User login
Interhospital Transfer and Receipt of Specialty Procedures
Patients who undergo interhospital transfer (IHT) are felt to benefit from receipt of unique specialty care at the receiving hospital.1 Although only 1.5% of all hospitalized Medicare patients undergo hospital transfer,2 the frequency of transfer is much greater within certain patient populations, as may be expected with diagnoses requiring specialty care.3,4 Existent data demonstrate that 5% of Medicare patients admitted to the intensive care unit (ICU)5 and up to 50% of patients presenting with acute myocardial infarction (AMI) undergo IHT.6
More recent data suggest variability in hospital transfer practices not accounted for by differences in patient or hospital characteristics.2 Although disease-specific guidelines for IHT exist for certain diagnoses,3,4 the process remains largely nonstandardized for many patients,7 leading to ambiguity surrounding indications for transfer. Because limited data suggest worse outcomes for transferred versus nontransferred patients,8 a better understanding of the specialized care patients actually receive across the transfer continuum may help to elucidate potential indications for transfer and ultimately help delineate which patients are most (or least) likely to benefit from transfer and why.
In this national study, we examined a select cohort of transferred patients with diagnoses associated with specific specialty procedural services to determine if they received these procedures and where along the transfer continuum they were performed.
METHODS
We performed a cross-sectional analysis using the Center for Medicare and Medicaid Services 2013 100% Master Beneficiary Summary and Inpatient claims files. Our study protocol was approved by the Partners Healthcare Human Subjects Review Committee.
Beneficiaries were eligible for inclusion if they were aged ≥65 years, continuously enrolled in Medicare A and B, and with an acute care hospitalization claim in 2013, excluding Medicare managed care and end stage renal disease beneficiaries due to incomplete claims data in these groups. We additionally excluded beneficiaries hospitalized at federal or nonacute care hospitals, or critical access hospitals given their mission to stabilize and then transfer patients to referral hospitals.9
Transferred patients were defined as beneficiaries with corresponding “transfer in” and “transfer out” claims, or those with either claim and a corresponding date of admission/discharge from another hospital within 1 day of the claim, as we used in our prior research.2 Beneficiaries transferred to the same hospital, those with greater than 1 transfer within the same hospitalization, or those cared for at hospitals with “outlier” transfer-in rates equal to 100% or transfer-out rates greater than 35% were excluded from analysis given the suggestion of nonstandard claims practices.
We first identified the top 15 primary diagnoses at time of transfer using International Classification of Diseases, Ninth Revision (ICD-9) codes (supplementary Appendix), and then identified those 4 most likely to require specialty procedural services: AMI, gastrointestinal bleed (GI bleed), renal failure, and hip fracture/dislocation. We then chose associated ICD-9 procedure codes for each diagnosis, via expert opinion (authors SM and JS, hospitalist physicians with greater than 20 years of combined clinical experience), erring on overinclusion of procedure codes. We then quantified receipt of associated procedures at transferring and receiving hospitals, stratified by diagnosis.
We further explored the cohort of patients with hip fracture/dislocation who underwent an associated procedure at the transferring but not receiving hospital, examining the frequency with which these patients had other (nonrelated) procedures at the receiving hospital, and identifying which procedures they received.
RESULTS
Of the 101,507 patients transferred to another hospital, 19,613 (19.3%) had a primary diagnosis of AMI, GI bleed, renal failure, or hip fracture/dislocation. Table 1 lists the ICD-9 procedure codes associated with each diagnosis.
Distribution of receipt of specialty procedures at the transferring and receiving hospitals varied by disease (Figure). With the exception of GI bleed, patients more often received specialty procedural care at the receiving than the transferring hospital. Depending on primary diagnosis, between 32.4% and 89.1% of patients did not receive any associated specialty procedure at the receiving hospital.
Of the 370 (22.1%) hip fracture/dislocation patients that received a specialty procedure at the transferring but not receiving hospital, 132 (35.7%) did not receive any procedure at the receiving hospital, whereas the remaining 238 (64.3%) received an unrelated (not associated with the primary diagnosis) procedure. There was great variety in the types of procedures received, the most common being transfusion of blood products (ICD-9 Clinical Modification 9904).
DISCUSSION
Among transferred patients with primary diagnoses that have clearly associated specialized procedural services, we found that patients received these procedures at varying frequency and locations across the transfer continuum. Across 4 diagnoses, receipt of associated procedures was more common at the receiving than the transferring hospital, with the exception being patients with GI bleed. We additionally found that many transferred patients did not receive any associated specialty procedure at the receiving hospital. These findings suggest the strong likelihood of more diverse underlying reasons for transfer rather than solely receipt of specialized procedural care.
Despite the frequency with which AMI patients are transferred,6 and American Heart Association guidelines directing hospitals to transfer AMI patients to institutions able to provide necessary invasive treatments,4 prior studies suggest these patients inconsistently receive specialty intervention following transfer, including stress testing, cardiac catheterization, or coronary artery bypass graft surgery.10,11 Our findings add to these data, demonstrating that only 47.3% of patients transferred with AMI received any cardiac-related procedure at the receiving hospital. Additionally, we found that 38.1% of AMI patients do not receive any specialty procedures at either the transferring or the receiving hospital. Taken together, these data suggest possible discrepancies in the perceived need for these procedures between transferring and receiving hospitals, reasons for transfer related to these conditions that don’t involve an associated procedure, or reasons for transfer unrelated to specialty care of the primary diagnosis (such as care of comorbidities, hospital location, prior relationships with that hospital, or desire for a second opinion). Although some of these alternate reasons for transfer likely still benefit the patient, some of these reasons may not justify the increased risks of discontinuity of care created by IHT.
Given limited data looking at IHT practices for patients with other diagnoses, the varying patterns of specialty procedural interventions we observed among transferred patients with GI bleed, renal failure, and hip fracture/dislocation are novel contributions to this topic. Notably, we found that among patients transferred with a primary diagnosis of renal failure, the vast majority (84.1%) did not receive any associated procedure at either the transferring or the receiving hospital. It is possible that although these patients carried the diagnosis of renal failure, their clinical phenotype is more heterogeneous, and they could still be managed conservatively without receipt of invasive procedures such as hemodialysis.
Conversely, patients transferred with primary diagnosis of hip fracture/dislocation were far more likely to receive associated specialty procedural intervention at the receiving hospital, presumably reflective of the evidence demonstrating improved outcomes with early surgical intervention.12 However, these data do not explain the reasoning behind the substantial minority of patients who received specialty intervention at the transferring hospital prior to transfer or those that did not receive any specialty intervention at either the transferring or receiving hospital. Our secondary analysis demonstrating great variety in receipt and type of nonassociated procedures provided at the receiving hospital did not help to elucidate potential underlying reasons for transfer.
Notably, among patients transferred with primary diagnosis of GI bleed, receipt of specialty procedures was more common at the transferring (77.7%) than receiving (63.2%) hospital, with nearly half (49.3%) undergoing specialty procedures at both hospitals. It is possible that these findings are reflective of the broad array of specialty procedures examined within this diagnosis. For example, it is reasonable to consider that a patient may be stabilized with receipt of a blood transfusion at the transferring hospital, then transferred to undergo a diagnostic/therapeutic procedure (ie, endoscopy/colonoscopy) at the receiving hospital, as is suggested by our results.
Our study is subject to several limitations. First, given the criteria we used to define transfer, it is possible that we included nontransferred patients within our transferred cohort if they were discharged from one hospital and admitted to a different hospital within 1 day, although quality assurance analyses we conducted in prior studies on these data support the validity of the criteria used.2 Second, we cannot exclude the possibility that patients received nonprocedural specialty care (ie, expert opinion, specialized imaging, medical management, management of secondary diagnoses, etc.) not available at the transferring hospital, although, arguably, in select patients, such input could be obtained without physical transfer of the patient (ie, tele-consult). And even in patients transferred with intent to receive procedural care who did not ultimately receive that care, there is likely an appropriate “nonprocedure” rate, where patients who might benefit from a procedure receive a timely evaluation to reduce the risk of missing the opportunity to receive it. This would be analogous to transferring a patient to an ICU even if they do not end up requiring intubation or pressor therapy. However, given the likelihood of higher risks of IHT compared with intrahospital transfers, one could argue that the threshold of perceived benefit might be different in patients being considered for IHT. Additionally, we limited our analyses to only 4 diagnoses; thus, our findings may not be generalizable to other diagnoses of transferred patients. However, because the diagnoses we examined were ones considered most effectively treated with specialty procedural interventions, it is reasonable to presume that the variability in receipt of specialty procedures observed within these diagnoses is also present, if not greater, across other diagnoses. Third, although we intentionally included a broad array of specialty procedures associated with each diagnosis, it is possible that we overlooked particular specialty interventions. For example, in assuming that patients are most likely to be transferred to receive procedural services associated with their primary diagnosis, we may have missed alternate indications for transfer, including need for procedural care related to secondary or subsequent diagnoses (ie, a patient may have presented with GI bleed
CONCLUSIONS
We found that Medicare patients who undergo IHT with primary diagnoses of AMI, GI bleed, renal failure, and hip fracture/dislocation receive associated specialty interventions at varying frequency and locations, and many patients do not receive any associated procedures at receiving hospitals. Our findings suggest that specialty procedural care of patients, even those with primary diagnoses that often warrant specialized intervention, may not be the primary driver of IHT as commonly suggested, although underlying reasons for transfer in these and other “nonprocedural” transferred patients remains obscure. Given known ambiguity in the transfer process,7 and unclear benefit of IHT,8 additional research is required to further identify and evaluate other potential underlying reasons for transfer and to examine these in the context of patient outcomes, in order to understand which patients may or may not benefit from transfer and why.
Disclosure
The authors have nothing to disclose.
1. Iwashyna TJ. The incomplete infrastructure for interhospital patient transfer. Crit Care Med. 2012;40(8):2470-2478. PubMed
2. Mueller SK, Zheng J, Orav EJ, Schnipper JL. Rates, Predictors and Variability of Interhospital Transfers: A National Evaluation. J Hosp Med. 2017;12(6):435-442. PubMed
3. Guidelines for the transfer of critically ill patients. Guidelines Committee of the American College of Critical Care Medicine; Society of Critical Care Medicine and American Association of Critical-Care Nurses Transfer Guidelines Task Force. Crit Care Med. 1993;21(6):931-937. PubMed
4. Anderson JL, Adams CD, Antman EM, et al. 2011 ACCF/AHA Focused Update Incorporated Into the ACC/AHA 2007 Guidelines for the Management of Patients With Unstable Angina/Non-ST-Elevation Myocardial Infarction: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. Circulation. 2011;123(18):e426-e579. PubMed
5. Iwashyna TJ, Christie JD, Moody J, Kahn JM, Asch DA. The structure of critical care transfer networks. Med Care. 2009;47(7):787-793. PubMed
6. Iwashyna TJ, Kahn JM, Hayward RA, Nallamothu BK. Interhospital transfers among Medicare beneficiaries admitted for acute myocardial infarction at nonrevascularization hospitals. Circ Cardiovasc Qual Outcomes. 2010;3(5):468-475. PubMed
7. Bosk EA, Veinot T, Iwashyna TJ. Which patients and where: a qualitative study of patient transfers from community hospitals. Med Care. 2011;49(6):592-598. PubMed
8. Sokol-Hessner L, White AA, Davis KF, Herzig SJ, Hohmann SF. Interhospital transfer patients discharged by academic hospitalists and general internists: Characteristics and outcomes. J Hosp Med. 2016;11(4):245-250. PubMed
9. Department of Health and Human Services, Center for Medicare & Medicaid Services: Critical Access Hospitals. https://www.cms.gov/Outreach-and-Education/Medicare-Learning-Network-MLN/MLNProducts/downloads/CritAccessHospfctsht.pdf. Accessed June 29, 2017. PubMed
10. Roe MT, Chen AY, Delong ER, et al. Patterns of transfer for patients with non-ST-segment elevation acute coronary syndrome from community to tertiary care hospitals. Am Heart J. 2008;156(1):185-192. PubMed
11. Barreto-Filho JA, Wang Y, Rathore SS, et al. Transfer rates from nonprocedure hospitals after initial admission and outcomes among elderly patients with acute myocardial infarction. JAMA Intern Med. 2014;174(2):213-222. PubMed
12. Doruk H, Mas MR, Yildiz C, Sonmez A, Kyrdemir V. The effect of the timing of hip fracture surgery on the activity of daily living and mortality in elderly. Arch Gerontol Geriatr. 2004;39(2):179-185. PubMed
Patients who undergo interhospital transfer (IHT) are felt to benefit from receipt of unique specialty care at the receiving hospital.1 Although only 1.5% of all hospitalized Medicare patients undergo hospital transfer,2 the frequency of transfer is much greater within certain patient populations, as may be expected with diagnoses requiring specialty care.3,4 Existent data demonstrate that 5% of Medicare patients admitted to the intensive care unit (ICU)5 and up to 50% of patients presenting with acute myocardial infarction (AMI) undergo IHT.6
More recent data suggest variability in hospital transfer practices not accounted for by differences in patient or hospital characteristics.2 Although disease-specific guidelines for IHT exist for certain diagnoses,3,4 the process remains largely nonstandardized for many patients,7 leading to ambiguity surrounding indications for transfer. Because limited data suggest worse outcomes for transferred versus nontransferred patients,8 a better understanding of the specialized care patients actually receive across the transfer continuum may help to elucidate potential indications for transfer and ultimately help delineate which patients are most (or least) likely to benefit from transfer and why.
In this national study, we examined a select cohort of transferred patients with diagnoses associated with specific specialty procedural services to determine if they received these procedures and where along the transfer continuum they were performed.
METHODS
We performed a cross-sectional analysis using the Center for Medicare and Medicaid Services 2013 100% Master Beneficiary Summary and Inpatient claims files. Our study protocol was approved by the Partners Healthcare Human Subjects Review Committee.
Beneficiaries were eligible for inclusion if they were aged ≥65 years, continuously enrolled in Medicare A and B, and with an acute care hospitalization claim in 2013, excluding Medicare managed care and end stage renal disease beneficiaries due to incomplete claims data in these groups. We additionally excluded beneficiaries hospitalized at federal or nonacute care hospitals, or critical access hospitals given their mission to stabilize and then transfer patients to referral hospitals.9
Transferred patients were defined as beneficiaries with corresponding “transfer in” and “transfer out” claims, or those with either claim and a corresponding date of admission/discharge from another hospital within 1 day of the claim, as we used in our prior research.2 Beneficiaries transferred to the same hospital, those with greater than 1 transfer within the same hospitalization, or those cared for at hospitals with “outlier” transfer-in rates equal to 100% or transfer-out rates greater than 35% were excluded from analysis given the suggestion of nonstandard claims practices.
We first identified the top 15 primary diagnoses at time of transfer using International Classification of Diseases, Ninth Revision (ICD-9) codes (supplementary Appendix), and then identified those 4 most likely to require specialty procedural services: AMI, gastrointestinal bleed (GI bleed), renal failure, and hip fracture/dislocation. We then chose associated ICD-9 procedure codes for each diagnosis, via expert opinion (authors SM and JS, hospitalist physicians with greater than 20 years of combined clinical experience), erring on overinclusion of procedure codes. We then quantified receipt of associated procedures at transferring and receiving hospitals, stratified by diagnosis.
We further explored the cohort of patients with hip fracture/dislocation who underwent an associated procedure at the transferring but not receiving hospital, examining the frequency with which these patients had other (nonrelated) procedures at the receiving hospital, and identifying which procedures they received.
RESULTS
Of the 101,507 patients transferred to another hospital, 19,613 (19.3%) had a primary diagnosis of AMI, GI bleed, renal failure, or hip fracture/dislocation. Table 1 lists the ICD-9 procedure codes associated with each diagnosis.
Distribution of receipt of specialty procedures at the transferring and receiving hospitals varied by disease (Figure). With the exception of GI bleed, patients more often received specialty procedural care at the receiving than the transferring hospital. Depending on primary diagnosis, between 32.4% and 89.1% of patients did not receive any associated specialty procedure at the receiving hospital.
Of the 370 (22.1%) hip fracture/dislocation patients that received a specialty procedure at the transferring but not receiving hospital, 132 (35.7%) did not receive any procedure at the receiving hospital, whereas the remaining 238 (64.3%) received an unrelated (not associated with the primary diagnosis) procedure. There was great variety in the types of procedures received, the most common being transfusion of blood products (ICD-9 Clinical Modification 9904).
DISCUSSION
Among transferred patients with primary diagnoses that have clearly associated specialized procedural services, we found that patients received these procedures at varying frequency and locations across the transfer continuum. Across 4 diagnoses, receipt of associated procedures was more common at the receiving than the transferring hospital, with the exception being patients with GI bleed. We additionally found that many transferred patients did not receive any associated specialty procedure at the receiving hospital. These findings suggest the strong likelihood of more diverse underlying reasons for transfer rather than solely receipt of specialized procedural care.
Despite the frequency with which AMI patients are transferred,6 and American Heart Association guidelines directing hospitals to transfer AMI patients to institutions able to provide necessary invasive treatments,4 prior studies suggest these patients inconsistently receive specialty intervention following transfer, including stress testing, cardiac catheterization, or coronary artery bypass graft surgery.10,11 Our findings add to these data, demonstrating that only 47.3% of patients transferred with AMI received any cardiac-related procedure at the receiving hospital. Additionally, we found that 38.1% of AMI patients do not receive any specialty procedures at either the transferring or the receiving hospital. Taken together, these data suggest possible discrepancies in the perceived need for these procedures between transferring and receiving hospitals, reasons for transfer related to these conditions that don’t involve an associated procedure, or reasons for transfer unrelated to specialty care of the primary diagnosis (such as care of comorbidities, hospital location, prior relationships with that hospital, or desire for a second opinion). Although some of these alternate reasons for transfer likely still benefit the patient, some of these reasons may not justify the increased risks of discontinuity of care created by IHT.
Given limited data looking at IHT practices for patients with other diagnoses, the varying patterns of specialty procedural interventions we observed among transferred patients with GI bleed, renal failure, and hip fracture/dislocation are novel contributions to this topic. Notably, we found that among patients transferred with a primary diagnosis of renal failure, the vast majority (84.1%) did not receive any associated procedure at either the transferring or the receiving hospital. It is possible that although these patients carried the diagnosis of renal failure, their clinical phenotype is more heterogeneous, and they could still be managed conservatively without receipt of invasive procedures such as hemodialysis.
Conversely, patients transferred with primary diagnosis of hip fracture/dislocation were far more likely to receive associated specialty procedural intervention at the receiving hospital, presumably reflective of the evidence demonstrating improved outcomes with early surgical intervention.12 However, these data do not explain the reasoning behind the substantial minority of patients who received specialty intervention at the transferring hospital prior to transfer or those that did not receive any specialty intervention at either the transferring or receiving hospital. Our secondary analysis demonstrating great variety in receipt and type of nonassociated procedures provided at the receiving hospital did not help to elucidate potential underlying reasons for transfer.
Notably, among patients transferred with primary diagnosis of GI bleed, receipt of specialty procedures was more common at the transferring (77.7%) than receiving (63.2%) hospital, with nearly half (49.3%) undergoing specialty procedures at both hospitals. It is possible that these findings are reflective of the broad array of specialty procedures examined within this diagnosis. For example, it is reasonable to consider that a patient may be stabilized with receipt of a blood transfusion at the transferring hospital, then transferred to undergo a diagnostic/therapeutic procedure (ie, endoscopy/colonoscopy) at the receiving hospital, as is suggested by our results.
Our study is subject to several limitations. First, given the criteria we used to define transfer, it is possible that we included nontransferred patients within our transferred cohort if they were discharged from one hospital and admitted to a different hospital within 1 day, although quality assurance analyses we conducted in prior studies on these data support the validity of the criteria used.2 Second, we cannot exclude the possibility that patients received nonprocedural specialty care (ie, expert opinion, specialized imaging, medical management, management of secondary diagnoses, etc.) not available at the transferring hospital, although, arguably, in select patients, such input could be obtained without physical transfer of the patient (ie, tele-consult). And even in patients transferred with intent to receive procedural care who did not ultimately receive that care, there is likely an appropriate “nonprocedure” rate, where patients who might benefit from a procedure receive a timely evaluation to reduce the risk of missing the opportunity to receive it. This would be analogous to transferring a patient to an ICU even if they do not end up requiring intubation or pressor therapy. However, given the likelihood of higher risks of IHT compared with intrahospital transfers, one could argue that the threshold of perceived benefit might be different in patients being considered for IHT. Additionally, we limited our analyses to only 4 diagnoses; thus, our findings may not be generalizable to other diagnoses of transferred patients. However, because the diagnoses we examined were ones considered most effectively treated with specialty procedural interventions, it is reasonable to presume that the variability in receipt of specialty procedures observed within these diagnoses is also present, if not greater, across other diagnoses. Third, although we intentionally included a broad array of specialty procedures associated with each diagnosis, it is possible that we overlooked particular specialty interventions. For example, in assuming that patients are most likely to be transferred to receive procedural services associated with their primary diagnosis, we may have missed alternate indications for transfer, including need for procedural care related to secondary or subsequent diagnoses (ie, a patient may have presented with GI bleed
CONCLUSIONS
We found that Medicare patients who undergo IHT with primary diagnoses of AMI, GI bleed, renal failure, and hip fracture/dislocation receive associated specialty interventions at varying frequency and locations, and many patients do not receive any associated procedures at receiving hospitals. Our findings suggest that specialty procedural care of patients, even those with primary diagnoses that often warrant specialized intervention, may not be the primary driver of IHT as commonly suggested, although underlying reasons for transfer in these and other “nonprocedural” transferred patients remains obscure. Given known ambiguity in the transfer process,7 and unclear benefit of IHT,8 additional research is required to further identify and evaluate other potential underlying reasons for transfer and to examine these in the context of patient outcomes, in order to understand which patients may or may not benefit from transfer and why.
Disclosure
The authors have nothing to disclose.
Patients who undergo interhospital transfer (IHT) are felt to benefit from receipt of unique specialty care at the receiving hospital.1 Although only 1.5% of all hospitalized Medicare patients undergo hospital transfer,2 the frequency of transfer is much greater within certain patient populations, as may be expected with diagnoses requiring specialty care.3,4 Existent data demonstrate that 5% of Medicare patients admitted to the intensive care unit (ICU)5 and up to 50% of patients presenting with acute myocardial infarction (AMI) undergo IHT.6
More recent data suggest variability in hospital transfer practices not accounted for by differences in patient or hospital characteristics.2 Although disease-specific guidelines for IHT exist for certain diagnoses,3,4 the process remains largely nonstandardized for many patients,7 leading to ambiguity surrounding indications for transfer. Because limited data suggest worse outcomes for transferred versus nontransferred patients,8 a better understanding of the specialized care patients actually receive across the transfer continuum may help to elucidate potential indications for transfer and ultimately help delineate which patients are most (or least) likely to benefit from transfer and why.
In this national study, we examined a select cohort of transferred patients with diagnoses associated with specific specialty procedural services to determine if they received these procedures and where along the transfer continuum they were performed.
METHODS
We performed a cross-sectional analysis using the Center for Medicare and Medicaid Services 2013 100% Master Beneficiary Summary and Inpatient claims files. Our study protocol was approved by the Partners Healthcare Human Subjects Review Committee.
Beneficiaries were eligible for inclusion if they were aged ≥65 years, continuously enrolled in Medicare A and B, and with an acute care hospitalization claim in 2013, excluding Medicare managed care and end stage renal disease beneficiaries due to incomplete claims data in these groups. We additionally excluded beneficiaries hospitalized at federal or nonacute care hospitals, or critical access hospitals given their mission to stabilize and then transfer patients to referral hospitals.9
Transferred patients were defined as beneficiaries with corresponding “transfer in” and “transfer out” claims, or those with either claim and a corresponding date of admission/discharge from another hospital within 1 day of the claim, as we used in our prior research.2 Beneficiaries transferred to the same hospital, those with greater than 1 transfer within the same hospitalization, or those cared for at hospitals with “outlier” transfer-in rates equal to 100% or transfer-out rates greater than 35% were excluded from analysis given the suggestion of nonstandard claims practices.
We first identified the top 15 primary diagnoses at time of transfer using International Classification of Diseases, Ninth Revision (ICD-9) codes (supplementary Appendix), and then identified those 4 most likely to require specialty procedural services: AMI, gastrointestinal bleed (GI bleed), renal failure, and hip fracture/dislocation. We then chose associated ICD-9 procedure codes for each diagnosis, via expert opinion (authors SM and JS, hospitalist physicians with greater than 20 years of combined clinical experience), erring on overinclusion of procedure codes. We then quantified receipt of associated procedures at transferring and receiving hospitals, stratified by diagnosis.
We further explored the cohort of patients with hip fracture/dislocation who underwent an associated procedure at the transferring but not receiving hospital, examining the frequency with which these patients had other (nonrelated) procedures at the receiving hospital, and identifying which procedures they received.
RESULTS
Of the 101,507 patients transferred to another hospital, 19,613 (19.3%) had a primary diagnosis of AMI, GI bleed, renal failure, or hip fracture/dislocation. Table 1 lists the ICD-9 procedure codes associated with each diagnosis.
Distribution of receipt of specialty procedures at the transferring and receiving hospitals varied by disease (Figure). With the exception of GI bleed, patients more often received specialty procedural care at the receiving than the transferring hospital. Depending on primary diagnosis, between 32.4% and 89.1% of patients did not receive any associated specialty procedure at the receiving hospital.
Of the 370 (22.1%) hip fracture/dislocation patients that received a specialty procedure at the transferring but not receiving hospital, 132 (35.7%) did not receive any procedure at the receiving hospital, whereas the remaining 238 (64.3%) received an unrelated (not associated with the primary diagnosis) procedure. There was great variety in the types of procedures received, the most common being transfusion of blood products (ICD-9 Clinical Modification 9904).
DISCUSSION
Among transferred patients with primary diagnoses that have clearly associated specialized procedural services, we found that patients received these procedures at varying frequency and locations across the transfer continuum. Across 4 diagnoses, receipt of associated procedures was more common at the receiving than the transferring hospital, with the exception being patients with GI bleed. We additionally found that many transferred patients did not receive any associated specialty procedure at the receiving hospital. These findings suggest the strong likelihood of more diverse underlying reasons for transfer rather than solely receipt of specialized procedural care.
Despite the frequency with which AMI patients are transferred,6 and American Heart Association guidelines directing hospitals to transfer AMI patients to institutions able to provide necessary invasive treatments,4 prior studies suggest these patients inconsistently receive specialty intervention following transfer, including stress testing, cardiac catheterization, or coronary artery bypass graft surgery.10,11 Our findings add to these data, demonstrating that only 47.3% of patients transferred with AMI received any cardiac-related procedure at the receiving hospital. Additionally, we found that 38.1% of AMI patients do not receive any specialty procedures at either the transferring or the receiving hospital. Taken together, these data suggest possible discrepancies in the perceived need for these procedures between transferring and receiving hospitals, reasons for transfer related to these conditions that don’t involve an associated procedure, or reasons for transfer unrelated to specialty care of the primary diagnosis (such as care of comorbidities, hospital location, prior relationships with that hospital, or desire for a second opinion). Although some of these alternate reasons for transfer likely still benefit the patient, some of these reasons may not justify the increased risks of discontinuity of care created by IHT.
Given limited data looking at IHT practices for patients with other diagnoses, the varying patterns of specialty procedural interventions we observed among transferred patients with GI bleed, renal failure, and hip fracture/dislocation are novel contributions to this topic. Notably, we found that among patients transferred with a primary diagnosis of renal failure, the vast majority (84.1%) did not receive any associated procedure at either the transferring or the receiving hospital. It is possible that although these patients carried the diagnosis of renal failure, their clinical phenotype is more heterogeneous, and they could still be managed conservatively without receipt of invasive procedures such as hemodialysis.
