User login
Opioids for persistent pain in older adults
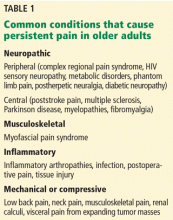
The use of opioid analgesics is widely accepted for treating severe acute pain, cancer pain, and pain at the end of life.1 However, their long-term use for other types of persistent pain (Table 1) remains controversial. Clinicians and regulators need to work together to achieve a balanced approach to the use of opioids, recognizing the legitimate medical need for these medications for persistent pain while acknowledging their increasing misuse and the morbidity and mortality related to them. Finding this balance is particularly challenging in older patients.2
PAIN IN OLDER PEOPLE: COMPLICATED, OFTEN UNDERTREATED
Persistent pain is a multifaceted manifestation of an unpleasant sensation that continues for a prolonged time and may or may not be related to a distinct disease process.3 (The term “persistent pain” is preferred as it does not have the negative connotations of “chronic pain.”4) “Older” has been defined as age 65 and older. As our population ages, especially to age 85 and older, more people will be living with persistent pain due to a variety of conditions.5
Persistent pain is more complicated in older than in younger patients. Many older people have more than one illness, making them more susceptible to adverse drug interactions such as altered pharmacokinetics and pharmacodynamics.6 Up to 40% of older outpatients report pain,7 and pain affects 70% to 80% of patients with advanced malignant disease.8 Pain is also prevalent in nonmalignant, progressive, life-limiting illnesses that are common in the geriatric population, affecting 41% to 77% of patients with advanced heart disease, 34% to 77% with advanced chronic obstructive pulmonary disease, and 47% to 50% with advanced renal disease.9
Pain is underrecognized in nursing home residents, who may have multiple somatic complaints and multiple causes of pain.10,11 From 27% to 83% of older adults in an institutionalized setting are affected by pain.12 Caregiver stress and attitudes towards pain may influence patients’ experiences with pain. This aspect should also be assessed and evaluated, if present.3
Pain in older adults is often undertreated, as evidenced by the findings of a study in which only one-third of older patients with persistent pain were receiving treatment that was consistent with current guidelines.13 Approximately 40% to 80% of older adults in the community with pain do not receive any treatment for it.14,15 Of those residing in institutions, 16% to 27% of older adults in pain do not receive any treatment for it.16,17 Inadequate treatment of persistent pain is associated with many adverse outcomes, including functional decline, falls, mood changes, decreased socialization, sleep and appetite difficulties, and increased healthcare utilization.18
GOALS: BETTER QUALITY OF LIFE AND FUNCTION
Persistent pain is multifactorial and so requires an approach that addresses a variety of causes and includes both nonpharmacologic and pharmacologic strategies. Opioids are part of a multipronged approach to pain management.
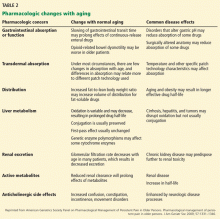
To avoid adverse effects, opioids for persistent pain in an older adult should be prescribed at the lowest possible dose that provides adequate analgesia. Due to age-related changes, finding the best treatments may be a challenge, and understanding the pharmacokinetic implications in this population is key (Table 2).
Complete pain relief is uncommon and is not the goal when using opioids in older patients. Rather, treatment goals should focus on quality of life and function. Patients need to be continually educated about these goals and regularly reassessed during treatment.
APPROACH TO PAIN MANAGEMENT
Initial steps in managing pain should always include a detailed pain assessment, ideally by an interdisciplinary team.19,20 Physical therapy, cognitive behavioral therapy, and patient and caregiver education are some effective nonpharmacologic strategies.3 If nonpharmacologic treatments are ineffective, pharmacologic strategies should be used. Often, both nonpharmacologic and pharmacologic treatments work well for persistent pain.
The World Health Organization’s three-step ladder approach, originally developed for cancer pain, has subsequently been adopted for all types of pain.
- Step 1 of the ladder is nonopioid analgesics, with or without adjuvant agents.
- Step 2 if the pain persists or increases, is a weak opioid (eg, codeine, tramadol), with or without a nonopioid analgesic and with or without an adjuvant agent.
- Step 3 is a strong opioid (eg, morphine, oxycodone, hydromorphone, fentanyl, or methadone), with or without nonopioid and adjuvant agents.
The European Association for Palliative Care recommendations state that there is no significant difference between morphine, oxycodone, and hydromorphone when given orally.21 Although this ladder has been modernized somewhat,22 it still provides a conceptual and practical guide.
FIRST STEP: NONOPIOID ANALGESICS
Acetaminophen is first-line
Acetaminophen is the first-line drug for persistent pain, as it is effective and safe. It does not have the same gastrointestinal and renal side effects that nonsteroidal anti-inflammatory drugs (NSAIDs) do. It also has fewer drug interactions, and its clearance does not decline with age.23
However, older adults should not take more than 3 g of acetaminophen in 24 hours.24 It should be used with extreme caution, if at all, in patients who have hepatic insufficiency or chronic alcohol abuse or dependence.
Topical therapies
Topical NSAIDs allow local analgesia with less risk of systemic side effects than with oral NSAIDs, which have a limited role in the older population.
Capsaicin, which depletes substance P, has primarily been studied for neuropathic pain.
Lidocaine 5% topical patch has been found effective for postherpetic neuralgia; however, there is limited evidence for using it in other painful conditions, such as osteoarthritis and back pain.25
Adjuvants
Duloxetine is a serotonin and norepinephrine reuptake inhibitor. Studies have found it effective in treating diabetic peripheral neuropathy, fibromyalgia, chronic low back pain, and osteoarthritis knee pain. However, except for the knee study, most of the patients enrolled were younger.
Antiepileptic medications. Gabapentin and pregabalin have been found to be effective in painful neuropathic conditions that commonly occur in older adults.25
Avoid oral NSAIDs
NSAIDs, both nonselective and cyclooxygenase 2-selective, should only rarely be considered for long-term use in older adults in view of increased risk of conditions such as congestive heart failure, acute kidney injury, and gastrointestinal bleeding.25 These adverse effects seem to be related to inhibition of prostaglandin, which plays a physiologic role in the gastrointestinal, renal, and cardiovascular systems.26 Oral NSAIDs should be used with extreme caution.
OPIOIDS
The American Geriatrics Society, American Pain Society, and American Academy of Pain Medicine made recommendations in 2009 supporting the use of opioids to treat persistent pain in patients who are carefully selected and monitored.4,6 An international expert panel in 2008 issued a consensus statement27 of evidence that also supported the use of opioids for those over age 65. The Federation of State Medical Boards of the United States also supports the use of opioids, particularly for adults who have refractory pain, and it recognizes undertreatment of pain as a public health issue.28
Clinicians are most comfortable with using opioids to manage cancer pain, but these drugs also provide an acceptable and effective means of analgesia in nonmalignant, persistent pain syndromes.24 The American Geriatrics Society Panel on Pharmacological Management of Persistent Pain in Older Persons recommends treatment with opioids in all patients with moderate-to-severe pain, pain-related functional impairment, or decreased quality of life due to pain, even though the evidence base is not robust.3
Unlike NSAIDs and acetaminophen, opioids do not have a presumed ceiling effect. However, in patients ages 15 to 64, the greatest benefits have been observed at lower doses of opioids, and the risk of death increases with dose.29 The dose can be raised gradually until pain is relieved.
Start low and go slow
When starting opioid therapy:
- Choose a short-acting agent
- Give it on a trial basis
- Start at a low dose and titrate up slowly.
No data are available to tell us how much to give an older adult, but a reasonable starting dose is 30% to 50% of the recommended dose for a younger adult.24 Short-acting opioids should be titrated by increasing the total daily dose by 25% to 50% every 24 hours until adequate analgesia is reached.24
Older adults who have frequent or continuous pain should receive scheduled (around-the-clock) dosing in an effort to achieve a steady state.3 The half-lives of opioids may be longer in older adults who have renal or hepatic insufficiency; therefore, their doses should be lower and the intervals between doses longer.27
When long-acting opioid preparations are used, it is important to also prescribe breakthrough (short-acting) pain management.2 Breakthrough pain includes end-of-dose failure, incident pain (ie, due to an identifiable cause, such as movement), and spontaneous pain; these can be prevented or treated with short-acting, immediate-release opioid formulations.3
Once therapy is initiated, its safety and efficacy should be continually monitored.2 With long-term use, patients should be reassessed for ongoing attainment of therapeutic goals, adverse effects, and safe and responsible medication use.3
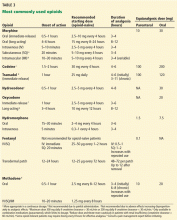
Table 3 lists common opioids and their initial dosing.
SIDE EFFECTS
Constipation
This is one of the most common side effects of opioids,30 and although many opioid side effects wane within days of starting as tolerance develops, this one does not.
A bowel regimen should be initiated when starting any opioid regimen. Although most of the evidence for bowel regimens is anecdotal, increasing fluid and fiber intake and taking stool softeners and laxatives are effective.31
For very difficult cases of opioid constipation, randomized trials suggest that specific agents with opioid antagonist activity that specifically target the gastrointestinal system can help.32,33 Opioid antagonists are not used as routine prophylaxis, but rather for constipation that is refractory to laxatives.34,35 A meta-analysis demonstrated that methylnaltrexone, naloxone, and alvimopan were generally well tolerated, with no significant difference in adverse effects compared with placebo.36
Sedation
Sedation due to opioids in opioid-naïve patients is well documented,37 but it decreases over time. When starting or changing the dose of opioids, it is important to counsel patients about driving and safety at work and home.
For persistent opioid-related sedation, three options are available: dose reduction, opioid rotation, and use of psychostimulants.38 Although it does not carry a US Food and Drug Administration indication for this use, methylphenidate has been studied in cancer patients, in whom it has been associated with less drowsiness, decreased pain, and less need for rescue doses of pain medications.39–41
Nausea and vomiting
Nausea and vomiting are common in opioid recipients. These adverse effects usually decrease over days to weeks with continued exposure.
A number of antiemetic therapies are available in oral, rectal, and intravenous formulations, but there is no evidence-based recommendation for antiemetic choice for opioid-induced nausea in patients with cancer.42 It is important to always rule out constipation as the cause of nausea. There is also some evidence that reducing the opioid dose or changing the route of administration may help with symptoms.42–45
Respiratory depression
Although respiratory depression is the most feared adverse effect of opioids, it is rare with low starting doses and appropriate dose titration. Sedation precedes respiratory depression, which occurs when initial opioid dosages are too high, titration is too rapid, or opioids are combined with other drugs associated with respiratory depression or that may potentiate opioid-induced respiratory depression, such as benzodiazepines.46–51
Patients with sleep apnea may be at higher risk. In addition, in a study that specifically reviewed patients who had persistent pain, specific factors that contributed to opioid-induced respiratory depression were use of methadone and transdermal fentanyl, renal impairment, and sensory deafferentation.52 Buprenorphine was found to have a ceiling effect for respiratory depression, but not for analgesia.49
Central sleep apnea
Chronic opioid use has been associated with sleep-disordered breathing, notably central sleep apnea. This is often unrecognized. The prevalence of central sleep apnea in this population is 24%.53
Although continuous positive airway pressure is the standard of care for obstructive sleep apnea, it is ineffective for central sleep apnea and possibly may make it worse. Adaptive servoventilation is a therapy that may be effective.54
Urinary retention
Opioids can cause urinary retention, which is most noted in a postoperative setting. Changes in bladder function have been found to be partially due to a peripheral opioid effect.55
Initial management: catheterize the bladder for prompt relief and try to reduce the dose of opioids.
