User login
Woman, 35, With Jaundice and Altered Mental Status
IN THIS ARTICLE
- Results of case patient's initial laboratory work-up
- Top 10 prescription medications associated with idiosyncratic disease
- Outcome for the case patient
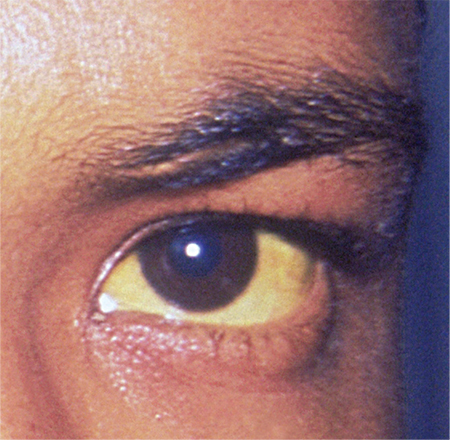
A 35-year-old African-American woman presented to the emergency department (ED) after being found disoriented and lethargic in her apartment by her friends. Given her altered mental status, the history of present illness was limited and informed mainly by her mother and friends. She had been unreachable by telephone for three days, and friends grew concerned when she was absent from work on two consecutive days. After obtaining access to her apartment, they found her in the bathroom jaundiced, incoherent, and surrounded by nonbloody, nonbilious vomit. She had no prior significant medical history, no documented daily medication, and no recent travel. Of note, previous medical contact was limited, and she did not have an established primary care provider. Additionally, there was no contributory family history, including autoimmune illness or liver disease.
ED presentation was marked by indications of grade 4 encephalopathy, including unresponsiveness to noxious stimuli. Initial laboratory work-up was notable for significantly elevated liver function test results (see Table 1). Based on her international normalized ratio (INR), total bilirubin, and creatinine, her initial Model for End-Stage Liver Disease score was 39, correlating to an 83% three-month mortality rate.1 Autoimmune marker testing revealed a positive antinuclear antibody (ANA), elevated immunoglobulin G (IgG), elevated smooth muscle antibody (IgG), normal antimitochondrial antibody, and normal anti-liver/kidney microsome antibody (IgG). Viral hepatitis serologies, including A, B, C, and E, were unremarkable. Ceruloplasmin and iron saturation were within normal limits. Acetaminophen, salicylate, and ethanol levels were negligible. Pregnancy testing and urine toxin testing were negative. Thyroid function tests were normal. Infectious work-up, including pan-culture, remained negative. Syphilis, herpes simplex virus (HSV), HIV, and varicella zoster testing were unremarkable.
CT of the head was not consistent with cerebral edema. CT of the abdomen and pelvis showed evidence of chronic pancreatitis and trace perihepatic ascites. She was intubated for airway protection and transferred to the medical ICU.
On liver biopsy, the patient was found to have acute hepatitis with centrilobular necrosis, approximately 30% to 40%, and prominent cholestasis. Histologically, these findings were reported as most consistent with drug-induced liver injury. Given her comatose state, coagulopathy, and extremely limited life expectancy without liver transplantation, the patient was listed for transplant as a status 1A candidate with fulminant hepatic failure.
She was placed on propofol and N-acetylcysteine infusions in addition to supportive IV resuscitation. The patient’s synthetic and neurocognitive function improved gradually over several weeks, and she was able to provide collateral history. She denied taking any prescription medications or having any ongoing medical issues. She did report that for two months prior to admission she had been taking an oral beauty supplement designed to enhance hair, skin, and nails. She obtained the supplement online. She could not recall the week leading up to admission, but she did note increasing malaise and fatigue beginning two weeks prior to admission. She denied any recreational drug or alcohol use.
Continue for discussion >>
DISCUSSION
Drug-induced liver injury (DILI) is a relatively uncommon occurrence in the United States.2 It is estimated to occur in approximately 20 individuals per 100,000 persons per year.2 However, DILI incidence secondary to herbal and dietary supplement use appears to be on the rise in the US. In a prospective study conducted by the Drug-Induced Liver Injury Network (DILIN) that included patients with liver injury referred to eight DILIN centers between 2004 and 2013, the proportion of DILI cases caused by herbal and dietary supplements increased from 7% to 20% over the study period.3
DILI can be subclassified into intrinsic and idiosyncratic. Intrinsic DILI results from substances causing a predictable time course and natural history. Substances causing a varied, unpredictable occurrence of DILI in susceptible individuals are idiosyncratic.4 Overall, acetaminophen overdose is the most common cause of DILI.2 However, the most common idiosyncratic offending agents, taken at FDA-approved dosages, are antimicrobials (see Table 2).5 The second most common offending agents are herbal and dietary supplements.5
In a retrospective cohort study evaluating all cases of acute liver failure (ALF) over a six-year period in an integrated health care system, the leading cause of ALF was DILI.6 Of the 32 patients with confirmed drug-induced ALF in this study, the majority of cases (18) were associated with acetaminophen. Herbal and dietary supplements were implicated in six cases, with miscellaneous medications accounting for the remaining eight cases.6 In terms of outcomes, 18.8% of patients with ALF due to DILI underwent liver transplantation, 68.8% were discharged, and 12.5% died during hospitalization.6
DILI disproportionately affects women and minorities7;although the etiology is unclear, it is hypothesized that increased use of antibiotics may play a role among women.2 Providers should be aware of the increased risk for DILI in these populations and consider this diagnosis in the appropriate setting.
