User login
How should you treat trochanteric bursitis?
Conservative measures—followed by corticosteroid injection, if necessary—are best. Conservative therapy includes rest, nonsteroidal anti-inflammatory drugs (NSAIDs), and stretching exercises focused on the lower back and sacroiliac joints (strength of recommendation [SOR]: C, usual practice). Patients whose symptoms persist despite conservative therapy are likely to benefit from an injection of 24 mg betamethasone and 1% lidocaine (or equivalent) into the inflamed bursa (SOR: B, limited-quality, patient-oriented evidence).
In rare cases of intractable symptoms, surgical procedures such as iliotibial band release, subgluteal bursectomy, and trochanteric reduction osteotomy are options (SOR: C, case studies).
Evidence summary
Trochanteric bursitis is characterized by chronic intermittent lateral hip pain caused by inflammation of the trochanteric bursae. The bursae can become inflamed at the gluteus medius tendon, iliotibial tract, or gluteus minimus during repetitive flexing of the hip. Several conditions are associated with trochanteric bursitis (TABLE).
Trochanteric bursitis peaks in the fourth to sixth decades of life. One retrospective cohort study found the prevalence to be 1.8 cases per 1000 patients per year in primary care; 79% of cases occurred in women.1
TABLE
Conditions associated with trochanteric bursitis
| Chronic mechanical low back pain |
| Degenerative arthritis or disc disease of lower lumbar spine |
| Degenerative joint disease of knees |
| Fibromyalgia |
| Iliotibial band syndrome |
| Inflammatory arthritis of the hip |
| Ipsilateral or contralateral hip arthritis |
| Leg length discrepancy |
| Obesity |
| Pes planus |
| Tendonitis of external hip rotators |
| Total hip arthroplasty |
| Source: Lievense A et al. Br J Gen Pract. 2005.1 |
No studies have compared conservative treatments
Most review articles refer to initial treatment with rest, physical therapy, stretching, and NSAIDs. These treatments were described in textbooks and articles from the 1940s and 1950s.
No studies comparing conservative treatments were found. Few reports discuss physical therapy for trochanteric bursitis.
Corticosteroid injection has the best evidential support
Corticosteroid injection for treating trochanteric bursitis is supported by the best evidence in the available literature. No controlled trials have compared injection with placebo, however.
A randomized, prospective, open comparison trial at a rheumatology clinic assigned patients with trochanteric bursitis to 6-, 12-, or 24-mg doses of betamethasone mixed with 1% lidocaine.2 Seventy-seven percent of patients had improved at 1 week, 69% at 6 weeks, and 61% at 26 weeks. Notably, a significant difference was found at 26 weeks in the number of patients with sustained pain improvement who had received 24 mg of steroid (P<.0123) compared with patients who received the lower doses. The authors didn’t report side effects or complications.
A prospective, noncomparative cohort study investigated 72 patients in a rheumatology clinic who hadn’t improved after at least 2 weeks of treatment with NSAIDs, analgesics, or ointments.3 Of the 59 patients who consented to steroid injections, 42 improved after 1 injection of 40 mg methylprednisolone with 2 mL of 2% lidocaine, 13 improved after a second injection 3 weeks later, and the remaining 4 improved after a third injection. Improvement was defined as disappearance of pain and disability. Six patients (8%) experienced a recurrence of bursitis during a 2-year follow-up period. No local or systemic complications were associated with the corticosteroids or anesthetic solution.
Two retrospective studies also documented the efficacy of corticosteroid injection. One investigated treatment of 36 patients in a rheumatology practice.4 All received methylprednisolone (40-80 mg) or triamcinolone (20-40 mg), and all improved. Two thirds of the patients were symptom free after 1 or 2 injections. Symptoms usually resolved within 2 days to several months (typically 1 or 2 weeks) postinjection. About 25% of the patients relapsed within 2 years.
Another retrospective cohort study of 164 British patients found that those who received a corticosteroid injection were 2.7 times more likely to have recovered at 5 years than patients who had not received an injection (odds ratio=0.4; 95% confidence interval, 0.1-1.0).1
When to consider surgery
Surgical treatment may be necessary for patients with refractory trochanteric bursitis. Several case studies5-7 demonstrate successful outcomes with a variety of surgical techniques, including trochanteric reduction osteotomy and iliotibial band release. Newer techniques involve arthroscopic bursectomy.
Recommendations
UpToDate8 recommends conservative treatment initially. For persistent cases, a corticosteroid injection should be given and repeated in 6 weeks if pain persists. Surgery may be considered if these measures don’t relieve symptoms and pain lasts longer than 1 year.
The American Academy of Orthopaedic Surgeons similarly recommends NSAIDs and activity modification followed by corticosteroid injection.9 Surgery is rarely indicated.
1. Lievense A, Bierma-Zeinstra S. Prognosis of trochanteric pain in primary care. Br J Gen Pract. 2005;55:199-204.
2. Shbeeb M, O’Duffy D, Michet CJ, Jr, et al. Evaluation of glucocorticosteroid injection for the treatment of trochanteric bursitis. J Rheumatol. 1996;23:2104-2106.
3. Schapira D, Nahir M, Scharf Y. Trochanteric bursitis: a common clinical problem. Arch Phys Med Rehabil. 1986;67:815-817.
4. Rasmussen K, Fan N. Trochanteric bursitis: treatment by corticosteroid injection. Scand J Rheumatol. 1985;14:417-420.
5. Slawski D, Howard R. Surgical management of refractory trochanteric bursitis. Am J Sports Med. 1997;25:86-89.
6. Fox J. The role of arthroscopic bursectomy in the treatment of trochanteric bursitis. Arthroscopy. 2002;18:E34.-
7. Govaert L, van der Vis R, Marti RK, et al. Trochanteric reduction osteotomy as a treatment for refractory trochanteric bursitis. J Bone Joint Surg Br. 2003;85:199-203.
8. Anderson B. Trochanteric bursitis. UpToDate [online database]. Version 17.2. Waltham, Mass: UpToDate; 2009.
9. Trochanteric bursitis. In: Griffin LY. Essentials of Musculoskeletal Care. 3rd ed. Rosemont, IL: American Academy of Orthopaedic Surgeons; 2005:461–463.
Conservative measures—followed by corticosteroid injection, if necessary—are best. Conservative therapy includes rest, nonsteroidal anti-inflammatory drugs (NSAIDs), and stretching exercises focused on the lower back and sacroiliac joints (strength of recommendation [SOR]: C, usual practice). Patients whose symptoms persist despite conservative therapy are likely to benefit from an injection of 24 mg betamethasone and 1% lidocaine (or equivalent) into the inflamed bursa (SOR: B, limited-quality, patient-oriented evidence).
In rare cases of intractable symptoms, surgical procedures such as iliotibial band release, subgluteal bursectomy, and trochanteric reduction osteotomy are options (SOR: C, case studies).
Evidence summary
Trochanteric bursitis is characterized by chronic intermittent lateral hip pain caused by inflammation of the trochanteric bursae. The bursae can become inflamed at the gluteus medius tendon, iliotibial tract, or gluteus minimus during repetitive flexing of the hip. Several conditions are associated with trochanteric bursitis (TABLE).
Trochanteric bursitis peaks in the fourth to sixth decades of life. One retrospective cohort study found the prevalence to be 1.8 cases per 1000 patients per year in primary care; 79% of cases occurred in women.1
TABLE
Conditions associated with trochanteric bursitis
| Chronic mechanical low back pain |
| Degenerative arthritis or disc disease of lower lumbar spine |
| Degenerative joint disease of knees |
| Fibromyalgia |
| Iliotibial band syndrome |
| Inflammatory arthritis of the hip |
| Ipsilateral or contralateral hip arthritis |
| Leg length discrepancy |
| Obesity |
| Pes planus |
| Tendonitis of external hip rotators |
| Total hip arthroplasty |
| Source: Lievense A et al. Br J Gen Pract. 2005.1 |
No studies have compared conservative treatments
Most review articles refer to initial treatment with rest, physical therapy, stretching, and NSAIDs. These treatments were described in textbooks and articles from the 1940s and 1950s.
No studies comparing conservative treatments were found. Few reports discuss physical therapy for trochanteric bursitis.
Corticosteroid injection has the best evidential support
Corticosteroid injection for treating trochanteric bursitis is supported by the best evidence in the available literature. No controlled trials have compared injection with placebo, however.
A randomized, prospective, open comparison trial at a rheumatology clinic assigned patients with trochanteric bursitis to 6-, 12-, or 24-mg doses of betamethasone mixed with 1% lidocaine.2 Seventy-seven percent of patients had improved at 1 week, 69% at 6 weeks, and 61% at 26 weeks. Notably, a significant difference was found at 26 weeks in the number of patients with sustained pain improvement who had received 24 mg of steroid (P<.0123) compared with patients who received the lower doses. The authors didn’t report side effects or complications.
A prospective, noncomparative cohort study investigated 72 patients in a rheumatology clinic who hadn’t improved after at least 2 weeks of treatment with NSAIDs, analgesics, or ointments.3 Of the 59 patients who consented to steroid injections, 42 improved after 1 injection of 40 mg methylprednisolone with 2 mL of 2% lidocaine, 13 improved after a second injection 3 weeks later, and the remaining 4 improved after a third injection. Improvement was defined as disappearance of pain and disability. Six patients (8%) experienced a recurrence of bursitis during a 2-year follow-up period. No local or systemic complications were associated with the corticosteroids or anesthetic solution.
Two retrospective studies also documented the efficacy of corticosteroid injection. One investigated treatment of 36 patients in a rheumatology practice.4 All received methylprednisolone (40-80 mg) or triamcinolone (20-40 mg), and all improved. Two thirds of the patients were symptom free after 1 or 2 injections. Symptoms usually resolved within 2 days to several months (typically 1 or 2 weeks) postinjection. About 25% of the patients relapsed within 2 years.
Another retrospective cohort study of 164 British patients found that those who received a corticosteroid injection were 2.7 times more likely to have recovered at 5 years than patients who had not received an injection (odds ratio=0.4; 95% confidence interval, 0.1-1.0).1
When to consider surgery
Surgical treatment may be necessary for patients with refractory trochanteric bursitis. Several case studies5-7 demonstrate successful outcomes with a variety of surgical techniques, including trochanteric reduction osteotomy and iliotibial band release. Newer techniques involve arthroscopic bursectomy.
Recommendations
UpToDate8 recommends conservative treatment initially. For persistent cases, a corticosteroid injection should be given and repeated in 6 weeks if pain persists. Surgery may be considered if these measures don’t relieve symptoms and pain lasts longer than 1 year.
The American Academy of Orthopaedic Surgeons similarly recommends NSAIDs and activity modification followed by corticosteroid injection.9 Surgery is rarely indicated.
Conservative measures—followed by corticosteroid injection, if necessary—are best. Conservative therapy includes rest, nonsteroidal anti-inflammatory drugs (NSAIDs), and stretching exercises focused on the lower back and sacroiliac joints (strength of recommendation [SOR]: C, usual practice). Patients whose symptoms persist despite conservative therapy are likely to benefit from an injection of 24 mg betamethasone and 1% lidocaine (or equivalent) into the inflamed bursa (SOR: B, limited-quality, patient-oriented evidence).
In rare cases of intractable symptoms, surgical procedures such as iliotibial band release, subgluteal bursectomy, and trochanteric reduction osteotomy are options (SOR: C, case studies).
Evidence summary
Trochanteric bursitis is characterized by chronic intermittent lateral hip pain caused by inflammation of the trochanteric bursae. The bursae can become inflamed at the gluteus medius tendon, iliotibial tract, or gluteus minimus during repetitive flexing of the hip. Several conditions are associated with trochanteric bursitis (TABLE).
Trochanteric bursitis peaks in the fourth to sixth decades of life. One retrospective cohort study found the prevalence to be 1.8 cases per 1000 patients per year in primary care; 79% of cases occurred in women.1
TABLE
Conditions associated with trochanteric bursitis
| Chronic mechanical low back pain |
| Degenerative arthritis or disc disease of lower lumbar spine |
| Degenerative joint disease of knees |
| Fibromyalgia |
| Iliotibial band syndrome |
| Inflammatory arthritis of the hip |
| Ipsilateral or contralateral hip arthritis |
| Leg length discrepancy |
| Obesity |
| Pes planus |
| Tendonitis of external hip rotators |
| Total hip arthroplasty |
| Source: Lievense A et al. Br J Gen Pract. 2005.1 |
No studies have compared conservative treatments
Most review articles refer to initial treatment with rest, physical therapy, stretching, and NSAIDs. These treatments were described in textbooks and articles from the 1940s and 1950s.
No studies comparing conservative treatments were found. Few reports discuss physical therapy for trochanteric bursitis.
Corticosteroid injection has the best evidential support
Corticosteroid injection for treating trochanteric bursitis is supported by the best evidence in the available literature. No controlled trials have compared injection with placebo, however.
A randomized, prospective, open comparison trial at a rheumatology clinic assigned patients with trochanteric bursitis to 6-, 12-, or 24-mg doses of betamethasone mixed with 1% lidocaine.2 Seventy-seven percent of patients had improved at 1 week, 69% at 6 weeks, and 61% at 26 weeks. Notably, a significant difference was found at 26 weeks in the number of patients with sustained pain improvement who had received 24 mg of steroid (P<.0123) compared with patients who received the lower doses. The authors didn’t report side effects or complications.
A prospective, noncomparative cohort study investigated 72 patients in a rheumatology clinic who hadn’t improved after at least 2 weeks of treatment with NSAIDs, analgesics, or ointments.3 Of the 59 patients who consented to steroid injections, 42 improved after 1 injection of 40 mg methylprednisolone with 2 mL of 2% lidocaine, 13 improved after a second injection 3 weeks later, and the remaining 4 improved after a third injection. Improvement was defined as disappearance of pain and disability. Six patients (8%) experienced a recurrence of bursitis during a 2-year follow-up period. No local or systemic complications were associated with the corticosteroids or anesthetic solution.
Two retrospective studies also documented the efficacy of corticosteroid injection. One investigated treatment of 36 patients in a rheumatology practice.4 All received methylprednisolone (40-80 mg) or triamcinolone (20-40 mg), and all improved. Two thirds of the patients were symptom free after 1 or 2 injections. Symptoms usually resolved within 2 days to several months (typically 1 or 2 weeks) postinjection. About 25% of the patients relapsed within 2 years.
