User login
Can recombinant growth hormone effectively treat idiopathic short stature?
Yes, treatment can increase a child’s final height. Injections of recombinant human growth hormone (rGH) at least 3 times a week for 4 to 6 years add 3.7 to 7.5 cm to final height in children between 8 and 16 years of age with idiopathic short stature (strength of recommendation [SOR]: B, 2 small, low-quality, randomized controlled trials [RCTs]). This population comprises children who are otherwise physically and developmentally normal with a height standard deviation score (SDS) of ≤–2.0—comparable to the bottom 2.5% percentile of height—and an adequate response to growth hormone stimulation testing.
Evidence summary
rGH has been available since 1985. The Food and Drug Administration has approved it for such conditions as growth hormone deficiency, chronic renal insufficiency, Turner syndrome, small size for gestational age, and Prader-Willi syndrome.1 The use of rGH to treat idiopathic short stature introduces many clinical, economic, and ethical questions. We have attempted to discern the clinical effectiveness of treatment by focusing on RCTs of rGH therapy while leaving the other substantive issues unexplored.
Final height is arguably the most important outcome measure for the effects of rGH and may be represented as actual height or as a standard deviation score (SDS)—actual height minus mean height for age divided by standard deviation of height for age.2 This measure standardizes height comparisons for different age groups and is comparable to the percentile values on growth charts.
Growth hormone increases height in girls and boys
A 2003 Cochrane systematic review identified 9 RCTs that evaluated treatment with rGH in children with idiopathic short stature. Only 1 used near final height as its main outcome. Inclusion criteria for this RCT comprised prepubertal girls in the bottom third percentile for height without a known cause.
Of the 40 subjects, only 18 provided consent for randomization. Seven of the 10 girls randomized to the treatment group and 6 of the 8 randomized to the control group completed the study to final height measurement. The average age of the treated girls at the start of therapy was 8.07 years; the average duration of treatment was 6.2 years. All participants reached stage 4 breast development, menarche, and a growth velocity of <2 cm per year in the year preceding final height measurement. Mean final height in the treatment group was 155.3 cm compared to 147.8 cm in the control group—a 7.5-cm difference (95% confidence interval [CI], 3.14-11.86 cm).2,3
A double-blind, placebo-controlled RCT published after the Cochrane review assessed final height in a peripubertal, predominantly male population with non-growth-hormone-deficient short stature.4 Inclusion criteria comprised a height SDS <–2.50, but 6 participants with a height SDS between –2.25 and –2.5 were included because of a change in the criteria.5 Sixty-eight children were initially randomized. Of the 37 randomized to treatment, 22 were available for final height measurement. The placebo group had a higher dropout rate—only 11 of 31 patients were available for final height measurement. In an attempt to reduce the dropout rate, the final height criterion for discontinuation of injections was changed from <0.5 to <1.5 cm per year. The mean age of the treatment group was 12.5 years at initiation of treatment; average duration of treatment was 4.6 years.
Intent-to-treat analysis of patients who received at least 6 months of treatment with final height assessment revealed a positive treatment effect on height (SDS) of 0.51. This is the equivalent of a 3.7-cm difference in final height for the treatment group compared with the placebo group (P<.02; 95% CI, 0.10-0.92 SDS).5
Recommendations
The FDA has approved rGH for use in children with height SDS ≤–2.25—equivalent to the lowest 1.2% of children. The Lawson-Wilkins Pediatric Endocrinology Society Drug and Therapeutics Committee states that rGH therapy should be considered only after accurate diagnosis, careful monitoring of growth velocity, and estimation of final height by a pediatric endocrinologist.6,7
1. Weise KL, Nahata MC. Growth hormone use in children with idiopathic short stature. Ann Pharmacother. 2004;38:1460-1468.
2. Bryant J, Cave C, Milne R. Recombinant growth hormone for idiopathic short stature in children and adolescents. Cochrane Database Syst Rev. 2003;(2):CD004440.-
3. McCaughey ES, Mulligan J, Voss LD, et al. Randomised trial of growth hormone in short normal girls. Lancet. 1998;351:940-944.
4. Leschek EW, Rose SR, Yanovski JA, et al. Effect of growth hormone treatment on adult height in peripubertal children with idiopathic short stature: a randomized, double-blind, placebo-controlled trial. J Clin Endocrinol Metab. 2004;89:3140-3148.
5. Humatrope for non-growth-hormone-deficient short stature. Briefing document from the Endocrinologic and Metabolic Drugs Advisory Committee. Lilly Research Laboratories, Eli Lilly and Company, Indianapolis, IN, June 10, 2003, volume 1.
6. FDA approves Humatrope for short stature. Available at: www.fda.gov/bbs/topics/ANSWERS/2003/ANS01242.html. Accessed June 12, 2008.
7. Wilson TA, Rose SR, Cohen P, et al. Update of guidelines for the use of growth hormone in children: the Lawson Wilkins Pediatric Endocrinology Society Drug and Therapeutics Committee. J Pediatr. 2003;143:415-421.
Yes, treatment can increase a child’s final height. Injections of recombinant human growth hormone (rGH) at least 3 times a week for 4 to 6 years add 3.7 to 7.5 cm to final height in children between 8 and 16 years of age with idiopathic short stature (strength of recommendation [SOR]: B, 2 small, low-quality, randomized controlled trials [RCTs]). This population comprises children who are otherwise physically and developmentally normal with a height standard deviation score (SDS) of ≤–2.0—comparable to the bottom 2.5% percentile of height—and an adequate response to growth hormone stimulation testing.
Evidence summary
rGH has been available since 1985. The Food and Drug Administration has approved it for such conditions as growth hormone deficiency, chronic renal insufficiency, Turner syndrome, small size for gestational age, and Prader-Willi syndrome.1 The use of rGH to treat idiopathic short stature introduces many clinical, economic, and ethical questions. We have attempted to discern the clinical effectiveness of treatment by focusing on RCTs of rGH therapy while leaving the other substantive issues unexplored.
Final height is arguably the most important outcome measure for the effects of rGH and may be represented as actual height or as a standard deviation score (SDS)—actual height minus mean height for age divided by standard deviation of height for age.2 This measure standardizes height comparisons for different age groups and is comparable to the percentile values on growth charts.
Growth hormone increases height in girls and boys
A 2003 Cochrane systematic review identified 9 RCTs that evaluated treatment with rGH in children with idiopathic short stature. Only 1 used near final height as its main outcome. Inclusion criteria for this RCT comprised prepubertal girls in the bottom third percentile for height without a known cause.
Of the 40 subjects, only 18 provided consent for randomization. Seven of the 10 girls randomized to the treatment group and 6 of the 8 randomized to the control group completed the study to final height measurement. The average age of the treated girls at the start of therapy was 8.07 years; the average duration of treatment was 6.2 years. All participants reached stage 4 breast development, menarche, and a growth velocity of <2 cm per year in the year preceding final height measurement. Mean final height in the treatment group was 155.3 cm compared to 147.8 cm in the control group—a 7.5-cm difference (95% confidence interval [CI], 3.14-11.86 cm).2,3
A double-blind, placebo-controlled RCT published after the Cochrane review assessed final height in a peripubertal, predominantly male population with non-growth-hormone-deficient short stature.4 Inclusion criteria comprised a height SDS <–2.50, but 6 participants with a height SDS between –2.25 and –2.5 were included because of a change in the criteria.5 Sixty-eight children were initially randomized. Of the 37 randomized to treatment, 22 were available for final height measurement. The placebo group had a higher dropout rate—only 11 of 31 patients were available for final height measurement. In an attempt to reduce the dropout rate, the final height criterion for discontinuation of injections was changed from <0.5 to <1.5 cm per year. The mean age of the treatment group was 12.5 years at initiation of treatment; average duration of treatment was 4.6 years.
Intent-to-treat analysis of patients who received at least 6 months of treatment with final height assessment revealed a positive treatment effect on height (SDS) of 0.51. This is the equivalent of a 3.7-cm difference in final height for the treatment group compared with the placebo group (P<.02; 95% CI, 0.10-0.92 SDS).5
Recommendations
The FDA has approved rGH for use in children with height SDS ≤–2.25—equivalent to the lowest 1.2% of children. The Lawson-Wilkins Pediatric Endocrinology Society Drug and Therapeutics Committee states that rGH therapy should be considered only after accurate diagnosis, careful monitoring of growth velocity, and estimation of final height by a pediatric endocrinologist.6,7
Yes, treatment can increase a child’s final height. Injections of recombinant human growth hormone (rGH) at least 3 times a week for 4 to 6 years add 3.7 to 7.5 cm to final height in children between 8 and 16 years of age with idiopathic short stature (strength of recommendation [SOR]: B, 2 small, low-quality, randomized controlled trials [RCTs]). This population comprises children who are otherwise physically and developmentally normal with a height standard deviation score (SDS) of ≤–2.0—comparable to the bottom 2.5% percentile of height—and an adequate response to growth hormone stimulation testing.
Evidence summary
rGH has been available since 1985. The Food and Drug Administration has approved it for such conditions as growth hormone deficiency, chronic renal insufficiency, Turner syndrome, small size for gestational age, and Prader-Willi syndrome.1 The use of rGH to treat idiopathic short stature introduces many clinical, economic, and ethical questions. We have attempted to discern the clinical effectiveness of treatment by focusing on RCTs of rGH therapy while leaving the other substantive issues unexplored.
Final height is arguably the most important outcome measure for the effects of rGH and may be represented as actual height or as a standard deviation score (SDS)—actual height minus mean height for age divided by standard deviation of height for age.2 This measure standardizes height comparisons for different age groups and is comparable to the percentile values on growth charts.
Growth hormone increases height in girls and boys
A 2003 Cochrane systematic review identified 9 RCTs that evaluated treatment with rGH in children with idiopathic short stature. Only 1 used near final height as its main outcome. Inclusion criteria for this RCT comprised prepubertal girls in the bottom third percentile for height without a known cause.
Of the 40 subjects, only 18 provided consent for randomization. Seven of the 10 girls randomized to the treatment group and 6 of the 8 randomized to the control group completed the study to final height measurement. The average age of the treated girls at the start of therapy was 8.07 years; the average duration of treatment was 6.2 years. All participants reached stage 4 breast development, menarche, and a growth velocity of <2 cm per year in the year preceding final height measurement. Mean final height in the treatment group was 155.3 cm compared to 147.8 cm in the control group—a 7.5-cm difference (95% confidence interval [CI], 3.14-11.86 cm).2,3
A double-blind, placebo-controlled RCT published after the Cochrane review assessed final height in a peripubertal, predominantly male population with non-growth-hormone-deficient short stature.4 Inclusion criteria comprised a height SDS <–2.50, but 6 participants with a height SDS between –2.25 and –2.5 were included because of a change in the criteria.5 Sixty-eight children were initially randomized. Of the 37 randomized to treatment, 22 were available for final height measurement. The placebo group had a higher dropout rate—only 11 of 31 patients were available for final height measurement. In an attempt to reduce the dropout rate, the final height criterion for discontinuation of injections was changed from <0.5 to <1.5 cm per year. The mean age of the treatment group was 12.5 years at initiation of treatment; average duration of treatment was 4.6 years.
Intent-to-treat analysis of patients who received at least 6 months of treatment with final height assessment revealed a positive treatment effect on height (SDS) of 0.51. This is the equivalent of a 3.7-cm difference in final height for the treatment group compared with the placebo group (P<.02; 95% CI, 0.10-0.92 SDS).5
Recommendations
The FDA has approved rGH for use in children with height SDS ≤–2.25—equivalent to the lowest 1.2% of children. The Lawson-Wilkins Pediatric Endocrinology Society Drug and Therapeutics Committee states that rGH therapy should be considered only after accurate diagnosis, careful monitoring of growth velocity, and estimation of final height by a pediatric endocrinologist.6,7
1. Weise KL, Nahata MC. Growth hormone use in children with idiopathic short stature. Ann Pharmacother. 2004;38:1460-1468.
2. Bryant J, Cave C, Milne R. Recombinant growth hormone for idiopathic short stature in children and adolescents. Cochrane Database Syst Rev. 2003;(2):CD004440.-
3. McCaughey ES, Mulligan J, Voss LD, et al. Randomised trial of growth hormone in short normal girls. Lancet. 1998;351:940-944.
4. Leschek EW, Rose SR, Yanovski JA, et al. Effect of growth hormone treatment on adult height in peripubertal children with idiopathic short stature: a randomized, double-blind, placebo-controlled trial. J Clin Endocrinol Metab. 2004;89:3140-3148.
5. Humatrope for non-growth-hormone-deficient short stature. Briefing document from the Endocrinologic and Metabolic Drugs Advisory Committee. Lilly Research Laboratories, Eli Lilly and Company, Indianapolis, IN, June 10, 2003, volume 1.
6. FDA approves Humatrope for short stature. Available at: www.fda.gov/bbs/topics/ANSWERS/2003/ANS01242.html. Accessed June 12, 2008.
7. Wilson TA, Rose SR, Cohen P, et al. Update of guidelines for the use of growth hormone in children: the Lawson Wilkins Pediatric Endocrinology Society Drug and Therapeutics Committee. J Pediatr. 2003;143:415-421.
1. Weise KL, Nahata MC. Growth hormone use in children with idiopathic short stature. Ann Pharmacother. 2004;38:1460-1468.
2. Bryant J, Cave C, Milne R. Recombinant growth hormone for idiopathic short stature in children and adolescents. Cochrane Database Syst Rev. 2003;(2):CD004440.-
3. McCaughey ES, Mulligan J, Voss LD, et al. Randomised trial of growth hormone in short normal girls. Lancet. 1998;351:940-944.
4. Leschek EW, Rose SR, Yanovski JA, et al. Effect of growth hormone treatment on adult height in peripubertal children with idiopathic short stature: a randomized, double-blind, placebo-controlled trial. J Clin Endocrinol Metab. 2004;89:3140-3148.
5. Humatrope for non-growth-hormone-deficient short stature. Briefing document from the Endocrinologic and Metabolic Drugs Advisory Committee. Lilly Research Laboratories, Eli Lilly and Company, Indianapolis, IN, June 10, 2003, volume 1.
6. FDA approves Humatrope for short stature. Available at: www.fda.gov/bbs/topics/ANSWERS/2003/ANS01242.html. Accessed June 12, 2008.
7. Wilson TA, Rose SR, Cohen P, et al. Update of guidelines for the use of growth hormone in children: the Lawson Wilkins Pediatric Endocrinology Society Drug and Therapeutics Committee. J Pediatr. 2003;143:415-421.
Evidence-based answers from the Family Physicians Inquiries Network
Does bed rest for preeclampsia improve neonatal outcomes?
No. strict bed rest in the hospital for pregnant women with preeclampsia does not appear to lower rates of perinatal mortality, neonatal mortality, or neonatal morbidity, including preterm birth, endotracheal intubations, or neonatal intensive care unit (NICU) admissions (strength of recommendation: B, based on 2 randomized controlled trials [RCT] and extrapolations from 2 RCTs of pregnant patients with nonproteinuric hypertension).
Ronald Januchowski, MD
Spartanburg Regional Medical Center, Spartanburg, SC
Changing long-standing practices is always a challenge We’ve said goodbye to magnesium for preterm labor, and now it looks like bed rest for preeclampsia is not far behind. Changing long-standing practices in response to stronger evidence-based information is always a challenge, especially when we’ve been relying on long-standing expert opinion or anecdotal evidence. Following these recommendations will be another challenge for us, even though we consider the relationship we have with our obstetrical nurses and physicians to be a good one.
Our plan to introduce these modifications will follow previous successful plans; the member of our group with the most “capital” in Obstetrics can bring others on board.
Evidence summary
Ten percent of preeclampsia occurs in pregnancies at less than 34 weeks of gestation. Traditionally, physicians often recommended bed rest to preterm, preeclamptic patients in the belief that it would improve neonatal outcomes.
RCTs find no difference between bed rest and ad lib movement
A single-center RCT investigated bed rest treatment for 105 patients with preeclampsia and gestational ages between 26 to 38 weeks. Patients were assigned to either strict bed rest with bathroom privileges in the hospital until delivery, or to bed rest with the ability to move freely around the hospital. Outcome assessors were not blinded to patient treatment allocation. There was no statistical difference between the 2 groups in perinatal or neonatal mortality, or in the neonatal morbidities of preterm births, endotracheal intubations, or NICU admissions.1
Similarly, a small, unblinded RCT of 40 preeclamptic patients treated in the hospital with strict bed confinement or without restrictions found no significant difference in fetal or perinatal mortality.2 No power calculations were reported for detecting differences in neonatal outcome rates in either of these studies.
Studies in nonproteinuric hypertension were no different
In addition to the studies in patients with preeclampsia, 2 RCTs measured neonatal outcomes with bed rest compared with normal activity in pregnancies complicated by nonproteinuric hypertension. These studies also found that bed rest did not improve neonatal outcomes.
The first trial was a multicenter RCT involving 218 patients between 28 to 38 weeks gestation with nonproteinuric hypertension (blood pressure >140/90 mm Hg). The patients were randomized to 2 groups: bed rest in the hospital but allowed to move around the ward, and normal activity at home without restrictions. The outcomes were measured by masked assessors. There were no statistical differences in perinatal or neonatal mortality, or in the neonatal morbidities of preterm birth, newborns small for their gestational age, or NICU admissions between the 2 groups.3
A second RCT of 135 nonproteinuric but hypertensive pregnant patients with diastolic blood pressures between 90 and 109 mm Hg also demonstrated no difference between patients treated with bed rest and sedation or normal activity in fetal or neonatal outcomes.4
Recommendations from others
An American College of Obstetrics and Gynecology practice bulletin on diagnosis and management of preeclampsia and eclampsia does not mention bed rest.5 The Canadian Hypertension Society Consensus Conference in 1997 stated that a “policy of hospital admission and strict bed rest is not advised for gestational hypertension with or without proteinuria.”6
1. Meher S, Abalos E, Carroli G. Bed rest with or without hospitalisation for hypertension during pregnancy. Cochrane Database Syst Rev 2005;CD(4):003-514.
2. Matthews DD, Agarwal V, Shuttleworth TP. A randomized controlled trial of complete bed rest versus ambulation in the management of proteinuric hypertension during pregnancy. Br J Obstet Gynecol 1982;89:128-131.
3. Crowther CA, Bouwmeester Am, Ashurst Hm. Does admission to hospital for bed rest prevent disease progression or improve fetal outcome in pregnancy complicated by non-proteinuric hyper-tension? Br J Obstet Gynecol 1992;99:13-17.
4. Matthews DD. A randomized controlled trial of bed rest and sedation or normal activity and non-sedation in the management of non-albuminuric hypertension in late pregnancy. Br J Obstet Gynecol 1977;84:108-114.
5. Diagnosis and management of preeclampsia and eclampsia. American College of obstetrics and Gynecology (ACoG) Practice bulletin, No. 33. Obstet Gynecol 2002;99:159-67.
6. Report of the Canadian Hypertension society Consensus Conference: Nonpharmacologic management and prevention of hypertensive disorders in pregnancy. Can Med Assoc J 1997;157:907-919.
No. strict bed rest in the hospital for pregnant women with preeclampsia does not appear to lower rates of perinatal mortality, neonatal mortality, or neonatal morbidity, including preterm birth, endotracheal intubations, or neonatal intensive care unit (NICU) admissions (strength of recommendation: B, based on 2 randomized controlled trials [RCT] and extrapolations from 2 RCTs of pregnant patients with nonproteinuric hypertension).
Ronald Januchowski, MD
Spartanburg Regional Medical Center, Spartanburg, SC
Changing long-standing practices is always a challenge We’ve said goodbye to magnesium for preterm labor, and now it looks like bed rest for preeclampsia is not far behind. Changing long-standing practices in response to stronger evidence-based information is always a challenge, especially when we’ve been relying on long-standing expert opinion or anecdotal evidence. Following these recommendations will be another challenge for us, even though we consider the relationship we have with our obstetrical nurses and physicians to be a good one.
Our plan to introduce these modifications will follow previous successful plans; the member of our group with the most “capital” in Obstetrics can bring others on board.
Evidence summary
Ten percent of preeclampsia occurs in pregnancies at less than 34 weeks of gestation. Traditionally, physicians often recommended bed rest to preterm, preeclamptic patients in the belief that it would improve neonatal outcomes.
RCTs find no difference between bed rest and ad lib movement
A single-center RCT investigated bed rest treatment for 105 patients with preeclampsia and gestational ages between 26 to 38 weeks. Patients were assigned to either strict bed rest with bathroom privileges in the hospital until delivery, or to bed rest with the ability to move freely around the hospital. Outcome assessors were not blinded to patient treatment allocation. There was no statistical difference between the 2 groups in perinatal or neonatal mortality, or in the neonatal morbidities of preterm births, endotracheal intubations, or NICU admissions.1
Similarly, a small, unblinded RCT of 40 preeclamptic patients treated in the hospital with strict bed confinement or without restrictions found no significant difference in fetal or perinatal mortality.2 No power calculations were reported for detecting differences in neonatal outcome rates in either of these studies.
Studies in nonproteinuric hypertension were no different
In addition to the studies in patients with preeclampsia, 2 RCTs measured neonatal outcomes with bed rest compared with normal activity in pregnancies complicated by nonproteinuric hypertension. These studies also found that bed rest did not improve neonatal outcomes.
The first trial was a multicenter RCT involving 218 patients between 28 to 38 weeks gestation with nonproteinuric hypertension (blood pressure >140/90 mm Hg). The patients were randomized to 2 groups: bed rest in the hospital but allowed to move around the ward, and normal activity at home without restrictions. The outcomes were measured by masked assessors. There were no statistical differences in perinatal or neonatal mortality, or in the neonatal morbidities of preterm birth, newborns small for their gestational age, or NICU admissions between the 2 groups.3
A second RCT of 135 nonproteinuric but hypertensive pregnant patients with diastolic blood pressures between 90 and 109 mm Hg also demonstrated no difference between patients treated with bed rest and sedation or normal activity in fetal or neonatal outcomes.4
Recommendations from others
An American College of Obstetrics and Gynecology practice bulletin on diagnosis and management of preeclampsia and eclampsia does not mention bed rest.5 The Canadian Hypertension Society Consensus Conference in 1997 stated that a “policy of hospital admission and strict bed rest is not advised for gestational hypertension with or without proteinuria.”6
No. strict bed rest in the hospital for pregnant women with preeclampsia does not appear to lower rates of perinatal mortality, neonatal mortality, or neonatal morbidity, including preterm birth, endotracheal intubations, or neonatal intensive care unit (NICU) admissions (strength of recommendation: B, based on 2 randomized controlled trials [RCT] and extrapolations from 2 RCTs of pregnant patients with nonproteinuric hypertension).
