User login
Interhospital Transfer and Receipt of Specialty Procedures
Patients who undergo interhospital transfer (IHT) are felt to benefit from receipt of unique specialty care at the receiving hospital.1 Although only 1.5% of all hospitalized Medicare patients undergo hospital transfer,2 the frequency of transfer is much greater within certain patient populations, as may be expected with diagnoses requiring specialty care.3,4 Existent data demonstrate that 5% of Medicare patients admitted to the intensive care unit (ICU)5 and up to 50% of patients presenting with acute myocardial infarction (AMI) undergo IHT.6
More recent data suggest variability in hospital transfer practices not accounted for by differences in patient or hospital characteristics.2 Although disease-specific guidelines for IHT exist for certain diagnoses,3,4 the process remains largely nonstandardized for many patients,7 leading to ambiguity surrounding indications for transfer. Because limited data suggest worse outcomes for transferred versus nontransferred patients,8 a better understanding of the specialized care patients actually receive across the transfer continuum may help to elucidate potential indications for transfer and ultimately help delineate which patients are most (or least) likely to benefit from transfer and why.
In this national study, we examined a select cohort of transferred patients with diagnoses associated with specific specialty procedural services to determine if they received these procedures and where along the transfer continuum they were performed.
METHODS
We performed a cross-sectional analysis using the Center for Medicare and Medicaid Services 2013 100% Master Beneficiary Summary and Inpatient claims files. Our study protocol was approved by the Partners Healthcare Human Subjects Review Committee.
Beneficiaries were eligible for inclusion if they were aged ≥65 years, continuously enrolled in Medicare A and B, and with an acute care hospitalization claim in 2013, excluding Medicare managed care and end stage renal disease beneficiaries due to incomplete claims data in these groups. We additionally excluded beneficiaries hospitalized at federal or nonacute care hospitals, or critical access hospitals given their mission to stabilize and then transfer patients to referral hospitals.9
Transferred patients were defined as beneficiaries with corresponding “transfer in” and “transfer out” claims, or those with either claim and a corresponding date of admission/discharge from another hospital within 1 day of the claim, as we used in our prior research.2 Beneficiaries transferred to the same hospital, those with greater than 1 transfer within the same hospitalization, or those cared for at hospitals with “outlier” transfer-in rates equal to 100% or transfer-out rates greater than 35% were excluded from analysis given the suggestion of nonstandard claims practices.
We first identified the top 15 primary diagnoses at time of transfer using International Classification of Diseases, Ninth Revision (ICD-9) codes (supplementary Appendix), and then identified those 4 most likely to require specialty procedural services: AMI, gastrointestinal bleed (GI bleed), renal failure, and hip fracture/dislocation. We then chose associated ICD-9 procedure codes for each diagnosis, via expert opinion (authors SM and JS, hospitalist physicians with greater than 20 years of combined clinical experience), erring on overinclusion of procedure codes. We then quantified receipt of associated procedures at transferring and receiving hospitals, stratified by diagnosis.
We further explored the cohort of patients with hip fracture/dislocation who underwent an associated procedure at the transferring but not receiving hospital, examining the frequency with which these patients had other (nonrelated) procedures at the receiving hospital, and identifying which procedures they received.
RESULTS
Of the 101,507 patients transferred to another hospital, 19,613 (19.3%) had a primary diagnosis of AMI, GI bleed, renal failure, or hip fracture/dislocation. Table 1 lists the ICD-9 procedure codes associated with each diagnosis.
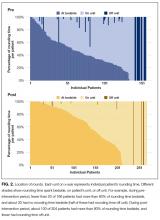
Distribution of receipt of specialty procedures at the transferring and receiving hospitals varied by disease (Figure). With the exception of GI bleed, patients more often received specialty procedural care at the receiving than the transferring hospital. Depending on primary diagnosis, between 32.4% and 89.1% of patients did not receive any associated specialty procedure at the receiving hospital.
Of the 370 (22.1%) hip fracture/dislocation patients that received a specialty procedure at the transferring but not receiving hospital, 132 (35.7%) did not receive any procedure at the receiving hospital, whereas the remaining 238 (64.3%) received an unrelated (not associated with the primary diagnosis) procedure. There was great variety in the types of procedures received, the most common being transfusion of blood products (ICD-9 Clinical Modification 9904).
DISCUSSION
Among transferred patients with primary diagnoses that have clearly associated specialized procedural services, we found that patients received these procedures at varying frequency and locations across the transfer continuum. Across 4 diagnoses, receipt of associated procedures was more common at the receiving than the transferring hospital, with the exception being patients with GI bleed. We additionally found that many transferred patients did not receive any associated specialty procedure at the receiving hospital. These findings suggest the strong likelihood of more diverse underlying reasons for transfer rather than solely receipt of specialized procedural care.
Despite the frequency with which AMI patients are transferred,6 and American Heart Association guidelines directing hospitals to transfer AMI patients to institutions able to provide necessary invasive treatments,4 prior studies suggest these patients inconsistently receive specialty intervention following transfer, including stress testing, cardiac catheterization, or coronary artery bypass graft surgery.10,11 Our findings add to these data, demonstrating that only 47.3% of patients transferred with AMI received any cardiac-related procedure at the receiving hospital. Additionally, we found that 38.1% of AMI patients do not receive any specialty procedures at either the transferring or the receiving hospital. Taken together, these data suggest possible discrepancies in the perceived need for these procedures between transferring and receiving hospitals, reasons for transfer related to these conditions that don’t involve an associated procedure, or reasons for transfer unrelated to specialty care of the primary diagnosis (such as care of comorbidities, hospital location, prior relationships with that hospital, or desire for a second opinion). Although some of these alternate reasons for transfer likely still benefit the patient, some of these reasons may not justify the increased risks of discontinuity of care created by IHT.
Given limited data looking at IHT practices for patients with other diagnoses, the varying patterns of specialty procedural interventions we observed among transferred patients with GI bleed, renal failure, and hip fracture/dislocation are novel contributions to this topic. Notably, we found that among patients transferred with a primary diagnosis of renal failure, the vast majority (84.1%) did not receive any associated procedure at either the transferring or the receiving hospital. It is possible that although these patients carried the diagnosis of renal failure, their clinical phenotype is more heterogeneous, and they could still be managed conservatively without receipt of invasive procedures such as hemodialysis.
Conversely, patients transferred with primary diagnosis of hip fracture/dislocation were far more likely to receive associated specialty procedural intervention at the receiving hospital, presumably reflective of the evidence demonstrating improved outcomes with early surgical intervention.12 However, these data do not explain the reasoning behind the substantial minority of patients who received specialty intervention at the transferring hospital prior to transfer or those that did not receive any specialty intervention at either the transferring or receiving hospital. Our secondary analysis demonstrating great variety in receipt and type of nonassociated procedures provided at the receiving hospital did not help to elucidate potential underlying reasons for transfer.
Notably, among patients transferred with primary diagnosis of GI bleed, receipt of specialty procedures was more common at the transferring (77.7%) than receiving (63.2%) hospital, with nearly half (49.3%) undergoing specialty procedures at both hospitals. It is possible that these findings are reflective of the broad array of specialty procedures examined within this diagnosis. For example, it is reasonable to consider that a patient may be stabilized with receipt of a blood transfusion at the transferring hospital, then transferred to undergo a diagnostic/therapeutic procedure (ie, endoscopy/colonoscopy) at the receiving hospital, as is suggested by our results.
Our study is subject to several limitations. First, given the criteria we used to define transfer, it is possible that we included nontransferred patients within our transferred cohort if they were discharged from one hospital and admitted to a different hospital within 1 day, although quality assurance analyses we conducted in prior studies on these data support the validity of the criteria used.2 Second, we cannot exclude the possibility that patients received nonprocedural specialty care (ie, expert opinion, specialized imaging, medical management, management of secondary diagnoses, etc.) not available at the transferring hospital, although, arguably, in select patients, such input could be obtained without physical transfer of the patient (ie, tele-consult). And even in patients transferred with intent to receive procedural care who did not ultimately receive that care, there is likely an appropriate “nonprocedure” rate, where patients who might benefit from a procedure receive a timely evaluation to reduce the risk of missing the opportunity to receive it. This would be analogous to transferring a patient to an ICU even if they do not end up requiring intubation or pressor therapy. However, given the likelihood of higher risks of IHT compared with intrahospital transfers, one could argue that the threshold of perceived benefit might be different in patients being considered for IHT. Additionally, we limited our analyses to only 4 diagnoses; thus, our findings may not be generalizable to other diagnoses of transferred patients. However, because the diagnoses we examined were ones considered most effectively treated with specialty procedural interventions, it is reasonable to presume that the variability in receipt of specialty procedures observed within these diagnoses is also present, if not greater, across other diagnoses. Third, although we intentionally included a broad array of specialty procedures associated with each diagnosis, it is possible that we overlooked particular specialty interventions. For example, in assuming that patients are most likely to be transferred to receive procedural services associated with their primary diagnosis, we may have missed alternate indications for transfer, including need for procedural care related to secondary or subsequent diagnoses (ie, a patient may have presented with GI bleed
CONCLUSIONS
We found that Medicare patients who undergo IHT with primary diagnoses of AMI, GI bleed, renal failure, and hip fracture/dislocation receive associated specialty interventions at varying frequency and locations, and many patients do not receive any associated procedures at receiving hospitals. Our findings suggest that specialty procedural care of patients, even those with primary diagnoses that often warrant specialized intervention, may not be the primary driver of IHT as commonly suggested, although underlying reasons for transfer in these and other “nonprocedural” transferred patients remains obscure. Given known ambiguity in the transfer process,7 and unclear benefit of IHT,8 additional research is required to further identify and evaluate other potential underlying reasons for transfer and to examine these in the context of patient outcomes, in order to understand which patients may or may not benefit from transfer and why.
Disclosure
The authors have nothing to disclose.
1. Iwashyna TJ. The incomplete infrastructure for interhospital patient transfer. Crit Care Med. 2012;40(8):2470-2478. PubMed
2. Mueller SK, Zheng J, Orav EJ, Schnipper JL. Rates, Predictors and Variability of Interhospital Transfers: A National Evaluation. J Hosp Med. 2017;12(6):435-442. PubMed
3. Guidelines for the transfer of critically ill patients. Guidelines Committee of the American College of Critical Care Medicine; Society of Critical Care Medicine and American Association of Critical-Care Nurses Transfer Guidelines Task Force. Crit Care Med. 1993;21(6):931-937. PubMed
4. Anderson JL, Adams CD, Antman EM, et al. 2011 ACCF/AHA Focused Update Incorporated Into the ACC/AHA 2007 Guidelines for the Management of Patients With Unstable Angina/Non-ST-Elevation Myocardial Infarction: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. Circulation. 2011;123(18):e426-e579. PubMed
5. Iwashyna TJ, Christie JD, Moody J, Kahn JM, Asch DA. The structure of critical care transfer networks. Med Care. 2009;47(7):787-793. PubMed
6. Iwashyna TJ, Kahn JM, Hayward RA, Nallamothu BK. Interhospital transfers among Medicare beneficiaries admitted for acute myocardial infarction at nonrevascularization hospitals. Circ Cardiovasc Qual Outcomes. 2010;3(5):468-475. PubMed
7. Bosk EA, Veinot T, Iwashyna TJ. Which patients and where: a qualitative study of patient transfers from community hospitals. Med Care. 2011;49(6):592-598. PubMed
8. Sokol-Hessner L, White AA, Davis KF, Herzig SJ, Hohmann SF. Interhospital transfer patients discharged by academic hospitalists and general internists: Characteristics and outcomes. J Hosp Med. 2016;11(4):245-250. PubMed
9. Department of Health and Human Services, Center for Medicare & Medicaid Services: Critical Access Hospitals. https://www.cms.gov/Outreach-and-Education/Medicare-Learning-Network-MLN/MLNProducts/downloads/CritAccessHospfctsht.pdf. Accessed June 29, 2017. PubMed
10. Roe MT, Chen AY, Delong ER, et al. Patterns of transfer for patients with non-ST-segment elevation acute coronary syndrome from community to tertiary care hospitals. Am Heart J. 2008;156(1):185-192. PubMed
11. Barreto-Filho JA, Wang Y, Rathore SS, et al. Transfer rates from nonprocedure hospitals after initial admission and outcomes among elderly patients with acute myocardial infarction. JAMA Intern Med. 2014;174(2):213-222. PubMed
12. Doruk H, Mas MR, Yildiz C, Sonmez A, Kyrdemir V. The effect of the timing of hip fracture surgery on the activity of daily living and mortality in elderly. Arch Gerontol Geriatr. 2004;39(2):179-185. PubMed
Patients who undergo interhospital transfer (IHT) are felt to benefit from receipt of unique specialty care at the receiving hospital.1 Although only 1.5% of all hospitalized Medicare patients undergo hospital transfer,2 the frequency of transfer is much greater within certain patient populations, as may be expected with diagnoses requiring specialty care.3,4 Existent data demonstrate that 5% of Medicare patients admitted to the intensive care unit (ICU)5 and up to 50% of patients presenting with acute myocardial infarction (AMI) undergo IHT.6
More recent data suggest variability in hospital transfer practices not accounted for by differences in patient or hospital characteristics.2 Although disease-specific guidelines for IHT exist for certain diagnoses,3,4 the process remains largely nonstandardized for many patients,7 leading to ambiguity surrounding indications for transfer. Because limited data suggest worse outcomes for transferred versus nontransferred patients,8 a better understanding of the specialized care patients actually receive across the transfer continuum may help to elucidate potential indications for transfer and ultimately help delineate which patients are most (or least) likely to benefit from transfer and why.
In this national study, we examined a select cohort of transferred patients with diagnoses associated with specific specialty procedural services to determine if they received these procedures and where along the transfer continuum they were performed.
METHODS
We performed a cross-sectional analysis using the Center for Medicare and Medicaid Services 2013 100% Master Beneficiary Summary and Inpatient claims files. Our study protocol was approved by the Partners Healthcare Human Subjects Review Committee.
Beneficiaries were eligible for inclusion if they were aged ≥65 years, continuously enrolled in Medicare A and B, and with an acute care hospitalization claim in 2013, excluding Medicare managed care and end stage renal disease beneficiaries due to incomplete claims data in these groups. We additionally excluded beneficiaries hospitalized at federal or nonacute care hospitals, or critical access hospitals given their mission to stabilize and then transfer patients to referral hospitals.9
Transferred patients were defined as beneficiaries with corresponding “transfer in” and “transfer out” claims, or those with either claim and a corresponding date of admission/discharge from another hospital within 1 day of the claim, as we used in our prior research.2 Beneficiaries transferred to the same hospital, those with greater than 1 transfer within the same hospitalization, or those cared for at hospitals with “outlier” transfer-in rates equal to 100% or transfer-out rates greater than 35% were excluded from analysis given the suggestion of nonstandard claims practices.
We first identified the top 15 primary diagnoses at time of transfer using International Classification of Diseases, Ninth Revision (ICD-9) codes (supplementary Appendix), and then identified those 4 most likely to require specialty procedural services: AMI, gastrointestinal bleed (GI bleed), renal failure, and hip fracture/dislocation. We then chose associated ICD-9 procedure codes for each diagnosis, via expert opinion (authors SM and JS, hospitalist physicians with greater than 20 years of combined clinical experience), erring on overinclusion of procedure codes. We then quantified receipt of associated procedures at transferring and receiving hospitals, stratified by diagnosis.
We further explored the cohort of patients with hip fracture/dislocation who underwent an associated procedure at the transferring but not receiving hospital, examining the frequency with which these patients had other (nonrelated) procedures at the receiving hospital, and identifying which procedures they received.
RESULTS
Of the 101,507 patients transferred to another hospital, 19,613 (19.3%) had a primary diagnosis of AMI, GI bleed, renal failure, or hip fracture/dislocation. Table 1 lists the ICD-9 procedure codes associated with each diagnosis.
Distribution of receipt of specialty procedures at the transferring and receiving hospitals varied by disease (Figure). With the exception of GI bleed, patients more often received specialty procedural care at the receiving than the transferring hospital. Depending on primary diagnosis, between 32.4% and 89.1% of patients did not receive any associated specialty procedure at the receiving hospital.
Of the 370 (22.1%) hip fracture/dislocation patients that received a specialty procedure at the transferring but not receiving hospital, 132 (35.7%) did not receive any procedure at the receiving hospital, whereas the remaining 238 (64.3%) received an unrelated (not associated with the primary diagnosis) procedure. There was great variety in the types of procedures received, the most common being transfusion of blood products (ICD-9 Clinical Modification 9904).
DISCUSSION
Among transferred patients with primary diagnoses that have clearly associated specialized procedural services, we found that patients received these procedures at varying frequency and locations across the transfer continuum. Across 4 diagnoses, receipt of associated procedures was more common at the receiving than the transferring hospital, with the exception being patients with GI bleed. We additionally found that many transferred patients did not receive any associated specialty procedure at the receiving hospital. These findings suggest the strong likelihood of more diverse underlying reasons for transfer rather than solely receipt of specialized procedural care.
Despite the frequency with which AMI patients are transferred,6 and American Heart Association guidelines directing hospitals to transfer AMI patients to institutions able to provide necessary invasive treatments,4 prior studies suggest these patients inconsistently receive specialty intervention following transfer, including stress testing, cardiac catheterization, or coronary artery bypass graft surgery.10,11 Our findings add to these data, demonstrating that only 47.3% of patients transferred with AMI received any cardiac-related procedure at the receiving hospital. Additionally, we found that 38.1% of AMI patients do not receive any specialty procedures at either the transferring or the receiving hospital. Taken together, these data suggest possible discrepancies in the perceived need for these procedures between transferring and receiving hospitals, reasons for transfer related to these conditions that don’t involve an associated procedure, or reasons for transfer unrelated to specialty care of the primary diagnosis (such as care of comorbidities, hospital location, prior relationships with that hospital, or desire for a second opinion). Although some of these alternate reasons for transfer likely still benefit the patient, some of these reasons may not justify the increased risks of discontinuity of care created by IHT.
Given limited data looking at IHT practices for patients with other diagnoses, the varying patterns of specialty procedural interventions we observed among transferred patients with GI bleed, renal failure, and hip fracture/dislocation are novel contributions to this topic. Notably, we found that among patients transferred with a primary diagnosis of renal failure, the vast majority (84.1%) did not receive any associated procedure at either the transferring or the receiving hospital. It is possible that although these patients carried the diagnosis of renal failure, their clinical phenotype is more heterogeneous, and they could still be managed conservatively without receipt of invasive procedures such as hemodialysis.
Conversely, patients transferred with primary diagnosis of hip fracture/dislocation were far more likely to receive associated specialty procedural intervention at the receiving hospital, presumably reflective of the evidence demonstrating improved outcomes with early surgical intervention.12 However, these data do not explain the reasoning behind the substantial minority of patients who received specialty intervention at the transferring hospital prior to transfer or those that did not receive any specialty intervention at either the transferring or receiving hospital. Our secondary analysis demonstrating great variety in receipt and type of nonassociated procedures provided at the receiving hospital did not help to elucidate potential underlying reasons for transfer.
Notably, among patients transferred with primary diagnosis of GI bleed, receipt of specialty procedures was more common at the transferring (77.7%) than receiving (63.2%) hospital, with nearly half (49.3%) undergoing specialty procedures at both hospitals. It is possible that these findings are reflective of the broad array of specialty procedures examined within this diagnosis. For example, it is reasonable to consider that a patient may be stabilized with receipt of a blood transfusion at the transferring hospital, then transferred to undergo a diagnostic/therapeutic procedure (ie, endoscopy/colonoscopy) at the receiving hospital, as is suggested by our results.
Our study is subject to several limitations. First, given the criteria we used to define transfer, it is possible that we included nontransferred patients within our transferred cohort if they were discharged from one hospital and admitted to a different hospital within 1 day, although quality assurance analyses we conducted in prior studies on these data support the validity of the criteria used.2 Second, we cannot exclude the possibility that patients received nonprocedural specialty care (ie, expert opinion, specialized imaging, medical management, management of secondary diagnoses, etc.) not available at the transferring hospital, although, arguably, in select patients, such input could be obtained without physical transfer of the patient (ie, tele-consult). And even in patients transferred with intent to receive procedural care who did not ultimately receive that care, there is likely an appropriate “nonprocedure” rate, where patients who might benefit from a procedure receive a timely evaluation to reduce the risk of missing the opportunity to receive it. This would be analogous to transferring a patient to an ICU even if they do not end up requiring intubation or pressor therapy. However, given the likelihood of higher risks of IHT compared with intrahospital transfers, one could argue that the threshold of perceived benefit might be different in patients being considered for IHT. Additionally, we limited our analyses to only 4 diagnoses; thus, our findings may not be generalizable to other diagnoses of transferred patients. However, because the diagnoses we examined were ones considered most effectively treated with specialty procedural interventions, it is reasonable to presume that the variability in receipt of specialty procedures observed within these diagnoses is also present, if not greater, across other diagnoses. Third, although we intentionally included a broad array of specialty procedures associated with each diagnosis, it is possible that we overlooked particular specialty interventions. For example, in assuming that patients are most likely to be transferred to receive procedural services associated with their primary diagnosis, we may have missed alternate indications for transfer, including need for procedural care related to secondary or subsequent diagnoses (ie, a patient may have presented with GI bleed
CONCLUSIONS
We found that Medicare patients who undergo IHT with primary diagnoses of AMI, GI bleed, renal failure, and hip fracture/dislocation receive associated specialty interventions at varying frequency and locations, and many patients do not receive any associated procedures at receiving hospitals. Our findings suggest that specialty procedural care of patients, even those with primary diagnoses that often warrant specialized intervention, may not be the primary driver of IHT as commonly suggested, although underlying reasons for transfer in these and other “nonprocedural” transferred patients remains obscure. Given known ambiguity in the transfer process,7 and unclear benefit of IHT,8 additional research is required to further identify and evaluate other potential underlying reasons for transfer and to examine these in the context of patient outcomes, in order to understand which patients may or may not benefit from transfer and why.
Disclosure
The authors have nothing to disclose.
Patients who undergo interhospital transfer (IHT) are felt to benefit from receipt of unique specialty care at the receiving hospital.1 Although only 1.5% of all hospitalized Medicare patients undergo hospital transfer,2 the frequency of transfer is much greater within certain patient populations, as may be expected with diagnoses requiring specialty care.3,4 Existent data demonstrate that 5% of Medicare patients admitted to the intensive care unit (ICU)5 and up to 50% of patients presenting with acute myocardial infarction (AMI) undergo IHT.6
More recent data suggest variability in hospital transfer practices not accounted for by differences in patient or hospital characteristics.2 Although disease-specific guidelines for IHT exist for certain diagnoses,3,4 the process remains largely nonstandardized for many patients,7 leading to ambiguity surrounding indications for transfer. Because limited data suggest worse outcomes for transferred versus nontransferred patients,8 a better understanding of the specialized care patients actually receive across the transfer continuum may help to elucidate potential indications for transfer and ultimately help delineate which patients are most (or least) likely to benefit from transfer and why.
In this national study, we examined a select cohort of transferred patients with diagnoses associated with specific specialty procedural services to determine if they received these procedures and where along the transfer continuum they were performed.
METHODS
We performed a cross-sectional analysis using the Center for Medicare and Medicaid Services 2013 100% Master Beneficiary Summary and Inpatient claims files. Our study protocol was approved by the Partners Healthcare Human Subjects Review Committee.
Beneficiaries were eligible for inclusion if they were aged ≥65 years, continuously enrolled in Medicare A and B, and with an acute care hospitalization claim in 2013, excluding Medicare managed care and end stage renal disease beneficiaries due to incomplete claims data in these groups. We additionally excluded beneficiaries hospitalized at federal or nonacute care hospitals, or critical access hospitals given their mission to stabilize and then transfer patients to referral hospitals.9
Transferred patients were defined as beneficiaries with corresponding “transfer in” and “transfer out” claims, or those with either claim and a corresponding date of admission/discharge from another hospital within 1 day of the claim, as we used in our prior research.2 Beneficiaries transferred to the same hospital, those with greater than 1 transfer within the same hospitalization, or those cared for at hospitals with “outlier” transfer-in rates equal to 100% or transfer-out rates greater than 35% were excluded from analysis given the suggestion of nonstandard claims practices.
We first identified the top 15 primary diagnoses at time of transfer using International Classification of Diseases, Ninth Revision (ICD-9) codes (supplementary Appendix), and then identified those 4 most likely to require specialty procedural services: AMI, gastrointestinal bleed (GI bleed), renal failure, and hip fracture/dislocation. We then chose associated ICD-9 procedure codes for each diagnosis, via expert opinion (authors SM and JS, hospitalist physicians with greater than 20 years of combined clinical experience), erring on overinclusion of procedure codes. We then quantified receipt of associated procedures at transferring and receiving hospitals, stratified by diagnosis.
We further explored the cohort of patients with hip fracture/dislocation who underwent an associated procedure at the transferring but not receiving hospital, examining the frequency with which these patients had other (nonrelated) procedures at the receiving hospital, and identifying which procedures they received.
RESULTS
Of the 101,507 patients transferred to another hospital, 19,613 (19.3%) had a primary diagnosis of AMI, GI bleed, renal failure, or hip fracture/dislocation. Table 1 lists the ICD-9 procedure codes associated with each diagnosis.
Distribution of receipt of specialty procedures at the transferring and receiving hospitals varied by disease (Figure). With the exception of GI bleed, patients more often received specialty procedural care at the receiving than the transferring hospital. Depending on primary diagnosis, between 32.4% and 89.1% of patients did not receive any associated specialty procedure at the receiving hospital.
Of the 370 (22.1%) hip fracture/dislocation patients that received a specialty procedure at the transferring but not receiving hospital, 132 (35.7%) did not receive any procedure at the receiving hospital, whereas the remaining 238 (64.3%) received an unrelated (not associated with the primary diagnosis) procedure. There was great variety in the types of procedures received, the most common being transfusion of blood products (ICD-9 Clinical Modification 9904).
DISCUSSION
Among transferred patients with primary diagnoses that have clearly associated specialized procedural services, we found that patients received these procedures at varying frequency and locations across the transfer continuum. Across 4 diagnoses, receipt of associated procedures was more common at the receiving than the transferring hospital, with the exception being patients with GI bleed. We additionally found that many transferred patients did not receive any associated specialty procedure at the receiving hospital. These findings suggest the strong likelihood of more diverse underlying reasons for transfer rather than solely receipt of specialized procedural care.
Despite the frequency with which AMI patients are transferred,6 and American Heart Association guidelines directing hospitals to transfer AMI patients to institutions able to provide necessary invasive treatments,4 prior studies suggest these patients inconsistently receive specialty intervention following transfer, including stress testing, cardiac catheterization, or coronary artery bypass graft surgery.10,11 Our findings add to these data, demonstrating that only 47.3% of patients transferred with AMI received any cardiac-related procedure at the receiving hospital. Additionally, we found that 38.1% of AMI patients do not receive any specialty procedures at either the transferring or the receiving hospital. Taken together, these data suggest possible discrepancies in the perceived need for these procedures between transferring and receiving hospitals, reasons for transfer related to these conditions that don’t involve an associated procedure, or reasons for transfer unrelated to specialty care of the primary diagnosis (such as care of comorbidities, hospital location, prior relationships with that hospital, or desire for a second opinion). Although some of these alternate reasons for transfer likely still benefit the patient, some of these reasons may not justify the increased risks of discontinuity of care created by IHT.
Given limited data looking at IHT practices for patients with other diagnoses, the varying patterns of specialty procedural interventions we observed among transferred patients with GI bleed, renal failure, and hip fracture/dislocation are novel contributions to this topic. Notably, we found that among patients transferred with a primary diagnosis of renal failure, the vast majority (84.1%) did not receive any associated procedure at either the transferring or the receiving hospital. It is possible that although these patients carried the diagnosis of renal failure, their clinical phenotype is more heterogeneous, and they could still be managed conservatively without receipt of invasive procedures such as hemodialysis.
Conversely, patients transferred with primary diagnosis of hip fracture/dislocation were far more likely to receive associated specialty procedural intervention at the receiving hospital, presumably reflective of the evidence demonstrating improved outcomes with early surgical intervention.12 However, these data do not explain the reasoning behind the substantial minority of patients who received specialty intervention at the transferring hospital prior to transfer or those that did not receive any specialty intervention at either the transferring or receiving hospital. Our secondary analysis demonstrating great variety in receipt and type of nonassociated procedures provided at the receiving hospital did not help to elucidate potential underlying reasons for transfer.
Notably, among patients transferred with primary diagnosis of GI bleed, receipt of specialty procedures was more common at the transferring (77.7%) than receiving (63.2%) hospital, with nearly half (49.3%) undergoing specialty procedures at both hospitals. It is possible that these findings are reflective of the broad array of specialty procedures examined within this diagnosis. For example, it is reasonable to consider that a patient may be stabilized with receipt of a blood transfusion at the transferring hospital, then transferred to undergo a diagnostic/therapeutic procedure (ie, endoscopy/colonoscopy) at the receiving hospital, as is suggested by our results.
Our study is subject to several limitations. First, given the criteria we used to define transfer, it is possible that we included nontransferred patients within our transferred cohort if they were discharged from one hospital and admitted to a different hospital within 1 day, although quality assurance analyses we conducted in prior studies on these data support the validity of the criteria used.2 Second, we cannot exclude the possibility that patients received nonprocedural specialty care (ie, expert opinion, specialized imaging, medical management, management of secondary diagnoses, etc.) not available at the transferring hospital, although, arguably, in select patients, such input could be obtained without physical transfer of the patient (ie, tele-consult). And even in patients transferred with intent to receive procedural care who did not ultimately receive that care, there is likely an appropriate “nonprocedure” rate, where patients who might benefit from a procedure receive a timely evaluation to reduce the risk of missing the opportunity to receive it. This would be analogous to transferring a patient to an ICU even if they do not end up requiring intubation or pressor therapy. However, given the likelihood of higher risks of IHT compared with intrahospital transfers, one could argue that the threshold of perceived benefit might be different in patients being considered for IHT. Additionally, we limited our analyses to only 4 diagnoses; thus, our findings may not be generalizable to other diagnoses of transferred patients. However, because the diagnoses we examined were ones considered most effectively treated with specialty procedural interventions, it is reasonable to presume that the variability in receipt of specialty procedures observed within these diagnoses is also present, if not greater, across other diagnoses. Third, although we intentionally included a broad array of specialty procedures associated with each diagnosis, it is possible that we overlooked particular specialty interventions. For example, in assuming that patients are most likely to be transferred to receive procedural services associated with their primary diagnosis, we may have missed alternate indications for transfer, including need for procedural care related to secondary or subsequent diagnoses (ie, a patient may have presented with GI bleed
CONCLUSIONS
We found that Medicare patients who undergo IHT with primary diagnoses of AMI, GI bleed, renal failure, and hip fracture/dislocation receive associated specialty interventions at varying frequency and locations, and many patients do not receive any associated procedures at receiving hospitals. Our findings suggest that specialty procedural care of patients, even those with primary diagnoses that often warrant specialized intervention, may not be the primary driver of IHT as commonly suggested, although underlying reasons for transfer in these and other “nonprocedural” transferred patients remains obscure. Given known ambiguity in the transfer process,7 and unclear benefit of IHT,8 additional research is required to further identify and evaluate other potential underlying reasons for transfer and to examine these in the context of patient outcomes, in order to understand which patients may or may not benefit from transfer and why.
Disclosure
The authors have nothing to disclose.
1. Iwashyna TJ. The incomplete infrastructure for interhospital patient transfer. Crit Care Med. 2012;40(8):2470-2478. PubMed
2. Mueller SK, Zheng J, Orav EJ, Schnipper JL. Rates, Predictors and Variability of Interhospital Transfers: A National Evaluation. J Hosp Med. 2017;12(6):435-442. PubMed
3. Guidelines for the transfer of critically ill patients. Guidelines Committee of the American College of Critical Care Medicine; Society of Critical Care Medicine and American Association of Critical-Care Nurses Transfer Guidelines Task Force. Crit Care Med. 1993;21(6):931-937. PubMed
4. Anderson JL, Adams CD, Antman EM, et al. 2011 ACCF/AHA Focused Update Incorporated Into the ACC/AHA 2007 Guidelines for the Management of Patients With Unstable Angina/Non-ST-Elevation Myocardial Infarction: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. Circulation. 2011;123(18):e426-e579. PubMed
5. Iwashyna TJ, Christie JD, Moody J, Kahn JM, Asch DA. The structure of critical care transfer networks. Med Care. 2009;47(7):787-793. PubMed
6. Iwashyna TJ, Kahn JM, Hayward RA, Nallamothu BK. Interhospital transfers among Medicare beneficiaries admitted for acute myocardial infarction at nonrevascularization hospitals. Circ Cardiovasc Qual Outcomes. 2010;3(5):468-475. PubMed
7. Bosk EA, Veinot T, Iwashyna TJ. Which patients and where: a qualitative study of patient transfers from community hospitals. Med Care. 2011;49(6):592-598. PubMed
8. Sokol-Hessner L, White AA, Davis KF, Herzig SJ, Hohmann SF. Interhospital transfer patients discharged by academic hospitalists and general internists: Characteristics and outcomes. J Hosp Med. 2016;11(4):245-250. PubMed
9. Department of Health and Human Services, Center for Medicare & Medicaid Services: Critical Access Hospitals. https://www.cms.gov/Outreach-and-Education/Medicare-Learning-Network-MLN/MLNProducts/downloads/CritAccessHospfctsht.pdf. Accessed June 29, 2017. PubMed
10. Roe MT, Chen AY, Delong ER, et al. Patterns of transfer for patients with non-ST-segment elevation acute coronary syndrome from community to tertiary care hospitals. Am Heart J. 2008;156(1):185-192. PubMed
11. Barreto-Filho JA, Wang Y, Rathore SS, et al. Transfer rates from nonprocedure hospitals after initial admission and outcomes among elderly patients with acute myocardial infarction. JAMA Intern Med. 2014;174(2):213-222. PubMed
12. Doruk H, Mas MR, Yildiz C, Sonmez A, Kyrdemir V. The effect of the timing of hip fracture surgery on the activity of daily living and mortality in elderly. Arch Gerontol Geriatr. 2004;39(2):179-185. PubMed
1. Iwashyna TJ. The incomplete infrastructure for interhospital patient transfer. Crit Care Med. 2012;40(8):2470-2478. PubMed
2. Mueller SK, Zheng J, Orav EJ, Schnipper JL. Rates, Predictors and Variability of Interhospital Transfers: A National Evaluation. J Hosp Med. 2017;12(6):435-442. PubMed
3. Guidelines for the transfer of critically ill patients. Guidelines Committee of the American College of Critical Care Medicine; Society of Critical Care Medicine and American Association of Critical-Care Nurses Transfer Guidelines Task Force. Crit Care Med. 1993;21(6):931-937. PubMed
4. Anderson JL, Adams CD, Antman EM, et al. 2011 ACCF/AHA Focused Update Incorporated Into the ACC/AHA 2007 Guidelines for the Management of Patients With Unstable Angina/Non-ST-Elevation Myocardial Infarction: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. Circulation. 2011;123(18):e426-e579. PubMed
5. Iwashyna TJ, Christie JD, Moody J, Kahn JM, Asch DA. The structure of critical care transfer networks. Med Care. 2009;47(7):787-793. PubMed
6. Iwashyna TJ, Kahn JM, Hayward RA, Nallamothu BK. Interhospital transfers among Medicare beneficiaries admitted for acute myocardial infarction at nonrevascularization hospitals. Circ Cardiovasc Qual Outcomes. 2010;3(5):468-475. PubMed
7. Bosk EA, Veinot T, Iwashyna TJ. Which patients and where: a qualitative study of patient transfers from community hospitals. Med Care. 2011;49(6):592-598. PubMed
8. Sokol-Hessner L, White AA, Davis KF, Herzig SJ, Hohmann SF. Interhospital transfer patients discharged by academic hospitalists and general internists: Characteristics and outcomes. J Hosp Med. 2016;11(4):245-250. PubMed
9. Department of Health and Human Services, Center for Medicare & Medicaid Services: Critical Access Hospitals. https://www.cms.gov/Outreach-and-Education/Medicare-Learning-Network-MLN/MLNProducts/downloads/CritAccessHospfctsht.pdf. Accessed June 29, 2017. PubMed
10. Roe MT, Chen AY, Delong ER, et al. Patterns of transfer for patients with non-ST-segment elevation acute coronary syndrome from community to tertiary care hospitals. Am Heart J. 2008;156(1):185-192. PubMed
11. Barreto-Filho JA, Wang Y, Rathore SS, et al. Transfer rates from nonprocedure hospitals after initial admission and outcomes among elderly patients with acute myocardial infarction. JAMA Intern Med. 2014;174(2):213-222. PubMed
12. Doruk H, Mas MR, Yildiz C, Sonmez A, Kyrdemir V. The effect of the timing of hip fracture surgery on the activity of daily living and mortality in elderly. Arch Gerontol Geriatr. 2004;39(2):179-185. PubMed
© 2017 Society of Hospital Medicine
617-732-7072; E-mail: smueller1@bwh.harvard.edu
Rates, predictors and variability of interhospital transfers: A national evaluation
Interhospital transfer (IHT) is defined as the transfer of hospitalized patients between acute care hospitals. Although cited reasons for transfer include providing patients access to unique specialty services,1 patterns and practices of IHT remain largely unstudied. Interhospital transfer is known to be common in certain patient populations, including selected patients presenting to the intensive care unit2 and those with acute myocardial infarction (AMI),3-5 but no recent studies have looked at frequency of IHT among a broader group of hospitalized patients nationally. Little is known about which patients are selected for transfer and why.6 Limited evidence suggests poor concordance between cited reason for transfer among patients, transferring physicians, and receiving physicians,7 indicating ambiguity in this care process.
Interhospital transfer exposes patients to the potential risks associated with discontinuity of care. Communication is particularly vulnerable to error during times of transition.8-10 Patients transferred between acute care hospitals are especially vulnerable, given the severity of illness in this patient population,11 and the absence of other factors to fill in gaps in communication, such as common electronic health records. Limited existing literature suggests transferred patients use more resources 12-13 and experience worse outcomes compared to nontransferred patients,11 although these data involved limited patient populations, and adjustment for illness severity and other factors was variably addressed.14-16
To improve the quality and safety of IHT, therefore, it is necessary to understand which patients benefit from IHT and identify best practices in the IHT process.17 A fundamental first step is to study patterns and practices of IHT, in particular with an eye towards identifying unwarranted variation.18 This is important to understand the prevalence of the issue, provide possible evidence of lack of standardization, and natural experiments with which to identify best practices.
To address this, we conducted a foundational study examining a national sample of Medicare patients to determine the nationwide frequency of IHT among elderly patients, patient and hospital-level predictors of transfer, and hospital variability in IHT practices.
METHODS
We performed a cross-sectional analysis using 2 nationally representative datasets: (1) Center for Medicare and Medicaid Services (CMS) 2013 100% Master Beneficiary Summary and Inpatient claims files, which contains data on all fee-for-service program Medicare enrollees’ demographic information, date of death, and hospitalization claims, including ICD-9 codes for diagnoses, diagnosis-related group (DRG), and dates of service; merged with (2) 2013 American Hospital Association (AHA) data,19 which contains hospital-level characteristics for all acute care hospitals in the U.S. Our study protocol was approved by the Partners Healthcare Human Subjects Review Committee.
Beneficiaries were eligible for inclusion if they were 65 years or older, continuously enrolled in Medicare A and B, with an acute care hospitalization claim in 2013, excluding Medicare managed care and end-stage renal disease (ESRD) beneficiaries. We additionally excluded beneficiaries hospitalized at federal or nonacute care hospitals, or critical access hospitals given their mission to stabilize and transfer patients to referral hospitals.20
Transferred patients were defined as: (1) beneficiaries with a “transfer out” claim and a corresponding “transfer in” claim at a different hospital; as well as (2) beneficiaries with a “transfer out” claim and a corresponding date of admission to another hospital within 1 day following the date of claim; and (3) beneficiaries with a “transfer in” claim and a corresponding date of discharge from another hospital within 1 day preceding the date of claim. Beneficiaries transferred to the same hospital, or cared for at hospitals with “outlier” transfer in rates equal to 100% or transfer out rates greater than 35%, were excluded from analysis given the suggestion of nonstandard claims practices. Beneficiaries with greater than 1 transfer within the same hospitalization were additionally excluded.
Patient Characteristics
Patient characteristics were obtained from the CMS data files and included: demographics (age, sex, race); DRG-weight, categorized into quartiles; primary diagnosis for the index hospitalization using ICD-9 codes; patient comorbidity using ICD-9 codes compiled into a CMS-Hierarchical Condition Category (HCC) risk score;21 presence of Medicaid co-insurance; number of hospitalizations in the past 12 months, categorized into 0, 1, 2-3, and 4 or more; season, defined as calendar quarters; and median income per household by census tract. These characteristics were chosen a priori given expert opinion in combination with prior research demonstrating association with IHT.11,22
Hospital Characteristics
Hospital characteristics were obtained from AHA data files and included hospitals’ size, categorized into small, medium, and large (less than 100, 100 to 399, 400 or more beds); geographic location; ownership; teaching status; setting (urban vs. rural); case mix index (CMI) for all patients cared for at the hospital; and presence of selected specialty services, including certified trauma center, medical intensive care unit, cardiac intensive care unit, cardiac surgery services, adult interventional cardiac catheterization, adult cardiac electrophysiology, and composite score of presence of 55 other specialty services (complete list in Appendix A). All characteristics were chosen a priori given expert opinion or relationship of characteristics with IHT, and prior research utilizing AHA data.23-24
Analysis
Descriptive statistics were used to evaluate the frequency of IHT, characteristics of transferred patients, and number of days to transfer. Patient and hospital characteristics of transferred vs. nontransferred patients were compared using chi-square analyses.
To analyze the effects of each patient and hospital characteristic on the odds of transfer, we used logistic regression models incorporating all patient and hospital characteristics, accounting for fixed effects for diagnosis, and utilizing generalized estimating equations (the GENMOD procedure in SAS statistical software, v 9.4; SAS Institute Inc., Cary, North Carolina) to account for the clustering of patients within hospitals.25 Indicator variables were created for missing covariate data and included in analyses when missing data accounted for greater than 10% of the total cohort.
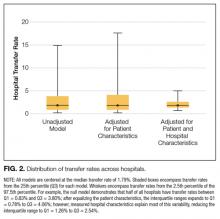
To measure the variability in transfer rates between hospitals, we used a sequence of random effects logistic regression models. We first ran a model with no covariates, representing the unadjusted differences in transfer rates between hospitals. We then added patient characteristics to see if the unadjusted differences in IHT rates were explained by differences in patient characteristics between hospitals. Lastly, we added hospital characteristics to determine if these explained the remaining differences in transfer rates. Each of the 3 models provided a measure of between-hospital variability, reflecting the degree to which IHT rates differed between hospitals. Additionally, we used the intercept from the unadjusted model and the measure of between-hospital variability from each model to calculate the 95% confidence intervals, illustrating the range of IHT rates spanning 95% of all hospitals. We used those same numbers to calculate the 25th and 75th percentiles, illustrating the range of IHT rates for the middle half of hospitals.
RESULTS
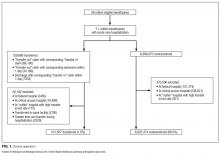
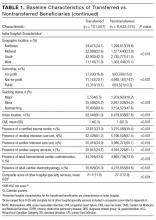
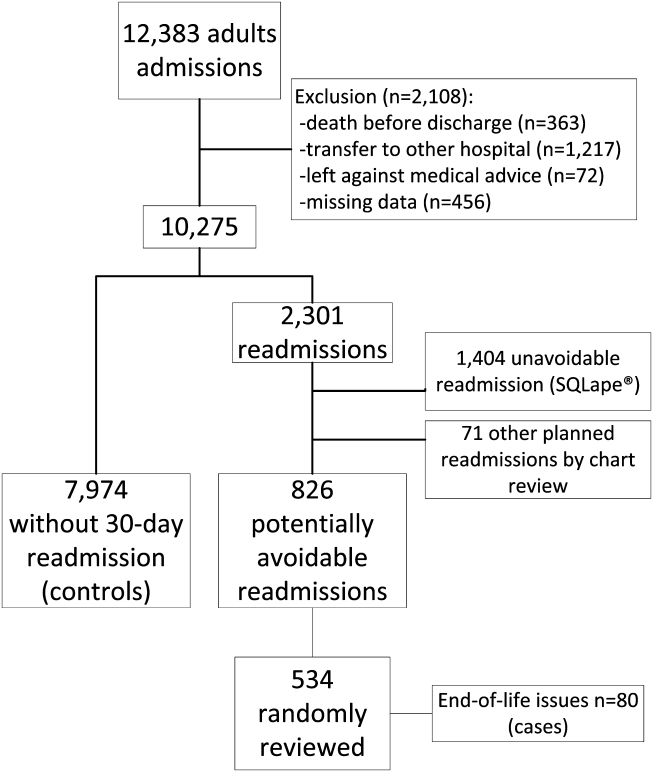
Among 28 million eligible beneficiaries, 6.6 million had an acute care hospitalization to nonfederal, noncritical access hospitals, and 107,741 met our defined criteria for IHT. An additional 3790 beneficiaries were excluded for being transferred to the same facility, 416 beneficiaries (115 transferred, 301 nontransferred) were excluded as they were cared for at 1 of the 11 hospitals with “outlier” transfer in/out rates, and 2329 were excluded because they had more than 1 transfer during hospitalization. Thus, the final cohort consisted of 101,507 transferred (1.5%) and 6,625,474 nontransferred beneficiaries (Figure 1). Of the 101,507 transferred beneficiaries, 2799 (2.8%) were included more than once (ie, experienced more than 1 IHT on separate hospitalizations throughout the study period; the vast majority of these had 2 separate hospitalizations resulting in IHT). Characteristics of transferred and nontransferred beneficiaries are shown (Table 1).
Among transferred patients, the top 5 primary diagnoses at time of transfer included AMI (12.2%), congestive heart failure (CHF) (7.2%), sepsis (6.6%), arrhythmia (6.6%), and pneumonia (3.4%). Comorbid conditions most commonly present in transferred patients included CHF (52.6%), renal failure (51.8%), arrhythmia (49.8%), and chronic obstructive pulmonary disease (COPD; 37.0%). The most common day of transfer was day after admission (hospital day 2, 24.7%), with 75% of transferred patients transferred before hospital day 6 (Appendix B).
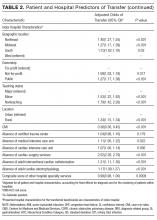
After adjusting for all other patient and hospital characteristics and clustering by hospital, the following variables were associated with greater odds of transfer: older age, male sex, nonblack race, non-Medicaid co-insurance, higher comorbidity (HCC score), lower DRG-weight, and fewer hospitalizations in the prior 12 months. Beneficiaries also had greater odds of transfer if initially hospitalized at smaller hospitals, nonteaching hospitals, public hospitals, at hospitals in the Northeast, those with fewer specialty services, and those with a low CMI (Table 2).
DISCUSSION
In this nationally representative study of 6.6 million Medicare beneficiaries, we found that 1.5% of patients were transferred between acute care facilities and were most often transferred prior to hospital day 6. Older age, male sex, nonblack race, higher medical comorbidity, lower DRG weight, and fewer recent hospitalizations were associated with greater odds of transfer. Initial hospitalization at smaller, nonteaching, public hospitals, with fewer specialty services were associated with greater odds of transfer, while higher CMI was associated with a lower odds of transfer. The most common comorbid conditions among transferred patients included CHF, renal failure, arrhythmia, and COPD; particularly notable was the very high prevalence of these conditions among transferred as compared with nontransferred patients. Importantly, we found significant variation in IHT by region and a large variation in transfer practices by hospital, with significant variability in transfer rates even after accounting for known patient and hospital characteristics.
Among our examined population, we found that a sizable number of patients undergo IHT—more than 100,000 per year. Primary diagnoses at time of transfer consist of common inpatient conditions, including AMI, CHF, sepsis, arrhythmia, and pneumonia. Limited prior data support our findings, with up to 50% of AMI patients reportedly undergoing IHT,3-5 and severe sepsis and respiratory illness reported as common diagnoses at transfer.11 Although knowledge of these primary diagnoses does not directly confer an understanding of reason for transfer, one can speculate based on our findings. For example, research demonstrates the majority of AMI patients who undergo IHT had further intervention, including stress testing, cardiac catheterization, and/or coronary artery bypass graft surgery.5,26 Thus, it is reasonable to presume that many of the beneficiaries
We additionally found that certain patient characteristics were associated with greater odds of transfer. Research suggests that transferred patients are “sicker” than nontransferred patients.1,11 Although our findings in part confirm these data, we paradoxically found that higher DRG-weight and 4 or more hospitalizations in the past year were actually associated with lower odds of transfer. In addition, the oldest patients in our cohort (85 years or older) were actually less likely to be transferred than their slightly younger counterparts (75 to 84 years). These variables may reflect extreme illness or frailty,27 and providers consciously (or subconsciously) may factor this in to their decision to transfer, considering a threshold past which transfer would confer more risk than benefit (eg, a patient may be “too sick” for transfer). Indeed, in a secondary analysis without hospital characteristics or comorbidities, and with fixed effects by hospital, we found the highest rates of IHT in patients in the middle 2 quartiles of DRG-weight, supporting this threshold hypothesis. It is also possible that patients with numerous hospitalizations may be less likely to be transferred because of familiarity and a strong sense of responsibility to continue to care for those patients (although we cannot confirm that those prior hospitalizations were all with the same index hospital).
It is also notable that odds of transfer differed by race, with black patients 17% less likely to undergo transfer compared to whites, similar to findings in other IHT studies.11 This finding, in combination with our demonstration that Medicaid patients also have lower odds of transfer, warrants further investigation to ensure the process of IHT does not bias against these populations, as with other well-documented health disparities.28-30
The hospital predictors of transfer were largely expected. However, interestingly, when we controlled for all other patient and hospital characteristics, regional variation persisted, with highest odds of transfer with hospitalization in the Northeast, indicating variability by region not explained by other factors, and findings supported by other limited data.31 This variability was further elucidated in our examination of change in variance estimates accounting for patient, then hospital, characteristics. Although we expected and found marked variability in hospital transfer rates in our null model (without accounting for any patient or hospital characteristics), we interestingly found that variability increased upon adjusting for patient characteristics. This result is presumably due to the fact that patients who are more likely to be transferred (ie, “sick” patients) are more often already at hospitals less likely to transfer patients, supported by our findings that hospital CMI is inversely associated with odds of transfer (in other words, hospitals that care for a less sick patient population are more likely to transfer their patients, and hospitals that care for a sicker patient population [higher CMI] are less likely to transfer). Adjusting solely for patient characteristics effectively equalizes these patients across hospitals, which would lead to even increased variability in transfer rates. Conversely, when we then adjusted for hospital characteristics, variability in hospital transfer rates decreased by 83% (in other words, hospital characteristics, rather than patient characteristics, explained much of the variability in transfer rates), although significant unexplained variability remained. We should note that although the observed reduction in variability was explained by the patient and hospital characteristics included in the model, these characteristics do not necessarily justify the variability they accounted for; although patients’ race or hospitals’ location may explain some of the observed variability, this does not reasonably justify it.
This observed variability in transfer practices is not surprising given the absence of standardization and clear guidelines to direct clinical IHT practice.17 Selection of patients that may benefit from transfer is often ambiguous and subjective.6 The Emergency Medical Treatment and Active Labor Act laws dictate that hospitals transfer patients requiring a more specialized service, or when “medical benefits ... outweigh the increased risks to the individual...,” although in practice this provides little guidance to practitioners.1 Thus, clearer guidelines may be necessary to achieve less variable practices.
Our study is subject to several limitations. First, although nationally representative, the Medicare population is not reflective of all hospitalized patients nationwide. Additionally, we excluded patients transferred from the emergency room. Thus, the total number of patients who undergo IHT nationally is expected to be much higher than reflected in our analysis. We also excluded patients who were transferred more than once during a given hospitalization. This enabled us to focus on the initial transfer decision but does not allow us to look at patients who are transferred to a referral center and then transferred back. Second, given the criteria we used to define transfer, it is possible that we included nontransferred patients within our transferred cohort if they were discharged from one hospital and admitted to a different hospital within 1 day. However, on quality assurance analyses where we limited our cohort to only those beneficiaries with corresponding “transfer in” and “transfer out” claims (87% of the total cohort), we found no marked differences in our results. Additionally, although we assume that patient transfer status was coded correctly within the Medicare dataset, we could not confirm by individually examining each patient we defined as “transferred.” However, on additional quality assurance analyses where we examined randomly selected excluded patients with greater than 1 transfer during hospitalization, we found differing provider numbers with each transfer, suggesting validity of the coding. Third, because there are likely many unmeasured patient confounders, we cannot be sure how much of the between-hospital variation is due to incomplete adjustment for patient characteristics. However, since adjusting for patient characteristics actually increased variability in hospital transfer rates, it is unlikely that residual patient confounders fully explain our observed results. Despite this, other variables that are not available within the CMS or AHA datasets may further elucidate hospital transfer practices, including variables reflective of the transfer process (eg, time of day of patient transfer, time delay between initiation of transfer and patient arrival at accepting hospital, accepting service on transfer, etc.); other markers of illness severity (eg, clinical service at the time of index admission, acute physiology score, utilization of critical care services on arrival at receiving hospital); and other hospital system variables (ie, membership in an accountable care organization and/or regional care network, the density of nearby tertiary referral centers (indicating possible supply-induced demand), other variables reflective of the “transfer culture” (such as the transfer rate at the hospital or region where the attending physician trained, etc.). Lastly, though our examination provides important foundational information regarding IHT nationally, this study did not examine patient outcomes in transferred and nontransferred patients, which may help to determine which patients benefit (or do not benefit) from transfer and why. Further investigation is needed to study these outcomes.
CONCLUSION
In this national study of IHT, we found that a sizable number of patients admitted to the hospital undergo transfer to another acute care facility. Patients are transferred with common medical conditions, including those requiring specialized care such as AMI, and a high rate of comorbid clinical conditions, and certain patient and hospital characteristics are associated with greater odds of transfer. Although many of the observed associations between characteristics and odds of transfer were expected based on limited existing literature, we found several unexpected findings, eg, suggesting the possibility of a threshold beyond which sicker patients are not transferred. Additionally, we found that black and Medicaid patients had lower odds of transfer, which warrants further investigation for potential health care disparity. Importantly, we found much variability in the practice of IHT, as evidenced by the inexplicable differences in transfer by hospital region, and by residual unexplained variability in hospital transfer rates after accounting for patient and hospital characteristics, which may be due to lack of standard guidelines to direct IHT practices. In conclusion, this study of hospitalized Medicare patients provides important foundational information regarding rates and predictors of IHT nationally, as well as unexplained variability that exists within this complex care transition. Further investigation will be essential to understand reasons for, processes related to, and outcomes of transferred patients, to help guide standardization in best practices in care.
Disclosure
Nothing to report.
1. Iwashyna TJ. The incomplete infrastructure for interhospital patient transfer. Crit Care Med. 2012;40(8):2470-2478. PubMed
2. Iwashyna TJ, Christie JD, Moody J, Kahn JM, Asch DA. The structure of critical care transfer networks. Med Care. 2009;47(7):787-793. PubMed
3. Mehta RH, Stalhandske EJ, McCargar PA, Ruane TJ, Eagle KA. Elderly patients at highest risk with acute myocardial infarction are more frequently transferred from community hospitals to tertiary centers: reality or myth? Am Heart J. 1999;138(4 Pt 1):688-695. PubMed
4. Iwashyna TJ, Kahn JM, Hayward RA, Nallamothu BK. Interhospital transfers among Medicare beneficiaries admitted for acute myocardial infarction at nonrevascularization hospitals. Circ Cardiovasc Qual Outcomes. 2010;3(5):468-475. PubMed
5. Roe MT, Chen AY, Delong ER, Boden WE, Calvin JE Jr, Cairns CB, et al. Patterns of transfer for patients with non-ST-segment elevation acute coronary syndrome from community to tertiary care hospitals. Am Heart J. 2008;156(1):185-192. PubMed
6. Bosk EA, Veinot T, Iwashyna TJ. Which patients and where: a qualitative study of patient transfers from community hospitals. Med Care. 2011;49(6):592-598. PubMed
7. Wagner J, Iwashyna TJ, Kahn JM. Reasons underlying interhospital transfers to an academic medical intensive care unit. J Crit Care. 2013;28(2):202-208. PubMed
8. Cohen MD, Hilligoss PB. The published literature on handoffs in hospitals: deficiencies identified in an extensive review. Qual Saf Health Care. 2010;19(6):493-497. PubMed
9. Riesenberg LA, Leitzsch J, Massucci JL, et al. Residents’ and attending physicians’ handoffs: a systematic review of the literature. Acad Med. 2009;84(12):1775-1787. PubMed
10. Arora V, Johnson J, Lovinger D, Humphrey HJ, Meltzer DO. Communication failures in patient sign-out and suggestions for improvement: a critical incident analysis. Qual Saf Health Care. 2005;14(6):401-407. PubMed
11. Sokol-Hessner L, White AA, Davis KF, Herzig SJ, Hohmann SF. Interhospital transfer patients discharged by academic hospitalists and general internists: characteristics and outcomes. J Hosp Med. 2016;11(4):245-250. PubMed
12. Bernard AM, Hayward RA, Rosevear J, Chun H, McMahon LF. Comparing the hospitalizations of transfer and non-transfer patients in an academic medical center. Acad Med. 1996;71(3):262-266. PubMed
13. Golestanian E, Scruggs JE, Gangnon RE, Mak RP, Wood KE. Effect of interhospital transfer on resource utilization and outcomes at a tertiary care referral center. Crit Care Med. 2007;35(6):1470-1476. PubMed
14. Durairaj L, Will JG, Torner JC, Doebbeling BN. Prognostic factors for mortality following interhospital transfers to the medical intensive care unit of a tertiary referral center. Crit Care Med. 2003;31(7):1981-1986. PubMed
15. Kerr HD, Byrd JC. Community hospital transfers to a VA Medical Center. JAMA. 1989;262(1):70-73. PubMed
16. Dragsted L, Jörgensen J, Jensen NH, et al. Interhospital comparisons of patient outcome from intensive care: importance of lead-time bias. Crit Care Med. 1989;17(5):418-422. PubMed
17. Gupta K, Mueller SK. Interhospital transfers: the need for standards. J Hosp Med. 2015;10(6):415-417. PubMed
18. The Dartmouth Atlas of Health Care: Understanding of the Efficiency and Effectiveness of the Health Care System. The Dartmouth Institute for Health Practice and Clinical Policy, Lebanon, NH. http://www.dartmouthatlas.org/. Accessed November 1, 2016.
19. American Hospital Association Annual Survey Database. American Hospital Association, Chicago, IL. http://www.ahadataviewer.com/book-cd-products/AHA-Survey/. Accessed July 1, 2013.
20. U.S. Department of Health and Human Services (HRSA): What are critical access hospitals (CAH)? http://www.hrsa.gov/healthit/toolbox/RuralHealthITtoolbox/Introduction/critical.html. Accessed June 9, 2016.
21. Li P, Kim MM, Doshi JA. Comparison of the performance of the CMS Hierarchical Condition Category (CMS-HCC) risk adjuster with the Charlson and Elixhauser comorbidity measures in predicting mortality. BMC Health Serv Res. 2010;10:245. PubMed
22. Hernandez-Boussard T, Davies S, McDonald K, Wang NE. Interhospital facility transfers in the United States: a nationwide outcomes study. J Patient Saf. Nov 13 2014. PubMed
23. Landon BE, Normand SL, Lessler A, et al. Quality of care for the treatment of acute medical conditions in US hospitals. Arch Intern Med. 2006;166(22):2511-2517. PubMed
24. Mueller SK, Lipsitz S, Hicks LS. Impact of hospital teaching intensity on quality of care and patient outcomes. Med Care.2013;51(7):567-574. PubMed
25. Lopez L, Hicks LS, Cohen AP, McKean S, Weissman JS. Hospitalists and the quality of care in hospitals. Arch Intern Med. 2009;169(15):1389-1394. PubMed
26. Barreto-Filho JA, Wang Y, Rathore SS, et al. Transfer rates from nonprocedure hospitals after initial admission and outcomes among elderly patients with acute myocardial infarction. JAMA Intern Med. 2014;174(2):213-222. PubMed
27. Carlson JE, Zocchi KA, Bettencourt DM, et al. Measuring frailty in the hospitalized elderly: concept of functional homeostasis. Am J Phys Med Rehabil. 1998;77(3):252-257. PubMed
28. Ward E, Jemal A, Cokkinides V, et al. Cancer disparities by race/ethnicity and socioeconomic status. CA Cancer J Clin. 2004;54(2):78-93. PubMed
29. Iribarren C, Tolstykh I, Somkin CP, et al. Sex and racial/ethnic disparities in outcomes after acute myocardial infarction: a cohort study among members of a large integrated health care delivery system in northern California. Arch Intern Med. 2005;165(18):2105-2113. PubMed
30. Kawachi I, Daniels N, Robinson DE. Health disparities by race and class: why both matter. Health Aff (Millwood). 2005;24(2):343-352. PubMed
31. Herrigel DJ, Carroll M, Fanning C, Steinberg MB, Parikh A, Usher M. Interhospital transfer handoff practices among US tertiary care centers: a descriptive survey. J Hosp Med. 2016;11(6):413-417. PubMed
Interhospital transfer (IHT) is defined as the transfer of hospitalized patients between acute care hospitals. Although cited reasons for transfer include providing patients access to unique specialty services,1 patterns and practices of IHT remain largely unstudied. Interhospital transfer is known to be common in certain patient populations, including selected patients presenting to the intensive care unit2 and those with acute myocardial infarction (AMI),3-5 but no recent studies have looked at frequency of IHT among a broader group of hospitalized patients nationally. Little is known about which patients are selected for transfer and why.6 Limited evidence suggests poor concordance between cited reason for transfer among patients, transferring physicians, and receiving physicians,7 indicating ambiguity in this care process.
Interhospital transfer exposes patients to the potential risks associated with discontinuity of care. Communication is particularly vulnerable to error during times of transition.8-10 Patients transferred between acute care hospitals are especially vulnerable, given the severity of illness in this patient population,11 and the absence of other factors to fill in gaps in communication, such as common electronic health records. Limited existing literature suggests transferred patients use more resources 12-13 and experience worse outcomes compared to nontransferred patients,11 although these data involved limited patient populations, and adjustment for illness severity and other factors was variably addressed.14-16
To improve the quality and safety of IHT, therefore, it is necessary to understand which patients benefit from IHT and identify best practices in the IHT process.17 A fundamental first step is to study patterns and practices of IHT, in particular with an eye towards identifying unwarranted variation.18 This is important to understand the prevalence of the issue, provide possible evidence of lack of standardization, and natural experiments with which to identify best practices.
To address this, we conducted a foundational study examining a national sample of Medicare patients to determine the nationwide frequency of IHT among elderly patients, patient and hospital-level predictors of transfer, and hospital variability in IHT practices.
METHODS
We performed a cross-sectional analysis using 2 nationally representative datasets: (1) Center for Medicare and Medicaid Services (CMS) 2013 100% Master Beneficiary Summary and Inpatient claims files, which contains data on all fee-for-service program Medicare enrollees’ demographic information, date of death, and hospitalization claims, including ICD-9 codes for diagnoses, diagnosis-related group (DRG), and dates of service; merged with (2) 2013 American Hospital Association (AHA) data,19 which contains hospital-level characteristics for all acute care hospitals in the U.S. Our study protocol was approved by the Partners Healthcare Human Subjects Review Committee.
Beneficiaries were eligible for inclusion if they were 65 years or older, continuously enrolled in Medicare A and B, with an acute care hospitalization claim in 2013, excluding Medicare managed care and end-stage renal disease (ESRD) beneficiaries. We additionally excluded beneficiaries hospitalized at federal or nonacute care hospitals, or critical access hospitals given their mission to stabilize and transfer patients to referral hospitals.20
Transferred patients were defined as: (1) beneficiaries with a “transfer out” claim and a corresponding “transfer in” claim at a different hospital; as well as (2) beneficiaries with a “transfer out” claim and a corresponding date of admission to another hospital within 1 day following the date of claim; and (3) beneficiaries with a “transfer in” claim and a corresponding date of discharge from another hospital within 1 day preceding the date of claim. Beneficiaries transferred to the same hospital, or cared for at hospitals with “outlier” transfer in rates equal to 100% or transfer out rates greater than 35%, were excluded from analysis given the suggestion of nonstandard claims practices. Beneficiaries with greater than 1 transfer within the same hospitalization were additionally excluded.
Patient Characteristics
Patient characteristics were obtained from the CMS data files and included: demographics (age, sex, race); DRG-weight, categorized into quartiles; primary diagnosis for the index hospitalization using ICD-9 codes; patient comorbidity using ICD-9 codes compiled into a CMS-Hierarchical Condition Category (HCC) risk score;21 presence of Medicaid co-insurance; number of hospitalizations in the past 12 months, categorized into 0, 1, 2-3, and 4 or more; season, defined as calendar quarters; and median income per household by census tract. These characteristics were chosen a priori given expert opinion in combination with prior research demonstrating association with IHT.11,22
Hospital Characteristics
Hospital characteristics were obtained from AHA data files and included hospitals’ size, categorized into small, medium, and large (less than 100, 100 to 399, 400 or more beds); geographic location; ownership; teaching status; setting (urban vs. rural); case mix index (CMI) for all patients cared for at the hospital; and presence of selected specialty services, including certified trauma center, medical intensive care unit, cardiac intensive care unit, cardiac surgery services, adult interventional cardiac catheterization, adult cardiac electrophysiology, and composite score of presence of 55 other specialty services (complete list in Appendix A). All characteristics were chosen a priori given expert opinion or relationship of characteristics with IHT, and prior research utilizing AHA data.23-24
Analysis
Descriptive statistics were used to evaluate the frequency of IHT, characteristics of transferred patients, and number of days to transfer. Patient and hospital characteristics of transferred vs. nontransferred patients were compared using chi-square analyses.
To analyze the effects of each patient and hospital characteristic on the odds of transfer, we used logistic regression models incorporating all patient and hospital characteristics, accounting for fixed effects for diagnosis, and utilizing generalized estimating equations (the GENMOD procedure in SAS statistical software, v 9.4; SAS Institute Inc., Cary, North Carolina) to account for the clustering of patients within hospitals.25 Indicator variables were created for missing covariate data and included in analyses when missing data accounted for greater than 10% of the total cohort.
To measure the variability in transfer rates between hospitals, we used a sequence of random effects logistic regression models. We first ran a model with no covariates, representing the unadjusted differences in transfer rates between hospitals. We then added patient characteristics to see if the unadjusted differences in IHT rates were explained by differences in patient characteristics between hospitals. Lastly, we added hospital characteristics to determine if these explained the remaining differences in transfer rates. Each of the 3 models provided a measure of between-hospital variability, reflecting the degree to which IHT rates differed between hospitals. Additionally, we used the intercept from the unadjusted model and the measure of between-hospital variability from each model to calculate the 95% confidence intervals, illustrating the range of IHT rates spanning 95% of all hospitals. We used those same numbers to calculate the 25th and 75th percentiles, illustrating the range of IHT rates for the middle half of hospitals.
RESULTS
Among 28 million eligible beneficiaries, 6.6 million had an acute care hospitalization to nonfederal, noncritical access hospitals, and 107,741 met our defined criteria for IHT. An additional 3790 beneficiaries were excluded for being transferred to the same facility, 416 beneficiaries (115 transferred, 301 nontransferred) were excluded as they were cared for at 1 of the 11 hospitals with “outlier” transfer in/out rates, and 2329 were excluded because they had more than 1 transfer during hospitalization. Thus, the final cohort consisted of 101,507 transferred (1.5%) and 6,625,474 nontransferred beneficiaries (Figure 1). Of the 101,507 transferred beneficiaries, 2799 (2.8%) were included more than once (ie, experienced more than 1 IHT on separate hospitalizations throughout the study period; the vast majority of these had 2 separate hospitalizations resulting in IHT). Characteristics of transferred and nontransferred beneficiaries are shown (Table 1).
Among transferred patients, the top 5 primary diagnoses at time of transfer included AMI (12.2%), congestive heart failure (CHF) (7.2%), sepsis (6.6%), arrhythmia (6.6%), and pneumonia (3.4%). Comorbid conditions most commonly present in transferred patients included CHF (52.6%), renal failure (51.8%), arrhythmia (49.8%), and chronic obstructive pulmonary disease (COPD; 37.0%). The most common day of transfer was day after admission (hospital day 2, 24.7%), with 75% of transferred patients transferred before hospital day 6 (Appendix B).
After adjusting for all other patient and hospital characteristics and clustering by hospital, the following variables were associated with greater odds of transfer: older age, male sex, nonblack race, non-Medicaid co-insurance, higher comorbidity (HCC score), lower DRG-weight, and fewer hospitalizations in the prior 12 months. Beneficiaries also had greater odds of transfer if initially hospitalized at smaller hospitals, nonteaching hospitals, public hospitals, at hospitals in the Northeast, those with fewer specialty services, and those with a low CMI (Table 2).
DISCUSSION
In this nationally representative study of 6.6 million Medicare beneficiaries, we found that 1.5% of patients were transferred between acute care facilities and were most often transferred prior to hospital day 6. Older age, male sex, nonblack race, higher medical comorbidity, lower DRG weight, and fewer recent hospitalizations were associated with greater odds of transfer. Initial hospitalization at smaller, nonteaching, public hospitals, with fewer specialty services were associated with greater odds of transfer, while higher CMI was associated with a lower odds of transfer. The most common comorbid conditions among transferred patients included CHF, renal failure, arrhythmia, and COPD; particularly notable was the very high prevalence of these conditions among transferred as compared with nontransferred patients. Importantly, we found significant variation in IHT by region and a large variation in transfer practices by hospital, with significant variability in transfer rates even after accounting for known patient and hospital characteristics.
Among our examined population, we found that a sizable number of patients undergo IHT—more than 100,000 per year. Primary diagnoses at time of transfer consist of common inpatient conditions, including AMI, CHF, sepsis, arrhythmia, and pneumonia. Limited prior data support our findings, with up to 50% of AMI patients reportedly undergoing IHT,3-5 and severe sepsis and respiratory illness reported as common diagnoses at transfer.11 Although knowledge of these primary diagnoses does not directly confer an understanding of reason for transfer, one can speculate based on our findings. For example, research demonstrates the majority of AMI patients who undergo IHT had further intervention, including stress testing, cardiac catheterization, and/or coronary artery bypass graft surgery.5,26 Thus, it is reasonable to presume that many of the beneficiaries
We additionally found that certain patient characteristics were associated with greater odds of transfer. Research suggests that transferred patients are “sicker” than nontransferred patients.1,11 Although our findings in part confirm these data, we paradoxically found that higher DRG-weight and 4 or more hospitalizations in the past year were actually associated with lower odds of transfer. In addition, the oldest patients in our cohort (85 years or older) were actually less likely to be transferred than their slightly younger counterparts (75 to 84 years). These variables may reflect extreme illness or frailty,27 and providers consciously (or subconsciously) may factor this in to their decision to transfer, considering a threshold past which transfer would confer more risk than benefit (eg, a patient may be “too sick” for transfer). Indeed, in a secondary analysis without hospital characteristics or comorbidities, and with fixed effects by hospital, we found the highest rates of IHT in patients in the middle 2 quartiles of DRG-weight, supporting this threshold hypothesis. It is also possible that patients with numerous hospitalizations may be less likely to be transferred because of familiarity and a strong sense of responsibility to continue to care for those patients (although we cannot confirm that those prior hospitalizations were all with the same index hospital).
It is also notable that odds of transfer differed by race, with black patients 17% less likely to undergo transfer compared to whites, similar to findings in other IHT studies.11 This finding, in combination with our demonstration that Medicaid patients also have lower odds of transfer, warrants further investigation to ensure the process of IHT does not bias against these populations, as with other well-documented health disparities.28-30
The hospital predictors of transfer were largely expected. However, interestingly, when we controlled for all other patient and hospital characteristics, regional variation persisted, with highest odds of transfer with hospitalization in the Northeast, indicating variability by region not explained by other factors, and findings supported by other limited data.31 This variability was further elucidated in our examination of change in variance estimates accounting for patient, then hospital, characteristics. Although we expected and found marked variability in hospital transfer rates in our null model (without accounting for any patient or hospital characteristics), we interestingly found that variability increased upon adjusting for patient characteristics. This result is presumably due to the fact that patients who are more likely to be transferred (ie, “sick” patients) are more often already at hospitals less likely to transfer patients, supported by our findings that hospital CMI is inversely associated with odds of transfer (in other words, hospitals that care for a less sick patient population are more likely to transfer their patients, and hospitals that care for a sicker patient population [higher CMI] are less likely to transfer). Adjusting solely for patient characteristics effectively equalizes these patients across hospitals, which would lead to even increased variability in transfer rates. Conversely, when we then adjusted for hospital characteristics, variability in hospital transfer rates decreased by 83% (in other words, hospital characteristics, rather than patient characteristics, explained much of the variability in transfer rates), although significant unexplained variability remained. We should note that although the observed reduction in variability was explained by the patient and hospital characteristics included in the model, these characteristics do not necessarily justify the variability they accounted for; although patients’ race or hospitals’ location may explain some of the observed variability, this does not reasonably justify it.
This observed variability in transfer practices is not surprising given the absence of standardization and clear guidelines to direct clinical IHT practice.17 Selection of patients that may benefit from transfer is often ambiguous and subjective.6 The Emergency Medical Treatment and Active Labor Act laws dictate that hospitals transfer patients requiring a more specialized service, or when “medical benefits ... outweigh the increased risks to the individual...,” although in practice this provides little guidance to practitioners.1 Thus, clearer guidelines may be necessary to achieve less variable practices.
Our study is subject to several limitations. First, although nationally representative, the Medicare population is not reflective of all hospitalized patients nationwide. Additionally, we excluded patients transferred from the emergency room. Thus, the total number of patients who undergo IHT nationally is expected to be much higher than reflected in our analysis. We also excluded patients who were transferred more than once during a given hospitalization. This enabled us to focus on the initial transfer decision but does not allow us to look at patients who are transferred to a referral center and then transferred back. Second, given the criteria we used to define transfer, it is possible that we included nontransferred patients within our transferred cohort if they were discharged from one hospital and admitted to a different hospital within 1 day. However, on quality assurance analyses where we limited our cohort to only those beneficiaries with corresponding “transfer in” and “transfer out” claims (87% of the total cohort), we found no marked differences in our results. Additionally, although we assume that patient transfer status was coded correctly within the Medicare dataset, we could not confirm by individually examining each patient we defined as “transferred.” However, on additional quality assurance analyses where we examined randomly selected excluded patients with greater than 1 transfer during hospitalization, we found differing provider numbers with each transfer, suggesting validity of the coding. Third, because there are likely many unmeasured patient confounders, we cannot be sure how much of the between-hospital variation is due to incomplete adjustment for patient characteristics. However, since adjusting for patient characteristics actually increased variability in hospital transfer rates, it is unlikely that residual patient confounders fully explain our observed results. Despite this, other variables that are not available within the CMS or AHA datasets may further elucidate hospital transfer practices, including variables reflective of the transfer process (eg, time of day of patient transfer, time delay between initiation of transfer and patient arrival at accepting hospital, accepting service on transfer, etc.); other markers of illness severity (eg, clinical service at the time of index admission, acute physiology score, utilization of critical care services on arrival at receiving hospital); and other hospital system variables (ie, membership in an accountable care organization and/or regional care network, the density of nearby tertiary referral centers (indicating possible supply-induced demand), other variables reflective of the “transfer culture” (such as the transfer rate at the hospital or region where the attending physician trained, etc.). Lastly, though our examination provides important foundational information regarding IHT nationally, this study did not examine patient outcomes in transferred and nontransferred patients, which may help to determine which patients benefit (or do not benefit) from transfer and why. Further investigation is needed to study these outcomes.
CONCLUSION
In this national study of IHT, we found that a sizable number of patients admitted to the hospital undergo transfer to another acute care facility. Patients are transferred with common medical conditions, including those requiring specialized care such as AMI, and a high rate of comorbid clinical conditions, and certain patient and hospital characteristics are associated with greater odds of transfer. Although many of the observed associations between characteristics and odds of transfer were expected based on limited existing literature, we found several unexpected findings, eg, suggesting the possibility of a threshold beyond which sicker patients are not transferred. Additionally, we found that black and Medicaid patients had lower odds of transfer, which warrants further investigation for potential health care disparity. Importantly, we found much variability in the practice of IHT, as evidenced by the inexplicable differences in transfer by hospital region, and by residual unexplained variability in hospital transfer rates after accounting for patient and hospital characteristics, which may be due to lack of standard guidelines to direct IHT practices. In conclusion, this study of hospitalized Medicare patients provides important foundational information regarding rates and predictors of IHT nationally, as well as unexplained variability that exists within this complex care transition. Further investigation will be essential to understand reasons for, processes related to, and outcomes of transferred patients, to help guide standardization in best practices in care.
Disclosure
Nothing to report.
Interhospital transfer (IHT) is defined as the transfer of hospitalized patients between acute care hospitals. Although cited reasons for transfer include providing patients access to unique specialty services,1 patterns and practices of IHT remain largely unstudied. Interhospital transfer is known to be common in certain patient populations, including selected patients presenting to the intensive care unit2 and those with acute myocardial infarction (AMI),3-5 but no recent studies have looked at frequency of IHT among a broader group of hospitalized patients nationally. Little is known about which patients are selected for transfer and why.6 Limited evidence suggests poor concordance between cited reason for transfer among patients, transferring physicians, and receiving physicians,7 indicating ambiguity in this care process.
Interhospital transfer exposes patients to the potential risks associated with discontinuity of care. Communication is particularly vulnerable to error during times of transition.8-10 Patients transferred between acute care hospitals are especially vulnerable, given the severity of illness in this patient population,11 and the absence of other factors to fill in gaps in communication, such as common electronic health records. Limited existing literature suggests transferred patients use more resources 12-13 and experience worse outcomes compared to nontransferred patients,11 although these data involved limited patient populations, and adjustment for illness severity and other factors was variably addressed.14-16
To improve the quality and safety of IHT, therefore, it is necessary to understand which patients benefit from IHT and identify best practices in the IHT process.17 A fundamental first step is to study patterns and practices of IHT, in particular with an eye towards identifying unwarranted variation.18 This is important to understand the prevalence of the issue, provide possible evidence of lack of standardization, and natural experiments with which to identify best practices.
To address this, we conducted a foundational study examining a national sample of Medicare patients to determine the nationwide frequency of IHT among elderly patients, patient and hospital-level predictors of transfer, and hospital variability in IHT practices.
METHODS
We performed a cross-sectional analysis using 2 nationally representative datasets: (1) Center for Medicare and Medicaid Services (CMS) 2013 100% Master Beneficiary Summary and Inpatient claims files, which contains data on all fee-for-service program Medicare enrollees’ demographic information, date of death, and hospitalization claims, including ICD-9 codes for diagnoses, diagnosis-related group (DRG), and dates of service; merged with (2) 2013 American Hospital Association (AHA) data,19 which contains hospital-level characteristics for all acute care hospitals in the U.S. Our study protocol was approved by the Partners Healthcare Human Subjects Review Committee.
Beneficiaries were eligible for inclusion if they were 65 years or older, continuously enrolled in Medicare A and B, with an acute care hospitalization claim in 2013, excluding Medicare managed care and end-stage renal disease (ESRD) beneficiaries. We additionally excluded beneficiaries hospitalized at federal or nonacute care hospitals, or critical access hospitals given their mission to stabilize and transfer patients to referral hospitals.20
Transferred patients were defined as: (1) beneficiaries with a “transfer out” claim and a corresponding “transfer in” claim at a different hospital; as well as (2) beneficiaries with a “transfer out” claim and a corresponding date of admission to another hospital within 1 day following the date of claim; and (3) beneficiaries with a “transfer in” claim and a corresponding date of discharge from another hospital within 1 day preceding the date of claim. Beneficiaries transferred to the same hospital, or cared for at hospitals with “outlier” transfer in rates equal to 100% or transfer out rates greater than 35%, were excluded from analysis given the suggestion of nonstandard claims practices. Beneficiaries with greater than 1 transfer within the same hospitalization were additionally excluded.
Patient Characteristics
Patient characteristics were obtained from the CMS data files and included: demographics (age, sex, race); DRG-weight, categorized into quartiles; primary diagnosis for the index hospitalization using ICD-9 codes; patient comorbidity using ICD-9 codes compiled into a CMS-Hierarchical Condition Category (HCC) risk score;21 presence of Medicaid co-insurance; number of hospitalizations in the past 12 months, categorized into 0, 1, 2-3, and 4 or more; season, defined as calendar quarters; and median income per household by census tract. These characteristics were chosen a priori given expert opinion in combination with prior research demonstrating association with IHT.11,22
Hospital Characteristics
Hospital characteristics were obtained from AHA data files and included hospitals’ size, categorized into small, medium, and large (less than 100, 100 to 399, 400 or more beds); geographic location; ownership; teaching status; setting (urban vs. rural); case mix index (CMI) for all patients cared for at the hospital; and presence of selected specialty services, including certified trauma center, medical intensive care unit, cardiac intensive care unit, cardiac surgery services, adult interventional cardiac catheterization, adult cardiac electrophysiology, and composite score of presence of 55 other specialty services (complete list in Appendix A). All characteristics were chosen a priori given expert opinion or relationship of characteristics with IHT, and prior research utilizing AHA data.23-24
Analysis
Descriptive statistics were used to evaluate the frequency of IHT, characteristics of transferred patients, and number of days to transfer. Patient and hospital characteristics of transferred vs. nontransferred patients were compared using chi-square analyses.
To analyze the effects of each patient and hospital characteristic on the odds of transfer, we used logistic regression models incorporating all patient and hospital characteristics, accounting for fixed effects for diagnosis, and utilizing generalized estimating equations (the GENMOD procedure in SAS statistical software, v 9.4; SAS Institute Inc., Cary, North Carolina) to account for the clustering of patients within hospitals.25 Indicator variables were created for missing covariate data and included in analyses when missing data accounted for greater than 10% of the total cohort.
