User login
What is the best initial treatment for orbital cellulitis in children?
Although antibiotics are the best initial treatment, surgical intervention is warranted when a child has:
- visual impairment, complete ophthalmoplegia, or well-defined abscess on presentation, or
- no clearly apparent clinical improvement by 24 hours (strength of recommendation [SOR]: C, based on patient-oriented case-series studies).
Target antimicrobial therapy toward the common pathogens associated with predisposing factors for orbital cellulitis, such as sinusitis—and pay attention to local resistance patterns (SOR: C, based on patient-oriented case series).
Rare but serious risk factors
Peter C. Smith, MD
Rose Family Medicine Residency, University of Colorado Health Sciences Center, Denver
The incidence of Haemophilus influenzae–related periorbital cellulitis appears to have plummeted with the advent of Hib vaccine. And while no national data have been published, case series support my clinical observation that the overall incidence of periorbital cellulitis has dropped as well.
The arrival of heptavalent pneumococcal vaccine may further contribute to its welcome scarcity. take this changing bacteriology—in conjunction with local resistance patterns—into account when considering antibiotic coverage.
When confronted by the rare case of periorbital cellulitis, I always consider risk factors that may change my management, such as immunization status and asplenia. Also, meningitis is a rare but serious complication, so I also keep meningitis risk factors in mind, such as immunosuppression, coincident trauma, or a poor response to initial medical therapy.
Finally, any question of orbital involvement should prompt an emergent consultation.
Evidence summary
Orbital cellulitis is a serious soft-tissue infection of childhood with very different etiologies.
- Periorbital (or preseptal) cellulitis is synonymous with stage I orbital cellulitis, in which there is induration, erythema, warmth, and tenderness of the periorbital soft tissues, usually secondary to external inoculation, but the inflammation does not extend into the bony orbit.
- Stages II, III, and IV orbital cellulitis are progressively more invasive infections that generally arise from the sinuses; they may involve the retro-orbital area. These stages of orbital cellulitis can cause proptosis, decrease visual acuity, or appear as abscesses on computed tomography scan.1,2
Staged treatment
Many retrospective studies of stage II–IV orbital cellulitis with relatively few subjects and small prospective case series have been published with common themes for management recommendations:
- early intravenous antibiotics (likely for an inpatient), and
- involvement of otolaryngology and ophthalmology specialists.
No head-to-head trials have been completed to evaluate efficacy of specific antimicrobial regimens.
Oral antibiotics. First, treat stage I orbital cellulitis with oral antibiotics.
IV antibiotics. Modify treatment to intravenous antibiotics when there is no improvement within 24 hours or if you discover any characteristic of more severe orbital cellulitis.
Medical management of stage II–IV orbital cellulitis with intravenous antibiotics is the current standard of care until it is clear that one of the following is present:
- no improvement by 24 to 48 hours
- visual impairment
- complete ophthalmoplegia, or
- well-defined periosteal abscess.1,2
Surgery. For refractory cases, surgical decompression will likely be required.
The evidence. A small case series (n=9) found 21 children admitted to hospital for preseptal cellulitis, of whom 4 later were diagnosed with orbital cellulitis. There was a total of 9 cases of orbital cellulitis; however, only 1 required operative management of orbital cellulitis.3 In a prospective study to evaluate medical management (n=23), 87% of patients responded to intravenous antibiotics.4 No statistically significant long-term difference in subperiosteal abscesses (as a complication of orbital cellulitis) was found in another retrospective study comparing medical to surgical management.5
Target the likely pathogens
Direct antimicrobial therapy toward common pathogens for likely sources of infection, paying attention to local resistance patterns and the pathogens usually associated with sinusitis (TABLE).1,2,6-8
A retrospective case series of 94 patients of all ages in China implicated Staphylococcus aureus and streptococcal species based on cultures taken from eye purulence and abscesses.6 Another retrospective case series from Vanderbilt (n=80) found streptococci as the most common cause, based on blood and wound cultures in the Hib vaccination era; however, only 12 wounds returned positive cultures.7
TABLE
Choose antibiotic based on cause and likely pathogen1,2,6-8
| ANTECEDENT EVENT | LIKELY PATHOGENS | BEST DRUGS |
|---|---|---|
| Acute sinusitis | Streptococcus pneumoniae Haemophilus influenzae Moraxella catarrhalis | Penicillinase-resistant penicillins |
| Trauma | Staphylococcus aureus Group A β-hernolytic streptococci Increasing concern for methicillin-resistant S aureus | Penicillinase-resistant penicillins First-generation cephalosporins Consider drugs appropriate for methicillin-resistant S aureus |
| Chronic sinusitis | Anaerobes | Metronidazole Clindamycin |
Steroids have no proven benefit
Systemic steroids have no proven benefit in the treatment of pediatric orbital cellulitis with subperiosteal abscess.
A small retrospective cohort study of the benefit of intravenous steroids in addition to antibiotics showed no decrease in hospital stay or need for surgical decompression (n=23, P=.26 and .20, respectively).9 Without prospective data and a power analysis, lack of benefit of steroids cannot be definitively shown.
Recommendations from others
Infectious Disease Society of America. The guidelines for the management of skin and soft-tissue infections implicate β-hemolytic streptococci as the most common cellulitis pathogen, but also recommend empiric coverage against S aureus.
Periorbital and orbital cellulitis are not specifically addressed in these guidelines, but oral dicloxacillin, cephalexin, clindamycin, or erythromycin are recommended for superficial cellulitis, provided there is no known resistance to these antibiotics.
Intravenous penicillinase-resistant penicillins (nafcillin) or a first-generation cephalosporin (cefazolin) may be used for more severe infections.
For penicillin-allergic patients, the IDSA recommends clindamycin or vancomycin.10
Sanford Guide to Antimicrobial Therapy. Nafcillin plus ceftriaxone and metronidazole is the recommended treatment for orbital cellulitis.
For patients allergic to penicillin, vancomycin plus levofloxacin and metronidazole are recommended.8
Neither the American Academy of Ophthalmology nor the International Council of Ophthalmology offers clinical statements on orbital cellulitis.
Acknowledgments
The opinions and assertions contained herein are the private views of the author and not to be construed as official, or as reflecting the views of the US Air Force Medical service or the US Air Force at large.
1. Nageswaran S, Woods CR, Benjamin DK, Jr, Givner LB, Shetty AK. Orbital cellulitis in children. Pediatr Infect Dis J 2006;25:695-699.
2. Vayalumkal JV, Jadavji T. Children hospitalized with skin and soft tissue infections: a guide to antibacterial selection and treatment. Paediatr Drugs 2006;8:99-111.
3. Starkey CR, Steele RW. Medical management of orbital cellulitis. Pediatr Infect Dis J 2001;20:1002-1005.
4. Noel LP, Clarke WN, MacDonald N. Clinical management of orbital cellulitis in children. Can J Ophthalmol 1990;25:11-16.
5. Greenberg MF, Pollard ZF. Medical treatment of pediatric subperiosteal orbital abscess secondary to sinusitis. J AAPOS 1998;2:351-355.
6. Liu IT, Kao SC, Wang AG, Tsai CC, Liang CK, Hsu WM. Preseptal and orbital cellulitis: a 10-year review of hospitalized patients. J Chin Med Assoc 2006;69:415-422.
7. Donohue SP, Schwartz G. Preseptal and orbital cellulitis in childhood. A changing microbiologic spectrum. Ophthalmology 1998;105:1902;1905.
8. Gilbert DM, Eliopoulos GM, Moellering RC, Sande MA. The Sanford Guide to Antimicrobial Therapy 2006 36th ed. Sperryville, Va: Antimicrobial Therapy; 2006;12.-
9. Yen MT, Yen KG. Effect of corticosteroids in the acute management of pediatric orbital cellulitis with subperiosteal abscess. Ophthal Plast Reconstr Surg 2005;21:363-366.
10. Stevens DL, Bisno AL, Chambers HF, et al. Infectious Diseases Society of America. Practice guidelines for the diagnosis and management of skin and soft-tissue infections. Clin Infect Dis 2005;41:1373-1406.
Although antibiotics are the best initial treatment, surgical intervention is warranted when a child has:
- visual impairment, complete ophthalmoplegia, or well-defined abscess on presentation, or
- no clearly apparent clinical improvement by 24 hours (strength of recommendation [SOR]: C, based on patient-oriented case-series studies).
Target antimicrobial therapy toward the common pathogens associated with predisposing factors for orbital cellulitis, such as sinusitis—and pay attention to local resistance patterns (SOR: C, based on patient-oriented case series).
