User login
Interhospital Transfer and Receipt of Specialty Procedures
Patients who undergo interhospital transfer (IHT) are felt to benefit from receipt of unique specialty care at the receiving hospital.1 Although only 1.5% of all hospitalized Medicare patients undergo hospital transfer,2 the frequency of transfer is much greater within certain patient populations, as may be expected with diagnoses requiring specialty care.3,4 Existent data demonstrate that 5% of Medicare patients admitted to the intensive care unit (ICU)5 and up to 50% of patients presenting with acute myocardial infarction (AMI) undergo IHT.6
More recent data suggest variability in hospital transfer practices not accounted for by differences in patient or hospital characteristics.2 Although disease-specific guidelines for IHT exist for certain diagnoses,3,4 the process remains largely nonstandardized for many patients,7 leading to ambiguity surrounding indications for transfer. Because limited data suggest worse outcomes for transferred versus nontransferred patients,8 a better understanding of the specialized care patients actually receive across the transfer continuum may help to elucidate potential indications for transfer and ultimately help delineate which patients are most (or least) likely to benefit from transfer and why.
In this national study, we examined a select cohort of transferred patients with diagnoses associated with specific specialty procedural services to determine if they received these procedures and where along the transfer continuum they were performed.
METHODS
We performed a cross-sectional analysis using the Center for Medicare and Medicaid Services 2013 100% Master Beneficiary Summary and Inpatient claims files. Our study protocol was approved by the Partners Healthcare Human Subjects Review Committee.
Beneficiaries were eligible for inclusion if they were aged ≥65 years, continuously enrolled in Medicare A and B, and with an acute care hospitalization claim in 2013, excluding Medicare managed care and end stage renal disease beneficiaries due to incomplete claims data in these groups. We additionally excluded beneficiaries hospitalized at federal or nonacute care hospitals, or critical access hospitals given their mission to stabilize and then transfer patients to referral hospitals.9
Transferred patients were defined as beneficiaries with corresponding “transfer in” and “transfer out” claims, or those with either claim and a corresponding date of admission/discharge from another hospital within 1 day of the claim, as we used in our prior research.2 Beneficiaries transferred to the same hospital, those with greater than 1 transfer within the same hospitalization, or those cared for at hospitals with “outlier” transfer-in rates equal to 100% or transfer-out rates greater than 35% were excluded from analysis given the suggestion of nonstandard claims practices.
We first identified the top 15 primary diagnoses at time of transfer using International Classification of Diseases, Ninth Revision (ICD-9) codes (supplementary Appendix), and then identified those 4 most likely to require specialty procedural services: AMI, gastrointestinal bleed (GI bleed), renal failure, and hip fracture/dislocation. We then chose associated ICD-9 procedure codes for each diagnosis, via expert opinion (authors SM and JS, hospitalist physicians with greater than 20 years of combined clinical experience), erring on overinclusion of procedure codes. We then quantified receipt of associated procedures at transferring and receiving hospitals, stratified by diagnosis.
We further explored the cohort of patients with hip fracture/dislocation who underwent an associated procedure at the transferring but not receiving hospital, examining the frequency with which these patients had other (nonrelated) procedures at the receiving hospital, and identifying which procedures they received.
RESULTS
Of the 101,507 patients transferred to another hospital, 19,613 (19.3%) had a primary diagnosis of AMI, GI bleed, renal failure, or hip fracture/dislocation. Table 1 lists the ICD-9 procedure codes associated with each diagnosis.
Distribution of receipt of specialty procedures at the transferring and receiving hospitals varied by disease (Figure). With the exception of GI bleed, patients more often received specialty procedural care at the receiving than the transferring hospital. Depending on primary diagnosis, between 32.4% and 89.1% of patients did not receive any associated specialty procedure at the receiving hospital.
Of the 370 (22.1%) hip fracture/dislocation patients that received a specialty procedure at the transferring but not receiving hospital, 132 (35.7%) did not receive any procedure at the receiving hospital, whereas the remaining 238 (64.3%) received an unrelated (not associated with the primary diagnosis) procedure. There was great variety in the types of procedures received, the most common being transfusion of blood products (ICD-9 Clinical Modification 9904).
DISCUSSION
Among transferred patients with primary diagnoses that have clearly associated specialized procedural services, we found that patients received these procedures at varying frequency and locations across the transfer continuum. Across 4 diagnoses, receipt of associated procedures was more common at the receiving than the transferring hospital, with the exception being patients with GI bleed. We additionally found that many transferred patients did not receive any associated specialty procedure at the receiving hospital. These findings suggest the strong likelihood of more diverse underlying reasons for transfer rather than solely receipt of specialized procedural care.
Despite the frequency with which AMI patients are transferred,6 and American Heart Association guidelines directing hospitals to transfer AMI patients to institutions able to provide necessary invasive treatments,4 prior studies suggest these patients inconsistently receive specialty intervention following transfer, including stress testing, cardiac catheterization, or coronary artery bypass graft surgery.10,11 Our findings add to these data, demonstrating that only 47.3% of patients transferred with AMI received any cardiac-related procedure at the receiving hospital. Additionally, we found that 38.1% of AMI patients do not receive any specialty procedures at either the transferring or the receiving hospital. Taken together, these data suggest possible discrepancies in the perceived need for these procedures between transferring and receiving hospitals, reasons for transfer related to these conditions that don’t involve an associated procedure, or reasons for transfer unrelated to specialty care of the primary diagnosis (such as care of comorbidities, hospital location, prior relationships with that hospital, or desire for a second opinion). Although some of these alternate reasons for transfer likely still benefit the patient, some of these reasons may not justify the increased risks of discontinuity of care created by IHT.
Given limited data looking at IHT practices for patients with other diagnoses, the varying patterns of specialty procedural interventions we observed among transferred patients with GI bleed, renal failure, and hip fracture/dislocation are novel contributions to this topic. Notably, we found that among patients transferred with a primary diagnosis of renal failure, the vast majority (84.1%) did not receive any associated procedure at either the transferring or the receiving hospital. It is possible that although these patients carried the diagnosis of renal failure, their clinical phenotype is more heterogeneous, and they could still be managed conservatively without receipt of invasive procedures such as hemodialysis.
Conversely, patients transferred with primary diagnosis of hip fracture/dislocation were far more likely to receive associated specialty procedural intervention at the receiving hospital, presumably reflective of the evidence demonstrating improved outcomes with early surgical intervention.12 However, these data do not explain the reasoning behind the substantial minority of patients who received specialty intervention at the transferring hospital prior to transfer or those that did not receive any specialty intervention at either the transferring or receiving hospital. Our secondary analysis demonstrating great variety in receipt and type of nonassociated procedures provided at the receiving hospital did not help to elucidate potential underlying reasons for transfer.
Notably, among patients transferred with primary diagnosis of GI bleed, receipt of specialty procedures was more common at the transferring (77.7%) than receiving (63.2%) hospital, with nearly half (49.3%) undergoing specialty procedures at both hospitals. It is possible that these findings are reflective of the broad array of specialty procedures examined within this diagnosis. For example, it is reasonable to consider that a patient may be stabilized with receipt of a blood transfusion at the transferring hospital, then transferred to undergo a diagnostic/therapeutic procedure (ie, endoscopy/colonoscopy) at the receiving hospital, as is suggested by our results.
Our study is subject to several limitations. First, given the criteria we used to define transfer, it is possible that we included nontransferred patients within our transferred cohort if they were discharged from one hospital and admitted to a different hospital within 1 day, although quality assurance analyses we conducted in prior studies on these data support the validity of the criteria used.2 Second, we cannot exclude the possibility that patients received nonprocedural specialty care (ie, expert opinion, specialized imaging, medical management, management of secondary diagnoses, etc.) not available at the transferring hospital, although, arguably, in select patients, such input could be obtained without physical transfer of the patient (ie, tele-consult). And even in patients transferred with intent to receive procedural care who did not ultimately receive that care, there is likely an appropriate “nonprocedure” rate, where patients who might benefit from a procedure receive a timely evaluation to reduce the risk of missing the opportunity to receive it. This would be analogous to transferring a patient to an ICU even if they do not end up requiring intubation or pressor therapy. However, given the likelihood of higher risks of IHT compared with intrahospital transfers, one could argue that the threshold of perceived benefit might be different in patients being considered for IHT. Additionally, we limited our analyses to only 4 diagnoses; thus, our findings may not be generalizable to other diagnoses of transferred patients. However, because the diagnoses we examined were ones considered most effectively treated with specialty procedural interventions, it is reasonable to presume that the variability in receipt of specialty procedures observed within these diagnoses is also present, if not greater, across other diagnoses. Third, although we intentionally included a broad array of specialty procedures associated with each diagnosis, it is possible that we overlooked particular specialty interventions. For example, in assuming that patients are most likely to be transferred to receive procedural services associated with their primary diagnosis, we may have missed alternate indications for transfer, including need for procedural care related to secondary or subsequent diagnoses (ie, a patient may have presented with GI bleed
CONCLUSIONS
We found that Medicare patients who undergo IHT with primary diagnoses of AMI, GI bleed, renal failure, and hip fracture/dislocation receive associated specialty interventions at varying frequency and locations, and many patients do not receive any associated procedures at receiving hospitals. Our findings suggest that specialty procedural care of patients, even those with primary diagnoses that often warrant specialized intervention, may not be the primary driver of IHT as commonly suggested, although underlying reasons for transfer in these and other “nonprocedural” transferred patients remains obscure. Given known ambiguity in the transfer process,7 and unclear benefit of IHT,8 additional research is required to further identify and evaluate other potential underlying reasons for transfer and to examine these in the context of patient outcomes, in order to understand which patients may or may not benefit from transfer and why.
Disclosure
The authors have nothing to disclose.
1. Iwashyna TJ. The incomplete infrastructure for interhospital patient transfer. Crit Care Med. 2012;40(8):2470-2478. PubMed
2. Mueller SK, Zheng J, Orav EJ, Schnipper JL. Rates, Predictors and Variability of Interhospital Transfers: A National Evaluation. J Hosp Med. 2017;12(6):435-442. PubMed
3. Guidelines for the transfer of critically ill patients. Guidelines Committee of the American College of Critical Care Medicine; Society of Critical Care Medicine and American Association of Critical-Care Nurses Transfer Guidelines Task Force. Crit Care Med. 1993;21(6):931-937. PubMed
4. Anderson JL, Adams CD, Antman EM, et al. 2011 ACCF/AHA Focused Update Incorporated Into the ACC/AHA 2007 Guidelines for the Management of Patients With Unstable Angina/Non-ST-Elevation Myocardial Infarction: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. Circulation. 2011;123(18):e426-e579. PubMed
5. Iwashyna TJ, Christie JD, Moody J, Kahn JM, Asch DA. The structure of critical care transfer networks. Med Care. 2009;47(7):787-793. PubMed
6. Iwashyna TJ, Kahn JM, Hayward RA, Nallamothu BK. Interhospital transfers among Medicare beneficiaries admitted for acute myocardial infarction at nonrevascularization hospitals. Circ Cardiovasc Qual Outcomes. 2010;3(5):468-475. PubMed
7. Bosk EA, Veinot T, Iwashyna TJ. Which patients and where: a qualitative study of patient transfers from community hospitals. Med Care. 2011;49(6):592-598. PubMed
8. Sokol-Hessner L, White AA, Davis KF, Herzig SJ, Hohmann SF. Interhospital transfer patients discharged by academic hospitalists and general internists: Characteristics and outcomes. J Hosp Med. 2016;11(4):245-250. PubMed
9. Department of Health and Human Services, Center for Medicare & Medicaid Services: Critical Access Hospitals. https://www.cms.gov/Outreach-and-Education/Medicare-Learning-Network-MLN/MLNProducts/downloads/CritAccessHospfctsht.pdf. Accessed June 29, 2017. PubMed
10. Roe MT, Chen AY, Delong ER, et al. Patterns of transfer for patients with non-ST-segment elevation acute coronary syndrome from community to tertiary care hospitals. Am Heart J. 2008;156(1):185-192. PubMed
11. Barreto-Filho JA, Wang Y, Rathore SS, et al. Transfer rates from nonprocedure hospitals after initial admission and outcomes among elderly patients with acute myocardial infarction. JAMA Intern Med. 2014;174(2):213-222. PubMed
12. Doruk H, Mas MR, Yildiz C, Sonmez A, Kyrdemir V. The effect of the timing of hip fracture surgery on the activity of daily living and mortality in elderly. Arch Gerontol Geriatr. 2004;39(2):179-185. PubMed
Patients who undergo interhospital transfer (IHT) are felt to benefit from receipt of unique specialty care at the receiving hospital.1 Although only 1.5% of all hospitalized Medicare patients undergo hospital transfer,2 the frequency of transfer is much greater within certain patient populations, as may be expected with diagnoses requiring specialty care.3,4 Existent data demonstrate that 5% of Medicare patients admitted to the intensive care unit (ICU)5 and up to 50% of patients presenting with acute myocardial infarction (AMI) undergo IHT.6
More recent data suggest variability in hospital transfer practices not accounted for by differences in patient or hospital characteristics.2 Although disease-specific guidelines for IHT exist for certain diagnoses,3,4 the process remains largely nonstandardized for many patients,7 leading to ambiguity surrounding indications for transfer. Because limited data suggest worse outcomes for transferred versus nontransferred patients,8 a better understanding of the specialized care patients actually receive across the transfer continuum may help to elucidate potential indications for transfer and ultimately help delineate which patients are most (or least) likely to benefit from transfer and why.
In this national study, we examined a select cohort of transferred patients with diagnoses associated with specific specialty procedural services to determine if they received these procedures and where along the transfer continuum they were performed.
METHODS
We performed a cross-sectional analysis using the Center for Medicare and Medicaid Services 2013 100% Master Beneficiary Summary and Inpatient claims files. Our study protocol was approved by the Partners Healthcare Human Subjects Review Committee.
Beneficiaries were eligible for inclusion if they were aged ≥65 years, continuously enrolled in Medicare A and B, and with an acute care hospitalization claim in 2013, excluding Medicare managed care and end stage renal disease beneficiaries due to incomplete claims data in these groups. We additionally excluded beneficiaries hospitalized at federal or nonacute care hospitals, or critical access hospitals given their mission to stabilize and then transfer patients to referral hospitals.9
Transferred patients were defined as beneficiaries with corresponding “transfer in” and “transfer out” claims, or those with either claim and a corresponding date of admission/discharge from another hospital within 1 day of the claim, as we used in our prior research.2 Beneficiaries transferred to the same hospital, those with greater than 1 transfer within the same hospitalization, or those cared for at hospitals with “outlier” transfer-in rates equal to 100% or transfer-out rates greater than 35% were excluded from analysis given the suggestion of nonstandard claims practices.
We first identified the top 15 primary diagnoses at time of transfer using International Classification of Diseases, Ninth Revision (ICD-9) codes (supplementary Appendix), and then identified those 4 most likely to require specialty procedural services: AMI, gastrointestinal bleed (GI bleed), renal failure, and hip fracture/dislocation. We then chose associated ICD-9 procedure codes for each diagnosis, via expert opinion (authors SM and JS, hospitalist physicians with greater than 20 years of combined clinical experience), erring on overinclusion of procedure codes. We then quantified receipt of associated procedures at transferring and receiving hospitals, stratified by diagnosis.
We further explored the cohort of patients with hip fracture/dislocation who underwent an associated procedure at the transferring but not receiving hospital, examining the frequency with which these patients had other (nonrelated) procedures at the receiving hospital, and identifying which procedures they received.
RESULTS
Of the 101,507 patients transferred to another hospital, 19,613 (19.3%) had a primary diagnosis of AMI, GI bleed, renal failure, or hip fracture/dislocation. Table 1 lists the ICD-9 procedure codes associated with each diagnosis.
Distribution of receipt of specialty procedures at the transferring and receiving hospitals varied by disease (Figure). With the exception of GI bleed, patients more often received specialty procedural care at the receiving than the transferring hospital. Depending on primary diagnosis, between 32.4% and 89.1% of patients did not receive any associated specialty procedure at the receiving hospital.
Of the 370 (22.1%) hip fracture/dislocation patients that received a specialty procedure at the transferring but not receiving hospital, 132 (35.7%) did not receive any procedure at the receiving hospital, whereas the remaining 238 (64.3%) received an unrelated (not associated with the primary diagnosis) procedure. There was great variety in the types of procedures received, the most common being transfusion of blood products (ICD-9 Clinical Modification 9904).
DISCUSSION
Among transferred patients with primary diagnoses that have clearly associated specialized procedural services, we found that patients received these procedures at varying frequency and locations across the transfer continuum. Across 4 diagnoses, receipt of associated procedures was more common at the receiving than the transferring hospital, with the exception being patients with GI bleed. We additionally found that many transferred patients did not receive any associated specialty procedure at the receiving hospital. These findings suggest the strong likelihood of more diverse underlying reasons for transfer rather than solely receipt of specialized procedural care.
Despite the frequency with which AMI patients are transferred,6 and American Heart Association guidelines directing hospitals to transfer AMI patients to institutions able to provide necessary invasive treatments,4 prior studies suggest these patients inconsistently receive specialty intervention following transfer, including stress testing, cardiac catheterization, or coronary artery bypass graft surgery.10,11 Our findings add to these data, demonstrating that only 47.3% of patients transferred with AMI received any cardiac-related procedure at the receiving hospital. Additionally, we found that 38.1% of AMI patients do not receive any specialty procedures at either the transferring or the receiving hospital. Taken together, these data suggest possible discrepancies in the perceived need for these procedures between transferring and receiving hospitals, reasons for transfer related to these conditions that don’t involve an associated procedure, or reasons for transfer unrelated to specialty care of the primary diagnosis (such as care of comorbidities, hospital location, prior relationships with that hospital, or desire for a second opinion). Although some of these alternate reasons for transfer likely still benefit the patient, some of these reasons may not justify the increased risks of discontinuity of care created by IHT.
Given limited data looking at IHT practices for patients with other diagnoses, the varying patterns of specialty procedural interventions we observed among transferred patients with GI bleed, renal failure, and hip fracture/dislocation are novel contributions to this topic. Notably, we found that among patients transferred with a primary diagnosis of renal failure, the vast majority (84.1%) did not receive any associated procedure at either the transferring or the receiving hospital. It is possible that although these patients carried the diagnosis of renal failure, their clinical phenotype is more heterogeneous, and they could still be managed conservatively without receipt of invasive procedures such as hemodialysis.
Conversely, patients transferred with primary diagnosis of hip fracture/dislocation were far more likely to receive associated specialty procedural intervention at the receiving hospital, presumably reflective of the evidence demonstrating improved outcomes with early surgical intervention.12 However, these data do not explain the reasoning behind the substantial minority of patients who received specialty intervention at the transferring hospital prior to transfer or those that did not receive any specialty intervention at either the transferring or receiving hospital. Our secondary analysis demonstrating great variety in receipt and type of nonassociated procedures provided at the receiving hospital did not help to elucidate potential underlying reasons for transfer.
Notably, among patients transferred with primary diagnosis of GI bleed, receipt of specialty procedures was more common at the transferring (77.7%) than receiving (63.2%) hospital, with nearly half (49.3%) undergoing specialty procedures at both hospitals. It is possible that these findings are reflective of the broad array of specialty procedures examined within this diagnosis. For example, it is reasonable to consider that a patient may be stabilized with receipt of a blood transfusion at the transferring hospital, then transferred to undergo a diagnostic/therapeutic procedure (ie, endoscopy/colonoscopy) at the receiving hospital, as is suggested by our results.
Our study is subject to several limitations. First, given the criteria we used to define transfer, it is possible that we included nontransferred patients within our transferred cohort if they were discharged from one hospital and admitted to a different hospital within 1 day, although quality assurance analyses we conducted in prior studies on these data support the validity of the criteria used.2 Second, we cannot exclude the possibility that patients received nonprocedural specialty care (ie, expert opinion, specialized imaging, medical management, management of secondary diagnoses, etc.) not available at the transferring hospital, although, arguably, in select patients, such input could be obtained without physical transfer of the patient (ie, tele-consult). And even in patients transferred with intent to receive procedural care who did not ultimately receive that care, there is likely an appropriate “nonprocedure” rate, where patients who might benefit from a procedure receive a timely evaluation to reduce the risk of missing the opportunity to receive it. This would be analogous to transferring a patient to an ICU even if they do not end up requiring intubation or pressor therapy. However, given the likelihood of higher risks of IHT compared with intrahospital transfers, one could argue that the threshold of perceived benefit might be different in patients being considered for IHT. Additionally, we limited our analyses to only 4 diagnoses; thus, our findings may not be generalizable to other diagnoses of transferred patients. However, because the diagnoses we examined were ones considered most effectively treated with specialty procedural interventions, it is reasonable to presume that the variability in receipt of specialty procedures observed within these diagnoses is also present, if not greater, across other diagnoses. Third, although we intentionally included a broad array of specialty procedures associated with each diagnosis, it is possible that we overlooked particular specialty interventions. For example, in assuming that patients are most likely to be transferred to receive procedural services associated with their primary diagnosis, we may have missed alternate indications for transfer, including need for procedural care related to secondary or subsequent diagnoses (ie, a patient may have presented with GI bleed
CONCLUSIONS
We found that Medicare patients who undergo IHT with primary diagnoses of AMI, GI bleed, renal failure, and hip fracture/dislocation receive associated specialty interventions at varying frequency and locations, and many patients do not receive any associated procedures at receiving hospitals. Our findings suggest that specialty procedural care of patients, even those with primary diagnoses that often warrant specialized intervention, may not be the primary driver of IHT as commonly suggested, although underlying reasons for transfer in these and other “nonprocedural” transferred patients remains obscure. Given known ambiguity in the transfer process,7 and unclear benefit of IHT,8 additional research is required to further identify and evaluate other potential underlying reasons for transfer and to examine these in the context of patient outcomes, in order to understand which patients may or may not benefit from transfer and why.
Disclosure
The authors have nothing to disclose.
Patients who undergo interhospital transfer (IHT) are felt to benefit from receipt of unique specialty care at the receiving hospital.1 Although only 1.5% of all hospitalized Medicare patients undergo hospital transfer,2 the frequency of transfer is much greater within certain patient populations, as may be expected with diagnoses requiring specialty care.3,4 Existent data demonstrate that 5% of Medicare patients admitted to the intensive care unit (ICU)5 and up to 50% of patients presenting with acute myocardial infarction (AMI) undergo IHT.6
More recent data suggest variability in hospital transfer practices not accounted for by differences in patient or hospital characteristics.2 Although disease-specific guidelines for IHT exist for certain diagnoses,3,4 the process remains largely nonstandardized for many patients,7 leading to ambiguity surrounding indications for transfer. Because limited data suggest worse outcomes for transferred versus nontransferred patients,8 a better understanding of the specialized care patients actually receive across the transfer continuum may help to elucidate potential indications for transfer and ultimately help delineate which patients are most (or least) likely to benefit from transfer and why.
In this national study, we examined a select cohort of transferred patients with diagnoses associated with specific specialty procedural services to determine if they received these procedures and where along the transfer continuum they were performed.
METHODS
We performed a cross-sectional analysis using the Center for Medicare and Medicaid Services 2013 100% Master Beneficiary Summary and Inpatient claims files. Our study protocol was approved by the Partners Healthcare Human Subjects Review Committee.
Beneficiaries were eligible for inclusion if they were aged ≥65 years, continuously enrolled in Medicare A and B, and with an acute care hospitalization claim in 2013, excluding Medicare managed care and end stage renal disease beneficiaries due to incomplete claims data in these groups. We additionally excluded beneficiaries hospitalized at federal or nonacute care hospitals, or critical access hospitals given their mission to stabilize and then transfer patients to referral hospitals.9
Transferred patients were defined as beneficiaries with corresponding “transfer in” and “transfer out” claims, or those with either claim and a corresponding date of admission/discharge from another hospital within 1 day of the claim, as we used in our prior research.2 Beneficiaries transferred to the same hospital, those with greater than 1 transfer within the same hospitalization, or those cared for at hospitals with “outlier” transfer-in rates equal to 100% or transfer-out rates greater than 35% were excluded from analysis given the suggestion of nonstandard claims practices.
We first identified the top 15 primary diagnoses at time of transfer using International Classification of Diseases, Ninth Revision (ICD-9) codes (supplementary Appendix), and then identified those 4 most likely to require specialty procedural services: AMI, gastrointestinal bleed (GI bleed), renal failure, and hip fracture/dislocation. We then chose associated ICD-9 procedure codes for each diagnosis, via expert opinion (authors SM and JS, hospitalist physicians with greater than 20 years of combined clinical experience), erring on overinclusion of procedure codes. We then quantified receipt of associated procedures at transferring and receiving hospitals, stratified by diagnosis.
We further explored the cohort of patients with hip fracture/dislocation who underwent an associated procedure at the transferring but not receiving hospital, examining the frequency with which these patients had other (nonrelated) procedures at the receiving hospital, and identifying which procedures they received.
RESULTS
Of the 101,507 patients transferred to another hospital, 19,613 (19.3%) had a primary diagnosis of AMI, GI bleed, renal failure, or hip fracture/dislocation. Table 1 lists the ICD-9 procedure codes associated with each diagnosis.
Distribution of receipt of specialty procedures at the transferring and receiving hospitals varied by disease (Figure). With the exception of GI bleed, patients more often received specialty procedural care at the receiving than the transferring hospital. Depending on primary diagnosis, between 32.4% and 89.1% of patients did not receive any associated specialty procedure at the receiving hospital.
