User login
Proton Pump Inhibitor-Associated Hypomagnesemia: A Retrospective Case-Control Study
In the U.S., proton pump inhibitors (PPIs) are one of the best-selling drug classes—more than $9 billion were spent on PPIs in 2012.1 These medications, available both by prescription and over-the-counter (OTC), are used to treat a variety of gastrointestinal conditions, including heartburn, gastroesophageal reflux disease, and peptic ulcer disease.1
Proton pump inhibitors are generally recognized as safe and effective. In 2011, however, the FDA reviewed Adverse Event Reporting System (AERS) reports, medical literature, and periodic safety updates and issued a safety communication outlining the risk for hypomagnesemia with prolonged PPI use.2 The FDA focused on 53 cases: 30 AERS cases, 15 in the literature, and 8 reported both through AERS and in the literature. The majority involved PPI use that continued for 1 year or longer, but in some cases hypomagnesemia developed after only 3 months. Labeling for prescription PPIs was updated with information about the hypomagnesemia risk, but labeling for the OTC drugs was not affected, as the FDA stated there is little risk with OTC use, and the label already indicated that use should be limited to 14 days at a time and up to 3 courses within 1 year.
Magnesium is an important intracellular cation that plays a role in multiple cellular activities. Low levels of magnesium can lead to a wide variety of adverse events (AEs), including vomiting, diarrhea, cramps, convulsions, bradycardia, and even death.3,4 The mechanism of PPI-associated hypomagnesemia is yet to be established but could be related to, as has been proposed, altered intestinal absorption of magnesium with long-term PPI use.4
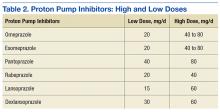
Results from investigations of PPI-associated hypomagnesemia have been inconclusive. In a study of PPI-associated AEs reported to the FDA, Luk and colleagues estimated that 1% of patients who experienced an AE reported hypomagnesemia and concluded that all PPIs are associated with hypomagnesemia, but the risk varies. Of the 6 PPIs that have been FDA approved, esomeprazole was associated with the lowest risk, pantoprazole with the most. Results also suggested that the risk was higher for elderly and male patients.
In another study of prior PPI use and its effects on magnesium levels among 11,490 intensive care unit admissions, Danziger and colleagues found that the association of PPI use and hypomagnesemia was limited to patients who concomitantly received a diuretic, and use of a histamine 2 receptor antagonist was not associated with hypomagnesemia.3 A third cross-sectional study of 402 adults with hypomagnesemia on hospital admission found no association between outpatient PPI regimens and hypomagnesemia.5 Other studies designed to investigate PPI-associated hypomagnesemia were limited by short-term PPI use, small samples, concurrent diseases, and confounding variables (eg, history of alcoholism).6,7
Need for Present Study
The evidence needed to establish the incidence of PPI-associated hypomagnesemia is limited. Hypomagnesemia can lead to serious AEs, as just outlined, and is a common indication for hospitalization.8 The hypomagnesemia rate is about 12% in hospitalized patients and sharply higher (60%-65%) in those who are critically ill. Proton pump inhibitor-associated hypomagnesemia is preventable, and monitoring parameters can be recommended to patients undergoing long-term therapy.
Ajumobi and colleagues found that 13,713 (23.4%) of 58,605 patients treated at a VA center over a 12-month period were receiving a PPI.9 Gawron and colleagues found that many veterans had been prescribed a PPI and were receiving high total daily doses for the treatment of gastroesophageal reflux disease.10 The majority of patients received a 90-day or longer supply and showed minimal evidence of step-down therapy or cessation of PPI therapy.
In the present study, the authors investigated the rate of PPI-associated hypomagnesemia in a veteran population at a facility where the majority of PPIs were by prescription, not OTC. The Captain James A. Lovell Federal Health Care Center (FHCC) is a combined DoD and VA facility where veterans and active military members and their dependents receive medical care and prescription drugs.
