User login
In Reference to: “Cost and Utility of Thrombophilia Testing”
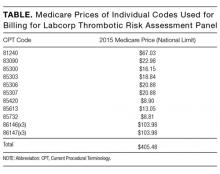
The article by Petrilli et al. points to the important but complicated issue of ordering laboratory testing for thrombophilia despite multiple guidelines that dispute the clinical utility of such testing for many indications.1 We question the basis of these authors’ assertion that Medicare spends $300 to $672 million for thrombophilia testing annually. They arrived at this figure by multiplying the price of a thrombophilia test panel (between $1100 and $2400) by the number of annual Medicare claims for thrombophilia analysis, which they estimated at 280,000. The price of the panel is derived from two papers: (1) a 2001 review2 that lists prices of various thrombophilia-related tests adding up to $1782, and (2) a 2006 evaluation by Somma et al.3 of thrombophilia screening at one hospital in New York in 2005. The latter paper refers to various thrombophilia panels from Quest Diagnostics with list prices ranging from $1311 to $2429. However, the repertoire of available test panels and their prices have changed over the last decade. The cost evaluation of thrombophilia testing should be based on actual current payments for tests, and not on list prices for laboratory offerings from over a decade ago. Several laboratories offer mutational analysis of 3 genes—F5, F2, and MTHFR—as a thrombophilia risk panel. Based on the Current Procedural Terminology (CPT) codes listed by the test suppliers (81240, 81241, and 81291), the average Medicare payment for the combination of these 3 markers in 2013 was $172.4 A broader panel of several biochemical, immunological, and genetic assays had a maximum Medicare payment in 2015 of $405 (Table).5
In conclusion, the cost evaluation of thrombophilia screening is more challenging than the calculation by Petrilli et al. suggests.1 Even if Medicare paid as much as $400 per individual tested and assuming up to 200,000 individuals underwent thrombophilia testing per year, the aggregate Medicare expenditure would have been no more than roughly $80 million. Thus, the estimated range in the article appears to have overstated actual Medicare expenditures by an order of magnitude. This does not take away from their overall conclusion that payers are burdened with significant expenditures for laboratory testing that may not present clinical value for many patients.6 We need research into the patterns of utilization as well as improvements in documentation of expenditures associated with these tests.
Disclosure
The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention, the Department of Veterans Affairs, or the United States government. The autho
1. Petrilli CM, Heidemann L, Mack M, Durance P, Chopra V. Inpatient inherited thrombophilia testing. J Hosp Med. 2016;11(11):801-804. PubMed
2. Abramson N, Abramson S. Hypercoagulability: clinical assessment and treatment. South Med J. 2001;94(10):1013-1020. PubMed
3. Somma J, Sussman, II, Rand JH. An evaluation of thrombophilia screening in an urban tertiary care medical center: A “real world” experience. Am J Clin Pathol. 2006;126(1):120-127. PubMed
4. Lynch JA, Berse B, Dotson WD, Khoury MJ, Coomer N, Kautter J. Utilization of genetic tests: Analysis of gene-specific billing in Medicare claims data [Published online ahead of print January 26, 2017]. Genet Med. 2017. doi: 10.1038/gim.2016.209. PubMed
5. Centers for Medicare and Medicaid Services. Clinical Laboratory Fee Schedule 2016. https://www.cms.gov/Medicare/Medicare-fee-for-service-Payment/clinicallabfeesched/index.html. Accessed on December 20, 2016.
6. Stevens SM, Woller SC, Bauer KA, et al. Guidance for the evaluation and treatment of hereditary and acquired thrombophilia. J Thromb Thrombolysis. 2016;41(1):154-164. PubMed
The article by Petrilli et al. points to the important but complicated issue of ordering laboratory testing for thrombophilia despite multiple guidelines that dispute the clinical utility of such testing for many indications.1 We question the basis of these authors’ assertion that Medicare spends $300 to $672 million for thrombophilia testing annually. They arrived at this figure by multiplying the price of a thrombophilia test panel (between $1100 and $2400) by the number of annual Medicare claims for thrombophilia analysis, which they estimated at 280,000. The price of the panel is derived from two papers: (1) a 2001 review2 that lists prices of various thrombophilia-related tests adding up to $1782, and (2) a 2006 evaluation by Somma et al.3 of thrombophilia screening at one hospital in New York in 2005. The latter paper refers to various thrombophilia panels from Quest Diagnostics with list prices ranging from $1311 to $2429. However, the repertoire of available test panels and their prices have changed over the last decade. The cost evaluation of thrombophilia testing should be based on actual current payments for tests, and not on list prices for laboratory offerings from over a decade ago. Several laboratories offer mutational analysis of 3 genes—F5, F2, and MTHFR—as a thrombophilia risk panel. Based on the Current Procedural Terminology (CPT) codes listed by the test suppliers (81240, 81241, and 81291), the average Medicare payment for the combination of these 3 markers in 2013 was $172.4 A broader panel of several biochemical, immunological, and genetic assays had a maximum Medicare payment in 2015 of $405 (Table).5
In conclusion, the cost evaluation of thrombophilia screening is more challenging than the calculation by Petrilli et al. suggests.1 Even if Medicare paid as much as $400 per individual tested and assuming up to 200,000 individuals underwent thrombophilia testing per year, the aggregate Medicare expenditure would have been no more than roughly $80 million. Thus, the estimated range in the article appears to have overstated actual Medicare expenditures by an order of magnitude. This does not take away from their overall conclusion that payers are burdened with significant expenditures for laboratory testing that may not present clinical value for many patients.6 We need research into the patterns of utilization as well as improvements in documentation of expenditures associated with these tests.