Of the 370 (22.1%) hip fracture/dislocation patients that received a specialty procedure at the transferring but not receiving hospital, 132 (35.7%) did not receive any procedure at the receiving hospital, whereas the remaining 238 (64.3%) received an unrelated (not associated with the primary diagnosis) procedure. There was great variety in the types of procedures received, the most common being transfusion of blood products (ICD-9 Clinical Modification 9904).
DISCUSSION
Among transferred patients with primary diagnoses that have clearly associated specialized procedural services, we found that patients received these procedures at varying frequency and locations across the transfer continuum. Across 4 diagnoses, receipt of associated procedures was more common at the receiving than the transferring hospital, with the exception being patients with GI bleed. We additionally found that many transferred patients did not receive any associated specialty procedure at the receiving hospital. These findings suggest the strong likelihood of more diverse underlying reasons for transfer rather than solely receipt of specialized procedural care.
Despite the frequency with which AMI patients are transferred,6 and American Heart Association guidelines directing hospitals to transfer AMI patients to institutions able to provide necessary invasive treatments,4 prior studies suggest these patients inconsistently receive specialty intervention following transfer, including stress testing, cardiac catheterization, or coronary artery bypass graft surgery.10,11 Our findings add to these data, demonstrating that only 47.3% of patients transferred with AMI received any cardiac-related procedure at the receiving hospital. Additionally, we found that 38.1% of AMI patients do not receive any specialty procedures at either the transferring or the receiving hospital. Taken together, these data suggest possible discrepancies in the perceived need for these procedures between transferring and receiving hospitals, reasons for transfer related to these conditions that don’t involve an associated procedure, or reasons for transfer unrelated to specialty care of the primary diagnosis (such as care of comorbidities, hospital location, prior relationships with that hospital, or desire for a second opinion). Although some of these alternate reasons for transfer likely still benefit the patient, some of these reasons may not justify the increased risks of discontinuity of care created by IHT.
Given limited data looking at IHT practices for patients with other diagnoses, the varying patterns of specialty procedural interventions we observed among transferred patients with GI bleed, renal failure, and hip fracture/dislocation are novel contributions to this topic. Notably, we found that among patients transferred with a primary diagnosis of renal failure, the vast majority (84.1%) did not receive any associated procedure at either the transferring or the receiving hospital. It is possible that although these patients carried the diagnosis of renal failure, their clinical phenotype is more heterogeneous, and they could still be managed conservatively without receipt of invasive procedures such as hemodialysis.
Conversely, patients transferred with primary diagnosis of hip fracture/dislocation were far more likely to receive associated specialty procedural intervention at the receiving hospital, presumably reflective of the evidence demonstrating improved outcomes with early surgical intervention.12 However, these data do not explain the reasoning behind the substantial minority of patients who received specialty intervention at the transferring hospital prior to transfer or those that did not receive any specialty intervention at either the transferring or receiving hospital. Our secondary analysis demonstrating great variety in receipt and type of nonassociated procedures provided at the receiving hospital did not help to elucidate potential underlying reasons for transfer.
Notably, among patients transferred with primary diagnosis of GI bleed, receipt of specialty procedures was more common at the transferring (77.7%) than receiving (63.2%) hospital, with nearly half (49.3%) undergoing specialty procedures at both hospitals. It is possible that these findings are reflective of the broad array of specialty procedures examined within this diagnosis. For example, it is reasonable to consider that a patient may be stabilized with receipt of a blood transfusion at the transferring hospital, then transferred to undergo a diagnostic/therapeutic procedure (ie, endoscopy/colonoscopy) at the receiving hospital, as is suggested by our results.
Our study is subject to several limitations. First, given the criteria we used to define transfer, it is possible that we included nontransferred patients within our transferred cohort if they were discharged from one hospital and admitted to a different hospital within 1 day, although quality assurance analyses we conducted in prior studies on these data support the validity of the criteria used.2 Second, we cannot exclude the possibility that patients received nonprocedural specialty care (ie, expert opinion, specialized imaging, medical management, management of secondary diagnoses, etc.) not available at the transferring hospital, although, arguably, in select patients, such input could be obtained without physical transfer of the patient (ie, tele-consult). And even in patients transferred with intent to receive procedural care who did not ultimately receive that care, there is likely an appropriate “nonprocedure” rate, where patients who might benefit from a procedure receive a timely evaluation to reduce the risk of missing the opportunity to receive it. This would be analogous to transferring a patient to an ICU even if they do not end up requiring intubation or pressor therapy. However, given the likelihood of higher risks of IHT compared with intrahospital transfers, one could argue that the threshold of perceived benefit might be different in patients being considered for IHT. Additionally, we limited our analyses to only 4 diagnoses; thus, our findings may not be generalizable to other diagnoses of transferred patients. However, because the diagnoses we examined were ones considered most effectively treated with specialty procedural interventions, it is reasonable to presume that the variability in receipt of specialty procedures observed within these diagnoses is also present, if not greater, across other diagnoses. Third, although we intentionally included a broad array of specialty procedures associated with each diagnosis, it is possible that we overlooked particular specialty interventions. For example, in assuming that patients are most likely to be transferred to receive procedural services associated with their primary diagnosis, we may have missed alternate indications for transfer, including need for procedural care related to secondary or subsequent diagnoses (ie, a patient may have presented with GI bleed
CONCLUSIONS
We found that Medicare patients who undergo IHT with primary diagnoses of AMI, GI bleed, renal failure, and hip fracture/dislocation receive associated specialty interventions at varying frequency and locations, and many patients do not receive any associated procedures at receiving hospitals. Our findings suggest that specialty procedural care of patients, even those with primary diagnoses that often warrant specialized intervention, may not be the primary driver of IHT as commonly suggested, although underlying reasons for transfer in these and other “nonprocedural” transferred patients remains obscure. Given known ambiguity in the transfer process,7 and unclear benefit of IHT,8 additional research is required to further identify and evaluate other potential underlying reasons for transfer and to examine these in the context of patient outcomes, in order to understand which patients may or may not benefit from transfer and why.
Disclosure
The authors have nothing to disclose.
1. Iwashyna TJ. The incomplete infrastructure for interhospital patient transfer. Crit Care Med. 2012;40(8):2470-2478. PubMed
2. Mueller SK, Zheng J, Orav EJ, Schnipper JL. Rates, Predictors and Variability of Interhospital Transfers: A National Evaluation. J Hosp Med. 2017;12(6):435-442. PubMed
3. Guidelines for the transfer of critically ill patients. Guidelines Committee of the American College of Critical Care Medicine; Society of Critical Care Medicine and American Association of Critical-Care Nurses Transfer Guidelines Task Force. Crit Care Med. 1993;21(6):931-937. PubMed
4. Anderson JL, Adams CD, Antman EM, et al. 2011 ACCF/AHA Focused Update Incorporated Into the ACC/AHA 2007 Guidelines for the Management of Patients With Unstable Angina/Non-ST-Elevation Myocardial Infarction: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. Circulation. 2011;123(18):e426-e579. PubMed
5. Iwashyna TJ, Christie JD, Moody J, Kahn JM, Asch DA. The structure of critical care transfer networks. Med Care. 2009;47(7):787-793. PubMed
6. Iwashyna TJ, Kahn JM, Hayward RA, Nallamothu BK. Interhospital transfers among Medicare beneficiaries admitted for acute myocardial infarction at nonrevascularization hospitals. Circ Cardiovasc Qual Outcomes. 2010;3(5):468-475. PubMed
7. Bosk EA, Veinot T, Iwashyna TJ. Which patients and where: a qualitative study of patient transfers from community hospitals. Med Care. 2011;49(6):592-598. PubMed
8. Sokol-Hessner L, White AA, Davis KF, Herzig SJ, Hohmann SF. Interhospital transfer patients discharged by academic hospitalists and general internists: Characteristics and outcomes. J Hosp Med. 2016;11(4):245-250. PubMed
9. Department of Health and Human Services, Center for Medicare & Medicaid Services: Critical Access Hospitals. https://www.cms.gov/Outreach-and-Education/Medicare-Learning-Network-MLN/MLNProducts/downloads/CritAccessHospfctsht.pdf. Accessed June 29, 2017. PubMed
10. Roe MT, Chen AY, Delong ER, et al. Patterns of transfer for patients with non-ST-segment elevation acute coronary syndrome from community to tertiary care hospitals. Am Heart J. 2008;156(1):185-192. PubMed
11. Barreto-Filho JA, Wang Y, Rathore SS, et al. Transfer rates from nonprocedure hospitals after initial admission and outcomes among elderly patients with acute myocardial infarction. JAMA Intern Med. 2014;174(2):213-222. PubMed
12. Doruk H, Mas MR, Yildiz C, Sonmez A, Kyrdemir V. The effect of the timing of hip fracture surgery on the activity of daily living and mortality in elderly. Arch Gerontol Geriatr. 2004;39(2):179-185. PubMed
1. Iwashyna TJ. The incomplete infrastructure for interhospital patient transfer. Crit Care Med. 2012;40(8):2470-2478. PubMed
2. Mueller SK, Zheng J, Orav EJ, Schnipper JL. Rates, Predictors and Variability of Interhospital Transfers: A National Evaluation. J Hosp Med. 2017;12(6):435-442. PubMed
3. Guidelines for the transfer of critically ill patients. Guidelines Committee of the American College of Critical Care Medicine; Society of Critical Care Medicine and American Association of Critical-Care Nurses Transfer Guidelines Task Force. Crit Care Med. 1993;21(6):931-937. PubMed
4. Anderson JL, Adams CD, Antman EM, et al. 2011 ACCF/AHA Focused Update Incorporated Into the ACC/AHA 2007 Guidelines for the Management of Patients With Unstable Angina/Non-ST-Elevation Myocardial Infarction: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. Circulation. 2011;123(18):e426-e579. PubMed
5. Iwashyna TJ, Christie JD, Moody J, Kahn JM, Asch DA. The structure of critical care transfer networks. Med Care. 2009;47(7):787-793. PubMed
6. Iwashyna TJ, Kahn JM, Hayward RA, Nallamothu BK. Interhospital transfers among Medicare beneficiaries admitted for acute myocardial infarction at nonrevascularization hospitals. Circ Cardiovasc Qual Outcomes. 2010;3(5):468-475. PubMed
7. Bosk EA, Veinot T, Iwashyna TJ. Which patients and where: a qualitative study of patient transfers from community hospitals. Med Care. 2011;49(6):592-598. PubMed
8. Sokol-Hessner L, White AA, Davis KF, Herzig SJ, Hohmann SF. Interhospital transfer patients discharged by academic hospitalists and general internists: Characteristics and outcomes. J Hosp Med. 2016;11(4):245-250. PubMed
9. Department of Health and Human Services, Center for Medicare & Medicaid Services: Critical Access Hospitals. https://www.cms.gov/Outreach-and-Education/Medicare-Learning-Network-MLN/MLNProducts/downloads/CritAccessHospfctsht.pdf. Accessed June 29, 2017. PubMed
10. Roe MT, Chen AY, Delong ER, et al. Patterns of transfer for patients with non-ST-segment elevation acute coronary syndrome from community to tertiary care hospitals. Am Heart J. 2008;156(1):185-192. PubMed
11. Barreto-Filho JA, Wang Y, Rathore SS, et al. Transfer rates from nonprocedure hospitals after initial admission and outcomes among elderly patients with acute myocardial infarction. JAMA Intern Med. 2014;174(2):213-222. PubMed
12. Doruk H, Mas MR, Yildiz C, Sonmez A, Kyrdemir V. The effect of the timing of hip fracture surgery on the activity of daily living and mortality in elderly. Arch Gerontol Geriatr. 2004;39(2):179-185. PubMed
© 2017 Society of Hospital Medicine
617-732-7072; E-mail: smueller1@bwh.harvard.edu
Rates, predictors and variability of interhospital transfers: A national evaluation
Interhospital transfer (IHT) is defined as the transfer of hospitalized patients between acute care hospitals. Although cited reasons for transfer include providing patients access to unique specialty services,1 patterns and practices of IHT remain largely unstudied. Interhospital transfer is known to be common in certain patient populations, including selected patients presenting to the intensive care unit2 and those with acute myocardial infarction (AMI),3-5 but no recent studies have looked at frequency of IHT among a broader group of hospitalized patients nationally. Little is known about which patients are selected for transfer and why.6 Limited evidence suggests poor concordance between cited reason for transfer among patients, transferring physicians, and receiving physicians,7 indicating ambiguity in this care process.
Interhospital transfer exposes patients to the potential risks associated with discontinuity of care. Communication is particularly vulnerable to error during times of transition.8-10 Patients transferred between acute care hospitals are especially vulnerable, given the severity of illness in this patient population,11 and the absence of other factors to fill in gaps in communication, such as common electronic health records. Limited existing literature suggests transferred patients use more resources 12-13 and experience worse outcomes compared to nontransferred patients,11 although these data involved limited patient populations, and adjustment for illness severity and other factors was variably addressed.14-16
To improve the quality and safety of IHT, therefore, it is necessary to understand which patients benefit from IHT and identify best practices in the IHT process.17 A fundamental first step is to study patterns and practices of IHT, in particular with an eye towards identifying unwarranted variation.18 This is important to understand the prevalence of the issue, provide possible evidence of lack of standardization, and natural experiments with which to identify best practices.
To address this, we conducted a foundational study examining a national sample of Medicare patients to determine the nationwide frequency of IHT among elderly patients, patient and hospital-level predictors of transfer, and hospital variability in IHT practices.
METHODS
We performed a cross-sectional analysis using 2 nationally representative datasets: (1) Center for Medicare and Medicaid Services (CMS) 2013 100% Master Beneficiary Summary and Inpatient claims files, which contains data on all fee-for-service program Medicare enrollees’ demographic information, date of death, and hospitalization claims, including ICD-9 codes for diagnoses, diagnosis-related group (DRG), and dates of service; merged with (2) 2013 American Hospital Association (AHA) data,19 which contains hospital-level characteristics for all acute care hospitals in the U.S. Our study protocol was approved by the Partners Healthcare Human Subjects Review Committee.
Beneficiaries were eligible for inclusion if they were 65 years or older, continuously enrolled in Medicare A and B, with an acute care hospitalization claim in 2013, excluding Medicare managed care and end-stage renal disease (ESRD) beneficiaries. We additionally excluded beneficiaries hospitalized at federal or nonacute care hospitals, or critical access hospitals given their mission to stabilize and transfer patients to referral hospitals.20
Transferred patients were defined as: (1) beneficiaries with a “transfer out” claim and a corresponding “transfer in” claim at a different hospital; as well as (2) beneficiaries with a “transfer out” claim and a corresponding date of admission to another hospital within 1 day following the date of claim; and (3) beneficiaries with a “transfer in” claim and a corresponding date of discharge from another hospital within 1 day preceding the date of claim. Beneficiaries transferred to the same hospital, or cared for at hospitals with “outlier” transfer in rates equal to 100% or transfer out rates greater than 35%, were excluded from analysis given the suggestion of nonstandard claims practices. Beneficiaries with greater than 1 transfer within the same hospitalization were additionally excluded.
Patient Characteristics
Patient characteristics were obtained from the CMS data files and included: demographics (age, sex, race); DRG-weight, categorized into quartiles; primary diagnosis for the index hospitalization using ICD-9 codes; patient comorbidity using ICD-9 codes compiled into a CMS-Hierarchical Condition Category (HCC) risk score;21 presence of Medicaid co-insurance; number of hospitalizations in the past 12 months, categorized into 0, 1, 2-3, and 4 or more; season, defined as calendar quarters; and median income per household by census tract. These characteristics were chosen a priori given expert opinion in combination with prior research demonstrating association with IHT.11,22
Hospital Characteristics
Hospital characteristics were obtained from AHA data files and included hospitals’ size, categorized into small, medium, and large (less than 100, 100 to 399, 400 or more beds); geographic location; ownership; teaching status; setting (urban vs. rural); case mix index (CMI) for all patients cared for at the hospital; and presence of selected specialty services, including certified trauma center, medical intensive care unit, cardiac intensive care unit, cardiac surgery services, adult interventional cardiac catheterization, adult cardiac electrophysiology, and composite score of presence of 55 other specialty services (complete list in Appendix A). All characteristics were chosen a priori given expert opinion or relationship of characteristics with IHT, and prior research utilizing AHA data.23-24
Analysis
Descriptive statistics were used to evaluate the frequency of IHT, characteristics of transferred patients, and number of days to transfer. Patient and hospital characteristics of transferred vs. nontransferred patients were compared using chi-square analyses.
To analyze the effects of each patient and hospital characteristic on the odds of transfer, we used logistic regression models incorporating all patient and hospital characteristics, accounting for fixed effects for diagnosis, and utilizing generalized estimating equations (the GENMOD procedure in SAS statistical software, v 9.4; SAS Institute Inc., Cary, North Carolina) to account for the clustering of patients within hospitals.25 Indicator variables were created for missing covariate data and included in analyses when missing data accounted for greater than 10% of the total cohort.
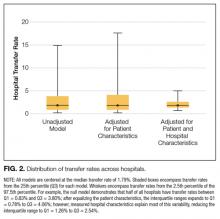
To measure the variability in transfer rates between hospitals, we used a sequence of random effects logistic regression models. We first ran a model with no covariates, representing the unadjusted differences in transfer rates between hospitals. We then added patient characteristics to see if the unadjusted differences in IHT rates were explained by differences in patient characteristics between hospitals. Lastly, we added hospital characteristics to determine if these explained the remaining differences in transfer rates. Each of the 3 models provided a measure of between-hospital variability, reflecting the degree to which IHT rates differed between hospitals. Additionally, we used the intercept from the unadjusted model and the measure of between-hospital variability from each model to calculate the 95% confidence intervals, illustrating the range of IHT rates spanning 95% of all hospitals. We used those same numbers to calculate the 25th and 75th percentiles, illustrating the range of IHT rates for the middle half of hospitals.
RESULTS
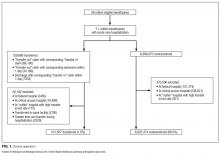
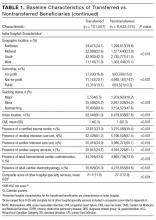
Among 28 million eligible beneficiaries, 6.6 million had an acute care hospitalization to nonfederal, noncritical access hospitals, and 107,741 met our defined criteria for IHT. An additional 3790 beneficiaries were excluded for being transferred to the same facility, 416 beneficiaries (115 transferred, 301 nontransferred) were excluded as they were cared for at 1 of the 11 hospitals with “outlier” transfer in/out rates, and 2329 were excluded because they had more than 1 transfer during hospitalization. Thus, the final cohort consisted of 101,507 transferred (1.5%) and 6,625,474 nontransferred beneficiaries (Figure 1). Of the 101,507 transferred beneficiaries, 2799 (2.8%) were included more than once (ie, experienced more than 1 IHT on separate hospitalizations throughout the study period; the vast majority of these had 2 separate hospitalizations resulting in IHT). Characteristics of transferred and nontransferred beneficiaries are shown (Table 1).
Among transferred patients, the top 5 primary diagnoses at time of transfer included AMI (12.2%), congestive heart failure (CHF) (7.2%), sepsis (6.6%), arrhythmia (6.6%), and pneumonia (3.4%). Comorbid conditions most commonly present in transferred patients included CHF (52.6%), renal failure (51.8%), arrhythmia (49.8%), and chronic obstructive pulmonary disease (COPD; 37.0%). The most common day of transfer was day after admission (hospital day 2, 24.7%), with 75% of transferred patients transferred before hospital day 6 (Appendix B).
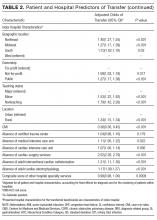
After adjusting for all other patient and hospital characteristics and clustering by hospital, the following variables were associated with greater odds of transfer: older age, male sex, nonblack race, non-Medicaid co-insurance, higher comorbidity (HCC score), lower DRG-weight, and fewer hospitalizations in the prior 12 months. Beneficiaries also had greater odds of transfer if initially hospitalized at smaller hospitals, nonteaching hospitals, public hospitals, at hospitals in the Northeast, those with fewer specialty services, and those with a low CMI (Table 2).
DISCUSSION
In this nationally representative study of 6.6 million Medicare beneficiaries, we found that 1.5% of patients were transferred between acute care facilities and were most often transferred prior to hospital day 6. Older age, male sex, nonblack race, higher medical comorbidity, lower DRG weight, and fewer recent hospitalizations were associated with greater odds of transfer. Initial hospitalization at smaller, nonteaching, public hospitals, with fewer specialty services were associated with greater odds of transfer, while higher CMI was associated with a lower odds of transfer. The most common comorbid conditions among transferred patients included CHF, renal failure, arrhythmia, and COPD; particularly notable was the very high prevalence of these conditions among transferred as compared with nontransferred patients. Importantly, we found significant variation in IHT by region and a large variation in transfer practices by hospital, with significant variability in transfer rates even after accounting for known patient and hospital characteristics.
Among our examined population, we found that a sizable number of patients undergo IHT—more than 100,000 per year. Primary diagnoses at time of transfer consist of common inpatient conditions, including AMI, CHF, sepsis, arrhythmia, and pneumonia. Limited prior data support our findings, with up to 50% of AMI patients reportedly undergoing IHT,3-5 and severe sepsis and respiratory illness reported as common diagnoses at transfer.11 Although knowledge of these primary diagnoses does not directly confer an understanding of reason for transfer, one can speculate based on our findings. For example, research demonstrates the majority of AMI patients who undergo IHT had further intervention, including stress testing, cardiac catheterization, and/or coronary artery bypass graft surgery.5,26 Thus, it is reasonable to presume that many of the beneficiaries
We additionally found that certain patient characteristics were associated with greater odds of transfer. Research suggests that transferred patients are “sicker” than nontransferred patients.1,11 Although our findings in part confirm these data, we paradoxically found that higher DRG-weight and 4 or more hospitalizations in the past year were actually associated with lower odds of transfer. In addition, the oldest patients in our cohort (85 years or older) were actually less likely to be transferred than their slightly younger counterparts (75 to 84 years). These variables may reflect extreme illness or frailty,27 and providers consciously (or subconsciously) may factor this in to their decision to transfer, considering a threshold past which transfer would confer more risk than benefit (eg, a patient may be “too sick” for transfer). Indeed, in a secondary analysis without hospital characteristics or comorbidities, and with fixed effects by hospital, we found the highest rates of IHT in patients in the middle 2 quartiles of DRG-weight, supporting this threshold hypothesis. It is also possible that patients with numerous hospitalizations may be less likely to be transferred because of familiarity and a strong sense of responsibility to continue to care for those patients (although we cannot confirm that those prior hospitalizations were all with the same index hospital).
It is also notable that odds of transfer differed by race, with black patients 17% less likely to undergo transfer compared to whites, similar to findings in other IHT studies.11 This finding, in combination with our demonstration that Medicaid patients also have lower odds of transfer, warrants further investigation to ensure the process of IHT does not bias against these populations, as with other well-documented health disparities.28-30
The hospital predictors of transfer were largely expected. However, interestingly, when we controlled for all other patient and hospital characteristics, regional variation persisted, with highest odds of transfer with hospitalization in the Northeast, indicating variability by region not explained by other factors, and findings supported by other limited data.31 This variability was further elucidated in our examination of change in variance estimates accounting for patient, then hospital, characteristics. Although we expected and found marked variability in hospital transfer rates in our null model (without accounting for any patient or hospital characteristics), we interestingly found that variability increased upon adjusting for patient characteristics. This result is presumably due to the fact that patients who are more likely to be transferred (ie, “sick” patients) are more often already at hospitals less likely to transfer patients, supported by our findings that hospital CMI is inversely associated with odds of transfer (in other words, hospitals that care for a less sick patient population are more likely to transfer their patients, and hospitals that care for a sicker patient population [higher CMI] are less likely to transfer). Adjusting solely for patient characteristics effectively equalizes these patients across hospitals, which would lead to even increased variability in transfer rates. Conversely, when we then adjusted for hospital characteristics, variability in hospital transfer rates decreased by 83% (in other words, hospital characteristics, rather than patient characteristics, explained much of the variability in transfer rates), although significant unexplained variability remained. We should note that although the observed reduction in variability was explained by the patient and hospital characteristics included in the model, these characteristics do not necessarily justify the variability they accounted for; although patients’ race or hospitals’ location may explain some of the observed variability, this does not reasonably justify it.
This observed variability in transfer practices is not surprising given the absence of standardization and clear guidelines to direct clinical IHT practice.17 Selection of patients that may benefit from transfer is often ambiguous and subjective.6 The Emergency Medical Treatment and Active Labor Act laws dictate that hospitals transfer patients requiring a more specialized service, or when “medical benefits ... outweigh the increased risks to the individual...,” although in practice this provides little guidance to practitioners.1 Thus, clearer guidelines may be necessary to achieve less variable practices.
