User login
Individualizing Treatment of Hyperglycemia in Type 2 Diabetes
From the University of Arizona College of Pharmacy and the University of Arizona College of Medicine-Tucson, Tucson, AZ.
Abstract
- Objective: To summarize key issues relevant to managing hyperglycemia in patients with type 2 diabetes mellitus (T2DM) and review a strategy for initiating and intensifying therapy.
- Methods: Review of the literature.
- Results: The 6 most widely used pharmacologic treatment options for hyperglycemia in T2DM are metformin, sulfonylureas, dipeptidyl peptidase-4 inhibitors, glucagon-like peptide-1 receptor agonists, sodium-glucose cotransporter-2 inhibitors, and insulin. Recent guidelines stress the importance of an individualized, patient-centered approach to managing hyperglycemia in T2DM, although sufficient guidance for nonspecialists on how to individualize treatment is often lacking. For patients with no contraindications, metformin should be recommended concurrent with lifestyle intervention at the time of diabetes diagnosis. Due to the progressive nature of T2DM, glycemic control on metformin monotherapy is likely to deteriorate over time, and there is no consensus as to what the second-line agent should be. A second agent should be selected based on glycemic goal and potential advantages and disadvantages of each agent for any given patient. If the patient progresses to the point where dual therapy does not provide adequate control, either a third non-insulin agent or insulin can be added.
- Conclusion: Although research is increasingly focusing on what the ideal number and sequence of drugs should be when managing T2DM, investigating all possible combinations in diverse patient populations is not feasible. Physicians therefore must continue to rely on clinical judgment to determine how to apply trial data to the treatment of individual patients.
Key words: type 2 diabetes; patient-centered care; antihyper-glycemic drugs; insulin; therapeutic decision-making.
Diabetes mellitus affects approximately 29.1 million people, or 9.3% of the U.S. population [1,2]. The high prevalence of diabetes and its associated multiple complications, including cardiovascular disease (CVD), blindness, renal failure, lower extremity amputations, and premature death, lead to a tremendous overall burden of disease. The financial cost is staggering as well, with more than 1 in 5 health care dollars spent on treating diabetes or its complications [3]. The goal of diabetes treatment is to prevent acute complications and reduce the risk of long-term complications. Interventions that have been shown to improve diabetes outcomes include medications for glycemic control and treatment of cardiovascular risk factors, nutrition and physical activity counseling, smoking cessation, immunizations, psychosocial care, and ongoing surveillance and early treatment for eye, kidney, and foot problems [4].
Glycemic management in type 2 diabetes mellitus (T2DM), the focus of this review, is growing increasingly complex and has been the subject of numerous extensive reviews [5,6] and published guidelines [4,7]. In the context of an increasing array of available pharmacologic options, there are mounting uncertainties regarding the benefits of intensive glycemic control as well as increasing concerns about potential adverse treatment effects, hypoglycemia in particular. While previous guidelines encouraged specific approaches for most patients, more recent guidelines stress the importance of a patient-centered approach with shared decision-making [4]. Less prescriptive guidelines are more appropriate, given the current state of science, but they also may be viewed as providing insufficient guidance to some providers. It can be overwhelming for a non-specialist to try to match the nuances of antihyperglycemic medications to the nuances of each patient’s preferences and medical characteristics.
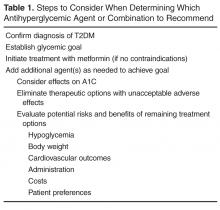
This article examines key issues faced by primary care providers when managing hyperglycemia in patients with T2DM and outlines a stepwise approach to determining the optimal antihyperglycemic agent(s) (Table 1).
Confirm Diagnosis of T2DM
It can be difficult to distinguish between type 1 diabetes mellitus and T2DM in some individuals due to overlapping characteristics. However, correctly classifying a patient’s diabetes at the outset is essential, as the classification helps determine the best treatment regimen and is rarely reconsidered [4,8]. Considerable evidence suggests that misclassification of diabetes occurs frequently [9,10], resulting in patients receiving inappropriate treatment. Clinical characteristics suggestive of T2DM include older age and features of insulin resistance such as obesity, hyper-tension, hypertriglyceridemia, and low high-density lipoprotein cholesterol. When these features are not present, an alternate diagnosis should be entertained.
Establish Glycemic Goal
Research over the past decade has led to a growing appreciation of the enormous complexity of hyperglycemia management. During the 1990s, landmark trials such as the Diabetes Control and Complications Trial (DCCT) [11] and UK Prospective Diabetes Study (UKPDS) [12] demonstrated that improving glucose control could reduce the incidence of microvascular complications [11,12], prompting a lower-is-better philosophy regarding glucose targets. Despite limited evidence to support such thinking, this viewpoint was adopted by the developers of many guidelines. During the following decade more research was devoted to determining whether aggressively lowering a patient’s glucose could also improve macrovascular outcomes. Table 2 summarizes microvascular and macrovascular effects of intensive glycemic control seen in major trials [11–23]. After several major trials [20,22] found only mild cardiovascular benefits and even suggested harm [18], experts and policy makers began to reconsider the value of tightly controlling glucose levels [24]. Since then, other studies have demonstrated that the potential benefits and risks of glucose control are strongly related to individual patient factors, such as age and duration of diabetes, and associated comorbidities, such as CVD and impaired renal function [6].
A one-size-fits-all glycemic goal is no longer recommended. Personalization is necessary, balancing the potential benefits and risks of treatments required to achieve that goal. Whereas an A1C of < 7% is an appropriate target for some individuals with diabetes, glycemic targets may be more or less stringent based on patient features including life expectancy, duration of diabetes, comorbidities, and patient attitude and support system (Table 3) [4].
A particular group in which less stringent goals should be considered is older patients, especially those with complex or poor health status [4,25]. The risk of intensive glycemic control may exceed the benefits in these patients, as they are at higher risk of hypoglycemia and polypharmacy [26]. A goal A1C of 7% to 7.5% is now recommended for healthy older adults, and less stringent A1C goals of 7.5% to 8% and 8% to 8.5% should be considered based on the presence and severity of multiple coexisting chronic illnesses, decreased self-care ability, or cognitive impairment [4,25]. Unfortunately, overtreatment is frequently seen in this group. In a recent study of patients over age 65 years, about 40% of those with complex or poor health status had tight glycemic control with A1C below 6.5% [26]. An analysis of U.S. Veterans Affairs administration data showed that only 27% of 12,917 patients older than 65 with very low A1C (< 6%) and about 21% of those with A1C of 6% to 6.5% underwent treatment deintensification [27].
Initiate Treatment with Metformin
There is strong consensus that metformin is the preferred drug for monotherapy due to its long proven safety record, low cost, weight-reduction benefit, and potential cardiovascular advantages [4,16]. As long as there are no contraindications, metformin should be recommended concurrent with lifestyle intervention at the time of diabetes diagnosis. The recommendation is based on the fact that adherence to diet, weight reduction, and regular exercise is not sustained in most patients, and most patients ultimately will require treatment. Since metformin is usually well-tolerated, does not cause hypoglycemia, has a favorable effect on body weight, and is relatively inexpensive, potential benefits of early initiation of medication appear to outweigh potential risks.
The U.S. Food and Drug Administration (FDA) recently relaxed prescribing polices to extend the use of this important medication to patients who have mild–moderate, but stable, chronic kidney disease (CKD) [28]. Metformin is recommended as first-line therapy and should be used unless it is contraindicated (ie, estimated glomerular filtration rate [eGFR] < 30 mL/min/1.73 m2)[4,7,29].
Add Additional Agent(s) as Needed to Achieve Goal
Other than metformin, evidence is limited for the optimal use of the burgeoning array of available agents, especially in dual or triple combinations [6,30]. Research is now starting to focus more on what the ideal number and sequence of drugs should be. The Glycemic Reduction Approach in Diabetes (GRADE) study, which will compare long-term benefits and risks of the 4 most widely used antihyperglycemic medications in combination with metformin, is now underway [31,32]. The 4 classes being studied are sulfonylurea, dipeptidyl peptidase-4 (DPP-4) inhibitors, glucagon-like peptide-1 (GLP-1) receptor agonists, and a basal,
Eleven classes of non-insulin medications are currently approved for treating hyperglycemia in T2DM [4]. Within each class, numerous agents are available. Six of these classes (ie, α-glucosidase inhibitors, colesevelam, bromocriptine, pramlintide, meglitinides, and thiazolidinediones) are not used frequently
Consider Effects on A1C
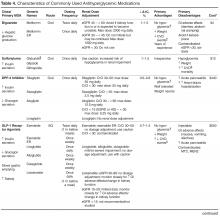
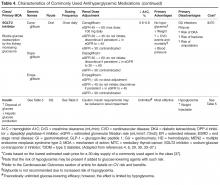
There is a paucity of high-quality, head-to-head comparison trials evaluating the ability of available agents to achieve recommended glycemic targets. This is important because the glucose-lowering effectiveness of individual medications is strongly influenced by baseline characteristics such as A1C, duration of diabetes, and previous therapy. With these limitations in mind, the relative glucose-lowering effectiveness of commonly used agents is shown in Table 4. When used as monotherapy, A1C reductions of approximately 1% to 1.5% are achieved with metformin, sulfonylureas, and GLP-1 receptor agonists [6,30,34,35,39]. DPP-4 inhibitors and SGLT-2 inhibitors have more modest glucose-lowering efficacy, with A1C reductions of approximately 0.5% to 1% [6,30,34,35,39]. Larger effects may be seen in individuals with higher baseline A1C and those who are drug naïve. Insulin is the most effective glucose-lowering agent—it can reduce virtually any level of A1C down to the normal range, with hypoglycemia being the only limiting factor. When a patient has uncontrolled hyperglycemia on metformin monotherapy, or if there is a contraindication or intolerance to metformin, clinicians should consider the potential glucose-lowering effects of other available options and should choose an agent that conceivably could bring a patient close to meeting their treatment goal.
Eliminate Options with Unacceptable Adverse Effects
When the pharmacologic options with acceptable A1C-lowering potential have been identified, the ones with contraindications and potential serious adverse effects for the individual patient can immediately be eliminated (Table 4). For example, if a patient has an eGFR < 30 mL/min/1.73 m2, metformin, sulfonylureas, GLP-1 receptor agonists, most DPP-4 inhibitors, and SGLT-2 inhibitors are either contraindicated or should be used with caution. In patients with severe osteoporosis, SGLT-2 inhibitors may not be the best option. In patients with a history of diabetic ketoacidosis (DKA), caution should be used with metformin and SGLT-2 inhibitors. There have been concerns of possible acute pancreatitis and neoplasia with the incretin-based agents, the DPP-4 inhibitors and GLP-1 receptor agonists [40,41], although other clinical trials and observational data have not found increased risk [42–45]. Nevertheless, these agents potentially should be avoided in patients with a history of pancreatitis or neoplasm. SGLT-2 inhibitors may be associated with genitourinary infections and volume depletion [46–48] and probably should be avoided in patients at high risk for these conditions.
If the adverse effects are not serious, changing the way the medication is administered may allow the patient to tolerate agents with high potential benefits. For example, metformin is commonly associated with gastrointestinal (GI) adverse effects, which can be reduced or avoided with slow titration of the dose [6] or by switching to an extended-release formulation [49]. GLP-1 receptor agonists are associated with GI adverse effects [6] and in most cases slow titration is recommended.
Evaluate Potential Risks/Benefits of Remaining Options
Hypoglycemia. The barrier of hypoglycemia generally precludes maintenance of euglycemia and full realization of the long-term benefits of good glucose control over a lifetime. Once considered a trivial issue, concerns about hypoglycemia in T2DM are increasingly being raised [19,50–55]. Clearly, hypoglycemia occurs more often as glycemic targets are lowered to near-normal values, especially in those with advanced age and multiple comorbidities [55]. Various comorbidities frequently encountered particularly as patients age also are associated with increasing propensity for experiencing hypoglycemia and untoward outcomes from it. These include coronary artery disease, heart failure, renal and liver disease, and dementia. Hypoglycemia, when it occurs, may lead to dysrhythmias, dizziness, accidents and falls, work disability, and decreased quality of life. In addition to relaxing blood glucose targets in high-risk patients, drug selection should favor agents that do not precipitate such events (Table 4).
Fortunately, the commonly used non-insulin agents are not associated with hypoglycemia unless they are used in combination with sulfonylureas or insulin. Sulfonylureas should be used with caution and other options considered in patients with high risk for hypoglycemia. When insulin is required, regimens which minimize risk of hypoglycemia should be used. For example, adding a GLP-1 receptor agonist to basal insulin as an alternative to mealtime insulin has been shown to be equally effective with a lower risk of hypoglycemia [4,6]. Also, premixed insulin preparations should be avoided or used cautiously in individuals who miss meals frequently. Additionally, newer basal insulins that exhibit longer duration of action are now available in the United States. Preliminary studies have shown that the newly FDA-approved longer-acting basal insulins, insulin degludec and glargine U-300, may be associated with a reduced risk for hypoglycemia [56,57]. However, it remains unclear how and when these newer agents will best be incorporated into a treatment regimen.
Body weight. Nearly 90% of people living with T2DM are overweight or obese. Given the close tie between obesity and T2DM, treating obesity is an obvious consideration in diabetes treatment. Major trials have shown the effectiveness of lifestyle modifications and weight reduction in delaying, prevention, and management of T2DM [4,58,59].With this in mind, clinicians should consider preferentially using antihyperglycemic agents with weight-lowering or weight-neutral effects. Among commonly used antihyperglycemic agents, metformin, GLP-1 receptor agonists, and SGLT-2 inhibitors have been shown to have weight-reduction benefits, and DPP-4 inhibitors are weight neutral. On the other hand, sulfonylureas and insulin are associated with weight gain. A systematic review and meta-analysis including 204 studies with study durations ranging from 3 months to 8 years showed comparative effects of diabetes medications with a differential effect on weight of up to 5 kg (Table 4) [60].
Metformin is associated with an average weight loss of 1.9 to 3.1 kg that was sustained with long-term use for at least 10 years in the Diabetes Prevention Program Outcomes Study [61].A systematic review of 7 randomized trials showed that in patients with T2DM, the SGLT-2 inhibitors dapagliflozin and canagliflozin were associated with weight loss (mean weighted difference of –1.81 kg and –2.3 kg, respectively) [62]. A systematic review and meta-analysis of 25 randomized controlled trials showed greater weight loss (mean weighted difference of –2.9 kg) in overweight or obese patients with or without T2DM using GLP-1 receptor agonists when compared to placebo, insulin, or oral antihyperglycemic agents [63]. Of note, the GLP-1 receptor agonist liraglutide is now approved for weight loss in patients with or without diabetes [64]. The maximum doses approved for diabetes and obesity treatment are 1.8 and 3.0 mg/day, respectively.
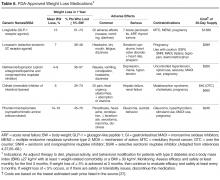
Since weight loss is associated with improved glycemic control, an area of emerging interest is the use of antiobesity medications for managing diabetes. Although most older weight-loss medications were only approved for short-term use, some newer agents are approved for longer-term use. Lorcaserin and the combination drugs topiramate/phentermine and naltrexone/bupropion are approved for chronic therapy, provided certain conditions are met. Patients on weight reduction agents should be monitored regularly.
An even more radical departure from conventional therapy for diabetes is the consideration of metabolic, or weight-loss, surgery, which has been found to be associated with rapid and dramatic improvements in blood glucose control. Metabolic surgery has been shown to improve glucose control more effectively than any known pharmaceutical or behavioral approach. For example, in an observational study of obese patients with T2DM, bariatric surgery led to diabetes remission rates of 72.3% 2 years after surgery and 30.4% 15 years after surgery compared to 16.4% and 6.5%, respectively, in control patients [69]. With long-term follow-up, significant decreases in microvascular and macrovascular complications were seen in the surgical group [69]. Compared with medical therapy alone, bariatric surgery plus medical therapy has been associated with more weight loss, better glycemic control, less need for diabetes medications, and improved quality of life [70]. A 2016 joint statement by numerous international diabetes organizations recommends considering metabolic surgery as a treatment for T2DM and obesity [71]. American Diabetes Association guidelines recommend consideration of bariatric surgery in individuals with T2DM who have a body mass index greater than 35 kg/m2,especially if achieving disease control is difficult by means of lifestyle modifications and medications [4].
Cardiovascular outcomes. Cardiovascular risk is about 2 to 4 times higher in patients with diabetes, and about half of patients with this condition develop heart failure [4,72]. CVD is responsible for most of the mortality in T2DM [72]. Therefore, prevention of cardiovascular morbidity and mortality is an important goal for diabetes treatment. Due to concerns about potential cardiovascular risks associated with glucose-lowering medications [73–76], the FDA has issued regulatory requirements for manufacturers to monitor the cardiovascular risk profile for these drugs [77]. Recent trials have led to a better understanding of potential cardiovascular benefits or harms of antihyperglycemic medications.
Metformin, the widely recommended first-line therapy for T2DM, carries a large body of evidence supporting its cardiovascular benefits. For example, the UKPDS found that compared to conventional therapy (mostly diet), metformin reduced cardiovascular events and mortality in obese patients with T2DM [15]. This result was supported in Hyperinsulinemia: the Outcome of its Metabolic Effect (HOME) study where, as an add-on to insulin, metformin decreased macrovascular complications when compared to placebo [78]. Research over the past decade also has assuaged concerns about metformin safety in heart failure [60]. A systematic review of observational studies involving 34,000 patients conducted in 2013 showed that metformin is as safe as other glucose-lowering medications in patients with diabetes and heart failure even in the presence of CKD [4,79]. Furthermore, numerous investigations have found metformin is not associated with increased hospitalizations or risk of lactic acidosis [80]. Metformin can be used safely in patients with diabetes and heart failure [60].
