User login
Cutaneous Protothecosis
To the Editor:
Protothecosis infections are caused by an achlorophyllic algae of the species Prototheca. Prototheca organisms are found mostly in soil and water.1 Human infections are rare and involve 2 species, Prototheca wickerhamii and Prototheca zopfii. The former most commonly is responsible for human infections, though P zopfii results in more serious systemic infections with a poor prognosis. There are various types of Prototheca infection presentations, with a 2007 review of 117 cases reporting that cutaneous infections are most common (66%), followed by systemic infections (19%), and olecranon bursitis (15%).2 Skin lesions most commonly occur on the extremities and face, and they present as vesiculobullous and ulcerative lesions with purulent drainage. The skin lesions also may appear as erythematous plaques or nodules, subcutaneous papules, verrucous or herpetiformis lesions, or pyogenic granuloma–like lesions.3 Protothecosis typically affects immunocompromised individuals, especially those with a history of chronic corticosteroid use, malignancy, diabetes mellitus, AIDS, and/or organ transplant.1 We present a case of cutaneous protothecosis on the dorsal distal extremity of a 94-year-old woman. History of exposure to soil while gardening was elicited from the patient, and no immunosuppressive history was present aside from the patient’s age. This case may prompt workup for malignancy or immunosuppression in this patient subset.
A 94-year-old woman with a medical history of cutaneous squamous cell carcinoma (SCC) presented with a growing lesion on the dorsal surface of the left fourth digit of 2 months’ duration. The patient reported the lesion was painful, and she noted preceding trauma to the area that was suspected to have occurred while gardening. Physical examination revealed an ulcerated, hypertrophic, erythematous nodule on the dorsal surface of the left fourth metacarpophalangeal joint. The differential diagnosis included SCC, inflamed cyst, verruca vulgaris, and orf virus due to the clinical presentation. A shave biopsy was performed, and the lesion subsequently was treated with electrodesiccation and curettage.
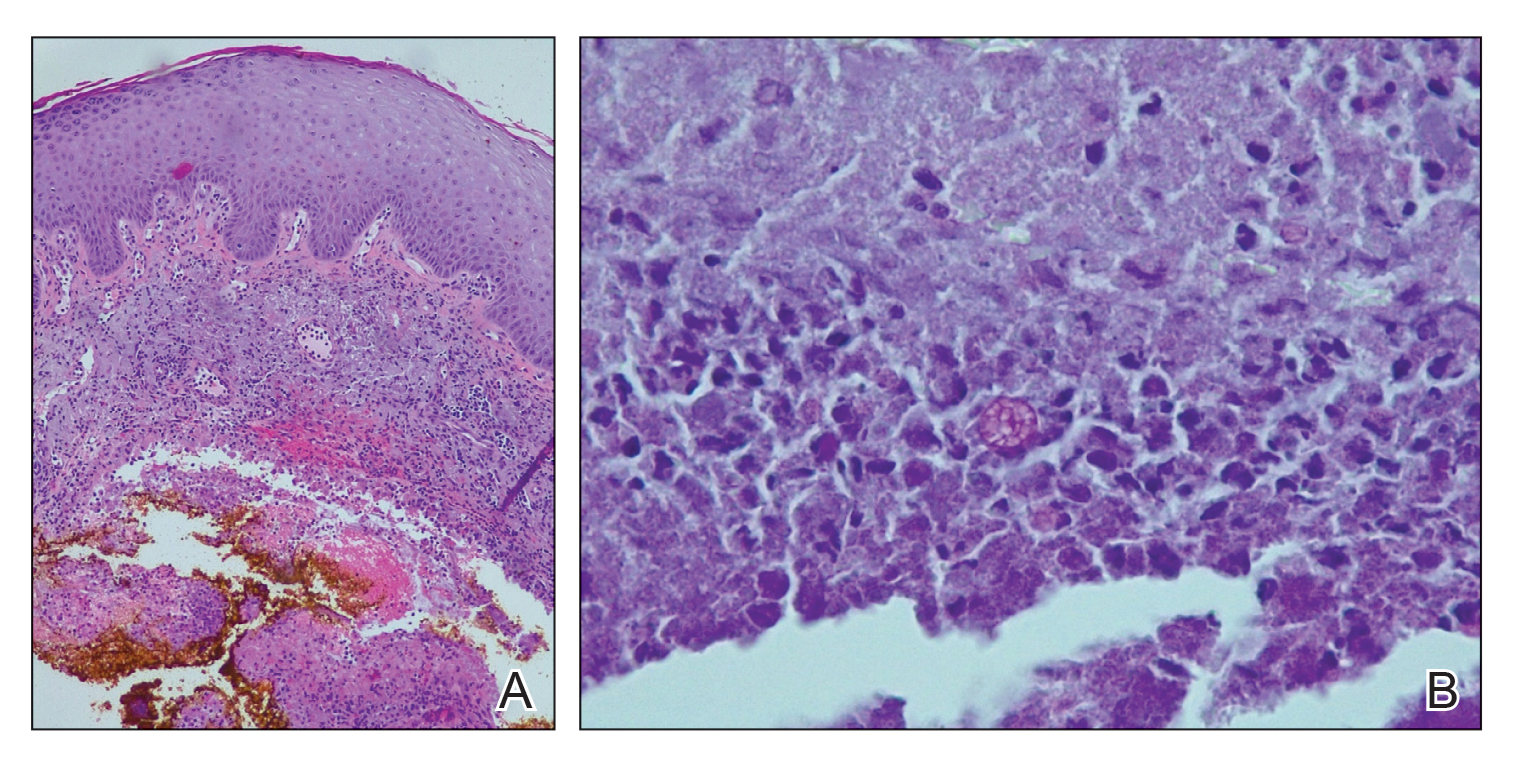
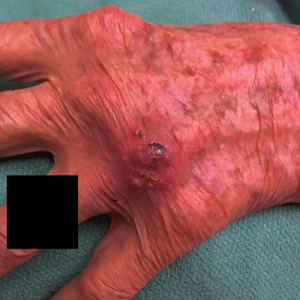
Histopathologic evaluation revealed pseudoepitheliomatous hyperplasia with a mixed inflammatory infiltrate including lymphocytes and histiocytes. A morula within the dermis was characteristic of a protothecosis infection (Figure 1). On follow-up visit 6 weeks later, the lesion had grown back to its original size and morphology (Figure 2). At this time, the lesion was again treated with shave removal, followed by electrodesiccation and curettage, and the patient was placed on oral fluconazole 200 mg daily for 1 month. When the lesion did not resolve with fluconazole, she was referred to infectious disease as well as general surgery for surgical removal and debridement of the lesion. Unfortunately, the patient was lost to follow-up.
Protothecosis is an infectious disease comprised of achlorophyllic algae found in soil and water that rarely affects humans. When it does affect humans, cutaneous infections are most common. All human cases in which organisms were identified to species level have been caused by P wickerhamii or P zopfii species.2 Inoculation is suspected to occur through trauma to affected skin, especially when in the context of contaminated water. Our patient reported history of trauma to the hand, with soil from gardening as the potential aquagenic source of the infection.
