User login
History of melanoma in situ • dyspnea • rib pain • Dx?
THE CASE
A 56-year-old woman with a history of melanoma in situ presented with progressive dyspnea on exertion, cough productive of clear sputum, and right-sided rib pain radiating to the upper back of 5 weeks’ duration.
Twenty-four years earlier, the patient had undergone excision of a skin lesion from the right side of her back. Pathology revealed melanoma in situ with no evidence of invasion of the underlying dermis. Because of close margins, she underwent wider excision 2 weeks later and no residual tumor was found. The patient subsequently underwent routine biannual dermatologic follow-up and transitioned (within the past few years) to annual dermatologic follow-up. At a recent dermatologic visit (9 months earlier), there were no suspicious skin lesions.
At current presentation, she denied fever, chills, night sweats, or unintentional weight loss. On examination, her vital signs were normal. Her pulse oximetry on room air was 95% at rest and 94% with ambulation. She had decreased breath sounds at the right lung base and a fixed 2 × 2-cm nontender, indurated mass in the right upper anterior chest wall, superior to the right breast. A skin examination was not performed at this time.
A complete blood count revealed a white blood cell count of 8220/mcL (reference range, 4500–11,000/mcL), hemoglobin of 13.6 g/dL (reference range, 14–17.5 g/dL), and a platelet count of 162 × 103/mcL (reference range, 150–350 × 103/mcL). The patient’s electrolytes were within normal limits, with a creatinine level of 0.67 mg/dL (reference range, 0.1–1.2 mg/dL) and a calcium level of 9.4 mg/dL (reference range, 8.2–10.2 mg/dL). Lactate dehydrogenase was elevated at 308 U/L (reference range, 100–200 U/L).
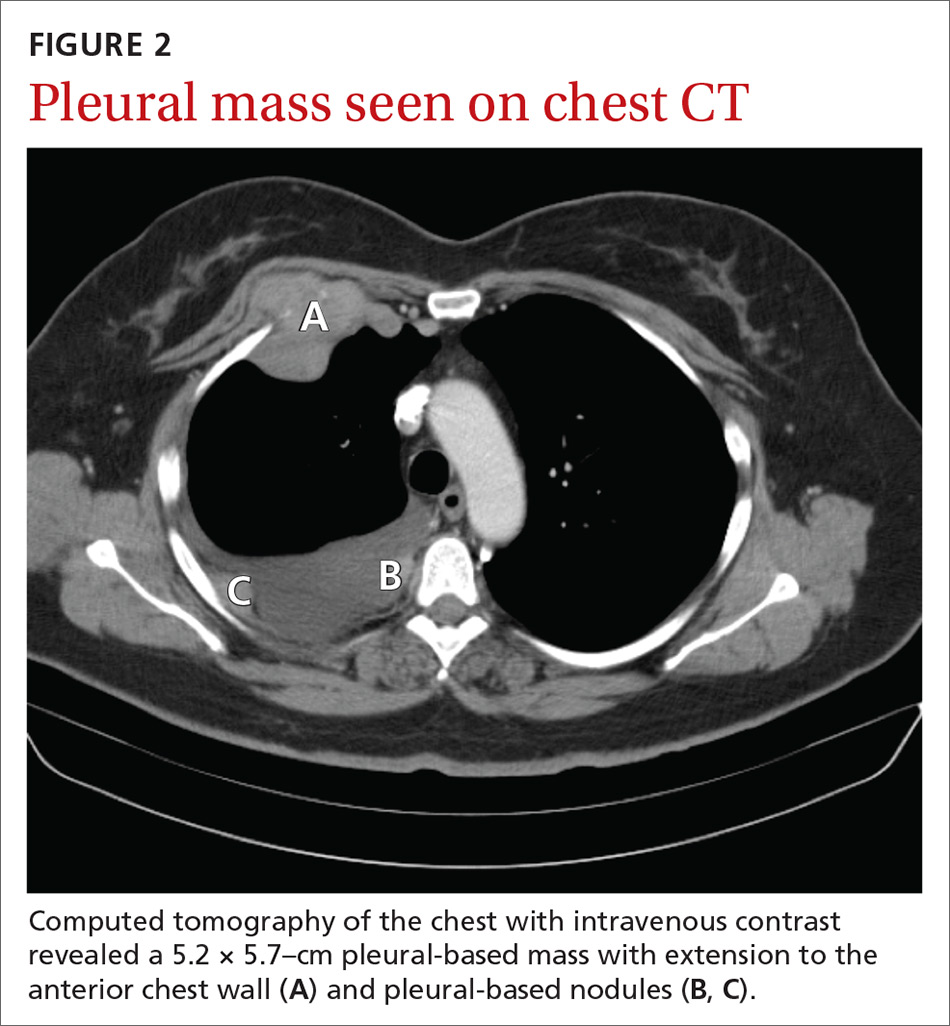
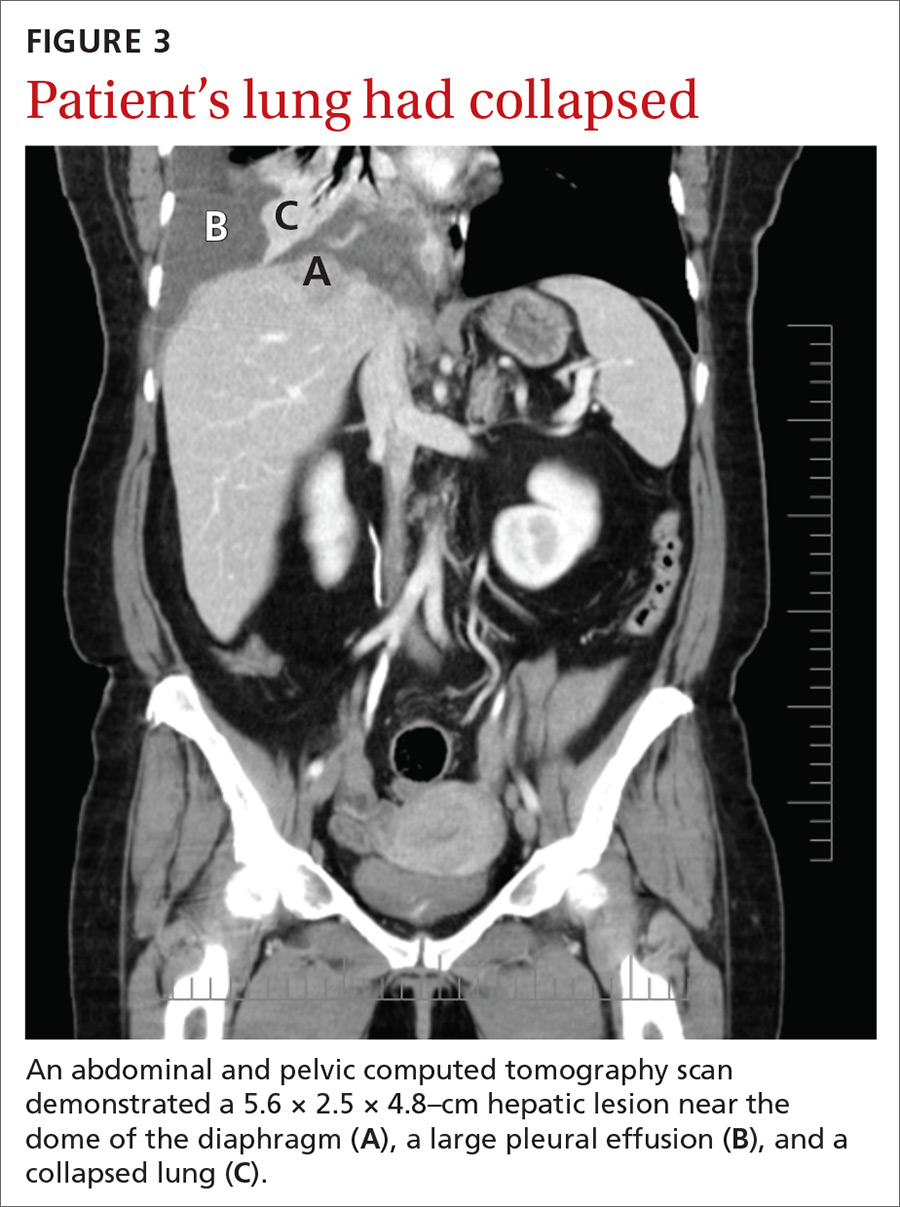
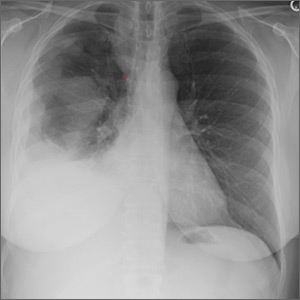
A chest radiograph revealed a right upper lobe mass, right lower lobe consolidation, and a large right-sided pleural effusion (FIGURE 1). Chest computed tomography (CT) with intravenous contrast revealed a 5.2 × 5.7–cm right pleural-based mass with extension to the anterior chest wall, 3 left-sided pulmonary nodules, numerous right-sided pleural-based nodules (FIGURE 2), and multiple low-density liver lesions. An abdominal and pelvic CT scan revealed a 5.6 × 2.5 × 4.8–cm hepatic lesion (FIGURE 3) with scattered hepatic cysts.
THE DIAGNOSIS
A diagnostic thoracentesis was performed, and pleural fluid cytology results revealed metastatic melanoma. Magnetic resonance imaging (MRI) of the brain showed no evidence of metastases.
