User login
What is the most effective and safe malaria prophylaxis during pregnancy?
Chloroquine and mefloquine have superior safety profiles in pregnancy, though all antimalarials are effective for prophylaxis. Antimalarials will decrease the severity of maternal malaria infection and malaria-associated anemia, while decreasing the incidence of low birth weight and perinatal death in women having their first or second baby (strength of recommendation [SOR]: A, based on systematic review of consistent, good-quality patient-oriented evidence).
You can determine malaria risk and sensitivity of Plasmodium species by country at wwwn.cdc.gov/travel/destinationlist.aspx.1 Urge women to delay travel until after pregnancy if possible2 (SOR: C, based on patient-oriented expert opinion).
Don’t forget to discuss mosquito netting and insect repellant
Meg Hayes, MD
Department of Family Medicine, Oregon Health and Science University, Portland
Adverse outcomes associated with malaria during pregnancy include restricted fetal growth, low birth weight, preterm delivery, congenital infection, spontaneous abortion, and perinatal death. You should counsel travelers to avoid travel to areas where malaria is endemic during pregnancy.
For those who are unable to avoid travel, or who reside in malaria-endemic areas during pregnancy, physicians should focus not only on chemoprophylaxis, but provide verbal and written counsel regarding malaria personal protection measures. Because mosquitoes usually feed at night, travelers should remain within screened areas after dusk, use permethrin-treated bed nets, wear protective clothing, and apply insect repellant. Advise patients who travel to malaria-endemic areas to quickly report febrile illnesses and to disclose their travel histories to healthcare providers.
Evidence summary
Malaria is a parasitic infection that causes significant morbidity and mortality worldwide, with more than 500 million people becoming severely ill every year.2 For pregnant women, malarial infection can be severe, with high fevers, chills, and anemia leading to increased risk of poor maternal and fetal outcomes—including death. Pregnant women are also more likely to become infected and to develop more severe disease—they attract twice as many mosquitoes as nonpregnant women and have a relative immuno-suppression.3
TABLE
Antimalarials for prophylaxis: Chloroquine, mefloquine are best choices during pregnancy
| DRUG | EFFICACY | SAFETY | PREGNANCY CLASS* | AVAILABILITY |
|---|---|---|---|---|
| Chloroquine | Good | Excellent | C | Worldwide |
| Chloroquine/proguanil | Good | Excellent | C | Worldwide |
| Mefloquine | Excellent | Good | C | Worldwide |
| Quinine | Excellent | Good | C† | Worldwide |
| Atovaquone/proguanil | Excellent | Good | C (poorly studied) | Worldwide |
| Artesunate | Excellent | Good | N/A | Asia, Africa, limited in UK, not in US |
| Primaquine | Good | Fair | C‡ | Worldwide |
| Doxycycline | Excellent | Fair | D (teratogenic) | Worldwide |
| Sulfadoxine/pyrimethamine | Fair | Poor | C | Worldwide, but restricted in US |
| Note: Prescribers and patients are urged to refer to the CDC reference about pregnancy in malaria (wwwn.cdc.gov/travel/contentMalariaPregnantPublic.aspx) and to specific country information regarding sensitivities of malaria (wwwn.cdc.gov/travel/destinationList.aspx). | ||||
| * Pregnancy class C: Animal reproduction studies have shown an adverse effect on the fetus and there are no adequate and well-controlled studies in humans, but potential benefits may warrant use of the drug in pregnant women, despite potential risks. | ||||
| * Pregnancy class D: There is positive evidence of human fetal risk based on adverse reaction data from investigational or marketing experience or studies in humans, but potential benefits may warrant use of the drug in pregnant women, despite potential risks. | ||||
| † Monitor patients for maternal hypoglycemia. | ||||
| ‡ There is no evidence of teratogenicity, but primaquine is associated with fetal intravascular hemolysis. | ||||
Chemoprophylaxis lowers rates of maternal infection
Although prophylaxis for pregnant patients traveling to malarial regions is a public concern, data for decision-making must be extrapolated from the available evidence, which is based primarily on women living in endemic areas. In a Cochrane systematic review, antimalarials were found to decrease the incidence of maternal infections (relative risk [RR]=0.27; 95% confidence interval [CI], 0.17–0.44) and reduce maternal anemia (RR=0.62; 95% CI, 0.50–0.78) in low-parity women—ie, during a first or second pregnancy.3
In low-parity women, these drugs were also found to decrease perinatal death (RR=0.73; 95% CI, 0.53–0.99) and low birth weight (RR=0.57; 95% CI, 0.46–0.72) associated with malarial infection. When used in all parity groups, antimalarials were somewhat less effective, yet still reduced maternal infections (RR=0.53; 95% CI, 0.33–0.86); the effects were similar with all antimalarials tested.3,4
Chloroquine, mefloquine are safe in pregnancy, doxycycline is not
While chemoprophylaxis in pregnancy appears efficacious, a major question remains—which agents are safest for both the woman and fetus? Some drugs routinely used in nonpregnant individuals should not be offered to pregnant women because of known direct effects on the fetus. Doxycycline is teratogenic, and primaquine poses a significant risk of fetal intravascular hemolysis in G6PD-deficient fetuses.5 Other drugs, such as atovaquone/proguanil and artesunate, are not well studied in pregnancy, and therefore are not recommended for use unless other options are not available.2,6
Among drugs that are well studied and without known direct fetal-damaging effects, adverse drug reaction profiles can guide use based on disease prevalence and drug-resistance patterns.
- Chloroquine is widely used because it is inexpensive and well tolerated, with only pruritus, mouth ulcers, and gastrointestinal upset as the most common adverse effects.
- Mefloquine is usually well tolerated, but can cause dose-related neuropsychiatric effects; it is contraindicated in those with a history of epilepsy or psychiatric disease.
- Sulfadoxine and pyrimethamine are not normally used as prophylaxis for any patient, due to the risk of toxic epidermal necrolysis and Stevens-Johnson syndrome, and the possible risk of jaundice and kernicterus if used in the third trimester of pregnancy.
- Quinine, which can be used for treatment or prophylaxis, may cause hypoglycemia, an effect that is more pronounced during pregnancy and requires close monitoring of blood glucose levels.5,7
Given these reaction profiles, chloroquine or mefloquine are usually the best choice with their superior safety and efficacy.