This study’s primary objective was to determine the rate of PPI-induced hypomagnesemia. The secondary objective was to identify any clinical factors (eg, PPI dose and therapy duration, concomitant use of a diuretic) that might further increase the risk of hypomagnesemia.
Methods
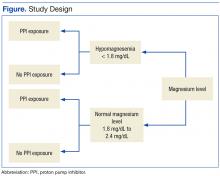
After the study protocol was approved by the Lovell FHCC institutional review board, the authors retrospectively compared patients with a low magnesium level (case group) with patients with a normal magnesium level (control group). In each group, the authors identified patients who underwent PPI therapy and those who did not (Figure).
Study inclusion criteria were low magnesium level (< 1.8 mg/dL) within the past 5 years for veterans in the case group and normal magnesium level (1.8-2.4 mg/dL) within the past 5 years for veterans in the control group. Exclusion criteria were nonveterans and no prior magnesium level for a veteran.
Patients were assigned in a ratio of 1 (case group) to 4 (control group) and were added only after confirmation that multiple magnesium levels had been recorded (January 2008-January 2013).
Patients who met the inclusion criteria were enrolled in the study. Patient’s Computerized Patient Record System charts were reviewed for demographics (sex, age, race); magnesium level; active order for PPI during same period magnesium level was drawn; PPI name, dose, and therapy duration; and concomitant use of a diuretic (yes or no) and, if yes, type of diuretic.
To assess a significance criterion (α) of 0.05 and a power of 80% 1,375 patients in a 1:4 ratio (275 cases, 1,100 controls) were required in order to detect a difference in rates of hypomagnesemia between patients who received a PPI and those who did not. Primary outcome data are reported as percentages and calculated odds ratios (ORs). Significance of ORs was determined with 95% confidence intervals (CIs). Secondary outcomes were PPI dose and therapy duration and concomitant use of a diuretic. Descriptive statistics were used for secondary outcomes.
Results
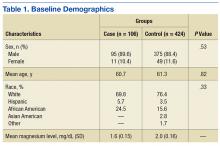
Five hundred thirty charts (106 cases, 424 controls) were included and reviewed. Table 1 lists the baseline demographics. There were no statistically significant differences in age, sex, or race between the case and control groups. Mean (SD) magnesium level was 1.6 (0.15)
The authors assessed for other clinical factors that might concurrently or
Discussion
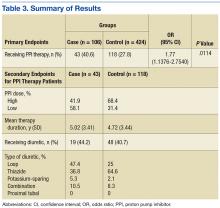
One of the most widely prescribed classes of medications, PPIs are often regarded as safe and effective and therefore continued as long-term therapy. Results of this study showed an association of PPI use and hypomagnesemia—thereby adding to the literature. Results for the secondary objective suggest that the association does not necessarily depend on PPI dose, but, given that a statistical analysis of the difference between the case and control groups was not conducted, the statistical significance is unknown.
Although the hypomagnesemia rate remains undetermined, the results of this NNH study suggest a rate higher than previously proposed. Other investigators have estimated the rate of PPI-associated hypomagnesemia at 1%, which does not correlate well with the NNH often calculated in this study. For 2 possible reasons, the poor correlation may be attributable to underreporting of hypomagnesemia: Magnesium levels are not commonly checked with a basic metabolic panel, and many patients who are mildly hypomagnesemic remain asymptomatic.
Future research directions include determining whether the risk for hypomagnesemia is related to patient status (eg, inpatient vs outpatient) and performing statistical analyses on the secondary objective to determine the clinical significance of potential risk factors. Other research directions might involve assessing PPI discontinuation rates in a hypomagnesemic population and assessing outcomes such as hospitalizations and AEs (eg, seizure, tetany, arrhythmia).