After the patient’s initial presentation, her dermatologist performed a biopsy on a pre-existing skin lesion on the patient’s left abdomen, which initially was thought to be a cherry angioma. This left abdominal skin lesion was in a location different from her previous melanoma in situ, which was located on the right side of the back. Biopsy results of the presumed cherry angioma revealed a nodular malignant melanoma (which was partially removed), adjacent to a cherry angioma (which was completely excised).
Continue to: Two primary melanomas
Two primary melanomas. Our patient had 2 different primary melanomas: a melanoma in situ on the right back diagnosed 24 years prior to the current presentation and the more recently identified melanoma on the left abdomen with metastases to the lung and liver.
We referred the patient to Oncology and she was enrolled in a clinical study with ipilimumab and nivolumab, monoclonal antibodies directed against negative regulators of T-cell activation.
DISCUSSION
In the United States, melanoma is the fifth leading cancer in men and the sixth leading cancer in women.1 A prior history of melanoma or melanoma in situ increases the risk for a second melanoma,2-4 and the risk remains elevated for more than 20 years after the initial diagnosis.2 One- and 2-year survival rates for metastatic melanoma are 32% to 65% and 18% to 40%, respectively5; the 5-year survival rate of metastatic melanoma to the lung is approximately 16%.6
Recommendations regarding the appropriate follow-up of patients with a history of melanoma in situ and melanoma vary widely.7 For patients with a history of melanoma in situ, the American Academy of Dermatology and the National Comprehensive Cancer Network recommend annual skin examination indefinitely and self-examination of the skin and lymph nodes monthly.4,7,8
Novel therapies are powerful allies in fight against melanoma
Previous standard treatment of metastatic melanoma included surgery, radiation, and cytotoxic chemotherapy. Resection rarely is curative in distant metastatic melanoma, and cytotoxic chemotherapy has low response rates, has a response duration of 4 to 6 months, and does not improve overall survival in advanced melanoma.9-12
Continue to: Novel therapies...
Novel therapies, such as immunotherapy and molecular-targeted therapies, are dramatically increasing survival rates in metastatic melanoma. Melanoma frequently is associated with somatic mutations, and each patient may have a unique collection of mutations resulting in the expression of antigens that bind to certain T-cell receptors, which serve as targets for inhibitor immunotherapy.
Ipilimumab and nivolumab are monoclonal antibodies directed against negative regulators of T-cell activation. When ipilimumab and nivolumab bind to their receptors, feedback inhibition is prevented, which results in an immune response against the tumor. In a trial of 53 patients with advanced melanoma treated with both drugs, the overall survival rate at 1 and 2 years was 94% and 88%, respectively.13
Dabrafenib and trametinib. Mutations that activate the serine/threonine kinase gene, BRAF, are present in approximately 40% to 60% of advanced melanomas and lead to clonal expansion and tumor progression.14,15 Inhibition of BRAF produces rapid tumor regression—even in extensive disease. Treatment with dabrafenib, a BRAF inhibitor, and trametinib, a mitogen-activated protein kinase inhibitor, has been shown to be superior to a BRAF inhibitor alone and is associated with a survival rate of 72% at 1 year.16
Our patient. Seven months after enrolling in the clinical trial with ipilimumab and nivolumab, our patient developed brain metastases and was withdrawn from the trial. A resection of her brain metastases and radiation therapy followed. The patient was then started on molecular-targeted therapy with dabrafenib and trametinib. Twelve weeks later, a repeat CT scan of the chest, abdomen, and pelvis demonstrated an interval decrease in the size of the majority of the metastatic lesions, and a repeat brain MRI showed no additional metastases.
More than 4 years after her diagnosis, our patient remains on dabrafenib and trametinib therapy and her metastatic lesions to the lung and liver remain stable.
Continue to: THE TAKEAWAY
THE TAKEAWAY
Patients with a prior melanoma in situ or invasive melanoma have a higher risk for a subsequent invasive melanoma, and this risk remains elevated for more than 20 years. While patients with a history of melanoma in situ do not require specific oncologic follow-up, they do require annual dermatologic follow-up indefinitely and should perform monthly self-examination of their skin and lymph nodes.
Heightened awareness of the risk for a second primary melanoma should prompt primary care physicians to conduct ongoing patient surveillance. Family physicians should also keep in mind that novel therapies for metastatic melanoma, such as molecular-targeted therapies and immunotherapy, are associated with a much higher survival rate than previous standard therapy.
CORRESPONDENCE
Iris Tong, MD, Associate Professor, Division of General Internal Medicine, Department of Medicine, Alpert Medical School of Brown University, 146 W River St, Providence, RI 02904; iris_tong@brown.edu
1. Siegel RL, Miller KD, Jemal A. Cancer statistics, 2018 [published online January 4, 2018]. Cancer J Clin. 2018;68:7-30.