Figure
Best protection: Avoidance
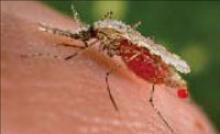
Chloroquine and mefloquine are the safest antimalarials for use in pregnant women, but personal protection measures are also critical. Above, an Anopheles stephensi mosquito expelling a droplet of blood from its abdomen after having engorged itself on its human host’s blood. (Source: CDC.)
Recommendations from others
The World Health Organization (WHO) recommends pregnant women avoid travel to malarial regions. If travel is required, WHO recommends chloroquine as first-line prophylaxis in pregnancy (plus proguanil if the region exhibits emerging chloroquine resistance). In areas with proven chloroquine resistance, mefloquine is the drug of choice. Other antimalarials—such as quinine, pyrimethamine, sulfadoxine, and artesunate—should not be withheld if the preferred drugs are not available, or if the infection is life-threatening.2
The Centers for Disease Control and Prevention (CDC) also recommends avoiding travel to malaria-endemic regions during pregnancy, but if travel is necessary, the CDC advises use of chloroquine (or mefloquine in regions with chloroquine resistance). The CDC discourages the use of atovaquone/proguanil, doxycycline, and primaquine, due to known adverse fetal effects or inadequate experience in pregnancy.6
Acknowledgments
The opinions and assertions contained herein are the private views of the authors and not to be construed as official, or as reflecting the views of the US Air Force Medical Service or the US Air Force at large.
1. Centers for Disease Control and Prevention Web site. Destinations: CDC Traveler’s Health. Available at: wwwn.cdc.gov/travel/destinationlist.aspx. Accessed on December 7, 2007.
2. Malaria. In: International Travel and Health. Geneva, Switzerland: World Health Organization; 2007. Available at: whqlibdoc.who.int/publications/2005/9241580364_chap7.pdf. Accessed on December 7, 2007.
3. Orton L, Garner P. Drugs for treating uncomplicated malaria in pregnant women. Cochrane Database Syst Rev 2005;(3):CD004912.-
4. Garner P, Gülmezoglu AM. Drugs for preventing malaria in pregnant women. Cochrane Database Syst Rev 2006;(4):CD000169.-
5. Phillips-Howard PA, Wood D. The safety of antimalarial drugs in pregnancy. Drug Saf 1996;14:131-145.
6. Centers for Disease Control and Prevention Web site. Diseases: Malaria: Prevention, Pregnant Women, Public Info. Available at: wwwn.cdc.gov/travel/ contentMalariaPregnantPublic.aspx. Accessed on December 7, 2007.
7. Taylor WR, White NJ. Antimalarial drug toxicity: a review. Drug Saf 2004;27:25-61.
Chloroquine and mefloquine have superior safety profiles in pregnancy, though all antimalarials are effective for prophylaxis. Antimalarials will decrease the severity of maternal malaria infection and malaria-associated anemia, while decreasing the incidence of low birth weight and perinatal death in women having their first or second baby (strength of recommendation [SOR]: A, based on systematic review of consistent, good-quality patient-oriented evidence).
You can determine malaria risk and sensitivity of Plasmodium species by country at wwwn.cdc.gov/travel/destinationlist.aspx.1 Urge women to delay travel until after pregnancy if possible2 (SOR: C, based on patient-oriented expert opinion).
Don’t forget to discuss mosquito netting and insect repellant
Meg Hayes, MD
Department of Family Medicine, Oregon Health and Science University, Portland
Adverse outcomes associated with malaria during pregnancy include restricted fetal growth, low birth weight, preterm delivery, congenital infection, spontaneous abortion, and perinatal death. You should counsel travelers to avoid travel to areas where malaria is endemic during pregnancy.
For those who are unable to avoid travel, or who reside in malaria-endemic areas during pregnancy, physicians should focus not only on chemoprophylaxis, but provide verbal and written counsel regarding malaria personal protection measures. Because mosquitoes usually feed at night, travelers should remain within screened areas after dusk, use permethrin-treated bed nets, wear protective clothing, and apply insect repellant. Advise patients who travel to malaria-endemic areas to quickly report febrile illnesses and to disclose their travel histories to healthcare providers.
Evidence summary
Malaria is a parasitic infection that causes significant morbidity and mortality worldwide, with more than 500 million people becoming severely ill every year.2 For pregnant women, malarial infection can be severe, with high fevers, chills, and anemia leading to increased risk of poor maternal and fetal outcomes—including death. Pregnant women are also more likely to become infected and to develop more severe disease—they attract twice as many mosquitoes as nonpregnant women and have a relative immuno-suppression.3
TABLE
Antimalarials for prophylaxis: Chloroquine, mefloquine are best choices during pregnancy
| DRUG | EFFICACY | SAFETY | PREGNANCY CLASS* | AVAILABILITY |
|---|---|---|---|---|
| Chloroquine | Good | Excellent | C | Worldwide |
| Chloroquine/proguanil | Good | Excellent | C | Worldwide |
| Mefloquine | Excellent | Good | C | Worldwide |
| Quinine | Excellent | Good | C† | Worldwide |
| Atovaquone/proguanil | Excellent | Good | C (poorly studied) | Worldwide |
| Artesunate | Excellent | Good | N/A | Asia, Africa, limited in UK, not in US |
| Primaquine | Good | Fair | C‡ | Worldwide |
| Doxycycline | Excellent | Fair | D (teratogenic) | Worldwide |
| Sulfadoxine/pyrimethamine | Fair | Poor | C | Worldwide, but restricted in US |
| Note: Prescribers and patients are urged to refer to the CDC reference about pregnancy in malaria (wwwn.cdc.gov/travel/contentMalariaPregnantPublic.aspx) and to specific country information regarding sensitivities of malaria (wwwn.cdc.gov/travel/destinationList.aspx). | ||||
| * Pregnancy class C: Animal reproduction studies have shown an adverse effect on the fetus and there are no adequate and well-controlled studies in humans, but potential benefits may warrant use of the drug in pregnant women, despite potential risks. | ||||
| * Pregnancy class D: There is positive evidence of human fetal risk based on adverse reaction data from investigational or marketing experience or studies in humans, but potential benefits may warrant use of the drug in pregnant women, despite potential risks. | ||||
| † Monitor patients for maternal hypoglycemia. | ||||
| ‡ There is no evidence of teratogenicity, but primaquine is associated with fetal intravascular hemolysis. | ||||
Chemoprophylaxis lowers rates of maternal infection
Although prophylaxis for pregnant patients traveling to malarial regions is a public concern, data for decision-making must be extrapolated from the available evidence, which is based primarily on women living in endemic areas. In a Cochrane systematic review, antimalarials were found to decrease the incidence of maternal infections (relative risk [RR]=0.27; 95% confidence interval [CI], 0.17–0.44) and reduce maternal anemia (RR=0.62; 95% CI, 0.50–0.78) in low-parity women—ie, during a first or second pregnancy.3
In low-parity women, these drugs were also found to decrease perinatal death (RR=0.73; 95% CI, 0.53–0.99) and low birth weight (RR=0.57; 95% CI, 0.46–0.72) associated with malarial infection. When used in all parity groups, antimalarials were somewhat less effective, yet still reduced maternal infections (RR=0.53; 95% CI, 0.33–0.86); the effects were similar with all antimalarials tested.3,4
Chloroquine, mefloquine are safe in pregnancy, doxycycline is not
While chemoprophylaxis in pregnancy appears efficacious, a major question remains—which agents are safest for both the woman and fetus? Some drugs routinely used in nonpregnant individuals should not be offered to pregnant women because of known direct effects on the fetus. Doxycycline is teratogenic, and primaquine poses a significant risk of fetal intravascular hemolysis in G6PD-deficient fetuses.5 Other drugs, such as atovaquone/proguanil and artesunate, are not well studied in pregnancy, and therefore are not recommended for use unless other options are not available.2,6
Among drugs that are well studied and without known direct fetal-damaging effects, adverse drug reaction profiles can guide use based on disease prevalence and drug-resistance patterns.