Limitations
This study had several limitations. First was the overall design. Study results described only a potential association of PPI use and hypomagnesemia, not definitive cause and effect. Results also depended on an assumed, previously reported rate of PPI-associated hypomagnesemia and a rate of exposure to PPIs, as these data were taken into account in the overall study design. In addition, patient adherence to prescribed therapy and accuracy of medication history were assumed from the medication and dispensing history, as not all medications obtained outside the Lovell FHCC were accurately documented. There also was an external validity limitation in that older men make up the typical FHCC patient population. Last, as inherent to all studies that use objective measures, there was the potential for laboratory magnesium level reporting errors.
Conclusion
The study results identified an association of PPI use and hypomagnesemia in a VA patient population of older men. More studies need to be conducted with non-VA patient populations to further assess the incidence of PPI-associated hypomagnesemia.
1. Consumers Union. Consumer Reports Best Buy Drugs: Using the proton pump inhibitors to treat heartburn and stomach acid reflux, comparing effectiveness, safety, and price. http://www.consumer reports.org/health/resources/pdf/best-buy-drugs/PPIsUpdate-FINAL.pdf. Updated July 2013. Accessed November 4, 2016.
2. U.S. Food and Drug Administration. FDA drug safety communication: low magnesium levels can be associated with long-term use of proton pump inhibitor drugs (PPIs). http://www.fda.gov/Drugs /DrugSafety/ucm245011.htm. Updated April 7, 2016. Accessed November 4, 2016.
3. Danziger J, William JH, Scott DJ, et al. Proton-pump inhibitor use is associated with low serum magnesium concentrations. Kidney Int. 2013;83(4):692-699.
4. Luk CP, Parsons R, Lee YP, Hughes JD. Proton pump inhibitor-associated hypomagnesemia: what do FDA data tell us? Ann Pharmacother. 2013;47(6):773-780.
5. Koulouridis I, Alfayez M, Tighiouart H, et al. Out-of-hospital use of proton pump inhibitors and hypomagnesemia at hospital admission: a nested case-control study. Am J Kidney Dis. 2013;62(4):730-737.
6. Mackay JD, Bladon PT. Hypomagnesaemia due to proton-pump inhibitor therapy: a clinical case series. QJM. 2010;103(6):387-395.
7. Faulhaber GA, Ascoli BA, Lubina A, et al. Serum magnesium and proton-pump inhibitors use: a cross-sectional study. Rev Assoc Med Bras (1992). 2013;59(3):276-279.
8. Yu ASL. Causes of hypomagnesemia. UpToDate. http://www.uptodate.com/contents/causes-of-hypomagnesemia. Updated February 4, 2016. Accessed November 4, 2016.
9. Ajumobi AB, Vuong R, Ahaneku H. Analysis of nonformulary use of PPIs and excess drug cost in a Veterans Affairs population. J Manag Care Pharm. 2012;18(1):63-67.
10. Gawron AJ, Pandolfino JE, Miskevics S, Lavela SL. Proton pump inhibitor prescriptions and subsequent use in US veterans diagnosed with gastroesophageal reflux disease. J Gen Intern Med. 2013;28(7):930-937.
In the U.S., proton pump inhibitors (PPIs) are one of the best-selling drug classes—more than $9 billion were spent on PPIs in 2012.1 These medications, available both by prescription and over-the-counter (OTC), are used to treat a variety of gastrointestinal conditions, including heartburn, gastroesophageal reflux disease, and peptic ulcer disease.1
Proton pump inhibitors are generally recognized as safe and effective. In 2011, however, the FDA reviewed Adverse Event Reporting System (AERS) reports, medical literature, and periodic safety updates and issued a safety communication outlining the risk for hypomagnesemia with prolonged PPI use.2 The FDA focused on 53 cases: 30 AERS cases, 15 in the literature, and 8 reported both through AERS and in the literature. The majority involved PPI use that continued for 1 year or longer, but in some cases hypomagnesemia developed after only 3 months. Labeling for prescription PPIs was updated with information about the hypomagnesemia risk, but labeling for the OTC drugs was not affected, as the FDA stated there is little risk with OTC use, and the label already indicated that use should be limited to 14 days at a time and up to 3 courses within 1 year.