Another retrospective cohort study of 164 British patients found that those who received a corticosteroid injection were 2.7 times more likely to have recovered at 5 years than patients who had not received an injection (odds ratio=0.4; 95% confidence interval, 0.1-1.0).1
When to consider surgery
Surgical treatment may be necessary for patients with refractory trochanteric bursitis. Several case studies5-7 demonstrate successful outcomes with a variety of surgical techniques, including trochanteric reduction osteotomy and iliotibial band release. Newer techniques involve arthroscopic bursectomy.
Recommendations
UpToDate8 recommends conservative treatment initially. For persistent cases, a corticosteroid injection should be given and repeated in 6 weeks if pain persists. Surgery may be considered if these measures don’t relieve symptoms and pain lasts longer than 1 year.
The American Academy of Orthopaedic Surgeons similarly recommends NSAIDs and activity modification followed by corticosteroid injection.9 Surgery is rarely indicated.
1. Lievense A, Bierma-Zeinstra S. Prognosis of trochanteric pain in primary care. Br J Gen Pract. 2005;55:199-204.
2. Shbeeb M, O’Duffy D, Michet CJ, Jr, et al. Evaluation of glucocorticosteroid injection for the treatment of trochanteric bursitis. J Rheumatol. 1996;23:2104-2106.
3. Schapira D, Nahir M, Scharf Y. Trochanteric bursitis: a common clinical problem. Arch Phys Med Rehabil. 1986;67:815-817.
4. Rasmussen K, Fan N. Trochanteric bursitis: treatment by corticosteroid injection. Scand J Rheumatol. 1985;14:417-420.
5. Slawski D, Howard R. Surgical management of refractory trochanteric bursitis. Am J Sports Med. 1997;25:86-89.
6. Fox J. The role of arthroscopic bursectomy in the treatment of trochanteric bursitis. Arthroscopy. 2002;18:E34.-
7. Govaert L, van der Vis R, Marti RK, et al. Trochanteric reduction osteotomy as a treatment for refractory trochanteric bursitis. J Bone Joint Surg Br. 2003;85:199-203.
8. Anderson B. Trochanteric bursitis. UpToDate [online database]. Version 17.2. Waltham, Mass: UpToDate; 2009.
9. Trochanteric bursitis. In: Griffin LY. Essentials of Musculoskeletal Care. 3rd ed. Rosemont, IL: American Academy of Orthopaedic Surgeons; 2005:461–463.
1. Lievense A, Bierma-Zeinstra S. Prognosis of trochanteric pain in primary care. Br J Gen Pract. 2005;55:199-204.
2. Shbeeb M, O’Duffy D, Michet CJ, Jr, et al. Evaluation of glucocorticosteroid injection for the treatment of trochanteric bursitis. J Rheumatol. 1996;23:2104-2106.
3. Schapira D, Nahir M, Scharf Y. Trochanteric bursitis: a common clinical problem. Arch Phys Med Rehabil. 1986;67:815-817.
4. Rasmussen K, Fan N. Trochanteric bursitis: treatment by corticosteroid injection. Scand J Rheumatol. 1985;14:417-420.
5. Slawski D, Howard R. Surgical management of refractory trochanteric bursitis. Am J Sports Med. 1997;25:86-89.
6. Fox J. The role of arthroscopic bursectomy in the treatment of trochanteric bursitis. Arthroscopy. 2002;18:E34.-
7. Govaert L, van der Vis R, Marti RK, et al. Trochanteric reduction osteotomy as a treatment for refractory trochanteric bursitis. J Bone Joint Surg Br. 2003;85:199-203.
8. Anderson B. Trochanteric bursitis. UpToDate [online database]. Version 17.2. Waltham, Mass: UpToDate; 2009.
9. Trochanteric bursitis. In: Griffin LY. Essentials of Musculoskeletal Care. 3rd ed. Rosemont, IL: American Academy of Orthopaedic Surgeons; 2005:461–463.
Evidence-based answers from the Family Physicians Inquiries Network
What is the diagnostic approach to a patient with leg cramps?
Leg cramps are very common (strength of recommendation [SOR]: C, case series), and most cases have no detectable cause (SOR: C, expert opinion). Arterial vascular disease and neurological diseases are more prevalent among male patients with leg cramps (SOR: C, small case series).
History and physical should focus on detecting precipitating factors for iron deficiency anemia (gastrointestinal bleeding, frequent blood donations, menorrhagia), electrolyte imbalance (renal disease, fluid losses), endocrine disorders (thyroid, Addison’s disease), neuromuscular disorders (neuropathies and myopathies), and medication use (antidepressants and diuretics). Laboratory testing is guided by the history and physical and may include ferritin, electrolytes, blood sugar, magnesium, zinc, creatinine, blood urea nitrogen, liver function test, and thyroid-stimulating hormone (SOR: C, expert opinion and nonsystematic review).
If a thorough search reveals no cause, keep your patient educated
Timothy E. Huber, MD, LCDR, MC, USNR
Department of Family Medicine, Naval Hospital Camp Pendleton
Leg cramps are a common nonspecific complaint that can have a significant impact on quality of life. The literature on the potential causes and treatments of leg cramps is limited to small studies and expert opinion. This leaves the clinician on the spot with their own knowledge of medicine and their relationship with the patient. A careful history and physical may suggest some avenues of inquiry while simultaneously excluding other serious causes. Lab and radiology testing can be useful when used in a thoughtful manner. A confusing clinical picture has frustrated me when I was too aggressive with studies. If a thorough search reveals no specific cause, I attempt to keep my patient educated regarding possible complications while keeping my differential diagnosis broad when addressing this problem in future visits.
Evidence summary
More than two thirds of people aged >50 years have experienced leg cramps.1 Though leg cramps are common, little is known about their actual causation.2,3
A small, retrospective chart review, limited to male patients, identified an association of vascular and neurologic diseases among patients taking quinine, presumably for leg cramps.2 Although commonly idiopathic, leg cramps are sometimes associated with various disorders including endocrine, metabolic, occupational, structural, neuromuscular, vascular, and congenital disorders, as well as toxin- and drug-related causes (TABLE).4,5 All reviews suggest that the best diagnostic approach to leg cramps is a thorough history, and careful physical and neurological examination.1,3,4 The health care provider should clarify the onset and duration of leg cramps, any precipitating activity, and factors that provide relief. A detailed history should focus on precipitating factors for iron deficiency anemia (gastro-intestinal bleeding, frequent blood donations, menorrhagia), a history of renal disease (especially end-stage renal failure) and medication use (antidepressants and diuretics).
The physical examination should include a search for obvious physical signs of symptoms noted in the history.6 Neurological examination can exclude most disorders that simulate leg cramps such as contractures, dystonia, myalgia and peripheral neuropathy.1,2,4
The choice of laboratory investigations such as ferritin, electrolytes, blood sugar, magnesium, zinc, creatinine, blood urea nitrogen, liver function test, and thyroid function test are largely governed by the findings from the history and physical examination.1 Though neurophysiological research shows that true muscle cramps are caused by explosive hyperactivity of motor nerves, using diagnostic tools such as electromyography, muscle biopsy, and muscle enzymes are seldom needed.7
Because of the lack of well-designed, randomized controlled studies, this diagnostic approach is based on nonsystematic reviews, and may differ for individuals based on history and clinical examination.
TABLE
Possible causes of leg cramps
| CATEGORY | DISEASES |
|---|---|
| Congenital | McArdle’s disease, “Glycogen storage disease,” autosomal dominant cramping disease |
| Endocrine disorder | Thyroid disease, diabetes mellitus, Addison’s disease |
| Fluid and electrolyte disorder | Hypocalcemia, hyponatremia, hypomagnesemia, hypokalemia, hyperkalemia, chronic diarrhea, hemodialysis |
| Neuromuscular | Nerve root compression, motor neuron disease, mononeuropathies, polyneuropathies, dystonias |
| Drugs | Calcium channel blockers (nifedipine), diuretics, phenothiazines, fibrates, selective estrogen receptive modulators, ethanol, morphine withdrawal |
| Vascular | Peripheral vascular disease |
| Toxins | Lead or strychnine poisoning, spider bites |
| Occupational | Focal dystonias (in writers, athletes, miners, and musicians) |
| Others | Diarrhea, liver cirrhosis, chronic alcoholism, sarcoidosis |
| Hematological | Iron deficiency anemia |
| Modified from Kanaan and Sawaya, Geriatrics2001.3 | |
Recommendations from others
UpToDate states, “a careful history and examination can exclude the majority of disorders in the differential diagnosis” of leg cramps.7
1. Hall AJ. Cramp and salt balance in ordinary life. Lancet 1947;3:231-233.
2. Haskell SG, Fiebach NH. Clinical epidemiology of nocturnal leg cramps in male veterans. Am J Med Sci 1997;313:210-214.
3. Kanaan N, Sawaya R. Nocturnal leg cramps. Clinically mysterious and painful—but manageable. Geriatrics 2001;56:34, 39-42.
4. Butler JV, Mulkerrin EC, O’Keeffe ST. Nocturnal leg cramps in older people. Postgrad Med J 2002;78:596-598
5. Riley JD, Antony SJ. Leg cramps: differential diagnosis and management. Am Fam Physician 1995;52:1794-1798.
6. Jansen PH, Joosten EM, Vingerhoets HM. Clinical diagnosis of muscle cramp and muscular cramp syndrome. Eur Arch Psychiatry Clin Neurosci 1991;241:98-101.
7. Sheon RP. Nocturnal leg cramps, night starts, and nocturnal myoclonus. UpToDate, version 13.1. Wellesley, Mass: UpToDate. Last updated December 2004.
Leg cramps are very common (strength of recommendation [SOR]: C, case series), and most cases have no detectable cause (SOR: C, expert opinion). Arterial vascular disease and neurological diseases are more prevalent among male patients with leg cramps (SOR: C, small case series).
History and physical should focus on detecting precipitating factors for iron deficiency anemia (gastrointestinal bleeding, frequent blood donations, menorrhagia), electrolyte imbalance (renal disease, fluid losses), endocrine disorders (thyroid, Addison’s disease), neuromuscular disorders (neuropathies and myopathies), and medication use (antidepressants and diuretics). Laboratory testing is guided by the history and physical and may include ferritin, electrolytes, blood sugar, magnesium, zinc, creatinine, blood urea nitrogen, liver function test, and thyroid-stimulating hormone (SOR: C, expert opinion and nonsystematic review).
If a thorough search reveals no cause, keep your patient educated
Timothy E. Huber, MD, LCDR, MC, USNR
Department of Family Medicine, Naval Hospital Camp Pendleton
Leg cramps are a common nonspecific complaint that can have a significant impact on quality of life. The literature on the potential causes and treatments of leg cramps is limited to small studies and expert opinion. This leaves the clinician on the spot with their own knowledge of medicine and their relationship with the patient. A careful history and physical may suggest some avenues of inquiry while simultaneously excluding other serious causes. Lab and radiology testing can be useful when used in a thoughtful manner. A confusing clinical picture has frustrated me when I was too aggressive with studies. If a thorough search reveals no specific cause, I attempt to keep my patient educated regarding possible complications while keeping my differential diagnosis broad when addressing this problem in future visits.
Evidence summary
More than two thirds of people aged >50 years have experienced leg cramps.1 Though leg cramps are common, little is known about their actual causation.2,3
A small, retrospective chart review, limited to male patients, identified an association of vascular and neurologic diseases among patients taking quinine, presumably for leg cramps.2 Although commonly idiopathic, leg cramps are sometimes associated with various disorders including endocrine, metabolic, occupational, structural, neuromuscular, vascular, and congenital disorders, as well as toxin- and drug-related causes (TABLE).4,5 All reviews suggest that the best diagnostic approach to leg cramps is a thorough history, and careful physical and neurological examination.1,3,4 The health care provider should clarify the onset and duration of leg cramps, any precipitating activity, and factors that provide relief. A detailed history should focus on precipitating factors for iron deficiency anemia (gastro-intestinal bleeding, frequent blood donations, menorrhagia), a history of renal disease (especially end-stage renal failure) and medication use (antidepressants and diuretics).
The physical examination should include a search for obvious physical signs of symptoms noted in the history.6 Neurological examination can exclude most disorders that simulate leg cramps such as contractures, dystonia, myalgia and peripheral neuropathy.1,2,4
The choice of laboratory investigations such as ferritin, electrolytes, blood sugar, magnesium, zinc, creatinine, blood urea nitrogen, liver function test, and thyroid function test are largely governed by the findings from the history and physical examination.1 Though neurophysiological research shows that true muscle cramps are caused by explosive hyperactivity of motor nerves, using diagnostic tools such as electromyography, muscle biopsy, and muscle enzymes are seldom needed.7
Because of the lack of well-designed, randomized controlled studies, this diagnostic approach is based on nonsystematic reviews, and may differ for individuals based on history and clinical examination.
TABLE
Possible causes of leg cramps
| CATEGORY | DISEASES |
|---|---|
| Congenital | McArdle’s disease, “Glycogen storage disease,” autosomal dominant cramping disease |
| Endocrine disorder | Thyroid disease, diabetes mellitus, Addison’s disease |
| Fluid and electrolyte disorder | Hypocalcemia, hyponatremia, hypomagnesemia, hypokalemia, hyperkalemia, chronic diarrhea, hemodialysis |
| Neuromuscular | Nerve root compression, motor neuron disease, mononeuropathies, polyneuropathies, dystonias |
| Drugs | Calcium channel blockers (nifedipine), diuretics, phenothiazines, fibrates, selective estrogen receptive modulators, ethanol, morphine withdrawal |
| Vascular | Peripheral vascular disease |
| Toxins | Lead or strychnine poisoning, spider bites |
| Occupational | Focal dystonias (in writers, athletes, miners, and musicians) |
| Others | Diarrhea, liver cirrhosis, chronic alcoholism, sarcoidosis |
| Hematological | Iron deficiency anemia |
| Modified from Kanaan and Sawaya, Geriatrics2001.3 | |
Recommendations from others
UpToDate states, “a careful history and examination can exclude the majority of disorders in the differential diagnosis” of leg cramps.7
Leg cramps are very common (strength of recommendation [SOR]: C, case series), and most cases have no detectable cause (SOR: C, expert opinion). Arterial vascular disease and neurological diseases are more prevalent among male patients with leg cramps (SOR: C, small case series).
