User login
Hospital Readmissions in End of Life
The need to improve end‐of‐life care is well recognized. Its quality is often poor, and its cost is enormous, with 30% of the Medicare expenditures used for medical treatments of the 6% of beneficiaries who die each year.[1, 2] Repeated hospitalizations are frequent toward the end of life,[3] where each admission should be viewed as an opportunity to initiate advance care planning to improve end‐of‐life care and possibly reduce future unnecessary readmissions.[4, 5] Identified problems include undertreatment of pain, lack of awareness of patient wishes or advance directives, and unwanted overtreatment.
To improve quality and reduce unnecessary hospital use near the end of life, there is an urgent need to help healthcare providers to better identify the most vulnerable and at‐risk patients to provide them with care coordination and supportive care services. We aimed to identify the risk factors for having a 30‐day potentially avoidable readmission (PAR) due to end‐of‐life care issues.
METHODS
Study Design and Population
A nested case‐control study was designed where potentially avoidable end‐of‐life readmissions were compared to nonreadmitted controls. We collected data on all consecutive adult patient admissions to any medical services of the Brigham and Women's Hospital with a discharge date between July 1, 2009 and June 30, 2010. Brigham and Women's Hospital is a 780‐bed academic medical center in Boston, Massachusetts. To avoid observation stays, only admissions with a length of stay of more than 1 day were included. We excluded patients who died before discharge, were transferred to another acute care hospital, and those who left against medical advice. We also excluded patients with no available data on medication treatment at discharge. The protocol was approved by the institutional review board of Brigham and Women's Hospital/Partners Healthcare.
Study Outcome
The study outcome was any 30‐day PAR due to end‐of‐life issues. To determine this outcome, first we identified all 30‐day readmissions to any service of 3 hospitals within the Partners network in Boston that followed the index hospitalization (prior studies have shown that these hospitals capture approximately 80% of readmissions after a Brigham and Women's Hospital medical hospitalization).[6, 7] These readmissions were subsequently differentiated as potentially avoidable or not using a validated algorithm (SQLape; SQLape, Corseaux, Switzerland).[8, 9] This algorithm uses administrative data and International Classification of Diseases, 9th Revision, Clinical Modification codes from the index and repeat hospitalization. Readmissions were considered potentially avoidable if they were: (1) readmissions related to previously known conditions during the index hospitalization, or (2) complications of treatment (eg, deep vein thrombosis, drug‐induced disorders). Conversely, readmissions were considered unavoidable if they were: (1) foreseen (such as readmissions for transplantation, delivery, chemo‐ or radiotherapy, and other specific surgical procedures), (2) follow‐up and rehabilitation treatments, or (3) readmissions for a new condition unknown during the preceding hospitalization. The algorithm has both a sensitivity and specificity of 96% compared with medical record review using the same criteria. Finally, a random sample of the 30‐day PARs was reviewed independently by 9 trained senior resident physicians to identify those due to end‐of‐life issues, defined by the following 2 criteria: (1) patient has a terminal clinical condition, such as malignancy, end stage renal disease, end stage congestive heart failure, or other condition with a life expectancy of 6 months or less; and (2) the readmission is part of the terminal disease process that was not adequately addressed during the index hospitalization. Examples of factors that were used when identifying cases included lack of healthcare proxy and lack of documentation of why end‐of‐life discussions did not take place during the index hospitalization. Training of adjudicators included a didactic session and review of standardized cases.
Risk Factors
We collected candidate risk factors based on a priori knowledge and according to the medical literature,[10, 11, 12] including demographic information, previous healthcare utilization, and index hospitalization characteristics from administrative data sources; procedures and chronic medical conditions from billing data; last laboratory values and medication information prior to discharge from the electronic medical record (Table 1). When laboratory values were missing (<1%), values were considered as normal.
| Characteristics | No 30‐Day Readmission, n=7,974 | 30‐Day PAR due to End of Life, n=80 | P Value |
|---|---|---|---|
| |||
| Age, y, mean (SD) | 61.5 (16.6) | 60.8 (11.9) | 0.69 |
| Male sex, n (%) | 3875 (48.6) | 37 (46.3) | 0.69 |
| Ethnicity, n (%) | 0.05 | ||
| Non‐Hispanic white* | 5772 (72.4) | 69 (86.3) | |
| Non‐Hispanic black | 1281 (16.1) | 4 (5.0) | |
| Hispanic | 666 (8.4) | 5 (6.3) | |
| Other | 255 (3.2) | 2 (2.5) | |
| Language, n (%) | 0.99 | ||
| English* | 7254 (91.0) | 73 (91.3) | |
| Spanish | 415 (5.2) | 4 (5.0) | |
| Other | 305 (3.8) | 3 (3.8) | |
| Marital status, n (%) | 0.37 | ||
| Currently married or partner* | 4107 (51.35) | 46 (57.5) | |
| Single/never married | 1967 (24.7) | 14 (17.5) | |
| Separated/divorced/widowed/no answer | 1900 (23.8) | 20 (25.0) | |
| Source of index admission, n (%) | 0.10 | ||
| Direct from home/outpatient clinic | 2456 (30.8) | 33 (41.3) | |
| Emergency department* | 4222 (53.0) | 34 (42.5) | |
| Nursing home/rehabilitation/other hospital | 1296 (16.3) | 13 (16.3) | |
| Length of stay of the index admission, median (IQR) | 4 (27) | 5.5 (38] | 0.13 |
| No. of hospital admissions in the past year, median (IQR) | 1 (02) | 2 (03) | <0.001 |
| Any procedure during the hospital stay, n (%) | 4809 (60.3) | 57 (71.3) | 0.05 |
| Identified caregiver at discharge | 7300 (91.6) | 76 (95.0) | 0.27 |
| No. of medications at discharge, mean (SD) | 10.6 (5.1) | 13.0 (5.0) | <0.001 |
| No. of opiate medication at discharge | <0.001 | ||
| 0 | 5297 (66.4) | 21 (26.3) | |
| 1 | 2677 (33.2) | 59 (73.8) | |
| Elixhauser, median (IQR) | 8 (215) | 23 (1442) | <0.001 |
| Selected comorbidities, n (%) | |||
| Diabetes mellitus | 1971 (24.7) | 20 (25.0) | 0.96 |
| Heart failure | 1756 (22.0) | 11 (13.8) | 0.10 |
| Atrial fibrillation | 1439 (18.1) | 10 (12.5) | 0.20 |
| COPD | 816 (10.2) | 7 (8.8) | 0.66 |
| Neoplasm | 2705 (33.9) | 69 (86.3) | <0.001 |
| Stroke | 294 (3.7) | 2 (2.5) | 0.57 |
| ESRD | 1258 (15.8) | 6 (7.5) | 0.04 |
| Liver disease | 328 (4.1) | 2 (2.5) | 0.47 |
Statistical Analysis
We first conducted a bivariate analysis on all collected potential risk factors, comparing admissions followed by a 30‐day PAR due to end‐of‐life care issues with admissions not followed by any 30‐day readmission, using the Pearson [2] test for categorical variables and Student t test for continuous variables. Then, we performed a multivariable logistic regression restricted to the variables that were found significantly associated with the outcome in the bivariate analysis. Age and Elixhauser comorbidity index were forced into the model as important potential confounders. Because a patient could have several outcomes over the study period, we used general estimating equations to cluster at the patient level. All tests were conducted as 2‐sided at a 0.05 level of significance. Analyses were performed using the SAS system for Windows, version 9.3 (SAS Institute Inc., Cary, NC).
RESULTS
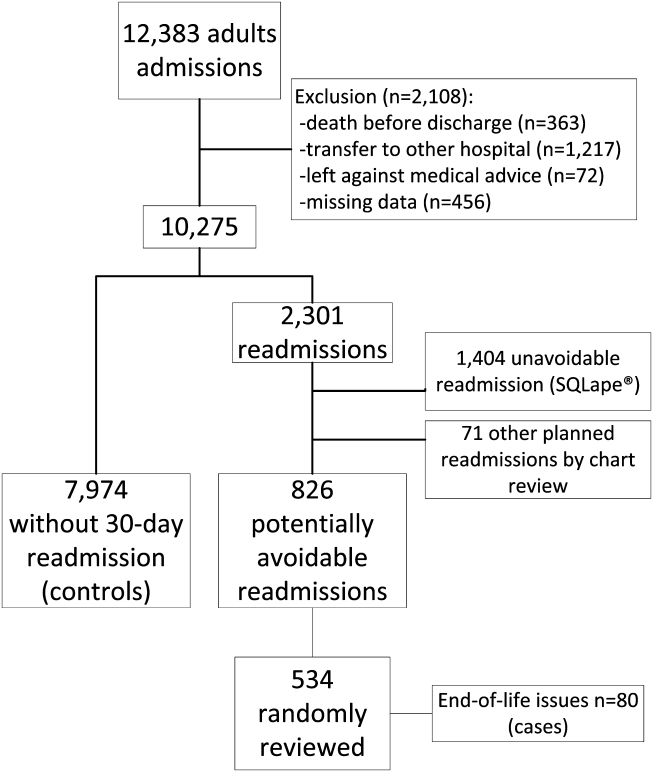
From the total of 12,383 patients who were discharged from the medical services of the Brigham and Women's Hospital during the study period, 2108 (17.0%) were excluded because of: (1) death before discharge, (2) transfer to another acute care hospital, (3) discharge against medical advice, or (4) missing data (Figure 1). Among the 10,275 eligible admissions, 22.3% (n=2301) were followed by a 30‐day readmission. Of these, 826 (8.0% of all admissions) were identified as potentially avoidable. Among a random sample of 534 PARs, 80 (15.0%) were related to end‐of‐life care issues (cases). Of note, only 16 (20%) of these patients received palliative care consultation during the index hospitalization. A total of 7974 discharges were not followed by any 30‐day readmission (controls).
Baseline characteristics are presented in Table 1. Among the combined cohort of cases plus controls, the patient's mean age at inclusion was 61.3 years, and about half were male. In bivariate analysis, demographics such as age and sex were similar between cases and controls. Cases had more hospitalizations in the previous year, a higher number of medications at discharge, and a higher Elixhauser comorbidity index. When looking at diseases more specifically, neoplasm was significantly associated with potentially avoidable 30‐day readmission due to end‐of‐life care issues. In contrast, end‐stage renal disease was associated with a significantly lower risk of 30‐day PAR due to end‐of‐life care issues.
In multivariate analysis, 4 factors remained significantly associated with 30‐day PAR due to end‐of‐life care issues (Table 2). Neoplasm was the strongest risk factor, with an odds ratio of 5.6 (95% confidence interval: 2.8511.0), followed by opiate medication use, Elixhauser score, and number of admissions in the previous 12 months.
| Variable | Odds Ratio (95% CI) |
|---|---|
| |
| Age, per 10 years | 1.04 (0.911.19) |
| No. of admissions in the previous 12 months, per admission | 1.10 (1.021.20)a |
| Total no. of medications at discharge, per medication | 1.04 (1.001.10) |
| Neoplasm | 5.60 (2.8511.0)a |
| Endstage renal disease | 0.60 (0.251.42) |
| Opiate medication at discharge | 2.29 (1.294.07)a |
| Elixhauser, per 5 unit increase | 1.16 (1.101.22)a |
The model, including all 4 variables, had an excellent discrimination power, with a C statistic of 0.85. Without the Elixhauser score, the C statistic remained very high, with a value of 0.82.
DISCUSSION
In a large medical population, potentially avoidable readmissions due to end‐of‐life care issues were not uncommon: 15% of all potentially avoidable readmissions (1.2% of all discharges). We identified 4 main risk factors for having a 30‐day potentially avoidable readmission due to end‐of‐life care issues: neoplasm, opiate use, Elixhauser comorbidity index, and number of admissions in the previous year. In a model that includes these 4 variables, the discrimination was very high with a C statistic of 0.85.
This study extends prior work indicating some risk factors for the need for palliative care. Neoplasm has been logically identified as a criterion for palliative care assessment at the time of admission.[13] Patients with neoplasm are not only at overall high risk for readmission,[14, 15, 16] but they obviously represent a fragile population whose condition is often terminal. Our results suggest that still more attention may be necessary to reduce the risk of readmission due to end‐of‐life care issues in this population (for example, only 20% of cases in our study received palliative care consultation during the index hospitalization). The overall comorbidity measured by the Elixhauser index was not surprisingly a significant risk factor. It probably accounts for the burden of comorbidities, but also for other advanced diseases besides neoplasm, like heart failure, chronic obstructive pulmonary disease, and others that may also be terminal. The number of previous hospital admissions in the past year is also an important risk factor, not only for the general population,[10, 11, 14, 17, 18] but also for patients with more advanced conditions,[19, 20, 21, 22] where admissions become more frequent as the disease progresses toward end stage. Opioid use was the final statistically significant risk factor, specific for this population, likely as a proxy for disease severity and progression toward terminal illness, especially in combination with the other risk factors such as cancer. Age was not a significant factor in either bivariate or multivariate analysis. Previous studies on the risk factors for readmission among patients receiving palliative care also failed to show age as a significant factor.[23, 24] Both of these studies looked at readmissions among patients who were already receiving palliative care. Our study asks a fundamentally different (and in many ways a more practical) question: who among a large population of medical patients might benefit from receiving input from palliative care in the first place. The number of medications at discharge was no longer significant in the multivariate analysis, likely due to its collinearity with the Elixhauser comorbidity index. An increased number of medications might be associated with a higher risk of adverse drug events and readmission, but they would not be necessarily considered to be end‐of‐life readmissions. Taken together, the 4 variables provide a very promising prediction model with high discrimination. To our knowledge, there is no previous existing list of risk factors for 30‐day potentially avoidable readmission due to end‐of‐life care issues, and no existing model to help prioritize palliative care to the most high‐risk patients. It is worth noting that the Elixhauser score might be difficult to calculate before the discharge of the patient (although hospitals with electronic capture of medical problem lists might be able to approximate it). However, even without the Elixhauser score, the C statistic remained very high at 0.82.
Our study has several limitations. Although we looked at readmissions at 2 other affiliated hospitals, some patients might have been readmitted to other acute care facilities outside our network. However, we would not expect the risk factors in these patients to be so different. The identification of end‐of‐life care issues by medical record review is based on a subjective judgment, although strict criteria were used. Furthermore, differentiation between potentially avoidable readmission and unavoidable readmission cannot be perfect. We used clear and logical criteria that were previously validated and allow large database management. Also, we did not analyze a comprehensive list of potential risk factors. It is probable that functional or cognitive status, for example, could also be important risk factors. We purposely chose a set of variables that could be easily obtained from administrative data sources. The small number of cases may have led to limited statistical power to identify less strongly associated risk factors. Last, the results may not be completely generalizable to small or community hospitals, in particular those that may care for less severely ill cancer patients.
Our findings have important implications. End‐of‐life care issues are not infrequent causes of readmission. Our study's findings could help prioritize palliative care resources to those patients at higher risk to improve the quality of end‐of‐life care. The risk factors identified in this study could be used informally by physicians at the bedside to identify such patients. In addition, a hospital could use these factors to provide a second‐level screen, beyond clinician recognition, to assist palliative care teams to identify patients who may not have otherwise been referred. This screen could be automated, for example, by using a list of medical problems from an electronic medical record to approximate an Elixhauser comorbidity score, or even leaving comorbidities out and simply relying on the other 3 easily identifiable risk factors. Such efforts could have a substantial effect on improving care near the end of life and potentially reducing unnecessary hospitalizations.
Acknowledgements
The authors thank Yves Eggli for having screened the database for potentially avoidable readmission using the algorithm SQLape.
Disclosures: Dr. Donz was supported by the Swiss National Science Foundation and the Swiss Foundation for MedicalBiological Scholarships. The Swiss Science National Foundation and the Swiss Foundation for MedicalBiological Scholarships had no role in the design and conduct of this study, the analysis or interpretation of the data, or the preparation of this manuscript. Dr. Schnipper is a consultant to QuantiaMD, for which he has helped create online educational materials for both providers and patients regarding patient safety, including medication safety during transitions in care. The findings of this study are not a part of those materials. Dr. Schnipper has received grant funding from Sanofi‐Aventis for an investigator‐initiated study to design and evaluate an intensive discharge and follow‐up intervention in patients with diabetes. The funder had had no role in the design of the study.
- , , , . Medicare beneficiaries' costs of care in the last year of life. Health Aff (Millwood). 2001;20(4):188–195.
- , , . Quality of End‐of‐Life Cancer Care for Medicare Beneficiaries: Regional and Hospital‐Specific Analyses. Lebanon, NH: The Dartmouth Institute for Health Policy and Clinical Practice; 2010.
- , , . Repeated hospitalizations predict mortality in the community population with heart failure. Am Heart J. 2007;154(2):260–266.
- . Perspectives on care at the close of life. Initiating end‐of‐life discussions with seriously ill patients: addressing the “elephant in the room.” JAMA. 2000;284(19):2502–2507.
- , , , . Advance care planning as a process: structuring the discussions in practice. J Am Geriatr Soc. 1995;43(4):440–446.
- , , , et al. Rationale and design of the Pharmacist Intervention for Low Literacy in Cardiovascular Disease (PILL‐CVD) study. Circ Cardiovasc Qual Outcomes. 2010;3(2):212–219.
- , , , et al. Effect of a pharmacist intervention on clinically important medication errors after hospital discharge: a randomized trial. Ann Intern Med. 2012;157(1):1–10.
- , , , , , . Measuring potentially avoidable hospital readmissions. J Clin Epidemiol. 2002;55(6):573–587.
- , , , , , . Validation of the potentially avoidable hospital readmission rate as a routine indicator of the quality of hospital care. Med Care. 2006;44(11):972–981.
- , , , et al. Hospital readmission in general medicine patients: a prediction model. J Gen Intern Med. 2010;25(3):211–219.
- , , , , , . Screening elders for risk of hospital admission. J Am Geriatr Soc. 1993;41(8):811–817.
- , , , , . Risk factors for 30‐day hospital readmission in patients ≥65 years of age. Proc (Bayl Univ Med Cent). 2008;21(4):363–372.
