User login
Predictors of Medication Adherence
In the outpatient setting, medication adherence (defined as percentage of prescribed medication doses taken by a patient during a specific time period) ranges between 40% and 80% for chronic conditions.1 During acute care hospitalization, changes are often made to patients' medication regimens, which can be confusing and contribute to nonadherence, medication errors, and harmful adverse events.2 Indeed, it is estimated that almost half of patients encounter a medication error after discharge, and approximately 12%17% experience an adverse drug event after returning home.36 It is likely that some of these adverse events may be the result of medication nonadherence.7 Improved patientprovider communication, systems to reconcile prehospitalization and posthospitalization medications, as well as development of mechanisms to enhance adherence, may prevent many of these errors and have become new targets for quality improvement.4, 8 Although postdischarge medication adherence is a crucial target for avoiding adverse events and rehospitalization, few studies have focused on understanding its incidence and predictors, in particular, patient demographic factors such as age and insurance status.911
In addition, few studies have looked at general and posthospital adherence in a population where health literacy is measured, an important area because medication changes during hospitalization may be particularly confusing for patients with low health literacy.11, 12 Health literacy is defined as the degree to which an individual has the capacity to obtain, process, and understand basic health information and services needed to make appropriate health decisions.13 Prior outpatient research shows that low health literacy is associated with poor patient understanding of the medication regimen and instructions for medication use, which may contribute to postdischarge medication nonadherence.14, 15 Understanding the factors associated with postdischarge medication adherence could help refine interventions that are oriented toward improving transitions in care, patient safety, and reducing unnecessary rehospitalization.
We report here on factors associated with postdischarge medication adherence using data from the Pharmacist Intervention for Low Literacy in Cardiovascular Disease (PILL‐CVD) study.16
METHODS
Study and Participants
PILL‐CVD was a federally funded, 2‐site randomized controlled trial using pharmacist‐assisted medication reconciliation, inpatient pharmacist counseling, low‐literacy adherence aids, and telephone follow‐up that aimed to decrease rates of serious medication errors after hospital discharge.16 The study targeted patients with cardiovascular disease (hospitalized on cardiology or general medical or geriatric services for acute coronary syndromes [ACS] or acute decompensated heart failure [ADHF]) at 2 large academic hospitals, Brigham and Women's Hospital (BWH) and Vanderbilt University Hospital (VUH).
Subjects were eligible for enrollment if they met criteria for ACS or ADHF, were likely to be discharged to home as determined by the primary medical team at the time of study enrollment, and took primary responsibility for administering their medications prior to admission (caregivers could be involved in medication management after discharge). Exclusion criteria included severe visual or hearing impairment, inability to communicate in English or Spanish, active psychiatric illness, dementia, delirium, illness too severe to participate, lack of a home phone number, being in police custody, or participation in another intensive medication adherence program (eg, due to renal transplant).
Out of 6416 patients originally screened for possible enrollment, 862 were randomly assigned to receive usual care or usual care plus the intervention, and 851 remained in the study.16 Both the main study and this secondary data analysis were approved by the Institutional Review Boards of each site.
Baseline Measures
Following informed consent and study enrollment, a variety of baseline data were collected on study participants from medical records and patient interview, including primary language, demographic information (age, race, insurance status, income, and education level), cognition (through administration of the 05‐point MiniCog scale),17 and level of health literacy (through use of the 036‐point short form of the Test of Functional Health Literacy in Adults [s‐TOFHLA] scale).18 Baseline information was also collected on medication use, including number of preadmission medications, measurement of self‐reported adherence prior to admission (using the Morisky scale, a validated 04‐point questionnaire shown to correlate with disease control and indicative of general patterns of adherence),19 and a medication understanding score, adapted from other instruments, which quantifies understanding of the indication, dose, and frequency of up to 5 randomly selected preadmission medications on a 03‐point scale.16, 20, 21
Outcome Measures
Outcomes were collected 30 days postdischarge through a structured questionnaire, administered by telephone. Only patients who completed this call are included in the present analysis. Postdischarge medication adherence was assessed by asking patients to report the number of days out of the previous week they had taken each medication from their postdischarge regimen exactly as prescribed.22 A score was calculated for each medication as the proportion of adherent days (eg, if a patient reported missing 2 days of a medication in the previous week, then adherence would be 5/7 or 71%). A global postdischarge adherence score was then derived for each patient by averaging the adherence score for all regularly scheduled medications. This quantitative measure focused on adherence to medications patients knew they should be taking and did not measure medication discrepancies (sometimes termed unintentional nonadherence).
Analysis
Patient characteristics were summarized and reported using simple descriptive statistics. Candidate predictors of postdischarge medication adherence were chosen a priori from patient characteristics assessed during hospital admission. These included patient age, gender, race, ethnicity, marital status, insurance, years of education, presence of primary care physician (PCP), study site, number of preadmission medications, medication understanding, baseline adherence, cognition, and health literacy. Unadjusted results were calculated using univariable linear regression, with each patient's adherence score as the dependent variable and each predictor as the independent variable. Adjusted results were then derived using multivariable linear regression with all the candidate predictors in the model.
Lastly, because of missing data for some predictors, in particular baseline adherence and medication understanding, multiple imputation techniques were used to impute missing data and increase statistical power.23 We used the Markov Chain Monte Carlo (MCMC) method for multiple imputation, which generally assumes that the data came from a normal distribution and that the missing data are missing at random. Because of the essentially normal distribution of the data, and because the amount of missing data was so small (<1% for almost all variables, 5% for baseline adherence, and 8% for medication understanding), we expected little bias and present the complete case analysis, which maximized statistical power.
Two‐sided P values <0.05 were considered significant, and SAS version 9.2 (Cary, NC) was used for all analyses.
RESULTS
Table 1 shows descriptive baseline patient characteristics of study sample (responders) as well as nonresponders at 30 days. For the responders, the mean age of the 646 patients was 61.2 years, 94.7% were insured, and 19.3% had inadequate or marginal health literacy. Patients were prescribed an average of 8 preadmission medications. Most patients (92.3%) had a regular PCP prior to admission. Nonresponders had nonsignificant trends towards having lower health literacy, medication understanding, and baseline medication adherence.
| Characteristic | Total N, 30‐Day Respondents | Value | Total N, Nonrespondents | Value |
|---|---|---|---|---|
| ||||
| Age, mean in yr (SD) | 646 | 61.2 (13.5) | 45 | 55.4 (14.3) |
| Gender, N (percentage) | 646 | 45 | ||
| Female | 272 (42.1) | 18 (40.0) | ||
| Male | 374 (57.9) | 27 (60.0) | ||
| Race, N (percentage) | 643 | 45 | ||
| White | 511 (79.5) | 32 (71.1) | ||
| Black | 104 (16.2) | 11 (24.4) | ||
| Other | 28 (4.4) | 2 (4.4) | ||
| Ethnicity, N (percentage) | 639 | 45 | ||
| Hispanic | 24 (3.8) | 1 (2.2) | ||
| Not Hispanic | 615 (96.2) | 44 (97.8) | ||
| Marital status, N (percentage) | 646 | 45 | ||
| Married/cohabitate | 382 (59.1) | 20 (44.4) | ||
| Separated/divorced | 118 (18.3) | 11 (24.4) | ||
| Widowed | 81 (12.5) | 5 (11.1) | ||
| Never married | 65 (10.1) | 9 (2.0) | ||
| Insurance type, N (percentage) | 646 | 45 | ||
| Medicaid | 53 (8.2) | 5 (11.1) | ||
| Medicare | 270 (41.8) | 13 (28.9) | ||
| Private | 289 (44.7) | 19 (42.2) | ||
| Self‐pay | 34 (5.3) | 8 (17.8) | ||
| Years of education, mean in yr (SD) | 643 | 14.0 (3.1) | 45 | 13.3 (2.7) |
| Presence of PCP prior to admission, N (percentage) | 646 | 45 | ||
| Yes | 596 (92.3) | 38 (84.4) | ||
| No | 50 (7.74) | 7 (15.6) | ||
| Site, N (percentage) | 646 | 45 | ||
| Site 1 | 358 (55.4) | 8 (17.8) | ||
| Site 2 | 288 (44.6) | 37 (82.2) | ||
| No. of preadmission medications, mean no. (SD) | 641 | 7.8 (4.8) | 45 | 7.7 (5.4) |
| Medication understanding score, mean (SD)* | 597 | 2.4 (0.5) | 40 | 2.2 (0.62) |
| Health literacy (s‐TOFHLA) score, mean (SD) | 642 | 29.1 (8.9) | 45 | 26.0 (12.0) |
| Baseline adherence (SD) | 613 | 2.7 (1.1) | 45 | 2.4 (1.2) |
| MiniCog score, N (percentage) | 646 | 45 | ||
| Demented | 63 (9.8) | 5 (11.1) | ||
| Not demented | 583 (90.2) | 40 (88.9) | ||
The average postdischarge adherence score was 95% (standard deviation [SD] = 10.2%), and less than 10% of patients had an adherence score of less than 85%; overall the distribution was left‐skewed. Table 2 illustrates crude and adjusted parameter estimates for variables in the model. Table 3 shows significant findings in the fully adjusted model, which used multiple imputation techniques to account for missing data.
| Predictor | Crude Parameter Estimate (Beta) With 95% Confidence Intervals | P Value | Adjusted Parameter Estimate (Beta) With 95% Confidence Intervals | P Value |
|---|---|---|---|---|
| ||||
| Age per 10 yr | 0.010 (0.007, 0.020) | <0.0001 | 0.010 (0.002, 0.020) | 0.018 |
| Male gender | 0.012 (0.004, 0.028) | 0.137 | 0.003 (0.014, 0.020) | 0.727 |
| Race/ethnicity | ||||
| White | 0.011 (0.009, 0.031) | 0.266 | Ref | Ref |
| Black | 0.017 (0.038, 0.005) | 0.13 | 0.006 (0.017, 0.030) | 0.598 |
| Other | 0.010 (0.029, 0.049) | 0.599 | 0.017 (0.027, 0.062) | 0.446 |
| Hispanic/Latino | 0.005 (0.037, 0.047) | 0.803 | 0.036 (0.013, 0.085) | 0.149 |
| Marital status | ||||
| Married/cohabitate | 0.006 (0.011, 0.022) | 0.500 | Ref | Ref |
| Separated/divorced | 0.005 (0.025, 0.016) | 0.664 | 0.009 (0.014, 0.031) | 0.446 |
| Widowed | 0.001 (0.023, 0.025) | 0.922 | 0.013 (0.039, 0.013) | 0.338 |
| Never married | 0.009 (0.035, 0.018) | 0.515 | 0.004 (0.033, 0.025) | 0.784 |
| Insurance type | ||||
| Private | 0.008 (0.008, 0.024) | 0.347 | Ref | Ref |
| Medicaid | 0.046 (0.075, 0.018) | 0.002 | 0.026 (0.058, 0.007) | 0.121 |
| Medicare | 0.012 (0.004, 0.028) | 0.138 | 0.002 (0.023, 0.018) | 0.844 |
| Self‐pay | 0.027 (0.062, 0.008) | 0.135 | 0.029 (0.073, 0.015) | 0.202 |
| Years of education | 0.003 (0.0003, 0.005) | 0.028 | 0.0001 (0.003, 0.003) | 0.949 |
| Presence of PCP prior to admission | 0.007 (0.022, 0.037) | 0.630 | 0.002 (0.032, 0.036) | 0.888 |
| Site | 0.050 (0.065, 0.034) | <0.0001 | 0.038 (0.056, 0.021) | <0.0001 |
| No. of preadmission medications | 0.0003 (0.002, 0.001) | 0.684 | 0.0001 (0.002, 0.002) | 0.918 |
| Medication understanding score per point | 0.007 (0.009, 0.023) | 0.390 | 0.006 (0.011, 0.023) | 0.513 |
| Health literacy (s‐TOFHLA) score per 10 points | 0.0006 (0.008, 0.01) | 0.897 | 0.003 (0.008, 0.01) | 0.644 |
| Baseline adherence per point | 0.023 (0.016, 0.031) | <0.0001 | 0.017 (0.009, 0.024) | <0.0001 |
| Cognitive function | 0.004 (0.022, 0.031) | 0.757 | 0.008 (0.019, 0.036) | 0.549 |
| Predictor | Parameter Estimate (Beta) With 95% Confidence Intervals | P Value |
|---|---|---|
| ||
| Age per 10 yr | 0.010 (0.004, 0.020) | 0.004 |
| Insurance type | ||
| Private | Ref | Ref |
| Medicaid | 0.045 (0.076, 0.014) | 0.005 |
| Medicare | 0.010 (0.030, 0.010) | 0.333 |
| Self‐pay | 0.013 (0.050, 0.025) | 0.512 |
| Site | 0.036 (0.053, 0.019) | <0.0001 |
| Baseline adherence per point | 0.016 (0.008, 0.024) | <0.0001 |
Intervention arm was of borderline statistical significance in predicting postdischarge adherence (P = 0.052), and so was removed from the final model. Study site, age, insurance, and baseline adherence were the only significant independent predictors of postdischarge adherence in the fully adjusted model (Table 3). For example, for every 10‐year increase in age, patients had, on average, an adjusted 1% absolute increase in their adherence score (95% confidence interval [CI] 0.4% to 2.0%). For every 1‐point increase in baseline medication adherence (based on the Morisky scale), there was a 1.6% absolute increase in medication adherence (95% CI 0.8% to 2.4%). In unadjusted analyses, patients with Medicaid were less adherent with medications after discharge than were patients with private insurance. This difference became nonsignificant in adjusted analyses, but when analyses were repeated using multiple imputation techniques, the results again became statistically significantMedicaid insurance was associated with a 4.5% absolute decrease in postdischarge adherence compared with private insurance (95% CI 7.6% to 1.4%). Study site (specifically, Brigham and Women's Hospital) was also a significant predictor of greater postdischarge medication adherence. Years of education was a significant predictor of adherence in unadjusted analyses, but was not an independent predictor when adjusted for other factors. When baseline adherence was removed from the multiple imputation model, there were no changes in which factors were significant predictors of adherence.
DISCUSSION
In this study, we found that low baseline adherence, younger age, Medicaid insurance, and study site were significant predictors of lower 30‐day medication adherence. Of particular interest is our finding regarding baseline adherence, a simple measure to obtain on hospitalized patients. It is notable that in our study, education was not an independent significant predictor of postdischarge adherence, even when baseline adherence was removed from the model. The same is true for medication understanding, cognitive function, and health literacy.
Older patients appeared more adherent with medications in the month after hospital discharge, perhaps reflecting increased interaction with the healthcare system (appointments, number of physician interactions), a greater belief in the importance of chronic medication management, or a higher level of experience with managing medications. A similar relationship between age and adherence has been shown in outpatient studies of patients with hypertension, diabetes, and other chronic diseases.2427
Medicaid patients may be less likely to remain adherent because of the plan's limited coverage of medications relative to patients' ability to pay. For example, Medicaid in Tennessee covers the first 5 generic medications at no cost to the patient but has co‐payments for additional medications and for brand name drugs. Medicaid in Massachusetts has co‐payments of $1 to $3 for each medication. Alternatively, Medicaid insurance may be a marker for other patient characteristics associated with low adherence for which we were not fully able to adjust.
Site differences were also notable in this study; these differences could have been due to differences in insurance coverage in Tennessee versus Massachusetts (which has near‐universal coverage), differences in types of insurance (eg, fewer patients at Brigham and Women's Hospital had Medicaid than at Vanderbilt), cultural and geographic differences between the 2 locations, or other differences in transitional care between the 2 sites.
This study corroborates previous literature on medication adherence (specifically unintentional nonadherence) in the outpatient setting,4, 811 for example, on the association of younger age with low adherence in certain populations. On the other hand, it may contrast with previous literature which has sometimes shown a relationship between patient education or health literacy and medication adherence.14, 15, 2835 However, previous studies have not focused on the transition from inpatient to outpatient settings. Perhaps intensive medication education in the hospital, even under usual care, mitigates the effects of these factors on postdischarge adherence. Finally, baseline adherence seems to correlate with postdischarge adherence, a finding which makes intuitive sense and has been previously reported for specific medications.36
There are several limitations to this study. Although large, the study was performed at only 2 clinical sites where most patients were white and fairly well‐educated, perhaps because patients admitted to a tertiary care center with ACS or ADHF are more affluent than general medical inpatients as a whole; this may limit generalizability. Postdischarge medication adherence might have been higher than in other patient populations given the nature of the population, possible loss‐to‐follow‐up bias, and the fact that half of the subjects received an intervention designed to improve medication management after discharge; such low rates of nonadherence in our study may have reduced our ability to detect important predictors in our models. In addition, the period of follow‐up was 30 days, thus limiting our findings to short‐term postdischarge medication adherence. Postdischarge medication adherence was based on patient self‐report, which not only assumed that the patient was still managing his/her own medications after discharge, but may also be susceptible to both recall and social acceptability bias, which might overestimate our adherence scores, again limiting our ability to detect important predictors of nonadherence. However, other studies have shown a good correlation between self‐reported medication adherence and other more objective measures,37, 38 and recall was only for 7 days, a measure used previously in the literature39, 40 and one designed to reduce recall bias. Systematic underreporting in certain patient populations is less likely but possible.
In the future, research should focus on targeting patients who have low baseline adherence to evaluate the effects of various interventions on postdischarge medication outcomes. Repeating the study in a population with a high prevalence of low health literacy might be illuminating, given that previous studies have shown that patients with low health literacy have less ability to identify their medications and have less refill adherence.29, 30
In conclusion, in patients hospitalized with cardiovascular disease, predictors of lower postdischarge adherence include younger age, Medicaid insurance, and low baseline adherence. It may be prudent to assess baseline adherence and insurance type in hospitalized patients in order to identify those who may benefit from additional assistance to improve medication adherence and medication safety during transitions in care.
Acknowledgements
Meeting Presentations: SGIM New England Regional Meeting, oral presentation, Boston, MA, March 4, 2011; and SGIM National Meeting, poster presentation, Phoenix, AZ, May 6, 2011. Dr Schnipper had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Disclosures: Financial support was provided by R01 HL089755 (NHLBI, Kripalani), K23 HL077597 (NHLBI, Kripalani), K08 HL072806 (NHLBI, Schnipper), T32HP10251‐02 (Cohen), and by the Division of General Medicine, Massachusetts General Hospital and the Harvard Medical School Fellowship in General Medicine and Primary Care (Cohen). Dr Kripalani is a consultant to and holds equity in PictureRx, LLC, which makes patient education tools to improve medication management. PictureRx did not provide materials or funding for this study. All other authors disclose no relevant or financial conflicts of interest.
- ,.Adherence to medication.N Engl J Med.2005;353(5):487–497.
- ,,,.Posthospital medication discrepancies: prevalence and contributing factors.Arch Intern Med.2005;165(16):1842–1847.
- ,.Medication use in the transition from hospital to home.Ann Acad Med Singapore.2008;37(2):136–141.
- ,,,.Medical errors related to discontinuity of care from an inpatient to an outpatient setting.J Gen Intern Med.2003;18(8):646–651.
- ,,,,.The incidence and severity of adverse events affecting patients after discharge from the hospital.Ann Intern Med.2003;138(3):161–167.
- ,,,,.Adverse drug events occurring following hospital discharge.J Gen Intern Med.2005;20(4):317–323.
- ,,, et al.Role of pharmacist counseling in preventing adverse drug events after hospitalization.Arch Intern Med.2006;166(5):565–571.
- ,,.Reconcilable differences: correcting medication errors at hospital admission and discharge.Qual Saf Health Care.2006;15(2):122–126.
- ,.Risk of rehospitalization among bipolar disorder patients who are nonadherent to antipsychotic therapy after hospital discharge.Am J Health Syst Pharm.2009;66(4):358–365.
- ,,,,.Continuity and adherence to long‐term drug treatment by geriatric patients after hospital discharge: a prospective cohort study.Drugs Aging.2008;25(10):861–870.
- ,,,.Medication use among inner‐city patients after hospital discharge: patient‐reported barriers and solutions.Mayo Clin Proc.2008;83(5):529–535.
- ,,,,,.Relationship of health literacy to intentional and unintentional non‐adherence of hospital discharge medications.J Gen Intern Med.2012;27(2):173–178.
- Office of Disease Prevention and Health Promotion, US Department of Health and Human Services.Healthy People 2010. Available at: http://www.healthypeople.gov/Document/pdf/uih/2010uih.pdf. Accessed February 15,2012.
- ,,, et al.Literacy and misunderstanding prescription drug labels.Ann Intern Med.2006;145(12):887–894.
- ,,,,,.Predictors of medication self‐management skill in a low‐literacy population.J Gen Intern Med.2006;21(8):852–856.
- ,,, et al;for the PILL‐CVD Study Group.Rationale and design of the Pharmacist Intervention for Low Literacy in Cardiovascular Disease (PILL‐CVD) study.Circ Cardiovasc Qual Outcomes.2010;3(2):212–219.
- ,,,,.Simplifying detection of cognitive impairment: comparison of the Mini‐Cog and Mini‐Mental State Examination in a multiethnic sample.J Am Geriatr Soc.2005;53(5):871–874.
- .Short Test of Functional Health Literacy in Adults.Snow Camp, NC:Peppercorn Books and Press;1998.
- ,,,.Predictive validity of a medication adherence measure in an outpatient setting.J Clin Hypertens (Greenwich).2008;10(5):348–354.
- ,,,,,.Health literacy and medication understanding among hospitalized adults.J Hosp Med. In press.
- ,,,,,.Health literacy and medication understanding among hospitalized adults.J Hosp Med.2011;6(9):488–493.
- ,,.The summary of diabetes self‐care activities measure: results from 7 studies and a revised scale.Diabetes Care.2000;23(7):943–950.
- .Multiple Imputation for Nonresponse in Surveys.New York, NY:John Wiley 1987.
- ,,, et al.Medication adherence in HIV‐infected adults: effect of patient age, cognitive status, and substance abuse.AIDS.2004;18(suppl 1):S19–S25.
- ,,.Factors associated with antihypertensive drug compliance in 83,884 Chinese patients: a cohort study.J Epidemiol Community Health.2010;64(10):895–901.
- ,,,,,.Adherence to oral hypoglycemic agents in 26,782 Chinese patients: a cohort study.J Clin Pharmacol.2011;51(10):1474–1482.
- ,,,,.Effect of a pharmacy‐based health literacy intervention and patient characteristics on medication refill adherence in an urban health system.Ann Pharmacother.2010;44(1):80–87.
- ,,.Adherence to combination antiretroviral therapies in HIV patients of low health literacy.J Gen Intern Med.1999;14(5):267–273.
- ,,,,,.Factors associated with medication refill adherence in cardiovascular‐related diseases: a focus on health literacy.J Gen Intern Med.2006;21(12):1215–1221.
