User login
Individualizing Treatment of Hyperglycemia in Type 2 Diabetes
From the University of Arizona College of Pharmacy and the University of Arizona College of Medicine-Tucson, Tucson, AZ.
Abstract
- Objective: To summarize key issues relevant to managing hyperglycemia in patients with type 2 diabetes mellitus (T2DM) and review a strategy for initiating and intensifying therapy.
- Methods: Review of the literature.
- Results: The 6 most widely used pharmacologic treatment options for hyperglycemia in T2DM are metformin, sulfonylureas, dipeptidyl peptidase-4 inhibitors, glucagon-like peptide-1 receptor agonists, sodium-glucose cotransporter-2 inhibitors, and insulin. Recent guidelines stress the importance of an individualized, patient-centered approach to managing hyperglycemia in T2DM, although sufficient guidance for nonspecialists on how to individualize treatment is often lacking. For patients with no contraindications, metformin should be recommended concurrent with lifestyle intervention at the time of diabetes diagnosis. Due to the progressive nature of T2DM, glycemic control on metformin monotherapy is likely to deteriorate over time, and there is no consensus as to what the second-line agent should be. A second agent should be selected based on glycemic goal and potential advantages and disadvantages of each agent for any given patient. If the patient progresses to the point where dual therapy does not provide adequate control, either a third non-insulin agent or insulin can be added.
- Conclusion: Although research is increasingly focusing on what the ideal number and sequence of drugs should be when managing T2DM, investigating all possible combinations in diverse patient populations is not feasible. Physicians therefore must continue to rely on clinical judgment to determine how to apply trial data to the treatment of individual patients.
Key words: type 2 diabetes; patient-centered care; antihyper-glycemic drugs; insulin; therapeutic decision-making.
Diabetes mellitus affects approximately 29.1 million people, or 9.3% of the U.S. population [1,2]. The high prevalence of diabetes and its associated multiple complications, including cardiovascular disease (CVD), blindness, renal failure, lower extremity amputations, and premature death, lead to a tremendous overall burden of disease. The financial cost is staggering as well, with more than 1 in 5 health care dollars spent on treating diabetes or its complications [3]. The goal of diabetes treatment is to prevent acute complications and reduce the risk of long-term complications. Interventions that have been shown to improve diabetes outcomes include medications for glycemic control and treatment of cardiovascular risk factors, nutrition and physical activity counseling, smoking cessation, immunizations, psychosocial care, and ongoing surveillance and early treatment for eye, kidney, and foot problems [4].
Glycemic management in type 2 diabetes mellitus (T2DM), the focus of this review, is growing increasingly complex and has been the subject of numerous extensive reviews [5,6] and published guidelines [4,7]. In the context of an increasing array of available pharmacologic options, there are mounting uncertainties regarding the benefits of intensive glycemic control as well as increasing concerns about potential adverse treatment effects, hypoglycemia in particular. While previous guidelines encouraged specific approaches for most patients, more recent guidelines stress the importance of a patient-centered approach with shared decision-making [4]. Less prescriptive guidelines are more appropriate, given the current state of science, but they also may be viewed as providing insufficient guidance to some providers. It can be overwhelming for a non-specialist to try to match the nuances of antihyperglycemic medications to the nuances of each patient’s preferences and medical characteristics.
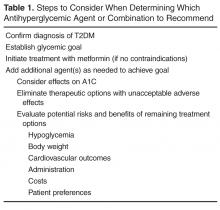
This article examines key issues faced by primary care providers when managing hyperglycemia in patients with T2DM and outlines a stepwise approach to determining the optimal antihyperglycemic agent(s) (Table 1).
Confirm Diagnosis of T2DM
It can be difficult to distinguish between type 1 diabetes mellitus and T2DM in some individuals due to overlapping characteristics. However, correctly classifying a patient’s diabetes at the outset is essential, as the classification helps determine the best treatment regimen and is rarely reconsidered [4,8]. Considerable evidence suggests that misclassification of diabetes occurs frequently [9,10], resulting in patients receiving inappropriate treatment. Clinical characteristics suggestive of T2DM include older age and features of insulin resistance such as obesity, hyper-tension, hypertriglyceridemia, and low high-density lipoprotein cholesterol. When these features are not present, an alternate diagnosis should be entertained.
Establish Glycemic Goal
Research over the past decade has led to a growing appreciation of the enormous complexity of hyperglycemia management. During the 1990s, landmark trials such as the Diabetes Control and Complications Trial (DCCT) [11] and UK Prospective Diabetes Study (UKPDS) [12] demonstrated that improving glucose control could reduce the incidence of microvascular complications [11,12], prompting a lower-is-better philosophy regarding glucose targets. Despite limited evidence to support such thinking, this viewpoint was adopted by the developers of many guidelines. During the following decade more research was devoted to determining whether aggressively lowering a patient’s glucose could also improve macrovascular outcomes. Table 2 summarizes microvascular and macrovascular effects of intensive glycemic control seen in major trials [11–23]. After several major trials [20,22] found only mild cardiovascular benefits and even suggested harm [18], experts and policy makers began to reconsider the value of tightly controlling glucose levels [24]. Since then, other studies have demonstrated that the potential benefits and risks of glucose control are strongly related to individual patient factors, such as age and duration of diabetes, and associated comorbidities, such as CVD and impaired renal function [6].
A one-size-fits-all glycemic goal is no longer recommended. Personalization is necessary, balancing the potential benefits and risks of treatments required to achieve that goal. Whereas an A1C of < 7% is an appropriate target for some individuals with diabetes, glycemic targets may be more or less stringent based on patient features including life expectancy, duration of diabetes, comorbidities, and patient attitude and support system (Table 3) [4].
A particular group in which less stringent goals should be considered is older patients, especially those with complex or poor health status [4,25]. The risk of intensive glycemic control may exceed the benefits in these patients, as they are at higher risk of hypoglycemia and polypharmacy [26]. A goal A1C of 7% to 7.5% is now recommended for healthy older adults, and less stringent A1C goals of 7.5% to 8% and 8% to 8.5% should be considered based on the presence and severity of multiple coexisting chronic illnesses, decreased self-care ability, or cognitive impairment [4,25]. Unfortunately, overtreatment is frequently seen in this group. In a recent study of patients over age 65 years, about 40% of those with complex or poor health status had tight glycemic control with A1C below 6.5% [26]. An analysis of U.S. Veterans Affairs administration data showed that only 27% of 12,917 patients older than 65 with very low A1C (< 6%) and about 21% of those with A1C of 6% to 6.5% underwent treatment deintensification [27].
Initiate Treatment with Metformin
There is strong consensus that metformin is the preferred drug for monotherapy due to its long proven safety record, low cost, weight-reduction benefit, and potential cardiovascular advantages [4,16]. As long as there are no contraindications, metformin should be recommended concurrent with lifestyle intervention at the time of diabetes diagnosis. The recommendation is based on the fact that adherence to diet, weight reduction, and regular exercise is not sustained in most patients, and most patients ultimately will require treatment. Since metformin is usually well-tolerated, does not cause hypoglycemia, has a favorable effect on body weight, and is relatively inexpensive, potential benefits of early initiation of medication appear to outweigh potential risks.
The U.S. Food and Drug Administration (FDA) recently relaxed prescribing polices to extend the use of this important medication to patients who have mild–moderate, but stable, chronic kidney disease (CKD) [28]. Metformin is recommended as first-line therapy and should be used unless it is contraindicated (ie, estimated glomerular filtration rate [eGFR] < 30 mL/min/1.73 m2)[4,7,29].
Add Additional Agent(s) as Needed to Achieve Goal
Other than metformin, evidence is limited for the optimal use of the burgeoning array of available agents, especially in dual or triple combinations [6,30]. Research is now starting to focus more on what the ideal number and sequence of drugs should be. The Glycemic Reduction Approach in Diabetes (GRADE) study, which will compare long-term benefits and risks of the 4 most widely used antihyperglycemic medications in combination with metformin, is now underway [31,32]. The 4 classes being studied are sulfonylurea, dipeptidyl peptidase-4 (DPP-4) inhibitors, glucagon-like peptide-1 (GLP-1) receptor agonists, and a basal,
Eleven classes of non-insulin medications are currently approved for treating hyperglycemia in T2DM [4]. Within each class, numerous agents are available. Six of these classes (ie, α-glucosidase inhibitors, colesevelam, bromocriptine, pramlintide, meglitinides, and thiazolidinediones) are not used frequently
Consider Effects on A1C
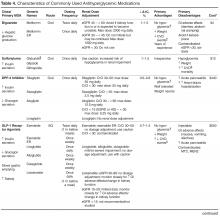
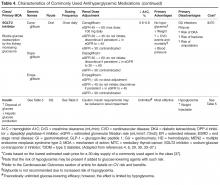
There is a paucity of high-quality, head-to-head comparison trials evaluating the ability of available agents to achieve recommended glycemic targets. This is important because the glucose-lowering effectiveness of individual medications is strongly influenced by baseline characteristics such as A1C, duration of diabetes, and previous therapy. With these limitations in mind, the relative glucose-lowering effectiveness of commonly used agents is shown in Table 4. When used as monotherapy, A1C reductions of approximately 1% to 1.5% are achieved with metformin, sulfonylureas, and GLP-1 receptor agonists [6,30,34,35,39]. DPP-4 inhibitors and SGLT-2 inhibitors have more modest glucose-lowering efficacy, with A1C reductions of approximately 0.5% to 1% [6,30,34,35,39]. Larger effects may be seen in individuals with higher baseline A1C and those who are drug naïve. Insulin is the most effective glucose-lowering agent—it can reduce virtually any level of A1C down to the normal range, with hypoglycemia being the only limiting factor. When a patient has uncontrolled hyperglycemia on metformin monotherapy, or if there is a contraindication or intolerance to metformin, clinicians should consider the potential glucose-lowering effects of other available options and should choose an agent that conceivably could bring a patient close to meeting their treatment goal.
Eliminate Options with Unacceptable Adverse Effects
When the pharmacologic options with acceptable A1C-lowering potential have been identified, the ones with contraindications and potential serious adverse effects for the individual patient can immediately be eliminated (Table 4). For example, if a patient has an eGFR < 30 mL/min/1.73 m2, metformin, sulfonylureas, GLP-1 receptor agonists, most DPP-4 inhibitors, and SGLT-2 inhibitors are either contraindicated or should be used with caution. In patients with severe osteoporosis, SGLT-2 inhibitors may not be the best option. In patients with a history of diabetic ketoacidosis (DKA), caution should be used with metformin and SGLT-2 inhibitors. There have been concerns of possible acute pancreatitis and neoplasia with the incretin-based agents, the DPP-4 inhibitors and GLP-1 receptor agonists [40,41], although other clinical trials and observational data have not found increased risk [42–45]. Nevertheless, these agents potentially should be avoided in patients with a history of pancreatitis or neoplasm. SGLT-2 inhibitors may be associated with genitourinary infections and volume depletion [46–48] and probably should be avoided in patients at high risk for these conditions.
If the adverse effects are not serious, changing the way the medication is administered may allow the patient to tolerate agents with high potential benefits. For example, metformin is commonly associated with gastrointestinal (GI) adverse effects, which can be reduced or avoided with slow titration of the dose [6] or by switching to an extended-release formulation [49]. GLP-1 receptor agonists are associated with GI adverse effects [6] and in most cases slow titration is recommended.
Evaluate Potential Risks/Benefits of Remaining Options
Hypoglycemia. The barrier of hypoglycemia generally precludes maintenance of euglycemia and full realization of the long-term benefits of good glucose control over a lifetime. Once considered a trivial issue, concerns about hypoglycemia in T2DM are increasingly being raised [19,50–55]. Clearly, hypoglycemia occurs more often as glycemic targets are lowered to near-normal values, especially in those with advanced age and multiple comorbidities [55]. Various comorbidities frequently encountered particularly as patients age also are associated with increasing propensity for experiencing hypoglycemia and untoward outcomes from it. These include coronary artery disease, heart failure, renal and liver disease, and dementia. Hypoglycemia, when it occurs, may lead to dysrhythmias, dizziness, accidents and falls, work disability, and decreased quality of life. In addition to relaxing blood glucose targets in high-risk patients, drug selection should favor agents that do not precipitate such events (Table 4).
Fortunately, the commonly used non-insulin agents are not associated with hypoglycemia unless they are used in combination with sulfonylureas or insulin. Sulfonylureas should be used with caution and other options considered in patients with high risk for hypoglycemia. When insulin is required, regimens which minimize risk of hypoglycemia should be used. For example, adding a GLP-1 receptor agonist to basal insulin as an alternative to mealtime insulin has been shown to be equally effective with a lower risk of hypoglycemia [4,6]. Also, premixed insulin preparations should be avoided or used cautiously in individuals who miss meals frequently. Additionally, newer basal insulins that exhibit longer duration of action are now available in the United States. Preliminary studies have shown that the newly FDA-approved longer-acting basal insulins, insulin degludec and glargine U-300, may be associated with a reduced risk for hypoglycemia [56,57]. However, it remains unclear how and when these newer agents will best be incorporated into a treatment regimen.
Body weight. Nearly 90% of people living with T2DM are overweight or obese. Given the close tie between obesity and T2DM, treating obesity is an obvious consideration in diabetes treatment. Major trials have shown the effectiveness of lifestyle modifications and weight reduction in delaying, prevention, and management of T2DM [4,58,59].With this in mind, clinicians should consider preferentially using antihyperglycemic agents with weight-lowering or weight-neutral effects. Among commonly used antihyperglycemic agents, metformin, GLP-1 receptor agonists, and SGLT-2 inhibitors have been shown to have weight-reduction benefits, and DPP-4 inhibitors are weight neutral. On the other hand, sulfonylureas and insulin are associated with weight gain. A systematic review and meta-analysis including 204 studies with study durations ranging from 3 months to 8 years showed comparative effects of diabetes medications with a differential effect on weight of up to 5 kg (Table 4) [60].
Metformin is associated with an average weight loss of 1.9 to 3.1 kg that was sustained with long-term use for at least 10 years in the Diabetes Prevention Program Outcomes Study [61].A systematic review of 7 randomized trials showed that in patients with T2DM, the SGLT-2 inhibitors dapagliflozin and canagliflozin were associated with weight loss (mean weighted difference of –1.81 kg and –2.3 kg, respectively) [62]. A systematic review and meta-analysis of 25 randomized controlled trials showed greater weight loss (mean weighted difference of –2.9 kg) in overweight or obese patients with or without T2DM using GLP-1 receptor agonists when compared to placebo, insulin, or oral antihyperglycemic agents [63]. Of note, the GLP-1 receptor agonist liraglutide is now approved for weight loss in patients with or without diabetes [64]. The maximum doses approved for diabetes and obesity treatment are 1.8 and 3.0 mg/day, respectively.
Since weight loss is associated with improved glycemic control, an area of emerging interest is the use of antiobesity medications for managing diabetes. Although most older weight-loss medications were only approved for short-term use, some newer agents are approved for longer-term use. Lorcaserin and the combination drugs topiramate/phentermine and naltrexone/bupropion are approved for chronic therapy, provided certain conditions are met. Patients on weight reduction agents should be monitored regularly.
An even more radical departure from conventional therapy for diabetes is the consideration of metabolic, or weight-loss, surgery, which has been found to be associated with rapid and dramatic improvements in blood glucose control. Metabolic surgery has been shown to improve glucose control more effectively than any known pharmaceutical or behavioral approach. For example, in an observational study of obese patients with T2DM, bariatric surgery led to diabetes remission rates of 72.3% 2 years after surgery and 30.4% 15 years after surgery compared to 16.4% and 6.5%, respectively, in control patients [69]. With long-term follow-up, significant decreases in microvascular and macrovascular complications were seen in the surgical group [69]. Compared with medical therapy alone, bariatric surgery plus medical therapy has been associated with more weight loss, better glycemic control, less need for diabetes medications, and improved quality of life [70]. A 2016 joint statement by numerous international diabetes organizations recommends considering metabolic surgery as a treatment for T2DM and obesity [71]. American Diabetes Association guidelines recommend consideration of bariatric surgery in individuals with T2DM who have a body mass index greater than 35 kg/m2,especially if achieving disease control is difficult by means of lifestyle modifications and medications [4].
Cardiovascular outcomes. Cardiovascular risk is about 2 to 4 times higher in patients with diabetes, and about half of patients with this condition develop heart failure [4,72]. CVD is responsible for most of the mortality in T2DM [72]. Therefore, prevention of cardiovascular morbidity and mortality is an important goal for diabetes treatment. Due to concerns about potential cardiovascular risks associated with glucose-lowering medications [73–76], the FDA has issued regulatory requirements for manufacturers to monitor the cardiovascular risk profile for these drugs [77]. Recent trials have led to a better understanding of potential cardiovascular benefits or harms of antihyperglycemic medications.
Metformin, the widely recommended first-line therapy for T2DM, carries a large body of evidence supporting its cardiovascular benefits. For example, the UKPDS found that compared to conventional therapy (mostly diet), metformin reduced cardiovascular events and mortality in obese patients with T2DM [15]. This result was supported in Hyperinsulinemia: the Outcome of its Metabolic Effect (HOME) study where, as an add-on to insulin, metformin decreased macrovascular complications when compared to placebo [78]. Research over the past decade also has assuaged concerns about metformin safety in heart failure [60]. A systematic review of observational studies involving 34,000 patients conducted in 2013 showed that metformin is as safe as other glucose-lowering medications in patients with diabetes and heart failure even in the presence of CKD [4,79]. Furthermore, numerous investigations have found metformin is not associated with increased hospitalizations or risk of lactic acidosis [80]. Metformin can be used safely in patients with diabetes and heart failure [60].
Although sulfonylureas have long been a mainstay of diabetes therapy, concerns about their potential adverse cardiovascular effects have been raised by numerous studies [81]. Tolbutamide, a first-generation sulfonylurea, was removed from the market after the University Group Diabetes Program study found increased CVD deaths with this agent versus placebo. Subsequently, the FDA issued a warning for all sulfonylureas [74]. The increased cardiovascular risk associated with sulfonylureas is thought to be due to their effect on cardiac mitochondrial potassium ATP channels. Sulfonylureas bind to these channels, preventing a protective phenomenon called ischemic preconditioning and resulting in a weakened defense against myocardial injury [76]. A recent study showed an increased risk of coronary heart disease associated with long-term use of sulfonylureas in women with diabetes [81].
GLP-1 receptor agonists have recently received much attention for their potential beneficial effects on cardiovascular outcomes. In a recent trial, lixisenatide was shown to be safe in patients with T2DM and acute coronary syndrome when compared to placebo [82]. More recently, the Liraglutide Effect and Action in Diabetes: Evaluation of cardiovascular outcome Results (LEADER) trial demonstrated significant cardiovascular benefits with liraglutide in patients with T2DM and established or high CVD risk [83]. The composite outcome of the first occurrence of death from cardiovascular causes, nonfatal myocardial infarction (MI), or nonfatal stroke, occurred less frequently in the liraglutide group compared to placebo (13% versus 14.9%, respectively), and there were fewer deaths from cardiovascular causes in the liraglutide group compared to placebo (4.7% and 6.0%, respectively) [83]. Other trials investigating the cardiovascular outcomes of this class [84,85] are in progress.
Another class with potential cardiovascular benefits is the SGLT-2 inhibitors. In a recent cardiovascular outcome study, empagliflozin significantly lowered the composite of cardiovascular death, nonfatal MI, or nonfatal stroke in T2DM patients with high cardiovascular risk compared to placebo (10.5% and 12.1%, respectively) [86]. There are several large ongoing studies evaluating the cardiovascular effects of other SGLT-2 inhibitors [87–89].
DPP-4 inhibitors were examined in recent studies and have shown no cardiovascular benefits [42,44,90].The studies showed mixed results regarding an association between DPP-4 inhibitors and heart failure. In one study, saxagliptin was associated with increased hospitalization for heart failure compared to placebo [44], while 2 noninferiority trials did not show a significant increase in heart failure hospitalizations associated with alogliptin and sitagliptin when compared to placebo [42,90].
Administration Considerations
Many patients with T2DM require multiple agents for glycemic control. Additional medications used for comorbid conditions add to this burden. When choosing antihyperglycemic agents, the route and frequency of administration, as well as the patients’ preferences and ability, should be considered. Either once or twice daily dosing is available for most agents, and once weekly dosing is available for some of the GLP-1 receptor agonists. Once daily or once weekly formulations may improve adherence and be more desirable than preparations that are dosed twice daily. Most of the commonly used medications are dosed orally. Although many patients find this route of administration preferable to insulin or GLP-1 receptor agonists, which require injections, some patients may prefer the risk/benefit of injectable agents. All GLP-1 receptor agonists come in a pen delivery system, which eliminates mixing and provides more convenient administration. Extended-release exenatide also is available as a single-dose tray that requires mixing and may be more cumbersome to inject.
Insulin requires special consideration. There has been an enormous increase in the number of insulin products on the market in the past 2 decades. These products include insulin analogs, concentrated insulins (U-200, U-300, and U-500), premixed insulin preparations, and ultra-long-acting insulin [91]. The availability of insulin options with different concentrations, onsets, and durations of actions has made decision making on which insulin to use difficult. Clinicians need to consider patient preference, dosing frequency, and timing with regard to meals, insulin dose, administration, as well as cost. For example, concentrated insulin is preferred for a patient on high doses of insulin requiring injecting a large volume of insulin. Rapid-acting insulin analogs would be more appropriate for patients who have difficulty administering their regular insulin 20 to 30 minutes before eating. Premixed insulin preparations make it impossible to independently adjust short- and long-acting components. However, these may be good choices in patients who have consistent meal schedules and who want to simplify administration. Despite a prevailing misconception that NPH must be given twice a day, it has long been recognized that in T2DM, a single daily injection of NPH yields improvements in control similar to those achieved with 2 daily injections [92].
Cost Considerations
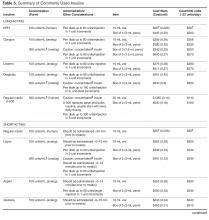
Treating T2DM imposes a great financial burden on individuals living with diabetes and their families due to the high cost of the medications. Table 4 and Table 5 provide information on the cost of non-insulin and insulin diabetes medications for patients who do not have prescription insurance coverage. From a practical standpoint, choice of diabetes agents is largely influenced by insurance formularies.
The older agents, metformin and the sulfonylureas, are available for a cash (no insurance) price of as little as $4 per month. This is in stark contrast to the SGLT-2 inhibitors, GLP-1 receptor agonists, and DPP-4 inhibitors, which range in cost between $400 and $600 per month. Of recent concern, the cost of insulin has been skyrocketing, with a more than 500% increase in the cost of certain insulins from 2001 to 2015 [93]. According to the Medical Expenditure Panel Survey (MEPS) from 2002 to 2013, the mean price of insulin increased by about 200% (from $4.34/mL to $12.92/mL) during this period, which was significantly higher than increases in the price of non-insulin comparators [94]. The introduction of biosimilar insulins to the market is expected to offer treatment options with lower cost. This will be tested when the biosimilar glargine, the first FDA-approved biosimilar insulin, becomes available in the U.S. market. However, a significant reduction in insulin prices is not expected soon [95].
When insulin is required, most patients with T2DM can be treated with older human insulins, which have similar efficacy and lower costs than the more expensive newer insulin analogs. A Cochrane review comparing basal insulin analogs to NPH showed similar efficacy in glycemic control with minimal clinical benefit in the form of less nocturnal hypoglycemia in the insulin analog arm [96]. Furthermore, similar glycemic control and risk of hypoglycemia was seen when regular insulin was compared with the rapid-acting insulin analogs [97]. The cost of human NPH insulin for a patient on a total daily dose of 60 units is approximately $52 per month. This contrasts with the most widely used insulin, insulin glargine, which has a cash price of about $500 per month for the same amount (Table 5). Insulin pens, which are convenient, are more expensive. Interestingly, human insulins do not require prescriptions, allowing underinsured, underfunded patients ongoing access to them.
Incorporating Patient Preferences
Research evidence is necessary but insufficient for making patient care decisions. Along with the potential benefits, harms, costs, and inconveniences of the management options, patient perspectives, beliefs, expectations, and health-related goals must be considered. Patients will undoubtedly have preferences regarding defining goals and ranking options. Clinicians should discuss therapeutic goals and treatment options and work collaboratively with patients in determining management strategies [98].
Summary
Potential treatment approaches for treating hyperglycemia in T2DM are summarized in Figure 1 and Figure 2 [4,7]. As long as there are no contraindications, metformin should be recommended concurrent with lifestyle intervention at the time of diabetes diagnosis. Even if metformin monotherapy is initially effective, glycemic control is likely to deteriorate over time due to progressive loss of β-cell function in T2DM.
There is no consensus as to what the second-line agent should be. Selection of a second agent should be made based on potential advantages and disadvantages of each agent for any given patient. A patient-centered approach is preferred over a fixed algorithm. If the patient progresses to the point where dual therapy does not provide adequate control, either a third non-insulin agent or insulin can be added. In patients with modestly elevated A1C (below ~8%), addition of a third non-insulin agent may be equally effective as (but more expensive than) addition of insulin.
Patients with significantly elevated A1C levels on non-insulin agents usually should have insulin added to their regimen. When insulin is added, metformin should be continued. DPP-4 inhibitors and sulfonylureas are typically stopped. If SGLT-2 inhibitors and/or GLP-1 receptor agonists are continued, this may aid with weight maintenance. However, continuing these agents is likely to be expensive and associated with problems associated with polypharmacy.
The most widely recommended strategy for initiating insulin in T2DM is to add a single bedtime injection of basal insulin (ie, NPH, glargine, detemir, or degludec) to the patient’s regimen. This regimen has been found to be effective in numerous studies and controls hyperglycemia in up to 60% of patients [99]. If the patient is treated with a single bedtime injection of insulin and the fasting glucose level is within the target range but the A1C level remains above goal, addition of mealtime insulin injections is likely to be beneficial. Alternatively, addition of a GLP-1 receptor agonist to basal insulin has been shown to be equally beneficial [4,6]. When adding mealtime insulin, a common strategy is to add a single injection of a rapid-acting insulin (eg, lispro, aspart, glulisine) before the patient’s largest meal of the day. Additional premeal injections of rapid-acting insulin may be added as needed, based on self-monitoring blood glucose results. If glycemia remains significantly uncontrolled on more than 200 units of insulin per day, switching to a concentrated form of insulin (eg, U-200, U-300, or U-500) should be considered.
Corresponding author: Maryam Fazel, PharmD, BCPS, BCACP, CDE, 1295 N. Martin Ave. (Room B211B), Tucson, Arizona 85721-0202, maryamfazel@pharmacy.arizona.edu.
Financial disclosures: None.
1. National diabetes statistics report: estimates of diabetes and its burden in the United States, 2014. Centers for Disease Control and Prevention Web site. www.cdc.gov/diabetes/pubs/statsreport14/national-diabetes-report-web.pdf. Accessed November 29, 2016.
2. Statistics about diabetes. American Diabetes Association Web site. www.diabetes.org/diabetes-basics/statistics/. Accessed November 29, 2016.
3. American Diabetes Association. Economic costs of diabetes in the U.S. in 2012. Diabetes Care 2013;36:1033–46.
4. American Diabetes Association. Standards of medical care in diabetes--2016. Diabetes Care 2016;39(Suppl. 1).
5. Raz I, Riddle MC, Rosenstock J, et al. Personalized management of hyperglycemia in type 2 diabetes: reflections from a Diabetes Care Editors’ Expert Forum. Diabetes Care 2013;36:1779–88.
6. Inzucchi SE, Bergenstal RM, Buse JB, et al. Management of hyperglycemia in type 2 diabetes, 2015: a patient-centered approach: update to a position statement of the American Diabetes Association and the European Association for the Study of Diabetes. Diabetes Care 2015;38:140–9.
7. Garber AJ, Abrahamson MJ, Barzilay JI, et al. Consensus Statement by the American Association of Clinical Endocrinologists and American College of Endocrinology on the Comprehensive Type 2 Diabetes Management Algorithm--2016 Executive Summary. Endocr Pract 2016;22:84–113.
8. Steenkamp DW, Alexanian SM, Sternthal E. Approach to the patient with atypical diabetes. CMAJ 2014;186:678–84.
9. de Lusignan S, Sadek N, Mulnier H, et al. Miscoding, misclassification and misdiagnosis of diabetes in primary care. Diabet Med 2012;29:181–9.
10. Tripathi A, Rizvi AA, Knight LM, Jerrell JM. Prevalence and impact of initial misclassification of pediatric type 1 diabetes mellitus. South Med J 2012;105:513–7.
11. The effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin-dependent diabetes mellitus. The Diabetes Control and Complications Trial Research Group. N Engl J Med 1993;329:977–86.
12. Intensive blood-glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33). UK Prospective Diabetes Study (UKPDS) Group. Lancet 1998;352:837–53.
13. Nathan DM, Cleary PA, Backlund JY, et al. Intensive diabetes treatment and cardiovascular disease in patients with type 1 diabetes. N Engl J Med 2005;353:2643–53.
14. Nathan DM, DCCT/EDIC Research Group. The diabetes control and complications trial/epidemiology of diabetes interventions and complications study at 30 years: overview. Diabetes Care 2014;37:9–16.
15. Effect of intensive blood-glucose control with metformin on complications in overweight patients with type 2 diabetes (UKPDS 34). UK Prospective Diabetes Study (UKPDS) Group. Lancet 1998;352:854–65.
16. Holman RR, Paul SK, Bethel MA, et al. 10-Year follow-up of intensive glucose control in type 2 diabetes. N Engl J Med 2008;359:1577–89.
17. Ohkubo Y, Kishikawa H, Araki E, et al. Intensive insulin therapy prevents the progression of diabetic microvascular complications in Japanese patients with non-insulin-dependent diabetes mellitus: a randomized prospective 6-year study. Diabetes Res Clin Pract 1995;28:103–17.
18. Action to Control Cardiovascular Risk in Diabetes Study Group, Gerstein HC, Miller ME, Byington RP, et al. Effects of intensive glucose lowering in type 2 diabetes. N Engl J Med 2008;358:2545–59.
19. ACCORD Study Group, Gerstein HC, Miller ME, Genuth S, et al. Long-term effects of intensive glucose lowering on cardiovascular outcomes. N Engl J Med 2011;364:818–28.
20. ADVANCE Collaborative Group, Patel A, MacMahon S, Chalmers J, et al. Intensive blood glucose control and vascular outcomes in patients with type 2 diabetes. N Engl J Med 2008;358:2560–72.
21. Wong MG, Perkovic V, Chalmers J, et al. Long-term Benefits of Intensive Glucose Control for Preventing End-Stage Kidney Disease: ADVANCE-ON. Diabetes Care 2016;39:694–700.
22. Duckworth W, Abraira C, Moritz T, et al. Glucose control and vascular complications in veterans with type 2 diabetes. N Engl J Med 2009;360:129–39.
23. Hayward RA, Reaven PD, Wiitala WL, et al. Follow-up of glycemic control and cardiovascular outcomes in type 2 diabetes. N Engl J Med 2015;372:2197–206.
24. American Diabetes Association. Standards of medical care in diabetes--2009. Diabetes Care 2009;32 Suppl 1:S13–61.
25. American Geriatrics Society Expert Panel on Care of Older Adults with Diabetes Mellitus, Moreno G, Mangione CM, Kimbro L, Vaisberg E. Guidelines abstracted from the American Geriatrics Society Guidelines for Improving the Care of Older Adults with Diabetes Mellitus: 2013 update. J Am Geriatr Soc 2013;61:2020–6.
26. Lipska KJ, Ross JS, Miao Y, et al. Potential overtreatment of diabetes mellitus in older adults with tight glycemic control. JAMA Intern Med 2015;175:356–62.
27. Sussman JB, Kerr EA, Saini SD, et al. Rates of deintensification of blood pressure and glycemic medication treatment based on levels of control and life expectancy in older patients with diabetes mellitus. JAMA Intern Med 2015;175:1942–9.
28. FDA Drug Safety Communication: FDA revises warnings regarding use of the diabetes medicine metformin in certain patients with reduced kidney function. FDA Web site. www.fda.gov/Drugs/DrugSafety/ucm493244.htm. Accessed December 1, 2016.
29. Inzucchi SE, Lipska KJ, Mayo H, et al. Metformin in patients with type 2 diabetes and kidney disease: a systematic review. JAMA 2014;312:2668–75.
30. Nathan DM. Diabetes: advances in diagnosis and treatment. JAMA 2015;314:1052–62.
31. Nathan DM, Buse JB, Kahn SE, et al. Rationale and design of the glycemia reduction approaches in diabetes: a comparative effectiveness study (GRADE). Diabetes Care 2013;36:2254–61.
32. NIH begins recruitment for long-term study of diabetes drug efficacy. NIH Web site. www.nih.gov/news-events/news-releases/nih-begins-recruitment-long-term-study-diabetes-drug-efficacy. Accessed December 1, 2016.
33. Hermayer KL, Dake A. Newer oral and noninsulin therapies to treat type 2 diabetes mellitus. Cleve Clin J Med 2016;83(5 Suppl 1):S18–26.
34. Bolen S, Wilson L, Vassy J, et al. Systematic review: comparative effectiveness and safety of oral medications for type 2 diabetes mellitus. Ann Intern Med 2007;147:386–99.
35. Bolen S, Tseng E, Hutfless S, et al. Oral diabetes medications for adults with type 2 diabetes: an update. Agency for Healthcare Research and Quality (US); 2011 Mar Report No: 11-EHC038-EF. AHRQ Comparative Effectiveness Reviews.
36. Metformin, glyburide, glipizide, glimeperide, sitagliptin, saxagliptin, linagliptin, lixisenatide, alogliptin, exenatide, liraglutide, albiglutide, dulaglutide, canagliflozin, danagliflozin, empagliflozin: drug information. Waltham (MA): UpToDate, Inc.; 2016. Accessed September 23, 2016.
37. GoodRx Web site. http://www.goodrx.com. Accessed August 6, 2016 and December 2016.
38. Insulins available in the United States. Diabetesforcast Web site. Accessed August 6, 2016. www.diabetesforecast.org/2016/mar-apr/images/2016-insulin-chart-new.pdf.
39. Inzucchi SE, Bergenstal RM, Buse JB, et al. Management of hyperglycaemia in type 2 diabetes: a patient-centered approach. Position statement of the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD). Diabetologia 2012;55:577–96.
40. Elashoff M, Matveyenko AV, Gier B, et al. Pancreatitis, pancreatic, and thyroid cancer with glucagon-like peptide-1-based therapies. Gastroenterology 2011;141:150–6.
41. Butler AE, Campbell-Thompson M, Gurlo T, et al. Marked expansion of exocrine and endocrine pancreas with incretin therapy in humans with increased exocrine pancreas dysplasia and the potential for glucagon-producing neuroendocrine tumors. Diabetes 2013;62:2595–604.
42. Green JB, Bethel MA, Armstrong PW, et al. Effect of sitagliptin on cardiovascular outcomes in type 2 diabetes. N Engl J Med 2015;373:232–42.
43. White WB, Cannon CP, Heller SR, et al. Alogliptin after acute coronary syndrome in patients with type 2 diabetes. N Engl J Med 2013;369:1327–35.
44. Scirica BM, Bhatt DL, Braunwald E, et al. Saxagliptin and cardiovascular outcomes in patients with type 2 diabetes mellitus. N Engl J Med 2013;369:1317–26.
45. Egan AG, Blind E, Dunder K, et al. Pancreatic safety of incretin-based drugs--FDA and EMA assessment. N Engl J Med 2014;370:794–7.
46. Vasilakou D, Karagiannis T, Athanasiadou E, et al. Sodium-glucose cotransporter 2 inhibitors for type 2 diabetes: a systematic review and meta-analysis. Ann Intern Med 2013;159:262–74.
47. Nyirjesy P, Sobel JD, Fung A, et al. Genital mycotic infections with canagliflozin, a sodium glucose co-transporter 2 inhibitor, in patients with type 2 diabetes mellitus: a pooled analysis of clinical studies. Curr Med Res Opin 2014;30:1109–19.
48. Schernthaner G, Gross JL, Rosenstock J, et al. Canagliflozin compared with sitagliptin for patients with type 2 diabetes who do not have adequate glycemic control with metformin plus sulfonylurea: a 52-week randomized trial. Diabetes Care 2013;36:2508–15.
49. Blonde L, Dailey G, Jabbour S, et al. Gastrointestinal tolerability of extended-release metformin tablets compared to immediate-release metformin tablets: results of a retrospective cohort study. Curr Med Res Opin 2004;20:562–72.
50. Kalra S, Mukherjee JJ, Venkataraman S, et al. Hypoglycemia: the neglected complication. Indian J Endocrinol Metab 2013;17:819–34.
51. Paty BW. The role of hypoglycemia in cardiovascular outcomes in diabetes. Can J Diabetes 2015;39 Suppl 5:S155–9.
52. Zoungas S, Patel A, Chalmers J, et al. Severe hypoglycemia and risks of vascular events and death. N Engl J Med 2010;363:1410–8.
53. Whitmer RA, Karter AJ, Yaffe K, et al. Hypoglycemic episodes and risk of dementia in older patients with type 2 diabetes mellitus. JAMA 2009;301:1565–72.
54. McCoy RG, Van Houten HK, Ziegenfuss JY, et al. Increased mortality of patients with diabetes reporting severe hypoglycemia. Diabetes Care 2012;35:1897–901.
55. McCoy RG, Lipska KJ, Yao X, et al. Intensive treatment and severe hypoglycemia among adults with type 2 diabetes. JAMA Intern Med 2016;176:969–78.
56. Rodbard HW, Gough S, Lane W, et al. Reduced risk of hypoglycemia with insulin degludec versus insulin glargine in patients with type 2 diabetes requiring high doses of basal insulin: a meta-analysis of 5 randomized begin trials. Endocr Pract 2014;20:285–92.
57. Yki-Jarvinen H, Bergenstal R, Ziemen M, et al. New insulin glargine 300 units/mL versus glargine 100 units/mL in people with type 2 diabetes using oral agents and basal insulin: glucose control and hypoglycemia in a 6-month randomized controlled trial (EDITION 2). Diabetes Care 2014;37:3235–43.
58. Tuomilehto J, Lindstrom J, Eriksson JG, et al. Prevention of type 2 diabetes mellitus by changes in lifestyle among subjects with impaired glucose tolerance. N Engl J Med 2001;344:1343–50.
59. Knowler WC, Barrett-Connor E, Fowler SE, et al. Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin. N Engl J Med 2002;346:393–403.
60. Maruthur NM, Tseng E, Hutfless S, et al. Diabetes medications as monotherapy or metformin-based combination therapy for type 2 diabetes: a systematic review and meta-analysis. Ann Intern Med 2016;164:740–51.
61. Diabetes Prevention Program Research Group. Long-term safety, tolerability, and weight loss associated with metformin in the Diabetes Prevention Program Outcomes Study. Diabetes Care 2012;35:731–7.
62. Clar C, Gill JA, Court R, Waugh N. Systematic review of SGLT2 receptor inhibitors in dual or triple therapy in type 2 diabetes. BMJ Open 2012;2:10.1136/bmjopen,2012-001007.
63. Vilsboll T, Christensen M, Junker AE, et al. Effects of glucagon-like peptide-1 receptor agonists on weight loss: systematic review and meta-analyses of randomised controlled trials. BMJ 2012;344:d7771.
64. FDA approves weight-management drug Saxenda. FDA Web site www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm427913.htm. Accessed September 22, 2016.
65. Apovian CM, Aronne LJ, Bessesen DH, et al. Pharmacological management of obesity: an endocrine Society clinical practice guideline. J Clin Endocrinol Metab 2015;100:342–62.
66. Liraglutide, lorcaserin, naltrexone/bupropion, orlistat, phentermine/topiramate: drug information. Greenwood Village (CO): Truven Health Analytics; 2016. www.micromedexsolutions.com. Accessed May 13, 2016.
67. Liraglutide, lorcaserin, naltrexone/bupropion, orlistat, phentermine/topiramate: drug information. Waltham (MA): UpToDate, Inc.; 2016. Accessed May 13, 2016.
68. Yanovski SZ, Yanovski JA. Long-term drug treatment for obesity: a systematic and clinical review. JAMA 2014;311:74–86.
69. Sjostrom L, Peltonen M, Jacobson P, et al. Association of bariatric surgery with long-term remission of type 2 diabetes and with microvascular and macrovascular complications. JAMA 2014;311:2297–304.
70. Schauer PR, Bhatt DL, Kirwan JP, et al. Bariatric surgery versus intensive medical therapy for diabetes--3-year outcomes. N Engl J Med 2014;370:2002–13.
71. Rubino F, Nathan DM, Eckel RH, et al. Metabolic surgery in the treatment algorithm for type 2 diabetes: a joint statement by international diabetes organizations. Diabetes Care 2016;39:861–77.
72. Lathief S, Inzucchi SE. Approach to diabetes management in patients with CVD. Trends Cardiovasc Med 2016;26:165–79.
73. Nissen SE, Wolski K. Effect of rosiglitazone on the risk of myocardial infarction and death from cardiovascular causes. N Engl J Med 2007;356:2457–71.
74. Knatterud GL, Klimt CR, Levin ME, et al. Effects of hypoglycemic agents on vascular complications in patients with adult-onset diabetes. VII. Mortality and selected nonfatal events with insulin treatment. JAMA 1978;240:37–42.
75. Masoudi FA, Inzucchi SE, Wang Y, et al. Thiazolidinediones, metformin, and outcomes in older patients with diabetes and heart failure: an observational study. Circulation 2005;111:583–90.
76. Klepzig H, Kober G, Matter C, et al. Sulfonylureas and ischaemic preconditioning; a double-blind, placebo-controlled evaluation of glimepiride and glibenclamide. Eur Heart J 1999;20:439–46.
77. FDA announces new recommendations on evaluating cardiovascular risk in drugs intended to treat type 2 diabetes. FDA Web site. www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/2008/ucm116994.htm. Accessed August 20, 2016.
78. Kooy A, de Jager J, Lehert P, et al. Long-term effects of metformin on metabolism and microvascular and macrovascular disease in patients with type 2 diabetes mellitus. Arch Intern Med 2009;169:616–25.
79. Eurich DT, Weir DL, Majumdar SR, et al. Comparative safety and effectiveness of metformin in patients with diabetes mellitus and heart failure: systematic review of observational studies involving 34,000 patients. Circ Heart Fail 2013;6:395–402.
80. Tahrani AA, Varughese GI, Scarpello JH, Hanna FW. Metformin, heart failure, and lactic acidosis: is metformin absolutely contraindicated? BMJ 2007;335:508–12.
81. Li Y, Hu Y, Ley SH, et al. Sulfonylurea use and incident cardiovascular disease among patients with type 2 diabetes: prospective cohort study among women. Diabetes Care 2014;37:3106–13.
82. Bentley-Lewis R, Aguilar D, Riddle MC, et al. Rationale, design, and baseline characteristics in Evaluation of LIXisenatide in Acute Coronary Syndrome, a long-term cardiovascular end point trial of lixisenatide versus placebo. Am Heart J 2015;169:631,638.e7.
83. Marso SP, Daniels GH, Brown-Frandsen K, et al. Liraglutide and cardiovascular outcomes in type 2 diabetes. N Engl J Med 2016;375:311–22.
84. Exenatide Study of Cardiovascular Event Lowering Trial (EXSCEL): A Trial To Evaluate Cardiovascular Outcomes After Treatment With Exenatide Once Weekly In Patients With Type 2 Diabetes Mellitus. clinicaltrials.gov Web site. https://clinicaltrials.gov/ct2/show/NCT01144338. 2016 Accessed September 23, 2016.
85. Researching Cardiovascular Events With a Weekly Incretin in Diabetes (REWIND). clinicaltrials.gov Web site. https://clinicaltrials.gov/ct2/show/NCT01394952. Accessed September 23, 2016.
86. Schernthaner G, Schernthaner-Reiter MH, Schernthaner GH. EMPA-REG and other cardiovascular outcome trials of glucose-lowering agents: implications for future treatment strategies in type 2 diabetes mellitus. Clin Ther 2016;38:1288–98.
87. CANVAS--CANagliflozin cardiovascular Assesssment Study (CANVAS). clinicaltrials.gov Web site. https://clinicaltrials.gov/ct2/show/NCT01032629. Accessed September 23, 2016.
88. Evaluation of the Effects of Canagliflozin on Renal and Cardiovascular Outcomes in Participants With Diabetic Nephropathy (CREDENCE). clinicaltrials.gov Web site. https://clinicaltrials.gov/ct2/show/NCT02065791. Accessed September 23, 2016.
89. Multicenter Trial to Evaluate the Effect of Dapagliflozin on the Incidence of Cardiovascular Events (DECLARE-TIMI58). clinicaltrials.gov Web site. https://clinicaltrials.gov/ct2/show/NCT01730534. Accessed September 23, 2016.
90. Zannad F, Cannon CP, Cushman WC, et al. Heart failure and mortality outcomes in patients with type 2 diabetes taking alogliptin versus placebo in EXAMINE: a multicentre, randomised, double-blind trial. Lancet 2015;385(9982):2067–76.
91. Van Klompenburg E, Heins JR. New insulin options for diabetic patients. S D Med 2016;69:84–5.
92. Rosenstock J, Schwartz SL, Clark CM Jr, et al. Basal insulin therapy in type 2 diabetes: 28-week comparison of insulin glargine (HOE 901) and NPH insulin. Diabetes Care 2001;24:631–6.
93. Tylee T, Hirsch IB. Costs associated with using different insulin preparations. JAMA 2015;314:665–6.
94. Hua X, Carvalho N, Tew M, et al. Expenditures and prices of antihyperglycemic medications in the United States: 2002-2013. JAMA 2016;315:1400–2.
95. Heinemann L. Biosimilar insulin and costs: what can we expect? J Diabetes Sci Technol 2016;10:457–62.
96. Horvath K, Jeitler K, Berghold A, et al. Long-acting insulin analogues versus NPH insulin (human isophane insulin) for type 2 diabetes mellitus. Cochrane Database Syst Rev 2007;(2)(2):CD005613.
97. Mannucci E, Monami M, Marchionni N. Short-acting insulin analogues vs. regular human insulin in type 2 diabetes: a meta-analysis. Diabetes Obes Metab 2009;11:53–9.
98. Powell PW, Corathers SD, Raymond J, Streisand R. New approaches to providing individualized diabetes care in the 21st century. Curr Diabetes Rev 2015;11:222–30.
99. Riddle MC, Rosenstock J, Gerich J, Insulin Glargine 4002 Study Investigators. The treat-to-target trial: randomized addition of glargine or human NPH insulin to oral therapy of type 2 diabetic patients. Diabetes Care 2003;26:3080–6.
From the University of Arizona College of Pharmacy and the University of Arizona College of Medicine-Tucson, Tucson, AZ.
Abstract
- Objective: To summarize key issues relevant to managing hyperglycemia in patients with type 2 diabetes mellitus (T2DM) and review a strategy for initiating and intensifying therapy.
- Methods: Review of the literature.
- Results: The 6 most widely used pharmacologic treatment options for hyperglycemia in T2DM are metformin, sulfonylureas, dipeptidyl peptidase-4 inhibitors, glucagon-like peptide-1 receptor agonists, sodium-glucose cotransporter-2 inhibitors, and insulin. Recent guidelines stress the importance of an individualized, patient-centered approach to managing hyperglycemia in T2DM, although sufficient guidance for nonspecialists on how to individualize treatment is often lacking. For patients with no contraindications, metformin should be recommended concurrent with lifestyle intervention at the time of diabetes diagnosis. Due to the progressive nature of T2DM, glycemic control on metformin monotherapy is likely to deteriorate over time, and there is no consensus as to what the second-line agent should be. A second agent should be selected based on glycemic goal and potential advantages and disadvantages of each agent for any given patient. If the patient progresses to the point where dual therapy does not provide adequate control, either a third non-insulin agent or insulin can be added.
- Conclusion: Although research is increasingly focusing on what the ideal number and sequence of drugs should be when managing T2DM, investigating all possible combinations in diverse patient populations is not feasible. Physicians therefore must continue to rely on clinical judgment to determine how to apply trial data to the treatment of individual patients.
Key words: type 2 diabetes; patient-centered care; antihyper-glycemic drugs; insulin; therapeutic decision-making.
Diabetes mellitus affects approximately 29.1 million people, or 9.3% of the U.S. population [1,2]. The high prevalence of diabetes and its associated multiple complications, including cardiovascular disease (CVD), blindness, renal failure, lower extremity amputations, and premature death, lead to a tremendous overall burden of disease. The financial cost is staggering as well, with more than 1 in 5 health care dollars spent on treating diabetes or its complications [3]. The goal of diabetes treatment is to prevent acute complications and reduce the risk of long-term complications. Interventions that have been shown to improve diabetes outcomes include medications for glycemic control and treatment of cardiovascular risk factors, nutrition and physical activity counseling, smoking cessation, immunizations, psychosocial care, and ongoing surveillance and early treatment for eye, kidney, and foot problems [4].