- Chloroquine is widely used because it is inexpensive and well tolerated, with only pruritus, mouth ulcers, and gastrointestinal upset as the most common adverse effects.
- Mefloquine is usually well tolerated, but can cause dose-related neuropsychiatric effects; it is contraindicated in those with a history of epilepsy or psychiatric disease.
- Sulfadoxine and pyrimethamine are not normally used as prophylaxis for any patient, due to the risk of toxic epidermal necrolysis and Stevens-Johnson syndrome, and the possible risk of jaundice and kernicterus if used in the third trimester of pregnancy.
- Quinine, which can be used for treatment or prophylaxis, may cause hypoglycemia, an effect that is more pronounced during pregnancy and requires close monitoring of blood glucose levels.5,7
Given these reaction profiles, chloroquine or mefloquine are usually the best choice with their superior safety and efficacy.
Figure
Best protection: Avoidance
Chloroquine and mefloquine are the safest antimalarials for use in pregnant women, but personal protection measures are also critical. Above, an Anopheles stephensi mosquito expelling a droplet of blood from its abdomen after having engorged itself on its human host’s blood. (Source: CDC.)
Recommendations from others
The World Health Organization (WHO) recommends pregnant women avoid travel to malarial regions. If travel is required, WHO recommends chloroquine as first-line prophylaxis in pregnancy (plus proguanil if the region exhibits emerging chloroquine resistance). In areas with proven chloroquine resistance, mefloquine is the drug of choice. Other antimalarials—such as quinine, pyrimethamine, sulfadoxine, and artesunate—should not be withheld if the preferred drugs are not available, or if the infection is life-threatening.2
The Centers for Disease Control and Prevention (CDC) also recommends avoiding travel to malaria-endemic regions during pregnancy, but if travel is necessary, the CDC advises use of chloroquine (or mefloquine in regions with chloroquine resistance). The CDC discourages the use of atovaquone/proguanil, doxycycline, and primaquine, due to known adverse fetal effects or inadequate experience in pregnancy.6
Acknowledgments
The opinions and assertions contained herein are the private views of the authors and not to be construed as official, or as reflecting the views of the US Air Force Medical Service or the US Air Force at large.
Chloroquine and mefloquine have superior safety profiles in pregnancy, though all antimalarials are effective for prophylaxis. Antimalarials will decrease the severity of maternal malaria infection and malaria-associated anemia, while decreasing the incidence of low birth weight and perinatal death in women having their first or second baby (strength of recommendation [SOR]: A, based on systematic review of consistent, good-quality patient-oriented evidence).
You can determine malaria risk and sensitivity of Plasmodium species by country at wwwn.cdc.gov/travel/destinationlist.aspx.1 Urge women to delay travel until after pregnancy if possible2 (SOR: C, based on patient-oriented expert opinion).
Don’t forget to discuss mosquito netting and insect repellant
Meg Hayes, MD
Department of Family Medicine, Oregon Health and Science University, Portland
Adverse outcomes associated with malaria during pregnancy include restricted fetal growth, low birth weight, preterm delivery, congenital infection, spontaneous abortion, and perinatal death. You should counsel travelers to avoid travel to areas where malaria is endemic during pregnancy.
For those who are unable to avoid travel, or who reside in malaria-endemic areas during pregnancy, physicians should focus not only on chemoprophylaxis, but provide verbal and written counsel regarding malaria personal protection measures. Because mosquitoes usually feed at night, travelers should remain within screened areas after dusk, use permethrin-treated bed nets, wear protective clothing, and apply insect repellant. Advise patients who travel to malaria-endemic areas to quickly report febrile illnesses and to disclose their travel histories to healthcare providers.