Although sulfonylureas have long been a mainstay of diabetes therapy, concerns about their potential adverse cardiovascular effects have been raised by numerous studies [81]. Tolbutamide, a first-generation sulfonylurea, was removed from the market after the University Group Diabetes Program study found increased CVD deaths with this agent versus placebo. Subsequently, the FDA issued a warning for all sulfonylureas [74]. The increased cardiovascular risk associated with sulfonylureas is thought to be due to their effect on cardiac mitochondrial potassium ATP channels. Sulfonylureas bind to these channels, preventing a protective phenomenon called ischemic preconditioning and resulting in a weakened defense against myocardial injury [76]. A recent study showed an increased risk of coronary heart disease associated with long-term use of sulfonylureas in women with diabetes [81].
GLP-1 receptor agonists have recently received much attention for their potential beneficial effects on cardiovascular outcomes. In a recent trial, lixisenatide was shown to be safe in patients with T2DM and acute coronary syndrome when compared to placebo [82]. More recently, the Liraglutide Effect and Action in Diabetes: Evaluation of cardiovascular outcome Results (LEADER) trial demonstrated significant cardiovascular benefits with liraglutide in patients with T2DM and established or high CVD risk [83]. The composite outcome of the first occurrence of death from cardiovascular causes, nonfatal myocardial infarction (MI), or nonfatal stroke, occurred less frequently in the liraglutide group compared to placebo (13% versus 14.9%, respectively), and there were fewer deaths from cardiovascular causes in the liraglutide group compared to placebo (4.7% and 6.0%, respectively) [83]. Other trials investigating the cardiovascular outcomes of this class [84,85] are in progress.
Another class with potential cardiovascular benefits is the SGLT-2 inhibitors. In a recent cardiovascular outcome study, empagliflozin significantly lowered the composite of cardiovascular death, nonfatal MI, or nonfatal stroke in T2DM patients with high cardiovascular risk compared to placebo (10.5% and 12.1%, respectively) [86]. There are several large ongoing studies evaluating the cardiovascular effects of other SGLT-2 inhibitors [87–89].
DPP-4 inhibitors were examined in recent studies and have shown no cardiovascular benefits [42,44,90].The studies showed mixed results regarding an association between DPP-4 inhibitors and heart failure. In one study, saxagliptin was associated with increased hospitalization for heart failure compared to placebo [44], while 2 noninferiority trials did not show a significant increase in heart failure hospitalizations associated with alogliptin and sitagliptin when compared to placebo [42,90].
Administration Considerations
Many patients with T2DM require multiple agents for glycemic control. Additional medications used for comorbid conditions add to this burden. When choosing antihyperglycemic agents, the route and frequency of administration, as well as the patients’ preferences and ability, should be considered. Either once or twice daily dosing is available for most agents, and once weekly dosing is available for some of the GLP-1 receptor agonists. Once daily or once weekly formulations may improve adherence and be more desirable than preparations that are dosed twice daily. Most of the commonly used medications are dosed orally. Although many patients find this route of administration preferable to insulin or GLP-1 receptor agonists, which require injections, some patients may prefer the risk/benefit of injectable agents. All GLP-1 receptor agonists come in a pen delivery system, which eliminates mixing and provides more convenient administration. Extended-release exenatide also is available as a single-dose tray that requires mixing and may be more cumbersome to inject.
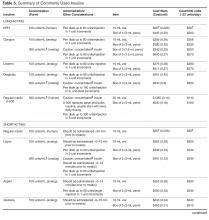
Insulin requires special consideration. There has been an enormous increase in the number of insulin products on the market in the past 2 decades. These products include insulin analogs, concentrated insulins (U-200, U-300, and U-500), premixed insulin preparations, and ultra-long-acting insulin [91]. The availability of insulin options with different concentrations, onsets, and durations of actions has made decision making on which insulin to use difficult. Clinicians need to consider patient preference, dosing frequency, and timing with regard to meals, insulin dose, administration, as well as cost. For example, concentrated insulin is preferred for a patient on high doses of insulin requiring injecting a large volume of insulin. Rapid-acting insulin analogs would be more appropriate for patients who have difficulty administering their regular insulin 20 to 30 minutes before eating. Premixed insulin preparations make it impossible to independently adjust short- and long-acting components. However, these may be good choices in patients who have consistent meal schedules and who want to simplify administration. Despite a prevailing misconception that NPH must be given twice a day, it has long been recognized that in T2DM, a single daily injection of NPH yields improvements in control similar to those achieved with 2 daily injections [92].
Cost Considerations
Treating T2DM imposes a great financial burden on individuals living with diabetes and their families due to the high cost of the medications. Table 4 and Table 5 provide information on the cost of non-insulin and insulin diabetes medications for patients who do not have prescription insurance coverage. From a practical standpoint, choice of diabetes agents is largely influenced by insurance formularies.
The older agents, metformin and the sulfonylureas, are available for a cash (no insurance) price of as little as $4 per month. This is in stark contrast to the SGLT-2 inhibitors, GLP-1 receptor agonists, and DPP-4 inhibitors, which range in cost between $400 and $600 per month. Of recent concern, the cost of insulin has been skyrocketing, with a more than 500% increase in the cost of certain insulins from 2001 to 2015 [93]. According to the Medical Expenditure Panel Survey (MEPS) from 2002 to 2013, the mean price of insulin increased by about 200% (from $4.34/mL to $12.92/mL) during this period, which was significantly higher than increases in the price of non-insulin comparators [94]. The introduction of biosimilar insulins to the market is expected to offer treatment options with lower cost. This will be tested when the biosimilar glargine, the first FDA-approved biosimilar insulin, becomes available in the U.S. market. However, a significant reduction in insulin prices is not expected soon [95].
When insulin is required, most patients with T2DM can be treated with older human insulins, which have similar efficacy and lower costs than the more expensive newer insulin analogs. A Cochrane review comparing basal insulin analogs to NPH showed similar efficacy in glycemic control with minimal clinical benefit in the form of less nocturnal hypoglycemia in the insulin analog arm [96]. Furthermore, similar glycemic control and risk of hypoglycemia was seen when regular insulin was compared with the rapid-acting insulin analogs [97]. The cost of human NPH insulin for a patient on a total daily dose of 60 units is approximately $52 per month. This contrasts with the most widely used insulin, insulin glargine, which has a cash price of about $500 per month for the same amount (Table 5). Insulin pens, which are convenient, are more expensive. Interestingly, human insulins do not require prescriptions, allowing underinsured, underfunded patients ongoing access to them.
Incorporating Patient Preferences
Research evidence is necessary but insufficient for making patient care decisions. Along with the potential benefits, harms, costs, and inconveniences of the management options, patient perspectives, beliefs, expectations, and health-related goals must be considered. Patients will undoubtedly have preferences regarding defining goals and ranking options. Clinicians should discuss therapeutic goals and treatment options and work collaboratively with patients in determining management strategies [98].
Summary
Potential treatment approaches for treating hyperglycemia in T2DM are summarized in Figure 1 and Figure 2 [4,7]. As long as there are no contraindications, metformin should be recommended concurrent with lifestyle intervention at the time of diabetes diagnosis. Even if metformin monotherapy is initially effective, glycemic control is likely to deteriorate over time due to progressive loss of β-cell function in T2DM.
There is no consensus as to what the second-line agent should be. Selection of a second agent should be made based on potential advantages and disadvantages of each agent for any given patient. A patient-centered approach is preferred over a fixed algorithm. If the patient progresses to the point where dual therapy does not provide adequate control, either a third non-insulin agent or insulin can be added. In patients with modestly elevated A1C (below ~8%), addition of a third non-insulin agent may be equally effective as (but more expensive than) addition of insulin.
Patients with significantly elevated A1C levels on non-insulin agents usually should have insulin added to their regimen. When insulin is added, metformin should be continued. DPP-4 inhibitors and sulfonylureas are typically stopped. If SGLT-2 inhibitors and/or GLP-1 receptor agonists are continued, this may aid with weight maintenance. However, continuing these agents is likely to be expensive and associated with problems associated with polypharmacy.
The most widely recommended strategy for initiating insulin in T2DM is to add a single bedtime injection of basal insulin (ie, NPH, glargine, detemir, or degludec) to the patient’s regimen. This regimen has been found to be effective in numerous studies and controls hyperglycemia in up to 60% of patients [99]. If the patient is treated with a single bedtime injection of insulin and the fasting glucose level is within the target range but the A1C level remains above goal, addition of mealtime insulin injections is likely to be beneficial. Alternatively, addition of a GLP-1 receptor agonist to basal insulin has been shown to be equally beneficial [4,6]. When adding mealtime insulin, a common strategy is to add a single injection of a rapid-acting insulin (eg, lispro, aspart, glulisine) before the patient’s largest meal of the day. Additional premeal injections of rapid-acting insulin may be added as needed, based on self-monitoring blood glucose results. If glycemia remains significantly uncontrolled on more than 200 units of insulin per day, switching to a concentrated form of insulin (eg, U-200, U-300, or U-500) should be considered.
Corresponding author: Maryam Fazel, PharmD, BCPS, BCACP, CDE, 1295 N. Martin Ave. (Room B211B), Tucson, Arizona 85721-0202, maryamfazel@pharmacy.arizona.edu.
Financial disclosures: None.
1. National diabetes statistics report: estimates of diabetes and its burden in the United States, 2014. Centers for Disease Control and Prevention Web site. www.cdc.gov/diabetes/pubs/statsreport14/national-diabetes-report-web.pdf. Accessed November 29, 2016.
2. Statistics about diabetes. American Diabetes Association Web site. www.diabetes.org/diabetes-basics/statistics/. Accessed November 29, 2016.
3. American Diabetes Association. Economic costs of diabetes in the U.S. in 2012. Diabetes Care 2013;36:1033–46.
4. American Diabetes Association. Standards of medical care in diabetes--2016. Diabetes Care 2016;39(Suppl. 1).
5. Raz I, Riddle MC, Rosenstock J, et al. Personalized management of hyperglycemia in type 2 diabetes: reflections from a Diabetes Care Editors’ Expert Forum. Diabetes Care 2013;36:1779–88.
6. Inzucchi SE, Bergenstal RM, Buse JB, et al. Management of hyperglycemia in type 2 diabetes, 2015: a patient-centered approach: update to a position statement of the American Diabetes Association and the European Association for the Study of Diabetes. Diabetes Care 2015;38:140–9.
7. Garber AJ, Abrahamson MJ, Barzilay JI, et al. Consensus Statement by the American Association of Clinical Endocrinologists and American College of Endocrinology on the Comprehensive Type 2 Diabetes Management Algorithm--2016 Executive Summary. Endocr Pract 2016;22:84–113.
8. Steenkamp DW, Alexanian SM, Sternthal E. Approach to the patient with atypical diabetes. CMAJ 2014;186:678–84.
9. de Lusignan S, Sadek N, Mulnier H, et al. Miscoding, misclassification and misdiagnosis of diabetes in primary care. Diabet Med 2012;29:181–9.
10. Tripathi A, Rizvi AA, Knight LM, Jerrell JM. Prevalence and impact of initial misclassification of pediatric type 1 diabetes mellitus. South Med J 2012;105:513–7.
11. The effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin-dependent diabetes mellitus. The Diabetes Control and Complications Trial Research Group. N Engl J Med 1993;329:977–86.
12. Intensive blood-glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33). UK Prospective Diabetes Study (UKPDS) Group. Lancet 1998;352:837–53.
13. Nathan DM, Cleary PA, Backlund JY, et al. Intensive diabetes treatment and cardiovascular disease in patients with type 1 diabetes. N Engl J Med 2005;353:2643–53.
14. Nathan DM, DCCT/EDIC Research Group. The diabetes control and complications trial/epidemiology of diabetes interventions and complications study at 30 years: overview. Diabetes Care 2014;37:9–16.
15. Effect of intensive blood-glucose control with metformin on complications in overweight patients with type 2 diabetes (UKPDS 34). UK Prospective Diabetes Study (UKPDS) Group. Lancet 1998;352:854–65.
16. Holman RR, Paul SK, Bethel MA, et al. 10-Year follow-up of intensive glucose control in type 2 diabetes. N Engl J Med 2008;359:1577–89.
17. Ohkubo Y, Kishikawa H, Araki E, et al. Intensive insulin therapy prevents the progression of diabetic microvascular complications in Japanese patients with non-insulin-dependent diabetes mellitus: a randomized prospective 6-year study. Diabetes Res Clin Pract 1995;28:103–17.
18. Action to Control Cardiovascular Risk in Diabetes Study Group, Gerstein HC, Miller ME, Byington RP, et al. Effects of intensive glucose lowering in type 2 diabetes. N Engl J Med 2008;358:2545–59.
19. ACCORD Study Group, Gerstein HC, Miller ME, Genuth S, et al. Long-term effects of intensive glucose lowering on cardiovascular outcomes. N Engl J Med 2011;364:818–28.
20. ADVANCE Collaborative Group, Patel A, MacMahon S, Chalmers J, et al. Intensive blood glucose control and vascular outcomes in patients with type 2 diabetes. N Engl J Med 2008;358:2560–72.
21. Wong MG, Perkovic V, Chalmers J, et al. Long-term Benefits of Intensive Glucose Control for Preventing End-Stage Kidney Disease: ADVANCE-ON. Diabetes Care 2016;39:694–700.
22. Duckworth W, Abraira C, Moritz T, et al. Glucose control and vascular complications in veterans with type 2 diabetes. N Engl J Med 2009;360:129–39.
23. Hayward RA, Reaven PD, Wiitala WL, et al. Follow-up of glycemic control and cardiovascular outcomes in type 2 diabetes. N Engl J Med 2015;372:2197–206.
24. American Diabetes Association. Standards of medical care in diabetes--2009. Diabetes Care 2009;32 Suppl 1:S13–61.
25. American Geriatrics Society Expert Panel on Care of Older Adults with Diabetes Mellitus, Moreno G, Mangione CM, Kimbro L, Vaisberg E. Guidelines abstracted from the American Geriatrics Society Guidelines for Improving the Care of Older Adults with Diabetes Mellitus: 2013 update. J Am Geriatr Soc 2013;61:2020–6.
26. Lipska KJ, Ross JS, Miao Y, et al. Potential overtreatment of diabetes mellitus in older adults with tight glycemic control. JAMA Intern Med 2015;175:356–62.
27. Sussman JB, Kerr EA, Saini SD, et al. Rates of deintensification of blood pressure and glycemic medication treatment based on levels of control and life expectancy in older patients with diabetes mellitus. JAMA Intern Med 2015;175:1942–9.
28. FDA Drug Safety Communication: FDA revises warnings regarding use of the diabetes medicine metformin in certain patients with reduced kidney function. FDA Web site. www.fda.gov/Drugs/DrugSafety/ucm493244.htm. Accessed December 1, 2016.
29. Inzucchi SE, Lipska KJ, Mayo H, et al. Metformin in patients with type 2 diabetes and kidney disease: a systematic review. JAMA 2014;312:2668–75.
30. Nathan DM. Diabetes: advances in diagnosis and treatment. JAMA 2015;314:1052–62.
31. Nathan DM, Buse JB, Kahn SE, et al. Rationale and design of the glycemia reduction approaches in diabetes: a comparative effectiveness study (GRADE). Diabetes Care 2013;36:2254–61.
32. NIH begins recruitment for long-term study of diabetes drug efficacy. NIH Web site. www.nih.gov/news-events/news-releases/nih-begins-recruitment-long-term-study-diabetes-drug-efficacy. Accessed December 1, 2016.
33. Hermayer KL, Dake A. Newer oral and noninsulin therapies to treat type 2 diabetes mellitus. Cleve Clin J Med 2016;83(5 Suppl 1):S18–26.
34. Bolen S, Wilson L, Vassy J, et al. Systematic review: comparative effectiveness and safety of oral medications for type 2 diabetes mellitus. Ann Intern Med 2007;147:386–99.
35. Bolen S, Tseng E, Hutfless S, et al. Oral diabetes medications for adults with type 2 diabetes: an update. Agency for Healthcare Research and Quality (US); 2011 Mar Report No: 11-EHC038-EF. AHRQ Comparative Effectiveness Reviews.
36. Metformin, glyburide, glipizide, glimeperide, sitagliptin, saxagliptin, linagliptin, lixisenatide, alogliptin, exenatide, liraglutide, albiglutide, dulaglutide, canagliflozin, danagliflozin, empagliflozin: drug information. Waltham (MA): UpToDate, Inc.; 2016. Accessed September 23, 2016.
37. GoodRx Web site. http://www.goodrx.com. Accessed August 6, 2016 and December 2016.
38. Insulins available in the United States. Diabetesforcast Web site. Accessed August 6, 2016. www.diabetesforecast.org/2016/mar-apr/images/2016-insulin-chart-new.pdf.
39. Inzucchi SE, Bergenstal RM, Buse JB, et al. Management of hyperglycaemia in type 2 diabetes: a patient-centered approach. Position statement of the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD). Diabetologia 2012;55:577–96.
40. Elashoff M, Matveyenko AV, Gier B, et al. Pancreatitis, pancreatic, and thyroid cancer with glucagon-like peptide-1-based therapies. Gastroenterology 2011;141:150–6.