Impaired balance and falls
Use of opioids, especially when combined with other medications active in the central nervous system, may lead to impaired balance and falls, especially in the elderly.56 In this group, all opioids are associated with falls except for buprenorphine.27,57 Older adults need to be assessed and educated about the risk of falls before they are given opioids. Physical therapy and mobility aids may help in these cases.
Dependence
The prevalence of dependence is low in patients who have no prior history of substance abuse.6 Older age is also associated with a significantly lower risk of opioid misuse and abuse.6
Opioid-induced hyperalgesia
Opioid-induced hyperalgesia should be considered if pain continues to worsen in spite of increasing doses, tolerance to opioids appears to develop rapidly, or pain becomes more diffuse and extends past the distribution of preexisting pain.58 Although the exact mechanism is unclear, exposure to opioids causes nociceptive sensitization, as measured by several techniques.59,60
Opioid-induced hyperalgesia is distinct from opioid analgesia tolerance. A key difference is that opioid tolerance can be overcome by increasing the dose, while opioid-induced hyperalgesia can be exacerbated by it.
Management of opioid-induced hyperalgesia includes decreasing the dose, switching to a different opioid, and maximizing nonopioid analgesia.58 The plan should be clearly communicated to patients and families to avoid misunderstanding.
Other adverse effects
Long-term use of opioids may suppress production of several hypothalamic, pituitary, gonadal, and adrenal hormones.3 Long-term use of opioids is also associated with bone loss.61 Opioids have also demonstrated immunodepressant effects.38,62
OPIOID ROTATION
Trying a different opioid (opioid rotation) may be required if pain remains poorly controlled despite increasing doses or if intolerable side effects occur.
According to consensus guidelines on opioid rotation,63 if the originally prescribed opioid is not providing the appropriate therapeutic effect or the patient cannot tolerate the regimen, an equianalgesic dose (Table 3) of the new opioid is calculated based on the original opioid and then decreased in two safety steps. The first safety step is a 25% to 50% reduction in the calculated equianalgesic dose to account for incomplete cross-tolerance. There are two exceptions: methadone requires a 75% to 90% reduction, and transdermal fentanyl does not require an adjustment. The next step is an adjustment of 15% to 30% based on pain severity and the patient’s medical or psychosocial aspects.63
SPECIAL POPULATION: PATIENTS WITH DEMENTIA
There is little scientific data on pain management in older adults with dementia. Many patients with mild to moderate dementia can verbally communicate pain reliably,64 but more challenging are those who are nonverbal, for whom providers depend on caregiver reports and observational scales.65
Prescribing in patients with dementia who are verbal and nonverbal mirrors the strategies used in those older adults who are cognitively intact,66 eg:
- Use scheduled (around-the-clock) dosing
- Start with nonopioid medications initially, but advance to opioids as needed, guided by the WHO ladder
- Carefully monitor the risks and benefits of pain treatment vs persistent pain.
When uncertain about whether a demented patient is in pain, a trial of analgesics is warranted. Signs of pain include not socializing, disturbed sleep, and a vegetative state.
SAFE PRESCRIBING PRACTICES
With the use of opioids to treat persistent pain comes the risk of abuse. A universal precautions approach helps establish reasonable limits before initiating therapy.
A thorough evaluation is required, including description and documentation of pain, disease processes, comorbidities, and effects on function; physical examination; and diagnostic testing. It is also important to inquire about a history of substance abuse. Tools such as the Opioid Risk Tool and the Screener and Opioid Assessment for Patients with Pain-Revised can help gauge risk of misuse or abuse.67,68
Ongoing screening and monitoring are necessary to minimize misuse and diversion. This also involves adhering to federal and state government regulatory policies and participating state prescription drug monitoring programs.69
- Chou R, Fanciullo GJ, Fine PG, et al; American Pain Society-American Academy of Pain Medicine Opioids Guidelines Panel. Clinical guidelines for the use of chronic opioid therapy in chronic noncancer pain. J Pain 2009; 10:113–130.
- West NA, Severtson SG, Green JL, Dart RC. Trends in abuse and misuse of prescription opioids among older adults. Drug Alcohol Depend 2015; 149:117–121.
- American Geriatrics Society Panel on Pharmacological Management of Persistent Pain in Older Persons. Pharmacological management of persistent pain in older persons. J Am Geriatr Soc 2009; 57:1331–1346.
- Weiner DK, Herr K. Comprehensive interdisciplinary assessment and treatment planning: an integrative overview. In: Weiner DK, Herr K, Rudy TE, editors. Persistent pain in older adults: an interdisciplinary guide for treatment. New York, NY: Springer Publishing Company; 2002.
- He W, Sengupta M, Velkoff V; US Census Bureau. 65+ in the United States: 2005. Washington, DC: US Government Printing Office; 2005. www.census.gov/prod/2006pubs/p23-209.pdf. Accessed March 30, 2016.
- American Geriatrics Society Panel on the Pharmacological Management of Persistent Pain in Older Persons. Pharmacological management of persistent pain in older persons. Pain Med 2009; 10:1062–1083.
- Thomas E, Peat G, Harris L, Wilkie R, Croft PR. The prevalence of pain and pain interference in a general population of older adults: cross-sectional findings from the North Staffordshire Osteoarthritis Project (NorStOP). Pain 2004; 110:361–368.
- Caraceni A, Hanks G, Kaasa S, et al; European Palliative Care Research Collaborative (EPCRC); European Association for Palliative Care (EAPC). Use of opioid analgesics in the treatment of cancer pain: evidence-based recommendations from the EAPC. Lancet Oncol 2012; 13:e58–e68.
- Solano JP, Gomes B, Higginson IJ. A comparison of symptom prevalence in far advanced cancer, AIDS, heart disease, chronic obstructive pulmonary disease and renal disease. J Pain Symptom Manage 2006; 31:58–69.
- Ferrell BA, Ferrell BR, Osterweil D. Pain in the nursing home. J Am Geriatr Soc 1990; 38:409–414.
- Ferrell BA, Ferrell BR, Rivera L. Pain in cognitively impaired nursing home patients. J Pain Symptom Manage 1995; 10:591–598.
- Fox PL, Raina P, Jadad AR. Prevalence and treatment of pain in older adults in nursing homes and other long-term care institutions: a systematic review. CMAJ 1999; 160:329–333.
- Stewart C, Leveille SG, Shmerling RH, Samelson EJ, Bean JF, Schofield P. Management of persistent pain in older adults: the MOBILIZE Boston Study. J Am Geriatr Soc 2012; 60:2081–2086.
- Woo J, Ho SC, Lau J, Leung PC. Musculoskeletal complaints and associated consequences in elderly Chinese aged 70 years and over. J Rheumatol 1994; 21:1927–1931.
- Pahor M, Guralnik JM, Wan JY, et al. Lower body osteoarticular pain and dose of analgesic medications in older disabled women: the Women’s Health and Aging Study. Am J Public Health 1999; 89:930–934.
- Marzinski LR. The tragedy of dementia: clinically assessing pain in the confused nonverbal elderly. J Gerontol Nurs 1991; 17:25–28.
- Roy R, Thomas M. A survey of chronic pain in an elderly population. Can Fam Physician 1986; 32:513–516.
- AGS Panel on Persistent Pain in Older Persons. The management of persistent pain in older persons. J Am Geriatr Soc 2002; 50(suppl 6): S205–S224.
- Stanos S, Houle TT. Multidisciplinary and interdisciplinary management of chronic pain. Phys Med Rehabil Clin N Am 2006; 17:435–450.
- Helme RD, Katz B, Gibson SJ, et al. Multidisciplinary pain clinics for older people. Do they have a role? Clin Geriatr Med 1996; 12:563–582.
- Harris DG. Management of pain in advanced disease. Br Med Bull 2014; 110:117–128.
- Raffa RB, Pergolizzi JV. A modern analgesics pain ‘pyramid’. J Clin Pharm Ther 2014; 39:4–6.
- Fine PG, Herr KA. Pharmacologic management of persistent pain in older persons. Clin Geriatr 2009; 17:25–32.
- Tracy B, Sean Morrison R. Pain management in older adults. Clin Ther 2013; 35:1659–1668.
- Malec M, Shega JW. Pain management in the elderly. Med Clin North Am 2015; 99:337–350.
- Abdulla A, Adams N, Bone M, et al; British Geriatric Society. Guidance on the management of pain in older people. Age Ageing 2013; 42(suppl 1):i1–i57.
- Pergolizzi J, Böger RH, Budd K, et al. Opioids and the management of chronic severe pain in the elderly: consensus statement of an International Expert Panel with focus on the six clinically most often used World Health Organization Step III opioids (buprenorphine, fentanyl, hydromorphone, methadone, morphine, oxycodone). Pain Pract 2008; 8:287–313.
- Gloth FM 3rd. Pharmacological management of persistent pain in older persons: focus on opioids and nonopioids. J Pain 2011; 12(suppl 1):S14–S20.
- Gomes T, Mamdani MM, Dhalla IA, Paterson JM, Juurlink DN. Opioid dose and drug-related mortality in patients with nonmalignant pain. Arch Intern Med 2011; 171:686–691.
- Moore RA, McQuay HJ. Prevalence of opioid adverse events in chronic non-malignant pain: systematic review of randomised trials of oral opioids. Arthritis Res Ther 2005; 7:R1046–R1051.
- Candy B, Jones L, Larkin PJ, Vickerstaff V, Tookman A, Stone P. Laxatives for the management of constipation in people receiving palliative care. Cochrane Database Syst Rev 2015; 5:CD003448.
- Webster LR, Butera PG, Moran LV, Wu N, Burns LH, Friedmann N. Oxytrex minimizes physical dependence while providing effective analgesia: a randomized controlled trial in low back pain. J Pain 2006; 7:937–946.
- Paulson DM, Kennedy DT, Donovick RA, et al. Alvimopan: an oral, peripherally acting, mu-opioid receptor antagonist for the treatment of opioid-induced bowel dysfunction—a 21-day treatment-randomized clinical trial. J Pain 2005; 6:184–192.