Teasing out the diagnosis
DILI is a diagnosis of exclusion, aided in large part by the history and physical exam.4 An extensive history may alert the health care provider to a potential offending substance as well as provide information on timing of exposure.4 DILI should be suspected in patients with persistently elevated liver enzymes, unremarkable work-up for all other underlying liver disease (including autoimmune and viral serologies), and negative abdominal imaging.4 In particular, acute hepatitis C virus (HCV) and hepatitis E virus (HEV) infection mimic the clinical presentation of DILI and should be excluded with HCV RNA and IgM anti-HEV testing, with reflex HEV RNA testing to confirm positive IgM anti-HEV results.8,9 Liver biopsy is rarely indicated for the diagnosis of DILI.2
The presentation of DILI ranges from asymptomatic, with mildly abnormal results on liver function testing, to fulminant hepatic failure. Acetaminophen is the most frequently reported cause of intrinsic DILI in the US, playing a role in approximately half of all ALF cases.10 DILI can be further subdivided according to the pattern of liver test abnormalities as hepatocellular, mixed, or cholestatic based on the ratio of ALT to alkaline phosphatase (R value).2 Utilizing the formula serum ALT/upper limit of normal (ULN) divided by the serum alkaline phosphatase/ULN to determine R value, liver test abnormalities are defined as hepatocellular (R > 5), mixed (R = 2-5), and cholestatic (R < 2).4 These liver test patterns can be used to predict prognosis (see “Prognosis: Hy’s law”). In a prospective, longitudinal study, DILIN found that chronic DILI was present in 18% of the study population at 6 months following onset.5 Patients with the cholestatic presentation were more likely to develop chronic DILI than were those with the hepatocellular or mixed pattern. Furthermore, the hepatocellular pattern on presentation was associated with greater mortality.5 Patients with the mixed pattern had the most favorable outcomes. Another prospective cohort study found that persistently elevated liver enzymes in DILI patients at 12 months is associated with older age and the cholestatic pattern of liver test abnormalities at presentation, in particular, alkaline phosphatase elevation.11 However, neither length of therapy nor type of offending medication was associated with long-term liver test abnormalities.11
Managing DILI and ALF
In all DILI cases, immediate discontinuation of the offending agent is the initial treatment recommendation.2 Patients presenting with DILI who have an accompanying bilirubin level > 2 mg/dL should be referred to a hepatology specialist due to an increased risk for ALF.2 ALF is defined as coagulopathy to INR ≥ 1.5 and hepatic encephalopathy within 26 weeks of initial symptom onset in individuals without known underlying liver disease, with the exception of autoimmune hepatitis, Wilson disease, and reactivation of hepatitis B.12-15 Fulminant hepatic failure is further specified as encephalopathy occurring within 8 weeks of jaundice onset.12
Patients presenting with ALF should be transferred to an intensive care setting, preferably within a liver transplant center, for supportive care and potential liver transplant evaluation.12 CT of the head should be used to rule out other etiologies for altered mental status.16N-Acetylcysteine is the treatment of choice for acetaminophen-induced ALF, and it has also been shown to improve transplant-free survival outcomes in patients with non-acetaminophen–related early ALF.17 Infectious work-up and continuous monitoring are essential in ALF care, since up to 80% of patients with ALF will develop a bacterial infection.18 A comprehensive infectious work-up should include pan-culture of blood, urine, and sputum in addition to assessment for Epstein-Barr virus, cytomegalovirus, and HSV.4,18 For irreversible ALF, liver transplantation remains the only validated treatment option.12,19
Prognosis: Hy’s law
Hy’s law refers to a method used in clinical trials to assess a drug’s likelihood of causing severe hepatotoxicity; it is also used to predict which patients with DILI will develop ALF.12,20 According to Hy’s law, patients with AST or ALT elevations three times ULN and total bilirubin elevations two times ULN are at increased risk for ALF.In a retrospective cohort study of more than 15,000 patients with DILI, the Hy’s law criteria were found to have high specificity but low sensitivity for detecting individuals at risk for ALF.15 An alternative model, the Drug-Induced Liver Toxicity ALF Score, uses platelet count and bilirubin level to identify patients at risk for ALF with high sensitivity.15
Patient education
Effective patient education is essential to decreasing DILI incidence at a time when herbal and dietary supplement consumption is increasing. Patients will often bring herbal and dietary supplements to their providers to obtain a safety profile prior to initiation. In these cases, it is essential to reinforce with patients the absence of federal regulation of these products. It should be stressed to patients that, due to the lack of government oversight, it is impossible to confidently identify the entirety and quantity of ingredients in these supplements. Furthermore, there is no existing protocol for surveillance or adverse event reporting for these products.21 Because these products are not routinely or systematically studied, even health care providers have no evidence on which to base monitoring or usage recommendations. Providers may direct patients to the National Institutes of Health’s LiverTox website (livertox.NIH.gov) to review prior case reports of hepatotoxicity for specific dietary and herbal supplements.
Level of education is associated with knowledge of the potential for overdose when taking OTC medications that contain acetaminophen.22 As a result, health care providers should strongly reinforce with patients the importance of reading all medication labels and abiding by the listed administration directions. In particular, providers should emphasize that the maximum daily dosage of acetaminophen is 4 g.23 For patients with chronic liver disease, a more conservative recommendation is warranted. Generally, patients with cirrhosis may be advised to consume up to 2 g/d of acetaminophen as a firstline treatment for pain. However, providers should ensure acetaminophen ingestion is limited to a brief period.24
Additionally, it is important to educate patients that many combination OTC medications contain acetaminophen. Of note, chronic opioid users are more likely to accurately identify OTC medications containing acetaminophen, compared with acute opioid users.22 These findings should compel health care providers to deliver in-depth education for all patients, particularly those with less education or experience with medications. Education on avoidance of offending medications, including medications within the same class, when appropriate, is essential for quality patient care.2
Continue to outcome for the case patient >>
OUTCOME FOR THE CASE PATIENT
Following discharge, the patient was monitored closely with regular clinic visits and blood work. Her liver test results improved gradually, with consideration of a repeat biopsy to evaluate for overlap or missed autoimmune disease. Her repeat ANA was negative and IgG was within normal limits. Within three months of admission, her liver tests normalized and repeat biopsy was deferred.
Upon review of the herbal beauty supplement the patient reported taking, shark cartilage was noted as a primary ingredient. In a case report, shark cartilage was identified as a hepatotoxin.25 The patient was advised never to ingest the offending supplement, or any other substances not regulated by the FDA, again. Furthermore, the offending medication was listed as a medication allergy in her electronic health record.
CONCLUSION
It is crucial to emphasize to patients the potential hepatotoxicity of medications and herbal and dietary supplements, especially OTC medications that pose an overdose risk. Patients should review all new supplements with their providers prior to therapy initiation. With known hepatotoxins, providers should closely monitor patients for liver injury while treatment is ongoing. In suspected cases of DILI, a thorough history and physical exam will greatly inform the diagnosis. In the majority of cases, the suspect medication should be discontinued immediately, with subsequent assessment of liver response. Identification of DILI early in the course increases the likelihood of full hepatic recovery and improves patient outcomes.
References
1. Kamath PS, Wiesner RH, Malinchoc M, et al. A model to predict survival in patients with end-stage liver disease. Hepatology. 2001;33(2):464-470.
