User login
Oral Lichen Planus With Malignant Transformation to Invasive Squamous Cell Carcinoma
To the Editor:
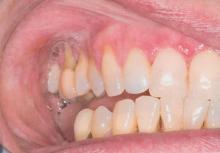
A 62-year-old woman with an extensive history of cutaneous and oral lichen planus (OLP) presented with gradual worsening of oral pain refractory to previously successful treatment regimens. The pains were described as sharp sensations originating in the right superior oral cavity, occurring almost constantly over the course of 2 months. On examination, the oral mucosa on the right side showed lacy, white, hyperkeratotic buccal lesions, as well as superficial erythematous erosion on the right upper alveolar ridge mucosa (Figure 1). On the left side, lacy, white, reticular patches were noted along the buccal mucosa. Gingival desquamation with superficial erosions were observed bilaterally, extending to the upper alveolar ridge in some locations. The skin examination revealed resolving, nonirritated, violaceous, flat-topped papules with a white-gray hue on the upper back and vulva.
The rest of the physical examination was benign, including a lack of appreciable lymphadenopathy, a cranial nerve examination without focal deficit, and the presence of fluent unaffected speech. On review of systems, the patient denied fevers, chills, weight loss, or night sweats. She had no history of skin cancer or oropharyngeal cancer. Family history revealed that her father had nonmelanoma skin cancer of the head and neck. She denied heavy alcohol use as well as history of smoking or other oral tobacco products. Laboratory tests revealed a complete blood cell count and comprehensive metabolic panel that was within reference range. Due to the refractory nature of the pain, which was out of character for OLP, the patient was referred to an oral maxillofacial surgeon who extracted right maxillary teeth adjacent to the erosion to obtain an adequate specimen for surgical biopsy of the lesion itself. Histopathology confirmed the diagnosis of chronic erosive OLP with malignant transformation to localized squamous cell carcinoma (SCC) of the right maxilla.
While awaiting treatment, she began to develop unremitting headaches and painful shooting sensations beginning in the right superior oral mucosa, radiating to the ipsilateral naris, nasolabial folds, malar cheek, and temple region. This clinical picture was consistent with neuralgia occurring along the maxillary nerve. A subsequent computed tomography scan revealed local bony destruction of the primary tumor and likely perineural involvement (Figure 2), without notable nodal involvement or metastasis (stage III: T4aN0M0). An otolaryngologist performed a wide alveolar and maxillary excision with lymph node dissection. Surgical margins were deemed as negative and there was no evidence of nodal disease. She was later seen by the oncology and radiation oncology teams and received several courses of chemoradiotherapy.
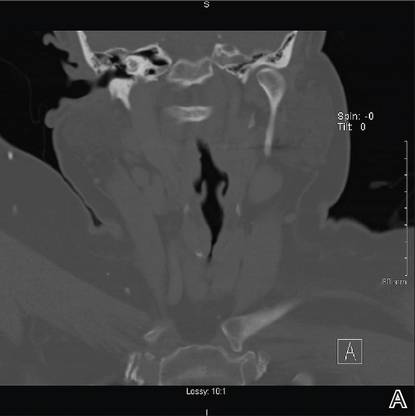 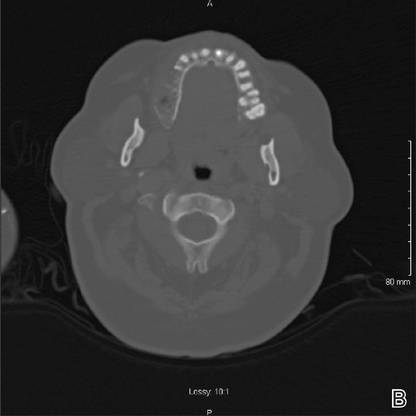 |
Figure 2. Coronal (A) and axial (B) computed tomography demonstrated right maxillary bony destruction. |
Seven months later, a new indurated ulcer was noted on the left lateral tongue. Biopsy revealed a new primary oral SCC (OSCC), which also was excised by an otolaryngologist. Recent computed tomography did not detect any recurrence or potential metastases, but the patient subsequently was lost to follow-up.