Conversely, patients transferred with primary diagnosis of hip fracture/dislocation were far more likely to receive associated specialty procedural intervention at the receiving hospital, presumably reflective of the evidence demonstrating improved outcomes with early surgical intervention.12 However, these data do not explain the reasoning behind the substantial minority of patients who received specialty intervention at the transferring hospital prior to transfer or those that did not receive any specialty intervention at either the transferring or receiving hospital. Our secondary analysis demonstrating great variety in receipt and type of nonassociated procedures provided at the receiving hospital did not help to elucidate potential underlying reasons for transfer.
Notably, among patients transferred with primary diagnosis of GI bleed, receipt of specialty procedures was more common at the transferring (77.7%) than receiving (63.2%) hospital, with nearly half (49.3%) undergoing specialty procedures at both hospitals. It is possible that these findings are reflective of the broad array of specialty procedures examined within this diagnosis. For example, it is reasonable to consider that a patient may be stabilized with receipt of a blood transfusion at the transferring hospital, then transferred to undergo a diagnostic/therapeutic procedure (ie, endoscopy/colonoscopy) at the receiving hospital, as is suggested by our results.
Our study is subject to several limitations. First, given the criteria we used to define transfer, it is possible that we included nontransferred patients within our transferred cohort if they were discharged from one hospital and admitted to a different hospital within 1 day, although quality assurance analyses we conducted in prior studies on these data support the validity of the criteria used.2 Second, we cannot exclude the possibility that patients received nonprocedural specialty care (ie, expert opinion, specialized imaging, medical management, management of secondary diagnoses, etc.) not available at the transferring hospital, although, arguably, in select patients, such input could be obtained without physical transfer of the patient (ie, tele-consult). And even in patients transferred with intent to receive procedural care who did not ultimately receive that care, there is likely an appropriate “nonprocedure” rate, where patients who might benefit from a procedure receive a timely evaluation to reduce the risk of missing the opportunity to receive it. This would be analogous to transferring a patient to an ICU even if they do not end up requiring intubation or pressor therapy. However, given the likelihood of higher risks of IHT compared with intrahospital transfers, one could argue that the threshold of perceived benefit might be different in patients being considered for IHT. Additionally, we limited our analyses to only 4 diagnoses; thus, our findings may not be generalizable to other diagnoses of transferred patients. However, because the diagnoses we examined were ones considered most effectively treated with specialty procedural interventions, it is reasonable to presume that the variability in receipt of specialty procedures observed within these diagnoses is also present, if not greater, across other diagnoses. Third, although we intentionally included a broad array of specialty procedures associated with each diagnosis, it is possible that we overlooked particular specialty interventions. For example, in assuming that patients are most likely to be transferred to receive procedural services associated with their primary diagnosis, we may have missed alternate indications for transfer, including need for procedural care related to secondary or subsequent diagnoses (ie, a patient may have presented with GI bleed
CONCLUSIONS
We found that Medicare patients who undergo IHT with primary diagnoses of AMI, GI bleed, renal failure, and hip fracture/dislocation receive associated specialty interventions at varying frequency and locations, and many patients do not receive any associated procedures at receiving hospitals. Our findings suggest that specialty procedural care of patients, even those with primary diagnoses that often warrant specialized intervention, may not be the primary driver of IHT as commonly suggested, although underlying reasons for transfer in these and other “nonprocedural” transferred patients remains obscure. Given known ambiguity in the transfer process,7 and unclear benefit of IHT,8 additional research is required to further identify and evaluate other potential underlying reasons for transfer and to examine these in the context of patient outcomes, in order to understand which patients may or may not benefit from transfer and why.
Disclosure
The authors have nothing to disclose.
1. Iwashyna TJ. The incomplete infrastructure for interhospital patient transfer. Crit Care Med. 2012;40(8):2470-2478. PubMed
2. Mueller SK, Zheng J, Orav EJ, Schnipper JL. Rates, Predictors and Variability of Interhospital Transfers: A National Evaluation. J Hosp Med. 2017;12(6):435-442. PubMed
3. Guidelines for the transfer of critically ill patients. Guidelines Committee of the American College of Critical Care Medicine; Society of Critical Care Medicine and American Association of Critical-Care Nurses Transfer Guidelines Task Force. Crit Care Med. 1993;21(6):931-937. PubMed
4. Anderson JL, Adams CD, Antman EM, et al. 2011 ACCF/AHA Focused Update Incorporated Into the ACC/AHA 2007 Guidelines for the Management of Patients With Unstable Angina/Non-ST-Elevation Myocardial Infarction: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. Circulation. 2011;123(18):e426-e579. PubMed
5. Iwashyna TJ, Christie JD, Moody J, Kahn JM, Asch DA. The structure of critical care transfer networks. Med Care. 2009;47(7):787-793. PubMed
6. Iwashyna TJ, Kahn JM, Hayward RA, Nallamothu BK. Interhospital transfers among Medicare beneficiaries admitted for acute myocardial infarction at nonrevascularization hospitals. Circ Cardiovasc Qual Outcomes. 2010;3(5):468-475. PubMed
7. Bosk EA, Veinot T, Iwashyna TJ. Which patients and where: a qualitative study of patient transfers from community hospitals. Med Care. 2011;49(6):592-598. PubMed
8. Sokol-Hessner L, White AA, Davis KF, Herzig SJ, Hohmann SF. Interhospital transfer patients discharged by academic hospitalists and general internists: Characteristics and outcomes. J Hosp Med. 2016;11(4):245-250. PubMed
9. Department of Health and Human Services, Center for Medicare & Medicaid Services: Critical Access Hospitals. https://www.cms.gov/Outreach-and-Education/Medicare-Learning-Network-MLN/MLNProducts/downloads/CritAccessHospfctsht.pdf. Accessed June 29, 2017. PubMed
10. Roe MT, Chen AY, Delong ER, et al. Patterns of transfer for patients with non-ST-segment elevation acute coronary syndrome from community to tertiary care hospitals. Am Heart J. 2008;156(1):185-192. PubMed
11. Barreto-Filho JA, Wang Y, Rathore SS, et al. Transfer rates from nonprocedure hospitals after initial admission and outcomes among elderly patients with acute myocardial infarction. JAMA Intern Med. 2014;174(2):213-222. PubMed
12. Doruk H, Mas MR, Yildiz C, Sonmez A, Kyrdemir V. The effect of the timing of hip fracture surgery on the activity of daily living and mortality in elderly. Arch Gerontol Geriatr. 2004;39(2):179-185. PubMed
1. Iwashyna TJ. The incomplete infrastructure for interhospital patient transfer. Crit Care Med. 2012;40(8):2470-2478. PubMed
2. Mueller SK, Zheng J, Orav EJ, Schnipper JL. Rates, Predictors and Variability of Interhospital Transfers: A National Evaluation. J Hosp Med. 2017;12(6):435-442. PubMed
3. Guidelines for the transfer of critically ill patients. Guidelines Committee of the American College of Critical Care Medicine; Society of Critical Care Medicine and American Association of Critical-Care Nurses Transfer Guidelines Task Force. Crit Care Med. 1993;21(6):931-937. PubMed
4. Anderson JL, Adams CD, Antman EM, et al. 2011 ACCF/AHA Focused Update Incorporated Into the ACC/AHA 2007 Guidelines for the Management of Patients With Unstable Angina/Non-ST-Elevation Myocardial Infarction: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. Circulation. 2011;123(18):e426-e579. PubMed
5. Iwashyna TJ, Christie JD, Moody J, Kahn JM, Asch DA. The structure of critical care transfer networks. Med Care. 2009;47(7):787-793. PubMed
6. Iwashyna TJ, Kahn JM, Hayward RA, Nallamothu BK. Interhospital transfers among Medicare beneficiaries admitted for acute myocardial infarction at nonrevascularization hospitals. Circ Cardiovasc Qual Outcomes. 2010;3(5):468-475. PubMed
7. Bosk EA, Veinot T, Iwashyna TJ. Which patients and where: a qualitative study of patient transfers from community hospitals. Med Care. 2011;49(6):592-598. PubMed
8. Sokol-Hessner L, White AA, Davis KF, Herzig SJ, Hohmann SF. Interhospital transfer patients discharged by academic hospitalists and general internists: Characteristics and outcomes. J Hosp Med. 2016;11(4):245-250. PubMed
9. Department of Health and Human Services, Center for Medicare & Medicaid Services: Critical Access Hospitals. https://www.cms.gov/Outreach-and-Education/Medicare-Learning-Network-MLN/MLNProducts/downloads/CritAccessHospfctsht.pdf. Accessed June 29, 2017. PubMed
10. Roe MT, Chen AY, Delong ER, et al. Patterns of transfer for patients with non-ST-segment elevation acute coronary syndrome from community to tertiary care hospitals. Am Heart J. 2008;156(1):185-192. PubMed
11. Barreto-Filho JA, Wang Y, Rathore SS, et al. Transfer rates from nonprocedure hospitals after initial admission and outcomes among elderly patients with acute myocardial infarction. JAMA Intern Med. 2014;174(2):213-222. PubMed
12. Doruk H, Mas MR, Yildiz C, Sonmez A, Kyrdemir V. The effect of the timing of hip fracture surgery on the activity of daily living and mortality in elderly. Arch Gerontol Geriatr. 2004;39(2):179-185. PubMed
© 2017 Society of Hospital Medicine
617-732-7072; E-mail: smueller1@bwh.harvard.edu
Rates, predictors and variability of interhospital transfers: A national evaluation
Interhospital transfer (IHT) is defined as the transfer of hospitalized patients between acute care hospitals. Although cited reasons for transfer include providing patients access to unique specialty services,1 patterns and practices of IHT remain largely unstudied. Interhospital transfer is known to be common in certain patient populations, including selected patients presenting to the intensive care unit2 and those with acute myocardial infarction (AMI),3-5 but no recent studies have looked at frequency of IHT among a broader group of hospitalized patients nationally. Little is known about which patients are selected for transfer and why.6 Limited evidence suggests poor concordance between cited reason for transfer among patients, transferring physicians, and receiving physicians,7 indicating ambiguity in this care process.
Interhospital transfer exposes patients to the potential risks associated with discontinuity of care. Communication is particularly vulnerable to error during times of transition.8-10 Patients transferred between acute care hospitals are especially vulnerable, given the severity of illness in this patient population,11 and the absence of other factors to fill in gaps in communication, such as common electronic health records. Limited existing literature suggests transferred patients use more resources 12-13 and experience worse outcomes compared to nontransferred patients,11 although these data involved limited patient populations, and adjustment for illness severity and other factors was variably addressed.14-16
To improve the quality and safety of IHT, therefore, it is necessary to understand which patients benefit from IHT and identify best practices in the IHT process.17 A fundamental first step is to study patterns and practices of IHT, in particular with an eye towards identifying unwarranted variation.18 This is important to understand the prevalence of the issue, provide possible evidence of lack of standardization, and natural experiments with which to identify best practices.
To address this, we conducted a foundational study examining a national sample of Medicare patients to determine the nationwide frequency of IHT among elderly patients, patient and hospital-level predictors of transfer, and hospital variability in IHT practices.
METHODS
We performed a cross-sectional analysis using 2 nationally representative datasets: (1) Center for Medicare and Medicaid Services (CMS) 2013 100% Master Beneficiary Summary and Inpatient claims files, which contains data on all fee-for-service program Medicare enrollees’ demographic information, date of death, and hospitalization claims, including ICD-9 codes for diagnoses, diagnosis-related group (DRG), and dates of service; merged with (2) 2013 American Hospital Association (AHA) data,19 which contains hospital-level characteristics for all acute care hospitals in the U.S. Our study protocol was approved by the Partners Healthcare Human Subjects Review Committee.
Beneficiaries were eligible for inclusion if they were 65 years or older, continuously enrolled in Medicare A and B, with an acute care hospitalization claim in 2013, excluding Medicare managed care and end-stage renal disease (ESRD) beneficiaries. We additionally excluded beneficiaries hospitalized at federal or nonacute care hospitals, or critical access hospitals given their mission to stabilize and transfer patients to referral hospitals.20
Transferred patients were defined as: (1) beneficiaries with a “transfer out” claim and a corresponding “transfer in” claim at a different hospital; as well as (2) beneficiaries with a “transfer out” claim and a corresponding date of admission to another hospital within 1 day following the date of claim; and (3) beneficiaries with a “transfer in” claim and a corresponding date of discharge from another hospital within 1 day preceding the date of claim. Beneficiaries transferred to the same hospital, or cared for at hospitals with “outlier” transfer in rates equal to 100% or transfer out rates greater than 35%, were excluded from analysis given the suggestion of nonstandard claims practices. Beneficiaries with greater than 1 transfer within the same hospitalization were additionally excluded.
Patient Characteristics
Patient characteristics were obtained from the CMS data files and included: demographics (age, sex, race); DRG-weight, categorized into quartiles; primary diagnosis for the index hospitalization using ICD-9 codes; patient comorbidity using ICD-9 codes compiled into a CMS-Hierarchical Condition Category (HCC) risk score;21 presence of Medicaid co-insurance; number of hospitalizations in the past 12 months, categorized into 0, 1, 2-3, and 4 or more; season, defined as calendar quarters; and median income per household by census tract. These characteristics were chosen a priori given expert opinion in combination with prior research demonstrating association with IHT.11,22
Hospital Characteristics
Hospital characteristics were obtained from AHA data files and included hospitals’ size, categorized into small, medium, and large (less than 100, 100 to 399, 400 or more beds); geographic location; ownership; teaching status; setting (urban vs. rural); case mix index (CMI) for all patients cared for at the hospital; and presence of selected specialty services, including certified trauma center, medical intensive care unit, cardiac intensive care unit, cardiac surgery services, adult interventional cardiac catheterization, adult cardiac electrophysiology, and composite score of presence of 55 other specialty services (complete list in Appendix A). All characteristics were chosen a priori given expert opinion or relationship of characteristics with IHT, and prior research utilizing AHA data.23-24
Analysis
Descriptive statistics were used to evaluate the frequency of IHT, characteristics of transferred patients, and number of days to transfer. Patient and hospital characteristics of transferred vs. nontransferred patients were compared using chi-square analyses.
To analyze the effects of each patient and hospital characteristic on the odds of transfer, we used logistic regression models incorporating all patient and hospital characteristics, accounting for fixed effects for diagnosis, and utilizing generalized estimating equations (the GENMOD procedure in SAS statistical software, v 9.4; SAS Institute Inc., Cary, North Carolina) to account for the clustering of patients within hospitals.25 Indicator variables were created for missing covariate data and included in analyses when missing data accounted for greater than 10% of the total cohort.
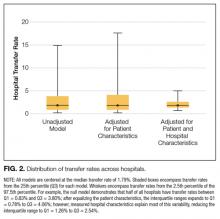
To measure the variability in transfer rates between hospitals, we used a sequence of random effects logistic regression models. We first ran a model with no covariates, representing the unadjusted differences in transfer rates between hospitals. We then added patient characteristics to see if the unadjusted differences in IHT rates were explained by differences in patient characteristics between hospitals. Lastly, we added hospital characteristics to determine if these explained the remaining differences in transfer rates. Each of the 3 models provided a measure of between-hospital variability, reflecting the degree to which IHT rates differed between hospitals. Additionally, we used the intercept from the unadjusted model and the measure of between-hospital variability from each model to calculate the 95% confidence intervals, illustrating the range of IHT rates spanning 95% of all hospitals. We used those same numbers to calculate the 25th and 75th percentiles, illustrating the range of IHT rates for the middle half of hospitals.
RESULTS
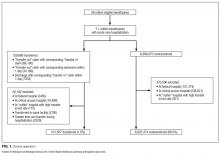
Among 28 million eligible beneficiaries, 6.6 million had an acute care hospitalization to nonfederal, noncritical access hospitals, and 107,741 met our defined criteria for IHT. An additional 3790 beneficiaries were excluded for being transferred to the same facility, 416 beneficiaries (115 transferred, 301 nontransferred) were excluded as they were cared for at 1 of the 11 hospitals with “outlier” transfer in/out rates, and 2329 were excluded because they had more than 1 transfer during hospitalization. Thus, the final cohort consisted of 101,507 transferred (1.5%) and 6,625,474 nontransferred beneficiaries (Figure 1). Of the 101,507 transferred beneficiaries, 2799 (2.8%) were included more than once (ie, experienced more than 1 IHT on separate hospitalizations throughout the study period; the vast majority of these had 2 separate hospitalizations resulting in IHT). Characteristics of transferred and nontransferred beneficiaries are shown (Table 1).
Among transferred patients, the top 5 primary diagnoses at time of transfer included AMI (12.2%), congestive heart failure (CHF) (7.2%), sepsis (6.6%), arrhythmia (6.6%), and pneumonia (3.4%). Comorbid conditions most commonly present in transferred patients included CHF (52.6%), renal failure (51.8%), arrhythmia (49.8%), and chronic obstructive pulmonary disease (COPD; 37.0%). The most common day of transfer was day after admission (hospital day 2, 24.7%), with 75% of transferred patients transferred before hospital day 6 (Appendix B).
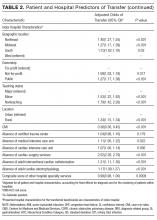
After adjusting for all other patient and hospital characteristics and clustering by hospital, the following variables were associated with greater odds of transfer: older age, male sex, nonblack race, non-Medicaid co-insurance, higher comorbidity (HCC score), lower DRG-weight, and fewer hospitalizations in the prior 12 months. Beneficiaries also had greater odds of transfer if initially hospitalized at smaller hospitals, nonteaching hospitals, public hospitals, at hospitals in the Northeast, those with fewer specialty services, and those with a low CMI (Table 2).
DISCUSSION
In this nationally representative study of 6.6 million Medicare beneficiaries, we found that 1.5% of patients were transferred between acute care facilities and were most often transferred prior to hospital day 6. Older age, male sex, nonblack race, higher medical comorbidity, lower DRG weight, and fewer recent hospitalizations were associated with greater odds of transfer. Initial hospitalization at smaller, nonteaching, public hospitals, with fewer specialty services were associated with greater odds of transfer, while higher CMI was associated with a lower odds of transfer. The most common comorbid conditions among transferred patients included CHF, renal failure, arrhythmia, and COPD; particularly notable was the very high prevalence of these conditions among transferred as compared with nontransferred patients. Importantly, we found significant variation in IHT by region and a large variation in transfer practices by hospital, with significant variability in transfer rates even after accounting for known patient and hospital characteristics.
Among our examined population, we found that a sizable number of patients undergo IHT—more than 100,000 per year. Primary diagnoses at time of transfer consist of common inpatient conditions, including AMI, CHF, sepsis, arrhythmia, and pneumonia. Limited prior data support our findings, with up to 50% of AMI patients reportedly undergoing IHT,3-5 and severe sepsis and respiratory illness reported as common diagnoses at transfer.11 Although knowledge of these primary diagnoses does not directly confer an understanding of reason for transfer, one can speculate based on our findings. For example, research demonstrates the majority of AMI patients who undergo IHT had further intervention, including stress testing, cardiac catheterization, and/or coronary artery bypass graft surgery.5,26 Thus, it is reasonable to presume that many of the beneficiaries
We additionally found that certain patient characteristics were associated with greater odds of transfer. Research suggests that transferred patients are “sicker” than nontransferred patients.1,11 Although our findings in part confirm these data, we paradoxically found that higher DRG-weight and 4 or more hospitalizations in the past year were actually associated with lower odds of transfer. In addition, the oldest patients in our cohort (85 years or older) were actually less likely to be transferred than their slightly younger counterparts (75 to 84 years). These variables may reflect extreme illness or frailty,27 and providers consciously (or subconsciously) may factor this in to their decision to transfer, considering a threshold past which transfer would confer more risk than benefit (eg, a patient may be “too sick” for transfer). Indeed, in a secondary analysis without hospital characteristics or comorbidities, and with fixed effects by hospital, we found the highest rates of IHT in patients in the middle 2 quartiles of DRG-weight, supporting this threshold hypothesis. It is also possible that patients with numerous hospitalizations may be less likely to be transferred because of familiarity and a strong sense of responsibility to continue to care for those patients (although we cannot confirm that those prior hospitalizations were all with the same index hospital).
It is also notable that odds of transfer differed by race, with black patients 17% less likely to undergo transfer compared to whites, similar to findings in other IHT studies.11 This finding, in combination with our demonstration that Medicaid patients also have lower odds of transfer, warrants further investigation to ensure the process of IHT does not bias against these populations, as with other well-documented health disparities.28-30
The hospital predictors of transfer were largely expected. However, interestingly, when we controlled for all other patient and hospital characteristics, regional variation persisted, with highest odds of transfer with hospitalization in the Northeast, indicating variability by region not explained by other factors, and findings supported by other limited data.31 This variability was further elucidated in our examination of change in variance estimates accounting for patient, then hospital, characteristics. Although we expected and found marked variability in hospital transfer rates in our null model (without accounting for any patient or hospital characteristics), we interestingly found that variability increased upon adjusting for patient characteristics. This result is presumably due to the fact that patients who are more likely to be transferred (ie, “sick” patients) are more often already at hospitals less likely to transfer patients, supported by our findings that hospital CMI is inversely associated with odds of transfer (in other words, hospitals that care for a less sick patient population are more likely to transfer their patients, and hospitals that care for a sicker patient population [higher CMI] are less likely to transfer). Adjusting solely for patient characteristics effectively equalizes these patients across hospitals, which would lead to even increased variability in transfer rates. Conversely, when we then adjusted for hospital characteristics, variability in hospital transfer rates decreased by 83% (in other words, hospital characteristics, rather than patient characteristics, explained much of the variability in transfer rates), although significant unexplained variability remained. We should note that although the observed reduction in variability was explained by the patient and hospital characteristics included in the model, these characteristics do not necessarily justify the variability they accounted for; although patients’ race or hospitals’ location may explain some of the observed variability, this does not reasonably justify it.
This observed variability in transfer practices is not surprising given the absence of standardization and clear guidelines to direct clinical IHT practice.17 Selection of patients that may benefit from transfer is often ambiguous and subjective.6 The Emergency Medical Treatment and Active Labor Act laws dictate that hospitals transfer patients requiring a more specialized service, or when “medical benefits ... outweigh the increased risks to the individual...,” although in practice this provides little guidance to practitioners.1 Thus, clearer guidelines may be necessary to achieve less variable practices.
Our study is subject to several limitations. First, although nationally representative, the Medicare population is not reflective of all hospitalized patients nationwide. Additionally, we excluded patients transferred from the emergency room. Thus, the total number of patients who undergo IHT nationally is expected to be much higher than reflected in our analysis. We also excluded patients who were transferred more than once during a given hospitalization. This enabled us to focus on the initial transfer decision but does not allow us to look at patients who are transferred to a referral center and then transferred back. Second, given the criteria we used to define transfer, it is possible that we included nontransferred patients within our transferred cohort if they were discharged from one hospital and admitted to a different hospital within 1 day. However, on quality assurance analyses where we limited our cohort to only those beneficiaries with corresponding “transfer in” and “transfer out” claims (87% of the total cohort), we found no marked differences in our results. Additionally, although we assume that patient transfer status was coded correctly within the Medicare dataset, we could not confirm by individually examining each patient we defined as “transferred.” However, on additional quality assurance analyses where we examined randomly selected excluded patients with greater than 1 transfer during hospitalization, we found differing provider numbers with each transfer, suggesting validity of the coding. Third, because there are likely many unmeasured patient confounders, we cannot be sure how much of the between-hospital variation is due to incomplete adjustment for patient characteristics. However, since adjusting for patient characteristics actually increased variability in hospital transfer rates, it is unlikely that residual patient confounders fully explain our observed results. Despite this, other variables that are not available within the CMS or AHA datasets may further elucidate hospital transfer practices, including variables reflective of the transfer process (eg, time of day of patient transfer, time delay between initiation of transfer and patient arrival at accepting hospital, accepting service on transfer, etc.); other markers of illness severity (eg, clinical service at the time of index admission, acute physiology score, utilization of critical care services on arrival at receiving hospital); and other hospital system variables (ie, membership in an accountable care organization and/or regional care network, the density of nearby tertiary referral centers (indicating possible supply-induced demand), other variables reflective of the “transfer culture” (such as the transfer rate at the hospital or region where the attending physician trained, etc.). Lastly, though our examination provides important foundational information regarding IHT nationally, this study did not examine patient outcomes in transferred and nontransferred patients, which may help to determine which patients benefit (or do not benefit) from transfer and why. Further investigation is needed to study these outcomes.
CONCLUSION
In this national study of IHT, we found that a sizable number of patients admitted to the hospital undergo transfer to another acute care facility. Patients are transferred with common medical conditions, including those requiring specialized care such as AMI, and a high rate of comorbid clinical conditions, and certain patient and hospital characteristics are associated with greater odds of transfer. Although many of the observed associations between characteristics and odds of transfer were expected based on limited existing literature, we found several unexpected findings, eg, suggesting the possibility of a threshold beyond which sicker patients are not transferred. Additionally, we found that black and Medicaid patients had lower odds of transfer, which warrants further investigation for potential health care disparity. Importantly, we found much variability in the practice of IHT, as evidenced by the inexplicable differences in transfer by hospital region, and by residual unexplained variability in hospital transfer rates after accounting for patient and hospital characteristics, which may be due to lack of standard guidelines to direct IHT practices. In conclusion, this study of hospitalized Medicare patients provides important foundational information regarding rates and predictors of IHT nationally, as well as unexplained variability that exists within this complex care transition. Further investigation will be essential to understand reasons for, processes related to, and outcomes of transferred patients, to help guide standardization in best practices in care.
Disclosure
Nothing to report.
