta-analysis: probiotics in antibiotic-associated diarrhoea. Aliment Pharmacol Ther. 2012;35:1355-1369.
11. Pillai A, Nelson RL. Probiotics for treatment of Clostridium difficile–associated colitis in adults. Cochrane Database Syst Rev. 2008;(1):CD004611.
12. Johnston BC, Ma SY, Goldenberg JZ, et al. Probiotics for the prevention of Clostridium difficile–associated diarrhea: a systematic review and meta-analysis. Ann Intern Med. 2012;157:878-888.
13. Szajewska H, Horvath A, Piwowarczyk A. Meta-analysis: the effects of Saccharomyces boulardii supplementation on Helicobacter pylori eradication rates and side effects during treatment. Aliment Pharmacol Ther. 2010;32:1069-1079.
14. Sachdeva A, Nagpal J. Effect of fermented milk-based probiotic preparations on Helicobacter pylori eradication: a systematic review and meta-analysis of randomized controlled trials. Eur J Gastroenterol Hepatol. 2009;21:45-53.
15. Wang ZH, Gao QY, Fang JY. Meta-analysis of the efficacy and safety of Lactobacillus-containing and Bifidobacterium-containing probiotic compound preparation in Helicobacter pylori eradication therapy. J Clin Gastroenterol. 2013;47:25-32.
16. Butterworth AD, Thomas AG, Akobeng AK. Probiotics for induction of remission in Crohn’s disease. Cochrane Database Syst Rev. 2008; 16:CD006634.
17. Rolfe VE, Fortun PJ, Hawkey CJ, et al. Probiotics for maintenance of remission in Crohn’s disease. Cochrane Database Syst Rev. 2006;(4):CD004826.
18. Mallon P, McKay D, Kirk SJ, et al. Probiotics for induction of remission in ulcerative colitis. Cochrane Database Syst Rev. 2007;(4):CD005573.
19. Naidoo K, Gordon M, Fagbemi AO, et al. Probiotics for maintenance of remission in ulcerative colitis. Cochrane Database Syst Rev. 2011;(12):CD007443.
20. Guglielmetti S, Mora D, Gschwender M, et al. Randomised clinical trial: Bifidobacterium bifidum MIMBb75 significantly alleviates irritable bowel syndrome and improves quality of life—a double-blind, placebo-controlled study. Aliment Pharmacol Ther. 2011;33:1123-1132.
21. Moayyedi P, Ford AC, Talley NJ, et al. The efficacy of probiotics in the treatment of irritable bowel syndrome: a systematic review. Gut. 2010; 59:325-332.
22. Allen SJ, Wareham K, Wang D, et al. Lactobacilli and bifidobacteria in the prevention of antibiotic-associated diarrhoea and Clostridium difficile diarrhoea in older inpatients (PLACIDE): a randomised, double-blind, placebo-controlled, multicentre trial. Lancet. 2013;382:1249-1257.
23. Roberts LM, McCahon D, Holder R, et al. A randomised controlled trial of a probiotic ‘functional food’ in the management of irritable bowel syndrome. BMC Gastroenterol. 2013;13:45.
Probiotics—live microorganisms that are consumed as supplements or food for purported health benefits—are a popular OTC remedy for various gastrointestinal (GI) ailments and other conditions, but the evidence supporting their use is mixed. Probiotics interact with the normal flora of the human body. They are believed to act by multiple mechanisms to deliver beneficial effects, including providing a protective barrier, altering intestinal pH to favor the growth of nonpathogenic bacteria, enhancing the host’s immunologic response, producing antimicrobial substances, and directly competing with pathogenic bacteria for receptors in the GI tract1 (see “The normal human intestinal flora,”).
In the United States, Lactobacillus and Bifidobacterium are the probiotic genera that are most commonly used. (For a list of the specific probiotic species found in five popular products, see Table 1.2-6) The review that follows examines the evidence for using probiotics for select GI ailments, including several types of diarrheal illness, inflammatory bowel disease (Crohn disease and ulcerative colitis), and irritable bowel syndrome (IBS). These findings are summarized in Table 2.1,7-21
Continue for probiotics may help with some types of diarrhea >>
PROBIOTICS MAY HELP WITH SOME TYPES OF DIARRHEA
Acute infectious diarrhea. Viruses, bacteria, and parasites cause acute infectious diarrhea, and probiotics are thought to act against these pathogens by competing for available nutrients and pattern-recognition receptors in the GI endothelium, acidifying the local environment, and increasing immune responses within the GI tract. In a meta-analysis of 63 studies (N = 8,014) that used multiple strains and dosages of probiotics, investigators found probiotics shortened the duration of acute infectious diarrhea by approximately 24 h.7 Probiotics also reduced both the risk for diarrhea lasting longer than four days (relative risk [RR], 0.41) and stool frequency on day 2 of illness (mean difference of 0.80 stools).
Traveler’s diarrhea. The incidence of traveler’s diarrhea is > 50% for travel to high-risk areas such as the Middle East, North Africa, Latin America, and Southeast Asia, and 5% to 10% when traveling to areas such as North America, Northern Europe, the United Kingdom, Australia, and New Zealand.8 Traveler’s diarrhea may be caused by ingesting food and liquids contaminated with fecal material. Symptoms include diarrhea, cramps, and nausea that, if untreated, typically last from two to six days but can last for as long as a month.8
In a meta-analysis of 12 studies (N = 5,171) that evaluated various probiotic strains, researchers found probiotics effectively prevented traveler’s diarrhea in US and European travelers who visited a variety of vacation spots (pooled RR, 0.85).8 No serious adverse events were reported.
Radiation-induced diarrhea. Radiation treatments to the abdomen and pelvis can damage the lower GI tract and cause diarrhea. The pooled results from a meta-analysis that included six studies (N = 1,449) significantly favored the use of probiotics over placebo for decreasing the incidence of radiation-induced diarrhea (odds ratio [OR], 0.44).9 Probiotic use also was associated with decreased loperamide use (OR, 0.29) and decreased incidence of watery stools (OR, 0.36), but these outcomes did not reach statistical significance.
Antibiotic-associated diarrhea. Antibiotic use has long been associated with the development of diarrheal illness, sometimes due to the acceleration of GI motility (eg, erythromycin) or by causing osmotic diarrhea by decreasing GI bacteria that assist in carbohydrate breakdown.11 A meta-analysis that evaluated 63 randomized controlled trials (RCTs) (N = 11,811) showed that probiotics are effective for treating and preventing antibiotic-associated diarrhea (AAD).1 There was a statistically significant reduction in AAD among patients who received probiotics (RR, 0.58; number needed to treat [NNT], 13). Most of the studies in this meta-analysis used a Lactobacillus probiotic alone or in combination with another probiotic. Researchers did not analyze whether the efficacy varied by patient population, probiotic used, causative antibiotic, or duration of treatment.1 Another meta-analysis of 34 studies (N = 4,138) also found probiotic therapy can prevent AAD.10 The pooled RR for AAD was 0.53 for patients treated with probiotics compared to placebo, with an NNT of 8. The effects remained significant when results were grouped by probiotic species, patient age, and duration of antibiotic treatment. Among a subgroup of patients in this meta-analysis who were being treated for Helicobacter pylori, the pooled RR of AAD was 0.37 and the NNT was 5.10 However, the 2013 PLACIDE trial (N = 17,420) found no significant decrease in AAD rates in hospitalized patients older than 65 being treated with antibiotics who received probiotics (RR, 1.04).22
Clostridium difficile–associated diarrhea. As we know, antibiotics can disrupt the normal GI flora and permit overgrowth of Clostridium difficile, which can result in C difficile–associated diarrhea (CDAD).12 This can occur with oral, parenteral, and even topical antibiotics.11 Researchers have investigated whether probiotics can prevent this opportunistic C difficile overgrowth.
A 2012 meta-analysis of 20 trials (N = 38,180) found probiotic prophylaxis prevented CDAD in both inpatients and outpatients while not increasing the incidence of significant adverse effects.12 Probiotics decreased the incidence of CDAD by 66% (pooled RR, 0.34).12 Adverse events occurred in 9.3% of patients taking probiotics, compared with 12.6% of controls (RR, 0.82).12
Conversely, a 2008 review of four studies (N = 336) concluded there is insufficient evidence for using probiotics to treat CDAD, either as monotherapy or adjunct therapy.11 One trial in this meta-analysis (N = 124) found patients who received the probiotic Saccharomyces boulardii in addition to antibiotic therapy were significantly less likely to experience CDAD recurrence than those who received placebo (RR, 0.59).11 However, this benefit was not found in the other trials in this meta-analysis.11
The PLACIDE trial found probiotics did not prevent CDAD in hospitalized patients older than 65; 0.8% of patients who received probiotics developed CDAD, compared to 1.2% in the placebo group (RR, 0.71).22
Helicobacter pylori infection. The triple-therapy regimen of a proton pump inhibitor plus the antibiotics clarithromycin and amoxicillin is the recommended treatment for H pylori infection.13 Associated adverse effects include diarrhea and decreased eradication rates, in part due to antibiotic resistance. Certain Lactobacillus species have been shown to inhibit or kill H pylori in vitro,13 and evidence from several meta-analyses suggests probiotics should be an adjunct therapy for the treatment of H pylori.
In a meta-analysis of 10 RCTs (N = 963), fermented milk-based probiotics improved H pylori eradication rates by 5% to 15%.14 In another meta-analysis that evaluated five RCTs (N = 1,307), S boulardii significantly increased the H pylori eradication rate when used as an adjunct to triple therapy (RR, 1.13) and reduced the rate of treatment related adverse effects (RR, 0.46).13 In a third meta-analysis of 10 trials (N = 1,469), Lactobacillus supplementation increased H pylori eradication rates (OR, 2.1) while decreasing the overall incidence of adverse effects (OR, 0.3).15
Next: For inflammatory bowel disease, probiotics are unlikely to help >>
FOR INFLAMMATORY BOWEL DISEASE, PROBIOTICS ARE UNLIKELY TO HELP
Current therapies for Crohn disease and ulcerative colitis, such as corticosteroids and other immunosuppressive agents, are effective but have significant adverse events.18 Researchers explored whether probiotics might help treat these diseases by improving immune response, the balance of microbes in the GI tract, and the intestinal barrier.18
Crohn disease. In a meta-analysis that was able to identify only one small RCT (N = 11), 80% of patients receiving probiotic treatment went into remission, compared to 83% in the placebo group (OR, 0.80).16 Researchers concluded there was insufficient evidence for the use of probiotics for inducing remission in Crohn disease.
Another meta-analysis of seven small studies (N = 160) found no significant evidence supporting probiotic use for maintaining remission in Crohn disease compared with aminosalicylates or azathioprine.17 One small study in this review found there was a benefit to combining S boulardii with a reduced level of standard maintenance therapy when compared to standard therapy alone, but this difference was not statistically significant.17
Ulcerative colitis. A systematic review of four RCTs (N = 244) that compared conventional treatment alone to conventional treatment plus probiotics for remission or clinical improvement in patients with active ulcerative colitis found no significant differences between groups.18 Another meta-analysis of four studies (N = 587) found that compared to placebo or treatment with mesalazine, probiotics had no benefit for maintaining remission in ulcerative colitis.19 The rate of relapse was 40.1% in the probiotics group, compared to 34.1% in the mesalazine group. The number of adverse effects was similar in both groups.
Continue for most evidence suggests probiotics are useful for IBS >>
MOST EVIDENCE SUGGESTS PROBIOTICS ARE USEFUL FOR IBS
In RCTs, probiotic supplements—but not yogurt containing probiotics—reduced IBS symptoms. Research suggests that imbalances in GI flora, along with subsequent dysfunction in intestinal barriers and translocation of intestinal flora, may play a role in symptoms associated with IBS, such as abdominal pain, bloating, and diarrhea/constipation.20 There are few effective therapeutic options for patients with IBS.
In a systematic review of 19 RCTs (N = 1,650), probiotics were significantly more effective than placebo for patients with IBS, with an NNT of 4.21 This review did not evaluate the difference between various probiotic species and strains.
In an RCT (N = 122), the probiotic strain Bifidobacterium bifidum MIMBb75 was found to be safe and beneficial for treating IBS symptoms and improving patients’ quality of life.20 On a 7-point scale of global assessment of IBS symptoms, the score was reduced by 0.88 points in the group that received B bifidum MIMBb75 and 0.16 points in the placebo group (P < .0001). Almost half (47%) of the patients who received B bifidum MIMBb75 reported adequate relief, compared to 11% in the placebo group (P < .0001).
An RCT (N = 179) that compared yogurt containing probiotics to nonprobiotic yogurt found that the former had no benefits for treating IBS symptoms.23 After four weeks, 57% of patients who ate the probiotic yogurt reported adequate relief, compared to 53% of those who ate nonprobiotic yogurt (P = .71). After eight weeks, those numbers were 47% and 68%, respectively.23
REFERENCES
1. Hempel S, Newberry SJ, Maher AR, et al. Probiotics for the prevention and treatment of antibiotic-associated diarrhea: a systematic review and meta-analysis. JAMA. 2012;307:1959-1969.
2. Procter & Gamble. Align product information. www.aligngi.com/information-on-Align-probiotic-supplement. Accessed May 19, 2015.
3. Bayer HealthCare. Phillip’s Colon Health product information. http://phillipspro.com/en/home/product-information/index.php. Accessed May 19, 2015.
4. Nature’s Bounty. Nature’s Bounty Acidophilus Probiotic product label. http://images.vitaminimages.com/cdn/sd/pdf/L002610-NB.PDF. Accessed May 19, 2015.
5. Dannon. Activia. http://activia.us.com/probiotic-yogurt/activia. Accessed May 19, 2015.
6. Lifeway. Lifeway Kefir frequently asked questions. http://lifewaykefir.com/faq/. Accessed May 19, 2015.
7. Allen SJ, Martinez EG, Gregorio GV, et al. Probiotics for treating acute infectious diarrhoea. Cochrane Database Syst Rev. 2010;(11):CD003048.
8. McFarland LV. Meta-analysis of probiotics for the prevention of traveler’s diarrhea. Travel Med Infect Dis. 2007;5:97-105.
9. Hamad A, Fragkos KC, Forbes A. A systemic review and meta-analysis of probiotics for the management of radiation induced bowel disease. Clin Nutr. 2013;32:353-360.
10. Videlock EJ, Cremonini F. Meta-analysis: probiotics in antibiotic-associated diarrhoea. Aliment Pharmacol Ther. 2012;35:1355-1369.
11. Pillai A, Nelson RL. Probiotics for treatment of Clostridium difficile–associated colitis in adults. Cochrane Database Syst Rev. 2008;(1):CD004611.
12. Johnston BC, Ma SY, Goldenberg JZ, et al. Probiotics for the prevention of Clostridium difficile–associated diarrhea: a systematic review and meta-analysis. Ann Intern Med. 2012;157:878-888.
13. Szajewska H, Horvath A, Piwowarczyk A. Meta-analysis: the effects of Saccharomyces boulardii supplementation on Helicobacter pylori eradication rates and side effects during treatment. Aliment Pharmacol Ther. 2010;32:1069-1079.
14. Sachdeva A, Nagpal J. Effect of fermented milk-based probiotic preparations on Helicobacter pylori eradication: a systematic review and meta-analysis of randomized controlled trials. Eur J Gastroenterol Hepatol. 2009;21:45-53.
15. Wang ZH, Gao QY, Fang JY. Meta-analysis of the efficacy and safety of Lactobacillus-containing and Bifidobacterium-containing probiotic compound preparation in Helicobacter pylori eradication therapy. J Clin Gastroenterol. 2013;47:25-32.
16. Butterworth AD, Thomas AG, Akobeng AK. Probiotics for induction of remission in Crohn’s disease. Cochrane Database Syst Rev. 2008; 16:CD006634.
17. Rolfe VE, Fortun PJ, Hawkey CJ, et al. Probiotics for maintenance of remission in Crohn’s disease. Cochrane Database Syst Rev. 2006;(4):CD004826.
18. Mallon P, McKay D, Kirk SJ, et al. Probiotics for induction of remission in ulcerative colitis. Cochrane Database Syst Rev. 2007;(4):CD005573.
19. Naidoo K, Gordon M, Fagbemi AO, et al. Probiotics for maintenance of remission in ulcerative colitis. Cochrane Database Syst Rev. 2011;(12):CD007443.
20. Guglielmetti S, Mora D, Gschwender M, et al. Randomised clinical trial: Bifidobacterium bifidum MIMBb75 significantly alleviates irritable bowel syndrome and improves quality of life—a double-blind, placebo-controlled study. Aliment Pharmacol Ther. 2011;33:1123-1132.
21. Moayyedi P, Ford AC, Talley NJ, et al. The efficacy of probiotics in the treatment of irritable bowel syndrome: a systematic review. Gut. 2010; 59:325-332.
22. Allen SJ, Wareham K, Wang D, et al. Lactobacilli and bifidobacteria in the prevention of antibiotic-associated diarrhoea and Clostridium difficile diarrhoea in older inpatients (PLACIDE): a randomised, double-blind, placebo-controlled, multicentre trial. Lancet. 2013;382:1249-1257.
23. Roberts LM, McCahon D, Holder R, et al. A randomised controlled trial of a probiotic ‘functional food’ in the management of irritable bowel syndrome. BMC Gastroenterol. 2013;13:45.
Probiotics—live microorganisms that are consumed as supplements or food for purported health benefits—are a popular OTC remedy for various gastrointestinal (GI) ailments and other conditions, but the evidence supporting their use is mixed. Probiotics interact with the normal flora of the human body. They are believed to act by multiple mechanisms to deliver beneficial effects, including providing a protective barrier, altering intestinal pH to favor the growth of nonpathogenic bacteria, enhancing the host’s immunologic response, producing antimicrobial substances, and directly competing with pathogenic bacteria for receptors in the GI tract1 (see “The normal human intestinal flora,”).
In the United States, Lactobacillus and Bifidobacterium are the probiotic genera that are most commonly used. (For a list of the specific probiotic species found in five popular products, see Table 1.2-6) The review that follows examines the evidence for using probiotics for select GI ailments, including several types of diarrheal illness, inflammatory bowel disease (Crohn disease and ulcerative colitis), and irritable bowel syndrome (IBS). These findings are summarized in Table 2.1,7-21
Continue for probiotics may help with some types of diarrhea >>
PROBIOTICS MAY HELP WITH SOME TYPES OF DIARRHEA
Acute infectious diarrhea. Viruses, bacteria, and parasites cause acute infectious diarrhea, and probiotics are thought to act against these pathogens by competing for available nutrients and pattern-recognition receptors in the GI endothelium, acidifying the local environment, and increasing immune responses within the GI tract. In a meta-analysis of 63 studies (N = 8,014) that used multiple strains and dosages of probiotics, investigators found probiotics shortened the duration of acute infectious diarrhea by approximately 24 h.7 Probiotics also reduced both the risk for diarrhea lasting longer than four days (relative risk [RR], 0.41) and stool frequency on day 2 of illness (mean difference of 0.80 stools).
Traveler’s diarrhea. The incidence of traveler’s diarrhea is > 50% for travel to high-risk areas such as the Middle East, North Africa, Latin America, and Southeast Asia, and 5% to 10% when traveling to areas such as North America, Northern Europe, the United Kingdom, Australia, and New Zealand.8 Traveler’s diarrhea may be caused by ingesting food and liquids contaminated with fecal material. Symptoms include diarrhea, cramps, and nausea that, if untreated, typically last from two to six days but can last for as long as a month.8
In a meta-analysis of 12 studies (N = 5,171) that evaluated various probiotic strains, researchers found probiotics effectively prevented traveler’s diarrhea in US and European travelers who visited a variety of vacation spots (pooled RR, 0.85).8 No serious adverse events were reported.
Radiation-induced diarrhea. Radiation treatments to the abdomen and pelvis can damage the lower GI tract and cause diarrhea. The pooled results from a meta-analysis that included six studies (N = 1,449) significantly favored the use of probiotics over placebo for decreasing the incidence of radiation-induced diarrhea (odds ratio [OR], 0.44).9 Probiotic use also was associated with decreased loperamide use (OR, 0.29) and decreased incidence of watery stools (OR, 0.36), but these outcomes did not reach statistical significance.
Antibiotic-associated diarrhea. Antibiotic use has long been associated with the development of diarrheal illness, sometimes due to the acceleration of GI motility (eg, erythromycin) or by causing osmotic diarrhea by decreasing GI bacteria that assist in carbohydrate breakdown.11 A meta-analysis that evaluated 63 randomized controlled trials (RCTs) (N = 11,811) showed that probiotics are effective for treating and preventing antibiotic-associated diarrhea (AAD).1 There was a statistically significant reduction in AAD among patients who received probiotics (RR, 0.58; number needed to treat [NNT], 13). Most of the studies in this meta-analysis used a Lactobacillus probiotic alone or in combination with another probiotic. Researchers did not analyze whether the efficacy varied by patient population, probiotic used, causative antibiotic, or duration of treatment.1 Another meta-analysis of 34 studies (N = 4,138) also found probiotic therapy can prevent AAD.10 The pooled RR for AAD was 0.53 for patients treated with probiotics compared to placebo, with an NNT of 8. The effects remained significant when results were grouped by probiotic species, patient age, and duration of antibiotic treatment. Among a subgroup of patients in this meta-analysis who were being treated for Helicobacter pylori, the pooled RR of AAD was 0.37 and the NNT was 5.10 However, the 2013 PLACIDE trial (N = 17,420) found no significant decrease in AAD rates in hospitalized patients older than 65 being treated with antibiotics who received probiotics (RR, 1.04).22
Clostridium difficile–associated diarrhea. As we know, antibiotics can disrupt the normal GI flora and permit overgrowth of Clostridium difficile, which can result in C difficile–associated diarrhea (CDAD).12 This can occur with oral, parenteral, and even topical antibiotics.11 Researchers have investigated whether probiotics can prevent this opportunistic C difficile overgrowth.
A 2012 meta-analysis of 20 trials (N = 38,180) found probiotic prophylaxis prevented CDAD in both inpatients and outpatients while not increasing the incidence of significant adverse effects.12 Probiotics decreased the incidence of CDAD by 66% (pooled RR, 0.34).12 Adverse events occurred in 9.3% of patients taking probiotics, compared with 12.6% of controls (RR, 0.82).12
Conversely, a 2008 review of four studies (N = 336) concluded there is insufficient evidence for using probiotics to treat CDAD, either as monotherapy or adjunct therapy.11 One trial in this meta-analysis (N = 124) found patients who received the probiotic Saccharomyces boulardii in addition to antibiotic therapy were significantly less likely to experience CDAD recurrence than those who received placebo (RR, 0.59).11 However, this benefit was not found in the other trials in this meta-analysis.11
The PLACIDE trial found probiotics did not prevent CDAD in hospitalized patients older than 65; 0.8% of patients who received probiotics developed CDAD, compared to 1.2% in the placebo group (RR, 0.71).22
Helicobacter pylori infection. The triple-therapy regimen of a proton pump inhibitor plus the antibiotics clarithromycin and amoxicillin is the recommended treatment for H pylori infection.13 Associated adverse effects include diarrhea and decreased eradication rates, in part due to antibiotic resistance. Certain Lactobacillus species have been shown to inhibit or kill H pylori in vitro,13 and evidence from several meta-analyses suggests probiotics should be an adjunct therapy for the treatment of H pylori.
In a meta-analysis of 10 RCTs (N = 963), fermented milk-based probiotics improved H pylori eradication rates by 5% to 15%.14 In another meta-analysis that evaluated five RCTs (N = 1,307), S boulardii significantly increased the H pylori eradication rate when used as an adjunct to triple therapy (RR, 1.13) and reduced the rate of treatment related adverse effects (RR, 0.46).13 In a third meta-analysis of 10 trials (N = 1,469), Lactobacillus supplementation increased H pylori eradication rates (OR, 2.1) while decreasing the overall incidence of adverse effects (OR, 0.3).15
Next: For inflammatory bowel disease, probiotics are unlikely to help >>
FOR INFLAMMATORY BOWEL DISEASE, PROBIOTICS ARE UNLIKELY TO HELP
Current therapies for Crohn disease and ulcerative colitis, such as corticosteroids and other immunosuppressive agents, are effective but have significant adverse events.18 Researchers explored whether probiotics might help treat these diseases by improving immune response, the balance of microbes in the GI tract, and the intestinal barrier.18
Crohn disease. In a meta-analysis that was able to identify only one small RCT (N = 11), 80% of patients receiving probiotic treatment went into remission, compared to 83% in the placebo group (OR, 0.80).16 Researchers concluded there was insufficient evidence for the use of probiotics for inducing remission in Crohn disease.
Another meta-analysis of seven small studies (N = 160) found no significant evidence supporting probiotic use for maintaining remission in Crohn disease compared with aminosalicylates or azathioprine.17 One small study in this review found there was a benefit to combining S boulardii with a reduced level of standard maintenance therapy when compared to standard therapy alone, but this difference was not statistically significant.17
Ulcerative colitis. A systematic review of four RCTs (N = 244) that compared conventional treatment alone to conventional treatment plus probiotics for remission or clinical improvement in patients with active ulcerative colitis found no significant differences between groups.18 Another meta-analysis of four studies (N = 587) found that compared to placebo or treatment with mesalazine, probiotics had no benefit for maintaining remission in ulcerative colitis.19 The rate of relapse was 40.1% in the probiotics group, compared to 34.1% in the mesalazine group. The number of adverse effects was similar in both groups.
Continue for most evidence suggests probiotics are useful for IBS >>
MOST EVIDENCE SUGGESTS PROBIOTICS ARE USEFUL FOR IBS
In RCTs, probiotic supplements—but not yogurt containing probiotics—reduced IBS symptoms. Research suggests that imbalances in GI flora, along with subsequent dysfunction in intestinal barriers and translocation of intestinal flora, may play a role in symptoms associated with IBS, such as abdominal pain, bloating, and diarrhea/constipation.20 There are few effective therapeutic options for patients with IBS.
In a systematic review of 19 RCTs (N = 1,650), probiotics were significantly more effective than placebo for patients with IBS, with an NNT of 4.21 This review did not evaluate the difference between various probiotic species and strains.
In an RCT (N = 122), the probiotic strain Bifidobacterium bifidum MIMBb75 was found to be safe and beneficial for treating IBS symptoms and improving patients’ quality of life.20 On a 7-point scale of global assessment of IBS symptoms, the score was reduced by 0.88 points in the group that received B bifidum MIMBb75 and 0.16 points in the placebo group (P < .0001). Almost half (47%) of the patients who received B bifidum MIMBb75 reported adequate relief, compared to 11% in the placebo group (P < .0001).
An RCT (N = 179) that compared yogurt containing probiotics to nonprobiotic yogurt found that the former had no benefits for treating IBS symptoms.23 After four weeks, 57% of patients who ate the probiotic yogurt reported adequate relief, compared to 53% of those who ate nonprobiotic yogurt (P = .71). After eight weeks, those numbers were 47% and 68%, respectively.23
REFERENCES
1. Hempel S, Newberry SJ, Maher AR, et al. Probiotics for the prevention and treatment of antibiotic-associated diarrhea: a systematic review and meta-analysis. JAMA. 2012;307:1959-1969.
2. Procter & Gamble. Align product information. www.aligngi.com/information-on-Align-probiotic-supplement. Accessed May 19, 2015.
3. Bayer HealthCare. Phillip’s Colon Health product information. http://phillipspro.com/en/home/product-information/index.php. Accessed May 19, 2015.
4. Nature’s Bounty. Nature’s Bounty Acidophilus Probiotic product label. http://images.vitaminimages.com/cdn/sd/pdf/L002610-NB.PDF. Accessed May 19, 2015.
5. Dannon. Activia. http://activia.us.com/probiotic-yogurt/activia. Accessed May 19, 2015.
6. Lifeway. Lifeway Kefir frequently asked questions. http://lifewaykefir.com/faq/. Accessed May 19, 2015.
7. Allen SJ, Martinez EG, Gregorio GV, et al. Probiotics for treating acute infectious diarrhoea. Cochrane Database Syst Rev. 2010;(11):CD003048.
8. McFarland LV. Meta-analysis of probiotics for the prevention of traveler’s diarrhea. Travel Med Infect Dis. 2007;5:97-105.
9. Hamad A, Fragkos KC, Forbes A. A systemic review and meta-analysis of probiotics for the management of radiation induced bowel disease. Clin Nutr. 2013;32:353-360.
10. Videlock EJ, Cremonini F. Meta-analysis: probiotics in antibiotic-associated diarrhoea. Aliment Pharmacol Ther. 2012;35:1355-1369.
11. Pillai A, Nelson RL. Probiotics for treatment of Clostridium difficile–associated colitis in adults. Cochrane Database Syst Rev. 2008;(1):CD004611.
12. Johnston BC, Ma SY, Goldenberg JZ, et al. Probiotics for the prevention of Clostridium difficile–associated diarrhea: a systematic review and meta-analysis. Ann Intern Med. 2012;157:878-888.
13. Szajewska H, Horvath A, Piwowarczyk A. Meta-analysis: the effects of Saccharomyces boulardii supplementation on Helicobacter pylori eradication rates and side effects during treatment. Aliment Pharmacol Ther. 2010;32:1069-1079.
14. Sachdeva A, Nagpal J. Effect of fermented milk-based probiotic preparations on Helicobacter pylori eradication: a systematic review and meta-analysis of randomized controlled trials. Eur J Gastroenterol Hepatol. 2009;21:45-53.
15. Wang ZH, Gao QY, Fang JY. Meta-analysis of the efficacy and safety of Lactobacillus-containing and Bifidobacterium-containing probiotic compound preparation in Helicobacter pylori eradication therapy. J Clin Gastroenterol. 2013;47:25-32.
16. Butterworth AD, Thomas AG, Akobeng AK. Probiotics for induction of remission in Crohn’s disease. Cochrane Database Syst Rev. 2008; 16:CD006634.
17. Rolfe VE, Fortun PJ, Hawkey CJ, et al. Probiotics for maintenance of remission in Crohn’s disease. Cochrane Database Syst Rev. 2006;(4):CD004826.
18. Mallon P, McKay D, Kirk SJ, et al. Probiotics for induction of remission in ulcerative colitis. Cochrane Database Syst Rev. 2007;(4):CD005573.
19. Naidoo K, Gordon M, Fagbemi AO, et al. Probiotics for maintenance of remission in ulcerative colitis. Cochrane Database Syst Rev. 2011;(12):CD007443.
20. Guglielmetti S, Mora D, Gschwender M, et al. Randomised clinical trial: Bifidobacterium bifidum MIMBb75 significantly alleviates irritable bowel syndrome and improves quality of life—a double-blind, placebo-controlled study. Aliment Pharmacol Ther. 2011;33:1123-1132.
21. Moayyedi P, Ford AC, Talley NJ, et al. The efficacy of probiotics in the treatment of irritable bowel syndrome: a systematic review. Gut. 2010; 59:325-332.
22. Allen SJ, Wareham K, Wang D, et al. Lactobacilli and bifidobacteria in the prevention of antibiotic-associated diarrhoea and Clostridium difficile diarrhoea in older inpatients (PLACIDE): a randomised, double-blind, placebo-controlled, multicentre trial. Lancet. 2013;382:1249-1257.
23. Roberts LM, McCahon D, Holder R, et al. A randomised controlled trial of a probiotic ‘functional food’ in the management of irritable bowel syndrome. BMC Gastroenterol. 2013;13:45.
What You Must Know Before You Recommend a Probiotic
› Consider probiotics for patients with acute infectious diarrhea, antibiotic-associated diarrhea, or Clostridium difficile-associated diarrhea. A
› Do not recommend probiotics for preventing or treating Crohn’s disease or ulcerative colitis. B
› Consider the probiotic Bifidobacterium bifidum MIMBb75 for patients with irritable bowel syndrome. B
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
Probiotics—live micoorganisms that are consumed as supplements or food for purported health benefits—are a popular over-the-counter remedy for various gastrointestinal (GI) ailments and other conditions, but the evidence supporting their use is mixed. Probiotics interact with the normal flora of the human body. They are believed to act by multiple mechanisms to deliver beneficial effects, including providing a protective barrier, altering intestinal pH to favor the growth of nonpathogenic bacteria, enhancing the host’s immunologic response, producing antimicrobial substances, and directly competing with pathogenic bacteria for receptors in the GI tract.1 (See “The normal human intestinal flora.”)
In the United States, Lactobacillus and Bifidobacterium are the probiotic genera that are most commonly used. (For a list of the specific probiotic species found in 5 popular products, see TABLE 1.2-6) The review that follows examines the evidence for using probiotics for select GI ailments, including several types of diarrheal illnesses, inflammatory bowel disease (Crohn’s disease and ulcerative colitis), and irritable bowel syndrome (IBS). These findings are summarized in TABLE 2.1,7-21
The human body contains approximately 1014 prokaryotic organisms, with a biomass of >1 kg. Most of these organisms are indigenous and stable, although transient members such as enteric pathogens can be found.
The gastrointestinal tract is sterile at birth but is colonized immediately, and each individual has marked variations in microbial composition. The complex symbiotic relationship between the normal intestinal flora and the human host is beneficial to both. These microbes utilize complex carbohydrates undigested by the host as energy. Fermentation results in the formation of short-chain fatty acids, which can provide up to 15% of human energy requirements.
In addition to these metabolic benefits, microbial flora dampen the human inflammatory response, induce immunosuppressive T cells (Tregs), and competitively exclude pathogens.
Colonic epithelium is nourished and proliferates in the presence of normal intestinal flora. Disruption of the normal flora can cause disease.
SOURCE: Neish AS. Microbes in gastrointestinal health and disease. Gastroenterology. 2009;136:65-80.
Probiotics may help with some types of diarrhea
Acute infectious diarrhea. Viruses, bacteria, and parasites cause acute infectious diarrhea, and probiotics are thought to act against these pathogens by competing for available nutrients and pattern recognition receptors in the GI endothelium, acidifying the local environment, and increasing immune responses within the GI tract. In a meta-analysis of 63 studies (N=8014) that used multiple strains and dosages of probiotics, investigators found probiotics shortened the duration of acute infectious diarrhea by approximately 24 hours (95% confidence interval [CI], 15.9-33.6 hours).7 Probiotics also reduced both the risk of diarrhea lasting longer than 4 days (relative risk [RR]=0.41; 95% CI, 0.32-0.53) and stool frequency on Day 2 of illness (mean difference of 0.80 stools; 95% CI, 0.45-1.14).