- ,,,,.Limited health literacy is a barrier to medication reconciliation in ambulatory care.J Gen Intern Med.2007;22(11):1523–1526.
- ,,,,.The impact of low health literacy on surgical practice.Am J Surg.2004;188(3):250–253.
- ,,,,.Relationships between beliefs about medications and adherence.Am J Health Syst Pharm.2009;66(7):657–664.
- ,,,.Health literacy and anticoagulation‐related outcomes among patients taking warfarin.J Gen Intern Med.2006;21(8):841–846.
- ,,,,,.Health literacy, antiretroviral adherence, and HIV‐RNA suppression: a longitudinal perspective.J Gen Intern Med.2006;21(8):835–840.
- ,,, et al.Risk factors for nonadherence to warfarin: results from the IN‐RANGE study.Pharmacoepidemiol Drug Saf.2008;17(9):853–860.
- ,,, et al.Predictors of low clopidogrel adherence following percutaneous coronary intervention.Am J Cardiol.2011;108(6):822–827.
- ,,,,,.Correlation between adherence rates measured by MEMS and self‐reported questionnaires: a meta‐analysis.Health Qual Life Outcomes.2010;8:99.
- ,,,,,.Concordance of adherence measurement using self‐reported adherence questionnaires and medication monitoring devices.Pharmacoeconomics.2010;28(12):1097–1107.
- ,,,.Polypharmacy and medication adherence in patients with type 2 diabetes.Diabetes Care.2003;26(5):1408–1412.
- ,,,.Improving adherence and reducing medication discrepancies in patients with diabetes.Ann Pharmacother.2003;37(7–8):962–969.
In the outpatient setting, medication adherence (defined as percentage of prescribed medication doses taken by a patient during a specific time period) ranges between 40% and 80% for chronic conditions.1 During acute care hospitalization, changes are often made to patients' medication regimens, which can be confusing and contribute to nonadherence, medication errors, and harmful adverse events.2 Indeed, it is estimated that almost half of patients encounter a medication error after discharge, and approximately 12%17% experience an adverse drug event after returning home.36 It is likely that some of these adverse events may be the result of medication nonadherence.7 Improved patientprovider communication, systems to reconcile prehospitalization and posthospitalization medications, as well as development of mechanisms to enhance adherence, may prevent many of these errors and have become new targets for quality improvement.4, 8 Although postdischarge medication adherence is a crucial target for avoiding adverse events and rehospitalization, few studies have focused on understanding its incidence and predictors, in particular, patient demographic factors such as age and insurance status.911
In addition, few studies have looked at general and posthospital adherence in a population where health literacy is measured, an important area because medication changes during hospitalization may be particularly confusing for patients with low health literacy.11, 12 Health literacy is defined as the degree to which an individual has the capacity to obtain, process, and understand basic health information and services needed to make appropriate health decisions.13 Prior outpatient research shows that low health literacy is associated with poor patient understanding of the medication regimen and instructions for medication use, which may contribute to postdischarge medication nonadherence.14, 15 Understanding the factors associated with postdischarge medication adherence could help refine interventions that are oriented toward improving transitions in care, patient safety, and reducing unnecessary rehospitalization.
We report here on factors associated with postdischarge medication adherence using data from the Pharmacist Intervention for Low Literacy in Cardiovascular Disease (PILL‐CVD) study.16
METHODS
Study and Participants
PILL‐CVD was a federally funded, 2‐site randomized controlled trial using pharmacist‐assisted medication reconciliation, inpatient pharmacist counseling, low‐literacy adherence aids, and telephone follow‐up that aimed to decrease rates of serious medication errors after hospital discharge.16 The study targeted patients with cardiovascular disease (hospitalized on cardiology or general medical or geriatric services for acute coronary syndromes [ACS] or acute decompensated heart failure [ADHF]) at 2 large academic hospitals, Brigham and Women's Hospital (BWH) and Vanderbilt University Hospital (VUH).
Subjects were eligible for enrollment if they met criteria for ACS or ADHF, were likely to be discharged to home as determined by the primary medical team at the time of study enrollment, and took primary responsibility for administering their medications prior to admission (caregivers could be involved in medication management after discharge). Exclusion criteria included severe visual or hearing impairment, inability to communicate in English or Spanish, active psychiatric illness, dementia, delirium, illness too severe to participate, lack of a home phone number, being in police custody, or participation in another intensive medication adherence program (eg, due to renal transplant).
Out of 6416 patients originally screened for possible enrollment, 862 were randomly assigned to receive usual care or usual care plus the intervention, and 851 remained in the study.16 Both the main study and this secondary data analysis were approved by the Institutional Review Boards of each site.
Baseline Measures
Following informed consent and study enrollment, a variety of baseline data were collected on study participants from medical records and patient interview, including primary language, demographic information (age, race, insurance status, income, and education level), cognition (through administration of the 05‐point MiniCog scale),17 and level of health literacy (through use of the 036‐point short form of the Test of Functional Health Literacy in Adults [s‐TOFHLA] scale).18 Baseline information was also collected on medication use, including number of preadmission medications, measurement of self‐reported adherence prior to admission (using the Morisky scale, a validated 04‐point questionnaire shown to correlate with disease control and indicative of general patterns of adherence),19 and a medication understanding score, adapted from other instruments, which quantifies understanding of the indication, dose, and frequency of up to 5 randomly selected preadmission medications on a 03‐point scale.16, 20, 21
Outcome Measures
Outcomes were collected 30 days postdischarge through a structured questionnaire, administered by telephone. Only patients who completed this call are included in the present analysis. Postdischarge medication adherence was assessed by asking patients to report the number of days out of the previous week they had taken each medication from their postdischarge regimen exactly as prescribed.22 A score was calculated for each medication as the proportion of adherent days (eg, if a patient reported missing 2 days of a medication in the previous week, then adherence would be 5/7 or 71%). A global postdischarge adherence score was then derived for each patient by averaging the adherence score for all regularly scheduled medications. This quantitative measure focused on adherence to medications patients knew they should be taking and did not measure medication discrepancies (sometimes termed unintentional nonadherence).
Analysis
Patient characteristics were summarized and reported using simple descriptive statistics. Candidate predictors of postdischarge medication adherence were chosen a priori from patient characteristics assessed during hospital admission. These included patient age, gender, race, ethnicity, marital status, insurance, years of education, presence of primary care physician (PCP), study site, number of preadmission medications, medication understanding, baseline adherence, cognition, and health literacy. Unadjusted results were calculated using univariable linear regression, with each patient's adherence score as the dependent variable and each predictor as the independent variable. Adjusted results were then derived using multivariable linear regression with all the candidate predictors in the model.
Lastly, because of missing data for some predictors, in particular baseline adherence and medication understanding, multiple imputation techniques were used to impute missing data and increase statistical power.23 We used the Markov Chain Monte Carlo (MCMC) method for multiple imputation, which generally assumes that the data came from a normal distribution and that the missing data are missing at random. Because of the essentially normal distribution of the data, and because the amount of missing data was so small (<1% for almost all variables, 5% for baseline adherence, and 8% for medication understanding), we expected little bias and present the complete case analysis, which maximized statistical power.
Two‐sided P values <0.05 were considered significant, and SAS version 9.2 (Cary, NC) was used for all analyses.
RESULTS
Table 1 shows descriptive baseline patient characteristics of study sample (responders) as well as nonresponders at 30 days. For the responders, the mean age of the 646 patients was 61.2 years, 94.7% were insured, and 19.3% had inadequate or marginal health literacy. Patients were prescribed an average of 8 preadmission medications. Most patients (92.3%) had a regular PCP prior to admission. Nonresponders had nonsignificant trends towards having lower health literacy, medication understanding, and baseline medication adherence.
| Characteristic | Total N, 30‐Day Respondents | Value | Total N, Nonrespondents | Value |
|---|---|---|---|---|
| ||||
| Age, mean in yr (SD) | 646 | 61.2 (13.5) | 45 | 55.4 (14.3) |
| Gender, N (percentage) | 646 | 45 | ||
| Female | 272 (42.1) | 18 (40.0) | ||
| Male | 374 (57.9) | 27 (60.0) | ||
| Race, N (percentage) | 643 | 45 | ||
| White | 511 (79.5) | 32 (71.1) | ||
| Black | 104 (16.2) | 11 (24.4) | ||
| Other | 28 (4.4) | 2 (4.4) | ||
| Ethnicity, N (percentage) | 639 | 45 | ||
| Hispanic | 24 (3.8) | 1 (2.2) | ||
| Not Hispanic | 615 (96.2) | 44 (97.8) | ||
| Marital status, N (percentage) | 646 | 45 | ||
| Married/cohabitate | 382 (59.1) | 20 (44.4) | ||
| Separated/divorced | 118 (18.3) | 11 (24.4) | ||
| Widowed | 81 (12.5) | 5 (11.1) | ||
| Never married | 65 (10.1) | 9 (2.0) | ||
| Insurance type, N (percentage) | 646 | 45 | ||
| Medicaid | 53 (8.2) | 5 (11.1) | ||
| Medicare | 270 (41.8) | 13 (28.9) | ||
| Private | 289 (44.7) | 19 (42.2) | ||
| Self‐pay | 34 (5.3) | 8 (17.8) | ||
| Years of education, mean in yr (SD) | 643 | 14.0 (3.1) | 45 | 13.3 (2.7) |
| Presence of PCP prior to admission, N (percentage) | 646 | 45 | ||
| Yes | 596 (92.3) | 38 (84.4) | ||
| No | 50 (7.74) | 7 (15.6) | ||
| Site, N (percentage) | 646 | 45 | ||
| Site 1 | 358 (55.4) | 8 (17.8) | ||
| Site 2 | 288 (44.6) | 37 (82.2) | ||
| No. of preadmission medications, mean no. (SD) | 641 | 7.8 (4.8) | 45 | 7.7 (5.4) |
| Medication understanding score, mean (SD)* | 597 | 2.4 (0.5) | 40 | 2.2 (0.62) |
| Health literacy (s‐TOFHLA) score, mean (SD) | 642 | 29.1 (8.9) | 45 | 26.0 (12.0) |
| Baseline adherence (SD) | 613 | 2.7 (1.1) | 45 | 2.4 (1.2) |
| MiniCog score, N (percentage) | 646 | 45 | ||
| Demented | 63 (9.8) | 5 (11.1) | ||
| Not demented | 583 (90.2) | 40 (88.9) | ||
The average postdischarge adherence score was 95% (standard deviation [SD] = 10.2%), and less than 10% of patients had an adherence score of less than 85%; overall the distribution was left‐skewed. Table 2 illustrates crude and adjusted parameter estimates for variables in the model. Table 3 shows significant findings in the fully adjusted model, which used multiple imputation techniques to account for missing data.
| Predictor | Crude Parameter Estimate (Beta) With 95% Confidence Intervals | P Value | Adjusted Parameter Estimate (Beta) With 95% Confidence Intervals | P Value |
|---|---|---|---|---|
| ||||
| Age per 10 yr | 0.010 (0.007, 0.020) | <0.0001 | 0.010 (0.002, 0.020) | 0.018 |
| Male gender | 0.012 (0.004, 0.028) | 0.137 | 0.003 (0.014, 0.020) | 0.727 |
| Race/ethnicity | ||||
| White | 0.011 (0.009, 0.031) | 0.266 | Ref | Ref |
| Black | 0.017 (0.038, 0.005) | 0.13 | 0.006 (0.017, 0.030) | 0.598 |
| Other | 0.010 (0.029, 0.049) | 0.599 | 0.017 (0.027, 0.062) | 0.446 |
| Hispanic/Latino | 0.005 (0.037, 0.047) | 0.803 | 0.036 (0.013, 0.085) | 0.149 |
| Marital status | ||||
| Married/cohabitate | 0.006 (0.011, 0.022) | 0.500 | Ref | Ref |
| Separated/divorced | 0.005 (0.025, 0.016) | 0.664 | 0.009 (0.014, 0.031) | 0.446 |
| Widowed | 0.001 (0.023, 0.025) | 0.922 | 0.013 (0.039, 0.013) | 0.338 |
| Never married | 0.009 (0.035, 0.018) | 0.515 | 0.004 (0.033, 0.025) | 0.784 |
| Insurance type | ||||
| Private | 0.008 (0.008, 0.024) | 0.347 | Ref | Ref |
| Medicaid | 0.046 (0.075, 0.018) | 0.002 | 0.026 (0.058, 0.007) | 0.121 |
| Medicare | 0.012 (0.004, 0.028) | 0.138 | 0.002 (0.023, 0.018) | 0.844 |
| Self‐pay | 0.027 (0.062, 0.008) | 0.135 | 0.029 (0.073, 0.015) | 0.202 |
| Years of education | 0.003 (0.0003, 0.005) | 0.028 | 0.0001 (0.003, 0.003) | 0.949 |
| Presence of PCP prior to admission | 0.007 (0.022, 0.037) | 0.630 | 0.002 (0.032, 0.036) | 0.888 |
| Site | 0.050 (0.065, 0.034) | <0.0001 | 0.038 (0.056, 0.021) | <0.0001 |
| No. of preadmission medications | 0.0003 (0.002, 0.001) | 0.684 | 0.0001 (0.002, 0.002) | 0.918 |
| Medication understanding score per point | 0.007 (0.009, 0.023) | 0.390 | 0.006 (0.011, 0.023) | 0.513 |
| Health literacy (s‐TOFHLA) score per 10 points | 0.0006 (0.008, 0.01) | 0.897 | 0.003 (0.008, 0.01) | 0.644 |
| Baseline adherence per point | 0.023 (0.016, 0.031) | <0.0001 | 0.017 (0.009, 0.024) | <0.0001 |
| Cognitive function | 0.004 (0.022, 0.031) | 0.757 | 0.008 (0.019, 0.036) | 0.549 |
| Predictor | Parameter Estimate (Beta) With 95% Confidence Intervals | P Value |
|---|---|---|
| ||
| Age per 10 yr | 0.010 (0.004, 0.020) | 0.004 |
| Insurance type | ||
| Private | Ref | Ref |
| Medicaid | 0.045 (0.076, 0.014) | 0.005 |
| Medicare | 0.010 (0.030, 0.010) | 0.333 |
| Self‐pay | 0.013 (0.050, 0.025) | 0.512 |
| Site | 0.036 (0.053, 0.019) | <0.0001 |
| Baseline adherence per point | 0.016 (0.008, 0.024) | <0.0001 |
Intervention arm was of borderline statistical significance in predicting postdischarge adherence (P = 0.052), and so was removed from the final model. Study site, age, insurance, and baseline adherence were the only significant independent predictors of postdischarge adherence in the fully adjusted model (Table 3). For example, for every 10‐year increase in age, patients had, on average, an adjusted 1% absolute increase in their adherence score (95% confidence interval [CI] 0.4% to 2.0%). For every 1‐point increase in baseline medication adherence (based on the Morisky scale), there was a 1.6% absolute increase in medication adherence (95% CI 0.8% to 2.4%). In unadjusted analyses, patients with Medicaid were less adherent with medications after discharge than were patients with private insurance. This difference became nonsignificant in adjusted analyses, but when analyses were repeated using multiple imputation techniques, the results again became statistically significantMedicaid insurance was associated with a 4.5% absolute decrease in postdischarge adherence compared with private insurance (95% CI 7.6% to 1.4%). Study site (specifically, Brigham and Women's Hospital) was also a significant predictor of greater postdischarge medication adherence. Years of education was a significant predictor of adherence in unadjusted analyses, but was not an independent predictor when adjusted for other factors. When baseline adherence was removed from the multiple imputation model, there were no changes in which factors were significant predictors of adherence.
DISCUSSION
In this study, we found that low baseline adherence, younger age, Medicaid insurance, and study site were significant predictors of lower 30‐day medication adherence. Of particular interest is our finding regarding baseline adherence, a simple measure to obtain on hospitalized patients. It is notable that in our study, education was not an independent significant predictor of postdischarge adherence, even when baseline adherence was removed from the model. The same is true for medication understanding, cognitive function, and health literacy.
Older patients appeared more adherent with medications in the month after hospital discharge, perhaps reflecting increased interaction with the healthcare system (appointments, number of physician interactions), a greater belief in the importance of chronic medication management, or a higher level of experience with managing medications. A similar relationship between age and adherence has been shown in outpatient studies of patients with hypertension, diabetes, and other chronic diseases.2427
Medicaid patients may be less likely to remain adherent because of the plan's limited coverage of medications relative to patients' ability to pay. For example, Medicaid in Tennessee covers the first 5 generic medications at no cost to the patient but has co‐payments for additional medications and for brand name drugs. Medicaid in Massachusetts has co‐payments of $1 to $3 for each medication. Alternatively, Medicaid insurance may be a marker for other patient characteristics associated with low adherence for which we were not fully able to adjust.
Site differences were also notable in this study; these differences could have been due to differences in insurance coverage in Tennessee versus Massachusetts (which has near‐universal coverage), differences in types of insurance (eg, fewer patients at Brigham and Women's Hospital had Medicaid than at Vanderbilt), cultural and geographic differences between the 2 locations, or other differences in transitional care between the 2 sites.
This study corroborates previous literature on medication adherence (specifically unintentional nonadherence) in the outpatient setting,4, 811 for example, on the association of younger age with low adherence in certain populations. On the other hand, it may contrast with previous literature which has sometimes shown a relationship between patient education or health literacy and medication adherence.14, 15, 2835 However, previous studies have not focused on the transition from inpatient to outpatient settings. Perhaps intensive medication education in the hospital, even under usual care, mitigates the effects of these factors on postdischarge adherence. Finally, baseline adherence seems to correlate with postdischarge adherence, a finding which makes intuitive sense and has been previously reported for specific medications.36
There are several limitations to this study. Although large, the study was performed at only 2 clinical sites where most patients were white and fairly well‐educated, perhaps because patients admitted to a tertiary care center with ACS or ADHF are more affluent than general medical inpatients as a whole; this may limit generalizability. Postdischarge medication adherence might have been higher than in other patient populations given the nature of the population, possible loss‐to‐follow‐up bias, and the fact that half of the subjects received an intervention designed to improve medication management after discharge; such low rates of nonadherence in our study may have reduced our ability to detect important predictors in our models. In addition, the period of follow‐up was 30 days, thus limiting our findings to short‐term postdischarge medication adherence. Postdischarge medication adherence was based on patient self‐report, which not only assumed that the patient was still managing his/her own medications after discharge, but may also be susceptible to both recall and social acceptability bias, which might overestimate our adherence scores, again limiting our ability to detect important predictors of nonadherence. However, other studies have shown a good correlation between self‐reported medication adherence and other more objective measures,37, 38 and recall was only for 7 days, a measure used previously in the literature39, 40 and one designed to reduce recall bias. Systematic underreporting in certain patient populations is less likely but possible.
In the future, research should focus on targeting patients who have low baseline adherence to evaluate the effects of various interventions on postdischarge medication outcomes. Repeating the study in a population with a high prevalence of low health literacy might be illuminating, given that previous studies have shown that patients with low health literacy have less ability to identify their medications and have less refill adherence.29, 30
In conclusion, in patients hospitalized with cardiovascular disease, predictors of lower postdischarge adherence include younger age, Medicaid insurance, and low baseline adherence. It may be prudent to assess baseline adherence and insurance type in hospitalized patients in order to identify those who may benefit from additional assistance to improve medication adherence and medication safety during transitions in care.
Acknowledgements
Meeting Presentations: SGIM New England Regional Meeting, oral presentation, Boston, MA, March 4, 2011; and SGIM National Meeting, poster presentation, Phoenix, AZ, May 6, 2011. Dr Schnipper had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Disclosures: Financial support was provided by R01 HL089755 (NHLBI, Kripalani), K23 HL077597 (NHLBI, Kripalani), K08 HL072806 (NHLBI, Schnipper), T32HP10251‐02 (Cohen), and by the Division of General Medicine, Massachusetts General Hospital and the Harvard Medical School Fellowship in General Medicine and Primary Care (Cohen). Dr Kripalani is a consultant to and holds equity in PictureRx, LLC, which makes patient education tools to improve medication management. PictureRx did not provide materials or funding for this study. All other authors disclose no relevant or financial conflicts of interest.
In the outpatient setting, medication adherence (defined as percentage of prescribed medication doses taken by a patient during a specific time period) ranges between 40% and 80% for chronic conditions.1 During acute care hospitalization, changes are often made to patients' medication regimens, which can be confusing and contribute to nonadherence, medication errors, and harmful adverse events.2 Indeed, it is estimated that almost half of patients encounter a medication error after discharge, and approximately 12%17% experience an adverse drug event after returning home.36 It is likely that some of these adverse events may be the result of medication nonadherence.7 Improved patientprovider communication, systems to reconcile prehospitalization and posthospitalization medications, as well as development of mechanisms to enhance adherence, may prevent many of these errors and have become new targets for quality improvement.4, 8 Although postdischarge medication adherence is a crucial target for avoiding adverse events and rehospitalization, few studies have focused on understanding its incidence and predictors, in particular, patient demographic factors such as age and insurance status.911
In addition, few studies have looked at general and posthospital adherence in a population where health literacy is measured, an important area because medication changes during hospitalization may be particularly confusing for patients with low health literacy.11, 12 Health literacy is defined as the degree to which an individual has the capacity to obtain, process, and understand basic health information and services needed to make appropriate health decisions.13 Prior outpatient research shows that low health literacy is associated with poor patient understanding of the medication regimen and instructions for medication use, which may contribute to postdischarge medication nonadherence.14, 15 Understanding the factors associated with postdischarge medication adherence could help refine interventions that are oriented toward improving transitions in care, patient safety, and reducing unnecessary rehospitalization.
We report here on factors associated with postdischarge medication adherence using data from the Pharmacist Intervention for Low Literacy in Cardiovascular Disease (PILL‐CVD) study.16
METHODS
Study and Participants
PILL‐CVD was a federally funded, 2‐site randomized controlled trial using pharmacist‐assisted medication reconciliation, inpatient pharmacist counseling, low‐literacy adherence aids, and telephone follow‐up that aimed to decrease rates of serious medication errors after hospital discharge.16 The study targeted patients with cardiovascular disease (hospitalized on cardiology or general medical or geriatric services for acute coronary syndromes [ACS] or acute decompensated heart failure [ADHF]) at 2 large academic hospitals, Brigham and Women's Hospital (BWH) and Vanderbilt University Hospital (VUH).
Subjects were eligible for enrollment if they met criteria for ACS or ADHF, were likely to be discharged to home as determined by the primary medical team at the time of study enrollment, and took primary responsibility for administering their medications prior to admission (caregivers could be involved in medication management after discharge). Exclusion criteria included severe visual or hearing impairment, inability to communicate in English or Spanish, active psychiatric illness, dementia, delirium, illness too severe to participate, lack of a home phone number, being in police custody, or participation in another intensive medication adherence program (eg, due to renal transplant).