To measure the variability in transfer rates between hospitals, we used a sequence of random effects logistic regression models. We first ran a model with no covariates, representing the unadjusted differences in transfer rates between hospitals. We then added patient characteristics to see if the unadjusted differences in IHT rates were explained by differences in patient characteristics between hospitals. Lastly, we added hospital characteristics to determine if these explained the remaining differences in transfer rates. Each of the 3 models provided a measure of between-hospital variability, reflecting the degree to which IHT rates differed between hospitals. Additionally, we used the intercept from the unadjusted model and the measure of between-hospital variability from each model to calculate the 95% confidence intervals, illustrating the range of IHT rates spanning 95% of all hospitals. We used those same numbers to calculate the 25th and 75th percentiles, illustrating the range of IHT rates for the middle half of hospitals.
RESULTS
Among 28 million eligible beneficiaries, 6.6 million had an acute care hospitalization to nonfederal, noncritical access hospitals, and 107,741 met our defined criteria for IHT. An additional 3790 beneficiaries were excluded for being transferred to the same facility, 416 beneficiaries (115 transferred, 301 nontransferred) were excluded as they were cared for at 1 of the 11 hospitals with “outlier” transfer in/out rates, and 2329 were excluded because they had more than 1 transfer during hospitalization. Thus, the final cohort consisted of 101,507 transferred (1.5%) and 6,625,474 nontransferred beneficiaries (Figure 1). Of the 101,507 transferred beneficiaries, 2799 (2.8%) were included more than once (ie, experienced more than 1 IHT on separate hospitalizations throughout the study period; the vast majority of these had 2 separate hospitalizations resulting in IHT). Characteristics of transferred and nontransferred beneficiaries are shown (Table 1).
Among transferred patients, the top 5 primary diagnoses at time of transfer included AMI (12.2%), congestive heart failure (CHF) (7.2%), sepsis (6.6%), arrhythmia (6.6%), and pneumonia (3.4%). Comorbid conditions most commonly present in transferred patients included CHF (52.6%), renal failure (51.8%), arrhythmia (49.8%), and chronic obstructive pulmonary disease (COPD; 37.0%). The most common day of transfer was day after admission (hospital day 2, 24.7%), with 75% of transferred patients transferred before hospital day 6 (Appendix B).
After adjusting for all other patient and hospital characteristics and clustering by hospital, the following variables were associated with greater odds of transfer: older age, male sex, nonblack race, non-Medicaid co-insurance, higher comorbidity (HCC score), lower DRG-weight, and fewer hospitalizations in the prior 12 months. Beneficiaries also had greater odds of transfer if initially hospitalized at smaller hospitals, nonteaching hospitals, public hospitals, at hospitals in the Northeast, those with fewer specialty services, and those with a low CMI (Table 2).
DISCUSSION
In this nationally representative study of 6.6 million Medicare beneficiaries, we found that 1.5% of patients were transferred between acute care facilities and were most often transferred prior to hospital day 6. Older age, male sex, nonblack race, higher medical comorbidity, lower DRG weight, and fewer recent hospitalizations were associated with greater odds of transfer. Initial hospitalization at smaller, nonteaching, public hospitals, with fewer specialty services were associated with greater odds of transfer, while higher CMI was associated with a lower odds of transfer. The most common comorbid conditions among transferred patients included CHF, renal failure, arrhythmia, and COPD; particularly notable was the very high prevalence of these conditions among transferred as compared with nontransferred patients. Importantly, we found significant variation in IHT by region and a large variation in transfer practices by hospital, with significant variability in transfer rates even after accounting for known patient and hospital characteristics.
Among our examined population, we found that a sizable number of patients undergo IHT—more than 100,000 per year. Primary diagnoses at time of transfer consist of common inpatient conditions, including AMI, CHF, sepsis, arrhythmia, and pneumonia. Limited prior data support our findings, with up to 50% of AMI patients reportedly undergoing IHT,3-5 and severe sepsis and respiratory illness reported as common diagnoses at transfer.11 Although knowledge of these primary diagnoses does not directly confer an understanding of reason for transfer, one can speculate based on our findings. For example, research demonstrates the majority of AMI patients who undergo IHT had further intervention, including stress testing, cardiac catheterization, and/or coronary artery bypass graft surgery.5,26 Thus, it is reasonable to presume that many of the beneficiaries
We additionally found that certain patient characteristics were associated with greater odds of transfer. Research suggests that transferred patients are “sicker” than nontransferred patients.1,11 Although our findings in part confirm these data, we paradoxically found that higher DRG-weight and 4 or more hospitalizations in the past year were actually associated with lower odds of transfer. In addition, the oldest patients in our cohort (85 years or older) were actually less likely to be transferred than their slightly younger counterparts (75 to 84 years). These variables may reflect extreme illness or frailty,27 and providers consciously (or subconsciously) may factor this in to their decision to transfer, considering a threshold past which transfer would confer more risk than benefit (eg, a patient may be “too sick” for transfer). Indeed, in a secondary analysis without hospital characteristics or comorbidities, and with fixed effects by hospital, we found the highest rates of IHT in patients in the middle 2 quartiles of DRG-weight, supporting this threshold hypothesis. It is also possible that patients with numerous hospitalizations may be less likely to be transferred because of familiarity and a strong sense of responsibility to continue to care for those patients (although we cannot confirm that those prior hospitalizations were all with the same index hospital).
It is also notable that odds of transfer differed by race, with black patients 17% less likely to undergo transfer compared to whites, similar to findings in other IHT studies.11 This finding, in combination with our demonstration that Medicaid patients also have lower odds of transfer, warrants further investigation to ensure the process of IHT does not bias against these populations, as with other well-documented health disparities.28-30
The hospital predictors of transfer were largely expected. However, interestingly, when we controlled for all other patient and hospital characteristics, regional variation persisted, with highest odds of transfer with hospitalization in the Northeast, indicating variability by region not explained by other factors, and findings supported by other limited data.31 This variability was further elucidated in our examination of change in variance estimates accounting for patient, then hospital, characteristics. Although we expected and found marked variability in hospital transfer rates in our null model (without accounting for any patient or hospital characteristics), we interestingly found that variability increased upon adjusting for patient characteristics. This result is presumably due to the fact that patients who are more likely to be transferred (ie, “sick” patients) are more often already at hospitals less likely to transfer patients, supported by our findings that hospital CMI is inversely associated with odds of transfer (in other words, hospitals that care for a less sick patient population are more likely to transfer their patients, and hospitals that care for a sicker patient population [higher CMI] are less likely to transfer). Adjusting solely for patient characteristics effectively equalizes these patients across hospitals, which would lead to even increased variability in transfer rates. Conversely, when we then adjusted for hospital characteristics, variability in hospital transfer rates decreased by 83% (in other words, hospital characteristics, rather than patient characteristics, explained much of the variability in transfer rates), although significant unexplained variability remained. We should note that although the observed reduction in variability was explained by the patient and hospital characteristics included in the model, these characteristics do not necessarily justify the variability they accounted for; although patients’ race or hospitals’ location may explain some of the observed variability, this does not reasonably justify it.
This observed variability in transfer practices is not surprising given the absence of standardization and clear guidelines to direct clinical IHT practice.17 Selection of patients that may benefit from transfer is often ambiguous and subjective.6 The Emergency Medical Treatment and Active Labor Act laws dictate that hospitals transfer patients requiring a more specialized service, or when “medical benefits ... outweigh the increased risks to the individual...,” although in practice this provides little guidance to practitioners.1 Thus, clearer guidelines may be necessary to achieve less variable practices.
Our study is subject to several limitations. First, although nationally representative, the Medicare population is not reflective of all hospitalized patients nationwide. Additionally, we excluded patients transferred from the emergency room. Thus, the total number of patients who undergo IHT nationally is expected to be much higher than reflected in our analysis. We also excluded patients who were transferred more than once during a given hospitalization. This enabled us to focus on the initial transfer decision but does not allow us to look at patients who are transferred to a referral center and then transferred back. Second, given the criteria we used to define transfer, it is possible that we included nontransferred patients within our transferred cohort if they were discharged from one hospital and admitted to a different hospital within 1 day. However, on quality assurance analyses where we limited our cohort to only those beneficiaries with corresponding “transfer in” and “transfer out” claims (87% of the total cohort), we found no marked differences in our results. Additionally, although we assume that patient transfer status was coded correctly within the Medicare dataset, we could not confirm by individually examining each patient we defined as “transferred.” However, on additional quality assurance analyses where we examined randomly selected excluded patients with greater than 1 transfer during hospitalization, we found differing provider numbers with each transfer, suggesting validity of the coding. Third, because there are likely many unmeasured patient confounders, we cannot be sure how much of the between-hospital variation is due to incomplete adjustment for patient characteristics. However, since adjusting for patient characteristics actually increased variability in hospital transfer rates, it is unlikely that residual patient confounders fully explain our observed results. Despite this, other variables that are not available within the CMS or AHA datasets may further elucidate hospital transfer practices, including variables reflective of the transfer process (eg, time of day of patient transfer, time delay between initiation of transfer and patient arrival at accepting hospital, accepting service on transfer, etc.); other markers of illness severity (eg, clinical service at the time of index admission, acute physiology score, utilization of critical care services on arrival at receiving hospital); and other hospital system variables (ie, membership in an accountable care organization and/or regional care network, the density of nearby tertiary referral centers (indicating possible supply-induced demand), other variables reflective of the “transfer culture” (such as the transfer rate at the hospital or region where the attending physician trained, etc.). Lastly, though our examination provides important foundational information regarding IHT nationally, this study did not examine patient outcomes in transferred and nontransferred patients, which may help to determine which patients benefit (or do not benefit) from transfer and why. Further investigation is needed to study these outcomes.
CONCLUSION
In this national study of IHT, we found that a sizable number of patients admitted to the hospital undergo transfer to another acute care facility. Patients are transferred with common medical conditions, including those requiring specialized care such as AMI, and a high rate of comorbid clinical conditions, and certain patient and hospital characteristics are associated with greater odds of transfer. Although many of the observed associations between characteristics and odds of transfer were expected based on limited existing literature, we found several unexpected findings, eg, suggesting the possibility of a threshold beyond which sicker patients are not transferred. Additionally, we found that black and Medicaid patients had lower odds of transfer, which warrants further investigation for potential health care disparity. Importantly, we found much variability in the practice of IHT, as evidenced by the inexplicable differences in transfer by hospital region, and by residual unexplained variability in hospital transfer rates after accounting for patient and hospital characteristics, which may be due to lack of standard guidelines to direct IHT practices. In conclusion, this study of hospitalized Medicare patients provides important foundational information regarding rates and predictors of IHT nationally, as well as unexplained variability that exists within this complex care transition. Further investigation will be essential to understand reasons for, processes related to, and outcomes of transferred patients, to help guide standardization in best practices in care.
Disclosure
Nothing to report.
1. Iwashyna TJ. The incomplete infrastructure for interhospital patient transfer. Crit Care Med. 2012;40(8):2470-2478. PubMed
2. Iwashyna TJ, Christie JD, Moody J, Kahn JM, Asch DA. The structure of critical care transfer networks. Med Care. 2009;47(7):787-793. PubMed
3. Mehta RH, Stalhandske EJ, McCargar PA, Ruane TJ, Eagle KA. Elderly patients at highest risk with acute myocardial infarction are more frequently transferred from community hospitals to tertiary centers: reality or myth? Am Heart J. 1999;138(4 Pt 1):688-695. PubMed
4. Iwashyna TJ, Kahn JM, Hayward RA, Nallamothu BK. Interhospital transfers among Medicare beneficiaries admitted for acute myocardial infarction at nonrevascularization hospitals. Circ Cardiovasc Qual Outcomes. 2010;3(5):468-475. PubMed
5. Roe MT, Chen AY, Delong ER, Boden WE, Calvin JE Jr, Cairns CB, et al. Patterns of transfer for patients with non-ST-segment elevation acute coronary syndrome from community to tertiary care hospitals. Am Heart J. 2008;156(1):185-192. PubMed
6. Bosk EA, Veinot T, Iwashyna TJ. Which patients and where: a qualitative study of patient transfers from community hospitals. Med Care. 2011;49(6):592-598. PubMed
7. Wagner J, Iwashyna TJ, Kahn JM. Reasons underlying interhospital transfers to an academic medical intensive care unit. J Crit Care. 2013;28(2):202-208. PubMed
8. Cohen MD, Hilligoss PB. The published literature on handoffs in hospitals: deficiencies identified in an extensive review. Qual Saf Health Care. 2010;19(6):493-497. PubMed
9. Riesenberg LA, Leitzsch J, Massucci JL, et al. Residents’ and attending physicians’ handoffs: a systematic review of the literature. Acad Med. 2009;84(12):1775-1787. PubMed
10. Arora V, Johnson J, Lovinger D, Humphrey HJ, Meltzer DO. Communication failures in patient sign-out and suggestions for improvement: a critical incident analysis. Qual Saf Health Care. 2005;14(6):401-407. PubMed
11. Sokol-Hessner L, White AA, Davis KF, Herzig SJ, Hohmann SF. Interhospital transfer patients discharged by academic hospitalists and general internists: characteristics and outcomes. J Hosp Med. 2016;11(4):245-250. PubMed
12. Bernard AM, Hayward RA, Rosevear J, Chun H, McMahon LF. Comparing the hospitalizations of transfer and non-transfer patients in an academic medical center. Acad Med. 1996;71(3):262-266. PubMed
13. Golestanian E, Scruggs JE, Gangnon RE, Mak RP, Wood KE. Effect of interhospital transfer on resource utilization and outcomes at a tertiary care referral center. Crit Care Med. 2007;35(6):1470-1476. PubMed
14. Durairaj L, Will JG, Torner JC, Doebbeling BN. Prognostic factors for mortality following interhospital transfers to the medical intensive care unit of a tertiary referral center. Crit Care Med. 2003;31(7):1981-1986. PubMed
15. Kerr HD, Byrd JC. Community hospital transfers to a VA Medical Center. JAMA. 1989;262(1):70-73. PubMed
16. Dragsted L, Jörgensen J, Jensen NH, et al. Interhospital comparisons of patient outcome from intensive care: importance of lead-time bias. Crit Care Med. 1989;17(5):418-422. PubMed
17. Gupta K, Mueller SK. Interhospital transfers: the need for standards. J Hosp Med. 2015;10(6):415-417. PubMed
18. The Dartmouth Atlas of Health Care: Understanding of the Efficiency and Effectiveness of the Health Care System. The Dartmouth Institute for Health Practice and Clinical Policy, Lebanon, NH. http://www.dartmouthatlas.org/. Accessed November 1, 2016.
19. American Hospital Association Annual Survey Database. American Hospital Association, Chicago, IL. http://www.ahadataviewer.com/book-cd-products/AHA-Survey/. Accessed July 1, 2013.
20. U.S. Department of Health and Human Services (HRSA): What are critical access hospitals (CAH)? http://www.hrsa.gov/healthit/toolbox/RuralHealthITtoolbox/Introduction/critical.html. Accessed June 9, 2016.
21. Li P, Kim MM, Doshi JA. Comparison of the performance of the CMS Hierarchical Condition Category (CMS-HCC) risk adjuster with the Charlson and Elixhauser comorbidity measures in predicting mortality. BMC Health Serv Res. 2010;10:245. PubMed
22. Hernandez-Boussard T, Davies S, McDonald K, Wang NE. Interhospital facility transfers in the United States: a nationwide outcomes study. J Patient Saf. Nov 13 2014. PubMed
23. Landon BE, Normand SL, Lessler A, et al. Quality of care for the treatment of acute medical conditions in US hospitals. Arch Intern Med. 2006;166(22):2511-2517. PubMed
24. Mueller SK, Lipsitz S, Hicks LS. Impact of hospital teaching intensity on quality of care and patient outcomes. Med Care.2013;51(7):567-574. PubMed
25. Lopez L, Hicks LS, Cohen AP, McKean S, Weissman JS. Hospitalists and the quality of care in hospitals. Arch Intern Med. 2009;169(15):1389-1394. PubMed
26. Barreto-Filho JA, Wang Y, Rathore SS, et al. Transfer rates from nonprocedure hospitals after initial admission and outcomes among elderly patients with acute myocardial infarction. JAMA Intern Med. 2014;174(2):213-222. PubMed
27. Carlson JE, Zocchi KA, Bettencourt DM, et al. Measuring frailty in the hospitalized elderly: concept of functional homeostasis. Am J Phys Med Rehabil. 1998;77(3):252-257. PubMed
28. Ward E, Jemal A, Cokkinides V, et al. Cancer disparities by race/ethnicity and socioeconomic status. CA Cancer J Clin. 2004;54(2):78-93. PubMed
29. Iribarren C, Tolstykh I, Somkin CP, et al. Sex and racial/ethnic disparities in outcomes after acute myocardial infarction: a cohort study among members of a large integrated health care delivery system in northern California. Arch Intern Med. 2005;165(18):2105-2113. PubMed
30. Kawachi I, Daniels N, Robinson DE. Health disparities by race and class: why both matter. Health Aff (Millwood). 2005;24(2):343-352. PubMed
31. Herrigel DJ, Carroll M, Fanning C, Steinberg MB, Parikh A, Usher M. Interhospital transfer handoff practices among US tertiary care centers: a descriptive survey. J Hosp Med. 2016;11(6):413-417. PubMed
1. Iwashyna TJ. The incomplete infrastructure for interhospital patient transfer. Crit Care Med. 2012;40(8):2470-2478. PubMed
2. Iwashyna TJ, Christie JD, Moody J, Kahn JM, Asch DA. The structure of critical care transfer networks. Med Care. 2009;47(7):787-793. PubMed
3. Mehta RH, Stalhandske EJ, McCargar PA, Ruane TJ, Eagle KA. Elderly patients at highest risk with acute myocardial infarction are more frequently transferred from community hospitals to tertiary centers: reality or myth? Am Heart J. 1999;138(4 Pt 1):688-695. PubMed
4. Iwashyna TJ, Kahn JM, Hayward RA, Nallamothu BK. Interhospital transfers among Medicare beneficiaries admitted for acute myocardial infarction at nonrevascularization hospitals. Circ Cardiovasc Qual Outcomes. 2010;3(5):468-475. PubMed
5. Roe MT, Chen AY, Delong ER, Boden WE, Calvin JE Jr, Cairns CB, et al. Patterns of transfer for patients with non-ST-segment elevation acute coronary syndrome from community to tertiary care hospitals. Am Heart J. 2008;156(1):185-192. PubMed
6. Bosk EA, Veinot T, Iwashyna TJ. Which patients and where: a qualitative study of patient transfers from community hospitals. Med Care. 2011;49(6):592-598. PubMed
7. Wagner J, Iwashyna TJ, Kahn JM. Reasons underlying interhospital transfers to an academic medical intensive care unit. J Crit Care. 2013;28(2):202-208. PubMed
8. Cohen MD, Hilligoss PB. The published literature on handoffs in hospitals: deficiencies identified in an extensive review. Qual Saf Health Care. 2010;19(6):493-497. PubMed
9. Riesenberg LA, Leitzsch J, Massucci JL, et al. Residents’ and attending physicians’ handoffs: a systematic review of the literature. Acad Med. 2009;84(12):1775-1787. PubMed
10. Arora V, Johnson J, Lovinger D, Humphrey HJ, Meltzer DO. Communication failures in patient sign-out and suggestions for improvement: a critical incident analysis. Qual Saf Health Care. 2005;14(6):401-407. PubMed
11. Sokol-Hessner L, White AA, Davis KF, Herzig SJ, Hohmann SF. Interhospital transfer patients discharged by academic hospitalists and general internists: characteristics and outcomes. J Hosp Med. 2016;11(4):245-250. PubMed
12. Bernard AM, Hayward RA, Rosevear J, Chun H, McMahon LF. Comparing the hospitalizations of transfer and non-transfer patients in an academic medical center. Acad Med. 1996;71(3):262-266. PubMed
13. Golestanian E, Scruggs JE, Gangnon RE, Mak RP, Wood KE. Effect of interhospital transfer on resource utilization and outcomes at a tertiary care referral center. Crit Care Med. 2007;35(6):1470-1476. PubMed
14. Durairaj L, Will JG, Torner JC, Doebbeling BN. Prognostic factors for mortality following interhospital transfers to the medical intensive care unit of a tertiary referral center. Crit Care Med. 2003;31(7):1981-1986. PubMed
15. Kerr HD, Byrd JC. Community hospital transfers to a VA Medical Center. JAMA. 1989;262(1):70-73. PubMed
16. Dragsted L, Jörgensen J, Jensen NH, et al. Interhospital comparisons of patient outcome from intensive care: importance of lead-time bias. Crit Care Med. 1989;17(5):418-422. PubMed
17. Gupta K, Mueller SK. Interhospital transfers: the need for standards. J Hosp Med. 2015;10(6):415-417. PubMed
18. The Dartmouth Atlas of Health Care: Understanding of the Efficiency and Effectiveness of the Health Care System. The Dartmouth Institute for Health Practice and Clinical Policy, Lebanon, NH. http://www.dartmouthatlas.org/. Accessed November 1, 2016.
19. American Hospital Association Annual Survey Database. American Hospital Association, Chicago, IL. http://www.ahadataviewer.com/book-cd-products/AHA-Survey/. Accessed July 1, 2013.
20. U.S. Department of Health and Human Services (HRSA): What are critical access hospitals (CAH)? http://www.hrsa.gov/healthit/toolbox/RuralHealthITtoolbox/Introduction/critical.html. Accessed June 9, 2016.
21. Li P, Kim MM, Doshi JA. Comparison of the performance of the CMS Hierarchical Condition Category (CMS-HCC) risk adjuster with the Charlson and Elixhauser comorbidity measures in predicting mortality. BMC Health Serv Res. 2010;10:245. PubMed
22. Hernandez-Boussard T, Davies S, McDonald K, Wang NE. Interhospital facility transfers in the United States: a nationwide outcomes study. J Patient Saf. Nov 13 2014. PubMed
23. Landon BE, Normand SL, Lessler A, et al. Quality of care for the treatment of acute medical conditions in US hospitals. Arch Intern Med. 2006;166(22):2511-2517. PubMed
24. Mueller SK, Lipsitz S, Hicks LS. Impact of hospital teaching intensity on quality of care and patient outcomes. Med Care.2013;51(7):567-574. PubMed
25. Lopez L, Hicks LS, Cohen AP, McKean S, Weissman JS. Hospitalists and the quality of care in hospitals. Arch Intern Med. 2009;169(15):1389-1394. PubMed
26. Barreto-Filho JA, Wang Y, Rathore SS, et al. Transfer rates from nonprocedure hospitals after initial admission and outcomes among elderly patients with acute myocardial infarction. JAMA Intern Med. 2014;174(2):213-222. PubMed
27. Carlson JE, Zocchi KA, Bettencourt DM, et al. Measuring frailty in the hospitalized elderly: concept of functional homeostasis. Am J Phys Med Rehabil. 1998;77(3):252-257. PubMed
28. Ward E, Jemal A, Cokkinides V, et al. Cancer disparities by race/ethnicity and socioeconomic status. CA Cancer J Clin. 2004;54(2):78-93. PubMed
29. Iribarren C, Tolstykh I, Somkin CP, et al. Sex and racial/ethnic disparities in outcomes after acute myocardial infarction: a cohort study among members of a large integrated health care delivery system in northern California. Arch Intern Med. 2005;165(18):2105-2113. PubMed
30. Kawachi I, Daniels N, Robinson DE. Health disparities by race and class: why both matter. Health Aff (Millwood). 2005;24(2):343-352. PubMed
31. Herrigel DJ, Carroll M, Fanning C, Steinberg MB, Parikh A, Usher M. Interhospital transfer handoff practices among US tertiary care centers: a descriptive survey. J Hosp Med. 2016;11(6):413-417. PubMed
© 2017 Society of Hospital Medicine
All together now: Impact of a regionalization and bedside rounding initiative on the efficiency and inclusiveness of clinical rounds
Attending rounds at academic medical centers are often disconnected from patients and non-physician care team members. Time spent bedside is consistently less than one third of total rounding time, with observational studies reporting a range of 9% to 33% over the past several decades.1-8 Rounds are often conducted outside patient rooms, denying patients, families, and nurses the opportunity to participate and offer valuable insights. Lack of bedside rounds thus limits patient and family engagement, patient input into the care plan, teaching of the physical examination, and communication and collaboration with nurses. In one study, physicians and nurses on rounds engaged in interprofessional communication in only 12% of patient cases.1 Studies have found interdisciplinary bedside rounds have several benefits, including subjectively improved communication and teamwork between physicians and nurses; increased patient satisfaction, including feeling more cared for by the medical team; and decreased length of stay and costs of care.2-10
However, there are many barriers to conducting interdisciplinary bedside rounds at large academic medical centers. Patients cared for by a single medical team are often geographically dispersed to several nursing units, and nurses are unable to predict when physicians will round on their patients. This situation limits nursing involvement on rounds and keeps doctors and nurses isolated from each other.2 Regionalization of care teams reduces this fragmentation by facilitating more interaction among doctors, patients, families, and nursing staff.
There are few data on how regionalized patients and interdisciplinary bedside rounds affect rounding time and the nature of rounds. This information is needed to understand how these structural changes mediate their effects, whether other steps are required to optimize outcomes, and how to maximize efficiency. We used time-motion analysis (TMA) to investigate how regionalization of medical teams, encouragement of bedside rounding, and systematic inclusion of nurses on ward rounds affect amount of time spent with patients, nursing presence on rounds, and total rounding time.
METHODS
Setting
This prospective interventional study, approved by the Institutional Review Board of Partners HealthCare, was conducted on the general medical wards at Brigham and Women’s Hospital, an academic 793-bed tertiary-care center in Boston, Massachusetts. Housestaff teams consist of 1 attending, 1 resident, and 2 interns with or without a medical student. Before June 20, 2013, daily rounds on medical inpatients were conducted largely on the patient unit but outside patient rooms. After completing most of a rounding discussion outside a patient’s room, the team might walk in to examine or speak with the patient. A typical medical team had patients dispersed over 7 medical units on average, and over as many as 13. As nurses were unit based, they did not consistently participate in rounds.
Intervention
In June 2013, as part of a general medical service care redesign initiative, the general medical teams were regionalized to specific inpatient units. The goal was to have teams admit patients predominantly to the team’s designated unit and to have all patients on a unit be cared for by the unit’s assigned team as often as possible, with an 85% goal for both. Toward those ends, the admitting structure was changed from a traditional 4-day call cycle to daily admitting for all teams, based on each unit’s bed availability.11
Teams were also expected to conduct rounds with nurses, and a system for facilitating these rounds was established. As physician and nurse care teams were now geographically co-located, it became possible for residents and nurses to check a rounding sheet for the planned patient rounding order, which had been set by the resident and nurse-in-charge before rounds. No more than about 5 minutes was needed to prepare each day’s order. The rounding sheet prioritized sick patients, newly admitted patients, and planned morning discharges, but patients were also always grouped by nurse. For example, the physician team rounded with the first nurse on all 3 of a nurse’s patients, and then proceeded to the next group of 3 patients with the next nurse, until all patients were seen.
Teams were encouraged to conduct patient- and family-centered rounds exclusively at bedside, except when bedside rounding was thought to be detrimental to a patient (eg, one with delirium). After an intern’s bedside presentation, which included a brief summary and details about overnight events and vital signs, the concerns of the patient, family, and nurse were shared, a focused physical examination performed, relevant data (eg, laboratory test results and imaging studies) reviewed, and the day’s plan formulated. The entire team, including the attending, was expected to have read new patients’ admission notes before rounds. Bedside rounds could thus be focused more on patient assessment and patient/family engagement and less on data transfer.
Several actions were taken to facilitate these changes. Residents, attendings, nurses, and other interdisciplinary team members participated in a series of focus groups and conferences to define workflows and share best practices for patient- and family-centered bedside rounds. Tips on bedside rounding were included in a general medicine rotation guidebook made available to residents and attendings. At the beginning of each post-intervention general medicine rotation, attendings and residents attended brief orientation sessions to review the new daily schedule, have interdisciplinary huddles, and share expectations for patient- and family-centered bedside rounds. On the general medicine units, new medical directors were hired to partner with existing nursing directors to support adoption of the workflows. Last, an interdisciplinary leadership team was formed to support the care redesign efforts. This team started meeting every 2 weeks.
Study Design
We used a pre–post analysis to study the effects of care redesign. Analysis was performed at the same time of year for 2 consecutive years to control for the stage of training and experience of the housestaff. TMA was performed by trained medical students using computer tablets linked to a customized Microsoft Access database form (Redmond, Washington). The form and the database were designed with specific buttons that, when pressed, recorded the time of particular events, such as the coming and going of each participant, the location of rounds, and the beginning and the end of rounding encounters with a patient. One research assistant using an Access entry form was able to dynamically track all events in real time, as they occurred. We collected data on 4 teams at baseline and 5 teams after the intervention. Each of the 4 baseline teams was followed for 4 consecutive weekdays—16 rounds total, April-June 2013—to capture the 4-day call cycle. Each of the 5 post-intervention teams was followed for 5 consecutive weekdays—25 rounds total, April–June 2014—to capture the 5-day cycle. (Because of technical difficulties, data from 1 rounding session were not captured.) For inclusion in the statistical analyses, TMA captured 166 on-service patients before the intervention and 304 afterward. Off-service patients, those with an attending other than the team attending, were excluded because their rounds were conducted separately.
We examined 2 primary outcomes, the proportion of time each clinical team member was present on rounds and the proportion of bedside rounding time. Secondary outcomes were round duration, rounding time per patient, and total non-patient time per rounding session (total rounding time minus total patient time).
Statistical Analysis
TMA data were organized in an Access database and analyzed with SAS Version 9.3 (SAS Institute, Cary, North Carolina). We analyzed the data by round session as well as by patient.
Data are presented as means with standard deviations, medians with interquartile ranges, and proportions, as appropriate. For analyses by round session, we used unadjusted linear regression; for patient-level analyses, we used general estimating equations to adjust for clustering of patients within each session; for nurse presence during any part of a round by patient, we used a χ2 test. Total non-patient time per round session was compared with use of patient-clustered general estimating equations using a γ distribution to account for the non-normality of the data.
RESULTS
Patient and Care Team Characteristics
Over the first year of the initiative, 85% of a team’s patients were on their assigned unit, and 87% of a unit’s patients were with the assigned team. Census numbers were 10.4 patients per general medicine team in April-June 2013 and 12.7 patients per team in April-June 2014, a 22% increase after care redesign. There were no statistically significant differences in patient characteristics, including age, sex, race, language, admission source, and comorbidity measure (Elixhauser score), between the pre-intervention and post-intervention study periods, except for a slightly higher proportion of patients admitted from home and fewer patients admitted directly from clinic (Table 1).
Primary Outcomes
Mean proportion of time the nurse was present on rounds per round session increased significantly (P < 0.001), from 24.1% to 67.8% (Figure 1A, Table 2). For individual patient encounters, the increased overall nursing presence was attributable to having more nurses on rounds and having nurses present for a larger proportion of individual rounding encounters (Figure 1B, Table 2). Nurses were present for at least some part of rounds for 53% of patients before the intervention and 93% afterward (P < 0.001). Mean proportion of round time by each of the 2 interns on each team decreased from 59.6% to 49.6% (P = 0.007).
Total bedside rounding time increased significantly ( P < 0.001), from 39.9% before the intervention to 55.8% afterward (Table 2). Meanwhile, percentage of rounding time spent on the unit but outside patient rooms decreased significantly ( P = 0.004), from 55.2% to 42.2%, as did rounding time on a unit completely different from the patient’s (4.9% before intervention, 2.0% afterward; P = 0.03). Again, patient-level results were similar (Figure 2, Table 2), but the decreased time spent on the unit, outside the patient rooms, was not significant.
Secondary Outcomes
Total rounding time decreased significantly, from a mean of 182 minutes (3.0 hours) at baseline to a mean of 146 minutes (2.4 hours) after the intervention, despite the higher post-intervention census. (When adjusted for patient census, the difference increased from 35.5 to 53.8 minutes; Table 2.) Mean rounding time per patient decreased significantly, from 14.7 minutes at baseline to 10.5 minutes after the intervention. For newly admitted patients, mean rounding time per patient decreased from 30.0 minutes before implementation to 16.3 minutes afterward. Mean rounding time also decreased, though much less, for subsequent-day patients (Table 2). For both new and existing patients, the decrease in rounding time largely was a reduction in time spent rounding outside patient rooms, with minimal impact on bedside time (Table 2). Mean time nurses were present during a patient’s rounds increased significantly, from 4.5 to 8.0 minutes (Table 2). Total nurse rounding time increased from 45.1 minutes per session to 98.8 minutes. Rounding time not related to patient discussion or evaluation decreased from 22.7 minutes per session to 13.3 minutes ( P = 0.003).
DISCUSSION
TMA of our care redesign initiative showed that this multipronged intervention, which included team regionalization, encouragement of bedside rounding with nurses, call structure changes, and attendings’ reading of admission notes before rounds, resulted in an increased proportion of rounding time spent with patients and an increased proportion of time nurses were present on rounds. Secondarily, round duration decreased even as patient census increased.
Regionalized teams have been found to improve interdisciplinary communication.1 The present study elaborates on that finding by demonstrating a dramatic increase in nursing presence on rounds, likely resulting from the unit’s use of rounding schedules and nurses’ prioritization of rounding orders, both of which were made possible by geographic co-localization. Other research has noted that one of the most significant barriers to interdisciplinary rounds is difficulty coordinating the start times of physician/nurse bedside rounding encounters. The system we have studied directly addresses this difficulty.9 Of note, nursing presence on rounds is necessary but not sufficient for true physician–nurse collaboration and effective communication,1 as reflected in a separate study of the intervention showing no significant difference in the concordance of the patient care plan between nurses and physicians before and after regionalization.12 Additional interventions may be needed to ensure that communication during bedside rounds is effective.
Our regionalized teams spent a significantly higher proportion of rounding time bedside, likely because of a cultural shift in expectations and the increased convenience of seeing patients on the team’s unit. Nevertheless, bedside time was not 100%. Structural barriers (eg, patients off-unit for dialysis) and cultural barriers likely contributed to the less than full adoption of bedside rounding. As described previously, cultural barriers to bedside rounding include trainees’ anxiety about being questioned in front of patients, the desire to freely exchange academic ideas in a conference room, and attendings’ doubts about their bedside teaching ability.1,9,13 Bedside rounds provide an important opportunity to apply the principles of patient- and family-centered care, including promotion of dignity and respect, information sharing, and collaboration. Thus, overcoming the concerns of housestaff and attendings and helping them feel prepared for bedside rounds can benefit the patient experience. More attention should be given to these practices as these types of interventions are implemented at Brigham and Women’s Hospital and elsewhere.1,13-15
Another primary concern about interdisciplinary bedside rounding is the perception that it takes more time.9 Therefore, it was important for us to measure round duration as a balancing measure to be considered for our intervention. Fortunately, we found round duration decreased with regionalization and encouragement of bedside rounding. This decrease was driven largely by a significant decrease in mean rounding time per new patient, which may be attributable at least in part to setting expectations that attendings and residents will read admission notes before rounds and that interns will summarize rather than recount information from admission notes. However, we also found rounding time decreases for subsequent-day patients, suggesting an underlying time savings. Spending a larger proportion of time bedside may therefore result in more efficient rounds. Bedside presentations can reduce redundancies, such as discussing a patient’s case outside his or her room and subsequently walking in and going over much of the same information with the patient. Our model de-emphasizes data transfer in favor of discussion of care plans. There was also a decrease in non-patient time, likely reflecting reduced transit time for regionalized teams. This decrease aligns with a recent finding that bedside rounding was at least as efficient as rounding outside the room.16
Of note, though a larger percentage of time was spent bedside after implementation of the care redesign, the absolute amount of bedside time did not change significantly. Our data showed that, even with shorter rounds, the same amount of absolute time can be spent bedside, face to face with the patient, by increasing the proportion of bedside rounding time. In other words, teams on average did not spend more time with patients, though the content and the structure of those encounters may have changed. This finding may be attributable to eliminating redundancy, forgoing the outside-the-room discussion, and thus the largest time reductions were realized there. In addition, teams incompletely adopted beside rounds, as reflected in the data. We expect that, with more complete adoption, an even larger proportion of time will be spent bedside, and absolute time bedside might increase as a result.
An unexpected result of the care redesign was that interns’ proportion of rounding time decreased after the intervention. This decrease most likely is attributable to interns’ being less likely to participate in rounds for a co-intern’s patient, and to their staying outside that patient’s room to give themselves more time to advance the care of their own patients. Before the intervention, when more rounding time was spent outside patient rooms, interns were more likely to join rounds for their co-intern’s patients because they could easily break away, as needed, to continue care of their own patients. The resident is now encouraged to use the morning huddle to identify which patients likely have the most educational value, and both interns are expected to join the bedside rounds for these patients.
This study had a few limitations. First, the pre–post design made it difficult to exclude the possibility that other temporal changes may have affected outcomes, though we did account for time-of-year effects by aligning our data-collection phases. In addition, the authors, including the director of the general medical service, are unaware of any co-interventions during the study period. Second, the multipronged intervention included care team regionalization, encouragement of bedside rounding with nurses, call structure changes (from 4 days to daily admitting), and attendings’ reading of admission notes before rounds. Thus, parsing which component(s) contributed to the results was difficult, though all the changes instituted likely were necessary for system redesign. For example, regionalization of clinicians to unit-based teams was made possible by switching to a daily admitting system.
Time that team members spent preparing for rounds was not recorded before or after the intervention. Thus, the decrease in total rounding time could have been accompanied by an increase in time spent preparing for rounds. However, admission notes were available in our electronic medical record before and after the intervention, and most residents and attendings were already reading them pre-intervention. After the intervention, pre-round note reading was more clearly defined as an expectation, and we were able to set the expectation that interns should use their presentations to summarize rather than recount information. In addition, in the post-intervention period, we did not include time spent preparing rounding orders; as already noted, however, preparation took only 5 minutes per day. Also, we did not analyze the content or the quality of the discussion on rounds, but simply recorded who was present where and when. Regarding the effect of the intervention on patient care, results were mixed. As reported in 2016, we saw no difference in frequency of adverse events with this intervention.12 However, a more sensitive measure of adverse events—used in a study on handoffs—showed our regionalization efforts had an additive effect on reducing overnight adverse events.17Researchers should now focus on the effects of care redesign on clinical outcomes, interdisciplinary care team communication, patient engagement and satisfaction, provider opinions of communication, workflow, patient care, and housestaff education. Our methodology can be used as a model to link structure, process, and outcome related to rounds and thereby better understand how best to optimize patient care and efficiency. Additional studies are needed to analyze the content of rounds and their association with patient and educational outcomes. Last, it will be important to conduct a study to see if the effects we have identified can be sustained. Such a study is already under way.
In conclusion, creating regionalized care teams and encouraging focused bedside rounds increased the proportion of bedside time and the presence of nurses on rounds. Rounds were shorter despite higher patient census. TMA revealed that regionalized care teams and bedside rounding at a large academic hospital are feasible, and are useful in establishing the necessary structures for increasing physician–nurse and provider–patient interactions.
Acknowledgments
The authors acknowledge Dr. Stan Ashley, Dr. Jacqueline Somerville, and Sheila Harris for their support of the regionalization initiative.
Disclosures
Dr. Schnipper received funding from Sanofi-aventis to conduct an investigator-initiated study to implement and evaluate a multi-faceted intervention to improve transitions of care in patients discharged home on insulin. The study was also supported by funding from the Marshall A. Wolf Medical Education Fund, Brigham and Women’s Hospital, and Dr. Stan Ashley, Chief Medical Officer, Brigham and Women’s Hospital. Some of the content of this article was orally presented at the annual meeting of the Society of Hospital Medicine; March 29-April 1, 2015; National Harbor, MD.
1. Crumlish CM, Yialamas MA, McMahon GT. Quantification of bedside teaching by an academic hospitalist group. J Hosp Med. 2009;4(5):304-307. PubMed
2. Gonzalo JD, Masters PA, Simons RJ, Chuang CH. Attending rounds and bedside case presentations: medical student and medicine resident experiences and attitudes. Teach Learn Med. 2009;21(2):105-110. PubMed
3. Elliot DL, Hickam DH. Attending rounds on in-patient units: differences between medical and non-medical services. Med Educ. 1993;27(6):503-508. PubMed
4. Payson HE, Barchas JD. A time study of medical teaching rounds. N Engl J Med. 1965;273(27):1468-1471. PubMed
5. Tremonti LP, Biddle WB. Teaching behaviors of residents and faculty members. J Med Educ. 1982;57(11):854-859. PubMed
6. Miller M, Johnson B, Greene HL, Baier M, Nowlin S. An observational study of attending rounds. J Gen Intern Med. 1992;7(6):646-648. PubMed
7. Collins GF, Cassie JM, Daggett CJ. The role of the attending physician in clinical training. J Med Educ. 1978;53(5):429-431. PubMed
8. Ward DR, Ghali WA, Graham A, Lemaire JB. A real-time locating system observes physician time-motion patterns during walk-rounds: a pilot study. BMC Med Educ. 2014;14:37. PubMed
9. Gonzalo JD, Kuperman E, Lehman E, Haidet P. Bedside interprofessional rounds: perceptions of benefits and barriers by internal medicine nursing staff, attending physicians, and housestaff physicians. J Hosp Med. 2014;9(10):646-651. PubMed
10. Stickrath C, Noble M, Prochazka A, et al. Attending rounds in the current era: what is and is not happening. JAMA Intern Med. 2013;173(12):1084-1089. PubMed
11. Boxer R, Vitale M, Gershanik EF, et al. 5th time’s a charm: creation of unit-based care teams in a high occupancy hospital [abstract]. J Hosp Med. 2015;10(suppl 2).
12. Mueller SK, Schnipper JL, Giannelli K, Roy CL, Boxer R. Impact of regionalized care on concordance of plan and preventable adverse events on general medicine services. J Hosp Med. 2016;11(9):620-627. PubMed
13. Chauke HL, Pattinson RC. Ward rounds—bedside or conference room? S Afr Med J. 2006;96(5):398-400. PubMed
14. Wang-Cheng RM, Barnas GP, Sigmann P, Riendl PA, Young MJ. Bedside case presentations: why patients like them but learners don’t. J Gen Intern Med. 1989;4(4):284-287. PubMed
15. Lehmann LS, Brancati FL, Chen MC, Roter D, Dobs AS. The effect of bedside case presentations on patients’ perceptions of their medical care. N Engl J Med. 1997;336(16):1150-1155. PubMed
16. Gonzalo JD, Chuang CH, Huang G, Smith C. The return of bedside rounds: an educational intervention. J Gen Intern Med. 2010;25(8):792-798. PubMed
17. Mueller SK, Yoon C, Schnipper JL. Association of a web-based handoff tool with rates of medical errors. JAMA Intern Med. 2016;176(9):1400-1402. PubMed
Attending rounds at academic medical centers are often disconnected from patients and non-physician care team members. Time spent bedside is consistently less than one third of total rounding time, with observational studies reporting a range of 9% to 33% over the past several decades.1-8 Rounds are often conducted outside patient rooms, denying patients, families, and nurses the opportunity to participate and offer valuable insights. Lack of bedside rounds thus limits patient and family engagement, patient input into the care plan, teaching of the physical examination, and communication and collaboration with nurses. In one study, physicians and nurses on rounds engaged in interprofessional communication in only 12% of patient cases.1 Studies have found interdisciplinary bedside rounds have several benefits, including subjectively improved communication and teamwork between physicians and nurses; increased patient satisfaction, including feeling more cared for by the medical team; and decreased length of stay and costs of care.2-10
However, there are many barriers to conducting interdisciplinary bedside rounds at large academic medical centers. Patients cared for by a single medical team are often geographically dispersed to several nursing units, and nurses are unable to predict when physicians will round on their patients. This situation limits nursing involvement on rounds and keeps doctors and nurses isolated from each other.2 Regionalization of care teams reduces this fragmentation by facilitating more interaction among doctors, patients, families, and nursing staff.
There are few data on how regionalized patients and interdisciplinary bedside rounds affect rounding time and the nature of rounds. This information is needed to understand how these structural changes mediate their effects, whether other steps are required to optimize outcomes, and how to maximize efficiency. We used time-motion analysis (TMA) to investigate how regionalization of medical teams, encouragement of bedside rounding, and systematic inclusion of nurses on ward rounds affect amount of time spent with patients, nursing presence on rounds, and total rounding time.
METHODS
Setting
This prospective interventional study, approved by the Institutional Review Board of Partners HealthCare, was conducted on the general medical wards at Brigham and Women’s Hospital, an academic 793-bed tertiary-care center in Boston, Massachusetts. Housestaff teams consist of 1 attending, 1 resident, and 2 interns with or without a medical student. Before June 20, 2013, daily rounds on medical inpatients were conducted largely on the patient unit but outside patient rooms. After completing most of a rounding discussion outside a patient’s room, the team might walk in to examine or speak with the patient. A typical medical team had patients dispersed over 7 medical units on average, and over as many as 13. As nurses were unit based, they did not consistently participate in rounds.
Intervention
In June 2013, as part of a general medical service care redesign initiative, the general medical teams were regionalized to specific inpatient units. The goal was to have teams admit patients predominantly to the team’s designated unit and to have all patients on a unit be cared for by the unit’s assigned team as often as possible, with an 85% goal for both. Toward those ends, the admitting structure was changed from a traditional 4-day call cycle to daily admitting for all teams, based on each unit’s bed availability.11
Teams were also expected to conduct rounds with nurses, and a system for facilitating these rounds was established. As physician and nurse care teams were now geographically co-located, it became possible for residents and nurses to check a rounding sheet for the planned patient rounding order, which had been set by the resident and nurse-in-charge before rounds. No more than about 5 minutes was needed to prepare each day’s order. The rounding sheet prioritized sick patients, newly admitted patients, and planned morning discharges, but patients were also always grouped by nurse. For example, the physician team rounded with the first nurse on all 3 of a nurse’s patients, and then proceeded to the next group of 3 patients with the next nurse, until all patients were seen.
Teams were encouraged to conduct patient- and family-centered rounds exclusively at bedside, except when bedside rounding was thought to be detrimental to a patient (eg, one with delirium). After an intern’s bedside presentation, which included a brief summary and details about overnight events and vital signs, the concerns of the patient, family, and nurse were shared, a focused physical examination performed, relevant data (eg, laboratory test results and imaging studies) reviewed, and the day’s plan formulated. The entire team, including the attending, was expected to have read new patients’ admission notes before rounds. Bedside rounds could thus be focused more on patient assessment and patient/family engagement and less on data transfer.
Several actions were taken to facilitate these changes. Residents, attendings, nurses, and other interdisciplinary team members participated in a series of focus groups and conferences to define workflows and share best practices for patient- and family-centered bedside rounds. Tips on bedside rounding were included in a general medicine rotation guidebook made available to residents and attendings. At the beginning of each post-intervention general medicine rotation, attendings and residents attended brief orientation sessions to review the new daily schedule, have interdisciplinary huddles, and share expectations for patient- and family-centered bedside rounds. On the general medicine units, new medical directors were hired to partner with existing nursing directors to support adoption of the workflows. Last, an interdisciplinary leadership team was formed to support the care redesign efforts. This team started meeting every 2 weeks.
Study Design
We used a pre–post analysis to study the effects of care redesign. Analysis was performed at the same time of year for 2 consecutive years to control for the stage of training and experience of the housestaff. TMA was performed by trained medical students using computer tablets linked to a customized Microsoft Access database form (Redmond, Washington). The form and the database were designed with specific buttons that, when pressed, recorded the time of particular events, such as the coming and going of each participant, the location of rounds, and the beginning and the end of rounding encounters with a patient. One research assistant using an Access entry form was able to dynamically track all events in real time, as they occurred. We collected data on 4 teams at baseline and 5 teams after the intervention. Each of the 4 baseline teams was followed for 4 consecutive weekdays—16 rounds total, April-June 2013—to capture the 4-day call cycle. Each of the 5 post-intervention teams was followed for 5 consecutive weekdays—25 rounds total, April–June 2014—to capture the 5-day cycle. (Because of technical difficulties, data from 1 rounding session were not captured.) For inclusion in the statistical analyses, TMA captured 166 on-service patients before the intervention and 304 afterward. Off-service patients, those with an attending other than the team attending, were excluded because their rounds were conducted separately.
We examined 2 primary outcomes, the proportion of time each clinical team member was present on rounds and the proportion of bedside rounding time. Secondary outcomes were round duration, rounding time per patient, and total non-patient time per rounding session (total rounding time minus total patient time).
Statistical Analysis
TMA data were organized in an Access database and analyzed with SAS Version 9.3 (SAS Institute, Cary, North Carolina). We analyzed the data by round session as well as by patient.
Data are presented as means with standard deviations, medians with interquartile ranges, and proportions, as appropriate. For analyses by round session, we used unadjusted linear regression; for patient-level analyses, we used general estimating equations to adjust for clustering of patients within each session; for nurse presence during any part of a round by patient, we used a χ2 test. Total non-patient time per round session was compared with use of patient-clustered general estimating equations using a γ distribution to account for the non-normality of the data.
RESULTS
Patient and Care Team Characteristics
Over the first year of the initiative, 85% of a team’s patients were on their assigned unit, and 87% of a unit’s patients were with the assigned team. Census numbers were 10.4 patients per general medicine team in April-June 2013 and 12.7 patients per team in April-June 2014, a 22% increase after care redesign. There were no statistically significant differences in patient characteristics, including age, sex, race, language, admission source, and comorbidity measure (Elixhauser score), between the pre-intervention and post-intervention study periods, except for a slightly higher proportion of patients admitted from home and fewer patients admitted directly from clinic (Table 1).
Primary Outcomes
Mean proportion of time the nurse was present on rounds per round session increased significantly (P < 0.001), from 24.1% to 67.8% (Figure 1A, Table 2). For individual patient encounters, the increased overall nursing presence was attributable to having more nurses on rounds and having nurses present for a larger proportion of individual rounding encounters (Figure 1B, Table 2). Nurses were present for at least some part of rounds for 53% of patients before the intervention and 93% afterward (P < 0.001). Mean proportion of round time by each of the 2 interns on each team decreased from 59.6% to 49.6% (P = 0.007).
Total bedside rounding time increased significantly ( P < 0.001), from 39.9% before the intervention to 55.8% afterward (Table 2). Meanwhile, percentage of rounding time spent on the unit but outside patient rooms decreased significantly ( P = 0.004), from 55.2% to 42.2%, as did rounding time on a unit completely different from the patient’s (4.9% before intervention, 2.0% afterward; P = 0.03). Again, patient-level results were similar (Figure 2, Table 2), but the decreased time spent on the unit, outside the patient rooms, was not significant.
Secondary Outcomes
Total rounding time decreased significantly, from a mean of 182 minutes (3.0 hours) at baseline to a mean of 146 minutes (2.4 hours) after the intervention, despite the higher post-intervention census. (When adjusted for patient census, the difference increased from 35.5 to 53.8 minutes; Table 2.) Mean rounding time per patient decreased significantly, from 14.7 minutes at baseline to 10.5 minutes after the intervention. For newly admitted patients, mean rounding time per patient decreased from 30.0 minutes before implementation to 16.3 minutes afterward. Mean rounding time also decreased, though much less, for subsequent-day patients (Table 2). For both new and existing patients, the decrease in rounding time largely was a reduction in time spent rounding outside patient rooms, with minimal impact on bedside time (Table 2). Mean time nurses were present during a patient’s rounds increased significantly, from 4.5 to 8.0 minutes (Table 2). Total nurse rounding time increased from 45.1 minutes per session to 98.8 minutes. Rounding time not related to patient discussion or evaluation decreased from 22.7 minutes per session to 13.3 minutes ( P = 0.003).
DISCUSSION
TMA of our care redesign initiative showed that this multipronged intervention, which included team regionalization, encouragement of bedside rounding with nurses, call structure changes, and attendings’ reading of admission notes before rounds, resulted in an increased proportion of rounding time spent with patients and an increased proportion of time nurses were present on rounds. Secondarily, round duration decreased even as patient census increased.
Regionalized teams have been found to improve interdisciplinary communication.1 The present study elaborates on that finding by demonstrating a dramatic increase in nursing presence on rounds, likely resulting from the unit’s use of rounding schedules and nurses’ prioritization of rounding orders, both of which were made possible by geographic co-localization. Other research has noted that one of the most significant barriers to interdisciplinary rounds is difficulty coordinating the start times of physician/nurse bedside rounding encounters. The system we have studied directly addresses this difficulty.9 Of note, nursing presence on rounds is necessary but not sufficient for true physician–nurse collaboration and effective communication,1 as reflected in a separate study of the intervention showing no significant difference in the concordance of the patient care plan between nurses and physicians before and after regionalization.12 Additional interventions may be needed to ensure that communication during bedside rounds is effective.
Our regionalized teams spent a significantly higher proportion of rounding time bedside, likely because of a cultural shift in expectations and the increased convenience of seeing patients on the team’s unit. Nevertheless, bedside time was not 100%. Structural barriers (eg, patients off-unit for dialysis) and cultural barriers likely contributed to the less than full adoption of bedside rounding. As described previously, cultural barriers to bedside rounding include trainees’ anxiety about being questioned in front of patients, the desire to freely exchange academic ideas in a conference room, and attendings’ doubts about their bedside teaching ability.1,9,13 Bedside rounds provide an important opportunity to apply the principles of patient- and family-centered care, including promotion of dignity and respect, information sharing, and collaboration. Thus, overcoming the concerns of housestaff and attendings and helping them feel prepared for bedside rounds can benefit the patient experience. More attention should be given to these practices as these types of interventions are implemented at Brigham and Women’s Hospital and elsewhere.1,13-15
Another primary concern about interdisciplinary bedside rounding is the perception that it takes more time.9 Therefore, it was important for us to measure round duration as a balancing measure to be considered for our intervention. Fortunately, we found round duration decreased with regionalization and encouragement of bedside rounding. This decrease was driven largely by a significant decrease in mean rounding time per new patient, which may be attributable at least in part to setting expectations that attendings and residents will read admission notes before rounds and that interns will summarize rather than recount information from admission notes. However, we also found rounding time decreases for subsequent-day patients, suggesting an underlying time savings. Spending a larger proportion of time bedside may therefore result in more efficient rounds. Bedside presentations can reduce redundancies, such as discussing a patient’s case outside his or her room and subsequently walking in and going over much of the same information with the patient. Our model de-emphasizes data transfer in favor of discussion of care plans. There was also a decrease in non-patient time, likely reflecting reduced transit time for regionalized teams. This decrease aligns with a recent finding that bedside rounding was at least as efficient as rounding outside the room.16
Of note, though a larger percentage of time was spent bedside after implementation of the care redesign, the absolute amount of bedside time did not change significantly. Our data showed that, even with shorter rounds, the same amount of absolute time can be spent bedside, face to face with the patient, by increasing the proportion of bedside rounding time. In other words, teams on average did not spend more time with patients, though the content and the structure of those encounters may have changed. This finding may be attributable to eliminating redundancy, forgoing the outside-the-room discussion, and thus the largest time reductions were realized there. In addition, teams incompletely adopted beside rounds, as reflected in the data. We expect that, with more complete adoption, an even larger proportion of time will be spent bedside, and absolute time bedside might increase as a result.
An unexpected result of the care redesign was that interns’ proportion of rounding time decreased after the intervention. This decrease most likely is attributable to interns’ being less likely to participate in rounds for a co-intern’s patient, and to their staying outside that patient’s room to give themselves more time to advance the care of their own patients. Before the intervention, when more rounding time was spent outside patient rooms, interns were more likely to join rounds for their co-intern’s patients because they could easily break away, as needed, to continue care of their own patients. The resident is now encouraged to use the morning huddle to identify which patients likely have the most educational value, and both interns are expected to join the bedside rounds for these patients.
This study had a few limitations. First, the pre–post design made it difficult to exclude the possibility that other temporal changes may have affected outcomes, though we did account for time-of-year effects by aligning our data-collection phases. In addition, the authors, including the director of the general medical service, are unaware of any co-interventions during the study period. Second, the multipronged intervention included care team regionalization, encouragement of bedside rounding with nurses, call structure changes (from 4 days to daily admitting), and attendings’ reading of admission notes before rounds. Thus, parsing which component(s) contributed to the results was difficult, though all the changes instituted likely were necessary for system redesign. For example, regionalization of clinicians to unit-based teams was made possible by switching to a daily admitting system.
Time that team members spent preparing for rounds was not recorded before or after the intervention. Thus, the decrease in total rounding time could have been accompanied by an increase in time spent preparing for rounds. However, admission notes were available in our electronic medical record before and after the intervention, and most residents and attendings were already reading them pre-intervention. After the intervention, pre-round note reading was more clearly defined as an expectation, and we were able to set the expectation that interns should use their presentations to summarize rather than recount information. In addition, in the post-intervention period, we did not include time spent preparing rounding orders; as already noted, however, preparation took only 5 minutes per day. Also, we did not analyze the content or the quality of the discussion on rounds, but simply recorded who was present where and when. Regarding the effect of the intervention on patient care, results were mixed. As reported in 2016, we saw no difference in frequency of adverse events with this intervention.12 However, a more sensitive measure of adverse events—used in a study on handoffs—showed our regionalization efforts had an additive effect on reducing overnight adverse events.17Researchers should now focus on the effects of care redesign on clinical outcomes, interdisciplinary care team communication, patient engagement and satisfaction, provider opinions of communication, workflow, patient care, and housestaff education. Our methodology can be used as a model to link structure, process, and outcome related to rounds and thereby better understand how best to optimize patient care and efficiency. Additional studies are needed to analyze the content of rounds and their association with patient and educational outcomes. Last, it will be important to conduct a study to see if the effects we have identified can be sustained. Such a study is already under way.
In conclusion, creating regionalized care teams and encouraging focused bedside rounds increased the proportion of bedside time and the presence of nurses on rounds. Rounds were shorter despite higher patient census. TMA revealed that regionalized care teams and bedside rounding at a large academic hospital are feasible, and are useful in establishing the necessary structures for increasing physician–nurse and provider–patient interactions.
Acknowledgments
The authors acknowledge Dr. Stan Ashley, Dr. Jacqueline Somerville, and Sheila Harris for their support of the regionalization initiative.
Disclosures
Dr. Schnipper received funding from Sanofi-aventis to conduct an investigator-initiated study to implement and evaluate a multi-faceted intervention to improve transitions of care in patients discharged home on insulin. The study was also supported by funding from the Marshall A. Wolf Medical Education Fund, Brigham and Women’s Hospital, and Dr. Stan Ashley, Chief Medical Officer, Brigham and Women’s Hospital. Some of the content of this article was orally presented at the annual meeting of the Society of Hospital Medicine; March 29-April 1, 2015; National Harbor, MD.
Attending rounds at academic medical centers are often disconnected from patients and non-physician care team members. Time spent bedside is consistently less than one third of total rounding time, with observational studies reporting a range of 9% to 33% over the past several decades.1-8 Rounds are often conducted outside patient rooms, denying patients, families, and nurses the opportunity to participate and offer valuable insights. Lack of bedside rounds thus limits patient and family engagement, patient input into the care plan, teaching of the physical examination, and communication and collaboration with nurses. In one study, physicians and nurses on rounds engaged in interprofessional communication in only 12% of patient cases.1 Studies have found interdisciplinary bedside rounds have several benefits, including subjectively improved communication and teamwork between physicians and nurses; increased patient satisfaction, including feeling more cared for by the medical team; and decreased length of stay and costs of care.2-10
However, there are many barriers to conducting interdisciplinary bedside rounds at large academic medical centers. Patients cared for by a single medical team are often geographically dispersed to several nursing units, and nurses are unable to predict when physicians will round on their patients. This situation limits nursing involvement on rounds and keeps doctors and nurses isolated from each other.2 Regionalization of care teams reduces this fragmentation by facilitating more interaction among doctors, patients, families, and nursing staff.
There are few data on how regionalized patients and interdisciplinary bedside rounds affect rounding time and the nature of rounds. This information is needed to understand how these structural changes mediate their effects, whether other steps are required to optimize outcomes, and how to maximize efficiency. We used time-motion analysis (TMA) to investigate how regionalization of medical teams, encouragement of bedside rounding, and systematic inclusion of nurses on ward rounds affect amount of time spent with patients, nursing presence on rounds, and total rounding time.
METHODS
Setting
This prospective interventional study, approved by the Institutional Review Board of Partners HealthCare, was conducted on the general medical wards at Brigham and Women’s Hospital, an academic 793-bed tertiary-care center in Boston, Massachusetts. Housestaff teams consist of 1 attending, 1 resident, and 2 interns with or without a medical student. Before June 20, 2013, daily rounds on medical inpatients were conducted largely on the patient unit but outside patient rooms. After completing most of a rounding discussion outside a patient’s room, the team might walk in to examine or speak with the patient. A typical medical team had patients dispersed over 7 medical units on average, and over as many as 13. As nurses were unit based, they did not consistently participate in rounds.
Intervention
In June 2013, as part of a general medical service care redesign initiative, the general medical teams were regionalized to specific inpatient units. The goal was to have teams admit patients predominantly to the team’s designated unit and to have all patients on a unit be cared for by the unit’s assigned team as often as possible, with an 85% goal for both. Toward those ends, the admitting structure was changed from a traditional 4-day call cycle to daily admitting for all teams, based on each unit’s bed availability.11
Teams were also expected to conduct rounds with nurses, and a system for facilitating these rounds was established. As physician and nurse care teams were now geographically co-located, it became possible for residents and nurses to check a rounding sheet for the planned patient rounding order, which had been set by the resident and nurse-in-charge before rounds. No more than about 5 minutes was needed to prepare each day’s order. The rounding sheet prioritized sick patients, newly admitted patients, and planned morning discharges, but patients were also always grouped by nurse. For example, the physician team rounded with the first nurse on all 3 of a nurse’s patients, and then proceeded to the next group of 3 patients with the next nurse, until all patients were seen.
Teams were encouraged to conduct patient- and family-centered rounds exclusively at bedside, except when bedside rounding was thought to be detrimental to a patient (eg, one with delirium). After an intern’s bedside presentation, which included a brief summary and details about overnight events and vital signs, the concerns of the patient, family, and nurse were shared, a focused physical examination performed, relevant data (eg, laboratory test results and imaging studies) reviewed, and the day’s plan formulated. The entire team, including the attending, was expected to have read new patients’ admission notes before rounds. Bedside rounds could thus be focused more on patient assessment and patient/family engagement and less on data transfer.
Several actions were taken to facilitate these changes. Residents, attendings, nurses, and other interdisciplinary team members participated in a series of focus groups and conferences to define workflows and share best practices for patient- and family-centered bedside rounds. Tips on bedside rounding were included in a general medicine rotation guidebook made available to residents and attendings. At the beginning of each post-intervention general medicine rotation, attendings and residents attended brief orientation sessions to review the new daily schedule, have interdisciplinary huddles, and share expectations for patient- and family-centered bedside rounds. On the general medicine units, new medical directors were hired to partner with existing nursing directors to support adoption of the workflows. Last, an interdisciplinary leadership team was formed to support the care redesign efforts. This team started meeting every 2 weeks.
Study Design
We used a pre–post analysis to study the effects of care redesign. Analysis was performed at the same time of year for 2 consecutive years to control for the stage of training and experience of the housestaff. TMA was performed by trained medical students using computer tablets linked to a customized Microsoft Access database form (Redmond, Washington). The form and the database were designed with specific buttons that, when pressed, recorded the time of particular events, such as the coming and going of each participant, the location of rounds, and the beginning and the end of rounding encounters with a patient. One research assistant using an Access entry form was able to dynamically track all events in real time, as they occurred. We collected data on 4 teams at baseline and 5 teams after the intervention. Each of the 4 baseline teams was followed for 4 consecutive weekdays—16 rounds total, April-June 2013—to capture the 4-day call cycle. Each of the 5 post-intervention teams was followed for 5 consecutive weekdays—25 rounds total, April–June 2014—to capture the 5-day cycle. (Because of technical difficulties, data from 1 rounding session were not captured.) For inclusion in the statistical analyses, TMA captured 166 on-service patients before the intervention and 304 afterward. Off-service patients, those with an attending other than the team attending, were excluded because their rounds were conducted separately.
We examined 2 primary outcomes, the proportion of time each clinical team member was present on rounds and the proportion of bedside rounding time. Secondary outcomes were round duration, rounding time per patient, and total non-patient time per rounding session (total rounding time minus total patient time).
Statistical Analysis
TMA data were organized in an Access database and analyzed with SAS Version 9.3 (SAS Institute, Cary, North Carolina). We analyzed the data by round session as well as by patient.
Data are presented as means with standard deviations, medians with interquartile ranges, and proportions, as appropriate. For analyses by round session, we used unadjusted linear regression; for patient-level analyses, we used general estimating equations to adjust for clustering of patients within each session; for nurse presence during any part of a round by patient, we used a χ2 test. Total non-patient time per round session was compared with use of patient-clustered general estimating equations using a γ distribution to account for the non-normality of the data.
RESULTS
Patient and Care Team Characteristics
Over the first year of the initiative, 85% of a team’s patients were on their assigned unit, and 87% of a unit’s patients were with the assigned team. Census numbers were 10.4 patients per general medicine team in April-June 2013 and 12.7 patients per team in April-June 2014, a 22% increase after care redesign. There were no statistically significant differences in patient characteristics, including age, sex, race, language, admission source, and comorbidity measure (Elixhauser score), between the pre-intervention and post-intervention study periods, except for a slightly higher proportion of patients admitted from home and fewer patients admitted directly from clinic (Table 1).
Primary Outcomes
Mean proportion of time the nurse was present on rounds per round session increased significantly (P < 0.001), from 24.1% to 67.8% (Figure 1A, Table 2). For individual patient encounters, the increased overall nursing presence was attributable to having more nurses on rounds and having nurses present for a larger proportion of individual rounding encounters (Figure 1B, Table 2). Nurses were present for at least some part of rounds for 53% of patients before the intervention and 93% afterward (P < 0.001). Mean proportion of round time by each of the 2 interns on each team decreased from 59.6% to 49.6% (P = 0.007).
Total bedside rounding time increased significantly ( P < 0.001), from 39.9% before the intervention to 55.8% afterward (Table 2). Meanwhile, percentage of rounding time spent on the unit but outside patient rooms decreased significantly ( P = 0.004), from 55.2% to 42.2%, as did rounding time on a unit completely different from the patient’s (4.9% before intervention, 2.0% afterward; P = 0.03). Again, patient-level results were similar (Figure 2, Table 2), but the decreased time spent on the unit, outside the patient rooms, was not significant.
Secondary Outcomes
Total rounding time decreased significantly, from a mean of 182 minutes (3.0 hours) at baseline to a mean of 146 minutes (2.4 hours) after the intervention, despite the higher post-intervention census. (When adjusted for patient census, the difference increased from 35.5 to 53.8 minutes; Table 2.) Mean rounding time per patient decreased significantly, from 14.7 minutes at baseline to 10.5 minutes after the intervention. For newly admitted patients, mean rounding time per patient decreased from 30.0 minutes before implementation to 16.3 minutes afterward. Mean rounding time also decreased, though much less, for subsequent-day patients (Table 2). For both new and existing patients, the decrease in rounding time largely was a reduction in time spent rounding outside patient rooms, with minimal impact on bedside time (Table 2). Mean time nurses were present during a patient’s rounds increased significantly, from 4.5 to 8.0 minutes (Table 2). Total nurse rounding time increased from 45.1 minutes per session to 98.8 minutes. Rounding time not related to patient discussion or evaluation decreased from 22.7 minutes per session to 13.3 minutes ( P = 0.003).
DISCUSSION
TMA of our care redesign initiative showed that this multipronged intervention, which included team regionalization, encouragement of bedside rounding with nurses, call structure changes, and attendings’ reading of admission notes before rounds, resulted in an increased proportion of rounding time spent with patients and an increased proportion of time nurses were present on rounds. Secondarily, round duration decreased even as patient census increased.
Regionalized teams have been found to improve interdisciplinary communication.1 The present study elaborates on that finding by demonstrating a dramatic increase in nursing presence on rounds, likely resulting from the unit’s use of rounding schedules and nurses’ prioritization of rounding orders, both of which were made possible by geographic co-localization. Other research has noted that one of the most significant barriers to interdisciplinary rounds is difficulty coordinating the start times of physician/nurse bedside rounding encounters. The system we have studied directly addresses this difficulty.9 Of note, nursing presence on rounds is necessary but not sufficient for true physician–nurse collaboration and effective communication,1 as reflected in a separate study of the intervention showing no significant difference in the concordance of the patient care plan between nurses and physicians before and after regionalization.12 Additional interventions may be needed to ensure that communication during bedside rounds is effective.
Our regionalized teams spent a significantly higher proportion of rounding time bedside, likely because of a cultural shift in expectations and the increased convenience of seeing patients on the team’s unit. Nevertheless, bedside time was not 100%. Structural barriers (eg, patients off-unit for dialysis) and cultural barriers likely contributed to the less than full adoption of bedside rounding. As described previously, cultural barriers to bedside rounding include trainees’ anxiety about being questioned in front of patients, the desire to freely exchange academic ideas in a conference room, and attendings’ doubts about their bedside teaching ability.1,9,13 Bedside rounds provide an important opportunity to apply the principles of patient- and family-centered care, including promotion of dignity and respect, information sharing, and collaboration. Thus, overcoming the concerns of housestaff and attendings and helping them feel prepared for bedside rounds can benefit the patient experience. More attention should be given to these practices as these types of interventions are implemented at Brigham and Women’s Hospital and elsewhere.1,13-15
Another primary concern about interdisciplinary bedside rounding is the perception that it takes more time.9 Therefore, it was important for us to measure round duration as a balancing measure to be considered for our intervention. Fortunately, we found round duration decreased with regionalization and encouragement of bedside rounding. This decrease was driven largely by a significant decrease in mean rounding time per new patient, which may be attributable at least in part to setting expectations that attendings and residents will read admission notes before rounds and that interns will summarize rather than recount information from admission notes. However, we also found rounding time decreases for subsequent-day patients, suggesting an underlying time savings. Spending a larger proportion of time bedside may therefore result in more efficient rounds. Bedside presentations can reduce redundancies, such as discussing a patient’s case outside his or her room and subsequently walking in and going over much of the same information with the patient. Our model de-emphasizes data transfer in favor of discussion of care plans. There was also a decrease in non-patient time, likely reflecting reduced transit time for regionalized teams. This decrease aligns with a recent finding that bedside rounding was at least as efficient as rounding outside the room.16
Of note, though a larger percentage of time was spent bedside after implementation of the care redesign, the absolute amount of bedside time did not change significantly. Our data showed that, even with shorter rounds, the same amount of absolute time can be spent bedside, face to face with the patient, by increasing the proportion of bedside rounding time. In other words, teams on average did not spend more time with patients, though the content and the structure of those encounters may have changed. This finding may be attributable to eliminating redundancy, forgoing the outside-the-room discussion, and thus the largest time reductions were realized there. In addition, teams incompletely adopted beside rounds, as reflected in the data. We expect that, with more complete adoption, an even larger proportion of time will be spent bedside, and absolute time bedside might increase as a result.
An unexpected result of the care redesign was that interns’ proportion of rounding time decreased after the intervention. This decrease most likely is attributable to interns’ being less likely to participate in rounds for a co-intern’s patient, and to their staying outside that patient’s room to give themselves more time to advance the care of their own patients. Before the intervention, when more rounding time was spent outside patient rooms, interns were more likely to join rounds for their co-intern’s patients because they could easily break away, as needed, to continue care of their own patients. The resident is now encouraged to use the morning huddle to identify which patients likely have the most educational value, and both interns are expected to join the bedside rounds for these patients.
This study had a few limitations. First, the pre–post design made it difficult to exclude the possibility that other temporal changes may have affected outcomes, though we did account for time-of-year effects by aligning our data-collection phases. In addition, the authors, including the director of the general medical service, are unaware of any co-interventions during the study period. Second, the multipronged intervention included care team regionalization, encouragement of bedside rounding with nurses, call structure changes (from 4 days to daily admitting), and attendings’ reading of admission notes before rounds. Thus, parsing which component(s) contributed to the results was difficult, though all the changes instituted likely were necessary for system redesign. For example, regionalization of clinicians to unit-based teams was made possible by switching to a daily admitting system.
Time that team members spent preparing for rounds was not recorded before or after the intervention. Thus, the decrease in total rounding time could have been accompanied by an increase in time spent preparing for rounds. However, admission notes were available in our electronic medical record before and after the intervention, and most residents and attendings were already reading them pre-intervention. After the intervention, pre-round note reading was more clearly defined as an expectation, and we were able to set the expectation that interns should use their presentations to summarize rather than recount information. In addition, in the post-intervention period, we did not include time spent preparing rounding orders; as already noted, however, preparation took only 5 minutes per day. Also, we did not analyze the content or the quality of the discussion on rounds, but simply recorded who was present where and when. Regarding the effect of the intervention on patient care, results were mixed. As reported in 2016, we saw no difference in frequency of adverse events with this intervention.12 However, a more sensitive measure of adverse events—used in a study on handoffs—showed our regionalization efforts had an additive effect on reducing overnight adverse events.17Researchers should now focus on the effects of care redesign on clinical outcomes, interdisciplinary care team communication, patient engagement and satisfaction, provider opinions of communication, workflow, patient care, and housestaff education. Our methodology can be used as a model to link structure, process, and outcome related to rounds and thereby better understand how best to optimize patient care and efficiency. Additional studies are needed to analyze the content of rounds and their association with patient and educational outcomes. Last, it will be important to conduct a study to see if the effects we have identified can be sustained. Such a study is already under way.
In conclusion, creating regionalized care teams and encouraging focused bedside rounds increased the proportion of bedside time and the presence of nurses on rounds. Rounds were shorter despite higher patient census. TMA revealed that regionalized care teams and bedside rounding at a large academic hospital are feasible, and are useful in establishing the necessary structures for increasing physician–nurse and provider–patient interactions.
Acknowledgments
The authors acknowledge Dr. Stan Ashley, Dr. Jacqueline Somerville, and Sheila Harris for their support of the regionalization initiative.
Disclosures
Dr. Schnipper received funding from Sanofi-aventis to conduct an investigator-initiated study to implement and evaluate a multi-faceted intervention to improve transitions of care in patients discharged home on insulin. The study was also supported by funding from the Marshall A. Wolf Medical Education Fund, Brigham and Women’s Hospital, and Dr. Stan Ashley, Chief Medical Officer, Brigham and Women’s Hospital. Some of the content of this article was orally presented at the annual meeting of the Society of Hospital Medicine; March 29-April 1, 2015; National Harbor, MD.
1. Crumlish CM, Yialamas MA, McMahon GT. Quantification of bedside teaching by an academic hospitalist group. J Hosp Med. 2009;4(5):304-307. PubMed
2. Gonzalo JD, Masters PA, Simons RJ, Chuang CH. Attending rounds and bedside case presentations: medical student and medicine resident experiences and attitudes. Teach Learn Med. 2009;21(2):105-110. PubMed
3. Elliot DL, Hickam DH. Attending rounds on in-patient units: differences between medical and non-medical services. Med Educ. 1993;27(6):503-508. PubMed
4. Payson HE, Barchas JD. A time study of medical teaching rounds. N Engl J Med. 1965;273(27):1468-1471. PubMed
5. Tremonti LP, Biddle WB. Teaching behaviors of residents and faculty members. J Med Educ. 1982;57(11):854-859. PubMed
6. Miller M, Johnson B, Greene HL, Baier M, Nowlin S. An observational study of attending rounds. J Gen Intern Med. 1992;7(6):646-648. PubMed
7. Collins GF, Cassie JM, Daggett CJ. The role of the attending physician in clinical training. J Med Educ. 1978;53(5):429-431. PubMed
8. Ward DR, Ghali WA, Graham A, Lemaire JB. A real-time locating system observes physician time-motion patterns during walk-rounds: a pilot study. BMC Med Educ. 2014;14:37. PubMed
9. Gonzalo JD, Kuperman E, Lehman E, Haidet P. Bedside interprofessional rounds: perceptions of benefits and barriers by internal medicine nursing staff, attending physicians, and housestaff physicians. J Hosp Med. 2014;9(10):646-651. PubMed
10. Stickrath C, Noble M, Prochazka A, et al. Attending rounds in the current era: what is and is not happening. JAMA Intern Med. 2013;173(12):1084-1089. PubMed
11. Boxer R, Vitale M, Gershanik EF, et al. 5th time’s a charm: creation of unit-based care teams in a high occupancy hospital [abstract]. J Hosp Med. 2015;10(suppl 2).
12. Mueller SK, Schnipper JL, Giannelli K, Roy CL, Boxer R. Impact of regionalized care on concordance of plan and preventable adverse events on general medicine services. J Hosp Med. 2016;11(9):620-627. PubMed
13. Chauke HL, Pattinson RC. Ward rounds—bedside or conference room? S Afr Med J. 2006;96(5):398-400. PubMed
14. Wang-Cheng RM, Barnas GP, Sigmann P, Riendl PA, Young MJ. Bedside case presentations: why patients like them but learners don’t. J Gen Intern Med. 1989;4(4):284-287. PubMed
15. Lehmann LS, Brancati FL, Chen MC, Roter D, Dobs AS. The effect of bedside case presentations on patients’ perceptions of their medical care. N Engl J Med. 1997;336(16):1150-1155. PubMed
16. Gonzalo JD, Chuang CH, Huang G, Smith C. The return of bedside rounds: an educational intervention. J Gen Intern Med. 2010;25(8):792-798. PubMed
17. Mueller SK, Yoon C, Schnipper JL. Association of a web-based handoff tool with rates of medical errors. JAMA Intern Med. 2016;176(9):1400-1402. PubMed
1. Crumlish CM, Yialamas MA, McMahon GT. Quantification of bedside teaching by an academic hospitalist group. J Hosp Med. 2009;4(5):304-307. PubMed
2. Gonzalo JD, Masters PA, Simons RJ, Chuang CH. Attending rounds and bedside case presentations: medical student and medicine resident experiences and attitudes. Teach Learn Med. 2009;21(2):105-110. PubMed
3. Elliot DL, Hickam DH. Attending rounds on in-patient units: differences between medical and non-medical services. Med Educ. 1993;27(6):503-508. PubMed
4. Payson HE, Barchas JD. A time study of medical teaching rounds. N Engl J Med. 1965;273(27):1468-1471. PubMed
5. Tremonti LP, Biddle WB. Teaching behaviors of residents and faculty members. J Med Educ. 1982;57(11):854-859. PubMed
6. Miller M, Johnson B, Greene HL, Baier M, Nowlin S. An observational study of attending rounds. J Gen Intern Med. 1992;7(6):646-648. PubMed
7. Collins GF, Cassie JM, Daggett CJ. The role of the attending physician in clinical training. J Med Educ. 1978;53(5):429-431. PubMed
8. Ward DR, Ghali WA, Graham A, Lemaire JB. A real-time locating system observes physician time-motion patterns during walk-rounds: a pilot study. BMC Med Educ. 2014;14:37. PubMed
9. Gonzalo JD, Kuperman E, Lehman E, Haidet P. Bedside interprofessional rounds: perceptions of benefits and barriers by internal medicine nursing staff, attending physicians, and housestaff physicians. J Hosp Med. 2014;9(10):646-651. PubMed
10. Stickrath C, Noble M, Prochazka A, et al. Attending rounds in the current era: what is and is not happening. JAMA Intern Med. 2013;173(12):1084-1089. PubMed
11. Boxer R, Vitale M, Gershanik EF, et al. 5th time’s a charm: creation of unit-based care teams in a high occupancy hospital [abstract]. J Hosp Med. 2015;10(suppl 2).
12. Mueller SK, Schnipper JL, Giannelli K, Roy CL, Boxer R. Impact of regionalized care on concordance of plan and preventable adverse events on general medicine services. J Hosp Med. 2016;11(9):620-627. PubMed
13. Chauke HL, Pattinson RC. Ward rounds—bedside or conference room? S Afr Med J. 2006;96(5):398-400. PubMed
14. Wang-Cheng RM, Barnas GP, Sigmann P, Riendl PA, Young MJ. Bedside case presentations: why patients like them but learners don’t. J Gen Intern Med. 1989;4(4):284-287. PubMed
15. Lehmann LS, Brancati FL, Chen MC, Roter D, Dobs AS. The effect of bedside case presentations on patients’ perceptions of their medical care. N Engl J Med. 1997;336(16):1150-1155. PubMed
16. Gonzalo JD, Chuang CH, Huang G, Smith C. The return of bedside rounds: an educational intervention. J Gen Intern Med. 2010;25(8):792-798. PubMed
17. Mueller SK, Yoon C, Schnipper JL. Association of a web-based handoff tool with rates of medical errors. JAMA Intern Med. 2016;176(9):1400-1402. PubMed
© 2017 Society of Hospital Medicine
Discordance Between Patient and Provider
Patient‐centered care has been recognized by the Institute of Medicine as an essential aim of the US healthcare system.[1] A fundamental component of patient‐centered care is to engage patients and caregivers in establishing preferences, needs, values, and overall goals regarding their care.[1] Prior studies have shown that delivering high‐quality patient‐centered care is associated with improved health outcomes, and in some cases, reduced costs.[2, 3, 4, 5, 6, 7] Payors, including the Centers for Medicare and Medicaid Services under the Hospital Value‐Based Purchasing program, are increasingly tying payments to measures of patient experience.[8, 9] As more emphasis is placed on public reporting of these patient‐reported outcomes, healthcare organizations are investing in efforts to engage patients and caregivers, including efforts at establishing patients' preferences for care.[10]
In the acute care setting, a prerequisite for high‐quality patient‐centered care is identifying a patient's primary goal for recovery and then delivering care consistent with that goal.[11, 12, 13] Haberle et al. previously validated patients' most common goals for recovery in the hospital setting into 7 broad categories: (1) be cured, (2) live longer, (3) improve or maintain health, (4) be comfortable, (5) accomplish a particular life goal, (6) provide support for a family member, or (7) other.[13] When providers' understanding of these recovery goals are not concordant with the patient's stated goals, patients may receive care inconsistent with their preferences; it is not uncommon for patients to receive aggressive curative treatments (eg, cardiopulmonary resuscitation) when they have expressed otherwise.[14] On the other hand, when patient goals and priorities are clearly established, patients may have better outcomes.[15] For example, earlier conversations about patient goals and priorities in serious illness can lead to realistic expectations of treatment, enhanced goal‐concordant care, improved quality of life, higher patient satisfaction, more and earlier hospice care, fewer hospitalizations, better patient and family coping, reduced burden of decision making for families, and improved bereavement outcomes.[16, 17, 18]
Although previous studies have suggested poor patient‐physician concordance with regard to the patient's plan of care,[19, 20, 21, 22, 23, 24] there are limited data regarding providers' understanding of the patient's primary recovery goal during hospitalization. The purpose of this study was to identify the patients' Haberle goal, and then determine the degree of concordance among patients and key hospital providers regarding this goal.