Lichen planus is an idiopathic inflammatory disease most commonly affecting the cutaneous skin as well as the oral mucosa, genital mucosa, nails, and scalp. Oral lichen planus is a relatively common manifestation, found in approximately 1% to 2% of individuals older than 15 years.1 Epidemiologic studies revealed that OLP is uncommon in children,2,3 it affects women more frequently than men (approximately 3:1 ratio),3 and its incidence peaks between 30 and 60 years of age.4 The literature on malignant transformation of OLP is varied and controversial, with some early investigations such as Krutchkoff et al5 concluding that the reported cases often fall short of supporting OLP as a premalignant source of OSCC due to insufficient evidence in claimed case reports supporting the diagnosis of OLP histopathologically, the occurrence of OSCC in sites where OLP lesions did not previously exist, and uncertainty regarding confounding factors such as carcinogen exposure.5 In contrast, a longitudinal cohort study reported malignant transformation in 2.4% of OLP cases (N=327), with a standardized incidence ratio of 17.7 (95% confidence interval, 8.8-35.3) when compared to a control group.6 Current literature has predominantly sided with the notion that OLP, especially the erosive variant, carries the risk for malignant potential6 as well as the World Health Organization’s classification of the disorder as precancerous.3
The pathophysiology of OLP and its potential for malignant transformation are unknown. It is believed that cell-mediated immunity, specifically CD8+ lymphocytes targeting stratum basale keratinocytes for apoptosis via the caspase cascade, plays a major role in the development of OLP, beginning with Langerhans cell recognition of an unknown basal cell antigen.3 Moreover, it is postulated that antigen expression is induced by certain drugs, infections, and contact allergens such as dental amalgams, explaining their known associations with OLP initiation and exacerbation. The etiology behind OLP developing into OSCC also is poorly understood and many different hypotheses have been suggested. Modified expression of p53, a 53-kd protein, in OLP patients has been demonstrated.6 Some investigators propose that a lack of the expected keratinocyte apoptotic response to the cell-mediated attack may be etiologic in cancerous transformation.3 Given their utility in treatment of OLP, there also has been apprehension over the potential for immunosuppressant medications leading to decreased expression of antitumor regulators and development of malignant cells, though it has not been substantiated by current literature.6 Finally, some cases of OSCC are believed to have been linked to N-nitrosobenzylmethylamine, a known carcinogen produced by colonized Candida albicans, which also may play a role in OLP treated with immunosuppressants.7
Clinically, OLP lesions are known to be more chronic in nature than cutaneous lichen planus.7 There are 6 classifications of OLP: reticular (lacy white with Wickham striae), plaquelike, papular, atrophic, bullous, and erosive. The latter 3 are known to be the more symptomatic manifestations.3,7 Of note, the atrophic and erosive forms are believed to account for the vast majority of cases of malignant transformation of OLP to OSCC. Approximately 90% of patients have involvement of multiple oral sites, with the most common affected areas being the buccal mucosa (90%), gingival margin (56%), and dorsal tongue (34%).7 Symptoms include increased sensitivity to foods, intense local pain, and coarse-feeling mucosa. The nature of the disease favors an active-quiescent-active course, with flares occurring after direct irritation (ie, dental procedures, Köbner phenomenon), emotional stress, medication use, and systemic illness.7 The differential diagnosis of OLP includes bite trauma, candidiasis, pemphigus, leukoplakia, lichenoid drug reaction, pemphigoid, and graft-versus-host disease.4 Red flags of malignant transformation include induration, worsening ulceration in the setting of previously effective therapy, and presence of constitutional symptoms.
Regarding the behavior of OSCC after malignant transformation, the literature seems to suggest a tendency for well-differentiated noninvasive tumors that most often occur on the buccal mucosa (43%), tongue (33%), gingiva (19%), and palate (4.8%).8 Interestingly, one study described that only 1 (4.8%) of 21 patients with OLP and OSCC was deemed as having stage II or higher disease at time of diagnosis. Likewise, 90% of the biopsied samples revealed well-differentiated carcinomas.8 These findings clearly contrast with our case in which the patient experienced rapid conversion of localized OSCC to more invasive disease. Also of consequence in this study was the finding that a relatively high proportion of patients (29% [6/21]) developed at least one other primary OSCC lesion over the course of follow-up.8 This finding is consistent with our patient.