Glycemic management in type 2 diabetes mellitus (T2DM), the focus of this review, is growing increasingly complex and has been the subject of numerous extensive reviews [5,6] and published guidelines [4,7]. In the context of an increasing array of available pharmacologic options, there are mounting uncertainties regarding the benefits of intensive glycemic control as well as increasing concerns about potential adverse treatment effects, hypoglycemia in particular. While previous guidelines encouraged specific approaches for most patients, more recent guidelines stress the importance of a patient-centered approach with shared decision-making [4]. Less prescriptive guidelines are more appropriate, given the current state of science, but they also may be viewed as providing insufficient guidance to some providers. It can be overwhelming for a non-specialist to try to match the nuances of antihyperglycemic medications to the nuances of each patient’s preferences and medical characteristics.
This article examines key issues faced by primary care providers when managing hyperglycemia in patients with T2DM and outlines a stepwise approach to determining the optimal antihyperglycemic agent(s) (Table 1).
Confirm Diagnosis of T2DM
It can be difficult to distinguish between type 1 diabetes mellitus and T2DM in some individuals due to overlapping characteristics. However, correctly classifying a patient’s diabetes at the outset is essential, as the classification helps determine the best treatment regimen and is rarely reconsidered [4,8]. Considerable evidence suggests that misclassification of diabetes occurs frequently [9,10], resulting in patients receiving inappropriate treatment. Clinical characteristics suggestive of T2DM include older age and features of insulin resistance such as obesity, hyper-tension, hypertriglyceridemia, and low high-density lipoprotein cholesterol. When these features are not present, an alternate diagnosis should be entertained.
Establish Glycemic Goal
Research over the past decade has led to a growing appreciation of the enormous complexity of hyperglycemia management. During the 1990s, landmark trials such as the Diabetes Control and Complications Trial (DCCT) [11] and UK Prospective Diabetes Study (UKPDS) [12] demonstrated that improving glucose control could reduce the incidence of microvascular complications [11,12], prompting a lower-is-better philosophy regarding glucose targets. Despite limited evidence to support such thinking, this viewpoint was adopted by the developers of many guidelines. During the following decade more research was devoted to determining whether aggressively lowering a patient’s glucose could also improve macrovascular outcomes. Table 2 summarizes microvascular and macrovascular effects of intensive glycemic control seen in major trials [11–23]. After several major trials [20,22] found only mild cardiovascular benefits and even suggested harm [18], experts and policy makers began to reconsider the value of tightly controlling glucose levels [24]. Since then, other studies have demonstrated that the potential benefits and risks of glucose control are strongly related to individual patient factors, such as age and duration of diabetes, and associated comorbidities, such as CVD and impaired renal function [6].
A one-size-fits-all glycemic goal is no longer recommended. Personalization is necessary, balancing the potential benefits and risks of treatments required to achieve that goal. Whereas an A1C of < 7% is an appropriate target for some individuals with diabetes, glycemic targets may be more or less stringent based on patient features including life expectancy, duration of diabetes, comorbidities, and patient attitude and support system (Table 3) [4].
A particular group in which less stringent goals should be considered is older patients, especially those with complex or poor health status [4,25]. The risk of intensive glycemic control may exceed the benefits in these patients, as they are at higher risk of hypoglycemia and polypharmacy [26]. A goal A1C of 7% to 7.5% is now recommended for healthy older adults, and less stringent A1C goals of 7.5% to 8% and 8% to 8.5% should be considered based on the presence and severity of multiple coexisting chronic illnesses, decreased self-care ability, or cognitive impairment [4,25]. Unfortunately, overtreatment is frequently seen in this group. In a recent study of patients over age 65 years, about 40% of those with complex or poor health status had tight glycemic control with A1C below 6.5% [26]. An analysis of U.S. Veterans Affairs administration data showed that only 27% of 12,917 patients older than 65 with very low A1C (< 6%) and about 21% of those with A1C of 6% to 6.5% underwent treatment deintensification [27].
Initiate Treatment with Metformin
There is strong consensus that metformin is the preferred drug for monotherapy due to its long proven safety record, low cost, weight-reduction benefit, and potential cardiovascular advantages [4,16]. As long as there are no contraindications, metformin should be recommended concurrent with lifestyle intervention at the time of diabetes diagnosis. The recommendation is based on the fact that adherence to diet, weight reduction, and regular exercise is not sustained in most patients, and most patients ultimately will require treatment. Since metformin is usually well-tolerated, does not cause hypoglycemia, has a favorable effect on body weight, and is relatively inexpensive, potential benefits of early initiation of medication appear to outweigh potential risks.
The U.S. Food and Drug Administration (FDA) recently relaxed prescribing polices to extend the use of this important medication to patients who have mild–moderate, but stable, chronic kidney disease (CKD) [28]. Metformin is recommended as first-line therapy and should be used unless it is contraindicated (ie, estimated glomerular filtration rate [eGFR] < 30 mL/min/1.73 m2)[4,7,29].
Add Additional Agent(s) as Needed to Achieve Goal
Other than metformin, evidence is limited for the optimal use of the burgeoning array of available agents, especially in dual or triple combinations [6,30]. Research is now starting to focus more on what the ideal number and sequence of drugs should be. The Glycemic Reduction Approach in Diabetes (GRADE) study, which will compare long-term benefits and risks of the 4 most widely used antihyperglycemic medications in combination with metformin, is now underway [31,32]. The 4 classes being studied are sulfonylurea, dipeptidyl peptidase-4 (DPP-4) inhibitors, glucagon-like peptide-1 (GLP-1) receptor agonists, and a basal,
Eleven classes of non-insulin medications are currently approved for treating hyperglycemia in T2DM [4]. Within each class, numerous agents are available. Six of these classes (ie, α-glucosidase inhibitors, colesevelam, bromocriptine, pramlintide, meglitinides, and thiazolidinediones) are not used frequently
Consider Effects on A1C
There is a paucity of high-quality, head-to-head comparison trials evaluating the ability of available agents to achieve recommended glycemic targets. This is important because the glucose-lowering effectiveness of individual medications is strongly influenced by baseline characteristics such as A1C, duration of diabetes, and previous therapy. With these limitations in mind, the relative glucose-lowering effectiveness of commonly used agents is shown in Table 4. When used as monotherapy, A1C reductions of approximately 1% to 1.5% are achieved with metformin, sulfonylureas, and GLP-1 receptor agonists [6,30,34,35,39]. DPP-4 inhibitors and SGLT-2 inhibitors have more modest glucose-lowering efficacy, with A1C reductions of approximately 0.5% to 1% [6,30,34,35,39]. Larger effects may be seen in individuals with higher baseline A1C and those who are drug naïve. Insulin is the most effective glucose-lowering agent—it can reduce virtually any level of A1C down to the normal range, with hypoglycemia being the only limiting factor. When a patient has uncontrolled hyperglycemia on metformin monotherapy, or if there is a contraindication or intolerance to metformin, clinicians should consider the potential glucose-lowering effects of other available options and should choose an agent that conceivably could bring a patient close to meeting their treatment goal.
Eliminate Options with Unacceptable Adverse Effects
When the pharmacologic options with acceptable A1C-lowering potential have been identified, the ones with contraindications and potential serious adverse effects for the individual patient can immediately be eliminated (Table 4). For example, if a patient has an eGFR < 30 mL/min/1.73 m2, metformin, sulfonylureas, GLP-1 receptor agonists, most DPP-4 inhibitors, and SGLT-2 inhibitors are either contraindicated or should be used with caution. In patients with severe osteoporosis, SGLT-2 inhibitors may not be the best option. In patients with a history of diabetic ketoacidosis (DKA), caution should be used with metformin and SGLT-2 inhibitors. There have been concerns of possible acute pancreatitis and neoplasia with the incretin-based agents, the DPP-4 inhibitors and GLP-1 receptor agonists [40,41], although other clinical trials and observational data have not found increased risk [42–45]. Nevertheless, these agents potentially should be avoided in patients with a history of pancreatitis or neoplasm. SGLT-2 inhibitors may be associated with genitourinary infections and volume depletion [46–48] and probably should be avoided in patients at high risk for these conditions.
If the adverse effects are not serious, changing the way the medication is administered may allow the patient to tolerate agents with high potential benefits. For example, metformin is commonly associated with gastrointestinal (GI) adverse effects, which can be reduced or avoided with slow titration of the dose [6] or by switching to an extended-release formulation [49]. GLP-1 receptor agonists are associated with GI adverse effects [6] and in most cases slow titration is recommended.
Evaluate Potential Risks/Benefits of Remaining Options
Hypoglycemia. The barrier of hypoglycemia generally precludes maintenance of euglycemia and full realization of the long-term benefits of good glucose control over a lifetime. Once considered a trivial issue, concerns about hypoglycemia in T2DM are increasingly being raised [19,50–55]. Clearly, hypoglycemia occurs more often as glycemic targets are lowered to near-normal values, especially in those with advanced age and multiple comorbidities [55]. Various comorbidities frequently encountered particularly as patients age also are associated with increasing propensity for experiencing hypoglycemia and untoward outcomes from it. These include coronary artery disease, heart failure, renal and liver disease, and dementia. Hypoglycemia, when it occurs, may lead to dysrhythmias, dizziness, accidents and falls, work disability, and decreased quality of life. In addition to relaxing blood glucose targets in high-risk patients, drug selection should favor agents that do not precipitate such events (Table 4).
Fortunately, the commonly used non-insulin agents are not associated with hypoglycemia unless they are used in combination with sulfonylureas or insulin. Sulfonylureas should be used with caution and other options considered in patients with high risk for hypoglycemia. When insulin is required, regimens which minimize risk of hypoglycemia should be used. For example, adding a GLP-1 receptor agonist to basal insulin as an alternative to mealtime insulin has been shown to be equally effective with a lower risk of hypoglycemia [4,6]. Also, premixed insulin preparations should be avoided or used cautiously in individuals who miss meals frequently. Additionally, newer basal insulins that exhibit longer duration of action are now available in the United States. Preliminary studies have shown that the newly FDA-approved longer-acting basal insulins, insulin degludec and glargine U-300, may be associated with a reduced risk for hypoglycemia [56,57]. However, it remains unclear how and when these newer agents will best be incorporated into a treatment regimen.
Body weight. Nearly 90% of people living with T2DM are overweight or obese. Given the close tie between obesity and T2DM, treating obesity is an obvious consideration in diabetes treatment. Major trials have shown the effectiveness of lifestyle modifications and weight reduction in delaying, prevention, and management of T2DM [4,58,59].With this in mind, clinicians should consider preferentially using antihyperglycemic agents with weight-lowering or weight-neutral effects. Among commonly used antihyperglycemic agents, metformin, GLP-1 receptor agonists, and SGLT-2 inhibitors have been shown to have weight-reduction benefits, and DPP-4 inhibitors are weight neutral. On the other hand, sulfonylureas and insulin are associated with weight gain. A systematic review and meta-analysis including 204 studies with study durations ranging from 3 months to 8 years showed comparative effects of diabetes medications with a differential effect on weight of up to 5 kg (Table 4) [60].
Metformin is associated with an average weight loss of 1.9 to 3.1 kg that was sustained with long-term use for at least 10 years in the Diabetes Prevention Program Outcomes Study [61].A systematic review of 7 randomized trials showed that in patients with T2DM, the SGLT-2 inhibitors dapagliflozin and canagliflozin were associated with weight loss (mean weighted difference of –1.81 kg and –2.3 kg, respectively) [62]. A systematic review and meta-analysis of 25 randomized controlled trials showed greater weight loss (mean weighted difference of –2.9 kg) in overweight or obese patients with or without T2DM using GLP-1 receptor agonists when compared to placebo, insulin, or oral antihyperglycemic agents [63]. Of note, the GLP-1 receptor agonist liraglutide is now approved for weight loss in patients with or without diabetes [64]. The maximum doses approved for diabetes and obesity treatment are 1.8 and 3.0 mg/day, respectively.
Since weight loss is associated with improved glycemic control, an area of emerging interest is the use of antiobesity medications for managing diabetes. Although most older weight-loss medications were only approved for short-term use, some newer agents are approved for longer-term use. Lorcaserin and the combination drugs topiramate/phentermine and naltrexone/bupropion are approved for chronic therapy, provided certain conditions are met. Patients on weight reduction agents should be monitored regularly.
An even more radical departure from conventional therapy for diabetes is the consideration of metabolic, or weight-loss, surgery, which has been found to be associated with rapid and dramatic improvements in blood glucose control. Metabolic surgery has been shown to improve glucose control more effectively than any known pharmaceutical or behavioral approach. For example, in an observational study of obese patients with T2DM, bariatric surgery led to diabetes remission rates of 72.3% 2 years after surgery and 30.4% 15 years after surgery compared to 16.4% and 6.5%, respectively, in control patients [69]. With long-term follow-up, significant decreases in microvascular and macrovascular complications were seen in the surgical group [69]. Compared with medical therapy alone, bariatric surgery plus medical therapy has been associated with more weight loss, better glycemic control, less need for diabetes medications, and improved quality of life [70]. A 2016 joint statement by numerous international diabetes organizations recommends considering metabolic surgery as a treatment for T2DM and obesity [71]. American Diabetes Association guidelines recommend consideration of bariatric surgery in individuals with T2DM who have a body mass index greater than 35 kg/m2,especially if achieving disease control is difficult by means of lifestyle modifications and medications [4].
Cardiovascular outcomes. Cardiovascular risk is about 2 to 4 times higher in patients with diabetes, and about half of patients with this condition develop heart failure [4,72]. CVD is responsible for most of the mortality in T2DM [72]. Therefore, prevention of cardiovascular morbidity and mortality is an important goal for diabetes treatment. Due to concerns about potential cardiovascular risks associated with glucose-lowering medications [73–76], the FDA has issued regulatory requirements for manufacturers to monitor the cardiovascular risk profile for these drugs [77]. Recent trials have led to a better understanding of potential cardiovascular benefits or harms of antihyperglycemic medications.
Metformin, the widely recommended first-line therapy for T2DM, carries a large body of evidence supporting its cardiovascular benefits. For example, the UKPDS found that compared to conventional therapy (mostly diet), metformin reduced cardiovascular events and mortality in obese patients with T2DM [15]. This result was supported in Hyperinsulinemia: the Outcome of its Metabolic Effect (HOME) study where, as an add-on to insulin, metformin decreased macrovascular complications when compared to placebo [78]. Research over the past decade also has assuaged concerns about metformin safety in heart failure [60]. A systematic review of observational studies involving 34,000 patients conducted in 2013 showed that metformin is as safe as other glucose-lowering medications in patients with diabetes and heart failure even in the presence of CKD [4,79]. Furthermore, numerous investigations have found metformin is not associated with increased hospitalizations or risk of lactic acidosis [80]. Metformin can be used safely in patients with diabetes and heart failure [60].
Although sulfonylureas have long been a mainstay of diabetes therapy, concerns about their potential adverse cardiovascular effects have been raised by numerous studies [81]. Tolbutamide, a first-generation sulfonylurea, was removed from the market after the University Group Diabetes Program study found increased CVD deaths with this agent versus placebo. Subsequently, the FDA issued a warning for all sulfonylureas [74]. The increased cardiovascular risk associated with sulfonylureas is thought to be due to their effect on cardiac mitochondrial potassium ATP channels. Sulfonylureas bind to these channels, preventing a protective phenomenon called ischemic preconditioning and resulting in a weakened defense against myocardial injury [76]. A recent study showed an increased risk of coronary heart disease associated with long-term use of sulfonylureas in women with diabetes [81].
GLP-1 receptor agonists have recently received much attention for their potential beneficial effects on cardiovascular outcomes. In a recent trial, lixisenatide was shown to be safe in patients with T2DM and acute coronary syndrome when compared to placebo [82]. More recently, the Liraglutide Effect and Action in Diabetes: Evaluation of cardiovascular outcome Results (LEADER) trial demonstrated significant cardiovascular benefits with liraglutide in patients with T2DM and established or high CVD risk [83]. The composite outcome of the first occurrence of death from cardiovascular causes, nonfatal myocardial infarction (MI), or nonfatal stroke, occurred less frequently in the liraglutide group compared to placebo (13% versus 14.9%, respectively), and there were fewer deaths from cardiovascular causes in the liraglutide group compared to placebo (4.7% and 6.0%, respectively) [83]. Other trials investigating the cardiovascular outcomes of this class [84,85] are in progress.
Another class with potential cardiovascular benefits is the SGLT-2 inhibitors. In a recent cardiovascular outcome study, empagliflozin significantly lowered the composite of cardiovascular death, nonfatal MI, or nonfatal stroke in T2DM patients with high cardiovascular risk compared to placebo (10.5% and 12.1%, respectively) [86]. There are several large ongoing studies evaluating the cardiovascular effects of other SGLT-2 inhibitors [87–89].
DPP-4 inhibitors were examined in recent studies and have shown no cardiovascular benefits [42,44,90].The studies showed mixed results regarding an association between DPP-4 inhibitors and heart failure. In one study, saxagliptin was associated with increased hospitalization for heart failure compared to placebo [44], while 2 noninferiority trials did not show a significant increase in heart failure hospitalizations associated with alogliptin and sitagliptin when compared to placebo [42,90].
Administration Considerations
Many patients with T2DM require multiple agents for glycemic control. Additional medications used for comorbid conditions add to this burden. When choosing antihyperglycemic agents, the route and frequency of administration, as well as the patients’ preferences and ability, should be considered. Either once or twice daily dosing is available for most agents, and once weekly dosing is available for some of the GLP-1 receptor agonists. Once daily or once weekly formulations may improve adherence and be more desirable than preparations that are dosed twice daily. Most of the commonly used medications are dosed orally. Although many patients find this route of administration preferable to insulin or GLP-1 receptor agonists, which require injections, some patients may prefer the risk/benefit of injectable agents. All GLP-1 receptor agonists come in a pen delivery system, which eliminates mixing and provides more convenient administration. Extended-release exenatide also is available as a single-dose tray that requires mixing and may be more cumbersome to inject.
Insulin requires special consideration. There has been an enormous increase in the number of insulin products on the market in the past 2 decades. These products include insulin analogs, concentrated insulins (U-200, U-300, and U-500), premixed insulin preparations, and ultra-long-acting insulin [91]. The availability of insulin options with different concentrations, onsets, and durations of actions has made decision making on which insulin to use difficult. Clinicians need to consider patient preference, dosing frequency, and timing with regard to meals, insulin dose, administration, as well as cost. For example, concentrated insulin is preferred for a patient on high doses of insulin requiring injecting a large volume of insulin. Rapid-acting insulin analogs would be more appropriate for patients who have difficulty administering their regular insulin 20 to 30 minutes before eating. Premixed insulin preparations make it impossible to independently adjust short- and long-acting components. However, these may be good choices in patients who have consistent meal schedules and who want to simplify administration. Despite a prevailing misconception that NPH must be given twice a day, it has long been recognized that in T2DM, a single daily injection of NPH yields improvements in control similar to those achieved with 2 daily injections [92].
Cost Considerations
Treating T2DM imposes a great financial burden on individuals living with diabetes and their families due to the high cost of the medications. Table 4 and Table 5 provide information on the cost of non-insulin and insulin diabetes medications for patients who do not have prescription insurance coverage. From a practical standpoint, choice of diabetes agents is largely influenced by insurance formularies.
The older agents, metformin and the sulfonylureas, are available for a cash (no insurance) price of as little as $4 per month. This is in stark contrast to the SGLT-2 inhibitors, GLP-1 receptor agonists, and DPP-4 inhibitors, which range in cost between $400 and $600 per month. Of recent concern, the cost of insulin has been skyrocketing, with a more than 500% increase in the cost of certain insulins from 2001 to 2015 [93]. According to the Medical Expenditure Panel Survey (MEPS) from 2002 to 2013, the mean price of insulin increased by about 200% (from $4.34/mL to $12.92/mL) during this period, which was significantly higher than increases in the price of non-insulin comparators [94]. The introduction of biosimilar insulins to the market is expected to offer treatment options with lower cost. This will be tested when the biosimilar glargine, the first FDA-approved biosimilar insulin, becomes available in the U.S. market. However, a significant reduction in insulin prices is not expected soon [95].
When insulin is required, most patients with T2DM can be treated with older human insulins, which have similar efficacy and lower costs than the more expensive newer insulin analogs. A Cochrane review comparing basal insulin analogs to NPH showed similar efficacy in glycemic control with minimal clinical benefit in the form of less nocturnal hypoglycemia in the insulin analog arm [96]. Furthermore, similar glycemic control and risk of hypoglycemia was seen when regular insulin was compared with the rapid-acting insulin analogs [97]. The cost of human NPH insulin for a patient on a total daily dose of 60 units is approximately $52 per month. This contrasts with the most widely used insulin, insulin glargine, which has a cash price of about $500 per month for the same amount (Table 5). Insulin pens, which are convenient, are more expensive. Interestingly, human insulins do not require prescriptions, allowing underinsured, underfunded patients ongoing access to them.
Incorporating Patient Preferences
Research evidence is necessary but insufficient for making patient care decisions. Along with the potential benefits, harms, costs, and inconveniences of the management options, patient perspectives, beliefs, expectations, and health-related goals must be considered. Patients will undoubtedly have preferences regarding defining goals and ranking options. Clinicians should discuss therapeutic goals and treatment options and work collaboratively with patients in determining management strategies [98].
Summary
Potential treatment approaches for treating hyperglycemia in T2DM are summarized in Figure 1 and Figure 2 [4,7]. As long as there are no contraindications, metformin should be recommended concurrent with lifestyle intervention at the time of diabetes diagnosis. Even if metformin monotherapy is initially effective, glycemic control is likely to deteriorate over time due to progressive loss of β-cell function in T2DM.
There is no consensus as to what the second-line agent should be. Selection of a second agent should be made based on potential advantages and disadvantages of each agent for any given patient. A patient-centered approach is preferred over a fixed algorithm. If the patient progresses to the point where dual therapy does not provide adequate control, either a third non-insulin agent or insulin can be added. In patients with modestly elevated A1C (below ~8%), addition of a third non-insulin agent may be equally effective as (but more expensive than) addition of insulin.
Patients with significantly elevated A1C levels on non-insulin agents usually should have insulin added to their regimen. When insulin is added, metformin should be continued. DPP-4 inhibitors and sulfonylureas are typically stopped. If SGLT-2 inhibitors and/or GLP-1 receptor agonists are continued, this may aid with weight maintenance. However, continuing these agents is likely to be expensive and associated with problems associated with polypharmacy.
The most widely recommended strategy for initiating insulin in T2DM is to add a single bedtime injection of basal insulin (ie, NPH, glargine, detemir, or degludec) to the patient’s regimen. This regimen has been found to be effective in numerous studies and controls hyperglycemia in up to 60% of patients [99]. If the patient is treated with a single bedtime injection of insulin and the fasting glucose level is within the target range but the A1C level remains above goal, addition of mealtime insulin injections is likely to be beneficial. Alternatively, addition of a GLP-1 receptor agonist to basal insulin has been shown to be equally beneficial [4,6]. When adding mealtime insulin, a common strategy is to add a single injection of a rapid-acting insulin (eg, lispro, aspart, glulisine) before the patient’s largest meal of the day. Additional premeal injections of rapid-acting insulin may be added as needed, based on self-monitoring blood glucose results. If glycemia remains significantly uncontrolled on more than 200 units of insulin per day, switching to a concentrated form of insulin (eg, U-200, U-300, or U-500) should be considered.
Corresponding author: Maryam Fazel, PharmD, BCPS, BCACP, CDE, 1295 N. Martin Ave. (Room B211B), Tucson, Arizona 85721-0202, maryamfazel@pharmacy.arizona.edu.
Financial disclosures: None.
From the University of Arizona College of Pharmacy and the University of Arizona College of Medicine-Tucson, Tucson, AZ.
Abstract
- Objective: To summarize key issues relevant to managing hyperglycemia in patients with type 2 diabetes mellitus (T2DM) and review a strategy for initiating and intensifying therapy.
- Methods: Review of the literature.
- Results: The 6 most widely used pharmacologic treatment options for hyperglycemia in T2DM are metformin, sulfonylureas, dipeptidyl peptidase-4 inhibitors, glucagon-like peptide-1 receptor agonists, sodium-glucose cotransporter-2 inhibitors, and insulin. Recent guidelines stress the importance of an individualized, patient-centered approach to managing hyperglycemia in T2DM, although sufficient guidance for nonspecialists on how to individualize treatment is often lacking. For patients with no contraindications, metformin should be recommended concurrent with lifestyle intervention at the time of diabetes diagnosis. Due to the progressive nature of T2DM, glycemic control on metformin monotherapy is likely to deteriorate over time, and there is no consensus as to what the second-line agent should be. A second agent should be selected based on glycemic goal and potential advantages and disadvantages of each agent for any given patient. If the patient progresses to the point where dual therapy does not provide adequate control, either a third non-insulin agent or insulin can be added.
- Conclusion: Although research is increasingly focusing on what the ideal number and sequence of drugs should be when managing T2DM, investigating all possible combinations in diverse patient populations is not feasible. Physicians therefore must continue to rely on clinical judgment to determine how to apply trial data to the treatment of individual patients.
Key words: type 2 diabetes; patient-centered care; antihyper-glycemic drugs; insulin; therapeutic decision-making.
Diabetes mellitus affects approximately 29.1 million people, or 9.3% of the U.S. population [1,2]. The high prevalence of diabetes and its associated multiple complications, including cardiovascular disease (CVD), blindness, renal failure, lower extremity amputations, and premature death, lead to a tremendous overall burden of disease. The financial cost is staggering as well, with more than 1 in 5 health care dollars spent on treating diabetes or its complications [3]. The goal of diabetes treatment is to prevent acute complications and reduce the risk of long-term complications. Interventions that have been shown to improve diabetes outcomes include medications for glycemic control and treatment of cardiovascular risk factors, nutrition and physical activity counseling, smoking cessation, immunizations, psychosocial care, and ongoing surveillance and early treatment for eye, kidney, and foot problems [4].
Glycemic management in type 2 diabetes mellitus (T2DM), the focus of this review, is growing increasingly complex and has been the subject of numerous extensive reviews [5,6] and published guidelines [4,7]. In the context of an increasing array of available pharmacologic options, there are mounting uncertainties regarding the benefits of intensive glycemic control as well as increasing concerns about potential adverse treatment effects, hypoglycemia in particular. While previous guidelines encouraged specific approaches for most patients, more recent guidelines stress the importance of a patient-centered approach with shared decision-making [4]. Less prescriptive guidelines are more appropriate, given the current state of science, but they also may be viewed as providing insufficient guidance to some providers. It can be overwhelming for a non-specialist to try to match the nuances of antihyperglycemic medications to the nuances of each patient’s preferences and medical characteristics.
This article examines key issues faced by primary care providers when managing hyperglycemia in patients with T2DM and outlines a stepwise approach to determining the optimal antihyperglycemic agent(s) (Table 1).
Confirm Diagnosis of T2DM
It can be difficult to distinguish between type 1 diabetes mellitus and T2DM in some individuals due to overlapping characteristics. However, correctly classifying a patient’s diabetes at the outset is essential, as the classification helps determine the best treatment regimen and is rarely reconsidered [4,8]. Considerable evidence suggests that misclassification of diabetes occurs frequently [9,10], resulting in patients receiving inappropriate treatment. Clinical characteristics suggestive of T2DM include older age and features of insulin resistance such as obesity, hyper-tension, hypertriglyceridemia, and low high-density lipoprotein cholesterol. When these features are not present, an alternate diagnosis should be entertained.
Establish Glycemic Goal
Research over the past decade has led to a growing appreciation of the enormous complexity of hyperglycemia management. During the 1990s, landmark trials such as the Diabetes Control and Complications Trial (DCCT) [11] and UK Prospective Diabetes Study (UKPDS) [12] demonstrated that improving glucose control could reduce the incidence of microvascular complications [11,12], prompting a lower-is-better philosophy regarding glucose targets. Despite limited evidence to support such thinking, this viewpoint was adopted by the developers of many guidelines. During the following decade more research was devoted to determining whether aggressively lowering a patient’s glucose could also improve macrovascular outcomes. Table 2 summarizes microvascular and macrovascular effects of intensive glycemic control seen in major trials [11–23]. After several major trials [20,22] found only mild cardiovascular benefits and even suggested harm [18], experts and policy makers began to reconsider the value of tightly controlling glucose levels [24]. Since then, other studies have demonstrated that the potential benefits and risks of glucose control are strongly related to individual patient factors, such as age and duration of diabetes, and associated comorbidities, such as CVD and impaired renal function [6].
A one-size-fits-all glycemic goal is no longer recommended. Personalization is necessary, balancing the potential benefits and risks of treatments required to achieve that goal. Whereas an A1C of < 7% is an appropriate target for some individuals with diabetes, glycemic targets may be more or less stringent based on patient features including life expectancy, duration of diabetes, comorbidities, and patient attitude and support system (Table 3) [4].
A particular group in which less stringent goals should be considered is older patients, especially those with complex or poor health status [4,25]. The risk of intensive glycemic control may exceed the benefits in these patients, as they are at higher risk of hypoglycemia and polypharmacy [26]. A goal A1C of 7% to 7.5% is now recommended for healthy older adults, and less stringent A1C goals of 7.5% to 8% and 8% to 8.5% should be considered based on the presence and severity of multiple coexisting chronic illnesses, decreased self-care ability, or cognitive impairment [4,25]. Unfortunately, overtreatment is frequently seen in this group. In a recent study of patients over age 65 years, about 40% of those with complex or poor health status had tight glycemic control with A1C below 6.5% [26]. An analysis of U.S. Veterans Affairs administration data showed that only 27% of 12,917 patients older than 65 with very low A1C (< 6%) and about 21% of those with A1C of 6% to 6.5% underwent treatment deintensification [27].
Initiate Treatment with Metformin
There is strong consensus that metformin is the preferred drug for monotherapy due to its long proven safety record, low cost, weight-reduction benefit, and potential cardiovascular advantages [4,16]. As long as there are no contraindications, metformin should be recommended concurrent with lifestyle intervention at the time of diabetes diagnosis. The recommendation is based on the fact that adherence to diet, weight reduction, and regular exercise is not sustained in most patients, and most patients ultimately will require treatment. Since metformin is usually well-tolerated, does not cause hypoglycemia, has a favorable effect on body weight, and is relatively inexpensive, potential benefits of early initiation of medication appear to outweigh potential risks.
The U.S. Food and Drug Administration (FDA) recently relaxed prescribing polices to extend the use of this important medication to patients who have mild–moderate, but stable, chronic kidney disease (CKD) [28]. Metformin is recommended as first-line therapy and should be used unless it is contraindicated (ie, estimated glomerular filtration rate [eGFR] < 30 mL/min/1.73 m2)[4,7,29].
Add Additional Agent(s) as Needed to Achieve Goal
Other than metformin, evidence is limited for the optimal use of the burgeoning array of available agents, especially in dual or triple combinations [6,30]. Research is now starting to focus more on what the ideal number and sequence of drugs should be. The Glycemic Reduction Approach in Diabetes (GRADE) study, which will compare long-term benefits and risks of the 4 most widely used antihyperglycemic medications in combination with metformin, is now underway [31,32]. The 4 classes being studied are sulfonylurea, dipeptidyl peptidase-4 (DPP-4) inhibitors, glucagon-like peptide-1 (GLP-1) receptor agonists, and a basal,
Eleven classes of non-insulin medications are currently approved for treating hyperglycemia in T2DM [4]. Within each class, numerous agents are available. Six of these classes (ie, α-glucosidase inhibitors, colesevelam, bromocriptine, pramlintide, meglitinides, and thiazolidinediones) are not used frequently
Consider Effects on A1C
There is a paucity of high-quality, head-to-head comparison trials evaluating the ability of available agents to achieve recommended glycemic targets. This is important because the glucose-lowering effectiveness of individual medications is strongly influenced by baseline characteristics such as A1C, duration of diabetes, and previous therapy. With these limitations in mind, the relative glucose-lowering effectiveness of commonly used agents is shown in Table 4. When used as monotherapy, A1C reductions of approximately 1% to 1.5% are achieved with metformin, sulfonylureas, and GLP-1 receptor agonists [6,30,34,35,39]. DPP-4 inhibitors and SGLT-2 inhibitors have more modest glucose-lowering efficacy, with A1C reductions of approximately 0.5% to 1% [6,30,34,35,39]. Larger effects may be seen in individuals with higher baseline A1C and those who are drug naïve. Insulin is the most effective glucose-lowering agent—it can reduce virtually any level of A1C down to the normal range, with hypoglycemia being the only limiting factor. When a patient has uncontrolled hyperglycemia on metformin monotherapy, or if there is a contraindication or intolerance to metformin, clinicians should consider the potential glucose-lowering effects of other available options and should choose an agent that conceivably could bring a patient close to meeting their treatment goal.
Eliminate Options with Unacceptable Adverse Effects
When the pharmacologic options with acceptable A1C-lowering potential have been identified, the ones with contraindications and potential serious adverse effects for the individual patient can immediately be eliminated (Table 4). For example, if a patient has an eGFR < 30 mL/min/1.73 m2, metformin, sulfonylureas, GLP-1 receptor agonists, most DPP-4 inhibitors, and SGLT-2 inhibitors are either contraindicated or should be used with caution. In patients with severe osteoporosis, SGLT-2 inhibitors may not be the best option. In patients with a history of diabetic ketoacidosis (DKA), caution should be used with metformin and SGLT-2 inhibitors. There have been concerns of possible acute pancreatitis and neoplasia with the incretin-based agents, the DPP-4 inhibitors and GLP-1 receptor agonists [40,41], although other clinical trials and observational data have not found increased risk [42–45]. Nevertheless, these agents potentially should be avoided in patients with a history of pancreatitis or neoplasm. SGLT-2 inhibitors may be associated with genitourinary infections and volume depletion [46–48] and probably should be avoided in patients at high risk for these conditions.
If the adverse effects are not serious, changing the way the medication is administered may allow the patient to tolerate agents with high potential benefits. For example, metformin is commonly associated with gastrointestinal (GI) adverse effects, which can be reduced or avoided with slow titration of the dose [6] or by switching to an extended-release formulation [49]. GLP-1 receptor agonists are associated with GI adverse effects [6] and in most cases slow titration is recommended.
Evaluate Potential Risks/Benefits of Remaining Options
Hypoglycemia. The barrier of hypoglycemia generally precludes maintenance of euglycemia and full realization of the long-term benefits of good glucose control over a lifetime. Once considered a trivial issue, concerns about hypoglycemia in T2DM are increasingly being raised [19,50–55]. Clearly, hypoglycemia occurs more often as glycemic targets are lowered to near-normal values, especially in those with advanced age and multiple comorbidities [55]. Various comorbidities frequently encountered particularly as patients age also are associated with increasing propensity for experiencing hypoglycemia and untoward outcomes from it. These include coronary artery disease, heart failure, renal and liver disease, and dementia. Hypoglycemia, when it occurs, may lead to dysrhythmias, dizziness, accidents and falls, work disability, and decreased quality of life. In addition to relaxing blood glucose targets in high-risk patients, drug selection should favor agents that do not precipitate such events (Table 4).
Fortunately, the commonly used non-insulin agents are not associated with hypoglycemia unless they are used in combination with sulfonylureas or insulin. Sulfonylureas should be used with caution and other options considered in patients with high risk for hypoglycemia. When insulin is required, regimens which minimize risk of hypoglycemia should be used. For example, adding a GLP-1 receptor agonist to basal insulin as an alternative to mealtime insulin has been shown to be equally effective with a lower risk of hypoglycemia [4,6]. Also, premixed insulin preparations should be avoided or used cautiously in individuals who miss meals frequently. Additionally, newer basal insulins that exhibit longer duration of action are now available in the United States. Preliminary studies have shown that the newly FDA-approved longer-acting basal insulins, insulin degludec and glargine U-300, may be associated with a reduced risk for hypoglycemia [56,57]. However, it remains unclear how and when these newer agents will best be incorporated into a treatment regimen.
Body weight. Nearly 90% of people living with T2DM are overweight or obese. Given the close tie between obesity and T2DM, treating obesity is an obvious consideration in diabetes treatment. Major trials have shown the effectiveness of lifestyle modifications and weight reduction in delaying, prevention, and management of T2DM [4,58,59].With this in mind, clinicians should consider preferentially using antihyperglycemic agents with weight-lowering or weight-neutral effects. Among commonly used antihyperglycemic agents, metformin, GLP-1 receptor agonists, and SGLT-2 inhibitors have been shown to have weight-reduction benefits, and DPP-4 inhibitors are weight neutral. On the other hand, sulfonylureas and insulin are associated with weight gain. A systematic review and meta-analysis including 204 studies with study durations ranging from 3 months to 8 years showed comparative effects of diabetes medications with a differential effect on weight of up to 5 kg (Table 4) [60].
Metformin is associated with an average weight loss of 1.9 to 3.1 kg that was sustained with long-term use for at least 10 years in the Diabetes Prevention Program Outcomes Study [61].A systematic review of 7 randomized trials showed that in patients with T2DM, the SGLT-2 inhibitors dapagliflozin and canagliflozin were associated with weight loss (mean weighted difference of –1.81 kg and –2.3 kg, respectively) [62]. A systematic review and meta-analysis of 25 randomized controlled trials showed greater weight loss (mean weighted difference of –2.9 kg) in overweight or obese patients with or without T2DM using GLP-1 receptor agonists when compared to placebo, insulin, or oral antihyperglycemic agents [63]. Of note, the GLP-1 receptor agonist liraglutide is now approved for weight loss in patients with or without diabetes [64]. The maximum doses approved for diabetes and obesity treatment are 1.8 and 3.0 mg/day, respectively.
Since weight loss is associated with improved glycemic control, an area of emerging interest is the use of antiobesity medications for managing diabetes. Although most older weight-loss medications were only approved for short-term use, some newer agents are approved for longer-term use. Lorcaserin and the combination drugs topiramate/phentermine and naltrexone/bupropion are approved for chronic therapy, provided certain conditions are met. Patients on weight reduction agents should be monitored regularly.
An even more radical departure from conventional therapy for diabetes is the consideration of metabolic, or weight-loss, surgery, which has been found to be associated with rapid and dramatic improvements in blood glucose control. Metabolic surgery has been shown to improve glucose control more effectively than any known pharmaceutical or behavioral approach. For example, in an observational study of obese patients with T2DM, bariatric surgery led to diabetes remission rates of 72.3% 2 years after surgery and 30.4% 15 years after surgery compared to 16.4% and 6.5%, respectively, in control patients [69]. With long-term follow-up, significant decreases in microvascular and macrovascular complications were seen in the surgical group [69]. Compared with medical therapy alone, bariatric surgery plus medical therapy has been associated with more weight loss, better glycemic control, less need for diabetes medications, and improved quality of life [70]. A 2016 joint statement by numerous international diabetes organizations recommends considering metabolic surgery as a treatment for T2DM and obesity [71]. American Diabetes Association guidelines recommend consideration of bariatric surgery in individuals with T2DM who have a body mass index greater than 35 kg/m2,especially if achieving disease control is difficult by means of lifestyle modifications and medications [4].
Cardiovascular outcomes. Cardiovascular risk is about 2 to 4 times higher in patients with diabetes, and about half of patients with this condition develop heart failure [4,72]. CVD is responsible for most of the mortality in T2DM [72]. Therefore, prevention of cardiovascular morbidity and mortality is an important goal for diabetes treatment. Due to concerns about potential cardiovascular risks associated with glucose-lowering medications [73–76], the FDA has issued regulatory requirements for manufacturers to monitor the cardiovascular risk profile for these drugs [77]. Recent trials have led to a better understanding of potential cardiovascular benefits or harms of antihyperglycemic medications.
Metformin, the widely recommended first-line therapy for T2DM, carries a large body of evidence supporting its cardiovascular benefits. For example, the UKPDS found that compared to conventional therapy (mostly diet), metformin reduced cardiovascular events and mortality in obese patients with T2DM [15]. This result was supported in Hyperinsulinemia: the Outcome of its Metabolic Effect (HOME) study where, as an add-on to insulin, metformin decreased macrovascular complications when compared to placebo [78]. Research over the past decade also has assuaged concerns about metformin safety in heart failure [60]. A systematic review of observational studies involving 34,000 patients conducted in 2013 showed that metformin is as safe as other glucose-lowering medications in patients with diabetes and heart failure even in the presence of CKD [4,79]. Furthermore, numerous investigations have found metformin is not associated with increased hospitalizations or risk of lactic acidosis [80]. Metformin can be used safely in patients with diabetes and heart failure [60].
Although sulfonylureas have long been a mainstay of diabetes therapy, concerns about their potential adverse cardiovascular effects have been raised by numerous studies [81]. Tolbutamide, a first-generation sulfonylurea, was removed from the market after the University Group Diabetes Program study found increased CVD deaths with this agent versus placebo. Subsequently, the FDA issued a warning for all sulfonylureas [74]. The increased cardiovascular risk associated with sulfonylureas is thought to be due to their effect on cardiac mitochondrial potassium ATP channels. Sulfonylureas bind to these channels, preventing a protective phenomenon called ischemic preconditioning and resulting in a weakened defense against myocardial injury [76]. A recent study showed an increased risk of coronary heart disease associated with long-term use of sulfonylureas in women with diabetes [81].
GLP-1 receptor agonists have recently received much attention for their potential beneficial effects on cardiovascular outcomes. In a recent trial, lixisenatide was shown to be safe in patients with T2DM and acute coronary syndrome when compared to placebo [82]. More recently, the Liraglutide Effect and Action in Diabetes: Evaluation of cardiovascular outcome Results (LEADER) trial demonstrated significant cardiovascular benefits with liraglutide in patients with T2DM and established or high CVD risk [83]. The composite outcome of the first occurrence of death from cardiovascular causes, nonfatal myocardial infarction (MI), or nonfatal stroke, occurred less frequently in the liraglutide group compared to placebo (13% versus 14.9%, respectively), and there were fewer deaths from cardiovascular causes in the liraglutide group compared to placebo (4.7% and 6.0%, respectively) [83]. Other trials investigating the cardiovascular outcomes of this class [84,85] are in progress.
Another class with potential cardiovascular benefits is the SGLT-2 inhibitors. In a recent cardiovascular outcome study, empagliflozin significantly lowered the composite of cardiovascular death, nonfatal MI, or nonfatal stroke in T2DM patients with high cardiovascular risk compared to placebo (10.5% and 12.1%, respectively) [86]. There are several large ongoing studies evaluating the cardiovascular effects of other SGLT-2 inhibitors [87–89].
DPP-4 inhibitors were examined in recent studies and have shown no cardiovascular benefits [42,44,90].The studies showed mixed results regarding an association between DPP-4 inhibitors and heart failure. In one study, saxagliptin was associated with increased hospitalization for heart failure compared to placebo [44], while 2 noninferiority trials did not show a significant increase in heart failure hospitalizations associated with alogliptin and sitagliptin when compared to placebo [42,90].
Administration Considerations
Many patients with T2DM require multiple agents for glycemic control. Additional medications used for comorbid conditions add to this burden. When choosing antihyperglycemic agents, the route and frequency of administration, as well as the patients’ preferences and ability, should be considered. Either once or twice daily dosing is available for most agents, and once weekly dosing is available for some of the GLP-1 receptor agonists. Once daily or once weekly formulations may improve adherence and be more desirable than preparations that are dosed twice daily. Most of the commonly used medications are dosed orally. Although many patients find this route of administration preferable to insulin or GLP-1 receptor agonists, which require injections, some patients may prefer the risk/benefit of injectable agents. All GLP-1 receptor agonists come in a pen delivery system, which eliminates mixing and provides more convenient administration. Extended-release exenatide also is available as a single-dose tray that requires mixing and may be more cumbersome to inject.
Insulin requires special consideration. There has been an enormous increase in the number of insulin products on the market in the past 2 decades. These products include insulin analogs, concentrated insulins (U-200, U-300, and U-500), premixed insulin preparations, and ultra-long-acting insulin [91]. The availability of insulin options with different concentrations, onsets, and durations of actions has made decision making on which insulin to use difficult. Clinicians need to consider patient preference, dosing frequency, and timing with regard to meals, insulin dose, administration, as well as cost. For example, concentrated insulin is preferred for a patient on high doses of insulin requiring injecting a large volume of insulin. Rapid-acting insulin analogs would be more appropriate for patients who have difficulty administering their regular insulin 20 to 30 minutes before eating. Premixed insulin preparations make it impossible to independently adjust short- and long-acting components. However, these may be good choices in patients who have consistent meal schedules and who want to simplify administration. Despite a prevailing misconception that NPH must be given twice a day, it has long been recognized that in T2DM, a single daily injection of NPH yields improvements in control similar to those achieved with 2 daily injections [92].
Cost Considerations
Treating T2DM imposes a great financial burden on individuals living with diabetes and their families due to the high cost of the medications. Table 4 and Table 5 provide information on the cost of non-insulin and insulin diabetes medications for patients who do not have prescription insurance coverage. From a practical standpoint, choice of diabetes agents is largely influenced by insurance formularies.
The older agents, metformin and the sulfonylureas, are available for a cash (no insurance) price of as little as $4 per month. This is in stark contrast to the SGLT-2 inhibitors, GLP-1 receptor agonists, and DPP-4 inhibitors, which range in cost between $400 and $600 per month. Of recent concern, the cost of insulin has been skyrocketing, with a more than 500% increase in the cost of certain insulins from 2001 to 2015 [93]. According to the Medical Expenditure Panel Survey (MEPS) from 2002 to 2013, the mean price of insulin increased by about 200% (from $4.34/mL to $12.92/mL) during this period, which was significantly higher than increases in the price of non-insulin comparators [94]. The introduction of biosimilar insulins to the market is expected to offer treatment options with lower cost. This will be tested when the biosimilar glargine, the first FDA-approved biosimilar insulin, becomes available in the U.S. market. However, a significant reduction in insulin prices is not expected soon [95].
When insulin is required, most patients with T2DM can be treated with older human insulins, which have similar efficacy and lower costs than the more expensive newer insulin analogs. A Cochrane review comparing basal insulin analogs to NPH showed similar efficacy in glycemic control with minimal clinical benefit in the form of less nocturnal hypoglycemia in the insulin analog arm [96]. Furthermore, similar glycemic control and risk of hypoglycemia was seen when regular insulin was compared with the rapid-acting insulin analogs [97]. The cost of human NPH insulin for a patient on a total daily dose of 60 units is approximately $52 per month. This contrasts with the most widely used insulin, insulin glargine, which has a cash price of about $500 per month for the same amount (Table 5). Insulin pens, which are convenient, are more expensive. Interestingly, human insulins do not require prescriptions, allowing underinsured, underfunded patients ongoing access to them.
Incorporating Patient Preferences
Research evidence is necessary but insufficient for making patient care decisions. Along with the potential benefits, harms, costs, and inconveniences of the management options, patient perspectives, beliefs, expectations, and health-related goals must be considered. Patients will undoubtedly have preferences regarding defining goals and ranking options. Clinicians should discuss therapeutic goals and treatment options and work collaboratively with patients in determining management strategies [98].
Summary
Potential treatment approaches for treating hyperglycemia in T2DM are summarized in Figure 1 and Figure 2 [4,7]. As long as there are no contraindications, metformin should be recommended concurrent with lifestyle intervention at the time of diabetes diagnosis. Even if metformin monotherapy is initially effective, glycemic control is likely to deteriorate over time due to progressive loss of β-cell function in T2DM.
There is no consensus as to what the second-line agent should be. Selection of a second agent should be made based on potential advantages and disadvantages of each agent for any given patient. A patient-centered approach is preferred over a fixed algorithm. If the patient progresses to the point where dual therapy does not provide adequate control, either a third non-insulin agent or insulin can be added. In patients with modestly elevated A1C (below ~8%), addition of a third non-insulin agent may be equally effective as (but more expensive than) addition of insulin.
Patients with significantly elevated A1C levels on non-insulin agents usually should have insulin added to their regimen. When insulin is added, metformin should be continued. DPP-4 inhibitors and sulfonylureas are typically stopped. If SGLT-2 inhibitors and/or GLP-1 receptor agonists are continued, this may aid with weight maintenance. However, continuing these agents is likely to be expensive and associated with problems associated with polypharmacy.
The most widely recommended strategy for initiating insulin in T2DM is to add a single bedtime injection of basal insulin (ie, NPH, glargine, detemir, or degludec) to the patient’s regimen. This regimen has been found to be effective in numerous studies and controls hyperglycemia in up to 60% of patients [99]. If the patient is treated with a single bedtime injection of insulin and the fasting glucose level is within the target range but the A1C level remains above goal, addition of mealtime insulin injections is likely to be beneficial. Alternatively, addition of a GLP-1 receptor agonist to basal insulin has been shown to be equally beneficial [4,6]. When adding mealtime insulin, a common strategy is to add a single injection of a rapid-acting insulin (eg, lispro, aspart, glulisine) before the patient’s largest meal of the day. Additional premeal injections of rapid-acting insulin may be added as needed, based on self-monitoring blood glucose results. If glycemia remains significantly uncontrolled on more than 200 units of insulin per day, switching to a concentrated form of insulin (eg, U-200, U-300, or U-500) should be considered.
