User login
Isobornyl Acrylate and Diabetic Devices Steal the Show for the 2020 American Contact Dermatitis Society Allergen of the Year
Each year, the American Contact Dermatitis Society names an Allergen of the Year with the purpose of promoting greater awareness of a key allergen and its impact on patients. Often, the Allergen of the Year is an emerging allergen that may represent an underrecognized or novel cause of allergic contact dermatitis (ACD).In 2020, the American Contact Dermatitis Society chose isobornyl acrylate as the Allergen of the Year.1 Not only has isobornyl acrylate been implicated in an epidemic of contact allergy to diabetic devices, but it also illustrates the challenges of investigating contact allergy to medical devices in general.
What Is Isobornyl Acrylate?
Isobornyl acrylate, also known as the isobornyl ester of acrylic acid, is a chemical used in glues, adhesives, coatings, sealants, inks, and paints. Similar to other acrylates, such as those involved in gel nail treatments, it is photopolymerizable; that is, when exposed to UV light, it can transform from a liquid monomer into a hard polymer, contributing to its utility as an adhesive. Prior to its recent implication in diabetic device contact allergy, isobornyl acrylate was not thought to be a common skin sensitizer. In a 2013 Dutch study of patients with acrylate allergy, only 1 of 14 patients with a contact allergy to other acrylates had a positive patch test reaction to isobornyl acrylate, which led the authors to conclude that adding it to their acrylate patch test series was not indicated.2
Isobornyl Acrylate in Diabetic Devices
Devices such as glucose monitoring systems and insulin pumps are used by millions of patients with diabetes worldwide. Not only are continuous glucose monitoring devices more convenient than self-monitoring of blood glucose, but they also are associated with a reduction in hemoglobin A1c levels and lower risk for hypoglycemia.3 However, these devices have been increasingly recognized as a source of irritant contact dermatitis and ACD.
Early cases of contact allergy to isobornyl acrylate in diabetic devices were reported in 1995 when 2 Belgian patients using insulin pumps developed ACD.4 The patients had positive patch test reactions to isobornyl acrylate 0.1% in petrolatum and other allergens including acrylates. In addition, patch testing with plastic scrapings from their insulin pumps also was positive, and it was determined that the glue affixing the needle to the plastic had diffused into the plastic. The patients were switched to insulin pumps produced by heat staking instead of glue, and their symptoms resolved. In retrospect, this case series may seem prescient, as it was written 2 decades before isobornyl acrylate became recognized as a widespread cause of ACD in users of diabetic devices. Admittedly, other acrylate components of the glue also were positive on patch testing in these patients, so it was not until much later that the focus turned more exclusively to isobornyl acrylate.4
Similar to the insulin pumps in the 1995 Belgian series, diffusion of glue to other parts of modern glucose sensors also appears to cause isobornyl acrylate contact allergy. This theory was supported by a 2017 study from Belgian and Swedish investigators in which gas chromatography–mass spectrometry was used to identify concentrations of isobornyl acrylate in various components of a popular continuous glucose monitoring sensor.5 The concentration of isobornyl acrylate was approximately 100-fold higher at the site where the top and bottom plastic components of the sensor were joined as compared to the adhesive patch in contact with the patient’s skin. Therefore, the adhesive patch itself was not the source of the isobornyl acrylate exposure; rather, the isobornyl acrylate diffused into the adhesive patch from the glue used to join the components of the sensor together.5 One ramification is that patients with diabetic device contact allergy can have a false-negative patch test result if the adhesive patch is tested by itself, whereas they may react to patch testing with the whole sensor or an acetonic extract thereof.
Frequency of Sensitization to Isobornyl Acrylate
It is difficult to estimate the frequency of sensitization to isobornyl acrylate among users of diabetic devices, in part because those with mild allergy may not seek medical treatment. Nevertheless, there are studies that demonstrate a high prevalence of sensitization among users with suspected allergy. In a 2019 Finnish study of 6567 patients using an isobornyl acrylate–containing glucose sensor, 63 were patch tested for suspected ACD.6 Of these 63 patients, 51 (81%) had positive patch test reactions to isobornyl acrylate 0.1% in petrolatum. These findings were consistent with the original 2017 study from Belgium and Sweden, in which 10 of 11 (91%) patients who used an isobornyl acrylate–containing glucose sensor and had suspected contact allergy had positive patch test reactions to isobornyl acrylate 0.1% in petrolatum compared to no positive reactions in the 14 control patients.5 Given that there are more than 1.5 million users of this isobornyl acrylate–containing glucose sensor across 46 countries,7 it requires no stretch of the imagination to understand why investigators refer to isobornyl acrylate allergy as an epidemic, even if only a small percentage of users are sensitized to the device.
The Journey to Discover Isobornyl Acrylate as a Culprit Allergen
Similar to the discoveries of radiography and penicillin, the discovery of isobornyl acrylate as a culprit allergen in a modern glucose sensor was purely accidental. In 2016, a 9-year-old boy with diabetes presented to a Belgian dermatology department with ACD to a glucose sensor.1 A patch test nurse serendipitously applied isobornyl acrylate—0.01%, 0.05%, and 0.1% in petrolatum—which was not intended to be applied as part of the typical acrylate series. The only positive patch test reactions in this patient were to isobornyl acrylate at all 3 concentrations. This lucky error inspired isobornyl acrylate to be tested at multiple other dermatology departments in Europe in patients with ACD to their glucose sensors, leading to its discovery as a culprit allergen.1
One challenge facing investigators was obtaining information and materials from the diabetic device industry. Medical device manufacturers are not required to disclose chemicals present in a device on its label.8 Therefore, for patients or investigators to determine whether a potential allergen is present in a given device, they must request that information from the manufacturer, which can be a time-consuming and frustrating effort. Luckily, investigators collaborated with one another, and Belgian investigators suggested that Swedish investigators performing chemical analyses on a glucose monitoring device should focus on isobornyl acrylate, which enabled its detection in an extract from the device.5
Testing for Isobornyl Acrylate Allergy in Your Clinic
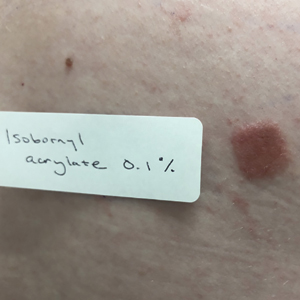
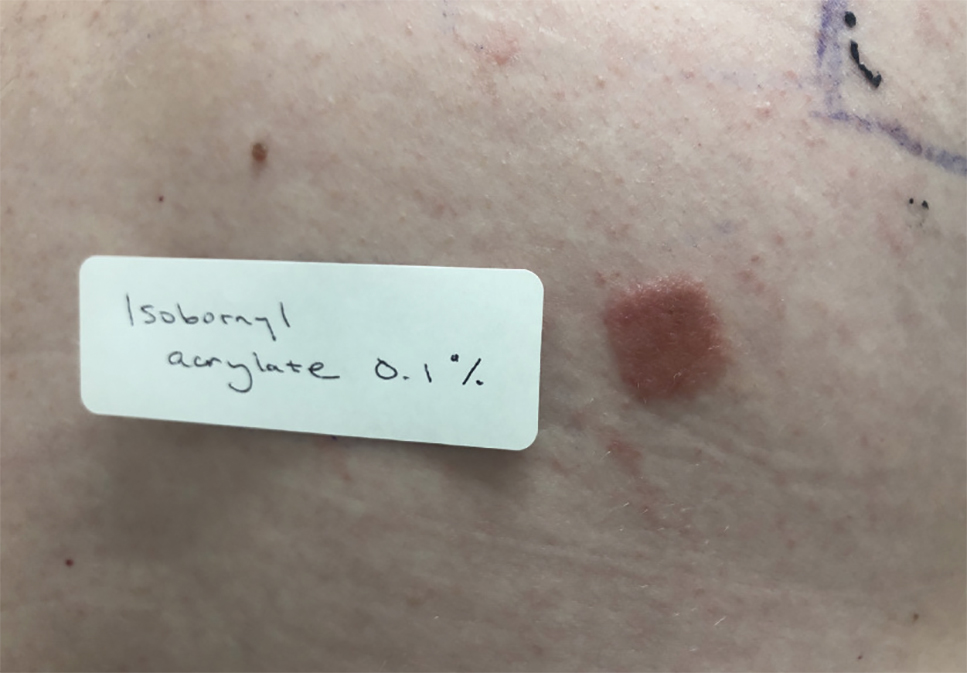
Patients with suspected ACD to a diabetic device—insulin pump or glucose sensor—should be patch tested with isobornyl acrylate, in addition to other previously reported allergens. The vehicle typically is petrolatum, and the commonly tested concentration is 0.1%. Testing with lower concentrations such as 0.01% can result in false-negative reactions,9 and testing at higher concentrations such as 0.3% can result in irritant skin reactions.2 Isobornyl acrylate 0.1% in petrolatum currently is available from one commercial allergen supplier (Chemotechnique Diagnostics). A positive patch test reaction to isobornyl acrylate 0.1% in petrolatum is shown in the Figure.
Management of Diabetic Device ACD
For patients with diabetic device ACD, there are several strategies that can reduce direct contact between the device and the patient’s skin. Methods that have been tried with varying success to allow patients to continue using their glucose sensors include barrier sprays (eg, Cavilon [3M], Silesse Skin Barrier [ConvaTec]); barrier pads (eg, Compeed [HRA Pharma], Surround skin protectors [Eakin], DuoDERM dressings [ConvaTec], Tegaderm dressings [3M]); and topical corticosteroids, calcineurin inhibitors, and phosphodiesterase 4 inhibitors. Nevertheless, a 2019 Finnish study showed that only 14 of 63 (22%) patients with ACD to their isobornyl acrylate–containing glucose sensor were able to continue using the device, with all 14 requiring use of a barrier agent. Despite using the barrier agent, 13 (93%) of these patients had residual dermatitis.6 There also is concern that use of barrier methods might hamper the proper functioning of glucose sensors and related devices.
Patients with known isobornyl acrylate contact allergy also may switch to a different diabetic device. A 2019 German study showed that in 5 patients with isobornyl acrylate ACD, none had reactions to the one particular system that has been shown by gas chromatography–mass spectrometry to not contain isobornyl acrylate.10 However, as a word of caution, the same device also has been associated with ACD11,12 but has been resolved by using heat staking during the production process.13 As manufacturers update device components, identification of other isobornyl acrylate–free devices may require a degree of trial and error, as neither isobornyl acrylate nor any other potential allergen is listed on device labels.
Final Interpretation
Isobornyl acrylate is not a common sensitizer in general patch test populations but is a recently identified major culprit in ACD to diabetic devices. Patch testing with isobornyl acrylate 0.1% in petrolatum is not necessary in standard screening panels but should be considered in patients with suspected ACD to glucose sensors or insulin pumps. If a patient with ACD wants to continue to experience the convenience provided by a diabetic device, options include using topical steroids or barrier agents and/or changing the brand of the diabetic device, though none of these methods are foolproof. Hopefully, the identification of isobornyl acrylate as a culprit allergen will help to improve the lives of patients who use diabetic devices worldwide.
- Aerts O, Herman A, Mowitz M, et al. Isobornyl acrylate. Dermatitis. 2020;31:4-12.
- Christoffers WA, Coenraads PJ, Schuttelaar ML. Two decades of occupational (meth)acrylate patch test results and focus on isobornyl acrylate. Contact Dermatitis. 2013;69:86-92.
- Pickup JC, Freeman SC, Sutton AJ. Glycaemic control in type 1 diabetes during real time continuous glucose monitoring compared with self monitoring of blood glucose: meta-analysis of randomised controlled trials using individual patient data. BMJ. 2011;343:d3805.
- Busschots AM, Meuleman V, Poesen N, et al. Contact allergy to components of glue in insulin pump infusion sets. Contact Dermatitis. 1995;33:205-206.
- Herman A, Aerts O, Baeck M, et al. Allergic contact dermatitis caused by isobornyl acrylate in Freestyle® Libre, a newly introduced glucose sensor. Contact Dermatitis. 2017;77:367-373.
- Hyry HSI, Liippo JP, Virtanen HM. Allergic contact dermatitis caused by glucose sensors in type 1 diabetes patients. Contact Dermatitis. 2019;81:161-166.
- Abbott’s Revolutionary FreeStyle® Libre system now reimbursed in the two largest provinces in Canada [press release]. Abbott Park, IL: Abbott; September 13, 2019. https://abbott.mediaroom.com/2019-09-13-Abbotts-Revolutionary-FreeStyle-R-Libre-System-Now-Reimbursed-in-the-Two-Largest-Provinces-in-Canada. Accessed May 14, 2020.
- Herman A, Goossens A. The need to disclose the composition of medical devices at the European level. Contact Dermatitis. 2019;81:159-160.
- Raison-Peyron N, Mowitz M, Bonardel N, et al. Allergic contact dermatitis caused by isobornyl acrylate in OmniPod, an innovative tubeless insulin pump. Contact Dermatitis. 2018;79:76-80.
- Oppel E, Kamann S, Reichl FX, et al. The Dexcom glucose monitoring system—an isobornyl acrylate-free alternative for diabetic patients. Contact Dermatitis. 2019;81:32-36.
- Peeters C, Herman A, Goossens A, et al. Allergic contact dermatitis caused by 2-ethyl cyanoacrylate contained in glucose sensor sets in two diabetic adults. Contact Dermatitis. 2017;77:426-429.
- Aschenbeck KA, Hylwa SA. A diabetic’s allergy: ethyl cyanoacrylate in glucose sensor adhesive. Dermatitis. 2017;28:289-291.
- Gisin V, Chan A, Welsh B. Manufacturing process changes and reduced skin irritations of an adhesive patch used for continuous glucose monitoring devices. J Diabetes Sci Technol. 2018;12:725-726.
Each year, the American Contact Dermatitis Society names an Allergen of the Year with the purpose of promoting greater awareness of a key allergen and its impact on patients. Often, the Allergen of the Year is an emerging allergen that may represent an underrecognized or novel cause of allergic contact dermatitis (ACD).In 2020, the American Contact Dermatitis Society chose isobornyl acrylate as the Allergen of the Year.1 Not only has isobornyl acrylate been implicated in an epidemic of contact allergy to diabetic devices, but it also illustrates the challenges of investigating contact allergy to medical devices in general.
What Is Isobornyl Acrylate?
Isobornyl acrylate, also known as the isobornyl ester of acrylic acid, is a chemical used in glues, adhesives, coatings, sealants, inks, and paints. Similar to other acrylates, such as those involved in gel nail treatments, it is photopolymerizable; that is, when exposed to UV light, it can transform from a liquid monomer into a hard polymer, contributing to its utility as an adhesive. Prior to its recent implication in diabetic device contact allergy, isobornyl acrylate was not thought to be a common skin sensitizer. In a 2013 Dutch study of patients with acrylate allergy, only 1 of 14 patients with a contact allergy to other acrylates had a positive patch test reaction to isobornyl acrylate, which led the authors to conclude that adding it to their acrylate patch test series was not indicated.2
Isobornyl Acrylate in Diabetic Devices
Devices such as glucose monitoring systems and insulin pumps are used by millions of patients with diabetes worldwide. Not only are continuous glucose monitoring devices more convenient than self-monitoring of blood glucose, but they also are associated with a reduction in hemoglobin A1c levels and lower risk for hypoglycemia.3 However, these devices have been increasingly recognized as a source of irritant contact dermatitis and ACD.
Early cases of contact allergy to isobornyl acrylate in diabetic devices were reported in 1995 when 2 Belgian patients using insulin pumps developed ACD.4 The patients had positive patch test reactions to isobornyl acrylate 0.1% in petrolatum and other allergens including acrylates. In addition, patch testing with plastic scrapings from their insulin pumps also was positive, and it was determined that the glue affixing the needle to the plastic had diffused into the plastic. The patients were switched to insulin pumps produced by heat staking instead of glue, and their symptoms resolved. In retrospect, this case series may seem prescient, as it was written 2 decades before isobornyl acrylate became recognized as a widespread cause of ACD in users of diabetic devices. Admittedly, other acrylate components of the glue also were positive on patch testing in these patients, so it was not until much later that the focus turned more exclusively to isobornyl acrylate.4
Similar to the insulin pumps in the 1995 Belgian series, diffusion of glue to other parts of modern glucose sensors also appears to cause isobornyl acrylate contact allergy. This theory was supported by a 2017 study from Belgian and Swedish investigators in which gas chromatography–mass spectrometry was used to identify concentrations of isobornyl acrylate in various components of a popular continuous glucose monitoring sensor.5 The concentration of isobornyl acrylate was approximately 100-fold higher at the site where the top and bottom plastic components of the sensor were joined as compared to the adhesive patch in contact with the patient’s skin. Therefore, the adhesive patch itself was not the source of the isobornyl acrylate exposure; rather, the isobornyl acrylate diffused into the adhesive patch from the glue used to join the components of the sensor together.5 One ramification is that patients with diabetic device contact allergy can have a false-negative patch test result if the adhesive patch is tested by itself, whereas they may react to patch testing with the whole sensor or an acetonic extract thereof.
Frequency of Sensitization to Isobornyl Acrylate
It is difficult to estimate the frequency of sensitization to isobornyl acrylate among users of diabetic devices, in part because those with mild allergy may not seek medical treatment. Nevertheless, there are studies that demonstrate a high prevalence of sensitization among users with suspected allergy. In a 2019 Finnish study of 6567 patients using an isobornyl acrylate–containing glucose sensor, 63 were patch tested for suspected ACD.6 Of these 63 patients, 51 (81%) had positive patch test reactions to isobornyl acrylate 0.1% in petrolatum. These findings were consistent with the original 2017 study from Belgium and Sweden, in which 10 of 11 (91%) patients who used an isobornyl acrylate–containing glucose sensor and had suspected contact allergy had positive patch test reactions to isobornyl acrylate 0.1% in petrolatum compared to no positive reactions in the 14 control patients.5 Given that there are more than 1.5 million users of this isobornyl acrylate–containing glucose sensor across 46 countries,7 it requires no stretch of the imagination to understand why investigators refer to isobornyl acrylate allergy as an epidemic, even if only a small percentage of users are sensitized to the device.
The Journey to Discover Isobornyl Acrylate as a Culprit Allergen
Similar to the discoveries of radiography and penicillin, the discovery of isobornyl acrylate as a culprit allergen in a modern glucose sensor was purely accidental. In 2016, a 9-year-old boy with diabetes presented to a Belgian dermatology department with ACD to a glucose sensor.1 A patch test nurse serendipitously applied isobornyl acrylate—0.01%, 0.05%, and 0.1% in petrolatum—which was not intended to be applied as part of the typical acrylate series. The only positive patch test reactions in this patient were to isobornyl acrylate at all 3 concentrations. This lucky error inspired isobornyl acrylate to be tested at multiple other dermatology departments in Europe in patients with ACD to their glucose sensors, leading to its discovery as a culprit allergen.1
One challenge facing investigators was obtaining information and materials from the diabetic device industry. Medical device manufacturers are not required to disclose chemicals present in a device on its label.8 Therefore, for patients or investigators to determine whether a potential allergen is present in a given device, they must request that information from the manufacturer, which can be a time-consuming and frustrating effort. Luckily, investigators collaborated with one another, and Belgian investigators suggested that Swedish investigators performing chemical analyses on a glucose monitoring device should focus on isobornyl acrylate, which enabled its detection in an extract from the device.5
Testing for Isobornyl Acrylate Allergy in Your Clinic
Patients with suspected ACD to a diabetic device—insulin pump or glucose sensor—should be patch tested with isobornyl acrylate, in addition to other previously reported allergens. The vehicle typically is petrolatum, and the commonly tested concentration is 0.1%. Testing with lower concentrations such as 0.01% can result in false-negative reactions,9 and testing at higher concentrations such as 0.3% can result in irritant skin reactions.2 Isobornyl acrylate 0.1% in petrolatum currently is available from one commercial allergen supplier (Chemotechnique Diagnostics). A positive patch test reaction to isobornyl acrylate 0.1% in petrolatum is shown in the Figure.

Management of Diabetic Device ACD
For patients with diabetic device ACD, there are several strategies that can reduce direct contact between the device and the patient’s skin. Methods that have been tried with varying success to allow patients to continue using their glucose sensors include barrier sprays (eg, Cavilon [3M], Silesse Skin Barrier [ConvaTec]); barrier pads (eg, Compeed [HRA Pharma], Surround skin protectors [Eakin], DuoDERM dressings [ConvaTec], Tegaderm dressings [3M]); and topical corticosteroids, calcineurin inhibitors, and phosphodiesterase 4 inhibitors. Nevertheless, a 2019 Finnish study showed that only 14 of 63 (22%) patients with ACD to their isobornyl acrylate–containing glucose sensor were able to continue using the device, with all 14 requiring use of a barrier agent. Despite using the barrier agent, 13 (93%) of these patients had residual dermatitis.6 There also is concern that use of barrier methods might hamper the proper functioning of glucose sensors and related devices.
Patients with known isobornyl acrylate contact allergy also may switch to a different diabetic device. A 2019 German study showed that in 5 patients with isobornyl acrylate ACD, none had reactions to the one particular system that has been shown by gas chromatography–mass spectrometry to not contain isobornyl acrylate.10 However, as a word of caution, the same device also has been associated with ACD11,12 but has been resolved by using heat staking during the production process.13 As manufacturers update device components, identification of other isobornyl acrylate–free devices may require a degree of trial and error, as neither isobornyl acrylate nor any other potential allergen is listed on device labels.
Final Interpretation
Isobornyl acrylate is not a common sensitizer in general patch test populations but is a recently identified major culprit in ACD to diabetic devices. Patch testing with isobornyl acrylate 0.1% in petrolatum is not necessary in standard screening panels but should be considered in patients with suspected ACD to glucose sensors or insulin pumps. If a patient with ACD wants to continue to experience the convenience provided by a diabetic device, options include using topical steroids or barrier agents and/or changing the brand of the diabetic device, though none of these methods are foolproof. Hopefully, the identification of isobornyl acrylate as a culprit allergen will help to improve the lives of patients who use diabetic devices worldwide.
Each year, the American Contact Dermatitis Society names an Allergen of the Year with the purpose of promoting greater awareness of a key allergen and its impact on patients. Often, the Allergen of the Year is an emerging allergen that may represent an underrecognized or novel cause of allergic contact dermatitis (ACD).In 2020, the American Contact Dermatitis Society chose isobornyl acrylate as the Allergen of the Year.1 Not only has isobornyl acrylate been implicated in an epidemic of contact allergy to diabetic devices, but it also illustrates the challenges of investigating contact allergy to medical devices in general.
What Is Isobornyl Acrylate?
Isobornyl acrylate, also known as the isobornyl ester of acrylic acid, is a chemical used in glues, adhesives, coatings, sealants, inks, and paints. Similar to other acrylates, such as those involved in gel nail treatments, it is photopolymerizable; that is, when exposed to UV light, it can transform from a liquid monomer into a hard polymer, contributing to its utility as an adhesive. Prior to its recent implication in diabetic device contact allergy, isobornyl acrylate was not thought to be a common skin sensitizer. In a 2013 Dutch study of patients with acrylate allergy, only 1 of 14 patients with a contact allergy to other acrylates had a positive patch test reaction to isobornyl acrylate, which led the authors to conclude that adding it to their acrylate patch test series was not indicated.2
Isobornyl Acrylate in Diabetic Devices
Devices such as glucose monitoring systems and insulin pumps are used by millions of patients with diabetes worldwide. Not only are continuous glucose monitoring devices more convenient than self-monitoring of blood glucose, but they also are associated with a reduction in hemoglobin A1c levels and lower risk for hypoglycemia.3 However, these devices have been increasingly recognized as a source of irritant contact dermatitis and ACD.
Early cases of contact allergy to isobornyl acrylate in diabetic devices were reported in 1995 when 2 Belgian patients using insulin pumps developed ACD.4 The patients had positive patch test reactions to isobornyl acrylate 0.1% in petrolatum and other allergens including acrylates. In addition, patch testing with plastic scrapings from their insulin pumps also was positive, and it was determined that the glue affixing the needle to the plastic had diffused into the plastic. The patients were switched to insulin pumps produced by heat staking instead of glue, and their symptoms resolved. In retrospect, this case series may seem prescient, as it was written 2 decades before isobornyl acrylate became recognized as a widespread cause of ACD in users of diabetic devices. Admittedly, other acrylate components of the glue also were positive on patch testing in these patients, so it was not until much later that the focus turned more exclusively to isobornyl acrylate.4
Similar to the insulin pumps in the 1995 Belgian series, diffusion of glue to other parts of modern glucose sensors also appears to cause isobornyl acrylate contact allergy. This theory was supported by a 2017 study from Belgian and Swedish investigators in which gas chromatography–mass spectrometry was used to identify concentrations of isobornyl acrylate in various components of a popular continuous glucose monitoring sensor.5 The concentration of isobornyl acrylate was approximately 100-fold higher at the site where the top and bottom plastic components of the sensor were joined as compared to the adhesive patch in contact with the patient’s skin. Therefore, the adhesive patch itself was not the source of the isobornyl acrylate exposure; rather, the isobornyl acrylate diffused into the adhesive patch from the glue used to join the components of the sensor together.5 One ramification is that patients with diabetic device contact allergy can have a false-negative patch test result if the adhesive patch is tested by itself, whereas they may react to patch testing with the whole sensor or an acetonic extract thereof.
Frequency of Sensitization to Isobornyl Acrylate
It is difficult to estimate the frequency of sensitization to isobornyl acrylate among users of diabetic devices, in part because those with mild allergy may not seek medical treatment. Nevertheless, there are studies that demonstrate a high prevalence of sensitization among users with suspected allergy. In a 2019 Finnish study of 6567 patients using an isobornyl acrylate–containing glucose sensor, 63 were patch tested for suspected ACD.6 Of these 63 patients, 51 (81%) had positive patch test reactions to isobornyl acrylate 0.1% in petrolatum. These findings were consistent with the original 2017 study from Belgium and Sweden, in which 10 of 11 (91%) patients who used an isobornyl acrylate–containing glucose sensor and had suspected contact allergy had positive patch test reactions to isobornyl acrylate 0.1% in petrolatum compared to no positive reactions in the 14 control patients.5 Given that there are more than 1.5 million users of this isobornyl acrylate–containing glucose sensor across 46 countries,7 it requires no stretch of the imagination to understand why investigators refer to isobornyl acrylate allergy as an epidemic, even if only a small percentage of users are sensitized to the device.
The Journey to Discover Isobornyl Acrylate as a Culprit Allergen
Similar to the discoveries of radiography and penicillin, the discovery of isobornyl acrylate as a culprit allergen in a modern glucose sensor was purely accidental. In 2016, a 9-year-old boy with diabetes presented to a Belgian dermatology department with ACD to a glucose sensor.1 A patch test nurse serendipitously applied isobornyl acrylate—0.01%, 0.05%, and 0.1% in petrolatum—which was not intended to be applied as part of the typical acrylate series. The only positive patch test reactions in this patient were to isobornyl acrylate at all 3 concentrations. This lucky error inspired isobornyl acrylate to be tested at multiple other dermatology departments in Europe in patients with ACD to their glucose sensors, leading to its discovery as a culprit allergen.1
One challenge facing investigators was obtaining information and materials from the diabetic device industry. Medical device manufacturers are not required to disclose chemicals present in a device on its label.8 Therefore, for patients or investigators to determine whether a potential allergen is present in a given device, they must request that information from the manufacturer, which can be a time-consuming and frustrating effort. Luckily, investigators collaborated with one another, and Belgian investigators suggested that Swedish investigators performing chemical analyses on a glucose monitoring device should focus on isobornyl acrylate, which enabled its detection in an extract from the device.5
Testing for Isobornyl Acrylate Allergy in Your Clinic
Patients with suspected ACD to a diabetic device—insulin pump or glucose sensor—should be patch tested with isobornyl acrylate, in addition to other previously reported allergens. The vehicle typically is petrolatum, and the commonly tested concentration is 0.1%. Testing with lower concentrations such as 0.01% can result in false-negative reactions,9 and testing at higher concentrations such as 0.3% can result in irritant skin reactions.2 Isobornyl acrylate 0.1% in petrolatum currently is available from one commercial allergen supplier (Chemotechnique Diagnostics). A positive patch test reaction to isobornyl acrylate 0.1% in petrolatum is shown in the Figure.

Management of Diabetic Device ACD
For patients with diabetic device ACD, there are several strategies that can reduce direct contact between the device and the patient’s skin. Methods that have been tried with varying success to allow patients to continue using their glucose sensors include barrier sprays (eg, Cavilon [3M], Silesse Skin Barrier [ConvaTec]); barrier pads (eg, Compeed [HRA Pharma], Surround skin protectors [Eakin], DuoDERM dressings [ConvaTec], Tegaderm dressings [3M]); and topical corticosteroids, calcineurin inhibitors, and phosphodiesterase 4 inhibitors. Nevertheless, a 2019 Finnish study showed that only 14 of 63 (22%) patients with ACD to their isobornyl acrylate–containing glucose sensor were able to continue using the device, with all 14 requiring use of a barrier agent. Despite using the barrier agent, 13 (93%) of these patients had residual dermatitis.6 There also is concern that use of barrier methods might hamper the proper functioning of glucose sensors and related devices.
Patients with known isobornyl acrylate contact allergy also may switch to a different diabetic device. A 2019 German study showed that in 5 patients with isobornyl acrylate ACD, none had reactions to the one particular system that has been shown by gas chromatography–mass spectrometry to not contain isobornyl acrylate.10 However, as a word of caution, the same device also has been associated with ACD11,12 but has been resolved by using heat staking during the production process.13 As manufacturers update device components, identification of other isobornyl acrylate–free devices may require a degree of trial and error, as neither isobornyl acrylate nor any other potential allergen is listed on device labels.
Final Interpretation
Isobornyl acrylate is not a common sensitizer in general patch test populations but is a recently identified major culprit in ACD to diabetic devices. Patch testing with isobornyl acrylate 0.1% in petrolatum is not necessary in standard screening panels but should be considered in patients with suspected ACD to glucose sensors or insulin pumps. If a patient with ACD wants to continue to experience the convenience provided by a diabetic device, options include using topical steroids or barrier agents and/or changing the brand of the diabetic device, though none of these methods are foolproof. Hopefully, the identification of isobornyl acrylate as a culprit allergen will help to improve the lives of patients who use diabetic devices worldwide.
- Aerts O, Herman A, Mowitz M, et al. Isobornyl acrylate. Dermatitis. 2020;31:4-12.
- Christoffers WA, Coenraads PJ, Schuttelaar ML. Two decades of occupational (meth)acrylate patch test results and focus on isobornyl acrylate. Contact Dermatitis. 2013;69:86-92.
- Pickup JC, Freeman SC, Sutton AJ. Glycaemic control in type 1 diabetes during real time continuous glucose monitoring compared with self monitoring of blood glucose: meta-analysis of randomised controlled trials using individual patient data. BMJ. 2011;343:d3805.
- Busschots AM, Meuleman V, Poesen N, et al. Contact allergy to components of glue in insulin pump infusion sets. Contact Dermatitis. 1995;33:205-206.
- Herman A, Aerts O, Baeck M, et al. Allergic contact dermatitis caused by isobornyl acrylate in Freestyle® Libre, a newly introduced glucose sensor. Contact Dermatitis. 2017;77:367-373.
- Hyry HSI, Liippo JP, Virtanen HM. Allergic contact dermatitis caused by glucose sensors in type 1 diabetes patients. Contact Dermatitis. 2019;81:161-166.
- Abbott’s Revolutionary FreeStyle® Libre system now reimbursed in the two largest provinces in Canada [press release]. Abbott Park, IL: Abbott; September 13, 2019. https://abbott.mediaroom.com/2019-09-13-Abbotts-Revolutionary-FreeStyle-R-Libre-System-Now-Reimbursed-in-the-Two-Largest-Provinces-in-Canada. Accessed May 14, 2020.
- Herman A, Goossens A. The need to disclose the composition of medical devices at the European level. Contact Dermatitis. 2019;81:159-160.
- Raison-Peyron N, Mowitz M, Bonardel N, et al. Allergic contact dermatitis caused by isobornyl acrylate in OmniPod, an innovative tubeless insulin pump. Contact Dermatitis. 2018;79:76-80.
- Oppel E, Kamann S, Reichl FX, et al. The Dexcom glucose monitoring system—an isobornyl acrylate-free alternative for diabetic patients. Contact Dermatitis. 2019;81:32-36.
- Peeters C, Herman A, Goossens A, et al. Allergic contact dermatitis caused by 2-ethyl cyanoacrylate contained in glucose sensor sets in two diabetic adults. Contact Dermatitis. 2017;77:426-429.
- Aschenbeck KA, Hylwa SA. A diabetic’s allergy: ethyl cyanoacrylate in glucose sensor adhesive. Dermatitis. 2017;28:289-291.
- Gisin V, Chan A, Welsh B. Manufacturing process changes and reduced skin irritations of an adhesive patch used for continuous glucose monitoring devices. J Diabetes Sci Technol. 2018;12:725-726.
- Aerts O, Herman A, Mowitz M, et al. Isobornyl acrylate. Dermatitis. 2020;31:4-12.
- Christoffers WA, Coenraads PJ, Schuttelaar ML. Two decades of occupational (meth)acrylate patch test results and focus on isobornyl acrylate. Contact Dermatitis. 2013;69:86-92.
- Pickup JC, Freeman SC, Sutton AJ. Glycaemic control in type 1 diabetes during real time continuous glucose monitoring compared with self monitoring of blood glucose: meta-analysis of randomised controlled trials using individual patient data. BMJ. 2011;343:d3805.
- Busschots AM, Meuleman V, Poesen N, et al. Contact allergy to components of glue in insulin pump infusion sets. Contact Dermatitis. 1995;33:205-206.
- Herman A, Aerts O, Baeck M, et al. Allergic contact dermatitis caused by isobornyl acrylate in Freestyle® Libre, a newly introduced glucose sensor. Contact Dermatitis. 2017;77:367-373.
- Hyry HSI, Liippo JP, Virtanen HM. Allergic contact dermatitis caused by glucose sensors in type 1 diabetes patients. Contact Dermatitis. 2019;81:161-166.
- Abbott’s Revolutionary FreeStyle® Libre system now reimbursed in the two largest provinces in Canada [press release]. Abbott Park, IL: Abbott; September 13, 2019. https://abbott.mediaroom.com/2019-09-13-Abbotts-Revolutionary-FreeStyle-R-Libre-System-Now-Reimbursed-in-the-Two-Largest-Provinces-in-Canada. Accessed May 14, 2020.
- Herman A, Goossens A. The need to disclose the composition of medical devices at the European level. Contact Dermatitis. 2019;81:159-160.
- Raison-Peyron N, Mowitz M, Bonardel N, et al. Allergic contact dermatitis caused by isobornyl acrylate in OmniPod, an innovative tubeless insulin pump. Contact Dermatitis. 2018;79:76-80.
- Oppel E, Kamann S, Reichl FX, et al. The Dexcom glucose monitoring system—an isobornyl acrylate-free alternative for diabetic patients. Contact Dermatitis. 2019;81:32-36.
- Peeters C, Herman A, Goossens A, et al. Allergic contact dermatitis caused by 2-ethyl cyanoacrylate contained in glucose sensor sets in two diabetic adults. Contact Dermatitis. 2017;77:426-429.
- Aschenbeck KA, Hylwa SA. A diabetic’s allergy: ethyl cyanoacrylate in glucose sensor adhesive. Dermatitis. 2017;28:289-291.
- Gisin V, Chan A, Welsh B. Manufacturing process changes and reduced skin irritations of an adhesive patch used for continuous glucose monitoring devices. J Diabetes Sci Technol. 2018;12:725-726.
Practice Points
- In patients with suspected allergic contact dermatitis (ACD) to a diabetic device, patch testing with isobornyl acrylate 0.1% in petrolatum should be considered.
- If patients with ACD to their diabetic device want to continue using the device, options include utilizing topical steroids or barrier agents and/or changing the brand of the diabetic device, though these steps may not be effective for every patient.
Essential Oils Debunked: Separating Fact From Myth
What is an essential oil?
An essential oil (EO) is defined by the International Organization for Standardization as a ‘‘product obtained from a natural raw material of plant origin, by steam distillation, by mechanical processes from the epicarp of citrus fruits, or by dry distillation, after separation of the aqueous phase—if any—by physical processes.’’1 Steam distillation is the primary method used for the production of commercial EOs,2 and believe it or not, most EOs contain 100 to 250 individual chemical components.3
The term essential oil often is incorrectly used for a variety of products obtained from plant material by methods other than distillation or cold-pressing, such as extraction. Products that are obtained via the extraction method include absolutes found in fine fragrances; hydrolates such as rose water; concretes such as jasmine or violet leaves; and vegetable oils including olive oil, coconut oil, and sesame oil.2 These products are not true EOs.
Where do EOs come from?
Essential oils are produced in many countries around the world.4 Individual oils may be obtained from species of different plants, from different parts of the same plant, or from various cultivars (plants selectively bred to obtain desirable levels of chemical constituents such as monoterpenes or sesquiterpenes and biochemical properties such as antibacterial or antioxidant activities).3,5 It is estimated that EOs can be obtained from approximately 30,000 plant species, but only 150 EOs are produced commercially.2,6
Why are people using EOs? What is their claim to fame?