METHODS
Study Setting
The Partners Human Research Committee approved the study. The study was conducted on an oncology and medical intensive care unit (MICU) at a major academic medical center in Boston, Massachusetts. The oncology unit was comprised of 2 non‐localized medical teams caring for patients admitted to that unit. The MICU was comprised of a single localized medical team. Medical teams working on these units consisted of a first responder (eg, intern or a physician assistant [PA]), medical residents, and an attending physician. Both units had dedicated nursing staff.
Study Participants
All adult patients (>17 years of age) admitted to the oncology and MICU units during the study period (November 2013 through May 2014) were eligible. These units were chosen because these patients are typically complex and have multiple medical comorbidities longer lengths of stay, and many procedures and tests. In addition, a standard method for asking patients to identify a primary recovery goal for hospitalization aligned well with ongoing institutional efforts to engage these patients in goals of care discussions.
Research assistants identified all patients admitted to each study unit for at least 48 hours and approached them in a random order with a daily target of 2 to 3 patients. Only patients who demonstrated capacity (determined by medical team), or had a legally designated healthcare proxy (who spoke English and was available to participate on their behalf) were included. Research assistants then approached the patient's nurse and a physician provider (defined for this study as housestaff physician, PA, or attending) from the primary medical team to participate in the interview (within 24 hours of patient's interview). We excluded eligible patients who did not have capacity or an available caregiver or declined to participate.
Data Collection Instrument and Interviews
Research assistants administered a validated questionnaire developed by Haberle et al. to participants after 48 hours into the patient's admission to provide time to establish mutual understanding of the diagnosis and prognosis.[13] We asked patients (or the designated healthcare proxy) to select their single, most important Haberle goal (see above). Specifically, as in the original validation study,[13] patients or proxies were asked the following question: Please tell me your most important goal of care for this hospitalization. If they did not understand this question, we asked a follow‐up question: What are you expecting will be accomplished during this hospitalization? Within 24 hours of the patient/proxy interview, we independently asked the patient's nurse and physician to select what they thought was the patient's most important goal for recovery using the same questionnaire, adapted for providers. In each case, all participants were blinded to the responses of others.
Measures
We measured the frequency that each participant (patient/proxy, nurse, and physician) selected a specific Haberle recovery goal across all patients. We measured the rate of pairwise concordance by recovery goal for each participant dyad (patient/proxy‐nurse, patient/proxy‐physician, and nurse‐physician). Finally, we calculated the frequency of cases for which all 3 participants selected the same recovery goal.
Statistical Analyses
Descriptive statistics were used to report patient demographic data. The frequencies of selected responses were calculated and reported as percentages for each type of participant. The differences in rate of responses for each Haberle goal were compared across each participant group using 2 analysis. We then performed 2‐way Kappa statistical tests to measure inter‐rater agreement for each dyad.
RESULTS
Of 1436 patients (882 oncology, 554 MICU) hospitalized during the study period, 341(156 oncology, 185 MICU) were admitted for <48 hours. Of 914 potentially eligible patients (617 oncology, 297 MICU), 191 (112 oncology and 79 MICU) were approached to participate based on our sampling strategy; of these, 8 (2 oncology and 6 MICU) did not have capacity (and no proxy was available) and 2 (1 oncology and 1 MICU) declined. Of the remaining 181 patients (109 oncology and 72 MICU), we obtained a completed questionnaire from all 3 interviewees on 109 (60.2% response rate).
Of the 109 study patients, 52 (47.7%) and 57 (52.3%) were admitted to the oncology and medical intensive care units, respectively (Table 1). Patients were predominantly middle aged, Caucasian, English‐speaking, and college‐educated. Healthcare proxies were frequently interviewed on behalf of patients in the MICU. Housestaff physicians were more often interviewed in the MICU, and PAs were interviewed only on oncology units. Compared to patient responders, nonresponders tended to be male and were admitted to oncology units (see Supporting Table 1 in the online version of this article).
| Characteristics | All Patients | Admitted to Medical Intensive Care Units | Admitted to Oncology Units |
|---|---|---|---|
| |||
| Total, no. (%) | 109 (100%) | 57 (52.3%) | 52 (47.7%) |
| Gender, no. (%) | |||
| Male | 55 (50.5%) | 28 (49.1%) | 26 (50.0%) |
| Female | 54 (49.5%) | 29 (50.9%) | 26 (50.0%) |
| Age, y, mean SD | 59.4 14 | 59.7 15 | 59.1 13 |
| Median | 61 | 61 | 60 |
| Range | 2188 | 2188 | 2285 |
| Race, no. (%) | |||
| White | 103 (94.5%) | 53 (93.0%) | 50 (96.2%) |
| Other | 6 (5.5%) | 4 (7.0%) | 2 (3.8%) |
| Language, no. (%) | |||
| English | 106 (97.2%) | 56 (98.1%) | 50 (96.2%) |
| Other | 3 (2.8%) | 1 (1.9%) | 2 (3.8%) |
| Education level, no. (%) | |||
| Less than high school | 30 (27.5%) | 17 (29.8%) | 13 (25.0%) |
| High school diploma | 27 (24.5%) | 18 (31.6%) | 9 (17.3%) |
| Some college or beyond | 52 (47.7%) | 22 (38.6%) | 30 (57.7%) |
| Patient or caregiver interviewed, no. (%) | |||
| Patient | 68 (62.4%) | 27 (47.4%) | 48 (92.3%) |
| Caregiver | 41 (37.6%) | 30 (52.6%) | 4 (7.7%) |
| Nurse interviewed, no. (unique) | 109 (75) | 57 (42) | 52 (33) |
| Physician provider interviewed, no. (%); no. unique | |||
| Attending | 27 (24.8%); 20 | 15 (26.3%); 10 | 12 (23.1%); 10 |
| Housestaff | 48 (44.0%); 39 | 42 (73.7%); 33 | 6 (11.5%); 6 |
| Physician assistant | 34 (31.2%); 25 | 0 (0%); 0 | 34 (65.4%); 25 |
The frequencies of selected Haberle recovery goals by participant type across all patients are listed in Table 2. Patients (or proxies) most often selected be cured (46.8%). Assigned nurses and physicians more commonly selected improve or maintain health (38.5% and 46.8%, respectively). Be comfortable was selected by nurses and physicians more frequently than by patients (16.5%, 16.5%, and 8.3%, respectively). The rate of responses for each Haberle goal was significantly different across all respondent groups (P < 0.0001). The frequencies of selected Haberle goals were not significantly different between patients or proxies (P = 0.67), or for patients admitted to the MICU compared to oncology units (P = 0.64).
| Haberle Recovery Goal | Patient/Caregiver, no. (%), n = 109 | Physician Provider, no. (%), n = 109* | Nurse, no. (%), n = 109 |
|---|---|---|---|
| |||
| Be cured | 51 (46.8%) | 20 (18.3%) | 20 (18.3%) |
| Be comfortable | 9 (8.3%) | 18 (16.5%) | 18 (16.5%) |
| Improve or maintain health | 32 (29.4%) | 42 (38.5%) | 51 (46.8%) |
| Live longer | 14 (12.8%) | 21 (19.3%) | 12 (11%) |
| Accomplish personal goal | 2 (1.8%) | 0 (0%) | 3 (2.8%) |
| Provide support for family | 1 (0.9%) | 1 (0.9%) | 1 (0.9%) |
| Other | 0 (0%) | 7 (6.4%) | 4 (3.7%) |
Inter‐rater agreement was poor to slight for the 3 participant dyads (kappa 0.09 [0.03‐0.19], 0.19 [0.08‐0.30], and 0.20 [0.08‐0.32] for patient‐physician, patient‐nurse, and nurse‐physician, respectively). The 3 participants selected the identical recovery goal in 22 (20.2%) cases, and each selected a distinct recovery goal in 32 (29.4%) cases. Pairwise concordance between nurses and physicians was 39.4%. There were no significant differences in agreement between patients admitted to the MICU compared to oncology units (P = 0.09).
DISCUSSION
We observed poor to slight concordance among patients and key hospital providers with regard to identifying the patient's primary recovery goal during acute hospitalization. The majority of patients (or proxies), chose be cured, whereas the majority of hospital providers chose improve or maintain health. Patients were twice as likely to select be cured and half as likely to choose be comfortable compared to nurses or physicians. Strikingly, the patient (or proxy), nurse, and physician identified the same recovery goal in just 20% of cases. These findings were similar for patients admitted to either the MICU or oncology units or when healthcare proxies participated on behalf of the patient (eg, when incapacitated in the MICU).
There are many reasons why hospital providers may not correctly identify the patients' primary recovery goals. First, we do not routinely ask patients to identify recovery goals upon admission in a structured and standardized manner. In fact, clinicians often do not elicit patients' needs, concerns, and expectations regarding their care in general.[25] Second, even when recovery goals are elicited at admission, they may not be communicated effectively to all members of the care team. This could be due to geographically non‐localized teams (although we did not observe a statistically significant difference between regionalized MICU and nonregionalized oncology care units), frequent provider‐to‐provider handoffs, and siloed electronic communication (eg, email, alphanumeric pages) regarding goals of care that inevitably leaves out key providers.[26] Third, healthcare proxies who are involved in decision making on the patient's behalf may not always be available to meet with the care team in person; consequently, their input may not be considered in a timely manner or reliably communicated to all members of the care team. We observed a large discrepancy in how often patients chose be cured compared to their hospital providers. This could be explained by clinicians' unwillingness to disclose bad news or divulge accurate prognostic information that causes patients to feel depressed or lose hope, particularly for those patients with the worst prognoses.[16, 27, 28] Patients may lack sophisticated knowledge of their conditions for a variety of reasons, including low health literacy, at times choosing to hope for the best even when it is not realistic. Additionally, there may be more subtle differences in what patients and hospital providers consider the primary recovery goal in context of the main reason for hospitalization and underlying medical illness. For example, a patient with metastatic lung cancer hospitalized with recurrent postobstructive pneumonia may choose be cured as his/her primary recovery goal (thinking of the pneumonia), whereas physicians may choose improve/maintain health or comfort (thinking of the cancer). We also cannot exclude the possibility that sometimes when patients state be cured and clinicians state improve health as the primary goal, that they are really saying the same thing in different ways. However, these are 2 different constructs (cure may not be possible for many patients) that may deserve an explicit discussion for patients to have realistic expectations for their health following hospitalization.
In short, our results underscore the importance of having an open and honest dialog with patients and caregivers throughout hospitalization, and the need to provide education about the potential futility of excessive care in situations where appropriate. Simply following patients' goals without discussing their feasibility and the consequences of aggressive treatments may result in unnecessary morbidity and misuse of healthcare resources. Once goals are clearly established, communicated, and refined in hospitalized patients with serious illness, there is much reason to believe that ongoing conversation will favorably impact outcomes.[29]
We found few studies that rigorously quantified the rate of concordance of hospital recovery goals among patients and key hospital providers; however, studies that measured overall plan of care agreement have demonstrated suboptimal concordance.[20, 30, 31] Shin et al. found significant underestimation of cancer patients' needs and poor concordance between patients and oncologists in assessing perceived needs of supportive care.[20] It is also notable that nurses and physicians had low levels of concordance in our study. O'Leary and colleagues found that nurses and physicians did not reliably communicate and often did not agree on the plan of care for hospitalized patients.[30] Although geographic regionalization of care teams and multidisciplinary rounds can improve the likelihood that key members of the care team are on the same page with regard to the plan of care, there is still much room for improvement.[26, 32, 33, 34] For example, although nurses and physicians in our study independently selected individual recovery goals with similar frequencies (Table 2), we observed suboptimal concordance between nurses and providers (36.8%) for specific patients, including on our regionalized care unit (MICU). This may be due to the reasons described above.
There are several implications of these findings. As payors continue to shift payments toward value‐based metrics, largely determined by patient experience and adequate advance care planning,[9] our findings suggest that more effort should be focused on delivering care consistent with patients' primary recovery goals. As a first step, healthcare organizations can focus on efforts to systematically identify and communicate recovery goals to all members of the care team, ensuring that patients' preferences, needs, and values are captured. In addition, as innovation in patient engagement and care delivery using Web‐based and mobile technology continues to grow,[35] using these tools to capture key goals for hospitalization and recovery can play an essential role. For example, as electronic health record vendors and institutions start to implement patient portals in the acute care setting, they should consider how to configure these tools to capture key goals for hospitalization and recovery, and then communicate them to the care team; preliminary work in this area is promising.[10]
Our study has several limitations to generalizability. First, the study was conducted on 2 services (MICU and oncology) at a single institution using a sampling strategy where research assistants enrolled 2 to 3 patients per day. Although the sampling was random, the availability of patients and proxies to be interviewed may have led to selection bias. Second, the sample size was small. Third, the patients who participated were predominantly white, English‐speaking, and well educated, possibly a consequence of our sampling strategy. However, this fact makes our findings more striking; although cultural and language barriers were generally not present in our study population, large discrepancies in goal concordance still existed. Fourth, in instances when patients were unable to participate themselves, we interviewed their healthcare proxy; therefore, it is possible that the proxies' responses did not reflect those of the patient. However, we note that concordance rates did not significantly differ between the 2 services despite the fact that the proportion of proxy interviews was much higher in the MICU. Similarly, we cannot exclude the possibility that patients altered their stated goals in the presence of proxies, but patients were given the option to be interviewed alone. Patients may also have misunderstood the timing of the goals (during this hospitalization as opposed to long term), although research assistants made every effort to clarify this during the interviews. Finally, our data‐collection instrument was previously validated in hospitalized general medicine patients and not oncology or MICU patients, and it has not been used to directly ask clinicians to identify patients' recovery goals. However, there is no reason to suspect that it could not be used for this purpose in critical care as well as noncritical care settings, as the survey was developed by a multidisciplinary team that included medical professionals and was validated by clinicians who successfully identified a single, very broad goal (eg, be cured) in each case.
CONCLUSION
We report poor to slight concordance among hospitalized patients and key hospital providers with regard to the main recovery goal. Future studies should assess whether patient satisfaction and experience is adversely impacted by patient‐provider discordance regarding key recovery goals. Additionally, institutions may consider future efforts to elicit and communicate patients' primary recovery goals more effectively to all members of the care team, and address discrepancies as soon as they are discovered.
Disclosures
This work was supported by a grant from the Gordon and Betty Moore Foundation (GBMF) (grant GBMF3914). GBMF had no role in the design or conduct of the study; collection, analysis, or interpretation of data; or preparation or review of the manuscript. The authors report no conflicts of interest.
- Institute of Medicine. Crossing the Quality Chasm: A New Health System for the 21st Century. Washington, DC: National Academy of Sciences; 2001.
- , , , , , . The effects of physician communications skills on patient satisfaction; recall, and adherence. J Chronic Dis. 1984;37(9–10):755–764.
- , , , et al. Observational study of effect of patient centredness and positive approach on outcomes of general practice consultations. BMJ. 2001;323(7318):908–911.
- . Reengineering hospital discharge: a protocol to improve patient safety, reduce costs, and boost patient satisfaction. Am J Med Qual. 2009;24(4):344–346.
- , , , , . Relationship between patient satisfaction with inpatient care and hospital readmission within 30 days. Am J Manag Care. 2011;17(1):41–48.
- , , , . Patients' perception of hospital care in the United States. N Engl J Med. 2008;359(18):1921–1931.
- , , . Enhanced support for shared decision making reduced costs of care for patients with preference‐sensitive conditions. Health Aff (Millwood). 2013;32(2):285–293.
- Centers for Medicare and Medicaid Services. Medicare program; hospital inpatient value‐based purchasing program. Final rule. Fed Regist. 2011;76(88):26490–26547.
- Centers for Medicare and Medicaid Services. CMS begins implementation of key payment legislation. Available at: https://www.cms.gov/Newsroom/MediaReleaseDatabase/Press‐releases/2015‐Press‐releases‐items/2015‐07‐08.html. Published July 8, 2015.
- , , , et al. A web‐based, patient‐centered toolkit to engage patients and caregivers in the acute care setting: a preliminary evaluation [published online August 2, 2015]. J Am Med Informatics Assoc. doi: 10.1093/jamia/ocv093.
- , , , et al. Effectiveness trial of an intensive communication structure for families of long‐stay ICU patients. Chest. 2010;138(6):1340–1348.
- , , , . Understanding goals of care statements and preferences among patients and their surrogates in the medical ICU. J Hosp Palliat Nurs. 2012;14(2):126–132.
- , , , . Goals of care among hospitalized patients: a validation study. Am J Hosp Palliat Care. 2011;28(5):335–341.
- , , , et al. Factors associated with use of cardiopulmonary resuscitation in seriously ill hospitalized adults. JAMA. 1999;282(24):2333–2339.
- , , , , . End‐of‐life discussions, goal attainment, and distress at the end of life: Predictors and outcomes of receipt of care consistent with preferences. J Clin Oncol. 2010;28(7):1203–1208.
- , . Reasons why physicians do not have discussions about poor prognosis, why it matters, and what can be improved. J Clin Oncol. 2012;30(22):2715–2717.
- , , , et al. Associations between end‐of‐life discussions, patient mental health, medical care near death, and caregiver bereavement adjustment. JAMA. 2008;300(14):1665–1673.
- , , , . Prior advance care planning is associated with less decisional conflict among surrogates for critically ill patients. Ann Am Thorac Soc. 2015;12(10):1528–1533.
- , , , et al. Hospitalized patients' understanding of their plan of care. Mayo Clin Proc. 2010;85(1):47–52.
- , , , et al. Discordance in perceived needs between patients and physicians in oncology practice: a nationwide survey in Korea. J Clin Oncol. 2011;29(33):4424–4429.
- , , , , , . Leveraging standards to support patient‐centric interdisciplinary plans of care. AMIA Annu Symp Proc. 2011;2011:356–363.
- , . Discordance between physician and patient self‐rated health and all‐cause mortality. Ochsner J. 2011;11(3):232–240.
- , , , , , . Determinants of discordance between patients and physicians in their assessment of lupus disease activity. J Rheumatol. 2003;30(9):1967–1976.
- , , , , , . Predictors of discordance between physicians' and patients' appraisals of health‐related quality of life in atrial fibrillation patients: Findings from the Angiotensin II Antagonist in Paroxysmal Atrial Fibrillation Trial. Am Heart J. 2013;166(3):589–596.
- , , , et al. Uncovering the blind spot of patient satisfaction: an international survey. BMJ Qual Saf. 2011;20(11):959–965.
- , , , , , . Improving teamwork: impact of structured interdisciplinary rounds on a hospitalist unit. J Hosp Med. 2011;6(2):88–93.
- , , , . Discrepancies between patient and physician estimates for the success of stem cell transplantation. JAMA. 2001;285(8):1034–1038.
- , , , , , . Optimistic expectations and survival after hematopoietic stem cell transplantation. Biol Blood Marrow Transplant. 2003;9(6):389–396.
- , , , et al. Development of the Serious Illness Care Program: a randomised controlled trial of a palliative care communication intervention. BMJ Open. 2015;5(10):e009032.
- , , , et al. Patterns of nurse‐physician communication and agreement on the plan of care. Qual Saf Health Care. 2010;19(3):195–199.
- , , , et al. Impact of localizing physicians to hospital units on nurse‐physician communication and agreement on the plan of care. J Gen Intern Med. 2009;24(11):1223–1227.
- , , , et al. Changes in medical errors after implementation of a handoff program. N Engl J Med. 2014;371(19):1803–1812.
- , , , et al. Reorganizing a hospital ward as an accountable care unit. J Hosp Med. 2015;10(1):36–40.
- , , , . Interdisciplinary teamwork in hospitals: a review and practical recommendations for improvement. J Hosp Med. 2012;7(1):48–54.
- , , , , . An evaluation of mobile health application tools. JMIR mHealth uHealth. 2014;2(2):e19.
Patient‐centered care has been recognized by the Institute of Medicine as an essential aim of the US healthcare system.[1] A fundamental component of patient‐centered care is to engage patients and caregivers in establishing preferences, needs, values, and overall goals regarding their care.[1] Prior studies have shown that delivering high‐quality patient‐centered care is associated with improved health outcomes, and in some cases, reduced costs.[2, 3, 4, 5, 6, 7] Payors, including the Centers for Medicare and Medicaid Services under the Hospital Value‐Based Purchasing program, are increasingly tying payments to measures of patient experience.[8, 9] As more emphasis is placed on public reporting of these patient‐reported outcomes, healthcare organizations are investing in efforts to engage patients and caregivers, including efforts at establishing patients' preferences for care.[10]
In the acute care setting, a prerequisite for high‐quality patient‐centered care is identifying a patient's primary goal for recovery and then delivering care consistent with that goal.[11, 12, 13] Haberle et al. previously validated patients' most common goals for recovery in the hospital setting into 7 broad categories: (1) be cured, (2) live longer, (3) improve or maintain health, (4) be comfortable, (5) accomplish a particular life goal, (6) provide support for a family member, or (7) other.[13] When providers' understanding of these recovery goals are not concordant with the patient's stated goals, patients may receive care inconsistent with their preferences; it is not uncommon for patients to receive aggressive curative treatments (eg, cardiopulmonary resuscitation) when they have expressed otherwise.[14] On the other hand, when patient goals and priorities are clearly established, patients may have better outcomes.[15] For example, earlier conversations about patient goals and priorities in serious illness can lead to realistic expectations of treatment, enhanced goal‐concordant care, improved quality of life, higher patient satisfaction, more and earlier hospice care, fewer hospitalizations, better patient and family coping, reduced burden of decision making for families, and improved bereavement outcomes.[16, 17, 18]
Although previous studies have suggested poor patient‐physician concordance with regard to the patient's plan of care,[19, 20, 21, 22, 23, 24] there are limited data regarding providers' understanding of the patient's primary recovery goal during hospitalization. The purpose of this study was to identify the patients' Haberle goal, and then determine the degree of concordance among patients and key hospital providers regarding this goal.
METHODS
Study Setting
The Partners Human Research Committee approved the study. The study was conducted on an oncology and medical intensive care unit (MICU) at a major academic medical center in Boston, Massachusetts. The oncology unit was comprised of 2 non‐localized medical teams caring for patients admitted to that unit. The MICU was comprised of a single localized medical team. Medical teams working on these units consisted of a first responder (eg, intern or a physician assistant [PA]), medical residents, and an attending physician. Both units had dedicated nursing staff.
Study Participants
All adult patients (>17 years of age) admitted to the oncology and MICU units during the study period (November 2013 through May 2014) were eligible. These units were chosen because these patients are typically complex and have multiple medical comorbidities longer lengths of stay, and many procedures and tests. In addition, a standard method for asking patients to identify a primary recovery goal for hospitalization aligned well with ongoing institutional efforts to engage these patients in goals of care discussions.
Research assistants identified all patients admitted to each study unit for at least 48 hours and approached them in a random order with a daily target of 2 to 3 patients. Only patients who demonstrated capacity (determined by medical team), or had a legally designated healthcare proxy (who spoke English and was available to participate on their behalf) were included. Research assistants then approached the patient's nurse and a physician provider (defined for this study as housestaff physician, PA, or attending) from the primary medical team to participate in the interview (within 24 hours of patient's interview). We excluded eligible patients who did not have capacity or an available caregiver or declined to participate.
Data Collection Instrument and Interviews
Research assistants administered a validated questionnaire developed by Haberle et al. to participants after 48 hours into the patient's admission to provide time to establish mutual understanding of the diagnosis and prognosis.[13] We asked patients (or the designated healthcare proxy) to select their single, most important Haberle goal (see above). Specifically, as in the original validation study,[13] patients or proxies were asked the following question: Please tell me your most important goal of care for this hospitalization. If they did not understand this question, we asked a follow‐up question: What are you expecting will be accomplished during this hospitalization? Within 24 hours of the patient/proxy interview, we independently asked the patient's nurse and physician to select what they thought was the patient's most important goal for recovery using the same questionnaire, adapted for providers. In each case, all participants were blinded to the responses of others.
Measures
We measured the frequency that each participant (patient/proxy, nurse, and physician) selected a specific Haberle recovery goal across all patients. We measured the rate of pairwise concordance by recovery goal for each participant dyad (patient/proxy‐nurse, patient/proxy‐physician, and nurse‐physician). Finally, we calculated the frequency of cases for which all 3 participants selected the same recovery goal.
Statistical Analyses
Descriptive statistics were used to report patient demographic data. The frequencies of selected responses were calculated and reported as percentages for each type of participant. The differences in rate of responses for each Haberle goal were compared across each participant group using 2 analysis. We then performed 2‐way Kappa statistical tests to measure inter‐rater agreement for each dyad.
RESULTS
Of 1436 patients (882 oncology, 554 MICU) hospitalized during the study period, 341(156 oncology, 185 MICU) were admitted for <48 hours. Of 914 potentially eligible patients (617 oncology, 297 MICU), 191 (112 oncology and 79 MICU) were approached to participate based on our sampling strategy; of these, 8 (2 oncology and 6 MICU) did not have capacity (and no proxy was available) and 2 (1 oncology and 1 MICU) declined. Of the remaining 181 patients (109 oncology and 72 MICU), we obtained a completed questionnaire from all 3 interviewees on 109 (60.2% response rate).
Of the 109 study patients, 52 (47.7%) and 57 (52.3%) were admitted to the oncology and medical intensive care units, respectively (Table 1). Patients were predominantly middle aged, Caucasian, English‐speaking, and college‐educated. Healthcare proxies were frequently interviewed on behalf of patients in the MICU. Housestaff physicians were more often interviewed in the MICU, and PAs were interviewed only on oncology units. Compared to patient responders, nonresponders tended to be male and were admitted to oncology units (see Supporting Table 1 in the online version of this article).
| Characteristics | All Patients | Admitted to Medical Intensive Care Units | Admitted to Oncology Units |
|---|---|---|---|
| |||
| Total, no. (%) | 109 (100%) | 57 (52.3%) | 52 (47.7%) |
| Gender, no. (%) | |||
| Male | 55 (50.5%) | 28 (49.1%) | 26 (50.0%) |
| Female | 54 (49.5%) | 29 (50.9%) | 26 (50.0%) |
| Age, y, mean SD | 59.4 14 | 59.7 15 | 59.1 13 |
| Median | 61 | 61 | 60 |
| Range | 2188 | 2188 | 2285 |
| Race, no. (%) | |||
| White | 103 (94.5%) | 53 (93.0%) | 50 (96.2%) |
| Other | 6 (5.5%) | 4 (7.0%) | 2 (3.8%) |
| Language, no. (%) | |||
| English | 106 (97.2%) | 56 (98.1%) | 50 (96.2%) |
| Other | 3 (2.8%) | 1 (1.9%) | 2 (3.8%) |
| Education level, no. (%) | |||
| Less than high school | 30 (27.5%) | 17 (29.8%) | 13 (25.0%) |
| High school diploma | 27 (24.5%) | 18 (31.6%) | 9 (17.3%) |
| Some college or beyond | 52 (47.7%) | 22 (38.6%) | 30 (57.7%) |
| Patient or caregiver interviewed, no. (%) | |||
| Patient | 68 (62.4%) | 27 (47.4%) | 48 (92.3%) |
| Caregiver | 41 (37.6%) | 30 (52.6%) | 4 (7.7%) |
| Nurse interviewed, no. (unique) | 109 (75) | 57 (42) | 52 (33) |
| Physician provider interviewed, no. (%); no. unique | |||
| Attending | 27 (24.8%); 20 | 15 (26.3%); 10 | 12 (23.1%); 10 |
| Housestaff | 48 (44.0%); 39 | 42 (73.7%); 33 | 6 (11.5%); 6 |
| Physician assistant | 34 (31.2%); 25 | 0 (0%); 0 | 34 (65.4%); 25 |
The frequencies of selected Haberle recovery goals by participant type across all patients are listed in Table 2. Patients (or proxies) most often selected be cured (46.8%). Assigned nurses and physicians more commonly selected improve or maintain health (38.5% and 46.8%, respectively). Be comfortable was selected by nurses and physicians more frequently than by patients (16.5%, 16.5%, and 8.3%, respectively). The rate of responses for each Haberle goal was significantly different across all respondent groups (P < 0.0001). The frequencies of selected Haberle goals were not significantly different between patients or proxies (P = 0.67), or for patients admitted to the MICU compared to oncology units (P = 0.64).
| Haberle Recovery Goal | Patient/Caregiver, no. (%), n = 109 | Physician Provider, no. (%), n = 109* | Nurse, no. (%), n = 109 |
|---|---|---|---|
| |||
| Be cured | 51 (46.8%) | 20 (18.3%) | 20 (18.3%) |
| Be comfortable | 9 (8.3%) | 18 (16.5%) | 18 (16.5%) |
| Improve or maintain health | 32 (29.4%) | 42 (38.5%) | 51 (46.8%) |
| Live longer | 14 (12.8%) | 21 (19.3%) | 12 (11%) |
| Accomplish personal goal | 2 (1.8%) | 0 (0%) | 3 (2.8%) |
| Provide support for family | 1 (0.9%) | 1 (0.9%) | 1 (0.9%) |
| Other | 0 (0%) | 7 (6.4%) | 4 (3.7%) |
Inter‐rater agreement was poor to slight for the 3 participant dyads (kappa 0.09 [0.03‐0.19], 0.19 [0.08‐0.30], and 0.20 [0.08‐0.32] for patient‐physician, patient‐nurse, and nurse‐physician, respectively). The 3 participants selected the identical recovery goal in 22 (20.2%) cases, and each selected a distinct recovery goal in 32 (29.4%) cases. Pairwise concordance between nurses and physicians was 39.4%. There were no significant differences in agreement between patients admitted to the MICU compared to oncology units (P = 0.09).
DISCUSSION
We observed poor to slight concordance among patients and key hospital providers with regard to identifying the patient's primary recovery goal during acute hospitalization. The majority of patients (or proxies), chose be cured, whereas the majority of hospital providers chose improve or maintain health. Patients were twice as likely to select be cured and half as likely to choose be comfortable compared to nurses or physicians. Strikingly, the patient (or proxy), nurse, and physician identified the same recovery goal in just 20% of cases. These findings were similar for patients admitted to either the MICU or oncology units or when healthcare proxies participated on behalf of the patient (eg, when incapacitated in the MICU).
There are many reasons why hospital providers may not correctly identify the patients' primary recovery goals. First, we do not routinely ask patients to identify recovery goals upon admission in a structured and standardized manner. In fact, clinicians often do not elicit patients' needs, concerns, and expectations regarding their care in general.[25] Second, even when recovery goals are elicited at admission, they may not be communicated effectively to all members of the care team. This could be due to geographically non‐localized teams (although we did not observe a statistically significant difference between regionalized MICU and nonregionalized oncology care units), frequent provider‐to‐provider handoffs, and siloed electronic communication (eg, email, alphanumeric pages) regarding goals of care that inevitably leaves out key providers.[26] Third, healthcare proxies who are involved in decision making on the patient's behalf may not always be available to meet with the care team in person; consequently, their input may not be considered in a timely manner or reliably communicated to all members of the care team. We observed a large discrepancy in how often patients chose be cured compared to their hospital providers. This could be explained by clinicians' unwillingness to disclose bad news or divulge accurate prognostic information that causes patients to feel depressed or lose hope, particularly for those patients with the worst prognoses.[16, 27, 28] Patients may lack sophisticated knowledge of their conditions for a variety of reasons, including low health literacy, at times choosing to hope for the best even when it is not realistic. Additionally, there may be more subtle differences in what patients and hospital providers consider the primary recovery goal in context of the main reason for hospitalization and underlying medical illness. For example, a patient with metastatic lung cancer hospitalized with recurrent postobstructive pneumonia may choose be cured as his/her primary recovery goal (thinking of the pneumonia), whereas physicians may choose improve/maintain health or comfort (thinking of the cancer). We also cannot exclude the possibility that sometimes when patients state be cured and clinicians state improve health as the primary goal, that they are really saying the same thing in different ways. However, these are 2 different constructs (cure may not be possible for many patients) that may deserve an explicit discussion for patients to have realistic expectations for their health following hospitalization.
In short, our results underscore the importance of having an open and honest dialog with patients and caregivers throughout hospitalization, and the need to provide education about the potential futility of excessive care in situations where appropriate. Simply following patients' goals without discussing their feasibility and the consequences of aggressive treatments may result in unnecessary morbidity and misuse of healthcare resources. Once goals are clearly established, communicated, and refined in hospitalized patients with serious illness, there is much reason to believe that ongoing conversation will favorably impact outcomes.[29]
We found few studies that rigorously quantified the rate of concordance of hospital recovery goals among patients and key hospital providers; however, studies that measured overall plan of care agreement have demonstrated suboptimal concordance.[20, 30, 31] Shin et al. found significant underestimation of cancer patients' needs and poor concordance between patients and oncologists in assessing perceived needs of supportive care.[20] It is also notable that nurses and physicians had low levels of concordance in our study. O'Leary and colleagues found that nurses and physicians did not reliably communicate and often did not agree on the plan of care for hospitalized patients.[30] Although geographic regionalization of care teams and multidisciplinary rounds can improve the likelihood that key members of the care team are on the same page with regard to the plan of care, there is still much room for improvement.[26, 32, 33, 34] For example, although nurses and physicians in our study independently selected individual recovery goals with similar frequencies (Table 2), we observed suboptimal concordance between nurses and providers (36.8%) for specific patients, including on our regionalized care unit (MICU). This may be due to the reasons described above.
There are several implications of these findings. As payors continue to shift payments toward value‐based metrics, largely determined by patient experience and adequate advance care planning,[9] our findings suggest that more effort should be focused on delivering care consistent with patients' primary recovery goals. As a first step, healthcare organizations can focus on efforts to systematically identify and communicate recovery goals to all members of the care team, ensuring that patients' preferences, needs, and values are captured. In addition, as innovation in patient engagement and care delivery using Web‐based and mobile technology continues to grow,[35] using these tools to capture key goals for hospitalization and recovery can play an essential role. For example, as electronic health record vendors and institutions start to implement patient portals in the acute care setting, they should consider how to configure these tools to capture key goals for hospitalization and recovery, and then communicate them to the care team; preliminary work in this area is promising.[10]
Our study has several limitations to generalizability. First, the study was conducted on 2 services (MICU and oncology) at a single institution using a sampling strategy where research assistants enrolled 2 to 3 patients per day. Although the sampling was random, the availability of patients and proxies to be interviewed may have led to selection bias. Second, the sample size was small. Third, the patients who participated were predominantly white, English‐speaking, and well educated, possibly a consequence of our sampling strategy. However, this fact makes our findings more striking; although cultural and language barriers were generally not present in our study population, large discrepancies in goal concordance still existed. Fourth, in instances when patients were unable to participate themselves, we interviewed their healthcare proxy; therefore, it is possible that the proxies' responses did not reflect those of the patient. However, we note that concordance rates did not significantly differ between the 2 services despite the fact that the proportion of proxy interviews was much higher in the MICU. Similarly, we cannot exclude the possibility that patients altered their stated goals in the presence of proxies, but patients were given the option to be interviewed alone. Patients may also have misunderstood the timing of the goals (during this hospitalization as opposed to long term), although research assistants made every effort to clarify this during the interviews. Finally, our data‐collection instrument was previously validated in hospitalized general medicine patients and not oncology or MICU patients, and it has not been used to directly ask clinicians to identify patients' recovery goals. However, there is no reason to suspect that it could not be used for this purpose in critical care as well as noncritical care settings, as the survey was developed by a multidisciplinary team that included medical professionals and was validated by clinicians who successfully identified a single, very broad goal (eg, be cured) in each case.
CONCLUSION
We report poor to slight concordance among hospitalized patients and key hospital providers with regard to the main recovery goal. Future studies should assess whether patient satisfaction and experience is adversely impacted by patient‐provider discordance regarding key recovery goals. Additionally, institutions may consider future efforts to elicit and communicate patients' primary recovery goals more effectively to all members of the care team, and address discrepancies as soon as they are discovered.
Disclosures
This work was supported by a grant from the Gordon and Betty Moore Foundation (GBMF) (grant GBMF3914). GBMF had no role in the design or conduct of the study; collection, analysis, or interpretation of data; or preparation or review of the manuscript. The authors report no conflicts of interest.
Patient‐centered care has been recognized by the Institute of Medicine as an essential aim of the US healthcare system.[1] A fundamental component of patient‐centered care is to engage patients and caregivers in establishing preferences, needs, values, and overall goals regarding their care.[1] Prior studies have shown that delivering high‐quality patient‐centered care is associated with improved health outcomes, and in some cases, reduced costs.[2, 3, 4, 5, 6, 7] Payors, including the Centers for Medicare and Medicaid Services under the Hospital Value‐Based Purchasing program, are increasingly tying payments to measures of patient experience.[8, 9] As more emphasis is placed on public reporting of these patient‐reported outcomes, healthcare organizations are investing in efforts to engage patients and caregivers, including efforts at establishing patients' preferences for care.[10]
In the acute care setting, a prerequisite for high‐quality patient‐centered care is identifying a patient's primary goal for recovery and then delivering care consistent with that goal.[11, 12, 13] Haberle et al. previously validated patients' most common goals for recovery in the hospital setting into 7 broad categories: (1) be cured, (2) live longer, (3) improve or maintain health, (4) be comfortable, (5) accomplish a particular life goal, (6) provide support for a family member, or (7) other.[13] When providers' understanding of these recovery goals are not concordant with the patient's stated goals, patients may receive care inconsistent with their preferences; it is not uncommon for patients to receive aggressive curative treatments (eg, cardiopulmonary resuscitation) when they have expressed otherwise.[14] On the other hand, when patient goals and priorities are clearly established, patients may have better outcomes.[15] For example, earlier conversations about patient goals and priorities in serious illness can lead to realistic expectations of treatment, enhanced goal‐concordant care, improved quality of life, higher patient satisfaction, more and earlier hospice care, fewer hospitalizations, better patient and family coping, reduced burden of decision making for families, and improved bereavement outcomes.[16, 17, 18]
Although previous studies have suggested poor patient‐physician concordance with regard to the patient's plan of care,[19, 20, 21, 22, 23, 24] there are limited data regarding providers' understanding of the patient's primary recovery goal during hospitalization. The purpose of this study was to identify the patients' Haberle goal, and then determine the degree of concordance among patients and key hospital providers regarding this goal.
METHODS
Study Setting
The Partners Human Research Committee approved the study. The study was conducted on an oncology and medical intensive care unit (MICU) at a major academic medical center in Boston, Massachusetts. The oncology unit was comprised of 2 non‐localized medical teams caring for patients admitted to that unit. The MICU was comprised of a single localized medical team. Medical teams working on these units consisted of a first responder (eg, intern or a physician assistant [PA]), medical residents, and an attending physician. Both units had dedicated nursing staff.
Study Participants
All adult patients (>17 years of age) admitted to the oncology and MICU units during the study period (November 2013 through May 2014) were eligible. These units were chosen because these patients are typically complex and have multiple medical comorbidities longer lengths of stay, and many procedures and tests. In addition, a standard method for asking patients to identify a primary recovery goal for hospitalization aligned well with ongoing institutional efforts to engage these patients in goals of care discussions.
Research assistants identified all patients admitted to each study unit for at least 48 hours and approached them in a random order with a daily target of 2 to 3 patients. Only patients who demonstrated capacity (determined by medical team), or had a legally designated healthcare proxy (who spoke English and was available to participate on their behalf) were included. Research assistants then approached the patient's nurse and a physician provider (defined for this study as housestaff physician, PA, or attending) from the primary medical team to participate in the interview (within 24 hours of patient's interview). We excluded eligible patients who did not have capacity or an available caregiver or declined to participate.
Data Collection Instrument and Interviews
Research assistants administered a validated questionnaire developed by Haberle et al. to participants after 48 hours into the patient's admission to provide time to establish mutual understanding of the diagnosis and prognosis.[13] We asked patients (or the designated healthcare proxy) to select their single, most important Haberle goal (see above). Specifically, as in the original validation study,[13] patients or proxies were asked the following question: Please tell me your most important goal of care for this hospitalization. If they did not understand this question, we asked a follow‐up question: What are you expecting will be accomplished during this hospitalization? Within 24 hours of the patient/proxy interview, we independently asked the patient's nurse and physician to select what they thought was the patient's most important goal for recovery using the same questionnaire, adapted for providers. In each case, all participants were blinded to the responses of others.
Measures
We measured the frequency that each participant (patient/proxy, nurse, and physician) selected a specific Haberle recovery goal across all patients. We measured the rate of pairwise concordance by recovery goal for each participant dyad (patient/proxy‐nurse, patient/proxy‐physician, and nurse‐physician). Finally, we calculated the frequency of cases for which all 3 participants selected the same recovery goal.
Statistical Analyses
Descriptive statistics were used to report patient demographic data. The frequencies of selected responses were calculated and reported as percentages for each type of participant. The differences in rate of responses for each Haberle goal were compared across each participant group using 2 analysis. We then performed 2‐way Kappa statistical tests to measure inter‐rater agreement for each dyad.
RESULTS
Of 1436 patients (882 oncology, 554 MICU) hospitalized during the study period, 341(156 oncology, 185 MICU) were admitted for <48 hours. Of 914 potentially eligible patients (617 oncology, 297 MICU), 191 (112 oncology and 79 MICU) were approached to participate based on our sampling strategy; of these, 8 (2 oncology and 6 MICU) did not have capacity (and no proxy was available) and 2 (1 oncology and 1 MICU) declined. Of the remaining 181 patients (109 oncology and 72 MICU), we obtained a completed questionnaire from all 3 interviewees on 109 (60.2% response rate).
Of the 109 study patients, 52 (47.7%) and 57 (52.3%) were admitted to the oncology and medical intensive care units, respectively (Table 1). Patients were predominantly middle aged, Caucasian, English‐speaking, and college‐educated. Healthcare proxies were frequently interviewed on behalf of patients in the MICU. Housestaff physicians were more often interviewed in the MICU, and PAs were interviewed only on oncology units. Compared to patient responders, nonresponders tended to be male and were admitted to oncology units (see Supporting Table 1 in the online version of this article).
| Characteristics | All Patients | Admitted to Medical Intensive Care Units | Admitted to Oncology Units |
|---|---|---|---|
| |||
| Total, no. (%) | 109 (100%) | 57 (52.3%) | 52 (47.7%) |
| Gender, no. (%) | |||
| Male | 55 (50.5%) | 28 (49.1%) | 26 (50.0%) |
| Female | 54 (49.5%) | 29 (50.9%) | 26 (50.0%) |
| Age, y, mean SD | 59.4 14 | 59.7 15 | 59.1 13 |
| Median | 61 | 61 | 60 |
| Range | 2188 | 2188 | 2285 |
| Race, no. (%) | |||
| White | 103 (94.5%) | 53 (93.0%) | 50 (96.2%) |
| Other | 6 (5.5%) | 4 (7.0%) | 2 (3.8%) |
| Language, no. (%) | |||
| English | 106 (97.2%) | 56 (98.1%) | 50 (96.2%) |
| Other | 3 (2.8%) | 1 (1.9%) | 2 (3.8%) |
| Education level, no. (%) | |||
| Less than high school | 30 (27.5%) | 17 (29.8%) | 13 (25.0%) |
| High school diploma | 27 (24.5%) | 18 (31.6%) | 9 (17.3%) |
| Some college or beyond | 52 (47.7%) | 22 (38.6%) | 30 (57.7%) |
| Patient or caregiver interviewed, no. (%) | |||
| Patient | 68 (62.4%) | 27 (47.4%) | 48 (92.3%) |
| Caregiver | 41 (37.6%) | 30 (52.6%) | 4 (7.7%) |
| Nurse interviewed, no. (unique) | 109 (75) | 57 (42) | 52 (33) |
| Physician provider interviewed, no. (%); no. unique | |||
| Attending | 27 (24.8%); 20 | 15 (26.3%); 10 | 12 (23.1%); 10 |
| Housestaff | 48 (44.0%); 39 | 42 (73.7%); 33 | 6 (11.5%); 6 |
| Physician assistant | 34 (31.2%); 25 | 0 (0%); 0 | 34 (65.4%); 25 |
The frequencies of selected Haberle recovery goals by participant type across all patients are listed in Table 2. Patients (or proxies) most often selected be cured (46.8%). Assigned nurses and physicians more commonly selected improve or maintain health (38.5% and 46.8%, respectively). Be comfortable was selected by nurses and physicians more frequently than by patients (16.5%, 16.5%, and 8.3%, respectively). The rate of responses for each Haberle goal was significantly different across all respondent groups (P < 0.0001). The frequencies of selected Haberle goals were not significantly different between patients or proxies (P = 0.67), or for patients admitted to the MICU compared to oncology units (P = 0.64).
| Haberle Recovery Goal | Patient/Caregiver, no. (%), n = 109 | Physician Provider, no. (%), n = 109* | Nurse, no. (%), n = 109 |
|---|---|---|---|
| |||
| Be cured | 51 (46.8%) | 20 (18.3%) | 20 (18.3%) |
| Be comfortable | 9 (8.3%) | 18 (16.5%) | 18 (16.5%) |
| Improve or maintain health | 32 (29.4%) | 42 (38.5%) | 51 (46.8%) |
| Live longer | 14 (12.8%) | 21 (19.3%) | 12 (11%) |
| Accomplish personal goal | 2 (1.8%) | 0 (0%) | 3 (2.8%) |
| Provide support for family | 1 (0.9%) | 1 (0.9%) | 1 (0.9%) |
| Other | 0 (0%) | 7 (6.4%) | 4 (3.7%) |
Inter‐rater agreement was poor to slight for the 3 participant dyads (kappa 0.09 [0.03‐0.19], 0.19 [0.08‐0.30], and 0.20 [0.08‐0.32] for patient‐physician, patient‐nurse, and nurse‐physician, respectively). The 3 participants selected the identical recovery goal in 22 (20.2%) cases, and each selected a distinct recovery goal in 32 (29.4%) cases. Pairwise concordance between nurses and physicians was 39.4%. There were no significant differences in agreement between patients admitted to the MICU compared to oncology units (P = 0.09).
DISCUSSION
We observed poor to slight concordance among patients and key hospital providers with regard to identifying the patient's primary recovery goal during acute hospitalization. The majority of patients (or proxies), chose be cured, whereas the majority of hospital providers chose improve or maintain health. Patients were twice as likely to select be cured and half as likely to choose be comfortable compared to nurses or physicians. Strikingly, the patient (or proxy), nurse, and physician identified the same recovery goal in just 20% of cases. These findings were similar for patients admitted to either the MICU or oncology units or when healthcare proxies participated on behalf of the patient (eg, when incapacitated in the MICU).
There are many reasons why hospital providers may not correctly identify the patients' primary recovery goals. First, we do not routinely ask patients to identify recovery goals upon admission in a structured and standardized manner. In fact, clinicians often do not elicit patients' needs, concerns, and expectations regarding their care in general.[25] Second, even when recovery goals are elicited at admission, they may not be communicated effectively to all members of the care team. This could be due to geographically non‐localized teams (although we did not observe a statistically significant difference between regionalized MICU and nonregionalized oncology care units), frequent provider‐to‐provider handoffs, and siloed electronic communication (eg, email, alphanumeric pages) regarding goals of care that inevitably leaves out key providers.[26] Third, healthcare proxies who are involved in decision making on the patient's behalf may not always be available to meet with the care team in person; consequently, their input may not be considered in a timely manner or reliably communicated to all members of the care team. We observed a large discrepancy in how often patients chose be cured compared to their hospital providers. This could be explained by clinicians' unwillingness to disclose bad news or divulge accurate prognostic information that causes patients to feel depressed or lose hope, particularly for those patients with the worst prognoses.[16, 27, 28] Patients may lack sophisticated knowledge of their conditions for a variety of reasons, including low health literacy, at times choosing to hope for the best even when it is not realistic. Additionally, there may be more subtle differences in what patients and hospital providers consider the primary recovery goal in context of the main reason for hospitalization and underlying medical illness. For example, a patient with metastatic lung cancer hospitalized with recurrent postobstructive pneumonia may choose be cured as his/her primary recovery goal (thinking of the pneumonia), whereas physicians may choose improve/maintain health or comfort (thinking of the cancer). We also cannot exclude the possibility that sometimes when patients state be cured and clinicians state improve health as the primary goal, that they are really saying the same thing in different ways. However, these are 2 different constructs (cure may not be possible for many patients) that may deserve an explicit discussion for patients to have realistic expectations for their health following hospitalization.
In short, our results underscore the importance of having an open and honest dialog with patients and caregivers throughout hospitalization, and the need to provide education about the potential futility of excessive care in situations where appropriate. Simply following patients' goals without discussing their feasibility and the consequences of aggressive treatments may result in unnecessary morbidity and misuse of healthcare resources. Once goals are clearly established, communicated, and refined in hospitalized patients with serious illness, there is much reason to believe that ongoing conversation will favorably impact outcomes.[29]
We found few studies that rigorously quantified the rate of concordance of hospital recovery goals among patients and key hospital providers; however, studies that measured overall plan of care agreement have demonstrated suboptimal concordance.[20, 30, 31] Shin et al. found significant underestimation of cancer patients' needs and poor concordance between patients and oncologists in assessing perceived needs of supportive care.[20] It is also notable that nurses and physicians had low levels of concordance in our study. O'Leary and colleagues found that nurses and physicians did not reliably communicate and often did not agree on the plan of care for hospitalized patients.[30] Although geographic regionalization of care teams and multidisciplinary rounds can improve the likelihood that key members of the care team are on the same page with regard to the plan of care, there is still much room for improvement.[26, 32, 33, 34] For example, although nurses and physicians in our study independently selected individual recovery goals with similar frequencies (Table 2), we observed suboptimal concordance between nurses and providers (36.8%) for specific patients, including on our regionalized care unit (MICU). This may be due to the reasons described above.
There are several implications of these findings. As payors continue to shift payments toward value‐based metrics, largely determined by patient experience and adequate advance care planning,[9] our findings suggest that more effort should be focused on delivering care consistent with patients' primary recovery goals. As a first step, healthcare organizations can focus on efforts to systematically identify and communicate recovery goals to all members of the care team, ensuring that patients' preferences, needs, and values are captured. In addition, as innovation in patient engagement and care delivery using Web‐based and mobile technology continues to grow,[35] using these tools to capture key goals for hospitalization and recovery can play an essential role. For example, as electronic health record vendors and institutions start to implement patient portals in the acute care setting, they should consider how to configure these tools to capture key goals for hospitalization and recovery, and then communicate them to the care team; preliminary work in this area is promising.[10]
Our study has several limitations to generalizability. First, the study was conducted on 2 services (MICU and oncology) at a single institution using a sampling strategy where research assistants enrolled 2 to 3 patients per day. Although the sampling was random, the availability of patients and proxies to be interviewed may have led to selection bias. Second, the sample size was small. Third, the patients who participated were predominantly white, English‐speaking, and well educated, possibly a consequence of our sampling strategy. However, this fact makes our findings more striking; although cultural and language barriers were generally not present in our study population, large discrepancies in goal concordance still existed. Fourth, in instances when patients were unable to participate themselves, we interviewed their healthcare proxy; therefore, it is possible that the proxies' responses did not reflect those of the patient. However, we note that concordance rates did not significantly differ between the 2 services despite the fact that the proportion of proxy interviews was much higher in the MICU. Similarly, we cannot exclude the possibility that patients altered their stated goals in the presence of proxies, but patients were given the option to be interviewed alone. Patients may also have misunderstood the timing of the goals (during this hospitalization as opposed to long term), although research assistants made every effort to clarify this during the interviews. Finally, our data‐collection instrument was previously validated in hospitalized general medicine patients and not oncology or MICU patients, and it has not been used to directly ask clinicians to identify patients' recovery goals. However, there is no reason to suspect that it could not be used for this purpose in critical care as well as noncritical care settings, as the survey was developed by a multidisciplinary team that included medical professionals and was validated by clinicians who successfully identified a single, very broad goal (eg, be cured) in each case.
CONCLUSION
We report poor to slight concordance among hospitalized patients and key hospital providers with regard to the main recovery goal. Future studies should assess whether patient satisfaction and experience is adversely impacted by patient‐provider discordance regarding key recovery goals. Additionally, institutions may consider future efforts to elicit and communicate patients' primary recovery goals more effectively to all members of the care team, and address discrepancies as soon as they are discovered.
Disclosures
This work was supported by a grant from the Gordon and Betty Moore Foundation (GBMF) (grant GBMF3914). GBMF had no role in the design or conduct of the study; collection, analysis, or interpretation of data; or preparation or review of the manuscript. The authors report no conflicts of interest.
- Institute of Medicine. Crossing the Quality Chasm: A New Health System for the 21st Century. Washington, DC: National Academy of Sciences; 2001.
- , , , , , . The effects of physician communications skills on patient satisfaction; recall, and adherence. J Chronic Dis. 1984;37(9–10):755–764.
- , , , et al. Observational study of effect of patient centredness and positive approach on outcomes of general practice consultations. BMJ. 2001;323(7318):908–911.
- . Reengineering hospital discharge: a protocol to improve patient safety, reduce costs, and boost patient satisfaction. Am J Med Qual. 2009;24(4):344–346.
- , , , , . Relationship between patient satisfaction with inpatient care and hospital readmission within 30 days. Am J Manag Care. 2011;17(1):41–48.
- , , , . Patients' perception of hospital care in the United States. N Engl J Med. 2008;359(18):1921–1931.
- , , . Enhanced support for shared decision making reduced costs of care for patients with preference‐sensitive conditions. Health Aff (Millwood). 2013;32(2):285–293.
- Centers for Medicare and Medicaid Services. Medicare program; hospital inpatient value‐based purchasing program. Final rule. Fed Regist. 2011;76(88):26490–26547.
- Centers for Medicare and Medicaid Services. CMS begins implementation of key payment legislation. Available at: https://www.cms.gov/Newsroom/MediaReleaseDatabase/Press‐releases/2015‐Press‐releases‐items/2015‐07‐08.html. Published July 8, 2015.
- , , , et al. A web‐based, patient‐centered toolkit to engage patients and caregivers in the acute care setting: a preliminary evaluation [published online August 2, 2015]. J Am Med Informatics Assoc. doi: 10.1093/jamia/ocv093.
- , , , et al. Effectiveness trial of an intensive communication structure for families of long‐stay ICU patients. Chest. 2010;138(6):1340–1348.
- , , , . Understanding goals of care statements and preferences among patients and their surrogates in the medical ICU. J Hosp Palliat Nurs. 2012;14(2):126–132.
- , , , . Goals of care among hospitalized patients: a validation study. Am J Hosp Palliat Care. 2011;28(5):335–341.
- , , , et al. Factors associated with use of cardiopulmonary resuscitation in seriously ill hospitalized adults. JAMA. 1999;282(24):2333–2339.
- , , , , . End‐of‐life discussions, goal attainment, and distress at the end of life: Predictors and outcomes of receipt of care consistent with preferences. J Clin Oncol. 2010;28(7):1203–1208.
- , . Reasons why physicians do not have discussions about poor prognosis, why it matters, and what can be improved. J Clin Oncol. 2012;30(22):2715–2717.
- , , , et al. Associations between end‐of‐life discussions, patient mental health, medical care near death, and caregiver bereavement adjustment. JAMA. 2008;300(14):1665–1673.
- , , , . Prior advance care planning is associated with less decisional conflict among surrogates for critically ill patients. Ann Am Thorac Soc. 2015;12(10):1528–1533.
- , , , et al. Hospitalized patients' understanding of their plan of care. Mayo Clin Proc. 2010;85(1):47–52.
- , , , et al. Discordance in perceived needs between patients and physicians in oncology practice: a nationwide survey in Korea. J Clin Oncol. 2011;29(33):4424–4429.
- , , , , , . Leveraging standards to support patient‐centric interdisciplinary plans of care. AMIA Annu Symp Proc. 2011;2011:356–363.
- , . Discordance between physician and patient self‐rated health and all‐cause mortality. Ochsner J. 2011;11(3):232–240.
- , , , , , . Determinants of discordance between patients and physicians in their assessment of lupus disease activity. J Rheumatol. 2003;30(9):1967–1976.
- , , , , , . Predictors of discordance between physicians' and patients' appraisals of health‐related quality of life in atrial fibrillation patients: Findings from the Angiotensin II Antagonist in Paroxysmal Atrial Fibrillation Trial. Am Heart J. 2013;166(3):589–596.
- , , , et al. Uncovering the blind spot of patient satisfaction: an international survey. BMJ Qual Saf. 2011;20(11):959–965.
- , , , , , . Improving teamwork: impact of structured interdisciplinary rounds on a hospitalist unit. J Hosp Med. 2011;6(2):88–93.
- , , , . Discrepancies between patient and physician estimates for the success of stem cell transplantation. JAMA. 2001;285(8):1034–1038.
- , , , , , . Optimistic expectations and survival after hematopoietic stem cell transplantation. Biol Blood Marrow Transplant. 2003;9(6):389–396.
- , , , et al. Development of the Serious Illness Care Program: a randomised controlled trial of a palliative care communication intervention. BMJ Open. 2015;5(10):e009032.
- , , , et al. Patterns of nurse‐physician communication and agreement on the plan of care. Qual Saf Health Care. 2010;19(3):195–199.
- , , , et al. Impact of localizing physicians to hospital units on nurse‐physician communication and agreement on the plan of care. J Gen Intern Med. 2009;24(11):1223–1227.
- , , , et al. Changes in medical errors after implementation of a handoff program. N Engl J Med. 2014;371(19):1803–1812.
- , , , et al. Reorganizing a hospital ward as an accountable care unit. J Hosp Med. 2015;10(1):36–40.
- , , , . Interdisciplinary teamwork in hospitals: a review and practical recommendations for improvement. J Hosp Med. 2012;7(1):48–54.
- , , , , . An evaluation of mobile health application tools. JMIR mHealth uHealth. 2014;2(2):e19.
- Institute of Medicine. Crossing the Quality Chasm: A New Health System for the 21st Century. Washington, DC: National Academy of Sciences; 2001.
- , , , , , . The effects of physician communications skills on patient satisfaction; recall, and adherence. J Chronic Dis. 1984;37(9–10):755–764.
- , , , et al. Observational study of effect of patient centredness and positive approach on outcomes of general practice consultations. BMJ. 2001;323(7318):908–911.
- . Reengineering hospital discharge: a protocol to improve patient safety, reduce costs, and boost patient satisfaction. Am J Med Qual. 2009;24(4):344–346.
- , , , , . Relationship between patient satisfaction with inpatient care and hospital readmission within 30 days. Am J Manag Care. 2011;17(1):41–48.
- , , , . Patients' perception of hospital care in the United States. N Engl J Med. 2008;359(18):1921–1931.
- , , . Enhanced support for shared decision making reduced costs of care for patients with preference‐sensitive conditions. Health Aff (Millwood). 2013;32(2):285–293.
- Centers for Medicare and Medicaid Services. Medicare program; hospital inpatient value‐based purchasing program. Final rule. Fed Regist. 2011;76(88):26490–26547.
- Centers for Medicare and Medicaid Services. CMS begins implementation of key payment legislation. Available at: https://www.cms.gov/Newsroom/MediaReleaseDatabase/Press‐releases/2015‐Press‐releases‐items/2015‐07‐08.html. Published July 8, 2015.
- , , , et al. A web‐based, patient‐centered toolkit to engage patients and caregivers in the acute care setting: a preliminary evaluation [published online August 2, 2015]. J Am Med Informatics Assoc. doi: 10.1093/jamia/ocv093.
- , , , et al. Effectiveness trial of an intensive communication structure for families of long‐stay ICU patients. Chest. 2010;138(6):1340–1348.
- , , , . Understanding goals of care statements and preferences among patients and their surrogates in the medical ICU. J Hosp Palliat Nurs. 2012;14(2):126–132.
- , , , . Goals of care among hospitalized patients: a validation study. Am J Hosp Palliat Care. 2011;28(5):335–341.
- , , , et al. Factors associated with use of cardiopulmonary resuscitation in seriously ill hospitalized adults. JAMA. 1999;282(24):2333–2339.
- , , , , . End‐of‐life discussions, goal attainment, and distress at the end of life: Predictors and outcomes of receipt of care consistent with preferences. J Clin Oncol. 2010;28(7):1203–1208.
- , . Reasons why physicians do not have discussions about poor prognosis, why it matters, and what can be improved. J Clin Oncol. 2012;30(22):2715–2717.
- , , , et al. Associations between end‐of‐life discussions, patient mental health, medical care near death, and caregiver bereavement adjustment. JAMA. 2008;300(14):1665–1673.
- , , , . Prior advance care planning is associated with less decisional conflict among surrogates for critically ill patients. Ann Am Thorac Soc. 2015;12(10):1528–1533.
- , , , et al. Hospitalized patients' understanding of their plan of care. Mayo Clin Proc. 2010;85(1):47–52.
- , , , et al. Discordance in perceived needs between patients and physicians in oncology practice: a nationwide survey in Korea. J Clin Oncol. 2011;29(33):4424–4429.
- , , , , , . Leveraging standards to support patient‐centric interdisciplinary plans of care. AMIA Annu Symp Proc. 2011;2011:356–363.
- , . Discordance between physician and patient self‐rated health and all‐cause mortality. Ochsner J. 2011;11(3):232–240.
- , , , , , . Determinants of discordance between patients and physicians in their assessment of lupus disease activity. J Rheumatol. 2003;30(9):1967–1976.
- , , , , , . Predictors of discordance between physicians' and patients' appraisals of health‐related quality of life in atrial fibrillation patients: Findings from the Angiotensin II Antagonist in Paroxysmal Atrial Fibrillation Trial. Am Heart J. 2013;166(3):589–596.
- , , , et al. Uncovering the blind spot of patient satisfaction: an international survey. BMJ Qual Saf. 2011;20(11):959–965.
- , , , , , . Improving teamwork: impact of structured interdisciplinary rounds on a hospitalist unit. J Hosp Med. 2011;6(2):88–93.
- , , , . Discrepancies between patient and physician estimates for the success of stem cell transplantation. JAMA. 2001;285(8):1034–1038.
- , , , , , . Optimistic expectations and survival after hematopoietic stem cell transplantation. Biol Blood Marrow Transplant. 2003;9(6):389–396.
- , , , et al. Development of the Serious Illness Care Program: a randomised controlled trial of a palliative care communication intervention. BMJ Open. 2015;5(10):e009032.
- , , , et al. Patterns of nurse‐physician communication and agreement on the plan of care. Qual Saf Health Care. 2010;19(3):195–199.
- , , , et al. Impact of localizing physicians to hospital units on nurse‐physician communication and agreement on the plan of care. J Gen Intern Med. 2009;24(11):1223–1227.
- , , , et al. Changes in medical errors after implementation of a handoff program. N Engl J Med. 2014;371(19):1803–1812.
- , , , et al. Reorganizing a hospital ward as an accountable care unit. J Hosp Med. 2015;10(1):36–40.
- , , , . Interdisciplinary teamwork in hospitals: a review and practical recommendations for improvement. J Hosp Med. 2012;7(1):48–54.
- , , , , . An evaluation of mobile health application tools. JMIR mHealth uHealth. 2014;2(2):e19.
Regionalized Care and Adverse Events
Failures in communication among healthcare professionals are known threats to patient safety. These failures account for over 60% of root causes of sentinel events, the most serious events reported to The Joint Commission.[1] As such, identifying both patterns of effective communication as well as barriers to successful communication has been a focus of efforts aimed at improving patient safety. However, to date, the majority of this work has centered on improving communication in settings such as the operating room and intensive care unit,[2, 3, 4] or at times of care transitions.[5, 6, 7, 8]
Unique barriers exist for effective interdisciplinary communication in the hospital setting, particularly physiciannurse communication regarding shared hospitalized patients.[9] Traditionally, care of hospitalized patients is provided by physicians, nurses, and other team members working in varied workflow patterns, leading to dispersed team membership, where each team member cares for different groups of patients in different locations across the hospital. This dispersion is further heightened on teaching services, where residents' rotation schedules lead to frequent changes of care team membership, leaving inpatient care teams particularly vulnerable to ineffective communication. Evidence suggests that communication between nurses and physicians is currently suboptimal, leading to frequent disagreement regarding the patient's plan of care.[9, 10] This divergence between physician and nursing perceptions of patients' care plans may leave patients at greater risk of adverse events (AEs).
Several studies have examined the effects of regionalized inpatient care teams, where multidisciplinary team members care for the same patients on the same hospital unit, on communication and patient outcomes.[4, 11, 12, 13, 14] Results of these studies have been inconsistent, perhaps due to the particular characteristics of the care teams or to the study methodology. Thus, further rigorously done studies are required to better understand the impact of team regionalization on patient care. The goal of this study was to examine whether the implementation of regionalized inpatient care teams was associated with improvements in care team communication and preventable AEs.
METHODS
Setting, Patients, and Study Design
We performed a cohort analysis of patients at a 700‐bed tertiary care center, pre‐ and postregionalization of inpatient general medicine care teams. Our study protocol was approved by the Partners Healthcare Human Subjects Review Committee. Patients were eligible for inclusion if they were 18 years of age or older and discharged from the general medicine service (GMS) from any of the 3 participating nursing units between April 1, 2012 and June 19, 2012 (preregionalization) or April 1, 2013 and June 19, 2013 (postregionalization).
Intervention
On June 20, 2012, regionalized care was implemented on the GMS such that each of 3 GMS teams was localized to 1 of 3, 15‐bed nursing units. Prior to regionalization, the GMS physician care teams, each consisting of 1 hospitalist attending, 1 medical resident, and 2 medical interns, would care for patients on an average of 7 and up to 13 different nursing units on a given day.
Regionalized care consisted of a multifaceted intervention codeveloped by hospitalist, residency, nursing, emergency department, and hospital leadership and included: (1) regionalizing GMS teams as much as possible; (2) change in resident call structure from a traditional 4‐day call cycle to daily admitting; (3) collaborative efforts to enhance GMS patient discharges before noon to promote regionalized placement of patients without prolonging time in the emergency department (ED); (4) daily morning and postround multidisciplinary huddles to prioritize sicker patients and discharges; (5) encouragement of daily rounds at patients' bedsides with presence of physician team, nurse, and team pharmacist if available; (6) creation of unit‐ and team‐level performance reports; and (7) creation of unit‐based physician and nursing co‐leadership (Figure 1).[15]
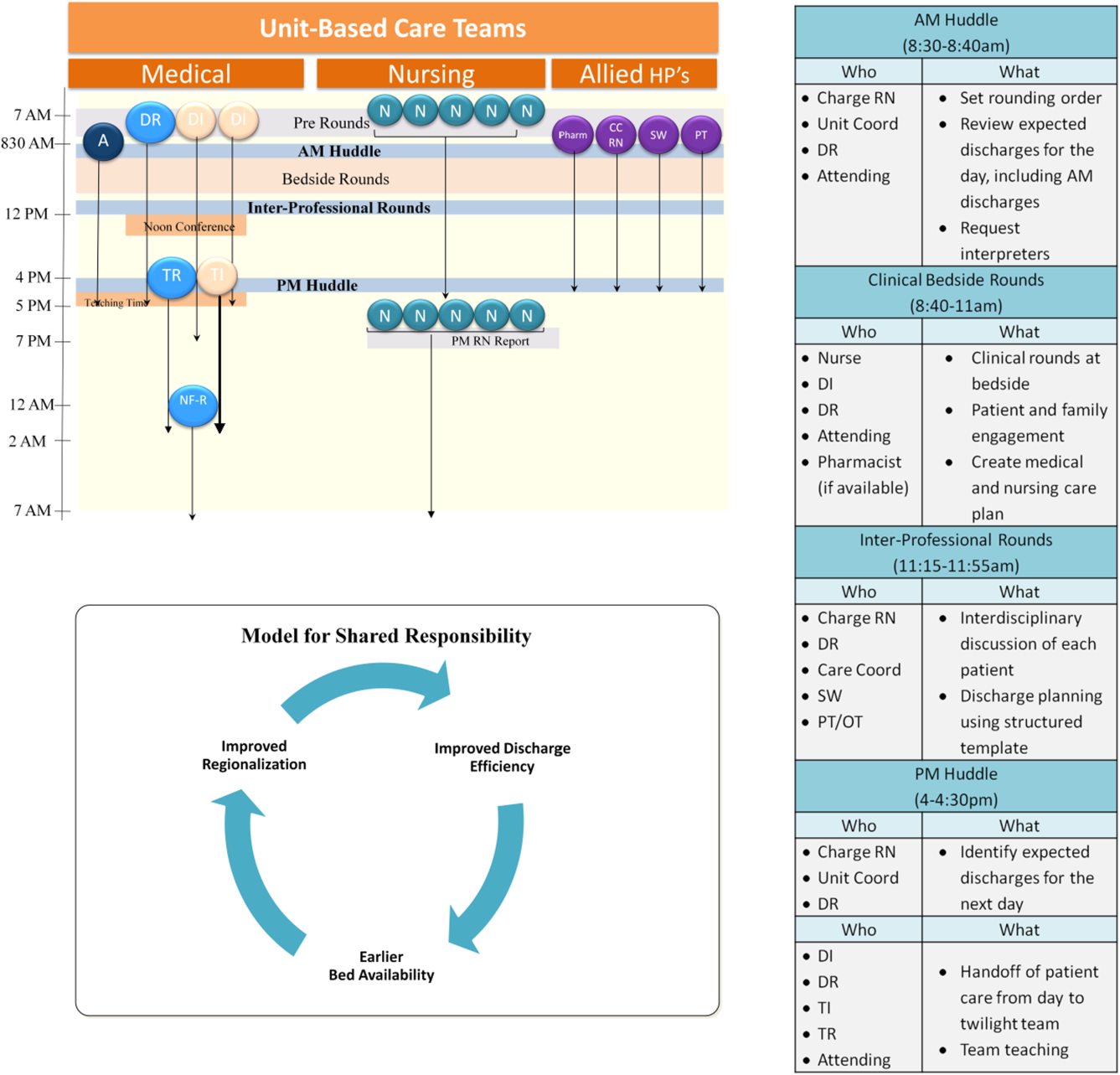
Concordance of Plan
Concordance of plan was measured via a 7‐question survey previously developed, pilot tested, and used to measure the impact of regionalized care on care team communication between inpatient nursephysician team members.[9] The survey was administered in‐person by 1 of 8 trained research assistants (RAs) (4/emntervention period) to nurse and intern pairs caring for patients on the study units pre‐ and postregionalization. GMS patients were eligible for inclusion if surveys could be administered to their nurse and intern within the first 24 hours of admission to the unit and within 48 hours of admission to the hospital, based on RA availability (thus excluding patients admitted on Fridays as surveys were not conducted over the weekend). Most often, all eligible patients admitted to the study units during time periods of data collection were included in the study. On limited occasions, the daily supply of patients surpassed RA capacity for inclusion, at which time computer‐generated randomization was utilized to randomly select patients for inclusion. Nurse and intern pairs were surveyed once during a patient's hospitalization, although they could be surveyed more than once about different patients, and patients could be included more than once if rehospitalized on the study unit and cared for by a different nurseintern pair. Of the 472 selected eligible patients, the nurses and interns of 418 patients were available and consented to survey administration, representing 361 unique nurse and intern pairs and 399 unique patients.
Each member of the pair was asked about 7 specific aspects of the patient's care plan for that day in isolation from the other team member, including: (1) the patient's primary diagnosis, (2) the patient's expressed chief concern, (3) the day's scheduled tests, (4) the day's scheduled procedures, (5) consulting services involved, (6) medication changes made that day, and (7) the patient's expected discharge date. In addition, each pair was asked the name of the other team member (ie, the nurse was asked the name of the intern and vice versa), and whether or not the patient care plan for the day had been discussed with the other team member, where concordance was defined as both members agreeing the plan had been discussed. All responses were recorded verbatim. Pairs were surveyed independently between 12 pm and 2 pm, limiting confounding by evolving plans of care over time.
Each set of surveys were then reviewed by 2 of 4 trained adjudicators, and responses to each question were scored as complete, partial, or no agreement. Rules for degree of agreement were based upon previously utilized parameters[9] as well as biweekly meetings during which common themes and disagreements in ratings were discussed, and rules generated to create consensus (see Supporting Information, Appendix, in the online version of this article).
Adverse Event Detection
Of the patients meeting eligibility criteria, 200 patients were randomly selected using computer‐generated randomization from each time period for AE outcome assessment, for a total of 400 patients.
Each patient's electronic medical record was retrospectively reviewed by a trained clinician using a previously validated screening tool to detect any possible AEs.[11] Any positive screen prompted documentation of a narrative summary including a short description of the possible AE and pertinent associated data. We defined AE as any injury due to medical management rather than the natural history of the illness, and further limited this definition to only include AEs that occurred on the study unit or as a result of care on that unit.
Two of 4 trained adjudicators, blinded to time period, then separately reviewed each narrative summary using previously validated 6‐point confidence scales to determine the presence and preventability of AE, with confidence ratings of 4 or greater used as cutoffs.[11] All AEs were also scored on a 4‐point severity scale (trivial, clinically significant, serious, or life threatening), with severe AE defined as serious or life threatening. Lastly, adjudicators grouped AEs into 1 of 10 prespecified categories.[11] Any disagreements in ratings or groupings were discussed by all 4 adjudicators to reach consensus.
Data Analysis
Patient characteristics are presented using descriptive statistics and were compared in the pre‐ and postregionalization time periods using 2 or t tests as appropriate.
To analyze whether regionalized care was associated with concordance of plan, adjudicated survey questions were assigned points of 1, 0.5, and 0 for complete, partial, and no agreement, respectively. Total mean concordance scores for any patient ranged from 0 to 7 points, and were divided by total number of answered questions (up to 7) for a range of 0 to 1. Total mean concordance scores as well as mean concordance score per survey question were compared pre‐ versus postregionalization using t tests. In sensitivity analyses, adjudicated survey responses were dichotomized with complete and partial agreement deemed concordant responses. Percent concordance for each question was then compared pre‐ versus postregionalization using 2 analysis. Questions about the name of the other team member and discussion of daily care plan with the other team member were excluded from total concordance score calculations and were compared individually pre‐ versus postregionalization, because they are not directly about the plan of care.
To analyze the association of regionalization with odds of preventable AE, we performed multivariable logistic regression adjusted for patient age, sex, race, language, and Elixhauser comorbidity score,[16] and utilized generalized estimating equations to account for clustering by hospital unit. Secondary outcomes included severe preventable AEs, nonpreventable AEs, and category of preventable AEs using similar methodology. Two‐sided P values <0.05 were considered significant, and SAS version 9.2 (SAS Institute Inc., Cary, NC) was used for all analyses.
RESULTS
The fidelity of the intervention in achieving its goal of regionalized care is discussed separately.[15] Briefly, the intervention was successful at achieving 85% regionalization by team (ie, average daily percentage of team's patients assigned to team's unit) and 87% regionalization by unit (ie, average daily percentage of unit's patients with assigned team) following implementation, compared to 20% regionalization by team and unit in the preintervention period. Importantly, the average daily census of physician care teams rose by 32%, from a mean of 10.8 patients/physician care team preregionalization to a mean of 14.3 patients/physician care team postregionalization.
Concordance of Plan
Of the 418 nurse and intern paired surveys, 4 surveys were excluded due to repeat surveys of the same patient during the same hospitalization, for a total of 197 distinct paired surveys preregionalization and 217 paired surveys postregionalization. There were no statistically significant differences in patients' age, sex, race, language, admission source, length of stay, Elixhauser comorbidity score and diagnosis‐related group weight pre‐ versus postregionalization (Table 1).
| Characteristic | Concordance of Care Plan | Adverse Events | ||||
|---|---|---|---|---|---|---|
| Pre, n = 197 | Post, n = 217 | P Value | Pre, n = 198 | Post, n = 194 | P Value | |
| ||||||
| Age, mean (SD) | 60.5 (19.4) | 57.6 (20.8) | 0.15 | 60.4 (18.9) | 58.0 (21.2) | 0.24 |
| Male, n (%) | 77 (39.1) | 92 (42.4) | 0.49 | 94 (47.5) | 85 (43.8) | 0.55 |
| Race/ethnicity, n (%) | 0.34 | 0.12 | ||||
| White | 134 (68.0) | 141 (65.0) | 132 (66.5) | 121 (62.4) | ||
| Black | 42 (21.3) | 45 (20.7) | 41 (20.8) | 54 (27.8) | ||
| Hispanic | 18 (9.1) | 21 (9.7) | 22 (11.3) | 13 (6.8) | ||
| Other/unknown | 3 (1.5) | 10 (4.6) | 3 (1.4) | 6 (2.9) | ||
| Language, n (%) | 0.30 | 0.73 | ||||
| English | 183 (92.9) | 203 (93.5) | 176 (88.7) | 175 (90.2) | ||
| Spanish | 6 (3.0) | 10 (4.6) | 10 (5.2) | 10 (5.3) | ||
| Other | 8 (4.1) | 4 (1.8) | 12 (6.1) | 9 (4.5) | ||
| Admitting source, n (%) | 1.00 | 0.10 | ||||
| Physician office | 13 (6.6) | 13 (6.0) | 13 (6.6) | 6 (3.1) | ||
| Emergency department | 136 (69.0) | 150 (69.1) | 126 (63.6) | 127 (65.5) | ||
| Transfer from different hospital | 40 (20.3) | 45 (20.7) | 54 (27.3) | 50 (25.8) | ||
| Transfer from skilled nursing facility | 8 (4.1) | 9 (4.2) | 5 (2.5) | 11 (5.6) | ||
| Length of stay, d, median (IQR) | 3.0 (4.0) | 3.0 (4.0) | 0.57 | 4.0 (5.0) | 3.0 (4.0) | 0.16 |
| Elixhauser Comorbidity Score, mean (SD) | 8.0 (8.8) | 8.3 (9.3) | 0.74 | 8.0 (8.6) | 7.8 (8.4) | 0.86 |
| DRG weight, mean (SD) | 1.6 (1.0) | 1.5 (1.0) | 0.37 | 1.5 (0.93) | 1.5 (1.1) | 0.96 |
Kappa scores for adjudications of concordance surveys (defined as both adjudicators scoring the same level of agreement (ie, both complete or partial agreement versus no agreement) ranged from 0.69 to 0.95, by question. There were no significant differences in total mean concordance scores in the care plan pre‐ versus postregionalization (0.65 vs 0.67, P = 0.26) (Table 2). Similarly, there were no significant differences in mean concordance score for each survey question, except agreement on expected date of discharge (0.56 vs 0.68, P = 0.003), knowledge of the other provider's name, and agreement that discussion of the daily plan had taken place with the other pair member. Similar results were seen when results were dichotomized (ie, partial or complete agreement vs no agreement) (Table 2).
| Concordance Outcome | Pre, n = 197 | Post, n = 217 | P Value |
|---|---|---|---|
| |||
| Concordance score* | |||
| Total concordance score, mean (SD) | 0.65 (0.17) | 0.67 (0.16) | 0.26 |
| Subgroups | |||
| Diagnosis | 0.77 (0.32) | 0.72 (0.35) | 0.11 |
| Patient's chief concern | 0.48 (0.44) | 0.48 (0.43) | 0.94 |
| Tests today | 0.67 (0.40) | 0.71 (0.42) | 0.36 |
| Procedures today | 0.93 (0.25) | 0.92 (0.25) | 0.71 |
| Medication changes today | 0.56 (0.44) | 0.59 (0.43) | 0.54 |
| Consulting services | 0.59 (0.44) | 0.60 (0.44) | 0.82 |
| Expected discharge date | 0.56 (0.44) | 0.68 (0.38) | 0.003 |
| Responding clinician knowledge of nurse's name | 0.56 (0.50) | 0.86 (0.35) | <0.001 |
| Nurse's knowledge of responding clinician's name | 0.56 (0.50) | 0.88 (0.33) | <0.001 |
| Plan discussed | 0.73 (0.45) | 0.88 (0.32) | <0.001 |
| Percent concordance, mean (SD) | |||
| Diagnosis | 92.0 (27.3) | 88.6 (31.9) | 0.25 |
| Patient's chief concern | 59.6 (49.1) | 60.6 (49.0) | 0.84 |
| Tests today | 78.9 (40.9) | 77.2 (42.1) | 0.67 |
| Procedures today | 93.5 (24.8) | 94.1 (23.7) | 0.80 |
| Medication changes today | 66.3 (33.6) | 69.9 (46.0) | 0.44 |
| Consulting services | 69.3 (46.2) | 68.9 (46.4) | 0.93 |
| Expected discharge date | 67.5 (47.0) | 82.6 (38.0) | <0.001 |
| Responding clinician knowledge of nurse's name | 55.7 (49.8) | 85.6 (35.2) | <0.001 |
| Nurse's knowledge of responding clinician's name | 55.9 (49.8) | 87.9 (32.8) | <0.001 |
| Plan discussed | 72.9 (44.6) | 88.2 (32.3) | <0.001 |
Adverse Events
Of the 400 patients screened for AEs, 8 were excluded due to missing medical record number (5) and discharge outside of study period (3). Of the final 392 patient screens (198 pre, 194 post), there were no significant differences in patients' age, sex, race, language, length of stay, or Elixhauser score pre‐ versus postregionalization (Table 1).