The clinical presentation of protothecosis ranges from localized cutaneous to disseminated systemic infections, with most reported cases of systemic disease occurring in immunocompromised individuals. The cutaneous lesions of protothecosis vary greatly in clinical appearance including ulcerative nodules (as in our case), papules, plaques, pustules, and vesicles with erosion or crusting.4
Cutaneous protothecosis has the potential to mimic many other skin diseases and lesions, and, given its rarity, it may not be on the radar of dermatologists. Our patient’s lesion was presumed to be a skin cancer and was treated as such because of the history of SCC and clinical presentation. Although excision of individual lesions of protothecosis can be curative, electrodesiccation and curettage does not appear to be an adequate treatment, as the lesion subsequently recurred. It also is possible that this case represents P zopfii infection, as it did not respond to treatment with oral fluconazole, though in vitro studies with fluconazole to both P zopfii and P wickerhamii had variable treatment success.2 Also, the histopathologic findings were most consistent with P wickerhamii, revealing small, round, symmetrical morula, compared to P zopfii, which typically will display oval or cylindrical, asymmetrical, random internal segmentation.5 This case may warrant determination of species, which can be accomplished by a culture on Sabouraud dextrose agar, carbohydrate and alcohol assimilation test, yeast biochemical card, serological typing by immunoblotting, immunofluorescence study using species-specific antibodies, or amplification by polymerase chain reaction for small subunit ribosomal DNA sequences.2,6-8
The natural history of isolated skin disease is an indolent progressive course; however, reports do exist noting spontaneous resolution.4,9 Treatment options for Prototheca infections can be disappointing and consist of both surgical and medical management, or a combination of the 2 approaches. Reports in the literature support the use of antifungals including ketoconazole, voriconazole, itraconazole, fluconazole, and amphotericin B, with the latter displaying the best activity against Prototheca species.2 Tetracycline has been used in combination with oral or topical amphotericin B and was found to be synergistic in vitro and in case reports at successfully treating cutaneous protothecosis infections. It is possible that our patient was not treated with fluconazole long enough for it to become therapeutic, as most reported treatment regimens are weeks to months in length. Conversely, it may have been of benefit to transition the patient to topical amphotericin B and tetracycline, as fluconazole failed in this patient. However, treatment successes and failures are limited to case reports/case series and in vitro studies, with prospective studies lacking. Due to the variability with in vitro susceptibility profiles for Prototheca species, it generally is not recommended to pursue in vitro susceptibility testing in the management of Prototheca skin infections due to the inconsistency demonstrated between in vitro activity and clinical response to therapy.2
- Silva PC, Costa e Silva SB, Lima RB, et al. Cutaneous protothecosis—case report. An Bras Dermatol. 2013;88:183-185.
- Lass-Flörl C, Mayr A. Human protothecosis. Clin Microbiol Rev. 2007;20:230-242.
- Seok JY, Lee Y, Lee H, et al. Human cutaneous protothecosis: report of a case and literature review. Korean J Pathol. 2013;47:575-578.
- Mayorga J, Barba-Gómez JF, Verduzco-Martínez AP, et al. Protothecosis. Clin Dermatol. 2012;30:432-436.
- Walsh SV, Johnson RA, Tahan SR. Protothecosis: an unusual cause of chronic subcutaneous and soft tissue infection. Am J Dermatopathol. 1998;20:379-382.
- Casal MJ, Gutierrez J. Simple new test for rapid differentiation of Prototheca wickerhamii from Prototheca zopfii. J Clin Microbiol. 1983;18:992-993.
- Arnold, P, Ahearn, DG. The systematics of the genus Prototheca with a description of a new species P. filamenta. Mycologia 1972;64:265-275.
- Roesler U, Scholz H, Hensel H. Emended phenotypic characterization of Prototheca zopfii: a proposal for three biotypes and standards for their identification. Int J Syst Evol Microbiol. 2003;53:1195-1199.
- Todd JR, King JW, Oberle A, et al. Protothecosis: report of a case with 20-year follow-up, and review of previously published cases. Med Mycol. 2012;50:673-689.
To the Editor:
Protothecosis infections are caused by an achlorophyllic algae of the species Prototheca. Prototheca organisms are found mostly in soil and water.1 Human infections are rare and involve 2 species, Prototheca wickerhamii and Prototheca zopfii. The former most commonly is responsible for human infections, though P zopfii results in more serious systemic infections with a poor prognosis. There are various types of Prototheca infection presentations, with a 2007 review of 117 cases reporting that cutaneous infections are most common (66%), followed by systemic infections (19%), and olecranon bursitis (15%).2 Skin lesions most commonly occur on the extremities and face, and they present as vesiculobullous and ulcerative lesions with purulent drainage. The skin lesions also may appear as erythematous plaques or nodules, subcutaneous papules, verrucous or herpetiformis lesions, or pyogenic granuloma–like lesions.3 Protothecosis typically affects immunocompromised individuals, especially those with a history of chronic corticosteroid use, malignancy, diabetes mellitus, AIDS, and/or organ transplant.1 We present a case of cutaneous protothecosis on the dorsal distal extremity of a 94-year-old woman. History of exposure to soil while gardening was elicited from the patient, and no immunosuppressive history was present aside from the patient’s age. This case may prompt workup for malignancy or immunosuppression in this patient subset.
A 94-year-old woman with a medical history of cutaneous squamous cell carcinoma (SCC) presented with a growing lesion on the dorsal surface of the left fourth digit of 2 months’ duration. The patient reported the lesion was painful, and she noted preceding trauma to the area that was suspected to have occurred while gardening. Physical examination revealed an ulcerated, hypertrophic, erythematous nodule on the dorsal surface of the left fourth metacarpophalangeal joint. The differential diagnosis included SCC, inflamed cyst, verruca vulgaris, and orf virus due to the clinical presentation. A shave biopsy was performed, and the lesion subsequently was treated with electrodesiccation and curettage.
Histopathologic evaluation revealed pseudoepitheliomatous hyperplasia with a mixed inflammatory infiltrate including lymphocytes and histiocytes. A morula within the dermis was characteristic of a protothecosis infection (Figure 1). On follow-up visit 6 weeks later, the lesion had grown back to its original size and morphology (Figure 2). At this time, the lesion was again treated with shave removal, followed by electrodesiccation and curettage, and the patient was placed on oral fluconazole 200 mg daily for 1 month. When the lesion did not resolve with fluconazole, she was referred to infectious disease as well as general surgery for surgical removal and debridement of the lesion. Unfortunately, the patient was lost to follow-up.
Protothecosis is an infectious disease comprised of achlorophyllic algae found in soil and water that rarely affects humans. When it does affect humans, cutaneous infections are most common. All human cases in which organisms were identified to species level have been caused by P wickerhamii or P zopfii species.2 Inoculation is suspected to occur through trauma to affected skin, especially when in the context of contaminated water. Our patient reported history of trauma to the hand, with soil from gardening as the potential aquagenic source of the infection.
The clinical presentation of protothecosis ranges from localized cutaneous to disseminated systemic infections, with most reported cases of systemic disease occurring in immunocompromised individuals. The cutaneous lesions of protothecosis vary greatly in clinical appearance including ulcerative nodules (as in our case), papules, plaques, pustules, and vesicles with erosion or crusting.4
Cutaneous protothecosis has the potential to mimic many other skin diseases and lesions, and, given its rarity, it may not be on the radar of dermatologists. Our patient’s lesion was presumed to be a skin cancer and was treated as such because of the history of SCC and clinical presentation. Although excision of individual lesions of protothecosis can be curative, electrodesiccation and curettage does not appear to be an adequate treatment, as the lesion subsequently recurred. It also is possible that this case represents P zopfii infection, as it did not respond to treatment with oral fluconazole, though in vitro studies with fluconazole to both P zopfii and P wickerhamii had variable treatment success.2 Also, the histopathologic findings were most consistent with P wickerhamii, revealing small, round, symmetrical morula, compared to P zopfii, which typically will display oval or cylindrical, asymmetrical, random internal segmentation.5 This case may warrant determination of species, which can be accomplished by a culture on Sabouraud dextrose agar, carbohydrate and alcohol assimilation test, yeast biochemical card, serological typing by immunoblotting, immunofluorescence study using species-specific antibodies, or amplification by polymerase chain reaction for small subunit ribosomal DNA sequences.2,6-8
The natural history of isolated skin disease is an indolent progressive course; however, reports do exist noting spontaneous resolution.4,9 Treatment options for Prototheca infections can be disappointing and consist of both surgical and medical management, or a combination of the 2 approaches. Reports in the literature support the use of antifungals including ketoconazole, voriconazole, itraconazole, fluconazole, and amphotericin B, with the latter displaying the best activity against Prototheca species.2 Tetracycline has been used in combination with oral or topical amphotericin B and was found to be synergistic in vitro and in case reports at successfully treating cutaneous protothecosis infections. It is possible that our patient was not treated with fluconazole long enough for it to become therapeutic, as most reported treatment regimens are weeks to months in length. Conversely, it may have been of benefit to transition the patient to topical amphotericin B and tetracycline, as fluconazole failed in this patient. However, treatment successes and failures are limited to case reports/case series and in vitro studies, with prospective studies lacking. Due to the variability with in vitro susceptibility profiles for Prototheca species, it generally is not recommended to pursue in vitro susceptibility testing in the management of Prototheca skin infections due to the inconsistency demonstrated between in vitro activity and clinical response to therapy.2
To the Editor:
Protothecosis infections are caused by an achlorophyllic algae of the species Prototheca. Prototheca organisms are found mostly in soil and water.1 Human infections are rare and involve 2 species, Prototheca wickerhamii and Prototheca zopfii. The former most commonly is responsible for human infections, though P zopfii results in more serious systemic infections with a poor prognosis. There are various types of Prototheca infection presentations, with a 2007 review of 117 cases reporting that cutaneous infections are most common (66%), followed by systemic infections (19%), and olecranon bursitis (15%).2 Skin lesions most commonly occur on the extremities and face, and they present as vesiculobullous and ulcerative lesions with purulent drainage. The skin lesions also may appear as erythematous plaques or nodules, subcutaneous papules, verrucous or herpetiformis lesions, or pyogenic granuloma–like lesions.3 Protothecosis typically affects immunocompromised individuals, especially those with a history of chronic corticosteroid use, malignancy, diabetes mellitus, AIDS, and/or organ transplant.1 We present a case of cutaneous protothecosis on the dorsal distal extremity of a 94-year-old woman. History of exposure to soil while gardening was elicited from the patient, and no immunosuppressive history was present aside from the patient’s age. This case may prompt workup for malignancy or immunosuppression in this patient subset.