2. Leise MD, Poterucha JJ, Talwalkar JA. Drug-induced liver injury. Mayo Clin Proc. 2014;89(1):95-106.
3. Navarro VJ, Barnhart H, Bonkovsky HL, et al. Liver injury from herbals and dietary supplements in the US Drug-Induced Liver Injury Network. Hepatology. 2014;60(4):1399-1408.
4. Chalasani NP, Hayashi PH, Bonkovsky HL, et al. ACG Clinical Guideline: the diagnosis and management of idiosyncratic drug-induced liver injury. Am J Gastroenterol. 2014;109(7):950-966.
5. Chalasani N, Bonkovsky HL, Fontana R, et al; United States Drug Induced Liver Injury Network. Features and outcomes of 899 patients with drug-induced liver injury: the DILIN prospective study. Gastroenterology. 2015;148(7):1340-1352.
6. Goldberg DS, Forde KA, Carbonari DM, et al. Population-representative incidence of drug-induced acute liver failure based on an analysis of an integrated health care system. Gastroenterology. 2015;148(7):1353-1361.
7. Reuben A, Koch DG, Lee WM. Drug-induced acute liver failure: results of a US multicenter, prospective study. Hepatology. 2010;52(6):2065-2076.
8. Davern TJ, Chalasani N, Fontana RJ, et al; Drug-Induced Liver Injury Network (DILIN). Acute hepatitis E infection accounts for some cases of suspected drug-induced liver injury. Gastroenterology. 2011;141(5):1665-1672.e1-9.
9. Chalasani N, Fontana RJ, Bonkovsky HL, et al. Causes, clinical features, and outcomes from a prospective study of drug-induced liver injury in the United States. Gastroenterology. 2008;135(6):1924-1934.
10. Fisher K, Vuppalanchi R, Saxena R. Drug-induced liver injury. Arch Pathol Lab Med. 2015;139(7):876-887.
11. Fontana RJ, Hayashi PH, Barnhart H, et al. Persistent liver biochemistry abnormalities are more common in older patients and those with cholestatic drug induced liver injury. Am J Gastroenterol. 2015;110(10):1450-1459.
12. Punzalan CS, Barry CT. Acute liver failure: diagnosis and management. J Intensive Care Med. 2015 Oct 6. [Epub ahead of print]
13. Bower WA, Johns M, Margolis HS, et al. Population-based surveillance for acute liver failure. Am J Gastroenterol. 2007;102(11):2459-2463.
14. O’Grady JG, Schalm SW, Williams R. Acute liver failure: redefining the syndromes. Lancet. 1993;342(8866):273-275.
15. Lo Re V III, Haynes K, Forde KA, et al. Risk of acute liver failure in patients with drug-induced liver injury: evaluation of Hy’s law and a new prognostic model. Clin Gastroenterol Hepatol. 2015;13(13):2360-2368.
16. Polson J, Lee WM; American Association for the Study of Liver Diseases. AASLD position paper: the management of acute liver failure. Hepatology. 2005;41:1179-1197.
17. Lee WM, Hynan LS, Rossaro L, et al. Intravenous N-acetylcysteine improves transplant-free survival in early stage non-acetaminophen acute liver failure. Gastroenterology. 2009;137(3):856-864.
18. Rolando N, Harvey F, Brahm J. Prospective study of bacterial infection in acute liver failure: an analysis of fifty patients. Hepatology. 1990;11(1):49-53.
19. Panackel C, Thomas R, Sebastian B, Mathai SK. Recent advances in management of acute liver failure. Indian J Crit Care Med. 2015;19(1):27-33.
20. Temple R. Hy’s law: predicting serious hepatotoxicity. Pharmacoepidemiol Drug Saf. 2006;15(4):241-243.
21. Bunchorntavakul C, Reddy K. Review article: herbal and dietary supplement hepatotoxicity. Aliment Pharmacol Ther. 2012;37(1):3-17.
22. Boudreau DM, Wirtz H, Von Korff M, et al. A survey of adult awareness and use of medicine containing acetaminophen. Pharmacoepidemiol Drug Saf. 2013;22(3):229-240.
23. Burns MJ, Friedman SL, Larson AM. Acetaminophen (paracetamol) poisoning in adults: pathophysiology, presentation, and diagnosis. UpToDate. www.uptodate.com/contents/acetaminophen-paracetamol-poisoning-in-adults-pathophysiology-presentation-and-diagnosis. Accessed May 20, 2016.
24. Lewis JH, Stine JG. Review article: prescribing medications in patients with cirrhosis—a practical guide. Aliment Pharmacol Ther. 2013;37(12):1132-1156.
25. Ashar B, Vargo E. Shark cartilage-induced hepatitis. Ann Intern Med. 1996;125(9):780-781.
IN THIS ARTICLE
- Results of case patient's initial laboratory work-up
- Top 10 prescription medications associated with idiosyncratic disease
- Outcome for the case patient
A 35-year-old African-American woman presented to the emergency department (ED) after being found disoriented and lethargic in her apartment by her friends. Given her altered mental status, the history of present illness was limited and informed mainly by her mother and friends. She had been unreachable by telephone for three days, and friends grew concerned when she was absent from work on two consecutive days. After obtaining access to her apartment, they found her in the bathroom jaundiced, incoherent, and surrounded by nonbloody, nonbilious vomit. She had no prior significant medical history, no documented daily medication, and no recent travel. Of note, previous medical contact was limited, and she did not have an established primary care provider. Additionally, there was no contributory family history, including autoimmune illness or liver disease.
ED presentation was marked by indications of grade 4 encephalopathy, including unresponsiveness to noxious stimuli. Initial laboratory work-up was notable for significantly elevated liver function test results (see Table 1). Based on her international normalized ratio (INR), total bilirubin, and creatinine, her initial Model for End-Stage Liver Disease score was 39, correlating to an 83% three-month mortality rate.1 Autoimmune marker testing revealed a positive antinuclear antibody (ANA), elevated immunoglobulin G (IgG), elevated smooth muscle antibody (IgG), normal antimitochondrial antibody, and normal anti-liver/kidney microsome antibody (IgG). Viral hepatitis serologies, including A, B, C, and E, were unremarkable. Ceruloplasmin and iron saturation were within normal limits. Acetaminophen, salicylate, and ethanol levels were negligible. Pregnancy testing and urine toxin testing were negative. Thyroid function tests were normal. Infectious work-up, including pan-culture, remained negative. Syphilis, herpes simplex virus (HSV), HIV, and varicella zoster testing were unremarkable.