- , . Identifying patients in need of a palliative care assessment in the hospital setting: a consensus report from the Center to Advance Palliative Care. J Palliat Med. 2011;14(1):17–23.
- , , , . Potentially avoidable 30‐day hospital readmissions in medical patients: derivation and validation of a prediction model. JAMA Intern Med. 2013;173(8):632–638.
- , , , . Redefining readmission risk factors for general medicine patients. J Hosp Med. 2011;6(2):54–60.
- , , . Patient and disease profile of emergency medical readmissions to an Irish teaching hospital. Postgrad Med J. 2004;80(946):470–474.
- , , , . Posthospital care transitions: patterns, complications, and risk identification. Health Serv Res. 2004;39(5):1449–1465.
- , . Factors predicting readmission of older general medicine patients. J Gen Intern Med. 1991;6(5):389–393.
- , , , , . Differences in health care utilization at the end of life among patients with chronic obstructive pulmonary disease and patients with lung cancer. Arch Intern Med. 2006;166(3):326–331.
- , , , , . Frequent hospital readmissions for acute exacerbation of COPD and their associated factors. Respirology. 2006;11(2):188–195.
- , , , et al. Consensus statement: Palliative and supportive care in advanced heart failure. J Card Fail. 2004;10(3):200–209.
- , , , , . Unplanned discharges from a surgical intensive care unit: readmissions and mortality. J Crit Care. 2010;25(3):375–381.
- , , , , . Evaluating causes for unplanned hospital readmissions of palliative care patients. Am J Hosp Palliat Care. 2010;27(8):526–531.
- , , . 30‐day readmissions among seriously ill older adults. J Palliat Med. 2012;15(12):1356–1361.
The need to improve end‐of‐life care is well recognized. Its quality is often poor, and its cost is enormous, with 30% of the Medicare expenditures used for medical treatments of the 6% of beneficiaries who die each year.[1, 2] Repeated hospitalizations are frequent toward the end of life,[3] where each admission should be viewed as an opportunity to initiate advance care planning to improve end‐of‐life care and possibly reduce future unnecessary readmissions.[4, 5] Identified problems include undertreatment of pain, lack of awareness of patient wishes or advance directives, and unwanted overtreatment.
To improve quality and reduce unnecessary hospital use near the end of life, there is an urgent need to help healthcare providers to better identify the most vulnerable and at‐risk patients to provide them with care coordination and supportive care services. We aimed to identify the risk factors for having a 30‐day potentially avoidable readmission (PAR) due to end‐of‐life care issues.
METHODS
Study Design and Population
A nested case‐control study was designed where potentially avoidable end‐of‐life readmissions were compared to nonreadmitted controls. We collected data on all consecutive adult patient admissions to any medical services of the Brigham and Women's Hospital with a discharge date between July 1, 2009 and June 30, 2010. Brigham and Women's Hospital is a 780‐bed academic medical center in Boston, Massachusetts. To avoid observation stays, only admissions with a length of stay of more than 1 day were included. We excluded patients who died before discharge, were transferred to another acute care hospital, and those who left against medical advice. We also excluded patients with no available data on medication treatment at discharge. The protocol was approved by the institutional review board of Brigham and Women's Hospital/Partners Healthcare.
Study Outcome
The study outcome was any 30‐day PAR due to end‐of‐life issues. To determine this outcome, first we identified all 30‐day readmissions to any service of 3 hospitals within the Partners network in Boston that followed the index hospitalization (prior studies have shown that these hospitals capture approximately 80% of readmissions after a Brigham and Women's Hospital medical hospitalization).[6, 7] These readmissions were subsequently differentiated as potentially avoidable or not using a validated algorithm (SQLape; SQLape, Corseaux, Switzerland).[8, 9] This algorithm uses administrative data and International Classification of Diseases, 9th Revision, Clinical Modification codes from the index and repeat hospitalization. Readmissions were considered potentially avoidable if they were: (1) readmissions related to previously known conditions during the index hospitalization, or (2) complications of treatment (eg, deep vein thrombosis, drug‐induced disorders). Conversely, readmissions were considered unavoidable if they were: (1) foreseen (such as readmissions for transplantation, delivery, chemo‐ or radiotherapy, and other specific surgical procedures), (2) follow‐up and rehabilitation treatments, or (3) readmissions for a new condition unknown during the preceding hospitalization. The algorithm has both a sensitivity and specificity of 96% compared with medical record review using the same criteria. Finally, a random sample of the 30‐day PARs was reviewed independently by 9 trained senior resident physicians to identify those due to end‐of‐life issues, defined by the following 2 criteria: (1) patient has a terminal clinical condition, such as malignancy, end stage renal disease, end stage congestive heart failure, or other condition with a life expectancy of 6 months or less; and (2) the readmission is part of the terminal disease process that was not adequately addressed during the index hospitalization. Examples of factors that were used when identifying cases included lack of healthcare proxy and lack of documentation of why end‐of‐life discussions did not take place during the index hospitalization. Training of adjudicators included a didactic session and review of standardized cases.
Risk Factors
We collected candidate risk factors based on a priori knowledge and according to the medical literature,[10, 11, 12] including demographic information, previous healthcare utilization, and index hospitalization characteristics from administrative data sources; procedures and chronic medical conditions from billing data; last laboratory values and medication information prior to discharge from the electronic medical record (Table 1). When laboratory values were missing (<1%), values were considered as normal.
| Characteristics | No 30‐Day Readmission, n=7,974 | 30‐Day PAR due to End of Life, n=80 | P Value |
|---|---|---|---|
| |||
| Age, y, mean (SD) | 61.5 (16.6) | 60.8 (11.9) | 0.69 |
| Male sex, n (%) | 3875 (48.6) | 37 (46.3) | 0.69 |
| Ethnicity, n (%) | 0.05 | ||
| Non‐Hispanic white* | 5772 (72.4) | 69 (86.3) | |
| Non‐Hispanic black | 1281 (16.1) | 4 (5.0) | |
| Hispanic | 666 (8.4) | 5 (6.3) | |
| Other | 255 (3.2) | 2 (2.5) | |
| Language, n (%) | 0.99 | ||
| English* | 7254 (91.0) | 73 (91.3) | |
| Spanish | 415 (5.2) | 4 (5.0) | |
| Other | 305 (3.8) | 3 (3.8) | |
| Marital status, n (%) | 0.37 | ||
| Currently married or partner* | 4107 (51.35) | 46 (57.5) | |
| Single/never married | 1967 (24.7) | 14 (17.5) | |
| Separated/divorced/widowed/no answer | 1900 (23.8) | 20 (25.0) | |
| Source of index admission, n (%) | 0.10 | ||
| Direct from home/outpatient clinic | 2456 (30.8) | 33 (41.3) | |
| Emergency department* | 4222 (53.0) | 34 (42.5) | |
| Nursing home/rehabilitation/other hospital | 1296 (16.3) | 13 (16.3) | |
| Length of stay of the index admission, median (IQR) | 4 (27) | 5.5 (38] | 0.13 |
| No. of hospital admissions in the past year, median (IQR) | 1 (02) | 2 (03) | <0.001 |
| Any procedure during the hospital stay, n (%) | 4809 (60.3) | 57 (71.3) | 0.05 |
| Identified caregiver at discharge | 7300 (91.6) | 76 (95.0) | 0.27 |
| No. of medications at discharge, mean (SD) | 10.6 (5.1) | 13.0 (5.0) | <0.001 |
| No. of opiate medication at discharge | <0.001 | ||
| 0 | 5297 (66.4) | 21 (26.3) | |
| 1 | 2677 (33.2) | 59 (73.8) | |
| Elixhauser, median (IQR) | 8 (215) | 23 (1442) | <0.001 |
| Selected comorbidities, n (%) | |||
| Diabetes mellitus | 1971 (24.7) | 20 (25.0) | 0.96 |
| Heart failure | 1756 (22.0) | 11 (13.8) | 0.10 |
| Atrial fibrillation | 1439 (18.1) | 10 (12.5) | 0.20 |
| COPD | 816 (10.2) | 7 (8.8) | 0.66 |
| Neoplasm | 2705 (33.9) | 69 (86.3) | <0.001 |
| Stroke | 294 (3.7) | 2 (2.5) | 0.57 |
| ESRD | 1258 (15.8) | 6 (7.5) | 0.04 |
| Liver disease | 328 (4.1) | 2 (2.5) | 0.47 |
Statistical Analysis
We first conducted a bivariate analysis on all collected potential risk factors, comparing admissions followed by a 30‐day PAR due to end‐of‐life care issues with admissions not followed by any 30‐day readmission, using the Pearson [2] test for categorical variables and Student t test for continuous variables. Then, we performed a multivariable logistic regression restricted to the variables that were found significantly associated with the outcome in the bivariate analysis. Age and Elixhauser comorbidity index were forced into the model as important potential confounders. Because a patient could have several outcomes over the study period, we used general estimating equations to cluster at the patient level. All tests were conducted as 2‐sided at a 0.05 level of significance. Analyses were performed using the SAS system for Windows, version 9.3 (SAS Institute Inc., Cary, NC).
RESULTS
From the total of 12,383 patients who were discharged from the medical services of the Brigham and Women's Hospital during the study period, 2108 (17.0%) were excluded because of: (1) death before discharge, (2) transfer to another acute care hospital, (3) discharge against medical advice, or (4) missing data (Figure 1). Among the 10,275 eligible admissions, 22.3% (n=2301) were followed by a 30‐day readmission. Of these, 826 (8.0% of all admissions) were identified as potentially avoidable. Among a random sample of 534 PARs, 80 (15.0%) were related to end‐of‐life care issues (cases). Of note, only 16 (20%) of these patients received palliative care consultation during the index hospitalization. A total of 7974 discharges were not followed by any 30‐day readmission (controls).

Baseline characteristics are presented in Table 1. Among the combined cohort of cases plus controls, the patient's mean age at inclusion was 61.3 years, and about half were male. In bivariate analysis, demographics such as age and sex were similar between cases and controls. Cases had more hospitalizations in the previous year, a higher number of medications at discharge, and a higher Elixhauser comorbidity index. When looking at diseases more specifically, neoplasm was significantly associated with potentially avoidable 30‐day readmission due to end‐of‐life care issues. In contrast, end‐stage renal disease was associated with a significantly lower risk of 30‐day PAR due to end‐of‐life care issues.
In multivariate analysis, 4 factors remained significantly associated with 30‐day PAR due to end‐of‐life care issues (Table 2). Neoplasm was the strongest risk factor, with an odds ratio of 5.6 (95% confidence interval: 2.8511.0), followed by opiate medication use, Elixhauser score, and number of admissions in the previous 12 months.
| Variable | Odds Ratio (95% CI) |
|---|---|
| |
| Age, per 10 years | 1.04 (0.911.19) |
| No. of admissions in the previous 12 months, per admission | 1.10 (1.021.20)a |
| Total no. of medications at discharge, per medication | 1.04 (1.001.10) |
| Neoplasm | 5.60 (2.8511.0)a |
| Endstage renal disease | 0.60 (0.251.42) |
| Opiate medication at discharge | 2.29 (1.294.07)a |
| Elixhauser, per 5 unit increase | 1.16 (1.101.22)a |
The model, including all 4 variables, had an excellent discrimination power, with a C statistic of 0.85. Without the Elixhauser score, the C statistic remained very high, with a value of 0.82.
DISCUSSION
In a large medical population, potentially avoidable readmissions due to end‐of‐life care issues were not uncommon: 15% of all potentially avoidable readmissions (1.2% of all discharges). We identified 4 main risk factors for having a 30‐day potentially avoidable readmission due to end‐of‐life care issues: neoplasm, opiate use, Elixhauser comorbidity index, and number of admissions in the previous year. In a model that includes these 4 variables, the discrimination was very high with a C statistic of 0.85.
This study extends prior work indicating some risk factors for the need for palliative care. Neoplasm has been logically identified as a criterion for palliative care assessment at the time of admission.[13] Patients with neoplasm are not only at overall high risk for readmission,[14, 15, 16] but they obviously represent a fragile population whose condition is often terminal. Our results suggest that still more attention may be necessary to reduce the risk of readmission due to end‐of‐life care issues in this population (for example, only 20% of cases in our study received palliative care consultation during the index hospitalization). The overall comorbidity measured by the Elixhauser index was not surprisingly a significant risk factor. It probably accounts for the burden of comorbidities, but also for other advanced diseases besides neoplasm, like heart failure, chronic obstructive pulmonary disease, and others that may also be terminal. The number of previous hospital admissions in the past year is also an important risk factor, not only for the general population,[10, 11, 14, 17, 18] but also for patients with more advanced conditions,[19, 20, 21, 22] where admissions become more frequent as the disease progresses toward end stage. Opioid use was the final statistically significant risk factor, specific for this population, likely as a proxy for disease severity and progression toward terminal illness, especially in combination with the other risk factors such as cancer. Age was not a significant factor in either bivariate or multivariate analysis. Previous studies on the risk factors for readmission among patients receiving palliative care also failed to show age as a significant factor.[23, 24] Both of these studies looked at readmissions among patients who were already receiving palliative care. Our study asks a fundamentally different (and in many ways a more practical) question: who among a large population of medical patients might benefit from receiving input from palliative care in the first place. The number of medications at discharge was no longer significant in the multivariate analysis, likely due to its collinearity with the Elixhauser comorbidity index. An increased number of medications might be associated with a higher risk of adverse drug events and readmission, but they would not be necessarily considered to be end‐of‐life readmissions. Taken together, the 4 variables provide a very promising prediction model with high discrimination. To our knowledge, there is no previous existing list of risk factors for 30‐day potentially avoidable readmission due to end‐of‐life care issues, and no existing model to help prioritize palliative care to the most high‐risk patients. It is worth noting that the Elixhauser score might be difficult to calculate before the discharge of the patient (although hospitals with electronic capture of medical problem lists might be able to approximate it). However, even without the Elixhauser score, the C statistic remained very high at 0.82.
Our study has several limitations. Although we looked at readmissions at 2 other affiliated hospitals, some patients might have been readmitted to other acute care facilities outside our network. However, we would not expect the risk factors in these patients to be so different. The identification of end‐of‐life care issues by medical record review is based on a subjective judgment, although strict criteria were used. Furthermore, differentiation between potentially avoidable readmission and unavoidable readmission cannot be perfect. We used clear and logical criteria that were previously validated and allow large database management. Also, we did not analyze a comprehensive list of potential risk factors. It is probable that functional or cognitive status, for example, could also be important risk factors. We purposely chose a set of variables that could be easily obtained from administrative data sources. The small number of cases may have led to limited statistical power to identify less strongly associated risk factors. Last, the results may not be completely generalizable to small or community hospitals, in particular those that may care for less severely ill cancer patients.
Our findings have important implications. End‐of‐life care issues are not infrequent causes of readmission. Our study's findings could help prioritize palliative care resources to those patients at higher risk to improve the quality of end‐of‐life care. The risk factors identified in this study could be used informally by physicians at the bedside to identify such patients. In addition, a hospital could use these factors to provide a second‐level screen, beyond clinician recognition, to assist palliative care teams to identify patients who may not have otherwise been referred. This screen could be automated, for example, by using a list of medical problems from an electronic medical record to approximate an Elixhauser comorbidity score, or even leaving comorbidities out and simply relying on the other 3 easily identifiable risk factors. Such efforts could have a substantial effect on improving care near the end of life and potentially reducing unnecessary hospitalizations.
Acknowledgements
The authors thank Yves Eggli for having screened the database for potentially avoidable readmission using the algorithm SQLape.
Disclosures: Dr. Donz was supported by the Swiss National Science Foundation and the Swiss Foundation for MedicalBiological Scholarships. The Swiss Science National Foundation and the Swiss Foundation for MedicalBiological Scholarships had no role in the design and conduct of this study, the analysis or interpretation of the data, or the preparation of this manuscript. Dr. Schnipper is a consultant to QuantiaMD, for which he has helped create online educational materials for both providers and patients regarding patient safety, including medication safety during transitions in care. The findings of this study are not a part of those materials. Dr. Schnipper has received grant funding from Sanofi‐Aventis for an investigator‐initiated study to design and evaluate an intensive discharge and follow‐up intervention in patients with diabetes. The funder had had no role in the design of the study.
The need to improve end‐of‐life care is well recognized. Its quality is often poor, and its cost is enormous, with 30% of the Medicare expenditures used for medical treatments of the 6% of beneficiaries who die each year.[1, 2] Repeated hospitalizations are frequent toward the end of life,[3] where each admission should be viewed as an opportunity to initiate advance care planning to improve end‐of‐life care and possibly reduce future unnecessary readmissions.[4, 5] Identified problems include undertreatment of pain, lack of awareness of patient wishes or advance directives, and unwanted overtreatment.
To improve quality and reduce unnecessary hospital use near the end of life, there is an urgent need to help healthcare providers to better identify the most vulnerable and at‐risk patients to provide them with care coordination and supportive care services. We aimed to identify the risk factors for having a 30‐day potentially avoidable readmission (PAR) due to end‐of‐life care issues.
METHODS
Study Design and Population
A nested case‐control study was designed where potentially avoidable end‐of‐life readmissions were compared to nonreadmitted controls. We collected data on all consecutive adult patient admissions to any medical services of the Brigham and Women's Hospital with a discharge date between July 1, 2009 and June 30, 2010. Brigham and Women's Hospital is a 780‐bed academic medical center in Boston, Massachusetts. To avoid observation stays, only admissions with a length of stay of more than 1 day were included. We excluded patients who died before discharge, were transferred to another acute care hospital, and those who left against medical advice. We also excluded patients with no available data on medication treatment at discharge. The protocol was approved by the institutional review board of Brigham and Women's Hospital/Partners Healthcare.