A 94-year-old woman with a medical history of cutaneous squamous cell carcinoma (SCC) presented with a growing lesion on the dorsal surface of the left fourth digit of 2 months’ duration. The patient reported the lesion was painful, and she noted preceding trauma to the area that was suspected to have occurred while gardening. Physical examination revealed an ulcerated, hypertrophic, erythematous nodule on the dorsal surface of the left fourth metacarpophalangeal joint. The differential diagnosis included SCC, inflamed cyst, verruca vulgaris, and orf virus due to the clinical presentation. A shave biopsy was performed, and the lesion subsequently was treated with electrodesiccation and curettage.
Histopathologic evaluation revealed pseudoepitheliomatous hyperplasia with a mixed inflammatory infiltrate including lymphocytes and histiocytes. A morula within the dermis was characteristic of a protothecosis infection (Figure 1). On follow-up visit 6 weeks later, the lesion had grown back to its original size and morphology (Figure 2). At this time, the lesion was again treated with shave removal, followed by electrodesiccation and curettage, and the patient was placed on oral fluconazole 200 mg daily for 1 month. When the lesion did not resolve with fluconazole, she was referred to infectious disease as well as general surgery for surgical removal and debridement of the lesion. Unfortunately, the patient was lost to follow-up.
Protothecosis is an infectious disease comprised of achlorophyllic algae found in soil and water that rarely affects humans. When it does affect humans, cutaneous infections are most common. All human cases in which organisms were identified to species level have been caused by P wickerhamii or P zopfii species.2 Inoculation is suspected to occur through trauma to affected skin, especially when in the context of contaminated water. Our patient reported history of trauma to the hand, with soil from gardening as the potential aquagenic source of the infection.
The clinical presentation of protothecosis ranges from localized cutaneous to disseminated systemic infections, with most reported cases of systemic disease occurring in immunocompromised individuals. The cutaneous lesions of protothecosis vary greatly in clinical appearance including ulcerative nodules (as in our case), papules, plaques, pustules, and vesicles with erosion or crusting.4
Cutaneous protothecosis has the potential to mimic many other skin diseases and lesions, and, given its rarity, it may not be on the radar of dermatologists. Our patient’s lesion was presumed to be a skin cancer and was treated as such because of the history of SCC and clinical presentation. Although excision of individual lesions of protothecosis can be curative, electrodesiccation and curettage does not appear to be an adequate treatment, as the lesion subsequently recurred. It also is possible that this case represents P zopfii infection, as it did not respond to treatment with oral fluconazole, though in vitro studies with fluconazole to both P zopfii and P wickerhamii had variable treatment success.2 Also, the histopathologic findings were most consistent with P wickerhamii, revealing small, round, symmetrical morula, compared to P zopfii, which typically will display oval or cylindrical, asymmetrical, random internal segmentation.5 This case may warrant determination of species, which can be accomplished by a culture on Sabouraud dextrose agar, carbohydrate and alcohol assimilation test, yeast biochemical card, serological typing by immunoblotting, immunofluorescence study using species-specific antibodies, or amplification by polymerase chain reaction for small subunit ribosomal DNA sequences.2,6-8
The natural history of isolated skin disease is an indolent progressive course; however, reports do exist noting spontaneous resolution.4,9 Treatment options for Prototheca infections can be disappointing and consist of both surgical and medical management, or a combination of the 2 approaches. Reports in the literature support the use of antifungals including ketoconazole, voriconazole, itraconazole, fluconazole, and amphotericin B, with the latter displaying the best activity against Prototheca species.2 Tetracycline has been used in combination with oral or topical amphotericin B and was found to be synergistic in vitro and in case reports at successfully treating cutaneous protothecosis infections. It is possible that our patient was not treated with fluconazole long enough for it to become therapeutic, as most reported treatment regimens are weeks to months in length. Conversely, it may have been of benefit to transition the patient to topical amphotericin B and tetracycline, as fluconazole failed in this patient. However, treatment successes and failures are limited to case reports/case series and in vitro studies, with prospective studies lacking. Due to the variability with in vitro susceptibility profiles for Prototheca species, it generally is not recommended to pursue in vitro susceptibility testing in the management of Prototheca skin infections due to the inconsistency demonstrated between in vitro activity and clinical response to therapy.2
- Silva PC, Costa e Silva SB, Lima RB, et al. Cutaneous protothecosis—case report. An Bras Dermatol. 2013;88:183-185.
- Lass-Flörl C, Mayr A. Human protothecosis. Clin Microbiol Rev. 2007;20:230-242.
- Seok JY, Lee Y, Lee H, et al. Human cutaneous protothecosis: report of a case and literature review. Korean J Pathol. 2013;47:575-578.
- Mayorga J, Barba-Gómez JF, Verduzco-Martínez AP, et al. Protothecosis. Clin Dermatol. 2012;30:432-436.
- Walsh SV, Johnson RA, Tahan SR. Protothecosis: an unusual cause of chronic subcutaneous and soft tissue infection. Am J Dermatopathol. 1998;20:379-382.
- Casal MJ, Gutierrez J. Simple new test for rapid differentiation of Prototheca wickerhamii from Prototheca zopfii. J Clin Microbiol. 1983;18:992-993.
- Arnold, P, Ahearn, DG. The systematics of the genus Prototheca with a description of a new species P. filamenta. Mycologia 1972;64:265-275.
- Roesler U, Scholz H, Hensel H. Emended phenotypic characterization of Prototheca zopfii: a proposal for three biotypes and standards for their identification. Int J Syst Evol Microbiol. 2003;53:1195-1199.
- Todd JR, King JW, Oberle A, et al. Protothecosis: report of a case with 20-year follow-up, and review of previously published cases. Med Mycol. 2012;50:673-689.
- Silva PC, Costa e Silva SB, Lima RB, et al. Cutaneous protothecosis—case report. An Bras Dermatol. 2013;88:183-185.
- Lass-Flörl C, Mayr A. Human protothecosis. Clin Microbiol Rev. 2007;20:230-242.
- Seok JY, Lee Y, Lee H, et al. Human cutaneous protothecosis: report of a case and literature review. Korean J Pathol. 2013;47:575-578.
- Mayorga J, Barba-Gómez JF, Verduzco-Martínez AP, et al. Protothecosis. Clin Dermatol. 2012;30:432-436.
- Walsh SV, Johnson RA, Tahan SR. Protothecosis: an unusual cause of chronic subcutaneous and soft tissue infection. Am J Dermatopathol. 1998;20:379-382.
- Casal MJ, Gutierrez J. Simple new test for rapid differentiation of Prototheca wickerhamii from Prototheca zopfii. J Clin Microbiol. 1983;18:992-993.