Essential oils are employed by the flavor, food (eg, soft drinks, milk, candies, chocolate, meats, sausages, alcoholic beverages, spices, herbs, tea, preservatives, animal foods), fragrance, cosmetic, tobacco, and pharmaceutical industries. They also are used in household products (eg, detergents, fabric softeners, air fresheners, candles, incense) and for medicinal purposes (eg, folk and traditional medicine, phytotherapy, balneotherapy, aromatherapy).2 The oils usually are applied to the skin but also can be administered orally, inhaled, diffused through the air, or used by other means.4 One 2019 survey of Minnesota State Fair attendees (N=282) found the most common reasons for using EOs were a desire for alternative treatments (53.4%), the opinion that EOs are safer than traditional therapies (47.6%), and/or failure of standard medical treatments (10.7%). The survey results also indicated that 46.7% of EO users utilized EOs to treat medical conditions or symptoms.7 Of note, review of the website of an international company that produces EOs confirmed that EOs are marketed not only for adults but also for children to help them concentrate,8 sleep,9 improve the appearance of their skin,10 soothe upset stomachs,11 and decrease sniffles due to colds.12
Why are people selling EOs to family and friends? They must be making major bucks!
In general, the cost of EOs depends on the complexity of cultivated plant species; the mode of harvesting, which is sometimes done by hand; and the yield of oil. Prices range from $4.50 to an incredible $150,000 per kilogram.2 On average, one bottle containing 5 to 15 mL of an EO or oil blend can cost anywhere from $7 to $251.13 In the United States, the consumer EO market is partially composed of multilevel/network marketing companies in which direct consumer sales occur via a hierarchy of individual distributors. Goodier et al7 found that 36.4% of participants who obtained EOs from family and friends purchased them through multilevel/network marketing companies. In 2018, individual distributors of an international EO-producing company made on average anywhere from $4 to as much as $1.54 million annually by selling the company’s EO products and enrolling additional members/individual distributors to purchase or sell the company’s EO products.14
Sometimes EOs are described as natural and pure, but are they really?
Just because a product is labeled as “pure” or “natural” does not ensure that it is a good-quality EO. Organically produced (ie, grown without the use of herbicides or pesticides) plant material can include up to 30% of extraneous herbs and weeds, which can change the composition of the oil.2
Lesser-quality EOs are the result of adulteration, contamination, inadequate oil production, or aging.2 Adulteration (eg, cutting, stretching, bouquetting) occurs when foreign substances are introduced into pure EOs for the benefit of a higher profit; to ensure a sufficient supply of oils; or to meet demands for cheaper oils by “stretching” a more expensive, pure oil by combining with a cheaper, less pure oil. Inadequate oil production leading to lower-quality oils can occur when a biomass is incorrectly distilled, either from too much steam or temperatures that are too high or due to lack of adequate cooling units. Aging occurs when the oils are not stored properly, resulting in a change in the chemical composition due to esterification, reduction, and oxidization of chemicals, which leads to the formation of peroxides and hydroperoxides that can be contact allergens.15
Can patients develop contact allergies to EOs?
The short answer is yes! Contact allergy to almost 80 EOs has been reported,15 including tea tree oil,16,17 ylang-ylang oil,17,18 lavender oil, peppermint oil,18 jasmine absolute,17 geranium oil, rose oil,18 turpentine oil,19,20 and sandalwood oil.18 The recent increased prevalence of allergic reactions to EOs likely is due to increased consumer use as well as increased detection from availability of commercial patch-test preparations.
Essential oils have many common ingredients. De Groot and Schmidt3 documented that 14 of 23 chemicals present in more than 80% of EOs have been reported to cause contact allergy. Interestingly, allergic patients often react to more than one EO, which may be explained by the many shared chemical components in EOs.
Essential oils are “natural” so they must be safe?
In general, most safety profiles are good, but rare toxic reactions from EOs have been observed.4 A recent Australian study reviewed EO exposure calls to the New South Wales Poisons Information Centre.21 The majority of EO poisonings were accidental or the result of therapeutic error such as mistaking EOs for liquid pharmaceuticals. Additionally, this study found that from July 2014 to June 2018, there was a 5% increase in the number of calls per year. More than half of EO poisoning calls involved children, with toddlers being the most frequent cases, suggesting the need for child-resistant top closures. The most frequently involved EOs in poisonings were eucalyptus (46.4% [n=2049]), tea tree (17% [n=749]), lavender (6.1% [n=271]), clove (4.1% [n=179]), and peppermint (3.5% [n=154]).21 Essential oils do not come without potential pitfalls.
What is the clinical presentation and workup?
The workup of EO allergic contact dermatitis begins with obtaining a history to evaluate for use of EO diffusers, perfumes, hygiene products, cosmetics, massage oils, toothpastes, and/or pharmaceutical products. Exploration of potential exposures through occupation, environment, and hobbies also is indicated. Clinical presentation is dependent on the mechanism of exposure. Contact allergy may result from direct application of an allergen to the skin or mucous membranes, contact with a contaminated environmental item (eg, lavender oil on a pillow), contact with EOs used by partners or coworkers (consort dermatitis), airborne exposure (EO diffusers), or systemic exposure (flavorings). Airborne dermatitis from EO diffusers may involve the exposed areas of the face, neck, forearms, arms, behind the earlobes, bilateral eyelids, nasolabial folds, and under the chin. History and clinical presentation can raise suspicion for allergic contact dermatitis, and patch testing is necessary to confirm the diagnosis.
How do we patch test for EO contact allergy?
There are many EOs commercially available for patch testing, and they typically are tested at 2% to 5% concentrations in petrolatum.15 A North American and European study of 62,354 patch-tested patients found that 7.4% of EO-positive individuals did not react to fragrance allergens in a standard screening series including fragrance mix I, fragrance mix II, and balsam of Peru, highlighting the importance of patch testing with specific EOs.22 Currently, only 3 EOs—tea tree oil, peppermint oil, and ylang-ylang oil—are included in the 2019-2020 North American Contact Dermatitis Group screening series, making supplemental testing for other EOs important if contact allergy is suspected; however, testing the patient’s own products is imperative, as there is strong variability in the composition of EOs. Additionally, aged oils may have been exposed to light, oxygen, or varying temperatures, which could result in the formation of additional allergenic chemicals not present in commercially available preparations.15 In addition to commercially available allergens, we test patient-provided EOs either as is in semi-open fashion (ie, EOs are applied to patient’s back with a cotton swab, allowed to dry, covered with adhesive tape, and read at the same interval as other patch tests23) or occasionally dilute them to 1% or 10% (in olive oil or mineral oil).
How should I manage a positive patch-test reaction to EOs?
Patients should avoid relevant EO allergens in their products and environment, which can be easily achieved with the use of the American Contact Dermatitis Society’s Contact Allergen Management Program or similar databases.
Final Interpretation
We are ubiquitously exposed to EOs every day—through the products we use at home, at work, and in our environment. Essential oils make their place in the world by providing sweet-smelling aromas in addition to their alleged therapeutic properties; however, beware, EOs may be the culprit of your next patient’s allergic contact dermatitis.
- International Organization for Standardization. ISO 9235:2013. aromatic natural raw materials—vocabulary. https://www.iso.org/obp/ui/#iso:std:iso:9235:ed-2:v1:en. Accessed March 24, 2020.
- De Groot AC, Schmidt E. Essential oils: part II: general aspects. Dermatitis. 2016;27:43-49.
De Groot AC, Schmidt E. Essential oils: part III: chemical composition. Dermatitis. 2016;27:161-169. - De Groot AC, Schmidt E. Essential oils: part I: introduction. Dermatitis. 2016;27:39-42.
- Insawang S, Pripdeevech P, Tanapichatsakul C, et al. Essential oil compositions and antibacterial and antioxidant activities of five Lavandula stoechas cultivars grown in Thailand. Chem Biodivers. 2019;16:e1900371.
- Lawrence BM. A preliminary report on the world production of some selected essential oils and countries. Perfum Flavor. 2009;34:38-44.
- Goodier MC, Zhang AJ, Nikle AB, et al. Use of essential oils: a general population survey. Contact Dermatitis. 2019;80:391-393.
- KidScents GeneYus. Young Living Essential Oils website. https://www.youngliving.com/en_US/products/kidscents-geneyus. Accessed March 25, 2020.
- KidScents SleepyIze. Young Living Essential Oils website. https://www.youngliving.com/en_US/products/kidscents-sleepyize-5ml. Accessed March 25, 2020.
- KidScents® Lotion. Young Living Essential Oils website. www.youngliving.com/en_US/products/kidscents-lotion. Accessed March 25, 2020.
- KidScents TummyGize. Young Living Essential Oils website. https://www.youngliving.com/en_US/products/kidscents-tummygize-5ml. Accessed March 25, 2020.
- KidScents SniffleEase. Young Living Essential Oils website. https://www.youngliving.com/en_US/products/kidscents-sniffleease. Accessed March 25, 2020.
- 2019 Product Guide. Young Living Essential Oils website. https://issuu.com/youngliving/docs/yl_productguide. Accessed March 25, 2020.
- 2018 Income Disclosure Statement. Young Living Essential Oils website. https://www.youngliving.com/en_US/opportunity/income-disclosure. Accessed March 25, 2020.
- De Groot AC, Schmidt E. Essential oils, part IV: contact allergy. Dermatitis. 2016;27:170-175.
- Pirker C, Hausen BM, Uter W, et al. Sensitization to tea tree oil in Germany and Austria. a multicenter study of the German Contact Dermatitis Group. J Dtsch Dermatol Ges. 2003;1:629-634.
- Larsen W, Nakayama H, Fischer T, et al. Fragrance contact dermatitis: a worldwide multicenter investigation (part II). Contact Dermatitis. 2001;44:344-346.
- Bleasel N, Tate B, Rademaker M. Allergic contact dermatitis following exposure to essential oils. Australas J Dermatol. 2002;43:211-213.
- Noiles K, Pratt M. Contact dermatitis to Vicks VapoRub. Dermatitis. 2010;21:167-169.
- Barchino-Ortiz L, Cabeza-Martinez R, Leis-Dosil VM, et al. Allergic contact hobby dermatitis from turpentine. Allergol Immunopathol (Madr). 2008;36:117-119.
- Lee KA, Harnett JE, Cairns R. Essential oil exposures in Australia: analysis of cases reported to the NSW Poisons Information Centre. Med J Aust. 2020;212:132-133.
- Warshaw EM, Zug KA, Belsito DV, et al. Positive patch test reactions to essential oils in consecutive patients: results from North America and central Europe. Dermatitis. 2017;28:246-252.
- Lazzarini R, Duarte I, Ferreira AL. Patch tests. An Bras Dermatol. 2013;88:879-888.
What is an essential oil?
An essential oil (EO) is defined by the International Organization for Standardization as a ‘‘product obtained from a natural raw material of plant origin, by steam distillation, by mechanical processes from the epicarp of citrus fruits, or by dry distillation, after separation of the aqueous phase—if any—by physical processes.’’1 Steam distillation is the primary method used for the production of commercial EOs,2 and believe it or not, most EOs contain 100 to 250 individual chemical components.3
The term essential oil often is incorrectly used for a variety of products obtained from plant material by methods other than distillation or cold-pressing, such as extraction. Products that are obtained via the extraction method include absolutes found in fine fragrances; hydrolates such as rose water; concretes such as jasmine or violet leaves; and vegetable oils including olive oil, coconut oil, and sesame oil.2 These products are not true EOs.
Where do EOs come from?
Essential oils are produced in many countries around the world.4 Individual oils may be obtained from species of different plants, from different parts of the same plant, or from various cultivars (plants selectively bred to obtain desirable levels of chemical constituents such as monoterpenes or sesquiterpenes and biochemical properties such as antibacterial or antioxidant activities).3,5 It is estimated that EOs can be obtained from approximately 30,000 plant species, but only 150 EOs are produced commercially.2,6
Why are people using EOs? What is their claim to fame?
Essential oils are employed by the flavor, food (eg, soft drinks, milk, candies, chocolate, meats, sausages, alcoholic beverages, spices, herbs, tea, preservatives, animal foods), fragrance, cosmetic, tobacco, and pharmaceutical industries. They also are used in household products (eg, detergents, fabric softeners, air fresheners, candles, incense) and for medicinal purposes (eg, folk and traditional medicine, phytotherapy, balneotherapy, aromatherapy).2 The oils usually are applied to the skin but also can be administered orally, inhaled, diffused through the air, or used by other means.4 One 2019 survey of Minnesota State Fair attendees (N=282) found the most common reasons for using EOs were a desire for alternative treatments (53.4%), the opinion that EOs are safer than traditional therapies (47.6%), and/or failure of standard medical treatments (10.7%). The survey results also indicated that 46.7% of EO users utilized EOs to treat medical conditions or symptoms.7 Of note, review of the website of an international company that produces EOs confirmed that EOs are marketed not only for adults but also for children to help them concentrate,8 sleep,9 improve the appearance of their skin,10 soothe upset stomachs,11 and decrease sniffles due to colds.12
Why are people selling EOs to family and friends? They must be making major bucks!
In general, the cost of EOs depends on the complexity of cultivated plant species; the mode of harvesting, which is sometimes done by hand; and the yield of oil. Prices range from $4.50 to an incredible $150,000 per kilogram.2 On average, one bottle containing 5 to 15 mL of an EO or oil blend can cost anywhere from $7 to $251.13 In the United States, the consumer EO market is partially composed of multilevel/network marketing companies in which direct consumer sales occur via a hierarchy of individual distributors. Goodier et al7 found that 36.4% of participants who obtained EOs from family and friends purchased them through multilevel/network marketing companies. In 2018, individual distributors of an international EO-producing company made on average anywhere from $4 to as much as $1.54 million annually by selling the company’s EO products and enrolling additional members/individual distributors to purchase or sell the company’s EO products.14
Sometimes EOs are described as natural and pure, but are they really?
Just because a product is labeled as “pure” or “natural” does not ensure that it is a good-quality EO. Organically produced (ie, grown without the use of herbicides or pesticides) plant material can include up to 30% of extraneous herbs and weeds, which can change the composition of the oil.2
Lesser-quality EOs are the result of adulteration, contamination, inadequate oil production, or aging.2 Adulteration (eg, cutting, stretching, bouquetting) occurs when foreign substances are introduced into pure EOs for the benefit of a higher profit; to ensure a sufficient supply of oils; or to meet demands for cheaper oils by “stretching” a more expensive, pure oil by combining with a cheaper, less pure oil. Inadequate oil production leading to lower-quality oils can occur when a biomass is incorrectly distilled, either from too much steam or temperatures that are too high or due to lack of adequate cooling units. Aging occurs when the oils are not stored properly, resulting in a change in the chemical composition due to esterification, reduction, and oxidization of chemicals, which leads to the formation of peroxides and hydroperoxides that can be contact allergens.15
Can patients develop contact allergies to EOs?
The short answer is yes! Contact allergy to almost 80 EOs has been reported,15 including tea tree oil,16,17 ylang-ylang oil,17,18 lavender oil, peppermint oil,18 jasmine absolute,17 geranium oil, rose oil,18 turpentine oil,19,20 and sandalwood oil.18 The recent increased prevalence of allergic reactions to EOs likely is due to increased consumer use as well as increased detection from availability of commercial patch-test preparations.
Essential oils have many common ingredients. De Groot and Schmidt3 documented that 14 of 23 chemicals present in more than 80% of EOs have been reported to cause contact allergy. Interestingly, allergic patients often react to more than one EO, which may be explained by the many shared chemical components in EOs.
Essential oils are “natural” so they must be safe?
In general, most safety profiles are good, but rare toxic reactions from EOs have been observed.4 A recent Australian study reviewed EO exposure calls to the New South Wales Poisons Information Centre.21 The majority of EO poisonings were accidental or the result of therapeutic error such as mistaking EOs for liquid pharmaceuticals. Additionally, this study found that from July 2014 to June 2018, there was a 5% increase in the number of calls per year. More than half of EO poisoning calls involved children, with toddlers being the most frequent cases, suggesting the need for child-resistant top closures. The most frequently involved EOs in poisonings were eucalyptus (46.4% [n=2049]), tea tree (17% [n=749]), lavender (6.1% [n=271]), clove (4.1% [n=179]), and peppermint (3.5% [n=154]).21 Essential oils do not come without potential pitfalls.
What is the clinical presentation and workup?
The workup of EO allergic contact dermatitis begins with obtaining a history to evaluate for use of EO diffusers, perfumes, hygiene products, cosmetics, massage oils, toothpastes, and/or pharmaceutical products. Exploration of potential exposures through occupation, environment, and hobbies also is indicated. Clinical presentation is dependent on the mechanism of exposure. Contact allergy may result from direct application of an allergen to the skin or mucous membranes, contact with a contaminated environmental item (eg, lavender oil on a pillow), contact with EOs used by partners or coworkers (consort dermatitis), airborne exposure (EO diffusers), or systemic exposure (flavorings). Airborne dermatitis from EO diffusers may involve the exposed areas of the face, neck, forearms, arms, behind the earlobes, bilateral eyelids, nasolabial folds, and under the chin. History and clinical presentation can raise suspicion for allergic contact dermatitis, and patch testing is necessary to confirm the diagnosis.
How do we patch test for EO contact allergy?
There are many EOs commercially available for patch testing, and they typically are tested at 2% to 5% concentrations in petrolatum.15 A North American and European study of 62,354 patch-tested patients found that 7.4% of EO-positive individuals did not react to fragrance allergens in a standard screening series including fragrance mix I, fragrance mix II, and balsam of Peru, highlighting the importance of patch testing with specific EOs.22 Currently, only 3 EOs—tea tree oil, peppermint oil, and ylang-ylang oil—are included in the 2019-2020 North American Contact Dermatitis Group screening series, making supplemental testing for other EOs important if contact allergy is suspected; however, testing the patient’s own products is imperative, as there is strong variability in the composition of EOs. Additionally, aged oils may have been exposed to light, oxygen, or varying temperatures, which could result in the formation of additional allergenic chemicals not present in commercially available preparations.15 In addition to commercially available allergens, we test patient-provided EOs either as is in semi-open fashion (ie, EOs are applied to patient’s back with a cotton swab, allowed to dry, covered with adhesive tape, and read at the same interval as other patch tests23) or occasionally dilute them to 1% or 10% (in olive oil or mineral oil).
How should I manage a positive patch-test reaction to EOs?
Patients should avoid relevant EO allergens in their products and environment, which can be easily achieved with the use of the American Contact Dermatitis Society’s Contact Allergen Management Program or similar databases.
Final Interpretation
We are ubiquitously exposed to EOs every day—through the products we use at home, at work, and in our environment. Essential oils make their place in the world by providing sweet-smelling aromas in addition to their alleged therapeutic properties; however, beware, EOs may be the culprit of your next patient’s allergic contact dermatitis.
What is an essential oil?
An essential oil (EO) is defined by the International Organization for Standardization as a ‘‘product obtained from a natural raw material of plant origin, by steam distillation, by mechanical processes from the epicarp of citrus fruits, or by dry distillation, after separation of the aqueous phase—if any—by physical processes.’’1 Steam distillation is the primary method used for the production of commercial EOs,2 and believe it or not, most EOs contain 100 to 250 individual chemical components.3
The term essential oil often is incorrectly used for a variety of products obtained from plant material by methods other than distillation or cold-pressing, such as extraction. Products that are obtained via the extraction method include absolutes found in fine fragrances; hydrolates such as rose water; concretes such as jasmine or violet leaves; and vegetable oils including olive oil, coconut oil, and sesame oil.2 These products are not true EOs.
Where do EOs come from?
Essential oils are produced in many countries around the world.4 Individual oils may be obtained from species of different plants, from different parts of the same plant, or from various cultivars (plants selectively bred to obtain desirable levels of chemical constituents such as monoterpenes or sesquiterpenes and biochemical properties such as antibacterial or antioxidant activities).3,5 It is estimated that EOs can be obtained from approximately 30,000 plant species, but only 150 EOs are produced commercially.2,6
Why are people using EOs? What is their claim to fame?
Essential oils are employed by the flavor, food (eg, soft drinks, milk, candies, chocolate, meats, sausages, alcoholic beverages, spices, herbs, tea, preservatives, animal foods), fragrance, cosmetic, tobacco, and pharmaceutical industries. They also are used in household products (eg, detergents, fabric softeners, air fresheners, candles, incense) and for medicinal purposes (eg, folk and traditional medicine, phytotherapy, balneotherapy, aromatherapy).2 The oils usually are applied to the skin but also can be administered orally, inhaled, diffused through the air, or used by other means.4 One 2019 survey of Minnesota State Fair attendees (N=282) found the most common reasons for using EOs were a desire for alternative treatments (53.4%), the opinion that EOs are safer than traditional therapies (47.6%), and/or failure of standard medical treatments (10.7%). The survey results also indicated that 46.7% of EO users utilized EOs to treat medical conditions or symptoms.7 Of note, review of the website of an international company that produces EOs confirmed that EOs are marketed not only for adults but also for children to help them concentrate,8 sleep,9 improve the appearance of their skin,10 soothe upset stomachs,11 and decrease sniffles due to colds.12
Why are people selling EOs to family and friends? They must be making major bucks!
In general, the cost of EOs depends on the complexity of cultivated plant species; the mode of harvesting, which is sometimes done by hand; and the yield of oil. Prices range from $4.50 to an incredible $150,000 per kilogram.2 On average, one bottle containing 5 to 15 mL of an EO or oil blend can cost anywhere from $7 to $251.13 In the United States, the consumer EO market is partially composed of multilevel/network marketing companies in which direct consumer sales occur via a hierarchy of individual distributors. Goodier et al7 found that 36.4% of participants who obtained EOs from family and friends purchased them through multilevel/network marketing companies. In 2018, individual distributors of an international EO-producing company made on average anywhere from $4 to as much as $1.54 million annually by selling the company’s EO products and enrolling additional members/individual distributors to purchase or sell the company’s EO products.14
Sometimes EOs are described as natural and pure, but are they really?
Just because a product is labeled as “pure” or “natural” does not ensure that it is a good-quality EO. Organically produced (ie, grown without the use of herbicides or pesticides) plant material can include up to 30% of extraneous herbs and weeds, which can change the composition of the oil.2
Lesser-quality EOs are the result of adulteration, contamination, inadequate oil production, or aging.2 Adulteration (eg, cutting, stretching, bouquetting) occurs when foreign substances are introduced into pure EOs for the benefit of a higher profit; to ensure a sufficient supply of oils; or to meet demands for cheaper oils by “stretching” a more expensive, pure oil by combining with a cheaper, less pure oil. Inadequate oil production leading to lower-quality oils can occur when a biomass is incorrectly distilled, either from too much steam or temperatures that are too high or due to lack of adequate cooling units. Aging occurs when the oils are not stored properly, resulting in a change in the chemical composition due to esterification, reduction, and oxidization of chemicals, which leads to the formation of peroxides and hydroperoxides that can be contact allergens.15
Can patients develop contact allergies to EOs?
The short answer is yes! Contact allergy to almost 80 EOs has been reported,15 including tea tree oil,16,17 ylang-ylang oil,17,18 lavender oil, peppermint oil,18 jasmine absolute,17 geranium oil, rose oil,18 turpentine oil,19,20 and sandalwood oil.18 The recent increased prevalence of allergic reactions to EOs likely is due to increased consumer use as well as increased detection from availability of commercial patch-test preparations.
Essential oils have many common ingredients. De Groot and Schmidt3 documented that 14 of 23 chemicals present in more than 80% of EOs have been reported to cause contact allergy. Interestingly, allergic patients often react to more than one EO, which may be explained by the many shared chemical components in EOs.
Essential oils are “natural” so they must be safe?
In general, most safety profiles are good, but rare toxic reactions from EOs have been observed.4 A recent Australian study reviewed EO exposure calls to the New South Wales Poisons Information Centre.21 The majority of EO poisonings were accidental or the result of therapeutic error such as mistaking EOs for liquid pharmaceuticals. Additionally, this study found that from July 2014 to June 2018, there was a 5% increase in the number of calls per year. More than half of EO poisoning calls involved children, with toddlers being the most frequent cases, suggesting the need for child-resistant top closures. The most frequently involved EOs in poisonings were eucalyptus (46.4% [n=2049]), tea tree (17% [n=749]), lavender (6.1% [n=271]), clove (4.1% [n=179]), and peppermint (3.5% [n=154]).21 Essential oils do not come without potential pitfalls.
What is the clinical presentation and workup?
The workup of EO allergic contact dermatitis begins with obtaining a history to evaluate for use of EO diffusers, perfumes, hygiene products, cosmetics, massage oils, toothpastes, and/or pharmaceutical products. Exploration of potential exposures through occupation, environment, and hobbies also is indicated. Clinical presentation is dependent on the mechanism of exposure. Contact allergy may result from direct application of an allergen to the skin or mucous membranes, contact with a contaminated environmental item (eg, lavender oil on a pillow), contact with EOs used by partners or coworkers (consort dermatitis), airborne exposure (EO diffusers), or systemic exposure (flavorings). Airborne dermatitis from EO diffusers may involve the exposed areas of the face, neck, forearms, arms, behind the earlobes, bilateral eyelids, nasolabial folds, and under the chin. History and clinical presentation can raise suspicion for allergic contact dermatitis, and patch testing is necessary to confirm the diagnosis.
How do we patch test for EO contact allergy?
There are many EOs commercially available for patch testing, and they typically are tested at 2% to 5% concentrations in petrolatum.15 A North American and European study of 62,354 patch-tested patients found that 7.4% of EO-positive individuals did not react to fragrance allergens in a standard screening series including fragrance mix I, fragrance mix II, and balsam of Peru, highlighting the importance of patch testing with specific EOs.22 Currently, only 3 EOs—tea tree oil, peppermint oil, and ylang-ylang oil—are included in the 2019-2020 North American Contact Dermatitis Group screening series, making supplemental testing for other EOs important if contact allergy is suspected; however, testing the patient’s own products is imperative, as there is strong variability in the composition of EOs. Additionally, aged oils may have been exposed to light, oxygen, or varying temperatures, which could result in the formation of additional allergenic chemicals not present in commercially available preparations.15 In addition to commercially available allergens, we test patient-provided EOs either as is in semi-open fashion (ie, EOs are applied to patient’s back with a cotton swab, allowed to dry, covered with adhesive tape, and read at the same interval as other patch tests23) or occasionally dilute them to 1% or 10% (in olive oil or mineral oil).
How should I manage a positive patch-test reaction to EOs?
Patients should avoid relevant EO allergens in their products and environment, which can be easily achieved with the use of the American Contact Dermatitis Society’s Contact Allergen Management Program or similar databases.
Final Interpretation
We are ubiquitously exposed to EOs every day—through the products we use at home, at work, and in our environment. Essential oils make their place in the world by providing sweet-smelling aromas in addition to their alleged therapeutic properties; however, beware, EOs may be the culprit of your next patient’s allergic contact dermatitis.
- International Organization for Standardization. ISO 9235:2013. aromatic natural raw materials—vocabulary. https://www.iso.org/obp/ui/#iso:std:iso:9235:ed-2:v1:en. Accessed March 24, 2020.
- De Groot AC, Schmidt E. Essential oils: part II: general aspects. Dermatitis. 2016;27:43-49.
De Groot AC, Schmidt E. Essential oils: part III: chemical composition. Dermatitis. 2016;27:161-169. - De Groot AC, Schmidt E. Essential oils: part I: introduction. Dermatitis. 2016;27:39-42.
- Insawang S, Pripdeevech P, Tanapichatsakul C, et al. Essential oil compositions and antibacterial and antioxidant activities of five Lavandula stoechas cultivars grown in Thailand. Chem Biodivers. 2019;16:e1900371.
- Lawrence BM. A preliminary report on the world production of some selected essential oils and countries. Perfum Flavor. 2009;34:38-44.
- Goodier MC, Zhang AJ, Nikle AB, et al. Use of essential oils: a general population survey. Contact Dermatitis. 2019;80:391-393.
- KidScents GeneYus. Young Living Essential Oils website. https://www.youngliving.com/en_US/products/kidscents-geneyus. Accessed March 25, 2020.
- KidScents SleepyIze. Young Living Essential Oils website. https://www.youngliving.com/en_US/products/kidscents-sleepyize-5ml. Accessed March 25, 2020.
- KidScents® Lotion. Young Living Essential Oils website. www.youngliving.com/en_US/products/kidscents-lotion. Accessed March 25, 2020.
- KidScents TummyGize. Young Living Essential Oils website. https://www.youngliving.com/en_US/products/kidscents-tummygize-5ml. Accessed March 25, 2020.
- KidScents SniffleEase. Young Living Essential Oils website. https://www.youngliving.com/en_US/products/kidscents-sniffleease. Accessed March 25, 2020.
- 2019 Product Guide. Young Living Essential Oils website. https://issuu.com/youngliving/docs/yl_productguide. Accessed March 25, 2020.
- 2018 Income Disclosure Statement. Young Living Essential Oils website. https://www.youngliving.com/en_US/opportunity/income-disclosure. Accessed March 25, 2020.
- De Groot AC, Schmidt E. Essential oils, part IV: contact allergy. Dermatitis. 2016;27:170-175.
- Pirker C, Hausen BM, Uter W, et al. Sensitization to tea tree oil in Germany and Austria. a multicenter study of the German Contact Dermatitis Group. J Dtsch Dermatol Ges. 2003;1:629-634.
- Larsen W, Nakayama H, Fischer T, et al. Fragrance contact dermatitis: a worldwide multicenter investigation (part II). Contact Dermatitis. 2001;44:344-346.
- Bleasel N, Tate B, Rademaker M. Allergic contact dermatitis following exposure to essential oils. Australas J Dermatol. 2002;43:211-213.
- Noiles K, Pratt M. Contact dermatitis to Vicks VapoRub. Dermatitis. 2010;21:167-169.
- Barchino-Ortiz L, Cabeza-Martinez R, Leis-Dosil VM, et al. Allergic contact hobby dermatitis from turpentine. Allergol Immunopathol (Madr). 2008;36:117-119.
- Lee KA, Harnett JE, Cairns R. Essential oil exposures in Australia: analysis of cases reported to the NSW Poisons Information Centre. Med J Aust. 2020;212:132-133.
- Warshaw EM, Zug KA, Belsito DV, et al. Positive patch test reactions to essential oils in consecutive patients: results from North America and central Europe. Dermatitis. 2017;28:246-252.
- Lazzarini R, Duarte I, Ferreira AL. Patch tests. An Bras Dermatol. 2013;88:879-888.
- International Organization for Standardization. ISO 9235:2013. aromatic natural raw materials—vocabulary. https://www.iso.org/obp/ui/#iso:std:iso:9235:ed-2:v1:en. Accessed March 24, 2020.
- De Groot AC, Schmidt E. Essential oils: part II: general aspects. Dermatitis. 2016;27:43-49.
De Groot AC, Schmidt E. Essential oils: part III: chemical composition. Dermatitis. 2016;27:161-169. - De Groot AC, Schmidt E. Essential oils: part I: introduction. Dermatitis. 2016;27:39-42.
- Insawang S, Pripdeevech P, Tanapichatsakul C, et al. Essential oil compositions and antibacterial and antioxidant activities of five Lavandula stoechas cultivars grown in Thailand. Chem Biodivers. 2019;16:e1900371.
- Lawrence BM. A preliminary report on the world production of some selected essential oils and countries. Perfum Flavor. 2009;34:38-44.
- Goodier MC, Zhang AJ, Nikle AB, et al. Use of essential oils: a general population survey. Contact Dermatitis. 2019;80:391-393.
- KidScents GeneYus. Young Living Essential Oils website. https://www.youngliving.com/en_US/products/kidscents-geneyus. Accessed March 25, 2020.
- KidScents SleepyIze. Young Living Essential Oils website. https://www.youngliving.com/en_US/products/kidscents-sleepyize-5ml. Accessed March 25, 2020.
- KidScents® Lotion. Young Living Essential Oils website. www.youngliving.com/en_US/products/kidscents-lotion. Accessed March 25, 2020.
- KidScents TummyGize. Young Living Essential Oils website. https://www.youngliving.com/en_US/products/kidscents-tummygize-5ml. Accessed March 25, 2020.
- KidScents SniffleEase. Young Living Essential Oils website. https://www.youngliving.com/en_US/products/kidscents-sniffleease. Accessed March 25, 2020.
- 2019 Product Guide. Young Living Essential Oils website. https://issuu.com/youngliving/docs/yl_productguide. Accessed March 25, 2020.
- 2018 Income Disclosure Statement. Young Living Essential Oils website. https://www.youngliving.com/en_US/opportunity/income-disclosure. Accessed March 25, 2020.
- De Groot AC, Schmidt E. Essential oils, part IV: contact allergy. Dermatitis. 2016;27:170-175.
- Pirker C, Hausen BM, Uter W, et al. Sensitization to tea tree oil in Germany and Austria. a multicenter study of the German Contact Dermatitis Group. J Dtsch Dermatol Ges. 2003;1:629-634.
- Larsen W, Nakayama H, Fischer T, et al. Fragrance contact dermatitis: a worldwide multicenter investigation (part II). Contact Dermatitis. 2001;44:344-346.
- Bleasel N, Tate B, Rademaker M. Allergic contact dermatitis following exposure to essential oils. Australas J Dermatol. 2002;43:211-213.
- Noiles K, Pratt M. Contact dermatitis to Vicks VapoRub. Dermatitis. 2010;21:167-169.
- Barchino-Ortiz L, Cabeza-Martinez R, Leis-Dosil VM, et al. Allergic contact hobby dermatitis from turpentine. Allergol Immunopathol (Madr). 2008;36:117-119.
- Lee KA, Harnett JE, Cairns R. Essential oil exposures in Australia: analysis of cases reported to the NSW Poisons Information Centre. Med J Aust. 2020;212:132-133.
- Warshaw EM, Zug KA, Belsito DV, et al. Positive patch test reactions to essential oils in consecutive patients: results from North America and central Europe. Dermatitis. 2017;28:246-252.
- Lazzarini R, Duarte I, Ferreira AL. Patch tests. An Bras Dermatol. 2013;88:879-888.
Practice Points
- Essential oils (EOs) are present in many consumer products, including foods, cosmetics, pharmaceuticals, and household products; patients can develop contact allergy to EOs.
- Common EO allergens include tea tree oil, ylang-ylang oil, lavender oil, peppermint oil, jasmine absolute, geranium oil, rose oil, turpentine oil, and sandalwood oil.
- In general, EOs have good safety profiles, but caution must be taken when storing them.
- When patch testing for potential EO contact allergy, supplemental testing with both commercially available EOs as well as a patient’s own products is necessary given there is strong variability in the composition of EO products.
Hypersensitivity Reactions to Orthopedic Implants: What’s All the Hype?
Hypersensitivity to metal implants remains a controversial field in contact dermatitis and patch testing. With positive reactions to nickel hovering around 20% in patch-tested populations,1 the question remains whether metal-allergic patients can safely receive metal implants. Unfortunately, large controlled studies are lacking, in part due to ethical concerns of knowingly placing a metal implant in a metal-allergic patient. Much of the focus of implant hypersensitivity reactions (IHRs) has been on orthopedic joints including hips, knees, and shoulders, as well as fixed orthopedic implanted materials such as screws and plates. However, there have been reports of IHRs to cardiac devices including defibrillators, pacemakers, and intracardiac devices; dental hardware including implants, crowns, dentures, and braces; and neurologic and gynecologic devices. For the purposes of this review, we will focus on IHRs to orthopedic implants.
Making the Case for IHRs
There are multiple case reports and series documenting likely orthopedic IHRs in the literature2-5; however, large prospective studies are lacking. Some of the largest series are from Danish registry studies. In 2009, Thyssen et al6 reviewed356 patients who had undergone both total hip arthroplasty and patch testing. Metal allergy frequencies were similar between patch-tested registry patients and patch test controls, showing no increase in positive patch tests to metals after receiving implants. Additionally, implant revision rates were comparable between registry patients with and without patch testing. The group concluded that the risk for revision after hip implantation in metal-allergic patients and the risk for development of metal allergy after implantation were both low.6 In 2015, Münch et al7 compared 327 patients who had undergone both total knee arthroplasty and patch testing and found that prevalence of allergy to nickel, cobalt, and chromium was similar between patients who had undergone revision surgery and those who had not; however, for patients who had 2 or more knee revisions, there was a higher prevalence of postimplant metal allergy. This study also showed that metal allergy identified before implantation did not increase the risk for postimplantation knee revision surgery or implant failure.7 These larger studies suggest that although individual cases of IHR exist, it is likely quite rare.
Patients have been found to have increased levels of chromium (serum and urine) and titanium (serum) following total hip arthroplasty.8 Additionally, metal wear particles have been identified in postmortem livers and spleens, which was more prevalent in patients with a history of failed hip arthroplasty.9 It is difficult to determine the meaning of this data, as the presence of metal ions does not necessarily indicate allergy or IHR. In 2001, Hallab et al10 pooled data from several implant cohort studies and concluded that in comparison to a baseline metal sensitivity prevalence of approximately 10%, patients with well-functioning implants had a metal sensitivity–weighted average of 25%, and those with poorly functioning implants had a weighted average of 60%. Again, positive patch testing to metals does not necessarily implicate allergy as the cause of implant failure.