Study Outcome
The study outcome was any 30‐day PAR due to end‐of‐life issues. To determine this outcome, first we identified all 30‐day readmissions to any service of 3 hospitals within the Partners network in Boston that followed the index hospitalization (prior studies have shown that these hospitals capture approximately 80% of readmissions after a Brigham and Women's Hospital medical hospitalization).[6, 7] These readmissions were subsequently differentiated as potentially avoidable or not using a validated algorithm (SQLape; SQLape, Corseaux, Switzerland).[8, 9] This algorithm uses administrative data and International Classification of Diseases, 9th Revision, Clinical Modification codes from the index and repeat hospitalization. Readmissions were considered potentially avoidable if they were: (1) readmissions related to previously known conditions during the index hospitalization, or (2) complications of treatment (eg, deep vein thrombosis, drug‐induced disorders). Conversely, readmissions were considered unavoidable if they were: (1) foreseen (such as readmissions for transplantation, delivery, chemo‐ or radiotherapy, and other specific surgical procedures), (2) follow‐up and rehabilitation treatments, or (3) readmissions for a new condition unknown during the preceding hospitalization. The algorithm has both a sensitivity and specificity of 96% compared with medical record review using the same criteria. Finally, a random sample of the 30‐day PARs was reviewed independently by 9 trained senior resident physicians to identify those due to end‐of‐life issues, defined by the following 2 criteria: (1) patient has a terminal clinical condition, such as malignancy, end stage renal disease, end stage congestive heart failure, or other condition with a life expectancy of 6 months or less; and (2) the readmission is part of the terminal disease process that was not adequately addressed during the index hospitalization. Examples of factors that were used when identifying cases included lack of healthcare proxy and lack of documentation of why end‐of‐life discussions did not take place during the index hospitalization. Training of adjudicators included a didactic session and review of standardized cases.
Risk Factors
We collected candidate risk factors based on a priori knowledge and according to the medical literature,[10, 11, 12] including demographic information, previous healthcare utilization, and index hospitalization characteristics from administrative data sources; procedures and chronic medical conditions from billing data; last laboratory values and medication information prior to discharge from the electronic medical record (Table 1). When laboratory values were missing (<1%), values were considered as normal.
| Characteristics | No 30‐Day Readmission, n=7,974 | 30‐Day PAR due to End of Life, n=80 | P Value |
|---|---|---|---|
| |||
| Age, y, mean (SD) | 61.5 (16.6) | 60.8 (11.9) | 0.69 |
| Male sex, n (%) | 3875 (48.6) | 37 (46.3) | 0.69 |
| Ethnicity, n (%) | 0.05 | ||
| Non‐Hispanic white* | 5772 (72.4) | 69 (86.3) | |
| Non‐Hispanic black | 1281 (16.1) | 4 (5.0) | |
| Hispanic | 666 (8.4) | 5 (6.3) | |
| Other | 255 (3.2) | 2 (2.5) | |
| Language, n (%) | 0.99 | ||
| English* | 7254 (91.0) | 73 (91.3) | |
| Spanish | 415 (5.2) | 4 (5.0) | |
| Other | 305 (3.8) | 3 (3.8) | |
| Marital status, n (%) | 0.37 | ||
| Currently married or partner* | 4107 (51.35) | 46 (57.5) | |
| Single/never married | 1967 (24.7) | 14 (17.5) | |
| Separated/divorced/widowed/no answer | 1900 (23.8) | 20 (25.0) | |
| Source of index admission, n (%) | 0.10 | ||
| Direct from home/outpatient clinic | 2456 (30.8) | 33 (41.3) | |
| Emergency department* | 4222 (53.0) | 34 (42.5) | |
| Nursing home/rehabilitation/other hospital | 1296 (16.3) | 13 (16.3) | |
| Length of stay of the index admission, median (IQR) | 4 (27) | 5.5 (38] | 0.13 |
| No. of hospital admissions in the past year, median (IQR) | 1 (02) | 2 (03) | <0.001 |
| Any procedure during the hospital stay, n (%) | 4809 (60.3) | 57 (71.3) | 0.05 |
| Identified caregiver at discharge | 7300 (91.6) | 76 (95.0) | 0.27 |
| No. of medications at discharge, mean (SD) | 10.6 (5.1) | 13.0 (5.0) | <0.001 |
| No. of opiate medication at discharge | <0.001 | ||
| 0 | 5297 (66.4) | 21 (26.3) | |
| 1 | 2677 (33.2) | 59 (73.8) | |
| Elixhauser, median (IQR) | 8 (215) | 23 (1442) | <0.001 |
| Selected comorbidities, n (%) | |||
| Diabetes mellitus | 1971 (24.7) | 20 (25.0) | 0.96 |
| Heart failure | 1756 (22.0) | 11 (13.8) | 0.10 |
| Atrial fibrillation | 1439 (18.1) | 10 (12.5) | 0.20 |
| COPD | 816 (10.2) | 7 (8.8) | 0.66 |
| Neoplasm | 2705 (33.9) | 69 (86.3) | <0.001 |
| Stroke | 294 (3.7) | 2 (2.5) | 0.57 |
| ESRD | 1258 (15.8) | 6 (7.5) | 0.04 |
| Liver disease | 328 (4.1) | 2 (2.5) | 0.47 |
Statistical Analysis
We first conducted a bivariate analysis on all collected potential risk factors, comparing admissions followed by a 30‐day PAR due to end‐of‐life care issues with admissions not followed by any 30‐day readmission, using the Pearson [2] test for categorical variables and Student t test for continuous variables. Then, we performed a multivariable logistic regression restricted to the variables that were found significantly associated with the outcome in the bivariate analysis. Age and Elixhauser comorbidity index were forced into the model as important potential confounders. Because a patient could have several outcomes over the study period, we used general estimating equations to cluster at the patient level. All tests were conducted as 2‐sided at a 0.05 level of significance. Analyses were performed using the SAS system for Windows, version 9.3 (SAS Institute Inc., Cary, NC).
RESULTS
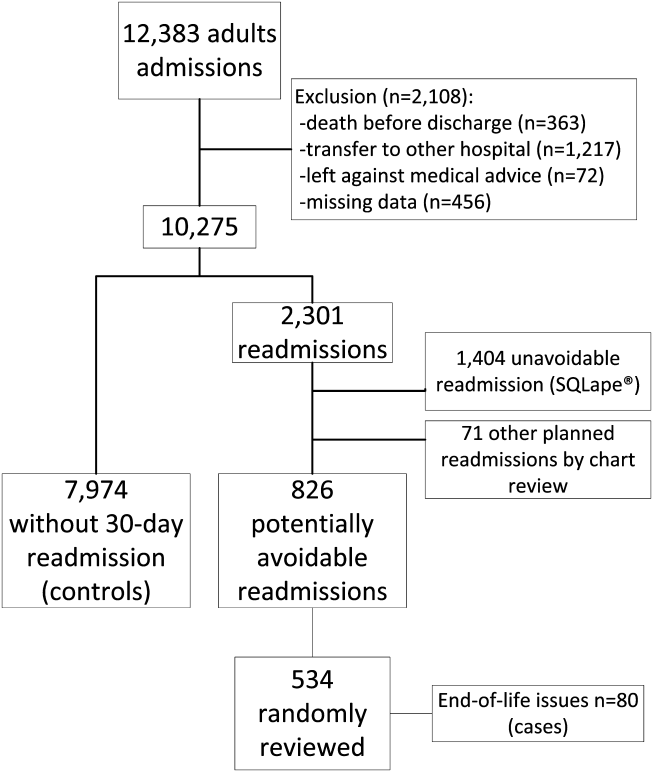
From the total of 12,383 patients who were discharged from the medical services of the Brigham and Women's Hospital during the study period, 2108 (17.0%) were excluded because of: (1) death before discharge, (2) transfer to another acute care hospital, (3) discharge against medical advice, or (4) missing data (Figure 1). Among the 10,275 eligible admissions, 22.3% (n=2301) were followed by a 30‐day readmission. Of these, 826 (8.0% of all admissions) were identified as potentially avoidable. Among a random sample of 534 PARs, 80 (15.0%) were related to end‐of‐life care issues (cases). Of note, only 16 (20%) of these patients received palliative care consultation during the index hospitalization. A total of 7974 discharges were not followed by any 30‐day readmission (controls).

Baseline characteristics are presented in Table 1. Among the combined cohort of cases plus controls, the patient's mean age at inclusion was 61.3 years, and about half were male. In bivariate analysis, demographics such as age and sex were similar between cases and controls. Cases had more hospitalizations in the previous year, a higher number of medications at discharge, and a higher Elixhauser comorbidity index. When looking at diseases more specifically, neoplasm was significantly associated with potentially avoidable 30‐day readmission due to end‐of‐life care issues. In contrast, end‐stage renal disease was associated with a significantly lower risk of 30‐day PAR due to end‐of‐life care issues.
In multivariate analysis, 4 factors remained significantly associated with 30‐day PAR due to end‐of‐life care issues (Table 2). Neoplasm was the strongest risk factor, with an odds ratio of 5.6 (95% confidence interval: 2.8511.0), followed by opiate medication use, Elixhauser score, and number of admissions in the previous 12 months.
| Variable | Odds Ratio (95% CI) |
|---|---|
| |
| Age, per 10 years | 1.04 (0.911.19) |
| No. of admissions in the previous 12 months, per admission | 1.10 (1.021.20)a |
| Total no. of medications at discharge, per medication | 1.04 (1.001.10) |
| Neoplasm | 5.60 (2.8511.0)a |
| Endstage renal disease | 0.60 (0.251.42) |
| Opiate medication at discharge | 2.29 (1.294.07)a |
| Elixhauser, per 5 unit increase | 1.16 (1.101.22)a |
The model, including all 4 variables, had an excellent discrimination power, with a C statistic of 0.85. Without the Elixhauser score, the C statistic remained very high, with a value of 0.82.
DISCUSSION
In a large medical population, potentially avoidable readmissions due to end‐of‐life care issues were not uncommon: 15% of all potentially avoidable readmissions (1.2% of all discharges). We identified 4 main risk factors for having a 30‐day potentially avoidable readmission due to end‐of‐life care issues: neoplasm, opiate use, Elixhauser comorbidity index, and number of admissions in the previous year. In a model that includes these 4 variables, the discrimination was very high with a C statistic of 0.85.
This study extends prior work indicating some risk factors for the need for palliative care. Neoplasm has been logically identified as a criterion for palliative care assessment at the time of admission.[13] Patients with neoplasm are not only at overall high risk for readmission,[14, 15, 16] but they obviously represent a fragile population whose condition is often terminal. Our results suggest that still more attention may be necessary to reduce the risk of readmission due to end‐of‐life care issues in this population (for example, only 20% of cases in our study received palliative care consultation during the index hospitalization). The overall comorbidity measured by the Elixhauser index was not surprisingly a significant risk factor. It probably accounts for the burden of comorbidities, but also for other advanced diseases besides neoplasm, like heart failure, chronic obstructive pulmonary disease, and others that may also be terminal. The number of previous hospital admissions in the past year is also an important risk factor, not only for the general population,[10, 11, 14, 17, 18] but also for patients with more advanced conditions,[19, 20, 21, 22] where admissions become more frequent as the disease progresses toward end stage. Opioid use was the final statistically significant risk factor, specific for this population, likely as a proxy for disease severity and progression toward terminal illness, especially in combination with the other risk factors such as cancer. Age was not a significant factor in either bivariate or multivariate analysis. Previous studies on the risk factors for readmission among patients receiving palliative care also failed to show age as a significant factor.[23, 24] Both of these studies looked at readmissions among patients who were already receiving palliative care. Our study asks a fundamentally different (and in many ways a more practical) question: who among a large population of medical patients might benefit from receiving input from palliative care in the first place. The number of medications at discharge was no longer significant in the multivariate analysis, likely due to its collinearity with the Elixhauser comorbidity index. An increased number of medications might be associated with a higher risk of adverse drug events and readmission, but they would not be necessarily considered to be end‐of‐life readmissions. Taken together, the 4 variables provide a very promising prediction model with high discrimination. To our knowledge, there is no previous existing list of risk factors for 30‐day potentially avoidable readmission due to end‐of‐life care issues, and no existing model to help prioritize palliative care to the most high‐risk patients. It is worth noting that the Elixhauser score might be difficult to calculate before the discharge of the patient (although hospitals with electronic capture of medical problem lists might be able to approximate it). However, even without the Elixhauser score, the C statistic remained very high at 0.82.
Our study has several limitations. Although we looked at readmissions at 2 other affiliated hospitals, some patients might have been readmitted to other acute care facilities outside our network. However, we would not expect the risk factors in these patients to be so different. The identification of end‐of‐life care issues by medical record review is based on a subjective judgment, although strict criteria were used. Furthermore, differentiation between potentially avoidable readmission and unavoidable readmission cannot be perfect. We used clear and logical criteria that were previously validated and allow large database management. Also, we did not analyze a comprehensive list of potential risk factors. It is probable that functional or cognitive status, for example, could also be important risk factors. We purposely chose a set of variables that could be easily obtained from administrative data sources. The small number of cases may have led to limited statistical power to identify less strongly associated risk factors. Last, the results may not be completely generalizable to small or community hospitals, in particular those that may care for less severely ill cancer patients.
Our findings have important implications. End‐of‐life care issues are not infrequent causes of readmission. Our study's findings could help prioritize palliative care resources to those patients at higher risk to improve the quality of end‐of‐life care. The risk factors identified in this study could be used informally by physicians at the bedside to identify such patients. In addition, a hospital could use these factors to provide a second‐level screen, beyond clinician recognition, to assist palliative care teams to identify patients who may not have otherwise been referred. This screen could be automated, for example, by using a list of medical problems from an electronic medical record to approximate an Elixhauser comorbidity score, or even leaving comorbidities out and simply relying on the other 3 easily identifiable risk factors. Such efforts could have a substantial effect on improving care near the end of life and potentially reducing unnecessary hospitalizations.
Acknowledgements
The authors thank Yves Eggli for having screened the database for potentially avoidable readmission using the algorithm SQLape.
Disclosures: Dr. Donz was supported by the Swiss National Science Foundation and the Swiss Foundation for MedicalBiological Scholarships. The Swiss Science National Foundation and the Swiss Foundation for MedicalBiological Scholarships had no role in the design and conduct of this study, the analysis or interpretation of the data, or the preparation of this manuscript. Dr. Schnipper is a consultant to QuantiaMD, for which he has helped create online educational materials for both providers and patients regarding patient safety, including medication safety during transitions in care. The findings of this study are not a part of those materials. Dr. Schnipper has received grant funding from Sanofi‐Aventis for an investigator‐initiated study to design and evaluate an intensive discharge and follow‐up intervention in patients with diabetes. The funder had had no role in the design of the study.
- , , , . Medicare beneficiaries' costs of care in the last year of life. Health Aff (Millwood). 2001;20(4):188–195.
- , , . Quality of End‐of‐Life Cancer Care for Medicare Beneficiaries: Regional and Hospital‐Specific Analyses. Lebanon, NH: The Dartmouth Institute for Health Policy and Clinical Practice; 2010.
- , , . Repeated hospitalizations predict mortality in the community population with heart failure. Am Heart J. 2007;154(2):260–266.
- . Perspectives on care at the close of life. Initiating end‐of‐life discussions with seriously ill patients: addressing the “elephant in the room.” JAMA. 2000;284(19):2502–2507.
- , , , . Advance care planning as a process: structuring the discussions in practice. J Am Geriatr Soc. 1995;43(4):440–446.
- , , , et al. Rationale and design of the Pharmacist Intervention for Low Literacy in Cardiovascular Disease (PILL‐CVD) study. Circ Cardiovasc Qual Outcomes. 2010;3(2):212–219.
- , , , et al. Effect of a pharmacist intervention on clinically important medication errors after hospital discharge: a randomized trial. Ann Intern Med. 2012;157(1):1–10.
- , , , , , . Measuring potentially avoidable hospital readmissions. J Clin Epidemiol. 2002;55(6):573–587.
- , , , , , . Validation of the potentially avoidable hospital readmission rate as a routine indicator of the quality of hospital care. Med Care. 2006;44(11):972–981.
- , , , et al. Hospital readmission in general medicine patients: a prediction model. J Gen Intern Med. 2010;25(3):211–219.
- , , , , , . Screening elders for risk of hospital admission. J Am Geriatr Soc. 1993;41(8):811–817.
- , , , , . Risk factors for 30‐day hospital readmission in patients ≥65 years of age. Proc (Bayl Univ Med Cent). 2008;21(4):363–372.
- , . Identifying patients in need of a palliative care assessment in the hospital setting: a consensus report from the Center to Advance Palliative Care. J Palliat Med. 2011;14(1):17–23.
- , , , . Potentially avoidable 30‐day hospital readmissions in medical patients: derivation and validation of a prediction model. JAMA Intern Med. 2013;173(8):632–638.
- , , , . Redefining readmission risk factors for general medicine patients. J Hosp Med. 2011;6(2):54–60.
- , , . Patient and disease profile of emergency medical readmissions to an Irish teaching hospital. Postgrad Med J. 2004;80(946):470–474.
- , , , . Posthospital care transitions: patterns, complications, and risk identification. Health Serv Res. 2004;39(5):1449–1465.
- , . Factors predicting readmission of older general medicine patients. J Gen Intern Med. 1991;6(5):389–393.
- , , , , . Differences in health care utilization at the end of life among patients with chronic obstructive pulmonary disease and patients with lung cancer. Arch Intern Med. 2006;166(3):326–331.
- , , , , . Frequent hospital readmissions for acute exacerbation of COPD and their associated factors. Respirology. 2006;11(2):188–195.
- , , , et al. Consensus statement: Palliative and supportive care in advanced heart failure. J Card Fail. 2004;10(3):200–209.
- , , , , . Unplanned discharges from a surgical intensive care unit: readmissions and mortality. J Crit Care. 2010;25(3):375–381.
- , , , , . Evaluating causes for unplanned hospital readmissions of palliative care patients. Am J Hosp Palliat Care. 2010;27(8):526–531.
- , , . 30‐day readmissions among seriously ill older adults. J Palliat Med. 2012;15(12):1356–1361.
- , , , . Medicare beneficiaries' costs of care in the last year of life. Health Aff (Millwood). 2001;20(4):188–195.
- , , . Quality of End‐of‐Life Cancer Care for Medicare Beneficiaries: Regional and Hospital‐Specific Analyses. Lebanon, NH: The Dartmouth Institute for Health Policy and Clinical Practice; 2010.