Traveler’s diarrhea. The incidence of traveler’s diarrhea is >50% when traveling to high-risk areas such as the Middle East, North Africa, Latin America, and Southeast Asia, and 5% to 10% when traveling to areas such as North America, Northern Europe, the United Kingdom, Australia, and New Zealand.8 Traveler’s diarrhea may be caused by ingesting food and liquids contaminated with fecal material. Symptoms include diarrhea, cramps, and nausea that if untreated typically last from 2 to 6 days but can last for as long as a month.8
In a meta-analysis of 12 studies (N=5171) that evaluated various probiotic strains, researchers found probiotics effectively prevented traveler’s diarrhea in US and European travelers who visited a variety of vacation spots (pooled RR=0.85; 95% CI, 0.79-0.91).8 No serious adverse events were reported.
Radiation-induced diarrhea. Radiation treatments to the abdomen and pelvis can damage the lower GI tract and cause diarrhea. The pooled results from a meta-analysis that included 6 studies (N=1449) significantly favored the use of probiotics over placebo for decreasing the incidence of radiation-induced diarrhea (odds ratio [OR]=0.44; 95% CI, 0.21-0.92).9 Probiotics use also was associated with decreased loperamide use (OR=0.29; 95% CI, 0.01-6.80) and decreased incidence of watery stools (OR=0.36; 95% CI, 0.05-2.81), but these outcomes did not reach statistical significance.
Antibiotic-associated diarrhea. Antibiotic use has long been associated with the development of diarrheal illness, sometimes due to the acceleration of GI motility (eg, erythromycin) or by causing osmotic diarrhea by decreasing GI bacteria that assist in carbohydrate breakdown.11 A meta-analysis that evaluated 63 randomized controlled trials (RCTs) (N=11,811) showed that probiotics are effective for treating and preventing antibiotic-associated diarrhea (AAD).1 There was a statistically significant reduction in AAD among patients who received probiotics (RR=0.58; 95% CI, 0.50-0.68; number needed to treat [NNT]=13). Most of the studies in this meta-analysis used a Lactobacillus probiotic alone or in combination with another probiotic. Researchers did not analyze whether the efficacy varied by patient population, probiotic used, causative antibiotic, or duration of treatment.1
Another meta-analysis of 34 studies (N=4138) also found probiotic therapy can prevent AAD.10 The pooled RR for AAD was 0.53 (95% CI, 0.44-0.63) for patients treated with probiotics compared to placebo, with an NNT of 8 (95% CI, 7-11). The effects remained significant when results were grouped by probiotic species, patient age, and duration of antibiotic treatment. Among a subgroup of patients in this meta-analysis who were being treated for Helicobacter pylori, the pooled RR of AAD was 0.37 (95% CI, 0.20-0.69) and the NNT was 5 (95% CI, 4-10).10 However, the 2013 PLACIDE trial (N=17,420) found no significant decrease in AAD rates in hospitalized patients over age 65 years being treated with antibiotics who received probiotics (RR=1.04; 95% CI, 0.84-1.28).22
Clostridium difficile-associated diarrhea. As we know, antibiotics can disrupt the normal GI flora and permit overgrow of Clostridium difficile, which can result in C. difficile-associated diarrhea (CDAD).12 This can occur with oral, parenteral, and even topical antibiotics.11 Researchers have investigated whether probiotics can prevent this opportunistic C. difficile overgrowth.
A 2012 meta-analysis of 20 trials (N=38,180) found probiotic prophylaxis prevented CDAD in both inpatients and outpatients while not increasing the incidence of significant adverse effects.12 Probiotics decreased the incidence of CDAD by 66% (pooled RR=0.34, 95% CI, 0.24-0.49).12 Adverse events occurred in 9.3% of patients taking probiotics, compared with 12.6% of controls (RR=0.82, 95% CI, 0.65-1.05).12
Conversely, a 2008 review of 4 studies (N=336) concluded there is insufficient evidence for using probiotics to treat CDAD, either as monotherapy or adjunct therapy.11 One trial in this meta-analysis (N=124) found patients who received the probiotic Saccharomyces boulardii in addition to antibiotic therapy were significantly less likely to experience CDAD recurrence than those who received placebo (RR=0.59; 95% CI, 0.35-0.98).11 However, this benefit was not found in the other trials in this meta-analysis.11
The PLACIDE trial found probiotics did not prevent CDAD in hospitalized patients over age 65 years; 0.8% of patients who received probiotics developed CDAD, compared to 1.2% in the placebo group (RR=0.71, 95% CI, 0.34-1.47).22
Helicobacter pylori infection. The triple therapy regimen of a proton pump inhibitor plus the antibiotics clarithromycin and amoxicillin is the recommended treatment for H. pylori infection.13 Problems with this treatment include adverse effects such as diarrhea and decreased eradication rates, in part due to antibiotic resistance. Certain Lactobacillus species have been shown to inhibit or kill H. pylori in vitro,13 and evidence from several meta-analyses suggests probiotics should be an adjunct therapy when treating H. pylori.
In a meta-analysis of 10 RCTs (N=963), fermented milk-based probiotics improved H. pylori eradication rates by 5% to 15%.14 In another meta-analysis that evaluated 5 RCTs (N=1307), S. boulardii significantly increased the H. pylori eradication rate when used as an adjunct to triple therapy (RR=1.13; 95% CI, 1.05-1.21) and reduced the rate of treatment-related adverse effects (RR=0.46; 95% CI, 0.3-0.7).13 In a third meta-analysis of 10 trials (N=1469), Lactobacillus supplementation increased H. pylori eradication rates (OR=2.1; 95% CI, 1.4-3.1) while decreasing the overall incidence of adverse effects (OR=0.3; 0.1-0.8).15
For inflammatory bowel disease, probiotics are unlikely to help
Current therapies for Crohn’s disease and ulcerative colitis, such as corticosteroids and other immunosuppressive agents, are effective but have significant adverse events.18 Researchers explored whether probiotics might help treat these diseases by improving immune response, the balance of microbes in the GI tract, and the intestinal barrier.18
Crohn’s disease. In a meta-analysis that was able to identify only one small RCT (N=11), 80% of patients receiving probiotic treatment went into remission, compared to 83% in the placebo group (OR=0.80; 95% CI, 0.04–17.20).16 Researchers concluded there was insufficient evidence for the use of probiotics for inducing remission in Crohn’s disease.
Another meta-analysis of 7 small studies (N=160) found no significant evidence supporting probiotic use for maintaining remission in Crohn’s disease compared with aminosalicylates or azathioprine.17 One small study in this review found there was a benefit to combining S. boulardii with a reduced level of standard maintenance therapy when compared to standard therapy alone, but this difference was not statistically significant.17
Ulcerative colitis. A systematic review of 4 RCTs (N=244) that compared conventional treatment alone to conventional treatment plus probiotics for remission or clinical improvement in patients with active ulcerative colitis found no significant differences between groups.18 Another meta-analysis of 4 studies (N=587) found that compared to placebo or treatment with mesalazine, probiotics had no benefit for maintaining remission in ulcerative colitis.19 The rate of relapse was 40.1% in the probiotics group compared to 34.1% in the mesalazine group. The number of adverse effects was similar in both groups.
Most evidence suggests probiotics are useful for IBS
Research suggests that imbalances in GI flora, along with subsequent dysfunction in intestinal barriers and translocation of intestinal flora, may play a role in symptoms associated with IBS, such as abdominal pain, bloating, and diarrhea/constipation.20 There are few effective therapeutic options for patients suffering with IBS.
In a systematic review of 19 RCTs (N=1650), probiotics were significantly more effective than placebo for patients with IBS, with an NNT of 4 (95% CI, 3-12.5).21 This review did not evaluate the difference between various probiotic species and strains.
In an RCT (N=122), the probiotic strain Bifidobacterium bifidum MIMBb75 was found to be safe and beneficial for treating IBS symptoms and improving patients’ quality of life.20 On a 7-point scale of global assessment of IBS symptoms, the score was reduced by 0.88 points (95% CI, 0.69-1.07) in the group that received B. bifidum MIMBb75 and 0.16 points (95% CI, -0.32-0.00) in the placebo group (P<0.0001). Almost half (47%) of the patients who received B. bifidum MIMBb75 reported adequate relief, compared to 11% in the placebo group (P<.0001).
An RCT (N=179) that compared yogurt that contained probiotics to non-probiotic yogurt found the probiotic yogurt had no benefits for treating IBS symptoms.23 After 4 weeks, 57% of patients who ate the probiotic yogurt reported adequate relief, compared to 53% of those who ate non-probiotic yogurt (P=0.71). After 8 weeks, those numbers were 47% and 68%, respectively.23
CORRESPONDENCE
Erik R. Clauson, DO, Nellis Family Medicine Residency, 99 MDOS/SGOF, 4700 Las Vegas Boulevard North, Nellis Air Force Base, NV 89191; erik.clauson.1@us.af.mil
REFERENCES
1. Hempel S, Newberry SJ, Maher AR, et al. Probiotics for the prevention and treatment of antibiotic-associated diarrhea: a systematic review and meta-analysis. JAMA. 2012;307:1959-1969.
2. Procter & Gamble. Align product information. Procter & Gamble Align Web site. Available at: http://www.aligngi.com/information-on-Align-probiotic-supplement. Accessed February 13, 2015.
3. Bayer HealthCare. Phillip’s Colon Health product information. Bayer HealthCare Phillip’s Colon Health Web site. Available at: http://phillipspro.com/en/home/product-information/index.php. Accessed February 13, 2015.
4. Nature’s Bounty. Nature’s Bounty Acidophilus Probiotic product label. Nature’s Bounty Web site. Available at: http://images.vitaminimages.com/cdn/sd/pdf/L002610-NB.PDF. Accessed February 13, 2015.
5. Dannon. Activia. Dannon Activia Web site. Available at: http://activia.us.com/probiotic-yogurt/activia. Accessed February 13, 2015.
6. Lifeway. Lifeway Kefir frequently asked questions. Lifeway Kefir Web site. Available at: http://lifewaykefir.com/faq/. Accessed February 13, 2015.
7. Allen SJ, Martinez EG, Gregorio GV, et al. Probiotics for treating acute infectious diarrhoea. Cochrane Database Syst Rev. 2010;(11):CD003048.
8. McFarland LV. Meta-analysis of probiotics for the prevention of traveler’s diarrhea. Travel Med Infect Dis. 2007;5:97-105.
9. Hamad A, Fragkos KC, Forbes A. A systemic review and meta-analysis of probiotics for the management of radiation induced bowel disease. Clin Nutr. 2013;32:353-360.
10. Videlock EJ, Cremonini F. Meta-analysis: probiotics in antibiotic-associated diarrhoea. Aliment Pharmacol Ther. 2012;35:1355-1369.
11. Pillai A, Nelson RL. Probiotics for treatment of Clostridium difficile-associated colitis in adults. Cochrane Database Syst Rev. 2008;(1):CD004611.
12. Johnston BC, Ma SY, Goldenberg JZ, et al. Probiotics for the prevention of Clostridium difficile-associated diarrhea: a systematic review and meta-analysis. Ann Intern Med. 2012;157:878-888.
13. Szajewska H, Horvath A, Piwowarczyk A. Meta-analysis: the effects of Saccharomyces boulardii supplementation on Helicobacter pylori eradication rates and side effects during treatment. Aliment Pharmacol Ther. 2010;32:1069-1079.
14. Sachdeva A, Nagpal J. Effect of fermented milk-based probiotic preparations on Helicobacter pylori eradication: a systematic review and meta-analysis of randomized-controlled trials. Eur J Gastroenterol Hepatol. 2009;21:45-53.
15. Wang ZH, Gao QY, Fang JY. Meta-analysis of the efficacy and safety of Lactobacillus-containing and Bifidobacterium-containing probiotic compound preparation in Helicobacter pylori eradication therapy. J Clin Gastroenterol. 2013;47:25-32.
16. Butterworth AD, Thomas AG, Akobeng AK. Probiotics for induction of remission in Crohn’s disease. Cochrane Database Syst Rev. 2008;16:CD006634.
17. Rolfe VE, Fortun PJ, Hawkey CJ, et al. Probiotics for maintenance of remission in Crohn’s disease. Cochrane Database Syst Rev. 2006;(4):CD004826.
18. Mallon P, McKay D, Kirk SJ, et al. Probiotics for induction of remission in ulcerative colitis. Cochrane Database Syst Rev. 2007;(4):CD005573.
19. Naidoo K, Gordon M, Fagbemi AO, et al. Probiotics for maintenance of remission in ulcerative colitis. Cochrane Database Syst Rev. 2011;(12):CD007443.
20. Guglielmetti S, Mora D, Gschwender M, et al. Randomised clinical trial: Bifidobacterium bifidum MIMBb75 significantly alleviates irritable bowel syndrome and improves quality of life–– a double-blind, placebo-controlled study. Aliment Pharmacol Ther. 2011;33:1123-1132.
21. Moayyedi P, Ford AC, Talley NJ, et al. The efficacy of probiotics in the treatment of irritable bowel syndrome: a systematic review. Gut. 2010;59:325-332.
22. Allen SJ, Wareham K, Wang D, et al. Lactobacilli and bifidobacteria in the prevention of antibiotic-associated diarrhoea and Clostridium difficile diarrhoea in older inpatients (PLACIDE): a randomised, double-blind, placebo-controlled, multicentre trial. Lancet. 2013;382:1249-1257.
23. Roberts LM, McCahon D, Holder R, et al. A randomised controlled trial of a probiotic ‘functional food’ in the management of irritable bowel syndrome. BMC Gastroenterol. 2013;13:45.
› Consider probiotics for patients with acute infectious diarrhea, antibiotic-associated diarrhea, or Clostridium difficile-associated diarrhea. A
› Do not recommend probiotics for preventing or treating Crohn’s disease or ulcerative colitis. B
› Consider the probiotic Bifidobacterium bifidum MIMBb75 for patients with irritable bowel syndrome. B
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
Probiotics—live micoorganisms that are consumed as supplements or food for purported health benefits—are a popular over-the-counter remedy for various gastrointestinal (GI) ailments and other conditions, but the evidence supporting their use is mixed. Probiotics interact with the normal flora of the human body. They are believed to act by multiple mechanisms to deliver beneficial effects, including providing a protective barrier, altering intestinal pH to favor the growth of nonpathogenic bacteria, enhancing the host’s immunologic response, producing antimicrobial substances, and directly competing with pathogenic bacteria for receptors in the GI tract.1 (See “The normal human intestinal flora.”)
In the United States, Lactobacillus and Bifidobacterium are the probiotic genera that are most commonly used. (For a list of the specific probiotic species found in 5 popular products, see TABLE 1.2-6) The review that follows examines the evidence for using probiotics for select GI ailments, including several types of diarrheal illnesses, inflammatory bowel disease (Crohn’s disease and ulcerative colitis), and irritable bowel syndrome (IBS). These findings are summarized in TABLE 2.1,7-21
The human body contains approximately 1014 prokaryotic organisms, with a biomass of >1 kg. Most of these organisms are indigenous and stable, although transient members such as enteric pathogens can be found.
The gastrointestinal tract is sterile at birth but is colonized immediately, and each individual has marked variations in microbial composition. The complex symbiotic relationship between the normal intestinal flora and the human host is beneficial to both. These microbes utilize complex carbohydrates undigested by the host as energy. Fermentation results in the formation of short-chain fatty acids, which can provide up to 15% of human energy requirements.
In addition to these metabolic benefits, microbial flora dampen the human inflammatory response, induce immunosuppressive T cells (Tregs), and competitively exclude pathogens.
Colonic epithelium is nourished and proliferates in the presence of normal intestinal flora. Disruption of the normal flora can cause disease.
SOURCE: Neish AS. Microbes in gastrointestinal health and disease. Gastroenterology. 2009;136:65-80.
Probiotics may help with some types of diarrhea
Acute infectious diarrhea. Viruses, bacteria, and parasites cause acute infectious diarrhea, and probiotics are thought to act against these pathogens by competing for available nutrients and pattern recognition receptors in the GI endothelium, acidifying the local environment, and increasing immune responses within the GI tract. In a meta-analysis of 63 studies (N=8014) that used multiple strains and dosages of probiotics, investigators found probiotics shortened the duration of acute infectious diarrhea by approximately 24 hours (95% confidence interval [CI], 15.9-33.6 hours).7 Probiotics also reduced both the risk of diarrhea lasting longer than 4 days (relative risk [RR]=0.41; 95% CI, 0.32-0.53) and stool frequency on Day 2 of illness (mean difference of 0.80 stools; 95% CI, 0.45-1.14).
Traveler’s diarrhea. The incidence of traveler’s diarrhea is >50% when traveling to high-risk areas such as the Middle East, North Africa, Latin America, and Southeast Asia, and 5% to 10% when traveling to areas such as North America, Northern Europe, the United Kingdom, Australia, and New Zealand.8 Traveler’s diarrhea may be caused by ingesting food and liquids contaminated with fecal material. Symptoms include diarrhea, cramps, and nausea that if untreated typically last from 2 to 6 days but can last for as long as a month.8
In a meta-analysis of 12 studies (N=5171) that evaluated various probiotic strains, researchers found probiotics effectively prevented traveler’s diarrhea in US and European travelers who visited a variety of vacation spots (pooled RR=0.85; 95% CI, 0.79-0.91).8 No serious adverse events were reported.
Radiation-induced diarrhea. Radiation treatments to the abdomen and pelvis can damage the lower GI tract and cause diarrhea. The pooled results from a meta-analysis that included 6 studies (N=1449) significantly favored the use of probiotics over placebo for decreasing the incidence of radiation-induced diarrhea (odds ratio [OR]=0.44; 95% CI, 0.21-0.92).9 Probiotics use also was associated with decreased loperamide use (OR=0.29; 95% CI, 0.01-6.80) and decreased incidence of watery stools (OR=0.36; 95% CI, 0.05-2.81), but these outcomes did not reach statistical significance.
Antibiotic-associated diarrhea. Antibiotic use has long been associated with the development of diarrheal illness, sometimes due to the acceleration of GI motility (eg, erythromycin) or by causing osmotic diarrhea by decreasing GI bacteria that assist in carbohydrate breakdown.11 A meta-analysis that evaluated 63 randomized controlled trials (RCTs) (N=11,811) showed that probiotics are effective for treating and preventing antibiotic-associated diarrhea (AAD).1 There was a statistically significant reduction in AAD among patients who received probiotics (RR=0.58; 95% CI, 0.50-0.68; number needed to treat [NNT]=13). Most of the studies in this meta-analysis used a Lactobacillus probiotic alone or in combination with another probiotic. Researchers did not analyze whether the efficacy varied by patient population, probiotic used, causative antibiotic, or duration of treatment.1
Another meta-analysis of 34 studies (N=4138) also found probiotic therapy can prevent AAD.10 The pooled RR for AAD was 0.53 (95% CI, 0.44-0.63) for patients treated with probiotics compared to placebo, with an NNT of 8 (95% CI, 7-11). The effects remained significant when results were grouped by probiotic species, patient age, and duration of antibiotic treatment. Among a subgroup of patients in this meta-analysis who were being treated for Helicobacter pylori, the pooled RR of AAD was 0.37 (95% CI, 0.20-0.69) and the NNT was 5 (95% CI, 4-10).10 However, the 2013 PLACIDE trial (N=17,420) found no significant decrease in AAD rates in hospitalized patients over age 65 years being treated with antibiotics who received probiotics (RR=1.04; 95% CI, 0.84-1.28).22
Clostridium difficile-associated diarrhea. As we know, antibiotics can disrupt the normal GI flora and permit overgrow of Clostridium difficile, which can result in C. difficile-associated diarrhea (CDAD).12 This can occur with oral, parenteral, and even topical antibiotics.11 Researchers have investigated whether probiotics can prevent this opportunistic C. difficile overgrowth.
A 2012 meta-analysis of 20 trials (N=38,180) found probiotic prophylaxis prevented CDAD in both inpatients and outpatients while not increasing the incidence of significant adverse effects.12 Probiotics decreased the incidence of CDAD by 66% (pooled RR=0.34, 95% CI, 0.24-0.49).12 Adverse events occurred in 9.3% of patients taking probiotics, compared with 12.6% of controls (RR=0.82, 95% CI, 0.65-1.05).12
Conversely, a 2008 review of 4 studies (N=336) concluded there is insufficient evidence for using probiotics to treat CDAD, either as monotherapy or adjunct therapy.11 One trial in this meta-analysis (N=124) found patients who received the probiotic Saccharomyces boulardii in addition to antibiotic therapy were significantly less likely to experience CDAD recurrence than those who received placebo (RR=0.59; 95% CI, 0.35-0.98).11 However, this benefit was not found in the other trials in this meta-analysis.11
The PLACIDE trial found probiotics did not prevent CDAD in hospitalized patients over age 65 years; 0.8% of patients who received probiotics developed CDAD, compared to 1.2% in the placebo group (RR=0.71, 95% CI, 0.34-1.47).22
Helicobacter pylori infection. The triple therapy regimen of a proton pump inhibitor plus the antibiotics clarithromycin and amoxicillin is the recommended treatment for H. pylori infection.13 Problems with this treatment include adverse effects such as diarrhea and decreased eradication rates, in part due to antibiotic resistance. Certain Lactobacillus species have been shown to inhibit or kill H. pylori in vitro,13 and evidence from several meta-analyses suggests probiotics should be an adjunct therapy when treating H. pylori.
In a meta-analysis of 10 RCTs (N=963), fermented milk-based probiotics improved H. pylori eradication rates by 5% to 15%.14 In another meta-analysis that evaluated 5 RCTs (N=1307), S. boulardii significantly increased the H. pylori eradication rate when used as an adjunct to triple therapy (RR=1.13; 95% CI, 1.05-1.21) and reduced the rate of treatment-related adverse effects (RR=0.46; 95% CI, 0.3-0.7).13 In a third meta-analysis of 10 trials (N=1469), Lactobacillus supplementation increased H. pylori eradication rates (OR=2.1; 95% CI, 1.4-3.1) while decreasing the overall incidence of adverse effects (OR=0.3; 0.1-0.8).15
For inflammatory bowel disease, probiotics are unlikely to help
Current therapies for Crohn’s disease and ulcerative colitis, such as corticosteroids and other immunosuppressive agents, are effective but have significant adverse events.18 Researchers explored whether probiotics might help treat these diseases by improving immune response, the balance of microbes in the GI tract, and the intestinal barrier.18
Crohn’s disease. In a meta-analysis that was able to identify only one small RCT (N=11), 80% of patients receiving probiotic treatment went into remission, compared to 83% in the placebo group (OR=0.80; 95% CI, 0.04–17.20).16 Researchers concluded there was insufficient evidence for the use of probiotics for inducing remission in Crohn’s disease.
Another meta-analysis of 7 small studies (N=160) found no significant evidence supporting probiotic use for maintaining remission in Crohn’s disease compared with aminosalicylates or azathioprine.17 One small study in this review found there was a benefit to combining S. boulardii with a reduced level of standard maintenance therapy when compared to standard therapy alone, but this difference was not statistically significant.17
Ulcerative colitis. A systematic review of 4 RCTs (N=244) that compared conventional treatment alone to conventional treatment plus probiotics for remission or clinical improvement in patients with active ulcerative colitis found no significant differences between groups.18 Another meta-analysis of 4 studies (N=587) found that compared to placebo or treatment with mesalazine, probiotics had no benefit for maintaining remission in ulcerative colitis.19 The rate of relapse was 40.1% in the probiotics group compared to 34.1% in the mesalazine group. The number of adverse effects was similar in both groups.
Most evidence suggests probiotics are useful for IBS
Research suggests that imbalances in GI flora, along with subsequent dysfunction in intestinal barriers and translocation of intestinal flora, may play a role in symptoms associated with IBS, such as abdominal pain, bloating, and diarrhea/constipation.20 There are few effective therapeutic options for patients suffering with IBS.
In a systematic review of 19 RCTs (N=1650), probiotics were significantly more effective than placebo for patients with IBS, with an NNT of 4 (95% CI, 3-12.5).21 This review did not evaluate the difference between various probiotic species and strains.
In an RCT (N=122), the probiotic strain Bifidobacterium bifidum MIMBb75 was found to be safe and beneficial for treating IBS symptoms and improving patients’ quality of life.20 On a 7-point scale of global assessment of IBS symptoms, the score was reduced by 0.88 points (95% CI, 0.69-1.07) in the group that received B. bifidum MIMBb75 and 0.16 points (95% CI, -0.32-0.00) in the placebo group (P<0.0001). Almost half (47%) of the patients who received B. bifidum MIMBb75 reported adequate relief, compared to 11% in the placebo group (P<.0001).
An RCT (N=179) that compared yogurt that contained probiotics to non-probiotic yogurt found the probiotic yogurt had no benefits for treating IBS symptoms.23 After 4 weeks, 57% of patients who ate the probiotic yogurt reported adequate relief, compared to 53% of those who ate non-probiotic yogurt (P=0.71). After 8 weeks, those numbers were 47% and 68%, respectively.23
CORRESPONDENCE
Erik R. Clauson, DO, Nellis Family Medicine Residency, 99 MDOS/SGOF, 4700 Las Vegas Boulevard North, Nellis Air Force Base, NV 89191; erik.clauson.1@us.af.mil
REFERENCES
1. Hempel S, Newberry SJ, Maher AR, et al. Probiotics for the prevention and treatment of antibiotic-associated diarrhea: a systematic review and meta-analysis. JAMA. 2012;307:1959-1969.
2. Procter & Gamble. Align product information. Procter & Gamble Align Web site. Available at: http://www.aligngi.com/information-on-Align-probiotic-supplement. Accessed February 13, 2015.
3. Bayer HealthCare. Phillip’s Colon Health product information. Bayer HealthCare Phillip’s Colon Health Web site. Available at: http://phillipspro.com/en/home/product-information/index.php. Accessed February 13, 2015.
4. Nature’s Bounty. Nature’s Bounty Acidophilus Probiotic product label. Nature’s Bounty Web site. Available at: http://images.vitaminimages.com/cdn/sd/pdf/L002610-NB.PDF. Accessed February 13, 2015.
5. Dannon. Activia. Dannon Activia Web site. Available at: http://activia.us.com/probiotic-yogurt/activia. Accessed February 13, 2015.
6. Lifeway. Lifeway Kefir frequently asked questions. Lifeway Kefir Web site. Available at: http://lifewaykefir.com/faq/. Accessed February 13, 2015.
7. Allen SJ, Martinez EG, Gregorio GV, et al. Probiotics for treating acute infectious diarrhoea. Cochrane Database Syst Rev. 2010;(11):CD003048.
8. McFarland LV. Meta-analysis of probiotics for the prevention of traveler’s diarrhea. Travel Med Infect Dis. 2007;5:97-105.
9. Hamad A, Fragkos KC, Forbes A. A systemic review and meta-analysis of probiotics for the management of radiation induced bowel disease. Clin Nutr. 2013;32:353-360.
10. Videlock EJ, Cremonini F. Meta-analysis: probiotics in antibiotic-associated diarrhoea. Aliment Pharmacol Ther. 2012;35:1355-1369.
11. Pillai A, Nelson RL. Probiotics for treatment of Clostridium difficile-associated colitis in adults. Cochrane Database Syst Rev. 2008;(1):CD004611.
12. Johnston BC, Ma SY, Goldenberg JZ, et al. Probiotics for the prevention of Clostridium difficile-associated diarrhea: a systematic review and meta-analysis. Ann Intern Med. 2012;157:878-888.
13. Szajewska H, Horvath A, Piwowarczyk A. Meta-analysis: the effects of Saccharomyces boulardii supplementation on Helicobacter pylori eradication rates and side effects during treatment. Aliment Pharmacol Ther. 2010;32:1069-1079.
14. Sachdeva A, Nagpal J. Effect of fermented milk-based probiotic preparations on Helicobacter pylori eradication: a systematic review and meta-analysis of randomized-controlled trials. Eur J Gastroenterol Hepatol. 2009;21:45-53.
15. Wang ZH, Gao QY, Fang JY. Meta-analysis of the efficacy and safety of Lactobacillus-containing and Bifidobacterium-containing probiotic compound preparation in Helicobacter pylori eradication therapy. J Clin Gastroenterol. 2013;47:25-32.
16. Butterworth AD, Thomas AG, Akobeng AK. Probiotics for induction of remission in Crohn’s disease. Cochrane Database Syst Rev. 2008;16:CD006634.
17. Rolfe VE, Fortun PJ, Hawkey CJ, et al. Probiotics for maintenance of remission in Crohn’s disease. Cochrane Database Syst Rev. 2006;(4):CD004826.
18. Mallon P, McKay D, Kirk SJ, et al. Probiotics for induction of remission in ulcerative colitis. Cochrane Database Syst Rev. 2007;(4):CD005573.
19. Naidoo K, Gordon M, Fagbemi AO, et al. Probiotics for maintenance of remission in ulcerative colitis. Cochrane Database Syst Rev. 2011;(12):CD007443.
20. Guglielmetti S, Mora D, Gschwender M, et al. Randomised clinical trial: Bifidobacterium bifidum MIMBb75 significantly alleviates irritable bowel syndrome and improves quality of life–– a double-blind, placebo-controlled study. Aliment Pharmacol Ther. 2011;33:1123-1132.
21. Moayyedi P, Ford AC, Talley NJ, et al. The efficacy of probiotics in the treatment of irritable bowel syndrome: a systematic review. Gut. 2010;59:325-332.
22. Allen SJ, Wareham K, Wang D, et al. Lactobacilli and bifidobacteria in the prevention of antibiotic-associated diarrhoea and Clostridium difficile diarrhoea in older inpatients (PLACIDE): a randomised, double-blind, placebo-controlled, multicentre trial. Lancet. 2013;382:1249-1257.
23. Roberts LM, McCahon D, Holder R, et al. A randomised controlled trial of a probiotic ‘functional food’ in the management of irritable bowel syndrome. BMC Gastroenterol. 2013;13:45.
› Consider probiotics for patients with acute infectious diarrhea, antibiotic-associated diarrhea, or Clostridium difficile-associated diarrhea. A
› Do not recommend probiotics for preventing or treating Crohn’s disease or ulcerative colitis. B
› Consider the probiotic Bifidobacterium bifidum MIMBb75 for patients with irritable bowel syndrome. B
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
Probiotics—live micoorganisms that are consumed as supplements or food for purported health benefits—are a popular over-the-counter remedy for various gastrointestinal (GI) ailments and other conditions, but the evidence supporting their use is mixed. Probiotics interact with the normal flora of the human body. They are believed to act by multiple mechanisms to deliver beneficial effects, including providing a protective barrier, altering intestinal pH to favor the growth of nonpathogenic bacteria, enhancing the host’s immunologic response, producing antimicrobial substances, and directly competing with pathogenic bacteria for receptors in the GI tract.1 (See “The normal human intestinal flora.”)
In the United States, Lactobacillus and Bifidobacterium are the probiotic genera that are most commonly used. (For a list of the specific probiotic species found in 5 popular products, see TABLE 1.2-6) The review that follows examines the evidence for using probiotics for select GI ailments, including several types of diarrheal illnesses, inflammatory bowel disease (Crohn’s disease and ulcerative colitis), and irritable bowel syndrome (IBS). These findings are summarized in TABLE 2.1,7-21
The human body contains approximately 1014 prokaryotic organisms, with a biomass of >1 kg. Most of these organisms are indigenous and stable, although transient members such as enteric pathogens can be found.
The gastrointestinal tract is sterile at birth but is colonized immediately, and each individual has marked variations in microbial composition. The complex symbiotic relationship between the normal intestinal flora and the human host is beneficial to both. These microbes utilize complex carbohydrates undigested by the host as energy. Fermentation results in the formation of short-chain fatty acids, which can provide up to 15% of human energy requirements.
In addition to these metabolic benefits, microbial flora dampen the human inflammatory response, induce immunosuppressive T cells (Tregs), and competitively exclude pathogens.
Colonic epithelium is nourished and proliferates in the presence of normal intestinal flora. Disruption of the normal flora can cause disease.
SOURCE: Neish AS. Microbes in gastrointestinal health and disease. Gastroenterology. 2009;136:65-80.
Probiotics may help with some types of diarrhea
Acute infectious diarrhea. Viruses, bacteria, and parasites cause acute infectious diarrhea, and probiotics are thought to act against these pathogens by competing for available nutrients and pattern recognition receptors in the GI endothelium, acidifying the local environment, and increasing immune responses within the GI tract. In a meta-analysis of 63 studies (N=8014) that used multiple strains and dosages of probiotics, investigators found probiotics shortened the duration of acute infectious diarrhea by approximately 24 hours (95% confidence interval [CI], 15.9-33.6 hours).7 Probiotics also reduced both the risk of diarrhea lasting longer than 4 days (relative risk [RR]=0.41; 95% CI, 0.32-0.53) and stool frequency on Day 2 of illness (mean difference of 0.80 stools; 95% CI, 0.45-1.14).
Traveler’s diarrhea. The incidence of traveler’s diarrhea is >50% when traveling to high-risk areas such as the Middle East, North Africa, Latin America, and Southeast Asia, and 5% to 10% when traveling to areas such as North America, Northern Europe, the United Kingdom, Australia, and New Zealand.8 Traveler’s diarrhea may be caused by ingesting food and liquids contaminated with fecal material. Symptoms include diarrhea, cramps, and nausea that if untreated typically last from 2 to 6 days but can last for as long as a month.8
In a meta-analysis of 12 studies (N=5171) that evaluated various probiotic strains, researchers found probiotics effectively prevented traveler’s diarrhea in US and European travelers who visited a variety of vacation spots (pooled RR=0.85; 95% CI, 0.79-0.91).8 No serious adverse events were reported.