History and physical should focus on detecting precipitating factors for iron deficiency anemia (gastrointestinal bleeding, frequent blood donations, menorrhagia), electrolyte imbalance (renal disease, fluid losses), endocrine disorders (thyroid, Addison’s disease), neuromuscular disorders (neuropathies and myopathies), and medication use (antidepressants and diuretics). Laboratory testing is guided by the history and physical and may include ferritin, electrolytes, blood sugar, magnesium, zinc, creatinine, blood urea nitrogen, liver function test, and thyroid-stimulating hormone (SOR: C, expert opinion and nonsystematic review).
If a thorough search reveals no cause, keep your patient educated
Timothy E. Huber, MD, LCDR, MC, USNR
Department of Family Medicine, Naval Hospital Camp Pendleton
Leg cramps are a common nonspecific complaint that can have a significant impact on quality of life. The literature on the potential causes and treatments of leg cramps is limited to small studies and expert opinion. This leaves the clinician on the spot with their own knowledge of medicine and their relationship with the patient. A careful history and physical may suggest some avenues of inquiry while simultaneously excluding other serious causes. Lab and radiology testing can be useful when used in a thoughtful manner. A confusing clinical picture has frustrated me when I was too aggressive with studies. If a thorough search reveals no specific cause, I attempt to keep my patient educated regarding possible complications while keeping my differential diagnosis broad when addressing this problem in future visits.
Evidence summary
More than two thirds of people aged >50 years have experienced leg cramps.1 Though leg cramps are common, little is known about their actual causation.2,3
A small, retrospective chart review, limited to male patients, identified an association of vascular and neurologic diseases among patients taking quinine, presumably for leg cramps.2 Although commonly idiopathic, leg cramps are sometimes associated with various disorders including endocrine, metabolic, occupational, structural, neuromuscular, vascular, and congenital disorders, as well as toxin- and drug-related causes (TABLE).4,5 All reviews suggest that the best diagnostic approach to leg cramps is a thorough history, and careful physical and neurological examination.1,3,4 The health care provider should clarify the onset and duration of leg cramps, any precipitating activity, and factors that provide relief. A detailed history should focus on precipitating factors for iron deficiency anemia (gastro-intestinal bleeding, frequent blood donations, menorrhagia), a history of renal disease (especially end-stage renal failure) and medication use (antidepressants and diuretics).
The physical examination should include a search for obvious physical signs of symptoms noted in the history.6 Neurological examination can exclude most disorders that simulate leg cramps such as contractures, dystonia, myalgia and peripheral neuropathy.1,2,4
The choice of laboratory investigations such as ferritin, electrolytes, blood sugar, magnesium, zinc, creatinine, blood urea nitrogen, liver function test, and thyroid function test are largely governed by the findings from the history and physical examination.1 Though neurophysiological research shows that true muscle cramps are caused by explosive hyperactivity of motor nerves, using diagnostic tools such as electromyography, muscle biopsy, and muscle enzymes are seldom needed.7
Because of the lack of well-designed, randomized controlled studies, this diagnostic approach is based on nonsystematic reviews, and may differ for individuals based on history and clinical examination.
TABLE
Possible causes of leg cramps
| CATEGORY | DISEASES |
|---|---|
| Congenital | McArdle’s disease, “Glycogen storage disease,” autosomal dominant cramping disease |
| Endocrine disorder | Thyroid disease, diabetes mellitus, Addison’s disease |
| Fluid and electrolyte disorder | Hypocalcemia, hyponatremia, hypomagnesemia, hypokalemia, hyperkalemia, chronic diarrhea, hemodialysis |
| Neuromuscular | Nerve root compression, motor neuron disease, mononeuropathies, polyneuropathies, dystonias |
| Drugs | Calcium channel blockers (nifedipine), diuretics, phenothiazines, fibrates, selective estrogen receptive modulators, ethanol, morphine withdrawal |
| Vascular | Peripheral vascular disease |
| Toxins | Lead or strychnine poisoning, spider bites |
| Occupational | Focal dystonias (in writers, athletes, miners, and musicians) |
| Others | Diarrhea, liver cirrhosis, chronic alcoholism, sarcoidosis |
| Hematological | Iron deficiency anemia |
| Modified from Kanaan and Sawaya, Geriatrics2001.3 | |
Recommendations from others
UpToDate states, “a careful history and examination can exclude the majority of disorders in the differential diagnosis” of leg cramps.7
1. Hall AJ. Cramp and salt balance in ordinary life. Lancet 1947;3:231-233.
2. Haskell SG, Fiebach NH. Clinical epidemiology of nocturnal leg cramps in male veterans. Am J Med Sci 1997;313:210-214.
3. Kanaan N, Sawaya R. Nocturnal leg cramps. Clinically mysterious and painful—but manageable. Geriatrics 2001;56:34, 39-42.
4. Butler JV, Mulkerrin EC, O’Keeffe ST. Nocturnal leg cramps in older people. Postgrad Med J 2002;78:596-598
5. Riley JD, Antony SJ. Leg cramps: differential diagnosis and management. Am Fam Physician 1995;52:1794-1798.
6. Jansen PH, Joosten EM, Vingerhoets HM. Clinical diagnosis of muscle cramp and muscular cramp syndrome. Eur Arch Psychiatry Clin Neurosci 1991;241:98-101.
7. Sheon RP. Nocturnal leg cramps, night starts, and nocturnal myoclonus. UpToDate, version 13.1. Wellesley, Mass: UpToDate. Last updated December 2004.
1. Hall AJ. Cramp and salt balance in ordinary life. Lancet 1947;3:231-233.
2. Haskell SG, Fiebach NH. Clinical epidemiology of nocturnal leg cramps in male veterans. Am J Med Sci 1997;313:210-214.
3. Kanaan N, Sawaya R. Nocturnal leg cramps. Clinically mysterious and painful—but manageable. Geriatrics 2001;56:34, 39-42.
4. Butler JV, Mulkerrin EC, O’Keeffe ST. Nocturnal leg cramps in older people. Postgrad Med J 2002;78:596-598
5. Riley JD, Antony SJ. Leg cramps: differential diagnosis and management. Am Fam Physician 1995;52:1794-1798.
6. Jansen PH, Joosten EM, Vingerhoets HM. Clinical diagnosis of muscle cramp and muscular cramp syndrome. Eur Arch Psychiatry Clin Neurosci 1991;241:98-101.
7. Sheon RP. Nocturnal leg cramps, night starts, and nocturnal myoclonus. UpToDate, version 13.1. Wellesley, Mass: UpToDate. Last updated December 2004.
Evidence-based answers from the Family Physicians Inquiries Network
Is an outpatient workup safe for patients with a transient ischemic attack?
There is no compelling evidence that outpatient diagnostic workup of patients with transient ischemic attack (TIA) is less safe than inpatient workup, or that hospitalization prevents stroke or improves stroke outcomes after TIA (strength of recommendation [SOR]: C, based on case series studies). Because the risk of stroke is substantial in the week following a TIA (SOR: A, based on a prospective cohort study), evaluation and treatment for reversible stroke risk factors should be initiated urgently and completed within a week of initial presentation (SOR: C, based on expert consensus opinion).
Risk factors for patients at highest risk for stroke or other cardiovascular events after TIA include age >60 years, diabetes, TIA lasting longer than 10 minutes, and a TIA associated with weakness or speech impairment (SOR: B, based on retrospective cohort study). Hospitalization may be prudent for patients at high risk for cardiovascular events or for those with mental status changes, an inadequate home situation, or the physician’s inability to obtain expedient evaluation (SOR: C, based on case series studies).
Evidence summary
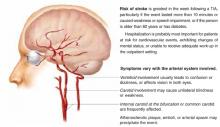
Transient ischemic attack (Figure) is a temporary, focal brain or retinal deficit caused by vascular disease that clears completely in less than 24 hours.1 A large prospective cohort study recently estimated the risk of stroke after a TIA or minor stroke to be 8% to 12% at 7 days and 11% to 15% at 1 month.2
In a large retrospective cohort study, 5% of TIA patients returned to the emergency department with a stroke within the first 2 days after TIA.3 Another 6% returned with a stroke within 90 days. Five independent risk factors were identified: age >60 years, diabetes mellitus, duration of TIA longer than 10 minutes, signs or symptoms of weakness, and speech impairment. Thirty-four percent of patients with all 5 risk factors, and none of the patients without any risk factors, had a stroke within 90 days. Of note, 13% of the TIA patients had an arrhythmia, congestive heart failure, unstable angina, myocardial infarction, stroke, or recurrent TIA within 4 days of initial presenting with a TIA. Twenty-five percent of the patients experienced 1 of these cardiovascular events during the 3 months of follow-up.
In a retrospective case review of TIA and stroke patients, the hospital admissions of 4 of 21 TIA patients were retrospectively categorized as medically justified.4 Admission was categorized as medically justified if the patient had 1 or more of the following criteria: another diagnosis that warranted admission, inadequate home situation, altered mental status, an adverse event during hospitalization including worsening of the deficit, and if the patient underwent some hospital-based treatment that could not be provided on an out-patient basis. Ease and rapidity of evaluation was not considered medically justifiable and outcome improvement (stroke prevention) was not studied.
Two retrospective chart reviews of TIA found considerable practice variability in the evaluation of TIA patient. In 1 study of TIA patients presenting to an emergency department, 81% had a computed tomography scan, 75% had electrocardiogram, and 74% had a complete blood count.5 Carotid Doppler imaging was performed in the emergency department in 16%, and 26% were referred for outpatient Doppler studies. One percent had an ECG in the emergency department, and 16% were given ECGs as outpatients. Seventy-five percent of patients were discharged home. Those hospitalized had a median length of stay of 1 day. In the second study, 31% of the TIA patients had no diagnostic studies performed during the first month after presenting to their primary care physician.6
FIGURE
Expeditious evaluation of TIA is imperative
Recommendations from others
The American Heart Association (AHA) recommends that physicians use a stepwise approach to TIA evaluation as outlined in the Table. The AHA also recommends that the diagnostic evaluation of patients seen within 7 days of a TIA should be completed within 1 week or less. The AHA leaves the decision whether to hospitalize a patient up to the physician based on a patient’s circumstances. The goals of diagnostic testing are to identify or exclude causes of TIA requiring specific therapy, to assess modifiable risk factors, and to determine prognosis.7
The National Stroke Association recommends that patients with known high-grade stenosis in a vascular territory appropriate to the symptoms, and patients with recurrent symptoms, undergo urgent evaluation. Evaluation includes imaging and ruling out other causes of TIA. Patients should be admitted to the hospital if imaging is not immediately available. If indicated, carotid endarterectomy should be performed without delay.8
TABLE
Stepwise diagnostic evaluation for patients with transient ischemic attack
Initial Evaluation
|
Second step (to resolve persistent diagnostic uncertainty as appropriate)
|
| Adapted from Feinberg et al 1994.7 |
Make the patient aware of the risks of TIA and quickly complete the work-up
Jon O. Neher, MD
Valley Medical Center, Renton, Wash
It is important to remember that a diagnosis of TIA can only be made retrospectively. All patients with ongoing focal neurologic signs must be evaluated immediately and (if the symptom duration is less than 3 hours) considered potential candidates for emergent thrombolytic therapy.
The vast majority of TIA patients are asymptomatic during their evaluation. Because they feel well and may have a considerable element of denial, it can be hard to get them to rapidly complete their evaluation in either the inpatient or outpatient setting. It is therefore critical that the patient be made aware that the highest risk period is soon after the TIA and that failure to quickly complete the work-up could have serious negative consequences.
1. Levy DE. How transient are transient ischemic attacks? Neurology 1988;38:674-677.
2. Coull AJ, Lovett JK, Rothwell PM. Oxford Vascular Study. Population based study of early risk of stroke after transient ischaemic attack or minor stroke: implications for public education and organisation of services. BMJ 2004;328:326.-
3. Johnston SC, Gress DR, Browner WS, Sidney S. Short-term prognosis after emergency department diagnosis of TIA. JAMA 2000;284:2901-2906.
4. Henneman PL, Lewis RJ. Is admission medically justified for all patients with acute stroke or transient ischemic attack? Ann Emerg Med 1995;25:458-463.
5. Chang E, Holroyd BR, Kochanski P, Kelly KD, Shuaib A, Rowe BH. Adherence to practice guidelines for transient ischemic attacks in an emergency department. Can J Neurol Sci 2002;29:358-363.
6. Goldstein LB, Bian J, Samsa GP, Bonito AJ, Lux LJ, Matchar DB. New transient ischemic attack and stroke: outpatient management by primary care physicians. Arch Intern Med 2000;160:2941-2946.
7. Feinberg WM, Albers GW, Barnett HJ, et al. Guidelines for the management of transient ischemic attacks. From the Ad Hoc Committee of Guidelines for the Management of Transient Ischemic Attacks of the Stroke Council of the American Heart Association. Circulation 1994;89:2950-2965.
8. Brott TG, Clark WM, Fagan SC, et al. Stroke: The First Hours: Guidelines for Acute Treatment. Englewood, Colo: National Stroke Association; 2000.
There is no compelling evidence that outpatient diagnostic workup of patients with transient ischemic attack (TIA) is less safe than inpatient workup, or that hospitalization prevents stroke or improves stroke outcomes after TIA (strength of recommendation [SOR]: C, based on case series studies). Because the risk of stroke is substantial in the week following a TIA (SOR: A, based on a prospective cohort study), evaluation and treatment for reversible stroke risk factors should be initiated urgently and completed within a week of initial presentation (SOR: C, based on expert consensus opinion).
Risk factors for patients at highest risk for stroke or other cardiovascular events after TIA include age >60 years, diabetes, TIA lasting longer than 10 minutes, and a TIA associated with weakness or speech impairment (SOR: B, based on retrospective cohort study). Hospitalization may be prudent for patients at high risk for cardiovascular events or for those with mental status changes, an inadequate home situation, or the physician’s inability to obtain expedient evaluation (SOR: C, based on case series studies).
Evidence summary
Transient ischemic attack (Figure) is a temporary, focal brain or retinal deficit caused by vascular disease that clears completely in less than 24 hours.1 A large prospective cohort study recently estimated the risk of stroke after a TIA or minor stroke to be 8% to 12% at 7 days and 11% to 15% at 1 month.2
In a large retrospective cohort study, 5% of TIA patients returned to the emergency department with a stroke within the first 2 days after TIA.3 Another 6% returned with a stroke within 90 days. Five independent risk factors were identified: age >60 years, diabetes mellitus, duration of TIA longer than 10 minutes, signs or symptoms of weakness, and speech impairment. Thirty-four percent of patients with all 5 risk factors, and none of the patients without any risk factors, had a stroke within 90 days. Of note, 13% of the TIA patients had an arrhythmia, congestive heart failure, unstable angina, myocardial infarction, stroke, or recurrent TIA within 4 days of initial presenting with a TIA. Twenty-five percent of the patients experienced 1 of these cardiovascular events during the 3 months of follow-up.