Ronald Januchowski, MD
Spartanburg Regional Medical Center, Spartanburg, SC
Changing long-standing practices is always a challenge We’ve said goodbye to magnesium for preterm labor, and now it looks like bed rest for preeclampsia is not far behind. Changing long-standing practices in response to stronger evidence-based information is always a challenge, especially when we’ve been relying on long-standing expert opinion or anecdotal evidence. Following these recommendations will be another challenge for us, even though we consider the relationship we have with our obstetrical nurses and physicians to be a good one.
Our plan to introduce these modifications will follow previous successful plans; the member of our group with the most “capital” in Obstetrics can bring others on board.
Evidence summary
Ten percent of preeclampsia occurs in pregnancies at less than 34 weeks of gestation. Traditionally, physicians often recommended bed rest to preterm, preeclamptic patients in the belief that it would improve neonatal outcomes.
RCTs find no difference between bed rest and ad lib movement
A single-center RCT investigated bed rest treatment for 105 patients with preeclampsia and gestational ages between 26 to 38 weeks. Patients were assigned to either strict bed rest with bathroom privileges in the hospital until delivery, or to bed rest with the ability to move freely around the hospital. Outcome assessors were not blinded to patient treatment allocation. There was no statistical difference between the 2 groups in perinatal or neonatal mortality, or in the neonatal morbidities of preterm births, endotracheal intubations, or NICU admissions.1
Similarly, a small, unblinded RCT of 40 preeclamptic patients treated in the hospital with strict bed confinement or without restrictions found no significant difference in fetal or perinatal mortality.2 No power calculations were reported for detecting differences in neonatal outcome rates in either of these studies.
Studies in nonproteinuric hypertension were no different
In addition to the studies in patients with preeclampsia, 2 RCTs measured neonatal outcomes with bed rest compared with normal activity in pregnancies complicated by nonproteinuric hypertension. These studies also found that bed rest did not improve neonatal outcomes.
The first trial was a multicenter RCT involving 218 patients between 28 to 38 weeks gestation with nonproteinuric hypertension (blood pressure >140/90 mm Hg). The patients were randomized to 2 groups: bed rest in the hospital but allowed to move around the ward, and normal activity at home without restrictions. The outcomes were measured by masked assessors. There were no statistical differences in perinatal or neonatal mortality, or in the neonatal morbidities of preterm birth, newborns small for their gestational age, or NICU admissions between the 2 groups.3
A second RCT of 135 nonproteinuric but hypertensive pregnant patients with diastolic blood pressures between 90 and 109 mm Hg also demonstrated no difference between patients treated with bed rest and sedation or normal activity in fetal or neonatal outcomes.4
Recommendations from others
An American College of Obstetrics and Gynecology practice bulletin on diagnosis and management of preeclampsia and eclampsia does not mention bed rest.5 The Canadian Hypertension Society Consensus Conference in 1997 stated that a “policy of hospital admission and strict bed rest is not advised for gestational hypertension with or without proteinuria.”6
1. Meher S, Abalos E, Carroli G. Bed rest with or without hospitalisation for hypertension during pregnancy. Cochrane Database Syst Rev 2005;CD(4):003-514.
2. Matthews DD, Agarwal V, Shuttleworth TP. A randomized controlled trial of complete bed rest versus ambulation in the management of proteinuric hypertension during pregnancy. Br J Obstet Gynecol 1982;89:128-131.
3. Crowther CA, Bouwmeester Am, Ashurst Hm. Does admission to hospital for bed rest prevent disease progression or improve fetal outcome in pregnancy complicated by non-proteinuric hyper-tension? Br J Obstet Gynecol 1992;99:13-17.
4. Matthews DD. A randomized controlled trial of bed rest and sedation or normal activity and non-sedation in the management of non-albuminuric hypertension in late pregnancy. Br J Obstet Gynecol 1977;84:108-114.
5. Diagnosis and management of preeclampsia and eclampsia. American College of obstetrics and Gynecology (ACoG) Practice bulletin, No. 33. Obstet Gynecol 2002;99:159-67.
6. Report of the Canadian Hypertension society Consensus Conference: Nonpharmacologic management and prevention of hypertensive disorders in pregnancy. Can Med Assoc J 1997;157:907-919.
1. Meher S, Abalos E, Carroli G. Bed rest with or without hospitalisation for hypertension during pregnancy. Cochrane Database Syst Rev 2005;CD(4):003-514.
2. Matthews DD, Agarwal V, Shuttleworth TP. A randomized controlled trial of complete bed rest versus ambulation in the management of proteinuric hypertension during pregnancy. Br J Obstet Gynecol 1982;89:128-131.
3. Crowther CA, Bouwmeester Am, Ashurst Hm. Does admission to hospital for bed rest prevent disease progression or improve fetal outcome in pregnancy complicated by non-proteinuric hyper-tension? Br J Obstet Gynecol 1992;99:13-17.
4. Matthews DD. A randomized controlled trial of bed rest and sedation or normal activity and non-sedation in the management of non-albuminuric hypertension in late pregnancy. Br J Obstet Gynecol 1977;84:108-114.
5. Diagnosis and management of preeclampsia and eclampsia. American College of obstetrics and Gynecology (ACoG) Practice bulletin, No. 33. Obstet Gynecol 2002;99:159-67.
6. Report of the Canadian Hypertension society Consensus Conference: Nonpharmacologic management and prevention of hypertensive disorders in pregnancy. Can Med Assoc J 1997;157:907-919.
Evidence-based answers from the Family Physicians Inquiries Network
What is the most effective management of acute fractures of the base of the fifth metatarsal?
For acute Jones’ fractures in recreationally active patients, early intramedullary screw fixation results in lower failure rates and shorter times to both clinical union and return to sports than non-weightbearing short leg casting (strength of recommendation [SOR]: A, based on 2 randomized controlled trials (RCT)]. Non-weightbearing short leg casting achieves union in 56% to 100% of patients but can require prolonged casting (SOR: B, based on 2 prospective cohorts and multiple retrospective, follow-up studies). Stress fractures were not included in this review.
For avulsion fractures of the fifth metatarsal tuberosity, a soft Jones–dressing allows earlier return to pre-injury levels of activity than rigid short leg casting (SOR: B, based on a lower-quality RCT).
For athletes, surgical correction of all Jones-type fractures usually preferred
Douglas F. Aukerman, MD
Family and Community Medicine, The Milton S. Hershey, Medical Center, Penn State University
Fifth metatarsal fractures are frequently seen in clinical practice. When faced with a fifth metatarsal fracture, determine its exact location, which influences treatment. Acute fractures to the proximal end of the bone within the cancellous bone area, if nondisplaced, do very well with closed treatment.
Fractures between the insertion of the peroneus brevis and tertius tendons, which marks a transition from mostly cancellous to relatively avascular cortical bone, can be problematic. This injury, often called a Jones fracture, needs to be identified as a chronic stress injury, which uniformly does not heal well, an acute or chronic stress injury, or a pure acute injury. For athletes, both young and old, I prefer surgical correction of all Jones-type fractures to ensure a more definitive return to athletics. For the non-athlete, I allow the patient to make an informed decision for immediate surgical correction or for an attempt at closed treatment if it is not a chronic stress failure of the bone. I find that patients who choose closed treatment and understand the possible prolonged treatment course are not upset if they need surgical treatment for nonunion and are pleased with the option and attempt of not having surgery.
Evidence summary
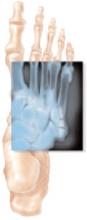
Fractures within 1.5 cm of the fifth metatarsal tuberosity, without extension distal to the fourth-fifth intermetatarsal articulation, occurring with less than 2-week symptom prodrome and without a history of previous fracture, are defined as “acute Jones’ fractures” (FIGURE). In a recent RCT by Mologne et al,1 37 active-duty military personnel with acute Jones’ fractures were randomized to either 8 weeks of no weight-bearing in a short leg casting, followed by a walking cast or hard-soled shoe until clinical union; or to early outpatient intramedullary screw fixation followed by no weight-bearing for 2 weeks, then weight-bearing as tolerated in a hard-soled shoe until clinical union. Screw fixation significantly reduced both time to clinical union and time to return to sports—by nearly 50% when compared with non-weightbearing short leg casting. Furthermore, at 26 weeks the casting group saw a significant 44% failure rate compared with only 5% in the surgical group (number needed to treat [NNT]=2.6). Six patients in the surgical group had mild discomfort from the screw head, and 3 needed the screw to be removed. Generalization of the results was limited by the mostly male military population.
The rates and times of union with short leg casting vary over a wide range in the research literature. The casting group in the RCT above had union rates of 56% and median time to union of 14.5 weeks (lower and upper quartile range, 10.5–18.5).1 A prospective registry of 68 consecutive acute Jones’ fractures in primarily young military service members showed a 72% union rate with non-weightbearing short leg casting with average time to union of 21.2 weeks.2 A heterogeneous group of 5 retrospective follow-up studies of short leg casting reported wide ranges in union rates of 72% to 100%, and in time to healing of 7 weeks to 21 months.3-7 These studies varied in average age, sex, and athletic ability of their samples as well as type of immobilization and weight-bearing status during treatment.
Tuberosity avulsion fractures are proximal fifth metatarsal styloid fractures resulting from a forceful pull of the lateral band of the long plantar ligament or the peroneus brevis tendon during ankle inversion. A 12-week RCT in 89 consecutive patients presenting to an emergency department with fifth metatarsal tuberosity avulsion fractures compared a nonrigid, soft Jones’ dressing consisting of alternating layers of cast padding and elastic bandages with a rigid short leg casting.8 The Jones’ dressing had a significant 28% reduction in time to return to pre-injury levels of activity. Other outcomes—time in treatment modality, time to radiographic healing, and functional foot score—were not different between intervention groups. Validity was limited by the 32% lost to follow-up rate.
FIGURE
Acute fracture of the fifth metatarsal
Acute Jones’ fractures are repaired with screw fixation of the broken bone using fluoroscopy. Patients may return to full activity when radiographs show that the bones were healing at the site of the fracture.
Recommendations from others
We were unable to locate any consensus statements or clinical guidelines regarding the treatment of Jones’ fractures.
DeLee and Drez’s Orthopaedic Sports Medicine recommends immobilization in a cast or below-the-knee boot with strict non-weightbearing for at least 6 weeks for acute Jones’ fractures.9 It recommends surgical treatment, followed by 6 weeks of cast immobilization, then progression to weight bearing based on radiographic findings, for nonoperative treatment failures or with desire to return high-performance athletes to activity.
In Fracture Management for Primary Care, the authors recommend posterior splinting and non-weightbearing with crutches for acute Jones’ fractures, followed by non-weightbearing short leg casting application at 3 to 5 days from injury.10 After a minimum of 6 to 8 weeks of casting, they recommend options of 4 additional weeks of casting or internal fixation for clinical or radiographic nonunion.
For tuberosity avulsion fractures, the authors recommend use of a firm-soled shoe for 4 to 8 weeks. For patients with discomfort at an initial 4- to 7-day follow-up, they give an option of using a walking short leg casting for 2 weeks, with follow-up every 2 to 4 weeks until clinical healing.
1. Mologne TS, Lundeen JM, Clapper MF, O’Brien TJ. Early screw fixation versus casting in the treatment of acute Jones fractures. Am J Sports Med 2005;33:970-975.
2. Clapper MF, O’Brien TJ, Lyons PM. Fractures of the fifth metatarsal: Analysis of a fracture registry. Clin Orthop Relat Res 1995;315:238-241.
3. Dameron TB. Fractures and anatomical variations of the proximal portion of the fifth metatarsal. J Bone Joint Surg Am 1975;57:788-792.
4. Torg JS, Balduini FC, Zelko RR, Pavlov H, Peff TC, Das M. Fractures of the base of the fifth metatarsal distal to the tuberosity. J Bone Joint Surg Am 1984;66:209-214.
5. Seitz WH, Grantham SA. The Jones’ fracture in the non-athlete. Foot Ankle 1985;6:97-100.
6. Josefsson PO, Karlsson M, Redlund-Johnell I, Wendeberg B. Closed treatment of Jones fracture: Good results in 40 cases after 11-26 years. Acta Orthop Scand 1994;65:545-547.
7. Josefsson PO, Karlsson M, Redlund-Johnell I, Wendeberg B. Jones fracture: Surgical versus nonsurgical treatment. Clin Orthop Related Res 1994;299:252-255.
8. Wiener BD, Linder JF, Giattini JF. Treatment of fractures of the fifth metatarsal: a prospective study. Foot Ankle Int 1997;18:267-269.
9. Brodsky JW, Krause JO. Stress fractures of the foot and ankle. In: Delee JC, Drez, D, Miller MD, eds. DeLee and Drez’s Orthopaedic Sports Medicine. Philadelphia, Pa: Saunders; 2003:2403-2406.
10. Metatarsal fractures. In: Eiff MP, Hatch RL, Calmbach WL. Fracture Management for Primary Care. Philadelphia, Pa: Saunders; 2003:345-349.
For acute Jones’ fractures in recreationally active patients, early intramedullary screw fixation results in lower failure rates and shorter times to both clinical union and return to sports than non-weightbearing short leg casting (strength of recommendation [SOR]: A, based on 2 randomized controlled trials (RCT)]. Non-weightbearing short leg casting achieves union in 56% to 100% of patients but can require prolonged casting (SOR: B, based on 2 prospective cohorts and multiple retrospective, follow-up studies). Stress fractures were not included in this review.
For avulsion fractures of the fifth metatarsal tuberosity, a soft Jones–dressing allows earlier return to pre-injury levels of activity than rigid short leg casting (SOR: B, based on a lower-quality RCT).
For athletes, surgical correction of all Jones-type fractures usually preferred
Douglas F. Aukerman, MD
Family and Community Medicine, The Milton S. Hershey, Medical Center, Penn State University
Fifth metatarsal fractures are frequently seen in clinical practice. When faced with a fifth metatarsal fracture, determine its exact location, which influences treatment. Acute fractures to the proximal end of the bone within the cancellous bone area, if nondisplaced, do very well with closed treatment.
Fractures between the insertion of the peroneus brevis and tertius tendons, which marks a transition from mostly cancellous to relatively avascular cortical bone, can be problematic. This injury, often called a Jones fracture, needs to be identified as a chronic stress injury, which uniformly does not heal well, an acute or chronic stress injury, or a pure acute injury. For athletes, both young and old, I prefer surgical correction of all Jones-type fractures to ensure a more definitive return to athletics. For the non-athlete, I allow the patient to make an informed decision for immediate surgical correction or for an attempt at closed treatment if it is not a chronic stress failure of the bone. I find that patients who choose closed treatment and understand the possible prolonged treatment course are not upset if they need surgical treatment for nonunion and are pleased with the option and attempt of not having surgery.
Evidence summary
Fractures within 1.5 cm of the fifth metatarsal tuberosity, without extension distal to the fourth-fifth intermetatarsal articulation, occurring with less than 2-week symptom prodrome and without a history of previous fracture, are defined as “acute Jones’ fractures” (FIGURE). In a recent RCT by Mologne et al,1 37 active-duty military personnel with acute Jones’ fractures were randomized to either 8 weeks of no weight-bearing in a short leg casting, followed by a walking cast or hard-soled shoe until clinical union; or to early outpatient intramedullary screw fixation followed by no weight-bearing for 2 weeks, then weight-bearing as tolerated in a hard-soled shoe until clinical union. Screw fixation significantly reduced both time to clinical union and time to return to sports—by nearly 50% when compared with non-weightbearing short leg casting. Furthermore, at 26 weeks the casting group saw a significant 44% failure rate compared with only 5% in the surgical group (number needed to treat [NNT]=2.6). Six patients in the surgical group had mild discomfort from the screw head, and 3 needed the screw to be removed. Generalization of the results was limited by the mostly male military population.
The rates and times of union with short leg casting vary over a wide range in the research literature. The casting group in the RCT above had union rates of 56% and median time to union of 14.5 weeks (lower and upper quartile range, 10.5–18.5).1 A prospective registry of 68 consecutive acute Jones’ fractures in primarily young military service members showed a 72% union rate with non-weightbearing short leg casting with average time to union of 21.2 weeks.2 A heterogeneous group of 5 retrospective follow-up studies of short leg casting reported wide ranges in union rates of 72% to 100%, and in time to healing of 7 weeks to 21 months.3-7 These studies varied in average age, sex, and athletic ability of their samples as well as type of immobilization and weight-bearing status during treatment.
Tuberosity avulsion fractures are proximal fifth metatarsal styloid fractures resulting from a forceful pull of the lateral band of the long plantar ligament or the peroneus brevis tendon during ankle inversion. A 12-week RCT in 89 consecutive patients presenting to an emergency department with fifth metatarsal tuberosity avulsion fractures compared a nonrigid, soft Jones’ dressing consisting of alternating layers of cast padding and elastic bandages with a rigid short leg casting.8 The Jones’ dressing had a significant 28% reduction in time to return to pre-injury levels of activity. Other outcomes—time in treatment modality, time to radiographic healing, and functional foot score—were not different between intervention groups. Validity was limited by the 32% lost to follow-up rate.
FIGURE
Acute fracture of the fifth metatarsal
Acute Jones’ fractures are repaired with screw fixation of the broken bone using fluoroscopy. Patients may return to full activity when radiographs show that the bones were healing at the site of the fracture.
Recommendations from others
We were unable to locate any consensus statements or clinical guidelines regarding the treatment of Jones’ fractures.
DeLee and Drez’s Orthopaedic Sports Medicine recommends immobilization in a cast or below-the-knee boot with strict non-weightbearing for at least 6 weeks for acute Jones’ fractures.9 It recommends surgical treatment, followed by 6 weeks of cast immobilization, then progression to weight bearing based on radiographic findings, for nonoperative treatment failures or with desire to return high-performance athletes to activity.
In Fracture Management for Primary Care, the authors recommend posterior splinting and non-weightbearing with crutches for acute Jones’ fractures, followed by non-weightbearing short leg casting application at 3 to 5 days from injury.10 After a minimum of 6 to 8 weeks of casting, they recommend options of 4 additional weeks of casting or internal fixation for clinical or radiographic nonunion.
For tuberosity avulsion fractures, the authors recommend use of a firm-soled shoe for 4 to 8 weeks. For patients with discomfort at an initial 4- to 7-day follow-up, they give an option of using a walking short leg casting for 2 weeks, with follow-up every 2 to 4 weeks until clinical healing.
For acute Jones’ fractures in recreationally active patients, early intramedullary screw fixation results in lower failure rates and shorter times to both clinical union and return to sports than non-weightbearing short leg casting (strength of recommendation [SOR]: A, based on 2 randomized controlled trials (RCT)]. Non-weightbearing short leg casting achieves union in 56% to 100% of patients but can require prolonged casting (SOR: B, based on 2 prospective cohorts and multiple retrospective, follow-up studies). Stress fractures were not included in this review.
For avulsion fractures of the fifth metatarsal tuberosity, a soft Jones–dressing allows earlier return to pre-injury levels of activity than rigid short leg casting (SOR: B, based on a lower-quality RCT).
For athletes, surgical correction of all Jones-type fractures usually preferred
Douglas F. Aukerman, MD
Family and Community Medicine, The Milton S. Hershey, Medical Center, Penn State University
Fifth metatarsal fractures are frequently seen in clinical practice. When faced with a fifth metatarsal fracture, determine its exact location, which influences treatment. Acute fractures to the proximal end of the bone within the cancellous bone area, if nondisplaced, do very well with closed treatment.
Fractures between the insertion of the peroneus brevis and tertius tendons, which marks a transition from mostly cancellous to relatively avascular cortical bone, can be problematic. This injury, often called a Jones fracture, needs to be identified as a chronic stress injury, which uniformly does not heal well, an acute or chronic stress injury, or a pure acute injury. For athletes, both young and old, I prefer surgical correction of all Jones-type fractures to ensure a more definitive return to athletics. For the non-athlete, I allow the patient to make an informed decision for immediate surgical correction or for an attempt at closed treatment if it is not a chronic stress failure of the bone. I find that patients who choose closed treatment and understand the possible prolonged treatment course are not upset if they need surgical treatment for nonunion and are pleased with the option and attempt of not having surgery.
Evidence summary
Fractures within 1.5 cm of the fifth metatarsal tuberosity, without extension distal to the fourth-fifth intermetatarsal articulation, occurring with less than 2-week symptom prodrome and without a history of previous fracture, are defined as “acute Jones’ fractures” (FIGURE). In a recent RCT by Mologne et al,1 37 active-duty military personnel with acute Jones’ fractures were randomized to either 8 weeks of no weight-bearing in a short leg casting, followed by a walking cast or hard-soled shoe until clinical union; or to early outpatient intramedullary screw fixation followed by no weight-bearing for 2 weeks, then weight-bearing as tolerated in a hard-soled shoe until clinical union. Screw fixation significantly reduced both time to clinical union and time to return to sports—by nearly 50% when compared with non-weightbearing short leg casting. Furthermore, at 26 weeks the casting group saw a significant 44% failure rate compared with only 5% in the surgical group (number needed to treat [NNT]=2.6). Six patients in the surgical group had mild discomfort from the screw head, and 3 needed the screw to be removed. Generalization of the results was limited by the mostly male military population.
The rates and times of union with short leg casting vary over a wide range in the research literature. The casting group in the RCT above had union rates of 56% and median time to union of 14.5 weeks (lower and upper quartile range, 10.5–18.5).1 A prospective registry of 68 consecutive acute Jones’ fractures in primarily young military service members showed a 72% union rate with non-weightbearing short leg casting with average time to union of 21.2 weeks.2 A heterogeneous group of 5 retrospective follow-up studies of short leg casting reported wide ranges in union rates of 72% to 100%, and in time to healing of 7 weeks to 21 months.3-7 These studies varied in average age, sex, and athletic ability of their samples as well as type of immobilization and weight-bearing status during treatment.
Tuberosity avulsion fractures are proximal fifth metatarsal styloid fractures resulting from a forceful pull of the lateral band of the long plantar ligament or the peroneus brevis tendon during ankle inversion. A 12-week RCT in 89 consecutive patients presenting to an emergency department with fifth metatarsal tuberosity avulsion fractures compared a nonrigid, soft Jones’ dressing consisting of alternating layers of cast padding and elastic bandages with a rigid short leg casting.8 The Jones’ dressing had a significant 28% reduction in time to return to pre-injury levels of activity. Other outcomes—time in treatment modality, time to radiographic healing, and functional foot score—were not different between intervention groups. Validity was limited by the 32% lost to follow-up rate.