1. Iwashyna TJ. The incomplete infrastructure for interhospital patient transfer. Crit Care Med. 2012;40(8):2470-2478. PubMed
2. Iwashyna TJ, Christie JD, Moody J, Kahn JM, Asch DA. The structure of critical care transfer networks. Med Care. 2009;47(7):787-793. PubMed
3. Mehta RH, Stalhandske EJ, McCargar PA, Ruane TJ, Eagle KA. Elderly patients at highest risk with acute myocardial infarction are more frequently transferred from community hospitals to tertiary centers: reality or myth? Am Heart J. 1999;138(4 Pt 1):688-695. PubMed
4. Iwashyna TJ, Kahn JM, Hayward RA, Nallamothu BK. Interhospital transfers among Medicare beneficiaries admitted for acute myocardial infarction at nonrevascularization hospitals. Circ Cardiovasc Qual Outcomes. 2010;3(5):468-475. PubMed
5. Roe MT, Chen AY, Delong ER, Boden WE, Calvin JE Jr, Cairns CB, et al. Patterns of transfer for patients with non-ST-segment elevation acute coronary syndrome from community to tertiary care hospitals. Am Heart J. 2008;156(1):185-192. PubMed
6. Bosk EA, Veinot T, Iwashyna TJ. Which patients and where: a qualitative study of patient transfers from community hospitals. Med Care. 2011;49(6):592-598. PubMed
7. Wagner J, Iwashyna TJ, Kahn JM. Reasons underlying interhospital transfers to an academic medical intensive care unit. J Crit Care. 2013;28(2):202-208. PubMed
8. Cohen MD, Hilligoss PB. The published literature on handoffs in hospitals: deficiencies identified in an extensive review. Qual Saf Health Care. 2010;19(6):493-497. PubMed
9. Riesenberg LA, Leitzsch J, Massucci JL, et al. Residents’ and attending physicians’ handoffs: a systematic review of the literature. Acad Med. 2009;84(12):1775-1787. PubMed
10. Arora V, Johnson J, Lovinger D, Humphrey HJ, Meltzer DO. Communication failures in patient sign-out and suggestions for improvement: a critical incident analysis. Qual Saf Health Care. 2005;14(6):401-407. PubMed
11. Sokol-Hessner L, White AA, Davis KF, Herzig SJ, Hohmann SF. Interhospital transfer patients discharged by academic hospitalists and general internists: characteristics and outcomes. J Hosp Med. 2016;11(4):245-250. PubMed
12. Bernard AM, Hayward RA, Rosevear J, Chun H, McMahon LF. Comparing the hospitalizations of transfer and non-transfer patients in an academic medical center. Acad Med. 1996;71(3):262-266. PubMed
13. Golestanian E, Scruggs JE, Gangnon RE, Mak RP, Wood KE. Effect of interhospital transfer on resource utilization and outcomes at a tertiary care referral center. Crit Care Med. 2007;35(6):1470-1476. PubMed
14. Durairaj L, Will JG, Torner JC, Doebbeling BN. Prognostic factors for mortality following interhospital transfers to the medical intensive care unit of a tertiary referral center. Crit Care Med. 2003;31(7):1981-1986. PubMed
15. Kerr HD, Byrd JC. Community hospital transfers to a VA Medical Center. JAMA. 1989;262(1):70-73. PubMed
16. Dragsted L, Jörgensen J, Jensen NH, et al. Interhospital comparisons of patient outcome from intensive care: importance of lead-time bias. Crit Care Med. 1989;17(5):418-422. PubMed
17. Gupta K, Mueller SK. Interhospital transfers: the need for standards. J Hosp Med. 2015;10(6):415-417. PubMed
18. The Dartmouth Atlas of Health Care: Understanding of the Efficiency and Effectiveness of the Health Care System. The Dartmouth Institute for Health Practice and Clinical Policy, Lebanon, NH. http://www.dartmouthatlas.org/. Accessed November 1, 2016.
19. American Hospital Association Annual Survey Database. American Hospital Association, Chicago, IL. http://www.ahadataviewer.com/book-cd-products/AHA-Survey/. Accessed July 1, 2013.
20. U.S. Department of Health and Human Services (HRSA): What are critical access hospitals (CAH)? http://www.hrsa.gov/healthit/toolbox/RuralHealthITtoolbox/Introduction/critical.html. Accessed June 9, 2016.
21. Li P, Kim MM, Doshi JA. Comparison of the performance of the CMS Hierarchical Condition Category (CMS-HCC) risk adjuster with the Charlson and Elixhauser comorbidity measures in predicting mortality. BMC Health Serv Res. 2010;10:245. PubMed
22. Hernandez-Boussard T, Davies S, McDonald K, Wang NE. Interhospital facility transfers in the United States: a nationwide outcomes study. J Patient Saf. Nov 13 2014. PubMed
23. Landon BE, Normand SL, Lessler A, et al. Quality of care for the treatment of acute medical conditions in US hospitals. Arch Intern Med. 2006;166(22):2511-2517. PubMed
24. Mueller SK, Lipsitz S, Hicks LS. Impact of hospital teaching intensity on quality of care and patient outcomes. Med Care.2013;51(7):567-574. PubMed
25. Lopez L, Hicks LS, Cohen AP, McKean S, Weissman JS. Hospitalists and the quality of care in hospitals. Arch Intern Med. 2009;169(15):1389-1394. PubMed
26. Barreto-Filho JA, Wang Y, Rathore SS, et al. Transfer rates from nonprocedure hospitals after initial admission and outcomes among elderly patients with acute myocardial infarction. JAMA Intern Med. 2014;174(2):213-222. PubMed
27. Carlson JE, Zocchi KA, Bettencourt DM, et al. Measuring frailty in the hospitalized elderly: concept of functional homeostasis. Am J Phys Med Rehabil. 1998;77(3):252-257. PubMed
28. Ward E, Jemal A, Cokkinides V, et al. Cancer disparities by race/ethnicity and socioeconomic status. CA Cancer J Clin. 2004;54(2):78-93. PubMed
29. Iribarren C, Tolstykh I, Somkin CP, et al. Sex and racial/ethnic disparities in outcomes after acute myocardial infarction: a cohort study among members of a large integrated health care delivery system in northern California. Arch Intern Med. 2005;165(18):2105-2113. PubMed
30. Kawachi I, Daniels N, Robinson DE. Health disparities by race and class: why both matter. Health Aff (Millwood). 2005;24(2):343-352. PubMed
31. Herrigel DJ, Carroll M, Fanning C, Steinberg MB, Parikh A, Usher M. Interhospital transfer handoff practices among US tertiary care centers: a descriptive survey. J Hosp Med. 2016;11(6):413-417. PubMed
Interhospital transfer (IHT) is defined as the transfer of hospitalized patients between acute care hospitals. Although cited reasons for transfer include providing patients access to unique specialty services,1 patterns and practices of IHT remain largely unstudied. Interhospital transfer is known to be common in certain patient populations, including selected patients presenting to the intensive care unit2 and those with acute myocardial infarction (AMI),3-5 but no recent studies have looked at frequency of IHT among a broader group of hospitalized patients nationally. Little is known about which patients are selected for transfer and why.6 Limited evidence suggests poor concordance between cited reason for transfer among patients, transferring physicians, and receiving physicians,7 indicating ambiguity in this care process.
Interhospital transfer exposes patients to the potential risks associated with discontinuity of care. Communication is particularly vulnerable to error during times of transition.8-10 Patients transferred between acute care hospitals are especially vulnerable, given the severity of illness in this patient population,11 and the absence of other factors to fill in gaps in communication, such as common electronic health records. Limited existing literature suggests transferred patients use more resources 12-13 and experience worse outcomes compared to nontransferred patients,11 although these data involved limited patient populations, and adjustment for illness severity and other factors was variably addressed.14-16
To improve the quality and safety of IHT, therefore, it is necessary to understand which patients benefit from IHT and identify best practices in the IHT process.17 A fundamental first step is to study patterns and practices of IHT, in particular with an eye towards identifying unwarranted variation.18 This is important to understand the prevalence of the issue, provide possible evidence of lack of standardization, and natural experiments with which to identify best practices.
To address this, we conducted a foundational study examining a national sample of Medicare patients to determine the nationwide frequency of IHT among elderly patients, patient and hospital-level predictors of transfer, and hospital variability in IHT practices.
METHODS
We performed a cross-sectional analysis using 2 nationally representative datasets: (1) Center for Medicare and Medicaid Services (CMS) 2013 100% Master Beneficiary Summary and Inpatient claims files, which contains data on all fee-for-service program Medicare enrollees’ demographic information, date of death, and hospitalization claims, including ICD-9 codes for diagnoses, diagnosis-related group (DRG), and dates of service; merged with (2) 2013 American Hospital Association (AHA) data,19 which contains hospital-level characteristics for all acute care hospitals in the U.S. Our study protocol was approved by the Partners Healthcare Human Subjects Review Committee.
Beneficiaries were eligible for inclusion if they were 65 years or older, continuously enrolled in Medicare A and B, with an acute care hospitalization claim in 2013, excluding Medicare managed care and end-stage renal disease (ESRD) beneficiaries. We additionally excluded beneficiaries hospitalized at federal or nonacute care hospitals, or critical access hospitals given their mission to stabilize and transfer patients to referral hospitals.20
Transferred patients were defined as: (1) beneficiaries with a “transfer out” claim and a corresponding “transfer in” claim at a different hospital; as well as (2) beneficiaries with a “transfer out” claim and a corresponding date of admission to another hospital within 1 day following the date of claim; and (3) beneficiaries with a “transfer in” claim and a corresponding date of discharge from another hospital within 1 day preceding the date of claim. Beneficiaries transferred to the same hospital, or cared for at hospitals with “outlier” transfer in rates equal to 100% or transfer out rates greater than 35%, were excluded from analysis given the suggestion of nonstandard claims practices. Beneficiaries with greater than 1 transfer within the same hospitalization were additionally excluded.
Patient Characteristics
Patient characteristics were obtained from the CMS data files and included: demographics (age, sex, race); DRG-weight, categorized into quartiles; primary diagnosis for the index hospitalization using ICD-9 codes; patient comorbidity using ICD-9 codes compiled into a CMS-Hierarchical Condition Category (HCC) risk score;21 presence of Medicaid co-insurance; number of hospitalizations in the past 12 months, categorized into 0, 1, 2-3, and 4 or more; season, defined as calendar quarters; and median income per household by census tract. These characteristics were chosen a priori given expert opinion in combination with prior research demonstrating association with IHT.11,22
Hospital Characteristics
Hospital characteristics were obtained from AHA data files and included hospitals’ size, categorized into small, medium, and large (less than 100, 100 to 399, 400 or more beds); geographic location; ownership; teaching status; setting (urban vs. rural); case mix index (CMI) for all patients cared for at the hospital; and presence of selected specialty services, including certified trauma center, medical intensive care unit, cardiac intensive care unit, cardiac surgery services, adult interventional cardiac catheterization, adult cardiac electrophysiology, and composite score of presence of 55 other specialty services (complete list in Appendix A). All characteristics were chosen a priori given expert opinion or relationship of characteristics with IHT, and prior research utilizing AHA data.23-24
Analysis
Descriptive statistics were used to evaluate the frequency of IHT, characteristics of transferred patients, and number of days to transfer. Patient and hospital characteristics of transferred vs. nontransferred patients were compared using chi-square analyses.
To analyze the effects of each patient and hospital characteristic on the odds of transfer, we used logistic regression models incorporating all patient and hospital characteristics, accounting for fixed effects for diagnosis, and utilizing generalized estimating equations (the GENMOD procedure in SAS statistical software, v 9.4; SAS Institute Inc., Cary, North Carolina) to account for the clustering of patients within hospitals.25 Indicator variables were created for missing covariate data and included in analyses when missing data accounted for greater than 10% of the total cohort.
To measure the variability in transfer rates between hospitals, we used a sequence of random effects logistic regression models. We first ran a model with no covariates, representing the unadjusted differences in transfer rates between hospitals. We then added patient characteristics to see if the unadjusted differences in IHT rates were explained by differences in patient characteristics between hospitals. Lastly, we added hospital characteristics to determine if these explained the remaining differences in transfer rates. Each of the 3 models provided a measure of between-hospital variability, reflecting the degree to which IHT rates differed between hospitals. Additionally, we used the intercept from the unadjusted model and the measure of between-hospital variability from each model to calculate the 95% confidence intervals, illustrating the range of IHT rates spanning 95% of all hospitals. We used those same numbers to calculate the 25th and 75th percentiles, illustrating the range of IHT rates for the middle half of hospitals.
RESULTS
Among 28 million eligible beneficiaries, 6.6 million had an acute care hospitalization to nonfederal, noncritical access hospitals, and 107,741 met our defined criteria for IHT. An additional 3790 beneficiaries were excluded for being transferred to the same facility, 416 beneficiaries (115 transferred, 301 nontransferred) were excluded as they were cared for at 1 of the 11 hospitals with “outlier” transfer in/out rates, and 2329 were excluded because they had more than 1 transfer during hospitalization. Thus, the final cohort consisted of 101,507 transferred (1.5%) and 6,625,474 nontransferred beneficiaries (Figure 1). Of the 101,507 transferred beneficiaries, 2799 (2.8%) were included more than once (ie, experienced more than 1 IHT on separate hospitalizations throughout the study period; the vast majority of these had 2 separate hospitalizations resulting in IHT). Characteristics of transferred and nontransferred beneficiaries are shown (Table 1).
Among transferred patients, the top 5 primary diagnoses at time of transfer included AMI (12.2%), congestive heart failure (CHF) (7.2%), sepsis (6.6%), arrhythmia (6.6%), and pneumonia (3.4%). Comorbid conditions most commonly present in transferred patients included CHF (52.6%), renal failure (51.8%), arrhythmia (49.8%), and chronic obstructive pulmonary disease (COPD; 37.0%). The most common day of transfer was day after admission (hospital day 2, 24.7%), with 75% of transferred patients transferred before hospital day 6 (Appendix B).
After adjusting for all other patient and hospital characteristics and clustering by hospital, the following variables were associated with greater odds of transfer: older age, male sex, nonblack race, non-Medicaid co-insurance, higher comorbidity (HCC score), lower DRG-weight, and fewer hospitalizations in the prior 12 months. Beneficiaries also had greater odds of transfer if initially hospitalized at smaller hospitals, nonteaching hospitals, public hospitals, at hospitals in the Northeast, those with fewer specialty services, and those with a low CMI (Table 2).
DISCUSSION
In this nationally representative study of 6.6 million Medicare beneficiaries, we found that 1.5% of patients were transferred between acute care facilities and were most often transferred prior to hospital day 6. Older age, male sex, nonblack race, higher medical comorbidity, lower DRG weight, and fewer recent hospitalizations were associated with greater odds of transfer. Initial hospitalization at smaller, nonteaching, public hospitals, with fewer specialty services were associated with greater odds of transfer, while higher CMI was associated with a lower odds of transfer. The most common comorbid conditions among transferred patients included CHF, renal failure, arrhythmia, and COPD; particularly notable was the very high prevalence of these conditions among transferred as compared with nontransferred patients. Importantly, we found significant variation in IHT by region and a large variation in transfer practices by hospital, with significant variability in transfer rates even after accounting for known patient and hospital characteristics.
Among our examined population, we found that a sizable number of patients undergo IHT—more than 100,000 per year. Primary diagnoses at time of transfer consist of common inpatient conditions, including AMI, CHF, sepsis, arrhythmia, and pneumonia. Limited prior data support our findings, with up to 50% of AMI patients reportedly undergoing IHT,3-5 and severe sepsis and respiratory illness reported as common diagnoses at transfer.11 Although knowledge of these primary diagnoses does not directly confer an understanding of reason for transfer, one can speculate based on our findings. For example, research demonstrates the majority of AMI patients who undergo IHT had further intervention, including stress testing, cardiac catheterization, and/or coronary artery bypass graft surgery.5,26 Thus, it is reasonable to presume that many of the beneficiaries
We additionally found that certain patient characteristics were associated with greater odds of transfer. Research suggests that transferred patients are “sicker” than nontransferred patients.1,11 Although our findings in part confirm these data, we paradoxically found that higher DRG-weight and 4 or more hospitalizations in the past year were actually associated with lower odds of transfer. In addition, the oldest patients in our cohort (85 years or older) were actually less likely to be transferred than their slightly younger counterparts (75 to 84 years). These variables may reflect extreme illness or frailty,27 and providers consciously (or subconsciously) may factor this in to their decision to transfer, considering a threshold past which transfer would confer more risk than benefit (eg, a patient may be “too sick” for transfer). Indeed, in a secondary analysis without hospital characteristics or comorbidities, and with fixed effects by hospital, we found the highest rates of IHT in patients in the middle 2 quartiles of DRG-weight, supporting this threshold hypothesis. It is also possible that patients with numerous hospitalizations may be less likely to be transferred because of familiarity and a strong sense of responsibility to continue to care for those patients (although we cannot confirm that those prior hospitalizations were all with the same index hospital).
It is also notable that odds of transfer differed by race, with black patients 17% less likely to undergo transfer compared to whites, similar to findings in other IHT studies.11 This finding, in combination with our demonstration that Medicaid patients also have lower odds of transfer, warrants further investigation to ensure the process of IHT does not bias against these populations, as with other well-documented health disparities.28-30
The hospital predictors of transfer were largely expected. However, interestingly, when we controlled for all other patient and hospital characteristics, regional variation persisted, with highest odds of transfer with hospitalization in the Northeast, indicating variability by region not explained by other factors, and findings supported by other limited data.31 This variability was further elucidated in our examination of change in variance estimates accounting for patient, then hospital, characteristics. Although we expected and found marked variability in hospital transfer rates in our null model (without accounting for any patient or hospital characteristics), we interestingly found that variability increased upon adjusting for patient characteristics. This result is presumably due to the fact that patients who are more likely to be transferred (ie, “sick” patients) are more often already at hospitals less likely to transfer patients, supported by our findings that hospital CMI is inversely associated with odds of transfer (in other words, hospitals that care for a less sick patient population are more likely to transfer their patients, and hospitals that care for a sicker patient population [higher CMI] are less likely to transfer). Adjusting solely for patient characteristics effectively equalizes these patients across hospitals, which would lead to even increased variability in transfer rates. Conversely, when we then adjusted for hospital characteristics, variability in hospital transfer rates decreased by 83% (in other words, hospital characteristics, rather than patient characteristics, explained much of the variability in transfer rates), although significant unexplained variability remained. We should note that although the observed reduction in variability was explained by the patient and hospital characteristics included in the model, these characteristics do not necessarily justify the variability they accounted for; although patients’ race or hospitals’ location may explain some of the observed variability, this does not reasonably justify it.
This observed variability in transfer practices is not surprising given the absence of standardization and clear guidelines to direct clinical IHT practice.17 Selection of patients that may benefit from transfer is often ambiguous and subjective.6 The Emergency Medical Treatment and Active Labor Act laws dictate that hospitals transfer patients requiring a more specialized service, or when “medical benefits ... outweigh the increased risks to the individual...,” although in practice this provides little guidance to practitioners.1 Thus, clearer guidelines may be necessary to achieve less variable practices.
Our study is subject to several limitations. First, although nationally representative, the Medicare population is not reflective of all hospitalized patients nationwide. Additionally, we excluded patients transferred from the emergency room. Thus, the total number of patients who undergo IHT nationally is expected to be much higher than reflected in our analysis. We also excluded patients who were transferred more than once during a given hospitalization. This enabled us to focus on the initial transfer decision but does not allow us to look at patients who are transferred to a referral center and then transferred back. Second, given the criteria we used to define transfer, it is possible that we included nontransferred patients within our transferred cohort if they were discharged from one hospital and admitted to a different hospital within 1 day. However, on quality assurance analyses where we limited our cohort to only those beneficiaries with corresponding “transfer in” and “transfer out” claims (87% of the total cohort), we found no marked differences in our results. Additionally, although we assume that patient transfer status was coded correctly within the Medicare dataset, we could not confirm by individually examining each patient we defined as “transferred.” However, on additional quality assurance analyses where we examined randomly selected excluded patients with greater than 1 transfer during hospitalization, we found differing provider numbers with each transfer, suggesting validity of the coding. Third, because there are likely many unmeasured patient confounders, we cannot be sure how much of the between-hospital variation is due to incomplete adjustment for patient characteristics. However, since adjusting for patient characteristics actually increased variability in hospital transfer rates, it is unlikely that residual patient confounders fully explain our observed results. Despite this, other variables that are not available within the CMS or AHA datasets may further elucidate hospital transfer practices, including variables reflective of the transfer process (eg, time of day of patient transfer, time delay between initiation of transfer and patient arrival at accepting hospital, accepting service on transfer, etc.); other markers of illness severity (eg, clinical service at the time of index admission, acute physiology score, utilization of critical care services on arrival at receiving hospital); and other hospital system variables (ie, membership in an accountable care organization and/or regional care network, the density of nearby tertiary referral centers (indicating possible supply-induced demand), other variables reflective of the “transfer culture” (such as the transfer rate at the hospital or region where the attending physician trained, etc.). Lastly, though our examination provides important foundational information regarding IHT nationally, this study did not examine patient outcomes in transferred and nontransferred patients, which may help to determine which patients benefit (or do not benefit) from transfer and why. Further investigation is needed to study these outcomes.
CONCLUSION
In this national study of IHT, we found that a sizable number of patients admitted to the hospital undergo transfer to another acute care facility. Patients are transferred with common medical conditions, including those requiring specialized care such as AMI, and a high rate of comorbid clinical conditions, and certain patient and hospital characteristics are associated with greater odds of transfer. Although many of the observed associations between characteristics and odds of transfer were expected based on limited existing literature, we found several unexpected findings, eg, suggesting the possibility of a threshold beyond which sicker patients are not transferred. Additionally, we found that black and Medicaid patients had lower odds of transfer, which warrants further investigation for potential health care disparity. Importantly, we found much variability in the practice of IHT, as evidenced by the inexplicable differences in transfer by hospital region, and by residual unexplained variability in hospital transfer rates after accounting for patient and hospital characteristics, which may be due to lack of standard guidelines to direct IHT practices. In conclusion, this study of hospitalized Medicare patients provides important foundational information regarding rates and predictors of IHT nationally, as well as unexplained variability that exists within this complex care transition. Further investigation will be essential to understand reasons for, processes related to, and outcomes of transferred patients, to help guide standardization in best practices in care.
Disclosure
Nothing to report.
Interhospital transfer (IHT) is defined as the transfer of hospitalized patients between acute care hospitals. Although cited reasons for transfer include providing patients access to unique specialty services,1 patterns and practices of IHT remain largely unstudied. Interhospital transfer is known to be common in certain patient populations, including selected patients presenting to the intensive care unit2 and those with acute myocardial infarction (AMI),3-5 but no recent studies have looked at frequency of IHT among a broader group of hospitalized patients nationally. Little is known about which patients are selected for transfer and why.6 Limited evidence suggests poor concordance between cited reason for transfer among patients, transferring physicians, and receiving physicians,7 indicating ambiguity in this care process.
Interhospital transfer exposes patients to the potential risks associated with discontinuity of care. Communication is particularly vulnerable to error during times of transition.8-10 Patients transferred between acute care hospitals are especially vulnerable, given the severity of illness in this patient population,11 and the absence of other factors to fill in gaps in communication, such as common electronic health records. Limited existing literature suggests transferred patients use more resources 12-13 and experience worse outcomes compared to nontransferred patients,11 although these data involved limited patient populations, and adjustment for illness severity and other factors was variably addressed.14-16
To improve the quality and safety of IHT, therefore, it is necessary to understand which patients benefit from IHT and identify best practices in the IHT process.17 A fundamental first step is to study patterns and practices of IHT, in particular with an eye towards identifying unwarranted variation.18 This is important to understand the prevalence of the issue, provide possible evidence of lack of standardization, and natural experiments with which to identify best practices.
To address this, we conducted a foundational study examining a national sample of Medicare patients to determine the nationwide frequency of IHT among elderly patients, patient and hospital-level predictors of transfer, and hospital variability in IHT practices.
METHODS
We performed a cross-sectional analysis using 2 nationally representative datasets: (1) Center for Medicare and Medicaid Services (CMS) 2013 100% Master Beneficiary Summary and Inpatient claims files, which contains data on all fee-for-service program Medicare enrollees’ demographic information, date of death, and hospitalization claims, including ICD-9 codes for diagnoses, diagnosis-related group (DRG), and dates of service; merged with (2) 2013 American Hospital Association (AHA) data,19 which contains hospital-level characteristics for all acute care hospitals in the U.S. Our study protocol was approved by the Partners Healthcare Human Subjects Review Committee.
Beneficiaries were eligible for inclusion if they were 65 years or older, continuously enrolled in Medicare A and B, with an acute care hospitalization claim in 2013, excluding Medicare managed care and end-stage renal disease (ESRD) beneficiaries. We additionally excluded beneficiaries hospitalized at federal or nonacute care hospitals, or critical access hospitals given their mission to stabilize and transfer patients to referral hospitals.20
Transferred patients were defined as: (1) beneficiaries with a “transfer out” claim and a corresponding “transfer in” claim at a different hospital; as well as (2) beneficiaries with a “transfer out” claim and a corresponding date of admission to another hospital within 1 day following the date of claim; and (3) beneficiaries with a “transfer in” claim and a corresponding date of discharge from another hospital within 1 day preceding the date of claim. Beneficiaries transferred to the same hospital, or cared for at hospitals with “outlier” transfer in rates equal to 100% or transfer out rates greater than 35%, were excluded from analysis given the suggestion of nonstandard claims practices. Beneficiaries with greater than 1 transfer within the same hospitalization were additionally excluded.
Patient Characteristics
Patient characteristics were obtained from the CMS data files and included: demographics (age, sex, race); DRG-weight, categorized into quartiles; primary diagnosis for the index hospitalization using ICD-9 codes; patient comorbidity using ICD-9 codes compiled into a CMS-Hierarchical Condition Category (HCC) risk score;21 presence of Medicaid co-insurance; number of hospitalizations in the past 12 months, categorized into 0, 1, 2-3, and 4 or more; season, defined as calendar quarters; and median income per household by census tract. These characteristics were chosen a priori given expert opinion in combination with prior research demonstrating association with IHT.11,22
Hospital Characteristics
Hospital characteristics were obtained from AHA data files and included hospitals’ size, categorized into small, medium, and large (less than 100, 100 to 399, 400 or more beds); geographic location; ownership; teaching status; setting (urban vs. rural); case mix index (CMI) for all patients cared for at the hospital; and presence of selected specialty services, including certified trauma center, medical intensive care unit, cardiac intensive care unit, cardiac surgery services, adult interventional cardiac catheterization, adult cardiac electrophysiology, and composite score of presence of 55 other specialty services (complete list in Appendix A). All characteristics were chosen a priori given expert opinion or relationship of characteristics with IHT, and prior research utilizing AHA data.23-24
Analysis
Descriptive statistics were used to evaluate the frequency of IHT, characteristics of transferred patients, and number of days to transfer. Patient and hospital characteristics of transferred vs. nontransferred patients were compared using chi-square analyses.
To analyze the effects of each patient and hospital characteristic on the odds of transfer, we used logistic regression models incorporating all patient and hospital characteristics, accounting for fixed effects for diagnosis, and utilizing generalized estimating equations (the GENMOD procedure in SAS statistical software, v 9.4; SAS Institute Inc., Cary, North Carolina) to account for the clustering of patients within hospitals.25 Indicator variables were created for missing covariate data and included in analyses when missing data accounted for greater than 10% of the total cohort.
To measure the variability in transfer rates between hospitals, we used a sequence of random effects logistic regression models. We first ran a model with no covariates, representing the unadjusted differences in transfer rates between hospitals. We then added patient characteristics to see if the unadjusted differences in IHT rates were explained by differences in patient characteristics between hospitals. Lastly, we added hospital characteristics to determine if these explained the remaining differences in transfer rates. Each of the 3 models provided a measure of between-hospital variability, reflecting the degree to which IHT rates differed between hospitals. Additionally, we used the intercept from the unadjusted model and the measure of between-hospital variability from each model to calculate the 95% confidence intervals, illustrating the range of IHT rates spanning 95% of all hospitals. We used those same numbers to calculate the 25th and 75th percentiles, illustrating the range of IHT rates for the middle half of hospitals.
RESULTS
Among 28 million eligible beneficiaries, 6.6 million had an acute care hospitalization to nonfederal, noncritical access hospitals, and 107,741 met our defined criteria for IHT. An additional 3790 beneficiaries were excluded for being transferred to the same facility, 416 beneficiaries (115 transferred, 301 nontransferred) were excluded as they were cared for at 1 of the 11 hospitals with “outlier” transfer in/out rates, and 2329 were excluded because they had more than 1 transfer during hospitalization. Thus, the final cohort consisted of 101,507 transferred (1.5%) and 6,625,474 nontransferred beneficiaries (Figure 1). Of the 101,507 transferred beneficiaries, 2799 (2.8%) were included more than once (ie, experienced more than 1 IHT on separate hospitalizations throughout the study period; the vast majority of these had 2 separate hospitalizations resulting in IHT). Characteristics of transferred and nontransferred beneficiaries are shown (Table 1).