Our study is subject to several limitations. First, although nationally representative, the Medicare population is not reflective of all hospitalized patients nationwide. Additionally, we excluded patients transferred from the emergency room. Thus, the total number of patients who undergo IHT nationally is expected to be much higher than reflected in our analysis. We also excluded patients who were transferred more than once during a given hospitalization. This enabled us to focus on the initial transfer decision but does not allow us to look at patients who are transferred to a referral center and then transferred back. Second, given the criteria we used to define transfer, it is possible that we included nontransferred patients within our transferred cohort if they were discharged from one hospital and admitted to a different hospital within 1 day. However, on quality assurance analyses where we limited our cohort to only those beneficiaries with corresponding “transfer in” and “transfer out” claims (87% of the total cohort), we found no marked differences in our results. Additionally, although we assume that patient transfer status was coded correctly within the Medicare dataset, we could not confirm by individually examining each patient we defined as “transferred.” However, on additional quality assurance analyses where we examined randomly selected excluded patients with greater than 1 transfer during hospitalization, we found differing provider numbers with each transfer, suggesting validity of the coding. Third, because there are likely many unmeasured patient confounders, we cannot be sure how much of the between-hospital variation is due to incomplete adjustment for patient characteristics. However, since adjusting for patient characteristics actually increased variability in hospital transfer rates, it is unlikely that residual patient confounders fully explain our observed results. Despite this, other variables that are not available within the CMS or AHA datasets may further elucidate hospital transfer practices, including variables reflective of the transfer process (eg, time of day of patient transfer, time delay between initiation of transfer and patient arrival at accepting hospital, accepting service on transfer, etc.); other markers of illness severity (eg, clinical service at the time of index admission, acute physiology score, utilization of critical care services on arrival at receiving hospital); and other hospital system variables (ie, membership in an accountable care organization and/or regional care network, the density of nearby tertiary referral centers (indicating possible supply-induced demand), other variables reflective of the “transfer culture” (such as the transfer rate at the hospital or region where the attending physician trained, etc.). Lastly, though our examination provides important foundational information regarding IHT nationally, this study did not examine patient outcomes in transferred and nontransferred patients, which may help to determine which patients benefit (or do not benefit) from transfer and why. Further investigation is needed to study these outcomes.
CONCLUSION
In this national study of IHT, we found that a sizable number of patients admitted to the hospital undergo transfer to another acute care facility. Patients are transferred with common medical conditions, including those requiring specialized care such as AMI, and a high rate of comorbid clinical conditions, and certain patient and hospital characteristics are associated with greater odds of transfer. Although many of the observed associations between characteristics and odds of transfer were expected based on limited existing literature, we found several unexpected findings, eg, suggesting the possibility of a threshold beyond which sicker patients are not transferred. Additionally, we found that black and Medicaid patients had lower odds of transfer, which warrants further investigation for potential health care disparity. Importantly, we found much variability in the practice of IHT, as evidenced by the inexplicable differences in transfer by hospital region, and by residual unexplained variability in hospital transfer rates after accounting for patient and hospital characteristics, which may be due to lack of standard guidelines to direct IHT practices. In conclusion, this study of hospitalized Medicare patients provides important foundational information regarding rates and predictors of IHT nationally, as well as unexplained variability that exists within this complex care transition. Further investigation will be essential to understand reasons for, processes related to, and outcomes of transferred patients, to help guide standardization in best practices in care.
Disclosure
Nothing to report.
1. Iwashyna TJ. The incomplete infrastructure for interhospital patient transfer. Crit Care Med. 2012;40(8):2470-2478. PubMed
2. Iwashyna TJ, Christie JD, Moody J, Kahn JM, Asch DA. The structure of critical care transfer networks. Med Care. 2009;47(7):787-793. PubMed
3. Mehta RH, Stalhandske EJ, McCargar PA, Ruane TJ, Eagle KA. Elderly patients at highest risk with acute myocardial infarction are more frequently transferred from community hospitals to tertiary centers: reality or myth? Am Heart J. 1999;138(4 Pt 1):688-695. PubMed
4. Iwashyna TJ, Kahn JM, Hayward RA, Nallamothu BK. Interhospital transfers among Medicare beneficiaries admitted for acute myocardial infarction at nonrevascularization hospitals. Circ Cardiovasc Qual Outcomes. 2010;3(5):468-475. PubMed
5. Roe MT, Chen AY, Delong ER, Boden WE, Calvin JE Jr, Cairns CB, et al. Patterns of transfer for patients with non-ST-segment elevation acute coronary syndrome from community to tertiary care hospitals. Am Heart J. 2008;156(1):185-192. PubMed
6. Bosk EA, Veinot T, Iwashyna TJ. Which patients and where: a qualitative study of patient transfers from community hospitals. Med Care. 2011;49(6):592-598. PubMed
7. Wagner J, Iwashyna TJ, Kahn JM. Reasons underlying interhospital transfers to an academic medical intensive care unit. J Crit Care. 2013;28(2):202-208. PubMed
8. Cohen MD, Hilligoss PB. The published literature on handoffs in hospitals: deficiencies identified in an extensive review. Qual Saf Health Care. 2010;19(6):493-497. PubMed
9. Riesenberg LA, Leitzsch J, Massucci JL, et al. Residents’ and attending physicians’ handoffs: a systematic review of the literature. Acad Med. 2009;84(12):1775-1787. PubMed
10. Arora V, Johnson J, Lovinger D, Humphrey HJ, Meltzer DO. Communication failures in patient sign-out and suggestions for improvement: a critical incident analysis. Qual Saf Health Care. 2005;14(6):401-407. PubMed
11. Sokol-Hessner L, White AA, Davis KF, Herzig SJ, Hohmann SF. Interhospital transfer patients discharged by academic hospitalists and general internists: characteristics and outcomes. J Hosp Med. 2016;11(4):245-250. PubMed
12. Bernard AM, Hayward RA, Rosevear J, Chun H, McMahon LF. Comparing the hospitalizations of transfer and non-transfer patients in an academic medical center. Acad Med. 1996;71(3):262-266. PubMed
13. Golestanian E, Scruggs JE, Gangnon RE, Mak RP, Wood KE. Effect of interhospital transfer on resource utilization and outcomes at a tertiary care referral center. Crit Care Med. 2007;35(6):1470-1476. PubMed
14. Durairaj L, Will JG, Torner JC, Doebbeling BN. Prognostic factors for mortality following interhospital transfers to the medical intensive care unit of a tertiary referral center. Crit Care Med. 2003;31(7):1981-1986. PubMed
15. Kerr HD, Byrd JC. Community hospital transfers to a VA Medical Center. JAMA. 1989;262(1):70-73. PubMed
16. Dragsted L, Jörgensen J, Jensen NH, et al. Interhospital comparisons of patient outcome from intensive care: importance of lead-time bias. Crit Care Med. 1989;17(5):418-422. PubMed
17. Gupta K, Mueller SK. Interhospital transfers: the need for standards. J Hosp Med. 2015;10(6):415-417. PubMed
18. The Dartmouth Atlas of Health Care: Understanding of the Efficiency and Effectiveness of the Health Care System. The Dartmouth Institute for Health Practice and Clinical Policy, Lebanon, NH. http://www.dartmouthatlas.org/. Accessed November 1, 2016.
19. American Hospital Association Annual Survey Database. American Hospital Association, Chicago, IL. http://www.ahadataviewer.com/book-cd-products/AHA-Survey/. Accessed July 1, 2013.
20. U.S. Department of Health and Human Services (HRSA): What are critical access hospitals (CAH)? http://www.hrsa.gov/healthit/toolbox/RuralHealthITtoolbox/Introduction/critical.html. Accessed June 9, 2016.
21. Li P, Kim MM, Doshi JA. Comparison of the performance of the CMS Hierarchical Condition Category (CMS-HCC) risk adjuster with the Charlson and Elixhauser comorbidity measures in predicting mortality. BMC Health Serv Res. 2010;10:245. PubMed
22. Hernandez-Boussard T, Davies S, McDonald K, Wang NE. Interhospital facility transfers in the United States: a nationwide outcomes study. J Patient Saf. Nov 13 2014. PubMed
23. Landon BE, Normand SL, Lessler A, et al. Quality of care for the treatment of acute medical conditions in US hospitals. Arch Intern Med. 2006;166(22):2511-2517. PubMed
24. Mueller SK, Lipsitz S, Hicks LS. Impact of hospital teaching intensity on quality of care and patient outcomes. Med Care.2013;51(7):567-574. PubMed
25. Lopez L, Hicks LS, Cohen AP, McKean S, Weissman JS. Hospitalists and the quality of care in hospitals. Arch Intern Med. 2009;169(15):1389-1394. PubMed
26. Barreto-Filho JA, Wang Y, Rathore SS, et al. Transfer rates from nonprocedure hospitals after initial admission and outcomes among elderly patients with acute myocardial infarction. JAMA Intern Med. 2014;174(2):213-222. PubMed
27. Carlson JE, Zocchi KA, Bettencourt DM, et al. Measuring frailty in the hospitalized elderly: concept of functional homeostasis. Am J Phys Med Rehabil. 1998;77(3):252-257. PubMed
28. Ward E, Jemal A, Cokkinides V, et al. Cancer disparities by race/ethnicity and socioeconomic status. CA Cancer J Clin. 2004;54(2):78-93. PubMed
29. Iribarren C, Tolstykh I, Somkin CP, et al. Sex and racial/ethnic disparities in outcomes after acute myocardial infarction: a cohort study among members of a large integrated health care delivery system in northern California. Arch Intern Med. 2005;165(18):2105-2113. PubMed
30. Kawachi I, Daniels N, Robinson DE. Health disparities by race and class: why both matter. Health Aff (Millwood). 2005;24(2):343-352. PubMed
31. Herrigel DJ, Carroll M, Fanning C, Steinberg MB, Parikh A, Usher M. Interhospital transfer handoff practices among US tertiary care centers: a descriptive survey. J Hosp Med. 2016;11(6):413-417. PubMed
Interhospital transfer (IHT) is defined as the transfer of hospitalized patients between acute care hospitals. Although cited reasons for transfer include providing patients access to unique specialty services,1 patterns and practices of IHT remain largely unstudied. Interhospital transfer is known to be common in certain patient populations, including selected patients presenting to the intensive care unit2 and those with acute myocardial infarction (AMI),3-5 but no recent studies have looked at frequency of IHT among a broader group of hospitalized patients nationally. Little is known about which patients are selected for transfer and why.6 Limited evidence suggests poor concordance between cited reason for transfer among patients, transferring physicians, and receiving physicians,7 indicating ambiguity in this care process.
Interhospital transfer exposes patients to the potential risks associated with discontinuity of care. Communication is particularly vulnerable to error during times of transition.8-10 Patients transferred between acute care hospitals are especially vulnerable, given the severity of illness in this patient population,11 and the absence of other factors to fill in gaps in communication, such as common electronic health records. Limited existing literature suggests transferred patients use more resources 12-13 and experience worse outcomes compared to nontransferred patients,11 although these data involved limited patient populations, and adjustment for illness severity and other factors was variably addressed.14-16
To improve the quality and safety of IHT, therefore, it is necessary to understand which patients benefit from IHT and identify best practices in the IHT process.17 A fundamental first step is to study patterns and practices of IHT, in particular with an eye towards identifying unwarranted variation.18 This is important to understand the prevalence of the issue, provide possible evidence of lack of standardization, and natural experiments with which to identify best practices.
To address this, we conducted a foundational study examining a national sample of Medicare patients to determine the nationwide frequency of IHT among elderly patients, patient and hospital-level predictors of transfer, and hospital variability in IHT practices.
METHODS
We performed a cross-sectional analysis using 2 nationally representative datasets: (1) Center for Medicare and Medicaid Services (CMS) 2013 100% Master Beneficiary Summary and Inpatient claims files, which contains data on all fee-for-service program Medicare enrollees’ demographic information, date of death, and hospitalization claims, including ICD-9 codes for diagnoses, diagnosis-related group (DRG), and dates of service; merged with (2) 2013 American Hospital Association (AHA) data,19 which contains hospital-level characteristics for all acute care hospitals in the U.S. Our study protocol was approved by the Partners Healthcare Human Subjects Review Committee.
Beneficiaries were eligible for inclusion if they were 65 years or older, continuously enrolled in Medicare A and B, with an acute care hospitalization claim in 2013, excluding Medicare managed care and end-stage renal disease (ESRD) beneficiaries. We additionally excluded beneficiaries hospitalized at federal or nonacute care hospitals, or critical access hospitals given their mission to stabilize and transfer patients to referral hospitals.20
Transferred patients were defined as: (1) beneficiaries with a “transfer out” claim and a corresponding “transfer in” claim at a different hospital; as well as (2) beneficiaries with a “transfer out” claim and a corresponding date of admission to another hospital within 1 day following the date of claim; and (3) beneficiaries with a “transfer in” claim and a corresponding date of discharge from another hospital within 1 day preceding the date of claim. Beneficiaries transferred to the same hospital, or cared for at hospitals with “outlier” transfer in rates equal to 100% or transfer out rates greater than 35%, were excluded from analysis given the suggestion of nonstandard claims practices. Beneficiaries with greater than 1 transfer within the same hospitalization were additionally excluded.
Patient Characteristics
Patient characteristics were obtained from the CMS data files and included: demographics (age, sex, race); DRG-weight, categorized into quartiles; primary diagnosis for the index hospitalization using ICD-9 codes; patient comorbidity using ICD-9 codes compiled into a CMS-Hierarchical Condition Category (HCC) risk score;21 presence of Medicaid co-insurance; number of hospitalizations in the past 12 months, categorized into 0, 1, 2-3, and 4 or more; season, defined as calendar quarters; and median income per household by census tract. These characteristics were chosen a priori given expert opinion in combination with prior research demonstrating association with IHT.11,22
Hospital Characteristics
Hospital characteristics were obtained from AHA data files and included hospitals’ size, categorized into small, medium, and large (less than 100, 100 to 399, 400 or more beds); geographic location; ownership; teaching status; setting (urban vs. rural); case mix index (CMI) for all patients cared for at the hospital; and presence of selected specialty services, including certified trauma center, medical intensive care unit, cardiac intensive care unit, cardiac surgery services, adult interventional cardiac catheterization, adult cardiac electrophysiology, and composite score of presence of 55 other specialty services (complete list in Appendix A). All characteristics were chosen a priori given expert opinion or relationship of characteristics with IHT, and prior research utilizing AHA data.23-24
Analysis
Descriptive statistics were used to evaluate the frequency of IHT, characteristics of transferred patients, and number of days to transfer. Patient and hospital characteristics of transferred vs. nontransferred patients were compared using chi-square analyses.
To analyze the effects of each patient and hospital characteristic on the odds of transfer, we used logistic regression models incorporating all patient and hospital characteristics, accounting for fixed effects for diagnosis, and utilizing generalized estimating equations (the GENMOD procedure in SAS statistical software, v 9.4; SAS Institute Inc., Cary, North Carolina) to account for the clustering of patients within hospitals.25 Indicator variables were created for missing covariate data and included in analyses when missing data accounted for greater than 10% of the total cohort.
To measure the variability in transfer rates between hospitals, we used a sequence of random effects logistic regression models. We first ran a model with no covariates, representing the unadjusted differences in transfer rates between hospitals. We then added patient characteristics to see if the unadjusted differences in IHT rates were explained by differences in patient characteristics between hospitals. Lastly, we added hospital characteristics to determine if these explained the remaining differences in transfer rates. Each of the 3 models provided a measure of between-hospital variability, reflecting the degree to which IHT rates differed between hospitals. Additionally, we used the intercept from the unadjusted model and the measure of between-hospital variability from each model to calculate the 95% confidence intervals, illustrating the range of IHT rates spanning 95% of all hospitals. We used those same numbers to calculate the 25th and 75th percentiles, illustrating the range of IHT rates for the middle half of hospitals.
RESULTS
Among 28 million eligible beneficiaries, 6.6 million had an acute care hospitalization to nonfederal, noncritical access hospitals, and 107,741 met our defined criteria for IHT. An additional 3790 beneficiaries were excluded for being transferred to the same facility, 416 beneficiaries (115 transferred, 301 nontransferred) were excluded as they were cared for at 1 of the 11 hospitals with “outlier” transfer in/out rates, and 2329 were excluded because they had more than 1 transfer during hospitalization. Thus, the final cohort consisted of 101,507 transferred (1.5%) and 6,625,474 nontransferred beneficiaries (Figure 1). Of the 101,507 transferred beneficiaries, 2799 (2.8%) were included more than once (ie, experienced more than 1 IHT on separate hospitalizations throughout the study period; the vast majority of these had 2 separate hospitalizations resulting in IHT). Characteristics of transferred and nontransferred beneficiaries are shown (Table 1).
Among transferred patients, the top 5 primary diagnoses at time of transfer included AMI (12.2%), congestive heart failure (CHF) (7.2%), sepsis (6.6%), arrhythmia (6.6%), and pneumonia (3.4%). Comorbid conditions most commonly present in transferred patients included CHF (52.6%), renal failure (51.8%), arrhythmia (49.8%), and chronic obstructive pulmonary disease (COPD; 37.0%). The most common day of transfer was day after admission (hospital day 2, 24.7%), with 75% of transferred patients transferred before hospital day 6 (Appendix B).
After adjusting for all other patient and hospital characteristics and clustering by hospital, the following variables were associated with greater odds of transfer: older age, male sex, nonblack race, non-Medicaid co-insurance, higher comorbidity (HCC score), lower DRG-weight, and fewer hospitalizations in the prior 12 months. Beneficiaries also had greater odds of transfer if initially hospitalized at smaller hospitals, nonteaching hospitals, public hospitals, at hospitals in the Northeast, those with fewer specialty services, and those with a low CMI (Table 2).
DISCUSSION
In this nationally representative study of 6.6 million Medicare beneficiaries, we found that 1.5% of patients were transferred between acute care facilities and were most often transferred prior to hospital day 6. Older age, male sex, nonblack race, higher medical comorbidity, lower DRG weight, and fewer recent hospitalizations were associated with greater odds of transfer. Initial hospitalization at smaller, nonteaching, public hospitals, with fewer specialty services were associated with greater odds of transfer, while higher CMI was associated with a lower odds of transfer. The most common comorbid conditions among transferred patients included CHF, renal failure, arrhythmia, and COPD; particularly notable was the very high prevalence of these conditions among transferred as compared with nontransferred patients. Importantly, we found significant variation in IHT by region and a large variation in transfer practices by hospital, with significant variability in transfer rates even after accounting for known patient and hospital characteristics.
Among our examined population, we found that a sizable number of patients undergo IHT—more than 100,000 per year. Primary diagnoses at time of transfer consist of common inpatient conditions, including AMI, CHF, sepsis, arrhythmia, and pneumonia. Limited prior data support our findings, with up to 50% of AMI patients reportedly undergoing IHT,3-5 and severe sepsis and respiratory illness reported as common diagnoses at transfer.11 Although knowledge of these primary diagnoses does not directly confer an understanding of reason for transfer, one can speculate based on our findings. For example, research demonstrates the majority of AMI patients who undergo IHT had further intervention, including stress testing, cardiac catheterization, and/or coronary artery bypass graft surgery.5,26 Thus, it is reasonable to presume that many of the beneficiaries
We additionally found that certain patient characteristics were associated with greater odds of transfer. Research suggests that transferred patients are “sicker” than nontransferred patients.1,11 Although our findings in part confirm these data, we paradoxically found that higher DRG-weight and 4 or more hospitalizations in the past year were actually associated with lower odds of transfer. In addition, the oldest patients in our cohort (85 years or older) were actually less likely to be transferred than their slightly younger counterparts (75 to 84 years). These variables may reflect extreme illness or frailty,27 and providers consciously (or subconsciously) may factor this in to their decision to transfer, considering a threshold past which transfer would confer more risk than benefit (eg, a patient may be “too sick” for transfer). Indeed, in a secondary analysis without hospital characteristics or comorbidities, and with fixed effects by hospital, we found the highest rates of IHT in patients in the middle 2 quartiles of DRG-weight, supporting this threshold hypothesis. It is also possible that patients with numerous hospitalizations may be less likely to be transferred because of familiarity and a strong sense of responsibility to continue to care for those patients (although we cannot confirm that those prior hospitalizations were all with the same index hospital).
It is also notable that odds of transfer differed by race, with black patients 17% less likely to undergo transfer compared to whites, similar to findings in other IHT studies.11 This finding, in combination with our demonstration that Medicaid patients also have lower odds of transfer, warrants further investigation to ensure the process of IHT does not bias against these populations, as with other well-documented health disparities.28-30
The hospital predictors of transfer were largely expected. However, interestingly, when we controlled for all other patient and hospital characteristics, regional variation persisted, with highest odds of transfer with hospitalization in the Northeast, indicating variability by region not explained by other factors, and findings supported by other limited data.31 This variability was further elucidated in our examination of change in variance estimates accounting for patient, then hospital, characteristics. Although we expected and found marked variability in hospital transfer rates in our null model (without accounting for any patient or hospital characteristics), we interestingly found that variability increased upon adjusting for patient characteristics. This result is presumably due to the fact that patients who are more likely to be transferred (ie, “sick” patients) are more often already at hospitals less likely to transfer patients, supported by our findings that hospital CMI is inversely associated with odds of transfer (in other words, hospitals that care for a less sick patient population are more likely to transfer their patients, and hospitals that care for a sicker patient population [higher CMI] are less likely to transfer). Adjusting solely for patient characteristics effectively equalizes these patients across hospitals, which would lead to even increased variability in transfer rates. Conversely, when we then adjusted for hospital characteristics, variability in hospital transfer rates decreased by 83% (in other words, hospital characteristics, rather than patient characteristics, explained much of the variability in transfer rates), although significant unexplained variability remained. We should note that although the observed reduction in variability was explained by the patient and hospital characteristics included in the model, these characteristics do not necessarily justify the variability they accounted for; although patients’ race or hospitals’ location may explain some of the observed variability, this does not reasonably justify it.
This observed variability in transfer practices is not surprising given the absence of standardization and clear guidelines to direct clinical IHT practice.17 Selection of patients that may benefit from transfer is often ambiguous and subjective.6 The Emergency Medical Treatment and Active Labor Act laws dictate that hospitals transfer patients requiring a more specialized service, or when “medical benefits ... outweigh the increased risks to the individual...,” although in practice this provides little guidance to practitioners.1 Thus, clearer guidelines may be necessary to achieve less variable practices.
Our study is subject to several limitations. First, although nationally representative, the Medicare population is not reflective of all hospitalized patients nationwide. Additionally, we excluded patients transferred from the emergency room. Thus, the total number of patients who undergo IHT nationally is expected to be much higher than reflected in our analysis. We also excluded patients who were transferred more than once during a given hospitalization. This enabled us to focus on the initial transfer decision but does not allow us to look at patients who are transferred to a referral center and then transferred back. Second, given the criteria we used to define transfer, it is possible that we included nontransferred patients within our transferred cohort if they were discharged from one hospital and admitted to a different hospital within 1 day. However, on quality assurance analyses where we limited our cohort to only those beneficiaries with corresponding “transfer in” and “transfer out” claims (87% of the total cohort), we found no marked differences in our results. Additionally, although we assume that patient transfer status was coded correctly within the Medicare dataset, we could not confirm by individually examining each patient we defined as “transferred.” However, on additional quality assurance analyses where we examined randomly selected excluded patients with greater than 1 transfer during hospitalization, we found differing provider numbers with each transfer, suggesting validity of the coding. Third, because there are likely many unmeasured patient confounders, we cannot be sure how much of the between-hospital variation is due to incomplete adjustment for patient characteristics. However, since adjusting for patient characteristics actually increased variability in hospital transfer rates, it is unlikely that residual patient confounders fully explain our observed results. Despite this, other variables that are not available within the CMS or AHA datasets may further elucidate hospital transfer practices, including variables reflective of the transfer process (eg, time of day of patient transfer, time delay between initiation of transfer and patient arrival at accepting hospital, accepting service on transfer, etc.); other markers of illness severity (eg, clinical service at the time of index admission, acute physiology score, utilization of critical care services on arrival at receiving hospital); and other hospital system variables (ie, membership in an accountable care organization and/or regional care network, the density of nearby tertiary referral centers (indicating possible supply-induced demand), other variables reflective of the “transfer culture” (such as the transfer rate at the hospital or region where the attending physician trained, etc.). Lastly, though our examination provides important foundational information regarding IHT nationally, this study did not examine patient outcomes in transferred and nontransferred patients, which may help to determine which patients benefit (or do not benefit) from transfer and why. Further investigation is needed to study these outcomes.
CONCLUSION
In this national study of IHT, we found that a sizable number of patients admitted to the hospital undergo transfer to another acute care facility. Patients are transferred with common medical conditions, including those requiring specialized care such as AMI, and a high rate of comorbid clinical conditions, and certain patient and hospital characteristics are associated with greater odds of transfer. Although many of the observed associations between characteristics and odds of transfer were expected based on limited existing literature, we found several unexpected findings, eg, suggesting the possibility of a threshold beyond which sicker patients are not transferred. Additionally, we found that black and Medicaid patients had lower odds of transfer, which warrants further investigation for potential health care disparity. Importantly, we found much variability in the practice of IHT, as evidenced by the inexplicable differences in transfer by hospital region, and by residual unexplained variability in hospital transfer rates after accounting for patient and hospital characteristics, which may be due to lack of standard guidelines to direct IHT practices. In conclusion, this study of hospitalized Medicare patients provides important foundational information regarding rates and predictors of IHT nationally, as well as unexplained variability that exists within this complex care transition. Further investigation will be essential to understand reasons for, processes related to, and outcomes of transferred patients, to help guide standardization in best practices in care.
Disclosure
Nothing to report.
Interhospital transfer (IHT) is defined as the transfer of hospitalized patients between acute care hospitals. Although cited reasons for transfer include providing patients access to unique specialty services,1 patterns and practices of IHT remain largely unstudied. Interhospital transfer is known to be common in certain patient populations, including selected patients presenting to the intensive care unit2 and those with acute myocardial infarction (AMI),3-5 but no recent studies have looked at frequency of IHT among a broader group of hospitalized patients nationally. Little is known about which patients are selected for transfer and why.6 Limited evidence suggests poor concordance between cited reason for transfer among patients, transferring physicians, and receiving physicians,7 indicating ambiguity in this care process.