CT of the head was not consistent with cerebral edema. CT of the abdomen and pelvis showed evidence of chronic pancreatitis and trace perihepatic ascites. She was intubated for airway protection and transferred to the medical ICU.
On liver biopsy, the patient was found to have acute hepatitis with centrilobular necrosis, approximately 30% to 40%, and prominent cholestasis. Histologically, these findings were reported as most consistent with drug-induced liver injury. Given her comatose state, coagulopathy, and extremely limited life expectancy without liver transplantation, the patient was listed for transplant as a status 1A candidate with fulminant hepatic failure.
She was placed on propofol and N-acetylcysteine infusions in addition to supportive IV resuscitation. The patient’s synthetic and neurocognitive function improved gradually over several weeks, and she was able to provide collateral history. She denied taking any prescription medications or having any ongoing medical issues. She did report that for two months prior to admission she had been taking an oral beauty supplement designed to enhance hair, skin, and nails. She obtained the supplement online. She could not recall the week leading up to admission, but she did note increasing malaise and fatigue beginning two weeks prior to admission. She denied any recreational drug or alcohol use.
Continue for discussion >>
DISCUSSION
Drug-induced liver injury (DILI) is a relatively uncommon occurrence in the United States.2 It is estimated to occur in approximately 20 individuals per 100,000 persons per year.2 However, DILI incidence secondary to herbal and dietary supplement use appears to be on the rise in the US. In a prospective study conducted by the Drug-Induced Liver Injury Network (DILIN) that included patients with liver injury referred to eight DILIN centers between 2004 and 2013, the proportion of DILI cases caused by herbal and dietary supplements increased from 7% to 20% over the study period.3
DILI can be subclassified into intrinsic and idiosyncratic. Intrinsic DILI results from substances causing a predictable time course and natural history. Substances causing a varied, unpredictable occurrence of DILI in susceptible individuals are idiosyncratic.4 Overall, acetaminophen overdose is the most common cause of DILI.2 However, the most common idiosyncratic offending agents, taken at FDA-approved dosages, are antimicrobials (see Table 2).5 The second most common offending agents are herbal and dietary supplements.5
In a retrospective cohort study evaluating all cases of acute liver failure (ALF) over a six-year period in an integrated health care system, the leading cause of ALF was DILI.6 Of the 32 patients with confirmed drug-induced ALF in this study, the majority of cases (18) were associated with acetaminophen. Herbal and dietary supplements were implicated in six cases, with miscellaneous medications accounting for the remaining eight cases.6 In terms of outcomes, 18.8% of patients with ALF due to DILI underwent liver transplantation, 68.8% were discharged, and 12.5% died during hospitalization.6
DILI disproportionately affects women and minorities7;although the etiology is unclear, it is hypothesized that increased use of antibiotics may play a role among women.2 Providers should be aware of the increased risk for DILI in these populations and consider this diagnosis in the appropriate setting.
Teasing out the diagnosis
DILI is a diagnosis of exclusion, aided in large part by the history and physical exam.4 An extensive history may alert the health care provider to a potential offending substance as well as provide information on timing of exposure.4 DILI should be suspected in patients with persistently elevated liver enzymes, unremarkable work-up for all other underlying liver disease (including autoimmune and viral serologies), and negative abdominal imaging.4 In particular, acute hepatitis C virus (HCV) and hepatitis E virus (HEV) infection mimic the clinical presentation of DILI and should be excluded with HCV RNA and IgM anti-HEV testing, with reflex HEV RNA testing to confirm positive IgM anti-HEV results.8,9 Liver biopsy is rarely indicated for the diagnosis of DILI.2
The presentation of DILI ranges from asymptomatic, with mildly abnormal results on liver function testing, to fulminant hepatic failure. Acetaminophen is the most frequently reported cause of intrinsic DILI in the US, playing a role in approximately half of all ALF cases.10 DILI can be further subdivided according to the pattern of liver test abnormalities as hepatocellular, mixed, or cholestatic based on the ratio of ALT to alkaline phosphatase (R value).2 Utilizing the formula serum ALT/upper limit of normal (ULN) divided by the serum alkaline phosphatase/ULN to determine R value, liver test abnormalities are defined as hepatocellular (R > 5), mixed (R = 2-5), and cholestatic (R < 2).4 These liver test patterns can be used to predict prognosis (see “Prognosis: Hy’s law”). In a prospective, longitudinal study, DILIN found that chronic DILI was present in 18% of the study population at 6 months following onset.5 Patients with the cholestatic presentation were more likely to develop chronic DILI than were those with the hepatocellular or mixed pattern. Furthermore, the hepatocellular pattern on presentation was associated with greater mortality.5 Patients with the mixed pattern had the most favorable outcomes. Another prospective cohort study found that persistently elevated liver enzymes in DILI patients at 12 months is associated with older age and the cholestatic pattern of liver test abnormalities at presentation, in particular, alkaline phosphatase elevation.11 However, neither length of therapy nor type of offending medication was associated with long-term liver test abnormalities.11
Managing DILI and ALF
In all DILI cases, immediate discontinuation of the offending agent is the initial treatment recommendation.2 Patients presenting with DILI who have an accompanying bilirubin level > 2 mg/dL should be referred to a hepatology specialist due to an increased risk for ALF.2 ALF is defined as coagulopathy to INR ≥ 1.5 and hepatic encephalopathy within 26 weeks of initial symptom onset in individuals without known underlying liver disease, with the exception of autoimmune hepatitis, Wilson disease, and reactivation of hepatitis B.12-15 Fulminant hepatic failure is further specified as encephalopathy occurring within 8 weeks of jaundice onset.12
Patients presenting with ALF should be transferred to an intensive care setting, preferably within a liver transplant center, for supportive care and potential liver transplant evaluation.12 CT of the head should be used to rule out other etiologies for altered mental status.16N-Acetylcysteine is the treatment of choice for acetaminophen-induced ALF, and it has also been shown to improve transplant-free survival outcomes in patients with non-acetaminophen–related early ALF.17 Infectious work-up and continuous monitoring are essential in ALF care, since up to 80% of patients with ALF will develop a bacterial infection.18 A comprehensive infectious work-up should include pan-culture of blood, urine, and sputum in addition to assessment for Epstein-Barr virus, cytomegalovirus, and HSV.4,18 For irreversible ALF, liver transplantation remains the only validated treatment option.12,19
Prognosis: Hy’s law
Hy’s law refers to a method used in clinical trials to assess a drug’s likelihood of causing severe hepatotoxicity; it is also used to predict which patients with DILI will develop ALF.12,20 According to Hy’s law, patients with AST or ALT elevations three times ULN and total bilirubin elevations two times ULN are at increased risk for ALF.In a retrospective cohort study of more than 15,000 patients with DILI, the Hy’s law criteria were found to have high specificity but low sensitivity for detecting individuals at risk for ALF.15 An alternative model, the Drug-Induced Liver Toxicity ALF Score, uses platelet count and bilirubin level to identify patients at risk for ALF with high sensitivity.15
Patient education
Effective patient education is essential to decreasing DILI incidence at a time when herbal and dietary supplement consumption is increasing. Patients will often bring herbal and dietary supplements to their providers to obtain a safety profile prior to initiation. In these cases, it is essential to reinforce with patients the absence of federal regulation of these products. It should be stressed to patients that, due to the lack of government oversight, it is impossible to confidently identify the entirety and quantity of ingredients in these supplements. Furthermore, there is no existing protocol for surveillance or adverse event reporting for these products.21 Because these products are not routinely or systematically studied, even health care providers have no evidence on which to base monitoring or usage recommendations. Providers may direct patients to the National Institutes of Health’s LiverTox website (livertox.NIH.gov) to review prior case reports of hepatotoxicity for specific dietary and herbal supplements.