Among transferred patients, the top 5 primary diagnoses at time of transfer included AMI (12.2%), congestive heart failure (CHF) (7.2%), sepsis (6.6%), arrhythmia (6.6%), and pneumonia (3.4%). Comorbid conditions most commonly present in transferred patients included CHF (52.6%), renal failure (51.8%), arrhythmia (49.8%), and chronic obstructive pulmonary disease (COPD; 37.0%). The most common day of transfer was day after admission (hospital day 2, 24.7%), with 75% of transferred patients transferred before hospital day 6 (Appendix B).
After adjusting for all other patient and hospital characteristics and clustering by hospital, the following variables were associated with greater odds of transfer: older age, male sex, nonblack race, non-Medicaid co-insurance, higher comorbidity (HCC score), lower DRG-weight, and fewer hospitalizations in the prior 12 months. Beneficiaries also had greater odds of transfer if initially hospitalized at smaller hospitals, nonteaching hospitals, public hospitals, at hospitals in the Northeast, those with fewer specialty services, and those with a low CMI (Table 2).
DISCUSSION
In this nationally representative study of 6.6 million Medicare beneficiaries, we found that 1.5% of patients were transferred between acute care facilities and were most often transferred prior to hospital day 6. Older age, male sex, nonblack race, higher medical comorbidity, lower DRG weight, and fewer recent hospitalizations were associated with greater odds of transfer. Initial hospitalization at smaller, nonteaching, public hospitals, with fewer specialty services were associated with greater odds of transfer, while higher CMI was associated with a lower odds of transfer. The most common comorbid conditions among transferred patients included CHF, renal failure, arrhythmia, and COPD; particularly notable was the very high prevalence of these conditions among transferred as compared with nontransferred patients. Importantly, we found significant variation in IHT by region and a large variation in transfer practices by hospital, with significant variability in transfer rates even after accounting for known patient and hospital characteristics.
Among our examined population, we found that a sizable number of patients undergo IHT—more than 100,000 per year. Primary diagnoses at time of transfer consist of common inpatient conditions, including AMI, CHF, sepsis, arrhythmia, and pneumonia. Limited prior data support our findings, with up to 50% of AMI patients reportedly undergoing IHT,3-5 and severe sepsis and respiratory illness reported as common diagnoses at transfer.11 Although knowledge of these primary diagnoses does not directly confer an understanding of reason for transfer, one can speculate based on our findings. For example, research demonstrates the majority of AMI patients who undergo IHT had further intervention, including stress testing, cardiac catheterization, and/or coronary artery bypass graft surgery.5,26 Thus, it is reasonable to presume that many of the beneficiaries
We additionally found that certain patient characteristics were associated with greater odds of transfer. Research suggests that transferred patients are “sicker” than nontransferred patients.1,11 Although our findings in part confirm these data, we paradoxically found that higher DRG-weight and 4 or more hospitalizations in the past year were actually associated with lower odds of transfer. In addition, the oldest patients in our cohort (85 years or older) were actually less likely to be transferred than their slightly younger counterparts (75 to 84 years). These variables may reflect extreme illness or frailty,27 and providers consciously (or subconsciously) may factor this in to their decision to transfer, considering a threshold past which transfer would confer more risk than benefit (eg, a patient may be “too sick” for transfer). Indeed, in a secondary analysis without hospital characteristics or comorbidities, and with fixed effects by hospital, we found the highest rates of IHT in patients in the middle 2 quartiles of DRG-weight, supporting this threshold hypothesis. It is also possible that patients with numerous hospitalizations may be less likely to be transferred because of familiarity and a strong sense of responsibility to continue to care for those patients (although we cannot confirm that those prior hospitalizations were all with the same index hospital).
It is also notable that odds of transfer differed by race, with black patients 17% less likely to undergo transfer compared to whites, similar to findings in other IHT studies.11 This finding, in combination with our demonstration that Medicaid patients also have lower odds of transfer, warrants further investigation to ensure the process of IHT does not bias against these populations, as with other well-documented health disparities.28-30
The hospital predictors of transfer were largely expected. However, interestingly, when we controlled for all other patient and hospital characteristics, regional variation persisted, with highest odds of transfer with hospitalization in the Northeast, indicating variability by region not explained by other factors, and findings supported by other limited data.31 This variability was further elucidated in our examination of change in variance estimates accounting for patient, then hospital, characteristics. Although we expected and found marked variability in hospital transfer rates in our null model (without accounting for any patient or hospital characteristics), we interestingly found that variability increased upon adjusting for patient characteristics. This result is presumably due to the fact that patients who are more likely to be transferred (ie, “sick” patients) are more often already at hospitals less likely to transfer patients, supported by our findings that hospital CMI is inversely associated with odds of transfer (in other words, hospitals that care for a less sick patient population are more likely to transfer their patients, and hospitals that care for a sicker patient population [higher CMI] are less likely to transfer). Adjusting solely for patient characteristics effectively equalizes these patients across hospitals, which would lead to even increased variability in transfer rates. Conversely, when we then adjusted for hospital characteristics, variability in hospital transfer rates decreased by 83% (in other words, hospital characteristics, rather than patient characteristics, explained much of the variability in transfer rates), although significant unexplained variability remained. We should note that although the observed reduction in variability was explained by the patient and hospital characteristics included in the model, these characteristics do not necessarily justify the variability they accounted for; although patients’ race or hospitals’ location may explain some of the observed variability, this does not reasonably justify it.
This observed variability in transfer practices is not surprising given the absence of standardization and clear guidelines to direct clinical IHT practice.17 Selection of patients that may benefit from transfer is often ambiguous and subjective.6 The Emergency Medical Treatment and Active Labor Act laws dictate that hospitals transfer patients requiring a more specialized service, or when “medical benefits ... outweigh the increased risks to the individual...,” although in practice this provides little guidance to practitioners.1 Thus, clearer guidelines may be necessary to achieve less variable practices.
Our study is subject to several limitations. First, although nationally representative, the Medicare population is not reflective of all hospitalized patients nationwide. Additionally, we excluded patients transferred from the emergency room. Thus, the total number of patients who undergo IHT nationally is expected to be much higher than reflected in our analysis. We also excluded patients who were transferred more than once during a given hospitalization. This enabled us to focus on the initial transfer decision but does not allow us to look at patients who are transferred to a referral center and then transferred back. Second, given the criteria we used to define transfer, it is possible that we included nontransferred patients within our transferred cohort if they were discharged from one hospital and admitted to a different hospital within 1 day. However, on quality assurance analyses where we limited our cohort to only those beneficiaries with corresponding “transfer in” and “transfer out” claims (87% of the total cohort), we found no marked differences in our results. Additionally, although we assume that patient transfer status was coded correctly within the Medicare dataset, we could not confirm by individually examining each patient we defined as “transferred.” However, on additional quality assurance analyses where we examined randomly selected excluded patients with greater than 1 transfer during hospitalization, we found differing provider numbers with each transfer, suggesting validity of the coding. Third, because there are likely many unmeasured patient confounders, we cannot be sure how much of the between-hospital variation is due to incomplete adjustment for patient characteristics. However, since adjusting for patient characteristics actually increased variability in hospital transfer rates, it is unlikely that residual patient confounders fully explain our observed results. Despite this, other variables that are not available within the CMS or AHA datasets may further elucidate hospital transfer practices, including variables reflective of the transfer process (eg, time of day of patient transfer, time delay between initiation of transfer and patient arrival at accepting hospital, accepting service on transfer, etc.); other markers of illness severity (eg, clinical service at the time of index admission, acute physiology score, utilization of critical care services on arrival at receiving hospital); and other hospital system variables (ie, membership in an accountable care organization and/or regional care network, the density of nearby tertiary referral centers (indicating possible supply-induced demand), other variables reflective of the “transfer culture” (such as the transfer rate at the hospital or region where the attending physician trained, etc.). Lastly, though our examination provides important foundational information regarding IHT nationally, this study did not examine patient outcomes in transferred and nontransferred patients, which may help to determine which patients benefit (or do not benefit) from transfer and why. Further investigation is needed to study these outcomes.
CONCLUSION
In this national study of IHT, we found that a sizable number of patients admitted to the hospital undergo transfer to another acute care facility. Patients are transferred with common medical conditions, including those requiring specialized care such as AMI, and a high rate of comorbid clinical conditions, and certain patient and hospital characteristics are associated with greater odds of transfer. Although many of the observed associations between characteristics and odds of transfer were expected based on limited existing literature, we found several unexpected findings, eg, suggesting the possibility of a threshold beyond which sicker patients are not transferred. Additionally, we found that black and Medicaid patients had lower odds of transfer, which warrants further investigation for potential health care disparity. Importantly, we found much variability in the practice of IHT, as evidenced by the inexplicable differences in transfer by hospital region, and by residual unexplained variability in hospital transfer rates after accounting for patient and hospital characteristics, which may be due to lack of standard guidelines to direct IHT practices. In conclusion, this study of hospitalized Medicare patients provides important foundational information regarding rates and predictors of IHT nationally, as well as unexplained variability that exists within this complex care transition. Further investigation will be essential to understand reasons for, processes related to, and outcomes of transferred patients, to help guide standardization in best practices in care.
Disclosure
Nothing to report.
1. Iwashyna TJ. The incomplete infrastructure for interhospital patient transfer. Crit Care Med. 2012;40(8):2470-2478. PubMed
2. Iwashyna TJ, Christie JD, Moody J, Kahn JM, Asch DA. The structure of critical care transfer networks. Med Care. 2009;47(7):787-793. PubMed
3. Mehta RH, Stalhandske EJ, McCargar PA, Ruane TJ, Eagle KA. Elderly patients at highest risk with acute myocardial infarction are more frequently transferred from community hospitals to tertiary centers: reality or myth? Am Heart J. 1999;138(4 Pt 1):688-695. PubMed
4. Iwashyna TJ, Kahn JM, Hayward RA, Nallamothu BK. Interhospital transfers among Medicare beneficiaries admitted for acute myocardial infarction at nonrevascularization hospitals. Circ Cardiovasc Qual Outcomes. 2010;3(5):468-475. PubMed
5. Roe MT, Chen AY, Delong ER, Boden WE, Calvin JE Jr, Cairns CB, et al. Patterns of transfer for patients with non-ST-segment elevation acute coronary syndrome from community to tertiary care hospitals. Am Heart J. 2008;156(1):185-192. PubMed
6. Bosk EA, Veinot T, Iwashyna TJ. Which patients and where: a qualitative study of patient transfers from community hospitals. Med Care. 2011;49(6):592-598. PubMed
7. Wagner J, Iwashyna TJ, Kahn JM. Reasons underlying interhospital transfers to an academic medical intensive care unit. J Crit Care. 2013;28(2):202-208. PubMed
8. Cohen MD, Hilligoss PB. The published literature on handoffs in hospitals: deficiencies identified in an extensive review. Qual Saf Health Care. 2010;19(6):493-497. PubMed
9. Riesenberg LA, Leitzsch J, Massucci JL, et al. Residents’ and attending physicians’ handoffs: a systematic review of the literature. Acad Med. 2009;84(12):1775-1787. PubMed
10. Arora V, Johnson J, Lovinger D, Humphrey HJ, Meltzer DO. Communication failures in patient sign-out and suggestions for improvement: a critical incident analysis. Qual Saf Health Care. 2005;14(6):401-407. PubMed
11. Sokol-Hessner L, White AA, Davis KF, Herzig SJ, Hohmann SF. Interhospital transfer patients discharged by academic hospitalists and general internists: characteristics and outcomes. J Hosp Med. 2016;11(4):245-250. PubMed
12. Bernard AM, Hayward RA, Rosevear J, Chun H, McMahon LF. Comparing the hospitalizations of transfer and non-transfer patients in an academic medical center. Acad Med. 1996;71(3):262-266. PubMed
13. Golestanian E, Scruggs JE, Gangnon RE, Mak RP, Wood KE. Effect of interhospital transfer on resource utilization and outcomes at a tertiary care referral center. Crit Care Med. 2007;35(6):1470-1476. PubMed
14. Durairaj L, Will JG, Torner JC, Doebbeling BN. Prognostic factors for mortality following interhospital transfers to the medical intensive care unit of a tertiary referral center. Crit Care Med. 2003;31(7):1981-1986. PubMed
15. Kerr HD, Byrd JC. Community hospital transfers to a VA Medical Center. JAMA. 1989;262(1):70-73. PubMed
16. Dragsted L, Jörgensen J, Jensen NH, et al. Interhospital comparisons of patient outcome from intensive care: importance of lead-time bias. Crit Care Med. 1989;17(5):418-422. PubMed
17. Gupta K, Mueller SK. Interhospital transfers: the need for standards. J Hosp Med. 2015;10(6):415-417. PubMed
18. The Dartmouth Atlas of Health Care: Understanding of the Efficiency and Effectiveness of the Health Care System. The Dartmouth Institute for Health Practice and Clinical Policy, Lebanon, NH. http://www.dartmouthatlas.org/. Accessed November 1, 2016.
19. American Hospital Association Annual Survey Database. American Hospital Association, Chicago, IL. http://www.ahadataviewer.com/book-cd-products/AHA-Survey/. Accessed July 1, 2013.
20. U.S. Department of Health and Human Services (HRSA): What are critical access hospitals (CAH)? http://www.hrsa.gov/healthit/toolbox/RuralHealthITtoolbox/Introduction/critical.html. Accessed June 9, 2016.
21. Li P, Kim MM, Doshi JA. Comparison of the performance of the CMS Hierarchical Condition Category (CMS-HCC) risk adjuster with the Charlson and Elixhauser comorbidity measures in predicting mortality. BMC Health Serv Res. 2010;10:245. PubMed
22. Hernandez-Boussard T, Davies S, McDonald K, Wang NE. Interhospital facility transfers in the United States: a nationwide outcomes study. J Patient Saf. Nov 13 2014. PubMed
23. Landon BE, Normand SL, Lessler A, et al. Quality of care for the treatment of acute medical conditions in US hospitals. Arch Intern Med. 2006;166(22):2511-2517. PubMed
24. Mueller SK, Lipsitz S, Hicks LS. Impact of hospital teaching intensity on quality of care and patient outcomes. Med Care.2013;51(7):567-574. PubMed
25. Lopez L, Hicks LS, Cohen AP, McKean S, Weissman JS. Hospitalists and the quality of care in hospitals. Arch Intern Med. 2009;169(15):1389-1394. PubMed
26. Barreto-Filho JA, Wang Y, Rathore SS, et al. Transfer rates from nonprocedure hospitals after initial admission and outcomes among elderly patients with acute myocardial infarction. JAMA Intern Med. 2014;174(2):213-222. PubMed
27. Carlson JE, Zocchi KA, Bettencourt DM, et al. Measuring frailty in the hospitalized elderly: concept of functional homeostasis. Am J Phys Med Rehabil. 1998;77(3):252-257. PubMed
28. Ward E, Jemal A, Cokkinides V, et al. Cancer disparities by race/ethnicity and socioeconomic status. CA Cancer J Clin. 2004;54(2):78-93. PubMed
29. Iribarren C, Tolstykh I, Somkin CP, et al. Sex and racial/ethnic disparities in outcomes after acute myocardial infarction: a cohort study among members of a large integrated health care delivery system in northern California. Arch Intern Med. 2005;165(18):2105-2113. PubMed
30. Kawachi I, Daniels N, Robinson DE. Health disparities by race and class: why both matter. Health Aff (Millwood). 2005;24(2):343-352. PubMed
31. Herrigel DJ, Carroll M, Fanning C, Steinberg MB, Parikh A, Usher M. Interhospital transfer handoff practices among US tertiary care centers: a descriptive survey. J Hosp Med. 2016;11(6):413-417. PubMed
1. Iwashyna TJ. The incomplete infrastructure for interhospital patient transfer. Crit Care Med. 2012;40(8):2470-2478. PubMed
2. Iwashyna TJ, Christie JD, Moody J, Kahn JM, Asch DA. The structure of critical care transfer networks. Med Care. 2009;47(7):787-793. PubMed
3. Mehta RH, Stalhandske EJ, McCargar PA, Ruane TJ, Eagle KA. Elderly patients at highest risk with acute myocardial infarction are more frequently transferred from community hospitals to tertiary centers: reality or myth? Am Heart J. 1999;138(4 Pt 1):688-695. PubMed
4. Iwashyna TJ, Kahn JM, Hayward RA, Nallamothu BK. Interhospital transfers among Medicare beneficiaries admitted for acute myocardial infarction at nonrevascularization hospitals. Circ Cardiovasc Qual Outcomes. 2010;3(5):468-475. PubMed
5. Roe MT, Chen AY, Delong ER, Boden WE, Calvin JE Jr, Cairns CB, et al. Patterns of transfer for patients with non-ST-segment elevation acute coronary syndrome from community to tertiary care hospitals. Am Heart J. 2008;156(1):185-192. PubMed
6. Bosk EA, Veinot T, Iwashyna TJ. Which patients and where: a qualitative study of patient transfers from community hospitals. Med Care. 2011;49(6):592-598. PubMed
7. Wagner J, Iwashyna TJ, Kahn JM. Reasons underlying interhospital transfers to an academic medical intensive care unit. J Crit Care. 2013;28(2):202-208. PubMed
8. Cohen MD, Hilligoss PB. The published literature on handoffs in hospitals: deficiencies identified in an extensive review. Qual Saf Health Care. 2010;19(6):493-497. PubMed
9. Riesenberg LA, Leitzsch J, Massucci JL, et al. Residents’ and attending physicians’ handoffs: a systematic review of the literature. Acad Med. 2009;84(12):1775-1787. PubMed
10. Arora V, Johnson J, Lovinger D, Humphrey HJ, Meltzer DO. Communication failures in patient sign-out and suggestions for improvement: a critical incident analysis. Qual Saf Health Care. 2005;14(6):401-407. PubMed
11. Sokol-Hessner L, White AA, Davis KF, Herzig SJ, Hohmann SF. Interhospital transfer patients discharged by academic hospitalists and general internists: characteristics and outcomes. J Hosp Med. 2016;11(4):245-250. PubMed
12. Bernard AM, Hayward RA, Rosevear J, Chun H, McMahon LF. Comparing the hospitalizations of transfer and non-transfer patients in an academic medical center. Acad Med. 1996;71(3):262-266. PubMed
13. Golestanian E, Scruggs JE, Gangnon RE, Mak RP, Wood KE. Effect of interhospital transfer on resource utilization and outcomes at a tertiary care referral center. Crit Care Med. 2007;35(6):1470-1476. PubMed
14. Durairaj L, Will JG, Torner JC, Doebbeling BN. Prognostic factors for mortality following interhospital transfers to the medical intensive care unit of a tertiary referral center. Crit Care Med. 2003;31(7):1981-1986. PubMed
15. Kerr HD, Byrd JC. Community hospital transfers to a VA Medical Center. JAMA. 1989;262(1):70-73. PubMed
16. Dragsted L, Jörgensen J, Jensen NH, et al. Interhospital comparisons of patient outcome from intensive care: importance of lead-time bias. Crit Care Med. 1989;17(5):418-422. PubMed
17. Gupta K, Mueller SK. Interhospital transfers: the need for standards. J Hosp Med. 2015;10(6):415-417. PubMed
18. The Dartmouth Atlas of Health Care: Understanding of the Efficiency and Effectiveness of the Health Care System. The Dartmouth Institute for Health Practice and Clinical Policy, Lebanon, NH. http://www.dartmouthatlas.org/. Accessed November 1, 2016.
19. American Hospital Association Annual Survey Database. American Hospital Association, Chicago, IL. http://www.ahadataviewer.com/book-cd-products/AHA-Survey/. Accessed July 1, 2013.
20. U.S. Department of Health and Human Services (HRSA): What are critical access hospitals (CAH)? http://www.hrsa.gov/healthit/toolbox/RuralHealthITtoolbox/Introduction/critical.html. Accessed June 9, 2016.
21. Li P, Kim MM, Doshi JA. Comparison of the performance of the CMS Hierarchical Condition Category (CMS-HCC) risk adjuster with the Charlson and Elixhauser comorbidity measures in predicting mortality. BMC Health Serv Res. 2010;10:245. PubMed
22. Hernandez-Boussard T, Davies S, McDonald K, Wang NE. Interhospital facility transfers in the United States: a nationwide outcomes study. J Patient Saf. Nov 13 2014. PubMed
23. Landon BE, Normand SL, Lessler A, et al. Quality of care for the treatment of acute medical conditions in US hospitals. Arch Intern Med. 2006;166(22):2511-2517. PubMed
24. Mueller SK, Lipsitz S, Hicks LS. Impact of hospital teaching intensity on quality of care and patient outcomes. Med Care.2013;51(7):567-574. PubMed
25. Lopez L, Hicks LS, Cohen AP, McKean S, Weissman JS. Hospitalists and the quality of care in hospitals. Arch Intern Med. 2009;169(15):1389-1394. PubMed
26. Barreto-Filho JA, Wang Y, Rathore SS, et al. Transfer rates from nonprocedure hospitals after initial admission and outcomes among elderly patients with acute myocardial infarction. JAMA Intern Med. 2014;174(2):213-222. PubMed
27. Carlson JE, Zocchi KA, Bettencourt DM, et al. Measuring frailty in the hospitalized elderly: concept of functional homeostasis. Am J Phys Med Rehabil. 1998;77(3):252-257. PubMed
28. Ward E, Jemal A, Cokkinides V, et al. Cancer disparities by race/ethnicity and socioeconomic status. CA Cancer J Clin. 2004;54(2):78-93. PubMed
29. Iribarren C, Tolstykh I, Somkin CP, et al. Sex and racial/ethnic disparities in outcomes after acute myocardial infarction: a cohort study among members of a large integrated health care delivery system in northern California. Arch Intern Med. 2005;165(18):2105-2113. PubMed
30. Kawachi I, Daniels N, Robinson DE. Health disparities by race and class: why both matter. Health Aff (Millwood). 2005;24(2):343-352. PubMed
31. Herrigel DJ, Carroll M, Fanning C, Steinberg MB, Parikh A, Usher M. Interhospital transfer handoff practices among US tertiary care centers: a descriptive survey. J Hosp Med. 2016;11(6):413-417. PubMed
© 2017 Society of Hospital Medicine
Regionalized Care and Adverse Events
Failures in communication among healthcare professionals are known threats to patient safety. These failures account for over 60% of root causes of sentinel events, the most serious events reported to The Joint Commission.[1] As such, identifying both patterns of effective communication as well as barriers to successful communication has been a focus of efforts aimed at improving patient safety. However, to date, the majority of this work has centered on improving communication in settings such as the operating room and intensive care unit,[2, 3, 4] or at times of care transitions.[5, 6, 7, 8]
Unique barriers exist for effective interdisciplinary communication in the hospital setting, particularly physiciannurse communication regarding shared hospitalized patients.[9] Traditionally, care of hospitalized patients is provided by physicians, nurses, and other team members working in varied workflow patterns, leading to dispersed team membership, where each team member cares for different groups of patients in different locations across the hospital. This dispersion is further heightened on teaching services, where residents' rotation schedules lead to frequent changes of care team membership, leaving inpatient care teams particularly vulnerable to ineffective communication. Evidence suggests that communication between nurses and physicians is currently suboptimal, leading to frequent disagreement regarding the patient's plan of care.[9, 10] This divergence between physician and nursing perceptions of patients' care plans may leave patients at greater risk of adverse events (AEs).
Several studies have examined the effects of regionalized inpatient care teams, where multidisciplinary team members care for the same patients on the same hospital unit, on communication and patient outcomes.[4, 11, 12, 13, 14] Results of these studies have been inconsistent, perhaps due to the particular characteristics of the care teams or to the study methodology. Thus, further rigorously done studies are required to better understand the impact of team regionalization on patient care. The goal of this study was to examine whether the implementation of regionalized inpatient care teams was associated with improvements in care team communication and preventable AEs.
METHODS
Setting, Patients, and Study Design
We performed a cohort analysis of patients at a 700‐bed tertiary care center, pre‐ and postregionalization of inpatient general medicine care teams. Our study protocol was approved by the Partners Healthcare Human Subjects Review Committee. Patients were eligible for inclusion if they were 18 years of age or older and discharged from the general medicine service (GMS) from any of the 3 participating nursing units between April 1, 2012 and June 19, 2012 (preregionalization) or April 1, 2013 and June 19, 2013 (postregionalization).
Intervention
On June 20, 2012, regionalized care was implemented on the GMS such that each of 3 GMS teams was localized to 1 of 3, 15‐bed nursing units. Prior to regionalization, the GMS physician care teams, each consisting of 1 hospitalist attending, 1 medical resident, and 2 medical interns, would care for patients on an average of 7 and up to 13 different nursing units on a given day.
Regionalized care consisted of a multifaceted intervention codeveloped by hospitalist, residency, nursing, emergency department, and hospital leadership and included: (1) regionalizing GMS teams as much as possible; (2) change in resident call structure from a traditional 4‐day call cycle to daily admitting; (3) collaborative efforts to enhance GMS patient discharges before noon to promote regionalized placement of patients without prolonging time in the emergency department (ED); (4) daily morning and postround multidisciplinary huddles to prioritize sicker patients and discharges; (5) encouragement of daily rounds at patients' bedsides with presence of physician team, nurse, and team pharmacist if available; (6) creation of unit‐ and team‐level performance reports; and (7) creation of unit‐based physician and nursing co‐leadership (Figure 1).[15]
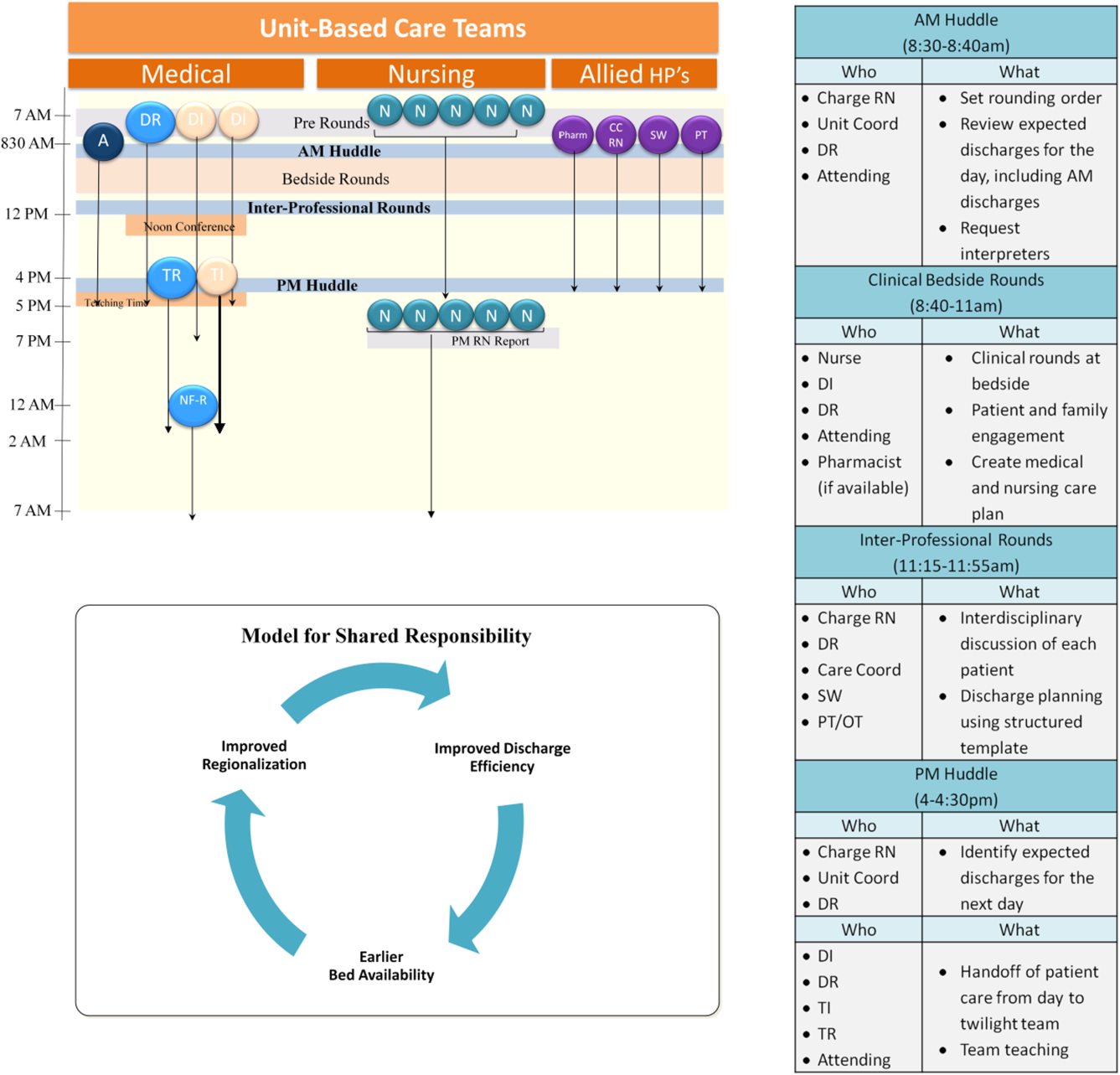
Concordance of Plan
Concordance of plan was measured via a 7‐question survey previously developed, pilot tested, and used to measure the impact of regionalized care on care team communication between inpatient nursephysician team members.[9] The survey was administered in‐person by 1 of 8 trained research assistants (RAs) (4/emntervention period) to nurse and intern pairs caring for patients on the study units pre‐ and postregionalization. GMS patients were eligible for inclusion if surveys could be administered to their nurse and intern within the first 24 hours of admission to the unit and within 48 hours of admission to the hospital, based on RA availability (thus excluding patients admitted on Fridays as surveys were not conducted over the weekend). Most often, all eligible patients admitted to the study units during time periods of data collection were included in the study. On limited occasions, the daily supply of patients surpassed RA capacity for inclusion, at which time computer‐generated randomization was utilized to randomly select patients for inclusion. Nurse and intern pairs were surveyed once during a patient's hospitalization, although they could be surveyed more than once about different patients, and patients could be included more than once if rehospitalized on the study unit and cared for by a different nurseintern pair. Of the 472 selected eligible patients, the nurses and interns of 418 patients were available and consented to survey administration, representing 361 unique nurse and intern pairs and 399 unique patients.