Some small studies have shown that patients with evidence of metal hypersensitivity improve with revision. Zondervan et al11 reviewed results of 46 orthopedic revisions following painful total knee arthroplasty. Patients with knee pain and lymphocyte transformation testing (LTT) positive for metals received hypoallergenic revisions, and those with LTT negative for metals received standard revisions. The group who received hypoallergenic revisions had more pain reduction compared to the standard revision group (37.8% reduction in pain vs 27%). However, this study was limited in that the diagnosis of metal allergy was made entirely on results of LTT.11 In 2012, Atanaskova Mesinkovska et al12 described 41 patients who underwent orthopedic patch testing following implantation for symptoms including pain, dermatitis, pruritus, joint loosening, edema, and impaired wound healing. Fifteen (37%) patients had positive patch test reactions to metals, and 10 (67%) of them had reactions to metals that were present in their implants. Six (60%) of these patients had their implants removed and their symptoms resolved; the remaining 4 continued to experience implant symptoms.12 These studies support the existence of rare metal-related orthopedic IHRs and support the concept of proceeding with orthopedic implant revision when indicated, safe, and agreed upon by the surgeon and patient. However, as noted in the series by Zondervan et al,11 not every patient with confirmed metal allergy who undergoes revision improves, so an informed conversation between the patient and surgeon is mandatory.
Types of Orthopedic Implants
Orthopedic implanted materials consist of either dynamic (knees, hips) or static (screws, plates) components. Several generations of hip implants have evolved since the 1960s. First-generation implanted hips were metal-on-metal and had high rates of metal release and sensitization. Metal-on-plastic implants may be less likely to release metal but instead release large polyethylene wear particles. Second-generation metal-on-metal implants reportedly have lower wear rates. With these implants, wear particles are generated but are reportedly smaller than first-generation particles.13
Allergens in IHRs
Metals
Metals are the most commonly implicated allergens in orthopedic IHRs. Potentially relevant metal alloys include 316L stainless steel, cobalt-chromium-molybdenum steel, Vitallium alloy, titanium alloy, titanium-tantalum-niobium alloy, and Oxinium (Smith & Nephew).14,15 Each alloy contains several metals, which can include nickel, chromium, cobalt, manganese, molybdenum, iron, titanium, aluminum, vanadium, niobium, tantalum, and zirconium, among others. For example, 316L stainless steel contains iron, nickel, chromium, manganese, molybdenum, nitrogen, carbon, sulfur, silicon, and phosphorus, whereas Oxinium contains only oxidized zirconium and niobium.
Bone Cement
Bone cement also has been reported in cases of orthopedic IHRs and can contain several chemicals, including methyl methacrylate, N,N-dimethyl-p-toluidine, benzoyl peroxide, hydroquinone, and gentamicin.14 Other potential exposures include adhesives (cyanoacrylates) and topical antibiotics.
Clinical Presentation
Several clinical presentations of orthopedic IHRs have been described. Perhaps the most commonly recognized is a localized cutaneous eczematous eruption, with dermatitis typically overlying the site of the implanted material.1,2,16 Generalized cutaneous eczematous IHRs also have been reported, including diffuse generalized dermatitis from a stainless steel orthopedic screw4 and nummular dermatitis attributed to vanadium in an orthopedic plate.5 Urticaria, vasculitis, and bullous cutaneous reactions, as well as extracutaneous complications, also have been reported.14,15 Pain, edema, joint loosening or failure, and poor wound healing have been reported,12 but it remains unclear whether these symptoms represent IHR.
Patch Testing for IHR
Several groups have published recommended patch test series for IHR.12,14,15 Common components of implant patch testing panels include metals, adhesives (acrylates, epoxy resins) and antibiotics. Importantly, obtaining product information from the manufacturer of the suspected implant can guide which allergens to include in patch testing. Implant and metal panels also are available for commercial purchase.
Other Diagnostic Tests
We rarely (almost never) order LTTs in the workup for potential IHRs. This is an in vitro test that includes lymphocytes, metal ions, and the radioactive marker methyl-3H-thymidine. The goal of the test is to evaluate if patient lymphocytes are reactive or responsive to metal ions. A positive LTT suggests that lymphocytes can respond to the presence of metal ions but does not confirm allergy or the presence of IHR.
Typically, skin or tissue biopsies are not required to make a diagnosis of IHR; however, if performed, histopathology suggestive of IHR can support a suspected diagnosis. Typical findings include but are not limited to spongiotic dermatitis. Eosinophils may or may not be present. Metal disc testing has been utilized for orthopedic IHR but is not currently recommended due to low diagnostic yield. Prick testing rarely is used and also is not a primary method for diagnosis of IHR.17
Preimplantation Patch Testing
Expert opinion guidelines published by the American Contact Dermatitis Society (ACDS) state that routine preimplantation patch testing is not necessary; however, for those patients with a clear history of contact reactions to metal, preimplantation patch testing can be considered.17
Patch test results can influence the orthopedic surgeon’s choice of implant material. In one study, when preimplantation patch testing showed a positive patch test reaction to metals, the results influenced the surgeon’s decision-making in all cases.12
Postimplantation Patch Testing: Diagnostic Criteria for Metal IHR After Implantation
From 2012 to 2013, Schalock and Thyssen18 surveyed expert attendees at meetings of the European Society of Contact Dermatitis and the ACDS for their opinions on proposed diagnostic criteria for metal IHRs. Based on these results (N=119), the authors stratified 4 major and 5 minor diagnostic criteria, which were defined based on overall responses of meeting attendees. Major criteria included (1) chronic dermatitis beginning weeks to months after metallic implantation, (2) complete recovery after removal of the offending implant, (3) eruption overlying the metal implant, and (4) positive patch test reaction to a metal used in the implant. Minor criteria included (1) histology consistent with allergic contact dermatitis, (2) morphology consistent with dermatitis (ie, erythema, induration, papules, vesicles), (3) positive in vitro test to metals (eg, lymphocyte transformation test), (4) systemic allergic dermatitis reaction, and (5) therapy-resistant dermatitis reaction. The authors did not describe a scoring system for evaluation and confirmation of a diagnosis of IHR. Instead, the criteria should be used as general guidelines when evaluating patients for possible IHRs. From a standpoint of available diagnostic tests for metal IHR, 86.1% of experts agreed that a positive patch test reaction to a metal used in the implant was suggestive of a diagnosis, whereas a positive in vitro test to metals (LTT) was suggestive of a diagnosis for only 32.2% of respondents. This study was designed specifically for metal IHRs and therefore is not necessarily generalizable for nonmetal IHRs.18
Final Interpretation
We follow the 2016 ACDS guidelines17 and complete preimplantation patch testing only in the setting of suspected metal allergy and postimplantation patch testing based on the guidelines described by Schalock and Thyssen.18 However, an extended conversation is warranted prior to patch testing to ensure the patient fully understands the limitations of the test. Although we have both ordered the LTT, interpretation remains murky, and until this test is standardized, routine use is unlikely to benefit the patient. Until we are more reliably able to predict who will develop hypersensitivity to implanted metals, the decision to remove or revise an implant is one that should be made by a multidisciplinary team that includes the surgeon and the patient.
- Dekoven JG, Warshaw EM, Zug KA, et al. North American Contact Dermatitis Group patch test results: 2015-2016. Dermatitis. 2018;29:297-309.
- Gao X, He RX, Yan SG, et al. Dermatitis associated with chromium following total knee arthroplasty. J Arthroplasty. 2011;26:665.E613-665.E616.
- Treudler R, Simon JC. Benzoyl peroxide: is it a relevant bone cement allergen in patients with orthopaedic implants? Contact Dermatitis. 2007;57:177-180.
- Barranco VP, Soloman H. Eczematous dermatitis from nickel. JAMA. 1972;220:1244.
- Engelhart S, Segal RJ. Allergic reaction to vanadium causes a diffuse eczematous eruption and titanium alloy orthopedic implant failure. Cutis. 2017;99:245-249.
- Thyssen JP, Jakobsen SS, Engkilde K, et al. The association between metal allergy, total hip arthroplasty, and revision. Acta Orthop. 2009;80:646-652.
- Münch HJ, Jacobsen SS, Olesen JT, et al. The association between metal allergy, total knee arthroplasty, and revision: study based on the Danish Knee Arthroplasty Register. Acta Orthop. 2015;86:378-383.
- Jacobs JJ, Skipor AK, Patterson LM, et al. Metal release in patients who have had a primary total hip arthroplasty. a prospective, controlled, longitudinal study. J Bone Joint Surg Am. 1998;80:1447-1458.
- Urban RM, Jacobs JJ, Tomlinson MJ, et al. Dissemination of wear particles to the liver, spleen, and abdominal lymph nodes of patients with hip or knee replacement. J Bone Joint Surg Am. 2000;82:457-476.
- Hallab N, Merritt K, Jacobs JJ. Metal sensitivity in patients with orthopaedic implants. J Bone Joint Surg Am. 2001;83:428-436.
- Zondervan RL, Vaux JJ, Blackmer MJ, et al. Improved outcomes in patients with positive metal sensitivity following revision total knee arthroplasty. J Orthop Surg Res. 2019;14:182.
- Atanaskova Mesinkovska N, Tellez A, Molina L, et al. The effect of patch testing on surgical practices and outcomes in orthopedic patients with metal implants. Arch Dermatol. 2012;148:687-693.
- Kovochich M, Fung ES, Donovan E, et al. Characterization of wear debris from metal-on-metal hip implants during normal wear versus edge-loading conditions. J Biomed Mater Res B Appl Biomater. 2018;106:986-996.
- Basko-Plluska JL, Thyssen JP, Schalock PC. Cutaneous and systemic hypersensitivity reactions to metallic implants. Dermatitis. 2011;22:65-79.
- Schalock PC, Menné T, Johansen JD, et al. Hypersensitivity reactions to metallic implants—diagnostic algorithm and suggested patch test series for clinical use. Contact Dermatitis. 2012;66:4-19.
- Thomas P, Gollwitzer H, Maier S, et al. Osteosynthesis associated contact dermatitis with unusual perpetuation of hyperreactivity in a nickel allergic patient. Contact Dermatitis. 2006;54:222-225.
- Schalock PC, Crawford G, Nedorost S, et al. Patch testing for evaluation of hypersensitivity to implanted metal devices: a perspective from the American Contact Dermatitis Society. Dermatitis. 2016;27:241-247.
- Schalock PC, Thyssen JP. Patch testers’ opinions regarding diagnostic criteria for metal hypersensitivity reactions to metallic implants. Dermatitis. 2013;24:183-185.
Hypersensitivity to metal implants remains a controversial field in contact dermatitis and patch testing. With positive reactions to nickel hovering around 20% in patch-tested populations,1 the question remains whether metal-allergic patients can safely receive metal implants. Unfortunately, large controlled studies are lacking, in part due to ethical concerns of knowingly placing a metal implant in a metal-allergic patient. Much of the focus of implant hypersensitivity reactions (IHRs) has been on orthopedic joints including hips, knees, and shoulders, as well as fixed orthopedic implanted materials such as screws and plates. However, there have been reports of IHRs to cardiac devices including defibrillators, pacemakers, and intracardiac devices; dental hardware including implants, crowns, dentures, and braces; and neurologic and gynecologic devices. For the purposes of this review, we will focus on IHRs to orthopedic implants.
Making the Case for IHRs
There are multiple case reports and series documenting likely orthopedic IHRs in the literature2-5; however, large prospective studies are lacking. Some of the largest series are from Danish registry studies. In 2009, Thyssen et al6 reviewed356 patients who had undergone both total hip arthroplasty and patch testing. Metal allergy frequencies were similar between patch-tested registry patients and patch test controls, showing no increase in positive patch tests to metals after receiving implants. Additionally, implant revision rates were comparable between registry patients with and without patch testing. The group concluded that the risk for revision after hip implantation in metal-allergic patients and the risk for development of metal allergy after implantation were both low.6 In 2015, Münch et al7 compared 327 patients who had undergone both total knee arthroplasty and patch testing and found that prevalence of allergy to nickel, cobalt, and chromium was similar between patients who had undergone revision surgery and those who had not; however, for patients who had 2 or more knee revisions, there was a higher prevalence of postimplant metal allergy. This study also showed that metal allergy identified before implantation did not increase the risk for postimplantation knee revision surgery or implant failure.7 These larger studies suggest that although individual cases of IHR exist, it is likely quite rare.
Patients have been found to have increased levels of chromium (serum and urine) and titanium (serum) following total hip arthroplasty.8 Additionally, metal wear particles have been identified in postmortem livers and spleens, which was more prevalent in patients with a history of failed hip arthroplasty.9 It is difficult to determine the meaning of this data, as the presence of metal ions does not necessarily indicate allergy or IHR. In 2001, Hallab et al10 pooled data from several implant cohort studies and concluded that in comparison to a baseline metal sensitivity prevalence of approximately 10%, patients with well-functioning implants had a metal sensitivity–weighted average of 25%, and those with poorly functioning implants had a weighted average of 60%. Again, positive patch testing to metals does not necessarily implicate allergy as the cause of implant failure.
Some small studies have shown that patients with evidence of metal hypersensitivity improve with revision. Zondervan et al11 reviewed results of 46 orthopedic revisions following painful total knee arthroplasty. Patients with knee pain and lymphocyte transformation testing (LTT) positive for metals received hypoallergenic revisions, and those with LTT negative for metals received standard revisions. The group who received hypoallergenic revisions had more pain reduction compared to the standard revision group (37.8% reduction in pain vs 27%). However, this study was limited in that the diagnosis of metal allergy was made entirely on results of LTT.11 In 2012, Atanaskova Mesinkovska et al12 described 41 patients who underwent orthopedic patch testing following implantation for symptoms including pain, dermatitis, pruritus, joint loosening, edema, and impaired wound healing. Fifteen (37%) patients had positive patch test reactions to metals, and 10 (67%) of them had reactions to metals that were present in their implants. Six (60%) of these patients had their implants removed and their symptoms resolved; the remaining 4 continued to experience implant symptoms.12 These studies support the existence of rare metal-related orthopedic IHRs and support the concept of proceeding with orthopedic implant revision when indicated, safe, and agreed upon by the surgeon and patient. However, as noted in the series by Zondervan et al,11 not every patient with confirmed metal allergy who undergoes revision improves, so an informed conversation between the patient and surgeon is mandatory.
Types of Orthopedic Implants
Orthopedic implanted materials consist of either dynamic (knees, hips) or static (screws, plates) components. Several generations of hip implants have evolved since the 1960s. First-generation implanted hips were metal-on-metal and had high rates of metal release and sensitization. Metal-on-plastic implants may be less likely to release metal but instead release large polyethylene wear particles. Second-generation metal-on-metal implants reportedly have lower wear rates. With these implants, wear particles are generated but are reportedly smaller than first-generation particles.13
Allergens in IHRs
Metals
Metals are the most commonly implicated allergens in orthopedic IHRs. Potentially relevant metal alloys include 316L stainless steel, cobalt-chromium-molybdenum steel, Vitallium alloy, titanium alloy, titanium-tantalum-niobium alloy, and Oxinium (Smith & Nephew).14,15 Each alloy contains several metals, which can include nickel, chromium, cobalt, manganese, molybdenum, iron, titanium, aluminum, vanadium, niobium, tantalum, and zirconium, among others. For example, 316L stainless steel contains iron, nickel, chromium, manganese, molybdenum, nitrogen, carbon, sulfur, silicon, and phosphorus, whereas Oxinium contains only oxidized zirconium and niobium.
Bone Cement
Bone cement also has been reported in cases of orthopedic IHRs and can contain several chemicals, including methyl methacrylate, N,N-dimethyl-p-toluidine, benzoyl peroxide, hydroquinone, and gentamicin.14 Other potential exposures include adhesives (cyanoacrylates) and topical antibiotics.
Clinical Presentation
Several clinical presentations of orthopedic IHRs have been described. Perhaps the most commonly recognized is a localized cutaneous eczematous eruption, with dermatitis typically overlying the site of the implanted material.1,2,16 Generalized cutaneous eczematous IHRs also have been reported, including diffuse generalized dermatitis from a stainless steel orthopedic screw4 and nummular dermatitis attributed to vanadium in an orthopedic plate.5 Urticaria, vasculitis, and bullous cutaneous reactions, as well as extracutaneous complications, also have been reported.14,15 Pain, edema, joint loosening or failure, and poor wound healing have been reported,12 but it remains unclear whether these symptoms represent IHR.
Patch Testing for IHR
Several groups have published recommended patch test series for IHR.12,14,15 Common components of implant patch testing panels include metals, adhesives (acrylates, epoxy resins) and antibiotics. Importantly, obtaining product information from the manufacturer of the suspected implant can guide which allergens to include in patch testing. Implant and metal panels also are available for commercial purchase.
Other Diagnostic Tests
We rarely (almost never) order LTTs in the workup for potential IHRs. This is an in vitro test that includes lymphocytes, metal ions, and the radioactive marker methyl-3H-thymidine. The goal of the test is to evaluate if patient lymphocytes are reactive or responsive to metal ions. A positive LTT suggests that lymphocytes can respond to the presence of metal ions but does not confirm allergy or the presence of IHR.
Typically, skin or tissue biopsies are not required to make a diagnosis of IHR; however, if performed, histopathology suggestive of IHR can support a suspected diagnosis. Typical findings include but are not limited to spongiotic dermatitis. Eosinophils may or may not be present. Metal disc testing has been utilized for orthopedic IHR but is not currently recommended due to low diagnostic yield. Prick testing rarely is used and also is not a primary method for diagnosis of IHR.17
Preimplantation Patch Testing
Expert opinion guidelines published by the American Contact Dermatitis Society (ACDS) state that routine preimplantation patch testing is not necessary; however, for those patients with a clear history of contact reactions to metal, preimplantation patch testing can be considered.17
Patch test results can influence the orthopedic surgeon’s choice of implant material. In one study, when preimplantation patch testing showed a positive patch test reaction to metals, the results influenced the surgeon’s decision-making in all cases.12
Postimplantation Patch Testing: Diagnostic Criteria for Metal IHR After Implantation
From 2012 to 2013, Schalock and Thyssen18 surveyed expert attendees at meetings of the European Society of Contact Dermatitis and the ACDS for their opinions on proposed diagnostic criteria for metal IHRs. Based on these results (N=119), the authors stratified 4 major and 5 minor diagnostic criteria, which were defined based on overall responses of meeting attendees. Major criteria included (1) chronic dermatitis beginning weeks to months after metallic implantation, (2) complete recovery after removal of the offending implant, (3) eruption overlying the metal implant, and (4) positive patch test reaction to a metal used in the implant. Minor criteria included (1) histology consistent with allergic contact dermatitis, (2) morphology consistent with dermatitis (ie, erythema, induration, papules, vesicles), (3) positive in vitro test to metals (eg, lymphocyte transformation test), (4) systemic allergic dermatitis reaction, and (5) therapy-resistant dermatitis reaction. The authors did not describe a scoring system for evaluation and confirmation of a diagnosis of IHR. Instead, the criteria should be used as general guidelines when evaluating patients for possible IHRs. From a standpoint of available diagnostic tests for metal IHR, 86.1% of experts agreed that a positive patch test reaction to a metal used in the implant was suggestive of a diagnosis, whereas a positive in vitro test to metals (LTT) was suggestive of a diagnosis for only 32.2% of respondents. This study was designed specifically for metal IHRs and therefore is not necessarily generalizable for nonmetal IHRs.18
Final Interpretation
We follow the 2016 ACDS guidelines17 and complete preimplantation patch testing only in the setting of suspected metal allergy and postimplantation patch testing based on the guidelines described by Schalock and Thyssen.18 However, an extended conversation is warranted prior to patch testing to ensure the patient fully understands the limitations of the test. Although we have both ordered the LTT, interpretation remains murky, and until this test is standardized, routine use is unlikely to benefit the patient. Until we are more reliably able to predict who will develop hypersensitivity to implanted metals, the decision to remove or revise an implant is one that should be made by a multidisciplinary team that includes the surgeon and the patient.
Hypersensitivity to metal implants remains a controversial field in contact dermatitis and patch testing. With positive reactions to nickel hovering around 20% in patch-tested populations,1 the question remains whether metal-allergic patients can safely receive metal implants. Unfortunately, large controlled studies are lacking, in part due to ethical concerns of knowingly placing a metal implant in a metal-allergic patient. Much of the focus of implant hypersensitivity reactions (IHRs) has been on orthopedic joints including hips, knees, and shoulders, as well as fixed orthopedic implanted materials such as screws and plates. However, there have been reports of IHRs to cardiac devices including defibrillators, pacemakers, and intracardiac devices; dental hardware including implants, crowns, dentures, and braces; and neurologic and gynecologic devices. For the purposes of this review, we will focus on IHRs to orthopedic implants.
Making the Case for IHRs
There are multiple case reports and series documenting likely orthopedic IHRs in the literature2-5; however, large prospective studies are lacking. Some of the largest series are from Danish registry studies. In 2009, Thyssen et al6 reviewed356 patients who had undergone both total hip arthroplasty and patch testing. Metal allergy frequencies were similar between patch-tested registry patients and patch test controls, showing no increase in positive patch tests to metals after receiving implants. Additionally, implant revision rates were comparable between registry patients with and without patch testing. The group concluded that the risk for revision after hip implantation in metal-allergic patients and the risk for development of metal allergy after implantation were both low.6 In 2015, Münch et al7 compared 327 patients who had undergone both total knee arthroplasty and patch testing and found that prevalence of allergy to nickel, cobalt, and chromium was similar between patients who had undergone revision surgery and those who had not; however, for patients who had 2 or more knee revisions, there was a higher prevalence of postimplant metal allergy. This study also showed that metal allergy identified before implantation did not increase the risk for postimplantation knee revision surgery or implant failure.7 These larger studies suggest that although individual cases of IHR exist, it is likely quite rare.
Patients have been found to have increased levels of chromium (serum and urine) and titanium (serum) following total hip arthroplasty.8 Additionally, metal wear particles have been identified in postmortem livers and spleens, which was more prevalent in patients with a history of failed hip arthroplasty.9 It is difficult to determine the meaning of this data, as the presence of metal ions does not necessarily indicate allergy or IHR. In 2001, Hallab et al10 pooled data from several implant cohort studies and concluded that in comparison to a baseline metal sensitivity prevalence of approximately 10%, patients with well-functioning implants had a metal sensitivity–weighted average of 25%, and those with poorly functioning implants had a weighted average of 60%. Again, positive patch testing to metals does not necessarily implicate allergy as the cause of implant failure.
Some small studies have shown that patients with evidence of metal hypersensitivity improve with revision. Zondervan et al11 reviewed results of 46 orthopedic revisions following painful total knee arthroplasty. Patients with knee pain and lymphocyte transformation testing (LTT) positive for metals received hypoallergenic revisions, and those with LTT negative for metals received standard revisions. The group who received hypoallergenic revisions had more pain reduction compared to the standard revision group (37.8% reduction in pain vs 27%). However, this study was limited in that the diagnosis of metal allergy was made entirely on results of LTT.11 In 2012, Atanaskova Mesinkovska et al12 described 41 patients who underwent orthopedic patch testing following implantation for symptoms including pain, dermatitis, pruritus, joint loosening, edema, and impaired wound healing. Fifteen (37%) patients had positive patch test reactions to metals, and 10 (67%) of them had reactions to metals that were present in their implants. Six (60%) of these patients had their implants removed and their symptoms resolved; the remaining 4 continued to experience implant symptoms.12 These studies support the existence of rare metal-related orthopedic IHRs and support the concept of proceeding with orthopedic implant revision when indicated, safe, and agreed upon by the surgeon and patient. However, as noted in the series by Zondervan et al,11 not every patient with confirmed metal allergy who undergoes revision improves, so an informed conversation between the patient and surgeon is mandatory.
Types of Orthopedic Implants
Orthopedic implanted materials consist of either dynamic (knees, hips) or static (screws, plates) components. Several generations of hip implants have evolved since the 1960s. First-generation implanted hips were metal-on-metal and had high rates of metal release and sensitization. Metal-on-plastic implants may be less likely to release metal but instead release large polyethylene wear particles. Second-generation metal-on-metal implants reportedly have lower wear rates. With these implants, wear particles are generated but are reportedly smaller than first-generation particles.13
Allergens in IHRs
Metals
Metals are the most commonly implicated allergens in orthopedic IHRs. Potentially relevant metal alloys include 316L stainless steel, cobalt-chromium-molybdenum steel, Vitallium alloy, titanium alloy, titanium-tantalum-niobium alloy, and Oxinium (Smith & Nephew).14,15 Each alloy contains several metals, which can include nickel, chromium, cobalt, manganese, molybdenum, iron, titanium, aluminum, vanadium, niobium, tantalum, and zirconium, among others. For example, 316L stainless steel contains iron, nickel, chromium, manganese, molybdenum, nitrogen, carbon, sulfur, silicon, and phosphorus, whereas Oxinium contains only oxidized zirconium and niobium.
Bone Cement
Bone cement also has been reported in cases of orthopedic IHRs and can contain several chemicals, including methyl methacrylate, N,N-dimethyl-p-toluidine, benzoyl peroxide, hydroquinone, and gentamicin.14 Other potential exposures include adhesives (cyanoacrylates) and topical antibiotics.
Clinical Presentation
Several clinical presentations of orthopedic IHRs have been described. Perhaps the most commonly recognized is a localized cutaneous eczematous eruption, with dermatitis typically overlying the site of the implanted material.1,2,16 Generalized cutaneous eczematous IHRs also have been reported, including diffuse generalized dermatitis from a stainless steel orthopedic screw4 and nummular dermatitis attributed to vanadium in an orthopedic plate.5 Urticaria, vasculitis, and bullous cutaneous reactions, as well as extracutaneous complications, also have been reported.14,15 Pain, edema, joint loosening or failure, and poor wound healing have been reported,12 but it remains unclear whether these symptoms represent IHR.
Patch Testing for IHR
Several groups have published recommended patch test series for IHR.12,14,15 Common components of implant patch testing panels include metals, adhesives (acrylates, epoxy resins) and antibiotics. Importantly, obtaining product information from the manufacturer of the suspected implant can guide which allergens to include in patch testing. Implant and metal panels also are available for commercial purchase.
Other Diagnostic Tests
We rarely (almost never) order LTTs in the workup for potential IHRs. This is an in vitro test that includes lymphocytes, metal ions, and the radioactive marker methyl-3H-thymidine. The goal of the test is to evaluate if patient lymphocytes are reactive or responsive to metal ions. A positive LTT suggests that lymphocytes can respond to the presence of metal ions but does not confirm allergy or the presence of IHR.
Typically, skin or tissue biopsies are not required to make a diagnosis of IHR; however, if performed, histopathology suggestive of IHR can support a suspected diagnosis. Typical findings include but are not limited to spongiotic dermatitis. Eosinophils may or may not be present. Metal disc testing has been utilized for orthopedic IHR but is not currently recommended due to low diagnostic yield. Prick testing rarely is used and also is not a primary method for diagnosis of IHR.17
Preimplantation Patch Testing
Expert opinion guidelines published by the American Contact Dermatitis Society (ACDS) state that routine preimplantation patch testing is not necessary; however, for those patients with a clear history of contact reactions to metal, preimplantation patch testing can be considered.17
Patch test results can influence the orthopedic surgeon’s choice of implant material. In one study, when preimplantation patch testing showed a positive patch test reaction to metals, the results influenced the surgeon’s decision-making in all cases.12
Postimplantation Patch Testing: Diagnostic Criteria for Metal IHR After Implantation
From 2012 to 2013, Schalock and Thyssen18 surveyed expert attendees at meetings of the European Society of Contact Dermatitis and the ACDS for their opinions on proposed diagnostic criteria for metal IHRs. Based on these results (N=119), the authors stratified 4 major and 5 minor diagnostic criteria, which were defined based on overall responses of meeting attendees. Major criteria included (1) chronic dermatitis beginning weeks to months after metallic implantation, (2) complete recovery after removal of the offending implant, (3) eruption overlying the metal implant, and (4) positive patch test reaction to a metal used in the implant. Minor criteria included (1) histology consistent with allergic contact dermatitis, (2) morphology consistent with dermatitis (ie, erythema, induration, papules, vesicles), (3) positive in vitro test to metals (eg, lymphocyte transformation test), (4) systemic allergic dermatitis reaction, and (5) therapy-resistant dermatitis reaction. The authors did not describe a scoring system for evaluation and confirmation of a diagnosis of IHR. Instead, the criteria should be used as general guidelines when evaluating patients for possible IHRs. From a standpoint of available diagnostic tests for metal IHR, 86.1% of experts agreed that a positive patch test reaction to a metal used in the implant was suggestive of a diagnosis, whereas a positive in vitro test to metals (LTT) was suggestive of a diagnosis for only 32.2% of respondents. This study was designed specifically for metal IHRs and therefore is not necessarily generalizable for nonmetal IHRs.18
Final Interpretation
We follow the 2016 ACDS guidelines17 and complete preimplantation patch testing only in the setting of suspected metal allergy and postimplantation patch testing based on the guidelines described by Schalock and Thyssen.18 However, an extended conversation is warranted prior to patch testing to ensure the patient fully understands the limitations of the test. Although we have both ordered the LTT, interpretation remains murky, and until this test is standardized, routine use is unlikely to benefit the patient. Until we are more reliably able to predict who will develop hypersensitivity to implanted metals, the decision to remove or revise an implant is one that should be made by a multidisciplinary team that includes the surgeon and the patient.
- Dekoven JG, Warshaw EM, Zug KA, et al. North American Contact Dermatitis Group patch test results: 2015-2016. Dermatitis. 2018;29:297-309.
- Gao X, He RX, Yan SG, et al. Dermatitis associated with chromium following total knee arthroplasty. J Arthroplasty. 2011;26:665.E613-665.E616.
- Treudler R, Simon JC. Benzoyl peroxide: is it a relevant bone cement allergen in patients with orthopaedic implants? Contact Dermatitis. 2007;57:177-180.
- Barranco VP, Soloman H. Eczematous dermatitis from nickel. JAMA. 1972;220:1244.
- Engelhart S, Segal RJ. Allergic reaction to vanadium causes a diffuse eczematous eruption and titanium alloy orthopedic implant failure. Cutis. 2017;99:245-249.
- Thyssen JP, Jakobsen SS, Engkilde K, et al. The association between metal allergy, total hip arthroplasty, and revision. Acta Orthop. 2009;80:646-652.
- Münch HJ, Jacobsen SS, Olesen JT, et al. The association between metal allergy, total knee arthroplasty, and revision: study based on the Danish Knee Arthroplasty Register. Acta Orthop. 2015;86:378-383.
- Jacobs JJ, Skipor AK, Patterson LM, et al. Metal release in patients who have had a primary total hip arthroplasty. a prospective, controlled, longitudinal study. J Bone Joint Surg Am. 1998;80:1447-1458.
- Urban RM, Jacobs JJ, Tomlinson MJ, et al. Dissemination of wear particles to the liver, spleen, and abdominal lymph nodes of patients with hip or knee replacement. J Bone Joint Surg Am. 2000;82:457-476.
- Hallab N, Merritt K, Jacobs JJ. Metal sensitivity in patients with orthopaedic implants. J Bone Joint Surg Am. 2001;83:428-436.
- Zondervan RL, Vaux JJ, Blackmer MJ, et al. Improved outcomes in patients with positive metal sensitivity following revision total knee arthroplasty. J Orthop Surg Res. 2019;14:182.
- Atanaskova Mesinkovska N, Tellez A, Molina L, et al. The effect of patch testing on surgical practices and outcomes in orthopedic patients with metal implants. Arch Dermatol. 2012;148:687-693.
- Kovochich M, Fung ES, Donovan E, et al. Characterization of wear debris from metal-on-metal hip implants during normal wear versus edge-loading conditions. J Biomed Mater Res B Appl Biomater. 2018;106:986-996.
- Basko-Plluska JL, Thyssen JP, Schalock PC. Cutaneous and systemic hypersensitivity reactions to metallic implants. Dermatitis. 2011;22:65-79.
- Schalock PC, Menné T, Johansen JD, et al. Hypersensitivity reactions to metallic implants—diagnostic algorithm and suggested patch test series for clinical use. Contact Dermatitis. 2012;66:4-19.
- Thomas P, Gollwitzer H, Maier S, et al. Osteosynthesis associated contact dermatitis with unusual perpetuation of hyperreactivity in a nickel allergic patient. Contact Dermatitis. 2006;54:222-225.
- Schalock PC, Crawford G, Nedorost S, et al. Patch testing for evaluation of hypersensitivity to implanted metal devices: a perspective from the American Contact Dermatitis Society. Dermatitis. 2016;27:241-247.
- Schalock PC, Thyssen JP. Patch testers’ opinions regarding diagnostic criteria for metal hypersensitivity reactions to metallic implants. Dermatitis. 2013;24:183-185.
- Dekoven JG, Warshaw EM, Zug KA, et al. North American Contact Dermatitis Group patch test results: 2015-2016. Dermatitis. 2018;29:297-309.
- Gao X, He RX, Yan SG, et al. Dermatitis associated with chromium following total knee arthroplasty. J Arthroplasty. 2011;26:665.E613-665.E616.
- Treudler R, Simon JC. Benzoyl peroxide: is it a relevant bone cement allergen in patients with orthopaedic implants? Contact Dermatitis. 2007;57:177-180.
- Barranco VP, Soloman H. Eczematous dermatitis from nickel. JAMA. 1972;220:1244.
- Engelhart S, Segal RJ. Allergic reaction to vanadium causes a diffuse eczematous eruption and titanium alloy orthopedic implant failure. Cutis. 2017;99:245-249.
- Thyssen JP, Jakobsen SS, Engkilde K, et al. The association between metal allergy, total hip arthroplasty, and revision. Acta Orthop. 2009;80:646-652.
- Münch HJ, Jacobsen SS, Olesen JT, et al. The association between metal allergy, total knee arthroplasty, and revision: study based on the Danish Knee Arthroplasty Register. Acta Orthop. 2015;86:378-383.
- Jacobs JJ, Skipor AK, Patterson LM, et al. Metal release in patients who have had a primary total hip arthroplasty. a prospective, controlled, longitudinal study. J Bone Joint Surg Am. 1998;80:1447-1458.
- Urban RM, Jacobs JJ, Tomlinson MJ, et al. Dissemination of wear particles to the liver, spleen, and abdominal lymph nodes of patients with hip or knee replacement. J Bone Joint Surg Am. 2000;82:457-476.
- Hallab N, Merritt K, Jacobs JJ. Metal sensitivity in patients with orthopaedic implants. J Bone Joint Surg Am. 2001;83:428-436.
- Zondervan RL, Vaux JJ, Blackmer MJ, et al. Improved outcomes in patients with positive metal sensitivity following revision total knee arthroplasty. J Orthop Surg Res. 2019;14:182.
- Atanaskova Mesinkovska N, Tellez A, Molina L, et al. The effect of patch testing on surgical practices and outcomes in orthopedic patients with metal implants. Arch Dermatol. 2012;148:687-693.
- Kovochich M, Fung ES, Donovan E, et al. Characterization of wear debris from metal-on-metal hip implants during normal wear versus edge-loading conditions. J Biomed Mater Res B Appl Biomater. 2018;106:986-996.
- Basko-Plluska JL, Thyssen JP, Schalock PC. Cutaneous and systemic hypersensitivity reactions to metallic implants. Dermatitis. 2011;22:65-79.
- Schalock PC, Menné T, Johansen JD, et al. Hypersensitivity reactions to metallic implants—diagnostic algorithm and suggested patch test series for clinical use. Contact Dermatitis. 2012;66:4-19.
- Thomas P, Gollwitzer H, Maier S, et al. Osteosynthesis associated contact dermatitis with unusual perpetuation of hyperreactivity in a nickel allergic patient. Contact Dermatitis. 2006;54:222-225.
- Schalock PC, Crawford G, Nedorost S, et al. Patch testing for evaluation of hypersensitivity to implanted metal devices: a perspective from the American Contact Dermatitis Society. Dermatitis. 2016;27:241-247.
- Schalock PC, Thyssen JP. Patch testers’ opinions regarding diagnostic criteria for metal hypersensitivity reactions to metallic implants. Dermatitis. 2013;24:183-185.
Practice Points
- Common clinical presentations of orthopedic implant hypersensitivity reactions include localized cutaneous eruptions, generalized cutaneous eruptions, and noncutaneous reactions.
- Allergens implicated in orthopedic implant hypersensitivity reactions include metals and bone cement components.
- Routine preimplant patch testing for orthopedic hypersensitivity reactions is not recommended but can be performed when there is strong concern for metal allergy.
- Postimplant patch testing should be performed when symptoms are consistent with potential orthopedic implant hypersensitivity reactions.