Rare but serious risk factors
Peter C. Smith, MD
Rose Family Medicine Residency, University of Colorado Health Sciences Center, Denver
The incidence of Haemophilus influenzae–related periorbital cellulitis appears to have plummeted with the advent of Hib vaccine. And while no national data have been published, case series support my clinical observation that the overall incidence of periorbital cellulitis has dropped as well.
The arrival of heptavalent pneumococcal vaccine may further contribute to its welcome scarcity. take this changing bacteriology—in conjunction with local resistance patterns—into account when considering antibiotic coverage.
When confronted by the rare case of periorbital cellulitis, I always consider risk factors that may change my management, such as immunization status and asplenia. Also, meningitis is a rare but serious complication, so I also keep meningitis risk factors in mind, such as immunosuppression, coincident trauma, or a poor response to initial medical therapy.
Finally, any question of orbital involvement should prompt an emergent consultation.
Evidence summary
Orbital cellulitis is a serious soft-tissue infection of childhood with very different etiologies.
- Periorbital (or preseptal) cellulitis is synonymous with stage I orbital cellulitis, in which there is induration, erythema, warmth, and tenderness of the periorbital soft tissues, usually secondary to external inoculation, but the inflammation does not extend into the bony orbit.
- Stages II, III, and IV orbital cellulitis are progressively more invasive infections that generally arise from the sinuses; they may involve the retro-orbital area. These stages of orbital cellulitis can cause proptosis, decrease visual acuity, or appear as abscesses on computed tomography scan.1,2
Staged treatment
Many retrospective studies of stage II–IV orbital cellulitis with relatively few subjects and small prospective case series have been published with common themes for management recommendations:
- early intravenous antibiotics (likely for an inpatient), and
- involvement of otolaryngology and ophthalmology specialists.
No head-to-head trials have been completed to evaluate efficacy of specific antimicrobial regimens.
Oral antibiotics. First, treat stage I orbital cellulitis with oral antibiotics.
IV antibiotics. Modify treatment to intravenous antibiotics when there is no improvement within 24 hours or if you discover any characteristic of more severe orbital cellulitis.
Medical management of stage II–IV orbital cellulitis with intravenous antibiotics is the current standard of care until it is clear that one of the following is present:
- no improvement by 24 to 48 hours
- visual impairment
- complete ophthalmoplegia, or
- well-defined periosteal abscess.1,2
Surgery. For refractory cases, surgical decompression will likely be required.
The evidence. A small case series (n=9) found 21 children admitted to hospital for preseptal cellulitis, of whom 4 later were diagnosed with orbital cellulitis. There was a total of 9 cases of orbital cellulitis; however, only 1 required operative management of orbital cellulitis.3 In a prospective study to evaluate medical management (n=23), 87% of patients responded to intravenous antibiotics.4 No statistically significant long-term difference in subperiosteal abscesses (as a complication of orbital cellulitis) was found in another retrospective study comparing medical to surgical management.5
Target the likely pathogens
Direct antimicrobial therapy toward common pathogens for likely sources of infection, paying attention to local resistance patterns and the pathogens usually associated with sinusitis (TABLE).1,2,6-8
A retrospective case series of 94 patients of all ages in China implicated Staphylococcus aureus and streptococcal species based on cultures taken from eye purulence and abscesses.6 Another retrospective case series from Vanderbilt (n=80) found streptococci as the most common cause, based on blood and wound cultures in the Hib vaccination era; however, only 12 wounds returned positive cultures.7
TABLE
Choose antibiotic based on cause and likely pathogen1,2,6-8
| ANTECEDENT EVENT | LIKELY PATHOGENS | BEST DRUGS |
|---|---|---|
| Acute sinusitis | Streptococcus pneumoniae Haemophilus influenzae Moraxella catarrhalis | Penicillinase-resistant penicillins |
| Trauma | Staphylococcus aureus Group A β-hernolytic streptococci Increasing concern for methicillin-resistant S aureus | Penicillinase-resistant penicillins First-generation cephalosporins Consider drugs appropriate for methicillin-resistant S aureus |
| Chronic sinusitis | Anaerobes | Metronidazole Clindamycin |
Steroids have no proven benefit
Systemic steroids have no proven benefit in the treatment of pediatric orbital cellulitis with subperiosteal abscess.
A small retrospective cohort study of the benefit of intravenous steroids in addition to antibiotics showed no decrease in hospital stay or need for surgical decompression (n=23, P=.26 and .20, respectively).9 Without prospective data and a power analysis, lack of benefit of steroids cannot be definitively shown.
Recommendations from others
Infectious Disease Society of America. The guidelines for the management of skin and soft-tissue infections implicate β-hemolytic streptococci as the most common cellulitis pathogen, but also recommend empiric coverage against S aureus.
Periorbital and orbital cellulitis are not specifically addressed in these guidelines, but oral dicloxacillin, cephalexin, clindamycin, or erythromycin are recommended for superficial cellulitis, provided there is no known resistance to these antibiotics.
Intravenous penicillinase-resistant penicillins (nafcillin) or a first-generation cephalosporin (cefazolin) may be used for more severe infections.
For penicillin-allergic patients, the IDSA recommends clindamycin or vancomycin.10
Sanford Guide to Antimicrobial Therapy. Nafcillin plus ceftriaxone and metronidazole is the recommended treatment for orbital cellulitis.
For patients allergic to penicillin, vancomycin plus levofloxacin and metronidazole are recommended.8
Neither the American Academy of Ophthalmology nor the International Council of Ophthalmology offers clinical statements on orbital cellulitis.
Acknowledgments
The opinions and assertions contained herein are the private views of the author and not to be construed as official, or as reflecting the views of the US Air Force Medical service or the US Air Force at large.
Although antibiotics are the best initial treatment, surgical intervention is warranted when a child has:
- visual impairment, complete ophthalmoplegia, or well-defined abscess on presentation, or
- no clearly apparent clinical improvement by 24 hours (strength of recommendation [SOR]: C, based on patient-oriented case-series studies).
Target antimicrobial therapy toward the common pathogens associated with predisposing factors for orbital cellulitis, such as sinusitis—and pay attention to local resistance patterns (SOR: C, based on patient-oriented case series).
Rare but serious risk factors
Peter C. Smith, MD
Rose Family Medicine Residency, University of Colorado Health Sciences Center, Denver
The incidence of Haemophilus influenzae–related periorbital cellulitis appears to have plummeted with the advent of Hib vaccine. And while no national data have been published, case series support my clinical observation that the overall incidence of periorbital cellulitis has dropped as well.
The arrival of heptavalent pneumococcal vaccine may further contribute to its welcome scarcity. take this changing bacteriology—in conjunction with local resistance patterns—into account when considering antibiotic coverage.
When confronted by the rare case of periorbital cellulitis, I always consider risk factors that may change my management, such as immunization status and asplenia. Also, meningitis is a rare but serious complication, so I also keep meningitis risk factors in mind, such as immunosuppression, coincident trauma, or a poor response to initial medical therapy.
Finally, any question of orbital involvement should prompt an emergent consultation.
Evidence summary
Orbital cellulitis is a serious soft-tissue infection of childhood with very different etiologies.
- Periorbital (or preseptal) cellulitis is synonymous with stage I orbital cellulitis, in which there is induration, erythema, warmth, and tenderness of the periorbital soft tissues, usually secondary to external inoculation, but the inflammation does not extend into the bony orbit.
- Stages II, III, and IV orbital cellulitis are progressively more invasive infections that generally arise from the sinuses; they may involve the retro-orbital area. These stages of orbital cellulitis can cause proptosis, decrease visual acuity, or appear as abscesses on computed tomography scan.1,2
Staged treatment
Many retrospective studies of stage II–IV orbital cellulitis with relatively few subjects and small prospective case series have been published with common themes for management recommendations:
- early intravenous antibiotics (likely for an inpatient), and
- involvement of otolaryngology and ophthalmology specialists.
No head-to-head trials have been completed to evaluate efficacy of specific antimicrobial regimens.
Oral antibiotics. First, treat stage I orbital cellulitis with oral antibiotics.
IV antibiotics. Modify treatment to intravenous antibiotics when there is no improvement within 24 hours or if you discover any characteristic of more severe orbital cellulitis.
Medical management of stage II–IV orbital cellulitis with intravenous antibiotics is the current standard of care until it is clear that one of the following is present:
- no improvement by 24 to 48 hours
- visual impairment
- complete ophthalmoplegia, or
- well-defined periosteal abscess.1,2
Surgery. For refractory cases, surgical decompression will likely be required.