FIGURE
Acute fracture of the fifth metatarsal
Acute Jones’ fractures are repaired with screw fixation of the broken bone using fluoroscopy. Patients may return to full activity when radiographs show that the bones were healing at the site of the fracture.
Recommendations from others
We were unable to locate any consensus statements or clinical guidelines regarding the treatment of Jones’ fractures.
DeLee and Drez’s Orthopaedic Sports Medicine recommends immobilization in a cast or below-the-knee boot with strict non-weightbearing for at least 6 weeks for acute Jones’ fractures.9 It recommends surgical treatment, followed by 6 weeks of cast immobilization, then progression to weight bearing based on radiographic findings, for nonoperative treatment failures or with desire to return high-performance athletes to activity.
In Fracture Management for Primary Care, the authors recommend posterior splinting and non-weightbearing with crutches for acute Jones’ fractures, followed by non-weightbearing short leg casting application at 3 to 5 days from injury.10 After a minimum of 6 to 8 weeks of casting, they recommend options of 4 additional weeks of casting or internal fixation for clinical or radiographic nonunion.
For tuberosity avulsion fractures, the authors recommend use of a firm-soled shoe for 4 to 8 weeks. For patients with discomfort at an initial 4- to 7-day follow-up, they give an option of using a walking short leg casting for 2 weeks, with follow-up every 2 to 4 weeks until clinical healing.
1. Mologne TS, Lundeen JM, Clapper MF, O’Brien TJ. Early screw fixation versus casting in the treatment of acute Jones fractures. Am J Sports Med 2005;33:970-975.
2. Clapper MF, O’Brien TJ, Lyons PM. Fractures of the fifth metatarsal: Analysis of a fracture registry. Clin Orthop Relat Res 1995;315:238-241.
3. Dameron TB. Fractures and anatomical variations of the proximal portion of the fifth metatarsal. J Bone Joint Surg Am 1975;57:788-792.
4. Torg JS, Balduini FC, Zelko RR, Pavlov H, Peff TC, Das M. Fractures of the base of the fifth metatarsal distal to the tuberosity. J Bone Joint Surg Am 1984;66:209-214.
5. Seitz WH, Grantham SA. The Jones’ fracture in the non-athlete. Foot Ankle 1985;6:97-100.
6. Josefsson PO, Karlsson M, Redlund-Johnell I, Wendeberg B. Closed treatment of Jones fracture: Good results in 40 cases after 11-26 years. Acta Orthop Scand 1994;65:545-547.
7. Josefsson PO, Karlsson M, Redlund-Johnell I, Wendeberg B. Jones fracture: Surgical versus nonsurgical treatment. Clin Orthop Related Res 1994;299:252-255.
8. Wiener BD, Linder JF, Giattini JF. Treatment of fractures of the fifth metatarsal: a prospective study. Foot Ankle Int 1997;18:267-269.
9. Brodsky JW, Krause JO. Stress fractures of the foot and ankle. In: Delee JC, Drez, D, Miller MD, eds. DeLee and Drez’s Orthopaedic Sports Medicine. Philadelphia, Pa: Saunders; 2003:2403-2406.
10. Metatarsal fractures. In: Eiff MP, Hatch RL, Calmbach WL. Fracture Management for Primary Care. Philadelphia, Pa: Saunders; 2003:345-349.
1. Mologne TS, Lundeen JM, Clapper MF, O’Brien TJ. Early screw fixation versus casting in the treatment of acute Jones fractures. Am J Sports Med 2005;33:970-975.
2. Clapper MF, O’Brien TJ, Lyons PM. Fractures of the fifth metatarsal: Analysis of a fracture registry. Clin Orthop Relat Res 1995;315:238-241.
3. Dameron TB. Fractures and anatomical variations of the proximal portion of the fifth metatarsal. J Bone Joint Surg Am 1975;57:788-792.
4. Torg JS, Balduini FC, Zelko RR, Pavlov H, Peff TC, Das M. Fractures of the base of the fifth metatarsal distal to the tuberosity. J Bone Joint Surg Am 1984;66:209-214.
5. Seitz WH, Grantham SA. The Jones’ fracture in the non-athlete. Foot Ankle 1985;6:97-100.
6. Josefsson PO, Karlsson M, Redlund-Johnell I, Wendeberg B. Closed treatment of Jones fracture: Good results in 40 cases after 11-26 years. Acta Orthop Scand 1994;65:545-547.
7. Josefsson PO, Karlsson M, Redlund-Johnell I, Wendeberg B. Jones fracture: Surgical versus nonsurgical treatment. Clin Orthop Related Res 1994;299:252-255.
8. Wiener BD, Linder JF, Giattini JF. Treatment of fractures of the fifth metatarsal: a prospective study. Foot Ankle Int 1997;18:267-269.
9. Brodsky JW, Krause JO. Stress fractures of the foot and ankle. In: Delee JC, Drez, D, Miller MD, eds. DeLee and Drez’s Orthopaedic Sports Medicine. Philadelphia, Pa: Saunders; 2003:2403-2406.
10. Metatarsal fractures. In: Eiff MP, Hatch RL, Calmbach WL. Fracture Management for Primary Care. Philadelphia, Pa: Saunders; 2003:345-349.
Evidence-based answers from the Family Physicians Inquiries Network
What is the addiction risk associated with tramadol?
Tramadol (Ultram, generic and with acetaminophen in Ultracet) carries a risk of substance abuse (strength of recommendation [SOR]: B, based on case report surveillance programs). While it appears that tramadol’s risk of substance abuse is low (SOR: B, based on case report surveillance programs), tramadol is associated with a withdrawal syndrome usually typical of opioid withdrawal (SOR: B, based on case report surveillance programs, and a prospective descriptive study).
Evidence summary
Tramadol is a novel, central-acting synthetic opioid with weak mu-opioid activity, and is approved for treatment of moderate to moderately severe pain in adults. Anecdotally, some clinicians have assumed this popular analgesic’s nonscheduled status under the Controlled Substance Act (CSA) means tramadol has no substance abuse potential. (The term “abuse” herein denotes substance abuse or dependence.)
Evidence of tramadol abuse in the US comes primarily from federally operated programs collecting adverse drug event (ADE) data. The MedWatch program of the Food and Drug Administration (FDA) provides a central depository for receiving and compiling postmarketing voluntary case reports. While passive reporting systems can significantly underestimate serious ADE numbers, these reports are often the first evidence of an ADE after a new drug’s release into the market.1 MedWatch has received 766 case reports of abuse associated with tramadol, as well as 482 cases of withdrawal associated with tramadol from the drug’s initial US marketing in 1995 through September 2004.2,3
The Drug Abuse Warning Network (DAWN) is a federally operated, national surveillance system that monitors trends in drug-related emergency department visits. Over the period from 1995 to 2002, DAWN reported drug-related emergency department visits mentioning tramadol in more than 12,000 cases. Tramadol case numbers significantly increased 165% during this time. For perspective, during the same period, DAWN found nalbuphine (Nubain, also not CSA scheduled) in 118 cases, propoxyphene drug combinations (CSA Class IV) in more than 45,000 cases, codeine drug combinations (CSA Classes III & V) in about 50,000 cases, and hydrocodone drug combinations (CSA Class III) in around 128,000 cases.4
Using data from observational postmarketing studies, investigators have extrapolated a tramadol abuse rate for the general tramadolexposed population.5,6 Ortho-McNeil, Ultram’s manufacturer, funded a surveillance program that compiled tramadol abuse and withdrawal case reports from 2 sources: (1) periodic surveys for tramadol abuse case reports from a group of 255 substance abuse experts studying and caring for addiction communities, and (2) voluntary ADE case reports from health care professionals and consumers received by Ortho-McNeil. Over 3 years of surveillance, the program received 454 case reports classified as tramadol abuse. Over 5 years of surveillance, 422 cases of substance withdrawal, with primarily opioid withdrawal symptoms, were reported. There are significant threats to the validity and generalizability of the investigators’ estimated abuse rate of 1 to 3 cases per 100,000 tramadol-exposed patients. The abuse cases were collected in nonrepresentative samples of the tramadol-exposed population. Tramadol exposure is likely suppressed in addiction communities with access to preferred, more potent or euphoriant opioids than tramadol. Voluntary case reports of tramadol abuse significantly underestimate the actual number of abuse cases in the tramadol-exposed population. In addition, the low survey return rate (49%) further decreases the accuracy of any estimation of tramadol abuse rates.
Prospective studies among patients with known abuse, or at high risk of abuse, reported a tramadol abuse rate, as well as subjective experiences of tramadol withdrawal. A 3-year post-marketing cohort study measured tramadol’s nonmedical misuse rates using urine drug testing for tramadol among 1601 participants in 4 US state monitoring programs for impaired healthcare professionals.7 Tramadol exposure occurred in 140 (8.7%) participants. Thirty-nine (28%) were classified as extensive experimentation or abuse of tramadol. Overall, the rate of extensive experimentation or abuse was 18 cases per thousand personyears. The Hawthorne effect, where awareness of being monitored alters a subject’s behavior, may threaten these measured frequency rates’ generalizability. Another prospective study assessed the subjective tramadol withdrawal experience in 219 patients with a diagnosis of “Tramadol misuse” who were attending 6 drug detoxification centers in China.8 Validated drug dependence symptom scales found that while the degree of physical dependence reported was uniformly mild, the majority of patients reported the psychic dependence symptom of tramadol craving.
The FDA’s Drug Abuse Advisory Committee performed a formal review of the tramadol abuse evidence in 1998, including the data from OrthoMcNeil’s surveillance studies and federal case reporting/surveillance programs. The FDA did not recommend changing tramadol’s unscheduled status.9 The FDA’s considered decision to not schedule tramadol as a controlled substance implies its abuse risk to the general population is low. in comparison to its novel analgesic benefit.
Recommendations from others
Ortho-McNeil’s revised 2001 product package insert for Ultram states, “Tramadol may induce psychic and physical dependence of the morphine type (mu-opioid). Dependence and abuse, including drug-seeking behavior and taking illicit actions to obtain the drug are not limited to those patients with prior history of opioid dependence.” (italics in original, emphasizing 2001 addition). The risk for patients with a history of substance abuse has been observed to be higher.10
Though it may not have high abuse potential, prescribe tramadol cautiously
David M. Schneider, MD
Sutter Medical Center Family Practice Residency Program, Santa Rosa, Calif
Although tramadol appears to have a low potential for abuse, the literature does reveal evidence of abuse, addiction, and withdrawal, even in patients without a history of such problems. We do not know if tramadol is less addictive than other narcotics in high-risk patients. For patients at risk for dependence, tramadol is a reasonable alternative to other opioids, but abuse appears more likely in these patients. Tramadol may be most appropriate for treatment of acute painful conditions, but it can be administered chronically under a watchful eye. Providers should prescribe it cautiously, particularly in patients with a history of abuse or addiction, at least until more definitive evidence surfaces.
1. Brewer T, Colditz GA. Postmarketing surveillance and adverse drug reactions: current perspectives and future needs. JAMA 1999;281:824-829.
2. Brinker A, Bonnel RA, Beitz J. Abuse, dependence, or withdrawal associated with tramadol. Am J Psychiatry 2002;159:881-882.
3. Adverse Event Reporting System. Freedom of Information Report. Rockville, Md: Office of Drug Safety, Food and Drug Administration: search November 1997 to September 2004.
4. Drug Abuse Warning Network. Emergency Department Trends From DAWN: Final Estimates 1995 to 2002. Available at: dawninfo.samhsa.gov. Accessed on August 25, 2004.
5. Cicero TJ, Adams EH, Geller A, et al. A postmarketing surveillance program to monitor Ultram (tramadol hydrochloride) abuse in the United States. Drug Alcohol Depend 1999;57:7-22.
6. Senay EC, Adams EH, Geller A, et al. Physical dependence on Ultram (tramadol hydrochloride): both opioid-like and atypical withdrawal symptoms occur. Drug Alcohol Depend 2003;69:233-241.
7. Knisely JS, Campbell ED, Dawson KS, Schnoll SH. Tramadol post-marketing surveillance in health care professionals. Drug Alcohol Depend 2002;68:15-22.
8. Liu ZM, Zhou WH, Lian Z, et al. Drug dependence and abuse potential of tramadol. Zhongguo Yao Li Xue Bao 1999;20:52-54.
9. FDA Drug Abuse Advisory Committee. The Scientific Evidence for Initiating a Scheduling Action for Ultrammadol hydrochloride). 1998. Available at: www.fda.gov.
10. Murray L, ed. Physicians’ Desk Reference. 58th ed. Montvale, NJ: Thomson PDR; 2004;2496.-
Tramadol (Ultram, generic and with acetaminophen in Ultracet) carries a risk of substance abuse (strength of recommendation [SOR]: B, based on case report surveillance programs). While it appears that tramadol’s risk of substance abuse is low (SOR: B, based on case report surveillance programs), tramadol is associated with a withdrawal syndrome usually typical of opioid withdrawal (SOR: B, based on case report surveillance programs, and a prospective descriptive study).
Evidence summary
Tramadol is a novel, central-acting synthetic opioid with weak mu-opioid activity, and is approved for treatment of moderate to moderately severe pain in adults. Anecdotally, some clinicians have assumed this popular analgesic’s nonscheduled status under the Controlled Substance Act (CSA) means tramadol has no substance abuse potential. (The term “abuse” herein denotes substance abuse or dependence.)
Evidence of tramadol abuse in the US comes primarily from federally operated programs collecting adverse drug event (ADE) data. The MedWatch program of the Food and Drug Administration (FDA) provides a central depository for receiving and compiling postmarketing voluntary case reports. While passive reporting systems can significantly underestimate serious ADE numbers, these reports are often the first evidence of an ADE after a new drug’s release into the market.1 MedWatch has received 766 case reports of abuse associated with tramadol, as well as 482 cases of withdrawal associated with tramadol from the drug’s initial US marketing in 1995 through September 2004.2,3
The Drug Abuse Warning Network (DAWN) is a federally operated, national surveillance system that monitors trends in drug-related emergency department visits. Over the period from 1995 to 2002, DAWN reported drug-related emergency department visits mentioning tramadol in more than 12,000 cases. Tramadol case numbers significantly increased 165% during this time. For perspective, during the same period, DAWN found nalbuphine (Nubain, also not CSA scheduled) in 118 cases, propoxyphene drug combinations (CSA Class IV) in more than 45,000 cases, codeine drug combinations (CSA Classes III & V) in about 50,000 cases, and hydrocodone drug combinations (CSA Class III) in around 128,000 cases.4
Using data from observational postmarketing studies, investigators have extrapolated a tramadol abuse rate for the general tramadolexposed population.5,6 Ortho-McNeil, Ultram’s manufacturer, funded a surveillance program that compiled tramadol abuse and withdrawal case reports from 2 sources: (1) periodic surveys for tramadol abuse case reports from a group of 255 substance abuse experts studying and caring for addiction communities, and (2) voluntary ADE case reports from health care professionals and consumers received by Ortho-McNeil. Over 3 years of surveillance, the program received 454 case reports classified as tramadol abuse. Over 5 years of surveillance, 422 cases of substance withdrawal, with primarily opioid withdrawal symptoms, were reported. There are significant threats to the validity and generalizability of the investigators’ estimated abuse rate of 1 to 3 cases per 100,000 tramadol-exposed patients. The abuse cases were collected in nonrepresentative samples of the tramadol-exposed population. Tramadol exposure is likely suppressed in addiction communities with access to preferred, more potent or euphoriant opioids than tramadol. Voluntary case reports of tramadol abuse significantly underestimate the actual number of abuse cases in the tramadol-exposed population. In addition, the low survey return rate (49%) further decreases the accuracy of any estimation of tramadol abuse rates.
Prospective studies among patients with known abuse, or at high risk of abuse, reported a tramadol abuse rate, as well as subjective experiences of tramadol withdrawal. A 3-year post-marketing cohort study measured tramadol’s nonmedical misuse rates using urine drug testing for tramadol among 1601 participants in 4 US state monitoring programs for impaired healthcare professionals.7 Tramadol exposure occurred in 140 (8.7%) participants. Thirty-nine (28%) were classified as extensive experimentation or abuse of tramadol. Overall, the rate of extensive experimentation or abuse was 18 cases per thousand personyears. The Hawthorne effect, where awareness of being monitored alters a subject’s behavior, may threaten these measured frequency rates’ generalizability. Another prospective study assessed the subjective tramadol withdrawal experience in 219 patients with a diagnosis of “Tramadol misuse” who were attending 6 drug detoxification centers in China.8 Validated drug dependence symptom scales found that while the degree of physical dependence reported was uniformly mild, the majority of patients reported the psychic dependence symptom of tramadol craving.
The FDA’s Drug Abuse Advisory Committee performed a formal review of the tramadol abuse evidence in 1998, including the data from OrthoMcNeil’s surveillance studies and federal case reporting/surveillance programs. The FDA did not recommend changing tramadol’s unscheduled status.9 The FDA’s considered decision to not schedule tramadol as a controlled substance implies its abuse risk to the general population is low. in comparison to its novel analgesic benefit.
Recommendations from others
Ortho-McNeil’s revised 2001 product package insert for Ultram states, “Tramadol may induce psychic and physical dependence of the morphine type (mu-opioid). Dependence and abuse, including drug-seeking behavior and taking illicit actions to obtain the drug are not limited to those patients with prior history of opioid dependence.” (italics in original, emphasizing 2001 addition). The risk for patients with a history of substance abuse has been observed to be higher.10
Though it may not have high abuse potential, prescribe tramadol cautiously
David M. Schneider, MD
Sutter Medical Center Family Practice Residency Program, Santa Rosa, Calif
Although tramadol appears to have a low potential for abuse, the literature does reveal evidence of abuse, addiction, and withdrawal, even in patients without a history of such problems. We do not know if tramadol is less addictive than other narcotics in high-risk patients. For patients at risk for dependence, tramadol is a reasonable alternative to other opioids, but abuse appears more likely in these patients. Tramadol may be most appropriate for treatment of acute painful conditions, but it can be administered chronically under a watchful eye. Providers should prescribe it cautiously, particularly in patients with a history of abuse or addiction, at least until more definitive evidence surfaces.
Tramadol (Ultram, generic and with acetaminophen in Ultracet) carries a risk of substance abuse (strength of recommendation [SOR]: B, based on case report surveillance programs). While it appears that tramadol’s risk of substance abuse is low (SOR: B, based on case report surveillance programs), tramadol is associated with a withdrawal syndrome usually typical of opioid withdrawal (SOR: B, based on case report surveillance programs, and a prospective descriptive study).
Evidence summary
Tramadol is a novel, central-acting synthetic opioid with weak mu-opioid activity, and is approved for treatment of moderate to moderately severe pain in adults. Anecdotally, some clinicians have assumed this popular analgesic’s nonscheduled status under the Controlled Substance Act (CSA) means tramadol has no substance abuse potential. (The term “abuse” herein denotes substance abuse or dependence.)
Evidence of tramadol abuse in the US comes primarily from federally operated programs collecting adverse drug event (ADE) data. The MedWatch program of the Food and Drug Administration (FDA) provides a central depository for receiving and compiling postmarketing voluntary case reports. While passive reporting systems can significantly underestimate serious ADE numbers, these reports are often the first evidence of an ADE after a new drug’s release into the market.1 MedWatch has received 766 case reports of abuse associated with tramadol, as well as 482 cases of withdrawal associated with tramadol from the drug’s initial US marketing in 1995 through September 2004.2,3
The Drug Abuse Warning Network (DAWN) is a federally operated, national surveillance system that monitors trends in drug-related emergency department visits. Over the period from 1995 to 2002, DAWN reported drug-related emergency department visits mentioning tramadol in more than 12,000 cases. Tramadol case numbers significantly increased 165% during this time. For perspective, during the same period, DAWN found nalbuphine (Nubain, also not CSA scheduled) in 118 cases, propoxyphene drug combinations (CSA Class IV) in more than 45,000 cases, codeine drug combinations (CSA Classes III & V) in about 50,000 cases, and hydrocodone drug combinations (CSA Class III) in around 128,000 cases.4
Using data from observational postmarketing studies, investigators have extrapolated a tramadol abuse rate for the general tramadolexposed population.5,6 Ortho-McNeil, Ultram’s manufacturer, funded a surveillance program that compiled tramadol abuse and withdrawal case reports from 2 sources: (1) periodic surveys for tramadol abuse case reports from a group of 255 substance abuse experts studying and caring for addiction communities, and (2) voluntary ADE case reports from health care professionals and consumers received by Ortho-McNeil. Over 3 years of surveillance, the program received 454 case reports classified as tramadol abuse. Over 5 years of surveillance, 422 cases of substance withdrawal, with primarily opioid withdrawal symptoms, were reported. There are significant threats to the validity and generalizability of the investigators’ estimated abuse rate of 1 to 3 cases per 100,000 tramadol-exposed patients. The abuse cases were collected in nonrepresentative samples of the tramadol-exposed population. Tramadol exposure is likely suppressed in addiction communities with access to preferred, more potent or euphoriant opioids than tramadol. Voluntary case reports of tramadol abuse significantly underestimate the actual number of abuse cases in the tramadol-exposed population. In addition, the low survey return rate (49%) further decreases the accuracy of any estimation of tramadol abuse rates.
Prospective studies among patients with known abuse, or at high risk of abuse, reported a tramadol abuse rate, as well as subjective experiences of tramadol withdrawal. A 3-year post-marketing cohort study measured tramadol’s nonmedical misuse rates using urine drug testing for tramadol among 1601 participants in 4 US state monitoring programs for impaired healthcare professionals.7 Tramadol exposure occurred in 140 (8.7%) participants. Thirty-nine (28%) were classified as extensive experimentation or abuse of tramadol. Overall, the rate of extensive experimentation or abuse was 18 cases per thousand personyears. The Hawthorne effect, where awareness of being monitored alters a subject’s behavior, may threaten these measured frequency rates’ generalizability. Another prospective study assessed the subjective tramadol withdrawal experience in 219 patients with a diagnosis of “Tramadol misuse” who were attending 6 drug detoxification centers in China.8 Validated drug dependence symptom scales found that while the degree of physical dependence reported was uniformly mild, the majority of patients reported the psychic dependence symptom of tramadol craving.
The FDA’s Drug Abuse Advisory Committee performed a formal review of the tramadol abuse evidence in 1998, including the data from OrthoMcNeil’s surveillance studies and federal case reporting/surveillance programs. The FDA did not recommend changing tramadol’s unscheduled status.9 The FDA’s considered decision to not schedule tramadol as a controlled substance implies its abuse risk to the general population is low. in comparison to its novel analgesic benefit.