- , , . Repeated hospitalizations predict mortality in the community population with heart failure. Am Heart J. 2007;154(2):260–266.
- . Perspectives on care at the close of life. Initiating end‐of‐life discussions with seriously ill patients: addressing the “elephant in the room.” JAMA. 2000;284(19):2502–2507.
- , , , . Advance care planning as a process: structuring the discussions in practice. J Am Geriatr Soc. 1995;43(4):440–446.
- , , , et al. Rationale and design of the Pharmacist Intervention for Low Literacy in Cardiovascular Disease (PILL‐CVD) study. Circ Cardiovasc Qual Outcomes. 2010;3(2):212–219.
- , , , et al. Effect of a pharmacist intervention on clinically important medication errors after hospital discharge: a randomized trial. Ann Intern Med. 2012;157(1):1–10.
- , , , , , . Measuring potentially avoidable hospital readmissions. J Clin Epidemiol. 2002;55(6):573–587.
- , , , , , . Validation of the potentially avoidable hospital readmission rate as a routine indicator of the quality of hospital care. Med Care. 2006;44(11):972–981.
- , , , et al. Hospital readmission in general medicine patients: a prediction model. J Gen Intern Med. 2010;25(3):211–219.
- , , , , , . Screening elders for risk of hospital admission. J Am Geriatr Soc. 1993;41(8):811–817.
- , , , , . Risk factors for 30‐day hospital readmission in patients ≥65 years of age. Proc (Bayl Univ Med Cent). 2008;21(4):363–372.
- , . Identifying patients in need of a palliative care assessment in the hospital setting: a consensus report from the Center to Advance Palliative Care. J Palliat Med. 2011;14(1):17–23.
- , , , . Potentially avoidable 30‐day hospital readmissions in medical patients: derivation and validation of a prediction model. JAMA Intern Med. 2013;173(8):632–638.
- , , , . Redefining readmission risk factors for general medicine patients. J Hosp Med. 2011;6(2):54–60.
- , , . Patient and disease profile of emergency medical readmissions to an Irish teaching hospital. Postgrad Med J. 2004;80(946):470–474.
- , , , . Posthospital care transitions: patterns, complications, and risk identification. Health Serv Res. 2004;39(5):1449–1465.
- , . Factors predicting readmission of older general medicine patients. J Gen Intern Med. 1991;6(5):389–393.
- , , , , . Differences in health care utilization at the end of life among patients with chronic obstructive pulmonary disease and patients with lung cancer. Arch Intern Med. 2006;166(3):326–331.
- , , , , . Frequent hospital readmissions for acute exacerbation of COPD and their associated factors. Respirology. 2006;11(2):188–195.
- , , , et al. Consensus statement: Palliative and supportive care in advanced heart failure. J Card Fail. 2004;10(3):200–209.
- , , , , . Unplanned discharges from a surgical intensive care unit: readmissions and mortality. J Crit Care. 2010;25(3):375–381.
- , , , , . Evaluating causes for unplanned hospital readmissions of palliative care patients. Am J Hosp Palliat Care. 2010;27(8):526–531.
- , , . 30‐day readmissions among seriously ill older adults. J Palliat Med. 2012;15(12):1356–1361.
© 2014 Society of Hospital Medicine
Patients at Risk for 30‐Day Readmission
Readmissions to the hospital are common and costly.[1] However, identifying patients prospectively who are likely to be readmitted and who may benefit from interventions to reduce readmission risk has proven challenging, with published risk scores having only moderate ability to discriminate between patients likely and unlikely to be readmitted.[2] One reason for this may be that published studies have not typically focused on patients who are cognitively impaired, psychiatrically ill, have low health or English literacy, or have poor social supports, all of whom may represent a substantial fraction of readmitted patients.[2, 3, 4, 5]
Psychiatric disease, in particular, may contribute to increased readmission risk for nonpsychiatric (medical) illness, and is associated with increased utilization of healthcare resources.[6, 7, 8, 9, 10, 11] For example, patients with mental illness who were discharged from New York hospitals were more likely to be rehospitalized and had more costly readmissions than patients without these comorbidities, including a length of stay nearly 1 day longer on average.[7] An unmet need for treatment of substance abuse was projected to cost Tennessee $772 million of excess healthcare costs in 2000, mostly incurred through repeat hospitalizations and emergency department (ED) visits.[10]
Despite this, few investigators have considered the role of psychiatric disease and/or substance abuse in medical readmission risk. The purpose of the current study was to evaluate the role of psychiatric illness and substance abuse in unselected medical patients to determine their relative contributions to 30‐day all‐cause readmissions (ACR) and potentially avoidable readmissions (PAR).
METHODS
Patients and Setting
We conducted a retrospective cohort study of consecutive adult patients discharged from medicine services at Brigham and Women's Hospital (BWH), a 747‐bed tertiary referral center and teaching hospital, between July 1, 2009 and June 30, 2010. Most patients are cared for by resident housestaff teams at BWH (approximately 25% are cared for by physician assistants working directly with attending physicians), and approximately half receive primary care in the Partners system, which has a shared electronic medical record (EMR). Outpatient mental health services are provided by Partners‐associated mental health professionals including those at McLean Hospital and MassHealth (Medicaid)‐associated sites through the Massachusetts Behavioral Health Partnership. Exclusion criteria were death in the hospital or discharge to another acute care facility. We also excluded patients who left against medical advice (AMA). The study protocol was approved by the Partners Institutional Review Board.
Outcome
The primary outcomes were ACR and PAR within 30 days of discharge. First, we identified all 30‐day readmissions to BWH or to 2 other hospitals in the Partners Healthcare Network (previous studies have shown that 80% of all readmitted patients are readmitted to 1 of these 3 hospitals).[12] For patients with multiple readmissions, only the first readmission was included in the dataset.
To find potentially avoidable readmissions, administrative and billing data for these patients were processed using the SQLape (SQLape s.a.r.l., Corseaux, Switzerland) algorithm, which identifies PAR by excluding patients who undergo planned follow‐up treatment (such as a cycle of planned chemotherapy) or are readmitted for conditions unrelated in any way to the index hospitalization.[13, 14] Common complications of treatment are categorized as potentially avoidable, such as development of a deep venous thrombosis, a decubitus ulcer after prolonged bed rest, or bleeding complications after starting anticoagulation. Although the algorithm identifies theoretically preventable readmissions, the algorithm does not quantify how preventable they are, and these are thus referred to as potentially avoidable. This is similar to other admission metrics, such as the Agency for Healthcare Research and Quality's prevention quality indicators, which are created from a list of ambulatory care‐sensitive conditions.[15] SQLape has the advantage of being a specific tool for readmissions. Patients with 30‐day readmissions identified by SQLape as planned or unlikely to be avoidable were excluded in the PAR analysis, although still included in ACR analysis. In each case, the comparison group is patients without any readmission.
Predictors
Our predictors of interest included the overall prevalence of a psychiatric diagnosis or diagnosis of substance abuse, the presence of specific psychiatric diagnoses, and prescription of psychiatric medications to help assess the independent contribution of these comorbidities to readmission risk.
We used a combination of easily obtainable inpatient and outpatient clinical and administrative data to identify relevant patients. Patients were considered likely to be psychiatrically ill if they: (1) had a psychiatric diagnosis on their Partners outpatient EMR problem list and were prescribed a medication to treat that condition as an outpatient, or (2) had an International Classification of Diseases, 9th Revision diagnosis of a psychiatric illness at hospital discharge. Patients were considered to have moderate probability of disease if they: (1) had a psychiatric diagnosis on their outpatient problem list, or (2) were prescribed a medication intended to treat a psychiatric condition as an outpatient. Patients were considered unlikely to have psychiatric disease if none of these criteria were met. Patients were considered likely to have a substance abuse disorder if they had this diagnosis on their outpatient EMR, or were prescribed a medication to treat this condition (eg, buprenorphine/naloxone), or received inpatient consultation from a substance abuse treatment team during their inpatient hospitalization, and were considered unlikely if none of these were true. We also evaluated individual categories of psychiatric illness (schizophrenia, depression, anxiety, bipolar disorder) and of psychotropic medications (antidepressants, antipsychotics, anxiolytics).
Potential Confounders
Data on potential confounders, based on prior literature,[16, 17] collected at the index admission were derived from electronic administrative, clinical, and billing sources, including the Brigham Integrated Computer System and the Partners Clinical Data Repository. They included patient age, gender, ethnicity, primary language, marital status, insurance status, living situation prior to admission, discharge location, length of stay, Elixhauser comorbidity index,[18] total number of medications prescribed, and number of prior admissions and ED visits in the prior year.
Statistical Analysis
Bivariate comparisons of each of the predictors of ACR and PAR risk (ie, patients with a 30‐day ACR or PAR vs those not readmitted within 30 days) were conducted using 2 trend tests for ordinal predictors (eg, likelihood of psychiatric disease), and 2 or Fisher exact test for dichotomous predictors (eg, receipt of inpatient substance abuse counseling).
We then used multivariate logistic regression analysis to adjust for all of the potential confounders noted above, entering each variable related to psychiatric illness into the model separately (eg, likely psychiatric illness, number of psychiatric medications). In a secondary analysis, we removed potentially collinear variables from the final model; as this did not alter the results, the full model is presented. We also conducted a secondary analysis where we included patients who left against medical advice (AMA), which also did not alter the results. Two‐sided P values <0.05 were considered significant, and all analyses were performed using the SAS version 9.2 (SAS Institute, Inc., Cary, NC).
RESULTS
There were 7984 unique patients discharged during the study period. Patients were generally white and English speaking; just over half of admissions came from the ED (Table 1). Of note, nearly all patients were insured, as are almost all patients in Massachusetts. They had high degrees of comorbid illness and large numbers of prescribed medications. Nearly 30% had at least 1 hospital admission within the prior year.
| Characteristic | All Patients, N (%) | Not Readmitted, N (%) | ACR, N (%) | PAR N (%)a |
|---|---|---|---|---|
| ||||
| Study cohort | 6987 (100) | 5727 (72) | 1260 (18) | 388 (5.6) |
| Age, y | ||||
| <50 | 1663 (23.8) | 1343 (23.5) | 320 (25.4) | 85 (21.9) |
| 5165 | 2273 (32.5) | 1859 (32.5) | 414 (32.9) | 136 (35.1) |
| 6679 | 1444 (20.7) | 1176 (20.5) | 268 (18.6) | 80 (20.6) |
| >80 | 1607 (23.0) | 1349 (23.6) | 258 (16.1) | 87 (22.4) |
| Female | 3604 (51.6) | 2967 (51.8) | 637 (50.6) | 206 (53.1) |
| Race | ||||
| White | 5126 (73.4) | 4153 (72.5) | 973 (77.2) | 300 (77.3) |
| Black | 1075 (15.4) | 899 (15.7) | 176 (14.0) | 53 (13.7) |
| Hispanic | 562 (8.0) | 477 (8.3) | 85 (6.8) | 28 (7.2) |
| Other | 224 (3.2) | 198 (3.5) | 26 (2.1) | 7 (1.8) |
| Primary language | ||||
| English | 6345 (90.8) | 5180 (90.5) | 1165 (92.5) | 356 (91.8) |
| Marital status | ||||
| Married | 3642 (52.1) | 2942 (51.4) | 702 (55.7) | 214 (55.2) |
| Single, never married | 1662 (23.8) | 1393 (24.3) | 269 (21.4) | 73 (18.8) |
| Previously married | 1683 (24.1) | 1386 (24.2) | 289 (22.9) | 101 (26.0) |
| Insurance | ||||
| Medicare | 3550 (50.8) | 2949 (51.5) | 601 (47.7) | 188 (48.5) |
| Medicaid | 539 (7.7) | 430 (7.5) | 109 (8.7) | 33 (8.5) |
| Private | 2892 (41.4) | 2344 (40.9) | 548 (43.5) | 167 (43.0) |
| Uninsured | 6 (0.1) | 4 (0.1) | 2 (0.1) | 0 (0) |
| Source of index admission | ||||
| Clinic or home | 2136 (30.6) | 1711 (29.9) | 425 (33.7) | 117 (30.2) |
| Emergency department | 3592 (51.4) | 2999 (52.4) | 593 (47.1) | 181 (46.7) |
| Nursing facility | 1204 (17.2) | 977 (17.1) | 227 (18.0) | 84 (21.7) |
| Other | 55 (0.1) | 40 (0.7) | 15 (1.1) | 6 (1.6) |
| Length of stay, d | ||||
| 02 | 1757 (25.2) | 1556 (27.2) | 201 (16.0) | 55 (14.2) |
| 34 | 2200 (31.5) | 1842 (32.2) | 358 (28.4) | 105 (27.1) |
| 57 | 1521 (21.8) | 1214 (21.2) | 307 (24.4) | 101 (26.0) |
| >7 | 1509 (21.6) | 1115 (19.5) | 394 (31.3) | 127 (32.7) |
| Elixhauser comorbidity index score | ||||
| 01 | 1987 (28.4) | 1729 (30.2) | 258 (20.5) | 66 (17.0) |
| 27 | 1773 (25.4) | 1541 (26.9) | 232 (18.4) | 67 (17.3) |
| 813 | 1535 (22.0) | 1212 (21.2) | 323 (25.6) | 86 (22.2) |
| >13 | 1692 (24.2) | 1245 (21.7) | 447 (35.5) | 169 (43.6) |
| Medications prescribed as outpatient | ||||
| 06 | 1684 (24.1) | 1410 (24.6) | 274 (21.8) | 72 (18.6) |
| 79 | 1601 (22.9) | 1349 (23.6) | 252 (20.0) | 77 (19.9) |
| 1013 | 1836 (26.3) | 1508 (26.3) | 328 (26.0) | 107 (27.6) |
| >13 | 1866 (26.7) | 1460 (25.5) | 406 (32.2) | 132 (34.0) |
| Number of admissions in past year | ||||
| 0 | 4816 (68.9) | 4032 (70.4) | 784 (62.2) | 279 (71.9) |
| 15 | 2075 (29.7) | 1640 (28.6) | 435 (34.5) | 107 (27.6) |
| >5 | 96 (1.4) | 55 (1.0) | 41 (3.3) | 2 (0.5) |
| Number of ED visits in past year | ||||
| 0 | 4661 (66.7) | 3862 (67.4) | 799 (63.4) | 261 (67.3) |
| 15 | 2326 (33.3) | 1865 (32.6) | 461 (36.6) | 127 (32.7) |
All‐Cause Readmissions
After exclusion of 997 patients who died, were discharged to skilled nursing or rehabilitation facilities, or left AMA, 6987 patients were included (Figure 1). Of these, 1260 had a readmission (18%). Approximately half were considered unlikely to be psychiatrically ill, 22% were considered moderately likely, and 29% likely (Table 2).
| All‐Cause Readmission Analysis | Potentially Avoidable Readmission Analysis | |||||
|---|---|---|---|---|---|---|
| No. in Cohort (%) | % of Patients With ACR | P Valuea | No. in Cohort (%) | % of Patients With PAR | P Valuea | |
| ||||||
| Entire cohort | 6987 | 18.0 | 6115 | 6.3 | ||
| Likelihood of psychiatric illness | ||||||
| Unlikely | 3424 (49) | 16.5 | 3026 (49) | 5.6 | ||
| Moderate | 1564 (22) | 23.5 | 1302 (21) | 7.1 | ||
| Likely | 1999 (29) | 16.4 | 1787 (29) | 6.4 | ||
| Likely versus unlikely | 0.87 | 0.20 | ||||
| Moderate+likely versus unlikely | 0.001 | 0.02 | ||||
| Likelihood of substance abuse | 0.01 | 0.20 | ||||
| Unlikely | 5804 (83) | 18.7 | 5104 (83) | 6.5 | ||
| Likely | 1183 (17) | 14.8 | 1011 (17) | 5.4 | 0.14 | |
| Number of prescribed outpatient psychotropic medications | <0.001 | 0.04 | ||||
| 0 | 4420 (63) | 16.3 | 3931 (64) | 5.9 | ||
| 1 | 1725 (25) | 20.4 | 1481 (24) | 7.2 | ||
| 2 | 781 (11) | 22.3 | 653 (11) | 7.0 | ||
| >2 | 61 (1) | 23.0 | 50 (1) | 6.0 | ||
| Prescribed antidepressant | 1474 (21) | 20.6 | 0.005 | 1248 (20) | 6.2 | 0.77 |
| Prescribed antipsychotic | 375 (5) | 22.4 | 0.02 | 315 (5) | 7.6 | 0.34 |
| Prescribed mood stabilizer | 81 (1) | 18.5 | 0.91 | 69 (1) | 4.4 | 0.49 |
| Prescribed anxiolytic | 1814 (26) | 21.8 | <0.001 | 1537 (25) | 7.7 | 0.01 |
| Prescribed stimulant | 101 (2) | 26.7 | 0.02 | 83 (1) | 10.8 | 0.09 |
| Prescribed pharmacologic treatment for substance abuse | 79 (1) | 25.3 | 0.09 | 60 (1) | 1.7 | 0.14 |
| Number of psychiatric diagnoses on outpatient problem list | 0.31 | 0.74 | ||||
| 0 | 6405 (92) | 18.2 | 5509 (90) | 6.3 | ||
| 1 or more | 582 (8) | 16.5 | 474 (8) | 7.0 | ||
| Outpatient diagnosis of substance abuse | 159 (2) | 13.2 | 0.11 | 144 (2) | 4.2 | 0.28 |
| Outpatient diagnosis of any psychiatric illness | 582 (8) | 16.5 | 0.31 | 517 (8) | 8.0 | 0.73 |
| Discharge diagnosis of depression | 774 (11) | 17.7 | 0.80 | 690 (11) | 7.7 | 0.13 |
| Discharge diagnosis of schizophrenia | 56 (1) | 23.2 | 0.31 | 50 (1) | 14 | 0.03 |
| Discharge diagnosis of bipolar disorder | 101 (1) | 10.9 | 0.06 | 92 (2) | 2.2 | 0.10 |
| Discharge diagnosis of anxiety | 1192 (17) | 15.0 | 0.003 | 1080 (18) | 6.2 | 0.83 |
| Discharge diagnosis of substance abuse | 885 (13) | 14.8 | 0.008 | 803 (13) | 6.1 | 0.76 |
| Discharge diagnosis of any psychiatric illness | 1839 (26) | 16.0 | 0.008 | 1654 (27) | 6.6 | 0.63 |
| Substance abuse consultation as inpatient | 284 (4) | 14.4 | 0.11 | 252 (4) | 3.6 | 0.07 |
In bivariate analysis (Table 2), likelihood of psychiatric illness (P<0.01) and increasing numbers of prescribed outpatient psychiatric medications (P<0.01) were significantly associated with ACR. In multivariate analysis, each additional prescribed outpatient psychiatric medication increased ACR risk (odds ratio [OR]: 1.10, 95% confidence interval [CI]: 1.01‐1.20) or any prescription of an anxiolytic in particular (OR: 1.16, 95% CI: 1.001.35) was associated with increased risk of ACR, whereas discharge diagnoses of anxiety (OR: 0.82, 95% CI: 0.68‐0.99) and substance abuse (OR: 0.80, 95% CI: 0.65‐0.99) were associated with lower risk of ACR (Table 3).