The evidence. A small case series (n=9) found 21 children admitted to hospital for preseptal cellulitis, of whom 4 later were diagnosed with orbital cellulitis. There was a total of 9 cases of orbital cellulitis; however, only 1 required operative management of orbital cellulitis.3 In a prospective study to evaluate medical management (n=23), 87% of patients responded to intravenous antibiotics.4 No statistically significant long-term difference in subperiosteal abscesses (as a complication of orbital cellulitis) was found in another retrospective study comparing medical to surgical management.5
Target the likely pathogens
Direct antimicrobial therapy toward common pathogens for likely sources of infection, paying attention to local resistance patterns and the pathogens usually associated with sinusitis (TABLE).1,2,6-8
A retrospective case series of 94 patients of all ages in China implicated Staphylococcus aureus and streptococcal species based on cultures taken from eye purulence and abscesses.6 Another retrospective case series from Vanderbilt (n=80) found streptococci as the most common cause, based on blood and wound cultures in the Hib vaccination era; however, only 12 wounds returned positive cultures.7
TABLE
Choose antibiotic based on cause and likely pathogen1,2,6-8
| ANTECEDENT EVENT | LIKELY PATHOGENS | BEST DRUGS |
|---|---|---|
| Acute sinusitis | Streptococcus pneumoniae Haemophilus influenzae Moraxella catarrhalis | Penicillinase-resistant penicillins |
| Trauma | Staphylococcus aureus Group A β-hernolytic streptococci Increasing concern for methicillin-resistant S aureus | Penicillinase-resistant penicillins First-generation cephalosporins Consider drugs appropriate for methicillin-resistant S aureus |
| Chronic sinusitis | Anaerobes | Metronidazole Clindamycin |
Steroids have no proven benefit
Systemic steroids have no proven benefit in the treatment of pediatric orbital cellulitis with subperiosteal abscess.
A small retrospective cohort study of the benefit of intravenous steroids in addition to antibiotics showed no decrease in hospital stay or need for surgical decompression (n=23, P=.26 and .20, respectively).9 Without prospective data and a power analysis, lack of benefit of steroids cannot be definitively shown.
Recommendations from others
Infectious Disease Society of America. The guidelines for the management of skin and soft-tissue infections implicate β-hemolytic streptococci as the most common cellulitis pathogen, but also recommend empiric coverage against S aureus.
Periorbital and orbital cellulitis are not specifically addressed in these guidelines, but oral dicloxacillin, cephalexin, clindamycin, or erythromycin are recommended for superficial cellulitis, provided there is no known resistance to these antibiotics.
Intravenous penicillinase-resistant penicillins (nafcillin) or a first-generation cephalosporin (cefazolin) may be used for more severe infections.
For penicillin-allergic patients, the IDSA recommends clindamycin or vancomycin.10
Sanford Guide to Antimicrobial Therapy. Nafcillin plus ceftriaxone and metronidazole is the recommended treatment for orbital cellulitis.
For patients allergic to penicillin, vancomycin plus levofloxacin and metronidazole are recommended.8
Neither the American Academy of Ophthalmology nor the International Council of Ophthalmology offers clinical statements on orbital cellulitis.
Acknowledgments
The opinions and assertions contained herein are the private views of the author and not to be construed as official, or as reflecting the views of the US Air Force Medical service or the US Air Force at large.
1. Nageswaran S, Woods CR, Benjamin DK, Jr, Givner LB, Shetty AK. Orbital cellulitis in children. Pediatr Infect Dis J 2006;25:695-699.
2. Vayalumkal JV, Jadavji T. Children hospitalized with skin and soft tissue infections: a guide to antibacterial selection and treatment. Paediatr Drugs 2006;8:99-111.
3. Starkey CR, Steele RW. Medical management of orbital cellulitis. Pediatr Infect Dis J 2001;20:1002-1005.
4. Noel LP, Clarke WN, MacDonald N. Clinical management of orbital cellulitis in children. Can J Ophthalmol 1990;25:11-16.
5. Greenberg MF, Pollard ZF. Medical treatment of pediatric subperiosteal orbital abscess secondary to sinusitis. J AAPOS 1998;2:351-355.
6. Liu IT, Kao SC, Wang AG, Tsai CC, Liang CK, Hsu WM. Preseptal and orbital cellulitis: a 10-year review of hospitalized patients. J Chin Med Assoc 2006;69:415-422.
7. Donohue SP, Schwartz G. Preseptal and orbital cellulitis in childhood. A changing microbiologic spectrum. Ophthalmology 1998;105:1902;1905.
8. Gilbert DM, Eliopoulos GM, Moellering RC, Sande MA. The Sanford Guide to Antimicrobial Therapy 2006 36th ed. Sperryville, Va: Antimicrobial Therapy; 2006;12.-
9. Yen MT, Yen KG. Effect of corticosteroids in the acute management of pediatric orbital cellulitis with subperiosteal abscess. Ophthal Plast Reconstr Surg 2005;21:363-366.
10. Stevens DL, Bisno AL, Chambers HF, et al. Infectious Diseases Society of America. Practice guidelines for the diagnosis and management of skin and soft-tissue infections. Clin Infect Dis 2005;41:1373-1406.
1. Nageswaran S, Woods CR, Benjamin DK, Jr, Givner LB, Shetty AK. Orbital cellulitis in children. Pediatr Infect Dis J 2006;25:695-699.
2. Vayalumkal JV, Jadavji T. Children hospitalized with skin and soft tissue infections: a guide to antibacterial selection and treatment. Paediatr Drugs 2006;8:99-111.
3. Starkey CR, Steele RW. Medical management of orbital cellulitis. Pediatr Infect Dis J 2001;20:1002-1005.
4. Noel LP, Clarke WN, MacDonald N. Clinical management of orbital cellulitis in children. Can J Ophthalmol 1990;25:11-16.
5. Greenberg MF, Pollard ZF. Medical treatment of pediatric subperiosteal orbital abscess secondary to sinusitis. J AAPOS 1998;2:351-355.
6. Liu IT, Kao SC, Wang AG, Tsai CC, Liang CK, Hsu WM. Preseptal and orbital cellulitis: a 10-year review of hospitalized patients. J Chin Med Assoc 2006;69:415-422.
7. Donohue SP, Schwartz G. Preseptal and orbital cellulitis in childhood. A changing microbiologic spectrum. Ophthalmology 1998;105:1902;1905.
8. Gilbert DM, Eliopoulos GM, Moellering RC, Sande MA. The Sanford Guide to Antimicrobial Therapy 2006 36th ed. Sperryville, Va: Antimicrobial Therapy; 2006;12.-
9. Yen MT, Yen KG. Effect of corticosteroids in the acute management of pediatric orbital cellulitis with subperiosteal abscess. Ophthal Plast Reconstr Surg 2005;21:363-366.
10. Stevens DL, Bisno AL, Chambers HF, et al. Infectious Diseases Society of America. Practice guidelines for the diagnosis and management of skin and soft-tissue infections. Clin Infect Dis 2005;41:1373-1406.
Evidence-based answers from the Family Physicians Inquiries Network
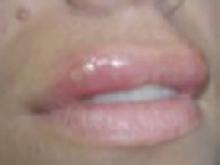
What are the best treatments for herpes labialis?
There are 3: valacyclovir, acyclovir, and topical penciclovir. Valacyclovir, 2 g twice in 1 day taken during the prodromal stage of herpes labialis, reduces the episode duration and time to healing. Acyclovir, 400 mg, taken 5 times a day for 5 days, decreases the pain duration and healing time to loss of crust (strength of recommendation [SOR]: A, based on randomized controlled trials [RCTs]). Topical penciclovir 1%, acyclovir 5%, or docosanol 10% also decrease the duration of pain and healing time (SOR: A, based on RCTs).
The best prophylaxis for herpes labialis is oral valacyclovir 500 mg daily; it reduces the frequency and severity of attacks (SOR: B, based on RCT). Sunscreen may be effective in sunlight-induced recurrence (SOR: B, based on 2 small crossover RCTs).
Let patients self-treat before breakouts
Tricia C. Elliott, MD, FAAFP
Kelsey-Seybold Family Medicine Residency Program, Houston, Texas
An effective management for the treatment of recurrent herpes labialis at the prodromal stage is a patient-initiated, self-treatment approach. In my experience, providing these patients with a prescription for valacyclovir prior to breakouts results in better overall outcomes. Patients are able to start self-treatment at the earliest signs of symptoms and feel more in control of their disease. With the lower pill burden and shorter treatment duration of valacyclovir, many patients report significantly shorter healing times, reduction in duration of pain, better compliance, and overall satisfaction.