Radiation-induced diarrhea. Radiation treatments to the abdomen and pelvis can damage the lower GI tract and cause diarrhea. The pooled results from a meta-analysis that included 6 studies (N=1449) significantly favored the use of probiotics over placebo for decreasing the incidence of radiation-induced diarrhea (odds ratio [OR]=0.44; 95% CI, 0.21-0.92).9 Probiotics use also was associated with decreased loperamide use (OR=0.29; 95% CI, 0.01-6.80) and decreased incidence of watery stools (OR=0.36; 95% CI, 0.05-2.81), but these outcomes did not reach statistical significance.
Antibiotic-associated diarrhea. Antibiotic use has long been associated with the development of diarrheal illness, sometimes due to the acceleration of GI motility (eg, erythromycin) or by causing osmotic diarrhea by decreasing GI bacteria that assist in carbohydrate breakdown.11 A meta-analysis that evaluated 63 randomized controlled trials (RCTs) (N=11,811) showed that probiotics are effective for treating and preventing antibiotic-associated diarrhea (AAD).1 There was a statistically significant reduction in AAD among patients who received probiotics (RR=0.58; 95% CI, 0.50-0.68; number needed to treat [NNT]=13). Most of the studies in this meta-analysis used a Lactobacillus probiotic alone or in combination with another probiotic. Researchers did not analyze whether the efficacy varied by patient population, probiotic used, causative antibiotic, or duration of treatment.1
Another meta-analysis of 34 studies (N=4138) also found probiotic therapy can prevent AAD.10 The pooled RR for AAD was 0.53 (95% CI, 0.44-0.63) for patients treated with probiotics compared to placebo, with an NNT of 8 (95% CI, 7-11). The effects remained significant when results were grouped by probiotic species, patient age, and duration of antibiotic treatment. Among a subgroup of patients in this meta-analysis who were being treated for Helicobacter pylori, the pooled RR of AAD was 0.37 (95% CI, 0.20-0.69) and the NNT was 5 (95% CI, 4-10).10 However, the 2013 PLACIDE trial (N=17,420) found no significant decrease in AAD rates in hospitalized patients over age 65 years being treated with antibiotics who received probiotics (RR=1.04; 95% CI, 0.84-1.28).22
Clostridium difficile-associated diarrhea. As we know, antibiotics can disrupt the normal GI flora and permit overgrow of Clostridium difficile, which can result in C. difficile-associated diarrhea (CDAD).12 This can occur with oral, parenteral, and even topical antibiotics.11 Researchers have investigated whether probiotics can prevent this opportunistic C. difficile overgrowth.
A 2012 meta-analysis of 20 trials (N=38,180) found probiotic prophylaxis prevented CDAD in both inpatients and outpatients while not increasing the incidence of significant adverse effects.12 Probiotics decreased the incidence of CDAD by 66% (pooled RR=0.34, 95% CI, 0.24-0.49).12 Adverse events occurred in 9.3% of patients taking probiotics, compared with 12.6% of controls (RR=0.82, 95% CI, 0.65-1.05).12
Conversely, a 2008 review of 4 studies (N=336) concluded there is insufficient evidence for using probiotics to treat CDAD, either as monotherapy or adjunct therapy.11 One trial in this meta-analysis (N=124) found patients who received the probiotic Saccharomyces boulardii in addition to antibiotic therapy were significantly less likely to experience CDAD recurrence than those who received placebo (RR=0.59; 95% CI, 0.35-0.98).11 However, this benefit was not found in the other trials in this meta-analysis.11
The PLACIDE trial found probiotics did not prevent CDAD in hospitalized patients over age 65 years; 0.8% of patients who received probiotics developed CDAD, compared to 1.2% in the placebo group (RR=0.71, 95% CI, 0.34-1.47).22
Helicobacter pylori infection. The triple therapy regimen of a proton pump inhibitor plus the antibiotics clarithromycin and amoxicillin is the recommended treatment for H. pylori infection.13 Problems with this treatment include adverse effects such as diarrhea and decreased eradication rates, in part due to antibiotic resistance. Certain Lactobacillus species have been shown to inhibit or kill H. pylori in vitro,13 and evidence from several meta-analyses suggests probiotics should be an adjunct therapy when treating H. pylori.
In a meta-analysis of 10 RCTs (N=963), fermented milk-based probiotics improved H. pylori eradication rates by 5% to 15%.14 In another meta-analysis that evaluated 5 RCTs (N=1307), S. boulardii significantly increased the H. pylori eradication rate when used as an adjunct to triple therapy (RR=1.13; 95% CI, 1.05-1.21) and reduced the rate of treatment-related adverse effects (RR=0.46; 95% CI, 0.3-0.7).13 In a third meta-analysis of 10 trials (N=1469), Lactobacillus supplementation increased H. pylori eradication rates (OR=2.1; 95% CI, 1.4-3.1) while decreasing the overall incidence of adverse effects (OR=0.3; 0.1-0.8).15
For inflammatory bowel disease, probiotics are unlikely to help
Current therapies for Crohn’s disease and ulcerative colitis, such as corticosteroids and other immunosuppressive agents, are effective but have significant adverse events.18 Researchers explored whether probiotics might help treat these diseases by improving immune response, the balance of microbes in the GI tract, and the intestinal barrier.18
Crohn’s disease. In a meta-analysis that was able to identify only one small RCT (N=11), 80% of patients receiving probiotic treatment went into remission, compared to 83% in the placebo group (OR=0.80; 95% CI, 0.04–17.20).16 Researchers concluded there was insufficient evidence for the use of probiotics for inducing remission in Crohn’s disease.
Another meta-analysis of 7 small studies (N=160) found no significant evidence supporting probiotic use for maintaining remission in Crohn’s disease compared with aminosalicylates or azathioprine.17 One small study in this review found there was a benefit to combining S. boulardii with a reduced level of standard maintenance therapy when compared to standard therapy alone, but this difference was not statistically significant.17
Ulcerative colitis. A systematic review of 4 RCTs (N=244) that compared conventional treatment alone to conventional treatment plus probiotics for remission or clinical improvement in patients with active ulcerative colitis found no significant differences between groups.18 Another meta-analysis of 4 studies (N=587) found that compared to placebo or treatment with mesalazine, probiotics had no benefit for maintaining remission in ulcerative colitis.19 The rate of relapse was 40.1% in the probiotics group compared to 34.1% in the mesalazine group. The number of adverse effects was similar in both groups.
Most evidence suggests probiotics are useful for IBS
Research suggests that imbalances in GI flora, along with subsequent dysfunction in intestinal barriers and translocation of intestinal flora, may play a role in symptoms associated with IBS, such as abdominal pain, bloating, and diarrhea/constipation.20 There are few effective therapeutic options for patients suffering with IBS.
In a systematic review of 19 RCTs (N=1650), probiotics were significantly more effective than placebo for patients with IBS, with an NNT of 4 (95% CI, 3-12.5).21 This review did not evaluate the difference between various probiotic species and strains.
In an RCT (N=122), the probiotic strain Bifidobacterium bifidum MIMBb75 was found to be safe and beneficial for treating IBS symptoms and improving patients’ quality of life.20 On a 7-point scale of global assessment of IBS symptoms, the score was reduced by 0.88 points (95% CI, 0.69-1.07) in the group that received B. bifidum MIMBb75 and 0.16 points (95% CI, -0.32-0.00) in the placebo group (P<0.0001). Almost half (47%) of the patients who received B. bifidum MIMBb75 reported adequate relief, compared to 11% in the placebo group (P<.0001).
An RCT (N=179) that compared yogurt that contained probiotics to non-probiotic yogurt found the probiotic yogurt had no benefits for treating IBS symptoms.23 After 4 weeks, 57% of patients who ate the probiotic yogurt reported adequate relief, compared to 53% of those who ate non-probiotic yogurt (P=0.71). After 8 weeks, those numbers were 47% and 68%, respectively.23
CORRESPONDENCE
Erik R. Clauson, DO, Nellis Family Medicine Residency, 99 MDOS/SGOF, 4700 Las Vegas Boulevard North, Nellis Air Force Base, NV 89191; erik.clauson.1@us.af.mil
REFERENCES
1. Hempel S, Newberry SJ, Maher AR, et al. Probiotics for the prevention and treatment of antibiotic-associated diarrhea: a systematic review and meta-analysis. JAMA. 2012;307:1959-1969.
2. Procter & Gamble. Align product information. Procter & Gamble Align Web site. Available at: http://www.aligngi.com/information-on-Align-probiotic-supplement. Accessed February 13, 2015.
3. Bayer HealthCare. Phillip’s Colon Health product information. Bayer HealthCare Phillip’s Colon Health Web site. Available at: http://phillipspro.com/en/home/product-information/index.php. Accessed February 13, 2015.
4. Nature’s Bounty. Nature’s Bounty Acidophilus Probiotic product label. Nature’s Bounty Web site. Available at: http://images.vitaminimages.com/cdn/sd/pdf/L002610-NB.PDF. Accessed February 13, 2015.
5. Dannon. Activia. Dannon Activia Web site. Available at: http://activia.us.com/probiotic-yogurt/activia. Accessed February 13, 2015.
6. Lifeway. Lifeway Kefir frequently asked questions. Lifeway Kefir Web site. Available at: http://lifewaykefir.com/faq/. Accessed February 13, 2015.
7. Allen SJ, Martinez EG, Gregorio GV, et al. Probiotics for treating acute infectious diarrhoea. Cochrane Database Syst Rev. 2010;(11):CD003048.
8. McFarland LV. Meta-analysis of probiotics for the prevention of traveler’s diarrhea. Travel Med Infect Dis. 2007;5:97-105.
9. Hamad A, Fragkos KC, Forbes A. A systemic review and meta-analysis of probiotics for the management of radiation induced bowel disease. Clin Nutr. 2013;32:353-360.
10. Videlock EJ, Cremonini F. Meta-analysis: probiotics in antibiotic-associated diarrhoea. Aliment Pharmacol Ther. 2012;35:1355-1369.
11. Pillai A, Nelson RL. Probiotics for treatment of Clostridium difficile-associated colitis in adults. Cochrane Database Syst Rev. 2008;(1):CD004611.
12. Johnston BC, Ma SY, Goldenberg JZ, et al. Probiotics for the prevention of Clostridium difficile-associated diarrhea: a systematic review and meta-analysis. Ann Intern Med. 2012;157:878-888.
13. Szajewska H, Horvath A, Piwowarczyk A. Meta-analysis: the effects of Saccharomyces boulardii supplementation on Helicobacter pylori eradication rates and side effects during treatment. Aliment Pharmacol Ther. 2010;32:1069-1079.
14. Sachdeva A, Nagpal J. Effect of fermented milk-based probiotic preparations on Helicobacter pylori eradication: a systematic review and meta-analysis of randomized-controlled trials. Eur J Gastroenterol Hepatol. 2009;21:45-53.
15. Wang ZH, Gao QY, Fang JY. Meta-analysis of the efficacy and safety of Lactobacillus-containing and Bifidobacterium-containing probiotic compound preparation in Helicobacter pylori eradication therapy. J Clin Gastroenterol. 2013;47:25-32.
16. Butterworth AD, Thomas AG, Akobeng AK. Probiotics for induction of remission in Crohn’s disease. Cochrane Database Syst Rev. 2008;16:CD006634.
17. Rolfe VE, Fortun PJ, Hawkey CJ, et al. Probiotics for maintenance of remission in Crohn’s disease. Cochrane Database Syst Rev. 2006;(4):CD004826.
18. Mallon P, McKay D, Kirk SJ, et al. Probiotics for induction of remission in ulcerative colitis. Cochrane Database Syst Rev. 2007;(4):CD005573.
19. Naidoo K, Gordon M, Fagbemi AO, et al. Probiotics for maintenance of remission in ulcerative colitis. Cochrane Database Syst Rev. 2011;(12):CD007443.
20. Guglielmetti S, Mora D, Gschwender M, et al. Randomised clinical trial: Bifidobacterium bifidum MIMBb75 significantly alleviates irritable bowel syndrome and improves quality of life–– a double-blind, placebo-controlled study. Aliment Pharmacol Ther. 2011;33:1123-1132.
21. Moayyedi P, Ford AC, Talley NJ, et al. The efficacy of probiotics in the treatment of irritable bowel syndrome: a systematic review. Gut. 2010;59:325-332.
22. Allen SJ, Wareham K, Wang D, et al. Lactobacilli and bifidobacteria in the prevention of antibiotic-associated diarrhoea and Clostridium difficile diarrhoea in older inpatients (PLACIDE): a randomised, double-blind, placebo-controlled, multicentre trial. Lancet. 2013;382:1249-1257.
23. Roberts LM, McCahon D, Holder R, et al. A randomised controlled trial of a probiotic ‘functional food’ in the management of irritable bowel syndrome. BMC Gastroenterol. 2013;13:45.
What you must know before you recommend a probiotic
› Consider probiotics for patients with acute infectious diarrhea, antibiotic-associated diarrhea, or Clostridium difficile-associated diarrhea. A
› Do not recommend probiotics for preventing or treating Crohn’s disease or ulcerative colitis. B
› Consider the probiotic Bifidobacterium bifidum MIMBb75 for patients with irritable bowel syndrome. B
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
Probiotics—live micoorganisms that are consumed as supplements or food for purported health benefits—are a popular over-the-counter remedy for various gastrointestinal (GI) ailments and other conditions, but the evidence supporting their use is mixed. Probiotics interact with the normal flora of the human body. They are believed to act by multiple mechanisms to deliver beneficial effects, including providing a protective barrier, altering intestinal pH to favor the growth of nonpathogenic bacteria, enhancing the host’s immunologic response, producing antimicrobial substances, and directly competing with pathogenic bacteria for receptors in the GI tract.1 (See “The normal human intestinal flora.”)
In the United States, Lactobacillus and Bifidobacterium are the probiotic genera that are most commonly used. (For a list of the specific probiotic species found in 5 popular products, see TABLE 1.2-6) The review that follows examines the evidence for using probiotics for select GI ailments, including several types of diarrheal illnesses, inflammatory bowel disease (Crohn’s disease and ulcerative colitis), and irritable bowel syndrome (IBS). These findings are summarized in TABLE 2.1,7-21
The human body contains approximately 1014 prokaryotic organisms, with a biomass of >1 kg. Most of these organisms are indigenous and stable, although transient members such as enteric pathogens can be found.
The gastrointestinal tract is sterile at birth but is colonized immediately, and each individual has marked variations in microbial composition. The complex symbiotic relationship between the normal intestinal flora and the human host is beneficial to both. These microbes utilize complex carbohydrates undigested by the host as energy. Fermentation results in the formation of short-chain fatty acids, which can provide up to 15% of human energy requirements.
In addition to these metabolic benefits, microbial flora dampen the human inflammatory response, induce immunosuppressive T cells (Tregs), and competitively exclude pathogens.
Colonic epithelium is nourished and proliferates in the presence of normal intestinal flora. Disruption of the normal flora can cause disease.
SOURCE: Neish AS. Microbes in gastrointestinal health and disease. Gastroenterology. 2009;136:65-80.
Probiotics may help with some types of diarrhea
Acute infectious diarrhea. Viruses, bacteria, and parasites cause acute infectious diarrhea, and probiotics are thought to act against these pathogens by competing for available nutrients and pattern recognition receptors in the GI endothelium, acidifying the local environment, and increasing immune responses within the GI tract. In a meta-analysis of 63 studies (N=8014) that used multiple strains and dosages of probiotics, investigators found probiotics shortened the duration of acute infectious diarrhea by approximately 24 hours (95% confidence interval [CI], 15.9-33.6 hours).7 Probiotics also reduced both the risk of diarrhea lasting longer than 4 days (relative risk [RR]=0.41; 95% CI, 0.32-0.53) and stool frequency on Day 2 of illness (mean difference of 0.80 stools; 95% CI, 0.45-1.14).
Traveler’s diarrhea. The incidence of traveler’s diarrhea is >50% when traveling to high-risk areas such as the Middle East, North Africa, Latin America, and Southeast Asia, and 5% to 10% when traveling to areas such as North America, Northern Europe, the United Kingdom, Australia, and New Zealand.8 Traveler’s diarrhea may be caused by ingesting food and liquids contaminated with fecal material. Symptoms include diarrhea, cramps, and nausea that if untreated typically last from 2 to 6 days but can last for as long as a month.8
In a meta-analysis of 12 studies (N=5171) that evaluated various probiotic strains, researchers found probiotics effectively prevented traveler’s diarrhea in US and European travelers who visited a variety of vacation spots (pooled RR=0.85; 95% CI, 0.79-0.91).8 No serious adverse events were reported.
Radiation-induced diarrhea. Radiation treatments to the abdomen and pelvis can damage the lower GI tract and cause diarrhea. The pooled results from a meta-analysis that included 6 studies (N=1449) significantly favored the use of probiotics over placebo for decreasing the incidence of radiation-induced diarrhea (odds ratio [OR]=0.44; 95% CI, 0.21-0.92).9 Probiotics use also was associated with decreased loperamide use (OR=0.29; 95% CI, 0.01-6.80) and decreased incidence of watery stools (OR=0.36; 95% CI, 0.05-2.81), but these outcomes did not reach statistical significance.
Antibiotic-associated diarrhea. Antibiotic use has long been associated with the development of diarrheal illness, sometimes due to the acceleration of GI motility (eg, erythromycin) or by causing osmotic diarrhea by decreasing GI bacteria that assist in carbohydrate breakdown.11 A meta-analysis that evaluated 63 randomized controlled trials (RCTs) (N=11,811) showed that probiotics are effective for treating and preventing antibiotic-associated diarrhea (AAD).1 There was a statistically significant reduction in AAD among patients who received probiotics (RR=0.58; 95% CI, 0.50-0.68; number needed to treat [NNT]=13). Most of the studies in this meta-analysis used a Lactobacillus probiotic alone or in combination with another probiotic. Researchers did not analyze whether the efficacy varied by patient population, probiotic used, causative antibiotic, or duration of treatment.1
Another meta-analysis of 34 studies (N=4138) also found probiotic therapy can prevent AAD.10 The pooled RR for AAD was 0.53 (95% CI, 0.44-0.63) for patients treated with probiotics compared to placebo, with an NNT of 8 (95% CI, 7-11). The effects remained significant when results were grouped by probiotic species, patient age, and duration of antibiotic treatment. Among a subgroup of patients in this meta-analysis who were being treated for Helicobacter pylori, the pooled RR of AAD was 0.37 (95% CI, 0.20-0.69) and the NNT was 5 (95% CI, 4-10).10 However, the 2013 PLACIDE trial (N=17,420) found no significant decrease in AAD rates in hospitalized patients over age 65 years being treated with antibiotics who received probiotics (RR=1.04; 95% CI, 0.84-1.28).22
Clostridium difficile-associated diarrhea. As we know, antibiotics can disrupt the normal GI flora and permit overgrow of Clostridium difficile, which can result in C. difficile-associated diarrhea (CDAD).12 This can occur with oral, parenteral, and even topical antibiotics.11 Researchers have investigated whether probiotics can prevent this opportunistic C. difficile overgrowth.
A 2012 meta-analysis of 20 trials (N=38,180) found probiotic prophylaxis prevented CDAD in both inpatients and outpatients while not increasing the incidence of significant adverse effects.12 Probiotics decreased the incidence of CDAD by 66% (pooled RR=0.34, 95% CI, 0.24-0.49).12 Adverse events occurred in 9.3% of patients taking probiotics, compared with 12.6% of controls (RR=0.82, 95% CI, 0.65-1.05).12
Conversely, a 2008 review of 4 studies (N=336) concluded there is insufficient evidence for using probiotics to treat CDAD, either as monotherapy or adjunct therapy.11 One trial in this meta-analysis (N=124) found patients who received the probiotic Saccharomyces boulardii in addition to antibiotic therapy were significantly less likely to experience CDAD recurrence than those who received placebo (RR=0.59; 95% CI, 0.35-0.98).11 However, this benefit was not found in the other trials in this meta-analysis.11
The PLACIDE trial found probiotics did not prevent CDAD in hospitalized patients over age 65 years; 0.8% of patients who received probiotics developed CDAD, compared to 1.2% in the placebo group (RR=0.71, 95% CI, 0.34-1.47).22
Helicobacter pylori infection. The triple therapy regimen of a proton pump inhibitor plus the antibiotics clarithromycin and amoxicillin is the recommended treatment for H. pylori infection.13 Problems with this treatment include adverse effects such as diarrhea and decreased eradication rates, in part due to antibiotic resistance. Certain Lactobacillus species have been shown to inhibit or kill H. pylori in vitro,13 and evidence from several meta-analyses suggests probiotics should be an adjunct therapy when treating H. pylori.
In a meta-analysis of 10 RCTs (N=963), fermented milk-based probiotics improved H. pylori eradication rates by 5% to 15%.14 In another meta-analysis that evaluated 5 RCTs (N=1307), S. boulardii significantly increased the H. pylori eradication rate when used as an adjunct to triple therapy (RR=1.13; 95% CI, 1.05-1.21) and reduced the rate of treatment-related adverse effects (RR=0.46; 95% CI, 0.3-0.7).13 In a third meta-analysis of 10 trials (N=1469), Lactobacillus supplementation increased H. pylori eradication rates (OR=2.1; 95% CI, 1.4-3.1) while decreasing the overall incidence of adverse effects (OR=0.3; 0.1-0.8).15
For inflammatory bowel disease, probiotics are unlikely to help
Current therapies for Crohn’s disease and ulcerative colitis, such as corticosteroids and other immunosuppressive agents, are effective but have significant adverse events.18 Researchers explored whether probiotics might help treat these diseases by improving immune response, the balance of microbes in the GI tract, and the intestinal barrier.18
Crohn’s disease. In a meta-analysis that was able to identify only one small RCT (N=11), 80% of patients receiving probiotic treatment went into remission, compared to 83% in the placebo group (OR=0.80; 95% CI, 0.04–17.20).16 Researchers concluded there was insufficient evidence for the use of probiotics for inducing remission in Crohn’s disease.
Another meta-analysis of 7 small studies (N=160) found no significant evidence supporting probiotic use for maintaining remission in Crohn’s disease compared with aminosalicylates or azathioprine.17 One small study in this review found there was a benefit to combining S. boulardii with a reduced level of standard maintenance therapy when compared to standard therapy alone, but this difference was not statistically significant.17
Ulcerative colitis. A systematic review of 4 RCTs (N=244) that compared conventional treatment alone to conventional treatment plus probiotics for remission or clinical improvement in patients with active ulcerative colitis found no significant differences between groups.18 Another meta-analysis of 4 studies (N=587) found that compared to placebo or treatment with mesalazine, probiotics had no benefit for maintaining remission in ulcerative colitis.19 The rate of relapse was 40.1% in the probiotics group compared to 34.1% in the mesalazine group. The number of adverse effects was similar in both groups.
Most evidence suggests probiotics are useful for IBS
Research suggests that imbalances in GI flora, along with subsequent dysfunction in intestinal barriers and translocation of intestinal flora, may play a role in symptoms associated with IBS, such as abdominal pain, bloating, and diarrhea/constipation.20 There are few effective therapeutic options for patients suffering with IBS.
In a systematic review of 19 RCTs (N=1650), probiotics were significantly more effective than placebo for patients with IBS, with an NNT of 4 (95% CI, 3-12.5).21 This review did not evaluate the difference between various probiotic species and strains.
In an RCT (N=122), the probiotic strain Bifidobacterium bifidum MIMBb75 was found to be safe and beneficial for treating IBS symptoms and improving patients’ quality of life.20 On a 7-point scale of global assessment of IBS symptoms, the score was reduced by 0.88 points (95% CI, 0.69-1.07) in the group that received B. bifidum MIMBb75 and 0.16 points (95% CI, -0.32-0.00) in the placebo group (P<0.0001). Almost half (47%) of the patients who received B. bifidum MIMBb75 reported adequate relief, compared to 11% in the placebo group (P<.0001).
An RCT (N=179) that compared yogurt that contained probiotics to non-probiotic yogurt found the probiotic yogurt had no benefits for treating IBS symptoms.23 After 4 weeks, 57% of patients who ate the probiotic yogurt reported adequate relief, compared to 53% of those who ate non-probiotic yogurt (P=0.71). After 8 weeks, those numbers were 47% and 68%, respectively.23
CORRESPONDENCE
Erik R. Clauson, DO, Nellis Family Medicine Residency, 99 MDOS/SGOF, 4700 Las Vegas Boulevard North, Nellis Air Force Base, NV 89191; erik.clauson.1@us.af.mil
1. Hempel S, Newberry SJ, Maher AR, et al. Probiotics for the prevention and treatment of antibiotic-associated diarrhea: a systematic review and meta-analysis. JAMA. 2012;307:1959-1969.
2. Procter & Gamble. Align product information. Procter & Gamble Align Web site. Available at: http://www.aligngi.com/information-on-Align-probiotic-supplement. Accessed February 13, 2015.
3. Bayer HealthCare. Phillip’s Colon Health product information. Bayer HealthCare Phillip’s Colon Health Web site. Available at: http://phillipspro.com/en/home/product-information/index.php. Accessed February 13, 2015.
4. Nature’s Bounty. Nature’s Bounty Acidophilus Probiotic product label. Nature’s Bounty Web site. Available at: http://images.vitaminimages.com/cdn/sd/pdf/L002610-NB.PDF. Accessed February 13, 2015.
5. Dannon. Activia. Dannon Activia Web site. Available at: http://activia.us.com/probiotic-yogurt/activia. Accessed February 13, 2015.
6. Lifeway. Lifeway Kefir frequently asked questions. Lifeway Kefir Web site. Available at: http://lifewaykefir.com/faq/. Accessed February 13, 2015.
7. Allen SJ, Martinez EG, Gregorio GV, et al. Probiotics for treating acute infectious diarrhoea. Cochrane Database Syst Rev. 2010;(11):CD003048.
8. McFarland LV. Meta-analysis of probiotics for the prevention of traveler’s diarrhea. Travel Med Infect Dis. 2007;5:97-105.
9. Hamad A, Fragkos KC, Forbes A. A systemic review and meta-analysis of probiotics for the management of radiation induced bowel disease. Clin Nutr. 2013;32:353-360.
10. Videlock EJ, Cremonini F. Meta-analysis: probiotics in antibiotic-associated diarrhoea. Aliment Pharmacol Ther. 2012;35:1355-1369.
11. Pillai A, Nelson RL. Probiotics for treatment of Clostridium difficile-associated colitis in adults. Cochrane Database Syst Rev. 2008;(1):CD004611.
12. Johnston BC, Ma SY, Goldenberg JZ, et al. Probiotics for the prevention of Clostridium difficile-associated diarrhea: a systematic review and meta-analysis. Ann Intern Med. 2012;157:878-888.
13. Szajewska H, Horvath A, Piwowarczyk A. Meta-analysis: the effects of Saccharomyces boulardii supplementation on Helicobacter pylori eradication rates and side effects during treatment. Aliment Pharmacol Ther. 2010;32:1069-1079.
14. Sachdeva A, Nagpal J. Effect of fermented milk-based probiotic preparations on Helicobacter pylori eradication: a systematic review and meta-analysis of randomized-controlled trials. Eur J Gastroenterol Hepatol. 2009;21:45-53.
15. Wang ZH, Gao QY, Fang JY. Meta-analysis of the efficacy and safety of Lactobacillus-containing and Bifidobacterium-containing probiotic compound preparation in Helicobacter pylori eradication therapy. J Clin Gastroenterol. 2013;47:25-32.
16. Butterworth AD, Thomas AG, Akobeng AK. Probiotics for induction of remission in Crohn’s disease. Cochrane Database Syst Rev. 2008;16:CD006634.
17. Rolfe VE, Fortun PJ, Hawkey CJ, et al. Probiotics for maintenance of remission in Crohn’s disease. Cochrane Database Syst Rev. 2006;(4):CD004826.
18. Mallon P, McKay D, Kirk SJ, et al. Probiotics for induction of remission in ulcerative colitis. Cochrane Database Syst Rev. 2007;(4):CD005573.
19. Naidoo K, Gordon M, Fagbemi AO, et al. Probiotics for maintenance of remission in ulcerative colitis. Cochrane Database Syst Rev. 2011;(12):CD007443.
20. Guglielmetti S, Mora D, Gschwender M, et al. Randomised clinical trial: Bifidobacterium bifidum MIMBb75 significantly alleviates irritable bowel syndrome and improves quality of life–– a double-blind, placebo-controlled study. Aliment Pharmacol Ther. 2011;33:1123-1132.
21. Moayyedi P, Ford AC, Talley NJ, et al. The efficacy of probiotics in the treatment of irritable bowel syndrome: a systematic review. Gut. 2010;59:325-332.
22. Allen SJ, Wareham K, Wang D, et al. Lactobacilli and bifidobacteria in the prevention of antibiotic-associated diarrhoea and Clostridium difficile diarrhoea in older inpatients (PLACIDE): a randomised, double-blind, placebo-controlled, multicentre trial. Lancet. 2013;382:1249-1257.
23. Roberts LM, McCahon D, Holder R, et al. A randomised controlled trial of a probiotic ‘functional food’ in the management of irritable bowel syndrome. BMC Gastroenterol. 2013;13:45.
› Consider probiotics for patients with acute infectious diarrhea, antibiotic-associated diarrhea, or Clostridium difficile-associated diarrhea. A
› Do not recommend probiotics for preventing or treating Crohn’s disease or ulcerative colitis. B
› Consider the probiotic Bifidobacterium bifidum MIMBb75 for patients with irritable bowel syndrome. B
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
Probiotics—live micoorganisms that are consumed as supplements or food for purported health benefits—are a popular over-the-counter remedy for various gastrointestinal (GI) ailments and other conditions, but the evidence supporting their use is mixed. Probiotics interact with the normal flora of the human body. They are believed to act by multiple mechanisms to deliver beneficial effects, including providing a protective barrier, altering intestinal pH to favor the growth of nonpathogenic bacteria, enhancing the host’s immunologic response, producing antimicrobial substances, and directly competing with pathogenic bacteria for receptors in the GI tract.1 (See “The normal human intestinal flora.”)
In the United States, Lactobacillus and Bifidobacterium are the probiotic genera that are most commonly used. (For a list of the specific probiotic species found in 5 popular products, see TABLE 1.2-6) The review that follows examines the evidence for using probiotics for select GI ailments, including several types of diarrheal illnesses, inflammatory bowel disease (Crohn’s disease and ulcerative colitis), and irritable bowel syndrome (IBS). These findings are summarized in TABLE 2.1,7-21
The human body contains approximately 1014 prokaryotic organisms, with a biomass of >1 kg. Most of these organisms are indigenous and stable, although transient members such as enteric pathogens can be found.
The gastrointestinal tract is sterile at birth but is colonized immediately, and each individual has marked variations in microbial composition. The complex symbiotic relationship between the normal intestinal flora and the human host is beneficial to both. These microbes utilize complex carbohydrates undigested by the host as energy. Fermentation results in the formation of short-chain fatty acids, which can provide up to 15% of human energy requirements.
In addition to these metabolic benefits, microbial flora dampen the human inflammatory response, induce immunosuppressive T cells (Tregs), and competitively exclude pathogens.
Colonic epithelium is nourished and proliferates in the presence of normal intestinal flora. Disruption of the normal flora can cause disease.
SOURCE: Neish AS. Microbes in gastrointestinal health and disease. Gastroenterology. 2009;136:65-80.
Probiotics may help with some types of diarrhea
Acute infectious diarrhea. Viruses, bacteria, and parasites cause acute infectious diarrhea, and probiotics are thought to act against these pathogens by competing for available nutrients and pattern recognition receptors in the GI endothelium, acidifying the local environment, and increasing immune responses within the GI tract. In a meta-analysis of 63 studies (N=8014) that used multiple strains and dosages of probiotics, investigators found probiotics shortened the duration of acute infectious diarrhea by approximately 24 hours (95% confidence interval [CI], 15.9-33.6 hours).7 Probiotics also reduced both the risk of diarrhea lasting longer than 4 days (relative risk [RR]=0.41; 95% CI, 0.32-0.53) and stool frequency on Day 2 of illness (mean difference of 0.80 stools; 95% CI, 0.45-1.14).
Traveler’s diarrhea. The incidence of traveler’s diarrhea is >50% when traveling to high-risk areas such as the Middle East, North Africa, Latin America, and Southeast Asia, and 5% to 10% when traveling to areas such as North America, Northern Europe, the United Kingdom, Australia, and New Zealand.8 Traveler’s diarrhea may be caused by ingesting food and liquids contaminated with fecal material. Symptoms include diarrhea, cramps, and nausea that if untreated typically last from 2 to 6 days but can last for as long as a month.8
In a meta-analysis of 12 studies (N=5171) that evaluated various probiotic strains, researchers found probiotics effectively prevented traveler’s diarrhea in US and European travelers who visited a variety of vacation spots (pooled RR=0.85; 95% CI, 0.79-0.91).8 No serious adverse events were reported.
Radiation-induced diarrhea. Radiation treatments to the abdomen and pelvis can damage the lower GI tract and cause diarrhea. The pooled results from a meta-analysis that included 6 studies (N=1449) significantly favored the use of probiotics over placebo for decreasing the incidence of radiation-induced diarrhea (odds ratio [OR]=0.44; 95% CI, 0.21-0.92).9 Probiotics use also was associated with decreased loperamide use (OR=0.29; 95% CI, 0.01-6.80) and decreased incidence of watery stools (OR=0.36; 95% CI, 0.05-2.81), but these outcomes did not reach statistical significance.