- Nalamachu SR, Pergolizzi J, Taylor R, et al. Efficacy and tolerability of subcutaneous methylnaltrexone in patients with advanced illness and opioid-induced constipation: a responder analysis of 2 randomized, placebo-controlled trials. Pain Pract 2015; 15:564–571.
- Brick N. Laxatives or methylnaltrexone for the management of constipation in palliative care patients. Clin J Oncol Nurs 2013; 17:91–92.
- Ford AC, Brenner DM, Schoenfeld PS. Efficacy of pharmacological therapies for the treatment of opioid-induced constipation: systematic review and meta-analysis. Am J Gastroenterolt 2013; 108:1566–1575.
- Byas-Smith MG, Chapman SL, Reed B, Cotsonis G. The effect of opioids on driving and psychomotor performance in patients with chronic pain. Clin J Pain 2005; 21:345–352.
- Benyamin R, Trescot AM, Datta S, et al. Opioid complications and side effects. Pain Physician 2008; 11(suppl 2):S105–S120.
- Wilwerding MB, Loprinzi CL, Mailliard JA, et al. A randomized, crossover evaluation of methylphenidate in cancer patients receiving strong narcotics. Support Care Cancer 1995; 3:135–138.
- Bruera E, Miller MJ, Macmillan K, Kuehn N. Neuropsychological effects of methylphenidate in patients receiving a continuous infusion of narcotics for cancer pain. Pain 1992; 48:163–166.
- Ahmedzai S. New approaches to pain control in patients with cancer. Eur J Cancer 1997; 33:S8–S14.
- Laugsand EA, Kaasa S, Klepstad P. Management of opioid-induced nausea and vomiting in cancer patients: systematic review and evidence-based recommendations. Palliat Med 2011; 25:442–453.
- Hardy J, Daly S, McQuade B, et al. A double-blind, randomised, parallel group, multinational, multicentre study comparing a single dose of ondansetron 24 mg p.o. with placebo and metoclopramide 10 mg t.d.s. p.o. in the treatment of opioid-induced nausea and emesis in cancer patients. Support Care Cancer 2002; 10:231–236.
- Apfel CC, Jalota L. Can central antiemetic effects of opioids counter-balance opioid-induced nausea and vomiting? Acta Anaesthesiol Scand 2010; 54:129–131.
- Okamoto Y, Tsuneto S, Matsuda Y, et al. A retrospective chart review of the antiemetic effectiveness of risperidone in refractory opioid-induced nausea and vomiting in advanced cancer patients. J Pain Symptom Manage 2007; 34:217–222.
- Overdyk F, Dahan A, Roozekrans M, van der Schrier R, Aarts L, Niesters M. Opioid-induced respiratory depression in the acute care setting: a compendium of case reports. Pain Manag 2014; 4:317–325.
- Niesters M, Overdyk F, Smith T, Aarts L, Dahan A. Buprenorphine-induced respiratory depression and involvement of ABCB1 SNPs in opioid-induced respiratory depression in paediatrics. Br J Anaesth 2013; 110:842–843.
- Niesters M, Mahajan RP, Aarts L, Dahan A. High-inspired oxygen concentration further impairs opioid-induced respiratory depression. Br J Anaesth 2013; 110:837–841.
- Dahan A, Yassen A, Romberg R, et al. Buprenorphine induces ceiling in respiratory depression but not in analgesia. Br J Anaesth 2006; 96:627–632.
- van Dorp E, Yassen A, Sarton E, et al. Naloxone reversal of buprenorphine-induced respiratory depression. Anesthesiology 2006; 105:51–57.
- Macintyre PE, Loadsman JA, Scott DA. Opioids, ventilation and acute pain management. Anaesth Intensive Care 2011; 39:545–558.
- Dahan A, Overdyk F, Smith T, Aarts L, Niesters M. Pharmacovigilance: a review of opioid-induced respiratory depression in chronic pain patients. Pain Physician 2013; 16:E85–E94.
- Correa D, Farney RJ, Chung F, Prasad A, Lam D, Wong J. Chronic opioid use and central sleep apnea: a review of the prevalence, mechanisms, and perioperative considerations. Anesth Analg 2015; 120:1273–1285.
- Randerath WJ, George S. Opioid-induced sleep apnea: is it a real problem? J Clin Sleep Med 2012; 8:577–578.
- Rosow CE, Gomery P, Chen TY, Stefanovich P, Stambler N, Israel R. Reversal of opioid-induced bladder dysfunction by intravenous naloxone and methylnaltrexone. Clin Pharmacol Ther 2007; 82:48–53.
- Weiner DK, Hanlon JT, Studenski SA. Effects of central nervous system polypharmacy on falls liability in community-dwelling elderly. Gerontology 1998; 44:217–221.
- Wolff ML, Kewley R, Hassett M, Collins J, Brodeur MR, Nokes S. Falls in skilled nursing facilities associated with opioid use. J Am Geriatr Soc 2012; 60:987.
- Zylicz Z, Twycross R. Opioid-induced hyperalgesia may be more frequent than previously thought. J Clin Oncol 2008; 26:1564; author reply 1565.
- Lee M, Silverman SM, Hansen H, Patel VB, Manchikanti L. A comprehensive review of opioid-induced hyperalgesia. Pain Physician 2011 2011; 14:145–161.
- Chen L, Sein M, Vo T, et al. Clinical interpretation of opioid tolerance versus opioid-induced hyperalgesia. J Opioid Manag 2014; 10:383–393.
- Vestergaard P, Hermann P, Jensen JE, Eiken P, Mosekilde L. Effects of paracetamol, non-steroidal anti-inflammatory drugs, acetylsalicylic acid, and opioids on bone mineral density and risk of fracture: results of the Danish Osteoporosis Prevention Study (DOPS). Osteoporos Int 2012; 23:1255–1265.
- Sacerdote P, Franchi S, Panerai AE. Non-analgesic effects of opioids: mechanisms and potential clinical relevance of opioid-induced immunodepression. Curr Pharm Des 2012; 18:6034–6042.
- Fine PG, Portenoy RK; Ad Hoc Expert Panel on Evidence Review and Guidelines for Opioid Rotation. Establishing “best practices” for opioid rotation: conclusions of an expert panel. J Pain Symptom Manage 2009; 38:418–425.
- Chibnall JT, Tait RC. Pain assessment in cognitively impaired and unimpaired older adults: a comparison of four scales. Pain 2001; 92:173–186.
- Andrade DC, Faria JW, Caramelli P, et al. The assessment and management of pain in the demented and non-demented elderly patient. Arq Neuropsiquiatr 2011; 69:387–394.
- Scherder E, Herr K, Pickering G, Gibson S, Benedetti F, Lautenbacher S. Pain in dementia. Pain 2009; 145:276–278.
- Chou R, Fanciullo GJ, Fine PG, Miaskowski C, Passik SD, Portenoy RK. Opioids for chronic noncancer pain: prediction and identification of aberrant drug-related behaviors: a review of the evidence for an American Pain Society and American Academy of Pain Medicine clinical practice guideline. J Pain 2009; 10:131–146.
- Butler SF, Budman SH, Fernandez KC, Fanciullo GJ, Jamison RN. Cross-validation of a screener to predict opioid misuse in chronic pain patients (SOAPP-R). J Addict Med 2009; 3:66–73.
- de Leon-Casasola OA. Opioids for chronic pain: new evidence, new strategies, safe prescribing. Am J Med 2013; 126(suppl 1):S3–S11.
- CDC guideline for prescribing opioids for chronic pain—United States, 2016. MMWR Recomm Rep 2016 Mar 18; 65(1):1–49.
The use of opioid analgesics is widely accepted for treating severe acute pain, cancer pain, and pain at the end of life.1 However, their long-term use for other types of persistent pain (Table 1) remains controversial. Clinicians and regulators need to work together to achieve a balanced approach to the use of opioids, recognizing the legitimate medical need for these medications for persistent pain while acknowledging their increasing misuse and the morbidity and mortality related to them. Finding this balance is particularly challenging in older patients.2
PAIN IN OLDER PEOPLE: COMPLICATED, OFTEN UNDERTREATED
Persistent pain is a multifaceted manifestation of an unpleasant sensation that continues for a prolonged time and may or may not be related to a distinct disease process.3 (The term “persistent pain” is preferred as it does not have the negative connotations of “chronic pain.”4) “Older” has been defined as age 65 and older. As our population ages, especially to age 85 and older, more people will be living with persistent pain due to a variety of conditions.5
Persistent pain is more complicated in older than in younger patients. Many older people have more than one illness, making them more susceptible to adverse drug interactions such as altered pharmacokinetics and pharmacodynamics.6 Up to 40% of older outpatients report pain,7 and pain affects 70% to 80% of patients with advanced malignant disease.8 Pain is also prevalent in nonmalignant, progressive, life-limiting illnesses that are common in the geriatric population, affecting 41% to 77% of patients with advanced heart disease, 34% to 77% with advanced chronic obstructive pulmonary disease, and 47% to 50% with advanced renal disease.9
Pain is underrecognized in nursing home residents, who may have multiple somatic complaints and multiple causes of pain.10,11 From 27% to 83% of older adults in an institutionalized setting are affected by pain.12 Caregiver stress and attitudes towards pain may influence patients’ experiences with pain. This aspect should also be assessed and evaluated, if present.3
Pain in older adults is often undertreated, as evidenced by the findings of a study in which only one-third of older patients with persistent pain were receiving treatment that was consistent with current guidelines.13 Approximately 40% to 80% of older adults in the community with pain do not receive any treatment for it.14,15 Of those residing in institutions, 16% to 27% of older adults in pain do not receive any treatment for it.16,17 Inadequate treatment of persistent pain is associated with many adverse outcomes, including functional decline, falls, mood changes, decreased socialization, sleep and appetite difficulties, and increased healthcare utilization.18
GOALS: BETTER QUALITY OF LIFE AND FUNCTION
Persistent pain is multifactorial and so requires an approach that addresses a variety of causes and includes both nonpharmacologic and pharmacologic strategies. Opioids are part of a multipronged approach to pain management.
To avoid adverse effects, opioids for persistent pain in an older adult should be prescribed at the lowest possible dose that provides adequate analgesia. Due to age-related changes, finding the best treatments may be a challenge, and understanding the pharmacokinetic implications in this population is key (Table 2).
Complete pain relief is uncommon and is not the goal when using opioids in older patients. Rather, treatment goals should focus on quality of life and function. Patients need to be continually educated about these goals and regularly reassessed during treatment.
APPROACH TO PAIN MANAGEMENT
Initial steps in managing pain should always include a detailed pain assessment, ideally by an interdisciplinary team.19,20 Physical therapy, cognitive behavioral therapy, and patient and caregiver education are some effective nonpharmacologic strategies.3 If nonpharmacologic treatments are ineffective, pharmacologic strategies should be used. Often, both nonpharmacologic and pharmacologic treatments work well for persistent pain.
The World Health Organization’s three-step ladder approach, originally developed for cancer pain, has subsequently been adopted for all types of pain.