| ACR, OR (95% CI) | PAR, OR (95% CI)a | |
|---|---|---|
| ||
| Likely psychiatric disease | 0.97 (0.82‐1.14) | 1.20 (0.92‐1.56) |
| Likely and possible psychiatric disease | 1.07 (0.94‐1.22) | 1.18 (0.94‐1.47) |
| Likely substance abuse | 0.83 (0.69‐0.99) | 0.85 (0.63‐1.16) |
| Psychiatric diagnosis on outpatient problem list | 0.97 (0.76‐1.23) | 1.04 (0.70‐1.55) |
| Substance abuse diagnosis on outpatient problem list | 0.63 (0.39‐1.02) | 0.65 (0.28‐1.52) |
| Increasing number of prescribed psychiatric medications | 1.10 (1.01‐1.20) | 1.00 (0.86‐1.16) |
| Outpatient prescription for antidepressant | 1.10 (0.94‐1.29) | 0.86 (0.66‐1.13) |
| Outpatient prescription for antipsychotic | 1.03 (0.79‐1.34) | 0.93 (0.59‐1.45) |
| Outpatient prescription for anxiolytic | 1.16 (1.001.35) | 1.13 (0.88‐1.44) |
| Outpatient prescription for methadone or buprenorphine | 1.15 (0.67‐1.98) | 0.18 (0.03‐1.36) |
| Discharge diagnosis of depression | 1.06 (0.86‐1.30) | 1.49 (1.09‐2.04) |
| Discharge diagnosis of schizophrenia | 1.43 (0.75‐2.74) | 2.63 (1.13‐6.13) |
| Discharge diagnosis of bipolar disorder | 0.53 (0.28‐1.02) | 0.35 (0.09‐1.45) |
| Discharge diagnosis of anxiety | 0.82 (0.68‐0.99) | 1.11 (0.83‐1.49) |
| Discharge diagnosis of substance abuse | 0.80 (0.65‐0.99) | 1.05 (0.75‐1.46) |
| Discharge diagnosis of any psychiatric illness | 0.88 (0.75‐1.02) | 1.22 (0.96‐1.56) |
| Addiction team consult while inpatient | 0.82 (0.58‐1.17) | 0.58 (0.29‐1.17) |
Potentially Avoidable Readmissions
After further exclusion of 872 patients who had unavoidable readmissions according to the SQLape algorithm, 6115 patients remained. Of these, 388 had a PAR within 30 days (6.3%, Table 1).
In bivariate analysis (Table 2), the likelihood of psychiatric illness (P=0.02), number of outpatient psychiatric medications (P=0.04), and prescription of anxiolytics (P=0.01) were significantly associated with PAR, as they were with ACR. A discharge diagnosis of schizophrenia was also associated with PAR (P=0.03).
In multivariate analysis, only discharge diagnoses of depression (OR: 1.49, 95% CI: 1.09‐2.04) and schizophrenia (OR: 2.63, 95% CI: 1.13‐6.13) were associated with PAR.
DISCUSSION
Comorbid psychiatric illness was common among patients admitted to the medicine wards. Patients with documented discharge diagnoses of depression or schizophrenia were at highest risk for a potentially avoidable 30‐day readmission, whereas those prescribed more psychiatric medications were at increased risk for ACR. These findings were independent of a comprehensive set of risk factors among medicine inpatients in this retrospective cohort study.
This study extends prior work indicating patients with psychiatric disease have increased healthcare utilization,[6, 7, 8, 9, 10, 11] by identifying at least 2 subpopulations of the psychiatrically ill (those with depression and schizophrenia) at particularly high risk for 30‐day PAR. To our knowledge, this is the first study to identify schizophrenia as a predictor of hospital readmission for medical illnesses. One prior study prospectively identified depression as increasing the 90‐day risk of readmission 3‐fold, although medication usage was not assessed,[6] and our report strengthens this association.
There are several possible explanations why these two subpopulations in particular would be more predisposed to readmissions that are potentially avoidable. It is known that patients with schizophrenia, for example, live on average 20 years less than the general population, and most of this excess mortality is due to medical illnesses.[20, 21] Reasons for this may include poor healthcare access, adverse effects of medication, and socioeconomic factors among others.[21, 22] All of these reasons may contribute to the increased PAR risk in this population, mediated, for example, by decreased ability to adhere to postdischarge care plans. Successful community‐based interventions to decrease these inequities have been described and could serve as a model for addressing the increased readmission risk in this population.[23]
Our finding that patients with a greater number of prescribed psychiatric medications are at increased risk for ACR may be expected, given other studies that have highlighted the crucial importance of medications in postdischarge adverse events, including readmissions.[24] Indeed, medication‐related errors and toxicities are the most common postdischarge adverse events experienced by patients.[25] Whether psychiatric medications are particularly prone to causing postdischarge adverse events or whether these medications represent greater psychiatric comorbidity cannot be answered by this study.
It was surprising but reassuring that substance abuse was not a predictor of short‐term readmissions as identified using our measures; in fact, a discharge diagnosis of substance abuse was associated with lower risk of ACR than comparator patients. It seems unlikely that we would have inadequate power to find such a result, as we found a statistically significant negative association in the ACR population, and 17% of our population overall was considered likely to have a substance abuse comorbidity. However, it is likely the burden of disease was underestimated given that we did not try to determine the contribution of long‐term substance abuse to medical diseases that may increase readmission risk (eg, liver cirrhosis from alcohol use). Unlike other conditions in our study, patients with substance abuse diagnoses at BWH can be seen by a dedicated multidisciplinary team while an inpatient to start treatment and plan for postdischarge follow‐up; this may have played a role in our findings.
A discharge diagnosis of anxiety was also somewhat protective against readmission, whereas a prescription of an anxiolytic (predominantly benzodiazepines) increased risk; many patients prescribed a benzodiazepine do not have a Diagnostic and Statistical Manual of Mental Disorders4th Edition (DSM‐IV) diagnosis of anxiety disorder, and thus these findings may reflect different patient populations. Discharging physicians may have used anxiety as a discharge diagnosis in patients in whom they suspected somatic complaints without organic basis; these patients may be at lower risk of readmission.
Discharge diagnoses of psychiatric illnesses were associated with ACR and PAR in our study, but outpatient diagnoses were not. This likely reflects greater severity of illness (documentation as a treated diagnosis on discharge indicates the illness was relevant during the hospitalization), but may also reflect inaccuracies of diagnosis and lack of assessment of severity in outpatient coding, which would bias toward null findings. Although many of the patients in our study were seen by primary care doctors within the Partners system, some patients had outside primary care physicians and we did not have access to these records. This may also have decreased our ability to find associations.
The findings of our study should be interpreted in the context of the study design. Our study was retrospective, which limited our ability to conclusively diagnose psychiatric disease presence or severity (as is true of most institutions, validated psychiatric screening was not routinely used at our institutions on hospital admission or discharge). However, we used a conservative scale to classify the likelihood of patients having psychiatric or substance abuse disorders, and we used other metrics to establish the presence of illness, such as the number of prescribed medications, inpatient consultation with a substance abuse service, and hospital discharge diagnoses. This approach also allowed us to quickly identify a large cohort unaffected by selection bias. Our study was single center, potentially limiting generalizability. Although we capture at least 80% of readmissions, we were not able to capture all readmissions, and we cannot rule out that patients readmitted elsewhere are different than those readmitted within the Partners system. Last, the SQLape algorithm is not perfectly sensitive or specific in identifying avoidable readmissions,[13] but it does eliminate many readmissions that are clearly unavoidable, creating an enriched cohort of patients whose readmissions are more likely to be avoidable and therefore potentially actionable.
We suggest that our study findings first be considered when risk stratifying patients before hospital discharge in terms of readmission risk. Patients with depression and schizophrenia would seem to merit postdischarge interventions to decrease their potentially avoidable readmissions. Compulsory community treatment (a feature of treatment in Canada and Australia that is ordered by clinicians) has been shown to decrease mortality due to medical illness in patients who have been hospitalized and are psychiatrically ill, and addition of these services to postdischarge care may be useful.[23] Inpatient physicians could work to ensure follow‐up not just with medical providers but with robust outpatient mental health programs to decrease potentially avoidable readmission risk, and administrators could work to ensure close linkages with these community resources. Studies evaluating the impact of these types of interventions would need to be conducted. Patients with polypharmacy, including psychiatric medications, may benefit from interventions to improve medication safety, such as enhanced medication reconciliation and pharmacist counseling.[26]
Our study suggests that patients with depression, those with schizophrenia, and those who have increased numbers of prescribed psychiatric medications should be considered at high risk for readmission for medical illnesses. Targeting interventions to these patients may be fruitful in preventing avoidable readmissions.
Acknowledgements
The authors thank Dr. Yves Eggli for screening the database for potentially avoidable readmissions using the SQLape algorithm.
Disclosures
Dr. Donz was supported by the Swiss National Science Foundation and the Swiss Foundation for MedicalBiological Scholarships. The authors otherwise have no conflicts of interest to disclose. The content is solely the responsibility of the authors and does not necessarily represent the official views of the US Department of Veterans Affairs.
- , , . Rehospitalizations among patients in the Medicare fee‐for‐service program. N Engl J Med. 2009;360(14):1418–1428.
- , , , et al. Risk prediction models for hospital readmission: a systematic review. JAMA. 2011;306(15):1688–1698.
- , . Hospital readmission as an accountability measure. JAMA. 2011;305(5):504–505.
- , , , , , . Postdischarge environmental and socioeconomic factors and the likelihood of early hospital readmission among community‐dwelling Medicare beneficiaries. Gerontologist. 2008;48(4):495–504.
- , , , , . Low health literacy and health outcomes: an updated systematic review. Ann Intern Med. 2011;155(2):97–107.
- , , , et al. Depression is a risk factor for rehospitalization in medical inpatients. Prim Care Companion J Clin Psychiatry. 2007;9(4):256–262.
- , , , . Mental illness and hospitalization for ambulatory care sensitive medical conditions. Med Care. 2008;46(12):1249–1256.
- , , , , , . Substance use treatment barriers for patients with frequent hospital admissions. J Subst Abuse Treat. 2010;38(1):22–30.
- , , , , . Managed care and the quality of substance abuse treatment. J Ment Health Policy Econ. 2002;5(4):163–174.
- , , , , . Unmet substance abuse treatment need, health services utilization, and cost: a population‐based emergency department study. Ann Emerg Med. 2005;45(2):118–127.
- , , , . Elderly Medicare inpatients with substance use disorders: characteristics and predictors of hospital readmissions over a four‐year interval. J Stud Alcohol. 2000;61(6):891–895.
- , , , et al. Rationale and design of the Pharmacist Intervention for Low Literacy in Cardiovascular Disease (PILL‐CVD) study. Circ Cardiovasc Qual Outcomes. 2010;3(2):212–219.
- , , , , , . Validation of the potentially avoidable hospital readmission rate as a routine indicator of the quality of hospital care. Med Care. 2006;44(11);972–981.
- , , , , , . Measuring potentially avoidable hospital readmissions. J Clin Epidemiol. 2002;55:573–587.
- Agency for Healthcare Research and Quality Quality Indicators. (April 7, 2006). Prevention Quality Indicators (PQI) Composite Measure Workgroup Final Report. Available at: http://www.qualityindicators.ahrq.gov/modules/pqi_resources.aspx. Accessed June 1, 2012.
- , , , et al. Hospital readmission in general medicine patients: a prediction model. J Gen Intern Med. 2010;25(3):211–219.
- , , , et al. Derivation and validation of an index to predict early death or unplanned readmission after discharge from hospital to the community. CMAJ. 2010;182(6):551–557.
- , , , . Comorbidity measures for use with administrative data. Med Care. Jan 1998;36(1):8–27.
- , , , , eds. Morbidity and mortality in people with serious mental illness. October 2006. National Association of State Mental Health Directors, Medical Directors Council. Available at: http://www.nasmhpd.org/docs/publications/MDCdocs/Mortality%20and%20 Mo rbidity%20Final%20Report%208.18.08.pdf. Accessed January 13, 2013.
- , , , . Mortality in individuals who have had psychiatric treatment: population‐based study in Nova Scotia. Br J Psychiat. 2005;187:552–558.
- , , , , , . Inequitable access for mentally ill patients to some medically necessary procedures. CMAJ. 2007;176(6):779–784.
- , , . Quality of medical care for people with and without comorbid mental illness and substance misuse: systematic review of comparative studies. Br J Psychiat. 2009;194:491–499.
- , , , , , . Reducing all‐cause mortality among patients with psychiatric disorders: a population‐based study. CMAJ. 2013;185(1):E50–E56.
- , , , et al. Incidence of potentially avoidable urgent readmissions and their relation to all‐cause urgent readmissions. CMAJ. 2011;183(14):E1067–E1072.
- , , , , . The incidence and severity of adverse events affecting patients after discharge from the hospital. Ann Intern Med. 2003;138(3):161–167.
- , , , . Hospital‐based medication reconciliation practices: a systematic review. Arch Intern Med. 2012;172(14):1057–1069.
Readmissions to the hospital are common and costly.[1] However, identifying patients prospectively who are likely to be readmitted and who may benefit from interventions to reduce readmission risk has proven challenging, with published risk scores having only moderate ability to discriminate between patients likely and unlikely to be readmitted.[2] One reason for this may be that published studies have not typically focused on patients who are cognitively impaired, psychiatrically ill, have low health or English literacy, or have poor social supports, all of whom may represent a substantial fraction of readmitted patients.[2, 3, 4, 5]
Psychiatric disease, in particular, may contribute to increased readmission risk for nonpsychiatric (medical) illness, and is associated with increased utilization of healthcare resources.[6, 7, 8, 9, 10, 11] For example, patients with mental illness who were discharged from New York hospitals were more likely to be rehospitalized and had more costly readmissions than patients without these comorbidities, including a length of stay nearly 1 day longer on average.[7] An unmet need for treatment of substance abuse was projected to cost Tennessee $772 million of excess healthcare costs in 2000, mostly incurred through repeat hospitalizations and emergency department (ED) visits.[10]
Despite this, few investigators have considered the role of psychiatric disease and/or substance abuse in medical readmission risk. The purpose of the current study was to evaluate the role of psychiatric illness and substance abuse in unselected medical patients to determine their relative contributions to 30‐day all‐cause readmissions (ACR) and potentially avoidable readmissions (PAR).
METHODS
Patients and Setting
We conducted a retrospective cohort study of consecutive adult patients discharged from medicine services at Brigham and Women's Hospital (BWH), a 747‐bed tertiary referral center and teaching hospital, between July 1, 2009 and June 30, 2010. Most patients are cared for by resident housestaff teams at BWH (approximately 25% are cared for by physician assistants working directly with attending physicians), and approximately half receive primary care in the Partners system, which has a shared electronic medical record (EMR). Outpatient mental health services are provided by Partners‐associated mental health professionals including those at McLean Hospital and MassHealth (Medicaid)‐associated sites through the Massachusetts Behavioral Health Partnership. Exclusion criteria were death in the hospital or discharge to another acute care facility. We also excluded patients who left against medical advice (AMA). The study protocol was approved by the Partners Institutional Review Board.
Outcome
The primary outcomes were ACR and PAR within 30 days of discharge. First, we identified all 30‐day readmissions to BWH or to 2 other hospitals in the Partners Healthcare Network (previous studies have shown that 80% of all readmitted patients are readmitted to 1 of these 3 hospitals).[12] For patients with multiple readmissions, only the first readmission was included in the dataset.
To find potentially avoidable readmissions, administrative and billing data for these patients were processed using the SQLape (SQLape s.a.r.l., Corseaux, Switzerland) algorithm, which identifies PAR by excluding patients who undergo planned follow‐up treatment (such as a cycle of planned chemotherapy) or are readmitted for conditions unrelated in any way to the index hospitalization.[13, 14] Common complications of treatment are categorized as potentially avoidable, such as development of a deep venous thrombosis, a decubitus ulcer after prolonged bed rest, or bleeding complications after starting anticoagulation. Although the algorithm identifies theoretically preventable readmissions, the algorithm does not quantify how preventable they are, and these are thus referred to as potentially avoidable. This is similar to other admission metrics, such as the Agency for Healthcare Research and Quality's prevention quality indicators, which are created from a list of ambulatory care‐sensitive conditions.[15] SQLape has the advantage of being a specific tool for readmissions. Patients with 30‐day readmissions identified by SQLape as planned or unlikely to be avoidable were excluded in the PAR analysis, although still included in ACR analysis. In each case, the comparison group is patients without any readmission.
Predictors
Our predictors of interest included the overall prevalence of a psychiatric diagnosis or diagnosis of substance abuse, the presence of specific psychiatric diagnoses, and prescription of psychiatric medications to help assess the independent contribution of these comorbidities to readmission risk.