Antibiotic-associated diarrhea. Antibiotic use has long been associated with the development of diarrheal illness, sometimes due to the acceleration of GI motility (eg, erythromycin) or by causing osmotic diarrhea by decreasing GI bacteria that assist in carbohydrate breakdown.11 A meta-analysis that evaluated 63 randomized controlled trials (RCTs) (N=11,811) showed that probiotics are effective for treating and preventing antibiotic-associated diarrhea (AAD).1 There was a statistically significant reduction in AAD among patients who received probiotics (RR=0.58; 95% CI, 0.50-0.68; number needed to treat [NNT]=13). Most of the studies in this meta-analysis used a Lactobacillus probiotic alone or in combination with another probiotic. Researchers did not analyze whether the efficacy varied by patient population, probiotic used, causative antibiotic, or duration of treatment.1
Another meta-analysis of 34 studies (N=4138) also found probiotic therapy can prevent AAD.10 The pooled RR for AAD was 0.53 (95% CI, 0.44-0.63) for patients treated with probiotics compared to placebo, with an NNT of 8 (95% CI, 7-11). The effects remained significant when results were grouped by probiotic species, patient age, and duration of antibiotic treatment. Among a subgroup of patients in this meta-analysis who were being treated for Helicobacter pylori, the pooled RR of AAD was 0.37 (95% CI, 0.20-0.69) and the NNT was 5 (95% CI, 4-10).10 However, the 2013 PLACIDE trial (N=17,420) found no significant decrease in AAD rates in hospitalized patients over age 65 years being treated with antibiotics who received probiotics (RR=1.04; 95% CI, 0.84-1.28).22
Clostridium difficile-associated diarrhea. As we know, antibiotics can disrupt the normal GI flora and permit overgrow of Clostridium difficile, which can result in C. difficile-associated diarrhea (CDAD).12 This can occur with oral, parenteral, and even topical antibiotics.11 Researchers have investigated whether probiotics can prevent this opportunistic C. difficile overgrowth.
A 2012 meta-analysis of 20 trials (N=38,180) found probiotic prophylaxis prevented CDAD in both inpatients and outpatients while not increasing the incidence of significant adverse effects.12 Probiotics decreased the incidence of CDAD by 66% (pooled RR=0.34, 95% CI, 0.24-0.49).12 Adverse events occurred in 9.3% of patients taking probiotics, compared with 12.6% of controls (RR=0.82, 95% CI, 0.65-1.05).12
Conversely, a 2008 review of 4 studies (N=336) concluded there is insufficient evidence for using probiotics to treat CDAD, either as monotherapy or adjunct therapy.11 One trial in this meta-analysis (N=124) found patients who received the probiotic Saccharomyces boulardii in addition to antibiotic therapy were significantly less likely to experience CDAD recurrence than those who received placebo (RR=0.59; 95% CI, 0.35-0.98).11 However, this benefit was not found in the other trials in this meta-analysis.11
The PLACIDE trial found probiotics did not prevent CDAD in hospitalized patients over age 65 years; 0.8% of patients who received probiotics developed CDAD, compared to 1.2% in the placebo group (RR=0.71, 95% CI, 0.34-1.47).22
Helicobacter pylori infection. The triple therapy regimen of a proton pump inhibitor plus the antibiotics clarithromycin and amoxicillin is the recommended treatment for H. pylori infection.13 Problems with this treatment include adverse effects such as diarrhea and decreased eradication rates, in part due to antibiotic resistance. Certain Lactobacillus species have been shown to inhibit or kill H. pylori in vitro,13 and evidence from several meta-analyses suggests probiotics should be an adjunct therapy when treating H. pylori.
In a meta-analysis of 10 RCTs (N=963), fermented milk-based probiotics improved H. pylori eradication rates by 5% to 15%.14 In another meta-analysis that evaluated 5 RCTs (N=1307), S. boulardii significantly increased the H. pylori eradication rate when used as an adjunct to triple therapy (RR=1.13; 95% CI, 1.05-1.21) and reduced the rate of treatment-related adverse effects (RR=0.46; 95% CI, 0.3-0.7).13 In a third meta-analysis of 10 trials (N=1469), Lactobacillus supplementation increased H. pylori eradication rates (OR=2.1; 95% CI, 1.4-3.1) while decreasing the overall incidence of adverse effects (OR=0.3; 0.1-0.8).15
For inflammatory bowel disease, probiotics are unlikely to help
Current therapies for Crohn’s disease and ulcerative colitis, such as corticosteroids and other immunosuppressive agents, are effective but have significant adverse events.18 Researchers explored whether probiotics might help treat these diseases by improving immune response, the balance of microbes in the GI tract, and the intestinal barrier.18
Crohn’s disease. In a meta-analysis that was able to identify only one small RCT (N=11), 80% of patients receiving probiotic treatment went into remission, compared to 83% in the placebo group (OR=0.80; 95% CI, 0.04–17.20).16 Researchers concluded there was insufficient evidence for the use of probiotics for inducing remission in Crohn’s disease.
Another meta-analysis of 7 small studies (N=160) found no significant evidence supporting probiotic use for maintaining remission in Crohn’s disease compared with aminosalicylates or azathioprine.17 One small study in this review found there was a benefit to combining S. boulardii with a reduced level of standard maintenance therapy when compared to standard therapy alone, but this difference was not statistically significant.17
Ulcerative colitis. A systematic review of 4 RCTs (N=244) that compared conventional treatment alone to conventional treatment plus probiotics for remission or clinical improvement in patients with active ulcerative colitis found no significant differences between groups.18 Another meta-analysis of 4 studies (N=587) found that compared to placebo or treatment with mesalazine, probiotics had no benefit for maintaining remission in ulcerative colitis.19 The rate of relapse was 40.1% in the probiotics group compared to 34.1% in the mesalazine group. The number of adverse effects was similar in both groups.
Most evidence suggests probiotics are useful for IBS
Research suggests that imbalances in GI flora, along with subsequent dysfunction in intestinal barriers and translocation of intestinal flora, may play a role in symptoms associated with IBS, such as abdominal pain, bloating, and diarrhea/constipation.20 There are few effective therapeutic options for patients suffering with IBS.
In a systematic review of 19 RCTs (N=1650), probiotics were significantly more effective than placebo for patients with IBS, with an NNT of 4 (95% CI, 3-12.5).21 This review did not evaluate the difference between various probiotic species and strains.
In an RCT (N=122), the probiotic strain Bifidobacterium bifidum MIMBb75 was found to be safe and beneficial for treating IBS symptoms and improving patients’ quality of life.20 On a 7-point scale of global assessment of IBS symptoms, the score was reduced by 0.88 points (95% CI, 0.69-1.07) in the group that received B. bifidum MIMBb75 and 0.16 points (95% CI, -0.32-0.00) in the placebo group (P<0.0001). Almost half (47%) of the patients who received B. bifidum MIMBb75 reported adequate relief, compared to 11% in the placebo group (P<.0001).
An RCT (N=179) that compared yogurt that contained probiotics to non-probiotic yogurt found the probiotic yogurt had no benefits for treating IBS symptoms.23 After 4 weeks, 57% of patients who ate the probiotic yogurt reported adequate relief, compared to 53% of those who ate non-probiotic yogurt (P=0.71). After 8 weeks, those numbers were 47% and 68%, respectively.23
CORRESPONDENCE
Erik R. Clauson, DO, Nellis Family Medicine Residency, 99 MDOS/SGOF, 4700 Las Vegas Boulevard North, Nellis Air Force Base, NV 89191; erik.clauson.1@us.af.mil
› Consider probiotics for patients with acute infectious diarrhea, antibiotic-associated diarrhea, or Clostridium difficile-associated diarrhea. A
› Do not recommend probiotics for preventing or treating Crohn’s disease or ulcerative colitis. B
› Consider the probiotic Bifidobacterium bifidum MIMBb75 for patients with irritable bowel syndrome. B
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
Probiotics—live micoorganisms that are consumed as supplements or food for purported health benefits—are a popular over-the-counter remedy for various gastrointestinal (GI) ailments and other conditions, but the evidence supporting their use is mixed. Probiotics interact with the normal flora of the human body. They are believed to act by multiple mechanisms to deliver beneficial effects, including providing a protective barrier, altering intestinal pH to favor the growth of nonpathogenic bacteria, enhancing the host’s immunologic response, producing antimicrobial substances, and directly competing with pathogenic bacteria for receptors in the GI tract.1 (See “The normal human intestinal flora.”)
In the United States, Lactobacillus and Bifidobacterium are the probiotic genera that are most commonly used. (For a list of the specific probiotic species found in 5 popular products, see TABLE 1.2-6) The review that follows examines the evidence for using probiotics for select GI ailments, including several types of diarrheal illnesses, inflammatory bowel disease (Crohn’s disease and ulcerative colitis), and irritable bowel syndrome (IBS). These findings are summarized in TABLE 2.1,7-21
The human body contains approximately 1014 prokaryotic organisms, with a biomass of >1 kg. Most of these organisms are indigenous and stable, although transient members such as enteric pathogens can be found.
The gastrointestinal tract is sterile at birth but is colonized immediately, and each individual has marked variations in microbial composition. The complex symbiotic relationship between the normal intestinal flora and the human host is beneficial to both. These microbes utilize complex carbohydrates undigested by the host as energy. Fermentation results in the formation of short-chain fatty acids, which can provide up to 15% of human energy requirements.
In addition to these metabolic benefits, microbial flora dampen the human inflammatory response, induce immunosuppressive T cells (Tregs), and competitively exclude pathogens.
Colonic epithelium is nourished and proliferates in the presence of normal intestinal flora. Disruption of the normal flora can cause disease.
SOURCE: Neish AS. Microbes in gastrointestinal health and disease. Gastroenterology. 2009;136:65-80.
Probiotics may help with some types of diarrhea
Acute infectious diarrhea. Viruses, bacteria, and parasites cause acute infectious diarrhea, and probiotics are thought to act against these pathogens by competing for available nutrients and pattern recognition receptors in the GI endothelium, acidifying the local environment, and increasing immune responses within the GI tract. In a meta-analysis of 63 studies (N=8014) that used multiple strains and dosages of probiotics, investigators found probiotics shortened the duration of acute infectious diarrhea by approximately 24 hours (95% confidence interval [CI], 15.9-33.6 hours).7 Probiotics also reduced both the risk of diarrhea lasting longer than 4 days (relative risk [RR]=0.41; 95% CI, 0.32-0.53) and stool frequency on Day 2 of illness (mean difference of 0.80 stools; 95% CI, 0.45-1.14).
Traveler’s diarrhea. The incidence of traveler’s diarrhea is >50% when traveling to high-risk areas such as the Middle East, North Africa, Latin America, and Southeast Asia, and 5% to 10% when traveling to areas such as North America, Northern Europe, the United Kingdom, Australia, and New Zealand.8 Traveler’s diarrhea may be caused by ingesting food and liquids contaminated with fecal material. Symptoms include diarrhea, cramps, and nausea that if untreated typically last from 2 to 6 days but can last for as long as a month.8
In a meta-analysis of 12 studies (N=5171) that evaluated various probiotic strains, researchers found probiotics effectively prevented traveler’s diarrhea in US and European travelers who visited a variety of vacation spots (pooled RR=0.85; 95% CI, 0.79-0.91).8 No serious adverse events were reported.
Radiation-induced diarrhea. Radiation treatments to the abdomen and pelvis can damage the lower GI tract and cause diarrhea. The pooled results from a meta-analysis that included 6 studies (N=1449) significantly favored the use of probiotics over placebo for decreasing the incidence of radiation-induced diarrhea (odds ratio [OR]=0.44; 95% CI, 0.21-0.92).9 Probiotics use also was associated with decreased loperamide use (OR=0.29; 95% CI, 0.01-6.80) and decreased incidence of watery stools (OR=0.36; 95% CI, 0.05-2.81), but these outcomes did not reach statistical significance.
Antibiotic-associated diarrhea. Antibiotic use has long been associated with the development of diarrheal illness, sometimes due to the acceleration of GI motility (eg, erythromycin) or by causing osmotic diarrhea by decreasing GI bacteria that assist in carbohydrate breakdown.11 A meta-analysis that evaluated 63 randomized controlled trials (RCTs) (N=11,811) showed that probiotics are effective for treating and preventing antibiotic-associated diarrhea (AAD).1 There was a statistically significant reduction in AAD among patients who received probiotics (RR=0.58; 95% CI, 0.50-0.68; number needed to treat [NNT]=13). Most of the studies in this meta-analysis used a Lactobacillus probiotic alone or in combination with another probiotic. Researchers did not analyze whether the efficacy varied by patient population, probiotic used, causative antibiotic, or duration of treatment.1
Another meta-analysis of 34 studies (N=4138) also found probiotic therapy can prevent AAD.10 The pooled RR for AAD was 0.53 (95% CI, 0.44-0.63) for patients treated with probiotics compared to placebo, with an NNT of 8 (95% CI, 7-11). The effects remained significant when results were grouped by probiotic species, patient age, and duration of antibiotic treatment. Among a subgroup of patients in this meta-analysis who were being treated for Helicobacter pylori, the pooled RR of AAD was 0.37 (95% CI, 0.20-0.69) and the NNT was 5 (95% CI, 4-10).10 However, the 2013 PLACIDE trial (N=17,420) found no significant decrease in AAD rates in hospitalized patients over age 65 years being treated with antibiotics who received probiotics (RR=1.04; 95% CI, 0.84-1.28).22
Clostridium difficile-associated diarrhea. As we know, antibiotics can disrupt the normal GI flora and permit overgrow of Clostridium difficile, which can result in C. difficile-associated diarrhea (CDAD).12 This can occur with oral, parenteral, and even topical antibiotics.11 Researchers have investigated whether probiotics can prevent this opportunistic C. difficile overgrowth.
A 2012 meta-analysis of 20 trials (N=38,180) found probiotic prophylaxis prevented CDAD in both inpatients and outpatients while not increasing the incidence of significant adverse effects.12 Probiotics decreased the incidence of CDAD by 66% (pooled RR=0.34, 95% CI, 0.24-0.49).12 Adverse events occurred in 9.3% of patients taking probiotics, compared with 12.6% of controls (RR=0.82, 95% CI, 0.65-1.05).12
Conversely, a 2008 review of 4 studies (N=336) concluded there is insufficient evidence for using probiotics to treat CDAD, either as monotherapy or adjunct therapy.11 One trial in this meta-analysis (N=124) found patients who received the probiotic Saccharomyces boulardii in addition to antibiotic therapy were significantly less likely to experience CDAD recurrence than those who received placebo (RR=0.59; 95% CI, 0.35-0.98).11 However, this benefit was not found in the other trials in this meta-analysis.11
The PLACIDE trial found probiotics did not prevent CDAD in hospitalized patients over age 65 years; 0.8% of patients who received probiotics developed CDAD, compared to 1.2% in the placebo group (RR=0.71, 95% CI, 0.34-1.47).22
Helicobacter pylori infection. The triple therapy regimen of a proton pump inhibitor plus the antibiotics clarithromycin and amoxicillin is the recommended treatment for H. pylori infection.13 Problems with this treatment include adverse effects such as diarrhea and decreased eradication rates, in part due to antibiotic resistance. Certain Lactobacillus species have been shown to inhibit or kill H. pylori in vitro,13 and evidence from several meta-analyses suggests probiotics should be an adjunct therapy when treating H. pylori.
In a meta-analysis of 10 RCTs (N=963), fermented milk-based probiotics improved H. pylori eradication rates by 5% to 15%.14 In another meta-analysis that evaluated 5 RCTs (N=1307), S. boulardii significantly increased the H. pylori eradication rate when used as an adjunct to triple therapy (RR=1.13; 95% CI, 1.05-1.21) and reduced the rate of treatment-related adverse effects (RR=0.46; 95% CI, 0.3-0.7).13 In a third meta-analysis of 10 trials (N=1469), Lactobacillus supplementation increased H. pylori eradication rates (OR=2.1; 95% CI, 1.4-3.1) while decreasing the overall incidence of adverse effects (OR=0.3; 0.1-0.8).15
For inflammatory bowel disease, probiotics are unlikely to help
Current therapies for Crohn’s disease and ulcerative colitis, such as corticosteroids and other immunosuppressive agents, are effective but have significant adverse events.18 Researchers explored whether probiotics might help treat these diseases by improving immune response, the balance of microbes in the GI tract, and the intestinal barrier.18
Crohn’s disease. In a meta-analysis that was able to identify only one small RCT (N=11), 80% of patients receiving probiotic treatment went into remission, compared to 83% in the placebo group (OR=0.80; 95% CI, 0.04–17.20).16 Researchers concluded there was insufficient evidence for the use of probiotics for inducing remission in Crohn’s disease.
Another meta-analysis of 7 small studies (N=160) found no significant evidence supporting probiotic use for maintaining remission in Crohn’s disease compared with aminosalicylates or azathioprine.17 One small study in this review found there was a benefit to combining S. boulardii with a reduced level of standard maintenance therapy when compared to standard therapy alone, but this difference was not statistically significant.17
Ulcerative colitis. A systematic review of 4 RCTs (N=244) that compared conventional treatment alone to conventional treatment plus probiotics for remission or clinical improvement in patients with active ulcerative colitis found no significant differences between groups.18 Another meta-analysis of 4 studies (N=587) found that compared to placebo or treatment with mesalazine, probiotics had no benefit for maintaining remission in ulcerative colitis.19 The rate of relapse was 40.1% in the probiotics group compared to 34.1% in the mesalazine group. The number of adverse effects was similar in both groups.
Most evidence suggests probiotics are useful for IBS
Research suggests that imbalances in GI flora, along with subsequent dysfunction in intestinal barriers and translocation of intestinal flora, may play a role in symptoms associated with IBS, such as abdominal pain, bloating, and diarrhea/constipation.20 There are few effective therapeutic options for patients suffering with IBS.
In a systematic review of 19 RCTs (N=1650), probiotics were significantly more effective than placebo for patients with IBS, with an NNT of 4 (95% CI, 3-12.5).21 This review did not evaluate the difference between various probiotic species and strains.
In an RCT (N=122), the probiotic strain Bifidobacterium bifidum MIMBb75 was found to be safe and beneficial for treating IBS symptoms and improving patients’ quality of life.20 On a 7-point scale of global assessment of IBS symptoms, the score was reduced by 0.88 points (95% CI, 0.69-1.07) in the group that received B. bifidum MIMBb75 and 0.16 points (95% CI, -0.32-0.00) in the placebo group (P<0.0001). Almost half (47%) of the patients who received B. bifidum MIMBb75 reported adequate relief, compared to 11% in the placebo group (P<.0001).
An RCT (N=179) that compared yogurt that contained probiotics to non-probiotic yogurt found the probiotic yogurt had no benefits for treating IBS symptoms.23 After 4 weeks, 57% of patients who ate the probiotic yogurt reported adequate relief, compared to 53% of those who ate non-probiotic yogurt (P=0.71). After 8 weeks, those numbers were 47% and 68%, respectively.23
CORRESPONDENCE
Erik R. Clauson, DO, Nellis Family Medicine Residency, 99 MDOS/SGOF, 4700 Las Vegas Boulevard North, Nellis Air Force Base, NV 89191; erik.clauson.1@us.af.mil
1. Hempel S, Newberry SJ, Maher AR, et al. Probiotics for the prevention and treatment of antibiotic-associated diarrhea: a systematic review and meta-analysis. JAMA. 2012;307:1959-1969.
2. Procter & Gamble. Align product information. Procter & Gamble Align Web site. Available at: http://www.aligngi.com/information-on-Align-probiotic-supplement. Accessed February 13, 2015.
3. Bayer HealthCare. Phillip’s Colon Health product information. Bayer HealthCare Phillip’s Colon Health Web site. Available at: http://phillipspro.com/en/home/product-information/index.php. Accessed February 13, 2015.
4. Nature’s Bounty. Nature’s Bounty Acidophilus Probiotic product label. Nature’s Bounty Web site. Available at: http://images.vitaminimages.com/cdn/sd/pdf/L002610-NB.PDF. Accessed February 13, 2015.
5. Dannon. Activia. Dannon Activia Web site. Available at: http://activia.us.com/probiotic-yogurt/activia. Accessed February 13, 2015.
6. Lifeway. Lifeway Kefir frequently asked questions. Lifeway Kefir Web site. Available at: http://lifewaykefir.com/faq/. Accessed February 13, 2015.
7. Allen SJ, Martinez EG, Gregorio GV, et al. Probiotics for treating acute infectious diarrhoea. Cochrane Database Syst Rev. 2010;(11):CD003048.
8. McFarland LV. Meta-analysis of probiotics for the prevention of traveler’s diarrhea. Travel Med Infect Dis. 2007;5:97-105.
9. Hamad A, Fragkos KC, Forbes A. A systemic review and meta-analysis of probiotics for the management of radiation induced bowel disease. Clin Nutr. 2013;32:353-360.
10. Videlock EJ, Cremonini F. Meta-analysis: probiotics in antibiotic-associated diarrhoea. Aliment Pharmacol Ther. 2012;35:1355-1369.
11. Pillai A, Nelson RL. Probiotics for treatment of Clostridium difficile-associated colitis in adults. Cochrane Database Syst Rev. 2008;(1):CD004611.
12. Johnston BC, Ma SY, Goldenberg JZ, et al. Probiotics for the prevention of Clostridium difficile-associated diarrhea: a systematic review and meta-analysis. Ann Intern Med. 2012;157:878-888.
13. Szajewska H, Horvath A, Piwowarczyk A. Meta-analysis: the effects of Saccharomyces boulardii supplementation on Helicobacter pylori eradication rates and side effects during treatment. Aliment Pharmacol Ther. 2010;32:1069-1079.
14. Sachdeva A, Nagpal J. Effect of fermented milk-based probiotic preparations on Helicobacter pylori eradication: a systematic review and meta-analysis of randomized-controlled trials. Eur J Gastroenterol Hepatol. 2009;21:45-53.
15. Wang ZH, Gao QY, Fang JY. Meta-analysis of the efficacy and safety of Lactobacillus-containing and Bifidobacterium-containing probiotic compound preparation in Helicobacter pylori eradication therapy. J Clin Gastroenterol. 2013;47:25-32.
16. Butterworth AD, Thomas AG, Akobeng AK. Probiotics for induction of remission in Crohn’s disease. Cochrane Database Syst Rev. 2008;16:CD006634.
17. Rolfe VE, Fortun PJ, Hawkey CJ, et al. Probiotics for maintenance of remission in Crohn’s disease. Cochrane Database Syst Rev. 2006;(4):CD004826.
18. Mallon P, McKay D, Kirk SJ, et al. Probiotics for induction of remission in ulcerative colitis. Cochrane Database Syst Rev. 2007;(4):CD005573.
19. Naidoo K, Gordon M, Fagbemi AO, et al. Probiotics for maintenance of remission in ulcerative colitis. Cochrane Database Syst Rev. 2011;(12):CD007443.
20. Guglielmetti S, Mora D, Gschwender M, et al. Randomised clinical trial: Bifidobacterium bifidum MIMBb75 significantly alleviates irritable bowel syndrome and improves quality of life–– a double-blind, placebo-controlled study. Aliment Pharmacol Ther. 2011;33:1123-1132.
21. Moayyedi P, Ford AC, Talley NJ, et al. The efficacy of probiotics in the treatment of irritable bowel syndrome: a systematic review. Gut. 2010;59:325-332.
22. Allen SJ, Wareham K, Wang D, et al. Lactobacilli and bifidobacteria in the prevention of antibiotic-associated diarrhoea and Clostridium difficile diarrhoea in older inpatients (PLACIDE): a randomised, double-blind, placebo-controlled, multicentre trial. Lancet. 2013;382:1249-1257.
23. Roberts LM, McCahon D, Holder R, et al. A randomised controlled trial of a probiotic ‘functional food’ in the management of irritable bowel syndrome. BMC Gastroenterol. 2013;13:45.
1. Hempel S, Newberry SJ, Maher AR, et al. Probiotics for the prevention and treatment of antibiotic-associated diarrhea: a systematic review and meta-analysis. JAMA. 2012;307:1959-1969.
2. Procter & Gamble. Align product information. Procter & Gamble Align Web site. Available at: http://www.aligngi.com/information-on-Align-probiotic-supplement. Accessed February 13, 2015.
3. Bayer HealthCare. Phillip’s Colon Health product information. Bayer HealthCare Phillip’s Colon Health Web site. Available at: http://phillipspro.com/en/home/product-information/index.php. Accessed February 13, 2015.
4. Nature’s Bounty. Nature’s Bounty Acidophilus Probiotic product label. Nature’s Bounty Web site. Available at: http://images.vitaminimages.com/cdn/sd/pdf/L002610-NB.PDF. Accessed February 13, 2015.
5. Dannon. Activia. Dannon Activia Web site. Available at: http://activia.us.com/probiotic-yogurt/activia. Accessed February 13, 2015.
6. Lifeway. Lifeway Kefir frequently asked questions. Lifeway Kefir Web site. Available at: http://lifewaykefir.com/faq/. Accessed February 13, 2015.
7. Allen SJ, Martinez EG, Gregorio GV, et al. Probiotics for treating acute infectious diarrhoea. Cochrane Database Syst Rev. 2010;(11):CD003048.
8. McFarland LV. Meta-analysis of probiotics for the prevention of traveler’s diarrhea. Travel Med Infect Dis. 2007;5:97-105.
9. Hamad A, Fragkos KC, Forbes A. A systemic review and meta-analysis of probiotics for the management of radiation induced bowel disease. Clin Nutr. 2013;32:353-360.
10. Videlock EJ, Cremonini F. Meta-analysis: probiotics in antibiotic-associated diarrhoea. Aliment Pharmacol Ther. 2012;35:1355-1369.
11. Pillai A, Nelson RL. Probiotics for treatment of Clostridium difficile-associated colitis in adults. Cochrane Database Syst Rev. 2008;(1):CD004611.
12. Johnston BC, Ma SY, Goldenberg JZ, et al. Probiotics for the prevention of Clostridium difficile-associated diarrhea: a systematic review and meta-analysis. Ann Intern Med. 2012;157:878-888.
13. Szajewska H, Horvath A, Piwowarczyk A. Meta-analysis: the effects of Saccharomyces boulardii supplementation on Helicobacter pylori eradication rates and side effects during treatment. Aliment Pharmacol Ther. 2010;32:1069-1079.
14. Sachdeva A, Nagpal J. Effect of fermented milk-based probiotic preparations on Helicobacter pylori eradication: a systematic review and meta-analysis of randomized-controlled trials. Eur J Gastroenterol Hepatol. 2009;21:45-53.
15. Wang ZH, Gao QY, Fang JY. Meta-analysis of the efficacy and safety of Lactobacillus-containing and Bifidobacterium-containing probiotic compound preparation in Helicobacter pylori eradication therapy. J Clin Gastroenterol. 2013;47:25-32.
16. Butterworth AD, Thomas AG, Akobeng AK. Probiotics for induction of remission in Crohn’s disease. Cochrane Database Syst Rev. 2008;16:CD006634.
17. Rolfe VE, Fortun PJ, Hawkey CJ, et al. Probiotics for maintenance of remission in Crohn’s disease. Cochrane Database Syst Rev. 2006;(4):CD004826.
18. Mallon P, McKay D, Kirk SJ, et al. Probiotics for induction of remission in ulcerative colitis. Cochrane Database Syst Rev. 2007;(4):CD005573.
19. Naidoo K, Gordon M, Fagbemi AO, et al. Probiotics for maintenance of remission in ulcerative colitis. Cochrane Database Syst Rev. 2011;(12):CD007443.
20. Guglielmetti S, Mora D, Gschwender M, et al. Randomised clinical trial: Bifidobacterium bifidum MIMBb75 significantly alleviates irritable bowel syndrome and improves quality of life–– a double-blind, placebo-controlled study. Aliment Pharmacol Ther. 2011;33:1123-1132.
21. Moayyedi P, Ford AC, Talley NJ, et al. The efficacy of probiotics in the treatment of irritable bowel syndrome: a systematic review. Gut. 2010;59:325-332.
22. Allen SJ, Wareham K, Wang D, et al. Lactobacilli and bifidobacteria in the prevention of antibiotic-associated diarrhoea and Clostridium difficile diarrhoea in older inpatients (PLACIDE): a randomised, double-blind, placebo-controlled, multicentre trial. Lancet. 2013;382:1249-1257.
23. Roberts LM, McCahon D, Holder R, et al. A randomised controlled trial of a probiotic ‘functional food’ in the management of irritable bowel syndrome. BMC Gastroenterol. 2013;13:45.
Does the presence of a trained support person during labor decrease C-section rates?
Sometimes. The continuous presence of a support person during labor slightly decreases (by about 2%) the likelihood of a cesarean section (C-section) but only when companions can’t be present and epidurals aren’t routine (strength of recommendation [SOR]: A, a well-done systematic review of randomized controlled trials [RCTs]). When the support person was neither hospital staff nor a member of the woman’s social network, C-section was significantly less likely (SOR A, a well-done systematic review of RCTs).
EVIDENCE SUMMARY
A 2012 Cochrane review of 22 multinational RCTs with a total of 15,288 patients investigated the effect of continuous support in labor on several outcomes, including C-section.1 All trials included pregnant women in labor. The study populations were heterogenous in terms of parity; most included only nulliparous women, but some included multiparous women. At least one study incorporated higher-risk groups such as mothers of twins, but several trials limited the study group to low-risk pregnancies.
The review found a small but significant decrease in risk of C-section in women receiving continuous support (absolute risk reduction [ARR]=2%; number needed to treat [NNT]=50; P=.0017).1 The average cost of trained childbirth support in 3 US metropolitan areas in October 2014 was about $875, according to a Web search of established businesses.
The effect only works in the absence of companions and epidurals…
A subgroup analysis of 22 studies investigated several variables to determine circumstances under which a support person decreased the risk of C-section.1 The support person’s presence was significant only when hospital policy prevented companions (such as the woman’s spouse) in the labor room and when epidurals were not routinely available. Eleven of the 22 studies (11,326 patients) permitted a companion; 11 studies (3849 patients) didn’t.
When policy allowed companions, the presence of a support person didn’t decrease C-section rates significantly (12.7% without support compared with 11.9% with support; P=.20).1 When the woman wasn’t permitted to have a companion, however, the presence of a support person significantly decreased C-section (ARR=5.4%; NNT=19; P<.01).
In 14 studies, with a total of 13,064 patients, epidurals were routinely available. In the other 8, with 2077 patients, epidurals weren’t available.1 These were older studies or studies conducted in developing countries. When epidurals were routinely available, the presence of a support person didn’t affect the C-section rate (13.8% rate without support, 12.9% with support; P=.12). But if epidural anesthesia wasn’t available, a support person decreased C-section (ARR=8.6%; NNT=12; P<.00001).
…And when the support person isn’t a hospital staffer or known to the patient
The Cochrane Review also evaluated different types of labor supporters: companions of the patient’s choice from her social network, hospital employees, and people who were neither. The support person conferred significant benefit only when that person was neither hospital staff nor a member of the woman’s social network.
Hospital staff members who provided support didn’t effectively decrease the C-section rate (12% rate in control group vs 11.3% in supported group; P=.28). Support people chosen by the patient likewise didn’t successfully reduce C-sections (19.4% control rate vs 15.5% supported rate; P=.062). When the support person was neither hospital staff nor someone well-known to the patient, the risk of C-section was significantly lower (ARR=6%; NNT=17; P=.0003).
RECOMMENDATIONS
In a Comparative Effectiveness Review published in October 2012, the Agency for Healthcare Research and Quality investigated 18 strategies to reduce C-section, one of which was psychosocial support from doulas and other providers. A trained support person was the only intervention that showed evidence of benefit in decreasing C-section, but the strength of evidence was low.2
An American College of Obstetricians and Gynecologists Practice Bulletin recommends continuous labor support, noting “the continuous presence of a support person may reduce the likelihood of…operative delivery” with no apparent harmful effects.3
1. Hodnett ED, Gates S, Hofmeyr GJ, et al. Continuous support for women during childbirth. Cochrane Database Syst Rev. 2012;(10):CD003766.
2. Agency for Healthcare Research and Quality. Strategies to reduce cesarean birth in low-risk women. Agency for Healthcare Research and Quality Web site. Available at: http://www.effectivehealthcare.ahrq.gov/ehc/products/263/1291/CER80_C-Section_ExecutiveSummary_20121018.pdf. Accessed February 22, 2015.
3. American College of Obstetrics and Gynecology Committee on Practice Bulletins—Obstetrics. ACOG Practice Bulletin Number 49, December 2003: Dystocia and augmentation of labor. Obstet Gynecol. 2003;102:1445-1454.
Sometimes. The continuous presence of a support person during labor slightly decreases (by about 2%) the likelihood of a cesarean section (C-section) but only when companions can’t be present and epidurals aren’t routine (strength of recommendation [SOR]: A, a well-done systematic review of randomized controlled trials [RCTs]). When the support person was neither hospital staff nor a member of the woman’s social network, C-section was significantly less likely (SOR A, a well-done systematic review of RCTs).
EVIDENCE SUMMARY
A 2012 Cochrane review of 22 multinational RCTs with a total of 15,288 patients investigated the effect of continuous support in labor on several outcomes, including C-section.1 All trials included pregnant women in labor. The study populations were heterogenous in terms of parity; most included only nulliparous women, but some included multiparous women. At least one study incorporated higher-risk groups such as mothers of twins, but several trials limited the study group to low-risk pregnancies.
The review found a small but significant decrease in risk of C-section in women receiving continuous support (absolute risk reduction [ARR]=2%; number needed to treat [NNT]=50; P=.0017).1 The average cost of trained childbirth support in 3 US metropolitan areas in October 2014 was about $875, according to a Web search of established businesses.
The effect only works in the absence of companions and epidurals…
A subgroup analysis of 22 studies investigated several variables to determine circumstances under which a support person decreased the risk of C-section.1 The support person’s presence was significant only when hospital policy prevented companions (such as the woman’s spouse) in the labor room and when epidurals were not routinely available. Eleven of the 22 studies (11,326 patients) permitted a companion; 11 studies (3849 patients) didn’t.
When policy allowed companions, the presence of a support person didn’t decrease C-section rates significantly (12.7% without support compared with 11.9% with support; P=.20).1 When the woman wasn’t permitted to have a companion, however, the presence of a support person significantly decreased C-section (ARR=5.4%; NNT=19; P<.01).
In 14 studies, with a total of 13,064 patients, epidurals were routinely available. In the other 8, with 2077 patients, epidurals weren’t available.1 These were older studies or studies conducted in developing countries. When epidurals were routinely available, the presence of a support person didn’t affect the C-section rate (13.8% rate without support, 12.9% with support; P=.12). But if epidural anesthesia wasn’t available, a support person decreased C-section (ARR=8.6%; NNT=12; P<.00001).
…And when the support person isn’t a hospital staffer or known to the patient
The Cochrane Review also evaluated different types of labor supporters: companions of the patient’s choice from her social network, hospital employees, and people who were neither. The support person conferred significant benefit only when that person was neither hospital staff nor a member of the woman’s social network.