Level of education is associated with knowledge of the potential for overdose when taking OTC medications that contain acetaminophen.22 As a result, health care providers should strongly reinforce with patients the importance of reading all medication labels and abiding by the listed administration directions. In particular, providers should emphasize that the maximum daily dosage of acetaminophen is 4 g.23 For patients with chronic liver disease, a more conservative recommendation is warranted. Generally, patients with cirrhosis may be advised to consume up to 2 g/d of acetaminophen as a firstline treatment for pain. However, providers should ensure acetaminophen ingestion is limited to a brief period.24
Additionally, it is important to educate patients that many combination OTC medications contain acetaminophen. Of note, chronic opioid users are more likely to accurately identify OTC medications containing acetaminophen, compared with acute opioid users.22 These findings should compel health care providers to deliver in-depth education for all patients, particularly those with less education or experience with medications. Education on avoidance of offending medications, including medications within the same class, when appropriate, is essential for quality patient care.2
Continue to outcome for the case patient >>
OUTCOME FOR THE CASE PATIENT
Following discharge, the patient was monitored closely with regular clinic visits and blood work. Her liver test results improved gradually, with consideration of a repeat biopsy to evaluate for overlap or missed autoimmune disease. Her repeat ANA was negative and IgG was within normal limits. Within three months of admission, her liver tests normalized and repeat biopsy was deferred.
Upon review of the herbal beauty supplement the patient reported taking, shark cartilage was noted as a primary ingredient. In a case report, shark cartilage was identified as a hepatotoxin.25 The patient was advised never to ingest the offending supplement, or any other substances not regulated by the FDA, again. Furthermore, the offending medication was listed as a medication allergy in her electronic health record.
CONCLUSION
It is crucial to emphasize to patients the potential hepatotoxicity of medications and herbal and dietary supplements, especially OTC medications that pose an overdose risk. Patients should review all new supplements with their providers prior to therapy initiation. With known hepatotoxins, providers should closely monitor patients for liver injury while treatment is ongoing. In suspected cases of DILI, a thorough history and physical exam will greatly inform the diagnosis. In the majority of cases, the suspect medication should be discontinued immediately, with subsequent assessment of liver response. Identification of DILI early in the course increases the likelihood of full hepatic recovery and improves patient outcomes.
References
1. Kamath PS, Wiesner RH, Malinchoc M, et al. A model to predict survival in patients with end-stage liver disease. Hepatology. 2001;33(2):464-470.
2. Leise MD, Poterucha JJ, Talwalkar JA. Drug-induced liver injury. Mayo Clin Proc. 2014;89(1):95-106.
3. Navarro VJ, Barnhart H, Bonkovsky HL, et al. Liver injury from herbals and dietary supplements in the US Drug-Induced Liver Injury Network. Hepatology. 2014;60(4):1399-1408.
4. Chalasani NP, Hayashi PH, Bonkovsky HL, et al. ACG Clinical Guideline: the diagnosis and management of idiosyncratic drug-induced liver injury. Am J Gastroenterol. 2014;109(7):950-966.
5. Chalasani N, Bonkovsky HL, Fontana R, et al; United States Drug Induced Liver Injury Network. Features and outcomes of 899 patients with drug-induced liver injury: the DILIN prospective study. Gastroenterology. 2015;148(7):1340-1352.
6. Goldberg DS, Forde KA, Carbonari DM, et al. Population-representative incidence of drug-induced acute liver failure based on an analysis of an integrated health care system. Gastroenterology. 2015;148(7):1353-1361.
7. Reuben A, Koch DG, Lee WM. Drug-induced acute liver failure: results of a US multicenter, prospective study. Hepatology. 2010;52(6):2065-2076.
8. Davern TJ, Chalasani N, Fontana RJ, et al; Drug-Induced Liver Injury Network (DILIN). Acute hepatitis E infection accounts for some cases of suspected drug-induced liver injury. Gastroenterology. 2011;141(5):1665-1672.e1-9.
9. Chalasani N, Fontana RJ, Bonkovsky HL, et al. Causes, clinical features, and outcomes from a prospective study of drug-induced liver injury in the United States. Gastroenterology. 2008;135(6):1924-1934.
10. Fisher K, Vuppalanchi R, Saxena R. Drug-induced liver injury. Arch Pathol Lab Med. 2015;139(7):876-887.
11. Fontana RJ, Hayashi PH, Barnhart H, et al. Persistent liver biochemistry abnormalities are more common in older patients and those with cholestatic drug induced liver injury. Am J Gastroenterol. 2015;110(10):1450-1459.
12. Punzalan CS, Barry CT. Acute liver failure: diagnosis and management. J Intensive Care Med. 2015 Oct 6. [Epub ahead of print]
13. Bower WA, Johns M, Margolis HS, et al. Population-based surveillance for acute liver failure. Am J Gastroenterol. 2007;102(11):2459-2463.