Each member of the pair was asked about 7 specific aspects of the patient's care plan for that day in isolation from the other team member, including: (1) the patient's primary diagnosis, (2) the patient's expressed chief concern, (3) the day's scheduled tests, (4) the day's scheduled procedures, (5) consulting services involved, (6) medication changes made that day, and (7) the patient's expected discharge date. In addition, each pair was asked the name of the other team member (ie, the nurse was asked the name of the intern and vice versa), and whether or not the patient care plan for the day had been discussed with the other team member, where concordance was defined as both members agreeing the plan had been discussed. All responses were recorded verbatim. Pairs were surveyed independently between 12 pm and 2 pm, limiting confounding by evolving plans of care over time.
Each set of surveys were then reviewed by 2 of 4 trained adjudicators, and responses to each question were scored as complete, partial, or no agreement. Rules for degree of agreement were based upon previously utilized parameters[9] as well as biweekly meetings during which common themes and disagreements in ratings were discussed, and rules generated to create consensus (see Supporting Information, Appendix, in the online version of this article).
Adverse Event Detection
Of the patients meeting eligibility criteria, 200 patients were randomly selected using computer‐generated randomization from each time period for AE outcome assessment, for a total of 400 patients.
Each patient's electronic medical record was retrospectively reviewed by a trained clinician using a previously validated screening tool to detect any possible AEs.[11] Any positive screen prompted documentation of a narrative summary including a short description of the possible AE and pertinent associated data. We defined AE as any injury due to medical management rather than the natural history of the illness, and further limited this definition to only include AEs that occurred on the study unit or as a result of care on that unit.
Two of 4 trained adjudicators, blinded to time period, then separately reviewed each narrative summary using previously validated 6‐point confidence scales to determine the presence and preventability of AE, with confidence ratings of 4 or greater used as cutoffs.[11] All AEs were also scored on a 4‐point severity scale (trivial, clinically significant, serious, or life threatening), with severe AE defined as serious or life threatening. Lastly, adjudicators grouped AEs into 1 of 10 prespecified categories.[11] Any disagreements in ratings or groupings were discussed by all 4 adjudicators to reach consensus.
Data Analysis
Patient characteristics are presented using descriptive statistics and were compared in the pre‐ and postregionalization time periods using 2 or t tests as appropriate.
To analyze whether regionalized care was associated with concordance of plan, adjudicated survey questions were assigned points of 1, 0.5, and 0 for complete, partial, and no agreement, respectively. Total mean concordance scores for any patient ranged from 0 to 7 points, and were divided by total number of answered questions (up to 7) for a range of 0 to 1. Total mean concordance scores as well as mean concordance score per survey question were compared pre‐ versus postregionalization using t tests. In sensitivity analyses, adjudicated survey responses were dichotomized with complete and partial agreement deemed concordant responses. Percent concordance for each question was then compared pre‐ versus postregionalization using 2 analysis. Questions about the name of the other team member and discussion of daily care plan with the other team member were excluded from total concordance score calculations and were compared individually pre‐ versus postregionalization, because they are not directly about the plan of care.
To analyze the association of regionalization with odds of preventable AE, we performed multivariable logistic regression adjusted for patient age, sex, race, language, and Elixhauser comorbidity score,[16] and utilized generalized estimating equations to account for clustering by hospital unit. Secondary outcomes included severe preventable AEs, nonpreventable AEs, and category of preventable AEs using similar methodology. Two‐sided P values <0.05 were considered significant, and SAS version 9.2 (SAS Institute Inc., Cary, NC) was used for all analyses.
RESULTS
The fidelity of the intervention in achieving its goal of regionalized care is discussed separately.[15] Briefly, the intervention was successful at achieving 85% regionalization by team (ie, average daily percentage of team's patients assigned to team's unit) and 87% regionalization by unit (ie, average daily percentage of unit's patients with assigned team) following implementation, compared to 20% regionalization by team and unit in the preintervention period. Importantly, the average daily census of physician care teams rose by 32%, from a mean of 10.8 patients/physician care team preregionalization to a mean of 14.3 patients/physician care team postregionalization.
Concordance of Plan
Of the 418 nurse and intern paired surveys, 4 surveys were excluded due to repeat surveys of the same patient during the same hospitalization, for a total of 197 distinct paired surveys preregionalization and 217 paired surveys postregionalization. There were no statistically significant differences in patients' age, sex, race, language, admission source, length of stay, Elixhauser comorbidity score and diagnosis‐related group weight pre‐ versus postregionalization (Table 1).
| Characteristic | Concordance of Care Plan | Adverse Events | ||||
|---|---|---|---|---|---|---|
| Pre, n = 197 | Post, n = 217 | P Value | Pre, n = 198 | Post, n = 194 | P Value | |
| ||||||
| Age, mean (SD) | 60.5 (19.4) | 57.6 (20.8) | 0.15 | 60.4 (18.9) | 58.0 (21.2) | 0.24 |
| Male, n (%) | 77 (39.1) | 92 (42.4) | 0.49 | 94 (47.5) | 85 (43.8) | 0.55 |
| Race/ethnicity, n (%) | 0.34 | 0.12 | ||||
| White | 134 (68.0) | 141 (65.0) | 132 (66.5) | 121 (62.4) | ||
| Black | 42 (21.3) | 45 (20.7) | 41 (20.8) | 54 (27.8) | ||
| Hispanic | 18 (9.1) | 21 (9.7) | 22 (11.3) | 13 (6.8) | ||
| Other/unknown | 3 (1.5) | 10 (4.6) | 3 (1.4) | 6 (2.9) | ||
| Language, n (%) | 0.30 | 0.73 | ||||
| English | 183 (92.9) | 203 (93.5) | 176 (88.7) | 175 (90.2) | ||
| Spanish | 6 (3.0) | 10 (4.6) | 10 (5.2) | 10 (5.3) | ||
| Other | 8 (4.1) | 4 (1.8) | 12 (6.1) | 9 (4.5) | ||
| Admitting source, n (%) | 1.00 | 0.10 | ||||
| Physician office | 13 (6.6) | 13 (6.0) | 13 (6.6) | 6 (3.1) | ||
| Emergency department | 136 (69.0) | 150 (69.1) | 126 (63.6) | 127 (65.5) | ||
| Transfer from different hospital | 40 (20.3) | 45 (20.7) | 54 (27.3) | 50 (25.8) | ||
| Transfer from skilled nursing facility | 8 (4.1) | 9 (4.2) | 5 (2.5) | 11 (5.6) | ||
| Length of stay, d, median (IQR) | 3.0 (4.0) | 3.0 (4.0) | 0.57 | 4.0 (5.0) | 3.0 (4.0) | 0.16 |
| Elixhauser Comorbidity Score, mean (SD) | 8.0 (8.8) | 8.3 (9.3) | 0.74 | 8.0 (8.6) | 7.8 (8.4) | 0.86 |
| DRG weight, mean (SD) | 1.6 (1.0) | 1.5 (1.0) | 0.37 | 1.5 (0.93) | 1.5 (1.1) | 0.96 |
Kappa scores for adjudications of concordance surveys (defined as both adjudicators scoring the same level of agreement (ie, both complete or partial agreement versus no agreement) ranged from 0.69 to 0.95, by question. There were no significant differences in total mean concordance scores in the care plan pre‐ versus postregionalization (0.65 vs 0.67, P = 0.26) (Table 2). Similarly, there were no significant differences in mean concordance score for each survey question, except agreement on expected date of discharge (0.56 vs 0.68, P = 0.003), knowledge of the other provider's name, and agreement that discussion of the daily plan had taken place with the other pair member. Similar results were seen when results were dichotomized (ie, partial or complete agreement vs no agreement) (Table 2).
| Concordance Outcome | Pre, n = 197 | Post, n = 217 | P Value |
|---|---|---|---|
| |||
| Concordance score* | |||
| Total concordance score, mean (SD) | 0.65 (0.17) | 0.67 (0.16) | 0.26 |
| Subgroups | |||
| Diagnosis | 0.77 (0.32) | 0.72 (0.35) | 0.11 |
| Patient's chief concern | 0.48 (0.44) | 0.48 (0.43) | 0.94 |
| Tests today | 0.67 (0.40) | 0.71 (0.42) | 0.36 |
| Procedures today | 0.93 (0.25) | 0.92 (0.25) | 0.71 |
| Medication changes today | 0.56 (0.44) | 0.59 (0.43) | 0.54 |
| Consulting services | 0.59 (0.44) | 0.60 (0.44) | 0.82 |
| Expected discharge date | 0.56 (0.44) | 0.68 (0.38) | 0.003 |
| Responding clinician knowledge of nurse's name | 0.56 (0.50) | 0.86 (0.35) | <0.001 |
| Nurse's knowledge of responding clinician's name | 0.56 (0.50) | 0.88 (0.33) | <0.001 |
| Plan discussed | 0.73 (0.45) | 0.88 (0.32) | <0.001 |
| Percent concordance, mean (SD) | |||
| Diagnosis | 92.0 (27.3) | 88.6 (31.9) | 0.25 |
| Patient's chief concern | 59.6 (49.1) | 60.6 (49.0) | 0.84 |
| Tests today | 78.9 (40.9) | 77.2 (42.1) | 0.67 |
| Procedures today | 93.5 (24.8) | 94.1 (23.7) | 0.80 |
| Medication changes today | 66.3 (33.6) | 69.9 (46.0) | 0.44 |
| Consulting services | 69.3 (46.2) | 68.9 (46.4) | 0.93 |
| Expected discharge date | 67.5 (47.0) | 82.6 (38.0) | <0.001 |
| Responding clinician knowledge of nurse's name | 55.7 (49.8) | 85.6 (35.2) | <0.001 |
| Nurse's knowledge of responding clinician's name | 55.9 (49.8) | 87.9 (32.8) | <0.001 |
| Plan discussed | 72.9 (44.6) | 88.2 (32.3) | <0.001 |
Adverse Events
Of the 400 patients screened for AEs, 8 were excluded due to missing medical record number (5) and discharge outside of study period (3). Of the final 392 patient screens (198 pre, 194 post), there were no significant differences in patients' age, sex, race, language, length of stay, or Elixhauser score pre‐ versus postregionalization (Table 1).
Kappa scores for adjudicator agreement were 0.35 for presence of AE and 0.34 for preventability of AE. Of the 392 reviewed patient records, there were 133 total AEs detected (66 pre, 67 post), 27 preventable AEs (13 pre, 14 post), and 9 severe preventable AEs (4 pre, 5 post) (Table 3). There was no significant difference in the adjusted odds of preventable AEs post‐ versus preregionalization (adjusted odds ratio: 1.37, 95% confidence interval: 0.69, 2.69). Although the low number of AEs rated as severe or life threatening precluded adjusted analysis, unadjusted results similarly demonstrated no difference in odds of severe preventable AEs pre‐ versus postregionalization. As expected, there was no significant difference in adjusted odds of nonpreventable AE after implementation of regionalized care (Table 3).
| Adverse Events | No. of Adverse Events | Adjusted Odds Ratio Post vs Pre (95% CI) | |
|---|---|---|---|
| Pre, n = 198 | Post, n = 194 | ||
| |||
| Preventable | 13 | 14 | 1.37 (0.69, 2.69) |
| Serious and preventable | 4 | 5 | |
| Nonpreventable | 47 | 50 | 1.20 (0.85, 1.75) |
Similarly, there were no significant differences in category of preventable AE pre‐ versus postregionalization. The most frequent preventable AEs in both time periods were those related to adverse drug events and to manifestations of poor glycemic control, examples of which are illustrated (Table 4).
| |
| Adverse drug event | 29‐year‐old male with history of alcohol abuse, complicated by prior withdrawal seizures/emntensive care unit admissions, presented with alcohol withdrawal. Started on standing and PRN lorazepam, kept on home medications including standing clonidine, gabapentin, citalopram, quetiapine. Became somnolent due to polypharmacy, ultimately discontinued quetiapine as discovered took only as needed at home for insomnia |
| Manifestations of poor glycemic control | 78‐year‐old male with recently diagnosed lymphoma, distant history of bladder and prostate cancer status post ileal loop diversion, presented status post syncopal event; during event, spilled boiling water on himself leading to second‐degree burns on 3% of his body. Initially admitted to trauma/burn service, ultimately transferred to medical service for ongoing multiple medical issues including obstructive uropathy, acute on chronic renal failure. Adverse event was hyperglycemia (>350 mg/dL on >2 consecutive readings) in the setting of holding his home insulin detemir and insulin aspart (had been placed on insulin aspart sliding scale alone). After hyperglycemic episodes, was placed back on weight‐based basal/nutritional insulin |
DISCUSSION
In this study of general medicine patients at a large academic medical center, we found that regionalization of care teams on general medicine services was associated with improved recognition of care team members and agreement on estimated date of patient discharge, but was not associated with improvement in overall nurse and physician concordance of the patient care plan, or the odds of preventable AEs.
This intervention importantly addresses the barrier of dispersion of team membership, a well‐recognized barrier to interdisciplinary collaboration,[17, 18] particularly with resident physician teams due to frequently changing team membership. Localization of all team members, in addition to encouragement of daily collaborative bedside rounds as part of the regionalization initiative, likely contributed to our observed improvement in team member identification and discussion of daily care plans. Similarly, regionalization resulted in improved agreement in estimations of date of patient discharge. Focus on early patient discharges was an integral part of the implementation efforts; we therefore hypothesize that mutual focus on discharge planning by both nurses and responding clinicians may have explained this observed result.
On the other hand, regionalization did not appreciably improve the overall concordance of care plan between nurses and interns, despite a significant increase in team members agreeing that the plan had been discussed. Our findings support similar prior research demonstrating that regionalizing hospitalist attendings to single nursing units had limited impact on agreement of care plan between physicians and nurses.[13] Similarly, in settings where physicians and nurses are inherently regionalized, such as the intensive care unit[4] or the operating room,[3] communication between physicians and nurses remains difficult. Collectively, our findings suggest that colocalization of physicians and nurses alone is likely insufficient to improve measured communication between care team members. Existing literature suggests that more standardized approaches to improve communication, such as structured communication tools used during daily inpatient care[19, 20] or formalized team training,[21, 22, 23] lead to improvements in communication and collaboration. Despite these findings, it is important to highlight that this study did not assess other measures of workplace culture, such as teamwork and care team cohesiveness, which may have been positively affected by this intervention, even without measurable effect on concordance of care plan. Additionally, as noted, the average daily census on each team increased by almost a third postintervention, which may have impeded improvements in care team communication.
In addition, we found that our intervention had no significant impact on preventable AEs or severe preventable AEs. Although we cannot exclude the possibility that more subtle AEs were missed with our methodology, our results indicate that regionalized care alone may be inadequate to improve major patient safety outcomes. As discussed, the volume of patients did increase postintervention; thus, another way to state our results is that we were able to increase the daily volume of patients without any significant decreases in patient safety. Nevertheless, the results on patient safety were less than desired. A recent review of interdisciplinary team care interventions on general medical wards similarly demonstrated underwhelming improvements in patient safety outcomes, although the reviewed interventions did not specifically address preventable AEs, a gap in the literature commented on by the authors.[24] Other albeit limited literature has demonstrated improvement in patient safety outcomes via multifaceted efforts aimed at improving care team member communication. Notably, these efforts include colocalization of care team members to single units but also involve additional measures to improve communication and collaboration between care team members, such as structured communication during interdisciplinary rounds, and certification of key interdisciplinary teamwork skills.[11, 14] Although our regionalized care intervention included many similar features to these accountable care units (ACUs) including unit‐based care teams, unit‐level performance reporting, and unit‐based physician and nursing coleadership, significant differences existed. Notably, in addition to the above features, the ACU model also incorporated highly structured communication models for interdisciplinary rounding, and certification processes to ensure an appropriate communication skill base among care team members.[14] Thus, although creation of regionalized care teams is likely a necessary precursor to implementation of these additional measures, alone it may be insufficient to improve patient safety outcomes.
Importantly, in our study we identified that adverse drug events and manifestations of poor glycemic control occurred in high frequency both before and following implementation of regionalized care, supporting other literature that describes the prevalence of these AEs.[11, 25, 26, 27] These results suggest that targeted interventions to address these specific AEs are likely necessary. Notably, the intervention units in our study did not consistently employ clinical pharmacists assigned specifically to that unit's care team to allow for integration within the care team. As prior research has suggested that greater collaboration with clinical pharmacists results in reduction of adverse drug events,[28] next steps may include improved integration of team‐based pharmacists into the activities of the regionalized care teams. Inpatient management of diabetes also requires specific interventions,[29, 30, 31] only some of which may be addressable by having regionalized care and better interdisciplinary communication.
Our findings are subject to several limitations. First, this was a single‐site study and thus our findings may not be generalizable to other institutions. However, regionalized care is increasingly encouraged to optimize communication between care team members.[17, 18] Therefore, our null findings may be pertinent to other institutions looking to improve patient safety outcomes, demonstrating that additional initiatives will likely be required. Second, our modes of outcome measurement possess limitations. In measuring concordance of care plan, although previously used survey techniques were employed,[9] the concordance survey has not been formally validated, and we believe some of the questions may have led to ambiguity on the part of the responders that may have resulted in less accurate responses, thus biasing toward the null. Similarly, in measuring AEs, the screening tool relied on retrospective chart review looking for specific AE types[11] and thus may not have captured more subtle AEs. Additionally, our study may have been underpowered to demonstrate significant reduction in preventable AEs, although other studies of similar methodology demonstrated significant results with similar sample size.[11] This was due in part to our lower‐than‐expected baseline AE rate (6.6% compared with approximately 10.3% in previous studies).[11] Lastly, our study solely examined the association of regionalization with concordance of care plan and preventable AEs, but importantly excluded other clinically important outcomes that may have been positively (or negatively) impacted by these regionalization efforts, such as ED wait times, provider efficiency (eg, fewer pages, less time in transit, more time at the bedside), interdisciplinary teamwork, or patient or provider satisfaction.
CONCLUSION
In summary, our findings suggest that regionalized care teams alone may be insufficient to effectively promote communication between care team members regarding the care plan or to lead to improvements in patient safety, although we recognize that there may have been benefits (or unintended harms) not measured in this study but are nonetheless important for clinical care and workplace culture. This is an important lesson, as many hospitals move toward regionalized care in an effort to improve patient safety outcomes. However, strengthening the infrastructure by colocalizing care team members to maximize opportunity for communication is likely a necessary first step toward facilitating implementation of additional initiatives that may lead to more robust patient safety improvements, such as structured interdisciplinary bedside rounds (eg, facilitating and training all team members to fulfill specific roles), teamwork training, and certification of key interdisciplinary teamwork skills. Additionally, close examination of identified prevalent and preventable AEs can help to determine which additional initiatives are most likely to have greatest impact in improving patient safety.
Disclosures: This research was supported by funds provided by Brigham and Women's Hospital (BWH) and by funds provided by the Department of Medicine at BWH. All authors had full access to all of the data in the study and were integrally involved in the design, implementation, data collection, and analyses. The first author, Dr. Stephanie Mueller, takes responsibility for the integrity for the data and the accuracy of the data analysis. Dr. Schnipper reports grants from Sanofi Aventis, outside the submitted work.
- Joint Commission on Accreditation of Healthcare Organizations. Understanding and Preventing Sentinel Events in Your Health Care Organization. Oak Brook, IL: Joint Commission; 2008.
- , , , et al. Communication failures in the operating room: an observational classification of recurrent types and effects. Qual Saf Health Care. 2004;13(5):330–334.
- , , , et al. Operating room teamwork among physicians and nurses: teamwork in the eye of the beholder. J Am Coll Surg. 2006;202(5):746–752.
- , , . Discrepant attitudes about teamwork among critical care nurses and physicians. Crit Care Med. 2003;31(3):956–959.
- , , , , . Communication failures in patient sign‐out and suggestions for improvement: a critical incident analysis. Qual Saf Health Care. 2005;14(6):401–407.
- , , , et al. Changes in medical errors after implementation of a handoff program. N Engl J Med. 2014;371(19):1803–1812.
- , , , et al. Communication and information deficits in patients discharged to rehabilitation facilities: an evaluation of five acute care hospitals. J Hosp Med. 2009;4(8):E28–E33.
- , , , , , . Deficits in communication and information transfer between hospital‐based and primary care physicians: implications for patient safety and continuity of care. JAMA. 2007;297(8):831–841.
- , , , et al. Patterns of nurse‐physician communication and agreement on the plan of care. Qual Saf Health Care. 2010;19(3):195–199.
- , , , , , . Can we talk? Priorities for patient care differed among health care providers. In: Henriksen K, Battles JB, Marks ES, Lewin DI, eds. Advances in Patient Safety: From Research to Implementation. Vol 1. Rockville, MD: Agency for Healthcare Research and Quality; 2005.
- , , , et al. Structured interdisciplinary rounds in a medical teaching unit: improving patient safety. Arch Intern Med. 2011;171(7):678–684.
- , , , , , . Improving teamwork: impact of structured interdisciplinary rounds on a hospitalist unit. J Hosp Med. 2011;6(2):88–93.
- , , , et al. Impact of localizing physicians to hospital units on nurse‐physician communication and agreement on the plan of care. J Gen Intern Med. 2009;24(11):1223–1227.
- , , , et al. Reorganizing a hospital ward as an accountable care unit. J Hosp Med. 2015;10(1):36–40.
- , , , et al. 5th time's a charm: creation of unit‐based care teams in a high occupancy hospital [abstract]. J Hosp Med. 2015;10 (suppl. 2). Available at: http://www.shmabstracts.com/abstract/5th‐times‐a‐charm‐creation‐of‐unit‐based‐care‐teams‐in‐a‐high‐occupancy‐hospital. Accessed July 28, 2015.
- , , , . Comorbidity measures for use with administrative data. Med Care. 1998;36(1):8–27.
- , , , , , . Teamwork on inpatient medical units: assessing attitudes and barriers. Qual Saf Health Care. 2010;19(2):117–121.
- , , , et al. A model for quality improvement programs in academic departments of medicine. Am J Med. 2008;121(10):922–929.
- , , , , . Improving nurse‐physician communication and satisfaction in the intensive care unit with a daily goals worksheet. Am J Crit Care. 2006;15(2):217–222.
- , , , , , . Improving communication in the ICU using daily goals. J Crit Care. 2003;18(2):71–75.
- , , , et al. Effect of crew resource management training in a multidisciplinary obstetrical setting. Int J Qual Health Care. 2008;20(4):254–263.
- , , , et al. Error reduction and performance improvement in the emergency department through formal teamwork training: evaluation results of the MedTeams project. Health Serv Res. 2002;37(6):1553–1581.
- , , , et al. Effects of teamwork training on adverse outcomes and process of care in labor and delivery: a randomized controlled trial. Obstet Gynecol. 2007;109(1):48–55.
- , , , et al. Effects of interdisciplinary team care interventions on general medical wards: a systematic review. JAMA Intern Med. 2015;175(8):1288–1298.
- , , , et al. Patient risk factors for adverse drug events in hospitalized patients. ADE Prevention Study Group. Arch Intern Med. 1999;159(21):2553–2560.
- , , , et al. Effect of a pharmacist intervention on clinically important medication errors after hospital discharge: a randomized trial. Ann Intern Med. 2012;157(1):1–10.
- , , , . Use of a standardized protocol to decrease medication errors and adverse events related to sliding scale insulin. Qual Saf Health Care. 2006;15(2):89–91.
- , , , . Clinical pharmacists and inpatient medical care: a systematic review. Arch Intern Med. 2006;166(9):955–964.
- , , , , . Improved inpatient use of basal insulin, reduced hypoglycemia, and improved glycemic control: effect of structured subcutaneous insulin orders and an insulin management algorithm. J Hosp Med. 2009;4(1):3–15.
- , , , . Effects of a computerized order set on the inpatient management of hyperglycemia: a cluster‐randomized controlled trial. Endocr Pract. 2010;16(2):209–218.
- , , , . Effects of a subcutaneous insulin protocol, clinical education, and computerized order set on the quality of inpatient management of hyperglycemia: results of a clinical trial. J Hosp Med. 2009;4(1):16–27.
Failures in communication among healthcare professionals are known threats to patient safety. These failures account for over 60% of root causes of sentinel events, the most serious events reported to The Joint Commission.[1] As such, identifying both patterns of effective communication as well as barriers to successful communication has been a focus of efforts aimed at improving patient safety. However, to date, the majority of this work has centered on improving communication in settings such as the operating room and intensive care unit,[2, 3, 4] or at times of care transitions.[5, 6, 7, 8]
Unique barriers exist for effective interdisciplinary communication in the hospital setting, particularly physiciannurse communication regarding shared hospitalized patients.[9] Traditionally, care of hospitalized patients is provided by physicians, nurses, and other team members working in varied workflow patterns, leading to dispersed team membership, where each team member cares for different groups of patients in different locations across the hospital. This dispersion is further heightened on teaching services, where residents' rotation schedules lead to frequent changes of care team membership, leaving inpatient care teams particularly vulnerable to ineffective communication. Evidence suggests that communication between nurses and physicians is currently suboptimal, leading to frequent disagreement regarding the patient's plan of care.[9, 10] This divergence between physician and nursing perceptions of patients' care plans may leave patients at greater risk of adverse events (AEs).