Corresponding author: Maryam Fazel, PharmD, BCPS, BCACP, CDE, 1295 N. Martin Ave. (Room B211B), Tucson, Arizona 85721-0202, maryamfazel@pharmacy.arizona.edu.
Financial disclosures: None.
1. National diabetes statistics report: estimates of diabetes and its burden in the United States, 2014. Centers for Disease Control and Prevention Web site. www.cdc.gov/diabetes/pubs/statsreport14/national-diabetes-report-web.pdf. Accessed November 29, 2016.
2. Statistics about diabetes. American Diabetes Association Web site. www.diabetes.org/diabetes-basics/statistics/. Accessed November 29, 2016.
3. American Diabetes Association. Economic costs of diabetes in the U.S. in 2012. Diabetes Care 2013;36:1033–46.
4. American Diabetes Association. Standards of medical care in diabetes--2016. Diabetes Care 2016;39(Suppl. 1).
5. Raz I, Riddle MC, Rosenstock J, et al. Personalized management of hyperglycemia in type 2 diabetes: reflections from a Diabetes Care Editors’ Expert Forum. Diabetes Care 2013;36:1779–88.
6. Inzucchi SE, Bergenstal RM, Buse JB, et al. Management of hyperglycemia in type 2 diabetes, 2015: a patient-centered approach: update to a position statement of the American Diabetes Association and the European Association for the Study of Diabetes. Diabetes Care 2015;38:140–9.
7. Garber AJ, Abrahamson MJ, Barzilay JI, et al. Consensus Statement by the American Association of Clinical Endocrinologists and American College of Endocrinology on the Comprehensive Type 2 Diabetes Management Algorithm--2016 Executive Summary. Endocr Pract 2016;22:84–113.
8. Steenkamp DW, Alexanian SM, Sternthal E. Approach to the patient with atypical diabetes. CMAJ 2014;186:678–84.
9. de Lusignan S, Sadek N, Mulnier H, et al. Miscoding, misclassification and misdiagnosis of diabetes in primary care. Diabet Med 2012;29:181–9.
10. Tripathi A, Rizvi AA, Knight LM, Jerrell JM. Prevalence and impact of initial misclassification of pediatric type 1 diabetes mellitus. South Med J 2012;105:513–7.
11. The effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin-dependent diabetes mellitus. The Diabetes Control and Complications Trial Research Group. N Engl J Med 1993;329:977–86.
12. Intensive blood-glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33). UK Prospective Diabetes Study (UKPDS) Group. Lancet 1998;352:837–53.
13. Nathan DM, Cleary PA, Backlund JY, et al. Intensive diabetes treatment and cardiovascular disease in patients with type 1 diabetes. N Engl J Med 2005;353:2643–53.
14. Nathan DM, DCCT/EDIC Research Group. The diabetes control and complications trial/epidemiology of diabetes interventions and complications study at 30 years: overview. Diabetes Care 2014;37:9–16.
15. Effect of intensive blood-glucose control with metformin on complications in overweight patients with type 2 diabetes (UKPDS 34). UK Prospective Diabetes Study (UKPDS) Group. Lancet 1998;352:854–65.
16. Holman RR, Paul SK, Bethel MA, et al. 10-Year follow-up of intensive glucose control in type 2 diabetes. N Engl J Med 2008;359:1577–89.
17. Ohkubo Y, Kishikawa H, Araki E, et al. Intensive insulin therapy prevents the progression of diabetic microvascular complications in Japanese patients with non-insulin-dependent diabetes mellitus: a randomized prospective 6-year study. Diabetes Res Clin Pract 1995;28:103–17.
18. Action to Control Cardiovascular Risk in Diabetes Study Group, Gerstein HC, Miller ME, Byington RP, et al. Effects of intensive glucose lowering in type 2 diabetes. N Engl J Med 2008;358:2545–59.
19. ACCORD Study Group, Gerstein HC, Miller ME, Genuth S, et al. Long-term effects of intensive glucose lowering on cardiovascular outcomes. N Engl J Med 2011;364:818–28.
20. ADVANCE Collaborative Group, Patel A, MacMahon S, Chalmers J, et al. Intensive blood glucose control and vascular outcomes in patients with type 2 diabetes. N Engl J Med 2008;358:2560–72.
21. Wong MG, Perkovic V, Chalmers J, et al. Long-term Benefits of Intensive Glucose Control for Preventing End-Stage Kidney Disease: ADVANCE-ON. Diabetes Care 2016;39:694–700.
22. Duckworth W, Abraira C, Moritz T, et al. Glucose control and vascular complications in veterans with type 2 diabetes. N Engl J Med 2009;360:129–39.
23. Hayward RA, Reaven PD, Wiitala WL, et al. Follow-up of glycemic control and cardiovascular outcomes in type 2 diabetes. N Engl J Med 2015;372:2197–206.
24. American Diabetes Association. Standards of medical care in diabetes--2009. Diabetes Care 2009;32 Suppl 1:S13–61.
25. American Geriatrics Society Expert Panel on Care of Older Adults with Diabetes Mellitus, Moreno G, Mangione CM, Kimbro L, Vaisberg E. Guidelines abstracted from the American Geriatrics Society Guidelines for Improving the Care of Older Adults with Diabetes Mellitus: 2013 update. J Am Geriatr Soc 2013;61:2020–6.
26. Lipska KJ, Ross JS, Miao Y, et al. Potential overtreatment of diabetes mellitus in older adults with tight glycemic control. JAMA Intern Med 2015;175:356–62.
27. Sussman JB, Kerr EA, Saini SD, et al. Rates of deintensification of blood pressure and glycemic medication treatment based on levels of control and life expectancy in older patients with diabetes mellitus. JAMA Intern Med 2015;175:1942–9.
28. FDA Drug Safety Communication: FDA revises warnings regarding use of the diabetes medicine metformin in certain patients with reduced kidney function. FDA Web site. www.fda.gov/Drugs/DrugSafety/ucm493244.htm. Accessed December 1, 2016.
29. Inzucchi SE, Lipska KJ, Mayo H, et al. Metformin in patients with type 2 diabetes and kidney disease: a systematic review. JAMA 2014;312:2668–75.
30. Nathan DM. Diabetes: advances in diagnosis and treatment. JAMA 2015;314:1052–62.
31. Nathan DM, Buse JB, Kahn SE, et al. Rationale and design of the glycemia reduction approaches in diabetes: a comparative effectiveness study (GRADE). Diabetes Care 2013;36:2254–61.
32. NIH begins recruitment for long-term study of diabetes drug efficacy. NIH Web site. www.nih.gov/news-events/news-releases/nih-begins-recruitment-long-term-study-diabetes-drug-efficacy. Accessed December 1, 2016.
33. Hermayer KL, Dake A. Newer oral and noninsulin therapies to treat type 2 diabetes mellitus. Cleve Clin J Med 2016;83(5 Suppl 1):S18–26.
34. Bolen S, Wilson L, Vassy J, et al. Systematic review: comparative effectiveness and safety of oral medications for type 2 diabetes mellitus. Ann Intern Med 2007;147:386–99.
35. Bolen S, Tseng E, Hutfless S, et al. Oral diabetes medications for adults with type 2 diabetes: an update. Agency for Healthcare Research and Quality (US); 2011 Mar Report No: 11-EHC038-EF. AHRQ Comparative Effectiveness Reviews.
36. Metformin, glyburide, glipizide, glimeperide, sitagliptin, saxagliptin, linagliptin, lixisenatide, alogliptin, exenatide, liraglutide, albiglutide, dulaglutide, canagliflozin, danagliflozin, empagliflozin: drug information. Waltham (MA): UpToDate, Inc.; 2016. Accessed September 23, 2016.
37. GoodRx Web site. http://www.goodrx.com. Accessed August 6, 2016 and December 2016.
38. Insulins available in the United States. Diabetesforcast Web site. Accessed August 6, 2016. www.diabetesforecast.org/2016/mar-apr/images/2016-insulin-chart-new.pdf.
39. Inzucchi SE, Bergenstal RM, Buse JB, et al. Management of hyperglycaemia in type 2 diabetes: a patient-centered approach. Position statement of the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD). Diabetologia 2012;55:577–96.
40. Elashoff M, Matveyenko AV, Gier B, et al. Pancreatitis, pancreatic, and thyroid cancer with glucagon-like peptide-1-based therapies. Gastroenterology 2011;141:150–6.
41. Butler AE, Campbell-Thompson M, Gurlo T, et al. Marked expansion of exocrine and endocrine pancreas with incretin therapy in humans with increased exocrine pancreas dysplasia and the potential for glucagon-producing neuroendocrine tumors. Diabetes 2013;62:2595–604.
42. Green JB, Bethel MA, Armstrong PW, et al. Effect of sitagliptin on cardiovascular outcomes in type 2 diabetes. N Engl J Med 2015;373:232–42.
43. White WB, Cannon CP, Heller SR, et al. Alogliptin after acute coronary syndrome in patients with type 2 diabetes. N Engl J Med 2013;369:1327–35.
44. Scirica BM, Bhatt DL, Braunwald E, et al. Saxagliptin and cardiovascular outcomes in patients with type 2 diabetes mellitus. N Engl J Med 2013;369:1317–26.
45. Egan AG, Blind E, Dunder K, et al. Pancreatic safety of incretin-based drugs--FDA and EMA assessment. N Engl J Med 2014;370:794–7.
46. Vasilakou D, Karagiannis T, Athanasiadou E, et al. Sodium-glucose cotransporter 2 inhibitors for type 2 diabetes: a systematic review and meta-analysis. Ann Intern Med 2013;159:262–74.
47. Nyirjesy P, Sobel JD, Fung A, et al. Genital mycotic infections with canagliflozin, a sodium glucose co-transporter 2 inhibitor, in patients with type 2 diabetes mellitus: a pooled analysis of clinical studies. Curr Med Res Opin 2014;30:1109–19.
48. Schernthaner G, Gross JL, Rosenstock J, et al. Canagliflozin compared with sitagliptin for patients with type 2 diabetes who do not have adequate glycemic control with metformin plus sulfonylurea: a 52-week randomized trial. Diabetes Care 2013;36:2508–15.
49. Blonde L, Dailey G, Jabbour S, et al. Gastrointestinal tolerability of extended-release metformin tablets compared to immediate-release metformin tablets: results of a retrospective cohort study. Curr Med Res Opin 2004;20:562–72.
50. Kalra S, Mukherjee JJ, Venkataraman S, et al. Hypoglycemia: the neglected complication. Indian J Endocrinol Metab 2013;17:819–34.
51. Paty BW. The role of hypoglycemia in cardiovascular outcomes in diabetes. Can J Diabetes 2015;39 Suppl 5:S155–9.
52. Zoungas S, Patel A, Chalmers J, et al. Severe hypoglycemia and risks of vascular events and death. N Engl J Med 2010;363:1410–8.
53. Whitmer RA, Karter AJ, Yaffe K, et al. Hypoglycemic episodes and risk of dementia in older patients with type 2 diabetes mellitus. JAMA 2009;301:1565–72.
54. McCoy RG, Van Houten HK, Ziegenfuss JY, et al. Increased mortality of patients with diabetes reporting severe hypoglycemia. Diabetes Care 2012;35:1897–901.
55. McCoy RG, Lipska KJ, Yao X, et al. Intensive treatment and severe hypoglycemia among adults with type 2 diabetes. JAMA Intern Med 2016;176:969–78.
56. Rodbard HW, Gough S, Lane W, et al. Reduced risk of hypoglycemia with insulin degludec versus insulin glargine in patients with type 2 diabetes requiring high doses of basal insulin: a meta-analysis of 5 randomized begin trials. Endocr Pract 2014;20:285–92.
57. Yki-Jarvinen H, Bergenstal R, Ziemen M, et al. New insulin glargine 300 units/mL versus glargine 100 units/mL in people with type 2 diabetes using oral agents and basal insulin: glucose control and hypoglycemia in a 6-month randomized controlled trial (EDITION 2). Diabetes Care 2014;37:3235–43.
58. Tuomilehto J, Lindstrom J, Eriksson JG, et al. Prevention of type 2 diabetes mellitus by changes in lifestyle among subjects with impaired glucose tolerance. N Engl J Med 2001;344:1343–50.
59. Knowler WC, Barrett-Connor E, Fowler SE, et al. Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin. N Engl J Med 2002;346:393–403.
60. Maruthur NM, Tseng E, Hutfless S, et al. Diabetes medications as monotherapy or metformin-based combination therapy for type 2 diabetes: a systematic review and meta-analysis. Ann Intern Med 2016;164:740–51.
61. Diabetes Prevention Program Research Group. Long-term safety, tolerability, and weight loss associated with metformin in the Diabetes Prevention Program Outcomes Study. Diabetes Care 2012;35:731–7.
62. Clar C, Gill JA, Court R, Waugh N. Systematic review of SGLT2 receptor inhibitors in dual or triple therapy in type 2 diabetes. BMJ Open 2012;2:10.1136/bmjopen,2012-001007.
63. Vilsboll T, Christensen M, Junker AE, et al. Effects of glucagon-like peptide-1 receptor agonists on weight loss: systematic review and meta-analyses of randomised controlled trials. BMJ 2012;344:d7771.
64. FDA approves weight-management drug Saxenda. FDA Web site www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm427913.htm. Accessed September 22, 2016.
65. Apovian CM, Aronne LJ, Bessesen DH, et al. Pharmacological management of obesity: an endocrine Society clinical practice guideline. J Clin Endocrinol Metab 2015;100:342–62.
66. Liraglutide, lorcaserin, naltrexone/bupropion, orlistat, phentermine/topiramate: drug information. Greenwood Village (CO): Truven Health Analytics; 2016. www.micromedexsolutions.com. Accessed May 13, 2016.
67. Liraglutide, lorcaserin, naltrexone/bupropion, orlistat, phentermine/topiramate: drug information. Waltham (MA): UpToDate, Inc.; 2016. Accessed May 13, 2016.
68. Yanovski SZ, Yanovski JA. Long-term drug treatment for obesity: a systematic and clinical review. JAMA 2014;311:74–86.
69. Sjostrom L, Peltonen M, Jacobson P, et al. Association of bariatric surgery with long-term remission of type 2 diabetes and with microvascular and macrovascular complications. JAMA 2014;311:2297–304.
70. Schauer PR, Bhatt DL, Kirwan JP, et al. Bariatric surgery versus intensive medical therapy for diabetes--3-year outcomes. N Engl J Med 2014;370:2002–13.
71. Rubino F, Nathan DM, Eckel RH, et al. Metabolic surgery in the treatment algorithm for type 2 diabetes: a joint statement by international diabetes organizations. Diabetes Care 2016;39:861–77.
72. Lathief S, Inzucchi SE. Approach to diabetes management in patients with CVD. Trends Cardiovasc Med 2016;26:165–79.
73. Nissen SE, Wolski K. Effect of rosiglitazone on the risk of myocardial infarction and death from cardiovascular causes. N Engl J Med 2007;356:2457–71.
74. Knatterud GL, Klimt CR, Levin ME, et al. Effects of hypoglycemic agents on vascular complications in patients with adult-onset diabetes. VII. Mortality and selected nonfatal events with insulin treatment. JAMA 1978;240:37–42.
75. Masoudi FA, Inzucchi SE, Wang Y, et al. Thiazolidinediones, metformin, and outcomes in older patients with diabetes and heart failure: an observational study. Circulation 2005;111:583–90.
76. Klepzig H, Kober G, Matter C, et al. Sulfonylureas and ischaemic preconditioning; a double-blind, placebo-controlled evaluation of glimepiride and glibenclamide. Eur Heart J 1999;20:439–46.
77. FDA announces new recommendations on evaluating cardiovascular risk in drugs intended to treat type 2 diabetes. FDA Web site. www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/2008/ucm116994.htm. Accessed August 20, 2016.
78. Kooy A, de Jager J, Lehert P, et al. Long-term effects of metformin on metabolism and microvascular and macrovascular disease in patients with type 2 diabetes mellitus. Arch Intern Med 2009;169:616–25.
79. Eurich DT, Weir DL, Majumdar SR, et al. Comparative safety and effectiveness of metformin in patients with diabetes mellitus and heart failure: systematic review of observational studies involving 34,000 patients. Circ Heart Fail 2013;6:395–402.
80. Tahrani AA, Varughese GI, Scarpello JH, Hanna FW. Metformin, heart failure, and lactic acidosis: is metformin absolutely contraindicated? BMJ 2007;335:508–12.
81. Li Y, Hu Y, Ley SH, et al. Sulfonylurea use and incident cardiovascular disease among patients with type 2 diabetes: prospective cohort study among women. Diabetes Care 2014;37:3106–13.
82. Bentley-Lewis R, Aguilar D, Riddle MC, et al. Rationale, design, and baseline characteristics in Evaluation of LIXisenatide in Acute Coronary Syndrome, a long-term cardiovascular end point trial of lixisenatide versus placebo. Am Heart J 2015;169:631,638.e7.
83. Marso SP, Daniels GH, Brown-Frandsen K, et al. Liraglutide and cardiovascular outcomes in type 2 diabetes. N Engl J Med 2016;375:311–22.
84. Exenatide Study of Cardiovascular Event Lowering Trial (EXSCEL): A Trial To Evaluate Cardiovascular Outcomes After Treatment With Exenatide Once Weekly In Patients With Type 2 Diabetes Mellitus. clinicaltrials.gov Web site. https://clinicaltrials.gov/ct2/show/NCT01144338. 2016 Accessed September 23, 2016.
85. Researching Cardiovascular Events With a Weekly Incretin in Diabetes (REWIND). clinicaltrials.gov Web site. https://clinicaltrials.gov/ct2/show/NCT01394952. Accessed September 23, 2016.
86. Schernthaner G, Schernthaner-Reiter MH, Schernthaner GH. EMPA-REG and other cardiovascular outcome trials of glucose-lowering agents: implications for future treatment strategies in type 2 diabetes mellitus. Clin Ther 2016;38:1288–98.
87. CANVAS--CANagliflozin cardiovascular Assesssment Study (CANVAS). clinicaltrials.gov Web site. https://clinicaltrials.gov/ct2/show/NCT01032629. Accessed September 23, 2016.
88. Evaluation of the Effects of Canagliflozin on Renal and Cardiovascular Outcomes in Participants With Diabetic Nephropathy (CREDENCE). clinicaltrials.gov Web site. https://clinicaltrials.gov/ct2/show/NCT02065791. Accessed September 23, 2016.
89. Multicenter Trial to Evaluate the Effect of Dapagliflozin on the Incidence of Cardiovascular Events (DECLARE-TIMI58). clinicaltrials.gov Web site. https://clinicaltrials.gov/ct2/show/NCT01730534. Accessed September 23, 2016.
90. Zannad F, Cannon CP, Cushman WC, et al. Heart failure and mortality outcomes in patients with type 2 diabetes taking alogliptin versus placebo in EXAMINE: a multicentre, randomised, double-blind trial. Lancet 2015;385(9982):2067–76.
91. Van Klompenburg E, Heins JR. New insulin options for diabetic patients. S D Med 2016;69:84–5.
92. Rosenstock J, Schwartz SL, Clark CM Jr, et al. Basal insulin therapy in type 2 diabetes: 28-week comparison of insulin glargine (HOE 901) and NPH insulin. Diabetes Care 2001;24:631–6.
93. Tylee T, Hirsch IB. Costs associated with using different insulin preparations. JAMA 2015;314:665–6.
94. Hua X, Carvalho N, Tew M, et al. Expenditures and prices of antihyperglycemic medications in the United States: 2002-2013. JAMA 2016;315:1400–2.
95. Heinemann L. Biosimilar insulin and costs: what can we expect? J Diabetes Sci Technol 2016;10:457–62.
96. Horvath K, Jeitler K, Berghold A, et al. Long-acting insulin analogues versus NPH insulin (human isophane insulin) for type 2 diabetes mellitus. Cochrane Database Syst Rev 2007;(2)(2):CD005613.
97. Mannucci E, Monami M, Marchionni N. Short-acting insulin analogues vs. regular human insulin in type 2 diabetes: a meta-analysis. Diabetes Obes Metab 2009;11:53–9.
98. Powell PW, Corathers SD, Raymond J, Streisand R. New approaches to providing individualized diabetes care in the 21st century. Curr Diabetes Rev 2015;11:222–30.
99. Riddle MC, Rosenstock J, Gerich J, Insulin Glargine 4002 Study Investigators. The treat-to-target trial: randomized addition of glargine or human NPH insulin to oral therapy of type 2 diabetic patients. Diabetes Care 2003;26:3080–6.
1. National diabetes statistics report: estimates of diabetes and its burden in the United States, 2014. Centers for Disease Control and Prevention Web site. www.cdc.gov/diabetes/pubs/statsreport14/national-diabetes-report-web.pdf. Accessed November 29, 2016.
2. Statistics about diabetes. American Diabetes Association Web site. www.diabetes.org/diabetes-basics/statistics/. Accessed November 29, 2016.
3. American Diabetes Association. Economic costs of diabetes in the U.S. in 2012. Diabetes Care 2013;36:1033–46.
4. American Diabetes Association. Standards of medical care in diabetes--2016. Diabetes Care 2016;39(Suppl. 1).
5. Raz I, Riddle MC, Rosenstock J, et al. Personalized management of hyperglycemia in type 2 diabetes: reflections from a Diabetes Care Editors’ Expert Forum. Diabetes Care 2013;36:1779–88.
6. Inzucchi SE, Bergenstal RM, Buse JB, et al. Management of hyperglycemia in type 2 diabetes, 2015: a patient-centered approach: update to a position statement of the American Diabetes Association and the European Association for the Study of Diabetes. Diabetes Care 2015;38:140–9.
7. Garber AJ, Abrahamson MJ, Barzilay JI, et al. Consensus Statement by the American Association of Clinical Endocrinologists and American College of Endocrinology on the Comprehensive Type 2 Diabetes Management Algorithm--2016 Executive Summary. Endocr Pract 2016;22:84–113.
8. Steenkamp DW, Alexanian SM, Sternthal E. Approach to the patient with atypical diabetes. CMAJ 2014;186:678–84.
9. de Lusignan S, Sadek N, Mulnier H, et al. Miscoding, misclassification and misdiagnosis of diabetes in primary care. Diabet Med 2012;29:181–9.
10. Tripathi A, Rizvi AA, Knight LM, Jerrell JM. Prevalence and impact of initial misclassification of pediatric type 1 diabetes mellitus. South Med J 2012;105:513–7.
11. The effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin-dependent diabetes mellitus. The Diabetes Control and Complications Trial Research Group. N Engl J Med 1993;329:977–86.
12. Intensive blood-glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33). UK Prospective Diabetes Study (UKPDS) Group. Lancet 1998;352:837–53.
13. Nathan DM, Cleary PA, Backlund JY, et al. Intensive diabetes treatment and cardiovascular disease in patients with type 1 diabetes. N Engl J Med 2005;353:2643–53.
14. Nathan DM, DCCT/EDIC Research Group. The diabetes control and complications trial/epidemiology of diabetes interventions and complications study at 30 years: overview. Diabetes Care 2014;37:9–16.
15. Effect of intensive blood-glucose control with metformin on complications in overweight patients with type 2 diabetes (UKPDS 34). UK Prospective Diabetes Study (UKPDS) Group. Lancet 1998;352:854–65.
16. Holman RR, Paul SK, Bethel MA, et al. 10-Year follow-up of intensive glucose control in type 2 diabetes. N Engl J Med 2008;359:1577–89.
17. Ohkubo Y, Kishikawa H, Araki E, et al. Intensive insulin therapy prevents the progression of diabetic microvascular complications in Japanese patients with non-insulin-dependent diabetes mellitus: a randomized prospective 6-year study. Diabetes Res Clin Pract 1995;28:103–17.
18. Action to Control Cardiovascular Risk in Diabetes Study Group, Gerstein HC, Miller ME, Byington RP, et al. Effects of intensive glucose lowering in type 2 diabetes. N Engl J Med 2008;358:2545–59.
19. ACCORD Study Group, Gerstein HC, Miller ME, Genuth S, et al. Long-term effects of intensive glucose lowering on cardiovascular outcomes. N Engl J Med 2011;364:818–28.
20. ADVANCE Collaborative Group, Patel A, MacMahon S, Chalmers J, et al. Intensive blood glucose control and vascular outcomes in patients with type 2 diabetes. N Engl J Med 2008;358:2560–72.
21. Wong MG, Perkovic V, Chalmers J, et al. Long-term Benefits of Intensive Glucose Control for Preventing End-Stage Kidney Disease: ADVANCE-ON. Diabetes Care 2016;39:694–700.
22. Duckworth W, Abraira C, Moritz T, et al. Glucose control and vascular complications in veterans with type 2 diabetes. N Engl J Med 2009;360:129–39.
23. Hayward RA, Reaven PD, Wiitala WL, et al. Follow-up of glycemic control and cardiovascular outcomes in type 2 diabetes. N Engl J Med 2015;372:2197–206.
24. American Diabetes Association. Standards of medical care in diabetes--2009. Diabetes Care 2009;32 Suppl 1:S13–61.
25. American Geriatrics Society Expert Panel on Care of Older Adults with Diabetes Mellitus, Moreno G, Mangione CM, Kimbro L, Vaisberg E. Guidelines abstracted from the American Geriatrics Society Guidelines for Improving the Care of Older Adults with Diabetes Mellitus: 2013 update. J Am Geriatr Soc 2013;61:2020–6.
26. Lipska KJ, Ross JS, Miao Y, et al. Potential overtreatment of diabetes mellitus in older adults with tight glycemic control. JAMA Intern Med 2015;175:356–62.
27. Sussman JB, Kerr EA, Saini SD, et al. Rates of deintensification of blood pressure and glycemic medication treatment based on levels of control and life expectancy in older patients with diabetes mellitus. JAMA Intern Med 2015;175:1942–9.
28. FDA Drug Safety Communication: FDA revises warnings regarding use of the diabetes medicine metformin in certain patients with reduced kidney function. FDA Web site. www.fda.gov/Drugs/DrugSafety/ucm493244.htm. Accessed December 1, 2016.
29. Inzucchi SE, Lipska KJ, Mayo H, et al. Metformin in patients with type 2 diabetes and kidney disease: a systematic review. JAMA 2014;312:2668–75.
30. Nathan DM. Diabetes: advances in diagnosis and treatment. JAMA 2015;314:1052–62.
31. Nathan DM, Buse JB, Kahn SE, et al. Rationale and design of the glycemia reduction approaches in diabetes: a comparative effectiveness study (GRADE). Diabetes Care 2013;36:2254–61.
32. NIH begins recruitment for long-term study of diabetes drug efficacy. NIH Web site. www.nih.gov/news-events/news-releases/nih-begins-recruitment-long-term-study-diabetes-drug-efficacy. Accessed December 1, 2016.
33. Hermayer KL, Dake A. Newer oral and noninsulin therapies to treat type 2 diabetes mellitus. Cleve Clin J Med 2016;83(5 Suppl 1):S18–26.
34. Bolen S, Wilson L, Vassy J, et al. Systematic review: comparative effectiveness and safety of oral medications for type 2 diabetes mellitus. Ann Intern Med 2007;147:386–99.
35. Bolen S, Tseng E, Hutfless S, et al. Oral diabetes medications for adults with type 2 diabetes: an update. Agency for Healthcare Research and Quality (US); 2011 Mar Report No: 11-EHC038-EF. AHRQ Comparative Effectiveness Reviews.
36. Metformin, glyburide, glipizide, glimeperide, sitagliptin, saxagliptin, linagliptin, lixisenatide, alogliptin, exenatide, liraglutide, albiglutide, dulaglutide, canagliflozin, danagliflozin, empagliflozin: drug information. Waltham (MA): UpToDate, Inc.; 2016. Accessed September 23, 2016.
37. GoodRx Web site. http://www.goodrx.com. Accessed August 6, 2016 and December 2016.
38. Insulins available in the United States. Diabetesforcast Web site. Accessed August 6, 2016. www.diabetesforecast.org/2016/mar-apr/images/2016-insulin-chart-new.pdf.
39. Inzucchi SE, Bergenstal RM, Buse JB, et al. Management of hyperglycaemia in type 2 diabetes: a patient-centered approach. Position statement of the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD). Diabetologia 2012;55:577–96.
40. Elashoff M, Matveyenko AV, Gier B, et al. Pancreatitis, pancreatic, and thyroid cancer with glucagon-like peptide-1-based therapies. Gastroenterology 2011;141:150–6.
41. Butler AE, Campbell-Thompson M, Gurlo T, et al. Marked expansion of exocrine and endocrine pancreas with incretin therapy in humans with increased exocrine pancreas dysplasia and the potential for glucagon-producing neuroendocrine tumors. Diabetes 2013;62:2595–604.
42. Green JB, Bethel MA, Armstrong PW, et al. Effect of sitagliptin on cardiovascular outcomes in type 2 diabetes. N Engl J Med 2015;373:232–42.
43. White WB, Cannon CP, Heller SR, et al. Alogliptin after acute coronary syndrome in patients with type 2 diabetes. N Engl J Med 2013;369:1327–35.
44. Scirica BM, Bhatt DL, Braunwald E, et al. Saxagliptin and cardiovascular outcomes in patients with type 2 diabetes mellitus. N Engl J Med 2013;369:1317–26.
45. Egan AG, Blind E, Dunder K, et al. Pancreatic safety of incretin-based drugs--FDA and EMA assessment. N Engl J Med 2014;370:794–7.
46. Vasilakou D, Karagiannis T, Athanasiadou E, et al. Sodium-glucose cotransporter 2 inhibitors for type 2 diabetes: a systematic review and meta-analysis. Ann Intern Med 2013;159:262–74.
47. Nyirjesy P, Sobel JD, Fung A, et al. Genital mycotic infections with canagliflozin, a sodium glucose co-transporter 2 inhibitor, in patients with type 2 diabetes mellitus: a pooled analysis of clinical studies. Curr Med Res Opin 2014;30:1109–19.
48. Schernthaner G, Gross JL, Rosenstock J, et al. Canagliflozin compared with sitagliptin for patients with type 2 diabetes who do not have adequate glycemic control with metformin plus sulfonylurea: a 52-week randomized trial. Diabetes Care 2013;36:2508–15.
49. Blonde L, Dailey G, Jabbour S, et al. Gastrointestinal tolerability of extended-release metformin tablets compared to immediate-release metformin tablets: results of a retrospective cohort study. Curr Med Res Opin 2004;20:562–72.
50. Kalra S, Mukherjee JJ, Venkataraman S, et al. Hypoglycemia: the neglected complication. Indian J Endocrinol Metab 2013;17:819–34.
51. Paty BW. The role of hypoglycemia in cardiovascular outcomes in diabetes. Can J Diabetes 2015;39 Suppl 5:S155–9.
52. Zoungas S, Patel A, Chalmers J, et al. Severe hypoglycemia and risks of vascular events and death. N Engl J Med 2010;363:1410–8.
53. Whitmer RA, Karter AJ, Yaffe K, et al. Hypoglycemic episodes and risk of dementia in older patients with type 2 diabetes mellitus. JAMA 2009;301:1565–72.
54. McCoy RG, Van Houten HK, Ziegenfuss JY, et al. Increased mortality of patients with diabetes reporting severe hypoglycemia. Diabetes Care 2012;35:1897–901.
55. McCoy RG, Lipska KJ, Yao X, et al. Intensive treatment and severe hypoglycemia among adults with type 2 diabetes. JAMA Intern Med 2016;176:969–78.
56. Rodbard HW, Gough S, Lane W, et al. Reduced risk of hypoglycemia with insulin degludec versus insulin glargine in patients with type 2 diabetes requiring high doses of basal insulin: a meta-analysis of 5 randomized begin trials. Endocr Pract 2014;20:285–92.
57. Yki-Jarvinen H, Bergenstal R, Ziemen M, et al. New insulin glargine 300 units/mL versus glargine 100 units/mL in people with type 2 diabetes using oral agents and basal insulin: glucose control and hypoglycemia in a 6-month randomized controlled trial (EDITION 2). Diabetes Care 2014;37:3235–43.
58. Tuomilehto J, Lindstrom J, Eriksson JG, et al. Prevention of type 2 diabetes mellitus by changes in lifestyle among subjects with impaired glucose tolerance. N Engl J Med 2001;344:1343–50.
59. Knowler WC, Barrett-Connor E, Fowler SE, et al. Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin. N Engl J Med 2002;346:393–403.
60. Maruthur NM, Tseng E, Hutfless S, et al. Diabetes medications as monotherapy or metformin-based combination therapy for type 2 diabetes: a systematic review and meta-analysis. Ann Intern Med 2016;164:740–51.
61. Diabetes Prevention Program Research Group. Long-term safety, tolerability, and weight loss associated with metformin in the Diabetes Prevention Program Outcomes Study. Diabetes Care 2012;35:731–7.
62. Clar C, Gill JA, Court R, Waugh N. Systematic review of SGLT2 receptor inhibitors in dual or triple therapy in type 2 diabetes. BMJ Open 2012;2:10.1136/bmjopen,2012-001007.
63. Vilsboll T, Christensen M, Junker AE, et al. Effects of glucagon-like peptide-1 receptor agonists on weight loss: systematic review and meta-analyses of randomised controlled trials. BMJ 2012;344:d7771.
64. FDA approves weight-management drug Saxenda. FDA Web site www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm427913.htm. Accessed September 22, 2016.
65. Apovian CM, Aronne LJ, Bessesen DH, et al. Pharmacological management of obesity: an endocrine Society clinical practice guideline. J Clin Endocrinol Metab 2015;100:342–62.
66. Liraglutide, lorcaserin, naltrexone/bupropion, orlistat, phentermine/topiramate: drug information. Greenwood Village (CO): Truven Health Analytics; 2016. www.micromedexsolutions.com. Accessed May 13, 2016.
67. Liraglutide, lorcaserin, naltrexone/bupropion, orlistat, phentermine/topiramate: drug information. Waltham (MA): UpToDate, Inc.; 2016. Accessed May 13, 2016.
68. Yanovski SZ, Yanovski JA. Long-term drug treatment for obesity: a systematic and clinical review. JAMA 2014;311:74–86.
69. Sjostrom L, Peltonen M, Jacobson P, et al. Association of bariatric surgery with long-term remission of type 2 diabetes and with microvascular and macrovascular complications. JAMA 2014;311:2297–304.
70. Schauer PR, Bhatt DL, Kirwan JP, et al. Bariatric surgery versus intensive medical therapy for diabetes--3-year outcomes. N Engl J Med 2014;370:2002–13.
71. Rubino F, Nathan DM, Eckel RH, et al. Metabolic surgery in the treatment algorithm for type 2 diabetes: a joint statement by international diabetes organizations. Diabetes Care 2016;39:861–77.
72. Lathief S, Inzucchi SE. Approach to diabetes management in patients with CVD. Trends Cardiovasc Med 2016;26:165–79.
73. Nissen SE, Wolski K. Effect of rosiglitazone on the risk of myocardial infarction and death from cardiovascular causes. N Engl J Med 2007;356:2457–71.
74. Knatterud GL, Klimt CR, Levin ME, et al. Effects of hypoglycemic agents on vascular complications in patients with adult-onset diabetes. VII. Mortality and selected nonfatal events with insulin treatment. JAMA 1978;240:37–42.
75. Masoudi FA, Inzucchi SE, Wang Y, et al. Thiazolidinediones, metformin, and outcomes in older patients with diabetes and heart failure: an observational study. Circulation 2005;111:583–90.
76. Klepzig H, Kober G, Matter C, et al. Sulfonylureas and ischaemic preconditioning; a double-blind, placebo-controlled evaluation of glimepiride and glibenclamide. Eur Heart J 1999;20:439–46.
77. FDA announces new recommendations on evaluating cardiovascular risk in drugs intended to treat type 2 diabetes. FDA Web site. www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/2008/ucm116994.htm. Accessed August 20, 2016.
78. Kooy A, de Jager J, Lehert P, et al. Long-term effects of metformin on metabolism and microvascular and macrovascular disease in patients with type 2 diabetes mellitus. Arch Intern Med 2009;169:616–25.
79. Eurich DT, Weir DL, Majumdar SR, et al. Comparative safety and effectiveness of metformin in patients with diabetes mellitus and heart failure: systematic review of observational studies involving 34,000 patients. Circ Heart Fail 2013;6:395–402.
80. Tahrani AA, Varughese GI, Scarpello JH, Hanna FW. Metformin, heart failure, and lactic acidosis: is metformin absolutely contraindicated? BMJ 2007;335:508–12.
81. Li Y, Hu Y, Ley SH, et al. Sulfonylurea use and incident cardiovascular disease among patients with type 2 diabetes: prospective cohort study among women. Diabetes Care 2014;37:3106–13.
82. Bentley-Lewis R, Aguilar D, Riddle MC, et al. Rationale, design, and baseline characteristics in Evaluation of LIXisenatide in Acute Coronary Syndrome, a long-term cardiovascular end point trial of lixisenatide versus placebo. Am Heart J 2015;169:631,638.e7.
83. Marso SP, Daniels GH, Brown-Frandsen K, et al. Liraglutide and cardiovascular outcomes in type 2 diabetes. N Engl J Med 2016;375:311–22.
84. Exenatide Study of Cardiovascular Event Lowering Trial (EXSCEL): A Trial To Evaluate Cardiovascular Outcomes After Treatment With Exenatide Once Weekly In Patients With Type 2 Diabetes Mellitus. clinicaltrials.gov Web site. https://clinicaltrials.gov/ct2/show/NCT01144338. 2016 Accessed September 23, 2016.
85. Researching Cardiovascular Events With a Weekly Incretin in Diabetes (REWIND). clinicaltrials.gov Web site. https://clinicaltrials.gov/ct2/show/NCT01394952. Accessed September 23, 2016.
86. Schernthaner G, Schernthaner-Reiter MH, Schernthaner GH. EMPA-REG and other cardiovascular outcome trials of glucose-lowering agents: implications for future treatment strategies in type 2 diabetes mellitus. Clin Ther 2016;38:1288–98.
87. CANVAS--CANagliflozin cardiovascular Assesssment Study (CANVAS). clinicaltrials.gov Web site. https://clinicaltrials.gov/ct2/show/NCT01032629. Accessed September 23, 2016.
88. Evaluation of the Effects of Canagliflozin on Renal and Cardiovascular Outcomes in Participants With Diabetic Nephropathy (CREDENCE). clinicaltrials.gov Web site. https://clinicaltrials.gov/ct2/show/NCT02065791. Accessed September 23, 2016.
89. Multicenter Trial to Evaluate the Effect of Dapagliflozin on the Incidence of Cardiovascular Events (DECLARE-TIMI58). clinicaltrials.gov Web site. https://clinicaltrials.gov/ct2/show/NCT01730534. Accessed September 23, 2016.
90. Zannad F, Cannon CP, Cushman WC, et al. Heart failure and mortality outcomes in patients with type 2 diabetes taking alogliptin versus placebo in EXAMINE: a multicentre, randomised, double-blind trial. Lancet 2015;385(9982):2067–76.
91. Van Klompenburg E, Heins JR. New insulin options for diabetic patients. S D Med 2016;69:84–5.
92. Rosenstock J, Schwartz SL, Clark CM Jr, et al. Basal insulin therapy in type 2 diabetes: 28-week comparison of insulin glargine (HOE 901) and NPH insulin. Diabetes Care 2001;24:631–6.
93. Tylee T, Hirsch IB. Costs associated with using different insulin preparations. JAMA 2015;314:665–6.
94. Hua X, Carvalho N, Tew M, et al. Expenditures and prices of antihyperglycemic medications in the United States: 2002-2013. JAMA 2016;315:1400–2.
95. Heinemann L. Biosimilar insulin and costs: what can we expect? J Diabetes Sci Technol 2016;10:457–62.
96. Horvath K, Jeitler K, Berghold A, et al. Long-acting insulin analogues versus NPH insulin (human isophane insulin) for type 2 diabetes mellitus. Cochrane Database Syst Rev 2007;(2)(2):CD005613.
97. Mannucci E, Monami M, Marchionni N. Short-acting insulin analogues vs. regular human insulin in type 2 diabetes: a meta-analysis. Diabetes Obes Metab 2009;11:53–9.
98. Powell PW, Corathers SD, Raymond J, Streisand R. New approaches to providing individualized diabetes care in the 21st century. Curr Diabetes Rev 2015;11:222–30.
99. Riddle MC, Rosenstock J, Gerich J, Insulin Glargine 4002 Study Investigators. The treat-to-target trial: randomized addition of glargine or human NPH insulin to oral therapy of type 2 diabetic patients. Diabetes Care 2003;26:3080–6.
Improving Inpatient Glycemic Control
Diabetes mellitus and/or inpatient hyperglycemia are common comorbid conditions in hospitalized patients. Recent surveys show that over 90% of hospitalized diabetic patients experience hyperglycemia (>200 mg/dL), and in nearly 1 in 5 of these patients hyperglycemia persists for 3 days or more.1 Hyperglycemia among inpatients without a previous history of diabetes mellitus is also very common.2 Observational studies have shown that hyperglycemia in hospitalized patients is associated with adverse outcomes including infectious complications, increased length of stay, and increased mortality.27 Recent randomized controlled trials have demonstrated that aggressive treatment of inpatient hyperglycemia improves outcomes in surgical and medical intensive care units.8, 9
Based on the available data, the American Diabetes Association (ADA) now advocates good metabolic control, defined as preprandial glucose levels of 90 to 130 mg/dL and peak postprandial glucose levels <180 mg/dL in hospitalized nonintensive care unit (ICU) patients.10 To reach these targets, the ADA and American College of Endocrinology (ACE) suggest that multidisciplinary teams develop and implement hyperglycemia management guidelines and protocols.11 Protocols should promote the use of continuous intravenous insulin infusions or scheduled basal‐bolus subcutaneous insulin regimens. Subcutaneous insulin protocols should include target glucose levels, basal, nutritional, and supplemental insulin, and daily dose adjustments.6 A recent randomized controlled trial of non‐ICU inpatients demonstrated that such a basal‐bolus insulin regimen results in improved glucose control compared with a sliding scale only regimen.12
To date, few published studies have investigated the best ways to implement such management protocols; those that have are often resource‐intensive, for example involving daily involvement of nurse practitioners or diabetologists.13, 14 It is therefore not known how best to implement an inpatient diabetes management program that is effective, efficient, and self‐perpetuating. At Brigham and Women's Hospital (BWH), we have been refining a subcutaneous insulin protocol, focused provider education, and more recently a computerized order set to overcome barriers related to fear of hypoglycemia, delays in insulin prescribing, and unfamiliarity with inpatient glucose management.15 The aims of this current trial were to evaluate the effects of these interventions on a geographically localized general medical service previously naive to these interventions to evaluate their effects on glycemic control, patient safety, and processes of care. We hypothesized that these interventions would improve glycemic control and increase use of basal‐bolus insulin orders without increasing the rate of hypoglycemia.
METHODS
Setting and Participants
This prospective, before‐after trial was conducted at BWH from July 15, 2005 through June 22, 2006. Eligible subjects were patients scheduled for admission to the BWH Physician Assistant/Clinician Educator (PACE) Service with either a known diagnosis of type 2 diabetes mellitus or inpatient hyperglycemia (at least 1 random laboratory glucose >180 mg/dL). The PACE service is a geographically‐localized general medicine service of up to 15 beds where patients are cared for by a single cadre of nurses, 2 physician's assistants (PAs), and 1 hospitalist attending. A moonlighter covers the service at night. The PACE service does not accept patients transferred from other acute care hospitals or from ICUs, but does not otherwise have triage guidelines related to diagnosis, complexity, or acuity. Patients were excluded if they had type 1 diabetes, presented with hyperosmolar hyperglycemic state (HHS) or diabetic ketoacidosis (DKA), received total parenteral nutrition (TPN), or were receiving palliative care. This study was approved by the BWH Institutional Review Board; patient consent was deemed not to be necessary for this study given the relatively nonsensitive nature of the data, noninvasive means of data collection, and the steps taken by research personnel to minimize any breach in patient confidentiality.
Intervention
The study intervention consisted of three components, initiated in January 2006:
Glycemic management protocol: a multidisciplinary team of a diabetologist (M.L.P.), a hospitalist (J.L.S.), and a pharmacist (Jennifer Trujillo) developed a subcutaneous insulin protocol based on ADA guidelines (Table 1; see the appendix for complete protocol). The protocol was approved by the BWH Pharmacy and Therapeutics Diabetes Subcommittee and refined through 6 months of pilot testing on other general medical services.15 The protocol consisted of a set of specific treatment recommendations, including: (1) bedside glucose monitoring; (2) stopping oral diabetes agents in most patients; (3) estimating total daily insulin requirements; (4) prescribing basal, nutritional, and supplemental insulin based on the patient's total insulin requirements, preadmission medication regimen, and nutritional status; (5) adjusting insulin on a daily basis as needed; (6) managing hypoglycemia; (7) suggestions for discharge orders; and (8) indications for an endocrinology consultation. The protocol was printed as a pocket guide, distributed to all members of the PACE service, and used to guide all other interventions.
Diabetes education: all PAs received 2 one‐hour educational sessions: a lecture by a diabetologist (M.L.P.) reviewing the rationale for tight glycemic control and general principles of management, and a workshop by a hospitalist (J.L.S.) in which specific cases were reviewed to illustrate how the protocol could be used in practice (eg, when oral agents could be safely continued, how to prescribe insulin on admission, and how to make subsequent adjustments in dose). All hospitalist attendings received a 1‐hour lecture summarizing the above material. All nurses on the service received a lecture that focused on issues unique to nursing care, such as insulin administration, glucose testing, managing patients with unpredictable oral (PO) intake, and patient education. (All materials are available from the authors upon request).
Order Set: an order set, built into BWH's proprietary computer provider order entry (CPOE) system, was created to parallel the glycemic management protocol and facilitate insulin orders for patients eating discrete meals, receiving continuous liquid enteral nutrition (tube feeds), or receiving nothing by mouth (NPO). Other components of the order set facilitated glucose monitoring and other laboratory tests and ordering consultation when appropriate.
| Oral Agents | Stop Oral Agents in Most Patients |
|---|---|
| |
| Glucose testing | Check bedside blood glucose before meals and at bedtime if eating, or every 6 hours if NPO |
| Insulin | |
| 1. Estimate total daily insulin dose | 0.5 to 0.7 units/kg/day, depending on patient's age, size, renal function, insulin sensitivity, history of hypoglycemia, and steroid use |
| 2. Start basal insulin | Patient's home dose or 50% of calculated total daily dose; NPH qAM/qHS or insulin glargine qHS; If NPO, use one‐half the home dose unless hyperglycemic |
| 3. Start nutritional insulin if not NPO | Patient's home dose or 50% of calculated total daily dose, less if poor or unknown intake; discrete meals: insulin aspart split over 3 meals, 0 to 15 minutes prior to eating; continuous tube feeds or IV dextrose: regular insulin every 6 hours |
| 4. Start correctional insulin | 1 of 3 scales provided based on total daily dose of insulin; same type as nutritional insulin; regular insulin if NPO |
| 5. Daily adjustment | Calculate total administered dose from prior day, adjust for degree of hyperglycemia or hypoglycemia, renal function, PO intake, steroid use, and degree of illness, and redistribute as 50% basal, 50% nutritional, or 100% basal if NPO |
| Hypoglycemia orders | Juice, IV dextrose, or IM glucagon depending on ability to take oral nutrition and IV access |
| Discharge orders | Based on A1C: either home regimen, titration of home regimen, or new insulin regimen (if latter, simple regimen with aggressive patient education and prompt follow‐up) |
| Indications for endocrine consultation | Labile blood sugars, poor control, prolonged NPO period, question of type 1 or type 2 diabetes |
Study Protocol and Data Collection
A research assistant prospectively identified eligible patients each weekday by screening all patients scheduled for admission to the PACE service using the daily computerized sign‐out system used on all general medical teams. Specifically, laboratory random glucose levels, inpatient medications, and medical histories were reviewed to determine if each patient met eligibility criteria. Eligibility criteria were then confirmed by medical record review and adjudicated by one study author (J.L.S.) if necessary. Further medical record review was performed to identify specific patient populations (eg, diet‐controlled, steroid‐induced, or previously undiagnosed diabetes), determine preadmission diabetes medications, and determine the patient's weight. Hospital computerized clinical and administrative records were abstracted to obtain patient demographics (age, sex, race, insurance status), laboratory data (glucose level on admission, A1C level [taken during or within 6 months prior to admission]), clinical data (length of stay, billing‐based Charlson comorbidity score,16 and diagnosis‐related group [DRG] case mix index), all inpatient insulin and oral diabetes medication orders, frequency of bedside glucose testing, and diet orders. Electronic medication administration record (eMAR) data were used to determine all doses and times of insulin administration.
Outcomes
The primary outcome was the mean percent of glucose readings between 60 and 180 mg/dL per patient (ie, calculated for each patient and averaged across all eligible patients in each study arm). Only bedside glucose readings were used given the lack of additional useful information typically provided by laboratory (venous plasma) glucose readings.17 Readings drawn within 1 hour of a previous reading were excluded to avoid ascertainment bias caused by follow‐up testing of abnormal glucose values. Only readings while on the study service were used. Readings on hospital day 1 were excluded because our intervention was expected to have little impact on the first day's glucose control; for patients with undiagnosed diabetes, data collection began the day following the first elevated glucose reading. Readings beyond hospital day 14 were also excluded to avoid biased data from patients with exceptionally long lengths of stay.