Recommendations from others
Ortho-McNeil’s revised 2001 product package insert for Ultram states, “Tramadol may induce psychic and physical dependence of the morphine type (mu-opioid). Dependence and abuse, including drug-seeking behavior and taking illicit actions to obtain the drug are not limited to those patients with prior history of opioid dependence.” (italics in original, emphasizing 2001 addition). The risk for patients with a history of substance abuse has been observed to be higher.10
Though it may not have high abuse potential, prescribe tramadol cautiously
David M. Schneider, MD
Sutter Medical Center Family Practice Residency Program, Santa Rosa, Calif
Although tramadol appears to have a low potential for abuse, the literature does reveal evidence of abuse, addiction, and withdrawal, even in patients without a history of such problems. We do not know if tramadol is less addictive than other narcotics in high-risk patients. For patients at risk for dependence, tramadol is a reasonable alternative to other opioids, but abuse appears more likely in these patients. Tramadol may be most appropriate for treatment of acute painful conditions, but it can be administered chronically under a watchful eye. Providers should prescribe it cautiously, particularly in patients with a history of abuse or addiction, at least until more definitive evidence surfaces.
1. Brewer T, Colditz GA. Postmarketing surveillance and adverse drug reactions: current perspectives and future needs. JAMA 1999;281:824-829.
2. Brinker A, Bonnel RA, Beitz J. Abuse, dependence, or withdrawal associated with tramadol. Am J Psychiatry 2002;159:881-882.
3. Adverse Event Reporting System. Freedom of Information Report. Rockville, Md: Office of Drug Safety, Food and Drug Administration: search November 1997 to September 2004.
4. Drug Abuse Warning Network. Emergency Department Trends From DAWN: Final Estimates 1995 to 2002. Available at: dawninfo.samhsa.gov. Accessed on August 25, 2004.
5. Cicero TJ, Adams EH, Geller A, et al. A postmarketing surveillance program to monitor Ultram (tramadol hydrochloride) abuse in the United States. Drug Alcohol Depend 1999;57:7-22.
6. Senay EC, Adams EH, Geller A, et al. Physical dependence on Ultram (tramadol hydrochloride): both opioid-like and atypical withdrawal symptoms occur. Drug Alcohol Depend 2003;69:233-241.
7. Knisely JS, Campbell ED, Dawson KS, Schnoll SH. Tramadol post-marketing surveillance in health care professionals. Drug Alcohol Depend 2002;68:15-22.
8. Liu ZM, Zhou WH, Lian Z, et al. Drug dependence and abuse potential of tramadol. Zhongguo Yao Li Xue Bao 1999;20:52-54.
9. FDA Drug Abuse Advisory Committee. The Scientific Evidence for Initiating a Scheduling Action for Ultrammadol hydrochloride). 1998. Available at: www.fda.gov.
10. Murray L, ed. Physicians’ Desk Reference. 58th ed. Montvale, NJ: Thomson PDR; 2004;2496.-
1. Brewer T, Colditz GA. Postmarketing surveillance and adverse drug reactions: current perspectives and future needs. JAMA 1999;281:824-829.
2. Brinker A, Bonnel RA, Beitz J. Abuse, dependence, or withdrawal associated with tramadol. Am J Psychiatry 2002;159:881-882.
3. Adverse Event Reporting System. Freedom of Information Report. Rockville, Md: Office of Drug Safety, Food and Drug Administration: search November 1997 to September 2004.
4. Drug Abuse Warning Network. Emergency Department Trends From DAWN: Final Estimates 1995 to 2002. Available at: dawninfo.samhsa.gov. Accessed on August 25, 2004.
5. Cicero TJ, Adams EH, Geller A, et al. A postmarketing surveillance program to monitor Ultram (tramadol hydrochloride) abuse in the United States. Drug Alcohol Depend 1999;57:7-22.
6. Senay EC, Adams EH, Geller A, et al. Physical dependence on Ultram (tramadol hydrochloride): both opioid-like and atypical withdrawal symptoms occur. Drug Alcohol Depend 2003;69:233-241.
7. Knisely JS, Campbell ED, Dawson KS, Schnoll SH. Tramadol post-marketing surveillance in health care professionals. Drug Alcohol Depend 2002;68:15-22.
8. Liu ZM, Zhou WH, Lian Z, et al. Drug dependence and abuse potential of tramadol. Zhongguo Yao Li Xue Bao 1999;20:52-54.
9. FDA Drug Abuse Advisory Committee. The Scientific Evidence for Initiating a Scheduling Action for Ultrammadol hydrochloride). 1998. Available at: www.fda.gov.
10. Murray L, ed. Physicians’ Desk Reference. 58th ed. Montvale, NJ: Thomson PDR; 2004;2496.-
Evidence-based answers from the Family Physicians Inquiries Network
Is an outpatient workup safe for patients with a transient ischemic attack?
There is no compelling evidence that outpatient diagnostic workup of patients with transient ischemic attack (TIA) is less safe than inpatient workup, or that hospitalization prevents stroke or improves stroke outcomes after TIA (strength of recommendation [SOR]: C, based on case series studies). Because the risk of stroke is substantial in the week following a TIA (SOR: A, based on a prospective cohort study), evaluation and treatment for reversible stroke risk factors should be initiated urgently and completed within a week of initial presentation (SOR: C, based on expert consensus opinion).
Risk factors for patients at highest risk for stroke or other cardiovascular events after TIA include age >60 years, diabetes, TIA lasting longer than 10 minutes, and a TIA associated with weakness or speech impairment (SOR: B, based on retrospective cohort study). Hospitalization may be prudent for patients at high risk for cardiovascular events or for those with mental status changes, an inadequate home situation, or the physician’s inability to obtain expedient evaluation (SOR: C, based on case series studies).
Evidence summary
Transient ischemic attack (Figure) is a temporary, focal brain or retinal deficit caused by vascular disease that clears completely in less than 24 hours.1 A large prospective cohort study recently estimated the risk of stroke after a TIA or minor stroke to be 8% to 12% at 7 days and 11% to 15% at 1 month.2
In a large retrospective cohort study, 5% of TIA patients returned to the emergency department with a stroke within the first 2 days after TIA.3 Another 6% returned with a stroke within 90 days. Five independent risk factors were identified: age >60 years, diabetes mellitus, duration of TIA longer than 10 minutes, signs or symptoms of weakness, and speech impairment. Thirty-four percent of patients with all 5 risk factors, and none of the patients without any risk factors, had a stroke within 90 days. Of note, 13% of the TIA patients had an arrhythmia, congestive heart failure, unstable angina, myocardial infarction, stroke, or recurrent TIA within 4 days of initial presenting with a TIA. Twenty-five percent of the patients experienced 1 of these cardiovascular events during the 3 months of follow-up.
In a retrospective case review of TIA and stroke patients, the hospital admissions of 4 of 21 TIA patients were retrospectively categorized as medically justified.4 Admission was categorized as medically justified if the patient had 1 or more of the following criteria: another diagnosis that warranted admission, inadequate home situation, altered mental status, an adverse event during hospitalization including worsening of the deficit, and if the patient underwent some hospital-based treatment that could not be provided on an out-patient basis. Ease and rapidity of evaluation was not considered medically justifiable and outcome improvement (stroke prevention) was not studied.
Two retrospective chart reviews of TIA found considerable practice variability in the evaluation of TIA patient. In 1 study of TIA patients presenting to an emergency department, 81% had a computed tomography scan, 75% had electrocardiogram, and 74% had a complete blood count.5 Carotid Doppler imaging was performed in the emergency department in 16%, and 26% were referred for outpatient Doppler studies. One percent had an ECG in the emergency department, and 16% were given ECGs as outpatients. Seventy-five percent of patients were discharged home. Those hospitalized had a median length of stay of 1 day. In the second study, 31% of the TIA patients had no diagnostic studies performed during the first month after presenting to their primary care physician.6
FIGURE
Expeditious evaluation of TIA is imperative
Recommendations from others
The American Heart Association (AHA) recommends that physicians use a stepwise approach to TIA evaluation as outlined in the Table. The AHA also recommends that the diagnostic evaluation of patients seen within 7 days of a TIA should be completed within 1 week or less. The AHA leaves the decision whether to hospitalize a patient up to the physician based on a patient’s circumstances. The goals of diagnostic testing are to identify or exclude causes of TIA requiring specific therapy, to assess modifiable risk factors, and to determine prognosis.7
The National Stroke Association recommends that patients with known high-grade stenosis in a vascular territory appropriate to the symptoms, and patients with recurrent symptoms, undergo urgent evaluation. Evaluation includes imaging and ruling out other causes of TIA. Patients should be admitted to the hospital if imaging is not immediately available. If indicated, carotid endarterectomy should be performed without delay.8
TABLE
Stepwise diagnostic evaluation for patients with transient ischemic attack
Initial Evaluation
|
Second step (to resolve persistent diagnostic uncertainty as appropriate)
|
| Adapted from Feinberg et al 1994.7 |
Make the patient aware of the risks of TIA and quickly complete the work-up
Jon O. Neher, MD
Valley Medical Center, Renton, Wash
It is important to remember that a diagnosis of TIA can only be made retrospectively. All patients with ongoing focal neurologic signs must be evaluated immediately and (if the symptom duration is less than 3 hours) considered potential candidates for emergent thrombolytic therapy.
The vast majority of TIA patients are asymptomatic during their evaluation. Because they feel well and may have a considerable element of denial, it can be hard to get them to rapidly complete their evaluation in either the inpatient or outpatient setting. It is therefore critical that the patient be made aware that the highest risk period is soon after the TIA and that failure to quickly complete the work-up could have serious negative consequences.
1. Levy DE. How transient are transient ischemic attacks? Neurology 1988;38:674-677.
2. Coull AJ, Lovett JK, Rothwell PM. Oxford Vascular Study. Population based study of early risk of stroke after transient ischaemic attack or minor stroke: implications for public education and organisation of services. BMJ 2004;328:326.-
3. Johnston SC, Gress DR, Browner WS, Sidney S. Short-term prognosis after emergency department diagnosis of TIA. JAMA 2000;284:2901-2906.
4. Henneman PL, Lewis RJ. Is admission medically justified for all patients with acute stroke or transient ischemic attack? Ann Emerg Med 1995;25:458-463.
5. Chang E, Holroyd BR, Kochanski P, Kelly KD, Shuaib A, Rowe BH. Adherence to practice guidelines for transient ischemic attacks in an emergency department. Can J Neurol Sci 2002;29:358-363.
6. Goldstein LB, Bian J, Samsa GP, Bonito AJ, Lux LJ, Matchar DB. New transient ischemic attack and stroke: outpatient management by primary care physicians. Arch Intern Med 2000;160:2941-2946.
7. Feinberg WM, Albers GW, Barnett HJ, et al. Guidelines for the management of transient ischemic attacks. From the Ad Hoc Committee of Guidelines for the Management of Transient Ischemic Attacks of the Stroke Council of the American Heart Association. Circulation 1994;89:2950-2965.
8. Brott TG, Clark WM, Fagan SC, et al. Stroke: The First Hours: Guidelines for Acute Treatment. Englewood, Colo: National Stroke Association; 2000.
There is no compelling evidence that outpatient diagnostic workup of patients with transient ischemic attack (TIA) is less safe than inpatient workup, or that hospitalization prevents stroke or improves stroke outcomes after TIA (strength of recommendation [SOR]: C, based on case series studies). Because the risk of stroke is substantial in the week following a TIA (SOR: A, based on a prospective cohort study), evaluation and treatment for reversible stroke risk factors should be initiated urgently and completed within a week of initial presentation (SOR: C, based on expert consensus opinion).
Risk factors for patients at highest risk for stroke or other cardiovascular events after TIA include age >60 years, diabetes, TIA lasting longer than 10 minutes, and a TIA associated with weakness or speech impairment (SOR: B, based on retrospective cohort study). Hospitalization may be prudent for patients at high risk for cardiovascular events or for those with mental status changes, an inadequate home situation, or the physician’s inability to obtain expedient evaluation (SOR: C, based on case series studies).
Evidence summary
Transient ischemic attack (Figure) is a temporary, focal brain or retinal deficit caused by vascular disease that clears completely in less than 24 hours.1 A large prospective cohort study recently estimated the risk of stroke after a TIA or minor stroke to be 8% to 12% at 7 days and 11% to 15% at 1 month.2
In a large retrospective cohort study, 5% of TIA patients returned to the emergency department with a stroke within the first 2 days after TIA.3 Another 6% returned with a stroke within 90 days. Five independent risk factors were identified: age >60 years, diabetes mellitus, duration of TIA longer than 10 minutes, signs or symptoms of weakness, and speech impairment. Thirty-four percent of patients with all 5 risk factors, and none of the patients without any risk factors, had a stroke within 90 days. Of note, 13% of the TIA patients had an arrhythmia, congestive heart failure, unstable angina, myocardial infarction, stroke, or recurrent TIA within 4 days of initial presenting with a TIA. Twenty-five percent of the patients experienced 1 of these cardiovascular events during the 3 months of follow-up.
In a retrospective case review of TIA and stroke patients, the hospital admissions of 4 of 21 TIA patients were retrospectively categorized as medically justified.4 Admission was categorized as medically justified if the patient had 1 or more of the following criteria: another diagnosis that warranted admission, inadequate home situation, altered mental status, an adverse event during hospitalization including worsening of the deficit, and if the patient underwent some hospital-based treatment that could not be provided on an out-patient basis. Ease and rapidity of evaluation was not considered medically justifiable and outcome improvement (stroke prevention) was not studied.
Two retrospective chart reviews of TIA found considerable practice variability in the evaluation of TIA patient. In 1 study of TIA patients presenting to an emergency department, 81% had a computed tomography scan, 75% had electrocardiogram, and 74% had a complete blood count.5 Carotid Doppler imaging was performed in the emergency department in 16%, and 26% were referred for outpatient Doppler studies. One percent had an ECG in the emergency department, and 16% were given ECGs as outpatients. Seventy-five percent of patients were discharged home. Those hospitalized had a median length of stay of 1 day. In the second study, 31% of the TIA patients had no diagnostic studies performed during the first month after presenting to their primary care physician.6
FIGURE
Expeditious evaluation of TIA is imperative
Recommendations from others
The American Heart Association (AHA) recommends that physicians use a stepwise approach to TIA evaluation as outlined in the Table. The AHA also recommends that the diagnostic evaluation of patients seen within 7 days of a TIA should be completed within 1 week or less. The AHA leaves the decision whether to hospitalize a patient up to the physician based on a patient’s circumstances. The goals of diagnostic testing are to identify or exclude causes of TIA requiring specific therapy, to assess modifiable risk factors, and to determine prognosis.7
The National Stroke Association recommends that patients with known high-grade stenosis in a vascular territory appropriate to the symptoms, and patients with recurrent symptoms, undergo urgent evaluation. Evaluation includes imaging and ruling out other causes of TIA. Patients should be admitted to the hospital if imaging is not immediately available. If indicated, carotid endarterectomy should be performed without delay.8
TABLE
Stepwise diagnostic evaluation for patients with transient ischemic attack
Initial Evaluation
|
Second step (to resolve persistent diagnostic uncertainty as appropriate)
|
| Adapted from Feinberg et al 1994.7 |
Make the patient aware of the risks of TIA and quickly complete the work-up
Jon O. Neher, MD
Valley Medical Center, Renton, Wash
It is important to remember that a diagnosis of TIA can only be made retrospectively. All patients with ongoing focal neurologic signs must be evaluated immediately and (if the symptom duration is less than 3 hours) considered potential candidates for emergent thrombolytic therapy.
The vast majority of TIA patients are asymptomatic during their evaluation. Because they feel well and may have a considerable element of denial, it can be hard to get them to rapidly complete their evaluation in either the inpatient or outpatient setting. It is therefore critical that the patient be made aware that the highest risk period is soon after the TIA and that failure to quickly complete the work-up could have serious negative consequences.
There is no compelling evidence that outpatient diagnostic workup of patients with transient ischemic attack (TIA) is less safe than inpatient workup, or that hospitalization prevents stroke or improves stroke outcomes after TIA (strength of recommendation [SOR]: C, based on case series studies). Because the risk of stroke is substantial in the week following a TIA (SOR: A, based on a prospective cohort study), evaluation and treatment for reversible stroke risk factors should be initiated urgently and completed within a week of initial presentation (SOR: C, based on expert consensus opinion).
Risk factors for patients at highest risk for stroke or other cardiovascular events after TIA include age >60 years, diabetes, TIA lasting longer than 10 minutes, and a TIA associated with weakness or speech impairment (SOR: B, based on retrospective cohort study). Hospitalization may be prudent for patients at high risk for cardiovascular events or for those with mental status changes, an inadequate home situation, or the physician’s inability to obtain expedient evaluation (SOR: C, based on case series studies).
Evidence summary
Transient ischemic attack (Figure) is a temporary, focal brain or retinal deficit caused by vascular disease that clears completely in less than 24 hours.1 A large prospective cohort study recently estimated the risk of stroke after a TIA or minor stroke to be 8% to 12% at 7 days and 11% to 15% at 1 month.2
In a large retrospective cohort study, 5% of TIA patients returned to the emergency department with a stroke within the first 2 days after TIA.3 Another 6% returned with a stroke within 90 days. Five independent risk factors were identified: age >60 years, diabetes mellitus, duration of TIA longer than 10 minutes, signs or symptoms of weakness, and speech impairment. Thirty-four percent of patients with all 5 risk factors, and none of the patients without any risk factors, had a stroke within 90 days. Of note, 13% of the TIA patients had an arrhythmia, congestive heart failure, unstable angina, myocardial infarction, stroke, or recurrent TIA within 4 days of initial presenting with a TIA. Twenty-five percent of the patients experienced 1 of these cardiovascular events during the 3 months of follow-up.
In a retrospective case review of TIA and stroke patients, the hospital admissions of 4 of 21 TIA patients were retrospectively categorized as medically justified.4 Admission was categorized as medically justified if the patient had 1 or more of the following criteria: another diagnosis that warranted admission, inadequate home situation, altered mental status, an adverse event during hospitalization including worsening of the deficit, and if the patient underwent some hospital-based treatment that could not be provided on an out-patient basis. Ease and rapidity of evaluation was not considered medically justifiable and outcome improvement (stroke prevention) was not studied.
Two retrospective chart reviews of TIA found considerable practice variability in the evaluation of TIA patient. In 1 study of TIA patients presenting to an emergency department, 81% had a computed tomography scan, 75% had electrocardiogram, and 74% had a complete blood count.5 Carotid Doppler imaging was performed in the emergency department in 16%, and 26% were referred for outpatient Doppler studies. One percent had an ECG in the emergency department, and 16% were given ECGs as outpatients. Seventy-five percent of patients were discharged home. Those hospitalized had a median length of stay of 1 day. In the second study, 31% of the TIA patients had no diagnostic studies performed during the first month after presenting to their primary care physician.6
FIGURE
Expeditious evaluation of TIA is imperative
Recommendations from others
The American Heart Association (AHA) recommends that physicians use a stepwise approach to TIA evaluation as outlined in the Table. The AHA also recommends that the diagnostic evaluation of patients seen within 7 days of a TIA should be completed within 1 week or less. The AHA leaves the decision whether to hospitalize a patient up to the physician based on a patient’s circumstances. The goals of diagnostic testing are to identify or exclude causes of TIA requiring specific therapy, to assess modifiable risk factors, and to determine prognosis.7
The National Stroke Association recommends that patients with known high-grade stenosis in a vascular territory appropriate to the symptoms, and patients with recurrent symptoms, undergo urgent evaluation. Evaluation includes imaging and ruling out other causes of TIA. Patients should be admitted to the hospital if imaging is not immediately available. If indicated, carotid endarterectomy should be performed without delay.8
TABLE
Stepwise diagnostic evaluation for patients with transient ischemic attack
Initial Evaluation
|
Second step (to resolve persistent diagnostic uncertainty as appropriate)
|
| Adapted from Feinberg et al 1994.7 |
Make the patient aware of the risks of TIA and quickly complete the work-up
Jon O. Neher, MD
Valley Medical Center, Renton, Wash
It is important to remember that a diagnosis of TIA can only be made retrospectively. All patients with ongoing focal neurologic signs must be evaluated immediately and (if the symptom duration is less than 3 hours) considered potential candidates for emergent thrombolytic therapy.
The vast majority of TIA patients are asymptomatic during their evaluation. Because they feel well and may have a considerable element of denial, it can be hard to get them to rapidly complete their evaluation in either the inpatient or outpatient setting. It is therefore critical that the patient be made aware that the highest risk period is soon after the TIA and that failure to quickly complete the work-up could have serious negative consequences.
1. Levy DE. How transient are transient ischemic attacks? Neurology 1988;38:674-677.
2. Coull AJ, Lovett JK, Rothwell PM. Oxford Vascular Study. Population based study of early risk of stroke after transient ischaemic attack or minor stroke: implications for public education and organisation of services. BMJ 2004;328:326.-
3. Johnston SC, Gress DR, Browner WS, Sidney S. Short-term prognosis after emergency department diagnosis of TIA. JAMA 2000;284:2901-2906.
4. Henneman PL, Lewis RJ. Is admission medically justified for all patients with acute stroke or transient ischemic attack? Ann Emerg Med 1995;25:458-463.
5. Chang E, Holroyd BR, Kochanski P, Kelly KD, Shuaib A, Rowe BH. Adherence to practice guidelines for transient ischemic attacks in an emergency department. Can J Neurol Sci 2002;29:358-363.
6. Goldstein LB, Bian J, Samsa GP, Bonito AJ, Lux LJ, Matchar DB. New transient ischemic attack and stroke: outpatient management by primary care physicians. Arch Intern Med 2000;160:2941-2946.
7. Feinberg WM, Albers GW, Barnett HJ, et al. Guidelines for the management of transient ischemic attacks. From the Ad Hoc Committee of Guidelines for the Management of Transient Ischemic Attacks of the Stroke Council of the American Heart Association. Circulation 1994;89:2950-2965.
8. Brott TG, Clark WM, Fagan SC, et al. Stroke: The First Hours: Guidelines for Acute Treatment. Englewood, Colo: National Stroke Association; 2000.
1. Levy DE. How transient are transient ischemic attacks? Neurology 1988;38:674-677.
2. Coull AJ, Lovett JK, Rothwell PM. Oxford Vascular Study. Population based study of early risk of stroke after transient ischaemic attack or minor stroke: implications for public education and organisation of services. BMJ 2004;328:326.-
3. Johnston SC, Gress DR, Browner WS, Sidney S. Short-term prognosis after emergency department diagnosis of TIA. JAMA 2000;284:2901-2906.