- Arnold, P, Ahearn, DG. The systematics of the genus Prototheca with a description of a new species P. filamenta. Mycologia 1972;64:265-275.
- Roesler U, Scholz H, Hensel H. Emended phenotypic characterization of Prototheca zopfii: a proposal for three biotypes and standards for their identification. Int J Syst Evol Microbiol. 2003;53:1195-1199.
- Todd JR, King JW, Oberle A, et al. Protothecosis: report of a case with 20-year follow-up, and review of previously published cases. Med Mycol. 2012;50:673-689.
Practice Points
- Cutaneous protothecosis is a rare skin infection most commonly reported in immunocompromised individuals with recent exposure to contaminated soil or water. Cutaneous protothecosis has the potential to mimic many other skin diseases and lesions, including eczema; nonmelanoma skin cancer; or bacterial, viral, and fungal skin infections.
- A skin biopsy is essential for diagnosis, and histopathology is characteristic with soccer ball–appearing morula noted in a mixed inflammatory infiltrate.
Calcipotriene 0.005%–Betamethasone Dipropionate 0.064% Ointment Versus Topical Suspension in the Treatment of Plaque Psoriasis: A Randomized Pilot Study of Patient Preference
Psoriasis is a chronic relapsing inflammatory skin and joint disease that affects 1% to 3% of the US population.1 In cases of mild to moderate disease, topical agents including corticosteroids, vitamin D analogues, and retinoids are the mainstay of therapy. The need for long-term treatment can be frustrating for patients and treatment adherence often is problematic, resulting in poor outcomes. Reported adherence rates to topical psoriasis treatments range from 27% to 73%.2-6
Topical agents for treatment of psoriasis are available in various formulations, including creams, lotions, gels, ointments, solutions, and shampoos. For topical psoriasis therapies, vehicle formulation plays a major role in both delivery of the active drug and treatment adherence. Patients often cite poor cosmetic characteristics (eg, product feels too sticky or greasy, product feels unpleasant/has a bad texture, product is too messy, product application is too time consuming/takes too long to rub in) as reasons for poor treatment adherence.2,6-9 Psoriasis patients tend to prefer formulations that are not as messy such as solutions and foams versus creams, gels, and ointments.10
Ointments have been favored by physicians for the treatment of psoriasis because of the belief that their occlusive properties result in greater potency; however, a systematic review of clinical trials of different formulations of clobetasol propionate did not find that ointments were more effective than other vehicles.11 Furthermore, if a patient finds an ointment to be cosmetically unacceptable, he/she will be less inclined to use the medication as prescribed, regardless of its potency.
The objective of this study was to conduct a preliminary assessment of patient preference for ointment versus topical suspension formulations of calcipotriene 0.005%–betamethasone dipropionate 0.064% for treatment of plaque psoriasis. The specific attributes that were found to be appealing or unappealing by participants for each formulation also were evaluated.
Methods
Study Design and Participants
This open-label, investigator-blinded, crossover, prospective, single-center study evaluated patient preference for ointment versus topical suspension formulations of calcipo-triene 0.005%–betamethasone dipropionate 0.064% in the treatment of plaque psoriasis. The study protocol was approved by the Wake Forest School of Medicine (Winston-Salem, North Carolina) institutional review board.
Participants were recruited from the Wake Forest School of Medicine dermatology clinic. Inclusion criteria included men and women with mild to moderate plaque-type psoriasis who were 18 years of age or older; participants also were required to have an investigator global assessment (IGA) score of 2 (mild) or 3 (moderate) on a 5-point scale and 1% to 10% body surface involvement on the trunk or extremities.
Exclusion criteria included use of a topical or systemic psoriasis treatment within 2 or 4 weeks of baseline, respectively. Women who were pregnant, breastfeeding, planning to become pregnant, or could potentially become pregnant and were not using a medically accepted form of contraception also were excluded from the study. Patients with other serious skin conditions or any other chronic medical conditions that were not well controlled also were considered ineligible.
As a pilot study, a sample size of 20 participants was needed based on available funding.
Assessments
At baseline, the diagnosis of plaque-type psoriasis in each participant was confirmed by the investigator. Each participant’s medical history was obtained and all prior and current medications were reviewed to ensure eligibility criteria were met. Female participants of childbearing potential also underwent a urine pregnancy test. Consent was obtained from all enrolled participants. Investigators assessed the severity of psoriasis at baseline using the IGA.
All participants then were randomized (1:1 randomization) for treatment with either calcipotriene 0.005%–betamethasone dipropionate 0.064% ointment or calcipotriene 0.005%–betamethasone dipropionate 0.064% topical suspension. A simple random sampling chart was prepared and used by the study staff to determine treatment group allotment. Participants were instructed to apply the assigned study drug to affected areas of the body, excluding the scalp, face, and intertriginous areas, once daily for 3 days. Participants and study staff were aware of the study product being used, while investigators remained blinded. Participants also were asked to maintain a daily medication diary noting when the medication was applied.
Participants returned on day 3 for crossover treatment. Participants were asked to complete a subjective participant preference survey and study staff reviewed concomitant medications and adverse events. The packaging for the initial study drug was collected and weighed, and the crossover drug was dispensed to each participant to be applied once daily for 3 days.
Participants returned on day 6 or 7 for follow-up and were again asked to complete the subjective participant preference survey; study staff reviewed concomitant medications and adverse events. The packaging for the crossover study drug was collected and weighed, and the participant’s medication diary also was collected.
Subjective Participant Preference Survey
The subjective participant preference survey consisted of 15 questions relating to the participant’s experience with the study drug (eg, how the product felt to touch, amount of greasiness, time it took to apply). The final survey question asked participants to rate the overall appeal of the vehicle. Participants responded to the questions using a 7-point grading scale (1=extremely unappealing; 4=neutral; 7=extremely appealing). Total preference scores could range from 15 to 105.
End Points
The primary end point was the mean total preference score for each study drug obtained from the subjective participant preference surveys. Secondary end points included median values for individual survey questions and treatment adherence, which was measured from self-reported medication diary entries.
Statistical Analysis
Participant characteristics were reported with percentages for dichotomous data and median and ranges for other data. Subjective participant preference survey scores were calculated by taking the mean (standard deviation [SD]) sum of the scores for each individual survey item for both products. A generalized linear model that accounted for possible carryover and period effects was used to compare the difference of individual participant scores for the 2 products using SAS software. The mean (SD) amount of product used was reported and correlated to the preference score using a Spearman rank correlation. Total and individual survey scores were compared between sexes using Wilcoxon rank sum tests.
Results
Participants were enrolled in and completed the study from January 2013 to March 2013. The Figure presents a diagram of the Consolidated Standards of Reporting Trials. Thirty patients were screened; 10 patients did not meet eligibility criteria. Twenty patients were enrolled in the study with 10 patients randomized to each study arm. All 20 participants completed the study.
Participant demographics are described in Table 1. The median age was 48 years (range 29–64 years). The majority of participants were male (13/20) and white (18/20). The median IGA score was 3 (range, 2–3).
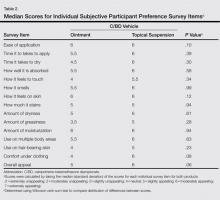
The mean (SD) total preference score for the calcipotriene 0.005%–betamethasone dipropionate 0.064% ointment formulation was 73.5 (19.4) and 80 (20.2) for the topical suspension formulation. The difference between means was -6.5 (95% confidence interval, -19.7-6.8; P=.32 after adjusting for possible carryover and period effects). Participants used a mean (SD) of 11 (11.4) g of product per study arm for the ointment formulation and 8.8 (6.6) g for the topical suspension formulation. There was no correlation between the amount of product used and preference for product (Spearman r=-0.01; P=.94). No statistically significant difference in product preference among men versus women was noted when considering total preference score or median scores of individual survey questions. Median overall appeal rating for the ointment formulation was 5 (slightly appealing) versus 6 (moderately appealing) for the topical suspension formulation, approaching statistical significance with P=.06 (Table 2). No significant carryover effects from one product to the other were noted (P=.64). The mean (SD) total preference scores were 81.1 (18.4) and 77.6 (21.2) in participants who used the topical suspension first followed by the ointment. In participants who used the ointment first followed by the topical suspension, the mean (SD) total preference scores were 69.4 (17.6) and 78.9 (22.8). Self-reported treatment adherence according to the participant’s daily medication diary was 100%.