Several studies have examined the effects of regionalized inpatient care teams, where multidisciplinary team members care for the same patients on the same hospital unit, on communication and patient outcomes.[4, 11, 12, 13, 14] Results of these studies have been inconsistent, perhaps due to the particular characteristics of the care teams or to the study methodology. Thus, further rigorously done studies are required to better understand the impact of team regionalization on patient care. The goal of this study was to examine whether the implementation of regionalized inpatient care teams was associated with improvements in care team communication and preventable AEs.
METHODS
Setting, Patients, and Study Design
We performed a cohort analysis of patients at a 700‐bed tertiary care center, pre‐ and postregionalization of inpatient general medicine care teams. Our study protocol was approved by the Partners Healthcare Human Subjects Review Committee. Patients were eligible for inclusion if they were 18 years of age or older and discharged from the general medicine service (GMS) from any of the 3 participating nursing units between April 1, 2012 and June 19, 2012 (preregionalization) or April 1, 2013 and June 19, 2013 (postregionalization).
Intervention
On June 20, 2012, regionalized care was implemented on the GMS such that each of 3 GMS teams was localized to 1 of 3, 15‐bed nursing units. Prior to regionalization, the GMS physician care teams, each consisting of 1 hospitalist attending, 1 medical resident, and 2 medical interns, would care for patients on an average of 7 and up to 13 different nursing units on a given day.
Regionalized care consisted of a multifaceted intervention codeveloped by hospitalist, residency, nursing, emergency department, and hospital leadership and included: (1) regionalizing GMS teams as much as possible; (2) change in resident call structure from a traditional 4‐day call cycle to daily admitting; (3) collaborative efforts to enhance GMS patient discharges before noon to promote regionalized placement of patients without prolonging time in the emergency department (ED); (4) daily morning and postround multidisciplinary huddles to prioritize sicker patients and discharges; (5) encouragement of daily rounds at patients' bedsides with presence of physician team, nurse, and team pharmacist if available; (6) creation of unit‐ and team‐level performance reports; and (7) creation of unit‐based physician and nursing co‐leadership (Figure 1).[15]

Concordance of Plan
Concordance of plan was measured via a 7‐question survey previously developed, pilot tested, and used to measure the impact of regionalized care on care team communication between inpatient nursephysician team members.[9] The survey was administered in‐person by 1 of 8 trained research assistants (RAs) (4/emntervention period) to nurse and intern pairs caring for patients on the study units pre‐ and postregionalization. GMS patients were eligible for inclusion if surveys could be administered to their nurse and intern within the first 24 hours of admission to the unit and within 48 hours of admission to the hospital, based on RA availability (thus excluding patients admitted on Fridays as surveys were not conducted over the weekend). Most often, all eligible patients admitted to the study units during time periods of data collection were included in the study. On limited occasions, the daily supply of patients surpassed RA capacity for inclusion, at which time computer‐generated randomization was utilized to randomly select patients for inclusion. Nurse and intern pairs were surveyed once during a patient's hospitalization, although they could be surveyed more than once about different patients, and patients could be included more than once if rehospitalized on the study unit and cared for by a different nurseintern pair. Of the 472 selected eligible patients, the nurses and interns of 418 patients were available and consented to survey administration, representing 361 unique nurse and intern pairs and 399 unique patients.
Each member of the pair was asked about 7 specific aspects of the patient's care plan for that day in isolation from the other team member, including: (1) the patient's primary diagnosis, (2) the patient's expressed chief concern, (3) the day's scheduled tests, (4) the day's scheduled procedures, (5) consulting services involved, (6) medication changes made that day, and (7) the patient's expected discharge date. In addition, each pair was asked the name of the other team member (ie, the nurse was asked the name of the intern and vice versa), and whether or not the patient care plan for the day had been discussed with the other team member, where concordance was defined as both members agreeing the plan had been discussed. All responses were recorded verbatim. Pairs were surveyed independently between 12 pm and 2 pm, limiting confounding by evolving plans of care over time.
Each set of surveys were then reviewed by 2 of 4 trained adjudicators, and responses to each question were scored as complete, partial, or no agreement. Rules for degree of agreement were based upon previously utilized parameters[9] as well as biweekly meetings during which common themes and disagreements in ratings were discussed, and rules generated to create consensus (see Supporting Information, Appendix, in the online version of this article).
Adverse Event Detection
Of the patients meeting eligibility criteria, 200 patients were randomly selected using computer‐generated randomization from each time period for AE outcome assessment, for a total of 400 patients.
Each patient's electronic medical record was retrospectively reviewed by a trained clinician using a previously validated screening tool to detect any possible AEs.[11] Any positive screen prompted documentation of a narrative summary including a short description of the possible AE and pertinent associated data. We defined AE as any injury due to medical management rather than the natural history of the illness, and further limited this definition to only include AEs that occurred on the study unit or as a result of care on that unit.
Two of 4 trained adjudicators, blinded to time period, then separately reviewed each narrative summary using previously validated 6‐point confidence scales to determine the presence and preventability of AE, with confidence ratings of 4 or greater used as cutoffs.[11] All AEs were also scored on a 4‐point severity scale (trivial, clinically significant, serious, or life threatening), with severe AE defined as serious or life threatening. Lastly, adjudicators grouped AEs into 1 of 10 prespecified categories.[11] Any disagreements in ratings or groupings were discussed by all 4 adjudicators to reach consensus.
Data Analysis
Patient characteristics are presented using descriptive statistics and were compared in the pre‐ and postregionalization time periods using 2 or t tests as appropriate.
To analyze whether regionalized care was associated with concordance of plan, adjudicated survey questions were assigned points of 1, 0.5, and 0 for complete, partial, and no agreement, respectively. Total mean concordance scores for any patient ranged from 0 to 7 points, and were divided by total number of answered questions (up to 7) for a range of 0 to 1. Total mean concordance scores as well as mean concordance score per survey question were compared pre‐ versus postregionalization using t tests. In sensitivity analyses, adjudicated survey responses were dichotomized with complete and partial agreement deemed concordant responses. Percent concordance for each question was then compared pre‐ versus postregionalization using 2 analysis. Questions about the name of the other team member and discussion of daily care plan with the other team member were excluded from total concordance score calculations and were compared individually pre‐ versus postregionalization, because they are not directly about the plan of care.
To analyze the association of regionalization with odds of preventable AE, we performed multivariable logistic regression adjusted for patient age, sex, race, language, and Elixhauser comorbidity score,[16] and utilized generalized estimating equations to account for clustering by hospital unit. Secondary outcomes included severe preventable AEs, nonpreventable AEs, and category of preventable AEs using similar methodology. Two‐sided P values <0.05 were considered significant, and SAS version 9.2 (SAS Institute Inc., Cary, NC) was used for all analyses.
RESULTS
The fidelity of the intervention in achieving its goal of regionalized care is discussed separately.[15] Briefly, the intervention was successful at achieving 85% regionalization by team (ie, average daily percentage of team's patients assigned to team's unit) and 87% regionalization by unit (ie, average daily percentage of unit's patients with assigned team) following implementation, compared to 20% regionalization by team and unit in the preintervention period. Importantly, the average daily census of physician care teams rose by 32%, from a mean of 10.8 patients/physician care team preregionalization to a mean of 14.3 patients/physician care team postregionalization.
Concordance of Plan
Of the 418 nurse and intern paired surveys, 4 surveys were excluded due to repeat surveys of the same patient during the same hospitalization, for a total of 197 distinct paired surveys preregionalization and 217 paired surveys postregionalization. There were no statistically significant differences in patients' age, sex, race, language, admission source, length of stay, Elixhauser comorbidity score and diagnosis‐related group weight pre‐ versus postregionalization (Table 1).
| Characteristic | Concordance of Care Plan | Adverse Events | ||||
|---|---|---|---|---|---|---|
| Pre, n = 197 | Post, n = 217 | P Value | Pre, n = 198 | Post, n = 194 | P Value | |
| ||||||
| Age, mean (SD) | 60.5 (19.4) | 57.6 (20.8) | 0.15 | 60.4 (18.9) | 58.0 (21.2) | 0.24 |
| Male, n (%) | 77 (39.1) | 92 (42.4) | 0.49 | 94 (47.5) | 85 (43.8) | 0.55 |
| Race/ethnicity, n (%) | 0.34 | 0.12 | ||||
| White | 134 (68.0) | 141 (65.0) | 132 (66.5) | 121 (62.4) | ||
| Black | 42 (21.3) | 45 (20.7) | 41 (20.8) | 54 (27.8) | ||
| Hispanic | 18 (9.1) | 21 (9.7) | 22 (11.3) | 13 (6.8) | ||
| Other/unknown | 3 (1.5) | 10 (4.6) | 3 (1.4) | 6 (2.9) | ||
| Language, n (%) | 0.30 | 0.73 | ||||
| English | 183 (92.9) | 203 (93.5) | 176 (88.7) | 175 (90.2) | ||
| Spanish | 6 (3.0) | 10 (4.6) | 10 (5.2) | 10 (5.3) | ||
| Other | 8 (4.1) | 4 (1.8) | 12 (6.1) | 9 (4.5) | ||
| Admitting source, n (%) | 1.00 | 0.10 | ||||
| Physician office | 13 (6.6) | 13 (6.0) | 13 (6.6) | 6 (3.1) | ||
| Emergency department | 136 (69.0) | 150 (69.1) | 126 (63.6) | 127 (65.5) | ||
| Transfer from different hospital | 40 (20.3) | 45 (20.7) | 54 (27.3) | 50 (25.8) | ||
| Transfer from skilled nursing facility | 8 (4.1) | 9 (4.2) | 5 (2.5) | 11 (5.6) | ||
| Length of stay, d, median (IQR) | 3.0 (4.0) | 3.0 (4.0) | 0.57 | 4.0 (5.0) | 3.0 (4.0) | 0.16 |
| Elixhauser Comorbidity Score, mean (SD) | 8.0 (8.8) | 8.3 (9.3) | 0.74 | 8.0 (8.6) | 7.8 (8.4) | 0.86 |
| DRG weight, mean (SD) | 1.6 (1.0) | 1.5 (1.0) | 0.37 | 1.5 (0.93) | 1.5 (1.1) | 0.96 |
Kappa scores for adjudications of concordance surveys (defined as both adjudicators scoring the same level of agreement (ie, both complete or partial agreement versus no agreement) ranged from 0.69 to 0.95, by question. There were no significant differences in total mean concordance scores in the care plan pre‐ versus postregionalization (0.65 vs 0.67, P = 0.26) (Table 2). Similarly, there were no significant differences in mean concordance score for each survey question, except agreement on expected date of discharge (0.56 vs 0.68, P = 0.003), knowledge of the other provider's name, and agreement that discussion of the daily plan had taken place with the other pair member. Similar results were seen when results were dichotomized (ie, partial or complete agreement vs no agreement) (Table 2).
| Concordance Outcome | Pre, n = 197 | Post, n = 217 | P Value |
|---|---|---|---|
| |||
| Concordance score* | |||
| Total concordance score, mean (SD) | 0.65 (0.17) | 0.67 (0.16) | 0.26 |
| Subgroups | |||
| Diagnosis | 0.77 (0.32) | 0.72 (0.35) | 0.11 |
| Patient's chief concern | 0.48 (0.44) | 0.48 (0.43) | 0.94 |
| Tests today | 0.67 (0.40) | 0.71 (0.42) | 0.36 |
| Procedures today | 0.93 (0.25) | 0.92 (0.25) | 0.71 |
| Medication changes today | 0.56 (0.44) | 0.59 (0.43) | 0.54 |
| Consulting services | 0.59 (0.44) | 0.60 (0.44) | 0.82 |
| Expected discharge date | 0.56 (0.44) | 0.68 (0.38) | 0.003 |
| Responding clinician knowledge of nurse's name | 0.56 (0.50) | 0.86 (0.35) | <0.001 |
| Nurse's knowledge of responding clinician's name | 0.56 (0.50) | 0.88 (0.33) | <0.001 |
| Plan discussed | 0.73 (0.45) | 0.88 (0.32) | <0.001 |
| Percent concordance, mean (SD) | |||
| Diagnosis | 92.0 (27.3) | 88.6 (31.9) | 0.25 |
| Patient's chief concern | 59.6 (49.1) | 60.6 (49.0) | 0.84 |
| Tests today | 78.9 (40.9) | 77.2 (42.1) | 0.67 |
| Procedures today | 93.5 (24.8) | 94.1 (23.7) | 0.80 |
| Medication changes today | 66.3 (33.6) | 69.9 (46.0) | 0.44 |
| Consulting services | 69.3 (46.2) | 68.9 (46.4) | 0.93 |
| Expected discharge date | 67.5 (47.0) | 82.6 (38.0) | <0.001 |
| Responding clinician knowledge of nurse's name | 55.7 (49.8) | 85.6 (35.2) | <0.001 |
| Nurse's knowledge of responding clinician's name | 55.9 (49.8) | 87.9 (32.8) | <0.001 |
| Plan discussed | 72.9 (44.6) | 88.2 (32.3) | <0.001 |
Adverse Events
Of the 400 patients screened for AEs, 8 were excluded due to missing medical record number (5) and discharge outside of study period (3). Of the final 392 patient screens (198 pre, 194 post), there were no significant differences in patients' age, sex, race, language, length of stay, or Elixhauser score pre‐ versus postregionalization (Table 1).
Kappa scores for adjudicator agreement were 0.35 for presence of AE and 0.34 for preventability of AE. Of the 392 reviewed patient records, there were 133 total AEs detected (66 pre, 67 post), 27 preventable AEs (13 pre, 14 post), and 9 severe preventable AEs (4 pre, 5 post) (Table 3). There was no significant difference in the adjusted odds of preventable AEs post‐ versus preregionalization (adjusted odds ratio: 1.37, 95% confidence interval: 0.69, 2.69). Although the low number of AEs rated as severe or life threatening precluded adjusted analysis, unadjusted results similarly demonstrated no difference in odds of severe preventable AEs pre‐ versus postregionalization. As expected, there was no significant difference in adjusted odds of nonpreventable AE after implementation of regionalized care (Table 3).
| Adverse Events | No. of Adverse Events | Adjusted Odds Ratio Post vs Pre (95% CI) | |
|---|---|---|---|
| Pre, n = 198 | Post, n = 194 | ||
| |||
| Preventable | 13 | 14 | 1.37 (0.69, 2.69) |
| Serious and preventable | 4 | 5 | |
| Nonpreventable | 47 | 50 | 1.20 (0.85, 1.75) |
Similarly, there were no significant differences in category of preventable AE pre‐ versus postregionalization. The most frequent preventable AEs in both time periods were those related to adverse drug events and to manifestations of poor glycemic control, examples of which are illustrated (Table 4).
| |
| Adverse drug event | 29‐year‐old male with history of alcohol abuse, complicated by prior withdrawal seizures/emntensive care unit admissions, presented with alcohol withdrawal. Started on standing and PRN lorazepam, kept on home medications including standing clonidine, gabapentin, citalopram, quetiapine. Became somnolent due to polypharmacy, ultimately discontinued quetiapine as discovered took only as needed at home for insomnia |
| Manifestations of poor glycemic control | 78‐year‐old male with recently diagnosed lymphoma, distant history of bladder and prostate cancer status post ileal loop diversion, presented status post syncopal event; during event, spilled boiling water on himself leading to second‐degree burns on 3% of his body. Initially admitted to trauma/burn service, ultimately transferred to medical service for ongoing multiple medical issues including obstructive uropathy, acute on chronic renal failure. Adverse event was hyperglycemia (>350 mg/dL on >2 consecutive readings) in the setting of holding his home insulin detemir and insulin aspart (had been placed on insulin aspart sliding scale alone). After hyperglycemic episodes, was placed back on weight‐based basal/nutritional insulin |
DISCUSSION
In this study of general medicine patients at a large academic medical center, we found that regionalization of care teams on general medicine services was associated with improved recognition of care team members and agreement on estimated date of patient discharge, but was not associated with improvement in overall nurse and physician concordance of the patient care plan, or the odds of preventable AEs.
This intervention importantly addresses the barrier of dispersion of team membership, a well‐recognized barrier to interdisciplinary collaboration,[17, 18] particularly with resident physician teams due to frequently changing team membership. Localization of all team members, in addition to encouragement of daily collaborative bedside rounds as part of the regionalization initiative, likely contributed to our observed improvement in team member identification and discussion of daily care plans. Similarly, regionalization resulted in improved agreement in estimations of date of patient discharge. Focus on early patient discharges was an integral part of the implementation efforts; we therefore hypothesize that mutual focus on discharge planning by both nurses and responding clinicians may have explained this observed result.
On the other hand, regionalization did not appreciably improve the overall concordance of care plan between nurses and interns, despite a significant increase in team members agreeing that the plan had been discussed. Our findings support similar prior research demonstrating that regionalizing hospitalist attendings to single nursing units had limited impact on agreement of care plan between physicians and nurses.[13] Similarly, in settings where physicians and nurses are inherently regionalized, such as the intensive care unit[4] or the operating room,[3] communication between physicians and nurses remains difficult. Collectively, our findings suggest that colocalization of physicians and nurses alone is likely insufficient to improve measured communication between care team members. Existing literature suggests that more standardized approaches to improve communication, such as structured communication tools used during daily inpatient care[19, 20] or formalized team training,[21, 22, 23] lead to improvements in communication and collaboration. Despite these findings, it is important to highlight that this study did not assess other measures of workplace culture, such as teamwork and care team cohesiveness, which may have been positively affected by this intervention, even without measurable effect on concordance of care plan. Additionally, as noted, the average daily census on each team increased by almost a third postintervention, which may have impeded improvements in care team communication.
In addition, we found that our intervention had no significant impact on preventable AEs or severe preventable AEs. Although we cannot exclude the possibility that more subtle AEs were missed with our methodology, our results indicate that regionalized care alone may be inadequate to improve major patient safety outcomes. As discussed, the volume of patients did increase postintervention; thus, another way to state our results is that we were able to increase the daily volume of patients without any significant decreases in patient safety. Nevertheless, the results on patient safety were less than desired. A recent review of interdisciplinary team care interventions on general medical wards similarly demonstrated underwhelming improvements in patient safety outcomes, although the reviewed interventions did not specifically address preventable AEs, a gap in the literature commented on by the authors.[24] Other albeit limited literature has demonstrated improvement in patient safety outcomes via multifaceted efforts aimed at improving care team member communication. Notably, these efforts include colocalization of care team members to single units but also involve additional measures to improve communication and collaboration between care team members, such as structured communication during interdisciplinary rounds, and certification of key interdisciplinary teamwork skills.[11, 14] Although our regionalized care intervention included many similar features to these accountable care units (ACUs) including unit‐based care teams, unit‐level performance reporting, and unit‐based physician and nursing coleadership, significant differences existed. Notably, in addition to the above features, the ACU model also incorporated highly structured communication models for interdisciplinary rounding, and certification processes to ensure an appropriate communication skill base among care team members.[14] Thus, although creation of regionalized care teams is likely a necessary precursor to implementation of these additional measures, alone it may be insufficient to improve patient safety outcomes.
Importantly, in our study we identified that adverse drug events and manifestations of poor glycemic control occurred in high frequency both before and following implementation of regionalized care, supporting other literature that describes the prevalence of these AEs.[11, 25, 26, 27] These results suggest that targeted interventions to address these specific AEs are likely necessary. Notably, the intervention units in our study did not consistently employ clinical pharmacists assigned specifically to that unit's care team to allow for integration within the care team. As prior research has suggested that greater collaboration with clinical pharmacists results in reduction of adverse drug events,[28] next steps may include improved integration of team‐based pharmacists into the activities of the regionalized care teams. Inpatient management of diabetes also requires specific interventions,[29, 30, 31] only some of which may be addressable by having regionalized care and better interdisciplinary communication.
Our findings are subject to several limitations. First, this was a single‐site study and thus our findings may not be generalizable to other institutions. However, regionalized care is increasingly encouraged to optimize communication between care team members.[17, 18] Therefore, our null findings may be pertinent to other institutions looking to improve patient safety outcomes, demonstrating that additional initiatives will likely be required. Second, our modes of outcome measurement possess limitations. In measuring concordance of care plan, although previously used survey techniques were employed,[9] the concordance survey has not been formally validated, and we believe some of the questions may have led to ambiguity on the part of the responders that may have resulted in less accurate responses, thus biasing toward the null. Similarly, in measuring AEs, the screening tool relied on retrospective chart review looking for specific AE types[11] and thus may not have captured more subtle AEs. Additionally, our study may have been underpowered to demonstrate significant reduction in preventable AEs, although other studies of similar methodology demonstrated significant results with similar sample size.[11] This was due in part to our lower‐than‐expected baseline AE rate (6.6% compared with approximately 10.3% in previous studies).[11] Lastly, our study solely examined the association of regionalization with concordance of care plan and preventable AEs, but importantly excluded other clinically important outcomes that may have been positively (or negatively) impacted by these regionalization efforts, such as ED wait times, provider efficiency (eg, fewer pages, less time in transit, more time at the bedside), interdisciplinary teamwork, or patient or provider satisfaction.
CONCLUSION
In summary, our findings suggest that regionalized care teams alone may be insufficient to effectively promote communication between care team members regarding the care plan or to lead to improvements in patient safety, although we recognize that there may have been benefits (or unintended harms) not measured in this study but are nonetheless important for clinical care and workplace culture. This is an important lesson, as many hospitals move toward regionalized care in an effort to improve patient safety outcomes. However, strengthening the infrastructure by colocalizing care team members to maximize opportunity for communication is likely a necessary first step toward facilitating implementation of additional initiatives that may lead to more robust patient safety improvements, such as structured interdisciplinary bedside rounds (eg, facilitating and training all team members to fulfill specific roles), teamwork training, and certification of key interdisciplinary teamwork skills. Additionally, close examination of identified prevalent and preventable AEs can help to determine which additional initiatives are most likely to have greatest impact in improving patient safety.
Disclosures: This research was supported by funds provided by Brigham and Women's Hospital (BWH) and by funds provided by the Department of Medicine at BWH. All authors had full access to all of the data in the study and were integrally involved in the design, implementation, data collection, and analyses. The first author, Dr. Stephanie Mueller, takes responsibility for the integrity for the data and the accuracy of the data analysis. Dr. Schnipper reports grants from Sanofi Aventis, outside the submitted work.
Failures in communication among healthcare professionals are known threats to patient safety. These failures account for over 60% of root causes of sentinel events, the most serious events reported to The Joint Commission.[1] As such, identifying both patterns of effective communication as well as barriers to successful communication has been a focus of efforts aimed at improving patient safety. However, to date, the majority of this work has centered on improving communication in settings such as the operating room and intensive care unit,[2, 3, 4] or at times of care transitions.[5, 6, 7, 8]
Unique barriers exist for effective interdisciplinary communication in the hospital setting, particularly physiciannurse communication regarding shared hospitalized patients.[9] Traditionally, care of hospitalized patients is provided by physicians, nurses, and other team members working in varied workflow patterns, leading to dispersed team membership, where each team member cares for different groups of patients in different locations across the hospital. This dispersion is further heightened on teaching services, where residents' rotation schedules lead to frequent changes of care team membership, leaving inpatient care teams particularly vulnerable to ineffective communication. Evidence suggests that communication between nurses and physicians is currently suboptimal, leading to frequent disagreement regarding the patient's plan of care.[9, 10] This divergence between physician and nursing perceptions of patients' care plans may leave patients at greater risk of adverse events (AEs).
Several studies have examined the effects of regionalized inpatient care teams, where multidisciplinary team members care for the same patients on the same hospital unit, on communication and patient outcomes.[4, 11, 12, 13, 14] Results of these studies have been inconsistent, perhaps due to the particular characteristics of the care teams or to the study methodology. Thus, further rigorously done studies are required to better understand the impact of team regionalization on patient care. The goal of this study was to examine whether the implementation of regionalized inpatient care teams was associated with improvements in care team communication and preventable AEs.
METHODS
Setting, Patients, and Study Design
We performed a cohort analysis of patients at a 700‐bed tertiary care center, pre‐ and postregionalization of inpatient general medicine care teams. Our study protocol was approved by the Partners Healthcare Human Subjects Review Committee. Patients were eligible for inclusion if they were 18 years of age or older and discharged from the general medicine service (GMS) from any of the 3 participating nursing units between April 1, 2012 and June 19, 2012 (preregionalization) or April 1, 2013 and June 19, 2013 (postregionalization).
Intervention
On June 20, 2012, regionalized care was implemented on the GMS such that each of 3 GMS teams was localized to 1 of 3, 15‐bed nursing units. Prior to regionalization, the GMS physician care teams, each consisting of 1 hospitalist attending, 1 medical resident, and 2 medical interns, would care for patients on an average of 7 and up to 13 different nursing units on a given day.
Regionalized care consisted of a multifaceted intervention codeveloped by hospitalist, residency, nursing, emergency department, and hospital leadership and included: (1) regionalizing GMS teams as much as possible; (2) change in resident call structure from a traditional 4‐day call cycle to daily admitting; (3) collaborative efforts to enhance GMS patient discharges before noon to promote regionalized placement of patients without prolonging time in the emergency department (ED); (4) daily morning and postround multidisciplinary huddles to prioritize sicker patients and discharges; (5) encouragement of daily rounds at patients' bedsides with presence of physician team, nurse, and team pharmacist if available; (6) creation of unit‐ and team‐level performance reports; and (7) creation of unit‐based physician and nursing co‐leadership (Figure 1).[15]

Concordance of Plan
Concordance of plan was measured via a 7‐question survey previously developed, pilot tested, and used to measure the impact of regionalized care on care team communication between inpatient nursephysician team members.[9] The survey was administered in‐person by 1 of 8 trained research assistants (RAs) (4/emntervention period) to nurse and intern pairs caring for patients on the study units pre‐ and postregionalization. GMS patients were eligible for inclusion if surveys could be administered to their nurse and intern within the first 24 hours of admission to the unit and within 48 hours of admission to the hospital, based on RA availability (thus excluding patients admitted on Fridays as surveys were not conducted over the weekend). Most often, all eligible patients admitted to the study units during time periods of data collection were included in the study. On limited occasions, the daily supply of patients surpassed RA capacity for inclusion, at which time computer‐generated randomization was utilized to randomly select patients for inclusion. Nurse and intern pairs were surveyed once during a patient's hospitalization, although they could be surveyed more than once about different patients, and patients could be included more than once if rehospitalized on the study unit and cared for by a different nurseintern pair. Of the 472 selected eligible patients, the nurses and interns of 418 patients were available and consented to survey administration, representing 361 unique nurse and intern pairs and 399 unique patients.
Each member of the pair was asked about 7 specific aspects of the patient's care plan for that day in isolation from the other team member, including: (1) the patient's primary diagnosis, (2) the patient's expressed chief concern, (3) the day's scheduled tests, (4) the day's scheduled procedures, (5) consulting services involved, (6) medication changes made that day, and (7) the patient's expected discharge date. In addition, each pair was asked the name of the other team member (ie, the nurse was asked the name of the intern and vice versa), and whether or not the patient care plan for the day had been discussed with the other team member, where concordance was defined as both members agreeing the plan had been discussed. All responses were recorded verbatim. Pairs were surveyed independently between 12 pm and 2 pm, limiting confounding by evolving plans of care over time.