Magnesium is an important intracellular cation that plays a role in multiple cellular activities. Low levels of magnesium can lead to a wide variety of adverse events (AEs), including vomiting, diarrhea, cramps, convulsions, bradycardia, and even death.3,4 The mechanism of PPI-associated hypomagnesemia is yet to be established but could be related to, as has been proposed, altered intestinal absorption of magnesium with long-term PPI use.4
Results from investigations of PPI-associated hypomagnesemia have been inconclusive. In a study of PPI-associated AEs reported to the FDA, Luk and colleagues estimated that 1% of patients who experienced an AE reported hypomagnesemia and concluded that all PPIs are associated with hypomagnesemia, but the risk varies. Of the 6 PPIs that have been FDA approved, esomeprazole was associated with the lowest risk, pantoprazole with the most. Results also suggested that the risk was higher for elderly and male patients.
In another study of prior PPI use and its effects on magnesium levels among 11,490 intensive care unit admissions, Danziger and colleagues found that the association of PPI use and hypomagnesemia was limited to patients who concomitantly received a diuretic, and use of a histamine 2 receptor antagonist was not associated with hypomagnesemia.3 A third cross-sectional study of 402 adults with hypomagnesemia on hospital admission found no association between outpatient PPI regimens and hypomagnesemia.5 Other studies designed to investigate PPI-associated hypomagnesemia were limited by short-term PPI use, small samples, concurrent diseases, and confounding variables (eg, history of alcoholism).6,7
Need for Present Study
The evidence needed to establish the incidence of PPI-associated hypomagnesemia is limited. Hypomagnesemia can lead to serious AEs, as just outlined, and is a common indication for hospitalization.8 The hypomagnesemia rate is about 12% in hospitalized patients and sharply higher (60%-65%) in those who are critically ill. Proton pump inhibitor-associated hypomagnesemia is preventable, and monitoring parameters can be recommended to patients undergoing long-term therapy.
Ajumobi and colleagues found that 13,713 (23.4%) of 58,605 patients treated at a VA center over a 12-month period were receiving a PPI.9 Gawron and colleagues found that many veterans had been prescribed a PPI and were receiving high total daily doses for the treatment of gastroesophageal reflux disease.10 The majority of patients received a 90-day or longer supply and showed minimal evidence of step-down therapy or cessation of PPI therapy.
In the present study, the authors investigated the rate of PPI-associated hypomagnesemia in a veteran population at a facility where the majority of PPIs were by prescription, not OTC. The Captain James A. Lovell Federal Health Care Center (FHCC) is a combined DoD and VA facility where veterans and active military members and their dependents receive medical care and prescription drugs.
This study’s primary objective was to determine the rate of PPI-induced hypomagnesemia. The secondary objective was to identify any clinical factors (eg, PPI dose and therapy duration, concomitant use of a diuretic) that might further increase the risk of hypomagnesemia.
Methods
After the study protocol was approved by the Lovell FHCC institutional review board, the authors retrospectively compared patients with a low magnesium level (case group) with patients with a normal magnesium level (control group). In each group, the authors identified patients who underwent PPI therapy and those who did not (Figure).
Study inclusion criteria were low magnesium level (< 1.8 mg/dL) within the past 5 years for veterans in the case group and normal magnesium level (1.8-2.4 mg/dL) within the past 5 years for veterans in the control group. Exclusion criteria were nonveterans and no prior magnesium level for a veteran.
Patients were assigned in a ratio of 1 (case group) to 4 (control group) and were added only after confirmation that multiple magnesium levels had been recorded (January 2008-January 2013).