Interhospital transfer exposes patients to the potential risks associated with discontinuity of care. Communication is particularly vulnerable to error during times of transition.8-10 Patients transferred between acute care hospitals are especially vulnerable, given the severity of illness in this patient population,11 and the absence of other factors to fill in gaps in communication, such as common electronic health records. Limited existing literature suggests transferred patients use more resources 12-13 and experience worse outcomes compared to nontransferred patients,11 although these data involved limited patient populations, and adjustment for illness severity and other factors was variably addressed.14-16
To improve the quality and safety of IHT, therefore, it is necessary to understand which patients benefit from IHT and identify best practices in the IHT process.17 A fundamental first step is to study patterns and practices of IHT, in particular with an eye towards identifying unwarranted variation.18 This is important to understand the prevalence of the issue, provide possible evidence of lack of standardization, and natural experiments with which to identify best practices.
To address this, we conducted a foundational study examining a national sample of Medicare patients to determine the nationwide frequency of IHT among elderly patients, patient and hospital-level predictors of transfer, and hospital variability in IHT practices.
METHODS
We performed a cross-sectional analysis using 2 nationally representative datasets: (1) Center for Medicare and Medicaid Services (CMS) 2013 100% Master Beneficiary Summary and Inpatient claims files, which contains data on all fee-for-service program Medicare enrollees’ demographic information, date of death, and hospitalization claims, including ICD-9 codes for diagnoses, diagnosis-related group (DRG), and dates of service; merged with (2) 2013 American Hospital Association (AHA) data,19 which contains hospital-level characteristics for all acute care hospitals in the U.S. Our study protocol was approved by the Partners Healthcare Human Subjects Review Committee.
Beneficiaries were eligible for inclusion if they were 65 years or older, continuously enrolled in Medicare A and B, with an acute care hospitalization claim in 2013, excluding Medicare managed care and end-stage renal disease (ESRD) beneficiaries. We additionally excluded beneficiaries hospitalized at federal or nonacute care hospitals, or critical access hospitals given their mission to stabilize and transfer patients to referral hospitals.20
Transferred patients were defined as: (1) beneficiaries with a “transfer out” claim and a corresponding “transfer in” claim at a different hospital; as well as (2) beneficiaries with a “transfer out” claim and a corresponding date of admission to another hospital within 1 day following the date of claim; and (3) beneficiaries with a “transfer in” claim and a corresponding date of discharge from another hospital within 1 day preceding the date of claim. Beneficiaries transferred to the same hospital, or cared for at hospitals with “outlier” transfer in rates equal to 100% or transfer out rates greater than 35%, were excluded from analysis given the suggestion of nonstandard claims practices. Beneficiaries with greater than 1 transfer within the same hospitalization were additionally excluded.
Patient Characteristics
Patient characteristics were obtained from the CMS data files and included: demographics (age, sex, race); DRG-weight, categorized into quartiles; primary diagnosis for the index hospitalization using ICD-9 codes; patient comorbidity using ICD-9 codes compiled into a CMS-Hierarchical Condition Category (HCC) risk score;21 presence of Medicaid co-insurance; number of hospitalizations in the past 12 months, categorized into 0, 1, 2-3, and 4 or more; season, defined as calendar quarters; and median income per household by census tract. These characteristics were chosen a priori given expert opinion in combination with prior research demonstrating association with IHT.11,22
Hospital Characteristics
Hospital characteristics were obtained from AHA data files and included hospitals’ size, categorized into small, medium, and large (less than 100, 100 to 399, 400 or more beds); geographic location; ownership; teaching status; setting (urban vs. rural); case mix index (CMI) for all patients cared for at the hospital; and presence of selected specialty services, including certified trauma center, medical intensive care unit, cardiac intensive care unit, cardiac surgery services, adult interventional cardiac catheterization, adult cardiac electrophysiology, and composite score of presence of 55 other specialty services (complete list in Appendix A). All characteristics were chosen a priori given expert opinion or relationship of characteristics with IHT, and prior research utilizing AHA data.23-24
Analysis
Descriptive statistics were used to evaluate the frequency of IHT, characteristics of transferred patients, and number of days to transfer. Patient and hospital characteristics of transferred vs. nontransferred patients were compared using chi-square analyses.
To analyze the effects of each patient and hospital characteristic on the odds of transfer, we used logistic regression models incorporating all patient and hospital characteristics, accounting for fixed effects for diagnosis, and utilizing generalized estimating equations (the GENMOD procedure in SAS statistical software, v 9.4; SAS Institute Inc., Cary, North Carolina) to account for the clustering of patients within hospitals.25 Indicator variables were created for missing covariate data and included in analyses when missing data accounted for greater than 10% of the total cohort.
To measure the variability in transfer rates between hospitals, we used a sequence of random effects logistic regression models. We first ran a model with no covariates, representing the unadjusted differences in transfer rates between hospitals. We then added patient characteristics to see if the unadjusted differences in IHT rates were explained by differences in patient characteristics between hospitals. Lastly, we added hospital characteristics to determine if these explained the remaining differences in transfer rates. Each of the 3 models provided a measure of between-hospital variability, reflecting the degree to which IHT rates differed between hospitals. Additionally, we used the intercept from the unadjusted model and the measure of between-hospital variability from each model to calculate the 95% confidence intervals, illustrating the range of IHT rates spanning 95% of all hospitals. We used those same numbers to calculate the 25th and 75th percentiles, illustrating the range of IHT rates for the middle half of hospitals.
RESULTS
Among 28 million eligible beneficiaries, 6.6 million had an acute care hospitalization to nonfederal, noncritical access hospitals, and 107,741 met our defined criteria for IHT. An additional 3790 beneficiaries were excluded for being transferred to the same facility, 416 beneficiaries (115 transferred, 301 nontransferred) were excluded as they were cared for at 1 of the 11 hospitals with “outlier” transfer in/out rates, and 2329 were excluded because they had more than 1 transfer during hospitalization. Thus, the final cohort consisted of 101,507 transferred (1.5%) and 6,625,474 nontransferred beneficiaries (Figure 1). Of the 101,507 transferred beneficiaries, 2799 (2.8%) were included more than once (ie, experienced more than 1 IHT on separate hospitalizations throughout the study period; the vast majority of these had 2 separate hospitalizations resulting in IHT). Characteristics of transferred and nontransferred beneficiaries are shown (Table 1).
Among transferred patients, the top 5 primary diagnoses at time of transfer included AMI (12.2%), congestive heart failure (CHF) (7.2%), sepsis (6.6%), arrhythmia (6.6%), and pneumonia (3.4%). Comorbid conditions most commonly present in transferred patients included CHF (52.6%), renal failure (51.8%), arrhythmia (49.8%), and chronic obstructive pulmonary disease (COPD; 37.0%). The most common day of transfer was day after admission (hospital day 2, 24.7%), with 75% of transferred patients transferred before hospital day 6 (Appendix B).
After adjusting for all other patient and hospital characteristics and clustering by hospital, the following variables were associated with greater odds of transfer: older age, male sex, nonblack race, non-Medicaid co-insurance, higher comorbidity (HCC score), lower DRG-weight, and fewer hospitalizations in the prior 12 months. Beneficiaries also had greater odds of transfer if initially hospitalized at smaller hospitals, nonteaching hospitals, public hospitals, at hospitals in the Northeast, those with fewer specialty services, and those with a low CMI (Table 2).
DISCUSSION
In this nationally representative study of 6.6 million Medicare beneficiaries, we found that 1.5% of patients were transferred between acute care facilities and were most often transferred prior to hospital day 6. Older age, male sex, nonblack race, higher medical comorbidity, lower DRG weight, and fewer recent hospitalizations were associated with greater odds of transfer. Initial hospitalization at smaller, nonteaching, public hospitals, with fewer specialty services were associated with greater odds of transfer, while higher CMI was associated with a lower odds of transfer. The most common comorbid conditions among transferred patients included CHF, renal failure, arrhythmia, and COPD; particularly notable was the very high prevalence of these conditions among transferred as compared with nontransferred patients. Importantly, we found significant variation in IHT by region and a large variation in transfer practices by hospital, with significant variability in transfer rates even after accounting for known patient and hospital characteristics.
Among our examined population, we found that a sizable number of patients undergo IHT—more than 100,000 per year. Primary diagnoses at time of transfer consist of common inpatient conditions, including AMI, CHF, sepsis, arrhythmia, and pneumonia. Limited prior data support our findings, with up to 50% of AMI patients reportedly undergoing IHT,3-5 and severe sepsis and respiratory illness reported as common diagnoses at transfer.11 Although knowledge of these primary diagnoses does not directly confer an understanding of reason for transfer, one can speculate based on our findings. For example, research demonstrates the majority of AMI patients who undergo IHT had further intervention, including stress testing, cardiac catheterization, and/or coronary artery bypass graft surgery.5,26 Thus, it is reasonable to presume that many of the beneficiaries
We additionally found that certain patient characteristics were associated with greater odds of transfer. Research suggests that transferred patients are “sicker” than nontransferred patients.1,11 Although our findings in part confirm these data, we paradoxically found that higher DRG-weight and 4 or more hospitalizations in the past year were actually associated with lower odds of transfer. In addition, the oldest patients in our cohort (85 years or older) were actually less likely to be transferred than their slightly younger counterparts (75 to 84 years). These variables may reflect extreme illness or frailty,27 and providers consciously (or subconsciously) may factor this in to their decision to transfer, considering a threshold past which transfer would confer more risk than benefit (eg, a patient may be “too sick” for transfer). Indeed, in a secondary analysis without hospital characteristics or comorbidities, and with fixed effects by hospital, we found the highest rates of IHT in patients in the middle 2 quartiles of DRG-weight, supporting this threshold hypothesis. It is also possible that patients with numerous hospitalizations may be less likely to be transferred because of familiarity and a strong sense of responsibility to continue to care for those patients (although we cannot confirm that those prior hospitalizations were all with the same index hospital).
It is also notable that odds of transfer differed by race, with black patients 17% less likely to undergo transfer compared to whites, similar to findings in other IHT studies.11 This finding, in combination with our demonstration that Medicaid patients also have lower odds of transfer, warrants further investigation to ensure the process of IHT does not bias against these populations, as with other well-documented health disparities.28-30
The hospital predictors of transfer were largely expected. However, interestingly, when we controlled for all other patient and hospital characteristics, regional variation persisted, with highest odds of transfer with hospitalization in the Northeast, indicating variability by region not explained by other factors, and findings supported by other limited data.31 This variability was further elucidated in our examination of change in variance estimates accounting for patient, then hospital, characteristics. Although we expected and found marked variability in hospital transfer rates in our null model (without accounting for any patient or hospital characteristics), we interestingly found that variability increased upon adjusting for patient characteristics. This result is presumably due to the fact that patients who are more likely to be transferred (ie, “sick” patients) are more often already at hospitals less likely to transfer patients, supported by our findings that hospital CMI is inversely associated with odds of transfer (in other words, hospitals that care for a less sick patient population are more likely to transfer their patients, and hospitals that care for a sicker patient population [higher CMI] are less likely to transfer). Adjusting solely for patient characteristics effectively equalizes these patients across hospitals, which would lead to even increased variability in transfer rates. Conversely, when we then adjusted for hospital characteristics, variability in hospital transfer rates decreased by 83% (in other words, hospital characteristics, rather than patient characteristics, explained much of the variability in transfer rates), although significant unexplained variability remained. We should note that although the observed reduction in variability was explained by the patient and hospital characteristics included in the model, these characteristics do not necessarily justify the variability they accounted for; although patients’ race or hospitals’ location may explain some of the observed variability, this does not reasonably justify it.
This observed variability in transfer practices is not surprising given the absence of standardization and clear guidelines to direct clinical IHT practice.17 Selection of patients that may benefit from transfer is often ambiguous and subjective.6 The Emergency Medical Treatment and Active Labor Act laws dictate that hospitals transfer patients requiring a more specialized service, or when “medical benefits ... outweigh the increased risks to the individual...,” although in practice this provides little guidance to practitioners.1 Thus, clearer guidelines may be necessary to achieve less variable practices.
Our study is subject to several limitations. First, although nationally representative, the Medicare population is not reflective of all hospitalized patients nationwide. Additionally, we excluded patients transferred from the emergency room. Thus, the total number of patients who undergo IHT nationally is expected to be much higher than reflected in our analysis. We also excluded patients who were transferred more than once during a given hospitalization. This enabled us to focus on the initial transfer decision but does not allow us to look at patients who are transferred to a referral center and then transferred back. Second, given the criteria we used to define transfer, it is possible that we included nontransferred patients within our transferred cohort if they were discharged from one hospital and admitted to a different hospital within 1 day. However, on quality assurance analyses where we limited our cohort to only those beneficiaries with corresponding “transfer in” and “transfer out” claims (87% of the total cohort), we found no marked differences in our results. Additionally, although we assume that patient transfer status was coded correctly within the Medicare dataset, we could not confirm by individually examining each patient we defined as “transferred.” However, on additional quality assurance analyses where we examined randomly selected excluded patients with greater than 1 transfer during hospitalization, we found differing provider numbers with each transfer, suggesting validity of the coding. Third, because there are likely many unmeasured patient confounders, we cannot be sure how much of the between-hospital variation is due to incomplete adjustment for patient characteristics. However, since adjusting for patient characteristics actually increased variability in hospital transfer rates, it is unlikely that residual patient confounders fully explain our observed results. Despite this, other variables that are not available within the CMS or AHA datasets may further elucidate hospital transfer practices, including variables reflective of the transfer process (eg, time of day of patient transfer, time delay between initiation of transfer and patient arrival at accepting hospital, accepting service on transfer, etc.); other markers of illness severity (eg, clinical service at the time of index admission, acute physiology score, utilization of critical care services on arrival at receiving hospital); and other hospital system variables (ie, membership in an accountable care organization and/or regional care network, the density of nearby tertiary referral centers (indicating possible supply-induced demand), other variables reflective of the “transfer culture” (such as the transfer rate at the hospital or region where the attending physician trained, etc.). Lastly, though our examination provides important foundational information regarding IHT nationally, this study did not examine patient outcomes in transferred and nontransferred patients, which may help to determine which patients benefit (or do not benefit) from transfer and why. Further investigation is needed to study these outcomes.
CONCLUSION
In this national study of IHT, we found that a sizable number of patients admitted to the hospital undergo transfer to another acute care facility. Patients are transferred with common medical conditions, including those requiring specialized care such as AMI, and a high rate of comorbid clinical conditions, and certain patient and hospital characteristics are associated with greater odds of transfer. Although many of the observed associations between characteristics and odds of transfer were expected based on limited existing literature, we found several unexpected findings, eg, suggesting the possibility of a threshold beyond which sicker patients are not transferred. Additionally, we found that black and Medicaid patients had lower odds of transfer, which warrants further investigation for potential health care disparity. Importantly, we found much variability in the practice of IHT, as evidenced by the inexplicable differences in transfer by hospital region, and by residual unexplained variability in hospital transfer rates after accounting for patient and hospital characteristics, which may be due to lack of standard guidelines to direct IHT practices. In conclusion, this study of hospitalized Medicare patients provides important foundational information regarding rates and predictors of IHT nationally, as well as unexplained variability that exists within this complex care transition. Further investigation will be essential to understand reasons for, processes related to, and outcomes of transferred patients, to help guide standardization in best practices in care.
Disclosure
Nothing to report.
1. Iwashyna TJ. The incomplete infrastructure for interhospital patient transfer. Crit Care Med. 2012;40(8):2470-2478. PubMed
2. Iwashyna TJ, Christie JD, Moody J, Kahn JM, Asch DA. The structure of critical care transfer networks. Med Care. 2009;47(7):787-793. PubMed
3. Mehta RH, Stalhandske EJ, McCargar PA, Ruane TJ, Eagle KA. Elderly patients at highest risk with acute myocardial infarction are more frequently transferred from community hospitals to tertiary centers: reality or myth? Am Heart J. 1999;138(4 Pt 1):688-695. PubMed
4. Iwashyna TJ, Kahn JM, Hayward RA, Nallamothu BK. Interhospital transfers among Medicare beneficiaries admitted for acute myocardial infarction at nonrevascularization hospitals. Circ Cardiovasc Qual Outcomes. 2010;3(5):468-475. PubMed
5. Roe MT, Chen AY, Delong ER, Boden WE, Calvin JE Jr, Cairns CB, et al. Patterns of transfer for patients with non-ST-segment elevation acute coronary syndrome from community to tertiary care hospitals. Am Heart J. 2008;156(1):185-192. PubMed
6. Bosk EA, Veinot T, Iwashyna TJ. Which patients and where: a qualitative study of patient transfers from community hospitals. Med Care. 2011;49(6):592-598. PubMed
7. Wagner J, Iwashyna TJ, Kahn JM. Reasons underlying interhospital transfers to an academic medical intensive care unit. J Crit Care. 2013;28(2):202-208. PubMed
8. Cohen MD, Hilligoss PB. The published literature on handoffs in hospitals: deficiencies identified in an extensive review. Qual Saf Health Care. 2010;19(6):493-497. PubMed
9. Riesenberg LA, Leitzsch J, Massucci JL, et al. Residents’ and attending physicians’ handoffs: a systematic review of the literature. Acad Med. 2009;84(12):1775-1787. PubMed
10. Arora V, Johnson J, Lovinger D, Humphrey HJ, Meltzer DO. Communication failures in patient sign-out and suggestions for improvement: a critical incident analysis. Qual Saf Health Care. 2005;14(6):401-407. PubMed
11. Sokol-Hessner L, White AA, Davis KF, Herzig SJ, Hohmann SF. Interhospital transfer patients discharged by academic hospitalists and general internists: characteristics and outcomes. J Hosp Med. 2016;11(4):245-250. PubMed
12. Bernard AM, Hayward RA, Rosevear J, Chun H, McMahon LF. Comparing the hospitalizations of transfer and non-transfer patients in an academic medical center. Acad Med. 1996;71(3):262-266. PubMed
13. Golestanian E, Scruggs JE, Gangnon RE, Mak RP, Wood KE. Effect of interhospital transfer on resource utilization and outcomes at a tertiary care referral center. Crit Care Med. 2007;35(6):1470-1476. PubMed
14. Durairaj L, Will JG, Torner JC, Doebbeling BN. Prognostic factors for mortality following interhospital transfers to the medical intensive care unit of a tertiary referral center. Crit Care Med. 2003;31(7):1981-1986. PubMed
15. Kerr HD, Byrd JC. Community hospital transfers to a VA Medical Center. JAMA. 1989;262(1):70-73. PubMed
16. Dragsted L, Jörgensen J, Jensen NH, et al. Interhospital comparisons of patient outcome from intensive care: importance of lead-time bias. Crit Care Med. 1989;17(5):418-422. PubMed
17. Gupta K, Mueller SK. Interhospital transfers: the need for standards. J Hosp Med. 2015;10(6):415-417. PubMed
18. The Dartmouth Atlas of Health Care: Understanding of the Efficiency and Effectiveness of the Health Care System. The Dartmouth Institute for Health Practice and Clinical Policy, Lebanon, NH. http://www.dartmouthatlas.org/. Accessed November 1, 2016.
19. American Hospital Association Annual Survey Database. American Hospital Association, Chicago, IL. http://www.ahadataviewer.com/book-cd-products/AHA-Survey/. Accessed July 1, 2013.
20. U.S. Department of Health and Human Services (HRSA): What are critical access hospitals (CAH)? http://www.hrsa.gov/healthit/toolbox/RuralHealthITtoolbox/Introduction/critical.html. Accessed June 9, 2016.
21. Li P, Kim MM, Doshi JA. Comparison of the performance of the CMS Hierarchical Condition Category (CMS-HCC) risk adjuster with the Charlson and Elixhauser comorbidity measures in predicting mortality. BMC Health Serv Res. 2010;10:245. PubMed
22. Hernandez-Boussard T, Davies S, McDonald K, Wang NE. Interhospital facility transfers in the United States: a nationwide outcomes study. J Patient Saf. Nov 13 2014. PubMed
23. Landon BE, Normand SL, Lessler A, et al. Quality of care for the treatment of acute medical conditions in US hospitals. Arch Intern Med. 2006;166(22):2511-2517. PubMed
24. Mueller SK, Lipsitz S, Hicks LS. Impact of hospital teaching intensity on quality of care and patient outcomes. Med Care.2013;51(7):567-574. PubMed
25. Lopez L, Hicks LS, Cohen AP, McKean S, Weissman JS. Hospitalists and the quality of care in hospitals. Arch Intern Med. 2009;169(15):1389-1394. PubMed
26. Barreto-Filho JA, Wang Y, Rathore SS, et al. Transfer rates from nonprocedure hospitals after initial admission and outcomes among elderly patients with acute myocardial infarction. JAMA Intern Med. 2014;174(2):213-222. PubMed
27. Carlson JE, Zocchi KA, Bettencourt DM, et al. Measuring frailty in the hospitalized elderly: concept of functional homeostasis. Am J Phys Med Rehabil. 1998;77(3):252-257. PubMed
28. Ward E, Jemal A, Cokkinides V, et al. Cancer disparities by race/ethnicity and socioeconomic status. CA Cancer J Clin. 2004;54(2):78-93. PubMed
29. Iribarren C, Tolstykh I, Somkin CP, et al. Sex and racial/ethnic disparities in outcomes after acute myocardial infarction: a cohort study among members of a large integrated health care delivery system in northern California. Arch Intern Med. 2005;165(18):2105-2113. PubMed
30. Kawachi I, Daniels N, Robinson DE. Health disparities by race and class: why both matter. Health Aff (Millwood). 2005;24(2):343-352. PubMed
31. Herrigel DJ, Carroll M, Fanning C, Steinberg MB, Parikh A, Usher M. Interhospital transfer handoff practices among US tertiary care centers: a descriptive survey. J Hosp Med. 2016;11(6):413-417. PubMed
1. Iwashyna TJ. The incomplete infrastructure for interhospital patient transfer. Crit Care Med. 2012;40(8):2470-2478. PubMed
2. Iwashyna TJ, Christie JD, Moody J, Kahn JM, Asch DA. The structure of critical care transfer networks. Med Care. 2009;47(7):787-793. PubMed
3. Mehta RH, Stalhandske EJ, McCargar PA, Ruane TJ, Eagle KA. Elderly patients at highest risk with acute myocardial infarction are more frequently transferred from community hospitals to tertiary centers: reality or myth? Am Heart J. 1999;138(4 Pt 1):688-695. PubMed
4. Iwashyna TJ, Kahn JM, Hayward RA, Nallamothu BK. Interhospital transfers among Medicare beneficiaries admitted for acute myocardial infarction at nonrevascularization hospitals. Circ Cardiovasc Qual Outcomes. 2010;3(5):468-475. PubMed
5. Roe MT, Chen AY, Delong ER, Boden WE, Calvin JE Jr, Cairns CB, et al. Patterns of transfer for patients with non-ST-segment elevation acute coronary syndrome from community to tertiary care hospitals. Am Heart J. 2008;156(1):185-192. PubMed
6. Bosk EA, Veinot T, Iwashyna TJ. Which patients and where: a qualitative study of patient transfers from community hospitals. Med Care. 2011;49(6):592-598. PubMed
7. Wagner J, Iwashyna TJ, Kahn JM. Reasons underlying interhospital transfers to an academic medical intensive care unit. J Crit Care. 2013;28(2):202-208. PubMed
8. Cohen MD, Hilligoss PB. The published literature on handoffs in hospitals: deficiencies identified in an extensive review. Qual Saf Health Care. 2010;19(6):493-497. PubMed
9. Riesenberg LA, Leitzsch J, Massucci JL, et al. Residents’ and attending physicians’ handoffs: a systematic review of the literature. Acad Med. 2009;84(12):1775-1787. PubMed
10. Arora V, Johnson J, Lovinger D, Humphrey HJ, Meltzer DO. Communication failures in patient sign-out and suggestions for improvement: a critical incident analysis. Qual Saf Health Care. 2005;14(6):401-407. PubMed
11. Sokol-Hessner L, White AA, Davis KF, Herzig SJ, Hohmann SF. Interhospital transfer patients discharged by academic hospitalists and general internists: characteristics and outcomes. J Hosp Med. 2016;11(4):245-250. PubMed
12. Bernard AM, Hayward RA, Rosevear J, Chun H, McMahon LF. Comparing the hospitalizations of transfer and non-transfer patients in an academic medical center. Acad Med. 1996;71(3):262-266. PubMed
13. Golestanian E, Scruggs JE, Gangnon RE, Mak RP, Wood KE. Effect of interhospital transfer on resource utilization and outcomes at a tertiary care referral center. Crit Care Med. 2007;35(6):1470-1476. PubMed
14. Durairaj L, Will JG, Torner JC, Doebbeling BN. Prognostic factors for mortality following interhospital transfers to the medical intensive care unit of a tertiary referral center. Crit Care Med. 2003;31(7):1981-1986. PubMed
15. Kerr HD, Byrd JC. Community hospital transfers to a VA Medical Center. JAMA. 1989;262(1):70-73. PubMed
16. Dragsted L, Jörgensen J, Jensen NH, et al. Interhospital comparisons of patient outcome from intensive care: importance of lead-time bias. Crit Care Med. 1989;17(5):418-422. PubMed
17. Gupta K, Mueller SK. Interhospital transfers: the need for standards. J Hosp Med. 2015;10(6):415-417. PubMed
18. The Dartmouth Atlas of Health Care: Understanding of the Efficiency and Effectiveness of the Health Care System. The Dartmouth Institute for Health Practice and Clinical Policy, Lebanon, NH. http://www.dartmouthatlas.org/. Accessed November 1, 2016.
19. American Hospital Association Annual Survey Database. American Hospital Association, Chicago, IL. http://www.ahadataviewer.com/book-cd-products/AHA-Survey/. Accessed July 1, 2013.