Systemic Contact Dermatitis: Sometimes It Is the Food
One of the perils of patch testing is fielding questions about which type of allergens will be used. Patients often ask if the patch test includes milk, foods, dander, mold, pets, and grass. Most patch testers spend a substantial amount of time explaining that the purpose of patch testing is to detect applied chemical allergens: It’s not what you eat; rather, it’s what touches your skin. However, the big caveat is that some oral, parenteral, inhaled, and even cutaneous allergens can produce systemic contact dermatitis (SCD), which represents a unique clinical scenario that we will review in this month’s Final Interpretation column.
There are many patterns of SCD. Familiarity with potential clinical presentations can aid in diagnosis and counseling. Systemic contact dermatitis tends to be symmetrical. Dyshidrotic hand dermatitis is a reported pattern for systemic metal allergy, most commonly nickel. Refractory eyelid or genital dermatitis can reflect a systemic exposure, particularly if the dermatitis is in areas not caused by direct skin contact with the allergen. Systemic drug-related intertriginous and flexural exanthema is, as the name describes, an eruption involving axillae, genital skin, and flexural sites. It usually is a type of drug reaction, but the culprit can be an ingested allergen. So-called baboon syndrome SCD can cause persistent genital and intertriginous dermatitis. Other clues to SCD include dermatitis flare at the patch test site and erythema multiforme. Some patients also describe systemic symptoms, including headache, fatigue, and malaise.
Rhus Dermatitis
Poison ivy is the most common cause of acute contact dermatitis but also can be a cause of SCD. From the family Anacardiaceae, this sneaky plant is common in many parts of the United States; most allergic patients are familiar with their allergy from prior exposure.
In 1982, 54 Little League baseball attendees developed diffuse vesicular dermatitis involving the flexures after ingesting packaged cashews contaminated with cashew shells.1 In the same family as poison ivy, the cashew nut tree (Anacardium occidentale) produces a cashew apple containing the cashew nut. The cashew shell is the site that contains the allergenic oils. Typically, cashews are processed to remove the shell and oil prior to consumption. Ingestion of raw cashews is more likely to lead to SCD than roasted cashews because the heat in the roasting process can break down any allergenic oil.2
Metals
Systemic exposures to nickel usually are dietary. Clinically, SCD from nickel most commonly presents as refractory dyshidrotic hand eczema or papular elbow dermatitis.3 Nickel is commonly found in vitamins and supplements as well as certain whole grains, vegetables, beans, coffee, chocolate, and tea.4 Sometimes, cookware also can be a source of nickel exposure, particularly with steel cookware, from which nickel can leach into food.
In general, a diet lower than 150 μg/d is needed to prevent flares.5 A point-based diet is available for nickel-allergic patients.5 Patients should ingest a restricted amount of nickel (15 points daily); those who are extremely allergic might need to limit nickel ingestion to less than 5 points daily. Because of the challenges associated with maintaining a low-nickel diet, chelation therapy has been recommended to prevent nickel absorption. Disulfiram3 and ascorbic acid5 have been recommended, but larger studies are lacking.
Cobalt and chromium are other metals that, when ingested, can lead to SCD; both can be found in multivitamins. Other sources of dietary chromium include vegetables, coffee, beans, certain meats, and seafood.4 For cobalt, the dietary exposures are similar with the addition of nuts, apricots, and whole-grain flour. A point-based cobalt avoidance diet has been published. This diet recommends less than 12 μg of cobalt daily; patients can ingest up to 12 cobalt points daily.6
Likewise, gold has been reported to cause SCD, with one case attributed to gold in a homeopathic cardiac medication.7 Gold SCD also should be considered in the setting of ingested gold-containing alcoholic beverages and historically has been associated with intramuscular gold sodium thiomalate for the treatment of rheumatoid arthritis.8
Metal implants, including prosthetic joints, stents, and other devices, have been implicated in SCD. (More to come on this topic soon; yes, dear reader, that is a teaser!)
Fragrances
Balsam of Peru
Secreted by the tree Myroxylon balsamum var pereirae, balsam of Peru (BOP) contains several potential allergens, including cinnamon oils (eg, eugenol, vanillin, cinnamates), coniferin derivatives, and benzoic acid derivatives.9 Foods and beverages associated with BOP include citrus, pickled vegetables, chocolate, ice cream, chili, pizza, tomatoes, wine, beer, gin, vermouth, flavored tea, and soft drinks.10 Flavoring agents, spices (eg, cloves, curry, vanilla, cinnamon, allspice, ginger, anise), and condiments (eg, ketchup, barbeque sauce) are potential sources, as are cough medicines, lozenges, and flavored tobacco.
Salam and Fowler10 described BOP-allergic patients whose condition improved with dietary restriction of BOP. Avoidance of tomatoes, citrus, spices, and cola most commonly contributed to improvement.10 Scheman et al9 proposed BOP subgroups, including the eugenol, vanillin, cinnamate, benzoate, ferulic acid, and coniferin groups. Targeted patch testing can identify relevant subgroups, and patients can focus dietary restrictions by subgroup.
Plants
Systemic contact dermatitis has been reported in association with a number of plants and herbals, including chamomile in tea,11 goldenrod in a medicated extract,12Hosta plantaginea roots,13 and garlic extract for hyperlipidemia.14 Many more have been described.
Propolis
Also known as bee glue, propolis comprises a mixture of balsams, resins, waxes, essential oils, pollen, cinnamic alcohol, and vitamins. It can be found in many cosmetic products, foods, and chewing gum.15 Propolis has been reported to be the source of SCD from ingestion of propolis capsules, which have been used to promote immune stimulation,15 and propolis solution as a natural tonic.16
Propylene Glycol
Propylene glycol (PG) can be found in (believe it or not) foods and medications. In foods, it typically is used for its softening, humectant, and preservative properties.17 Common food sources of PG include sauces, desserts, snack foods, and salad dressings.
Many topical prescription medications, including corticosteroids and newer nonsteroidal anti-inflammatory topicals, might contain PG; providers must specifically request PG-free products for PG-allergic patients. A detailed PG-avoidance diet lists products to avoid and products that are PG free.18
Preservatives
Sulfites
These compounds are preservatives found in cosmetics, hair dyes, and certain foods. Systemic contact dermatitis caused by sulfites in food has been described in numerous patients. One unfortunate vacationer developed axillary and groin dermatitis after ingesting large amounts of grapes, wine, shrimp, and french fries while vacationing in Italy.19 Among dietary sources, beer and wine contain higher levels of sulfites. Sulfites also can be found in some pickled foods; bottled citrus juice; dried fruits; and commercial prepared foods, such as powdered potatoes and gravy mixes. Other reports of SCD from sulfites include an enema preparation20 and anesthetics21 as the source of the allergen.
Formaldehyde
Formaldehyde can cause SCD after ingestion of aspartame, which is hydrolyzed to phenylalanine, aspartic acid, and aspartic acid methyl ester in the intestine.22 The methyl ester is converted to methyl alcohol, which is transported to the liver and oxidized to formaldehyde, which is then converted to formic acid. Hill and Belsito22 reported a case of SCD presenting as eyelid dermatitis after ingestion of an aspartame-based artificial sweetener. A similar case of eyelid, neck, and leg dermatitis was reported after ingestion of drinks and candy sweetened with aspartame.23
Parabens
Although parabens are rare contact sensitizers, there are a few reports of paraben SCD. Cases include a predominantly flexural pattern from ingestion of a mucolytic-containing methylparaben,24 a generalized eczematous eruption after intramuscular injection of ampicillin preserved with methylparaben and propylparaben,25 and diffuse dermatitis from methylparaben in a local anesthetic.26
Sorbic Acid
Sorbic acid is utilized as a preservative in foods and occurs naturally in red fruit, such as strawberries and cranberries.27 It is a rare allergen, but several cases of sorbic acid SCD have been reported, including perianal and buttock dermatitis,27 hand dermatitis in an infant,28 and hand-and-foot dermatitis in a storekeeper.29
Carmine
Carmine, or cochineal extract, is a red dye derived from dried pulverized scale insects of the family Coccidae. This chemical can be used in a multitude of foods and medications, including candies, yogurt, red velvet items, popsicles, food coloring, frozen meat and fish, ice cream, syrups, ketchup, sausage, donuts, cake pops, applesauce, canned fruits, soups, and drinks.30 Machler and Jacob31 described a child with recurrent episodes of erythroderma and periorbital edema in whom patch testing revealed a reaction to carmine. The patient’s mother connected the flares with ingestion of red velvet cupcakes.31 Ferris et al32 reported a likely case of SCD attributed to carmine in a multivitamin.
Steroids
Ingested and injected corticosteroids have been associated with SCD, which is illustrated by a case of a generalized cutaneous eruption several days after joint injection with triamcinolone acetonide.33 In another report, a patient developed an eruption in the body folds, later generalized, after topical application of a corticosteroid, first in ear drops and later in nasal spray.34 Traditional corticosteroid classification systems might be less reliable in predicting relevant allergens in corticosteroid SCD; comprehensive testing, including oral challenge, might be necessary to identify alternatives.33
Ethylenediamine
Ethylenediamine is an uncommon allergen in patch test populations. It is present in aminophylline35 and is utilized in the production of hydroxyzine36 and other piperazine-derived medications, such as cetirizine, levocetirizine, meclizine, and olanzapine. Several cases of SCD caused by aminophylline,35 cetirizine,36 and hydroxyzine37 have been reported, all in the setting of a positive patch test reaction to ethylenediamine.
When to Counsel About Systemic Exposures
In general, we usually do not counsel on systemic exposures to allergens at the final patch test reading unless the pattern of dermatitis or clinical history strongly suggests systemic exposure. In most cases, we find that counseling on topical allergen avoidance alone is sufficient to treat allergic contact dermatitis. Because of the restrictive nature of allergen-avoidance diets, counseling all patients on the potential for SCD might cause undue stress without much benefit. However, if a patient experiences persistent dermatitis on follow-up with topical avoidance alone, we often will delve into systemic exposures and counsel on further avoidance strategies, including medication and diet.
Final Interpretation
A multitude of chemicals have been reported as the source of SCD; these exposures can occur through ingestion, injection, and inhaled and cutaneous routes. Chemicals present in foods, medications, and beverages have been implicated. Systemic contact dermatitis is rare and should be considered when traditional avoidance of contact allergens is unsuccessful and the clinical pattern is consistent with SCD.
- Marks JG, DeMelfi T, McCarthy MA, et al. Dermatitis from cashew nuts. J Am Acad Dermatol. 1984;10:627-631.
- Hamilton TK, Zug KA. Systemic contact dermatitis to raw cashew nuts in a pesto sauce. Am J Contact Dermat. 1998;9:51-54.
- Fabbro SK, Zirwas MJ. Systemic contact dermatitis to foods: nickel, BOP, and more. Curr Allergy Asthma Rep. 2014;14:463.
- American Contact Dermatitis Society. Contact Allergy Management Program (CAMP). https://www.contactderm.org/resources/acds-camp. Accessed October 23, 2019.
- Mislankar M, Zirwas MJ. Low-nickel diet scoring system for systemic nickel allergy. Dermatitis. 2013;24:190-195.
- Stuckert J, Nedorost S. Low-cobalt diet for dyshidrotic eczema patients. Contact Dermatitis. 2008;59:361-365.
- Malinauskiene L, Isaksson M, Bruze M. Systemic contact dermatitis in a gold-allergic patient after treatment with an oral homeopathic drug. J Am Acad Dermatol. 2013;68:e58.
- Wicks IP, Wong D, McCullagh RB, et al. Contact allergy to gold after systemic administration of gold for rheumatoid arthritis. Ann Rheum Dis. 1988;47:421-422.
- Scheman A, Rakowski EM, Chou V, et al. Balsam of Peru: past and future. Dermatitis. 2013;24:153-160.
- Salam TN, Fowler JF Jr. Balsam-related systemic contact dermatitis. J Am Acad Dermatol. 2001;45:377-381.
- Rodríguez-Serna M, Sánchez-Motilla JM, Ramón R, et al. Allergic and systemic contact dermatitis from Matricaria chamomilla tea. Contact Dermatitis. 1998;39:192-193.
- Schätzle M, Agathos M, Breit R. Allergic contact dermatitis from goldenrod (Herba solidaginis) after systemic administration. Contact Dermatitis. 1998;39:271-272.
- Yun SJ, Lee JY, Kim GH, et al. Systemic contact dermatitis induced by roots of Hosta plantaginea. J Eur Acad Dermatol Venereol. 2018;32:e28-e29.
- Burden AD, Wilkinson SM, Beck MH, et al. Garlic-induced systemic contact dermatitis. Contact Dermatitis. 1994;30:299-300.
- Komericki P, Kränke B. Maculopapular exanthem from propolis: case report and review of systemic cutaneous and non-cutaneous reactions. Contact Dermatitis. 2009;61:353-355.
- Cho E, Lee JD, Cho SH. Systemic contact dermatitis from propolis ingestion. Ann Dermatol. 2011;23:85-88.
- Lowther A, McCormick T, Nedorost S. Systemic contact dermatitis from propylene glycol. Dermatitis. 2008;19:105-108.
- Scheman A, Cha C, Jacob SE, et al. Food avoidance diets for systemic, lip, and oral contact allergy: an American Contact Alternatives Group article. Dermatitis. 2012;23:248-257.
- Cussans A, McFadden J, Ostlere L. Systemic sodium metabisulfite allergy. Contact Dermatitis. 2015;73:316-317.
- Borges AS, Valejo Coelho MM, Fernandes C, et al. Systemic allergic dermatitis caused by sodium metabisulfite in rectal enemas. Contact Dermatitis. 2018;78:429-430.
- Guha-Niyogi B, Sabroe R, Holden C. An unusual case of a systemic delayed hypersensitivity reaction to sodium metabisulfite. Contact Dermatitis. 2018;79:246-247.
- Hill AM, Belsito DV. Systemic contact dermatitis of the eyelids caused by formaldehyde derived from aspartame? Contact Dermatitis. 2003;49:258-259.
- Veien NK, Lomholt HB. Systemic allergic dermatitis presumably caused by formaldehyde derived from aspartame. Contact Dermatitis. 2012;67:315-316.
- Sánchez-Pérez J, Diez MB, Pérez AA, et al. Allergic and systemic contact dermatitis to methylparaben. Contact Dermatitis. 2006;54:117-118.
- Carradori S, Peluso AM, Faccioli M. Systemic contact dermatitis due to parabens. Contact Dermatitis. 1990;22:238-239.
- Aeling JL, Nuss DD. Systemic eczematous “contact-type” dermatitis medicamentosa caused by parabens. Arch Dermatol. 1974;110:640.
- Giordano-Labadie F, Pech-Ormieres C, Bazex J. Systemic contact dermatitis from sorbic acid. Contact Dermatitis. 1996;34:61-62.
- Raison-Peyron N, Meynadier JM, Meynadier J. Sorbic acid: an unusual cause of systemic contact dermatitis in an infant. Contact Dermatitis. 2000;43:247-248.
- Dejobert Y, Delaporte E, Piette F, et al. Vesicular eczema and systemic contact dermatitis from sorbic acid. Contact Dermatitis. 2001;45:291.
- Rundle CW, Jacob SE, Machler BC. Contact dermatitis to carmine. Dermatitis. 2018;29:244-249.
- Machler BC, Jacob SE. Carmine red: a potentially overlooked allergen in children. Dermatitis. 2018;29:92-93.
- Ferris GJ, Wat M, Nedorost S. Multifactorial dermatitis with probable systemic contact dermatitis to carmine. Dermatitis. 2017;28:293-294.
- Santos-Alarcón S, Benavente-Villegas FC, Farzanegan-Miñano R, et al. Delayed hypersensitivity to topical and systemic corticosteroids. Contact Dermatitis. 2018;78:86-88.
- Faber MA, Sabato V, Ebo DG, et al. Systemic allergic dermatitis caused by prednisone derivatives in nose and ear drops. Contact Dermatitis. 2015;73:317-320.
- Isaksson M, Ljunggren B. Systemic contact dermatitis from ethylenediamine in an aminophylline preparation presenting as the baboon syndrome. Acta Derm Venereol. 2003;83:69-70.
- Cusano F, Ferrara G, Crisman G, et al. Clinicopathologic features of systemic contact dermatitis from ethylenediamine in cetirizine and levocetirizine. Dermatology. 2006;213:353-355.
- Ash S, Scheman AJ. Systemic contact dermatitis to hydroxyzine. Am J Contact Dermat. 1997;8:2-5.
One of the perils of patch testing is fielding questions about which type of allergens will be used. Patients often ask if the patch test includes milk, foods, dander, mold, pets, and grass. Most patch testers spend a substantial amount of time explaining that the purpose of patch testing is to detect applied chemical allergens: It’s not what you eat; rather, it’s what touches your skin. However, the big caveat is that some oral, parenteral, inhaled, and even cutaneous allergens can produce systemic contact dermatitis (SCD), which represents a unique clinical scenario that we will review in this month’s Final Interpretation column.
There are many patterns of SCD. Familiarity with potential clinical presentations can aid in diagnosis and counseling. Systemic contact dermatitis tends to be symmetrical. Dyshidrotic hand dermatitis is a reported pattern for systemic metal allergy, most commonly nickel. Refractory eyelid or genital dermatitis can reflect a systemic exposure, particularly if the dermatitis is in areas not caused by direct skin contact with the allergen. Systemic drug-related intertriginous and flexural exanthema is, as the name describes, an eruption involving axillae, genital skin, and flexural sites. It usually is a type of drug reaction, but the culprit can be an ingested allergen. So-called baboon syndrome SCD can cause persistent genital and intertriginous dermatitis. Other clues to SCD include dermatitis flare at the patch test site and erythema multiforme. Some patients also describe systemic symptoms, including headache, fatigue, and malaise.
Rhus Dermatitis
Poison ivy is the most common cause of acute contact dermatitis but also can be a cause of SCD. From the family Anacardiaceae, this sneaky plant is common in many parts of the United States; most allergic patients are familiar with their allergy from prior exposure.
In 1982, 54 Little League baseball attendees developed diffuse vesicular dermatitis involving the flexures after ingesting packaged cashews contaminated with cashew shells.1 In the same family as poison ivy, the cashew nut tree (Anacardium occidentale) produces a cashew apple containing the cashew nut. The cashew shell is the site that contains the allergenic oils. Typically, cashews are processed to remove the shell and oil prior to consumption. Ingestion of raw cashews is more likely to lead to SCD than roasted cashews because the heat in the roasting process can break down any allergenic oil.2
Metals
Systemic exposures to nickel usually are dietary. Clinically, SCD from nickel most commonly presents as refractory dyshidrotic hand eczema or papular elbow dermatitis.3 Nickel is commonly found in vitamins and supplements as well as certain whole grains, vegetables, beans, coffee, chocolate, and tea.4 Sometimes, cookware also can be a source of nickel exposure, particularly with steel cookware, from which nickel can leach into food.
In general, a diet lower than 150 μg/d is needed to prevent flares.5 A point-based diet is available for nickel-allergic patients.5 Patients should ingest a restricted amount of nickel (15 points daily); those who are extremely allergic might need to limit nickel ingestion to less than 5 points daily. Because of the challenges associated with maintaining a low-nickel diet, chelation therapy has been recommended to prevent nickel absorption. Disulfiram3 and ascorbic acid5 have been recommended, but larger studies are lacking.
Cobalt and chromium are other metals that, when ingested, can lead to SCD; both can be found in multivitamins. Other sources of dietary chromium include vegetables, coffee, beans, certain meats, and seafood.4 For cobalt, the dietary exposures are similar with the addition of nuts, apricots, and whole-grain flour. A point-based cobalt avoidance diet has been published. This diet recommends less than 12 μg of cobalt daily; patients can ingest up to 12 cobalt points daily.6
Likewise, gold has been reported to cause SCD, with one case attributed to gold in a homeopathic cardiac medication.7 Gold SCD also should be considered in the setting of ingested gold-containing alcoholic beverages and historically has been associated with intramuscular gold sodium thiomalate for the treatment of rheumatoid arthritis.8
Metal implants, including prosthetic joints, stents, and other devices, have been implicated in SCD. (More to come on this topic soon; yes, dear reader, that is a teaser!)
Fragrances
Balsam of Peru
Secreted by the tree Myroxylon balsamum var pereirae, balsam of Peru (BOP) contains several potential allergens, including cinnamon oils (eg, eugenol, vanillin, cinnamates), coniferin derivatives, and benzoic acid derivatives.9 Foods and beverages associated with BOP include citrus, pickled vegetables, chocolate, ice cream, chili, pizza, tomatoes, wine, beer, gin, vermouth, flavored tea, and soft drinks.10 Flavoring agents, spices (eg, cloves, curry, vanilla, cinnamon, allspice, ginger, anise), and condiments (eg, ketchup, barbeque sauce) are potential sources, as are cough medicines, lozenges, and flavored tobacco.
Salam and Fowler10 described BOP-allergic patients whose condition improved with dietary restriction of BOP. Avoidance of tomatoes, citrus, spices, and cola most commonly contributed to improvement.10 Scheman et al9 proposed BOP subgroups, including the eugenol, vanillin, cinnamate, benzoate, ferulic acid, and coniferin groups. Targeted patch testing can identify relevant subgroups, and patients can focus dietary restrictions by subgroup.
Plants
Systemic contact dermatitis has been reported in association with a number of plants and herbals, including chamomile in tea,11 goldenrod in a medicated extract,12Hosta plantaginea roots,13 and garlic extract for hyperlipidemia.14 Many more have been described.
Propolis
Also known as bee glue, propolis comprises a mixture of balsams, resins, waxes, essential oils, pollen, cinnamic alcohol, and vitamins. It can be found in many cosmetic products, foods, and chewing gum.15 Propolis has been reported to be the source of SCD from ingestion of propolis capsules, which have been used to promote immune stimulation,15 and propolis solution as a natural tonic.16
Propylene Glycol
Propylene glycol (PG) can be found in (believe it or not) foods and medications. In foods, it typically is used for its softening, humectant, and preservative properties.17 Common food sources of PG include sauces, desserts, snack foods, and salad dressings.
Many topical prescription medications, including corticosteroids and newer nonsteroidal anti-inflammatory topicals, might contain PG; providers must specifically request PG-free products for PG-allergic patients. A detailed PG-avoidance diet lists products to avoid and products that are PG free.18
Preservatives
Sulfites
These compounds are preservatives found in cosmetics, hair dyes, and certain foods. Systemic contact dermatitis caused by sulfites in food has been described in numerous patients. One unfortunate vacationer developed axillary and groin dermatitis after ingesting large amounts of grapes, wine, shrimp, and french fries while vacationing in Italy.19 Among dietary sources, beer and wine contain higher levels of sulfites. Sulfites also can be found in some pickled foods; bottled citrus juice; dried fruits; and commercial prepared foods, such as powdered potatoes and gravy mixes. Other reports of SCD from sulfites include an enema preparation20 and anesthetics21 as the source of the allergen.
Formaldehyde
Formaldehyde can cause SCD after ingestion of aspartame, which is hydrolyzed to phenylalanine, aspartic acid, and aspartic acid methyl ester in the intestine.22 The methyl ester is converted to methyl alcohol, which is transported to the liver and oxidized to formaldehyde, which is then converted to formic acid. Hill and Belsito22 reported a case of SCD presenting as eyelid dermatitis after ingestion of an aspartame-based artificial sweetener. A similar case of eyelid, neck, and leg dermatitis was reported after ingestion of drinks and candy sweetened with aspartame.23
Parabens
Although parabens are rare contact sensitizers, there are a few reports of paraben SCD. Cases include a predominantly flexural pattern from ingestion of a mucolytic-containing methylparaben,24 a generalized eczematous eruption after intramuscular injection of ampicillin preserved with methylparaben and propylparaben,25 and diffuse dermatitis from methylparaben in a local anesthetic.26
Sorbic Acid
Sorbic acid is utilized as a preservative in foods and occurs naturally in red fruit, such as strawberries and cranberries.27 It is a rare allergen, but several cases of sorbic acid SCD have been reported, including perianal and buttock dermatitis,27 hand dermatitis in an infant,28 and hand-and-foot dermatitis in a storekeeper.29
Carmine
Carmine, or cochineal extract, is a red dye derived from dried pulverized scale insects of the family Coccidae. This chemical can be used in a multitude of foods and medications, including candies, yogurt, red velvet items, popsicles, food coloring, frozen meat and fish, ice cream, syrups, ketchup, sausage, donuts, cake pops, applesauce, canned fruits, soups, and drinks.30 Machler and Jacob31 described a child with recurrent episodes of erythroderma and periorbital edema in whom patch testing revealed a reaction to carmine. The patient’s mother connected the flares with ingestion of red velvet cupcakes.31 Ferris et al32 reported a likely case of SCD attributed to carmine in a multivitamin.
Steroids
Ingested and injected corticosteroids have been associated with SCD, which is illustrated by a case of a generalized cutaneous eruption several days after joint injection with triamcinolone acetonide.33 In another report, a patient developed an eruption in the body folds, later generalized, after topical application of a corticosteroid, first in ear drops and later in nasal spray.34 Traditional corticosteroid classification systems might be less reliable in predicting relevant allergens in corticosteroid SCD; comprehensive testing, including oral challenge, might be necessary to identify alternatives.33
Ethylenediamine
Ethylenediamine is an uncommon allergen in patch test populations. It is present in aminophylline35 and is utilized in the production of hydroxyzine36 and other piperazine-derived medications, such as cetirizine, levocetirizine, meclizine, and olanzapine. Several cases of SCD caused by aminophylline,35 cetirizine,36 and hydroxyzine37 have been reported, all in the setting of a positive patch test reaction to ethylenediamine.
When to Counsel About Systemic Exposures
In general, we usually do not counsel on systemic exposures to allergens at the final patch test reading unless the pattern of dermatitis or clinical history strongly suggests systemic exposure. In most cases, we find that counseling on topical allergen avoidance alone is sufficient to treat allergic contact dermatitis. Because of the restrictive nature of allergen-avoidance diets, counseling all patients on the potential for SCD might cause undue stress without much benefit. However, if a patient experiences persistent dermatitis on follow-up with topical avoidance alone, we often will delve into systemic exposures and counsel on further avoidance strategies, including medication and diet.
Final Interpretation
A multitude of chemicals have been reported as the source of SCD; these exposures can occur through ingestion, injection, and inhaled and cutaneous routes. Chemicals present in foods, medications, and beverages have been implicated. Systemic contact dermatitis is rare and should be considered when traditional avoidance of contact allergens is unsuccessful and the clinical pattern is consistent with SCD.
One of the perils of patch testing is fielding questions about which type of allergens will be used. Patients often ask if the patch test includes milk, foods, dander, mold, pets, and grass. Most patch testers spend a substantial amount of time explaining that the purpose of patch testing is to detect applied chemical allergens: It’s not what you eat; rather, it’s what touches your skin. However, the big caveat is that some oral, parenteral, inhaled, and even cutaneous allergens can produce systemic contact dermatitis (SCD), which represents a unique clinical scenario that we will review in this month’s Final Interpretation column.
There are many patterns of SCD. Familiarity with potential clinical presentations can aid in diagnosis and counseling. Systemic contact dermatitis tends to be symmetrical. Dyshidrotic hand dermatitis is a reported pattern for systemic metal allergy, most commonly nickel. Refractory eyelid or genital dermatitis can reflect a systemic exposure, particularly if the dermatitis is in areas not caused by direct skin contact with the allergen. Systemic drug-related intertriginous and flexural exanthema is, as the name describes, an eruption involving axillae, genital skin, and flexural sites. It usually is a type of drug reaction, but the culprit can be an ingested allergen. So-called baboon syndrome SCD can cause persistent genital and intertriginous dermatitis. Other clues to SCD include dermatitis flare at the patch test site and erythema multiforme. Some patients also describe systemic symptoms, including headache, fatigue, and malaise.
Rhus Dermatitis
Poison ivy is the most common cause of acute contact dermatitis but also can be a cause of SCD. From the family Anacardiaceae, this sneaky plant is common in many parts of the United States; most allergic patients are familiar with their allergy from prior exposure.
In 1982, 54 Little League baseball attendees developed diffuse vesicular dermatitis involving the flexures after ingesting packaged cashews contaminated with cashew shells.1 In the same family as poison ivy, the cashew nut tree (Anacardium occidentale) produces a cashew apple containing the cashew nut. The cashew shell is the site that contains the allergenic oils. Typically, cashews are processed to remove the shell and oil prior to consumption. Ingestion of raw cashews is more likely to lead to SCD than roasted cashews because the heat in the roasting process can break down any allergenic oil.2
Metals
Systemic exposures to nickel usually are dietary. Clinically, SCD from nickel most commonly presents as refractory dyshidrotic hand eczema or papular elbow dermatitis.3 Nickel is commonly found in vitamins and supplements as well as certain whole grains, vegetables, beans, coffee, chocolate, and tea.4 Sometimes, cookware also can be a source of nickel exposure, particularly with steel cookware, from which nickel can leach into food.
In general, a diet lower than 150 μg/d is needed to prevent flares.5 A point-based diet is available for nickel-allergic patients.5 Patients should ingest a restricted amount of nickel (15 points daily); those who are extremely allergic might need to limit nickel ingestion to less than 5 points daily. Because of the challenges associated with maintaining a low-nickel diet, chelation therapy has been recommended to prevent nickel absorption. Disulfiram3 and ascorbic acid5 have been recommended, but larger studies are lacking.
Cobalt and chromium are other metals that, when ingested, can lead to SCD; both can be found in multivitamins. Other sources of dietary chromium include vegetables, coffee, beans, certain meats, and seafood.4 For cobalt, the dietary exposures are similar with the addition of nuts, apricots, and whole-grain flour. A point-based cobalt avoidance diet has been published. This diet recommends less than 12 μg of cobalt daily; patients can ingest up to 12 cobalt points daily.6
Likewise, gold has been reported to cause SCD, with one case attributed to gold in a homeopathic cardiac medication.7 Gold SCD also should be considered in the setting of ingested gold-containing alcoholic beverages and historically has been associated with intramuscular gold sodium thiomalate for the treatment of rheumatoid arthritis.8
Metal implants, including prosthetic joints, stents, and other devices, have been implicated in SCD. (More to come on this topic soon; yes, dear reader, that is a teaser!)
Fragrances
Balsam of Peru
Secreted by the tree Myroxylon balsamum var pereirae, balsam of Peru (BOP) contains several potential allergens, including cinnamon oils (eg, eugenol, vanillin, cinnamates), coniferin derivatives, and benzoic acid derivatives.9 Foods and beverages associated with BOP include citrus, pickled vegetables, chocolate, ice cream, chili, pizza, tomatoes, wine, beer, gin, vermouth, flavored tea, and soft drinks.10 Flavoring agents, spices (eg, cloves, curry, vanilla, cinnamon, allspice, ginger, anise), and condiments (eg, ketchup, barbeque sauce) are potential sources, as are cough medicines, lozenges, and flavored tobacco.
Salam and Fowler10 described BOP-allergic patients whose condition improved with dietary restriction of BOP. Avoidance of tomatoes, citrus, spices, and cola most commonly contributed to improvement.10 Scheman et al9 proposed BOP subgroups, including the eugenol, vanillin, cinnamate, benzoate, ferulic acid, and coniferin groups. Targeted patch testing can identify relevant subgroups, and patients can focus dietary restrictions by subgroup.
Plants
Systemic contact dermatitis has been reported in association with a number of plants and herbals, including chamomile in tea,11 goldenrod in a medicated extract,12Hosta plantaginea roots,13 and garlic extract for hyperlipidemia.14 Many more have been described.
Propolis
Also known as bee glue, propolis comprises a mixture of balsams, resins, waxes, essential oils, pollen, cinnamic alcohol, and vitamins. It can be found in many cosmetic products, foods, and chewing gum.15 Propolis has been reported to be the source of SCD from ingestion of propolis capsules, which have been used to promote immune stimulation,15 and propolis solution as a natural tonic.16
Propylene Glycol
Propylene glycol (PG) can be found in (believe it or not) foods and medications. In foods, it typically is used for its softening, humectant, and preservative properties.17 Common food sources of PG include sauces, desserts, snack foods, and salad dressings.
Many topical prescription medications, including corticosteroids and newer nonsteroidal anti-inflammatory topicals, might contain PG; providers must specifically request PG-free products for PG-allergic patients. A detailed PG-avoidance diet lists products to avoid and products that are PG free.18
Preservatives
Sulfites
These compounds are preservatives found in cosmetics, hair dyes, and certain foods. Systemic contact dermatitis caused by sulfites in food has been described in numerous patients. One unfortunate vacationer developed axillary and groin dermatitis after ingesting large amounts of grapes, wine, shrimp, and french fries while vacationing in Italy.19 Among dietary sources, beer and wine contain higher levels of sulfites. Sulfites also can be found in some pickled foods; bottled citrus juice; dried fruits; and commercial prepared foods, such as powdered potatoes and gravy mixes. Other reports of SCD from sulfites include an enema preparation20 and anesthetics21 as the source of the allergen.
Formaldehyde
Formaldehyde can cause SCD after ingestion of aspartame, which is hydrolyzed to phenylalanine, aspartic acid, and aspartic acid methyl ester in the intestine.22 The methyl ester is converted to methyl alcohol, which is transported to the liver and oxidized to formaldehyde, which is then converted to formic acid. Hill and Belsito22 reported a case of SCD presenting as eyelid dermatitis after ingestion of an aspartame-based artificial sweetener. A similar case of eyelid, neck, and leg dermatitis was reported after ingestion of drinks and candy sweetened with aspartame.23
Parabens
Although parabens are rare contact sensitizers, there are a few reports of paraben SCD. Cases include a predominantly flexural pattern from ingestion of a mucolytic-containing methylparaben,24 a generalized eczematous eruption after intramuscular injection of ampicillin preserved with methylparaben and propylparaben,25 and diffuse dermatitis from methylparaben in a local anesthetic.26
Sorbic Acid
Sorbic acid is utilized as a preservative in foods and occurs naturally in red fruit, such as strawberries and cranberries.27 It is a rare allergen, but several cases of sorbic acid SCD have been reported, including perianal and buttock dermatitis,27 hand dermatitis in an infant,28 and hand-and-foot dermatitis in a storekeeper.29
Carmine
Carmine, or cochineal extract, is a red dye derived from dried pulverized scale insects of the family Coccidae. This chemical can be used in a multitude of foods and medications, including candies, yogurt, red velvet items, popsicles, food coloring, frozen meat and fish, ice cream, syrups, ketchup, sausage, donuts, cake pops, applesauce, canned fruits, soups, and drinks.30 Machler and Jacob31 described a child with recurrent episodes of erythroderma and periorbital edema in whom patch testing revealed a reaction to carmine. The patient’s mother connected the flares with ingestion of red velvet cupcakes.31 Ferris et al32 reported a likely case of SCD attributed to carmine in a multivitamin.
Steroids
Ingested and injected corticosteroids have been associated with SCD, which is illustrated by a case of a generalized cutaneous eruption several days after joint injection with triamcinolone acetonide.33 In another report, a patient developed an eruption in the body folds, later generalized, after topical application of a corticosteroid, first in ear drops and later in nasal spray.34 Traditional corticosteroid classification systems might be less reliable in predicting relevant allergens in corticosteroid SCD; comprehensive testing, including oral challenge, might be necessary to identify alternatives.33
Ethylenediamine
Ethylenediamine is an uncommon allergen in patch test populations. It is present in aminophylline35 and is utilized in the production of hydroxyzine36 and other piperazine-derived medications, such as cetirizine, levocetirizine, meclizine, and olanzapine. Several cases of SCD caused by aminophylline,35 cetirizine,36 and hydroxyzine37 have been reported, all in the setting of a positive patch test reaction to ethylenediamine.
When to Counsel About Systemic Exposures
In general, we usually do not counsel on systemic exposures to allergens at the final patch test reading unless the pattern of dermatitis or clinical history strongly suggests systemic exposure. In most cases, we find that counseling on topical allergen avoidance alone is sufficient to treat allergic contact dermatitis. Because of the restrictive nature of allergen-avoidance diets, counseling all patients on the potential for SCD might cause undue stress without much benefit. However, if a patient experiences persistent dermatitis on follow-up with topical avoidance alone, we often will delve into systemic exposures and counsel on further avoidance strategies, including medication and diet.
Final Interpretation
A multitude of chemicals have been reported as the source of SCD; these exposures can occur through ingestion, injection, and inhaled and cutaneous routes. Chemicals present in foods, medications, and beverages have been implicated. Systemic contact dermatitis is rare and should be considered when traditional avoidance of contact allergens is unsuccessful and the clinical pattern is consistent with SCD.
- Marks JG, DeMelfi T, McCarthy MA, et al. Dermatitis from cashew nuts. J Am Acad Dermatol. 1984;10:627-631.
- Hamilton TK, Zug KA. Systemic contact dermatitis to raw cashew nuts in a pesto sauce. Am J Contact Dermat. 1998;9:51-54.
- Fabbro SK, Zirwas MJ. Systemic contact dermatitis to foods: nickel, BOP, and more. Curr Allergy Asthma Rep. 2014;14:463.
- American Contact Dermatitis Society. Contact Allergy Management Program (CAMP). https://www.contactderm.org/resources/acds-camp. Accessed October 23, 2019.