Evidence summary
Malaria is a parasitic infection that causes significant morbidity and mortality worldwide, with more than 500 million people becoming severely ill every year.2 For pregnant women, malarial infection can be severe, with high fevers, chills, and anemia leading to increased risk of poor maternal and fetal outcomes—including death. Pregnant women are also more likely to become infected and to develop more severe disease—they attract twice as many mosquitoes as nonpregnant women and have a relative immuno-suppression.3
TABLE
Antimalarials for prophylaxis: Chloroquine, mefloquine are best choices during pregnancy
| DRUG | EFFICACY | SAFETY | PREGNANCY CLASS* | AVAILABILITY |
|---|---|---|---|---|
| Chloroquine | Good | Excellent | C | Worldwide |
| Chloroquine/proguanil | Good | Excellent | C | Worldwide |
| Mefloquine | Excellent | Good | C | Worldwide |
| Quinine | Excellent | Good | C† | Worldwide |
| Atovaquone/proguanil | Excellent | Good | C (poorly studied) | Worldwide |
| Artesunate | Excellent | Good | N/A | Asia, Africa, limited in UK, not in US |
| Primaquine | Good | Fair | C‡ | Worldwide |
| Doxycycline | Excellent | Fair | D (teratogenic) | Worldwide |
| Sulfadoxine/pyrimethamine | Fair | Poor | C | Worldwide, but restricted in US |
| Note: Prescribers and patients are urged to refer to the CDC reference about pregnancy in malaria (wwwn.cdc.gov/travel/contentMalariaPregnantPublic.aspx) and to specific country information regarding sensitivities of malaria (wwwn.cdc.gov/travel/destinationList.aspx). | ||||
| * Pregnancy class C: Animal reproduction studies have shown an adverse effect on the fetus and there are no adequate and well-controlled studies in humans, but potential benefits may warrant use of the drug in pregnant women, despite potential risks. | ||||
| * Pregnancy class D: There is positive evidence of human fetal risk based on adverse reaction data from investigational or marketing experience or studies in humans, but potential benefits may warrant use of the drug in pregnant women, despite potential risks. | ||||
| † Monitor patients for maternal hypoglycemia. | ||||
| ‡ There is no evidence of teratogenicity, but primaquine is associated with fetal intravascular hemolysis. | ||||
Chemoprophylaxis lowers rates of maternal infection
Although prophylaxis for pregnant patients traveling to malarial regions is a public concern, data for decision-making must be extrapolated from the available evidence, which is based primarily on women living in endemic areas. In a Cochrane systematic review, antimalarials were found to decrease the incidence of maternal infections (relative risk [RR]=0.27; 95% confidence interval [CI], 0.17–0.44) and reduce maternal anemia (RR=0.62; 95% CI, 0.50–0.78) in low-parity women—ie, during a first or second pregnancy.3
In low-parity women, these drugs were also found to decrease perinatal death (RR=0.73; 95% CI, 0.53–0.99) and low birth weight (RR=0.57; 95% CI, 0.46–0.72) associated with malarial infection. When used in all parity groups, antimalarials were somewhat less effective, yet still reduced maternal infections (RR=0.53; 95% CI, 0.33–0.86); the effects were similar with all antimalarials tested.3,4
Chloroquine, mefloquine are safe in pregnancy, doxycycline is not
While chemoprophylaxis in pregnancy appears efficacious, a major question remains—which agents are safest for both the woman and fetus? Some drugs routinely used in nonpregnant individuals should not be offered to pregnant women because of known direct effects on the fetus. Doxycycline is teratogenic, and primaquine poses a significant risk of fetal intravascular hemolysis in G6PD-deficient fetuses.5 Other drugs, such as atovaquone/proguanil and artesunate, are not well studied in pregnancy, and therefore are not recommended for use unless other options are not available.2,6
Among drugs that are well studied and without known direct fetal-damaging effects, adverse drug reaction profiles can guide use based on disease prevalence and drug-resistance patterns.
- Chloroquine is widely used because it is inexpensive and well tolerated, with only pruritus, mouth ulcers, and gastrointestinal upset as the most common adverse effects.
- Mefloquine is usually well tolerated, but can cause dose-related neuropsychiatric effects; it is contraindicated in those with a history of epilepsy or psychiatric disease.
- Sulfadoxine and pyrimethamine are not normally used as prophylaxis for any patient, due to the risk of toxic epidermal necrolysis and Stevens-Johnson syndrome, and the possible risk of jaundice and kernicterus if used in the third trimester of pregnancy.
- Quinine, which can be used for treatment or prophylaxis, may cause hypoglycemia, an effect that is more pronounced during pregnancy and requires close monitoring of blood glucose levels.5,7
Given these reaction profiles, chloroquine or mefloquine are usually the best choice with their superior safety and efficacy.
Figure
Best protection: Avoidance
Chloroquine and mefloquine are the safest antimalarials for use in pregnant women, but personal protection measures are also critical. Above, an Anopheles stephensi mosquito expelling a droplet of blood from its abdomen after having engorged itself on its human host’s blood. (Source: CDC.)
Recommendations from others
The World Health Organization (WHO) recommends pregnant women avoid travel to malarial regions. If travel is required, WHO recommends chloroquine as first-line prophylaxis in pregnancy (plus proguanil if the region exhibits emerging chloroquine resistance). In areas with proven chloroquine resistance, mefloquine is the drug of choice. Other antimalarials—such as quinine, pyrimethamine, sulfadoxine, and artesunate—should not be withheld if the preferred drugs are not available, or if the infection is life-threatening.2
The Centers for Disease Control and Prevention (CDC) also recommends avoiding travel to malaria-endemic regions during pregnancy, but if travel is necessary, the CDC advises use of chloroquine (or mefloquine in regions with chloroquine resistance). The CDC discourages the use of atovaquone/proguanil, doxycycline, and primaquine, due to known adverse fetal effects or inadequate experience in pregnancy.6
Acknowledgments
The opinions and assertions contained herein are the private views of the authors and not to be construed as official, or as reflecting the views of the US Air Force Medical Service or the US Air Force at large.
1. Centers for Disease Control and Prevention Web site. Destinations: CDC Traveler’s Health. Available at: wwwn.cdc.gov/travel/destinationlist.aspx. Accessed on December 7, 2007.
2. Malaria. In: International Travel and Health. Geneva, Switzerland: World Health Organization; 2007. Available at: whqlibdoc.who.int/publications/2005/9241580364_chap7.pdf. Accessed on December 7, 2007.
3. Orton L, Garner P. Drugs for treating uncomplicated malaria in pregnant women. Cochrane Database Syst Rev 2005;(3):CD004912.-
4. Garner P, Gülmezoglu AM. Drugs for preventing malaria in pregnant women. Cochrane Database Syst Rev 2006;(4):CD000169.-
5. Phillips-Howard PA, Wood D. The safety of antimalarial drugs in pregnancy. Drug Saf 1996;14:131-145.
6. Centers for Disease Control and Prevention Web site. Diseases: Malaria: Prevention, Pregnant Women, Public Info. Available at: wwwn.cdc.gov/travel/ contentMalariaPregnantPublic.aspx. Accessed on December 7, 2007.
7. Taylor WR, White NJ. Antimalarial drug toxicity: a review. Drug Saf 2004;27:25-61.
1. Centers for Disease Control and Prevention Web site. Destinations: CDC Traveler’s Health. Available at: wwwn.cdc.gov/travel/destinationlist.aspx. Accessed on December 7, 2007.