4. Henneman PL, Lewis RJ. Is admission medically justified for all patients with acute stroke or transient ischemic attack? Ann Emerg Med 1995;25:458-463.
5. Chang E, Holroyd BR, Kochanski P, Kelly KD, Shuaib A, Rowe BH. Adherence to practice guidelines for transient ischemic attacks in an emergency department. Can J Neurol Sci 2002;29:358-363.
6. Goldstein LB, Bian J, Samsa GP, Bonito AJ, Lux LJ, Matchar DB. New transient ischemic attack and stroke: outpatient management by primary care physicians. Arch Intern Med 2000;160:2941-2946.
7. Feinberg WM, Albers GW, Barnett HJ, et al. Guidelines for the management of transient ischemic attacks. From the Ad Hoc Committee of Guidelines for the Management of Transient Ischemic Attacks of the Stroke Council of the American Heart Association. Circulation 1994;89:2950-2965.
8. Brott TG, Clark WM, Fagan SC, et al. Stroke: The First Hours: Guidelines for Acute Treatment. Englewood, Colo: National Stroke Association; 2000.
Evidence-based answers from the Family Physicians Inquiries Network
Do systemic corticosteroids lessen symptoms in acute exacerbations of COPD?
Systemic corticosteroids improve measures of dyspnea in patients with acute exacerbations of chronic obstructive pulmonary disease (COPD) (strength of recommendation [SOR]: A, meta-analysis of 2 small randomized controlled trials). The optimal dose of systemic corticosteroids to achieve these benefits is uncertain. An international consensus panel recommended 30 to 40 mg of oral prednisone daily for 10 to 14 days as a reasonable compromise of efficacy and safety (SOR: C, consensus expert opinion).
Evidence summary
Three systematic reviews addressing the efficacy of systemic corticosteroids in managing acute exacerbations of COPD found consistent, good-quality evidence supporting short courses of systemic steroids. The improvement in outcomes included decreases in airflow obstruction, treatment failure, and length of hospital stay.1-3
The optimal initial doses of systemic corticosteroids to achieve these benefits are uncertain. Variable study designs limit combining study results into a dose-response curve, and there are no comparative trials of high- vs low-dose regimens. A panel consensus judgment from a collaboration of the National Heart, Lung, and Blood Institute and the World Health Organization recommended 30–40 mg of oral prednisone daily for 10 to 14 days.4
A Cochrane systematic review analyzed 7 randomized, placebo-controlled trials of systemic steroids for acute exacerbations of COPD.1 While most of the studies reporting symptom outcomes used disparate methods of measurement, 2 small studies5,6 reported changes in quality of life using validated visual analogue scales. This allowed their results to be combined into a summary estimate of the effect of corticosteroids compared with placebo. Combining the visual analogue scales using a standardized mean difference showed a significant improvement of this summary quality of life measure in the steroid-treated group.
Other small randomized controlled trials of systemic steroids1 demonstrated trends towards improvement in symptom outcomes. A Taiwanese study randomized 138 patients presenting to an emergency department to treatment with 100 mg intravenous hydrocortisone or placebo within 15 minutes of arrival.1 Using a 6-point scale, patients gave self-assessments of the severity of their attack on arrival and at 6 hours. Compared with placebo, the steroid group showed a 6-hour improvement of uncertain significance.
Similarly, a British trial of 30 mg prednisone vs placebo in 56 inpatients with acute exacerbations of COPD measured a daily composite symptom score based on 7 pulmonary and functional symptoms.8 There was a nonsignificant trend towards greater improvement in the steroid-treated group.
Finally, a multicenter, 3-armed, placebo-controlled, double-blinded, parallel design study enrolled 199 COPD inpatients, who were randomized to oral prednisone, inhaled budesonide, or placebo treatment groups.9 Dyspnea was assessed using a validated, modified Borg scale every 12 hours for 72 hours. The reduction in the modified Borg scale rating was of comparable magnitude in the 3 groups, but again there was a nonsignificant greater reduction in the systemic steroid group compared with both the placebo and inhaled budesonide groups. Power calculations were not provided, so it is unclear whether sample size in this study was sufficient to detect important differences in outcomes.
Three randomized controlled trials prospectively measured adverse events rates of systemic steroids in acute exacerbations of COPD.9-11 Hyperglycemia or glycosuria was more common in the steroid-treated groups. The SCCOPE study, the largest of the 3 trials, found hyperglycemia requiring treatment occurred in a greater proportion of the steroid-treated group than placebo (15% vs 4%; P=.002; number needed to harm=9).
Recommendations from others
A recent review provides a concise summary of practice guidelines for the management of acute exacerbations of COPD from widely recognized professional societies.12 Systemic steroids are endorsed in the evidence-based systematic review guidelines from the American College of Chest Physicians–American Society of Internal Medicine, along with the National Heart, Lung, and Blood Institute with the World Health Organization cosponsored Global Initiative for Chronic Obstructive Lung Disease (GOLD), and the consensus guidelines of the American Thoracic Society.13
Lack of long-term benefits emphasize need for prevention
Donald Briscoe, MD
CHRISTUS St. Joseph Family Practice Residency, Houston, TX
It is reassuring to see that there is good evidence to support what most practicing physicians already do—use steroids for acute exacerbations of COPD. Along with inhaled anticholinergics, beta-agonists and (sometimes) antibiotics, short-term measures of patient oriented outcomes seem to be improved. Questions still remain regarding the optimal dosing, route of administration, and length of therapy needed. The lack of evidence of long-term outcome benefits emphasizes, to me, the need for improved efforts at primary and secondary prevention, such as smoking prevention and cessation interventions, annual influenza vaccination, and routine pneumococcal vaccination in our COPD patients.
1. Wood-Baker R, Walters EH, Gibson P. Oral corticos-teroids for acute exacerbations of chronic obstructive pulmonary disease (Cochrane Review). The Cochrane Library, issue 4, 2003. Updated January 12, 2001.
2. McCrory DC, Brown C, Gelfand SE, Bach PB. Management of acute exacerbations of COPD: a summary and appraisal of published evidence. Chest 2001;119:1190-1209.
3. Singh JM, Palda VA, Stanbrook MB, Chapman KR. Corticosteroid therapy for patients with acute exacerbations of chronic obstructive pulmonary disease. Arch Intern Med 2002;162:2527-2536.
4. Pauwels RA, Buist AS, Calverley PM, Jenkins CR, Hurd SS. GOLD Scientific Committee. Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease. NHLBI/WHO Global Initiative for Chronic Obstructive Lung Disease (GOLD) Workshop Summary. Am J Respir Crit Care Med 2001;163:1256-1276.
5. Thompson WH, Nielson CP, Carvalho P, Charan NB, Crowley JJ. Controlled trial of oral prednisone in outpatients with acute COPD exacerbation. Am J Respir Crit Care Med 1996;154:407-412.
6. Wood-Baker R, Wilinson J, Pearce M, Ryan G. A double-blind, placebo-controlled trial of corticosteroids for acute exacerbations of chronic obstructive pulmonary disease. Aust N Z J Med 1998;28:262.-
7. Bullard MJ, Liaw SJ, Tsai YH, Min HP. Early corticosteroid use in acute exacerbations of chronic airflow obstruction. Am J Emerg Med 1996;14:139-143.
8. Davies L, Angus RM, Calverley PM. Oral corticosteroids in patients admitted to hospital with exacerbations of chronic obstructive pulmonary disease: a prospective randomised controlled trial. Lancet 1999;354:456-460.
9. Maltais F, Ostinelli J, Bourbeau J, et al. Comparison of nebulized budesonide and oral prednisolone with placebo in the treatment of acute exacerbations of chronic obstructive pulmonary disease: a randomized controlled trial. Am J Respir Crit Care Med 2002;165:698-703.
10. Albert RK, Martin TR, Lewis SW. Controlled clinical trial of methylprednisolone in patients with chronic bronchitis and acute respiratory insufficiency. Ann Intern Med 1980;92:753-758.
11. Niewoehner DE, Erbland ML, Deupree RH, et al. Effect of systemic glucocorticoids on exacerbations of chronic obstructive pulmonary disease. Department of Veterans Affairs Cooperative Study Group. N Engl J Med 1999;340:1941-1947.
12. Stoller JK. Clinical practice. Acute exacerbations of chronic obstructive pulmonary disease. N Engl J Med 2002;346:988-994.
13. Niewoehner DE, Erbland M, Collins D. Glucocorticoids for chronic obstructive pulmonary disease [letter]. N Engl J Med 1999;341:1772-1773.
Systemic corticosteroids improve measures of dyspnea in patients with acute exacerbations of chronic obstructive pulmonary disease (COPD) (strength of recommendation [SOR]: A, meta-analysis of 2 small randomized controlled trials). The optimal dose of systemic corticosteroids to achieve these benefits is uncertain. An international consensus panel recommended 30 to 40 mg of oral prednisone daily for 10 to 14 days as a reasonable compromise of efficacy and safety (SOR: C, consensus expert opinion).
Evidence summary
Three systematic reviews addressing the efficacy of systemic corticosteroids in managing acute exacerbations of COPD found consistent, good-quality evidence supporting short courses of systemic steroids. The improvement in outcomes included decreases in airflow obstruction, treatment failure, and length of hospital stay.1-3
The optimal initial doses of systemic corticosteroids to achieve these benefits are uncertain. Variable study designs limit combining study results into a dose-response curve, and there are no comparative trials of high- vs low-dose regimens. A panel consensus judgment from a collaboration of the National Heart, Lung, and Blood Institute and the World Health Organization recommended 30–40 mg of oral prednisone daily for 10 to 14 days.4
A Cochrane systematic review analyzed 7 randomized, placebo-controlled trials of systemic steroids for acute exacerbations of COPD.1 While most of the studies reporting symptom outcomes used disparate methods of measurement, 2 small studies5,6 reported changes in quality of life using validated visual analogue scales. This allowed their results to be combined into a summary estimate of the effect of corticosteroids compared with placebo. Combining the visual analogue scales using a standardized mean difference showed a significant improvement of this summary quality of life measure in the steroid-treated group.
Other small randomized controlled trials of systemic steroids1 demonstrated trends towards improvement in symptom outcomes. A Taiwanese study randomized 138 patients presenting to an emergency department to treatment with 100 mg intravenous hydrocortisone or placebo within 15 minutes of arrival.1 Using a 6-point scale, patients gave self-assessments of the severity of their attack on arrival and at 6 hours. Compared with placebo, the steroid group showed a 6-hour improvement of uncertain significance.
Similarly, a British trial of 30 mg prednisone vs placebo in 56 inpatients with acute exacerbations of COPD measured a daily composite symptom score based on 7 pulmonary and functional symptoms.8 There was a nonsignificant trend towards greater improvement in the steroid-treated group.
Finally, a multicenter, 3-armed, placebo-controlled, double-blinded, parallel design study enrolled 199 COPD inpatients, who were randomized to oral prednisone, inhaled budesonide, or placebo treatment groups.9 Dyspnea was assessed using a validated, modified Borg scale every 12 hours for 72 hours. The reduction in the modified Borg scale rating was of comparable magnitude in the 3 groups, but again there was a nonsignificant greater reduction in the systemic steroid group compared with both the placebo and inhaled budesonide groups. Power calculations were not provided, so it is unclear whether sample size in this study was sufficient to detect important differences in outcomes.
Three randomized controlled trials prospectively measured adverse events rates of systemic steroids in acute exacerbations of COPD.9-11 Hyperglycemia or glycosuria was more common in the steroid-treated groups. The SCCOPE study, the largest of the 3 trials, found hyperglycemia requiring treatment occurred in a greater proportion of the steroid-treated group than placebo (15% vs 4%; P=.002; number needed to harm=9).
Recommendations from others
A recent review provides a concise summary of practice guidelines for the management of acute exacerbations of COPD from widely recognized professional societies.12 Systemic steroids are endorsed in the evidence-based systematic review guidelines from the American College of Chest Physicians–American Society of Internal Medicine, along with the National Heart, Lung, and Blood Institute with the World Health Organization cosponsored Global Initiative for Chronic Obstructive Lung Disease (GOLD), and the consensus guidelines of the American Thoracic Society.13
Lack of long-term benefits emphasize need for prevention
Donald Briscoe, MD
CHRISTUS St. Joseph Family Practice Residency, Houston, TX
It is reassuring to see that there is good evidence to support what most practicing physicians already do—use steroids for acute exacerbations of COPD. Along with inhaled anticholinergics, beta-agonists and (sometimes) antibiotics, short-term measures of patient oriented outcomes seem to be improved. Questions still remain regarding the optimal dosing, route of administration, and length of therapy needed. The lack of evidence of long-term outcome benefits emphasizes, to me, the need for improved efforts at primary and secondary prevention, such as smoking prevention and cessation interventions, annual influenza vaccination, and routine pneumococcal vaccination in our COPD patients.
Systemic corticosteroids improve measures of dyspnea in patients with acute exacerbations of chronic obstructive pulmonary disease (COPD) (strength of recommendation [SOR]: A, meta-analysis of 2 small randomized controlled trials). The optimal dose of systemic corticosteroids to achieve these benefits is uncertain. An international consensus panel recommended 30 to 40 mg of oral prednisone daily for 10 to 14 days as a reasonable compromise of efficacy and safety (SOR: C, consensus expert opinion).
Evidence summary
Three systematic reviews addressing the efficacy of systemic corticosteroids in managing acute exacerbations of COPD found consistent, good-quality evidence supporting short courses of systemic steroids. The improvement in outcomes included decreases in airflow obstruction, treatment failure, and length of hospital stay.1-3
The optimal initial doses of systemic corticosteroids to achieve these benefits are uncertain. Variable study designs limit combining study results into a dose-response curve, and there are no comparative trials of high- vs low-dose regimens. A panel consensus judgment from a collaboration of the National Heart, Lung, and Blood Institute and the World Health Organization recommended 30–40 mg of oral prednisone daily for 10 to 14 days.4
A Cochrane systematic review analyzed 7 randomized, placebo-controlled trials of systemic steroids for acute exacerbations of COPD.1 While most of the studies reporting symptom outcomes used disparate methods of measurement, 2 small studies5,6 reported changes in quality of life using validated visual analogue scales. This allowed their results to be combined into a summary estimate of the effect of corticosteroids compared with placebo. Combining the visual analogue scales using a standardized mean difference showed a significant improvement of this summary quality of life measure in the steroid-treated group.
Other small randomized controlled trials of systemic steroids1 demonstrated trends towards improvement in symptom outcomes. A Taiwanese study randomized 138 patients presenting to an emergency department to treatment with 100 mg intravenous hydrocortisone or placebo within 15 minutes of arrival.1 Using a 6-point scale, patients gave self-assessments of the severity of their attack on arrival and at 6 hours. Compared with placebo, the steroid group showed a 6-hour improvement of uncertain significance.
Similarly, a British trial of 30 mg prednisone vs placebo in 56 inpatients with acute exacerbations of COPD measured a daily composite symptom score based on 7 pulmonary and functional symptoms.8 There was a nonsignificant trend towards greater improvement in the steroid-treated group.
Finally, a multicenter, 3-armed, placebo-controlled, double-blinded, parallel design study enrolled 199 COPD inpatients, who were randomized to oral prednisone, inhaled budesonide, or placebo treatment groups.9 Dyspnea was assessed using a validated, modified Borg scale every 12 hours for 72 hours. The reduction in the modified Borg scale rating was of comparable magnitude in the 3 groups, but again there was a nonsignificant greater reduction in the systemic steroid group compared with both the placebo and inhaled budesonide groups. Power calculations were not provided, so it is unclear whether sample size in this study was sufficient to detect important differences in outcomes.
Three randomized controlled trials prospectively measured adverse events rates of systemic steroids in acute exacerbations of COPD.9-11 Hyperglycemia or glycosuria was more common in the steroid-treated groups. The SCCOPE study, the largest of the 3 trials, found hyperglycemia requiring treatment occurred in a greater proportion of the steroid-treated group than placebo (15% vs 4%; P=.002; number needed to harm=9).
Recommendations from others
A recent review provides a concise summary of practice guidelines for the management of acute exacerbations of COPD from widely recognized professional societies.12 Systemic steroids are endorsed in the evidence-based systematic review guidelines from the American College of Chest Physicians–American Society of Internal Medicine, along with the National Heart, Lung, and Blood Institute with the World Health Organization cosponsored Global Initiative for Chronic Obstructive Lung Disease (GOLD), and the consensus guidelines of the American Thoracic Society.13
Lack of long-term benefits emphasize need for prevention
Donald Briscoe, MD
CHRISTUS St. Joseph Family Practice Residency, Houston, TX
It is reassuring to see that there is good evidence to support what most practicing physicians already do—use steroids for acute exacerbations of COPD. Along with inhaled anticholinergics, beta-agonists and (sometimes) antibiotics, short-term measures of patient oriented outcomes seem to be improved. Questions still remain regarding the optimal dosing, route of administration, and length of therapy needed. The lack of evidence of long-term outcome benefits emphasizes, to me, the need for improved efforts at primary and secondary prevention, such as smoking prevention and cessation interventions, annual influenza vaccination, and routine pneumococcal vaccination in our COPD patients.
1. Wood-Baker R, Walters EH, Gibson P. Oral corticos-teroids for acute exacerbations of chronic obstructive pulmonary disease (Cochrane Review). The Cochrane Library, issue 4, 2003. Updated January 12, 2001.
2. McCrory DC, Brown C, Gelfand SE, Bach PB. Management of acute exacerbations of COPD: a summary and appraisal of published evidence. Chest 2001;119:1190-1209.
3. Singh JM, Palda VA, Stanbrook MB, Chapman KR. Corticosteroid therapy for patients with acute exacerbations of chronic obstructive pulmonary disease. Arch Intern Med 2002;162:2527-2536.
4. Pauwels RA, Buist AS, Calverley PM, Jenkins CR, Hurd SS. GOLD Scientific Committee. Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease. NHLBI/WHO Global Initiative for Chronic Obstructive Lung Disease (GOLD) Workshop Summary. Am J Respir Crit Care Med 2001;163:1256-1276.
5. Thompson WH, Nielson CP, Carvalho P, Charan NB, Crowley JJ. Controlled trial of oral prednisone in outpatients with acute COPD exacerbation. Am J Respir Crit Care Med 1996;154:407-412.
6. Wood-Baker R, Wilinson J, Pearce M, Ryan G. A double-blind, placebo-controlled trial of corticosteroids for acute exacerbations of chronic obstructive pulmonary disease. Aust N Z J Med 1998;28:262.-
7. Bullard MJ, Liaw SJ, Tsai YH, Min HP. Early corticosteroid use in acute exacerbations of chronic airflow obstruction. Am J Emerg Med 1996;14:139-143.
8. Davies L, Angus RM, Calverley PM. Oral corticosteroids in patients admitted to hospital with exacerbations of chronic obstructive pulmonary disease: a prospective randomised controlled trial. Lancet 1999;354:456-460.
9. Maltais F, Ostinelli J, Bourbeau J, et al. Comparison of nebulized budesonide and oral prednisolone with placebo in the treatment of acute exacerbations of chronic obstructive pulmonary disease: a randomized controlled trial. Am J Respir Crit Care Med 2002;165:698-703.
10. Albert RK, Martin TR, Lewis SW. Controlled clinical trial of methylprednisolone in patients with chronic bronchitis and acute respiratory insufficiency. Ann Intern Med 1980;92:753-758.
11. Niewoehner DE, Erbland ML, Deupree RH, et al. Effect of systemic glucocorticoids on exacerbations of chronic obstructive pulmonary disease. Department of Veterans Affairs Cooperative Study Group. N Engl J Med 1999;340:1941-1947.
12. Stoller JK. Clinical practice. Acute exacerbations of chronic obstructive pulmonary disease. N Engl J Med 2002;346:988-994.
13. Niewoehner DE, Erbland M, Collins D. Glucocorticoids for chronic obstructive pulmonary disease [letter]. N Engl J Med 1999;341:1772-1773.
1. Wood-Baker R, Walters EH, Gibson P. Oral corticos-teroids for acute exacerbations of chronic obstructive pulmonary disease (Cochrane Review). The Cochrane Library, issue 4, 2003. Updated January 12, 2001.
2. McCrory DC, Brown C, Gelfand SE, Bach PB. Management of acute exacerbations of COPD: a summary and appraisal of published evidence. Chest 2001;119:1190-1209.
3. Singh JM, Palda VA, Stanbrook MB, Chapman KR. Corticosteroid therapy for patients with acute exacerbations of chronic obstructive pulmonary disease. Arch Intern Med 2002;162:2527-2536.
4. Pauwels RA, Buist AS, Calverley PM, Jenkins CR, Hurd SS. GOLD Scientific Committee. Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease. NHLBI/WHO Global Initiative for Chronic Obstructive Lung Disease (GOLD) Workshop Summary. Am J Respir Crit Care Med 2001;163:1256-1276.
5. Thompson WH, Nielson CP, Carvalho P, Charan NB, Crowley JJ. Controlled trial of oral prednisone in outpatients with acute COPD exacerbation. Am J Respir Crit Care Med 1996;154:407-412.
6. Wood-Baker R, Wilinson J, Pearce M, Ryan G. A double-blind, placebo-controlled trial of corticosteroids for acute exacerbations of chronic obstructive pulmonary disease. Aust N Z J Med 1998;28:262.-
7. Bullard MJ, Liaw SJ, Tsai YH, Min HP. Early corticosteroid use in acute exacerbations of chronic airflow obstruction. Am J Emerg Med 1996;14:139-143.
8. Davies L, Angus RM, Calverley PM. Oral corticosteroids in patients admitted to hospital with exacerbations of chronic obstructive pulmonary disease: a prospective randomised controlled trial. Lancet 1999;354:456-460.
9. Maltais F, Ostinelli J, Bourbeau J, et al. Comparison of nebulized budesonide and oral prednisolone with placebo in the treatment of acute exacerbations of chronic obstructive pulmonary disease: a randomized controlled trial. Am J Respir Crit Care Med 2002;165:698-703.
10. Albert RK, Martin TR, Lewis SW. Controlled clinical trial of methylprednisolone in patients with chronic bronchitis and acute respiratory insufficiency. Ann Intern Med 1980;92:753-758.
11. Niewoehner DE, Erbland ML, Deupree RH, et al. Effect of systemic glucocorticoids on exacerbations of chronic obstructive pulmonary disease. Department of Veterans Affairs Cooperative Study Group. N Engl J Med 1999;340:1941-1947.