41. Butler AE, Campbell-Thompson M, Gurlo T, et al. Marked expansion of exocrine and endocrine pancreas with incretin therapy in humans with increased exocrine pancreas dysplasia and the potential for glucagon-producing neuroendocrine tumors. Diabetes 2013;62:2595–604.
42. Green JB, Bethel MA, Armstrong PW, et al. Effect of sitagliptin on cardiovascular outcomes in type 2 diabetes. N Engl J Med 2015;373:232–42.
43. White WB, Cannon CP, Heller SR, et al. Alogliptin after acute coronary syndrome in patients with type 2 diabetes. N Engl J Med 2013;369:1327–35.
44. Scirica BM, Bhatt DL, Braunwald E, et al. Saxagliptin and cardiovascular outcomes in patients with type 2 diabetes mellitus. N Engl J Med 2013;369:1317–26.
45. Egan AG, Blind E, Dunder K, et al. Pancreatic safety of incretin-based drugs--FDA and EMA assessment. N Engl J Med 2014;370:794–7.
46. Vasilakou D, Karagiannis T, Athanasiadou E, et al. Sodium-glucose cotransporter 2 inhibitors for type 2 diabetes: a systematic review and meta-analysis. Ann Intern Med 2013;159:262–74.
47. Nyirjesy P, Sobel JD, Fung A, et al. Genital mycotic infections with canagliflozin, a sodium glucose co-transporter 2 inhibitor, in patients with type 2 diabetes mellitus: a pooled analysis of clinical studies. Curr Med Res Opin 2014;30:1109–19.
48. Schernthaner G, Gross JL, Rosenstock J, et al. Canagliflozin compared with sitagliptin for patients with type 2 diabetes who do not have adequate glycemic control with metformin plus sulfonylurea: a 52-week randomized trial. Diabetes Care 2013;36:2508–15.
49. Blonde L, Dailey G, Jabbour S, et al. Gastrointestinal tolerability of extended-release metformin tablets compared to immediate-release metformin tablets: results of a retrospective cohort study. Curr Med Res Opin 2004;20:562–72.
50. Kalra S, Mukherjee JJ, Venkataraman S, et al. Hypoglycemia: the neglected complication. Indian J Endocrinol Metab 2013;17:819–34.
51. Paty BW. The role of hypoglycemia in cardiovascular outcomes in diabetes. Can J Diabetes 2015;39 Suppl 5:S155–9.
52. Zoungas S, Patel A, Chalmers J, et al. Severe hypoglycemia and risks of vascular events and death. N Engl J Med 2010;363:1410–8.
53. Whitmer RA, Karter AJ, Yaffe K, et al. Hypoglycemic episodes and risk of dementia in older patients with type 2 diabetes mellitus. JAMA 2009;301:1565–72.
54. McCoy RG, Van Houten HK, Ziegenfuss JY, et al. Increased mortality of patients with diabetes reporting severe hypoglycemia. Diabetes Care 2012;35:1897–901.
55. McCoy RG, Lipska KJ, Yao X, et al. Intensive treatment and severe hypoglycemia among adults with type 2 diabetes. JAMA Intern Med 2016;176:969–78.
56. Rodbard HW, Gough S, Lane W, et al. Reduced risk of hypoglycemia with insulin degludec versus insulin glargine in patients with type 2 diabetes requiring high doses of basal insulin: a meta-analysis of 5 randomized begin trials. Endocr Pract 2014;20:285–92.
57. Yki-Jarvinen H, Bergenstal R, Ziemen M, et al. New insulin glargine 300 units/mL versus glargine 100 units/mL in people with type 2 diabetes using oral agents and basal insulin: glucose control and hypoglycemia in a 6-month randomized controlled trial (EDITION 2). Diabetes Care 2014;37:3235–43.
58. Tuomilehto J, Lindstrom J, Eriksson JG, et al. Prevention of type 2 diabetes mellitus by changes in lifestyle among subjects with impaired glucose tolerance. N Engl J Med 2001;344:1343–50.
59. Knowler WC, Barrett-Connor E, Fowler SE, et al. Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin. N Engl J Med 2002;346:393–403.
60. Maruthur NM, Tseng E, Hutfless S, et al. Diabetes medications as monotherapy or metformin-based combination therapy for type 2 diabetes: a systematic review and meta-analysis. Ann Intern Med 2016;164:740–51.
61. Diabetes Prevention Program Research Group. Long-term safety, tolerability, and weight loss associated with metformin in the Diabetes Prevention Program Outcomes Study. Diabetes Care 2012;35:731–7.
62. Clar C, Gill JA, Court R, Waugh N. Systematic review of SGLT2 receptor inhibitors in dual or triple therapy in type 2 diabetes. BMJ Open 2012;2:10.1136/bmjopen,2012-001007.
63. Vilsboll T, Christensen M, Junker AE, et al. Effects of glucagon-like peptide-1 receptor agonists on weight loss: systematic review and meta-analyses of randomised controlled trials. BMJ 2012;344:d7771.
64. FDA approves weight-management drug Saxenda. FDA Web site www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm427913.htm. Accessed September 22, 2016.
65. Apovian CM, Aronne LJ, Bessesen DH, et al. Pharmacological management of obesity: an endocrine Society clinical practice guideline. J Clin Endocrinol Metab 2015;100:342–62.
66. Liraglutide, lorcaserin, naltrexone/bupropion, orlistat, phentermine/topiramate: drug information. Greenwood Village (CO): Truven Health Analytics; 2016. www.micromedexsolutions.com. Accessed May 13, 2016.
67. Liraglutide, lorcaserin, naltrexone/bupropion, orlistat, phentermine/topiramate: drug information. Waltham (MA): UpToDate, Inc.; 2016. Accessed May 13, 2016.
68. Yanovski SZ, Yanovski JA. Long-term drug treatment for obesity: a systematic and clinical review. JAMA 2014;311:74–86.
69. Sjostrom L, Peltonen M, Jacobson P, et al. Association of bariatric surgery with long-term remission of type 2 diabetes and with microvascular and macrovascular complications. JAMA 2014;311:2297–304.
70. Schauer PR, Bhatt DL, Kirwan JP, et al. Bariatric surgery versus intensive medical therapy for diabetes--3-year outcomes. N Engl J Med 2014;370:2002–13.
71. Rubino F, Nathan DM, Eckel RH, et al. Metabolic surgery in the treatment algorithm for type 2 diabetes: a joint statement by international diabetes organizations. Diabetes Care 2016;39:861–77.
72. Lathief S, Inzucchi SE. Approach to diabetes management in patients with CVD. Trends Cardiovasc Med 2016;26:165–79.
73. Nissen SE, Wolski K. Effect of rosiglitazone on the risk of myocardial infarction and death from cardiovascular causes. N Engl J Med 2007;356:2457–71.
74. Knatterud GL, Klimt CR, Levin ME, et al. Effects of hypoglycemic agents on vascular complications in patients with adult-onset diabetes. VII. Mortality and selected nonfatal events with insulin treatment. JAMA 1978;240:37–42.
75. Masoudi FA, Inzucchi SE, Wang Y, et al. Thiazolidinediones, metformin, and outcomes in older patients with diabetes and heart failure: an observational study. Circulation 2005;111:583–90.
76. Klepzig H, Kober G, Matter C, et al. Sulfonylureas and ischaemic preconditioning; a double-blind, placebo-controlled evaluation of glimepiride and glibenclamide. Eur Heart J 1999;20:439–46.
77. FDA announces new recommendations on evaluating cardiovascular risk in drugs intended to treat type 2 diabetes. FDA Web site. www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/2008/ucm116994.htm. Accessed August 20, 2016.
78. Kooy A, de Jager J, Lehert P, et al. Long-term effects of metformin on metabolism and microvascular and macrovascular disease in patients with type 2 diabetes mellitus. Arch Intern Med 2009;169:616–25.
79. Eurich DT, Weir DL, Majumdar SR, et al. Comparative safety and effectiveness of metformin in patients with diabetes mellitus and heart failure: systematic review of observational studies involving 34,000 patients. Circ Heart Fail 2013;6:395–402.
80. Tahrani AA, Varughese GI, Scarpello JH, Hanna FW. Metformin, heart failure, and lactic acidosis: is metformin absolutely contraindicated? BMJ 2007;335:508–12.
81. Li Y, Hu Y, Ley SH, et al. Sulfonylurea use and incident cardiovascular disease among patients with type 2 diabetes: prospective cohort study among women. Diabetes Care 2014;37:3106–13.
82. Bentley-Lewis R, Aguilar D, Riddle MC, et al. Rationale, design, and baseline characteristics in Evaluation of LIXisenatide in Acute Coronary Syndrome, a long-term cardiovascular end point trial of lixisenatide versus placebo. Am Heart J 2015;169:631,638.e7.
83. Marso SP, Daniels GH, Brown-Frandsen K, et al. Liraglutide and cardiovascular outcomes in type 2 diabetes. N Engl J Med 2016;375:311–22.
84. Exenatide Study of Cardiovascular Event Lowering Trial (EXSCEL): A Trial To Evaluate Cardiovascular Outcomes After Treatment With Exenatide Once Weekly In Patients With Type 2 Diabetes Mellitus. clinicaltrials.gov Web site. https://clinicaltrials.gov/ct2/show/NCT01144338. 2016 Accessed September 23, 2016.
85. Researching Cardiovascular Events With a Weekly Incretin in Diabetes (REWIND). clinicaltrials.gov Web site. https://clinicaltrials.gov/ct2/show/NCT01394952. Accessed September 23, 2016.
86. Schernthaner G, Schernthaner-Reiter MH, Schernthaner GH. EMPA-REG and other cardiovascular outcome trials of glucose-lowering agents: implications for future treatment strategies in type 2 diabetes mellitus. Clin Ther 2016;38:1288–98.
87. CANVAS--CANagliflozin cardiovascular Assesssment Study (CANVAS). clinicaltrials.gov Web site. https://clinicaltrials.gov/ct2/show/NCT01032629. Accessed September 23, 2016.
88. Evaluation of the Effects of Canagliflozin on Renal and Cardiovascular Outcomes in Participants With Diabetic Nephropathy (CREDENCE). clinicaltrials.gov Web site. https://clinicaltrials.gov/ct2/show/NCT02065791. Accessed September 23, 2016.
89. Multicenter Trial to Evaluate the Effect of Dapagliflozin on the Incidence of Cardiovascular Events (DECLARE-TIMI58). clinicaltrials.gov Web site. https://clinicaltrials.gov/ct2/show/NCT01730534. Accessed September 23, 2016.
90. Zannad F, Cannon CP, Cushman WC, et al. Heart failure and mortality outcomes in patients with type 2 diabetes taking alogliptin versus placebo in EXAMINE: a multicentre, randomised, double-blind trial. Lancet 2015;385(9982):2067–76.
91. Van Klompenburg E, Heins JR. New insulin options for diabetic patients. S D Med 2016;69:84–5.
92. Rosenstock J, Schwartz SL, Clark CM Jr, et al. Basal insulin therapy in type 2 diabetes: 28-week comparison of insulin glargine (HOE 901) and NPH insulin. Diabetes Care 2001;24:631–6.
93. Tylee T, Hirsch IB. Costs associated with using different insulin preparations. JAMA 2015;314:665–6.
94. Hua X, Carvalho N, Tew M, et al. Expenditures and prices of antihyperglycemic medications in the United States: 2002-2013. JAMA 2016;315:1400–2.
95. Heinemann L. Biosimilar insulin and costs: what can we expect? J Diabetes Sci Technol 2016;10:457–62.
96. Horvath K, Jeitler K, Berghold A, et al. Long-acting insulin analogues versus NPH insulin (human isophane insulin) for type 2 diabetes mellitus. Cochrane Database Syst Rev 2007;(2)(2):CD005613.
97. Mannucci E, Monami M, Marchionni N. Short-acting insulin analogues vs. regular human insulin in type 2 diabetes: a meta-analysis. Diabetes Obes Metab 2009;11:53–9.
98. Powell PW, Corathers SD, Raymond J, Streisand R. New approaches to providing individualized diabetes care in the 21st century. Curr Diabetes Rev 2015;11:222–30.
99. Riddle MC, Rosenstock J, Gerich J, Insulin Glargine 4002 Study Investigators. The treat-to-target trial: randomized addition of glargine or human NPH insulin to oral therapy of type 2 diabetic patients. Diabetes Care 2003;26:3080–6.
From the University of Arizona College of Pharmacy and the University of Arizona College of Medicine-Tucson, Tucson, AZ.
Abstract
- Objective: To summarize key issues relevant to managing hyperglycemia in patients with type 2 diabetes mellitus (T2DM) and review a strategy for initiating and intensifying therapy.
- Methods: Review of the literature.
- Results: The 6 most widely used pharmacologic treatment options for hyperglycemia in T2DM are metformin, sulfonylureas, dipeptidyl peptidase-4 inhibitors, glucagon-like peptide-1 receptor agonists, sodium-glucose cotransporter-2 inhibitors, and insulin. Recent guidelines stress the importance of an individualized, patient-centered approach to managing hyperglycemia in T2DM, although sufficient guidance for nonspecialists on how to individualize treatment is often lacking. For patients with no contraindications, metformin should be recommended concurrent with lifestyle intervention at the time of diabetes diagnosis. Due to the progressive nature of T2DM, glycemic control on metformin monotherapy is likely to deteriorate over time, and there is no consensus as to what the second-line agent should be. A second agent should be selected based on glycemic goal and potential advantages and disadvantages of each agent for any given patient. If the patient progresses to the point where dual therapy does not provide adequate control, either a third non-insulin agent or insulin can be added.
- Conclusion: Although research is increasingly focusing on what the ideal number and sequence of drugs should be when managing T2DM, investigating all possible combinations in diverse patient populations is not feasible. Physicians therefore must continue to rely on clinical judgment to determine how to apply trial data to the treatment of individual patients.
Key words: type 2 diabetes; patient-centered care; antihyper-glycemic drugs; insulin; therapeutic decision-making.
Diabetes mellitus affects approximately 29.1 million people, or 9.3% of the U.S. population [1,2]. The high prevalence of diabetes and its associated multiple complications, including cardiovascular disease (CVD), blindness, renal failure, lower extremity amputations, and premature death, lead to a tremendous overall burden of disease. The financial cost is staggering as well, with more than 1 in 5 health care dollars spent on treating diabetes or its complications [3]. The goal of diabetes treatment is to prevent acute complications and reduce the risk of long-term complications. Interventions that have been shown to improve diabetes outcomes include medications for glycemic control and treatment of cardiovascular risk factors, nutrition and physical activity counseling, smoking cessation, immunizations, psychosocial care, and ongoing surveillance and early treatment for eye, kidney, and foot problems [4].
Glycemic management in type 2 diabetes mellitus (T2DM), the focus of this review, is growing increasingly complex and has been the subject of numerous extensive reviews [5,6] and published guidelines [4,7]. In the context of an increasing array of available pharmacologic options, there are mounting uncertainties regarding the benefits of intensive glycemic control as well as increasing concerns about potential adverse treatment effects, hypoglycemia in particular. While previous guidelines encouraged specific approaches for most patients, more recent guidelines stress the importance of a patient-centered approach with shared decision-making [4]. Less prescriptive guidelines are more appropriate, given the current state of science, but they also may be viewed as providing insufficient guidance to some providers. It can be overwhelming for a non-specialist to try to match the nuances of antihyperglycemic medications to the nuances of each patient’s preferences and medical characteristics.
This article examines key issues faced by primary care providers when managing hyperglycemia in patients with T2DM and outlines a stepwise approach to determining the optimal antihyperglycemic agent(s) (Table 1).
Confirm Diagnosis of T2DM
It can be difficult to distinguish between type 1 diabetes mellitus and T2DM in some individuals due to overlapping characteristics. However, correctly classifying a patient’s diabetes at the outset is essential, as the classification helps determine the best treatment regimen and is rarely reconsidered [4,8]. Considerable evidence suggests that misclassification of diabetes occurs frequently [9,10], resulting in patients receiving inappropriate treatment. Clinical characteristics suggestive of T2DM include older age and features of insulin resistance such as obesity, hyper-tension, hypertriglyceridemia, and low high-density lipoprotein cholesterol. When these features are not present, an alternate diagnosis should be entertained.
Establish Glycemic Goal
Research over the past decade has led to a growing appreciation of the enormous complexity of hyperglycemia management. During the 1990s, landmark trials such as the Diabetes Control and Complications Trial (DCCT) [11] and UK Prospective Diabetes Study (UKPDS) [12] demonstrated that improving glucose control could reduce the incidence of microvascular complications [11,12], prompting a lower-is-better philosophy regarding glucose targets. Despite limited evidence to support such thinking, this viewpoint was adopted by the developers of many guidelines. During the following decade more research was devoted to determining whether aggressively lowering a patient’s glucose could also improve macrovascular outcomes. Table 2 summarizes microvascular and macrovascular effects of intensive glycemic control seen in major trials [11–23]. After several major trials [20,22] found only mild cardiovascular benefits and even suggested harm [18], experts and policy makers began to reconsider the value of tightly controlling glucose levels [24]. Since then, other studies have demonstrated that the potential benefits and risks of glucose control are strongly related to individual patient factors, such as age and duration of diabetes, and associated comorbidities, such as CVD and impaired renal function [6].
A one-size-fits-all glycemic goal is no longer recommended. Personalization is necessary, balancing the potential benefits and risks of treatments required to achieve that goal. Whereas an A1C of < 7% is an appropriate target for some individuals with diabetes, glycemic targets may be more or less stringent based on patient features including life expectancy, duration of diabetes, comorbidities, and patient attitude and support system (Table 3) [4].