2. Malaria. In: International Travel and Health. Geneva, Switzerland: World Health Organization; 2007. Available at: whqlibdoc.who.int/publications/2005/9241580364_chap7.pdf. Accessed on December 7, 2007.
3. Orton L, Garner P. Drugs for treating uncomplicated malaria in pregnant women. Cochrane Database Syst Rev 2005;(3):CD004912.-
4. Garner P, Gülmezoglu AM. Drugs for preventing malaria in pregnant women. Cochrane Database Syst Rev 2006;(4):CD000169.-
5. Phillips-Howard PA, Wood D. The safety of antimalarial drugs in pregnancy. Drug Saf 1996;14:131-145.
6. Centers for Disease Control and Prevention Web site. Diseases: Malaria: Prevention, Pregnant Women, Public Info. Available at: wwwn.cdc.gov/travel/ contentMalariaPregnantPublic.aspx. Accessed on December 7, 2007.
7. Taylor WR, White NJ. Antimalarial drug toxicity: a review. Drug Saf 2004;27:25-61.
Evidence-based answers from the Family Physicians Inquiries Network
What is the best portable method of purifying water to prevent infectious disease?
There isn’t a single best method, but there are 5 that adequately purify water according to environmental Protection agency (EPA) standards. These include 1) boiling for 1 minute if below 2000 m (6562 feet) and 3 minutes if above, 2) chlorine dioxide tablets, 3) MIoX purifier, 4) ultraviolet light (steriPEN), and 5) portable filtration with a absolute pore size <1 micrometer combined with halogenation or charcoal filtration (strength of recommendation [SOR]: C, based on expert opinion and microbiological testing). Halogenation alone (ie, chlorine and iodine) is not effective against Cryptosporidium (SOR: C, based on microbiological testing).
Why boil water when there are so many other options?
Timothy Mott, MD, FAAFP
US Naval Hospital, Sigonella, Italy
These days, “boil it, peel it, or forget it” only goes so far with the unencumbered traveler. Experience tells me that most hear “Boil it” and instantly go right to “Forget it!” Fortunately, there is an excellent resource to assist patients in choosing a personally acceptable portable water purification system. It’s called the Water Purification Database at usachppm.apgea.army.mil/WPD/CompareDevices.aspx.1
This outstanding database was developed by an impartial third-party for the US Army and gives clear, well-organized guidance on over 60 purifiers. For each purifier, the guide covers efficacy against primary pathogens, purification mechanism, links to manufacturers, and an advantages/ disadvantages breakdown (such as weight, cost, and ease of use). Add this site to your Internet “favorites” folder.
Evidence summary
With the rise in international travel and adventure sports, individuals are at increased risk of acquiring infections by drinking water from impure water sources. Common waterborne infections that back-country and international travelers may contract include bacterial diarrhea, viruses, protozoa (such as Giardia and Cryptosporidium), and parasites (such as schistosoma). The risk of infection varies based on travel location.
To prevent illness, travelers may seek medical guidance regarding safe water practice. In one study, 36% of travelers sought advice from a physician prior to international travel.2 Preventing waterborne infections should be a component of traveler education, in addition to other standard advice, such as mosquito avoidance and immunizations.3 (For more on travel safety, see these Clinical Inquiries: “When should travelers begin malaria prophylaxis?” in the November 2007 Journal of Family Practice, pages 950–952, and “What is the most effective and safe malaria prophylaxis during pregnancy?” on page 51 of this issue.)
Which devices meet EPA standards?
The EPA has established a “minimal microbiological hazard” allowed for a portable water purification system to be considered safe. Water purifiers must reduce bacteria by 99.9999%, viruses by 99.99%, and protozoa (such as Cryptosporidium parvum) by 99.9% to receive an EPA certification number.4
There are no head-to-head trials comparing the effectiveness of different methods of purification to prevent infectious disease. The majority of the evidence is based on data provided by manufacturers to the EPA, with some independent studies and expert opinion (TABLE).
Expert opinion recommends bringing water to a rapid boil for at least 3 minutes and letting it cool as an effective means of water purification.5 Chlorine dioxide tablets, the MIOX purifier, and UV light (SteriPEN) have all met EPA standards for lower pathogen counts under ideal conditions. Halogenation does not reduce Cryptosporidium below the microbiological hazard of 99.9%, but it is generally accepted to effectively treat viruses, bacteria, and other protozoa after filtering through a cloth to remove large particles.6
Filtration with an absolute pore size of <0.1 micrometer (10 times smaller than the EPA standard) has been generally accepted as effective against protozoa and bacteria, but it is not effective against viruses because of their small size.7 When combined with either halogenation or charcoal filters, filtration can be effective against all pathogens.8
TABLE
Portable water purification: How do these 6 methods compare?
| METHOD | EFFECTIVENESS | ADVANTAGES | DISADVANTAGES |
|---|---|---|---|
| Boiling with cooling* | Kills viruses, bacteria, protozoa, and parasites | simple, universally accepted, no special equipment required | Time-consuming, may require large amounts of fuel |
chlorine dioxide* | Kills bacteria, viruses, protozoa, and parasites | same as chlorine/iodine treatment but also treats Cryptosporidium, good palatability | Must wait up to 4 hours to treat Cryptosporidium, costs more than iodine/chlorine ($13 for 30 tabs) |
Chlorine/iodine | Kills bacteria, viruses, protozoa (not Cryptosporidium), and parasites | Inexpensive, easy, lightweight, treats large quantities | Does not kill Cryptosporidium, poor taste, must wait for water to be treated; contraindicated in pregnancy, thyroid disease; not recommended beyond few weeks of use |
Filtration † | Removes parasites, Giardia, Cryptosporidium, and bacteria | Able to use water immediately, removes sediment, many have combination of activated carbon, chemical disinfectant, or both | Can potentially be expensive, filters may clog easily, heavy, not effective against small particle viruses, therefore should supplement with chlorine or iodine |
MIOX Purifier* | Kills bacteria, viruses, protozoa, and parasites | light (8 oz), sturdy, treats large quantities; requires camera batteries and salt | Cost $130, must wait for 4 hours and treat with higher strength to treat Cryptosporidium; requires 30 minutes to treat viruses, bacteria, and Giardia |
 UV light (steriPEN) ‡ | Kills bacteria, viruses, protozoa, parasites in clear water | Light (8 oz), quick (treats 16 oz of water in 1 minute) | Cost $100, does not work in turbid conditions |
| * Meets EPA standards. | |||
| † some filtration systems meet EPA standards. See chppm-www.apgea.army.mil/WPD/CompareDevices.aspx for testing results of individual filters.1 | |||
| ‡ Meets EPA standards in clear water. | |||
Recommendations from others
The US Army Center for Health Promotion and Preventive Medicine (USACHPPM) published a report in 2006 on the efficacy of commercial off-the-shelf individual water purifiers.8 Using National Sanitation Foundation Protocol P248 and applying it to “real-world” emergency military operational conditions, USACHPPM found that no device scored high on every attribute, and that overall scores for most devices were in the moderate range. The top score for any device was 79 (out of 100).8
The overall top 3 scoring products were: 1) the SweetWater Purifier from Mountain Safety Research; 2) the Micropur MP 1 tablets from Katadyn North America, Inc; and 3) the First Need Deluxe water purifier from General Ecology, Inc.