Patients who met the inclusion criteria were enrolled in the study. Patient’s Computerized Patient Record System charts were reviewed for demographics (sex, age, race); magnesium level; active order for PPI during same period magnesium level was drawn; PPI name, dose, and therapy duration; and concomitant use of a diuretic (yes or no) and, if yes, type of diuretic.
To assess a significance criterion (α) of 0.05 and a power of 80% 1,375 patients in a 1:4 ratio (275 cases, 1,100 controls) were required in order to detect a difference in rates of hypomagnesemia between patients who received a PPI and those who did not. Primary outcome data are reported as percentages and calculated odds ratios (ORs). Significance of ORs was determined with 95% confidence intervals (CIs). Secondary outcomes were PPI dose and therapy duration and concomitant use of a diuretic. Descriptive statistics were used for secondary outcomes.
Results
Five hundred thirty charts (106 cases, 424 controls) were included and reviewed. Table 1 lists the baseline demographics. There were no statistically significant differences in age, sex, or race between the case and control groups. Mean (SD) magnesium level was 1.6 (0.15)
The authors assessed for other clinical factors that might concurrently or
Discussion
One of the most widely prescribed classes of medications, PPIs are often regarded as safe and effective and therefore continued as long-term therapy. Results of this study showed an association of PPI use and hypomagnesemia—thereby adding to the literature. Results for the secondary objective suggest that the association does not necessarily depend on PPI dose, but, given that a statistical analysis of the difference between the case and control groups was not conducted, the statistical significance is unknown.
Although the hypomagnesemia rate remains undetermined, the results of this NNH study suggest a rate higher than previously proposed. Other investigators have estimated the rate of PPI-associated hypomagnesemia at 1%, which does not correlate well with the NNH often calculated in this study. For 2 possible reasons, the poor correlation may be attributable to underreporting of hypomagnesemia: Magnesium levels are not commonly checked with a basic metabolic panel, and many patients who are mildly hypomagnesemic remain asymptomatic.
Future research directions include determining whether the risk for hypomagnesemia is related to patient status (eg, inpatient vs outpatient) and performing statistical analyses on the secondary objective to determine the clinical significance of potential risk factors. Other research directions might involve assessing PPI discontinuation rates in a hypomagnesemic population and assessing outcomes such as hospitalizations and AEs (eg, seizure, tetany, arrhythmia).
Limitations
This study had several limitations. First was the overall design. Study results described only a potential association of PPI use and hypomagnesemia, not definitive cause and effect. Results also depended on an assumed, previously reported rate of PPI-associated hypomagnesemia and a rate of exposure to PPIs, as these data were taken into account in the overall study design. In addition, patient adherence to prescribed therapy and accuracy of medication history were assumed from the medication and dispensing history, as not all medications obtained outside the Lovell FHCC were accurately documented. There also was an external validity limitation in that older men make up the typical FHCC patient population. Last, as inherent to all studies that use objective measures, there was the potential for laboratory magnesium level reporting errors.
Conclusion
The study results identified an association of PPI use and hypomagnesemia in a VA patient population of older men. More studies need to be conducted with non-VA patient populations to further assess the incidence of PPI-associated hypomagnesemia.
In the U.S., proton pump inhibitors (PPIs) are one of the best-selling drug classes—more than $9 billion were spent on PPIs in 2012.1 These medications, available both by prescription and over-the-counter (OTC), are used to treat a variety of gastrointestinal conditions, including heartburn, gastroesophageal reflux disease, and peptic ulcer disease.1
Proton pump inhibitors are generally recognized as safe and effective. In 2011, however, the FDA reviewed Adverse Event Reporting System (AERS) reports, medical literature, and periodic safety updates and issued a safety communication outlining the risk for hypomagnesemia with prolonged PPI use.2 The FDA focused on 53 cases: 30 AERS cases, 15 in the literature, and 8 reported both through AERS and in the literature. The majority involved PPI use that continued for 1 year or longer, but in some cases hypomagnesemia developed after only 3 months. Labeling for prescription PPIs was updated with information about the hypomagnesemia risk, but labeling for the OTC drugs was not affected, as the FDA stated there is little risk with OTC use, and the label already indicated that use should be limited to 14 days at a time and up to 3 courses within 1 year.