14. O’Grady JG, Schalm SW, Williams R. Acute liver failure: redefining the syndromes. Lancet. 1993;342(8866):273-275.
15. Lo Re V III, Haynes K, Forde KA, et al. Risk of acute liver failure in patients with drug-induced liver injury: evaluation of Hy’s law and a new prognostic model. Clin Gastroenterol Hepatol. 2015;13(13):2360-2368.
16. Polson J, Lee WM; American Association for the Study of Liver Diseases. AASLD position paper: the management of acute liver failure. Hepatology. 2005;41:1179-1197.
17. Lee WM, Hynan LS, Rossaro L, et al. Intravenous N-acetylcysteine improves transplant-free survival in early stage non-acetaminophen acute liver failure. Gastroenterology. 2009;137(3):856-864.
18. Rolando N, Harvey F, Brahm J. Prospective study of bacterial infection in acute liver failure: an analysis of fifty patients. Hepatology. 1990;11(1):49-53.
19. Panackel C, Thomas R, Sebastian B, Mathai SK. Recent advances in management of acute liver failure. Indian J Crit Care Med. 2015;19(1):27-33.
20. Temple R. Hy’s law: predicting serious hepatotoxicity. Pharmacoepidemiol Drug Saf. 2006;15(4):241-243.
21. Bunchorntavakul C, Reddy K. Review article: herbal and dietary supplement hepatotoxicity. Aliment Pharmacol Ther. 2012;37(1):3-17.
22. Boudreau DM, Wirtz H, Von Korff M, et al. A survey of adult awareness and use of medicine containing acetaminophen. Pharmacoepidemiol Drug Saf. 2013;22(3):229-240.
23. Burns MJ, Friedman SL, Larson AM. Acetaminophen (paracetamol) poisoning in adults: pathophysiology, presentation, and diagnosis. UpToDate. www.uptodate.com/contents/acetaminophen-paracetamol-poisoning-in-adults-pathophysiology-presentation-and-diagnosis. Accessed May 20, 2016.
24. Lewis JH, Stine JG. Review article: prescribing medications in patients with cirrhosis—a practical guide. Aliment Pharmacol Ther. 2013;37(12):1132-1156.
25. Ashar B, Vargo E. Shark cartilage-induced hepatitis. Ann Intern Med. 1996;125(9):780-781.
IN THIS ARTICLE
- Results of case patient's initial laboratory work-up
- Top 10 prescription medications associated with idiosyncratic disease
- Outcome for the case patient
A 35-year-old African-American woman presented to the emergency department (ED) after being found disoriented and lethargic in her apartment by her friends. Given her altered mental status, the history of present illness was limited and informed mainly by her mother and friends. She had been unreachable by telephone for three days, and friends grew concerned when she was absent from work on two consecutive days. After obtaining access to her apartment, they found her in the bathroom jaundiced, incoherent, and surrounded by nonbloody, nonbilious vomit. She had no prior significant medical history, no documented daily medication, and no recent travel. Of note, previous medical contact was limited, and she did not have an established primary care provider. Additionally, there was no contributory family history, including autoimmune illness or liver disease.
ED presentation was marked by indications of grade 4 encephalopathy, including unresponsiveness to noxious stimuli. Initial laboratory work-up was notable for significantly elevated liver function test results (see Table 1). Based on her international normalized ratio (INR), total bilirubin, and creatinine, her initial Model for End-Stage Liver Disease score was 39, correlating to an 83% three-month mortality rate.1 Autoimmune marker testing revealed a positive antinuclear antibody (ANA), elevated immunoglobulin G (IgG), elevated smooth muscle antibody (IgG), normal antimitochondrial antibody, and normal anti-liver/kidney microsome antibody (IgG). Viral hepatitis serologies, including A, B, C, and E, were unremarkable. Ceruloplasmin and iron saturation were within normal limits. Acetaminophen, salicylate, and ethanol levels were negligible. Pregnancy testing and urine toxin testing were negative. Thyroid function tests were normal. Infectious work-up, including pan-culture, remained negative. Syphilis, herpes simplex virus (HSV), HIV, and varicella zoster testing were unremarkable.
CT of the head was not consistent with cerebral edema. CT of the abdomen and pelvis showed evidence of chronic pancreatitis and trace perihepatic ascites. She was intubated for airway protection and transferred to the medical ICU.
On liver biopsy, the patient was found to have acute hepatitis with centrilobular necrosis, approximately 30% to 40%, and prominent cholestasis. Histologically, these findings were reported as most consistent with drug-induced liver injury. Given her comatose state, coagulopathy, and extremely limited life expectancy without liver transplantation, the patient was listed for transplant as a status 1A candidate with fulminant hepatic failure.
She was placed on propofol and N-acetylcysteine infusions in addition to supportive IV resuscitation. The patient’s synthetic and neurocognitive function improved gradually over several weeks, and she was able to provide collateral history. She denied taking any prescription medications or having any ongoing medical issues. She did report that for two months prior to admission she had been taking an oral beauty supplement designed to enhance hair, skin, and nails. She obtained the supplement online. She could not recall the week leading up to admission, but she did note increasing malaise and fatigue beginning two weeks prior to admission. She denied any recreational drug or alcohol use.
Continue for discussion >>
DISCUSSION
Drug-induced liver injury (DILI) is a relatively uncommon occurrence in the United States.2 It is estimated to occur in approximately 20 individuals per 100,000 persons per year.2 However, DILI incidence secondary to herbal and dietary supplement use appears to be on the rise in the US. In a prospective study conducted by the Drug-Induced Liver Injury Network (DILIN) that included patients with liver injury referred to eight DILIN centers between 2004 and 2013, the proportion of DILI cases caused by herbal and dietary supplements increased from 7% to 20% over the study period.3
DILI can be subclassified into intrinsic and idiosyncratic. Intrinsic DILI results from substances causing a predictable time course and natural history. Substances causing a varied, unpredictable occurrence of DILI in susceptible individuals are idiosyncratic.4 Overall, acetaminophen overdose is the most common cause of DILI.2 However, the most common idiosyncratic offending agents, taken at FDA-approved dosages, are antimicrobials (see Table 2).5 The second most common offending agents are herbal and dietary supplements.5
In a retrospective cohort study evaluating all cases of acute liver failure (ALF) over a six-year period in an integrated health care system, the leading cause of ALF was DILI.6 Of the 32 patients with confirmed drug-induced ALF in this study, the majority of cases (18) were associated with acetaminophen. Herbal and dietary supplements were implicated in six cases, with miscellaneous medications accounting for the remaining eight cases.6 In terms of outcomes, 18.8% of patients with ALF due to DILI underwent liver transplantation, 68.8% were discharged, and 12.5% died during hospitalization.6
DILI disproportionately affects women and minorities7;although the etiology is unclear, it is hypothesized that increased use of antibiotics may play a role among women.2 Providers should be aware of the increased risk for DILI in these populations and consider this diagnosis in the appropriate setting.