Acknowledgments
The opinions and assertions contained herein are the private views of the authors and not to be construed as official, or as reflecting the views of the US Air Force Medical Service or the US air Force at large.
1. Commercially available individual water purifiers. Water Purification Database, US Army Center for Health Promotion and Preventive Medicine Web site. Available at: usachppm.apgea.army.mil/WPD/CompareDevices.aspx. Accessed on December 7, 2007.
2. Hamer DH, Connor Ba. Travel health knowledge, attitudes and practices among united states travelers. J Travel Med 2004;11:23-26.
3. Hill DR, Ericsson CD, Pearson RD, et al. The Practice of Travel Medicine: Guidelines by the Infectious Diseases Society of America. Clin Infect Dis 2006;43:1499-1539.
4. US Environmental Protection agency: Guide standard and Protocol for Testing Microbiological Water Purifiers: Report to Task Force. Cincinnati, OH: US environmental Protection agency; 1987.
5. Centers for Disease Control and Prevention Water treatment methods. Available at: wwwn.cdc.gov/travel/contentWaterTreatment.aspx. Accessed on December 7, 2007.
6. Gerba CP, Johnson DC, Hasan MN. efficacy of iodine water purification tablets against Cryptosporidium oocysts and Giardia cysts. Wilderness Environ Med 1997;8:96-100.
7. Centers for Disease Control and Prevention Division of Parasitic Diseases Preventing Cryptosporidiosis: a guide to water filters and bottled water. Available at: www.cdc.gov/ncidod/dpd/parasites/cryptosporidiosis/factsht_crypto_prevent_water.htm. Accessed on December 7, 2007.
8. Water supply Management Program Project No 31-EC-03E0. Performance and health risk assessment of commercial-off-the-shelf individual water purifiers. Aberdeen Proving Ground, MD: US Army Center for Health Promotion and Preventive Medicine; 2006. Available at: usachppm.apgea.army.mil/WPD/PDFDocs/Finalreport.pdf. Accessed on December 7, 2007.
There isn’t a single best method, but there are 5 that adequately purify water according to environmental Protection agency (EPA) standards. These include 1) boiling for 1 minute if below 2000 m (6562 feet) and 3 minutes if above, 2) chlorine dioxide tablets, 3) MIoX purifier, 4) ultraviolet light (steriPEN), and 5) portable filtration with a absolute pore size <1 micrometer combined with halogenation or charcoal filtration (strength of recommendation [SOR]: C, based on expert opinion and microbiological testing). Halogenation alone (ie, chlorine and iodine) is not effective against Cryptosporidium (SOR: C, based on microbiological testing).
Why boil water when there are so many other options?
Timothy Mott, MD, FAAFP
US Naval Hospital, Sigonella, Italy
These days, “boil it, peel it, or forget it” only goes so far with the unencumbered traveler. Experience tells me that most hear “Boil it” and instantly go right to “Forget it!” Fortunately, there is an excellent resource to assist patients in choosing a personally acceptable portable water purification system. It’s called the Water Purification Database at usachppm.apgea.army.mil/WPD/CompareDevices.aspx.1
This outstanding database was developed by an impartial third-party for the US Army and gives clear, well-organized guidance on over 60 purifiers. For each purifier, the guide covers efficacy against primary pathogens, purification mechanism, links to manufacturers, and an advantages/ disadvantages breakdown (such as weight, cost, and ease of use). Add this site to your Internet “favorites” folder.
Evidence summary
With the rise in international travel and adventure sports, individuals are at increased risk of acquiring infections by drinking water from impure water sources. Common waterborne infections that back-country and international travelers may contract include bacterial diarrhea, viruses, protozoa (such as Giardia and Cryptosporidium), and parasites (such as schistosoma). The risk of infection varies based on travel location.
To prevent illness, travelers may seek medical guidance regarding safe water practice. In one study, 36% of travelers sought advice from a physician prior to international travel.2 Preventing waterborne infections should be a component of traveler education, in addition to other standard advice, such as mosquito avoidance and immunizations.3 (For more on travel safety, see these Clinical Inquiries: “When should travelers begin malaria prophylaxis?” in the November 2007 Journal of Family Practice, pages 950–952, and “What is the most effective and safe malaria prophylaxis during pregnancy?” on page 51 of this issue.)
Which devices meet EPA standards?
The EPA has established a “minimal microbiological hazard” allowed for a portable water purification system to be considered safe. Water purifiers must reduce bacteria by 99.9999%, viruses by 99.99%, and protozoa (such as Cryptosporidium parvum) by 99.9% to receive an EPA certification number.4
There are no head-to-head trials comparing the effectiveness of different methods of purification to prevent infectious disease. The majority of the evidence is based on data provided by manufacturers to the EPA, with some independent studies and expert opinion (TABLE).