A particular group in which less stringent goals should be considered is older patients, especially those with complex or poor health status [4,25]. The risk of intensive glycemic control may exceed the benefits in these patients, as they are at higher risk of hypoglycemia and polypharmacy [26]. A goal A1C of 7% to 7.5% is now recommended for healthy older adults, and less stringent A1C goals of 7.5% to 8% and 8% to 8.5% should be considered based on the presence and severity of multiple coexisting chronic illnesses, decreased self-care ability, or cognitive impairment [4,25]. Unfortunately, overtreatment is frequently seen in this group. In a recent study of patients over age 65 years, about 40% of those with complex or poor health status had tight glycemic control with A1C below 6.5% [26]. An analysis of U.S. Veterans Affairs administration data showed that only 27% of 12,917 patients older than 65 with very low A1C (< 6%) and about 21% of those with A1C of 6% to 6.5% underwent treatment deintensification [27].
Initiate Treatment with Metformin
There is strong consensus that metformin is the preferred drug for monotherapy due to its long proven safety record, low cost, weight-reduction benefit, and potential cardiovascular advantages [4,16]. As long as there are no contraindications, metformin should be recommended concurrent with lifestyle intervention at the time of diabetes diagnosis. The recommendation is based on the fact that adherence to diet, weight reduction, and regular exercise is not sustained in most patients, and most patients ultimately will require treatment. Since metformin is usually well-tolerated, does not cause hypoglycemia, has a favorable effect on body weight, and is relatively inexpensive, potential benefits of early initiation of medication appear to outweigh potential risks.
The U.S. Food and Drug Administration (FDA) recently relaxed prescribing polices to extend the use of this important medication to patients who have mild–moderate, but stable, chronic kidney disease (CKD) [28]. Metformin is recommended as first-line therapy and should be used unless it is contraindicated (ie, estimated glomerular filtration rate [eGFR] < 30 mL/min/1.73 m2)[4,7,29].
Add Additional Agent(s) as Needed to Achieve Goal
Other than metformin, evidence is limited for the optimal use of the burgeoning array of available agents, especially in dual or triple combinations [6,30]. Research is now starting to focus more on what the ideal number and sequence of drugs should be. The Glycemic Reduction Approach in Diabetes (GRADE) study, which will compare long-term benefits and risks of the 4 most widely used antihyperglycemic medications in combination with metformin, is now underway [31,32]. The 4 classes being studied are sulfonylurea, dipeptidyl peptidase-4 (DPP-4) inhibitors, glucagon-like peptide-1 (GLP-1) receptor agonists, and a basal,
Eleven classes of non-insulin medications are currently approved for treating hyperglycemia in T2DM [4]. Within each class, numerous agents are available. Six of these classes (ie, α-glucosidase inhibitors, colesevelam, bromocriptine, pramlintide, meglitinides, and thiazolidinediones) are not used frequently
Consider Effects on A1C
There is a paucity of high-quality, head-to-head comparison trials evaluating the ability of available agents to achieve recommended glycemic targets. This is important because the glucose-lowering effectiveness of individual medications is strongly influenced by baseline characteristics such as A1C, duration of diabetes, and previous therapy. With these limitations in mind, the relative glucose-lowering effectiveness of commonly used agents is shown in Table 4. When used as monotherapy, A1C reductions of approximately 1% to 1.5% are achieved with metformin, sulfonylureas, and GLP-1 receptor agonists [6,30,34,35,39]. DPP-4 inhibitors and SGLT-2 inhibitors have more modest glucose-lowering efficacy, with A1C reductions of approximately 0.5% to 1% [6,30,34,35,39]. Larger effects may be seen in individuals with higher baseline A1C and those who are drug naïve. Insulin is the most effective glucose-lowering agent—it can reduce virtually any level of A1C down to the normal range, with hypoglycemia being the only limiting factor. When a patient has uncontrolled hyperglycemia on metformin monotherapy, or if there is a contraindication or intolerance to metformin, clinicians should consider the potential glucose-lowering effects of other available options and should choose an agent that conceivably could bring a patient close to meeting their treatment goal.
Eliminate Options with Unacceptable Adverse Effects
When the pharmacologic options with acceptable A1C-lowering potential have been identified, the ones with contraindications and potential serious adverse effects for the individual patient can immediately be eliminated (Table 4). For example, if a patient has an eGFR < 30 mL/min/1.73 m2, metformin, sulfonylureas, GLP-1 receptor agonists, most DPP-4 inhibitors, and SGLT-2 inhibitors are either contraindicated or should be used with caution. In patients with severe osteoporosis, SGLT-2 inhibitors may not be the best option. In patients with a history of diabetic ketoacidosis (DKA), caution should be used with metformin and SGLT-2 inhibitors. There have been concerns of possible acute pancreatitis and neoplasia with the incretin-based agents, the DPP-4 inhibitors and GLP-1 receptor agonists [40,41], although other clinical trials and observational data have not found increased risk [42–45]. Nevertheless, these agents potentially should be avoided in patients with a history of pancreatitis or neoplasm. SGLT-2 inhibitors may be associated with genitourinary infections and volume depletion [46–48] and probably should be avoided in patients at high risk for these conditions.
If the adverse effects are not serious, changing the way the medication is administered may allow the patient to tolerate agents with high potential benefits. For example, metformin is commonly associated with gastrointestinal (GI) adverse effects, which can be reduced or avoided with slow titration of the dose [6] or by switching to an extended-release formulation [49]. GLP-1 receptor agonists are associated with GI adverse effects [6] and in most cases slow titration is recommended.
Evaluate Potential Risks/Benefits of Remaining Options
Hypoglycemia. The barrier of hypoglycemia generally precludes maintenance of euglycemia and full realization of the long-term benefits of good glucose control over a lifetime. Once considered a trivial issue, concerns about hypoglycemia in T2DM are increasingly being raised [19,50–55]. Clearly, hypoglycemia occurs more often as glycemic targets are lowered to near-normal values, especially in those with advanced age and multiple comorbidities [55]. Various comorbidities frequently encountered particularly as patients age also are associated with increasing propensity for experiencing hypoglycemia and untoward outcomes from it. These include coronary artery disease, heart failure, renal and liver disease, and dementia. Hypoglycemia, when it occurs, may lead to dysrhythmias, dizziness, accidents and falls, work disability, and decreased quality of life. In addition to relaxing blood glucose targets in high-risk patients, drug selection should favor agents that do not precipitate such events (Table 4).
Fortunately, the commonly used non-insulin agents are not associated with hypoglycemia unless they are used in combination with sulfonylureas or insulin. Sulfonylureas should be used with caution and other options considered in patients with high risk for hypoglycemia. When insulin is required, regimens which minimize risk of hypoglycemia should be used. For example, adding a GLP-1 receptor agonist to basal insulin as an alternative to mealtime insulin has been shown to be equally effective with a lower risk of hypoglycemia [4,6]. Also, premixed insulin preparations should be avoided or used cautiously in individuals who miss meals frequently. Additionally, newer basal insulins that exhibit longer duration of action are now available in the United States. Preliminary studies have shown that the newly FDA-approved longer-acting basal insulins, insulin degludec and glargine U-300, may be associated with a reduced risk for hypoglycemia [56,57]. However, it remains unclear how and when these newer agents will best be incorporated into a treatment regimen.
Body weight. Nearly 90% of people living with T2DM are overweight or obese. Given the close tie between obesity and T2DM, treating obesity is an obvious consideration in diabetes treatment. Major trials have shown the effectiveness of lifestyle modifications and weight reduction in delaying, prevention, and management of T2DM [4,58,59].With this in mind, clinicians should consider preferentially using antihyperglycemic agents with weight-lowering or weight-neutral effects. Among commonly used antihyperglycemic agents, metformin, GLP-1 receptor agonists, and SGLT-2 inhibitors have been shown to have weight-reduction benefits, and DPP-4 inhibitors are weight neutral. On the other hand, sulfonylureas and insulin are associated with weight gain. A systematic review and meta-analysis including 204 studies with study durations ranging from 3 months to 8 years showed comparative effects of diabetes medications with a differential effect on weight of up to 5 kg (Table 4) [60].
Metformin is associated with an average weight loss of 1.9 to 3.1 kg that was sustained with long-term use for at least 10 years in the Diabetes Prevention Program Outcomes Study [61].A systematic review of 7 randomized trials showed that in patients with T2DM, the SGLT-2 inhibitors dapagliflozin and canagliflozin were associated with weight loss (mean weighted difference of –1.81 kg and –2.3 kg, respectively) [62]. A systematic review and meta-analysis of 25 randomized controlled trials showed greater weight loss (mean weighted difference of –2.9 kg) in overweight or obese patients with or without T2DM using GLP-1 receptor agonists when compared to placebo, insulin, or oral antihyperglycemic agents [63]. Of note, the GLP-1 receptor agonist liraglutide is now approved for weight loss in patients with or without diabetes [64]. The maximum doses approved for diabetes and obesity treatment are 1.8 and 3.0 mg/day, respectively.
Since weight loss is associated with improved glycemic control, an area of emerging interest is the use of antiobesity medications for managing diabetes. Although most older weight-loss medications were only approved for short-term use, some newer agents are approved for longer-term use. Lorcaserin and the combination drugs topiramate/phentermine and naltrexone/bupropion are approved for chronic therapy, provided certain conditions are met. Patients on weight reduction agents should be monitored regularly.
An even more radical departure from conventional therapy for diabetes is the consideration of metabolic, or weight-loss, surgery, which has been found to be associated with rapid and dramatic improvements in blood glucose control. Metabolic surgery has been shown to improve glucose control more effectively than any known pharmaceutical or behavioral approach. For example, in an observational study of obese patients with T2DM, bariatric surgery led to diabetes remission rates of 72.3% 2 years after surgery and 30.4% 15 years after surgery compared to 16.4% and 6.5%, respectively, in control patients [69]. With long-term follow-up, significant decreases in microvascular and macrovascular complications were seen in the surgical group [69]. Compared with medical therapy alone, bariatric surgery plus medical therapy has been associated with more weight loss, better glycemic control, less need for diabetes medications, and improved quality of life [70]. A 2016 joint statement by numerous international diabetes organizations recommends considering metabolic surgery as a treatment for T2DM and obesity [71]. American Diabetes Association guidelines recommend consideration of bariatric surgery in individuals with T2DM who have a body mass index greater than 35 kg/m2,especially if achieving disease control is difficult by means of lifestyle modifications and medications [4].
Cardiovascular outcomes. Cardiovascular risk is about 2 to 4 times higher in patients with diabetes, and about half of patients with this condition develop heart failure [4,72]. CVD is responsible for most of the mortality in T2DM [72]. Therefore, prevention of cardiovascular morbidity and mortality is an important goal for diabetes treatment. Due to concerns about potential cardiovascular risks associated with glucose-lowering medications [73–76], the FDA has issued regulatory requirements for manufacturers to monitor the cardiovascular risk profile for these drugs [77]. Recent trials have led to a better understanding of potential cardiovascular benefits or harms of antihyperglycemic medications.
Metformin, the widely recommended first-line therapy for T2DM, carries a large body of evidence supporting its cardiovascular benefits. For example, the UKPDS found that compared to conventional therapy (mostly diet), metformin reduced cardiovascular events and mortality in obese patients with T2DM [15]. This result was supported in Hyperinsulinemia: the Outcome of its Metabolic Effect (HOME) study where, as an add-on to insulin, metformin decreased macrovascular complications when compared to placebo [78]. Research over the past decade also has assuaged concerns about metformin safety in heart failure [60]. A systematic review of observational studies involving 34,000 patients conducted in 2013 showed that metformin is as safe as other glucose-lowering medications in patients with diabetes and heart failure even in the presence of CKD [4,79]. Furthermore, numerous investigations have found metformin is not associated with increased hospitalizations or risk of lactic acidosis [80]. Metformin can be used safely in patients with diabetes and heart failure [60].
Although sulfonylureas have long been a mainstay of diabetes therapy, concerns about their potential adverse cardiovascular effects have been raised by numerous studies [81]. Tolbutamide, a first-generation sulfonylurea, was removed from the market after the University Group Diabetes Program study found increased CVD deaths with this agent versus placebo. Subsequently, the FDA issued a warning for all sulfonylureas [74]. The increased cardiovascular risk associated with sulfonylureas is thought to be due to their effect on cardiac mitochondrial potassium ATP channels. Sulfonylureas bind to these channels, preventing a protective phenomenon called ischemic preconditioning and resulting in a weakened defense against myocardial injury [76]. A recent study showed an increased risk of coronary heart disease associated with long-term use of sulfonylureas in women with diabetes [81].
GLP-1 receptor agonists have recently received much attention for their potential beneficial effects on cardiovascular outcomes. In a recent trial, lixisenatide was shown to be safe in patients with T2DM and acute coronary syndrome when compared to placebo [82]. More recently, the Liraglutide Effect and Action in Diabetes: Evaluation of cardiovascular outcome Results (LEADER) trial demonstrated significant cardiovascular benefits with liraglutide in patients with T2DM and established or high CVD risk [83]. The composite outcome of the first occurrence of death from cardiovascular causes, nonfatal myocardial infarction (MI), or nonfatal stroke, occurred less frequently in the liraglutide group compared to placebo (13% versus 14.9%, respectively), and there were fewer deaths from cardiovascular causes in the liraglutide group compared to placebo (4.7% and 6.0%, respectively) [83]. Other trials investigating the cardiovascular outcomes of this class [84,85] are in progress.
Another class with potential cardiovascular benefits is the SGLT-2 inhibitors. In a recent cardiovascular outcome study, empagliflozin significantly lowered the composite of cardiovascular death, nonfatal MI, or nonfatal stroke in T2DM patients with high cardiovascular risk compared to placebo (10.5% and 12.1%, respectively) [86]. There are several large ongoing studies evaluating the cardiovascular effects of other SGLT-2 inhibitors [87–89].
DPP-4 inhibitors were examined in recent studies and have shown no cardiovascular benefits [42,44,90].The studies showed mixed results regarding an association between DPP-4 inhibitors and heart failure. In one study, saxagliptin was associated with increased hospitalization for heart failure compared to placebo [44], while 2 noninferiority trials did not show a significant increase in heart failure hospitalizations associated with alogliptin and sitagliptin when compared to placebo [42,90].
Administration Considerations
Many patients with T2DM require multiple agents for glycemic control. Additional medications used for comorbid conditions add to this burden. When choosing antihyperglycemic agents, the route and frequency of administration, as well as the patients’ preferences and ability, should be considered. Either once or twice daily dosing is available for most agents, and once weekly dosing is available for some of the GLP-1 receptor agonists. Once daily or once weekly formulations may improve adherence and be more desirable than preparations that are dosed twice daily. Most of the commonly used medications are dosed orally. Although many patients find this route of administration preferable to insulin or GLP-1 receptor agonists, which require injections, some patients may prefer the risk/benefit of injectable agents. All GLP-1 receptor agonists come in a pen delivery system, which eliminates mixing and provides more convenient administration. Extended-release exenatide also is available as a single-dose tray that requires mixing and may be more cumbersome to inject.
Insulin requires special consideration. There has been an enormous increase in the number of insulin products on the market in the past 2 decades. These products include insulin analogs, concentrated insulins (U-200, U-300, and U-500), premixed insulin preparations, and ultra-long-acting insulin [91]. The availability of insulin options with different concentrations, onsets, and durations of actions has made decision making on which insulin to use difficult. Clinicians need to consider patient preference, dosing frequency, and timing with regard to meals, insulin dose, administration, as well as cost. For example, concentrated insulin is preferred for a patient on high doses of insulin requiring injecting a large volume of insulin. Rapid-acting insulin analogs would be more appropriate for patients who have difficulty administering their regular insulin 20 to 30 minutes before eating. Premixed insulin preparations make it impossible to independently adjust short- and long-acting components. However, these may be good choices in patients who have consistent meal schedules and who want to simplify administration. Despite a prevailing misconception that NPH must be given twice a day, it has long been recognized that in T2DM, a single daily injection of NPH yields improvements in control similar to those achieved with 2 daily injections [92].
Cost Considerations
Treating T2DM imposes a great financial burden on individuals living with diabetes and their families due to the high cost of the medications. Table 4 and Table 5 provide information on the cost of non-insulin and insulin diabetes medications for patients who do not have prescription insurance coverage. From a practical standpoint, choice of diabetes agents is largely influenced by insurance formularies.
The older agents, metformin and the sulfonylureas, are available for a cash (no insurance) price of as little as $4 per month. This is in stark contrast to the SGLT-2 inhibitors, GLP-1 receptor agonists, and DPP-4 inhibitors, which range in cost between $400 and $600 per month. Of recent concern, the cost of insulin has been skyrocketing, with a more than 500% increase in the cost of certain insulins from 2001 to 2015 [93]. According to the Medical Expenditure Panel Survey (MEPS) from 2002 to 2013, the mean price of insulin increased by about 200% (from $4.34/mL to $12.92/mL) during this period, which was significantly higher than increases in the price of non-insulin comparators [94]. The introduction of biosimilar insulins to the market is expected to offer treatment options with lower cost. This will be tested when the biosimilar glargine, the first FDA-approved biosimilar insulin, becomes available in the U.S. market. However, a significant reduction in insulin prices is not expected soon [95].