12. Stoller JK. Clinical practice. Acute exacerbations of chronic obstructive pulmonary disease. N Engl J Med 2002;346:988-994.
13. Niewoehner DE, Erbland M, Collins D. Glucocorticoids for chronic obstructive pulmonary disease [letter]. N Engl J Med 1999;341:1772-1773.
Evidence-based answers from the Family Physicians Inquiries Network
What medication best prevents migraine in children?
Propranolol, valproic acid, and amitriptyline are effective prophylaxis for migraine in children to varying degrees, are widely available, and have a reasonable safety profile (strength of recommendation [SOR]: B, based on either single randomized controlled trial, prospective or retrospective cohort studies, or trials with conflicting evidence).
Flunarizine and nimodipine have the best evidence of benefit in children; however, availability, cost, and side effects limit their usefulness (SOR: B, based on multiple small randomized controlled trials).
Evidence summary
Amitryptyline was moderately efficacious in 3 small nonblinded trials.1,2 The largest and best-designed prospective cohort trial studied 192 children. Of the 146 patients available for the first follow-up visit, 84% noted subjective improvement of symptoms. Headache frequency decreased from 17.1 ± 10.1 to 9.2 ± 10.0 days/month (P<.001).1
Propranolol, although widely used in children, has conflicting evidence regarding effectiveness. One small randomized controlled trial showed reduced headache frequency in children when compared with placebo.3 However, these results were not duplicated in a larger randomized controlled trial using slightly smaller doses.4
A comparative randomized controlled trial with multiple crossovers involving 33 children found that a self-hypnosis placebo decreased mean headache frequency from 13.3 per 3-month interval to 5.8 (P=.045), but found propranolol no different than placebo.5 Propranolol was also studied in a 3-armed randomized controlled trial in comparison with flunarizine—a drug likely to be efficacious—and placebo. Both drugs were equally efficacious and superior to placebo according to reviews; however, these results were not published in English and could not be critiqued by this author.2
In 2 small retrospective case studies, valproic acid demonstrated >50% improvement in symptoms in 65%6 and 78%7 of subjects. A single uncontrolled interventional trial of valproic acid in 10 children showed a significant trend of improvement in frequency (mean of 6 attacks/month to 0.8 attacks/month) and duration (mean 5.5 hours per attack to 1.1 hour).8
Two similar vasodilatory calcium channel blockers, flunarizine and nimodipine, have the best evidence as migraine prophylactics in children. Flunarizine was found to be effective in multiple well-designed randomized controlled trials and case series, as well as in multiple comparative trials with other agents.2
In a double-blinded, placebo-controlled randomized controlled trial of 48 children, flunarizine decreased mean headache frequency (3.0 attacks/3 months vs 6.5 [P<.001]).9 A repeat randomized controlled trial in 70 children had similar outcomes.10
Nimodipine, in a single randomized controlled trial with crossover design in 37 children decreased headache frequency from a mean of ~2.7 attacks/month to ~1.9 vs. no change for placebo (P<.05).11 A small, prospective, nonblinded comparative trial found that nimodipine and flunarizine have similar efficacy and are superior to placebo.12
Cyproheptadine is widely used in children but is not as effective as amitriptyline and propranolol.2 In adults it is not considered a first-line agent due to lack of evidence of efficacy.13 Nonsteroidal anti-inflammatory drugs have insufficient data to recommend them as prophylactic medications in children.2
RECOMMENDATIONS FROM OTHERS
Nelson Textbook of Pediatrics recommends propranolol as a first-line agent for prevention.14
A recent review article15 recommends cyproheptadine as an initial agent in children <10 years of age. This article also has a patient handout discussing nonpharmacologic prophylactics such as regular sleep, exercise, stress reduction, and avoiding certain foods.
UpToDate recommends propranolol, cyproheptadine, valproate, and amitriptyline as prophylactic options based on patient parameters such as age, sex, and comorbid conditions.16
Propranolol has fewest side effects
Ra Nae Stanton, MD
Southern Illinois University, Carbondale; Quincy Family Practice Residency, Quincy, Ill
Migraines in children are not as well studied as the same problem in adults. I like to stick with older medications known to have fewer side effects. Propranolol is my first choice for any age, since it has been well studied and has very few side effects. Amitriptyline would be second because it is well known, but it does have a sedating effect. If both of these fail to control the migraines, I would consider calcium channel blockers, which are newer in the prevention of migraines.
1. Hershey AD, Powers SW, Vockell AL, et al. Effectiveness of amitriptyline in the prophylactic management of childhood headaches. Headache 2000;40:539-549.
2. Evers S. Drug treatment of migraine in children. Paediatr Drugs 1999;1:7-18.
3. Ludvigsson J. Propranolol used in prophylaxis of migraine in children. Acta Neurol Scand 1974;50:109-115.
4. Forsythe W, Gillies D, Sills M. Propranolol (‘Inderal’) in the treatment of childhood migraine. Dev Med Child Neurol 1984;26:737-741.
5. Olness K, MacDonald JT, Uden DL. Comparison of self-hypnosis and propranolol in the treatment of juvenile classic migraine. Pediatrics 1987;79:593-597.
6. Pakalnis A, Greenburg G, Drake ME, Jr, Paolichi J. Pediatric migraine prophylaxis with divalproex. J Child Neurol 2001;16:731-734.
7. Caruso JM, Brown WD, Exil G, Gascon GG. The efficacy of divalproex sodium in the prophylactic treatment of children with migraine. Headache 2000;40:672-676.
8. Serdaroglu G, Erhan E, Tekgul H, et al. Sodium valproate prophylaxis in childhood migraine. Headache 2002;42:819-822.
9. Sorge F, Marano E, Flunarizine v. placebo in childhood migraine. A double-blind study. Cephalagia. 1985;5(suppl 2):145-148.
10. Sorge F, De Simone R, Marano E, Nolana M, Orefice G, Carrieri P. Flunarizine in prophylaxis of childhood migraine. A double-blind, placebo-controlled, crossover study. Cephalagia 1988;8:1-6.
11. Battistella PA, Ruffilli R, Moro R, et al. A placebo-controlled crossover trial of nimodipine in pediatric migraine. Headache 1990;30:264-268.
12. Castellana M, Carini U, Capirci G, Mazzocchi B. Calcium entry blockers in the treatment of primary headache in children: our experience with flunarizine and nimodipine. In: Lanzi G, Balottin U, Cernibori A, eds. Headache in children and adolescents. Amsterdam: Elsevier; 1989;349-352.
13. Ramadan NM, Silberstein SD, Freitag FG, Gilbert TT, Frishberg BM. Evidence-based guidelines for migraine headache in the primary care setting: pharmacological management for prevention of migraine. American College of Neurology, April 2000. Available at: www.aan.com/professionals/practice/pdfs/gl0090.pdf. Accessed on August 7, 2003.
14. Behrman RE, Kliegman R, Jenson HB. Nelson Textbook of Pediatrics. 16th ed. Philadelphia: W.B. Saunders; 2000;16:1832-1834.
15. Lewis DW. Headaches in children and adolescents. Am Fam Physician 2002;65:625-632.
16. Cruse, RP. Management of migraine headache in children. UpToDate. Last update October 15, 2002. Available at: www.uptodate.com. Accessed on July 22, 2003.
Propranolol, valproic acid, and amitriptyline are effective prophylaxis for migraine in children to varying degrees, are widely available, and have a reasonable safety profile (strength of recommendation [SOR]: B, based on either single randomized controlled trial, prospective or retrospective cohort studies, or trials with conflicting evidence).
Flunarizine and nimodipine have the best evidence of benefit in children; however, availability, cost, and side effects limit their usefulness (SOR: B, based on multiple small randomized controlled trials).
Evidence summary
Amitryptyline was moderately efficacious in 3 small nonblinded trials.1,2 The largest and best-designed prospective cohort trial studied 192 children. Of the 146 patients available for the first follow-up visit, 84% noted subjective improvement of symptoms. Headache frequency decreased from 17.1 ± 10.1 to 9.2 ± 10.0 days/month (P<.001).1
Propranolol, although widely used in children, has conflicting evidence regarding effectiveness. One small randomized controlled trial showed reduced headache frequency in children when compared with placebo.3 However, these results were not duplicated in a larger randomized controlled trial using slightly smaller doses.4
A comparative randomized controlled trial with multiple crossovers involving 33 children found that a self-hypnosis placebo decreased mean headache frequency from 13.3 per 3-month interval to 5.8 (P=.045), but found propranolol no different than placebo.5 Propranolol was also studied in a 3-armed randomized controlled trial in comparison with flunarizine—a drug likely to be efficacious—and placebo. Both drugs were equally efficacious and superior to placebo according to reviews; however, these results were not published in English and could not be critiqued by this author.2
In 2 small retrospective case studies, valproic acid demonstrated >50% improvement in symptoms in 65%6 and 78%7 of subjects. A single uncontrolled interventional trial of valproic acid in 10 children showed a significant trend of improvement in frequency (mean of 6 attacks/month to 0.8 attacks/month) and duration (mean 5.5 hours per attack to 1.1 hour).8
Two similar vasodilatory calcium channel blockers, flunarizine and nimodipine, have the best evidence as migraine prophylactics in children. Flunarizine was found to be effective in multiple well-designed randomized controlled trials and case series, as well as in multiple comparative trials with other agents.2
In a double-blinded, placebo-controlled randomized controlled trial of 48 children, flunarizine decreased mean headache frequency (3.0 attacks/3 months vs 6.5 [P<.001]).9 A repeat randomized controlled trial in 70 children had similar outcomes.10
Nimodipine, in a single randomized controlled trial with crossover design in 37 children decreased headache frequency from a mean of ~2.7 attacks/month to ~1.9 vs. no change for placebo (P<.05).11 A small, prospective, nonblinded comparative trial found that nimodipine and flunarizine have similar efficacy and are superior to placebo.12
Cyproheptadine is widely used in children but is not as effective as amitriptyline and propranolol.2 In adults it is not considered a first-line agent due to lack of evidence of efficacy.13 Nonsteroidal anti-inflammatory drugs have insufficient data to recommend them as prophylactic medications in children.2
RECOMMENDATIONS FROM OTHERS
Nelson Textbook of Pediatrics recommends propranolol as a first-line agent for prevention.14
A recent review article15 recommends cyproheptadine as an initial agent in children <10 years of age. This article also has a patient handout discussing nonpharmacologic prophylactics such as regular sleep, exercise, stress reduction, and avoiding certain foods.
UpToDate recommends propranolol, cyproheptadine, valproate, and amitriptyline as prophylactic options based on patient parameters such as age, sex, and comorbid conditions.16
Propranolol has fewest side effects
Ra Nae Stanton, MD
Southern Illinois University, Carbondale; Quincy Family Practice Residency, Quincy, Ill
Migraines in children are not as well studied as the same problem in adults. I like to stick with older medications known to have fewer side effects. Propranolol is my first choice for any age, since it has been well studied and has very few side effects. Amitriptyline would be second because it is well known, but it does have a sedating effect. If both of these fail to control the migraines, I would consider calcium channel blockers, which are newer in the prevention of migraines.
Propranolol, valproic acid, and amitriptyline are effective prophylaxis for migraine in children to varying degrees, are widely available, and have a reasonable safety profile (strength of recommendation [SOR]: B, based on either single randomized controlled trial, prospective or retrospective cohort studies, or trials with conflicting evidence).
Flunarizine and nimodipine have the best evidence of benefit in children; however, availability, cost, and side effects limit their usefulness (SOR: B, based on multiple small randomized controlled trials).
Evidence summary
Amitryptyline was moderately efficacious in 3 small nonblinded trials.1,2 The largest and best-designed prospective cohort trial studied 192 children. Of the 146 patients available for the first follow-up visit, 84% noted subjective improvement of symptoms. Headache frequency decreased from 17.1 ± 10.1 to 9.2 ± 10.0 days/month (P<.001).1
Propranolol, although widely used in children, has conflicting evidence regarding effectiveness. One small randomized controlled trial showed reduced headache frequency in children when compared with placebo.3 However, these results were not duplicated in a larger randomized controlled trial using slightly smaller doses.4
A comparative randomized controlled trial with multiple crossovers involving 33 children found that a self-hypnosis placebo decreased mean headache frequency from 13.3 per 3-month interval to 5.8 (P=.045), but found propranolol no different than placebo.5 Propranolol was also studied in a 3-armed randomized controlled trial in comparison with flunarizine—a drug likely to be efficacious—and placebo. Both drugs were equally efficacious and superior to placebo according to reviews; however, these results were not published in English and could not be critiqued by this author.2
In 2 small retrospective case studies, valproic acid demonstrated >50% improvement in symptoms in 65%6 and 78%7 of subjects. A single uncontrolled interventional trial of valproic acid in 10 children showed a significant trend of improvement in frequency (mean of 6 attacks/month to 0.8 attacks/month) and duration (mean 5.5 hours per attack to 1.1 hour).8
Two similar vasodilatory calcium channel blockers, flunarizine and nimodipine, have the best evidence as migraine prophylactics in children. Flunarizine was found to be effective in multiple well-designed randomized controlled trials and case series, as well as in multiple comparative trials with other agents.2
In a double-blinded, placebo-controlled randomized controlled trial of 48 children, flunarizine decreased mean headache frequency (3.0 attacks/3 months vs 6.5 [P<.001]).9 A repeat randomized controlled trial in 70 children had similar outcomes.10
Nimodipine, in a single randomized controlled trial with crossover design in 37 children decreased headache frequency from a mean of ~2.7 attacks/month to ~1.9 vs. no change for placebo (P<.05).11 A small, prospective, nonblinded comparative trial found that nimodipine and flunarizine have similar efficacy and are superior to placebo.12
Cyproheptadine is widely used in children but is not as effective as amitriptyline and propranolol.2 In adults it is not considered a first-line agent due to lack of evidence of efficacy.13 Nonsteroidal anti-inflammatory drugs have insufficient data to recommend them as prophylactic medications in children.2
RECOMMENDATIONS FROM OTHERS
Nelson Textbook of Pediatrics recommends propranolol as a first-line agent for prevention.14
A recent review article15 recommends cyproheptadine as an initial agent in children <10 years of age. This article also has a patient handout discussing nonpharmacologic prophylactics such as regular sleep, exercise, stress reduction, and avoiding certain foods.
UpToDate recommends propranolol, cyproheptadine, valproate, and amitriptyline as prophylactic options based on patient parameters such as age, sex, and comorbid conditions.16
Propranolol has fewest side effects
Ra Nae Stanton, MD
Southern Illinois University, Carbondale; Quincy Family Practice Residency, Quincy, Ill
Migraines in children are not as well studied as the same problem in adults. I like to stick with older medications known to have fewer side effects. Propranolol is my first choice for any age, since it has been well studied and has very few side effects. Amitriptyline would be second because it is well known, but it does have a sedating effect. If both of these fail to control the migraines, I would consider calcium channel blockers, which are newer in the prevention of migraines.
1. Hershey AD, Powers SW, Vockell AL, et al. Effectiveness of amitriptyline in the prophylactic management of childhood headaches. Headache 2000;40:539-549.
2. Evers S. Drug treatment of migraine in children. Paediatr Drugs 1999;1:7-18.
3. Ludvigsson J. Propranolol used in prophylaxis of migraine in children. Acta Neurol Scand 1974;50:109-115.
4. Forsythe W, Gillies D, Sills M. Propranolol (‘Inderal’) in the treatment of childhood migraine. Dev Med Child Neurol 1984;26:737-741.
5. Olness K, MacDonald JT, Uden DL. Comparison of self-hypnosis and propranolol in the treatment of juvenile classic migraine. Pediatrics 1987;79:593-597.
6. Pakalnis A, Greenburg G, Drake ME, Jr, Paolichi J. Pediatric migraine prophylaxis with divalproex. J Child Neurol 2001;16:731-734.
7. Caruso JM, Brown WD, Exil G, Gascon GG. The efficacy of divalproex sodium in the prophylactic treatment of children with migraine. Headache 2000;40:672-676.
8. Serdaroglu G, Erhan E, Tekgul H, et al. Sodium valproate prophylaxis in childhood migraine. Headache 2002;42:819-822.
9. Sorge F, Marano E, Flunarizine v. placebo in childhood migraine. A double-blind study. Cephalagia. 1985;5(suppl 2):145-148.
10. Sorge F, De Simone R, Marano E, Nolana M, Orefice G, Carrieri P. Flunarizine in prophylaxis of childhood migraine. A double-blind, placebo-controlled, crossover study. Cephalagia 1988;8:1-6.
11. Battistella PA, Ruffilli R, Moro R, et al. A placebo-controlled crossover trial of nimodipine in pediatric migraine. Headache 1990;30:264-268.
12. Castellana M, Carini U, Capirci G, Mazzocchi B. Calcium entry blockers in the treatment of primary headache in children: our experience with flunarizine and nimodipine. In: Lanzi G, Balottin U, Cernibori A, eds. Headache in children and adolescents. Amsterdam: Elsevier; 1989;349-352.
13. Ramadan NM, Silberstein SD, Freitag FG, Gilbert TT, Frishberg BM. Evidence-based guidelines for migraine headache in the primary care setting: pharmacological management for prevention of migraine. American College of Neurology, April 2000. Available at: www.aan.com/professionals/practice/pdfs/gl0090.pdf. Accessed on August 7, 2003.
14. Behrman RE, Kliegman R, Jenson HB. Nelson Textbook of Pediatrics. 16th ed. Philadelphia: W.B. Saunders; 2000;16:1832-1834.
15. Lewis DW. Headaches in children and adolescents. Am Fam Physician 2002;65:625-632.
16. Cruse, RP. Management of migraine headache in children. UpToDate. Last update October 15, 2002. Available at: www.uptodate.com. Accessed on July 22, 2003.
1. Hershey AD, Powers SW, Vockell AL, et al. Effectiveness of amitriptyline in the prophylactic management of childhood headaches. Headache 2000;40:539-549.
2. Evers S. Drug treatment of migraine in children. Paediatr Drugs 1999;1:7-18.
3. Ludvigsson J. Propranolol used in prophylaxis of migraine in children. Acta Neurol Scand 1974;50:109-115.
4. Forsythe W, Gillies D, Sills M. Propranolol (‘Inderal’) in the treatment of childhood migraine. Dev Med Child Neurol 1984;26:737-741.
5. Olness K, MacDonald JT, Uden DL. Comparison of self-hypnosis and propranolol in the treatment of juvenile classic migraine. Pediatrics 1987;79:593-597.
6. Pakalnis A, Greenburg G, Drake ME, Jr, Paolichi J. Pediatric migraine prophylaxis with divalproex. J Child Neurol 2001;16:731-734.
7. Caruso JM, Brown WD, Exil G, Gascon GG. The efficacy of divalproex sodium in the prophylactic treatment of children with migraine. Headache 2000;40:672-676.
8. Serdaroglu G, Erhan E, Tekgul H, et al. Sodium valproate prophylaxis in childhood migraine. Headache 2002;42:819-822.
9. Sorge F, Marano E, Flunarizine v. placebo in childhood migraine. A double-blind study. Cephalagia. 1985;5(suppl 2):145-148.
10. Sorge F, De Simone R, Marano E, Nolana M, Orefice G, Carrieri P. Flunarizine in prophylaxis of childhood migraine. A double-blind, placebo-controlled, crossover study. Cephalagia 1988;8:1-6.
11. Battistella PA, Ruffilli R, Moro R, et al. A placebo-controlled crossover trial of nimodipine in pediatric migraine. Headache 1990;30:264-268.
12. Castellana M, Carini U, Capirci G, Mazzocchi B. Calcium entry blockers in the treatment of primary headache in children: our experience with flunarizine and nimodipine. In: Lanzi G, Balottin U, Cernibori A, eds. Headache in children and adolescents. Amsterdam: Elsevier; 1989;349-352.
13. Ramadan NM, Silberstein SD, Freitag FG, Gilbert TT, Frishberg BM. Evidence-based guidelines for migraine headache in the primary care setting: pharmacological management for prevention of migraine. American College of Neurology, April 2000. Available at: www.aan.com/professionals/practice/pdfs/gl0090.pdf. Accessed on August 7, 2003.
14. Behrman RE, Kliegman R, Jenson HB. Nelson Textbook of Pediatrics. 16th ed. Philadelphia: W.B. Saunders; 2000;16:1832-1834.
15. Lewis DW. Headaches in children and adolescents. Am Fam Physician 2002;65:625-632.
16. Cruse, RP. Management of migraine headache in children. UpToDate. Last update October 15, 2002. Available at: www.uptodate.com. Accessed on July 22, 2003.
Evidence-based answers from the Family Physicians Inquiries Network
Do glucosamine or chondroitin cause regeneration of cartilage in osteoarthritis?
No direct evidence suggests glucosamine or chrondroitin cause regeneration of cartilage in osteoarthritis. Use of glucosamine sulfate in knee osteoarthritis prevents joint space narrowing on radiographs (strength of recommendation [SOR]: B, based on 1 randomized controlled trial).
Intramuscular chondroitin polysulfate prevents radiographic progression of finger osteoarthritis (SOR: B, based on 1 randomized controlled trial).
Both chondroitin sulfate and glucosamine sulfate stimulate chondrocyte growth in vitro and in animal models (SOR: D, based on several bench research studies).
Evidence summary
A systematic review of glucosamine sulfate use for osteoarthritis, based on early research (1956–1991), found that it has anti-inflammatory properties and rebuilds damaged cartilage.1 These studies evaluated chondrocytes grown in culture and animal models.1,2 Chondroitin sulfate also stimulates chondrocyte biosynthesis in both animal and in vitro studies. There is insufficient evidence to demonstrate glucosamine sulfate or chondroitin sulfate stimulates chrondrocyte growth in humans with osteoarthritis.2,3
Joint space narrowing on radiographs suggests progression of osteoarthritis. This narrowing is thought to imply cartilage destruction or loss due to osteoarthritis. A double-blinded randomized controlled trial studied the effect of glucosamine sulfate on tibial-femoral compartment joint space narrowing in 212 patients older than 50 with mild to moderate knee osteoarthritis.4 Patients took either 1500 mg/day of glucosamine sulfate or placebo over 3 years. Knee radiographs in a standing anterior-posterior view, using visual and digital analysis, were used to assess joint space narrowing.5 The average mean joint space loss was 0.31 mm in the placebo group and 0.07 mm in the treatment group (P<.05; 95% confidence interval, 0.13–0.48).