20. U.S. Department of Health and Human Services (HRSA): What are critical access hospitals (CAH)? http://www.hrsa.gov/healthit/toolbox/RuralHealthITtoolbox/Introduction/critical.html. Accessed June 9, 2016.
21. Li P, Kim MM, Doshi JA. Comparison of the performance of the CMS Hierarchical Condition Category (CMS-HCC) risk adjuster with the Charlson and Elixhauser comorbidity measures in predicting mortality. BMC Health Serv Res. 2010;10:245. PubMed
22. Hernandez-Boussard T, Davies S, McDonald K, Wang NE. Interhospital facility transfers in the United States: a nationwide outcomes study. J Patient Saf. Nov 13 2014. PubMed
23. Landon BE, Normand SL, Lessler A, et al. Quality of care for the treatment of acute medical conditions in US hospitals. Arch Intern Med. 2006;166(22):2511-2517. PubMed
24. Mueller SK, Lipsitz S, Hicks LS. Impact of hospital teaching intensity on quality of care and patient outcomes. Med Care.2013;51(7):567-574. PubMed
25. Lopez L, Hicks LS, Cohen AP, McKean S, Weissman JS. Hospitalists and the quality of care in hospitals. Arch Intern Med. 2009;169(15):1389-1394. PubMed
26. Barreto-Filho JA, Wang Y, Rathore SS, et al. Transfer rates from nonprocedure hospitals after initial admission and outcomes among elderly patients with acute myocardial infarction. JAMA Intern Med. 2014;174(2):213-222. PubMed
27. Carlson JE, Zocchi KA, Bettencourt DM, et al. Measuring frailty in the hospitalized elderly: concept of functional homeostasis. Am J Phys Med Rehabil. 1998;77(3):252-257. PubMed
28. Ward E, Jemal A, Cokkinides V, et al. Cancer disparities by race/ethnicity and socioeconomic status. CA Cancer J Clin. 2004;54(2):78-93. PubMed
29. Iribarren C, Tolstykh I, Somkin CP, et al. Sex and racial/ethnic disparities in outcomes after acute myocardial infarction: a cohort study among members of a large integrated health care delivery system in northern California. Arch Intern Med. 2005;165(18):2105-2113. PubMed
30. Kawachi I, Daniels N, Robinson DE. Health disparities by race and class: why both matter. Health Aff (Millwood). 2005;24(2):343-352. PubMed
31. Herrigel DJ, Carroll M, Fanning C, Steinberg MB, Parikh A, Usher M. Interhospital transfer handoff practices among US tertiary care centers: a descriptive survey. J Hosp Med. 2016;11(6):413-417. PubMed
© 2017 Society of Hospital Medicine
Insurance Status and Hospital Care
With about 1 in 5 working‐age Americans (age 18‐64 years) currently uninsured and a large number relying on Medicaid, adequate access to quality health care services is becoming increasingly difficult.1 Substantial literature has accumulated over the years suggesting that access and quality in health care are closely linked to an individual's health insurance status.211 Some studies indicate that being uninsured or publicly insured is associated with negative health consequences.12, 13 Although the Medicaid program has improved access for qualifying low‐income individuals, significant gaps in access and quality remain.2, 5, 11, 1419 These issues are likely to become more pervasive should there be further modifications to state Medicaid funding in response to the unfolding economic crisis.
Although numerous studies have focused on insurance‐related disparities in the outpatient setting, few nationally representative studies have examined such disparities among hospitalized patients. A cross‐sectional study of a large hospital database from 1987 reported higher risk‐adjusted in‐hospital mortality, shorter length of stay (LOS), and lower procedure use among uninsured patients.9 A more recent analysis, limited to patients admitted with stroke, reported significant variation in hospital care associated with insurance status.15 Other studies reporting myocardial infarction registry and quality improvement program data are biased by the self‐selection of large urban teaching hospitals.1618 To our knowledge, no nationally representative study has focused on the impact of insurance coverage on hospital care for common medical conditions among working‐age Americans, the fastest growing segment of the uninsured population.
To address this gap in knowledge, we analyzed a nationally representative hospital database to determine whether there are significant insurance‐related disparities in in‐hospital mortality, LOS, and cost per hospitalization for 3 common medical conditions among working‐age adults, and, if present, to determine whether these disparities are due to differences in disease severity and comorbidities, and whether these disparities are affected by the proportion of uninsured and Medicaid patients receiving care in each hospital.
Methods
Design and Subjects
We examined data from the 2005 Nationwide Inpatient Sample (NIS), a nationally representative database of hospital inpatient stays maintained by the Agency for Healthcare Research and Quality (AHRQ) as part of the Healthcare Cost and Utilization Project (HCUP).20, 21 The NIS is a stratified probability sample of 20% of all US community hospitals, including public hospitals, academic medical centers, and specialty hospitals. Long‐term care hospitals, psychiatric hospitals, and alcoholism/chemical‐dependency treatment facilities are excluded. The 2005 NIS contains data on 7,995,048 discharges from 1054 hospitals located in 37 States and is designed to be representative of all acute care discharges from all US community hospitals.21
We identified discharges with a principal diagnosis of acute myocardial infarction (AMI), stroke, and pneumonia using International Classification of Diseases, 9th Revision, Clinical Modification (ICD‐9‐CM) codes specified in the AHRQ definitions of Inpatient Quality Indicators (Supporting Information Appendix).22 These 3 conditions are among the leading causes of noncancer inpatient deaths in patients under 65 years old,23 and evidence suggests that high mortality may be associated with deficiencies in the quality of inpatient care.24
We confined our analysis to patients 18 to 64 years of age, since this population is most at risk of being uninsured or underinsured.25 We excluded pregnant women because they account for an unusually high proportion of uninsured discharges and were relatively few in our cohort.26 In addition, we excluded patients transferred to another acute care hospital and patients missing payer source and discharge disposition. Our study protocol was approved by the Partners Human Research Committee.
Study Variables
We categorized insurance status as privately insured, uninsured, Medicaid, or Medicare. We defined privately insured patients as those having either Blue Cross or another commercial carrier listed as the primary payer and uninsured patients as those having either no charge or self‐pay listed as the primary payer.27 Other governmental payer categories were noted to share several characteristics with Medicare patients and comprised only a small proportion of the sample, and were thus included with Medicare. In order to account for NIS's sampling scheme and accurately apply sample weights in our analysis, we used Medicare as a separate category. However, since Medicare patients age 18 to 64 years represent a fundamentally different population that is primarily disabled or very ill, only results of privately insured, uninsured, and Medicaid patients are reported.
We selected in‐hospital mortality as the outcome measure and LOS and cost per hospitalization as measures of resource utilization. The NIS includes a binary indicator variable for in‐hospital mortality and specifies inpatient LOS in integers, with same‐day stays coded as 0. NIS's cost estimates are based on hospital cost reports submitted to the Centers for Medicare and Medicaid Services. To test the validity of our cost analyses, we performed parallel analyses using hospital charges as a measure of utilization (charges include hospital overhead, charity care, and bad debt). The resulting adjusted ratios differed little from cost ratios and we opted to report only the details of our cost analyses.
In order to assess the independent association between insurance status and the outcome measures listed above, we selected covariates for inclusion in multivariable models based on the existing literature. Patient covariates included: age group (18‐34 years, 35‐49 years, 50‐64 years), sex (male/female), race/ethnicity (non‐Hispanic white, non‐Hispanic black, Hispanic, other, missing), median income by zip code of residence (less than $37,000, $37,000‐$45,999, $46,000‐$60,999, $61,000 or more), admission through the emergency department (yes/no), admission on a weekend (yes/no), measures of disease severity, and comorbidity indicators. Measures of disease severity specific to each outcome are assigned in the NIS using criteria developed by Medstat (Medstat Disease Staging Software Version 5.2, Thomson Medstat Inc., Ann Arbor, MI). Severity is categorized into 5 levels, with a higher level indicating greater risk. We recorded comorbidities for each patient in our sample using HCUP Comorbidity Software, Version 3.2 (
Hospital covariates included: bed size (small, medium, large), ownership/control (private, government, private or government), geographic region (northeast, midwest, south, west), teaching status (teaching, non‐teaching), and the proportion of uninsured and Medicaid patients (combined) admitted to each hospital for AMI, stroke, or pneumonia. The actual number of hospital beds in each bed size category varies according to a hospital's geographic region and teaching status.27 Ownership/control, geographic region, and teaching status are assigned according to information from the American Hospital Association Annual Survey of Hospitals. The proportion of uninsured and Medicaid patients admitted to each hospital was found to have a nonmonotonic relationship with the outcomes being assessed and was thus treated as a 6‐level categorical variable with the following levels: 0% to 10%, 11% to 20%, 21% to 30%, 31% to 40%, 41% to 50%, and 51% to 100%.
Statistical Analysis
Summary statistics were constructed at the patient level and differences in proportions were evaluated with the chi‐square test. We employed direct standardization, using the age and sex distribution of the entire cohort, to compute age‐standardized and sex‐standardized estimates for each insurance group and compared them using the chi‐square test for in‐hospital mortality and t test for log transformed LOS and cost per hospitalization. For each condition, we developed multivariable logistic regression models for in‐hospital mortality and multivariable linear regression models for log transformed LOS and cost. The patient was the unit of analysis in all models.
In order to elucidate the contribution of disease severity and comorbidities and the proportion of uninsured and Medicaid patients admitted to each hospital, we fitted 3 sequential models for each outcome measure: Model 1 adjusted for patient sociodemographic characteristics and hospital characteristics with the exception of the covariate for the proportion of uninsured and Medicaid patients, Model 2 adjusted for all covariates in the preceding model as well as patients' comorbidities and severity of principal diagnosis, and Model 3 adjusted for all previously mentioned covariates as well as the proportion of uninsured and Medicaid patients admitted to each hospital. We excluded patients who died during hospitalization from the models for LOS and cost. We exponentiated the effect estimates from the log transformed linear regression models so that the adjusted ratio represents the percentage change in the mean LOS or mean cost.
To determine whether the association between patients' insurance status and in‐hospital mortality was modified by seeking care in hospitals treating a smaller or larger proportion of uninsured and Medicaid patients, we entered an interaction term for insurance status and proportion of uninsured and Medicaid patients in the final models (Model 3) for our primary outcome of in‐hospital mortality. However, since no significant interaction was found for any of the 3 conditions, this term was removed from the models and results from the interaction models are not described. In order to assess model specification for the linear regression models, we evaluated the normality of model residuals and found that these were approximately normally distributed. Lastly, we attempted to test the robustness of our analyses by creating fixed effects models that controlled for hospital site but were unable to do so due to the computational limitations of available software packages that could not render fixed effects models with more than 1000 hospital sites.
For all variables except race/ethnicity, data were missing for less than 3% of patients, so we excluded these individuals from adjusted analyses. However, race/ethnicity data were missing for 29% of the sample and were analyzed in 3 different ways, namely, with the missing data treated as a separate covariate level, with the missing data removed from the analysis, and with the missing data assigned to the majority covariate level (white race). The results of our analysis were unchanged no matter how the missing values were assigned. As a result, missing values for race/ethnicity were treated as a separate covariate level in the final analysis.15 Sociodemographic characteristics of patients with missing race/ethnicity information were similar to those with complete data.
We used SUDAAN (Release 9.0.1, Research Triangle Institute, NC) to account for NIS's sampling scheme and generalized estimating equations to adjust for the clustering of patients within hospitals and hospitals within sampling strata.29 In order to account for NIS's stratified probability sampling scheme, SUDAAN uses Taylor series linearization for robust variance estimation of descriptive statistics and regression parameters.30, 31 We present 2‐tailed P values or 95% confidence intervals (CIs) for all statistical comparisons.
Results
Patient and Hospital Characteristics
The final cohort comprised of 154,381 patients discharged from 1018 hospitals in 37 states during calendar year 2005 (Table 1). This cohort was representative of 755,346 working‐age Americans, representing approximately 225,947 cases of AMI (29.9%), 151,812 cases of stroke (20.1%), and 377,588 cases of pneumonia (50.0%). Of these patients, 47.5% were privately insured, 12.0% were uninsured, 17.0% received Medicaid, and 23.5% were assigned to Medicare. Compared with the privately insured, uninsured and Medicaid patients were generally younger, less likely to be white, more likely to have lower income, and more likely to be admitted through the emergency department. Of the 1018 hospitals included in our study, close to half (44.3%) were small, with bedsize ranging from 24 to 249. A large number of hospitals were located in the South (39.9%), and 14.9% were designated teaching hospitals.
| Characteristic* | Privately insured (n = 73,256) | Uninsured (n = 18,531) | Medicaid (n = 26,222) |
|---|---|---|---|
| |||
| Principal diagnosis (%) | |||
| Acute myocardial infarction | 36.7 | 31.2 | 19.7 |
| Stroke | 20.6 | 23.7 | 19.9 |
| Pneumonia | 42.7 | 45.2 | 60.4 |
| Age group (%) | |||
| 18‐34 years | 6.8 | 13.0 | 13.7 |
| 35‐49 years | 27.6 | 36.9 | 33.2 |
| 50‐64 years | 65.7 | 50.1 | 53.2 |
| Male sex (%) | 59.3 | 62.3 | 46.6 |
| Race or ethnicity (%) | |||
| White | 55.7 | 41.5 | 38.0 |
| Black | 7.6 | 14.8 | 16.6 |
| Hispanic | 4.8 | 10.5 | 10.4 |
| Other race | 3.6 | 4.7 | 5.2 |
| Missing | 28.4 | 29.0 | 29.7 |
| Median income by ZIP code (%) | |||
| <$37,000 | 21.5 | 36.7 | 43.0 |
| $37,000‐$45,999 | 25.2 | 27.8 | 27.1 |
| $46,000‐$60,999 | 26.3 | 20.3 | 17.6 |
| $61,000 | 24.8 | 11.5 | 8.4 |
| Emergency admission (%) | 63.3 | 75.6 | 72.9 |
| Weekend admission (%) | 24.5 | 26.2 | 25.1 |
| Hospital bed size (%) | |||
| Small | 8.9 | 10.3 | 11.4 |
| Medium | 24.0 | 22.3 | 25.9 |
| Large | 67.1 | 67.5 | 62.8 |
| Hospital control (%) | |||
| Private | 33.8 | 34.8 | 34.4 |
| Government (nonfederal) | 6.7 | 9.7 | 8.3 |
| Private or government | 59.5 | 55.5 | 57.3 |
| Hospital region (%) | |||
| Northeast | 17.4 | 12.5 | 17.6 |
| Midwest | 25.7 | 19.4 | 20.9 |
| South | 39.5 | 56.8 | 42.4 |
| West | 17.4 | 11.3 | 19.2 |
| Teaching hospital (%) | 41.7 | 43.8 | 43.3 |
Compared with privately insured patients, a larger proportion of uninsured and Medicaid patients had higher predicted mortality levels (Table 2). Medicaid patients had a disproportionately higher predicted LOS, whereas predicted resource demand was higher among privately insured patients. Hypertension (48%), chronic pulmonary disease (29.5%), and uncomplicated diabetes (21.5%) were the 3 most common comorbidities in the study cohort, with a generally higher prevalence of comorbidities among Medicaid patients.
| Characteristic* | Privately insured (n = 73,256) | Uninsured (n = 18,531) | Medicaid (n = 26,222) |
|---|---|---|---|
| |||
| Medstat disease staging (%) | |||
| Mortality level 1 | 50.8 | 45.4 | 36.7 |
| Mortality level 2 | 44.0 | 49.1 | 56.7 |
| Mortality level 3 | 5.3 | 5.5 | 6.7 |
| Length of stay level 1 | 66.8 | 71.6 | 53.8 |
| Length of stay level 2 | 28.5 | 24.5 | 39.3 |
| Length of stay level 3 | 4.8 | 3.8 | 6.9 |
| Resource demand level 1 | 45.2 | 54.2 | 48.5 |
| Resource demand level 2 | 40.5 | 34.2 | 39.2 |
| Resource demand level 3 | 14.2 | 11.7 | 12.3 |
| Coexisting medical conditions (%) | |||
| Congestive heart failure | 4.7 | 4.8 | 10.1 |
| Valvular disease | 2.8 | 2.0 | 2.7 |
| Pulmonary circulation disease | 0.8 | 0.6 | 1.5 |
| Peripheral vascular disease | 3.2 | 2.2 | 3.2 |
| Paralysis | 1.2 | 0.8 | 3.5 |
| Other neurological disorders | 2.4 | 1.9 | 7.3 |
| Chronic pulmonary disease | 23.6 | 22.4 | 37.7 |
| Uncomplicated diabetes | 19.6 | 18.6 | 23.4 |
| Complicated diabetes | 3.3 | 2.1 | 4.9 |
| Hypothyroidism | 5.6 | 2.7 | 4.7 |
| Renal failure | 3.0 | 1.9 | 5.6 |
| Liver disease | 1.6 | 2.5 | 4.4 |
| Peptic ulcer disease | <0.5 | <0.5 | <0.5 |
| AIDS | 0.1 | 0.1 | 0.4 |
| Lymphoma | 1.1 | 0.4 | 0.6 |
| Metastatic cancer | 2.1 | 0.7 | 2.2 |
| Non‐metastatic solid tumor | 1.5 | 0.8 | 2.1 |
| Collagen vascular diseases | 2.3 | 0.9 | 2.3 |
| Coagulopathy | 2.7 | 2.4 | 3.4 |
| Obesity | 10.3 | 8.2 | 9.3 |
| Weight loss | 1.6 | 1.8 | 3.3 |
| Fluid and electrolyte disorders | 18.3 | 19.4 | 23.8 |
| Chronic blood loss anemia | 0.6 | 0.6 | 0.8 |
| Deficiency anemias | 8.6 | 8.5 | 13.4 |
| Alcohol abuse | 3.3 | 9.8 | 8.3 |
| Drug abuse | 1.9 | 10.2 | 9.8 |
| Psychoses | 1.5 | 1.9 | 6.8 |
| Depression | 7.2 | 4.8 | 9.9 |
| Hypertension | 48.0 | 44.1 | 45.7 |
In‐Hospital Mortality
Compared with the privately insured, age‐standardized and sex‐standardized in‐hospital mortality for AMI and stroke was significantly higher for uninsured and Medicaid patients (Table 3). Among pneumonia patients, Medicaid recipients had significantly higher in‐hospital mortality compared with privately insured and uninsured patients.
| Privately Insured | Uninsured | Medicaid | |
|---|---|---|---|
| |||
| In‐hospital mortality, rate per 100 discharges (SE) | |||
| Acute myocardial infarction | 2.22 (0.10) | 4.03 (0.31)* | 4.57 (0.34)* |
| Stroke | 7.49 (0.27) | 10.46 (0.64)* | 9.89 (0.45)* |
| Pneumonia | 1.75 (0.09) | 1.74 (0.18) | 2.48 (0.14)* |
| Length of stay, mean (SE), days | |||
| Acute myocardial infarction | 4.17 (0.06) | 4.46 (0.09) | 5.85 (0.16) |
| Stroke | 6.37 (0.13) | 7.15 (0.25) | 9.28 (0.30) |
| Pneumonia | 4.89 (0.05) | 4.64 (0.10) | 5.80 (0.08) |
| Cost per episode, mean (SE), dollars | |||
| Acute myocardial infarction | 21,077 (512) | 19,977 (833) | 22,452 (841) |
| Stroke | 16,022 (679) | 14,571 (1,036) | 18,462 (824) |
| Pneumonia | 8,223 (192) | 7,086 (293) | 9,479 (271) |
After multivariable adjustment for additional patient and hospital characteristics, uninsured AMI and stroke patients continued to have significantly higher in‐hospital mortality compared with the privately insured (Table 4). Among pneumonia patients, Medicaid recipients persisted in having significantly higher in‐hospital mortality than the privately insured.
| Model 1* | Model 2 | Model 3 | |
|---|---|---|---|
| |||
| In‐hospital mortality, adjusted odds ratio (95% CI) | |||
| Acute Myocardial Infarction | |||
| Uninsured vs. privately insured | 1.59 (1.35‐1.88) | 1.58 (1.30‐1.93) | 1.52 (1.24‐1.85) |
| Medicaid vs. privately insured | 1.83 (1.54‐2.18) | 1.22 (0.99‐1.50) | 1.15 (0.94‐1.42) |
| Stroke | |||
| Uninsured vs. privately insured | 1.56 (1.35‐1.80) | 1.50 (1.30‐1.73) | 1.49 (1.29‐1.72) |
| Medicaid vs. privately insured | 1.32 (1.15‐1.52) | 1.09 (0.93‐1.27) | 1.08 (0.93‐1.26) |
| Pneumonia | |||
| Uninsured vs. privately insured | 0.99 (0.81‐1.21) | 1.12 (0.91‐1.39) | 1.10 (0.89‐1.36) |
| Medicaid vs. privately insured | 1.41 (1.20‐1.65) | 1.24 (1.04‐1.48) | 1.21 (1.01‐1.45) |
| Length of stay, adjusted ratio (95% CI)| | |||
| Acute Myocardial Infarction | |||
| Uninsured vs. privately insured | 1.00 (0.98‐1.02) | 1.00 (0.98‐1.02) | 1.00 (0.98‐1.02) |
| Medicaid vs. privately insured | 1.17 (1.14‐1.21) | 1.07 (1.05‐1.09) | 1.07 (1.05‐1.09) |
| Stroke | |||
| Uninsured vs. privately insured | 1.06 (1.02‐1.10) | 1.08 (1.04‐1.11) | 1.07 (1.04‐1.11) |
| Medicaid vs. privately insured | 1.30 (1.26‐1.34) | 1.17 (1.14‐1.20) | 1.17 (1.14‐1.20) |
| Pneumonia | |||
| Uninsured vs. privately insured | 0.95 (0.93‐0.97) | 0.96 (0.94‐0.99) | 0.96 (0.94‐0.98) |
| Medicaid vs. privately insured | 1.15 (1.13‐1.17) | 1.04 (1.03‐1.06) | 1.04 (1.03‐1.06) |
| Cost per episode, adjusted ratio (95% CI)| | |||
| Acute Myocardial Infarction | |||
| Uninsured vs. privately insured | 0.97 (0.95‐0.99) | 0.99 (0.97‐1.00) | 0.99 (0.97‐1.00) |
| Medicaid vs. privately insured | 1.01 (0.98‐1.04) | 0.99 (0.97‐1.01) | 0.99 (0.97‐1.01) |
| Stroke | |||
| Uninsured vs. privately insured | 0.97 (0.93‐1.02) | 1.00 (0.96‐1.03) | 1.00 (0.97‐1.03) |
| Medicaid vs. privately insured | 1.17 (1.13‐1.21) | 1.06 (1.04‐1.09) | 1.06 (1.04‐1.09) |
| Pneumonia | |||
| Uninsured vs. privately insured | 0.95 (0.92‐0.97) | 0.98 (0.96‐1.00) | 0.98 (0.96‐1.00) |
| Medicaid vs. privately insured | 1.17 (1.15‐1.19) | 1.05 (1.04‐1.07) | 1.05 (1.04‐1.07) |
LOS
Among AMI and stroke patients, age‐standardized and sex‐standardized mean LOS was significantly longer for the uninsured and Medicaid recipients compared with the privately insured (Table 3). Among pneumonia patients, the uninsured had a slightly shorter mean LOS compared with the privately insured whereas Medicaid recipients averaged the longest LOS.
These insurance‐related disparities in LOS among pneumonia patients persisted after multivariable adjustment (Table 4). Among AMI patients, only Medicaid recipients persisted in having a significantly longer LOS than the privately insured. Among stroke patients, both the uninsured and Medicaid recipients averaged a longer LOS compared with the privately insured.
Cost per Episode
For all 3 conditions, the uninsured had significantly lower age‐standardized and sex‐standardized costs compared with the privately insured (Table 3). However, Medicaid patients had higher costs than the privately insured for all three conditions, significantly so among patients with stroke and pneumonia.
These insurance‐related disparities in costs persisted in multivariable analyses (Table 4). The uninsured continued to have lower costs compared with the privately insured, significantly so for patients with AMI and pneumonia. Among stroke and pneumonia patients, Medicaid recipients continued to accrue higher costs than the uninsured or privately insured.
Discussion
In this nationally representative study of working‐age Americans hospitalized for 3 common medical conditions, we found that insurance status was associated with significant variations in in‐hospital mortality and resource use. Whereas privately insured patients experienced comparatively lower in‐hospital mortality in most cases, mortality risk was highest among the uninsured for 2 of the 3 common causes of noncancer inpatient deaths. Although previous studies have examined insurance‐related disparities in inpatient care for individual diagnoses and specific populations, no broad overview of this important issue has been published in the past decade. In light of the current economic recession and national healthcare debate, these findings may be a prescient indication of a widening insurance gap in the quality of hospital care.
There are several potential mechanisms for these disparities. For instance, Hadley et al.9 reported significant underuse of high‐cost or high‐discretion procedures among the uninsured in their analysis of a nationally representative sample of 592,598 hospitalized patients. Similarly, Burstin et al.10 found that among a population of 30,195 hospitalized patients with diverse diagnoses, the uninsured were at greater risk for receiving substandard care regardless of hospital characteristics. These, and other similar findings,7, 8, 19 are suggestive of differences in the way uninsured patients are generally managed in the hospital that may partly explain the disparities reported herein.
More specifically, analyses of national registries of AMI have documented lower rates of utilization of invasive, potentially life‐saving, cardiac interventions among the uninsured.16, 17 Similarly, a lower rate of carotid endarterectomy was reported among uninsured stroke patients from an analysis of the 2002 NIS.15 Other differences in inpatient management unmeasured by administrative data, such as the use of subspecialists and allied health professionals, may also contribute.32 Unfortunately, limitations in the available data prevented us from being able to appropriately address the important issue of insurance related differences in the utilization of specific inpatient procedures.