When insulin is required, most patients with T2DM can be treated with older human insulins, which have similar efficacy and lower costs than the more expensive newer insulin analogs. A Cochrane review comparing basal insulin analogs to NPH showed similar efficacy in glycemic control with minimal clinical benefit in the form of less nocturnal hypoglycemia in the insulin analog arm [96]. Furthermore, similar glycemic control and risk of hypoglycemia was seen when regular insulin was compared with the rapid-acting insulin analogs [97]. The cost of human NPH insulin for a patient on a total daily dose of 60 units is approximately $52 per month. This contrasts with the most widely used insulin, insulin glargine, which has a cash price of about $500 per month for the same amount (Table 5). Insulin pens, which are convenient, are more expensive. Interestingly, human insulins do not require prescriptions, allowing underinsured, underfunded patients ongoing access to them.
Incorporating Patient Preferences
Research evidence is necessary but insufficient for making patient care decisions. Along with the potential benefits, harms, costs, and inconveniences of the management options, patient perspectives, beliefs, expectations, and health-related goals must be considered. Patients will undoubtedly have preferences regarding defining goals and ranking options. Clinicians should discuss therapeutic goals and treatment options and work collaboratively with patients in determining management strategies [98].
Summary
Potential treatment approaches for treating hyperglycemia in T2DM are summarized in Figure 1 and Figure 2 [4,7]. As long as there are no contraindications, metformin should be recommended concurrent with lifestyle intervention at the time of diabetes diagnosis. Even if metformin monotherapy is initially effective, glycemic control is likely to deteriorate over time due to progressive loss of β-cell function in T2DM.
There is no consensus as to what the second-line agent should be. Selection of a second agent should be made based on potential advantages and disadvantages of each agent for any given patient. A patient-centered approach is preferred over a fixed algorithm. If the patient progresses to the point where dual therapy does not provide adequate control, either a third non-insulin agent or insulin can be added. In patients with modestly elevated A1C (below ~8%), addition of a third non-insulin agent may be equally effective as (but more expensive than) addition of insulin.
Patients with significantly elevated A1C levels on non-insulin agents usually should have insulin added to their regimen. When insulin is added, metformin should be continued. DPP-4 inhibitors and sulfonylureas are typically stopped. If SGLT-2 inhibitors and/or GLP-1 receptor agonists are continued, this may aid with weight maintenance. However, continuing these agents is likely to be expensive and associated with problems associated with polypharmacy.
The most widely recommended strategy for initiating insulin in T2DM is to add a single bedtime injection of basal insulin (ie, NPH, glargine, detemir, or degludec) to the patient’s regimen. This regimen has been found to be effective in numerous studies and controls hyperglycemia in up to 60% of patients [99]. If the patient is treated with a single bedtime injection of insulin and the fasting glucose level is within the target range but the A1C level remains above goal, addition of mealtime insulin injections is likely to be beneficial. Alternatively, addition of a GLP-1 receptor agonist to basal insulin has been shown to be equally beneficial [4,6]. When adding mealtime insulin, a common strategy is to add a single injection of a rapid-acting insulin (eg, lispro, aspart, glulisine) before the patient’s largest meal of the day. Additional premeal injections of rapid-acting insulin may be added as needed, based on self-monitoring blood glucose results. If glycemia remains significantly uncontrolled on more than 200 units of insulin per day, switching to a concentrated form of insulin (eg, U-200, U-300, or U-500) should be considered.
Corresponding author: Maryam Fazel, PharmD, BCPS, BCACP, CDE, 1295 N. Martin Ave. (Room B211B), Tucson, Arizona 85721-0202, maryamfazel@pharmacy.arizona.edu.
Financial disclosures: None.
From the University of Arizona College of Pharmacy and the University of Arizona College of Medicine-Tucson, Tucson, AZ.
Abstract
- Objective: To summarize key issues relevant to managing hyperglycemia in patients with type 2 diabetes mellitus (T2DM) and review a strategy for initiating and intensifying therapy.
- Methods: Review of the literature.
- Results: The 6 most widely used pharmacologic treatment options for hyperglycemia in T2DM are metformin, sulfonylureas, dipeptidyl peptidase-4 inhibitors, glucagon-like peptide-1 receptor agonists, sodium-glucose cotransporter-2 inhibitors, and insulin. Recent guidelines stress the importance of an individualized, patient-centered approach to managing hyperglycemia in T2DM, although sufficient guidance for nonspecialists on how to individualize treatment is often lacking. For patients with no contraindications, metformin should be recommended concurrent with lifestyle intervention at the time of diabetes diagnosis. Due to the progressive nature of T2DM, glycemic control on metformin monotherapy is likely to deteriorate over time, and there is no consensus as to what the second-line agent should be. A second agent should be selected based on glycemic goal and potential advantages and disadvantages of each agent for any given patient. If the patient progresses to the point where dual therapy does not provide adequate control, either a third non-insulin agent or insulin can be added.
- Conclusion: Although research is increasingly focusing on what the ideal number and sequence of drugs should be when managing T2DM, investigating all possible combinations in diverse patient populations is not feasible. Physicians therefore must continue to rely on clinical judgment to determine how to apply trial data to the treatment of individual patients.
Key words: type 2 diabetes; patient-centered care; antihyper-glycemic drugs; insulin; therapeutic decision-making.
Diabetes mellitus affects approximately 29.1 million people, or 9.3% of the U.S. population [1,2]. The high prevalence of diabetes and its associated multiple complications, including cardiovascular disease (CVD), blindness, renal failure, lower extremity amputations, and premature death, lead to a tremendous overall burden of disease. The financial cost is staggering as well, with more than 1 in 5 health care dollars spent on treating diabetes or its complications [3]. The goal of diabetes treatment is to prevent acute complications and reduce the risk of long-term complications. Interventions that have been shown to improve diabetes outcomes include medications for glycemic control and treatment of cardiovascular risk factors, nutrition and physical activity counseling, smoking cessation, immunizations, psychosocial care, and ongoing surveillance and early treatment for eye, kidney, and foot problems [4].
Glycemic management in type 2 diabetes mellitus (T2DM), the focus of this review, is growing increasingly complex and has been the subject of numerous extensive reviews [5,6] and published guidelines [4,7]. In the context of an increasing array of available pharmacologic options, there are mounting uncertainties regarding the benefits of intensive glycemic control as well as increasing concerns about potential adverse treatment effects, hypoglycemia in particular. While previous guidelines encouraged specific approaches for most patients, more recent guidelines stress the importance of a patient-centered approach with shared decision-making [4]. Less prescriptive guidelines are more appropriate, given the current state of science, but they also may be viewed as providing insufficient guidance to some providers. It can be overwhelming for a non-specialist to try to match the nuances of antihyperglycemic medications to the nuances of each patient’s preferences and medical characteristics.
This article examines key issues faced by primary care providers when managing hyperglycemia in patients with T2DM and outlines a stepwise approach to determining the optimal antihyperglycemic agent(s) (Table 1).
Confirm Diagnosis of T2DM
It can be difficult to distinguish between type 1 diabetes mellitus and T2DM in some individuals due to overlapping characteristics. However, correctly classifying a patient’s diabetes at the outset is essential, as the classification helps determine the best treatment regimen and is rarely reconsidered [4,8]. Considerable evidence suggests that misclassification of diabetes occurs frequently [9,10], resulting in patients receiving inappropriate treatment. Clinical characteristics suggestive of T2DM include older age and features of insulin resistance such as obesity, hyper-tension, hypertriglyceridemia, and low high-density lipoprotein cholesterol. When these features are not present, an alternate diagnosis should be entertained.
Establish Glycemic Goal
Research over the past decade has led to a growing appreciation of the enormous complexity of hyperglycemia management. During the 1990s, landmark trials such as the Diabetes Control and Complications Trial (DCCT) [11] and UK Prospective Diabetes Study (UKPDS) [12] demonstrated that improving glucose control could reduce the incidence of microvascular complications [11,12], prompting a lower-is-better philosophy regarding glucose targets. Despite limited evidence to support such thinking, this viewpoint was adopted by the developers of many guidelines. During the following decade more research was devoted to determining whether aggressively lowering a patient’s glucose could also improve macrovascular outcomes. Table 2 summarizes microvascular and macrovascular effects of intensive glycemic control seen in major trials [11–23]. After several major trials [20,22] found only mild cardiovascular benefits and even suggested harm [18], experts and policy makers began to reconsider the value of tightly controlling glucose levels [24]. Since then, other studies have demonstrated that the potential benefits and risks of glucose control are strongly related to individual patient factors, such as age and duration of diabetes, and associated comorbidities, such as CVD and impaired renal function [6].
A one-size-fits-all glycemic goal is no longer recommended. Personalization is necessary, balancing the potential benefits and risks of treatments required to achieve that goal. Whereas an A1C of < 7% is an appropriate target for some individuals with diabetes, glycemic targets may be more or less stringent based on patient features including life expectancy, duration of diabetes, comorbidities, and patient attitude and support system (Table 3) [4].
A particular group in which less stringent goals should be considered is older patients, especially those with complex or poor health status [4,25]. The risk of intensive glycemic control may exceed the benefits in these patients, as they are at higher risk of hypoglycemia and polypharmacy [26]. A goal A1C of 7% to 7.5% is now recommended for healthy older adults, and less stringent A1C goals of 7.5% to 8% and 8% to 8.5% should be considered based on the presence and severity of multiple coexisting chronic illnesses, decreased self-care ability, or cognitive impairment [4,25]. Unfortunately, overtreatment is frequently seen in this group. In a recent study of patients over age 65 years, about 40% of those with complex or poor health status had tight glycemic control with A1C below 6.5% [26]. An analysis of U.S. Veterans Affairs administration data showed that only 27% of 12,917 patients older than 65 with very low A1C (< 6%) and about 21% of those with A1C of 6% to 6.5% underwent treatment deintensification [27].
Initiate Treatment with Metformin
There is strong consensus that metformin is the preferred drug for monotherapy due to its long proven safety record, low cost, weight-reduction benefit, and potential cardiovascular advantages [4,16]. As long as there are no contraindications, metformin should be recommended concurrent with lifestyle intervention at the time of diabetes diagnosis. The recommendation is based on the fact that adherence to diet, weight reduction, and regular exercise is not sustained in most patients, and most patients ultimately will require treatment. Since metformin is usually well-tolerated, does not cause hypoglycemia, has a favorable effect on body weight, and is relatively inexpensive, potential benefits of early initiation of medication appear to outweigh potential risks.
The U.S. Food and Drug Administration (FDA) recently relaxed prescribing polices to extend the use of this important medication to patients who have mild–moderate, but stable, chronic kidney disease (CKD) [28]. Metformin is recommended as first-line therapy and should be used unless it is contraindicated (ie, estimated glomerular filtration rate [eGFR] < 30 mL/min/1.73 m2)[4,7,29].
Add Additional Agent(s) as Needed to Achieve Goal
Other than metformin, evidence is limited for the optimal use of the burgeoning array of available agents, especially in dual or triple combinations [6,30]. Research is now starting to focus more on what the ideal number and sequence of drugs should be. The Glycemic Reduction Approach in Diabetes (GRADE) study, which will compare long-term benefits and risks of the 4 most widely used antihyperglycemic medications in combination with metformin, is now underway [31,32]. The 4 classes being studied are sulfonylurea, dipeptidyl peptidase-4 (DPP-4) inhibitors, glucagon-like peptide-1 (GLP-1) receptor agonists, and a basal,
Eleven classes of non-insulin medications are currently approved for treating hyperglycemia in T2DM [4]. Within each class, numerous agents are available. Six of these classes (ie, α-glucosidase inhibitors, colesevelam, bromocriptine, pramlintide, meglitinides, and thiazolidinediones) are not used frequently
Consider Effects on A1C
There is a paucity of high-quality, head-to-head comparison trials evaluating the ability of available agents to achieve recommended glycemic targets. This is important because the glucose-lowering effectiveness of individual medications is strongly influenced by baseline characteristics such as A1C, duration of diabetes, and previous therapy. With these limitations in mind, the relative glucose-lowering effectiveness of commonly used agents is shown in Table 4. When used as monotherapy, A1C reductions of approximately 1% to 1.5% are achieved with metformin, sulfonylureas, and GLP-1 receptor agonists [6,30,34,35,39]. DPP-4 inhibitors and SGLT-2 inhibitors have more modest glucose-lowering efficacy, with A1C reductions of approximately 0.5% to 1% [6,30,34,35,39]. Larger effects may be seen in individuals with higher baseline A1C and those who are drug naïve. Insulin is the most effective glucose-lowering agent—it can reduce virtually any level of A1C down to the normal range, with hypoglycemia being the only limiting factor. When a patient has uncontrolled hyperglycemia on metformin monotherapy, or if there is a contraindication or intolerance to metformin, clinicians should consider the potential glucose-lowering effects of other available options and should choose an agent that conceivably could bring a patient close to meeting their treatment goal.
Eliminate Options with Unacceptable Adverse Effects
When the pharmacologic options with acceptable A1C-lowering potential have been identified, the ones with contraindications and potential serious adverse effects for the individual patient can immediately be eliminated (Table 4). For example, if a patient has an eGFR < 30 mL/min/1.73 m2, metformin, sulfonylureas, GLP-1 receptor agonists, most DPP-4 inhibitors, and SGLT-2 inhibitors are either contraindicated or should be used with caution. In patients with severe osteoporosis, SGLT-2 inhibitors may not be the best option. In patients with a history of diabetic ketoacidosis (DKA), caution should be used with metformin and SGLT-2 inhibitors. There have been concerns of possible acute pancreatitis and neoplasia with the incretin-based agents, the DPP-4 inhibitors and GLP-1 receptor agonists [40,41], although other clinical trials and observational data have not found increased risk [42–45]. Nevertheless, these agents potentially should be avoided in patients with a history of pancreatitis or neoplasm. SGLT-2 inhibitors may be associated with genitourinary infections and volume depletion [46–48] and probably should be avoided in patients at high risk for these conditions.
If the adverse effects are not serious, changing the way the medication is administered may allow the patient to tolerate agents with high potential benefits. For example, metformin is commonly associated with gastrointestinal (GI) adverse effects, which can be reduced or avoided with slow titration of the dose [6] or by switching to an extended-release formulation [49]. GLP-1 receptor agonists are associated with GI adverse effects [6] and in most cases slow titration is recommended.
Evaluate Potential Risks/Benefits of Remaining Options
Hypoglycemia. The barrier of hypoglycemia generally precludes maintenance of euglycemia and full realization of the long-term benefits of good glucose control over a lifetime. Once considered a trivial issue, concerns about hypoglycemia in T2DM are increasingly being raised [19,50–55]. Clearly, hypoglycemia occurs more often as glycemic targets are lowered to near-normal values, especially in those with advanced age and multiple comorbidities [55]. Various comorbidities frequently encountered particularly as patients age also are associated with increasing propensity for experiencing hypoglycemia and untoward outcomes from it. These include coronary artery disease, heart failure, renal and liver disease, and dementia. Hypoglycemia, when it occurs, may lead to dysrhythmias, dizziness, accidents and falls, work disability, and decreased quality of life. In addition to relaxing blood glucose targets in high-risk patients, drug selection should favor agents that do not precipitate such events (Table 4).
Fortunately, the commonly used non-insulin agents are not associated with hypoglycemia unless they are used in combination with sulfonylureas or insulin. Sulfonylureas should be used with caution and other options considered in patients with high risk for hypoglycemia. When insulin is required, regimens which minimize risk of hypoglycemia should be used. For example, adding a GLP-1 receptor agonist to basal insulin as an alternative to mealtime insulin has been shown to be equally effective with a lower risk of hypoglycemia [4,6]. Also, premixed insulin preparations should be avoided or used cautiously in individuals who miss meals frequently. Additionally, newer basal insulins that exhibit longer duration of action are now available in the United States. Preliminary studies have shown that the newly FDA-approved longer-acting basal insulins, insulin degludec and glargine U-300, may be associated with a reduced risk for hypoglycemia [56,57]. However, it remains unclear how and when these newer agents will best be incorporated into a treatment regimen.
Body weight. Nearly 90% of people living with T2DM are overweight or obese. Given the close tie between obesity and T2DM, treating obesity is an obvious consideration in diabetes treatment. Major trials have shown the effectiveness of lifestyle modifications and weight reduction in delaying, prevention, and management of T2DM [4,58,59].With this in mind, clinicians should consider preferentially using antihyperglycemic agents with weight-lowering or weight-neutral effects. Among commonly used antihyperglycemic agents, metformin, GLP-1 receptor agonists, and SGLT-2 inhibitors have been shown to have weight-reduction benefits, and DPP-4 inhibitors are weight neutral. On the other hand, sulfonylureas and insulin are associated with weight gain. A systematic review and meta-analysis including 204 studies with study durations ranging from 3 months to 8 years showed comparative effects of diabetes medications with a differential effect on weight of up to 5 kg (Table 4) [60].
Metformin is associated with an average weight loss of 1.9 to 3.1 kg that was sustained with long-term use for at least 10 years in the Diabetes Prevention Program Outcomes Study [61].A systematic review of 7 randomized trials showed that in patients with T2DM, the SGLT-2 inhibitors dapagliflozin and canagliflozin were associated with weight loss (mean weighted difference of –1.81 kg and –2.3 kg, respectively) [62]. A systematic review and meta-analysis of 25 randomized controlled trials showed greater weight loss (mean weighted difference of –2.9 kg) in overweight or obese patients with or without T2DM using GLP-1 receptor agonists when compared to placebo, insulin, or oral antihyperglycemic agents [63]. Of note, the GLP-1 receptor agonist liraglutide is now approved for weight loss in patients with or without diabetes [64]. The maximum doses approved for diabetes and obesity treatment are 1.8 and 3.0 mg/day, respectively.