This approach is particularly useful for patients like medical personnel and daycare workers, for whom outbreaks can pose significant adverse outcomes, such as loss of work days and increased risk of infecting others. If breakouts are frequent and risk of infecting others is high, consider daily valacyclovir as prophylaxis for these patients.
Evidence summary
Herpes labialis is the most common presentation of herpes simplex virus 1 (HSV-1) infection and generally represents reactivation. The disease progresses quickly; therefore, early treatment is required.
Patient-initiated treatment can be effective. TABLE 1 shows the comparison of oral (valacyclovir and acyclovir) and topical (penciclovir, acyclovir, and docosanol) antiviral agents for treatment of herpes labialis.1-5
TABLE 1
Antiviral agents for herpes labialis: A comparison
| DRUG | REGIMEN (OR PLACEBO) | N | OUTCOME (VS PLACEBO) | ||
|---|---|---|---|---|---|
| HEALING TIME | PAIN DURATION | ||||
| Oral | Valacyclovir* | 2 g twice daily for 1 day | 603 | 1.3 days ↓ (95% CI, –1.9 to –0.7) (4.8 vs 6.1 days)1 | |
| 615 | 1.3 days ↓ (95% CI, –1.8 to –0.7) (5.1 vs 6.4 days)1 | ||||
| Acyclovir | 400 mg 5 times a day for 5 days | 174 | 1.3 days ↓ (2.5 vs 3.8 days)2 | ||
| Topical | Penciclovir* 1% | Every 2 hours during waking hours for 4 days | 3057 | 31% ↓ (HR=1.31; 95% CI, 1.20–1.42)3 | 28% ↓ (HR=1.28; 95% CI, 1.17–1.39)3 |
| 1573 | 0.7 days ↓ (4.8 vs 5.5)3 | 0.6 days ↓ (3.5 vs 4.1)3 | |||
| Acyclovir 5% | 5 times a day for 4 days | 689 | 0.5 days ↓ (4.3 vs 4.8) (HR=1.23; 95% CI, 1.06–1.44)4 | 0.3 days ↓ (2.9 vs 3.2 days, HR=1.20; 95% CI, 1.03–1.40)4 | |
| Docosanol* 10% (available OTC) | 5 times daily | 737 | 0.7 days ↓ (95% CI, 0.08–0.92 days) (4.1 vs 4.8 days)5 | 0.56 days ↓ (95% CI, 0.125–0.69 days) (2.18 vs 2.74 days)5 | |
| * FDA approved | |||||
| CI, confidence interval; ↓, decrease; HR, hazard ratio. | |||||
Oral treatments: Shortening episodes by a day
Two RCTs have shown that valacyclovir (the prodrug of acyclovir, which has 3 to 5 times greater bioavailability) at a dosage of 2 g twice in 1 day significantly decreased the episode duration and time to lesion healing compared with placebo. In the first study (n=603), the mean episode duration was decreased by 1.1 days (5.0 vs 6.1 days; 95% confidence interval [CI], –1.6 to –0.6); in the second study (n=615) by 1.0 day (5.3 vs 6.3 days; 95% CI, –1.0 to –0.5).1
Oral acyclovir has also been shown to be effective in a well-done RCT (TABLE 1). For a subgroup of patients who started acyclovir in the prodrome or erythema stage, the duration decreased (2.5 vs 3.9 days, P=.02), but in the papular stage, it did not decrease significantly (2.5 vs. 3.6 days, P=.36).2
Topical treatments speed healing, reduce pain
Topical penciclovir 1% cream decreases the duration of lesion healing and pain compared with a vehicle control, as shown by 2 RCTs (n=3057, 1573). Patients initiated self-treatment every 2 hours during waking hours for 4 days. In one RCT, the treatment patients lost classic lesions 31% faster than the placebo group. In another trial, healing of classical lesions was faster by 0.7 days (4.8 vs 5.5). Benefits were achieved in both the early (P=.001) and later stages (P=.0055) of recurrence.3
Two RCTs of topical acyclovir 5% cream, 5 times a day for 4 days (n=689, 699) showed that topical acyclovir, compared with placebo, shortened the duration of an outbreak by 0.5 day (4.3 vs 4.8) and 0.6 day (4.6 vs 5.2), respectively.4 When it comes to prophylaxis, several studies have shown that oral valacyclovir and sunscreen may be effective for prophylaxis of herpes labialis (TABLE 2).6-8
TABLE 2
Valacyclovir and sunscreen: Helpful in preventing a herpes labialis outbreak
| DRUG | REGIMEN | N | OUTCOME (VS PLACEBO) |
|---|---|---|---|
| Valacyclovir (oral) | 500 mg daily | 98 | 24%↓; attack rate, 38% vs 62%; NNT=46 |
| Sunscreen | Various | 19 | Attack rate, 0% vs 71%; NNT=17 |
| Sunscreen | Various | 19 | Attack rate, 5% vs 58%; NNT=28 |
| ↓, decrease; NNT, number needed to treat | |||
Recommendations from others
The BMJ Clinical Evidence Guideline reiterates that oral agents (acyclovir or valacyclovir) and topical agents (acyclovir or penciclovir) slightly reduce healing time and duration of pain in treating recurrent attack. As prophylaxis, oral acyclovir or sunscreen are likely to be beneficial.9
UpToDate reports that recurrent herpes labialis is usually not treated with antivirals unless a prodromal stage can be identified. In these cases, oral acyclovir or penciclovir cream can be prescribed for 4 days’ duration. Chronic suppressive therapy can be useful in immunocompetent patients with more than 2 episodes in 4 months, and for recurrences associated with systemic complications or those that affect job performance. As prophylaxis, oral acyclovir (200 mg 3–5 times a day) is generally used, but valacyclovir (500 mg once daily) is also effective.10
1. Spruance SL, Jones TM, Blatter MM, et al. High-dose, short-duration, early valacyclovir therapy for episodic treatment of cold sores: results of two randomized, placebo-controlled, multicenter studies. Antimicrob Agents Chemother 2003;47:1072-1080.
2. Spruance SL, Stewart JC, Rowe NH, et al. Treatment of recurrent herpes simplex labialis with oral acyclovir. J Infect Dis 1990;161:181-190.
3. Spruance SL, Rea TL, Thoming C, et al. Penciclovir cream for the treatment of herpes simplex labialis. JAMA 1997;277:1374-1379.
4. Spruance SL, Nett R, Marbury T, et al. Acyclovir cream for treatment of herpes simplex labialis: results of two randomized, double-blind, vehicle-controlled multicenter clinical trials. Antimicrob Agents Chemother 2002;46:2238-2243.
5. Sacks SL, Thisted RA, Jones TM, et al. Clinical efficacy of topical docosanol 10% cream for herpes simplex labialis: a multicenter, randomized, placebo-controlled trial. J Am Acad Dermatol 2001;45:222-230.
6. Baker D, Eisen D. Valacyclovir for prevention of recurrent herpes labialis: 2 double-blind, placebo-controlled studies. Cutis 2003;71:239-242.
7. Rooney JF, Bryson Y, Mannix ML, et al. Prevention of ultraviolet-light-induced herpes labialis by sunscreen. Lancet 1991;338:1419-1421.
8. Duteil L, Queille-Roussel C, Loesche C, et al. Assessment of the effect of a sunblock stick in the prevention of solar-simulating ultraviolet light-induced herpes labialis. J Dermatol Treat 1998;9:11-14.
9. Graham Warrall G. Interventions of herpes labialis. Search date April 2005. Available at: clinicalevidence.com. Accessed on April 10, 2006.
10. Klein R. Treatment and prevention of herpes simplex virus type 1 infection. UpToDate version 14.1, last updated September 20, 2005. Available at: UpToDate.com. Accessed on April 24, 2006.
There are 3: valacyclovir, acyclovir, and topical penciclovir. Valacyclovir, 2 g twice in 1 day taken during the prodromal stage of herpes labialis, reduces the episode duration and time to healing. Acyclovir, 400 mg, taken 5 times a day for 5 days, decreases the pain duration and healing time to loss of crust (strength of recommendation [SOR]: A, based on randomized controlled trials [RCTs]). Topical penciclovir 1%, acyclovir 5%, or docosanol 10% also decrease the duration of pain and healing time (SOR: A, based on RCTs).
The best prophylaxis for herpes labialis is oral valacyclovir 500 mg daily; it reduces the frequency and severity of attacks (SOR: B, based on RCT). Sunscreen may be effective in sunlight-induced recurrence (SOR: B, based on 2 small crossover RCTs).