Each set of surveys were then reviewed by 2 of 4 trained adjudicators, and responses to each question were scored as complete, partial, or no agreement. Rules for degree of agreement were based upon previously utilized parameters[9] as well as biweekly meetings during which common themes and disagreements in ratings were discussed, and rules generated to create consensus (see Supporting Information, Appendix, in the online version of this article).
Adverse Event Detection
Of the patients meeting eligibility criteria, 200 patients were randomly selected using computer‐generated randomization from each time period for AE outcome assessment, for a total of 400 patients.
Each patient's electronic medical record was retrospectively reviewed by a trained clinician using a previously validated screening tool to detect any possible AEs.[11] Any positive screen prompted documentation of a narrative summary including a short description of the possible AE and pertinent associated data. We defined AE as any injury due to medical management rather than the natural history of the illness, and further limited this definition to only include AEs that occurred on the study unit or as a result of care on that unit.
Two of 4 trained adjudicators, blinded to time period, then separately reviewed each narrative summary using previously validated 6‐point confidence scales to determine the presence and preventability of AE, with confidence ratings of 4 or greater used as cutoffs.[11] All AEs were also scored on a 4‐point severity scale (trivial, clinically significant, serious, or life threatening), with severe AE defined as serious or life threatening. Lastly, adjudicators grouped AEs into 1 of 10 prespecified categories.[11] Any disagreements in ratings or groupings were discussed by all 4 adjudicators to reach consensus.
Data Analysis
Patient characteristics are presented using descriptive statistics and were compared in the pre‐ and postregionalization time periods using 2 or t tests as appropriate.
To analyze whether regionalized care was associated with concordance of plan, adjudicated survey questions were assigned points of 1, 0.5, and 0 for complete, partial, and no agreement, respectively. Total mean concordance scores for any patient ranged from 0 to 7 points, and were divided by total number of answered questions (up to 7) for a range of 0 to 1. Total mean concordance scores as well as mean concordance score per survey question were compared pre‐ versus postregionalization using t tests. In sensitivity analyses, adjudicated survey responses were dichotomized with complete and partial agreement deemed concordant responses. Percent concordance for each question was then compared pre‐ versus postregionalization using 2 analysis. Questions about the name of the other team member and discussion of daily care plan with the other team member were excluded from total concordance score calculations and were compared individually pre‐ versus postregionalization, because they are not directly about the plan of care.
To analyze the association of regionalization with odds of preventable AE, we performed multivariable logistic regression adjusted for patient age, sex, race, language, and Elixhauser comorbidity score,[16] and utilized generalized estimating equations to account for clustering by hospital unit. Secondary outcomes included severe preventable AEs, nonpreventable AEs, and category of preventable AEs using similar methodology. Two‐sided P values <0.05 were considered significant, and SAS version 9.2 (SAS Institute Inc., Cary, NC) was used for all analyses.
RESULTS
The fidelity of the intervention in achieving its goal of regionalized care is discussed separately.[15] Briefly, the intervention was successful at achieving 85% regionalization by team (ie, average daily percentage of team's patients assigned to team's unit) and 87% regionalization by unit (ie, average daily percentage of unit's patients with assigned team) following implementation, compared to 20% regionalization by team and unit in the preintervention period. Importantly, the average daily census of physician care teams rose by 32%, from a mean of 10.8 patients/physician care team preregionalization to a mean of 14.3 patients/physician care team postregionalization.
Concordance of Plan
Of the 418 nurse and intern paired surveys, 4 surveys were excluded due to repeat surveys of the same patient during the same hospitalization, for a total of 197 distinct paired surveys preregionalization and 217 paired surveys postregionalization. There were no statistically significant differences in patients' age, sex, race, language, admission source, length of stay, Elixhauser comorbidity score and diagnosis‐related group weight pre‐ versus postregionalization (Table 1).
| Characteristic | Concordance of Care Plan | Adverse Events | ||||
|---|---|---|---|---|---|---|
| Pre, n = 197 | Post, n = 217 | P Value | Pre, n = 198 | Post, n = 194 | P Value | |
| ||||||
| Age, mean (SD) | 60.5 (19.4) | 57.6 (20.8) | 0.15 | 60.4 (18.9) | 58.0 (21.2) | 0.24 |
| Male, n (%) | 77 (39.1) | 92 (42.4) | 0.49 | 94 (47.5) | 85 (43.8) | 0.55 |
| Race/ethnicity, n (%) | 0.34 | 0.12 | ||||
| White | 134 (68.0) | 141 (65.0) | 132 (66.5) | 121 (62.4) | ||
| Black | 42 (21.3) | 45 (20.7) | 41 (20.8) | 54 (27.8) | ||
| Hispanic | 18 (9.1) | 21 (9.7) | 22 (11.3) | 13 (6.8) | ||
| Other/unknown | 3 (1.5) | 10 (4.6) | 3 (1.4) | 6 (2.9) | ||
| Language, n (%) | 0.30 | 0.73 | ||||
| English | 183 (92.9) | 203 (93.5) | 176 (88.7) | 175 (90.2) | ||
| Spanish | 6 (3.0) | 10 (4.6) | 10 (5.2) | 10 (5.3) | ||
| Other | 8 (4.1) | 4 (1.8) | 12 (6.1) | 9 (4.5) | ||
| Admitting source, n (%) | 1.00 | 0.10 | ||||
| Physician office | 13 (6.6) | 13 (6.0) | 13 (6.6) | 6 (3.1) | ||
| Emergency department | 136 (69.0) | 150 (69.1) | 126 (63.6) | 127 (65.5) | ||
| Transfer from different hospital | 40 (20.3) | 45 (20.7) | 54 (27.3) | 50 (25.8) | ||
| Transfer from skilled nursing facility | 8 (4.1) | 9 (4.2) | 5 (2.5) | 11 (5.6) | ||
| Length of stay, d, median (IQR) | 3.0 (4.0) | 3.0 (4.0) | 0.57 | 4.0 (5.0) | 3.0 (4.0) | 0.16 |
| Elixhauser Comorbidity Score, mean (SD) | 8.0 (8.8) | 8.3 (9.3) | 0.74 | 8.0 (8.6) | 7.8 (8.4) | 0.86 |
| DRG weight, mean (SD) | 1.6 (1.0) | 1.5 (1.0) | 0.37 | 1.5 (0.93) | 1.5 (1.1) | 0.96 |
Kappa scores for adjudications of concordance surveys (defined as both adjudicators scoring the same level of agreement (ie, both complete or partial agreement versus no agreement) ranged from 0.69 to 0.95, by question. There were no significant differences in total mean concordance scores in the care plan pre‐ versus postregionalization (0.65 vs 0.67, P = 0.26) (Table 2). Similarly, there were no significant differences in mean concordance score for each survey question, except agreement on expected date of discharge (0.56 vs 0.68, P = 0.003), knowledge of the other provider's name, and agreement that discussion of the daily plan had taken place with the other pair member. Similar results were seen when results were dichotomized (ie, partial or complete agreement vs no agreement) (Table 2).
| Concordance Outcome | Pre, n = 197 | Post, n = 217 | P Value |
|---|---|---|---|
| |||
| Concordance score* | |||
| Total concordance score, mean (SD) | 0.65 (0.17) | 0.67 (0.16) | 0.26 |
| Subgroups | |||
| Diagnosis | 0.77 (0.32) | 0.72 (0.35) | 0.11 |
| Patient's chief concern | 0.48 (0.44) | 0.48 (0.43) | 0.94 |
| Tests today | 0.67 (0.40) | 0.71 (0.42) | 0.36 |
| Procedures today | 0.93 (0.25) | 0.92 (0.25) | 0.71 |
| Medication changes today | 0.56 (0.44) | 0.59 (0.43) | 0.54 |
| Consulting services | 0.59 (0.44) | 0.60 (0.44) | 0.82 |
| Expected discharge date | 0.56 (0.44) | 0.68 (0.38) | 0.003 |
| Responding clinician knowledge of nurse's name | 0.56 (0.50) | 0.86 (0.35) | <0.001 |
| Nurse's knowledge of responding clinician's name | 0.56 (0.50) | 0.88 (0.33) | <0.001 |
| Plan discussed | 0.73 (0.45) | 0.88 (0.32) | <0.001 |
| Percent concordance, mean (SD) | |||
| Diagnosis | 92.0 (27.3) | 88.6 (31.9) | 0.25 |
| Patient's chief concern | 59.6 (49.1) | 60.6 (49.0) | 0.84 |
| Tests today | 78.9 (40.9) | 77.2 (42.1) | 0.67 |
| Procedures today | 93.5 (24.8) | 94.1 (23.7) | 0.80 |
| Medication changes today | 66.3 (33.6) | 69.9 (46.0) | 0.44 |
| Consulting services | 69.3 (46.2) | 68.9 (46.4) | 0.93 |
| Expected discharge date | 67.5 (47.0) | 82.6 (38.0) | <0.001 |
| Responding clinician knowledge of nurse's name | 55.7 (49.8) | 85.6 (35.2) | <0.001 |
| Nurse's knowledge of responding clinician's name | 55.9 (49.8) | 87.9 (32.8) | <0.001 |
| Plan discussed | 72.9 (44.6) | 88.2 (32.3) | <0.001 |
Adverse Events
Of the 400 patients screened for AEs, 8 were excluded due to missing medical record number (5) and discharge outside of study period (3). Of the final 392 patient screens (198 pre, 194 post), there were no significant differences in patients' age, sex, race, language, length of stay, or Elixhauser score pre‐ versus postregionalization (Table 1).
Kappa scores for adjudicator agreement were 0.35 for presence of AE and 0.34 for preventability of AE. Of the 392 reviewed patient records, there were 133 total AEs detected (66 pre, 67 post), 27 preventable AEs (13 pre, 14 post), and 9 severe preventable AEs (4 pre, 5 post) (Table 3). There was no significant difference in the adjusted odds of preventable AEs post‐ versus preregionalization (adjusted odds ratio: 1.37, 95% confidence interval: 0.69, 2.69). Although the low number of AEs rated as severe or life threatening precluded adjusted analysis, unadjusted results similarly demonstrated no difference in odds of severe preventable AEs pre‐ versus postregionalization. As expected, there was no significant difference in adjusted odds of nonpreventable AE after implementation of regionalized care (Table 3).
| Adverse Events | No. of Adverse Events | Adjusted Odds Ratio Post vs Pre (95% CI) | |
|---|---|---|---|
| Pre, n = 198 | Post, n = 194 | ||
| |||
| Preventable | 13 | 14 | 1.37 (0.69, 2.69) |
| Serious and preventable | 4 | 5 | |
| Nonpreventable | 47 | 50 | 1.20 (0.85, 1.75) |
Similarly, there were no significant differences in category of preventable AE pre‐ versus postregionalization. The most frequent preventable AEs in both time periods were those related to adverse drug events and to manifestations of poor glycemic control, examples of which are illustrated (Table 4).
| |
| Adverse drug event | 29‐year‐old male with history of alcohol abuse, complicated by prior withdrawal seizures/emntensive care unit admissions, presented with alcohol withdrawal. Started on standing and PRN lorazepam, kept on home medications including standing clonidine, gabapentin, citalopram, quetiapine. Became somnolent due to polypharmacy, ultimately discontinued quetiapine as discovered took only as needed at home for insomnia |
| Manifestations of poor glycemic control | 78‐year‐old male with recently diagnosed lymphoma, distant history of bladder and prostate cancer status post ileal loop diversion, presented status post syncopal event; during event, spilled boiling water on himself leading to second‐degree burns on 3% of his body. Initially admitted to trauma/burn service, ultimately transferred to medical service for ongoing multiple medical issues including obstructive uropathy, acute on chronic renal failure. Adverse event was hyperglycemia (>350 mg/dL on >2 consecutive readings) in the setting of holding his home insulin detemir and insulin aspart (had been placed on insulin aspart sliding scale alone). After hyperglycemic episodes, was placed back on weight‐based basal/nutritional insulin |
DISCUSSION
In this study of general medicine patients at a large academic medical center, we found that regionalization of care teams on general medicine services was associated with improved recognition of care team members and agreement on estimated date of patient discharge, but was not associated with improvement in overall nurse and physician concordance of the patient care plan, or the odds of preventable AEs.
This intervention importantly addresses the barrier of dispersion of team membership, a well‐recognized barrier to interdisciplinary collaboration,[17, 18] particularly with resident physician teams due to frequently changing team membership. Localization of all team members, in addition to encouragement of daily collaborative bedside rounds as part of the regionalization initiative, likely contributed to our observed improvement in team member identification and discussion of daily care plans. Similarly, regionalization resulted in improved agreement in estimations of date of patient discharge. Focus on early patient discharges was an integral part of the implementation efforts; we therefore hypothesize that mutual focus on discharge planning by both nurses and responding clinicians may have explained this observed result.
On the other hand, regionalization did not appreciably improve the overall concordance of care plan between nurses and interns, despite a significant increase in team members agreeing that the plan had been discussed. Our findings support similar prior research demonstrating that regionalizing hospitalist attendings to single nursing units had limited impact on agreement of care plan between physicians and nurses.[13] Similarly, in settings where physicians and nurses are inherently regionalized, such as the intensive care unit[4] or the operating room,[3] communication between physicians and nurses remains difficult. Collectively, our findings suggest that colocalization of physicians and nurses alone is likely insufficient to improve measured communication between care team members. Existing literature suggests that more standardized approaches to improve communication, such as structured communication tools used during daily inpatient care[19, 20] or formalized team training,[21, 22, 23] lead to improvements in communication and collaboration. Despite these findings, it is important to highlight that this study did not assess other measures of workplace culture, such as teamwork and care team cohesiveness, which may have been positively affected by this intervention, even without measurable effect on concordance of care plan. Additionally, as noted, the average daily census on each team increased by almost a third postintervention, which may have impeded improvements in care team communication.
In addition, we found that our intervention had no significant impact on preventable AEs or severe preventable AEs. Although we cannot exclude the possibility that more subtle AEs were missed with our methodology, our results indicate that regionalized care alone may be inadequate to improve major patient safety outcomes. As discussed, the volume of patients did increase postintervention; thus, another way to state our results is that we were able to increase the daily volume of patients without any significant decreases in patient safety. Nevertheless, the results on patient safety were less than desired. A recent review of interdisciplinary team care interventions on general medical wards similarly demonstrated underwhelming improvements in patient safety outcomes, although the reviewed interventions did not specifically address preventable AEs, a gap in the literature commented on by the authors.[24] Other albeit limited literature has demonstrated improvement in patient safety outcomes via multifaceted efforts aimed at improving care team member communication. Notably, these efforts include colocalization of care team members to single units but also involve additional measures to improve communication and collaboration between care team members, such as structured communication during interdisciplinary rounds, and certification of key interdisciplinary teamwork skills.[11, 14] Although our regionalized care intervention included many similar features to these accountable care units (ACUs) including unit‐based care teams, unit‐level performance reporting, and unit‐based physician and nursing coleadership, significant differences existed. Notably, in addition to the above features, the ACU model also incorporated highly structured communication models for interdisciplinary rounding, and certification processes to ensure an appropriate communication skill base among care team members.[14] Thus, although creation of regionalized care teams is likely a necessary precursor to implementation of these additional measures, alone it may be insufficient to improve patient safety outcomes.
Importantly, in our study we identified that adverse drug events and manifestations of poor glycemic control occurred in high frequency both before and following implementation of regionalized care, supporting other literature that describes the prevalence of these AEs.[11, 25, 26, 27] These results suggest that targeted interventions to address these specific AEs are likely necessary. Notably, the intervention units in our study did not consistently employ clinical pharmacists assigned specifically to that unit's care team to allow for integration within the care team. As prior research has suggested that greater collaboration with clinical pharmacists results in reduction of adverse drug events,[28] next steps may include improved integration of team‐based pharmacists into the activities of the regionalized care teams. Inpatient management of diabetes also requires specific interventions,[29, 30, 31] only some of which may be addressable by having regionalized care and better interdisciplinary communication.
Our findings are subject to several limitations. First, this was a single‐site study and thus our findings may not be generalizable to other institutions. However, regionalized care is increasingly encouraged to optimize communication between care team members.[17, 18] Therefore, our null findings may be pertinent to other institutions looking to improve patient safety outcomes, demonstrating that additional initiatives will likely be required. Second, our modes of outcome measurement possess limitations. In measuring concordance of care plan, although previously used survey techniques were employed,[9] the concordance survey has not been formally validated, and we believe some of the questions may have led to ambiguity on the part of the responders that may have resulted in less accurate responses, thus biasing toward the null. Similarly, in measuring AEs, the screening tool relied on retrospective chart review looking for specific AE types[11] and thus may not have captured more subtle AEs. Additionally, our study may have been underpowered to demonstrate significant reduction in preventable AEs, although other studies of similar methodology demonstrated significant results with similar sample size.[11] This was due in part to our lower‐than‐expected baseline AE rate (6.6% compared with approximately 10.3% in previous studies).[11] Lastly, our study solely examined the association of regionalization with concordance of care plan and preventable AEs, but importantly excluded other clinically important outcomes that may have been positively (or negatively) impacted by these regionalization efforts, such as ED wait times, provider efficiency (eg, fewer pages, less time in transit, more time at the bedside), interdisciplinary teamwork, or patient or provider satisfaction.
CONCLUSION
In summary, our findings suggest that regionalized care teams alone may be insufficient to effectively promote communication between care team members regarding the care plan or to lead to improvements in patient safety, although we recognize that there may have been benefits (or unintended harms) not measured in this study but are nonetheless important for clinical care and workplace culture. This is an important lesson, as many hospitals move toward regionalized care in an effort to improve patient safety outcomes. However, strengthening the infrastructure by colocalizing care team members to maximize opportunity for communication is likely a necessary first step toward facilitating implementation of additional initiatives that may lead to more robust patient safety improvements, such as structured interdisciplinary bedside rounds (eg, facilitating and training all team members to fulfill specific roles), teamwork training, and certification of key interdisciplinary teamwork skills. Additionally, close examination of identified prevalent and preventable AEs can help to determine which additional initiatives are most likely to have greatest impact in improving patient safety.
Disclosures: This research was supported by funds provided by Brigham and Women's Hospital (BWH) and by funds provided by the Department of Medicine at BWH. All authors had full access to all of the data in the study and were integrally involved in the design, implementation, data collection, and analyses. The first author, Dr. Stephanie Mueller, takes responsibility for the integrity for the data and the accuracy of the data analysis. Dr. Schnipper reports grants from Sanofi Aventis, outside the submitted work.
- Joint Commission on Accreditation of Healthcare Organizations. Understanding and Preventing Sentinel Events in Your Health Care Organization. Oak Brook, IL: Joint Commission; 2008.
- , , , et al. Communication failures in the operating room: an observational classification of recurrent types and effects. Qual Saf Health Care. 2004;13(5):330–334.
- , , , et al. Operating room teamwork among physicians and nurses: teamwork in the eye of the beholder. J Am Coll Surg. 2006;202(5):746–752.
- , , . Discrepant attitudes about teamwork among critical care nurses and physicians. Crit Care Med. 2003;31(3):956–959.
- , , , , . Communication failures in patient sign‐out and suggestions for improvement: a critical incident analysis. Qual Saf Health Care. 2005;14(6):401–407.
- , , , et al. Changes in medical errors after implementation of a handoff program. N Engl J Med. 2014;371(19):1803–1812.
- , , , et al. Communication and information deficits in patients discharged to rehabilitation facilities: an evaluation of five acute care hospitals. J Hosp Med. 2009;4(8):E28–E33.
- , , , , , . Deficits in communication and information transfer between hospital‐based and primary care physicians: implications for patient safety and continuity of care. JAMA. 2007;297(8):831–841.
- , , , et al. Patterns of nurse‐physician communication and agreement on the plan of care. Qual Saf Health Care. 2010;19(3):195–199.
- , , , , , . Can we talk? Priorities for patient care differed among health care providers. In: Henriksen K, Battles JB, Marks ES, Lewin DI, eds. Advances in Patient Safety: From Research to Implementation. Vol 1. Rockville, MD: Agency for Healthcare Research and Quality; 2005.
- , , , et al. Structured interdisciplinary rounds in a medical teaching unit: improving patient safety. Arch Intern Med. 2011;171(7):678–684.
- , , , , , . Improving teamwork: impact of structured interdisciplinary rounds on a hospitalist unit. J Hosp Med. 2011;6(2):88–93.
- , , , et al. Impact of localizing physicians to hospital units on nurse‐physician communication and agreement on the plan of care. J Gen Intern Med. 2009;24(11):1223–1227.
- , , , et al. Reorganizing a hospital ward as an accountable care unit. J Hosp Med. 2015;10(1):36–40.
- , , , et al. 5th time's a charm: creation of unit‐based care teams in a high occupancy hospital [abstract]. J Hosp Med. 2015;10 (suppl. 2). Available at: http://www.shmabstracts.com/abstract/5th‐times‐a‐charm‐creation‐of‐unit‐based‐care‐teams‐in‐a‐high‐occupancy‐hospital. Accessed July 28, 2015.
- , , , . Comorbidity measures for use with administrative data. Med Care. 1998;36(1):8–27.
- , , , , , . Teamwork on inpatient medical units: assessing attitudes and barriers. Qual Saf Health Care. 2010;19(2):117–121.
- , , , et al. A model for quality improvement programs in academic departments of medicine. Am J Med. 2008;121(10):922–929.
- , , , , . Improving nurse‐physician communication and satisfaction in the intensive care unit with a daily goals worksheet. Am J Crit Care. 2006;15(2):217–222.
- , , , , , . Improving communication in the ICU using daily goals. J Crit Care. 2003;18(2):71–75.
- , , , et al. Effect of crew resource management training in a multidisciplinary obstetrical setting. Int J Qual Health Care. 2008;20(4):254–263.
- , , , et al. Error reduction and performance improvement in the emergency department through formal teamwork training: evaluation results of the MedTeams project. Health Serv Res. 2002;37(6):1553–1581.
- , , , et al. Effects of teamwork training on adverse outcomes and process of care in labor and delivery: a randomized controlled trial. Obstet Gynecol. 2007;109(1):48–55.
- , , , et al. Effects of interdisciplinary team care interventions on general medical wards: a systematic review. JAMA Intern Med. 2015;175(8):1288–1298.
- , , , et al. Patient risk factors for adverse drug events in hospitalized patients. ADE Prevention Study Group. Arch Intern Med. 1999;159(21):2553–2560.
- , , , et al. Effect of a pharmacist intervention on clinically important medication errors after hospital discharge: a randomized trial. Ann Intern Med. 2012;157(1):1–10.
- , , , . Use of a standardized protocol to decrease medication errors and adverse events related to sliding scale insulin. Qual Saf Health Care. 2006;15(2):89–91.
- , , , . Clinical pharmacists and inpatient medical care: a systematic review. Arch Intern Med. 2006;166(9):955–964.
- , , , , . Improved inpatient use of basal insulin, reduced hypoglycemia, and improved glycemic control: effect of structured subcutaneous insulin orders and an insulin management algorithm. J Hosp Med. 2009;4(1):3–15.
- , , , . Effects of a computerized order set on the inpatient management of hyperglycemia: a cluster‐randomized controlled trial. Endocr Pract. 2010;16(2):209–218.
- , , , . Effects of a subcutaneous insulin protocol, clinical education, and computerized order set on the quality of inpatient management of hyperglycemia: results of a clinical trial. J Hosp Med. 2009;4(1):16–27.
- Joint Commission on Accreditation of Healthcare Organizations. Understanding and Preventing Sentinel Events in Your Health Care Organization. Oak Brook, IL: Joint Commission; 2008.
- , , , et al. Communication failures in the operating room: an observational classification of recurrent types and effects. Qual Saf Health Care. 2004;13(5):330–334.
- , , , et al. Operating room teamwork among physicians and nurses: teamwork in the eye of the beholder. J Am Coll Surg. 2006;202(5):746–752.
- , , . Discrepant attitudes about teamwork among critical care nurses and physicians. Crit Care Med. 2003;31(3):956–959.
- , , , , . Communication failures in patient sign‐out and suggestions for improvement: a critical incident analysis. Qual Saf Health Care. 2005;14(6):401–407.
- , , , et al. Changes in medical errors after implementation of a handoff program. N Engl J Med. 2014;371(19):1803–1812.
- , , , et al. Communication and information deficits in patients discharged to rehabilitation facilities: an evaluation of five acute care hospitals. J Hosp Med. 2009;4(8):E28–E33.
- , , , , , . Deficits in communication and information transfer between hospital‐based and primary care physicians: implications for patient safety and continuity of care. JAMA. 2007;297(8):831–841.
- , , , et al. Patterns of nurse‐physician communication and agreement on the plan of care. Qual Saf Health Care. 2010;19(3):195–199.
- , , , , , . Can we talk? Priorities for patient care differed among health care providers. In: Henriksen K, Battles JB, Marks ES, Lewin DI, eds. Advances in Patient Safety: From Research to Implementation. Vol 1. Rockville, MD: Agency for Healthcare Research and Quality; 2005.
- , , , et al. Structured interdisciplinary rounds in a medical teaching unit: improving patient safety. Arch Intern Med. 2011;171(7):678–684.
- , , , , , . Improving teamwork: impact of structured interdisciplinary rounds on a hospitalist unit. J Hosp Med. 2011;6(2):88–93.
- , , , et al. Impact of localizing physicians to hospital units on nurse‐physician communication and agreement on the plan of care. J Gen Intern Med. 2009;24(11):1223–1227.
- , , , et al. Reorganizing a hospital ward as an accountable care unit. J Hosp Med. 2015;10(1):36–40.
- , , , et al. 5th time's a charm: creation of unit‐based care teams in a high occupancy hospital [abstract]. J Hosp Med. 2015;10 (suppl. 2). Available at: http://www.shmabstracts.com/abstract/5th‐times‐a‐charm‐creation‐of‐unit‐based‐care‐teams‐in‐a‐high‐occupancy‐hospital. Accessed July 28, 2015.
- , , , . Comorbidity measures for use with administrative data. Med Care. 1998;36(1):8–27.
- , , , , , . Teamwork on inpatient medical units: assessing attitudes and barriers. Qual Saf Health Care. 2010;19(2):117–121.
- , , , et al. A model for quality improvement programs in academic departments of medicine. Am J Med. 2008;121(10):922–929.
- , , , , . Improving nurse‐physician communication and satisfaction in the intensive care unit with a daily goals worksheet. Am J Crit Care. 2006;15(2):217–222.
- , , , , , . Improving communication in the ICU using daily goals. J Crit Care. 2003;18(2):71–75.
- , , , et al. Effect of crew resource management training in a multidisciplinary obstetrical setting. Int J Qual Health Care. 2008;20(4):254–263.
- , , , et al. Error reduction and performance improvement in the emergency department through formal teamwork training: evaluation results of the MedTeams project. Health Serv Res. 2002;37(6):1553–1581.
- , , , et al. Effects of teamwork training on adverse outcomes and process of care in labor and delivery: a randomized controlled trial. Obstet Gynecol. 2007;109(1):48–55.
- , , , et al. Effects of interdisciplinary team care interventions on general medical wards: a systematic review. JAMA Intern Med. 2015;175(8):1288–1298.
- , , , et al. Patient risk factors for adverse drug events in hospitalized patients. ADE Prevention Study Group. Arch Intern Med. 1999;159(21):2553–2560.
- , , , et al. Effect of a pharmacist intervention on clinically important medication errors after hospital discharge: a randomized trial. Ann Intern Med. 2012;157(1):1–10.
- , , , . Use of a standardized protocol to decrease medication errors and adverse events related to sliding scale insulin. Qual Saf Health Care. 2006;15(2):89–91.
- , , , . Clinical pharmacists and inpatient medical care: a systematic review. Arch Intern Med. 2006;166(9):955–964.