Magnesium is an important intracellular cation that plays a role in multiple cellular activities. Low levels of magnesium can lead to a wide variety of adverse events (AEs), including vomiting, diarrhea, cramps, convulsions, bradycardia, and even death.3,4 The mechanism of PPI-associated hypomagnesemia is yet to be established but could be related to, as has been proposed, altered intestinal absorption of magnesium with long-term PPI use.4
Results from investigations of PPI-associated hypomagnesemia have been inconclusive. In a study of PPI-associated AEs reported to the FDA, Luk and colleagues estimated that 1% of patients who experienced an AE reported hypomagnesemia and concluded that all PPIs are associated with hypomagnesemia, but the risk varies. Of the 6 PPIs that have been FDA approved, esomeprazole was associated with the lowest risk, pantoprazole with the most. Results also suggested that the risk was higher for elderly and male patients.
In another study of prior PPI use and its effects on magnesium levels among 11,490 intensive care unit admissions, Danziger and colleagues found that the association of PPI use and hypomagnesemia was limited to patients who concomitantly received a diuretic, and use of a histamine 2 receptor antagonist was not associated with hypomagnesemia.3 A third cross-sectional study of 402 adults with hypomagnesemia on hospital admission found no association between outpatient PPI regimens and hypomagnesemia.5 Other studies designed to investigate PPI-associated hypomagnesemia were limited by short-term PPI use, small samples, concurrent diseases, and confounding variables (eg, history of alcoholism).6,7
Need for Present Study
The evidence needed to establish the incidence of PPI-associated hypomagnesemia is limited. Hypomagnesemia can lead to serious AEs, as just outlined, and is a common indication for hospitalization.8 The hypomagnesemia rate is about 12% in hospitalized patients and sharply higher (60%-65%) in those who are critically ill. Proton pump inhibitor-associated hypomagnesemia is preventable, and monitoring parameters can be recommended to patients undergoing long-term therapy.
Ajumobi and colleagues found that 13,713 (23.4%) of 58,605 patients treated at a VA center over a 12-month period were receiving a PPI.9 Gawron and colleagues found that many veterans had been prescribed a PPI and were receiving high total daily doses for the treatment of gastroesophageal reflux disease.10 The majority of patients received a 90-day or longer supply and showed minimal evidence of step-down therapy or cessation of PPI therapy.
In the present study, the authors investigated the rate of PPI-associated hypomagnesemia in a veteran population at a facility where the majority of PPIs were by prescription, not OTC. The Captain James A. Lovell Federal Health Care Center (FHCC) is a combined DoD and VA facility where veterans and active military members and their dependents receive medical care and prescription drugs.
This study’s primary objective was to determine the rate of PPI-induced hypomagnesemia. The secondary objective was to identify any clinical factors (eg, PPI dose and therapy duration, concomitant use of a diuretic) that might further increase the risk of hypomagnesemia.
Methods
After the study protocol was approved by the Lovell FHCC institutional review board, the authors retrospectively compared patients with a low magnesium level (case group) with patients with a normal magnesium level (control group). In each group, the authors identified patients who underwent PPI therapy and those who did not (Figure).
Study inclusion criteria were low magnesium level (< 1.8 mg/dL) within the past 5 years for veterans in the case group and normal magnesium level (1.8-2.4 mg/dL) within the past 5 years for veterans in the control group. Exclusion criteria were nonveterans and no prior magnesium level for a veteran.
Patients were assigned in a ratio of 1 (case group) to 4 (control group) and were added only after confirmation that multiple magnesium levels had been recorded (January 2008-January 2013).