Secondary outcomes included the following:
Glycemic control:
Patient‐day weighted mean glucose (ie, mean glucose for each patient‐day, averaged across all patient days);
Mean glucose per patient for each hospital day (days 17).
Patient safety:
Proportion of patient‐days with any glucose reading <60 mg/dL (hypoglycemia) and <40 mg/dL (severe hypoglycemia).
Processes of care:
Use of any NPH insulin or insulin glargine (basal) insulin during the hospitalization if 2 or more glucose readings were >180 mg/dL.
Adequacy of basal dose on day first prescribed: for patients prescribed a diet, within 20% of preadmission basal dose or 0.20 to 0.42 units/kg if not known or not taken prior to admission. If not eating, half the above calculations.
Use of any scheduled nutritional insulin during the hospitalization if ever prescribed a diet and 2 or more glucose readings were greater than 180 mg/dL.
Adequacy of nutritional dose on day first prescribed: for patients prescribed a diet, within 20% of preadmission nutritional dose or 0.20 to 0.42 units/kg/day if not known or not taken prior to admission. Patients on clear liquid diets, enteral feeds, or receiving glucocorticoids were excluded from this analysis.
Correct type of nutritional insulin: if eating discrete meals, insulin aspart (the rapid‐acting insulin on formulary at BWH); if prescribed tube feeds, regular insulin.
Use of supplemental insulin by itself (without scheduled basal or nutritional insulin), a marker of poor care.
A1C testing within 1 month prior to or during hospitalization.
Clinical inertia: if at least two glucose readings <60 mg/dL or >180 mg/dL on a patient‐day, lack of any change to any insulin order the following day if still on the study service.
Healthcare utilization:
Hospital length of stay in hours, calculated from the exact time of admission until the exact time of discharge, using hospital administrative data.
Analyses
Study results were compared prior to the intervention (July 15 through December 12, 2005) with those during the intervention (January 18 through June 20, 2006). Patient data and clinical outcomes were analyzed descriptively using proportions, means with standard deviations (SDs), or medians with interquartile ranges (IQRs) as appropriate. Comparisons between groups were calculated using Fisher's exact test for dichotomous and categorical variables, and Student t test or Wilcoxon rank sum test for continuous variables as appropriate. The primary outcome was first analyzed using linear regression with study group as the independent variable and percent of glucose readings within range per patient as the dependent variable. We then adjusted for potential confounders by putting each covariate into the model, one at a time. All significant predictors of the outcome at a P value <0.10 were retained in the final model. We used general estimating equations to adjust for clustering of results by each PA. Similar analyses were performed for hospital length of stay per patient using a negative binomial model, so chosen because it fit the data distribution much better than the typically used Poisson model. With a planned sample size of 115 patients and 1250 glucose readings per arm, an intraclass correlation coefficient of 0.10, and an alpha of 0.05, the study had 90% power to detect an increase in percent of glucose readings in range from 67% to 75%. All analyses were based on the intention‐to‐treat principle. Except as above, 2‐sided P values <0.05 were considered significant. SAS version 9.1 (SAS Institute, Cary, NC) was used for all analyses.
RESULTS
We prospectively identified 248 potential patients for the study. We subsequently excluded 79 patients for the following reasons: no glucose readings beyond hospital day 1 while on PACE service (34 patients); never admitted to PACE service (15 patients); no diabetes or inpatient hyperglycemia (9 patients, mostly patients prescribed an insulin sliding scale prophylactically to avoid steroid‐induced hyperglycemia); type 1 diabetes (13 patients); TPN, DKA, or HHS (5 patients); and palliative care (3 patients). The remaining 169 patients included 63 from the preintervention period(out of 489 total admissions to the PACE service; 13%) and 106 patients in the postintervention period (out of 565 admissions; 19%). These patients had 2447 glucose readings, or an average of 3.6 glucose readings per monitored patient‐day in the preintervention period and 3.3 glucose readings per patient‐day in the postintervention period. Even including the 34 patients who were excluded for lack of glucose readings, glucose data were still available for 717 out of a potential 775 patient‐days (93%). Characteristics for all included patients are shown in Table 2. The mean admission glucose was 197 mg/dL, mean A1C was 8.4%, 54% of the patients were prescribed insulin prior to admission, and 7% had no prior diagnosis of diabetes. There were no significant differences in baseline characteristics between the 2 patient groups except for Charlson score, which was higher in the preintervention group (87% versus 74% with score 2 or higher; Table 2). The top diagnosis‐related groups for the entire cohort included: heart failure and shock (12 patients); kidney and urinary tract infections (12 patients); esophagitis, gastroenteritis, and miscellaneous digestive disorders (11 patients); chronic obstructive pulmonary disease (10 patients); renal failure (10 patients); simple pneumonia and pleurisy (7 patients); disorders of the pancreas except malignancy (6 patients); chest pain (5 patients); and cellulitis (5 patients).
| Preintervention (n = 63) | Postintervention (n = 106) | P Value | |
|---|---|---|---|
| |||
| Mean age, year (SD) | 63.0 (15.7) | 64.7 (14.3) | 0.52 |
| Male, n (%) | 25 (40) | 52 (49) | 0.27 |
| Race, n (%) | 0.33 | ||
| White | 29 (46) | 42 (40) | |
| Black | 21 (33) | 28 (26) | |
| Hispanic | 11 (17) | 30 (28) | |
| Unknown | 2 (3) | 6 (6) | |
| Admission glucose, mg/dL (SD) | 188 (90.9) | 203 (96.1) | 0.33 |
| A1C, % (SD) | 8.5 (2.4) | 8.3 (2.4) | 0.85 |
| Insulin use prior to admission, n (%) | 38 (60) | 54 (51) | 0.48 |
| Case mix index, median (IQR) | 0.89 (0.781.11) | 0.91 (0.841.22) | 0.33 |
| Charlson index, n (%) | 0.03 | ||
| 01 | 8 (13) | 28 (26) | |
| 23 | 29 (46) | 27 (26) | |
| 45 | 15 (24) | 29 (27) | |
| >5 | 11 (17) | 22 (21) | |
| Known history of diabetes, n (%) | 62 (98) | 96 (91) | 0.06 |
With respect to insulin ordering practices, there was no significant difference in the use of basal insulin in hyperglycemic patients between the preintervention period and postintervention period (81% versus 91%; P = 0.17), nor in the dose of basal insulin prescribed (results not shown), but there was an increase in the use of scheduled nutritional insulin for those patients with hyperglycemia receiving nutrition: 40% versus 75%, P < 0.001 (Table 3). The percent of patients receiving supplemental (sliding scale) insulin by itself (ie, without ever receiving basal or nutritional insulin) was lower during the postintervention period (29% versus 8%, P < 0.001). Nonsignificant differences were seen in the rates of prescribing an appropriate dose and type of nutritional insulin. Notably, there was no difference at all in the proportion of patient‐days in which insulin adjustments were made when 2 or more episodes of hyperglycemia or hypoglycemia were present during the previous day (56% of patient‐days in both groups; P = 0.90).
| Preintervention (n = 63) | Postintervention (n = 106) | Unadjusted Effect Size (95% CI) | Adjusted Effect Size (95% CI) | |
|---|---|---|---|---|
| ||||
| Mean percent glucose readings 60180 mg/dL per patient (SD) | 59.1 (0.28) | 64.7 (0.27) | +5.6 (3.0 to +14.3) | +9.7 (+0.6 to +18.8)*, |
| Patient‐day weighted mean glucose, mg/dL (SD) | 174.7 (60.0) | 164.6 (54.2) | 10.1 (1.6 to 18.5) | 15.6 (6.4 to 24.9), |
| Percent patient‐days with any glucose <60 mg/dL | 16/293 (5.5%) | 26/424 (6.1%) | 1.1 (0.6 to 2.1) | 1.1 (0.6 to 2.1) |
| Percent patient‐days with any glucose <40 mg/dL | 3/293 (1.0%) | 5/424 (1.2%) | 1.3 (0.3 to 5.9) | 1.1 (0.3 to 5.1) |
| Hospital length of stay, hours, mean (SD) | 112.2 (63.3) | 86.0 (89.6) | 30% (5% to 51%) | 25% (6% to 44%),∥ |
| Basal insulin if inpatient hyperglycemia (2 or more readings >180 mg/dL) | 39/48 (81%) | 67/74 (91%) | 2.2 (0.8 to 6.4) | |
| Nutritional insulin if inpatient hyperglycemia and PO intake | 19/48 (40%) | 53/71 (75%) | 4.5 (2.0 to 9.9), | |
| Adequate initial dose of nutritional insulin (home dose or 0.200.42 units/kg/day)# | 2/9 (22%) | 22/49 (45%) | 2.9 (0.5 to 15.1) | |
| Supplemental insulin alone (without basal or nutritional insulin) | 16/56 (29%) | 7/92 (8%) | 0.2 (0.08 to 0.5), | |
| Insulin changed if previous day's glucose out of range (2 or more values <60 or >180 mg/dL) | 70/126 (56%) | 76/135 (56%) | 1.0 (0.6 to 1.6) | |
| A1C tested during hospitalization if not available within 30 days prior | 38/63 (60%) | 74/106 (70%) | 1.5 (0.8 to 2.9) | |
The primary outcome, the mean percent of glucose readings between 60 and 180 mg/dL per patient, was 59.1% in the preintervention period and 64.7% in the postintervention (P = 0.13 in unadjusted analysis; Table 3). When adjusted for A1C, admission glucose, and insulin use prior to admission, the adjusted absolute difference in the percent of glucose readings within range was 9.7% (95% confidence interval [CI], 0.6%‐18.8%; P = 0.04; Table 3). Regarding other measures of glucose control, the patient‐day weighted mean glucose was 174.7 mg/dL in the preintervention period and 164.6 mg/dL postintervention (P = 0.02), and there was no significant difference in the percent of patient‐days with any hypoglycemia (glucose <60 mg/dL) or severe hypoglycemia (glucose <40 mg/dL; Table 3). There were also no significant differences in the mean number of hypoglycemic events per patient‐day (6.8 versus 6.6 per 100 patient‐days; relative risk, 0.95; 95% CI, 0.541.67; P = 0.87) or severe hypoglycemic events per patient‐day (1.0 versus 1.4 per 100 patient‐days; relative risk, 1.38; 95% CI, 0.355.53; P = 0.65).
We also compared hospital length of stay in hours between the study groups (Table 3). Length of stay (LOS) was shorter in the postintervention arm in unadjusted analyses (112 versus 86 hours; P < 0.001), and this difference persisted when adjusted for patient insurance, race, gender, and Charlson comorbidity score (25% shorter; 95% CI, 6%‐44%). A comparison of LOS among nonstudy patients on the PACE service during these 2 time periods revealed no difference (105 versus 101 hours). When the length of stay analysis was limited to study patients with a known diagnosis of diabetes, the adjusted effect size was a 31% relative decrease in length of stay.
Figure 1A shows the percent glucose readings within range per patient by hospital day. The greatest differences between groups can be seen on hospital days 2 and 3 (11% absolute differences on both days). Similarly, Figure 1B shows the mean glucose per patient by hospital day. Again, the biggest differences are seen on hospital days 2 and 3 (20 and 23 mg/dL difference between groups, respectively). In both cases, only the day 3 comparisons were significantly different between study groups.
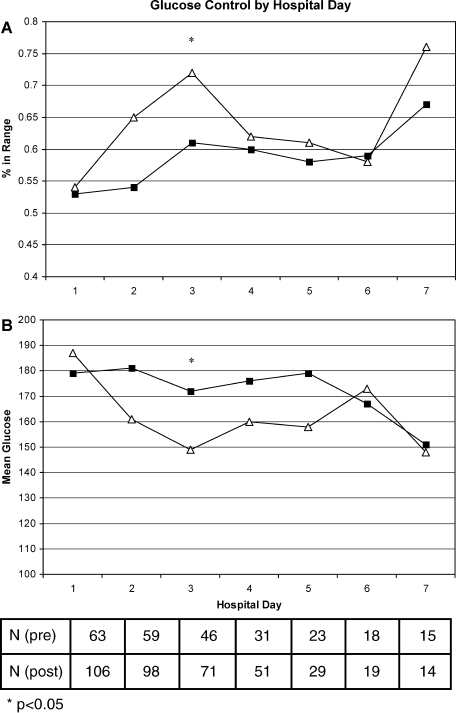
DISCUSSION
In this before‐after study, we found that a multifaceted intervention consisting of a subcutaneous insulin protocol, focused education, and an order set built into the hospital's CPOE system was associated with a significantly higher percentage of glucose readings within range per patient in analyses adjusted for patient demographics and severity of diabetes. We also found a significant decrease in patient‐day weighted mean glucose, a marked increase in appropriate use of scheduled nutritional insulin, and a concomitant decrease in sliding scale insulin only regimens during the postintervention period. Moreover, we found a shorter length of stay during the postintervention period that persisted after adjustment for several clinical factors. Importantly, the interventions described in this study require very few resources to continue indefinitely: printing costs for the management protocol, 4 hours of education delivered per year, and routine upkeep of an electronic order set.
Because this was a before‐after study, we cannot exclude the possibility that these improvements in process and outcome were due to cointerventions and/or temporal trends. However, we know of no other interventions aimed at improving diabetes care in this self‐contained service of nurses, PAs, and hospitalists. Moreover, the process improvements, especially the increase in scheduled nutritional insulin, were rather marked, unlikely to be due to temporal trends alone, and likely capable of producing the corresponding improvements in glucose control. That glucose control stopped improving after hospital day 3 may be due to the fact that subsequent adjustment to insulin orders occurred infrequently and no more often than prior to the intervention. That we did not see greater improvements in glycemic control overall may also reflect the fact that 81% of study patients with inpatient hyperglycemia received basal insulin prior to the intervention.
The reduction in patient LOS was somewhat surprising given the relatively small sample size. However, the results are consistent with those of other studies linking hyperglycemia to LOS18, 19 and we found no evidence for a temporal trend toward lower LOS on the PACE service as a whole during the same time period. While a greater proportion of patients on the PACE service were in the study in the post‐intervention period compared with the preintervention period, we found no evidence that the difference in length of stay was due to increased surveillance for nondiabetics, especially because eligibility criteria depended on phlebotomy glucose values, which were uniformly tested in all inpatients. Also, effects on length of stay were actually stronger when limited to patients with known diabetes. Finally, we controlled for several predictors of length of stay, although we still cannot exclude the possibility of unmeasured confounding between groups.
Since ADA and ACE issued guidelines for inpatient management of diabetes and hyperglycemia, many institutions have developed subcutaneous insulin algorithms, educational curricula, and/or order sets to increase compliance with these guidelines and improve glycemic control. Some of these efforts have been studied and some have been successful in their efforts.13, 14, 2023 Unfortunately, most of these programs have not rigorously assessed their impact on process and outcomes, and the most effective studies published to date have involved interventions much more intensive than those described here. For example, Rush University's intervention was associated with a 50 mg/dL decrease in mean blood glucose but involved an endocrinologist rounding twice daily with house officers for 2 weeks at a time.13 At Northwestern University, a diabetes management service run by nurse practitioners was established, and the focus was on the conversion from intravenous to subcutaneous insulin regimens.14 The RABBIT 2 study that demonstrated the benefits of a basal‐bolus insulin regimen used daily rounding with an endocrinologist.12 More modestly, a program in Pitt County Memorial Hospital in Greenville, NC, relied mostly on diabetes nurse case managers, a strategy which reduced hospital‐wide mean glucose levels as well as LOS, although the greatest improvements in glycemic control were seen in the ICU.19 Our findings are much more consistent with those from University of California San Diego, as yet unpublished, which also used an algorithm, computerized order set, education, as well as continuous quality improvement methods to achieve its aims.22
Our study has several limitations, including being conducted on 1 general medicine service at 1 academic medical center. Moreover, this service, using a physician assistant/hospitalist model, a closed geographic unit, and fairly generous staffing ratio, is likely different from those in many settings and may limit the generalizability of our findings. However, this model allowed us to conduct the study in a laboratory relatively untouched by other cointerventions. Furthermore, the use of PAs in this way may become more common as both academic and community hospitals rely more on mid‐level providers. Our study had a relatively low percentage of patients without a known diagnosis of diabetes compared with other studies, again potentially but not necessarily limiting generalizability. This finding has been shown in other studies at our institution24 and may be due to the high rate of screening for diabetes in the community. Another limitation is that this was a nonrandomized, before‐after trial. However, all subjects were prospectively enrolled to improve comparability, and we performed rigorous adjustment for multiple potential confounding factors. Also, this study had limited statistical power to detect differences in hypoglycemia rates. The preintervention arm was smaller than planned due to fewer diabetic patients than expected on the service and a higher number of exclusions; we prolonged the postintervention period to achieve the desired sample size for that arm of the study.
Our study also has several strengths, including electronic capture of many processes of care and a methodology to operationalize them into measures of protocol adherence. Our metrics of glycemic control were rigorously designed and based on a national task force on inpatient glycemic control sponsored by the Society of Hospital Medicine, with representation from the ADA and AACE.25
Potential future improvements to this intervention include modifications to the daily adjustment algorithm to improve its usability and ability to improve glucose control. Another is the use of high‐reliability methods to improve order set use and daily insulin adjustment, including alerts within the CPOE system and nurse empowerment to contact medical teams if glucose levels are out of range (eg, if greater than 180 mg/dL, not just if greater than 350 or 400 mg/dL). Future research directions include multicenter, randomized controlled trials of these types of interventions and an analysis of more distal patient outcomes including total healthcare utilization, infection rates, end‐organ damage, and mortality.
In conclusion, we found a relationship between a relatively low‐cost quality improvement intervention and improved glycemic control in the non‐ICU general medical setting. Such a finding suggests the benefits of the algorithm itself to improve glucose control and of our implementation strategy. Other institutions may find this intervention a useful starting point for their own quality improvement efforts. Both the algorithm and implementation strategy are deserving of further improvements and future study.
Acknowledgements
We thank Paul Szumita, Karen Fiumara, Jennifer Trujillo, and the other members of the BWH Diabetes Pharmacy and Therapeutics Subcommittee for their help designing and implementing the intervention; Aubre McClendon, Nicole Auclair, Emily Dattwyler, Mariya Fiman, and Alison Pietras for valuable research assistance; Deborah Williams for data analysis; Amy Bloom for project support; and Stuart Lipsitz for biostatistical expertise.
APPENDIX
INPATIENT DIABETES MANAGEMENT PROTOCOL
Management of Diabetes and Hyperglycemia in Hospitalized Non‐ICU Patients
Rationale
Increasing data show a strong association between hyperglycemia and adverse inpatient outcomes. The American Diabetes Association and the American College of Clinical Endocrinology recommend all glucose levels be below 180 mg/dL in non‐ICU patients. Because hospitalizations are unstable situations, even patients who are well controlled on oral agents as outpatients are usually best managed with insulin.
Insulin may be safely administered even to patients without previously diagnosed diabetes. As long as the prescribed doses are below what is normally produced by the pancreas, the patient will not become hypoglycemic. If the glucose level drops, endogenous insulin secretion will reduce to compensate.
Total insulin requirements in insulin‐sensitive patients (eg, type 1 diabetes mellitus) is 0.50.7/units/kg/day. Insulin requirements in insulin‐resistant type 2 diabetic patients may vary greatly, and can exceed 12 units/kg/day. A conservative estimate for initial insulin therapy in any patient with diabetes is to start with the type 1 diabetes mellitus dose, 0.50.7 units/kg/day.
Overview
Effective inpatient insulin regimens typically include 3 components:
Basal insulin (eg, scheduled NPH or insulin glargine [Lantus]), which is used to manage fasting and premeal hyperglycemia.
Nutritional or prandial insulin (eg, scheduled regular insulin, insulin lispro [Humalog] or insulin aspart [Novolog]) which controls hyperglycemia from nutritional (eg, discrete meals, TPN, IV dextrose) sources.
Supplemental or correctional insulin (eg, regular insulin, insulin lispro, or insulin aspart), which is used in addition to scheduled insulin to meet unexpected basal hyperglycemia that is not covered by the scheduled insulin.
Sample Orders (Not for Patients with Uncontrolled Type 1 Diabetes, DKA, Hyperglycemic Hyperosmolar State, or Other Absolute Need for IV Insulin)
Check (fingerstick) capillary blood glucose qAC, qHS.
NPH insulin subcutaneously (SC) ___ units qAM, ___ units qHS.
Insulin aspart SC ___ units pre‐breakfast, ___ units pre‐lunch, ___ units pre‐dinner, hold if NPO or premeal BS <60 mg/dL; give 015 minutes before meals.
Insulin aspart SC sliding scale (see Table 6) qAC, in addition to standing nutritional insulin, 015 minutes before meals.
For BS <60 mg/dL:
If patient can take PO
Give 15 g of fast acting carbohydrate (4 oz fruit juice/nondiet soda, 8 oz nonfat milk, or 34 glucose tablets).
Repeat finger capillary glucose every 15 (q15) minutes and repeat above (5.a.i.) if BG <60 mg/dL.
When BG >60 mg/dL, give snack or meal in a half‐hour.
If patient cannot take PO
Give 25 mL of 50% dextrose (D50) as an IV push.;
Repeat finger capillary glucose q15 minutes and repeat above (5.b.i.) if BG <80 mg/dL.
Guidelines
Stop oral diabetes agents in most patients (see Table 7 for list of contraindications and precautions).
Check bedside blood glucose (BBG or fingerstick) qAC and qHS (or at 0600 hours, 1200 hours, 1800 hours, and 2400 hours if no discrete meals).
Estimate total daily insulin requirement:
For most patients, conservative estimate is 0.50.7 units/kg/day, but may be much higher.
Reasons for lower end of the range: renal insufficiency, small size, insulin sensitive (eg, type 1), recent hypoglycemia, decreasing doses of steroids, older age.
Reasons for higher end of the range: obese, initiation or increasing doses of steroids, marked hyperglycemia.
Start basal insulin if any premeal BG >140 mg/dL and no recent glucose <60 mg/dL off insulin (Table 5).
Start nutritional or prandial insulinhold if nutrition is stopped/held or premeal BS <60 (Table 5).
Start supplemental/correctional insulin in addition to nutritional (prandial) insulin (Table 6):
Discrete meals: Insulin aspart qAC (with nutritional insulin). 0
No discrete meals: Regular insulin q6h.
On a daily basis, adjust scheduled insulin based on previous days' blood sugars:
Add up total insulin given the previous day, including scheduled and supplemental insulin, to determine new total daily insulin requirement.
Adjust total daily insulin requirement based on clinical considerations (eg, give more if marked hyperglycemia, eating more, improving renal function, increasing steroids; give less if eating less, worsening renal function, tapering steroids, recovering from severe illness).
Give 50% of requirement as basal and 50% as nutritional, as above (may need proportionately less nutritional insulin if appetite poor or unknown).
Adjust sliding scale if needed based on total scheduled insulin dose (see step 6, above).
For BG <60 mg/dL:
If patient can take PO, give 15 g of fast acting carbohydrate.
(4 oz fruit juice/nondiet soda, 8 oz nonfat milk, or 34 glucose tablets; not juice plus sugar).
Repeat finger capillary glucose q15 minutes and repeat above if BG <60.
When BG >60, give snack or meal in half an hour.
If patient cannot take PO, give 25 mL of D50 as IV push.
Check finger capillary glucose q15 minutes and repeat above if BG <80.
Discharge orders:
Patient should be discharged home on a medication regimen that was similar to the admission regimen (ie, the regimen prescribed by their PCP). Exceptions include
The patient has a contraindication to an admission medication.
There is evidence of severe hyperglycemia (eg, very high A1C) or hypoglycemia on admission regimen.
If a patient is admitted with no insulin, and requires insulin to be continued as an outpatient (eg, newly‐diagnosed type 1 diabetes, A1C very high, and contraindication to or on maximum oral regimen), limit discharge insulin regimen to no more than 1 injection per day (eg, hs NPH; an exception to this is for type 1 diabetic patients, who are optimally treated with 34 injections/day). Make sure the patient has prompt follow‐up with their primary care provider (PCP).
Avoid discharging home on sliding scale.
If a patient is going to require insulin injections and self‐monitoring blood glucose as an outpatient, make sure they are instructed about how to perform these.
Indications for calling an endocrine consult:
Labile blood sugars.
Prolonged periods of NPO, eg, for procedures, especially in patients with type 1 diabetes
Marked hyperglycemia despite following this guideline.
Question of type 1 versus type 2 versus other type of diabetes. 0
Basil Insulin Guidelines Home Insulin Regimen Starting Dose of Basal Insulin Considerations NOTE: Patients with T1DM require basal insulin at all times! Basal never should be held!
Abbreviations: NPO, nothing by mouth.
On basal (eg, NPH or glargine) insulin at home Patient's home dose of NPH or glargine If NPO, consider starting half of NPH or glargine home dose, unless hyperglycemic at home. Not on basal (eg, NPH or glargine) insulin at home NPH 50% of total daily insulin requirement, given qHS or split qAM/qHS (maximum starting dose 20 units/day) Same dose if patient has previously diagnosed or undiagnosed diabetes Nutritional Insulin Guidelines Type of Nutrition Common Nutritional Regimens Sample Starting Doses Abbreviation: qAM, every morning; qHS, at bed time.
If receiving cycled tube feeds at night, give nutritional NPH qHS only.
Discrete meals Aspart given 015 minutes before meals Home dose, if known or 50% of total insulin requirement, split over 3 meals, may need less if poor or unknown appetite Continuous tube feeding,* IV dextrose NPH qHS or qAM/qHS 50% of total insulin requirement (in addition to basal dose), may need less if not at goal caloric intake Glargine given every day (qd), anytime Regular every 6 hours (q6h) Sample Supplemental/Correctional Insulin Scales Blood Glucose Scheduled Insulin < 40 Units/Day Scheduled Insulin of 4080 Units/Day Scheduled Insulin > 80 Units/Day Individualized NOTE: Avoid supplemental insulin qHS unless patient is very hyperglycemic and obese.
150199 1 unit 1 unit 2 units ____ units 200249 2 units 3 units 4 units ____ units 250299 3 units 5 units 7 units ____ units 300349 4 units 7 units 10 units ____ units >349 5 units + call HO 8 units + call HO 12 units + call HO ___ units + call HO Notes on Oral Agents Agents Considerations Metabolism Sulfonylureas/secretagogues: glyburide, glipizide, glimeperide (Amaryl); repaglinide (Prandin); nateglinide (Starlix) Risk for hypoglycemia Metabolized in liver; Glyburide metabolized to active metabolites; 50% renally eliminated Metformin Contraindicated in heart failure and renal dysfunction (creatinine [Cr] >1.5 mg/dL in men and 1.4 mg/dL in women) Eliminated renally Should be held at time of iodinated contrast studies. (May be restarted after normal postcontrast renal function is confirmed) Adverse effects include diarrhea, nausea, and anorexia Thiazolidinediones: pioglitazone (Actos), rosiglitazone (Avandia) Contraindicated in class III and IV heart failure Metabolized in liver Use with caution in patients with edema Adverse effects include increased intravascular volume Slow onset of action Avoid in hepatic dysfunction Glucosidease inhibitors: acarbose (Precose), miglitol (Glycet) Gastrointestinal intolerance Acarbose eliminated in gut and renally
- ,,,,.Prevalence of hyper‐ and hypoglycemia among inpatients with diabetes: a national survey of 44 U.S. hospitals.Diabetes Care.2007;30:367–369.
- ,,,,,.Hyperglycemia: an independent marker of in‐hospital mortality in patients with undiagnosed diabetes.J Clin Endocrinol Metab.2002;87:978–982.
- ,,, et al.Hyperglycaemia is associated with poor outcomes in patients admitted to hospital with acute exacerbations of chronic obstructive pulmonary disease.Thorax.2006;61:284–289.
- ,,,,.Stress hyperglycemia and prognosis of stroke in nondiabetic and diabetic patients: a systematic overview.Stroke.2001;32:2426–2432.
- ,,,.Hyperglycemia is associated with adverse outcomes in patients receiving total parenteral nutrition.Diabetes Care.2005;28:2367–2371.
- ,,, et al.Management of diabetes and hyperglycemia in hospitals.Diabetes Care.2004;27:553–597.
- ,,,,,.The relation between hyperglycemia and outcomes in 2471 patients admitted to the hospital with community‐acquired pneumonia.Diabetes Care.2005;28:810–815.
- ,,, et al.Intensive insulin therapy in the medical ICU.N Engl J Med.2006;354:449–461.
- ,,, et al.Intensive insulin therapy in the critically ill patients.N Engl J Med.2001;345:1359–1367.
- Standards of medical care in diabetes, 2007.Diabetes Care.2007;30(Suppl 1):S4–S41.
- American College of Endocrinology and American Diabetes Association Consensus statement on inpatient diabetes and glycemic control: a call to action.Diabetes Care.2006;29:1955–1962.
- ,,, et al.Randomized study of basal‐bolus insulin therapy in the inpatient management of patients with type 2 diabetes (RABBIT 2 trial).Diabetes Care.2007;30:2181–2186.
- ,,,.Eliminating inpatient sliding‐scale insulin: a reeducation project with medical house staff.Diabetes Care.2005;28:1008–1011.
- ,,, et al.Inpatient management of hyperglycemia: the Northwestern experience.Endocr Pract.2006;12:491–505.
- ,,, et al.Improving glycemic control in medical inpatients: a pilot study.J Hosp Med.2008;3:55–63.
- ,,.Adapting a clinical comorbidity index for use with ICD‐9‐CM administrative databases.J Clin Epidemiol.1992;45:613–619.
- ,,, et al.“Glucometrics”—assessing the quality of inpatient glucose management.Diabetes Technol Ther.2006;8:560–569.
- ,,,.Hyperglycemia and length of stay in patients hospitalized for bone marrow transplantation.Diabetes Care.2007;30:993–994.
- ,.Financial implications of glycemic control: results of an inpatient diabetes management program.Endocr Pract.2006;12(Suppl 3):43–48.
- ,,, et al.In‐hospital treatment of hyperglycemia: effects of intensified subcutaneous insulin treatment.Curr Med Res Opin.2007;23:757–765.
- ,,,.Impact of endocrine and diabetes team consultation on hospital length of stay for patients with diabetes.Am J Med.1995;99:22–28.
- ,,,.Effect of a standardized insulin order set and an insulin management algorithm on inpatient glycemic control and hypoglycemia. Society of Hospital Medicine Annual Meeting, 2007; Dallas, TX;2007.
- ,,, et al.Trends in bed occupancy for inpatients with diabetes before and after the introduction of a diabetes inpatient specialist nurse service.Diabet Med.2006;23:1008–1015.
- ,,,,.Inpatient management of diabetes and hyperglycemia among general medicine patients at a large teaching hospital.J Hosp Med.2006;1:145–150.
- ,,, et al. Improving glycemic control, preventing hypoglycemia, and optimizing care of the inpatient with hyperglycemia and diabetes, 2006. Available at:http://www.hospitalmedicine.org/ResourceRoomRedesign/html/GC_Imp_Guide.cfm. Accessed October 2008.
Diabetes mellitus and/or inpatient hyperglycemia are common comorbid conditions in hospitalized patients. Recent surveys show that over 90% of hospitalized diabetic patients experience hyperglycemia (>200 mg/dL), and in nearly 1 in 5 of these patients hyperglycemia persists for 3 days or more.1 Hyperglycemia among inpatients without a previous history of diabetes mellitus is also very common.2 Observational studies have shown that hyperglycemia in hospitalized patients is associated with adverse outcomes including infectious complications, increased length of stay, and increased mortality.27 Recent randomized controlled trials have demonstrated that aggressive treatment of inpatient hyperglycemia improves outcomes in surgical and medical intensive care units.8, 9
Based on the available data, the American Diabetes Association (ADA) now advocates good metabolic control, defined as preprandial glucose levels of 90 to 130 mg/dL and peak postprandial glucose levels <180 mg/dL in hospitalized nonintensive care unit (ICU) patients.10 To reach these targets, the ADA and American College of Endocrinology (ACE) suggest that multidisciplinary teams develop and implement hyperglycemia management guidelines and protocols.11 Protocols should promote the use of continuous intravenous insulin infusions or scheduled basal‐bolus subcutaneous insulin regimens. Subcutaneous insulin protocols should include target glucose levels, basal, nutritional, and supplemental insulin, and daily dose adjustments.6 A recent randomized controlled trial of non‐ICU inpatients demonstrated that such a basal‐bolus insulin regimen results in improved glucose control compared with a sliding scale only regimen.12
To date, few published studies have investigated the best ways to implement such management protocols; those that have are often resource‐intensive, for example involving daily involvement of nurse practitioners or diabetologists.13, 14 It is therefore not known how best to implement an inpatient diabetes management program that is effective, efficient, and self‐perpetuating. At Brigham and Women's Hospital (BWH), we have been refining a subcutaneous insulin protocol, focused provider education, and more recently a computerized order set to overcome barriers related to fear of hypoglycemia, delays in insulin prescribing, and unfamiliarity with inpatient glucose management.15 The aims of this current trial were to evaluate the effects of these interventions on a geographically localized general medical service previously naive to these interventions to evaluate their effects on glycemic control, patient safety, and processes of care. We hypothesized that these interventions would improve glycemic control and increase use of basal‐bolus insulin orders without increasing the rate of hypoglycemia.
METHODS
Setting and Participants
This prospective, before‐after trial was conducted at BWH from July 15, 2005 through June 22, 2006. Eligible subjects were patients scheduled for admission to the BWH Physician Assistant/Clinician Educator (PACE) Service with either a known diagnosis of type 2 diabetes mellitus or inpatient hyperglycemia (at least 1 random laboratory glucose >180 mg/dL). The PACE service is a geographically‐localized general medicine service of up to 15 beds where patients are cared for by a single cadre of nurses, 2 physician's assistants (PAs), and 1 hospitalist attending. A moonlighter covers the service at night. The PACE service does not accept patients transferred from other acute care hospitals or from ICUs, but does not otherwise have triage guidelines related to diagnosis, complexity, or acuity. Patients were excluded if they had type 1 diabetes, presented with hyperosmolar hyperglycemic state (HHS) or diabetic ketoacidosis (DKA), received total parenteral nutrition (TPN), or were receiving palliative care. This study was approved by the BWH Institutional Review Board; patient consent was deemed not to be necessary for this study given the relatively nonsensitive nature of the data, noninvasive means of data collection, and the steps taken by research personnel to minimize any breach in patient confidentiality.
Intervention
The study intervention consisted of three components, initiated in January 2006:
Glycemic management protocol: a multidisciplinary team of a diabetologist (M.L.P.), a hospitalist (J.L.S.), and a pharmacist (Jennifer Trujillo) developed a subcutaneous insulin protocol based on ADA guidelines (Table 1; see the appendix for complete protocol). The protocol was approved by the BWH Pharmacy and Therapeutics Diabetes Subcommittee and refined through 6 months of pilot testing on other general medical services.15 The protocol consisted of a set of specific treatment recommendations, including: (1) bedside glucose monitoring; (2) stopping oral diabetes agents in most patients; (3) estimating total daily insulin requirements; (4) prescribing basal, nutritional, and supplemental insulin based on the patient's total insulin requirements, preadmission medication regimen, and nutritional status; (5) adjusting insulin on a daily basis as needed; (6) managing hypoglycemia; (7) suggestions for discharge orders; and (8) indications for an endocrinology consultation. The protocol was printed as a pocket guide, distributed to all members of the PACE service, and used to guide all other interventions.
Diabetes education: all PAs received 2 one‐hour educational sessions: a lecture by a diabetologist (M.L.P.) reviewing the rationale for tight glycemic control and general principles of management, and a workshop by a hospitalist (J.L.S.) in which specific cases were reviewed to illustrate how the protocol could be used in practice (eg, when oral agents could be safely continued, how to prescribe insulin on admission, and how to make subsequent adjustments in dose). All hospitalist attendings received a 1‐hour lecture summarizing the above material. All nurses on the service received a lecture that focused on issues unique to nursing care, such as insulin administration, glucose testing, managing patients with unpredictable oral (PO) intake, and patient education. (All materials are available from the authors upon request).
Order Set: an order set, built into BWH's proprietary computer provider order entry (CPOE) system, was created to parallel the glycemic management protocol and facilitate insulin orders for patients eating discrete meals, receiving continuous liquid enteral nutrition (tube feeds), or receiving nothing by mouth (NPO). Other components of the order set facilitated glucose monitoring and other laboratory tests and ordering consultation when appropriate.
| Oral Agents | Stop Oral Agents in Most Patients |
|---|---|
| |
| Glucose testing | Check bedside blood glucose before meals and at bedtime if eating, or every 6 hours if NPO |
| Insulin | |
| 1. Estimate total daily insulin dose | 0.5 to 0.7 units/kg/day, depending on patient's age, size, renal function, insulin sensitivity, history of hypoglycemia, and steroid use |
| 2. Start basal insulin | Patient's home dose or 50% of calculated total daily dose; NPH qAM/qHS or insulin glargine qHS; If NPO, use one‐half the home dose unless hyperglycemic |
| 3. Start nutritional insulin if not NPO | Patient's home dose or 50% of calculated total daily dose, less if poor or unknown intake; discrete meals: insulin aspart split over 3 meals, 0 to 15 minutes prior to eating; continuous tube feeds or IV dextrose: regular insulin every 6 hours |
| 4. Start correctional insulin | 1 of 3 scales provided based on total daily dose of insulin; same type as nutritional insulin; regular insulin if NPO |
| 5. Daily adjustment | Calculate total administered dose from prior day, adjust for degree of hyperglycemia or hypoglycemia, renal function, PO intake, steroid use, and degree of illness, and redistribute as 50% basal, 50% nutritional, or 100% basal if NPO |
| Hypoglycemia orders | Juice, IV dextrose, or IM glucagon depending on ability to take oral nutrition and IV access |
| Discharge orders | Based on A1C: either home regimen, titration of home regimen, or new insulin regimen (if latter, simple regimen with aggressive patient education and prompt follow‐up) |
| Indications for endocrine consultation | Labile blood sugars, poor control, prolonged NPO period, question of type 1 or type 2 diabetes |
Study Protocol and Data Collection
A research assistant prospectively identified eligible patients each weekday by screening all patients scheduled for admission to the PACE service using the daily computerized sign‐out system used on all general medical teams. Specifically, laboratory random glucose levels, inpatient medications, and medical histories were reviewed to determine if each patient met eligibility criteria. Eligibility criteria were then confirmed by medical record review and adjudicated by one study author (J.L.S.) if necessary. Further medical record review was performed to identify specific patient populations (eg, diet‐controlled, steroid‐induced, or previously undiagnosed diabetes), determine preadmission diabetes medications, and determine the patient's weight. Hospital computerized clinical and administrative records were abstracted to obtain patient demographics (age, sex, race, insurance status), laboratory data (glucose level on admission, A1C level [taken during or within 6 months prior to admission]), clinical data (length of stay, billing‐based Charlson comorbidity score,16 and diagnosis‐related group [DRG] case mix index), all inpatient insulin and oral diabetes medication orders, frequency of bedside glucose testing, and diet orders. Electronic medication administration record (eMAR) data were used to determine all doses and times of insulin administration.
Outcomes
The primary outcome was the mean percent of glucose readings between 60 and 180 mg/dL per patient (ie, calculated for each patient and averaged across all eligible patients in each study arm). Only bedside glucose readings were used given the lack of additional useful information typically provided by laboratory (venous plasma) glucose readings.17 Readings drawn within 1 hour of a previous reading were excluded to avoid ascertainment bias caused by follow‐up testing of abnormal glucose values. Only readings while on the study service were used. Readings on hospital day 1 were excluded because our intervention was expected to have little impact on the first day's glucose control; for patients with undiagnosed diabetes, data collection began the day following the first elevated glucose reading. Readings beyond hospital day 14 were also excluded to avoid biased data from patients with exceptionally long lengths of stay.
Secondary outcomes included the following:
Glycemic control:
Patient‐day weighted mean glucose (ie, mean glucose for each patient‐day, averaged across all patient days);
Mean glucose per patient for each hospital day (days 17).
Patient safety:
Proportion of patient‐days with any glucose reading <60 mg/dL (hypoglycemia) and <40 mg/dL (severe hypoglycemia).
Processes of care:
Use of any NPH insulin or insulin glargine (basal) insulin during the hospitalization if 2 or more glucose readings were >180 mg/dL.
Adequacy of basal dose on day first prescribed: for patients prescribed a diet, within 20% of preadmission basal dose or 0.20 to 0.42 units/kg if not known or not taken prior to admission. If not eating, half the above calculations.
Use of any scheduled nutritional insulin during the hospitalization if ever prescribed a diet and 2 or more glucose readings were greater than 180 mg/dL.
Adequacy of nutritional dose on day first prescribed: for patients prescribed a diet, within 20% of preadmission nutritional dose or 0.20 to 0.42 units/kg/day if not known or not taken prior to admission. Patients on clear liquid diets, enteral feeds, or receiving glucocorticoids were excluded from this analysis.
Correct type of nutritional insulin: if eating discrete meals, insulin aspart (the rapid‐acting insulin on formulary at BWH); if prescribed tube feeds, regular insulin.
Use of supplemental insulin by itself (without scheduled basal or nutritional insulin), a marker of poor care.
A1C testing within 1 month prior to or during hospitalization.
Clinical inertia: if at least two glucose readings <60 mg/dL or >180 mg/dL on a patient‐day, lack of any change to any insulin order the following day if still on the study service.
Healthcare utilization:
Hospital length of stay in hours, calculated from the exact time of admission until the exact time of discharge, using hospital administrative data.
Analyses
Study results were compared prior to the intervention (July 15 through December 12, 2005) with those during the intervention (January 18 through June 20, 2006). Patient data and clinical outcomes were analyzed descriptively using proportions, means with standard deviations (SDs), or medians with interquartile ranges (IQRs) as appropriate. Comparisons between groups were calculated using Fisher's exact test for dichotomous and categorical variables, and Student t test or Wilcoxon rank sum test for continuous variables as appropriate. The primary outcome was first analyzed using linear regression with study group as the independent variable and percent of glucose readings within range per patient as the dependent variable. We then adjusted for potential confounders by putting each covariate into the model, one at a time. All significant predictors of the outcome at a P value <0.10 were retained in the final model. We used general estimating equations to adjust for clustering of results by each PA. Similar analyses were performed for hospital length of stay per patient using a negative binomial model, so chosen because it fit the data distribution much better than the typically used Poisson model. With a planned sample size of 115 patients and 1250 glucose readings per arm, an intraclass correlation coefficient of 0.10, and an alpha of 0.05, the study had 90% power to detect an increase in percent of glucose readings in range from 67% to 75%. All analyses were based on the intention‐to‐treat principle. Except as above, 2‐sided P values <0.05 were considered significant. SAS version 9.1 (SAS Institute, Cary, NC) was used for all analyses.
RESULTS
We prospectively identified 248 potential patients for the study. We subsequently excluded 79 patients for the following reasons: no glucose readings beyond hospital day 1 while on PACE service (34 patients); never admitted to PACE service (15 patients); no diabetes or inpatient hyperglycemia (9 patients, mostly patients prescribed an insulin sliding scale prophylactically to avoid steroid‐induced hyperglycemia); type 1 diabetes (13 patients); TPN, DKA, or HHS (5 patients); and palliative care (3 patients). The remaining 169 patients included 63 from the preintervention period(out of 489 total admissions to the PACE service; 13%) and 106 patients in the postintervention period (out of 565 admissions; 19%). These patients had 2447 glucose readings, or an average of 3.6 glucose readings per monitored patient‐day in the preintervention period and 3.3 glucose readings per patient‐day in the postintervention period. Even including the 34 patients who were excluded for lack of glucose readings, glucose data were still available for 717 out of a potential 775 patient‐days (93%). Characteristics for all included patients are shown in Table 2. The mean admission glucose was 197 mg/dL, mean A1C was 8.4%, 54% of the patients were prescribed insulin prior to admission, and 7% had no prior diagnosis of diabetes. There were no significant differences in baseline characteristics between the 2 patient groups except for Charlson score, which was higher in the preintervention group (87% versus 74% with score 2 or higher; Table 2). The top diagnosis‐related groups for the entire cohort included: heart failure and shock (12 patients); kidney and urinary tract infections (12 patients); esophagitis, gastroenteritis, and miscellaneous digestive disorders (11 patients); chronic obstructive pulmonary disease (10 patients); renal failure (10 patients); simple pneumonia and pleurisy (7 patients); disorders of the pancreas except malignancy (6 patients); chest pain (5 patients); and cellulitis (5 patients).
| Preintervention (n = 63) | Postintervention (n = 106) | P Value | |
|---|---|---|---|
| |||
| Mean age, year (SD) | 63.0 (15.7) | 64.7 (14.3) | 0.52 |
| Male, n (%) | 25 (40) | 52 (49) | 0.27 |
| Race, n (%) | 0.33 | ||
| White | 29 (46) | 42 (40) | |
| Black | 21 (33) | 28 (26) | |
| Hispanic | 11 (17) | 30 (28) | |
| Unknown | 2 (3) | 6 (6) | |
| Admission glucose, mg/dL (SD) | 188 (90.9) | 203 (96.1) | 0.33 |
| A1C, % (SD) | 8.5 (2.4) | 8.3 (2.4) | 0.85 |
| Insulin use prior to admission, n (%) | 38 (60) | 54 (51) | 0.48 |
| Case mix index, median (IQR) | 0.89 (0.781.11) | 0.91 (0.841.22) | 0.33 |
| Charlson index, n (%) | 0.03 | ||
| 01 | 8 (13) | 28 (26) | |
| 23 | 29 (46) | 27 (26) | |
| 45 | 15 (24) | 29 (27) | |
| >5 | 11 (17) | 22 (21) | |
| Known history of diabetes, n (%) | 62 (98) | 96 (91) | 0.06 |
With respect to insulin ordering practices, there was no significant difference in the use of basal insulin in hyperglycemic patients between the preintervention period and postintervention period (81% versus 91%; P = 0.17), nor in the dose of basal insulin prescribed (results not shown), but there was an increase in the use of scheduled nutritional insulin for those patients with hyperglycemia receiving nutrition: 40% versus 75%, P < 0.001 (Table 3). The percent of patients receiving supplemental (sliding scale) insulin by itself (ie, without ever receiving basal or nutritional insulin) was lower during the postintervention period (29% versus 8%, P < 0.001). Nonsignificant differences were seen in the rates of prescribing an appropriate dose and type of nutritional insulin. Notably, there was no difference at all in the proportion of patient‐days in which insulin adjustments were made when 2 or more episodes of hyperglycemia or hypoglycemia were present during the previous day (56% of patient‐days in both groups; P = 0.90).
| Preintervention (n = 63) | Postintervention (n = 106) | Unadjusted Effect Size (95% CI) | Adjusted Effect Size (95% CI) | |
|---|---|---|---|---|
| ||||
| Mean percent glucose readings 60180 mg/dL per patient (SD) | 59.1 (0.28) | 64.7 (0.27) | +5.6 (3.0 to +14.3) | +9.7 (+0.6 to +18.8)*, |
| Patient‐day weighted mean glucose, mg/dL (SD) | 174.7 (60.0) | 164.6 (54.2) | 10.1 (1.6 to 18.5) | 15.6 (6.4 to 24.9), |
| Percent patient‐days with any glucose <60 mg/dL | 16/293 (5.5%) | 26/424 (6.1%) | 1.1 (0.6 to 2.1) | 1.1 (0.6 to 2.1) |
| Percent patient‐days with any glucose <40 mg/dL | 3/293 (1.0%) | 5/424 (1.2%) | 1.3 (0.3 to 5.9) | 1.1 (0.3 to 5.1) |
| Hospital length of stay, hours, mean (SD) | 112.2 (63.3) | 86.0 (89.6) | 30% (5% to 51%) | 25% (6% to 44%),∥ |
| Basal insulin if inpatient hyperglycemia (2 or more readings >180 mg/dL) | 39/48 (81%) | 67/74 (91%) | 2.2 (0.8 to 6.4) | |
| Nutritional insulin if inpatient hyperglycemia and PO intake | 19/48 (40%) | 53/71 (75%) | 4.5 (2.0 to 9.9), | |
| Adequate initial dose of nutritional insulin (home dose or 0.200.42 units/kg/day)# | 2/9 (22%) | 22/49 (45%) | 2.9 (0.5 to 15.1) | |
| Supplemental insulin alone (without basal or nutritional insulin) | 16/56 (29%) | 7/92 (8%) | 0.2 (0.08 to 0.5), | |
| Insulin changed if previous day's glucose out of range (2 or more values <60 or >180 mg/dL) | 70/126 (56%) | 76/135 (56%) | 1.0 (0.6 to 1.6) | |
| A1C tested during hospitalization if not available within 30 days prior | 38/63 (60%) | 74/106 (70%) | 1.5 (0.8 to 2.9) | |
The primary outcome, the mean percent of glucose readings between 60 and 180 mg/dL per patient, was 59.1% in the preintervention period and 64.7% in the postintervention (P = 0.13 in unadjusted analysis; Table 3). When adjusted for A1C, admission glucose, and insulin use prior to admission, the adjusted absolute difference in the percent of glucose readings within range was 9.7% (95% confidence interval [CI], 0.6%‐18.8%; P = 0.04; Table 3). Regarding other measures of glucose control, the patient‐day weighted mean glucose was 174.7 mg/dL in the preintervention period and 164.6 mg/dL postintervention (P = 0.02), and there was no significant difference in the percent of patient‐days with any hypoglycemia (glucose <60 mg/dL) or severe hypoglycemia (glucose <40 mg/dL; Table 3). There were also no significant differences in the mean number of hypoglycemic events per patient‐day (6.8 versus 6.6 per 100 patient‐days; relative risk, 0.95; 95% CI, 0.541.67; P = 0.87) or severe hypoglycemic events per patient‐day (1.0 versus 1.4 per 100 patient‐days; relative risk, 1.38; 95% CI, 0.355.53; P = 0.65).