2. Bradford PT, Freedman DM, Goldstein AM, et al. Increased risk of second primary cancers after diagnosis of melanoma. Arch Dermatol. 2010;146:265-272.
3. Balamurugan A, Rees JR, Kosary C, et al. Subsequent primary cancers among men and women with in situ and invasive melanoma of the skin. J Am Acad Dermatol. 2011;65(5) (suppl 1):S69-S77.
4. Pomerantz H, Huang D, Weinstock MA. Risk of subsequent melanoma after melanoma in situ and invasive melanoma: a population-based study from 1973 to 2011. J Am Acad Dermatol. 2015;72:794-800.
5. Balch CM, Gershenwald JE, Soong SJ, et al. Final version of 2009 AJCC melanoma staging and classification. J Clin Oncol. 2009;27:6199-6206.
6. American Cancer Society. Cancer facts & figures 2015. www.cancer.org/research/cancerfactsstatistics/cancerfactsfigures2015/index. Accessed November 26, 2018.
7. Coit DG, Andtbacka R, Anker CJ, et al. Melanoma: clinical practice guidelines in oncology. J Natl Compr Canc Netw. 2012;3:366-400.
8. Bichakjian CK, Halpern AC, Johnson TM. Guidelines of care for the management of primary cutaneous melanoma. J Am Acad Dermatol. 2011;5:1032-1047.
9. Atkins MB. The role of cytotoxic chemotherapeutic agents either alone or in combination with biological response modifiers. In: Kirkwood JK, ed. Molecular Diagnosis, Prevention & Therapy of Melanoma. New York, NY: Marcel Dekker; 1997:219.
10. Patel PM, Suciu S, Mortier L, et al; EORTC Melanoma Group. Extended schedule, escalated dose temozolomide versus dacarbazine in stage IV melanoma: final results of a randomised phase III study (EORTC 18032) [published online May 18, 2011]. Eur J Cancer. 2011;47:1476-1483.
11. Flaherty KT, Lee SJ, Zhao F, et al. Phase III trial of carboplatin and paclitaxel with or without sorafenib in metastatic melanoma [published online December 17, 2012]. J Clin Oncol. 2013;31:373-379.
12. Sosman JA, Moon J, Tuthill RJ, et al. A phase 2 trial of complete resection for stage IV melanoma: results of Southwest Oncology Group Clinical Trial S9430 [published online March 31, 2011]. Cancer. 2011;117:4740-4746.
13. Sznol M, Kluger HM, Callahan MK, et al. Abstract LBA9003. Presented at: 2014 American Society of Clinical Oncology (ASCO) Annual Meeting; May 30–June 3, 2014; Chicago, IL.
14. Omholt K, Platz A, Kanter L, et al. NRAS and BRAF mutations arise early during melanoma pathogenesis and are preserved throughout tumor progression. Clin Cancer Res. 2003;9:6483-6488.
15. Long GV, Menzies AM, Nagrial AM, et al. Prognostic and clinicopathologic associations of oncogenic BRAF in metastatic melanoma. J Clin Oncol. 2011;29:1239-1246.
16. Robert C, Karaszewska B, Schachter J, et al. Improved overall survival in melanoma with combined dabrafenib and trametinib. N Engl J Med. 2015;372:30-39.
THE CASE
A 56-year-old woman with a history of melanoma in situ presented with progressive dyspnea on exertion, cough productive of clear sputum, and right-sided rib pain radiating to the upper back of 5 weeks’ duration.
Twenty-four years earlier, the patient had undergone excision of a skin lesion from the right side of her back. Pathology revealed melanoma in situ with no evidence of invasion of the underlying dermis. Because of close margins, she underwent wider excision 2 weeks later and no residual tumor was found. The patient subsequently underwent routine biannual dermatologic follow-up and transitioned (within the past few years) to annual dermatologic follow-up. At a recent dermatologic visit (9 months earlier), there were no suspicious skin lesions.
At current presentation, she denied fever, chills, night sweats, or unintentional weight loss. On examination, her vital signs were normal. Her pulse oximetry on room air was 95% at rest and 94% with ambulation. She had decreased breath sounds at the right lung base and a fixed 2 × 2-cm nontender, indurated mass in the right upper anterior chest wall, superior to the right breast. A skin examination was not performed at this time.
A complete blood count revealed a white blood cell count of 8220/mcL (reference range, 4500–11,000/mcL), hemoglobin of 13.6 g/dL (reference range, 14–17.5 g/dL), and a platelet count of 162 × 103/mcL (reference range, 150–350 × 103/mcL). The patient’s electrolytes were within normal limits, with a creatinine level of 0.67 mg/dL (reference range, 0.1–1.2 mg/dL) and a calcium level of 9.4 mg/dL (reference range, 8.2–10.2 mg/dL). Lactate dehydrogenase was elevated at 308 U/L (reference range, 100–200 U/L).