In a retrospective case review of TIA and stroke patients, the hospital admissions of 4 of 21 TIA patients were retrospectively categorized as medically justified.4 Admission was categorized as medically justified if the patient had 1 or more of the following criteria: another diagnosis that warranted admission, inadequate home situation, altered mental status, an adverse event during hospitalization including worsening of the deficit, and if the patient underwent some hospital-based treatment that could not be provided on an out-patient basis. Ease and rapidity of evaluation was not considered medically justifiable and outcome improvement (stroke prevention) was not studied.
Two retrospective chart reviews of TIA found considerable practice variability in the evaluation of TIA patient. In 1 study of TIA patients presenting to an emergency department, 81% had a computed tomography scan, 75% had electrocardiogram, and 74% had a complete blood count.5 Carotid Doppler imaging was performed in the emergency department in 16%, and 26% were referred for outpatient Doppler studies. One percent had an ECG in the emergency department, and 16% were given ECGs as outpatients. Seventy-five percent of patients were discharged home. Those hospitalized had a median length of stay of 1 day. In the second study, 31% of the TIA patients had no diagnostic studies performed during the first month after presenting to their primary care physician.6
FIGURE
Expeditious evaluation of TIA is imperative
Recommendations from others
The American Heart Association (AHA) recommends that physicians use a stepwise approach to TIA evaluation as outlined in the Table. The AHA also recommends that the diagnostic evaluation of patients seen within 7 days of a TIA should be completed within 1 week or less. The AHA leaves the decision whether to hospitalize a patient up to the physician based on a patient’s circumstances. The goals of diagnostic testing are to identify or exclude causes of TIA requiring specific therapy, to assess modifiable risk factors, and to determine prognosis.7
The National Stroke Association recommends that patients with known high-grade stenosis in a vascular territory appropriate to the symptoms, and patients with recurrent symptoms, undergo urgent evaluation. Evaluation includes imaging and ruling out other causes of TIA. Patients should be admitted to the hospital if imaging is not immediately available. If indicated, carotid endarterectomy should be performed without delay.8
TABLE
Stepwise diagnostic evaluation for patients with transient ischemic attack
Initial Evaluation
|
Second step (to resolve persistent diagnostic uncertainty as appropriate)
|
| Adapted from Feinberg et al 1994.7 |
Make the patient aware of the risks of TIA and quickly complete the work-up
Jon O. Neher, MD
Valley Medical Center, Renton, Wash
It is important to remember that a diagnosis of TIA can only be made retrospectively. All patients with ongoing focal neurologic signs must be evaluated immediately and (if the symptom duration is less than 3 hours) considered potential candidates for emergent thrombolytic therapy.
The vast majority of TIA patients are asymptomatic during their evaluation. Because they feel well and may have a considerable element of denial, it can be hard to get them to rapidly complete their evaluation in either the inpatient or outpatient setting. It is therefore critical that the patient be made aware that the highest risk period is soon after the TIA and that failure to quickly complete the work-up could have serious negative consequences.
There is no compelling evidence that outpatient diagnostic workup of patients with transient ischemic attack (TIA) is less safe than inpatient workup, or that hospitalization prevents stroke or improves stroke outcomes after TIA (strength of recommendation [SOR]: C, based on case series studies). Because the risk of stroke is substantial in the week following a TIA (SOR: A, based on a prospective cohort study), evaluation and treatment for reversible stroke risk factors should be initiated urgently and completed within a week of initial presentation (SOR: C, based on expert consensus opinion).
Risk factors for patients at highest risk for stroke or other cardiovascular events after TIA include age >60 years, diabetes, TIA lasting longer than 10 minutes, and a TIA associated with weakness or speech impairment (SOR: B, based on retrospective cohort study). Hospitalization may be prudent for patients at high risk for cardiovascular events or for those with mental status changes, an inadequate home situation, or the physician’s inability to obtain expedient evaluation (SOR: C, based on case series studies).
Evidence summary
Transient ischemic attack (Figure) is a temporary, focal brain or retinal deficit caused by vascular disease that clears completely in less than 24 hours.1 A large prospective cohort study recently estimated the risk of stroke after a TIA or minor stroke to be 8% to 12% at 7 days and 11% to 15% at 1 month.2
In a large retrospective cohort study, 5% of TIA patients returned to the emergency department with a stroke within the first 2 days after TIA.3 Another 6% returned with a stroke within 90 days. Five independent risk factors were identified: age >60 years, diabetes mellitus, duration of TIA longer than 10 minutes, signs or symptoms of weakness, and speech impairment. Thirty-four percent of patients with all 5 risk factors, and none of the patients without any risk factors, had a stroke within 90 days. Of note, 13% of the TIA patients had an arrhythmia, congestive heart failure, unstable angina, myocardial infarction, stroke, or recurrent TIA within 4 days of initial presenting with a TIA. Twenty-five percent of the patients experienced 1 of these cardiovascular events during the 3 months of follow-up.
In a retrospective case review of TIA and stroke patients, the hospital admissions of 4 of 21 TIA patients were retrospectively categorized as medically justified.4 Admission was categorized as medically justified if the patient had 1 or more of the following criteria: another diagnosis that warranted admission, inadequate home situation, altered mental status, an adverse event during hospitalization including worsening of the deficit, and if the patient underwent some hospital-based treatment that could not be provided on an out-patient basis. Ease and rapidity of evaluation was not considered medically justifiable and outcome improvement (stroke prevention) was not studied.
Two retrospective chart reviews of TIA found considerable practice variability in the evaluation of TIA patient. In 1 study of TIA patients presenting to an emergency department, 81% had a computed tomography scan, 75% had electrocardiogram, and 74% had a complete blood count.5 Carotid Doppler imaging was performed in the emergency department in 16%, and 26% were referred for outpatient Doppler studies. One percent had an ECG in the emergency department, and 16% were given ECGs as outpatients. Seventy-five percent of patients were discharged home. Those hospitalized had a median length of stay of 1 day. In the second study, 31% of the TIA patients had no diagnostic studies performed during the first month after presenting to their primary care physician.6
FIGURE
Expeditious evaluation of TIA is imperative
Recommendations from others
The American Heart Association (AHA) recommends that physicians use a stepwise approach to TIA evaluation as outlined in the Table. The AHA also recommends that the diagnostic evaluation of patients seen within 7 days of a TIA should be completed within 1 week or less. The AHA leaves the decision whether to hospitalize a patient up to the physician based on a patient’s circumstances. The goals of diagnostic testing are to identify or exclude causes of TIA requiring specific therapy, to assess modifiable risk factors, and to determine prognosis.7
The National Stroke Association recommends that patients with known high-grade stenosis in a vascular territory appropriate to the symptoms, and patients with recurrent symptoms, undergo urgent evaluation. Evaluation includes imaging and ruling out other causes of TIA. Patients should be admitted to the hospital if imaging is not immediately available. If indicated, carotid endarterectomy should be performed without delay.8
TABLE
Stepwise diagnostic evaluation for patients with transient ischemic attack
Initial Evaluation
|
Second step (to resolve persistent diagnostic uncertainty as appropriate)
|
| Adapted from Feinberg et al 1994.7 |
Make the patient aware of the risks of TIA and quickly complete the work-up
Jon O. Neher, MD
Valley Medical Center, Renton, Wash
It is important to remember that a diagnosis of TIA can only be made retrospectively. All patients with ongoing focal neurologic signs must be evaluated immediately and (if the symptom duration is less than 3 hours) considered potential candidates for emergent thrombolytic therapy.
The vast majority of TIA patients are asymptomatic during their evaluation. Because they feel well and may have a considerable element of denial, it can be hard to get them to rapidly complete their evaluation in either the inpatient or outpatient setting. It is therefore critical that the patient be made aware that the highest risk period is soon after the TIA and that failure to quickly complete the work-up could have serious negative consequences.
1. Levy DE. How transient are transient ischemic attacks? Neurology 1988;38:674-677.
2. Coull AJ, Lovett JK, Rothwell PM. Oxford Vascular Study. Population based study of early risk of stroke after transient ischaemic attack or minor stroke: implications for public education and organisation of services. BMJ 2004;328:326.-
3. Johnston SC, Gress DR, Browner WS, Sidney S. Short-term prognosis after emergency department diagnosis of TIA. JAMA 2000;284:2901-2906.
4. Henneman PL, Lewis RJ. Is admission medically justified for all patients with acute stroke or transient ischemic attack? Ann Emerg Med 1995;25:458-463.
5. Chang E, Holroyd BR, Kochanski P, Kelly KD, Shuaib A, Rowe BH. Adherence to practice guidelines for transient ischemic attacks in an emergency department. Can J Neurol Sci 2002;29:358-363.
6. Goldstein LB, Bian J, Samsa GP, Bonito AJ, Lux LJ, Matchar DB. New transient ischemic attack and stroke: outpatient management by primary care physicians. Arch Intern Med 2000;160:2941-2946.
7. Feinberg WM, Albers GW, Barnett HJ, et al. Guidelines for the management of transient ischemic attacks. From the Ad Hoc Committee of Guidelines for the Management of Transient Ischemic Attacks of the Stroke Council of the American Heart Association. Circulation 1994;89:2950-2965.
8. Brott TG, Clark WM, Fagan SC, et al. Stroke: The First Hours: Guidelines for Acute Treatment. Englewood, Colo: National Stroke Association; 2000.
1. Levy DE. How transient are transient ischemic attacks? Neurology 1988;38:674-677.
2. Coull AJ, Lovett JK, Rothwell PM. Oxford Vascular Study. Population based study of early risk of stroke after transient ischaemic attack or minor stroke: implications for public education and organisation of services. BMJ 2004;328:326.-
3. Johnston SC, Gress DR, Browner WS, Sidney S. Short-term prognosis after emergency department diagnosis of TIA. JAMA 2000;284:2901-2906.
4. Henneman PL, Lewis RJ. Is admission medically justified for all patients with acute stroke or transient ischemic attack? Ann Emerg Med 1995;25:458-463.
5. Chang E, Holroyd BR, Kochanski P, Kelly KD, Shuaib A, Rowe BH. Adherence to practice guidelines for transient ischemic attacks in an emergency department. Can J Neurol Sci 2002;29:358-363.
6. Goldstein LB, Bian J, Samsa GP, Bonito AJ, Lux LJ, Matchar DB. New transient ischemic attack and stroke: outpatient management by primary care physicians. Arch Intern Med 2000;160:2941-2946.
7. Feinberg WM, Albers GW, Barnett HJ, et al. Guidelines for the management of transient ischemic attacks. From the Ad Hoc Committee of Guidelines for the Management of Transient Ischemic Attacks of the Stroke Council of the American Heart Association. Circulation 1994;89:2950-2965.
8. Brott TG, Clark WM, Fagan SC, et al. Stroke: The First Hours: Guidelines for Acute Treatment. Englewood, Colo: National Stroke Association; 2000.
Evidence-based answers from the Family Physicians Inquiries Network
Does warfarin prevent deep venous thrombosis in high-risk patients?
Warfarin (Coumadin) is effective in preventing deep venous thrombosis (DVT) among patients with a history of DVT. Conventional dosing and longer durations are the most effective, but the ideal length of therapy is unknown (strength of recommendation [SOR]: A, based on large randomized controlled trials and meta-analysis).
Warfarin is useful in preventing DVT in patients with cancer, specifically those treated with chemotherapy (SOR: B, based on small randomized controlled trials). Warfarin may be effective in pre-venting DVT in immobilized patients such as those with trauma, spinal cord injury, or stroke (SOR: B, based on an underpowered randomized controlled trial and uncontrolled studies).
Evidence summary
Warfarin, at both low and conventional doses, has been shown to be effective in preventing recurrence of DVT. A large, 4-year placebo-controlled randomized controlled trial showed that long-term low-dose warfarin (international normalized ratio [INR], 1.5-1.9) was more effective than placebo for prevention of DVT (hazard ratio=0.36; 95% confidence interval [CI], 0.19-0.67).1
A double-blind randomized controlled trial of 738 patients demonstrated that conventional-intensity warfarin therapy (INR=2.0-3.0) was more effective than low-intensity therapy (INR=1.5-1.9) in prevention of recurrent DVT. There were 1.9 vs 0.7 DVTs per 100 person-years in the low-intensity vs conventional-intensity therapy groups (hazard ratio=2.8; 95% CI, 1.1-7.0; number needed to treat [NNT]=37). No significant difference was seen in the frequency of bleeding complications between the groups.2 This and other studies suggest that low-intensity warfarin therapy reduces the relative risk of thrombosis by about 75%, and conventional-intensity therapy reduces this risk by over 90%.2
Several studies have examined the duration of warfarin therapy. A meta-analysis found treatment with warfarin for 12 to 24 weeks decreased DVT recurrence compared with 2- to 6-week regimens (relative risk [RR]=0.60; 95% CI, 0.45-0.79; NNT=21).3 A multicenter randomized controlled trial found extending warfarin treatment for 12 months vs 3 months resulted in a 95% relative risk reduction (RRR) in risk of DVT recurrence (95% CI, 63-99; NNT=5).4 A multicenter randomized trial showed similar results, but risk for recurrence was the same after treatment was stopped, regardless of the length of treatment.5
In patients with cancer, warfarin was shown to be more effective than placebo in prevention of DVT. In a trial of 311 breast cancer patients receiving chemotherapy, treatment with very-low-dose warfarin (INR=1.3-1.9) decreased thrombotic events compared with placebo, with no increase in bleeding complications (RRR=85%; P=.031; NNT=27).6 A later cost analysis showed that very-low-dose warfarin can be used in prevention of DVT in breast cancer patients on chemotherapy without an increase in health care costs.7
Although immobilized patients are at high risk for DVT, no randomized controlled trials exist for the use of warfarin in these patients. A few small studies suggest that warfarin reduces DVT rates in spinal-cord-injured patients.8 A small trial randomized stroke patients undergoing rehabilitation to placebo or fixed 1- or 2-mg doses of warfarin. This underpowered study showed a nonsignificant decrease in the risk of development of DVT (RR=0.39; 95% CI, 0.13-1.37).8
Recommendations from others
The 6th American College of Chest Physicians Consensus Conference on Antithrombotic Therapy makes these recommendations:9
Prior DVT: Oral anticoagulation therapy (INR=2.0-3.0) is indicated for at least 3 months for patients with proximal DVT or for at least 6 months in those with idiopathic proximal vein thrombosis or recurrent venous thrombosis. Indefinite anticoagulation is indicated for patients with more than 1 episode of idiopathic proximal vein thrombosis or pulmonary embolus.