The clinical relevance of knee joint space narrowing is undetermined. Radiographic evaluation of a weight-bearing joint space may not be an accurate or reproducible technique. A study of 15 patients with mild to moderate knee osteoarthritis used standing and semi-flexed radiographic views after an analgesic and nonsteroid anti-inflammatory drug washout period, and 1 to 12 weeks after resumption of analgesic therapy (mean 6.0 weeks).6 Knee pain significantly decreased radiographic joint space in the standing anteriorposterior position, but not in the semiflexed position. Using the standing anterior-posterior method may confound accurate interpretation of joint space narrowing and changes in articular cartilage since glucosamine may have an anti-inflammatory effect.6
One double-blinded randomized controlled trial, comparing chondroitin sulfate with placebo, evaluated joint space in patients with symptomatic hand osteoarthritis.7 One hundred sixty-five Caucasian patients, aged 40 to 70 years, were randomized to receive either a 50-mg intramuscular injection of chondroitin polysulfate, twice weekly, for 8 weeks, every 4 months, versus placebo, or 400 mg of oral chondroitin sulfate, 3 times a day, versus placebo.
Osteoarthritis progression in the metacarpalphalangeal and interphalangeal joints was assessed with radiographs over 3 years. Evaluators used the Anatomic Lesion Progression Scale to assess the development of osteophytes and joint space narrowing, with or without subchondral bone changes, to determine osteoarthritis progression. This scale makes it very difficult to determine whether improvements are clinically significant.
Chondroitin sulfate and polysulfate did not prevent osteoarthritis from occurring in previously normal joints. In joints already affected, intramuscular chondroitin polysulfate significantly reduced progression of distal interphalangeal, proximal interpharangeal, and metacarpophalangeal joint space narrowing (P<.013), using the progression scale. Oral chondroitin sulfate did not prevent progression.7
Recommendations from others
The American College of Rheumatology stated in 2000 that recommending glucosamine sulfate or chondroitin sulfate for osteoarthritis might be premature due to the methodology, lack of standardization, and insufficient information on study designs. More research was recommended.8
These products are sold as supplements in the United States. Their purity is often questionable and thus may affect study results. When studying glucosamine, the National Institutes of Health was forced to manufacture the drug itself due to lack of a reliable amount present in commercial products.9
Fred Tudiver, MD
East Tennessee State University, Johnson City
Most family physicians see many patients with osteoarthritis, which can be difficult to treat. My patients typically want improvement in their symptoms, function, and disease progression. Although there is good evidence that the use of glucosamine sulphate (but not chondroitin sulphate) can improve the common symptoms and functional problems of osteoarthritis, this review states it is unclear whether these substances can alter disease progression through regeneration of cartilage.
I tell my patients with osteoarthritis that glucosamine sulfate can help problems like joint pain and function, but that we do not have a safe and reliable treatment for reversing the disease or the joint damage resulting from it.
1. Towheed TE, Anastassiades TP, Shea B, Houpt J, Welch V, Hochberg MC. Glucosamine therapy for treating osteoarthritis. Cochrane Database Syst Rev 2001;CD002946.-
2. Hauselmann HJ. Nutripharmaceuticals for osteoarthritis. Best Pract Res Clin Rheumatol 2001;15:595-607.
3. de los Reyes GC, Koda RT, Lien EJ. Glucosamine and chondroitin sulfates in the treatment of osteoarthritis: a survey. Prog Drug Res 2000;155:81-103.
4. Jubb RW. Oral and intra-articular remedies: review of papers published from March 2001 to February 2002. Curr Opin Rheumatol 2002;14:597-602.
5. Reginster JY, Deroisy R, Rovati LC, et al. Long-term effects of glucosamine sulphate on osteoarthritis progression: a randomised, placebo-controlled clinical trial. Lancet 2001;357:251-256.
6. Mazzuca SA, Brandt KD, Lane KA, Katz BP. Knee pain reduces joint space width in conventional standing anteroposterior radiographs of osteoarthritic knees. Arthritis Rheum 2002;46:1223-1227.
7. Verbruggen G, Goemaere S, Veys EM. Systems to assess the progression of finger joint osteoarthritis and the effects of disease modifying osteoarthritis drugs. Clin Rheumatol 2002;21:231-243.
8. Recommendations for the medical management of osteoarthritis of the hip and knee: 2000 update. American College of Rheumatology Subcommittee on Osteoarthritis Guidelines. Arthritis Rheum 2000;43:1905-1915.
9. Problems with dietary supplements. Med Lett Drugs Ther 2002;44:84-86.
No direct evidence suggests glucosamine or chrondroitin cause regeneration of cartilage in osteoarthritis. Use of glucosamine sulfate in knee osteoarthritis prevents joint space narrowing on radiographs (strength of recommendation [SOR]: B, based on 1 randomized controlled trial).
Intramuscular chondroitin polysulfate prevents radiographic progression of finger osteoarthritis (SOR: B, based on 1 randomized controlled trial).
Both chondroitin sulfate and glucosamine sulfate stimulate chondrocyte growth in vitro and in animal models (SOR: D, based on several bench research studies).
Evidence summary
A systematic review of glucosamine sulfate use for osteoarthritis, based on early research (1956–1991), found that it has anti-inflammatory properties and rebuilds damaged cartilage.1 These studies evaluated chondrocytes grown in culture and animal models.1,2 Chondroitin sulfate also stimulates chondrocyte biosynthesis in both animal and in vitro studies. There is insufficient evidence to demonstrate glucosamine sulfate or chondroitin sulfate stimulates chrondrocyte growth in humans with osteoarthritis.2,3
Joint space narrowing on radiographs suggests progression of osteoarthritis. This narrowing is thought to imply cartilage destruction or loss due to osteoarthritis. A double-blinded randomized controlled trial studied the effect of glucosamine sulfate on tibial-femoral compartment joint space narrowing in 212 patients older than 50 with mild to moderate knee osteoarthritis.4 Patients took either 1500 mg/day of glucosamine sulfate or placebo over 3 years. Knee radiographs in a standing anterior-posterior view, using visual and digital analysis, were used to assess joint space narrowing.5 The average mean joint space loss was 0.31 mm in the placebo group and 0.07 mm in the treatment group (P<.05; 95% confidence interval, 0.13–0.48).
The clinical relevance of knee joint space narrowing is undetermined. Radiographic evaluation of a weight-bearing joint space may not be an accurate or reproducible technique. A study of 15 patients with mild to moderate knee osteoarthritis used standing and semi-flexed radiographic views after an analgesic and nonsteroid anti-inflammatory drug washout period, and 1 to 12 weeks after resumption of analgesic therapy (mean 6.0 weeks).6 Knee pain significantly decreased radiographic joint space in the standing anteriorposterior position, but not in the semiflexed position. Using the standing anterior-posterior method may confound accurate interpretation of joint space narrowing and changes in articular cartilage since glucosamine may have an anti-inflammatory effect.6
One double-blinded randomized controlled trial, comparing chondroitin sulfate with placebo, evaluated joint space in patients with symptomatic hand osteoarthritis.7 One hundred sixty-five Caucasian patients, aged 40 to 70 years, were randomized to receive either a 50-mg intramuscular injection of chondroitin polysulfate, twice weekly, for 8 weeks, every 4 months, versus placebo, or 400 mg of oral chondroitin sulfate, 3 times a day, versus placebo.
Osteoarthritis progression in the metacarpalphalangeal and interphalangeal joints was assessed with radiographs over 3 years. Evaluators used the Anatomic Lesion Progression Scale to assess the development of osteophytes and joint space narrowing, with or without subchondral bone changes, to determine osteoarthritis progression. This scale makes it very difficult to determine whether improvements are clinically significant.
Chondroitin sulfate and polysulfate did not prevent osteoarthritis from occurring in previously normal joints. In joints already affected, intramuscular chondroitin polysulfate significantly reduced progression of distal interphalangeal, proximal interpharangeal, and metacarpophalangeal joint space narrowing (P<.013), using the progression scale. Oral chondroitin sulfate did not prevent progression.7
Recommendations from others
The American College of Rheumatology stated in 2000 that recommending glucosamine sulfate or chondroitin sulfate for osteoarthritis might be premature due to the methodology, lack of standardization, and insufficient information on study designs. More research was recommended.8
These products are sold as supplements in the United States. Their purity is often questionable and thus may affect study results. When studying glucosamine, the National Institutes of Health was forced to manufacture the drug itself due to lack of a reliable amount present in commercial products.9
Fred Tudiver, MD
East Tennessee State University, Johnson City
Most family physicians see many patients with osteoarthritis, which can be difficult to treat. My patients typically want improvement in their symptoms, function, and disease progression. Although there is good evidence that the use of glucosamine sulphate (but not chondroitin sulphate) can improve the common symptoms and functional problems of osteoarthritis, this review states it is unclear whether these substances can alter disease progression through regeneration of cartilage.
I tell my patients with osteoarthritis that glucosamine sulfate can help problems like joint pain and function, but that we do not have a safe and reliable treatment for reversing the disease or the joint damage resulting from it.
No direct evidence suggests glucosamine or chrondroitin cause regeneration of cartilage in osteoarthritis. Use of glucosamine sulfate in knee osteoarthritis prevents joint space narrowing on radiographs (strength of recommendation [SOR]: B, based on 1 randomized controlled trial).
Intramuscular chondroitin polysulfate prevents radiographic progression of finger osteoarthritis (SOR: B, based on 1 randomized controlled trial).
Both chondroitin sulfate and glucosamine sulfate stimulate chondrocyte growth in vitro and in animal models (SOR: D, based on several bench research studies).
Evidence summary
A systematic review of glucosamine sulfate use for osteoarthritis, based on early research (1956–1991), found that it has anti-inflammatory properties and rebuilds damaged cartilage.1 These studies evaluated chondrocytes grown in culture and animal models.1,2 Chondroitin sulfate also stimulates chondrocyte biosynthesis in both animal and in vitro studies. There is insufficient evidence to demonstrate glucosamine sulfate or chondroitin sulfate stimulates chrondrocyte growth in humans with osteoarthritis.2,3
Joint space narrowing on radiographs suggests progression of osteoarthritis. This narrowing is thought to imply cartilage destruction or loss due to osteoarthritis. A double-blinded randomized controlled trial studied the effect of glucosamine sulfate on tibial-femoral compartment joint space narrowing in 212 patients older than 50 with mild to moderate knee osteoarthritis.4 Patients took either 1500 mg/day of glucosamine sulfate or placebo over 3 years. Knee radiographs in a standing anterior-posterior view, using visual and digital analysis, were used to assess joint space narrowing.5 The average mean joint space loss was 0.31 mm in the placebo group and 0.07 mm in the treatment group (P<.05; 95% confidence interval, 0.13–0.48).
The clinical relevance of knee joint space narrowing is undetermined. Radiographic evaluation of a weight-bearing joint space may not be an accurate or reproducible technique. A study of 15 patients with mild to moderate knee osteoarthritis used standing and semi-flexed radiographic views after an analgesic and nonsteroid anti-inflammatory drug washout period, and 1 to 12 weeks after resumption of analgesic therapy (mean 6.0 weeks).6 Knee pain significantly decreased radiographic joint space in the standing anteriorposterior position, but not in the semiflexed position. Using the standing anterior-posterior method may confound accurate interpretation of joint space narrowing and changes in articular cartilage since glucosamine may have an anti-inflammatory effect.6
One double-blinded randomized controlled trial, comparing chondroitin sulfate with placebo, evaluated joint space in patients with symptomatic hand osteoarthritis.7 One hundred sixty-five Caucasian patients, aged 40 to 70 years, were randomized to receive either a 50-mg intramuscular injection of chondroitin polysulfate, twice weekly, for 8 weeks, every 4 months, versus placebo, or 400 mg of oral chondroitin sulfate, 3 times a day, versus placebo.
Osteoarthritis progression in the metacarpalphalangeal and interphalangeal joints was assessed with radiographs over 3 years. Evaluators used the Anatomic Lesion Progression Scale to assess the development of osteophytes and joint space narrowing, with or without subchondral bone changes, to determine osteoarthritis progression. This scale makes it very difficult to determine whether improvements are clinically significant.
Chondroitin sulfate and polysulfate did not prevent osteoarthritis from occurring in previously normal joints. In joints already affected, intramuscular chondroitin polysulfate significantly reduced progression of distal interphalangeal, proximal interpharangeal, and metacarpophalangeal joint space narrowing (P<.013), using the progression scale. Oral chondroitin sulfate did not prevent progression.7
Recommendations from others
The American College of Rheumatology stated in 2000 that recommending glucosamine sulfate or chondroitin sulfate for osteoarthritis might be premature due to the methodology, lack of standardization, and insufficient information on study designs. More research was recommended.8
These products are sold as supplements in the United States. Their purity is often questionable and thus may affect study results. When studying glucosamine, the National Institutes of Health was forced to manufacture the drug itself due to lack of a reliable amount present in commercial products.9
Fred Tudiver, MD
East Tennessee State University, Johnson City
Most family physicians see many patients with osteoarthritis, which can be difficult to treat. My patients typically want improvement in their symptoms, function, and disease progression. Although there is good evidence that the use of glucosamine sulphate (but not chondroitin sulphate) can improve the common symptoms and functional problems of osteoarthritis, this review states it is unclear whether these substances can alter disease progression through regeneration of cartilage.
I tell my patients with osteoarthritis that glucosamine sulfate can help problems like joint pain and function, but that we do not have a safe and reliable treatment for reversing the disease or the joint damage resulting from it.
1. Towheed TE, Anastassiades TP, Shea B, Houpt J, Welch V, Hochberg MC. Glucosamine therapy for treating osteoarthritis. Cochrane Database Syst Rev 2001;CD002946.-
2. Hauselmann HJ. Nutripharmaceuticals for osteoarthritis. Best Pract Res Clin Rheumatol 2001;15:595-607.
3. de los Reyes GC, Koda RT, Lien EJ. Glucosamine and chondroitin sulfates in the treatment of osteoarthritis: a survey. Prog Drug Res 2000;155:81-103.
4. Jubb RW. Oral and intra-articular remedies: review of papers published from March 2001 to February 2002. Curr Opin Rheumatol 2002;14:597-602.
5. Reginster JY, Deroisy R, Rovati LC, et al. Long-term effects of glucosamine sulphate on osteoarthritis progression: a randomised, placebo-controlled clinical trial. Lancet 2001;357:251-256.
6. Mazzuca SA, Brandt KD, Lane KA, Katz BP. Knee pain reduces joint space width in conventional standing anteroposterior radiographs of osteoarthritic knees. Arthritis Rheum 2002;46:1223-1227.
7. Verbruggen G, Goemaere S, Veys EM. Systems to assess the progression of finger joint osteoarthritis and the effects of disease modifying osteoarthritis drugs. Clin Rheumatol 2002;21:231-243.
8. Recommendations for the medical management of osteoarthritis of the hip and knee: 2000 update. American College of Rheumatology Subcommittee on Osteoarthritis Guidelines. Arthritis Rheum 2000;43:1905-1915.
9. Problems with dietary supplements. Med Lett Drugs Ther 2002;44:84-86.
1. Towheed TE, Anastassiades TP, Shea B, Houpt J, Welch V, Hochberg MC. Glucosamine therapy for treating osteoarthritis. Cochrane Database Syst Rev 2001;CD002946.-
2. Hauselmann HJ. Nutripharmaceuticals for osteoarthritis. Best Pract Res Clin Rheumatol 2001;15:595-607.
3. de los Reyes GC, Koda RT, Lien EJ. Glucosamine and chondroitin sulfates in the treatment of osteoarthritis: a survey. Prog Drug Res 2000;155:81-103.
4. Jubb RW. Oral and intra-articular remedies: review of papers published from March 2001 to February 2002. Curr Opin Rheumatol 2002;14:597-602.
5. Reginster JY, Deroisy R, Rovati LC, et al. Long-term effects of glucosamine sulphate on osteoarthritis progression: a randomised, placebo-controlled clinical trial. Lancet 2001;357:251-256.
6. Mazzuca SA, Brandt KD, Lane KA, Katz BP. Knee pain reduces joint space width in conventional standing anteroposterior radiographs of osteoarthritic knees. Arthritis Rheum 2002;46:1223-1227.
7. Verbruggen G, Goemaere S, Veys EM. Systems to assess the progression of finger joint osteoarthritis and the effects of disease modifying osteoarthritis drugs. Clin Rheumatol 2002;21:231-243.
8. Recommendations for the medical management of osteoarthritis of the hip and knee: 2000 update. American College of Rheumatology Subcommittee on Osteoarthritis Guidelines. Arthritis Rheum 2000;43:1905-1915.
9. Problems with dietary supplements. Med Lett Drugs Ther 2002;44:84-86.
Evidence-based answers from the Family Physicians Inquiries Network
Do antioxidants (vitamins C, E) improve outcomes in patients with coronary artery disease?
EVIDENCE-BASED ANSWER
Antioxidant supplements of vitamins E and C do not reduce cardiovascular death in people with coronary artery disease. Vitamin E supplementation, in a variety of doses, does not decrease the incidence of cardiovascular or all-cause mortality (grade of recommendation: A, 4 high quality randomized controlled trials [RCTs]). There is no evidence that vitamin C decreases mortality in patients at risk for coronary disease (grade of recommendation: A, meta-analysis of 3 small RCTs). Combination antioxidant regimens (Vitamins E, C, and betacarotene) seem safe, but do not decrease mortality or incidence of major coronary and vascular events (grade recommendation: A, 1 high-quality RCT).
Evidence summary
Four large, well-designed RCTs with a combined enrollment of nearly 25,000 individuals with known coronary artery disease (CAD) or high risk for CAD receiving vitamin E (50–800 IU/d) collectively demonstrated no change in all-cause mortality or incidence of total cardiovascular events.1 Three of these studies were double-blind, placebo-controlled and the fourth was an open-label design with central randomization and 4 treatment arms.2-5 Two of the studies did suggest that vitamin E may reduce the incidence of non-fatal myocardial infarctions. One study of 2002 persons receiving 400–800 IU/d showed a statistically significant reduction of non-fatal coronary events (relative risk [RR], 0.62)2 In a subgroup analysis of another, 1862 men with history of MI also had reduced risk of non-fatal MI (RR, 0.23).3 However, in both of these groups, the increase in coronary death was not significant.1
Three small RCTs enrolling a total of 1034 geriatric patients, with follow-up of less than 2 years, evaluated vitamin C (50–200 mg/d) versus placebo and showed no mortality benefit.1 Meta-analysis of these studies showed a non-significant increase in the relative risk of death (RR, 1.08).6
A randomized, placebo-controlled study of simvastatin 40 mg and antioxidants (vitamin E 600 mg, vitamin C 250 mg, beta-carotene 20 mg) enrolled 20,536 adults aged 40 to 80 years with known CAD or high risk for CAD. No significant difference was found in all-cause mortality (RR, 1.04), major coronary events (RR, 1.02), any stroke (RR, 0.99), or any major vascular event (RR, 1.00).7 The investigators found no evidence of an adverse affect of the antioxidants on the substantial outcome benefits demonstrated with 40 mg daily of simvastatin. This finding eases some concern from a smaller prior study, which had suggested a negative interaction between simvastatin plus niacin and antioxidant supplementation (composed of vitamins E and C, beta-carotene, and selenium).8
Recommendations from others
A 2002 systematic review of antioxidant vitamins (carotene, tocopherol, and ascorbic acid) in primary and secondary prevention of cardiovascular disease concluded simply that “antioxidant vitamins as food supplements cannot be recommended in the primary or secondary prevention against cardiovascular disease.”9
The American Heart Association guidelines do not advocate antioxidant vitamin supplements, rather a well-balanced diet “with emphasis on anti-oxidant rich fruits and vegetables and whole grains.”10
1. Antioxidant vitamins. Clin Evid Issue 6, December 2001 130-2.
2. Stephens NG, Parsons A, Schofield PM, et al. Lancet 1996;347:781-6.
3. Rapola JM, Virtamo J, Ripatti S, et al. Lancet 1997;349:1715-7.
4. The Heart Outcomes Prevention Evaluation Study Investigators. N Eng J Med 2000;342:154-60.
5. GISSI-Prevezione Investigators. Lancet 1999;354:447-55.
6. Ness A, Egger M, Smith GD. BMJ 1999;319:577.-
7. Heart Protection Study Collaborative Group. Lancet 2002;360:23-33.
8. Brown BG, Xue-Qiao Z, Chait A, et al. N Engl J Med 2001;345:1583-92.
9. Asplund K. J Int Med 2002;251:372-392
10. Tribble DL. Circulation 1999;99:591-95.
EVIDENCE-BASED ANSWER
Antioxidant supplements of vitamins E and C do not reduce cardiovascular death in people with coronary artery disease. Vitamin E supplementation, in a variety of doses, does not decrease the incidence of cardiovascular or all-cause mortality (grade of recommendation: A, 4 high quality randomized controlled trials [RCTs]). There is no evidence that vitamin C decreases mortality in patients at risk for coronary disease (grade of recommendation: A, meta-analysis of 3 small RCTs). Combination antioxidant regimens (Vitamins E, C, and betacarotene) seem safe, but do not decrease mortality or incidence of major coronary and vascular events (grade recommendation: A, 1 high-quality RCT).
Evidence summary
Four large, well-designed RCTs with a combined enrollment of nearly 25,000 individuals with known coronary artery disease (CAD) or high risk for CAD receiving vitamin E (50–800 IU/d) collectively demonstrated no change in all-cause mortality or incidence of total cardiovascular events.1 Three of these studies were double-blind, placebo-controlled and the fourth was an open-label design with central randomization and 4 treatment arms.2-5 Two of the studies did suggest that vitamin E may reduce the incidence of non-fatal myocardial infarctions. One study of 2002 persons receiving 400–800 IU/d showed a statistically significant reduction of non-fatal coronary events (relative risk [RR], 0.62)2 In a subgroup analysis of another, 1862 men with history of MI also had reduced risk of non-fatal MI (RR, 0.23).3 However, in both of these groups, the increase in coronary death was not significant.1
Three small RCTs enrolling a total of 1034 geriatric patients, with follow-up of less than 2 years, evaluated vitamin C (50–200 mg/d) versus placebo and showed no mortality benefit.1 Meta-analysis of these studies showed a non-significant increase in the relative risk of death (RR, 1.08).6
A randomized, placebo-controlled study of simvastatin 40 mg and antioxidants (vitamin E 600 mg, vitamin C 250 mg, beta-carotene 20 mg) enrolled 20,536 adults aged 40 to 80 years with known CAD or high risk for CAD. No significant difference was found in all-cause mortality (RR, 1.04), major coronary events (RR, 1.02), any stroke (RR, 0.99), or any major vascular event (RR, 1.00).7 The investigators found no evidence of an adverse affect of the antioxidants on the substantial outcome benefits demonstrated with 40 mg daily of simvastatin. This finding eases some concern from a smaller prior study, which had suggested a negative interaction between simvastatin plus niacin and antioxidant supplementation (composed of vitamins E and C, beta-carotene, and selenium).8
Recommendations from others
A 2002 systematic review of antioxidant vitamins (carotene, tocopherol, and ascorbic acid) in primary and secondary prevention of cardiovascular disease concluded simply that “antioxidant vitamins as food supplements cannot be recommended in the primary or secondary prevention against cardiovascular disease.”9
The American Heart Association guidelines do not advocate antioxidant vitamin supplements, rather a well-balanced diet “with emphasis on anti-oxidant rich fruits and vegetables and whole grains.”10
EVIDENCE-BASED ANSWER
Antioxidant supplements of vitamins E and C do not reduce cardiovascular death in people with coronary artery disease. Vitamin E supplementation, in a variety of doses, does not decrease the incidence of cardiovascular or all-cause mortality (grade of recommendation: A, 4 high quality randomized controlled trials [RCTs]). There is no evidence that vitamin C decreases mortality in patients at risk for coronary disease (grade of recommendation: A, meta-analysis of 3 small RCTs). Combination antioxidant regimens (Vitamins E, C, and betacarotene) seem safe, but do not decrease mortality or incidence of major coronary and vascular events (grade recommendation: A, 1 high-quality RCT).