Teasing out the diagnosis
DILI is a diagnosis of exclusion, aided in large part by the history and physical exam.4 An extensive history may alert the health care provider to a potential offending substance as well as provide information on timing of exposure.4 DILI should be suspected in patients with persistently elevated liver enzymes, unremarkable work-up for all other underlying liver disease (including autoimmune and viral serologies), and negative abdominal imaging.4 In particular, acute hepatitis C virus (HCV) and hepatitis E virus (HEV) infection mimic the clinical presentation of DILI and should be excluded with HCV RNA and IgM anti-HEV testing, with reflex HEV RNA testing to confirm positive IgM anti-HEV results.8,9 Liver biopsy is rarely indicated for the diagnosis of DILI.2
The presentation of DILI ranges from asymptomatic, with mildly abnormal results on liver function testing, to fulminant hepatic failure. Acetaminophen is the most frequently reported cause of intrinsic DILI in the US, playing a role in approximately half of all ALF cases.10 DILI can be further subdivided according to the pattern of liver test abnormalities as hepatocellular, mixed, or cholestatic based on the ratio of ALT to alkaline phosphatase (R value).2 Utilizing the formula serum ALT/upper limit of normal (ULN) divided by the serum alkaline phosphatase/ULN to determine R value, liver test abnormalities are defined as hepatocellular (R > 5), mixed (R = 2-5), and cholestatic (R < 2).4 These liver test patterns can be used to predict prognosis (see “Prognosis: Hy’s law”). In a prospective, longitudinal study, DILIN found that chronic DILI was present in 18% of the study population at 6 months following onset.5 Patients with the cholestatic presentation were more likely to develop chronic DILI than were those with the hepatocellular or mixed pattern. Furthermore, the hepatocellular pattern on presentation was associated with greater mortality.5 Patients with the mixed pattern had the most favorable outcomes. Another prospective cohort study found that persistently elevated liver enzymes in DILI patients at 12 months is associated with older age and the cholestatic pattern of liver test abnormalities at presentation, in particular, alkaline phosphatase elevation.11 However, neither length of therapy nor type of offending medication was associated with long-term liver test abnormalities.11
Managing DILI and ALF
In all DILI cases, immediate discontinuation of the offending agent is the initial treatment recommendation.2 Patients presenting with DILI who have an accompanying bilirubin level > 2 mg/dL should be referred to a hepatology specialist due to an increased risk for ALF.2 ALF is defined as coagulopathy to INR ≥ 1.5 and hepatic encephalopathy within 26 weeks of initial symptom onset in individuals without known underlying liver disease, with the exception of autoimmune hepatitis, Wilson disease, and reactivation of hepatitis B.12-15 Fulminant hepatic failure is further specified as encephalopathy occurring within 8 weeks of jaundice onset.12
Patients presenting with ALF should be transferred to an intensive care setting, preferably within a liver transplant center, for supportive care and potential liver transplant evaluation.12 CT of the head should be used to rule out other etiologies for altered mental status.16N-Acetylcysteine is the treatment of choice for acetaminophen-induced ALF, and it has also been shown to improve transplant-free survival outcomes in patients with non-acetaminophen–related early ALF.17 Infectious work-up and continuous monitoring are essential in ALF care, since up to 80% of patients with ALF will develop a bacterial infection.18 A comprehensive infectious work-up should include pan-culture of blood, urine, and sputum in addition to assessment for Epstein-Barr virus, cytomegalovirus, and HSV.4,18 For irreversible ALF, liver transplantation remains the only validated treatment option.12,19
Prognosis: Hy’s law
Hy’s law refers to a method used in clinical trials to assess a drug’s likelihood of causing severe hepatotoxicity; it is also used to predict which patients with DILI will develop ALF.12,20 According to Hy’s law, patients with AST or ALT elevations three times ULN and total bilirubin elevations two times ULN are at increased risk for ALF.In a retrospective cohort study of more than 15,000 patients with DILI, the Hy’s law criteria were found to have high specificity but low sensitivity for detecting individuals at risk for ALF.15 An alternative model, the Drug-Induced Liver Toxicity ALF Score, uses platelet count and bilirubin level to identify patients at risk for ALF with high sensitivity.15
Patient education
Effective patient education is essential to decreasing DILI incidence at a time when herbal and dietary supplement consumption is increasing. Patients will often bring herbal and dietary supplements to their providers to obtain a safety profile prior to initiation. In these cases, it is essential to reinforce with patients the absence of federal regulation of these products. It should be stressed to patients that, due to the lack of government oversight, it is impossible to confidently identify the entirety and quantity of ingredients in these supplements. Furthermore, there is no existing protocol for surveillance or adverse event reporting for these products.21 Because these products are not routinely or systematically studied, even health care providers have no evidence on which to base monitoring or usage recommendations. Providers may direct patients to the National Institutes of Health’s LiverTox website (livertox.NIH.gov) to review prior case reports of hepatotoxicity for specific dietary and herbal supplements.
Level of education is associated with knowledge of the potential for overdose when taking OTC medications that contain acetaminophen.22 As a result, health care providers should strongly reinforce with patients the importance of reading all medication labels and abiding by the listed administration directions. In particular, providers should emphasize that the maximum daily dosage of acetaminophen is 4 g.23 For patients with chronic liver disease, a more conservative recommendation is warranted. Generally, patients with cirrhosis may be advised to consume up to 2 g/d of acetaminophen as a firstline treatment for pain. However, providers should ensure acetaminophen ingestion is limited to a brief period.24
Additionally, it is important to educate patients that many combination OTC medications contain acetaminophen. Of note, chronic opioid users are more likely to accurately identify OTC medications containing acetaminophen, compared with acute opioid users.22 These findings should compel health care providers to deliver in-depth education for all patients, particularly those with less education or experience with medications. Education on avoidance of offending medications, including medications within the same class, when appropriate, is essential for quality patient care.2
Continue to outcome for the case patient >>
OUTCOME FOR THE CASE PATIENT
Following discharge, the patient was monitored closely with regular clinic visits and blood work. Her liver test results improved gradually, with consideration of a repeat biopsy to evaluate for overlap or missed autoimmune disease. Her repeat ANA was negative and IgG was within normal limits. Within three months of admission, her liver tests normalized and repeat biopsy was deferred.