- Step 1 of the ladder is nonopioid analgesics, with or without adjuvant agents.
- Step 2 if the pain persists or increases, is a weak opioid (eg, codeine, tramadol), with or without a nonopioid analgesic and with or without an adjuvant agent.
- Step 3 is a strong opioid (eg, morphine, oxycodone, hydromorphone, fentanyl, or methadone), with or without nonopioid and adjuvant agents.
The European Association for Palliative Care recommendations state that there is no significant difference between morphine, oxycodone, and hydromorphone when given orally.21 Although this ladder has been modernized somewhat,22 it still provides a conceptual and practical guide.
FIRST STEP: NONOPIOID ANALGESICS
Acetaminophen is first-line
Acetaminophen is the first-line drug for persistent pain, as it is effective and safe. It does not have the same gastrointestinal and renal side effects that nonsteroidal anti-inflammatory drugs (NSAIDs) do. It also has fewer drug interactions, and its clearance does not decline with age.23
However, older adults should not take more than 3 g of acetaminophen in 24 hours.24 It should be used with extreme caution, if at all, in patients who have hepatic insufficiency or chronic alcohol abuse or dependence.
Topical therapies
Topical NSAIDs allow local analgesia with less risk of systemic side effects than with oral NSAIDs, which have a limited role in the older population.
Capsaicin, which depletes substance P, has primarily been studied for neuropathic pain.
Lidocaine 5% topical patch has been found effective for postherpetic neuralgia; however, there is limited evidence for using it in other painful conditions, such as osteoarthritis and back pain.25
Adjuvants
Duloxetine is a serotonin and norepinephrine reuptake inhibitor. Studies have found it effective in treating diabetic peripheral neuropathy, fibromyalgia, chronic low back pain, and osteoarthritis knee pain. However, except for the knee study, most of the patients enrolled were younger.
Antiepileptic medications. Gabapentin and pregabalin have been found to be effective in painful neuropathic conditions that commonly occur in older adults.25
Avoid oral NSAIDs
NSAIDs, both nonselective and cyclooxygenase 2-selective, should only rarely be considered for long-term use in older adults in view of increased risk of conditions such as congestive heart failure, acute kidney injury, and gastrointestinal bleeding.25 These adverse effects seem to be related to inhibition of prostaglandin, which plays a physiologic role in the gastrointestinal, renal, and cardiovascular systems.26 Oral NSAIDs should be used with extreme caution.
OPIOIDS
The American Geriatrics Society, American Pain Society, and American Academy of Pain Medicine made recommendations in 2009 supporting the use of opioids to treat persistent pain in patients who are carefully selected and monitored.4,6 An international expert panel in 2008 issued a consensus statement27 of evidence that also supported the use of opioids for those over age 65. The Federation of State Medical Boards of the United States also supports the use of opioids, particularly for adults who have refractory pain, and it recognizes undertreatment of pain as a public health issue.28
Clinicians are most comfortable with using opioids to manage cancer pain, but these drugs also provide an acceptable and effective means of analgesia in nonmalignant, persistent pain syndromes.24 The American Geriatrics Society Panel on Pharmacological Management of Persistent Pain in Older Persons recommends treatment with opioids in all patients with moderate-to-severe pain, pain-related functional impairment, or decreased quality of life due to pain, even though the evidence base is not robust.3
Unlike NSAIDs and acetaminophen, opioids do not have a presumed ceiling effect. However, in patients ages 15 to 64, the greatest benefits have been observed at lower doses of opioids, and the risk of death increases with dose.29 The dose can be raised gradually until pain is relieved.
Start low and go slow
When starting opioid therapy:
- Choose a short-acting agent
- Give it on a trial basis
- Start at a low dose and titrate up slowly.
No data are available to tell us how much to give an older adult, but a reasonable starting dose is 30% to 50% of the recommended dose for a younger adult.24 Short-acting opioids should be titrated by increasing the total daily dose by 25% to 50% every 24 hours until adequate analgesia is reached.24
Older adults who have frequent or continuous pain should receive scheduled (around-the-clock) dosing in an effort to achieve a steady state.3 The half-lives of opioids may be longer in older adults who have renal or hepatic insufficiency; therefore, their doses should be lower and the intervals between doses longer.27
When long-acting opioid preparations are used, it is important to also prescribe breakthrough (short-acting) pain management.2 Breakthrough pain includes end-of-dose failure, incident pain (ie, due to an identifiable cause, such as movement), and spontaneous pain; these can be prevented or treated with short-acting, immediate-release opioid formulations.3
Once therapy is initiated, its safety and efficacy should be continually monitored.2 With long-term use, patients should be reassessed for ongoing attainment of therapeutic goals, adverse effects, and safe and responsible medication use.3
Table 3 lists common opioids and their initial dosing.
SIDE EFFECTS
Constipation
This is one of the most common side effects of opioids,30 and although many opioid side effects wane within days of starting as tolerance develops, this one does not.
A bowel regimen should be initiated when starting any opioid regimen. Although most of the evidence for bowel regimens is anecdotal, increasing fluid and fiber intake and taking stool softeners and laxatives are effective.31
For very difficult cases of opioid constipation, randomized trials suggest that specific agents with opioid antagonist activity that specifically target the gastrointestinal system can help.32,33 Opioid antagonists are not used as routine prophylaxis, but rather for constipation that is refractory to laxatives.34,35 A meta-analysis demonstrated that methylnaltrexone, naloxone, and alvimopan were generally well tolerated, with no significant difference in adverse effects compared with placebo.36
Sedation
Sedation due to opioids in opioid-naïve patients is well documented,37 but it decreases over time. When starting or changing the dose of opioids, it is important to counsel patients about driving and safety at work and home.
For persistent opioid-related sedation, three options are available: dose reduction, opioid rotation, and use of psychostimulants.38 Although it does not carry a US Food and Drug Administration indication for this use, methylphenidate has been studied in cancer patients, in whom it has been associated with less drowsiness, decreased pain, and less need for rescue doses of pain medications.39–41
Nausea and vomiting
Nausea and vomiting are common in opioid recipients. These adverse effects usually decrease over days to weeks with continued exposure.
A number of antiemetic therapies are available in oral, rectal, and intravenous formulations, but there is no evidence-based recommendation for antiemetic choice for opioid-induced nausea in patients with cancer.42 It is important to always rule out constipation as the cause of nausea. There is also some evidence that reducing the opioid dose or changing the route of administration may help with symptoms.42–45
Respiratory depression
Although respiratory depression is the most feared adverse effect of opioids, it is rare with low starting doses and appropriate dose titration. Sedation precedes respiratory depression, which occurs when initial opioid dosages are too high, titration is too rapid, or opioids are combined with other drugs associated with respiratory depression or that may potentiate opioid-induced respiratory depression, such as benzodiazepines.46–51
Patients with sleep apnea may be at higher risk. In addition, in a study that specifically reviewed patients who had persistent pain, specific factors that contributed to opioid-induced respiratory depression were use of methadone and transdermal fentanyl, renal impairment, and sensory deafferentation.52 Buprenorphine was found to have a ceiling effect for respiratory depression, but not for analgesia.49
Central sleep apnea
Chronic opioid use has been associated with sleep-disordered breathing, notably central sleep apnea. This is often unrecognized. The prevalence of central sleep apnea in this population is 24%.53
Although continuous positive airway pressure is the standard of care for obstructive sleep apnea, it is ineffective for central sleep apnea and possibly may make it worse. Adaptive servoventilation is a therapy that may be effective.54
Urinary retention
Opioids can cause urinary retention, which is most noted in a postoperative setting. Changes in bladder function have been found to be partially due to a peripheral opioid effect.55
Initial management: catheterize the bladder for prompt relief and try to reduce the dose of opioids.
Impaired balance and falls
Use of opioids, especially when combined with other medications active in the central nervous system, may lead to impaired balance and falls, especially in the elderly.56 In this group, all opioids are associated with falls except for buprenorphine.27,57 Older adults need to be assessed and educated about the risk of falls before they are given opioids. Physical therapy and mobility aids may help in these cases.
Dependence
The prevalence of dependence is low in patients who have no prior history of substance abuse.6 Older age is also associated with a significantly lower risk of opioid misuse and abuse.6
Opioid-induced hyperalgesia
Opioid-induced hyperalgesia should be considered if pain continues to worsen in spite of increasing doses, tolerance to opioids appears to develop rapidly, or pain becomes more diffuse and extends past the distribution of preexisting pain.58 Although the exact mechanism is unclear, exposure to opioids causes nociceptive sensitization, as measured by several techniques.59,60
Opioid-induced hyperalgesia is distinct from opioid analgesia tolerance. A key difference is that opioid tolerance can be overcome by increasing the dose, while opioid-induced hyperalgesia can be exacerbated by it.
Management of opioid-induced hyperalgesia includes decreasing the dose, switching to a different opioid, and maximizing nonopioid analgesia.58 The plan should be clearly communicated to patients and families to avoid misunderstanding.
Other adverse effects
Long-term use of opioids may suppress production of several hypothalamic, pituitary, gonadal, and adrenal hormones.3 Long-term use of opioids is also associated with bone loss.61 Opioids have also demonstrated immunodepressant effects.38,62
OPIOID ROTATION
Trying a different opioid (opioid rotation) may be required if pain remains poorly controlled despite increasing doses or if intolerable side effects occur.
According to consensus guidelines on opioid rotation,63 if the originally prescribed opioid is not providing the appropriate therapeutic effect or the patient cannot tolerate the regimen, an equianalgesic dose (Table 3) of the new opioid is calculated based on the original opioid and then decreased in two safety steps. The first safety step is a 25% to 50% reduction in the calculated equianalgesic dose to account for incomplete cross-tolerance. There are two exceptions: methadone requires a 75% to 90% reduction, and transdermal fentanyl does not require an adjustment. The next step is an adjustment of 15% to 30% based on pain severity and the patient’s medical or psychosocial aspects.63
SPECIAL POPULATION: PATIENTS WITH DEMENTIA
There is little scientific data on pain management in older adults with dementia. Many patients with mild to moderate dementia can verbally communicate pain reliably,64 but more challenging are those who are nonverbal, for whom providers depend on caregiver reports and observational scales.65
Prescribing in patients with dementia who are verbal and nonverbal mirrors the strategies used in those older adults who are cognitively intact,66 eg:
- Use scheduled (around-the-clock) dosing
- Start with nonopioid medications initially, but advance to opioids as needed, guided by the WHO ladder
- Carefully monitor the risks and benefits of pain treatment vs persistent pain.
When uncertain about whether a demented patient is in pain, a trial of analgesics is warranted. Signs of pain include not socializing, disturbed sleep, and a vegetative state.
SAFE PRESCRIBING PRACTICES
With the use of opioids to treat persistent pain comes the risk of abuse. A universal precautions approach helps establish reasonable limits before initiating therapy.