Out of 6416 patients originally screened for possible enrollment, 862 were randomly assigned to receive usual care or usual care plus the intervention, and 851 remained in the study.16 Both the main study and this secondary data analysis were approved by the Institutional Review Boards of each site.
Baseline Measures
Following informed consent and study enrollment, a variety of baseline data were collected on study participants from medical records and patient interview, including primary language, demographic information (age, race, insurance status, income, and education level), cognition (through administration of the 05‐point MiniCog scale),17 and level of health literacy (through use of the 036‐point short form of the Test of Functional Health Literacy in Adults [s‐TOFHLA] scale).18 Baseline information was also collected on medication use, including number of preadmission medications, measurement of self‐reported adherence prior to admission (using the Morisky scale, a validated 04‐point questionnaire shown to correlate with disease control and indicative of general patterns of adherence),19 and a medication understanding score, adapted from other instruments, which quantifies understanding of the indication, dose, and frequency of up to 5 randomly selected preadmission medications on a 03‐point scale.16, 20, 21
Outcome Measures
Outcomes were collected 30 days postdischarge through a structured questionnaire, administered by telephone. Only patients who completed this call are included in the present analysis. Postdischarge medication adherence was assessed by asking patients to report the number of days out of the previous week they had taken each medication from their postdischarge regimen exactly as prescribed.22 A score was calculated for each medication as the proportion of adherent days (eg, if a patient reported missing 2 days of a medication in the previous week, then adherence would be 5/7 or 71%). A global postdischarge adherence score was then derived for each patient by averaging the adherence score for all regularly scheduled medications. This quantitative measure focused on adherence to medications patients knew they should be taking and did not measure medication discrepancies (sometimes termed unintentional nonadherence).
Analysis
Patient characteristics were summarized and reported using simple descriptive statistics. Candidate predictors of postdischarge medication adherence were chosen a priori from patient characteristics assessed during hospital admission. These included patient age, gender, race, ethnicity, marital status, insurance, years of education, presence of primary care physician (PCP), study site, number of preadmission medications, medication understanding, baseline adherence, cognition, and health literacy. Unadjusted results were calculated using univariable linear regression, with each patient's adherence score as the dependent variable and each predictor as the independent variable. Adjusted results were then derived using multivariable linear regression with all the candidate predictors in the model.
Lastly, because of missing data for some predictors, in particular baseline adherence and medication understanding, multiple imputation techniques were used to impute missing data and increase statistical power.23 We used the Markov Chain Monte Carlo (MCMC) method for multiple imputation, which generally assumes that the data came from a normal distribution and that the missing data are missing at random. Because of the essentially normal distribution of the data, and because the amount of missing data was so small (<1% for almost all variables, 5% for baseline adherence, and 8% for medication understanding), we expected little bias and present the complete case analysis, which maximized statistical power.
Two‐sided P values <0.05 were considered significant, and SAS version 9.2 (Cary, NC) was used for all analyses.
RESULTS
Table 1 shows descriptive baseline patient characteristics of study sample (responders) as well as nonresponders at 30 days. For the responders, the mean age of the 646 patients was 61.2 years, 94.7% were insured, and 19.3% had inadequate or marginal health literacy. Patients were prescribed an average of 8 preadmission medications. Most patients (92.3%) had a regular PCP prior to admission. Nonresponders had nonsignificant trends towards having lower health literacy, medication understanding, and baseline medication adherence.
| Characteristic | Total N, 30‐Day Respondents | Value | Total N, Nonrespondents | Value |
|---|---|---|---|---|
| ||||
| Age, mean in yr (SD) | 646 | 61.2 (13.5) | 45 | 55.4 (14.3) |
| Gender, N (percentage) | 646 | 45 | ||
| Female | 272 (42.1) | 18 (40.0) | ||
| Male | 374 (57.9) | 27 (60.0) | ||
| Race, N (percentage) | 643 | 45 | ||
| White | 511 (79.5) | 32 (71.1) | ||
| Black | 104 (16.2) | 11 (24.4) | ||
| Other | 28 (4.4) | 2 (4.4) | ||
| Ethnicity, N (percentage) | 639 | 45 | ||
| Hispanic | 24 (3.8) | 1 (2.2) | ||
| Not Hispanic | 615 (96.2) | 44 (97.8) | ||
| Marital status, N (percentage) | 646 | 45 | ||
| Married/cohabitate | 382 (59.1) | 20 (44.4) | ||
| Separated/divorced | 118 (18.3) | 11 (24.4) | ||
| Widowed | 81 (12.5) | 5 (11.1) | ||
| Never married | 65 (10.1) | 9 (2.0) | ||
| Insurance type, N (percentage) | 646 | 45 | ||
| Medicaid | 53 (8.2) | 5 (11.1) | ||
| Medicare | 270 (41.8) | 13 (28.9) | ||
| Private | 289 (44.7) | 19 (42.2) | ||
| Self‐pay | 34 (5.3) | 8 (17.8) | ||
| Years of education, mean in yr (SD) | 643 | 14.0 (3.1) | 45 | 13.3 (2.7) |
| Presence of PCP prior to admission, N (percentage) | 646 | 45 | ||
| Yes | 596 (92.3) | 38 (84.4) | ||
| No | 50 (7.74) | 7 (15.6) | ||
| Site, N (percentage) | 646 | 45 | ||
| Site 1 | 358 (55.4) | 8 (17.8) | ||
| Site 2 | 288 (44.6) | 37 (82.2) | ||
| No. of preadmission medications, mean no. (SD) | 641 | 7.8 (4.8) | 45 | 7.7 (5.4) |
| Medication understanding score, mean (SD)* | 597 | 2.4 (0.5) | 40 | 2.2 (0.62) |
| Health literacy (s‐TOFHLA) score, mean (SD) | 642 | 29.1 (8.9) | 45 | 26.0 (12.0) |
| Baseline adherence (SD) | 613 | 2.7 (1.1) | 45 | 2.4 (1.2) |
| MiniCog score, N (percentage) | 646 | 45 | ||
| Demented | 63 (9.8) | 5 (11.1) | ||
| Not demented | 583 (90.2) | 40 (88.9) | ||
The average postdischarge adherence score was 95% (standard deviation [SD] = 10.2%), and less than 10% of patients had an adherence score of less than 85%; overall the distribution was left‐skewed. Table 2 illustrates crude and adjusted parameter estimates for variables in the model. Table 3 shows significant findings in the fully adjusted model, which used multiple imputation techniques to account for missing data.
| Predictor | Crude Parameter Estimate (Beta) With 95% Confidence Intervals | P Value | Adjusted Parameter Estimate (Beta) With 95% Confidence Intervals | P Value |
|---|---|---|---|---|
| ||||
| Age per 10 yr | 0.010 (0.007, 0.020) | <0.0001 | 0.010 (0.002, 0.020) | 0.018 |
| Male gender | 0.012 (0.004, 0.028) | 0.137 | 0.003 (0.014, 0.020) | 0.727 |
| Race/ethnicity | ||||
| White | 0.011 (0.009, 0.031) | 0.266 | Ref | Ref |
| Black | 0.017 (0.038, 0.005) | 0.13 | 0.006 (0.017, 0.030) | 0.598 |
| Other | 0.010 (0.029, 0.049) | 0.599 | 0.017 (0.027, 0.062) | 0.446 |
| Hispanic/Latino | 0.005 (0.037, 0.047) | 0.803 | 0.036 (0.013, 0.085) | 0.149 |
| Marital status | ||||
| Married/cohabitate | 0.006 (0.011, 0.022) | 0.500 | Ref | Ref |
| Separated/divorced | 0.005 (0.025, 0.016) | 0.664 | 0.009 (0.014, 0.031) | 0.446 |
| Widowed | 0.001 (0.023, 0.025) | 0.922 | 0.013 (0.039, 0.013) | 0.338 |
| Never married | 0.009 (0.035, 0.018) | 0.515 | 0.004 (0.033, 0.025) | 0.784 |
| Insurance type | ||||
| Private | 0.008 (0.008, 0.024) | 0.347 | Ref | Ref |
| Medicaid | 0.046 (0.075, 0.018) | 0.002 | 0.026 (0.058, 0.007) | 0.121 |
| Medicare | 0.012 (0.004, 0.028) | 0.138 | 0.002 (0.023, 0.018) | 0.844 |
| Self‐pay | 0.027 (0.062, 0.008) | 0.135 | 0.029 (0.073, 0.015) | 0.202 |
| Years of education | 0.003 (0.0003, 0.005) | 0.028 | 0.0001 (0.003, 0.003) | 0.949 |
| Presence of PCP prior to admission | 0.007 (0.022, 0.037) | 0.630 | 0.002 (0.032, 0.036) | 0.888 |
| Site | 0.050 (0.065, 0.034) | <0.0001 | 0.038 (0.056, 0.021) | <0.0001 |
| No. of preadmission medications | 0.0003 (0.002, 0.001) | 0.684 | 0.0001 (0.002, 0.002) | 0.918 |
| Medication understanding score per point | 0.007 (0.009, 0.023) | 0.390 | 0.006 (0.011, 0.023) | 0.513 |
| Health literacy (s‐TOFHLA) score per 10 points | 0.0006 (0.008, 0.01) | 0.897 | 0.003 (0.008, 0.01) | 0.644 |
| Baseline adherence per point | 0.023 (0.016, 0.031) | <0.0001 | 0.017 (0.009, 0.024) | <0.0001 |
| Cognitive function | 0.004 (0.022, 0.031) | 0.757 | 0.008 (0.019, 0.036) | 0.549 |
| Predictor | Parameter Estimate (Beta) With 95% Confidence Intervals | P Value |
|---|---|---|
| ||
| Age per 10 yr | 0.010 (0.004, 0.020) | 0.004 |
| Insurance type | ||
| Private | Ref | Ref |
| Medicaid | 0.045 (0.076, 0.014) | 0.005 |
| Medicare | 0.010 (0.030, 0.010) | 0.333 |
| Self‐pay | 0.013 (0.050, 0.025) | 0.512 |
| Site | 0.036 (0.053, 0.019) | <0.0001 |
| Baseline adherence per point | 0.016 (0.008, 0.024) | <0.0001 |
Intervention arm was of borderline statistical significance in predicting postdischarge adherence (P = 0.052), and so was removed from the final model. Study site, age, insurance, and baseline adherence were the only significant independent predictors of postdischarge adherence in the fully adjusted model (Table 3). For example, for every 10‐year increase in age, patients had, on average, an adjusted 1% absolute increase in their adherence score (95% confidence interval [CI] 0.4% to 2.0%). For every 1‐point increase in baseline medication adherence (based on the Morisky scale), there was a 1.6% absolute increase in medication adherence (95% CI 0.8% to 2.4%). In unadjusted analyses, patients with Medicaid were less adherent with medications after discharge than were patients with private insurance. This difference became nonsignificant in adjusted analyses, but when analyses were repeated using multiple imputation techniques, the results again became statistically significantMedicaid insurance was associated with a 4.5% absolute decrease in postdischarge adherence compared with private insurance (95% CI 7.6% to 1.4%). Study site (specifically, Brigham and Women's Hospital) was also a significant predictor of greater postdischarge medication adherence. Years of education was a significant predictor of adherence in unadjusted analyses, but was not an independent predictor when adjusted for other factors. When baseline adherence was removed from the multiple imputation model, there were no changes in which factors were significant predictors of adherence.
DISCUSSION
In this study, we found that low baseline adherence, younger age, Medicaid insurance, and study site were significant predictors of lower 30‐day medication adherence. Of particular interest is our finding regarding baseline adherence, a simple measure to obtain on hospitalized patients. It is notable that in our study, education was not an independent significant predictor of postdischarge adherence, even when baseline adherence was removed from the model. The same is true for medication understanding, cognitive function, and health literacy.
Older patients appeared more adherent with medications in the month after hospital discharge, perhaps reflecting increased interaction with the healthcare system (appointments, number of physician interactions), a greater belief in the importance of chronic medication management, or a higher level of experience with managing medications. A similar relationship between age and adherence has been shown in outpatient studies of patients with hypertension, diabetes, and other chronic diseases.2427
Medicaid patients may be less likely to remain adherent because of the plan's limited coverage of medications relative to patients' ability to pay. For example, Medicaid in Tennessee covers the first 5 generic medications at no cost to the patient but has co‐payments for additional medications and for brand name drugs. Medicaid in Massachusetts has co‐payments of $1 to $3 for each medication. Alternatively, Medicaid insurance may be a marker for other patient characteristics associated with low adherence for which we were not fully able to adjust.
Site differences were also notable in this study; these differences could have been due to differences in insurance coverage in Tennessee versus Massachusetts (which has near‐universal coverage), differences in types of insurance (eg, fewer patients at Brigham and Women's Hospital had Medicaid than at Vanderbilt), cultural and geographic differences between the 2 locations, or other differences in transitional care between the 2 sites.
This study corroborates previous literature on medication adherence (specifically unintentional nonadherence) in the outpatient setting,4, 811 for example, on the association of younger age with low adherence in certain populations. On the other hand, it may contrast with previous literature which has sometimes shown a relationship between patient education or health literacy and medication adherence.14, 15, 2835 However, previous studies have not focused on the transition from inpatient to outpatient settings. Perhaps intensive medication education in the hospital, even under usual care, mitigates the effects of these factors on postdischarge adherence. Finally, baseline adherence seems to correlate with postdischarge adherence, a finding which makes intuitive sense and has been previously reported for specific medications.36
There are several limitations to this study. Although large, the study was performed at only 2 clinical sites where most patients were white and fairly well‐educated, perhaps because patients admitted to a tertiary care center with ACS or ADHF are more affluent than general medical inpatients as a whole; this may limit generalizability. Postdischarge medication adherence might have been higher than in other patient populations given the nature of the population, possible loss‐to‐follow‐up bias, and the fact that half of the subjects received an intervention designed to improve medication management after discharge; such low rates of nonadherence in our study may have reduced our ability to detect important predictors in our models. In addition, the period of follow‐up was 30 days, thus limiting our findings to short‐term postdischarge medication adherence. Postdischarge medication adherence was based on patient self‐report, which not only assumed that the patient was still managing his/her own medications after discharge, but may also be susceptible to both recall and social acceptability bias, which might overestimate our adherence scores, again limiting our ability to detect important predictors of nonadherence. However, other studies have shown a good correlation between self‐reported medication adherence and other more objective measures,37, 38 and recall was only for 7 days, a measure used previously in the literature39, 40 and one designed to reduce recall bias. Systematic underreporting in certain patient populations is less likely but possible.
In the future, research should focus on targeting patients who have low baseline adherence to evaluate the effects of various interventions on postdischarge medication outcomes. Repeating the study in a population with a high prevalence of low health literacy might be illuminating, given that previous studies have shown that patients with low health literacy have less ability to identify their medications and have less refill adherence.29, 30
In conclusion, in patients hospitalized with cardiovascular disease, predictors of lower postdischarge adherence include younger age, Medicaid insurance, and low baseline adherence. It may be prudent to assess baseline adherence and insurance type in hospitalized patients in order to identify those who may benefit from additional assistance to improve medication adherence and medication safety during transitions in care.
Acknowledgements
Meeting Presentations: SGIM New England Regional Meeting, oral presentation, Boston, MA, March 4, 2011; and SGIM National Meeting, poster presentation, Phoenix, AZ, May 6, 2011. Dr Schnipper had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Disclosures: Financial support was provided by R01 HL089755 (NHLBI, Kripalani), K23 HL077597 (NHLBI, Kripalani), K08 HL072806 (NHLBI, Schnipper), T32HP10251‐02 (Cohen), and by the Division of General Medicine, Massachusetts General Hospital and the Harvard Medical School Fellowship in General Medicine and Primary Care (Cohen). Dr Kripalani is a consultant to and holds equity in PictureRx, LLC, which makes patient education tools to improve medication management. PictureRx did not provide materials or funding for this study. All other authors disclose no relevant or financial conflicts of interest.
- ,.Adherence to medication.N Engl J Med.2005;353(5):487–497.
- ,,,.Posthospital medication discrepancies: prevalence and contributing factors.Arch Intern Med.2005;165(16):1842–1847.
- ,.Medication use in the transition from hospital to home.Ann Acad Med Singapore.2008;37(2):136–141.
- ,,,.Medical errors related to discontinuity of care from an inpatient to an outpatient setting.J Gen Intern Med.2003;18(8):646–651.
- ,,,,.The incidence and severity of adverse events affecting patients after discharge from the hospital.Ann Intern Med.2003;138(3):161–167.
- ,,,,.Adverse drug events occurring following hospital discharge.J Gen Intern Med.2005;20(4):317–323.
- ,,, et al.Role of pharmacist counseling in preventing adverse drug events after hospitalization.Arch Intern Med.2006;166(5):565–571.
- ,,.Reconcilable differences: correcting medication errors at hospital admission and discharge.Qual Saf Health Care.2006;15(2):122–126.
- ,.Risk of rehospitalization among bipolar disorder patients who are nonadherent to antipsychotic therapy after hospital discharge.Am J Health Syst Pharm.2009;66(4):358–365.
- ,,,,.Continuity and adherence to long‐term drug treatment by geriatric patients after hospital discharge: a prospective cohort study.Drugs Aging.2008;25(10):861–870.
- ,,,.Medication use among inner‐city patients after hospital discharge: patient‐reported barriers and solutions.Mayo Clin Proc.2008;83(5):529–535.
- ,,,,,.Relationship of health literacy to intentional and unintentional non‐adherence of hospital discharge medications.J Gen Intern Med.2012;27(2):173–178.
- Office of Disease Prevention and Health Promotion, US Department of Health and Human Services.Healthy People 2010. Available at: http://www.healthypeople.gov/Document/pdf/uih/2010uih.pdf. Accessed February 15,2012.
- ,,, et al.Literacy and misunderstanding prescription drug labels.Ann Intern Med.2006;145(12):887–894.
- ,,,,,.Predictors of medication self‐management skill in a low‐literacy population.J Gen Intern Med.2006;21(8):852–856.
- ,,, et al;for the PILL‐CVD Study Group.Rationale and design of the Pharmacist Intervention for Low Literacy in Cardiovascular Disease (PILL‐CVD) study.Circ Cardiovasc Qual Outcomes.2010;3(2):212–219.
- ,,,,.Simplifying detection of cognitive impairment: comparison of the Mini‐Cog and Mini‐Mental State Examination in a multiethnic sample.J Am Geriatr Soc.2005;53(5):871–874.
- .Short Test of Functional Health Literacy in Adults.Snow Camp, NC:Peppercorn Books and Press;1998.
- ,,,.Predictive validity of a medication adherence measure in an outpatient setting.J Clin Hypertens (Greenwich).2008;10(5):348–354.
- ,,,,,.Health literacy and medication understanding among hospitalized adults.J Hosp Med. In press.
- ,,,,,.Health literacy and medication understanding among hospitalized adults.J Hosp Med.2011;6(9):488–493.
- ,,.The summary of diabetes self‐care activities measure: results from 7 studies and a revised scale.Diabetes Care.2000;23(7):943–950.
- .Multiple Imputation for Nonresponse in Surveys.New York, NY:John Wiley 1987.
- ,,, et al.Medication adherence in HIV‐infected adults: effect of patient age, cognitive status, and substance abuse.AIDS.2004;18(suppl 1):S19–S25.
- ,,.Factors associated with antihypertensive drug compliance in 83,884 Chinese patients: a cohort study.J Epidemiol Community Health.2010;64(10):895–901.
- ,,,,,.Adherence to oral hypoglycemic agents in 26,782 Chinese patients: a cohort study.J Clin Pharmacol.2011;51(10):1474–1482.
- ,,,,.Effect of a pharmacy‐based health literacy intervention and patient characteristics on medication refill adherence in an urban health system.Ann Pharmacother.2010;44(1):80–87.
- ,,.Adherence to combination antiretroviral therapies in HIV patients of low health literacy.J Gen Intern Med.1999;14(5):267–273.
- ,,,,,.Factors associated with medication refill adherence in cardiovascular‐related diseases: a focus on health literacy.J Gen Intern Med.2006;21(12):1215–1221.
- ,,,,.Limited health literacy is a barrier to medication reconciliation in ambulatory care.J Gen Intern Med.2007;22(11):1523–1526.
- ,,,,.The impact of low health literacy on surgical practice.Am J Surg.2004;188(3):250–253.
- ,,,,.Relationships between beliefs about medications and adherence.Am J Health Syst Pharm.2009;66(7):657–664.
- ,,,.Health literacy and anticoagulation‐related outcomes among patients taking warfarin.J Gen Intern Med.2006;21(8):841–846.
- ,,,,,.Health literacy, antiretroviral adherence, and HIV‐RNA suppression: a longitudinal perspective.J Gen Intern Med.2006;21(8):835–840.
- ,,, et al.Risk factors for nonadherence to warfarin: results from the IN‐RANGE study.Pharmacoepidemiol Drug Saf.2008;17(9):853–860.
- ,,, et al.Predictors of low clopidogrel adherence following percutaneous coronary intervention.Am J Cardiol.2011;108(6):822–827.
- ,,,,,.Correlation between adherence rates measured by MEMS and self‐reported questionnaires: a meta‐analysis.Health Qual Life Outcomes.2010;8:99.
- ,,,,,.Concordance of adherence measurement using self‐reported adherence questionnaires and medication monitoring devices.Pharmacoeconomics.2010;28(12):1097–1107.
- ,,,.Polypharmacy and medication adherence in patients with type 2 diabetes.Diabetes Care.2003;26(5):1408–1412.
- ,,,.Improving adherence and reducing medication discrepancies in patients with diabetes.Ann Pharmacother.2003;37(7–8):962–969.
- ,.Adherence to medication.N Engl J Med.2005;353(5):487–497.
- ,,,.Posthospital medication discrepancies: prevalence and contributing factors.Arch Intern Med.2005;165(16):1842–1847.
- ,.Medication use in the transition from hospital to home.Ann Acad Med Singapore.2008;37(2):136–141.
- ,,,.Medical errors related to discontinuity of care from an inpatient to an outpatient setting.J Gen Intern Med.2003;18(8):646–651.
- ,,,,.The incidence and severity of adverse events affecting patients after discharge from the hospital.Ann Intern Med.2003;138(3):161–167.
- ,,,,.Adverse drug events occurring following hospital discharge.J Gen Intern Med.2005;20(4):317–323.
- ,,, et al.Role of pharmacist counseling in preventing adverse drug events after hospitalization.Arch Intern Med.2006;166(5):565–571.
- ,,.Reconcilable differences: correcting medication errors at hospital admission and discharge.Qual Saf Health Care.2006;15(2):122–126.
- ,.Risk of rehospitalization among bipolar disorder patients who are nonadherent to antipsychotic therapy after hospital discharge.Am J Health Syst Pharm.2009;66(4):358–365.
- ,,,,.Continuity and adherence to long‐term drug treatment by geriatric patients after hospital discharge: a prospective cohort study.Drugs Aging.2008;25(10):861–870.