- Mislankar M, Zirwas MJ. Low-nickel diet scoring system for systemic nickel allergy. Dermatitis. 2013;24:190-195.
- Stuckert J, Nedorost S. Low-cobalt diet for dyshidrotic eczema patients. Contact Dermatitis. 2008;59:361-365.
- Malinauskiene L, Isaksson M, Bruze M. Systemic contact dermatitis in a gold-allergic patient after treatment with an oral homeopathic drug. J Am Acad Dermatol. 2013;68:e58.
- Wicks IP, Wong D, McCullagh RB, et al. Contact allergy to gold after systemic administration of gold for rheumatoid arthritis. Ann Rheum Dis. 1988;47:421-422.
- Scheman A, Rakowski EM, Chou V, et al. Balsam of Peru: past and future. Dermatitis. 2013;24:153-160.
- Salam TN, Fowler JF Jr. Balsam-related systemic contact dermatitis. J Am Acad Dermatol. 2001;45:377-381.
- Rodríguez-Serna M, Sánchez-Motilla JM, Ramón R, et al. Allergic and systemic contact dermatitis from Matricaria chamomilla tea. Contact Dermatitis. 1998;39:192-193.
- Schätzle M, Agathos M, Breit R. Allergic contact dermatitis from goldenrod (Herba solidaginis) after systemic administration. Contact Dermatitis. 1998;39:271-272.
- Yun SJ, Lee JY, Kim GH, et al. Systemic contact dermatitis induced by roots of Hosta plantaginea. J Eur Acad Dermatol Venereol. 2018;32:e28-e29.
- Burden AD, Wilkinson SM, Beck MH, et al. Garlic-induced systemic contact dermatitis. Contact Dermatitis. 1994;30:299-300.
- Komericki P, Kränke B. Maculopapular exanthem from propolis: case report and review of systemic cutaneous and non-cutaneous reactions. Contact Dermatitis. 2009;61:353-355.
- Cho E, Lee JD, Cho SH. Systemic contact dermatitis from propolis ingestion. Ann Dermatol. 2011;23:85-88.
- Lowther A, McCormick T, Nedorost S. Systemic contact dermatitis from propylene glycol. Dermatitis. 2008;19:105-108.
- Scheman A, Cha C, Jacob SE, et al. Food avoidance diets for systemic, lip, and oral contact allergy: an American Contact Alternatives Group article. Dermatitis. 2012;23:248-257.
- Cussans A, McFadden J, Ostlere L. Systemic sodium metabisulfite allergy. Contact Dermatitis. 2015;73:316-317.
- Borges AS, Valejo Coelho MM, Fernandes C, et al. Systemic allergic dermatitis caused by sodium metabisulfite in rectal enemas. Contact Dermatitis. 2018;78:429-430.
- Guha-Niyogi B, Sabroe R, Holden C. An unusual case of a systemic delayed hypersensitivity reaction to sodium metabisulfite. Contact Dermatitis. 2018;79:246-247.
- Hill AM, Belsito DV. Systemic contact dermatitis of the eyelids caused by formaldehyde derived from aspartame? Contact Dermatitis. 2003;49:258-259.
- Veien NK, Lomholt HB. Systemic allergic dermatitis presumably caused by formaldehyde derived from aspartame. Contact Dermatitis. 2012;67:315-316.
- Sánchez-Pérez J, Diez MB, Pérez AA, et al. Allergic and systemic contact dermatitis to methylparaben. Contact Dermatitis. 2006;54:117-118.
- Carradori S, Peluso AM, Faccioli M. Systemic contact dermatitis due to parabens. Contact Dermatitis. 1990;22:238-239.
- Aeling JL, Nuss DD. Systemic eczematous “contact-type” dermatitis medicamentosa caused by parabens. Arch Dermatol. 1974;110:640.
- Giordano-Labadie F, Pech-Ormieres C, Bazex J. Systemic contact dermatitis from sorbic acid. Contact Dermatitis. 1996;34:61-62.
- Raison-Peyron N, Meynadier JM, Meynadier J. Sorbic acid: an unusual cause of systemic contact dermatitis in an infant. Contact Dermatitis. 2000;43:247-248.
- Dejobert Y, Delaporte E, Piette F, et al. Vesicular eczema and systemic contact dermatitis from sorbic acid. Contact Dermatitis. 2001;45:291.
- Rundle CW, Jacob SE, Machler BC. Contact dermatitis to carmine. Dermatitis. 2018;29:244-249.
- Machler BC, Jacob SE. Carmine red: a potentially overlooked allergen in children. Dermatitis. 2018;29:92-93.
- Ferris GJ, Wat M, Nedorost S. Multifactorial dermatitis with probable systemic contact dermatitis to carmine. Dermatitis. 2017;28:293-294.
- Santos-Alarcón S, Benavente-Villegas FC, Farzanegan-Miñano R, et al. Delayed hypersensitivity to topical and systemic corticosteroids. Contact Dermatitis. 2018;78:86-88.
- Faber MA, Sabato V, Ebo DG, et al. Systemic allergic dermatitis caused by prednisone derivatives in nose and ear drops. Contact Dermatitis. 2015;73:317-320.
- Isaksson M, Ljunggren B. Systemic contact dermatitis from ethylenediamine in an aminophylline preparation presenting as the baboon syndrome. Acta Derm Venereol. 2003;83:69-70.
- Cusano F, Ferrara G, Crisman G, et al. Clinicopathologic features of systemic contact dermatitis from ethylenediamine in cetirizine and levocetirizine. Dermatology. 2006;213:353-355.
- Ash S, Scheman AJ. Systemic contact dermatitis to hydroxyzine. Am J Contact Dermat. 1997;8:2-5.
- Marks JG, DeMelfi T, McCarthy MA, et al. Dermatitis from cashew nuts. J Am Acad Dermatol. 1984;10:627-631.
- Hamilton TK, Zug KA. Systemic contact dermatitis to raw cashew nuts in a pesto sauce. Am J Contact Dermat. 1998;9:51-54.
- Fabbro SK, Zirwas MJ. Systemic contact dermatitis to foods: nickel, BOP, and more. Curr Allergy Asthma Rep. 2014;14:463.
- American Contact Dermatitis Society. Contact Allergy Management Program (CAMP). https://www.contactderm.org/resources/acds-camp. Accessed October 23, 2019.
- Mislankar M, Zirwas MJ. Low-nickel diet scoring system for systemic nickel allergy. Dermatitis. 2013;24:190-195.
- Stuckert J, Nedorost S. Low-cobalt diet for dyshidrotic eczema patients. Contact Dermatitis. 2008;59:361-365.
- Malinauskiene L, Isaksson M, Bruze M. Systemic contact dermatitis in a gold-allergic patient after treatment with an oral homeopathic drug. J Am Acad Dermatol. 2013;68:e58.
- Wicks IP, Wong D, McCullagh RB, et al. Contact allergy to gold after systemic administration of gold for rheumatoid arthritis. Ann Rheum Dis. 1988;47:421-422.
- Scheman A, Rakowski EM, Chou V, et al. Balsam of Peru: past and future. Dermatitis. 2013;24:153-160.
- Salam TN, Fowler JF Jr. Balsam-related systemic contact dermatitis. J Am Acad Dermatol. 2001;45:377-381.
- Rodríguez-Serna M, Sánchez-Motilla JM, Ramón R, et al. Allergic and systemic contact dermatitis from Matricaria chamomilla tea. Contact Dermatitis. 1998;39:192-193.
- Schätzle M, Agathos M, Breit R. Allergic contact dermatitis from goldenrod (Herba solidaginis) after systemic administration. Contact Dermatitis. 1998;39:271-272.
- Yun SJ, Lee JY, Kim GH, et al. Systemic contact dermatitis induced by roots of Hosta plantaginea. J Eur Acad Dermatol Venereol. 2018;32:e28-e29.
- Burden AD, Wilkinson SM, Beck MH, et al. Garlic-induced systemic contact dermatitis. Contact Dermatitis. 1994;30:299-300.
- Komericki P, Kränke B. Maculopapular exanthem from propolis: case report and review of systemic cutaneous and non-cutaneous reactions. Contact Dermatitis. 2009;61:353-355.
- Cho E, Lee JD, Cho SH. Systemic contact dermatitis from propolis ingestion. Ann Dermatol. 2011;23:85-88.
- Lowther A, McCormick T, Nedorost S. Systemic contact dermatitis from propylene glycol. Dermatitis. 2008;19:105-108.
- Scheman A, Cha C, Jacob SE, et al. Food avoidance diets for systemic, lip, and oral contact allergy: an American Contact Alternatives Group article. Dermatitis. 2012;23:248-257.
- Cussans A, McFadden J, Ostlere L. Systemic sodium metabisulfite allergy. Contact Dermatitis. 2015;73:316-317.
- Borges AS, Valejo Coelho MM, Fernandes C, et al. Systemic allergic dermatitis caused by sodium metabisulfite in rectal enemas. Contact Dermatitis. 2018;78:429-430.
- Guha-Niyogi B, Sabroe R, Holden C. An unusual case of a systemic delayed hypersensitivity reaction to sodium metabisulfite. Contact Dermatitis. 2018;79:246-247.
- Hill AM, Belsito DV. Systemic contact dermatitis of the eyelids caused by formaldehyde derived from aspartame? Contact Dermatitis. 2003;49:258-259.
- Veien NK, Lomholt HB. Systemic allergic dermatitis presumably caused by formaldehyde derived from aspartame. Contact Dermatitis. 2012;67:315-316.
- Sánchez-Pérez J, Diez MB, Pérez AA, et al. Allergic and systemic contact dermatitis to methylparaben. Contact Dermatitis. 2006;54:117-118.
- Carradori S, Peluso AM, Faccioli M. Systemic contact dermatitis due to parabens. Contact Dermatitis. 1990;22:238-239.
- Aeling JL, Nuss DD. Systemic eczematous “contact-type” dermatitis medicamentosa caused by parabens. Arch Dermatol. 1974;110:640.
- Giordano-Labadie F, Pech-Ormieres C, Bazex J. Systemic contact dermatitis from sorbic acid. Contact Dermatitis. 1996;34:61-62.
- Raison-Peyron N, Meynadier JM, Meynadier J. Sorbic acid: an unusual cause of systemic contact dermatitis in an infant. Contact Dermatitis. 2000;43:247-248.
- Dejobert Y, Delaporte E, Piette F, et al. Vesicular eczema and systemic contact dermatitis from sorbic acid. Contact Dermatitis. 2001;45:291.
- Rundle CW, Jacob SE, Machler BC. Contact dermatitis to carmine. Dermatitis. 2018;29:244-249.
- Machler BC, Jacob SE. Carmine red: a potentially overlooked allergen in children. Dermatitis. 2018;29:92-93.
- Ferris GJ, Wat M, Nedorost S. Multifactorial dermatitis with probable systemic contact dermatitis to carmine. Dermatitis. 2017;28:293-294.
- Santos-Alarcón S, Benavente-Villegas FC, Farzanegan-Miñano R, et al. Delayed hypersensitivity to topical and systemic corticosteroids. Contact Dermatitis. 2018;78:86-88.
- Faber MA, Sabato V, Ebo DG, et al. Systemic allergic dermatitis caused by prednisone derivatives in nose and ear drops. Contact Dermatitis. 2015;73:317-320.
- Isaksson M, Ljunggren B. Systemic contact dermatitis from ethylenediamine in an aminophylline preparation presenting as the baboon syndrome. Acta Derm Venereol. 2003;83:69-70.
- Cusano F, Ferrara G, Crisman G, et al. Clinicopathologic features of systemic contact dermatitis from ethylenediamine in cetirizine and levocetirizine. Dermatology. 2006;213:353-355.
- Ash S, Scheman AJ. Systemic contact dermatitis to hydroxyzine. Am J Contact Dermat. 1997;8:2-5.
Practice Points
- Although most cases of allergic contact dermatitis are from direct skin contact, systemic contact dermatitis (SCD) can occur from ingesting certain allergens.
- Systemic contact dermatitis tends to present as a symmetric pruritic eruption, which may involve the flexural or intertriginous surfaces, eyelids, hands, or genital skin.
- Allergens known to cause SCD include certain plants, fragrances, metals, preservatives, and medications.
Patch Testing in Children: Not Just Little Adults
The pediatric population has a unique product exposure profile due to the many care products specifically marketed for use in children. In fact, the prevalence of allergic contact dermatitis (ACD) in children may be as high as 24.5% in the United States.1 In patch tested children, relevant positive reaction rates of 56.7% and 48% have been reported by the North American Contact Dermatitis Group and the Pediatric Contact Dermatitis Registry, respectively.2,3 In this article, we provide an overview of current trends in pediatric patch testing as well as specific considerations in this patient population.
Patch Test Reactions in Children
Several publications have documented pediatric patch test reactions. The North American Contact Dermatitis Group reported patch test results in 883 children from the United States and Canada (2005-2012).2 The most common reactions were nickel (28.1%), cobalt (12.3%), neomycin (7.1%), balsam of Peru (5.7%), lanolin (5.5%), and fragrance mix I (5.2%). When compared to adults, children were more likely to have relevant positive patch tests to nickel, cobalt, and compositae mix.2 In comparison, data from the Pediatric Contact Dermatitis Registry showed that the most common reactions in 1142 children in the United States (2015-2016) were nickel (22%), fragrance mix I (11%), cobalt (9.1%), balsam of Peru (8.4%), neomycin (7.2%), and propylene glycol (6.8%).3
Allergen sensitivities may vary based on geographic region. In Spain, children showed the highest sensitivities to thiomersal (10.2%), cobalt (9.1%), colophony (9.1%), paraphenylenediamine (8.3%), mercury (7.9%), potassium dichromate (7.9%), and nickel (6.4%).4
Pediatric Patch Testing Pearls
History of Product Use
From diapers to drama club, pediatric exposures and sources of ACD are not the same as those seen in adults. Because obtaining a medical history from a toddler can be exasperating, the patient’s caregivers should be asked about potential exposures, ranging from personal care products and diapers to school activities, hobbies, and sports.5,6 It is important to keep in mind that the patient’s primary caregiver may not be the only individual who applies products to the child.7
Application of Allergens
Children are not merely small adults, but they usually do have smaller backs than adult patients. This reduced surface area means that the patch tester must carefully select the allergens to be patch tested. For reference, the back of a typical 6-year-old child can fit 40 to 60 allergens during patch testing.8
Patch Test Chambers
In children, the use of plastic patch test chambers may be preferred over aluminum chambers. Children with persistent pruritic subcutaneous nodules induced by aluminum-based vaccines also may have delayed-type sensitivity reactions to aluminum.9 These patients could react to the aluminum present in some patch test chambers, making interpretation of the results difficult. The authors (A.R.A. and M.R.) typically use plastic chambers in the pediatric population.
Managing Expectations
As with other procedures in the pediatric population, patch testing can elicit emotions of fear, anxiety, and distrust. Video distraction and/or role-playing games may help capture the attention of children and can be particularly helpful during patch application. Children may be apprehensive about the term allergy testing if they are familiar with the term needle testing from previous allergies.5
Securing Patches
Young children can be quite active, posing another challenge for keeping patches in place. We recommend using extra tape to secure the patches in place on a child’s back. In addition, a large transparent film dressing (ie, 12×8 in) can be used if quick application is needed. For extra precaution, the use of a tight T-shirt or favorite onesie during the patch test process may be helpful, making it more difficult for little fingers to remove tape edges.
Duration of Patch Testing
Some authors have proposed application of patch tests for 24 hours in pediatric patients, as compared to 48 hours in adults.10 This recommendation is based on a theory that the reduced application period will decrease the risk for irritant reactions in pediatric patients.
Pediatric Patch Test Screening Series
A summary of the published screening series for patch testing in the pediatric population is provided (Table).
The T.R.U.E. Test (SmartPractice) is approved by the US Food and Drug Administration for use in patients 6 years and older11; however, it may not adequately represent allergen exposures in the pediatric population. Brankov and Jacob14 found that 10 (40%) of their proposed top 25 pediatric allergens were not detected using the T.R.U.E. Test.
In 2014, the North American Pediatric Patch Test Series was proposed as a basic screening panel for children aged 6 to 12 years.12 This series of 20 allergens was developed based on a literature review of pediatric patch test results and case reports as well as a database review. The authors proposed additional allergens to be considered based on patient history.12
More recently, a 2017 American Contact Dermatitis Society physician work group proposed the Pediatric Baseline Patch Test Series. This series of 38 allergens for children aged 6 to 18 years was developed based on expert consensus.8 Studies to determine the efficacy of this series have yet to be conducted, but it may have high sensitivity in detecting relevant allergens in children as demonstrated by a theoretical detection rate of 84%.14
There are 2 recommended patch test series for allergic diaper dermatitis.15 The first series focuses on 23 potential allergens found in wet wipes and topical diaper preparations. The second series contains 10 potential allergens found in diapers. These series contain common topical medications for children including corticosteroids, antimicrobials, and sensitizers specific to diapers such as rubbers and adhesives.15
Similar to adults, it may be difficult to designate one screening panel that can identify all relevant allergens in children; thus, it is always important to obtain a thorough exposure history and customize testing to suspected allergens and/or patient products based on history and clinical relevance.
Unique Pediatric Allergens
Hobbies
Sports gear such as shin guards and splints often contain allergens such as formaldehyde resin, thiuram mix, and dialkyl thioureas.16 Perioral dermatitis may be caused by musical instrument mouthpieces containing nickel.6
Preservatives
Commonly reported causes of ACD in children include methylisothiazolinone (MI) and methylchloroisothiazolinone (MCI) found in wet wipes. A 2016 analysis of diaper wipes showed a low prevalence of MI (6.3%) and MCI (1.6%) in these products, which may reflect the industry’s awareness of these potential allergens and a subsequent change in the preservatives they utilize.17 However, the prevalence of MCI/MI contact allergy may be on the rise due to the popularity of homemade slime, which is made from common household products such as laundry detergent, dishwashing soap, and liquid glue. The Pediatric Baseline Patch Test Series captures most of the potential allergens in these homemade slime recipes and is recommended for use in pediatric patients suspected of having dermatitis secondary to playing with slime.8,18
Toilet Seat Dermatitis
Toilet seat dermatitis presents as a pruritic dermatitis on the posterior upper thighs and buttocks. Although most cases of toilet seat dermatitis are irritant rather than allergic, potential allergens include plastics, fragrances, and components of cleaning products. Thus, physicians should maintain a high index of suspicion for ACD to toilet seats.19
Fragrance and Natural Ingredients
A 2018 study evaluating personal care products marketed specifically for infants and children found that 55% of products (294/533) contained at least 1 common allergen, with fragrance being the most common (48% [255/533]). Other common allergens include betaines (18%), propylene glycol (9%), lanolin (6%), and MCI/MI (3%).20 Caregivers should be advised against the myth that natural products are safer and less allergenic and should be provided with resources such as the Contact Allergen Management Program (CAMP) database (https://www.contactderm.org/resources/acds-camp) for safe alternative personal care products.
Metal Allergens
Nickel, the American Contact Dermatitis Society 2008 Allergen of the Year, is another common allergen that affects children. Nickel allergy, commonly thought to affect the ears due to jewelry and ear piercing, may actually be found in a wide range of daily items such as braces, eyeglasses, keys, zippers, school chairs, electronics, toys, and even food.3,6,21,22 With increased use of electronics in children of all ages, nickel found in mobile phones and other devices may be of particular concern. Caregivers can use a case or cover for metallic-appearing electronics.
Final Interpretation
Pediatric ACD is common. With limited surface area for patch testing in children, we recommend customized panels based on patient history and exposure. It is important for clinicians to recognize the unique causes of ACD in children and develop age-appropriate management plans.
- Bruckner AL, Weston WL, Morelli JG. Does sensitization to contact allergens begin in infancy? Pediatrics. 2000;105:e3.
- Zug KA, Pham AK, Belsito DV, et al. Patch testing in children from 2005 to 2012: results from the North American contact dermatitis group. Dermatitis. 2014;25:345-355.
- Goldenberg A, Mousdicas N, Silverberg N, et al. Pediatric Contact Dermatitis Registry inaugural case data. Dermatitis. 2016;27:293-302.
- Ortiz Salvador JM, Esteve Martinez A, Subiabre Ferrer D, et al. Pediatric allergic contact dermatitis: clinical and epidemiological study in a tertiary hospital. Actas Dermosifiliogr. 2017;108:571-578.
- Jacob SE, Steele T, Brod B, et al. Dispelling the myths behind pediatric patch testing—experience from our tertiary care patch testing centers. Pediatr Dermatol. 2008;25:296-300.
- Brod BA, Treat JR, Rothe MJ, et al. Allergic contact dermatitis: kids are not just little people. Clin Dermatol. 2015;33:605-612.
- Elliott JF, Ramzy A, Nilsson U, et al. Severe intractable eyelid dermatitis probably caused by exposure to hydroperoxides of linalool in a heavily fragranced shampoo. Contact Dermatitis. 2017;76:114-115.
- Yu J, Atwater AR, Brod B, et al. Pediatric Baseline Patch Test Series: Pediatric Contact Dermatitis Workgroup. Dermatitis. 2018;29:206-212.
- Bergfors E, Inerot A, Falk L, et al. Patch testing children with aluminium chloride hexahydrate in petrolatum: a review and a recommendation. Contact Dermatitis. 2019;81:81-88.
- Worm M, Aberer W, Agathos M, et al. Patch testing in children—recommendations of the German Contact Dermatitis Research Group (DKG). J Dtsch Dermatol Ges. 2007;5:107-109.
- T.R.U.E. Test (Thin-Layer Rapid Use Epicutaneous Patch Test) [package insert]. Hillerød, Denmark: SmartPractice Denmark ApS; 2017.
- Jacob SE, Admani S, Herro EM. Invited commentary: recommendation for a North American pediatric patch test series. Curr Allergy Asthma Rep. 2014;14:444.
- Castanedo-Tardana MP, Zug KA. Methylisothiazolinone. Dermatitis. 2013;24:2-6.
- Brankov N, Jacob SE. Pre-emptive avoidance strategy 2016: update on pediatric contact dermatitis allergens. Expert Rev Clin Immunol. 2017;13:93-95.
- Yu J, Treat J, Brod B. Patch test series for allergic perineal dermatitis in the diapered infant. Dermatitis. 2017;28:70-75.
- Sung CT, McGowan MA, Jacob SE. Allergic contact dermatitis evaluation: strategies for the preschooler. Curr Allergy Asthma Rep. 2018;18:49.
- Yu J, Treat J, Chaney K, et al. Potential allergens in disposable diaper wipes, topical diaper preparations, and disposable diapers: under-recognized etiology of pediatric perineal dermatitis. Dermatitis. 2016;27:110-118.
- Anderson LE, Treat JR, Brod BA, et al. “Slime” contact dermatitis: case report and review of relevant allergens. Pediatr Dermatol. 2019;36:335-337.
- Dorfman CO, Barros MA, Zaenglein AL. Contact dermatitis to training toilet seat (potty seat dermatitis). Pediatr Dermatol. 2018;35:e251-e252.
- Bonchak JG, Prouty ME, de la Feld SF. Prevalence of contact allergens in personal care products for babies and children. Dermatitis. 2018;29:81-84.
- Chen JK, Jacob SE, Nedorost ST, et al. A pragmatic approach to patch testing atopic dermatitis patients: clinical recommendations based on expert consensus opinion. Dermatitis. 2016;27:186-192.
- Goldenberg A, Silverberg N, Silverberg JI, et al. Pediatric allergic contact dermatitis: lessons for better care. J Allergy Clin Immunol Pract. 2015;3:661-667; quiz 668.
The pediatric population has a unique product exposure profile due to the many care products specifically marketed for use in children. In fact, the prevalence of allergic contact dermatitis (ACD) in children may be as high as 24.5% in the United States.1 In patch tested children, relevant positive reaction rates of 56.7% and 48% have been reported by the North American Contact Dermatitis Group and the Pediatric Contact Dermatitis Registry, respectively.2,3 In this article, we provide an overview of current trends in pediatric patch testing as well as specific considerations in this patient population.
Patch Test Reactions in Children
Several publications have documented pediatric patch test reactions. The North American Contact Dermatitis Group reported patch test results in 883 children from the United States and Canada (2005-2012).2 The most common reactions were nickel (28.1%), cobalt (12.3%), neomycin (7.1%), balsam of Peru (5.7%), lanolin (5.5%), and fragrance mix I (5.2%). When compared to adults, children were more likely to have relevant positive patch tests to nickel, cobalt, and compositae mix.2 In comparison, data from the Pediatric Contact Dermatitis Registry showed that the most common reactions in 1142 children in the United States (2015-2016) were nickel (22%), fragrance mix I (11%), cobalt (9.1%), balsam of Peru (8.4%), neomycin (7.2%), and propylene glycol (6.8%).3
Allergen sensitivities may vary based on geographic region. In Spain, children showed the highest sensitivities to thiomersal (10.2%), cobalt (9.1%), colophony (9.1%), paraphenylenediamine (8.3%), mercury (7.9%), potassium dichromate (7.9%), and nickel (6.4%).4
Pediatric Patch Testing Pearls
History of Product Use
From diapers to drama club, pediatric exposures and sources of ACD are not the same as those seen in adults. Because obtaining a medical history from a toddler can be exasperating, the patient’s caregivers should be asked about potential exposures, ranging from personal care products and diapers to school activities, hobbies, and sports.5,6 It is important to keep in mind that the patient’s primary caregiver may not be the only individual who applies products to the child.7
Application of Allergens
Children are not merely small adults, but they usually do have smaller backs than adult patients. This reduced surface area means that the patch tester must carefully select the allergens to be patch tested. For reference, the back of a typical 6-year-old child can fit 40 to 60 allergens during patch testing.8
Patch Test Chambers
In children, the use of plastic patch test chambers may be preferred over aluminum chambers. Children with persistent pruritic subcutaneous nodules induced by aluminum-based vaccines also may have delayed-type sensitivity reactions to aluminum.9 These patients could react to the aluminum present in some patch test chambers, making interpretation of the results difficult. The authors (A.R.A. and M.R.) typically use plastic chambers in the pediatric population.
Managing Expectations
As with other procedures in the pediatric population, patch testing can elicit emotions of fear, anxiety, and distrust. Video distraction and/or role-playing games may help capture the attention of children and can be particularly helpful during patch application. Children may be apprehensive about the term allergy testing if they are familiar with the term needle testing from previous allergies.5
Securing Patches
Young children can be quite active, posing another challenge for keeping patches in place. We recommend using extra tape to secure the patches in place on a child’s back. In addition, a large transparent film dressing (ie, 12×8 in) can be used if quick application is needed. For extra precaution, the use of a tight T-shirt or favorite onesie during the patch test process may be helpful, making it more difficult for little fingers to remove tape edges.
Duration of Patch Testing
Some authors have proposed application of patch tests for 24 hours in pediatric patients, as compared to 48 hours in adults.10 This recommendation is based on a theory that the reduced application period will decrease the risk for irritant reactions in pediatric patients.
Pediatric Patch Test Screening Series
A summary of the published screening series for patch testing in the pediatric population is provided (Table).
The T.R.U.E. Test (SmartPractice) is approved by the US Food and Drug Administration for use in patients 6 years and older11; however, it may not adequately represent allergen exposures in the pediatric population. Brankov and Jacob14 found that 10 (40%) of their proposed top 25 pediatric allergens were not detected using the T.R.U.E. Test.
In 2014, the North American Pediatric Patch Test Series was proposed as a basic screening panel for children aged 6 to 12 years.12 This series of 20 allergens was developed based on a literature review of pediatric patch test results and case reports as well as a database review. The authors proposed additional allergens to be considered based on patient history.12
More recently, a 2017 American Contact Dermatitis Society physician work group proposed the Pediatric Baseline Patch Test Series. This series of 38 allergens for children aged 6 to 18 years was developed based on expert consensus.8 Studies to determine the efficacy of this series have yet to be conducted, but it may have high sensitivity in detecting relevant allergens in children as demonstrated by a theoretical detection rate of 84%.14
There are 2 recommended patch test series for allergic diaper dermatitis.15 The first series focuses on 23 potential allergens found in wet wipes and topical diaper preparations. The second series contains 10 potential allergens found in diapers. These series contain common topical medications for children including corticosteroids, antimicrobials, and sensitizers specific to diapers such as rubbers and adhesives.15
Similar to adults, it may be difficult to designate one screening panel that can identify all relevant allergens in children; thus, it is always important to obtain a thorough exposure history and customize testing to suspected allergens and/or patient products based on history and clinical relevance.
Unique Pediatric Allergens
Hobbies
Sports gear such as shin guards and splints often contain allergens such as formaldehyde resin, thiuram mix, and dialkyl thioureas.16 Perioral dermatitis may be caused by musical instrument mouthpieces containing nickel.6
Preservatives
Commonly reported causes of ACD in children include methylisothiazolinone (MI) and methylchloroisothiazolinone (MCI) found in wet wipes. A 2016 analysis of diaper wipes showed a low prevalence of MI (6.3%) and MCI (1.6%) in these products, which may reflect the industry’s awareness of these potential allergens and a subsequent change in the preservatives they utilize.17 However, the prevalence of MCI/MI contact allergy may be on the rise due to the popularity of homemade slime, which is made from common household products such as laundry detergent, dishwashing soap, and liquid glue. The Pediatric Baseline Patch Test Series captures most of the potential allergens in these homemade slime recipes and is recommended for use in pediatric patients suspected of having dermatitis secondary to playing with slime.8,18
Toilet Seat Dermatitis
Toilet seat dermatitis presents as a pruritic dermatitis on the posterior upper thighs and buttocks. Although most cases of toilet seat dermatitis are irritant rather than allergic, potential allergens include plastics, fragrances, and components of cleaning products. Thus, physicians should maintain a high index of suspicion for ACD to toilet seats.19
Fragrance and Natural Ingredients
A 2018 study evaluating personal care products marketed specifically for infants and children found that 55% of products (294/533) contained at least 1 common allergen, with fragrance being the most common (48% [255/533]). Other common allergens include betaines (18%), propylene glycol (9%), lanolin (6%), and MCI/MI (3%).20 Caregivers should be advised against the myth that natural products are safer and less allergenic and should be provided with resources such as the Contact Allergen Management Program (CAMP) database (https://www.contactderm.org/resources/acds-camp) for safe alternative personal care products.
Metal Allergens
Nickel, the American Contact Dermatitis Society 2008 Allergen of the Year, is another common allergen that affects children. Nickel allergy, commonly thought to affect the ears due to jewelry and ear piercing, may actually be found in a wide range of daily items such as braces, eyeglasses, keys, zippers, school chairs, electronics, toys, and even food.3,6,21,22 With increased use of electronics in children of all ages, nickel found in mobile phones and other devices may be of particular concern. Caregivers can use a case or cover for metallic-appearing electronics.
Final Interpretation
Pediatric ACD is common. With limited surface area for patch testing in children, we recommend customized panels based on patient history and exposure. It is important for clinicians to recognize the unique causes of ACD in children and develop age-appropriate management plans.
The pediatric population has a unique product exposure profile due to the many care products specifically marketed for use in children. In fact, the prevalence of allergic contact dermatitis (ACD) in children may be as high as 24.5% in the United States.1 In patch tested children, relevant positive reaction rates of 56.7% and 48% have been reported by the North American Contact Dermatitis Group and the Pediatric Contact Dermatitis Registry, respectively.2,3 In this article, we provide an overview of current trends in pediatric patch testing as well as specific considerations in this patient population.
Patch Test Reactions in Children
Several publications have documented pediatric patch test reactions. The North American Contact Dermatitis Group reported patch test results in 883 children from the United States and Canada (2005-2012).2 The most common reactions were nickel (28.1%), cobalt (12.3%), neomycin (7.1%), balsam of Peru (5.7%), lanolin (5.5%), and fragrance mix I (5.2%). When compared to adults, children were more likely to have relevant positive patch tests to nickel, cobalt, and compositae mix.2 In comparison, data from the Pediatric Contact Dermatitis Registry showed that the most common reactions in 1142 children in the United States (2015-2016) were nickel (22%), fragrance mix I (11%), cobalt (9.1%), balsam of Peru (8.4%), neomycin (7.2%), and propylene glycol (6.8%).3
Allergen sensitivities may vary based on geographic region. In Spain, children showed the highest sensitivities to thiomersal (10.2%), cobalt (9.1%), colophony (9.1%), paraphenylenediamine (8.3%), mercury (7.9%), potassium dichromate (7.9%), and nickel (6.4%).4
Pediatric Patch Testing Pearls
History of Product Use
From diapers to drama club, pediatric exposures and sources of ACD are not the same as those seen in adults. Because obtaining a medical history from a toddler can be exasperating, the patient’s caregivers should be asked about potential exposures, ranging from personal care products and diapers to school activities, hobbies, and sports.5,6 It is important to keep in mind that the patient’s primary caregiver may not be the only individual who applies products to the child.7
Application of Allergens
Children are not merely small adults, but they usually do have smaller backs than adult patients. This reduced surface area means that the patch tester must carefully select the allergens to be patch tested. For reference, the back of a typical 6-year-old child can fit 40 to 60 allergens during patch testing.8
Patch Test Chambers
In children, the use of plastic patch test chambers may be preferred over aluminum chambers. Children with persistent pruritic subcutaneous nodules induced by aluminum-based vaccines also may have delayed-type sensitivity reactions to aluminum.9 These patients could react to the aluminum present in some patch test chambers, making interpretation of the results difficult. The authors (A.R.A. and M.R.) typically use plastic chambers in the pediatric population.
Managing Expectations
As with other procedures in the pediatric population, patch testing can elicit emotions of fear, anxiety, and distrust. Video distraction and/or role-playing games may help capture the attention of children and can be particularly helpful during patch application. Children may be apprehensive about the term allergy testing if they are familiar with the term needle testing from previous allergies.5
Securing Patches
Young children can be quite active, posing another challenge for keeping patches in place. We recommend using extra tape to secure the patches in place on a child’s back. In addition, a large transparent film dressing (ie, 12×8 in) can be used if quick application is needed. For extra precaution, the use of a tight T-shirt or favorite onesie during the patch test process may be helpful, making it more difficult for little fingers to remove tape edges.
Duration of Patch Testing
Some authors have proposed application of patch tests for 24 hours in pediatric patients, as compared to 48 hours in adults.10 This recommendation is based on a theory that the reduced application period will decrease the risk for irritant reactions in pediatric patients.
Pediatric Patch Test Screening Series
A summary of the published screening series for patch testing in the pediatric population is provided (Table).
The T.R.U.E. Test (SmartPractice) is approved by the US Food and Drug Administration for use in patients 6 years and older11; however, it may not adequately represent allergen exposures in the pediatric population. Brankov and Jacob14 found that 10 (40%) of their proposed top 25 pediatric allergens were not detected using the T.R.U.E. Test.
In 2014, the North American Pediatric Patch Test Series was proposed as a basic screening panel for children aged 6 to 12 years.12 This series of 20 allergens was developed based on a literature review of pediatric patch test results and case reports as well as a database review. The authors proposed additional allergens to be considered based on patient history.12
More recently, a 2017 American Contact Dermatitis Society physician work group proposed the Pediatric Baseline Patch Test Series. This series of 38 allergens for children aged 6 to 18 years was developed based on expert consensus.8 Studies to determine the efficacy of this series have yet to be conducted, but it may have high sensitivity in detecting relevant allergens in children as demonstrated by a theoretical detection rate of 84%.14
There are 2 recommended patch test series for allergic diaper dermatitis.15 The first series focuses on 23 potential allergens found in wet wipes and topical diaper preparations. The second series contains 10 potential allergens found in diapers. These series contain common topical medications for children including corticosteroids, antimicrobials, and sensitizers specific to diapers such as rubbers and adhesives.15
Similar to adults, it may be difficult to designate one screening panel that can identify all relevant allergens in children; thus, it is always important to obtain a thorough exposure history and customize testing to suspected allergens and/or patient products based on history and clinical relevance.
Unique Pediatric Allergens
Hobbies
Sports gear such as shin guards and splints often contain allergens such as formaldehyde resin, thiuram mix, and dialkyl thioureas.16 Perioral dermatitis may be caused by musical instrument mouthpieces containing nickel.6
Preservatives
Commonly reported causes of ACD in children include methylisothiazolinone (MI) and methylchloroisothiazolinone (MCI) found in wet wipes. A 2016 analysis of diaper wipes showed a low prevalence of MI (6.3%) and MCI (1.6%) in these products, which may reflect the industry’s awareness of these potential allergens and a subsequent change in the preservatives they utilize.17 However, the prevalence of MCI/MI contact allergy may be on the rise due to the popularity of homemade slime, which is made from common household products such as laundry detergent, dishwashing soap, and liquid glue. The Pediatric Baseline Patch Test Series captures most of the potential allergens in these homemade slime recipes and is recommended for use in pediatric patients suspected of having dermatitis secondary to playing with slime.8,18
Toilet Seat Dermatitis
Toilet seat dermatitis presents as a pruritic dermatitis on the posterior upper thighs and buttocks. Although most cases of toilet seat dermatitis are irritant rather than allergic, potential allergens include plastics, fragrances, and components of cleaning products. Thus, physicians should maintain a high index of suspicion for ACD to toilet seats.19
Fragrance and Natural Ingredients
A 2018 study evaluating personal care products marketed specifically for infants and children found that 55% of products (294/533) contained at least 1 common allergen, with fragrance being the most common (48% [255/533]). Other common allergens include betaines (18%), propylene glycol (9%), lanolin (6%), and MCI/MI (3%).20 Caregivers should be advised against the myth that natural products are safer and less allergenic and should be provided with resources such as the Contact Allergen Management Program (CAMP) database (https://www.contactderm.org/resources/acds-camp) for safe alternative personal care products.