We used a combination of easily obtainable inpatient and outpatient clinical and administrative data to identify relevant patients. Patients were considered likely to be psychiatrically ill if they: (1) had a psychiatric diagnosis on their Partners outpatient EMR problem list and were prescribed a medication to treat that condition as an outpatient, or (2) had an International Classification of Diseases, 9th Revision diagnosis of a psychiatric illness at hospital discharge. Patients were considered to have moderate probability of disease if they: (1) had a psychiatric diagnosis on their outpatient problem list, or (2) were prescribed a medication intended to treat a psychiatric condition as an outpatient. Patients were considered unlikely to have psychiatric disease if none of these criteria were met. Patients were considered likely to have a substance abuse disorder if they had this diagnosis on their outpatient EMR, or were prescribed a medication to treat this condition (eg, buprenorphine/naloxone), or received inpatient consultation from a substance abuse treatment team during their inpatient hospitalization, and were considered unlikely if none of these were true. We also evaluated individual categories of psychiatric illness (schizophrenia, depression, anxiety, bipolar disorder) and of psychotropic medications (antidepressants, antipsychotics, anxiolytics).
Potential Confounders
Data on potential confounders, based on prior literature,[16, 17] collected at the index admission were derived from electronic administrative, clinical, and billing sources, including the Brigham Integrated Computer System and the Partners Clinical Data Repository. They included patient age, gender, ethnicity, primary language, marital status, insurance status, living situation prior to admission, discharge location, length of stay, Elixhauser comorbidity index,[18] total number of medications prescribed, and number of prior admissions and ED visits in the prior year.
Statistical Analysis
Bivariate comparisons of each of the predictors of ACR and PAR risk (ie, patients with a 30‐day ACR or PAR vs those not readmitted within 30 days) were conducted using 2 trend tests for ordinal predictors (eg, likelihood of psychiatric disease), and 2 or Fisher exact test for dichotomous predictors (eg, receipt of inpatient substance abuse counseling).
We then used multivariate logistic regression analysis to adjust for all of the potential confounders noted above, entering each variable related to psychiatric illness into the model separately (eg, likely psychiatric illness, number of psychiatric medications). In a secondary analysis, we removed potentially collinear variables from the final model; as this did not alter the results, the full model is presented. We also conducted a secondary analysis where we included patients who left against medical advice (AMA), which also did not alter the results. Two‐sided P values <0.05 were considered significant, and all analyses were performed using the SAS version 9.2 (SAS Institute, Inc., Cary, NC).
RESULTS
There were 7984 unique patients discharged during the study period. Patients were generally white and English speaking; just over half of admissions came from the ED (Table 1). Of note, nearly all patients were insured, as are almost all patients in Massachusetts. They had high degrees of comorbid illness and large numbers of prescribed medications. Nearly 30% had at least 1 hospital admission within the prior year.
| Characteristic | All Patients, N (%) | Not Readmitted, N (%) | ACR, N (%) | PAR N (%)a |
|---|---|---|---|---|
| ||||
| Study cohort | 6987 (100) | 5727 (72) | 1260 (18) | 388 (5.6) |
| Age, y | ||||
| <50 | 1663 (23.8) | 1343 (23.5) | 320 (25.4) | 85 (21.9) |
| 5165 | 2273 (32.5) | 1859 (32.5) | 414 (32.9) | 136 (35.1) |
| 6679 | 1444 (20.7) | 1176 (20.5) | 268 (18.6) | 80 (20.6) |
| >80 | 1607 (23.0) | 1349 (23.6) | 258 (16.1) | 87 (22.4) |
| Female | 3604 (51.6) | 2967 (51.8) | 637 (50.6) | 206 (53.1) |
| Race | ||||
| White | 5126 (73.4) | 4153 (72.5) | 973 (77.2) | 300 (77.3) |
| Black | 1075 (15.4) | 899 (15.7) | 176 (14.0) | 53 (13.7) |
| Hispanic | 562 (8.0) | 477 (8.3) | 85 (6.8) | 28 (7.2) |
| Other | 224 (3.2) | 198 (3.5) | 26 (2.1) | 7 (1.8) |
| Primary language | ||||
| English | 6345 (90.8) | 5180 (90.5) | 1165 (92.5) | 356 (91.8) |
| Marital status | ||||
| Married | 3642 (52.1) | 2942 (51.4) | 702 (55.7) | 214 (55.2) |
| Single, never married | 1662 (23.8) | 1393 (24.3) | 269 (21.4) | 73 (18.8) |
| Previously married | 1683 (24.1) | 1386 (24.2) | 289 (22.9) | 101 (26.0) |
| Insurance | ||||
| Medicare | 3550 (50.8) | 2949 (51.5) | 601 (47.7) | 188 (48.5) |
| Medicaid | 539 (7.7) | 430 (7.5) | 109 (8.7) | 33 (8.5) |
| Private | 2892 (41.4) | 2344 (40.9) | 548 (43.5) | 167 (43.0) |
| Uninsured | 6 (0.1) | 4 (0.1) | 2 (0.1) | 0 (0) |
| Source of index admission | ||||
| Clinic or home | 2136 (30.6) | 1711 (29.9) | 425 (33.7) | 117 (30.2) |
| Emergency department | 3592 (51.4) | 2999 (52.4) | 593 (47.1) | 181 (46.7) |
| Nursing facility | 1204 (17.2) | 977 (17.1) | 227 (18.0) | 84 (21.7) |
| Other | 55 (0.1) | 40 (0.7) | 15 (1.1) | 6 (1.6) |
| Length of stay, d | ||||
| 02 | 1757 (25.2) | 1556 (27.2) | 201 (16.0) | 55 (14.2) |
| 34 | 2200 (31.5) | 1842 (32.2) | 358 (28.4) | 105 (27.1) |
| 57 | 1521 (21.8) | 1214 (21.2) | 307 (24.4) | 101 (26.0) |
| >7 | 1509 (21.6) | 1115 (19.5) | 394 (31.3) | 127 (32.7) |
| Elixhauser comorbidity index score | ||||
| 01 | 1987 (28.4) | 1729 (30.2) | 258 (20.5) | 66 (17.0) |
| 27 | 1773 (25.4) | 1541 (26.9) | 232 (18.4) | 67 (17.3) |
| 813 | 1535 (22.0) | 1212 (21.2) | 323 (25.6) | 86 (22.2) |
| >13 | 1692 (24.2) | 1245 (21.7) | 447 (35.5) | 169 (43.6) |
| Medications prescribed as outpatient | ||||
| 06 | 1684 (24.1) | 1410 (24.6) | 274 (21.8) | 72 (18.6) |
| 79 | 1601 (22.9) | 1349 (23.6) | 252 (20.0) | 77 (19.9) |
| 1013 | 1836 (26.3) | 1508 (26.3) | 328 (26.0) | 107 (27.6) |
| >13 | 1866 (26.7) | 1460 (25.5) | 406 (32.2) | 132 (34.0) |
| Number of admissions in past year | ||||
| 0 | 4816 (68.9) | 4032 (70.4) | 784 (62.2) | 279 (71.9) |
| 15 | 2075 (29.7) | 1640 (28.6) | 435 (34.5) | 107 (27.6) |
| >5 | 96 (1.4) | 55 (1.0) | 41 (3.3) | 2 (0.5) |
| Number of ED visits in past year | ||||
| 0 | 4661 (66.7) | 3862 (67.4) | 799 (63.4) | 261 (67.3) |
| 15 | 2326 (33.3) | 1865 (32.6) | 461 (36.6) | 127 (32.7) |
All‐Cause Readmissions
After exclusion of 997 patients who died, were discharged to skilled nursing or rehabilitation facilities, or left AMA, 6987 patients were included (Figure 1). Of these, 1260 had a readmission (18%). Approximately half were considered unlikely to be psychiatrically ill, 22% were considered moderately likely, and 29% likely (Table 2).
| All‐Cause Readmission Analysis | Potentially Avoidable Readmission Analysis | |||||
|---|---|---|---|---|---|---|
| No. in Cohort (%) | % of Patients With ACR | P Valuea | No. in Cohort (%) | % of Patients With PAR | P Valuea | |
| ||||||
| Entire cohort | 6987 | 18.0 | 6115 | 6.3 | ||
| Likelihood of psychiatric illness | ||||||
| Unlikely | 3424 (49) | 16.5 | 3026 (49) | 5.6 | ||
| Moderate | 1564 (22) | 23.5 | 1302 (21) | 7.1 | ||
| Likely | 1999 (29) | 16.4 | 1787 (29) | 6.4 | ||
| Likely versus unlikely | 0.87 | 0.20 | ||||
| Moderate+likely versus unlikely | 0.001 | 0.02 | ||||
| Likelihood of substance abuse | 0.01 | 0.20 | ||||
| Unlikely | 5804 (83) | 18.7 | 5104 (83) | 6.5 | ||
| Likely | 1183 (17) | 14.8 | 1011 (17) | 5.4 | 0.14 | |
| Number of prescribed outpatient psychotropic medications | <0.001 | 0.04 | ||||
| 0 | 4420 (63) | 16.3 | 3931 (64) | 5.9 | ||
| 1 | 1725 (25) | 20.4 | 1481 (24) | 7.2 | ||
| 2 | 781 (11) | 22.3 | 653 (11) | 7.0 | ||
| >2 | 61 (1) | 23.0 | 50 (1) | 6.0 | ||
| Prescribed antidepressant | 1474 (21) | 20.6 | 0.005 | 1248 (20) | 6.2 | 0.77 |
| Prescribed antipsychotic | 375 (5) | 22.4 | 0.02 | 315 (5) | 7.6 | 0.34 |
| Prescribed mood stabilizer | 81 (1) | 18.5 | 0.91 | 69 (1) | 4.4 | 0.49 |
| Prescribed anxiolytic | 1814 (26) | 21.8 | <0.001 | 1537 (25) | 7.7 | 0.01 |
| Prescribed stimulant | 101 (2) | 26.7 | 0.02 | 83 (1) | 10.8 | 0.09 |
| Prescribed pharmacologic treatment for substance abuse | 79 (1) | 25.3 | 0.09 | 60 (1) | 1.7 | 0.14 |
| Number of psychiatric diagnoses on outpatient problem list | 0.31 | 0.74 | ||||
| 0 | 6405 (92) | 18.2 | 5509 (90) | 6.3 | ||
| 1 or more | 582 (8) | 16.5 | 474 (8) | 7.0 | ||
| Outpatient diagnosis of substance abuse | 159 (2) | 13.2 | 0.11 | 144 (2) | 4.2 | 0.28 |
| Outpatient diagnosis of any psychiatric illness | 582 (8) | 16.5 | 0.31 | 517 (8) | 8.0 | 0.73 |
| Discharge diagnosis of depression | 774 (11) | 17.7 | 0.80 | 690 (11) | 7.7 | 0.13 |
| Discharge diagnosis of schizophrenia | 56 (1) | 23.2 | 0.31 | 50 (1) | 14 | 0.03 |
| Discharge diagnosis of bipolar disorder | 101 (1) | 10.9 | 0.06 | 92 (2) | 2.2 | 0.10 |
| Discharge diagnosis of anxiety | 1192 (17) | 15.0 | 0.003 | 1080 (18) | 6.2 | 0.83 |
| Discharge diagnosis of substance abuse | 885 (13) | 14.8 | 0.008 | 803 (13) | 6.1 | 0.76 |
| Discharge diagnosis of any psychiatric illness | 1839 (26) | 16.0 | 0.008 | 1654 (27) | 6.6 | 0.63 |
| Substance abuse consultation as inpatient | 284 (4) | 14.4 | 0.11 | 252 (4) | 3.6 | 0.07 |
In bivariate analysis (Table 2), likelihood of psychiatric illness (P<0.01) and increasing numbers of prescribed outpatient psychiatric medications (P<0.01) were significantly associated with ACR. In multivariate analysis, each additional prescribed outpatient psychiatric medication increased ACR risk (odds ratio [OR]: 1.10, 95% confidence interval [CI]: 1.01‐1.20) or any prescription of an anxiolytic in particular (OR: 1.16, 95% CI: 1.001.35) was associated with increased risk of ACR, whereas discharge diagnoses of anxiety (OR: 0.82, 95% CI: 0.68‐0.99) and substance abuse (OR: 0.80, 95% CI: 0.65‐0.99) were associated with lower risk of ACR (Table 3).
| ACR, OR (95% CI) | PAR, OR (95% CI)a | |
|---|---|---|
| ||
| Likely psychiatric disease | 0.97 (0.82‐1.14) | 1.20 (0.92‐1.56) |
| Likely and possible psychiatric disease | 1.07 (0.94‐1.22) | 1.18 (0.94‐1.47) |
| Likely substance abuse | 0.83 (0.69‐0.99) | 0.85 (0.63‐1.16) |
| Psychiatric diagnosis on outpatient problem list | 0.97 (0.76‐1.23) | 1.04 (0.70‐1.55) |
| Substance abuse diagnosis on outpatient problem list | 0.63 (0.39‐1.02) | 0.65 (0.28‐1.52) |
| Increasing number of prescribed psychiatric medications | 1.10 (1.01‐1.20) | 1.00 (0.86‐1.16) |
| Outpatient prescription for antidepressant | 1.10 (0.94‐1.29) | 0.86 (0.66‐1.13) |
| Outpatient prescription for antipsychotic | 1.03 (0.79‐1.34) | 0.93 (0.59‐1.45) |
| Outpatient prescription for anxiolytic | 1.16 (1.001.35) | 1.13 (0.88‐1.44) |
| Outpatient prescription for methadone or buprenorphine | 1.15 (0.67‐1.98) | 0.18 (0.03‐1.36) |
| Discharge diagnosis of depression | 1.06 (0.86‐1.30) | 1.49 (1.09‐2.04) |
| Discharge diagnosis of schizophrenia | 1.43 (0.75‐2.74) | 2.63 (1.13‐6.13) |
| Discharge diagnosis of bipolar disorder | 0.53 (0.28‐1.02) | 0.35 (0.09‐1.45) |
| Discharge diagnosis of anxiety | 0.82 (0.68‐0.99) | 1.11 (0.83‐1.49) |
| Discharge diagnosis of substance abuse | 0.80 (0.65‐0.99) | 1.05 (0.75‐1.46) |
| Discharge diagnosis of any psychiatric illness | 0.88 (0.75‐1.02) | 1.22 (0.96‐1.56) |
| Addiction team consult while inpatient | 0.82 (0.58‐1.17) | 0.58 (0.29‐1.17) |
Potentially Avoidable Readmissions
After further exclusion of 872 patients who had unavoidable readmissions according to the SQLape algorithm, 6115 patients remained. Of these, 388 had a PAR within 30 days (6.3%, Table 1).
In bivariate analysis (Table 2), the likelihood of psychiatric illness (P=0.02), number of outpatient psychiatric medications (P=0.04), and prescription of anxiolytics (P=0.01) were significantly associated with PAR, as they were with ACR. A discharge diagnosis of schizophrenia was also associated with PAR (P=0.03).
In multivariate analysis, only discharge diagnoses of depression (OR: 1.49, 95% CI: 1.09‐2.04) and schizophrenia (OR: 2.63, 95% CI: 1.13‐6.13) were associated with PAR.
DISCUSSION
Comorbid psychiatric illness was common among patients admitted to the medicine wards. Patients with documented discharge diagnoses of depression or schizophrenia were at highest risk for a potentially avoidable 30‐day readmission, whereas those prescribed more psychiatric medications were at increased risk for ACR. These findings were independent of a comprehensive set of risk factors among medicine inpatients in this retrospective cohort study.
This study extends prior work indicating patients with psychiatric disease have increased healthcare utilization,[6, 7, 8, 9, 10, 11] by identifying at least 2 subpopulations of the psychiatrically ill (those with depression and schizophrenia) at particularly high risk for 30‐day PAR. To our knowledge, this is the first study to identify schizophrenia as a predictor of hospital readmission for medical illnesses. One prior study prospectively identified depression as increasing the 90‐day risk of readmission 3‐fold, although medication usage was not assessed,[6] and our report strengthens this association.
There are several possible explanations why these two subpopulations in particular would be more predisposed to readmissions that are potentially avoidable. It is known that patients with schizophrenia, for example, live on average 20 years less than the general population, and most of this excess mortality is due to medical illnesses.[20, 21] Reasons for this may include poor healthcare access, adverse effects of medication, and socioeconomic factors among others.[21, 22] All of these reasons may contribute to the increased PAR risk in this population, mediated, for example, by decreased ability to adhere to postdischarge care plans. Successful community‐based interventions to decrease these inequities have been described and could serve as a model for addressing the increased readmission risk in this population.[23]
Our finding that patients with a greater number of prescribed psychiatric medications are at increased risk for ACR may be expected, given other studies that have highlighted the crucial importance of medications in postdischarge adverse events, including readmissions.[24] Indeed, medication‐related errors and toxicities are the most common postdischarge adverse events experienced by patients.[25] Whether psychiatric medications are particularly prone to causing postdischarge adverse events or whether these medications represent greater psychiatric comorbidity cannot be answered by this study.
It was surprising but reassuring that substance abuse was not a predictor of short‐term readmissions as identified using our measures; in fact, a discharge diagnosis of substance abuse was associated with lower risk of ACR than comparator patients. It seems unlikely that we would have inadequate power to find such a result, as we found a statistically significant negative association in the ACR population, and 17% of our population overall was considered likely to have a substance abuse comorbidity. However, it is likely the burden of disease was underestimated given that we did not try to determine the contribution of long‐term substance abuse to medical diseases that may increase readmission risk (eg, liver cirrhosis from alcohol use). Unlike other conditions in our study, patients with substance abuse diagnoses at BWH can be seen by a dedicated multidisciplinary team while an inpatient to start treatment and plan for postdischarge follow‐up; this may have played a role in our findings.
A discharge diagnosis of anxiety was also somewhat protective against readmission, whereas a prescription of an anxiolytic (predominantly benzodiazepines) increased risk; many patients prescribed a benzodiazepine do not have a Diagnostic and Statistical Manual of Mental Disorders4th Edition (DSM‐IV) diagnosis of anxiety disorder, and thus these findings may reflect different patient populations. Discharging physicians may have used anxiety as a discharge diagnosis in patients in whom they suspected somatic complaints without organic basis; these patients may be at lower risk of readmission.