- ,,,.Medication use among inner‐city patients after hospital discharge: patient‐reported barriers and solutions.Mayo Clin Proc.2008;83(5):529–535.
- ,,,,,.Relationship of health literacy to intentional and unintentional non‐adherence of hospital discharge medications.J Gen Intern Med.2012;27(2):173–178.
- Office of Disease Prevention and Health Promotion, US Department of Health and Human Services.Healthy People 2010. Available at: http://www.healthypeople.gov/Document/pdf/uih/2010uih.pdf. Accessed February 15,2012.
- ,,, et al.Literacy and misunderstanding prescription drug labels.Ann Intern Med.2006;145(12):887–894.
- ,,,,,.Predictors of medication self‐management skill in a low‐literacy population.J Gen Intern Med.2006;21(8):852–856.
- ,,, et al;for the PILL‐CVD Study Group.Rationale and design of the Pharmacist Intervention for Low Literacy in Cardiovascular Disease (PILL‐CVD) study.Circ Cardiovasc Qual Outcomes.2010;3(2):212–219.
- ,,,,.Simplifying detection of cognitive impairment: comparison of the Mini‐Cog and Mini‐Mental State Examination in a multiethnic sample.J Am Geriatr Soc.2005;53(5):871–874.
- .Short Test of Functional Health Literacy in Adults.Snow Camp, NC:Peppercorn Books and Press;1998.
- ,,,.Predictive validity of a medication adherence measure in an outpatient setting.J Clin Hypertens (Greenwich).2008;10(5):348–354.
- ,,,,,.Health literacy and medication understanding among hospitalized adults.J Hosp Med. In press.
- ,,,,,.Health literacy and medication understanding among hospitalized adults.J Hosp Med.2011;6(9):488–493.
- ,,.The summary of diabetes self‐care activities measure: results from 7 studies and a revised scale.Diabetes Care.2000;23(7):943–950.
- .Multiple Imputation for Nonresponse in Surveys.New York, NY:John Wiley 1987.
- ,,, et al.Medication adherence in HIV‐infected adults: effect of patient age, cognitive status, and substance abuse.AIDS.2004;18(suppl 1):S19–S25.
- ,,.Factors associated with antihypertensive drug compliance in 83,884 Chinese patients: a cohort study.J Epidemiol Community Health.2010;64(10):895–901.
- ,,,,,.Adherence to oral hypoglycemic agents in 26,782 Chinese patients: a cohort study.J Clin Pharmacol.2011;51(10):1474–1482.
- ,,,,.Effect of a pharmacy‐based health literacy intervention and patient characteristics on medication refill adherence in an urban health system.Ann Pharmacother.2010;44(1):80–87.
- ,,.Adherence to combination antiretroviral therapies in HIV patients of low health literacy.J Gen Intern Med.1999;14(5):267–273.
- ,,,,,.Factors associated with medication refill adherence in cardiovascular‐related diseases: a focus on health literacy.J Gen Intern Med.2006;21(12):1215–1221.
- ,,,,.Limited health literacy is a barrier to medication reconciliation in ambulatory care.J Gen Intern Med.2007;22(11):1523–1526.
- ,,,,.The impact of low health literacy on surgical practice.Am J Surg.2004;188(3):250–253.
- ,,,,.Relationships between beliefs about medications and adherence.Am J Health Syst Pharm.2009;66(7):657–664.
- ,,,.Health literacy and anticoagulation‐related outcomes among patients taking warfarin.J Gen Intern Med.2006;21(8):841–846.
- ,,,,,.Health literacy, antiretroviral adherence, and HIV‐RNA suppression: a longitudinal perspective.J Gen Intern Med.2006;21(8):835–840.
- ,,, et al.Risk factors for nonadherence to warfarin: results from the IN‐RANGE study.Pharmacoepidemiol Drug Saf.2008;17(9):853–860.
- ,,, et al.Predictors of low clopidogrel adherence following percutaneous coronary intervention.Am J Cardiol.2011;108(6):822–827.
- ,,,,,.Correlation between adherence rates measured by MEMS and self‐reported questionnaires: a meta‐analysis.Health Qual Life Outcomes.2010;8:99.
- ,,,,,.Concordance of adherence measurement using self‐reported adherence questionnaires and medication monitoring devices.Pharmacoeconomics.2010;28(12):1097–1107.
- ,,,.Polypharmacy and medication adherence in patients with type 2 diabetes.Diabetes Care.2003;26(5):1408–1412.
- ,,,.Improving adherence and reducing medication discrepancies in patients with diabetes.Ann Pharmacother.2003;37(7–8):962–969.
Copyright © 2012 Society of Hospital Medicine
Health literacy and medication understanding among hospitalized adults
If you wish to receive credit for this activity, please refer to the website:
Accreditation and Designation Statement
Blackwell Futura Media Services designates this journal‐based CME activity for a maximum of 1 AMA PRA Category 1 Credit. Physicians should only claim credit commensurate with the extent of their participation in the activity.
Blackwell Futura Media Services is accredited by the Accreditation Council for Continuing Medical Education to provide continuing medical education for physicians.
Educational Objectives
Upon completion of this activity, participants will be better able to:
-
Assess the factors associated with reduced medication adherence.
-
Distinguish which components of medication understanding are assessed by the Medication Understanding Questionnaire.
This manuscript underwent peer review in line with the standards of editorial integrity and publication ethics maintained by Journal of Hospital Medicine. The peer reviewers have no relevant financial relationships. The peer review process for Journal of Hospital Medicine is single‐blinded. As such, the identities of the reviewers are not disclosed in line with the standard accepted practices of medical journal peer review.
Conflicts of interest have been identified and resolved in accordance with Blackwell Futura Media Services's Policy on Activity Disclosure and Conflict of Interest. The primary resolution method used was peer review and review by a non‐conflicted expert.
Instructions on Receiving Credit
For information on applicability and acceptance of CME credit for this activity, please consult your professional licensing board.
This activity is designed to be completed within an hour; physicians should claim only those credits that reflect the time actually spent in the activity. To successfully earn credit, participants must complete the activity during the valid credit period, which is up to two years from initial publication.
Follow these steps to earn credit:
-
Log on to www.wileyblackwellcme.com
-
Read the target audience, learning objectives, and author disclosures.
-
Read the article in print or online format.
-
Reflect on the article.
-
Access the CME Exam, and choose the best answer to each question.
-
Complete the required evaluation component of the activity.
This activity will be available for CME credit for twelve months following its publication date. At that time, it will be reviewed and potentially updated and extended for an additional twelve months.
If you wish to receive credit for this activity, please refer to the website:
Accreditation and Designation Statement
Blackwell Futura Media Services designates this journal‐based CME activity for a maximum of 1 AMA PRA Category 1 Credit. Physicians should only claim credit commensurate with the extent of their participation in the activity.
Blackwell Futura Media Services is accredited by the Accreditation Council for Continuing Medical Education to provide continuing medical education for physicians.
Educational Objectives
Upon completion of this activity, participants will be better able to:
-
Assess the factors associated with reduced medication adherence.
-
Distinguish which components of medication understanding are assessed by the Medication Understanding Questionnaire.
This manuscript underwent peer review in line with the standards of editorial integrity and publication ethics maintained by Journal of Hospital Medicine. The peer reviewers have no relevant financial relationships. The peer review process for Journal of Hospital Medicine is single‐blinded. As such, the identities of the reviewers are not disclosed in line with the standard accepted practices of medical journal peer review.
Conflicts of interest have been identified and resolved in accordance with Blackwell Futura Media Services's Policy on Activity Disclosure and Conflict of Interest. The primary resolution method used was peer review and review by a non‐conflicted expert.
Instructions on Receiving Credit
For information on applicability and acceptance of CME credit for this activity, please consult your professional licensing board.
This activity is designed to be completed within an hour; physicians should claim only those credits that reflect the time actually spent in the activity. To successfully earn credit, participants must complete the activity during the valid credit period, which is up to two years from initial publication.
Follow these steps to earn credit:
-
Log on to www.wileyblackwellcme.com
-
Read the target audience, learning objectives, and author disclosures.
-
Read the article in print or online format.
-
Reflect on the article.
-
Access the CME Exam, and choose the best answer to each question.
-
Complete the required evaluation component of the activity.
This activity will be available for CME credit for twelve months following its publication date. At that time, it will be reviewed and potentially updated and extended for an additional twelve months.
If you wish to receive credit for this activity, please refer to the website:
Accreditation and Designation Statement
Blackwell Futura Media Services designates this journal‐based CME activity for a maximum of 1 AMA PRA Category 1 Credit. Physicians should only claim credit commensurate with the extent of their participation in the activity.
Blackwell Futura Media Services is accredited by the Accreditation Council for Continuing Medical Education to provide continuing medical education for physicians.
Educational Objectives
Upon completion of this activity, participants will be better able to:
-
Assess the factors associated with reduced medication adherence.
-
Distinguish which components of medication understanding are assessed by the Medication Understanding Questionnaire.
This manuscript underwent peer review in line with the standards of editorial integrity and publication ethics maintained by Journal of Hospital Medicine. The peer reviewers have no relevant financial relationships. The peer review process for Journal of Hospital Medicine is single‐blinded. As such, the identities of the reviewers are not disclosed in line with the standard accepted practices of medical journal peer review.
Conflicts of interest have been identified and resolved in accordance with Blackwell Futura Media Services's Policy on Activity Disclosure and Conflict of Interest. The primary resolution method used was peer review and review by a non‐conflicted expert.
Instructions on Receiving Credit
For information on applicability and acceptance of CME credit for this activity, please consult your professional licensing board.
This activity is designed to be completed within an hour; physicians should claim only those credits that reflect the time actually spent in the activity. To successfully earn credit, participants must complete the activity during the valid credit period, which is up to two years from initial publication.
Follow these steps to earn credit:
-
Log on to www.wileyblackwellcme.com
-
Read the target audience, learning objectives, and author disclosures.
-
Read the article in print or online format.
-
Reflect on the article.
-
Access the CME Exam, and choose the best answer to each question.
-
Complete the required evaluation component of the activity.
This activity will be available for CME credit for twelve months following its publication date. At that time, it will be reviewed and potentially updated and extended for an additional twelve months.
Health Literacy and Medication Use
With the aging of the US population, complex medication regimens to treat multiple comorbidities are increasingly common.1 Nevertheless, patients often do not fully understand the instructions for safe and effective medication use. Aspects of medication understanding include knowledge of the drug indication, dose, frequency, and for certain medications, special instructions.2 Medication understanding is associated with better medication adherence, fewer drug‐related problems, and fewer emergency department visits.3 Among patients with chronic conditions, such as cardiovascular disease (CVD), understanding and adherence to the medication regimen are critical for successful disease control and clinical outcomes.4
Patients' understanding of their medication regimen is also vitally important upon admission to the hospital. Patients often are the main source of information for the admission medication history and subsequent medication reconciliation.5 Poor patient understanding of the preadmission medication regimen can contribute to errors in inpatient and postdischarge medication orders, and adversely affect patient safety.6 However, little research has examined patients' understanding of the preadmission medication regimen and factors that affect it.
In the outpatient setting, previous investigations have suggested that low health literacy, advanced age, and impaired cognitive function adversely affect patients' understanding of medication instructions.2, 7, 8 These studies were limited by a small sample size, single site, or focus on a specific population, such as inner‐city patients. Additionally, the measures used to assess medication understanding were time‐consuming and required patients' medications to be present for testing, thus limiting their utility.2
To address these gaps in the literature, we developed and implemented the Medication Understanding Questionnaire (MUQ), an original and relatively rapid measure that does not require patients' medications be present for testing. In a study of adults at 2 large teaching hospitals, we examined the association of health literacy, age, cognitive function, number of preadmission medications, and other factors on patients' understanding of their preadmission medication regimen. We hypothesized that lower health literacy would be independently associated with lower medication understanding as assessed using the MUQ.
METHODS
The present study was a cross‐sectional assessment conducted using baseline interview data from the Pharmacist Intervention for Low Literacy in Cardiovascular Disease (PILL‐CVD) Study (ClinicalTrials.gov Registration #NCT00632021; available at:
Population
The PILL‐CVD study protocol and eligibility criteria has been previously published.9 Briefly, patients were eligible if they were at least 18 years old and admitted with acute coronary syndrome or acute decompensated heart failure. Patients were excluded if they: were too ill to complete an interview; were not oriented to person, place, or time; had a corrected visual acuity worse than 20/200; had impaired hearing; could not communicate in English or Spanish; were not responsible for managing their own medications; had no phone; were unlikely to be discharged to home; were in police custody; or had been previously enrolled in the study. For the present analysis, we also excluded any patient who was not on at least 1 prescription medication prior to admission. Saline nasal spray, saline eye drops, herbal products, nutritional supplements, vitamins, and over the counter (OTC) lotions and creams were not counted as prescription medications. Oral medications available both OTC and by prescription (eg, aspirin, nonsteroidal anti‐inflammatory drugs, and acid reflux medications) were counted as prescription medications.
Measures
At enrollment, which was usually within 24 to 48 hours of admission, participants completed the short form of the Test of Functional Health Literacy in Adults (s‐TOFHLA) in English or Spanish,10 the Mini‐Cog test of cognition,11 and the Medication Understanding Questionnaire (MUQ), as well as demographic information. The number of prescription medications prior to hospital admission was abstracted from the best available reference listthat documented by the treating physicians in the electronic health record (EHR). The EHR at each site was a home‐grown system and included both inpatient and outpatient records, which facilitated physicians' documentation of the medication list.
The s‐TOFHLA consists of 2 short reading‐comprehension passages. Scores on the s‐TOFHLA range from 0 to 36, and can be categorized as inadequate (0‐16), marginal (17‐22), or adequate (23‐36) health literacy.10 The Mini‐Cog includes 3‐item recall and clock‐drawing tests. It provides a brief measure of cognitive function and performs well among patients with limited literacy or educational attainment.11 Scores range from 0 to 5, with a score <3 indicating possible dementia.
The MUQ was administered verbally and assessed patients' understanding of their own preadmission medication regimen. It was developed for this study, based on published measures of medication understanding.2, 12 To administer the MUQ, research assistants (RAs) accessed the patient's preadmission medication list from the EHR and used a random number table to select up to 5 prescription medications from the list. If the patient was taking 5 or fewer medications, all of their medications were selected. Saline nasal spray, saline eye drops, herbal products, nutritional supplements, vitamins, and OTC lotions and creams were excluded from testing. The RA provided the brand and generic name of each medication, and then asked the patient for the drug's purpose, strength per unit (eg, 20 mg tablet), number of units taken at a time (eg, 2 tablets), and dosing frequency (eg, twice a day). For drugs prescribed on an as‐needed basis, the RA asked patients for the maximum allowable dose and frequency. Patients were instructed to not refer to a medication list or bottles when responding. The RA documented the patient's responses on the MUQ, along with the dosing information from the EHR for each selected medication.
One clinical pharmacist (MM) scored all MUQ forms by applying a set of scoring rules. Each medication score could range from 0 to 3. The components of the score included indication (1 point), strength (0.5 point), units (0.5 point), and frequency (1 point). The patient's overall MUQ score was an average of the MUQ scores for each tested medication.
Statistical Analysis
We summarized patient characteristics, number of preadmission medications, and MUQ scores using median and interquartile range (IRQ) for continuous variables, and frequencies and proportions for categorical variables. We conducted proportional odds logistic regression (ordinal regression) to estimate the effect of s‐TOFHLA score, other patient characteristics, and number of medications on MUQ scores.13
Important covariates were selected a priori based on clinical significance. These included age (continuous), gender, patient self‐reported race (white, black, other nonwhite), Mini‐Cog score (continuous), primary language (English or Spanish), years of education (continuous), number of preadmission medications (continuous), income (ordinal categories), insurance type (categorical), and study site. Covariates with missing data (household income, health literacy, and years of education) were imputed using multiple imputation techniques.14 The relationship between number of preadmission medications and MUQ scores was found to be nonlinear, and it was modeled using restricted cubic splines.14 We also fit models which treated health literacy and cognition as categorical variables. Results are reported as odds ratios (OR) with 95% confidence intervals (CI). Wald tests were used to test for the statistical significance of predictor variables. Two‐sided P values less than 0.05 were considered statistically significant. All analyses were performed using statistical language R (R Foundation, available at:
RESULTS
Baseline Characteristics
Among the 862 patients enrolled in PILL‐CVD, 790 (91.7 %) had at least 1 preadmission medication and were included in this analysis (Table 1). Forty‐seven percent were admitted to VUH (N = 373) and 53% to BWH (N = 417). The median age was 61 (interquartile range [IQR] 52, 71), 77% were white, and 57% were male. Inadequate or marginal health literacy was identified among 11% and 9% of patients, respectively. The median number of preadmission medications was 8 (IQR 5, 11). Patients excluded from the analysis for not having preadmission medications were similar to included patients, except they were more likely to be male (76% vs 57%) and less likely to have health insurance (23% self‐pay vs 4%). (Data available upon request.)
| Characteristic | N = 790 |
|---|---|
| |
| Study hospital, N (%) | |
| Vanderbilt University Hospital | 373 (47.2) |
| Brigham and Women's Hospital | 417 (52.8) |
| Age in years, median (IQR) | 61 (52, 71) |
| Gender, N (%) | |
| Male | 452 (57.2) |
| Female | 338 (42.8) |
| Primary language, N (%) | |
| English | 779 (98.6) |
| Spanish | 11 (1.4) |
| Race, N (%) | |
| White | 610 (77.2) |
| Black or African American | 136 (17.2) |
| Other | 44 (5.6) |
| Health literacy, s‐TOFHLA score, median (IQR) | 33 (25, 35) |
| Health literacy, N (%)& | |
| Inadequate | 84 (10.6) |
| Marginal | 74 (9.4) |
| Adequate | 613 (77.6) |
| Mini‐Cog score, median (IQR) | 4 (3, 5) |
| Dementia, N (%) | |
| No | 692 (87.6) |
| Yes | 98 (12.4) |
| Number of medications, median (IQR) | 8 (5, 11) |
| Health insurance type, N (%) | |
| Medicaid | 74 (9.4) |
| Medicare | 337 (42.6) |
| Private | 334 (42.3) |
| Self‐pay | 35 (4.4) |
| Other | 10 (1.3) |
| Self‐reported household income, N (%)& | |
| <$10,000 | 38 (4.8) |
| $10,000 to <$15,000 | 45 (5.7) |
| $15,000 to <$20,000 | 42 (5.3) |
| $20,000 to <$25,000 | 105 (13.3) |
| $25,000 to <$35,000 | 99 (12.5) |
| $35,000 to <$50,000 | 112 (14.2) |
| $50,000 to <$75,000 | 118 (14.9) |
| $75,000+ | 227 (28.7) |
| Years of school, median (IQR)& | 14 (12, 16) |
MUQ Scores
The MUQ was administered in approximately 5 minutes. The median MUQ score was 2.5 (IQR 2.2, 2.8) (Table 2); 16.3% of patients scored less than 2. Subjects typically achieved high scores for the domains of indication, units, and frequency, while scores on the strength domain were lower (median = 0.2 [IQR 0.1, 0.4], maximum possible = 0.5).
| Median (IQR) | |
|---|---|
| |
| No. of drugs tested | 5 (4, 5) |
| MUQ score* | 2.5 (2.2, 2.8) |
| Indication | 1.0 (0.8, 1.0) |
| Strength | 0.2 (0.1, 0.4) |
| Units | 0.5 (0.4, 0.5) |
| Frequency | 1.0 (0.8, 1.0) |
Predictors of Medication Understanding
Unadjusted relationships of health literacy, cognition, and number of medications with medication understanding are shown in Figure 1 (panels A, B, and C, respectively). The figure demonstrates a linear relationship with both health literacy (Figure 1A) and cognition (Figure 1B), and a nonlinear relationship between number of preadmission medications and MUQ score (Figure 1C).
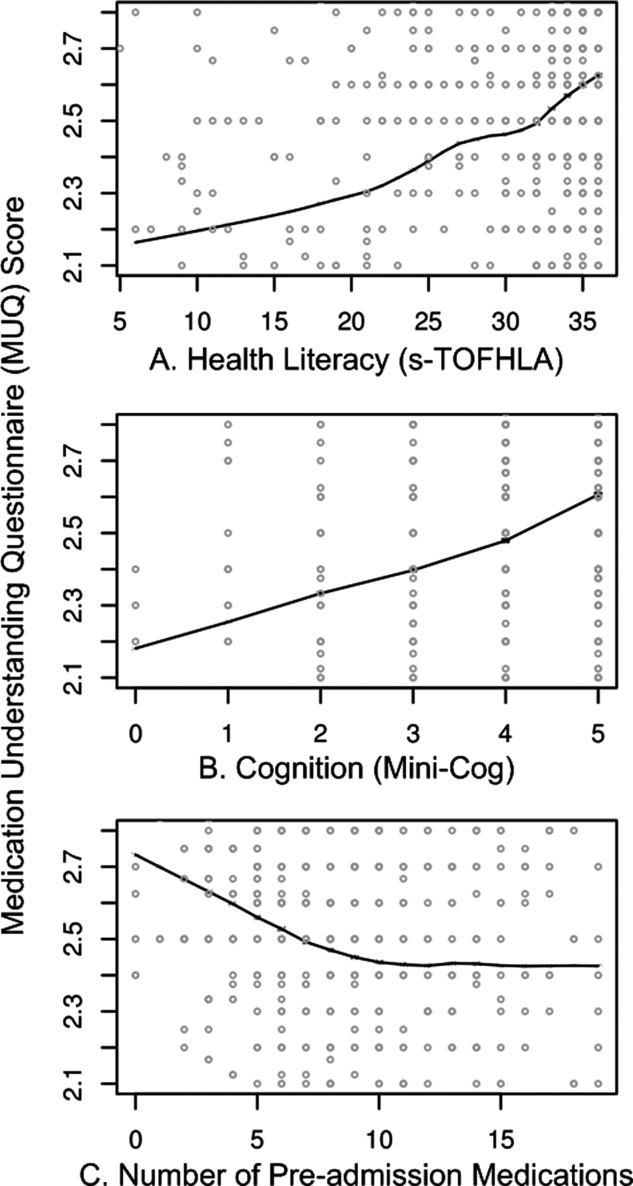
Adjusted relationships using imputed data for missing covariates are shown in Figure 2. Lower health literacy, cognitive impairment, male gender, and black race were independently associated with lower understanding of preadmission medications. Each 1 point increase in s‐TOFHLA or Mini‐Cog score led to an increase in medication understanding (OR = 1.04; 95% CI, 1.02 to 1.06; P = 0.0001; and OR = 1.24; 95% CI, 1.1 to 1.4; P = 0.001; respectively). Patients with marginal or inadequate health literacy had lower odds of understanding their regimen (OR = 0.53; 95% CI, 0.34 to 0.84; and OR = 0.49; 95% CI, 0.31 to 0.78, respectively) compared to those with adequate health literacy. Impaired cognitive function (Mini‐Cog score <3, indicating dementia) was also associated with lower odds of medication understanding (OR = 0.57; 95% CI, 0.38 to 0.86) compared to those with no cognitive impairment. An increase in the number of preadmission medications (up to 10) was also strongly associated with lower MUQ scores. For each increase by 1 medication, there was a significant decrease in medication understanding, up to 10 medications, beyond which understanding did not significantly decrease further. Patients on 6 medications were about half as likely to understand their medication regimen as patients on only 1 medication (OR = 0.52; 95% CI, 0.36 to 0.75). For patients on 11 medications, the odds of medication understanding were 24% lower than for patients on 6 medications (OR = 0.76; 95% CI, 0.65 to 0.89). Patients' age, years of schooling, and household income were not independently associated with medication understanding. Results were similar using data without multiple imputation.