Malignancy: Indefinite anticoagulation (INR= 2.0-3.0) is indicated for patients with thrombosis complicating malignancy. Prophylaxis with low-intensity warfarin in ambulatory patients with cancer to prevent initial DVT warrants further study.
Acute spinal cord injuries: Low-molecular-weight heparin or switch to full-dose oral anti-coagulation (INR=2.0-3.0) for the duration of the rehabilitation phase.
Routine prophylaxis dramatically reduces DVT cases
John P. Langlois, MD
MAHEC Family Practice Residency, Asheville, NC
I can clearly recall the dramatic reduction in the number of our patients who developed DVT when our orthopedic colleagues embraced routine prophylaxis for the high-risk surgical patients with hip surgery and knee replacements. This answer indicates that we may also be able to reduce the risk of DVT in our high-risk nonsurgical patients with previous DVT or breast cancer. Note that much of the evidence is based on the use of low-dose and very-low-dose warfarin. This may help mitigate our fear of substituting bleeding complications for the prevention of clots.
1. Ridker PM, Goldhaber SZ, Danielson E, et al. Long-term, low-intensity warfarin therapy for the prevention of recurrent venous thromboembolism. N Engl J Med 2003;348:1425-1434.
2. Kearon C, Ginsberg JS, Kovacs MJ, et al. Comparison of low-intensity warfarin therapy with conventional-intensity war-farin therapy for long-term prevention of recurrent venous thromboembolism. N Engl J Med 2003;349:631-639.
3. Pinede L, Duhaut P, Cucherat M, Ninet J, Pasquier J, Boissel JP. Comparison of long versus short duration of anticoagulant therapy after a first episode of venous thromboembolism: a meta-analysis of randomized, controlled trials. J Intern Med 2000;247:553-562.
4. Kearon C, Gent M, Hirsh J, et al. A comparison of three months of anticoagulation with extended anticoagulation for a first episode of idiopathic venous thromboembolism. N Engl J Med 1999;340:901-907.
5. Agnelli G, Prandoni P, Santamaria MG, et al. Three months versus one year of oral anticoagulant therapy for idiopathic deep venous thrombosis. Warfarin Optimal Duration Italian Trial Investigators. N Engl J Med 2001;345:165-169.
6. Levine M, Hirsh J, Gent M, et al. Double-blind randomised trial of a very-low-dose warfarin for prevention of throm-boembolism in stage IV breast cancer. Lancet 1994;343:886-889.
7. Rajan R, Gafni A, Levine M, Hirsh J, Gent M. Very low-dose warfarin prophylaxis to prevent thromboembolism in women with metastatic breast cancer receiving chemotherapy: an economic evaluation. J Clin Oncol 1995;13:42-46.
8. Ginsberg JS, Bates SM, Oczkowski W, et al. Low-dose warfarin in rehabilitating stroke survivors. Thromb Res 2002;107:287-290.
9. Hirsh J, Dalen J, Guyatt G. American College of Chest Physicians. The sixth (2000) ACCP guidelines for antithrombotic therapy for prevention and treatment of thrombosis. American College of Chest Physicians. Chest 2001;119(1 Suppl):132S-193S.
Warfarin (Coumadin) is effective in preventing deep venous thrombosis (DVT) among patients with a history of DVT. Conventional dosing and longer durations are the most effective, but the ideal length of therapy is unknown (strength of recommendation [SOR]: A, based on large randomized controlled trials and meta-analysis).
Warfarin is useful in preventing DVT in patients with cancer, specifically those treated with chemotherapy (SOR: B, based on small randomized controlled trials). Warfarin may be effective in pre-venting DVT in immobilized patients such as those with trauma, spinal cord injury, or stroke (SOR: B, based on an underpowered randomized controlled trial and uncontrolled studies).
Evidence summary
Warfarin, at both low and conventional doses, has been shown to be effective in preventing recurrence of DVT. A large, 4-year placebo-controlled randomized controlled trial showed that long-term low-dose warfarin (international normalized ratio [INR], 1.5-1.9) was more effective than placebo for prevention of DVT (hazard ratio=0.36; 95% confidence interval [CI], 0.19-0.67).1
A double-blind randomized controlled trial of 738 patients demonstrated that conventional-intensity warfarin therapy (INR=2.0-3.0) was more effective than low-intensity therapy (INR=1.5-1.9) in prevention of recurrent DVT. There were 1.9 vs 0.7 DVTs per 100 person-years in the low-intensity vs conventional-intensity therapy groups (hazard ratio=2.8; 95% CI, 1.1-7.0; number needed to treat [NNT]=37). No significant difference was seen in the frequency of bleeding complications between the groups.2 This and other studies suggest that low-intensity warfarin therapy reduces the relative risk of thrombosis by about 75%, and conventional-intensity therapy reduces this risk by over 90%.2
Several studies have examined the duration of warfarin therapy. A meta-analysis found treatment with warfarin for 12 to 24 weeks decreased DVT recurrence compared with 2- to 6-week regimens (relative risk [RR]=0.60; 95% CI, 0.45-0.79; NNT=21).3 A multicenter randomized controlled trial found extending warfarin treatment for 12 months vs 3 months resulted in a 95% relative risk reduction (RRR) in risk of DVT recurrence (95% CI, 63-99; NNT=5).4 A multicenter randomized trial showed similar results, but risk for recurrence was the same after treatment was stopped, regardless of the length of treatment.5
In patients with cancer, warfarin was shown to be more effective than placebo in prevention of DVT. In a trial of 311 breast cancer patients receiving chemotherapy, treatment with very-low-dose warfarin (INR=1.3-1.9) decreased thrombotic events compared with placebo, with no increase in bleeding complications (RRR=85%; P=.031; NNT=27).6 A later cost analysis showed that very-low-dose warfarin can be used in prevention of DVT in breast cancer patients on chemotherapy without an increase in health care costs.7
Although immobilized patients are at high risk for DVT, no randomized controlled trials exist for the use of warfarin in these patients. A few small studies suggest that warfarin reduces DVT rates in spinal-cord-injured patients.8 A small trial randomized stroke patients undergoing rehabilitation to placebo or fixed 1- or 2-mg doses of warfarin. This underpowered study showed a nonsignificant decrease in the risk of development of DVT (RR=0.39; 95% CI, 0.13-1.37).8
Recommendations from others
The 6th American College of Chest Physicians Consensus Conference on Antithrombotic Therapy makes these recommendations:9
Prior DVT: Oral anticoagulation therapy (INR=2.0-3.0) is indicated for at least 3 months for patients with proximal DVT or for at least 6 months in those with idiopathic proximal vein thrombosis or recurrent venous thrombosis. Indefinite anticoagulation is indicated for patients with more than 1 episode of idiopathic proximal vein thrombosis or pulmonary embolus.
Malignancy: Indefinite anticoagulation (INR= 2.0-3.0) is indicated for patients with thrombosis complicating malignancy. Prophylaxis with low-intensity warfarin in ambulatory patients with cancer to prevent initial DVT warrants further study.
Acute spinal cord injuries: Low-molecular-weight heparin or switch to full-dose oral anti-coagulation (INR=2.0-3.0) for the duration of the rehabilitation phase.
Routine prophylaxis dramatically reduces DVT cases
John P. Langlois, MD
MAHEC Family Practice Residency, Asheville, NC
I can clearly recall the dramatic reduction in the number of our patients who developed DVT when our orthopedic colleagues embraced routine prophylaxis for the high-risk surgical patients with hip surgery and knee replacements. This answer indicates that we may also be able to reduce the risk of DVT in our high-risk nonsurgical patients with previous DVT or breast cancer. Note that much of the evidence is based on the use of low-dose and very-low-dose warfarin. This may help mitigate our fear of substituting bleeding complications for the prevention of clots.
Warfarin (Coumadin) is effective in preventing deep venous thrombosis (DVT) among patients with a history of DVT. Conventional dosing and longer durations are the most effective, but the ideal length of therapy is unknown (strength of recommendation [SOR]: A, based on large randomized controlled trials and meta-analysis).
Warfarin is useful in preventing DVT in patients with cancer, specifically those treated with chemotherapy (SOR: B, based on small randomized controlled trials). Warfarin may be effective in pre-venting DVT in immobilized patients such as those with trauma, spinal cord injury, or stroke (SOR: B, based on an underpowered randomized controlled trial and uncontrolled studies).
Evidence summary
Warfarin, at both low and conventional doses, has been shown to be effective in preventing recurrence of DVT. A large, 4-year placebo-controlled randomized controlled trial showed that long-term low-dose warfarin (international normalized ratio [INR], 1.5-1.9) was more effective than placebo for prevention of DVT (hazard ratio=0.36; 95% confidence interval [CI], 0.19-0.67).1
A double-blind randomized controlled trial of 738 patients demonstrated that conventional-intensity warfarin therapy (INR=2.0-3.0) was more effective than low-intensity therapy (INR=1.5-1.9) in prevention of recurrent DVT. There were 1.9 vs 0.7 DVTs per 100 person-years in the low-intensity vs conventional-intensity therapy groups (hazard ratio=2.8; 95% CI, 1.1-7.0; number needed to treat [NNT]=37). No significant difference was seen in the frequency of bleeding complications between the groups.2 This and other studies suggest that low-intensity warfarin therapy reduces the relative risk of thrombosis by about 75%, and conventional-intensity therapy reduces this risk by over 90%.2
Several studies have examined the duration of warfarin therapy. A meta-analysis found treatment with warfarin for 12 to 24 weeks decreased DVT recurrence compared with 2- to 6-week regimens (relative risk [RR]=0.60; 95% CI, 0.45-0.79; NNT=21).3 A multicenter randomized controlled trial found extending warfarin treatment for 12 months vs 3 months resulted in a 95% relative risk reduction (RRR) in risk of DVT recurrence (95% CI, 63-99; NNT=5).4 A multicenter randomized trial showed similar results, but risk for recurrence was the same after treatment was stopped, regardless of the length of treatment.5
In patients with cancer, warfarin was shown to be more effective than placebo in prevention of DVT. In a trial of 311 breast cancer patients receiving chemotherapy, treatment with very-low-dose warfarin (INR=1.3-1.9) decreased thrombotic events compared with placebo, with no increase in bleeding complications (RRR=85%; P=.031; NNT=27).6 A later cost analysis showed that very-low-dose warfarin can be used in prevention of DVT in breast cancer patients on chemotherapy without an increase in health care costs.7
Although immobilized patients are at high risk for DVT, no randomized controlled trials exist for the use of warfarin in these patients. A few small studies suggest that warfarin reduces DVT rates in spinal-cord-injured patients.8 A small trial randomized stroke patients undergoing rehabilitation to placebo or fixed 1- or 2-mg doses of warfarin. This underpowered study showed a nonsignificant decrease in the risk of development of DVT (RR=0.39; 95% CI, 0.13-1.37).8
Recommendations from others
The 6th American College of Chest Physicians Consensus Conference on Antithrombotic Therapy makes these recommendations:9
Prior DVT: Oral anticoagulation therapy (INR=2.0-3.0) is indicated for at least 3 months for patients with proximal DVT or for at least 6 months in those with idiopathic proximal vein thrombosis or recurrent venous thrombosis. Indefinite anticoagulation is indicated for patients with more than 1 episode of idiopathic proximal vein thrombosis or pulmonary embolus.
Malignancy: Indefinite anticoagulation (INR= 2.0-3.0) is indicated for patients with thrombosis complicating malignancy. Prophylaxis with low-intensity warfarin in ambulatory patients with cancer to prevent initial DVT warrants further study.
Acute spinal cord injuries: Low-molecular-weight heparin or switch to full-dose oral anti-coagulation (INR=2.0-3.0) for the duration of the rehabilitation phase.
Routine prophylaxis dramatically reduces DVT cases
John P. Langlois, MD
MAHEC Family Practice Residency, Asheville, NC
I can clearly recall the dramatic reduction in the number of our patients who developed DVT when our orthopedic colleagues embraced routine prophylaxis for the high-risk surgical patients with hip surgery and knee replacements. This answer indicates that we may also be able to reduce the risk of DVT in our high-risk nonsurgical patients with previous DVT or breast cancer. Note that much of the evidence is based on the use of low-dose and very-low-dose warfarin. This may help mitigate our fear of substituting bleeding complications for the prevention of clots.
1. Ridker PM, Goldhaber SZ, Danielson E, et al. Long-term, low-intensity warfarin therapy for the prevention of recurrent venous thromboembolism. N Engl J Med 2003;348:1425-1434.
2. Kearon C, Ginsberg JS, Kovacs MJ, et al. Comparison of low-intensity warfarin therapy with conventional-intensity war-farin therapy for long-term prevention of recurrent venous thromboembolism. N Engl J Med 2003;349:631-639.
3. Pinede L, Duhaut P, Cucherat M, Ninet J, Pasquier J, Boissel JP. Comparison of long versus short duration of anticoagulant therapy after a first episode of venous thromboembolism: a meta-analysis of randomized, controlled trials. J Intern Med 2000;247:553-562.
4. Kearon C, Gent M, Hirsh J, et al. A comparison of three months of anticoagulation with extended anticoagulation for a first episode of idiopathic venous thromboembolism. N Engl J Med 1999;340:901-907.
5. Agnelli G, Prandoni P, Santamaria MG, et al. Three months versus one year of oral anticoagulant therapy for idiopathic deep venous thrombosis. Warfarin Optimal Duration Italian Trial Investigators. N Engl J Med 2001;345:165-169.
6. Levine M, Hirsh J, Gent M, et al. Double-blind randomised trial of a very-low-dose warfarin for prevention of throm-boembolism in stage IV breast cancer. Lancet 1994;343:886-889.