Discharge diagnoses of psychiatric illnesses were associated with ACR and PAR in our study, but outpatient diagnoses were not. This likely reflects greater severity of illness (documentation as a treated diagnosis on discharge indicates the illness was relevant during the hospitalization), but may also reflect inaccuracies of diagnosis and lack of assessment of severity in outpatient coding, which would bias toward null findings. Although many of the patients in our study were seen by primary care doctors within the Partners system, some patients had outside primary care physicians and we did not have access to these records. This may also have decreased our ability to find associations.
The findings of our study should be interpreted in the context of the study design. Our study was retrospective, which limited our ability to conclusively diagnose psychiatric disease presence or severity (as is true of most institutions, validated psychiatric screening was not routinely used at our institutions on hospital admission or discharge). However, we used a conservative scale to classify the likelihood of patients having psychiatric or substance abuse disorders, and we used other metrics to establish the presence of illness, such as the number of prescribed medications, inpatient consultation with a substance abuse service, and hospital discharge diagnoses. This approach also allowed us to quickly identify a large cohort unaffected by selection bias. Our study was single center, potentially limiting generalizability. Although we capture at least 80% of readmissions, we were not able to capture all readmissions, and we cannot rule out that patients readmitted elsewhere are different than those readmitted within the Partners system. Last, the SQLape algorithm is not perfectly sensitive or specific in identifying avoidable readmissions,[13] but it does eliminate many readmissions that are clearly unavoidable, creating an enriched cohort of patients whose readmissions are more likely to be avoidable and therefore potentially actionable.
We suggest that our study findings first be considered when risk stratifying patients before hospital discharge in terms of readmission risk. Patients with depression and schizophrenia would seem to merit postdischarge interventions to decrease their potentially avoidable readmissions. Compulsory community treatment (a feature of treatment in Canada and Australia that is ordered by clinicians) has been shown to decrease mortality due to medical illness in patients who have been hospitalized and are psychiatrically ill, and addition of these services to postdischarge care may be useful.[23] Inpatient physicians could work to ensure follow‐up not just with medical providers but with robust outpatient mental health programs to decrease potentially avoidable readmission risk, and administrators could work to ensure close linkages with these community resources. Studies evaluating the impact of these types of interventions would need to be conducted. Patients with polypharmacy, including psychiatric medications, may benefit from interventions to improve medication safety, such as enhanced medication reconciliation and pharmacist counseling.[26]
Our study suggests that patients with depression, those with schizophrenia, and those who have increased numbers of prescribed psychiatric medications should be considered at high risk for readmission for medical illnesses. Targeting interventions to these patients may be fruitful in preventing avoidable readmissions.
Acknowledgements
The authors thank Dr. Yves Eggli for screening the database for potentially avoidable readmissions using the SQLape algorithm.
Disclosures
Dr. Donz was supported by the Swiss National Science Foundation and the Swiss Foundation for MedicalBiological Scholarships. The authors otherwise have no conflicts of interest to disclose. The content is solely the responsibility of the authors and does not necessarily represent the official views of the US Department of Veterans Affairs.
Readmissions to the hospital are common and costly.[1] However, identifying patients prospectively who are likely to be readmitted and who may benefit from interventions to reduce readmission risk has proven challenging, with published risk scores having only moderate ability to discriminate between patients likely and unlikely to be readmitted.[2] One reason for this may be that published studies have not typically focused on patients who are cognitively impaired, psychiatrically ill, have low health or English literacy, or have poor social supports, all of whom may represent a substantial fraction of readmitted patients.[2, 3, 4, 5]
Psychiatric disease, in particular, may contribute to increased readmission risk for nonpsychiatric (medical) illness, and is associated with increased utilization of healthcare resources.[6, 7, 8, 9, 10, 11] For example, patients with mental illness who were discharged from New York hospitals were more likely to be rehospitalized and had more costly readmissions than patients without these comorbidities, including a length of stay nearly 1 day longer on average.[7] An unmet need for treatment of substance abuse was projected to cost Tennessee $772 million of excess healthcare costs in 2000, mostly incurred through repeat hospitalizations and emergency department (ED) visits.[10]
Despite this, few investigators have considered the role of psychiatric disease and/or substance abuse in medical readmission risk. The purpose of the current study was to evaluate the role of psychiatric illness and substance abuse in unselected medical patients to determine their relative contributions to 30‐day all‐cause readmissions (ACR) and potentially avoidable readmissions (PAR).
METHODS
Patients and Setting
We conducted a retrospective cohort study of consecutive adult patients discharged from medicine services at Brigham and Women's Hospital (BWH), a 747‐bed tertiary referral center and teaching hospital, between July 1, 2009 and June 30, 2010. Most patients are cared for by resident housestaff teams at BWH (approximately 25% are cared for by physician assistants working directly with attending physicians), and approximately half receive primary care in the Partners system, which has a shared electronic medical record (EMR). Outpatient mental health services are provided by Partners‐associated mental health professionals including those at McLean Hospital and MassHealth (Medicaid)‐associated sites through the Massachusetts Behavioral Health Partnership. Exclusion criteria were death in the hospital or discharge to another acute care facility. We also excluded patients who left against medical advice (AMA). The study protocol was approved by the Partners Institutional Review Board.
Outcome
The primary outcomes were ACR and PAR within 30 days of discharge. First, we identified all 30‐day readmissions to BWH or to 2 other hospitals in the Partners Healthcare Network (previous studies have shown that 80% of all readmitted patients are readmitted to 1 of these 3 hospitals).[12] For patients with multiple readmissions, only the first readmission was included in the dataset.
To find potentially avoidable readmissions, administrative and billing data for these patients were processed using the SQLape (SQLape s.a.r.l., Corseaux, Switzerland) algorithm, which identifies PAR by excluding patients who undergo planned follow‐up treatment (such as a cycle of planned chemotherapy) or are readmitted for conditions unrelated in any way to the index hospitalization.[13, 14] Common complications of treatment are categorized as potentially avoidable, such as development of a deep venous thrombosis, a decubitus ulcer after prolonged bed rest, or bleeding complications after starting anticoagulation. Although the algorithm identifies theoretically preventable readmissions, the algorithm does not quantify how preventable they are, and these are thus referred to as potentially avoidable. This is similar to other admission metrics, such as the Agency for Healthcare Research and Quality's prevention quality indicators, which are created from a list of ambulatory care‐sensitive conditions.[15] SQLape has the advantage of being a specific tool for readmissions. Patients with 30‐day readmissions identified by SQLape as planned or unlikely to be avoidable were excluded in the PAR analysis, although still included in ACR analysis. In each case, the comparison group is patients without any readmission.
Predictors
Our predictors of interest included the overall prevalence of a psychiatric diagnosis or diagnosis of substance abuse, the presence of specific psychiatric diagnoses, and prescription of psychiatric medications to help assess the independent contribution of these comorbidities to readmission risk.
We used a combination of easily obtainable inpatient and outpatient clinical and administrative data to identify relevant patients. Patients were considered likely to be psychiatrically ill if they: (1) had a psychiatric diagnosis on their Partners outpatient EMR problem list and were prescribed a medication to treat that condition as an outpatient, or (2) had an International Classification of Diseases, 9th Revision diagnosis of a psychiatric illness at hospital discharge. Patients were considered to have moderate probability of disease if they: (1) had a psychiatric diagnosis on their outpatient problem list, or (2) were prescribed a medication intended to treat a psychiatric condition as an outpatient. Patients were considered unlikely to have psychiatric disease if none of these criteria were met. Patients were considered likely to have a substance abuse disorder if they had this diagnosis on their outpatient EMR, or were prescribed a medication to treat this condition (eg, buprenorphine/naloxone), or received inpatient consultation from a substance abuse treatment team during their inpatient hospitalization, and were considered unlikely if none of these were true. We also evaluated individual categories of psychiatric illness (schizophrenia, depression, anxiety, bipolar disorder) and of psychotropic medications (antidepressants, antipsychotics, anxiolytics).
Potential Confounders
Data on potential confounders, based on prior literature,[16, 17] collected at the index admission were derived from electronic administrative, clinical, and billing sources, including the Brigham Integrated Computer System and the Partners Clinical Data Repository. They included patient age, gender, ethnicity, primary language, marital status, insurance status, living situation prior to admission, discharge location, length of stay, Elixhauser comorbidity index,[18] total number of medications prescribed, and number of prior admissions and ED visits in the prior year.
Statistical Analysis
Bivariate comparisons of each of the predictors of ACR and PAR risk (ie, patients with a 30‐day ACR or PAR vs those not readmitted within 30 days) were conducted using 2 trend tests for ordinal predictors (eg, likelihood of psychiatric disease), and 2 or Fisher exact test for dichotomous predictors (eg, receipt of inpatient substance abuse counseling).
We then used multivariate logistic regression analysis to adjust for all of the potential confounders noted above, entering each variable related to psychiatric illness into the model separately (eg, likely psychiatric illness, number of psychiatric medications). In a secondary analysis, we removed potentially collinear variables from the final model; as this did not alter the results, the full model is presented. We also conducted a secondary analysis where we included patients who left against medical advice (AMA), which also did not alter the results. Two‐sided P values <0.05 were considered significant, and all analyses were performed using the SAS version 9.2 (SAS Institute, Inc., Cary, NC).
RESULTS
There were 7984 unique patients discharged during the study period. Patients were generally white and English speaking; just over half of admissions came from the ED (Table 1). Of note, nearly all patients were insured, as are almost all patients in Massachusetts. They had high degrees of comorbid illness and large numbers of prescribed medications. Nearly 30% had at least 1 hospital admission within the prior year.
| Characteristic | All Patients, N (%) | Not Readmitted, N (%) | ACR, N (%) | PAR N (%)a |
|---|---|---|---|---|
| ||||
| Study cohort | 6987 (100) | 5727 (72) | 1260 (18) | 388 (5.6) |
| Age, y | ||||
| <50 | 1663 (23.8) | 1343 (23.5) | 320 (25.4) | 85 (21.9) |
| 5165 | 2273 (32.5) | 1859 (32.5) | 414 (32.9) | 136 (35.1) |
| 6679 | 1444 (20.7) | 1176 (20.5) | 268 (18.6) | 80 (20.6) |
| >80 | 1607 (23.0) | 1349 (23.6) | 258 (16.1) | 87 (22.4) |
| Female | 3604 (51.6) | 2967 (51.8) | 637 (50.6) | 206 (53.1) |
| Race | ||||
| White | 5126 (73.4) | 4153 (72.5) | 973 (77.2) | 300 (77.3) |
| Black | 1075 (15.4) | 899 (15.7) | 176 (14.0) | 53 (13.7) |
| Hispanic | 562 (8.0) | 477 (8.3) | 85 (6.8) | 28 (7.2) |
| Other | 224 (3.2) | 198 (3.5) | 26 (2.1) | 7 (1.8) |
| Primary language | ||||
| English | 6345 (90.8) | 5180 (90.5) | 1165 (92.5) | 356 (91.8) |
| Marital status | ||||
| Married | 3642 (52.1) | 2942 (51.4) | 702 (55.7) | 214 (55.2) |
| Single, never married | 1662 (23.8) | 1393 (24.3) | 269 (21.4) | 73 (18.8) |
| Previously married | 1683 (24.1) | 1386 (24.2) | 289 (22.9) | 101 (26.0) |
| Insurance | ||||
| Medicare | 3550 (50.8) | 2949 (51.5) | 601 (47.7) | 188 (48.5) |
| Medicaid | 539 (7.7) | 430 (7.5) | 109 (8.7) | 33 (8.5) |
| Private | 2892 (41.4) | 2344 (40.9) | 548 (43.5) | 167 (43.0) |
| Uninsured | 6 (0.1) | 4 (0.1) | 2 (0.1) | 0 (0) |
| Source of index admission | ||||
| Clinic or home | 2136 (30.6) | 1711 (29.9) | 425 (33.7) | 117 (30.2) |
| Emergency department | 3592 (51.4) | 2999 (52.4) | 593 (47.1) | 181 (46.7) |
| Nursing facility | 1204 (17.2) | 977 (17.1) | 227 (18.0) | 84 (21.7) |
| Other | 55 (0.1) | 40 (0.7) | 15 (1.1) | 6 (1.6) |
| Length of stay, d | ||||
| 02 | 1757 (25.2) | 1556 (27.2) | 201 (16.0) | 55 (14.2) |
| 34 | 2200 (31.5) | 1842 (32.2) | 358 (28.4) | 105 (27.1) |
| 57 | 1521 (21.8) | 1214 (21.2) | 307 (24.4) | 101 (26.0) |
| >7 | 1509 (21.6) | 1115 (19.5) | 394 (31.3) | 127 (32.7) |
| Elixhauser comorbidity index score | ||||
| 01 | 1987 (28.4) | 1729 (30.2) | 258 (20.5) | 66 (17.0) |
| 27 | 1773 (25.4) | 1541 (26.9) | 232 (18.4) | 67 (17.3) |
| 813 | 1535 (22.0) | 1212 (21.2) | 323 (25.6) | 86 (22.2) |
| >13 | 1692 (24.2) | 1245 (21.7) | 447 (35.5) | 169 (43.6) |
| Medications prescribed as outpatient | ||||
| 06 | 1684 (24.1) | 1410 (24.6) | 274 (21.8) | 72 (18.6) |
| 79 | 1601 (22.9) | 1349 (23.6) | 252 (20.0) | 77 (19.9) |
| 1013 | 1836 (26.3) | 1508 (26.3) | 328 (26.0) | 107 (27.6) |
| >13 | 1866 (26.7) | 1460 (25.5) | 406 (32.2) | 132 (34.0) |
| Number of admissions in past year | ||||
| 0 | 4816 (68.9) | 4032 (70.4) | 784 (62.2) | 279 (71.9) |
| 15 | 2075 (29.7) | 1640 (28.6) | 435 (34.5) | 107 (27.6) |
| >5 | 96 (1.4) | 55 (1.0) | 41 (3.3) | 2 (0.5) |
| Number of ED visits in past year | ||||
| 0 | 4661 (66.7) | 3862 (67.4) | 799 (63.4) | 261 (67.3) |
| 15 | 2326 (33.3) | 1865 (32.6) | 461 (36.6) | 127 (32.7) |
All‐Cause Readmissions
After exclusion of 997 patients who died, were discharged to skilled nursing or rehabilitation facilities, or left AMA, 6987 patients were included (Figure 1). Of these, 1260 had a readmission (18%). Approximately half were considered unlikely to be psychiatrically ill, 22% were considered moderately likely, and 29% likely (Table 2).
| All‐Cause Readmission Analysis | Potentially Avoidable Readmission Analysis | |||||
|---|---|---|---|---|---|---|
| No. in Cohort (%) | % of Patients With ACR | P Valuea | No. in Cohort (%) | % of Patients With PAR | P Valuea | |
| ||||||
| Entire cohort | 6987 | 18.0 | 6115 | 6.3 | ||
| Likelihood of psychiatric illness | ||||||
| Unlikely | 3424 (49) | 16.5 | 3026 (49) | 5.6 | ||
| Moderate | 1564 (22) | 23.5 | 1302 (21) | 7.1 | ||
| Likely | 1999 (29) | 16.4 | 1787 (29) | 6.4 | ||
| Likely versus unlikely | 0.87 | 0.20 | ||||
| Moderate+likely versus unlikely | 0.001 | 0.02 | ||||
| Likelihood of substance abuse | 0.01 | 0.20 | ||||
| Unlikely | 5804 (83) | 18.7 | 5104 (83) | 6.5 | ||
| Likely | 1183 (17) | 14.8 | 1011 (17) | 5.4 | 0.14 | |
| Number of prescribed outpatient psychotropic medications | <0.001 | 0.04 | ||||
| 0 | 4420 (63) | 16.3 | 3931 (64) | 5.9 | ||
| 1 | 1725 (25) | 20.4 | 1481 (24) | 7.2 | ||
| 2 | 781 (11) | 22.3 | 653 (11) | 7.0 | ||
| >2 | 61 (1) | 23.0 | 50 (1) | 6.0 | ||
| Prescribed antidepressant | 1474 (21) | 20.6 | 0.005 | 1248 (20) | 6.2 | 0.77 |
| Prescribed antipsychotic | 375 (5) | 22.4 | 0.02 | 315 (5) | 7.6 | 0.34 |
| Prescribed mood stabilizer | 81 (1) | 18.5 | 0.91 | 69 (1) | 4.4 | 0.49 |
| Prescribed anxiolytic | 1814 (26) | 21.8 | <0.001 | 1537 (25) | 7.7 | 0.01 |
| Prescribed stimulant | 101 (2) | 26.7 | 0.02 | 83 (1) | 10.8 | 0.09 |
| Prescribed pharmacologic treatment for substance abuse | 79 (1) | 25.3 | 0.09 | 60 (1) | 1.7 | 0.14 |
| Number of psychiatric diagnoses on outpatient problem list | 0.31 | 0.74 | ||||
| 0 | 6405 (92) | 18.2 | 5509 (90) | 6.3 | ||
| 1 or more | 582 (8) | 16.5 | 474 (8) | 7.0 | ||
| Outpatient diagnosis of substance abuse | 159 (2) | 13.2 | 0.11 | 144 (2) | 4.2 | 0.28 |
| Outpatient diagnosis of any psychiatric illness | 582 (8) | 16.5 | 0.31 | 517 (8) | 8.0 | 0.73 |
| Discharge diagnosis of depression | 774 (11) | 17.7 | 0.80 | 690 (11) | 7.7 | 0.13 |
| Discharge diagnosis of schizophrenia | 56 (1) | 23.2 | 0.31 | 50 (1) | 14 | 0.03 |
| Discharge diagnosis of bipolar disorder | 101 (1) | 10.9 | 0.06 | 92 (2) | 2.2 | 0.10 |
| Discharge diagnosis of anxiety | 1192 (17) | 15.0 | 0.003 | 1080 (18) | 6.2 | 0.83 |
| Discharge diagnosis of substance abuse | 885 (13) | 14.8 | 0.008 | 803 (13) | 6.1 | 0.76 |
| Discharge diagnosis of any psychiatric illness | 1839 (26) | 16.0 | 0.008 | 1654 (27) | 6.6 | 0.63 |
| Substance abuse consultation as inpatient | 284 (4) | 14.4 | 0.11 | 252 (4) | 3.6 | 0.07 |
In bivariate analysis (Table 2), likelihood of psychiatric illness (P<0.01) and increasing numbers of prescribed outpatient psychiatric medications (P<0.01) were significantly associated with ACR. In multivariate analysis, each additional prescribed outpatient psychiatric medication increased ACR risk (odds ratio [OR]: 1.10, 95% confidence interval [CI]: 1.01‐1.20) or any prescription of an anxiolytic in particular (OR: 1.16, 95% CI: 1.001.35) was associated with increased risk of ACR, whereas discharge diagnoses of anxiety (OR: 0.82, 95% CI: 0.68‐0.99) and substance abuse (OR: 0.80, 95% CI: 0.65‐0.99) were associated with lower risk of ACR (Table 3).