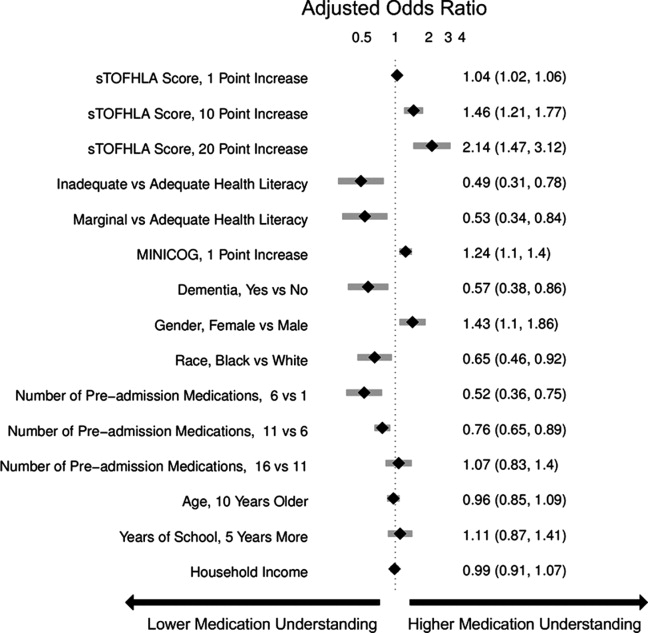
Examples of Misunderstanding of Common Medications
Table 3 provides examples of incorrect patient responses for several commonly prescribed medications or drug classes, including aspirin, digoxin, nitroglycerin, and HMG‐CoA reductase inhibitors (statins). For aspirin, many patients were not aware of the strength. For digoxin, several participants reported splitting a higher‐strength pill to obtain the prescribed dose, which should not be done given the imprecision of splitting and narrow therapeutic index of this drug. Patients prescribed nitroglycerin sublingual tablets were commonly unable to report the correct dosing and frequency for angina treatment. Medications for cholesterol were often reported as being taken in the morning; this was scored strictly as a frequency error if the medication timing in the EHR was listed as evening or bedtime. We also identified many patients with poor understanding of opioid analgesics, particularly regarding their dosing and frequency.
| Medications | Common Incorrect Responses | Correct Information | Coded Error |
|---|---|---|---|
| |||
| Aspirin | tablet twice a day | 1 tablet once a day | Units and frequency |
| I am not aware what aspirin I am taking | 81 mg once a day | Strength | |
| I am taking 6‐something every day | 81 mg once a day | Strength | |
| 31 mg a day | 81 mg once a day | Strength | |
| 180 mg a day | 81 mg once a day | Strength | |
| 1 low‐dose daily | 325 mg once a day | Strength | |
| 125 mg a day | 325 mg once a day | Strength | |
| I am taking it for my blood pressure | Heart medication | Indication | |
| Nitroglycerin sublingual | As needed, I have taken up to 4 a day | Dissolve 1 tablet under the tongue, every 5 min as needed, up to 3 doses | Frequency |
| As needed every 15 min | Frequency | ||
| As needed up to 4 doses every 10 min | Frequency | ||
| Dissolve couple units under the tongue, as needed | Units and frequency | ||
| As many as I want, every 5 min | Frequency | ||
| Digoxin | tablet daily | 1 tablet daily | Units |
| 1 tablet daily | 1 tablet every other day | Frequency | |
| I am taking it for my blood pressure | Heart medication | Indication | |
| HMG‐CoA reductase inhibitors | 1 tablet every morning | 1 tablet every evening | Frequency |
| tablet twice a day | 1 tablet once a day | Units and frequency | |
| I do not know the indication | High cholesterol | Indication | |
| Propoxyphene/acetaminophen | tablet as needed | 1 tablet every 4‐6 hr as needed | Units and frequency |
| Hydrocodone/acetaminophen | I do not know the strength of this medication | 5 mg/500 mg | Strength |
| 1 tablet as I need it | 1 tablet every 4‐6 hr as needed | Frequency | |
DISCUSSION
We used a novel four‐component medication understanding questionnaire, developed for this study, to assess patients' understanding of up to 5 drugs selected randomly from the participant's preadmission medication list. The MUQ proved to be easy to administer by nonmedical staff within a short period of time (approximately 5 minutes per patient). It was well understood by patients. By limiting the assessment to 5 or fewer medications, the MUQ has a distinct advantage over existing measures of medication understanding that require testing the entire regimen. We did not find any limitations related to cutting off the assessment at 5 medications. In addition, this tool affords assessment of medication understanding without requiring medication bottles be present, enhancing its utility in the inpatient setting.
MUQ scores were associated with health literacy and other patient characteristics in an expected manner. We demonstrated that inadequate or marginal health literacy, as well as impaired cognitive function, were associated with low medication understanding. We also were able to demonstrate a relationship between increasing number of medications and lower medication understanding. Interestingly, in our patient population, understanding continued to decrease until reaching 10 medications, beyond which further increases in the number of medications had no additional detrimental effect on medication understanding. This nonlinear relationship between number of medications and medication understanding has potential implications for prescribing practice.
Our findings which utilize the MUQ among inpatients are consistent with prior literature in other settings.2, 7, 8 In a previous outpatient study, we identified that health literacy plays an important role in a patient's ability to successfully report and manage their daily medications.2 Other studies have also shown that patients with low health literacy have more difficulty understanding prescription drug information, and that they often experience medication‐related problems after hospital discharge.15, 16 The number and often the types of medications an individual takes have also been shown to increase the risk for adverse events and nonadherence to the treatment plan.1720 We postulate that this risk of adverse drug events is related at least in part to a patient's understanding of their medication regimen.
There are several limitations to this study. First, the MUQ did not assess certain aspects of medication understanding, such as knowledge of pill appearance or side effects, nor did it assess components of patients' actual drug‐taking behavior, such as organization of medications or behavioral cues. Thus, adaptive behaviors that patients may perform to improve their medication management, such as writing on labels or memory cues, are not captured by this test. Second, in administering and scoring the MUQ, we used the patient's preadmission medication list documented in the EHR as the reference standard. This was the best available reference list, and was generally accurate, as both hospitals had medication reconciliation systems in use at the time of the study21; nevertheless, it may contain inaccuracies. Documentation for certain medications, such as warfarin, in which dose can change frequently, often did not reflect the latest prescribed dose. In such cases, we scored the patient's answer as correct if the dose appeared reasonable and appropriate to the clinical pharmacist. As a result, a patient's MUQ score may have been overestimated in these cases.
Additional research will be needed to further validate the MUQ in other settings. In particular, studies should establish the relationship between the MUQ, serious medication errors after discharge, and potential to benefit from educational interventions. Also, as noted above, the nonlinear relationship between number of medications and medication understanding should be confirmed in other studies.
In conclusion, we demonstrated that patients with low health literacy, impaired cognition, or a higher number of medications had significantly poorer understanding of their preadmission medication regimen. These findings have important clinical implications. It would be appropriate to exercise greater caution when taking a medication history from patients who cannot readily provide the purpose, strength, units, and frequency of their medications. Attempts to validate the information obtained from patients with other sources of data, such as family members, inpatient or outpatient health records, and community pharmacy records should be considered. Patients at high risk for poor medication understanding, either measured directly using the MUQ or identified via risk factors such as polypharmacy, low cognition, or low health literacy, may warrant more intensive medication reconciliation interventions and/or educational counseling and follow‐up to prevent postdischarge adverse drug events. Further research is needed to determine if targeting these populations for interventions improves medication safety during transitions in care.
- ,,.Prevalence, expenditures, and complications of multiple chronic conditions in the elderly.Arch Intern Med.2002;162(20):2269–2276.
- ,,,,,.Predictors of medication self‐management skill in a low‐literacy population.J Gen Intern Med.2006;21(8):852–856.
- ,.Adherence to medication.N Engl J Med.2005;353(5):487–497.
- ,,.Medication adherence: its importance in cardiovascular outcomes.Circulation.2009;119(23):3028–3035.
- ,,, et al.Classifying and predicting errors of inpatient medication reconciliation.J Gen Intern Med.2008;23(9):1414–1422.
- ,.Addressing postdischarge adverse events: a neglected area.Jt Comm J Qual Patient Saf.2008;34(2):85–97.
- ,,.Medication management capacity in highly functioning community‐living older adults: detection of early deficits.J Am Geriatr Soc.1999;47(5):592–596.
- ,,.Variation in medication understanding among the elderly.Am J Health‐Syst Pharm.2004;61(4):373–380.
- ,,, et al.The rationale and design of the Pharmacist Intervention for Low Literacy in Cardiovascular Disease (PILL‐CVD) study.Circ Cardiovasc Qual Outcomes.2010;3:212–219.
- ,,,.Short Test of Functional Health Literacy in Adults.Snow Camp, NC:Peppercorn Books and Press;1998.
- ,,,,.Simplifying detection of cognitive impairment: comparison of the Mini‐Cog and Mini‐Mental State Examination in a multiethnic sample.J Am Geriatr Soc.2005;53(5):871–874.
- ,.Instruments assessing capacity to manage medications.Ann Pharmacother.2008;42(7):1026–1036.
- ,.Estimation of the probability of an event as a function of several independent variables.Biometrika.1967;54(1):167–179.
- ,.Using full probability models to compute probabilities of actual interest to decision makers.Int J Technol Assess Health Care.2001;17(1):17–26.
- ,,, et al.Literacy and misunderstanding prescription drug labels.Ann Intern Med.2006;145(12):887–894.
- ,,,.Medication use among inner‐city patients after hospital discharge: patient reported barriers and solutions.Mayo Clin Proc.2008;83(5):529–535.
- ,,,,,.National surveillance of emergency department visits for outpatient adverse drug events.JAMA.2006;296(15):1858–1866.
- ,,,.Medication use leading to emergency department visits for adverse drug events in older adults.Ann Intern Med.2007;147(11):755–765.
- ,,,,.Adverse drug events occurring following hospital discharge.J Gen Intern Med.2005;20:317–323.
- ,,, et al.Adverse drug events in ambulatory care.N Engl J Med.2003;348(16):1556–1564.
- ,,, et al.Effect of an electronic medication reconciliation application and process redesign on potential adverse drug events: a cluster‐randomized trial.Arch Intern Med.2009;169(8):771–780.
With the aging of the US population, complex medication regimens to treat multiple comorbidities are increasingly common.1 Nevertheless, patients often do not fully understand the instructions for safe and effective medication use. Aspects of medication understanding include knowledge of the drug indication, dose, frequency, and for certain medications, special instructions.2 Medication understanding is associated with better medication adherence, fewer drug‐related problems, and fewer emergency department visits.3 Among patients with chronic conditions, such as cardiovascular disease (CVD), understanding and adherence to the medication regimen are critical for successful disease control and clinical outcomes.4
Patients' understanding of their medication regimen is also vitally important upon admission to the hospital. Patients often are the main source of information for the admission medication history and subsequent medication reconciliation.5 Poor patient understanding of the preadmission medication regimen can contribute to errors in inpatient and postdischarge medication orders, and adversely affect patient safety.6 However, little research has examined patients' understanding of the preadmission medication regimen and factors that affect it.
In the outpatient setting, previous investigations have suggested that low health literacy, advanced age, and impaired cognitive function adversely affect patients' understanding of medication instructions.2, 7, 8 These studies were limited by a small sample size, single site, or focus on a specific population, such as inner‐city patients. Additionally, the measures used to assess medication understanding were time‐consuming and required patients' medications to be present for testing, thus limiting their utility.2
To address these gaps in the literature, we developed and implemented the Medication Understanding Questionnaire (MUQ), an original and relatively rapid measure that does not require patients' medications be present for testing. In a study of adults at 2 large teaching hospitals, we examined the association of health literacy, age, cognitive function, number of preadmission medications, and other factors on patients' understanding of their preadmission medication regimen. We hypothesized that lower health literacy would be independently associated with lower medication understanding as assessed using the MUQ.
METHODS
The present study was a cross‐sectional assessment conducted using baseline interview data from the Pharmacist Intervention for Low Literacy in Cardiovascular Disease (PILL‐CVD) Study (ClinicalTrials.gov Registration #NCT00632021; available at:
Population
The PILL‐CVD study protocol and eligibility criteria has been previously published.9 Briefly, patients were eligible if they were at least 18 years old and admitted with acute coronary syndrome or acute decompensated heart failure. Patients were excluded if they: were too ill to complete an interview; were not oriented to person, place, or time; had a corrected visual acuity worse than 20/200; had impaired hearing; could not communicate in English or Spanish; were not responsible for managing their own medications; had no phone; were unlikely to be discharged to home; were in police custody; or had been previously enrolled in the study. For the present analysis, we also excluded any patient who was not on at least 1 prescription medication prior to admission. Saline nasal spray, saline eye drops, herbal products, nutritional supplements, vitamins, and over the counter (OTC) lotions and creams were not counted as prescription medications. Oral medications available both OTC and by prescription (eg, aspirin, nonsteroidal anti‐inflammatory drugs, and acid reflux medications) were counted as prescription medications.
Measures
At enrollment, which was usually within 24 to 48 hours of admission, participants completed the short form of the Test of Functional Health Literacy in Adults (s‐TOFHLA) in English or Spanish,10 the Mini‐Cog test of cognition,11 and the Medication Understanding Questionnaire (MUQ), as well as demographic information. The number of prescription medications prior to hospital admission was abstracted from the best available reference listthat documented by the treating physicians in the electronic health record (EHR). The EHR at each site was a home‐grown system and included both inpatient and outpatient records, which facilitated physicians' documentation of the medication list.
The s‐TOFHLA consists of 2 short reading‐comprehension passages. Scores on the s‐TOFHLA range from 0 to 36, and can be categorized as inadequate (0‐16), marginal (17‐22), or adequate (23‐36) health literacy.10 The Mini‐Cog includes 3‐item recall and clock‐drawing tests. It provides a brief measure of cognitive function and performs well among patients with limited literacy or educational attainment.11 Scores range from 0 to 5, with a score <3 indicating possible dementia.
The MUQ was administered verbally and assessed patients' understanding of their own preadmission medication regimen. It was developed for this study, based on published measures of medication understanding.2, 12 To administer the MUQ, research assistants (RAs) accessed the patient's preadmission medication list from the EHR and used a random number table to select up to 5 prescription medications from the list. If the patient was taking 5 or fewer medications, all of their medications were selected. Saline nasal spray, saline eye drops, herbal products, nutritional supplements, vitamins, and OTC lotions and creams were excluded from testing. The RA provided the brand and generic name of each medication, and then asked the patient for the drug's purpose, strength per unit (eg, 20 mg tablet), number of units taken at a time (eg, 2 tablets), and dosing frequency (eg, twice a day). For drugs prescribed on an as‐needed basis, the RA asked patients for the maximum allowable dose and frequency. Patients were instructed to not refer to a medication list or bottles when responding. The RA documented the patient's responses on the MUQ, along with the dosing information from the EHR for each selected medication.
One clinical pharmacist (MM) scored all MUQ forms by applying a set of scoring rules. Each medication score could range from 0 to 3. The components of the score included indication (1 point), strength (0.5 point), units (0.5 point), and frequency (1 point). The patient's overall MUQ score was an average of the MUQ scores for each tested medication.
Statistical Analysis
We summarized patient characteristics, number of preadmission medications, and MUQ scores using median and interquartile range (IRQ) for continuous variables, and frequencies and proportions for categorical variables. We conducted proportional odds logistic regression (ordinal regression) to estimate the effect of s‐TOFHLA score, other patient characteristics, and number of medications on MUQ scores.13
Important covariates were selected a priori based on clinical significance. These included age (continuous), gender, patient self‐reported race (white, black, other nonwhite), Mini‐Cog score (continuous), primary language (English or Spanish), years of education (continuous), number of preadmission medications (continuous), income (ordinal categories), insurance type (categorical), and study site. Covariates with missing data (household income, health literacy, and years of education) were imputed using multiple imputation techniques.14 The relationship between number of preadmission medications and MUQ scores was found to be nonlinear, and it was modeled using restricted cubic splines.14 We also fit models which treated health literacy and cognition as categorical variables. Results are reported as odds ratios (OR) with 95% confidence intervals (CI). Wald tests were used to test for the statistical significance of predictor variables. Two‐sided P values less than 0.05 were considered statistically significant. All analyses were performed using statistical language R (R Foundation, available at:
RESULTS
Baseline Characteristics
Among the 862 patients enrolled in PILL‐CVD, 790 (91.7 %) had at least 1 preadmission medication and were included in this analysis (Table 1). Forty‐seven percent were admitted to VUH (N = 373) and 53% to BWH (N = 417). The median age was 61 (interquartile range [IQR] 52, 71), 77% were white, and 57% were male. Inadequate or marginal health literacy was identified among 11% and 9% of patients, respectively. The median number of preadmission medications was 8 (IQR 5, 11). Patients excluded from the analysis for not having preadmission medications were similar to included patients, except they were more likely to be male (76% vs 57%) and less likely to have health insurance (23% self‐pay vs 4%). (Data available upon request.)
| Characteristic | N = 790 |
|---|---|
| |
| Study hospital, N (%) | |
| Vanderbilt University Hospital | 373 (47.2) |
| Brigham and Women's Hospital | 417 (52.8) |
| Age in years, median (IQR) | 61 (52, 71) |
| Gender, N (%) | |
| Male | 452 (57.2) |
| Female | 338 (42.8) |
| Primary language, N (%) | |
| English | 779 (98.6) |
| Spanish | 11 (1.4) |
| Race, N (%) | |
| White | 610 (77.2) |
| Black or African American | 136 (17.2) |
| Other | 44 (5.6) |
| Health literacy, s‐TOFHLA score, median (IQR) | 33 (25, 35) |
| Health literacy, N (%)& | |
| Inadequate | 84 (10.6) |
| Marginal | 74 (9.4) |
| Adequate | 613 (77.6) |
| Mini‐Cog score, median (IQR) | 4 (3, 5) |
| Dementia, N (%) | |
| No | 692 (87.6) |
| Yes | 98 (12.4) |
| Number of medications, median (IQR) | 8 (5, 11) |
| Health insurance type, N (%) | |
| Medicaid | 74 (9.4) |
| Medicare | 337 (42.6) |
| Private | 334 (42.3) |
| Self‐pay | 35 (4.4) |
| Other | 10 (1.3) |
| Self‐reported household income, N (%)& | |
| <$10,000 | 38 (4.8) |
| $10,000 to <$15,000 | 45 (5.7) |
| $15,000 to <$20,000 | 42 (5.3) |
| $20,000 to <$25,000 | 105 (13.3) |
| $25,000 to <$35,000 | 99 (12.5) |
| $35,000 to <$50,000 | 112 (14.2) |
| $50,000 to <$75,000 | 118 (14.9) |
| $75,000+ | 227 (28.7) |
| Years of school, median (IQR)& | 14 (12, 16) |
MUQ Scores
The MUQ was administered in approximately 5 minutes. The median MUQ score was 2.5 (IQR 2.2, 2.8) (Table 2); 16.3% of patients scored less than 2. Subjects typically achieved high scores for the domains of indication, units, and frequency, while scores on the strength domain were lower (median = 0.2 [IQR 0.1, 0.4], maximum possible = 0.5).
| Median (IQR) | |
|---|---|
| |
| No. of drugs tested | 5 (4, 5) |
| MUQ score* | 2.5 (2.2, 2.8) |
| Indication | 1.0 (0.8, 1.0) |
| Strength | 0.2 (0.1, 0.4) |
| Units | 0.5 (0.4, 0.5) |
| Frequency | 1.0 (0.8, 1.0) |
Predictors of Medication Understanding
Unadjusted relationships of health literacy, cognition, and number of medications with medication understanding are shown in Figure 1 (panels A, B, and C, respectively). The figure demonstrates a linear relationship with both health literacy (Figure 1A) and cognition (Figure 1B), and a nonlinear relationship between number of preadmission medications and MUQ score (Figure 1C).

Adjusted relationships using imputed data for missing covariates are shown in Figure 2. Lower health literacy, cognitive impairment, male gender, and black race were independently associated with lower understanding of preadmission medications. Each 1 point increase in s‐TOFHLA or Mini‐Cog score led to an increase in medication understanding (OR = 1.04; 95% CI, 1.02 to 1.06; P = 0.0001; and OR = 1.24; 95% CI, 1.1 to 1.4; P = 0.001; respectively). Patients with marginal or inadequate health literacy had lower odds of understanding their regimen (OR = 0.53; 95% CI, 0.34 to 0.84; and OR = 0.49; 95% CI, 0.31 to 0.78, respectively) compared to those with adequate health literacy. Impaired cognitive function (Mini‐Cog score <3, indicating dementia) was also associated with lower odds of medication understanding (OR = 0.57; 95% CI, 0.38 to 0.86) compared to those with no cognitive impairment. An increase in the number of preadmission medications (up to 10) was also strongly associated with lower MUQ scores. For each increase by 1 medication, there was a significant decrease in medication understanding, up to 10 medications, beyond which understanding did not significantly decrease further. Patients on 6 medications were about half as likely to understand their medication regimen as patients on only 1 medication (OR = 0.52; 95% CI, 0.36 to 0.75). For patients on 11 medications, the odds of medication understanding were 24% lower than for patients on 6 medications (OR = 0.76; 95% CI, 0.65 to 0.89). Patients' age, years of schooling, and household income were not independently associated with medication understanding. Results were similar using data without multiple imputation.