7. Rajan R, Gafni A, Levine M, Hirsh J, Gent M. Very low-dose warfarin prophylaxis to prevent thromboembolism in women with metastatic breast cancer receiving chemotherapy: an economic evaluation. J Clin Oncol 1995;13:42-46.
8. Ginsberg JS, Bates SM, Oczkowski W, et al. Low-dose warfarin in rehabilitating stroke survivors. Thromb Res 2002;107:287-290.
9. Hirsh J, Dalen J, Guyatt G. American College of Chest Physicians. The sixth (2000) ACCP guidelines for antithrombotic therapy for prevention and treatment of thrombosis. American College of Chest Physicians. Chest 2001;119(1 Suppl):132S-193S.
1. Ridker PM, Goldhaber SZ, Danielson E, et al. Long-term, low-intensity warfarin therapy for the prevention of recurrent venous thromboembolism. N Engl J Med 2003;348:1425-1434.
2. Kearon C, Ginsberg JS, Kovacs MJ, et al. Comparison of low-intensity warfarin therapy with conventional-intensity war-farin therapy for long-term prevention of recurrent venous thromboembolism. N Engl J Med 2003;349:631-639.
3. Pinede L, Duhaut P, Cucherat M, Ninet J, Pasquier J, Boissel JP. Comparison of long versus short duration of anticoagulant therapy after a first episode of venous thromboembolism: a meta-analysis of randomized, controlled trials. J Intern Med 2000;247:553-562.
4. Kearon C, Gent M, Hirsh J, et al. A comparison of three months of anticoagulation with extended anticoagulation for a first episode of idiopathic venous thromboembolism. N Engl J Med 1999;340:901-907.
5. Agnelli G, Prandoni P, Santamaria MG, et al. Three months versus one year of oral anticoagulant therapy for idiopathic deep venous thrombosis. Warfarin Optimal Duration Italian Trial Investigators. N Engl J Med 2001;345:165-169.
6. Levine M, Hirsh J, Gent M, et al. Double-blind randomised trial of a very-low-dose warfarin for prevention of throm-boembolism in stage IV breast cancer. Lancet 1994;343:886-889.
7. Rajan R, Gafni A, Levine M, Hirsh J, Gent M. Very low-dose warfarin prophylaxis to prevent thromboembolism in women with metastatic breast cancer receiving chemotherapy: an economic evaluation. J Clin Oncol 1995;13:42-46.
8. Ginsberg JS, Bates SM, Oczkowski W, et al. Low-dose warfarin in rehabilitating stroke survivors. Thromb Res 2002;107:287-290.
9. Hirsh J, Dalen J, Guyatt G. American College of Chest Physicians. The sixth (2000) ACCP guidelines for antithrombotic therapy for prevention and treatment of thrombosis. American College of Chest Physicians. Chest 2001;119(1 Suppl):132S-193S.
Evidence-based answers from the Family Physicians Inquiries Network
Do systemic corticosteroids lessen symptoms in acute exacerbations of COPD?
Systemic corticosteroids improve measures of dyspnea in patients with acute exacerbations of chronic obstructive pulmonary disease (COPD) (strength of recommendation [SOR]: A, meta-analysis of 2 small randomized controlled trials). The optimal dose of systemic corticosteroids to achieve these benefits is uncertain. An international consensus panel recommended 30 to 40 mg of oral prednisone daily for 10 to 14 days as a reasonable compromise of efficacy and safety (SOR: C, consensus expert opinion).
Evidence summary
Three systematic reviews addressing the efficacy of systemic corticosteroids in managing acute exacerbations of COPD found consistent, good-quality evidence supporting short courses of systemic steroids. The improvement in outcomes included decreases in airflow obstruction, treatment failure, and length of hospital stay.1-3
The optimal initial doses of systemic corticosteroids to achieve these benefits are uncertain. Variable study designs limit combining study results into a dose-response curve, and there are no comparative trials of high- vs low-dose regimens. A panel consensus judgment from a collaboration of the National Heart, Lung, and Blood Institute and the World Health Organization recommended 30–40 mg of oral prednisone daily for 10 to 14 days.4
A Cochrane systematic review analyzed 7 randomized, placebo-controlled trials of systemic steroids for acute exacerbations of COPD.1 While most of the studies reporting symptom outcomes used disparate methods of measurement, 2 small studies5,6 reported changes in quality of life using validated visual analogue scales. This allowed their results to be combined into a summary estimate of the effect of corticosteroids compared with placebo. Combining the visual analogue scales using a standardized mean difference showed a significant improvement of this summary quality of life measure in the steroid-treated group.
Other small randomized controlled trials of systemic steroids1 demonstrated trends towards improvement in symptom outcomes. A Taiwanese study randomized 138 patients presenting to an emergency department to treatment with 100 mg intravenous hydrocortisone or placebo within 15 minutes of arrival.1 Using a 6-point scale, patients gave self-assessments of the severity of their attack on arrival and at 6 hours. Compared with placebo, the steroid group showed a 6-hour improvement of uncertain significance.
Similarly, a British trial of 30 mg prednisone vs placebo in 56 inpatients with acute exacerbations of COPD measured a daily composite symptom score based on 7 pulmonary and functional symptoms.8 There was a nonsignificant trend towards greater improvement in the steroid-treated group.
Finally, a multicenter, 3-armed, placebo-controlled, double-blinded, parallel design study enrolled 199 COPD inpatients, who were randomized to oral prednisone, inhaled budesonide, or placebo treatment groups.9 Dyspnea was assessed using a validated, modified Borg scale every 12 hours for 72 hours. The reduction in the modified Borg scale rating was of comparable magnitude in the 3 groups, but again there was a nonsignificant greater reduction in the systemic steroid group compared with both the placebo and inhaled budesonide groups. Power calculations were not provided, so it is unclear whether sample size in this study was sufficient to detect important differences in outcomes.
Three randomized controlled trials prospectively measured adverse events rates of systemic steroids in acute exacerbations of COPD.9-11 Hyperglycemia or glycosuria was more common in the steroid-treated groups. The SCCOPE study, the largest of the 3 trials, found hyperglycemia requiring treatment occurred in a greater proportion of the steroid-treated group than placebo (15% vs 4%; P=.002; number needed to harm=9).
Recommendations from others
A recent review provides a concise summary of practice guidelines for the management of acute exacerbations of COPD from widely recognized professional societies.12 Systemic steroids are endorsed in the evidence-based systematic review guidelines from the American College of Chest Physicians–American Society of Internal Medicine, along with the National Heart, Lung, and Blood Institute with the World Health Organization cosponsored Global Initiative for Chronic Obstructive Lung Disease (GOLD), and the consensus guidelines of the American Thoracic Society.13
Lack of long-term benefits emphasize need for prevention
Donald Briscoe, MD
CHRISTUS St. Joseph Family Practice Residency, Houston, TX
It is reassuring to see that there is good evidence to support what most practicing physicians already do—use steroids for acute exacerbations of COPD. Along with inhaled anticholinergics, beta-agonists and (sometimes) antibiotics, short-term measures of patient oriented outcomes seem to be improved. Questions still remain regarding the optimal dosing, route of administration, and length of therapy needed. The lack of evidence of long-term outcome benefits emphasizes, to me, the need for improved efforts at primary and secondary prevention, such as smoking prevention and cessation interventions, annual influenza vaccination, and routine pneumococcal vaccination in our COPD patients.
1. Wood-Baker R, Walters EH, Gibson P. Oral corticos-teroids for acute exacerbations of chronic obstructive pulmonary disease (Cochrane Review). The Cochrane Library, issue 4, 2003. Updated January 12, 2001.
2. McCrory DC, Brown C, Gelfand SE, Bach PB. Management of acute exacerbations of COPD: a summary and appraisal of published evidence. Chest 2001;119:1190-1209.
3. Singh JM, Palda VA, Stanbrook MB, Chapman KR. Corticosteroid therapy for patients with acute exacerbations of chronic obstructive pulmonary disease. Arch Intern Med 2002;162:2527-2536.
4. Pauwels RA, Buist AS, Calverley PM, Jenkins CR, Hurd SS. GOLD Scientific Committee. Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease. NHLBI/WHO Global Initiative for Chronic Obstructive Lung Disease (GOLD) Workshop Summary. Am J Respir Crit Care Med 2001;163:1256-1276.
5. Thompson WH, Nielson CP, Carvalho P, Charan NB, Crowley JJ. Controlled trial of oral prednisone in outpatients with acute COPD exacerbation. Am J Respir Crit Care Med 1996;154:407-412.
6. Wood-Baker R, Wilinson J, Pearce M, Ryan G. A double-blind, placebo-controlled trial of corticosteroids for acute exacerbations of chronic obstructive pulmonary disease. Aust N Z J Med 1998;28:262.-
7. Bullard MJ, Liaw SJ, Tsai YH, Min HP. Early corticosteroid use in acute exacerbations of chronic airflow obstruction. Am J Emerg Med 1996;14:139-143.
8. Davies L, Angus RM, Calverley PM. Oral corticosteroids in patients admitted to hospital with exacerbations of chronic obstructive pulmonary disease: a prospective randomised controlled trial. Lancet 1999;354:456-460.
9. Maltais F, Ostinelli J, Bourbeau J, et al. Comparison of nebulized budesonide and oral prednisolone with placebo in the treatment of acute exacerbations of chronic obstructive pulmonary disease: a randomized controlled trial. Am J Respir Crit Care Med 2002;165:698-703.
10. Albert RK, Martin TR, Lewis SW. Controlled clinical trial of methylprednisolone in patients with chronic bronchitis and acute respiratory insufficiency. Ann Intern Med 1980;92:753-758.
11. Niewoehner DE, Erbland ML, Deupree RH, et al. Effect of systemic glucocorticoids on exacerbations of chronic obstructive pulmonary disease. Department of Veterans Affairs Cooperative Study Group. N Engl J Med 1999;340:1941-1947.
12. Stoller JK. Clinical practice. Acute exacerbations of chronic obstructive pulmonary disease. N Engl J Med 2002;346:988-994.
13. Niewoehner DE, Erbland M, Collins D. Glucocorticoids for chronic obstructive pulmonary disease [letter]. N Engl J Med 1999;341:1772-1773.
Systemic corticosteroids improve measures of dyspnea in patients with acute exacerbations of chronic obstructive pulmonary disease (COPD) (strength of recommendation [SOR]: A, meta-analysis of 2 small randomized controlled trials). The optimal dose of systemic corticosteroids to achieve these benefits is uncertain. An international consensus panel recommended 30 to 40 mg of oral prednisone daily for 10 to 14 days as a reasonable compromise of efficacy and safety (SOR: C, consensus expert opinion).
Evidence summary
Three systematic reviews addressing the efficacy of systemic corticosteroids in managing acute exacerbations of COPD found consistent, good-quality evidence supporting short courses of systemic steroids. The improvement in outcomes included decreases in airflow obstruction, treatment failure, and length of hospital stay.1-3
The optimal initial doses of systemic corticosteroids to achieve these benefits are uncertain. Variable study designs limit combining study results into a dose-response curve, and there are no comparative trials of high- vs low-dose regimens. A panel consensus judgment from a collaboration of the National Heart, Lung, and Blood Institute and the World Health Organization recommended 30–40 mg of oral prednisone daily for 10 to 14 days.4
A Cochrane systematic review analyzed 7 randomized, placebo-controlled trials of systemic steroids for acute exacerbations of COPD.1 While most of the studies reporting symptom outcomes used disparate methods of measurement, 2 small studies5,6 reported changes in quality of life using validated visual analogue scales. This allowed their results to be combined into a summary estimate of the effect of corticosteroids compared with placebo. Combining the visual analogue scales using a standardized mean difference showed a significant improvement of this summary quality of life measure in the steroid-treated group.
Other small randomized controlled trials of systemic steroids1 demonstrated trends towards improvement in symptom outcomes. A Taiwanese study randomized 138 patients presenting to an emergency department to treatment with 100 mg intravenous hydrocortisone or placebo within 15 minutes of arrival.1 Using a 6-point scale, patients gave self-assessments of the severity of their attack on arrival and at 6 hours. Compared with placebo, the steroid group showed a 6-hour improvement of uncertain significance.
Similarly, a British trial of 30 mg prednisone vs placebo in 56 inpatients with acute exacerbations of COPD measured a daily composite symptom score based on 7 pulmonary and functional symptoms.8 There was a nonsignificant trend towards greater improvement in the steroid-treated group.
Finally, a multicenter, 3-armed, placebo-controlled, double-blinded, parallel design study enrolled 199 COPD inpatients, who were randomized to oral prednisone, inhaled budesonide, or placebo treatment groups.9 Dyspnea was assessed using a validated, modified Borg scale every 12 hours for 72 hours. The reduction in the modified Borg scale rating was of comparable magnitude in the 3 groups, but again there was a nonsignificant greater reduction in the systemic steroid group compared with both the placebo and inhaled budesonide groups. Power calculations were not provided, so it is unclear whether sample size in this study was sufficient to detect important differences in outcomes.
Three randomized controlled trials prospectively measured adverse events rates of systemic steroids in acute exacerbations of COPD.9-11 Hyperglycemia or glycosuria was more common in the steroid-treated groups. The SCCOPE study, the largest of the 3 trials, found hyperglycemia requiring treatment occurred in a greater proportion of the steroid-treated group than placebo (15% vs 4%; P=.002; number needed to harm=9).
Recommendations from others
A recent review provides a concise summary of practice guidelines for the management of acute exacerbations of COPD from widely recognized professional societies.12 Systemic steroids are endorsed in the evidence-based systematic review guidelines from the American College of Chest Physicians–American Society of Internal Medicine, along with the National Heart, Lung, and Blood Institute with the World Health Organization cosponsored Global Initiative for Chronic Obstructive Lung Disease (GOLD), and the consensus guidelines of the American Thoracic Society.13
Lack of long-term benefits emphasize need for prevention
Donald Briscoe, MD
CHRISTUS St. Joseph Family Practice Residency, Houston, TX
It is reassuring to see that there is good evidence to support what most practicing physicians already do—use steroids for acute exacerbations of COPD. Along with inhaled anticholinergics, beta-agonists and (sometimes) antibiotics, short-term measures of patient oriented outcomes seem to be improved. Questions still remain regarding the optimal dosing, route of administration, and length of therapy needed. The lack of evidence of long-term outcome benefits emphasizes, to me, the need for improved efforts at primary and secondary prevention, such as smoking prevention and cessation interventions, annual influenza vaccination, and routine pneumococcal vaccination in our COPD patients.