Last, management of OLP lesions is most commonly accomplished with topical steroids such as fluocinolone acetonide or triamcinolone acetonide.3 Treatment of gingival disease may be enhanced with the use of form-fitting trays.2 For refractory erosive disease, tacrolimus ointment has been demonstrated as a useful backup therapy but may actually be associated with the development of OSCC through alteration of MAPK and p53.3 Some investigators suggest regular 4-month follow-up of OLP patients to detect if acute worsening and or refractoriness to treatment have signified early dysplastic change. Various scoring systems also have been suggested for following up on the severity of OLP lesions.3
The management of OSCC usually is accomplished via surgery, radiation, or both. The decision is dependent on tumor stage and the patient’s individual limitations. It is highly recommended that patients with OSCC arising from OLP be closely followed after diagnosis of cancer, with some sources suggesting follow-up every 2 months for the first 6 to 9 months after diagnosis due to the relatively high rate of discovery of nodal metastases and new primary lesions in that critical time span.8 Thereafter, an examination every 4 months is suggested as sufficient for detecting future complications.
1. van der Meij EH, Schepman KP, Smeele LE, et al. A review of the recent literature regarding malignant transformation of oral lichen planus. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 1999;88:307-310.
2. Scattarella A, Petruzzi M, Ballini A, et al. Oral lichen planus and dental hygiene: a case report [published online ahead of print September 1, 2010]. Int J Dent Hyg. 2011;9:163-166.
3. Ismail SB, Kumar SK, Zain RB. Oral lichen planus and lichenoid reactions: etiopathogenesis, diagnosis, management and malignant transformation. J Oral Sci. 2007;49:89-106.
4. Usatine RP, Tinitigan M. Diagnosis and treatment of lichen planus. Am Fam Physician. 2011;84:53-60.
5. Krutchkoff DJ, Cutler L, Laskowski S. Oral lichen planus: the evidence regarding potential malignant transformation. J Oral Pathol. 1978;7:1-7.
6. Bombeccari GP, Guzzi G, Tettamanti M, et al. Oral lichen planus and malignant transformation: a longitudinal cohort study [published online ahead of print July 22, 2011]. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2011;112:328-334.
7. Eisen D. The clinical features, malignant potential, and systemic associations of oral lichen planus: a study of 723 patients. J Am Acad Dermatol. 2002;46:207-214.
8. Mignogna MD, Lo Russo L, Fedele S, et al. Clinical behaviour of malignant transforming oral lichen planus. Eur J Surg Oncol. 2002;28:838-843.
To the Editor:
A 62-year-old woman with an extensive history of cutaneous and oral lichen planus (OLP) presented with gradual worsening of oral pain refractory to previously successful treatment regimens. The pains were described as sharp sensations originating in the right superior oral cavity, occurring almost constantly over the course of 2 months. On examination, the oral mucosa on the right side showed lacy, white, hyperkeratotic buccal lesions, as well as superficial erythematous erosion on the right upper alveolar ridge mucosa (Figure 1). On the left side, lacy, white, reticular patches were noted along the buccal mucosa. Gingival desquamation with superficial erosions were observed bilaterally, extending to the upper alveolar ridge in some locations. The skin examination revealed resolving, nonirritated, violaceous, flat-topped papules with a white-gray hue on the upper back and vulva.
The rest of the physical examination was benign, including a lack of appreciable lymphadenopathy, a cranial nerve examination without focal deficit, and the presence of fluent unaffected speech. On review of systems, the patient denied fevers, chills, weight loss, or night sweats. She had no history of skin cancer or oropharyngeal cancer. Family history revealed that her father had nonmelanoma skin cancer of the head and neck. She denied heavy alcohol use as well as history of smoking or other oral tobacco products. Laboratory tests revealed a complete blood cell count and comprehensive metabolic panel that was within reference range. Due to the refractory nature of the pain, which was out of character for OLP, the patient was referred to an oral maxillofacial surgeon who extracted right maxillary teeth adjacent to the erosion to obtain an adequate specimen for surgical biopsy of the lesion itself. Histopathology confirmed the diagnosis of chronic erosive OLP with malignant transformation to localized squamous cell carcinoma (SCC) of the right maxilla.