Examples of Misunderstanding of Common Medications
Table 3 provides examples of incorrect patient responses for several commonly prescribed medications or drug classes, including aspirin, digoxin, nitroglycerin, and HMG‐CoA reductase inhibitors (statins). For aspirin, many patients were not aware of the strength. For digoxin, several participants reported splitting a higher‐strength pill to obtain the prescribed dose, which should not be done given the imprecision of splitting and narrow therapeutic index of this drug. Patients prescribed nitroglycerin sublingual tablets were commonly unable to report the correct dosing and frequency for angina treatment. Medications for cholesterol were often reported as being taken in the morning; this was scored strictly as a frequency error if the medication timing in the EHR was listed as evening or bedtime. We also identified many patients with poor understanding of opioid analgesics, particularly regarding their dosing and frequency.
| Medications | Common Incorrect Responses | Correct Information | Coded Error |
|---|---|---|---|
| |||
| Aspirin | tablet twice a day | 1 tablet once a day | Units and frequency |
| I am not aware what aspirin I am taking | 81 mg once a day | Strength | |
| I am taking 6‐something every day | 81 mg once a day | Strength | |
| 31 mg a day | 81 mg once a day | Strength | |
| 180 mg a day | 81 mg once a day | Strength | |
| 1 low‐dose daily | 325 mg once a day | Strength | |
| 125 mg a day | 325 mg once a day | Strength | |
| I am taking it for my blood pressure | Heart medication | Indication | |
| Nitroglycerin sublingual | As needed, I have taken up to 4 a day | Dissolve 1 tablet under the tongue, every 5 min as needed, up to 3 doses | Frequency |
| As needed every 15 min | Frequency | ||
| As needed up to 4 doses every 10 min | Frequency | ||
| Dissolve couple units under the tongue, as needed | Units and frequency | ||
| As many as I want, every 5 min | Frequency | ||
| Digoxin | tablet daily | 1 tablet daily | Units |
| 1 tablet daily | 1 tablet every other day | Frequency | |
| I am taking it for my blood pressure | Heart medication | Indication | |
| HMG‐CoA reductase inhibitors | 1 tablet every morning | 1 tablet every evening | Frequency |
| tablet twice a day | 1 tablet once a day | Units and frequency | |
| I do not know the indication | High cholesterol | Indication | |
| Propoxyphene/acetaminophen | tablet as needed | 1 tablet every 4‐6 hr as needed | Units and frequency |
| Hydrocodone/acetaminophen | I do not know the strength of this medication | 5 mg/500 mg | Strength |
| 1 tablet as I need it | 1 tablet every 4‐6 hr as needed | Frequency | |
DISCUSSION
We used a novel four‐component medication understanding questionnaire, developed for this study, to assess patients' understanding of up to 5 drugs selected randomly from the participant's preadmission medication list. The MUQ proved to be easy to administer by nonmedical staff within a short period of time (approximately 5 minutes per patient). It was well understood by patients. By limiting the assessment to 5 or fewer medications, the MUQ has a distinct advantage over existing measures of medication understanding that require testing the entire regimen. We did not find any limitations related to cutting off the assessment at 5 medications. In addition, this tool affords assessment of medication understanding without requiring medication bottles be present, enhancing its utility in the inpatient setting.
MUQ scores were associated with health literacy and other patient characteristics in an expected manner. We demonstrated that inadequate or marginal health literacy, as well as impaired cognitive function, were associated with low medication understanding. We also were able to demonstrate a relationship between increasing number of medications and lower medication understanding. Interestingly, in our patient population, understanding continued to decrease until reaching 10 medications, beyond which further increases in the number of medications had no additional detrimental effect on medication understanding. This nonlinear relationship between number of medications and medication understanding has potential implications for prescribing practice.
Our findings which utilize the MUQ among inpatients are consistent with prior literature in other settings.2, 7, 8 In a previous outpatient study, we identified that health literacy plays an important role in a patient's ability to successfully report and manage their daily medications.2 Other studies have also shown that patients with low health literacy have more difficulty understanding prescription drug information, and that they often experience medication‐related problems after hospital discharge.15, 16 The number and often the types of medications an individual takes have also been shown to increase the risk for adverse events and nonadherence to the treatment plan.1720 We postulate that this risk of adverse drug events is related at least in part to a patient's understanding of their medication regimen.
There are several limitations to this study. First, the MUQ did not assess certain aspects of medication understanding, such as knowledge of pill appearance or side effects, nor did it assess components of patients' actual drug‐taking behavior, such as organization of medications or behavioral cues. Thus, adaptive behaviors that patients may perform to improve their medication management, such as writing on labels or memory cues, are not captured by this test. Second, in administering and scoring the MUQ, we used the patient's preadmission medication list documented in the EHR as the reference standard. This was the best available reference list, and was generally accurate, as both hospitals had medication reconciliation systems in use at the time of the study21; nevertheless, it may contain inaccuracies. Documentation for certain medications, such as warfarin, in which dose can change frequently, often did not reflect the latest prescribed dose. In such cases, we scored the patient's answer as correct if the dose appeared reasonable and appropriate to the clinical pharmacist. As a result, a patient's MUQ score may have been overestimated in these cases.
Additional research will be needed to further validate the MUQ in other settings. In particular, studies should establish the relationship between the MUQ, serious medication errors after discharge, and potential to benefit from educational interventions. Also, as noted above, the nonlinear relationship between number of medications and medication understanding should be confirmed in other studies.
In conclusion, we demonstrated that patients with low health literacy, impaired cognition, or a higher number of medications had significantly poorer understanding of their preadmission medication regimen. These findings have important clinical implications. It would be appropriate to exercise greater caution when taking a medication history from patients who cannot readily provide the purpose, strength, units, and frequency of their medications. Attempts to validate the information obtained from patients with other sources of data, such as family members, inpatient or outpatient health records, and community pharmacy records should be considered. Patients at high risk for poor medication understanding, either measured directly using the MUQ or identified via risk factors such as polypharmacy, low cognition, or low health literacy, may warrant more intensive medication reconciliation interventions and/or educational counseling and follow‐up to prevent postdischarge adverse drug events. Further research is needed to determine if targeting these populations for interventions improves medication safety during transitions in care.
With the aging of the US population, complex medication regimens to treat multiple comorbidities are increasingly common.1 Nevertheless, patients often do not fully understand the instructions for safe and effective medication use. Aspects of medication understanding include knowledge of the drug indication, dose, frequency, and for certain medications, special instructions.2 Medication understanding is associated with better medication adherence, fewer drug‐related problems, and fewer emergency department visits.3 Among patients with chronic conditions, such as cardiovascular disease (CVD), understanding and adherence to the medication regimen are critical for successful disease control and clinical outcomes.4
Patients' understanding of their medication regimen is also vitally important upon admission to the hospital. Patients often are the main source of information for the admission medication history and subsequent medication reconciliation.5 Poor patient understanding of the preadmission medication regimen can contribute to errors in inpatient and postdischarge medication orders, and adversely affect patient safety.6 However, little research has examined patients' understanding of the preadmission medication regimen and factors that affect it.
In the outpatient setting, previous investigations have suggested that low health literacy, advanced age, and impaired cognitive function adversely affect patients' understanding of medication instructions.2, 7, 8 These studies were limited by a small sample size, single site, or focus on a specific population, such as inner‐city patients. Additionally, the measures used to assess medication understanding were time‐consuming and required patients' medications to be present for testing, thus limiting their utility.2
To address these gaps in the literature, we developed and implemented the Medication Understanding Questionnaire (MUQ), an original and relatively rapid measure that does not require patients' medications be present for testing. In a study of adults at 2 large teaching hospitals, we examined the association of health literacy, age, cognitive function, number of preadmission medications, and other factors on patients' understanding of their preadmission medication regimen. We hypothesized that lower health literacy would be independently associated with lower medication understanding as assessed using the MUQ.
METHODS
The present study was a cross‐sectional assessment conducted using baseline interview data from the Pharmacist Intervention for Low Literacy in Cardiovascular Disease (PILL‐CVD) Study (ClinicalTrials.gov Registration #NCT00632021; available at:
Population
The PILL‐CVD study protocol and eligibility criteria has been previously published.9 Briefly, patients were eligible if they were at least 18 years old and admitted with acute coronary syndrome or acute decompensated heart failure. Patients were excluded if they: were too ill to complete an interview; were not oriented to person, place, or time; had a corrected visual acuity worse than 20/200; had impaired hearing; could not communicate in English or Spanish; were not responsible for managing their own medications; had no phone; were unlikely to be discharged to home; were in police custody; or had been previously enrolled in the study. For the present analysis, we also excluded any patient who was not on at least 1 prescription medication prior to admission. Saline nasal spray, saline eye drops, herbal products, nutritional supplements, vitamins, and over the counter (OTC) lotions and creams were not counted as prescription medications. Oral medications available both OTC and by prescription (eg, aspirin, nonsteroidal anti‐inflammatory drugs, and acid reflux medications) were counted as prescription medications.
Measures
At enrollment, which was usually within 24 to 48 hours of admission, participants completed the short form of the Test of Functional Health Literacy in Adults (s‐TOFHLA) in English or Spanish,10 the Mini‐Cog test of cognition,11 and the Medication Understanding Questionnaire (MUQ), as well as demographic information. The number of prescription medications prior to hospital admission was abstracted from the best available reference listthat documented by the treating physicians in the electronic health record (EHR). The EHR at each site was a home‐grown system and included both inpatient and outpatient records, which facilitated physicians' documentation of the medication list.
The s‐TOFHLA consists of 2 short reading‐comprehension passages. Scores on the s‐TOFHLA range from 0 to 36, and can be categorized as inadequate (0‐16), marginal (17‐22), or adequate (23‐36) health literacy.10 The Mini‐Cog includes 3‐item recall and clock‐drawing tests. It provides a brief measure of cognitive function and performs well among patients with limited literacy or educational attainment.11 Scores range from 0 to 5, with a score <3 indicating possible dementia.
The MUQ was administered verbally and assessed patients' understanding of their own preadmission medication regimen. It was developed for this study, based on published measures of medication understanding.2, 12 To administer the MUQ, research assistants (RAs) accessed the patient's preadmission medication list from the EHR and used a random number table to select up to 5 prescription medications from the list. If the patient was taking 5 or fewer medications, all of their medications were selected. Saline nasal spray, saline eye drops, herbal products, nutritional supplements, vitamins, and OTC lotions and creams were excluded from testing. The RA provided the brand and generic name of each medication, and then asked the patient for the drug's purpose, strength per unit (eg, 20 mg tablet), number of units taken at a time (eg, 2 tablets), and dosing frequency (eg, twice a day). For drugs prescribed on an as‐needed basis, the RA asked patients for the maximum allowable dose and frequency. Patients were instructed to not refer to a medication list or bottles when responding. The RA documented the patient's responses on the MUQ, along with the dosing information from the EHR for each selected medication.
One clinical pharmacist (MM) scored all MUQ forms by applying a set of scoring rules. Each medication score could range from 0 to 3. The components of the score included indication (1 point), strength (0.5 point), units (0.5 point), and frequency (1 point). The patient's overall MUQ score was an average of the MUQ scores for each tested medication.
Statistical Analysis
We summarized patient characteristics, number of preadmission medications, and MUQ scores using median and interquartile range (IRQ) for continuous variables, and frequencies and proportions for categorical variables. We conducted proportional odds logistic regression (ordinal regression) to estimate the effect of s‐TOFHLA score, other patient characteristics, and number of medications on MUQ scores.13
Important covariates were selected a priori based on clinical significance. These included age (continuous), gender, patient self‐reported race (white, black, other nonwhite), Mini‐Cog score (continuous), primary language (English or Spanish), years of education (continuous), number of preadmission medications (continuous), income (ordinal categories), insurance type (categorical), and study site. Covariates with missing data (household income, health literacy, and years of education) were imputed using multiple imputation techniques.14 The relationship between number of preadmission medications and MUQ scores was found to be nonlinear, and it was modeled using restricted cubic splines.14 We also fit models which treated health literacy and cognition as categorical variables. Results are reported as odds ratios (OR) with 95% confidence intervals (CI). Wald tests were used to test for the statistical significance of predictor variables. Two‐sided P values less than 0.05 were considered statistically significant. All analyses were performed using statistical language R (R Foundation, available at:
RESULTS
Baseline Characteristics
Among the 862 patients enrolled in PILL‐CVD, 790 (91.7 %) had at least 1 preadmission medication and were included in this analysis (Table 1). Forty‐seven percent were admitted to VUH (N = 373) and 53% to BWH (N = 417). The median age was 61 (interquartile range [IQR] 52, 71), 77% were white, and 57% were male. Inadequate or marginal health literacy was identified among 11% and 9% of patients, respectively. The median number of preadmission medications was 8 (IQR 5, 11). Patients excluded from the analysis for not having preadmission medications were similar to included patients, except they were more likely to be male (76% vs 57%) and less likely to have health insurance (23% self‐pay vs 4%). (Data available upon request.)
| Characteristic | N = 790 |
|---|---|
| |
| Study hospital, N (%) | |
| Vanderbilt University Hospital | 373 (47.2) |
| Brigham and Women's Hospital | 417 (52.8) |
| Age in years, median (IQR) | 61 (52, 71) |
| Gender, N (%) | |
| Male | 452 (57.2) |
| Female | 338 (42.8) |
| Primary language, N (%) | |
| English | 779 (98.6) |
| Spanish | 11 (1.4) |
| Race, N (%) | |
| White | 610 (77.2) |
| Black or African American | 136 (17.2) |
| Other | 44 (5.6) |
| Health literacy, s‐TOFHLA score, median (IQR) | 33 (25, 35) |
| Health literacy, N (%)& | |
| Inadequate | 84 (10.6) |
| Marginal | 74 (9.4) |
| Adequate | 613 (77.6) |
| Mini‐Cog score, median (IQR) | 4 (3, 5) |
| Dementia, N (%) | |
| No | 692 (87.6) |
| Yes | 98 (12.4) |
| Number of medications, median (IQR) | 8 (5, 11) |
| Health insurance type, N (%) | |
| Medicaid | 74 (9.4) |
| Medicare | 337 (42.6) |
| Private | 334 (42.3) |
| Self‐pay | 35 (4.4) |
| Other | 10 (1.3) |
| Self‐reported household income, N (%)& | |
| <$10,000 | 38 (4.8) |
| $10,000 to <$15,000 | 45 (5.7) |
| $15,000 to <$20,000 | 42 (5.3) |
| $20,000 to <$25,000 | 105 (13.3) |
| $25,000 to <$35,000 | 99 (12.5) |
| $35,000 to <$50,000 | 112 (14.2) |
| $50,000 to <$75,000 | 118 (14.9) |
| $75,000+ | 227 (28.7) |
| Years of school, median (IQR)& | 14 (12, 16) |
MUQ Scores
The MUQ was administered in approximately 5 minutes. The median MUQ score was 2.5 (IQR 2.2, 2.8) (Table 2); 16.3% of patients scored less than 2. Subjects typically achieved high scores for the domains of indication, units, and frequency, while scores on the strength domain were lower (median = 0.2 [IQR 0.1, 0.4], maximum possible = 0.5).
| Median (IQR) | |
|---|---|
| |
| No. of drugs tested | 5 (4, 5) |
| MUQ score* | 2.5 (2.2, 2.8) |
| Indication | 1.0 (0.8, 1.0) |
| Strength | 0.2 (0.1, 0.4) |
| Units | 0.5 (0.4, 0.5) |
| Frequency | 1.0 (0.8, 1.0) |
Predictors of Medication Understanding
Unadjusted relationships of health literacy, cognition, and number of medications with medication understanding are shown in Figure 1 (panels A, B, and C, respectively). The figure demonstrates a linear relationship with both health literacy (Figure 1A) and cognition (Figure 1B), and a nonlinear relationship between number of preadmission medications and MUQ score (Figure 1C).

Adjusted relationships using imputed data for missing covariates are shown in Figure 2. Lower health literacy, cognitive impairment, male gender, and black race were independently associated with lower understanding of preadmission medications. Each 1 point increase in s‐TOFHLA or Mini‐Cog score led to an increase in medication understanding (OR = 1.04; 95% CI, 1.02 to 1.06; P = 0.0001; and OR = 1.24; 95% CI, 1.1 to 1.4; P = 0.001; respectively). Patients with marginal or inadequate health literacy had lower odds of understanding their regimen (OR = 0.53; 95% CI, 0.34 to 0.84; and OR = 0.49; 95% CI, 0.31 to 0.78, respectively) compared to those with adequate health literacy. Impaired cognitive function (Mini‐Cog score <3, indicating dementia) was also associated with lower odds of medication understanding (OR = 0.57; 95% CI, 0.38 to 0.86) compared to those with no cognitive impairment. An increase in the number of preadmission medications (up to 10) was also strongly associated with lower MUQ scores. For each increase by 1 medication, there was a significant decrease in medication understanding, up to 10 medications, beyond which understanding did not significantly decrease further. Patients on 6 medications were about half as likely to understand their medication regimen as patients on only 1 medication (OR = 0.52; 95% CI, 0.36 to 0.75). For patients on 11 medications, the odds of medication understanding were 24% lower than for patients on 6 medications (OR = 0.76; 95% CI, 0.65 to 0.89). Patients' age, years of schooling, and household income were not independently associated with medication understanding. Results were similar using data without multiple imputation.

Examples of Misunderstanding of Common Medications
Table 3 provides examples of incorrect patient responses for several commonly prescribed medications or drug classes, including aspirin, digoxin, nitroglycerin, and HMG‐CoA reductase inhibitors (statins). For aspirin, many patients were not aware of the strength. For digoxin, several participants reported splitting a higher‐strength pill to obtain the prescribed dose, which should not be done given the imprecision of splitting and narrow therapeutic index of this drug. Patients prescribed nitroglycerin sublingual tablets were commonly unable to report the correct dosing and frequency for angina treatment. Medications for cholesterol were often reported as being taken in the morning; this was scored strictly as a frequency error if the medication timing in the EHR was listed as evening or bedtime. We also identified many patients with poor understanding of opioid analgesics, particularly regarding their dosing and frequency.
| Medications | Common Incorrect Responses | Correct Information | Coded Error |
|---|---|---|---|
| |||
| Aspirin | tablet twice a day | 1 tablet once a day | Units and frequency |
| I am not aware what aspirin I am taking | 81 mg once a day | Strength | |
| I am taking 6‐something every day | 81 mg once a day | Strength | |
| 31 mg a day | 81 mg once a day | Strength | |
| 180 mg a day | 81 mg once a day | Strength | |
| 1 low‐dose daily | 325 mg once a day | Strength | |
| 125 mg a day | 325 mg once a day | Strength | |
| I am taking it for my blood pressure | Heart medication | Indication | |
| Nitroglycerin sublingual | As needed, I have taken up to 4 a day | Dissolve 1 tablet under the tongue, every 5 min as needed, up to 3 doses | Frequency |
| As needed every 15 min | Frequency | ||
| As needed up to 4 doses every 10 min | Frequency | ||
| Dissolve couple units under the tongue, as needed | Units and frequency | ||
| As many as I want, every 5 min | Frequency | ||
| Digoxin | tablet daily | 1 tablet daily | Units |
| 1 tablet daily | 1 tablet every other day | Frequency | |
| I am taking it for my blood pressure | Heart medication | Indication | |
| HMG‐CoA reductase inhibitors | 1 tablet every morning | 1 tablet every evening | Frequency |
| tablet twice a day | 1 tablet once a day | Units and frequency | |
| I do not know the indication | High cholesterol | Indication | |
| Propoxyphene/acetaminophen | tablet as needed | 1 tablet every 4‐6 hr as needed | Units and frequency |
| Hydrocodone/acetaminophen | I do not know the strength of this medication | 5 mg/500 mg | Strength |
| 1 tablet as I need it | 1 tablet every 4‐6 hr as needed | Frequency | |
DISCUSSION
We used a novel four‐component medication understanding questionnaire, developed for this study, to assess patients' understanding of up to 5 drugs selected randomly from the participant's preadmission medication list. The MUQ proved to be easy to administer by nonmedical staff within a short period of time (approximately 5 minutes per patient). It was well understood by patients. By limiting the assessment to 5 or fewer medications, the MUQ has a distinct advantage over existing measures of medication understanding that require testing the entire regimen. We did not find any limitations related to cutting off the assessment at 5 medications. In addition, this tool affords assessment of medication understanding without requiring medication bottles be present, enhancing its utility in the inpatient setting.
MUQ scores were associated with health literacy and other patient characteristics in an expected manner. We demonstrated that inadequate or marginal health literacy, as well as impaired cognitive function, were associated with low medication understanding. We also were able to demonstrate a relationship between increasing number of medications and lower medication understanding. Interestingly, in our patient population, understanding continued to decrease until reaching 10 medications, beyond which further increases in the number of medications had no additional detrimental effect on medication understanding. This nonlinear relationship between number of medications and medication understanding has potential implications for prescribing practice.
Our findings which utilize the MUQ among inpatients are consistent with prior literature in other settings.2, 7, 8 In a previous outpatient study, we identified that health literacy plays an important role in a patient's ability to successfully report and manage their daily medications.2 Other studies have also shown that patients with low health literacy have more difficulty understanding prescription drug information, and that they often experience medication‐related problems after hospital discharge.15, 16 The number and often the types of medications an individual takes have also been shown to increase the risk for adverse events and nonadherence to the treatment plan.1720 We postulate that this risk of adverse drug events is related at least in part to a patient's understanding of their medication regimen.
There are several limitations to this study. First, the MUQ did not assess certain aspects of medication understanding, such as knowledge of pill appearance or side effects, nor did it assess components of patients' actual drug‐taking behavior, such as organization of medications or behavioral cues. Thus, adaptive behaviors that patients may perform to improve their medication management, such as writing on labels or memory cues, are not captured by this test. Second, in administering and scoring the MUQ, we used the patient's preadmission medication list documented in the EHR as the reference standard. This was the best available reference list, and was generally accurate, as both hospitals had medication reconciliation systems in use at the time of the study21; nevertheless, it may contain inaccuracies. Documentation for certain medications, such as warfarin, in which dose can change frequently, often did not reflect the latest prescribed dose. In such cases, we scored the patient's answer as correct if the dose appeared reasonable and appropriate to the clinical pharmacist. As a result, a patient's MUQ score may have been overestimated in these cases.
Additional research will be needed to further validate the MUQ in other settings. In particular, studies should establish the relationship between the MUQ, serious medication errors after discharge, and potential to benefit from educational interventions. Also, as noted above, the nonlinear relationship between number of medications and medication understanding should be confirmed in other studies.
In conclusion, we demonstrated that patients with low health literacy, impaired cognition, or a higher number of medications had significantly poorer understanding of their preadmission medication regimen. These findings have important clinical implications. It would be appropriate to exercise greater caution when taking a medication history from patients who cannot readily provide the purpose, strength, units, and frequency of their medications. Attempts to validate the information obtained from patients with other sources of data, such as family members, inpatient or outpatient health records, and community pharmacy records should be considered. Patients at high risk for poor medication understanding, either measured directly using the MUQ or identified via risk factors such as polypharmacy, low cognition, or low health literacy, may warrant more intensive medication reconciliation interventions and/or educational counseling and follow‐up to prevent postdischarge adverse drug events. Further research is needed to determine if targeting these populations for interventions improves medication safety during transitions in care.
- ,,.Prevalence, expenditures, and complications of multiple chronic conditions in the elderly.Arch Intern Med.2002;162(20):2269–2276.