Kappa scores for adjudicator agreement were 0.35 for presence of AE and 0.34 for preventability of AE. Of the 392 reviewed patient records, there were 133 total AEs detected (66 pre, 67 post), 27 preventable AEs (13 pre, 14 post), and 9 severe preventable AEs (4 pre, 5 post) (Table 3). There was no significant difference in the adjusted odds of preventable AEs post‐ versus preregionalization (adjusted odds ratio: 1.37, 95% confidence interval: 0.69, 2.69). Although the low number of AEs rated as severe or life threatening precluded adjusted analysis, unadjusted results similarly demonstrated no difference in odds of severe preventable AEs pre‐ versus postregionalization. As expected, there was no significant difference in adjusted odds of nonpreventable AE after implementation of regionalized care (Table 3).
| Adverse Events | No. of Adverse Events | Adjusted Odds Ratio Post vs Pre (95% CI) | |
|---|---|---|---|
| Pre, n = 198 | Post, n = 194 | ||
| |||
| Preventable | 13 | 14 | 1.37 (0.69, 2.69) |
| Serious and preventable | 4 | 5 | |
| Nonpreventable | 47 | 50 | 1.20 (0.85, 1.75) |
Similarly, there were no significant differences in category of preventable AE pre‐ versus postregionalization. The most frequent preventable AEs in both time periods were those related to adverse drug events and to manifestations of poor glycemic control, examples of which are illustrated (Table 4).
| |
| Adverse drug event | 29‐year‐old male with history of alcohol abuse, complicated by prior withdrawal seizures/emntensive care unit admissions, presented with alcohol withdrawal. Started on standing and PRN lorazepam, kept on home medications including standing clonidine, gabapentin, citalopram, quetiapine. Became somnolent due to polypharmacy, ultimately discontinued quetiapine as discovered took only as needed at home for insomnia |
| Manifestations of poor glycemic control | 78‐year‐old male with recently diagnosed lymphoma, distant history of bladder and prostate cancer status post ileal loop diversion, presented status post syncopal event; during event, spilled boiling water on himself leading to second‐degree burns on 3% of his body. Initially admitted to trauma/burn service, ultimately transferred to medical service for ongoing multiple medical issues including obstructive uropathy, acute on chronic renal failure. Adverse event was hyperglycemia (>350 mg/dL on >2 consecutive readings) in the setting of holding his home insulin detemir and insulin aspart (had been placed on insulin aspart sliding scale alone). After hyperglycemic episodes, was placed back on weight‐based basal/nutritional insulin |
DISCUSSION
In this study of general medicine patients at a large academic medical center, we found that regionalization of care teams on general medicine services was associated with improved recognition of care team members and agreement on estimated date of patient discharge, but was not associated with improvement in overall nurse and physician concordance of the patient care plan, or the odds of preventable AEs.
This intervention importantly addresses the barrier of dispersion of team membership, a well‐recognized barrier to interdisciplinary collaboration,[17, 18] particularly with resident physician teams due to frequently changing team membership. Localization of all team members, in addition to encouragement of daily collaborative bedside rounds as part of the regionalization initiative, likely contributed to our observed improvement in team member identification and discussion of daily care plans. Similarly, regionalization resulted in improved agreement in estimations of date of patient discharge. Focus on early patient discharges was an integral part of the implementation efforts; we therefore hypothesize that mutual focus on discharge planning by both nurses and responding clinicians may have explained this observed result.
On the other hand, regionalization did not appreciably improve the overall concordance of care plan between nurses and interns, despite a significant increase in team members agreeing that the plan had been discussed. Our findings support similar prior research demonstrating that regionalizing hospitalist attendings to single nursing units had limited impact on agreement of care plan between physicians and nurses.[13] Similarly, in settings where physicians and nurses are inherently regionalized, such as the intensive care unit[4] or the operating room,[3] communication between physicians and nurses remains difficult. Collectively, our findings suggest that colocalization of physicians and nurses alone is likely insufficient to improve measured communication between care team members. Existing literature suggests that more standardized approaches to improve communication, such as structured communication tools used during daily inpatient care[19, 20] or formalized team training,[21, 22, 23] lead to improvements in communication and collaboration. Despite these findings, it is important to highlight that this study did not assess other measures of workplace culture, such as teamwork and care team cohesiveness, which may have been positively affected by this intervention, even without measurable effect on concordance of care plan. Additionally, as noted, the average daily census on each team increased by almost a third postintervention, which may have impeded improvements in care team communication.
In addition, we found that our intervention had no significant impact on preventable AEs or severe preventable AEs. Although we cannot exclude the possibility that more subtle AEs were missed with our methodology, our results indicate that regionalized care alone may be inadequate to improve major patient safety outcomes. As discussed, the volume of patients did increase postintervention; thus, another way to state our results is that we were able to increase the daily volume of patients without any significant decreases in patient safety. Nevertheless, the results on patient safety were less than desired. A recent review of interdisciplinary team care interventions on general medical wards similarly demonstrated underwhelming improvements in patient safety outcomes, although the reviewed interventions did not specifically address preventable AEs, a gap in the literature commented on by the authors.[24] Other albeit limited literature has demonstrated improvement in patient safety outcomes via multifaceted efforts aimed at improving care team member communication. Notably, these efforts include colocalization of care team members to single units but also involve additional measures to improve communication and collaboration between care team members, such as structured communication during interdisciplinary rounds, and certification of key interdisciplinary teamwork skills.[11, 14] Although our regionalized care intervention included many similar features to these accountable care units (ACUs) including unit‐based care teams, unit‐level performance reporting, and unit‐based physician and nursing coleadership, significant differences existed. Notably, in addition to the above features, the ACU model also incorporated highly structured communication models for interdisciplinary rounding, and certification processes to ensure an appropriate communication skill base among care team members.[14] Thus, although creation of regionalized care teams is likely a necessary precursor to implementation of these additional measures, alone it may be insufficient to improve patient safety outcomes.
Importantly, in our study we identified that adverse drug events and manifestations of poor glycemic control occurred in high frequency both before and following implementation of regionalized care, supporting other literature that describes the prevalence of these AEs.[11, 25, 26, 27] These results suggest that targeted interventions to address these specific AEs are likely necessary. Notably, the intervention units in our study did not consistently employ clinical pharmacists assigned specifically to that unit's care team to allow for integration within the care team. As prior research has suggested that greater collaboration with clinical pharmacists results in reduction of adverse drug events,[28] next steps may include improved integration of team‐based pharmacists into the activities of the regionalized care teams. Inpatient management of diabetes also requires specific interventions,[29, 30, 31] only some of which may be addressable by having regionalized care and better interdisciplinary communication.
Our findings are subject to several limitations. First, this was a single‐site study and thus our findings may not be generalizable to other institutions. However, regionalized care is increasingly encouraged to optimize communication between care team members.[17, 18] Therefore, our null findings may be pertinent to other institutions looking to improve patient safety outcomes, demonstrating that additional initiatives will likely be required. Second, our modes of outcome measurement possess limitations. In measuring concordance of care plan, although previously used survey techniques were employed,[9] the concordance survey has not been formally validated, and we believe some of the questions may have led to ambiguity on the part of the responders that may have resulted in less accurate responses, thus biasing toward the null. Similarly, in measuring AEs, the screening tool relied on retrospective chart review looking for specific AE types[11] and thus may not have captured more subtle AEs. Additionally, our study may have been underpowered to demonstrate significant reduction in preventable AEs, although other studies of similar methodology demonstrated significant results with similar sample size.[11] This was due in part to our lower‐than‐expected baseline AE rate (6.6% compared with approximately 10.3% in previous studies).[11] Lastly, our study solely examined the association of regionalization with concordance of care plan and preventable AEs, but importantly excluded other clinically important outcomes that may have been positively (or negatively) impacted by these regionalization efforts, such as ED wait times, provider efficiency (eg, fewer pages, less time in transit, more time at the bedside), interdisciplinary teamwork, or patient or provider satisfaction.
CONCLUSION
In summary, our findings suggest that regionalized care teams alone may be insufficient to effectively promote communication between care team members regarding the care plan or to lead to improvements in patient safety, although we recognize that there may have been benefits (or unintended harms) not measured in this study but are nonetheless important for clinical care and workplace culture. This is an important lesson, as many hospitals move toward regionalized care in an effort to improve patient safety outcomes. However, strengthening the infrastructure by colocalizing care team members to maximize opportunity for communication is likely a necessary first step toward facilitating implementation of additional initiatives that may lead to more robust patient safety improvements, such as structured interdisciplinary bedside rounds (eg, facilitating and training all team members to fulfill specific roles), teamwork training, and certification of key interdisciplinary teamwork skills. Additionally, close examination of identified prevalent and preventable AEs can help to determine which additional initiatives are most likely to have greatest impact in improving patient safety.
Disclosures: This research was supported by funds provided by Brigham and Women's Hospital (BWH) and by funds provided by the Department of Medicine at BWH. All authors had full access to all of the data in the study and were integrally involved in the design, implementation, data collection, and analyses. The first author, Dr. Stephanie Mueller, takes responsibility for the integrity for the data and the accuracy of the data analysis. Dr. Schnipper reports grants from Sanofi Aventis, outside the submitted work.
- Joint Commission on Accreditation of Healthcare Organizations. Understanding and Preventing Sentinel Events in Your Health Care Organization. Oak Brook, IL: Joint Commission; 2008.
- , , , et al. Communication failures in the operating room: an observational classification of recurrent types and effects. Qual Saf Health Care. 2004;13(5):330–334.
- , , , et al. Operating room teamwork among physicians and nurses: teamwork in the eye of the beholder. J Am Coll Surg. 2006;202(5):746–752.
- , , . Discrepant attitudes about teamwork among critical care nurses and physicians. Crit Care Med. 2003;31(3):956–959.
- , , , , . Communication failures in patient sign‐out and suggestions for improvement: a critical incident analysis. Qual Saf Health Care. 2005;14(6):401–407.
- , , , et al. Changes in medical errors after implementation of a handoff program. N Engl J Med. 2014;371(19):1803–1812.
- , , , et al. Communication and information deficits in patients discharged to rehabilitation facilities: an evaluation of five acute care hospitals. J Hosp Med. 2009;4(8):E28–E33.
- , , , , , . Deficits in communication and information transfer between hospital‐based and primary care physicians: implications for patient safety and continuity of care. JAMA. 2007;297(8):831–841.
- , , , et al. Patterns of nurse‐physician communication and agreement on the plan of care. Qual Saf Health Care. 2010;19(3):195–199.
- , , , , , . Can we talk? Priorities for patient care differed among health care providers. In: Henriksen K, Battles JB, Marks ES, Lewin DI, eds. Advances in Patient Safety: From Research to Implementation. Vol 1. Rockville, MD: Agency for Healthcare Research and Quality; 2005.
- , , , et al. Structured interdisciplinary rounds in a medical teaching unit: improving patient safety. Arch Intern Med. 2011;171(7):678–684.
- , , , , , . Improving teamwork: impact of structured interdisciplinary rounds on a hospitalist unit. J Hosp Med. 2011;6(2):88–93.
- , , , et al. Impact of localizing physicians to hospital units on nurse‐physician communication and agreement on the plan of care. J Gen Intern Med. 2009;24(11):1223–1227.
- , , , et al. Reorganizing a hospital ward as an accountable care unit. J Hosp Med. 2015;10(1):36–40.
- , , , et al. 5th time's a charm: creation of unit‐based care teams in a high occupancy hospital [abstract]. J Hosp Med. 2015;10 (suppl. 2). Available at: http://www.shmabstracts.com/abstract/5th‐times‐a‐charm‐creation‐of‐unit‐based‐care‐teams‐in‐a‐high‐occupancy‐hospital. Accessed July 28, 2015.
- , , , . Comorbidity measures for use with administrative data. Med Care. 1998;36(1):8–27.
- , , , , , . Teamwork on inpatient medical units: assessing attitudes and barriers. Qual Saf Health Care. 2010;19(2):117–121.
- , , , et al. A model for quality improvement programs in academic departments of medicine. Am J Med. 2008;121(10):922–929.
- , , , , . Improving nurse‐physician communication and satisfaction in the intensive care unit with a daily goals worksheet. Am J Crit Care. 2006;15(2):217–222.
- , , , , , . Improving communication in the ICU using daily goals. J Crit Care. 2003;18(2):71–75.
- , , , et al. Effect of crew resource management training in a multidisciplinary obstetrical setting. Int J Qual Health Care. 2008;20(4):254–263.
- , , , et al. Error reduction and performance improvement in the emergency department through formal teamwork training: evaluation results of the MedTeams project. Health Serv Res. 2002;37(6):1553–1581.
- , , , et al. Effects of teamwork training on adverse outcomes and process of care in labor and delivery: a randomized controlled trial. Obstet Gynecol. 2007;109(1):48–55.
- , , , et al. Effects of interdisciplinary team care interventions on general medical wards: a systematic review. JAMA Intern Med. 2015;175(8):1288–1298.
- , , , et al. Patient risk factors for adverse drug events in hospitalized patients. ADE Prevention Study Group. Arch Intern Med. 1999;159(21):2553–2560.
- , , , et al. Effect of a pharmacist intervention on clinically important medication errors after hospital discharge: a randomized trial. Ann Intern Med. 2012;157(1):1–10.
- , , , . Use of a standardized protocol to decrease medication errors and adverse events related to sliding scale insulin. Qual Saf Health Care. 2006;15(2):89–91.
- , , , . Clinical pharmacists and inpatient medical care: a systematic review. Arch Intern Med. 2006;166(9):955–964.
- , , , , . Improved inpatient use of basal insulin, reduced hypoglycemia, and improved glycemic control: effect of structured subcutaneous insulin orders and an insulin management algorithm. J Hosp Med. 2009;4(1):3–15.
- , , , . Effects of a computerized order set on the inpatient management of hyperglycemia: a cluster‐randomized controlled trial. Endocr Pract. 2010;16(2):209–218.
- , , , . Effects of a subcutaneous insulin protocol, clinical education, and computerized order set on the quality of inpatient management of hyperglycemia: results of a clinical trial. J Hosp Med. 2009;4(1):16–27.
Failures in communication among healthcare professionals are known threats to patient safety. These failures account for over 60% of root causes of sentinel events, the most serious events reported to The Joint Commission.[1] As such, identifying both patterns of effective communication as well as barriers to successful communication has been a focus of efforts aimed at improving patient safety. However, to date, the majority of this work has centered on improving communication in settings such as the operating room and intensive care unit,[2, 3, 4] or at times of care transitions.[5, 6, 7, 8]
Unique barriers exist for effective interdisciplinary communication in the hospital setting, particularly physiciannurse communication regarding shared hospitalized patients.[9] Traditionally, care of hospitalized patients is provided by physicians, nurses, and other team members working in varied workflow patterns, leading to dispersed team membership, where each team member cares for different groups of patients in different locations across the hospital. This dispersion is further heightened on teaching services, where residents' rotation schedules lead to frequent changes of care team membership, leaving inpatient care teams particularly vulnerable to ineffective communication. Evidence suggests that communication between nurses and physicians is currently suboptimal, leading to frequent disagreement regarding the patient's plan of care.[9, 10] This divergence between physician and nursing perceptions of patients' care plans may leave patients at greater risk of adverse events (AEs).
Several studies have examined the effects of regionalized inpatient care teams, where multidisciplinary team members care for the same patients on the same hospital unit, on communication and patient outcomes.[4, 11, 12, 13, 14] Results of these studies have been inconsistent, perhaps due to the particular characteristics of the care teams or to the study methodology. Thus, further rigorously done studies are required to better understand the impact of team regionalization on patient care. The goal of this study was to examine whether the implementation of regionalized inpatient care teams was associated with improvements in care team communication and preventable AEs.
METHODS
Setting, Patients, and Study Design
We performed a cohort analysis of patients at a 700‐bed tertiary care center, pre‐ and postregionalization of inpatient general medicine care teams. Our study protocol was approved by the Partners Healthcare Human Subjects Review Committee. Patients were eligible for inclusion if they were 18 years of age or older and discharged from the general medicine service (GMS) from any of the 3 participating nursing units between April 1, 2012 and June 19, 2012 (preregionalization) or April 1, 2013 and June 19, 2013 (postregionalization).
Intervention
On June 20, 2012, regionalized care was implemented on the GMS such that each of 3 GMS teams was localized to 1 of 3, 15‐bed nursing units. Prior to regionalization, the GMS physician care teams, each consisting of 1 hospitalist attending, 1 medical resident, and 2 medical interns, would care for patients on an average of 7 and up to 13 different nursing units on a given day.
Regionalized care consisted of a multifaceted intervention codeveloped by hospitalist, residency, nursing, emergency department, and hospital leadership and included: (1) regionalizing GMS teams as much as possible; (2) change in resident call structure from a traditional 4‐day call cycle to daily admitting; (3) collaborative efforts to enhance GMS patient discharges before noon to promote regionalized placement of patients without prolonging time in the emergency department (ED); (4) daily morning and postround multidisciplinary huddles to prioritize sicker patients and discharges; (5) encouragement of daily rounds at patients' bedsides with presence of physician team, nurse, and team pharmacist if available; (6) creation of unit‐ and team‐level performance reports; and (7) creation of unit‐based physician and nursing co‐leadership (Figure 1).[15]

Concordance of Plan
Concordance of plan was measured via a 7‐question survey previously developed, pilot tested, and used to measure the impact of regionalized care on care team communication between inpatient nursephysician team members.[9] The survey was administered in‐person by 1 of 8 trained research assistants (RAs) (4/emntervention period) to nurse and intern pairs caring for patients on the study units pre‐ and postregionalization. GMS patients were eligible for inclusion if surveys could be administered to their nurse and intern within the first 24 hours of admission to the unit and within 48 hours of admission to the hospital, based on RA availability (thus excluding patients admitted on Fridays as surveys were not conducted over the weekend). Most often, all eligible patients admitted to the study units during time periods of data collection were included in the study. On limited occasions, the daily supply of patients surpassed RA capacity for inclusion, at which time computer‐generated randomization was utilized to randomly select patients for inclusion. Nurse and intern pairs were surveyed once during a patient's hospitalization, although they could be surveyed more than once about different patients, and patients could be included more than once if rehospitalized on the study unit and cared for by a different nurseintern pair. Of the 472 selected eligible patients, the nurses and interns of 418 patients were available and consented to survey administration, representing 361 unique nurse and intern pairs and 399 unique patients.
Each member of the pair was asked about 7 specific aspects of the patient's care plan for that day in isolation from the other team member, including: (1) the patient's primary diagnosis, (2) the patient's expressed chief concern, (3) the day's scheduled tests, (4) the day's scheduled procedures, (5) consulting services involved, (6) medication changes made that day, and (7) the patient's expected discharge date. In addition, each pair was asked the name of the other team member (ie, the nurse was asked the name of the intern and vice versa), and whether or not the patient care plan for the day had been discussed with the other team member, where concordance was defined as both members agreeing the plan had been discussed. All responses were recorded verbatim. Pairs were surveyed independently between 12 pm and 2 pm, limiting confounding by evolving plans of care over time.
Each set of surveys were then reviewed by 2 of 4 trained adjudicators, and responses to each question were scored as complete, partial, or no agreement. Rules for degree of agreement were based upon previously utilized parameters[9] as well as biweekly meetings during which common themes and disagreements in ratings were discussed, and rules generated to create consensus (see Supporting Information, Appendix, in the online version of this article).
Adverse Event Detection
Of the patients meeting eligibility criteria, 200 patients were randomly selected using computer‐generated randomization from each time period for AE outcome assessment, for a total of 400 patients.
Each patient's electronic medical record was retrospectively reviewed by a trained clinician using a previously validated screening tool to detect any possible AEs.[11] Any positive screen prompted documentation of a narrative summary including a short description of the possible AE and pertinent associated data. We defined AE as any injury due to medical management rather than the natural history of the illness, and further limited this definition to only include AEs that occurred on the study unit or as a result of care on that unit.
Two of 4 trained adjudicators, blinded to time period, then separately reviewed each narrative summary using previously validated 6‐point confidence scales to determine the presence and preventability of AE, with confidence ratings of 4 or greater used as cutoffs.[11] All AEs were also scored on a 4‐point severity scale (trivial, clinically significant, serious, or life threatening), with severe AE defined as serious or life threatening. Lastly, adjudicators grouped AEs into 1 of 10 prespecified categories.[11] Any disagreements in ratings or groupings were discussed by all 4 adjudicators to reach consensus.
Data Analysis
Patient characteristics are presented using descriptive statistics and were compared in the pre‐ and postregionalization time periods using 2 or t tests as appropriate.
To analyze whether regionalized care was associated with concordance of plan, adjudicated survey questions were assigned points of 1, 0.5, and 0 for complete, partial, and no agreement, respectively. Total mean concordance scores for any patient ranged from 0 to 7 points, and were divided by total number of answered questions (up to 7) for a range of 0 to 1. Total mean concordance scores as well as mean concordance score per survey question were compared pre‐ versus postregionalization using t tests. In sensitivity analyses, adjudicated survey responses were dichotomized with complete and partial agreement deemed concordant responses. Percent concordance for each question was then compared pre‐ versus postregionalization using 2 analysis. Questions about the name of the other team member and discussion of daily care plan with the other team member were excluded from total concordance score calculations and were compared individually pre‐ versus postregionalization, because they are not directly about the plan of care.
To analyze the association of regionalization with odds of preventable AE, we performed multivariable logistic regression adjusted for patient age, sex, race, language, and Elixhauser comorbidity score,[16] and utilized generalized estimating equations to account for clustering by hospital unit. Secondary outcomes included severe preventable AEs, nonpreventable AEs, and category of preventable AEs using similar methodology. Two‐sided P values <0.05 were considered significant, and SAS version 9.2 (SAS Institute Inc., Cary, NC) was used for all analyses.
RESULTS
The fidelity of the intervention in achieving its goal of regionalized care is discussed separately.[15] Briefly, the intervention was successful at achieving 85% regionalization by team (ie, average daily percentage of team's patients assigned to team's unit) and 87% regionalization by unit (ie, average daily percentage of unit's patients with assigned team) following implementation, compared to 20% regionalization by team and unit in the preintervention period. Importantly, the average daily census of physician care teams rose by 32%, from a mean of 10.8 patients/physician care team preregionalization to a mean of 14.3 patients/physician care team postregionalization.
Concordance of Plan
Of the 418 nurse and intern paired surveys, 4 surveys were excluded due to repeat surveys of the same patient during the same hospitalization, for a total of 197 distinct paired surveys preregionalization and 217 paired surveys postregionalization. There were no statistically significant differences in patients' age, sex, race, language, admission source, length of stay, Elixhauser comorbidity score and diagnosis‐related group weight pre‐ versus postregionalization (Table 1).
| Characteristic | Concordance of Care Plan | Adverse Events | ||||
|---|---|---|---|---|---|---|
| Pre, n = 197 | Post, n = 217 | P Value | Pre, n = 198 | Post, n = 194 | P Value | |
| ||||||
| Age, mean (SD) | 60.5 (19.4) | 57.6 (20.8) | 0.15 | 60.4 (18.9) | 58.0 (21.2) | 0.24 |
| Male, n (%) | 77 (39.1) | 92 (42.4) | 0.49 | 94 (47.5) | 85 (43.8) | 0.55 |
| Race/ethnicity, n (%) | 0.34 | 0.12 | ||||
| White | 134 (68.0) | 141 (65.0) | 132 (66.5) | 121 (62.4) | ||
| Black | 42 (21.3) | 45 (20.7) | 41 (20.8) | 54 (27.8) | ||
| Hispanic | 18 (9.1) | 21 (9.7) | 22 (11.3) | 13 (6.8) | ||
| Other/unknown | 3 (1.5) | 10 (4.6) | 3 (1.4) | 6 (2.9) | ||
| Language, n (%) | 0.30 | 0.73 | ||||
| English | 183 (92.9) | 203 (93.5) | 176 (88.7) | 175 (90.2) | ||
| Spanish | 6 (3.0) | 10 (4.6) | 10 (5.2) | 10 (5.3) | ||
| Other | 8 (4.1) | 4 (1.8) | 12 (6.1) | 9 (4.5) | ||
| Admitting source, n (%) | 1.00 | 0.10 | ||||
| Physician office | 13 (6.6) | 13 (6.0) | 13 (6.6) | 6 (3.1) | ||
| Emergency department | 136 (69.0) | 150 (69.1) | 126 (63.6) | 127 (65.5) | ||
| Transfer from different hospital | 40 (20.3) | 45 (20.7) | 54 (27.3) | 50 (25.8) | ||
| Transfer from skilled nursing facility | 8 (4.1) | 9 (4.2) | 5 (2.5) | 11 (5.6) | ||
| Length of stay, d, median (IQR) | 3.0 (4.0) | 3.0 (4.0) | 0.57 | 4.0 (5.0) | 3.0 (4.0) | 0.16 |
| Elixhauser Comorbidity Score, mean (SD) | 8.0 (8.8) | 8.3 (9.3) | 0.74 | 8.0 (8.6) | 7.8 (8.4) | 0.86 |
| DRG weight, mean (SD) | 1.6 (1.0) | 1.5 (1.0) | 0.37 | 1.5 (0.93) | 1.5 (1.1) | 0.96 |
Kappa scores for adjudications of concordance surveys (defined as both adjudicators scoring the same level of agreement (ie, both complete or partial agreement versus no agreement) ranged from 0.69 to 0.95, by question. There were no significant differences in total mean concordance scores in the care plan pre‐ versus postregionalization (0.65 vs 0.67, P = 0.26) (Table 2). Similarly, there were no significant differences in mean concordance score for each survey question, except agreement on expected date of discharge (0.56 vs 0.68, P = 0.003), knowledge of the other provider's name, and agreement that discussion of the daily plan had taken place with the other pair member. Similar results were seen when results were dichotomized (ie, partial or complete agreement vs no agreement) (Table 2).
| Concordance Outcome | Pre, n = 197 | Post, n = 217 | P Value |
|---|---|---|---|
| |||
| Concordance score* | |||
| Total concordance score, mean (SD) | 0.65 (0.17) | 0.67 (0.16) | 0.26 |
| Subgroups | |||
| Diagnosis | 0.77 (0.32) | 0.72 (0.35) | 0.11 |
| Patient's chief concern | 0.48 (0.44) | 0.48 (0.43) | 0.94 |
| Tests today | 0.67 (0.40) | 0.71 (0.42) | 0.36 |
| Procedures today | 0.93 (0.25) | 0.92 (0.25) | 0.71 |
| Medication changes today | 0.56 (0.44) | 0.59 (0.43) | 0.54 |
| Consulting services | 0.59 (0.44) | 0.60 (0.44) | 0.82 |
| Expected discharge date | 0.56 (0.44) | 0.68 (0.38) | 0.003 |
| Responding clinician knowledge of nurse's name | 0.56 (0.50) | 0.86 (0.35) | <0.001 |
| Nurse's knowledge of responding clinician's name | 0.56 (0.50) | 0.88 (0.33) | <0.001 |
| Plan discussed | 0.73 (0.45) | 0.88 (0.32) | <0.001 |
| Percent concordance, mean (SD) | |||
| Diagnosis | 92.0 (27.3) | 88.6 (31.9) | 0.25 |
| Patient's chief concern | 59.6 (49.1) | 60.6 (49.0) | 0.84 |
| Tests today | 78.9 (40.9) | 77.2 (42.1) | 0.67 |
| Procedures today | 93.5 (24.8) | 94.1 (23.7) | 0.80 |
| Medication changes today | 66.3 (33.6) | 69.9 (46.0) | 0.44 |
| Consulting services | 69.3 (46.2) | 68.9 (46.4) | 0.93 |
| Expected discharge date | 67.5 (47.0) | 82.6 (38.0) | <0.001 |
| Responding clinician knowledge of nurse's name | 55.7 (49.8) | 85.6 (35.2) | <0.001 |
| Nurse's knowledge of responding clinician's name | 55.9 (49.8) | 87.9 (32.8) | <0.001 |
| Plan discussed | 72.9 (44.6) | 88.2 (32.3) | <0.001 |
Adverse Events
Of the 400 patients screened for AEs, 8 were excluded due to missing medical record number (5) and discharge outside of study period (3). Of the final 392 patient screens (198 pre, 194 post), there were no significant differences in patients' age, sex, race, language, length of stay, or Elixhauser score pre‐ versus postregionalization (Table 1).
Kappa scores for adjudicator agreement were 0.35 for presence of AE and 0.34 for preventability of AE. Of the 392 reviewed patient records, there were 133 total AEs detected (66 pre, 67 post), 27 preventable AEs (13 pre, 14 post), and 9 severe preventable AEs (4 pre, 5 post) (Table 3). There was no significant difference in the adjusted odds of preventable AEs post‐ versus preregionalization (adjusted odds ratio: 1.37, 95% confidence interval: 0.69, 2.69). Although the low number of AEs rated as severe or life threatening precluded adjusted analysis, unadjusted results similarly demonstrated no difference in odds of severe preventable AEs pre‐ versus postregionalization. As expected, there was no significant difference in adjusted odds of nonpreventable AE after implementation of regionalized care (Table 3).
| Adverse Events | No. of Adverse Events | Adjusted Odds Ratio Post vs Pre (95% CI) | |
|---|---|---|---|
| Pre, n = 198 | Post, n = 194 | ||
| |||
| Preventable | 13 | 14 | 1.37 (0.69, 2.69) |
| Serious and preventable | 4 | 5 | |
| Nonpreventable | 47 | 50 | 1.20 (0.85, 1.75) |
Similarly, there were no significant differences in category of preventable AE pre‐ versus postregionalization. The most frequent preventable AEs in both time periods were those related to adverse drug events and to manifestations of poor glycemic control, examples of which are illustrated (Table 4).
| |
| Adverse drug event | 29‐year‐old male with history of alcohol abuse, complicated by prior withdrawal seizures/emntensive care unit admissions, presented with alcohol withdrawal. Started on standing and PRN lorazepam, kept on home medications including standing clonidine, gabapentin, citalopram, quetiapine. Became somnolent due to polypharmacy, ultimately discontinued quetiapine as discovered took only as needed at home for insomnia |
| Manifestations of poor glycemic control | 78‐year‐old male with recently diagnosed lymphoma, distant history of bladder and prostate cancer status post ileal loop diversion, presented status post syncopal event; during event, spilled boiling water on himself leading to second‐degree burns on 3% of his body. Initially admitted to trauma/burn service, ultimately transferred to medical service for ongoing multiple medical issues including obstructive uropathy, acute on chronic renal failure. Adverse event was hyperglycemia (>350 mg/dL on >2 consecutive readings) in the setting of holding his home insulin detemir and insulin aspart (had been placed on insulin aspart sliding scale alone). After hyperglycemic episodes, was placed back on weight‐based basal/nutritional insulin |
DISCUSSION
In this study of general medicine patients at a large academic medical center, we found that regionalization of care teams on general medicine services was associated with improved recognition of care team members and agreement on estimated date of patient discharge, but was not associated with improvement in overall nurse and physician concordance of the patient care plan, or the odds of preventable AEs.
This intervention importantly addresses the barrier of dispersion of team membership, a well‐recognized barrier to interdisciplinary collaboration,[17, 18] particularly with resident physician teams due to frequently changing team membership. Localization of all team members, in addition to encouragement of daily collaborative bedside rounds as part of the regionalization initiative, likely contributed to our observed improvement in team member identification and discussion of daily care plans. Similarly, regionalization resulted in improved agreement in estimations of date of patient discharge. Focus on early patient discharges was an integral part of the implementation efforts; we therefore hypothesize that mutual focus on discharge planning by both nurses and responding clinicians may have explained this observed result.
On the other hand, regionalization did not appreciably improve the overall concordance of care plan between nurses and interns, despite a significant increase in team members agreeing that the plan had been discussed. Our findings support similar prior research demonstrating that regionalizing hospitalist attendings to single nursing units had limited impact on agreement of care plan between physicians and nurses.[13] Similarly, in settings where physicians and nurses are inherently regionalized, such as the intensive care unit[4] or the operating room,[3] communication between physicians and nurses remains difficult. Collectively, our findings suggest that colocalization of physicians and nurses alone is likely insufficient to improve measured communication between care team members. Existing literature suggests that more standardized approaches to improve communication, such as structured communication tools used during daily inpatient care[19, 20] or formalized team training,[21, 22, 23] lead to improvements in communication and collaboration. Despite these findings, it is important to highlight that this study did not assess other measures of workplace culture, such as teamwork and care team cohesiveness, which may have been positively affected by this intervention, even without measurable effect on concordance of care plan. Additionally, as noted, the average daily census on each team increased by almost a third postintervention, which may have impeded improvements in care team communication.
In addition, we found that our intervention had no significant impact on preventable AEs or severe preventable AEs. Although we cannot exclude the possibility that more subtle AEs were missed with our methodology, our results indicate that regionalized care alone may be inadequate to improve major patient safety outcomes. As discussed, the volume of patients did increase postintervention; thus, another way to state our results is that we were able to increase the daily volume of patients without any significant decreases in patient safety. Nevertheless, the results on patient safety were less than desired. A recent review of interdisciplinary team care interventions on general medical wards similarly demonstrated underwhelming improvements in patient safety outcomes, although the reviewed interventions did not specifically address preventable AEs, a gap in the literature commented on by the authors.[24] Other albeit limited literature has demonstrated improvement in patient safety outcomes via multifaceted efforts aimed at improving care team member communication. Notably, these efforts include colocalization of care team members to single units but also involve additional measures to improve communication and collaboration between care team members, such as structured communication during interdisciplinary rounds, and certification of key interdisciplinary teamwork skills.[11, 14] Although our regionalized care intervention included many similar features to these accountable care units (ACUs) including unit‐based care teams, unit‐level performance reporting, and unit‐based physician and nursing coleadership, significant differences existed. Notably, in addition to the above features, the ACU model also incorporated highly structured communication models for interdisciplinary rounding, and certification processes to ensure an appropriate communication skill base among care team members.[14] Thus, although creation of regionalized care teams is likely a necessary precursor to implementation of these additional measures, alone it may be insufficient to improve patient safety outcomes.
Importantly, in our study we identified that adverse drug events and manifestations of poor glycemic control occurred in high frequency both before and following implementation of regionalized care, supporting other literature that describes the prevalence of these AEs.[11, 25, 26, 27] These results suggest that targeted interventions to address these specific AEs are likely necessary. Notably, the intervention units in our study did not consistently employ clinical pharmacists assigned specifically to that unit's care team to allow for integration within the care team. As prior research has suggested that greater collaboration with clinical pharmacists results in reduction of adverse drug events,[28] next steps may include improved integration of team‐based pharmacists into the activities of the regionalized care teams. Inpatient management of diabetes also requires specific interventions,[29, 30, 31] only some of which may be addressable by having regionalized care and better interdisciplinary communication.
Our findings are subject to several limitations. First, this was a single‐site study and thus our findings may not be generalizable to other institutions. However, regionalized care is increasingly encouraged to optimize communication between care team members.[17, 18] Therefore, our null findings may be pertinent to other institutions looking to improve patient safety outcomes, demonstrating that additional initiatives will likely be required. Second, our modes of outcome measurement possess limitations. In measuring concordance of care plan, although previously used survey techniques were employed,[9] the concordance survey has not been formally validated, and we believe some of the questions may have led to ambiguity on the part of the responders that may have resulted in less accurate responses, thus biasing toward the null. Similarly, in measuring AEs, the screening tool relied on retrospective chart review looking for specific AE types[11] and thus may not have captured more subtle AEs. Additionally, our study may have been underpowered to demonstrate significant reduction in preventable AEs, although other studies of similar methodology demonstrated significant results with similar sample size.[11] This was due in part to our lower‐than‐expected baseline AE rate (6.6% compared with approximately 10.3% in previous studies).[11] Lastly, our study solely examined the association of regionalization with concordance of care plan and preventable AEs, but importantly excluded other clinically important outcomes that may have been positively (or negatively) impacted by these regionalization efforts, such as ED wait times, provider efficiency (eg, fewer pages, less time in transit, more time at the bedside), interdisciplinary teamwork, or patient or provider satisfaction.
CONCLUSION
In summary, our findings suggest that regionalized care teams alone may be insufficient to effectively promote communication between care team members regarding the care plan or to lead to improvements in patient safety, although we recognize that there may have been benefits (or unintended harms) not measured in this study but are nonetheless important for clinical care and workplace culture. This is an important lesson, as many hospitals move toward regionalized care in an effort to improve patient safety outcomes. However, strengthening the infrastructure by colocalizing care team members to maximize opportunity for communication is likely a necessary first step toward facilitating implementation of additional initiatives that may lead to more robust patient safety improvements, such as structured interdisciplinary bedside rounds (eg, facilitating and training all team members to fulfill specific roles), teamwork training, and certification of key interdisciplinary teamwork skills. Additionally, close examination of identified prevalent and preventable AEs can help to determine which additional initiatives are most likely to have greatest impact in improving patient safety.
Disclosures: This research was supported by funds provided by Brigham and Women's Hospital (BWH) and by funds provided by the Department of Medicine at BWH. All authors had full access to all of the data in the study and were integrally involved in the design, implementation, data collection, and analyses. The first author, Dr. Stephanie Mueller, takes responsibility for the integrity for the data and the accuracy of the data analysis. Dr. Schnipper reports grants from Sanofi Aventis, outside the submitted work.
Failures in communication among healthcare professionals are known threats to patient safety. These failures account for over 60% of root causes of sentinel events, the most serious events reported to The Joint Commission.[1] As such, identifying both patterns of effective communication as well as barriers to successful communication has been a focus of efforts aimed at improving patient safety. However, to date, the majority of this work has centered on improving communication in settings such as the operating room and intensive care unit,[2, 3, 4] or at times of care transitions.[5, 6, 7, 8]
Unique barriers exist for effective interdisciplinary communication in the hospital setting, particularly physiciannurse communication regarding shared hospitalized patients.[9] Traditionally, care of hospitalized patients is provided by physicians, nurses, and other team members working in varied workflow patterns, leading to dispersed team membership, where each team member cares for different groups of patients in different locations across the hospital. This dispersion is further heightened on teaching services, where residents' rotation schedules lead to frequent changes of care team membership, leaving inpatient care teams particularly vulnerable to ineffective communication. Evidence suggests that communication between nurses and physicians is currently suboptimal, leading to frequent disagreement regarding the patient's plan of care.[9, 10] This divergence between physician and nursing perceptions of patients' care plans may leave patients at greater risk of adverse events (AEs).
Several studies have examined the effects of regionalized inpatient care teams, where multidisciplinary team members care for the same patients on the same hospital unit, on communication and patient outcomes.[4, 11, 12, 13, 14] Results of these studies have been inconsistent, perhaps due to the particular characteristics of the care teams or to the study methodology. Thus, further rigorously done studies are required to better understand the impact of team regionalization on patient care. The goal of this study was to examine whether the implementation of regionalized inpatient care teams was associated with improvements in care team communication and preventable AEs.
METHODS
Setting, Patients, and Study Design
We performed a cohort analysis of patients at a 700‐bed tertiary care center, pre‐ and postregionalization of inpatient general medicine care teams. Our study protocol was approved by the Partners Healthcare Human Subjects Review Committee. Patients were eligible for inclusion if they were 18 years of age or older and discharged from the general medicine service (GMS) from any of the 3 participating nursing units between April 1, 2012 and June 19, 2012 (preregionalization) or April 1, 2013 and June 19, 2013 (postregionalization).
Intervention
On June 20, 2012, regionalized care was implemented on the GMS such that each of 3 GMS teams was localized to 1 of 3, 15‐bed nursing units. Prior to regionalization, the GMS physician care teams, each consisting of 1 hospitalist attending, 1 medical resident, and 2 medical interns, would care for patients on an average of 7 and up to 13 different nursing units on a given day.
Regionalized care consisted of a multifaceted intervention codeveloped by hospitalist, residency, nursing, emergency department, and hospital leadership and included: (1) regionalizing GMS teams as much as possible; (2) change in resident call structure from a traditional 4‐day call cycle to daily admitting; (3) collaborative efforts to enhance GMS patient discharges before noon to promote regionalized placement of patients without prolonging time in the emergency department (ED); (4) daily morning and postround multidisciplinary huddles to prioritize sicker patients and discharges; (5) encouragement of daily rounds at patients' bedsides with presence of physician team, nurse, and team pharmacist if available; (6) creation of unit‐ and team‐level performance reports; and (7) creation of unit‐based physician and nursing co‐leadership (Figure 1).[15]

Concordance of Plan
Concordance of plan was measured via a 7‐question survey previously developed, pilot tested, and used to measure the impact of regionalized care on care team communication between inpatient nursephysician team members.[9] The survey was administered in‐person by 1 of 8 trained research assistants (RAs) (4/emntervention period) to nurse and intern pairs caring for patients on the study units pre‐ and postregionalization. GMS patients were eligible for inclusion if surveys could be administered to their nurse and intern within the first 24 hours of admission to the unit and within 48 hours of admission to the hospital, based on RA availability (thus excluding patients admitted on Fridays as surveys were not conducted over the weekend). Most often, all eligible patients admitted to the study units during time periods of data collection were included in the study. On limited occasions, the daily supply of patients surpassed RA capacity for inclusion, at which time computer‐generated randomization was utilized to randomly select patients for inclusion. Nurse and intern pairs were surveyed once during a patient's hospitalization, although they could be surveyed more than once about different patients, and patients could be included more than once if rehospitalized on the study unit and cared for by a different nurseintern pair. Of the 472 selected eligible patients, the nurses and interns of 418 patients were available and consented to survey administration, representing 361 unique nurse and intern pairs and 399 unique patients.
Each member of the pair was asked about 7 specific aspects of the patient's care plan for that day in isolation from the other team member, including: (1) the patient's primary diagnosis, (2) the patient's expressed chief concern, (3) the day's scheduled tests, (4) the day's scheduled procedures, (5) consulting services involved, (6) medication changes made that day, and (7) the patient's expected discharge date. In addition, each pair was asked the name of the other team member (ie, the nurse was asked the name of the intern and vice versa), and whether or not the patient care plan for the day had been discussed with the other team member, where concordance was defined as both members agreeing the plan had been discussed. All responses were recorded verbatim. Pairs were surveyed independently between 12 pm and 2 pm, limiting confounding by evolving plans of care over time.
Each set of surveys were then reviewed by 2 of 4 trained adjudicators, and responses to each question were scored as complete, partial, or no agreement. Rules for degree of agreement were based upon previously utilized parameters[9] as well as biweekly meetings during which common themes and disagreements in ratings were discussed, and rules generated to create consensus (see Supporting Information, Appendix, in the online version of this article).
Adverse Event Detection
Of the patients meeting eligibility criteria, 200 patients were randomly selected using computer‐generated randomization from each time period for AE outcome assessment, for a total of 400 patients.
Each patient's electronic medical record was retrospectively reviewed by a trained clinician using a previously validated screening tool to detect any possible AEs.[11] Any positive screen prompted documentation of a narrative summary including a short description of the possible AE and pertinent associated data. We defined AE as any injury due to medical management rather than the natural history of the illness, and further limited this definition to only include AEs that occurred on the study unit or as a result of care on that unit.
Two of 4 trained adjudicators, blinded to time period, then separately reviewed each narrative summary using previously validated 6‐point confidence scales to determine the presence and preventability of AE, with confidence ratings of 4 or greater used as cutoffs.[11] All AEs were also scored on a 4‐point severity scale (trivial, clinically significant, serious, or life threatening), with severe AE defined as serious or life threatening. Lastly, adjudicators grouped AEs into 1 of 10 prespecified categories.[11] Any disagreements in ratings or groupings were discussed by all 4 adjudicators to reach consensus.
Data Analysis
Patient characteristics are presented using descriptive statistics and were compared in the pre‐ and postregionalization time periods using 2 or t tests as appropriate.
To analyze whether regionalized care was associated with concordance of plan, adjudicated survey questions were assigned points of 1, 0.5, and 0 for complete, partial, and no agreement, respectively. Total mean concordance scores for any patient ranged from 0 to 7 points, and were divided by total number of answered questions (up to 7) for a range of 0 to 1. Total mean concordance scores as well as mean concordance score per survey question were compared pre‐ versus postregionalization using t tests. In sensitivity analyses, adjudicated survey responses were dichotomized with complete and partial agreement deemed concordant responses. Percent concordance for each question was then compared pre‐ versus postregionalization using 2 analysis. Questions about the name of the other team member and discussion of daily care plan with the other team member were excluded from total concordance score calculations and were compared individually pre‐ versus postregionalization, because they are not directly about the plan of care.
To analyze the association of regionalization with odds of preventable AE, we performed multivariable logistic regression adjusted for patient age, sex, race, language, and Elixhauser comorbidity score,[16] and utilized generalized estimating equations to account for clustering by hospital unit. Secondary outcomes included severe preventable AEs, nonpreventable AEs, and category of preventable AEs using similar methodology. Two‐sided P values <0.05 were considered significant, and SAS version 9.2 (SAS Institute Inc., Cary, NC) was used for all analyses.
RESULTS
The fidelity of the intervention in achieving its goal of regionalized care is discussed separately.[15] Briefly, the intervention was successful at achieving 85% regionalization by team (ie, average daily percentage of team's patients assigned to team's unit) and 87% regionalization by unit (ie, average daily percentage of unit's patients with assigned team) following implementation, compared to 20% regionalization by team and unit in the preintervention period. Importantly, the average daily census of physician care teams rose by 32%, from a mean of 10.8 patients/physician care team preregionalization to a mean of 14.3 patients/physician care team postregionalization.
Concordance of Plan
Of the 418 nurse and intern paired surveys, 4 surveys were excluded due to repeat surveys of the same patient during the same hospitalization, for a total of 197 distinct paired surveys preregionalization and 217 paired surveys postregionalization. There were no statistically significant differences in patients' age, sex, race, language, admission source, length of stay, Elixhauser comorbidity score and diagnosis‐related group weight pre‐ versus postregionalization (Table 1).
| Characteristic | Concordance of Care Plan | Adverse Events | ||||
|---|---|---|---|---|---|---|
| Pre, n = 197 | Post, n = 217 | P Value | Pre, n = 198 | Post, n = 194 | P Value | |
| ||||||
| Age, mean (SD) | 60.5 (19.4) | 57.6 (20.8) | 0.15 | 60.4 (18.9) | 58.0 (21.2) | 0.24 |
| Male, n (%) | 77 (39.1) | 92 (42.4) | 0.49 | 94 (47.5) | 85 (43.8) | 0.55 |
| Race/ethnicity, n (%) | 0.34 | 0.12 | ||||
| White | 134 (68.0) | 141 (65.0) | 132 (66.5) | 121 (62.4) | ||
| Black | 42 (21.3) | 45 (20.7) | 41 (20.8) | 54 (27.8) | ||
| Hispanic | 18 (9.1) | 21 (9.7) | 22 (11.3) | 13 (6.8) | ||
| Other/unknown | 3 (1.5) | 10 (4.6) | 3 (1.4) | 6 (2.9) | ||
| Language, n (%) | 0.30 | 0.73 | ||||
| English | 183 (92.9) | 203 (93.5) | 176 (88.7) | 175 (90.2) | ||
| Spanish | 6 (3.0) | 10 (4.6) | 10 (5.2) | 10 (5.3) | ||
| Other | 8 (4.1) | 4 (1.8) | 12 (6.1) | 9 (4.5) | ||
| Admitting source, n (%) | 1.00 | 0.10 | ||||
| Physician office | 13 (6.6) | 13 (6.0) | 13 (6.6) | 6 (3.1) | ||
| Emergency department | 136 (69.0) | 150 (69.1) | 126 (63.6) | 127 (65.5) | ||
| Transfer from different hospital | 40 (20.3) | 45 (20.7) | 54 (27.3) | 50 (25.8) | ||
| Transfer from skilled nursing facility | 8 (4.1) | 9 (4.2) | 5 (2.5) | 11 (5.6) | ||
| Length of stay, d, median (IQR) | 3.0 (4.0) | 3.0 (4.0) | 0.57 | 4.0 (5.0) | 3.0 (4.0) | 0.16 |
| Elixhauser Comorbidity Score, mean (SD) | 8.0 (8.8) | 8.3 (9.3) | 0.74 | 8.0 (8.6) | 7.8 (8.4) | 0.86 |
| DRG weight, mean (SD) | 1.6 (1.0) | 1.5 (1.0) | 0.37 | 1.5 (0.93) | 1.5 (1.1) | 0.96 |
Kappa scores for adjudications of concordance surveys (defined as both adjudicators scoring the same level of agreement (ie, both complete or partial agreement versus no agreement) ranged from 0.69 to 0.95, by question. There were no significant differences in total mean concordance scores in the care plan pre‐ versus postregionalization (0.65 vs 0.67, P = 0.26) (Table 2). Similarly, there were no significant differences in mean concordance score for each survey question, except agreement on expected date of discharge (0.56 vs 0.68, P = 0.003), knowledge of the other provider's name, and agreement that discussion of the daily plan had taken place with the other pair member. Similar results were seen when results were dichotomized (ie, partial or complete agreement vs no agreement) (Table 2).
| Concordance Outcome | Pre, n = 197 | Post, n = 217 | P Value |
|---|---|---|---|
| |||
| Concordance score* | |||
| Total concordance score, mean (SD) | 0.65 (0.17) | 0.67 (0.16) | 0.26 |
| Subgroups | |||
| Diagnosis | 0.77 (0.32) | 0.72 (0.35) | 0.11 |
| Patient's chief concern | 0.48 (0.44) | 0.48 (0.43) | 0.94 |
| Tests today | 0.67 (0.40) | 0.71 (0.42) | 0.36 |
| Procedures today | 0.93 (0.25) | 0.92 (0.25) | 0.71 |
| Medication changes today | 0.56 (0.44) | 0.59 (0.43) | 0.54 |
| Consulting services | 0.59 (0.44) | 0.60 (0.44) | 0.82 |
| Expected discharge date | 0.56 (0.44) | 0.68 (0.38) | 0.003 |
| Responding clinician knowledge of nurse's name | 0.56 (0.50) | 0.86 (0.35) | <0.001 |
| Nurse's knowledge of responding clinician's name | 0.56 (0.50) | 0.88 (0.33) | <0.001 |
| Plan discussed | 0.73 (0.45) | 0.88 (0.32) | <0.001 |
| Percent concordance, mean (SD) | |||
| Diagnosis | 92.0 (27.3) | 88.6 (31.9) | 0.25 |
| Patient's chief concern | 59.6 (49.1) | 60.6 (49.0) | 0.84 |
| Tests today | 78.9 (40.9) | 77.2 (42.1) | 0.67 |
| Procedures today | 93.5 (24.8) | 94.1 (23.7) | 0.80 |
| Medication changes today | 66.3 (33.6) | 69.9 (46.0) | 0.44 |
| Consulting services | 69.3 (46.2) | 68.9 (46.4) | 0.93 |
| Expected discharge date | 67.5 (47.0) | 82.6 (38.0) | <0.001 |
| Responding clinician knowledge of nurse's name | 55.7 (49.8) | 85.6 (35.2) | <0.001 |
| Nurse's knowledge of responding clinician's name | 55.9 (49.8) | 87.9 (32.8) | <0.001 |
| Plan discussed | 72.9 (44.6) | 88.2 (32.3) | <0.001 |
Adverse Events
Of the 400 patients screened for AEs, 8 were excluded due to missing medical record number (5) and discharge outside of study period (3). Of the final 392 patient screens (198 pre, 194 post), there were no significant differences in patients' age, sex, race, language, length of stay, or Elixhauser score pre‐ versus postregionalization (Table 1).
Kappa scores for adjudicator agreement were 0.35 for presence of AE and 0.34 for preventability of AE. Of the 392 reviewed patient records, there were 133 total AEs detected (66 pre, 67 post), 27 preventable AEs (13 pre, 14 post), and 9 severe preventable AEs (4 pre, 5 post) (Table 3). There was no significant difference in the adjusted odds of preventable AEs post‐ versus preregionalization (adjusted odds ratio: 1.37, 95% confidence interval: 0.69, 2.69). Although the low number of AEs rated as severe or life threatening precluded adjusted analysis, unadjusted results similarly demonstrated no difference in odds of severe preventable AEs pre‐ versus postregionalization. As expected, there was no significant difference in adjusted odds of nonpreventable AE after implementation of regionalized care (Table 3).
| Adverse Events | No. of Adverse Events | Adjusted Odds Ratio Post vs Pre (95% CI) | |
|---|---|---|---|
| Pre, n = 198 | Post, n = 194 | ||
| |||
| Preventable | 13 | 14 | 1.37 (0.69, 2.69) |
| Serious and preventable | 4 | 5 | |
| Nonpreventable | 47 | 50 | 1.20 (0.85, 1.75) |
Similarly, there were no significant differences in category of preventable AE pre‐ versus postregionalization. The most frequent preventable AEs in both time periods were those related to adverse drug events and to manifestations of poor glycemic control, examples of which are illustrated (Table 4).
| |
| Adverse drug event | 29‐year‐old male with history of alcohol abuse, complicated by prior withdrawal seizures/emntensive care unit admissions, presented with alcohol withdrawal. Started on standing and PRN lorazepam, kept on home medications including standing clonidine, gabapentin, citalopram, quetiapine. Became somnolent due to polypharmacy, ultimately discontinued quetiapine as discovered took only as needed at home for insomnia |
| Manifestations of poor glycemic control | 78‐year‐old male with recently diagnosed lymphoma, distant history of bladder and prostate cancer status post ileal loop diversion, presented status post syncopal event; during event, spilled boiling water on himself leading to second‐degree burns on 3% of his body. Initially admitted to trauma/burn service, ultimately transferred to medical service for ongoing multiple medical issues including obstructive uropathy, acute on chronic renal failure. Adverse event was hyperglycemia (>350 mg/dL on >2 consecutive readings) in the setting of holding his home insulin detemir and insulin aspart (had been placed on insulin aspart sliding scale alone). After hyperglycemic episodes, was placed back on weight‐based basal/nutritional insulin |
DISCUSSION
In this study of general medicine patients at a large academic medical center, we found that regionalization of care teams on general medicine services was associated with improved recognition of care team members and agreement on estimated date of patient discharge, but was not associated with improvement in overall nurse and physician concordance of the patient care plan, or the odds of preventable AEs.
This intervention importantly addresses the barrier of dispersion of team membership, a well‐recognized barrier to interdisciplinary collaboration,[17, 18] particularly with resident physician teams due to frequently changing team membership. Localization of all team members, in addition to encouragement of daily collaborative bedside rounds as part of the regionalization initiative, likely contributed to our observed improvement in team member identification and discussion of daily care plans. Similarly, regionalization resulted in improved agreement in estimations of date of patient discharge. Focus on early patient discharges was an integral part of the implementation efforts; we therefore hypothesize that mutual focus on discharge planning by both nurses and responding clinicians may have explained this observed result.
On the other hand, regionalization did not appreciably improve the overall concordance of care plan between nurses and interns, despite a significant increase in team members agreeing that the plan had been discussed. Our findings support similar prior research demonstrating that regionalizing hospitalist attendings to single nursing units had limited impact on agreement of care plan between physicians and nurses.[13] Similarly, in settings where physicians and nurses are inherently regionalized, such as the intensive care unit[4] or the operating room,[3] communication between physicians and nurses remains difficult. Collectively, our findings suggest that colocalization of physicians and nurses alone is likely insufficient to improve measured communication between care team members. Existing literature suggests that more standardized approaches to improve communication, such as structured communication tools used during daily inpatient care[19, 20] or formalized team training,[21, 22, 23] lead to improvements in communication and collaboration. Despite these findings, it is important to highlight that this study did not assess other measures of workplace culture, such as teamwork and care team cohesiveness, which may have been positively affected by this intervention, even without measurable effect on concordance of care plan. Additionally, as noted, the average daily census on each team increased by almost a third postintervention, which may have impeded improvements in care team communication.
In addition, we found that our intervention had no significant impact on preventable AEs or severe preventable AEs. Although we cannot exclude the possibility that more subtle AEs were missed with our methodology, our results indicate that regionalized care alone may be inadequate to improve major patient safety outcomes. As discussed, the volume of patients did increase postintervention; thus, another way to state our results is that we were able to increase the daily volume of patients without any significant decreases in patient safety. Nevertheless, the results on patient safety were less than desired. A recent review of interdisciplinary team care interventions on general medical wards similarly demonstrated underwhelming improvements in patient safety outcomes, although the reviewed interventions did not specifically address preventable AEs, a gap in the literature commented on by the authors.[24] Other albeit limited literature has demonstrated improvement in patient safety outcomes via multifaceted efforts aimed at improving care team member communication. Notably, these efforts include colocalization of care team members to single units but also involve additional measures to improve communication and collaboration between care team members, such as structured communication during interdisciplinary rounds, and certification of key interdisciplinary teamwork skills.[11, 14] Although our regionalized care intervention included many similar features to these accountable care units (ACUs) including unit‐based care teams, unit‐level performance reporting, and unit‐based physician and nursing coleadership, significant differences existed. Notably, in addition to the above features, the ACU model also incorporated highly structured communication models for interdisciplinary rounding, and certification processes to ensure an appropriate communication skill base among care team members.[14] Thus, although creation of regionalized care teams is likely a necessary precursor to implementation of these additional measures, alone it may be insufficient to improve patient safety outcomes.
Importantly, in our study we identified that adverse drug events and manifestations of poor glycemic control occurred in high frequency both before and following implementation of regionalized care, supporting other literature that describes the prevalence of these AEs.[11, 25, 26, 27] These results suggest that targeted interventions to address these specific AEs are likely necessary. Notably, the intervention units in our study did not consistently employ clinical pharmacists assigned specifically to that unit's care team to allow for integration within the care team. As prior research has suggested that greater collaboration with clinical pharmacists results in reduction of adverse drug events,[28] next steps may include improved integration of team‐based pharmacists into the activities of the regionalized care teams. Inpatient management of diabetes also requires specific interventions,[29, 30, 31] only some of which may be addressable by having regionalized care and better interdisciplinary communication.
Our findings are subject to several limitations. First, this was a single‐site study and thus our findings may not be generalizable to other institutions. However, regionalized care is increasingly encouraged to optimize communication between care team members.[17, 18] Therefore, our null findings may be pertinent to other institutions looking to improve patient safety outcomes, demonstrating that additional initiatives will likely be required. Second, our modes of outcome measurement possess limitations. In measuring concordance of care plan, although previously used survey techniques were employed,[9] the concordance survey has not been formally validated, and we believe some of the questions may have led to ambiguity on the part of the responders that may have resulted in less accurate responses, thus biasing toward the null. Similarly, in measuring AEs, the screening tool relied on retrospective chart review looking for specific AE types[11] and thus may not have captured more subtle AEs. Additionally, our study may have been underpowered to demonstrate significant reduction in preventable AEs, although other studies of similar methodology demonstrated significant results with similar sample size.[11] This was due in part to our lower‐than‐expected baseline AE rate (6.6% compared with approximately 10.3% in previous studies).[11] Lastly, our study solely examined the association of regionalization with concordance of care plan and preventable AEs, but importantly excluded other clinically important outcomes that may have been positively (or negatively) impacted by these regionalization efforts, such as ED wait times, provider efficiency (eg, fewer pages, less time in transit, more time at the bedside), interdisciplinary teamwork, or patient or provider satisfaction.
CONCLUSION
In summary, our findings suggest that regionalized care teams alone may be insufficient to effectively promote communication between care team members regarding the care plan or to lead to improvements in patient safety, although we recognize that there may have been benefits (or unintended harms) not measured in this study but are nonetheless important for clinical care and workplace culture. This is an important lesson, as many hospitals move toward regionalized care in an effort to improve patient safety outcomes. However, strengthening the infrastructure by colocalizing care team members to maximize opportunity for communication is likely a necessary first step toward facilitating implementation of additional initiatives that may lead to more robust patient safety improvements, such as structured interdisciplinary bedside rounds (eg, facilitating and training all team members to fulfill specific roles), teamwork training, and certification of key interdisciplinary teamwork skills. Additionally, close examination of identified prevalent and preventable AEs can help to determine which additional initiatives are most likely to have greatest impact in improving patient safety.
Disclosures: This research was supported by funds provided by Brigham and Women's Hospital (BWH) and by funds provided by the Department of Medicine at BWH. All authors had full access to all of the data in the study and were integrally involved in the design, implementation, data collection, and analyses. The first author, Dr. Stephanie Mueller, takes responsibility for the integrity for the data and the accuracy of the data analysis. Dr. Schnipper reports grants from Sanofi Aventis, outside the submitted work.
- Joint Commission on Accreditation of Healthcare Organizations. Understanding and Preventing Sentinel Events in Your Health Care Organization. Oak Brook, IL: Joint Commission; 2008.
- , , , et al. Communication failures in the operating room: an observational classification of recurrent types and effects. Qual Saf Health Care. 2004;13(5):330–334.
- , , , et al. Operating room teamwork among physicians and nurses: teamwork in the eye of the beholder. J Am Coll Surg. 2006;202(5):746–752.
- , , . Discrepant attitudes about teamwork among critical care nurses and physicians. Crit Care Med. 2003;31(3):956–959.
- , , , , . Communication failures in patient sign‐out and suggestions for improvement: a critical incident analysis. Qual Saf Health Care. 2005;14(6):401–407.
- , , , et al. Changes in medical errors after implementation of a handoff program. N Engl J Med. 2014;371(19):1803–1812.
- , , , et al. Communication and information deficits in patients discharged to rehabilitation facilities: an evaluation of five acute care hospitals. J Hosp Med. 2009;4(8):E28–E33.
- , , , , , . Deficits in communication and information transfer between hospital‐based and primary care physicians: implications for patient safety and continuity of care. JAMA. 2007;297(8):831–841.
- , , , et al. Patterns of nurse‐physician communication and agreement on the plan of care. Qual Saf Health Care. 2010;19(3):195–199.
- , , , , , . Can we talk? Priorities for patient care differed among health care providers. In: Henriksen K, Battles JB, Marks ES, Lewin DI, eds. Advances in Patient Safety: From Research to Implementation. Vol 1. Rockville, MD: Agency for Healthcare Research and Quality; 2005.
- , , , et al. Structured interdisciplinary rounds in a medical teaching unit: improving patient safety. Arch Intern Med. 2011;171(7):678–684.
- , , , , , . Improving teamwork: impact of structured interdisciplinary rounds on a hospitalist unit. J Hosp Med. 2011;6(2):88–93.
- , , , et al. Impact of localizing physicians to hospital units on nurse‐physician communication and agreement on the plan of care. J Gen Intern Med. 2009;24(11):1223–1227.
- , , , et al. Reorganizing a hospital ward as an accountable care unit. J Hosp Med. 2015;10(1):36–40.
- , , , et al. 5th time's a charm: creation of unit‐based care teams in a high occupancy hospital [abstract]. J Hosp Med. 2015;10 (suppl. 2). Available at: http://www.shmabstracts.com/abstract/5th‐times‐a‐charm‐creation‐of‐unit‐based‐care‐teams‐in‐a‐high‐occupancy‐hospital. Accessed July 28, 2015.
- , , , . Comorbidity measures for use with administrative data. Med Care. 1998;36(1):8–27.
- , , , , , . Teamwork on inpatient medical units: assessing attitudes and barriers. Qual Saf Health Care. 2010;19(2):117–121.
- , , , et al. A model for quality improvement programs in academic departments of medicine. Am J Med. 2008;121(10):922–929.
- , , , , . Improving nurse‐physician communication and satisfaction in the intensive care unit with a daily goals worksheet. Am J Crit Care. 2006;15(2):217–222.
- , , , , , . Improving communication in the ICU using daily goals. J Crit Care. 2003;18(2):71–75.
- , , , et al. Effect of crew resource management training in a multidisciplinary obstetrical setting. Int J Qual Health Care. 2008;20(4):254–263.
- , , , et al. Error reduction and performance improvement in the emergency department through formal teamwork training: evaluation results of the MedTeams project. Health Serv Res. 2002;37(6):1553–1581.
- , , , et al. Effects of teamwork training on adverse outcomes and process of care in labor and delivery: a randomized controlled trial. Obstet Gynecol. 2007;109(1):48–55.
- , , , et al. Effects of interdisciplinary team care interventions on general medical wards: a systematic review. JAMA Intern Med. 2015;175(8):1288–1298.
- , , , et al. Patient risk factors for adverse drug events in hospitalized patients. ADE Prevention Study Group. Arch Intern Med. 1999;159(21):2553–2560.
- , , , et al. Effect of a pharmacist intervention on clinically important medication errors after hospital discharge: a randomized trial. Ann Intern Med. 2012;157(1):1–10.
- , , , . Use of a standardized protocol to decrease medication errors and adverse events related to sliding scale insulin. Qual Saf Health Care. 2006;15(2):89–91.
- , , , . Clinical pharmacists and inpatient medical care: a systematic review. Arch Intern Med. 2006;166(9):955–964.
- , , , , . Improved inpatient use of basal insulin, reduced hypoglycemia, and improved glycemic control: effect of structured subcutaneous insulin orders and an insulin management algorithm. J Hosp Med. 2009;4(1):3–15.
- , , , . Effects of a computerized order set on the inpatient management of hyperglycemia: a cluster‐randomized controlled trial. Endocr Pract. 2010;16(2):209–218.
- , , , . Effects of a subcutaneous insulin protocol, clinical education, and computerized order set on the quality of inpatient management of hyperglycemia: results of a clinical trial. J Hosp Med. 2009;4(1):16–27.
- Joint Commission on Accreditation of Healthcare Organizations. Understanding and Preventing Sentinel Events in Your Health Care Organization. Oak Brook, IL: Joint Commission; 2008.
- , , , et al. Communication failures in the operating room: an observational classification of recurrent types and effects. Qual Saf Health Care. 2004;13(5):330–334.
- , , , et al. Operating room teamwork among physicians and nurses: teamwork in the eye of the beholder. J Am Coll Surg. 2006;202(5):746–752.
- , , . Discrepant attitudes about teamwork among critical care nurses and physicians. Crit Care Med. 2003;31(3):956–959.
- , , , , . Communication failures in patient sign‐out and suggestions for improvement: a critical incident analysis. Qual Saf Health Care. 2005;14(6):401–407.
- , , , et al. Changes in medical errors after implementation of a handoff program. N Engl J Med. 2014;371(19):1803–1812.
- , , , et al. Communication and information deficits in patients discharged to rehabilitation facilities: an evaluation of five acute care hospitals. J Hosp Med. 2009;4(8):E28–E33.
- , , , , , . Deficits in communication and information transfer between hospital‐based and primary care physicians: implications for patient safety and continuity of care. JAMA. 2007;297(8):831–841.
- , , , et al. Patterns of nurse‐physician communication and agreement on the plan of care. Qual Saf Health Care. 2010;19(3):195–199.
- , , , , , . Can we talk? Priorities for patient care differed among health care providers. In: Henriksen K, Battles JB, Marks ES, Lewin DI, eds. Advances in Patient Safety: From Research to Implementation. Vol 1. Rockville, MD: Agency for Healthcare Research and Quality; 2005.
- , , , et al. Structured interdisciplinary rounds in a medical teaching unit: improving patient safety. Arch Intern Med. 2011;171(7):678–684.
- , , , , , . Improving teamwork: impact of structured interdisciplinary rounds on a hospitalist unit. J Hosp Med. 2011;6(2):88–93.
- , , , et al. Impact of localizing physicians to hospital units on nurse‐physician communication and agreement on the plan of care. J Gen Intern Med. 2009;24(11):1223–1227.
- , , , et al. Reorganizing a hospital ward as an accountable care unit. J Hosp Med. 2015;10(1):36–40.
- , , , et al. 5th time's a charm: creation of unit‐based care teams in a high occupancy hospital [abstract]. J Hosp Med. 2015;10 (suppl. 2). Available at: http://www.shmabstracts.com/abstract/5th‐times‐a‐charm‐creation‐of‐unit‐based‐care‐teams‐in‐a‐high‐occupancy‐hospital. Accessed July 28, 2015.
- , , , . Comorbidity measures for use with administrative data. Med Care. 1998;36(1):8–27.
- , , , , , . Teamwork on inpatient medical units: assessing attitudes and barriers. Qual Saf Health Care. 2010;19(2):117–121.
- , , , et al. A model for quality improvement programs in academic departments of medicine. Am J Med. 2008;121(10):922–929.
- , , , , . Improving nurse‐physician communication and satisfaction in the intensive care unit with a daily goals worksheet. Am J Crit Care. 2006;15(2):217–222.
- , , , , , . Improving communication in the ICU using daily goals. J Crit Care. 2003;18(2):71–75.
- , , , et al. Effect of crew resource management training in a multidisciplinary obstetrical setting. Int J Qual Health Care. 2008;20(4):254–263.
- , , , et al. Error reduction and performance improvement in the emergency department through formal teamwork training: evaluation results of the MedTeams project. Health Serv Res. 2002;37(6):1553–1581.
- , , , et al. Effects of teamwork training on adverse outcomes and process of care in labor and delivery: a randomized controlled trial. Obstet Gynecol. 2007;109(1):48–55.
- , , , et al. Effects of interdisciplinary team care interventions on general medical wards: a systematic review. JAMA Intern Med. 2015;175(8):1288–1298.
- , , , et al. Patient risk factors for adverse drug events in hospitalized patients. ADE Prevention Study Group. Arch Intern Med. 1999;159(21):2553–2560.
- , , , et al. Effect of a pharmacist intervention on clinically important medication errors after hospital discharge: a randomized trial. Ann Intern Med. 2012;157(1):1–10.
- , , , . Use of a standardized protocol to decrease medication errors and adverse events related to sliding scale insulin. Qual Saf Health Care. 2006;15(2):89–91.
- , , , . Clinical pharmacists and inpatient medical care: a systematic review. Arch Intern Med. 2006;166(9):955–964.
- , , , , . Improved inpatient use of basal insulin, reduced hypoglycemia, and improved glycemic control: effect of structured subcutaneous insulin orders and an insulin management algorithm. J Hosp Med. 2009;4(1):3–15.
- , , , . Effects of a computerized order set on the inpatient management of hyperglycemia: a cluster‐randomized controlled trial. Endocr Pract. 2010;16(2):209–218.
- , , , . Effects of a subcutaneous insulin protocol, clinical education, and computerized order set on the quality of inpatient management of hyperglycemia: results of a clinical trial. J Hosp Med. 2009;4(1):16–27.
Care Team Identification
Patient‐centered communication is a strategy that is used to promote shared understanding of the plan of care among providers and patients.[1, 2, 3] Caring for hospitalized patients is a collaborative effort that requires seamless patient‐centered communication among a rapidly changing care team to safely progress a patient from admission through discharge. Yet, hospitals continue to struggle with improving the complex and increasingly electronic conversation patterns among care team members and patients to achieve effective patient‐centered communication.[4, 5] When members of the care team operate in this environment, patients often receive conflicting information regarding their plan of care, medications, and test results. Ineffective communication can lead to a suboptimal patient experience, additional costs, medical errors, and preventable adverse events.[6, 7, 8, 9, 10]
A critical first step to improving patient‐centered communication is identifying the care team.[11, 12] Accurate and reliable identification of all care team members is a pressing information need; it is fundamental to efficiently conveying information about the plan of care to those who know the patient the best, must make timely decisions, or will assume care once the patient leaves the hospital.[13] Furthermore, it has implications for engaging patients more meaningfully in their care.[14, 15, 16, 17] Ideally, the process of identifying an individual caring for the patient in a specific role is quickly and reliably determined from the electronic health record, the single source of truth where any provider can quickly identify other team members. This source of truth can be updated manually when individual members assign and remove themselves from the care team, or automatically when accessing the patient's record, writing a note, placing an order, or adding a patient to a coverage list. When providers correctly identify other team members in this way, hospital paging directories and secure messaging tools that link to the electronic health record become more effective at supporting care team communication.[18]
In general, the process of identifying care teams is difficult,[19] and maintaining role assignments in the electronic health record is equally challenging. Vawdrey et al. previously reported that care team lists are inaccurate and cannot be used to reliably identify other members at any given moment.[18] The inability to identify team members often leads to incorrectly routed pages, e‐mail messages, and phone calls.[20] Consequently, the potential to reliably manage the care team and improve electronic communication remains untapped, rendering team collaboration and care coordination less effective.[18, 21, 22]
In recent years, the trend toward restructuring inpatient teamsgeographical localization, structured communication interventions, teamwork training, and interdisciplinary roundswould seem to diminish the need for electronic care team identification, as those efforts have already made a positive impact with regard to interprofessional communication and collaboration, team satisfaction, and adverse events.[23, 24, 25, 26] Nonetheless, interdisciplinary teamwork, though critically important for patient‐centered communication, does not completely obviate the need for accurate and reliable care team identification.[26] Although care teams are statically located on units, the plan of care is dynamic; it evolves when the patient's status changes, when new information becomes available, and when key longitudinal providers (eg, primary care physician, subspecialty consultant) make recommendations. Thus, information conveyed as a team on rounds quickly becomes out of date, requiring additional forms of communication. Furthermore, due to frequent ad hoc coverage among team members, the identity of providers covering the patient at any given moment is often not clear.[27] This is particularly problematic for nonunit‐based providers who try to communicate with unit‐based care team members. These providers, in particular, have valuable knowledge and insight that can aid the primary team in decision making.[28, 29] However, they typically do not participate in rounds, often waste time identifying responsible providers,[20] and may communicate their recommendations directly with the patient without discussing with the primary team. These factors in part explain why geographic localization has shown limited improvement in shared understanding of the plan of care.[23]
From the perspective of patients and caregivers, identification of the providers entering and leaving their room is also challenging; only 11% to 51% of patients identify their providers correctly.[30] This adds to confusion regarding who is responsible for which aspects of the patient's care and can negatively affect the perception of the quality of care received.[31] Use of whiteboards has been shown to improve the proportion of patients who could identify key providers,[32] but these are not reliably updated and generally cannot accommodate all team members. When face cards are used, patients and caregivers report that they are more likely to identify their providers correctly.[14, 33, 34] However, potential confusion may ensue when another provider assumes care of the patient in the same role. Finally, use of technology to display team members at the bedside is typically a feature that patients like and can improve identification of care team members.[14, 15, 16] Yet, patient engagement technologies are not readily available in the hospital setting,[35] and ideally should be linked to the electronic health record, which again must be reliably updated.[11, 12, 15, 16]
If care team identification is so critical for delivering effective patient‐centered communication, why is maintaining role assignment problematic? At the individual level, reasons include discontinuity of the care team due to changing clinical rotations and intrateam coverage, shift‐based schedules, and lack of awareness and underutilization of functionality. Additionally, clinicians may have different ways to maintain lists of patients. At the institutional level, functionality to enforce role assignment when accessing patient records may be disabled (to avoid perceived burdens on clinical staff or nonclinical personnel who require access for administrative functions). Finally, electronic health record vendors currently have no incentive to adopt functionality that supports more effective care coordination across settings.[22]
However, more than technical solutions and policy changes are required; care team identification in the electronic health record requires a change in institutional culture. Maintaining an accurate relationship to each patient requires work without tangible benefitsthe benefits accrue only when everyone else identifies their role on the teama tragedy of the commons. This can be illustrated by our own experience. We conducted a quality improvement initiative (Table 1) as a part of 2 concurrent research initiatives that serve to promote patient‐centered communication:[12] PCORI (Patient‐Centered Outcomes Research Institute Transitions), the goal of which is to improve care transitions within the Partners' Pioneer Accountable Care Organization; and the PROSPECT (Promoting Respect and Ongoing Safety Through Patient‐Centeredness, Engagement, Communication, and Technology) project, an initiative funded by the Gordon and Betty Moore Foundation to eliminate preventable harms in the acute care environment.[29] Our goal was to electronically manage the care team with a high degree of fidelity. We enhanced a home‐grown application, which was developed to improve management of team lists for inpatient providers, accessible from our electronic health record, to facilitate role assignment. Specifically, we leveraged existing care processes (eg, nursing log‐on to the electronic medication administration system) to automatically assign certain providers to the care team at change of shift, added functionality to make it easy to assign a provider to all patients on a list for a defined period of time, and encouraged providers to assign their role by demonstrating benefits including quick access to patient‐specific group e‐mail and secure messaging tools (Table 1, Key Facets). The initiative was well‐received by most disciplines, but uptake was suboptimal. Our research assistants routinely assigned residents and others to the care team because our proactive attempts at advertising and reinforcing use of the application failed to reach a critical mass. Most did not see immediate benefits because it was an added step to their busy day, had other methods of managing team lists, and only saw benefit if everyone else participated. Key facets of our care team identification initiative, successes, and challenges are outlined in Table 1.
| Key Facets | Successes | Challenges |
|---|---|---|
| Linked electronic role assignment to administrative processes and clinical workflows | Leveraged existing processes to identify attending provider by routinely reviewing online schedules Linked role assignment to electronic medication administration system sign‐in process for nurses at the start of their shift |
Difficult to generate buy‐in from administrators and specific clinician groups to incorporate routine use of role assignment functionality into existing and/or new workflows No institutional policy mandating role assignments for members of extended care team |
| Incorporated default functionality to specify length of role assignment (eg, stop date) | Used by trainees (residents, fellows) to automate team list role assignments for a prespecified period of time according to online schedules | Underutilized by subspecialty consultants, many of who were unaware or did not fully appreciate the added value of this functionality Research assistants regularly verified that default role assignments were accurately maintained for trainees |
| Linked role assignment to patient‐specific group e‐mail and messaging tools | Clinicians acknowledged clear efficiency benefits (eg, automated patient identification within messages, correct routing of e‐mails) Used by specific members of the care team tasked with facilitating coordination of care (eg, nurse practitioner trained as discharge advocate for research study) |
Difficult to promote use of patient‐specific messaging, particularly for nonunit‐based providers (eg, consultants, primary care physicians) Required access to an application not typically used for clinical messaging Difficult to change culture of network e‐mail use for clinical messaging |
| Advertised new functionality and demonstrated potential efficiencies for care team communication | Unit‐based clinicians (hospitalists, nurses, housestaff) typically understood benefits when demonstrated and were easier to engage | Some nonunit‐based clinicians (eg, consulting attendings, primary care physicians) did not see benefits and/or were difficult to engage |
| Some nonunit‐based provider groups (eg, social workers, nutritionists, subspecialty fellows) considered the initiative worthwhile, and were open to learning about new functionality to improve communication | Clinicians had several options for managing team lists prior to implementation of new electronic health record | |
| Institutional effort toward implementing new electronic health record detracted from efforts at demonstrating enhanced functionality of existing applications |
There were a few glimmers of hope, however. On several PROSPECT units, we displayed team members on a tablet‐based patient portal so that patients would recognize their providers.[11, 17, 36] Similar to recent work by O'Leary et al.,[14] patients on PROSPECT units were able to correctly identify several care team members, but regularly asked why other providers (eg, consulting fellow) were not listed. Those providers asked the same question, and some eventually learned to assign their role via the application. As part of PROSEPCT, we visited other institutions and learned of an effort to display team members on high‐definition televisions in the patient's room. Several providers, wondering why they were not listed, learned to assign their role and their picture then appeared. Social pressure was the driving force.
Coincidently, we recently implemented a new electronic health record at our institution. Anecdotally, although no formal policy was established, many providers (eg, attendings, first responders, nurses, care coordinators, and other unit‐based providers) appear to be assigning their roles. Other providers (eg, dieticians, physical therapists, residents) also assign their role, but often fail to end role assignments upon completing their rotation or when the patient transfers to another service. Finally, even when actively involved, most subspecialists still do not designate their role. Despite these gaps and inconsistencies, we have made progress toward improving care team identification. The reasons for this progress are straightforward; during required training for the new electronic health record, all inpatient providers were taught to assign their role on the treatment team when assuming care of patients and now have 1 option for managing team lists. However, most providers were not trained to end their role assignments, and many have learned that role assignment is not required to access the patient's record; functionality to enforce this was disabled. Based on lessons learned from our experience,[12] we offer several strategies that hospitalists can employ to improve care team identification in the electronic health record (Table 2).
| Goal | Strategies to Achieve Goal |
|---|---|
| Identify and/or establish reliable processes that administrative staff can use to ensure accurate care team role assignments | Identify databases that serve as the source of truth for provider schedules and routinely access those databases |
| Access resident scheduling application (eg, Amion) that is routinely updated by training program staff | |
| Work with clinical and administrative staff to maintain care team role assignments | |
| Engage affiliated ambulatory practices to ensure patient's primary care physician is updated in the electronic health record | |
| Engage admissions office to improve reliability of attending assignments based on online clinical schedules when patients are admitted | |
| Integrate role assignment into established workflows for specific provider groups when administrative processes not feasible | Link routine care processes to care team role assignment |
| Train nurses, interns, physician assistants to assign role on care team when assuming care of patient at shift change | |
| Train residents, fellows to use default functionality to automatically assign their role on care team at the beginning of a clinical rotation | |
| Demonstrate value of maintaining role assignments in the electronic health record to the unit‐based care team | Emphasize how accurate and reliable care team role assignment can facilitate correct routing of information (eg, test results, discharge summaries) |
| Helps to maintain patient coverage lists (eg, fellows, consultants, social workers) | |
| Facilitates patient‐specific communication (eg, via group email and messaging tools linked to the electronic health record's care team functionality) | |
| Align with concurrent institutional initiatives that enforce or incentivize care team role assignment | Mandate role assignment when writing a note, placing an order, or adding a patient to a coverage list in the electronic health record |
| Provide patients and caregivers the ability to identify the care team via patient portalcreates social pressure for those providers who do not identify themselves on the care team | |
| Incentivize providers to maintain role assignments during patient's hospitalization in order to receive notifications if patients are readmitted | |
| Automate role assignments for all members of the care team whenever possible | Work with clinical informatics/emnformation system staff to determine feasibility of linking online scheduling systems or log‐in process to other systems routinely accessed by specific providers to automatically assign/unassign specific providers at the beginning/end of a shift (eg, nurses automatically assigned to care team when they access the electronic medication administration record system at beginning of shift) |
| Explore availability of default functionality to assign and unassign providers to and from the care team in a specific role by team, service, or unit‐based patient lists | |
| Require a stop time/date for role assignments or set a default if none entered |
In the future, care team identification in the electronic health record can be automated by integrating directly with electronic workflows, online scheduling applications, and provider directories. Hospitals could then leverage care team lists to facilitate patient‐centered communication via secure web‐based and mobile messaging applications configured to simultaneously update all team members (eg, group messaging apps, microblogs).[11, 37, 38] By synchronizing with the electronic health record, role assignments can be automatically updated via these applications, further increasing fidelity of care team identification.[12] Finally, as hospitals implement acute care patient portals, team lists can be leveraged to display all care team members correctly so that patients and caregivers can communicate more easily with providers.[17] The potential ramifications for patient‐centered communication are tremendous.
Disclosures
This work was funded by the Patient‐Centered Outcomes Research Institute and the Gordon and Betty Moore Foundation (GBMF3914). The authors report no conflicts of interest.
- . Patient‐centered communication. Annu Rev Nurs Res. 1999;17:85–104.
- , , , . Facilitating patient‐centered cancer communication: a road map. Patient Educ Couns. 2009;77:319–321.
- , . Patient‐Centered Communication in Cancer Care: Promoting Healing and Reducing Suffering. NIH Publication No. 07–6225. Bethesda, MD: National Cancer Institute; 2007.
- . When conversation is better than computation. J Am Med Inform Assoc. 2000;7:277–286.
- . Communication systems in healthcare. Clin Biochem Rev. 2006;27:89–98.