| ACR, OR (95% CI) | PAR, OR (95% CI)a | |
|---|---|---|
| ||
| Likely psychiatric disease | 0.97 (0.82‐1.14) | 1.20 (0.92‐1.56) |
| Likely and possible psychiatric disease | 1.07 (0.94‐1.22) | 1.18 (0.94‐1.47) |
| Likely substance abuse | 0.83 (0.69‐0.99) | 0.85 (0.63‐1.16) |
| Psychiatric diagnosis on outpatient problem list | 0.97 (0.76‐1.23) | 1.04 (0.70‐1.55) |
| Substance abuse diagnosis on outpatient problem list | 0.63 (0.39‐1.02) | 0.65 (0.28‐1.52) |
| Increasing number of prescribed psychiatric medications | 1.10 (1.01‐1.20) | 1.00 (0.86‐1.16) |
| Outpatient prescription for antidepressant | 1.10 (0.94‐1.29) | 0.86 (0.66‐1.13) |
| Outpatient prescription for antipsychotic | 1.03 (0.79‐1.34) | 0.93 (0.59‐1.45) |
| Outpatient prescription for anxiolytic | 1.16 (1.001.35) | 1.13 (0.88‐1.44) |
| Outpatient prescription for methadone or buprenorphine | 1.15 (0.67‐1.98) | 0.18 (0.03‐1.36) |
| Discharge diagnosis of depression | 1.06 (0.86‐1.30) | 1.49 (1.09‐2.04) |
| Discharge diagnosis of schizophrenia | 1.43 (0.75‐2.74) | 2.63 (1.13‐6.13) |
| Discharge diagnosis of bipolar disorder | 0.53 (0.28‐1.02) | 0.35 (0.09‐1.45) |
| Discharge diagnosis of anxiety | 0.82 (0.68‐0.99) | 1.11 (0.83‐1.49) |
| Discharge diagnosis of substance abuse | 0.80 (0.65‐0.99) | 1.05 (0.75‐1.46) |
| Discharge diagnosis of any psychiatric illness | 0.88 (0.75‐1.02) | 1.22 (0.96‐1.56) |
| Addiction team consult while inpatient | 0.82 (0.58‐1.17) | 0.58 (0.29‐1.17) |
Potentially Avoidable Readmissions
After further exclusion of 872 patients who had unavoidable readmissions according to the SQLape algorithm, 6115 patients remained. Of these, 388 had a PAR within 30 days (6.3%, Table 1).
In bivariate analysis (Table 2), the likelihood of psychiatric illness (P=0.02), number of outpatient psychiatric medications (P=0.04), and prescription of anxiolytics (P=0.01) were significantly associated with PAR, as they were with ACR. A discharge diagnosis of schizophrenia was also associated with PAR (P=0.03).
In multivariate analysis, only discharge diagnoses of depression (OR: 1.49, 95% CI: 1.09‐2.04) and schizophrenia (OR: 2.63, 95% CI: 1.13‐6.13) were associated with PAR.
DISCUSSION
Comorbid psychiatric illness was common among patients admitted to the medicine wards. Patients with documented discharge diagnoses of depression or schizophrenia were at highest risk for a potentially avoidable 30‐day readmission, whereas those prescribed more psychiatric medications were at increased risk for ACR. These findings were independent of a comprehensive set of risk factors among medicine inpatients in this retrospective cohort study.
This study extends prior work indicating patients with psychiatric disease have increased healthcare utilization,[6, 7, 8, 9, 10, 11] by identifying at least 2 subpopulations of the psychiatrically ill (those with depression and schizophrenia) at particularly high risk for 30‐day PAR. To our knowledge, this is the first study to identify schizophrenia as a predictor of hospital readmission for medical illnesses. One prior study prospectively identified depression as increasing the 90‐day risk of readmission 3‐fold, although medication usage was not assessed,[6] and our report strengthens this association.
There are several possible explanations why these two subpopulations in particular would be more predisposed to readmissions that are potentially avoidable. It is known that patients with schizophrenia, for example, live on average 20 years less than the general population, and most of this excess mortality is due to medical illnesses.[20, 21] Reasons for this may include poor healthcare access, adverse effects of medication, and socioeconomic factors among others.[21, 22] All of these reasons may contribute to the increased PAR risk in this population, mediated, for example, by decreased ability to adhere to postdischarge care plans. Successful community‐based interventions to decrease these inequities have been described and could serve as a model for addressing the increased readmission risk in this population.[23]
Our finding that patients with a greater number of prescribed psychiatric medications are at increased risk for ACR may be expected, given other studies that have highlighted the crucial importance of medications in postdischarge adverse events, including readmissions.[24] Indeed, medication‐related errors and toxicities are the most common postdischarge adverse events experienced by patients.[25] Whether psychiatric medications are particularly prone to causing postdischarge adverse events or whether these medications represent greater psychiatric comorbidity cannot be answered by this study.
It was surprising but reassuring that substance abuse was not a predictor of short‐term readmissions as identified using our measures; in fact, a discharge diagnosis of substance abuse was associated with lower risk of ACR than comparator patients. It seems unlikely that we would have inadequate power to find such a result, as we found a statistically significant negative association in the ACR population, and 17% of our population overall was considered likely to have a substance abuse comorbidity. However, it is likely the burden of disease was underestimated given that we did not try to determine the contribution of long‐term substance abuse to medical diseases that may increase readmission risk (eg, liver cirrhosis from alcohol use). Unlike other conditions in our study, patients with substance abuse diagnoses at BWH can be seen by a dedicated multidisciplinary team while an inpatient to start treatment and plan for postdischarge follow‐up; this may have played a role in our findings.
A discharge diagnosis of anxiety was also somewhat protective against readmission, whereas a prescription of an anxiolytic (predominantly benzodiazepines) increased risk; many patients prescribed a benzodiazepine do not have a Diagnostic and Statistical Manual of Mental Disorders4th Edition (DSM‐IV) diagnosis of anxiety disorder, and thus these findings may reflect different patient populations. Discharging physicians may have used anxiety as a discharge diagnosis in patients in whom they suspected somatic complaints without organic basis; these patients may be at lower risk of readmission.
Discharge diagnoses of psychiatric illnesses were associated with ACR and PAR in our study, but outpatient diagnoses were not. This likely reflects greater severity of illness (documentation as a treated diagnosis on discharge indicates the illness was relevant during the hospitalization), but may also reflect inaccuracies of diagnosis and lack of assessment of severity in outpatient coding, which would bias toward null findings. Although many of the patients in our study were seen by primary care doctors within the Partners system, some patients had outside primary care physicians and we did not have access to these records. This may also have decreased our ability to find associations.
The findings of our study should be interpreted in the context of the study design. Our study was retrospective, which limited our ability to conclusively diagnose psychiatric disease presence or severity (as is true of most institutions, validated psychiatric screening was not routinely used at our institutions on hospital admission or discharge). However, we used a conservative scale to classify the likelihood of patients having psychiatric or substance abuse disorders, and we used other metrics to establish the presence of illness, such as the number of prescribed medications, inpatient consultation with a substance abuse service, and hospital discharge diagnoses. This approach also allowed us to quickly identify a large cohort unaffected by selection bias. Our study was single center, potentially limiting generalizability. Although we capture at least 80% of readmissions, we were not able to capture all readmissions, and we cannot rule out that patients readmitted elsewhere are different than those readmitted within the Partners system. Last, the SQLape algorithm is not perfectly sensitive or specific in identifying avoidable readmissions,[13] but it does eliminate many readmissions that are clearly unavoidable, creating an enriched cohort of patients whose readmissions are more likely to be avoidable and therefore potentially actionable.
We suggest that our study findings first be considered when risk stratifying patients before hospital discharge in terms of readmission risk. Patients with depression and schizophrenia would seem to merit postdischarge interventions to decrease their potentially avoidable readmissions. Compulsory community treatment (a feature of treatment in Canada and Australia that is ordered by clinicians) has been shown to decrease mortality due to medical illness in patients who have been hospitalized and are psychiatrically ill, and addition of these services to postdischarge care may be useful.[23] Inpatient physicians could work to ensure follow‐up not just with medical providers but with robust outpatient mental health programs to decrease potentially avoidable readmission risk, and administrators could work to ensure close linkages with these community resources. Studies evaluating the impact of these types of interventions would need to be conducted. Patients with polypharmacy, including psychiatric medications, may benefit from interventions to improve medication safety, such as enhanced medication reconciliation and pharmacist counseling.[26]
Our study suggests that patients with depression, those with schizophrenia, and those who have increased numbers of prescribed psychiatric medications should be considered at high risk for readmission for medical illnesses. Targeting interventions to these patients may be fruitful in preventing avoidable readmissions.
Acknowledgements
The authors thank Dr. Yves Eggli for screening the database for potentially avoidable readmissions using the SQLape algorithm.
Disclosures
Dr. Donz was supported by the Swiss National Science Foundation and the Swiss Foundation for MedicalBiological Scholarships. The authors otherwise have no conflicts of interest to disclose. The content is solely the responsibility of the authors and does not necessarily represent the official views of the US Department of Veterans Affairs.
- , , . Rehospitalizations among patients in the Medicare fee‐for‐service program. N Engl J Med. 2009;360(14):1418–1428.
- , , , et al. Risk prediction models for hospital readmission: a systematic review. JAMA. 2011;306(15):1688–1698.
- , . Hospital readmission as an accountability measure. JAMA. 2011;305(5):504–505.
- , , , , , . Postdischarge environmental and socioeconomic factors and the likelihood of early hospital readmission among community‐dwelling Medicare beneficiaries. Gerontologist. 2008;48(4):495–504.
- , , , , . Low health literacy and health outcomes: an updated systematic review. Ann Intern Med. 2011;155(2):97–107.
- , , , et al. Depression is a risk factor for rehospitalization in medical inpatients. Prim Care Companion J Clin Psychiatry. 2007;9(4):256–262.
- , , , . Mental illness and hospitalization for ambulatory care sensitive medical conditions. Med Care. 2008;46(12):1249–1256.
- , , , , , . Substance use treatment barriers for patients with frequent hospital admissions. J Subst Abuse Treat. 2010;38(1):22–30.
- , , , , . Managed care and the quality of substance abuse treatment. J Ment Health Policy Econ. 2002;5(4):163–174.
- , , , , . Unmet substance abuse treatment need, health services utilization, and cost: a population‐based emergency department study. Ann Emerg Med. 2005;45(2):118–127.
- , , , . Elderly Medicare inpatients with substance use disorders: characteristics and predictors of hospital readmissions over a four‐year interval. J Stud Alcohol. 2000;61(6):891–895.
- , , , et al. Rationale and design of the Pharmacist Intervention for Low Literacy in Cardiovascular Disease (PILL‐CVD) study. Circ Cardiovasc Qual Outcomes. 2010;3(2):212–219.
- , , , , , . Validation of the potentially avoidable hospital readmission rate as a routine indicator of the quality of hospital care. Med Care. 2006;44(11);972–981.
- , , , , , . Measuring potentially avoidable hospital readmissions. J Clin Epidemiol. 2002;55:573–587.
- Agency for Healthcare Research and Quality Quality Indicators. (April 7, 2006). Prevention Quality Indicators (PQI) Composite Measure Workgroup Final Report. Available at: http://www.qualityindicators.ahrq.gov/modules/pqi_resources.aspx. Accessed June 1, 2012.
- , , , et al. Hospital readmission in general medicine patients: a prediction model. J Gen Intern Med. 2010;25(3):211–219.
- , , , et al. Derivation and validation of an index to predict early death or unplanned readmission after discharge from hospital to the community. CMAJ. 2010;182(6):551–557.
- , , , . Comorbidity measures for use with administrative data. Med Care. Jan 1998;36(1):8–27.
- , , , , eds. Morbidity and mortality in people with serious mental illness. October 2006. National Association of State Mental Health Directors, Medical Directors Council. Available at: http://www.nasmhpd.org/docs/publications/MDCdocs/Mortality%20and%20 Mo rbidity%20Final%20Report%208.18.08.pdf. Accessed January 13, 2013.
- , , , . Mortality in individuals who have had psychiatric treatment: population‐based study in Nova Scotia. Br J Psychiat. 2005;187:552–558.
- , , , , , . Inequitable access for mentally ill patients to some medically necessary procedures. CMAJ. 2007;176(6):779–784.
- , , . Quality of medical care for people with and without comorbid mental illness and substance misuse: systematic review of comparative studies. Br J Psychiat. 2009;194:491–499.
- , , , , , . Reducing all‐cause mortality among patients with psychiatric disorders: a population‐based study. CMAJ. 2013;185(1):E50–E56.
- , , , et al. Incidence of potentially avoidable urgent readmissions and their relation to all‐cause urgent readmissions. CMAJ. 2011;183(14):E1067–E1072.
- , , , , . The incidence and severity of adverse events affecting patients after discharge from the hospital. Ann Intern Med. 2003;138(3):161–167.
- , , , . Hospital‐based medication reconciliation practices: a systematic review. Arch Intern Med. 2012;172(14):1057–1069.
- , , . Rehospitalizations among patients in the Medicare fee‐for‐service program. N Engl J Med. 2009;360(14):1418–1428.
- , , , et al. Risk prediction models for hospital readmission: a systematic review. JAMA. 2011;306(15):1688–1698.
- , . Hospital readmission as an accountability measure. JAMA. 2011;305(5):504–505.
- , , , , , . Postdischarge environmental and socioeconomic factors and the likelihood of early hospital readmission among community‐dwelling Medicare beneficiaries. Gerontologist. 2008;48(4):495–504.
- , , , , . Low health literacy and health outcomes: an updated systematic review. Ann Intern Med. 2011;155(2):97–107.
- , , , et al. Depression is a risk factor for rehospitalization in medical inpatients. Prim Care Companion J Clin Psychiatry. 2007;9(4):256–262.
- , , , . Mental illness and hospitalization for ambulatory care sensitive medical conditions. Med Care. 2008;46(12):1249–1256.
- , , , , , . Substance use treatment barriers for patients with frequent hospital admissions. J Subst Abuse Treat. 2010;38(1):22–30.
- , , , , . Managed care and the quality of substance abuse treatment. J Ment Health Policy Econ. 2002;5(4):163–174.
- , , , , . Unmet substance abuse treatment need, health services utilization, and cost: a population‐based emergency department study. Ann Emerg Med. 2005;45(2):118–127.
- , , , . Elderly Medicare inpatients with substance use disorders: characteristics and predictors of hospital readmissions over a four‐year interval. J Stud Alcohol. 2000;61(6):891–895.
- , , , et al. Rationale and design of the Pharmacist Intervention for Low Literacy in Cardiovascular Disease (PILL‐CVD) study. Circ Cardiovasc Qual Outcomes. 2010;3(2):212–219.
- , , , , , . Validation of the potentially avoidable hospital readmission rate as a routine indicator of the quality of hospital care. Med Care. 2006;44(11);972–981.
- , , , , , . Measuring potentially avoidable hospital readmissions. J Clin Epidemiol. 2002;55:573–587.
- Agency for Healthcare Research and Quality Quality Indicators. (April 7, 2006). Prevention Quality Indicators (PQI) Composite Measure Workgroup Final Report. Available at: http://www.qualityindicators.ahrq.gov/modules/pqi_resources.aspx. Accessed June 1, 2012.
- , , , et al. Hospital readmission in general medicine patients: a prediction model. J Gen Intern Med. 2010;25(3):211–219.
- , , , et al. Derivation and validation of an index to predict early death or unplanned readmission after discharge from hospital to the community. CMAJ. 2010;182(6):551–557.
- , , , . Comorbidity measures for use with administrative data. Med Care. Jan 1998;36(1):8–27.
- , , , , eds. Morbidity and mortality in people with serious mental illness. October 2006. National Association of State Mental Health Directors, Medical Directors Council. Available at: http://www.nasmhpd.org/docs/publications/MDCdocs/Mortality%20and%20 Mo rbidity%20Final%20Report%208.18.08.pdf. Accessed January 13, 2013.
- , , , . Mortality in individuals who have had psychiatric treatment: population‐based study in Nova Scotia. Br J Psychiat. 2005;187:552–558.
- , , , , , . Inequitable access for mentally ill patients to some medically necessary procedures. CMAJ. 2007;176(6):779–784.
- , , . Quality of medical care for people with and without comorbid mental illness and substance misuse: systematic review of comparative studies. Br J Psychiat. 2009;194:491–499.
- , , , , , . Reducing all‐cause mortality among patients with psychiatric disorders: a population‐based study. CMAJ. 2013;185(1):E50–E56.
- , , , et al. Incidence of potentially avoidable urgent readmissions and their relation to all‐cause urgent readmissions. CMAJ. 2011;183(14):E1067–E1072.
- , , , , . The incidence and severity of adverse events affecting patients after discharge from the hospital. Ann Intern Med. 2003;138(3):161–167.
- , , , . Hospital‐based medication reconciliation practices: a systematic review. Arch Intern Med. 2012;172(14):1057–1069.
Copyright © 2013 Society of Hospital Medicine