- ,,,,,.Predictors of medication self‐management skill in a low‐literacy population.J Gen Intern Med.2006;21(8):852–856.
- ,.Adherence to medication.N Engl J Med.2005;353(5):487–497.
- ,,.Medication adherence: its importance in cardiovascular outcomes.Circulation.2009;119(23):3028–3035.
- ,,, et al.Classifying and predicting errors of inpatient medication reconciliation.J Gen Intern Med.2008;23(9):1414–1422.
- ,.Addressing postdischarge adverse events: a neglected area.Jt Comm J Qual Patient Saf.2008;34(2):85–97.
- ,,.Medication management capacity in highly functioning community‐living older adults: detection of early deficits.J Am Geriatr Soc.1999;47(5):592–596.
- ,,.Variation in medication understanding among the elderly.Am J Health‐Syst Pharm.2004;61(4):373–380.
- ,,, et al.The rationale and design of the Pharmacist Intervention for Low Literacy in Cardiovascular Disease (PILL‐CVD) study.Circ Cardiovasc Qual Outcomes.2010;3:212–219.
- ,,,.Short Test of Functional Health Literacy in Adults.Snow Camp, NC:Peppercorn Books and Press;1998.
- ,,,,.Simplifying detection of cognitive impairment: comparison of the Mini‐Cog and Mini‐Mental State Examination in a multiethnic sample.J Am Geriatr Soc.2005;53(5):871–874.
- ,.Instruments assessing capacity to manage medications.Ann Pharmacother.2008;42(7):1026–1036.
- ,.Estimation of the probability of an event as a function of several independent variables.Biometrika.1967;54(1):167–179.
- ,.Using full probability models to compute probabilities of actual interest to decision makers.Int J Technol Assess Health Care.2001;17(1):17–26.
- ,,, et al.Literacy and misunderstanding prescription drug labels.Ann Intern Med.2006;145(12):887–894.
- ,,,.Medication use among inner‐city patients after hospital discharge: patient reported barriers and solutions.Mayo Clin Proc.2008;83(5):529–535.
- ,,,,,.National surveillance of emergency department visits for outpatient adverse drug events.JAMA.2006;296(15):1858–1866.
- ,,,.Medication use leading to emergency department visits for adverse drug events in older adults.Ann Intern Med.2007;147(11):755–765.
- ,,,,.Adverse drug events occurring following hospital discharge.J Gen Intern Med.2005;20:317–323.
- ,,, et al.Adverse drug events in ambulatory care.N Engl J Med.2003;348(16):1556–1564.
- ,,, et al.Effect of an electronic medication reconciliation application and process redesign on potential adverse drug events: a cluster‐randomized trial.Arch Intern Med.2009;169(8):771–780.
- ,,.Prevalence, expenditures, and complications of multiple chronic conditions in the elderly.Arch Intern Med.2002;162(20):2269–2276.
- ,,,,,.Predictors of medication self‐management skill in a low‐literacy population.J Gen Intern Med.2006;21(8):852–856.
- ,.Adherence to medication.N Engl J Med.2005;353(5):487–497.
- ,,.Medication adherence: its importance in cardiovascular outcomes.Circulation.2009;119(23):3028–3035.
- ,,, et al.Classifying and predicting errors of inpatient medication reconciliation.J Gen Intern Med.2008;23(9):1414–1422.
- ,.Addressing postdischarge adverse events: a neglected area.Jt Comm J Qual Patient Saf.2008;34(2):85–97.
- ,,.Medication management capacity in highly functioning community‐living older adults: detection of early deficits.J Am Geriatr Soc.1999;47(5):592–596.
- ,,.Variation in medication understanding among the elderly.Am J Health‐Syst Pharm.2004;61(4):373–380.
- ,,, et al.The rationale and design of the Pharmacist Intervention for Low Literacy in Cardiovascular Disease (PILL‐CVD) study.Circ Cardiovasc Qual Outcomes.2010;3:212–219.
- ,,,.Short Test of Functional Health Literacy in Adults.Snow Camp, NC:Peppercorn Books and Press;1998.
- ,,,,.Simplifying detection of cognitive impairment: comparison of the Mini‐Cog and Mini‐Mental State Examination in a multiethnic sample.J Am Geriatr Soc.2005;53(5):871–874.
- ,.Instruments assessing capacity to manage medications.Ann Pharmacother.2008;42(7):1026–1036.
- ,.Estimation of the probability of an event as a function of several independent variables.Biometrika.1967;54(1):167–179.
- ,.Using full probability models to compute probabilities of actual interest to decision makers.Int J Technol Assess Health Care.2001;17(1):17–26.
- ,,, et al.Literacy and misunderstanding prescription drug labels.Ann Intern Med.2006;145(12):887–894.
- ,,,.Medication use among inner‐city patients after hospital discharge: patient reported barriers and solutions.Mayo Clin Proc.2008;83(5):529–535.
- ,,,,,.National surveillance of emergency department visits for outpatient adverse drug events.JAMA.2006;296(15):1858–1866.
- ,,,.Medication use leading to emergency department visits for adverse drug events in older adults.Ann Intern Med.2007;147(11):755–765.
- ,,,,.Adverse drug events occurring following hospital discharge.J Gen Intern Med.2005;20:317–323.
- ,,, et al.Adverse drug events in ambulatory care.N Engl J Med.2003;348(16):1556–1564.
- ,,, et al.Effect of an electronic medication reconciliation application and process redesign on potential adverse drug events: a cluster‐randomized trial.Arch Intern Med.2009;169(8):771–780.
Copyright © 2011 Society of Hospital Medicine
Improving Inpatient Glycemic Control
Diabetes mellitus and/or inpatient hyperglycemia are common comorbid conditions in hospitalized patients. Recent surveys show that over 90% of hospitalized diabetic patients experience hyperglycemia (>200 mg/dL), and in nearly 1 in 5 of these patients hyperglycemia persists for 3 days or more.1 Hyperglycemia among inpatients without a previous history of diabetes mellitus is also very common.2 Observational studies have shown that hyperglycemia in hospitalized patients is associated with adverse outcomes including infectious complications, increased length of stay, and increased mortality.27 Recent randomized controlled trials have demonstrated that aggressive treatment of inpatient hyperglycemia improves outcomes in surgical and medical intensive care units.8, 9
Based on the available data, the American Diabetes Association (ADA) now advocates good metabolic control, defined as preprandial glucose levels of 90 to 130 mg/dL and peak postprandial glucose levels <180 mg/dL in hospitalized nonintensive care unit (ICU) patients.10 To reach these targets, the ADA and American College of Endocrinology (ACE) suggest that multidisciplinary teams develop and implement hyperglycemia management guidelines and protocols.11 Protocols should promote the use of continuous intravenous insulin infusions or scheduled basal‐bolus subcutaneous insulin regimens. Subcutaneous insulin protocols should include target glucose levels, basal, nutritional, and supplemental insulin, and daily dose adjustments.6 A recent randomized controlled trial of non‐ICU inpatients demonstrated that such a basal‐bolus insulin regimen results in improved glucose control compared with a sliding scale only regimen.12
To date, few published studies have investigated the best ways to implement such management protocols; those that have are often resource‐intensive, for example involving daily involvement of nurse practitioners or diabetologists.13, 14 It is therefore not known how best to implement an inpatient diabetes management program that is effective, efficient, and self‐perpetuating. At Brigham and Women's Hospital (BWH), we have been refining a subcutaneous insulin protocol, focused provider education, and more recently a computerized order set to overcome barriers related to fear of hypoglycemia, delays in insulin prescribing, and unfamiliarity with inpatient glucose management.15 The aims of this current trial were to evaluate the effects of these interventions on a geographically localized general medical service previously naive to these interventions to evaluate their effects on glycemic control, patient safety, and processes of care. We hypothesized that these interventions would improve glycemic control and increase use of basal‐bolus insulin orders without increasing the rate of hypoglycemia.
METHODS
Setting and Participants
This prospective, before‐after trial was conducted at BWH from July 15, 2005 through June 22, 2006. Eligible subjects were patients scheduled for admission to the BWH Physician Assistant/Clinician Educator (PACE) Service with either a known diagnosis of type 2 diabetes mellitus or inpatient hyperglycemia (at least 1 random laboratory glucose >180 mg/dL). The PACE service is a geographically‐localized general medicine service of up to 15 beds where patients are cared for by a single cadre of nurses, 2 physician's assistants (PAs), and 1 hospitalist attending. A moonlighter covers the service at night. The PACE service does not accept patients transferred from other acute care hospitals or from ICUs, but does not otherwise have triage guidelines related to diagnosis, complexity, or acuity. Patients were excluded if they had type 1 diabetes, presented with hyperosmolar hyperglycemic state (HHS) or diabetic ketoacidosis (DKA), received total parenteral nutrition (TPN), or were receiving palliative care. This study was approved by the BWH Institutional Review Board; patient consent was deemed not to be necessary for this study given the relatively nonsensitive nature of the data, noninvasive means of data collection, and the steps taken by research personnel to minimize any breach in patient confidentiality.
Intervention
The study intervention consisted of three components, initiated in January 2006:
Glycemic management protocol: a multidisciplinary team of a diabetologist (M.L.P.), a hospitalist (J.L.S.), and a pharmacist (Jennifer Trujillo) developed a subcutaneous insulin protocol based on ADA guidelines (Table 1; see the appendix for complete protocol). The protocol was approved by the BWH Pharmacy and Therapeutics Diabetes Subcommittee and refined through 6 months of pilot testing on other general medical services.15 The protocol consisted of a set of specific treatment recommendations, including: (1) bedside glucose monitoring; (2) stopping oral diabetes agents in most patients; (3) estimating total daily insulin requirements; (4) prescribing basal, nutritional, and supplemental insulin based on the patient's total insulin requirements, preadmission medication regimen, and nutritional status; (5) adjusting insulin on a daily basis as needed; (6) managing hypoglycemia; (7) suggestions for discharge orders; and (8) indications for an endocrinology consultation. The protocol was printed as a pocket guide, distributed to all members of the PACE service, and used to guide all other interventions.
Diabetes education: all PAs received 2 one‐hour educational sessions: a lecture by a diabetologist (M.L.P.) reviewing the rationale for tight glycemic control and general principles of management, and a workshop by a hospitalist (J.L.S.) in which specific cases were reviewed to illustrate how the protocol could be used in practice (eg, when oral agents could be safely continued, how to prescribe insulin on admission, and how to make subsequent adjustments in dose). All hospitalist attendings received a 1‐hour lecture summarizing the above material. All nurses on the service received a lecture that focused on issues unique to nursing care, such as insulin administration, glucose testing, managing patients with unpredictable oral (PO) intake, and patient education. (All materials are available from the authors upon request).
Order Set: an order set, built into BWH's proprietary computer provider order entry (CPOE) system, was created to parallel the glycemic management protocol and facilitate insulin orders for patients eating discrete meals, receiving continuous liquid enteral nutrition (tube feeds), or receiving nothing by mouth (NPO). Other components of the order set facilitated glucose monitoring and other laboratory tests and ordering consultation when appropriate.
| Oral Agents | Stop Oral Agents in Most Patients |
|---|---|
| |
| Glucose testing | Check bedside blood glucose before meals and at bedtime if eating, or every 6 hours if NPO |
| Insulin | |
| 1. Estimate total daily insulin dose | 0.5 to 0.7 units/kg/day, depending on patient's age, size, renal function, insulin sensitivity, history of hypoglycemia, and steroid use |
| 2. Start basal insulin | Patient's home dose or 50% of calculated total daily dose; NPH qAM/qHS or insulin glargine qHS; If NPO, use one‐half the home dose unless hyperglycemic |
| 3. Start nutritional insulin if not NPO | Patient's home dose or 50% of calculated total daily dose, less if poor or unknown intake; discrete meals: insulin aspart split over 3 meals, 0 to 15 minutes prior to eating; continuous tube feeds or IV dextrose: regular insulin every 6 hours |
| 4. Start correctional insulin | 1 of 3 scales provided based on total daily dose of insulin; same type as nutritional insulin; regular insulin if NPO |
| 5. Daily adjustment | Calculate total administered dose from prior day, adjust for degree of hyperglycemia or hypoglycemia, renal function, PO intake, steroid use, and degree of illness, and redistribute as 50% basal, 50% nutritional, or 100% basal if NPO |
| Hypoglycemia orders | Juice, IV dextrose, or IM glucagon depending on ability to take oral nutrition and IV access |
| Discharge orders | Based on A1C: either home regimen, titration of home regimen, or new insulin regimen (if latter, simple regimen with aggressive patient education and prompt follow‐up) |
| Indications for endocrine consultation | Labile blood sugars, poor control, prolonged NPO period, question of type 1 or type 2 diabetes |
Study Protocol and Data Collection
A research assistant prospectively identified eligible patients each weekday by screening all patients scheduled for admission to the PACE service using the daily computerized sign‐out system used on all general medical teams. Specifically, laboratory random glucose levels, inpatient medications, and medical histories were reviewed to determine if each patient met eligibility criteria. Eligibility criteria were then confirmed by medical record review and adjudicated by one study author (J.L.S.) if necessary. Further medical record review was performed to identify specific patient populations (eg, diet‐controlled, steroid‐induced, or previously undiagnosed diabetes), determine preadmission diabetes medications, and determine the patient's weight. Hospital computerized clinical and administrative records were abstracted to obtain patient demographics (age, sex, race, insurance status), laboratory data (glucose level on admission, A1C level [taken during or within 6 months prior to admission]), clinical data (length of stay, billing‐based Charlson comorbidity score,16 and diagnosis‐related group [DRG] case mix index), all inpatient insulin and oral diabetes medication orders, frequency of bedside glucose testing, and diet orders. Electronic medication administration record (eMAR) data were used to determine all doses and times of insulin administration.
Outcomes
The primary outcome was the mean percent of glucose readings between 60 and 180 mg/dL per patient (ie, calculated for each patient and averaged across all eligible patients in each study arm). Only bedside glucose readings were used given the lack of additional useful information typically provided by laboratory (venous plasma) glucose readings.17 Readings drawn within 1 hour of a previous reading were excluded to avoid ascertainment bias caused by follow‐up testing of abnormal glucose values. Only readings while on the study service were used. Readings on hospital day 1 were excluded because our intervention was expected to have little impact on the first day's glucose control; for patients with undiagnosed diabetes, data collection began the day following the first elevated glucose reading. Readings beyond hospital day 14 were also excluded to avoid biased data from patients with exceptionally long lengths of stay.
Secondary outcomes included the following:
Glycemic control:
Patient‐day weighted mean glucose (ie, mean glucose for each patient‐day, averaged across all patient days);
Mean glucose per patient for each hospital day (days 17).
Patient safety:
Proportion of patient‐days with any glucose reading <60 mg/dL (hypoglycemia) and <40 mg/dL (severe hypoglycemia).
Processes of care:
Use of any NPH insulin or insulin glargine (basal) insulin during the hospitalization if 2 or more glucose readings were >180 mg/dL.
Adequacy of basal dose on day first prescribed: for patients prescribed a diet, within 20% of preadmission basal dose or 0.20 to 0.42 units/kg if not known or not taken prior to admission. If not eating, half the above calculations.
Use of any scheduled nutritional insulin during the hospitalization if ever prescribed a diet and 2 or more glucose readings were greater than 180 mg/dL.
Adequacy of nutritional dose on day first prescribed: for patients prescribed a diet, within 20% of preadmission nutritional dose or 0.20 to 0.42 units/kg/day if not known or not taken prior to admission. Patients on clear liquid diets, enteral feeds, or receiving glucocorticoids were excluded from this analysis.
Correct type of nutritional insulin: if eating discrete meals, insulin aspart (the rapid‐acting insulin on formulary at BWH); if prescribed tube feeds, regular insulin.
Use of supplemental insulin by itself (without scheduled basal or nutritional insulin), a marker of poor care.
A1C testing within 1 month prior to or during hospitalization.
Clinical inertia: if at least two glucose readings <60 mg/dL or >180 mg/dL on a patient‐day, lack of any change to any insulin order the following day if still on the study service.
Healthcare utilization:
Hospital length of stay in hours, calculated from the exact time of admission until the exact time of discharge, using hospital administrative data.
Analyses
Study results were compared prior to the intervention (July 15 through December 12, 2005) with those during the intervention (January 18 through June 20, 2006). Patient data and clinical outcomes were analyzed descriptively using proportions, means with standard deviations (SDs), or medians with interquartile ranges (IQRs) as appropriate. Comparisons between groups were calculated using Fisher's exact test for dichotomous and categorical variables, and Student t test or Wilcoxon rank sum test for continuous variables as appropriate. The primary outcome was first analyzed using linear regression with study group as the independent variable and percent of glucose readings within range per patient as the dependent variable. We then adjusted for potential confounders by putting each covariate into the model, one at a time. All significant predictors of the outcome at a P value <0.10 were retained in the final model. We used general estimating equations to adjust for clustering of results by each PA. Similar analyses were performed for hospital length of stay per patient using a negative binomial model, so chosen because it fit the data distribution much better than the typically used Poisson model. With a planned sample size of 115 patients and 1250 glucose readings per arm, an intraclass correlation coefficient of 0.10, and an alpha of 0.05, the study had 90% power to detect an increase in percent of glucose readings in range from 67% to 75%. All analyses were based on the intention‐to‐treat principle. Except as above, 2‐sided P values <0.05 were considered significant. SAS version 9.1 (SAS Institute, Cary, NC) was used for all analyses.
RESULTS
We prospectively identified 248 potential patients for the study. We subsequently excluded 79 patients for the following reasons: no glucose readings beyond hospital day 1 while on PACE service (34 patients); never admitted to PACE service (15 patients); no diabetes or inpatient hyperglycemia (9 patients, mostly patients prescribed an insulin sliding scale prophylactically to avoid steroid‐induced hyperglycemia); type 1 diabetes (13 patients); TPN, DKA, or HHS (5 patients); and palliative care (3 patients). The remaining 169 patients included 63 from the preintervention period(out of 489 total admissions to the PACE service; 13%) and 106 patients in the postintervention period (out of 565 admissions; 19%). These patients had 2447 glucose readings, or an average of 3.6 glucose readings per monitored patient‐day in the preintervention period and 3.3 glucose readings per patient‐day in the postintervention period. Even including the 34 patients who were excluded for lack of glucose readings, glucose data were still available for 717 out of a potential 775 patient‐days (93%). Characteristics for all included patients are shown in Table 2. The mean admission glucose was 197 mg/dL, mean A1C was 8.4%, 54% of the patients were prescribed insulin prior to admission, and 7% had no prior diagnosis of diabetes. There were no significant differences in baseline characteristics between the 2 patient groups except for Charlson score, which was higher in the preintervention group (87% versus 74% with score 2 or higher; Table 2). The top diagnosis‐related groups for the entire cohort included: heart failure and shock (12 patients); kidney and urinary tract infections (12 patients); esophagitis, gastroenteritis, and miscellaneous digestive disorders (11 patients); chronic obstructive pulmonary disease (10 patients); renal failure (10 patients); simple pneumonia and pleurisy (7 patients); disorders of the pancreas except malignancy (6 patients); chest pain (5 patients); and cellulitis (5 patients).
| Preintervention (n = 63) | Postintervention (n = 106) | P Value | |
|---|---|---|---|
| |||
| Mean age, year (SD) | 63.0 (15.7) | 64.7 (14.3) | 0.52 |
| Male, n (%) | 25 (40) | 52 (49) | 0.27 |
| Race, n (%) | 0.33 | ||
| White | 29 (46) | 42 (40) | |
| Black | 21 (33) | 28 (26) | |
| Hispanic | 11 (17) | 30 (28) | |
| Unknown | 2 (3) | 6 (6) | |
| Admission glucose, mg/dL (SD) | 188 (90.9) | 203 (96.1) | 0.33 |
| A1C, % (SD) | 8.5 (2.4) | 8.3 (2.4) | 0.85 |
| Insulin use prior to admission, n (%) | 38 (60) | 54 (51) | 0.48 |
| Case mix index, median (IQR) | 0.89 (0.781.11) | 0.91 (0.841.22) | 0.33 |
| Charlson index, n (%) | 0.03 | ||
| 01 | 8 (13) | 28 (26) | |
| 23 | 29 (46) | 27 (26) | |
| 45 | 15 (24) | 29 (27) | |
| >5 | 11 (17) | 22 (21) | |
| Known history of diabetes, n (%) | 62 (98) | 96 (91) | 0.06 |
With respect to insulin ordering practices, there was no significant difference in the use of basal insulin in hyperglycemic patients between the preintervention period and postintervention period (81% versus 91%; P = 0.17), nor in the dose of basal insulin prescribed (results not shown), but there was an increase in the use of scheduled nutritional insulin for those patients with hyperglycemia receiving nutrition: 40% versus 75%, P < 0.001 (Table 3). The percent of patients receiving supplemental (sliding scale) insulin by itself (ie, without ever receiving basal or nutritional insulin) was lower during the postintervention period (29% versus 8%, P < 0.001). Nonsignificant differences were seen in the rates of prescribing an appropriate dose and type of nutritional insulin. Notably, there was no difference at all in the proportion of patient‐days in which insulin adjustments were made when 2 or more episodes of hyperglycemia or hypoglycemia were present during the previous day (56% of patient‐days in both groups; P = 0.90).
| Preintervention (n = 63) | Postintervention (n = 106) | Unadjusted Effect Size (95% CI) | Adjusted Effect Size (95% CI) | |
|---|---|---|---|---|
| ||||
| Mean percent glucose readings 60180 mg/dL per patient (SD) | 59.1 (0.28) | 64.7 (0.27) | +5.6 (3.0 to +14.3) | +9.7 (+0.6 to +18.8)*, |
| Patient‐day weighted mean glucose, mg/dL (SD) | 174.7 (60.0) | 164.6 (54.2) | 10.1 (1.6 to 18.5) | 15.6 (6.4 to 24.9), |
| Percent patient‐days with any glucose <60 mg/dL | 16/293 (5.5%) | 26/424 (6.1%) | 1.1 (0.6 to 2.1) | 1.1 (0.6 to 2.1) |
| Percent patient‐days with any glucose <40 mg/dL | 3/293 (1.0%) | 5/424 (1.2%) | 1.3 (0.3 to 5.9) | 1.1 (0.3 to 5.1) |
| Hospital length of stay, hours, mean (SD) | 112.2 (63.3) | 86.0 (89.6) | 30% (5% to 51%) | 25% (6% to 44%),∥ |
| Basal insulin if inpatient hyperglycemia (2 or more readings >180 mg/dL) | 39/48 (81%) | 67/74 (91%) | 2.2 (0.8 to 6.4) | |
| Nutritional insulin if inpatient hyperglycemia and PO intake | 19/48 (40%) | 53/71 (75%) | 4.5 (2.0 to 9.9), | |
| Adequate initial dose of nutritional insulin (home dose or 0.200.42 units/kg/day)# | 2/9 (22%) | 22/49 (45%) | 2.9 (0.5 to 15.1) | |
| Supplemental insulin alone (without basal or nutritional insulin) | 16/56 (29%) | 7/92 (8%) | 0.2 (0.08 to 0.5), | |
| Insulin changed if previous day's glucose out of range (2 or more values <60 or >180 mg/dL) | 70/126 (56%) | 76/135 (56%) | 1.0 (0.6 to 1.6) | |
| A1C tested during hospitalization if not available within 30 days prior | 38/63 (60%) | 74/106 (70%) | 1.5 (0.8 to 2.9) | |
The primary outcome, the mean percent of glucose readings between 60 and 180 mg/dL per patient, was 59.1% in the preintervention period and 64.7% in the postintervention (P = 0.13 in unadjusted analysis; Table 3). When adjusted for A1C, admission glucose, and insulin use prior to admission, the adjusted absolute difference in the percent of glucose readings within range was 9.7% (95% confidence interval [CI], 0.6%‐18.8%; P = 0.04; Table 3). Regarding other measures of glucose control, the patient‐day weighted mean glucose was 174.7 mg/dL in the preintervention period and 164.6 mg/dL postintervention (P = 0.02), and there was no significant difference in the percent of patient‐days with any hypoglycemia (glucose <60 mg/dL) or severe hypoglycemia (glucose <40 mg/dL; Table 3). There were also no significant differences in the mean number of hypoglycemic events per patient‐day (6.8 versus 6.6 per 100 patient‐days; relative risk, 0.95; 95% CI, 0.541.67; P = 0.87) or severe hypoglycemic events per patient‐day (1.0 versus 1.4 per 100 patient‐days; relative risk, 1.38; 95% CI, 0.355.53; P = 0.65).