- , , , et al. The nature of adverse events in hospitalized patients. results of the Harvard Medical Practice Study II. N Engl J Med. 1991;324:377–384.
- , , , et al. A look into the nature and causes of human errors in the intensive care unit. Crit Care Med. 1995;23:294–300.
- , , . Communication failures: an insidious contributor to medical mishaps. Acad Med. 2004;79:186–194.
- , . Interdisciplinary communication: an uncharted source of medical error? J Crit Care. 2006;21:236–242; discussion 242.
- , , . Quantifying the economic impact of communication inefficiencies in U.S. hospitals. J Healthc Manag. 2010;55:265–281; discussion 281–282.
- , , , . Transforming the acute care environment: a web‐based patient‐centered toolkit [abstract]. J Hosp Med. 2014;9(suppl 2):694.
- , , , et al. Creating a culture of patient‐centered care team communication at a large academic medical center [Abstract]. J Hosp Med. 2015;10 (suppl 2). Available at: http://www.shmabstracts.com/abstract/creating‐a‐culture‐of‐patient‐centered‐care‐team‐communication‐at‐a‐large‐academic‐medical‐center. Accessed April 24, 2015.
- , , , , . Perceived information needs and communication difficulties of inpatient physicians and nurses. Proc AMIA Symp. 2001:453–457.
- , , , , , . The effect of tablet computers with a mobile patient portal application on hospitalized patients' knowledge and activation [published online June 15, 2015]. J Am Med Inform Assoc. doi: 10.1093/jamia/ocv058.
- , , , , . Bedside information technology to support patient‐centered care. Int J Med Inform. 2012;81(7):442–451.
- , , , et al. Building and testing a patient‐centric electronic bedside communication center. J Gerontol Nurs. 2013;39:15–19.
- , , , et al. A web‐based, patient‐centered toolkit to engage patients and caregivers in the acute care setting: a preliminary evaluation [published online August 2, 2015]. J Am Med Inform Assoc. doi: 10.1093/jamia/ocv093.
- , , , et al. Awareness of the care team in electronic health records. Appl Clin Inform. 2011;2:395–405.
- , , , , , . Teamwork on inpatient medical units: assessing attitudes and barriers. Qual Saf Health Care. 2010;19:117–121.
- , , , et al. Frequency and clinical importance of pages sent to the wrong physician. Arch Intern Med. 2009;169:1072–1073.
- , . Patient experiences with coordination of care: the benefit of continuity and primary care physician as referral source. J Gen Intern Med. 2009;24:170–177.
- , , , , . Are electronic medical records helpful for care coordination? Experiences of physician practices. J Gen Intern Med. 2010;25:177–185.
- , , , et al. Impact of localizing physicians to hospital units on nurse‐physician communication and agreement on the plan of care. J Gen Intern Med. 2009;24:1223–1227.
- , , , , , . Improving teamwork: impact of structured interdisciplinary rounds on a hospitalist unit. J Hosp Med. 2011;6:88–93.
- , , , et al. Structured interdisciplinary rounds in a medical teaching unit: improving patient safety. Arch Intern Med. 2011;171:678–684.
- , , , ; High Performance Teams and the Hospital of the Future Project Team. Interdisciplinary teamwork in hospitals: a review and practical recommendations for improvement. J Hosp Med. 2012;7:48–54.
- , , . Intrateam coverage is common, intrateam handoffs are not. J Hosp Med. 2014;9:734–736.
- . A primary care physician's ideal transitions of care? Where's the evidence? J Hosp Med. 2013;8:472–477.
- , , , , . Primary care physician communication at hospital discharge reduces medication discrepancies. J Hosp Med. 2013;8:672–677.
- , . Let's “face” it: time to introduce yourself to patients. J Hosp Med. 2014;9:199–200.
- , , . Patient perceptions of coordinated care: the importance of organized communication in hospitals. J Healthc Qual. 1999;21:18–23.
- , , , . Patient whiteboards to improve patient‐centred care in the hospital. Postgrad Med J. 2013;89:604–609.
- , , , , , . Effect of a face sheet tool on medical team provider identification and family satisfaction. J Hosp Med. 2014;9:186–188.
- , , , , , . The impact of facecards on patients' knowledge, satisfaction, trust, and agreement with hospital physicians: a pilot study. J Hosp Med. 2014;9:137–141.
- , , , et al. Patient engagement in the inpatient setting: a systematic review. J Am Med Inform Assoc. 2014;21:742–750.
- PROSPECT: Promoting Respect and Ongoing Safety Through Patient‐centeredness, Engagement, Communication, and Technology. Available at: http://www.partners.org/cird/PROSPECT/Index.htm. Accessed May 3, 2015.
- , , , , , . Smarter hospital communication: secure smartphone text messaging improves provider satisfaction and perception of efficacy, workflow. J Hosp Med. 2014;9:573–578.
- , , , et al. Engaging patients, providers, and institutional stakeholders in developing a patient‐centered microblog. Paper presented at: Proceeding of the American Medical Informatics Association Annual Fall Symposium; November 16–19, 2014; Washington, DC.
Patient‐centered communication is a strategy that is used to promote shared understanding of the plan of care among providers and patients.[1, 2, 3] Caring for hospitalized patients is a collaborative effort that requires seamless patient‐centered communication among a rapidly changing care team to safely progress a patient from admission through discharge. Yet, hospitals continue to struggle with improving the complex and increasingly electronic conversation patterns among care team members and patients to achieve effective patient‐centered communication.[4, 5] When members of the care team operate in this environment, patients often receive conflicting information regarding their plan of care, medications, and test results. Ineffective communication can lead to a suboptimal patient experience, additional costs, medical errors, and preventable adverse events.[6, 7, 8, 9, 10]
A critical first step to improving patient‐centered communication is identifying the care team.[11, 12] Accurate and reliable identification of all care team members is a pressing information need; it is fundamental to efficiently conveying information about the plan of care to those who know the patient the best, must make timely decisions, or will assume care once the patient leaves the hospital.[13] Furthermore, it has implications for engaging patients more meaningfully in their care.[14, 15, 16, 17] Ideally, the process of identifying an individual caring for the patient in a specific role is quickly and reliably determined from the electronic health record, the single source of truth where any provider can quickly identify other team members. This source of truth can be updated manually when individual members assign and remove themselves from the care team, or automatically when accessing the patient's record, writing a note, placing an order, or adding a patient to a coverage list. When providers correctly identify other team members in this way, hospital paging directories and secure messaging tools that link to the electronic health record become more effective at supporting care team communication.[18]
In general, the process of identifying care teams is difficult,[19] and maintaining role assignments in the electronic health record is equally challenging. Vawdrey et al. previously reported that care team lists are inaccurate and cannot be used to reliably identify other members at any given moment.[18] The inability to identify team members often leads to incorrectly routed pages, e‐mail messages, and phone calls.[20] Consequently, the potential to reliably manage the care team and improve electronic communication remains untapped, rendering team collaboration and care coordination less effective.[18, 21, 22]
In recent years, the trend toward restructuring inpatient teamsgeographical localization, structured communication interventions, teamwork training, and interdisciplinary roundswould seem to diminish the need for electronic care team identification, as those efforts have already made a positive impact with regard to interprofessional communication and collaboration, team satisfaction, and adverse events.[23, 24, 25, 26] Nonetheless, interdisciplinary teamwork, though critically important for patient‐centered communication, does not completely obviate the need for accurate and reliable care team identification.[26] Although care teams are statically located on units, the plan of care is dynamic; it evolves when the patient's status changes, when new information becomes available, and when key longitudinal providers (eg, primary care physician, subspecialty consultant) make recommendations. Thus, information conveyed as a team on rounds quickly becomes out of date, requiring additional forms of communication. Furthermore, due to frequent ad hoc coverage among team members, the identity of providers covering the patient at any given moment is often not clear.[27] This is particularly problematic for nonunit‐based providers who try to communicate with unit‐based care team members. These providers, in particular, have valuable knowledge and insight that can aid the primary team in decision making.[28, 29] However, they typically do not participate in rounds, often waste time identifying responsible providers,[20] and may communicate their recommendations directly with the patient without discussing with the primary team. These factors in part explain why geographic localization has shown limited improvement in shared understanding of the plan of care.[23]
From the perspective of patients and caregivers, identification of the providers entering and leaving their room is also challenging; only 11% to 51% of patients identify their providers correctly.[30] This adds to confusion regarding who is responsible for which aspects of the patient's care and can negatively affect the perception of the quality of care received.[31] Use of whiteboards has been shown to improve the proportion of patients who could identify key providers,[32] but these are not reliably updated and generally cannot accommodate all team members. When face cards are used, patients and caregivers report that they are more likely to identify their providers correctly.[14, 33, 34] However, potential confusion may ensue when another provider assumes care of the patient in the same role. Finally, use of technology to display team members at the bedside is typically a feature that patients like and can improve identification of care team members.[14, 15, 16] Yet, patient engagement technologies are not readily available in the hospital setting,[35] and ideally should be linked to the electronic health record, which again must be reliably updated.[11, 12, 15, 16]
If care team identification is so critical for delivering effective patient‐centered communication, why is maintaining role assignment problematic? At the individual level, reasons include discontinuity of the care team due to changing clinical rotations and intrateam coverage, shift‐based schedules, and lack of awareness and underutilization of functionality. Additionally, clinicians may have different ways to maintain lists of patients. At the institutional level, functionality to enforce role assignment when accessing patient records may be disabled (to avoid perceived burdens on clinical staff or nonclinical personnel who require access for administrative functions). Finally, electronic health record vendors currently have no incentive to adopt functionality that supports more effective care coordination across settings.[22]
However, more than technical solutions and policy changes are required; care team identification in the electronic health record requires a change in institutional culture. Maintaining an accurate relationship to each patient requires work without tangible benefitsthe benefits accrue only when everyone else identifies their role on the teama tragedy of the commons. This can be illustrated by our own experience. We conducted a quality improvement initiative (Table 1) as a part of 2 concurrent research initiatives that serve to promote patient‐centered communication:[12] PCORI (Patient‐Centered Outcomes Research Institute Transitions), the goal of which is to improve care transitions within the Partners' Pioneer Accountable Care Organization; and the PROSPECT (Promoting Respect and Ongoing Safety Through Patient‐Centeredness, Engagement, Communication, and Technology) project, an initiative funded by the Gordon and Betty Moore Foundation to eliminate preventable harms in the acute care environment.[29] Our goal was to electronically manage the care team with a high degree of fidelity. We enhanced a home‐grown application, which was developed to improve management of team lists for inpatient providers, accessible from our electronic health record, to facilitate role assignment. Specifically, we leveraged existing care processes (eg, nursing log‐on to the electronic medication administration system) to automatically assign certain providers to the care team at change of shift, added functionality to make it easy to assign a provider to all patients on a list for a defined period of time, and encouraged providers to assign their role by demonstrating benefits including quick access to patient‐specific group e‐mail and secure messaging tools (Table 1, Key Facets). The initiative was well‐received by most disciplines, but uptake was suboptimal. Our research assistants routinely assigned residents and others to the care team because our proactive attempts at advertising and reinforcing use of the application failed to reach a critical mass. Most did not see immediate benefits because it was an added step to their busy day, had other methods of managing team lists, and only saw benefit if everyone else participated. Key facets of our care team identification initiative, successes, and challenges are outlined in Table 1.
| Key Facets | Successes | Challenges |
|---|---|---|
| Linked electronic role assignment to administrative processes and clinical workflows | Leveraged existing processes to identify attending provider by routinely reviewing online schedules Linked role assignment to electronic medication administration system sign‐in process for nurses at the start of their shift |
Difficult to generate buy‐in from administrators and specific clinician groups to incorporate routine use of role assignment functionality into existing and/or new workflows No institutional policy mandating role assignments for members of extended care team |
| Incorporated default functionality to specify length of role assignment (eg, stop date) | Used by trainees (residents, fellows) to automate team list role assignments for a prespecified period of time according to online schedules | Underutilized by subspecialty consultants, many of who were unaware or did not fully appreciate the added value of this functionality Research assistants regularly verified that default role assignments were accurately maintained for trainees |
| Linked role assignment to patient‐specific group e‐mail and messaging tools | Clinicians acknowledged clear efficiency benefits (eg, automated patient identification within messages, correct routing of e‐mails) Used by specific members of the care team tasked with facilitating coordination of care (eg, nurse practitioner trained as discharge advocate for research study) |
Difficult to promote use of patient‐specific messaging, particularly for nonunit‐based providers (eg, consultants, primary care physicians) Required access to an application not typically used for clinical messaging Difficult to change culture of network e‐mail use for clinical messaging |
| Advertised new functionality and demonstrated potential efficiencies for care team communication | Unit‐based clinicians (hospitalists, nurses, housestaff) typically understood benefits when demonstrated and were easier to engage | Some nonunit‐based clinicians (eg, consulting attendings, primary care physicians) did not see benefits and/or were difficult to engage |
| Some nonunit‐based provider groups (eg, social workers, nutritionists, subspecialty fellows) considered the initiative worthwhile, and were open to learning about new functionality to improve communication | Clinicians had several options for managing team lists prior to implementation of new electronic health record | |
| Institutional effort toward implementing new electronic health record detracted from efforts at demonstrating enhanced functionality of existing applications |
There were a few glimmers of hope, however. On several PROSPECT units, we displayed team members on a tablet‐based patient portal so that patients would recognize their providers.[11, 17, 36] Similar to recent work by O'Leary et al.,[14] patients on PROSPECT units were able to correctly identify several care team members, but regularly asked why other providers (eg, consulting fellow) were not listed. Those providers asked the same question, and some eventually learned to assign their role via the application. As part of PROSEPCT, we visited other institutions and learned of an effort to display team members on high‐definition televisions in the patient's room. Several providers, wondering why they were not listed, learned to assign their role and their picture then appeared. Social pressure was the driving force.
Coincidently, we recently implemented a new electronic health record at our institution. Anecdotally, although no formal policy was established, many providers (eg, attendings, first responders, nurses, care coordinators, and other unit‐based providers) appear to be assigning their roles. Other providers (eg, dieticians, physical therapists, residents) also assign their role, but often fail to end role assignments upon completing their rotation or when the patient transfers to another service. Finally, even when actively involved, most subspecialists still do not designate their role. Despite these gaps and inconsistencies, we have made progress toward improving care team identification. The reasons for this progress are straightforward; during required training for the new electronic health record, all inpatient providers were taught to assign their role on the treatment team when assuming care of patients and now have 1 option for managing team lists. However, most providers were not trained to end their role assignments, and many have learned that role assignment is not required to access the patient's record; functionality to enforce this was disabled. Based on lessons learned from our experience,[12] we offer several strategies that hospitalists can employ to improve care team identification in the electronic health record (Table 2).
| Goal | Strategies to Achieve Goal |
|---|---|
| Identify and/or establish reliable processes that administrative staff can use to ensure accurate care team role assignments | Identify databases that serve as the source of truth for provider schedules and routinely access those databases |
| Access resident scheduling application (eg, Amion) that is routinely updated by training program staff | |
| Work with clinical and administrative staff to maintain care team role assignments | |
| Engage affiliated ambulatory practices to ensure patient's primary care physician is updated in the electronic health record | |
| Engage admissions office to improve reliability of attending assignments based on online clinical schedules when patients are admitted | |
| Integrate role assignment into established workflows for specific provider groups when administrative processes not feasible | Link routine care processes to care team role assignment |
| Train nurses, interns, physician assistants to assign role on care team when assuming care of patient at shift change | |
| Train residents, fellows to use default functionality to automatically assign their role on care team at the beginning of a clinical rotation | |
| Demonstrate value of maintaining role assignments in the electronic health record to the unit‐based care team | Emphasize how accurate and reliable care team role assignment can facilitate correct routing of information (eg, test results, discharge summaries) |
| Helps to maintain patient coverage lists (eg, fellows, consultants, social workers) | |
| Facilitates patient‐specific communication (eg, via group email and messaging tools linked to the electronic health record's care team functionality) | |
| Align with concurrent institutional initiatives that enforce or incentivize care team role assignment | Mandate role assignment when writing a note, placing an order, or adding a patient to a coverage list in the electronic health record |
| Provide patients and caregivers the ability to identify the care team via patient portalcreates social pressure for those providers who do not identify themselves on the care team | |
| Incentivize providers to maintain role assignments during patient's hospitalization in order to receive notifications if patients are readmitted | |
| Automate role assignments for all members of the care team whenever possible | Work with clinical informatics/emnformation system staff to determine feasibility of linking online scheduling systems or log‐in process to other systems routinely accessed by specific providers to automatically assign/unassign specific providers at the beginning/end of a shift (eg, nurses automatically assigned to care team when they access the electronic medication administration record system at beginning of shift) |
| Explore availability of default functionality to assign and unassign providers to and from the care team in a specific role by team, service, or unit‐based patient lists | |
| Require a stop time/date for role assignments or set a default if none entered |
In the future, care team identification in the electronic health record can be automated by integrating directly with electronic workflows, online scheduling applications, and provider directories. Hospitals could then leverage care team lists to facilitate patient‐centered communication via secure web‐based and mobile messaging applications configured to simultaneously update all team members (eg, group messaging apps, microblogs).[11, 37, 38] By synchronizing with the electronic health record, role assignments can be automatically updated via these applications, further increasing fidelity of care team identification.[12] Finally, as hospitals implement acute care patient portals, team lists can be leveraged to display all care team members correctly so that patients and caregivers can communicate more easily with providers.[17] The potential ramifications for patient‐centered communication are tremendous.
Disclosures
This work was funded by the Patient‐Centered Outcomes Research Institute and the Gordon and Betty Moore Foundation (GBMF3914). The authors report no conflicts of interest.
Patient‐centered communication is a strategy that is used to promote shared understanding of the plan of care among providers and patients.[1, 2, 3] Caring for hospitalized patients is a collaborative effort that requires seamless patient‐centered communication among a rapidly changing care team to safely progress a patient from admission through discharge. Yet, hospitals continue to struggle with improving the complex and increasingly electronic conversation patterns among care team members and patients to achieve effective patient‐centered communication.[4, 5] When members of the care team operate in this environment, patients often receive conflicting information regarding their plan of care, medications, and test results. Ineffective communication can lead to a suboptimal patient experience, additional costs, medical errors, and preventable adverse events.[6, 7, 8, 9, 10]
A critical first step to improving patient‐centered communication is identifying the care team.[11, 12] Accurate and reliable identification of all care team members is a pressing information need; it is fundamental to efficiently conveying information about the plan of care to those who know the patient the best, must make timely decisions, or will assume care once the patient leaves the hospital.[13] Furthermore, it has implications for engaging patients more meaningfully in their care.[14, 15, 16, 17] Ideally, the process of identifying an individual caring for the patient in a specific role is quickly and reliably determined from the electronic health record, the single source of truth where any provider can quickly identify other team members. This source of truth can be updated manually when individual members assign and remove themselves from the care team, or automatically when accessing the patient's record, writing a note, placing an order, or adding a patient to a coverage list. When providers correctly identify other team members in this way, hospital paging directories and secure messaging tools that link to the electronic health record become more effective at supporting care team communication.[18]
In general, the process of identifying care teams is difficult,[19] and maintaining role assignments in the electronic health record is equally challenging. Vawdrey et al. previously reported that care team lists are inaccurate and cannot be used to reliably identify other members at any given moment.[18] The inability to identify team members often leads to incorrectly routed pages, e‐mail messages, and phone calls.[20] Consequently, the potential to reliably manage the care team and improve electronic communication remains untapped, rendering team collaboration and care coordination less effective.[18, 21, 22]
In recent years, the trend toward restructuring inpatient teamsgeographical localization, structured communication interventions, teamwork training, and interdisciplinary roundswould seem to diminish the need for electronic care team identification, as those efforts have already made a positive impact with regard to interprofessional communication and collaboration, team satisfaction, and adverse events.[23, 24, 25, 26] Nonetheless, interdisciplinary teamwork, though critically important for patient‐centered communication, does not completely obviate the need for accurate and reliable care team identification.[26] Although care teams are statically located on units, the plan of care is dynamic; it evolves when the patient's status changes, when new information becomes available, and when key longitudinal providers (eg, primary care physician, subspecialty consultant) make recommendations. Thus, information conveyed as a team on rounds quickly becomes out of date, requiring additional forms of communication. Furthermore, due to frequent ad hoc coverage among team members, the identity of providers covering the patient at any given moment is often not clear.[27] This is particularly problematic for nonunit‐based providers who try to communicate with unit‐based care team members. These providers, in particular, have valuable knowledge and insight that can aid the primary team in decision making.[28, 29] However, they typically do not participate in rounds, often waste time identifying responsible providers,[20] and may communicate their recommendations directly with the patient without discussing with the primary team. These factors in part explain why geographic localization has shown limited improvement in shared understanding of the plan of care.[23]
From the perspective of patients and caregivers, identification of the providers entering and leaving their room is also challenging; only 11% to 51% of patients identify their providers correctly.[30] This adds to confusion regarding who is responsible for which aspects of the patient's care and can negatively affect the perception of the quality of care received.[31] Use of whiteboards has been shown to improve the proportion of patients who could identify key providers,[32] but these are not reliably updated and generally cannot accommodate all team members. When face cards are used, patients and caregivers report that they are more likely to identify their providers correctly.[14, 33, 34] However, potential confusion may ensue when another provider assumes care of the patient in the same role. Finally, use of technology to display team members at the bedside is typically a feature that patients like and can improve identification of care team members.[14, 15, 16] Yet, patient engagement technologies are not readily available in the hospital setting,[35] and ideally should be linked to the electronic health record, which again must be reliably updated.[11, 12, 15, 16]
If care team identification is so critical for delivering effective patient‐centered communication, why is maintaining role assignment problematic? At the individual level, reasons include discontinuity of the care team due to changing clinical rotations and intrateam coverage, shift‐based schedules, and lack of awareness and underutilization of functionality. Additionally, clinicians may have different ways to maintain lists of patients. At the institutional level, functionality to enforce role assignment when accessing patient records may be disabled (to avoid perceived burdens on clinical staff or nonclinical personnel who require access for administrative functions). Finally, electronic health record vendors currently have no incentive to adopt functionality that supports more effective care coordination across settings.[22]
However, more than technical solutions and policy changes are required; care team identification in the electronic health record requires a change in institutional culture. Maintaining an accurate relationship to each patient requires work without tangible benefitsthe benefits accrue only when everyone else identifies their role on the teama tragedy of the commons. This can be illustrated by our own experience. We conducted a quality improvement initiative (Table 1) as a part of 2 concurrent research initiatives that serve to promote patient‐centered communication:[12] PCORI (Patient‐Centered Outcomes Research Institute Transitions), the goal of which is to improve care transitions within the Partners' Pioneer Accountable Care Organization; and the PROSPECT (Promoting Respect and Ongoing Safety Through Patient‐Centeredness, Engagement, Communication, and Technology) project, an initiative funded by the Gordon and Betty Moore Foundation to eliminate preventable harms in the acute care environment.[29] Our goal was to electronically manage the care team with a high degree of fidelity. We enhanced a home‐grown application, which was developed to improve management of team lists for inpatient providers, accessible from our electronic health record, to facilitate role assignment. Specifically, we leveraged existing care processes (eg, nursing log‐on to the electronic medication administration system) to automatically assign certain providers to the care team at change of shift, added functionality to make it easy to assign a provider to all patients on a list for a defined period of time, and encouraged providers to assign their role by demonstrating benefits including quick access to patient‐specific group e‐mail and secure messaging tools (Table 1, Key Facets). The initiative was well‐received by most disciplines, but uptake was suboptimal. Our research assistants routinely assigned residents and others to the care team because our proactive attempts at advertising and reinforcing use of the application failed to reach a critical mass. Most did not see immediate benefits because it was an added step to their busy day, had other methods of managing team lists, and only saw benefit if everyone else participated. Key facets of our care team identification initiative, successes, and challenges are outlined in Table 1.
| Key Facets | Successes | Challenges |
|---|---|---|
| Linked electronic role assignment to administrative processes and clinical workflows | Leveraged existing processes to identify attending provider by routinely reviewing online schedules Linked role assignment to electronic medication administration system sign‐in process for nurses at the start of their shift |
Difficult to generate buy‐in from administrators and specific clinician groups to incorporate routine use of role assignment functionality into existing and/or new workflows No institutional policy mandating role assignments for members of extended care team |
| Incorporated default functionality to specify length of role assignment (eg, stop date) | Used by trainees (residents, fellows) to automate team list role assignments for a prespecified period of time according to online schedules | Underutilized by subspecialty consultants, many of who were unaware or did not fully appreciate the added value of this functionality Research assistants regularly verified that default role assignments were accurately maintained for trainees |
| Linked role assignment to patient‐specific group e‐mail and messaging tools | Clinicians acknowledged clear efficiency benefits (eg, automated patient identification within messages, correct routing of e‐mails) Used by specific members of the care team tasked with facilitating coordination of care (eg, nurse practitioner trained as discharge advocate for research study) |
Difficult to promote use of patient‐specific messaging, particularly for nonunit‐based providers (eg, consultants, primary care physicians) Required access to an application not typically used for clinical messaging Difficult to change culture of network e‐mail use for clinical messaging |
| Advertised new functionality and demonstrated potential efficiencies for care team communication | Unit‐based clinicians (hospitalists, nurses, housestaff) typically understood benefits when demonstrated and were easier to engage | Some nonunit‐based clinicians (eg, consulting attendings, primary care physicians) did not see benefits and/or were difficult to engage |
| Some nonunit‐based provider groups (eg, social workers, nutritionists, subspecialty fellows) considered the initiative worthwhile, and were open to learning about new functionality to improve communication | Clinicians had several options for managing team lists prior to implementation of new electronic health record | |
| Institutional effort toward implementing new electronic health record detracted from efforts at demonstrating enhanced functionality of existing applications |
There were a few glimmers of hope, however. On several PROSPECT units, we displayed team members on a tablet‐based patient portal so that patients would recognize their providers.[11, 17, 36] Similar to recent work by O'Leary et al.,[14] patients on PROSPECT units were able to correctly identify several care team members, but regularly asked why other providers (eg, consulting fellow) were not listed. Those providers asked the same question, and some eventually learned to assign their role via the application. As part of PROSEPCT, we visited other institutions and learned of an effort to display team members on high‐definition televisions in the patient's room. Several providers, wondering why they were not listed, learned to assign their role and their picture then appeared. Social pressure was the driving force.
Coincidently, we recently implemented a new electronic health record at our institution. Anecdotally, although no formal policy was established, many providers (eg, attendings, first responders, nurses, care coordinators, and other unit‐based providers) appear to be assigning their roles. Other providers (eg, dieticians, physical therapists, residents) also assign their role, but often fail to end role assignments upon completing their rotation or when the patient transfers to another service. Finally, even when actively involved, most subspecialists still do not designate their role. Despite these gaps and inconsistencies, we have made progress toward improving care team identification. The reasons for this progress are straightforward; during required training for the new electronic health record, all inpatient providers were taught to assign their role on the treatment team when assuming care of patients and now have 1 option for managing team lists. However, most providers were not trained to end their role assignments, and many have learned that role assignment is not required to access the patient's record; functionality to enforce this was disabled. Based on lessons learned from our experience,[12] we offer several strategies that hospitalists can employ to improve care team identification in the electronic health record (Table 2).
| Goal | Strategies to Achieve Goal |
|---|---|
| Identify and/or establish reliable processes that administrative staff can use to ensure accurate care team role assignments | Identify databases that serve as the source of truth for provider schedules and routinely access those databases |
| Access resident scheduling application (eg, Amion) that is routinely updated by training program staff | |
| Work with clinical and administrative staff to maintain care team role assignments | |
| Engage affiliated ambulatory practices to ensure patient's primary care physician is updated in the electronic health record | |
| Engage admissions office to improve reliability of attending assignments based on online clinical schedules when patients are admitted | |
| Integrate role assignment into established workflows for specific provider groups when administrative processes not feasible | Link routine care processes to care team role assignment |
| Train nurses, interns, physician assistants to assign role on care team when assuming care of patient at shift change | |
| Train residents, fellows to use default functionality to automatically assign their role on care team at the beginning of a clinical rotation | |
| Demonstrate value of maintaining role assignments in the electronic health record to the unit‐based care team | Emphasize how accurate and reliable care team role assignment can facilitate correct routing of information (eg, test results, discharge summaries) |
| Helps to maintain patient coverage lists (eg, fellows, consultants, social workers) | |
| Facilitates patient‐specific communication (eg, via group email and messaging tools linked to the electronic health record's care team functionality) | |
| Align with concurrent institutional initiatives that enforce or incentivize care team role assignment | Mandate role assignment when writing a note, placing an order, or adding a patient to a coverage list in the electronic health record |
| Provide patients and caregivers the ability to identify the care team via patient portalcreates social pressure for those providers who do not identify themselves on the care team | |
| Incentivize providers to maintain role assignments during patient's hospitalization in order to receive notifications if patients are readmitted | |
| Automate role assignments for all members of the care team whenever possible | Work with clinical informatics/emnformation system staff to determine feasibility of linking online scheduling systems or log‐in process to other systems routinely accessed by specific providers to automatically assign/unassign specific providers at the beginning/end of a shift (eg, nurses automatically assigned to care team when they access the electronic medication administration record system at beginning of shift) |
| Explore availability of default functionality to assign and unassign providers to and from the care team in a specific role by team, service, or unit‐based patient lists | |
| Require a stop time/date for role assignments or set a default if none entered |
In the future, care team identification in the electronic health record can be automated by integrating directly with electronic workflows, online scheduling applications, and provider directories. Hospitals could then leverage care team lists to facilitate patient‐centered communication via secure web‐based and mobile messaging applications configured to simultaneously update all team members (eg, group messaging apps, microblogs).[11, 37, 38] By synchronizing with the electronic health record, role assignments can be automatically updated via these applications, further increasing fidelity of care team identification.[12] Finally, as hospitals implement acute care patient portals, team lists can be leveraged to display all care team members correctly so that patients and caregivers can communicate more easily with providers.[17] The potential ramifications for patient‐centered communication are tremendous.
Disclosures
This work was funded by the Patient‐Centered Outcomes Research Institute and the Gordon and Betty Moore Foundation (GBMF3914). The authors report no conflicts of interest.
- . Patient‐centered communication. Annu Rev Nurs Res. 1999;17:85–104.
- , , , . Facilitating patient‐centered cancer communication: a road map. Patient Educ Couns. 2009;77:319–321.
- , . Patient‐Centered Communication in Cancer Care: Promoting Healing and Reducing Suffering. NIH Publication No. 07–6225. Bethesda, MD: National Cancer Institute; 2007.
- . When conversation is better than computation. J Am Med Inform Assoc. 2000;7:277–286.
- . Communication systems in healthcare. Clin Biochem Rev. 2006;27:89–98.
- , , , et al. The nature of adverse events in hospitalized patients. results of the Harvard Medical Practice Study II. N Engl J Med. 1991;324:377–384.
- , , , et al. A look into the nature and causes of human errors in the intensive care unit. Crit Care Med. 1995;23:294–300.
- , , . Communication failures: an insidious contributor to medical mishaps. Acad Med. 2004;79:186–194.
- , . Interdisciplinary communication: an uncharted source of medical error? J Crit Care. 2006;21:236–242; discussion 242.
- , , . Quantifying the economic impact of communication inefficiencies in U.S. hospitals. J Healthc Manag. 2010;55:265–281; discussion 281–282.
- , , , . Transforming the acute care environment: a web‐based patient‐centered toolkit [abstract]. J Hosp Med. 2014;9(suppl 2):694.
- , , , et al. Creating a culture of patient‐centered care team communication at a large academic medical center [Abstract]. J Hosp Med. 2015;10 (suppl 2). Available at: http://www.shmabstracts.com/abstract/creating‐a‐culture‐of‐patient‐centered‐care‐team‐communication‐at‐a‐large‐academic‐medical‐center. Accessed April 24, 2015.
- , , , , . Perceived information needs and communication difficulties of inpatient physicians and nurses. Proc AMIA Symp. 2001:453–457.
- , , , , , . The effect of tablet computers with a mobile patient portal application on hospitalized patients' knowledge and activation [published online June 15, 2015]. J Am Med Inform Assoc. doi: 10.1093/jamia/ocv058.
- , , , , . Bedside information technology to support patient‐centered care. Int J Med Inform. 2012;81(7):442–451.
- , , , et al. Building and testing a patient‐centric electronic bedside communication center. J Gerontol Nurs. 2013;39:15–19.
- , , , et al. A web‐based, patient‐centered toolkit to engage patients and caregivers in the acute care setting: a preliminary evaluation [published online August 2, 2015]. J Am Med Inform Assoc. doi: 10.1093/jamia/ocv093.
- , , , et al. Awareness of the care team in electronic health records. Appl Clin Inform. 2011;2:395–405.
- , , , , , . Teamwork on inpatient medical units: assessing attitudes and barriers. Qual Saf Health Care. 2010;19:117–121.
- , , , et al. Frequency and clinical importance of pages sent to the wrong physician. Arch Intern Med. 2009;169:1072–1073.
- , . Patient experiences with coordination of care: the benefit of continuity and primary care physician as referral source. J Gen Intern Med. 2009;24:170–177.
- , , , , . Are electronic medical records helpful for care coordination? Experiences of physician practices. J Gen Intern Med. 2010;25:177–185.
- , , , et al. Impact of localizing physicians to hospital units on nurse‐physician communication and agreement on the plan of care. J Gen Intern Med. 2009;24:1223–1227.
- , , , , , . Improving teamwork: impact of structured interdisciplinary rounds on a hospitalist unit. J Hosp Med. 2011;6:88–93.
- , , , et al. Structured interdisciplinary rounds in a medical teaching unit: improving patient safety. Arch Intern Med. 2011;171:678–684.
- , , , ; High Performance Teams and the Hospital of the Future Project Team. Interdisciplinary teamwork in hospitals: a review and practical recommendations for improvement. J Hosp Med. 2012;7:48–54.
- , , . Intrateam coverage is common, intrateam handoffs are not. J Hosp Med. 2014;9:734–736.
- . A primary care physician's ideal transitions of care? Where's the evidence? J Hosp Med. 2013;8:472–477.
- , , , , . Primary care physician communication at hospital discharge reduces medication discrepancies. J Hosp Med. 2013;8:672–677.
- , . Let's “face” it: time to introduce yourself to patients. J Hosp Med. 2014;9:199–200.
- , , . Patient perceptions of coordinated care: the importance of organized communication in hospitals. J Healthc Qual. 1999;21:18–23.
- , , , . Patient whiteboards to improve patient‐centred care in the hospital. Postgrad Med J. 2013;89:604–609.
- , , , , , . Effect of a face sheet tool on medical team provider identification and family satisfaction. J Hosp Med. 2014;9:186–188.
- , , , , , . The impact of facecards on patients' knowledge, satisfaction, trust, and agreement with hospital physicians: a pilot study. J Hosp Med. 2014;9:137–141.
- , , , et al. Patient engagement in the inpatient setting: a systematic review. J Am Med Inform Assoc. 2014;21:742–750.
- PROSPECT: Promoting Respect and Ongoing Safety Through Patient‐centeredness, Engagement, Communication, and Technology. Available at: http://www.partners.org/cird/PROSPECT/Index.htm. Accessed May 3, 2015.
- , , , , , . Smarter hospital communication: secure smartphone text messaging improves provider satisfaction and perception of efficacy, workflow. J Hosp Med. 2014;9:573–578.
- , , , et al. Engaging patients, providers, and institutional stakeholders in developing a patient‐centered microblog. Paper presented at: Proceeding of the American Medical Informatics Association Annual Fall Symposium; November 16–19, 2014; Washington, DC.
- . Patient‐centered communication. Annu Rev Nurs Res. 1999;17:85–104.
- , , , . Facilitating patient‐centered cancer communication: a road map. Patient Educ Couns. 2009;77:319–321.
- , . Patient‐Centered Communication in Cancer Care: Promoting Healing and Reducing Suffering. NIH Publication No. 07–6225. Bethesda, MD: National Cancer Institute; 2007.
- . When conversation is better than computation. J Am Med Inform Assoc. 2000;7:277–286.
- . Communication systems in healthcare. Clin Biochem Rev. 2006;27:89–98.
- , , , et al. The nature of adverse events in hospitalized patients. results of the Harvard Medical Practice Study II. N Engl J Med. 1991;324:377–384.
- , , , et al. A look into the nature and causes of human errors in the intensive care unit. Crit Care Med. 1995;23:294–300.
- , , . Communication failures: an insidious contributor to medical mishaps. Acad Med. 2004;79:186–194.
- , . Interdisciplinary communication: an uncharted source of medical error? J Crit Care. 2006;21:236–242; discussion 242.
- , , . Quantifying the economic impact of communication inefficiencies in U.S. hospitals. J Healthc Manag. 2010;55:265–281; discussion 281–282.
- , , , . Transforming the acute care environment: a web‐based patient‐centered toolkit [abstract]. J Hosp Med. 2014;9(suppl 2):694.
- , , , et al. Creating a culture of patient‐centered care team communication at a large academic medical center [Abstract]. J Hosp Med. 2015;10 (suppl 2). Available at: http://www.shmabstracts.com/abstract/creating‐a‐culture‐of‐patient‐centered‐care‐team‐communication‐at‐a‐large‐academic‐medical‐center. Accessed April 24, 2015.
- , , , , . Perceived information needs and communication difficulties of inpatient physicians and nurses. Proc AMIA Symp. 2001:453–457.
- , , , , , . The effect of tablet computers with a mobile patient portal application on hospitalized patients' knowledge and activation [published online June 15, 2015]. J Am Med Inform Assoc. doi: 10.1093/jamia/ocv058.
- , , , , . Bedside information technology to support patient‐centered care. Int J Med Inform. 2012;81(7):442–451.
- , , , et al. Building and testing a patient‐centric electronic bedside communication center. J Gerontol Nurs. 2013;39:15–19.
- , , , et al. A web‐based, patient‐centered toolkit to engage patients and caregivers in the acute care setting: a preliminary evaluation [published online August 2, 2015]. J Am Med Inform Assoc. doi: 10.1093/jamia/ocv093.
- , , , et al. Awareness of the care team in electronic health records. Appl Clin Inform. 2011;2:395–405.
- , , , , , . Teamwork on inpatient medical units: assessing attitudes and barriers. Qual Saf Health Care. 2010;19:117–121.
- , , , et al. Frequency and clinical importance of pages sent to the wrong physician. Arch Intern Med. 2009;169:1072–1073.
- , . Patient experiences with coordination of care: the benefit of continuity and primary care physician as referral source. J Gen Intern Med. 2009;24:170–177.
- , , , , . Are electronic medical records helpful for care coordination? Experiences of physician practices. J Gen Intern Med. 2010;25:177–185.
- , , , et al. Impact of localizing physicians to hospital units on nurse‐physician communication and agreement on the plan of care. J Gen Intern Med. 2009;24:1223–1227.
- , , , , , . Improving teamwork: impact of structured interdisciplinary rounds on a hospitalist unit. J Hosp Med. 2011;6:88–93.
- , , , et al. Structured interdisciplinary rounds in a medical teaching unit: improving patient safety. Arch Intern Med. 2011;171:678–684.
- , , , ; High Performance Teams and the Hospital of the Future Project Team. Interdisciplinary teamwork in hospitals: a review and practical recommendations for improvement. J Hosp Med. 2012;7:48–54.
- , , . Intrateam coverage is common, intrateam handoffs are not. J Hosp Med. 2014;9:734–736.
- . A primary care physician's ideal transitions of care? Where's the evidence? J Hosp Med. 2013;8:472–477.
- , , , , . Primary care physician communication at hospital discharge reduces medication discrepancies. J Hosp Med. 2013;8:672–677.
- , . Let's “face” it: time to introduce yourself to patients. J Hosp Med. 2014;9:199–200.
- , , . Patient perceptions of coordinated care: the importance of organized communication in hospitals. J Healthc Qual. 1999;21:18–23.
- , , , . Patient whiteboards to improve patient‐centred care in the hospital. Postgrad Med J. 2013;89:604–609.
- , , , , , . Effect of a face sheet tool on medical team provider identification and family satisfaction. J Hosp Med. 2014;9:186–188.
- , , , , , . The impact of facecards on patients' knowledge, satisfaction, trust, and agreement with hospital physicians: a pilot study. J Hosp Med. 2014;9:137–141.
- , , , et al. Patient engagement in the inpatient setting: a systematic review. J Am Med Inform Assoc. 2014;21:742–750.
- PROSPECT: Promoting Respect and Ongoing Safety Through Patient‐centeredness, Engagement, Communication, and Technology. Available at: http://www.partners.org/cird/PROSPECT/Index.htm. Accessed May 3, 2015.
- , , , , , . Smarter hospital communication: secure smartphone text messaging improves provider satisfaction and perception of efficacy, workflow. J Hosp Med. 2014;9:573–578.
- , , , et al. Engaging patients, providers, and institutional stakeholders in developing a patient‐centered microblog. Paper presented at: Proceeding of the American Medical Informatics Association Annual Fall Symposium; November 16–19, 2014; Washington, DC.
Primary Medication Nonadherence
Medication nonadherence after hospital discharge impacts morbidity and mortality in patients with cardiovascular disease.[1] Primary nonadherence, part of the spectrum of medication underuse, occurs when a patient receives a prescription but does not fill it.[1] Prior studies utilizing retrospective administrative data have found a prevalence of postdischarge primary nonadherence between 24% and 28%,[1, 2] similar to findings in a variety of outpatient populations.[3, 4]
One strategy for reduction in nonadherence is discharge medication counseling, which has been associated with improved postdischarge outcomes.[1] We evaluated the prevalence and predictors of refractory primary nonadherence in a cohort of patients hospitalized for acute cardiovascular conditions who received pharmacist counseling prior to discharge to guide future adherence interventions.
METHODS
Setting and Participants
The present study represents a secondary analysis of data from the Pharmacist Intervention for Low Literacy in Cardiovascular Disease (PILL‐CVD) study. PILL‐CVD was a randomized controlled trial that evaluated the effect of a tailored intervention consisting of pharmacist‐assisted medication reconciliation, discharge counseling, low‐literacy adherence aids, and follow‐up phone calls in adults hospitalized for acute coronary syndromes or acute decompensated heart failure. Patients likely to be discharged home taking primary responsibility for their medication management were eligible. Full study methods and results, including inclusion and exclusion criteria, can be found elsewhere.[5] The institutional review boards of each site approved the study.
For the present analysis, patients were included if they had any new discharge prescriptions to fill and received the study intervention, including a postdischarge follow‐up phone call with questions about filling discharge prescriptions.
Baseline Measures
Baseline data were obtained from medical records and patient interviews, including demographic information as well as survey data for cognitive impairment (Mini‐Cog) and health literacy (Short Test of Functional Health Literacy in Adults).[6, 7]
Data were also collected related to medication use, including the number of scheduled and as‐needed medications listed at discharge, self‐reported preadmission adherence, medication understanding, and medication management practices (eg, use of a pillbox, refill reminders). Self‐reported medication adherence was measured with the 4‐item Morisky scale.[8] Medication understanding was assessed with a tool previously developed by Marvanova et al.[9]
Outcome Measures
The primary outcome was the percentage of patients who reported not filling at least 1 discharge prescription on a telephone call that was conducted 1 to 4 days postdischarge. Patients were asked a dichotomous question about whether or not they filled all of their discharge prescriptions. Further characterization of the class or number of medications not filled was not performed. Patients were asked to provide a reason for not filling the prescriptions.
Analysis
We evaluated the prevalence and possible predictors of primary nonadherence including age, gender, race, marital status, education and income levels, insurance type, health literacy, cognition, presence of a primary care physician, number of listed discharge medications, prehospital medication adherence, medication understanding, and medication management practices using Pearson 2, Fisher exact, or Wilcoxon rank sum tests as appropriate. Multiple logistic regression with backward elimination was performed to identify independent predictors, selected with P values<0.1. We also evaluated reasons that patients cited for not filling prescriptions. Two‐sided P values<0.05 were considered statistically significant. All analyses were conducted using Stata version 13.1 (StataCorp LP, College Station, TX).
RESULTS
Of 851 patients in the PILL‐CVD study, the present sample includes 341 patients who received the intervention, completed the postdischarge follow‐up call, and had new discharge prescriptions to be filled. This represents 85% of patients who received the intervention.
The mean age of participants was 61.3 years, and 59.5% were male (Table 1). The majority were white (75.1%), and 88% had at least a high school education. Married or cohabitating patients represented 54.3% of the group. Just over half of the patients (54%) had an income of $35K or greater. The primary source of insurance for 82.5% of patients was either Medicare or private insurance, and 7.4% of patients were self‐pay. Most patients (80%) had adequate health literacy. The median Mini‐Cog score was 4 out of 5 (interquartile range [IQR]=35), and 11% of patients had scores indicating cognitive impairment. Just less than one‐fourth of the patients (24.1%) had a Morisky score of 8, indicating high self‐reported adherence, and the median score of patients' understanding of medications (range of 03) was 2.5 (IQR=2.22.8), reflecting relatively high understanding. The median number of prescriptions on patients' discharge medications lists was 10 (IQR=813).
| Variable | Overall 341 (100.0%) | Filled Prescription309 (90.6%) | Did Not Fill 32 (9.4%) | P Value |
|---|---|---|---|---|
| ||||
| Age, y, N (%) | 0.745a | |||
| 1849 | 69 | 63 (91.3) | 6 (8.7) | |
| 5064 | 128 | 114 (89.1) | 14 (10.9) | |
| 65+ | 144 | 132 (91.7) | 12 (8.3) | |
| Gender, N (%) | 0.056a | |||
| Male | 203 | 189 (93.1) | 14 (6.9) | |
| Female | 138 | 120 (87.0) | 18 (13.0) | |
| Race, N (%) | 0.712a | |||
| White | 256 | 234 (91.4) | 22 (8.6) | |
| African American | 60 | 54 (90.0) | 6 (10.0) | |
| Other | 22 | 19 (86.4) | 3 (13.6) | |
| Education, N (%) | 0.054a | |||
| Less than high school | 40 | 32 (80.0) | 8 (20.0) | |
| High school | 99 | 91 (91.9) | 8 (8.1) | |
| 1315 years | 93 | 83 (89.2) | 10 (10.8) | |
| 16 years | 109 | 103 (94.5) | 6 (5.5) | |
| Marital status, N (%) | ||||
| Separated/divorced/widowed/never married | 156 | 135 (86.5) | 21 (13.5) | 0.018a, b |
| Married/cohabitating | 185 | 174 (94.1) | 11 (5.9) | |
| Income, N (%) | 0.040a, b | |||
| <10K<20K | 58 | 48 (82.8) | 10 (17.2) | |
| 20K35K | 86 | 76 (88.4) | 10 (11.6) | |
| 35K<50K | 40 | 36 (90.0) | 4 (10.0) | |
| 50K<75K | 46 | 43 (93.5) | 3 (6.5) | |
| 75K+ | 83 | 81 (97.6) | 2 (2.4) | |
| Primary source of payment, N (%) | 0.272a | |||
| Medicaid | 34 | 28 (82.4) | 6 (17.6) | |
| Medicare | 145 | 131 (90.3) | 14 (9.7) | |
| Private | 132 | 123 (93.2) | 9 (6.8) | |
| Self‐pay | 25 | 22 (88.0) | 3 (12.0) | |
| Primary care physician, N (%) | 1.000c | |||
| None/do not know | 28 | 26 (92.9) | 2 (7.1) | |
| Yes | 313 | 283 (90.4) | 30 (9.6) | |
| Site, N (%) | 0.071a | |||
| Nashville, TN | 172 | 151 (87.8) | 21 (12.2) | |
| Boston, MA | 169 | 158 (93.5) | 11 (6.5) | |
The prevalence of refractory primary nonadherence was 9.4%. In univariate analysis, single marital status, lower income, and having more than 10 total discharge medications were significantly associated with not filling medications (P=0.018, 0.04, 0.016, respectively; Table 1). In multivariable analysis, single marital status and having more than 10 total discharge medications maintained significance when controlling for other patient characteristics. Patients who were single had higher odds of failing to fill discharge prescriptions compared to married or cohabitating individuals (odds ratio [OR]: 2.2, 95% confidence interval [CI]: 1.014.8, P=0.047). Patients with more than 10 discharge medications also had higher odds of failing to fill compared with patients who had fewer total medications (OR: 2.3, 95% CI: 1.054.98, P=0.036).
Filling discharge prescriptions was not associated with health literacy, cognition, prehospital adherence, patients' medication understanding, or any of the surveyed medication management practices (Table 2). Patients' reasons for not filling included lack of time to go to the pharmacy, medications not being delivered or dispensed, or inability to afford prescriptions. Prescription cost was cited by 23.5% of patients who did not fill their prescriptions and provided a reason.
| Variable | Overall 341 (100.0%) | Filled Prescription 309 (90.6%) | Did Not Fill 32 (9.4%) | P Value |
|---|---|---|---|---|
| ||||
| s‐TOFHLA score, range 036, N (%) | 0.443a | |||
| Inadequate, 016 | 40 | 34 (85.0) | 6 (15.0) | |
| Marginal, 1722 | 27 | 25 (92.6) | 2 (7.4) | |
| Adequate, 2336 | 268 | 244 (91.0) | 24 (9.0) | |
| MiniCog score, range 05, N (%) | 0.764b | |||
| Not impaired, 35 | 304 | 276 (90.8) | 28 (9.2) | |
| Impaired, 02 | 37 | 33 (89.2) | 4 (10.8) | |
| Morisky score, range 48, N (%) | 0.517a | |||
| Low/moderate self‐reported adherence, 47 | 249 | 224 (90.0) | 25 (10.0) | |
| High self‐reported adherence, 8 | 79 | 73 (92.4) | 6 (7.6) | |
| No. of discharge medications, range 126, N (%)c | 0.016a | |||
| 010 medications | 186 | 175 (94.1) | 11 (5.9) | |
| 11+medications | 155 | 134 (86.5) | 21 (13.5) | |
| Patient responses to medication behavior questions | ||||
| Patient associates medication taking time with daily events | 253 | 229 (90.5) | 24 (9.5) | 0.913a |
| Patient uses a pillbox to organize medicine | 180 | 162 (90.0) | 18 (10.0) | 0.680a |
| Friends of family help remind patient when it is time to take medicine | 89 | 79 (88.8) | 10 (11.2) | 0.486a |
| Patient writes down instructions for when to take medicine | 60 | 55 (91.7) | 5 (8.3) | 0.758a |
| Patient uses an alarm or a reminder that beeps when it is time to take medicine | 8 | 6 (75.0) | 2 (25.0) | 0.167a |
| Patient marks refill date on calendar | 38 | 35 (92.1) | 3 (7.9) | 1.000b |
| Pharmacy gives or sends patient a reminder when it is time to refill medicine | 94 | 84 (89.4) | 10 (10.6) | 0.624a |
| Friends or family help patient to refill medicine | 60 | 53 (88.3) | 7 (11.7) | 0.504a |
DISCUSSION
Almost 1 in 10 patients hospitalized with cardiovascular disease demonstrated primary nonadherence refractory to an intervention including pharmacist discharge medication counseling. Being unmarried and having greater than 10 medications at discharge were significantly associated with higher primary nonadherence when controlling for other patient factors.
Patients with a cohabitant partner were significantly less likely to exhibit primary nonadherence, which may reflect higher levels of social support, including encouragement for disease self‐management and/or support with tasks such as picking up medications from the pharmacy. Previous research has demonstrated that social support mediates outpatient medication adherence for heart failure patients.[10]
Similar to Jackevicius et al., we found that patients with more medications at discharge were less likely to fill their prescriptions.[1] These findings may reflect the challenges that patients face in adhering to complex treatment plans, which are associated with increased coordination and cost. Conversely, some prior studies have found that patients with fewer prescriptions were less likely to fill.[11, 12] These patients were often younger, thus potentially less conditioned to fill prescriptions, and unlike our cohort, these populations had consistent prescription coverage. Interventions for polypharmacy, which have been shown to improve outcomes and decrease costs, especially in the geriatric population, may be of benefit for primary nonadherence as well.[13]
Additionally, patients with lower household incomes had higher rates of primary nonadherence, at least in univariate analysis. Medication cost and transportation limitations, which are more pronounced in lower‐income patients, likely play influential roles in this group. These findings build on prior literature that has found lower prescription cost to be associated with better medication adherence in a variety of settings.[3, 4, 14]
Because the prevalence of primary nonadherence in this cohort is less than half of historical rates, we suspect the intervention did reduce unintentional nonadherence. However, regimen cost and complexity, transportation challenges, and ingrained medication beliefs likely remained barriers. It may be that a postdischarge phone call is able address unintended primary nonadherence in many cases. Meds to beds programs, where a supply of medications is provided to patients prior to discharge, could assist patients with limited transportation. Prior studies have also found reduced primary nonadherence when e‐prescriptions are utilized.[3]
Establishing outpatient follow‐up at discharge provides additional opportunities to address unanticipated adherence barriers. Because the efficacy of any adherence intervention depends on individual patient barriers, we recommend combining medication counseling with a targeted approach for patient‐specific needs.
We note several limitations to our study. First, because we studied primary nonadherence that persisted despite an intervention, this cohort likely underestimates the prevalence of primary nonadherence and alters the associated patient characteristics found in routine practice (although counseling is becoming more common). Second, patient reporting is subject to biases that underestimate nonadherence, although this approach has been validated previously.[15] Third, our outcome measure was unable to capture the spectrum of non‐adherence that could provide a more nuanced look at predictors of postdischarge nonadherence. Fourth, we did not have patient copayment data to better characterize whether out of pocket costs or pharmacologic classes drove nonadherence. Finally, sample size may have limited the detection of other important factors, and the university setting may limit generalizability to cardiovascular patients in other practice environments. Future research should focus on intervention strategies that assess patients' individual adherence barriers for a targeted or multimodal approach to improve adherence.
In conclusion, we found a prevalence of primary nonadherence of almost 1 in 10 patients who received pharmacist counseling. Nonadherence was associated with being single and those discharged with longer medication lists. Our results support existing literature that primary nonadherence is a significant problem in the postdischarge setting and substantiate the need for ongoing efforts to study and implement interventions for adherence after hospital discharge.
Disclosures
This material is based on work supported by the Office of Academic Affiliations, Department of Veterans Affairs, Veterans Affairs National Quality Scholars Program, and with use of facilities at Veterans Affairs Tennessee Valley Healthcare System, Nashville, Tennessee (Dr. Wooldridge). The funding agency supported the work indirectly through provision of salary support and training for the primary author, but had no specific role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; or decision to submit the manuscript for publication. This work was also supported by R01 HL089755 (Dr. Kripalani) and in part by K23 HL077597 (Dr. Kripalani), K08 HL072806 (Dr. Schnipper), and the Center for Clinical Quality and Implementation Research at Vanderbilt University Medical Center. A preliminary version of this research was presented at the AcademyHealth Annual Research Meeting, June 16, 2015, Minneapolis, Minnesota. The authors report the following potential conflicts of interest: Jeffrey Schnipper: PI, investigator‐initiated study funded by Sanofi‐Aventis to develop, implement, and evaluate a multifaceted intervention to improve transitions of care in patients with diabetes mellitus discharged on insulin. Robert Dittus: passive co‐owner, Medical Decision Modeling, Inc.; Bayer HealthCare. One‐day consultation and panelist on educational video for population health (consultant fee); GlaxoSmithKline. One‐day consultant for population health, envisioning the future (consultant fee). Sunil Kripalani: Bioscape Digital, stock ownership
- , , . Prevalence, predictors, and outcomes of primary nonadherence after acute myocardial infarction. Circulation. 2008;117(8):1028–1036.
- , , , . Primary medication non‐adherence after discharge from a general internal medicine service. PloS One. 2013;8(5):e61735.
- , , , et al. Trouble getting started: predictors of primary medication nonadherence. Am J Med. 2011;124(11):1081.e9–22.
- , , , , . The incidence and determinants of primary nonadherence with prescribed medication in primary care: a cohort study. Ann Intern Med. 2014;160(7):441–450.
- , , , et al. Effect of a pharmacist intervention on clinically important medication errors after hospital discharge: a randomized trial. Ann Intern Med. 2012;157(1):1–10.
- , , , . Short Test of Functional Health Literacy in Adults. Snow Camp, NC: Peppercorn Books and Press; 1998.
- , , , , . Simplifying detection of cognitive impairment: comparison of the Mini‐Cog and Mini‐Mental State Examination in a multiethnic sample. J Am Geriatr Soc. 2005;53(5):871–874.
- , , . Concurrent and predictive validity of a self‐reported measure of medication adherence. Med Care. 1986;24(1):67–74.
- , , , , , . Health literacy and medication understanding among hospitalized adults. J Hosp Med. 2011;6(9):488–493.
- , , , , , . Medication adherence, social support, and event‐free survival in patients with heart failure. Health Psychol. 2013;32(6):637–646.
- , , , , , . Effect of patient comorbidities on filling of antihypertensive prescriptions. Am J Manag Care. 2009;15(1):24–30.
- , , , et al. Primary nonadherence to statin medications in a managed care organization. J Manag Care Pharm. 2013;19(5):367–373.
- , , , et al. Reducing cost by reducing polypharmacy: the polypharmacy outcomes project. J Am Med Dir Assoc. 2012;13(9):818.e811–815.
- , , , et al. The epidemiology of prescriptions abandoned at the pharmacy. Ann Intern Med. 2010;153(10):633–640.
- , , , , , . Can simple clinical measurements detect patient noncompliance? Hypertension. 1980;2(6):757–764.
Medication nonadherence after hospital discharge impacts morbidity and mortality in patients with cardiovascular disease.[1] Primary nonadherence, part of the spectrum of medication underuse, occurs when a patient receives a prescription but does not fill it.[1] Prior studies utilizing retrospective administrative data have found a prevalence of postdischarge primary nonadherence between 24% and 28%,[1, 2] similar to findings in a variety of outpatient populations.[3, 4]
One strategy for reduction in nonadherence is discharge medication counseling, which has been associated with improved postdischarge outcomes.[1] We evaluated the prevalence and predictors of refractory primary nonadherence in a cohort of patients hospitalized for acute cardiovascular conditions who received pharmacist counseling prior to discharge to guide future adherence interventions.
METHODS
Setting and Participants
The present study represents a secondary analysis of data from the Pharmacist Intervention for Low Literacy in Cardiovascular Disease (PILL‐CVD) study. PILL‐CVD was a randomized controlled trial that evaluated the effect of a tailored intervention consisting of pharmacist‐assisted medication reconciliation, discharge counseling, low‐literacy adherence aids, and follow‐up phone calls in adults hospitalized for acute coronary syndromes or acute decompensated heart failure. Patients likely to be discharged home taking primary responsibility for their medication management were eligible. Full study methods and results, including inclusion and exclusion criteria, can be found elsewhere.[5] The institutional review boards of each site approved the study.
For the present analysis, patients were included if they had any new discharge prescriptions to fill and received the study intervention, including a postdischarge follow‐up phone call with questions about filling discharge prescriptions.
Baseline Measures
Baseline data were obtained from medical records and patient interviews, including demographic information as well as survey data for cognitive impairment (Mini‐Cog) and health literacy (Short Test of Functional Health Literacy in Adults).[6, 7]
Data were also collected related to medication use, including the number of scheduled and as‐needed medications listed at discharge, self‐reported preadmission adherence, medication understanding, and medication management practices (eg, use of a pillbox, refill reminders). Self‐reported medication adherence was measured with the 4‐item Morisky scale.[8] Medication understanding was assessed with a tool previously developed by Marvanova et al.[9]
Outcome Measures
The primary outcome was the percentage of patients who reported not filling at least 1 discharge prescription on a telephone call that was conducted 1 to 4 days postdischarge. Patients were asked a dichotomous question about whether or not they filled all of their discharge prescriptions. Further characterization of the class or number of medications not filled was not performed. Patients were asked to provide a reason for not filling the prescriptions.
Analysis
We evaluated the prevalence and possible predictors of primary nonadherence including age, gender, race, marital status, education and income levels, insurance type, health literacy, cognition, presence of a primary care physician, number of listed discharge medications, prehospital medication adherence, medication understanding, and medication management practices using Pearson 2, Fisher exact, or Wilcoxon rank sum tests as appropriate. Multiple logistic regression with backward elimination was performed to identify independent predictors, selected with P values<0.1. We also evaluated reasons that patients cited for not filling prescriptions. Two‐sided P values<0.05 were considered statistically significant. All analyses were conducted using Stata version 13.1 (StataCorp LP, College Station, TX).
RESULTS
Of 851 patients in the PILL‐CVD study, the present sample includes 341 patients who received the intervention, completed the postdischarge follow‐up call, and had new discharge prescriptions to be filled. This represents 85% of patients who received the intervention.
The mean age of participants was 61.3 years, and 59.5% were male (Table 1). The majority were white (75.1%), and 88% had at least a high school education. Married or cohabitating patients represented 54.3% of the group. Just over half of the patients (54%) had an income of $35K or greater. The primary source of insurance for 82.5% of patients was either Medicare or private insurance, and 7.4% of patients were self‐pay. Most patients (80%) had adequate health literacy. The median Mini‐Cog score was 4 out of 5 (interquartile range [IQR]=35), and 11% of patients had scores indicating cognitive impairment. Just less than one‐fourth of the patients (24.1%) had a Morisky score of 8, indicating high self‐reported adherence, and the median score of patients' understanding of medications (range of 03) was 2.5 (IQR=2.22.8), reflecting relatively high understanding. The median number of prescriptions on patients' discharge medications lists was 10 (IQR=813).
| Variable | Overall 341 (100.0%) | Filled Prescription309 (90.6%) | Did Not Fill 32 (9.4%) | P Value |
|---|---|---|---|---|
| ||||
| Age, y, N (%) | 0.745a | |||
| 1849 | 69 | 63 (91.3) | 6 (8.7) | |
| 5064 | 128 | 114 (89.1) | 14 (10.9) | |
| 65+ | 144 | 132 (91.7) | 12 (8.3) | |
| Gender, N (%) | 0.056a | |||
| Male | 203 | 189 (93.1) | 14 (6.9) | |
| Female | 138 | 120 (87.0) | 18 (13.0) | |
| Race, N (%) | 0.712a | |||
| White | 256 | 234 (91.4) | 22 (8.6) | |
| African American | 60 | 54 (90.0) | 6 (10.0) | |
| Other | 22 | 19 (86.4) | 3 (13.6) | |
| Education, N (%) | 0.054a | |||
| Less than high school | 40 | 32 (80.0) | 8 (20.0) | |
| High school | 99 | 91 (91.9) | 8 (8.1) | |
| 1315 years | 93 | 83 (89.2) | 10 (10.8) | |
| 16 years | 109 | 103 (94.5) | 6 (5.5) | |
| Marital status, N (%) | ||||
| Separated/divorced/widowed/never married | 156 | 135 (86.5) | 21 (13.5) | 0.018a, b |
| Married/cohabitating | 185 | 174 (94.1) | 11 (5.9) | |
| Income, N (%) | 0.040a, b | |||
| <10K<20K | 58 | 48 (82.8) | 10 (17.2) | |
| 20K35K | 86 | 76 (88.4) | 10 (11.6) | |
| 35K<50K | 40 | 36 (90.0) | 4 (10.0) | |
| 50K<75K | 46 | 43 (93.5) | 3 (6.5) | |
| 75K+ | 83 | 81 (97.6) | 2 (2.4) | |
| Primary source of payment, N (%) | 0.272a | |||
| Medicaid | 34 | 28 (82.4) | 6 (17.6) | |
| Medicare | 145 | 131 (90.3) | 14 (9.7) | |
| Private | 132 | 123 (93.2) | 9 (6.8) | |
| Self‐pay | 25 | 22 (88.0) | 3 (12.0) | |
| Primary care physician, N (%) | 1.000c | |||
| None/do not know | 28 | 26 (92.9) | 2 (7.1) | |
| Yes | 313 | 283 (90.4) | 30 (9.6) | |
| Site, N (%) | 0.071a | |||
| Nashville, TN | 172 | 151 (87.8) | 21 (12.2) | |
| Boston, MA | 169 | 158 (93.5) | 11 (6.5) | |
The prevalence of refractory primary nonadherence was 9.4%. In univariate analysis, single marital status, lower income, and having more than 10 total discharge medications were significantly associated with not filling medications (P=0.018, 0.04, 0.016, respectively; Table 1). In multivariable analysis, single marital status and having more than 10 total discharge medications maintained significance when controlling for other patient characteristics. Patients who were single had higher odds of failing to fill discharge prescriptions compared to married or cohabitating individuals (odds ratio [OR]: 2.2, 95% confidence interval [CI]: 1.014.8, P=0.047). Patients with more than 10 discharge medications also had higher odds of failing to fill compared with patients who had fewer total medications (OR: 2.3, 95% CI: 1.054.98, P=0.036).
Filling discharge prescriptions was not associated with health literacy, cognition, prehospital adherence, patients' medication understanding, or any of the surveyed medication management practices (Table 2). Patients' reasons for not filling included lack of time to go to the pharmacy, medications not being delivered or dispensed, or inability to afford prescriptions. Prescription cost was cited by 23.5% of patients who did not fill their prescriptions and provided a reason.
| Variable | Overall 341 (100.0%) | Filled Prescription 309 (90.6%) | Did Not Fill 32 (9.4%) | P Value |
|---|---|---|---|---|
| ||||
| s‐TOFHLA score, range 036, N (%) | 0.443a | |||
| Inadequate, 016 | 40 | 34 (85.0) | 6 (15.0) | |
| Marginal, 1722 | 27 | 25 (92.6) | 2 (7.4) | |
| Adequate, 2336 | 268 | 244 (91.0) | 24 (9.0) | |
| MiniCog score, range 05, N (%) | 0.764b | |||
| Not impaired, 35 | 304 | 276 (90.8) | 28 (9.2) | |
| Impaired, 02 | 37 | 33 (89.2) | 4 (10.8) | |
| Morisky score, range 48, N (%) | 0.517a | |||
| Low/moderate self‐reported adherence, 47 | 249 | 224 (90.0) | 25 (10.0) | |
| High self‐reported adherence, 8 | 79 | 73 (92.4) | 6 (7.6) | |
| No. of discharge medications, range 126, N (%)c | 0.016a | |||
| 010 medications | 186 | 175 (94.1) | 11 (5.9) | |
| 11+medications | 155 | 134 (86.5) | 21 (13.5) | |
| Patient responses to medication behavior questions | ||||
| Patient associates medication taking time with daily events | 253 | 229 (90.5) | 24 (9.5) | 0.913a |
| Patient uses a pillbox to organize medicine | 180 | 162 (90.0) | 18 (10.0) | 0.680a |
| Friends of family help remind patient when it is time to take medicine | 89 | 79 (88.8) | 10 (11.2) | 0.486a |
| Patient writes down instructions for when to take medicine | 60 | 55 (91.7) | 5 (8.3) | 0.758a |
| Patient uses an alarm or a reminder that beeps when it is time to take medicine | 8 | 6 (75.0) | 2 (25.0) | 0.167a |
| Patient marks refill date on calendar | 38 | 35 (92.1) | 3 (7.9) | 1.000b |
| Pharmacy gives or sends patient a reminder when it is time to refill medicine | 94 | 84 (89.4) | 10 (10.6) | 0.624a |
| Friends or family help patient to refill medicine | 60 | 53 (88.3) | 7 (11.7) | 0.504a |
DISCUSSION
Almost 1 in 10 patients hospitalized with cardiovascular disease demonstrated primary nonadherence refractory to an intervention including pharmacist discharge medication counseling. Being unmarried and having greater than 10 medications at discharge were significantly associated with higher primary nonadherence when controlling for other patient factors.
Patients with a cohabitant partner were significantly less likely to exhibit primary nonadherence, which may reflect higher levels of social support, including encouragement for disease self‐management and/or support with tasks such as picking up medications from the pharmacy. Previous research has demonstrated that social support mediates outpatient medication adherence for heart failure patients.[10]
Similar to Jackevicius et al., we found that patients with more medications at discharge were less likely to fill their prescriptions.[1] These findings may reflect the challenges that patients face in adhering to complex treatment plans, which are associated with increased coordination and cost. Conversely, some prior studies have found that patients with fewer prescriptions were less likely to fill.[11, 12] These patients were often younger, thus potentially less conditioned to fill prescriptions, and unlike our cohort, these populations had consistent prescription coverage. Interventions for polypharmacy, which have been shown to improve outcomes and decrease costs, especially in the geriatric population, may be of benefit for primary nonadherence as well.[13]
Additionally, patients with lower household incomes had higher rates of primary nonadherence, at least in univariate analysis. Medication cost and transportation limitations, which are more pronounced in lower‐income patients, likely play influential roles in this group. These findings build on prior literature that has found lower prescription cost to be associated with better medication adherence in a variety of settings.[3, 4, 14]
Because the prevalence of primary nonadherence in this cohort is less than half of historical rates, we suspect the intervention did reduce unintentional nonadherence. However, regimen cost and complexity, transportation challenges, and ingrained medication beliefs likely remained barriers. It may be that a postdischarge phone call is able address unintended primary nonadherence in many cases. Meds to beds programs, where a supply of medications is provided to patients prior to discharge, could assist patients with limited transportation. Prior studies have also found reduced primary nonadherence when e‐prescriptions are utilized.[3]
Establishing outpatient follow‐up at discharge provides additional opportunities to address unanticipated adherence barriers. Because the efficacy of any adherence intervention depends on individual patient barriers, we recommend combining medication counseling with a targeted approach for patient‐specific needs.
We note several limitations to our study. First, because we studied primary nonadherence that persisted despite an intervention, this cohort likely underestimates the prevalence of primary nonadherence and alters the associated patient characteristics found in routine practice (although counseling is becoming more common). Second, patient reporting is subject to biases that underestimate nonadherence, although this approach has been validated previously.[15] Third, our outcome measure was unable to capture the spectrum of non‐adherence that could provide a more nuanced look at predictors of postdischarge nonadherence. Fourth, we did not have patient copayment data to better characterize whether out of pocket costs or pharmacologic classes drove nonadherence. Finally, sample size may have limited the detection of other important factors, and the university setting may limit generalizability to cardiovascular patients in other practice environments. Future research should focus on intervention strategies that assess patients' individual adherence barriers for a targeted or multimodal approach to improve adherence.
In conclusion, we found a prevalence of primary nonadherence of almost 1 in 10 patients who received pharmacist counseling. Nonadherence was associated with being single and those discharged with longer medication lists. Our results support existing literature that primary nonadherence is a significant problem in the postdischarge setting and substantiate the need for ongoing efforts to study and implement interventions for adherence after hospital discharge.
Disclosures
This material is based on work supported by the Office of Academic Affiliations, Department of Veterans Affairs, Veterans Affairs National Quality Scholars Program, and with use of facilities at Veterans Affairs Tennessee Valley Healthcare System, Nashville, Tennessee (Dr. Wooldridge). The funding agency supported the work indirectly through provision of salary support and training for the primary author, but had no specific role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; or decision to submit the manuscript for publication. This work was also supported by R01 HL089755 (Dr. Kripalani) and in part by K23 HL077597 (Dr. Kripalani), K08 HL072806 (Dr. Schnipper), and the Center for Clinical Quality and Implementation Research at Vanderbilt University Medical Center. A preliminary version of this research was presented at the AcademyHealth Annual Research Meeting, June 16, 2015, Minneapolis, Minnesota. The authors report the following potential conflicts of interest: Jeffrey Schnipper: PI, investigator‐initiated study funded by Sanofi‐Aventis to develop, implement, and evaluate a multifaceted intervention to improve transitions of care in patients with diabetes mellitus discharged on insulin. Robert Dittus: passive co‐owner, Medical Decision Modeling, Inc.; Bayer HealthCare. One‐day consultation and panelist on educational video for population health (consultant fee); GlaxoSmithKline. One‐day consultant for population health, envisioning the future (consultant fee). Sunil Kripalani: Bioscape Digital, stock ownership
Medication nonadherence after hospital discharge impacts morbidity and mortality in patients with cardiovascular disease.[1] Primary nonadherence, part of the spectrum of medication underuse, occurs when a patient receives a prescription but does not fill it.[1] Prior studies utilizing retrospective administrative data have found a prevalence of postdischarge primary nonadherence between 24% and 28%,[1, 2] similar to findings in a variety of outpatient populations.[3, 4]
One strategy for reduction in nonadherence is discharge medication counseling, which has been associated with improved postdischarge outcomes.[1] We evaluated the prevalence and predictors of refractory primary nonadherence in a cohort of patients hospitalized for acute cardiovascular conditions who received pharmacist counseling prior to discharge to guide future adherence interventions.
METHODS
Setting and Participants
The present study represents a secondary analysis of data from the Pharmacist Intervention for Low Literacy in Cardiovascular Disease (PILL‐CVD) study. PILL‐CVD was a randomized controlled trial that evaluated the effect of a tailored intervention consisting of pharmacist‐assisted medication reconciliation, discharge counseling, low‐literacy adherence aids, and follow‐up phone calls in adults hospitalized for acute coronary syndromes or acute decompensated heart failure. Patients likely to be discharged home taking primary responsibility for their medication management were eligible. Full study methods and results, including inclusion and exclusion criteria, can be found elsewhere.[5] The institutional review boards of each site approved the study.
For the present analysis, patients were included if they had any new discharge prescriptions to fill and received the study intervention, including a postdischarge follow‐up phone call with questions about filling discharge prescriptions.
Baseline Measures
Baseline data were obtained from medical records and patient interviews, including demographic information as well as survey data for cognitive impairment (Mini‐Cog) and health literacy (Short Test of Functional Health Literacy in Adults).[6, 7]
Data were also collected related to medication use, including the number of scheduled and as‐needed medications listed at discharge, self‐reported preadmission adherence, medication understanding, and medication management practices (eg, use of a pillbox, refill reminders). Self‐reported medication adherence was measured with the 4‐item Morisky scale.[8] Medication understanding was assessed with a tool previously developed by Marvanova et al.[9]
Outcome Measures
The primary outcome was the percentage of patients who reported not filling at least 1 discharge prescription on a telephone call that was conducted 1 to 4 days postdischarge. Patients were asked a dichotomous question about whether or not they filled all of their discharge prescriptions. Further characterization of the class or number of medications not filled was not performed. Patients were asked to provide a reason for not filling the prescriptions.
Analysis
We evaluated the prevalence and possible predictors of primary nonadherence including age, gender, race, marital status, education and income levels, insurance type, health literacy, cognition, presence of a primary care physician, number of listed discharge medications, prehospital medication adherence, medication understanding, and medication management practices using Pearson 2, Fisher exact, or Wilcoxon rank sum tests as appropriate. Multiple logistic regression with backward elimination was performed to identify independent predictors, selected with P values<0.1. We also evaluated reasons that patients cited for not filling prescriptions. Two‐sided P values<0.05 were considered statistically significant. All analyses were conducted using Stata version 13.1 (StataCorp LP, College Station, TX).
RESULTS
Of 851 patients in the PILL‐CVD study, the present sample includes 341 patients who received the intervention, completed the postdischarge follow‐up call, and had new discharge prescriptions to be filled. This represents 85% of patients who received the intervention.
The mean age of participants was 61.3 years, and 59.5% were male (Table 1). The majority were white (75.1%), and 88% had at least a high school education. Married or cohabitating patients represented 54.3% of the group. Just over half of the patients (54%) had an income of $35K or greater. The primary source of insurance for 82.5% of patients was either Medicare or private insurance, and 7.4% of patients were self‐pay. Most patients (80%) had adequate health literacy. The median Mini‐Cog score was 4 out of 5 (interquartile range [IQR]=35), and 11% of patients had scores indicating cognitive impairment. Just less than one‐fourth of the patients (24.1%) had a Morisky score of 8, indicating high self‐reported adherence, and the median score of patients' understanding of medications (range of 03) was 2.5 (IQR=2.22.8), reflecting relatively high understanding. The median number of prescriptions on patients' discharge medications lists was 10 (IQR=813).
| Variable | Overall 341 (100.0%) | Filled Prescription309 (90.6%) | Did Not Fill 32 (9.4%) | P Value |
|---|---|---|---|---|
| ||||
| Age, y, N (%) | 0.745a | |||
| 1849 | 69 | 63 (91.3) | 6 (8.7) | |
| 5064 | 128 | 114 (89.1) | 14 (10.9) | |
| 65+ | 144 | 132 (91.7) | 12 (8.3) | |
| Gender, N (%) | 0.056a | |||
| Male | 203 | 189 (93.1) | 14 (6.9) | |
| Female | 138 | 120 (87.0) | 18 (13.0) | |
| Race, N (%) | 0.712a | |||
| White | 256 | 234 (91.4) | 22 (8.6) | |
| African American | 60 | 54 (90.0) | 6 (10.0) | |
| Other | 22 | 19 (86.4) | 3 (13.6) | |
| Education, N (%) | 0.054a | |||
| Less than high school | 40 | 32 (80.0) | 8 (20.0) | |
| High school | 99 | 91 (91.9) | 8 (8.1) | |
| 1315 years | 93 | 83 (89.2) | 10 (10.8) | |
| 16 years | 109 | 103 (94.5) | 6 (5.5) | |
| Marital status, N (%) | ||||
| Separated/divorced/widowed/never married | 156 | 135 (86.5) | 21 (13.5) | 0.018a, b |
| Married/cohabitating | 185 | 174 (94.1) | 11 (5.9) | |
| Income, N (%) | 0.040a, b | |||
| <10K<20K | 58 | 48 (82.8) | 10 (17.2) | |
| 20K35K | 86 | 76 (88.4) | 10 (11.6) | |
| 35K<50K | 40 | 36 (90.0) | 4 (10.0) | |
| 50K<75K | 46 | 43 (93.5) | 3 (6.5) | |
| 75K+ | 83 | 81 (97.6) | 2 (2.4) | |
| Primary source of payment, N (%) | 0.272a | |||
| Medicaid | 34 | 28 (82.4) | 6 (17.6) | |
| Medicare | 145 | 131 (90.3) | 14 (9.7) | |
| Private | 132 | 123 (93.2) | 9 (6.8) | |
| Self‐pay | 25 | 22 (88.0) | 3 (12.0) | |
| Primary care physician, N (%) | 1.000c | |||
| None/do not know | 28 | 26 (92.9) | 2 (7.1) | |
| Yes | 313 | 283 (90.4) | 30 (9.6) | |
| Site, N (%) | 0.071a | |||
| Nashville, TN | 172 | 151 (87.8) | 21 (12.2) | |
| Boston, MA | 169 | 158 (93.5) | 11 (6.5) | |
The prevalence of refractory primary nonadherence was 9.4%. In univariate analysis, single marital status, lower income, and having more than 10 total discharge medications were significantly associated with not filling medications (P=0.018, 0.04, 0.016, respectively; Table 1). In multivariable analysis, single marital status and having more than 10 total discharge medications maintained significance when controlling for other patient characteristics. Patients who were single had higher odds of failing to fill discharge prescriptions compared to married or cohabitating individuals (odds ratio [OR]: 2.2, 95% confidence interval [CI]: 1.014.8, P=0.047). Patients with more than 10 discharge medications also had higher odds of failing to fill compared with patients who had fewer total medications (OR: 2.3, 95% CI: 1.054.98, P=0.036).
Filling discharge prescriptions was not associated with health literacy, cognition, prehospital adherence, patients' medication understanding, or any of the surveyed medication management practices (Table 2). Patients' reasons for not filling included lack of time to go to the pharmacy, medications not being delivered or dispensed, or inability to afford prescriptions. Prescription cost was cited by 23.5% of patients who did not fill their prescriptions and provided a reason.
| Variable | Overall 341 (100.0%) | Filled Prescription 309 (90.6%) | Did Not Fill 32 (9.4%) | P Value |
|---|---|---|---|---|
| ||||
| s‐TOFHLA score, range 036, N (%) | 0.443a | |||
| Inadequate, 016 | 40 | 34 (85.0) | 6 (15.0) | |
| Marginal, 1722 | 27 | 25 (92.6) | 2 (7.4) | |
| Adequate, 2336 | 268 | 244 (91.0) | 24 (9.0) | |
| MiniCog score, range 05, N (%) | 0.764b | |||
| Not impaired, 35 | 304 | 276 (90.8) | 28 (9.2) | |
| Impaired, 02 | 37 | 33 (89.2) | 4 (10.8) | |
| Morisky score, range 48, N (%) | 0.517a | |||
| Low/moderate self‐reported adherence, 47 | 249 | 224 (90.0) | 25 (10.0) | |
| High self‐reported adherence, 8 | 79 | 73 (92.4) | 6 (7.6) | |
| No. of discharge medications, range 126, N (%)c | 0.016a | |||
| 010 medications | 186 | 175 (94.1) | 11 (5.9) | |
| 11+medications | 155 | 134 (86.5) | 21 (13.5) | |
| Patient responses to medication behavior questions | ||||
| Patient associates medication taking time with daily events | 253 | 229 (90.5) | 24 (9.5) | 0.913a |
| Patient uses a pillbox to organize medicine | 180 | 162 (90.0) | 18 (10.0) | 0.680a |
| Friends of family help remind patient when it is time to take medicine | 89 | 79 (88.8) | 10 (11.2) | 0.486a |
| Patient writes down instructions for when to take medicine | 60 | 55 (91.7) | 5 (8.3) | 0.758a |
| Patient uses an alarm or a reminder that beeps when it is time to take medicine | 8 | 6 (75.0) | 2 (25.0) | 0.167a |
| Patient marks refill date on calendar | 38 | 35 (92.1) | 3 (7.9) | 1.000b |
| Pharmacy gives or sends patient a reminder when it is time to refill medicine | 94 | 84 (89.4) | 10 (10.6) | 0.624a |
| Friends or family help patient to refill medicine | 60 | 53 (88.3) | 7 (11.7) | 0.504a |
DISCUSSION
Almost 1 in 10 patients hospitalized with cardiovascular disease demonstrated primary nonadherence refractory to an intervention including pharmacist discharge medication counseling. Being unmarried and having greater than 10 medications at discharge were significantly associated with higher primary nonadherence when controlling for other patient factors.