Metal Allergens
Nickel, the American Contact Dermatitis Society 2008 Allergen of the Year, is another common allergen that affects children. Nickel allergy, commonly thought to affect the ears due to jewelry and ear piercing, may actually be found in a wide range of daily items such as braces, eyeglasses, keys, zippers, school chairs, electronics, toys, and even food.3,6,21,22 With increased use of electronics in children of all ages, nickel found in mobile phones and other devices may be of particular concern. Caregivers can use a case or cover for metallic-appearing electronics.
Final Interpretation
Pediatric ACD is common. With limited surface area for patch testing in children, we recommend customized panels based on patient history and exposure. It is important for clinicians to recognize the unique causes of ACD in children and develop age-appropriate management plans.
- Bruckner AL, Weston WL, Morelli JG. Does sensitization to contact allergens begin in infancy? Pediatrics. 2000;105:e3.
- Zug KA, Pham AK, Belsito DV, et al. Patch testing in children from 2005 to 2012: results from the North American contact dermatitis group. Dermatitis. 2014;25:345-355.
- Goldenberg A, Mousdicas N, Silverberg N, et al. Pediatric Contact Dermatitis Registry inaugural case data. Dermatitis. 2016;27:293-302.
- Ortiz Salvador JM, Esteve Martinez A, Subiabre Ferrer D, et al. Pediatric allergic contact dermatitis: clinical and epidemiological study in a tertiary hospital. Actas Dermosifiliogr. 2017;108:571-578.
- Jacob SE, Steele T, Brod B, et al. Dispelling the myths behind pediatric patch testing—experience from our tertiary care patch testing centers. Pediatr Dermatol. 2008;25:296-300.
- Brod BA, Treat JR, Rothe MJ, et al. Allergic contact dermatitis: kids are not just little people. Clin Dermatol. 2015;33:605-612.
- Elliott JF, Ramzy A, Nilsson U, et al. Severe intractable eyelid dermatitis probably caused by exposure to hydroperoxides of linalool in a heavily fragranced shampoo. Contact Dermatitis. 2017;76:114-115.
- Yu J, Atwater AR, Brod B, et al. Pediatric Baseline Patch Test Series: Pediatric Contact Dermatitis Workgroup. Dermatitis. 2018;29:206-212.
- Bergfors E, Inerot A, Falk L, et al. Patch testing children with aluminium chloride hexahydrate in petrolatum: a review and a recommendation. Contact Dermatitis. 2019;81:81-88.
- Worm M, Aberer W, Agathos M, et al. Patch testing in children—recommendations of the German Contact Dermatitis Research Group (DKG). J Dtsch Dermatol Ges. 2007;5:107-109.
- T.R.U.E. Test (Thin-Layer Rapid Use Epicutaneous Patch Test) [package insert]. Hillerød, Denmark: SmartPractice Denmark ApS; 2017.
- Jacob SE, Admani S, Herro EM. Invited commentary: recommendation for a North American pediatric patch test series. Curr Allergy Asthma Rep. 2014;14:444.
- Castanedo-Tardana MP, Zug KA. Methylisothiazolinone. Dermatitis. 2013;24:2-6.
- Brankov N, Jacob SE. Pre-emptive avoidance strategy 2016: update on pediatric contact dermatitis allergens. Expert Rev Clin Immunol. 2017;13:93-95.
- Yu J, Treat J, Brod B. Patch test series for allergic perineal dermatitis in the diapered infant. Dermatitis. 2017;28:70-75.
- Sung CT, McGowan MA, Jacob SE. Allergic contact dermatitis evaluation: strategies for the preschooler. Curr Allergy Asthma Rep. 2018;18:49.
- Yu J, Treat J, Chaney K, et al. Potential allergens in disposable diaper wipes, topical diaper preparations, and disposable diapers: under-recognized etiology of pediatric perineal dermatitis. Dermatitis. 2016;27:110-118.
- Anderson LE, Treat JR, Brod BA, et al. “Slime” contact dermatitis: case report and review of relevant allergens. Pediatr Dermatol. 2019;36:335-337.
- Dorfman CO, Barros MA, Zaenglein AL. Contact dermatitis to training toilet seat (potty seat dermatitis). Pediatr Dermatol. 2018;35:e251-e252.
- Bonchak JG, Prouty ME, de la Feld SF. Prevalence of contact allergens in personal care products for babies and children. Dermatitis. 2018;29:81-84.
- Chen JK, Jacob SE, Nedorost ST, et al. A pragmatic approach to patch testing atopic dermatitis patients: clinical recommendations based on expert consensus opinion. Dermatitis. 2016;27:186-192.
- Goldenberg A, Silverberg N, Silverberg JI, et al. Pediatric allergic contact dermatitis: lessons for better care. J Allergy Clin Immunol Pract. 2015;3:661-667; quiz 668.
- Bruckner AL, Weston WL, Morelli JG. Does sensitization to contact allergens begin in infancy? Pediatrics. 2000;105:e3.
- Zug KA, Pham AK, Belsito DV, et al. Patch testing in children from 2005 to 2012: results from the North American contact dermatitis group. Dermatitis. 2014;25:345-355.
- Goldenberg A, Mousdicas N, Silverberg N, et al. Pediatric Contact Dermatitis Registry inaugural case data. Dermatitis. 2016;27:293-302.
- Ortiz Salvador JM, Esteve Martinez A, Subiabre Ferrer D, et al. Pediatric allergic contact dermatitis: clinical and epidemiological study in a tertiary hospital. Actas Dermosifiliogr. 2017;108:571-578.
- Jacob SE, Steele T, Brod B, et al. Dispelling the myths behind pediatric patch testing—experience from our tertiary care patch testing centers. Pediatr Dermatol. 2008;25:296-300.
- Brod BA, Treat JR, Rothe MJ, et al. Allergic contact dermatitis: kids are not just little people. Clin Dermatol. 2015;33:605-612.
- Elliott JF, Ramzy A, Nilsson U, et al. Severe intractable eyelid dermatitis probably caused by exposure to hydroperoxides of linalool in a heavily fragranced shampoo. Contact Dermatitis. 2017;76:114-115.
- Yu J, Atwater AR, Brod B, et al. Pediatric Baseline Patch Test Series: Pediatric Contact Dermatitis Workgroup. Dermatitis. 2018;29:206-212.
- Bergfors E, Inerot A, Falk L, et al. Patch testing children with aluminium chloride hexahydrate in petrolatum: a review and a recommendation. Contact Dermatitis. 2019;81:81-88.
- Worm M, Aberer W, Agathos M, et al. Patch testing in children—recommendations of the German Contact Dermatitis Research Group (DKG). J Dtsch Dermatol Ges. 2007;5:107-109.
- T.R.U.E. Test (Thin-Layer Rapid Use Epicutaneous Patch Test) [package insert]. Hillerød, Denmark: SmartPractice Denmark ApS; 2017.
- Jacob SE, Admani S, Herro EM. Invited commentary: recommendation for a North American pediatric patch test series. Curr Allergy Asthma Rep. 2014;14:444.
- Castanedo-Tardana MP, Zug KA. Methylisothiazolinone. Dermatitis. 2013;24:2-6.
- Brankov N, Jacob SE. Pre-emptive avoidance strategy 2016: update on pediatric contact dermatitis allergens. Expert Rev Clin Immunol. 2017;13:93-95.
- Yu J, Treat J, Brod B. Patch test series for allergic perineal dermatitis in the diapered infant. Dermatitis. 2017;28:70-75.
- Sung CT, McGowan MA, Jacob SE. Allergic contact dermatitis evaluation: strategies for the preschooler. Curr Allergy Asthma Rep. 2018;18:49.
- Yu J, Treat J, Chaney K, et al. Potential allergens in disposable diaper wipes, topical diaper preparations, and disposable diapers: under-recognized etiology of pediatric perineal dermatitis. Dermatitis. 2016;27:110-118.
- Anderson LE, Treat JR, Brod BA, et al. “Slime” contact dermatitis: case report and review of relevant allergens. Pediatr Dermatol. 2019;36:335-337.
- Dorfman CO, Barros MA, Zaenglein AL. Contact dermatitis to training toilet seat (potty seat dermatitis). Pediatr Dermatol. 2018;35:e251-e252.
- Bonchak JG, Prouty ME, de la Feld SF. Prevalence of contact allergens in personal care products for babies and children. Dermatitis. 2018;29:81-84.
- Chen JK, Jacob SE, Nedorost ST, et al. A pragmatic approach to patch testing atopic dermatitis patients: clinical recommendations based on expert consensus opinion. Dermatitis. 2016;27:186-192.
- Goldenberg A, Silverberg N, Silverberg JI, et al. Pediatric allergic contact dermatitis: lessons for better care. J Allergy Clin Immunol Pract. 2015;3:661-667; quiz 668.
Practice Points
- Pediatric allergic contact dermatitis (ACD) is common with children having unique product exposures.
- Children suspected to have ACD should be patch tested with customized panels based on history and exposure.
- Common pediatric allergens have been identified in personal care products, household products, and recreational gear and toys.
Methylisothiazolinone and Isothiazolinone Allergy
Unless you have been living under a rock, you probably already know that the preservative methylisothiazolinone (MI) has caused an epidemic of allergic contact dermatitis (ACD) and was named the 2013 American Contact Dermatitis Society Allergen of the Year.1 Methylisothiazolinone is not new on the market, but its solo use as a preservative is relatively new. In this article, we review the emergence of MI as a common allergen, discuss North American MI patch test results, and describe common and uncommon sources of MI exposure. We also explore the related isothiazolinones, benzisothiazolinone (BIT) and octylisothiazolinone (OIT).
Background
Methylchloroisothiazolinone (MCI) and MI have been utilized as a preservative in a 3:1 ratio since the 1980s. In 2005, MI was first used alone as a preservative in personal care products in concentrations of up to 100 ppm, which represented a 25-fold increase in exposure to MI in personal care products and thus unleashed an epidemic of ACD.1 In the 2015 to 2016 cycle of the North American Contact Dermatitis Group (NACDG) patch testing results, MI was found to be positive in 13.4% of patch tested patients (N=5597) and also had the highest significance-prevalence index number, a calculation that represents the relevance of positive reactions in relationship to prevalence.2 In Europe, MI is banned in leave-on products and is allowed in rinse-off products in concentrations of up to 15 ppm. In the United States, the Cosmetic Ingredient Review panel concluded that MI is safe at a maximum concentration up to 100 ppm in rinse-off products and safe in leave-on products when formulated to be nonsensitizing, which may be determined based on a quantitative risk assessment.3
It is recommended that MI be patch tested at a concentration of 2000 ppm (0.2% aqueous).4 Testing at lower concentrations may result in missed positives. In addition, it should be noted that MCI/MI is present in the T.R.U.E. Test (SmartPractice), but MI alone is not.
Sources of MI Exposure
The first few case reports of MI contact allergy were associated with occupational exposures. In 2004, Isaksson et al5 reported 2 cases of MI allergy following exposure to wallpaper glue and a chemical burn from a biocide, respectively. Soon after, Thyssen et al6 reported 4 occupational cases of MI allergy at a paint manufacturing plant.
An early case series of MI contact allergy associated with personal care products was published in 2010 in which the authors described adults with ACD from wet wipes and a makeup remover that contained MI.7 A more recent report indicated that MI is now an infrequent ingredient in wet wipes but is still found in a wide variety of household and personal care products.8 A 2017 query of the American Contact Dermatitis Society’s Contact Allergy Management Program (CAMP) database revealed that 12.9% of all products contained MI. Furthermore, CAMP data revealed that MI was the most commonly found preservative in both hair care and household products.9 An additional CAMP database study revealed that 53% of shampoos and 45% of conditioners contained MI, and it also was commonly found in hair dyes, soaps and cleansers, hand cleaners and sanitizers, vaginal hygiene products, sunscreens, and moisturizers.10
Household products represent an important source of MI exposure. A chemical analysis of water-based paints identified the presence of isothiazolinones. Contact allergy from isothiazolinones in paint can present as either direct or airborne-pattern contact dermatitis.11 Sodium bisulfite has been used to inactivate MCI/MI in wall paint and could be utilized in severe cases of airborne contact dermatitis.12 Off-gassing may take up to 5.5 weeks before the paint cures and the isothiazolinone level decreases.13 A 2016 analysis of household products in the CAMP database revealed that MI commonly was found in dishwashing soap (64%), followed by household cleaners (47%), laundry softeners/additives (30%), surface disinfectants (27%), and laundry detergents (13%).10 Because certain chemical ingredients are not always listed on household product labels, patients with MI contact allergy may be at higher risk for unanticipated exposure to this allergen.
Dear reader, we know that you know all of this. We know that you have been watching the MI epidemic and have followed its every turn. But something that may be new to you are the unique MI exposures identified over the last several years.
In 2017, MI was identified in the glue used to affix 3 layers of the upper portion of a shoe.14 In addition, a recent chemical analysis of US consumer adhesives confirmed the presence of isothiazolinones in 50% (19/38) of products; 44.7% (17/38) specifically contained MI.15 Slime, the sticky play substance that children concoct out of household materials, has caused ACD, and not surprisingly, MI has been identified as a culprit allergen. In one case report, contact allergy was caused by MI present in a slime mixture made up of laundry detergent, dish soap, shampoo, and hand cream.16 In another case series, 3 children with MI contact allergy had played with slime that included dishwashing liquid, which contained MI, along with polyvinyl acetate glue and liquid soap components.17 Another case report documented slime made from MI-containing school glue as the source of ACD.18 Isothiazolinones also have been identified as causative allergens in “noise putty,” another homemade play item.19
Additionally, there has been a report of contact allergy to MI in a designer eyeglass frame.20 There also have been several documented cases of ACD to MCI/MI aerosolized from water used during ironing.21,22
There also have been several reports of photoaggravated ACD and possible photoallergic contact dermatitis from MI.23,24 In such cases, patients also may have transient photosensitivity even when MI exposure is discontinued; therefore, MI should be considered for inclusion in photopatch test panels when relevant.
Methylisothiazolinone contact allergy also should be considered for products that do not list MI on the label, which presents another potential exposure. In products that do not list MI as an ingredient on the label, its presence may be due to inclusion of the preservative in raw materials used in production. For example, a patient who reacted to a facial mask gel had a positive patch test reaction to MI, the facial mask gel, and sodium hyaluronate, the raw ingredient in the gel. Further analysis revealed that MI was unexpectedly present in the sodium hyaluronate.25 Similar scenarios have been reported in association with facial wet wipes,26 an exfoliating facial sponge,27 and a polyurethane sponge from a wound vacuum pump,28 among others.
Other Isothiazolinones
Other isothiazolinones also are known to cause ACD, albeit less commonly than MI. Benzisothiazolinone has been identified in glues, cleaning agents, paints, and industrial chemicals; unlike MI, the presence of BIT is infrequent in personal care products.15,29 This chemical is not commonly included in patch test screening series in the United States but is currently present in the NACDG screening series as BIT 0.1% in petrolatum.
Octylisothiazolinone (OIT) has been reported in leather furniture, belts, shoes, and watchbands, as well as industrial chemicals.30,31 Similar to BIT, OIT is not commonly tested in screening series in the United States; the NACDG tests this chemical as OIT 0.025% in petrolatum.
The cross-reaction patterns between the isothiazolinones remain uncertain. A study in mice supported cross-reactivity between MI, OIT, and BIT32; however, several clinical epidemiologic studies suggested that although there is evidence that there may be cross-reactivity between OIT and MI, concomitant positive BIT and MI reactions more likely represent cosensitization.33-35
Final Interpretation
Methylisothiazolinone continues to have high positive patch test rates in North American patch test populations and should be tested at a concentration of 2000 ppm (0.2% aqueous). Methylisothiazolinone may now be rare in wet wipes, but it is still present in numerous personal care products including hair care products, liquid soaps, and cleaning products. Novel exposures to MI include paint, slime, and glues. It also is important to remember that MI can cause photoaggravated or photoallergic contact dermatitis and might be a worthy addition to photopatch test trays. Finally, keep a look out for BIT and OIT, which may be present in industrial chemicals, glues, paints, cleaning products, and leather items
- Castanedo-Tardana MP, Zug KA. Methylisothiazolinone. Dermatitis. 2013;24:2-6. 2. DeKoven JG, Warshaw EM, Zug KA, et al. North American Contact Dermatitis Group patch test results: 2015-2016. Dermatitis. 2018;29:297-309.
- DeKoven JG, Warshaw EM, Zug KA, et al. North American Contact Dermatitis Group patch test results: 2015-2016. Dermatitis. 2018;29:297-309.
- Cosmetic Ingredient Review. Amended safety assessment of methylisothiazolinone as used in cosmetics. https://www.cir-safety.org/sites/default/files/mthiaz092014FR_final.pdf. Released October 8, 2014. Accessed July 9, 2019.
- Isaksson M, Ale I, Andersen KE, et al. Multicenter patch testing with methylisothiazolinone and methylchloroisothiazolinone/methylisothiazolinone within the International Contact Dermatitis Research Group. Dermatitis. 2017;28:210-214.
- Isaksson M, Gruvberger B, Bruze M. Occupational contact allergy and dermatitis from methylisothiazolinone after contact with wallcovering glue and after a chemical burn from a biocide. Dermatitis. 2004;15:201-205.
- Thyssen JP, Sederberg-Olsen N, Thomsen JF, et al. Contact dermatitis from methylisothiazolinone in a paint factory. Contact Dermatitis. 2006;54:322-324.
- García-Gavín J, Vansina S, Kerre S, et al. Methylisothiazolinone, an emerging allergen in cosmetics? Contact Dermatitis. 2010;63:96-101.
- Hamann CR, Sahni S, Zug KA. Methylisothiazolinone: still on leave-on products, but no longer on baby wipes. Dermatitis. 2019;30:173-174.
- Beene KM, Scheman A, Severson D, et al. Prevalence of preservatives across all product types in the Contact Allergen Management Program. Dermatitis. 2017;28:81-87.
- Scheman A, Severson D. American Contact Dermatitis Society Contact Allergy Management Program: an epidemiologic tool to quantify ingredient usage. Dermatitis. 2016;27:11-13.
- Goodier MC, Siegel PD, Zang LY, et al. Isothiazolinone in residential interior wall paint: a high-performance liquid chromatographic-mass spectrometry analysis. Dermatitis. 2018;29:332-338.
- Bohn S, Niederer M, Brehm K, et al. Airborne contact dermatitis from methylchloroisothiazolinone in wall paint. abolition of symptoms by chemical allergen inactivation. Contact Dermatitis. 2000;42:196-201.
- Amsler E, Aerts O, Raison-Peyron N, et al; Dermatology Allergy Group (DAG) of the French Society of Dermatology. Airborne allergic contact dermatitis caused by isothiazolinones in water-based paints: a retrospective study of 44 cases. Contact Dermatitis. 2017;77:163-170.
- Silva CA, El-Houri RB, Christensen LP, et al. Contact allergy caused by methylisothiazolinone in shoe glue. Contact Dermatitis. 2017;77:175-176.
- Goodier MC, Zang LY, Siegel PD, et al. Isothiazolinone content of US consumer adhesives: ultrahigh-performance liquid chromatographic mass spectrometry analysis. Dermatitis. 2019;30:129-134.
- Anderson LE, Treat JR, Brod BA, et al. “Slime” contact dermatitis: case report and review of relevant allergens. Pediatr Dermatol. 2019;36:335-337.
- Salman A, Demir G, Apti O. “Slime”: a trending cause of isothiazolinone contact allergy in children. Contact Dermatitis. 2019;80:409-411.
- Zhang AJ, Boyd AH, Asch S, et al. Allergic contact dermatitis to slime: the epidemic of isothiazolinone allergy encompasses school glue. Pediatr Dermatol. 2019;36:e37-e38.
- Ducharme O, Labadie M, Briand SM, et al. Allergic contact dermatitis in a child caused by isothiazolinones in a “noise putty.” Contact Dermatitis. 2018;79:393-394.
- El-Houri RB, Christensen LP, Persson C, et al. Methylisothiazolinone in a designer spectacle frame—a surprising finding. Contact Dermatitis. 2016;75:310-312.
- Atkar R, Todd P. Four cases of allergic contact dermatitis caused by methylchloroisothiazolinone/methylisothiazolinone in ironing water. Contact Dermatitis. 2016;75:316-317.
- Hunter KJ, Shelley JC, Haworth AE. Airborne allergic contact dermatitis to methylchloroisothiazolinone/methylisothiazolinone in ironing water. Contact Dermatitis. 2008;58:183-184.
- Aerts O, Goossens A, Marguery MC, et al. Photoaggravated allergic contact dermatitis and transient photosensitivity caused by methylisothiazolinone. Contact Dermatitis. 2018;78:241-245.
- Trokoudes D, Banerjee P, Fityan A, et al. Photoaggravated contact dermatitis caused by methylisothiazolinone. Contact Dermatitis. 2017;76:303-304.
- Kerre S, Naessens T, Theunis M, et al. Facial dermatitis caused by undeclared methylisothiazolinone in a gel mask: is the preservation of raw materials in cosmetics a cause of concern? Contact Dermatitis. 2018;78:421-424.
- Isaksson M, Persson L. ‘Mislabelled’ make-up remover wet wipes as a cause of severe, recalcitrant facial eczema [published online March 27, 2015]. Contact Dermatitis. 2015;73:56-59.
- Madsen JT, Andersen KE, Nielsen DT, et al. Undisclosed presence of methylisothiazolinone in ‘100% natural’ Konjac® sponge. Contact Dermatitis. 2016;75:308-309.
- Schliemann S, Isaksson M, Persson C, et al. Allergic contact dermatitis caused by methylchloroisothiazolinone/methylisothiazolinone in a medical device. Contact Dermatitis. 2016;75:312-314.
- Kaur-Knudsen D, Menné T, Christina Carlsen B. Systemic allergic dermatitis following airborne exposure to 1,2-benzisothiazolin-3-one. Contact Dermatitis. 2012;67:310-312.
- Aerts O, Meert H, Romaen E, et al. Octylisothiazolinone, an additional cause of allergic contact dermatitis caused by leather: case series and potential implications for the study of cross-reactivity with methylisothiazolinone. Contact Dermatitis. 2016;75:276-284.
- Alipour Tehrany Y, Quenan S, Bugey A, et al. Allergic contact dermatitis caused by octylisothiazolinone in a leather sofa. Contact Dermatitis. 2018;79:188-189.
- Schwensen JF, Menné Bonefeld C, Zachariae C, et al. Cross-reactivity between methylisothiazolinone, octylisothiazolinone and benzisothiazolinone using a modified local lymph node assay. Br J Dermatol. 2017;176:176-183.
- Aalto-Korte K, Suuronen K. Patterns of concomitant allergic reactions in patients suggest cross-sensitization between octylisothiazolinone and methylisothiazolinone. Contact Dermatitis. 2017;77:385-389.
- Craig S, Urwin R, Latheef F, et al. Patch test clinic experience of potential cross-reactivity of isothiazolinones. Contact Dermatitis. 2017;76:299-300.
- Geier J, Lessmann H, Schnuch A, et al. Concomitant reactivity to methylisothiazolinone, benzisothiazolinone, and octylisothiazolinone. International Network of Departments of Dermatology data, 2009-2013. Contact Dermatitis. 2015;72:337-339.
Unless you have been living under a rock, you probably already know that the preservative methylisothiazolinone (MI) has caused an epidemic of allergic contact dermatitis (ACD) and was named the 2013 American Contact Dermatitis Society Allergen of the Year.1 Methylisothiazolinone is not new on the market, but its solo use as a preservative is relatively new. In this article, we review the emergence of MI as a common allergen, discuss North American MI patch test results, and describe common and uncommon sources of MI exposure. We also explore the related isothiazolinones, benzisothiazolinone (BIT) and octylisothiazolinone (OIT).
Background
Methylchloroisothiazolinone (MCI) and MI have been utilized as a preservative in a 3:1 ratio since the 1980s. In 2005, MI was first used alone as a preservative in personal care products in concentrations of up to 100 ppm, which represented a 25-fold increase in exposure to MI in personal care products and thus unleashed an epidemic of ACD.1 In the 2015 to 2016 cycle of the North American Contact Dermatitis Group (NACDG) patch testing results, MI was found to be positive in 13.4% of patch tested patients (N=5597) and also had the highest significance-prevalence index number, a calculation that represents the relevance of positive reactions in relationship to prevalence.2 In Europe, MI is banned in leave-on products and is allowed in rinse-off products in concentrations of up to 15 ppm. In the United States, the Cosmetic Ingredient Review panel concluded that MI is safe at a maximum concentration up to 100 ppm in rinse-off products and safe in leave-on products when formulated to be nonsensitizing, which may be determined based on a quantitative risk assessment.3
It is recommended that MI be patch tested at a concentration of 2000 ppm (0.2% aqueous).4 Testing at lower concentrations may result in missed positives. In addition, it should be noted that MCI/MI is present in the T.R.U.E. Test (SmartPractice), but MI alone is not.
Sources of MI Exposure
The first few case reports of MI contact allergy were associated with occupational exposures. In 2004, Isaksson et al5 reported 2 cases of MI allergy following exposure to wallpaper glue and a chemical burn from a biocide, respectively. Soon after, Thyssen et al6 reported 4 occupational cases of MI allergy at a paint manufacturing plant.
An early case series of MI contact allergy associated with personal care products was published in 2010 in which the authors described adults with ACD from wet wipes and a makeup remover that contained MI.7 A more recent report indicated that MI is now an infrequent ingredient in wet wipes but is still found in a wide variety of household and personal care products.8 A 2017 query of the American Contact Dermatitis Society’s Contact Allergy Management Program (CAMP) database revealed that 12.9% of all products contained MI. Furthermore, CAMP data revealed that MI was the most commonly found preservative in both hair care and household products.9 An additional CAMP database study revealed that 53% of shampoos and 45% of conditioners contained MI, and it also was commonly found in hair dyes, soaps and cleansers, hand cleaners and sanitizers, vaginal hygiene products, sunscreens, and moisturizers.10
Household products represent an important source of MI exposure. A chemical analysis of water-based paints identified the presence of isothiazolinones. Contact allergy from isothiazolinones in paint can present as either direct or airborne-pattern contact dermatitis.11 Sodium bisulfite has been used to inactivate MCI/MI in wall paint and could be utilized in severe cases of airborne contact dermatitis.12 Off-gassing may take up to 5.5 weeks before the paint cures and the isothiazolinone level decreases.13 A 2016 analysis of household products in the CAMP database revealed that MI commonly was found in dishwashing soap (64%), followed by household cleaners (47%), laundry softeners/additives (30%), surface disinfectants (27%), and laundry detergents (13%).10 Because certain chemical ingredients are not always listed on household product labels, patients with MI contact allergy may be at higher risk for unanticipated exposure to this allergen.
Dear reader, we know that you know all of this. We know that you have been watching the MI epidemic and have followed its every turn. But something that may be new to you are the unique MI exposures identified over the last several years.
In 2017, MI was identified in the glue used to affix 3 layers of the upper portion of a shoe.14 In addition, a recent chemical analysis of US consumer adhesives confirmed the presence of isothiazolinones in 50% (19/38) of products; 44.7% (17/38) specifically contained MI.15 Slime, the sticky play substance that children concoct out of household materials, has caused ACD, and not surprisingly, MI has been identified as a culprit allergen. In one case report, contact allergy was caused by MI present in a slime mixture made up of laundry detergent, dish soap, shampoo, and hand cream.16 In another case series, 3 children with MI contact allergy had played with slime that included dishwashing liquid, which contained MI, along with polyvinyl acetate glue and liquid soap components.17 Another case report documented slime made from MI-containing school glue as the source of ACD.18 Isothiazolinones also have been identified as causative allergens in “noise putty,” another homemade play item.19
Additionally, there has been a report of contact allergy to MI in a designer eyeglass frame.20 There also have been several documented cases of ACD to MCI/MI aerosolized from water used during ironing.21,22
There also have been several reports of photoaggravated ACD and possible photoallergic contact dermatitis from MI.23,24 In such cases, patients also may have transient photosensitivity even when MI exposure is discontinued; therefore, MI should be considered for inclusion in photopatch test panels when relevant.
Methylisothiazolinone contact allergy also should be considered for products that do not list MI on the label, which presents another potential exposure. In products that do not list MI as an ingredient on the label, its presence may be due to inclusion of the preservative in raw materials used in production. For example, a patient who reacted to a facial mask gel had a positive patch test reaction to MI, the facial mask gel, and sodium hyaluronate, the raw ingredient in the gel. Further analysis revealed that MI was unexpectedly present in the sodium hyaluronate.25 Similar scenarios have been reported in association with facial wet wipes,26 an exfoliating facial sponge,27 and a polyurethane sponge from a wound vacuum pump,28 among others.
Other Isothiazolinones
Other isothiazolinones also are known to cause ACD, albeit less commonly than MI. Benzisothiazolinone has been identified in glues, cleaning agents, paints, and industrial chemicals; unlike MI, the presence of BIT is infrequent in personal care products.15,29 This chemical is not commonly included in patch test screening series in the United States but is currently present in the NACDG screening series as BIT 0.1% in petrolatum.
Octylisothiazolinone (OIT) has been reported in leather furniture, belts, shoes, and watchbands, as well as industrial chemicals.30,31 Similar to BIT, OIT is not commonly tested in screening series in the United States; the NACDG tests this chemical as OIT 0.025% in petrolatum.
The cross-reaction patterns between the isothiazolinones remain uncertain. A study in mice supported cross-reactivity between MI, OIT, and BIT32; however, several clinical epidemiologic studies suggested that although there is evidence that there may be cross-reactivity between OIT and MI, concomitant positive BIT and MI reactions more likely represent cosensitization.33-35
Final Interpretation
Methylisothiazolinone continues to have high positive patch test rates in North American patch test populations and should be tested at a concentration of 2000 ppm (0.2% aqueous). Methylisothiazolinone may now be rare in wet wipes, but it is still present in numerous personal care products including hair care products, liquid soaps, and cleaning products. Novel exposures to MI include paint, slime, and glues. It also is important to remember that MI can cause photoaggravated or photoallergic contact dermatitis and might be a worthy addition to photopatch test trays. Finally, keep a look out for BIT and OIT, which may be present in industrial chemicals, glues, paints, cleaning products, and leather items
Unless you have been living under a rock, you probably already know that the preservative methylisothiazolinone (MI) has caused an epidemic of allergic contact dermatitis (ACD) and was named the 2013 American Contact Dermatitis Society Allergen of the Year.1 Methylisothiazolinone is not new on the market, but its solo use as a preservative is relatively new. In this article, we review the emergence of MI as a common allergen, discuss North American MI patch test results, and describe common and uncommon sources of MI exposure. We also explore the related isothiazolinones, benzisothiazolinone (BIT) and octylisothiazolinone (OIT).
Background
Methylchloroisothiazolinone (MCI) and MI have been utilized as a preservative in a 3:1 ratio since the 1980s. In 2005, MI was first used alone as a preservative in personal care products in concentrations of up to 100 ppm, which represented a 25-fold increase in exposure to MI in personal care products and thus unleashed an epidemic of ACD.1 In the 2015 to 2016 cycle of the North American Contact Dermatitis Group (NACDG) patch testing results, MI was found to be positive in 13.4% of patch tested patients (N=5597) and also had the highest significance-prevalence index number, a calculation that represents the relevance of positive reactions in relationship to prevalence.2 In Europe, MI is banned in leave-on products and is allowed in rinse-off products in concentrations of up to 15 ppm. In the United States, the Cosmetic Ingredient Review panel concluded that MI is safe at a maximum concentration up to 100 ppm in rinse-off products and safe in leave-on products when formulated to be nonsensitizing, which may be determined based on a quantitative risk assessment.3
It is recommended that MI be patch tested at a concentration of 2000 ppm (0.2% aqueous).4 Testing at lower concentrations may result in missed positives. In addition, it should be noted that MCI/MI is present in the T.R.U.E. Test (SmartPractice), but MI alone is not.
Sources of MI Exposure
The first few case reports of MI contact allergy were associated with occupational exposures. In 2004, Isaksson et al5 reported 2 cases of MI allergy following exposure to wallpaper glue and a chemical burn from a biocide, respectively. Soon after, Thyssen et al6 reported 4 occupational cases of MI allergy at a paint manufacturing plant.
An early case series of MI contact allergy associated with personal care products was published in 2010 in which the authors described adults with ACD from wet wipes and a makeup remover that contained MI.7 A more recent report indicated that MI is now an infrequent ingredient in wet wipes but is still found in a wide variety of household and personal care products.8 A 2017 query of the American Contact Dermatitis Society’s Contact Allergy Management Program (CAMP) database revealed that 12.9% of all products contained MI. Furthermore, CAMP data revealed that MI was the most commonly found preservative in both hair care and household products.9 An additional CAMP database study revealed that 53% of shampoos and 45% of conditioners contained MI, and it also was commonly found in hair dyes, soaps and cleansers, hand cleaners and sanitizers, vaginal hygiene products, sunscreens, and moisturizers.10
Household products represent an important source of MI exposure. A chemical analysis of water-based paints identified the presence of isothiazolinones. Contact allergy from isothiazolinones in paint can present as either direct or airborne-pattern contact dermatitis.11 Sodium bisulfite has been used to inactivate MCI/MI in wall paint and could be utilized in severe cases of airborne contact dermatitis.12 Off-gassing may take up to 5.5 weeks before the paint cures and the isothiazolinone level decreases.13 A 2016 analysis of household products in the CAMP database revealed that MI commonly was found in dishwashing soap (64%), followed by household cleaners (47%), laundry softeners/additives (30%), surface disinfectants (27%), and laundry detergents (13%).10 Because certain chemical ingredients are not always listed on household product labels, patients with MI contact allergy may be at higher risk for unanticipated exposure to this allergen.
Dear reader, we know that you know all of this. We know that you have been watching the MI epidemic and have followed its every turn. But something that may be new to you are the unique MI exposures identified over the last several years.
In 2017, MI was identified in the glue used to affix 3 layers of the upper portion of a shoe.14 In addition, a recent chemical analysis of US consumer adhesives confirmed the presence of isothiazolinones in 50% (19/38) of products; 44.7% (17/38) specifically contained MI.15 Slime, the sticky play substance that children concoct out of household materials, has caused ACD, and not surprisingly, MI has been identified as a culprit allergen. In one case report, contact allergy was caused by MI present in a slime mixture made up of laundry detergent, dish soap, shampoo, and hand cream.16 In another case series, 3 children with MI contact allergy had played with slime that included dishwashing liquid, which contained MI, along with polyvinyl acetate glue and liquid soap components.17 Another case report documented slime made from MI-containing school glue as the source of ACD.18 Isothiazolinones also have been identified as causative allergens in “noise putty,” another homemade play item.19
Additionally, there has been a report of contact allergy to MI in a designer eyeglass frame.20 There also have been several documented cases of ACD to MCI/MI aerosolized from water used during ironing.21,22
There also have been several reports of photoaggravated ACD and possible photoallergic contact dermatitis from MI.23,24 In such cases, patients also may have transient photosensitivity even when MI exposure is discontinued; therefore, MI should be considered for inclusion in photopatch test panels when relevant.
Methylisothiazolinone contact allergy also should be considered for products that do not list MI on the label, which presents another potential exposure. In products that do not list MI as an ingredient on the label, its presence may be due to inclusion of the preservative in raw materials used in production. For example, a patient who reacted to a facial mask gel had a positive patch test reaction to MI, the facial mask gel, and sodium hyaluronate, the raw ingredient in the gel. Further analysis revealed that MI was unexpectedly present in the sodium hyaluronate.25 Similar scenarios have been reported in association with facial wet wipes,26 an exfoliating facial sponge,27 and a polyurethane sponge from a wound vacuum pump,28 among others.
Other Isothiazolinones
Other isothiazolinones also are known to cause ACD, albeit less commonly than MI. Benzisothiazolinone has been identified in glues, cleaning agents, paints, and industrial chemicals; unlike MI, the presence of BIT is infrequent in personal care products.15,29 This chemical is not commonly included in patch test screening series in the United States but is currently present in the NACDG screening series as BIT 0.1% in petrolatum.
Octylisothiazolinone (OIT) has been reported in leather furniture, belts, shoes, and watchbands, as well as industrial chemicals.30,31 Similar to BIT, OIT is not commonly tested in screening series in the United States; the NACDG tests this chemical as OIT 0.025% in petrolatum.