While awaiting treatment, she began to develop unremitting headaches and painful shooting sensations beginning in the right superior oral mucosa, radiating to the ipsilateral naris, nasolabial folds, malar cheek, and temple region. This clinical picture was consistent with neuralgia occurring along the maxillary nerve. A subsequent computed tomography scan revealed local bony destruction of the primary tumor and likely perineural involvement (Figure 2), without notable nodal involvement or metastasis (stage III: T4aN0M0). An otolaryngologist performed a wide alveolar and maxillary excision with lymph node dissection. Surgical margins were deemed as negative and there was no evidence of nodal disease. She was later seen by the oncology and radiation oncology teams and received several courses of chemoradiotherapy.
  |
Figure 2. Coronal (A) and axial (B) computed tomography demonstrated right maxillary bony destruction. |
Seven months later, a new indurated ulcer was noted on the left lateral tongue. Biopsy revealed a new primary oral SCC (OSCC), which also was excised by an otolaryngologist. Recent computed tomography did not detect any recurrence or potential metastases, but the patient subsequently was lost to follow-up.
Lichen planus is an idiopathic inflammatory disease most commonly affecting the cutaneous skin as well as the oral mucosa, genital mucosa, nails, and scalp. Oral lichen planus is a relatively common manifestation, found in approximately 1% to 2% of individuals older than 15 years.1 Epidemiologic studies revealed that OLP is uncommon in children,2,3 it affects women more frequently than men (approximately 3:1 ratio),3 and its incidence peaks between 30 and 60 years of age.4 The literature on malignant transformation of OLP is varied and controversial, with some early investigations such as Krutchkoff et al5 concluding that the reported cases often fall short of supporting OLP as a premalignant source of OSCC due to insufficient evidence in claimed case reports supporting the diagnosis of OLP histopathologically, the occurrence of OSCC in sites where OLP lesions did not previously exist, and uncertainty regarding confounding factors such as carcinogen exposure.5 In contrast, a longitudinal cohort study reported malignant transformation in 2.4% of OLP cases (N=327), with a standardized incidence ratio of 17.7 (95% confidence interval, 8.8-35.3) when compared to a control group.6 Current literature has predominantly sided with the notion that OLP, especially the erosive variant, carries the risk for malignant potential6 as well as the World Health Organization’s classification of the disorder as precancerous.3
The pathophysiology of OLP and its potential for malignant transformation are unknown. It is believed that cell-mediated immunity, specifically CD8+ lymphocytes targeting stratum basale keratinocytes for apoptosis via the caspase cascade, plays a major role in the development of OLP, beginning with Langerhans cell recognition of an unknown basal cell antigen.3 Moreover, it is postulated that antigen expression is induced by certain drugs, infections, and contact allergens such as dental amalgams, explaining their known associations with OLP initiation and exacerbation. The etiology behind OLP developing into OSCC also is poorly understood and many different hypotheses have been suggested. Modified expression of p53, a 53-kd protein, in OLP patients has been demonstrated.6 Some investigators propose that a lack of the expected keratinocyte apoptotic response to the cell-mediated attack may be etiologic in cancerous transformation.3 Given their utility in treatment of OLP, there also has been apprehension over the potential for immunosuppressant medications leading to decreased expression of antitumor regulators and development of malignant cells, though it has not been substantiated by current literature.6 Finally, some cases of OSCC are believed to have been linked to N-nitrosobenzylmethylamine, a known carcinogen produced by colonized Candida albicans, which also may play a role in OLP treated with immunosuppressants.7
Clinically, OLP lesions are known to be more chronic in nature than cutaneous lichen planus.7 There are 6 classifications of OLP: reticular (lacy white with Wickham striae), plaquelike, papular, atrophic, bullous, and erosive. The latter 3 are known to be the more symptomatic manifestations.3,7 Of note, the atrophic and erosive forms are believed to account for the vast majority of cases of malignant transformation of OLP to OSCC. Approximately 90% of patients have involvement of multiple oral sites, with the most common affected areas being the buccal mucosa (90%), gingival margin (56%), and dorsal tongue (34%).7 Symptoms include increased sensitivity to foods, intense local pain, and coarse-feeling mucosa. The nature of the disease favors an active-quiescent-active course, with flares occurring after direct irritation (ie, dental procedures, Köbner phenomenon), emotional stress, medication use, and systemic illness.7 The differential diagnosis of OLP includes bite trauma, candidiasis, pemphigus, leukoplakia, lichenoid drug reaction, pemphigoid, and graft-versus-host disease.4 Red flags of malignant transformation include induration, worsening ulceration in the setting of previously effective therapy, and presence of constitutional symptoms.