Upon review of the herbal beauty supplement the patient reported taking, shark cartilage was noted as a primary ingredient. In a case report, shark cartilage was identified as a hepatotoxin.25 The patient was advised never to ingest the offending supplement, or any other substances not regulated by the FDA, again. Furthermore, the offending medication was listed as a medication allergy in her electronic health record.
CONCLUSION
It is crucial to emphasize to patients the potential hepatotoxicity of medications and herbal and dietary supplements, especially OTC medications that pose an overdose risk. Patients should review all new supplements with their providers prior to therapy initiation. With known hepatotoxins, providers should closely monitor patients for liver injury while treatment is ongoing. In suspected cases of DILI, a thorough history and physical exam will greatly inform the diagnosis. In the majority of cases, the suspect medication should be discontinued immediately, with subsequent assessment of liver response. Identification of DILI early in the course increases the likelihood of full hepatic recovery and improves patient outcomes.
References
1. Kamath PS, Wiesner RH, Malinchoc M, et al. A model to predict survival in patients with end-stage liver disease. Hepatology. 2001;33(2):464-470.
2. Leise MD, Poterucha JJ, Talwalkar JA. Drug-induced liver injury. Mayo Clin Proc. 2014;89(1):95-106.
3. Navarro VJ, Barnhart H, Bonkovsky HL, et al. Liver injury from herbals and dietary supplements in the US Drug-Induced Liver Injury Network. Hepatology. 2014;60(4):1399-1408.
4. Chalasani NP, Hayashi PH, Bonkovsky HL, et al. ACG Clinical Guideline: the diagnosis and management of idiosyncratic drug-induced liver injury. Am J Gastroenterol. 2014;109(7):950-966.
5. Chalasani N, Bonkovsky HL, Fontana R, et al; United States Drug Induced Liver Injury Network. Features and outcomes of 899 patients with drug-induced liver injury: the DILIN prospective study. Gastroenterology. 2015;148(7):1340-1352.
6. Goldberg DS, Forde KA, Carbonari DM, et al. Population-representative incidence of drug-induced acute liver failure based on an analysis of an integrated health care system. Gastroenterology. 2015;148(7):1353-1361.
7. Reuben A, Koch DG, Lee WM. Drug-induced acute liver failure: results of a US multicenter, prospective study. Hepatology. 2010;52(6):2065-2076.
8. Davern TJ, Chalasani N, Fontana RJ, et al; Drug-Induced Liver Injury Network (DILIN). Acute hepatitis E infection accounts for some cases of suspected drug-induced liver injury. Gastroenterology. 2011;141(5):1665-1672.e1-9.
9. Chalasani N, Fontana RJ, Bonkovsky HL, et al. Causes, clinical features, and outcomes from a prospective study of drug-induced liver injury in the United States. Gastroenterology. 2008;135(6):1924-1934.
10. Fisher K, Vuppalanchi R, Saxena R. Drug-induced liver injury. Arch Pathol Lab Med. 2015;139(7):876-887.
11. Fontana RJ, Hayashi PH, Barnhart H, et al. Persistent liver biochemistry abnormalities are more common in older patients and those with cholestatic drug induced liver injury. Am J Gastroenterol. 2015;110(10):1450-1459.
12. Punzalan CS, Barry CT. Acute liver failure: diagnosis and management. J Intensive Care Med. 2015 Oct 6. [Epub ahead of print]
13. Bower WA, Johns M, Margolis HS, et al. Population-based surveillance for acute liver failure. Am J Gastroenterol. 2007;102(11):2459-2463.
14. O’Grady JG, Schalm SW, Williams R. Acute liver failure: redefining the syndromes. Lancet. 1993;342(8866):273-275.
15. Lo Re V III, Haynes K, Forde KA, et al. Risk of acute liver failure in patients with drug-induced liver injury: evaluation of Hy’s law and a new prognostic model. Clin Gastroenterol Hepatol. 2015;13(13):2360-2368.
16. Polson J, Lee WM; American Association for the Study of Liver Diseases. AASLD position paper: the management of acute liver failure. Hepatology. 2005;41:1179-1197.
17. Lee WM, Hynan LS, Rossaro L, et al. Intravenous N-acetylcysteine improves transplant-free survival in early stage non-acetaminophen acute liver failure. Gastroenterology. 2009;137(3):856-864.
18. Rolando N, Harvey F, Brahm J. Prospective study of bacterial infection in acute liver failure: an analysis of fifty patients. Hepatology. 1990;11(1):49-53.
19. Panackel C, Thomas R, Sebastian B, Mathai SK. Recent advances in management of acute liver failure. Indian J Crit Care Med. 2015;19(1):27-33.
20. Temple R. Hy’s law: predicting serious hepatotoxicity. Pharmacoepidemiol Drug Saf. 2006;15(4):241-243.
21. Bunchorntavakul C, Reddy K. Review article: herbal and dietary supplement hepatotoxicity. Aliment Pharmacol Ther. 2012;37(1):3-17.
22. Boudreau DM, Wirtz H, Von Korff M, et al. A survey of adult awareness and use of medicine containing acetaminophen. Pharmacoepidemiol Drug Saf. 2013;22(3):229-240.
23. Burns MJ, Friedman SL, Larson AM. Acetaminophen (paracetamol) poisoning in adults: pathophysiology, presentation, and diagnosis. UpToDate. www.uptodate.com/contents/acetaminophen-paracetamol-poisoning-in-adults-pathophysiology-presentation-and-diagnosis. Accessed May 20, 2016.
24. Lewis JH, Stine JG. Review article: prescribing medications in patients with cirrhosis—a practical guide. Aliment Pharmacol Ther. 2013;37(12):1132-1156.
25. Ashar B, Vargo E. Shark cartilage-induced hepatitis. Ann Intern Med. 1996;125(9):780-781.