A chest radiograph revealed a right upper lobe mass, right lower lobe consolidation, and a large right-sided pleural effusion (FIGURE 1). Chest computed tomography (CT) with intravenous contrast revealed a 5.2 × 5.7–cm right pleural-based mass with extension to the anterior chest wall, 3 left-sided pulmonary nodules, numerous right-sided pleural-based nodules (FIGURE 2), and multiple low-density liver lesions. An abdominal and pelvic CT scan revealed a 5.6 × 2.5 × 4.8–cm hepatic lesion (FIGURE 3) with scattered hepatic cysts.
THE DIAGNOSIS
A diagnostic thoracentesis was performed, and pleural fluid cytology results revealed metastatic melanoma. Magnetic resonance imaging (MRI) of the brain showed no evidence of metastases.
After the patient’s initial presentation, her dermatologist performed a biopsy on a pre-existing skin lesion on the patient’s left abdomen, which initially was thought to be a cherry angioma. This left abdominal skin lesion was in a location different from her previous melanoma in situ, which was located on the right side of the back. Biopsy results of the presumed cherry angioma revealed a nodular malignant melanoma (which was partially removed), adjacent to a cherry angioma (which was completely excised).
Continue to: Two primary melanomas
Two primary melanomas. Our patient had 2 different primary melanomas: a melanoma in situ on the right back diagnosed 24 years prior to the current presentation and the more recently identified melanoma on the left abdomen with metastases to the lung and liver.
We referred the patient to Oncology and she was enrolled in a clinical study with ipilimumab and nivolumab, monoclonal antibodies directed against negative regulators of T-cell activation.
DISCUSSION
In the United States, melanoma is the fifth leading cancer in men and the sixth leading cancer in women.1 A prior history of melanoma or melanoma in situ increases the risk for a second melanoma,2-4 and the risk remains elevated for more than 20 years after the initial diagnosis.2 One- and 2-year survival rates for metastatic melanoma are 32% to 65% and 18% to 40%, respectively5; the 5-year survival rate of metastatic melanoma to the lung is approximately 16%.6
Recommendations regarding the appropriate follow-up of patients with a history of melanoma in situ and melanoma vary widely.7 For patients with a history of melanoma in situ, the American Academy of Dermatology and the National Comprehensive Cancer Network recommend annual skin examination indefinitely and self-examination of the skin and lymph nodes monthly.4,7,8
Novel therapies are powerful allies in fight against melanoma
Previous standard treatment of metastatic melanoma included surgery, radiation, and cytotoxic chemotherapy. Resection rarely is curative in distant metastatic melanoma, and cytotoxic chemotherapy has low response rates, has a response duration of 4 to 6 months, and does not improve overall survival in advanced melanoma.9-12
Continue to: Novel therapies...
Novel therapies, such as immunotherapy and molecular-targeted therapies, are dramatically increasing survival rates in metastatic melanoma. Melanoma frequently is associated with somatic mutations, and each patient may have a unique collection of mutations resulting in the expression of antigens that bind to certain T-cell receptors, which serve as targets for inhibitor immunotherapy.
Ipilimumab and nivolumab are monoclonal antibodies directed against negative regulators of T-cell activation. When ipilimumab and nivolumab bind to their receptors, feedback inhibition is prevented, which results in an immune response against the tumor. In a trial of 53 patients with advanced melanoma treated with both drugs, the overall survival rate at 1 and 2 years was 94% and 88%, respectively.13
Dabrafenib and trametinib. Mutations that activate the serine/threonine kinase gene, BRAF, are present in approximately 40% to 60% of advanced melanomas and lead to clonal expansion and tumor progression.14,15 Inhibition of BRAF produces rapid tumor regression—even in extensive disease. Treatment with dabrafenib, a BRAF inhibitor, and trametinib, a mitogen-activated protein kinase inhibitor, has been shown to be superior to a BRAF inhibitor alone and is associated with a survival rate of 72% at 1 year.16
Our patient. Seven months after enrolling in the clinical trial with ipilimumab and nivolumab, our patient developed brain metastases and was withdrawn from the trial. A resection of her brain metastases and radiation therapy followed. The patient was then started on molecular-targeted therapy with dabrafenib and trametinib. Twelve weeks later, a repeat CT scan of the chest, abdomen, and pelvis demonstrated an interval decrease in the size of the majority of the metastatic lesions, and a repeat brain MRI showed no additional metastases.
More than 4 years after her diagnosis, our patient remains on dabrafenib and trametinib therapy and her metastatic lesions to the lung and liver remain stable.
Continue to: THE TAKEAWAY
THE TAKEAWAY
Patients with a prior melanoma in situ or invasive melanoma have a higher risk for a subsequent invasive melanoma, and this risk remains elevated for more than 20 years. While patients with a history of melanoma in situ do not require specific oncologic follow-up, they do require annual dermatologic follow-up indefinitely and should perform monthly self-examination of their skin and lymph nodes.