Regarding the behavior of OSCC after malignant transformation, the literature seems to suggest a tendency for well-differentiated noninvasive tumors that most often occur on the buccal mucosa (43%), tongue (33%), gingiva (19%), and palate (4.8%).8 Interestingly, one study described that only 1 (4.8%) of 21 patients with OLP and OSCC was deemed as having stage II or higher disease at time of diagnosis. Likewise, 90% of the biopsied samples revealed well-differentiated carcinomas.8 These findings clearly contrast with our case in which the patient experienced rapid conversion of localized OSCC to more invasive disease. Also of consequence in this study was the finding that a relatively high proportion of patients (29% [6/21]) developed at least one other primary OSCC lesion over the course of follow-up.8 This finding is consistent with our patient.
Last, management of OLP lesions is most commonly accomplished with topical steroids such as fluocinolone acetonide or triamcinolone acetonide.3 Treatment of gingival disease may be enhanced with the use of form-fitting trays.2 For refractory erosive disease, tacrolimus ointment has been demonstrated as a useful backup therapy but may actually be associated with the development of OSCC through alteration of MAPK and p53.3 Some investigators suggest regular 4-month follow-up of OLP patients to detect if acute worsening and or refractoriness to treatment have signified early dysplastic change. Various scoring systems also have been suggested for following up on the severity of OLP lesions.3
The management of OSCC usually is accomplished via surgery, radiation, or both. The decision is dependent on tumor stage and the patient’s individual limitations. It is highly recommended that patients with OSCC arising from OLP be closely followed after diagnosis of cancer, with some sources suggesting follow-up every 2 months for the first 6 to 9 months after diagnosis due to the relatively high rate of discovery of nodal metastases and new primary lesions in that critical time span.8 Thereafter, an examination every 4 months is suggested as sufficient for detecting future complications.
To the Editor:
A 62-year-old woman with an extensive history of cutaneous and oral lichen planus (OLP) presented with gradual worsening of oral pain refractory to previously successful treatment regimens. The pains were described as sharp sensations originating in the right superior oral cavity, occurring almost constantly over the course of 2 months. On examination, the oral mucosa on the right side showed lacy, white, hyperkeratotic buccal lesions, as well as superficial erythematous erosion on the right upper alveolar ridge mucosa (Figure 1). On the left side, lacy, white, reticular patches were noted along the buccal mucosa. Gingival desquamation with superficial erosions were observed bilaterally, extending to the upper alveolar ridge in some locations. The skin examination revealed resolving, nonirritated, violaceous, flat-topped papules with a white-gray hue on the upper back and vulva.
The rest of the physical examination was benign, including a lack of appreciable lymphadenopathy, a cranial nerve examination without focal deficit, and the presence of fluent unaffected speech. On review of systems, the patient denied fevers, chills, weight loss, or night sweats. She had no history of skin cancer or oropharyngeal cancer. Family history revealed that her father had nonmelanoma skin cancer of the head and neck. She denied heavy alcohol use as well as history of smoking or other oral tobacco products. Laboratory tests revealed a complete blood cell count and comprehensive metabolic panel that was within reference range. Due to the refractory nature of the pain, which was out of character for OLP, the patient was referred to an oral maxillofacial surgeon who extracted right maxillary teeth adjacent to the erosion to obtain an adequate specimen for surgical biopsy of the lesion itself. Histopathology confirmed the diagnosis of chronic erosive OLP with malignant transformation to localized squamous cell carcinoma (SCC) of the right maxilla.
While awaiting treatment, she began to develop unremitting headaches and painful shooting sensations beginning in the right superior oral mucosa, radiating to the ipsilateral naris, nasolabial folds, malar cheek, and temple region. This clinical picture was consistent with neuralgia occurring along the maxillary nerve. A subsequent computed tomography scan revealed local bony destruction of the primary tumor and likely perineural involvement (Figure 2), without notable nodal involvement or metastasis (stage III: T4aN0M0). An otolaryngologist performed a wide alveolar and maxillary excision with lymph node dissection. Surgical margins were deemed as negative and there was no evidence of nodal disease. She was later seen by the oncology and radiation oncology teams and received several courses of chemoradiotherapy.
  |
Figure 2. Coronal (A) and axial (B) computed tomography demonstrated right maxillary bony destruction. |
Seven months later, a new indurated ulcer was noted on the left lateral tongue. Biopsy revealed a new primary oral SCC (OSCC), which also was excised by an otolaryngologist. Recent computed tomography did not detect any recurrence or potential metastases, but the patient subsequently was lost to follow-up.