A thorough evaluation is required, including description and documentation of pain, disease processes, comorbidities, and effects on function; physical examination; and diagnostic testing. It is also important to inquire about a history of substance abuse. Tools such as the Opioid Risk Tool and the Screener and Opioid Assessment for Patients with Pain-Revised can help gauge risk of misuse or abuse.67,68
Ongoing screening and monitoring are necessary to minimize misuse and diversion. This also involves adhering to federal and state government regulatory policies and participating state prescription drug monitoring programs.69
The use of opioid analgesics is widely accepted for treating severe acute pain, cancer pain, and pain at the end of life.1 However, their long-term use for other types of persistent pain (Table 1) remains controversial. Clinicians and regulators need to work together to achieve a balanced approach to the use of opioids, recognizing the legitimate medical need for these medications for persistent pain while acknowledging their increasing misuse and the morbidity and mortality related to them. Finding this balance is particularly challenging in older patients.2
PAIN IN OLDER PEOPLE: COMPLICATED, OFTEN UNDERTREATED
Persistent pain is a multifaceted manifestation of an unpleasant sensation that continues for a prolonged time and may or may not be related to a distinct disease process.3 (The term “persistent pain” is preferred as it does not have the negative connotations of “chronic pain.”4) “Older” has been defined as age 65 and older. As our population ages, especially to age 85 and older, more people will be living with persistent pain due to a variety of conditions.5
Persistent pain is more complicated in older than in younger patients. Many older people have more than one illness, making them more susceptible to adverse drug interactions such as altered pharmacokinetics and pharmacodynamics.6 Up to 40% of older outpatients report pain,7 and pain affects 70% to 80% of patients with advanced malignant disease.8 Pain is also prevalent in nonmalignant, progressive, life-limiting illnesses that are common in the geriatric population, affecting 41% to 77% of patients with advanced heart disease, 34% to 77% with advanced chronic obstructive pulmonary disease, and 47% to 50% with advanced renal disease.9
Pain is underrecognized in nursing home residents, who may have multiple somatic complaints and multiple causes of pain.10,11 From 27% to 83% of older adults in an institutionalized setting are affected by pain.12 Caregiver stress and attitudes towards pain may influence patients’ experiences with pain. This aspect should also be assessed and evaluated, if present.3
Pain in older adults is often undertreated, as evidenced by the findings of a study in which only one-third of older patients with persistent pain were receiving treatment that was consistent with current guidelines.13 Approximately 40% to 80% of older adults in the community with pain do not receive any treatment for it.14,15 Of those residing in institutions, 16% to 27% of older adults in pain do not receive any treatment for it.16,17 Inadequate treatment of persistent pain is associated with many adverse outcomes, including functional decline, falls, mood changes, decreased socialization, sleep and appetite difficulties, and increased healthcare utilization.18
GOALS: BETTER QUALITY OF LIFE AND FUNCTION
Persistent pain is multifactorial and so requires an approach that addresses a variety of causes and includes both nonpharmacologic and pharmacologic strategies. Opioids are part of a multipronged approach to pain management.
To avoid adverse effects, opioids for persistent pain in an older adult should be prescribed at the lowest possible dose that provides adequate analgesia. Due to age-related changes, finding the best treatments may be a challenge, and understanding the pharmacokinetic implications in this population is key (Table 2).
Complete pain relief is uncommon and is not the goal when using opioids in older patients. Rather, treatment goals should focus on quality of life and function. Patients need to be continually educated about these goals and regularly reassessed during treatment.
APPROACH TO PAIN MANAGEMENT
Initial steps in managing pain should always include a detailed pain assessment, ideally by an interdisciplinary team.19,20 Physical therapy, cognitive behavioral therapy, and patient and caregiver education are some effective nonpharmacologic strategies.3 If nonpharmacologic treatments are ineffective, pharmacologic strategies should be used. Often, both nonpharmacologic and pharmacologic treatments work well for persistent pain.
The World Health Organization’s three-step ladder approach, originally developed for cancer pain, has subsequently been adopted for all types of pain.
- Step 1 of the ladder is nonopioid analgesics, with or without adjuvant agents.
- Step 2 if the pain persists or increases, is a weak opioid (eg, codeine, tramadol), with or without a nonopioid analgesic and with or without an adjuvant agent.
- Step 3 is a strong opioid (eg, morphine, oxycodone, hydromorphone, fentanyl, or methadone), with or without nonopioid and adjuvant agents.
The European Association for Palliative Care recommendations state that there is no significant difference between morphine, oxycodone, and hydromorphone when given orally.21 Although this ladder has been modernized somewhat,22 it still provides a conceptual and practical guide.
FIRST STEP: NONOPIOID ANALGESICS
Acetaminophen is first-line
Acetaminophen is the first-line drug for persistent pain, as it is effective and safe. It does not have the same gastrointestinal and renal side effects that nonsteroidal anti-inflammatory drugs (NSAIDs) do. It also has fewer drug interactions, and its clearance does not decline with age.23
However, older adults should not take more than 3 g of acetaminophen in 24 hours.24 It should be used with extreme caution, if at all, in patients who have hepatic insufficiency or chronic alcohol abuse or dependence.
Topical therapies
Topical NSAIDs allow local analgesia with less risk of systemic side effects than with oral NSAIDs, which have a limited role in the older population.
Capsaicin, which depletes substance P, has primarily been studied for neuropathic pain.
Lidocaine 5% topical patch has been found effective for postherpetic neuralgia; however, there is limited evidence for using it in other painful conditions, such as osteoarthritis and back pain.25
Adjuvants
Duloxetine is a serotonin and norepinephrine reuptake inhibitor. Studies have found it effective in treating diabetic peripheral neuropathy, fibromyalgia, chronic low back pain, and osteoarthritis knee pain. However, except for the knee study, most of the patients enrolled were younger.
Antiepileptic medications. Gabapentin and pregabalin have been found to be effective in painful neuropathic conditions that commonly occur in older adults.25
Avoid oral NSAIDs
NSAIDs, both nonselective and cyclooxygenase 2-selective, should only rarely be considered for long-term use in older adults in view of increased risk of conditions such as congestive heart failure, acute kidney injury, and gastrointestinal bleeding.25 These adverse effects seem to be related to inhibition of prostaglandin, which plays a physiologic role in the gastrointestinal, renal, and cardiovascular systems.26 Oral NSAIDs should be used with extreme caution.
OPIOIDS
The American Geriatrics Society, American Pain Society, and American Academy of Pain Medicine made recommendations in 2009 supporting the use of opioids to treat persistent pain in patients who are carefully selected and monitored.4,6 An international expert panel in 2008 issued a consensus statement27 of evidence that also supported the use of opioids for those over age 65. The Federation of State Medical Boards of the United States also supports the use of opioids, particularly for adults who have refractory pain, and it recognizes undertreatment of pain as a public health issue.28
Clinicians are most comfortable with using opioids to manage cancer pain, but these drugs also provide an acceptable and effective means of analgesia in nonmalignant, persistent pain syndromes.24 The American Geriatrics Society Panel on Pharmacological Management of Persistent Pain in Older Persons recommends treatment with opioids in all patients with moderate-to-severe pain, pain-related functional impairment, or decreased quality of life due to pain, even though the evidence base is not robust.3
Unlike NSAIDs and acetaminophen, opioids do not have a presumed ceiling effect. However, in patients ages 15 to 64, the greatest benefits have been observed at lower doses of opioids, and the risk of death increases with dose.29 The dose can be raised gradually until pain is relieved.
Start low and go slow
When starting opioid therapy:
- Choose a short-acting agent
- Give it on a trial basis
- Start at a low dose and titrate up slowly.
No data are available to tell us how much to give an older adult, but a reasonable starting dose is 30% to 50% of the recommended dose for a younger adult.24 Short-acting opioids should be titrated by increasing the total daily dose by 25% to 50% every 24 hours until adequate analgesia is reached.24
Older adults who have frequent or continuous pain should receive scheduled (around-the-clock) dosing in an effort to achieve a steady state.3 The half-lives of opioids may be longer in older adults who have renal or hepatic insufficiency; therefore, their doses should be lower and the intervals between doses longer.27
When long-acting opioid preparations are used, it is important to also prescribe breakthrough (short-acting) pain management.2 Breakthrough pain includes end-of-dose failure, incident pain (ie, due to an identifiable cause, such as movement), and spontaneous pain; these can be prevented or treated with short-acting, immediate-release opioid formulations.3
Once therapy is initiated, its safety and efficacy should be continually monitored.2 With long-term use, patients should be reassessed for ongoing attainment of therapeutic goals, adverse effects, and safe and responsible medication use.3
Table 3 lists common opioids and their initial dosing.
SIDE EFFECTS
Constipation
This is one of the most common side effects of opioids,30 and although many opioid side effects wane within days of starting as tolerance develops, this one does not.
A bowel regimen should be initiated when starting any opioid regimen. Although most of the evidence for bowel regimens is anecdotal, increasing fluid and fiber intake and taking stool softeners and laxatives are effective.31
For very difficult cases of opioid constipation, randomized trials suggest that specific agents with opioid antagonist activity that specifically target the gastrointestinal system can help.32,33 Opioid antagonists are not used as routine prophylaxis, but rather for constipation that is refractory to laxatives.34,35 A meta-analysis demonstrated that methylnaltrexone, naloxone, and alvimopan were generally well tolerated, with no significant difference in adverse effects compared with placebo.36
Sedation
Sedation due to opioids in opioid-naïve patients is well documented,37 but it decreases over time. When starting or changing the dose of opioids, it is important to counsel patients about driving and safety at work and home.
For persistent opioid-related sedation, three options are available: dose reduction, opioid rotation, and use of psychostimulants.38 Although it does not carry a US Food and Drug Administration indication for this use, methylphenidate has been studied in cancer patients, in whom it has been associated with less drowsiness, decreased pain, and less need for rescue doses of pain medications.39–41
Nausea and vomiting
Nausea and vomiting are common in opioid recipients. These adverse effects usually decrease over days to weeks with continued exposure.
A number of antiemetic therapies are available in oral, rectal, and intravenous formulations, but there is no evidence-based recommendation for antiemetic choice for opioid-induced nausea in patients with cancer.42 It is important to always rule out constipation as the cause of nausea. There is also some evidence that reducing the opioid dose or changing the route of administration may help with symptoms.42–45
Respiratory depression
Although respiratory depression is the most feared adverse effect of opioids, it is rare with low starting doses and appropriate dose titration. Sedation precedes respiratory depression, which occurs when initial opioid dosages are too high, titration is too rapid, or opioids are combined with other drugs associated with respiratory depression or that may potentiate opioid-induced respiratory depression, such as benzodiazepines.46–51
Patients with sleep apnea may be at higher risk. In addition, in a study that specifically reviewed patients who had persistent pain, specific factors that contributed to opioid-induced respiratory depression were use of methadone and transdermal fentanyl, renal impairment, and sensory deafferentation.52 Buprenorphine was found to have a ceiling effect for respiratory depression, but not for analgesia.49
Central sleep apnea
Chronic opioid use has been associated with sleep-disordered breathing, notably central sleep apnea. This is often unrecognized. The prevalence of central sleep apnea in this population is 24%.53
Although continuous positive airway pressure is the standard of care for obstructive sleep apnea, it is ineffective for central sleep apnea and possibly may make it worse. Adaptive servoventilation is a therapy that may be effective.54
Urinary retention
Opioids can cause urinary retention, which is most noted in a postoperative setting. Changes in bladder function have been found to be partially due to a peripheral opioid effect.55
Initial management: catheterize the bladder for prompt relief and try to reduce the dose of opioids.