The cross-reaction patterns between the isothiazolinones remain uncertain. A study in mice supported cross-reactivity between MI, OIT, and BIT32; however, several clinical epidemiologic studies suggested that although there is evidence that there may be cross-reactivity between OIT and MI, concomitant positive BIT and MI reactions more likely represent cosensitization.33-35
Final Interpretation
Methylisothiazolinone continues to have high positive patch test rates in North American patch test populations and should be tested at a concentration of 2000 ppm (0.2% aqueous). Methylisothiazolinone may now be rare in wet wipes, but it is still present in numerous personal care products including hair care products, liquid soaps, and cleaning products. Novel exposures to MI include paint, slime, and glues. It also is important to remember that MI can cause photoaggravated or photoallergic contact dermatitis and might be a worthy addition to photopatch test trays. Finally, keep a look out for BIT and OIT, which may be present in industrial chemicals, glues, paints, cleaning products, and leather items
- Castanedo-Tardana MP, Zug KA. Methylisothiazolinone. Dermatitis. 2013;24:2-6. 2. DeKoven JG, Warshaw EM, Zug KA, et al. North American Contact Dermatitis Group patch test results: 2015-2016. Dermatitis. 2018;29:297-309.
- DeKoven JG, Warshaw EM, Zug KA, et al. North American Contact Dermatitis Group patch test results: 2015-2016. Dermatitis. 2018;29:297-309.
- Cosmetic Ingredient Review. Amended safety assessment of methylisothiazolinone as used in cosmetics. https://www.cir-safety.org/sites/default/files/mthiaz092014FR_final.pdf. Released October 8, 2014. Accessed July 9, 2019.
- Isaksson M, Ale I, Andersen KE, et al. Multicenter patch testing with methylisothiazolinone and methylchloroisothiazolinone/methylisothiazolinone within the International Contact Dermatitis Research Group. Dermatitis. 2017;28:210-214.
- Isaksson M, Gruvberger B, Bruze M. Occupational contact allergy and dermatitis from methylisothiazolinone after contact with wallcovering glue and after a chemical burn from a biocide. Dermatitis. 2004;15:201-205.
- Thyssen JP, Sederberg-Olsen N, Thomsen JF, et al. Contact dermatitis from methylisothiazolinone in a paint factory. Contact Dermatitis. 2006;54:322-324.
- García-Gavín J, Vansina S, Kerre S, et al. Methylisothiazolinone, an emerging allergen in cosmetics? Contact Dermatitis. 2010;63:96-101.
- Hamann CR, Sahni S, Zug KA. Methylisothiazolinone: still on leave-on products, but no longer on baby wipes. Dermatitis. 2019;30:173-174.
- Beene KM, Scheman A, Severson D, et al. Prevalence of preservatives across all product types in the Contact Allergen Management Program. Dermatitis. 2017;28:81-87.
- Scheman A, Severson D. American Contact Dermatitis Society Contact Allergy Management Program: an epidemiologic tool to quantify ingredient usage. Dermatitis. 2016;27:11-13.
- Goodier MC, Siegel PD, Zang LY, et al. Isothiazolinone in residential interior wall paint: a high-performance liquid chromatographic-mass spectrometry analysis. Dermatitis. 2018;29:332-338.
- Bohn S, Niederer M, Brehm K, et al. Airborne contact dermatitis from methylchloroisothiazolinone in wall paint. abolition of symptoms by chemical allergen inactivation. Contact Dermatitis. 2000;42:196-201.
- Amsler E, Aerts O, Raison-Peyron N, et al; Dermatology Allergy Group (DAG) of the French Society of Dermatology. Airborne allergic contact dermatitis caused by isothiazolinones in water-based paints: a retrospective study of 44 cases. Contact Dermatitis. 2017;77:163-170.
- Silva CA, El-Houri RB, Christensen LP, et al. Contact allergy caused by methylisothiazolinone in shoe glue. Contact Dermatitis. 2017;77:175-176.
- Goodier MC, Zang LY, Siegel PD, et al. Isothiazolinone content of US consumer adhesives: ultrahigh-performance liquid chromatographic mass spectrometry analysis. Dermatitis. 2019;30:129-134.
- Anderson LE, Treat JR, Brod BA, et al. “Slime” contact dermatitis: case report and review of relevant allergens. Pediatr Dermatol. 2019;36:335-337.
- Salman A, Demir G, Apti O. “Slime”: a trending cause of isothiazolinone contact allergy in children. Contact Dermatitis. 2019;80:409-411.
- Zhang AJ, Boyd AH, Asch S, et al. Allergic contact dermatitis to slime: the epidemic of isothiazolinone allergy encompasses school glue. Pediatr Dermatol. 2019;36:e37-e38.
- Ducharme O, Labadie M, Briand SM, et al. Allergic contact dermatitis in a child caused by isothiazolinones in a “noise putty.” Contact Dermatitis. 2018;79:393-394.
- El-Houri RB, Christensen LP, Persson C, et al. Methylisothiazolinone in a designer spectacle frame—a surprising finding. Contact Dermatitis. 2016;75:310-312.
- Atkar R, Todd P. Four cases of allergic contact dermatitis caused by methylchloroisothiazolinone/methylisothiazolinone in ironing water. Contact Dermatitis. 2016;75:316-317.
- Hunter KJ, Shelley JC, Haworth AE. Airborne allergic contact dermatitis to methylchloroisothiazolinone/methylisothiazolinone in ironing water. Contact Dermatitis. 2008;58:183-184.
- Aerts O, Goossens A, Marguery MC, et al. Photoaggravated allergic contact dermatitis and transient photosensitivity caused by methylisothiazolinone. Contact Dermatitis. 2018;78:241-245.
- Trokoudes D, Banerjee P, Fityan A, et al. Photoaggravated contact dermatitis caused by methylisothiazolinone. Contact Dermatitis. 2017;76:303-304.
- Kerre S, Naessens T, Theunis M, et al. Facial dermatitis caused by undeclared methylisothiazolinone in a gel mask: is the preservation of raw materials in cosmetics a cause of concern? Contact Dermatitis. 2018;78:421-424.
- Isaksson M, Persson L. ‘Mislabelled’ make-up remover wet wipes as a cause of severe, recalcitrant facial eczema [published online March 27, 2015]. Contact Dermatitis. 2015;73:56-59.
- Madsen JT, Andersen KE, Nielsen DT, et al. Undisclosed presence of methylisothiazolinone in ‘100% natural’ Konjac® sponge. Contact Dermatitis. 2016;75:308-309.
- Schliemann S, Isaksson M, Persson C, et al. Allergic contact dermatitis caused by methylchloroisothiazolinone/methylisothiazolinone in a medical device. Contact Dermatitis. 2016;75:312-314.
- Kaur-Knudsen D, Menné T, Christina Carlsen B. Systemic allergic dermatitis following airborne exposure to 1,2-benzisothiazolin-3-one. Contact Dermatitis. 2012;67:310-312.
- Aerts O, Meert H, Romaen E, et al. Octylisothiazolinone, an additional cause of allergic contact dermatitis caused by leather: case series and potential implications for the study of cross-reactivity with methylisothiazolinone. Contact Dermatitis. 2016;75:276-284.
- Alipour Tehrany Y, Quenan S, Bugey A, et al. Allergic contact dermatitis caused by octylisothiazolinone in a leather sofa. Contact Dermatitis. 2018;79:188-189.
- Schwensen JF, Menné Bonefeld C, Zachariae C, et al. Cross-reactivity between methylisothiazolinone, octylisothiazolinone and benzisothiazolinone using a modified local lymph node assay. Br J Dermatol. 2017;176:176-183.
- Aalto-Korte K, Suuronen K. Patterns of concomitant allergic reactions in patients suggest cross-sensitization between octylisothiazolinone and methylisothiazolinone. Contact Dermatitis. 2017;77:385-389.
- Craig S, Urwin R, Latheef F, et al. Patch test clinic experience of potential cross-reactivity of isothiazolinones. Contact Dermatitis. 2017;76:299-300.
- Geier J, Lessmann H, Schnuch A, et al. Concomitant reactivity to methylisothiazolinone, benzisothiazolinone, and octylisothiazolinone. International Network of Departments of Dermatology data, 2009-2013. Contact Dermatitis. 2015;72:337-339.
- Castanedo-Tardana MP, Zug KA. Methylisothiazolinone. Dermatitis. 2013;24:2-6. 2. DeKoven JG, Warshaw EM, Zug KA, et al. North American Contact Dermatitis Group patch test results: 2015-2016. Dermatitis. 2018;29:297-309.
- DeKoven JG, Warshaw EM, Zug KA, et al. North American Contact Dermatitis Group patch test results: 2015-2016. Dermatitis. 2018;29:297-309.
- Cosmetic Ingredient Review. Amended safety assessment of methylisothiazolinone as used in cosmetics. https://www.cir-safety.org/sites/default/files/mthiaz092014FR_final.pdf. Released October 8, 2014. Accessed July 9, 2019.
- Isaksson M, Ale I, Andersen KE, et al. Multicenter patch testing with methylisothiazolinone and methylchloroisothiazolinone/methylisothiazolinone within the International Contact Dermatitis Research Group. Dermatitis. 2017;28:210-214.
- Isaksson M, Gruvberger B, Bruze M. Occupational contact allergy and dermatitis from methylisothiazolinone after contact with wallcovering glue and after a chemical burn from a biocide. Dermatitis. 2004;15:201-205.
- Thyssen JP, Sederberg-Olsen N, Thomsen JF, et al. Contact dermatitis from methylisothiazolinone in a paint factory. Contact Dermatitis. 2006;54:322-324.
- García-Gavín J, Vansina S, Kerre S, et al. Methylisothiazolinone, an emerging allergen in cosmetics? Contact Dermatitis. 2010;63:96-101.
- Hamann CR, Sahni S, Zug KA. Methylisothiazolinone: still on leave-on products, but no longer on baby wipes. Dermatitis. 2019;30:173-174.
- Beene KM, Scheman A, Severson D, et al. Prevalence of preservatives across all product types in the Contact Allergen Management Program. Dermatitis. 2017;28:81-87.
- Scheman A, Severson D. American Contact Dermatitis Society Contact Allergy Management Program: an epidemiologic tool to quantify ingredient usage. Dermatitis. 2016;27:11-13.
- Goodier MC, Siegel PD, Zang LY, et al. Isothiazolinone in residential interior wall paint: a high-performance liquid chromatographic-mass spectrometry analysis. Dermatitis. 2018;29:332-338.
- Bohn S, Niederer M, Brehm K, et al. Airborne contact dermatitis from methylchloroisothiazolinone in wall paint. abolition of symptoms by chemical allergen inactivation. Contact Dermatitis. 2000;42:196-201.
- Amsler E, Aerts O, Raison-Peyron N, et al; Dermatology Allergy Group (DAG) of the French Society of Dermatology. Airborne allergic contact dermatitis caused by isothiazolinones in water-based paints: a retrospective study of 44 cases. Contact Dermatitis. 2017;77:163-170.
- Silva CA, El-Houri RB, Christensen LP, et al. Contact allergy caused by methylisothiazolinone in shoe glue. Contact Dermatitis. 2017;77:175-176.
- Goodier MC, Zang LY, Siegel PD, et al. Isothiazolinone content of US consumer adhesives: ultrahigh-performance liquid chromatographic mass spectrometry analysis. Dermatitis. 2019;30:129-134.
- Anderson LE, Treat JR, Brod BA, et al. “Slime” contact dermatitis: case report and review of relevant allergens. Pediatr Dermatol. 2019;36:335-337.
- Salman A, Demir G, Apti O. “Slime”: a trending cause of isothiazolinone contact allergy in children. Contact Dermatitis. 2019;80:409-411.
- Zhang AJ, Boyd AH, Asch S, et al. Allergic contact dermatitis to slime: the epidemic of isothiazolinone allergy encompasses school glue. Pediatr Dermatol. 2019;36:e37-e38.
- Ducharme O, Labadie M, Briand SM, et al. Allergic contact dermatitis in a child caused by isothiazolinones in a “noise putty.” Contact Dermatitis. 2018;79:393-394.
- El-Houri RB, Christensen LP, Persson C, et al. Methylisothiazolinone in a designer spectacle frame—a surprising finding. Contact Dermatitis. 2016;75:310-312.
- Atkar R, Todd P. Four cases of allergic contact dermatitis caused by methylchloroisothiazolinone/methylisothiazolinone in ironing water. Contact Dermatitis. 2016;75:316-317.
- Hunter KJ, Shelley JC, Haworth AE. Airborne allergic contact dermatitis to methylchloroisothiazolinone/methylisothiazolinone in ironing water. Contact Dermatitis. 2008;58:183-184.
- Aerts O, Goossens A, Marguery MC, et al. Photoaggravated allergic contact dermatitis and transient photosensitivity caused by methylisothiazolinone. Contact Dermatitis. 2018;78:241-245.
- Trokoudes D, Banerjee P, Fityan A, et al. Photoaggravated contact dermatitis caused by methylisothiazolinone. Contact Dermatitis. 2017;76:303-304.
- Kerre S, Naessens T, Theunis M, et al. Facial dermatitis caused by undeclared methylisothiazolinone in a gel mask: is the preservation of raw materials in cosmetics a cause of concern? Contact Dermatitis. 2018;78:421-424.
- Isaksson M, Persson L. ‘Mislabelled’ make-up remover wet wipes as a cause of severe, recalcitrant facial eczema [published online March 27, 2015]. Contact Dermatitis. 2015;73:56-59.
- Madsen JT, Andersen KE, Nielsen DT, et al. Undisclosed presence of methylisothiazolinone in ‘100% natural’ Konjac® sponge. Contact Dermatitis. 2016;75:308-309.
- Schliemann S, Isaksson M, Persson C, et al. Allergic contact dermatitis caused by methylchloroisothiazolinone/methylisothiazolinone in a medical device. Contact Dermatitis. 2016;75:312-314.
- Kaur-Knudsen D, Menné T, Christina Carlsen B. Systemic allergic dermatitis following airborne exposure to 1,2-benzisothiazolin-3-one. Contact Dermatitis. 2012;67:310-312.
- Aerts O, Meert H, Romaen E, et al. Octylisothiazolinone, an additional cause of allergic contact dermatitis caused by leather: case series and potential implications for the study of cross-reactivity with methylisothiazolinone. Contact Dermatitis. 2016;75:276-284.
- Alipour Tehrany Y, Quenan S, Bugey A, et al. Allergic contact dermatitis caused by octylisothiazolinone in a leather sofa. Contact Dermatitis. 2018;79:188-189.
- Schwensen JF, Menné Bonefeld C, Zachariae C, et al. Cross-reactivity between methylisothiazolinone, octylisothiazolinone and benzisothiazolinone using a modified local lymph node assay. Br J Dermatol. 2017;176:176-183.
- Aalto-Korte K, Suuronen K. Patterns of concomitant allergic reactions in patients suggest cross-sensitization between octylisothiazolinone and methylisothiazolinone. Contact Dermatitis. 2017;77:385-389.
- Craig S, Urwin R, Latheef F, et al. Patch test clinic experience of potential cross-reactivity of isothiazolinones. Contact Dermatitis. 2017;76:299-300.
- Geier J, Lessmann H, Schnuch A, et al. Concomitant reactivity to methylisothiazolinone, benzisothiazolinone, and octylisothiazolinone. International Network of Departments of Dermatology data, 2009-2013. Contact Dermatitis. 2015;72:337-339.
Practice Points
- Methylisothiazolinone (MI) is a preservative found in water-based personal care products and is a common allergen in patch-tested populations.
- Methylisothiazolinone also has been identified in household products, industrial chemicals, paint, adhesives, and other unique sources.
- Benzisothiazolinone and octylisothiazolinone are structurally similar to MI, and a subset of MI-allergic patients may need to avoid them.
Trends in Nail Services May Cause Dermatitis: Not Your Mother’s Nail Polish
In 2017, consumers spent an average of $8.53 billion on nail services.1 This booming industry is set to grow to more than $15.5 billion by 2024.2 Nail polishes and other nail cosmetic trends can present new exposures for consumers, including chemicals that can elicit allergic contact dermatitis. In this article, we discuss new nail trends and their associated allergens, the acrylates.
Tosylamide/Formaldehyde Resin
Traditionally, the most widely recognized nail polish allergen has been tosylamide/formaldehyde resin (TSFR). However, there now are many touted TSFR-free nail polishes on the market, and the rate of positive reactions to this chemical has been declining in recent years. The North American Contact Dermatitis Group reported a positive reaction rate of 1.3% from 2005 through 2006,3 and rates decreased to 0.9% from 2015 through 2016.4 An Australian study demonstrated a similar reduction in positive reaction rates to nail polish chemicals, with only 0.7% of patients reacting to TSFR from 2014 to 2016 and 0% in 2017. It is theorized that this reduction occurred from replacing TSFR in traditional nail polishes with other chemicals such as polyester resins and cellulose acetate butyrate.5
Acrylate-Based Nail Treatments
Consumers recently have been gravitating toward acrylate-based nail treatments vs traditional nail polishes for a variety of reasons. Often referred to as gels, dips, or shellac, acrylate-based nail treatments represent a hot new trend in nail cosmetics. These manicures are resistant to chipping and scratches, creating a like-new look that lasts for weeks after application. The long-lasting nature of acrylate-based nail polishes has made them wildly popular with consumers.
Traditional acrylic nails consist of a powder polymer mixed with a liquid monomer, which polymerizes when a catalyst is added.6 The procedure is time consuming and can take up to 2 hours for application. In contrast, the newer gel manicure can be completed faster and includes application of acrylate-based nail polish, including a base coat, 2 coats of color, and a top coat. Exposure to either a light-emitting diode (30–60 seconds) or UVA (2 minutes) lamp is necessary after each coat is applied for polymerization (Figure 1).6 This long-lasting, semipermanent manicure typically is what patients are referring to when they say they have “gel nails.”
Gel dipping powders (referred to as dips) are another long-lasting acrylate-based nail treatment. This type of polish uses ethyl cyanoacrylate, a slightly different acrylate (yes, that IS super glue). After the nail is prepared, a base polish is applied to three-quarters of the nail and it is dipped into a natural color dip powder. The base polish is then applied to the entire nail, followed by a dip into the polish color of choice. This process is completed twice, followed by shaping and application of a top coat (Figure 2).
base coat. B, Application of dip powder to gel polish. Note the entire
distal finger and nail are dipped into the powder. C, Shaping of the
nail after the second coat of color is applied.
Finally, there are nail wraps, which are similar to stickers placed over or extending the nail plate. The wraps can be made from linen, silk, vinyl, or other material. Ethyl cyanoacrylate and isopropyl-2-cyanoacrylates have been identified in nail wrap adhesive.7 The heated product is directly applied to the prepared nail, and the excess wrap is filed off. Additional nail polish and a top coat usually are applied to finish the nail. Many of these products are available for in-salon use as well as online purchase and home application by consumers.
Acrylate Allergy
Patients who are allergic to acrylates can present with different patterns of dermatitis. Although the majority of patients present with dermatitis on the hands, fingers, or wrists, up to 10% may only have facial and neck dermatitis.8 Less commonly, the abdomen and thighs can be involved.6,8 Nail technicians most commonly present with pulpitis with cutaneous fissures.8 Other symptoms can include subungual hyperkeratosis, onycholysis, and nail dystrophy. Paresthesia, urticaria, and upper respiratory tract symptoms can occur but are less common.6,8
Acrylate allergy typically is the result of sensitization to the acrylate monomers. In theory, gel nail acrylate materials are polymerized following exposure to a light-emitting diode or UVA lamp; however, there likely is some incomplete polymerization, which can increase the risk for development of allergy. Allergen exposure can occur due to incorrect application of the light source; inadvertent monomer exposure, which occurs when nail technicians wipe extra acrylate off of a client’s finger(s); or inadvertent application of acrylate monomers to objects in the nail technician’s work environment.6,8
Several acrylate nail allergens have been reported. Many studies have identified 2-hydroxyethyl methacrylate (HEMA) as the most common nail acrylate allergen.8,9 At least one study identified 2-hydroxypropyl methacrylate as the most common, with HEMA in second place.6 Other reported acrylate allergens have included ethylene glycol dimethacrylate, triethylene glycol dimethacrylate, methyl methacrylate, ethyl cyanoacrylate, 1,4-butanediol diacrylate, hydroxypropyl acrylate, and 2-hydroxyethyl acrylate.8,9
The American Contact Dermatitis Society Core Allergen Series and the North American Contact Dermatitis Group screening series currently include HEMA, methyl methacrylate, ethyl acrylate, ethyl cyanoacrylate, and TSFR.4,10 Of note, acrylates are not included in the thin-layer rapid use epicutaneous (T.R.U.E.) patch test (SmartPractice), so they will be missed if this series is used.11 In the setting of suspected nail acrylate allergy, some authors recommend initial screening with HEMA and ethyl cyanoacrylate, with extended acrylate testing if both are negative.8
Upon patch testing with an acrylate series, patients frequently react to 2 or more acrylates and the reactions can be strong (++) or extreme (+++), which may represent cosensitization or cross-sensitization.8 The likelihood of cross-reactivity between acrylates is not clear, though it has been postulated that it is theoretically possible.6
An important pearl for patch testers using the chamber method is proper storage of acrylate allergens and assembly of trays prior to patch testing. Similar to all haptens, manufacturers recommend that acrylates should be stored in a refrigerator, but some authors suggest that acrylates should be stored in the freezer.12 Acrylates are volatile chemicals and rapidly degrade when exposed to air. A methyl methacrylate preparation loaded into an inert quadrate (IQ) chamber and stored at room temperature showed a nearly undetectable amount of any residual methyl methacrylate 24 hours later. Refrigeration of allergens in chambers slowed but did not stop eventual degradation, with nearly all acrylate preparations reaching an undetectable level of allergen by day 8.13 Acrylates, along with other volatile allergens, should only be loaded into chambers immediately prior to placement on the patient.
Allergy Prevention
Prevention of nail acrylate allergy among consumers is simple: avoid contact with the offending allergen. Acrylate spillover (ie, applying the acrylate onto the skin) and direct contact with objects and working surfaces contaminated with acrylate-based nail products should be avoided.8 Avoidance is more complicated for nail technicians, but it is thought that nitrile gloves allow for the best dexterity and allergen avoidance when acrylate exposure is brief.14 Allowable exposure times with nitrile gloves may be 15 to 30 minutes. After this times passes, a glove change is required to avoid exposure.14 Wearing nitrile gloves for longer than 15 to 30 minutes will result in cutaneous exposure and risk for dermatitis in sensitized patients. If longer wear is desired, one option includes cutting the fingertips off of Silver Shield/4H gloves (Honeywell Safety Products USA, Inc), applying them to the distal fingers, and wearing a standard nitrile glove over top, known as the finger stall technique.6 In one study, this technique was recommended to nail technicians with acrylate allergy. A telephone survey conducted 4 to 43 months later confirmed that 36% (8/22) of participants were using the technique without symptoms. In this same study, 73% (16/22) had continued working as nail technicians.6
Acrylates are used for other medical purposes, including dental procedures, orthopedic procedures, surgical glues, wound dressings, and contact and intraocular lenses. They also have additional cosmetic applications, including eyelash and hair extensions.8 Therefore, it is vital that patients disclose any history of acrylate allergy to both their medical and cosmetic providers.
Our Final Interpretation
Acrylate allergy has become increasingly common, and long-lasting nail treatments often are the culprit. Whether through gels, dips, or shellac, repeated exposure to acrylates through nail treatments can increase the risk for allergy. The T.R.U.E. test alone will not make the diagnosis, as acrylates are not present in this patch test system. It is important to remind your allergic patients that acrylates are present in other compounds used for medical and cosmetic purposes. Avoidance is key, and for allergic patients who love to bedazzle their nails, we suggest less-permanent, acrylate-free nail polishes as alternatives.
- 2017-2018 industry statistics highlights. Nails Magazine. http://files.nailsmag.com/handouts/nabb2017-18stats-lr.pdf. Accessed May 17, 2019.
- Nail polish market size worth $15.55 billion by 2024. Grand View Research website. https://www.grandviewresearch.com/press-release/global-nail-polish-market. Published October 2017. Accessed May 17, 2019.
- Zug KA, Warshaw EM, Fowler JF, et al. Patch-test results of the North American Contact Dermatitis Group 2005-2006. Dermatitis. 2009;20:149-160.
- DeKoven J, Warshaw EM, Zug KA, et al. North American Contact Dermatitis Group patch test results: 2015-2016. Dermatitis. 2018;29:297-309.
- Lee S, Maor D, Palmer A, et al. Declining prevalence of allergic contact dermatitis caused by tosylamide/formaldehyde in nail polish. Contact Dermatitis. 2018;79:184-185.
- Gatica-Ortega ME, Pastor-Nieto MA, Mercader-García P, et al. Allergic contact dermatitis caused by (meth)acrylates in long-lasting nail polish: are we facing a new epidemic in the beauty industry? Contact Dermatitis. 2017;7:360-366.
- Fitzgerald DA, Bhaggoe R, English JS. Contact sensitivity to cyanoacrylate nail-adhesive with dermatitis at remote sites. Contact Dermatitis. 1995;32:175-176.
- Goncalo M, Pinho A, Agner T et al. Allergic contact dermatitis caused by nail acrylates in Europe. an EECDRG study. Contact Dermatitis. 2017;78:254-260.
- Fisch A, Hamnerius N, Isaksson M. Dermatitis and occupational (meth)acrylate contact allergy in nail technicians—a 10-year study [published online January 14, 2019]. Contact Dermatitis. doi:10.1111/cod.13216.
- Schalock PC, Dunnick CA, Nedorost S, et al. American Contact Dermatitis Society core allergen series: 2017 update. Dermatitis. 2017;28:141-143.
- T.R.U.E. TEST ready-to-use patch test panels. Smart Practice website. https://www.smartpractice.com/shop/wa/category?cn=T.R.U.E.-TEST%C2%AE-Ready-to-Use-Patch-Test-Panels&id=508222&m=SPA. Accessed May 17, 2019.
- Good AT, Bruze M, Zimerson E, et al. Variation in allergen content over time of acrylates/methylacrylates in patch test preparations. Br J Dermatol. 2011;164:116-124.
- Goon A, Bruze M, Zimerson E, et al. Variation in allergen content over time of acrylates/methacrylates in patch test preparations. Br J Dermatol. 2011;164:116-124.
- Morgado F, Batista M, Gonçalo M. Short exposures and glove protection against (meth)acrylates in nail beauticians—thoughts on a rising concern [published online January 17, 2019]. Contact Dermatitis. doi:10.1111/cod.13222.
In 2017, consumers spent an average of $8.53 billion on nail services.1 This booming industry is set to grow to more than $15.5 billion by 2024.2 Nail polishes and other nail cosmetic trends can present new exposures for consumers, including chemicals that can elicit allergic contact dermatitis. In this article, we discuss new nail trends and their associated allergens, the acrylates.
Tosylamide/Formaldehyde Resin
Traditionally, the most widely recognized nail polish allergen has been tosylamide/formaldehyde resin (TSFR). However, there now are many touted TSFR-free nail polishes on the market, and the rate of positive reactions to this chemical has been declining in recent years. The North American Contact Dermatitis Group reported a positive reaction rate of 1.3% from 2005 through 2006,3 and rates decreased to 0.9% from 2015 through 2016.4 An Australian study demonstrated a similar reduction in positive reaction rates to nail polish chemicals, with only 0.7% of patients reacting to TSFR from 2014 to 2016 and 0% in 2017. It is theorized that this reduction occurred from replacing TSFR in traditional nail polishes with other chemicals such as polyester resins and cellulose acetate butyrate.5
Acrylate-Based Nail Treatments
Consumers recently have been gravitating toward acrylate-based nail treatments vs traditional nail polishes for a variety of reasons. Often referred to as gels, dips, or shellac, acrylate-based nail treatments represent a hot new trend in nail cosmetics. These manicures are resistant to chipping and scratches, creating a like-new look that lasts for weeks after application. The long-lasting nature of acrylate-based nail polishes has made them wildly popular with consumers.
Traditional acrylic nails consist of a powder polymer mixed with a liquid monomer, which polymerizes when a catalyst is added.6 The procedure is time consuming and can take up to 2 hours for application. In contrast, the newer gel manicure can be completed faster and includes application of acrylate-based nail polish, including a base coat, 2 coats of color, and a top coat. Exposure to either a light-emitting diode (30–60 seconds) or UVA (2 minutes) lamp is necessary after each coat is applied for polymerization (Figure 1).6 This long-lasting, semipermanent manicure typically is what patients are referring to when they say they have “gel nails.”
Gel dipping powders (referred to as dips) are another long-lasting acrylate-based nail treatment. This type of polish uses ethyl cyanoacrylate, a slightly different acrylate (yes, that IS super glue). After the nail is prepared, a base polish is applied to three-quarters of the nail and it is dipped into a natural color dip powder. The base polish is then applied to the entire nail, followed by a dip into the polish color of choice. This process is completed twice, followed by shaping and application of a top coat (Figure 2).
base coat. B, Application of dip powder to gel polish. Note the entire
distal finger and nail are dipped into the powder. C, Shaping of the
nail after the second coat of color is applied.
Finally, there are nail wraps, which are similar to stickers placed over or extending the nail plate. The wraps can be made from linen, silk, vinyl, or other material. Ethyl cyanoacrylate and isopropyl-2-cyanoacrylates have been identified in nail wrap adhesive.7 The heated product is directly applied to the prepared nail, and the excess wrap is filed off. Additional nail polish and a top coat usually are applied to finish the nail. Many of these products are available for in-salon use as well as online purchase and home application by consumers.
Acrylate Allergy
Patients who are allergic to acrylates can present with different patterns of dermatitis. Although the majority of patients present with dermatitis on the hands, fingers, or wrists, up to 10% may only have facial and neck dermatitis.8 Less commonly, the abdomen and thighs can be involved.6,8 Nail technicians most commonly present with pulpitis with cutaneous fissures.8 Other symptoms can include subungual hyperkeratosis, onycholysis, and nail dystrophy. Paresthesia, urticaria, and upper respiratory tract symptoms can occur but are less common.6,8
Acrylate allergy typically is the result of sensitization to the acrylate monomers. In theory, gel nail acrylate materials are polymerized following exposure to a light-emitting diode or UVA lamp; however, there likely is some incomplete polymerization, which can increase the risk for development of allergy. Allergen exposure can occur due to incorrect application of the light source; inadvertent monomer exposure, which occurs when nail technicians wipe extra acrylate off of a client’s finger(s); or inadvertent application of acrylate monomers to objects in the nail technician’s work environment.6,8
Several acrylate nail allergens have been reported. Many studies have identified 2-hydroxyethyl methacrylate (HEMA) as the most common nail acrylate allergen.8,9 At least one study identified 2-hydroxypropyl methacrylate as the most common, with HEMA in second place.6 Other reported acrylate allergens have included ethylene glycol dimethacrylate, triethylene glycol dimethacrylate, methyl methacrylate, ethyl cyanoacrylate, 1,4-butanediol diacrylate, hydroxypropyl acrylate, and 2-hydroxyethyl acrylate.8,9
The American Contact Dermatitis Society Core Allergen Series and the North American Contact Dermatitis Group screening series currently include HEMA, methyl methacrylate, ethyl acrylate, ethyl cyanoacrylate, and TSFR.4,10 Of note, acrylates are not included in the thin-layer rapid use epicutaneous (T.R.U.E.) patch test (SmartPractice), so they will be missed if this series is used.11 In the setting of suspected nail acrylate allergy, some authors recommend initial screening with HEMA and ethyl cyanoacrylate, with extended acrylate testing if both are negative.8
Upon patch testing with an acrylate series, patients frequently react to 2 or more acrylates and the reactions can be strong (++) or extreme (+++), which may represent cosensitization or cross-sensitization.8 The likelihood of cross-reactivity between acrylates is not clear, though it has been postulated that it is theoretically possible.6
An important pearl for patch testers using the chamber method is proper storage of acrylate allergens and assembly of trays prior to patch testing. Similar to all haptens, manufacturers recommend that acrylates should be stored in a refrigerator, but some authors suggest that acrylates should be stored in the freezer.12 Acrylates are volatile chemicals and rapidly degrade when exposed to air. A methyl methacrylate preparation loaded into an inert quadrate (IQ) chamber and stored at room temperature showed a nearly undetectable amount of any residual methyl methacrylate 24 hours later. Refrigeration of allergens in chambers slowed but did not stop eventual degradation, with nearly all acrylate preparations reaching an undetectable level of allergen by day 8.13 Acrylates, along with other volatile allergens, should only be loaded into chambers immediately prior to placement on the patient.
Allergy Prevention
Prevention of nail acrylate allergy among consumers is simple: avoid contact with the offending allergen. Acrylate spillover (ie, applying the acrylate onto the skin) and direct contact with objects and working surfaces contaminated with acrylate-based nail products should be avoided.8 Avoidance is more complicated for nail technicians, but it is thought that nitrile gloves allow for the best dexterity and allergen avoidance when acrylate exposure is brief.14 Allowable exposure times with nitrile gloves may be 15 to 30 minutes. After this times passes, a glove change is required to avoid exposure.14 Wearing nitrile gloves for longer than 15 to 30 minutes will result in cutaneous exposure and risk for dermatitis in sensitized patients. If longer wear is desired, one option includes cutting the fingertips off of Silver Shield/4H gloves (Honeywell Safety Products USA, Inc), applying them to the distal fingers, and wearing a standard nitrile glove over top, known as the finger stall technique.6 In one study, this technique was recommended to nail technicians with acrylate allergy. A telephone survey conducted 4 to 43 months later confirmed that 36% (8/22) of participants were using the technique without symptoms. In this same study, 73% (16/22) had continued working as nail technicians.6
Acrylates are used for other medical purposes, including dental procedures, orthopedic procedures, surgical glues, wound dressings, and contact and intraocular lenses. They also have additional cosmetic applications, including eyelash and hair extensions.8 Therefore, it is vital that patients disclose any history of acrylate allergy to both their medical and cosmetic providers.
Our Final Interpretation
Acrylate allergy has become increasingly common, and long-lasting nail treatments often are the culprit. Whether through gels, dips, or shellac, repeated exposure to acrylates through nail treatments can increase the risk for allergy. The T.R.U.E. test alone will not make the diagnosis, as acrylates are not present in this patch test system. It is important to remind your allergic patients that acrylates are present in other compounds used for medical and cosmetic purposes. Avoidance is key, and for allergic patients who love to bedazzle their nails, we suggest less-permanent, acrylate-free nail polishes as alternatives.
In 2017, consumers spent an average of $8.53 billion on nail services.1 This booming industry is set to grow to more than $15.5 billion by 2024.2 Nail polishes and other nail cosmetic trends can present new exposures for consumers, including chemicals that can elicit allergic contact dermatitis. In this article, we discuss new nail trends and their associated allergens, the acrylates.
Tosylamide/Formaldehyde Resin
Traditionally, the most widely recognized nail polish allergen has been tosylamide/formaldehyde resin (TSFR). However, there now are many touted TSFR-free nail polishes on the market, and the rate of positive reactions to this chemical has been declining in recent years. The North American Contact Dermatitis Group reported a positive reaction rate of 1.3% from 2005 through 2006,3 and rates decreased to 0.9% from 2015 through 2016.4 An Australian study demonstrated a similar reduction in positive reaction rates to nail polish chemicals, with only 0.7% of patients reacting to TSFR from 2014 to 2016 and 0% in 2017. It is theorized that this reduction occurred from replacing TSFR in traditional nail polishes with other chemicals such as polyester resins and cellulose acetate butyrate.5
Acrylate-Based Nail Treatments
Consumers recently have been gravitating toward acrylate-based nail treatments vs traditional nail polishes for a variety of reasons. Often referred to as gels, dips, or shellac, acrylate-based nail treatments represent a hot new trend in nail cosmetics. These manicures are resistant to chipping and scratches, creating a like-new look that lasts for weeks after application. The long-lasting nature of acrylate-based nail polishes has made them wildly popular with consumers.
Traditional acrylic nails consist of a powder polymer mixed with a liquid monomer, which polymerizes when a catalyst is added.6 The procedure is time consuming and can take up to 2 hours for application. In contrast, the newer gel manicure can be completed faster and includes application of acrylate-based nail polish, including a base coat, 2 coats of color, and a top coat. Exposure to either a light-emitting diode (30–60 seconds) or UVA (2 minutes) lamp is necessary after each coat is applied for polymerization (Figure 1).6 This long-lasting, semipermanent manicure typically is what patients are referring to when they say they have “gel nails.”
Gel dipping powders (referred to as dips) are another long-lasting acrylate-based nail treatment. This type of polish uses ethyl cyanoacrylate, a slightly different acrylate (yes, that IS super glue). After the nail is prepared, a base polish is applied to three-quarters of the nail and it is dipped into a natural color dip powder. The base polish is then applied to the entire nail, followed by a dip into the polish color of choice. This process is completed twice, followed by shaping and application of a top coat (Figure 2).
base coat. B, Application of dip powder to gel polish. Note the entire
distal finger and nail are dipped into the powder. C, Shaping of the
nail after the second coat of color is applied.