Lichen planus is an idiopathic inflammatory disease most commonly affecting the cutaneous skin as well as the oral mucosa, genital mucosa, nails, and scalp. Oral lichen planus is a relatively common manifestation, found in approximately 1% to 2% of individuals older than 15 years.1 Epidemiologic studies revealed that OLP is uncommon in children,2,3 it affects women more frequently than men (approximately 3:1 ratio),3 and its incidence peaks between 30 and 60 years of age.4 The literature on malignant transformation of OLP is varied and controversial, with some early investigations such as Krutchkoff et al5 concluding that the reported cases often fall short of supporting OLP as a premalignant source of OSCC due to insufficient evidence in claimed case reports supporting the diagnosis of OLP histopathologically, the occurrence of OSCC in sites where OLP lesions did not previously exist, and uncertainty regarding confounding factors such as carcinogen exposure.5 In contrast, a longitudinal cohort study reported malignant transformation in 2.4% of OLP cases (N=327), with a standardized incidence ratio of 17.7 (95% confidence interval, 8.8-35.3) when compared to a control group.6 Current literature has predominantly sided with the notion that OLP, especially the erosive variant, carries the risk for malignant potential6 as well as the World Health Organization’s classification of the disorder as precancerous.3
The pathophysiology of OLP and its potential for malignant transformation are unknown. It is believed that cell-mediated immunity, specifically CD8+ lymphocytes targeting stratum basale keratinocytes for apoptosis via the caspase cascade, plays a major role in the development of OLP, beginning with Langerhans cell recognition of an unknown basal cell antigen.3 Moreover, it is postulated that antigen expression is induced by certain drugs, infections, and contact allergens such as dental amalgams, explaining their known associations with OLP initiation and exacerbation. The etiology behind OLP developing into OSCC also is poorly understood and many different hypotheses have been suggested. Modified expression of p53, a 53-kd protein, in OLP patients has been demonstrated.6 Some investigators propose that a lack of the expected keratinocyte apoptotic response to the cell-mediated attack may be etiologic in cancerous transformation.3 Given their utility in treatment of OLP, there also has been apprehension over the potential for immunosuppressant medications leading to decreased expression of antitumor regulators and development of malignant cells, though it has not been substantiated by current literature.6 Finally, some cases of OSCC are believed to have been linked to N-nitrosobenzylmethylamine, a known carcinogen produced by colonized Candida albicans, which also may play a role in OLP treated with immunosuppressants.7
Clinically, OLP lesions are known to be more chronic in nature than cutaneous lichen planus.7 There are 6 classifications of OLP: reticular (lacy white with Wickham striae), plaquelike, papular, atrophic, bullous, and erosive. The latter 3 are known to be the more symptomatic manifestations.3,7 Of note, the atrophic and erosive forms are believed to account for the vast majority of cases of malignant transformation of OLP to OSCC. Approximately 90% of patients have involvement of multiple oral sites, with the most common affected areas being the buccal mucosa (90%), gingival margin (56%), and dorsal tongue (34%).7 Symptoms include increased sensitivity to foods, intense local pain, and coarse-feeling mucosa. The nature of the disease favors an active-quiescent-active course, with flares occurring after direct irritation (ie, dental procedures, Köbner phenomenon), emotional stress, medication use, and systemic illness.7 The differential diagnosis of OLP includes bite trauma, candidiasis, pemphigus, leukoplakia, lichenoid drug reaction, pemphigoid, and graft-versus-host disease.4 Red flags of malignant transformation include induration, worsening ulceration in the setting of previously effective therapy, and presence of constitutional symptoms.
Regarding the behavior of OSCC after malignant transformation, the literature seems to suggest a tendency for well-differentiated noninvasive tumors that most often occur on the buccal mucosa (43%), tongue (33%), gingiva (19%), and palate (4.8%).8 Interestingly, one study described that only 1 (4.8%) of 21 patients with OLP and OSCC was deemed as having stage II or higher disease at time of diagnosis. Likewise, 90% of the biopsied samples revealed well-differentiated carcinomas.8 These findings clearly contrast with our case in which the patient experienced rapid conversion of localized OSCC to more invasive disease. Also of consequence in this study was the finding that a relatively high proportion of patients (29% [6/21]) developed at least one other primary OSCC lesion over the course of follow-up.8 This finding is consistent with our patient.