Adverse effects during the study included 1 report of neck and back muscle pain and 1 report of sinusitis; neither was considered to be related to the study drugs.
Comment
Psoriasis is a chronic disease that can be difficult to treat, and treatment compliance often is poor. Multiple topical agents often are needed for adequate disease control, and adherence can be an even greater hurdle than with monotherapy. Combination products such as calcipotriene–betamethasone dipropionate offer the potential advantage of once-daily application. Adherence to once-daily application regimens for treatment of psoriasis has been shown to be greater than twice-daily application (82% vs 44%).4 However, the vehicle may be an adherence barrier for some patients.
Calcipotriene 0.005%–betamethasone dipropionate 0.064% topical suspension originally was indicated for the treatment of psoriasis of the scalp; however, it is now also indicated for treatment of psoriasis of the body.12 This topical suspension formulation is less messy, which could potentially be more cosmetically appealing and useful for improving treatment adherence.
Overall, the participants in our small pilot study showed a preference for the topical suspension versus the ointment formulation. The difference was substantial but was not statistically significant. This result is consistent with a previous study in which patients were found to prefer solutions that were less greasy compared to messy sticky ointments.10 Although the topical suspension received a higher average rating from participants for how it felt to touch and how it felt under clothing than the ointment and the ointment was rated on average as more greasy (none of these individual items achieved statistical significance), the calcipotriene–betamethasone dipropionate ointment was still rated as slightly appealing overall (Table 2). This result supports the need for physicians to discuss individual patient preferences when choosing the most appropriate vehicle for topical psoriasis treatment.
In our study, participants were found to use less product during treatment with the topical suspension versus the ointment, likely because the topical suspension formulation is thinner and spreads easier; however, participants rated the ease of application of the 2 products equally (Table 2). Ease of application was rated moderately appealing and time to apply was rated slightly to moderately appealing, which is important because patients often cite these factors as barriers to treatment adherence.2,6,13
The occlusive properties of ointment formulations provide moisturization by preventing water loss, a property that can be desirable when treating psoriatic plaques. Unlike the ointment, which was formulated with petrolatum and mineral oil, the calcipotriene–betamethasone dipropionate topical suspension was formulated with hydrogenated castor oil and mineral oil to provide moisturization. Nevertheless, participants found both the ointment and topical suspension to be moderately appealing (median score, 6; P=.94) for moisturization.
Limitations of this pilot study include the small sample size, which restricted the extent of subgroup analyses and the generalizability of our results. The small sample size also may or may not have contributed to the lack of statistical significance in the majority of the outcomes. This study provides pilot data that can be used to define a larger study; however, we do not think that a larger study is needed, as patients can be offered both vehicles in a practical clinical setting and can choose the product that is right for them. The short treatment duration of 3 days for each formulation also is a limitation, as a patient’s preference may change over time with longer use of the product. Treatment efficacy, which was not measured in this study, also could have an effect on patient preference, which could be assessed over a longer treatment period. Additionally, the study drugs may not be representative of all ointments or topical suspensions in their cosmetic appeal.
Conclusion
In this small cohort of plaque psoriasis patients, a calcipotriene–betamethasone dipropionate topical suspension was preferred over an ointment formulation, but in clinical practice it may be best to allow patients to choose the vehicle formulation that is most desirable on an individual basis. The topical suspension provides clinicians with an alternative that not only has the benefits of a combination product but also has been found to be appealing to patients.
1. Parisi R, Symmons DP, Griffiths CE, et al. Global epidemiology of psoriasis: a systematic review of incidence and prevalence. J Invest Dermatol. 2013;133:377-385.
2. Brown KK, Rehmus WE, Kimball AB. Determining the relative importance of patient motivations for nonadherence to topical corticosteroid therapy in psoriasis. J Am Acad Dermatol. 2006;55:607-613.
3. Carroll CL, Feldman SR, Camacho FT, et al. Adherence to topical therapy decreases during the course of an 8-week psoriasis clinical trial: commonly used methods of measuring adherence to topical therapy overestimate actual use. J Am Acad Dermatol. 2004;51:212-216.
4. Zaghloul SS, Goodfield MJ. Objective assessment of compliance with psoriasis treatment. Arch Dermatol. 2004;140:408-414.
5. Richards HL, Fortune DG, O’Sullivan TM, et al. Patients with psoriasis and their compliance with medication. J Am Acad Dermatol. 1999;41:581-583.
6. Fouere S, Adjadj L, Pawin H. How patients experience psoriasis: results from a European survey. J Eur Acad Dermatol Venereol. 2005;19:S2-S6.
7. van de Kerkhof PC, Steegers-Theunissen RP, Kuipers MV. Evaluation of topical drug treatment in psoriasis. Dermatology. 1998;197:31-36.
8. Devaux S, Castela A, Archier E, et al. Adherence to topical treatment in psoriasis: a systematic literature review. J Eur Acad Dermatol Venereol. 2012;26(suppl 3):S61-S67.
9. Chan SA, Hussain F, Lawson LG, et al. Factors affecting adherence to treatment of psoriasis: comparing biologic therapy to other modalities. J Dermatolog Treat. 2013;24:64-69.
10. Housman TS, Mellen BG, Rapp SR, et al. Patients with psoriasis prefer solution and foam vehicles: a quantitative assessment of vehicle preference. Cutis. 2002;70:327-332.
11. Warino L, Balkrishnan R, Feldman SR. Clobetasol propionate for psoriasis: are ointments really more potent? J Drugs Dermatol. 2006;5:527-532.
12. Menter A, Gold LS, Bukhalo M, et al. Calcipotriene plus betamethasone dipropionate topical suspension for the treatment of mild to moderate psoriasis vulgaris on the body: a randomized, double-blind, vehicle-controlled trial. J Drugs Dermatol. 2013;12:92-98.
13. Krueger G, Koo J, Lebwohl M, et al. The impact of psoriasis on quality of life: results of a 1998 National Psoriasis Foundation patient-membership survey. Arch Dermatol. 2001;137:280-284.
Psoriasis is a chronic relapsing inflammatory skin and joint disease that affects 1% to 3% of the US population.1 In cases of mild to moderate disease, topical agents including corticosteroids, vitamin D analogues, and retinoids are the mainstay of therapy. The need for long-term treatment can be frustrating for patients and treatment adherence often is problematic, resulting in poor outcomes. Reported adherence rates to topical psoriasis treatments range from 27% to 73%.2-6
Topical agents for treatment of psoriasis are available in various formulations, including creams, lotions, gels, ointments, solutions, and shampoos. For topical psoriasis therapies, vehicle formulation plays a major role in both delivery of the active drug and treatment adherence. Patients often cite poor cosmetic characteristics (eg, product feels too sticky or greasy, product feels unpleasant/has a bad texture, product is too messy, product application is too time consuming/takes too long to rub in) as reasons for poor treatment adherence.2,6-9 Psoriasis patients tend to prefer formulations that are not as messy such as solutions and foams versus creams, gels, and ointments.10
Ointments have been favored by physicians for the treatment of psoriasis because of the belief that their occlusive properties result in greater potency; however, a systematic review of clinical trials of different formulations of clobetasol propionate did not find that ointments were more effective than other vehicles.11 Furthermore, if a patient finds an ointment to be cosmetically unacceptable, he/she will be less inclined to use the medication as prescribed, regardless of its potency.
The objective of this study was to conduct a preliminary assessment of patient preference for ointment versus topical suspension formulations of calcipotriene 0.005%–betamethasone dipropionate 0.064% for treatment of plaque psoriasis. The specific attributes that were found to be appealing or unappealing by participants for each formulation also were evaluated.
Methods
Study Design and Participants
This open-label, investigator-blinded, crossover, prospective, single-center study evaluated patient preference for ointment versus topical suspension formulations of calcipo-triene 0.005%–betamethasone dipropionate 0.064% in the treatment of plaque psoriasis. The study protocol was approved by the Wake Forest School of Medicine (Winston-Salem, North Carolina) institutional review board.