Heightened awareness of the risk for a second primary melanoma should prompt primary care physicians to conduct ongoing patient surveillance. Family physicians should also keep in mind that novel therapies for metastatic melanoma, such as molecular-targeted therapies and immunotherapy, are associated with a much higher survival rate than previous standard therapy.
CORRESPONDENCE
Iris Tong, MD, Associate Professor, Division of General Internal Medicine, Department of Medicine, Alpert Medical School of Brown University, 146 W River St, Providence, RI 02904; iris_tong@brown.edu
THE CASE
A 56-year-old woman with a history of melanoma in situ presented with progressive dyspnea on exertion, cough productive of clear sputum, and right-sided rib pain radiating to the upper back of 5 weeks’ duration.
Twenty-four years earlier, the patient had undergone excision of a skin lesion from the right side of her back. Pathology revealed melanoma in situ with no evidence of invasion of the underlying dermis. Because of close margins, she underwent wider excision 2 weeks later and no residual tumor was found. The patient subsequently underwent routine biannual dermatologic follow-up and transitioned (within the past few years) to annual dermatologic follow-up. At a recent dermatologic visit (9 months earlier), there were no suspicious skin lesions.
At current presentation, she denied fever, chills, night sweats, or unintentional weight loss. On examination, her vital signs were normal. Her pulse oximetry on room air was 95% at rest and 94% with ambulation. She had decreased breath sounds at the right lung base and a fixed 2 × 2-cm nontender, indurated mass in the right upper anterior chest wall, superior to the right breast. A skin examination was not performed at this time.
A complete blood count revealed a white blood cell count of 8220/mcL (reference range, 4500–11,000/mcL), hemoglobin of 13.6 g/dL (reference range, 14–17.5 g/dL), and a platelet count of 162 × 103/mcL (reference range, 150–350 × 103/mcL). The patient’s electrolytes were within normal limits, with a creatinine level of 0.67 mg/dL (reference range, 0.1–1.2 mg/dL) and a calcium level of 9.4 mg/dL (reference range, 8.2–10.2 mg/dL). Lactate dehydrogenase was elevated at 308 U/L (reference range, 100–200 U/L).
A chest radiograph revealed a right upper lobe mass, right lower lobe consolidation, and a large right-sided pleural effusion (FIGURE 1). Chest computed tomography (CT) with intravenous contrast revealed a 5.2 × 5.7–cm right pleural-based mass with extension to the anterior chest wall, 3 left-sided pulmonary nodules, numerous right-sided pleural-based nodules (FIGURE 2), and multiple low-density liver lesions. An abdominal and pelvic CT scan revealed a 5.6 × 2.5 × 4.8–cm hepatic lesion (FIGURE 3) with scattered hepatic cysts.
THE DIAGNOSIS
A diagnostic thoracentesis was performed, and pleural fluid cytology results revealed metastatic melanoma. Magnetic resonance imaging (MRI) of the brain showed no evidence of metastases.
After the patient’s initial presentation, her dermatologist performed a biopsy on a pre-existing skin lesion on the patient’s left abdomen, which initially was thought to be a cherry angioma. This left abdominal skin lesion was in a location different from her previous melanoma in situ, which was located on the right side of the back. Biopsy results of the presumed cherry angioma revealed a nodular malignant melanoma (which was partially removed), adjacent to a cherry angioma (which was completely excised).
Continue to: Two primary melanomas
Two primary melanomas. Our patient had 2 different primary melanomas: a melanoma in situ on the right back diagnosed 24 years prior to the current presentation and the more recently identified melanoma on the left abdomen with metastases to the lung and liver.
We referred the patient to Oncology and she was enrolled in a clinical study with ipilimumab and nivolumab, monoclonal antibodies directed against negative regulators of T-cell activation.
DISCUSSION
In the United States, melanoma is the fifth leading cancer in men and the sixth leading cancer in women.1 A prior history of melanoma or melanoma in situ increases the risk for a second melanoma,2-4 and the risk remains elevated for more than 20 years after the initial diagnosis.2 One- and 2-year survival rates for metastatic melanoma are 32% to 65% and 18% to 40%, respectively5; the 5-year survival rate of metastatic melanoma to the lung is approximately 16%.6
Recommendations regarding the appropriate follow-up of patients with a history of melanoma in situ and melanoma vary widely.7 For patients with a history of melanoma in situ, the American Academy of Dermatology and the National Comprehensive Cancer Network recommend annual skin examination indefinitely and self-examination of the skin and lymph nodes monthly.4,7,8
Novel therapies are powerful allies in fight against melanoma
Previous standard treatment of metastatic melanoma included surgery, radiation, and cytotoxic chemotherapy. Resection rarely is curative in distant metastatic melanoma, and cytotoxic chemotherapy has low response rates, has a response duration of 4 to 6 months, and does not improve overall survival in advanced melanoma.9-12
Continue to: Novel therapies...