Last, management of OLP lesions is most commonly accomplished with topical steroids such as fluocinolone acetonide or triamcinolone acetonide.3 Treatment of gingival disease may be enhanced with the use of form-fitting trays.2 For refractory erosive disease, tacrolimus ointment has been demonstrated as a useful backup therapy but may actually be associated with the development of OSCC through alteration of MAPK and p53.3 Some investigators suggest regular 4-month follow-up of OLP patients to detect if acute worsening and or refractoriness to treatment have signified early dysplastic change. Various scoring systems also have been suggested for following up on the severity of OLP lesions.3
The management of OSCC usually is accomplished via surgery, radiation, or both. The decision is dependent on tumor stage and the patient’s individual limitations. It is highly recommended that patients with OSCC arising from OLP be closely followed after diagnosis of cancer, with some sources suggesting follow-up every 2 months for the first 6 to 9 months after diagnosis due to the relatively high rate of discovery of nodal metastases and new primary lesions in that critical time span.8 Thereafter, an examination every 4 months is suggested as sufficient for detecting future complications.
1. van der Meij EH, Schepman KP, Smeele LE, et al. A review of the recent literature regarding malignant transformation of oral lichen planus. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 1999;88:307-310.
2. Scattarella A, Petruzzi M, Ballini A, et al. Oral lichen planus and dental hygiene: a case report [published online ahead of print September 1, 2010]. Int J Dent Hyg. 2011;9:163-166.
3. Ismail SB, Kumar SK, Zain RB. Oral lichen planus and lichenoid reactions: etiopathogenesis, diagnosis, management and malignant transformation. J Oral Sci. 2007;49:89-106.
4. Usatine RP, Tinitigan M. Diagnosis and treatment of lichen planus. Am Fam Physician. 2011;84:53-60.
5. Krutchkoff DJ, Cutler L, Laskowski S. Oral lichen planus: the evidence regarding potential malignant transformation. J Oral Pathol. 1978;7:1-7.
6. Bombeccari GP, Guzzi G, Tettamanti M, et al. Oral lichen planus and malignant transformation: a longitudinal cohort study [published online ahead of print July 22, 2011]. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2011;112:328-334.
7. Eisen D. The clinical features, malignant potential, and systemic associations of oral lichen planus: a study of 723 patients. J Am Acad Dermatol. 2002;46:207-214.
8. Mignogna MD, Lo Russo L, Fedele S, et al. Clinical behaviour of malignant transforming oral lichen planus. Eur J Surg Oncol. 2002;28:838-843.
1. van der Meij EH, Schepman KP, Smeele LE, et al. A review of the recent literature regarding malignant transformation of oral lichen planus. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 1999;88:307-310.
2. Scattarella A, Petruzzi M, Ballini A, et al. Oral lichen planus and dental hygiene: a case report [published online ahead of print September 1, 2010]. Int J Dent Hyg. 2011;9:163-166.
3. Ismail SB, Kumar SK, Zain RB. Oral lichen planus and lichenoid reactions: etiopathogenesis, diagnosis, management and malignant transformation. J Oral Sci. 2007;49:89-106.
4. Usatine RP, Tinitigan M. Diagnosis and treatment of lichen planus. Am Fam Physician. 2011;84:53-60.
5. Krutchkoff DJ, Cutler L, Laskowski S. Oral lichen planus: the evidence regarding potential malignant transformation. J Oral Pathol. 1978;7:1-7.
6. Bombeccari GP, Guzzi G, Tettamanti M, et al. Oral lichen planus and malignant transformation: a longitudinal cohort study [published online ahead of print July 22, 2011]. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2011;112:328-334.
7. Eisen D. The clinical features, malignant potential, and systemic associations of oral lichen planus: a study of 723 patients. J Am Acad Dermatol. 2002;46:207-214.
8. Mignogna MD, Lo Russo L, Fedele S, et al. Clinical behaviour of malignant transforming oral lichen planus. Eur J Surg Oncol. 2002;28:838-843.