- , , , , . Improved inpatient use of basal insulin, reduced hypoglycemia, and improved glycemic control: effect of structured subcutaneous insulin orders and an insulin management algorithm. J Hosp Med. 2009;4(1):3–15.
- , , , . Effects of a computerized order set on the inpatient management of hyperglycemia: a cluster‐randomized controlled trial. Endocr Pract. 2010;16(2):209–218.
- , , , . Effects of a subcutaneous insulin protocol, clinical education, and computerized order set on the quality of inpatient management of hyperglycemia: results of a clinical trial. J Hosp Med. 2009;4(1):16–27.
Improving Interhospital Transfers
Mrs. S arrived to the medicine service at our hospital by ambulance transport at 9:00 pm. The intern on call received a page from the nurse, Mrs. S has arrived. She is confused. Please assess. As is often the case, the intern had no prior knowledge of the patient's arrival, and review of medical records indicated that Mrs. S had never been seen at our hospital before.
The intern went to the bedside to assess the patient and found an elderly woman who appeared confused and was unable to provider her medical history, reason for the transfer, or details about her recent hospital course.
A few minutes later, the patient's son arrived at the bedside asking about her plan of care. The intern looked through the stack of papers in the envelope by her chart, and was able to locate reports of a recent chest x‐ray and abdominal computed tomography, as well as copies of brief progress notes, but was unable to find a transfer summary detailing her prior 5 days of hospitalization or reason for transfer. The patient's son was able to give some information, but he had just returned from a business trip and was not up to date on the details of his mother's hospital stay. Based on her son's input, the intern concluded the patient's somnolence was not her baseline; he performed an arterial blood gas and blood work, revealing profound acidemia and hyponatremia of unclear acuity. Mrs. S became hypotensive, requiring transfer to the intensive care unit. Several days later, she died.
This scenario highlights the potential dangers associated with patient transfers between acute care hospitals, known as interhospital transfer (IHT). Unfortunately, the described scenario is not a rare event.[1, 2] Most providers who care for transferred patients can recount similar challenges when caring for IHT patients.[3]
Patient transfers from 1 hospital to another are common, affecting nearly 1 in 20 Medicare patients admitted to the intensive care unit[4] and up to 50% of patients presenting with acute myocardial infarction,[5] although reasons for transfer remain largely unstudied. The Emergency Medical Treatment and Active Labor Act requires a hospital to transfer patients who require a more specialized service unavailable at the subject institution, or when medical benefits outweigh the increased risks to the individual.[6] Yet, this broad standard provides little guidance to clinicians in practice.
Identifying which patients may benefit from transfer is an ambiguous and subjective process. Studies show little agreement between the reasons cited for transfer among patients, transferring physicians, and receiving physicians,[7] and incentives for transfer are often different between each stakeholder. For example, patients or families might initiate transfer for a second opinion from a fresh set of eyes because of a grim or uncertain prognosis or in the hope of a more promising or definitive medical opinion. Similarly, referring physicians may initiate transfer for particular procedures, surgeries, or consultations that the receiving physician may ultimately decide will be of little clinical benefit to the patient. Such heightened expectations and changes to the care plan as agreed on by the patient and referring physician may affect the patient's perceptions of care at the receiving institution, although exactly how remains unknown. Alternatively, patients and families may desire transfer because of previously established relationships with providers at another institution, or they may be dissatisfied with certain aspects of care at the referring institution. Referring institutions may initiate transfer for a variety of reasons, including inability to provide a needed procedure or test, patient/family preference, or protocol, among others. Receiving hospitals usually have an interest in maintaining a large referral base for the sake of both revenue and reputation, but may also view accepting transfers as part of their larger mission to provide expert consultation and specialty services that may not be available at the referring institution. Additional proposed benefits include strengthening provider networks, promoting clinical diversity, and improving the educational experience of trainees often present at the accepting institution. Although patients, providers, and referring and accepting hospitals all undoubtedly benefit from various aspects of the IHT, further research is needed to more clearly identify which patients are most likely to benefit from transfer and why.
Once the decision to transfer/accept a patient has been made, there are no clear guidelines over how this process should be executed. For this reason, care providers at community hospitals describe IHT as frustrating and time consuming.[8] Referring providers may face challenges identifying an accepting hospital due to the limited capacity of the receiving institution, reaching the correct receiving physician, and managing delays in transfer once the patient is accepted.[8] Similarly, accepting physicians may be frustrated by the time waste associated with accepting a patient that ends up transferred to another facility, limited authority to triage the patient to the most appropriate accepting service, inability to predict time of patient arrival, and missing pieces of critical information at time of patient arrival, among other reasons. Furthermore, incompatible electronic health records make access to data from the referring institution difficult. For example, without standards for transferring imaging, patients may undergo unnecessary and costly duplicate imaging leading to delay in needed procedures. Existing guidelines are largely focused on equipment and expertise required for the physical transfer of the patient, but fail to consider other aspects of the transfer process that may be critical for patient safety such as protocols for communication of patient information and transfer of completed imaging. As such, hospitals are largely left to devise their own protocols for IHT, which often differ between hospitals as well as between different services within 1 hospital.[1, 3]
Although it is true that many patients benefit from IHT, the process introduces inherent vulnerability into healthcare delivery. Moving a patient between facilities exposes that individual to risks associated with discontinuity of care, well described in the literature on intrahospital patient handoffs (ie, the transfer of patient care responsibility from 1 provider to another within 1 hospital), which can lead to excessive costs and poor patient outcomes.[9] Presumably, such risks are even greater for patients transferred between hospitals than for those transferred between providers within 1 hospital, because system factors like electronic health records, nursing and ancillary staff continuity, and accessibility of transferring provider are not in place to mitigate communication gaps. Furthermore, unlike discharges home or to subacute care facilities, also known to be error prone and lead to adverse events,[10, 11] in the case of IHT, patients are often more acutely ill and less stable. In fact, limited data suggest that aside from a select subset of patients requiring specialized care, individuals transferred may have increased resource utilization and greater‐than‐expected mortality than those who are not transferred.[1, 2, 12] Moreover, these findings may not be entirely attributable to medical complexity among transferred patients.
Today, the process of IHT varies tremendously across US hospitals,[1] differences that may have significant implications for both cost and patient safety outcomes. Standardization of IHT, including patient selection and information exchange between transferring and accepting providers/emnstitutions, is imperative to improve the quality and safety of this process. As demonstrated with other common, high‐risk care transitions, such as intrahospital patient handoffs and patient discharge, creating basic guidelines of practice (such as including important data elements at time of care transfer)[13, 14] is necessary to improve quality of the care transition.
However, to achieve high‐quality standardization, we must first methodologically conduct rigorous clinical research to understand fundamental issues of the IHT process, including why patients are transferred (from the perspective of patients and transferring and accepting institutions), which patients benefit most from transfer and why, and how various IHT processes impact health outcomes. Interventions such as communication and data transfer tools, feedback mechanisms between referring and accepting institutions, and other evidence‐based guidelines can then be designed to improve IHT based on the findings of this research while still allowing for flexibility of individual patient needs. Additional work is then needed to implement and rigorously evaluate the effects of such interventions on patient and provider outcomes including, but not limited to, length of stay, adverse events, mortality, readmissions, and patient satisfaction measures. In summary, by focusing research and quality improvement initiatives on these vital questions, we can begin to improve the quality of care we provide to patients during this critical transition of care.
Disclosure
Disclosure: Nothing to report.
- , , , . Patients transferred from outside hospitals to academic hospitalists and general internists have higher mortality and costs than patients from the ED. Paper presented at: Society of Hospital Medicine National Conference; May 2013; Washington, DC.
- , , , . Interhospital facility transfers in the united states: a nationwide outcomes study [published online ahead of print November 13, 2014]. J Patient Saf. doi: 10.1097/PTS.0000000000000148.
- , . Physician perspectives on inter‐hospital transfers. Paper presented at: Society of Hospital Medicine National Conference; March 2014; Las Vegas, NV.
- , , , , . The structure of critical care transfer networks. Med Care. 2009;47(7):787–793.
- , , , . Interhospital transfers among Medicare beneficiaries admitted for acute myocardial infarction at nonrevascularization hospitals. Circ Cardiovasc Qual Outcomes. 2010;3(5):468–475.
- U.S. House of Representatives. Office of the Law Revision Counsel. Examination and treatment for emergency medical conditions and women in labor. Title 42 USC §1395dd. Available at: http://www.gpo.gov/fdsys/granule/USCODE‐2010‐title42/USCODE‐2010‐title42‐chap7‐subchapXVIII‐partE‐sec1395dd. Accessed October 29 2014.
- , , . Reasons underlying interhospital transfers to an academic medical intensive care unit. J Crit Care. 2013;28(2):202–208.
- , , . Which patients and where: a qualitative study of patient transfers from community hospitals. Med Care. 2011;49(6):592–598.
- , , , . Conceptualizing handover strategies at change of shift in the emergency department: a grounded theory study. BMC Health Serv Res. 2008;8:256.
- , , , , . The incidence and severity of adverse events affecting patients after discharge from the hospital. Ann Intern Med. 2003;138(3):161–167.
- , , , , . Pending laboratory tests and the hospital discharge summary in patients discharged to sub‐acute care. J Gen Intern Med. 2011;26(4):393–398.
- , , , , . Comparing the hospitalizations of transfer and non‐transfer patients in an academic medical center. Acad Med. 1996;71(3):262–266.
- , , , , , . I‐pass, a mnemonic to standardize verbal handoffs. Pediatrics. 2012;129(2):201–204.
- Hospital Medicine Reengineering Network (HOMERUN) Collaborative. Executive summary. Available at: https://members.aamc.org/eweb/upload/HOMERUN%20summary%202012.pdf. Accessed July 23, 2013.
Mrs. S arrived to the medicine service at our hospital by ambulance transport at 9:00 pm. The intern on call received a page from the nurse, Mrs. S has arrived. She is confused. Please assess. As is often the case, the intern had no prior knowledge of the patient's arrival, and review of medical records indicated that Mrs. S had never been seen at our hospital before.
The intern went to the bedside to assess the patient and found an elderly woman who appeared confused and was unable to provider her medical history, reason for the transfer, or details about her recent hospital course.
A few minutes later, the patient's son arrived at the bedside asking about her plan of care. The intern looked through the stack of papers in the envelope by her chart, and was able to locate reports of a recent chest x‐ray and abdominal computed tomography, as well as copies of brief progress notes, but was unable to find a transfer summary detailing her prior 5 days of hospitalization or reason for transfer. The patient's son was able to give some information, but he had just returned from a business trip and was not up to date on the details of his mother's hospital stay. Based on her son's input, the intern concluded the patient's somnolence was not her baseline; he performed an arterial blood gas and blood work, revealing profound acidemia and hyponatremia of unclear acuity. Mrs. S became hypotensive, requiring transfer to the intensive care unit. Several days later, she died.
This scenario highlights the potential dangers associated with patient transfers between acute care hospitals, known as interhospital transfer (IHT). Unfortunately, the described scenario is not a rare event.[1, 2] Most providers who care for transferred patients can recount similar challenges when caring for IHT patients.[3]
Patient transfers from 1 hospital to another are common, affecting nearly 1 in 20 Medicare patients admitted to the intensive care unit[4] and up to 50% of patients presenting with acute myocardial infarction,[5] although reasons for transfer remain largely unstudied. The Emergency Medical Treatment and Active Labor Act requires a hospital to transfer patients who require a more specialized service unavailable at the subject institution, or when medical benefits outweigh the increased risks to the individual.[6] Yet, this broad standard provides little guidance to clinicians in practice.
Identifying which patients may benefit from transfer is an ambiguous and subjective process. Studies show little agreement between the reasons cited for transfer among patients, transferring physicians, and receiving physicians,[7] and incentives for transfer are often different between each stakeholder. For example, patients or families might initiate transfer for a second opinion from a fresh set of eyes because of a grim or uncertain prognosis or in the hope of a more promising or definitive medical opinion. Similarly, referring physicians may initiate transfer for particular procedures, surgeries, or consultations that the receiving physician may ultimately decide will be of little clinical benefit to the patient. Such heightened expectations and changes to the care plan as agreed on by the patient and referring physician may affect the patient's perceptions of care at the receiving institution, although exactly how remains unknown. Alternatively, patients and families may desire transfer because of previously established relationships with providers at another institution, or they may be dissatisfied with certain aspects of care at the referring institution. Referring institutions may initiate transfer for a variety of reasons, including inability to provide a needed procedure or test, patient/family preference, or protocol, among others. Receiving hospitals usually have an interest in maintaining a large referral base for the sake of both revenue and reputation, but may also view accepting transfers as part of their larger mission to provide expert consultation and specialty services that may not be available at the referring institution. Additional proposed benefits include strengthening provider networks, promoting clinical diversity, and improving the educational experience of trainees often present at the accepting institution. Although patients, providers, and referring and accepting hospitals all undoubtedly benefit from various aspects of the IHT, further research is needed to more clearly identify which patients are most likely to benefit from transfer and why.
Once the decision to transfer/accept a patient has been made, there are no clear guidelines over how this process should be executed. For this reason, care providers at community hospitals describe IHT as frustrating and time consuming.[8] Referring providers may face challenges identifying an accepting hospital due to the limited capacity of the receiving institution, reaching the correct receiving physician, and managing delays in transfer once the patient is accepted.[8] Similarly, accepting physicians may be frustrated by the time waste associated with accepting a patient that ends up transferred to another facility, limited authority to triage the patient to the most appropriate accepting service, inability to predict time of patient arrival, and missing pieces of critical information at time of patient arrival, among other reasons. Furthermore, incompatible electronic health records make access to data from the referring institution difficult. For example, without standards for transferring imaging, patients may undergo unnecessary and costly duplicate imaging leading to delay in needed procedures. Existing guidelines are largely focused on equipment and expertise required for the physical transfer of the patient, but fail to consider other aspects of the transfer process that may be critical for patient safety such as protocols for communication of patient information and transfer of completed imaging. As such, hospitals are largely left to devise their own protocols for IHT, which often differ between hospitals as well as between different services within 1 hospital.[1, 3]
Although it is true that many patients benefit from IHT, the process introduces inherent vulnerability into healthcare delivery. Moving a patient between facilities exposes that individual to risks associated with discontinuity of care, well described in the literature on intrahospital patient handoffs (ie, the transfer of patient care responsibility from 1 provider to another within 1 hospital), which can lead to excessive costs and poor patient outcomes.[9] Presumably, such risks are even greater for patients transferred between hospitals than for those transferred between providers within 1 hospital, because system factors like electronic health records, nursing and ancillary staff continuity, and accessibility of transferring provider are not in place to mitigate communication gaps. Furthermore, unlike discharges home or to subacute care facilities, also known to be error prone and lead to adverse events,[10, 11] in the case of IHT, patients are often more acutely ill and less stable. In fact, limited data suggest that aside from a select subset of patients requiring specialized care, individuals transferred may have increased resource utilization and greater‐than‐expected mortality than those who are not transferred.[1, 2, 12] Moreover, these findings may not be entirely attributable to medical complexity among transferred patients.
Today, the process of IHT varies tremendously across US hospitals,[1] differences that may have significant implications for both cost and patient safety outcomes. Standardization of IHT, including patient selection and information exchange between transferring and accepting providers/emnstitutions, is imperative to improve the quality and safety of this process. As demonstrated with other common, high‐risk care transitions, such as intrahospital patient handoffs and patient discharge, creating basic guidelines of practice (such as including important data elements at time of care transfer)[13, 14] is necessary to improve quality of the care transition.
However, to achieve high‐quality standardization, we must first methodologically conduct rigorous clinical research to understand fundamental issues of the IHT process, including why patients are transferred (from the perspective of patients and transferring and accepting institutions), which patients benefit most from transfer and why, and how various IHT processes impact health outcomes. Interventions such as communication and data transfer tools, feedback mechanisms between referring and accepting institutions, and other evidence‐based guidelines can then be designed to improve IHT based on the findings of this research while still allowing for flexibility of individual patient needs. Additional work is then needed to implement and rigorously evaluate the effects of such interventions on patient and provider outcomes including, but not limited to, length of stay, adverse events, mortality, readmissions, and patient satisfaction measures. In summary, by focusing research and quality improvement initiatives on these vital questions, we can begin to improve the quality of care we provide to patients during this critical transition of care.
Disclosure
Disclosure: Nothing to report.
Mrs. S arrived to the medicine service at our hospital by ambulance transport at 9:00 pm. The intern on call received a page from the nurse, Mrs. S has arrived. She is confused. Please assess. As is often the case, the intern had no prior knowledge of the patient's arrival, and review of medical records indicated that Mrs. S had never been seen at our hospital before.
The intern went to the bedside to assess the patient and found an elderly woman who appeared confused and was unable to provider her medical history, reason for the transfer, or details about her recent hospital course.
A few minutes later, the patient's son arrived at the bedside asking about her plan of care. The intern looked through the stack of papers in the envelope by her chart, and was able to locate reports of a recent chest x‐ray and abdominal computed tomography, as well as copies of brief progress notes, but was unable to find a transfer summary detailing her prior 5 days of hospitalization or reason for transfer. The patient's son was able to give some information, but he had just returned from a business trip and was not up to date on the details of his mother's hospital stay. Based on her son's input, the intern concluded the patient's somnolence was not her baseline; he performed an arterial blood gas and blood work, revealing profound acidemia and hyponatremia of unclear acuity. Mrs. S became hypotensive, requiring transfer to the intensive care unit. Several days later, she died.
This scenario highlights the potential dangers associated with patient transfers between acute care hospitals, known as interhospital transfer (IHT). Unfortunately, the described scenario is not a rare event.[1, 2] Most providers who care for transferred patients can recount similar challenges when caring for IHT patients.[3]
Patient transfers from 1 hospital to another are common, affecting nearly 1 in 20 Medicare patients admitted to the intensive care unit[4] and up to 50% of patients presenting with acute myocardial infarction,[5] although reasons for transfer remain largely unstudied. The Emergency Medical Treatment and Active Labor Act requires a hospital to transfer patients who require a more specialized service unavailable at the subject institution, or when medical benefits outweigh the increased risks to the individual.[6] Yet, this broad standard provides little guidance to clinicians in practice.
Identifying which patients may benefit from transfer is an ambiguous and subjective process. Studies show little agreement between the reasons cited for transfer among patients, transferring physicians, and receiving physicians,[7] and incentives for transfer are often different between each stakeholder. For example, patients or families might initiate transfer for a second opinion from a fresh set of eyes because of a grim or uncertain prognosis or in the hope of a more promising or definitive medical opinion. Similarly, referring physicians may initiate transfer for particular procedures, surgeries, or consultations that the receiving physician may ultimately decide will be of little clinical benefit to the patient. Such heightened expectations and changes to the care plan as agreed on by the patient and referring physician may affect the patient's perceptions of care at the receiving institution, although exactly how remains unknown. Alternatively, patients and families may desire transfer because of previously established relationships with providers at another institution, or they may be dissatisfied with certain aspects of care at the referring institution. Referring institutions may initiate transfer for a variety of reasons, including inability to provide a needed procedure or test, patient/family preference, or protocol, among others. Receiving hospitals usually have an interest in maintaining a large referral base for the sake of both revenue and reputation, but may also view accepting transfers as part of their larger mission to provide expert consultation and specialty services that may not be available at the referring institution. Additional proposed benefits include strengthening provider networks, promoting clinical diversity, and improving the educational experience of trainees often present at the accepting institution. Although patients, providers, and referring and accepting hospitals all undoubtedly benefit from various aspects of the IHT, further research is needed to more clearly identify which patients are most likely to benefit from transfer and why.
Once the decision to transfer/accept a patient has been made, there are no clear guidelines over how this process should be executed. For this reason, care providers at community hospitals describe IHT as frustrating and time consuming.[8] Referring providers may face challenges identifying an accepting hospital due to the limited capacity of the receiving institution, reaching the correct receiving physician, and managing delays in transfer once the patient is accepted.[8] Similarly, accepting physicians may be frustrated by the time waste associated with accepting a patient that ends up transferred to another facility, limited authority to triage the patient to the most appropriate accepting service, inability to predict time of patient arrival, and missing pieces of critical information at time of patient arrival, among other reasons. Furthermore, incompatible electronic health records make access to data from the referring institution difficult. For example, without standards for transferring imaging, patients may undergo unnecessary and costly duplicate imaging leading to delay in needed procedures. Existing guidelines are largely focused on equipment and expertise required for the physical transfer of the patient, but fail to consider other aspects of the transfer process that may be critical for patient safety such as protocols for communication of patient information and transfer of completed imaging. As such, hospitals are largely left to devise their own protocols for IHT, which often differ between hospitals as well as between different services within 1 hospital.[1, 3]
Although it is true that many patients benefit from IHT, the process introduces inherent vulnerability into healthcare delivery. Moving a patient between facilities exposes that individual to risks associated with discontinuity of care, well described in the literature on intrahospital patient handoffs (ie, the transfer of patient care responsibility from 1 provider to another within 1 hospital), which can lead to excessive costs and poor patient outcomes.[9] Presumably, such risks are even greater for patients transferred between hospitals than for those transferred between providers within 1 hospital, because system factors like electronic health records, nursing and ancillary staff continuity, and accessibility of transferring provider are not in place to mitigate communication gaps. Furthermore, unlike discharges home or to subacute care facilities, also known to be error prone and lead to adverse events,[10, 11] in the case of IHT, patients are often more acutely ill and less stable. In fact, limited data suggest that aside from a select subset of patients requiring specialized care, individuals transferred may have increased resource utilization and greater‐than‐expected mortality than those who are not transferred.[1, 2, 12] Moreover, these findings may not be entirely attributable to medical complexity among transferred patients.
Today, the process of IHT varies tremendously across US hospitals,[1] differences that may have significant implications for both cost and patient safety outcomes. Standardization of IHT, including patient selection and information exchange between transferring and accepting providers/emnstitutions, is imperative to improve the quality and safety of this process. As demonstrated with other common, high‐risk care transitions, such as intrahospital patient handoffs and patient discharge, creating basic guidelines of practice (such as including important data elements at time of care transfer)[13, 14] is necessary to improve quality of the care transition.
However, to achieve high‐quality standardization, we must first methodologically conduct rigorous clinical research to understand fundamental issues of the IHT process, including why patients are transferred (from the perspective of patients and transferring and accepting institutions), which patients benefit most from transfer and why, and how various IHT processes impact health outcomes. Interventions such as communication and data transfer tools, feedback mechanisms between referring and accepting institutions, and other evidence‐based guidelines can then be designed to improve IHT based on the findings of this research while still allowing for flexibility of individual patient needs. Additional work is then needed to implement and rigorously evaluate the effects of such interventions on patient and provider outcomes including, but not limited to, length of stay, adverse events, mortality, readmissions, and patient satisfaction measures. In summary, by focusing research and quality improvement initiatives on these vital questions, we can begin to improve the quality of care we provide to patients during this critical transition of care.
Disclosure
Disclosure: Nothing to report.
- , , , . Patients transferred from outside hospitals to academic hospitalists and general internists have higher mortality and costs than patients from the ED. Paper presented at: Society of Hospital Medicine National Conference; May 2013; Washington, DC.
- , , , . Interhospital facility transfers in the united states: a nationwide outcomes study [published online ahead of print November 13, 2014]. J Patient Saf. doi: 10.1097/PTS.0000000000000148.
- , . Physician perspectives on inter‐hospital transfers. Paper presented at: Society of Hospital Medicine National Conference; March 2014; Las Vegas, NV.
- , , , , . The structure of critical care transfer networks. Med Care. 2009;47(7):787–793.
- , , , . Interhospital transfers among Medicare beneficiaries admitted for acute myocardial infarction at nonrevascularization hospitals. Circ Cardiovasc Qual Outcomes. 2010;3(5):468–475.
- U.S. House of Representatives. Office of the Law Revision Counsel. Examination and treatment for emergency medical conditions and women in labor. Title 42 USC §1395dd. Available at: http://www.gpo.gov/fdsys/granule/USCODE‐2010‐title42/USCODE‐2010‐title42‐chap7‐subchapXVIII‐partE‐sec1395dd. Accessed October 29 2014.
- , , . Reasons underlying interhospital transfers to an academic medical intensive care unit. J Crit Care. 2013;28(2):202–208.
- , , . Which patients and where: a qualitative study of patient transfers from community hospitals. Med Care. 2011;49(6):592–598.
- , , , . Conceptualizing handover strategies at change of shift in the emergency department: a grounded theory study. BMC Health Serv Res. 2008;8:256.
- , , , , . The incidence and severity of adverse events affecting patients after discharge from the hospital. Ann Intern Med. 2003;138(3):161–167.
- , , , , . Pending laboratory tests and the hospital discharge summary in patients discharged to sub‐acute care. J Gen Intern Med. 2011;26(4):393–398.
- , , , , . Comparing the hospitalizations of transfer and non‐transfer patients in an academic medical center. Acad Med. 1996;71(3):262–266.
- , , , , , . I‐pass, a mnemonic to standardize verbal handoffs. Pediatrics. 2012;129(2):201–204.
- Hospital Medicine Reengineering Network (HOMERUN) Collaborative. Executive summary. Available at: https://members.aamc.org/eweb/upload/HOMERUN%20summary%202012.pdf. Accessed July 23, 2013.
- , , , . Patients transferred from outside hospitals to academic hospitalists and general internists have higher mortality and costs than patients from the ED. Paper presented at: Society of Hospital Medicine National Conference; May 2013; Washington, DC.
- , , , . Interhospital facility transfers in the united states: a nationwide outcomes study [published online ahead of print November 13, 2014]. J Patient Saf. doi: 10.1097/PTS.0000000000000148.
- , . Physician perspectives on inter‐hospital transfers. Paper presented at: Society of Hospital Medicine National Conference; March 2014; Las Vegas, NV.
- , , , , . The structure of critical care transfer networks. Med Care. 2009;47(7):787–793.
- , , , . Interhospital transfers among Medicare beneficiaries admitted for acute myocardial infarction at nonrevascularization hospitals. Circ Cardiovasc Qual Outcomes. 2010;3(5):468–475.
- U.S. House of Representatives. Office of the Law Revision Counsel. Examination and treatment for emergency medical conditions and women in labor. Title 42 USC §1395dd. Available at: http://www.gpo.gov/fdsys/granule/USCODE‐2010‐title42/USCODE‐2010‐title42‐chap7‐subchapXVIII‐partE‐sec1395dd. Accessed October 29 2014.
- , , . Reasons underlying interhospital transfers to an academic medical intensive care unit. J Crit Care. 2013;28(2):202–208.
- , , . Which patients and where: a qualitative study of patient transfers from community hospitals. Med Care. 2011;49(6):592–598.
- , , , . Conceptualizing handover strategies at change of shift in the emergency department: a grounded theory study. BMC Health Serv Res. 2008;8:256.
- , , , , . The incidence and severity of adverse events affecting patients after discharge from the hospital. Ann Intern Med. 2003;138(3):161–167.
- , , , , . Pending laboratory tests and the hospital discharge summary in patients discharged to sub‐acute care. J Gen Intern Med. 2011;26(4):393–398.
- , , , , . Comparing the hospitalizations of transfer and non‐transfer patients in an academic medical center. Acad Med. 1996;71(3):262–266.
- , , , , , . I‐pass, a mnemonic to standardize verbal handoffs. Pediatrics. 2012;129(2):201–204.
- Hospital Medicine Reengineering Network (HOMERUN) Collaborative. Executive summary. Available at: https://members.aamc.org/eweb/upload/HOMERUN%20summary%202012.pdf. Accessed July 23, 2013.