Finally, there are nail wraps, which are similar to stickers placed over or extending the nail plate. The wraps can be made from linen, silk, vinyl, or other material. Ethyl cyanoacrylate and isopropyl-2-cyanoacrylates have been identified in nail wrap adhesive.7 The heated product is directly applied to the prepared nail, and the excess wrap is filed off. Additional nail polish and a top coat usually are applied to finish the nail. Many of these products are available for in-salon use as well as online purchase and home application by consumers.
Acrylate Allergy
Patients who are allergic to acrylates can present with different patterns of dermatitis. Although the majority of patients present with dermatitis on the hands, fingers, or wrists, up to 10% may only have facial and neck dermatitis.8 Less commonly, the abdomen and thighs can be involved.6,8 Nail technicians most commonly present with pulpitis with cutaneous fissures.8 Other symptoms can include subungual hyperkeratosis, onycholysis, and nail dystrophy. Paresthesia, urticaria, and upper respiratory tract symptoms can occur but are less common.6,8
Acrylate allergy typically is the result of sensitization to the acrylate monomers. In theory, gel nail acrylate materials are polymerized following exposure to a light-emitting diode or UVA lamp; however, there likely is some incomplete polymerization, which can increase the risk for development of allergy. Allergen exposure can occur due to incorrect application of the light source; inadvertent monomer exposure, which occurs when nail technicians wipe extra acrylate off of a client’s finger(s); or inadvertent application of acrylate monomers to objects in the nail technician’s work environment.6,8
Several acrylate nail allergens have been reported. Many studies have identified 2-hydroxyethyl methacrylate (HEMA) as the most common nail acrylate allergen.8,9 At least one study identified 2-hydroxypropyl methacrylate as the most common, with HEMA in second place.6 Other reported acrylate allergens have included ethylene glycol dimethacrylate, triethylene glycol dimethacrylate, methyl methacrylate, ethyl cyanoacrylate, 1,4-butanediol diacrylate, hydroxypropyl acrylate, and 2-hydroxyethyl acrylate.8,9
The American Contact Dermatitis Society Core Allergen Series and the North American Contact Dermatitis Group screening series currently include HEMA, methyl methacrylate, ethyl acrylate, ethyl cyanoacrylate, and TSFR.4,10 Of note, acrylates are not included in the thin-layer rapid use epicutaneous (T.R.U.E.) patch test (SmartPractice), so they will be missed if this series is used.11 In the setting of suspected nail acrylate allergy, some authors recommend initial screening with HEMA and ethyl cyanoacrylate, with extended acrylate testing if both are negative.8
Upon patch testing with an acrylate series, patients frequently react to 2 or more acrylates and the reactions can be strong (++) or extreme (+++), which may represent cosensitization or cross-sensitization.8 The likelihood of cross-reactivity between acrylates is not clear, though it has been postulated that it is theoretically possible.6
An important pearl for patch testers using the chamber method is proper storage of acrylate allergens and assembly of trays prior to patch testing. Similar to all haptens, manufacturers recommend that acrylates should be stored in a refrigerator, but some authors suggest that acrylates should be stored in the freezer.12 Acrylates are volatile chemicals and rapidly degrade when exposed to air. A methyl methacrylate preparation loaded into an inert quadrate (IQ) chamber and stored at room temperature showed a nearly undetectable amount of any residual methyl methacrylate 24 hours later. Refrigeration of allergens in chambers slowed but did not stop eventual degradation, with nearly all acrylate preparations reaching an undetectable level of allergen by day 8.13 Acrylates, along with other volatile allergens, should only be loaded into chambers immediately prior to placement on the patient.
Allergy Prevention
Prevention of nail acrylate allergy among consumers is simple: avoid contact with the offending allergen. Acrylate spillover (ie, applying the acrylate onto the skin) and direct contact with objects and working surfaces contaminated with acrylate-based nail products should be avoided.8 Avoidance is more complicated for nail technicians, but it is thought that nitrile gloves allow for the best dexterity and allergen avoidance when acrylate exposure is brief.14 Allowable exposure times with nitrile gloves may be 15 to 30 minutes. After this times passes, a glove change is required to avoid exposure.14 Wearing nitrile gloves for longer than 15 to 30 minutes will result in cutaneous exposure and risk for dermatitis in sensitized patients. If longer wear is desired, one option includes cutting the fingertips off of Silver Shield/4H gloves (Honeywell Safety Products USA, Inc), applying them to the distal fingers, and wearing a standard nitrile glove over top, known as the finger stall technique.6 In one study, this technique was recommended to nail technicians with acrylate allergy. A telephone survey conducted 4 to 43 months later confirmed that 36% (8/22) of participants were using the technique without symptoms. In this same study, 73% (16/22) had continued working as nail technicians.6
Acrylates are used for other medical purposes, including dental procedures, orthopedic procedures, surgical glues, wound dressings, and contact and intraocular lenses. They also have additional cosmetic applications, including eyelash and hair extensions.8 Therefore, it is vital that patients disclose any history of acrylate allergy to both their medical and cosmetic providers.
Our Final Interpretation
Acrylate allergy has become increasingly common, and long-lasting nail treatments often are the culprit. Whether through gels, dips, or shellac, repeated exposure to acrylates through nail treatments can increase the risk for allergy. The T.R.U.E. test alone will not make the diagnosis, as acrylates are not present in this patch test system. It is important to remind your allergic patients that acrylates are present in other compounds used for medical and cosmetic purposes. Avoidance is key, and for allergic patients who love to bedazzle their nails, we suggest less-permanent, acrylate-free nail polishes as alternatives.
- 2017-2018 industry statistics highlights. Nails Magazine. http://files.nailsmag.com/handouts/nabb2017-18stats-lr.pdf. Accessed May 17, 2019.
- Nail polish market size worth $15.55 billion by 2024. Grand View Research website. https://www.grandviewresearch.com/press-release/global-nail-polish-market. Published October 2017. Accessed May 17, 2019.
- Zug KA, Warshaw EM, Fowler JF, et al. Patch-test results of the North American Contact Dermatitis Group 2005-2006. Dermatitis. 2009;20:149-160.
- DeKoven J, Warshaw EM, Zug KA, et al. North American Contact Dermatitis Group patch test results: 2015-2016. Dermatitis. 2018;29:297-309.
- Lee S, Maor D, Palmer A, et al. Declining prevalence of allergic contact dermatitis caused by tosylamide/formaldehyde in nail polish. Contact Dermatitis. 2018;79:184-185.
- Gatica-Ortega ME, Pastor-Nieto MA, Mercader-García P, et al. Allergic contact dermatitis caused by (meth)acrylates in long-lasting nail polish: are we facing a new epidemic in the beauty industry? Contact Dermatitis. 2017;7:360-366.
- Fitzgerald DA, Bhaggoe R, English JS. Contact sensitivity to cyanoacrylate nail-adhesive with dermatitis at remote sites. Contact Dermatitis. 1995;32:175-176.
- Goncalo M, Pinho A, Agner T et al. Allergic contact dermatitis caused by nail acrylates in Europe. an EECDRG study. Contact Dermatitis. 2017;78:254-260.
- Fisch A, Hamnerius N, Isaksson M. Dermatitis and occupational (meth)acrylate contact allergy in nail technicians—a 10-year study [published online January 14, 2019]. Contact Dermatitis. doi:10.1111/cod.13216.
- Schalock PC, Dunnick CA, Nedorost S, et al. American Contact Dermatitis Society core allergen series: 2017 update. Dermatitis. 2017;28:141-143.
- T.R.U.E. TEST ready-to-use patch test panels. Smart Practice website. https://www.smartpractice.com/shop/wa/category?cn=T.R.U.E.-TEST%C2%AE-Ready-to-Use-Patch-Test-Panels&id=508222&m=SPA. Accessed May 17, 2019.
- Good AT, Bruze M, Zimerson E, et al. Variation in allergen content over time of acrylates/methylacrylates in patch test preparations. Br J Dermatol. 2011;164:116-124.
- Goon A, Bruze M, Zimerson E, et al. Variation in allergen content over time of acrylates/methacrylates in patch test preparations. Br J Dermatol. 2011;164:116-124.
- Morgado F, Batista M, Gonçalo M. Short exposures and glove protection against (meth)acrylates in nail beauticians—thoughts on a rising concern [published online January 17, 2019]. Contact Dermatitis. doi:10.1111/cod.13222.
- 2017-2018 industry statistics highlights. Nails Magazine. http://files.nailsmag.com/handouts/nabb2017-18stats-lr.pdf. Accessed May 17, 2019.
- Nail polish market size worth $15.55 billion by 2024. Grand View Research website. https://www.grandviewresearch.com/press-release/global-nail-polish-market. Published October 2017. Accessed May 17, 2019.
- Zug KA, Warshaw EM, Fowler JF, et al. Patch-test results of the North American Contact Dermatitis Group 2005-2006. Dermatitis. 2009;20:149-160.
- DeKoven J, Warshaw EM, Zug KA, et al. North American Contact Dermatitis Group patch test results: 2015-2016. Dermatitis. 2018;29:297-309.
- Lee S, Maor D, Palmer A, et al. Declining prevalence of allergic contact dermatitis caused by tosylamide/formaldehyde in nail polish. Contact Dermatitis. 2018;79:184-185.
- Gatica-Ortega ME, Pastor-Nieto MA, Mercader-García P, et al. Allergic contact dermatitis caused by (meth)acrylates in long-lasting nail polish: are we facing a new epidemic in the beauty industry? Contact Dermatitis. 2017;7:360-366.
- Fitzgerald DA, Bhaggoe R, English JS. Contact sensitivity to cyanoacrylate nail-adhesive with dermatitis at remote sites. Contact Dermatitis. 1995;32:175-176.
- Goncalo M, Pinho A, Agner T et al. Allergic contact dermatitis caused by nail acrylates in Europe. an EECDRG study. Contact Dermatitis. 2017;78:254-260.
- Fisch A, Hamnerius N, Isaksson M. Dermatitis and occupational (meth)acrylate contact allergy in nail technicians—a 10-year study [published online January 14, 2019]. Contact Dermatitis. doi:10.1111/cod.13216.
- Schalock PC, Dunnick CA, Nedorost S, et al. American Contact Dermatitis Society core allergen series: 2017 update. Dermatitis. 2017;28:141-143.
- T.R.U.E. TEST ready-to-use patch test panels. Smart Practice website. https://www.smartpractice.com/shop/wa/category?cn=T.R.U.E.-TEST%C2%AE-Ready-to-Use-Patch-Test-Panels&id=508222&m=SPA. Accessed May 17, 2019.
- Good AT, Bruze M, Zimerson E, et al. Variation in allergen content over time of acrylates/methylacrylates in patch test preparations. Br J Dermatol. 2011;164:116-124.
- Goon A, Bruze M, Zimerson E, et al. Variation in allergen content over time of acrylates/methacrylates in patch test preparations. Br J Dermatol. 2011;164:116-124.
- Morgado F, Batista M, Gonçalo M. Short exposures and glove protection against (meth)acrylates in nail beauticians—thoughts on a rising concern [published online January 17, 2019]. Contact Dermatitis. doi:10.1111/cod.13222.
Practice Points
- Changing trends in nail services mean new exposures for consumers. Traditional nail polish has been replaced by semipermanent nail polish, which contains acrylates.
- Acrylates are a common cause of allergic contact dermatitis from nail polish. Acrylates can be found in gel, dip, and shellac nail polishes, among others.
- Patch testing with 2-hydroxyethyl methacrylate and ethyl cyanoacrylate can screen many patients for allergy due to nail services.
Parabens: The 2019 Nonallergen of the Year
Each year, the American Contact Dermatitis Society (ACDS) names an allergen of the year with the purpose of promoting greater awareness of a key allergen and its impact on patients. Often, the allergen of the year is an emerging allergen that may represent an underrecognized or novel cause of allergic contact dermatitis (ACD). In 2019, the ACDS chose parabens as the “nonallergen” of the year to draw attention to their low rate of associated ACD despite high public interest in limiting exposure to parabens.1
What types of products contain parabens?
Parabens are preservatives commonly found in many different categories of personal care products. Preservatives inhibit microbial growth and are necessary ingredients in water-based products. The 4 most common parabens used in personal care products are methylparaben, ethylparaben, propylparaben, and butylparaben.1 Parabens are metabolized to 4-hydroxybenzoic acid and are excreted in urine. When parabens are applied topically, there is minimal penetration through intact human skin.2 In the United States, parabens are allowed as preservatives in cosmetics at concentrations up to 0.4% when used alone or up to 0.8% when used in combination with other parabens.3
Consumers are exposed to parabens in a wide variety of personal care products. The Contact Allergen Management Program (CAMP) is a system owned and managed by the ACDS that typically is used to generate lists of safe personal care products for patients and also can be queried for the presence of individual chemicals in products. According to a 2018 query of the CAMP, parabens were found in 19% of all products.1 A more recent query of CAMP (http://www.contactderm.org/resources/acds-camp) in March 2019 showed parabens were present in 39.3% of makeup products, especially in eye products, foundations, and concealers; parabens also were found in 34% of moisturizers, 11.5% of soaps, and 19% of sunscreens. Notably, 14.8% of prescription topical steroids listed in the CAMP contained a paraben. Another method for evaluating chemical contents of personal care products is a review of the Voluntary Cosmetic Registration Program, a US Food and Drug Administration–based registry for cosmetic products. Survey data from the Voluntary Cosmetic Registration Program in 2018 documented methylparaben in 11,626 formulations.4 Other parabens included propylparaben (8885 products), butylparaben (3915 products), and ethylparaben (3860 products). Parabens were reported more frequently in leave-on rather than rinse-off products.4
In medications, parabens are recommended at concentrations of no more than 0.1%.1 Fransway et al1 compiled a list of medications that contain parabens, including commonly prescribed dermatologic topical medications such as corticosteroids, several acne preparations, eflornithine, fluorouracil, hydroquinone, imiquimod, urea, and sertaconazole. Oral and parenteral medications including local anesthetics and corticosteroids also may contain parabens.
Consumers also may be exposed to parabens through foodstuffs. Methylparaben and propylparaben have been classified as generally recognized as safe in foods by the US Food and Drug Administration.5 The acceptable daily intake of parabens in food is 0 to 10 mg/kg of body weight,1 and the estimated dietary intake for a typical adult is 307 mg/kg of body weight daily.6 Several studies on paraben content in foodstuffs have confirmed their presence in both natural and processed foods.1,6 Systemic contact dermatitis caused by ingestion of parabens is rare. In general, individuals with positive patch test reactions to parabens should not routinely avoid them in foods or oral medications,1 but they should, of course, be avoided in topical medications.
What is the rate of ACD with parabens?
One of the main reasons that parabens were designated as the ACDS nonallergen of the year is the very low rate of ACD associated with parabens. The North American Contact Dermatitis Group, a research group with members in the United States and Canada, reported a 0.6% positive reaction rate when patch testing with paraben mix 12%,7 which closely compares with a 0.8% positive reaction rate when patch testing with paraben mix 16% using the Mayo Clinic standard series.8 From the standpoint of ACD, this very low patch test reaction rate makes parabens one of the safest preservative options for use in cosmetic products.
Are there health risks associated with parabens?
The paraben controversy in the scientific literature and in the lay press centers around potential health risks and endocrine disruption. We will focus on the conversation regarding parabens and the risk for endocrine disruption and association with breast cancer.
Parabens have been reported to have estrogenic effects; however, the bulk of the data is limited to in vitro and animal studies, with less evidence of endocrine disruption in humans.2 In vitro studies have demonstrated that the estrogenic potency of parabens is much less than that of estrogen. In one study, parabens were shown to be 10,000-fold less potent than 17β-estradiol9; in a separate study, they had a maximum potency of only 1/4000 that of estrogen.10 Additionally, an in vitro study showed varying ability for parabens to bind estrogen receptors, with a greater ability to bind with longer alkyl side chains.11 The result is decreased or increased estrogen activity, dependent on side chain length and type of receptor.2 Finally, some studies add conflicting results that parabens may actually create an antiestrogenic effect in human breast cancer cells.12 From the standpoint of estrogen mimicry, there are no known studies in humans confirming harmful effects associated with paraben exposure.
The reported association between parabens and breast cancer is closely related to their theoretical estrogenic effects. The conversation regarding parabens and breast cancer has been fueled by the identification of parabens in human breast tumors and their presence in concentrations similar to what is needed to stimulate in vitro breast cancer cells.2 The existing data do not confirm causation. An association with parabens in topical axillary personal care products has been theorized but not confirmed; for example, it was shown that paraben levels were highest in the axillary region of breast cancer tissue, including women who had never used deodorant. It was concluded that the presence of axillary parabens was due to sources other than topical axillary personal care products.13 Another study confirmed there was not an increased risk for breast cancer in patients who applied personal care products to the axillary area within an hour of shaving.14 The existing data do not support topical paraben exposure as a risk for breast cancer.
Final Thoughts
Parabens are preservatives frequently found in personal care products and exhibit a very low rate of associated ACD. Consumers may be exposed to parabens through foods, cosmetics, and medications. Although there have been consumer concerns regarding endocrine disruption or carcinogenicity associated with parabens, definite evidence of their harm is lacking in the scientific literature, and many studies confirm their safety.2 With their high prevalence in personal care products and low rates of associated contact allergy, parabens remain ideal preservative agents.
Ultimately, contact dermatitis is a common yet often underrecognized dermatologic condition. To address this knowledge gap in clinical practice, we are proud to launch Final Interpretation, a new column in Cutis covering emerging trends in contact dermatitis. We will address pearls, pitfalls, and updates in contact dermatitis. Although our primary focus will be ACD, other important causes of contact dermatitis will be highlighted. Look for the inaugural column in the June 2019 issue of Cutis.
- Fransway AF, Fransway PJ, Belsito DV, et al. Parabens: contact (non)allergen of the year. Dermatitis. 2019;30:3-31.
- Fransway AF, Fransway PJ, Belsito DV, et al. Paraben toxicology. Dermatitis. 2019;30:32-45.
- Final amended report on the safety assessment of methylparaben, ethylparaben, propylparaben, isopropylparaben, butylparaben, isobutylparaben, and benzylparaben as used in cosmetic products. Int J Toxicol. 2008;27(suppl 4):1-82.
- Cosmetic Ingredient Review. Amended safety assessment of parabens as used in cosmetics. https://www.cir-safety.org/sites/default/files/Parabens.pdf. Published August 29, 2018. Accessed March 12, 2019.
- Methylparaben. Fed Regist. 2018;21(3):1490. To be codified at 21 CFR §184.
- Liao C, Liu F, Kannan K. Occurrence of and dietary exposure to parabens in foodstuffs from the United States. Environ Sci Technol. 2013;47:3918-3925.
- DeKoven JG, Warshaw EM, Zug KA, et al. North American Contact Dermatitis Group Patch Test Results: 2015-2016. Dermatitis. 2018;29:297-309.
- Veverka KK, Hall MR, Yiannias JA, et al. Trends in patch testing with the Mayo Clinic standard series, 2011-2015. Dermatitis. 2018;29:310-315.
- Routledge EJ, Parker J, Odum J, et al. Some alkyl hydroxy benzoate preservatives (parabens) are estrogenic. Toxicol Appl Pharmacol. 1998;153:12-19.
- Miller D, Brian B, Wheals BB, et al. Estrogenic activity of phenolic additives determined by an in vitro yeast bioassay. Environ Health Perspect. 2001;109:133-138.
- Blair RM, Fang H, Branham WS. The estrogen receptor relative binding affinities of 188 natural and xenochemicals: structural diversity of ligands. Toxicol Sci. 2000;54:138-153.
- van Meeuwen JA, van Son O, Piersma AH, et al. Aromatase inhibiting and combined estrogenic effects of parabens and estrogenic effects of other additives in cosmetics. Toxicol Appl Pharmacol. 2008;230:372-382.
- Barr L, Metaxas G, Harbach CA, et al. Measurement of paraben concentrations in human breast tissue at serial locations across the breast from axilla to sternum. J Appl Toxicol. 2012;32:219-232.
- Mirick DK, Davis S, Thomas DB. Antiperspirant use and the risk of breast cancer. J Natl Cancer Inst. 2002;94:1578-1580
.
Each year, the American Contact Dermatitis Society (ACDS) names an allergen of the year with the purpose of promoting greater awareness of a key allergen and its impact on patients. Often, the allergen of the year is an emerging allergen that may represent an underrecognized or novel cause of allergic contact dermatitis (ACD). In 2019, the ACDS chose parabens as the “nonallergen” of the year to draw attention to their low rate of associated ACD despite high public interest in limiting exposure to parabens.1
What types of products contain parabens?
Parabens are preservatives commonly found in many different categories of personal care products. Preservatives inhibit microbial growth and are necessary ingredients in water-based products. The 4 most common parabens used in personal care products are methylparaben, ethylparaben, propylparaben, and butylparaben.1 Parabens are metabolized to 4-hydroxybenzoic acid and are excreted in urine. When parabens are applied topically, there is minimal penetration through intact human skin.2 In the United States, parabens are allowed as preservatives in cosmetics at concentrations up to 0.4% when used alone or up to 0.8% when used in combination with other parabens.3
Consumers are exposed to parabens in a wide variety of personal care products. The Contact Allergen Management Program (CAMP) is a system owned and managed by the ACDS that typically is used to generate lists of safe personal care products for patients and also can be queried for the presence of individual chemicals in products. According to a 2018 query of the CAMP, parabens were found in 19% of all products.1 A more recent query of CAMP (http://www.contactderm.org/resources/acds-camp) in March 2019 showed parabens were present in 39.3% of makeup products, especially in eye products, foundations, and concealers; parabens also were found in 34% of moisturizers, 11.5% of soaps, and 19% of sunscreens. Notably, 14.8% of prescription topical steroids listed in the CAMP contained a paraben. Another method for evaluating chemical contents of personal care products is a review of the Voluntary Cosmetic Registration Program, a US Food and Drug Administration–based registry for cosmetic products. Survey data from the Voluntary Cosmetic Registration Program in 2018 documented methylparaben in 11,626 formulations.4 Other parabens included propylparaben (8885 products), butylparaben (3915 products), and ethylparaben (3860 products). Parabens were reported more frequently in leave-on rather than rinse-off products.4
In medications, parabens are recommended at concentrations of no more than 0.1%.1 Fransway et al1 compiled a list of medications that contain parabens, including commonly prescribed dermatologic topical medications such as corticosteroids, several acne preparations, eflornithine, fluorouracil, hydroquinone, imiquimod, urea, and sertaconazole. Oral and parenteral medications including local anesthetics and corticosteroids also may contain parabens.
Consumers also may be exposed to parabens through foodstuffs. Methylparaben and propylparaben have been classified as generally recognized as safe in foods by the US Food and Drug Administration.5 The acceptable daily intake of parabens in food is 0 to 10 mg/kg of body weight,1 and the estimated dietary intake for a typical adult is 307 mg/kg of body weight daily.6 Several studies on paraben content in foodstuffs have confirmed their presence in both natural and processed foods.1,6 Systemic contact dermatitis caused by ingestion of parabens is rare. In general, individuals with positive patch test reactions to parabens should not routinely avoid them in foods or oral medications,1 but they should, of course, be avoided in topical medications.
What is the rate of ACD with parabens?
One of the main reasons that parabens were designated as the ACDS nonallergen of the year is the very low rate of ACD associated with parabens. The North American Contact Dermatitis Group, a research group with members in the United States and Canada, reported a 0.6% positive reaction rate when patch testing with paraben mix 12%,7 which closely compares with a 0.8% positive reaction rate when patch testing with paraben mix 16% using the Mayo Clinic standard series.8 From the standpoint of ACD, this very low patch test reaction rate makes parabens one of the safest preservative options for use in cosmetic products.
Are there health risks associated with parabens?
The paraben controversy in the scientific literature and in the lay press centers around potential health risks and endocrine disruption. We will focus on the conversation regarding parabens and the risk for endocrine disruption and association with breast cancer.
Parabens have been reported to have estrogenic effects; however, the bulk of the data is limited to in vitro and animal studies, with less evidence of endocrine disruption in humans.2 In vitro studies have demonstrated that the estrogenic potency of parabens is much less than that of estrogen. In one study, parabens were shown to be 10,000-fold less potent than 17β-estradiol9; in a separate study, they had a maximum potency of only 1/4000 that of estrogen.10 Additionally, an in vitro study showed varying ability for parabens to bind estrogen receptors, with a greater ability to bind with longer alkyl side chains.11 The result is decreased or increased estrogen activity, dependent on side chain length and type of receptor.2 Finally, some studies add conflicting results that parabens may actually create an antiestrogenic effect in human breast cancer cells.12 From the standpoint of estrogen mimicry, there are no known studies in humans confirming harmful effects associated with paraben exposure.
The reported association between parabens and breast cancer is closely related to their theoretical estrogenic effects. The conversation regarding parabens and breast cancer has been fueled by the identification of parabens in human breast tumors and their presence in concentrations similar to what is needed to stimulate in vitro breast cancer cells.2 The existing data do not confirm causation. An association with parabens in topical axillary personal care products has been theorized but not confirmed; for example, it was shown that paraben levels were highest in the axillary region of breast cancer tissue, including women who had never used deodorant. It was concluded that the presence of axillary parabens was due to sources other than topical axillary personal care products.13 Another study confirmed there was not an increased risk for breast cancer in patients who applied personal care products to the axillary area within an hour of shaving.14 The existing data do not support topical paraben exposure as a risk for breast cancer.
Final Thoughts
Parabens are preservatives frequently found in personal care products and exhibit a very low rate of associated ACD. Consumers may be exposed to parabens through foods, cosmetics, and medications. Although there have been consumer concerns regarding endocrine disruption or carcinogenicity associated with parabens, definite evidence of their harm is lacking in the scientific literature, and many studies confirm their safety.2 With their high prevalence in personal care products and low rates of associated contact allergy, parabens remain ideal preservative agents.
Ultimately, contact dermatitis is a common yet often underrecognized dermatologic condition. To address this knowledge gap in clinical practice, we are proud to launch Final Interpretation, a new column in Cutis covering emerging trends in contact dermatitis. We will address pearls, pitfalls, and updates in contact dermatitis. Although our primary focus will be ACD, other important causes of contact dermatitis will be highlighted. Look for the inaugural column in the June 2019 issue of Cutis.
Each year, the American Contact Dermatitis Society (ACDS) names an allergen of the year with the purpose of promoting greater awareness of a key allergen and its impact on patients. Often, the allergen of the year is an emerging allergen that may represent an underrecognized or novel cause of allergic contact dermatitis (ACD). In 2019, the ACDS chose parabens as the “nonallergen” of the year to draw attention to their low rate of associated ACD despite high public interest in limiting exposure to parabens.1
What types of products contain parabens?
Parabens are preservatives commonly found in many different categories of personal care products. Preservatives inhibit microbial growth and are necessary ingredients in water-based products. The 4 most common parabens used in personal care products are methylparaben, ethylparaben, propylparaben, and butylparaben.1 Parabens are metabolized to 4-hydroxybenzoic acid and are excreted in urine. When parabens are applied topically, there is minimal penetration through intact human skin.2 In the United States, parabens are allowed as preservatives in cosmetics at concentrations up to 0.4% when used alone or up to 0.8% when used in combination with other parabens.3
Consumers are exposed to parabens in a wide variety of personal care products. The Contact Allergen Management Program (CAMP) is a system owned and managed by the ACDS that typically is used to generate lists of safe personal care products for patients and also can be queried for the presence of individual chemicals in products. According to a 2018 query of the CAMP, parabens were found in 19% of all products.1 A more recent query of CAMP (http://www.contactderm.org/resources/acds-camp) in March 2019 showed parabens were present in 39.3% of makeup products, especially in eye products, foundations, and concealers; parabens also were found in 34% of moisturizers, 11.5% of soaps, and 19% of sunscreens. Notably, 14.8% of prescription topical steroids listed in the CAMP contained a paraben. Another method for evaluating chemical contents of personal care products is a review of the Voluntary Cosmetic Registration Program, a US Food and Drug Administration–based registry for cosmetic products. Survey data from the Voluntary Cosmetic Registration Program in 2018 documented methylparaben in 11,626 formulations.4 Other parabens included propylparaben (8885 products), butylparaben (3915 products), and ethylparaben (3860 products). Parabens were reported more frequently in leave-on rather than rinse-off products.4
In medications, parabens are recommended at concentrations of no more than 0.1%.1 Fransway et al1 compiled a list of medications that contain parabens, including commonly prescribed dermatologic topical medications such as corticosteroids, several acne preparations, eflornithine, fluorouracil, hydroquinone, imiquimod, urea, and sertaconazole. Oral and parenteral medications including local anesthetics and corticosteroids also may contain parabens.
Consumers also may be exposed to parabens through foodstuffs. Methylparaben and propylparaben have been classified as generally recognized as safe in foods by the US Food and Drug Administration.5 The acceptable daily intake of parabens in food is 0 to 10 mg/kg of body weight,1 and the estimated dietary intake for a typical adult is 307 mg/kg of body weight daily.6 Several studies on paraben content in foodstuffs have confirmed their presence in both natural and processed foods.1,6 Systemic contact dermatitis caused by ingestion of parabens is rare. In general, individuals with positive patch test reactions to parabens should not routinely avoid them in foods or oral medications,1 but they should, of course, be avoided in topical medications.
What is the rate of ACD with parabens?
One of the main reasons that parabens were designated as the ACDS nonallergen of the year is the very low rate of ACD associated with parabens. The North American Contact Dermatitis Group, a research group with members in the United States and Canada, reported a 0.6% positive reaction rate when patch testing with paraben mix 12%,7 which closely compares with a 0.8% positive reaction rate when patch testing with paraben mix 16% using the Mayo Clinic standard series.8 From the standpoint of ACD, this very low patch test reaction rate makes parabens one of the safest preservative options for use in cosmetic products.
Are there health risks associated with parabens?
The paraben controversy in the scientific literature and in the lay press centers around potential health risks and endocrine disruption. We will focus on the conversation regarding parabens and the risk for endocrine disruption and association with breast cancer.
Parabens have been reported to have estrogenic effects; however, the bulk of the data is limited to in vitro and animal studies, with less evidence of endocrine disruption in humans.2 In vitro studies have demonstrated that the estrogenic potency of parabens is much less than that of estrogen. In one study, parabens were shown to be 10,000-fold less potent than 17β-estradiol9; in a separate study, they had a maximum potency of only 1/4000 that of estrogen.10 Additionally, an in vitro study showed varying ability for parabens to bind estrogen receptors, with a greater ability to bind with longer alkyl side chains.11 The result is decreased or increased estrogen activity, dependent on side chain length and type of receptor.2 Finally, some studies add conflicting results that parabens may actually create an antiestrogenic effect in human breast cancer cells.12 From the standpoint of estrogen mimicry, there are no known studies in humans confirming harmful effects associated with paraben exposure.
The reported association between parabens and breast cancer is closely related to their theoretical estrogenic effects. The conversation regarding parabens and breast cancer has been fueled by the identification of parabens in human breast tumors and their presence in concentrations similar to what is needed to stimulate in vitro breast cancer cells.2 The existing data do not confirm causation. An association with parabens in topical axillary personal care products has been theorized but not confirmed; for example, it was shown that paraben levels were highest in the axillary region of breast cancer tissue, including women who had never used deodorant. It was concluded that the presence of axillary parabens was due to sources other than topical axillary personal care products.13 Another study confirmed there was not an increased risk for breast cancer in patients who applied personal care products to the axillary area within an hour of shaving.14 The existing data do not support topical paraben exposure as a risk for breast cancer.
Final Thoughts
Parabens are preservatives frequently found in personal care products and exhibit a very low rate of associated ACD. Consumers may be exposed to parabens through foods, cosmetics, and medications. Although there have been consumer concerns regarding endocrine disruption or carcinogenicity associated with parabens, definite evidence of their harm is lacking in the scientific literature, and many studies confirm their safety.2 With their high prevalence in personal care products and low rates of associated contact allergy, parabens remain ideal preservative agents.
Ultimately, contact dermatitis is a common yet often underrecognized dermatologic condition. To address this knowledge gap in clinical practice, we are proud to launch Final Interpretation, a new column in Cutis covering emerging trends in contact dermatitis. We will address pearls, pitfalls, and updates in contact dermatitis. Although our primary focus will be ACD, other important causes of contact dermatitis will be highlighted. Look for the inaugural column in the June 2019 issue of Cutis.
- Fransway AF, Fransway PJ, Belsito DV, et al. Parabens: contact (non)allergen of the year. Dermatitis. 2019;30:3-31.
- Fransway AF, Fransway PJ, Belsito DV, et al. Paraben toxicology. Dermatitis. 2019;30:32-45.
- Final amended report on the safety assessment of methylparaben, ethylparaben, propylparaben, isopropylparaben, butylparaben, isobutylparaben, and benzylparaben as used in cosmetic products. Int J Toxicol. 2008;27(suppl 4):1-82.
- Cosmetic Ingredient Review. Amended safety assessment of parabens as used in cosmetics. https://www.cir-safety.org/sites/default/files/Parabens.pdf. Published August 29, 2018. Accessed March 12, 2019.
- Methylparaben. Fed Regist. 2018;21(3):1490. To be codified at 21 CFR §184.
- Liao C, Liu F, Kannan K. Occurrence of and dietary exposure to parabens in foodstuffs from the United States. Environ Sci Technol. 2013;47:3918-3925.
- DeKoven JG, Warshaw EM, Zug KA, et al. North American Contact Dermatitis Group Patch Test Results: 2015-2016. Dermatitis. 2018;29:297-309.
- Veverka KK, Hall MR, Yiannias JA, et al. Trends in patch testing with the Mayo Clinic standard series, 2011-2015. Dermatitis. 2018;29:310-315.
- Routledge EJ, Parker J, Odum J, et al. Some alkyl hydroxy benzoate preservatives (parabens) are estrogenic. Toxicol Appl Pharmacol. 1998;153:12-19.
- Miller D, Brian B, Wheals BB, et al. Estrogenic activity of phenolic additives determined by an in vitro yeast bioassay. Environ Health Perspect. 2001;109:133-138.
- Blair RM, Fang H, Branham WS. The estrogen receptor relative binding affinities of 188 natural and xenochemicals: structural diversity of ligands. Toxicol Sci. 2000;54:138-153.
- van Meeuwen JA, van Son O, Piersma AH, et al. Aromatase inhibiting and combined estrogenic effects of parabens and estrogenic effects of other additives in cosmetics. Toxicol Appl Pharmacol. 2008;230:372-382.
- Barr L, Metaxas G, Harbach CA, et al. Measurement of paraben concentrations in human breast tissue at serial locations across the breast from axilla to sternum. J Appl Toxicol. 2012;32:219-232.
- Mirick DK, Davis S, Thomas DB. Antiperspirant use and the risk of breast cancer. J Natl Cancer Inst. 2002;94:1578-1580
.
- Fransway AF, Fransway PJ, Belsito DV, et al. Parabens: contact (non)allergen of the year. Dermatitis. 2019;30:3-31.
- Fransway AF, Fransway PJ, Belsito DV, et al. Paraben toxicology. Dermatitis. 2019;30:32-45.
- Final amended report on the safety assessment of methylparaben, ethylparaben, propylparaben, isopropylparaben, butylparaben, isobutylparaben, and benzylparaben as used in cosmetic products. Int J Toxicol. 2008;27(suppl 4):1-82.
- Cosmetic Ingredient Review. Amended safety assessment of parabens as used in cosmetics. https://www.cir-safety.org/sites/default/files/Parabens.pdf. Published August 29, 2018. Accessed March 12, 2019.
- Methylparaben. Fed Regist. 2018;21(3):1490. To be codified at 21 CFR §184.
- Liao C, Liu F, Kannan K. Occurrence of and dietary exposure to parabens in foodstuffs from the United States. Environ Sci Technol. 2013;47:3918-3925.
- DeKoven JG, Warshaw EM, Zug KA, et al. North American Contact Dermatitis Group Patch Test Results: 2015-2016. Dermatitis. 2018;29:297-309.
- Veverka KK, Hall MR, Yiannias JA, et al. Trends in patch testing with the Mayo Clinic standard series, 2011-2015. Dermatitis. 2018;29:310-315.
- Routledge EJ, Parker J, Odum J, et al. Some alkyl hydroxy benzoate preservatives (parabens) are estrogenic. Toxicol Appl Pharmacol. 1998;153:12-19.
- Miller D, Brian B, Wheals BB, et al. Estrogenic activity of phenolic additives determined by an in vitro yeast bioassay. Environ Health Perspect. 2001;109:133-138.
- Blair RM, Fang H, Branham WS. The estrogen receptor relative binding affinities of 188 natural and xenochemicals: structural diversity of ligands. Toxicol Sci. 2000;54:138-153.
- van Meeuwen JA, van Son O, Piersma AH, et al. Aromatase inhibiting and combined estrogenic effects of parabens and estrogenic effects of other additives in cosmetics. Toxicol Appl Pharmacol. 2008;230:372-382.
- Barr L, Metaxas G, Harbach CA, et al. Measurement of paraben concentrations in human breast tissue at serial locations across the breast from axilla to sternum. J Appl Toxicol. 2012;32:219-232.
- Mirick DK, Davis S, Thomas DB. Antiperspirant use and the risk of breast cancer. J Natl Cancer Inst. 2002;94:1578-1580
.