Expert opinion recommends bringing water to a rapid boil for at least 3 minutes and letting it cool as an effective means of water purification.5 Chlorine dioxide tablets, the MIOX purifier, and UV light (SteriPEN) have all met EPA standards for lower pathogen counts under ideal conditions. Halogenation does not reduce Cryptosporidium below the microbiological hazard of 99.9%, but it is generally accepted to effectively treat viruses, bacteria, and other protozoa after filtering through a cloth to remove large particles.6
Filtration with an absolute pore size of <0.1 micrometer (10 times smaller than the EPA standard) has been generally accepted as effective against protozoa and bacteria, but it is not effective against viruses because of their small size.7 When combined with either halogenation or charcoal filters, filtration can be effective against all pathogens.8
TABLE
Portable water purification: How do these 6 methods compare?
| METHOD | EFFECTIVENESS | ADVANTAGES | DISADVANTAGES |
|---|---|---|---|
| Boiling with cooling* | Kills viruses, bacteria, protozoa, and parasites | simple, universally accepted, no special equipment required | Time-consuming, may require large amounts of fuel |
chlorine dioxide* | Kills bacteria, viruses, protozoa, and parasites | same as chlorine/iodine treatment but also treats Cryptosporidium, good palatability | Must wait up to 4 hours to treat Cryptosporidium, costs more than iodine/chlorine ($13 for 30 tabs) |
Chlorine/iodine | Kills bacteria, viruses, protozoa (not Cryptosporidium), and parasites | Inexpensive, easy, lightweight, treats large quantities | Does not kill Cryptosporidium, poor taste, must wait for water to be treated; contraindicated in pregnancy, thyroid disease; not recommended beyond few weeks of use |
Filtration † | Removes parasites, Giardia, Cryptosporidium, and bacteria | Able to use water immediately, removes sediment, many have combination of activated carbon, chemical disinfectant, or both | Can potentially be expensive, filters may clog easily, heavy, not effective against small particle viruses, therefore should supplement with chlorine or iodine |
MIOX Purifier* | Kills bacteria, viruses, protozoa, and parasites | light (8 oz), sturdy, treats large quantities; requires camera batteries and salt | Cost $130, must wait for 4 hours and treat with higher strength to treat Cryptosporidium; requires 30 minutes to treat viruses, bacteria, and Giardia |
 UV light (steriPEN) ‡ | Kills bacteria, viruses, protozoa, parasites in clear water | Light (8 oz), quick (treats 16 oz of water in 1 minute) | Cost $100, does not work in turbid conditions |
| * Meets EPA standards. | |||
| † some filtration systems meet EPA standards. See chppm-www.apgea.army.mil/WPD/CompareDevices.aspx for testing results of individual filters.1 | |||
| ‡ Meets EPA standards in clear water. | |||
Recommendations from others
The US Army Center for Health Promotion and Preventive Medicine (USACHPPM) published a report in 2006 on the efficacy of commercial off-the-shelf individual water purifiers.8 Using National Sanitation Foundation Protocol P248 and applying it to “real-world” emergency military operational conditions, USACHPPM found that no device scored high on every attribute, and that overall scores for most devices were in the moderate range. The top score for any device was 79 (out of 100).8
The overall top 3 scoring products were: 1) the SweetWater Purifier from Mountain Safety Research; 2) the Micropur MP 1 tablets from Katadyn North America, Inc; and 3) the First Need Deluxe water purifier from General Ecology, Inc.
Acknowledgments
The opinions and assertions contained herein are the private views of the authors and not to be construed as official, or as reflecting the views of the US Air Force Medical Service or the US air Force at large.
There isn’t a single best method, but there are 5 that adequately purify water according to environmental Protection agency (EPA) standards. These include 1) boiling for 1 minute if below 2000 m (6562 feet) and 3 minutes if above, 2) chlorine dioxide tablets, 3) MIoX purifier, 4) ultraviolet light (steriPEN), and 5) portable filtration with a absolute pore size <1 micrometer combined with halogenation or charcoal filtration (strength of recommendation [SOR]: C, based on expert opinion and microbiological testing). Halogenation alone (ie, chlorine and iodine) is not effective against Cryptosporidium (SOR: C, based on microbiological testing).
Why boil water when there are so many other options?
Timothy Mott, MD, FAAFP
US Naval Hospital, Sigonella, Italy
These days, “boil it, peel it, or forget it” only goes so far with the unencumbered traveler. Experience tells me that most hear “Boil it” and instantly go right to “Forget it!” Fortunately, there is an excellent resource to assist patients in choosing a personally acceptable portable water purification system. It’s called the Water Purification Database at usachppm.apgea.army.mil/WPD/CompareDevices.aspx.1
This outstanding database was developed by an impartial third-party for the US Army and gives clear, well-organized guidance on over 60 purifiers. For each purifier, the guide covers efficacy against primary pathogens, purification mechanism, links to manufacturers, and an advantages/ disadvantages breakdown (such as weight, cost, and ease of use). Add this site to your Internet “favorites” folder.
Evidence summary
With the rise in international travel and adventure sports, individuals are at increased risk of acquiring infections by drinking water from impure water sources. Common waterborne infections that back-country and international travelers may contract include bacterial diarrhea, viruses, protozoa (such as Giardia and Cryptosporidium), and parasites (such as schistosoma). The risk of infection varies based on travel location.
To prevent illness, travelers may seek medical guidance regarding safe water practice. In one study, 36% of travelers sought advice from a physician prior to international travel.2 Preventing waterborne infections should be a component of traveler education, in addition to other standard advice, such as mosquito avoidance and immunizations.3 (For more on travel safety, see these Clinical Inquiries: “When should travelers begin malaria prophylaxis?” in the November 2007 Journal of Family Practice, pages 950–952, and “What is the most effective and safe malaria prophylaxis during pregnancy?” on page 51 of this issue.)
Which devices meet EPA standards?
The EPA has established a “minimal microbiological hazard” allowed for a portable water purification system to be considered safe. Water purifiers must reduce bacteria by 99.9999%, viruses by 99.99%, and protozoa (such as Cryptosporidium parvum) by 99.9% to receive an EPA certification number.4
There are no head-to-head trials comparing the effectiveness of different methods of purification to prevent infectious disease. The majority of the evidence is based on data provided by manufacturers to the EPA, with some independent studies and expert opinion (TABLE).