Since weight loss is associated with improved glycemic control, an area of emerging interest is the use of antiobesity medications for managing diabetes. Although most older weight-loss medications were only approved for short-term use, some newer agents are approved for longer-term use. Lorcaserin and the combination drugs topiramate/phentermine and naltrexone/bupropion are approved for chronic therapy, provided certain conditions are met. Patients on weight reduction agents should be monitored regularly.
An even more radical departure from conventional therapy for diabetes is the consideration of metabolic, or weight-loss, surgery, which has been found to be associated with rapid and dramatic improvements in blood glucose control. Metabolic surgery has been shown to improve glucose control more effectively than any known pharmaceutical or behavioral approach. For example, in an observational study of obese patients with T2DM, bariatric surgery led to diabetes remission rates of 72.3% 2 years after surgery and 30.4% 15 years after surgery compared to 16.4% and 6.5%, respectively, in control patients [69]. With long-term follow-up, significant decreases in microvascular and macrovascular complications were seen in the surgical group [69]. Compared with medical therapy alone, bariatric surgery plus medical therapy has been associated with more weight loss, better glycemic control, less need for diabetes medications, and improved quality of life [70]. A 2016 joint statement by numerous international diabetes organizations recommends considering metabolic surgery as a treatment for T2DM and obesity [71]. American Diabetes Association guidelines recommend consideration of bariatric surgery in individuals with T2DM who have a body mass index greater than 35 kg/m2,especially if achieving disease control is difficult by means of lifestyle modifications and medications [4].
Cardiovascular outcomes. Cardiovascular risk is about 2 to 4 times higher in patients with diabetes, and about half of patients with this condition develop heart failure [4,72]. CVD is responsible for most of the mortality in T2DM [72]. Therefore, prevention of cardiovascular morbidity and mortality is an important goal for diabetes treatment. Due to concerns about potential cardiovascular risks associated with glucose-lowering medications [73–76], the FDA has issued regulatory requirements for manufacturers to monitor the cardiovascular risk profile for these drugs [77]. Recent trials have led to a better understanding of potential cardiovascular benefits or harms of antihyperglycemic medications.
Metformin, the widely recommended first-line therapy for T2DM, carries a large body of evidence supporting its cardiovascular benefits. For example, the UKPDS found that compared to conventional therapy (mostly diet), metformin reduced cardiovascular events and mortality in obese patients with T2DM [15]. This result was supported in Hyperinsulinemia: the Outcome of its Metabolic Effect (HOME) study where, as an add-on to insulin, metformin decreased macrovascular complications when compared to placebo [78]. Research over the past decade also has assuaged concerns about metformin safety in heart failure [60]. A systematic review of observational studies involving 34,000 patients conducted in 2013 showed that metformin is as safe as other glucose-lowering medications in patients with diabetes and heart failure even in the presence of CKD [4,79]. Furthermore, numerous investigations have found metformin is not associated with increased hospitalizations or risk of lactic acidosis [80]. Metformin can be used safely in patients with diabetes and heart failure [60].
Although sulfonylureas have long been a mainstay of diabetes therapy, concerns about their potential adverse cardiovascular effects have been raised by numerous studies [81]. Tolbutamide, a first-generation sulfonylurea, was removed from the market after the University Group Diabetes Program study found increased CVD deaths with this agent versus placebo. Subsequently, the FDA issued a warning for all sulfonylureas [74]. The increased cardiovascular risk associated with sulfonylureas is thought to be due to their effect on cardiac mitochondrial potassium ATP channels. Sulfonylureas bind to these channels, preventing a protective phenomenon called ischemic preconditioning and resulting in a weakened defense against myocardial injury [76]. A recent study showed an increased risk of coronary heart disease associated with long-term use of sulfonylureas in women with diabetes [81].
GLP-1 receptor agonists have recently received much attention for their potential beneficial effects on cardiovascular outcomes. In a recent trial, lixisenatide was shown to be safe in patients with T2DM and acute coronary syndrome when compared to placebo [82]. More recently, the Liraglutide Effect and Action in Diabetes: Evaluation of cardiovascular outcome Results (LEADER) trial demonstrated significant cardiovascular benefits with liraglutide in patients with T2DM and established or high CVD risk [83]. The composite outcome of the first occurrence of death from cardiovascular causes, nonfatal myocardial infarction (MI), or nonfatal stroke, occurred less frequently in the liraglutide group compared to placebo (13% versus 14.9%, respectively), and there were fewer deaths from cardiovascular causes in the liraglutide group compared to placebo (4.7% and 6.0%, respectively) [83]. Other trials investigating the cardiovascular outcomes of this class [84,85] are in progress.
Another class with potential cardiovascular benefits is the SGLT-2 inhibitors. In a recent cardiovascular outcome study, empagliflozin significantly lowered the composite of cardiovascular death, nonfatal MI, or nonfatal stroke in T2DM patients with high cardiovascular risk compared to placebo (10.5% and 12.1%, respectively) [86]. There are several large ongoing studies evaluating the cardiovascular effects of other SGLT-2 inhibitors [87–89].
DPP-4 inhibitors were examined in recent studies and have shown no cardiovascular benefits [42,44,90].The studies showed mixed results regarding an association between DPP-4 inhibitors and heart failure. In one study, saxagliptin was associated with increased hospitalization for heart failure compared to placebo [44], while 2 noninferiority trials did not show a significant increase in heart failure hospitalizations associated with alogliptin and sitagliptin when compared to placebo [42,90].
Administration Considerations
Many patients with T2DM require multiple agents for glycemic control. Additional medications used for comorbid conditions add to this burden. When choosing antihyperglycemic agents, the route and frequency of administration, as well as the patients’ preferences and ability, should be considered. Either once or twice daily dosing is available for most agents, and once weekly dosing is available for some of the GLP-1 receptor agonists. Once daily or once weekly formulations may improve adherence and be more desirable than preparations that are dosed twice daily. Most of the commonly used medications are dosed orally. Although many patients find this route of administration preferable to insulin or GLP-1 receptor agonists, which require injections, some patients may prefer the risk/benefit of injectable agents. All GLP-1 receptor agonists come in a pen delivery system, which eliminates mixing and provides more convenient administration. Extended-release exenatide also is available as a single-dose tray that requires mixing and may be more cumbersome to inject.
Insulin requires special consideration. There has been an enormous increase in the number of insulin products on the market in the past 2 decades. These products include insulin analogs, concentrated insulins (U-200, U-300, and U-500), premixed insulin preparations, and ultra-long-acting insulin [91]. The availability of insulin options with different concentrations, onsets, and durations of actions has made decision making on which insulin to use difficult. Clinicians need to consider patient preference, dosing frequency, and timing with regard to meals, insulin dose, administration, as well as cost. For example, concentrated insulin is preferred for a patient on high doses of insulin requiring injecting a large volume of insulin. Rapid-acting insulin analogs would be more appropriate for patients who have difficulty administering their regular insulin 20 to 30 minutes before eating. Premixed insulin preparations make it impossible to independently adjust short- and long-acting components. However, these may be good choices in patients who have consistent meal schedules and who want to simplify administration. Despite a prevailing misconception that NPH must be given twice a day, it has long been recognized that in T2DM, a single daily injection of NPH yields improvements in control similar to those achieved with 2 daily injections [92].
Cost Considerations
Treating T2DM imposes a great financial burden on individuals living with diabetes and their families due to the high cost of the medications. Table 4 and Table 5 provide information on the cost of non-insulin and insulin diabetes medications for patients who do not have prescription insurance coverage. From a practical standpoint, choice of diabetes agents is largely influenced by insurance formularies.
The older agents, metformin and the sulfonylureas, are available for a cash (no insurance) price of as little as $4 per month. This is in stark contrast to the SGLT-2 inhibitors, GLP-1 receptor agonists, and DPP-4 inhibitors, which range in cost between $400 and $600 per month. Of recent concern, the cost of insulin has been skyrocketing, with a more than 500% increase in the cost of certain insulins from 2001 to 2015 [93]. According to the Medical Expenditure Panel Survey (MEPS) from 2002 to 2013, the mean price of insulin increased by about 200% (from $4.34/mL to $12.92/mL) during this period, which was significantly higher than increases in the price of non-insulin comparators [94]. The introduction of biosimilar insulins to the market is expected to offer treatment options with lower cost. This will be tested when the biosimilar glargine, the first FDA-approved biosimilar insulin, becomes available in the U.S. market. However, a significant reduction in insulin prices is not expected soon [95].
When insulin is required, most patients with T2DM can be treated with older human insulins, which have similar efficacy and lower costs than the more expensive newer insulin analogs. A Cochrane review comparing basal insulin analogs to NPH showed similar efficacy in glycemic control with minimal clinical benefit in the form of less nocturnal hypoglycemia in the insulin analog arm [96]. Furthermore, similar glycemic control and risk of hypoglycemia was seen when regular insulin was compared with the rapid-acting insulin analogs [97]. The cost of human NPH insulin for a patient on a total daily dose of 60 units is approximately $52 per month. This contrasts with the most widely used insulin, insulin glargine, which has a cash price of about $500 per month for the same amount (Table 5). Insulin pens, which are convenient, are more expensive. Interestingly, human insulins do not require prescriptions, allowing underinsured, underfunded patients ongoing access to them.
Incorporating Patient Preferences
Research evidence is necessary but insufficient for making patient care decisions. Along with the potential benefits, harms, costs, and inconveniences of the management options, patient perspectives, beliefs, expectations, and health-related goals must be considered. Patients will undoubtedly have preferences regarding defining goals and ranking options. Clinicians should discuss therapeutic goals and treatment options and work collaboratively with patients in determining management strategies [98].
Summary
Potential treatment approaches for treating hyperglycemia in T2DM are summarized in Figure 1 and Figure 2 [4,7]. As long as there are no contraindications, metformin should be recommended concurrent with lifestyle intervention at the time of diabetes diagnosis. Even if metformin monotherapy is initially effective, glycemic control is likely to deteriorate over time due to progressive loss of β-cell function in T2DM.
There is no consensus as to what the second-line agent should be. Selection of a second agent should be made based on potential advantages and disadvantages of each agent for any given patient. A patient-centered approach is preferred over a fixed algorithm. If the patient progresses to the point where dual therapy does not provide adequate control, either a third non-insulin agent or insulin can be added. In patients with modestly elevated A1C (below ~8%), addition of a third non-insulin agent may be equally effective as (but more expensive than) addition of insulin.
Patients with significantly elevated A1C levels on non-insulin agents usually should have insulin added to their regimen. When insulin is added, metformin should be continued. DPP-4 inhibitors and sulfonylureas are typically stopped. If SGLT-2 inhibitors and/or GLP-1 receptor agonists are continued, this may aid with weight maintenance. However, continuing these agents is likely to be expensive and associated with problems associated with polypharmacy.
The most widely recommended strategy for initiating insulin in T2DM is to add a single bedtime injection of basal insulin (ie, NPH, glargine, detemir, or degludec) to the patient’s regimen. This regimen has been found to be effective in numerous studies and controls hyperglycemia in up to 60% of patients [99]. If the patient is treated with a single bedtime injection of insulin and the fasting glucose level is within the target range but the A1C level remains above goal, addition of mealtime insulin injections is likely to be beneficial. Alternatively, addition of a GLP-1 receptor agonist to basal insulin has been shown to be equally beneficial [4,6]. When adding mealtime insulin, a common strategy is to add a single injection of a rapid-acting insulin (eg, lispro, aspart, glulisine) before the patient’s largest meal of the day. Additional premeal injections of rapid-acting insulin may be added as needed, based on self-monitoring blood glucose results. If glycemia remains significantly uncontrolled on more than 200 units of insulin per day, switching to a concentrated form of insulin (eg, U-200, U-300, or U-500) should be considered.
Corresponding author: Maryam Fazel, PharmD, BCPS, BCACP, CDE, 1295 N. Martin Ave. (Room B211B), Tucson, Arizona 85721-0202, maryamfazel@pharmacy.arizona.edu.
Financial disclosures: None.
1. National diabetes statistics report: estimates of diabetes and its burden in the United States, 2014. Centers for Disease Control and Prevention Web site. www.cdc.gov/diabetes/pubs/statsreport14/national-diabetes-report-web.pdf. Accessed November 29, 2016.
2. Statistics about diabetes. American Diabetes Association Web site. www.diabetes.org/diabetes-basics/statistics/. Accessed November 29, 2016.
3. American Diabetes Association. Economic costs of diabetes in the U.S. in 2012. Diabetes Care 2013;36:1033–46.
4. American Diabetes Association. Standards of medical care in diabetes--2016. Diabetes Care 2016;39(Suppl. 1).
5. Raz I, Riddle MC, Rosenstock J, et al. Personalized management of hyperglycemia in type 2 diabetes: reflections from a Diabetes Care Editors’ Expert Forum. Diabetes Care 2013;36:1779–88.
6. Inzucchi SE, Bergenstal RM, Buse JB, et al. Management of hyperglycemia in type 2 diabetes, 2015: a patient-centered approach: update to a position statement of the American Diabetes Association and the European Association for the Study of Diabetes. Diabetes Care 2015;38:140–9.
7. Garber AJ, Abrahamson MJ, Barzilay JI, et al. Consensus Statement by the American Association of Clinical Endocrinologists and American College of Endocrinology on the Comprehensive Type 2 Diabetes Management Algorithm--2016 Executive Summary. Endocr Pract 2016;22:84–113.
8. Steenkamp DW, Alexanian SM, Sternthal E. Approach to the patient with atypical diabetes. CMAJ 2014;186:678–84.
9. de Lusignan S, Sadek N, Mulnier H, et al. Miscoding, misclassification and misdiagnosis of diabetes in primary care. Diabet Med 2012;29:181–9.
10. Tripathi A, Rizvi AA, Knight LM, Jerrell JM. Prevalence and impact of initial misclassification of pediatric type 1 diabetes mellitus. South Med J 2012;105:513–7.
11. The effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin-dependent diabetes mellitus. The Diabetes Control and Complications Trial Research Group. N Engl J Med 1993;329:977–86.
12. Intensive blood-glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33). UK Prospective Diabetes Study (UKPDS) Group. Lancet 1998;352:837–53.
13. Nathan DM, Cleary PA, Backlund JY, et al. Intensive diabetes treatment and cardiovascular disease in patients with type 1 diabetes. N Engl J Med 2005;353:2643–53.
14. Nathan DM, DCCT/EDIC Research Group. The diabetes control and complications trial/epidemiology of diabetes interventions and complications study at 30 years: overview. Diabetes Care 2014;37:9–16.
15. Effect of intensive blood-glucose control with metformin on complications in overweight patients with type 2 diabetes (UKPDS 34). UK Prospective Diabetes Study (UKPDS) Group. Lancet 1998;352:854–65.
16. Holman RR, Paul SK, Bethel MA, et al. 10-Year follow-up of intensive glucose control in type 2 diabetes. N Engl J Med 2008;359:1577–89.
17. Ohkubo Y, Kishikawa H, Araki E, et al. Intensive insulin therapy prevents the progression of diabetic microvascular complications in Japanese patients with non-insulin-dependent diabetes mellitus: a randomized prospective 6-year study. Diabetes Res Clin Pract 1995;28:103–17.
18. Action to Control Cardiovascular Risk in Diabetes Study Group, Gerstein HC, Miller ME, Byington RP, et al. Effects of intensive glucose lowering in type 2 diabetes. N Engl J Med 2008;358:2545–59.
19. ACCORD Study Group, Gerstein HC, Miller ME, Genuth S, et al. Long-term effects of intensive glucose lowering on cardiovascular outcomes. N Engl J Med 2011;364:818–28.
20. ADVANCE Collaborative Group, Patel A, MacMahon S, Chalmers J, et al. Intensive blood glucose control and vascular outcomes in patients with type 2 diabetes. N Engl J Med 2008;358:2560–72.
21. Wong MG, Perkovic V, Chalmers J, et al. Long-term Benefits of Intensive Glucose Control for Preventing End-Stage Kidney Disease: ADVANCE-ON. Diabetes Care 2016;39:694–700.
22. Duckworth W, Abraira C, Moritz T, et al. Glucose control and vascular complications in veterans with type 2 diabetes. N Engl J Med 2009;360:129–39.
23. Hayward RA, Reaven PD, Wiitala WL, et al. Follow-up of glycemic control and cardiovascular outcomes in type 2 diabetes. N Engl J Med 2015;372:2197–206.
24. American Diabetes Association. Standards of medical care in diabetes--2009. Diabetes Care 2009;32 Suppl 1:S13–61.
25. American Geriatrics Society Expert Panel on Care of Older Adults with Diabetes Mellitus, Moreno G, Mangione CM, Kimbro L, Vaisberg E. Guidelines abstracted from the American Geriatrics Society Guidelines for Improving the Care of Older Adults with Diabetes Mellitus: 2013 update. J Am Geriatr Soc 2013;61:2020–6.
26. Lipska KJ, Ross JS, Miao Y, et al. Potential overtreatment of diabetes mellitus in older adults with tight glycemic control. JAMA Intern Med 2015;175:356–62.
27. Sussman JB, Kerr EA, Saini SD, et al. Rates of deintensification of blood pressure and glycemic medication treatment based on levels of control and life expectancy in older patients with diabetes mellitus. JAMA Intern Med 2015;175:1942–9.
28. FDA Drug Safety Communication: FDA revises warnings regarding use of the diabetes medicine metformin in certain patients with reduced kidney function. FDA Web site. www.fda.gov/Drugs/DrugSafety/ucm493244.htm. Accessed December 1, 2016.