Participants were recruited from the Wake Forest School of Medicine dermatology clinic. Inclusion criteria included men and women with mild to moderate plaque-type psoriasis who were 18 years of age or older; participants also were required to have an investigator global assessment (IGA) score of 2 (mild) or 3 (moderate) on a 5-point scale and 1% to 10% body surface involvement on the trunk or extremities.
Exclusion criteria included use of a topical or systemic psoriasis treatment within 2 or 4 weeks of baseline, respectively. Women who were pregnant, breastfeeding, planning to become pregnant, or could potentially become pregnant and were not using a medically accepted form of contraception also were excluded from the study. Patients with other serious skin conditions or any other chronic medical conditions that were not well controlled also were considered ineligible.
As a pilot study, a sample size of 20 participants was needed based on available funding.
Assessments
At baseline, the diagnosis of plaque-type psoriasis in each participant was confirmed by the investigator. Each participant’s medical history was obtained and all prior and current medications were reviewed to ensure eligibility criteria were met. Female participants of childbearing potential also underwent a urine pregnancy test. Consent was obtained from all enrolled participants. Investigators assessed the severity of psoriasis at baseline using the IGA.
All participants then were randomized (1:1 randomization) for treatment with either calcipotriene 0.005%–betamethasone dipropionate 0.064% ointment or calcipotriene 0.005%–betamethasone dipropionate 0.064% topical suspension. A simple random sampling chart was prepared and used by the study staff to determine treatment group allotment. Participants were instructed to apply the assigned study drug to affected areas of the body, excluding the scalp, face, and intertriginous areas, once daily for 3 days. Participants and study staff were aware of the study product being used, while investigators remained blinded. Participants also were asked to maintain a daily medication diary noting when the medication was applied.
Participants returned on day 3 for crossover treatment. Participants were asked to complete a subjective participant preference survey and study staff reviewed concomitant medications and adverse events. The packaging for the initial study drug was collected and weighed, and the crossover drug was dispensed to each participant to be applied once daily for 3 days.
Participants returned on day 6 or 7 for follow-up and were again asked to complete the subjective participant preference survey; study staff reviewed concomitant medications and adverse events. The packaging for the crossover study drug was collected and weighed, and the participant’s medication diary also was collected.
Subjective Participant Preference Survey
The subjective participant preference survey consisted of 15 questions relating to the participant’s experience with the study drug (eg, how the product felt to touch, amount of greasiness, time it took to apply). The final survey question asked participants to rate the overall appeal of the vehicle. Participants responded to the questions using a 7-point grading scale (1=extremely unappealing; 4=neutral; 7=extremely appealing). Total preference scores could range from 15 to 105.
End Points
The primary end point was the mean total preference score for each study drug obtained from the subjective participant preference surveys. Secondary end points included median values for individual survey questions and treatment adherence, which was measured from self-reported medication diary entries.
Statistical Analysis
Participant characteristics were reported with percentages for dichotomous data and median and ranges for other data. Subjective participant preference survey scores were calculated by taking the mean (standard deviation [SD]) sum of the scores for each individual survey item for both products. A generalized linear model that accounted for possible carryover and period effects was used to compare the difference of individual participant scores for the 2 products using SAS software. The mean (SD) amount of product used was reported and correlated to the preference score using a Spearman rank correlation. Total and individual survey scores were compared between sexes using Wilcoxon rank sum tests.
Results
Participants were enrolled in and completed the study from January 2013 to March 2013. The Figure presents a diagram of the Consolidated Standards of Reporting Trials. Thirty patients were screened; 10 patients did not meet eligibility criteria. Twenty patients were enrolled in the study with 10 patients randomized to each study arm. All 20 participants completed the study.
Participant demographics are described in Table 1. The median age was 48 years (range 29–64 years). The majority of participants were male (13/20) and white (18/20). The median IGA score was 3 (range, 2–3).
The mean (SD) total preference score for the calcipotriene 0.005%–betamethasone dipropionate 0.064% ointment formulation was 73.5 (19.4) and 80 (20.2) for the topical suspension formulation. The difference between means was -6.5 (95% confidence interval, -19.7-6.8; P=.32 after adjusting for possible carryover and period effects). Participants used a mean (SD) of 11 (11.4) g of product per study arm for the ointment formulation and 8.8 (6.6) g for the topical suspension formulation. There was no correlation between the amount of product used and preference for product (Spearman r=-0.01; P=.94). No statistically significant difference in product preference among men versus women was noted when considering total preference score or median scores of individual survey questions. Median overall appeal rating for the ointment formulation was 5 (slightly appealing) versus 6 (moderately appealing) for the topical suspension formulation, approaching statistical significance with P=.06 (Table 2). No significant carryover effects from one product to the other were noted (P=.64). The mean (SD) total preference scores were 81.1 (18.4) and 77.6 (21.2) in participants who used the topical suspension first followed by the ointment. In participants who used the ointment first followed by the topical suspension, the mean (SD) total preference scores were 69.4 (17.6) and 78.9 (22.8). Self-reported treatment adherence according to the participant’s daily medication diary was 100%.
Adverse effects during the study included 1 report of neck and back muscle pain and 1 report of sinusitis; neither was considered to be related to the study drugs.
Comment
Psoriasis is a chronic disease that can be difficult to treat, and treatment compliance often is poor. Multiple topical agents often are needed for adequate disease control, and adherence can be an even greater hurdle than with monotherapy. Combination products such as calcipotriene–betamethasone dipropionate offer the potential advantage of once-daily application. Adherence to once-daily application regimens for treatment of psoriasis has been shown to be greater than twice-daily application (82% vs 44%).4 However, the vehicle may be an adherence barrier for some patients.
Calcipotriene 0.005%–betamethasone dipropionate 0.064% topical suspension originally was indicated for the treatment of psoriasis of the scalp; however, it is now also indicated for treatment of psoriasis of the body.12 This topical suspension formulation is less messy, which could potentially be more cosmetically appealing and useful for improving treatment adherence.
Overall, the participants in our small pilot study showed a preference for the topical suspension versus the ointment formulation. The difference was substantial but was not statistically significant. This result is consistent with a previous study in which patients were found to prefer solutions that were less greasy compared to messy sticky ointments.10 Although the topical suspension received a higher average rating from participants for how it felt to touch and how it felt under clothing than the ointment and the ointment was rated on average as more greasy (none of these individual items achieved statistical significance), the calcipotriene–betamethasone dipropionate ointment was still rated as slightly appealing overall (Table 2). This result supports the need for physicians to discuss individual patient preferences when choosing the most appropriate vehicle for topical psoriasis treatment.
In our study, participants were found to use less product during treatment with the topical suspension versus the ointment, likely because the topical suspension formulation is thinner and spreads easier; however, participants rated the ease of application of the 2 products equally (Table 2). Ease of application was rated moderately appealing and time to apply was rated slightly to moderately appealing, which is important because patients often cite these factors as barriers to treatment adherence.2,6,13
The occlusive properties of ointment formulations provide moisturization by preventing water loss, a property that can be desirable when treating psoriatic plaques. Unlike the ointment, which was formulated with petrolatum and mineral oil, the calcipotriene–betamethasone dipropionate topical suspension was formulated with hydrogenated castor oil and mineral oil to provide moisturization. Nevertheless, participants found both the ointment and topical suspension to be moderately appealing (median score, 6; P=.94) for moisturization.
Limitations of this pilot study include the small sample size, which restricted the extent of subgroup analyses and the generalizability of our results. The small sample size also may or may not have contributed to the lack of statistical significance in the majority of the outcomes. This study provides pilot data that can be used to define a larger study; however, we do not think that a larger study is needed, as patients can be offered both vehicles in a practical clinical setting and can choose the product that is right for them. The short treatment duration of 3 days for each formulation also is a limitation, as a patient’s preference may change over time with longer use of the product. Treatment efficacy, which was not measured in this study, also could have an effect on patient preference, which could be assessed over a longer treatment period. Additionally, the study drugs may not be representative of all ointments or topical suspensions in their cosmetic appeal.