Systemic corticosteroids improve measures of dyspnea in patients with acute exacerbations of chronic obstructive pulmonary disease (COPD) (strength of recommendation [SOR]: A, meta-analysis of 2 small randomized controlled trials). The optimal dose of systemic corticosteroids to achieve these benefits is uncertain. An international consensus panel recommended 30 to 40 mg of oral prednisone daily for 10 to 14 days as a reasonable compromise of efficacy and safety (SOR: C, consensus expert opinion).
Evidence summary
Three systematic reviews addressing the efficacy of systemic corticosteroids in managing acute exacerbations of COPD found consistent, good-quality evidence supporting short courses of systemic steroids. The improvement in outcomes included decreases in airflow obstruction, treatment failure, and length of hospital stay.1-3
The optimal initial doses of systemic corticosteroids to achieve these benefits are uncertain. Variable study designs limit combining study results into a dose-response curve, and there are no comparative trials of high- vs low-dose regimens. A panel consensus judgment from a collaboration of the National Heart, Lung, and Blood Institute and the World Health Organization recommended 30–40 mg of oral prednisone daily for 10 to 14 days.4
A Cochrane systematic review analyzed 7 randomized, placebo-controlled trials of systemic steroids for acute exacerbations of COPD.1 While most of the studies reporting symptom outcomes used disparate methods of measurement, 2 small studies5,6 reported changes in quality of life using validated visual analogue scales. This allowed their results to be combined into a summary estimate of the effect of corticosteroids compared with placebo. Combining the visual analogue scales using a standardized mean difference showed a significant improvement of this summary quality of life measure in the steroid-treated group.
Other small randomized controlled trials of systemic steroids1 demonstrated trends towards improvement in symptom outcomes. A Taiwanese study randomized 138 patients presenting to an emergency department to treatment with 100 mg intravenous hydrocortisone or placebo within 15 minutes of arrival.1 Using a 6-point scale, patients gave self-assessments of the severity of their attack on arrival and at 6 hours. Compared with placebo, the steroid group showed a 6-hour improvement of uncertain significance.
Similarly, a British trial of 30 mg prednisone vs placebo in 56 inpatients with acute exacerbations of COPD measured a daily composite symptom score based on 7 pulmonary and functional symptoms.8 There was a nonsignificant trend towards greater improvement in the steroid-treated group.
Finally, a multicenter, 3-armed, placebo-controlled, double-blinded, parallel design study enrolled 199 COPD inpatients, who were randomized to oral prednisone, inhaled budesonide, or placebo treatment groups.9 Dyspnea was assessed using a validated, modified Borg scale every 12 hours for 72 hours. The reduction in the modified Borg scale rating was of comparable magnitude in the 3 groups, but again there was a nonsignificant greater reduction in the systemic steroid group compared with both the placebo and inhaled budesonide groups. Power calculations were not provided, so it is unclear whether sample size in this study was sufficient to detect important differences in outcomes.
Three randomized controlled trials prospectively measured adverse events rates of systemic steroids in acute exacerbations of COPD.9-11 Hyperglycemia or glycosuria was more common in the steroid-treated groups. The SCCOPE study, the largest of the 3 trials, found hyperglycemia requiring treatment occurred in a greater proportion of the steroid-treated group than placebo (15% vs 4%; P=.002; number needed to harm=9).
Recommendations from others
A recent review provides a concise summary of practice guidelines for the management of acute exacerbations of COPD from widely recognized professional societies.12 Systemic steroids are endorsed in the evidence-based systematic review guidelines from the American College of Chest Physicians–American Society of Internal Medicine, along with the National Heart, Lung, and Blood Institute with the World Health Organization cosponsored Global Initiative for Chronic Obstructive Lung Disease (GOLD), and the consensus guidelines of the American Thoracic Society.13
Lack of long-term benefits emphasize need for prevention
Donald Briscoe, MD
CHRISTUS St. Joseph Family Practice Residency, Houston, TX
It is reassuring to see that there is good evidence to support what most practicing physicians already do—use steroids for acute exacerbations of COPD. Along with inhaled anticholinergics, beta-agonists and (sometimes) antibiotics, short-term measures of patient oriented outcomes seem to be improved. Questions still remain regarding the optimal dosing, route of administration, and length of therapy needed. The lack of evidence of long-term outcome benefits emphasizes, to me, the need for improved efforts at primary and secondary prevention, such as smoking prevention and cessation interventions, annual influenza vaccination, and routine pneumococcal vaccination in our COPD patients.
1. Wood-Baker R, Walters EH, Gibson P. Oral corticos-teroids for acute exacerbations of chronic obstructive pulmonary disease (Cochrane Review). The Cochrane Library, issue 4, 2003. Updated January 12, 2001.
2. McCrory DC, Brown C, Gelfand SE, Bach PB. Management of acute exacerbations of COPD: a summary and appraisal of published evidence. Chest 2001;119:1190-1209.
3. Singh JM, Palda VA, Stanbrook MB, Chapman KR. Corticosteroid therapy for patients with acute exacerbations of chronic obstructive pulmonary disease. Arch Intern Med 2002;162:2527-2536.
4. Pauwels RA, Buist AS, Calverley PM, Jenkins CR, Hurd SS. GOLD Scientific Committee. Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease. NHLBI/WHO Global Initiative for Chronic Obstructive Lung Disease (GOLD) Workshop Summary. Am J Respir Crit Care Med 2001;163:1256-1276.
5. Thompson WH, Nielson CP, Carvalho P, Charan NB, Crowley JJ. Controlled trial of oral prednisone in outpatients with acute COPD exacerbation. Am J Respir Crit Care Med 1996;154:407-412.
6. Wood-Baker R, Wilinson J, Pearce M, Ryan G. A double-blind, placebo-controlled trial of corticosteroids for acute exacerbations of chronic obstructive pulmonary disease. Aust N Z J Med 1998;28:262.-
7. Bullard MJ, Liaw SJ, Tsai YH, Min HP. Early corticosteroid use in acute exacerbations of chronic airflow obstruction. Am J Emerg Med 1996;14:139-143.
8. Davies L, Angus RM, Calverley PM. Oral corticosteroids in patients admitted to hospital with exacerbations of chronic obstructive pulmonary disease: a prospective randomised controlled trial. Lancet 1999;354:456-460.
9. Maltais F, Ostinelli J, Bourbeau J, et al. Comparison of nebulized budesonide and oral prednisolone with placebo in the treatment of acute exacerbations of chronic obstructive pulmonary disease: a randomized controlled trial. Am J Respir Crit Care Med 2002;165:698-703.
10. Albert RK, Martin TR, Lewis SW. Controlled clinical trial of methylprednisolone in patients with chronic bronchitis and acute respiratory insufficiency. Ann Intern Med 1980;92:753-758.
11. Niewoehner DE, Erbland ML, Deupree RH, et al. Effect of systemic glucocorticoids on exacerbations of chronic obstructive pulmonary disease. Department of Veterans Affairs Cooperative Study Group. N Engl J Med 1999;340:1941-1947.
12. Stoller JK. Clinical practice. Acute exacerbations of chronic obstructive pulmonary disease. N Engl J Med 2002;346:988-994.
13. Niewoehner DE, Erbland M, Collins D. Glucocorticoids for chronic obstructive pulmonary disease [letter]. N Engl J Med 1999;341:1772-1773.
1. Wood-Baker R, Walters EH, Gibson P. Oral corticos-teroids for acute exacerbations of chronic obstructive pulmonary disease (Cochrane Review). The Cochrane Library, issue 4, 2003. Updated January 12, 2001.
2. McCrory DC, Brown C, Gelfand SE, Bach PB. Management of acute exacerbations of COPD: a summary and appraisal of published evidence. Chest 2001;119:1190-1209.
3. Singh JM, Palda VA, Stanbrook MB, Chapman KR. Corticosteroid therapy for patients with acute exacerbations of chronic obstructive pulmonary disease. Arch Intern Med 2002;162:2527-2536.
4. Pauwels RA, Buist AS, Calverley PM, Jenkins CR, Hurd SS. GOLD Scientific Committee. Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease. NHLBI/WHO Global Initiative for Chronic Obstructive Lung Disease (GOLD) Workshop Summary. Am J Respir Crit Care Med 2001;163:1256-1276.
5. Thompson WH, Nielson CP, Carvalho P, Charan NB, Crowley JJ. Controlled trial of oral prednisone in outpatients with acute COPD exacerbation. Am J Respir Crit Care Med 1996;154:407-412.
6. Wood-Baker R, Wilinson J, Pearce M, Ryan G. A double-blind, placebo-controlled trial of corticosteroids for acute exacerbations of chronic obstructive pulmonary disease. Aust N Z J Med 1998;28:262.-
7. Bullard MJ, Liaw SJ, Tsai YH, Min HP. Early corticosteroid use in acute exacerbations of chronic airflow obstruction. Am J Emerg Med 1996;14:139-143.
8. Davies L, Angus RM, Calverley PM. Oral corticosteroids in patients admitted to hospital with exacerbations of chronic obstructive pulmonary disease: a prospective randomised controlled trial. Lancet 1999;354:456-460.
9. Maltais F, Ostinelli J, Bourbeau J, et al. Comparison of nebulized budesonide and oral prednisolone with placebo in the treatment of acute exacerbations of chronic obstructive pulmonary disease: a randomized controlled trial. Am J Respir Crit Care Med 2002;165:698-703.
10. Albert RK, Martin TR, Lewis SW. Controlled clinical trial of methylprednisolone in patients with chronic bronchitis and acute respiratory insufficiency. Ann Intern Med 1980;92:753-758.
11. Niewoehner DE, Erbland ML, Deupree RH, et al. Effect of systemic glucocorticoids on exacerbations of chronic obstructive pulmonary disease. Department of Veterans Affairs Cooperative Study Group. N Engl J Med 1999;340:1941-1947.
12. Stoller JK. Clinical practice. Acute exacerbations of chronic obstructive pulmonary disease. N Engl J Med 2002;346:988-994.
13. Niewoehner DE, Erbland M, Collins D. Glucocorticoids for chronic obstructive pulmonary disease [letter]. N Engl J Med 1999;341:1772-1773.
Evidence-based answers from the Family Physicians Inquiries Network
Do glucosamine or chondroitin cause regeneration of cartilage in osteoarthritis?
No direct evidence suggests glucosamine or chrondroitin cause regeneration of cartilage in osteoarthritis. Use of glucosamine sulfate in knee osteoarthritis prevents joint space narrowing on radiographs (strength of recommendation [SOR]: B, based on 1 randomized controlled trial).
Intramuscular chondroitin polysulfate prevents radiographic progression of finger osteoarthritis (SOR: B, based on 1 randomized controlled trial).
Both chondroitin sulfate and glucosamine sulfate stimulate chondrocyte growth in vitro and in animal models (SOR: D, based on several bench research studies).
Evidence summary
A systematic review of glucosamine sulfate use for osteoarthritis, based on early research (1956–1991), found that it has anti-inflammatory properties and rebuilds damaged cartilage.1 These studies evaluated chondrocytes grown in culture and animal models.1,2 Chondroitin sulfate also stimulates chondrocyte biosynthesis in both animal and in vitro studies. There is insufficient evidence to demonstrate glucosamine sulfate or chondroitin sulfate stimulates chrondrocyte growth in humans with osteoarthritis.2,3
Joint space narrowing on radiographs suggests progression of osteoarthritis. This narrowing is thought to imply cartilage destruction or loss due to osteoarthritis. A double-blinded randomized controlled trial studied the effect of glucosamine sulfate on tibial-femoral compartment joint space narrowing in 212 patients older than 50 with mild to moderate knee osteoarthritis.4 Patients took either 1500 mg/day of glucosamine sulfate or placebo over 3 years. Knee radiographs in a standing anterior-posterior view, using visual and digital analysis, were used to assess joint space narrowing.5 The average mean joint space loss was 0.31 mm in the placebo group and 0.07 mm in the treatment group (P<.05; 95% confidence interval, 0.13–0.48).
The clinical relevance of knee joint space narrowing is undetermined. Radiographic evaluation of a weight-bearing joint space may not be an accurate or reproducible technique. A study of 15 patients with mild to moderate knee osteoarthritis used standing and semi-flexed radiographic views after an analgesic and nonsteroid anti-inflammatory drug washout period, and 1 to 12 weeks after resumption of analgesic therapy (mean 6.0 weeks).6 Knee pain significantly decreased radiographic joint space in the standing anteriorposterior position, but not in the semiflexed position. Using the standing anterior-posterior method may confound accurate interpretation of joint space narrowing and changes in articular cartilage since glucosamine may have an anti-inflammatory effect.6
One double-blinded randomized controlled trial, comparing chondroitin sulfate with placebo, evaluated joint space in patients with symptomatic hand osteoarthritis.7 One hundred sixty-five Caucasian patients, aged 40 to 70 years, were randomized to receive either a 50-mg intramuscular injection of chondroitin polysulfate, twice weekly, for 8 weeks, every 4 months, versus placebo, or 400 mg of oral chondroitin sulfate, 3 times a day, versus placebo.
Osteoarthritis progression in the metacarpalphalangeal and interphalangeal joints was assessed with radiographs over 3 years. Evaluators used the Anatomic Lesion Progression Scale to assess the development of osteophytes and joint space narrowing, with or without subchondral bone changes, to determine osteoarthritis progression. This scale makes it very difficult to determine whether improvements are clinically significant.
Chondroitin sulfate and polysulfate did not prevent osteoarthritis from occurring in previously normal joints. In joints already affected, intramuscular chondroitin polysulfate significantly reduced progression of distal interphalangeal, proximal interpharangeal, and metacarpophalangeal joint space narrowing (P<.013), using the progression scale. Oral chondroitin sulfate did not prevent progression.7
Recommendations from others
The American College of Rheumatology stated in 2000 that recommending glucosamine sulfate or chondroitin sulfate for osteoarthritis might be premature due to the methodology, lack of standardization, and insufficient information on study designs. More research was recommended.8
These products are sold as supplements in the United States. Their purity is often questionable and thus may affect study results. When studying glucosamine, the National Institutes of Health was forced to manufacture the drug itself due to lack of a reliable amount present in commercial products.9
Fred Tudiver, MD
East Tennessee State University, Johnson City
Most family physicians see many patients with osteoarthritis, which can be difficult to treat. My patients typically want improvement in their symptoms, function, and disease progression. Although there is good evidence that the use of glucosamine sulphate (but not chondroitin sulphate) can improve the common symptoms and functional problems of osteoarthritis, this review states it is unclear whether these substances can alter disease progression through regeneration of cartilage.