Patients with a cohabitant partner were significantly less likely to exhibit primary nonadherence, which may reflect higher levels of social support, including encouragement for disease self‐management and/or support with tasks such as picking up medications from the pharmacy. Previous research has demonstrated that social support mediates outpatient medication adherence for heart failure patients.[10]
Similar to Jackevicius et al., we found that patients with more medications at discharge were less likely to fill their prescriptions.[1] These findings may reflect the challenges that patients face in adhering to complex treatment plans, which are associated with increased coordination and cost. Conversely, some prior studies have found that patients with fewer prescriptions were less likely to fill.[11, 12] These patients were often younger, thus potentially less conditioned to fill prescriptions, and unlike our cohort, these populations had consistent prescription coverage. Interventions for polypharmacy, which have been shown to improve outcomes and decrease costs, especially in the geriatric population, may be of benefit for primary nonadherence as well.[13]
Additionally, patients with lower household incomes had higher rates of primary nonadherence, at least in univariate analysis. Medication cost and transportation limitations, which are more pronounced in lower‐income patients, likely play influential roles in this group. These findings build on prior literature that has found lower prescription cost to be associated with better medication adherence in a variety of settings.[3, 4, 14]
Because the prevalence of primary nonadherence in this cohort is less than half of historical rates, we suspect the intervention did reduce unintentional nonadherence. However, regimen cost and complexity, transportation challenges, and ingrained medication beliefs likely remained barriers. It may be that a postdischarge phone call is able address unintended primary nonadherence in many cases. Meds to beds programs, where a supply of medications is provided to patients prior to discharge, could assist patients with limited transportation. Prior studies have also found reduced primary nonadherence when e‐prescriptions are utilized.[3]
Establishing outpatient follow‐up at discharge provides additional opportunities to address unanticipated adherence barriers. Because the efficacy of any adherence intervention depends on individual patient barriers, we recommend combining medication counseling with a targeted approach for patient‐specific needs.
We note several limitations to our study. First, because we studied primary nonadherence that persisted despite an intervention, this cohort likely underestimates the prevalence of primary nonadherence and alters the associated patient characteristics found in routine practice (although counseling is becoming more common). Second, patient reporting is subject to biases that underestimate nonadherence, although this approach has been validated previously.[15] Third, our outcome measure was unable to capture the spectrum of non‐adherence that could provide a more nuanced look at predictors of postdischarge nonadherence. Fourth, we did not have patient copayment data to better characterize whether out of pocket costs or pharmacologic classes drove nonadherence. Finally, sample size may have limited the detection of other important factors, and the university setting may limit generalizability to cardiovascular patients in other practice environments. Future research should focus on intervention strategies that assess patients' individual adherence barriers for a targeted or multimodal approach to improve adherence.
In conclusion, we found a prevalence of primary nonadherence of almost 1 in 10 patients who received pharmacist counseling. Nonadherence was associated with being single and those discharged with longer medication lists. Our results support existing literature that primary nonadherence is a significant problem in the postdischarge setting and substantiate the need for ongoing efforts to study and implement interventions for adherence after hospital discharge.
Disclosures
This material is based on work supported by the Office of Academic Affiliations, Department of Veterans Affairs, Veterans Affairs National Quality Scholars Program, and with use of facilities at Veterans Affairs Tennessee Valley Healthcare System, Nashville, Tennessee (Dr. Wooldridge). The funding agency supported the work indirectly through provision of salary support and training for the primary author, but had no specific role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; or decision to submit the manuscript for publication. This work was also supported by R01 HL089755 (Dr. Kripalani) and in part by K23 HL077597 (Dr. Kripalani), K08 HL072806 (Dr. Schnipper), and the Center for Clinical Quality and Implementation Research at Vanderbilt University Medical Center. A preliminary version of this research was presented at the AcademyHealth Annual Research Meeting, June 16, 2015, Minneapolis, Minnesota. The authors report the following potential conflicts of interest: Jeffrey Schnipper: PI, investigator‐initiated study funded by Sanofi‐Aventis to develop, implement, and evaluate a multifaceted intervention to improve transitions of care in patients with diabetes mellitus discharged on insulin. Robert Dittus: passive co‐owner, Medical Decision Modeling, Inc.; Bayer HealthCare. One‐day consultation and panelist on educational video for population health (consultant fee); GlaxoSmithKline. One‐day consultant for population health, envisioning the future (consultant fee). Sunil Kripalani: Bioscape Digital, stock ownership
- , , . Prevalence, predictors, and outcomes of primary nonadherence after acute myocardial infarction. Circulation. 2008;117(8):1028–1036.
- , , , . Primary medication non‐adherence after discharge from a general internal medicine service. PloS One. 2013;8(5):e61735.
- , , , et al. Trouble getting started: predictors of primary medication nonadherence. Am J Med. 2011;124(11):1081.e9–22.
- , , , , . The incidence and determinants of primary nonadherence with prescribed medication in primary care: a cohort study. Ann Intern Med. 2014;160(7):441–450.
- , , , et al. Effect of a pharmacist intervention on clinically important medication errors after hospital discharge: a randomized trial. Ann Intern Med. 2012;157(1):1–10.
- , , , . Short Test of Functional Health Literacy in Adults. Snow Camp, NC: Peppercorn Books and Press; 1998.
- , , , , . Simplifying detection of cognitive impairment: comparison of the Mini‐Cog and Mini‐Mental State Examination in a multiethnic sample. J Am Geriatr Soc. 2005;53(5):871–874.
- , , . Concurrent and predictive validity of a self‐reported measure of medication adherence. Med Care. 1986;24(1):67–74.
- , , , , , . Health literacy and medication understanding among hospitalized adults. J Hosp Med. 2011;6(9):488–493.
- , , , , , . Medication adherence, social support, and event‐free survival in patients with heart failure. Health Psychol. 2013;32(6):637–646.
- , , , , , . Effect of patient comorbidities on filling of antihypertensive prescriptions. Am J Manag Care. 2009;15(1):24–30.
- , , , et al. Primary nonadherence to statin medications in a managed care organization. J Manag Care Pharm. 2013;19(5):367–373.
- , , , et al. Reducing cost by reducing polypharmacy: the polypharmacy outcomes project. J Am Med Dir Assoc. 2012;13(9):818.e811–815.
- , , , et al. The epidemiology of prescriptions abandoned at the pharmacy. Ann Intern Med. 2010;153(10):633–640.
- , , , , , . Can simple clinical measurements detect patient noncompliance? Hypertension. 1980;2(6):757–764.
- , , . Prevalence, predictors, and outcomes of primary nonadherence after acute myocardial infarction. Circulation. 2008;117(8):1028–1036.
- , , , . Primary medication non‐adherence after discharge from a general internal medicine service. PloS One. 2013;8(5):e61735.
- , , , et al. Trouble getting started: predictors of primary medication nonadherence. Am J Med. 2011;124(11):1081.e9–22.
- , , , , . The incidence and determinants of primary nonadherence with prescribed medication in primary care: a cohort study. Ann Intern Med. 2014;160(7):441–450.
- , , , et al. Effect of a pharmacist intervention on clinically important medication errors after hospital discharge: a randomized trial. Ann Intern Med. 2012;157(1):1–10.
- , , , . Short Test of Functional Health Literacy in Adults. Snow Camp, NC: Peppercorn Books and Press; 1998.
- , , , , . Simplifying detection of cognitive impairment: comparison of the Mini‐Cog and Mini‐Mental State Examination in a multiethnic sample. J Am Geriatr Soc. 2005;53(5):871–874.
- , , . Concurrent and predictive validity of a self‐reported measure of medication adherence. Med Care. 1986;24(1):67–74.
- , , , , , . Health literacy and medication understanding among hospitalized adults. J Hosp Med. 2011;6(9):488–493.
- , , , , , . Medication adherence, social support, and event‐free survival in patients with heart failure. Health Psychol. 2013;32(6):637–646.
- , , , , , . Effect of patient comorbidities on filling of antihypertensive prescriptions. Am J Manag Care. 2009;15(1):24–30.
- , , , et al. Primary nonadherence to statin medications in a managed care organization. J Manag Care Pharm. 2013;19(5):367–373.
- , , , et al. Reducing cost by reducing polypharmacy: the polypharmacy outcomes project. J Am Med Dir Assoc. 2012;13(9):818.e811–815.
- , , , et al. The epidemiology of prescriptions abandoned at the pharmacy. Ann Intern Med. 2010;153(10):633–640.
- , , , , , . Can simple clinical measurements detect patient noncompliance? Hypertension. 1980;2(6):757–764.
Hospital Readmissions in End of Life
The need to improve end‐of‐life care is well recognized. Its quality is often poor, and its cost is enormous, with 30% of the Medicare expenditures used for medical treatments of the 6% of beneficiaries who die each year.[1, 2] Repeated hospitalizations are frequent toward the end of life,[3] where each admission should be viewed as an opportunity to initiate advance care planning to improve end‐of‐life care and possibly reduce future unnecessary readmissions.[4, 5] Identified problems include undertreatment of pain, lack of awareness of patient wishes or advance directives, and unwanted overtreatment.
To improve quality and reduce unnecessary hospital use near the end of life, there is an urgent need to help healthcare providers to better identify the most vulnerable and at‐risk patients to provide them with care coordination and supportive care services. We aimed to identify the risk factors for having a 30‐day potentially avoidable readmission (PAR) due to end‐of‐life care issues.
METHODS
Study Design and Population
A nested case‐control study was designed where potentially avoidable end‐of‐life readmissions were compared to nonreadmitted controls. We collected data on all consecutive adult patient admissions to any medical services of the Brigham and Women's Hospital with a discharge date between July 1, 2009 and June 30, 2010. Brigham and Women's Hospital is a 780‐bed academic medical center in Boston, Massachusetts. To avoid observation stays, only admissions with a length of stay of more than 1 day were included. We excluded patients who died before discharge, were transferred to another acute care hospital, and those who left against medical advice. We also excluded patients with no available data on medication treatment at discharge. The protocol was approved by the institutional review board of Brigham and Women's Hospital/Partners Healthcare.
Study Outcome
The study outcome was any 30‐day PAR due to end‐of‐life issues. To determine this outcome, first we identified all 30‐day readmissions to any service of 3 hospitals within the Partners network in Boston that followed the index hospitalization (prior studies have shown that these hospitals capture approximately 80% of readmissions after a Brigham and Women's Hospital medical hospitalization).[6, 7] These readmissions were subsequently differentiated as potentially avoidable or not using a validated algorithm (SQLape; SQLape, Corseaux, Switzerland).[8, 9] This algorithm uses administrative data and International Classification of Diseases, 9th Revision, Clinical Modification codes from the index and repeat hospitalization. Readmissions were considered potentially avoidable if they were: (1) readmissions related to previously known conditions during the index hospitalization, or (2) complications of treatment (eg, deep vein thrombosis, drug‐induced disorders). Conversely, readmissions were considered unavoidable if they were: (1) foreseen (such as readmissions for transplantation, delivery, chemo‐ or radiotherapy, and other specific surgical procedures), (2) follow‐up and rehabilitation treatments, or (3) readmissions for a new condition unknown during the preceding hospitalization. The algorithm has both a sensitivity and specificity of 96% compared with medical record review using the same criteria. Finally, a random sample of the 30‐day PARs was reviewed independently by 9 trained senior resident physicians to identify those due to end‐of‐life issues, defined by the following 2 criteria: (1) patient has a terminal clinical condition, such as malignancy, end stage renal disease, end stage congestive heart failure, or other condition with a life expectancy of 6 months or less; and (2) the readmission is part of the terminal disease process that was not adequately addressed during the index hospitalization. Examples of factors that were used when identifying cases included lack of healthcare proxy and lack of documentation of why end‐of‐life discussions did not take place during the index hospitalization. Training of adjudicators included a didactic session and review of standardized cases.
Risk Factors
We collected candidate risk factors based on a priori knowledge and according to the medical literature,[10, 11, 12] including demographic information, previous healthcare utilization, and index hospitalization characteristics from administrative data sources; procedures and chronic medical conditions from billing data; last laboratory values and medication information prior to discharge from the electronic medical record (Table 1). When laboratory values were missing (<1%), values were considered as normal.
| Characteristics | No 30‐Day Readmission, n=7,974 | 30‐Day PAR due to End of Life, n=80 | P Value |
|---|---|---|---|
| |||
| Age, y, mean (SD) | 61.5 (16.6) | 60.8 (11.9) | 0.69 |
| Male sex, n (%) | 3875 (48.6) | 37 (46.3) | 0.69 |
| Ethnicity, n (%) | 0.05 | ||
| Non‐Hispanic white* | 5772 (72.4) | 69 (86.3) | |
| Non‐Hispanic black | 1281 (16.1) | 4 (5.0) | |
| Hispanic | 666 (8.4) | 5 (6.3) | |
| Other | 255 (3.2) | 2 (2.5) | |
| Language, n (%) | 0.99 | ||
| English* | 7254 (91.0) | 73 (91.3) | |
| Spanish | 415 (5.2) | 4 (5.0) | |
| Other | 305 (3.8) | 3 (3.8) | |
| Marital status, n (%) | 0.37 | ||
| Currently married or partner* | 4107 (51.35) | 46 (57.5) | |
| Single/never married | 1967 (24.7) | 14 (17.5) | |
| Separated/divorced/widowed/no answer | 1900 (23.8) | 20 (25.0) | |
| Source of index admission, n (%) | 0.10 | ||
| Direct from home/outpatient clinic | 2456 (30.8) | 33 (41.3) | |
| Emergency department* | 4222 (53.0) | 34 (42.5) | |
| Nursing home/rehabilitation/other hospital | 1296 (16.3) | 13 (16.3) | |
| Length of stay of the index admission, median (IQR) | 4 (27) | 5.5 (38] | 0.13 |
| No. of hospital admissions in the past year, median (IQR) | 1 (02) | 2 (03) | <0.001 |
| Any procedure during the hospital stay, n (%) | 4809 (60.3) | 57 (71.3) | 0.05 |
| Identified caregiver at discharge | 7300 (91.6) | 76 (95.0) | 0.27 |
| No. of medications at discharge, mean (SD) | 10.6 (5.1) | 13.0 (5.0) | <0.001 |
| No. of opiate medication at discharge | <0.001 | ||
| 0 | 5297 (66.4) | 21 (26.3) | |
| 1 | 2677 (33.2) | 59 (73.8) | |
| Elixhauser, median (IQR) | 8 (215) | 23 (1442) | <0.001 |
| Selected comorbidities, n (%) | |||
| Diabetes mellitus | 1971 (24.7) | 20 (25.0) | 0.96 |
| Heart failure | 1756 (22.0) | 11 (13.8) | 0.10 |
| Atrial fibrillation | 1439 (18.1) | 10 (12.5) | 0.20 |
| COPD | 816 (10.2) | 7 (8.8) | 0.66 |
| Neoplasm | 2705 (33.9) | 69 (86.3) | <0.001 |
| Stroke | 294 (3.7) | 2 (2.5) | 0.57 |
| ESRD | 1258 (15.8) | 6 (7.5) | 0.04 |
| Liver disease | 328 (4.1) | 2 (2.5) | 0.47 |
Statistical Analysis
We first conducted a bivariate analysis on all collected potential risk factors, comparing admissions followed by a 30‐day PAR due to end‐of‐life care issues with admissions not followed by any 30‐day readmission, using the Pearson [2] test for categorical variables and Student t test for continuous variables. Then, we performed a multivariable logistic regression restricted to the variables that were found significantly associated with the outcome in the bivariate analysis. Age and Elixhauser comorbidity index were forced into the model as important potential confounders. Because a patient could have several outcomes over the study period, we used general estimating equations to cluster at the patient level. All tests were conducted as 2‐sided at a 0.05 level of significance. Analyses were performed using the SAS system for Windows, version 9.3 (SAS Institute Inc., Cary, NC).
RESULTS
From the total of 12,383 patients who were discharged from the medical services of the Brigham and Women's Hospital during the study period, 2108 (17.0%) were excluded because of: (1) death before discharge, (2) transfer to another acute care hospital, (3) discharge against medical advice, or (4) missing data (Figure 1). Among the 10,275 eligible admissions, 22.3% (n=2301) were followed by a 30‐day readmission. Of these, 826 (8.0% of all admissions) were identified as potentially avoidable. Among a random sample of 534 PARs, 80 (15.0%) were related to end‐of‐life care issues (cases). Of note, only 16 (20%) of these patients received palliative care consultation during the index hospitalization. A total of 7974 discharges were not followed by any 30‐day readmission (controls).
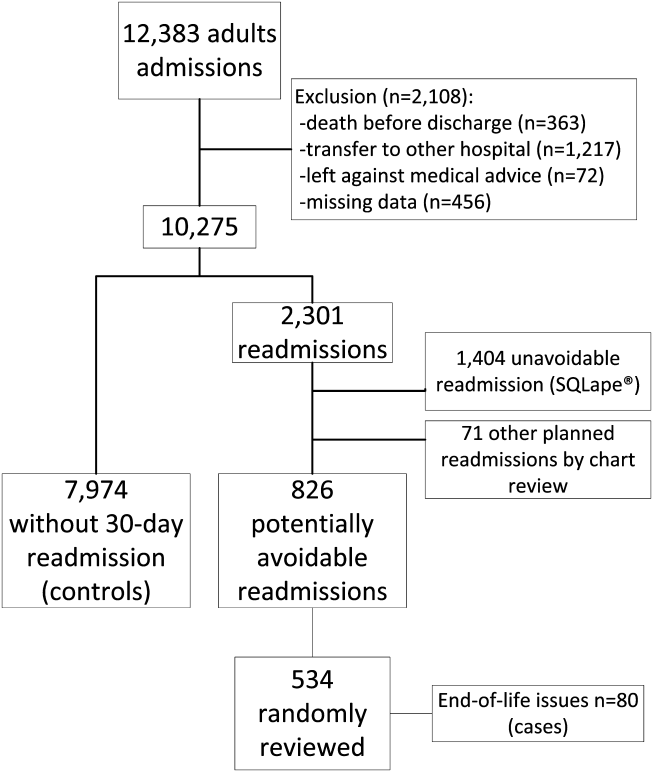
Baseline characteristics are presented in Table 1. Among the combined cohort of cases plus controls, the patient's mean age at inclusion was 61.3 years, and about half were male. In bivariate analysis, demographics such as age and sex were similar between cases and controls. Cases had more hospitalizations in the previous year, a higher number of medications at discharge, and a higher Elixhauser comorbidity index. When looking at diseases more specifically, neoplasm was significantly associated with potentially avoidable 30‐day readmission due to end‐of‐life care issues. In contrast, end‐stage renal disease was associated with a significantly lower risk of 30‐day PAR due to end‐of‐life care issues.
In multivariate analysis, 4 factors remained significantly associated with 30‐day PAR due to end‐of‐life care issues (Table 2). Neoplasm was the strongest risk factor, with an odds ratio of 5.6 (95% confidence interval: 2.8511.0), followed by opiate medication use, Elixhauser score, and number of admissions in the previous 12 months.
| Variable | Odds Ratio (95% CI) |
|---|---|
| |
| Age, per 10 years | 1.04 (0.911.19) |
| No. of admissions in the previous 12 months, per admission | 1.10 (1.021.20)a |
| Total no. of medications at discharge, per medication | 1.04 (1.001.10) |
| Neoplasm | 5.60 (2.8511.0)a |
| Endstage renal disease | 0.60 (0.251.42) |
| Opiate medication at discharge | 2.29 (1.294.07)a |
| Elixhauser, per 5 unit increase | 1.16 (1.101.22)a |
The model, including all 4 variables, had an excellent discrimination power, with a C statistic of 0.85. Without the Elixhauser score, the C statistic remained very high, with a value of 0.82.
DISCUSSION
In a large medical population, potentially avoidable readmissions due to end‐of‐life care issues were not uncommon: 15% of all potentially avoidable readmissions (1.2% of all discharges). We identified 4 main risk factors for having a 30‐day potentially avoidable readmission due to end‐of‐life care issues: neoplasm, opiate use, Elixhauser comorbidity index, and number of admissions in the previous year. In a model that includes these 4 variables, the discrimination was very high with a C statistic of 0.85.
This study extends prior work indicating some risk factors for the need for palliative care. Neoplasm has been logically identified as a criterion for palliative care assessment at the time of admission.[13] Patients with neoplasm are not only at overall high risk for readmission,[14, 15, 16] but they obviously represent a fragile population whose condition is often terminal. Our results suggest that still more attention may be necessary to reduce the risk of readmission due to end‐of‐life care issues in this population (for example, only 20% of cases in our study received palliative care consultation during the index hospitalization). The overall comorbidity measured by the Elixhauser index was not surprisingly a significant risk factor. It probably accounts for the burden of comorbidities, but also for other advanced diseases besides neoplasm, like heart failure, chronic obstructive pulmonary disease, and others that may also be terminal. The number of previous hospital admissions in the past year is also an important risk factor, not only for the general population,[10, 11, 14, 17, 18] but also for patients with more advanced conditions,[19, 20, 21, 22] where admissions become more frequent as the disease progresses toward end stage. Opioid use was the final statistically significant risk factor, specific for this population, likely as a proxy for disease severity and progression toward terminal illness, especially in combination with the other risk factors such as cancer. Age was not a significant factor in either bivariate or multivariate analysis. Previous studies on the risk factors for readmission among patients receiving palliative care also failed to show age as a significant factor.[23, 24] Both of these studies looked at readmissions among patients who were already receiving palliative care. Our study asks a fundamentally different (and in many ways a more practical) question: who among a large population of medical patients might benefit from receiving input from palliative care in the first place. The number of medications at discharge was no longer significant in the multivariate analysis, likely due to its collinearity with the Elixhauser comorbidity index. An increased number of medications might be associated with a higher risk of adverse drug events and readmission, but they would not be necessarily considered to be end‐of‐life readmissions. Taken together, the 4 variables provide a very promising prediction model with high discrimination. To our knowledge, there is no previous existing list of risk factors for 30‐day potentially avoidable readmission due to end‐of‐life care issues, and no existing model to help prioritize palliative care to the most high‐risk patients. It is worth noting that the Elixhauser score might be difficult to calculate before the discharge of the patient (although hospitals with electronic capture of medical problem lists might be able to approximate it). However, even without the Elixhauser score, the C statistic remained very high at 0.82.
Our study has several limitations. Although we looked at readmissions at 2 other affiliated hospitals, some patients might have been readmitted to other acute care facilities outside our network. However, we would not expect the risk factors in these patients to be so different. The identification of end‐of‐life care issues by medical record review is based on a subjective judgment, although strict criteria were used. Furthermore, differentiation between potentially avoidable readmission and unavoidable readmission cannot be perfect. We used clear and logical criteria that were previously validated and allow large database management. Also, we did not analyze a comprehensive list of potential risk factors. It is probable that functional or cognitive status, for example, could also be important risk factors. We purposely chose a set of variables that could be easily obtained from administrative data sources. The small number of cases may have led to limited statistical power to identify less strongly associated risk factors. Last, the results may not be completely generalizable to small or community hospitals, in particular those that may care for less severely ill cancer patients.
Our findings have important implications. End‐of‐life care issues are not infrequent causes of readmission. Our study's findings could help prioritize palliative care resources to those patients at higher risk to improve the quality of end‐of‐life care. The risk factors identified in this study could be used informally by physicians at the bedside to identify such patients. In addition, a hospital could use these factors to provide a second‐level screen, beyond clinician recognition, to assist palliative care teams to identify patients who may not have otherwise been referred. This screen could be automated, for example, by using a list of medical problems from an electronic medical record to approximate an Elixhauser comorbidity score, or even leaving comorbidities out and simply relying on the other 3 easily identifiable risk factors. Such efforts could have a substantial effect on improving care near the end of life and potentially reducing unnecessary hospitalizations.
Acknowledgements
The authors thank Yves Eggli for having screened the database for potentially avoidable readmission using the algorithm SQLape.
Disclosures: Dr. Donz was supported by the Swiss National Science Foundation and the Swiss Foundation for MedicalBiological Scholarships. The Swiss Science National Foundation and the Swiss Foundation for MedicalBiological Scholarships had no role in the design and conduct of this study, the analysis or interpretation of the data, or the preparation of this manuscript. Dr. Schnipper is a consultant to QuantiaMD, for which he has helped create online educational materials for both providers and patients regarding patient safety, including medication safety during transitions in care. The findings of this study are not a part of those materials. Dr. Schnipper has received grant funding from Sanofi‐Aventis for an investigator‐initiated study to design and evaluate an intensive discharge and follow‐up intervention in patients with diabetes. The funder had had no role in the design of the study.
- , , , . Medicare beneficiaries' costs of care in the last year of life. Health Aff (Millwood). 2001;20(4):188–195.
- , , . Quality of End‐of‐Life Cancer Care for Medicare Beneficiaries: Regional and Hospital‐Specific Analyses. Lebanon, NH: The Dartmouth Institute for Health Policy and Clinical Practice; 2010.
- , , . Repeated hospitalizations predict mortality in the community population with heart failure. Am Heart J. 2007;154(2):260–266.
- . Perspectives on care at the close of life. Initiating end‐of‐life discussions with seriously ill patients: addressing the “elephant in the room.” JAMA. 2000;284(19):2502–2507.
- , , , . Advance care planning as a process: structuring the discussions in practice. J Am Geriatr Soc. 1995;43(4):440–446.
- , , , et al. Rationale and design of the Pharmacist Intervention for Low Literacy in Cardiovascular Disease (PILL‐CVD) study. Circ Cardiovasc Qual Outcomes. 2010;3(2):212–219.
- , , , et al. Effect of a pharmacist intervention on clinically important medication errors after hospital discharge: a randomized trial. Ann Intern Med. 2012;157(1):1–10.
- , , , , , . Measuring potentially avoidable hospital readmissions. J Clin Epidemiol. 2002;55(6):573–587.
- , , , , , . Validation of the potentially avoidable hospital readmission rate as a routine indicator of the quality of hospital care. Med Care. 2006;44(11):972–981.
- , , , et al. Hospital readmission in general medicine patients: a prediction model. J Gen Intern Med. 2010;25(3):211–219.
- , , , , , . Screening elders for risk of hospital admission. J Am Geriatr Soc. 1993;41(8):811–817.
- , , , , . Risk factors for 30‐day hospital readmission in patients ≥65 years of age. Proc (Bayl Univ Med Cent). 2008;21(4):363–372.
- , . Identifying patients in need of a palliative care assessment in the hospital setting: a consensus report from the Center to Advance Palliative Care. J Palliat Med. 2011;14(1):17–23.
- , , , . Potentially avoidable 30‐day hospital readmissions in medical patients: derivation and validation of a prediction model. JAMA Intern Med. 2013;173(8):632–638.
- , , , . Redefining readmission risk factors for general medicine patients. J Hosp Med. 2011;6(2):54–60.
- , , . Patient and disease profile of emergency medical readmissions to an Irish teaching hospital. Postgrad Med J. 2004;80(946):470–474.
- , , , . Posthospital care transitions: patterns, complications, and risk identification. Health Serv Res. 2004;39(5):1449–1465.
- , . Factors predicting readmission of older general medicine patients. J Gen Intern Med. 1991;6(5):389–393.
- , , , , . Differences in health care utilization at the end of life among patients with chronic obstructive pulmonary disease and patients with lung cancer. Arch Intern Med. 2006;166(3):326–331.
- , , , , . Frequent hospital readmissions for acute exacerbation of COPD and their associated factors. Respirology. 2006;11(2):188–195.
- , , , et al. Consensus statement: Palliative and supportive care in advanced heart failure. J Card Fail. 2004;10(3):200–209.
- , , , , . Unplanned discharges from a surgical intensive care unit: readmissions and mortality. J Crit Care. 2010;25(3):375–381.
- , , , , . Evaluating causes for unplanned hospital readmissions of palliative care patients. Am J Hosp Palliat Care. 2010;27(8):526–531.
- , , . 30‐day readmissions among seriously ill older adults. J Palliat Med. 2012;15(12):1356–1361.
The need to improve end‐of‐life care is well recognized. Its quality is often poor, and its cost is enormous, with 30% of the Medicare expenditures used for medical treatments of the 6% of beneficiaries who die each year.[1, 2] Repeated hospitalizations are frequent toward the end of life,[3] where each admission should be viewed as an opportunity to initiate advance care planning to improve end‐of‐life care and possibly reduce future unnecessary readmissions.[4, 5] Identified problems include undertreatment of pain, lack of awareness of patient wishes or advance directives, and unwanted overtreatment.
To improve quality and reduce unnecessary hospital use near the end of life, there is an urgent need to help healthcare providers to better identify the most vulnerable and at‐risk patients to provide them with care coordination and supportive care services. We aimed to identify the risk factors for having a 30‐day potentially avoidable readmission (PAR) due to end‐of‐life care issues.
METHODS
Study Design and Population
A nested case‐control study was designed where potentially avoidable end‐of‐life readmissions were compared to nonreadmitted controls. We collected data on all consecutive adult patient admissions to any medical services of the Brigham and Women's Hospital with a discharge date between July 1, 2009 and June 30, 2010. Brigham and Women's Hospital is a 780‐bed academic medical center in Boston, Massachusetts. To avoid observation stays, only admissions with a length of stay of more than 1 day were included. We excluded patients who died before discharge, were transferred to another acute care hospital, and those who left against medical advice. We also excluded patients with no available data on medication treatment at discharge. The protocol was approved by the institutional review board of Brigham and Women's Hospital/Partners Healthcare.
Study Outcome
The study outcome was any 30‐day PAR due to end‐of‐life issues. To determine this outcome, first we identified all 30‐day readmissions to any service of 3 hospitals within the Partners network in Boston that followed the index hospitalization (prior studies have shown that these hospitals capture approximately 80% of readmissions after a Brigham and Women's Hospital medical hospitalization).[6, 7] These readmissions were subsequently differentiated as potentially avoidable or not using a validated algorithm (SQLape; SQLape, Corseaux, Switzerland).[8, 9] This algorithm uses administrative data and International Classification of Diseases, 9th Revision, Clinical Modification codes from the index and repeat hospitalization. Readmissions were considered potentially avoidable if they were: (1) readmissions related to previously known conditions during the index hospitalization, or (2) complications of treatment (eg, deep vein thrombosis, drug‐induced disorders). Conversely, readmissions were considered unavoidable if they were: (1) foreseen (such as readmissions for transplantation, delivery, chemo‐ or radiotherapy, and other specific surgical procedures), (2) follow‐up and rehabilitation treatments, or (3) readmissions for a new condition unknown during the preceding hospitalization. The algorithm has both a sensitivity and specificity of 96% compared with medical record review using the same criteria. Finally, a random sample of the 30‐day PARs was reviewed independently by 9 trained senior resident physicians to identify those due to end‐of‐life issues, defined by the following 2 criteria: (1) patient has a terminal clinical condition, such as malignancy, end stage renal disease, end stage congestive heart failure, or other condition with a life expectancy of 6 months or less; and (2) the readmission is part of the terminal disease process that was not adequately addressed during the index hospitalization. Examples of factors that were used when identifying cases included lack of healthcare proxy and lack of documentation of why end‐of‐life discussions did not take place during the index hospitalization. Training of adjudicators included a didactic session and review of standardized cases.
Risk Factors
We collected candidate risk factors based on a priori knowledge and according to the medical literature,[10, 11, 12] including demographic information, previous healthcare utilization, and index hospitalization characteristics from administrative data sources; procedures and chronic medical conditions from billing data; last laboratory values and medication information prior to discharge from the electronic medical record (Table 1). When laboratory values were missing (<1%), values were considered as normal.
| Characteristics | No 30‐Day Readmission, n=7,974 | 30‐Day PAR due to End of Life, n=80 | P Value |
|---|---|---|---|
| |||
| Age, y, mean (SD) | 61.5 (16.6) | 60.8 (11.9) | 0.69 |
| Male sex, n (%) | 3875 (48.6) | 37 (46.3) | 0.69 |
| Ethnicity, n (%) | 0.05 | ||
| Non‐Hispanic white* | 5772 (72.4) | 69 (86.3) | |
| Non‐Hispanic black | 1281 (16.1) | 4 (5.0) | |
| Hispanic | 666 (8.4) | 5 (6.3) | |
| Other | 255 (3.2) | 2 (2.5) | |
| Language, n (%) | 0.99 | ||
| English* | 7254 (91.0) | 73 (91.3) | |
| Spanish | 415 (5.2) | 4 (5.0) | |
| Other | 305 (3.8) | 3 (3.8) | |
| Marital status, n (%) | 0.37 | ||
| Currently married or partner* | 4107 (51.35) | 46 (57.5) | |
| Single/never married | 1967 (24.7) | 14 (17.5) | |
| Separated/divorced/widowed/no answer | 1900 (23.8) | 20 (25.0) | |
| Source of index admission, n (%) | 0.10 | ||
| Direct from home/outpatient clinic | 2456 (30.8) | 33 (41.3) | |
| Emergency department* | 4222 (53.0) | 34 (42.5) | |
| Nursing home/rehabilitation/other hospital | 1296 (16.3) | 13 (16.3) | |
| Length of stay of the index admission, median (IQR) | 4 (27) | 5.5 (38] | 0.13 |
| No. of hospital admissions in the past year, median (IQR) | 1 (02) | 2 (03) | <0.001 |
| Any procedure during the hospital stay, n (%) | 4809 (60.3) | 57 (71.3) | 0.05 |
| Identified caregiver at discharge | 7300 (91.6) | 76 (95.0) | 0.27 |
| No. of medications at discharge, mean (SD) | 10.6 (5.1) | 13.0 (5.0) | <0.001 |
| No. of opiate medication at discharge | <0.001 | ||
| 0 | 5297 (66.4) | 21 (26.3) | |
| 1 | 2677 (33.2) | 59 (73.8) | |
| Elixhauser, median (IQR) | 8 (215) | 23 (1442) | <0.001 |
| Selected comorbidities, n (%) | |||
| Diabetes mellitus | 1971 (24.7) | 20 (25.0) | 0.96 |
| Heart failure | 1756 (22.0) | 11 (13.8) | 0.10 |
| Atrial fibrillation | 1439 (18.1) | 10 (12.5) | 0.20 |
| COPD | 816 (10.2) | 7 (8.8) | 0.66 |
| Neoplasm | 2705 (33.9) | 69 (86.3) | <0.001 |
| Stroke | 294 (3.7) | 2 (2.5) | 0.57 |
| ESRD | 1258 (15.8) | 6 (7.5) | 0.04 |
| Liver disease | 328 (4.1) | 2 (2.5) | 0.47 |
Statistical Analysis
We first conducted a bivariate analysis on all collected potential risk factors, comparing admissions followed by a 30‐day PAR due to end‐of‐life care issues with admissions not followed by any 30‐day readmission, using the Pearson [2] test for categorical variables and Student t test for continuous variables. Then, we performed a multivariable logistic regression restricted to the variables that were found significantly associated with the outcome in the bivariate analysis. Age and Elixhauser comorbidity index were forced into the model as important potential confounders. Because a patient could have several outcomes over the study period, we used general estimating equations to cluster at the patient level. All tests were conducted as 2‐sided at a 0.05 level of significance. Analyses were performed using the SAS system for Windows, version 9.3 (SAS Institute Inc., Cary, NC).
RESULTS
From the total of 12,383 patients who were discharged from the medical services of the Brigham and Women's Hospital during the study period, 2108 (17.0%) were excluded because of: (1) death before discharge, (2) transfer to another acute care hospital, (3) discharge against medical advice, or (4) missing data (Figure 1). Among the 10,275 eligible admissions, 22.3% (n=2301) were followed by a 30‐day readmission. Of these, 826 (8.0% of all admissions) were identified as potentially avoidable. Among a random sample of 534 PARs, 80 (15.0%) were related to end‐of‐life care issues (cases). Of note, only 16 (20%) of these patients received palliative care consultation during the index hospitalization. A total of 7974 discharges were not followed by any 30‐day readmission (controls).

Baseline characteristics are presented in Table 1. Among the combined cohort of cases plus controls, the patient's mean age at inclusion was 61.3 years, and about half were male. In bivariate analysis, demographics such as age and sex were similar between cases and controls. Cases had more hospitalizations in the previous year, a higher number of medications at discharge, and a higher Elixhauser comorbidity index. When looking at diseases more specifically, neoplasm was significantly associated with potentially avoidable 30‐day readmission due to end‐of‐life care issues. In contrast, end‐stage renal disease was associated with a significantly lower risk of 30‐day PAR due to end‐of‐life care issues.
In multivariate analysis, 4 factors remained significantly associated with 30‐day PAR due to end‐of‐life care issues (Table 2). Neoplasm was the strongest risk factor, with an odds ratio of 5.6 (95% confidence interval: 2.8511.0), followed by opiate medication use, Elixhauser score, and number of admissions in the previous 12 months.
| Variable | Odds Ratio (95% CI) |
|---|---|
| |
| Age, per 10 years | 1.04 (0.911.19) |
| No. of admissions in the previous 12 months, per admission | 1.10 (1.021.20)a |
| Total no. of medications at discharge, per medication | 1.04 (1.001.10) |
| Neoplasm | 5.60 (2.8511.0)a |
| Endstage renal disease | 0.60 (0.251.42) |
| Opiate medication at discharge | 2.29 (1.294.07)a |
| Elixhauser, per 5 unit increase | 1.16 (1.101.22)a |
The model, including all 4 variables, had an excellent discrimination power, with a C statistic of 0.85. Without the Elixhauser score, the C statistic remained very high, with a value of 0.82.
DISCUSSION
In a large medical population, potentially avoidable readmissions due to end‐of‐life care issues were not uncommon: 15% of all potentially avoidable readmissions (1.2% of all discharges). We identified 4 main risk factors for having a 30‐day potentially avoidable readmission due to end‐of‐life care issues: neoplasm, opiate use, Elixhauser comorbidity index, and number of admissions in the previous year. In a model that includes these 4 variables, the discrimination was very high with a C statistic of 0.85.
This study extends prior work indicating some risk factors for the need for palliative care. Neoplasm has been logically identified as a criterion for palliative care assessment at the time of admission.[13] Patients with neoplasm are not only at overall high risk for readmission,[14, 15, 16] but they obviously represent a fragile population whose condition is often terminal. Our results suggest that still more attention may be necessary to reduce the risk of readmission due to end‐of‐life care issues in this population (for example, only 20% of cases in our study received palliative care consultation during the index hospitalization). The overall comorbidity measured by the Elixhauser index was not surprisingly a significant risk factor. It probably accounts for the burden of comorbidities, but also for other advanced diseases besides neoplasm, like heart failure, chronic obstructive pulmonary disease, and others that may also be terminal. The number of previous hospital admissions in the past year is also an important risk factor, not only for the general population,[10, 11, 14, 17, 18] but also for patients with more advanced conditions,[19, 20, 21, 22] where admissions become more frequent as the disease progresses toward end stage. Opioid use was the final statistically significant risk factor, specific for this population, likely as a proxy for disease severity and progression toward terminal illness, especially in combination with the other risk factors such as cancer. Age was not a significant factor in either bivariate or multivariate analysis. Previous studies on the risk factors for readmission among patients receiving palliative care also failed to show age as a significant factor.[23, 24] Both of these studies looked at readmissions among patients who were already receiving palliative care. Our study asks a fundamentally different (and in many ways a more practical) question: who among a large population of medical patients might benefit from receiving input from palliative care in the first place. The number of medications at discharge was no longer significant in the multivariate analysis, likely due to its collinearity with the Elixhauser comorbidity index. An increased number of medications might be associated with a higher risk of adverse drug events and readmission, but they would not be necessarily considered to be end‐of‐life readmissions. Taken together, the 4 variables provide a very promising prediction model with high discrimination. To our knowledge, there is no previous existing list of risk factors for 30‐day potentially avoidable readmission due to end‐of‐life care issues, and no existing model to help prioritize palliative care to the most high‐risk patients. It is worth noting that the Elixhauser score might be difficult to calculate before the discharge of the patient (although hospitals with electronic capture of medical problem lists might be able to approximate it). However, even without the Elixhauser score, the C statistic remained very high at 0.82.
Our study has several limitations. Although we looked at readmissions at 2 other affiliated hospitals, some patients might have been readmitted to other acute care facilities outside our network. However, we would not expect the risk factors in these patients to be so different. The identification of end‐of‐life care issues by medical record review is based on a subjective judgment, although strict criteria were used. Furthermore, differentiation between potentially avoidable readmission and unavoidable readmission cannot be perfect. We used clear and logical criteria that were previously validated and allow large database management. Also, we did not analyze a comprehensive list of potential risk factors. It is probable that functional or cognitive status, for example, could also be important risk factors. We purposely chose a set of variables that could be easily obtained from administrative data sources. The small number of cases may have led to limited statistical power to identify less strongly associated risk factors. Last, the results may not be completely generalizable to small or community hospitals, in particular those that may care for less severely ill cancer patients.
Our findings have important implications. End‐of‐life care issues are not infrequent causes of readmission. Our study's findings could help prioritize palliative care resources to those patients at higher risk to improve the quality of end‐of‐life care. The risk factors identified in this study could be used informally by physicians at the bedside to identify such patients. In addition, a hospital could use these factors to provide a second‐level screen, beyond clinician recognition, to assist palliative care teams to identify patients who may not have otherwise been referred. This screen could be automated, for example, by using a list of medical problems from an electronic medical record to approximate an Elixhauser comorbidity score, or even leaving comorbidities out and simply relying on the other 3 easily identifiable risk factors. Such efforts could have a substantial effect on improving care near the end of life and potentially reducing unnecessary hospitalizations.
Acknowledgements
The authors thank Yves Eggli for having screened the database for potentially avoidable readmission using the algorithm SQLape.
Disclosures: Dr. Donz was supported by the Swiss National Science Foundation and the Swiss Foundation for MedicalBiological Scholarships. The Swiss Science National Foundation and the Swiss Foundation for MedicalBiological Scholarships had no role in the design and conduct of this study, the analysis or interpretation of the data, or the preparation of this manuscript. Dr. Schnipper is a consultant to QuantiaMD, for which he has helped create online educational materials for both providers and patients regarding patient safety, including medication safety during transitions in care. The findings of this study are not a part of those materials. Dr. Schnipper has received grant funding from Sanofi‐Aventis for an investigator‐initiated study to design and evaluate an intensive discharge and follow‐up intervention in patients with diabetes. The funder had had no role in the design of the study.
The need to improve end‐of‐life care is well recognized. Its quality is often poor, and its cost is enormous, with 30% of the Medicare expenditures used for medical treatments of the 6% of beneficiaries who die each year.[1, 2] Repeated hospitalizations are frequent toward the end of life,[3] where each admission should be viewed as an opportunity to initiate advance care planning to improve end‐of‐life care and possibly reduce future unnecessary readmissions.[4, 5] Identified problems include undertreatment of pain, lack of awareness of patient wishes or advance directives, and unwanted overtreatment.
To improve quality and reduce unnecessary hospital use near the end of life, there is an urgent need to help healthcare providers to better identify the most vulnerable and at‐risk patients to provide them with care coordination and supportive care services. We aimed to identify the risk factors for having a 30‐day potentially avoidable readmission (PAR) due to end‐of‐life care issues.
METHODS
Study Design and Population
A nested case‐control study was designed where potentially avoidable end‐of‐life readmissions were compared to nonreadmitted controls. We collected data on all consecutive adult patient admissions to any medical services of the Brigham and Women's Hospital with a discharge date between July 1, 2009 and June 30, 2010. Brigham and Women's Hospital is a 780‐bed academic medical center in Boston, Massachusetts. To avoid observation stays, only admissions with a length of stay of more than 1 day were included. We excluded patients who died before discharge, were transferred to another acute care hospital, and those who left against medical advice. We also excluded patients with no available data on medication treatment at discharge. The protocol was approved by the institutional review board of Brigham and Women's Hospital/Partners Healthcare.
Study Outcome
The study outcome was any 30‐day PAR due to end‐of‐life issues. To determine this outcome, first we identified all 30‐day readmissions to any service of 3 hospitals within the Partners network in Boston that followed the index hospitalization (prior studies have shown that these hospitals capture approximately 80% of readmissions after a Brigham and Women's Hospital medical hospitalization).[6, 7] These readmissions were subsequently differentiated as potentially avoidable or not using a validated algorithm (SQLape; SQLape, Corseaux, Switzerland).[8, 9] This algorithm uses administrative data and International Classification of Diseases, 9th Revision, Clinical Modification codes from the index and repeat hospitalization. Readmissions were considered potentially avoidable if they were: (1) readmissions related to previously known conditions during the index hospitalization, or (2) complications of treatment (eg, deep vein thrombosis, drug‐induced disorders). Conversely, readmissions were considered unavoidable if they were: (1) foreseen (such as readmissions for transplantation, delivery, chemo‐ or radiotherapy, and other specific surgical procedures), (2) follow‐up and rehabilitation treatments, or (3) readmissions for a new condition unknown during the preceding hospitalization. The algorithm has both a sensitivity and specificity of 96% compared with medical record review using the same criteria. Finally, a random sample of the 30‐day PARs was reviewed independently by 9 trained senior resident physicians to identify those due to end‐of‐life issues, defined by the following 2 criteria: (1) patient has a terminal clinical condition, such as malignancy, end stage renal disease, end stage congestive heart failure, or other condition with a life expectancy of 6 months or less; and (2) the readmission is part of the terminal disease process that was not adequately addressed during the index hospitalization. Examples of factors that were used when identifying cases included lack of healthcare proxy and lack of documentation of why end‐of‐life discussions did not take place during the index hospitalization. Training of adjudicators included a didactic session and review of standardized cases.
Risk Factors
We collected candidate risk factors based on a priori knowledge and according to the medical literature,[10, 11, 12] including demographic information, previous healthcare utilization, and index hospitalization characteristics from administrative data sources; procedures and chronic medical conditions from billing data; last laboratory values and medication information prior to discharge from the electronic medical record (Table 1). When laboratory values were missing (<1%), values were considered as normal.
| Characteristics | No 30‐Day Readmission, n=7,974 | 30‐Day PAR due to End of Life, n=80 | P Value |
|---|---|---|---|
| |||
| Age, y, mean (SD) | 61.5 (16.6) | 60.8 (11.9) | 0.69 |
| Male sex, n (%) | 3875 (48.6) | 37 (46.3) | 0.69 |
| Ethnicity, n (%) | 0.05 | ||
| Non‐Hispanic white* | 5772 (72.4) | 69 (86.3) | |
| Non‐Hispanic black | 1281 (16.1) | 4 (5.0) | |
| Hispanic | 666 (8.4) | 5 (6.3) | |
| Other | 255 (3.2) | 2 (2.5) | |
| Language, n (%) | 0.99 | ||
| English* | 7254 (91.0) | 73 (91.3) | |
| Spanish | 415 (5.2) | 4 (5.0) | |
| Other | 305 (3.8) | 3 (3.8) | |
| Marital status, n (%) | 0.37 | ||
| Currently married or partner* | 4107 (51.35) | 46 (57.5) | |
| Single/never married | 1967 (24.7) | 14 (17.5) | |
| Separated/divorced/widowed/no answer | 1900 (23.8) | 20 (25.0) | |
| Source of index admission, n (%) | 0.10 | ||
| Direct from home/outpatient clinic | 2456 (30.8) | 33 (41.3) | |
| Emergency department* | 4222 (53.0) | 34 (42.5) | |
| Nursing home/rehabilitation/other hospital | 1296 (16.3) | 13 (16.3) | |
| Length of stay of the index admission, median (IQR) | 4 (27) | 5.5 (38] | 0.13 |
| No. of hospital admissions in the past year, median (IQR) | 1 (02) | 2 (03) | <0.001 |
| Any procedure during the hospital stay, n (%) | 4809 (60.3) | 57 (71.3) | 0.05 |
| Identified caregiver at discharge | 7300 (91.6) | 76 (95.0) | 0.27 |
| No. of medications at discharge, mean (SD) | 10.6 (5.1) | 13.0 (5.0) | <0.001 |
| No. of opiate medication at discharge | <0.001 | ||
| 0 | 5297 (66.4) | 21 (26.3) | |
| 1 | 2677 (33.2) | 59 (73.8) | |
| Elixhauser, median (IQR) | 8 (215) | 23 (1442) | <0.001 |
| Selected comorbidities, n (%) | |||
| Diabetes mellitus | 1971 (24.7) | 20 (25.0) | 0.96 |
| Heart failure | 1756 (22.0) | 11 (13.8) | 0.10 |
| Atrial fibrillation | 1439 (18.1) | 10 (12.5) | 0.20 |
| COPD | 816 (10.2) | 7 (8.8) | 0.66 |
| Neoplasm | 2705 (33.9) | 69 (86.3) | <0.001 |
| Stroke | 294 (3.7) | 2 (2.5) | 0.57 |
| ESRD | 1258 (15.8) | 6 (7.5) | 0.04 |
| Liver disease | 328 (4.1) | 2 (2.5) | 0.47 |
Statistical Analysis
We first conducted a bivariate analysis on all collected potential risk factors, comparing admissions followed by a 30‐day PAR due to end‐of‐life care issues with admissions not followed by any 30‐day readmission, using the Pearson [2] test for categorical variables and Student t test for continuous variables. Then, we performed a multivariable logistic regression restricted to the variables that were found significantly associated with the outcome in the bivariate analysis. Age and Elixhauser comorbidity index were forced into the model as important potential confounders. Because a patient could have several outcomes over the study period, we used general estimating equations to cluster at the patient level. All tests were conducted as 2‐sided at a 0.05 level of significance. Analyses were performed using the SAS system for Windows, version 9.3 (SAS Institute Inc., Cary, NC).
RESULTS
From the total of 12,383 patients who were discharged from the medical services of the Brigham and Women's Hospital during the study period, 2108 (17.0%) were excluded because of: (1) death before discharge, (2) transfer to another acute care hospital, (3) discharge against medical advice, or (4) missing data (Figure 1). Among the 10,275 eligible admissions, 22.3% (n=2301) were followed by a 30‐day readmission. Of these, 826 (8.0% of all admissions) were identified as potentially avoidable. Among a random sample of 534 PARs, 80 (15.0%) were related to end‐of‐life care issues (cases). Of note, only 16 (20%) of these patients received palliative care consultation during the index hospitalization. A total of 7974 discharges were not followed by any 30‐day readmission (controls).

Baseline characteristics are presented in Table 1. Among the combined cohort of cases plus controls, the patient's mean age at inclusion was 61.3 years, and about half were male. In bivariate analysis, demographics such as age and sex were similar between cases and controls. Cases had more hospitalizations in the previous year, a higher number of medications at discharge, and a higher Elixhauser comorbidity index. When looking at diseases more specifically, neoplasm was significantly associated with potentially avoidable 30‐day readmission due to end‐of‐life care issues. In contrast, end‐stage renal disease was associated with a significantly lower risk of 30‐day PAR due to end‐of‐life care issues.
In multivariate analysis, 4 factors remained significantly associated with 30‐day PAR due to end‐of‐life care issues (Table 2). Neoplasm was the strongest risk factor, with an odds ratio of 5.6 (95% confidence interval: 2.8511.0), followed by opiate medication use, Elixhauser score, and number of admissions in the previous 12 months.
| Variable | Odds Ratio (95% CI) |
|---|---|
| |
| Age, per 10 years | 1.04 (0.911.19) |
| No. of admissions in the previous 12 months, per admission | 1.10 (1.021.20)a |
| Total no. of medications at discharge, per medication | 1.04 (1.001.10) |
| Neoplasm | 5.60 (2.8511.0)a |
| Endstage renal disease | 0.60 (0.251.42) |
| Opiate medication at discharge | 2.29 (1.294.07)a |
| Elixhauser, per 5 unit increase | 1.16 (1.101.22)a |
The model, including all 4 variables, had an excellent discrimination power, with a C statistic of 0.85. Without the Elixhauser score, the C statistic remained very high, with a value of 0.82.
DISCUSSION
In a large medical population, potentially avoidable readmissions due to end‐of‐life care issues were not uncommon: 15% of all potentially avoidable readmissions (1.2% of all discharges). We identified 4 main risk factors for having a 30‐day potentially avoidable readmission due to end‐of‐life care issues: neoplasm, opiate use, Elixhauser comorbidity index, and number of admissions in the previous year. In a model that includes these 4 variables, the discrimination was very high with a C statistic of 0.85.
This study extends prior work indicating some risk factors for the need for palliative care. Neoplasm has been logically identified as a criterion for palliative care assessment at the time of admission.[13] Patients with neoplasm are not only at overall high risk for readmission,[14, 15, 16] but they obviously represent a fragile population whose condition is often terminal. Our results suggest that still more attention may be necessary to reduce the risk of readmission due to end‐of‐life care issues in this population (for example, only 20% of cases in our study received palliative care consultation during the index hospitalization). The overall comorbidity measured by the Elixhauser index was not surprisingly a significant risk factor. It probably accounts for the burden of comorbidities, but also for other advanced diseases besides neoplasm, like heart failure, chronic obstructive pulmonary disease, and others that may also be terminal. The number of previous hospital admissions in the past year is also an important risk factor, not only for the general population,[10, 11, 14, 17, 18] but also for patients with more advanced conditions,[19, 20, 21, 22] where admissions become more frequent as the disease progresses toward end stage. Opioid use was the final statistically significant risk factor, specific for this population, likely as a proxy for disease severity and progression toward terminal illness, especially in combination with the other risk factors such as cancer. Age was not a significant factor in either bivariate or multivariate analysis. Previous studies on the risk factors for readmission among patients receiving palliative care also failed to show age as a significant factor.[23, 24] Both of these studies looked at readmissions among patients who were already receiving palliative care. Our study asks a fundamentally different (and in many ways a more practical) question: who among a large population of medical patients might benefit from receiving input from palliative care in the first place. The number of medications at discharge was no longer significant in the multivariate analysis, likely due to its collinearity with the Elixhauser comorbidity index. An increased number of medications might be associated with a higher risk of adverse drug events and readmission, but they would not be necessarily considered to be end‐of‐life readmissions. Taken together, the 4 variables provide a very promising prediction model with high discrimination. To our knowledge, there is no previous existing list of risk factors for 30‐day potentially avoidable readmission due to end‐of‐life care issues, and no existing model to help prioritize palliative care to the most high‐risk patients. It is worth noting that the Elixhauser score might be difficult to calculate before the discharge of the patient (although hospitals with electronic capture of medical problem lists might be able to approximate it). However, even without the Elixhauser score, the C statistic remained very high at 0.82.
Our study has several limitations. Although we looked at readmissions at 2 other affiliated hospitals, some patients might have been readmitted to other acute care facilities outside our network. However, we would not expect the risk factors in these patients to be so different. The identification of end‐of‐life care issues by medical record review is based on a subjective judgment, although strict criteria were used. Furthermore, differentiation between potentially avoidable readmission and unavoidable readmission cannot be perfect. We used clear and logical criteria that were previously validated and allow large database management. Also, we did not analyze a comprehensive list of potential risk factors. It is probable that functional or cognitive status, for example, could also be important risk factors. We purposely chose a set of variables that could be easily obtained from administrative data sources. The small number of cases may have led to limited statistical power to identify less strongly associated risk factors. Last, the results may not be completely generalizable to small or community hospitals, in particular those that may care for less severely ill cancer patients.
Our findings have important implications. End‐of‐life care issues are not infrequent causes of readmission. Our study's findings could help prioritize palliative care resources to those patients at higher risk to improve the quality of end‐of‐life care. The risk factors identified in this study could be used informally by physicians at the bedside to identify such patients. In addition, a hospital could use these factors to provide a second‐level screen, beyond clinician recognition, to assist palliative care teams to identify patients who may not have otherwise been referred. This screen could be automated, for example, by using a list of medical problems from an electronic medical record to approximate an Elixhauser comorbidity score, or even leaving comorbidities out and simply relying on the other 3 easily identifiable risk factors. Such efforts could have a substantial effect on improving care near the end of life and potentially reducing unnecessary hospitalizations.
Acknowledgements
The authors thank Yves Eggli for having screened the database for potentially avoidable readmission using the algorithm SQLape.
Disclosures: Dr. Donz was supported by the Swiss National Science Foundation and the Swiss Foundation for MedicalBiological Scholarships. The Swiss Science National Foundation and the Swiss Foundation for MedicalBiological Scholarships had no role in the design and conduct of this study, the analysis or interpretation of the data, or the preparation of this manuscript. Dr. Schnipper is a consultant to QuantiaMD, for which he has helped create online educational materials for both providers and patients regarding patient safety, including medication safety during transitions in care. The findings of this study are not a part of those materials. Dr. Schnipper has received grant funding from Sanofi‐Aventis for an investigator‐initiated study to design and evaluate an intensive discharge and follow‐up intervention in patients with diabetes. The funder had had no role in the design of the study.
- , , , . Medicare beneficiaries' costs of care in the last year of life. Health Aff (Millwood). 2001;20(4):188–195.
- , , . Quality of End‐of‐Life Cancer Care for Medicare Beneficiaries: Regional and Hospital‐Specific Analyses. Lebanon, NH: The Dartmouth Institute for Health Policy and Clinical Practice; 2010.
- , , . Repeated hospitalizations predict mortality in the community population with heart failure. Am Heart J. 2007;154(2):260–266.
- . Perspectives on care at the close of life. Initiating end‐of‐life discussions with seriously ill patients: addressing the “elephant in the room.” JAMA. 2000;284(19):2502–2507.
- , , , . Advance care planning as a process: structuring the discussions in practice. J Am Geriatr Soc. 1995;43(4):440–446.
- , , , et al. Rationale and design of the Pharmacist Intervention for Low Literacy in Cardiovascular Disease (PILL‐CVD) study. Circ Cardiovasc Qual Outcomes. 2010;3(2):212–219.
- , , , et al. Effect of a pharmacist intervention on clinically important medication errors after hospital discharge: a randomized trial. Ann Intern Med. 2012;157(1):1–10.
- , , , , , . Measuring potentially avoidable hospital readmissions. J Clin Epidemiol. 2002;55(6):573–587.
- , , , , , . Validation of the potentially avoidable hospital readmission rate as a routine indicator of the quality of hospital care. Med Care. 2006;44(11):972–981.
- , , , et al. Hospital readmission in general medicine patients: a prediction model. J Gen Intern Med. 2010;25(3):211–219.
- , , , , , . Screening elders for risk of hospital admission. J Am Geriatr Soc. 1993;41(8):811–817.
- , , , , . Risk factors for 30‐day hospital readmission in patients ≥65 years of age. Proc (Bayl Univ Med Cent). 2008;21(4):363–372.
- , . Identifying patients in need of a palliative care assessment in the hospital setting: a consensus report from the Center to Advance Palliative Care. J Palliat Med. 2011;14(1):17–23.
- , , , . Potentially avoidable 30‐day hospital readmissions in medical patients: derivation and validation of a prediction model. JAMA Intern Med. 2013;173(8):632–638.
- , , , . Redefining readmission risk factors for general medicine patients. J Hosp Med. 2011;6(2):54–60.
- , , . Patient and disease profile of emergency medical readmissions to an Irish teaching hospital. Postgrad Med J. 2004;80(946):470–474.
- , , , . Posthospital care transitions: patterns, complications, and risk identification. Health Serv Res. 2004;39(5):1449–1465.
- , . Factors predicting readmission of older general medicine patients. J Gen Intern Med. 1991;6(5):389–393.
- , , , , . Differences in health care utilization at the end of life among patients with chronic obstructive pulmonary disease and patients with lung cancer. Arch Intern Med. 2006;166(3):326–331.
- , , , , . Frequent hospital readmissions for acute exacerbation of COPD and their associated factors. Respirology. 2006;11(2):188–195.
- , , , et al. Consensus statement: Palliative and supportive care in advanced heart failure. J Card Fail. 2004;10(3):200–209.
- , , , , . Unplanned discharges from a surgical intensive care unit: readmissions and mortality. J Crit Care. 2010;25(3):375–381.
- , , , , . Evaluating causes for unplanned hospital readmissions of palliative care patients. Am J Hosp Palliat Care. 2010;27(8):526–531.
- , , . 30‐day readmissions among seriously ill older adults. J Palliat Med. 2012;15(12):1356–1361.
- , , , . Medicare beneficiaries' costs of care in the last year of life. Health Aff (Millwood). 2001;20(4):188–195.
- , , . Quality of End‐of‐Life Cancer Care for Medicare Beneficiaries: Regional and Hospital‐Specific Analyses. Lebanon, NH: The Dartmouth Institute for Health Policy and Clinical Practice; 2010.
- , , . Repeated hospitalizations predict mortality in the community population with heart failure. Am Heart J. 2007;154(2):260–266.
- . Perspectives on care at the close of life. Initiating end‐of‐life discussions with seriously ill patients: addressing the “elephant in the room.” JAMA. 2000;284(19):2502–2507.
- , , , . Advance care planning as a process: structuring the discussions in practice. J Am Geriatr Soc. 1995;43(4):440–446.
- , , , et al. Rationale and design of the Pharmacist Intervention for Low Literacy in Cardiovascular Disease (PILL‐CVD) study. Circ Cardiovasc Qual Outcomes. 2010;3(2):212–219.
- , , , et al. Effect of a pharmacist intervention on clinically important medication errors after hospital discharge: a randomized trial. Ann Intern Med. 2012;157(1):1–10.
- , , , , , . Measuring potentially avoidable hospital readmissions. J Clin Epidemiol. 2002;55(6):573–587.
- , , , , , . Validation of the potentially avoidable hospital readmission rate as a routine indicator of the quality of hospital care. Med Care. 2006;44(11):972–981.
- , , , et al. Hospital readmission in general medicine patients: a prediction model. J Gen Intern Med. 2010;25(3):211–219.
- , , , , , . Screening elders for risk of hospital admission. J Am Geriatr Soc. 1993;41(8):811–817.
- , , , , . Risk factors for 30‐day hospital readmission in patients ≥65 years of age. Proc (Bayl Univ Med Cent). 2008;21(4):363–372.
- , . Identifying patients in need of a palliative care assessment in the hospital setting: a consensus report from the Center to Advance Palliative Care. J Palliat Med. 2011;14(1):17–23.
- , , , . Potentially avoidable 30‐day hospital readmissions in medical patients: derivation and validation of a prediction model. JAMA Intern Med. 2013;173(8):632–638.
- , , , . Redefining readmission risk factors for general medicine patients. J Hosp Med. 2011;6(2):54–60.
- , , . Patient and disease profile of emergency medical readmissions to an Irish teaching hospital. Postgrad Med J. 2004;80(946):470–474.
- , , , . Posthospital care transitions: patterns, complications, and risk identification. Health Serv Res. 2004;39(5):1449–1465.
- , . Factors predicting readmission of older general medicine patients. J Gen Intern Med. 1991;6(5):389–393.
- , , , , . Differences in health care utilization at the end of life among patients with chronic obstructive pulmonary disease and patients with lung cancer. Arch Intern Med. 2006;166(3):326–331.
- , , , , . Frequent hospital readmissions for acute exacerbation of COPD and their associated factors. Respirology. 2006;11(2):188–195.
- , , , et al. Consensus statement: Palliative and supportive care in advanced heart failure. J Card Fail. 2004;10(3):200–209.
- , , , , . Unplanned discharges from a surgical intensive care unit: readmissions and mortality. J Crit Care. 2010;25(3):375–381.
- , , , , . Evaluating causes for unplanned hospital readmissions of palliative care patients. Am J Hosp Palliat Care. 2010;27(8):526–531.
- , , . 30‐day readmissions among seriously ill older adults. J Palliat Med. 2012;15(12):1356–1361.
© 2014 Society of Hospital Medicine
Patients at Risk for 30‐Day Readmission
Readmissions to the hospital are common and costly.[1] However, identifying patients prospectively who are likely to be readmitted and who may benefit from interventions to reduce readmission risk has proven challenging, with published risk scores having only moderate ability to discriminate between patients likely and unlikely to be readmitted.[2] One reason for this may be that published studies have not typically focused on patients who are cognitively impaired, psychiatrically ill, have low health or English literacy, or have poor social supports, all of whom may represent a substantial fraction of readmitted patients.[2, 3, 4, 5]
Psychiatric disease, in particular, may contribute to increased readmission risk for nonpsychiatric (medical) illness, and is associated with increased utilization of healthcare resources.[6, 7, 8, 9, 10, 11] For example, patients with mental illness who were discharged from New York hospitals were more likely to be rehospitalized and had more costly readmissions than patients without these comorbidities, including a length of stay nearly 1 day longer on average.[7] An unmet need for treatment of substance abuse was projected to cost Tennessee $772 million of excess healthcare costs in 2000, mostly incurred through repeat hospitalizations and emergency department (ED) visits.[10]
Despite this, few investigators have considered the role of psychiatric disease and/or substance abuse in medical readmission risk. The purpose of the current study was to evaluate the role of psychiatric illness and substance abuse in unselected medical patients to determine their relative contributions to 30‐day all‐cause readmissions (ACR) and potentially avoidable readmissions (PAR).
METHODS
Patients and Setting
We conducted a retrospective cohort study of consecutive adult patients discharged from medicine services at Brigham and Women's Hospital (BWH), a 747‐bed tertiary referral center and teaching hospital, between July 1, 2009 and June 30, 2010. Most patients are cared for by resident housestaff teams at BWH (approximately 25% are cared for by physician assistants working directly with attending physicians), and approximately half receive primary care in the Partners system, which has a shared electronic medical record (EMR). Outpatient mental health services are provided by Partners‐associated mental health professionals including those at McLean Hospital and MassHealth (Medicaid)‐associated sites through the Massachusetts Behavioral Health Partnership. Exclusion criteria were death in the hospital or discharge to another acute care facility. We also excluded patients who left against medical advice (AMA). The study protocol was approved by the Partners Institutional Review Board.
Outcome
The primary outcomes were ACR and PAR within 30 days of discharge. First, we identified all 30‐day readmissions to BWH or to 2 other hospitals in the Partners Healthcare Network (previous studies have shown that 80% of all readmitted patients are readmitted to 1 of these 3 hospitals).[12] For patients with multiple readmissions, only the first readmission was included in the dataset.
To find potentially avoidable readmissions, administrative and billing data for these patients were processed using the SQLape (SQLape s.a.r.l., Corseaux, Switzerland) algorithm, which identifies PAR by excluding patients who undergo planned follow‐up treatment (such as a cycle of planned chemotherapy) or are readmitted for conditions unrelated in any way to the index hospitalization.[13, 14] Common complications of treatment are categorized as potentially avoidable, such as development of a deep venous thrombosis, a decubitus ulcer after prolonged bed rest, or bleeding complications after starting anticoagulation. Although the algorithm identifies theoretically preventable readmissions, the algorithm does not quantify how preventable they are, and these are thus referred to as potentially avoidable. This is similar to other admission metrics, such as the Agency for Healthcare Research and Quality's prevention quality indicators, which are created from a list of ambulatory care‐sensitive conditions.[15] SQLape has the advantage of being a specific tool for readmissions. Patients with 30‐day readmissions identified by SQLape as planned or unlikely to be avoidable were excluded in the PAR analysis, although still included in ACR analysis. In each case, the comparison group is patients without any readmission.
Predictors
Our predictors of interest included the overall prevalence of a psychiatric diagnosis or diagnosis of substance abuse, the presence of specific psychiatric diagnoses, and prescription of psychiatric medications to help assess the independent contribution of these comorbidities to readmission risk.
We used a combination of easily obtainable inpatient and outpatient clinical and administrative data to identify relevant patients. Patients were considered likely to be psychiatrically ill if they: (1) had a psychiatric diagnosis on their Partners outpatient EMR problem list and were prescribed a medication to treat that condition as an outpatient, or (2) had an International Classification of Diseases, 9th Revision diagnosis of a psychiatric illness at hospital discharge. Patients were considered to have moderate probability of disease if they: (1) had a psychiatric diagnosis on their outpatient problem list, or (2) were prescribed a medication intended to treat a psychiatric condition as an outpatient. Patients were considered unlikely to have psychiatric disease if none of these criteria were met. Patients were considered likely to have a substance abuse disorder if they had this diagnosis on their outpatient EMR, or were prescribed a medication to treat this condition (eg, buprenorphine/naloxone), or received inpatient consultation from a substance abuse treatment team during their inpatient hospitalization, and were considered unlikely if none of these were true. We also evaluated individual categories of psychiatric illness (schizophrenia, depression, anxiety, bipolar disorder) and of psychotropic medications (antidepressants, antipsychotics, anxiolytics).
Potential Confounders
Data on potential confounders, based on prior literature,[16, 17] collected at the index admission were derived from electronic administrative, clinical, and billing sources, including the Brigham Integrated Computer System and the Partners Clinical Data Repository. They included patient age, gender, ethnicity, primary language, marital status, insurance status, living situation prior to admission, discharge location, length of stay, Elixhauser comorbidity index,[18] total number of medications prescribed, and number of prior admissions and ED visits in the prior year.
Statistical Analysis
Bivariate comparisons of each of the predictors of ACR and PAR risk (ie, patients with a 30‐day ACR or PAR vs those not readmitted within 30 days) were conducted using 2 trend tests for ordinal predictors (eg, likelihood of psychiatric disease), and 2 or Fisher exact test for dichotomous predictors (eg, receipt of inpatient substance abuse counseling).
We then used multivariate logistic regression analysis to adjust for all of the potential confounders noted above, entering each variable related to psychiatric illness into the model separately (eg, likely psychiatric illness, number of psychiatric medications). In a secondary analysis, we removed potentially collinear variables from the final model; as this did not alter the results, the full model is presented. We also conducted a secondary analysis where we included patients who left against medical advice (AMA), which also did not alter the results. Two‐sided P values <0.05 were considered significant, and all analyses were performed using the SAS version 9.2 (SAS Institute, Inc., Cary, NC).
RESULTS
There were 7984 unique patients discharged during the study period. Patients were generally white and English speaking; just over half of admissions came from the ED (Table 1). Of note, nearly all patients were insured, as are almost all patients in Massachusetts. They had high degrees of comorbid illness and large numbers of prescribed medications. Nearly 30% had at least 1 hospital admission within the prior year.
| Characteristic | All Patients, N (%) | Not Readmitted, N (%) | ACR, N (%) | PAR N (%)a |
|---|---|---|---|---|
| ||||
| Study cohort | 6987 (100) | 5727 (72) | 1260 (18) | 388 (5.6) |
| Age, y | ||||
| <50 | 1663 (23.8) | 1343 (23.5) | 320 (25.4) | 85 (21.9) |
| 5165 | 2273 (32.5) | 1859 (32.5) | 414 (32.9) | 136 (35.1) |
| 6679 | 1444 (20.7) | 1176 (20.5) | 268 (18.6) | 80 (20.6) |
| >80 | 1607 (23.0) | 1349 (23.6) | 258 (16.1) | 87 (22.4) |
| Female | 3604 (51.6) | 2967 (51.8) | 637 (50.6) | 206 (53.1) |
| Race | ||||
| White | 5126 (73.4) | 4153 (72.5) | 973 (77.2) | 300 (77.3) |
| Black | 1075 (15.4) | 899 (15.7) | 176 (14.0) | 53 (13.7) |
| Hispanic | 562 (8.0) | 477 (8.3) | 85 (6.8) | 28 (7.2) |
| Other | 224 (3.2) | 198 (3.5) | 26 (2.1) | 7 (1.8) |
| Primary language | ||||
| English | 6345 (90.8) | 5180 (90.5) | 1165 (92.5) | 356 (91.8) |
| Marital status | ||||
| Married | 3642 (52.1) | 2942 (51.4) | 702 (55.7) | 214 (55.2) |
| Single, never married | 1662 (23.8) | 1393 (24.3) | 269 (21.4) | 73 (18.8) |
| Previously married | 1683 (24.1) | 1386 (24.2) | 289 (22.9) | 101 (26.0) |
| Insurance | ||||
| Medicare | 3550 (50.8) | 2949 (51.5) | 601 (47.7) | 188 (48.5) |
| Medicaid | 539 (7.7) | 430 (7.5) | 109 (8.7) | 33 (8.5) |
| Private | 2892 (41.4) | 2344 (40.9) | 548 (43.5) | 167 (43.0) |
| Uninsured | 6 (0.1) | 4 (0.1) | 2 (0.1) | 0 (0) |
| Source of index admission | ||||
| Clinic or home | 2136 (30.6) | 1711 (29.9) | 425 (33.7) | 117 (30.2) |
| Emergency department | 3592 (51.4) | 2999 (52.4) | 593 (47.1) | 181 (46.7) |
| Nursing facility | 1204 (17.2) | 977 (17.1) | 227 (18.0) | 84 (21.7) |
| Other | 55 (0.1) | 40 (0.7) | 15 (1.1) | 6 (1.6) |
| Length of stay, d | ||||
| 02 | 1757 (25.2) | 1556 (27.2) | 201 (16.0) | 55 (14.2) |
| 34 | 2200 (31.5) | 1842 (32.2) | 358 (28.4) | 105 (27.1) |
| 57 | 1521 (21.8) | 1214 (21.2) | 307 (24.4) | 101 (26.0) |
| >7 | 1509 (21.6) | 1115 (19.5) | 394 (31.3) | 127 (32.7) |
| Elixhauser comorbidity index score | ||||
| 01 | 1987 (28.4) | 1729 (30.2) | 258 (20.5) | 66 (17.0) |
| 27 | 1773 (25.4) | 1541 (26.9) | 232 (18.4) | 67 (17.3) |
| 813 | 1535 (22.0) | 1212 (21.2) | 323 (25.6) | 86 (22.2) |
| >13 | 1692 (24.2) | 1245 (21.7) | 447 (35.5) | 169 (43.6) |
| Medications prescribed as outpatient | ||||
| 06 | 1684 (24.1) | 1410 (24.6) | 274 (21.8) | 72 (18.6) |
| 79 | 1601 (22.9) | 1349 (23.6) | 252 (20.0) | 77 (19.9) |
| 1013 | 1836 (26.3) | 1508 (26.3) | 328 (26.0) | 107 (27.6) |
| >13 | 1866 (26.7) | 1460 (25.5) | 406 (32.2) | 132 (34.0) |
| Number of admissions in past year | ||||
| 0 | 4816 (68.9) | 4032 (70.4) | 784 (62.2) | 279 (71.9) |
| 15 | 2075 (29.7) | 1640 (28.6) | 435 (34.5) | 107 (27.6) |
| >5 | 96 (1.4) | 55 (1.0) | 41 (3.3) | 2 (0.5) |
| Number of ED visits in past year | ||||
| 0 | 4661 (66.7) | 3862 (67.4) | 799 (63.4) | 261 (67.3) |
| 15 | 2326 (33.3) | 1865 (32.6) | 461 (36.6) | 127 (32.7) |
All‐Cause Readmissions
After exclusion of 997 patients who died, were discharged to skilled nursing or rehabilitation facilities, or left AMA, 6987 patients were included (Figure 1). Of these, 1260 had a readmission (18%). Approximately half were considered unlikely to be psychiatrically ill, 22% were considered moderately likely, and 29% likely (Table 2).
| All‐Cause Readmission Analysis | Potentially Avoidable Readmission Analysis | |||||
|---|---|---|---|---|---|---|
| No. in Cohort (%) | % of Patients With ACR | P Valuea | No. in Cohort (%) | % of Patients With PAR | P Valuea | |
| ||||||
| Entire cohort | 6987 | 18.0 | 6115 | 6.3 | ||
| Likelihood of psychiatric illness | ||||||
| Unlikely | 3424 (49) | 16.5 | 3026 (49) | 5.6 | ||
| Moderate | 1564 (22) | 23.5 | 1302 (21) | 7.1 | ||
| Likely | 1999 (29) | 16.4 | 1787 (29) | 6.4 | ||
| Likely versus unlikely | 0.87 | 0.20 | ||||
| Moderate+likely versus unlikely | 0.001 | 0.02 | ||||
| Likelihood of substance abuse | 0.01 | 0.20 | ||||
| Unlikely | 5804 (83) | 18.7 | 5104 (83) | 6.5 | ||
| Likely | 1183 (17) | 14.8 | 1011 (17) | 5.4 | 0.14 | |
| Number of prescribed outpatient psychotropic medications | <0.001 | 0.04 | ||||
| 0 | 4420 (63) | 16.3 | 3931 (64) | 5.9 | ||
| 1 | 1725 (25) | 20.4 | 1481 (24) | 7.2 | ||
| 2 | 781 (11) | 22.3 | 653 (11) | 7.0 | ||
| >2 | 61 (1) | 23.0 | 50 (1) | 6.0 | ||
| Prescribed antidepressant | 1474 (21) | 20.6 | 0.005 | 1248 (20) | 6.2 | 0.77 |
| Prescribed antipsychotic | 375 (5) | 22.4 | 0.02 | 315 (5) | 7.6 | 0.34 |
| Prescribed mood stabilizer | 81 (1) | 18.5 | 0.91 | 69 (1) | 4.4 | 0.49 |
| Prescribed anxiolytic | 1814 (26) | 21.8 | <0.001 | 1537 (25) | 7.7 | 0.01 |
| Prescribed stimulant | 101 (2) | 26.7 | 0.02 | 83 (1) | 10.8 | 0.09 |
| Prescribed pharmacologic treatment for substance abuse | 79 (1) | 25.3 | 0.09 | 60 (1) | 1.7 | 0.14 |
| Number of psychiatric diagnoses on outpatient problem list | 0.31 | 0.74 | ||||
| 0 | 6405 (92) | 18.2 | 5509 (90) | 6.3 | ||
| 1 or more | 582 (8) | 16.5 | 474 (8) | 7.0 | ||
| Outpatient diagnosis of substance abuse | 159 (2) | 13.2 | 0.11 | 144 (2) | 4.2 | 0.28 |
| Outpatient diagnosis of any psychiatric illness | 582 (8) | 16.5 | 0.31 | 517 (8) | 8.0 | 0.73 |
| Discharge diagnosis of depression | 774 (11) | 17.7 | 0.80 | 690 (11) | 7.7 | 0.13 |
| Discharge diagnosis of schizophrenia | 56 (1) | 23.2 | 0.31 | 50 (1) | 14 | 0.03 |
| Discharge diagnosis of bipolar disorder | 101 (1) | 10.9 | 0.06 | 92 (2) | 2.2 | 0.10 |
| Discharge diagnosis of anxiety | 1192 (17) | 15.0 | 0.003 | 1080 (18) | 6.2 | 0.83 |
| Discharge diagnosis of substance abuse | 885 (13) | 14.8 | 0.008 | 803 (13) | 6.1 | 0.76 |
| Discharge diagnosis of any psychiatric illness | 1839 (26) | 16.0 | 0.008 | 1654 (27) | 6.6 | 0.63 |
| Substance abuse consultation as inpatient | 284 (4) | 14.4 | 0.11 | 252 (4) | 3.6 | 0.07 |
In bivariate analysis (Table 2), likelihood of psychiatric illness (P<0.01) and increasing numbers of prescribed outpatient psychiatric medications (P<0.01) were significantly associated with ACR. In multivariate analysis, each additional prescribed outpatient psychiatric medication increased ACR risk (odds ratio [OR]: 1.10, 95% confidence interval [CI]: 1.01‐1.20) or any prescription of an anxiolytic in particular (OR: 1.16, 95% CI: 1.001.35) was associated with increased risk of ACR, whereas discharge diagnoses of anxiety (OR: 0.82, 95% CI: 0.68‐0.99) and substance abuse (OR: 0.80, 95% CI: 0.65‐0.99) were associated with lower risk of ACR (Table 3).
| ACR, OR (95% CI) | PAR, OR (95% CI)a | |
|---|---|---|
| ||
| Likely psychiatric disease | 0.97 (0.82‐1.14) | 1.20 (0.92‐1.56) |
| Likely and possible psychiatric disease | 1.07 (0.94‐1.22) | 1.18 (0.94‐1.47) |
| Likely substance abuse | 0.83 (0.69‐0.99) | 0.85 (0.63‐1.16) |
| Psychiatric diagnosis on outpatient problem list | 0.97 (0.76‐1.23) | 1.04 (0.70‐1.55) |
| Substance abuse diagnosis on outpatient problem list | 0.63 (0.39‐1.02) | 0.65 (0.28‐1.52) |
| Increasing number of prescribed psychiatric medications | 1.10 (1.01‐1.20) | 1.00 (0.86‐1.16) |
| Outpatient prescription for antidepressant | 1.10 (0.94‐1.29) | 0.86 (0.66‐1.13) |
| Outpatient prescription for antipsychotic | 1.03 (0.79‐1.34) | 0.93 (0.59‐1.45) |
| Outpatient prescription for anxiolytic | 1.16 (1.001.35) | 1.13 (0.88‐1.44) |
| Outpatient prescription for methadone or buprenorphine | 1.15 (0.67‐1.98) | 0.18 (0.03‐1.36) |
| Discharge diagnosis of depression | 1.06 (0.86‐1.30) | 1.49 (1.09‐2.04) |
| Discharge diagnosis of schizophrenia | 1.43 (0.75‐2.74) | 2.63 (1.13‐6.13) |
| Discharge diagnosis of bipolar disorder | 0.53 (0.28‐1.02) | 0.35 (0.09‐1.45) |
| Discharge diagnosis of anxiety | 0.82 (0.68‐0.99) | 1.11 (0.83‐1.49) |
| Discharge diagnosis of substance abuse | 0.80 (0.65‐0.99) | 1.05 (0.75‐1.46) |
| Discharge diagnosis of any psychiatric illness | 0.88 (0.75‐1.02) | 1.22 (0.96‐1.56) |
| Addiction team consult while inpatient | 0.82 (0.58‐1.17) | 0.58 (0.29‐1.17) |
Potentially Avoidable Readmissions
After further exclusion of 872 patients who had unavoidable readmissions according to the SQLape algorithm, 6115 patients remained. Of these, 388 had a PAR within 30 days (6.3%, Table 1).
In bivariate analysis (Table 2), the likelihood of psychiatric illness (P=0.02), number of outpatient psychiatric medications (P=0.04), and prescription of anxiolytics (P=0.01) were significantly associated with PAR, as they were with ACR. A discharge diagnosis of schizophrenia was also associated with PAR (P=0.03).
In multivariate analysis, only discharge diagnoses of depression (OR: 1.49, 95% CI: 1.09‐2.04) and schizophrenia (OR: 2.63, 95% CI: 1.13‐6.13) were associated with PAR.
DISCUSSION
Comorbid psychiatric illness was common among patients admitted to the medicine wards. Patients with documented discharge diagnoses of depression or schizophrenia were at highest risk for a potentially avoidable 30‐day readmission, whereas those prescribed more psychiatric medications were at increased risk for ACR. These findings were independent of a comprehensive set of risk factors among medicine inpatients in this retrospective cohort study.
This study extends prior work indicating patients with psychiatric disease have increased healthcare utilization,[6, 7, 8, 9, 10, 11] by identifying at least 2 subpopulations of the psychiatrically ill (those with depression and schizophrenia) at particularly high risk for 30‐day PAR. To our knowledge, this is the first study to identify schizophrenia as a predictor of hospital readmission for medical illnesses. One prior study prospectively identified depression as increasing the 90‐day risk of readmission 3‐fold, although medication usage was not assessed,[6] and our report strengthens this association.