Impaired balance and falls
Use of opioids, especially when combined with other medications active in the central nervous system, may lead to impaired balance and falls, especially in the elderly.56 In this group, all opioids are associated with falls except for buprenorphine.27,57 Older adults need to be assessed and educated about the risk of falls before they are given opioids. Physical therapy and mobility aids may help in these cases.
Dependence
The prevalence of dependence is low in patients who have no prior history of substance abuse.6 Older age is also associated with a significantly lower risk of opioid misuse and abuse.6
Opioid-induced hyperalgesia
Opioid-induced hyperalgesia should be considered if pain continues to worsen in spite of increasing doses, tolerance to opioids appears to develop rapidly, or pain becomes more diffuse and extends past the distribution of preexisting pain.58 Although the exact mechanism is unclear, exposure to opioids causes nociceptive sensitization, as measured by several techniques.59,60
Opioid-induced hyperalgesia is distinct from opioid analgesia tolerance. A key difference is that opioid tolerance can be overcome by increasing the dose, while opioid-induced hyperalgesia can be exacerbated by it.
Management of opioid-induced hyperalgesia includes decreasing the dose, switching to a different opioid, and maximizing nonopioid analgesia.58 The plan should be clearly communicated to patients and families to avoid misunderstanding.
Other adverse effects
Long-term use of opioids may suppress production of several hypothalamic, pituitary, gonadal, and adrenal hormones.3 Long-term use of opioids is also associated with bone loss.61 Opioids have also demonstrated immunodepressant effects.38,62
OPIOID ROTATION
Trying a different opioid (opioid rotation) may be required if pain remains poorly controlled despite increasing doses or if intolerable side effects occur.
According to consensus guidelines on opioid rotation,63 if the originally prescribed opioid is not providing the appropriate therapeutic effect or the patient cannot tolerate the regimen, an equianalgesic dose (Table 3) of the new opioid is calculated based on the original opioid and then decreased in two safety steps. The first safety step is a 25% to 50% reduction in the calculated equianalgesic dose to account for incomplete cross-tolerance. There are two exceptions: methadone requires a 75% to 90% reduction, and transdermal fentanyl does not require an adjustment. The next step is an adjustment of 15% to 30% based on pain severity and the patient’s medical or psychosocial aspects.63
SPECIAL POPULATION: PATIENTS WITH DEMENTIA
There is little scientific data on pain management in older adults with dementia. Many patients with mild to moderate dementia can verbally communicate pain reliably,64 but more challenging are those who are nonverbal, for whom providers depend on caregiver reports and observational scales.65
Prescribing in patients with dementia who are verbal and nonverbal mirrors the strategies used in those older adults who are cognitively intact,66 eg:
- Use scheduled (around-the-clock) dosing
- Start with nonopioid medications initially, but advance to opioids as needed, guided by the WHO ladder
- Carefully monitor the risks and benefits of pain treatment vs persistent pain.
When uncertain about whether a demented patient is in pain, a trial of analgesics is warranted. Signs of pain include not socializing, disturbed sleep, and a vegetative state.
SAFE PRESCRIBING PRACTICES
With the use of opioids to treat persistent pain comes the risk of abuse. A universal precautions approach helps establish reasonable limits before initiating therapy.
A thorough evaluation is required, including description and documentation of pain, disease processes, comorbidities, and effects on function; physical examination; and diagnostic testing. It is also important to inquire about a history of substance abuse. Tools such as the Opioid Risk Tool and the Screener and Opioid Assessment for Patients with Pain-Revised can help gauge risk of misuse or abuse.67,68
Ongoing screening and monitoring are necessary to minimize misuse and diversion. This also involves adhering to federal and state government regulatory policies and participating state prescription drug monitoring programs.69
- Chou R, Fanciullo GJ, Fine PG, et al; American Pain Society-American Academy of Pain Medicine Opioids Guidelines Panel. Clinical guidelines for the use of chronic opioid therapy in chronic noncancer pain. J Pain 2009; 10:113–130.
- West NA, Severtson SG, Green JL, Dart RC. Trends in abuse and misuse of prescription opioids among older adults. Drug Alcohol Depend 2015; 149:117–121.
- American Geriatrics Society Panel on Pharmacological Management of Persistent Pain in Older Persons. Pharmacological management of persistent pain in older persons. J Am Geriatr Soc 2009; 57:1331–1346.
- Weiner DK, Herr K. Comprehensive interdisciplinary assessment and treatment planning: an integrative overview. In: Weiner DK, Herr K, Rudy TE, editors. Persistent pain in older adults: an interdisciplinary guide for treatment. New York, NY: Springer Publishing Company; 2002.
- He W, Sengupta M, Velkoff V; US Census Bureau. 65+ in the United States: 2005. Washington, DC: US Government Printing Office; 2005. www.census.gov/prod/2006pubs/p23-209.pdf. Accessed March 30, 2016.
- American Geriatrics Society Panel on the Pharmacological Management of Persistent Pain in Older Persons. Pharmacological management of persistent pain in older persons. Pain Med 2009; 10:1062–1083.
- Thomas E, Peat G, Harris L, Wilkie R, Croft PR. The prevalence of pain and pain interference in a general population of older adults: cross-sectional findings from the North Staffordshire Osteoarthritis Project (NorStOP). Pain 2004; 110:361–368.
- Caraceni A, Hanks G, Kaasa S, et al; European Palliative Care Research Collaborative (EPCRC); European Association for Palliative Care (EAPC). Use of opioid analgesics in the treatment of cancer pain: evidence-based recommendations from the EAPC. Lancet Oncol 2012; 13:e58–e68.
- Solano JP, Gomes B, Higginson IJ. A comparison of symptom prevalence in far advanced cancer, AIDS, heart disease, chronic obstructive pulmonary disease and renal disease. J Pain Symptom Manage 2006; 31:58–69.
- Ferrell BA, Ferrell BR, Osterweil D. Pain in the nursing home. J Am Geriatr Soc 1990; 38:409–414.
- Ferrell BA, Ferrell BR, Rivera L. Pain in cognitively impaired nursing home patients. J Pain Symptom Manage 1995; 10:591–598.
- Fox PL, Raina P, Jadad AR. Prevalence and treatment of pain in older adults in nursing homes and other long-term care institutions: a systematic review. CMAJ 1999; 160:329–333.
- Stewart C, Leveille SG, Shmerling RH, Samelson EJ, Bean JF, Schofield P. Management of persistent pain in older adults: the MOBILIZE Boston Study. J Am Geriatr Soc 2012; 60:2081–2086.
- Woo J, Ho SC, Lau J, Leung PC. Musculoskeletal complaints and associated consequences in elderly Chinese aged 70 years and over. J Rheumatol 1994; 21:1927–1931.
- Pahor M, Guralnik JM, Wan JY, et al. Lower body osteoarticular pain and dose of analgesic medications in older disabled women: the Women’s Health and Aging Study. Am J Public Health 1999; 89:930–934.
- Marzinski LR. The tragedy of dementia: clinically assessing pain in the confused nonverbal elderly. J Gerontol Nurs 1991; 17:25–28.
- Roy R, Thomas M. A survey of chronic pain in an elderly population. Can Fam Physician 1986; 32:513–516.
- AGS Panel on Persistent Pain in Older Persons. The management of persistent pain in older persons. J Am Geriatr Soc 2002; 50(suppl 6): S205–S224.
- Stanos S, Houle TT. Multidisciplinary and interdisciplinary management of chronic pain. Phys Med Rehabil Clin N Am 2006; 17:435–450.
- Helme RD, Katz B, Gibson SJ, et al. Multidisciplinary pain clinics for older people. Do they have a role? Clin Geriatr Med 1996; 12:563–582.
- Harris DG. Management of pain in advanced disease. Br Med Bull 2014; 110:117–128.
- Raffa RB, Pergolizzi JV. A modern analgesics pain ‘pyramid’. J Clin Pharm Ther 2014; 39:4–6.
- Fine PG, Herr KA. Pharmacologic management of persistent pain in older persons. Clin Geriatr 2009; 17:25–32.
- Tracy B, Sean Morrison R. Pain management in older adults. Clin Ther 2013; 35:1659–1668.
- Malec M, Shega JW. Pain management in the elderly. Med Clin North Am 2015; 99:337–350.
- Abdulla A, Adams N, Bone M, et al; British Geriatric Society. Guidance on the management of pain in older people. Age Ageing 2013; 42(suppl 1):i1–i57.
- Pergolizzi J, Böger RH, Budd K, et al. Opioids and the management of chronic severe pain in the elderly: consensus statement of an International Expert Panel with focus on the six clinically most often used World Health Organization Step III opioids (buprenorphine, fentanyl, hydromorphone, methadone, morphine, oxycodone). Pain Pract 2008; 8:287–313.
- Gloth FM 3rd. Pharmacological management of persistent pain in older persons: focus on opioids and nonopioids. J Pain 2011; 12(suppl 1):S14–S20.
- Gomes T, Mamdani MM, Dhalla IA, Paterson JM, Juurlink DN. Opioid dose and drug-related mortality in patients with nonmalignant pain. Arch Intern Med 2011; 171:686–691.
- Moore RA, McQuay HJ. Prevalence of opioid adverse events in chronic non-malignant pain: systematic review of randomised trials of oral opioids. Arthritis Res Ther 2005; 7:R1046–R1051.
- Candy B, Jones L, Larkin PJ, Vickerstaff V, Tookman A, Stone P. Laxatives for the management of constipation in people receiving palliative care. Cochrane Database Syst Rev 2015; 5:CD003448.
- Webster LR, Butera PG, Moran LV, Wu N, Burns LH, Friedmann N. Oxytrex minimizes physical dependence while providing effective analgesia: a randomized controlled trial in low back pain. J Pain 2006; 7:937–946.
- Paulson DM, Kennedy DT, Donovick RA, et al. Alvimopan: an oral, peripherally acting, mu-opioid receptor antagonist for the treatment of opioid-induced bowel dysfunction—a 21-day treatment-randomized clinical trial. J Pain 2005; 6:184–192.
- Nalamachu SR, Pergolizzi J, Taylor R, et al. Efficacy and tolerability of subcutaneous methylnaltrexone in patients with advanced illness and opioid-induced constipation: a responder analysis of 2 randomized, placebo-controlled trials. Pain Pract 2015; 15:564–571.