Hospital staff members who provided support didn’t effectively decrease the C-section rate (12% rate in control group vs 11.3% in supported group; P=.28). Support people chosen by the patient likewise didn’t successfully reduce C-sections (19.4% control rate vs 15.5% supported rate; P=.062). When the support person was neither hospital staff nor someone well-known to the patient, the risk of C-section was significantly lower (ARR=6%; NNT=17; P=.0003).
RECOMMENDATIONS
In a Comparative Effectiveness Review published in October 2012, the Agency for Healthcare Research and Quality investigated 18 strategies to reduce C-section, one of which was psychosocial support from doulas and other providers. A trained support person was the only intervention that showed evidence of benefit in decreasing C-section, but the strength of evidence was low.2
An American College of Obstetricians and Gynecologists Practice Bulletin recommends continuous labor support, noting “the continuous presence of a support person may reduce the likelihood of…operative delivery” with no apparent harmful effects.3
Sometimes. The continuous presence of a support person during labor slightly decreases (by about 2%) the likelihood of a cesarean section (C-section) but only when companions can’t be present and epidurals aren’t routine (strength of recommendation [SOR]: A, a well-done systematic review of randomized controlled trials [RCTs]). When the support person was neither hospital staff nor a member of the woman’s social network, C-section was significantly less likely (SOR A, a well-done systematic review of RCTs).
EVIDENCE SUMMARY
A 2012 Cochrane review of 22 multinational RCTs with a total of 15,288 patients investigated the effect of continuous support in labor on several outcomes, including C-section.1 All trials included pregnant women in labor. The study populations were heterogenous in terms of parity; most included only nulliparous women, but some included multiparous women. At least one study incorporated higher-risk groups such as mothers of twins, but several trials limited the study group to low-risk pregnancies.
The review found a small but significant decrease in risk of C-section in women receiving continuous support (absolute risk reduction [ARR]=2%; number needed to treat [NNT]=50; P=.0017).1 The average cost of trained childbirth support in 3 US metropolitan areas in October 2014 was about $875, according to a Web search of established businesses.
The effect only works in the absence of companions and epidurals…
A subgroup analysis of 22 studies investigated several variables to determine circumstances under which a support person decreased the risk of C-section.1 The support person’s presence was significant only when hospital policy prevented companions (such as the woman’s spouse) in the labor room and when epidurals were not routinely available. Eleven of the 22 studies (11,326 patients) permitted a companion; 11 studies (3849 patients) didn’t.
When policy allowed companions, the presence of a support person didn’t decrease C-section rates significantly (12.7% without support compared with 11.9% with support; P=.20).1 When the woman wasn’t permitted to have a companion, however, the presence of a support person significantly decreased C-section (ARR=5.4%; NNT=19; P<.01).
In 14 studies, with a total of 13,064 patients, epidurals were routinely available. In the other 8, with 2077 patients, epidurals weren’t available.1 These were older studies or studies conducted in developing countries. When epidurals were routinely available, the presence of a support person didn’t affect the C-section rate (13.8% rate without support, 12.9% with support; P=.12). But if epidural anesthesia wasn’t available, a support person decreased C-section (ARR=8.6%; NNT=12; P<.00001).
…And when the support person isn’t a hospital staffer or known to the patient
The Cochrane Review also evaluated different types of labor supporters: companions of the patient’s choice from her social network, hospital employees, and people who were neither. The support person conferred significant benefit only when that person was neither hospital staff nor a member of the woman’s social network.
Hospital staff members who provided support didn’t effectively decrease the C-section rate (12% rate in control group vs 11.3% in supported group; P=.28). Support people chosen by the patient likewise didn’t successfully reduce C-sections (19.4% control rate vs 15.5% supported rate; P=.062). When the support person was neither hospital staff nor someone well-known to the patient, the risk of C-section was significantly lower (ARR=6%; NNT=17; P=.0003).
RECOMMENDATIONS
In a Comparative Effectiveness Review published in October 2012, the Agency for Healthcare Research and Quality investigated 18 strategies to reduce C-section, one of which was psychosocial support from doulas and other providers. A trained support person was the only intervention that showed evidence of benefit in decreasing C-section, but the strength of evidence was low.2
An American College of Obstetricians and Gynecologists Practice Bulletin recommends continuous labor support, noting “the continuous presence of a support person may reduce the likelihood of…operative delivery” with no apparent harmful effects.3
1. Hodnett ED, Gates S, Hofmeyr GJ, et al. Continuous support for women during childbirth. Cochrane Database Syst Rev. 2012;(10):CD003766.
2. Agency for Healthcare Research and Quality. Strategies to reduce cesarean birth in low-risk women. Agency for Healthcare Research and Quality Web site. Available at: http://www.effectivehealthcare.ahrq.gov/ehc/products/263/1291/CER80_C-Section_ExecutiveSummary_20121018.pdf. Accessed February 22, 2015.
3. American College of Obstetrics and Gynecology Committee on Practice Bulletins—Obstetrics. ACOG Practice Bulletin Number 49, December 2003: Dystocia and augmentation of labor. Obstet Gynecol. 2003;102:1445-1454.
1. Hodnett ED, Gates S, Hofmeyr GJ, et al. Continuous support for women during childbirth. Cochrane Database Syst Rev. 2012;(10):CD003766.
2. Agency for Healthcare Research and Quality. Strategies to reduce cesarean birth in low-risk women. Agency for Healthcare Research and Quality Web site. Available at: http://www.effectivehealthcare.ahrq.gov/ehc/products/263/1291/CER80_C-Section_ExecutiveSummary_20121018.pdf. Accessed February 22, 2015.
3. American College of Obstetrics and Gynecology Committee on Practice Bulletins—Obstetrics. ACOG Practice Bulletin Number 49, December 2003: Dystocia and augmentation of labor. Obstet Gynecol. 2003;102:1445-1454.
Evidence-based answers from the Family Physicians Inquiries Network
Sore throat and left ear pain
A 79 year-old man sought care at our clinic for pain in his left ear and a severe sore throat that had been bothering him for the past 2 days. He also complained of pain when he swallowed, a decreased appetite, and dizziness. He denied weight loss, fever, tinnitus, subjective hearing loss, unilateral facial droop, or weakness.
On physical exam, we noted vesicles on an erythematous base on his hard palate. They were on the left side and didn’t cross the midline (FIGURE 1). The left pinna was mildly erythematous and swollen (FIGURE 2) without obvious vesicles, although we noted vesicles in the external auditory canal on otoscopic examination. The tympanic membrane was normal, as was the patient’s right ear.
WHAT IS YOUR DIAGNOSIS?
HOW WOULD YOU TREAT THIS PATIENT?
Diagnosis: Ramsay Hunt syndrome
Based on our patient’s clinical presentation, we diagnosed herpes zoster oticus—also known as Ramsay Hunt syndrome. This syndrome is a rare complication of herpes zoster that occurs when latent varicella zoster virus (VZV) infection reactivates and spreads to affect the geniculate ganglion.1 An estimated 5 out of every 100,000 people develop Ramsay Hunt syndrome each year in the United States; men and women are equally affected.1 Any patient who’s had VZV infection runs the risk of developing Ramsay Hunt syndrome, but it most often develops in individuals older than age 60.1
Ramsay Hunt syndrome classically presents with unilateral facial paralysis and erythematous vesicles located ipsilaterally on the ear and/or in the mouth. Vesicles in the mouth usually develop on the tongue or hard palate. Other symptoms may include tinnitus, hearing loss, nausea, vomiting, vertigo, and nystagmus.2
Several types of infection are in the differential diagnosis
Because the symptoms of Ramsay Hunt syndrome suggest a possible infection, the differential diagnosis should include herpes simplex virus type 1 (HSV-1), Epstein-Barr virus (EBV), group A Streptococcus (GAS), and measles.
HSV-1 can cause oral symptoms similar to Ramsay Hunt syndrome. However,
HSV-1 doesn’t cause vesicles in the ear. Also worth noting: Recurrent HSV-1 infections normally involve keratinized surfaces such as the vermilion border and gums, but rarely the hard palate.3
EBV can cause multiple systemic symptoms. It can cause leukoplakia in the mouth— most often on the sides of the tongue—but does not cause vesicles.4
GAS presents as a sore throat, fever, anterior cervical lymphadenitis, and a scarlatiniform rash. Oral manifestations can include tonsillar erythema with or without exudate, soft palate petechiae, and a red swollen uvula.5 Use of validated clinical prediction tools, such as the sore throat tool found at http://www.ncbi.nlm.nih.gov/pmc/articles/PMC1228750/pdf/cmaj_158_1_75.pdf, can help distinguish GAS infection from other conditions.6-8
Measles typically occurs in children and young adults. Infection in immunized individuals is rare. It presents with fever and the “3 Cs”—cough, coryza, and conjunctivitis. Koplik’s spots are blue to white ulcerated lesions on the buccal mucosa, typically opposite the first and second molars, although they can occur anywhere in the mouth. They precede the generalized maculopapular rash of measles.9
Although it’s a clinical Dx, lab testing can provide confirmation
Diagnosis of Ramsay Hunt syndrome is typically made clinically, but can be confirmed with direct fluorescent antibody (DFA) analysis,10 polymerase chain reaction (PCR) testing,11 or viral culture of vesicular exudates. DFA for VZV has an 87% sensitivity.10 PCR has a higher sensitivity (92%),11 is widely available, and is the diagnostic test of choice according to the Centers for Disease Control and Prevention.12
For our patient, we obtained swabs of the oral vesicles and ordered a DFA analysis; however, the sample didn’t show VZV. This may have been due to inadequate sampling. (Proper sampling requires that there be an adequate collection of cells from the base of the vesicles.)
Oral antivirals, steroids are mainstays of treatment
Treatment with an oral steroid such as prednisone in addition to an antiviral such as acyclovir or valacyclovir may reduce the likelihood of postherpetic neuralgia and improve facial motor function; however, these benefits have not been demonstrated in randomized controlled trials.13
Our patient was treated with oral valacyclovir 1 g 3 times a day for 7 days and oral prednisone 50 mg/d for 5 days. After one week of treatment, his symptoms resolved and the vesicles in his mouth crusted over. He did not experience postherpetic neuralgia or have a recurrence.
CORRESPONDENCE
David A. Moss, MD, 4700 North Las Vegas Boulevard, Nellis AFB, NV 89191; david.moss.3@us.af.mil
1. National Organization for Rare Disorders. Ramsay Hunt Syndrome. National Organization for Rare Disorders Web site. Available at: http://www.rarediseases.org/rare-disease-information/rare-diseases/byID/1153/viewFullReport. Accessed December 30, 2014.
2. Sweeney CJ, Gilden DH. Ramsay Hunt syndrome. J Neurol Neurosurg Psychiatry. 2001;71:149-154.
3. Habif TP. Warts, herpes simplex, and other viral infections. Clinical Dermatology. A Color Guide to Diagnosis and Therapy. 5th ed. Maryland Heights, Missouri: Mosby Elsevier; 2010: 467-471.
4. Habif TP. Premalignant and malignant nonmelanoma skin tumors. Clinical Dermatology: A Color Guide to Diagnosis and Therapy. 5th ed. Maryland Heights, Missouri: Mosby; 2010:829.
5. Bope ET, Kellerman RD. Pharyngitis. Conn’s Current Therapy 2012. Philadelphia, PA: Saunders; 2012:32.
6. Centor RM, Witherspoon JM, Dalton HP, et al. The diagnosis of strep throat in adults in the emergency room. Med Decis Making. 1981;1:239-246.
7. McIsaac WJ, Goel V, To T, et al. The validity of a sore throat score in family practice. CMAJ. 2000;163:811-815.
8. McIsaac WJ, White D, Tannenbaum D, et al. A clinical score to reduce unnecessary antibiotic use in patients with sore throat. CMAJ. 1998;158:75-83.
9. Habif TP. Exanthems and drug eruptions. Clinical Dermatology: A Color Guide to Diagnosis and Therapy. 5th ed. Maryland Heights, Missouri: Mosby; 2010:544-547.
10. Chan EL, Brandt K, Horsman GB. Comparison of Chemicon SimulFluor direct fluorescent antibody staining with cell culture and shell vial direct immunoperoxidase staining for detection of herpes simplex virus and with cytospin direct immunofluorescence staining for detection of varicella-zoster virus. Clin Diagn Lab Immunol. 2001;8:909-912.
11. Harbecke R, Oxman MN, Arnold BA, et al; Shingles Prevention Study Group. A real-time PCR assay to identify and discriminate among wild-type and vaccine strains of varicella-zoster virus and herpes simplex virus in clinical specimens, and comparison with the clinical diagnoses. J Med Virol. 2009;81: 1310-1322.
12. Lopez A, Schmid S, Bialek S. Varicella. In: Roush SW, McIntyre L, Baldy LM, eds. Manual for the Surveillance of Vaccine-Preventable Diseases. 5th ed. Atlanta, GA: Centers for Disease Control and Prevention; 2011.
13. Murakami S, Hato N, Horiuchi J, et al. Treatment of Ramsay Hunt syndrome with acyclovir-prednisone: significance of early diagnosis and treatment. Ann Neurol. 1997;41:353-357.
A 79 year-old man sought care at our clinic for pain in his left ear and a severe sore throat that had been bothering him for the past 2 days. He also complained of pain when he swallowed, a decreased appetite, and dizziness. He denied weight loss, fever, tinnitus, subjective hearing loss, unilateral facial droop, or weakness.
On physical exam, we noted vesicles on an erythematous base on his hard palate. They were on the left side and didn’t cross the midline (FIGURE 1). The left pinna was mildly erythematous and swollen (FIGURE 2) without obvious vesicles, although we noted vesicles in the external auditory canal on otoscopic examination. The tympanic membrane was normal, as was the patient’s right ear.


WHAT IS YOUR DIAGNOSIS?
HOW WOULD YOU TREAT THIS PATIENT?
Diagnosis: Ramsay Hunt syndrome
Based on our patient’s clinical presentation, we diagnosed herpes zoster oticus—also known as Ramsay Hunt syndrome. This syndrome is a rare complication of herpes zoster that occurs when latent varicella zoster virus (VZV) infection reactivates and spreads to affect the geniculate ganglion.1 An estimated 5 out of every 100,000 people develop Ramsay Hunt syndrome each year in the United States; men and women are equally affected.1 Any patient who’s had VZV infection runs the risk of developing Ramsay Hunt syndrome, but it most often develops in individuals older than age 60.1
Ramsay Hunt syndrome classically presents with unilateral facial paralysis and erythematous vesicles located ipsilaterally on the ear and/or in the mouth. Vesicles in the mouth usually develop on the tongue or hard palate. Other symptoms may include tinnitus, hearing loss, nausea, vomiting, vertigo, and nystagmus.2
Several types of infection are in the differential diagnosis
Because the symptoms of Ramsay Hunt syndrome suggest a possible infection, the differential diagnosis should include herpes simplex virus type 1 (HSV-1), Epstein-Barr virus (EBV), group A Streptococcus (GAS), and measles.
HSV-1 can cause oral symptoms similar to Ramsay Hunt syndrome. However,
HSV-1 doesn’t cause vesicles in the ear. Also worth noting: Recurrent HSV-1 infections normally involve keratinized surfaces such as the vermilion border and gums, but rarely the hard palate.3
EBV can cause multiple systemic symptoms. It can cause leukoplakia in the mouth— most often on the sides of the tongue—but does not cause vesicles.4
GAS presents as a sore throat, fever, anterior cervical lymphadenitis, and a scarlatiniform rash. Oral manifestations can include tonsillar erythema with or without exudate, soft palate petechiae, and a red swollen uvula.5 Use of validated clinical prediction tools, such as the sore throat tool found at http://www.ncbi.nlm.nih.gov/pmc/articles/PMC1228750/pdf/cmaj_158_1_75.pdf, can help distinguish GAS infection from other conditions.6-8
Measles typically occurs in children and young adults. Infection in immunized individuals is rare. It presents with fever and the “3 Cs”—cough, coryza, and conjunctivitis. Koplik’s spots are blue to white ulcerated lesions on the buccal mucosa, typically opposite the first and second molars, although they can occur anywhere in the mouth. They precede the generalized maculopapular rash of measles.9
Although it’s a clinical Dx, lab testing can provide confirmation
Diagnosis of Ramsay Hunt syndrome is typically made clinically, but can be confirmed with direct fluorescent antibody (DFA) analysis,10 polymerase chain reaction (PCR) testing,11 or viral culture of vesicular exudates. DFA for VZV has an 87% sensitivity.10 PCR has a higher sensitivity (92%),11 is widely available, and is the diagnostic test of choice according to the Centers for Disease Control and Prevention.12
For our patient, we obtained swabs of the oral vesicles and ordered a DFA analysis; however, the sample didn’t show VZV. This may have been due to inadequate sampling. (Proper sampling requires that there be an adequate collection of cells from the base of the vesicles.)
Oral antivirals, steroids are mainstays of treatment
Treatment with an oral steroid such as prednisone in addition to an antiviral such as acyclovir or valacyclovir may reduce the likelihood of postherpetic neuralgia and improve facial motor function; however, these benefits have not been demonstrated in randomized controlled trials.13
Our patient was treated with oral valacyclovir 1 g 3 times a day for 7 days and oral prednisone 50 mg/d for 5 days. After one week of treatment, his symptoms resolved and the vesicles in his mouth crusted over. He did not experience postherpetic neuralgia or have a recurrence.
CORRESPONDENCE
David A. Moss, MD, 4700 North Las Vegas Boulevard, Nellis AFB, NV 89191; david.moss.3@us.af.mil
A 79 year-old man sought care at our clinic for pain in his left ear and a severe sore throat that had been bothering him for the past 2 days. He also complained of pain when he swallowed, a decreased appetite, and dizziness. He denied weight loss, fever, tinnitus, subjective hearing loss, unilateral facial droop, or weakness.
On physical exam, we noted vesicles on an erythematous base on his hard palate. They were on the left side and didn’t cross the midline (FIGURE 1). The left pinna was mildly erythematous and swollen (FIGURE 2) without obvious vesicles, although we noted vesicles in the external auditory canal on otoscopic examination. The tympanic membrane was normal, as was the patient’s right ear.


WHAT IS YOUR DIAGNOSIS?
HOW WOULD YOU TREAT THIS PATIENT?
Diagnosis: Ramsay Hunt syndrome
Based on our patient’s clinical presentation, we diagnosed herpes zoster oticus—also known as Ramsay Hunt syndrome. This syndrome is a rare complication of herpes zoster that occurs when latent varicella zoster virus (VZV) infection reactivates and spreads to affect the geniculate ganglion.1 An estimated 5 out of every 100,000 people develop Ramsay Hunt syndrome each year in the United States; men and women are equally affected.1 Any patient who’s had VZV infection runs the risk of developing Ramsay Hunt syndrome, but it most often develops in individuals older than age 60.1
Ramsay Hunt syndrome classically presents with unilateral facial paralysis and erythematous vesicles located ipsilaterally on the ear and/or in the mouth. Vesicles in the mouth usually develop on the tongue or hard palate. Other symptoms may include tinnitus, hearing loss, nausea, vomiting, vertigo, and nystagmus.2
Several types of infection are in the differential diagnosis
Because the symptoms of Ramsay Hunt syndrome suggest a possible infection, the differential diagnosis should include herpes simplex virus type 1 (HSV-1), Epstein-Barr virus (EBV), group A Streptococcus (GAS), and measles.
HSV-1 can cause oral symptoms similar to Ramsay Hunt syndrome. However,
HSV-1 doesn’t cause vesicles in the ear. Also worth noting: Recurrent HSV-1 infections normally involve keratinized surfaces such as the vermilion border and gums, but rarely the hard palate.3
EBV can cause multiple systemic symptoms. It can cause leukoplakia in the mouth— most often on the sides of the tongue—but does not cause vesicles.4
GAS presents as a sore throat, fever, anterior cervical lymphadenitis, and a scarlatiniform rash. Oral manifestations can include tonsillar erythema with or without exudate, soft palate petechiae, and a red swollen uvula.5 Use of validated clinical prediction tools, such as the sore throat tool found at http://www.ncbi.nlm.nih.gov/pmc/articles/PMC1228750/pdf/cmaj_158_1_75.pdf, can help distinguish GAS infection from other conditions.6-8
Measles typically occurs in children and young adults. Infection in immunized individuals is rare. It presents with fever and the “3 Cs”—cough, coryza, and conjunctivitis. Koplik’s spots are blue to white ulcerated lesions on the buccal mucosa, typically opposite the first and second molars, although they can occur anywhere in the mouth. They precede the generalized maculopapular rash of measles.9
Although it’s a clinical Dx, lab testing can provide confirmation
Diagnosis of Ramsay Hunt syndrome is typically made clinically, but can be confirmed with direct fluorescent antibody (DFA) analysis,10 polymerase chain reaction (PCR) testing,11 or viral culture of vesicular exudates. DFA for VZV has an 87% sensitivity.10 PCR has a higher sensitivity (92%),11 is widely available, and is the diagnostic test of choice according to the Centers for Disease Control and Prevention.12
For our patient, we obtained swabs of the oral vesicles and ordered a DFA analysis; however, the sample didn’t show VZV. This may have been due to inadequate sampling. (Proper sampling requires that there be an adequate collection of cells from the base of the vesicles.)
Oral antivirals, steroids are mainstays of treatment
Treatment with an oral steroid such as prednisone in addition to an antiviral such as acyclovir or valacyclovir may reduce the likelihood of postherpetic neuralgia and improve facial motor function; however, these benefits have not been demonstrated in randomized controlled trials.13
Our patient was treated with oral valacyclovir 1 g 3 times a day for 7 days and oral prednisone 50 mg/d for 5 days. After one week of treatment, his symptoms resolved and the vesicles in his mouth crusted over. He did not experience postherpetic neuralgia or have a recurrence.
CORRESPONDENCE
David A. Moss, MD, 4700 North Las Vegas Boulevard, Nellis AFB, NV 89191; david.moss.3@us.af.mil
1. National Organization for Rare Disorders. Ramsay Hunt Syndrome. National Organization for Rare Disorders Web site. Available at: http://www.rarediseases.org/rare-disease-information/rare-diseases/byID/1153/viewFullReport. Accessed December 30, 2014.
2. Sweeney CJ, Gilden DH. Ramsay Hunt syndrome. J Neurol Neurosurg Psychiatry. 2001;71:149-154.
3. Habif TP. Warts, herpes simplex, and other viral infections. Clinical Dermatology. A Color Guide to Diagnosis and Therapy. 5th ed. Maryland Heights, Missouri: Mosby Elsevier; 2010: 467-471.
4. Habif TP. Premalignant and malignant nonmelanoma skin tumors. Clinical Dermatology: A Color Guide to Diagnosis and Therapy. 5th ed. Maryland Heights, Missouri: Mosby; 2010:829.
5. Bope ET, Kellerman RD. Pharyngitis. Conn’s Current Therapy 2012. Philadelphia, PA: Saunders; 2012:32.
6. Centor RM, Witherspoon JM, Dalton HP, et al. The diagnosis of strep throat in adults in the emergency room. Med Decis Making. 1981;1:239-246.
7. McIsaac WJ, Goel V, To T, et al. The validity of a sore throat score in family practice. CMAJ. 2000;163:811-815.
8. McIsaac WJ, White D, Tannenbaum D, et al. A clinical score to reduce unnecessary antibiotic use in patients with sore throat. CMAJ. 1998;158:75-83.
9. Habif TP. Exanthems and drug eruptions. Clinical Dermatology: A Color Guide to Diagnosis and Therapy. 5th ed. Maryland Heights, Missouri: Mosby; 2010:544-547.
10. Chan EL, Brandt K, Horsman GB. Comparison of Chemicon SimulFluor direct fluorescent antibody staining with cell culture and shell vial direct immunoperoxidase staining for detection of herpes simplex virus and with cytospin direct immunofluorescence staining for detection of varicella-zoster virus. Clin Diagn Lab Immunol. 2001;8:909-912.
11. Harbecke R, Oxman MN, Arnold BA, et al; Shingles Prevention Study Group. A real-time PCR assay to identify and discriminate among wild-type and vaccine strains of varicella-zoster virus and herpes simplex virus in clinical specimens, and comparison with the clinical diagnoses. J Med Virol. 2009;81: 1310-1322.
12. Lopez A, Schmid S, Bialek S. Varicella. In: Roush SW, McIntyre L, Baldy LM, eds. Manual for the Surveillance of Vaccine-Preventable Diseases. 5th ed. Atlanta, GA: Centers for Disease Control and Prevention; 2011.
13. Murakami S, Hato N, Horiuchi J, et al. Treatment of Ramsay Hunt syndrome with acyclovir-prednisone: significance of early diagnosis and treatment. Ann Neurol. 1997;41:353-357.
1. National Organization for Rare Disorders. Ramsay Hunt Syndrome. National Organization for Rare Disorders Web site. Available at: http://www.rarediseases.org/rare-disease-information/rare-diseases/byID/1153/viewFullReport. Accessed December 30, 2014.
2. Sweeney CJ, Gilden DH. Ramsay Hunt syndrome. J Neurol Neurosurg Psychiatry. 2001;71:149-154.
3. Habif TP. Warts, herpes simplex, and other viral infections. Clinical Dermatology. A Color Guide to Diagnosis and Therapy. 5th ed. Maryland Heights, Missouri: Mosby Elsevier; 2010: 467-471.
4. Habif TP. Premalignant and malignant nonmelanoma skin tumors. Clinical Dermatology: A Color Guide to Diagnosis and Therapy. 5th ed. Maryland Heights, Missouri: Mosby; 2010:829.
5. Bope ET, Kellerman RD. Pharyngitis. Conn’s Current Therapy 2012. Philadelphia, PA: Saunders; 2012:32.
6. Centor RM, Witherspoon JM, Dalton HP, et al. The diagnosis of strep throat in adults in the emergency room. Med Decis Making. 1981;1:239-246.
7. McIsaac WJ, Goel V, To T, et al. The validity of a sore throat score in family practice. CMAJ. 2000;163:811-815.
8. McIsaac WJ, White D, Tannenbaum D, et al. A clinical score to reduce unnecessary antibiotic use in patients with sore throat. CMAJ. 1998;158:75-83.
9. Habif TP. Exanthems and drug eruptions. Clinical Dermatology: A Color Guide to Diagnosis and Therapy. 5th ed. Maryland Heights, Missouri: Mosby; 2010:544-547.
10. Chan EL, Brandt K, Horsman GB. Comparison of Chemicon SimulFluor direct fluorescent antibody staining with cell culture and shell vial direct immunoperoxidase staining for detection of herpes simplex virus and with cytospin direct immunofluorescence staining for detection of varicella-zoster virus. Clin Diagn Lab Immunol. 2001;8:909-912.
11. Harbecke R, Oxman MN, Arnold BA, et al; Shingles Prevention Study Group. A real-time PCR assay to identify and discriminate among wild-type and vaccine strains of varicella-zoster virus and herpes simplex virus in clinical specimens, and comparison with the clinical diagnoses. J Med Virol. 2009;81: 1310-1322.
12. Lopez A, Schmid S, Bialek S. Varicella. In: Roush SW, McIntyre L, Baldy LM, eds. Manual for the Surveillance of Vaccine-Preventable Diseases. 5th ed. Atlanta, GA: Centers for Disease Control and Prevention; 2011.
13. Murakami S, Hato N, Horiuchi J, et al. Treatment of Ramsay Hunt syndrome with acyclovir-prednisone: significance of early diagnosis and treatment. Ann Neurol. 1997;41:353-357.
Which risk factors and signs and symptoms are associated with coccidioidomycosis?
EVIDENCE-BASED ANSWER:
Risk factors for coccidioidomycosis, or valley fever, include lower respiratory tract symptoms lasting longer than 14 days, chest pain, rash, having lived in endemic areas fewer than 10 years, and diabetes mellitus or immunosuppressive conditions (strength of recommendation [SOR]: B, several prospective cohort and case-control studies).
The most common signs and symptoms include cough (74%), fever (56%), night sweats (35%), pleuritic chest pain (33%), chills (28%), dyspnea (27%), weight loss (21%), and rash (14%) (SOR: B, retrospective cohort study).
EVIDENCE SUMMARY
A 2013 surveillance report by the Centers for Disease Control and Prevention that included 111,717 patients in 28 states and the District of Columbia found an 8-fold increase in reported coccidioidomycosis in endemic areas from 1998 to 2011 (age-adjusted incidence rates: 5.3 per 100,000 in 1998 and 42.6 per 100,000 in 2011). Cases in nonendemic states increased 40-fold in the same time period, from 6 cases to 240.1 The disease is endemic in the southwest United States and northwest Mexico.
Risk factors include persistent symptoms, chest pain, diabetes, immunosuppression
A 2008 case-control study of 136 patients in Phoenix, Arizona (an endemic area) found that 15% of the patients diagnosed with community-acquired pneumonia (CAP) had coccidioidomycosis on serologic testing. Risk factors for CAP caused by coccidioidomycosis in this population were symptom duration longer than 14 days (odds ratio [OR]=5.0; 95% confidence interval [CI], 2.1-15.7), age younger than 18 years (OR=5.5; 95% CI, 2.1-15.3), chest pain (OR=4.6; 95% CI, 1.8-11.8), and diabetes mellitus or an immunosuppressive condition (OR=3.8; 95% CI, 1.0-16.5).2
Abnormal chest X-rays, myalgia— and a rash
A 2006 prospective cohort study of 55 patients in Tucson, Arizona, which is part of the endemic area, found that 29% of patients diagnosed with CAP tested serologically positive for coccidioidomycosis. Risk factors included fewer than 10 years of exposure to an endemic area (OR=4.11; 95% CI, 1.01-16.8). Chest radiograph abnormalities were more common in patients with CAP caused by coccidioidomycosis than patients without coccidioidomycosis (75% vs 25%, P=.005). Myalgia is more common when coccidioidal pneumonia is present (69% vs 23%, P=.0022).3
A 2009 prospective cohort study of 35 patients with CAP in Phoenix, Arizona found that 6 patients (17%) tested positive for coccidioidomycosis. Only 1 statistically significant risk factor was identified—half of patients with coccidioidomycosis exhibited a rash, while there were no rashes in the group without the disease (P=.002).4
Other common signs and symptoms
A retrospective cohort study in San Diego, California in 2004 evaluated and stratified 223 patients with known coccidioidomycosis for presenting symptoms, exam findings, and radiographic findings. The most common signs and symptoms at time of seropositive testing were cough (74%), fever (56%), night sweats (35%), pleuritic chest pain (33%), chills (28%), weight loss (21%), rash (14%), and arthralgia or myalgia (13% and 12%, respectively).5
Airspace opacity was the most common radiographic abnormality (58.8%); the second most common was pulmonary nodules (22.8%).5 The study didn’t compare the frequency of these findings with noncoccidioidal pneumonia.
RECOMMENDATIONS
In 2005 guidelines, the Infectious Diseases Society of America (IDSA) stated that the “management of coccidioidomycosis first involves recognizing that a coccidioidal infection exists, defining the extent of infection, and identifying host factors that predispose to disease severity.”6 The IDSA didn’t give specific recommendations regarding how to diagnose or differentiate coccidioidal infection from CAP.
1. Centers for Disease Control and Prevention (CDC). Increase in reported coccidioidomycosis—United States, 1998-2011. MMWR Morb Mortal Wkly Rep. 2013;62:217-221.
2. Chang DC, Anderson S, Wannemuehler K, et al. Testing for coccidioidomycosis among patients with community-acquired pneumonia. Emerg Infect Dis. 2008;14: 1053-1059.
3. Valdivia L, Nix D, Wright M, et al. Coccidioidomycosis as a common cause of community-acquired pneumonia. Emerg Infect Dis. 2006;12:958-962.
4. Kim MM, Blair JE, Carey EJ, et al. Coccidioidal pneumonia, Phoenix, Arizona, USA, 2000-2004. Emerg Infect Dis. 2009;15:397-401.
5. Crum NF, Lederman ER, Stafford CM, et al. Coccidioidomycosis: a descriptive survey of a reemerging disease. Clinical characteristics and current controversies. Medicine (Baltimore). 2004;83:149-175.
6. Galgiani JN, Ampel NM, Blair JE, et al; Infectious Disease Society of America. Coccidioidomycosis. Clin Infect Dis. 2005;41:1217-1223.
EVIDENCE-BASED ANSWER:
Risk factors for coccidioidomycosis, or valley fever, include lower respiratory tract symptoms lasting longer than 14 days, chest pain, rash, having lived in endemic areas fewer than 10 years, and diabetes mellitus or immunosuppressive conditions (strength of recommendation [SOR]: B, several prospective cohort and case-control studies).
The most common signs and symptoms include cough (74%), fever (56%), night sweats (35%), pleuritic chest pain (33%), chills (28%), dyspnea (27%), weight loss (21%), and rash (14%) (SOR: B, retrospective cohort study).
EVIDENCE SUMMARY
A 2013 surveillance report by the Centers for Disease Control and Prevention that included 111,717 patients in 28 states and the District of Columbia found an 8-fold increase in reported coccidioidomycosis in endemic areas from 1998 to 2011 (age-adjusted incidence rates: 5.3 per 100,000 in 1998 and 42.6 per 100,000 in 2011). Cases in nonendemic states increased 40-fold in the same time period, from 6 cases to 240.1 The disease is endemic in the southwest United States and northwest Mexico.