Evidence summary
Four large, well-designed RCTs with a combined enrollment of nearly 25,000 individuals with known coronary artery disease (CAD) or high risk for CAD receiving vitamin E (50–800 IU/d) collectively demonstrated no change in all-cause mortality or incidence of total cardiovascular events.1 Three of these studies were double-blind, placebo-controlled and the fourth was an open-label design with central randomization and 4 treatment arms.2-5 Two of the studies did suggest that vitamin E may reduce the incidence of non-fatal myocardial infarctions. One study of 2002 persons receiving 400–800 IU/d showed a statistically significant reduction of non-fatal coronary events (relative risk [RR], 0.62)2 In a subgroup analysis of another, 1862 men with history of MI also had reduced risk of non-fatal MI (RR, 0.23).3 However, in both of these groups, the increase in coronary death was not significant.1
Three small RCTs enrolling a total of 1034 geriatric patients, with follow-up of less than 2 years, evaluated vitamin C (50–200 mg/d) versus placebo and showed no mortality benefit.1 Meta-analysis of these studies showed a non-significant increase in the relative risk of death (RR, 1.08).6
A randomized, placebo-controlled study of simvastatin 40 mg and antioxidants (vitamin E 600 mg, vitamin C 250 mg, beta-carotene 20 mg) enrolled 20,536 adults aged 40 to 80 years with known CAD or high risk for CAD. No significant difference was found in all-cause mortality (RR, 1.04), major coronary events (RR, 1.02), any stroke (RR, 0.99), or any major vascular event (RR, 1.00).7 The investigators found no evidence of an adverse affect of the antioxidants on the substantial outcome benefits demonstrated with 40 mg daily of simvastatin. This finding eases some concern from a smaller prior study, which had suggested a negative interaction between simvastatin plus niacin and antioxidant supplementation (composed of vitamins E and C, beta-carotene, and selenium).8
Recommendations from others
A 2002 systematic review of antioxidant vitamins (carotene, tocopherol, and ascorbic acid) in primary and secondary prevention of cardiovascular disease concluded simply that “antioxidant vitamins as food supplements cannot be recommended in the primary or secondary prevention against cardiovascular disease.”9
The American Heart Association guidelines do not advocate antioxidant vitamin supplements, rather a well-balanced diet “with emphasis on anti-oxidant rich fruits and vegetables and whole grains.”10
1. Antioxidant vitamins. Clin Evid Issue 6, December 2001 130-2.
2. Stephens NG, Parsons A, Schofield PM, et al. Lancet 1996;347:781-6.
3. Rapola JM, Virtamo J, Ripatti S, et al. Lancet 1997;349:1715-7.
4. The Heart Outcomes Prevention Evaluation Study Investigators. N Eng J Med 2000;342:154-60.
5. GISSI-Prevezione Investigators. Lancet 1999;354:447-55.
6. Ness A, Egger M, Smith GD. BMJ 1999;319:577.-
7. Heart Protection Study Collaborative Group. Lancet 2002;360:23-33.
8. Brown BG, Xue-Qiao Z, Chait A, et al. N Engl J Med 2001;345:1583-92.
9. Asplund K. J Int Med 2002;251:372-392
10. Tribble DL. Circulation 1999;99:591-95.
1. Antioxidant vitamins. Clin Evid Issue 6, December 2001 130-2.
2. Stephens NG, Parsons A, Schofield PM, et al. Lancet 1996;347:781-6.
3. Rapola JM, Virtamo J, Ripatti S, et al. Lancet 1997;349:1715-7.
4. The Heart Outcomes Prevention Evaluation Study Investigators. N Eng J Med 2000;342:154-60.
5. GISSI-Prevezione Investigators. Lancet 1999;354:447-55.
6. Ness A, Egger M, Smith GD. BMJ 1999;319:577.-
7. Heart Protection Study Collaborative Group. Lancet 2002;360:23-33.
8. Brown BG, Xue-Qiao Z, Chait A, et al. N Engl J Med 2001;345:1583-92.
9. Asplund K. J Int Med 2002;251:372-392
10. Tribble DL. Circulation 1999;99:591-95.
Evidence-based answers from the Family Physicians Inquiries Network
Are any oral iron formulations better tolerated than ferrous sulfate?
Ferrous salt preparations (ferrous sulfate, ferrous gluconate, and ferrous fumarate) are equally tolerable. (Grade of recommendation: A, based on randomized controlled trial.) Controlled-release iron preparations cause less nausea and epigastric pain than conventional ferrous sulfate (grade of recommendation: A, based on randomized controlled trials), although the discontinuation rates between the 2 iron formulations were similar. Ferrous sulfate remains the standard first-line treatment of iron-deficiency anemia given its general tolerability, effectiveness, and low cost.
Evidence summary
A randomized, double-blinded, placebo-controlled study in 1496 subjects examined side-effect rates of 3 iron salt formulations using equal dosages of elemental iron (Table).1 Gastrointestinal (GI) side-effect rates were not significantly different. The side-effect rate in the ferrous sulfate group (23%) was significantly different from that of the placebo group (14%); thus, for every 11 patients treated with ferrous sulfate, 1 patient would have GI side effects attributable to the iron salt (number needed to harm [NNH] = 11).
Two formulations—controlled-release iron preparations and polysaccharide–iron complexes—decrease the amount of iron presented to the proximal GI tract. Three large randomized trials assessed tolerability of controlled-release iron preparations compared with ferrous sulfate.2–4 The only double-blinded study found a lower rate of nausea and epigastric pain in the controlled-release iron formulation among 1376 blood donors receiving 200 mg/day elemental iron (3.3% vs 6.4%, P < .05, NNH = ~32).2 A nonblinded randomized trial of 543 non-anemic adult patients taking 50 mg/day elemental iron also found a lower rate of stomach-related side effects in the controlled-release group (12.2% vs 27.2%, P < .001, NNH = ~7).3 However, none of the 3 studies showed a difference in the discontinuation rates between the 2 iron formulations. Comparative constipation rates among the trials were conflicting.
Two small, nonblinded, randomized trials of polysaccharide–iron complexes reported conflicting results. A study of 159 subjects found fewer subjects discontinuing the polysaccharide–iron complex taken with meals than ferrous sulfate taken on an empty stomach.5 A study of 60 subjects taking both preparations on an empty stomach found no difference in side-effect rates.6 Two small, randomized, blinded studies found no difference in rates of GI side effects between carbonyl iron and ferrous sulfate.7,8
TABLE
Representative average wholesale prices* for various iron supplement formulations
| Iron supplement group | Generic or brand name | Dosage | Cost of 1-month course |
|---|---|---|---|
| Ferrous salts | Ferrous sulfate (generic) | Tablet: 325 mg po tid | $0.63 to $5.11 (90 tabs) |
| Ferrous fumarate (generic) | Tablet: 300 mg (99 mg iron) po bid | $1.80 (60 tabs) | |
| Ferrous gluconate (generic) | Tablet: 325 mg (36 mg iron) po tid | $2.70 to $5.00 (90 tabs) | |
| Controlled-release | Slow FE (Novartis) | Tablet: 160 mg (50 mg iron) po tid | $18.92 (90 tabs) |
| Ferro-Grad-500 (Abbott) | Tablet: 105 mg iron po bid | $31.84 (60 tabs) | |
| Polysaccharide–iron complex | Niferex-150 (Schwarz Pharma) | Capsule: 150 mg iron po qd | $10.50 (30 caps) |
| Carbonyl iron | Feosol (SmithKline Beecham) | Tablet: 50 mg iron po tid | $18.38 (90 tabs) |
| *2001 Drug Topics, Red Book. Daily dosages given here deliver 150 to 210 mg of elemental iron and are for comparison of average costs. Actual dosage should be adjusted according to the calculated need for iron replacement and the results of laboratory monitoring. | |||
Recommendations from others
Wintrobe’s Clinical Hematology9 and Williams Hematology10 recommend ferrous sulfate as the standard oral iron preparation, and assert that claims of improved tolerability of one oral iron preparation over another have not been substantiated.
Clinical Commentary by Andrea Gordon, MD, at http://www.fpin.org.
1. Hallberg L, Ryttinger L, Solvell L. Side-effects of oral iron therapy. A double-blind study of different iron compounds in tablet form. Acta Med Scand Suppl 1966;459:3-10.
2. Rybo G, Solvell L. Side-effect studies on a new sustained release iron preparation. Scand J Haematol 1971;8:257-64.
3. Brock C, Curry H, Hanna C, Knipfer M, Taylor L. Adverse effects of iron supplementation: a comparative trial of a wax-matrix iron preparation and conventional ferrous sulfate tablets. Clin Ther 1985;7:568-73.
4. Elwood PC, Williams G. A comparative trial of slow-release and conventional iron preparations. Practitioner 1970;204:812-5.
5. Jacobs P, Coghlan P. Comparative bioavailability of ferric polymaltose and ferrous sulphate in iron-deficient blood donors. J Clin Apheresis 1993;8:89-95.
6. Sas G, Nemesanszky E, Brauer H, Scheffer K. On the therapeutic effects of trivalent and divalent iron in iron deficiency anaemia. Arzneimittel-Forschung 1984;34:1575-9.
7. Gordeuk VR, Brittenham GM, Hughes M, Keating LJ, Opplt JJ. High-dose carbonyl iron for iron deficiency anemia: a randomized double-blind trial. Am J Clin Nutr 1987;46:1029-34.
8. Devasthali SD, Gordeuk VR, Brittenham GM, Bravo JR, Hughes MA, Keating LJ. Bioavailability of carbonyl iron: a randomized, double-blind study. Eur J Haematol 1991;46:272-8.
9. Richard LG. Wintrobe’s Clinical Hematology. 10th ed. Baltimore: Williams &; Wilkins; 1999;979-1010.
10. Fairbanks VF, Beutler E. Williams Hematology. 6th ed. New York: McGraw-Hill; 2001;447-70.
Ferrous salt preparations (ferrous sulfate, ferrous gluconate, and ferrous fumarate) are equally tolerable. (Grade of recommendation: A, based on randomized controlled trial.) Controlled-release iron preparations cause less nausea and epigastric pain than conventional ferrous sulfate (grade of recommendation: A, based on randomized controlled trials), although the discontinuation rates between the 2 iron formulations were similar. Ferrous sulfate remains the standard first-line treatment of iron-deficiency anemia given its general tolerability, effectiveness, and low cost.
Evidence summary
A randomized, double-blinded, placebo-controlled study in 1496 subjects examined side-effect rates of 3 iron salt formulations using equal dosages of elemental iron (Table).1 Gastrointestinal (GI) side-effect rates were not significantly different. The side-effect rate in the ferrous sulfate group (23%) was significantly different from that of the placebo group (14%); thus, for every 11 patients treated with ferrous sulfate, 1 patient would have GI side effects attributable to the iron salt (number needed to harm [NNH] = 11).
Two formulations—controlled-release iron preparations and polysaccharide–iron complexes—decrease the amount of iron presented to the proximal GI tract. Three large randomized trials assessed tolerability of controlled-release iron preparations compared with ferrous sulfate.2–4 The only double-blinded study found a lower rate of nausea and epigastric pain in the controlled-release iron formulation among 1376 blood donors receiving 200 mg/day elemental iron (3.3% vs 6.4%, P < .05, NNH = ~32).2 A nonblinded randomized trial of 543 non-anemic adult patients taking 50 mg/day elemental iron also found a lower rate of stomach-related side effects in the controlled-release group (12.2% vs 27.2%, P < .001, NNH = ~7).3 However, none of the 3 studies showed a difference in the discontinuation rates between the 2 iron formulations. Comparative constipation rates among the trials were conflicting.
Two small, nonblinded, randomized trials of polysaccharide–iron complexes reported conflicting results. A study of 159 subjects found fewer subjects discontinuing the polysaccharide–iron complex taken with meals than ferrous sulfate taken on an empty stomach.5 A study of 60 subjects taking both preparations on an empty stomach found no difference in side-effect rates.6 Two small, randomized, blinded studies found no difference in rates of GI side effects between carbonyl iron and ferrous sulfate.7,8
TABLE
Representative average wholesale prices* for various iron supplement formulations
| Iron supplement group | Generic or brand name | Dosage | Cost of 1-month course |
|---|---|---|---|
| Ferrous salts | Ferrous sulfate (generic) | Tablet: 325 mg po tid | $0.63 to $5.11 (90 tabs) |
| Ferrous fumarate (generic) | Tablet: 300 mg (99 mg iron) po bid | $1.80 (60 tabs) | |
| Ferrous gluconate (generic) | Tablet: 325 mg (36 mg iron) po tid | $2.70 to $5.00 (90 tabs) | |
| Controlled-release | Slow FE (Novartis) | Tablet: 160 mg (50 mg iron) po tid | $18.92 (90 tabs) |
| Ferro-Grad-500 (Abbott) | Tablet: 105 mg iron po bid | $31.84 (60 tabs) | |
| Polysaccharide–iron complex | Niferex-150 (Schwarz Pharma) | Capsule: 150 mg iron po qd | $10.50 (30 caps) |
| Carbonyl iron | Feosol (SmithKline Beecham) | Tablet: 50 mg iron po tid | $18.38 (90 tabs) |
| *2001 Drug Topics, Red Book. Daily dosages given here deliver 150 to 210 mg of elemental iron and are for comparison of average costs. Actual dosage should be adjusted according to the calculated need for iron replacement and the results of laboratory monitoring. | |||
Recommendations from others
Wintrobe’s Clinical Hematology9 and Williams Hematology10 recommend ferrous sulfate as the standard oral iron preparation, and assert that claims of improved tolerability of one oral iron preparation over another have not been substantiated.
Clinical Commentary by Andrea Gordon, MD, at http://www.fpin.org.
Ferrous salt preparations (ferrous sulfate, ferrous gluconate, and ferrous fumarate) are equally tolerable. (Grade of recommendation: A, based on randomized controlled trial.) Controlled-release iron preparations cause less nausea and epigastric pain than conventional ferrous sulfate (grade of recommendation: A, based on randomized controlled trials), although the discontinuation rates between the 2 iron formulations were similar. Ferrous sulfate remains the standard first-line treatment of iron-deficiency anemia given its general tolerability, effectiveness, and low cost.
Evidence summary
A randomized, double-blinded, placebo-controlled study in 1496 subjects examined side-effect rates of 3 iron salt formulations using equal dosages of elemental iron (Table).1 Gastrointestinal (GI) side-effect rates were not significantly different. The side-effect rate in the ferrous sulfate group (23%) was significantly different from that of the placebo group (14%); thus, for every 11 patients treated with ferrous sulfate, 1 patient would have GI side effects attributable to the iron salt (number needed to harm [NNH] = 11).
Two formulations—controlled-release iron preparations and polysaccharide–iron complexes—decrease the amount of iron presented to the proximal GI tract. Three large randomized trials assessed tolerability of controlled-release iron preparations compared with ferrous sulfate.2–4 The only double-blinded study found a lower rate of nausea and epigastric pain in the controlled-release iron formulation among 1376 blood donors receiving 200 mg/day elemental iron (3.3% vs 6.4%, P < .05, NNH = ~32).2 A nonblinded randomized trial of 543 non-anemic adult patients taking 50 mg/day elemental iron also found a lower rate of stomach-related side effects in the controlled-release group (12.2% vs 27.2%, P < .001, NNH = ~7).3 However, none of the 3 studies showed a difference in the discontinuation rates between the 2 iron formulations. Comparative constipation rates among the trials were conflicting.
Two small, nonblinded, randomized trials of polysaccharide–iron complexes reported conflicting results. A study of 159 subjects found fewer subjects discontinuing the polysaccharide–iron complex taken with meals than ferrous sulfate taken on an empty stomach.5 A study of 60 subjects taking both preparations on an empty stomach found no difference in side-effect rates.6 Two small, randomized, blinded studies found no difference in rates of GI side effects between carbonyl iron and ferrous sulfate.7,8
TABLE
Representative average wholesale prices* for various iron supplement formulations
| Iron supplement group | Generic or brand name | Dosage | Cost of 1-month course |
|---|---|---|---|
| Ferrous salts | Ferrous sulfate (generic) | Tablet: 325 mg po tid | $0.63 to $5.11 (90 tabs) |
| Ferrous fumarate (generic) | Tablet: 300 mg (99 mg iron) po bid | $1.80 (60 tabs) | |
| Ferrous gluconate (generic) | Tablet: 325 mg (36 mg iron) po tid | $2.70 to $5.00 (90 tabs) | |
| Controlled-release | Slow FE (Novartis) | Tablet: 160 mg (50 mg iron) po tid | $18.92 (90 tabs) |
| Ferro-Grad-500 (Abbott) | Tablet: 105 mg iron po bid | $31.84 (60 tabs) | |
| Polysaccharide–iron complex | Niferex-150 (Schwarz Pharma) | Capsule: 150 mg iron po qd | $10.50 (30 caps) |
| Carbonyl iron | Feosol (SmithKline Beecham) | Tablet: 50 mg iron po tid | $18.38 (90 tabs) |
| *2001 Drug Topics, Red Book. Daily dosages given here deliver 150 to 210 mg of elemental iron and are for comparison of average costs. Actual dosage should be adjusted according to the calculated need for iron replacement and the results of laboratory monitoring. | |||
Recommendations from others
Wintrobe’s Clinical Hematology9 and Williams Hematology10 recommend ferrous sulfate as the standard oral iron preparation, and assert that claims of improved tolerability of one oral iron preparation over another have not been substantiated.
Clinical Commentary by Andrea Gordon, MD, at http://www.fpin.org.
1. Hallberg L, Ryttinger L, Solvell L. Side-effects of oral iron therapy. A double-blind study of different iron compounds in tablet form. Acta Med Scand Suppl 1966;459:3-10.
2. Rybo G, Solvell L. Side-effect studies on a new sustained release iron preparation. Scand J Haematol 1971;8:257-64.
3. Brock C, Curry H, Hanna C, Knipfer M, Taylor L. Adverse effects of iron supplementation: a comparative trial of a wax-matrix iron preparation and conventional ferrous sulfate tablets. Clin Ther 1985;7:568-73.
4. Elwood PC, Williams G. A comparative trial of slow-release and conventional iron preparations. Practitioner 1970;204:812-5.
5. Jacobs P, Coghlan P. Comparative bioavailability of ferric polymaltose and ferrous sulphate in iron-deficient blood donors. J Clin Apheresis 1993;8:89-95.
6. Sas G, Nemesanszky E, Brauer H, Scheffer K. On the therapeutic effects of trivalent and divalent iron in iron deficiency anaemia. Arzneimittel-Forschung 1984;34:1575-9.
7. Gordeuk VR, Brittenham GM, Hughes M, Keating LJ, Opplt JJ. High-dose carbonyl iron for iron deficiency anemia: a randomized double-blind trial. Am J Clin Nutr 1987;46:1029-34.
8. Devasthali SD, Gordeuk VR, Brittenham GM, Bravo JR, Hughes MA, Keating LJ. Bioavailability of carbonyl iron: a randomized, double-blind study. Eur J Haematol 1991;46:272-8.
9. Richard LG. Wintrobe’s Clinical Hematology. 10th ed. Baltimore: Williams &; Wilkins; 1999;979-1010.
10. Fairbanks VF, Beutler E. Williams Hematology. 6th ed. New York: McGraw-Hill; 2001;447-70.
1. Hallberg L, Ryttinger L, Solvell L. Side-effects of oral iron therapy. A double-blind study of different iron compounds in tablet form. Acta Med Scand Suppl 1966;459:3-10.
2. Rybo G, Solvell L. Side-effect studies on a new sustained release iron preparation. Scand J Haematol 1971;8:257-64.
3. Brock C, Curry H, Hanna C, Knipfer M, Taylor L. Adverse effects of iron supplementation: a comparative trial of a wax-matrix iron preparation and conventional ferrous sulfate tablets. Clin Ther 1985;7:568-73.
4. Elwood PC, Williams G. A comparative trial of slow-release and conventional iron preparations. Practitioner 1970;204:812-5.
5. Jacobs P, Coghlan P. Comparative bioavailability of ferric polymaltose and ferrous sulphate in iron-deficient blood donors. J Clin Apheresis 1993;8:89-95.
6. Sas G, Nemesanszky E, Brauer H, Scheffer K. On the therapeutic effects of trivalent and divalent iron in iron deficiency anaemia. Arzneimittel-Forschung 1984;34:1575-9.
7. Gordeuk VR, Brittenham GM, Hughes M, Keating LJ, Opplt JJ. High-dose carbonyl iron for iron deficiency anemia: a randomized double-blind trial. Am J Clin Nutr 1987;46:1029-34.
8. Devasthali SD, Gordeuk VR, Brittenham GM, Bravo JR, Hughes MA, Keating LJ. Bioavailability of carbonyl iron: a randomized, double-blind study. Eur J Haematol 1991;46:272-8.
9. Richard LG. Wintrobe’s Clinical Hematology. 10th ed. Baltimore: Williams &; Wilkins; 1999;979-1010.
10. Fairbanks VF, Beutler E. Williams Hematology. 6th ed. New York: McGraw-Hill; 2001;447-70.
Evidence-based answers from the Family Physicians Inquiries Network