These disparities may also be indicative of differences in severity of illness that are not captured fully by the MedStat disease staging criteria. The uninsured might have more severe illness at admission, either due to the presence of more advanced chronic disease or delay in seeking care for the acute episode. AMI and stroke are usually the culmination of longstanding atherosclerosis that is amenable to improvement through timely and consistent risk‐factor modification. Not having a usual source of medical care,6, 33 inadequate screening and management of known risk‐factors,3, 34 and difficulties in obtaining specialty care5 among the uninsured likely increases their risk of being hospitalized with more advanced disease. The higher likelihood of being admitted through the emergency department19 and on weekends9 among the uninsured lends credence to the possibility of delays in seeking care. All of these are potential mediators of higher AMI and stroke mortality in uninsured patients.
Finally, these mortality differences could also be due to the additional risks imposed by poorly managed comorbidities among uninsured patients. Although we controlled for the presence of comorbidities in our analysis, we lacked data about the severity of individual comorbidities. A recent study reported significant lapses in follow‐up care after the onset of a chronic condition in uninsured individuals under 65 years of age.34 Other studies have also documented insurance related disparities in the care of chronic diseases3, 35 that were among the most common comorbidities in our cohort.
Most of the reasons for insurance‐related disparities noted above for the uninsured are also applicable to Medicaid patients. Differences in the intensity of inpatient care,7, 8, 1519 limited access to health care services,2, 14 unmet health needs,5 and suboptimal management of chronic medical conditions35 were also reported for Medicaid patients in prior research. These factors likely contributed to the higher in‐hospital mortality in this patient population, evidenced by the sequential decrease in odds after adjusting for comorbidities and disease severity. Medicaid patients hospitalized for stroke were noted to have significantly longer LOS, which could plausibly be due to difficulties with arranging appropriate discharge disposition; the higher likelihood of paralysis among these patients15 would likely necessitate a higher frequency of rehabilitation facility placement. The higher costs for Medicaid patients with stroke and pneumonia may potentially be the result of these patients longer LOS. Although cost differences between the uninsured and privately insured were statistically significant, these were not large enough to be of material significance.
Limitations
Our study has several limitations. Since the NIS does not assign unique patient identifiers that would permit tracking of readmissions, we excluded patients transferred to another acute‐care hospital from our study to avoid counting the same patient twice. However, only 10% of hospitalized patients underwent transfer for cardiac procedures in the National Registry of Myocardial Infarction, with privately insured patients more likely to be transferred than other insurance groups.17 Since these patients are also more likely to have better survival, their exclusion likely biased our study toward the null. The same is probable for stroke patients as well.
Some uninsured patients begin Medicaid coverage during hospitalization and should ideally be counted as uninsured but were included under Medicaid in our analysis. They are also likely to be state‐ and plan‐specific variations in Medicaid and private payer coverage that we could not incorporate into our analysis. In addition, we were unable to include deaths that may have occurred shortly after discharge, even though these may have been related to the quality of hospital care. Furthermore, although the 3 conditions we studied are common and responsible for a large number of hospital deaths, they make up about 8% of total annual hospital discharges,23 and caution should be exercised in generalizing our findings to the full spectrum of hospitalizations. Lastly, it is possible that unmeasured confounding could be responsible for the observed associations. Uninsured and Medicaid patients are likely to have more severe disease, which may not be adequately captured by the administrative data available in the NIS. If so, this would explain the mortality association rather than insurance status.36, 37
Conclusions
Significant insurance‐related differences in mortality exist for 2 of the leading causes of noncancer inpatient deaths among working‐age Americans. Further studies are needed to determine whether provider sensitivity to insurance status or unmeasured sociodemographic and clinical prognostic factors are responsible for these disparities. Policy makers, hospital administrators, and physicians should be cognizant of these disparities and consider policies to address potential insurance related gaps in the quality of inpatient care.
- , .The U.S. economy and changes in health insurance coverage, 2000‐2006.Health Aff (Millwood).2008;27(2):w135‐w144.
- , , .Rates of avoidable hospitalization by insurance status in Massachusetts and Maryland.JAMA.1992;268(17):2388‐2394.
- , , , et al.Unmet health needs of uninsured adults in the United States.JAMA.2000;284(16):2061‐2069.
- , , .Health insurance and access to care for symptomatic conditions.Arch Intern Med.2000;160(9):1269‐1274.
- , , , et al.Access to specialty care and medical services in community health centers.Health Aff (Millwood).2007;26(5):1459‐1468.
- , , , et al.A national study of chronic disease prevalence and access to care in uninsured U.S. adults.Ann Intern Med.2008;149:170–176.
- , , , .Relationship between patient source of payment and the intensity of hospital services.Med Care.1988;26(11):1111‐1114.
- , , .The association of payer with utilization of cardiac procedures in Massachusetts.JAMA.1990;264(10):1255‐1260.
- , , .Comparison of uninsured and privately insured hospital patients: condition on admission, resource use, and outcome.JAMA.1991;265:374–379.
- , , .Socioeconomic status and risk for substandard medical care.JAMA.1992;268(17):2383‐2387.
- , , , .The relation between health insurance coverage and clinical outcomes among women with breast cancer.N Engl J Med.1993;329(5):326‐331.
- , , .Health insurance and mortality. Evidence from a national cohort.JAMA.1993;270(6):737‐741.
- , , , .Mortality in the uninsured compared with that in persons with public and private health insurance.Arch Intern Med.1994;154(21):2409‐2416.
- .Medicaid policy and the substitution of hospital outpatient care for physician care.Health Serv Res.1989;24:33–66.
- , .Disparities in outcomes among patients with stroke associated with insurance status.Stroke.2007;38(3):1010‐1016.
- , , , et al.Influence of payor on use of invasive cardiac procedures and patient outcome after myocardial infarction in the United States. Participants in the National Registry of Myocardial Infarction.J Am Coll Cardiol.1998;31(7):1474‐1480.
- , , , et al.Payer status and the utilization of hospital resources in acute myocardial infarction: a report from the National Registry of Myocardial Infarction 2.Arch Intern Med.2000;160(6):817–823.
- , , , et al.Insurance coverage and care of patients with non‐ST‐segment elevation acute coronary syndromes.Ann Intern Med.2006;145(10):739–748.
- , , .Comparing uninsured and privately insured hospital patients: Admission severity, health outcomes and resource use.Health Serv Manage Res.2001;14(3):203–210.
- Healthcare Cost and Utilization Project. Introduction to the HCUP Nationwide Inpatient Sample (NIS) 2005. Rockville, MD: Agency for Healthcare Research and Quality; 2007:6. Available at: www.hcup‐us.ahrq.gov/db/nation/nis/NIS_Introduction_2005.pdf. Accessed February2010.
- Healthcare Cost and Utilization Project. Design of the Nationwide Inpatient Sample (NIS) 2005. Rockville, MD: Agency for Healthcare Research and Quality; 2007. Available at: www.hcup‐us.ahrq.gov/db/nation/nis/reports/NIS_2005_Design_Report.pdf. Accessed February2010.
- AHRQ Quality Indicators. Inpatient Quality Indicators: Technical Specifications. Version 3.1 (March 12, 2007). Available at: www.qualityindicators.ahrq.gov/downloads/iqi/iqi_technical_specs_v31.pdf. Accessed February2010.
- , , . National Hospital Discharge Survey: 2005 annual summary with detailed diagnosis and procedure data. Washington, DC: National Center for Health Statistics; 2007. Vital and Health Statistics 13(165). Available at: www.cdc.gov/nchs/data/series/sr_13/sr13_165.pdf. Accessed February2010.
- AHRQ Quality Indicators. Guide to Inpatient Quality Indicators: Quality of Care in Hospitals—Volume, Mortality, and Utilization. Version 3.1 (March 12, 2007). Available at: www.qualityindicators.ahrq.gov/downloads/iqi/iqi_guide_v31.pdf. Accessed February2010.
- , , .Income, poverty, and health insurance coverage in the United States: 2006.Washington, DC:US Census Bureau. Current Population Reports;2007:60–233.
- , . Conditions Related to Uninsured Hospitalizations, 2003. HCUP Statistical Brief #8. Rockville, MD: Agency for Healthcare Research and Quality; 2006:6. Available at: www.hcup‐us.ahrq.gov/reports/statbriefs/sb8.pdf. Accessed February2010.
- Healthcare Cost and Utilization Project. NIS Description of Data Elements. Available at: www.hcup‐us.ahrq.gov/db/nation/nis/nisdde.jsp. Accessed February2010.
- , , , .Comorbidity measures for use with administrative data.Med Care.1998;36:8–27.
- SUDAAN User's Manual, Release 9.0. Research TrianglePark, NC:Research Triangle Institute;2006.
- , . Final Report on Calculating Nationwide Inpatient Sample (NIS) Variances, 2001. HCUP Methods Series Report #2003‐2. Online June 2005 (revised June 6, 2005). U.S. Agency for Healthcare Research and Quality. Available at: www.hcup‐us.ahrq.gov/reports/CalculatingNISVariances200106092005.pdf. Accessed February2010.
- .On the variances of asymptotically normal estimators from complex surveys.Int Stat Rev.1983;51:279–292.
- , , , et al.Patient characteristics associated with care by a cardiologist among adults hospitalized with severe congestive heart failure.J Am Coll Cardiol.2000;36:2119–2125.
- , . Summary health statistics for U.S. adults: National Health Interview Survey, 2006. Washington, DC: National Center for Health Statistics; 2007:12. Vital and Health Statistics 10(235). Available at: www.cdc.gov/nchs/data/series/sr_10/sr10_235.pdf. Accessed February2010.
- .Insurance coverage, medical care use, and short‐term health changes following an unintentional injury or the onset of a chronic condition.JAMA.2007;297(10):1073–1084.
- , , , et al.The quality of chronic disease care in U.S. community health centers.Health Aff (Millwood).2006;25(6):1712–1723.
- , , , , , .Risk‐adjusting hospital inpatient mortality using automated inpatient, outpatient, and laboratory databases.Med Care.2008;46(3):232–239.
- , , , .The Kaiser Permanente inpatient risk adjustment methodology was valid in an external patient population.J Clin Epidemiol. [E‐pub ahead of print].
With about 1 in 5 working‐age Americans (age 18‐64 years) currently uninsured and a large number relying on Medicaid, adequate access to quality health care services is becoming increasingly difficult.1 Substantial literature has accumulated over the years suggesting that access and quality in health care are closely linked to an individual's health insurance status.211 Some studies indicate that being uninsured or publicly insured is associated with negative health consequences.12, 13 Although the Medicaid program has improved access for qualifying low‐income individuals, significant gaps in access and quality remain.2, 5, 11, 1419 These issues are likely to become more pervasive should there be further modifications to state Medicaid funding in response to the unfolding economic crisis.
Although numerous studies have focused on insurance‐related disparities in the outpatient setting, few nationally representative studies have examined such disparities among hospitalized patients. A cross‐sectional study of a large hospital database from 1987 reported higher risk‐adjusted in‐hospital mortality, shorter length of stay (LOS), and lower procedure use among uninsured patients.9 A more recent analysis, limited to patients admitted with stroke, reported significant variation in hospital care associated with insurance status.15 Other studies reporting myocardial infarction registry and quality improvement program data are biased by the self‐selection of large urban teaching hospitals.1618 To our knowledge, no nationally representative study has focused on the impact of insurance coverage on hospital care for common medical conditions among working‐age Americans, the fastest growing segment of the uninsured population.
To address this gap in knowledge, we analyzed a nationally representative hospital database to determine whether there are significant insurance‐related disparities in in‐hospital mortality, LOS, and cost per hospitalization for 3 common medical conditions among working‐age adults, and, if present, to determine whether these disparities are due to differences in disease severity and comorbidities, and whether these disparities are affected by the proportion of uninsured and Medicaid patients receiving care in each hospital.
Methods
Design and Subjects
We examined data from the 2005 Nationwide Inpatient Sample (NIS), a nationally representative database of hospital inpatient stays maintained by the Agency for Healthcare Research and Quality (AHRQ) as part of the Healthcare Cost and Utilization Project (HCUP).20, 21 The NIS is a stratified probability sample of 20% of all US community hospitals, including public hospitals, academic medical centers, and specialty hospitals. Long‐term care hospitals, psychiatric hospitals, and alcoholism/chemical‐dependency treatment facilities are excluded. The 2005 NIS contains data on 7,995,048 discharges from 1054 hospitals located in 37 States and is designed to be representative of all acute care discharges from all US community hospitals.21
We identified discharges with a principal diagnosis of acute myocardial infarction (AMI), stroke, and pneumonia using International Classification of Diseases, 9th Revision, Clinical Modification (ICD‐9‐CM) codes specified in the AHRQ definitions of Inpatient Quality Indicators (Supporting Information Appendix).22 These 3 conditions are among the leading causes of noncancer inpatient deaths in patients under 65 years old,23 and evidence suggests that high mortality may be associated with deficiencies in the quality of inpatient care.24
We confined our analysis to patients 18 to 64 years of age, since this population is most at risk of being uninsured or underinsured.25 We excluded pregnant women because they account for an unusually high proportion of uninsured discharges and were relatively few in our cohort.26 In addition, we excluded patients transferred to another acute care hospital and patients missing payer source and discharge disposition. Our study protocol was approved by the Partners Human Research Committee.
Study Variables
We categorized insurance status as privately insured, uninsured, Medicaid, or Medicare. We defined privately insured patients as those having either Blue Cross or another commercial carrier listed as the primary payer and uninsured patients as those having either no charge or self‐pay listed as the primary payer.27 Other governmental payer categories were noted to share several characteristics with Medicare patients and comprised only a small proportion of the sample, and were thus included with Medicare. In order to account for NIS's sampling scheme and accurately apply sample weights in our analysis, we used Medicare as a separate category. However, since Medicare patients age 18 to 64 years represent a fundamentally different population that is primarily disabled or very ill, only results of privately insured, uninsured, and Medicaid patients are reported.
We selected in‐hospital mortality as the outcome measure and LOS and cost per hospitalization as measures of resource utilization. The NIS includes a binary indicator variable for in‐hospital mortality and specifies inpatient LOS in integers, with same‐day stays coded as 0. NIS's cost estimates are based on hospital cost reports submitted to the Centers for Medicare and Medicaid Services. To test the validity of our cost analyses, we performed parallel analyses using hospital charges as a measure of utilization (charges include hospital overhead, charity care, and bad debt). The resulting adjusted ratios differed little from cost ratios and we opted to report only the details of our cost analyses.
In order to assess the independent association between insurance status and the outcome measures listed above, we selected covariates for inclusion in multivariable models based on the existing literature. Patient covariates included: age group (18‐34 years, 35‐49 years, 50‐64 years), sex (male/female), race/ethnicity (non‐Hispanic white, non‐Hispanic black, Hispanic, other, missing), median income by zip code of residence (less than $37,000, $37,000‐$45,999, $46,000‐$60,999, $61,000 or more), admission through the emergency department (yes/no), admission on a weekend (yes/no), measures of disease severity, and comorbidity indicators. Measures of disease severity specific to each outcome are assigned in the NIS using criteria developed by Medstat (Medstat Disease Staging Software Version 5.2, Thomson Medstat Inc., Ann Arbor, MI). Severity is categorized into 5 levels, with a higher level indicating greater risk. We recorded comorbidities for each patient in our sample using HCUP Comorbidity Software, Version 3.2 (
Hospital covariates included: bed size (small, medium, large), ownership/control (private, government, private or government), geographic region (northeast, midwest, south, west), teaching status (teaching, non‐teaching), and the proportion of uninsured and Medicaid patients (combined) admitted to each hospital for AMI, stroke, or pneumonia. The actual number of hospital beds in each bed size category varies according to a hospital's geographic region and teaching status.27 Ownership/control, geographic region, and teaching status are assigned according to information from the American Hospital Association Annual Survey of Hospitals. The proportion of uninsured and Medicaid patients admitted to each hospital was found to have a nonmonotonic relationship with the outcomes being assessed and was thus treated as a 6‐level categorical variable with the following levels: 0% to 10%, 11% to 20%, 21% to 30%, 31% to 40%, 41% to 50%, and 51% to 100%.
Statistical Analysis
Summary statistics were constructed at the patient level and differences in proportions were evaluated with the chi‐square test. We employed direct standardization, using the age and sex distribution of the entire cohort, to compute age‐standardized and sex‐standardized estimates for each insurance group and compared them using the chi‐square test for in‐hospital mortality and t test for log transformed LOS and cost per hospitalization. For each condition, we developed multivariable logistic regression models for in‐hospital mortality and multivariable linear regression models for log transformed LOS and cost. The patient was the unit of analysis in all models.
In order to elucidate the contribution of disease severity and comorbidities and the proportion of uninsured and Medicaid patients admitted to each hospital, we fitted 3 sequential models for each outcome measure: Model 1 adjusted for patient sociodemographic characteristics and hospital characteristics with the exception of the covariate for the proportion of uninsured and Medicaid patients, Model 2 adjusted for all covariates in the preceding model as well as patients' comorbidities and severity of principal diagnosis, and Model 3 adjusted for all previously mentioned covariates as well as the proportion of uninsured and Medicaid patients admitted to each hospital. We excluded patients who died during hospitalization from the models for LOS and cost. We exponentiated the effect estimates from the log transformed linear regression models so that the adjusted ratio represents the percentage change in the mean LOS or mean cost.
To determine whether the association between patients' insurance status and in‐hospital mortality was modified by seeking care in hospitals treating a smaller or larger proportion of uninsured and Medicaid patients, we entered an interaction term for insurance status and proportion of uninsured and Medicaid patients in the final models (Model 3) for our primary outcome of in‐hospital mortality. However, since no significant interaction was found for any of the 3 conditions, this term was removed from the models and results from the interaction models are not described. In order to assess model specification for the linear regression models, we evaluated the normality of model residuals and found that these were approximately normally distributed. Lastly, we attempted to test the robustness of our analyses by creating fixed effects models that controlled for hospital site but were unable to do so due to the computational limitations of available software packages that could not render fixed effects models with more than 1000 hospital sites.
For all variables except race/ethnicity, data were missing for less than 3% of patients, so we excluded these individuals from adjusted analyses. However, race/ethnicity data were missing for 29% of the sample and were analyzed in 3 different ways, namely, with the missing data treated as a separate covariate level, with the missing data removed from the analysis, and with the missing data assigned to the majority covariate level (white race). The results of our analysis were unchanged no matter how the missing values were assigned. As a result, missing values for race/ethnicity were treated as a separate covariate level in the final analysis.15 Sociodemographic characteristics of patients with missing race/ethnicity information were similar to those with complete data.
We used SUDAAN (Release 9.0.1, Research Triangle Institute, NC) to account for NIS's sampling scheme and generalized estimating equations to adjust for the clustering of patients within hospitals and hospitals within sampling strata.29 In order to account for NIS's stratified probability sampling scheme, SUDAAN uses Taylor series linearization for robust variance estimation of descriptive statistics and regression parameters.30, 31 We present 2‐tailed P values or 95% confidence intervals (CIs) for all statistical comparisons.
Results
Patient and Hospital Characteristics
The final cohort comprised of 154,381 patients discharged from 1018 hospitals in 37 states during calendar year 2005 (Table 1). This cohort was representative of 755,346 working‐age Americans, representing approximately 225,947 cases of AMI (29.9%), 151,812 cases of stroke (20.1%), and 377,588 cases of pneumonia (50.0%). Of these patients, 47.5% were privately insured, 12.0% were uninsured, 17.0% received Medicaid, and 23.5% were assigned to Medicare. Compared with the privately insured, uninsured and Medicaid patients were generally younger, less likely to be white, more likely to have lower income, and more likely to be admitted through the emergency department. Of the 1018 hospitals included in our study, close to half (44.3%) were small, with bedsize ranging from 24 to 249. A large number of hospitals were located in the South (39.9%), and 14.9% were designated teaching hospitals.
| Characteristic* | Privately insured (n = 73,256) | Uninsured (n = 18,531) | Medicaid (n = 26,222) |
|---|---|---|---|
| |||
| Principal diagnosis (%) | |||
| Acute myocardial infarction | 36.7 | 31.2 | 19.7 |
| Stroke | 20.6 | 23.7 | 19.9 |
| Pneumonia | 42.7 | 45.2 | 60.4 |
| Age group (%) | |||
| 18‐34 years | 6.8 | 13.0 | 13.7 |
| 35‐49 years | 27.6 | 36.9 | 33.2 |
| 50‐64 years | 65.7 | 50.1 | 53.2 |
| Male sex (%) | 59.3 | 62.3 | 46.6 |
| Race or ethnicity (%) | |||
| White | 55.7 | 41.5 | 38.0 |
| Black | 7.6 | 14.8 | 16.6 |
| Hispanic | 4.8 | 10.5 | 10.4 |
| Other race | 3.6 | 4.7 | 5.2 |
| Missing | 28.4 | 29.0 | 29.7 |
| Median income by ZIP code (%) | |||
| <$37,000 | 21.5 | 36.7 | 43.0 |
| $37,000‐$45,999 | 25.2 | 27.8 | 27.1 |
| $46,000‐$60,999 | 26.3 | 20.3 | 17.6 |
| $61,000 | 24.8 | 11.5 | 8.4 |
| Emergency admission (%) | 63.3 | 75.6 | 72.9 |
| Weekend admission (%) | 24.5 | 26.2 | 25.1 |
| Hospital bed size (%) | |||
| Small | 8.9 | 10.3 | 11.4 |
| Medium | 24.0 | 22.3 | 25.9 |
| Large | 67.1 | 67.5 | 62.8 |
| Hospital control (%) | |||
| Private | 33.8 | 34.8 | 34.4 |
| Government (nonfederal) | 6.7 | 9.7 | 8.3 |
| Private or government | 59.5 | 55.5 | 57.3 |
| Hospital region (%) | |||
| Northeast | 17.4 | 12.5 | 17.6 |
| Midwest | 25.7 | 19.4 | 20.9 |
| South | 39.5 | 56.8 | 42.4 |
| West | 17.4 | 11.3 | 19.2 |
| Teaching hospital (%) | 41.7 | 43.8 | 43.3 |
Compared with privately insured patients, a larger proportion of uninsured and Medicaid patients had higher predicted mortality levels (Table 2). Medicaid patients had a disproportionately higher predicted LOS, whereas predicted resource demand was higher among privately insured patients. Hypertension (48%), chronic pulmonary disease (29.5%), and uncomplicated diabetes (21.5%) were the 3 most common comorbidities in the study cohort, with a generally higher prevalence of comorbidities among Medicaid patients.
| Characteristic* | Privately insured (n = 73,256) | Uninsured (n = 18,531) | Medicaid (n = 26,222) |
|---|---|---|---|
| |||
| Medstat disease staging (%) | |||
| Mortality level 1 | 50.8 | 45.4 | 36.7 |
| Mortality level 2 | 44.0 | 49.1 | 56.7 |
| Mortality level 3 | 5.3 | 5.5 | 6.7 |
| Length of stay level 1 | 66.8 | 71.6 | 53.8 |
| Length of stay level 2 | 28.5 | 24.5 | 39.3 |
| Length of stay level 3 | 4.8 | 3.8 | 6.9 |
| Resource demand level 1 | 45.2 | 54.2 | 48.5 |
| Resource demand level 2 | 40.5 | 34.2 | 39.2 |
| Resource demand level 3 | 14.2 | 11.7 | 12.3 |
| Coexisting medical conditions (%) | |||
| Congestive heart failure | 4.7 | 4.8 | 10.1 |
| Valvular disease | 2.8 | 2.0 | 2.7 |
| Pulmonary circulation disease | 0.8 | 0.6 | 1.5 |
| Peripheral vascular disease | 3.2 | 2.2 | 3.2 |
| Paralysis | 1.2 | 0.8 | 3.5 |
| Other neurological disorders | 2.4 | 1.9 | 7.3 |
| Chronic pulmonary disease | 23.6 | 22.4 | 37.7 |
| Uncomplicated diabetes | 19.6 | 18.6 | 23.4 |
| Complicated diabetes | 3.3 | 2.1 | 4.9 |
| Hypothyroidism | 5.6 | 2.7 | 4.7 |
| Renal failure | 3.0 | 1.9 | 5.6 |
| Liver disease | 1.6 | 2.5 | 4.4 |
| Peptic ulcer disease | <0.5 | <0.5 | <0.5 |
| AIDS | 0.1 | 0.1 | 0.4 |
| Lymphoma | 1.1 | 0.4 | 0.6 |
| Metastatic cancer | 2.1 | 0.7 | 2.2 |
| Non‐metastatic solid tumor | 1.5 | 0.8 | 2.1 |
| Collagen vascular diseases | 2.3 | 0.9 | 2.3 |
| Coagulopathy | 2.7 | 2.4 | 3.4 |
| Obesity | 10.3 | 8.2 | 9.3 |
| Weight loss | 1.6 | 1.8 | 3.3 |
| Fluid and electrolyte disorders | 18.3 | 19.4 | 23.8 |
| Chronic blood loss anemia | 0.6 | 0.6 | 0.8 |
| Deficiency anemias | 8.6 | 8.5 | 13.4 |
| Alcohol abuse | 3.3 | 9.8 | 8.3 |
| Drug abuse | 1.9 | 10.2 | 9.8 |
| Psychoses | 1.5 | 1.9 | 6.8 |
| Depression | 7.2 | 4.8 | 9.9 |
| Hypertension | 48.0 | 44.1 | 45.7 |
In‐Hospital Mortality
Compared with the privately insured, age‐standardized and sex‐standardized in‐hospital mortality for AMI and stroke was significantly higher for uninsured and Medicaid patients (Table 3). Among pneumonia patients, Medicaid recipients had significantly higher in‐hospital mortality compared with privately insured and uninsured patients.