29. Inzucchi SE, Lipska KJ, Mayo H, et al. Metformin in patients with type 2 diabetes and kidney disease: a systematic review. JAMA 2014;312:2668–75.
30. Nathan DM. Diabetes: advances in diagnosis and treatment. JAMA 2015;314:1052–62.
31. Nathan DM, Buse JB, Kahn SE, et al. Rationale and design of the glycemia reduction approaches in diabetes: a comparative effectiveness study (GRADE). Diabetes Care 2013;36:2254–61.
32. NIH begins recruitment for long-term study of diabetes drug efficacy. NIH Web site. www.nih.gov/news-events/news-releases/nih-begins-recruitment-long-term-study-diabetes-drug-efficacy. Accessed December 1, 2016.
33. Hermayer KL, Dake A. Newer oral and noninsulin therapies to treat type 2 diabetes mellitus. Cleve Clin J Med 2016;83(5 Suppl 1):S18–26.
34. Bolen S, Wilson L, Vassy J, et al. Systematic review: comparative effectiveness and safety of oral medications for type 2 diabetes mellitus. Ann Intern Med 2007;147:386–99.
35. Bolen S, Tseng E, Hutfless S, et al. Oral diabetes medications for adults with type 2 diabetes: an update. Agency for Healthcare Research and Quality (US); 2011 Mar Report No: 11-EHC038-EF. AHRQ Comparative Effectiveness Reviews.
36. Metformin, glyburide, glipizide, glimeperide, sitagliptin, saxagliptin, linagliptin, lixisenatide, alogliptin, exenatide, liraglutide, albiglutide, dulaglutide, canagliflozin, danagliflozin, empagliflozin: drug information. Waltham (MA): UpToDate, Inc.; 2016. Accessed September 23, 2016.
37. GoodRx Web site. http://www.goodrx.com. Accessed August 6, 2016 and December 2016.
38. Insulins available in the United States. Diabetesforcast Web site. Accessed August 6, 2016. www.diabetesforecast.org/2016/mar-apr/images/2016-insulin-chart-new.pdf.
39. Inzucchi SE, Bergenstal RM, Buse JB, et al. Management of hyperglycaemia in type 2 diabetes: a patient-centered approach. Position statement of the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD). Diabetologia 2012;55:577–96.
40. Elashoff M, Matveyenko AV, Gier B, et al. Pancreatitis, pancreatic, and thyroid cancer with glucagon-like peptide-1-based therapies. Gastroenterology 2011;141:150–6.
41. Butler AE, Campbell-Thompson M, Gurlo T, et al. Marked expansion of exocrine and endocrine pancreas with incretin therapy in humans with increased exocrine pancreas dysplasia and the potential for glucagon-producing neuroendocrine tumors. Diabetes 2013;62:2595–604.
42. Green JB, Bethel MA, Armstrong PW, et al. Effect of sitagliptin on cardiovascular outcomes in type 2 diabetes. N Engl J Med 2015;373:232–42.
43. White WB, Cannon CP, Heller SR, et al. Alogliptin after acute coronary syndrome in patients with type 2 diabetes. N Engl J Med 2013;369:1327–35.
44. Scirica BM, Bhatt DL, Braunwald E, et al. Saxagliptin and cardiovascular outcomes in patients with type 2 diabetes mellitus. N Engl J Med 2013;369:1317–26.
45. Egan AG, Blind E, Dunder K, et al. Pancreatic safety of incretin-based drugs--FDA and EMA assessment. N Engl J Med 2014;370:794–7.
46. Vasilakou D, Karagiannis T, Athanasiadou E, et al. Sodium-glucose cotransporter 2 inhibitors for type 2 diabetes: a systematic review and meta-analysis. Ann Intern Med 2013;159:262–74.
47. Nyirjesy P, Sobel JD, Fung A, et al. Genital mycotic infections with canagliflozin, a sodium glucose co-transporter 2 inhibitor, in patients with type 2 diabetes mellitus: a pooled analysis of clinical studies. Curr Med Res Opin 2014;30:1109–19.
48. Schernthaner G, Gross JL, Rosenstock J, et al. Canagliflozin compared with sitagliptin for patients with type 2 diabetes who do not have adequate glycemic control with metformin plus sulfonylurea: a 52-week randomized trial. Diabetes Care 2013;36:2508–15.
49. Blonde L, Dailey G, Jabbour S, et al. Gastrointestinal tolerability of extended-release metformin tablets compared to immediate-release metformin tablets: results of a retrospective cohort study. Curr Med Res Opin 2004;20:562–72.
50. Kalra S, Mukherjee JJ, Venkataraman S, et al. Hypoglycemia: the neglected complication. Indian J Endocrinol Metab 2013;17:819–34.
51. Paty BW. The role of hypoglycemia in cardiovascular outcomes in diabetes. Can J Diabetes 2015;39 Suppl 5:S155–9.
52. Zoungas S, Patel A, Chalmers J, et al. Severe hypoglycemia and risks of vascular events and death. N Engl J Med 2010;363:1410–8.
53. Whitmer RA, Karter AJ, Yaffe K, et al. Hypoglycemic episodes and risk of dementia in older patients with type 2 diabetes mellitus. JAMA 2009;301:1565–72.
54. McCoy RG, Van Houten HK, Ziegenfuss JY, et al. Increased mortality of patients with diabetes reporting severe hypoglycemia. Diabetes Care 2012;35:1897–901.
55. McCoy RG, Lipska KJ, Yao X, et al. Intensive treatment and severe hypoglycemia among adults with type 2 diabetes. JAMA Intern Med 2016;176:969–78.
56. Rodbard HW, Gough S, Lane W, et al. Reduced risk of hypoglycemia with insulin degludec versus insulin glargine in patients with type 2 diabetes requiring high doses of basal insulin: a meta-analysis of 5 randomized begin trials. Endocr Pract 2014;20:285–92.
57. Yki-Jarvinen H, Bergenstal R, Ziemen M, et al. New insulin glargine 300 units/mL versus glargine 100 units/mL in people with type 2 diabetes using oral agents and basal insulin: glucose control and hypoglycemia in a 6-month randomized controlled trial (EDITION 2). Diabetes Care 2014;37:3235–43.
58. Tuomilehto J, Lindstrom J, Eriksson JG, et al. Prevention of type 2 diabetes mellitus by changes in lifestyle among subjects with impaired glucose tolerance. N Engl J Med 2001;344:1343–50.
59. Knowler WC, Barrett-Connor E, Fowler SE, et al. Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin. N Engl J Med 2002;346:393–403.
60. Maruthur NM, Tseng E, Hutfless S, et al. Diabetes medications as monotherapy or metformin-based combination therapy for type 2 diabetes: a systematic review and meta-analysis. Ann Intern Med 2016;164:740–51.
61. Diabetes Prevention Program Research Group. Long-term safety, tolerability, and weight loss associated with metformin in the Diabetes Prevention Program Outcomes Study. Diabetes Care 2012;35:731–7.
62. Clar C, Gill JA, Court R, Waugh N. Systematic review of SGLT2 receptor inhibitors in dual or triple therapy in type 2 diabetes. BMJ Open 2012;2:10.1136/bmjopen,2012-001007.
63. Vilsboll T, Christensen M, Junker AE, et al. Effects of glucagon-like peptide-1 receptor agonists on weight loss: systematic review and meta-analyses of randomised controlled trials. BMJ 2012;344:d7771.
64. FDA approves weight-management drug Saxenda. FDA Web site www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm427913.htm. Accessed September 22, 2016.
65. Apovian CM, Aronne LJ, Bessesen DH, et al. Pharmacological management of obesity: an endocrine Society clinical practice guideline. J Clin Endocrinol Metab 2015;100:342–62.
66. Liraglutide, lorcaserin, naltrexone/bupropion, orlistat, phentermine/topiramate: drug information. Greenwood Village (CO): Truven Health Analytics; 2016. www.micromedexsolutions.com. Accessed May 13, 2016.
67. Liraglutide, lorcaserin, naltrexone/bupropion, orlistat, phentermine/topiramate: drug information. Waltham (MA): UpToDate, Inc.; 2016. Accessed May 13, 2016.
68. Yanovski SZ, Yanovski JA. Long-term drug treatment for obesity: a systematic and clinical review. JAMA 2014;311:74–86.
69. Sjostrom L, Peltonen M, Jacobson P, et al. Association of bariatric surgery with long-term remission of type 2 diabetes and with microvascular and macrovascular complications. JAMA 2014;311:2297–304.
70. Schauer PR, Bhatt DL, Kirwan JP, et al. Bariatric surgery versus intensive medical therapy for diabetes--3-year outcomes. N Engl J Med 2014;370:2002–13.
71. Rubino F, Nathan DM, Eckel RH, et al. Metabolic surgery in the treatment algorithm for type 2 diabetes: a joint statement by international diabetes organizations. Diabetes Care 2016;39:861–77.
72. Lathief S, Inzucchi SE. Approach to diabetes management in patients with CVD. Trends Cardiovasc Med 2016;26:165–79.
73. Nissen SE, Wolski K. Effect of rosiglitazone on the risk of myocardial infarction and death from cardiovascular causes. N Engl J Med 2007;356:2457–71.
74. Knatterud GL, Klimt CR, Levin ME, et al. Effects of hypoglycemic agents on vascular complications in patients with adult-onset diabetes. VII. Mortality and selected nonfatal events with insulin treatment. JAMA 1978;240:37–42.
75. Masoudi FA, Inzucchi SE, Wang Y, et al. Thiazolidinediones, metformin, and outcomes in older patients with diabetes and heart failure: an observational study. Circulation 2005;111:583–90.
76. Klepzig H, Kober G, Matter C, et al. Sulfonylureas and ischaemic preconditioning; a double-blind, placebo-controlled evaluation of glimepiride and glibenclamide. Eur Heart J 1999;20:439–46.
77. FDA announces new recommendations on evaluating cardiovascular risk in drugs intended to treat type 2 diabetes. FDA Web site. www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/2008/ucm116994.htm. Accessed August 20, 2016.
78. Kooy A, de Jager J, Lehert P, et al. Long-term effects of metformin on metabolism and microvascular and macrovascular disease in patients with type 2 diabetes mellitus. Arch Intern Med 2009;169:616–25.
79. Eurich DT, Weir DL, Majumdar SR, et al. Comparative safety and effectiveness of metformin in patients with diabetes mellitus and heart failure: systematic review of observational studies involving 34,000 patients. Circ Heart Fail 2013;6:395–402.
80. Tahrani AA, Varughese GI, Scarpello JH, Hanna FW. Metformin, heart failure, and lactic acidosis: is metformin absolutely contraindicated? BMJ 2007;335:508–12.
81. Li Y, Hu Y, Ley SH, et al. Sulfonylurea use and incident cardiovascular disease among patients with type 2 diabetes: prospective cohort study among women. Diabetes Care 2014;37:3106–13.
82. Bentley-Lewis R, Aguilar D, Riddle MC, et al. Rationale, design, and baseline characteristics in Evaluation of LIXisenatide in Acute Coronary Syndrome, a long-term cardiovascular end point trial of lixisenatide versus placebo. Am Heart J 2015;169:631,638.e7.
83. Marso SP, Daniels GH, Brown-Frandsen K, et al. Liraglutide and cardiovascular outcomes in type 2 diabetes. N Engl J Med 2016;375:311–22.
84. Exenatide Study of Cardiovascular Event Lowering Trial (EXSCEL): A Trial To Evaluate Cardiovascular Outcomes After Treatment With Exenatide Once Weekly In Patients With Type 2 Diabetes Mellitus. clinicaltrials.gov Web site. https://clinicaltrials.gov/ct2/show/NCT01144338. 2016 Accessed September 23, 2016.
85. Researching Cardiovascular Events With a Weekly Incretin in Diabetes (REWIND). clinicaltrials.gov Web site. https://clinicaltrials.gov/ct2/show/NCT01394952. Accessed September 23, 2016.
86. Schernthaner G, Schernthaner-Reiter MH, Schernthaner GH. EMPA-REG and other cardiovascular outcome trials of glucose-lowering agents: implications for future treatment strategies in type 2 diabetes mellitus. Clin Ther 2016;38:1288–98.
87. CANVAS--CANagliflozin cardiovascular Assesssment Study (CANVAS). clinicaltrials.gov Web site. https://clinicaltrials.gov/ct2/show/NCT01032629. Accessed September 23, 2016.
88. Evaluation of the Effects of Canagliflozin on Renal and Cardiovascular Outcomes in Participants With Diabetic Nephropathy (CREDENCE). clinicaltrials.gov Web site. https://clinicaltrials.gov/ct2/show/NCT02065791. Accessed September 23, 2016.
89. Multicenter Trial to Evaluate the Effect of Dapagliflozin on the Incidence of Cardiovascular Events (DECLARE-TIMI58). clinicaltrials.gov Web site. https://clinicaltrials.gov/ct2/show/NCT01730534. Accessed September 23, 2016.
90. Zannad F, Cannon CP, Cushman WC, et al. Heart failure and mortality outcomes in patients with type 2 diabetes taking alogliptin versus placebo in EXAMINE: a multicentre, randomised, double-blind trial. Lancet 2015;385(9982):2067–76.
91. Van Klompenburg E, Heins JR. New insulin options for diabetic patients. S D Med 2016;69:84–5.
92. Rosenstock J, Schwartz SL, Clark CM Jr, et al. Basal insulin therapy in type 2 diabetes: 28-week comparison of insulin glargine (HOE 901) and NPH insulin. Diabetes Care 2001;24:631–6.
93. Tylee T, Hirsch IB. Costs associated with using different insulin preparations. JAMA 2015;314:665–6.
94. Hua X, Carvalho N, Tew M, et al. Expenditures and prices of antihyperglycemic medications in the United States: 2002-2013. JAMA 2016;315:1400–2.
95. Heinemann L. Biosimilar insulin and costs: what can we expect? J Diabetes Sci Technol 2016;10:457–62.
96. Horvath K, Jeitler K, Berghold A, et al. Long-acting insulin analogues versus NPH insulin (human isophane insulin) for type 2 diabetes mellitus. Cochrane Database Syst Rev 2007;(2)(2):CD005613.
97. Mannucci E, Monami M, Marchionni N. Short-acting insulin analogues vs. regular human insulin in type 2 diabetes: a meta-analysis. Diabetes Obes Metab 2009;11:53–9.
98. Powell PW, Corathers SD, Raymond J, Streisand R. New approaches to providing individualized diabetes care in the 21st century. Curr Diabetes Rev 2015;11:222–30.
99. Riddle MC, Rosenstock J, Gerich J, Insulin Glargine 4002 Study Investigators. The treat-to-target trial: randomized addition of glargine or human NPH insulin to oral therapy of type 2 diabetic patients. Diabetes Care 2003;26:3080–6.
1. National diabetes statistics report: estimates of diabetes and its burden in the United States, 2014. Centers for Disease Control and Prevention Web site. www.cdc.gov/diabetes/pubs/statsreport14/national-diabetes-report-web.pdf. Accessed November 29, 2016.
2. Statistics about diabetes. American Diabetes Association Web site. www.diabetes.org/diabetes-basics/statistics/. Accessed November 29, 2016.
3. American Diabetes Association. Economic costs of diabetes in the U.S. in 2012. Diabetes Care 2013;36:1033–46.
4. American Diabetes Association. Standards of medical care in diabetes--2016. Diabetes Care 2016;39(Suppl. 1).
5. Raz I, Riddle MC, Rosenstock J, et al. Personalized management of hyperglycemia in type 2 diabetes: reflections from a Diabetes Care Editors’ Expert Forum. Diabetes Care 2013;36:1779–88.
6. Inzucchi SE, Bergenstal RM, Buse JB, et al. Management of hyperglycemia in type 2 diabetes, 2015: a patient-centered approach: update to a position statement of the American Diabetes Association and the European Association for the Study of Diabetes. Diabetes Care 2015;38:140–9.
7. Garber AJ, Abrahamson MJ, Barzilay JI, et al. Consensus Statement by the American Association of Clinical Endocrinologists and American College of Endocrinology on the Comprehensive Type 2 Diabetes Management Algorithm--2016 Executive Summary. Endocr Pract 2016;22:84–113.
8. Steenkamp DW, Alexanian SM, Sternthal E. Approach to the patient with atypical diabetes. CMAJ 2014;186:678–84.
9. de Lusignan S, Sadek N, Mulnier H, et al. Miscoding, misclassification and misdiagnosis of diabetes in primary care. Diabet Med 2012;29:181–9.
10. Tripathi A, Rizvi AA, Knight LM, Jerrell JM. Prevalence and impact of initial misclassification of pediatric type 1 diabetes mellitus. South Med J 2012;105:513–7.
11. The effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin-dependent diabetes mellitus. The Diabetes Control and Complications Trial Research Group. N Engl J Med 1993;329:977–86.
12. Intensive blood-glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33). UK Prospective Diabetes Study (UKPDS) Group. Lancet 1998;352:837–53.
13. Nathan DM, Cleary PA, Backlund JY, et al. Intensive diabetes treatment and cardiovascular disease in patients with type 1 diabetes. N Engl J Med 2005;353:2643–53.
14. Nathan DM, DCCT/EDIC Research Group. The diabetes control and complications trial/epidemiology of diabetes interventions and complications study at 30 years: overview. Diabetes Care 2014;37:9–16.
15. Effect of intensive blood-glucose control with metformin on complications in overweight patients with type 2 diabetes (UKPDS 34). UK Prospective Diabetes Study (UKPDS) Group. Lancet 1998;352:854–65.