Novel therapies, such as immunotherapy and molecular-targeted therapies, are dramatically increasing survival rates in metastatic melanoma. Melanoma frequently is associated with somatic mutations, and each patient may have a unique collection of mutations resulting in the expression of antigens that bind to certain T-cell receptors, which serve as targets for inhibitor immunotherapy.
Ipilimumab and nivolumab are monoclonal antibodies directed against negative regulators of T-cell activation. When ipilimumab and nivolumab bind to their receptors, feedback inhibition is prevented, which results in an immune response against the tumor. In a trial of 53 patients with advanced melanoma treated with both drugs, the overall survival rate at 1 and 2 years was 94% and 88%, respectively.13
Dabrafenib and trametinib. Mutations that activate the serine/threonine kinase gene, BRAF, are present in approximately 40% to 60% of advanced melanomas and lead to clonal expansion and tumor progression.14,15 Inhibition of BRAF produces rapid tumor regression—even in extensive disease. Treatment with dabrafenib, a BRAF inhibitor, and trametinib, a mitogen-activated protein kinase inhibitor, has been shown to be superior to a BRAF inhibitor alone and is associated with a survival rate of 72% at 1 year.16
Our patient. Seven months after enrolling in the clinical trial with ipilimumab and nivolumab, our patient developed brain metastases and was withdrawn from the trial. A resection of her brain metastases and radiation therapy followed. The patient was then started on molecular-targeted therapy with dabrafenib and trametinib. Twelve weeks later, a repeat CT scan of the chest, abdomen, and pelvis demonstrated an interval decrease in the size of the majority of the metastatic lesions, and a repeat brain MRI showed no additional metastases.
More than 4 years after her diagnosis, our patient remains on dabrafenib and trametinib therapy and her metastatic lesions to the lung and liver remain stable.
Continue to: THE TAKEAWAY
THE TAKEAWAY
Patients with a prior melanoma in situ or invasive melanoma have a higher risk for a subsequent invasive melanoma, and this risk remains elevated for more than 20 years. While patients with a history of melanoma in situ do not require specific oncologic follow-up, they do require annual dermatologic follow-up indefinitely and should perform monthly self-examination of their skin and lymph nodes.
Heightened awareness of the risk for a second primary melanoma should prompt primary care physicians to conduct ongoing patient surveillance. Family physicians should also keep in mind that novel therapies for metastatic melanoma, such as molecular-targeted therapies and immunotherapy, are associated with a much higher survival rate than previous standard therapy.
CORRESPONDENCE
Iris Tong, MD, Associate Professor, Division of General Internal Medicine, Department of Medicine, Alpert Medical School of Brown University, 146 W River St, Providence, RI 02904; iris_tong@brown.edu
1. Siegel RL, Miller KD, Jemal A. Cancer statistics, 2018 [published online January 4, 2018]. Cancer J Clin. 2018;68:7-30.
2. Bradford PT, Freedman DM, Goldstein AM, et al. Increased risk of second primary cancers after diagnosis of melanoma. Arch Dermatol. 2010;146:265-272.
3. Balamurugan A, Rees JR, Kosary C, et al. Subsequent primary cancers among men and women with in situ and invasive melanoma of the skin. J Am Acad Dermatol. 2011;65(5) (suppl 1):S69-S77.
4. Pomerantz H, Huang D, Weinstock MA. Risk of subsequent melanoma after melanoma in situ and invasive melanoma: a population-based study from 1973 to 2011. J Am Acad Dermatol. 2015;72:794-800.
5. Balch CM, Gershenwald JE, Soong SJ, et al. Final version of 2009 AJCC melanoma staging and classification. J Clin Oncol. 2009;27:6199-6206.
6. American Cancer Society. Cancer facts & figures 2015. www.cancer.org/research/cancerfactsstatistics/cancerfactsfigures2015/index. Accessed November 26, 2018.
7. Coit DG, Andtbacka R, Anker CJ, et al. Melanoma: clinical practice guidelines in oncology. J Natl Compr Canc Netw. 2012;3:366-400.
8. Bichakjian CK, Halpern AC, Johnson TM. Guidelines of care for the management of primary cutaneous melanoma. J Am Acad Dermatol. 2011;5:1032-1047.
9. Atkins MB. The role of cytotoxic chemotherapeutic agents either alone or in combination with biological response modifiers. In: Kirkwood JK, ed. Molecular Diagnosis, Prevention & Therapy of Melanoma. New York, NY: Marcel Dekker; 1997:219.
10. Patel PM, Suciu S, Mortier L, et al; EORTC Melanoma Group. Extended schedule, escalated dose temozolomide versus dacarbazine in stage IV melanoma: final results of a randomised phase III study (EORTC 18032) [published online May 18, 2011]. Eur J Cancer. 2011;47:1476-1483.