Risk factors include persistent symptoms, chest pain, diabetes, immunosuppression
A 2008 case-control study of 136 patients in Phoenix, Arizona (an endemic area) found that 15% of the patients diagnosed with community-acquired pneumonia (CAP) had coccidioidomycosis on serologic testing. Risk factors for CAP caused by coccidioidomycosis in this population were symptom duration longer than 14 days (odds ratio [OR]=5.0; 95% confidence interval [CI], 2.1-15.7), age younger than 18 years (OR=5.5; 95% CI, 2.1-15.3), chest pain (OR=4.6; 95% CI, 1.8-11.8), and diabetes mellitus or an immunosuppressive condition (OR=3.8; 95% CI, 1.0-16.5).2
Abnormal chest X-rays, myalgia— and a rash
A 2006 prospective cohort study of 55 patients in Tucson, Arizona, which is part of the endemic area, found that 29% of patients diagnosed with CAP tested serologically positive for coccidioidomycosis. Risk factors included fewer than 10 years of exposure to an endemic area (OR=4.11; 95% CI, 1.01-16.8). Chest radiograph abnormalities were more common in patients with CAP caused by coccidioidomycosis than patients without coccidioidomycosis (75% vs 25%, P=.005). Myalgia is more common when coccidioidal pneumonia is present (69% vs 23%, P=.0022).3
A 2009 prospective cohort study of 35 patients with CAP in Phoenix, Arizona found that 6 patients (17%) tested positive for coccidioidomycosis. Only 1 statistically significant risk factor was identified—half of patients with coccidioidomycosis exhibited a rash, while there were no rashes in the group without the disease (P=.002).4
Other common signs and symptoms
A retrospective cohort study in San Diego, California in 2004 evaluated and stratified 223 patients with known coccidioidomycosis for presenting symptoms, exam findings, and radiographic findings. The most common signs and symptoms at time of seropositive testing were cough (74%), fever (56%), night sweats (35%), pleuritic chest pain (33%), chills (28%), weight loss (21%), rash (14%), and arthralgia or myalgia (13% and 12%, respectively).5
Airspace opacity was the most common radiographic abnormality (58.8%); the second most common was pulmonary nodules (22.8%).5 The study didn’t compare the frequency of these findings with noncoccidioidal pneumonia.
RECOMMENDATIONS
In 2005 guidelines, the Infectious Diseases Society of America (IDSA) stated that the “management of coccidioidomycosis first involves recognizing that a coccidioidal infection exists, defining the extent of infection, and identifying host factors that predispose to disease severity.”6 The IDSA didn’t give specific recommendations regarding how to diagnose or differentiate coccidioidal infection from CAP.
EVIDENCE-BASED ANSWER:
Risk factors for coccidioidomycosis, or valley fever, include lower respiratory tract symptoms lasting longer than 14 days, chest pain, rash, having lived in endemic areas fewer than 10 years, and diabetes mellitus or immunosuppressive conditions (strength of recommendation [SOR]: B, several prospective cohort and case-control studies).
The most common signs and symptoms include cough (74%), fever (56%), night sweats (35%), pleuritic chest pain (33%), chills (28%), dyspnea (27%), weight loss (21%), and rash (14%) (SOR: B, retrospective cohort study).
EVIDENCE SUMMARY
A 2013 surveillance report by the Centers for Disease Control and Prevention that included 111,717 patients in 28 states and the District of Columbia found an 8-fold increase in reported coccidioidomycosis in endemic areas from 1998 to 2011 (age-adjusted incidence rates: 5.3 per 100,000 in 1998 and 42.6 per 100,000 in 2011). Cases in nonendemic states increased 40-fold in the same time period, from 6 cases to 240.1 The disease is endemic in the southwest United States and northwest Mexico.
Risk factors include persistent symptoms, chest pain, diabetes, immunosuppression
A 2008 case-control study of 136 patients in Phoenix, Arizona (an endemic area) found that 15% of the patients diagnosed with community-acquired pneumonia (CAP) had coccidioidomycosis on serologic testing. Risk factors for CAP caused by coccidioidomycosis in this population were symptom duration longer than 14 days (odds ratio [OR]=5.0; 95% confidence interval [CI], 2.1-15.7), age younger than 18 years (OR=5.5; 95% CI, 2.1-15.3), chest pain (OR=4.6; 95% CI, 1.8-11.8), and diabetes mellitus or an immunosuppressive condition (OR=3.8; 95% CI, 1.0-16.5).2
Abnormal chest X-rays, myalgia— and a rash
A 2006 prospective cohort study of 55 patients in Tucson, Arizona, which is part of the endemic area, found that 29% of patients diagnosed with CAP tested serologically positive for coccidioidomycosis. Risk factors included fewer than 10 years of exposure to an endemic area (OR=4.11; 95% CI, 1.01-16.8). Chest radiograph abnormalities were more common in patients with CAP caused by coccidioidomycosis than patients without coccidioidomycosis (75% vs 25%, P=.005). Myalgia is more common when coccidioidal pneumonia is present (69% vs 23%, P=.0022).3
A 2009 prospective cohort study of 35 patients with CAP in Phoenix, Arizona found that 6 patients (17%) tested positive for coccidioidomycosis. Only 1 statistically significant risk factor was identified—half of patients with coccidioidomycosis exhibited a rash, while there were no rashes in the group without the disease (P=.002).4
Other common signs and symptoms
A retrospective cohort study in San Diego, California in 2004 evaluated and stratified 223 patients with known coccidioidomycosis for presenting symptoms, exam findings, and radiographic findings. The most common signs and symptoms at time of seropositive testing were cough (74%), fever (56%), night sweats (35%), pleuritic chest pain (33%), chills (28%), weight loss (21%), rash (14%), and arthralgia or myalgia (13% and 12%, respectively).5
Airspace opacity was the most common radiographic abnormality (58.8%); the second most common was pulmonary nodules (22.8%).5 The study didn’t compare the frequency of these findings with noncoccidioidal pneumonia.
RECOMMENDATIONS
In 2005 guidelines, the Infectious Diseases Society of America (IDSA) stated that the “management of coccidioidomycosis first involves recognizing that a coccidioidal infection exists, defining the extent of infection, and identifying host factors that predispose to disease severity.”6 The IDSA didn’t give specific recommendations regarding how to diagnose or differentiate coccidioidal infection from CAP.
1. Centers for Disease Control and Prevention (CDC). Increase in reported coccidioidomycosis—United States, 1998-2011. MMWR Morb Mortal Wkly Rep. 2013;62:217-221.
2. Chang DC, Anderson S, Wannemuehler K, et al. Testing for coccidioidomycosis among patients with community-acquired pneumonia. Emerg Infect Dis. 2008;14: 1053-1059.
3. Valdivia L, Nix D, Wright M, et al. Coccidioidomycosis as a common cause of community-acquired pneumonia. Emerg Infect Dis. 2006;12:958-962.
4. Kim MM, Blair JE, Carey EJ, et al. Coccidioidal pneumonia, Phoenix, Arizona, USA, 2000-2004. Emerg Infect Dis. 2009;15:397-401.
5. Crum NF, Lederman ER, Stafford CM, et al. Coccidioidomycosis: a descriptive survey of a reemerging disease. Clinical characteristics and current controversies. Medicine (Baltimore). 2004;83:149-175.
6. Galgiani JN, Ampel NM, Blair JE, et al; Infectious Disease Society of America. Coccidioidomycosis. Clin Infect Dis. 2005;41:1217-1223.
1. Centers for Disease Control and Prevention (CDC). Increase in reported coccidioidomycosis—United States, 1998-2011. MMWR Morb Mortal Wkly Rep. 2013;62:217-221.
2. Chang DC, Anderson S, Wannemuehler K, et al. Testing for coccidioidomycosis among patients with community-acquired pneumonia. Emerg Infect Dis. 2008;14: 1053-1059.
3. Valdivia L, Nix D, Wright M, et al. Coccidioidomycosis as a common cause of community-acquired pneumonia. Emerg Infect Dis. 2006;12:958-962.
4. Kim MM, Blair JE, Carey EJ, et al. Coccidioidal pneumonia, Phoenix, Arizona, USA, 2000-2004. Emerg Infect Dis. 2009;15:397-401.
5. Crum NF, Lederman ER, Stafford CM, et al. Coccidioidomycosis: a descriptive survey of a reemerging disease. Clinical characteristics and current controversies. Medicine (Baltimore). 2004;83:149-175.
6. Galgiani JN, Ampel NM, Blair JE, et al; Infectious Disease Society of America. Coccidioidomycosis. Clin Infect Dis. 2005;41:1217-1223.
Evidence-based answers from the Family Physicians Inquiries Network
What is the best treatment for plant-induced contact dermatitis?
IT’S UNCLEAR which treatment is best, because there have been no head-to-head comparisons of treatments for Rhus (plant-induced) contact dermatitis. That said, topical high-potency steroids slightly improve pruritus and the appearance of the rash (strength of recommendation [SOR]: B, small cohort studies).
Neither topical pimecrolimus (an immunomodulatory drug) nor jewelweed extract are helpful (SOR: B, 1 small randomized controlled trial [RCT]).
Oral steroids improve symptoms in severe cases (SOR: C, expert opinion).
It’s unclear which treatment is best, because there have been no head-to-head comparisons of treatments for Rhus (plant-induced) contact dermatitis. That said, topical high-potency steroids slightly improve pruritus and the appearance of the rash (strength of recommendation [SOR]: B, small cohort studies).
Neither topical pimecrolimus (an immunomodulatory drug) nor jewelweed extract are helpful (SOR: B, 1 small randomized controlled trial [RCT]).
Oral steroids improve symptoms in severe cases (SOR: C, expert opinion).
Evidence summary
Two prospective, self-controlled cohort studies (N=30) showed that high-potency topical steroids improved symptoms associated with artificially induced Rhus dermatitis in a group with a history of that type of dermatitis.
The first study found that 0.05% clobetasol propionate ointment applied twice a day significantly reduced overall vesiculation, erythema, induration, and pruritus compared with the control (P<.05, .01, .01, and .05, respectively).1 Investigators evaluated erythema, induration, and pruritus on a scale of 0 to 3 (absent, mild, moderate, or severe) and graded vesiculation on a similar 0- to 3-point scale (a frank bulla was graded 3). They started treatment at 12, 24, and 48 hours after exposure and followed patients for 14 days. The greatest difference in mean scores—a reduction in vesiculation scores of approximately 1 point—occurred between 2 and 7 days of therapy.
The second study compared improvement in symptoms of Rhus dermatitis with daily application of topical steroids of different potencies and a control ointment.2 Investigators evaluated healing using a 0- to 4-point scale (0=clearing and 4=marked edema, erythema, and vesiculation). They found that lower-potency topical steroids such as 1% hydrocortisone and 0.1% triamcinolone were equivalent to the control ointment, but high-potency (class IV) steroid ointments produced significant improvement in symptoms (by a mean of 1.07 points vs the control ointment; supporting statistics not given).
A systematic review of contact dermatitis treatment and prevention identified 4 “good-quality” RCTs that evaluated effective remedies for nickel-induced allergic contact dermatitis in a predominantly female Caucasian population.3 All found that moderately high-potency topical steroid therapy improved symptoms, but heterogeneity among the studies made it impossible to determine the best agent.
Topical immunomodulatory drugs and jewelweed are no help
In a double-blinded RCT of 12 adults with a history of Rhus dermatitis and a significant reaction to tincture of poison ivy, topical pimecrolimus didn’t improve the duration or severity of symptoms (P=nonsignificant).4
A similar RCT from a dermatology clinic of 10 adults with confirmed sensitivity to poison oak or ivy found that topical jewelweed extract didn’t improve symptoms of artificially induced Rhus dermatitis. Investigators didn’t report P values.5
Oral steroids haven’t been studied
No studies have evaluated the effectiveness of oral steroids for Rhus dermatitis. Expert opinion recommends prednisone (60 mg daily, tapered over 14 days) for severe and widespread cases of poison ivy dermatitis.6,7
Recommendations
The American Academy of Allergy, Asthma, and Immunology and the American College of Allergy, Asthma, and Immunology jointly recommend topical corticosteroids as firstline treatment for localized allergic contact dermatitis. They advise giving systemic corticosteroids for lesions covering more than 20% of body surface area (for example, prednisone 0.5-1 mg/kg per day for 5-7 days, then 50% of the dose for another 5-7 days).6
The American Academy of Dermatology hasn’t issued guidelines on plant-induced dermatitis.
A dermatology textbook states that topical steroids are effective during the early stages of an outbreak, when vesicles and blisters aren’t yet present, and that systemic steroids are extremely effective for severe outbreaks. The authors recommend treating weepy lesions with tepid baths, wet to dry soaks, or calamine lotion to dry the lesions.7
1. Vernon HJ, Olsen EA. A controlled trial of clobetasol propionate ointment 0.05% in the treatment of experimentally induced Rhus dermatitis. J Am Acad Dermatol. 1990;23:829-832.
2. Kaidbey KH, Kligman AM. Assay of topical corticosteroids: efficacy of suppression of experimental Rhus dermatitis in humans. Arch Dermatol. 1976;112:808-813.
3. Saary J, Qureshi R, Palda V, et al. A systematic review of contact dermatitis treatment and prevention. J Am Acad Dermatol. 2005;53:845.
4. Amrol D, Keitel D, Hagaman D, et al. Topical pimecrolimus in the treatment of human allergic contact dermatitis. Ann Allergy Asthma Immunol. 2003;91:563-566.
5. Long D, Ballentine NH, Marks JG Jr. Treatment of poison ivy/ oak allergic contact dermatitis with an extract of jewelweed. Am J Contact Dermatol. 1997;8:150-153.
6. American Academy of Allergy, Asthma and Immunology; American College of Allergy, Asthma and Immunology. Contact dermatitis: a practice parameter. Ann Allergy Asthma Immunol. 2006;97(suppl 2):S1-S38.
7. Habif TP. Contact dermatitis and patch testing. In: Habif TP. Clinical Dermatology: A Color Guide to Diagnosis and Therapy. 5th ed. St. Louis, Mo: Mosby; 2010:130-153.
IT’S UNCLEAR which treatment is best, because there have been no head-to-head comparisons of treatments for Rhus (plant-induced) contact dermatitis. That said, topical high-potency steroids slightly improve pruritus and the appearance of the rash (strength of recommendation [SOR]: B, small cohort studies).
Neither topical pimecrolimus (an immunomodulatory drug) nor jewelweed extract are helpful (SOR: B, 1 small randomized controlled trial [RCT]).
Oral steroids improve symptoms in severe cases (SOR: C, expert opinion).
It’s unclear which treatment is best, because there have been no head-to-head comparisons of treatments for Rhus (plant-induced) contact dermatitis. That said, topical high-potency steroids slightly improve pruritus and the appearance of the rash (strength of recommendation [SOR]: B, small cohort studies).
Neither topical pimecrolimus (an immunomodulatory drug) nor jewelweed extract are helpful (SOR: B, 1 small randomized controlled trial [RCT]).
Oral steroids improve symptoms in severe cases (SOR: C, expert opinion).
Evidence summary
Two prospective, self-controlled cohort studies (N=30) showed that high-potency topical steroids improved symptoms associated with artificially induced Rhus dermatitis in a group with a history of that type of dermatitis.
The first study found that 0.05% clobetasol propionate ointment applied twice a day significantly reduced overall vesiculation, erythema, induration, and pruritus compared with the control (P<.05, .01, .01, and .05, respectively).1 Investigators evaluated erythema, induration, and pruritus on a scale of 0 to 3 (absent, mild, moderate, or severe) and graded vesiculation on a similar 0- to 3-point scale (a frank bulla was graded 3). They started treatment at 12, 24, and 48 hours after exposure and followed patients for 14 days. The greatest difference in mean scores—a reduction in vesiculation scores of approximately 1 point—occurred between 2 and 7 days of therapy.
The second study compared improvement in symptoms of Rhus dermatitis with daily application of topical steroids of different potencies and a control ointment.2 Investigators evaluated healing using a 0- to 4-point scale (0=clearing and 4=marked edema, erythema, and vesiculation). They found that lower-potency topical steroids such as 1% hydrocortisone and 0.1% triamcinolone were equivalent to the control ointment, but high-potency (class IV) steroid ointments produced significant improvement in symptoms (by a mean of 1.07 points vs the control ointment; supporting statistics not given).
A systematic review of contact dermatitis treatment and prevention identified 4 “good-quality” RCTs that evaluated effective remedies for nickel-induced allergic contact dermatitis in a predominantly female Caucasian population.3 All found that moderately high-potency topical steroid therapy improved symptoms, but heterogeneity among the studies made it impossible to determine the best agent.
Topical immunomodulatory drugs and jewelweed are no help
In a double-blinded RCT of 12 adults with a history of Rhus dermatitis and a significant reaction to tincture of poison ivy, topical pimecrolimus didn’t improve the duration or severity of symptoms (P=nonsignificant).4
A similar RCT from a dermatology clinic of 10 adults with confirmed sensitivity to poison oak or ivy found that topical jewelweed extract didn’t improve symptoms of artificially induced Rhus dermatitis. Investigators didn’t report P values.5
Oral steroids haven’t been studied
No studies have evaluated the effectiveness of oral steroids for Rhus dermatitis. Expert opinion recommends prednisone (60 mg daily, tapered over 14 days) for severe and widespread cases of poison ivy dermatitis.6,7
Recommendations
The American Academy of Allergy, Asthma, and Immunology and the American College of Allergy, Asthma, and Immunology jointly recommend topical corticosteroids as firstline treatment for localized allergic contact dermatitis. They advise giving systemic corticosteroids for lesions covering more than 20% of body surface area (for example, prednisone 0.5-1 mg/kg per day for 5-7 days, then 50% of the dose for another 5-7 days).6
The American Academy of Dermatology hasn’t issued guidelines on plant-induced dermatitis.
A dermatology textbook states that topical steroids are effective during the early stages of an outbreak, when vesicles and blisters aren’t yet present, and that systemic steroids are extremely effective for severe outbreaks. The authors recommend treating weepy lesions with tepid baths, wet to dry soaks, or calamine lotion to dry the lesions.7
IT’S UNCLEAR which treatment is best, because there have been no head-to-head comparisons of treatments for Rhus (plant-induced) contact dermatitis. That said, topical high-potency steroids slightly improve pruritus and the appearance of the rash (strength of recommendation [SOR]: B, small cohort studies).
Neither topical pimecrolimus (an immunomodulatory drug) nor jewelweed extract are helpful (SOR: B, 1 small randomized controlled trial [RCT]).
Oral steroids improve symptoms in severe cases (SOR: C, expert opinion).
It’s unclear which treatment is best, because there have been no head-to-head comparisons of treatments for Rhus (plant-induced) contact dermatitis. That said, topical high-potency steroids slightly improve pruritus and the appearance of the rash (strength of recommendation [SOR]: B, small cohort studies).
Neither topical pimecrolimus (an immunomodulatory drug) nor jewelweed extract are helpful (SOR: B, 1 small randomized controlled trial [RCT]).
Oral steroids improve symptoms in severe cases (SOR: C, expert opinion).
Evidence summary
Two prospective, self-controlled cohort studies (N=30) showed that high-potency topical steroids improved symptoms associated with artificially induced Rhus dermatitis in a group with a history of that type of dermatitis.
The first study found that 0.05% clobetasol propionate ointment applied twice a day significantly reduced overall vesiculation, erythema, induration, and pruritus compared with the control (P<.05, .01, .01, and .05, respectively).1 Investigators evaluated erythema, induration, and pruritus on a scale of 0 to 3 (absent, mild, moderate, or severe) and graded vesiculation on a similar 0- to 3-point scale (a frank bulla was graded 3). They started treatment at 12, 24, and 48 hours after exposure and followed patients for 14 days. The greatest difference in mean scores—a reduction in vesiculation scores of approximately 1 point—occurred between 2 and 7 days of therapy.
The second study compared improvement in symptoms of Rhus dermatitis with daily application of topical steroids of different potencies and a control ointment.2 Investigators evaluated healing using a 0- to 4-point scale (0=clearing and 4=marked edema, erythema, and vesiculation). They found that lower-potency topical steroids such as 1% hydrocortisone and 0.1% triamcinolone were equivalent to the control ointment, but high-potency (class IV) steroid ointments produced significant improvement in symptoms (by a mean of 1.07 points vs the control ointment; supporting statistics not given).
A systematic review of contact dermatitis treatment and prevention identified 4 “good-quality” RCTs that evaluated effective remedies for nickel-induced allergic contact dermatitis in a predominantly female Caucasian population.3 All found that moderately high-potency topical steroid therapy improved symptoms, but heterogeneity among the studies made it impossible to determine the best agent.
Topical immunomodulatory drugs and jewelweed are no help
In a double-blinded RCT of 12 adults with a history of Rhus dermatitis and a significant reaction to tincture of poison ivy, topical pimecrolimus didn’t improve the duration or severity of symptoms (P=nonsignificant).4
A similar RCT from a dermatology clinic of 10 adults with confirmed sensitivity to poison oak or ivy found that topical jewelweed extract didn’t improve symptoms of artificially induced Rhus dermatitis. Investigators didn’t report P values.5
Oral steroids haven’t been studied
No studies have evaluated the effectiveness of oral steroids for Rhus dermatitis. Expert opinion recommends prednisone (60 mg daily, tapered over 14 days) for severe and widespread cases of poison ivy dermatitis.6,7
Recommendations
The American Academy of Allergy, Asthma, and Immunology and the American College of Allergy, Asthma, and Immunology jointly recommend topical corticosteroids as firstline treatment for localized allergic contact dermatitis. They advise giving systemic corticosteroids for lesions covering more than 20% of body surface area (for example, prednisone 0.5-1 mg/kg per day for 5-7 days, then 50% of the dose for another 5-7 days).6
The American Academy of Dermatology hasn’t issued guidelines on plant-induced dermatitis.
A dermatology textbook states that topical steroids are effective during the early stages of an outbreak, when vesicles and blisters aren’t yet present, and that systemic steroids are extremely effective for severe outbreaks. The authors recommend treating weepy lesions with tepid baths, wet to dry soaks, or calamine lotion to dry the lesions.7
1. Vernon HJ, Olsen EA. A controlled trial of clobetasol propionate ointment 0.05% in the treatment of experimentally induced Rhus dermatitis. J Am Acad Dermatol. 1990;23:829-832.
2. Kaidbey KH, Kligman AM. Assay of topical corticosteroids: efficacy of suppression of experimental Rhus dermatitis in humans. Arch Dermatol. 1976;112:808-813.
3. Saary J, Qureshi R, Palda V, et al. A systematic review of contact dermatitis treatment and prevention. J Am Acad Dermatol. 2005;53:845.
4. Amrol D, Keitel D, Hagaman D, et al. Topical pimecrolimus in the treatment of human allergic contact dermatitis. Ann Allergy Asthma Immunol. 2003;91:563-566.
5. Long D, Ballentine NH, Marks JG Jr. Treatment of poison ivy/ oak allergic contact dermatitis with an extract of jewelweed. Am J Contact Dermatol. 1997;8:150-153.
6. American Academy of Allergy, Asthma and Immunology; American College of Allergy, Asthma and Immunology. Contact dermatitis: a practice parameter. Ann Allergy Asthma Immunol. 2006;97(suppl 2):S1-S38.
7. Habif TP. Contact dermatitis and patch testing. In: Habif TP. Clinical Dermatology: A Color Guide to Diagnosis and Therapy. 5th ed. St. Louis, Mo: Mosby; 2010:130-153.
1. Vernon HJ, Olsen EA. A controlled trial of clobetasol propionate ointment 0.05% in the treatment of experimentally induced Rhus dermatitis. J Am Acad Dermatol. 1990;23:829-832.
2. Kaidbey KH, Kligman AM. Assay of topical corticosteroids: efficacy of suppression of experimental Rhus dermatitis in humans. Arch Dermatol. 1976;112:808-813.
3. Saary J, Qureshi R, Palda V, et al. A systematic review of contact dermatitis treatment and prevention. J Am Acad Dermatol. 2005;53:845.
4. Amrol D, Keitel D, Hagaman D, et al. Topical pimecrolimus in the treatment of human allergic contact dermatitis. Ann Allergy Asthma Immunol. 2003;91:563-566.
5. Long D, Ballentine NH, Marks JG Jr. Treatment of poison ivy/ oak allergic contact dermatitis with an extract of jewelweed. Am J Contact Dermatol. 1997;8:150-153.
6. American Academy of Allergy, Asthma and Immunology; American College of Allergy, Asthma and Immunology. Contact dermatitis: a practice parameter. Ann Allergy Asthma Immunol. 2006;97(suppl 2):S1-S38.
7. Habif TP. Contact dermatitis and patch testing. In: Habif TP. Clinical Dermatology: A Color Guide to Diagnosis and Therapy. 5th ed. St. Louis, Mo: Mosby; 2010:130-153.
Evidence-based answers from the Family Physicians Inquiries Network
Which combination drug therapies are most effective for hypertension?
INSUFFICIENT EVIDENCE exists to determine which specific combinations most effectively decrease cardiovascular morbidity and mortality, although combinations of hypertension medications at lower doses generally reduce cardiovascular outcomes (stroke, coronary heart disease) more than monotherapy (strength of recommendation [SOR]: A, large meta-analyses).
The combination of benazepril and amlodipine reduces the composite endpoint of cardiovascular events and deaths more than benazepril plus hydrochlorothiazide with similar rates of adverse effects (SOR: A, randomized controlled trial [RCT]).
Combining an angiotensin converting enzyme inhibitor (ACE-I) with a thiazide, ß-blocker, or calcium channel blocker produces side effects similar to monotherapy, as does combining an angiotensin receptor blocker (ARB) with a thiazide or calcium channel blocker (SOR: A, meta-analyses). However, an ACE-I combined with an ARB increases the risk of renal complications and death more than monotherapy (SOR: A, RCT).
Evidence summary
A meta-analysis of 147 RCTs with a total of 464,000 patients demonstrated better cardiovascular outcomes for combination therapy vs monotherapy among patients 60 to 69 years of age with diastolic blood pressures 90 mm Hg or higher. Investigators randomized participants with no history of vascular disease, a history of coronary heart disease, or a history of stroke to monotherapy or a combination of 3 drugs from any class at half-standard doses. Combination therapy reduced both coronary heart disease and stroke (number needed to treat [NNT] to prevent 1 new case of coronary heart disease=4, NNT to prevent 1 stroke=3).1
Another meta-analysis of 61 prospective observational studies with a total of 1 million patients showed that for every coronary event or stroke prevented by doubling the dose of a single drug, 4 events were prevented by using combination therapy.2 A 3-point reduction in systolic blood pressure resulted in a 5% to 10% reduction in heart disease and stroke.1
A meta-analysis of 42 trials with a total of almost 11,000 patients found that combining any 2 drug classes at low doses decreased diastolic blood pressure more than doubling the dose of a single drug (9 mm Hg vs 6 mm Hg).3
ACE-I plus ß-blocker or calcium channnel blocker outperforms thiazide combos
The combination of an ACE-I plus a ß-blocker lowered systolic blood pressure more than ACE-I monotherapy (22.9 mm Hg vs 12.5 mm Hg) in an RCT with 48 patients.4 More patients taking an ACE-I plus a calcium channel blocker achieved the primary end point (reductions in systolic blood pressure ≥25 mm Hg) than did patients randomized to monotherapy (74.2% vs 53.9%; NNT=5).5
In an RCT of 11,506 patients, benazepril plus amlodipine decreased blood pressure more than benazepril plus hydrochlorothiazide (difference=0.9 mm Hg systolic, 1.1 mm Hg diastolic) and improved the composite outcome of cardiovascular events and deaths (absolute risk reduction=2.2%; NNT=45).6 Rates of adverse drug reactions were similar among patients taking ACE-I monotherapy and combinations of benazepril plus amlodipine or benazepril plus hydrochlorothiazide.4-6
ARB plus a thiazide lowers BP more than monotherapy
Five short-term RCTs comparing ARB-thiazide combinations with monotherapy measured changes in blood pressure rather than morbidity and mortality. In these studies, sponsored by pharmaceutical companies, combination treatment more often produced blood pressures within the goals of the Seventh Report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure (JNC-VII) than monotherapy (62% vs 37%; NNT to reach goal=4 [approximately]).7-8 An ARB plus hydrochlorothiazide lowered blood pressure more effectively than either drug alone but produced more dizziness (8.5% vs 4.7%; P=.002).7
In an RCT of 926 patients who had failed monotherapy with an ARB, 74.8% treated with an ARB plus a calcium channel blocker achieved blood pressures <140/90.9 Adding a calcium channel blocker decreased blood pressures by about 19 mm Hg systolic and 11 mm Hg diastolic with few adverse drug reactions.
How safe is combination therapy?
Participants in a 6-year RCT of 25,260 patients had more adverse outcomes with an ARB plus ACE-I combination than monotherapy (number needed to harm=100 to cause composite endpoint of death, dialysis, or creatinine doubling).10 For most other combinations, the safety profile is unknown or similar to monotherapy.
The TABLE summarizes the efficacy and safety profiles of antihypertensive drug combinations.4-10
TABLE
Efficacy and safety of drug combinations for essential hypertension*
| Combined with | |||||
|---|---|---|---|---|---|
| ACE-I | ARB | ß-blocker | Calcium channel blocker | Thiazide | |
| ACE-I efficacy | N/A | 16-27 mm Hg systolic BP drop (based on RCT, N=25,260)10 | 22.9 mm Hg systolic BP drop (based on RCT, N=48)4 | 13.7-20.9 mm Hg systolic BP drop† (based on RCT, N= >10,000)5 | 12.9 mm Hg systolic BP drop (based on RCT, N=11,506)6,7 |
| ACE-I safety | N/A | Increased risk of death, dialysis, doubled creatinine (NNH=100 for combined endpoint)10 | Side effects similar to ACE-I monotherapy4 | Side effects similar to ACE-I monotherapy5,6 | Side effects similar to ACE-I monotherapy6,7 |
| ARB efficacy | 16-27 mm Hg systolic BP drop (based on RCT, N=25,260)10 | N/A | Unknown | 12-20 mm Hg systolic BP drop (based on RCT, N=926)9 | 14-25 mm Hg systolic BP drop (based on subgroup analysis of large RCT and RCT, N=261)8 |
| ARB safety | Increased risk of death, dialysis, doubled creatinine (NNH=100 for combined endpoint)10 | N/A | Unknown | Side effects similar to ARB monotherapy9 | Combination increased dizziness more than ARB monotherapy (NNH=33)8 |
| ACE-I, angiotensin converting enzyme inhibitor; ARB, angiotensin receptor blocker; BP, blood pressure; N/A, not applicable; NNH, number needed to harm; RCT, randomized controlled trial. *The efficacy and safety of pairing the drugs in the column at left with those in the row at top. All combinations used approximately half the maximum dose of each component. †Significant decrease in cardiovascular mortality. | |||||
Recommendations
Both the 2003 JNC-VII and the 2008 Canadian Hypertension Education Program recommendations for managing hypertension advise lowering blood pressure to <140/90 mm Hg in all patients and <130/80 mm Hg in patients with diabetes and chronic kidney disease.11,12 Both guidelines also suggest starting therapy with 2 drugs when blood pressure is more than 20 mm Hg above systolic goal or 10 mm Hg above diastolic goal, but they do not endorse specific combinations.
1. Law MR, Morris JK, Wald NJ. Use of blood pressure lowering drugs in the prevention of cardiovascular disease: meta-analysis of 147 randomised trials in the context of expectations from prospective epidemiological studies. BMJ. 2009;338:b1665.-Available at: www.ncbi.nlm.nih.gov/pmc/articles/PMC2684577/?tool=pubmed. Accessed May 5, 2011.
2. Prospective Studies Collaboration. Age-specific relevance of usual blood pressure to vascular mortality: a meta-analysis of individual data for one million adults in 61 prospective studies. Lancet. 2002;360:1903-1913.
3. Wald DS, Law M, Morris JK, et al. Combination therapy versus monotherapy in reducing blood pressure: meta-analysis on 11,000 participants from 42 trials. Am J Med. 2009;122:290-300.
4. Wald DS, Law M, Mills S, et al. A 16-week, randomized, double-blind, placebo-controlled, crossover trial to quantify the combined effect of an angiotensin-converting enzyme inhibitor and a beta-blocker on blood pressure reduction. Clin Ther. 2008;30:2030-2039.
5. Jamerson KA, Nwose O, Jean-Louis L, et al. Initial angiotensin-converting enzyme inhibitor/calcium channel blocker combination therapy achieves superior blood pressure control compared with calcium channel blocker monotherapy in patients with stage 2 hypertension. Am J Hypertens. 2004;17:495-501.
6. Jamerson K, Weber MA, Bakris GL, et al. Benazepril plus amlodipine or hydrochlorothiazide for hypertension in high-risk patients. N Engl J Med. 2008;359:2417-2428.
7. Everett BM, Glynn RJ, Danielson E, et al. Combination therapy versus monotherapy as initial treatment for stage 2 hypertension: a prespecified subgroup analysis of a community-based, randomized, open-label trial. Clin Ther. 2008;30:661-672.
8. Oparil S, Abate N, Chen E, et al. A double-blind, randomized study evaluating losartan potassium monotherapy or in combination with hydrochlorothiazide versus placebo in obese patients with hypertension. Curr Med Res Opin. 2008;24:1101-1114.
9. Allemann Y, Fraile B, Lambert M, et al. Efficacy of the combination of amlodipine and valsartan in patients with hypertension uncontrolled with previous monotherapy: the Exforge in failure after single therapy (EX-FAST) study. J Clin Hypertens. 2008;10:185-194.
10. Mann JF, Schmieder RE, McQueen M, et al. Renal outcomes with telmisartan, ramipril, or both, in people at high vascular risk (the ONTARGET study): a multicentre, randomised, double-blind, controlled trial. Lancet. 2008;372:547-553.
11. Chobanian AV, Bakris GL, Black HR, et al. Seventh report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure. Hypertension. 2003;42:1206-1252.Available at: www.nhlbi.nih.gov/guidelines/hypertension/jnc7full.pdf. Accessed May 5, 2011.
12. Khan NA, Hemmelgarn B, Herman RJ, et al. The 2008 Canadian hypertension education program recommendations for the management of hypertension: part 2—therapy. Can J Cardiol. 2008;24:465-475.Available at: www.ncbi.nlm.nih.gov/pmc/articles/PMC2643190/?tool=pubmed. Accessed February 18, 2011.
INSUFFICIENT EVIDENCE exists to determine which specific combinations most effectively decrease cardiovascular morbidity and mortality, although combinations of hypertension medications at lower doses generally reduce cardiovascular outcomes (stroke, coronary heart disease) more than monotherapy (strength of recommendation [SOR]: A, large meta-analyses).
The combination of benazepril and amlodipine reduces the composite endpoint of cardiovascular events and deaths more than benazepril plus hydrochlorothiazide with similar rates of adverse effects (SOR: A, randomized controlled trial [RCT]).