Conclusion
In this small cohort of plaque psoriasis patients, a calcipotriene–betamethasone dipropionate topical suspension was preferred over an ointment formulation, but in clinical practice it may be best to allow patients to choose the vehicle formulation that is most desirable on an individual basis. The topical suspension provides clinicians with an alternative that not only has the benefits of a combination product but also has been found to be appealing to patients.
Psoriasis is a chronic relapsing inflammatory skin and joint disease that affects 1% to 3% of the US population.1 In cases of mild to moderate disease, topical agents including corticosteroids, vitamin D analogues, and retinoids are the mainstay of therapy. The need for long-term treatment can be frustrating for patients and treatment adherence often is problematic, resulting in poor outcomes. Reported adherence rates to topical psoriasis treatments range from 27% to 73%.2-6
Topical agents for treatment of psoriasis are available in various formulations, including creams, lotions, gels, ointments, solutions, and shampoos. For topical psoriasis therapies, vehicle formulation plays a major role in both delivery of the active drug and treatment adherence. Patients often cite poor cosmetic characteristics (eg, product feels too sticky or greasy, product feels unpleasant/has a bad texture, product is too messy, product application is too time consuming/takes too long to rub in) as reasons for poor treatment adherence.2,6-9 Psoriasis patients tend to prefer formulations that are not as messy such as solutions and foams versus creams, gels, and ointments.10
Ointments have been favored by physicians for the treatment of psoriasis because of the belief that their occlusive properties result in greater potency; however, a systematic review of clinical trials of different formulations of clobetasol propionate did not find that ointments were more effective than other vehicles.11 Furthermore, if a patient finds an ointment to be cosmetically unacceptable, he/she will be less inclined to use the medication as prescribed, regardless of its potency.
The objective of this study was to conduct a preliminary assessment of patient preference for ointment versus topical suspension formulations of calcipotriene 0.005%–betamethasone dipropionate 0.064% for treatment of plaque psoriasis. The specific attributes that were found to be appealing or unappealing by participants for each formulation also were evaluated.
Methods
Study Design and Participants
This open-label, investigator-blinded, crossover, prospective, single-center study evaluated patient preference for ointment versus topical suspension formulations of calcipo-triene 0.005%–betamethasone dipropionate 0.064% in the treatment of plaque psoriasis. The study protocol was approved by the Wake Forest School of Medicine (Winston-Salem, North Carolina) institutional review board.
Participants were recruited from the Wake Forest School of Medicine dermatology clinic. Inclusion criteria included men and women with mild to moderate plaque-type psoriasis who were 18 years of age or older; participants also were required to have an investigator global assessment (IGA) score of 2 (mild) or 3 (moderate) on a 5-point scale and 1% to 10% body surface involvement on the trunk or extremities.
Exclusion criteria included use of a topical or systemic psoriasis treatment within 2 or 4 weeks of baseline, respectively. Women who were pregnant, breastfeeding, planning to become pregnant, or could potentially become pregnant and were not using a medically accepted form of contraception also were excluded from the study. Patients with other serious skin conditions or any other chronic medical conditions that were not well controlled also were considered ineligible.
As a pilot study, a sample size of 20 participants was needed based on available funding.
Assessments
At baseline, the diagnosis of plaque-type psoriasis in each participant was confirmed by the investigator. Each participant’s medical history was obtained and all prior and current medications were reviewed to ensure eligibility criteria were met. Female participants of childbearing potential also underwent a urine pregnancy test. Consent was obtained from all enrolled participants. Investigators assessed the severity of psoriasis at baseline using the IGA.
All participants then were randomized (1:1 randomization) for treatment with either calcipotriene 0.005%–betamethasone dipropionate 0.064% ointment or calcipotriene 0.005%–betamethasone dipropionate 0.064% topical suspension. A simple random sampling chart was prepared and used by the study staff to determine treatment group allotment. Participants were instructed to apply the assigned study drug to affected areas of the body, excluding the scalp, face, and intertriginous areas, once daily for 3 days. Participants and study staff were aware of the study product being used, while investigators remained blinded. Participants also were asked to maintain a daily medication diary noting when the medication was applied.
Participants returned on day 3 for crossover treatment. Participants were asked to complete a subjective participant preference survey and study staff reviewed concomitant medications and adverse events. The packaging for the initial study drug was collected and weighed, and the crossover drug was dispensed to each participant to be applied once daily for 3 days.
Participants returned on day 6 or 7 for follow-up and were again asked to complete the subjective participant preference survey; study staff reviewed concomitant medications and adverse events. The packaging for the crossover study drug was collected and weighed, and the participant’s medication diary also was collected.
Subjective Participant Preference Survey
The subjective participant preference survey consisted of 15 questions relating to the participant’s experience with the study drug (eg, how the product felt to touch, amount of greasiness, time it took to apply). The final survey question asked participants to rate the overall appeal of the vehicle. Participants responded to the questions using a 7-point grading scale (1=extremely unappealing; 4=neutral; 7=extremely appealing). Total preference scores could range from 15 to 105.
End Points
The primary end point was the mean total preference score for each study drug obtained from the subjective participant preference surveys. Secondary end points included median values for individual survey questions and treatment adherence, which was measured from self-reported medication diary entries.
Statistical Analysis
Participant characteristics were reported with percentages for dichotomous data and median and ranges for other data. Subjective participant preference survey scores were calculated by taking the mean (standard deviation [SD]) sum of the scores for each individual survey item for both products. A generalized linear model that accounted for possible carryover and period effects was used to compare the difference of individual participant scores for the 2 products using SAS software. The mean (SD) amount of product used was reported and correlated to the preference score using a Spearman rank correlation. Total and individual survey scores were compared between sexes using Wilcoxon rank sum tests.
Results
Participants were enrolled in and completed the study from January 2013 to March 2013. The Figure presents a diagram of the Consolidated Standards of Reporting Trials. Thirty patients were screened; 10 patients did not meet eligibility criteria. Twenty patients were enrolled in the study with 10 patients randomized to each study arm. All 20 participants completed the study.
Participant demographics are described in Table 1. The median age was 48 years (range 29–64 years). The majority of participants were male (13/20) and white (18/20). The median IGA score was 3 (range, 2–3).
The mean (SD) total preference score for the calcipotriene 0.005%–betamethasone dipropionate 0.064% ointment formulation was 73.5 (19.4) and 80 (20.2) for the topical suspension formulation. The difference between means was -6.5 (95% confidence interval, -19.7-6.8; P=.32 after adjusting for possible carryover and period effects). Participants used a mean (SD) of 11 (11.4) g of product per study arm for the ointment formulation and 8.8 (6.6) g for the topical suspension formulation. There was no correlation between the amount of product used and preference for product (Spearman r=-0.01; P=.94). No statistically significant difference in product preference among men versus women was noted when considering total preference score or median scores of individual survey questions. Median overall appeal rating for the ointment formulation was 5 (slightly appealing) versus 6 (moderately appealing) for the topical suspension formulation, approaching statistical significance with P=.06 (Table 2). No significant carryover effects from one product to the other were noted (P=.64). The mean (SD) total preference scores were 81.1 (18.4) and 77.6 (21.2) in participants who used the topical suspension first followed by the ointment. In participants who used the ointment first followed by the topical suspension, the mean (SD) total preference scores were 69.4 (17.6) and 78.9 (22.8). Self-reported treatment adherence according to the participant’s daily medication diary was 100%.
Adverse effects during the study included 1 report of neck and back muscle pain and 1 report of sinusitis; neither was considered to be related to the study drugs.
Comment
Psoriasis is a chronic disease that can be difficult to treat, and treatment compliance often is poor. Multiple topical agents often are needed for adequate disease control, and adherence can be an even greater hurdle than with monotherapy. Combination products such as calcipotriene–betamethasone dipropionate offer the potential advantage of once-daily application. Adherence to once-daily application regimens for treatment of psoriasis has been shown to be greater than twice-daily application (82% vs 44%).4 However, the vehicle may be an adherence barrier for some patients.
Calcipotriene 0.005%–betamethasone dipropionate 0.064% topical suspension originally was indicated for the treatment of psoriasis of the scalp; however, it is now also indicated for treatment of psoriasis of the body.12 This topical suspension formulation is less messy, which could potentially be more cosmetically appealing and useful for improving treatment adherence.