Patients who met the inclusion criteria were enrolled in the study. Patient’s Computerized Patient Record System charts were reviewed for demographics (sex, age, race); magnesium level; active order for PPI during same period magnesium level was drawn; PPI name, dose, and therapy duration; and concomitant use of a diuretic (yes or no) and, if yes, type of diuretic.
To assess a significance criterion (α) of 0.05 and a power of 80% 1,375 patients in a 1:4 ratio (275 cases, 1,100 controls) were required in order to detect a difference in rates of hypomagnesemia between patients who received a PPI and those who did not. Primary outcome data are reported as percentages and calculated odds ratios (ORs). Significance of ORs was determined with 95% confidence intervals (CIs). Secondary outcomes were PPI dose and therapy duration and concomitant use of a diuretic. Descriptive statistics were used for secondary outcomes.
Results
Five hundred thirty charts (106 cases, 424 controls) were included and reviewed. Table 1 lists the baseline demographics. There were no statistically significant differences in age, sex, or race between the case and control groups. Mean (SD) magnesium level was 1.6 (0.15)
The authors assessed for other clinical factors that might concurrently or
Discussion
One of the most widely prescribed classes of medications, PPIs are often regarded as safe and effective and therefore continued as long-term therapy. Results of this study showed an association of PPI use and hypomagnesemia—thereby adding to the literature. Results for the secondary objective suggest that the association does not necessarily depend on PPI dose, but, given that a statistical analysis of the difference between the case and control groups was not conducted, the statistical significance is unknown.
Although the hypomagnesemia rate remains undetermined, the results of this NNH study suggest a rate higher than previously proposed. Other investigators have estimated the rate of PPI-associated hypomagnesemia at 1%, which does not correlate well with the NNH often calculated in this study. For 2 possible reasons, the poor correlation may be attributable to underreporting of hypomagnesemia: Magnesium levels are not commonly checked with a basic metabolic panel, and many patients who are mildly hypomagnesemic remain asymptomatic.
Future research directions include determining whether the risk for hypomagnesemia is related to patient status (eg, inpatient vs outpatient) and performing statistical analyses on the secondary objective to determine the clinical significance of potential risk factors. Other research directions might involve assessing PPI discontinuation rates in a hypomagnesemic population and assessing outcomes such as hospitalizations and AEs (eg, seizure, tetany, arrhythmia).
Limitations
This study had several limitations. First was the overall design. Study results described only a potential association of PPI use and hypomagnesemia, not definitive cause and effect. Results also depended on an assumed, previously reported rate of PPI-associated hypomagnesemia and a rate of exposure to PPIs, as these data were taken into account in the overall study design. In addition, patient adherence to prescribed therapy and accuracy of medication history were assumed from the medication and dispensing history, as not all medications obtained outside the Lovell FHCC were accurately documented. There also was an external validity limitation in that older men make up the typical FHCC patient population. Last, as inherent to all studies that use objective measures, there was the potential for laboratory magnesium level reporting errors.
Conclusion
The study results identified an association of PPI use and hypomagnesemia in a VA patient population of older men. More studies need to be conducted with non-VA patient populations to further assess the incidence of PPI-associated hypomagnesemia.
1. Consumers Union. Consumer Reports Best Buy Drugs: Using the proton pump inhibitors to treat heartburn and stomach acid reflux, comparing effectiveness, safety, and price. http://www.consumer reports.org/health/resources/pdf/best-buy-drugs/PPIsUpdate-FINAL.pdf. Updated July 2013. Accessed November 4, 2016.
2. U.S. Food and Drug Administration. FDA drug safety communication: low magnesium levels can be associated with long-term use of proton pump inhibitor drugs (PPIs). http://www.fda.gov/Drugs /DrugSafety/ucm245011.htm. Updated April 7, 2016. Accessed November 4, 2016.
3. Danziger J, William JH, Scott DJ, et al. Proton-pump inhibitor use is associated with low serum magnesium concentrations. Kidney Int. 2013;83(4):692-699.