Let patients self-treat before breakouts
Tricia C. Elliott, MD, FAAFP
Kelsey-Seybold Family Medicine Residency Program, Houston, Texas
An effective management for the treatment of recurrent herpes labialis at the prodromal stage is a patient-initiated, self-treatment approach. In my experience, providing these patients with a prescription for valacyclovir prior to breakouts results in better overall outcomes. Patients are able to start self-treatment at the earliest signs of symptoms and feel more in control of their disease. With the lower pill burden and shorter treatment duration of valacyclovir, many patients report significantly shorter healing times, reduction in duration of pain, better compliance, and overall satisfaction.
This approach is particularly useful for patients like medical personnel and daycare workers, for whom outbreaks can pose significant adverse outcomes, such as loss of work days and increased risk of infecting others. If breakouts are frequent and risk of infecting others is high, consider daily valacyclovir as prophylaxis for these patients.
Evidence summary
Herpes labialis is the most common presentation of herpes simplex virus 1 (HSV-1) infection and generally represents reactivation. The disease progresses quickly; therefore, early treatment is required.
Patient-initiated treatment can be effective. TABLE 1 shows the comparison of oral (valacyclovir and acyclovir) and topical (penciclovir, acyclovir, and docosanol) antiviral agents for treatment of herpes labialis.1-5
TABLE 1
Antiviral agents for herpes labialis: A comparison
| DRUG | REGIMEN (OR PLACEBO) | N | OUTCOME (VS PLACEBO) | ||
|---|---|---|---|---|---|
| HEALING TIME | PAIN DURATION | ||||
| Oral | Valacyclovir* | 2 g twice daily for 1 day | 603 | 1.3 days ↓ (95% CI, –1.9 to –0.7) (4.8 vs 6.1 days)1 | |
| 615 | 1.3 days ↓ (95% CI, –1.8 to –0.7) (5.1 vs 6.4 days)1 | ||||
| Acyclovir | 400 mg 5 times a day for 5 days | 174 | 1.3 days ↓ (2.5 vs 3.8 days)2 | ||
| Topical | Penciclovir* 1% | Every 2 hours during waking hours for 4 days | 3057 | 31% ↓ (HR=1.31; 95% CI, 1.20–1.42)3 | 28% ↓ (HR=1.28; 95% CI, 1.17–1.39)3 |
| 1573 | 0.7 days ↓ (4.8 vs 5.5)3 | 0.6 days ↓ (3.5 vs 4.1)3 | |||
| Acyclovir 5% | 5 times a day for 4 days | 689 | 0.5 days ↓ (4.3 vs 4.8) (HR=1.23; 95% CI, 1.06–1.44)4 | 0.3 days ↓ (2.9 vs 3.2 days, HR=1.20; 95% CI, 1.03–1.40)4 | |
| Docosanol* 10% (available OTC) | 5 times daily | 737 | 0.7 days ↓ (95% CI, 0.08–0.92 days) (4.1 vs 4.8 days)5 | 0.56 days ↓ (95% CI, 0.125–0.69 days) (2.18 vs 2.74 days)5 | |
| * FDA approved | |||||
| CI, confidence interval; ↓, decrease; HR, hazard ratio. | |||||
Oral treatments: Shortening episodes by a day
Two RCTs have shown that valacyclovir (the prodrug of acyclovir, which has 3 to 5 times greater bioavailability) at a dosage of 2 g twice in 1 day significantly decreased the episode duration and time to lesion healing compared with placebo. In the first study (n=603), the mean episode duration was decreased by 1.1 days (5.0 vs 6.1 days; 95% confidence interval [CI], –1.6 to –0.6); in the second study (n=615) by 1.0 day (5.3 vs 6.3 days; 95% CI, –1.0 to –0.5).1
Oral acyclovir has also been shown to be effective in a well-done RCT (TABLE 1). For a subgroup of patients who started acyclovir in the prodrome or erythema stage, the duration decreased (2.5 vs 3.9 days, P=.02), but in the papular stage, it did not decrease significantly (2.5 vs. 3.6 days, P=.36).2
Topical treatments speed healing, reduce pain
Topical penciclovir 1% cream decreases the duration of lesion healing and pain compared with a vehicle control, as shown by 2 RCTs (n=3057, 1573). Patients initiated self-treatment every 2 hours during waking hours for 4 days. In one RCT, the treatment patients lost classic lesions 31% faster than the placebo group. In another trial, healing of classical lesions was faster by 0.7 days (4.8 vs 5.5). Benefits were achieved in both the early (P=.001) and later stages (P=.0055) of recurrence.3
Two RCTs of topical acyclovir 5% cream, 5 times a day for 4 days (n=689, 699) showed that topical acyclovir, compared with placebo, shortened the duration of an outbreak by 0.5 day (4.3 vs 4.8) and 0.6 day (4.6 vs 5.2), respectively.4 When it comes to prophylaxis, several studies have shown that oral valacyclovir and sunscreen may be effective for prophylaxis of herpes labialis (TABLE 2).6-8
TABLE 2
Valacyclovir and sunscreen: Helpful in preventing a herpes labialis outbreak
| DRUG | REGIMEN | N | OUTCOME (VS PLACEBO) |
|---|---|---|---|
| Valacyclovir (oral) | 500 mg daily | 98 | 24%↓; attack rate, 38% vs 62%; NNT=46 |
| Sunscreen | Various | 19 | Attack rate, 0% vs 71%; NNT=17 |
| Sunscreen | Various | 19 | Attack rate, 5% vs 58%; NNT=28 |
| ↓, decrease; NNT, number needed to treat | |||
Recommendations from others
The BMJ Clinical Evidence Guideline reiterates that oral agents (acyclovir or valacyclovir) and topical agents (acyclovir or penciclovir) slightly reduce healing time and duration of pain in treating recurrent attack. As prophylaxis, oral acyclovir or sunscreen are likely to be beneficial.9
UpToDate reports that recurrent herpes labialis is usually not treated with antivirals unless a prodromal stage can be identified. In these cases, oral acyclovir or penciclovir cream can be prescribed for 4 days’ duration. Chronic suppressive therapy can be useful in immunocompetent patients with more than 2 episodes in 4 months, and for recurrences associated with systemic complications or those that affect job performance. As prophylaxis, oral acyclovir (200 mg 3–5 times a day) is generally used, but valacyclovir (500 mg once daily) is also effective.10
There are 3: valacyclovir, acyclovir, and topical penciclovir. Valacyclovir, 2 g twice in 1 day taken during the prodromal stage of herpes labialis, reduces the episode duration and time to healing. Acyclovir, 400 mg, taken 5 times a day for 5 days, decreases the pain duration and healing time to loss of crust (strength of recommendation [SOR]: A, based on randomized controlled trials [RCTs]). Topical penciclovir 1%, acyclovir 5%, or docosanol 10% also decrease the duration of pain and healing time (SOR: A, based on RCTs).
The best prophylaxis for herpes labialis is oral valacyclovir 500 mg daily; it reduces the frequency and severity of attacks (SOR: B, based on RCT). Sunscreen may be effective in sunlight-induced recurrence (SOR: B, based on 2 small crossover RCTs).
Let patients self-treat before breakouts
Tricia C. Elliott, MD, FAAFP
Kelsey-Seybold Family Medicine Residency Program, Houston, Texas
An effective management for the treatment of recurrent herpes labialis at the prodromal stage is a patient-initiated, self-treatment approach. In my experience, providing these patients with a prescription for valacyclovir prior to breakouts results in better overall outcomes. Patients are able to start self-treatment at the earliest signs of symptoms and feel more in control of their disease. With the lower pill burden and shorter treatment duration of valacyclovir, many patients report significantly shorter healing times, reduction in duration of pain, better compliance, and overall satisfaction.
This approach is particularly useful for patients like medical personnel and daycare workers, for whom outbreaks can pose significant adverse outcomes, such as loss of work days and increased risk of infecting others. If breakouts are frequent and risk of infecting others is high, consider daily valacyclovir as prophylaxis for these patients.
Evidence summary
Herpes labialis is the most common presentation of herpes simplex virus 1 (HSV-1) infection and generally represents reactivation. The disease progresses quickly; therefore, early treatment is required.