| Privately Insured | Uninsured | Medicaid | |
|---|---|---|---|
| |||
| In‐hospital mortality, rate per 100 discharges (SE) | |||
| Acute myocardial infarction | 2.22 (0.10) | 4.03 (0.31)* | 4.57 (0.34)* |
| Stroke | 7.49 (0.27) | 10.46 (0.64)* | 9.89 (0.45)* |
| Pneumonia | 1.75 (0.09) | 1.74 (0.18) | 2.48 (0.14)* |
| Length of stay, mean (SE), days | |||
| Acute myocardial infarction | 4.17 (0.06) | 4.46 (0.09) | 5.85 (0.16) |
| Stroke | 6.37 (0.13) | 7.15 (0.25) | 9.28 (0.30) |
| Pneumonia | 4.89 (0.05) | 4.64 (0.10) | 5.80 (0.08) |
| Cost per episode, mean (SE), dollars | |||
| Acute myocardial infarction | 21,077 (512) | 19,977 (833) | 22,452 (841) |
| Stroke | 16,022 (679) | 14,571 (1,036) | 18,462 (824) |
| Pneumonia | 8,223 (192) | 7,086 (293) | 9,479 (271) |
After multivariable adjustment for additional patient and hospital characteristics, uninsured AMI and stroke patients continued to have significantly higher in‐hospital mortality compared with the privately insured (Table 4). Among pneumonia patients, Medicaid recipients persisted in having significantly higher in‐hospital mortality than the privately insured.
| Model 1* | Model 2 | Model 3 | |
|---|---|---|---|
| |||
| In‐hospital mortality, adjusted odds ratio (95% CI) | |||
| Acute Myocardial Infarction | |||
| Uninsured vs. privately insured | 1.59 (1.35‐1.88) | 1.58 (1.30‐1.93) | 1.52 (1.24‐1.85) |
| Medicaid vs. privately insured | 1.83 (1.54‐2.18) | 1.22 (0.99‐1.50) | 1.15 (0.94‐1.42) |
| Stroke | |||
| Uninsured vs. privately insured | 1.56 (1.35‐1.80) | 1.50 (1.30‐1.73) | 1.49 (1.29‐1.72) |
| Medicaid vs. privately insured | 1.32 (1.15‐1.52) | 1.09 (0.93‐1.27) | 1.08 (0.93‐1.26) |
| Pneumonia | |||
| Uninsured vs. privately insured | 0.99 (0.81‐1.21) | 1.12 (0.91‐1.39) | 1.10 (0.89‐1.36) |
| Medicaid vs. privately insured | 1.41 (1.20‐1.65) | 1.24 (1.04‐1.48) | 1.21 (1.01‐1.45) |
| Length of stay, adjusted ratio (95% CI)| | |||
| Acute Myocardial Infarction | |||
| Uninsured vs. privately insured | 1.00 (0.98‐1.02) | 1.00 (0.98‐1.02) | 1.00 (0.98‐1.02) |
| Medicaid vs. privately insured | 1.17 (1.14‐1.21) | 1.07 (1.05‐1.09) | 1.07 (1.05‐1.09) |
| Stroke | |||
| Uninsured vs. privately insured | 1.06 (1.02‐1.10) | 1.08 (1.04‐1.11) | 1.07 (1.04‐1.11) |
| Medicaid vs. privately insured | 1.30 (1.26‐1.34) | 1.17 (1.14‐1.20) | 1.17 (1.14‐1.20) |
| Pneumonia | |||
| Uninsured vs. privately insured | 0.95 (0.93‐0.97) | 0.96 (0.94‐0.99) | 0.96 (0.94‐0.98) |
| Medicaid vs. privately insured | 1.15 (1.13‐1.17) | 1.04 (1.03‐1.06) | 1.04 (1.03‐1.06) |
| Cost per episode, adjusted ratio (95% CI)| | |||
| Acute Myocardial Infarction | |||
| Uninsured vs. privately insured | 0.97 (0.95‐0.99) | 0.99 (0.97‐1.00) | 0.99 (0.97‐1.00) |
| Medicaid vs. privately insured | 1.01 (0.98‐1.04) | 0.99 (0.97‐1.01) | 0.99 (0.97‐1.01) |
| Stroke | |||
| Uninsured vs. privately insured | 0.97 (0.93‐1.02) | 1.00 (0.96‐1.03) | 1.00 (0.97‐1.03) |
| Medicaid vs. privately insured | 1.17 (1.13‐1.21) | 1.06 (1.04‐1.09) | 1.06 (1.04‐1.09) |
| Pneumonia | |||
| Uninsured vs. privately insured | 0.95 (0.92‐0.97) | 0.98 (0.96‐1.00) | 0.98 (0.96‐1.00) |
| Medicaid vs. privately insured | 1.17 (1.15‐1.19) | 1.05 (1.04‐1.07) | 1.05 (1.04‐1.07) |
LOS
Among AMI and stroke patients, age‐standardized and sex‐standardized mean LOS was significantly longer for the uninsured and Medicaid recipients compared with the privately insured (Table 3). Among pneumonia patients, the uninsured had a slightly shorter mean LOS compared with the privately insured whereas Medicaid recipients averaged the longest LOS.
These insurance‐related disparities in LOS among pneumonia patients persisted after multivariable adjustment (Table 4). Among AMI patients, only Medicaid recipients persisted in having a significantly longer LOS than the privately insured. Among stroke patients, both the uninsured and Medicaid recipients averaged a longer LOS compared with the privately insured.
Cost per Episode
For all 3 conditions, the uninsured had significantly lower age‐standardized and sex‐standardized costs compared with the privately insured (Table 3). However, Medicaid patients had higher costs than the privately insured for all three conditions, significantly so among patients with stroke and pneumonia.
These insurance‐related disparities in costs persisted in multivariable analyses (Table 4). The uninsured continued to have lower costs compared with the privately insured, significantly so for patients with AMI and pneumonia. Among stroke and pneumonia patients, Medicaid recipients continued to accrue higher costs than the uninsured or privately insured.
Discussion
In this nationally representative study of working‐age Americans hospitalized for 3 common medical conditions, we found that insurance status was associated with significant variations in in‐hospital mortality and resource use. Whereas privately insured patients experienced comparatively lower in‐hospital mortality in most cases, mortality risk was highest among the uninsured for 2 of the 3 common causes of noncancer inpatient deaths. Although previous studies have examined insurance‐related disparities in inpatient care for individual diagnoses and specific populations, no broad overview of this important issue has been published in the past decade. In light of the current economic recession and national healthcare debate, these findings may be a prescient indication of a widening insurance gap in the quality of hospital care.
There are several potential mechanisms for these disparities. For instance, Hadley et al.9 reported significant underuse of high‐cost or high‐discretion procedures among the uninsured in their analysis of a nationally representative sample of 592,598 hospitalized patients. Similarly, Burstin et al.10 found that among a population of 30,195 hospitalized patients with diverse diagnoses, the uninsured were at greater risk for receiving substandard care regardless of hospital characteristics. These, and other similar findings,7, 8, 19 are suggestive of differences in the way uninsured patients are generally managed in the hospital that may partly explain the disparities reported herein.
More specifically, analyses of national registries of AMI have documented lower rates of utilization of invasive, potentially life‐saving, cardiac interventions among the uninsured.16, 17 Similarly, a lower rate of carotid endarterectomy was reported among uninsured stroke patients from an analysis of the 2002 NIS.15 Other differences in inpatient management unmeasured by administrative data, such as the use of subspecialists and allied health professionals, may also contribute.32 Unfortunately, limitations in the available data prevented us from being able to appropriately address the important issue of insurance related differences in the utilization of specific inpatient procedures.
These disparities may also be indicative of differences in severity of illness that are not captured fully by the MedStat disease staging criteria. The uninsured might have more severe illness at admission, either due to the presence of more advanced chronic disease or delay in seeking care for the acute episode. AMI and stroke are usually the culmination of longstanding atherosclerosis that is amenable to improvement through timely and consistent risk‐factor modification. Not having a usual source of medical care,6, 33 inadequate screening and management of known risk‐factors,3, 34 and difficulties in obtaining specialty care5 among the uninsured likely increases their risk of being hospitalized with more advanced disease. The higher likelihood of being admitted through the emergency department19 and on weekends9 among the uninsured lends credence to the possibility of delays in seeking care. All of these are potential mediators of higher AMI and stroke mortality in uninsured patients.
Finally, these mortality differences could also be due to the additional risks imposed by poorly managed comorbidities among uninsured patients. Although we controlled for the presence of comorbidities in our analysis, we lacked data about the severity of individual comorbidities. A recent study reported significant lapses in follow‐up care after the onset of a chronic condition in uninsured individuals under 65 years of age.34 Other studies have also documented insurance related disparities in the care of chronic diseases3, 35 that were among the most common comorbidities in our cohort.
Most of the reasons for insurance‐related disparities noted above for the uninsured are also applicable to Medicaid patients. Differences in the intensity of inpatient care,7, 8, 1519 limited access to health care services,2, 14 unmet health needs,5 and suboptimal management of chronic medical conditions35 were also reported for Medicaid patients in prior research. These factors likely contributed to the higher in‐hospital mortality in this patient population, evidenced by the sequential decrease in odds after adjusting for comorbidities and disease severity. Medicaid patients hospitalized for stroke were noted to have significantly longer LOS, which could plausibly be due to difficulties with arranging appropriate discharge disposition; the higher likelihood of paralysis among these patients15 would likely necessitate a higher frequency of rehabilitation facility placement. The higher costs for Medicaid patients with stroke and pneumonia may potentially be the result of these patients longer LOS. Although cost differences between the uninsured and privately insured were statistically significant, these were not large enough to be of material significance.
Limitations
Our study has several limitations. Since the NIS does not assign unique patient identifiers that would permit tracking of readmissions, we excluded patients transferred to another acute‐care hospital from our study to avoid counting the same patient twice. However, only 10% of hospitalized patients underwent transfer for cardiac procedures in the National Registry of Myocardial Infarction, with privately insured patients more likely to be transferred than other insurance groups.17 Since these patients are also more likely to have better survival, their exclusion likely biased our study toward the null. The same is probable for stroke patients as well.
Some uninsured patients begin Medicaid coverage during hospitalization and should ideally be counted as uninsured but were included under Medicaid in our analysis. They are also likely to be state‐ and plan‐specific variations in Medicaid and private payer coverage that we could not incorporate into our analysis. In addition, we were unable to include deaths that may have occurred shortly after discharge, even though these may have been related to the quality of hospital care. Furthermore, although the 3 conditions we studied are common and responsible for a large number of hospital deaths, they make up about 8% of total annual hospital discharges,23 and caution should be exercised in generalizing our findings to the full spectrum of hospitalizations. Lastly, it is possible that unmeasured confounding could be responsible for the observed associations. Uninsured and Medicaid patients are likely to have more severe disease, which may not be adequately captured by the administrative data available in the NIS. If so, this would explain the mortality association rather than insurance status.36, 37
Conclusions
Significant insurance‐related differences in mortality exist for 2 of the leading causes of noncancer inpatient deaths among working‐age Americans. Further studies are needed to determine whether provider sensitivity to insurance status or unmeasured sociodemographic and clinical prognostic factors are responsible for these disparities. Policy makers, hospital administrators, and physicians should be cognizant of these disparities and consider policies to address potential insurance related gaps in the quality of inpatient care.
With about 1 in 5 working‐age Americans (age 18‐64 years) currently uninsured and a large number relying on Medicaid, adequate access to quality health care services is becoming increasingly difficult.1 Substantial literature has accumulated over the years suggesting that access and quality in health care are closely linked to an individual's health insurance status.211 Some studies indicate that being uninsured or publicly insured is associated with negative health consequences.12, 13 Although the Medicaid program has improved access for qualifying low‐income individuals, significant gaps in access and quality remain.2, 5, 11, 1419 These issues are likely to become more pervasive should there be further modifications to state Medicaid funding in response to the unfolding economic crisis.
Although numerous studies have focused on insurance‐related disparities in the outpatient setting, few nationally representative studies have examined such disparities among hospitalized patients. A cross‐sectional study of a large hospital database from 1987 reported higher risk‐adjusted in‐hospital mortality, shorter length of stay (LOS), and lower procedure use among uninsured patients.9 A more recent analysis, limited to patients admitted with stroke, reported significant variation in hospital care associated with insurance status.15 Other studies reporting myocardial infarction registry and quality improvement program data are biased by the self‐selection of large urban teaching hospitals.1618 To our knowledge, no nationally representative study has focused on the impact of insurance coverage on hospital care for common medical conditions among working‐age Americans, the fastest growing segment of the uninsured population.
To address this gap in knowledge, we analyzed a nationally representative hospital database to determine whether there are significant insurance‐related disparities in in‐hospital mortality, LOS, and cost per hospitalization for 3 common medical conditions among working‐age adults, and, if present, to determine whether these disparities are due to differences in disease severity and comorbidities, and whether these disparities are affected by the proportion of uninsured and Medicaid patients receiving care in each hospital.
Methods
Design and Subjects
We examined data from the 2005 Nationwide Inpatient Sample (NIS), a nationally representative database of hospital inpatient stays maintained by the Agency for Healthcare Research and Quality (AHRQ) as part of the Healthcare Cost and Utilization Project (HCUP).20, 21 The NIS is a stratified probability sample of 20% of all US community hospitals, including public hospitals, academic medical centers, and specialty hospitals. Long‐term care hospitals, psychiatric hospitals, and alcoholism/chemical‐dependency treatment facilities are excluded. The 2005 NIS contains data on 7,995,048 discharges from 1054 hospitals located in 37 States and is designed to be representative of all acute care discharges from all US community hospitals.21
We identified discharges with a principal diagnosis of acute myocardial infarction (AMI), stroke, and pneumonia using International Classification of Diseases, 9th Revision, Clinical Modification (ICD‐9‐CM) codes specified in the AHRQ definitions of Inpatient Quality Indicators (Supporting Information Appendix).22 These 3 conditions are among the leading causes of noncancer inpatient deaths in patients under 65 years old,23 and evidence suggests that high mortality may be associated with deficiencies in the quality of inpatient care.24
We confined our analysis to patients 18 to 64 years of age, since this population is most at risk of being uninsured or underinsured.25 We excluded pregnant women because they account for an unusually high proportion of uninsured discharges and were relatively few in our cohort.26 In addition, we excluded patients transferred to another acute care hospital and patients missing payer source and discharge disposition. Our study protocol was approved by the Partners Human Research Committee.
Study Variables
We categorized insurance status as privately insured, uninsured, Medicaid, or Medicare. We defined privately insured patients as those having either Blue Cross or another commercial carrier listed as the primary payer and uninsured patients as those having either no charge or self‐pay listed as the primary payer.27 Other governmental payer categories were noted to share several characteristics with Medicare patients and comprised only a small proportion of the sample, and were thus included with Medicare. In order to account for NIS's sampling scheme and accurately apply sample weights in our analysis, we used Medicare as a separate category. However, since Medicare patients age 18 to 64 years represent a fundamentally different population that is primarily disabled or very ill, only results of privately insured, uninsured, and Medicaid patients are reported.
We selected in‐hospital mortality as the outcome measure and LOS and cost per hospitalization as measures of resource utilization. The NIS includes a binary indicator variable for in‐hospital mortality and specifies inpatient LOS in integers, with same‐day stays coded as 0. NIS's cost estimates are based on hospital cost reports submitted to the Centers for Medicare and Medicaid Services. To test the validity of our cost analyses, we performed parallel analyses using hospital charges as a measure of utilization (charges include hospital overhead, charity care, and bad debt). The resulting adjusted ratios differed little from cost ratios and we opted to report only the details of our cost analyses.
In order to assess the independent association between insurance status and the outcome measures listed above, we selected covariates for inclusion in multivariable models based on the existing literature. Patient covariates included: age group (18‐34 years, 35‐49 years, 50‐64 years), sex (male/female), race/ethnicity (non‐Hispanic white, non‐Hispanic black, Hispanic, other, missing), median income by zip code of residence (less than $37,000, $37,000‐$45,999, $46,000‐$60,999, $61,000 or more), admission through the emergency department (yes/no), admission on a weekend (yes/no), measures of disease severity, and comorbidity indicators. Measures of disease severity specific to each outcome are assigned in the NIS using criteria developed by Medstat (Medstat Disease Staging Software Version 5.2, Thomson Medstat Inc., Ann Arbor, MI). Severity is categorized into 5 levels, with a higher level indicating greater risk. We recorded comorbidities for each patient in our sample using HCUP Comorbidity Software, Version 3.2 (
Hospital covariates included: bed size (small, medium, large), ownership/control (private, government, private or government), geographic region (northeast, midwest, south, west), teaching status (teaching, non‐teaching), and the proportion of uninsured and Medicaid patients (combined) admitted to each hospital for AMI, stroke, or pneumonia. The actual number of hospital beds in each bed size category varies according to a hospital's geographic region and teaching status.27 Ownership/control, geographic region, and teaching status are assigned according to information from the American Hospital Association Annual Survey of Hospitals. The proportion of uninsured and Medicaid patients admitted to each hospital was found to have a nonmonotonic relationship with the outcomes being assessed and was thus treated as a 6‐level categorical variable with the following levels: 0% to 10%, 11% to 20%, 21% to 30%, 31% to 40%, 41% to 50%, and 51% to 100%.
Statistical Analysis
Summary statistics were constructed at the patient level and differences in proportions were evaluated with the chi‐square test. We employed direct standardization, using the age and sex distribution of the entire cohort, to compute age‐standardized and sex‐standardized estimates for each insurance group and compared them using the chi‐square test for in‐hospital mortality and t test for log transformed LOS and cost per hospitalization. For each condition, we developed multivariable logistic regression models for in‐hospital mortality and multivariable linear regression models for log transformed LOS and cost. The patient was the unit of analysis in all models.
In order to elucidate the contribution of disease severity and comorbidities and the proportion of uninsured and Medicaid patients admitted to each hospital, we fitted 3 sequential models for each outcome measure: Model 1 adjusted for patient sociodemographic characteristics and hospital characteristics with the exception of the covariate for the proportion of uninsured and Medicaid patients, Model 2 adjusted for all covariates in the preceding model as well as patients' comorbidities and severity of principal diagnosis, and Model 3 adjusted for all previously mentioned covariates as well as the proportion of uninsured and Medicaid patients admitted to each hospital. We excluded patients who died during hospitalization from the models for LOS and cost. We exponentiated the effect estimates from the log transformed linear regression models so that the adjusted ratio represents the percentage change in the mean LOS or mean cost.
To determine whether the association between patients' insurance status and in‐hospital mortality was modified by seeking care in hospitals treating a smaller or larger proportion of uninsured and Medicaid patients, we entered an interaction term for insurance status and proportion of uninsured and Medicaid patients in the final models (Model 3) for our primary outcome of in‐hospital mortality. However, since no significant interaction was found for any of the 3 conditions, this term was removed from the models and results from the interaction models are not described. In order to assess model specification for the linear regression models, we evaluated the normality of model residuals and found that these were approximately normally distributed. Lastly, we attempted to test the robustness of our analyses by creating fixed effects models that controlled for hospital site but were unable to do so due to the computational limitations of available software packages that could not render fixed effects models with more than 1000 hospital sites.
For all variables except race/ethnicity, data were missing for less than 3% of patients, so we excluded these individuals from adjusted analyses. However, race/ethnicity data were missing for 29% of the sample and were analyzed in 3 different ways, namely, with the missing data treated as a separate covariate level, with the missing data removed from the analysis, and with the missing data assigned to the majority covariate level (white race). The results of our analysis were unchanged no matter how the missing values were assigned. As a result, missing values for race/ethnicity were treated as a separate covariate level in the final analysis.15 Sociodemographic characteristics of patients with missing race/ethnicity information were similar to those with complete data.
We used SUDAAN (Release 9.0.1, Research Triangle Institute, NC) to account for NIS's sampling scheme and generalized estimating equations to adjust for the clustering of patients within hospitals and hospitals within sampling strata.29 In order to account for NIS's stratified probability sampling scheme, SUDAAN uses Taylor series linearization for robust variance estimation of descriptive statistics and regression parameters.30, 31 We present 2‐tailed P values or 95% confidence intervals (CIs) for all statistical comparisons.
Results
Patient and Hospital Characteristics
The final cohort comprised of 154,381 patients discharged from 1018 hospitals in 37 states during calendar year 2005 (Table 1). This cohort was representative of 755,346 working‐age Americans, representing approximately 225,947 cases of AMI (29.9%), 151,812 cases of stroke (20.1%), and 377,588 cases of pneumonia (50.0%). Of these patients, 47.5% were privately insured, 12.0% were uninsured, 17.0% received Medicaid, and 23.5% were assigned to Medicare. Compared with the privately insured, uninsured and Medicaid patients were generally younger, less likely to be white, more likely to have lower income, and more likely to be admitted through the emergency department. Of the 1018 hospitals included in our study, close to half (44.3%) were small, with bedsize ranging from 24 to 249. A large number of hospitals were located in the South (39.9%), and 14.9% were designated teaching hospitals.
| Characteristic* | Privately insured (n = 73,256) | Uninsured (n = 18,531) | Medicaid (n = 26,222) |
|---|---|---|---|
| |||
| Principal diagnosis (%) | |||
| Acute myocardial infarction | 36.7 | 31.2 | 19.7 |
| Stroke | 20.6 | 23.7 | 19.9 |
| Pneumonia | 42.7 | 45.2 | 60.4 |
| Age group (%) | |||
| 18‐34 years | 6.8 | 13.0 | 13.7 |
| 35‐49 years | 27.6 | 36.9 | 33.2 |
| 50‐64 years | 65.7 | 50.1 | 53.2 |
| Male sex (%) | 59.3 | 62.3 | 46.6 |
| Race or ethnicity (%) | |||
| White | 55.7 | 41.5 | 38.0 |
| Black | 7.6 | 14.8 | 16.6 |
| Hispanic | 4.8 | 10.5 | 10.4 |
| Other race | 3.6 | 4.7 | 5.2 |
| Missing | 28.4 | 29.0 | 29.7 |
| Median income by ZIP code (%) | |||
| <$37,000 | 21.5 | 36.7 | 43.0 |
| $37,000‐$45,999 | 25.2 | 27.8 | 27.1 |
| $46,000‐$60,999 | 26.3 | 20.3 | 17.6 |
| $61,000 | 24.8 | 11.5 | 8.4 |
| Emergency admission (%) | 63.3 | 75.6 | 72.9 |
| Weekend admission (%) | 24.5 | 26.2 | 25.1 |
| Hospital bed size (%) | |||
| Small | 8.9 | 10.3 | 11.4 |
| Medium | 24.0 | 22.3 | 25.9 |
| Large | 67.1 | 67.5 | 62.8 |
| Hospital control (%) | |||
| Private | 33.8 | 34.8 | 34.4 |
| Government (nonfederal) | 6.7 | 9.7 | 8.3 |
| Private or government | 59.5 | 55.5 | 57.3 |
| Hospital region (%) | |||
| Northeast | 17.4 | 12.5 | 17.6 |
| Midwest | 25.7 | 19.4 | 20.9 |
| South | 39.5 | 56.8 | 42.4 |
| West | 17.4 | 11.3 | 19.2 |
| Teaching hospital (%) | 41.7 | 43.8 | 43.3 |
Compared with privately insured patients, a larger proportion of uninsured and Medicaid patients had higher predicted mortality levels (Table 2). Medicaid patients had a disproportionately higher predicted LOS, whereas predicted resource demand was higher among privately insured patients. Hypertension (48%), chronic pulmonary disease (29.5%), and uncomplicated diabetes (21.5%) were the 3 most common comorbidities in the study cohort, with a generally higher prevalence of comorbidities among Medicaid patients.