We also compared hospital length of stay in hours between the study groups (Table 3). Length of stay (LOS) was shorter in the postintervention arm in unadjusted analyses (112 versus 86 hours; P < 0.001), and this difference persisted when adjusted for patient insurance, race, gender, and Charlson comorbidity score (25% shorter; 95% CI, 6%‐44%). A comparison of LOS among nonstudy patients on the PACE service during these 2 time periods revealed no difference (105 versus 101 hours). When the length of stay analysis was limited to study patients with a known diagnosis of diabetes, the adjusted effect size was a 31% relative decrease in length of stay.
Figure 1A shows the percent glucose readings within range per patient by hospital day. The greatest differences between groups can be seen on hospital days 2 and 3 (11% absolute differences on both days). Similarly, Figure 1B shows the mean glucose per patient by hospital day. Again, the biggest differences are seen on hospital days 2 and 3 (20 and 23 mg/dL difference between groups, respectively). In both cases, only the day 3 comparisons were significantly different between study groups.

DISCUSSION
In this before‐after study, we found that a multifaceted intervention consisting of a subcutaneous insulin protocol, focused education, and an order set built into the hospital's CPOE system was associated with a significantly higher percentage of glucose readings within range per patient in analyses adjusted for patient demographics and severity of diabetes. We also found a significant decrease in patient‐day weighted mean glucose, a marked increase in appropriate use of scheduled nutritional insulin, and a concomitant decrease in sliding scale insulin only regimens during the postintervention period. Moreover, we found a shorter length of stay during the postintervention period that persisted after adjustment for several clinical factors. Importantly, the interventions described in this study require very few resources to continue indefinitely: printing costs for the management protocol, 4 hours of education delivered per year, and routine upkeep of an electronic order set.
Because this was a before‐after study, we cannot exclude the possibility that these improvements in process and outcome were due to cointerventions and/or temporal trends. However, we know of no other interventions aimed at improving diabetes care in this self‐contained service of nurses, PAs, and hospitalists. Moreover, the process improvements, especially the increase in scheduled nutritional insulin, were rather marked, unlikely to be due to temporal trends alone, and likely capable of producing the corresponding improvements in glucose control. That glucose control stopped improving after hospital day 3 may be due to the fact that subsequent adjustment to insulin orders occurred infrequently and no more often than prior to the intervention. That we did not see greater improvements in glycemic control overall may also reflect the fact that 81% of study patients with inpatient hyperglycemia received basal insulin prior to the intervention.
The reduction in patient LOS was somewhat surprising given the relatively small sample size. However, the results are consistent with those of other studies linking hyperglycemia to LOS18, 19 and we found no evidence for a temporal trend toward lower LOS on the PACE service as a whole during the same time period. While a greater proportion of patients on the PACE service were in the study in the post‐intervention period compared with the preintervention period, we found no evidence that the difference in length of stay was due to increased surveillance for nondiabetics, especially because eligibility criteria depended on phlebotomy glucose values, which were uniformly tested in all inpatients. Also, effects on length of stay were actually stronger when limited to patients with known diabetes. Finally, we controlled for several predictors of length of stay, although we still cannot exclude the possibility of unmeasured confounding between groups.
Since ADA and ACE issued guidelines for inpatient management of diabetes and hyperglycemia, many institutions have developed subcutaneous insulin algorithms, educational curricula, and/or order sets to increase compliance with these guidelines and improve glycemic control. Some of these efforts have been studied and some have been successful in their efforts.13, 14, 2023 Unfortunately, most of these programs have not rigorously assessed their impact on process and outcomes, and the most effective studies published to date have involved interventions much more intensive than those described here. For example, Rush University's intervention was associated with a 50 mg/dL decrease in mean blood glucose but involved an endocrinologist rounding twice daily with house officers for 2 weeks at a time.13 At Northwestern University, a diabetes management service run by nurse practitioners was established, and the focus was on the conversion from intravenous to subcutaneous insulin regimens.14 The RABBIT 2 study that demonstrated the benefits of a basal‐bolus insulin regimen used daily rounding with an endocrinologist.12 More modestly, a program in Pitt County Memorial Hospital in Greenville, NC, relied mostly on diabetes nurse case managers, a strategy which reduced hospital‐wide mean glucose levels as well as LOS, although the greatest improvements in glycemic control were seen in the ICU.19 Our findings are much more consistent with those from University of California San Diego, as yet unpublished, which also used an algorithm, computerized order set, education, as well as continuous quality improvement methods to achieve its aims.22
Our study has several limitations, including being conducted on 1 general medicine service at 1 academic medical center. Moreover, this service, using a physician assistant/hospitalist model, a closed geographic unit, and fairly generous staffing ratio, is likely different from those in many settings and may limit the generalizability of our findings. However, this model allowed us to conduct the study in a laboratory relatively untouched by other cointerventions. Furthermore, the use of PAs in this way may become more common as both academic and community hospitals rely more on mid‐level providers. Our study had a relatively low percentage of patients without a known diagnosis of diabetes compared with other studies, again potentially but not necessarily limiting generalizability. This finding has been shown in other studies at our institution24 and may be due to the high rate of screening for diabetes in the community. Another limitation is that this was a nonrandomized, before‐after trial. However, all subjects were prospectively enrolled to improve comparability, and we performed rigorous adjustment for multiple potential confounding factors. Also, this study had limited statistical power to detect differences in hypoglycemia rates. The preintervention arm was smaller than planned due to fewer diabetic patients than expected on the service and a higher number of exclusions; we prolonged the postintervention period to achieve the desired sample size for that arm of the study.
Our study also has several strengths, including electronic capture of many processes of care and a methodology to operationalize them into measures of protocol adherence. Our metrics of glycemic control were rigorously designed and based on a national task force on inpatient glycemic control sponsored by the Society of Hospital Medicine, with representation from the ADA and AACE.25
Potential future improvements to this intervention include modifications to the daily adjustment algorithm to improve its usability and ability to improve glucose control. Another is the use of high‐reliability methods to improve order set use and daily insulin adjustment, including alerts within the CPOE system and nurse empowerment to contact medical teams if glucose levels are out of range (eg, if greater than 180 mg/dL, not just if greater than 350 or 400 mg/dL). Future research directions include multicenter, randomized controlled trials of these types of interventions and an analysis of more distal patient outcomes including total healthcare utilization, infection rates, end‐organ damage, and mortality.
In conclusion, we found a relationship between a relatively low‐cost quality improvement intervention and improved glycemic control in the non‐ICU general medical setting. Such a finding suggests the benefits of the algorithm itself to improve glucose control and of our implementation strategy. Other institutions may find this intervention a useful starting point for their own quality improvement efforts. Both the algorithm and implementation strategy are deserving of further improvements and future study.
Acknowledgements
We thank Paul Szumita, Karen Fiumara, Jennifer Trujillo, and the other members of the BWH Diabetes Pharmacy and Therapeutics Subcommittee for their help designing and implementing the intervention; Aubre McClendon, Nicole Auclair, Emily Dattwyler, Mariya Fiman, and Alison Pietras for valuable research assistance; Deborah Williams for data analysis; Amy Bloom for project support; and Stuart Lipsitz for biostatistical expertise.
APPENDIX
INPATIENT DIABETES MANAGEMENT PROTOCOL
Management of Diabetes and Hyperglycemia in Hospitalized Non‐ICU Patients
Rationale
Increasing data show a strong association between hyperglycemia and adverse inpatient outcomes. The American Diabetes Association and the American College of Clinical Endocrinology recommend all glucose levels be below 180 mg/dL in non‐ICU patients. Because hospitalizations are unstable situations, even patients who are well controlled on oral agents as outpatients are usually best managed with insulin.
Insulin may be safely administered even to patients without previously diagnosed diabetes. As long as the prescribed doses are below what is normally produced by the pancreas, the patient will not become hypoglycemic. If the glucose level drops, endogenous insulin secretion will reduce to compensate.
Total insulin requirements in insulin‐sensitive patients (eg, type 1 diabetes mellitus) is 0.50.7/units/kg/day. Insulin requirements in insulin‐resistant type 2 diabetic patients may vary greatly, and can exceed 12 units/kg/day. A conservative estimate for initial insulin therapy in any patient with diabetes is to start with the type 1 diabetes mellitus dose, 0.50.7 units/kg/day.
Overview
Effective inpatient insulin regimens typically include 3 components:
Basal insulin (eg, scheduled NPH or insulin glargine [Lantus]), which is used to manage fasting and premeal hyperglycemia.
Nutritional or prandial insulin (eg, scheduled regular insulin, insulin lispro [Humalog] or insulin aspart [Novolog]) which controls hyperglycemia from nutritional (eg, discrete meals, TPN, IV dextrose) sources.
Supplemental or correctional insulin (eg, regular insulin, insulin lispro, or insulin aspart), which is used in addition to scheduled insulin to meet unexpected basal hyperglycemia that is not covered by the scheduled insulin.
Sample Orders (Not for Patients with Uncontrolled Type 1 Diabetes, DKA, Hyperglycemic Hyperosmolar State, or Other Absolute Need for IV Insulin)
Check (fingerstick) capillary blood glucose qAC, qHS.
NPH insulin subcutaneously (SC) ___ units qAM, ___ units qHS.
Insulin aspart SC ___ units pre‐breakfast, ___ units pre‐lunch, ___ units pre‐dinner, hold if NPO or premeal BS <60 mg/dL; give 015 minutes before meals.
Insulin aspart SC sliding scale (see Table 6) qAC, in addition to standing nutritional insulin, 015 minutes before meals.
For BS <60 mg/dL:
If patient can take PO
Give 15 g of fast acting carbohydrate (4 oz fruit juice/nondiet soda, 8 oz nonfat milk, or 34 glucose tablets).
Repeat finger capillary glucose every 15 (q15) minutes and repeat above (5.a.i.) if BG <60 mg/dL.
When BG >60 mg/dL, give snack or meal in a half‐hour.
If patient cannot take PO
Give 25 mL of 50% dextrose (D50) as an IV push.;
Repeat finger capillary glucose q15 minutes and repeat above (5.b.i.) if BG <80 mg/dL.
Guidelines
Stop oral diabetes agents in most patients (see Table 7 for list of contraindications and precautions).
Check bedside blood glucose (BBG or fingerstick) qAC and qHS (or at 0600 hours, 1200 hours, 1800 hours, and 2400 hours if no discrete meals).
Estimate total daily insulin requirement:
For most patients, conservative estimate is 0.50.7 units/kg/day, but may be much higher.
Reasons for lower end of the range: renal insufficiency, small size, insulin sensitive (eg, type 1), recent hypoglycemia, decreasing doses of steroids, older age.
Reasons for higher end of the range: obese, initiation or increasing doses of steroids, marked hyperglycemia.
Start basal insulin if any premeal BG >140 mg/dL and no recent glucose <60 mg/dL off insulin (Table 5).
Start nutritional or prandial insulinhold if nutrition is stopped/held or premeal BS <60 (Table 5).
Start supplemental/correctional insulin in addition to nutritional (prandial) insulin (Table 6):
Discrete meals: Insulin aspart qAC (with nutritional insulin). 0
No discrete meals: Regular insulin q6h.
On a daily basis, adjust scheduled insulin based on previous days' blood sugars:
Add up total insulin given the previous day, including scheduled and supplemental insulin, to determine new total daily insulin requirement.
Adjust total daily insulin requirement based on clinical considerations (eg, give more if marked hyperglycemia, eating more, improving renal function, increasing steroids; give less if eating less, worsening renal function, tapering steroids, recovering from severe illness).
Give 50% of requirement as basal and 50% as nutritional, as above (may need proportionately less nutritional insulin if appetite poor or unknown).
Adjust sliding scale if needed based on total scheduled insulin dose (see step 6, above).
For BG <60 mg/dL:
If patient can take PO, give 15 g of fast acting carbohydrate.
(4 oz fruit juice/nondiet soda, 8 oz nonfat milk, or 34 glucose tablets; not juice plus sugar).
Repeat finger capillary glucose q15 minutes and repeat above if BG <60.
When BG >60, give snack or meal in half an hour.
If patient cannot take PO, give 25 mL of D50 as IV push.
Check finger capillary glucose q15 minutes and repeat above if BG <80.
Discharge orders:
Patient should be discharged home on a medication regimen that was similar to the admission regimen (ie, the regimen prescribed by their PCP). Exceptions include
The patient has a contraindication to an admission medication.
There is evidence of severe hyperglycemia (eg, very high A1C) or hypoglycemia on admission regimen.
If a patient is admitted with no insulin, and requires insulin to be continued as an outpatient (eg, newly‐diagnosed type 1 diabetes, A1C very high, and contraindication to or on maximum oral regimen), limit discharge insulin regimen to no more than 1 injection per day (eg, hs NPH; an exception to this is for type 1 diabetic patients, who are optimally treated with 34 injections/day). Make sure the patient has prompt follow‐up with their primary care provider (PCP).
Avoid discharging home on sliding scale.
If a patient is going to require insulin injections and self‐monitoring blood glucose as an outpatient, make sure they are instructed about how to perform these.
Indications for calling an endocrine consult:
Labile blood sugars.
Prolonged periods of NPO, eg, for procedures, especially in patients with type 1 diabetes
Marked hyperglycemia despite following this guideline.
Question of type 1 versus type 2 versus other type of diabetes. 0
Basil Insulin Guidelines Home Insulin Regimen Starting Dose of Basal Insulin Considerations NOTE: Patients with T1DM require basal insulin at all times! Basal never should be held!
Abbreviations: NPO, nothing by mouth.
On basal (eg, NPH or glargine) insulin at home Patient's home dose of NPH or glargine If NPO, consider starting half of NPH or glargine home dose, unless hyperglycemic at home. Not on basal (eg, NPH or glargine) insulin at home NPH 50% of total daily insulin requirement, given qHS or split qAM/qHS (maximum starting dose 20 units/day) Same dose if patient has previously diagnosed or undiagnosed diabetes Nutritional Insulin Guidelines Type of Nutrition Common Nutritional Regimens Sample Starting Doses Abbreviation: qAM, every morning; qHS, at bed time.
If receiving cycled tube feeds at night, give nutritional NPH qHS only.
Discrete meals Aspart given 015 minutes before meals Home dose, if known or 50% of total insulin requirement, split over 3 meals, may need less if poor or unknown appetite Continuous tube feeding,* IV dextrose NPH qHS or qAM/qHS 50% of total insulin requirement (in addition to basal dose), may need less if not at goal caloric intake Glargine given every day (qd), anytime Regular every 6 hours (q6h) Sample Supplemental/Correctional Insulin Scales Blood Glucose Scheduled Insulin < 40 Units/Day Scheduled Insulin of 4080 Units/Day Scheduled Insulin > 80 Units/Day Individualized NOTE: Avoid supplemental insulin qHS unless patient is very hyperglycemic and obese.
150199 1 unit 1 unit 2 units ____ units 200249 2 units 3 units 4 units ____ units 250299 3 units 5 units 7 units ____ units 300349 4 units 7 units 10 units ____ units >349 5 units + call HO 8 units + call HO 12 units + call HO ___ units + call HO Notes on Oral Agents Agents Considerations Metabolism Sulfonylureas/secretagogues: glyburide, glipizide, glimeperide (Amaryl); repaglinide (Prandin); nateglinide (Starlix) Risk for hypoglycemia Metabolized in liver; Glyburide metabolized to active metabolites; 50% renally eliminated Metformin Contraindicated in heart failure and renal dysfunction (creatinine [Cr] >1.5 mg/dL in men and 1.4 mg/dL in women) Eliminated renally Should be held at time of iodinated contrast studies. (May be restarted after normal postcontrast renal function is confirmed) Adverse effects include diarrhea, nausea, and anorexia Thiazolidinediones: pioglitazone (Actos), rosiglitazone (Avandia) Contraindicated in class III and IV heart failure Metabolized in liver Use with caution in patients with edema Adverse effects include increased intravascular volume Slow onset of action Avoid in hepatic dysfunction Glucosidease inhibitors: acarbose (Precose), miglitol (Glycet) Gastrointestinal intolerance Acarbose eliminated in gut and renally
Diabetes mellitus and/or inpatient hyperglycemia are common comorbid conditions in hospitalized patients. Recent surveys show that over 90% of hospitalized diabetic patients experience hyperglycemia (>200 mg/dL), and in nearly 1 in 5 of these patients hyperglycemia persists for 3 days or more.1 Hyperglycemia among inpatients without a previous history of diabetes mellitus is also very common.2 Observational studies have shown that hyperglycemia in hospitalized patients is associated with adverse outcomes including infectious complications, increased length of stay, and increased mortality.27 Recent randomized controlled trials have demonstrated that aggressive treatment of inpatient hyperglycemia improves outcomes in surgical and medical intensive care units.8, 9
Based on the available data, the American Diabetes Association (ADA) now advocates good metabolic control, defined as preprandial glucose levels of 90 to 130 mg/dL and peak postprandial glucose levels <180 mg/dL in hospitalized nonintensive care unit (ICU) patients.10 To reach these targets, the ADA and American College of Endocrinology (ACE) suggest that multidisciplinary teams develop and implement hyperglycemia management guidelines and protocols.11 Protocols should promote the use of continuous intravenous insulin infusions or scheduled basal‐bolus subcutaneous insulin regimens. Subcutaneous insulin protocols should include target glucose levels, basal, nutritional, and supplemental insulin, and daily dose adjustments.6 A recent randomized controlled trial of non‐ICU inpatients demonstrated that such a basal‐bolus insulin regimen results in improved glucose control compared with a sliding scale only regimen.12
To date, few published studies have investigated the best ways to implement such management protocols; those that have are often resource‐intensive, for example involving daily involvement of nurse practitioners or diabetologists.13, 14 It is therefore not known how best to implement an inpatient diabetes management program that is effective, efficient, and self‐perpetuating. At Brigham and Women's Hospital (BWH), we have been refining a subcutaneous insulin protocol, focused provider education, and more recently a computerized order set to overcome barriers related to fear of hypoglycemia, delays in insulin prescribing, and unfamiliarity with inpatient glucose management.15 The aims of this current trial were to evaluate the effects of these interventions on a geographically localized general medical service previously naive to these interventions to evaluate their effects on glycemic control, patient safety, and processes of care. We hypothesized that these interventions would improve glycemic control and increase use of basal‐bolus insulin orders without increasing the rate of hypoglycemia.
METHODS
Setting and Participants
This prospective, before‐after trial was conducted at BWH from July 15, 2005 through June 22, 2006. Eligible subjects were patients scheduled for admission to the BWH Physician Assistant/Clinician Educator (PACE) Service with either a known diagnosis of type 2 diabetes mellitus or inpatient hyperglycemia (at least 1 random laboratory glucose >180 mg/dL). The PACE service is a geographically‐localized general medicine service of up to 15 beds where patients are cared for by a single cadre of nurses, 2 physician's assistants (PAs), and 1 hospitalist attending. A moonlighter covers the service at night. The PACE service does not accept patients transferred from other acute care hospitals or from ICUs, but does not otherwise have triage guidelines related to diagnosis, complexity, or acuity. Patients were excluded if they had type 1 diabetes, presented with hyperosmolar hyperglycemic state (HHS) or diabetic ketoacidosis (DKA), received total parenteral nutrition (TPN), or were receiving palliative care. This study was approved by the BWH Institutional Review Board; patient consent was deemed not to be necessary for this study given the relatively nonsensitive nature of the data, noninvasive means of data collection, and the steps taken by research personnel to minimize any breach in patient confidentiality.
Intervention
The study intervention consisted of three components, initiated in January 2006:
Glycemic management protocol: a multidisciplinary team of a diabetologist (M.L.P.), a hospitalist (J.L.S.), and a pharmacist (Jennifer Trujillo) developed a subcutaneous insulin protocol based on ADA guidelines (Table 1; see the appendix for complete protocol). The protocol was approved by the BWH Pharmacy and Therapeutics Diabetes Subcommittee and refined through 6 months of pilot testing on other general medical services.15 The protocol consisted of a set of specific treatment recommendations, including: (1) bedside glucose monitoring; (2) stopping oral diabetes agents in most patients; (3) estimating total daily insulin requirements; (4) prescribing basal, nutritional, and supplemental insulin based on the patient's total insulin requirements, preadmission medication regimen, and nutritional status; (5) adjusting insulin on a daily basis as needed; (6) managing hypoglycemia; (7) suggestions for discharge orders; and (8) indications for an endocrinology consultation. The protocol was printed as a pocket guide, distributed to all members of the PACE service, and used to guide all other interventions.
Diabetes education: all PAs received 2 one‐hour educational sessions: a lecture by a diabetologist (M.L.P.) reviewing the rationale for tight glycemic control and general principles of management, and a workshop by a hospitalist (J.L.S.) in which specific cases were reviewed to illustrate how the protocol could be used in practice (eg, when oral agents could be safely continued, how to prescribe insulin on admission, and how to make subsequent adjustments in dose). All hospitalist attendings received a 1‐hour lecture summarizing the above material. All nurses on the service received a lecture that focused on issues unique to nursing care, such as insulin administration, glucose testing, managing patients with unpredictable oral (PO) intake, and patient education. (All materials are available from the authors upon request).
Order Set: an order set, built into BWH's proprietary computer provider order entry (CPOE) system, was created to parallel the glycemic management protocol and facilitate insulin orders for patients eating discrete meals, receiving continuous liquid enteral nutrition (tube feeds), or receiving nothing by mouth (NPO). Other components of the order set facilitated glucose monitoring and other laboratory tests and ordering consultation when appropriate.
| Oral Agents | Stop Oral Agents in Most Patients |
|---|---|
| |
| Glucose testing | Check bedside blood glucose before meals and at bedtime if eating, or every 6 hours if NPO |
| Insulin | |
| 1. Estimate total daily insulin dose | 0.5 to 0.7 units/kg/day, depending on patient's age, size, renal function, insulin sensitivity, history of hypoglycemia, and steroid use |
| 2. Start basal insulin | Patient's home dose or 50% of calculated total daily dose; NPH qAM/qHS or insulin glargine qHS; If NPO, use one‐half the home dose unless hyperglycemic |
| 3. Start nutritional insulin if not NPO | Patient's home dose or 50% of calculated total daily dose, less if poor or unknown intake; discrete meals: insulin aspart split over 3 meals, 0 to 15 minutes prior to eating; continuous tube feeds or IV dextrose: regular insulin every 6 hours |
| 4. Start correctional insulin | 1 of 3 scales provided based on total daily dose of insulin; same type as nutritional insulin; regular insulin if NPO |
| 5. Daily adjustment | Calculate total administered dose from prior day, adjust for degree of hyperglycemia or hypoglycemia, renal function, PO intake, steroid use, and degree of illness, and redistribute as 50% basal, 50% nutritional, or 100% basal if NPO |
| Hypoglycemia orders | Juice, IV dextrose, or IM glucagon depending on ability to take oral nutrition and IV access |
| Discharge orders | Based on A1C: either home regimen, titration of home regimen, or new insulin regimen (if latter, simple regimen with aggressive patient education and prompt follow‐up) |
| Indications for endocrine consultation | Labile blood sugars, poor control, prolonged NPO period, question of type 1 or type 2 diabetes |
Study Protocol and Data Collection
A research assistant prospectively identified eligible patients each weekday by screening all patients scheduled for admission to the PACE service using the daily computerized sign‐out system used on all general medical teams. Specifically, laboratory random glucose levels, inpatient medications, and medical histories were reviewed to determine if each patient met eligibility criteria. Eligibility criteria were then confirmed by medical record review and adjudicated by one study author (J.L.S.) if necessary. Further medical record review was performed to identify specific patient populations (eg, diet‐controlled, steroid‐induced, or previously undiagnosed diabetes), determine preadmission diabetes medications, and determine the patient's weight. Hospital computerized clinical and administrative records were abstracted to obtain patient demographics (age, sex, race, insurance status), laboratory data (glucose level on admission, A1C level [taken during or within 6 months prior to admission]), clinical data (length of stay, billing‐based Charlson comorbidity score,16 and diagnosis‐related group [DRG] case mix index), all inpatient insulin and oral diabetes medication orders, frequency of bedside glucose testing, and diet orders. Electronic medication administration record (eMAR) data were used to determine all doses and times of insulin administration.
Outcomes
The primary outcome was the mean percent of glucose readings between 60 and 180 mg/dL per patient (ie, calculated for each patient and averaged across all eligible patients in each study arm). Only bedside glucose readings were used given the lack of additional useful information typically provided by laboratory (venous plasma) glucose readings.17 Readings drawn within 1 hour of a previous reading were excluded to avoid ascertainment bias caused by follow‐up testing of abnormal glucose values. Only readings while on the study service were used. Readings on hospital day 1 were excluded because our intervention was expected to have little impact on the first day's glucose control; for patients with undiagnosed diabetes, data collection began the day following the first elevated glucose reading. Readings beyond hospital day 14 were also excluded to avoid biased data from patients with exceptionally long lengths of stay.
Secondary outcomes included the following:
Glycemic control:
Patient‐day weighted mean glucose (ie, mean glucose for each patient‐day, averaged across all patient days);
Mean glucose per patient for each hospital day (days 17).
Patient safety:
Proportion of patient‐days with any glucose reading <60 mg/dL (hypoglycemia) and <40 mg/dL (severe hypoglycemia).
Processes of care:
Use of any NPH insulin or insulin glargine (basal) insulin during the hospitalization if 2 or more glucose readings were >180 mg/dL.
Adequacy of basal dose on day first prescribed: for patients prescribed a diet, within 20% of preadmission basal dose or 0.20 to 0.42 units/kg if not known or not taken prior to admission. If not eating, half the above calculations.
Use of any scheduled nutritional insulin during the hospitalization if ever prescribed a diet and 2 or more glucose readings were greater than 180 mg/dL.
Adequacy of nutritional dose on day first prescribed: for patients prescribed a diet, within 20% of preadmission nutritional dose or 0.20 to 0.42 units/kg/day if not known or not taken prior to admission. Patients on clear liquid diets, enteral feeds, or receiving glucocorticoids were excluded from this analysis.
Correct type of nutritional insulin: if eating discrete meals, insulin aspart (the rapid‐acting insulin on formulary at BWH); if prescribed tube feeds, regular insulin.
Use of supplemental insulin by itself (without scheduled basal or nutritional insulin), a marker of poor care.
A1C testing within 1 month prior to or during hospitalization.
Clinical inertia: if at least two glucose readings <60 mg/dL or >180 mg/dL on a patient‐day, lack of any change to any insulin order the following day if still on the study service.
Healthcare utilization:
Hospital length of stay in hours, calculated from the exact time of admission until the exact time of discharge, using hospital administrative data.
Analyses
Study results were compared prior to the intervention (July 15 through December 12, 2005) with those during the intervention (January 18 through June 20, 2006). Patient data and clinical outcomes were analyzed descriptively using proportions, means with standard deviations (SDs), or medians with interquartile ranges (IQRs) as appropriate. Comparisons between groups were calculated using Fisher's exact test for dichotomous and categorical variables, and Student t test or Wilcoxon rank sum test for continuous variables as appropriate. The primary outcome was first analyzed using linear regression with study group as the independent variable and percent of glucose readings within range per patient as the dependent variable. We then adjusted for potential confounders by putting each covariate into the model, one at a time. All significant predictors of the outcome at a P value <0.10 were retained in the final model. We used general estimating equations to adjust for clustering of results by each PA. Similar analyses were performed for hospital length of stay per patient using a negative binomial model, so chosen because it fit the data distribution much better than the typically used Poisson model. With a planned sample size of 115 patients and 1250 glucose readings per arm, an intraclass correlation coefficient of 0.10, and an alpha of 0.05, the study had 90% power to detect an increase in percent of glucose readings in range from 67% to 75%. All analyses were based on the intention‐to‐treat principle. Except as above, 2‐sided P values <0.05 were considered significant. SAS version 9.1 (SAS Institute, Cary, NC) was used for all analyses.
RESULTS
We prospectively identified 248 potential patients for the study. We subsequently excluded 79 patients for the following reasons: no glucose readings beyond hospital day 1 while on PACE service (34 patients); never admitted to PACE service (15 patients); no diabetes or inpatient hyperglycemia (9 patients, mostly patients prescribed an insulin sliding scale prophylactically to avoid steroid‐induced hyperglycemia); type 1 diabetes (13 patients); TPN, DKA, or HHS (5 patients); and palliative care (3 patients). The remaining 169 patients included 63 from the preintervention period(out of 489 total admissions to the PACE service; 13%) and 106 patients in the postintervention period (out of 565 admissions; 19%). These patients had 2447 glucose readings, or an average of 3.6 glucose readings per monitored patient‐day in the preintervention period and 3.3 glucose readings per patient‐day in the postintervention period. Even including the 34 patients who were excluded for lack of glucose readings, glucose data were still available for 717 out of a potential 775 patient‐days (93%). Characteristics for all included patients are shown in Table 2. The mean admission glucose was 197 mg/dL, mean A1C was 8.4%, 54% of the patients were prescribed insulin prior to admission, and 7% had no prior diagnosis of diabetes. There were no significant differences in baseline characteristics between the 2 patient groups except for Charlson score, which was higher in the preintervention group (87% versus 74% with score 2 or higher; Table 2). The top diagnosis‐related groups for the entire cohort included: heart failure and shock (12 patients); kidney and urinary tract infections (12 patients); esophagitis, gastroenteritis, and miscellaneous digestive disorders (11 patients); chronic obstructive pulmonary disease (10 patients); renal failure (10 patients); simple pneumonia and pleurisy (7 patients); disorders of the pancreas except malignancy (6 patients); chest pain (5 patients); and cellulitis (5 patients).
| Preintervention (n = 63) | Postintervention (n = 106) | P Value | |
|---|---|---|---|
| |||
| Mean age, year (SD) | 63.0 (15.7) | 64.7 (14.3) | 0.52 |
| Male, n (%) | 25 (40) | 52 (49) | 0.27 |
| Race, n (%) | 0.33 | ||
| White | 29 (46) | 42 (40) | |
| Black | 21 (33) | 28 (26) | |
| Hispanic | 11 (17) | 30 (28) | |
| Unknown | 2 (3) | 6 (6) | |
| Admission glucose, mg/dL (SD) | 188 (90.9) | 203 (96.1) | 0.33 |
| A1C, % (SD) | 8.5 (2.4) | 8.3 (2.4) | 0.85 |
| Insulin use prior to admission, n (%) | 38 (60) | 54 (51) | 0.48 |
| Case mix index, median (IQR) | 0.89 (0.781.11) | 0.91 (0.841.22) | 0.33 |
| Charlson index, n (%) | 0.03 | ||
| 01 | 8 (13) | 28 (26) | |
| 23 | 29 (46) | 27 (26) | |
| 45 | 15 (24) | 29 (27) | |
| >5 | 11 (17) | 22 (21) | |
| Known history of diabetes, n (%) | 62 (98) | 96 (91) | 0.06 |
With respect to insulin ordering practices, there was no significant difference in the use of basal insulin in hyperglycemic patients between the preintervention period and postintervention period (81% versus 91%; P = 0.17), nor in the dose of basal insulin prescribed (results not shown), but there was an increase in the use of scheduled nutritional insulin for those patients with hyperglycemia receiving nutrition: 40% versus 75%, P < 0.001 (Table 3). The percent of patients receiving supplemental (sliding scale) insulin by itself (ie, without ever receiving basal or nutritional insulin) was lower during the postintervention period (29% versus 8%, P < 0.001). Nonsignificant differences were seen in the rates of prescribing an appropriate dose and type of nutritional insulin. Notably, there was no difference at all in the proportion of patient‐days in which insulin adjustments were made when 2 or more episodes of hyperglycemia or hypoglycemia were present during the previous day (56% of patient‐days in both groups; P = 0.90).
| Preintervention (n = 63) | Postintervention (n = 106) | Unadjusted Effect Size (95% CI) | Adjusted Effect Size (95% CI) | |
|---|---|---|---|---|
| ||||
| Mean percent glucose readings 60180 mg/dL per patient (SD) | 59.1 (0.28) | 64.7 (0.27) | +5.6 (3.0 to +14.3) | +9.7 (+0.6 to +18.8)*, |
| Patient‐day weighted mean glucose, mg/dL (SD) | 174.7 (60.0) | 164.6 (54.2) | 10.1 (1.6 to 18.5) | 15.6 (6.4 to 24.9), |
| Percent patient‐days with any glucose <60 mg/dL | 16/293 (5.5%) | 26/424 (6.1%) | 1.1 (0.6 to 2.1) | 1.1 (0.6 to 2.1) |
| Percent patient‐days with any glucose <40 mg/dL | 3/293 (1.0%) | 5/424 (1.2%) | 1.3 (0.3 to 5.9) | 1.1 (0.3 to 5.1) |
| Hospital length of stay, hours, mean (SD) | 112.2 (63.3) | 86.0 (89.6) | 30% (5% to 51%) | 25% (6% to 44%),∥ |
| Basal insulin if inpatient hyperglycemia (2 or more readings >180 mg/dL) | 39/48 (81%) | 67/74 (91%) | 2.2 (0.8 to 6.4) | |
| Nutritional insulin if inpatient hyperglycemia and PO intake | 19/48 (40%) | 53/71 (75%) | 4.5 (2.0 to 9.9), | |
| Adequate initial dose of nutritional insulin (home dose or 0.200.42 units/kg/day)# | 2/9 (22%) | 22/49 (45%) | 2.9 (0.5 to 15.1) | |
| Supplemental insulin alone (without basal or nutritional insulin) | 16/56 (29%) | 7/92 (8%) | 0.2 (0.08 to 0.5), | |
| Insulin changed if previous day's glucose out of range (2 or more values <60 or >180 mg/dL) | 70/126 (56%) | 76/135 (56%) | 1.0 (0.6 to 1.6) | |
| A1C tested during hospitalization if not available within 30 days prior | 38/63 (60%) | 74/106 (70%) | 1.5 (0.8 to 2.9) | |
The primary outcome, the mean percent of glucose readings between 60 and 180 mg/dL per patient, was 59.1% in the preintervention period and 64.7% in the postintervention (P = 0.13 in unadjusted analysis; Table 3). When adjusted for A1C, admission glucose, and insulin use prior to admission, the adjusted absolute difference in the percent of glucose readings within range was 9.7% (95% confidence interval [CI], 0.6%‐18.8%; P = 0.04; Table 3). Regarding other measures of glucose control, the patient‐day weighted mean glucose was 174.7 mg/dL in the preintervention period and 164.6 mg/dL postintervention (P = 0.02), and there was no significant difference in the percent of patient‐days with any hypoglycemia (glucose <60 mg/dL) or severe hypoglycemia (glucose <40 mg/dL; Table 3). There were also no significant differences in the mean number of hypoglycemic events per patient‐day (6.8 versus 6.6 per 100 patient‐days; relative risk, 0.95; 95% CI, 0.541.67; P = 0.87) or severe hypoglycemic events per patient‐day (1.0 versus 1.4 per 100 patient‐days; relative risk, 1.38; 95% CI, 0.355.53; P = 0.65).
We also compared hospital length of stay in hours between the study groups (Table 3). Length of stay (LOS) was shorter in the postintervention arm in unadjusted analyses (112 versus 86 hours; P < 0.001), and this difference persisted when adjusted for patient insurance, race, gender, and Charlson comorbidity score (25% shorter; 95% CI, 6%‐44%). A comparison of LOS among nonstudy patients on the PACE service during these 2 time periods revealed no difference (105 versus 101 hours). When the length of stay analysis was limited to study patients with a known diagnosis of diabetes, the adjusted effect size was a 31% relative decrease in length of stay.
Figure 1A shows the percent glucose readings within range per patient by hospital day. The greatest differences between groups can be seen on hospital days 2 and 3 (11% absolute differences on both days). Similarly, Figure 1B shows the mean glucose per patient by hospital day. Again, the biggest differences are seen on hospital days 2 and 3 (20 and 23 mg/dL difference between groups, respectively). In both cases, only the day 3 comparisons were significantly different between study groups.

DISCUSSION
In this before‐after study, we found that a multifaceted intervention consisting of a subcutaneous insulin protocol, focused education, and an order set built into the hospital's CPOE system was associated with a significantly higher percentage of glucose readings within range per patient in analyses adjusted for patient demographics and severity of diabetes. We also found a significant decrease in patient‐day weighted mean glucose, a marked increase in appropriate use of scheduled nutritional insulin, and a concomitant decrease in sliding scale insulin only regimens during the postintervention period. Moreover, we found a shorter length of stay during the postintervention period that persisted after adjustment for several clinical factors. Importantly, the interventions described in this study require very few resources to continue indefinitely: printing costs for the management protocol, 4 hours of education delivered per year, and routine upkeep of an electronic order set.
Because this was a before‐after study, we cannot exclude the possibility that these improvements in process and outcome were due to cointerventions and/or temporal trends. However, we know of no other interventions aimed at improving diabetes care in this self‐contained service of nurses, PAs, and hospitalists. Moreover, the process improvements, especially the increase in scheduled nutritional insulin, were rather marked, unlikely to be due to temporal trends alone, and likely capable of producing the corresponding improvements in glucose control. That glucose control stopped improving after hospital day 3 may be due to the fact that subsequent adjustment to insulin orders occurred infrequently and no more often than prior to the intervention. That we did not see greater improvements in glycemic control overall may also reflect the fact that 81% of study patients with inpatient hyperglycemia received basal insulin prior to the intervention.
The reduction in patient LOS was somewhat surprising given the relatively small sample size. However, the results are consistent with those of other studies linking hyperglycemia to LOS18, 19 and we found no evidence for a temporal trend toward lower LOS on the PACE service as a whole during the same time period. While a greater proportion of patients on the PACE service were in the study in the post‐intervention period compared with the preintervention period, we found no evidence that the difference in length of stay was due to increased surveillance for nondiabetics, especially because eligibility criteria depended on phlebotomy glucose values, which were uniformly tested in all inpatients. Also, effects on length of stay were actually stronger when limited to patients with known diabetes. Finally, we controlled for several predictors of length of stay, although we still cannot exclude the possibility of unmeasured confounding between groups.
Since ADA and ACE issued guidelines for inpatient management of diabetes and hyperglycemia, many institutions have developed subcutaneous insulin algorithms, educational curricula, and/or order sets to increase compliance with these guidelines and improve glycemic control. Some of these efforts have been studied and some have been successful in their efforts.13, 14, 2023 Unfortunately, most of these programs have not rigorously assessed their impact on process and outcomes, and the most effective studies published to date have involved interventions much more intensive than those described here. For example, Rush University's intervention was associated with a 50 mg/dL decrease in mean blood glucose but involved an endocrinologist rounding twice daily with house officers for 2 weeks at a time.13 At Northwestern University, a diabetes management service run by nurse practitioners was established, and the focus was on the conversion from intravenous to subcutaneous insulin regimens.14 The RABBIT 2 study that demonstrated the benefits of a basal‐bolus insulin regimen used daily rounding with an endocrinologist.12 More modestly, a program in Pitt County Memorial Hospital in Greenville, NC, relied mostly on diabetes nurse case managers, a strategy which reduced hospital‐wide mean glucose levels as well as LOS, although the greatest improvements in glycemic control were seen in the ICU.19 Our findings are much more consistent with those from University of California San Diego, as yet unpublished, which also used an algorithm, computerized order set, education, as well as continuous quality improvement methods to achieve its aims.22
Our study has several limitations, including being conducted on 1 general medicine service at 1 academic medical center. Moreover, this service, using a physician assistant/hospitalist model, a closed geographic unit, and fairly generous staffing ratio, is likely different from those in many settings and may limit the generalizability of our findings. However, this model allowed us to conduct the study in a laboratory relatively untouched by other cointerventions. Furthermore, the use of PAs in this way may become more common as both academic and community hospitals rely more on mid‐level providers. Our study had a relatively low percentage of patients without a known diagnosis of diabetes compared with other studies, again potentially but not necessarily limiting generalizability. This finding has been shown in other studies at our institution24 and may be due to the high rate of screening for diabetes in the community. Another limitation is that this was a nonrandomized, before‐after trial. However, all subjects were prospectively enrolled to improve comparability, and we performed rigorous adjustment for multiple potential confounding factors. Also, this study had limited statistical power to detect differences in hypoglycemia rates. The preintervention arm was smaller than planned due to fewer diabetic patients than expected on the service and a higher number of exclusions; we prolonged the postintervention period to achieve the desired sample size for that arm of the study.
Our study also has several strengths, including electronic capture of many processes of care and a methodology to operationalize them into measures of protocol adherence. Our metrics of glycemic control were rigorously designed and based on a national task force on inpatient glycemic control sponsored by the Society of Hospital Medicine, with representation from the ADA and AACE.25
Potential future improvements to this intervention include modifications to the daily adjustment algorithm to improve its usability and ability to improve glucose control. Another is the use of high‐reliability methods to improve order set use and daily insulin adjustment, including alerts within the CPOE system and nurse empowerment to contact medical teams if glucose levels are out of range (eg, if greater than 180 mg/dL, not just if greater than 350 or 400 mg/dL). Future research directions include multicenter, randomized controlled trials of these types of interventions and an analysis of more distal patient outcomes including total healthcare utilization, infection rates, end‐organ damage, and mortality.
In conclusion, we found a relationship between a relatively low‐cost quality improvement intervention and improved glycemic control in the non‐ICU general medical setting. Such a finding suggests the benefits of the algorithm itself to improve glucose control and of our implementation strategy. Other institutions may find this intervention a useful starting point for their own quality improvement efforts. Both the algorithm and implementation strategy are deserving of further improvements and future study.
Acknowledgements
We thank Paul Szumita, Karen Fiumara, Jennifer Trujillo, and the other members of the BWH Diabetes Pharmacy and Therapeutics Subcommittee for their help designing and implementing the intervention; Aubre McClendon, Nicole Auclair, Emily Dattwyler, Mariya Fiman, and Alison Pietras for valuable research assistance; Deborah Williams for data analysis; Amy Bloom for project support; and Stuart Lipsitz for biostatistical expertise.
APPENDIX
INPATIENT DIABETES MANAGEMENT PROTOCOL
Management of Diabetes and Hyperglycemia in Hospitalized Non‐ICU Patients
Rationale
Increasing data show a strong association between hyperglycemia and adverse inpatient outcomes. The American Diabetes Association and the American College of Clinical Endocrinology recommend all glucose levels be below 180 mg/dL in non‐ICU patients. Because hospitalizations are unstable situations, even patients who are well controlled on oral agents as outpatients are usually best managed with insulin.
Insulin may be safely administered even to patients without previously diagnosed diabetes. As long as the prescribed doses are below what is normally produced by the pancreas, the patient will not become hypoglycemic. If the glucose level drops, endogenous insulin secretion will reduce to compensate.
Total insulin requirements in insulin‐sensitive patients (eg, type 1 diabetes mellitus) is 0.50.7/units/kg/day. Insulin requirements in insulin‐resistant type 2 diabetic patients may vary greatly, and can exceed 12 units/kg/day. A conservative estimate for initial insulin therapy in any patient with diabetes is to start with the type 1 diabetes mellitus dose, 0.50.7 units/kg/day.
Overview
Effective inpatient insulin regimens typically include 3 components:
Basal insulin (eg, scheduled NPH or insulin glargine [Lantus]), which is used to manage fasting and premeal hyperglycemia.
Nutritional or prandial insulin (eg, scheduled regular insulin, insulin lispro [Humalog] or insulin aspart [Novolog]) which controls hyperglycemia from nutritional (eg, discrete meals, TPN, IV dextrose) sources.
Supplemental or correctional insulin (eg, regular insulin, insulin lispro, or insulin aspart), which is used in addition to scheduled insulin to meet unexpected basal hyperglycemia that is not covered by the scheduled insulin.
Sample Orders (Not for Patients with Uncontrolled Type 1 Diabetes, DKA, Hyperglycemic Hyperosmolar State, or Other Absolute Need for IV Insulin)
Check (fingerstick) capillary blood glucose qAC, qHS.
NPH insulin subcutaneously (SC) ___ units qAM, ___ units qHS.
Insulin aspart SC ___ units pre‐breakfast, ___ units pre‐lunch, ___ units pre‐dinner, hold if NPO or premeal BS <60 mg/dL; give 015 minutes before meals.
Insulin aspart SC sliding scale (see Table 6) qAC, in addition to standing nutritional insulin, 015 minutes before meals.
For BS <60 mg/dL:
If patient can take PO
Give 15 g of fast acting carbohydrate (4 oz fruit juice/nondiet soda, 8 oz nonfat milk, or 34 glucose tablets).
Repeat finger capillary glucose every 15 (q15) minutes and repeat above (5.a.i.) if BG <60 mg/dL.
When BG >60 mg/dL, give snack or meal in a half‐hour.
If patient cannot take PO
Give 25 mL of 50% dextrose (D50) as an IV push.;
Repeat finger capillary glucose q15 minutes and repeat above (5.b.i.) if BG <80 mg/dL.
Guidelines
Stop oral diabetes agents in most patients (see Table 7 for list of contraindications and precautions).
Check bedside blood glucose (BBG or fingerstick) qAC and qHS (or at 0600 hours, 1200 hours, 1800 hours, and 2400 hours if no discrete meals).
Estimate total daily insulin requirement:
For most patients, conservative estimate is 0.50.7 units/kg/day, but may be much higher.
Reasons for lower end of the range: renal insufficiency, small size, insulin sensitive (eg, type 1), recent hypoglycemia, decreasing doses of steroids, older age.
Reasons for higher end of the range: obese, initiation or increasing doses of steroids, marked hyperglycemia.
Start basal insulin if any premeal BG >140 mg/dL and no recent glucose <60 mg/dL off insulin (Table 5).
Start nutritional or prandial insulinhold if nutrition is stopped/held or premeal BS <60 (Table 5).
Start supplemental/correctional insulin in addition to nutritional (prandial) insulin (Table 6):
Discrete meals: Insulin aspart qAC (with nutritional insulin). 0
No discrete meals: Regular insulin q6h.
On a daily basis, adjust scheduled insulin based on previous days' blood sugars:
Add up total insulin given the previous day, including scheduled and supplemental insulin, to determine new total daily insulin requirement.
Adjust total daily insulin requirement based on clinical considerations (eg, give more if marked hyperglycemia, eating more, improving renal function, increasing steroids; give less if eating less, worsening renal function, tapering steroids, recovering from severe illness).
Give 50% of requirement as basal and 50% as nutritional, as above (may need proportionately less nutritional insulin if appetite poor or unknown).
Adjust sliding scale if needed based on total scheduled insulin dose (see step 6, above).
For BG <60 mg/dL:
If patient can take PO, give 15 g of fast acting carbohydrate.
(4 oz fruit juice/nondiet soda, 8 oz nonfat milk, or 34 glucose tablets; not juice plus sugar).
Repeat finger capillary glucose q15 minutes and repeat above if BG <60.
When BG >60, give snack or meal in half an hour.
If patient cannot take PO, give 25 mL of D50 as IV push.
Check finger capillary glucose q15 minutes and repeat above if BG <80.
Discharge orders:
Patient should be discharged home on a medication regimen that was similar to the admission regimen (ie, the regimen prescribed by their PCP). Exceptions include
The patient has a contraindication to an admission medication.
There is evidence of severe hyperglycemia (eg, very high A1C) or hypoglycemia on admission regimen.
If a patient is admitted with no insulin, and requires insulin to be continued as an outpatient (eg, newly‐diagnosed type 1 diabetes, A1C very high, and contraindication to or on maximum oral regimen), limit discharge insulin regimen to no more than 1 injection per day (eg, hs NPH; an exception to this is for type 1 diabetic patients, who are optimally treated with 34 injections/day). Make sure the patient has prompt follow‐up with their primary care provider (PCP).
Avoid discharging home on sliding scale.
If a patient is going to require insulin injections and self‐monitoring blood glucose as an outpatient, make sure they are instructed about how to perform these.
Indications for calling an endocrine consult:
Labile blood sugars.
Prolonged periods of NPO, eg, for procedures, especially in patients with type 1 diabetes
Marked hyperglycemia despite following this guideline.