Patient-initiated treatment can be effective. TABLE 1 shows the comparison of oral (valacyclovir and acyclovir) and topical (penciclovir, acyclovir, and docosanol) antiviral agents for treatment of herpes labialis.1-5
TABLE 1
Antiviral agents for herpes labialis: A comparison
| DRUG | REGIMEN (OR PLACEBO) | N | OUTCOME (VS PLACEBO) | ||
|---|---|---|---|---|---|
| HEALING TIME | PAIN DURATION | ||||
| Oral | Valacyclovir* | 2 g twice daily for 1 day | 603 | 1.3 days ↓ (95% CI, –1.9 to –0.7) (4.8 vs 6.1 days)1 | |
| 615 | 1.3 days ↓ (95% CI, –1.8 to –0.7) (5.1 vs 6.4 days)1 | ||||
| Acyclovir | 400 mg 5 times a day for 5 days | 174 | 1.3 days ↓ (2.5 vs 3.8 days)2 | ||
| Topical | Penciclovir* 1% | Every 2 hours during waking hours for 4 days | 3057 | 31% ↓ (HR=1.31; 95% CI, 1.20–1.42)3 | 28% ↓ (HR=1.28; 95% CI, 1.17–1.39)3 |
| 1573 | 0.7 days ↓ (4.8 vs 5.5)3 | 0.6 days ↓ (3.5 vs 4.1)3 | |||
| Acyclovir 5% | 5 times a day for 4 days | 689 | 0.5 days ↓ (4.3 vs 4.8) (HR=1.23; 95% CI, 1.06–1.44)4 | 0.3 days ↓ (2.9 vs 3.2 days, HR=1.20; 95% CI, 1.03–1.40)4 | |
| Docosanol* 10% (available OTC) | 5 times daily | 737 | 0.7 days ↓ (95% CI, 0.08–0.92 days) (4.1 vs 4.8 days)5 | 0.56 days ↓ (95% CI, 0.125–0.69 days) (2.18 vs 2.74 days)5 | |
| * FDA approved | |||||
| CI, confidence interval; ↓, decrease; HR, hazard ratio. | |||||
Oral treatments: Shortening episodes by a day
Two RCTs have shown that valacyclovir (the prodrug of acyclovir, which has 3 to 5 times greater bioavailability) at a dosage of 2 g twice in 1 day significantly decreased the episode duration and time to lesion healing compared with placebo. In the first study (n=603), the mean episode duration was decreased by 1.1 days (5.0 vs 6.1 days; 95% confidence interval [CI], –1.6 to –0.6); in the second study (n=615) by 1.0 day (5.3 vs 6.3 days; 95% CI, –1.0 to –0.5).1
Oral acyclovir has also been shown to be effective in a well-done RCT (TABLE 1). For a subgroup of patients who started acyclovir in the prodrome or erythema stage, the duration decreased (2.5 vs 3.9 days, P=.02), but in the papular stage, it did not decrease significantly (2.5 vs. 3.6 days, P=.36).2
Topical treatments speed healing, reduce pain
Topical penciclovir 1% cream decreases the duration of lesion healing and pain compared with a vehicle control, as shown by 2 RCTs (n=3057, 1573). Patients initiated self-treatment every 2 hours during waking hours for 4 days. In one RCT, the treatment patients lost classic lesions 31% faster than the placebo group. In another trial, healing of classical lesions was faster by 0.7 days (4.8 vs 5.5). Benefits were achieved in both the early (P=.001) and later stages (P=.0055) of recurrence.3
Two RCTs of topical acyclovir 5% cream, 5 times a day for 4 days (n=689, 699) showed that topical acyclovir, compared with placebo, shortened the duration of an outbreak by 0.5 day (4.3 vs 4.8) and 0.6 day (4.6 vs 5.2), respectively.4 When it comes to prophylaxis, several studies have shown that oral valacyclovir and sunscreen may be effective for prophylaxis of herpes labialis (TABLE 2).6-8
TABLE 2
Valacyclovir and sunscreen: Helpful in preventing a herpes labialis outbreak
| DRUG | REGIMEN | N | OUTCOME (VS PLACEBO) |
|---|---|---|---|
| Valacyclovir (oral) | 500 mg daily | 98 | 24%↓; attack rate, 38% vs 62%; NNT=46 |
| Sunscreen | Various | 19 | Attack rate, 0% vs 71%; NNT=17 |
| Sunscreen | Various | 19 | Attack rate, 5% vs 58%; NNT=28 |
| ↓, decrease; NNT, number needed to treat | |||
Recommendations from others
The BMJ Clinical Evidence Guideline reiterates that oral agents (acyclovir or valacyclovir) and topical agents (acyclovir or penciclovir) slightly reduce healing time and duration of pain in treating recurrent attack. As prophylaxis, oral acyclovir or sunscreen are likely to be beneficial.9
UpToDate reports that recurrent herpes labialis is usually not treated with antivirals unless a prodromal stage can be identified. In these cases, oral acyclovir or penciclovir cream can be prescribed for 4 days’ duration. Chronic suppressive therapy can be useful in immunocompetent patients with more than 2 episodes in 4 months, and for recurrences associated with systemic complications or those that affect job performance. As prophylaxis, oral acyclovir (200 mg 3–5 times a day) is generally used, but valacyclovir (500 mg once daily) is also effective.10
1. Spruance SL, Jones TM, Blatter MM, et al. High-dose, short-duration, early valacyclovir therapy for episodic treatment of cold sores: results of two randomized, placebo-controlled, multicenter studies. Antimicrob Agents Chemother 2003;47:1072-1080.
2. Spruance SL, Stewart JC, Rowe NH, et al. Treatment of recurrent herpes simplex labialis with oral acyclovir. J Infect Dis 1990;161:181-190.
3. Spruance SL, Rea TL, Thoming C, et al. Penciclovir cream for the treatment of herpes simplex labialis. JAMA 1997;277:1374-1379.
4. Spruance SL, Nett R, Marbury T, et al. Acyclovir cream for treatment of herpes simplex labialis: results of two randomized, double-blind, vehicle-controlled multicenter clinical trials. Antimicrob Agents Chemother 2002;46:2238-2243.
5. Sacks SL, Thisted RA, Jones TM, et al. Clinical efficacy of topical docosanol 10% cream for herpes simplex labialis: a multicenter, randomized, placebo-controlled trial. J Am Acad Dermatol 2001;45:222-230.
6. Baker D, Eisen D. Valacyclovir for prevention of recurrent herpes labialis: 2 double-blind, placebo-controlled studies. Cutis 2003;71:239-242.
7. Rooney JF, Bryson Y, Mannix ML, et al. Prevention of ultraviolet-light-induced herpes labialis by sunscreen. Lancet 1991;338:1419-1421.
8. Duteil L, Queille-Roussel C, Loesche C, et al. Assessment of the effect of a sunblock stick in the prevention of solar-simulating ultraviolet light-induced herpes labialis. J Dermatol Treat 1998;9:11-14.
9. Graham Warrall G. Interventions of herpes labialis. Search date April 2005. Available at: clinicalevidence.com. Accessed on April 10, 2006.
10. Klein R. Treatment and prevention of herpes simplex virus type 1 infection. UpToDate version 14.1, last updated September 20, 2005. Available at: UpToDate.com. Accessed on April 24, 2006.
1. Spruance SL, Jones TM, Blatter MM, et al. High-dose, short-duration, early valacyclovir therapy for episodic treatment of cold sores: results of two randomized, placebo-controlled, multicenter studies. Antimicrob Agents Chemother 2003;47:1072-1080.
2. Spruance SL, Stewart JC, Rowe NH, et al. Treatment of recurrent herpes simplex labialis with oral acyclovir. J Infect Dis 1990;161:181-190.
3. Spruance SL, Rea TL, Thoming C, et al. Penciclovir cream for the treatment of herpes simplex labialis. JAMA 1997;277:1374-1379.
4. Spruance SL, Nett R, Marbury T, et al. Acyclovir cream for treatment of herpes simplex labialis: results of two randomized, double-blind, vehicle-controlled multicenter clinical trials. Antimicrob Agents Chemother 2002;46:2238-2243.
5. Sacks SL, Thisted RA, Jones TM, et al. Clinical efficacy of topical docosanol 10% cream for herpes simplex labialis: a multicenter, randomized, placebo-controlled trial. J Am Acad Dermatol 2001;45:222-230.
6. Baker D, Eisen D. Valacyclovir for prevention of recurrent herpes labialis: 2 double-blind, placebo-controlled studies. Cutis 2003;71:239-242.
7. Rooney JF, Bryson Y, Mannix ML, et al. Prevention of ultraviolet-light-induced herpes labialis by sunscreen. Lancet 1991;338:1419-1421.