Disclosure
The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention, the Department of Veterans Affairs, or the United States government. The autho
The article by Petrilli et al. points to the important but complicated issue of ordering laboratory testing for thrombophilia despite multiple guidelines that dispute the clinical utility of such testing for many indications.1 We question the basis of these authors’ assertion that Medicare spends $300 to $672 million for thrombophilia testing annually. They arrived at this figure by multiplying the price of a thrombophilia test panel (between $1100 and $2400) by the number of annual Medicare claims for thrombophilia analysis, which they estimated at 280,000. The price of the panel is derived from two papers: (1) a 2001 review2 that lists prices of various thrombophilia-related tests adding up to $1782, and (2) a 2006 evaluation by Somma et al.3 of thrombophilia screening at one hospital in New York in 2005. The latter paper refers to various thrombophilia panels from Quest Diagnostics with list prices ranging from $1311 to $2429. However, the repertoire of available test panels and their prices have changed over the last decade. The cost evaluation of thrombophilia testing should be based on actual current payments for tests, and not on list prices for laboratory offerings from over a decade ago. Several laboratories offer mutational analysis of 3 genes—F5, F2, and MTHFR—as a thrombophilia risk panel. Based on the Current Procedural Terminology (CPT) codes listed by the test suppliers (81240, 81241, and 81291), the average Medicare payment for the combination of these 3 markers in 2013 was $172.4 A broader panel of several biochemical, immunological, and genetic assays had a maximum Medicare payment in 2015 of $405 (Table).5
In conclusion, the cost evaluation of thrombophilia screening is more challenging than the calculation by Petrilli et al. suggests.1 Even if Medicare paid as much as $400 per individual tested and assuming up to 200,000 individuals underwent thrombophilia testing per year, the aggregate Medicare expenditure would have been no more than roughly $80 million. Thus, the estimated range in the article appears to have overstated actual Medicare expenditures by an order of magnitude. This does not take away from their overall conclusion that payers are burdened with significant expenditures for laboratory testing that may not present clinical value for many patients.6 We need research into the patterns of utilization as well as improvements in documentation of expenditures associated with these tests.
Disclosure
The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention, the Department of Veterans Affairs, or the United States government. The autho
1. Petrilli CM, Heidemann L, Mack M, Durance P, Chopra V. Inpatient inherited thrombophilia testing. J Hosp Med. 2016;11(11):801-804. PubMed
2. Abramson N, Abramson S. Hypercoagulability: clinical assessment and treatment. South Med J. 2001;94(10):1013-1020. PubMed
3. Somma J, Sussman, II, Rand JH. An evaluation of thrombophilia screening in an urban tertiary care medical center: A “real world” experience. Am J Clin Pathol. 2006;126(1):120-127. PubMed
4. Lynch JA, Berse B, Dotson WD, Khoury MJ, Coomer N, Kautter J. Utilization of genetic tests: Analysis of gene-specific billing in Medicare claims data [Published online ahead of print January 26, 2017]. Genet Med. 2017. doi: 10.1038/gim.2016.209. PubMed
5. Centers for Medicare and Medicaid Services. Clinical Laboratory Fee Schedule 2016. https://www.cms.gov/Medicare/Medicare-fee-for-service-Payment/clinicallabfeesched/index.html. Accessed on December 20, 2016.
6. Stevens SM, Woller SC, Bauer KA, et al. Guidance for the evaluation and treatment of hereditary and acquired thrombophilia. J Thromb Thrombolysis. 2016;41(1):154-164. PubMed
1. Petrilli CM, Heidemann L, Mack M, Durance P, Chopra V. Inpatient inherited thrombophilia testing. J Hosp Med. 2016;11(11):801-804. PubMed
2. Abramson N, Abramson S. Hypercoagulability: clinical assessment and treatment. South Med J. 2001;94(10):1013-1020. PubMed
3. Somma J, Sussman, II, Rand JH. An evaluation of thrombophilia screening in an urban tertiary care medical center: A “real world” experience. Am J Clin Pathol. 2006;126(1):120-127. PubMed
4. Lynch JA, Berse B, Dotson WD, Khoury MJ, Coomer N, Kautter J. Utilization of genetic tests: Analysis of gene-specific billing in Medicare claims data [Published online ahead of print January 26, 2017]. Genet Med. 2017. doi: 10.1038/gim.2016.209. PubMed
5. Centers for Medicare and Medicaid Services. Clinical Laboratory Fee Schedule 2016. https://www.cms.gov/Medicare/Medicare-fee-for-service-Payment/clinicallabfeesched/index.html. Accessed on December 20, 2016.
6. Stevens SM, Woller SC, Bauer KA, et al. Guidance for the evaluation and treatment of hereditary and acquired thrombophilia. J Thromb Thrombolysis. 2016;41(1):154-164. PubMed
© 2017 Society of Hospital Medicine