Combining an angiotensin converting enzyme inhibitor (ACE-I) with a thiazide, ß-blocker, or calcium channel blocker produces side effects similar to monotherapy, as does combining an angiotensin receptor blocker (ARB) with a thiazide or calcium channel blocker (SOR: A, meta-analyses). However, an ACE-I combined with an ARB increases the risk of renal complications and death more than monotherapy (SOR: A, RCT).
Evidence summary
A meta-analysis of 147 RCTs with a total of 464,000 patients demonstrated better cardiovascular outcomes for combination therapy vs monotherapy among patients 60 to 69 years of age with diastolic blood pressures 90 mm Hg or higher. Investigators randomized participants with no history of vascular disease, a history of coronary heart disease, or a history of stroke to monotherapy or a combination of 3 drugs from any class at half-standard doses. Combination therapy reduced both coronary heart disease and stroke (number needed to treat [NNT] to prevent 1 new case of coronary heart disease=4, NNT to prevent 1 stroke=3).1
Another meta-analysis of 61 prospective observational studies with a total of 1 million patients showed that for every coronary event or stroke prevented by doubling the dose of a single drug, 4 events were prevented by using combination therapy.2 A 3-point reduction in systolic blood pressure resulted in a 5% to 10% reduction in heart disease and stroke.1
A meta-analysis of 42 trials with a total of almost 11,000 patients found that combining any 2 drug classes at low doses decreased diastolic blood pressure more than doubling the dose of a single drug (9 mm Hg vs 6 mm Hg).3
ACE-I plus ß-blocker or calcium channnel blocker outperforms thiazide combos
The combination of an ACE-I plus a ß-blocker lowered systolic blood pressure more than ACE-I monotherapy (22.9 mm Hg vs 12.5 mm Hg) in an RCT with 48 patients.4 More patients taking an ACE-I plus a calcium channel blocker achieved the primary end point (reductions in systolic blood pressure ≥25 mm Hg) than did patients randomized to monotherapy (74.2% vs 53.9%; NNT=5).5
In an RCT of 11,506 patients, benazepril plus amlodipine decreased blood pressure more than benazepril plus hydrochlorothiazide (difference=0.9 mm Hg systolic, 1.1 mm Hg diastolic) and improved the composite outcome of cardiovascular events and deaths (absolute risk reduction=2.2%; NNT=45).6 Rates of adverse drug reactions were similar among patients taking ACE-I monotherapy and combinations of benazepril plus amlodipine or benazepril plus hydrochlorothiazide.4-6
ARB plus a thiazide lowers BP more than monotherapy
Five short-term RCTs comparing ARB-thiazide combinations with monotherapy measured changes in blood pressure rather than morbidity and mortality. In these studies, sponsored by pharmaceutical companies, combination treatment more often produced blood pressures within the goals of the Seventh Report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure (JNC-VII) than monotherapy (62% vs 37%; NNT to reach goal=4 [approximately]).7-8 An ARB plus hydrochlorothiazide lowered blood pressure more effectively than either drug alone but produced more dizziness (8.5% vs 4.7%; P=.002).7
In an RCT of 926 patients who had failed monotherapy with an ARB, 74.8% treated with an ARB plus a calcium channel blocker achieved blood pressures <140/90.9 Adding a calcium channel blocker decreased blood pressures by about 19 mm Hg systolic and 11 mm Hg diastolic with few adverse drug reactions.
How safe is combination therapy?
Participants in a 6-year RCT of 25,260 patients had more adverse outcomes with an ARB plus ACE-I combination than monotherapy (number needed to harm=100 to cause composite endpoint of death, dialysis, or creatinine doubling).10 For most other combinations, the safety profile is unknown or similar to monotherapy.
The TABLE summarizes the efficacy and safety profiles of antihypertensive drug combinations.4-10
TABLE
Efficacy and safety of drug combinations for essential hypertension*
| Combined with | |||||
|---|---|---|---|---|---|
| ACE-I | ARB | ß-blocker | Calcium channel blocker | Thiazide | |
| ACE-I efficacy | N/A | 16-27 mm Hg systolic BP drop (based on RCT, N=25,260)10 | 22.9 mm Hg systolic BP drop (based on RCT, N=48)4 | 13.7-20.9 mm Hg systolic BP drop† (based on RCT, N= >10,000)5 | 12.9 mm Hg systolic BP drop (based on RCT, N=11,506)6,7 |
| ACE-I safety | N/A | Increased risk of death, dialysis, doubled creatinine (NNH=100 for combined endpoint)10 | Side effects similar to ACE-I monotherapy4 | Side effects similar to ACE-I monotherapy5,6 | Side effects similar to ACE-I monotherapy6,7 |
| ARB efficacy | 16-27 mm Hg systolic BP drop (based on RCT, N=25,260)10 | N/A | Unknown | 12-20 mm Hg systolic BP drop (based on RCT, N=926)9 | 14-25 mm Hg systolic BP drop (based on subgroup analysis of large RCT and RCT, N=261)8 |
| ARB safety | Increased risk of death, dialysis, doubled creatinine (NNH=100 for combined endpoint)10 | N/A | Unknown | Side effects similar to ARB monotherapy9 | Combination increased dizziness more than ARB monotherapy (NNH=33)8 |
| ACE-I, angiotensin converting enzyme inhibitor; ARB, angiotensin receptor blocker; BP, blood pressure; N/A, not applicable; NNH, number needed to harm; RCT, randomized controlled trial. *The efficacy and safety of pairing the drugs in the column at left with those in the row at top. All combinations used approximately half the maximum dose of each component. †Significant decrease in cardiovascular mortality. | |||||
Recommendations
Both the 2003 JNC-VII and the 2008 Canadian Hypertension Education Program recommendations for managing hypertension advise lowering blood pressure to <140/90 mm Hg in all patients and <130/80 mm Hg in patients with diabetes and chronic kidney disease.11,12 Both guidelines also suggest starting therapy with 2 drugs when blood pressure is more than 20 mm Hg above systolic goal or 10 mm Hg above diastolic goal, but they do not endorse specific combinations.
INSUFFICIENT EVIDENCE exists to determine which specific combinations most effectively decrease cardiovascular morbidity and mortality, although combinations of hypertension medications at lower doses generally reduce cardiovascular outcomes (stroke, coronary heart disease) more than monotherapy (strength of recommendation [SOR]: A, large meta-analyses).
The combination of benazepril and amlodipine reduces the composite endpoint of cardiovascular events and deaths more than benazepril plus hydrochlorothiazide with similar rates of adverse effects (SOR: A, randomized controlled trial [RCT]).
Combining an angiotensin converting enzyme inhibitor (ACE-I) with a thiazide, ß-blocker, or calcium channel blocker produces side effects similar to monotherapy, as does combining an angiotensin receptor blocker (ARB) with a thiazide or calcium channel blocker (SOR: A, meta-analyses). However, an ACE-I combined with an ARB increases the risk of renal complications and death more than monotherapy (SOR: A, RCT).
Evidence summary
A meta-analysis of 147 RCTs with a total of 464,000 patients demonstrated better cardiovascular outcomes for combination therapy vs monotherapy among patients 60 to 69 years of age with diastolic blood pressures 90 mm Hg or higher. Investigators randomized participants with no history of vascular disease, a history of coronary heart disease, or a history of stroke to monotherapy or a combination of 3 drugs from any class at half-standard doses. Combination therapy reduced both coronary heart disease and stroke (number needed to treat [NNT] to prevent 1 new case of coronary heart disease=4, NNT to prevent 1 stroke=3).1
Another meta-analysis of 61 prospective observational studies with a total of 1 million patients showed that for every coronary event or stroke prevented by doubling the dose of a single drug, 4 events were prevented by using combination therapy.2 A 3-point reduction in systolic blood pressure resulted in a 5% to 10% reduction in heart disease and stroke.1
A meta-analysis of 42 trials with a total of almost 11,000 patients found that combining any 2 drug classes at low doses decreased diastolic blood pressure more than doubling the dose of a single drug (9 mm Hg vs 6 mm Hg).3
ACE-I plus ß-blocker or calcium channnel blocker outperforms thiazide combos
The combination of an ACE-I plus a ß-blocker lowered systolic blood pressure more than ACE-I monotherapy (22.9 mm Hg vs 12.5 mm Hg) in an RCT with 48 patients.4 More patients taking an ACE-I plus a calcium channel blocker achieved the primary end point (reductions in systolic blood pressure ≥25 mm Hg) than did patients randomized to monotherapy (74.2% vs 53.9%; NNT=5).5
In an RCT of 11,506 patients, benazepril plus amlodipine decreased blood pressure more than benazepril plus hydrochlorothiazide (difference=0.9 mm Hg systolic, 1.1 mm Hg diastolic) and improved the composite outcome of cardiovascular events and deaths (absolute risk reduction=2.2%; NNT=45).6 Rates of adverse drug reactions were similar among patients taking ACE-I monotherapy and combinations of benazepril plus amlodipine or benazepril plus hydrochlorothiazide.4-6
ARB plus a thiazide lowers BP more than monotherapy
Five short-term RCTs comparing ARB-thiazide combinations with monotherapy measured changes in blood pressure rather than morbidity and mortality. In these studies, sponsored by pharmaceutical companies, combination treatment more often produced blood pressures within the goals of the Seventh Report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure (JNC-VII) than monotherapy (62% vs 37%; NNT to reach goal=4 [approximately]).7-8 An ARB plus hydrochlorothiazide lowered blood pressure more effectively than either drug alone but produced more dizziness (8.5% vs 4.7%; P=.002).7
In an RCT of 926 patients who had failed monotherapy with an ARB, 74.8% treated with an ARB plus a calcium channel blocker achieved blood pressures <140/90.9 Adding a calcium channel blocker decreased blood pressures by about 19 mm Hg systolic and 11 mm Hg diastolic with few adverse drug reactions.
How safe is combination therapy?
Participants in a 6-year RCT of 25,260 patients had more adverse outcomes with an ARB plus ACE-I combination than monotherapy (number needed to harm=100 to cause composite endpoint of death, dialysis, or creatinine doubling).10 For most other combinations, the safety profile is unknown or similar to monotherapy.
The TABLE summarizes the efficacy and safety profiles of antihypertensive drug combinations.4-10
TABLE
Efficacy and safety of drug combinations for essential hypertension*
| Combined with | |||||
|---|---|---|---|---|---|
| ACE-I | ARB | ß-blocker | Calcium channel blocker | Thiazide | |
| ACE-I efficacy | N/A | 16-27 mm Hg systolic BP drop (based on RCT, N=25,260)10 | 22.9 mm Hg systolic BP drop (based on RCT, N=48)4 | 13.7-20.9 mm Hg systolic BP drop† (based on RCT, N= >10,000)5 | 12.9 mm Hg systolic BP drop (based on RCT, N=11,506)6,7 |
| ACE-I safety | N/A | Increased risk of death, dialysis, doubled creatinine (NNH=100 for combined endpoint)10 | Side effects similar to ACE-I monotherapy4 | Side effects similar to ACE-I monotherapy5,6 | Side effects similar to ACE-I monotherapy6,7 |
| ARB efficacy | 16-27 mm Hg systolic BP drop (based on RCT, N=25,260)10 | N/A | Unknown | 12-20 mm Hg systolic BP drop (based on RCT, N=926)9 | 14-25 mm Hg systolic BP drop (based on subgroup analysis of large RCT and RCT, N=261)8 |
| ARB safety | Increased risk of death, dialysis, doubled creatinine (NNH=100 for combined endpoint)10 | N/A | Unknown | Side effects similar to ARB monotherapy9 | Combination increased dizziness more than ARB monotherapy (NNH=33)8 |
| ACE-I, angiotensin converting enzyme inhibitor; ARB, angiotensin receptor blocker; BP, blood pressure; N/A, not applicable; NNH, number needed to harm; RCT, randomized controlled trial. *The efficacy and safety of pairing the drugs in the column at left with those in the row at top. All combinations used approximately half the maximum dose of each component. †Significant decrease in cardiovascular mortality. | |||||
Recommendations
Both the 2003 JNC-VII and the 2008 Canadian Hypertension Education Program recommendations for managing hypertension advise lowering blood pressure to <140/90 mm Hg in all patients and <130/80 mm Hg in patients with diabetes and chronic kidney disease.11,12 Both guidelines also suggest starting therapy with 2 drugs when blood pressure is more than 20 mm Hg above systolic goal or 10 mm Hg above diastolic goal, but they do not endorse specific combinations.
1. Law MR, Morris JK, Wald NJ. Use of blood pressure lowering drugs in the prevention of cardiovascular disease: meta-analysis of 147 randomised trials in the context of expectations from prospective epidemiological studies. BMJ. 2009;338:b1665.-Available at: www.ncbi.nlm.nih.gov/pmc/articles/PMC2684577/?tool=pubmed. Accessed May 5, 2011.
2. Prospective Studies Collaboration. Age-specific relevance of usual blood pressure to vascular mortality: a meta-analysis of individual data for one million adults in 61 prospective studies. Lancet. 2002;360:1903-1913.
3. Wald DS, Law M, Morris JK, et al. Combination therapy versus monotherapy in reducing blood pressure: meta-analysis on 11,000 participants from 42 trials. Am J Med. 2009;122:290-300.
4. Wald DS, Law M, Mills S, et al. A 16-week, randomized, double-blind, placebo-controlled, crossover trial to quantify the combined effect of an angiotensin-converting enzyme inhibitor and a beta-blocker on blood pressure reduction. Clin Ther. 2008;30:2030-2039.
5. Jamerson KA, Nwose O, Jean-Louis L, et al. Initial angiotensin-converting enzyme inhibitor/calcium channel blocker combination therapy achieves superior blood pressure control compared with calcium channel blocker monotherapy in patients with stage 2 hypertension. Am J Hypertens. 2004;17:495-501.
6. Jamerson K, Weber MA, Bakris GL, et al. Benazepril plus amlodipine or hydrochlorothiazide for hypertension in high-risk patients. N Engl J Med. 2008;359:2417-2428.
7. Everett BM, Glynn RJ, Danielson E, et al. Combination therapy versus monotherapy as initial treatment for stage 2 hypertension: a prespecified subgroup analysis of a community-based, randomized, open-label trial. Clin Ther. 2008;30:661-672.
8. Oparil S, Abate N, Chen E, et al. A double-blind, randomized study evaluating losartan potassium monotherapy or in combination with hydrochlorothiazide versus placebo in obese patients with hypertension. Curr Med Res Opin. 2008;24:1101-1114.
9. Allemann Y, Fraile B, Lambert M, et al. Efficacy of the combination of amlodipine and valsartan in patients with hypertension uncontrolled with previous monotherapy: the Exforge in failure after single therapy (EX-FAST) study. J Clin Hypertens. 2008;10:185-194.
10. Mann JF, Schmieder RE, McQueen M, et al. Renal outcomes with telmisartan, ramipril, or both, in people at high vascular risk (the ONTARGET study): a multicentre, randomised, double-blind, controlled trial. Lancet. 2008;372:547-553.
11. Chobanian AV, Bakris GL, Black HR, et al. Seventh report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure. Hypertension. 2003;42:1206-1252.Available at: www.nhlbi.nih.gov/guidelines/hypertension/jnc7full.pdf. Accessed May 5, 2011.
12. Khan NA, Hemmelgarn B, Herman RJ, et al. The 2008 Canadian hypertension education program recommendations for the management of hypertension: part 2—therapy. Can J Cardiol. 2008;24:465-475.Available at: www.ncbi.nlm.nih.gov/pmc/articles/PMC2643190/?tool=pubmed. Accessed February 18, 2011.
1. Law MR, Morris JK, Wald NJ. Use of blood pressure lowering drugs in the prevention of cardiovascular disease: meta-analysis of 147 randomised trials in the context of expectations from prospective epidemiological studies. BMJ. 2009;338:b1665.-Available at: www.ncbi.nlm.nih.gov/pmc/articles/PMC2684577/?tool=pubmed. Accessed May 5, 2011.
2. Prospective Studies Collaboration. Age-specific relevance of usual blood pressure to vascular mortality: a meta-analysis of individual data for one million adults in 61 prospective studies. Lancet. 2002;360:1903-1913.
3. Wald DS, Law M, Morris JK, et al. Combination therapy versus monotherapy in reducing blood pressure: meta-analysis on 11,000 participants from 42 trials. Am J Med. 2009;122:290-300.
4. Wald DS, Law M, Mills S, et al. A 16-week, randomized, double-blind, placebo-controlled, crossover trial to quantify the combined effect of an angiotensin-converting enzyme inhibitor and a beta-blocker on blood pressure reduction. Clin Ther. 2008;30:2030-2039.
5. Jamerson KA, Nwose O, Jean-Louis L, et al. Initial angiotensin-converting enzyme inhibitor/calcium channel blocker combination therapy achieves superior blood pressure control compared with calcium channel blocker monotherapy in patients with stage 2 hypertension. Am J Hypertens. 2004;17:495-501.
6. Jamerson K, Weber MA, Bakris GL, et al. Benazepril plus amlodipine or hydrochlorothiazide for hypertension in high-risk patients. N Engl J Med. 2008;359:2417-2428.
7. Everett BM, Glynn RJ, Danielson E, et al. Combination therapy versus monotherapy as initial treatment for stage 2 hypertension: a prespecified subgroup analysis of a community-based, randomized, open-label trial. Clin Ther. 2008;30:661-672.
8. Oparil S, Abate N, Chen E, et al. A double-blind, randomized study evaluating losartan potassium monotherapy or in combination with hydrochlorothiazide versus placebo in obese patients with hypertension. Curr Med Res Opin. 2008;24:1101-1114.
9. Allemann Y, Fraile B, Lambert M, et al. Efficacy of the combination of amlodipine and valsartan in patients with hypertension uncontrolled with previous monotherapy: the Exforge in failure after single therapy (EX-FAST) study. J Clin Hypertens. 2008;10:185-194.
10. Mann JF, Schmieder RE, McQueen M, et al. Renal outcomes with telmisartan, ramipril, or both, in people at high vascular risk (the ONTARGET study): a multicentre, randomised, double-blind, controlled trial. Lancet. 2008;372:547-553.
11. Chobanian AV, Bakris GL, Black HR, et al. Seventh report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure. Hypertension. 2003;42:1206-1252.Available at: www.nhlbi.nih.gov/guidelines/hypertension/jnc7full.pdf. Accessed May 5, 2011.
12. Khan NA, Hemmelgarn B, Herman RJ, et al. The 2008 Canadian hypertension education program recommendations for the management of hypertension: part 2—therapy. Can J Cardiol. 2008;24:465-475.Available at: www.ncbi.nlm.nih.gov/pmc/articles/PMC2643190/?tool=pubmed. Accessed February 18, 2011.
Evidence-based answers from the Family Physicians Inquiries Network
What is the most effective and safe malaria prophylaxis during pregnancy?
Chloroquine and mefloquine have superior safety profiles in pregnancy, though all antimalarials are effective for prophylaxis. Antimalarials will decrease the severity of maternal malaria infection and malaria-associated anemia, while decreasing the incidence of low birth weight and perinatal death in women having their first or second baby (strength of recommendation [SOR]: A, based on systematic review of consistent, good-quality patient-oriented evidence).
You can determine malaria risk and sensitivity of Plasmodium species by country at wwwn.cdc.gov/travel/destinationlist.aspx.1 Urge women to delay travel until after pregnancy if possible2 (SOR: C, based on patient-oriented expert opinion).
Don’t forget to discuss mosquito netting and insect repellant
Meg Hayes, MD
Department of Family Medicine, Oregon Health and Science University, Portland
Adverse outcomes associated with malaria during pregnancy include restricted fetal growth, low birth weight, preterm delivery, congenital infection, spontaneous abortion, and perinatal death. You should counsel travelers to avoid travel to areas where malaria is endemic during pregnancy.
For those who are unable to avoid travel, or who reside in malaria-endemic areas during pregnancy, physicians should focus not only on chemoprophylaxis, but provide verbal and written counsel regarding malaria personal protection measures. Because mosquitoes usually feed at night, travelers should remain within screened areas after dusk, use permethrin-treated bed nets, wear protective clothing, and apply insect repellant. Advise patients who travel to malaria-endemic areas to quickly report febrile illnesses and to disclose their travel histories to healthcare providers.
Evidence summary
Malaria is a parasitic infection that causes significant morbidity and mortality worldwide, with more than 500 million people becoming severely ill every year.2 For pregnant women, malarial infection can be severe, with high fevers, chills, and anemia leading to increased risk of poor maternal and fetal outcomes—including death. Pregnant women are also more likely to become infected and to develop more severe disease—they attract twice as many mosquitoes as nonpregnant women and have a relative immuno-suppression.3
TABLE
Antimalarials for prophylaxis: Chloroquine, mefloquine are best choices during pregnancy
| DRUG | EFFICACY | SAFETY | PREGNANCY CLASS* | AVAILABILITY |
|---|---|---|---|---|
| Chloroquine | Good | Excellent | C | Worldwide |
| Chloroquine/proguanil | Good | Excellent | C | Worldwide |
| Mefloquine | Excellent | Good | C | Worldwide |
| Quinine | Excellent | Good | C† | Worldwide |
| Atovaquone/proguanil | Excellent | Good | C (poorly studied) | Worldwide |
| Artesunate | Excellent | Good | N/A | Asia, Africa, limited in UK, not in US |
| Primaquine | Good | Fair | C‡ | Worldwide |
| Doxycycline | Excellent | Fair | D (teratogenic) | Worldwide |
| Sulfadoxine/pyrimethamine | Fair | Poor | C | Worldwide, but restricted in US |
| Note: Prescribers and patients are urged to refer to the CDC reference about pregnancy in malaria (wwwn.cdc.gov/travel/contentMalariaPregnantPublic.aspx) and to specific country information regarding sensitivities of malaria (wwwn.cdc.gov/travel/destinationList.aspx). | ||||
| * Pregnancy class C: Animal reproduction studies have shown an adverse effect on the fetus and there are no adequate and well-controlled studies in humans, but potential benefits may warrant use of the drug in pregnant women, despite potential risks. | ||||
| * Pregnancy class D: There is positive evidence of human fetal risk based on adverse reaction data from investigational or marketing experience or studies in humans, but potential benefits may warrant use of the drug in pregnant women, despite potential risks. | ||||
| † Monitor patients for maternal hypoglycemia. | ||||
| ‡ There is no evidence of teratogenicity, but primaquine is associated with fetal intravascular hemolysis. | ||||
Chemoprophylaxis lowers rates of maternal infection
Although prophylaxis for pregnant patients traveling to malarial regions is a public concern, data for decision-making must be extrapolated from the available evidence, which is based primarily on women living in endemic areas. In a Cochrane systematic review, antimalarials were found to decrease the incidence of maternal infections (relative risk [RR]=0.27; 95% confidence interval [CI], 0.17–0.44) and reduce maternal anemia (RR=0.62; 95% CI, 0.50–0.78) in low-parity women—ie, during a first or second pregnancy.3
In low-parity women, these drugs were also found to decrease perinatal death (RR=0.73; 95% CI, 0.53–0.99) and low birth weight (RR=0.57; 95% CI, 0.46–0.72) associated with malarial infection. When used in all parity groups, antimalarials were somewhat less effective, yet still reduced maternal infections (RR=0.53; 95% CI, 0.33–0.86); the effects were similar with all antimalarials tested.3,4
Chloroquine, mefloquine are safe in pregnancy, doxycycline is not
While chemoprophylaxis in pregnancy appears efficacious, a major question remains—which agents are safest for both the woman and fetus? Some drugs routinely used in nonpregnant individuals should not be offered to pregnant women because of known direct effects on the fetus. Doxycycline is teratogenic, and primaquine poses a significant risk of fetal intravascular hemolysis in G6PD-deficient fetuses.5 Other drugs, such as atovaquone/proguanil and artesunate, are not well studied in pregnancy, and therefore are not recommended for use unless other options are not available.2,6
Among drugs that are well studied and without known direct fetal-damaging effects, adverse drug reaction profiles can guide use based on disease prevalence and drug-resistance patterns.
- Chloroquine is widely used because it is inexpensive and well tolerated, with only pruritus, mouth ulcers, and gastrointestinal upset as the most common adverse effects.
- Mefloquine is usually well tolerated, but can cause dose-related neuropsychiatric effects; it is contraindicated in those with a history of epilepsy or psychiatric disease.
- Sulfadoxine and pyrimethamine are not normally used as prophylaxis for any patient, due to the risk of toxic epidermal necrolysis and Stevens-Johnson syndrome, and the possible risk of jaundice and kernicterus if used in the third trimester of pregnancy.
- Quinine, which can be used for treatment or prophylaxis, may cause hypoglycemia, an effect that is more pronounced during pregnancy and requires close monitoring of blood glucose levels.5,7
Given these reaction profiles, chloroquine or mefloquine are usually the best choice with their superior safety and efficacy.
Figure
Best protection: Avoidance
Chloroquine and mefloquine are the safest antimalarials for use in pregnant women, but personal protection measures are also critical. Above, an Anopheles stephensi mosquito expelling a droplet of blood from its abdomen after having engorged itself on its human host’s blood. (Source: CDC.)
Recommendations from others
The World Health Organization (WHO) recommends pregnant women avoid travel to malarial regions. If travel is required, WHO recommends chloroquine as first-line prophylaxis in pregnancy (plus proguanil if the region exhibits emerging chloroquine resistance). In areas with proven chloroquine resistance, mefloquine is the drug of choice. Other antimalarials—such as quinine, pyrimethamine, sulfadoxine, and artesunate—should not be withheld if the preferred drugs are not available, or if the infection is life-threatening.2
The Centers for Disease Control and Prevention (CDC) also recommends avoiding travel to malaria-endemic regions during pregnancy, but if travel is necessary, the CDC advises use of chloroquine (or mefloquine in regions with chloroquine resistance). The CDC discourages the use of atovaquone/proguanil, doxycycline, and primaquine, due to known adverse fetal effects or inadequate experience in pregnancy.6
Acknowledgments
The opinions and assertions contained herein are the private views of the authors and not to be construed as official, or as reflecting the views of the US Air Force Medical Service or the US Air Force at large.
1. Centers for Disease Control and Prevention Web site. Destinations: CDC Traveler’s Health. Available at: wwwn.cdc.gov/travel/destinationlist.aspx. Accessed on December 7, 2007.
2. Malaria. In: International Travel and Health. Geneva, Switzerland: World Health Organization; 2007. Available at: whqlibdoc.who.int/publications/2005/9241580364_chap7.pdf. Accessed on December 7, 2007.
3. Orton L, Garner P. Drugs for treating uncomplicated malaria in pregnant women. Cochrane Database Syst Rev 2005;(3):CD004912.-
4. Garner P, Gülmezoglu AM. Drugs for preventing malaria in pregnant women. Cochrane Database Syst Rev 2006;(4):CD000169.-
5. Phillips-Howard PA, Wood D. The safety of antimalarial drugs in pregnancy. Drug Saf 1996;14:131-145.
6. Centers for Disease Control and Prevention Web site. Diseases: Malaria: Prevention, Pregnant Women, Public Info. Available at: wwwn.cdc.gov/travel/ contentMalariaPregnantPublic.aspx. Accessed on December 7, 2007.
7. Taylor WR, White NJ. Antimalarial drug toxicity: a review. Drug Saf 2004;27:25-61.
Chloroquine and mefloquine have superior safety profiles in pregnancy, though all antimalarials are effective for prophylaxis. Antimalarials will decrease the severity of maternal malaria infection and malaria-associated anemia, while decreasing the incidence of low birth weight and perinatal death in women having their first or second baby (strength of recommendation [SOR]: A, based on systematic review of consistent, good-quality patient-oriented evidence).
You can determine malaria risk and sensitivity of Plasmodium species by country at wwwn.cdc.gov/travel/destinationlist.aspx.1 Urge women to delay travel until after pregnancy if possible2 (SOR: C, based on patient-oriented expert opinion).
Don’t forget to discuss mosquito netting and insect repellant
Meg Hayes, MD
Department of Family Medicine, Oregon Health and Science University, Portland
Adverse outcomes associated with malaria during pregnancy include restricted fetal growth, low birth weight, preterm delivery, congenital infection, spontaneous abortion, and perinatal death. You should counsel travelers to avoid travel to areas where malaria is endemic during pregnancy.
For those who are unable to avoid travel, or who reside in malaria-endemic areas during pregnancy, physicians should focus not only on chemoprophylaxis, but provide verbal and written counsel regarding malaria personal protection measures. Because mosquitoes usually feed at night, travelers should remain within screened areas after dusk, use permethrin-treated bed nets, wear protective clothing, and apply insect repellant. Advise patients who travel to malaria-endemic areas to quickly report febrile illnesses and to disclose their travel histories to healthcare providers.
Evidence summary
Malaria is a parasitic infection that causes significant morbidity and mortality worldwide, with more than 500 million people becoming severely ill every year.2 For pregnant women, malarial infection can be severe, with high fevers, chills, and anemia leading to increased risk of poor maternal and fetal outcomes—including death. Pregnant women are also more likely to become infected and to develop more severe disease—they attract twice as many mosquitoes as nonpregnant women and have a relative immuno-suppression.3
TABLE
Antimalarials for prophylaxis: Chloroquine, mefloquine are best choices during pregnancy
| DRUG | EFFICACY | SAFETY | PREGNANCY CLASS* | AVAILABILITY |
|---|---|---|---|---|
| Chloroquine | Good | Excellent | C | Worldwide |
| Chloroquine/proguanil | Good | Excellent | C | Worldwide |
| Mefloquine | Excellent | Good | C | Worldwide |
| Quinine | Excellent | Good | C† | Worldwide |
| Atovaquone/proguanil | Excellent | Good | C (poorly studied) | Worldwide |
| Artesunate | Excellent | Good | N/A | Asia, Africa, limited in UK, not in US |
| Primaquine | Good | Fair | C‡ | Worldwide |
| Doxycycline | Excellent | Fair | D (teratogenic) | Worldwide |
| Sulfadoxine/pyrimethamine | Fair | Poor | C | Worldwide, but restricted in US |
| Note: Prescribers and patients are urged to refer to the CDC reference about pregnancy in malaria (wwwn.cdc.gov/travel/contentMalariaPregnantPublic.aspx) and to specific country information regarding sensitivities of malaria (wwwn.cdc.gov/travel/destinationList.aspx). | ||||
| * Pregnancy class C: Animal reproduction studies have shown an adverse effect on the fetus and there are no adequate and well-controlled studies in humans, but potential benefits may warrant use of the drug in pregnant women, despite potential risks. | ||||
| * Pregnancy class D: There is positive evidence of human fetal risk based on adverse reaction data from investigational or marketing experience or studies in humans, but potential benefits may warrant use of the drug in pregnant women, despite potential risks. | ||||
| † Monitor patients for maternal hypoglycemia. | ||||
| ‡ There is no evidence of teratogenicity, but primaquine is associated with fetal intravascular hemolysis. | ||||
Chemoprophylaxis lowers rates of maternal infection
Although prophylaxis for pregnant patients traveling to malarial regions is a public concern, data for decision-making must be extrapolated from the available evidence, which is based primarily on women living in endemic areas. In a Cochrane systematic review, antimalarials were found to decrease the incidence of maternal infections (relative risk [RR]=0.27; 95% confidence interval [CI], 0.17–0.44) and reduce maternal anemia (RR=0.62; 95% CI, 0.50–0.78) in low-parity women—ie, during a first or second pregnancy.3
In low-parity women, these drugs were also found to decrease perinatal death (RR=0.73; 95% CI, 0.53–0.99) and low birth weight (RR=0.57; 95% CI, 0.46–0.72) associated with malarial infection. When used in all parity groups, antimalarials were somewhat less effective, yet still reduced maternal infections (RR=0.53; 95% CI, 0.33–0.86); the effects were similar with all antimalarials tested.3,4
Chloroquine, mefloquine are safe in pregnancy, doxycycline is not
While chemoprophylaxis in pregnancy appears efficacious, a major question remains—which agents are safest for both the woman and fetus? Some drugs routinely used in nonpregnant individuals should not be offered to pregnant women because of known direct effects on the fetus. Doxycycline is teratogenic, and primaquine poses a significant risk of fetal intravascular hemolysis in G6PD-deficient fetuses.5 Other drugs, such as atovaquone/proguanil and artesunate, are not well studied in pregnancy, and therefore are not recommended for use unless other options are not available.2,6
Among drugs that are well studied and without known direct fetal-damaging effects, adverse drug reaction profiles can guide use based on disease prevalence and drug-resistance patterns.
- Chloroquine is widely used because it is inexpensive and well tolerated, with only pruritus, mouth ulcers, and gastrointestinal upset as the most common adverse effects.
- Mefloquine is usually well tolerated, but can cause dose-related neuropsychiatric effects; it is contraindicated in those with a history of epilepsy or psychiatric disease.
- Sulfadoxine and pyrimethamine are not normally used as prophylaxis for any patient, due to the risk of toxic epidermal necrolysis and Stevens-Johnson syndrome, and the possible risk of jaundice and kernicterus if used in the third trimester of pregnancy.
- Quinine, which can be used for treatment or prophylaxis, may cause hypoglycemia, an effect that is more pronounced during pregnancy and requires close monitoring of blood glucose levels.5,7
Given these reaction profiles, chloroquine or mefloquine are usually the best choice with their superior safety and efficacy.
Figure
Best protection: Avoidance
Chloroquine and mefloquine are the safest antimalarials for use in pregnant women, but personal protection measures are also critical. Above, an Anopheles stephensi mosquito expelling a droplet of blood from its abdomen after having engorged itself on its human host’s blood. (Source: CDC.)
Recommendations from others
The World Health Organization (WHO) recommends pregnant women avoid travel to malarial regions. If travel is required, WHO recommends chloroquine as first-line prophylaxis in pregnancy (plus proguanil if the region exhibits emerging chloroquine resistance). In areas with proven chloroquine resistance, mefloquine is the drug of choice. Other antimalarials—such as quinine, pyrimethamine, sulfadoxine, and artesunate—should not be withheld if the preferred drugs are not available, or if the infection is life-threatening.2
The Centers for Disease Control and Prevention (CDC) also recommends avoiding travel to malaria-endemic regions during pregnancy, but if travel is necessary, the CDC advises use of chloroquine (or mefloquine in regions with chloroquine resistance). The CDC discourages the use of atovaquone/proguanil, doxycycline, and primaquine, due to known adverse fetal effects or inadequate experience in pregnancy.6
Acknowledgments
The opinions and assertions contained herein are the private views of the authors and not to be construed as official, or as reflecting the views of the US Air Force Medical Service or the US Air Force at large.