I tell my patients with osteoarthritis that glucosamine sulfate can help problems like joint pain and function, but that we do not have a safe and reliable treatment for reversing the disease or the joint damage resulting from it.
1. Towheed TE, Anastassiades TP, Shea B, Houpt J, Welch V, Hochberg MC. Glucosamine therapy for treating osteoarthritis. Cochrane Database Syst Rev 2001;CD002946.-
2. Hauselmann HJ. Nutripharmaceuticals for osteoarthritis. Best Pract Res Clin Rheumatol 2001;15:595-607.
3. de los Reyes GC, Koda RT, Lien EJ. Glucosamine and chondroitin sulfates in the treatment of osteoarthritis: a survey. Prog Drug Res 2000;155:81-103.
4. Jubb RW. Oral and intra-articular remedies: review of papers published from March 2001 to February 2002. Curr Opin Rheumatol 2002;14:597-602.
5. Reginster JY, Deroisy R, Rovati LC, et al. Long-term effects of glucosamine sulphate on osteoarthritis progression: a randomised, placebo-controlled clinical trial. Lancet 2001;357:251-256.
6. Mazzuca SA, Brandt KD, Lane KA, Katz BP. Knee pain reduces joint space width in conventional standing anteroposterior radiographs of osteoarthritic knees. Arthritis Rheum 2002;46:1223-1227.
7. Verbruggen G, Goemaere S, Veys EM. Systems to assess the progression of finger joint osteoarthritis and the effects of disease modifying osteoarthritis drugs. Clin Rheumatol 2002;21:231-243.
8. Recommendations for the medical management of osteoarthritis of the hip and knee: 2000 update. American College of Rheumatology Subcommittee on Osteoarthritis Guidelines. Arthritis Rheum 2000;43:1905-1915.
9. Problems with dietary supplements. Med Lett Drugs Ther 2002;44:84-86.
No direct evidence suggests glucosamine or chrondroitin cause regeneration of cartilage in osteoarthritis. Use of glucosamine sulfate in knee osteoarthritis prevents joint space narrowing on radiographs (strength of recommendation [SOR]: B, based on 1 randomized controlled trial).
Intramuscular chondroitin polysulfate prevents radiographic progression of finger osteoarthritis (SOR: B, based on 1 randomized controlled trial).
Both chondroitin sulfate and glucosamine sulfate stimulate chondrocyte growth in vitro and in animal models (SOR: D, based on several bench research studies).
Evidence summary
A systematic review of glucosamine sulfate use for osteoarthritis, based on early research (1956–1991), found that it has anti-inflammatory properties and rebuilds damaged cartilage.1 These studies evaluated chondrocytes grown in culture and animal models.1,2 Chondroitin sulfate also stimulates chondrocyte biosynthesis in both animal and in vitro studies. There is insufficient evidence to demonstrate glucosamine sulfate or chondroitin sulfate stimulates chrondrocyte growth in humans with osteoarthritis.2,3
Joint space narrowing on radiographs suggests progression of osteoarthritis. This narrowing is thought to imply cartilage destruction or loss due to osteoarthritis. A double-blinded randomized controlled trial studied the effect of glucosamine sulfate on tibial-femoral compartment joint space narrowing in 212 patients older than 50 with mild to moderate knee osteoarthritis.4 Patients took either 1500 mg/day of glucosamine sulfate or placebo over 3 years. Knee radiographs in a standing anterior-posterior view, using visual and digital analysis, were used to assess joint space narrowing.5 The average mean joint space loss was 0.31 mm in the placebo group and 0.07 mm in the treatment group (P<.05; 95% confidence interval, 0.13–0.48).
The clinical relevance of knee joint space narrowing is undetermined. Radiographic evaluation of a weight-bearing joint space may not be an accurate or reproducible technique. A study of 15 patients with mild to moderate knee osteoarthritis used standing and semi-flexed radiographic views after an analgesic and nonsteroid anti-inflammatory drug washout period, and 1 to 12 weeks after resumption of analgesic therapy (mean 6.0 weeks).6 Knee pain significantly decreased radiographic joint space in the standing anteriorposterior position, but not in the semiflexed position. Using the standing anterior-posterior method may confound accurate interpretation of joint space narrowing and changes in articular cartilage since glucosamine may have an anti-inflammatory effect.6
One double-blinded randomized controlled trial, comparing chondroitin sulfate with placebo, evaluated joint space in patients with symptomatic hand osteoarthritis.7 One hundred sixty-five Caucasian patients, aged 40 to 70 years, were randomized to receive either a 50-mg intramuscular injection of chondroitin polysulfate, twice weekly, for 8 weeks, every 4 months, versus placebo, or 400 mg of oral chondroitin sulfate, 3 times a day, versus placebo.
Osteoarthritis progression in the metacarpalphalangeal and interphalangeal joints was assessed with radiographs over 3 years. Evaluators used the Anatomic Lesion Progression Scale to assess the development of osteophytes and joint space narrowing, with or without subchondral bone changes, to determine osteoarthritis progression. This scale makes it very difficult to determine whether improvements are clinically significant.
Chondroitin sulfate and polysulfate did not prevent osteoarthritis from occurring in previously normal joints. In joints already affected, intramuscular chondroitin polysulfate significantly reduced progression of distal interphalangeal, proximal interpharangeal, and metacarpophalangeal joint space narrowing (P<.013), using the progression scale. Oral chondroitin sulfate did not prevent progression.7
Recommendations from others
The American College of Rheumatology stated in 2000 that recommending glucosamine sulfate or chondroitin sulfate for osteoarthritis might be premature due to the methodology, lack of standardization, and insufficient information on study designs. More research was recommended.8
These products are sold as supplements in the United States. Their purity is often questionable and thus may affect study results. When studying glucosamine, the National Institutes of Health was forced to manufacture the drug itself due to lack of a reliable amount present in commercial products.9
Fred Tudiver, MD
East Tennessee State University, Johnson City
Most family physicians see many patients with osteoarthritis, which can be difficult to treat. My patients typically want improvement in their symptoms, function, and disease progression. Although there is good evidence that the use of glucosamine sulphate (but not chondroitin sulphate) can improve the common symptoms and functional problems of osteoarthritis, this review states it is unclear whether these substances can alter disease progression through regeneration of cartilage.
I tell my patients with osteoarthritis that glucosamine sulfate can help problems like joint pain and function, but that we do not have a safe and reliable treatment for reversing the disease or the joint damage resulting from it.
No direct evidence suggests glucosamine or chrondroitin cause regeneration of cartilage in osteoarthritis. Use of glucosamine sulfate in knee osteoarthritis prevents joint space narrowing on radiographs (strength of recommendation [SOR]: B, based on 1 randomized controlled trial).
Intramuscular chondroitin polysulfate prevents radiographic progression of finger osteoarthritis (SOR: B, based on 1 randomized controlled trial).
Both chondroitin sulfate and glucosamine sulfate stimulate chondrocyte growth in vitro and in animal models (SOR: D, based on several bench research studies).
Evidence summary
A systematic review of glucosamine sulfate use for osteoarthritis, based on early research (1956–1991), found that it has anti-inflammatory properties and rebuilds damaged cartilage.1 These studies evaluated chondrocytes grown in culture and animal models.1,2 Chondroitin sulfate also stimulates chondrocyte biosynthesis in both animal and in vitro studies. There is insufficient evidence to demonstrate glucosamine sulfate or chondroitin sulfate stimulates chrondrocyte growth in humans with osteoarthritis.2,3
Joint space narrowing on radiographs suggests progression of osteoarthritis. This narrowing is thought to imply cartilage destruction or loss due to osteoarthritis. A double-blinded randomized controlled trial studied the effect of glucosamine sulfate on tibial-femoral compartment joint space narrowing in 212 patients older than 50 with mild to moderate knee osteoarthritis.4 Patients took either 1500 mg/day of glucosamine sulfate or placebo over 3 years. Knee radiographs in a standing anterior-posterior view, using visual and digital analysis, were used to assess joint space narrowing.5 The average mean joint space loss was 0.31 mm in the placebo group and 0.07 mm in the treatment group (P<.05; 95% confidence interval, 0.13–0.48).
The clinical relevance of knee joint space narrowing is undetermined. Radiographic evaluation of a weight-bearing joint space may not be an accurate or reproducible technique. A study of 15 patients with mild to moderate knee osteoarthritis used standing and semi-flexed radiographic views after an analgesic and nonsteroid anti-inflammatory drug washout period, and 1 to 12 weeks after resumption of analgesic therapy (mean 6.0 weeks).6 Knee pain significantly decreased radiographic joint space in the standing anteriorposterior position, but not in the semiflexed position. Using the standing anterior-posterior method may confound accurate interpretation of joint space narrowing and changes in articular cartilage since glucosamine may have an anti-inflammatory effect.6
One double-blinded randomized controlled trial, comparing chondroitin sulfate with placebo, evaluated joint space in patients with symptomatic hand osteoarthritis.7 One hundred sixty-five Caucasian patients, aged 40 to 70 years, were randomized to receive either a 50-mg intramuscular injection of chondroitin polysulfate, twice weekly, for 8 weeks, every 4 months, versus placebo, or 400 mg of oral chondroitin sulfate, 3 times a day, versus placebo.
Osteoarthritis progression in the metacarpalphalangeal and interphalangeal joints was assessed with radiographs over 3 years. Evaluators used the Anatomic Lesion Progression Scale to assess the development of osteophytes and joint space narrowing, with or without subchondral bone changes, to determine osteoarthritis progression. This scale makes it very difficult to determine whether improvements are clinically significant.
Chondroitin sulfate and polysulfate did not prevent osteoarthritis from occurring in previously normal joints. In joints already affected, intramuscular chondroitin polysulfate significantly reduced progression of distal interphalangeal, proximal interpharangeal, and metacarpophalangeal joint space narrowing (P<.013), using the progression scale. Oral chondroitin sulfate did not prevent progression.7
Recommendations from others
The American College of Rheumatology stated in 2000 that recommending glucosamine sulfate or chondroitin sulfate for osteoarthritis might be premature due to the methodology, lack of standardization, and insufficient information on study designs. More research was recommended.8
These products are sold as supplements in the United States. Their purity is often questionable and thus may affect study results. When studying glucosamine, the National Institutes of Health was forced to manufacture the drug itself due to lack of a reliable amount present in commercial products.9
Fred Tudiver, MD
East Tennessee State University, Johnson City
Most family physicians see many patients with osteoarthritis, which can be difficult to treat. My patients typically want improvement in their symptoms, function, and disease progression. Although there is good evidence that the use of glucosamine sulphate (but not chondroitin sulphate) can improve the common symptoms and functional problems of osteoarthritis, this review states it is unclear whether these substances can alter disease progression through regeneration of cartilage.
I tell my patients with osteoarthritis that glucosamine sulfate can help problems like joint pain and function, but that we do not have a safe and reliable treatment for reversing the disease or the joint damage resulting from it.
1. Towheed TE, Anastassiades TP, Shea B, Houpt J, Welch V, Hochberg MC. Glucosamine therapy for treating osteoarthritis. Cochrane Database Syst Rev 2001;CD002946.-
2. Hauselmann HJ. Nutripharmaceuticals for osteoarthritis. Best Pract Res Clin Rheumatol 2001;15:595-607.
3. de los Reyes GC, Koda RT, Lien EJ. Glucosamine and chondroitin sulfates in the treatment of osteoarthritis: a survey. Prog Drug Res 2000;155:81-103.
4. Jubb RW. Oral and intra-articular remedies: review of papers published from March 2001 to February 2002. Curr Opin Rheumatol 2002;14:597-602.
5. Reginster JY, Deroisy R, Rovati LC, et al. Long-term effects of glucosamine sulphate on osteoarthritis progression: a randomised, placebo-controlled clinical trial. Lancet 2001;357:251-256.
6. Mazzuca SA, Brandt KD, Lane KA, Katz BP. Knee pain reduces joint space width in conventional standing anteroposterior radiographs of osteoarthritic knees. Arthritis Rheum 2002;46:1223-1227.
7. Verbruggen G, Goemaere S, Veys EM. Systems to assess the progression of finger joint osteoarthritis and the effects of disease modifying osteoarthritis drugs. Clin Rheumatol 2002;21:231-243.
8. Recommendations for the medical management of osteoarthritis of the hip and knee: 2000 update. American College of Rheumatology Subcommittee on Osteoarthritis Guidelines. Arthritis Rheum 2000;43:1905-1915.
9. Problems with dietary supplements. Med Lett Drugs Ther 2002;44:84-86.
1. Towheed TE, Anastassiades TP, Shea B, Houpt J, Welch V, Hochberg MC. Glucosamine therapy for treating osteoarthritis. Cochrane Database Syst Rev 2001;CD002946.-
2. Hauselmann HJ. Nutripharmaceuticals for osteoarthritis. Best Pract Res Clin Rheumatol 2001;15:595-607.
3. de los Reyes GC, Koda RT, Lien EJ. Glucosamine and chondroitin sulfates in the treatment of osteoarthritis: a survey. Prog Drug Res 2000;155:81-103.
4. Jubb RW. Oral and intra-articular remedies: review of papers published from March 2001 to February 2002. Curr Opin Rheumatol 2002;14:597-602.
5. Reginster JY, Deroisy R, Rovati LC, et al. Long-term effects of glucosamine sulphate on osteoarthritis progression: a randomised, placebo-controlled clinical trial. Lancet 2001;357:251-256.
6. Mazzuca SA, Brandt KD, Lane KA, Katz BP. Knee pain reduces joint space width in conventional standing anteroposterior radiographs of osteoarthritic knees. Arthritis Rheum 2002;46:1223-1227.
7. Verbruggen G, Goemaere S, Veys EM. Systems to assess the progression of finger joint osteoarthritis and the effects of disease modifying osteoarthritis drugs. Clin Rheumatol 2002;21:231-243.
8. Recommendations for the medical management of osteoarthritis of the hip and knee: 2000 update. American College of Rheumatology Subcommittee on Osteoarthritis Guidelines. Arthritis Rheum 2000;43:1905-1915.
9. Problems with dietary supplements. Med Lett Drugs Ther 2002;44:84-86.
Evidence-based answers from the Family Physicians Inquiries Network