8. Duteil L, Queille-Roussel C, Loesche C, et al. Assessment of the effect of a sunblock stick in the prevention of solar-simulating ultraviolet light-induced herpes labialis. J Dermatol Treat 1998;9:11-14.
9. Graham Warrall G. Interventions of herpes labialis. Search date April 2005. Available at: clinicalevidence.com. Accessed on April 10, 2006.
10. Klein R. Treatment and prevention of herpes simplex virus type 1 infection. UpToDate version 14.1, last updated September 20, 2005. Available at: UpToDate.com. Accessed on April 24, 2006.
Evidence-based answers from the Family Physicians Inquiries Network
What is the role of herpes virus serology in sexually transmitted disease screening?
Screening for herpes simplex virus type 2 (HSV-2) infection with antibody testing is not indicated for asymptomatic adults (strength of recommendation [SOR]: B, prevalence studies and predictive value of testing). Screening with serology testing is not indicated for asymptomatic pregnant women (SOR: B, 1 cohort study).
You may consider offering testing to asymptomatic patients with an HSV-positive partner, patients with HIV infection, and those with current or recent sexually transmitted infection or high-risk behavior (SOR: C, expert opinion and 1 case control study with extrapolation of result).
Counsel patients that the diagnostic gold standard remains viral culture or PCR testing of active lesions
John Mercer, MD, FAAFP
Baylor Family Medicine Residency, Garland, Tex
Early in my practice, a couple came to my office demanding serology testing for HSV after resolution of a new genital lesion. The results of the non-type-specific HSV serology led to more questions than answers due to cross-reactivity between virus types. Even with the newer type-specific glycoprotein enzyme immunoassays for HSV 1 and 2, I reserve serologic testing for specific situations, as outlined in this review, and when recurrent genital signs or symptoms of unclear cause present with negative viral culture results. I counsel patients that the diagnostic gold standard remains viral culture or PCR testing of active lesions. The best course of action for most asymptomatic patients remains sexually transmitted disease counseling and returning to the clinic for viral culture if a suspicious lesion returns.
Evidence summary
An effective screening test for HSV would need to identify those with HSV infection before substantial morbidity resulted, and effective interventions would need to be available for use in the asymptomatic stage. Screening for HSV-2 must also consider the psychosocial impact of serologic diagnosis in those without symptoms, as a qualitative study showed both negative and positive emotional responses in those with positive serology, with short-term emotional responses described as surprise, denial, confusion, distress, disappointment, and sense of relief.1 Patients also expressed fear of partner notification, concern for transmission to newborns, and concern for social stigma.
Pre- and post-test counseling must accompany testing as negative emotional or psychological responses are amenable to this intervention. A consideration for screening decisions is the positive predictive value (PPV) of testing for the specific patient, which ranges from 58% (in a British population with 4% prevalence) to 90% (in a population with 22% prevalence taken from sexually transmitted disease clinics in the Netherlands).2 (A PPV of 58% means that only 58% of women with a positive test actually had the disease, and 42% were false-positive).
The primary goal for screening pregnant women is prevention of neonatal transmission of HSV. A prospective observational study3 of 7046 women found that acquisition of HSV-2 during pregnancy was asymptomatic in 74% of 94 cases. No increase in neonatal or pregnancy-related morbidity was seen for those patients who had seroconverted by the time of labor. The main benefit of serology testing during pregnancy has been to identify patients with asymptomatic infection and counsel them on reporting new symptoms for evaluation and treatment.
Another prospective cohort study4 identified seropositive pregnant women with no history of genital herpes. Forty-three of 264 (16%) of these women were able to identify and report clinical HSV to their physician during the pregnancy.
Testing of asymptomatic patients with HSV-2 serology and counseling has been recommended by some experts5 for motivated patients with current or recent sexually transmitted infection or HIV infection and for partners of HSV-positive patients.6 Screening could give those identified the opportunity to learn to recognize symptoms, decrease transmission, and understand risks of acquiring HIV or other sexually transmitted infections. Patients screening negative might have heightened awareness to susceptibility and reinforce lifestyle changes.6 Success of HSV prevention strategies is reviewed elsewhere.7
Recommendations from others
The Centers for Disease Control and Prevention, the United States Preventive Services Task Force, and the American Academy of Family Physicians do not recommend screening asymptomatic adults for HSV infection.7,8 The American College of Obstetricians and Gynecologists does not recommend routine screening of pregnant women for HSV.9
1. Melville J, Sniffen S, Salazar L, et al. Psychosocial impact of serological diagnosis of herpes simplex virus type 2: a qualitative assessment. Sex Trans Infect 2003;79:280-285.
2. Krantz I, Lowhagen G, Ahlberg B, Nilstun T. Ethics of screening for asymptomatic herpes virus type 2 infection. BMJ 2004;329:618-621.
3. Brown Z, Selke S, Zeh J, et al. The acquisition of herpes simplex virus during pregnancy. N Engl J Med 1997;337:509-516.
4. Frenkel L, Garratty E, Ping S, et al. Clinical reactivation of herpes simplex virus type 2 infection in seropositive pregnant women with no history of genital herpes. Ann Int Med 1993;118:414-418.
5. Centers for Disease Control and Prevention. Incorporating HIV prevention into medical care of persons living with HIV: recommendations of CDC, the Health Resources and Services Administration, the National Institutes of Health, and the HIV Medicine Association of the Infectious Diseases Society of America. MMWR Recomm Rep 2003;52(RR-12).-
6. Centers for Disease Control and Prevention. Diseases Characterized by Genital Ulcers. Sexually Transmitted Diseases Guidelines. MMWR Recomm Rep 2002;51(RR-6):11-25.
7. Screening for genital herpes simplex. Rockville, Md: US Preventive Task Force updated March 2005. Available at: www.ahrq.gov/clinic/uspstf05/herpes/herpesup.htm. Accessed on April 18, 2006.
8. American Academy of Family Physicians. Summary of policy recommendations for periodic health examinations. Leawood, Kan: American Academy of Family Physicians; 2004. 15pp.
9. American College of Obstetricians and Gynecologists. Guidelines for Perinatal Care. 5th ed. Elk Grove, Ill: AAP; Washington, DC: ACOG; 2002.
Screening for herpes simplex virus type 2 (HSV-2) infection with antibody testing is not indicated for asymptomatic adults (strength of recommendation [SOR]: B, prevalence studies and predictive value of testing). Screening with serology testing is not indicated for asymptomatic pregnant women (SOR: B, 1 cohort study).
You may consider offering testing to asymptomatic patients with an HSV-positive partner, patients with HIV infection, and those with current or recent sexually transmitted infection or high-risk behavior (SOR: C, expert opinion and 1 case control study with extrapolation of result).
Counsel patients that the diagnostic gold standard remains viral culture or PCR testing of active lesions
John Mercer, MD, FAAFP
Baylor Family Medicine Residency, Garland, Tex
Early in my practice, a couple came to my office demanding serology testing for HSV after resolution of a new genital lesion. The results of the non-type-specific HSV serology led to more questions than answers due to cross-reactivity between virus types. Even with the newer type-specific glycoprotein enzyme immunoassays for HSV 1 and 2, I reserve serologic testing for specific situations, as outlined in this review, and when recurrent genital signs or symptoms of unclear cause present with negative viral culture results. I counsel patients that the diagnostic gold standard remains viral culture or PCR testing of active lesions. The best course of action for most asymptomatic patients remains sexually transmitted disease counseling and returning to the clinic for viral culture if a suspicious lesion returns.
Evidence summary
An effective screening test for HSV would need to identify those with HSV infection before substantial morbidity resulted, and effective interventions would need to be available for use in the asymptomatic stage. Screening for HSV-2 must also consider the psychosocial impact of serologic diagnosis in those without symptoms, as a qualitative study showed both negative and positive emotional responses in those with positive serology, with short-term emotional responses described as surprise, denial, confusion, distress, disappointment, and sense of relief.1 Patients also expressed fear of partner notification, concern for transmission to newborns, and concern for social stigma.
Pre- and post-test counseling must accompany testing as negative emotional or psychological responses are amenable to this intervention. A consideration for screening decisions is the positive predictive value (PPV) of testing for the specific patient, which ranges from 58% (in a British population with 4% prevalence) to 90% (in a population with 22% prevalence taken from sexually transmitted disease clinics in the Netherlands).2 (A PPV of 58% means that only 58% of women with a positive test actually had the disease, and 42% were false-positive).