- Brick N. Laxatives or methylnaltrexone for the management of constipation in palliative care patients. Clin J Oncol Nurs 2013; 17:91–92.
- Ford AC, Brenner DM, Schoenfeld PS. Efficacy of pharmacological therapies for the treatment of opioid-induced constipation: systematic review and meta-analysis. Am J Gastroenterolt 2013; 108:1566–1575.
- Byas-Smith MG, Chapman SL, Reed B, Cotsonis G. The effect of opioids on driving and psychomotor performance in patients with chronic pain. Clin J Pain 2005; 21:345–352.
- Benyamin R, Trescot AM, Datta S, et al. Opioid complications and side effects. Pain Physician 2008; 11(suppl 2):S105–S120.
- Wilwerding MB, Loprinzi CL, Mailliard JA, et al. A randomized, crossover evaluation of methylphenidate in cancer patients receiving strong narcotics. Support Care Cancer 1995; 3:135–138.
- Bruera E, Miller MJ, Macmillan K, Kuehn N. Neuropsychological effects of methylphenidate in patients receiving a continuous infusion of narcotics for cancer pain. Pain 1992; 48:163–166.
- Ahmedzai S. New approaches to pain control in patients with cancer. Eur J Cancer 1997; 33:S8–S14.
- Laugsand EA, Kaasa S, Klepstad P. Management of opioid-induced nausea and vomiting in cancer patients: systematic review and evidence-based recommendations. Palliat Med 2011; 25:442–453.
- Hardy J, Daly S, McQuade B, et al. A double-blind, randomised, parallel group, multinational, multicentre study comparing a single dose of ondansetron 24 mg p.o. with placebo and metoclopramide 10 mg t.d.s. p.o. in the treatment of opioid-induced nausea and emesis in cancer patients. Support Care Cancer 2002; 10:231–236.
- Apfel CC, Jalota L. Can central antiemetic effects of opioids counter-balance opioid-induced nausea and vomiting? Acta Anaesthesiol Scand 2010; 54:129–131.
- Okamoto Y, Tsuneto S, Matsuda Y, et al. A retrospective chart review of the antiemetic effectiveness of risperidone in refractory opioid-induced nausea and vomiting in advanced cancer patients. J Pain Symptom Manage 2007; 34:217–222.
- Overdyk F, Dahan A, Roozekrans M, van der Schrier R, Aarts L, Niesters M. Opioid-induced respiratory depression in the acute care setting: a compendium of case reports. Pain Manag 2014; 4:317–325.
- Niesters M, Overdyk F, Smith T, Aarts L, Dahan A. Buprenorphine-induced respiratory depression and involvement of ABCB1 SNPs in opioid-induced respiratory depression in paediatrics. Br J Anaesth 2013; 110:842–843.
- Niesters M, Mahajan RP, Aarts L, Dahan A. High-inspired oxygen concentration further impairs opioid-induced respiratory depression. Br J Anaesth 2013; 110:837–841.
- Dahan A, Yassen A, Romberg R, et al. Buprenorphine induces ceiling in respiratory depression but not in analgesia. Br J Anaesth 2006; 96:627–632.
- van Dorp E, Yassen A, Sarton E, et al. Naloxone reversal of buprenorphine-induced respiratory depression. Anesthesiology 2006; 105:51–57.
- Macintyre PE, Loadsman JA, Scott DA. Opioids, ventilation and acute pain management. Anaesth Intensive Care 2011; 39:545–558.
- Dahan A, Overdyk F, Smith T, Aarts L, Niesters M. Pharmacovigilance: a review of opioid-induced respiratory depression in chronic pain patients. Pain Physician 2013; 16:E85–E94.
- Correa D, Farney RJ, Chung F, Prasad A, Lam D, Wong J. Chronic opioid use and central sleep apnea: a review of the prevalence, mechanisms, and perioperative considerations. Anesth Analg 2015; 120:1273–1285.
- Randerath WJ, George S. Opioid-induced sleep apnea: is it a real problem? J Clin Sleep Med 2012; 8:577–578.
- Rosow CE, Gomery P, Chen TY, Stefanovich P, Stambler N, Israel R. Reversal of opioid-induced bladder dysfunction by intravenous naloxone and methylnaltrexone. Clin Pharmacol Ther 2007; 82:48–53.
- Weiner DK, Hanlon JT, Studenski SA. Effects of central nervous system polypharmacy on falls liability in community-dwelling elderly. Gerontology 1998; 44:217–221.
- Wolff ML, Kewley R, Hassett M, Collins J, Brodeur MR, Nokes S. Falls in skilled nursing facilities associated with opioid use. J Am Geriatr Soc 2012; 60:987.
- Zylicz Z, Twycross R. Opioid-induced hyperalgesia may be more frequent than previously thought. J Clin Oncol 2008; 26:1564; author reply 1565.
- Lee M, Silverman SM, Hansen H, Patel VB, Manchikanti L. A comprehensive review of opioid-induced hyperalgesia. Pain Physician 2011 2011; 14:145–161.
- Chen L, Sein M, Vo T, et al. Clinical interpretation of opioid tolerance versus opioid-induced hyperalgesia. J Opioid Manag 2014; 10:383–393.
- Vestergaard P, Hermann P, Jensen JE, Eiken P, Mosekilde L. Effects of paracetamol, non-steroidal anti-inflammatory drugs, acetylsalicylic acid, and opioids on bone mineral density and risk of fracture: results of the Danish Osteoporosis Prevention Study (DOPS). Osteoporos Int 2012; 23:1255–1265.
- Sacerdote P, Franchi S, Panerai AE. Non-analgesic effects of opioids: mechanisms and potential clinical relevance of opioid-induced immunodepression. Curr Pharm Des 2012; 18:6034–6042.
- Fine PG, Portenoy RK; Ad Hoc Expert Panel on Evidence Review and Guidelines for Opioid Rotation. Establishing “best practices” for opioid rotation: conclusions of an expert panel. J Pain Symptom Manage 2009; 38:418–425.
- Chibnall JT, Tait RC. Pain assessment in cognitively impaired and unimpaired older adults: a comparison of four scales. Pain 2001; 92:173–186.
- Andrade DC, Faria JW, Caramelli P, et al. The assessment and management of pain in the demented and non-demented elderly patient. Arq Neuropsiquiatr 2011; 69:387–394.
- Scherder E, Herr K, Pickering G, Gibson S, Benedetti F, Lautenbacher S. Pain in dementia. Pain 2009; 145:276–278.
- Chou R, Fanciullo GJ, Fine PG, Miaskowski C, Passik SD, Portenoy RK. Opioids for chronic noncancer pain: prediction and identification of aberrant drug-related behaviors: a review of the evidence for an American Pain Society and American Academy of Pain Medicine clinical practice guideline. J Pain 2009; 10:131–146.
- Butler SF, Budman SH, Fernandez KC, Fanciullo GJ, Jamison RN. Cross-validation of a screener to predict opioid misuse in chronic pain patients (SOAPP-R). J Addict Med 2009; 3:66–73.
- de Leon-Casasola OA. Opioids for chronic pain: new evidence, new strategies, safe prescribing. Am J Med 2013; 126(suppl 1):S3–S11.
- CDC guideline for prescribing opioids for chronic pain—United States, 2016. MMWR Recomm Rep 2016 Mar 18; 65(1):1–49.
- Chou R, Fanciullo GJ, Fine PG, et al; American Pain Society-American Academy of Pain Medicine Opioids Guidelines Panel. Clinical guidelines for the use of chronic opioid therapy in chronic noncancer pain. J Pain 2009; 10:113–130.
- West NA, Severtson SG, Green JL, Dart RC. Trends in abuse and misuse of prescription opioids among older adults. Drug Alcohol Depend 2015; 149:117–121.
- American Geriatrics Society Panel on Pharmacological Management of Persistent Pain in Older Persons. Pharmacological management of persistent pain in older persons. J Am Geriatr Soc 2009; 57:1331–1346.
- Weiner DK, Herr K. Comprehensive interdisciplinary assessment and treatment planning: an integrative overview. In: Weiner DK, Herr K, Rudy TE, editors. Persistent pain in older adults: an interdisciplinary guide for treatment. New York, NY: Springer Publishing Company; 2002.
- He W, Sengupta M, Velkoff V; US Census Bureau. 65+ in the United States: 2005. Washington, DC: US Government Printing Office; 2005. www.census.gov/prod/2006pubs/p23-209.pdf. Accessed March 30, 2016.
- American Geriatrics Society Panel on the Pharmacological Management of Persistent Pain in Older Persons. Pharmacological management of persistent pain in older persons. Pain Med 2009; 10:1062–1083.
- Thomas E, Peat G, Harris L, Wilkie R, Croft PR. The prevalence of pain and pain interference in a general population of older adults: cross-sectional findings from the North Staffordshire Osteoarthritis Project (NorStOP). Pain 2004; 110:361–368.
- Caraceni A, Hanks G, Kaasa S, et al; European Palliative Care Research Collaborative (EPCRC); European Association for Palliative Care (EAPC). Use of opioid analgesics in the treatment of cancer pain: evidence-based recommendations from the EAPC. Lancet Oncol 2012; 13:e58–e68.
- Solano JP, Gomes B, Higginson IJ. A comparison of symptom prevalence in far advanced cancer, AIDS, heart disease, chronic obstructive pulmonary disease and renal disease. J Pain Symptom Manage 2006; 31:58–69.
- Ferrell BA, Ferrell BR, Osterweil D. Pain in the nursing home. J Am Geriatr Soc 1990; 38:409–414.
- Ferrell BA, Ferrell BR, Rivera L. Pain in cognitively impaired nursing home patients. J Pain Symptom Manage 1995; 10:591–598.
- Fox PL, Raina P, Jadad AR. Prevalence and treatment of pain in older adults in nursing homes and other long-term care institutions: a systematic review. CMAJ 1999; 160:329–333.
- Stewart C, Leveille SG, Shmerling RH, Samelson EJ, Bean JF, Schofield P. Management of persistent pain in older adults: the MOBILIZE Boston Study. J Am Geriatr Soc 2012; 60:2081–2086.
- Woo J, Ho SC, Lau J, Leung PC. Musculoskeletal complaints and associated consequences in elderly Chinese aged 70 years and over. J Rheumatol 1994; 21:1927–1931.
- Pahor M, Guralnik JM, Wan JY, et al. Lower body osteoarticular pain and dose of analgesic medications in older disabled women: the Women’s Health and Aging Study. Am J Public Health 1999; 89:930–934.
- Marzinski LR. The tragedy of dementia: clinically assessing pain in the confused nonverbal elderly. J Gerontol Nurs 1991; 17:25–28.
- Roy R, Thomas M. A survey of chronic pain in an elderly population. Can Fam Physician 1986; 32:513–516.