Overall, the participants in our small pilot study showed a preference for the topical suspension versus the ointment formulation. The difference was substantial but was not statistically significant. This result is consistent with a previous study in which patients were found to prefer solutions that were less greasy compared to messy sticky ointments.10 Although the topical suspension received a higher average rating from participants for how it felt to touch and how it felt under clothing than the ointment and the ointment was rated on average as more greasy (none of these individual items achieved statistical significance), the calcipotriene–betamethasone dipropionate ointment was still rated as slightly appealing overall (Table 2). This result supports the need for physicians to discuss individual patient preferences when choosing the most appropriate vehicle for topical psoriasis treatment.
In our study, participants were found to use less product during treatment with the topical suspension versus the ointment, likely because the topical suspension formulation is thinner and spreads easier; however, participants rated the ease of application of the 2 products equally (Table 2). Ease of application was rated moderately appealing and time to apply was rated slightly to moderately appealing, which is important because patients often cite these factors as barriers to treatment adherence.2,6,13
The occlusive properties of ointment formulations provide moisturization by preventing water loss, a property that can be desirable when treating psoriatic plaques. Unlike the ointment, which was formulated with petrolatum and mineral oil, the calcipotriene–betamethasone dipropionate topical suspension was formulated with hydrogenated castor oil and mineral oil to provide moisturization. Nevertheless, participants found both the ointment and topical suspension to be moderately appealing (median score, 6; P=.94) for moisturization.
Limitations of this pilot study include the small sample size, which restricted the extent of subgroup analyses and the generalizability of our results. The small sample size also may or may not have contributed to the lack of statistical significance in the majority of the outcomes. This study provides pilot data that can be used to define a larger study; however, we do not think that a larger study is needed, as patients can be offered both vehicles in a practical clinical setting and can choose the product that is right for them. The short treatment duration of 3 days for each formulation also is a limitation, as a patient’s preference may change over time with longer use of the product. Treatment efficacy, which was not measured in this study, also could have an effect on patient preference, which could be assessed over a longer treatment period. Additionally, the study drugs may not be representative of all ointments or topical suspensions in their cosmetic appeal.
Conclusion
In this small cohort of plaque psoriasis patients, a calcipotriene–betamethasone dipropionate topical suspension was preferred over an ointment formulation, but in clinical practice it may be best to allow patients to choose the vehicle formulation that is most desirable on an individual basis. The topical suspension provides clinicians with an alternative that not only has the benefits of a combination product but also has been found to be appealing to patients.
1. Parisi R, Symmons DP, Griffiths CE, et al. Global epidemiology of psoriasis: a systematic review of incidence and prevalence. J Invest Dermatol. 2013;133:377-385.
2. Brown KK, Rehmus WE, Kimball AB. Determining the relative importance of patient motivations for nonadherence to topical corticosteroid therapy in psoriasis. J Am Acad Dermatol. 2006;55:607-613.
3. Carroll CL, Feldman SR, Camacho FT, et al. Adherence to topical therapy decreases during the course of an 8-week psoriasis clinical trial: commonly used methods of measuring adherence to topical therapy overestimate actual use. J Am Acad Dermatol. 2004;51:212-216.
4. Zaghloul SS, Goodfield MJ. Objective assessment of compliance with psoriasis treatment. Arch Dermatol. 2004;140:408-414.
5. Richards HL, Fortune DG, O’Sullivan TM, et al. Patients with psoriasis and their compliance with medication. J Am Acad Dermatol. 1999;41:581-583.
6. Fouere S, Adjadj L, Pawin H. How patients experience psoriasis: results from a European survey. J Eur Acad Dermatol Venereol. 2005;19:S2-S6.
7. van de Kerkhof PC, Steegers-Theunissen RP, Kuipers MV. Evaluation of topical drug treatment in psoriasis. Dermatology. 1998;197:31-36.
8. Devaux S, Castela A, Archier E, et al. Adherence to topical treatment in psoriasis: a systematic literature review. J Eur Acad Dermatol Venereol. 2012;26(suppl 3):S61-S67.
9. Chan SA, Hussain F, Lawson LG, et al. Factors affecting adherence to treatment of psoriasis: comparing biologic therapy to other modalities. J Dermatolog Treat. 2013;24:64-69.
10. Housman TS, Mellen BG, Rapp SR, et al. Patients with psoriasis prefer solution and foam vehicles: a quantitative assessment of vehicle preference. Cutis. 2002;70:327-332.
11. Warino L, Balkrishnan R, Feldman SR. Clobetasol propionate for psoriasis: are ointments really more potent? J Drugs Dermatol. 2006;5:527-532.
12. Menter A, Gold LS, Bukhalo M, et al. Calcipotriene plus betamethasone dipropionate topical suspension for the treatment of mild to moderate psoriasis vulgaris on the body: a randomized, double-blind, vehicle-controlled trial. J Drugs Dermatol. 2013;12:92-98.
13. Krueger G, Koo J, Lebwohl M, et al. The impact of psoriasis on quality of life: results of a 1998 National Psoriasis Foundation patient-membership survey. Arch Dermatol. 2001;137:280-284.
1. Parisi R, Symmons DP, Griffiths CE, et al. Global epidemiology of psoriasis: a systematic review of incidence and prevalence. J Invest Dermatol. 2013;133:377-385.
2. Brown KK, Rehmus WE, Kimball AB. Determining the relative importance of patient motivations for nonadherence to topical corticosteroid therapy in psoriasis. J Am Acad Dermatol. 2006;55:607-613.
3. Carroll CL, Feldman SR, Camacho FT, et al. Adherence to topical therapy decreases during the course of an 8-week psoriasis clinical trial: commonly used methods of measuring adherence to topical therapy overestimate actual use. J Am Acad Dermatol. 2004;51:212-216.
4. Zaghloul SS, Goodfield MJ. Objective assessment of compliance with psoriasis treatment. Arch Dermatol. 2004;140:408-414.
5. Richards HL, Fortune DG, O’Sullivan TM, et al. Patients with psoriasis and their compliance with medication. J Am Acad Dermatol. 1999;41:581-583.
6. Fouere S, Adjadj L, Pawin H. How patients experience psoriasis: results from a European survey. J Eur Acad Dermatol Venereol. 2005;19:S2-S6.
7. van de Kerkhof PC, Steegers-Theunissen RP, Kuipers MV. Evaluation of topical drug treatment in psoriasis. Dermatology. 1998;197:31-36.
8. Devaux S, Castela A, Archier E, et al. Adherence to topical treatment in psoriasis: a systematic literature review. J Eur Acad Dermatol Venereol. 2012;26(suppl 3):S61-S67.
9. Chan SA, Hussain F, Lawson LG, et al. Factors affecting adherence to treatment of psoriasis: comparing biologic therapy to other modalities. J Dermatolog Treat. 2013;24:64-69.
10. Housman TS, Mellen BG, Rapp SR, et al. Patients with psoriasis prefer solution and foam vehicles: a quantitative assessment of vehicle preference. Cutis. 2002;70:327-332.
11. Warino L, Balkrishnan R, Feldman SR. Clobetasol propionate for psoriasis: are ointments really more potent? J Drugs Dermatol. 2006;5:527-532.
12. Menter A, Gold LS, Bukhalo M, et al. Calcipotriene plus betamethasone dipropionate topical suspension for the treatment of mild to moderate psoriasis vulgaris on the body: a randomized, double-blind, vehicle-controlled trial. J Drugs Dermatol. 2013;12:92-98.
13. Krueger G, Koo J, Lebwohl M, et al. The impact of psoriasis on quality of life: results of a 1998 National Psoriasis Foundation patient-membership survey. Arch Dermatol. 2001;137:280-284.
Practice Points
- Patient preference plays an important role in adherence to treatment regimens in chronic skin diseases such as psoriasis.
- The topical suspension formulation of calcipotriene 0.005%–betamethasone dipropionate 0.064% was preferred by psoriasis patients over the ointment; however, the availability of the 2 formulations provides patients with options and allows them to choose which product they prefer.