The primary goal for screening pregnant women is prevention of neonatal transmission of HSV. A prospective observational study3 of 7046 women found that acquisition of HSV-2 during pregnancy was asymptomatic in 74% of 94 cases. No increase in neonatal or pregnancy-related morbidity was seen for those patients who had seroconverted by the time of labor. The main benefit of serology testing during pregnancy has been to identify patients with asymptomatic infection and counsel them on reporting new symptoms for evaluation and treatment.
Another prospective cohort study4 identified seropositive pregnant women with no history of genital herpes. Forty-three of 264 (16%) of these women were able to identify and report clinical HSV to their physician during the pregnancy.
Testing of asymptomatic patients with HSV-2 serology and counseling has been recommended by some experts5 for motivated patients with current or recent sexually transmitted infection or HIV infection and for partners of HSV-positive patients.6 Screening could give those identified the opportunity to learn to recognize symptoms, decrease transmission, and understand risks of acquiring HIV or other sexually transmitted infections. Patients screening negative might have heightened awareness to susceptibility and reinforce lifestyle changes.6 Success of HSV prevention strategies is reviewed elsewhere.7
Recommendations from others
The Centers for Disease Control and Prevention, the United States Preventive Services Task Force, and the American Academy of Family Physicians do not recommend screening asymptomatic adults for HSV infection.7,8 The American College of Obstetricians and Gynecologists does not recommend routine screening of pregnant women for HSV.9
Screening for herpes simplex virus type 2 (HSV-2) infection with antibody testing is not indicated for asymptomatic adults (strength of recommendation [SOR]: B, prevalence studies and predictive value of testing). Screening with serology testing is not indicated for asymptomatic pregnant women (SOR: B, 1 cohort study).
You may consider offering testing to asymptomatic patients with an HSV-positive partner, patients with HIV infection, and those with current or recent sexually transmitted infection or high-risk behavior (SOR: C, expert opinion and 1 case control study with extrapolation of result).
Counsel patients that the diagnostic gold standard remains viral culture or PCR testing of active lesions
John Mercer, MD, FAAFP
Baylor Family Medicine Residency, Garland, Tex
Early in my practice, a couple came to my office demanding serology testing for HSV after resolution of a new genital lesion. The results of the non-type-specific HSV serology led to more questions than answers due to cross-reactivity between virus types. Even with the newer type-specific glycoprotein enzyme immunoassays for HSV 1 and 2, I reserve serologic testing for specific situations, as outlined in this review, and when recurrent genital signs or symptoms of unclear cause present with negative viral culture results. I counsel patients that the diagnostic gold standard remains viral culture or PCR testing of active lesions. The best course of action for most asymptomatic patients remains sexually transmitted disease counseling and returning to the clinic for viral culture if a suspicious lesion returns.
Evidence summary
An effective screening test for HSV would need to identify those with HSV infection before substantial morbidity resulted, and effective interventions would need to be available for use in the asymptomatic stage. Screening for HSV-2 must also consider the psychosocial impact of serologic diagnosis in those without symptoms, as a qualitative study showed both negative and positive emotional responses in those with positive serology, with short-term emotional responses described as surprise, denial, confusion, distress, disappointment, and sense of relief.1 Patients also expressed fear of partner notification, concern for transmission to newborns, and concern for social stigma.
Pre- and post-test counseling must accompany testing as negative emotional or psychological responses are amenable to this intervention. A consideration for screening decisions is the positive predictive value (PPV) of testing for the specific patient, which ranges from 58% (in a British population with 4% prevalence) to 90% (in a population with 22% prevalence taken from sexually transmitted disease clinics in the Netherlands).2 (A PPV of 58% means that only 58% of women with a positive test actually had the disease, and 42% were false-positive).
The primary goal for screening pregnant women is prevention of neonatal transmission of HSV. A prospective observational study3 of 7046 women found that acquisition of HSV-2 during pregnancy was asymptomatic in 74% of 94 cases. No increase in neonatal or pregnancy-related morbidity was seen for those patients who had seroconverted by the time of labor. The main benefit of serology testing during pregnancy has been to identify patients with asymptomatic infection and counsel them on reporting new symptoms for evaluation and treatment.
Another prospective cohort study4 identified seropositive pregnant women with no history of genital herpes. Forty-three of 264 (16%) of these women were able to identify and report clinical HSV to their physician during the pregnancy.
Testing of asymptomatic patients with HSV-2 serology and counseling has been recommended by some experts5 for motivated patients with current or recent sexually transmitted infection or HIV infection and for partners of HSV-positive patients.6 Screening could give those identified the opportunity to learn to recognize symptoms, decrease transmission, and understand risks of acquiring HIV or other sexually transmitted infections. Patients screening negative might have heightened awareness to susceptibility and reinforce lifestyle changes.6 Success of HSV prevention strategies is reviewed elsewhere.7
Recommendations from others
The Centers for Disease Control and Prevention, the United States Preventive Services Task Force, and the American Academy of Family Physicians do not recommend screening asymptomatic adults for HSV infection.7,8 The American College of Obstetricians and Gynecologists does not recommend routine screening of pregnant women for HSV.9
1. Melville J, Sniffen S, Salazar L, et al. Psychosocial impact of serological diagnosis of herpes simplex virus type 2: a qualitative assessment. Sex Trans Infect 2003;79:280-285.
2. Krantz I, Lowhagen G, Ahlberg B, Nilstun T. Ethics of screening for asymptomatic herpes virus type 2 infection. BMJ 2004;329:618-621.
3. Brown Z, Selke S, Zeh J, et al. The acquisition of herpes simplex virus during pregnancy. N Engl J Med 1997;337:509-516.
4. Frenkel L, Garratty E, Ping S, et al. Clinical reactivation of herpes simplex virus type 2 infection in seropositive pregnant women with no history of genital herpes. Ann Int Med 1993;118:414-418.
5. Centers for Disease Control and Prevention. Incorporating HIV prevention into medical care of persons living with HIV: recommendations of CDC, the Health Resources and Services Administration, the National Institutes of Health, and the HIV Medicine Association of the Infectious Diseases Society of America. MMWR Recomm Rep 2003;52(RR-12).-
6. Centers for Disease Control and Prevention. Diseases Characterized by Genital Ulcers. Sexually Transmitted Diseases Guidelines. MMWR Recomm Rep 2002;51(RR-6):11-25.
7. Screening for genital herpes simplex. Rockville, Md: US Preventive Task Force updated March 2005. Available at: www.ahrq.gov/clinic/uspstf05/herpes/herpesup.htm. Accessed on April 18, 2006.
8. American Academy of Family Physicians. Summary of policy recommendations for periodic health examinations. Leawood, Kan: American Academy of Family Physicians; 2004. 15pp.
9. American College of Obstetricians and Gynecologists. Guidelines for Perinatal Care. 5th ed. Elk Grove, Ill: AAP; Washington, DC: ACOG; 2002.
1. Melville J, Sniffen S, Salazar L, et al. Psychosocial impact of serological diagnosis of herpes simplex virus type 2: a qualitative assessment. Sex Trans Infect 2003;79:280-285.
2. Krantz I, Lowhagen G, Ahlberg B, Nilstun T. Ethics of screening for asymptomatic herpes virus type 2 infection. BMJ 2004;329:618-621.
3. Brown Z, Selke S, Zeh J, et al. The acquisition of herpes simplex virus during pregnancy. N Engl J Med 1997;337:509-516.
4. Frenkel L, Garratty E, Ping S, et al. Clinical reactivation of herpes simplex virus type 2 infection in seropositive pregnant women with no history of genital herpes. Ann Int Med 1993;118:414-418.
5. Centers for Disease Control and Prevention. Incorporating HIV prevention into medical care of persons living with HIV: recommendations of CDC, the Health Resources and Services Administration, the National Institutes of Health, and the HIV Medicine Association of the Infectious Diseases Society of America. MMWR Recomm Rep 2003;52(RR-12).-
6. Centers for Disease Control and Prevention. Diseases Characterized by Genital Ulcers. Sexually Transmitted Diseases Guidelines. MMWR Recomm Rep 2002;51(RR-6):11-25.
7. Screening for genital herpes simplex. Rockville, Md: US Preventive Task Force updated March 2005. Available at: www.ahrq.gov/clinic/uspstf05/herpes/herpesup.htm. Accessed on April 18, 2006.
8. American Academy of Family Physicians. Summary of policy recommendations for periodic health examinations. Leawood, Kan: American Academy of Family Physicians; 2004. 15pp.
9. American College of Obstetricians and Gynecologists. Guidelines for Perinatal Care. 5th ed. Elk Grove, Ill: AAP; Washington, DC: ACOG; 2002.
Evidence-based answers from the Family Physicians Inquiries Network