Expert opinion recommends bringing water to a rapid boil for at least 3 minutes and letting it cool as an effective means of water purification.5 Chlorine dioxide tablets, the MIOX purifier, and UV light (SteriPEN) have all met EPA standards for lower pathogen counts under ideal conditions. Halogenation does not reduce Cryptosporidium below the microbiological hazard of 99.9%, but it is generally accepted to effectively treat viruses, bacteria, and other protozoa after filtering through a cloth to remove large particles.6
Filtration with an absolute pore size of <0.1 micrometer (10 times smaller than the EPA standard) has been generally accepted as effective against protozoa and bacteria, but it is not effective against viruses because of their small size.7 When combined with either halogenation or charcoal filters, filtration can be effective against all pathogens.8
TABLE
Portable water purification: How do these 6 methods compare?
| METHOD | EFFECTIVENESS | ADVANTAGES | DISADVANTAGES |
|---|---|---|---|
| Boiling with cooling* | Kills viruses, bacteria, protozoa, and parasites | simple, universally accepted, no special equipment required | Time-consuming, may require large amounts of fuel |
chlorine dioxide* | Kills bacteria, viruses, protozoa, and parasites | same as chlorine/iodine treatment but also treats Cryptosporidium, good palatability | Must wait up to 4 hours to treat Cryptosporidium, costs more than iodine/chlorine ($13 for 30 tabs) |
Chlorine/iodine | Kills bacteria, viruses, protozoa (not Cryptosporidium), and parasites | Inexpensive, easy, lightweight, treats large quantities | Does not kill Cryptosporidium, poor taste, must wait for water to be treated; contraindicated in pregnancy, thyroid disease; not recommended beyond few weeks of use |
Filtration † | Removes parasites, Giardia, Cryptosporidium, and bacteria | Able to use water immediately, removes sediment, many have combination of activated carbon, chemical disinfectant, or both | Can potentially be expensive, filters may clog easily, heavy, not effective against small particle viruses, therefore should supplement with chlorine or iodine |
MIOX Purifier* | Kills bacteria, viruses, protozoa, and parasites | light (8 oz), sturdy, treats large quantities; requires camera batteries and salt | Cost $130, must wait for 4 hours and treat with higher strength to treat Cryptosporidium; requires 30 minutes to treat viruses, bacteria, and Giardia |
 UV light (steriPEN) ‡ | Kills bacteria, viruses, protozoa, parasites in clear water | Light (8 oz), quick (treats 16 oz of water in 1 minute) | Cost $100, does not work in turbid conditions |
| * Meets EPA standards. | |||
| † some filtration systems meet EPA standards. See chppm-www.apgea.army.mil/WPD/CompareDevices.aspx for testing results of individual filters.1 | |||
| ‡ Meets EPA standards in clear water. | |||
Recommendations from others
The US Army Center for Health Promotion and Preventive Medicine (USACHPPM) published a report in 2006 on the efficacy of commercial off-the-shelf individual water purifiers.8 Using National Sanitation Foundation Protocol P248 and applying it to “real-world” emergency military operational conditions, USACHPPM found that no device scored high on every attribute, and that overall scores for most devices were in the moderate range. The top score for any device was 79 (out of 100).8
The overall top 3 scoring products were: 1) the SweetWater Purifier from Mountain Safety Research; 2) the Micropur MP 1 tablets from Katadyn North America, Inc; and 3) the First Need Deluxe water purifier from General Ecology, Inc.
Acknowledgments
The opinions and assertions contained herein are the private views of the authors and not to be construed as official, or as reflecting the views of the US Air Force Medical Service or the US air Force at large.
1. Commercially available individual water purifiers. Water Purification Database, US Army Center for Health Promotion and Preventive Medicine Web site. Available at: usachppm.apgea.army.mil/WPD/CompareDevices.aspx. Accessed on December 7, 2007.
2. Hamer DH, Connor Ba. Travel health knowledge, attitudes and practices among united states travelers. J Travel Med 2004;11:23-26.
3. Hill DR, Ericsson CD, Pearson RD, et al. The Practice of Travel Medicine: Guidelines by the Infectious Diseases Society of America. Clin Infect Dis 2006;43:1499-1539.
4. US Environmental Protection agency: Guide standard and Protocol for Testing Microbiological Water Purifiers: Report to Task Force. Cincinnati, OH: US environmental Protection agency; 1987.
5. Centers for Disease Control and Prevention Water treatment methods. Available at: wwwn.cdc.gov/travel/contentWaterTreatment.aspx. Accessed on December 7, 2007.
6. Gerba CP, Johnson DC, Hasan MN. efficacy of iodine water purification tablets against Cryptosporidium oocysts and Giardia cysts. Wilderness Environ Med 1997;8:96-100.
7. Centers for Disease Control and Prevention Division of Parasitic Diseases Preventing Cryptosporidiosis: a guide to water filters and bottled water. Available at: www.cdc.gov/ncidod/dpd/parasites/cryptosporidiosis/factsht_crypto_prevent_water.htm. Accessed on December 7, 2007.
8. Water supply Management Program Project No 31-EC-03E0. Performance and health risk assessment of commercial-off-the-shelf individual water purifiers. Aberdeen Proving Ground, MD: US Army Center for Health Promotion and Preventive Medicine; 2006. Available at: usachppm.apgea.army.mil/WPD/PDFDocs/Finalreport.pdf. Accessed on December 7, 2007.
1. Commercially available individual water purifiers. Water Purification Database, US Army Center for Health Promotion and Preventive Medicine Web site. Available at: usachppm.apgea.army.mil/WPD/CompareDevices.aspx. Accessed on December 7, 2007.
2. Hamer DH, Connor Ba. Travel health knowledge, attitudes and practices among united states travelers. J Travel Med 2004;11:23-26.
3. Hill DR, Ericsson CD, Pearson RD, et al. The Practice of Travel Medicine: Guidelines by the Infectious Diseases Society of America. Clin Infect Dis 2006;43:1499-1539.
4. US Environmental Protection agency: Guide standard and Protocol for Testing Microbiological Water Purifiers: Report to Task Force. Cincinnati, OH: US environmental Protection agency; 1987.
5. Centers for Disease Control and Prevention Water treatment methods. Available at: wwwn.cdc.gov/travel/contentWaterTreatment.aspx. Accessed on December 7, 2007.
6. Gerba CP, Johnson DC, Hasan MN. efficacy of iodine water purification tablets against Cryptosporidium oocysts and Giardia cysts. Wilderness Environ Med 1997;8:96-100.
7. Centers for Disease Control and Prevention Division of Parasitic Diseases Preventing Cryptosporidiosis: a guide to water filters and bottled water. Available at: www.cdc.gov/ncidod/dpd/parasites/cryptosporidiosis/factsht_crypto_prevent_water.htm. Accessed on December 7, 2007.
8. Water supply Management Program Project No 31-EC-03E0. Performance and health risk assessment of commercial-off-the-shelf individual water purifiers. Aberdeen Proving Ground, MD: US Army Center for Health Promotion and Preventive Medicine; 2006. Available at: usachppm.apgea.army.mil/WPD/PDFDocs/Finalreport.pdf. Accessed on December 7, 2007.
Evidence-based answers from the Family Physicians Inquiries Network