There are several possible explanations why these two subpopulations in particular would be more predisposed to readmissions that are potentially avoidable. It is known that patients with schizophrenia, for example, live on average 20 years less than the general population, and most of this excess mortality is due to medical illnesses.[20, 21] Reasons for this may include poor healthcare access, adverse effects of medication, and socioeconomic factors among others.[21, 22] All of these reasons may contribute to the increased PAR risk in this population, mediated, for example, by decreased ability to adhere to postdischarge care plans. Successful community‐based interventions to decrease these inequities have been described and could serve as a model for addressing the increased readmission risk in this population.[23]
Our finding that patients with a greater number of prescribed psychiatric medications are at increased risk for ACR may be expected, given other studies that have highlighted the crucial importance of medications in postdischarge adverse events, including readmissions.[24] Indeed, medication‐related errors and toxicities are the most common postdischarge adverse events experienced by patients.[25] Whether psychiatric medications are particularly prone to causing postdischarge adverse events or whether these medications represent greater psychiatric comorbidity cannot be answered by this study.
It was surprising but reassuring that substance abuse was not a predictor of short‐term readmissions as identified using our measures; in fact, a discharge diagnosis of substance abuse was associated with lower risk of ACR than comparator patients. It seems unlikely that we would have inadequate power to find such a result, as we found a statistically significant negative association in the ACR population, and 17% of our population overall was considered likely to have a substance abuse comorbidity. However, it is likely the burden of disease was underestimated given that we did not try to determine the contribution of long‐term substance abuse to medical diseases that may increase readmission risk (eg, liver cirrhosis from alcohol use). Unlike other conditions in our study, patients with substance abuse diagnoses at BWH can be seen by a dedicated multidisciplinary team while an inpatient to start treatment and plan for postdischarge follow‐up; this may have played a role in our findings.
A discharge diagnosis of anxiety was also somewhat protective against readmission, whereas a prescription of an anxiolytic (predominantly benzodiazepines) increased risk; many patients prescribed a benzodiazepine do not have a Diagnostic and Statistical Manual of Mental Disorders4th Edition (DSM‐IV) diagnosis of anxiety disorder, and thus these findings may reflect different patient populations. Discharging physicians may have used anxiety as a discharge diagnosis in patients in whom they suspected somatic complaints without organic basis; these patients may be at lower risk of readmission.
Discharge diagnoses of psychiatric illnesses were associated with ACR and PAR in our study, but outpatient diagnoses were not. This likely reflects greater severity of illness (documentation as a treated diagnosis on discharge indicates the illness was relevant during the hospitalization), but may also reflect inaccuracies of diagnosis and lack of assessment of severity in outpatient coding, which would bias toward null findings. Although many of the patients in our study were seen by primary care doctors within the Partners system, some patients had outside primary care physicians and we did not have access to these records. This may also have decreased our ability to find associations.
The findings of our study should be interpreted in the context of the study design. Our study was retrospective, which limited our ability to conclusively diagnose psychiatric disease presence or severity (as is true of most institutions, validated psychiatric screening was not routinely used at our institutions on hospital admission or discharge). However, we used a conservative scale to classify the likelihood of patients having psychiatric or substance abuse disorders, and we used other metrics to establish the presence of illness, such as the number of prescribed medications, inpatient consultation with a substance abuse service, and hospital discharge diagnoses. This approach also allowed us to quickly identify a large cohort unaffected by selection bias. Our study was single center, potentially limiting generalizability. Although we capture at least 80% of readmissions, we were not able to capture all readmissions, and we cannot rule out that patients readmitted elsewhere are different than those readmitted within the Partners system. Last, the SQLape algorithm is not perfectly sensitive or specific in identifying avoidable readmissions,[13] but it does eliminate many readmissions that are clearly unavoidable, creating an enriched cohort of patients whose readmissions are more likely to be avoidable and therefore potentially actionable.
We suggest that our study findings first be considered when risk stratifying patients before hospital discharge in terms of readmission risk. Patients with depression and schizophrenia would seem to merit postdischarge interventions to decrease their potentially avoidable readmissions. Compulsory community treatment (a feature of treatment in Canada and Australia that is ordered by clinicians) has been shown to decrease mortality due to medical illness in patients who have been hospitalized and are psychiatrically ill, and addition of these services to postdischarge care may be useful.[23] Inpatient physicians could work to ensure follow‐up not just with medical providers but with robust outpatient mental health programs to decrease potentially avoidable readmission risk, and administrators could work to ensure close linkages with these community resources. Studies evaluating the impact of these types of interventions would need to be conducted. Patients with polypharmacy, including psychiatric medications, may benefit from interventions to improve medication safety, such as enhanced medication reconciliation and pharmacist counseling.[26]
Our study suggests that patients with depression, those with schizophrenia, and those who have increased numbers of prescribed psychiatric medications should be considered at high risk for readmission for medical illnesses. Targeting interventions to these patients may be fruitful in preventing avoidable readmissions.
Acknowledgements
The authors thank Dr. Yves Eggli for screening the database for potentially avoidable readmissions using the SQLape algorithm.
Disclosures
Dr. Donz was supported by the Swiss National Science Foundation and the Swiss Foundation for MedicalBiological Scholarships. The authors otherwise have no conflicts of interest to disclose. The content is solely the responsibility of the authors and does not necessarily represent the official views of the US Department of Veterans Affairs.
- , , . Rehospitalizations among patients in the Medicare fee‐for‐service program. N Engl J Med. 2009;360(14):1418–1428.
- , , , et al. Risk prediction models for hospital readmission: a systematic review. JAMA. 2011;306(15):1688–1698.
- , . Hospital readmission as an accountability measure. JAMA. 2011;305(5):504–505.
- , , , , , . Postdischarge environmental and socioeconomic factors and the likelihood of early hospital readmission among community‐dwelling Medicare beneficiaries. Gerontologist. 2008;48(4):495–504.
- , , , , . Low health literacy and health outcomes: an updated systematic review. Ann Intern Med. 2011;155(2):97–107.
- , , , et al. Depression is a risk factor for rehospitalization in medical inpatients. Prim Care Companion J Clin Psychiatry. 2007;9(4):256–262.
- , , , . Mental illness and hospitalization for ambulatory care sensitive medical conditions. Med Care. 2008;46(12):1249–1256.
- , , , , , . Substance use treatment barriers for patients with frequent hospital admissions. J Subst Abuse Treat. 2010;38(1):22–30.
- , , , , . Managed care and the quality of substance abuse treatment. J Ment Health Policy Econ. 2002;5(4):163–174.
- , , , , . Unmet substance abuse treatment need, health services utilization, and cost: a population‐based emergency department study. Ann Emerg Med. 2005;45(2):118–127.
- , , , . Elderly Medicare inpatients with substance use disorders: characteristics and predictors of hospital readmissions over a four‐year interval. J Stud Alcohol. 2000;61(6):891–895.
- , , , et al. Rationale and design of the Pharmacist Intervention for Low Literacy in Cardiovascular Disease (PILL‐CVD) study. Circ Cardiovasc Qual Outcomes. 2010;3(2):212–219.
- , , , , , . Validation of the potentially avoidable hospital readmission rate as a routine indicator of the quality of hospital care. Med Care. 2006;44(11);972–981.
- , , , , , . Measuring potentially avoidable hospital readmissions. J Clin Epidemiol. 2002;55:573–587.
- Agency for Healthcare Research and Quality Quality Indicators. (April 7, 2006). Prevention Quality Indicators (PQI) Composite Measure Workgroup Final Report. Available at: http://www.qualityindicators.ahrq.gov/modules/pqi_resources.aspx. Accessed June 1, 2012.
- , , , et al. Hospital readmission in general medicine patients: a prediction model. J Gen Intern Med. 2010;25(3):211–219.
- , , , et al. Derivation and validation of an index to predict early death or unplanned readmission after discharge from hospital to the community. CMAJ. 2010;182(6):551–557.
- , , , . Comorbidity measures for use with administrative data. Med Care. Jan 1998;36(1):8–27.
- , , , , eds. Morbidity and mortality in people with serious mental illness. October 2006. National Association of State Mental Health Directors, Medical Directors Council. Available at: http://www.nasmhpd.org/docs/publications/MDCdocs/Mortality%20and%20 Mo rbidity%20Final%20Report%208.18.08.pdf. Accessed January 13, 2013.
- , , , . Mortality in individuals who have had psychiatric treatment: population‐based study in Nova Scotia. Br J Psychiat. 2005;187:552–558.
- , , , , , . Inequitable access for mentally ill patients to some medically necessary procedures. CMAJ. 2007;176(6):779–784.
- , , . Quality of medical care for people with and without comorbid mental illness and substance misuse: systematic review of comparative studies. Br J Psychiat. 2009;194:491–499.
- , , , , , . Reducing all‐cause mortality among patients with psychiatric disorders: a population‐based study. CMAJ. 2013;185(1):E50–E56.
- , , , et al. Incidence of potentially avoidable urgent readmissions and their relation to all‐cause urgent readmissions. CMAJ. 2011;183(14):E1067–E1072.
- , , , , . The incidence and severity of adverse events affecting patients after discharge from the hospital. Ann Intern Med. 2003;138(3):161–167.
- , , , . Hospital‐based medication reconciliation practices: a systematic review. Arch Intern Med. 2012;172(14):1057–1069.
Readmissions to the hospital are common and costly.[1] However, identifying patients prospectively who are likely to be readmitted and who may benefit from interventions to reduce readmission risk has proven challenging, with published risk scores having only moderate ability to discriminate between patients likely and unlikely to be readmitted.[2] One reason for this may be that published studies have not typically focused on patients who are cognitively impaired, psychiatrically ill, have low health or English literacy, or have poor social supports, all of whom may represent a substantial fraction of readmitted patients.[2, 3, 4, 5]
Psychiatric disease, in particular, may contribute to increased readmission risk for nonpsychiatric (medical) illness, and is associated with increased utilization of healthcare resources.[6, 7, 8, 9, 10, 11] For example, patients with mental illness who were discharged from New York hospitals were more likely to be rehospitalized and had more costly readmissions than patients without these comorbidities, including a length of stay nearly 1 day longer on average.[7] An unmet need for treatment of substance abuse was projected to cost Tennessee $772 million of excess healthcare costs in 2000, mostly incurred through repeat hospitalizations and emergency department (ED) visits.[10]
Despite this, few investigators have considered the role of psychiatric disease and/or substance abuse in medical readmission risk. The purpose of the current study was to evaluate the role of psychiatric illness and substance abuse in unselected medical patients to determine their relative contributions to 30‐day all‐cause readmissions (ACR) and potentially avoidable readmissions (PAR).
METHODS
Patients and Setting
We conducted a retrospective cohort study of consecutive adult patients discharged from medicine services at Brigham and Women's Hospital (BWH), a 747‐bed tertiary referral center and teaching hospital, between July 1, 2009 and June 30, 2010. Most patients are cared for by resident housestaff teams at BWH (approximately 25% are cared for by physician assistants working directly with attending physicians), and approximately half receive primary care in the Partners system, which has a shared electronic medical record (EMR). Outpatient mental health services are provided by Partners‐associated mental health professionals including those at McLean Hospital and MassHealth (Medicaid)‐associated sites through the Massachusetts Behavioral Health Partnership. Exclusion criteria were death in the hospital or discharge to another acute care facility. We also excluded patients who left against medical advice (AMA). The study protocol was approved by the Partners Institutional Review Board.
Outcome
The primary outcomes were ACR and PAR within 30 days of discharge. First, we identified all 30‐day readmissions to BWH or to 2 other hospitals in the Partners Healthcare Network (previous studies have shown that 80% of all readmitted patients are readmitted to 1 of these 3 hospitals).[12] For patients with multiple readmissions, only the first readmission was included in the dataset.
To find potentially avoidable readmissions, administrative and billing data for these patients were processed using the SQLape (SQLape s.a.r.l., Corseaux, Switzerland) algorithm, which identifies PAR by excluding patients who undergo planned follow‐up treatment (such as a cycle of planned chemotherapy) or are readmitted for conditions unrelated in any way to the index hospitalization.[13, 14] Common complications of treatment are categorized as potentially avoidable, such as development of a deep venous thrombosis, a decubitus ulcer after prolonged bed rest, or bleeding complications after starting anticoagulation. Although the algorithm identifies theoretically preventable readmissions, the algorithm does not quantify how preventable they are, and these are thus referred to as potentially avoidable. This is similar to other admission metrics, such as the Agency for Healthcare Research and Quality's prevention quality indicators, which are created from a list of ambulatory care‐sensitive conditions.[15] SQLape has the advantage of being a specific tool for readmissions. Patients with 30‐day readmissions identified by SQLape as planned or unlikely to be avoidable were excluded in the PAR analysis, although still included in ACR analysis. In each case, the comparison group is patients without any readmission.
Predictors
Our predictors of interest included the overall prevalence of a psychiatric diagnosis or diagnosis of substance abuse, the presence of specific psychiatric diagnoses, and prescription of psychiatric medications to help assess the independent contribution of these comorbidities to readmission risk.
We used a combination of easily obtainable inpatient and outpatient clinical and administrative data to identify relevant patients. Patients were considered likely to be psychiatrically ill if they: (1) had a psychiatric diagnosis on their Partners outpatient EMR problem list and were prescribed a medication to treat that condition as an outpatient, or (2) had an International Classification of Diseases, 9th Revision diagnosis of a psychiatric illness at hospital discharge. Patients were considered to have moderate probability of disease if they: (1) had a psychiatric diagnosis on their outpatient problem list, or (2) were prescribed a medication intended to treat a psychiatric condition as an outpatient. Patients were considered unlikely to have psychiatric disease if none of these criteria were met. Patients were considered likely to have a substance abuse disorder if they had this diagnosis on their outpatient EMR, or were prescribed a medication to treat this condition (eg, buprenorphine/naloxone), or received inpatient consultation from a substance abuse treatment team during their inpatient hospitalization, and were considered unlikely if none of these were true. We also evaluated individual categories of psychiatric illness (schizophrenia, depression, anxiety, bipolar disorder) and of psychotropic medications (antidepressants, antipsychotics, anxiolytics).
Potential Confounders
Data on potential confounders, based on prior literature,[16, 17] collected at the index admission were derived from electronic administrative, clinical, and billing sources, including the Brigham Integrated Computer System and the Partners Clinical Data Repository. They included patient age, gender, ethnicity, primary language, marital status, insurance status, living situation prior to admission, discharge location, length of stay, Elixhauser comorbidity index,[18] total number of medications prescribed, and number of prior admissions and ED visits in the prior year.
Statistical Analysis
Bivariate comparisons of each of the predictors of ACR and PAR risk (ie, patients with a 30‐day ACR or PAR vs those not readmitted within 30 days) were conducted using 2 trend tests for ordinal predictors (eg, likelihood of psychiatric disease), and 2 or Fisher exact test for dichotomous predictors (eg, receipt of inpatient substance abuse counseling).
We then used multivariate logistic regression analysis to adjust for all of the potential confounders noted above, entering each variable related to psychiatric illness into the model separately (eg, likely psychiatric illness, number of psychiatric medications). In a secondary analysis, we removed potentially collinear variables from the final model; as this did not alter the results, the full model is presented. We also conducted a secondary analysis where we included patients who left against medical advice (AMA), which also did not alter the results. Two‐sided P values <0.05 were considered significant, and all analyses were performed using the SAS version 9.2 (SAS Institute, Inc., Cary, NC).
RESULTS
There were 7984 unique patients discharged during the study period. Patients were generally white and English speaking; just over half of admissions came from the ED (Table 1). Of note, nearly all patients were insured, as are almost all patients in Massachusetts. They had high degrees of comorbid illness and large numbers of prescribed medications. Nearly 30% had at least 1 hospital admission within the prior year.
| Characteristic | All Patients, N (%) | Not Readmitted, N (%) | ACR, N (%) | PAR N (%)a |
|---|---|---|---|---|
| ||||
| Study cohort | 6987 (100) | 5727 (72) | 1260 (18) | 388 (5.6) |
| Age, y | ||||
| <50 | 1663 (23.8) | 1343 (23.5) | 320 (25.4) | 85 (21.9) |
| 5165 | 2273 (32.5) | 1859 (32.5) | 414 (32.9) | 136 (35.1) |
| 6679 | 1444 (20.7) | 1176 (20.5) | 268 (18.6) | 80 (20.6) |
| >80 | 1607 (23.0) | 1349 (23.6) | 258 (16.1) | 87 (22.4) |
| Female | 3604 (51.6) | 2967 (51.8) | 637 (50.6) | 206 (53.1) |
| Race | ||||
| White | 5126 (73.4) | 4153 (72.5) | 973 (77.2) | 300 (77.3) |
| Black | 1075 (15.4) | 899 (15.7) | 176 (14.0) | 53 (13.7) |
| Hispanic | 562 (8.0) | 477 (8.3) | 85 (6.8) | 28 (7.2) |
| Other | 224 (3.2) | 198 (3.5) | 26 (2.1) | 7 (1.8) |
| Primary language | ||||
| English | 6345 (90.8) | 5180 (90.5) | 1165 (92.5) | 356 (91.8) |
| Marital status | ||||
| Married | 3642 (52.1) | 2942 (51.4) | 702 (55.7) | 214 (55.2) |
| Single, never married | 1662 (23.8) | 1393 (24.3) | 269 (21.4) | 73 (18.8) |
| Previously married | 1683 (24.1) | 1386 (24.2) | 289 (22.9) | 101 (26.0) |
| Insurance | ||||
| Medicare | 3550 (50.8) | 2949 (51.5) | 601 (47.7) | 188 (48.5) |
| Medicaid | 539 (7.7) | 430 (7.5) | 109 (8.7) | 33 (8.5) |
| Private | 2892 (41.4) | 2344 (40.9) | 548 (43.5) | 167 (43.0) |
| Uninsured | 6 (0.1) | 4 (0.1) | 2 (0.1) | 0 (0) |
| Source of index admission | ||||
| Clinic or home | 2136 (30.6) | 1711 (29.9) | 425 (33.7) | 117 (30.2) |
| Emergency department | 3592 (51.4) | 2999 (52.4) | 593 (47.1) | 181 (46.7) |
| Nursing facility | 1204 (17.2) | 977 (17.1) | 227 (18.0) | 84 (21.7) |
| Other | 55 (0.1) | 40 (0.7) | 15 (1.1) | 6 (1.6) |
| Length of stay, d | ||||
| 02 | 1757 (25.2) | 1556 (27.2) | 201 (16.0) | 55 (14.2) |
| 34 | 2200 (31.5) | 1842 (32.2) | 358 (28.4) | 105 (27.1) |
| 57 | 1521 (21.8) | 1214 (21.2) | 307 (24.4) | 101 (26.0) |
| >7 | 1509 (21.6) | 1115 (19.5) | 394 (31.3) | 127 (32.7) |
| Elixhauser comorbidity index score | ||||
| 01 | 1987 (28.4) | 1729 (30.2) | 258 (20.5) | 66 (17.0) |
| 27 | 1773 (25.4) | 1541 (26.9) | 232 (18.4) | 67 (17.3) |
| 813 | 1535 (22.0) | 1212 (21.2) | 323 (25.6) | 86 (22.2) |
| >13 | 1692 (24.2) | 1245 (21.7) | 447 (35.5) | 169 (43.6) |
| Medications prescribed as outpatient | ||||
| 06 | 1684 (24.1) | 1410 (24.6) | 274 (21.8) | 72 (18.6) |
| 79 | 1601 (22.9) | 1349 (23.6) | 252 (20.0) | 77 (19.9) |
| 1013 | 1836 (26.3) | 1508 (26.3) | 328 (26.0) | 107 (27.6) |
| >13 | 1866 (26.7) | 1460 (25.5) | 406 (32.2) | 132 (34.0) |
| Number of admissions in past year | ||||
| 0 | 4816 (68.9) | 4032 (70.4) | 784 (62.2) | 279 (71.9) |
| 15 | 2075 (29.7) | 1640 (28.6) | 435 (34.5) | 107 (27.6) |
| >5 | 96 (1.4) | 55 (1.0) | 41 (3.3) | 2 (0.5) |
| Number of ED visits in past year | ||||
| 0 | 4661 (66.7) | 3862 (67.4) | 799 (63.4) | 261 (67.3) |
| 15 | 2326 (33.3) | 1865 (32.6) | 461 (36.6) | 127 (32.7) |
All‐Cause Readmissions
After exclusion of 997 patients who died, were discharged to skilled nursing or rehabilitation facilities, or left AMA, 6987 patients were included (Figure 1). Of these, 1260 had a readmission (18%). Approximately half were considered unlikely to be psychiatrically ill, 22% were considered moderately likely, and 29% likely (Table 2).
| All‐Cause Readmission Analysis | Potentially Avoidable Readmission Analysis | |||||
|---|---|---|---|---|---|---|
| No. in Cohort (%) | % of Patients With ACR | P Valuea | No. in Cohort (%) | % of Patients With PAR | P Valuea | |
| ||||||
| Entire cohort | 6987 | 18.0 | 6115 | 6.3 | ||
| Likelihood of psychiatric illness | ||||||
| Unlikely | 3424 (49) | 16.5 | 3026 (49) | 5.6 | ||
| Moderate | 1564 (22) | 23.5 | 1302 (21) | 7.1 | ||
| Likely | 1999 (29) | 16.4 | 1787 (29) | 6.4 | ||
| Likely versus unlikely | 0.87 | 0.20 | ||||
| Moderate+likely versus unlikely | 0.001 | 0.02 | ||||
| Likelihood of substance abuse | 0.01 | 0.20 | ||||
| Unlikely | 5804 (83) | 18.7 | 5104 (83) | 6.5 | ||
| Likely | 1183 (17) | 14.8 | 1011 (17) | 5.4 | 0.14 | |
| Number of prescribed outpatient psychotropic medications | <0.001 | 0.04 | ||||
| 0 | 4420 (63) | 16.3 | 3931 (64) | 5.9 | ||
| 1 | 1725 (25) | 20.4 | 1481 (24) | 7.2 | ||
| 2 | 781 (11) | 22.3 | 653 (11) | 7.0 | ||
| >2 | 61 (1) | 23.0 | 50 (1) | 6.0 | ||
| Prescribed antidepressant | 1474 (21) | 20.6 | 0.005 | 1248 (20) | 6.2 | 0.77 |
| Prescribed antipsychotic | 375 (5) | 22.4 | 0.02 | 315 (5) | 7.6 | 0.34 |
| Prescribed mood stabilizer | 81 (1) | 18.5 | 0.91 | 69 (1) | 4.4 | 0.49 |
| Prescribed anxiolytic | 1814 (26) | 21.8 | <0.001 | 1537 (25) | 7.7 | 0.01 |
| Prescribed stimulant | 101 (2) | 26.7 | 0.02 | 83 (1) | 10.8 | 0.09 |
| Prescribed pharmacologic treatment for substance abuse | 79 (1) | 25.3 | 0.09 | 60 (1) | 1.7 | 0.14 |
| Number of psychiatric diagnoses on outpatient problem list | 0.31 | 0.74 | ||||
| 0 | 6405 (92) | 18.2 | 5509 (90) | 6.3 | ||
| 1 or more | 582 (8) | 16.5 | 474 (8) | 7.0 | ||
| Outpatient diagnosis of substance abuse | 159 (2) | 13.2 | 0.11 | 144 (2) | 4.2 | 0.28 |
| Outpatient diagnosis of any psychiatric illness | 582 (8) | 16.5 | 0.31 | 517 (8) | 8.0 | 0.73 |
| Discharge diagnosis of depression | 774 (11) | 17.7 | 0.80 | 690 (11) | 7.7 | 0.13 |
| Discharge diagnosis of schizophrenia | 56 (1) | 23.2 | 0.31 | 50 (1) | 14 | 0.03 |
| Discharge diagnosis of bipolar disorder | 101 (1) | 10.9 | 0.06 | 92 (2) | 2.2 | 0.10 |
| Discharge diagnosis of anxiety | 1192 (17) | 15.0 | 0.003 | 1080 (18) | 6.2 | 0.83 |
| Discharge diagnosis of substance abuse | 885 (13) | 14.8 | 0.008 | 803 (13) | 6.1 | 0.76 |
| Discharge diagnosis of any psychiatric illness | 1839 (26) | 16.0 | 0.008 | 1654 (27) | 6.6 | 0.63 |
| Substance abuse consultation as inpatient | 284 (4) | 14.4 | 0.11 | 252 (4) | 3.6 | 0.07 |
In bivariate analysis (Table 2), likelihood of psychiatric illness (P<0.01) and increasing numbers of prescribed outpatient psychiatric medications (P<0.01) were significantly associated with ACR. In multivariate analysis, each additional prescribed outpatient psychiatric medication increased ACR risk (odds ratio [OR]: 1.10, 95% confidence interval [CI]: 1.01‐1.20) or any prescription of an anxiolytic in particular (OR: 1.16, 95% CI: 1.001.35) was associated with increased risk of ACR, whereas discharge diagnoses of anxiety (OR: 0.82, 95% CI: 0.68‐0.99) and substance abuse (OR: 0.80, 95% CI: 0.65‐0.99) were associated with lower risk of ACR (Table 3).
| ACR, OR (95% CI) | PAR, OR (95% CI)a | |
|---|---|---|
| ||
| Likely psychiatric disease | 0.97 (0.82‐1.14) | 1.20 (0.92‐1.56) |
| Likely and possible psychiatric disease | 1.07 (0.94‐1.22) | 1.18 (0.94‐1.47) |
| Likely substance abuse | 0.83 (0.69‐0.99) | 0.85 (0.63‐1.16) |
| Psychiatric diagnosis on outpatient problem list | 0.97 (0.76‐1.23) | 1.04 (0.70‐1.55) |
| Substance abuse diagnosis on outpatient problem list | 0.63 (0.39‐1.02) | 0.65 (0.28‐1.52) |
| Increasing number of prescribed psychiatric medications | 1.10 (1.01‐1.20) | 1.00 (0.86‐1.16) |
| Outpatient prescription for antidepressant | 1.10 (0.94‐1.29) | 0.86 (0.66‐1.13) |
| Outpatient prescription for antipsychotic | 1.03 (0.79‐1.34) | 0.93 (0.59‐1.45) |
| Outpatient prescription for anxiolytic | 1.16 (1.001.35) | 1.13 (0.88‐1.44) |
| Outpatient prescription for methadone or buprenorphine | 1.15 (0.67‐1.98) | 0.18 (0.03‐1.36) |
| Discharge diagnosis of depression | 1.06 (0.86‐1.30) | 1.49 (1.09‐2.04) |
| Discharge diagnosis of schizophrenia | 1.43 (0.75‐2.74) | 2.63 (1.13‐6.13) |
| Discharge diagnosis of bipolar disorder | 0.53 (0.28‐1.02) | 0.35 (0.09‐1.45) |
| Discharge diagnosis of anxiety | 0.82 (0.68‐0.99) | 1.11 (0.83‐1.49) |
| Discharge diagnosis of substance abuse | 0.80 (0.65‐0.99) | 1.05 (0.75‐1.46) |
| Discharge diagnosis of any psychiatric illness | 0.88 (0.75‐1.02) | 1.22 (0.96‐1.56) |
| Addiction team consult while inpatient | 0.82 (0.58‐1.17) | 0.58 (0.29‐1.17) |
Potentially Avoidable Readmissions
After further exclusion of 872 patients who had unavoidable readmissions according to the SQLape algorithm, 6115 patients remained. Of these, 388 had a PAR within 30 days (6.3%, Table 1).
In bivariate analysis (Table 2), the likelihood of psychiatric illness (P=0.02), number of outpatient psychiatric medications (P=0.04), and prescription of anxiolytics (P=0.01) were significantly associated with PAR, as they were with ACR. A discharge diagnosis of schizophrenia was also associated with PAR (P=0.03).
In multivariate analysis, only discharge diagnoses of depression (OR: 1.49, 95% CI: 1.09‐2.04) and schizophrenia (OR: 2.63, 95% CI: 1.13‐6.13) were associated with PAR.
DISCUSSION
Comorbid psychiatric illness was common among patients admitted to the medicine wards. Patients with documented discharge diagnoses of depression or schizophrenia were at highest risk for a potentially avoidable 30‐day readmission, whereas those prescribed more psychiatric medications were at increased risk for ACR. These findings were independent of a comprehensive set of risk factors among medicine inpatients in this retrospective cohort study.
This study extends prior work indicating patients with psychiatric disease have increased healthcare utilization,[6, 7, 8, 9, 10, 11] by identifying at least 2 subpopulations of the psychiatrically ill (those with depression and schizophrenia) at particularly high risk for 30‐day PAR. To our knowledge, this is the first study to identify schizophrenia as a predictor of hospital readmission for medical illnesses. One prior study prospectively identified depression as increasing the 90‐day risk of readmission 3‐fold, although medication usage was not assessed,[6] and our report strengthens this association.
There are several possible explanations why these two subpopulations in particular would be more predisposed to readmissions that are potentially avoidable. It is known that patients with schizophrenia, for example, live on average 20 years less than the general population, and most of this excess mortality is due to medical illnesses.[20, 21] Reasons for this may include poor healthcare access, adverse effects of medication, and socioeconomic factors among others.[21, 22] All of these reasons may contribute to the increased PAR risk in this population, mediated, for example, by decreased ability to adhere to postdischarge care plans. Successful community‐based interventions to decrease these inequities have been described and could serve as a model for addressing the increased readmission risk in this population.[23]
Our finding that patients with a greater number of prescribed psychiatric medications are at increased risk for ACR may be expected, given other studies that have highlighted the crucial importance of medications in postdischarge adverse events, including readmissions.[24] Indeed, medication‐related errors and toxicities are the most common postdischarge adverse events experienced by patients.[25] Whether psychiatric medications are particularly prone to causing postdischarge adverse events or whether these medications represent greater psychiatric comorbidity cannot be answered by this study.
It was surprising but reassuring that substance abuse was not a predictor of short‐term readmissions as identified using our measures; in fact, a discharge diagnosis of substance abuse was associated with lower risk of ACR than comparator patients. It seems unlikely that we would have inadequate power to find such a result, as we found a statistically significant negative association in the ACR population, and 17% of our population overall was considered likely to have a substance abuse comorbidity. However, it is likely the burden of disease was underestimated given that we did not try to determine the contribution of long‐term substance abuse to medical diseases that may increase readmission risk (eg, liver cirrhosis from alcohol use). Unlike other conditions in our study, patients with substance abuse diagnoses at BWH can be seen by a dedicated multidisciplinary team while an inpatient to start treatment and plan for postdischarge follow‐up; this may have played a role in our findings.
A discharge diagnosis of anxiety was also somewhat protective against readmission, whereas a prescription of an anxiolytic (predominantly benzodiazepines) increased risk; many patients prescribed a benzodiazepine do not have a Diagnostic and Statistical Manual of Mental Disorders4th Edition (DSM‐IV) diagnosis of anxiety disorder, and thus these findings may reflect different patient populations. Discharging physicians may have used anxiety as a discharge diagnosis in patients in whom they suspected somatic complaints without organic basis; these patients may be at lower risk of readmission.
Discharge diagnoses of psychiatric illnesses were associated with ACR and PAR in our study, but outpatient diagnoses were not. This likely reflects greater severity of illness (documentation as a treated diagnosis on discharge indicates the illness was relevant during the hospitalization), but may also reflect inaccuracies of diagnosis and lack of assessment of severity in outpatient coding, which would bias toward null findings. Although many of the patients in our study were seen by primary care doctors within the Partners system, some patients had outside primary care physicians and we did not have access to these records. This may also have decreased our ability to find associations.
The findings of our study should be interpreted in the context of the study design. Our study was retrospective, which limited our ability to conclusively diagnose psychiatric disease presence or severity (as is true of most institutions, validated psychiatric screening was not routinely used at our institutions on hospital admission or discharge). However, we used a conservative scale to classify the likelihood of patients having psychiatric or substance abuse disorders, and we used other metrics to establish the presence of illness, such as the number of prescribed medications, inpatient consultation with a substance abuse service, and hospital discharge diagnoses. This approach also allowed us to quickly identify a large cohort unaffected by selection bias. Our study was single center, potentially limiting generalizability. Although we capture at least 80% of readmissions, we were not able to capture all readmissions, and we cannot rule out that patients readmitted elsewhere are different than those readmitted within the Partners system. Last, the SQLape algorithm is not perfectly sensitive or specific in identifying avoidable readmissions,[13] but it does eliminate many readmissions that are clearly unavoidable, creating an enriched cohort of patients whose readmissions are more likely to be avoidable and therefore potentially actionable.
We suggest that our study findings first be considered when risk stratifying patients before hospital discharge in terms of readmission risk. Patients with depression and schizophrenia would seem to merit postdischarge interventions to decrease their potentially avoidable readmissions. Compulsory community treatment (a feature of treatment in Canada and Australia that is ordered by clinicians) has been shown to decrease mortality due to medical illness in patients who have been hospitalized and are psychiatrically ill, and addition of these services to postdischarge care may be useful.[23] Inpatient physicians could work to ensure follow‐up not just with medical providers but with robust outpatient mental health programs to decrease potentially avoidable readmission risk, and administrators could work to ensure close linkages with these community resources. Studies evaluating the impact of these types of interventions would need to be conducted. Patients with polypharmacy, including psychiatric medications, may benefit from interventions to improve medication safety, such as enhanced medication reconciliation and pharmacist counseling.[26]
Our study suggests that patients with depression, those with schizophrenia, and those who have increased numbers of prescribed psychiatric medications should be considered at high risk for readmission for medical illnesses. Targeting interventions to these patients may be fruitful in preventing avoidable readmissions.
Acknowledgements
The authors thank Dr. Yves Eggli for screening the database for potentially avoidable readmissions using the SQLape algorithm.
Disclosures
Dr. Donz was supported by the Swiss National Science Foundation and the Swiss Foundation for MedicalBiological Scholarships. The authors otherwise have no conflicts of interest to disclose. The content is solely the responsibility of the authors and does not necessarily represent the official views of the US Department of Veterans Affairs.
Readmissions to the hospital are common and costly.[1] However, identifying patients prospectively who are likely to be readmitted and who may benefit from interventions to reduce readmission risk has proven challenging, with published risk scores having only moderate ability to discriminate between patients likely and unlikely to be readmitted.[2] One reason for this may be that published studies have not typically focused on patients who are cognitively impaired, psychiatrically ill, have low health or English literacy, or have poor social supports, all of whom may represent a substantial fraction of readmitted patients.[2, 3, 4, 5]
Psychiatric disease, in particular, may contribute to increased readmission risk for nonpsychiatric (medical) illness, and is associated with increased utilization of healthcare resources.[6, 7, 8, 9, 10, 11] For example, patients with mental illness who were discharged from New York hospitals were more likely to be rehospitalized and had more costly readmissions than patients without these comorbidities, including a length of stay nearly 1 day longer on average.[7] An unmet need for treatment of substance abuse was projected to cost Tennessee $772 million of excess healthcare costs in 2000, mostly incurred through repeat hospitalizations and emergency department (ED) visits.[10]
Despite this, few investigators have considered the role of psychiatric disease and/or substance abuse in medical readmission risk. The purpose of the current study was to evaluate the role of psychiatric illness and substance abuse in unselected medical patients to determine their relative contributions to 30‐day all‐cause readmissions (ACR) and potentially avoidable readmissions (PAR).
METHODS
Patients and Setting
We conducted a retrospective cohort study of consecutive adult patients discharged from medicine services at Brigham and Women's Hospital (BWH), a 747‐bed tertiary referral center and teaching hospital, between July 1, 2009 and June 30, 2010. Most patients are cared for by resident housestaff teams at BWH (approximately 25% are cared for by physician assistants working directly with attending physicians), and approximately half receive primary care in the Partners system, which has a shared electronic medical record (EMR). Outpatient mental health services are provided by Partners‐associated mental health professionals including those at McLean Hospital and MassHealth (Medicaid)‐associated sites through the Massachusetts Behavioral Health Partnership. Exclusion criteria were death in the hospital or discharge to another acute care facility. We also excluded patients who left against medical advice (AMA). The study protocol was approved by the Partners Institutional Review Board.
Outcome
The primary outcomes were ACR and PAR within 30 days of discharge. First, we identified all 30‐day readmissions to BWH or to 2 other hospitals in the Partners Healthcare Network (previous studies have shown that 80% of all readmitted patients are readmitted to 1 of these 3 hospitals).[12] For patients with multiple readmissions, only the first readmission was included in the dataset.
To find potentially avoidable readmissions, administrative and billing data for these patients were processed using the SQLape (SQLape s.a.r.l., Corseaux, Switzerland) algorithm, which identifies PAR by excluding patients who undergo planned follow‐up treatment (such as a cycle of planned chemotherapy) or are readmitted for conditions unrelated in any way to the index hospitalization.[13, 14] Common complications of treatment are categorized as potentially avoidable, such as development of a deep venous thrombosis, a decubitus ulcer after prolonged bed rest, or bleeding complications after starting anticoagulation. Although the algorithm identifies theoretically preventable readmissions, the algorithm does not quantify how preventable they are, and these are thus referred to as potentially avoidable. This is similar to other admission metrics, such as the Agency for Healthcare Research and Quality's prevention quality indicators, which are created from a list of ambulatory care‐sensitive conditions.[15] SQLape has the advantage of being a specific tool for readmissions. Patients with 30‐day readmissions identified by SQLape as planned or unlikely to be avoidable were excluded in the PAR analysis, although still included in ACR analysis. In each case, the comparison group is patients without any readmission.
Predictors
Our predictors of interest included the overall prevalence of a psychiatric diagnosis or diagnosis of substance abuse, the presence of specific psychiatric diagnoses, and prescription of psychiatric medications to help assess the independent contribution of these comorbidities to readmission risk.
We used a combination of easily obtainable inpatient and outpatient clinical and administrative data to identify relevant patients. Patients were considered likely to be psychiatrically ill if they: (1) had a psychiatric diagnosis on their Partners outpatient EMR problem list and were prescribed a medication to treat that condition as an outpatient, or (2) had an International Classification of Diseases, 9th Revision diagnosis of a psychiatric illness at hospital discharge. Patients were considered to have moderate probability of disease if they: (1) had a psychiatric diagnosis on their outpatient problem list, or (2) were prescribed a medication intended to treat a psychiatric condition as an outpatient. Patients were considered unlikely to have psychiatric disease if none of these criteria were met. Patients were considered likely to have a substance abuse disorder if they had this diagnosis on their outpatient EMR, or were prescribed a medication to treat this condition (eg, buprenorphine/naloxone), or received inpatient consultation from a substance abuse treatment team during their inpatient hospitalization, and were considered unlikely if none of these were true. We also evaluated individual categories of psychiatric illness (schizophrenia, depression, anxiety, bipolar disorder) and of psychotropic medications (antidepressants, antipsychotics, anxiolytics).
Potential Confounders
Data on potential confounders, based on prior literature,[16, 17] collected at the index admission were derived from electronic administrative, clinical, and billing sources, including the Brigham Integrated Computer System and the Partners Clinical Data Repository. They included patient age, gender, ethnicity, primary language, marital status, insurance status, living situation prior to admission, discharge location, length of stay, Elixhauser comorbidity index,[18] total number of medications prescribed, and number of prior admissions and ED visits in the prior year.
Statistical Analysis
Bivariate comparisons of each of the predictors of ACR and PAR risk (ie, patients with a 30‐day ACR or PAR vs those not readmitted within 30 days) were conducted using 2 trend tests for ordinal predictors (eg, likelihood of psychiatric disease), and 2 or Fisher exact test for dichotomous predictors (eg, receipt of inpatient substance abuse counseling).
We then used multivariate logistic regression analysis to adjust for all of the potential confounders noted above, entering each variable related to psychiatric illness into the model separately (eg, likely psychiatric illness, number of psychiatric medications). In a secondary analysis, we removed potentially collinear variables from the final model; as this did not alter the results, the full model is presented. We also conducted a secondary analysis where we included patients who left against medical advice (AMA), which also did not alter the results. Two‐sided P values <0.05 were considered significant, and all analyses were performed using the SAS version 9.2 (SAS Institute, Inc., Cary, NC).
RESULTS
There were 7984 unique patients discharged during the study period. Patients were generally white and English speaking; just over half of admissions came from the ED (Table 1). Of note, nearly all patients were insured, as are almost all patients in Massachusetts. They had high degrees of comorbid illness and large numbers of prescribed medications. Nearly 30% had at least 1 hospital admission within the prior year.
| Characteristic | All Patients, N (%) | Not Readmitted, N (%) | ACR, N (%) | PAR N (%)a |
|---|---|---|---|---|
| ||||
| Study cohort | 6987 (100) | 5727 (72) | 1260 (18) | 388 (5.6) |
| Age, y | ||||
| <50 | 1663 (23.8) | 1343 (23.5) | 320 (25.4) | 85 (21.9) |
| 5165 | 2273 (32.5) | 1859 (32.5) | 414 (32.9) | 136 (35.1) |
| 6679 | 1444 (20.7) | 1176 (20.5) | 268 (18.6) | 80 (20.6) |
| >80 | 1607 (23.0) | 1349 (23.6) | 258 (16.1) | 87 (22.4) |
| Female | 3604 (51.6) | 2967 (51.8) | 637 (50.6) | 206 (53.1) |
| Race | ||||
| White | 5126 (73.4) | 4153 (72.5) | 973 (77.2) | 300 (77.3) |
| Black | 1075 (15.4) | 899 (15.7) | 176 (14.0) | 53 (13.7) |
| Hispanic | 562 (8.0) | 477 (8.3) | 85 (6.8) | 28 (7.2) |
| Other | 224 (3.2) | 198 (3.5) | 26 (2.1) | 7 (1.8) |
| Primary language | ||||
| English | 6345 (90.8) | 5180 (90.5) | 1165 (92.5) | 356 (91.8) |
| Marital status | ||||
| Married | 3642 (52.1) | 2942 (51.4) | 702 (55.7) | 214 (55.2) |
| Single, never married | 1662 (23.8) | 1393 (24.3) | 269 (21.4) | 73 (18.8) |
| Previously married | 1683 (24.1) | 1386 (24.2) | 289 (22.9) | 101 (26.0) |
| Insurance | ||||
| Medicare | 3550 (50.8) | 2949 (51.5) | 601 (47.7) | 188 (48.5) |
| Medicaid | 539 (7.7) | 430 (7.5) | 109 (8.7) | 33 (8.5) |
| Private | 2892 (41.4) | 2344 (40.9) | 548 (43.5) | 167 (43.0) |
| Uninsured | 6 (0.1) | 4 (0.1) | 2 (0.1) | 0 (0) |
| Source of index admission | ||||
| Clinic or home | 2136 (30.6) | 1711 (29.9) | 425 (33.7) | 117 (30.2) |
| Emergency department | 3592 (51.4) | 2999 (52.4) | 593 (47.1) | 181 (46.7) |
| Nursing facility | 1204 (17.2) | 977 (17.1) | 227 (18.0) | 84 (21.7) |
| Other | 55 (0.1) | 40 (0.7) | 15 (1.1) | 6 (1.6) |
| Length of stay, d | ||||
| 02 | 1757 (25.2) | 1556 (27.2) | 201 (16.0) | 55 (14.2) |
| 34 | 2200 (31.5) | 1842 (32.2) | 358 (28.4) | 105 (27.1) |
| 57 | 1521 (21.8) | 1214 (21.2) | 307 (24.4) | 101 (26.0) |
| >7 | 1509 (21.6) | 1115 (19.5) | 394 (31.3) | 127 (32.7) |
| Elixhauser comorbidity index score | ||||
| 01 | 1987 (28.4) | 1729 (30.2) | 258 (20.5) | 66 (17.0) |
| 27 | 1773 (25.4) | 1541 (26.9) | 232 (18.4) | 67 (17.3) |
| 813 | 1535 (22.0) | 1212 (21.2) | 323 (25.6) | 86 (22.2) |
| >13 | 1692 (24.2) | 1245 (21.7) | 447 (35.5) | 169 (43.6) |
| Medications prescribed as outpatient | ||||
| 06 | 1684 (24.1) | 1410 (24.6) | 274 (21.8) | 72 (18.6) |
| 79 | 1601 (22.9) | 1349 (23.6) | 252 (20.0) | 77 (19.9) |
| 1013 | 1836 (26.3) | 1508 (26.3) | 328 (26.0) | 107 (27.6) |
| >13 | 1866 (26.7) | 1460 (25.5) | 406 (32.2) | 132 (34.0) |
| Number of admissions in past year | ||||
| 0 | 4816 (68.9) | 4032 (70.4) | 784 (62.2) | 279 (71.9) |
| 15 | 2075 (29.7) | 1640 (28.6) | 435 (34.5) | 107 (27.6) |
| >5 | 96 (1.4) | 55 (1.0) | 41 (3.3) | 2 (0.5) |
| Number of ED visits in past year | ||||
| 0 | 4661 (66.7) | 3862 (67.4) | 799 (63.4) | 261 (67.3) |
| 15 | 2326 (33.3) | 1865 (32.6) | 461 (36.6) | 127 (32.7) |
All‐Cause Readmissions
After exclusion of 997 patients who died, were discharged to skilled nursing or rehabilitation facilities, or left AMA, 6987 patients were included (Figure 1). Of these, 1260 had a readmission (18%). Approximately half were considered unlikely to be psychiatrically ill, 22% were considered moderately likely, and 29% likely (Table 2).
| All‐Cause Readmission Analysis | Potentially Avoidable Readmission Analysis | |||||
|---|---|---|---|---|---|---|
| No. in Cohort (%) | % of Patients With ACR | P Valuea | No. in Cohort (%) | % of Patients With PAR | P Valuea | |
| ||||||
| Entire cohort | 6987 | 18.0 | 6115 | 6.3 | ||
| Likelihood of psychiatric illness | ||||||
| Unlikely | 3424 (49) | 16.5 | 3026 (49) | 5.6 | ||
| Moderate | 1564 (22) | 23.5 | 1302 (21) | 7.1 | ||
| Likely | 1999 (29) | 16.4 | 1787 (29) | 6.4 | ||
| Likely versus unlikely | 0.87 | 0.20 | ||||
| Moderate+likely versus unlikely | 0.001 | 0.02 | ||||
| Likelihood of substance abuse | 0.01 | 0.20 | ||||
| Unlikely | 5804 (83) | 18.7 | 5104 (83) | 6.5 | ||
| Likely | 1183 (17) | 14.8 | 1011 (17) | 5.4 | 0.14 | |
| Number of prescribed outpatient psychotropic medications | <0.001 | 0.04 | ||||
| 0 | 4420 (63) | 16.3 | 3931 (64) | 5.9 | ||
| 1 | 1725 (25) | 20.4 | 1481 (24) | 7.2 | ||
| 2 | 781 (11) | 22.3 | 653 (11) | 7.0 | ||
| >2 | 61 (1) | 23.0 | 50 (1) | 6.0 | ||
| Prescribed antidepressant | 1474 (21) | 20.6 | 0.005 | 1248 (20) | 6.2 | 0.77 |
| Prescribed antipsychotic | 375 (5) | 22.4 | 0.02 | 315 (5) | 7.6 | 0.34 |
| Prescribed mood stabilizer | 81 (1) | 18.5 | 0.91 | 69 (1) | 4.4 | 0.49 |
| Prescribed anxiolytic | 1814 (26) | 21.8 | <0.001 | 1537 (25) | 7.7 | 0.01 |
| Prescribed stimulant | 101 (2) | 26.7 | 0.02 | 83 (1) | 10.8 | 0.09 |
| Prescribed pharmacologic treatment for substance abuse | 79 (1) | 25.3 | 0.09 | 60 (1) | 1.7 | 0.14 |
| Number of psychiatric diagnoses on outpatient problem list | 0.31 | 0.74 | ||||
| 0 | 6405 (92) | 18.2 | 5509 (90) | 6.3 | ||
| 1 or more | 582 (8) | 16.5 | 474 (8) | 7.0 | ||
| Outpatient diagnosis of substance abuse | 159 (2) | 13.2 | 0.11 | 144 (2) | 4.2 | 0.28 |
| Outpatient diagnosis of any psychiatric illness | 582 (8) | 16.5 | 0.31 | 517 (8) | 8.0 | 0.73 |
| Discharge diagnosis of depression | 774 (11) | 17.7 | 0.80 | 690 (11) | 7.7 | 0.13 |
| Discharge diagnosis of schizophrenia | 56 (1) | 23.2 | 0.31 | 50 (1) | 14 | 0.03 |
| Discharge diagnosis of bipolar disorder | 101 (1) | 10.9 | 0.06 | 92 (2) | 2.2 | 0.10 |
| Discharge diagnosis of anxiety | 1192 (17) | 15.0 | 0.003 | 1080 (18) | 6.2 | 0.83 |
| Discharge diagnosis of substance abuse | 885 (13) | 14.8 | 0.008 | 803 (13) | 6.1 | 0.76 |
| Discharge diagnosis of any psychiatric illness | 1839 (26) | 16.0 | 0.008 | 1654 (27) | 6.6 | 0.63 |
| Substance abuse consultation as inpatient | 284 (4) | 14.4 | 0.11 | 252 (4) | 3.6 | 0.07 |
In bivariate analysis (Table 2), likelihood of psychiatric illness (P<0.01) and increasing numbers of prescribed outpatient psychiatric medications (P<0.01) were significantly associated with ACR. In multivariate analysis, each additional prescribed outpatient psychiatric medication increased ACR risk (odds ratio [OR]: 1.10, 95% confidence interval [CI]: 1.01‐1.20) or any prescription of an anxiolytic in particular (OR: 1.16, 95% CI: 1.001.35) was associated with increased risk of ACR, whereas discharge diagnoses of anxiety (OR: 0.82, 95% CI: 0.68‐0.99) and substance abuse (OR: 0.80, 95% CI: 0.65‐0.99) were associated with lower risk of ACR (Table 3).
| ACR, OR (95% CI) | PAR, OR (95% CI)a | |
|---|---|---|
| ||
| Likely psychiatric disease | 0.97 (0.82‐1.14) | 1.20 (0.92‐1.56) |
| Likely and possible psychiatric disease | 1.07 (0.94‐1.22) | 1.18 (0.94‐1.47) |
| Likely substance abuse | 0.83 (0.69‐0.99) | 0.85 (0.63‐1.16) |
| Psychiatric diagnosis on outpatient problem list | 0.97 (0.76‐1.23) | 1.04 (0.70‐1.55) |
| Substance abuse diagnosis on outpatient problem list | 0.63 (0.39‐1.02) | 0.65 (0.28‐1.52) |
| Increasing number of prescribed psychiatric medications | 1.10 (1.01‐1.20) | 1.00 (0.86‐1.16) |
| Outpatient prescription for antidepressant | 1.10 (0.94‐1.29) | 0.86 (0.66‐1.13) |
| Outpatient prescription for antipsychotic | 1.03 (0.79‐1.34) | 0.93 (0.59‐1.45) |
| Outpatient prescription for anxiolytic | 1.16 (1.001.35) | 1.13 (0.88‐1.44) |
| Outpatient prescription for methadone or buprenorphine | 1.15 (0.67‐1.98) | 0.18 (0.03‐1.36) |
| Discharge diagnosis of depression | 1.06 (0.86‐1.30) | 1.49 (1.09‐2.04) |
| Discharge diagnosis of schizophrenia | 1.43 (0.75‐2.74) | 2.63 (1.13‐6.13) |
| Discharge diagnosis of bipolar disorder | 0.53 (0.28‐1.02) | 0.35 (0.09‐1.45) |
| Discharge diagnosis of anxiety | 0.82 (0.68‐0.99) | 1.11 (0.83‐1.49) |
| Discharge diagnosis of substance abuse | 0.80 (0.65‐0.99) | 1.05 (0.75‐1.46) |
| Discharge diagnosis of any psychiatric illness | 0.88 (0.75‐1.02) | 1.22 (0.96‐1.56) |
| Addiction team consult while inpatient | 0.82 (0.58‐1.17) | 0.58 (0.29‐1.17) |
Potentially Avoidable Readmissions
After further exclusion of 872 patients who had unavoidable readmissions according to the SQLape algorithm, 6115 patients remained. Of these, 388 had a PAR within 30 days (6.3%, Table 1).
In bivariate analysis (Table 2), the likelihood of psychiatric illness (P=0.02), number of outpatient psychiatric medications (P=0.04), and prescription of anxiolytics (P=0.01) were significantly associated with PAR, as they were with ACR. A discharge diagnosis of schizophrenia was also associated with PAR (P=0.03).
In multivariate analysis, only discharge diagnoses of depression (OR: 1.49, 95% CI: 1.09‐2.04) and schizophrenia (OR: 2.63, 95% CI: 1.13‐6.13) were associated with PAR.
DISCUSSION
Comorbid psychiatric illness was common among patients admitted to the medicine wards. Patients with documented discharge diagnoses of depression or schizophrenia were at highest risk for a potentially avoidable 30‐day readmission, whereas those prescribed more psychiatric medications were at increased risk for ACR. These findings were independent of a comprehensive set of risk factors among medicine inpatients in this retrospective cohort study.
This study extends prior work indicating patients with psychiatric disease have increased healthcare utilization,[6, 7, 8, 9, 10, 11] by identifying at least 2 subpopulations of the psychiatrically ill (those with depression and schizophrenia) at particularly high risk for 30‐day PAR. To our knowledge, this is the first study to identify schizophrenia as a predictor of hospital readmission for medical illnesses. One prior study prospectively identified depression as increasing the 90‐day risk of readmission 3‐fold, although medication usage was not assessed,[6] and our report strengthens this association.
There are several possible explanations why these two subpopulations in particular would be more predisposed to readmissions that are potentially avoidable. It is known that patients with schizophrenia, for example, live on average 20 years less than the general population, and most of this excess mortality is due to medical illnesses.[20, 21] Reasons for this may include poor healthcare access, adverse effects of medication, and socioeconomic factors among others.[21, 22] All of these reasons may contribute to the increased PAR risk in this population, mediated, for example, by decreased ability to adhere to postdischarge care plans. Successful community‐based interventions to decrease these inequities have been described and could serve as a model for addressing the increased readmission risk in this population.[23]
Our finding that patients with a greater number of prescribed psychiatric medications are at increased risk for ACR may be expected, given other studies that have highlighted the crucial importance of medications in postdischarge adverse events, including readmissions.[24] Indeed, medication‐related errors and toxicities are the most common postdischarge adverse events experienced by patients.[25] Whether psychiatric medications are particularly prone to causing postdischarge adverse events or whether these medications represent greater psychiatric comorbidity cannot be answered by this study.
It was surprising but reassuring that substance abuse was not a predictor of short‐term readmissions as identified using our measures; in fact, a discharge diagnosis of substance abuse was associated with lower risk of ACR than comparator patients. It seems unlikely that we would have inadequate power to find such a result, as we found a statistically significant negative association in the ACR population, and 17% of our population overall was considered likely to have a substance abuse comorbidity. However, it is likely the burden of disease was underestimated given that we did not try to determine the contribution of long‐term substance abuse to medical diseases that may increase readmission risk (eg, liver cirrhosis from alcohol use). Unlike other conditions in our study, patients with substance abuse diagnoses at BWH can be seen by a dedicated multidisciplinary team while an inpatient to start treatment and plan for postdischarge follow‐up; this may have played a role in our findings.
A discharge diagnosis of anxiety was also somewhat protective against readmission, whereas a prescription of an anxiolytic (predominantly benzodiazepines) increased risk; many patients prescribed a benzodiazepine do not have a Diagnostic and Statistical Manual of Mental Disorders4th Edition (DSM‐IV) diagnosis of anxiety disorder, and thus these findings may reflect different patient populations. Discharging physicians may have used anxiety as a discharge diagnosis in patients in whom they suspected somatic complaints without organic basis; these patients may be at lower risk of readmission.
Discharge diagnoses of psychiatric illnesses were associated with ACR and PAR in our study, but outpatient diagnoses were not. This likely reflects greater severity of illness (documentation as a treated diagnosis on discharge indicates the illness was relevant during the hospitalization), but may also reflect inaccuracies of diagnosis and lack of assessment of severity in outpatient coding, which would bias toward null findings. Although many of the patients in our study were seen by primary care doctors within the Partners system, some patients had outside primary care physicians and we did not have access to these records. This may also have decreased our ability to find associations.
The findings of our study should be interpreted in the context of the study design. Our study was retrospective, which limited our ability to conclusively diagnose psychiatric disease presence or severity (as is true of most institutions, validated psychiatric screening was not routinely used at our institutions on hospital admission or discharge). However, we used a conservative scale to classify the likelihood of patients having psychiatric or substance abuse disorders, and we used other metrics to establish the presence of illness, such as the number of prescribed medications, inpatient consultation with a substance abuse service, and hospital discharge diagnoses. This approach also allowed us to quickly identify a large cohort unaffected by selection bias. Our study was single center, potentially limiting generalizability. Although we capture at least 80% of readmissions, we were not able to capture all readmissions, and we cannot rule out that patients readmitted elsewhere are different than those readmitted within the Partners system. Last, the SQLape algorithm is not perfectly sensitive or specific in identifying avoidable readmissions,[13] but it does eliminate many readmissions that are clearly unavoidable, creating an enriched cohort of patients whose readmissions are more likely to be avoidable and therefore potentially actionable.
We suggest that our study findings first be considered when risk stratifying patients before hospital discharge in terms of readmission risk. Patients with depression and schizophrenia would seem to merit postdischarge interventions to decrease their potentially avoidable readmissions. Compulsory community treatment (a feature of treatment in Canada and Australia that is ordered by clinicians) has been shown to decrease mortality due to medical illness in patients who have been hospitalized and are psychiatrically ill, and addition of these services to postdischarge care may be useful.[23] Inpatient physicians could work to ensure follow‐up not just with medical providers but with robust outpatient mental health programs to decrease potentially avoidable readmission risk, and administrators could work to ensure close linkages with these community resources. Studies evaluating the impact of these types of interventions would need to be conducted. Patients with polypharmacy, including psychiatric medications, may benefit from interventions to improve medication safety, such as enhanced medication reconciliation and pharmacist counseling.[26]
Our study suggests that patients with depression, those with schizophrenia, and those who have increased numbers of prescribed psychiatric medications should be considered at high risk for readmission for medical illnesses. Targeting interventions to these patients may be fruitful in preventing avoidable readmissions.
Acknowledgements
The authors thank Dr. Yves Eggli for screening the database for potentially avoidable readmissions using the SQLape algorithm.
Disclosures
Dr. Donz was supported by the Swiss National Science Foundation and the Swiss Foundation for MedicalBiological Scholarships. The authors otherwise have no conflicts of interest to disclose. The content is solely the responsibility of the authors and does not necessarily represent the official views of the US Department of Veterans Affairs.
- , , . Rehospitalizations among patients in the Medicare fee‐for‐service program. N Engl J Med. 2009;360(14):1418–1428.
- , , , et al. Risk prediction models for hospital readmission: a systematic review. JAMA. 2011;306(15):1688–1698.
- , . Hospital readmission as an accountability measure. JAMA. 2011;305(5):504–505.
- , , , , , . Postdischarge environmental and socioeconomic factors and the likelihood of early hospital readmission among community‐dwelling Medicare beneficiaries. Gerontologist. 2008;48(4):495–504.
- , , , , . Low health literacy and health outcomes: an updated systematic review. Ann Intern Med. 2011;155(2):97–107.
- , , , et al. Depression is a risk factor for rehospitalization in medical inpatients. Prim Care Companion J Clin Psychiatry. 2007;9(4):256–262.
- , , , . Mental illness and hospitalization for ambulatory care sensitive medical conditions. Med Care. 2008;46(12):1249–1256.
- , , , , , . Substance use treatment barriers for patients with frequent hospital admissions. J Subst Abuse Treat. 2010;38(1):22–30.
- , , , , . Managed care and the quality of substance abuse treatment. J Ment Health Policy Econ. 2002;5(4):163–174.
- , , , , . Unmet substance abuse treatment need, health services utilization, and cost: a population‐based emergency department study. Ann Emerg Med. 2005;45(2):118–127.
- , , , . Elderly Medicare inpatients with substance use disorders: characteristics and predictors of hospital readmissions over a four‐year interval. J Stud Alcohol. 2000;61(6):891–895.
- , , , et al. Rationale and design of the Pharmacist Intervention for Low Literacy in Cardiovascular Disease (PILL‐CVD) study. Circ Cardiovasc Qual Outcomes. 2010;3(2):212–219.
- , , , , , . Validation of the potentially avoidable hospital readmission rate as a routine indicator of the quality of hospital care. Med Care. 2006;44(11);972–981.
- , , , , , . Measuring potentially avoidable hospital readmissions. J Clin Epidemiol. 2002;55:573–587.
- Agency for Healthcare Research and Quality Quality Indicators. (April 7, 2006). Prevention Quality Indicators (PQI) Composite Measure Workgroup Final Report. Available at: http://www.qualityindicators.ahrq.gov/modules/pqi_resources.aspx. Accessed June 1, 2012.
- , , , et al. Hospital readmission in general medicine patients: a prediction model. J Gen Intern Med. 2010;25(3):211–219.
- , , , et al. Derivation and validation of an index to predict early death or unplanned readmission after discharge from hospital to the community. CMAJ. 2010;182(6):551–557.
- , , , . Comorbidity measures for use with administrative data. Med Care. Jan 1998;36(1):8–27.
- , , , , eds. Morbidity and mortality in people with serious mental illness. October 2006. National Association of State Mental Health Directors, Medical Directors Council. Available at: http://www.nasmhpd.org/docs/publications/MDCdocs/Mortality%20and%20 Mo rbidity%20Final%20Report%208.18.08.pdf. Accessed January 13, 2013.
- , , , . Mortality in individuals who have had psychiatric treatment: population‐based study in Nova Scotia. Br J Psychiat. 2005;187:552–558.
- , , , , , . Inequitable access for mentally ill patients to some medically necessary procedures. CMAJ. 2007;176(6):779–784.
- , , . Quality of medical care for people with and without comorbid mental illness and substance misuse: systematic review of comparative studies. Br J Psychiat. 2009;194:491–499.
- , , , , , . Reducing all‐cause mortality among patients with psychiatric disorders: a population‐based study. CMAJ. 2013;185(1):E50–E56.
- , , , et al. Incidence of potentially avoidable urgent readmissions and their relation to all‐cause urgent readmissions. CMAJ. 2011;183(14):E1067–E1072.
- , , , , . The incidence and severity of adverse events affecting patients after discharge from the hospital. Ann Intern Med. 2003;138(3):161–167.
- , , , . Hospital‐based medication reconciliation practices: a systematic review. Arch Intern Med. 2012;172(14):1057–1069.
- , , . Rehospitalizations among patients in the Medicare fee‐for‐service program. N Engl J Med. 2009;360(14):1418–1428.
- , , , et al. Risk prediction models for hospital readmission: a systematic review. JAMA. 2011;306(15):1688–1698.
- , . Hospital readmission as an accountability measure. JAMA. 2011;305(5):504–505.
- , , , , , . Postdischarge environmental and socioeconomic factors and the likelihood of early hospital readmission among community‐dwelling Medicare beneficiaries. Gerontologist. 2008;48(4):495–504.
- , , , , . Low health literacy and health outcomes: an updated systematic review. Ann Intern Med. 2011;155(2):97–107.
- , , , et al. Depression is a risk factor for rehospitalization in medical inpatients. Prim Care Companion J Clin Psychiatry. 2007;9(4):256–262.
- , , , . Mental illness and hospitalization for ambulatory care sensitive medical conditions. Med Care. 2008;46(12):1249–1256.
- , , , , , . Substance use treatment barriers for patients with frequent hospital admissions. J Subst Abuse Treat. 2010;38(1):22–30.
- , , , , . Managed care and the quality of substance abuse treatment. J Ment Health Policy Econ. 2002;5(4):163–174.
- , , , , . Unmet substance abuse treatment need, health services utilization, and cost: a population‐based emergency department study. Ann Emerg Med. 2005;45(2):118–127.
- , , , . Elderly Medicare inpatients with substance use disorders: characteristics and predictors of hospital readmissions over a four‐year interval. J Stud Alcohol. 2000;61(6):891–895.
- , , , et al. Rationale and design of the Pharmacist Intervention for Low Literacy in Cardiovascular Disease (PILL‐CVD) study. Circ Cardiovasc Qual Outcomes. 2010;3(2):212–219.
- , , , , , . Validation of the potentially avoidable hospital readmission rate as a routine indicator of the quality of hospital care. Med Care. 2006;44(11);972–981.
- , , , , , . Measuring potentially avoidable hospital readmissions. J Clin Epidemiol. 2002;55:573–587.
- Agency for Healthcare Research and Quality Quality Indicators. (April 7, 2006). Prevention Quality Indicators (PQI) Composite Measure Workgroup Final Report. Available at: http://www.qualityindicators.ahrq.gov/modules/pqi_resources.aspx. Accessed June 1, 2012.
- , , , et al. Hospital readmission in general medicine patients: a prediction model. J Gen Intern Med. 2010;25(3):211–219.
- , , , et al. Derivation and validation of an index to predict early death or unplanned readmission after discharge from hospital to the community. CMAJ. 2010;182(6):551–557.
- , , , . Comorbidity measures for use with administrative data. Med Care. Jan 1998;36(1):8–27.
- , , , , eds. Morbidity and mortality in people with serious mental illness. October 2006. National Association of State Mental Health Directors, Medical Directors Council. Available at: http://www.nasmhpd.org/docs/publications/MDCdocs/Mortality%20and%20 Mo rbidity%20Final%20Report%208.18.08.pdf. Accessed January 13, 2013.
- , , , . Mortality in individuals who have had psychiatric treatment: population‐based study in Nova Scotia. Br J Psychiat. 2005;187:552–558.
- , , , , , . Inequitable access for mentally ill patients to some medically necessary procedures. CMAJ. 2007;176(6):779–784.
- , , . Quality of medical care for people with and without comorbid mental illness and substance misuse: systematic review of comparative studies. Br J Psychiat. 2009;194:491–499.
- , , , , , . Reducing all‐cause mortality among patients with psychiatric disorders: a population‐based study. CMAJ. 2013;185(1):E50–E56.
- , , , et al. Incidence of potentially avoidable urgent readmissions and their relation to all‐cause urgent readmissions. CMAJ. 2011;183(14):E1067–E1072.
- , , , , . The incidence and severity of adverse events affecting patients after discharge from the hospital. Ann Intern Med. 2003;138(3):161–167.
- , , , . Hospital‐based medication reconciliation practices: a systematic review. Arch Intern Med. 2012;172(14):1057–1069.
Copyright © 2013 Society of Hospital Medicine
Moving Beyond Readmission Penalties
Containing the rise of healthcare costs has taken on a new sense of urgency in the wake of the recent economic recession and continued growth in the cost of healthcare. Accordingly, many stakeholders seek solutions to improve value (reducing costs while improving care)[1]; hospital readmissions, which are common and costly,[2] have emerged as a key target. The Centers for Medicare and Medicaid Services (CMS) have instituted several programs intended to reduce readmissions, including funding for community‐based, care‐transition programs; penalties for hospitals with elevated risk‐adjusted readmission rates for selected diagnoses; pioneer Accountable Care Organizations (ACOs) with incentives to reduce global costs of care; and Hospital Engagement Networks (HENs) through the Partnership for Patients.[3] A primary aim of these initiatives is to enhance the quality of care transitions as patients are discharged from the hospital.
Though the recent focus on hospital readmissions has appropriately drawn attention to transitions in care, some have expressed concerns. Among these are questions about: 1) the extent to which readmissions truly reflect the quality of hospital care[4]; 2) the preventability of readmissions[5]; 3) limitations in risk‐adjustment techniques[6]; and 4) best practices for preventing readmissions.[7] We believe these concerns stem in part from deficiencies in the state of the science of transitional care, and that future efforts in this area will be hindered without a clear vision of an ideal transition in care. We propose the key components of an ideal transition in care and discuss the implications of this concept as it pertains to hospital readmissions.
THE IDEAL TRANSITION IN CARE
We propose the key components of an ideal transition in care in Figure 1 and Table 1. Figure 1 represents 10 domains described more fully below as structural supports of the bridge patients must cross from one care environment to another during a care transition. This figure highlights key domains and suggests that lack of a domain makes the bridge weaker and more prone to gaps in care and poor outcomes. It also implies that the more components are missing, the less safe is the bridge or transition. Those domains that mainly take place prior to discharge are placed closer to the hospital side of the bridge, those that mainly take place after discharge are placed closer to the community side of the bridge, while those that take place both prior to and after discharge are in the middle. Table 1 provides descriptions of the key content for each of these domains, as well as guidance about which personnel might be involved and where in the transition process that domain should be implemented. We support these domains with supporting evidence where available.
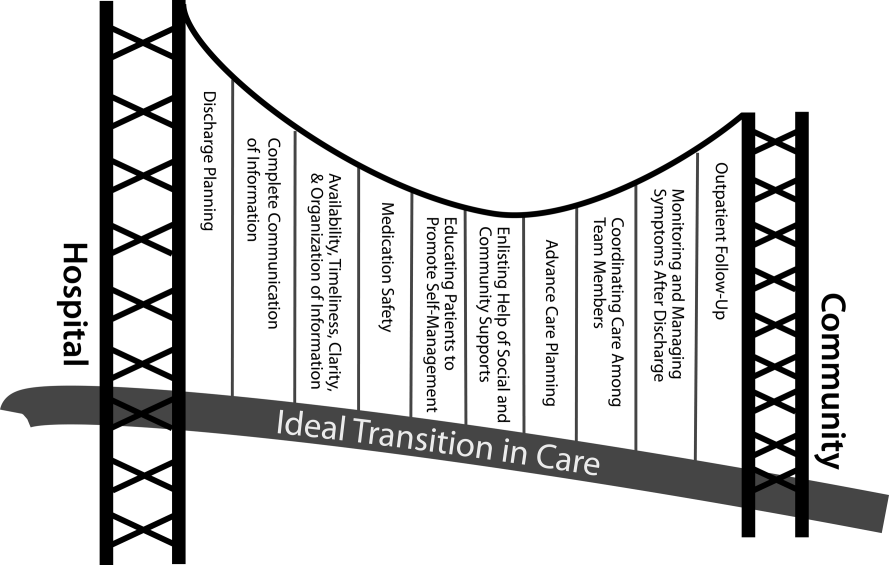
| Domain | Who | When | References |
|---|---|---|---|
| |||
| Discharge planning | |||
| Use a multidisciplinary team to create a discharge plan | Discharging clinician | Predischarge | 911 |
| Collaborate with PCP regarding discharge and follow‐up plan | Care managers/discharge planners | ||
| Arrange follow‐up appointments prior to discharge | Nurses | ||
| Make timely appointments for follow‐up care | |||
| Make appointments that take patient and caregiver's schedules and transportation needs into account | |||
| Complete communication of information | |||
| Includes: | Discharging clinician | Time of discharge | 1214 |
| Patient's full name | |||
| Age | |||
| Dates of admission and discharge | |||
| Names of responsible hospital physicians | |||
| Name of physician preparing discharge summary | |||
| Name of PCP | |||
| Main diagnosis | |||
| Other relevant diagnoses, procedures, and complications | |||
| Relevant findings at admission | |||
| Treatment and response for each active problem | |||
| Results of procedures and abnormal laboratory test results | |||
| Recommendations of any subspecialty consultants | |||
| Patient's functional status at discharge | |||
| Discharge medications | |||
| Follow‐up appointments made and those to be made | |||
| Tests to be ordered and pending tests to be followed‐up | |||
| Counseling provided to patient and caregiver, when applicable | |||
| Contingency planning | |||
| Code status | |||
| Availability, timeliness, clarity, and organization of information | |||
| Timely communication with postdischarge providers verbally (preferred) or by fax/e‐mail | Discharging clinician | Time of discharge | 1214 |
| Timely completion of discharge summary and reliable transmission to postdischarge providers | |||
| Availability of information in medical record | |||
| Use of a structured template with subheadings in discharge communication | |||
| Medication safety | |||
| Take an accurate preadmission medication history | Clinicians | Admission | 1521 |
| Reconcile preadmission medications with all ordered medications at all transfers in care, including discharge | Pharmacists | Throughout hospitalization | |
| Communicate discharge medications to all outpatient providers, including all changes and rationale for those changes | Nurses | Time of discharge | |
| Educating patients, promoting self‐management | |||
| Focus discharge counseling on major diagnoses, medication changes, dates of follow‐up appointments, self‐care instructions, warning signs and symptoms, and who to contact for problems | Clinicians | Daily | 911, 2228, 30 |
| Include caregivers as appropriate | Nurses | Time of discharge | |
| Ensure staff members provide consistent messages | Care managers/discharge planners | Postdischarge | |
| Provide simply written patient‐centered materials with instructions | Transition coaches | ||
| Use teach‐back methods to confirm understanding | |||
| Encourage questions | |||
| Continue teaching during postdischarge follow‐up | |||
| Use transition coaches in high‐risk patients: focus on medication management, keeping a personal medical record, follow‐up appointments, and knowledge of red flags | |||
| Enlisting help of social and community supports | |||
| Assess needs and appropriately arrange for home services | Clinicians | Predischarge and postdischarge | 29, 30 |
| Enlist help of caregivers | Nurses | ||
| Enlist help of community supports | Care managers | ||
| Home health staff | |||
| Advanced care planning | |||
| Establish healthcare proxy | Clinicians | Predischarge and postdischarge | 31, 32 |
| Discuss goals of care | Palliative care staff | ||
| Palliative care consultation (if appropriate) | Social workers | ||
| Enlist hospice services (if appropriate) | Nurses | ||
| Hospice workers | |||
| Coordinating care among team members | |||
| Share medical records | Clinicians | Predischarge and postdischarge | 33 |
| Communicate involving all team members | Nurses | ||
| Optimize continuity of providers and formal handoffs of care | Office personnel | ||
| IT staff | |||
| Monitoring and managing symptoms after discharge | |||
| Monitor for: | Clinicians | Postdischarge | 1113, 28, 3436 |
| Worsening disease control | Nurses | ||
| Medication side effects, discrepancies, nonadherence | Pharmacists | ||
| Therapeutic drug monitoring | Care managers | ||
| Inability to manage conditions at home | Visiting nurses and other home health staff | ||
| Via: | |||
| Postdischarge phone calls | |||
| Home visits | |||
| Postdischarge clinic visits | |||
| Patient hotline | |||
| Availability of inpatient providers after discharge | |||
| Follow‐up with outpatient providers | |||
| Within an appropriate time frame (eg, 7 d or sooner for high‐risk patients) | Clinicians | Postdischarge | 3740 |
| With appropriate providers (eg, most related to reasons for hospitalization, who manage least stable conditions, and/or PCP) | Nurses Pharmacists | ||
| Utilize multidisciplinary teams as appropriate | Care managers | ||
| Ensure appropriate progress along plan of care and safe transition | Office personnel | ||
| Other clinical staff as appropriate | |||
Our concept of an ideal transition in care began with work by Naylor, who described several important components of a safe transition in care, including complete communication of information, patient education, enlisting the help of social and community supports, ensuring continuity of care, and coordinating care among team members.[8] It is supplemented by the Transitions of Care Consensus Policy Statement proposed by representatives from hospital medicine, primary care, and emergency medicine, which emphasized aspects of timeliness and content of communication between providers.[9] Our present articulation of these key components includes 10 organizing domains.
The Discharge Planning domain highlights the important principle of planning ahead for hospital discharge while the patient is still being treated in the hospital, a paradigm espoused by Project RED[10] and other successful care transitions interventions.[11, 12] Collaborating with the outpatient provider and taking the patient and caregiver's preferences for appointment scheduling into account can help ensure optimal outpatient follow‐up.
Complete Communication of Information refers to the content that should be included in discharge summaries and other means of information transfer from hospital to postdischarge care. The specific content areas are based on the Society of Hospital Medicine and Society of General Internal Medicine Continuity of Care Task Force systematic review and recommendations,[13] which takes into account information requested by primary care physicians after discharge.
Availability, Timeliness, Clarity, and Organization of that information is as important as the content because postdischarge providers must be able to access and quickly understand the information they have been provided before assuming care of the patient.[14, 15]
The Medication Safety domain is of central importance because medications are responsible for most postdischarge adverse events.[16] Taking an accurate medication history,[17] reconciling changes throughout the hospitalization,[18] and communicating the reconciled medication regimen to patients and providers across transitions of care can reduce medication errors and improve patient safety.[19, 20, 21, 22]
The Patient Education and Promotion of Self‐Management domain involves teaching patients and their caregivers about the main hospital diagnoses and instructions for self‐care, including medication changes, appointments, and whom to contact if issues arise. Confirming comprehension of instructions through assessments of acute (delirium) and chronic (dementia) cognitive impairments[23, 24, 25, 26] and teach‐back from the patient (or caregiver) is an important aspect of such counseling, as is providing patients and caregivers with educational materials that are appropriate for their level of health literacy and preferred language.[14] High‐risk patients may benefit from patient coaching to improve their self‐management skills.[12] These recommendations are based on years of health literacy research,[27, 28, 29] and such elements are generally included in effective interventions (including Project RED,[10] Naylor and colleagues' Transitional Care Model,[11] and Coleman and colleagues' Care Transitions Intervention[12]).
Enlisting the help of Social and Community Supports is an important adjunct to medical care and is the rationale for the recent increase in CMS funding for community‐based, care‐transition programs. These programs are crucial for assisting patients with household activities, meals, and other necessities during the period of recovery, though they should be distinguished from care management or care coordination interventions, which have not been found to be helpful in preventing readmissions unless high touch in nature.[30, 31]
The Advanced Care Planning domain may begin in the hospital or outpatient setting, and involves establishing goals of care and healthcare proxies, as well as engaging with palliative care or hospice services if appropriate. Emerging evidence supports the intuitive conclusion that this approach prevents readmissions, particularly in patients who do not benefit from hospital readmission.[32, 33]
Attention to the Coordinating Care Among Team Members domain is needed to synchronize efforts across settings and providers. Clearly, many healthcare professionals as well as other involved parties can be involved in helping a single patient during transitions in care. It is vital that they coordinate information, assessments, and plans as a team.[34]
We recognize the domain of Monitoring and Managing Symptoms After Discharge as increasingly crucial as reflected in our growing understanding of the reasons for readmission, especially among patients with fragile conditions such as heart failure, chronic lung disease, gastrointestinal disorders, dementia,[23, 24, 25, 26] and vascular disease.[35] Monitoring for new or worsening symptoms; medication side effects, discrepancies, or nonadherence; and other self‐management challenges will allow problems to be detected and addressed early, before they result in unplanned healthcare utilization. It is noteworthy that successful interventions in this regard rely on in‐home evaluation[13, 14, 29] by nurses rather than telemonitoring, which in isolation has not been effective to date.[36, 37]
Finally, optimal Outpatient Follow‐Up with appropriate postdischarge providers is crucial for providing ideal transitions. These appointments need to be prompt[38, 39] (eg, within 7 days if not sooner for high‐risk patients) and with providers who have a longitudinal relationship to the patient, as prior work has shown increased readmissions when the provider is unfamiliar with the patient.[40] The advantages and disadvantages of hospitalist‐run postdischarge clinics as one way to increase access and expedite follow‐up are currently being explored. Although the optimal content of a postdischarge visit has not been defined, logical tasks to be completed are myriad and imply the need for checklists, adequate time, and a multidisciplinary team of providers.[41]
IMPLICATIONS OF THE IDEAL TRANSITION IN CARE
Our conceptualization of an ideal transition in care provides insight for hospital and healthcare system leadership, policymakers, researchers, clinicians, and educators seeking to improve transitions of care and reduce hospital readmissions. In the sections below, we briefly review commonly cited concerns about the recent focus on readmissions as a quality measure, illustrate how the Ideal Transition in Care addresses these concerns, and propose fruitful areas for future work.
How Does the Framework Address the Extent to Which Readmissions Reflect Hospital Quality?
One of the chief problems with readmissionrates as a hospital quality measure is that many of the factors that influence readmission may not currently be under the hospital's control. The healthcare environment to which a patient is being discharged (and was admitted from in the first place) is an important determinant of readmission.[42] In this context, it is noteworthy that successful interventions to reduce readmission are generally those that focus on outpatient follow‐up, while inpatient‐only interventions have had less success.[7] This is reflected in our framework above, informed by the literature, highlighting the importance of coordination between inpatient and outpatient providers and the importance of postdischarge care, including monitoring and managing symptoms after discharge, prompt follow‐up appointments, the continuation of patient self‐management activities, monitoring for drug‐related problems after discharge, and the effective utilization of community supports. Accountable care organizations, once established, would be responsible for several components of this environment, including the provision of prompt and effective follow‐up care.
The implication of the framework is that if a hospital does not have control over most of the factors that influence its readmission rate, it should see financial incentives to reduce readmission rates as an opportunity to invest in relationships with the outpatient environment from which their patients are admitted and to which they are discharged. One can envision hospitals growing ever‐closer relationships with their network of primary care physician groups, community agencies, and home health services, rehabilitation facilities, and nursing homes through coordinated discharge planning, medication management, patient education, shared electronic medical records, structured handoffs in care, and systems of intensive outpatient monitoring. Our proposed framework, in other words, emphasizes that hospitals cannot reduce their readmission rates by focusing on aspects of care within their walls. They must forge new and stronger relationships with their communities if they are to be successful.
How Does the Framework Help Us Understand Which Readmissions Are Preventable?
Public reporting and financial penalties are currently tied to all‐cause readmission, but preventable readmissions are a more appealing outcome to target. In one study, the ranking of hospitals by all‐cause readmission rate had very little correlation with the ranking by preventable readmission rate.[5] However, researchers have struggled to establish standardized, valid, and reliable measures for determining what proportion of readmissions are in fact preventable, with estimates ranging from 5% to 79% in the published literature.[43]
The difficulty of accurately determining preventability stems from an inadequate understanding of the roles that patient comorbidities, transitional processes of care, individual patient behaviors, and social and environmental determinants of health play in the complex process of hospital recidivism. Our proposed elements of an ideal transition in care provide a structure to frame this discussion and suggest future research opportunities to allow a more accurate and reliable understanding of the spectrum of preventability. Care system leadership can use the framework to rigorously evaluate their readmissions and determine the extent to which the transitions process approached the ideal. For example, if a readmission occurs despite care processes that addressed most of the domains with high fidelity, it becomes much less likely that the readmission was preventable. It should be noted that the converse is not always true: When a transition falls well short of the ideal, it does not always imply that provision of a more ideal transition would necessarily have prevented the readmission, but it does make it more likely.