16. Holman RR, Paul SK, Bethel MA, et al. 10-Year follow-up of intensive glucose control in type 2 diabetes. N Engl J Med 2008;359:1577–89.
17. Ohkubo Y, Kishikawa H, Araki E, et al. Intensive insulin therapy prevents the progression of diabetic microvascular complications in Japanese patients with non-insulin-dependent diabetes mellitus: a randomized prospective 6-year study. Diabetes Res Clin Pract 1995;28:103–17.
18. Action to Control Cardiovascular Risk in Diabetes Study Group, Gerstein HC, Miller ME, Byington RP, et al. Effects of intensive glucose lowering in type 2 diabetes. N Engl J Med 2008;358:2545–59.
19. ACCORD Study Group, Gerstein HC, Miller ME, Genuth S, et al. Long-term effects of intensive glucose lowering on cardiovascular outcomes. N Engl J Med 2011;364:818–28.
20. ADVANCE Collaborative Group, Patel A, MacMahon S, Chalmers J, et al. Intensive blood glucose control and vascular outcomes in patients with type 2 diabetes. N Engl J Med 2008;358:2560–72.
21. Wong MG, Perkovic V, Chalmers J, et al. Long-term Benefits of Intensive Glucose Control for Preventing End-Stage Kidney Disease: ADVANCE-ON. Diabetes Care 2016;39:694–700.
22. Duckworth W, Abraira C, Moritz T, et al. Glucose control and vascular complications in veterans with type 2 diabetes. N Engl J Med 2009;360:129–39.
23. Hayward RA, Reaven PD, Wiitala WL, et al. Follow-up of glycemic control and cardiovascular outcomes in type 2 diabetes. N Engl J Med 2015;372:2197–206.
24. American Diabetes Association. Standards of medical care in diabetes--2009. Diabetes Care 2009;32 Suppl 1:S13–61.
25. American Geriatrics Society Expert Panel on Care of Older Adults with Diabetes Mellitus, Moreno G, Mangione CM, Kimbro L, Vaisberg E. Guidelines abstracted from the American Geriatrics Society Guidelines for Improving the Care of Older Adults with Diabetes Mellitus: 2013 update. J Am Geriatr Soc 2013;61:2020–6.
26. Lipska KJ, Ross JS, Miao Y, et al. Potential overtreatment of diabetes mellitus in older adults with tight glycemic control. JAMA Intern Med 2015;175:356–62.
27. Sussman JB, Kerr EA, Saini SD, et al. Rates of deintensification of blood pressure and glycemic medication treatment based on levels of control and life expectancy in older patients with diabetes mellitus. JAMA Intern Med 2015;175:1942–9.
28. FDA Drug Safety Communication: FDA revises warnings regarding use of the diabetes medicine metformin in certain patients with reduced kidney function. FDA Web site. www.fda.gov/Drugs/DrugSafety/ucm493244.htm. Accessed December 1, 2016.
29. Inzucchi SE, Lipska KJ, Mayo H, et al. Metformin in patients with type 2 diabetes and kidney disease: a systematic review. JAMA 2014;312:2668–75.
30. Nathan DM. Diabetes: advances in diagnosis and treatment. JAMA 2015;314:1052–62.
31. Nathan DM, Buse JB, Kahn SE, et al. Rationale and design of the glycemia reduction approaches in diabetes: a comparative effectiveness study (GRADE). Diabetes Care 2013;36:2254–61.
32. NIH begins recruitment for long-term study of diabetes drug efficacy. NIH Web site. www.nih.gov/news-events/news-releases/nih-begins-recruitment-long-term-study-diabetes-drug-efficacy. Accessed December 1, 2016.
33. Hermayer KL, Dake A. Newer oral and noninsulin therapies to treat type 2 diabetes mellitus. Cleve Clin J Med 2016;83(5 Suppl 1):S18–26.
34. Bolen S, Wilson L, Vassy J, et al. Systematic review: comparative effectiveness and safety of oral medications for type 2 diabetes mellitus. Ann Intern Med 2007;147:386–99.
35. Bolen S, Tseng E, Hutfless S, et al. Oral diabetes medications for adults with type 2 diabetes: an update. Agency for Healthcare Research and Quality (US); 2011 Mar Report No: 11-EHC038-EF. AHRQ Comparative Effectiveness Reviews.
36. Metformin, glyburide, glipizide, glimeperide, sitagliptin, saxagliptin, linagliptin, lixisenatide, alogliptin, exenatide, liraglutide, albiglutide, dulaglutide, canagliflozin, danagliflozin, empagliflozin: drug information. Waltham (MA): UpToDate, Inc.; 2016. Accessed September 23, 2016.
37. GoodRx Web site. http://www.goodrx.com. Accessed August 6, 2016 and December 2016.
38. Insulins available in the United States. Diabetesforcast Web site. Accessed August 6, 2016. www.diabetesforecast.org/2016/mar-apr/images/2016-insulin-chart-new.pdf.
39. Inzucchi SE, Bergenstal RM, Buse JB, et al. Management of hyperglycaemia in type 2 diabetes: a patient-centered approach. Position statement of the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD). Diabetologia 2012;55:577–96.
40. Elashoff M, Matveyenko AV, Gier B, et al. Pancreatitis, pancreatic, and thyroid cancer with glucagon-like peptide-1-based therapies. Gastroenterology 2011;141:150–6.
41. Butler AE, Campbell-Thompson M, Gurlo T, et al. Marked expansion of exocrine and endocrine pancreas with incretin therapy in humans with increased exocrine pancreas dysplasia and the potential for glucagon-producing neuroendocrine tumors. Diabetes 2013;62:2595–604.
42. Green JB, Bethel MA, Armstrong PW, et al. Effect of sitagliptin on cardiovascular outcomes in type 2 diabetes. N Engl J Med 2015;373:232–42.
43. White WB, Cannon CP, Heller SR, et al. Alogliptin after acute coronary syndrome in patients with type 2 diabetes. N Engl J Med 2013;369:1327–35.
44. Scirica BM, Bhatt DL, Braunwald E, et al. Saxagliptin and cardiovascular outcomes in patients with type 2 diabetes mellitus. N Engl J Med 2013;369:1317–26.
45. Egan AG, Blind E, Dunder K, et al. Pancreatic safety of incretin-based drugs--FDA and EMA assessment. N Engl J Med 2014;370:794–7.
46. Vasilakou D, Karagiannis T, Athanasiadou E, et al. Sodium-glucose cotransporter 2 inhibitors for type 2 diabetes: a systematic review and meta-analysis. Ann Intern Med 2013;159:262–74.
47. Nyirjesy P, Sobel JD, Fung A, et al. Genital mycotic infections with canagliflozin, a sodium glucose co-transporter 2 inhibitor, in patients with type 2 diabetes mellitus: a pooled analysis of clinical studies. Curr Med Res Opin 2014;30:1109–19.
48. Schernthaner G, Gross JL, Rosenstock J, et al. Canagliflozin compared with sitagliptin for patients with type 2 diabetes who do not have adequate glycemic control with metformin plus sulfonylurea: a 52-week randomized trial. Diabetes Care 2013;36:2508–15.
49. Blonde L, Dailey G, Jabbour S, et al. Gastrointestinal tolerability of extended-release metformin tablets compared to immediate-release metformin tablets: results of a retrospective cohort study. Curr Med Res Opin 2004;20:562–72.
50. Kalra S, Mukherjee JJ, Venkataraman S, et al. Hypoglycemia: the neglected complication. Indian J Endocrinol Metab 2013;17:819–34.
51. Paty BW. The role of hypoglycemia in cardiovascular outcomes in diabetes. Can J Diabetes 2015;39 Suppl 5:S155–9.
52. Zoungas S, Patel A, Chalmers J, et al. Severe hypoglycemia and risks of vascular events and death. N Engl J Med 2010;363:1410–8.
53. Whitmer RA, Karter AJ, Yaffe K, et al. Hypoglycemic episodes and risk of dementia in older patients with type 2 diabetes mellitus. JAMA 2009;301:1565–72.
54. McCoy RG, Van Houten HK, Ziegenfuss JY, et al. Increased mortality of patients with diabetes reporting severe hypoglycemia. Diabetes Care 2012;35:1897–901.
55. McCoy RG, Lipska KJ, Yao X, et al. Intensive treatment and severe hypoglycemia among adults with type 2 diabetes. JAMA Intern Med 2016;176:969–78.
56. Rodbard HW, Gough S, Lane W, et al. Reduced risk of hypoglycemia with insulin degludec versus insulin glargine in patients with type 2 diabetes requiring high doses of basal insulin: a meta-analysis of 5 randomized begin trials. Endocr Pract 2014;20:285–92.
57. Yki-Jarvinen H, Bergenstal R, Ziemen M, et al. New insulin glargine 300 units/mL versus glargine 100 units/mL in people with type 2 diabetes using oral agents and basal insulin: glucose control and hypoglycemia in a 6-month randomized controlled trial (EDITION 2). Diabetes Care 2014;37:3235–43.
58. Tuomilehto J, Lindstrom J, Eriksson JG, et al. Prevention of type 2 diabetes mellitus by changes in lifestyle among subjects with impaired glucose tolerance. N Engl J Med 2001;344:1343–50.
59. Knowler WC, Barrett-Connor E, Fowler SE, et al. Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin. N Engl J Med 2002;346:393–403.
60. Maruthur NM, Tseng E, Hutfless S, et al. Diabetes medications as monotherapy or metformin-based combination therapy for type 2 diabetes: a systematic review and meta-analysis. Ann Intern Med 2016;164:740–51.
61. Diabetes Prevention Program Research Group. Long-term safety, tolerability, and weight loss associated with metformin in the Diabetes Prevention Program Outcomes Study. Diabetes Care 2012;35:731–7.
62. Clar C, Gill JA, Court R, Waugh N. Systematic review of SGLT2 receptor inhibitors in dual or triple therapy in type 2 diabetes. BMJ Open 2012;2:10.1136/bmjopen,2012-001007.
63. Vilsboll T, Christensen M, Junker AE, et al. Effects of glucagon-like peptide-1 receptor agonists on weight loss: systematic review and meta-analyses of randomised controlled trials. BMJ 2012;344:d7771.
64. FDA approves weight-management drug Saxenda. FDA Web site www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm427913.htm. Accessed September 22, 2016.
65. Apovian CM, Aronne LJ, Bessesen DH, et al. Pharmacological management of obesity: an endocrine Society clinical practice guideline. J Clin Endocrinol Metab 2015;100:342–62.
66. Liraglutide, lorcaserin, naltrexone/bupropion, orlistat, phentermine/topiramate: drug information. Greenwood Village (CO): Truven Health Analytics; 2016. www.micromedexsolutions.com. Accessed May 13, 2016.
67. Liraglutide, lorcaserin, naltrexone/bupropion, orlistat, phentermine/topiramate: drug information. Waltham (MA): UpToDate, Inc.; 2016. Accessed May 13, 2016.
68. Yanovski SZ, Yanovski JA. Long-term drug treatment for obesity: a systematic and clinical review. JAMA 2014;311:74–86.
69. Sjostrom L, Peltonen M, Jacobson P, et al. Association of bariatric surgery with long-term remission of type 2 diabetes and with microvascular and macrovascular complications. JAMA 2014;311:2297–304.
70. Schauer PR, Bhatt DL, Kirwan JP, et al. Bariatric surgery versus intensive medical therapy for diabetes--3-year outcomes. N Engl J Med 2014;370:2002–13.
71. Rubino F, Nathan DM, Eckel RH, et al. Metabolic surgery in the treatment algorithm for type 2 diabetes: a joint statement by international diabetes organizations. Diabetes Care 2016;39:861–77.
72. Lathief S, Inzucchi SE. Approach to diabetes management in patients with CVD. Trends Cardiovasc Med 2016;26:165–79.
73. Nissen SE, Wolski K. Effect of rosiglitazone on the risk of myocardial infarction and death from cardiovascular causes. N Engl J Med 2007;356:2457–71.
74. Knatterud GL, Klimt CR, Levin ME, et al. Effects of hypoglycemic agents on vascular complications in patients with adult-onset diabetes. VII. Mortality and selected nonfatal events with insulin treatment. JAMA 1978;240:37–42.
75. Masoudi FA, Inzucchi SE, Wang Y, et al. Thiazolidinediones, metformin, and outcomes in older patients with diabetes and heart failure: an observational study. Circulation 2005;111:583–90.
76. Klepzig H, Kober G, Matter C, et al. Sulfonylureas and ischaemic preconditioning; a double-blind, placebo-controlled evaluation of glimepiride and glibenclamide. Eur Heart J 1999;20:439–46.
77. FDA announces new recommendations on evaluating cardiovascular risk in drugs intended to treat type 2 diabetes. FDA Web site. www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/2008/ucm116994.htm. Accessed August 20, 2016.
78. Kooy A, de Jager J, Lehert P, et al. Long-term effects of metformin on metabolism and microvascular and macrovascular disease in patients with type 2 diabetes mellitus. Arch Intern Med 2009;169:616–25.
79. Eurich DT, Weir DL, Majumdar SR, et al. Comparative safety and effectiveness of metformin in patients with diabetes mellitus and heart failure: systematic review of observational studies involving 34,000 patients. Circ Heart Fail 2013;6:395–402.
80. Tahrani AA, Varughese GI, Scarpello JH, Hanna FW. Metformin, heart failure, and lactic acidosis: is metformin absolutely contraindicated? BMJ 2007;335:508–12.
81. Li Y, Hu Y, Ley SH, et al. Sulfonylurea use and incident cardiovascular disease among patients with type 2 diabetes: prospective cohort study among women. Diabetes Care 2014;37:3106–13.
82. Bentley-Lewis R, Aguilar D, Riddle MC, et al. Rationale, design, and baseline characteristics in Evaluation of LIXisenatide in Acute Coronary Syndrome, a long-term cardiovascular end point trial of lixisenatide versus placebo. Am Heart J 2015;169:631,638.e7.
83. Marso SP, Daniels GH, Brown-Frandsen K, et al. Liraglutide and cardiovascular outcomes in type 2 diabetes. N Engl J Med 2016;375:311–22.
84. Exenatide Study of Cardiovascular Event Lowering Trial (EXSCEL): A Trial To Evaluate Cardiovascular Outcomes After Treatment With Exenatide Once Weekly In Patients With Type 2 Diabetes Mellitus. clinicaltrials.gov Web site. https://clinicaltrials.gov/ct2/show/NCT01144338. 2016 Accessed September 23, 2016.
85. Researching Cardiovascular Events With a Weekly Incretin in Diabetes (REWIND). clinicaltrials.gov Web site. https://clinicaltrials.gov/ct2/show/NCT01394952. Accessed September 23, 2016.
86. Schernthaner G, Schernthaner-Reiter MH, Schernthaner GH. EMPA-REG and other cardiovascular outcome trials of glucose-lowering agents: implications for future treatment strategies in type 2 diabetes mellitus. Clin Ther 2016;38:1288–98.
87. CANVAS--CANagliflozin cardiovascular Assesssment Study (CANVAS). clinicaltrials.gov Web site. https://clinicaltrials.gov/ct2/show/NCT01032629. Accessed September 23, 2016.
88. Evaluation of the Effects of Canagliflozin on Renal and Cardiovascular Outcomes in Participants With Diabetic Nephropathy (CREDENCE). clinicaltrials.gov Web site. https://clinicaltrials.gov/ct2/show/NCT02065791. Accessed September 23, 2016.
89. Multicenter Trial to Evaluate the Effect of Dapagliflozin on the Incidence of Cardiovascular Events (DECLARE-TIMI58). clinicaltrials.gov Web site. https://clinicaltrials.gov/ct2/show/NCT01730534. Accessed September 23, 2016.
90. Zannad F, Cannon CP, Cushman WC, et al. Heart failure and mortality outcomes in patients with type 2 diabetes taking alogliptin versus placebo in EXAMINE: a multicentre, randomised, double-blind trial. Lancet 2015;385(9982):2067–76.
91. Van Klompenburg E, Heins JR. New insulin options for diabetic patients. S D Med 2016;69:84–5.
92. Rosenstock J, Schwartz SL, Clark CM Jr, et al. Basal insulin therapy in type 2 diabetes: 28-week comparison of insulin glargine (HOE 901) and NPH insulin. Diabetes Care 2001;24:631–6.
93. Tylee T, Hirsch IB. Costs associated with using different insulin preparations. JAMA 2015;314:665–6.
94. Hua X, Carvalho N, Tew M, et al. Expenditures and prices of antihyperglycemic medications in the United States: 2002-2013. JAMA 2016;315:1400–2.
95. Heinemann L. Biosimilar insulin and costs: what can we expect? J Diabetes Sci Technol 2016;10:457–62.
96. Horvath K, Jeitler K, Berghold A, et al. Long-acting insulin analogues versus NPH insulin (human isophane insulin) for type 2 diabetes mellitus. Cochrane Database Syst Rev 2007;(2)(2):CD005613.
97. Mannucci E, Monami M, Marchionni N. Short-acting insulin analogues vs. regular human insulin in type 2 diabetes: a meta-analysis. Diabetes Obes Metab 2009;11:53–9.
98. Powell PW, Corathers SD, Raymond J, Streisand R. New approaches to providing individualized diabetes care in the 21st century. Curr Diabetes Rev 2015;11:222–30.
99. Riddle MC, Rosenstock J, Gerich J, Insulin Glargine 4002 Study Investigators. The treat-to-target trial: randomized addition of glargine or human NPH insulin to oral therapy of type 2 diabetic patients. Diabetes Care 2003;26:3080–6.