4. Luk CP, Parsons R, Lee YP, Hughes JD. Proton pump inhibitor-associated hypomagnesemia: what do FDA data tell us? Ann Pharmacother. 2013;47(6):773-780.
5. Koulouridis I, Alfayez M, Tighiouart H, et al. Out-of-hospital use of proton pump inhibitors and hypomagnesemia at hospital admission: a nested case-control study. Am J Kidney Dis. 2013;62(4):730-737.
6. Mackay JD, Bladon PT. Hypomagnesaemia due to proton-pump inhibitor therapy: a clinical case series. QJM. 2010;103(6):387-395.
7. Faulhaber GA, Ascoli BA, Lubina A, et al. Serum magnesium and proton-pump inhibitors use: a cross-sectional study. Rev Assoc Med Bras (1992). 2013;59(3):276-279.
8. Yu ASL. Causes of hypomagnesemia. UpToDate. http://www.uptodate.com/contents/causes-of-hypomagnesemia. Updated February 4, 2016. Accessed November 4, 2016.
9. Ajumobi AB, Vuong R, Ahaneku H. Analysis of nonformulary use of PPIs and excess drug cost in a Veterans Affairs population. J Manag Care Pharm. 2012;18(1):63-67.
10. Gawron AJ, Pandolfino JE, Miskevics S, Lavela SL. Proton pump inhibitor prescriptions and subsequent use in US veterans diagnosed with gastroesophageal reflux disease. J Gen Intern Med. 2013;28(7):930-937.
1. Consumers Union. Consumer Reports Best Buy Drugs: Using the proton pump inhibitors to treat heartburn and stomach acid reflux, comparing effectiveness, safety, and price. http://www.consumer reports.org/health/resources/pdf/best-buy-drugs/PPIsUpdate-FINAL.pdf. Updated July 2013. Accessed November 4, 2016.
2. U.S. Food and Drug Administration. FDA drug safety communication: low magnesium levels can be associated with long-term use of proton pump inhibitor drugs (PPIs). http://www.fda.gov/Drugs /DrugSafety/ucm245011.htm. Updated April 7, 2016. Accessed November 4, 2016.
3. Danziger J, William JH, Scott DJ, et al. Proton-pump inhibitor use is associated with low serum magnesium concentrations. Kidney Int. 2013;83(4):692-699.
4. Luk CP, Parsons R, Lee YP, Hughes JD. Proton pump inhibitor-associated hypomagnesemia: what do FDA data tell us? Ann Pharmacother. 2013;47(6):773-780.
5. Koulouridis I, Alfayez M, Tighiouart H, et al. Out-of-hospital use of proton pump inhibitors and hypomagnesemia at hospital admission: a nested case-control study. Am J Kidney Dis. 2013;62(4):730-737.
6. Mackay JD, Bladon PT. Hypomagnesaemia due to proton-pump inhibitor therapy: a clinical case series. QJM. 2010;103(6):387-395.
7. Faulhaber GA, Ascoli BA, Lubina A, et al. Serum magnesium and proton-pump inhibitors use: a cross-sectional study. Rev Assoc Med Bras (1992). 2013;59(3):276-279.
8. Yu ASL. Causes of hypomagnesemia. UpToDate. http://www.uptodate.com/contents/causes-of-hypomagnesemia. Updated February 4, 2016. Accessed November 4, 2016.
9. Ajumobi AB, Vuong R, Ahaneku H. Analysis of nonformulary use of PPIs and excess drug cost in a Veterans Affairs population. J Manag Care Pharm. 2012;18(1):63-67.
10. Gawron AJ, Pandolfino JE, Miskevics S, Lavela SL. Proton pump inhibitor prescriptions and subsequent use in US veterans diagnosed with gastroesophageal reflux disease. J Gen Intern Med. 2013;28(7):930-937.