11. Flaherty KT, Lee SJ, Zhao F, et al. Phase III trial of carboplatin and paclitaxel with or without sorafenib in metastatic melanoma [published online December 17, 2012]. J Clin Oncol. 2013;31:373-379.
12. Sosman JA, Moon J, Tuthill RJ, et al. A phase 2 trial of complete resection for stage IV melanoma: results of Southwest Oncology Group Clinical Trial S9430 [published online March 31, 2011]. Cancer. 2011;117:4740-4746.
13. Sznol M, Kluger HM, Callahan MK, et al. Abstract LBA9003. Presented at: 2014 American Society of Clinical Oncology (ASCO) Annual Meeting; May 30–June 3, 2014; Chicago, IL.
14. Omholt K, Platz A, Kanter L, et al. NRAS and BRAF mutations arise early during melanoma pathogenesis and are preserved throughout tumor progression. Clin Cancer Res. 2003;9:6483-6488.
15. Long GV, Menzies AM, Nagrial AM, et al. Prognostic and clinicopathologic associations of oncogenic BRAF in metastatic melanoma. J Clin Oncol. 2011;29:1239-1246.
16. Robert C, Karaszewska B, Schachter J, et al. Improved overall survival in melanoma with combined dabrafenib and trametinib. N Engl J Med. 2015;372:30-39.
1. Siegel RL, Miller KD, Jemal A. Cancer statistics, 2018 [published online January 4, 2018]. Cancer J Clin. 2018;68:7-30.
2. Bradford PT, Freedman DM, Goldstein AM, et al. Increased risk of second primary cancers after diagnosis of melanoma. Arch Dermatol. 2010;146:265-272.
3. Balamurugan A, Rees JR, Kosary C, et al. Subsequent primary cancers among men and women with in situ and invasive melanoma of the skin. J Am Acad Dermatol. 2011;65(5) (suppl 1):S69-S77.
4. Pomerantz H, Huang D, Weinstock MA. Risk of subsequent melanoma after melanoma in situ and invasive melanoma: a population-based study from 1973 to 2011. J Am Acad Dermatol. 2015;72:794-800.
5. Balch CM, Gershenwald JE, Soong SJ, et al. Final version of 2009 AJCC melanoma staging and classification. J Clin Oncol. 2009;27:6199-6206.
6. American Cancer Society. Cancer facts & figures 2015. www.cancer.org/research/cancerfactsstatistics/cancerfactsfigures2015/index. Accessed November 26, 2018.
7. Coit DG, Andtbacka R, Anker CJ, et al. Melanoma: clinical practice guidelines in oncology. J Natl Compr Canc Netw. 2012;3:366-400.
8. Bichakjian CK, Halpern AC, Johnson TM. Guidelines of care for the management of primary cutaneous melanoma. J Am Acad Dermatol. 2011;5:1032-1047.
9. Atkins MB. The role of cytotoxic chemotherapeutic agents either alone or in combination with biological response modifiers. In: Kirkwood JK, ed. Molecular Diagnosis, Prevention & Therapy of Melanoma. New York, NY: Marcel Dekker; 1997:219.
10. Patel PM, Suciu S, Mortier L, et al; EORTC Melanoma Group. Extended schedule, escalated dose temozolomide versus dacarbazine in stage IV melanoma: final results of a randomised phase III study (EORTC 18032) [published online May 18, 2011]. Eur J Cancer. 2011;47:1476-1483.
11. Flaherty KT, Lee SJ, Zhao F, et al. Phase III trial of carboplatin and paclitaxel with or without sorafenib in metastatic melanoma [published online December 17, 2012]. J Clin Oncol. 2013;31:373-379.
12. Sosman JA, Moon J, Tuthill RJ, et al. A phase 2 trial of complete resection for stage IV melanoma: results of Southwest Oncology Group Clinical Trial S9430 [published online March 31, 2011]. Cancer. 2011;117:4740-4746.
13. Sznol M, Kluger HM, Callahan MK, et al. Abstract LBA9003. Presented at: 2014 American Society of Clinical Oncology (ASCO) Annual Meeting; May 30–June 3, 2014; Chicago, IL.
14. Omholt K, Platz A, Kanter L, et al. NRAS and BRAF mutations arise early during melanoma pathogenesis and are preserved throughout tumor progression. Clin Cancer Res. 2003;9:6483-6488.
15. Long GV, Menzies AM, Nagrial AM, et al. Prognostic and clinicopathologic associations of oncogenic BRAF in metastatic melanoma. J Clin Oncol. 2011;29:1239-1246.
16. Robert C, Karaszewska B, Schachter J, et al. Improved overall survival in melanoma with combined dabrafenib and trametinib. N Engl J Med. 2015;372:30-39.