Question of type 1 versus type 2 versus other type of diabetes. 0
Basil Insulin Guidelines Home Insulin Regimen Starting Dose of Basal Insulin Considerations NOTE: Patients with T1DM require basal insulin at all times! Basal never should be held!
Abbreviations: NPO, nothing by mouth.
On basal (eg, NPH or glargine) insulin at home Patient's home dose of NPH or glargine If NPO, consider starting half of NPH or glargine home dose, unless hyperglycemic at home. Not on basal (eg, NPH or glargine) insulin at home NPH 50% of total daily insulin requirement, given qHS or split qAM/qHS (maximum starting dose 20 units/day) Same dose if patient has previously diagnosed or undiagnosed diabetes Nutritional Insulin Guidelines Type of Nutrition Common Nutritional Regimens Sample Starting Doses Abbreviation: qAM, every morning; qHS, at bed time.
If receiving cycled tube feeds at night, give nutritional NPH qHS only.
Discrete meals Aspart given 015 minutes before meals Home dose, if known or 50% of total insulin requirement, split over 3 meals, may need less if poor or unknown appetite Continuous tube feeding,* IV dextrose NPH qHS or qAM/qHS 50% of total insulin requirement (in addition to basal dose), may need less if not at goal caloric intake Glargine given every day (qd), anytime Regular every 6 hours (q6h) Sample Supplemental/Correctional Insulin Scales Blood Glucose Scheduled Insulin < 40 Units/Day Scheduled Insulin of 4080 Units/Day Scheduled Insulin > 80 Units/Day Individualized NOTE: Avoid supplemental insulin qHS unless patient is very hyperglycemic and obese.
150199 1 unit 1 unit 2 units ____ units 200249 2 units 3 units 4 units ____ units 250299 3 units 5 units 7 units ____ units 300349 4 units 7 units 10 units ____ units >349 5 units + call HO 8 units + call HO 12 units + call HO ___ units + call HO Notes on Oral Agents Agents Considerations Metabolism Sulfonylureas/secretagogues: glyburide, glipizide, glimeperide (Amaryl); repaglinide (Prandin); nateglinide (Starlix) Risk for hypoglycemia Metabolized in liver; Glyburide metabolized to active metabolites; 50% renally eliminated Metformin Contraindicated in heart failure and renal dysfunction (creatinine [Cr] >1.5 mg/dL in men and 1.4 mg/dL in women) Eliminated renally Should be held at time of iodinated contrast studies. (May be restarted after normal postcontrast renal function is confirmed) Adverse effects include diarrhea, nausea, and anorexia Thiazolidinediones: pioglitazone (Actos), rosiglitazone (Avandia) Contraindicated in class III and IV heart failure Metabolized in liver Use with caution in patients with edema Adverse effects include increased intravascular volume Slow onset of action Avoid in hepatic dysfunction Glucosidease inhibitors: acarbose (Precose), miglitol (Glycet) Gastrointestinal intolerance Acarbose eliminated in gut and renally
- ,,,,.Prevalence of hyper‐ and hypoglycemia among inpatients with diabetes: a national survey of 44 U.S. hospitals.Diabetes Care.2007;30:367–369.
- ,,,,,.Hyperglycemia: an independent marker of in‐hospital mortality in patients with undiagnosed diabetes.J Clin Endocrinol Metab.2002;87:978–982.
- ,,, et al.Hyperglycaemia is associated with poor outcomes in patients admitted to hospital with acute exacerbations of chronic obstructive pulmonary disease.Thorax.2006;61:284–289.
- ,,,,.Stress hyperglycemia and prognosis of stroke in nondiabetic and diabetic patients: a systematic overview.Stroke.2001;32:2426–2432.
- ,,,.Hyperglycemia is associated with adverse outcomes in patients receiving total parenteral nutrition.Diabetes Care.2005;28:2367–2371.
- ,,, et al.Management of diabetes and hyperglycemia in hospitals.Diabetes Care.2004;27:553–597.
- ,,,,,.The relation between hyperglycemia and outcomes in 2471 patients admitted to the hospital with community‐acquired pneumonia.Diabetes Care.2005;28:810–815.
- ,,, et al.Intensive insulin therapy in the medical ICU.N Engl J Med.2006;354:449–461.
- ,,, et al.Intensive insulin therapy in the critically ill patients.N Engl J Med.2001;345:1359–1367.
- Standards of medical care in diabetes, 2007.Diabetes Care.2007;30(Suppl 1):S4–S41.
- American College of Endocrinology and American Diabetes Association Consensus statement on inpatient diabetes and glycemic control: a call to action.Diabetes Care.2006;29:1955–1962.
- ,,, et al.Randomized study of basal‐bolus insulin therapy in the inpatient management of patients with type 2 diabetes (RABBIT 2 trial).Diabetes Care.2007;30:2181–2186.
- ,,,.Eliminating inpatient sliding‐scale insulin: a reeducation project with medical house staff.Diabetes Care.2005;28:1008–1011.
- ,,, et al.Inpatient management of hyperglycemia: the Northwestern experience.Endocr Pract.2006;12:491–505.
- ,,, et al.Improving glycemic control in medical inpatients: a pilot study.J Hosp Med.2008;3:55–63.
- ,,.Adapting a clinical comorbidity index for use with ICD‐9‐CM administrative databases.J Clin Epidemiol.1992;45:613–619.
- ,,, et al.“Glucometrics”—assessing the quality of inpatient glucose management.Diabetes Technol Ther.2006;8:560–569.
- ,,,.Hyperglycemia and length of stay in patients hospitalized for bone marrow transplantation.Diabetes Care.2007;30:993–994.
- ,.Financial implications of glycemic control: results of an inpatient diabetes management program.Endocr Pract.2006;12(Suppl 3):43–48.
- ,,, et al.In‐hospital treatment of hyperglycemia: effects of intensified subcutaneous insulin treatment.Curr Med Res Opin.2007;23:757–765.
- ,,,.Impact of endocrine and diabetes team consultation on hospital length of stay for patients with diabetes.Am J Med.1995;99:22–28.
- ,,,.Effect of a standardized insulin order set and an insulin management algorithm on inpatient glycemic control and hypoglycemia. Society of Hospital Medicine Annual Meeting, 2007; Dallas, TX;2007.
- ,,, et al.Trends in bed occupancy for inpatients with diabetes before and after the introduction of a diabetes inpatient specialist nurse service.Diabet Med.2006;23:1008–1015.
- ,,,,.Inpatient management of diabetes and hyperglycemia among general medicine patients at a large teaching hospital.J Hosp Med.2006;1:145–150.
- ,,, et al. Improving glycemic control, preventing hypoglycemia, and optimizing care of the inpatient with hyperglycemia and diabetes, 2006. Available at:http://www.hospitalmedicine.org/ResourceRoomRedesign/html/GC_Imp_Guide.cfm. Accessed October 2008.
- ,,,,.Prevalence of hyper‐ and hypoglycemia among inpatients with diabetes: a national survey of 44 U.S. hospitals.Diabetes Care.2007;30:367–369.
- ,,,,,.Hyperglycemia: an independent marker of in‐hospital mortality in patients with undiagnosed diabetes.J Clin Endocrinol Metab.2002;87:978–982.
- ,,, et al.Hyperglycaemia is associated with poor outcomes in patients admitted to hospital with acute exacerbations of chronic obstructive pulmonary disease.Thorax.2006;61:284–289.
- ,,,,.Stress hyperglycemia and prognosis of stroke in nondiabetic and diabetic patients: a systematic overview.Stroke.2001;32:2426–2432.
- ,,,.Hyperglycemia is associated with adverse outcomes in patients receiving total parenteral nutrition.Diabetes Care.2005;28:2367–2371.
- ,,, et al.Management of diabetes and hyperglycemia in hospitals.Diabetes Care.2004;27:553–597.
- ,,,,,.The relation between hyperglycemia and outcomes in 2471 patients admitted to the hospital with community‐acquired pneumonia.Diabetes Care.2005;28:810–815.
- ,,, et al.Intensive insulin therapy in the medical ICU.N Engl J Med.2006;354:449–461.
- ,,, et al.Intensive insulin therapy in the critically ill patients.N Engl J Med.2001;345:1359–1367.
- Standards of medical care in diabetes, 2007.Diabetes Care.2007;30(Suppl 1):S4–S41.
- American College of Endocrinology and American Diabetes Association Consensus statement on inpatient diabetes and glycemic control: a call to action.Diabetes Care.2006;29:1955–1962.
- ,,, et al.Randomized study of basal‐bolus insulin therapy in the inpatient management of patients with type 2 diabetes (RABBIT 2 trial).Diabetes Care.2007;30:2181–2186.
- ,,,.Eliminating inpatient sliding‐scale insulin: a reeducation project with medical house staff.Diabetes Care.2005;28:1008–1011.
- ,,, et al.Inpatient management of hyperglycemia: the Northwestern experience.Endocr Pract.2006;12:491–505.
- ,,, et al.Improving glycemic control in medical inpatients: a pilot study.J Hosp Med.2008;3:55–63.
- ,,.Adapting a clinical comorbidity index for use with ICD‐9‐CM administrative databases.J Clin Epidemiol.1992;45:613–619.
- ,,, et al.“Glucometrics”—assessing the quality of inpatient glucose management.Diabetes Technol Ther.2006;8:560–569.
- ,,,.Hyperglycemia and length of stay in patients hospitalized for bone marrow transplantation.Diabetes Care.2007;30:993–994.
- ,.Financial implications of glycemic control: results of an inpatient diabetes management program.Endocr Pract.2006;12(Suppl 3):43–48.
- ,,, et al.In‐hospital treatment of hyperglycemia: effects of intensified subcutaneous insulin treatment.Curr Med Res Opin.2007;23:757–765.
- ,,,.Impact of endocrine and diabetes team consultation on hospital length of stay for patients with diabetes.Am J Med.1995;99:22–28.
- ,,,.Effect of a standardized insulin order set and an insulin management algorithm on inpatient glycemic control and hypoglycemia. Society of Hospital Medicine Annual Meeting, 2007; Dallas, TX;2007.
- ,,, et al.Trends in bed occupancy for inpatients with diabetes before and after the introduction of a diabetes inpatient specialist nurse service.Diabet Med.2006;23:1008–1015.
- ,,,,.Inpatient management of diabetes and hyperglycemia among general medicine patients at a large teaching hospital.J Hosp Med.2006;1:145–150.
- ,,, et al. Improving glycemic control, preventing hypoglycemia, and optimizing care of the inpatient with hyperglycemia and diabetes, 2006. Available at:http://www.hospitalmedicine.org/ResourceRoomRedesign/html/GC_Imp_Guide.cfm. Accessed October 2008.
Copyright © 2009 Society of Hospital Medicine
Glycemic Control in Medical Inpatients
Diabetes mellitus is a common comorbid condition in hospitalized patients. In 2003, diabetes was listed as a diagnosis in 17.2% of hospital discharges in the United States.1 Because these diagnosis codes do not account for undiagnosed diabetes or hospital‐related hyperglycemia, the true prevalence of diabetes or hyperglycemia in hospitalized patients is likely higher and has been estimated to be as great as 38%.2 Hyperglycemia has been associated with adverse outcomes among hospitalized patients, including infectious complications, increased length of stay, and increased mortality.27 However, because hyperglycemia is not usually the primary reason patients with diabetes are hospitalized, its management is often not a focus in the inpatient setting. Sliding‐scale insulin alone continues to be commonly prescribed despite clinical evidence showing it to be ineffective in achieving glycemic control.8, 9
Recent randomized controlled trials have demonstrated that aggressive treatment of inpatient hyperglycemia improves outcomes in surgical and medical intensive care units10, 11 and in patients admitted for myocardial infarction.12, 13 Based on this clinical evidence and strong observational data linking hyperglycemia to poor patient outcomes in the non‐ICU setting,27 the American Diabetes Association (ADA) now advocates good metabolic control, defined as preprandial glucose levels of 90‐130 mg/dL and peak postprandial glucose levels < 180 mg/dL in hospitalized non‐ICU patients with hyperglycemia14 (note that these targets are less aggressive than those for ICU patients, for whom randomized controlled trials showed the benefits of reduced mortality provided by tight glucose control).11 To reach these targets, the ADA and American College of Endocrinology suggest that multidisciplinary teams develop and implement hyperglycemia management guidelines and protocols.15 Protocols should promote the use of continuous intravenous insulin or scheduled subcutaneous insulin as opposed to the use of sliding‐scale insulin alone. Subcutaneous insulin protocols should include target glucose levels; basal, nutritional, and supplemental insulin; and daily adjustments based on previous glucose levels, insulin sensitivity, nutritional intake, illness, and medications.6, 15 To date, few published protocols or algorithms for inpatient subcutaneous insulin have been shown to be effective.16, 17 It is therefore not known how best to design and implement an inpatient diabetes management protocol that is effective, efficient, and self‐perpetuating. The aims of our pilot study were to develop and implement a subcutaneous insulin protocol on a general medicine service, to identify barriers to implementation, and to determine the effect of this protocol on glycemic control.
METHODS
Setting and Participants
This prospective quality‐improvement pilot study was conducted at Brigham and Women's Hospital (BWH) from January 10, 2005, through June 23, 2005. Patients were eligible to participate if they were admitted to either of 2 General Medicine Service (GMS) teams with either a known diagnosis of type 2 diabetes or inpatient hyperglycemia (random laboratory glucose level > 180 mg/dL) and at least 1 fasting point‐of‐care glucose reading > 140 mg/dL. Patients were excluded if they had diabetic ketoacidosis, hyperosmolar hyperglycemic state, another absolute indication for intravenous insulin, or fasting glucose < 60 mg/dL on no insulin or if they were pregnant. Each GMS team consisted of a teaching attending, a junior or senior resident, 2 interns, and a clinical pharmacist. Twenty‐six physicians attended on these 2 teams during the study period, 13 of whom were hospitalists. This study was approved by the BWH Institutional Review Board; patient consent to participate in this study was deemed not necessary because of the relatively nonsensitive nature of the data (eg, glucose control, insulin orders), the noninvasive means of data collection (eg, chart review), and the steps taken by research personnel to minimize any breach in patient confidentiality.
Intervention
A multidisciplinary team composed of a diabetologist (M.L.P.), a hospitalist (J.L.S.), and a pharmacist (J.M.T.) developed a subcutaneous insulin protocol that was approved by the BWH Pharmacy and Therapeutics Diabetes Subcommittee. The protocol consisted of a set of treatment recommendations made by a pharmacist to be carried out by the medical team. The primary components are shown in Table 1 (a full description can be found in the Appendix). The main emphasis of the protocol was on discontinuing oral antihyperglycemic agents during hospitalization, initiating basal insulin in most patients, and adjusting basal insulin daily as needed.
|
| Oral agents |
| 1. Stop oral agents in most patients |
| Glucose testing |
| 2. Check bedside blood glucose before meals and at bedtime if eating, or every 6 hours if not eating |
| Insulin |
| 3. Start basal insulin Patient's home dose or NPH 0.1 units/kg before breakfast and at bedtime or insulin glargine 0.2 units/kg at bedtime (max dose 20 units) If NPO, consider half dose unless hyperglycemic |
| 4. Start nutritional insulin Discrete meals: insulin aspart 0.05‐0.1 units/kg per meal or home dose 0‐15 minutes prior to eating Continuous tube feeds: regular insulin every 6 hours or NPH every morning and at bedtime (0.1‐0.2 units/kg per day in addition to basal insulin) Hold if NPO |
| 5. Start correctional insulin Scale provided based on blood glucose and daily scheduled insulin requirements |
| Daily Adjustments |
6. Adjust scheduled insulin daily
|
| Other Considerations |
| 7. Hypoglycemia management (protocols for fruit juice, glucagons, IV dextrose, and when to call physician) |
| 8. Discharge orders (recommendations to discharge most patients on admission medication regimen, avoid sliding scale insulin, simplify dosing for patients requiring new insulin regimens, ensure adequate patient education and prompt outpatient follow‐up) |
All medical residents received general instructions regarding inpatient diabetes control by the research team's diabetologist (M.L.P.) through a 1‐hour department‐wide didactic lecture. The standards of care taught were identical to those in the protocol. In addition, the research team's hospitalist (J.L.S.) contacted each medical resident assigned to the 2 GMS teams electronically to introduce the protocol and describe the purpose and logistics of the pilot study.
A research assistant prospectively identified eligible patients each weekday by screening all patients admitted to the 2 GMS teams using the daily computerized sign‐out system used by all medical residents. Specifically, laboratory random glucose levels, inpatient medications, and medical history were reviewed to determine if each patient met eligibility criteria. Eligibility criteria were confirmed by medical record review. The pharmacist recommended to the primary team that the protocol be initiated for eligible patients. In addition, the pharmacist recommended daily adjustment of the insulin dose according to the protocol as appropriate. A chronologically organized summary of clinical data relevant to glycemic management for each patient, including bedside blood glucose measurements, general dietary intake, use of intravenous dextrose solutions, and administration of systemic steroids, oral diabetes medications, and all insulins, was provided to the team each day by the research assistant.
Measurements
The resident's acceptance of the protocol or reasons for declining it were recorded by the pharmacist on the day the protocol was recommended. Protocol acceptance was categorized as yes, no, or partial. Partial acceptance was defined as resident agreement to use the protocol, but with stated caveats or modifications. Clinical data were collected on each eligible patient for up to 7 days on GMS. Several data sources were used, including physician admission notes, the hospital's computerized clinical data system, vital‐sign sheets, medication administration records, and personal communication with nurses regarding any missing or discrepant data.
All insulin use (prescribed drug, dose, route, schedule and actual administered drug, dose, route, and time) was recorded each day by the research assistant. Use of basal and nutritional insulin and daily dose adjustments if previous hypo‐ or hyperglycemia (categorized as yes, no, or not applicable for each patient each day) were determined by the study pharmacist (J.M.T.) through retrospective review of all orders.
Up to 4 routine bedside blood glucose measurements were recorded each day: for patients eating discrete meals, these were the measurements taken before meals and at bedtime; for patients not eating or receiving continuous nutrition, these were the measurements taken closest to 6 AM, noon, 6 PM, and midnight. Additional measurements were not recorded to avoid ascertainment bias caused by follow‐up testing of abnormal glucose values. Glucose readings on the day of admission were excluded from analysis because these values are not amenable to inpatient ordering practices.
Study outcomes included overall protocol acceptance rate, insulin prescribing practices including use of basal insulin (ie, long‐acting agents such as NPH and insulin glargine), nutritional insulin (ie, scheduled regular, lispro, or aspart insulin given before each meal), daily dose adjustments under the protocol, and mean percentage of glucose readings per person greater than 180 mg/dL (hyperglycemia) and below 60 mg/dL (hypoglycemia). Comparable data from a previous cohort study of 91 GMS patients were used as baseline data for comparisons with the results of the present study.9
Other patient data collected included age, sex, weight, baseline A1C (taken at or within 6 months of admission), diabetic medications used prior to admission (none, oral agents only, or any insulin use); daily inpatient use of oral or intravenous steroids, oral diabetic medications, dextrose‐containing intravenous fluids, tube feeds, total parenteral nutrition, and general nutritional intake (nothing by mouth, clear diet, low carbohydrate diet, house diet).
Statistical Analysis
Characteristics of the study subjects and process and outcome measures were analyzed descriptively using rates, means, and standard deviations or medians with interquartile ranges as appropriate. Comparisons between the pilot study and baseline cohorts were performed using Fisher's exact test for dichotomous outcomes (eg, use of basal insulin). For rates of hyperglycemia (ie, fraction of readings > 180 mg/dL), we used binomial logistic regression, accounting for potential correlation among repeated events by individual patients with a dispersion parameter18 (note that we did not use the same analysis for rates of hypoglycemia because it was such a rare event; for analysis of hypoglycemia, the variables were dichotomized). We also analyzed outcomes by hospital day (through hospital day 5, the limit used in the baseline study) to determine daily trends during the course of hospitalization; for these analyses we used the Mantel‐Haenszel chi‐square test for dichotomous variables and binomial logistic regression with hospital day as the independent variable for rates of hyperglycemia. Two‐sided P values < .05 were considered significant. SAS version 9.1 (Cary, NC) was used for all analyses.
RESULTS
After screening all 785 admissions to the 2 medical teams during the study period, we prospectively identified 109 patients (14%) for the pilot study. Twenty patients were subsequently excluded: 7 patients who were discharged the same day they were identified, 4 who did not have a fasting blood glucose value greater than 140 mg/dL, 4 patients who had type 1 diabetes, 2 patients who were admitted with diabetic ketoacidosis, and 3 patients whose data could not be accessed because of repeated unavailability of the medical record. Characteristics of the remaining 89 study subjects are shown in Table 2 and are compared to 91 baseline subjects. The mean age of the study subjects was 68.7 years; 45% were men. Five patients (6%) did not have a previous diagnosis of diabetes, and 51% were taking insulin prior to admission; the median A1C was 6.8%.
| Characteristic | Baseline (n = 91) | Pilot (n = 89) |
|---|---|---|
| ||
| Age (years), mean (SD) | 66.0 (14.5) | 68.7 (14.7) |
| Male | 53/91 (58%) | 40/89 (45%) |
| No diagnosis of diabetes at admission | 7/91 (8%) | 5/89 (6%) |
| Preadmission diabetes regimen | ||
| None | 15/91 (16%) | 14/78 (18%) |
| Oral medications only | 32/91 (35%) | 24/78 (31%) |
| Insulin | 44/91 (48%) | 40/78 (51%) |
| A1C (IQR) | 7.0 (6.0, 8.0) | 6.8 (6.3, 7.8) |
| Hospital length of stay (days), median (IQR) | 5 (3, 7) | 5 (3, 7) |
The medical residents agreed, at least in theory, to follow the subcutaneous insulin protocol for 50 patients (56%), partially accepted it for 8 (9%), and declined for 31 (35%). Reasons for declining the protocol included fear of hypoglycemia, severity of patient's other disease states or overall poor health of patient, concern for the effects of renal insufficiency on insulin clearance, concern for the effect of steroid tapers on glucose levels, desire to titrate oral medications, and anticipation of patient's imminent discharge. Other reasons such as the glucose levels are not that bad and let's watch the glucose levels for one more day suggest that some residents did not view hyperglycemia as an acute problem requiring immediate attention.
Regarding insulin‐ordering practices (Table 3), basal insulin was prescribed for 57 patients (64%) in the pilot group compared to 45 patients (49%) in the baseline group (P = .05). Nutritional insulin was prescribed to 12 patients (13%) in the pilot group compared to no patients in the baseline group (P < .001). Oral hypoglycemic agents were prescribed less often in the pilot study than at baseline (20% vs. 38%, P = .01). The use of a standard default sliding scale from the hospital computer order set was high and was not significantly different in the pilot study compared with that at baseline (93% vs. 90%, P = .78). Twenty‐four of the 83 patients in the pilot group (29%) received sliding‐scale insulin without ever receiving basal or nutritional insulin during hospitalization compared to 45 of 91 patients in the baseline group (49%; P = .01 for comparison). Among patients started on basal insulin, 42% (24 of 57) were started after the first full hospital day. The initial basal insulin dose was appropriate according to the protocol (within 20%) in 38 of 57 patients (67%). Only 20 of 61 patients (33%) who had any hypo‐ or hyperglycemia had any change to their insulin regimen made during days 2 through 7 of their hospitalization on GMS, similar to the rate noted at baseline (36%).
| Measure | Baseline | Pilot | P value |
|---|---|---|---|
| |||
| Process | |||
| Any basal insulin during hospitalization | 45/91 (49%) | 57/89 (64%) | 0.05 |
| Any nutritional insulin during hospitalization | 0/91 (0%) | 12/89 (13%) | < 0.001 |
| Change in dose to any insulin order during hospitalization | 24/66 (36%) | 20/61 (33%) | 0.71 |
| Standard sliding scale from hospital computer order set | 75/83 (90%) | 76/82 (93%) | 0.78 |
| Any oral antihyperglycemic agents during hospitalization | 35/91 (38%) | 18/89 (20%) | 0.01 |
| Outcome | |||
| Mean percentage of glucose readings > 180 mg/dL (SD) | 33.3% (33.3%) | 31.6% (29.6%) | 0.85 |
| Any hyperglycemia (glucose > 180 mg/dL) | 66/89 (74%) | 59/78 (76%) | 0.86 |
| 1%‐20% of readings | 17/89 (19%) | 15/78 (19%) | 0.85 for trend |
| 20%‐40% | 15/89 (17%) | 15/78 (19%) | |
| 40%‐60% | 15/89 (17%) | 15/78 (19%) | |
| 60%‐80% | 7/89 (8%) | 6/78 (8%) | |
| >80% | 12/89 (13%) | 8/78 (10%) | |
| Any hypoglycemia (glucose < 60 mg/dL) | 6/89 (7%) | 10/78 (13%) | 0.20 |
Regarding glucose control (Table 3), the mean percentage of glucose readings per patient greater than 180 mg/dL was not significantly different in the pilot study compared to baseline (31.6% vs. 33.3%, P = .85). Despite implementation of the protocol and increased use of basal and nutritional insulin, 76% of patients had at least 1 routine glucose reading greater than 180 mg/dL, and 37% of patients had at least 40% of their routine glucose readings greater than 180 mg/dL, comparable to baseline (74% and 38%, respectively, P = NS for both comparisons). At least 1 hypoglycemic event (glucose reading below 60 mg/dL) occurred in 7% of patients at baseline and 13% during the pilot study (P = .20). Eleven hypoglycemic events in the pilot study were between 50 and 59 mg/dL (55%), 6 were between 40 and 49 mg/dL (30%), 3 were between 30 and 39 mg/dL (15%), and none were less than 30 mg/dL. Nine occurred before breakfast (45%), 5 before dinner (25%), 3 before lunch (15%), and 3 at bedtime (15%).
During the pilot study, the use of basal insulin did improve over the first 5 days of hospitalization (Fig. 1), in both the percentage of patients prescribed any basal insulin and the percentage of each patient's total insulin dose (basal, nutritional, and supplemental) given as basal (both P < .001 for trend). Hyperglycemia rates also improved during hospitalization (Fig. 1), decreasing from 48% on hospital day 1 to 34% on hospital day 5 (P = .004 for trend). These trends were not observed in the baseline group, with hyperglycemia rates of 37% on hospital day 1 and 34% on hospital day 5 (P = .16 for trend).
Patients for whom the resident accepted or partially accepted the protocol had higher use of basal insulin (91% vs. 13%, P < .0001), higher use of nutritional insulin (21% vs. 0%, P = .01), and more frequent dose adjustments (47% vs. 7%, P = .01) compared with patients for whom the resident declined the protocol. However, the rate of hyperglycemia was higher in patients for whom the protocol was accepted or partially accepted than in patients for whom the protocol was declined (37% vs. 20%, P = .02).
DISCUSSION
Our subcutaneous insulin protocol focused on increasing the use of basal and nutritional insulin, avoiding the use of sliding‐scale insulin by itself, and performing daily insulin adjustments in response to the hypo‐ or hyperglycemia of general medical inpatients with diabetes or hyperglycemia.
The most notable finding of our pilot study was that residents were resistant to using the protocol, both in general and in its specific recommendations. Despite receiving education about inpatient diabetes control and protocol recommendations from the team pharmacist, and despite being on a hospitalist‐run medical service, the residents accepted use of the protocol for only half the eligible patients. Patients who were started on basal insulin were often underdosed or started after the first day of hospitalization, and daily dose adjustments were not consistently made despite persistent hypo‐ or hyperglycemia. Although the use of nutritional insulin was greater compared with that in the baseline group, it was still only prescribed for 13% of patients. Use of a standard sliding scale from the hospital computer order set was common in the pilot study and similar to that in the baseline group. These results suggest significant resistance to changing the current standard of practice.
Despite this lack of adherence to the protocol, some modest improvements in processes of care were seen. Basal insulin was ordered more often during the pilot study than at baseline, especially over the course of a hospital stay. Nutritional insulin was also ordered more often during the pilot study than at baseline, but was still infrequent. Oral antihyperglycemic agents were ordered less often during the pilot study than at baseline. This demonstrates that use of the protocol may be able to improve process outcomes. However, the modest improvements in process outcomes could have simply been a result of increased awareness and education, not the protocol itself.
Regarding patient outcomes, the overall hyperglycemia rate did not improve in the pilot study relative to that at baseline. Importantly, hypoglycemia rates did not increase significantly compared with those at baseline. However, because of the small number of hypoglycemia events, the sample size may not have been sufficient to detect a true difference between groups.
The most likely reason that the protocol did not show an effect on glycemic control was that its recommendations were not adhered to. In turn, this may have been a result of incomplete education, training, and implementation measures and/or inherent problems with the protocol that made its recommendations difficult to follow. Another possibility is that the protocol itself may not have been capable of improving glucose control, even when properly used. However, we do know that resident agreement to use the protocol did lead to higher rates of recommended best practices being carried out, such as basal insulin use and daily insulin dose adjustments, and that use of the protocol was associated with improvements in glucose control over the hospital stay. A larger study with a higher degree of protocol adherence would be better able to evaluate the merits of the protocol itself, as would a randomized controlled trial using instrumental variables to measure treatment efficacy. Another possibility explanation for the lack of effect is that glucose control on admission happened to be worse in the pilot group than in the control group: rates of hyperglycemia on day 1 were 48% in the pilot group compared with 37% in the baseline group (Fig. 1). Also, the decreased use of oral agents in the pilot group, a purposeful change to decrease the risk of hypoglycemia, may have counteracted the beneficial effects of more appropriate insulin use. Finally, there were few patients with poorly controlled diabetes at baseline (18 patients with A1C 8.0 in the baseline group and 12 such patients in the pilot group), arguably those most likely to benefit.
There is a pressing need to identify protocols that can improve glucose control in the non‐ICU inpatient setting and successfully implement these protocols with a minimum of resources and effort. To date, most studies that have improved glucose control in the non‐ICU setting have relied on frequent input from diabetologists or nurse‐practitioners.14, 15
The results of this study should be viewed in light of its limitations, including its relatively small sample size (thus limiting our ability to detect possible significant differences between groups) and that it was conducted at a single institution (thus limiting its generalizability). Patients were enrolled on weekdays, so patients admitted and discharged over a weekend or on a holiday may have been missed. Also, because of the nonrandomized design of the study, we cannot exclude the possibility that the improvements noted in the pilot study were a result of the increased education provided or of increased awareness and general improvement in diabetes management over the course of the study. Finally, implementation of the protocol was somewhat labor intensive and required staff support that could be difficult to replicate in other institutions. However, most of the study staff's effort was necessary either to implement the protocol in the absence of an order set or to evaluate barriers to implementation. Widespread implementation of a protocol with an order set, education, and the use of highly reliable tools should be possible with much less effort and resources. The strengths of this study include its prospective data collection methods, which included rigorous inclusion criteria and collection of detailed clinical data.
Our study findings suggest several approaches to improve care in the future. To combat resistance to change, the American Association of Clinical Endocrinologists strongly recommends that each institution ensure that all its clinicians involved agree about general philosophies of diabetes management.19 A more expansive, hospital‐wide educational and promotional plan may increase the initial acceptance of the protocol. Interviews with residents also indicated there was unfamiliarity with diabetes management and significant concerns about the harmful affects of tight glucose control (ie, risk of hypoglycemia), especially in certain patient subgroups. These results confirmed the need for more practical individualized training and sparked the implementation of small‐group, case‐based educational sessions on inpatient diabetes management for all house officers, with a particular focus on patients with multiple comorbidities, on steroid tapers, and/or with renal failure.
The lack of nutritional insulin orders, delays in ordering basal insulin, and use of inadequate doses of insulin may be counteracted by the use of an order set, in our case built into our computer physician order entry (CPOE) system. The use of CPOE also allows reminders to be automatically sent to clinicians if eligible patients are not started on these orders. Clinical inertia (eg, failure to adjust the insulin doses of specific patients despite hyperglycemia) is more difficult to combat but may be addressed through better organization of clinical data, individualized, case‐based education, and CPOE reminders and eventually through culture change.
As a result of our pilot study, additional revisions were made to the protocol in hopes of increasing protocol adherence. For example, for patients eating discrete meals who are not taking insulin at home, the pilot protocol had suggested a starting insulin dose range for basal and nutritional insulin that required 2 separate calculations. The revised protocol was simplified to recommend a total daily insulin dose to be split evenly between basal and nutritional insulin. The daily adjustment instructions were also simplified. The pilot protocol had included a complicated table of adjustment recommendations based on bedside glucose trends. The revised protocol recommends adjusting the new daily dose by adding the total units of insulin given the previous day (including supplemental doses), making minor adjustments for hyper‐ or hypoglycemia and other clinical factors (like renal failure), and splitting this dose evenly between scheduled basal and nutritional insulin. In addition, 3 order sets were built into our computerized physician order entry system to facilitate early and appropriate insulin orders for patients with different diets (discrete meals, continuous tube feeds, and nothing by mouth); 3 different insulin sliding scales were created for patients with different degrees of insulin resistance; a diabetes management page for our electronic medication administration record is being developed to better organize clinical data; and hospital‐wide education and individualized training are ongoing.
In conclusion, the adherence to an inpatient glycemic management protocol that focused on increasing use of basal insulin and performing daily insulin adjustments was only fair. Barriers to successful implementation included clinical inertia regarding individual patients, unfamiliarity with inpatient diabetes management strategies, fear of hypoglycemia, and resistance to changing the current standard of practice. Targeted education, standard order sets, better organization of clinical data, protocol simplification, and institutional culture changes may be necessary for successful protocol implementation and improved inpatient glucose control.
- Agency for Healthcare Research and Quality. HCUPnet, Healthcare Cost and Utilization Project. 8/17/05; http://www.ahrq.gove/HCUPnet/. Accessed 7/17/06,2006.
- ,,,,,.Hyperglycemia: an independent marker of in‐hospital mortality in patients with undiagnosed diabetes.J Clin Endocrinol Metab.2002;87:978–982.
- ,,, et al.Hyperglycaemia is associated with poor outcomes in patients admitted to hospital with acute exacerbations of chronic obstructive pulmonary disease.Thorax2006;61:284–289.
- ,,,,.Stress hyperglycemia and prognosis of stroke in nondiabetic and diabetic patients: a systematic overview.Stroke.2001;32:2426–2432.
- ,,,.Hyperglycemia is associated with adverse outcomes in patients receiving total parenteral nutrition.Diabetes Care.2005;28:2367–2371.
- ,,, et al.Management of diabetes and hyperglycemia in hospitals.Diabetes Care.2004;27:553–597.
- ,,,,,.The relation between hyperglycemia and outcomes in 2,471 patients admitted to the hospital with community‐acquired pneumonia.Diabetes Care.2005;28:810–815.
- ,,.Glycemic control and sliding scale insulin use in medical inpatients with diabetes mellitus.Arch Intern Med.1997;157:545–552.
- ,,,,.Inpatient management of diabetes and hyperglycemia among general medicine patients at a large teaching hospital.J Hosp Med.2006;1:145–150.
- ,,, et al.Intensive insulin therapy in the medical ICU.N Engl J Med.2006;354:449–461.
- ,,, et al.Intensive insulin therapy in the critically ill patients.N Engl J Med.2001;345:1359–1367.
- .Prospective randomised study of intensive insulin treatment on long term survival after acute myocardial infarction in patients with diabetes mellitus.DIGAMI (Diabetes Mellitus, Insulin Glucose Infusion in Acute Myocardial Infarction) Study Group.BMJ.1997;314:1512–1515.
- ,,, et al.Intense metabolic control by means of insulin in patients with diabetes mellitus and acute myocardial infarction (DIGAMI 2): effects on mortality and morbidity.Eur Heart J.2005;26:650–661.
- American Diabetes Association.Standards of Medical Care in Diabetes ‐ 2006.Diabetes Care.2006;29:S4–S42.
- ACE/ADA Task Force on Inpatient Diabetes.American College of Endocrinology and American Diabetes Association consensus statement on inpatient diabetes and glycemic control: A call to action.Diabetes Care.2006;29:1955–1962.
- ,,,.Eliminating inpatient sliding‐scale insulin: a reeducation project with medical house staff.Diabetes Care.2005;28:1008–11.
- ,,, et al.Randomized study of basal‐bolus insulin therapy in the inpatient management of patients with type 2 diabetes (RABBIT 2 Trial).Diabetes Care.2007;30:2181–2186.
- .Extra‐binomial variation in logistic linear models.Appl Stat.1982;31:144–148.
- ,.Hospital management of diabetes.Endocrinol Metab Clin North Am.2005;34:99–116.
Diabetes mellitus is a common comorbid condition in hospitalized patients. In 2003, diabetes was listed as a diagnosis in 17.2% of hospital discharges in the United States.1 Because these diagnosis codes do not account for undiagnosed diabetes or hospital‐related hyperglycemia, the true prevalence of diabetes or hyperglycemia in hospitalized patients is likely higher and has been estimated to be as great as 38%.2 Hyperglycemia has been associated with adverse outcomes among hospitalized patients, including infectious complications, increased length of stay, and increased mortality.27 However, because hyperglycemia is not usually the primary reason patients with diabetes are hospitalized, its management is often not a focus in the inpatient setting. Sliding‐scale insulin alone continues to be commonly prescribed despite clinical evidence showing it to be ineffective in achieving glycemic control.8, 9
Recent randomized controlled trials have demonstrated that aggressive treatment of inpatient hyperglycemia improves outcomes in surgical and medical intensive care units10, 11 and in patients admitted for myocardial infarction.12, 13 Based on this clinical evidence and strong observational data linking hyperglycemia to poor patient outcomes in the non‐ICU setting,27 the American Diabetes Association (ADA) now advocates good metabolic control, defined as preprandial glucose levels of 90‐130 mg/dL and peak postprandial glucose levels < 180 mg/dL in hospitalized non‐ICU patients with hyperglycemia14 (note that these targets are less aggressive than those for ICU patients, for whom randomized controlled trials showed the benefits of reduced mortality provided by tight glucose control).11 To reach these targets, the ADA and American College of Endocrinology suggest that multidisciplinary teams develop and implement hyperglycemia management guidelines and protocols.15 Protocols should promote the use of continuous intravenous insulin or scheduled subcutaneous insulin as opposed to the use of sliding‐scale insulin alone. Subcutaneous insulin protocols should include target glucose levels; basal, nutritional, and supplemental insulin; and daily adjustments based on previous glucose levels, insulin sensitivity, nutritional intake, illness, and medications.6, 15 To date, few published protocols or algorithms for inpatient subcutaneous insulin have been shown to be effective.16, 17 It is therefore not known how best to design and implement an inpatient diabetes management protocol that is effective, efficient, and self‐perpetuating. The aims of our pilot study were to develop and implement a subcutaneous insulin protocol on a general medicine service, to identify barriers to implementation, and to determine the effect of this protocol on glycemic control.
METHODS
Setting and Participants
This prospective quality‐improvement pilot study was conducted at Brigham and Women's Hospital (BWH) from January 10, 2005, through June 23, 2005. Patients were eligible to participate if they were admitted to either of 2 General Medicine Service (GMS) teams with either a known diagnosis of type 2 diabetes or inpatient hyperglycemia (random laboratory glucose level > 180 mg/dL) and at least 1 fasting point‐of‐care glucose reading > 140 mg/dL. Patients were excluded if they had diabetic ketoacidosis, hyperosmolar hyperglycemic state, another absolute indication for intravenous insulin, or fasting glucose < 60 mg/dL on no insulin or if they were pregnant. Each GMS team consisted of a teaching attending, a junior or senior resident, 2 interns, and a clinical pharmacist. Twenty‐six physicians attended on these 2 teams during the study period, 13 of whom were hospitalists. This study was approved by the BWH Institutional Review Board; patient consent to participate in this study was deemed not necessary because of the relatively nonsensitive nature of the data (eg, glucose control, insulin orders), the noninvasive means of data collection (eg, chart review), and the steps taken by research personnel to minimize any breach in patient confidentiality.
Intervention
A multidisciplinary team composed of a diabetologist (M.L.P.), a hospitalist (J.L.S.), and a pharmacist (J.M.T.) developed a subcutaneous insulin protocol that was approved by the BWH Pharmacy and Therapeutics Diabetes Subcommittee. The protocol consisted of a set of treatment recommendations made by a pharmacist to be carried out by the medical team. The primary components are shown in Table 1 (a full description can be found in the Appendix). The main emphasis of the protocol was on discontinuing oral antihyperglycemic agents during hospitalization, initiating basal insulin in most patients, and adjusting basal insulin daily as needed.
|
| Oral agents |
| 1. Stop oral agents in most patients |
| Glucose testing |
| 2. Check bedside blood glucose before meals and at bedtime if eating, or every 6 hours if not eating |
| Insulin |
| 3. Start basal insulin Patient's home dose or NPH 0.1 units/kg before breakfast and at bedtime or insulin glargine 0.2 units/kg at bedtime (max dose 20 units) If NPO, consider half dose unless hyperglycemic |
| 4. Start nutritional insulin Discrete meals: insulin aspart 0.05‐0.1 units/kg per meal or home dose 0‐15 minutes prior to eating Continuous tube feeds: regular insulin every 6 hours or NPH every morning and at bedtime (0.1‐0.2 units/kg per day in addition to basal insulin) Hold if NPO |
| 5. Start correctional insulin Scale provided based on blood glucose and daily scheduled insulin requirements |
| Daily Adjustments |
6. Adjust scheduled insulin daily
|
| Other Considerations |
| 7. Hypoglycemia management (protocols for fruit juice, glucagons, IV dextrose, and when to call physician) |
| 8. Discharge orders (recommendations to discharge most patients on admission medication regimen, avoid sliding scale insulin, simplify dosing for patients requiring new insulin regimens, ensure adequate patient education and prompt outpatient follow‐up) |
All medical residents received general instructions regarding inpatient diabetes control by the research team's diabetologist (M.L.P.) through a 1‐hour department‐wide didactic lecture. The standards of care taught were identical to those in the protocol. In addition, the research team's hospitalist (J.L.S.) contacted each medical resident assigned to the 2 GMS teams electronically to introduce the protocol and describe the purpose and logistics of the pilot study.
A research assistant prospectively identified eligible patients each weekday by screening all patients admitted to the 2 GMS teams using the daily computerized sign‐out system used by all medical residents. Specifically, laboratory random glucose levels, inpatient medications, and medical history were reviewed to determine if each patient met eligibility criteria. Eligibility criteria were confirmed by medical record review. The pharmacist recommended to the primary team that the protocol be initiated for eligible patients. In addition, the pharmacist recommended daily adjustment of the insulin dose according to the protocol as appropriate. A chronologically organized summary of clinical data relevant to glycemic management for each patient, including bedside blood glucose measurements, general dietary intake, use of intravenous dextrose solutions, and administration of systemic steroids, oral diabetes medications, and all insulins, was provided to the team each day by the research assistant.
Measurements
The resident's acceptance of the protocol or reasons for declining it were recorded by the pharmacist on the day the protocol was recommended. Protocol acceptance was categorized as yes, no, or partial. Partial acceptance was defined as resident agreement to use the protocol, but with stated caveats or modifications. Clinical data were collected on each eligible patient for up to 7 days on GMS. Several data sources were used, including physician admission notes, the hospital's computerized clinical data system, vital‐sign sheets, medication administration records, and personal communication with nurses regarding any missing or discrepant data.
All insulin use (prescribed drug, dose, route, schedule and actual administered drug, dose, route, and time) was recorded each day by the research assistant. Use of basal and nutritional insulin and daily dose adjustments if previous hypo‐ or hyperglycemia (categorized as yes, no, or not applicable for each patient each day) were determined by the study pharmacist (J.M.T.) through retrospective review of all orders.
Up to 4 routine bedside blood glucose measurements were recorded each day: for patients eating discrete meals, these were the measurements taken before meals and at bedtime; for patients not eating or receiving continuous nutrition, these were the measurements taken closest to 6 AM, noon, 6 PM, and midnight. Additional measurements were not recorded to avoid ascertainment bias caused by follow‐up testing of abnormal glucose values. Glucose readings on the day of admission were excluded from analysis because these values are not amenable to inpatient ordering practices.
Study outcomes included overall protocol acceptance rate, insulin prescribing practices including use of basal insulin (ie, long‐acting agents such as NPH and insulin glargine), nutritional insulin (ie, scheduled regular, lispro, or aspart insulin given before each meal), daily dose adjustments under the protocol, and mean percentage of glucose readings per person greater than 180 mg/dL (hyperglycemia) and below 60 mg/dL (hypoglycemia). Comparable data from a previous cohort study of 91 GMS patients were used as baseline data for comparisons with the results of the present study.9
Other patient data collected included age, sex, weight, baseline A1C (taken at or within 6 months of admission), diabetic medications used prior to admission (none, oral agents only, or any insulin use); daily inpatient use of oral or intravenous steroids, oral diabetic medications, dextrose‐containing intravenous fluids, tube feeds, total parenteral nutrition, and general nutritional intake (nothing by mouth, clear diet, low carbohydrate diet, house diet).
Statistical Analysis
Characteristics of the study subjects and process and outcome measures were analyzed descriptively using rates, means, and standard deviations or medians with interquartile ranges as appropriate. Comparisons between the pilot study and baseline cohorts were performed using Fisher's exact test for dichotomous outcomes (eg, use of basal insulin). For rates of hyperglycemia (ie, fraction of readings > 180 mg/dL), we used binomial logistic regression, accounting for potential correlation among repeated events by individual patients with a dispersion parameter18 (note that we did not use the same analysis for rates of hypoglycemia because it was such a rare event; for analysis of hypoglycemia, the variables were dichotomized). We also analyzed outcomes by hospital day (through hospital day 5, the limit used in the baseline study) to determine daily trends during the course of hospitalization; for these analyses we used the Mantel‐Haenszel chi‐square test for dichotomous variables and binomial logistic regression with hospital day as the independent variable for rates of hyperglycemia. Two‐sided P values < .05 were considered significant. SAS version 9.1 (Cary, NC) was used for all analyses.
RESULTS
After screening all 785 admissions to the 2 medical teams during the study period, we prospectively identified 109 patients (14%) for the pilot study. Twenty patients were subsequently excluded: 7 patients who were discharged the same day they were identified, 4 who did not have a fasting blood glucose value greater than 140 mg/dL, 4 patients who had type 1 diabetes, 2 patients who were admitted with diabetic ketoacidosis, and 3 patients whose data could not be accessed because of repeated unavailability of the medical record. Characteristics of the remaining 89 study subjects are shown in Table 2 and are compared to 91 baseline subjects. The mean age of the study subjects was 68.7 years; 45% were men. Five patients (6%) did not have a previous diagnosis of diabetes, and 51% were taking insulin prior to admission; the median A1C was 6.8%.
| Characteristic | Baseline (n = 91) | Pilot (n = 89) |
|---|---|---|
| ||
| Age (years), mean (SD) | 66.0 (14.5) | 68.7 (14.7) |
| Male | 53/91 (58%) | 40/89 (45%) |
| No diagnosis of diabetes at admission | 7/91 (8%) | 5/89 (6%) |
| Preadmission diabetes regimen | ||
| None | 15/91 (16%) | 14/78 (18%) |
| Oral medications only | 32/91 (35%) | 24/78 (31%) |
| Insulin | 44/91 (48%) | 40/78 (51%) |
| A1C (IQR) | 7.0 (6.0, 8.0) | 6.8 (6.3, 7.8) |
| Hospital length of stay (days), median (IQR) | 5 (3, 7) | 5 (3, 7) |
The medical residents agreed, at least in theory, to follow the subcutaneous insulin protocol for 50 patients (56%), partially accepted it for 8 (9%), and declined for 31 (35%). Reasons for declining the protocol included fear of hypoglycemia, severity of patient's other disease states or overall poor health of patient, concern for the effects of renal insufficiency on insulin clearance, concern for the effect of steroid tapers on glucose levels, desire to titrate oral medications, and anticipation of patient's imminent discharge. Other reasons such as the glucose levels are not that bad and let's watch the glucose levels for one more day suggest that some residents did not view hyperglycemia as an acute problem requiring immediate attention.