- AGS Panel on Persistent Pain in Older Persons. The management of persistent pain in older persons. J Am Geriatr Soc 2002; 50(suppl 6): S205–S224.
- Stanos S, Houle TT. Multidisciplinary and interdisciplinary management of chronic pain. Phys Med Rehabil Clin N Am 2006; 17:435–450.
- Helme RD, Katz B, Gibson SJ, et al. Multidisciplinary pain clinics for older people. Do they have a role? Clin Geriatr Med 1996; 12:563–582.
- Harris DG. Management of pain in advanced disease. Br Med Bull 2014; 110:117–128.
- Raffa RB, Pergolizzi JV. A modern analgesics pain ‘pyramid’. J Clin Pharm Ther 2014; 39:4–6.
- Fine PG, Herr KA. Pharmacologic management of persistent pain in older persons. Clin Geriatr 2009; 17:25–32.
- Tracy B, Sean Morrison R. Pain management in older adults. Clin Ther 2013; 35:1659–1668.
- Malec M, Shega JW. Pain management in the elderly. Med Clin North Am 2015; 99:337–350.
- Abdulla A, Adams N, Bone M, et al; British Geriatric Society. Guidance on the management of pain in older people. Age Ageing 2013; 42(suppl 1):i1–i57.
- Pergolizzi J, Böger RH, Budd K, et al. Opioids and the management of chronic severe pain in the elderly: consensus statement of an International Expert Panel with focus on the six clinically most often used World Health Organization Step III opioids (buprenorphine, fentanyl, hydromorphone, methadone, morphine, oxycodone). Pain Pract 2008; 8:287–313.
- Gloth FM 3rd. Pharmacological management of persistent pain in older persons: focus on opioids and nonopioids. J Pain 2011; 12(suppl 1):S14–S20.
- Gomes T, Mamdani MM, Dhalla IA, Paterson JM, Juurlink DN. Opioid dose and drug-related mortality in patients with nonmalignant pain. Arch Intern Med 2011; 171:686–691.
- Moore RA, McQuay HJ. Prevalence of opioid adverse events in chronic non-malignant pain: systematic review of randomised trials of oral opioids. Arthritis Res Ther 2005; 7:R1046–R1051.
- Candy B, Jones L, Larkin PJ, Vickerstaff V, Tookman A, Stone P. Laxatives for the management of constipation in people receiving palliative care. Cochrane Database Syst Rev 2015; 5:CD003448.
- Webster LR, Butera PG, Moran LV, Wu N, Burns LH, Friedmann N. Oxytrex minimizes physical dependence while providing effective analgesia: a randomized controlled trial in low back pain. J Pain 2006; 7:937–946.
- Paulson DM, Kennedy DT, Donovick RA, et al. Alvimopan: an oral, peripherally acting, mu-opioid receptor antagonist for the treatment of opioid-induced bowel dysfunction—a 21-day treatment-randomized clinical trial. J Pain 2005; 6:184–192.
- Nalamachu SR, Pergolizzi J, Taylor R, et al. Efficacy and tolerability of subcutaneous methylnaltrexone in patients with advanced illness and opioid-induced constipation: a responder analysis of 2 randomized, placebo-controlled trials. Pain Pract 2015; 15:564–571.
- Brick N. Laxatives or methylnaltrexone for the management of constipation in palliative care patients. Clin J Oncol Nurs 2013; 17:91–92.
- Ford AC, Brenner DM, Schoenfeld PS. Efficacy of pharmacological therapies for the treatment of opioid-induced constipation: systematic review and meta-analysis. Am J Gastroenterolt 2013; 108:1566–1575.
- Byas-Smith MG, Chapman SL, Reed B, Cotsonis G. The effect of opioids on driving and psychomotor performance in patients with chronic pain. Clin J Pain 2005; 21:345–352.
- Benyamin R, Trescot AM, Datta S, et al. Opioid complications and side effects. Pain Physician 2008; 11(suppl 2):S105–S120.
- Wilwerding MB, Loprinzi CL, Mailliard JA, et al. A randomized, crossover evaluation of methylphenidate in cancer patients receiving strong narcotics. Support Care Cancer 1995; 3:135–138.
- Bruera E, Miller MJ, Macmillan K, Kuehn N. Neuropsychological effects of methylphenidate in patients receiving a continuous infusion of narcotics for cancer pain. Pain 1992; 48:163–166.
- Ahmedzai S. New approaches to pain control in patients with cancer. Eur J Cancer 1997; 33:S8–S14.
- Laugsand EA, Kaasa S, Klepstad P. Management of opioid-induced nausea and vomiting in cancer patients: systematic review and evidence-based recommendations. Palliat Med 2011; 25:442–453.
- Hardy J, Daly S, McQuade B, et al. A double-blind, randomised, parallel group, multinational, multicentre study comparing a single dose of ondansetron 24 mg p.o. with placebo and metoclopramide 10 mg t.d.s. p.o. in the treatment of opioid-induced nausea and emesis in cancer patients. Support Care Cancer 2002; 10:231–236.
- Apfel CC, Jalota L. Can central antiemetic effects of opioids counter-balance opioid-induced nausea and vomiting? Acta Anaesthesiol Scand 2010; 54:129–131.
- Okamoto Y, Tsuneto S, Matsuda Y, et al. A retrospective chart review of the antiemetic effectiveness of risperidone in refractory opioid-induced nausea and vomiting in advanced cancer patients. J Pain Symptom Manage 2007; 34:217–222.
- Overdyk F, Dahan A, Roozekrans M, van der Schrier R, Aarts L, Niesters M. Opioid-induced respiratory depression in the acute care setting: a compendium of case reports. Pain Manag 2014; 4:317–325.
- Niesters M, Overdyk F, Smith T, Aarts L, Dahan A. Buprenorphine-induced respiratory depression and involvement of ABCB1 SNPs in opioid-induced respiratory depression in paediatrics. Br J Anaesth 2013; 110:842–843.
- Niesters M, Mahajan RP, Aarts L, Dahan A. High-inspired oxygen concentration further impairs opioid-induced respiratory depression. Br J Anaesth 2013; 110:837–841.
- Dahan A, Yassen A, Romberg R, et al. Buprenorphine induces ceiling in respiratory depression but not in analgesia. Br J Anaesth 2006; 96:627–632.
- van Dorp E, Yassen A, Sarton E, et al. Naloxone reversal of buprenorphine-induced respiratory depression. Anesthesiology 2006; 105:51–57.
- Macintyre PE, Loadsman JA, Scott DA. Opioids, ventilation and acute pain management. Anaesth Intensive Care 2011; 39:545–558.
- Dahan A, Overdyk F, Smith T, Aarts L, Niesters M. Pharmacovigilance: a review of opioid-induced respiratory depression in chronic pain patients. Pain Physician 2013; 16:E85–E94.
- Correa D, Farney RJ, Chung F, Prasad A, Lam D, Wong J. Chronic opioid use and central sleep apnea: a review of the prevalence, mechanisms, and perioperative considerations. Anesth Analg 2015; 120:1273–1285.
- Randerath WJ, George S. Opioid-induced sleep apnea: is it a real problem? J Clin Sleep Med 2012; 8:577–578.
- Rosow CE, Gomery P, Chen TY, Stefanovich P, Stambler N, Israel R. Reversal of opioid-induced bladder dysfunction by intravenous naloxone and methylnaltrexone. Clin Pharmacol Ther 2007; 82:48–53.
- Weiner DK, Hanlon JT, Studenski SA. Effects of central nervous system polypharmacy on falls liability in community-dwelling elderly. Gerontology 1998; 44:217–221.
- Wolff ML, Kewley R, Hassett M, Collins J, Brodeur MR, Nokes S. Falls in skilled nursing facilities associated with opioid use. J Am Geriatr Soc 2012; 60:987.
- Zylicz Z, Twycross R. Opioid-induced hyperalgesia may be more frequent than previously thought. J Clin Oncol 2008; 26:1564; author reply 1565.
- Lee M, Silverman SM, Hansen H, Patel VB, Manchikanti L. A comprehensive review of opioid-induced hyperalgesia. Pain Physician 2011 2011; 14:145–161.
- Chen L, Sein M, Vo T, et al. Clinical interpretation of opioid tolerance versus opioid-induced hyperalgesia. J Opioid Manag 2014; 10:383–393.
- Vestergaard P, Hermann P, Jensen JE, Eiken P, Mosekilde L. Effects of paracetamol, non-steroidal anti-inflammatory drugs, acetylsalicylic acid, and opioids on bone mineral density and risk of fracture: results of the Danish Osteoporosis Prevention Study (DOPS). Osteoporos Int 2012; 23:1255–1265.
- Sacerdote P, Franchi S, Panerai AE. Non-analgesic effects of opioids: mechanisms and potential clinical relevance of opioid-induced immunodepression. Curr Pharm Des 2012; 18:6034–6042.
- Fine PG, Portenoy RK; Ad Hoc Expert Panel on Evidence Review and Guidelines for Opioid Rotation. Establishing “best practices” for opioid rotation: conclusions of an expert panel. J Pain Symptom Manage 2009; 38:418–425.
- Chibnall JT, Tait RC. Pain assessment in cognitively impaired and unimpaired older adults: a comparison of four scales. Pain 2001; 92:173–186.
- Andrade DC, Faria JW, Caramelli P, et al. The assessment and management of pain in the demented and non-demented elderly patient. Arq Neuropsiquiatr 2011; 69:387–394.
- Scherder E, Herr K, Pickering G, Gibson S, Benedetti F, Lautenbacher S. Pain in dementia. Pain 2009; 145:276–278.
- Chou R, Fanciullo GJ, Fine PG, Miaskowski C, Passik SD, Portenoy RK. Opioids for chronic noncancer pain: prediction and identification of aberrant drug-related behaviors: a review of the evidence for an American Pain Society and American Academy of Pain Medicine clinical practice guideline. J Pain 2009; 10:131–146.
- Butler SF, Budman SH, Fernandez KC, Fanciullo GJ, Jamison RN. Cross-validation of a screener to predict opioid misuse in chronic pain patients (SOAPP-R). J Addict Med 2009; 3:66–73.
- de Leon-Casasola OA. Opioids for chronic pain: new evidence, new strategies, safe prescribing. Am J Med 2013; 126(suppl 1):S3–S11.
- CDC guideline for prescribing opioids for chronic pain—United States, 2016. MMWR Recomm Rep 2016 Mar 18; 65(1):1–49.
KEY POINTS
- Treatment of persistent pain in older adults presents several challenges.
- Often, persistent pain is underrecognized and undertreated, impairing function and reducing quality of life.
- A combination of pharmacologic and nonpharmacologic strategies is needed to address the multiple factors contributing to pain and manage it effectively.
- The World Health Organization’s three-step ladder is valuable for treating persistent pain in older adults.
- Although nonopioids are the first-line treatments for persistent pain, opioids are also important to provide safe and effective pain management in older adults.