| Characteristic* | Privately insured (n = 73,256) | Uninsured (n = 18,531) | Medicaid (n = 26,222) |
|---|---|---|---|
| |||
| Medstat disease staging (%) | |||
| Mortality level 1 | 50.8 | 45.4 | 36.7 |
| Mortality level 2 | 44.0 | 49.1 | 56.7 |
| Mortality level 3 | 5.3 | 5.5 | 6.7 |
| Length of stay level 1 | 66.8 | 71.6 | 53.8 |
| Length of stay level 2 | 28.5 | 24.5 | 39.3 |
| Length of stay level 3 | 4.8 | 3.8 | 6.9 |
| Resource demand level 1 | 45.2 | 54.2 | 48.5 |
| Resource demand level 2 | 40.5 | 34.2 | 39.2 |
| Resource demand level 3 | 14.2 | 11.7 | 12.3 |
| Coexisting medical conditions (%) | |||
| Congestive heart failure | 4.7 | 4.8 | 10.1 |
| Valvular disease | 2.8 | 2.0 | 2.7 |
| Pulmonary circulation disease | 0.8 | 0.6 | 1.5 |
| Peripheral vascular disease | 3.2 | 2.2 | 3.2 |
| Paralysis | 1.2 | 0.8 | 3.5 |
| Other neurological disorders | 2.4 | 1.9 | 7.3 |
| Chronic pulmonary disease | 23.6 | 22.4 | 37.7 |
| Uncomplicated diabetes | 19.6 | 18.6 | 23.4 |
| Complicated diabetes | 3.3 | 2.1 | 4.9 |
| Hypothyroidism | 5.6 | 2.7 | 4.7 |
| Renal failure | 3.0 | 1.9 | 5.6 |
| Liver disease | 1.6 | 2.5 | 4.4 |
| Peptic ulcer disease | <0.5 | <0.5 | <0.5 |
| AIDS | 0.1 | 0.1 | 0.4 |
| Lymphoma | 1.1 | 0.4 | 0.6 |
| Metastatic cancer | 2.1 | 0.7 | 2.2 |
| Non‐metastatic solid tumor | 1.5 | 0.8 | 2.1 |
| Collagen vascular diseases | 2.3 | 0.9 | 2.3 |
| Coagulopathy | 2.7 | 2.4 | 3.4 |
| Obesity | 10.3 | 8.2 | 9.3 |
| Weight loss | 1.6 | 1.8 | 3.3 |
| Fluid and electrolyte disorders | 18.3 | 19.4 | 23.8 |
| Chronic blood loss anemia | 0.6 | 0.6 | 0.8 |
| Deficiency anemias | 8.6 | 8.5 | 13.4 |
| Alcohol abuse | 3.3 | 9.8 | 8.3 |
| Drug abuse | 1.9 | 10.2 | 9.8 |
| Psychoses | 1.5 | 1.9 | 6.8 |
| Depression | 7.2 | 4.8 | 9.9 |
| Hypertension | 48.0 | 44.1 | 45.7 |
In‐Hospital Mortality
Compared with the privately insured, age‐standardized and sex‐standardized in‐hospital mortality for AMI and stroke was significantly higher for uninsured and Medicaid patients (Table 3). Among pneumonia patients, Medicaid recipients had significantly higher in‐hospital mortality compared with privately insured and uninsured patients.
| Privately Insured | Uninsured | Medicaid | |
|---|---|---|---|
| |||
| In‐hospital mortality, rate per 100 discharges (SE) | |||
| Acute myocardial infarction | 2.22 (0.10) | 4.03 (0.31)* | 4.57 (0.34)* |
| Stroke | 7.49 (0.27) | 10.46 (0.64)* | 9.89 (0.45)* |
| Pneumonia | 1.75 (0.09) | 1.74 (0.18) | 2.48 (0.14)* |
| Length of stay, mean (SE), days | |||
| Acute myocardial infarction | 4.17 (0.06) | 4.46 (0.09) | 5.85 (0.16) |
| Stroke | 6.37 (0.13) | 7.15 (0.25) | 9.28 (0.30) |
| Pneumonia | 4.89 (0.05) | 4.64 (0.10) | 5.80 (0.08) |
| Cost per episode, mean (SE), dollars | |||
| Acute myocardial infarction | 21,077 (512) | 19,977 (833) | 22,452 (841) |
| Stroke | 16,022 (679) | 14,571 (1,036) | 18,462 (824) |
| Pneumonia | 8,223 (192) | 7,086 (293) | 9,479 (271) |
After multivariable adjustment for additional patient and hospital characteristics, uninsured AMI and stroke patients continued to have significantly higher in‐hospital mortality compared with the privately insured (Table 4). Among pneumonia patients, Medicaid recipients persisted in having significantly higher in‐hospital mortality than the privately insured.
| Model 1* | Model 2 | Model 3 | |
|---|---|---|---|
| |||
| In‐hospital mortality, adjusted odds ratio (95% CI) | |||
| Acute Myocardial Infarction | |||
| Uninsured vs. privately insured | 1.59 (1.35‐1.88) | 1.58 (1.30‐1.93) | 1.52 (1.24‐1.85) |
| Medicaid vs. privately insured | 1.83 (1.54‐2.18) | 1.22 (0.99‐1.50) | 1.15 (0.94‐1.42) |
| Stroke | |||
| Uninsured vs. privately insured | 1.56 (1.35‐1.80) | 1.50 (1.30‐1.73) | 1.49 (1.29‐1.72) |
| Medicaid vs. privately insured | 1.32 (1.15‐1.52) | 1.09 (0.93‐1.27) | 1.08 (0.93‐1.26) |
| Pneumonia | |||
| Uninsured vs. privately insured | 0.99 (0.81‐1.21) | 1.12 (0.91‐1.39) | 1.10 (0.89‐1.36) |
| Medicaid vs. privately insured | 1.41 (1.20‐1.65) | 1.24 (1.04‐1.48) | 1.21 (1.01‐1.45) |
| Length of stay, adjusted ratio (95% CI)| | |||
| Acute Myocardial Infarction | |||
| Uninsured vs. privately insured | 1.00 (0.98‐1.02) | 1.00 (0.98‐1.02) | 1.00 (0.98‐1.02) |
| Medicaid vs. privately insured | 1.17 (1.14‐1.21) | 1.07 (1.05‐1.09) | 1.07 (1.05‐1.09) |
| Stroke | |||
| Uninsured vs. privately insured | 1.06 (1.02‐1.10) | 1.08 (1.04‐1.11) | 1.07 (1.04‐1.11) |
| Medicaid vs. privately insured | 1.30 (1.26‐1.34) | 1.17 (1.14‐1.20) | 1.17 (1.14‐1.20) |
| Pneumonia | |||
| Uninsured vs. privately insured | 0.95 (0.93‐0.97) | 0.96 (0.94‐0.99) | 0.96 (0.94‐0.98) |
| Medicaid vs. privately insured | 1.15 (1.13‐1.17) | 1.04 (1.03‐1.06) | 1.04 (1.03‐1.06) |
| Cost per episode, adjusted ratio (95% CI)| | |||
| Acute Myocardial Infarction | |||
| Uninsured vs. privately insured | 0.97 (0.95‐0.99) | 0.99 (0.97‐1.00) | 0.99 (0.97‐1.00) |
| Medicaid vs. privately insured | 1.01 (0.98‐1.04) | 0.99 (0.97‐1.01) | 0.99 (0.97‐1.01) |
| Stroke | |||
| Uninsured vs. privately insured | 0.97 (0.93‐1.02) | 1.00 (0.96‐1.03) | 1.00 (0.97‐1.03) |
| Medicaid vs. privately insured | 1.17 (1.13‐1.21) | 1.06 (1.04‐1.09) | 1.06 (1.04‐1.09) |
| Pneumonia | |||
| Uninsured vs. privately insured | 0.95 (0.92‐0.97) | 0.98 (0.96‐1.00) | 0.98 (0.96‐1.00) |
| Medicaid vs. privately insured | 1.17 (1.15‐1.19) | 1.05 (1.04‐1.07) | 1.05 (1.04‐1.07) |
LOS
Among AMI and stroke patients, age‐standardized and sex‐standardized mean LOS was significantly longer for the uninsured and Medicaid recipients compared with the privately insured (Table 3). Among pneumonia patients, the uninsured had a slightly shorter mean LOS compared with the privately insured whereas Medicaid recipients averaged the longest LOS.
These insurance‐related disparities in LOS among pneumonia patients persisted after multivariable adjustment (Table 4). Among AMI patients, only Medicaid recipients persisted in having a significantly longer LOS than the privately insured. Among stroke patients, both the uninsured and Medicaid recipients averaged a longer LOS compared with the privately insured.
Cost per Episode
For all 3 conditions, the uninsured had significantly lower age‐standardized and sex‐standardized costs compared with the privately insured (Table 3). However, Medicaid patients had higher costs than the privately insured for all three conditions, significantly so among patients with stroke and pneumonia.
These insurance‐related disparities in costs persisted in multivariable analyses (Table 4). The uninsured continued to have lower costs compared with the privately insured, significantly so for patients with AMI and pneumonia. Among stroke and pneumonia patients, Medicaid recipients continued to accrue higher costs than the uninsured or privately insured.
Discussion
In this nationally representative study of working‐age Americans hospitalized for 3 common medical conditions, we found that insurance status was associated with significant variations in in‐hospital mortality and resource use. Whereas privately insured patients experienced comparatively lower in‐hospital mortality in most cases, mortality risk was highest among the uninsured for 2 of the 3 common causes of noncancer inpatient deaths. Although previous studies have examined insurance‐related disparities in inpatient care for individual diagnoses and specific populations, no broad overview of this important issue has been published in the past decade. In light of the current economic recession and national healthcare debate, these findings may be a prescient indication of a widening insurance gap in the quality of hospital care.
There are several potential mechanisms for these disparities. For instance, Hadley et al.9 reported significant underuse of high‐cost or high‐discretion procedures among the uninsured in their analysis of a nationally representative sample of 592,598 hospitalized patients. Similarly, Burstin et al.10 found that among a population of 30,195 hospitalized patients with diverse diagnoses, the uninsured were at greater risk for receiving substandard care regardless of hospital characteristics. These, and other similar findings,7, 8, 19 are suggestive of differences in the way uninsured patients are generally managed in the hospital that may partly explain the disparities reported herein.
More specifically, analyses of national registries of AMI have documented lower rates of utilization of invasive, potentially life‐saving, cardiac interventions among the uninsured.16, 17 Similarly, a lower rate of carotid endarterectomy was reported among uninsured stroke patients from an analysis of the 2002 NIS.15 Other differences in inpatient management unmeasured by administrative data, such as the use of subspecialists and allied health professionals, may also contribute.32 Unfortunately, limitations in the available data prevented us from being able to appropriately address the important issue of insurance related differences in the utilization of specific inpatient procedures.
These disparities may also be indicative of differences in severity of illness that are not captured fully by the MedStat disease staging criteria. The uninsured might have more severe illness at admission, either due to the presence of more advanced chronic disease or delay in seeking care for the acute episode. AMI and stroke are usually the culmination of longstanding atherosclerosis that is amenable to improvement through timely and consistent risk‐factor modification. Not having a usual source of medical care,6, 33 inadequate screening and management of known risk‐factors,3, 34 and difficulties in obtaining specialty care5 among the uninsured likely increases their risk of being hospitalized with more advanced disease. The higher likelihood of being admitted through the emergency department19 and on weekends9 among the uninsured lends credence to the possibility of delays in seeking care. All of these are potential mediators of higher AMI and stroke mortality in uninsured patients.
Finally, these mortality differences could also be due to the additional risks imposed by poorly managed comorbidities among uninsured patients. Although we controlled for the presence of comorbidities in our analysis, we lacked data about the severity of individual comorbidities. A recent study reported significant lapses in follow‐up care after the onset of a chronic condition in uninsured individuals under 65 years of age.34 Other studies have also documented insurance related disparities in the care of chronic diseases3, 35 that were among the most common comorbidities in our cohort.
Most of the reasons for insurance‐related disparities noted above for the uninsured are also applicable to Medicaid patients. Differences in the intensity of inpatient care,7, 8, 1519 limited access to health care services,2, 14 unmet health needs,5 and suboptimal management of chronic medical conditions35 were also reported for Medicaid patients in prior research. These factors likely contributed to the higher in‐hospital mortality in this patient population, evidenced by the sequential decrease in odds after adjusting for comorbidities and disease severity. Medicaid patients hospitalized for stroke were noted to have significantly longer LOS, which could plausibly be due to difficulties with arranging appropriate discharge disposition; the higher likelihood of paralysis among these patients15 would likely necessitate a higher frequency of rehabilitation facility placement. The higher costs for Medicaid patients with stroke and pneumonia may potentially be the result of these patients longer LOS. Although cost differences between the uninsured and privately insured were statistically significant, these were not large enough to be of material significance.
Limitations
Our study has several limitations. Since the NIS does not assign unique patient identifiers that would permit tracking of readmissions, we excluded patients transferred to another acute‐care hospital from our study to avoid counting the same patient twice. However, only 10% of hospitalized patients underwent transfer for cardiac procedures in the National Registry of Myocardial Infarction, with privately insured patients more likely to be transferred than other insurance groups.17 Since these patients are also more likely to have better survival, their exclusion likely biased our study toward the null. The same is probable for stroke patients as well.
Some uninsured patients begin Medicaid coverage during hospitalization and should ideally be counted as uninsured but were included under Medicaid in our analysis. They are also likely to be state‐ and plan‐specific variations in Medicaid and private payer coverage that we could not incorporate into our analysis. In addition, we were unable to include deaths that may have occurred shortly after discharge, even though these may have been related to the quality of hospital care. Furthermore, although the 3 conditions we studied are common and responsible for a large number of hospital deaths, they make up about 8% of total annual hospital discharges,23 and caution should be exercised in generalizing our findings to the full spectrum of hospitalizations. Lastly, it is possible that unmeasured confounding could be responsible for the observed associations. Uninsured and Medicaid patients are likely to have more severe disease, which may not be adequately captured by the administrative data available in the NIS. If so, this would explain the mortality association rather than insurance status.36, 37
Conclusions
Significant insurance‐related differences in mortality exist for 2 of the leading causes of noncancer inpatient deaths among working‐age Americans. Further studies are needed to determine whether provider sensitivity to insurance status or unmeasured sociodemographic and clinical prognostic factors are responsible for these disparities. Policy makers, hospital administrators, and physicians should be cognizant of these disparities and consider policies to address potential insurance related gaps in the quality of inpatient care.
- , .The U.S. economy and changes in health insurance coverage, 2000‐2006.Health Aff (Millwood).2008;27(2):w135‐w144.
- , , .Rates of avoidable hospitalization by insurance status in Massachusetts and Maryland.JAMA.1992;268(17):2388‐2394.
- , , , et al.Unmet health needs of uninsured adults in the United States.JAMA.2000;284(16):2061‐2069.
- , , .Health insurance and access to care for symptomatic conditions.Arch Intern Med.2000;160(9):1269‐1274.
- , , , et al.Access to specialty care and medical services in community health centers.Health Aff (Millwood).2007;26(5):1459‐1468.
- , , , et al.A national study of chronic disease prevalence and access to care in uninsured U.S. adults.Ann Intern Med.2008;149:170–176.
- , , , .Relationship between patient source of payment and the intensity of hospital services.Med Care.1988;26(11):1111‐1114.
- , , .The association of payer with utilization of cardiac procedures in Massachusetts.JAMA.1990;264(10):1255‐1260.
- , , .Comparison of uninsured and privately insured hospital patients: condition on admission, resource use, and outcome.JAMA.1991;265:374–379.
- , , .Socioeconomic status and risk for substandard medical care.JAMA.1992;268(17):2383‐2387.
- , , , .The relation between health insurance coverage and clinical outcomes among women with breast cancer.N Engl J Med.1993;329(5):326‐331.
- , , .Health insurance and mortality. Evidence from a national cohort.JAMA.1993;270(6):737‐741.
- , , , .Mortality in the uninsured compared with that in persons with public and private health insurance.Arch Intern Med.1994;154(21):2409‐2416.
- .Medicaid policy and the substitution of hospital outpatient care for physician care.Health Serv Res.1989;24:33–66.
- , .Disparities in outcomes among patients with stroke associated with insurance status.Stroke.2007;38(3):1010‐1016.
- , , , et al.Influence of payor on use of invasive cardiac procedures and patient outcome after myocardial infarction in the United States. Participants in the National Registry of Myocardial Infarction.J Am Coll Cardiol.1998;31(7):1474‐1480.
- , , , et al.Payer status and the utilization of hospital resources in acute myocardial infarction: a report from the National Registry of Myocardial Infarction 2.Arch Intern Med.2000;160(6):817–823.
- , , , et al.Insurance coverage and care of patients with non‐ST‐segment elevation acute coronary syndromes.Ann Intern Med.2006;145(10):739–748.
- , , .Comparing uninsured and privately insured hospital patients: Admission severity, health outcomes and resource use.Health Serv Manage Res.2001;14(3):203–210.
- Healthcare Cost and Utilization Project. Introduction to the HCUP Nationwide Inpatient Sample (NIS) 2005. Rockville, MD: Agency for Healthcare Research and Quality; 2007:6. Available at: www.hcup‐us.ahrq.gov/db/nation/nis/NIS_Introduction_2005.pdf. Accessed February2010.
- Healthcare Cost and Utilization Project. Design of the Nationwide Inpatient Sample (NIS) 2005. Rockville, MD: Agency for Healthcare Research and Quality; 2007. Available at: www.hcup‐us.ahrq.gov/db/nation/nis/reports/NIS_2005_Design_Report.pdf. Accessed February2010.
- AHRQ Quality Indicators. Inpatient Quality Indicators: Technical Specifications. Version 3.1 (March 12, 2007). Available at: www.qualityindicators.ahrq.gov/downloads/iqi/iqi_technical_specs_v31.pdf. Accessed February2010.
- , , . National Hospital Discharge Survey: 2005 annual summary with detailed diagnosis and procedure data. Washington, DC: National Center for Health Statistics; 2007. Vital and Health Statistics 13(165). Available at: www.cdc.gov/nchs/data/series/sr_13/sr13_165.pdf. Accessed February2010.
- AHRQ Quality Indicators. Guide to Inpatient Quality Indicators: Quality of Care in Hospitals—Volume, Mortality, and Utilization. Version 3.1 (March 12, 2007). Available at: www.qualityindicators.ahrq.gov/downloads/iqi/iqi_guide_v31.pdf. Accessed February2010.
- , , .Income, poverty, and health insurance coverage in the United States: 2006.Washington, DC:US Census Bureau. Current Population Reports;2007:60–233.
- , . Conditions Related to Uninsured Hospitalizations, 2003. HCUP Statistical Brief #8. Rockville, MD: Agency for Healthcare Research and Quality; 2006:6. Available at: www.hcup‐us.ahrq.gov/reports/statbriefs/sb8.pdf. Accessed February2010.
- Healthcare Cost and Utilization Project. NIS Description of Data Elements. Available at: www.hcup‐us.ahrq.gov/db/nation/nis/nisdde.jsp. Accessed February2010.
- , , , .Comorbidity measures for use with administrative data.Med Care.1998;36:8–27.
- SUDAAN User's Manual, Release 9.0. Research TrianglePark, NC:Research Triangle Institute;2006.
- , . Final Report on Calculating Nationwide Inpatient Sample (NIS) Variances, 2001. HCUP Methods Series Report #2003‐2. Online June 2005 (revised June 6, 2005). U.S. Agency for Healthcare Research and Quality. Available at: www.hcup‐us.ahrq.gov/reports/CalculatingNISVariances200106092005.pdf. Accessed February2010.
- .On the variances of asymptotically normal estimators from complex surveys.Int Stat Rev.1983;51:279–292.
- , , , et al.Patient characteristics associated with care by a cardiologist among adults hospitalized with severe congestive heart failure.J Am Coll Cardiol.2000;36:2119–2125.
- , . Summary health statistics for U.S. adults: National Health Interview Survey, 2006. Washington, DC: National Center for Health Statistics; 2007:12. Vital and Health Statistics 10(235). Available at: www.cdc.gov/nchs/data/series/sr_10/sr10_235.pdf. Accessed February2010.
- .Insurance coverage, medical care use, and short‐term health changes following an unintentional injury or the onset of a chronic condition.JAMA.2007;297(10):1073–1084.
- , , , et al.The quality of chronic disease care in U.S. community health centers.Health Aff (Millwood).2006;25(6):1712–1723.
- , , , , , .Risk‐adjusting hospital inpatient mortality using automated inpatient, outpatient, and laboratory databases.Med Care.2008;46(3):232–239.
- , , , .The Kaiser Permanente inpatient risk adjustment methodology was valid in an external patient population.J Clin Epidemiol. [E‐pub ahead of print].
- , .The U.S. economy and changes in health insurance coverage, 2000‐2006.Health Aff (Millwood).2008;27(2):w135‐w144.
- , , .Rates of avoidable hospitalization by insurance status in Massachusetts and Maryland.JAMA.1992;268(17):2388‐2394.
- , , , et al.Unmet health needs of uninsured adults in the United States.JAMA.2000;284(16):2061‐2069.
- , , .Health insurance and access to care for symptomatic conditions.Arch Intern Med.2000;160(9):1269‐1274.
- , , , et al.Access to specialty care and medical services in community health centers.Health Aff (Millwood).2007;26(5):1459‐1468.
- , , , et al.A national study of chronic disease prevalence and access to care in uninsured U.S. adults.Ann Intern Med.2008;149:170–176.
- , , , .Relationship between patient source of payment and the intensity of hospital services.Med Care.1988;26(11):1111‐1114.
- , , .The association of payer with utilization of cardiac procedures in Massachusetts.JAMA.1990;264(10):1255‐1260.
- , , .Comparison of uninsured and privately insured hospital patients: condition on admission, resource use, and outcome.JAMA.1991;265:374–379.
- , , .Socioeconomic status and risk for substandard medical care.JAMA.1992;268(17):2383‐2387.
- , , , .The relation between health insurance coverage and clinical outcomes among women with breast cancer.N Engl J Med.1993;329(5):326‐331.
- , , .Health insurance and mortality. Evidence from a national cohort.JAMA.1993;270(6):737‐741.
- , , , .Mortality in the uninsured compared with that in persons with public and private health insurance.Arch Intern Med.1994;154(21):2409‐2416.
- .Medicaid policy and the substitution of hospital outpatient care for physician care.Health Serv Res.1989;24:33–66.
- , .Disparities in outcomes among patients with stroke associated with insurance status.Stroke.2007;38(3):1010‐1016.
- , , , et al.Influence of payor on use of invasive cardiac procedures and patient outcome after myocardial infarction in the United States. Participants in the National Registry of Myocardial Infarction.J Am Coll Cardiol.1998;31(7):1474‐1480.
- , , , et al.Payer status and the utilization of hospital resources in acute myocardial infarction: a report from the National Registry of Myocardial Infarction 2.Arch Intern Med.2000;160(6):817–823.
- , , , et al.Insurance coverage and care of patients with non‐ST‐segment elevation acute coronary syndromes.Ann Intern Med.2006;145(10):739–748.
- , , .Comparing uninsured and privately insured hospital patients: Admission severity, health outcomes and resource use.Health Serv Manage Res.2001;14(3):203–210.
- Healthcare Cost and Utilization Project. Introduction to the HCUP Nationwide Inpatient Sample (NIS) 2005. Rockville, MD: Agency for Healthcare Research and Quality; 2007:6. Available at: www.hcup‐us.ahrq.gov/db/nation/nis/NIS_Introduction_2005.pdf. Accessed February2010.
- Healthcare Cost and Utilization Project. Design of the Nationwide Inpatient Sample (NIS) 2005. Rockville, MD: Agency for Healthcare Research and Quality; 2007. Available at: www.hcup‐us.ahrq.gov/db/nation/nis/reports/NIS_2005_Design_Report.pdf. Accessed February2010.
- AHRQ Quality Indicators. Inpatient Quality Indicators: Technical Specifications. Version 3.1 (March 12, 2007). Available at: www.qualityindicators.ahrq.gov/downloads/iqi/iqi_technical_specs_v31.pdf. Accessed February2010.
- , , . National Hospital Discharge Survey: 2005 annual summary with detailed diagnosis and procedure data. Washington, DC: National Center for Health Statistics; 2007. Vital and Health Statistics 13(165). Available at: www.cdc.gov/nchs/data/series/sr_13/sr13_165.pdf. Accessed February2010.
- AHRQ Quality Indicators. Guide to Inpatient Quality Indicators: Quality of Care in Hospitals—Volume, Mortality, and Utilization. Version 3.1 (March 12, 2007). Available at: www.qualityindicators.ahrq.gov/downloads/iqi/iqi_guide_v31.pdf. Accessed February2010.
- , , .Income, poverty, and health insurance coverage in the United States: 2006.Washington, DC:US Census Bureau. Current Population Reports;2007:60–233.
- , . Conditions Related to Uninsured Hospitalizations, 2003. HCUP Statistical Brief #8. Rockville, MD: Agency for Healthcare Research and Quality; 2006:6. Available at: www.hcup‐us.ahrq.gov/reports/statbriefs/sb8.pdf. Accessed February2010.
- Healthcare Cost and Utilization Project. NIS Description of Data Elements. Available at: www.hcup‐us.ahrq.gov/db/nation/nis/nisdde.jsp. Accessed February2010.
- , , , .Comorbidity measures for use with administrative data.Med Care.1998;36:8–27.
- SUDAAN User's Manual, Release 9.0. Research TrianglePark, NC:Research Triangle Institute;2006.
- , . Final Report on Calculating Nationwide Inpatient Sample (NIS) Variances, 2001. HCUP Methods Series Report #2003‐2. Online June 2005 (revised June 6, 2005). U.S. Agency for Healthcare Research and Quality. Available at: www.hcup‐us.ahrq.gov/reports/CalculatingNISVariances200106092005.pdf. Accessed February2010.
- .On the variances of asymptotically normal estimators from complex surveys.Int Stat Rev.1983;51:279–292.
- , , , et al.Patient characteristics associated with care by a cardiologist among adults hospitalized with severe congestive heart failure.J Am Coll Cardiol.2000;36:2119–2125.
- , . Summary health statistics for U.S. adults: National Health Interview Survey, 2006. Washington, DC: National Center for Health Statistics; 2007:12. Vital and Health Statistics 10(235). Available at: www.cdc.gov/nchs/data/series/sr_10/sr10_235.pdf. Accessed February2010.
- .Insurance coverage, medical care use, and short‐term health changes following an unintentional injury or the onset of a chronic condition.JAMA.2007;297(10):1073–1084.
- , , , et al.The quality of chronic disease care in U.S. community health centers.Health Aff (Millwood).2006;25(6):1712–1723.
- , , , , , .Risk‐adjusting hospital inpatient mortality using automated inpatient, outpatient, and laboratory databases.Med Care.2008;46(3):232–239.
- , , , .The Kaiser Permanente inpatient risk adjustment methodology was valid in an external patient population.J Clin Epidemiol. [E‐pub ahead of print].
Copyright © 2010 Society of Hospital Medicine