Regarding insulin‐ordering practices (Table 3), basal insulin was prescribed for 57 patients (64%) in the pilot group compared to 45 patients (49%) in the baseline group (P = .05). Nutritional insulin was prescribed to 12 patients (13%) in the pilot group compared to no patients in the baseline group (P < .001). Oral hypoglycemic agents were prescribed less often in the pilot study than at baseline (20% vs. 38%, P = .01). The use of a standard default sliding scale from the hospital computer order set was high and was not significantly different in the pilot study compared with that at baseline (93% vs. 90%, P = .78). Twenty‐four of the 83 patients in the pilot group (29%) received sliding‐scale insulin without ever receiving basal or nutritional insulin during hospitalization compared to 45 of 91 patients in the baseline group (49%; P = .01 for comparison). Among patients started on basal insulin, 42% (24 of 57) were started after the first full hospital day. The initial basal insulin dose was appropriate according to the protocol (within 20%) in 38 of 57 patients (67%). Only 20 of 61 patients (33%) who had any hypo‐ or hyperglycemia had any change to their insulin regimen made during days 2 through 7 of their hospitalization on GMS, similar to the rate noted at baseline (36%).
| Measure | Baseline | Pilot | P value |
|---|---|---|---|
| |||
| Process | |||
| Any basal insulin during hospitalization | 45/91 (49%) | 57/89 (64%) | 0.05 |
| Any nutritional insulin during hospitalization | 0/91 (0%) | 12/89 (13%) | < 0.001 |
| Change in dose to any insulin order during hospitalization | 24/66 (36%) | 20/61 (33%) | 0.71 |
| Standard sliding scale from hospital computer order set | 75/83 (90%) | 76/82 (93%) | 0.78 |
| Any oral antihyperglycemic agents during hospitalization | 35/91 (38%) | 18/89 (20%) | 0.01 |
| Outcome | |||
| Mean percentage of glucose readings > 180 mg/dL (SD) | 33.3% (33.3%) | 31.6% (29.6%) | 0.85 |
| Any hyperglycemia (glucose > 180 mg/dL) | 66/89 (74%) | 59/78 (76%) | 0.86 |
| 1%‐20% of readings | 17/89 (19%) | 15/78 (19%) | 0.85 for trend |
| 20%‐40% | 15/89 (17%) | 15/78 (19%) | |
| 40%‐60% | 15/89 (17%) | 15/78 (19%) | |
| 60%‐80% | 7/89 (8%) | 6/78 (8%) | |
| >80% | 12/89 (13%) | 8/78 (10%) | |
| Any hypoglycemia (glucose < 60 mg/dL) | 6/89 (7%) | 10/78 (13%) | 0.20 |
Regarding glucose control (Table 3), the mean percentage of glucose readings per patient greater than 180 mg/dL was not significantly different in the pilot study compared to baseline (31.6% vs. 33.3%, P = .85). Despite implementation of the protocol and increased use of basal and nutritional insulin, 76% of patients had at least 1 routine glucose reading greater than 180 mg/dL, and 37% of patients had at least 40% of their routine glucose readings greater than 180 mg/dL, comparable to baseline (74% and 38%, respectively, P = NS for both comparisons). At least 1 hypoglycemic event (glucose reading below 60 mg/dL) occurred in 7% of patients at baseline and 13% during the pilot study (P = .20). Eleven hypoglycemic events in the pilot study were between 50 and 59 mg/dL (55%), 6 were between 40 and 49 mg/dL (30%), 3 were between 30 and 39 mg/dL (15%), and none were less than 30 mg/dL. Nine occurred before breakfast (45%), 5 before dinner (25%), 3 before lunch (15%), and 3 at bedtime (15%).
During the pilot study, the use of basal insulin did improve over the first 5 days of hospitalization (Fig. 1), in both the percentage of patients prescribed any basal insulin and the percentage of each patient's total insulin dose (basal, nutritional, and supplemental) given as basal (both P < .001 for trend). Hyperglycemia rates also improved during hospitalization (Fig. 1), decreasing from 48% on hospital day 1 to 34% on hospital day 5 (P = .004 for trend). These trends were not observed in the baseline group, with hyperglycemia rates of 37% on hospital day 1 and 34% on hospital day 5 (P = .16 for trend).

Patients for whom the resident accepted or partially accepted the protocol had higher use of basal insulin (91% vs. 13%, P < .0001), higher use of nutritional insulin (21% vs. 0%, P = .01), and more frequent dose adjustments (47% vs. 7%, P = .01) compared with patients for whom the resident declined the protocol. However, the rate of hyperglycemia was higher in patients for whom the protocol was accepted or partially accepted than in patients for whom the protocol was declined (37% vs. 20%, P = .02).
DISCUSSION
Our subcutaneous insulin protocol focused on increasing the use of basal and nutritional insulin, avoiding the use of sliding‐scale insulin by itself, and performing daily insulin adjustments in response to the hypo‐ or hyperglycemia of general medical inpatients with diabetes or hyperglycemia.
The most notable finding of our pilot study was that residents were resistant to using the protocol, both in general and in its specific recommendations. Despite receiving education about inpatient diabetes control and protocol recommendations from the team pharmacist, and despite being on a hospitalist‐run medical service, the residents accepted use of the protocol for only half the eligible patients. Patients who were started on basal insulin were often underdosed or started after the first day of hospitalization, and daily dose adjustments were not consistently made despite persistent hypo‐ or hyperglycemia. Although the use of nutritional insulin was greater compared with that in the baseline group, it was still only prescribed for 13% of patients. Use of a standard sliding scale from the hospital computer order set was common in the pilot study and similar to that in the baseline group. These results suggest significant resistance to changing the current standard of practice.
Despite this lack of adherence to the protocol, some modest improvements in processes of care were seen. Basal insulin was ordered more often during the pilot study than at baseline, especially over the course of a hospital stay. Nutritional insulin was also ordered more often during the pilot study than at baseline, but was still infrequent. Oral antihyperglycemic agents were ordered less often during the pilot study than at baseline. This demonstrates that use of the protocol may be able to improve process outcomes. However, the modest improvements in process outcomes could have simply been a result of increased awareness and education, not the protocol itself.
Regarding patient outcomes, the overall hyperglycemia rate did not improve in the pilot study relative to that at baseline. Importantly, hypoglycemia rates did not increase significantly compared with those at baseline. However, because of the small number of hypoglycemia events, the sample size may not have been sufficient to detect a true difference between groups.
The most likely reason that the protocol did not show an effect on glycemic control was that its recommendations were not adhered to. In turn, this may have been a result of incomplete education, training, and implementation measures and/or inherent problems with the protocol that made its recommendations difficult to follow. Another possibility is that the protocol itself may not have been capable of improving glucose control, even when properly used. However, we do know that resident agreement to use the protocol did lead to higher rates of recommended best practices being carried out, such as basal insulin use and daily insulin dose adjustments, and that use of the protocol was associated with improvements in glucose control over the hospital stay. A larger study with a higher degree of protocol adherence would be better able to evaluate the merits of the protocol itself, as would a randomized controlled trial using instrumental variables to measure treatment efficacy. Another possibility explanation for the lack of effect is that glucose control on admission happened to be worse in the pilot group than in the control group: rates of hyperglycemia on day 1 were 48% in the pilot group compared with 37% in the baseline group (Fig. 1). Also, the decreased use of oral agents in the pilot group, a purposeful change to decrease the risk of hypoglycemia, may have counteracted the beneficial effects of more appropriate insulin use. Finally, there were few patients with poorly controlled diabetes at baseline (18 patients with A1C 8.0 in the baseline group and 12 such patients in the pilot group), arguably those most likely to benefit.
There is a pressing need to identify protocols that can improve glucose control in the non‐ICU inpatient setting and successfully implement these protocols with a minimum of resources and effort. To date, most studies that have improved glucose control in the non‐ICU setting have relied on frequent input from diabetologists or nurse‐practitioners.14, 15
The results of this study should be viewed in light of its limitations, including its relatively small sample size (thus limiting our ability to detect possible significant differences between groups) and that it was conducted at a single institution (thus limiting its generalizability). Patients were enrolled on weekdays, so patients admitted and discharged over a weekend or on a holiday may have been missed. Also, because of the nonrandomized design of the study, we cannot exclude the possibility that the improvements noted in the pilot study were a result of the increased education provided or of increased awareness and general improvement in diabetes management over the course of the study. Finally, implementation of the protocol was somewhat labor intensive and required staff support that could be difficult to replicate in other institutions. However, most of the study staff's effort was necessary either to implement the protocol in the absence of an order set or to evaluate barriers to implementation. Widespread implementation of a protocol with an order set, education, and the use of highly reliable tools should be possible with much less effort and resources. The strengths of this study include its prospective data collection methods, which included rigorous inclusion criteria and collection of detailed clinical data.
Our study findings suggest several approaches to improve care in the future. To combat resistance to change, the American Association of Clinical Endocrinologists strongly recommends that each institution ensure that all its clinicians involved agree about general philosophies of diabetes management.19 A more expansive, hospital‐wide educational and promotional plan may increase the initial acceptance of the protocol. Interviews with residents also indicated there was unfamiliarity with diabetes management and significant concerns about the harmful affects of tight glucose control (ie, risk of hypoglycemia), especially in certain patient subgroups. These results confirmed the need for more practical individualized training and sparked the implementation of small‐group, case‐based educational sessions on inpatient diabetes management for all house officers, with a particular focus on patients with multiple comorbidities, on steroid tapers, and/or with renal failure.
The lack of nutritional insulin orders, delays in ordering basal insulin, and use of inadequate doses of insulin may be counteracted by the use of an order set, in our case built into our computer physician order entry (CPOE) system. The use of CPOE also allows reminders to be automatically sent to clinicians if eligible patients are not started on these orders. Clinical inertia (eg, failure to adjust the insulin doses of specific patients despite hyperglycemia) is more difficult to combat but may be addressed through better organization of clinical data, individualized, case‐based education, and CPOE reminders and eventually through culture change.
As a result of our pilot study, additional revisions were made to the protocol in hopes of increasing protocol adherence. For example, for patients eating discrete meals who are not taking insulin at home, the pilot protocol had suggested a starting insulin dose range for basal and nutritional insulin that required 2 separate calculations. The revised protocol was simplified to recommend a total daily insulin dose to be split evenly between basal and nutritional insulin. The daily adjustment instructions were also simplified. The pilot protocol had included a complicated table of adjustment recommendations based on bedside glucose trends. The revised protocol recommends adjusting the new daily dose by adding the total units of insulin given the previous day (including supplemental doses), making minor adjustments for hyper‐ or hypoglycemia and other clinical factors (like renal failure), and splitting this dose evenly between scheduled basal and nutritional insulin. In addition, 3 order sets were built into our computerized physician order entry system to facilitate early and appropriate insulin orders for patients with different diets (discrete meals, continuous tube feeds, and nothing by mouth); 3 different insulin sliding scales were created for patients with different degrees of insulin resistance; a diabetes management page for our electronic medication administration record is being developed to better organize clinical data; and hospital‐wide education and individualized training are ongoing.
In conclusion, the adherence to an inpatient glycemic management protocol that focused on increasing use of basal insulin and performing daily insulin adjustments was only fair. Barriers to successful implementation included clinical inertia regarding individual patients, unfamiliarity with inpatient diabetes management strategies, fear of hypoglycemia, and resistance to changing the current standard of practice. Targeted education, standard order sets, better organization of clinical data, protocol simplification, and institutional culture changes may be necessary for successful protocol implementation and improved inpatient glucose control.
Diabetes mellitus is a common comorbid condition in hospitalized patients. In 2003, diabetes was listed as a diagnosis in 17.2% of hospital discharges in the United States.1 Because these diagnosis codes do not account for undiagnosed diabetes or hospital‐related hyperglycemia, the true prevalence of diabetes or hyperglycemia in hospitalized patients is likely higher and has been estimated to be as great as 38%.2 Hyperglycemia has been associated with adverse outcomes among hospitalized patients, including infectious complications, increased length of stay, and increased mortality.27 However, because hyperglycemia is not usually the primary reason patients with diabetes are hospitalized, its management is often not a focus in the inpatient setting. Sliding‐scale insulin alone continues to be commonly prescribed despite clinical evidence showing it to be ineffective in achieving glycemic control.8, 9
Recent randomized controlled trials have demonstrated that aggressive treatment of inpatient hyperglycemia improves outcomes in surgical and medical intensive care units10, 11 and in patients admitted for myocardial infarction.12, 13 Based on this clinical evidence and strong observational data linking hyperglycemia to poor patient outcomes in the non‐ICU setting,27 the American Diabetes Association (ADA) now advocates good metabolic control, defined as preprandial glucose levels of 90‐130 mg/dL and peak postprandial glucose levels < 180 mg/dL in hospitalized non‐ICU patients with hyperglycemia14 (note that these targets are less aggressive than those for ICU patients, for whom randomized controlled trials showed the benefits of reduced mortality provided by tight glucose control).11 To reach these targets, the ADA and American College of Endocrinology suggest that multidisciplinary teams develop and implement hyperglycemia management guidelines and protocols.15 Protocols should promote the use of continuous intravenous insulin or scheduled subcutaneous insulin as opposed to the use of sliding‐scale insulin alone. Subcutaneous insulin protocols should include target glucose levels; basal, nutritional, and supplemental insulin; and daily adjustments based on previous glucose levels, insulin sensitivity, nutritional intake, illness, and medications.6, 15 To date, few published protocols or algorithms for inpatient subcutaneous insulin have been shown to be effective.16, 17 It is therefore not known how best to design and implement an inpatient diabetes management protocol that is effective, efficient, and self‐perpetuating. The aims of our pilot study were to develop and implement a subcutaneous insulin protocol on a general medicine service, to identify barriers to implementation, and to determine the effect of this protocol on glycemic control.
METHODS
Setting and Participants
This prospective quality‐improvement pilot study was conducted at Brigham and Women's Hospital (BWH) from January 10, 2005, through June 23, 2005. Patients were eligible to participate if they were admitted to either of 2 General Medicine Service (GMS) teams with either a known diagnosis of type 2 diabetes or inpatient hyperglycemia (random laboratory glucose level > 180 mg/dL) and at least 1 fasting point‐of‐care glucose reading > 140 mg/dL. Patients were excluded if they had diabetic ketoacidosis, hyperosmolar hyperglycemic state, another absolute indication for intravenous insulin, or fasting glucose < 60 mg/dL on no insulin or if they were pregnant. Each GMS team consisted of a teaching attending, a junior or senior resident, 2 interns, and a clinical pharmacist. Twenty‐six physicians attended on these 2 teams during the study period, 13 of whom were hospitalists. This study was approved by the BWH Institutional Review Board; patient consent to participate in this study was deemed not necessary because of the relatively nonsensitive nature of the data (eg, glucose control, insulin orders), the noninvasive means of data collection (eg, chart review), and the steps taken by research personnel to minimize any breach in patient confidentiality.
Intervention
A multidisciplinary team composed of a diabetologist (M.L.P.), a hospitalist (J.L.S.), and a pharmacist (J.M.T.) developed a subcutaneous insulin protocol that was approved by the BWH Pharmacy and Therapeutics Diabetes Subcommittee. The protocol consisted of a set of treatment recommendations made by a pharmacist to be carried out by the medical team. The primary components are shown in Table 1 (a full description can be found in the Appendix). The main emphasis of the protocol was on discontinuing oral antihyperglycemic agents during hospitalization, initiating basal insulin in most patients, and adjusting basal insulin daily as needed.
|
| Oral agents |
| 1. Stop oral agents in most patients |
| Glucose testing |
| 2. Check bedside blood glucose before meals and at bedtime if eating, or every 6 hours if not eating |
| Insulin |
| 3. Start basal insulin Patient's home dose or NPH 0.1 units/kg before breakfast and at bedtime or insulin glargine 0.2 units/kg at bedtime (max dose 20 units) If NPO, consider half dose unless hyperglycemic |
| 4. Start nutritional insulin Discrete meals: insulin aspart 0.05‐0.1 units/kg per meal or home dose 0‐15 minutes prior to eating Continuous tube feeds: regular insulin every 6 hours or NPH every morning and at bedtime (0.1‐0.2 units/kg per day in addition to basal insulin) Hold if NPO |
| 5. Start correctional insulin Scale provided based on blood glucose and daily scheduled insulin requirements |
| Daily Adjustments |
6. Adjust scheduled insulin daily
|
| Other Considerations |
| 7. Hypoglycemia management (protocols for fruit juice, glucagons, IV dextrose, and when to call physician) |
| 8. Discharge orders (recommendations to discharge most patients on admission medication regimen, avoid sliding scale insulin, simplify dosing for patients requiring new insulin regimens, ensure adequate patient education and prompt outpatient follow‐up) |
All medical residents received general instructions regarding inpatient diabetes control by the research team's diabetologist (M.L.P.) through a 1‐hour department‐wide didactic lecture. The standards of care taught were identical to those in the protocol. In addition, the research team's hospitalist (J.L.S.) contacted each medical resident assigned to the 2 GMS teams electronically to introduce the protocol and describe the purpose and logistics of the pilot study.
A research assistant prospectively identified eligible patients each weekday by screening all patients admitted to the 2 GMS teams using the daily computerized sign‐out system used by all medical residents. Specifically, laboratory random glucose levels, inpatient medications, and medical history were reviewed to determine if each patient met eligibility criteria. Eligibility criteria were confirmed by medical record review. The pharmacist recommended to the primary team that the protocol be initiated for eligible patients. In addition, the pharmacist recommended daily adjustment of the insulin dose according to the protocol as appropriate. A chronologically organized summary of clinical data relevant to glycemic management for each patient, including bedside blood glucose measurements, general dietary intake, use of intravenous dextrose solutions, and administration of systemic steroids, oral diabetes medications, and all insulins, was provided to the team each day by the research assistant.
Measurements
The resident's acceptance of the protocol or reasons for declining it were recorded by the pharmacist on the day the protocol was recommended. Protocol acceptance was categorized as yes, no, or partial. Partial acceptance was defined as resident agreement to use the protocol, but with stated caveats or modifications. Clinical data were collected on each eligible patient for up to 7 days on GMS. Several data sources were used, including physician admission notes, the hospital's computerized clinical data system, vital‐sign sheets, medication administration records, and personal communication with nurses regarding any missing or discrepant data.
All insulin use (prescribed drug, dose, route, schedule and actual administered drug, dose, route, and time) was recorded each day by the research assistant. Use of basal and nutritional insulin and daily dose adjustments if previous hypo‐ or hyperglycemia (categorized as yes, no, or not applicable for each patient each day) were determined by the study pharmacist (J.M.T.) through retrospective review of all orders.
Up to 4 routine bedside blood glucose measurements were recorded each day: for patients eating discrete meals, these were the measurements taken before meals and at bedtime; for patients not eating or receiving continuous nutrition, these were the measurements taken closest to 6 AM, noon, 6 PM, and midnight. Additional measurements were not recorded to avoid ascertainment bias caused by follow‐up testing of abnormal glucose values. Glucose readings on the day of admission were excluded from analysis because these values are not amenable to inpatient ordering practices.
Study outcomes included overall protocol acceptance rate, insulin prescribing practices including use of basal insulin (ie, long‐acting agents such as NPH and insulin glargine), nutritional insulin (ie, scheduled regular, lispro, or aspart insulin given before each meal), daily dose adjustments under the protocol, and mean percentage of glucose readings per person greater than 180 mg/dL (hyperglycemia) and below 60 mg/dL (hypoglycemia). Comparable data from a previous cohort study of 91 GMS patients were used as baseline data for comparisons with the results of the present study.9
Other patient data collected included age, sex, weight, baseline A1C (taken at or within 6 months of admission), diabetic medications used prior to admission (none, oral agents only, or any insulin use); daily inpatient use of oral or intravenous steroids, oral diabetic medications, dextrose‐containing intravenous fluids, tube feeds, total parenteral nutrition, and general nutritional intake (nothing by mouth, clear diet, low carbohydrate diet, house diet).
Statistical Analysis
Characteristics of the study subjects and process and outcome measures were analyzed descriptively using rates, means, and standard deviations or medians with interquartile ranges as appropriate. Comparisons between the pilot study and baseline cohorts were performed using Fisher's exact test for dichotomous outcomes (eg, use of basal insulin). For rates of hyperglycemia (ie, fraction of readings > 180 mg/dL), we used binomial logistic regression, accounting for potential correlation among repeated events by individual patients with a dispersion parameter18 (note that we did not use the same analysis for rates of hypoglycemia because it was such a rare event; for analysis of hypoglycemia, the variables were dichotomized). We also analyzed outcomes by hospital day (through hospital day 5, the limit used in the baseline study) to determine daily trends during the course of hospitalization; for these analyses we used the Mantel‐Haenszel chi‐square test for dichotomous variables and binomial logistic regression with hospital day as the independent variable for rates of hyperglycemia. Two‐sided P values < .05 were considered significant. SAS version 9.1 (Cary, NC) was used for all analyses.
RESULTS
After screening all 785 admissions to the 2 medical teams during the study period, we prospectively identified 109 patients (14%) for the pilot study. Twenty patients were subsequently excluded: 7 patients who were discharged the same day they were identified, 4 who did not have a fasting blood glucose value greater than 140 mg/dL, 4 patients who had type 1 diabetes, 2 patients who were admitted with diabetic ketoacidosis, and 3 patients whose data could not be accessed because of repeated unavailability of the medical record. Characteristics of the remaining 89 study subjects are shown in Table 2 and are compared to 91 baseline subjects. The mean age of the study subjects was 68.7 years; 45% were men. Five patients (6%) did not have a previous diagnosis of diabetes, and 51% were taking insulin prior to admission; the median A1C was 6.8%.
| Characteristic | Baseline (n = 91) | Pilot (n = 89) |
|---|---|---|
| ||
| Age (years), mean (SD) | 66.0 (14.5) | 68.7 (14.7) |
| Male | 53/91 (58%) | 40/89 (45%) |
| No diagnosis of diabetes at admission | 7/91 (8%) | 5/89 (6%) |
| Preadmission diabetes regimen | ||
| None | 15/91 (16%) | 14/78 (18%) |
| Oral medications only | 32/91 (35%) | 24/78 (31%) |
| Insulin | 44/91 (48%) | 40/78 (51%) |
| A1C (IQR) | 7.0 (6.0, 8.0) | 6.8 (6.3, 7.8) |
| Hospital length of stay (days), median (IQR) | 5 (3, 7) | 5 (3, 7) |
The medical residents agreed, at least in theory, to follow the subcutaneous insulin protocol for 50 patients (56%), partially accepted it for 8 (9%), and declined for 31 (35%). Reasons for declining the protocol included fear of hypoglycemia, severity of patient's other disease states or overall poor health of patient, concern for the effects of renal insufficiency on insulin clearance, concern for the effect of steroid tapers on glucose levels, desire to titrate oral medications, and anticipation of patient's imminent discharge. Other reasons such as the glucose levels are not that bad and let's watch the glucose levels for one more day suggest that some residents did not view hyperglycemia as an acute problem requiring immediate attention.
Regarding insulin‐ordering practices (Table 3), basal insulin was prescribed for 57 patients (64%) in the pilot group compared to 45 patients (49%) in the baseline group (P = .05). Nutritional insulin was prescribed to 12 patients (13%) in the pilot group compared to no patients in the baseline group (P < .001). Oral hypoglycemic agents were prescribed less often in the pilot study than at baseline (20% vs. 38%, P = .01). The use of a standard default sliding scale from the hospital computer order set was high and was not significantly different in the pilot study compared with that at baseline (93% vs. 90%, P = .78). Twenty‐four of the 83 patients in the pilot group (29%) received sliding‐scale insulin without ever receiving basal or nutritional insulin during hospitalization compared to 45 of 91 patients in the baseline group (49%; P = .01 for comparison). Among patients started on basal insulin, 42% (24 of 57) were started after the first full hospital day. The initial basal insulin dose was appropriate according to the protocol (within 20%) in 38 of 57 patients (67%). Only 20 of 61 patients (33%) who had any hypo‐ or hyperglycemia had any change to their insulin regimen made during days 2 through 7 of their hospitalization on GMS, similar to the rate noted at baseline (36%).
| Measure | Baseline | Pilot | P value |
|---|---|---|---|
| |||
| Process | |||
| Any basal insulin during hospitalization | 45/91 (49%) | 57/89 (64%) | 0.05 |
| Any nutritional insulin during hospitalization | 0/91 (0%) | 12/89 (13%) | < 0.001 |
| Change in dose to any insulin order during hospitalization | 24/66 (36%) | 20/61 (33%) | 0.71 |
| Standard sliding scale from hospital computer order set | 75/83 (90%) | 76/82 (93%) | 0.78 |
| Any oral antihyperglycemic agents during hospitalization | 35/91 (38%) | 18/89 (20%) | 0.01 |
| Outcome | |||
| Mean percentage of glucose readings > 180 mg/dL (SD) | 33.3% (33.3%) | 31.6% (29.6%) | 0.85 |
| Any hyperglycemia (glucose > 180 mg/dL) | 66/89 (74%) | 59/78 (76%) | 0.86 |
| 1%‐20% of readings | 17/89 (19%) | 15/78 (19%) | 0.85 for trend |
| 20%‐40% | 15/89 (17%) | 15/78 (19%) | |
| 40%‐60% | 15/89 (17%) | 15/78 (19%) | |
| 60%‐80% | 7/89 (8%) | 6/78 (8%) | |
| >80% | 12/89 (13%) | 8/78 (10%) | |
| Any hypoglycemia (glucose < 60 mg/dL) | 6/89 (7%) | 10/78 (13%) | 0.20 |
Regarding glucose control (Table 3), the mean percentage of glucose readings per patient greater than 180 mg/dL was not significantly different in the pilot study compared to baseline (31.6% vs. 33.3%, P = .85). Despite implementation of the protocol and increased use of basal and nutritional insulin, 76% of patients had at least 1 routine glucose reading greater than 180 mg/dL, and 37% of patients had at least 40% of their routine glucose readings greater than 180 mg/dL, comparable to baseline (74% and 38%, respectively, P = NS for both comparisons). At least 1 hypoglycemic event (glucose reading below 60 mg/dL) occurred in 7% of patients at baseline and 13% during the pilot study (P = .20). Eleven hypoglycemic events in the pilot study were between 50 and 59 mg/dL (55%), 6 were between 40 and 49 mg/dL (30%), 3 were between 30 and 39 mg/dL (15%), and none were less than 30 mg/dL. Nine occurred before breakfast (45%), 5 before dinner (25%), 3 before lunch (15%), and 3 at bedtime (15%).
During the pilot study, the use of basal insulin did improve over the first 5 days of hospitalization (Fig. 1), in both the percentage of patients prescribed any basal insulin and the percentage of each patient's total insulin dose (basal, nutritional, and supplemental) given as basal (both P < .001 for trend). Hyperglycemia rates also improved during hospitalization (Fig. 1), decreasing from 48% on hospital day 1 to 34% on hospital day 5 (P = .004 for trend). These trends were not observed in the baseline group, with hyperglycemia rates of 37% on hospital day 1 and 34% on hospital day 5 (P = .16 for trend).

Patients for whom the resident accepted or partially accepted the protocol had higher use of basal insulin (91% vs. 13%, P < .0001), higher use of nutritional insulin (21% vs. 0%, P = .01), and more frequent dose adjustments (47% vs. 7%, P = .01) compared with patients for whom the resident declined the protocol. However, the rate of hyperglycemia was higher in patients for whom the protocol was accepted or partially accepted than in patients for whom the protocol was declined (37% vs. 20%, P = .02).
DISCUSSION
Our subcutaneous insulin protocol focused on increasing the use of basal and nutritional insulin, avoiding the use of sliding‐scale insulin by itself, and performing daily insulin adjustments in response to the hypo‐ or hyperglycemia of general medical inpatients with diabetes or hyperglycemia.
The most notable finding of our pilot study was that residents were resistant to using the protocol, both in general and in its specific recommendations. Despite receiving education about inpatient diabetes control and protocol recommendations from the team pharmacist, and despite being on a hospitalist‐run medical service, the residents accepted use of the protocol for only half the eligible patients. Patients who were started on basal insulin were often underdosed or started after the first day of hospitalization, and daily dose adjustments were not consistently made despite persistent hypo‐ or hyperglycemia. Although the use of nutritional insulin was greater compared with that in the baseline group, it was still only prescribed for 13% of patients. Use of a standard sliding scale from the hospital computer order set was common in the pilot study and similar to that in the baseline group. These results suggest significant resistance to changing the current standard of practice.
Despite this lack of adherence to the protocol, some modest improvements in processes of care were seen. Basal insulin was ordered more often during the pilot study than at baseline, especially over the course of a hospital stay. Nutritional insulin was also ordered more often during the pilot study than at baseline, but was still infrequent. Oral antihyperglycemic agents were ordered less often during the pilot study than at baseline. This demonstrates that use of the protocol may be able to improve process outcomes. However, the modest improvements in process outcomes could have simply been a result of increased awareness and education, not the protocol itself.
Regarding patient outcomes, the overall hyperglycemia rate did not improve in the pilot study relative to that at baseline. Importantly, hypoglycemia rates did not increase significantly compared with those at baseline. However, because of the small number of hypoglycemia events, the sample size may not have been sufficient to detect a true difference between groups.
The most likely reason that the protocol did not show an effect on glycemic control was that its recommendations were not adhered to. In turn, this may have been a result of incomplete education, training, and implementation measures and/or inherent problems with the protocol that made its recommendations difficult to follow. Another possibility is that the protocol itself may not have been capable of improving glucose control, even when properly used. However, we do know that resident agreement to use the protocol did lead to higher rates of recommended best practices being carried out, such as basal insulin use and daily insulin dose adjustments, and that use of the protocol was associated with improvements in glucose control over the hospital stay. A larger study with a higher degree of protocol adherence would be better able to evaluate the merits of the protocol itself, as would a randomized controlled trial using instrumental variables to measure treatment efficacy. Another possibility explanation for the lack of effect is that glucose control on admission happened to be worse in the pilot group than in the control group: rates of hyperglycemia on day 1 were 48% in the pilot group compared with 37% in the baseline group (Fig. 1). Also, the decreased use of oral agents in the pilot group, a purposeful change to decrease the risk of hypoglycemia, may have counteracted the beneficial effects of more appropriate insulin use. Finally, there were few patients with poorly controlled diabetes at baseline (18 patients with A1C 8.0 in the baseline group and 12 such patients in the pilot group), arguably those most likely to benefit.
There is a pressing need to identify protocols that can improve glucose control in the non‐ICU inpatient setting and successfully implement these protocols with a minimum of resources and effort. To date, most studies that have improved glucose control in the non‐ICU setting have relied on frequent input from diabetologists or nurse‐practitioners.14, 15
The results of this study should be viewed in light of its limitations, including its relatively small sample size (thus limiting our ability to detect possible significant differences between groups) and that it was conducted at a single institution (thus limiting its generalizability). Patients were enrolled on weekdays, so patients admitted and discharged over a weekend or on a holiday may have been missed. Also, because of the nonrandomized design of the study, we cannot exclude the possibility that the improvements noted in the pilot study were a result of the increased education provided or of increased awareness and general improvement in diabetes management over the course of the study. Finally, implementation of the protocol was somewhat labor intensive and required staff support that could be difficult to replicate in other institutions. However, most of the study staff's effort was necessary either to implement the protocol in the absence of an order set or to evaluate barriers to implementation. Widespread implementation of a protocol with an order set, education, and the use of highly reliable tools should be possible with much less effort and resources. The strengths of this study include its prospective data collection methods, which included rigorous inclusion criteria and collection of detailed clinical data.
Our study findings suggest several approaches to improve care in the future. To combat resistance to change, the American Association of Clinical Endocrinologists strongly recommends that each institution ensure that all its clinicians involved agree about general philosophies of diabetes management.19 A more expansive, hospital‐wide educational and promotional plan may increase the initial acceptance of the protocol. Interviews with residents also indicated there was unfamiliarity with diabetes management and significant concerns about the harmful affects of tight glucose control (ie, risk of hypoglycemia), especially in certain patient subgroups. These results confirmed the need for more practical individualized training and sparked the implementation of small‐group, case‐based educational sessions on inpatient diabetes management for all house officers, with a particular focus on patients with multiple comorbidities, on steroid tapers, and/or with renal failure.
The lack of nutritional insulin orders, delays in ordering basal insulin, and use of inadequate doses of insulin may be counteracted by the use of an order set, in our case built into our computer physician order entry (CPOE) system. The use of CPOE also allows reminders to be automatically sent to clinicians if eligible patients are not started on these orders. Clinical inertia (eg, failure to adjust the insulin doses of specific patients despite hyperglycemia) is more difficult to combat but may be addressed through better organization of clinical data, individualized, case‐based education, and CPOE reminders and eventually through culture change.
As a result of our pilot study, additional revisions were made to the protocol in hopes of increasing protocol adherence. For example, for patients eating discrete meals who are not taking insulin at home, the pilot protocol had suggested a starting insulin dose range for basal and nutritional insulin that required 2 separate calculations. The revised protocol was simplified to recommend a total daily insulin dose to be split evenly between basal and nutritional insulin. The daily adjustment instructions were also simplified. The pilot protocol had included a complicated table of adjustment recommendations based on bedside glucose trends. The revised protocol recommends adjusting the new daily dose by adding the total units of insulin given the previous day (including supplemental doses), making minor adjustments for hyper‐ or hypoglycemia and other clinical factors (like renal failure), and splitting this dose evenly between scheduled basal and nutritional insulin. In addition, 3 order sets were built into our computerized physician order entry system to facilitate early and appropriate insulin orders for patients with different diets (discrete meals, continuous tube feeds, and nothing by mouth); 3 different insulin sliding scales were created for patients with different degrees of insulin resistance; a diabetes management page for our electronic medication administration record is being developed to better organize clinical data; and hospital‐wide education and individualized training are ongoing.
In conclusion, the adherence to an inpatient glycemic management protocol that focused on increasing use of basal insulin and performing daily insulin adjustments was only fair. Barriers to successful implementation included clinical inertia regarding individual patients, unfamiliarity with inpatient diabetes management strategies, fear of hypoglycemia, and resistance to changing the current standard of practice. Targeted education, standard order sets, better organization of clinical data, protocol simplification, and institutional culture changes may be necessary for successful protocol implementation and improved inpatient glucose control.
- Agency for Healthcare Research and Quality. HCUPnet, Healthcare Cost and Utilization Project. 8/17/05; http://www.ahrq.gove/HCUPnet/. Accessed 7/17/06,2006.
- ,,,,,.Hyperglycemia: an independent marker of in‐hospital mortality in patients with undiagnosed diabetes.J Clin Endocrinol Metab.2002;87:978–982.
- ,,, et al.Hyperglycaemia is associated with poor outcomes in patients admitted to hospital with acute exacerbations of chronic obstructive pulmonary disease.Thorax2006;61:284–289.
- ,,,,.Stress hyperglycemia and prognosis of stroke in nondiabetic and diabetic patients: a systematic overview.Stroke.2001;32:2426–2432.
- ,,,.Hyperglycemia is associated with adverse outcomes in patients receiving total parenteral nutrition.Diabetes Care.2005;28:2367–2371.
- ,,, et al.Management of diabetes and hyperglycemia in hospitals.Diabetes Care.2004;27:553–597.
- ,,,,,.The relation between hyperglycemia and outcomes in 2,471 patients admitted to the hospital with community‐acquired pneumonia.Diabetes Care.2005;28:810–815.
- ,,.Glycemic control and sliding scale insulin use in medical inpatients with diabetes mellitus.Arch Intern Med.1997;157:545–552.
- ,,,,.Inpatient management of diabetes and hyperglycemia among general medicine patients at a large teaching hospital.J Hosp Med.2006;1:145–150.
- ,,, et al.Intensive insulin therapy in the medical ICU.N Engl J Med.2006;354:449–461.
- ,,, et al.Intensive insulin therapy in the critically ill patients.N Engl J Med.2001;345:1359–1367.
- .Prospective randomised study of intensive insulin treatment on long term survival after acute myocardial infarction in patients with diabetes mellitus.DIGAMI (Diabetes Mellitus, Insulin Glucose Infusion in Acute Myocardial Infarction) Study Group.BMJ.1997;314:1512–1515.
- ,,, et al.Intense metabolic control by means of insulin in patients with diabetes mellitus and acute myocardial infarction (DIGAMI 2): effects on mortality and morbidity.Eur Heart J.2005;26:650–661.
- American Diabetes Association.Standards of Medical Care in Diabetes ‐ 2006.Diabetes Care.2006;29:S4–S42.
- ACE/ADA Task Force on Inpatient Diabetes.American College of Endocrinology and American Diabetes Association consensus statement on inpatient diabetes and glycemic control: A call to action.Diabetes Care.2006;29:1955–1962.
- ,,,.Eliminating inpatient sliding‐scale insulin: a reeducation project with medical house staff.Diabetes Care.2005;28:1008–11.
- ,,, et al.Randomized study of basal‐bolus insulin therapy in the inpatient management of patients with type 2 diabetes (RABBIT 2 Trial).Diabetes Care.2007;30:2181–2186.
- .Extra‐binomial variation in logistic linear models.Appl Stat.1982;31:144–148.
- ,.Hospital management of diabetes.Endocrinol Metab Clin North Am.2005;34:99–116.
- Agency for Healthcare Research and Quality. HCUPnet, Healthcare Cost and Utilization Project. 8/17/05; http://www.ahrq.gove/HCUPnet/. Accessed 7/17/06,2006.
- ,,,,,.Hyperglycemia: an independent marker of in‐hospital mortality in patients with undiagnosed diabetes.J Clin Endocrinol Metab.2002;87:978–982.
- ,,, et al.Hyperglycaemia is associated with poor outcomes in patients admitted to hospital with acute exacerbations of chronic obstructive pulmonary disease.Thorax2006;61:284–289.
- ,,,,.Stress hyperglycemia and prognosis of stroke in nondiabetic and diabetic patients: a systematic overview.Stroke.2001;32:2426–2432.
- ,,,.Hyperglycemia is associated with adverse outcomes in patients receiving total parenteral nutrition.Diabetes Care.2005;28:2367–2371.
- ,,, et al.Management of diabetes and hyperglycemia in hospitals.Diabetes Care.2004;27:553–597.
- ,,,,,.The relation between hyperglycemia and outcomes in 2,471 patients admitted to the hospital with community‐acquired pneumonia.Diabetes Care.2005;28:810–815.
- ,,.Glycemic control and sliding scale insulin use in medical inpatients with diabetes mellitus.Arch Intern Med.1997;157:545–552.
- ,,,,.Inpatient management of diabetes and hyperglycemia among general medicine patients at a large teaching hospital.J Hosp Med.2006;1:145–150.
- ,,, et al.Intensive insulin therapy in the medical ICU.N Engl J Med.2006;354:449–461.
- ,,, et al.Intensive insulin therapy in the critically ill patients.N Engl J Med.2001;345:1359–1367.
- .Prospective randomised study of intensive insulin treatment on long term survival after acute myocardial infarction in patients with diabetes mellitus.DIGAMI (Diabetes Mellitus, Insulin Glucose Infusion in Acute Myocardial Infarction) Study Group.BMJ.1997;314:1512–1515.
- ,,, et al.Intense metabolic control by means of insulin in patients with diabetes mellitus and acute myocardial infarction (DIGAMI 2): effects on mortality and morbidity.Eur Heart J.2005;26:650–661.
- American Diabetes Association.Standards of Medical Care in Diabetes ‐ 2006.Diabetes Care.2006;29:S4–S42.
- ACE/ADA Task Force on Inpatient Diabetes.American College of Endocrinology and American Diabetes Association consensus statement on inpatient diabetes and glycemic control: A call to action.Diabetes Care.2006;29:1955–1962.
- ,,,.Eliminating inpatient sliding‐scale insulin: a reeducation project with medical house staff.Diabetes Care.2005;28:1008–11.
- ,,, et al.Randomized study of basal‐bolus insulin therapy in the inpatient management of patients with type 2 diabetes (RABBIT 2 Trial).Diabetes Care.2007;30:2181–2186.
- .Extra‐binomial variation in logistic linear models.Appl Stat.1982;31:144–148.
- ,.Hospital management of diabetes.Endocrinol Metab Clin North Am.2005;34:99–116.
Quality of Inpatient Diabetes Management
Diabetes mellitus is a common comorbidity of hospitalization; in 2003 diabetes was a secondary diagnosis in 17.8% of all adult hospital discharges.1 When undiagnosed diabetes is included, the prevalence of inpatient diabetes or hyperglycemia may be as high as 38%.2 Recent studies show that hyperglycemia in hospitalized patients complicates numerous illnesses and is an independent predictor of adverse outcomes.3 Treatment of inpatient hyperglycemia improves outcomes, including mortality, for patients in surgical intensive care units4 and possibly for those admitted for myocardial infarction.5, 6 For these reasons, the American Diabetes Association and the American College of Endocrinology now recommend that glucose levels of all patients admitted to non‐critical‐care units be maintained below 180 mg/dL.3, 7
Evidence‐based recommendations for achieving these goals include effective protocols for subcutaneous insulin therapy for patients who do not require continuous intravenous insulin infusion. Components of these protocols include use of basal insulin and scheduled nutritional insulin, avoiding use of supplemental (sliding‐scale) insulin alone (which has been shown to be ineffective and possibly deleterious in prior studies),8 and adjustment of insulin orders to reflect nutritional intake, insulin sensitivity, and previous response to therapy.7
We conducted this study to evaluate the current state of glycemic control and adherence to current recommendations on a general medicine service run by hospitalists in a busy teaching hospital. We also sought to correlate insulin‐ordering practices with the quality of glycemic control in these patients.
METHODS
Setting and Participants
This prospective cohort study was conducted at Brigham and Women's Hospital (BWH) from August 1 through September 30, 2004. Eligible subjects were patients admitted to 3 General Medicine Service (GMS) teams with either a known diagnosis of diabetes or inpatient hyperglycemia (random glucose > 200 mg/dL). Patients admitted for diabetic ketoacidosis, hyperosmolar hyperglycemic state, or gestational diabetes were excluded. Members of the BWH/Faulkner Hospitalist Service are the teaching attendings on these 3 teams (each consisting of 2 interns and 1 junior or senior resident) and are the attendings of record for approximately 90% of the patients on these teams. A research assistant identified potential subjects each weekday from the daily computerized sign‐out system used by all medical residents by searching for diabetes on the patient summary, a diabetic medication in the automatically abstracted medication list, or a laboratory glucose value greater than 200 mg/dL from automatically abstracted daily laboratory results. Eligibility criteria were confirmed by medical record review, and any question of eligibility was reviewed with the principal investigator. This study was approved by the BWH Institutional Review Board; patient consent was not deemed necessary for this study given the relatively nonsensitive nature of the data (eg, glucose control, insulin orders), the noninvasive means of collecting it (eg, chart review), and the steps taken by research personnel to minimize any breach in patient confidentiality.
Measurements
We abstracted clinical data on each eligible subject for up to 5 days on GMS. Several data sources were used, including physician admission notes, the hospital's computerized clinical data system, nursing notes, vital sign sheets, the medication administration record, and personal communication with nurses about any missing or discrepant data. Up to 4 routine bedside blood glucose measurements were recorded each day: the measurements taken before meals and at bedtime for patients eating discrete meals or the measurements closest to 6 AM, noon, 6 PM, and midnight for patients not eating or receiving continuous nutrition. Additional measurements were not recorded to avoid ascertainment bias caused by follow‐up testing of abnormal glucose values.
Study outcomes included the percentage of glucose readings below 60 mg/dL (hypoglycemia) and greater than 180 mg/dL (hyperglycemia). Use of several types of insulin ordering practices were also recorded: use of basal insulin (ie, long‐acting agents such as NPH and insulin glargine), scheduled prandial insulin (eg, regular insulin, insulin lispro, and insulin aspart given before each meal), daily adjustments to insulin orders, use of different insulin sliding scales for patients with different daily insulin requirements, orders to hold or adjust insulin doses in patients not eating, and the percentage of the total daily insulin dose given as basal insulin.
Other patient variables collected were age, sex, weight; medical comorbidities (using a modified Charlson score)9; severity of illness (using a simplified APACHE III score)8; admission diagnosis; baseline HbA1C (taken at or within 6 months of admission); severe complications of diabetes (blindness, dialysis, renal transplant, amputation due to peripheral vascular disease, vascular bypass surgery); diabetic medications prior to admission (none, oral agents only, or any insulin use); daily inpatient use of oral or intravenous steroids, oral diabetic medications, dextrose‐containing intravenous fluids, tube feeds, inpatient total parenteral nutrition, and general nutritional intake (all, most, some, little, or none for each meal).
Statistical Analysis
Characteristics of the study subjects and process and outcome measures were analyzed descriptively using rates, means with standard deviations, and medians with interquartile ranges as appropriate. We also analyzed outcomes by patient‐day to determine daily trends during the course of hospitalization. In these analyses, we used the Mantel‐Haenszel chi‐square test for the dichotomous variables (eg, daily use of any basal insulin) and univariable linear regression with general linear models clustered by patient, that is, repeated‐measures analysis, for the continuous variables. We used an arcsin(square‐root) transformation for those continuous outcomes that were percentages (eg, percentage of glucose readings > 180 mg/dL) and logarithmic transformation for right‐tailed continuous variables (eg, total number of units of insulin administered).
To determine the effects of various insulin‐ordering practices on glucose control, we also performed multivariable analysis of mean glucose levels per patient‐day. We chose mean glucose rather than rates of specific glucose ranges as the outcome because of the low rate of hypoglycemia and the additional sensitivity of this method. First, univariable analysis was performed using the Student's t test, analysis of variance, or Spearman correlation as appropriate for each predictor. Multiple linear regression models were then constructed, using variables significant in the univariable testing at the P < .10 level. Confounding variables that changed beta coefficients by 10% or more were retained, whereas collinear terms were removed by hand; patient age and sex were also retained in the models as a priori selected confounding variables.
As with the repeated‐measures analysis, we used general linear models, accounting for within‐patient clustering, with an exchangeable correlation structure. In addition, standard regression techniques could not be applied to the basal insulin variable because use of basal insulin is a mediator of subsequently lower glucose levels but often is the result of previously elevated glucose levels. Instead, we used a marginal structural model,10, 11 weighting the usual regression analysis to statistically remove the effect of confounding by indication. The weights for this analysis were based on the inverse probability of use of basal insulin, given previous glucose levels and prior use of basal insulin and were estimated from a separate logistic regression analysis. Results were considered significant at P < .05 except as noted above. SAS version 8.1 (Cary, NC) was used for all analyses.
RESULTS
We prospectively identified 123 patients for the study. Subsequently, 16 patients were excluded, 11 who did not have diabetes or inpatient hyperglycemia (most of whom had been placed on insulin prophylactically to avoid steroid‐induced hyperglycemia), 2 who were admitted for diabetic ketoacidosis, 2 who were not on GMS teams 13, and 1 whose data could not be accessed. Characteristics of the remaining 107 study subjects are shown in Table 1. The mean age of the subjects was 65.2 years; 55% were men. Nine patients had no previous diagnosis of diabetes, 43% were taking insulin prior to admission, 14% had severe diabetic complications, and the median HbA1C was 7.
| Characteristic | |
|---|---|
| |
| Age, mean (SD), y | 65.2 (14.5) |
| Male | 59/107 (55) |
| No diagnosis of diabetes at admission | 9/107 (8) |
| Preadmission diabetes medication regimen: | |
| None | 24/106 (22) |
| Oral medications only | 36/106 (34) |
| Insulin | 46/106 (43) |
| HbA1C, median (IQR) | 7 (6, 8) |
| Diabetic complications | 15/107 (14) |
| Hospital length of stay, median (IQR), d | 5 (3, 7) |
| Charlson score, mean (SD) | 5.3 (3.0) |
| APACHE III score, mean (SD) | 36.9 (15.6) |
Regarding insulin‐ordering practices (Table 2), 47 patients (43%) had basal insulin prescribed, while 4% of patients had an order for scheduled mealtime short‐ or rapid‐acting insulin. Of the 89 patients on sliding‐scale insulin, 80 (90%) had orders written for the default sliding scale built into the computerized physician order entry system at BWH. There was no correlation between intensity of the sliding scale and the patient's total daily insulin dose (data not shown). Of the patients on sliding‐scale insulin, 47% were prescribed basal insulin, 39% were prescribed oral agents, and 23% were prescribed neither.
| Measure | |
|---|---|
| |
| Process | |
| Any basal insulin during hospitalization | 47/107 (43) |
| Separate nutritional insulin order | 4/107 (3.7) |
| Change in dose to any insulin order during hospitalization if any hyper‐ or hypoglycemia | 26/75 (35) |
| Standard sliding scale from hospital computer order set | 80/89 (90) |
| Any oral diabetic agents during hospitalization | 39/107 (36) |
| Outcomes | |
| Any hyperglycemia (glucose > 180 mg/dL) | 74/98 (76) |
| 0%20% of readings | 20/98 (20) |
| 20%40% | 19/98 (19) |
| 40%60% | 19/98 (19) |
| 60%80% | 6/98 (6.1) |
| Greater than 80% | 10/98 (10) |
| Any hypoglycemia (glucose < 60 mg/dL) | 11/98 (11) |
| 0%20% of readings | 9/98 (9.2) |
| 20%40% | 2/98 (2.0) |
Regarding glucose control, 317 of 1022 glucose meter readings (31%) were greater than 180 mg/dL, and the mean rate of glucose readings greater than 180 mg/dL per patient was also 31%. Approximately three quarters of all patients had at least one routine glucose reading greater than 180 mg/dL, and 35% of patients had at least 40% of their routine glucose readings greater than 180 mg/dL (Table 2). Twelve of 1022 readings (1.2%) were less than 60 mg/dL, and 11% of patients had at least one glucose reading less than 60 mg/dL (Table 2).