For educators, the framework may provide insights for trainees into the complexity of the transitions process and vulnerability of patients during this time, highlighting preventable aspects of readmissions that are within the grasp of the discharging clinician or team. It highlights the importance of medication reconciliation, synchronous communication, and predischarge teaching, which are measurable and teachable skills for non‐physician providers, housestaff, and medical students. It also may allow for more structured feedback, for example, on the quality of discharge summaries produced by trainees.
How Could the Framework Improve Risk Adjustment for Between‐Hospital Comparisons?
Under the Patient Protection and Affordable Care Act (PPACA), hospitals will be compared to one another using risk‐standardized readmission rates as a way to penalize poorly performing hospitals. However, risk‐adjustment models have only modest ability to predict hospital readmission.[6] Moreover, current approaches predominantly adjust for patients' medical comorbidities (which are easily measurable), but they do not adequately take into account the growing literature on other factors that influence readmission rates, including a patient's health literacy, visual or cognitive impairment, functional status, language barriers, and community‐level factors such as social supports.[44, 45]
The Ideal Transition of Care provides a comprehensive framework of hospital discharge quality that provides additional process measures on which hospitals could be compared rather than focusing solely on (inadequately) risk‐adjusted readmission rates. Indeed, most other quality and safety measures (such as the National Quality Forum's Safe Practices[46] and The Joint Commission's National Patient Safety Goals),[47] emphasize process over outcome, in part because of issues of fairness. Process measures are less subject to differences in patient populations and also change the focus from simply reducing readmissions to improving transitional care more broadly. These process measures should be based on our framework and should attempt to capture as many dimensions of an optimal care transition as possible.
Possible examples of process measures include: the accuracy of medication reconciliation at admission and discharge; provision of prompt outpatient follow‐up; provision of adequate systems to monitor and manage symptoms after discharge; advanced care planning in appropriate patients; and the quality of discharge education, incorporating measurements of the patient's understanding and ability to self‐manage their illness. At least some of these could be used now as part of a performance measurement set that highlights opportunities for immediate system change and can serve as performance milestones.
The framework could also be used to validate risk‐adjustment techniques. After accounting for patient factors, the remaining variability in outcomes should be accounted for by processes of care that are in the transitions framework. Once these processes are accurately measured, one can determine if indeed the remaining variability is due to transitions processes, or rather unaccounted factors that are not being measured and that hospitals may have little control over. Such work can lead to iterative refinement of patient risk‐adjustment models.
What Does the Framework Imply About Best Practices for Reducing Readmission Rates?
Despite the limitations of readmission rates as a quality measure noted above, hospitals presently face potentially large financial penalties for readmissions and are allocating resources to readmission reduction efforts. However, hospitals currently may not have enough guidance to know what actions to take to reduce readmissions, and thus could be spending money inefficiently and reducing the value proposition of focusing on readmissions.
A recent systematic review of interventions hospitals could employ to reduce readmissions identified several positive studies, but also many negative studies, and there were significant barriers to understanding what works to reduce readmissions.[7] For example, most of the interventions described in both positive and negative studies were multifaceted, and the authors were unable to identify which components of the intervention were most effective. Also, while several studies have identified risk factors for readmission,[6, 48, 49] very few studies have identified which subgroups of patients benefit most from specific interventions. Few of the studies described key contextual factors that may have led to successful or failed implementation, or the fidelity with which the intervention was implemented.[50, 51, 52]
Few if any of the studies were guided by a concept of the ideal transition in care.[10] Such a framework will better guide development of multifaceted interventions and provide an improved means for interpreting the results. Clearly, rigorously conducted, multicenter studies of readmission prevention interventions are needed to move the field forward. These studies should: 1) correlate implementation of specific intervention components with reductions in readmission rates to better understand the most effective components; 2) be adequately powered to show effect modification, ie, which patients benefit most from these interventions; and 3) rigorously measure environmental context and intervention fidelity, and employ mixed methods to better understand predictors of implementation success and failure.
Our framework can be used in the design and evaluation of such interventions. For example, interventions could be designed that incorporate as many of the domains of an ideal transition as possible, in particular those that span the inpatient and outpatient settings. Processes of care metrics can be developed that measure the extent to which each domain is delivered, analogous to the way the Joint Commission might aggregate individual scores on the 10 items in Acute Myocardial Infarction Core Measure Set[53] to provide a composite of the quality of care provided to patients with this diagnosis. These can be used to correlate certain intervention components with success in reducing readmissions and also in measuring intervention fidelity.
NEXT STEPS
For hospital and healthcare system leaders, who need to take action now to avoid financial penalties, we recommend starting with proven, high‐touch interventions such as Project RED and the Care Transitions Intervention, which are durable, cost‐effective, robustly address multiple domains of the Ideal Transition in Care, and have been implemented at numerous sites.[54, 55] Each hospital or group will need to decide on a bundle of interventions and customize them based on local workflow, resources, and culture.
Risk‐stratification, to match the intensity of the intervention to the risk of readmission of the patient, will undoubtedly be a key component for the efficient use of resources. We anticipate future research will allow risk stratification to be a robust part of any implementation plan. However, as noted above, current risk prediction models are imperfect,[6] and more work is needed to determine which patients benefit most from which interventions. Few if any studies have described interventions tailored to risk for this reason.
Based on our ideal transition in care, our collective experience, and published evidence,[7, 10, 11, 12] potential elements to start with include: early discharge planning; medication reconciliation[56]; patient/caregiver education using health literacy principles, cognitive assessments, and teach‐back to confirm understanding; synchronous communication (eg, by phone) between inpatient and postdischarge providers; follow‐up phone calls to patients within 72 hours of discharge; 24/7 availability of a responsible inpatient provider to address questions and problems (both from the patient/caregiver and from postdischarge providers); and prompt appointments for patients discharged home. High‐risk patients will likely require additional interventions, including in‐home assessments, disease‐monitoring programs, and/or patient coaching. Lastly, patients with certain conditions prone to readmission (such as heart failure and chronic obstructive pulmonary disease) may benefit from disease‐specific programs, including patient education, outpatient disease management, and monitoring protocols.
It is likely that the most effective interventions are those that come from combined, coordinated interventions shared between inpatient and outpatient settings, and are intensive in nature. We expect that the more domains in the framework that are addressed, the safer and more seamless transitions in care will be, with improvement in patient outcomes. To the extent that fragmentation of care has been a barrier to the implementation of these types of interventions in the past, ACOs, perhaps with imbedded Patient‐Centered Medical Homes, may be in the best position to take advantage of newly aligned financial incentives to design comprehensive transitional care. Indeed, we anticipate that Figure 1 may provide substrate for a discussion of postdischarge care and division of responsibilities between inpatient and outpatient care teams at the time of transition, so effort is not duplicated and multiple domains are addressed.
Other barriers to implementation of ideal transitions in care will continue to be an issue for most healthcare systems. Financial constraints that have been a barrier up until now will be partially overcome by penalties for high readmission rates and by ACOs, bundled payments, and alternative care contracts (ie, global payments), but the extent to which each institution feels rewarded for investing in transitional interventions will vary greatly. Healthcare leadership that sees the value of improving transitions in care will be critical to overcoming this barrier. Competing demands (such as lowering hospital length of stay and carrying out other patient care responsibilities),[57] lack of coordination and diffusion of responsibility among various clinical personnel, and lack of standards are other barriers[58] that will require clear prioritization from leadership, policy changes, team‐based care, provider education and feedback, and adequate allocation of personnel resources. In short, process redesign using continuous quality improvement efforts and effective tools will be required to maximize the possibility of success.
CONCLUSIONS
Readmissions are costly and undesirable. Intuition suggests they are a marker of poor care and that hospitals should be capable of reducing them, thereby improving care and decreasing costs. In a potential future world of ACOs based on global payments, financial incentives would be aligned for each system to reduce readmissions below their current baseline, therefore obviating the need for external financial rewards and penalties. In the meantime, financial penalties do exist, and controversy exists over their fairness and likelihood of driving appropriate behavior. To address these controversies and promote better transitional care, we call for the development and use of multifaceted, collaborative transitions interventions that span settings, risk‐adjustment models that allow for fairer comparisons among hospitals, better and more widespread measurement of processes of transitional care, a better understanding of what interventions are most effective and in whom, and better guidance in how to implement these interventions. Our conceptualization of an ideal transition of care serves as a guide and provides a common vocabulary for these efforts. Such research is likely to produce the knowledge needed for healthcare systems to improve transitions in care, reduce readmissions, and reduce costs.
Disclosure
Funding for Dr Vasilevskis has been provided by the National Institutes of Health (K23AG040157) and the VA Tennessee Valley Geriatric Research, Education and Clinical Center (GRECC). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute on Aging, the National Institutes of Health, or the US Department of Veterans Affairs.
- , . Redefining Health Care: Creating Value‐Based Competition on Results. Boston, MA:Harvard Business School Press;2006.
- , , . Rehospitalizations among patients in the Medicare fee‐for‐service program. N Engl J Med. 2009;360(14):1418–1428.
- Patient Protection and Affordable Care Act (PPACA). Public Law 111–148; 2010. Available at: http://www.gpo.gov/fdsys/pkg/PLAW‐111publ148/pdf/PLAW‐111publ148.pdf. Accessed on June 4, 2012.
- , . Hospital readmission as an accountability measure. JAMA. 2011;305(5):504–505.
- , , , et al. Incidence of potentially avoidable urgent readmissions and their relation to all‐cause urgent readmissions. Can Med Assoc J. 2011;183(14):E1067–E1072.
- , , , et al. Risk prediction models for hospital readmission: a systematic review. JAMA. 2011;306(15):1688–1698.
- , , , , . Interventions to reduce 30‐day rehospitalization: a systematic review. Ann Intern Med. 2011;155(8):520–528.
- . A decade of transitional care research with vulnerable elders. J Cardiovasc Nurs. 2000;14(3):1–14.
- , , , et al;for the American College of Physicians; Society of General Internal Medicine; Society of Hospital Medicine; American Geriatrics Society; American College of Emergency Physicians; Society of Academic Emergency Medicine. Transitions of Care Consensus Policy Statement American College of Physicians–Society of General Internal Medicine–Society of Hospital Medicine–American Geriatrics Society–American College of Emergency Physicians–Society of Academic Emergency Medicine. J Gen Intern Med. 2009;24(8):971–976.
- , , , et al. A reengineered hospital discharge program to decrease rehospitalization: a randomized trial. Ann Intern Med. 2009;150(3):178–187.
- , , , et al. Comprehensive discharge planning and home follow‐up of hospitalized elders: a randomized clinical trial. JAMA. 1999;281(7):613–620.
- , , , . The care transitions intervention: results of a randomized controlled trial. Arch Intern Med. 2006;166(17):1822–1828.
- , , , , , . Deficits in communication and information transfer between hospital‐based and primary care physicians: implications for patient safety and continuity of care. JAMA. 2007;297(8):831–841.
- , , , . Promoting effective transitions of care at hospital discharge: a review of key issues for hospitalists. J Hosp Med. 2007;2(5):314–323.
- , , , , , . Discharge documentation of patients discharged to subacute facilities: a three‐year quality improvement process across an integrated health care system. Jt Comm J Qual Patient Saf. 2010;36(6):243–251.
- , , , , . The incidence and severity of adverse events affecting patients after discharge from the hospital. Ann Intern Med. 2003;138(3):161–167.
- , , , , , . Frequency, type and clinical importance of medication history errors at admission to hospital: a systematic review. Can Med Assoc J. 2005;173(5):510–515.
- , , , et al. Classifying and predicting errors of inpatient medication reconciliation. J Gen Intern Med. 2008;23(9):1414–1422.
- , , et al;for the PILL‐CVD (Pharmacist Intervention for Low Literacy in Cardiovascular Disease) Study Group. Effect of a pharmacist intervention on clinically important medication errors after hospital discharge: a randomized trial. Ann Intern Med. 2012;157(1):1–10.
- , , , . Hospital‐based medication reconciliation practices: a systematic review. Arch Intern Med. 2012;172(14):1057–1069.
- , , , et al. Effect of an electronic medication reconciliation application and process redesign on potential adverse drug events: a cluster‐randomized trial. Arch Intern Med. 2009;169(8):771–780.
- , , , et al. Role of pharmacist counseling in preventing adverse drug events after hospitalization. Arch Intern Med. 2006;166(5):565–571.
- , , , , . Insufficient help for activity of daily living disabilities and risk of all–cause hospitalization. J Am Geriatr Soc. 2012;60(5):927–933.
- , , , et al. Transitions in care for older adults with and without dementia. J Am Geriatr Soc. 2012;60(5):813–820.
- , , , , . Association of incident dementia with hospitalizations. JAMA. 2012;307(2):165–172.
- , , , et al. Potentially avoidable hospitalizations of dually eligible Medicare and Medicaid beneficiaries from nursing facility and home– and community–based services waiver programs. J Am Geriatr Soc. 2012;60(5):821–829.
- , . Teaching about health literacy and clear communication. J Gen Intern Med. 2006;21(8):888–890.
- , , , et al. Health literacy and outcomes among patients with heart failure. JAMA. 2011;305(16):1695–1701.
- , , , , . Patient experiences of transitioning from hospital to home: an ethnographic quality improvement project. J Hosp Med. 2012;7(5):382–387.
- , , , . Effects of care coordination on hospitalization, quality of care, and health care expenditures among Medicare beneficiaries: 15 randomized trials. JAMA. 2009;301(6):603–618.
- , , , , . How changes in Washington University's Medicare coordinated care demonstration pilot ultimately achieved savings. Health Aff (Millwood). 2012;31(6):1216–1226.
- , , , et al. Quality of care and rehospitalization rate in the last stage of disease in brain tumor patients assisted at home: a cost effectiveness study. J Palliat Med. 2012;15(2):225–227.
- , , , , . Inpatient palliative care consults and the probability of hospital readmission. Perm J. 2011;15(2):48–51.
- , , , et al. TeamSTEPPS™: team strategies and tools to enhance performance and patient safety. In: Henriksen K, Battles JB, Keyes MA, Grady ML, ed. Advances in Patient Safety: New Directions and Alternative Approaches. Vol 3: Performance and Tools. Rockville, MD:Agency for Healthcare Research and Quality; August2008.
- , , , et al. Factors contributing to all‐cause 30‐day readmissions: a structured case series across 18 hospitals. Med Care. 2012;50(7):599–605.
- , , , et al. Telemonitoring in patients with heart failure [erratum, N Engl J Med. 2011;364(5):490]. N Engl J Med. 2010;363(24):2301–2309.
- , , , et al. A randomized controlled trial of telemonitoring in older adults with multiple health issues to prevent hospitalizations and emergency department visits. Arch Intern Med. 2012;172(10):773–779.
- , , , et al. Relationship between early physician follow‐up and 30‐day readmission among Medicare beneficiaries hospitalized for heart failure. JAMA. 2010;303(17):1716–1722.
- , , . Post‐hospitalization transitions: examining the effects of timing of primary care provider follow‐up. J Hosp Med. 2010;5(7):392–397.
- , , . Does increased access to primary care reduce hospital readmissions? Veterans Affairs Cooperative Study Group on Primary Care and Hospital Readmission. N Engl J Med. 1996;334(22):1441–1447.
- . The Post‐Hospital Follow‐Up Visit: A Physician Checklist to Reduce Readmissions. California Healthcare Foundation; October 2010. Available at: http://www.chcf.org/publications/2010/10/the‐post‐hospital‐follow‐up‐visit‐a‐physician‐checklist. Accessed on January 10, 2012.
- , , . Thirty‐day readmission rates for Medicare beneficiaries by race and site of care. JAMA. 2011;305(7):675–681.
- , , , , . Proportion of hospital readmissions deemed avoidable: a systematic review. Can Med Assoc J. 2011;183(7):E391–E402.
- , , , et al. Postdischarge environmental and socioeconomic factors and the likelihood of early hospital readmission among community‐dwelling Medicare beneficiaries. Gerontologist. 2008;48(4):495–504.
- , , , , . Low health literacy and health outcomes: an updated systematic review. Ann Intern Med. 2011;155(2):97–107.
- National Quality Forum. Safe Practices for Better Healthcare—2010 Update: A Consensus Report. Washington, DC;2010.
- Joint Commission on Accreditation of Healthcare Organizations. Accreditation Program: Hospital 2010 National Patient Safety Goals (NPSGs). 2010. Available at: http://www.jointcommission.org/PatientSafety/NationalPatientSafetyGoals/. Accessed on March 20, 2012.
- , , , et al. Hospital readmission in general medicine patients: a prediction model. J Gen Intern Med. 2010;25(3):211–219.
- , , , et al. Derivation and validation of an index to predict early death or unplanned readmission after discharge from hospital to the community. Can Med Assoc J. 2010;182(6):551–557.
- , . Evaluating service delivery interventions to enhance patient safety. BMJ. 2008;337:a2764.
- , , . Assessing the Evidence for Context‐Sensitive Effectiveness and Safety of Patient Safety Practices: Developing Criteria. Rockville, MD:Agency for Healthcare Research and Quality; December2010.
- , , , et al. Advancing the science of patient safety. Ann Intern Med. 2011;154(10):693–696.
- The Joint Commission. Acute Myocardial Infarction Core Measure Set. Available at: http://www.jointcommission.org/assets/1/6/Acute%20Myocardial%20Infarction.pdf. Accessed August 20,2012.
- , , , , , . The care transitions intervention: translating from efficacy to effectiveness. Arch Intern Med. 2011;171(14):1232–1237.
- Project RED toolkit, AHRQ Innovations Exchange. Available at:http://www.innovations.ahrq.gov/content.aspx?id=2180. Accessed on July 2, 2012.
- , , , et al. A comprehensive pharmacist intervention to reduce morbidity in patients 80 years or older: a randomized controlled trial. Arch Intern Med. 2009;169(9):894–900.
- , . Thirty‐day readmissions—truth and consequences. N Engl J Med. 2012;366(15):1366–1369.
- , , , , . “Out of sight, out of mind”: housestaff perceptions of quality‐limiting factors in discharge care at teaching hospitals. J Hosp Med. 2012;7(5):376–381.
Containing the rise of healthcare costs has taken on a new sense of urgency in the wake of the recent economic recession and continued growth in the cost of healthcare. Accordingly, many stakeholders seek solutions to improve value (reducing costs while improving care)[1]; hospital readmissions, which are common and costly,[2] have emerged as a key target. The Centers for Medicare and Medicaid Services (CMS) have instituted several programs intended to reduce readmissions, including funding for community‐based, care‐transition programs; penalties for hospitals with elevated risk‐adjusted readmission rates for selected diagnoses; pioneer Accountable Care Organizations (ACOs) with incentives to reduce global costs of care; and Hospital Engagement Networks (HENs) through the Partnership for Patients.[3] A primary aim of these initiatives is to enhance the quality of care transitions as patients are discharged from the hospital.
Though the recent focus on hospital readmissions has appropriately drawn attention to transitions in care, some have expressed concerns. Among these are questions about: 1) the extent to which readmissions truly reflect the quality of hospital care[4]; 2) the preventability of readmissions[5]; 3) limitations in risk‐adjustment techniques[6]; and 4) best practices for preventing readmissions.[7] We believe these concerns stem in part from deficiencies in the state of the science of transitional care, and that future efforts in this area will be hindered without a clear vision of an ideal transition in care. We propose the key components of an ideal transition in care and discuss the implications of this concept as it pertains to hospital readmissions.
THE IDEAL TRANSITION IN CARE
We propose the key components of an ideal transition in care in Figure 1 and Table 1. Figure 1 represents 10 domains described more fully below as structural supports of the bridge patients must cross from one care environment to another during a care transition. This figure highlights key domains and suggests that lack of a domain makes the bridge weaker and more prone to gaps in care and poor outcomes. It also implies that the more components are missing, the less safe is the bridge or transition. Those domains that mainly take place prior to discharge are placed closer to the hospital side of the bridge, those that mainly take place after discharge are placed closer to the community side of the bridge, while those that take place both prior to and after discharge are in the middle. Table 1 provides descriptions of the key content for each of these domains, as well as guidance about which personnel might be involved and where in the transition process that domain should be implemented. We support these domains with supporting evidence where available.

| Domain | Who | When | References |
|---|---|---|---|
| |||
| Discharge planning | |||
| Use a multidisciplinary team to create a discharge plan | Discharging clinician | Predischarge | 911 |
| Collaborate with PCP regarding discharge and follow‐up plan | Care managers/discharge planners | ||
| Arrange follow‐up appointments prior to discharge | Nurses | ||
| Make timely appointments for follow‐up care | |||
| Make appointments that take patient and caregiver's schedules and transportation needs into account | |||
| Complete communication of information | |||
| Includes: | Discharging clinician | Time of discharge | 1214 |
| Patient's full name | |||
| Age | |||
| Dates of admission and discharge | |||
| Names of responsible hospital physicians | |||
| Name of physician preparing discharge summary | |||
| Name of PCP | |||
| Main diagnosis | |||
| Other relevant diagnoses, procedures, and complications | |||
| Relevant findings at admission | |||
| Treatment and response for each active problem | |||
| Results of procedures and abnormal laboratory test results | |||
| Recommendations of any subspecialty consultants | |||
| Patient's functional status at discharge | |||
| Discharge medications | |||
| Follow‐up appointments made and those to be made | |||
| Tests to be ordered and pending tests to be followed‐up | |||
| Counseling provided to patient and caregiver, when applicable | |||
| Contingency planning | |||
| Code status | |||
| Availability, timeliness, clarity, and organization of information | |||
| Timely communication with postdischarge providers verbally (preferred) or by fax/e‐mail | Discharging clinician | Time of discharge | 1214 |
| Timely completion of discharge summary and reliable transmission to postdischarge providers | |||
| Availability of information in medical record | |||
| Use of a structured template with subheadings in discharge communication | |||
| Medication safety | |||
| Take an accurate preadmission medication history | Clinicians | Admission | 1521 |
| Reconcile preadmission medications with all ordered medications at all transfers in care, including discharge | Pharmacists | Throughout hospitalization | |
| Communicate discharge medications to all outpatient providers, including all changes and rationale for those changes | Nurses | Time of discharge | |
| Educating patients, promoting self‐management | |||
| Focus discharge counseling on major diagnoses, medication changes, dates of follow‐up appointments, self‐care instructions, warning signs and symptoms, and who to contact for problems | Clinicians | Daily | 911, 2228, 30 |
| Include caregivers as appropriate | Nurses | Time of discharge | |
| Ensure staff members provide consistent messages | Care managers/discharge planners | Postdischarge | |
| Provide simply written patient‐centered materials with instructions | Transition coaches | ||
| Use teach‐back methods to confirm understanding | |||
| Encourage questions | |||
| Continue teaching during postdischarge follow‐up | |||
| Use transition coaches in high‐risk patients: focus on medication management, keeping a personal medical record, follow‐up appointments, and knowledge of red flags | |||
| Enlisting help of social and community supports | |||
| Assess needs and appropriately arrange for home services | Clinicians | Predischarge and postdischarge | 29, 30 |
| Enlist help of caregivers | Nurses | ||
| Enlist help of community supports | Care managers | ||
| Home health staff | |||
| Advanced care planning | |||
| Establish healthcare proxy | Clinicians | Predischarge and postdischarge | 31, 32 |
| Discuss goals of care | Palliative care staff | ||
| Palliative care consultation (if appropriate) | Social workers | ||
| Enlist hospice services (if appropriate) | Nurses | ||
| Hospice workers | |||
| Coordinating care among team members | |||
| Share medical records | Clinicians | Predischarge and postdischarge | 33 |
| Communicate involving all team members | Nurses | ||
| Optimize continuity of providers and formal handoffs of care | Office personnel | ||
| IT staff | |||
| Monitoring and managing symptoms after discharge | |||
| Monitor for: | Clinicians | Postdischarge | 1113, 28, 3436 |
| Worsening disease control | Nurses | ||
| Medication side effects, discrepancies, nonadherence | Pharmacists | ||
| Therapeutic drug monitoring | Care managers | ||
| Inability to manage conditions at home | Visiting nurses and other home health staff | ||
| Via: | |||
| Postdischarge phone calls | |||
| Home visits | |||
| Postdischarge clinic visits | |||
| Patient hotline | |||
| Availability of inpatient providers after discharge | |||
| Follow‐up with outpatient providers | |||
| Within an appropriate time frame (eg, 7 d or sooner for high‐risk patients) | Clinicians | Postdischarge | 3740 |
| With appropriate providers (eg, most related to reasons for hospitalization, who manage least stable conditions, and/or PCP) | Nurses Pharmacists | ||
| Utilize multidisciplinary teams as appropriate | Care managers | ||
| Ensure appropriate progress along plan of care and safe transition | Office personnel | ||
| Other clinical staff as appropriate | |||
Our concept of an ideal transition in care began with work by Naylor, who described several important components of a safe transition in care, including complete communication of information, patient education, enlisting the help of social and community supports, ensuring continuity of care, and coordinating care among team members.[8] It is supplemented by the Transitions of Care Consensus Policy Statement proposed by representatives from hospital medicine, primary care, and emergency medicine, which emphasized aspects of timeliness and content of communication between providers.[9] Our present articulation of these key components includes 10 organizing domains.
The Discharge Planning domain highlights the important principle of planning ahead for hospital discharge while the patient is still being treated in the hospital, a paradigm espoused by Project RED[10] and other successful care transitions interventions.[11, 12] Collaborating with the outpatient provider and taking the patient and caregiver's preferences for appointment scheduling into account can help ensure optimal outpatient follow‐up.
Complete Communication of Information refers to the content that should be included in discharge summaries and other means of information transfer from hospital to postdischarge care. The specific content areas are based on the Society of Hospital Medicine and Society of General Internal Medicine Continuity of Care Task Force systematic review and recommendations,[13] which takes into account information requested by primary care physicians after discharge.
Availability, Timeliness, Clarity, and Organization of that information is as important as the content because postdischarge providers must be able to access and quickly understand the information they have been provided before assuming care of the patient.[14, 15]
The Medication Safety domain is of central importance because medications are responsible for most postdischarge adverse events.[16] Taking an accurate medication history,[17] reconciling changes throughout the hospitalization,[18] and communicating the reconciled medication regimen to patients and providers across transitions of care can reduce medication errors and improve patient safety.[19, 20, 21, 22]
The Patient Education and Promotion of Self‐Management domain involves teaching patients and their caregivers about the main hospital diagnoses and instructions for self‐care, including medication changes, appointments, and whom to contact if issues arise. Confirming comprehension of instructions through assessments of acute (delirium) and chronic (dementia) cognitive impairments[23, 24, 25, 26] and teach‐back from the patient (or caregiver) is an important aspect of such counseling, as is providing patients and caregivers with educational materials that are appropriate for their level of health literacy and preferred language.[14] High‐risk patients may benefit from patient coaching to improve their self‐management skills.[12] These recommendations are based on years of health literacy research,[27, 28, 29] and such elements are generally included in effective interventions (including Project RED,[10] Naylor and colleagues' Transitional Care Model,[11] and Coleman and colleagues' Care Transitions Intervention[12]).
Enlisting the help of Social and Community Supports is an important adjunct to medical care and is the rationale for the recent increase in CMS funding for community‐based, care‐transition programs. These programs are crucial for assisting patients with household activities, meals, and other necessities during the period of recovery, though they should be distinguished from care management or care coordination interventions, which have not been found to be helpful in preventing readmissions unless high touch in nature.[30, 31]
The Advanced Care Planning domain may begin in the hospital or outpatient setting, and involves establishing goals of care and healthcare proxies, as well as engaging with palliative care or hospice services if appropriate. Emerging evidence supports the intuitive conclusion that this approach prevents readmissions, particularly in patients who do not benefit from hospital readmission.[32, 33]
Attention to the Coordinating Care Among Team Members domain is needed to synchronize efforts across settings and providers. Clearly, many healthcare professionals as well as other involved parties can be involved in helping a single patient during transitions in care. It is vital that they coordinate information, assessments, and plans as a team.[34]
We recognize the domain of Monitoring and Managing Symptoms After Discharge as increasingly crucial as reflected in our growing understanding of the reasons for readmission, especially among patients with fragile conditions such as heart failure, chronic lung disease, gastrointestinal disorders, dementia,[23, 24, 25, 26] and vascular disease.[35] Monitoring for new or worsening symptoms; medication side effects, discrepancies, or nonadherence; and other self‐management challenges will allow problems to be detected and addressed early, before they result in unplanned healthcare utilization. It is noteworthy that successful interventions in this regard rely on in‐home evaluation[13, 14, 29] by nurses rather than telemonitoring, which in isolation has not been effective to date.[36, 37]
Finally, optimal Outpatient Follow‐Up with appropriate postdischarge providers is crucial for providing ideal transitions. These appointments need to be prompt[38, 39] (eg, within 7 days if not sooner for high‐risk patients) and with providers who have a longitudinal relationship to the patient, as prior work has shown increased readmissions when the provider is unfamiliar with the patient.[40] The advantages and disadvantages of hospitalist‐run postdischarge clinics as one way to increase access and expedite follow‐up are currently being explored. Although the optimal content of a postdischarge visit has not been defined, logical tasks to be completed are myriad and imply the need for checklists, adequate time, and a multidisciplinary team of providers.[41]
IMPLICATIONS OF THE IDEAL TRANSITION IN CARE
Our conceptualization of an ideal transition in care provides insight for hospital and healthcare system leadership, policymakers, researchers, clinicians, and educators seeking to improve transitions of care and reduce hospital readmissions. In the sections below, we briefly review commonly cited concerns about the recent focus on readmissions as a quality measure, illustrate how the Ideal Transition in Care addresses these concerns, and propose fruitful areas for future work.
How Does the Framework Address the Extent to Which Readmissions Reflect Hospital Quality?
One of the chief problems with readmissionrates as a hospital quality measure is that many of the factors that influence readmission may not currently be under the hospital's control. The healthcare environment to which a patient is being discharged (and was admitted from in the first place) is an important determinant of readmission.[42] In this context, it is noteworthy that successful interventions to reduce readmission are generally those that focus on outpatient follow‐up, while inpatient‐only interventions have had less success.[7] This is reflected in our framework above, informed by the literature, highlighting the importance of coordination between inpatient and outpatient providers and the importance of postdischarge care, including monitoring and managing symptoms after discharge, prompt follow‐up appointments, the continuation of patient self‐management activities, monitoring for drug‐related problems after discharge, and the effective utilization of community supports. Accountable care organizations, once established, would be responsible for several components of this environment, including the provision of prompt and effective follow‐up care.
The implication of the framework is that if a hospital does not have control over most of the factors that influence its readmission rate, it should see financial incentives to reduce readmission rates as an opportunity to invest in relationships with the outpatient environment from which their patients are admitted and to which they are discharged. One can envision hospitals growing ever‐closer relationships with their network of primary care physician groups, community agencies, and home health services, rehabilitation facilities, and nursing homes through coordinated discharge planning, medication management, patient education, shared electronic medical records, structured handoffs in care, and systems of intensive outpatient monitoring. Our proposed framework, in other words, emphasizes that hospitals cannot reduce their readmission rates by focusing on aspects of care within their walls. They must forge new and stronger relationships with their communities if they are to be successful.
How Does the Framework Help Us Understand Which Readmissions Are Preventable?
Public reporting and financial penalties are currently tied to all‐cause readmission, but preventable readmissions are a more appealing outcome to target. In one study, the ranking of hospitals by all‐cause readmission rate had very little correlation with the ranking by preventable readmission rate.[5] However, researchers have struggled to establish standardized, valid, and reliable measures for determining what proportion of readmissions are in fact preventable, with estimates ranging from 5% to 79% in the published literature.[43]
The difficulty of accurately determining preventability stems from an inadequate understanding of the roles that patient comorbidities, transitional processes of care, individual patient behaviors, and social and environmental determinants of health play in the complex process of hospital recidivism. Our proposed elements of an ideal transition in care provide a structure to frame this discussion and suggest future research opportunities to allow a more accurate and reliable understanding of the spectrum of preventability. Care system leadership can use the framework to rigorously evaluate their readmissions and determine the extent to which the transitions process approached the ideal. For example, if a readmission occurs despite care processes that addressed most of the domains with high fidelity, it becomes much less likely that the readmission was preventable. It should be noted that the converse is not always true: When a transition falls well short of the ideal, it does not always imply that provision of a more ideal transition would necessarily have prevented the readmission, but it does make it more likely.
For educators, the framework may provide insights for trainees into the complexity of the transitions process and vulnerability of patients during this time, highlighting preventable aspects of readmissions that are within the grasp of the discharging clinician or team. It highlights the importance of medication reconciliation, synchronous communication, and predischarge teaching, which are measurable and teachable skills for non‐physician providers, housestaff, and medical students. It also may allow for more structured feedback, for example, on the quality of discharge summaries produced by trainees.
How Could the Framework Improve Risk Adjustment for Between‐Hospital Comparisons?
Under the Patient Protection and Affordable Care Act (PPACA), hospitals will be compared to one another using risk‐standardized readmission rates as a way to penalize poorly performing hospitals. However, risk‐adjustment models have only modest ability to predict hospital readmission.[6] Moreover, current approaches predominantly adjust for patients' medical comorbidities (which are easily measurable), but they do not adequately take into account the growing literature on other factors that influence readmission rates, including a patient's health literacy, visual or cognitive impairment, functional status, language barriers, and community‐level factors such as social supports.[44, 45]
The Ideal Transition of Care provides a comprehensive framework of hospital discharge quality that provides additional process measures on which hospitals could be compared rather than focusing solely on (inadequately) risk‐adjusted readmission rates. Indeed, most other quality and safety measures (such as the National Quality Forum's Safe Practices[46] and The Joint Commission's National Patient Safety Goals),[47] emphasize process over outcome, in part because of issues of fairness. Process measures are less subject to differences in patient populations and also change the focus from simply reducing readmissions to improving transitional care more broadly. These process measures should be based on our framework and should attempt to capture as many dimensions of an optimal care transition as possible.
Possible examples of process measures include: the accuracy of medication reconciliation at admission and discharge; provision of prompt outpatient follow‐up; provision of adequate systems to monitor and manage symptoms after discharge; advanced care planning in appropriate patients; and the quality of discharge education, incorporating measurements of the patient's understanding and ability to self‐manage their illness. At least some of these could be used now as part of a performance measurement set that highlights opportunities for immediate system change and can serve as performance milestones.
The framework could also be used to validate risk‐adjustment techniques. After accounting for patient factors, the remaining variability in outcomes should be accounted for by processes of care that are in the transitions framework. Once these processes are accurately measured, one can determine if indeed the remaining variability is due to transitions processes, or rather unaccounted factors that are not being measured and that hospitals may have little control over. Such work can lead to iterative refinement of patient risk‐adjustment models.
What Does the Framework Imply About Best Practices for Reducing Readmission Rates?
Despite the limitations of readmission rates as a quality measure noted above, hospitals presently face potentially large financial penalties for readmissions and are allocating resources to readmission reduction efforts. However, hospitals currently may not have enough guidance to know what actions to take to reduce readmissions, and thus could be spending money inefficiently and reducing the value proposition of focusing on readmissions.
A recent systematic review of interventions hospitals could employ to reduce readmissions identified several positive studies, but also many negative studies, and there were significant barriers to understanding what works to reduce readmissions.[7] For example, most of the interventions described in both positive and negative studies were multifaceted, and the authors were unable to identify which components of the intervention were most effective. Also, while several studies have identified risk factors for readmission,[6, 48, 49] very few studies have identified which subgroups of patients benefit most from specific interventions. Few of the studies described key contextual factors that may have led to successful or failed implementation, or the fidelity with which the intervention was implemented.[50, 51, 52]
Few if any of the studies were guided by a concept of the ideal transition in care.[10] Such a framework will better guide development of multifaceted interventions and provide an improved means for interpreting the results. Clearly, rigorously conducted, multicenter studies of readmission prevention interventions are needed to move the field forward. These studies should: 1) correlate implementation of specific intervention components with reductions in readmission rates to better understand the most effective components; 2) be adequately powered to show effect modification, ie, which patients benefit most from these interventions; and 3) rigorously measure environmental context and intervention fidelity, and employ mixed methods to better understand predictors of implementation success and failure.
Our framework can be used in the design and evaluation of such interventions. For example, interventions could be designed that incorporate as many of the domains of an ideal transition as possible, in particular those that span the inpatient and outpatient settings. Processes of care metrics can be developed that measure the extent to which each domain is delivered, analogous to the way the Joint Commission might aggregate individual scores on the 10 items in Acute Myocardial Infarction Core Measure Set[53] to provide a composite of the quality of care provided to patients with this diagnosis. These can be used to correlate certain intervention components with success in reducing readmissions and also in measuring intervention fidelity.
NEXT STEPS
For hospital and healthcare system leaders, who need to take action now to avoid financial penalties, we recommend starting with proven, high‐touch interventions such as Project RED and the Care Transitions Intervention, which are durable, cost‐effective, robustly address multiple domains of the Ideal Transition in Care, and have been implemented at numerous sites.[54, 55] Each hospital or group will need to decide on a bundle of interventions and customize them based on local workflow, resources, and culture.
Risk‐stratification, to match the intensity of the intervention to the risk of readmission of the patient, will undoubtedly be a key component for the efficient use of resources. We anticipate future research will allow risk stratification to be a robust part of any implementation plan. However, as noted above, current risk prediction models are imperfect,[6] and more work is needed to determine which patients benefit most from which interventions. Few if any studies have described interventions tailored to risk for this reason.
Based on our ideal transition in care, our collective experience, and published evidence,[7, 10, 11, 12] potential elements to start with include: early discharge planning; medication reconciliation[56]; patient/caregiver education using health literacy principles, cognitive assessments, and teach‐back to confirm understanding; synchronous communication (eg, by phone) between inpatient and postdischarge providers; follow‐up phone calls to patients within 72 hours of discharge; 24/7 availability of a responsible inpatient provider to address questions and problems (both from the patient/caregiver and from postdischarge providers); and prompt appointments for patients discharged home. High‐risk patients will likely require additional interventions, including in‐home assessments, disease‐monitoring programs, and/or patient coaching. Lastly, patients with certain conditions prone to readmission (such as heart failure and chronic obstructive pulmonary disease) may benefit from disease‐specific programs, including patient education, outpatient disease management, and monitoring protocols.
It is likely that the most effective interventions are those that come from combined, coordinated interventions shared between inpatient and outpatient settings, and are intensive in nature. We expect that the more domains in the framework that are addressed, the safer and more seamless transitions in care will be, with improvement in patient outcomes. To the extent that fragmentation of care has been a barrier to the implementation of these types of interventions in the past, ACOs, perhaps with imbedded Patient‐Centered Medical Homes, may be in the best position to take advantage of newly aligned financial incentives to design comprehensive transitional care. Indeed, we anticipate that Figure 1 may provide substrate for a discussion of postdischarge care and division of responsibilities between inpatient and outpatient care teams at the time of transition, so effort is not duplicated and multiple domains are addressed.
Other barriers to implementation of ideal transitions in care will continue to be an issue for most healthcare systems. Financial constraints that have been a barrier up until now will be partially overcome by penalties for high readmission rates and by ACOs, bundled payments, and alternative care contracts (ie, global payments), but the extent to which each institution feels rewarded for investing in transitional interventions will vary greatly. Healthcare leadership that sees the value of improving transitions in care will be critical to overcoming this barrier. Competing demands (such as lowering hospital length of stay and carrying out other patient care responsibilities),[57] lack of coordination and diffusion of responsibility among various clinical personnel, and lack of standards are other barriers[58] that will require clear prioritization from leadership, policy changes, team‐based care, provider education and feedback, and adequate allocation of personnel resources. In short, process redesign using continuous quality improvement efforts and effective tools will be required to maximize the possibility of success.
CONCLUSIONS
Readmissions are costly and undesirable. Intuition suggests they are a marker of poor care and that hospitals should be capable of reducing them, thereby improving care and decreasing costs. In a potential future world of ACOs based on global payments, financial incentives would be aligned for each system to reduce readmissions below their current baseline, therefore obviating the need for external financial rewards and penalties. In the meantime, financial penalties do exist, and controversy exists over their fairness and likelihood of driving appropriate behavior. To address these controversies and promote better transitional care, we call for the development and use of multifaceted, collaborative transitions interventions that span settings, risk‐adjustment models that allow for fairer comparisons among hospitals, better and more widespread measurement of processes of transitional care, a better understanding of what interventions are most effective and in whom, and better guidance in how to implement these interventions. Our conceptualization of an ideal transition of care serves as a guide and provides a common vocabulary for these efforts. Such research is likely to produce the knowledge needed for healthcare systems to improve transitions in care, reduce readmissions, and reduce costs.
Disclosure
Funding for Dr Vasilevskis has been provided by the National Institutes of Health (K23AG040157) and the VA Tennessee Valley Geriatric Research, Education and Clinical Center (GRECC). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute on Aging, the National Institutes of Health, or the US Department of Veterans Affairs.
Containing the rise of healthcare costs has taken on a new sense of urgency in the wake of the recent economic recession and continued growth in the cost of healthcare. Accordingly, many stakeholders seek solutions to improve value (reducing costs while improving care)[1]; hospital readmissions, which are common and costly,[2] have emerged as a key target. The Centers for Medicare and Medicaid Services (CMS) have instituted several programs intended to reduce readmissions, including funding for community‐based, care‐transition programs; penalties for hospitals with elevated risk‐adjusted readmission rates for selected diagnoses; pioneer Accountable Care Organizations (ACOs) with incentives to reduce global costs of care; and Hospital Engagement Networks (HENs) through the Partnership for Patients.[3] A primary aim of these initiatives is to enhance the quality of care transitions as patients are discharged from the hospital.
Though the recent focus on hospital readmissions has appropriately drawn attention to transitions in care, some have expressed concerns. Among these are questions about: 1) the extent to which readmissions truly reflect the quality of hospital care[4]; 2) the preventability of readmissions[5]; 3) limitations in risk‐adjustment techniques[6]; and 4) best practices for preventing readmissions.[7] We believe these concerns stem in part from deficiencies in the state of the science of transitional care, and that future efforts in this area will be hindered without a clear vision of an ideal transition in care. We propose the key components of an ideal transition in care and discuss the implications of this concept as it pertains to hospital readmissions.
THE IDEAL TRANSITION IN CARE
We propose the key components of an ideal transition in care in Figure 1 and Table 1. Figure 1 represents 10 domains described more fully below as structural supports of the bridge patients must cross from one care environment to another during a care transition. This figure highlights key domains and suggests that lack of a domain makes the bridge weaker and more prone to gaps in care and poor outcomes. It also implies that the more components are missing, the less safe is the bridge or transition. Those domains that mainly take place prior to discharge are placed closer to the hospital side of the bridge, those that mainly take place after discharge are placed closer to the community side of the bridge, while those that take place both prior to and after discharge are in the middle. Table 1 provides descriptions of the key content for each of these domains, as well as guidance about which personnel might be involved and where in the transition process that domain should be implemented. We support these domains with supporting evidence where available.

| Domain | Who | When | References |
|---|---|---|---|
| |||
| Discharge planning | |||
| Use a multidisciplinary team to create a discharge plan | Discharging clinician | Predischarge | 911 |
| Collaborate with PCP regarding discharge and follow‐up plan | Care managers/discharge planners | ||
| Arrange follow‐up appointments prior to discharge | Nurses | ||
| Make timely appointments for follow‐up care | |||
| Make appointments that take patient and caregiver's schedules and transportation needs into account | |||
| Complete communication of information | |||
| Includes: | Discharging clinician | Time of discharge | 1214 |
| Patient's full name | |||
| Age | |||
| Dates of admission and discharge | |||
| Names of responsible hospital physicians | |||
| Name of physician preparing discharge summary | |||
| Name of PCP | |||
| Main diagnosis | |||
| Other relevant diagnoses, procedures, and complications | |||
| Relevant findings at admission | |||
| Treatment and response for each active problem | |||
| Results of procedures and abnormal laboratory test results | |||
| Recommendations of any subspecialty consultants | |||
| Patient's functional status at discharge | |||
| Discharge medications | |||
| Follow‐up appointments made and those to be made | |||
| Tests to be ordered and pending tests to be followed‐up | |||
| Counseling provided to patient and caregiver, when applicable | |||
| Contingency planning | |||
| Code status | |||
| Availability, timeliness, clarity, and organization of information | |||
| Timely communication with postdischarge providers verbally (preferred) or by fax/e‐mail | Discharging clinician | Time of discharge | 1214 |
| Timely completion of discharge summary and reliable transmission to postdischarge providers | |||
| Availability of information in medical record | |||
| Use of a structured template with subheadings in discharge communication | |||
| Medication safety | |||
| Take an accurate preadmission medication history | Clinicians | Admission | 1521 |
| Reconcile preadmission medications with all ordered medications at all transfers in care, including discharge | Pharmacists | Throughout hospitalization | |
| Communicate discharge medications to all outpatient providers, including all changes and rationale for those changes | Nurses | Time of discharge | |
| Educating patients, promoting self‐management | |||
| Focus discharge counseling on major diagnoses, medication changes, dates of follow‐up appointments, self‐care instructions, warning signs and symptoms, and who to contact for problems | Clinicians | Daily | 911, 2228, 30 |
| Include caregivers as appropriate | Nurses | Time of discharge | |
| Ensure staff members provide consistent messages | Care managers/discharge planners | Postdischarge | |
| Provide simply written patient‐centered materials with instructions | Transition coaches | ||
| Use teach‐back methods to confirm understanding | |||
| Encourage questions | |||
| Continue teaching during postdischarge follow‐up | |||
| Use transition coaches in high‐risk patients: focus on medication management, keeping a personal medical record, follow‐up appointments, and knowledge of red flags | |||
| Enlisting help of social and community supports | |||
| Assess needs and appropriately arrange for home services | Clinicians | Predischarge and postdischarge | 29, 30 |
| Enlist help of caregivers | Nurses | ||
| Enlist help of community supports | Care managers | ||
| Home health staff | |||
| Advanced care planning | |||
| Establish healthcare proxy | Clinicians | Predischarge and postdischarge | 31, 32 |
| Discuss goals of care | Palliative care staff | ||
| Palliative care consultation (if appropriate) | Social workers | ||
| Enlist hospice services (if appropriate) | Nurses | ||
| Hospice workers | |||
| Coordinating care among team members | |||
| Share medical records | Clinicians | Predischarge and postdischarge | 33 |
| Communicate involving all team members | Nurses | ||
| Optimize continuity of providers and formal handoffs of care | Office personnel | ||
| IT staff | |||
| Monitoring and managing symptoms after discharge | |||
| Monitor for: | Clinicians | Postdischarge | 1113, 28, 3436 |
| Worsening disease control | Nurses | ||
| Medication side effects, discrepancies, nonadherence | Pharmacists | ||
| Therapeutic drug monitoring | Care managers | ||
| Inability to manage conditions at home | Visiting nurses and other home health staff | ||
| Via: | |||
| Postdischarge phone calls | |||
| Home visits | |||
| Postdischarge clinic visits | |||
| Patient hotline | |||
| Availability of inpatient providers after discharge | |||
| Follow‐up with outpatient providers | |||
| Within an appropriate time frame (eg, 7 d or sooner for high‐risk patients) | Clinicians | Postdischarge | 3740 |
| With appropriate providers (eg, most related to reasons for hospitalization, who manage least stable conditions, and/or PCP) | Nurses Pharmacists | ||
| Utilize multidisciplinary teams as appropriate | Care managers | ||
| Ensure appropriate progress along plan of care and safe transition | Office personnel | ||
| Other clinical staff as appropriate | |||
Our concept of an ideal transition in care began with work by Naylor, who described several important components of a safe transition in care, including complete communication of information, patient education, enlisting the help of social and community supports, ensuring continuity of care, and coordinating care among team members.[8] It is supplemented by the Transitions of Care Consensus Policy Statement proposed by representatives from hospital medicine, primary care, and emergency medicine, which emphasized aspects of timeliness and content of communication between providers.[9] Our present articulation of these key components includes 10 organizing domains.
The Discharge Planning domain highlights the important principle of planning ahead for hospital discharge while the patient is still being treated in the hospital, a paradigm espoused by Project RED[10] and other successful care transitions interventions.[11, 12] Collaborating with the outpatient provider and taking the patient and caregiver's preferences for appointment scheduling into account can help ensure optimal outpatient follow‐up.
Complete Communication of Information refers to the content that should be included in discharge summaries and other means of information transfer from hospital to postdischarge care. The specific content areas are based on the Society of Hospital Medicine and Society of General Internal Medicine Continuity of Care Task Force systematic review and recommendations,[13] which takes into account information requested by primary care physicians after discharge.
Availability, Timeliness, Clarity, and Organization of that information is as important as the content because postdischarge providers must be able to access and quickly understand the information they have been provided before assuming care of the patient.[14, 15]
The Medication Safety domain is of central importance because medications are responsible for most postdischarge adverse events.[16] Taking an accurate medication history,[17] reconciling changes throughout the hospitalization,[18] and communicating the reconciled medication regimen to patients and providers across transitions of care can reduce medication errors and improve patient safety.[19, 20, 21, 22]
The Patient Education and Promotion of Self‐Management domain involves teaching patients and their caregivers about the main hospital diagnoses and instructions for self‐care, including medication changes, appointments, and whom to contact if issues arise. Confirming comprehension of instructions through assessments of acute (delirium) and chronic (dementia) cognitive impairments[23, 24, 25, 26] and teach‐back from the patient (or caregiver) is an important aspect of such counseling, as is providing patients and caregivers with educational materials that are appropriate for their level of health literacy and preferred language.[14] High‐risk patients may benefit from patient coaching to improve their self‐management skills.[12] These recommendations are based on years of health literacy research,[27, 28, 29] and such elements are generally included in effective interventions (including Project RED,[10] Naylor and colleagues' Transitional Care Model,[11] and Coleman and colleagues' Care Transitions Intervention[12]).
Enlisting the help of Social and Community Supports is an important adjunct to medical care and is the rationale for the recent increase in CMS funding for community‐based, care‐transition programs. These programs are crucial for assisting patients with household activities, meals, and other necessities during the period of recovery, though they should be distinguished from care management or care coordination interventions, which have not been found to be helpful in preventing readmissions unless high touch in nature.[30, 31]
The Advanced Care Planning domain may begin in the hospital or outpatient setting, and involves establishing goals of care and healthcare proxies, as well as engaging with palliative care or hospice services if appropriate. Emerging evidence supports the intuitive conclusion that this approach prevents readmissions, particularly in patients who do not benefit from hospital readmission.[32, 33]
Attention to the Coordinating Care Among Team Members domain is needed to synchronize efforts across settings and providers. Clearly, many healthcare professionals as well as other involved parties can be involved in helping a single patient during transitions in care. It is vital that they coordinate information, assessments, and plans as a team.[34]
We recognize the domain of Monitoring and Managing Symptoms After Discharge as increasingly crucial as reflected in our growing understanding of the reasons for readmission, especially among patients with fragile conditions such as heart failure, chronic lung disease, gastrointestinal disorders, dementia,[23, 24, 25, 26] and vascular disease.[35] Monitoring for new or worsening symptoms; medication side effects, discrepancies, or nonadherence; and other self‐management challenges will allow problems to be detected and addressed early, before they result in unplanned healthcare utilization. It is noteworthy that successful interventions in this regard rely on in‐home evaluation[13, 14, 29] by nurses rather than telemonitoring, which in isolation has not been effective to date.[36, 37]
Finally, optimal Outpatient Follow‐Up with appropriate postdischarge providers is crucial for providing ideal transitions. These appointments need to be prompt[38, 39] (eg, within 7 days if not sooner for high‐risk patients) and with providers who have a longitudinal relationship to the patient, as prior work has shown increased readmissions when the provider is unfamiliar with the patient.[40] The advantages and disadvantages of hospitalist‐run postdischarge clinics as one way to increase access and expedite follow‐up are currently being explored. Although the optimal content of a postdischarge visit has not been defined, logical tasks to be completed are myriad and imply the need for checklists, adequate time, and a multidisciplinary team of providers.[41]
IMPLICATIONS OF THE IDEAL TRANSITION IN CARE
Our conceptualization of an ideal transition in care provides insight for hospital and healthcare system leadership, policymakers, researchers, clinicians, and educators seeking to improve transitions of care and reduce hospital readmissions. In the sections below, we briefly review commonly cited concerns about the recent focus on readmissions as a quality measure, illustrate how the Ideal Transition in Care addresses these concerns, and propose fruitful areas for future work.
How Does the Framework Address the Extent to Which Readmissions Reflect Hospital Quality?
One of the chief problems with readmissionrates as a hospital quality measure is that many of the factors that influence readmission may not currently be under the hospital's control. The healthcare environment to which a patient is being discharged (and was admitted from in the first place) is an important determinant of readmission.[42] In this context, it is noteworthy that successful interventions to reduce readmission are generally those that focus on outpatient follow‐up, while inpatient‐only interventions have had less success.[7] This is reflected in our framework above, informed by the literature, highlighting the importance of coordination between inpatient and outpatient providers and the importance of postdischarge care, including monitoring and managing symptoms after discharge, prompt follow‐up appointments, the continuation of patient self‐management activities, monitoring for drug‐related problems after discharge, and the effective utilization of community supports. Accountable care organizations, once established, would be responsible for several components of this environment, including the provision of prompt and effective follow‐up care.
The implication of the framework is that if a hospital does not have control over most of the factors that influence its readmission rate, it should see financial incentives to reduce readmission rates as an opportunity to invest in relationships with the outpatient environment from which their patients are admitted and to which they are discharged. One can envision hospitals growing ever‐closer relationships with their network of primary care physician groups, community agencies, and home health services, rehabilitation facilities, and nursing homes through coordinated discharge planning, medication management, patient education, shared electronic medical records, structured handoffs in care, and systems of intensive outpatient monitoring. Our proposed framework, in other words, emphasizes that hospitals cannot reduce their readmission rates by focusing on aspects of care within their walls. They must forge new and stronger relationships with their communities if they are to be successful.
How Does the Framework Help Us Understand Which Readmissions Are Preventable?
Public reporting and financial penalties are currently tied to all‐cause readmission, but preventable readmissions are a more appealing outcome to target. In one study, the ranking of hospitals by all‐cause readmission rate had very little correlation with the ranking by preventable readmission rate.[5] However, researchers have struggled to establish standardized, valid, and reliable measures for determining what proportion of readmissions are in fact preventable, with estimates ranging from 5% to 79% in the published literature.[43]
The difficulty of accurately determining preventability stems from an inadequate understanding of the roles that patient comorbidities, transitional processes of care, individual patient behaviors, and social and environmental determinants of health play in the complex process of hospital recidivism. Our proposed elements of an ideal transition in care provide a structure to frame this discussion and suggest future research opportunities to allow a more accurate and reliable understanding of the spectrum of preventability. Care system leadership can use the framework to rigorously evaluate their readmissions and determine the extent to which the transitions process approached the ideal. For example, if a readmission occurs despite care processes that addressed most of the domains with high fidelity, it becomes much less likely that the readmission was preventable. It should be noted that the converse is not always true: When a transition falls well short of the ideal, it does not always imply that provision of a more ideal transition would necessarily have prevented the readmission, but it does make it more likely.
For educators, the framework may provide insights for trainees into the complexity of the transitions process and vulnerability of patients during this time, highlighting preventable aspects of readmissions that are within the grasp of the discharging clinician or team. It highlights the importance of medication reconciliation, synchronous communication, and predischarge teaching, which are measurable and teachable skills for non‐physician providers, housestaff, and medical students. It also may allow for more structured feedback, for example, on the quality of discharge summaries produced by trainees.
How Could the Framework Improve Risk Adjustment for Between‐Hospital Comparisons?
Under the Patient Protection and Affordable Care Act (PPACA), hospitals will be compared to one another using risk‐standardized readmission rates as a way to penalize poorly performing hospitals. However, risk‐adjustment models have only modest ability to predict hospital readmission.[6] Moreover, current approaches predominantly adjust for patients' medical comorbidities (which are easily measurable), but they do not adequately take into account the growing literature on other factors that influence readmission rates, including a patient's health literacy, visual or cognitive impairment, functional status, language barriers, and community‐level factors such as social supports.[44, 45]
The Ideal Transition of Care provides a comprehensive framework of hospital discharge quality that provides additional process measures on which hospitals could be compared rather than focusing solely on (inadequately) risk‐adjusted readmission rates. Indeed, most other quality and safety measures (such as the National Quality Forum's Safe Practices[46] and The Joint Commission's National Patient Safety Goals),[47] emphasize process over outcome, in part because of issues of fairness. Process measures are less subject to differences in patient populations and also change the focus from simply reducing readmissions to improving transitional care more broadly. These process measures should be based on our framework and should attempt to capture as many dimensions of an optimal care transition as possible.
Possible examples of process measures include: the accuracy of medication reconciliation at admission and discharge; provision of prompt outpatient follow‐up; provision of adequate systems to monitor and manage symptoms after discharge; advanced care planning in appropriate patients; and the quality of discharge education, incorporating measurements of the patient's understanding and ability to self‐manage their illness. At least some of these could be used now as part of a performance measurement set that highlights opportunities for immediate system change and can serve as performance milestones.
The framework could also be used to validate risk‐adjustment techniques. After accounting for patient factors, the remaining variability in outcomes should be accounted for by processes of care that are in the transitions framework. Once these processes are accurately measured, one can determine if indeed the remaining variability is due to transitions processes, or rather unaccounted factors that are not being measured and that hospitals may have little control over. Such work can lead to iterative refinement of patient risk‐adjustment models.
What Does the Framework Imply About Best Practices for Reducing Readmission Rates?
Despite the limitations of readmission rates as a quality measure noted above, hospitals presently face potentially large financial penalties for readmissions and are allocating resources to readmission reduction efforts. However, hospitals currently may not have enough guidance to know what actions to take to reduce readmissions, and thus could be spending money inefficiently and reducing the value proposition of focusing on readmissions.
A recent systematic review of interventions hospitals could employ to reduce readmissions identified several positive studies, but also many negative studies, and there were significant barriers to understanding what works to reduce readmissions.[7] For example, most of the interventions described in both positive and negative studies were multifaceted, and the authors were unable to identify which components of the intervention were most effective. Also, while several studies have identified risk factors for readmission,[6, 48, 49] very few studies have identified which subgroups of patients benefit most from specific interventions. Few of the studies described key contextual factors that may have led to successful or failed implementation, or the fidelity with which the intervention was implemented.[50, 51, 52]
Few if any of the studies were guided by a concept of the ideal transition in care.[10] Such a framework will better guide development of multifaceted interventions and provide an improved means for interpreting the results. Clearly, rigorously conducted, multicenter studies of readmission prevention interventions are needed to move the field forward. These studies should: 1) correlate implementation of specific intervention components with reductions in readmission rates to better understand the most effective components; 2) be adequately powered to show effect modification, ie, which patients benefit most from these interventions; and 3) rigorously measure environmental context and intervention fidelity, and employ mixed methods to better understand predictors of implementation success and failure.
Our framework can be used in the design and evaluation of such interventions. For example, interventions could be designed that incorporate as many of the domains of an ideal transition as possible, in particular those that span the inpatient and outpatient settings. Processes of care metrics can be developed that measure the extent to which each domain is delivered, analogous to the way the Joint Commission might aggregate individual scores on the 10 items in Acute Myocardial Infarction Core Measure Set[53] to provide a composite of the quality of care provided to patients with this diagnosis. These can be used to correlate certain intervention components with success in reducing readmissions and also in measuring intervention fidelity.
NEXT STEPS
For hospital and healthcare system leaders, who need to take action now to avoid financial penalties, we recommend starting with proven, high‐touch interventions such as Project RED and the Care Transitions Intervention, which are durable, cost‐effective, robustly address multiple domains of the Ideal Transition in Care, and have been implemented at numerous sites.[54, 55] Each hospital or group will need to decide on a bundle of interventions and customize them based on local workflow, resources, and culture.
Risk‐stratification, to match the intensity of the intervention to the risk of readmission of the patient, will undoubtedly be a key component for the efficient use of resources. We anticipate future research will allow risk stratification to be a robust part of any implementation plan. However, as noted above, current risk prediction models are imperfect,[6] and more work is needed to determine which patients benefit most from which interventions. Few if any studies have described interventions tailored to risk for this reason.
Based on our ideal transition in care, our collective experience, and published evidence,[7, 10, 11, 12] potential elements to start with include: early discharge planning; medication reconciliation[56]; patient/caregiver education using health literacy principles, cognitive assessments, and teach‐back to confirm understanding; synchronous communication (eg, by phone) between inpatient and postdischarge providers; follow‐up phone calls to patients within 72 hours of discharge; 24/7 availability of a responsible inpatient provider to address questions and problems (both from the patient/caregiver and from postdischarge providers); and prompt appointments for patients discharged home. High‐risk patients will likely require additional interventions, including in‐home assessments, disease‐monitoring programs, and/or patient coaching. Lastly, patients with certain conditions prone to readmission (such as heart failure and chronic obstructive pulmonary disease) may benefit from disease‐specific programs, including patient education, outpatient disease management, and monitoring protocols.
It is likely that the most effective interventions are those that come from combined, coordinated interventions shared between inpatient and outpatient settings, and are intensive in nature. We expect that the more domains in the framework that are addressed, the safer and more seamless transitions in care will be, with improvement in patient outcomes. To the extent that fragmentation of care has been a barrier to the implementation of these types of interventions in the past, ACOs, perhaps with imbedded Patient‐Centered Medical Homes, may be in the best position to take advantage of newly aligned financial incentives to design comprehensive transitional care. Indeed, we anticipate that Figure 1 may provide substrate for a discussion of postdischarge care and division of responsibilities between inpatient and outpatient care teams at the time of transition, so effort is not duplicated and multiple domains are addressed.
Other barriers to implementation of ideal transitions in care will continue to be an issue for most healthcare systems. Financial constraints that have been a barrier up until now will be partially overcome by penalties for high readmission rates and by ACOs, bundled payments, and alternative care contracts (ie, global payments), but the extent to which each institution feels rewarded for investing in transitional interventions will vary greatly. Healthcare leadership that sees the value of improving transitions in care will be critical to overcoming this barrier. Competing demands (such as lowering hospital length of stay and carrying out other patient care responsibilities),[57] lack of coordination and diffusion of responsibility among various clinical personnel, and lack of standards are other barriers[58] that will require clear prioritization from leadership, policy changes, team‐based care, provider education and feedback, and adequate allocation of personnel resources. In short, process redesign using continuous quality improvement efforts and effective tools will be required to maximize the possibility of success.
CONCLUSIONS
Readmissions are costly and undesirable. Intuition suggests they are a marker of poor care and that hospitals should be capable of reducing them, thereby improving care and decreasing costs. In a potential future world of ACOs based on global payments, financial incentives would be aligned for each system to reduce readmissions below their current baseline, therefore obviating the need for external financial rewards and penalties. In the meantime, financial penalties do exist, and controversy exists over their fairness and likelihood of driving appropriate behavior. To address these controversies and promote better transitional care, we call for the development and use of multifaceted, collaborative transitions interventions that span settings, risk‐adjustment models that allow for fairer comparisons among hospitals, better and more widespread measurement of processes of transitional care, a better understanding of what interventions are most effective and in whom, and better guidance in how to implement these interventions. Our conceptualization of an ideal transition of care serves as a guide and provides a common vocabulary for these efforts. Such research is likely to produce the knowledge needed for healthcare systems to improve transitions in care, reduce readmissions, and reduce costs.
Disclosure
Funding for Dr Vasilevskis has been provided by the National Institutes of Health (K23AG040157) and the VA Tennessee Valley Geriatric Research, Education and Clinical Center (GRECC). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute on Aging, the National Institutes of Health, or the US Department of Veterans Affairs.
- , . Redefining Health Care: Creating Value‐Based Competition on Results. Boston, MA:Harvard Business School Press;2006.
- , , . Rehospitalizations among patients in the Medicare fee‐for‐service program. N Engl J Med. 2009;360(14):1418–1428.
- Patient Protection and Affordable Care Act (PPACA). Public Law 111–148; 2010. Available at: http://www.gpo.gov/fdsys/pkg/PLAW‐111publ148/pdf/PLAW‐111publ148.pdf. Accessed on June 4, 2012.
- , . Hospital readmission as an accountability measure. JAMA. 2011;305(5):504–505.
- , , , et al. Incidence of potentially avoidable urgent readmissions and their relation to all‐cause urgent readmissions. Can Med Assoc J. 2011;183(14):E1067–E1072.
- , , , et al. Risk prediction models for hospital readmission: a systematic review. JAMA. 2011;306(15):1688–1698.
- , , , , . Interventions to reduce 30‐day rehospitalization: a systematic review. Ann Intern Med. 2011;155(8):520–528.
- . A decade of transitional care research with vulnerable elders. J Cardiovasc Nurs. 2000;14(3):1–14.
- , , , et al;for the American College of Physicians; Society of General Internal Medicine; Society of Hospital Medicine; American Geriatrics Society; American College of Emergency Physicians; Society of Academic Emergency Medicine. Transitions of Care Consensus Policy Statement American College of Physicians–Society of General Internal Medicine–Society of Hospital Medicine–American Geriatrics Society–American College of Emergency Physicians–Society of Academic Emergency Medicine. J Gen Intern Med. 2009;24(8):971–976.
- , , , et al. A reengineered hospital discharge program to decrease rehospitalization: a randomized trial. Ann Intern Med. 2009;150(3):178–187.
- , , , et al. Comprehensive discharge planning and home follow‐up of hospitalized elders: a randomized clinical trial. JAMA. 1999;281(7):613–620.
- , , , . The care transitions intervention: results of a randomized controlled trial. Arch Intern Med. 2006;166(17):1822–1828.
- , , , , , . Deficits in communication and information transfer between hospital‐based and primary care physicians: implications for patient safety and continuity of care. JAMA. 2007;297(8):831–841.
- , , , . Promoting effective transitions of care at hospital discharge: a review of key issues for hospitalists. J Hosp Med. 2007;2(5):314–323.
- , , , , , . Discharge documentation of patients discharged to subacute facilities: a three‐year quality improvement process across an integrated health care system. Jt Comm J Qual Patient Saf. 2010;36(6):243–251.
- , , , , . The incidence and severity of adverse events affecting patients after discharge from the hospital. Ann Intern Med. 2003;138(3):161–167.
- , , , , , . Frequency, type and clinical importance of medication history errors at admission to hospital: a systematic review. Can Med Assoc J. 2005;173(5):510–515.
- , , , et al. Classifying and predicting errors of inpatient medication reconciliation. J Gen Intern Med. 2008;23(9):1414–1422.
- , , et al;for the PILL‐CVD (Pharmacist Intervention for Low Literacy in Cardiovascular Disease) Study Group. Effect of a pharmacist intervention on clinically important medication errors after hospital discharge: a randomized trial. Ann Intern Med. 2012;157(1):1–10.
- , , , . Hospital‐based medication reconciliation practices: a systematic review. Arch Intern Med. 2012;172(14):1057–1069.
- , , , et al. Effect of an electronic medication reconciliation application and process redesign on potential adverse drug events: a cluster‐randomized trial. Arch Intern Med. 2009;169(8):771–780.
- , , , et al. Role of pharmacist counseling in preventing adverse drug events after hospitalization. Arch Intern Med. 2006;166(5):565–571.
- , , , , . Insufficient help for activity of daily living disabilities and risk of all–cause hospitalization. J Am Geriatr Soc. 2012;60(5):927–933.
- , , , et al. Transitions in care for older adults with and without dementia. J Am Geriatr Soc. 2012;60(5):813–820.
- , , , , . Association of incident dementia with hospitalizations. JAMA. 2012;307(2):165–172.
- , , , et al. Potentially avoidable hospitalizations of dually eligible Medicare and Medicaid beneficiaries from nursing facility and home– and community–based services waiver programs. J Am Geriatr Soc. 2012;60(5):821–829.
- , . Teaching about health literacy and clear communication. J Gen Intern Med. 2006;21(8):888–890.
- , , , et al. Health literacy and outcomes among patients with heart failure. JAMA. 2011;305(16):1695–1701.
- , , , , . Patient experiences of transitioning from hospital to home: an ethnographic quality improvement project. J Hosp Med. 2012;7(5):382–387.
- , , , . Effects of care coordination on hospitalization, quality of care, and health care expenditures among Medicare beneficiaries: 15 randomized trials. JAMA. 2009;301(6):603–618.
- , , , , . How changes in Washington University's Medicare coordinated care demonstration pilot ultimately achieved savings. Health Aff (Millwood). 2012;31(6):1216–1226.
- , , , et al. Quality of care and rehospitalization rate in the last stage of disease in brain tumor patients assisted at home: a cost effectiveness study. J Palliat Med. 2012;15(2):225–227.
- , , , , . Inpatient palliative care consults and the probability of hospital readmission. Perm J. 2011;15(2):48–51.
- , , , et al. TeamSTEPPS™: team strategies and tools to enhance performance and patient safety. In: Henriksen K, Battles JB, Keyes MA, Grady ML, ed. Advances in Patient Safety: New Directions and Alternative Approaches. Vol 3: Performance and Tools. Rockville, MD:Agency for Healthcare Research and Quality; August2008.
- , , , et al. Factors contributing to all‐cause 30‐day readmissions: a structured case series across 18 hospitals. Med Care. 2012;50(7):599–605.
- , , , et al. Telemonitoring in patients with heart failure [erratum, N Engl J Med. 2011;364(5):490]. N Engl J Med. 2010;363(24):2301–2309.
- , , , et al. A randomized controlled trial of telemonitoring in older adults with multiple health issues to prevent hospitalizations and emergency department visits. Arch Intern Med. 2012;172(10):773–779.
- , , , et al. Relationship between early physician follow‐up and 30‐day readmission among Medicare beneficiaries hospitalized for heart failure. JAMA. 2010;303(17):1716–1722.
- , , . Post‐hospitalization transitions: examining the effects of timing of primary care provider follow‐up. J Hosp Med. 2010;5(7):392–397.
- , , . Does increased access to primary care reduce hospital readmissions? Veterans Affairs Cooperative Study Group on Primary Care and Hospital Readmission. N Engl J Med. 1996;334(22):1441–1447.
- . The Post‐Hospital Follow‐Up Visit: A Physician Checklist to Reduce Readmissions. California Healthcare Foundation; October 2010. Available at: http://www.chcf.org/publications/2010/10/the‐post‐hospital‐follow‐up‐visit‐a‐physician‐checklist. Accessed on January 10, 2012.
- , , . Thirty‐day readmission rates for Medicare beneficiaries by race and site of care. JAMA. 2011;305(7):675–681.
- , , , , . Proportion of hospital readmissions deemed avoidable: a systematic review. Can Med Assoc J. 2011;183(7):E391–E402.
- , , , et al. Postdischarge environmental and socioeconomic factors and the likelihood of early hospital readmission among community‐dwelling Medicare beneficiaries. Gerontologist. 2008;48(4):495–504.
- , , , , . Low health literacy and health outcomes: an updated systematic review. Ann Intern Med. 2011;155(2):97–107.
- National Quality Forum. Safe Practices for Better Healthcare—2010 Update: A Consensus Report. Washington, DC;2010.
- Joint Commission on Accreditation of Healthcare Organizations. Accreditation Program: Hospital 2010 National Patient Safety Goals (NPSGs). 2010. Available at: http://www.jointcommission.org/PatientSafety/NationalPatientSafetyGoals/. Accessed on March 20, 2012.
- , , , et al. Hospital readmission in general medicine patients: a prediction model. J Gen Intern Med. 2010;25(3):211–219.
- , , , et al. Derivation and validation of an index to predict early death or unplanned readmission after discharge from hospital to the community. Can Med Assoc J. 2010;182(6):551–557.
- , . Evaluating service delivery interventions to enhance patient safety. BMJ. 2008;337:a2764.
- , , . Assessing the Evidence for Context‐Sensitive Effectiveness and Safety of Patient Safety Practices: Developing Criteria. Rockville, MD:Agency for Healthcare Research and Quality; December2010.
- , , , et al. Advancing the science of patient safety. Ann Intern Med. 2011;154(10):693–696.
- The Joint Commission. Acute Myocardial Infarction Core Measure Set. Available at: http://www.jointcommission.org/assets/1/6/Acute%20Myocardial%20Infarction.pdf. Accessed August 20,2012.
- , , , , , . The care transitions intervention: translating from efficacy to effectiveness. Arch Intern Med. 2011;171(14):1232–1237.
- Project RED toolkit, AHRQ Innovations Exchange. Available at:http://www.innovations.ahrq.gov/content.aspx?id=2180. Accessed on July 2, 2012.
- , , , et al. A comprehensive pharmacist intervention to reduce morbidity in patients 80 years or older: a randomized controlled trial. Arch Intern Med. 2009;169(9):894–900.
- , . Thirty‐day readmissions—truth and consequences. N Engl J Med. 2012;366(15):1366–1369.
- , , , , . “Out of sight, out of mind”: housestaff perceptions of quality‐limiting factors in discharge care at teaching hospitals. J Hosp Med. 2012;7(5):376–381.
- , . Redefining Health Care: Creating Value‐Based Competition on Results. Boston, MA:Harvard Business School Press;2006.
- , , . Rehospitalizations among patients in the Medicare fee‐for‐service program. N Engl J Med. 2009;360(14):1418–1428.
- Patient Protection and Affordable Care Act (PPACA). Public Law 111–148; 2010. Available at: http://www.gpo.gov/fdsys/pkg/PLAW‐111publ148/pdf/PLAW‐111publ148.pdf. Accessed on June 4, 2012.
- , . Hospital readmission as an accountability measure. JAMA. 2011;305(5):504–505.
- , , , et al. Incidence of potentially avoidable urgent readmissions and their relation to all‐cause urgent readmissions. Can Med Assoc J. 2011;183(14):E1067–E1072.
- , , , et al. Risk prediction models for hospital readmission: a systematic review. JAMA. 2011;306(15):1688–1698.
- , , , , . Interventions to reduce 30‐day rehospitalization: a systematic review. Ann Intern Med. 2011;155(8):520–528.
- . A decade of transitional care research with vulnerable elders. J Cardiovasc Nurs. 2000;14(3):1–14.
- , , , et al;for the American College of Physicians; Society of General Internal Medicine; Society of Hospital Medicine; American Geriatrics Society; American College of Emergency Physicians; Society of Academic Emergency Medicine. Transitions of Care Consensus Policy Statement American College of Physicians–Society of General Internal Medicine–Society of Hospital Medicine–American Geriatrics Society–American College of Emergency Physicians–Society of Academic Emergency Medicine. J Gen Intern Med. 2009;24(8):971–976.
- , , , et al. A reengineered hospital discharge program to decrease rehospitalization: a randomized trial. Ann Intern Med. 2009;150(3):178–187.
- , , , et al. Comprehensive discharge planning and home follow‐up of hospitalized elders: a randomized clinical trial. JAMA. 1999;281(7):613–620.
- , , , . The care transitions intervention: results of a randomized controlled trial. Arch Intern Med. 2006;166(17):1822–1828.
- , , , , , . Deficits in communication and information transfer between hospital‐based and primary care physicians: implications for patient safety and continuity of care. JAMA. 2007;297(8):831–841.
- , , , . Promoting effective transitions of care at hospital discharge: a review of key issues for hospitalists. J Hosp Med. 2007;2(5):314–323.
- , , , , , . Discharge documentation of patients discharged to subacute facilities: a three‐year quality improvement process across an integrated health care system. Jt Comm J Qual Patient Saf. 2010;36(6):243–251.
- , , , , . The incidence and severity of adverse events affecting patients after discharge from the hospital. Ann Intern Med. 2003;138(3):161–167.
- , , , , , . Frequency, type and clinical importance of medication history errors at admission to hospital: a systematic review. Can Med Assoc J. 2005;173(5):510–515.
- , , , et al. Classifying and predicting errors of inpatient medication reconciliation. J Gen Intern Med. 2008;23(9):1414–1422.
- , , et al;for the PILL‐CVD (Pharmacist Intervention for Low Literacy in Cardiovascular Disease) Study Group. Effect of a pharmacist intervention on clinically important medication errors after hospital discharge: a randomized trial. Ann Intern Med. 2012;157(1):1–10.
- , , , . Hospital‐based medication reconciliation practices: a systematic review. Arch Intern Med. 2012;172(14):1057–1069.
- , , , et al. Effect of an electronic medication reconciliation application and process redesign on potential adverse drug events: a cluster‐randomized trial. Arch Intern Med. 2009;169(8):771–780.
- , , , et al. Role of pharmacist counseling in preventing adverse drug events after hospitalization. Arch Intern Med. 2006;166(5):565–571.
- , , , , . Insufficient help for activity of daily living disabilities and risk of all–cause hospitalization. J Am Geriatr Soc. 2012;60(5):927–933.
- , , , et al. Transitions in care for older adults with and without dementia. J Am Geriatr Soc. 2012;60(5):813–820.
- , , , , . Association of incident dementia with hospitalizations. JAMA. 2012;307(2):165–172.
- , , , et al. Potentially avoidable hospitalizations of dually eligible Medicare and Medicaid beneficiaries from nursing facility and home– and community–based services waiver programs. J Am Geriatr Soc. 2012;60(5):821–829.
- , . Teaching about health literacy and clear communication. J Gen Intern Med. 2006;21(8):888–890.
- , , , et al. Health literacy and outcomes among patients with heart failure. JAMA. 2011;305(16):1695–1701.
- , , , , . Patient experiences of transitioning from hospital to home: an ethnographic quality improvement project. J Hosp Med. 2012;7(5):382–387.
- , , , . Effects of care coordination on hospitalization, quality of care, and health care expenditures among Medicare beneficiaries: 15 randomized trials. JAMA. 2009;301(6):603–618.
- , , , , . How changes in Washington University's Medicare coordinated care demonstration pilot ultimately achieved savings. Health Aff (Millwood). 2012;31(6):1216–1226.
- , , , et al. Quality of care and rehospitalization rate in the last stage of disease in brain tumor patients assisted at home: a cost effectiveness study. J Palliat Med. 2012;15(2):225–227.
- , , , , . Inpatient palliative care consults and the probability of hospital readmission. Perm J. 2011;15(2):48–51.
- , , , et al. TeamSTEPPS™: team strategies and tools to enhance performance and patient safety. In: Henriksen K, Battles JB, Keyes MA, Grady ML, ed. Advances in Patient Safety: New Directions and Alternative Approaches. Vol 3: Performance and Tools. Rockville, MD:Agency for Healthcare Research and Quality; August2008.
- , , , et al. Factors contributing to all‐cause 30‐day readmissions: a structured case series across 18 hospitals. Med Care. 2012;50(7):599–605.
- , , , et al. Telemonitoring in patients with heart failure [erratum, N Engl J Med. 2011;364(5):490]. N Engl J Med. 2010;363(24):2301–2309.
- , , , et al. A randomized controlled trial of telemonitoring in older adults with multiple health issues to prevent hospitalizations and emergency department visits. Arch Intern Med. 2012;172(10):773–779.
- , , , et al. Relationship between early physician follow‐up and 30‐day readmission among Medicare beneficiaries hospitalized for heart failure. JAMA. 2010;303(17):1716–1722.
- , , . Post‐hospitalization transitions: examining the effects of timing of primary care provider follow‐up. J Hosp Med. 2010;5(7):392–397.
- , , . Does increased access to primary care reduce hospital readmissions? Veterans Affairs Cooperative Study Group on Primary Care and Hospital Readmission. N Engl J Med. 1996;334(22):1441–1447.
- . The Post‐Hospital Follow‐Up Visit: A Physician Checklist to Reduce Readmissions. California Healthcare Foundation; October 2010. Available at: http://www.chcf.org/publications/2010/10/the‐post‐hospital‐follow‐up‐visit‐a‐physician‐checklist. Accessed on January 10, 2012.
- , , . Thirty‐day readmission rates for Medicare beneficiaries by race and site of care. JAMA. 2011;305(7):675–681.
- , , , , . Proportion of hospital readmissions deemed avoidable: a systematic review. Can Med Assoc J. 2011;183(7):E391–E402.
- , , , et al. Postdischarge environmental and socioeconomic factors and the likelihood of early hospital readmission among community‐dwelling Medicare beneficiaries. Gerontologist. 2008;48(4):495–504.
- , , , , . Low health literacy and health outcomes: an updated systematic review. Ann Intern Med. 2011;155(2):97–107.
- National Quality Forum. Safe Practices for Better Healthcare—2010 Update: A Consensus Report. Washington, DC;2010.
- Joint Commission on Accreditation of Healthcare Organizations. Accreditation Program: Hospital 2010 National Patient Safety Goals (NPSGs). 2010. Available at: http://www.jointcommission.org/PatientSafety/NationalPatientSafetyGoals/. Accessed on March 20, 2012.
- , , , et al. Hospital readmission in general medicine patients: a prediction model. J Gen Intern Med. 2010;25(3):211–219.
- , , , et al. Derivation and validation of an index to predict early death or unplanned readmission after discharge from hospital to the community. Can Med Assoc J. 2010;182(6):551–557.
- , . Evaluating service delivery interventions to enhance patient safety. BMJ. 2008;337:a2764.
- , , . Assessing the Evidence for Context‐Sensitive Effectiveness and Safety of Patient Safety Practices: Developing Criteria. Rockville, MD:Agency for Healthcare Research and Quality; December2010.
- , , , et al. Advancing the science of patient safety. Ann Intern Med. 2011;154(10):693–696.
- The Joint Commission. Acute Myocardial Infarction Core Measure Set. Available at: http://www.jointcommission.org/assets/1/6/Acute%20Myocardial%20Infarction.pdf. Accessed August 20,2012.
- , , , , , . The care transitions intervention: translating from efficacy to effectiveness. Arch Intern Med. 2011;171(14):1232–1237.
- Project RED toolkit, AHRQ Innovations Exchange. Available at:http://www.innovations.ahrq.gov/content.aspx?id=2180. Accessed on July 2, 2012.
- , , , et al. A comprehensive pharmacist intervention to reduce morbidity in patients 80 years or older: a randomized controlled trial. Arch Intern Med. 2009;169(9):894–900.
- , . Thirty‐day readmissions—truth and consequences. N Engl J Med. 2012;366(15):1366–1369.
- , , , , . “Out of sight, out of mind”: housestaff perceptions of quality‐limiting factors in discharge care at teaching hospitals. J Hosp Med. 2012;7(5):376–381.