Chloroquine and mefloquine have superior safety profiles in pregnancy, though all antimalarials are effective for prophylaxis. Antimalarials will decrease the severity of maternal malaria infection and malaria-associated anemia, while decreasing the incidence of low birth weight and perinatal death in women having their first or second baby (strength of recommendation [SOR]: A, based on systematic review of consistent, good-quality patient-oriented evidence).
You can determine malaria risk and sensitivity of Plasmodium species by country at wwwn.cdc.gov/travel/destinationlist.aspx.1 Urge women to delay travel until after pregnancy if possible2 (SOR: C, based on patient-oriented expert opinion).
Don’t forget to discuss mosquito netting and insect repellant
Meg Hayes, MD
Department of Family Medicine, Oregon Health and Science University, Portland
Adverse outcomes associated with malaria during pregnancy include restricted fetal growth, low birth weight, preterm delivery, congenital infection, spontaneous abortion, and perinatal death. You should counsel travelers to avoid travel to areas where malaria is endemic during pregnancy.
For those who are unable to avoid travel, or who reside in malaria-endemic areas during pregnancy, physicians should focus not only on chemoprophylaxis, but provide verbal and written counsel regarding malaria personal protection measures. Because mosquitoes usually feed at night, travelers should remain within screened areas after dusk, use permethrin-treated bed nets, wear protective clothing, and apply insect repellant. Advise patients who travel to malaria-endemic areas to quickly report febrile illnesses and to disclose their travel histories to healthcare providers.
Evidence summary
Malaria is a parasitic infection that causes significant morbidity and mortality worldwide, with more than 500 million people becoming severely ill every year.2 For pregnant women, malarial infection can be severe, with high fevers, chills, and anemia leading to increased risk of poor maternal and fetal outcomes—including death. Pregnant women are also more likely to become infected and to develop more severe disease—they attract twice as many mosquitoes as nonpregnant women and have a relative immuno-suppression.3
TABLE
Antimalarials for prophylaxis: Chloroquine, mefloquine are best choices during pregnancy
| DRUG | EFFICACY | SAFETY | PREGNANCY CLASS* | AVAILABILITY |
|---|---|---|---|---|
| Chloroquine | Good | Excellent | C | Worldwide |
| Chloroquine/proguanil | Good | Excellent | C | Worldwide |
| Mefloquine | Excellent | Good | C | Worldwide |
| Quinine | Excellent | Good | C† | Worldwide |
| Atovaquone/proguanil | Excellent | Good | C (poorly studied) | Worldwide |
| Artesunate | Excellent | Good | N/A | Asia, Africa, limited in UK, not in US |
| Primaquine | Good | Fair | C‡ | Worldwide |
| Doxycycline | Excellent | Fair | D (teratogenic) | Worldwide |
| Sulfadoxine/pyrimethamine | Fair | Poor | C | Worldwide, but restricted in US |
| Note: Prescribers and patients are urged to refer to the CDC reference about pregnancy in malaria (wwwn.cdc.gov/travel/contentMalariaPregnantPublic.aspx) and to specific country information regarding sensitivities of malaria (wwwn.cdc.gov/travel/destinationList.aspx). | ||||
| * Pregnancy class C: Animal reproduction studies have shown an adverse effect on the fetus and there are no adequate and well-controlled studies in humans, but potential benefits may warrant use of the drug in pregnant women, despite potential risks. | ||||
| * Pregnancy class D: There is positive evidence of human fetal risk based on adverse reaction data from investigational or marketing experience or studies in humans, but potential benefits may warrant use of the drug in pregnant women, despite potential risks. | ||||
| † Monitor patients for maternal hypoglycemia. | ||||
| ‡ There is no evidence of teratogenicity, but primaquine is associated with fetal intravascular hemolysis. | ||||
Chemoprophylaxis lowers rates of maternal infection
Although prophylaxis for pregnant patients traveling to malarial regions is a public concern, data for decision-making must be extrapolated from the available evidence, which is based primarily on women living in endemic areas. In a Cochrane systematic review, antimalarials were found to decrease the incidence of maternal infections (relative risk [RR]=0.27; 95% confidence interval [CI], 0.17–0.44) and reduce maternal anemia (RR=0.62; 95% CI, 0.50–0.78) in low-parity women—ie, during a first or second pregnancy.3
In low-parity women, these drugs were also found to decrease perinatal death (RR=0.73; 95% CI, 0.53–0.99) and low birth weight (RR=0.57; 95% CI, 0.46–0.72) associated with malarial infection. When used in all parity groups, antimalarials were somewhat less effective, yet still reduced maternal infections (RR=0.53; 95% CI, 0.33–0.86); the effects were similar with all antimalarials tested.3,4
Chloroquine, mefloquine are safe in pregnancy, doxycycline is not
While chemoprophylaxis in pregnancy appears efficacious, a major question remains—which agents are safest for both the woman and fetus? Some drugs routinely used in nonpregnant individuals should not be offered to pregnant women because of known direct effects on the fetus. Doxycycline is teratogenic, and primaquine poses a significant risk of fetal intravascular hemolysis in G6PD-deficient fetuses.5 Other drugs, such as atovaquone/proguanil and artesunate, are not well studied in pregnancy, and therefore are not recommended for use unless other options are not available.2,6
Among drugs that are well studied and without known direct fetal-damaging effects, adverse drug reaction profiles can guide use based on disease prevalence and drug-resistance patterns.
- Chloroquine is widely used because it is inexpensive and well tolerated, with only pruritus, mouth ulcers, and gastrointestinal upset as the most common adverse effects.
- Mefloquine is usually well tolerated, but can cause dose-related neuropsychiatric effects; it is contraindicated in those with a history of epilepsy or psychiatric disease.
- Sulfadoxine and pyrimethamine are not normally used as prophylaxis for any patient, due to the risk of toxic epidermal necrolysis and Stevens-Johnson syndrome, and the possible risk of jaundice and kernicterus if used in the third trimester of pregnancy.
- Quinine, which can be used for treatment or prophylaxis, may cause hypoglycemia, an effect that is more pronounced during pregnancy and requires close monitoring of blood glucose levels.5,7
Given these reaction profiles, chloroquine or mefloquine are usually the best choice with their superior safety and efficacy.
Figure
Best protection: Avoidance
Chloroquine and mefloquine are the safest antimalarials for use in pregnant women, but personal protection measures are also critical. Above, an Anopheles stephensi mosquito expelling a droplet of blood from its abdomen after having engorged itself on its human host’s blood. (Source: CDC.)
Recommendations from others
The World Health Organization (WHO) recommends pregnant women avoid travel to malarial regions. If travel is required, WHO recommends chloroquine as first-line prophylaxis in pregnancy (plus proguanil if the region exhibits emerging chloroquine resistance). In areas with proven chloroquine resistance, mefloquine is the drug of choice. Other antimalarials—such as quinine, pyrimethamine, sulfadoxine, and artesunate—should not be withheld if the preferred drugs are not available, or if the infection is life-threatening.2
The Centers for Disease Control and Prevention (CDC) also recommends avoiding travel to malaria-endemic regions during pregnancy, but if travel is necessary, the CDC advises use of chloroquine (or mefloquine in regions with chloroquine resistance). The CDC discourages the use of atovaquone/proguanil, doxycycline, and primaquine, due to known adverse fetal effects or inadequate experience in pregnancy.6
Acknowledgments
The opinions and assertions contained herein are the private views of the authors and not to be construed as official, or as reflecting the views of the US Air Force Medical Service or the US Air Force at large.
1. Centers for Disease Control and Prevention Web site. Destinations: CDC Traveler’s Health. Available at: wwwn.cdc.gov/travel/destinationlist.aspx. Accessed on December 7, 2007.
2. Malaria. In: International Travel and Health. Geneva, Switzerland: World Health Organization; 2007. Available at: whqlibdoc.who.int/publications/2005/9241580364_chap7.pdf. Accessed on December 7, 2007.
3. Orton L, Garner P. Drugs for treating uncomplicated malaria in pregnant women. Cochrane Database Syst Rev 2005;(3):CD004912.-
4. Garner P, Gülmezoglu AM. Drugs for preventing malaria in pregnant women. Cochrane Database Syst Rev 2006;(4):CD000169.-
5. Phillips-Howard PA, Wood D. The safety of antimalarial drugs in pregnancy. Drug Saf 1996;14:131-145.
6. Centers for Disease Control and Prevention Web site. Diseases: Malaria: Prevention, Pregnant Women, Public Info. Available at: wwwn.cdc.gov/travel/ contentMalariaPregnantPublic.aspx. Accessed on December 7, 2007.
7. Taylor WR, White NJ. Antimalarial drug toxicity: a review. Drug Saf 2004;27:25-61.
1. Centers for Disease Control and Prevention Web site. Destinations: CDC Traveler’s Health. Available at: wwwn.cdc.gov/travel/destinationlist.aspx. Accessed on December 7, 2007.
2. Malaria. In: International Travel and Health. Geneva, Switzerland: World Health Organization; 2007. Available at: whqlibdoc.who.int/publications/2005/9241580364_chap7.pdf. Accessed on December 7, 2007.
3. Orton L, Garner P. Drugs for treating uncomplicated malaria in pregnant women. Cochrane Database Syst Rev 2005;(3):CD004912.-
4. Garner P, Gülmezoglu AM. Drugs for preventing malaria in pregnant women. Cochrane Database Syst Rev 2006;(4):CD000169.-
5. Phillips-Howard PA, Wood D. The safety of antimalarial drugs in pregnancy. Drug Saf 1996;14:131-145.
6. Centers for Disease Control and Prevention Web site. Diseases: Malaria: Prevention, Pregnant Women, Public Info. Available at: wwwn.cdc.gov/travel/ contentMalariaPregnantPublic.aspx. Accessed on December 7, 2007.
7. Taylor WR, White NJ. Antimalarial drug toxicity: a review. Drug Saf 2004;27:25-61.
Evidence-based answers from the Family Physicians Inquiries Network
What is the best portable method of purifying water to prevent infectious disease?
There isn’t a single best method, but there are 5 that adequately purify water according to environmental Protection agency (EPA) standards. These include 1) boiling for 1 minute if below 2000 m (6562 feet) and 3 minutes if above, 2) chlorine dioxide tablets, 3) MIoX purifier, 4) ultraviolet light (steriPEN), and 5) portable filtration with a absolute pore size <1 micrometer combined with halogenation or charcoal filtration (strength of recommendation [SOR]: C, based on expert opinion and microbiological testing). Halogenation alone (ie, chlorine and iodine) is not effective against Cryptosporidium (SOR: C, based on microbiological testing).
Why boil water when there are so many other options?
Timothy Mott, MD, FAAFP
US Naval Hospital, Sigonella, Italy
These days, “boil it, peel it, or forget it” only goes so far with the unencumbered traveler. Experience tells me that most hear “Boil it” and instantly go right to “Forget it!” Fortunately, there is an excellent resource to assist patients in choosing a personally acceptable portable water purification system. It’s called the Water Purification Database at usachppm.apgea.army.mil/WPD/CompareDevices.aspx.1
This outstanding database was developed by an impartial third-party for the US Army and gives clear, well-organized guidance on over 60 purifiers. For each purifier, the guide covers efficacy against primary pathogens, purification mechanism, links to manufacturers, and an advantages/ disadvantages breakdown (such as weight, cost, and ease of use). Add this site to your Internet “favorites” folder.
Evidence summary
With the rise in international travel and adventure sports, individuals are at increased risk of acquiring infections by drinking water from impure water sources. Common waterborne infections that back-country and international travelers may contract include bacterial diarrhea, viruses, protozoa (such as Giardia and Cryptosporidium), and parasites (such as schistosoma). The risk of infection varies based on travel location.
To prevent illness, travelers may seek medical guidance regarding safe water practice. In one study, 36% of travelers sought advice from a physician prior to international travel.2 Preventing waterborne infections should be a component of traveler education, in addition to other standard advice, such as mosquito avoidance and immunizations.3 (For more on travel safety, see these Clinical Inquiries: “When should travelers begin malaria prophylaxis?” in the November 2007 Journal of Family Practice, pages 950–952, and “What is the most effective and safe malaria prophylaxis during pregnancy?” on page 51 of this issue.)
Which devices meet EPA standards?
The EPA has established a “minimal microbiological hazard” allowed for a portable water purification system to be considered safe. Water purifiers must reduce bacteria by 99.9999%, viruses by 99.99%, and protozoa (such as Cryptosporidium parvum) by 99.9% to receive an EPA certification number.4
There are no head-to-head trials comparing the effectiveness of different methods of purification to prevent infectious disease. The majority of the evidence is based on data provided by manufacturers to the EPA, with some independent studies and expert opinion (TABLE).
Expert opinion recommends bringing water to a rapid boil for at least 3 minutes and letting it cool as an effective means of water purification.5 Chlorine dioxide tablets, the MIOX purifier, and UV light (SteriPEN) have all met EPA standards for lower pathogen counts under ideal conditions. Halogenation does not reduce Cryptosporidium below the microbiological hazard of 99.9%, but it is generally accepted to effectively treat viruses, bacteria, and other protozoa after filtering through a cloth to remove large particles.6
Filtration with an absolute pore size of <0.1 micrometer (10 times smaller than the EPA standard) has been generally accepted as effective against protozoa and bacteria, but it is not effective against viruses because of their small size.7 When combined with either halogenation or charcoal filters, filtration can be effective against all pathogens.8
TABLE
Portable water purification: How do these 6 methods compare?
| METHOD | EFFECTIVENESS | ADVANTAGES | DISADVANTAGES |
|---|---|---|---|
| Boiling with cooling* | Kills viruses, bacteria, protozoa, and parasites | simple, universally accepted, no special equipment required | Time-consuming, may require large amounts of fuel |
chlorine dioxide* | Kills bacteria, viruses, protozoa, and parasites | same as chlorine/iodine treatment but also treats Cryptosporidium, good palatability | Must wait up to 4 hours to treat Cryptosporidium, costs more than iodine/chlorine ($13 for 30 tabs) |
Chlorine/iodine | Kills bacteria, viruses, protozoa (not Cryptosporidium), and parasites | Inexpensive, easy, lightweight, treats large quantities | Does not kill Cryptosporidium, poor taste, must wait for water to be treated; contraindicated in pregnancy, thyroid disease; not recommended beyond few weeks of use |
Filtration † | Removes parasites, Giardia, Cryptosporidium, and bacteria | Able to use water immediately, removes sediment, many have combination of activated carbon, chemical disinfectant, or both | Can potentially be expensive, filters may clog easily, heavy, not effective against small particle viruses, therefore should supplement with chlorine or iodine |
MIOX Purifier* | Kills bacteria, viruses, protozoa, and parasites | light (8 oz), sturdy, treats large quantities; requires camera batteries and salt | Cost $130, must wait for 4 hours and treat with higher strength to treat Cryptosporidium; requires 30 minutes to treat viruses, bacteria, and Giardia |
 UV light (steriPEN) ‡ | Kills bacteria, viruses, protozoa, parasites in clear water | Light (8 oz), quick (treats 16 oz of water in 1 minute) | Cost $100, does not work in turbid conditions |
| * Meets EPA standards. | |||
| † some filtration systems meet EPA standards. See chppm-www.apgea.army.mil/WPD/CompareDevices.aspx for testing results of individual filters.1 | |||
| ‡ Meets EPA standards in clear water. | |||
Recommendations from others
The US Army Center for Health Promotion and Preventive Medicine (USACHPPM) published a report in 2006 on the efficacy of commercial off-the-shelf individual water purifiers.8 Using National Sanitation Foundation Protocol P248 and applying it to “real-world” emergency military operational conditions, USACHPPM found that no device scored high on every attribute, and that overall scores for most devices were in the moderate range. The top score for any device was 79 (out of 100).8
The overall top 3 scoring products were: 1) the SweetWater Purifier from Mountain Safety Research; 2) the Micropur MP 1 tablets from Katadyn North America, Inc; and 3) the First Need Deluxe water purifier from General Ecology, Inc.
Acknowledgments
The opinions and assertions contained herein are the private views of the authors and not to be construed as official, or as reflecting the views of the US Air Force Medical Service or the US air Force at large.
1. Commercially available individual water purifiers. Water Purification Database, US Army Center for Health Promotion and Preventive Medicine Web site. Available at: usachppm.apgea.army.mil/WPD/CompareDevices.aspx. Accessed on December 7, 2007.
2. Hamer DH, Connor Ba. Travel health knowledge, attitudes and practices among united states travelers. J Travel Med 2004;11:23-26.
3. Hill DR, Ericsson CD, Pearson RD, et al. The Practice of Travel Medicine: Guidelines by the Infectious Diseases Society of America. Clin Infect Dis 2006;43:1499-1539.
4. US Environmental Protection agency: Guide standard and Protocol for Testing Microbiological Water Purifiers: Report to Task Force. Cincinnati, OH: US environmental Protection agency; 1987.
5. Centers for Disease Control and Prevention Water treatment methods. Available at: wwwn.cdc.gov/travel/contentWaterTreatment.aspx. Accessed on December 7, 2007.
6. Gerba CP, Johnson DC, Hasan MN. efficacy of iodine water purification tablets against Cryptosporidium oocysts and Giardia cysts. Wilderness Environ Med 1997;8:96-100.
7. Centers for Disease Control and Prevention Division of Parasitic Diseases Preventing Cryptosporidiosis: a guide to water filters and bottled water. Available at: www.cdc.gov/ncidod/dpd/parasites/cryptosporidiosis/factsht_crypto_prevent_water.htm. Accessed on December 7, 2007.
8. Water supply Management Program Project No 31-EC-03E0. Performance and health risk assessment of commercial-off-the-shelf individual water purifiers. Aberdeen Proving Ground, MD: US Army Center for Health Promotion and Preventive Medicine; 2006. Available at: usachppm.apgea.army.mil/WPD/PDFDocs/Finalreport.pdf. Accessed on December 7, 2007.
There isn’t a single best method, but there are 5 that adequately purify water according to environmental Protection agency (EPA) standards. These include 1) boiling for 1 minute if below 2000 m (6562 feet) and 3 minutes if above, 2) chlorine dioxide tablets, 3) MIoX purifier, 4) ultraviolet light (steriPEN), and 5) portable filtration with a absolute pore size <1 micrometer combined with halogenation or charcoal filtration (strength of recommendation [SOR]: C, based on expert opinion and microbiological testing). Halogenation alone (ie, chlorine and iodine) is not effective against Cryptosporidium (SOR: C, based on microbiological testing).
Why boil water when there are so many other options?
Timothy Mott, MD, FAAFP
US Naval Hospital, Sigonella, Italy
These days, “boil it, peel it, or forget it” only goes so far with the unencumbered traveler. Experience tells me that most hear “Boil it” and instantly go right to “Forget it!” Fortunately, there is an excellent resource to assist patients in choosing a personally acceptable portable water purification system. It’s called the Water Purification Database at usachppm.apgea.army.mil/WPD/CompareDevices.aspx.1
This outstanding database was developed by an impartial third-party for the US Army and gives clear, well-organized guidance on over 60 purifiers. For each purifier, the guide covers efficacy against primary pathogens, purification mechanism, links to manufacturers, and an advantages/ disadvantages breakdown (such as weight, cost, and ease of use). Add this site to your Internet “favorites” folder.
Evidence summary
With the rise in international travel and adventure sports, individuals are at increased risk of acquiring infections by drinking water from impure water sources. Common waterborne infections that back-country and international travelers may contract include bacterial diarrhea, viruses, protozoa (such as Giardia and Cryptosporidium), and parasites (such as schistosoma). The risk of infection varies based on travel location.
To prevent illness, travelers may seek medical guidance regarding safe water practice. In one study, 36% of travelers sought advice from a physician prior to international travel.2 Preventing waterborne infections should be a component of traveler education, in addition to other standard advice, such as mosquito avoidance and immunizations.3 (For more on travel safety, see these Clinical Inquiries: “When should travelers begin malaria prophylaxis?” in the November 2007 Journal of Family Practice, pages 950–952, and “What is the most effective and safe malaria prophylaxis during pregnancy?” on page 51 of this issue.)
Which devices meet EPA standards?
The EPA has established a “minimal microbiological hazard” allowed for a portable water purification system to be considered safe. Water purifiers must reduce bacteria by 99.9999%, viruses by 99.99%, and protozoa (such as Cryptosporidium parvum) by 99.9% to receive an EPA certification number.4
There are no head-to-head trials comparing the effectiveness of different methods of purification to prevent infectious disease. The majority of the evidence is based on data provided by manufacturers to the EPA, with some independent studies and expert opinion (TABLE).
Expert opinion recommends bringing water to a rapid boil for at least 3 minutes and letting it cool as an effective means of water purification.5 Chlorine dioxide tablets, the MIOX purifier, and UV light (SteriPEN) have all met EPA standards for lower pathogen counts under ideal conditions. Halogenation does not reduce Cryptosporidium below the microbiological hazard of 99.9%, but it is generally accepted to effectively treat viruses, bacteria, and other protozoa after filtering through a cloth to remove large particles.6
Filtration with an absolute pore size of <0.1 micrometer (10 times smaller than the EPA standard) has been generally accepted as effective against protozoa and bacteria, but it is not effective against viruses because of their small size.7 When combined with either halogenation or charcoal filters, filtration can be effective against all pathogens.8
TABLE
Portable water purification: How do these 6 methods compare?
| METHOD | EFFECTIVENESS | ADVANTAGES | DISADVANTAGES |
|---|---|---|---|
| Boiling with cooling* | Kills viruses, bacteria, protozoa, and parasites | simple, universally accepted, no special equipment required | Time-consuming, may require large amounts of fuel |
chlorine dioxide* | Kills bacteria, viruses, protozoa, and parasites | same as chlorine/iodine treatment but also treats Cryptosporidium, good palatability | Must wait up to 4 hours to treat Cryptosporidium, costs more than iodine/chlorine ($13 for 30 tabs) |
Chlorine/iodine | Kills bacteria, viruses, protozoa (not Cryptosporidium), and parasites | Inexpensive, easy, lightweight, treats large quantities | Does not kill Cryptosporidium, poor taste, must wait for water to be treated; contraindicated in pregnancy, thyroid disease; not recommended beyond few weeks of use |
Filtration † | Removes parasites, Giardia, Cryptosporidium, and bacteria | Able to use water immediately, removes sediment, many have combination of activated carbon, chemical disinfectant, or both | Can potentially be expensive, filters may clog easily, heavy, not effective against small particle viruses, therefore should supplement with chlorine or iodine |
MIOX Purifier* | Kills bacteria, viruses, protozoa, and parasites | light (8 oz), sturdy, treats large quantities; requires camera batteries and salt | Cost $130, must wait for 4 hours and treat with higher strength to treat Cryptosporidium; requires 30 minutes to treat viruses, bacteria, and Giardia |
 UV light (steriPEN) ‡ | Kills bacteria, viruses, protozoa, parasites in clear water | Light (8 oz), quick (treats 16 oz of water in 1 minute) | Cost $100, does not work in turbid conditions |
| * Meets EPA standards. | |||
| † some filtration systems meet EPA standards. See chppm-www.apgea.army.mil/WPD/CompareDevices.aspx for testing results of individual filters.1 | |||
| ‡ Meets EPA standards in clear water. | |||
Recommendations from others
The US Army Center for Health Promotion and Preventive Medicine (USACHPPM) published a report in 2006 on the efficacy of commercial off-the-shelf individual water purifiers.8 Using National Sanitation Foundation Protocol P248 and applying it to “real-world” emergency military operational conditions, USACHPPM found that no device scored high on every attribute, and that overall scores for most devices were in the moderate range. The top score for any device was 79 (out of 100).8
The overall top 3 scoring products were: 1) the SweetWater Purifier from Mountain Safety Research; 2) the Micropur MP 1 tablets from Katadyn North America, Inc; and 3) the First Need Deluxe water purifier from General Ecology, Inc.
Acknowledgments
The opinions and assertions contained herein are the private views of the authors and not to be construed as official, or as reflecting the views of the US Air Force Medical Service or the US air Force at large.
There isn’t a single best method, but there are 5 that adequately purify water according to environmental Protection agency (EPA) standards. These include 1) boiling for 1 minute if below 2000 m (6562 feet) and 3 minutes if above, 2) chlorine dioxide tablets, 3) MIoX purifier, 4) ultraviolet light (steriPEN), and 5) portable filtration with a absolute pore size <1 micrometer combined with halogenation or charcoal filtration (strength of recommendation [SOR]: C, based on expert opinion and microbiological testing). Halogenation alone (ie, chlorine and iodine) is not effective against Cryptosporidium (SOR: C, based on microbiological testing).
Why boil water when there are so many other options?
Timothy Mott, MD, FAAFP
US Naval Hospital, Sigonella, Italy
These days, “boil it, peel it, or forget it” only goes so far with the unencumbered traveler. Experience tells me that most hear “Boil it” and instantly go right to “Forget it!” Fortunately, there is an excellent resource to assist patients in choosing a personally acceptable portable water purification system. It’s called the Water Purification Database at usachppm.apgea.army.mil/WPD/CompareDevices.aspx.1
This outstanding database was developed by an impartial third-party for the US Army and gives clear, well-organized guidance on over 60 purifiers. For each purifier, the guide covers efficacy against primary pathogens, purification mechanism, links to manufacturers, and an advantages/ disadvantages breakdown (such as weight, cost, and ease of use). Add this site to your Internet “favorites” folder.
Evidence summary
With the rise in international travel and adventure sports, individuals are at increased risk of acquiring infections by drinking water from impure water sources. Common waterborne infections that back-country and international travelers may contract include bacterial diarrhea, viruses, protozoa (such as Giardia and Cryptosporidium), and parasites (such as schistosoma). The risk of infection varies based on travel location.
To prevent illness, travelers may seek medical guidance regarding safe water practice. In one study, 36% of travelers sought advice from a physician prior to international travel.2 Preventing waterborne infections should be a component of traveler education, in addition to other standard advice, such as mosquito avoidance and immunizations.3 (For more on travel safety, see these Clinical Inquiries: “When should travelers begin malaria prophylaxis?” in the November 2007 Journal of Family Practice, pages 950–952, and “What is the most effective and safe malaria prophylaxis during pregnancy?” on page 51 of this issue.)
Which devices meet EPA standards?
The EPA has established a “minimal microbiological hazard” allowed for a portable water purification system to be considered safe. Water purifiers must reduce bacteria by 99.9999%, viruses by 99.99%, and protozoa (such as Cryptosporidium parvum) by 99.9% to receive an EPA certification number.4
There are no head-to-head trials comparing the effectiveness of different methods of purification to prevent infectious disease. The majority of the evidence is based on data provided by manufacturers to the EPA, with some independent studies and expert opinion (TABLE).
Expert opinion recommends bringing water to a rapid boil for at least 3 minutes and letting it cool as an effective means of water purification.5 Chlorine dioxide tablets, the MIOX purifier, and UV light (SteriPEN) have all met EPA standards for lower pathogen counts under ideal conditions. Halogenation does not reduce Cryptosporidium below the microbiological hazard of 99.9%, but it is generally accepted to effectively treat viruses, bacteria, and other protozoa after filtering through a cloth to remove large particles.6
Filtration with an absolute pore size of <0.1 micrometer (10 times smaller than the EPA standard) has been generally accepted as effective against protozoa and bacteria, but it is not effective against viruses because of their small size.7 When combined with either halogenation or charcoal filters, filtration can be effective against all pathogens.8
TABLE
Portable water purification: How do these 6 methods compare?
| METHOD | EFFECTIVENESS | ADVANTAGES | DISADVANTAGES |
|---|---|---|---|
| Boiling with cooling* | Kills viruses, bacteria, protozoa, and parasites | simple, universally accepted, no special equipment required | Time-consuming, may require large amounts of fuel |
chlorine dioxide* | Kills bacteria, viruses, protozoa, and parasites | same as chlorine/iodine treatment but also treats Cryptosporidium, good palatability | Must wait up to 4 hours to treat Cryptosporidium, costs more than iodine/chlorine ($13 for 30 tabs) |
Chlorine/iodine | Kills bacteria, viruses, protozoa (not Cryptosporidium), and parasites | Inexpensive, easy, lightweight, treats large quantities | Does not kill Cryptosporidium, poor taste, must wait for water to be treated; contraindicated in pregnancy, thyroid disease; not recommended beyond few weeks of use |
Filtration † | Removes parasites, Giardia, Cryptosporidium, and bacteria | Able to use water immediately, removes sediment, many have combination of activated carbon, chemical disinfectant, or both | Can potentially be expensive, filters may clog easily, heavy, not effective against small particle viruses, therefore should supplement with chlorine or iodine |
MIOX Purifier* | Kills bacteria, viruses, protozoa, and parasites | light (8 oz), sturdy, treats large quantities; requires camera batteries and salt | Cost $130, must wait for 4 hours and treat with higher strength to treat Cryptosporidium; requires 30 minutes to treat viruses, bacteria, and Giardia |
 UV light (steriPEN) ‡ | Kills bacteria, viruses, protozoa, parasites in clear water | Light (8 oz), quick (treats 16 oz of water in 1 minute) | Cost $100, does not work in turbid conditions |
| * Meets EPA standards. | |||
| † some filtration systems meet EPA standards. See chppm-www.apgea.army.mil/WPD/CompareDevices.aspx for testing results of individual filters.1 | |||
| ‡ Meets EPA standards in clear water. | |||
Recommendations from others
The US Army Center for Health Promotion and Preventive Medicine (USACHPPM) published a report in 2006 on the efficacy of commercial off-the-shelf individual water purifiers.8 Using National Sanitation Foundation Protocol P248 and applying it to “real-world” emergency military operational conditions, USACHPPM found that no device scored high on every attribute, and that overall scores for most devices were in the moderate range. The top score for any device was 79 (out of 100).8
The overall top 3 scoring products were: 1) the SweetWater Purifier from Mountain Safety Research; 2) the Micropur MP 1 tablets from Katadyn North America, Inc; and 3) the First Need Deluxe water purifier from General Ecology, Inc.
Acknowledgments
The opinions and assertions contained herein are the private views of the authors and not to be construed as official, or as reflecting the views of the US Air Force Medical Service or the US air Force at large.
1. Commercially available individual water purifiers. Water Purification Database, US Army Center for Health Promotion and Preventive Medicine Web site. Available at: usachppm.apgea.army.mil/WPD/CompareDevices.aspx. Accessed on December 7, 2007.
2. Hamer DH, Connor Ba. Travel health knowledge, attitudes and practices among united states travelers. J Travel Med 2004;11:23-26.
3. Hill DR, Ericsson CD, Pearson RD, et al. The Practice of Travel Medicine: Guidelines by the Infectious Diseases Society of America. Clin Infect Dis 2006;43:1499-1539.
4. US Environmental Protection agency: Guide standard and Protocol for Testing Microbiological Water Purifiers: Report to Task Force. Cincinnati, OH: US environmental Protection agency; 1987.
5. Centers for Disease Control and Prevention Water treatment methods. Available at: wwwn.cdc.gov/travel/contentWaterTreatment.aspx. Accessed on December 7, 2007.
6. Gerba CP, Johnson DC, Hasan MN. efficacy of iodine water purification tablets against Cryptosporidium oocysts and Giardia cysts. Wilderness Environ Med 1997;8:96-100.
7. Centers for Disease Control and Prevention Division of Parasitic Diseases Preventing Cryptosporidiosis: a guide to water filters and bottled water. Available at: www.cdc.gov/ncidod/dpd/parasites/cryptosporidiosis/factsht_crypto_prevent_water.htm. Accessed on December 7, 2007.
8. Water supply Management Program Project No 31-EC-03E0. Performance and health risk assessment of commercial-off-the-shelf individual water purifiers. Aberdeen Proving Ground, MD: US Army Center for Health Promotion and Preventive Medicine; 2006. Available at: usachppm.apgea.army.mil/WPD/PDFDocs/Finalreport.pdf. Accessed on December 7, 2007.
1. Commercially available individual water purifiers. Water Purification Database, US Army Center for Health Promotion and Preventive Medicine Web site. Available at: usachppm.apgea.army.mil/WPD/CompareDevices.aspx. Accessed on December 7, 2007.
2. Hamer DH, Connor Ba. Travel health knowledge, attitudes and practices among united states travelers. J Travel Med 2004;11:23-26.
3. Hill DR, Ericsson CD, Pearson RD, et al. The Practice of Travel Medicine: Guidelines by the Infectious Diseases Society of America. Clin Infect Dis 2006;43:1499-1539.
4. US Environmental Protection agency: Guide standard and Protocol for Testing Microbiological Water Purifiers: Report to Task Force. Cincinnati, OH: US environmental Protection agency; 1987.
5. Centers for Disease Control and Prevention Water treatment methods. Available at: wwwn.cdc.gov/travel/contentWaterTreatment.aspx. Accessed on December 7, 2007.
6. Gerba CP, Johnson DC, Hasan MN. efficacy of iodine water purification tablets against Cryptosporidium oocysts and Giardia cysts. Wilderness Environ Med 1997;8:96-100.
7. Centers for Disease Control and Prevention Division of Parasitic Diseases Preventing Cryptosporidiosis: a guide to water filters and bottled water. Available at: www.cdc.gov/ncidod/dpd/parasites/cryptosporidiosis/factsht_crypto_prevent_water.htm. Accessed on December 7, 2007.
8. Water supply Management Program Project No 31-EC-03E0. Performance and health risk assessment of commercial-off-the-shelf individual water purifiers. Aberdeen Proving Ground, MD: US Army Center for Health Promotion and Preventive Medicine; 2006. Available at: usachppm.apgea.army.mil/WPD/PDFDocs/Finalreport.pdf. Accessed on December 7, 2007.
Evidence-based answers from the Family Physicians Inquiries Network