Despite a relatively constant percentage of glucose readings greater than 180 mg/dL per patient over the first 5 days of hospitalization (25%36% each day), we found no evidence of change in the percentage of patients prescribed basal insulin or the percentage of insulin given as basal insulin, and there was a small but significant increase in the total amount of insulin prescribed (Table 3). Of the 75 patients with at least one episode of hypo‐ or hyperglycemia, 43 (57%) were ever prescribed basal insulin, 29 (39%) were prescribed oral diabetes agents, and only 26 (35%) had any change to their insulin regimen during the first 5 days of their hospitalization on GMS. Of the 47 patients prescribed basal insulin in the hospital, 41 had been taking insulin prior to admission.
| Hospital day | P value for Trend | |||||
|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | ||
| ||||||
| Number of patients | 107 | 105 | 85 | 66 | 48 | |
| Mean adjusted total daily insulin units* | 17 | 22 | 23 | 20 | 27 | 0.03 |
| Patients prescribed any basal insulin (%) | 29/79 (37) | 41/93 (44) | 33/74 (45) | 27/57 (47) | 20/43 (47) | 0.18 |
| Mean % of total insulin dose consisting of basal insulin | 35 | 42 | 38 | 39 | 33 | 0.80 |
| Mean % glucose readings < 60 mg/dL | 2 | 1 | 1 | 0 | 1 | 0.13 |
| Mean % glucose readings> 180 mg/dL | 36 | 34 | 29 | 25 | 32 | 0.13 |
In a multivariable analysis of the mean glucose reading per patient‐day, we found several predictors of lower glucose readings, including diet‐controlled diabetes prior to admission and prescription of oral hypoglycemic medications in the hospital. We also found several predictors of higher glucose readings, including severe diabetic complications and higher glucose level at admission. Finally, we noted variation both by medical team (each composed of 1 medical attending, 1 resident, and 2 interns) and by floor of the hospital (each staffed by a different cadre of nurses). Adjusting for these factors (as well as for the daily use of dextrose‐containing intravenous fluids and steroids, sex, age, Charlson comorbidity score, APACHE 3 score, prior diagnosis of diabetes, HbA1C level, and length of hospital stay) use of sliding‐scale insulin alone (eg, without scheduled basal or nutritional insulin) was associated with a daily average glucose reading that was 20 mg/dL higher than that for those prescribed scheduled insulin or those not prescribed a sliding scale at all (95% confidence interval, 5.035 mg/dL; Table 4). In a separate analysis, adjusting for the same clinical factors, we could find no relationship between change in daily dose of basal insulin and change in daily average glucose level (data not shown).
| Characteristic | Effect size (95% CI)* | P value |
|---|---|---|
| ||
| Sliding scale insulin alone | 20 (5.035) | 0.01 |
| Oral diabetes regimen during hospitalization | 22 (413.0) | 0.02 |
| Diet‐controlled diabetes prior to admission | 32 (577.6) | 0.01 |
| No prior diagnosis of diabetes | 28 (3.260) | 0.08 |
| Complications of diabetes | 44 (2167) | < 0.001 |
| HbA1C | 6.1 (120.0073) | 0.05 |
| Admission glucose | 0.19 (0.0670.31) | 0.002 |
| Medical Team‖ | 47 (6727) | < 0.001 |
| Hospital Floor | 46 (6824) | < 0.001 |
DISCUSSION
In this observational study, we found several deficiencies in the management of diabetes and hyperglycemia among hospitalized patients on a hospitalist‐run general medical service. These deficiencies were both in processes of care (eg, limited use of basal and especially nutritional insulin) and in outcomes (ie, glycemic control) compared with national guidelines. We also found evidence of clinical inertia when comparing outpatient to inpatient regimens, when evaluating daily changes in management, and when evaluating responses to previous hyperglycemia. Finally, we demonstrated that use of an insulin sliding scale by itself was associated with worse glycemic control after extensive adjustment for a variety of clinical factors.
Of note, other than the use of sliding‐scale insulin by itself, we could not find a relationship between specific daily adjustments to insulin orders and daily glycemic control in this study. However, we did find differences in glycemic control by medical team and by floor (the latter a proxy for nursing staff). This suggests that glycemic control depends on the exact details of how insulin is managed, rather than on crude measures of insulin adjustment such as change in dose in response to hyperglycemia. These findings also suggest that interventions focused on medical and nursing staff may be able to improve inpatient glycemic control.
The association between the use of oral diabetic agents and improved glucose control was notable and could represent an actual benefit of these agents (especially when added to sliding‐scale insulin by itself) and/or the result of uncontrolled confounding (ie, as a marker of well‐controlled diabetes). Further study is needed to distinguish among these possibilities.
Previous studies have shown evidence of poor inpatient glycemic control as well as the deleterious effects of sliding‐scale insulin by itself.8 This study is perhaps most notable for the suggestion that little, if anything, has changed over the previous decade in this area, despite recent well‐done observational and randomized controlled trials demonstrating the hazards of inpatient hyperglycemia and the publication of expert consensus statements on inpatient glucose management. Strategies to improve glucose control have been investigated to a greater extent in intensive care units12, 13 than on general medical wards,14 perhaps because the strength of evidence is strongest in this setting. Without such strong evidence for general medical patients, factors such as clinician fear of hypoglycemia, clinical inertia, and resistance to institutional change may play predominant roles.
Clinical inertia (ie, recognition of the problem but failure to act)15 has been demonstrated previously in the outpatient management of diabetes16, 17; this study provides evidence of the phenomenon in the inpatient setting. Work by Phillips and colleagues15 has shown that clinical inertia results from at least 3 problems: overestimation of care provided; use of soft reasons to avoid intensification of therapy; and lack of education, training, and practice organization aimed at achieving specific goals. All 3 problems likely contribute to clinical inertia in inpatient diabetes management. Revised educational programs; systems for improving care such as reminders, flow sheets, and order sets; and performance feedback can help address clinical inertia and improve care.15
This study should be viewed in light of its limitations, including relatively small sample size, thus limiting our ability to detect other possible significant predictors of glycemic control, and the use of a single institution, thus limiting generalizability. However, recent data from the University HealthSystem Consortium revealed that our institution was typical of the 37 participating academic medical centers in that study.18 In addition, only 9 patients were identified without a prior diagnosis of diabetes, raising the possibility that some patients with undiagnosed diabetes were missed in our study. However, our search strategy included a daily review of automatically abstracted laboratory values, making this possibility less likely. Strengths of this study include its prospective data collection methods with rigorous inclusion criteria, collection of detailed clinical data, and use of a novel statistical technique to more accurately assess the complex relationship between insulin use and glycemic control, appropriately adjusting for confounding by indication caused by prior glucose measurements.
Future research should focus on patient, clinician, and system barriers to improving inpatient glycemic management, using the clinical inertia framework as a starting point, and on the creation of insulin protocols that can be used and proven effective in the non‐ICU inpatient setting. Also needed are improved measures of the quality of glycemic control, insulin orders, and daily insulin adjustment.
In conclusion, inpatient glycemic management was shown to be in need of improvement. Institutionwide quality improvement efforts should probably target both physician and nursing behavior and should focus on increasing use of basal and nutritional insulin, as proposed in recent guidelines, avoiding use of sliding‐scale insulin by itself, and performing daily insulin adjustment in response to previous hypo‐ or hyperglycemia. Hospitalists can play a major role in these institutionwide quality improvement efforts.
Acknowledgements
We thank Paul Szumita, PharmD, and LeRoi Hicks, MD, MPH, for assistance with the conception of this project and E. John Orav, PhD, for statistical assistance.
- Agency for Healthcare Research and Quality. HCUPnet, Healthcare Cost and Utilization Project. 8/17/2005. Available at: http://www.ahrq.gov/HCUPnet/. Accessed November 29,2005.
- ,,,,,.Hyperglycemia: an independent marker of in‐hospital mortality in patients with undiagnosed diabetes.J Clin Endocrinol Metab.2002;87:978–982.
- ,,, et al.Management of diabetes and hyperglycemia in hospitals.Diabetes Care.2004;27:553–597.
- ,,, et al.Intensive insulin therapy in the critically ill patients.N Engl J Med.2001;345:1359–1367.
- .Prospective randomised study of intensive insulin treatment on long term survival after acute myocardial infarction in patients with diabetes mellitus. DIGAMI (Diabetes Mellitus, Insulin Glucose Infusion in Acute Myocardial Infarction) Study Group.Br Med J.1997;314:1512–1515.
- ,,, et al.Intense metabolic control by means of insulin in patients with diabetes mellitus and acute myocardial infarction (DIGAMI 2): effects on mortality and morbidity.Eur Heart J2005;26:650–661.
- ,,, et al.American College of Endocrinology position statement on inpatient diabetes and metabolic control.Endocr Pract.2004;10(Suppl2):4–9.
- ,,.Glycemic control and sliding scale insulin use in medical inpatients with diabetes mellitus.Arch Intern Med.1997;157:545–552.
- ,,,,.Can comorbidity be measured by questionnaire rather than medical record review?Med Care.1996;34:73–84.
- ,,.Marginal structural models to estimate the joint causal effect of nonrandomized treatments.J Am Stat Assoc—App Case Stud.2001;96:440–448.
- .Correction for non‐compliance in equivalence trials.Stat Med.1998;17:269–302; discussion87–89.
- ,,, et al.Implementation of a safe and effective insulin infusion protocol in a medical intensive care unit.Diabetes Care.2004;27:461–7.
- .Effect of an intensive glucose management protocol on the mortality of critically ill adult patients.Mayo Clin Proc.2004;79:992–1000.
- ,,,.Eliminating inpatient sliding‐scale insulin: a reeducation project with medical house staff.Diabetes Care.2005;28:1008–1011.
- ,,, et al.Clinical inertia.Ann Intern Med.2001;135:825–834.
- ,,,,.Diabetes in urban African‐Americans. XV. Identification of barriers to provider adherence to management protocols.Diabetes Care.1999;22:1617–1620.
- ,,, et al.Clinical inertia in the management of type 2 diabetes metabolic risk factors.Diabet Med.2004;21:150–155.
- University HealthSystem Consortium.Glycemic control 2005 findings and conclusions. Paper presented at Glycemic Control 2005 Knowledge Transfer Meeting. August 19,2005, Chicago, IL.
Diabetes mellitus is a common comorbidity of hospitalization; in 2003 diabetes was a secondary diagnosis in 17.8% of all adult hospital discharges.1 When undiagnosed diabetes is included, the prevalence of inpatient diabetes or hyperglycemia may be as high as 38%.2 Recent studies show that hyperglycemia in hospitalized patients complicates numerous illnesses and is an independent predictor of adverse outcomes.3 Treatment of inpatient hyperglycemia improves outcomes, including mortality, for patients in surgical intensive care units4 and possibly for those admitted for myocardial infarction.5, 6 For these reasons, the American Diabetes Association and the American College of Endocrinology now recommend that glucose levels of all patients admitted to non‐critical‐care units be maintained below 180 mg/dL.3, 7
Evidence‐based recommendations for achieving these goals include effective protocols for subcutaneous insulin therapy for patients who do not require continuous intravenous insulin infusion. Components of these protocols include use of basal insulin and scheduled nutritional insulin, avoiding use of supplemental (sliding‐scale) insulin alone (which has been shown to be ineffective and possibly deleterious in prior studies),8 and adjustment of insulin orders to reflect nutritional intake, insulin sensitivity, and previous response to therapy.7
We conducted this study to evaluate the current state of glycemic control and adherence to current recommendations on a general medicine service run by hospitalists in a busy teaching hospital. We also sought to correlate insulin‐ordering practices with the quality of glycemic control in these patients.
METHODS
Setting and Participants
This prospective cohort study was conducted at Brigham and Women's Hospital (BWH) from August 1 through September 30, 2004. Eligible subjects were patients admitted to 3 General Medicine Service (GMS) teams with either a known diagnosis of diabetes or inpatient hyperglycemia (random glucose > 200 mg/dL). Patients admitted for diabetic ketoacidosis, hyperosmolar hyperglycemic state, or gestational diabetes were excluded. Members of the BWH/Faulkner Hospitalist Service are the teaching attendings on these 3 teams (each consisting of 2 interns and 1 junior or senior resident) and are the attendings of record for approximately 90% of the patients on these teams. A research assistant identified potential subjects each weekday from the daily computerized sign‐out system used by all medical residents by searching for diabetes on the patient summary, a diabetic medication in the automatically abstracted medication list, or a laboratory glucose value greater than 200 mg/dL from automatically abstracted daily laboratory results. Eligibility criteria were confirmed by medical record review, and any question of eligibility was reviewed with the principal investigator. This study was approved by the BWH Institutional Review Board; patient consent was not deemed necessary for this study given the relatively nonsensitive nature of the data (eg, glucose control, insulin orders), the noninvasive means of collecting it (eg, chart review), and the steps taken by research personnel to minimize any breach in patient confidentiality.
Measurements
We abstracted clinical data on each eligible subject for up to 5 days on GMS. Several data sources were used, including physician admission notes, the hospital's computerized clinical data system, nursing notes, vital sign sheets, the medication administration record, and personal communication with nurses about any missing or discrepant data. Up to 4 routine bedside blood glucose measurements were recorded each day: the measurements taken before meals and at bedtime for patients eating discrete meals or the measurements closest to 6 AM, noon, 6 PM, and midnight for patients not eating or receiving continuous nutrition. Additional measurements were not recorded to avoid ascertainment bias caused by follow‐up testing of abnormal glucose values.
Study outcomes included the percentage of glucose readings below 60 mg/dL (hypoglycemia) and greater than 180 mg/dL (hyperglycemia). Use of several types of insulin ordering practices were also recorded: use of basal insulin (ie, long‐acting agents such as NPH and insulin glargine), scheduled prandial insulin (eg, regular insulin, insulin lispro, and insulin aspart given before each meal), daily adjustments to insulin orders, use of different insulin sliding scales for patients with different daily insulin requirements, orders to hold or adjust insulin doses in patients not eating, and the percentage of the total daily insulin dose given as basal insulin.
Other patient variables collected were age, sex, weight; medical comorbidities (using a modified Charlson score)9; severity of illness (using a simplified APACHE III score)8; admission diagnosis; baseline HbA1C (taken at or within 6 months of admission); severe complications of diabetes (blindness, dialysis, renal transplant, amputation due to peripheral vascular disease, vascular bypass surgery); diabetic medications prior to admission (none, oral agents only, or any insulin use); daily inpatient use of oral or intravenous steroids, oral diabetic medications, dextrose‐containing intravenous fluids, tube feeds, inpatient total parenteral nutrition, and general nutritional intake (all, most, some, little, or none for each meal).
Statistical Analysis
Characteristics of the study subjects and process and outcome measures were analyzed descriptively using rates, means with standard deviations, and medians with interquartile ranges as appropriate. We also analyzed outcomes by patient‐day to determine daily trends during the course of hospitalization. In these analyses, we used the Mantel‐Haenszel chi‐square test for the dichotomous variables (eg, daily use of any basal insulin) and univariable linear regression with general linear models clustered by patient, that is, repeated‐measures analysis, for the continuous variables. We used an arcsin(square‐root) transformation for those continuous outcomes that were percentages (eg, percentage of glucose readings > 180 mg/dL) and logarithmic transformation for right‐tailed continuous variables (eg, total number of units of insulin administered).
To determine the effects of various insulin‐ordering practices on glucose control, we also performed multivariable analysis of mean glucose levels per patient‐day. We chose mean glucose rather than rates of specific glucose ranges as the outcome because of the low rate of hypoglycemia and the additional sensitivity of this method. First, univariable analysis was performed using the Student's t test, analysis of variance, or Spearman correlation as appropriate for each predictor. Multiple linear regression models were then constructed, using variables significant in the univariable testing at the P < .10 level. Confounding variables that changed beta coefficients by 10% or more were retained, whereas collinear terms were removed by hand; patient age and sex were also retained in the models as a priori selected confounding variables.
As with the repeated‐measures analysis, we used general linear models, accounting for within‐patient clustering, with an exchangeable correlation structure. In addition, standard regression techniques could not be applied to the basal insulin variable because use of basal insulin is a mediator of subsequently lower glucose levels but often is the result of previously elevated glucose levels. Instead, we used a marginal structural model,10, 11 weighting the usual regression analysis to statistically remove the effect of confounding by indication. The weights for this analysis were based on the inverse probability of use of basal insulin, given previous glucose levels and prior use of basal insulin and were estimated from a separate logistic regression analysis. Results were considered significant at P < .05 except as noted above. SAS version 8.1 (Cary, NC) was used for all analyses.
RESULTS
We prospectively identified 123 patients for the study. Subsequently, 16 patients were excluded, 11 who did not have diabetes or inpatient hyperglycemia (most of whom had been placed on insulin prophylactically to avoid steroid‐induced hyperglycemia), 2 who were admitted for diabetic ketoacidosis, 2 who were not on GMS teams 13, and 1 whose data could not be accessed. Characteristics of the remaining 107 study subjects are shown in Table 1. The mean age of the subjects was 65.2 years; 55% were men. Nine patients had no previous diagnosis of diabetes, 43% were taking insulin prior to admission, 14% had severe diabetic complications, and the median HbA1C was 7.
| Characteristic | |
|---|---|
| |
| Age, mean (SD), y | 65.2 (14.5) |
| Male | 59/107 (55) |
| No diagnosis of diabetes at admission | 9/107 (8) |
| Preadmission diabetes medication regimen: | |
| None | 24/106 (22) |
| Oral medications only | 36/106 (34) |
| Insulin | 46/106 (43) |
| HbA1C, median (IQR) | 7 (6, 8) |
| Diabetic complications | 15/107 (14) |
| Hospital length of stay, median (IQR), d | 5 (3, 7) |
| Charlson score, mean (SD) | 5.3 (3.0) |
| APACHE III score, mean (SD) | 36.9 (15.6) |
Regarding insulin‐ordering practices (Table 2), 47 patients (43%) had basal insulin prescribed, while 4% of patients had an order for scheduled mealtime short‐ or rapid‐acting insulin. Of the 89 patients on sliding‐scale insulin, 80 (90%) had orders written for the default sliding scale built into the computerized physician order entry system at BWH. There was no correlation between intensity of the sliding scale and the patient's total daily insulin dose (data not shown). Of the patients on sliding‐scale insulin, 47% were prescribed basal insulin, 39% were prescribed oral agents, and 23% were prescribed neither.
| Measure | |
|---|---|
| |
| Process | |
| Any basal insulin during hospitalization | 47/107 (43) |
| Separate nutritional insulin order | 4/107 (3.7) |
| Change in dose to any insulin order during hospitalization if any hyper‐ or hypoglycemia | 26/75 (35) |
| Standard sliding scale from hospital computer order set | 80/89 (90) |
| Any oral diabetic agents during hospitalization | 39/107 (36) |
| Outcomes | |
| Any hyperglycemia (glucose > 180 mg/dL) | 74/98 (76) |
| 0%20% of readings | 20/98 (20) |
| 20%40% | 19/98 (19) |
| 40%60% | 19/98 (19) |
| 60%80% | 6/98 (6.1) |
| Greater than 80% | 10/98 (10) |
| Any hypoglycemia (glucose < 60 mg/dL) | 11/98 (11) |
| 0%20% of readings | 9/98 (9.2) |
| 20%40% | 2/98 (2.0) |
Regarding glucose control, 317 of 1022 glucose meter readings (31%) were greater than 180 mg/dL, and the mean rate of glucose readings greater than 180 mg/dL per patient was also 31%. Approximately three quarters of all patients had at least one routine glucose reading greater than 180 mg/dL, and 35% of patients had at least 40% of their routine glucose readings greater than 180 mg/dL (Table 2). Twelve of 1022 readings (1.2%) were less than 60 mg/dL, and 11% of patients had at least one glucose reading less than 60 mg/dL (Table 2).
Despite a relatively constant percentage of glucose readings greater than 180 mg/dL per patient over the first 5 days of hospitalization (25%36% each day), we found no evidence of change in the percentage of patients prescribed basal insulin or the percentage of insulin given as basal insulin, and there was a small but significant increase in the total amount of insulin prescribed (Table 3). Of the 75 patients with at least one episode of hypo‐ or hyperglycemia, 43 (57%) were ever prescribed basal insulin, 29 (39%) were prescribed oral diabetes agents, and only 26 (35%) had any change to their insulin regimen during the first 5 days of their hospitalization on GMS. Of the 47 patients prescribed basal insulin in the hospital, 41 had been taking insulin prior to admission.
| Hospital day | P value for Trend | |||||
|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | ||
| ||||||
| Number of patients | 107 | 105 | 85 | 66 | 48 | |
| Mean adjusted total daily insulin units* | 17 | 22 | 23 | 20 | 27 | 0.03 |
| Patients prescribed any basal insulin (%) | 29/79 (37) | 41/93 (44) | 33/74 (45) | 27/57 (47) | 20/43 (47) | 0.18 |
| Mean % of total insulin dose consisting of basal insulin | 35 | 42 | 38 | 39 | 33 | 0.80 |
| Mean % glucose readings < 60 mg/dL | 2 | 1 | 1 | 0 | 1 | 0.13 |
| Mean % glucose readings> 180 mg/dL | 36 | 34 | 29 | 25 | 32 | 0.13 |
In a multivariable analysis of the mean glucose reading per patient‐day, we found several predictors of lower glucose readings, including diet‐controlled diabetes prior to admission and prescription of oral hypoglycemic medications in the hospital. We also found several predictors of higher glucose readings, including severe diabetic complications and higher glucose level at admission. Finally, we noted variation both by medical team (each composed of 1 medical attending, 1 resident, and 2 interns) and by floor of the hospital (each staffed by a different cadre of nurses). Adjusting for these factors (as well as for the daily use of dextrose‐containing intravenous fluids and steroids, sex, age, Charlson comorbidity score, APACHE 3 score, prior diagnosis of diabetes, HbA1C level, and length of hospital stay) use of sliding‐scale insulin alone (eg, without scheduled basal or nutritional insulin) was associated with a daily average glucose reading that was 20 mg/dL higher than that for those prescribed scheduled insulin or those not prescribed a sliding scale at all (95% confidence interval, 5.035 mg/dL; Table 4). In a separate analysis, adjusting for the same clinical factors, we could find no relationship between change in daily dose of basal insulin and change in daily average glucose level (data not shown).
| Characteristic | Effect size (95% CI)* | P value |
|---|---|---|
| ||
| Sliding scale insulin alone | 20 (5.035) | 0.01 |
| Oral diabetes regimen during hospitalization | 22 (413.0) | 0.02 |
| Diet‐controlled diabetes prior to admission | 32 (577.6) | 0.01 |
| No prior diagnosis of diabetes | 28 (3.260) | 0.08 |
| Complications of diabetes | 44 (2167) | < 0.001 |
| HbA1C | 6.1 (120.0073) | 0.05 |
| Admission glucose | 0.19 (0.0670.31) | 0.002 |
| Medical Team‖ | 47 (6727) | < 0.001 |
| Hospital Floor | 46 (6824) | < 0.001 |
DISCUSSION
In this observational study, we found several deficiencies in the management of diabetes and hyperglycemia among hospitalized patients on a hospitalist‐run general medical service. These deficiencies were both in processes of care (eg, limited use of basal and especially nutritional insulin) and in outcomes (ie, glycemic control) compared with national guidelines. We also found evidence of clinical inertia when comparing outpatient to inpatient regimens, when evaluating daily changes in management, and when evaluating responses to previous hyperglycemia. Finally, we demonstrated that use of an insulin sliding scale by itself was associated with worse glycemic control after extensive adjustment for a variety of clinical factors.
Of note, other than the use of sliding‐scale insulin by itself, we could not find a relationship between specific daily adjustments to insulin orders and daily glycemic control in this study. However, we did find differences in glycemic control by medical team and by floor (the latter a proxy for nursing staff). This suggests that glycemic control depends on the exact details of how insulin is managed, rather than on crude measures of insulin adjustment such as change in dose in response to hyperglycemia. These findings also suggest that interventions focused on medical and nursing staff may be able to improve inpatient glycemic control.
The association between the use of oral diabetic agents and improved glucose control was notable and could represent an actual benefit of these agents (especially when added to sliding‐scale insulin by itself) and/or the result of uncontrolled confounding (ie, as a marker of well‐controlled diabetes). Further study is needed to distinguish among these possibilities.
Previous studies have shown evidence of poor inpatient glycemic control as well as the deleterious effects of sliding‐scale insulin by itself.8 This study is perhaps most notable for the suggestion that little, if anything, has changed over the previous decade in this area, despite recent well‐done observational and randomized controlled trials demonstrating the hazards of inpatient hyperglycemia and the publication of expert consensus statements on inpatient glucose management. Strategies to improve glucose control have been investigated to a greater extent in intensive care units12, 13 than on general medical wards,14 perhaps because the strength of evidence is strongest in this setting. Without such strong evidence for general medical patients, factors such as clinician fear of hypoglycemia, clinical inertia, and resistance to institutional change may play predominant roles.
Clinical inertia (ie, recognition of the problem but failure to act)15 has been demonstrated previously in the outpatient management of diabetes16, 17; this study provides evidence of the phenomenon in the inpatient setting. Work by Phillips and colleagues15 has shown that clinical inertia results from at least 3 problems: overestimation of care provided; use of soft reasons to avoid intensification of therapy; and lack of education, training, and practice organization aimed at achieving specific goals. All 3 problems likely contribute to clinical inertia in inpatient diabetes management. Revised educational programs; systems for improving care such as reminders, flow sheets, and order sets; and performance feedback can help address clinical inertia and improve care.15
This study should be viewed in light of its limitations, including relatively small sample size, thus limiting our ability to detect other possible significant predictors of glycemic control, and the use of a single institution, thus limiting generalizability. However, recent data from the University HealthSystem Consortium revealed that our institution was typical of the 37 participating academic medical centers in that study.18 In addition, only 9 patients were identified without a prior diagnosis of diabetes, raising the possibility that some patients with undiagnosed diabetes were missed in our study. However, our search strategy included a daily review of automatically abstracted laboratory values, making this possibility less likely. Strengths of this study include its prospective data collection methods with rigorous inclusion criteria, collection of detailed clinical data, and use of a novel statistical technique to more accurately assess the complex relationship between insulin use and glycemic control, appropriately adjusting for confounding by indication caused by prior glucose measurements.
Future research should focus on patient, clinician, and system barriers to improving inpatient glycemic management, using the clinical inertia framework as a starting point, and on the creation of insulin protocols that can be used and proven effective in the non‐ICU inpatient setting. Also needed are improved measures of the quality of glycemic control, insulin orders, and daily insulin adjustment.
In conclusion, inpatient glycemic management was shown to be in need of improvement. Institutionwide quality improvement efforts should probably target both physician and nursing behavior and should focus on increasing use of basal and nutritional insulin, as proposed in recent guidelines, avoiding use of sliding‐scale insulin by itself, and performing daily insulin adjustment in response to previous hypo‐ or hyperglycemia. Hospitalists can play a major role in these institutionwide quality improvement efforts.
Acknowledgements
We thank Paul Szumita, PharmD, and LeRoi Hicks, MD, MPH, for assistance with the conception of this project and E. John Orav, PhD, for statistical assistance.
Diabetes mellitus is a common comorbidity of hospitalization; in 2003 diabetes was a secondary diagnosis in 17.8% of all adult hospital discharges.1 When undiagnosed diabetes is included, the prevalence of inpatient diabetes or hyperglycemia may be as high as 38%.2 Recent studies show that hyperglycemia in hospitalized patients complicates numerous illnesses and is an independent predictor of adverse outcomes.3 Treatment of inpatient hyperglycemia improves outcomes, including mortality, for patients in surgical intensive care units4 and possibly for those admitted for myocardial infarction.5, 6 For these reasons, the American Diabetes Association and the American College of Endocrinology now recommend that glucose levels of all patients admitted to non‐critical‐care units be maintained below 180 mg/dL.3, 7
Evidence‐based recommendations for achieving these goals include effective protocols for subcutaneous insulin therapy for patients who do not require continuous intravenous insulin infusion. Components of these protocols include use of basal insulin and scheduled nutritional insulin, avoiding use of supplemental (sliding‐scale) insulin alone (which has been shown to be ineffective and possibly deleterious in prior studies),8 and adjustment of insulin orders to reflect nutritional intake, insulin sensitivity, and previous response to therapy.7
We conducted this study to evaluate the current state of glycemic control and adherence to current recommendations on a general medicine service run by hospitalists in a busy teaching hospital. We also sought to correlate insulin‐ordering practices with the quality of glycemic control in these patients.
METHODS
Setting and Participants
This prospective cohort study was conducted at Brigham and Women's Hospital (BWH) from August 1 through September 30, 2004. Eligible subjects were patients admitted to 3 General Medicine Service (GMS) teams with either a known diagnosis of diabetes or inpatient hyperglycemia (random glucose > 200 mg/dL). Patients admitted for diabetic ketoacidosis, hyperosmolar hyperglycemic state, or gestational diabetes were excluded. Members of the BWH/Faulkner Hospitalist Service are the teaching attendings on these 3 teams (each consisting of 2 interns and 1 junior or senior resident) and are the attendings of record for approximately 90% of the patients on these teams. A research assistant identified potential subjects each weekday from the daily computerized sign‐out system used by all medical residents by searching for diabetes on the patient summary, a diabetic medication in the automatically abstracted medication list, or a laboratory glucose value greater than 200 mg/dL from automatically abstracted daily laboratory results. Eligibility criteria were confirmed by medical record review, and any question of eligibility was reviewed with the principal investigator. This study was approved by the BWH Institutional Review Board; patient consent was not deemed necessary for this study given the relatively nonsensitive nature of the data (eg, glucose control, insulin orders), the noninvasive means of collecting it (eg, chart review), and the steps taken by research personnel to minimize any breach in patient confidentiality.
Measurements
We abstracted clinical data on each eligible subject for up to 5 days on GMS. Several data sources were used, including physician admission notes, the hospital's computerized clinical data system, nursing notes, vital sign sheets, the medication administration record, and personal communication with nurses about any missing or discrepant data. Up to 4 routine bedside blood glucose measurements were recorded each day: the measurements taken before meals and at bedtime for patients eating discrete meals or the measurements closest to 6 AM, noon, 6 PM, and midnight for patients not eating or receiving continuous nutrition. Additional measurements were not recorded to avoid ascertainment bias caused by follow‐up testing of abnormal glucose values.
Study outcomes included the percentage of glucose readings below 60 mg/dL (hypoglycemia) and greater than 180 mg/dL (hyperglycemia). Use of several types of insulin ordering practices were also recorded: use of basal insulin (ie, long‐acting agents such as NPH and insulin glargine), scheduled prandial insulin (eg, regular insulin, insulin lispro, and insulin aspart given before each meal), daily adjustments to insulin orders, use of different insulin sliding scales for patients with different daily insulin requirements, orders to hold or adjust insulin doses in patients not eating, and the percentage of the total daily insulin dose given as basal insulin.
Other patient variables collected were age, sex, weight; medical comorbidities (using a modified Charlson score)9; severity of illness (using a simplified APACHE III score)8; admission diagnosis; baseline HbA1C (taken at or within 6 months of admission); severe complications of diabetes (blindness, dialysis, renal transplant, amputation due to peripheral vascular disease, vascular bypass surgery); diabetic medications prior to admission (none, oral agents only, or any insulin use); daily inpatient use of oral or intravenous steroids, oral diabetic medications, dextrose‐containing intravenous fluids, tube feeds, inpatient total parenteral nutrition, and general nutritional intake (all, most, some, little, or none for each meal).
Statistical Analysis
Characteristics of the study subjects and process and outcome measures were analyzed descriptively using rates, means with standard deviations, and medians with interquartile ranges as appropriate. We also analyzed outcomes by patient‐day to determine daily trends during the course of hospitalization. In these analyses, we used the Mantel‐Haenszel chi‐square test for the dichotomous variables (eg, daily use of any basal insulin) and univariable linear regression with general linear models clustered by patient, that is, repeated‐measures analysis, for the continuous variables. We used an arcsin(square‐root) transformation for those continuous outcomes that were percentages (eg, percentage of glucose readings > 180 mg/dL) and logarithmic transformation for right‐tailed continuous variables (eg, total number of units of insulin administered).
To determine the effects of various insulin‐ordering practices on glucose control, we also performed multivariable analysis of mean glucose levels per patient‐day. We chose mean glucose rather than rates of specific glucose ranges as the outcome because of the low rate of hypoglycemia and the additional sensitivity of this method. First, univariable analysis was performed using the Student's t test, analysis of variance, or Spearman correlation as appropriate for each predictor. Multiple linear regression models were then constructed, using variables significant in the univariable testing at the P < .10 level. Confounding variables that changed beta coefficients by 10% or more were retained, whereas collinear terms were removed by hand; patient age and sex were also retained in the models as a priori selected confounding variables.
As with the repeated‐measures analysis, we used general linear models, accounting for within‐patient clustering, with an exchangeable correlation structure. In addition, standard regression techniques could not be applied to the basal insulin variable because use of basal insulin is a mediator of subsequently lower glucose levels but often is the result of previously elevated glucose levels. Instead, we used a marginal structural model,10, 11 weighting the usual regression analysis to statistically remove the effect of confounding by indication. The weights for this analysis were based on the inverse probability of use of basal insulin, given previous glucose levels and prior use of basal insulin and were estimated from a separate logistic regression analysis. Results were considered significant at P < .05 except as noted above. SAS version 8.1 (Cary, NC) was used for all analyses.
RESULTS
We prospectively identified 123 patients for the study. Subsequently, 16 patients were excluded, 11 who did not have diabetes or inpatient hyperglycemia (most of whom had been placed on insulin prophylactically to avoid steroid‐induced hyperglycemia), 2 who were admitted for diabetic ketoacidosis, 2 who were not on GMS teams 13, and 1 whose data could not be accessed. Characteristics of the remaining 107 study subjects are shown in Table 1. The mean age of the subjects was 65.2 years; 55% were men. Nine patients had no previous diagnosis of diabetes, 43% were taking insulin prior to admission, 14% had severe diabetic complications, and the median HbA1C was 7.
| Characteristic | |
|---|---|
| |
| Age, mean (SD), y | 65.2 (14.5) |
| Male | 59/107 (55) |
| No diagnosis of diabetes at admission | 9/107 (8) |
| Preadmission diabetes medication regimen: | |
| None | 24/106 (22) |
| Oral medications only | 36/106 (34) |
| Insulin | 46/106 (43) |
| HbA1C, median (IQR) | 7 (6, 8) |
| Diabetic complications | 15/107 (14) |
| Hospital length of stay, median (IQR), d | 5 (3, 7) |
| Charlson score, mean (SD) | 5.3 (3.0) |
| APACHE III score, mean (SD) | 36.9 (15.6) |
Regarding insulin‐ordering practices (Table 2), 47 patients (43%) had basal insulin prescribed, while 4% of patients had an order for scheduled mealtime short‐ or rapid‐acting insulin. Of the 89 patients on sliding‐scale insulin, 80 (90%) had orders written for the default sliding scale built into the computerized physician order entry system at BWH. There was no correlation between intensity of the sliding scale and the patient's total daily insulin dose (data not shown). Of the patients on sliding‐scale insulin, 47% were prescribed basal insulin, 39% were prescribed oral agents, and 23% were prescribed neither.
| Measure | |
|---|---|
| |
| Process | |
| Any basal insulin during hospitalization | 47/107 (43) |
| Separate nutritional insulin order | 4/107 (3.7) |
| Change in dose to any insulin order during hospitalization if any hyper‐ or hypoglycemia | 26/75 (35) |
| Standard sliding scale from hospital computer order set | 80/89 (90) |
| Any oral diabetic agents during hospitalization | 39/107 (36) |
| Outcomes | |
| Any hyperglycemia (glucose > 180 mg/dL) | 74/98 (76) |
| 0%20% of readings | 20/98 (20) |
| 20%40% | 19/98 (19) |
| 40%60% | 19/98 (19) |
| 60%80% | 6/98 (6.1) |
| Greater than 80% | 10/98 (10) |
| Any hypoglycemia (glucose < 60 mg/dL) | 11/98 (11) |
| 0%20% of readings | 9/98 (9.2) |
| 20%40% | 2/98 (2.0) |
Regarding glucose control, 317 of 1022 glucose meter readings (31%) were greater than 180 mg/dL, and the mean rate of glucose readings greater than 180 mg/dL per patient was also 31%. Approximately three quarters of all patients had at least one routine glucose reading greater than 180 mg/dL, and 35% of patients had at least 40% of their routine glucose readings greater than 180 mg/dL (Table 2). Twelve of 1022 readings (1.2%) were less than 60 mg/dL, and 11% of patients had at least one glucose reading less than 60 mg/dL (Table 2).
Despite a relatively constant percentage of glucose readings greater than 180 mg/dL per patient over the first 5 days of hospitalization (25%36% each day), we found no evidence of change in the percentage of patients prescribed basal insulin or the percentage of insulin given as basal insulin, and there was a small but significant increase in the total amount of insulin prescribed (Table 3). Of the 75 patients with at least one episode of hypo‐ or hyperglycemia, 43 (57%) were ever prescribed basal insulin, 29 (39%) were prescribed oral diabetes agents, and only 26 (35%) had any change to their insulin regimen during the first 5 days of their hospitalization on GMS. Of the 47 patients prescribed basal insulin in the hospital, 41 had been taking insulin prior to admission.
| Hospital day | P value for Trend | |||||
|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | ||
| ||||||
| Number of patients | 107 | 105 | 85 | 66 | 48 | |
| Mean adjusted total daily insulin units* | 17 | 22 | 23 | 20 | 27 | 0.03 |
| Patients prescribed any basal insulin (%) | 29/79 (37) | 41/93 (44) | 33/74 (45) | 27/57 (47) | 20/43 (47) | 0.18 |
| Mean % of total insulin dose consisting of basal insulin | 35 | 42 | 38 | 39 | 33 | 0.80 |
| Mean % glucose readings < 60 mg/dL | 2 | 1 | 1 | 0 | 1 | 0.13 |
| Mean % glucose readings> 180 mg/dL | 36 | 34 | 29 | 25 | 32 | 0.13 |
In a multivariable analysis of the mean glucose reading per patient‐day, we found several predictors of lower glucose readings, including diet‐controlled diabetes prior to admission and prescription of oral hypoglycemic medications in the hospital. We also found several predictors of higher glucose readings, including severe diabetic complications and higher glucose level at admission. Finally, we noted variation both by medical team (each composed of 1 medical attending, 1 resident, and 2 interns) and by floor of the hospital (each staffed by a different cadre of nurses). Adjusting for these factors (as well as for the daily use of dextrose‐containing intravenous fluids and steroids, sex, age, Charlson comorbidity score, APACHE 3 score, prior diagnosis of diabetes, HbA1C level, and length of hospital stay) use of sliding‐scale insulin alone (eg, without scheduled basal or nutritional insulin) was associated with a daily average glucose reading that was 20 mg/dL higher than that for those prescribed scheduled insulin or those not prescribed a sliding scale at all (95% confidence interval, 5.035 mg/dL; Table 4). In a separate analysis, adjusting for the same clinical factors, we could find no relationship between change in daily dose of basal insulin and change in daily average glucose level (data not shown).
| Characteristic | Effect size (95% CI)* | P value |
|---|---|---|
| ||
| Sliding scale insulin alone | 20 (5.035) | 0.01 |
| Oral diabetes regimen during hospitalization | 22 (413.0) | 0.02 |
| Diet‐controlled diabetes prior to admission | 32 (577.6) | 0.01 |
| No prior diagnosis of diabetes | 28 (3.260) | 0.08 |
| Complications of diabetes | 44 (2167) | < 0.001 |
| HbA1C | 6.1 (120.0073) | 0.05 |
| Admission glucose | 0.19 (0.0670.31) | 0.002 |
| Medical Team‖ | 47 (6727) | < 0.001 |
| Hospital Floor | 46 (6824) | < 0.001 |
DISCUSSION
In this observational study, we found several deficiencies in the management of diabetes and hyperglycemia among hospitalized patients on a hospitalist‐run general medical service. These deficiencies were both in processes of care (eg, limited use of basal and especially nutritional insulin) and in outcomes (ie, glycemic control) compared with national guidelines. We also found evidence of clinical inertia when comparing outpatient to inpatient regimens, when evaluating daily changes in management, and when evaluating responses to previous hyperglycemia. Finally, we demonstrated that use of an insulin sliding scale by itself was associated with worse glycemic control after extensive adjustment for a variety of clinical factors.
Of note, other than the use of sliding‐scale insulin by itself, we could not find a relationship between specific daily adjustments to insulin orders and daily glycemic control in this study. However, we did find differences in glycemic control by medical team and by floor (the latter a proxy for nursing staff). This suggests that glycemic control depends on the exact details of how insulin is managed, rather than on crude measures of insulin adjustment such as change in dose in response to hyperglycemia. These findings also suggest that interventions focused on medical and nursing staff may be able to improve inpatient glycemic control.
The association between the use of oral diabetic agents and improved glucose control was notable and could represent an actual benefit of these agents (especially when added to sliding‐scale insulin by itself) and/or the result of uncontrolled confounding (ie, as a marker of well‐controlled diabetes). Further study is needed to distinguish among these possibilities.
Previous studies have shown evidence of poor inpatient glycemic control as well as the deleterious effects of sliding‐scale insulin by itself.8 This study is perhaps most notable for the suggestion that little, if anything, has changed over the previous decade in this area, despite recent well‐done observational and randomized controlled trials demonstrating the hazards of inpatient hyperglycemia and the publication of expert consensus statements on inpatient glucose management. Strategies to improve glucose control have been investigated to a greater extent in intensive care units12, 13 than on general medical wards,14 perhaps because the strength of evidence is strongest in this setting. Without such strong evidence for general medical patients, factors such as clinician fear of hypoglycemia, clinical inertia, and resistance to institutional change may play predominant roles.
Clinical inertia (ie, recognition of the problem but failure to act)15 has been demonstrated previously in the outpatient management of diabetes16, 17; this study provides evidence of the phenomenon in the inpatient setting. Work by Phillips and colleagues15 has shown that clinical inertia results from at least 3 problems: overestimation of care provided; use of soft reasons to avoid intensification of therapy; and lack of education, training, and practice organization aimed at achieving specific goals. All 3 problems likely contribute to clinical inertia in inpatient diabetes management. Revised educational programs; systems for improving care such as reminders, flow sheets, and order sets; and performance feedback can help address clinical inertia and improve care.15
This study should be viewed in light of its limitations, including relatively small sample size, thus limiting our ability to detect other possible significant predictors of glycemic control, and the use of a single institution, thus limiting generalizability. However, recent data from the University HealthSystem Consortium revealed that our institution was typical of the 37 participating academic medical centers in that study.18 In addition, only 9 patients were identified without a prior diagnosis of diabetes, raising the possibility that some patients with undiagnosed diabetes were missed in our study. However, our search strategy included a daily review of automatically abstracted laboratory values, making this possibility less likely. Strengths of this study include its prospective data collection methods with rigorous inclusion criteria, collection of detailed clinical data, and use of a novel statistical technique to more accurately assess the complex relationship between insulin use and glycemic control, appropriately adjusting for confounding by indication caused by prior glucose measurements.
Future research should focus on patient, clinician, and system barriers to improving inpatient glycemic management, using the clinical inertia framework as a starting point, and on the creation of insulin protocols that can be used and proven effective in the non‐ICU inpatient setting. Also needed are improved measures of the quality of glycemic control, insulin orders, and daily insulin adjustment.
In conclusion, inpatient glycemic management was shown to be in need of improvement. Institutionwide quality improvement efforts should probably target both physician and nursing behavior and should focus on increasing use of basal and nutritional insulin, as proposed in recent guidelines, avoiding use of sliding‐scale insulin by itself, and performing daily insulin adjustment in response to previous hypo‐ or hyperglycemia. Hospitalists can play a major role in these institutionwide quality improvement efforts.
Acknowledgements
We thank Paul Szumita, PharmD, and LeRoi Hicks, MD, MPH, for assistance with the conception of this project and E. John Orav, PhD, for statistical assistance.
- Agency for Healthcare Research and Quality. HCUPnet, Healthcare Cost and Utilization Project. 8/17/2005. Available at: http://www.ahrq.gov/HCUPnet/. Accessed November 29,2005.
- ,,,,,.Hyperglycemia: an independent marker of in‐hospital mortality in patients with undiagnosed diabetes.J Clin Endocrinol Metab.2002;87:978–982.
- ,,, et al.Management of diabetes and hyperglycemia in hospitals.Diabetes Care.2004;27:553–597.
- ,,, et al.Intensive insulin therapy in the critically ill patients.N Engl J Med.2001;345:1359–1367.
- .Prospective randomised study of intensive insulin treatment on long term survival after acute myocardial infarction in patients with diabetes mellitus. DIGAMI (Diabetes Mellitus, Insulin Glucose Infusion in Acute Myocardial Infarction) Study Group.Br Med J.1997;314:1512–1515.
- ,,, et al.Intense metabolic control by means of insulin in patients with diabetes mellitus and acute myocardial infarction (DIGAMI 2): effects on mortality and morbidity.Eur Heart J2005;26:650–661.
- ,,, et al.American College of Endocrinology position statement on inpatient diabetes and metabolic control.Endocr Pract.2004;10(Suppl2):4–9.
- ,,.Glycemic control and sliding scale insulin use in medical inpatients with diabetes mellitus.Arch Intern Med.1997;157:545–552.
- ,,,,.Can comorbidity be measured by questionnaire rather than medical record review?Med Care.1996;34:73–84.
- ,,.Marginal structural models to estimate the joint causal effect of nonrandomized treatments.J Am Stat Assoc—App Case Stud.2001;96:440–448.
- .Correction for non‐compliance in equivalence trials.Stat Med.1998;17:269–302; discussion87–89.
- ,,, et al.Implementation of a safe and effective insulin infusion protocol in a medical intensive care unit.Diabetes Care.2004;27:461–7.
- .Effect of an intensive glucose management protocol on the mortality of critically ill adult patients.Mayo Clin Proc.2004;79:992–1000.
- ,,,.Eliminating inpatient sliding‐scale insulin: a reeducation project with medical house staff.Diabetes Care.2005;28:1008–1011.
- ,,, et al.Clinical inertia.Ann Intern Med.2001;135:825–834.
- ,,,,.Diabetes in urban African‐Americans. XV. Identification of barriers to provider adherence to management protocols.Diabetes Care.1999;22:1617–1620.
- ,,, et al.Clinical inertia in the management of type 2 diabetes metabolic risk factors.Diabet Med.2004;21:150–155.
- University HealthSystem Consortium.Glycemic control 2005 findings and conclusions. Paper presented at Glycemic Control 2005 Knowledge Transfer Meeting. August 19,2005, Chicago, IL.
- Agency for Healthcare Research and Quality. HCUPnet, Healthcare Cost and Utilization Project. 8/17/2005. Available at: http://www.ahrq.gov/HCUPnet/. Accessed November 29,2005.
- ,,,,,.Hyperglycemia: an independent marker of in‐hospital mortality in patients with undiagnosed diabetes.J Clin Endocrinol Metab.2002;87:978–982.
- ,,, et al.Management of diabetes and hyperglycemia in hospitals.Diabetes Care.2004;27:553–597.
- ,,, et al.Intensive insulin therapy in the critically ill patients.N Engl J Med.2001;345:1359–1367.
- .Prospective randomised study of intensive insulin treatment on long term survival after acute myocardial infarction in patients with diabetes mellitus. DIGAMI (Diabetes Mellitus, Insulin Glucose Infusion in Acute Myocardial Infarction) Study Group.Br Med J.1997;314:1512–1515.
- ,,, et al.Intense metabolic control by means of insulin in patients with diabetes mellitus and acute myocardial infarction (DIGAMI 2): effects on mortality and morbidity.Eur Heart J2005;26:650–661.
- ,,, et al.American College of Endocrinology position statement on inpatient diabetes and metabolic control.Endocr Pract.2004;10(Suppl2):4–9.
- ,,.Glycemic control and sliding scale insulin use in medical inpatients with diabetes mellitus.Arch Intern Med.1997;157:545–552.
- ,,,,.Can comorbidity be measured by questionnaire rather than medical record review?Med Care.1996;34:73–84.
- ,,.Marginal structural models to estimate the joint causal effect of nonrandomized treatments.J Am Stat Assoc—App Case Stud.2001;96:440–448.
- .Correction for non‐compliance in equivalence trials.Stat Med.1998;17:269–302; discussion87–89.
- ,,, et al.Implementation of a safe and effective insulin infusion protocol in a medical intensive care unit.Diabetes Care.2004;27:461–7.
- .Effect of an intensive glucose management protocol on the mortality of critically ill adult patients.Mayo Clin Proc.2004;79:992–1000.
- ,,,.Eliminating inpatient sliding‐scale insulin: a reeducation project with medical house staff.Diabetes Care.2005;28:1008–1011.
- ,,, et al.Clinical inertia.Ann Intern Med.2001;135:825–834.
- ,,,,.Diabetes in urban African‐Americans. XV. Identification of barriers to provider adherence to management protocols.Diabetes Care.1999;22:1617–1620.
- ,,, et al.Clinical inertia in the management of type 2 diabetes metabolic risk factors.Diabet Med.2004;21:150–155.
- University HealthSystem Consortium.Glycemic control 2005 findings and conclusions. Paper presented at Glycemic Control 2005 Knowledge Transfer Meeting. August 19,2005, Chicago, IL.
Copyright © 2006 Society of Hospital Medicine