We also compared hospital length of stay in hours between the study groups (Table 3). Length of stay (LOS) was shorter in the postintervention arm in unadjusted analyses (112 versus 86 hours; P < 0.001), and this difference persisted when adjusted for patient insurance, race, gender, and Charlson comorbidity score (25% shorter; 95% CI, 6%‐44%). A comparison of LOS among nonstudy patients on the PACE service during these 2 time periods revealed no difference (105 versus 101 hours). When the length of stay analysis was limited to study patients with a known diagnosis of diabetes, the adjusted effect size was a 31% relative decrease in length of stay.
Figure 1A shows the percent glucose readings within range per patient by hospital day. The greatest differences between groups can be seen on hospital days 2 and 3 (11% absolute differences on both days). Similarly, Figure 1B shows the mean glucose per patient by hospital day. Again, the biggest differences are seen on hospital days 2 and 3 (20 and 23 mg/dL difference between groups, respectively). In both cases, only the day 3 comparisons were significantly different between study groups.
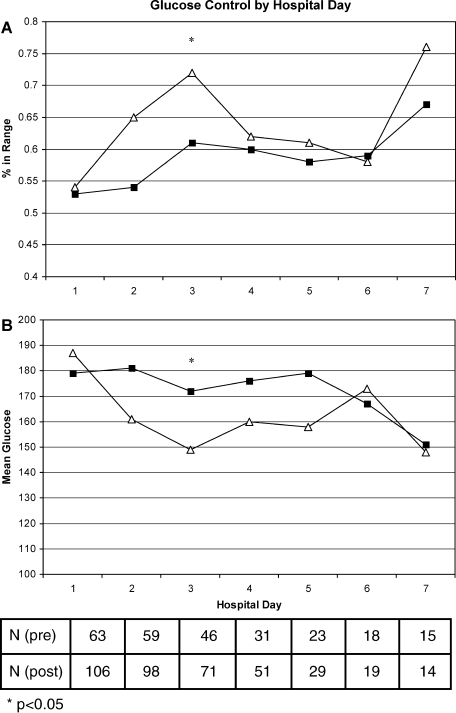
DISCUSSION
In this before‐after study, we found that a multifaceted intervention consisting of a subcutaneous insulin protocol, focused education, and an order set built into the hospital's CPOE system was associated with a significantly higher percentage of glucose readings within range per patient in analyses adjusted for patient demographics and severity of diabetes. We also found a significant decrease in patient‐day weighted mean glucose, a marked increase in appropriate use of scheduled nutritional insulin, and a concomitant decrease in sliding scale insulin only regimens during the postintervention period. Moreover, we found a shorter length of stay during the postintervention period that persisted after adjustment for several clinical factors. Importantly, the interventions described in this study require very few resources to continue indefinitely: printing costs for the management protocol, 4 hours of education delivered per year, and routine upkeep of an electronic order set.
Because this was a before‐after study, we cannot exclude the possibility that these improvements in process and outcome were due to cointerventions and/or temporal trends. However, we know of no other interventions aimed at improving diabetes care in this self‐contained service of nurses, PAs, and hospitalists. Moreover, the process improvements, especially the increase in scheduled nutritional insulin, were rather marked, unlikely to be due to temporal trends alone, and likely capable of producing the corresponding improvements in glucose control. That glucose control stopped improving after hospital day 3 may be due to the fact that subsequent adjustment to insulin orders occurred infrequently and no more often than prior to the intervention. That we did not see greater improvements in glycemic control overall may also reflect the fact that 81% of study patients with inpatient hyperglycemia received basal insulin prior to the intervention.
The reduction in patient LOS was somewhat surprising given the relatively small sample size. However, the results are consistent with those of other studies linking hyperglycemia to LOS18, 19 and we found no evidence for a temporal trend toward lower LOS on the PACE service as a whole during the same time period. While a greater proportion of patients on the PACE service were in the study in the post‐intervention period compared with the preintervention period, we found no evidence that the difference in length of stay was due to increased surveillance for nondiabetics, especially because eligibility criteria depended on phlebotomy glucose values, which were uniformly tested in all inpatients. Also, effects on length of stay were actually stronger when limited to patients with known diabetes. Finally, we controlled for several predictors of length of stay, although we still cannot exclude the possibility of unmeasured confounding between groups.
Since ADA and ACE issued guidelines for inpatient management of diabetes and hyperglycemia, many institutions have developed subcutaneous insulin algorithms, educational curricula, and/or order sets to increase compliance with these guidelines and improve glycemic control. Some of these efforts have been studied and some have been successful in their efforts.13, 14, 2023 Unfortunately, most of these programs have not rigorously assessed their impact on process and outcomes, and the most effective studies published to date have involved interventions much more intensive than those described here. For example, Rush University's intervention was associated with a 50 mg/dL decrease in mean blood glucose but involved an endocrinologist rounding twice daily with house officers for 2 weeks at a time.13 At Northwestern University, a diabetes management service run by nurse practitioners was established, and the focus was on the conversion from intravenous to subcutaneous insulin regimens.14 The RABBIT 2 study that demonstrated the benefits of a basal‐bolus insulin regimen used daily rounding with an endocrinologist.12 More modestly, a program in Pitt County Memorial Hospital in Greenville, NC, relied mostly on diabetes nurse case managers, a strategy which reduced hospital‐wide mean glucose levels as well as LOS, although the greatest improvements in glycemic control were seen in the ICU.19 Our findings are much more consistent with those from University of California San Diego, as yet unpublished, which also used an algorithm, computerized order set, education, as well as continuous quality improvement methods to achieve its aims.22
Our study has several limitations, including being conducted on 1 general medicine service at 1 academic medical center. Moreover, this service, using a physician assistant/hospitalist model, a closed geographic unit, and fairly generous staffing ratio, is likely different from those in many settings and may limit the generalizability of our findings. However, this model allowed us to conduct the study in a laboratory relatively untouched by other cointerventions. Furthermore, the use of PAs in this way may become more common as both academic and community hospitals rely more on mid‐level providers. Our study had a relatively low percentage of patients without a known diagnosis of diabetes compared with other studies, again potentially but not necessarily limiting generalizability. This finding has been shown in other studies at our institution24 and may be due to the high rate of screening for diabetes in the community. Another limitation is that this was a nonrandomized, before‐after trial. However, all subjects were prospectively enrolled to improve comparability, and we performed rigorous adjustment for multiple potential confounding factors. Also, this study had limited statistical power to detect differences in hypoglycemia rates. The preintervention arm was smaller than planned due to fewer diabetic patients than expected on the service and a higher number of exclusions; we prolonged the postintervention period to achieve the desired sample size for that arm of the study.
Our study also has several strengths, including electronic capture of many processes of care and a methodology to operationalize them into measures of protocol adherence. Our metrics of glycemic control were rigorously designed and based on a national task force on inpatient glycemic control sponsored by the Society of Hospital Medicine, with representation from the ADA and AACE.25
Potential future improvements to this intervention include modifications to the daily adjustment algorithm to improve its usability and ability to improve glucose control. Another is the use of high‐reliability methods to improve order set use and daily insulin adjustment, including alerts within the CPOE system and nurse empowerment to contact medical teams if glucose levels are out of range (eg, if greater than 180 mg/dL, not just if greater than 350 or 400 mg/dL). Future research directions include multicenter, randomized controlled trials of these types of interventions and an analysis of more distal patient outcomes including total healthcare utilization, infection rates, end‐organ damage, and mortality.
In conclusion, we found a relationship between a relatively low‐cost quality improvement intervention and improved glycemic control in the non‐ICU general medical setting. Such a finding suggests the benefits of the algorithm itself to improve glucose control and of our implementation strategy. Other institutions may find this intervention a useful starting point for their own quality improvement efforts. Both the algorithm and implementation strategy are deserving of further improvements and future study.
Acknowledgements
We thank Paul Szumita, Karen Fiumara, Jennifer Trujillo, and the other members of the BWH Diabetes Pharmacy and Therapeutics Subcommittee for their help designing and implementing the intervention; Aubre McClendon, Nicole Auclair, Emily Dattwyler, Mariya Fiman, and Alison Pietras for valuable research assistance; Deborah Williams for data analysis; Amy Bloom for project support; and Stuart Lipsitz for biostatistical expertise.
APPENDIX
INPATIENT DIABETES MANAGEMENT PROTOCOL
Management of Diabetes and Hyperglycemia in Hospitalized Non‐ICU Patients
Rationale
Increasing data show a strong association between hyperglycemia and adverse inpatient outcomes. The American Diabetes Association and the American College of Clinical Endocrinology recommend all glucose levels be below 180 mg/dL in non‐ICU patients. Because hospitalizations are unstable situations, even patients who are well controlled on oral agents as outpatients are usually best managed with insulin.
Insulin may be safely administered even to patients without previously diagnosed diabetes. As long as the prescribed doses are below what is normally produced by the pancreas, the patient will not become hypoglycemic. If the glucose level drops, endogenous insulin secretion will reduce to compensate.
Total insulin requirements in insulin‐sensitive patients (eg, type 1 diabetes mellitus) is 0.50.7/units/kg/day. Insulin requirements in insulin‐resistant type 2 diabetic patients may vary greatly, and can exceed 12 units/kg/day. A conservative estimate for initial insulin therapy in any patient with diabetes is to start with the type 1 diabetes mellitus dose, 0.50.7 units/kg/day.
Overview
Effective inpatient insulin regimens typically include 3 components:
Basal insulin (eg, scheduled NPH or insulin glargine [Lantus]), which is used to manage fasting and premeal hyperglycemia.
Nutritional or prandial insulin (eg, scheduled regular insulin, insulin lispro [Humalog] or insulin aspart [Novolog]) which controls hyperglycemia from nutritional (eg, discrete meals, TPN, IV dextrose) sources.
Supplemental or correctional insulin (eg, regular insulin, insulin lispro, or insulin aspart), which is used in addition to scheduled insulin to meet unexpected basal hyperglycemia that is not covered by the scheduled insulin.
Sample Orders (Not for Patients with Uncontrolled Type 1 Diabetes, DKA, Hyperglycemic Hyperosmolar State, or Other Absolute Need for IV Insulin)
Check (fingerstick) capillary blood glucose qAC, qHS.
NPH insulin subcutaneously (SC) ___ units qAM, ___ units qHS.
Insulin aspart SC ___ units pre‐breakfast, ___ units pre‐lunch, ___ units pre‐dinner, hold if NPO or premeal BS <60 mg/dL; give 015 minutes before meals.
Insulin aspart SC sliding scale (see Table 6) qAC, in addition to standing nutritional insulin, 015 minutes before meals.
For BS <60 mg/dL:
If patient can take PO
Give 15 g of fast acting carbohydrate (4 oz fruit juice/nondiet soda, 8 oz nonfat milk, or 34 glucose tablets).
Repeat finger capillary glucose every 15 (q15) minutes and repeat above (5.a.i.) if BG <60 mg/dL.
When BG >60 mg/dL, give snack or meal in a half‐hour.
If patient cannot take PO
Give 25 mL of 50% dextrose (D50) as an IV push.;
Repeat finger capillary glucose q15 minutes and repeat above (5.b.i.) if BG <80 mg/dL.
Guidelines
Stop oral diabetes agents in most patients (see Table 7 for list of contraindications and precautions).
Check bedside blood glucose (BBG or fingerstick) qAC and qHS (or at 0600 hours, 1200 hours, 1800 hours, and 2400 hours if no discrete meals).
Estimate total daily insulin requirement:
For most patients, conservative estimate is 0.50.7 units/kg/day, but may be much higher.
Reasons for lower end of the range: renal insufficiency, small size, insulin sensitive (eg, type 1), recent hypoglycemia, decreasing doses of steroids, older age.
Reasons for higher end of the range: obese, initiation or increasing doses of steroids, marked hyperglycemia.
Start basal insulin if any premeal BG >140 mg/dL and no recent glucose <60 mg/dL off insulin (Table 5).
Start nutritional or prandial insulinhold if nutrition is stopped/held or premeal BS <60 (Table 5).
Start supplemental/correctional insulin in addition to nutritional (prandial) insulin (Table 6):
Discrete meals: Insulin aspart qAC (with nutritional insulin). 0
No discrete meals: Regular insulin q6h.
On a daily basis, adjust scheduled insulin based on previous days' blood sugars:
Add up total insulin given the previous day, including scheduled and supplemental insulin, to determine new total daily insulin requirement.
Adjust total daily insulin requirement based on clinical considerations (eg, give more if marked hyperglycemia, eating more, improving renal function, increasing steroids; give less if eating less, worsening renal function, tapering steroids, recovering from severe illness).
Give 50% of requirement as basal and 50% as nutritional, as above (may need proportionately less nutritional insulin if appetite poor or unknown).
Adjust sliding scale if needed based on total scheduled insulin dose (see step 6, above).
For BG <60 mg/dL:
If patient can take PO, give 15 g of fast acting carbohydrate.
(4 oz fruit juice/nondiet soda, 8 oz nonfat milk, or 34 glucose tablets; not juice plus sugar).
Repeat finger capillary glucose q15 minutes and repeat above if BG <60.
When BG >60, give snack or meal in half an hour.
If patient cannot take PO, give 25 mL of D50 as IV push.
Check finger capillary glucose q15 minutes and repeat above if BG <80.
Discharge orders:
Patient should be discharged home on a medication regimen that was similar to the admission regimen (ie, the regimen prescribed by their PCP). Exceptions include
The patient has a contraindication to an admission medication.
There is evidence of severe hyperglycemia (eg, very high A1C) or hypoglycemia on admission regimen.
If a patient is admitted with no insulin, and requires insulin to be continued as an outpatient (eg, newly‐diagnosed type 1 diabetes, A1C very high, and contraindication to or on maximum oral regimen), limit discharge insulin regimen to no more than 1 injection per day (eg, hs NPH; an exception to this is for type 1 diabetic patients, who are optimally treated with 34 injections/day). Make sure the patient has prompt follow‐up with their primary care provider (PCP).
Avoid discharging home on sliding scale.
If a patient is going to require insulin injections and self‐monitoring blood glucose as an outpatient, make sure they are instructed about how to perform these.
Indications for calling an endocrine consult:
Labile blood sugars.
Prolonged periods of NPO, eg, for procedures, especially in patients with type 1 diabetes
Marked hyperglycemia despite following this guideline.
Question of type 1 versus type 2 versus other type of diabetes. 0
Basil Insulin Guidelines Home Insulin Regimen Starting Dose of Basal Insulin Considerations NOTE: Patients with T1DM require basal insulin at all times! Basal never should be held!
Abbreviations: NPO, nothing by mouth.
On basal (eg, NPH or glargine) insulin at home Patient's home dose of NPH or glargine If NPO, consider starting half of NPH or glargine home dose, unless hyperglycemic at home. Not on basal (eg, NPH or glargine) insulin at home NPH 50% of total daily insulin requirement, given qHS or split qAM/qHS (maximum starting dose 20 units/day) Same dose if patient has previously diagnosed or undiagnosed diabetes Nutritional Insulin Guidelines Type of Nutrition Common Nutritional Regimens Sample Starting Doses Abbreviation: qAM, every morning; qHS, at bed time.
If receiving cycled tube feeds at night, give nutritional NPH qHS only.
Discrete meals Aspart given 015 minutes before meals Home dose, if known or 50% of total insulin requirement, split over 3 meals, may need less if poor or unknown appetite Continuous tube feeding,* IV dextrose NPH qHS or qAM/qHS 50% of total insulin requirement (in addition to basal dose), may need less if not at goal caloric intake Glargine given every day (qd), anytime Regular every 6 hours (q6h) Sample Supplemental/Correctional Insulin Scales Blood Glucose Scheduled Insulin < 40 Units/Day Scheduled Insulin of 4080 Units/Day Scheduled Insulin > 80 Units/Day Individualized NOTE: Avoid supplemental insulin qHS unless patient is very hyperglycemic and obese.
150199 1 unit 1 unit 2 units ____ units 200249 2 units 3 units 4 units ____ units 250299 3 units 5 units 7 units ____ units 300349 4 units 7 units 10 units ____ units >349 5 units + call HO 8 units + call HO 12 units + call HO ___ units + call HO Notes on Oral Agents Agents Considerations Metabolism Sulfonylureas/secretagogues: glyburide, glipizide, glimeperide (Amaryl); repaglinide (Prandin); nateglinide (Starlix) Risk for hypoglycemia Metabolized in liver; Glyburide metabolized to active metabolites; 50% renally eliminated Metformin Contraindicated in heart failure and renal dysfunction (creatinine [Cr] >1.5 mg/dL in men and 1.4 mg/dL in women) Eliminated renally Should be held at time of iodinated contrast studies. (May be restarted after normal postcontrast renal function is confirmed) Adverse effects include diarrhea, nausea, and anorexia Thiazolidinediones: pioglitazone (Actos), rosiglitazone (Avandia) Contraindicated in class III and IV heart failure Metabolized in liver Use with caution in patients with edema Adverse effects include increased intravascular volume Slow onset of action Avoid in hepatic dysfunction Glucosidease inhibitors: acarbose (Precose), miglitol (Glycet) Gastrointestinal intolerance Acarbose eliminated in gut and renally
- ,,,,.Prevalence of hyper‐ and hypoglycemia among inpatients with diabetes: a national survey of 44 U.S. hospitals.Diabetes Care.2007;30:367–369.
- ,,,,,.Hyperglycemia: an independent marker of in‐hospital mortality in patients with undiagnosed diabetes.J Clin Endocrinol Metab.2002;87:978–982.
- ,,, et al.Hyperglycaemia is associated with poor outcomes in patients admitted to hospital with acute exacerbations of chronic obstructive pulmonary disease.Thorax.2006;61:284–289.
- ,,,,.Stress hyperglycemia and prognosis of stroke in nondiabetic and diabetic patients: a systematic overview.Stroke.2001;32:2426–2432.
- ,,,.Hyperglycemia is associated with adverse outcomes in patients receiving total parenteral nutrition.Diabetes Care.2005;28:2367–2371.
- ,,, et al.Management of diabetes and hyperglycemia in hospitals.Diabetes Care.2004;27:553–597.
- ,,,,,.The relation between hyperglycemia and outcomes in 2471 patients admitted to the hospital with community‐acquired pneumonia.Diabetes Care.2005;28:810–815.
- ,,, et al.Intensive insulin therapy in the medical ICU.N Engl J Med.2006;354:449–461.
- ,,, et al.Intensive insulin therapy in the critically ill patients.N Engl J Med.2001;345:1359–1367.
- Standards of medical care in diabetes, 2007.Diabetes Care.2007;30(Suppl 1):S4–S41.
- American College of Endocrinology and American Diabetes Association Consensus statement on inpatient diabetes and glycemic control: a call to action.Diabetes Care.2006;29:1955–1962.
- ,,, et al.Randomized study of basal‐bolus insulin therapy in the inpatient management of patients with type 2 diabetes (RABBIT 2 trial).Diabetes Care.2007;30:2181–2186.
- ,,,.Eliminating inpatient sliding‐scale insulin: a reeducation project with medical house staff.Diabetes Care.2005;28:1008–1011.
- ,,, et al.Inpatient management of hyperglycemia: the Northwestern experience.Endocr Pract.2006;12:491–505.
- ,,, et al.Improving glycemic control in medical inpatients: a pilot study.J Hosp Med.2008;3:55–63.
- ,,.Adapting a clinical comorbidity index for use with ICD‐9‐CM administrative databases.J Clin Epidemiol.1992;45:613–619.
- ,,, et al.“Glucometrics”—assessing the quality of inpatient glucose management.Diabetes Technol Ther.2006;8:560–569.
- ,,,.Hyperglycemia and length of stay in patients hospitalized for bone marrow transplantation.Diabetes Care.2007;30:993–994.
- ,.Financial implications of glycemic control: results of an inpatient diabetes management program.Endocr Pract.2006;12(Suppl 3):43–48.
- ,,, et al.In‐hospital treatment of hyperglycemia: effects of intensified subcutaneous insulin treatment.Curr Med Res Opin.2007;23:757–765.
- ,,,.Impact of endocrine and diabetes team consultation on hospital length of stay for patients with diabetes.Am J Med.1995;99:22–28.
- ,,,.Effect of a standardized insulin order set and an insulin management algorithm on inpatient glycemic control and hypoglycemia. Society of Hospital Medicine Annual Meeting, 2007; Dallas, TX;2007.
- ,,, et al.Trends in bed occupancy for inpatients with diabetes before and after the introduction of a diabetes inpatient specialist nurse service.Diabet Med.2006;23:1008–1015.
- ,,,,.Inpatient management of diabetes and hyperglycemia among general medicine patients at a large teaching hospital.J Hosp Med.2006;1:145–150.
- ,,, et al. Improving glycemic control, preventing hypoglycemia, and optimizing care of the inpatient with hyperglycemia and diabetes, 2006. Available at:http://www.hospitalmedicine.org/ResourceRoomRedesign/html/GC_Imp_Guide.cfm. Accessed October 2008.
Diabetes mellitus and/or inpatient hyperglycemia are common comorbid conditions in hospitalized patients. Recent surveys show that over 90% of hospitalized diabetic patients experience hyperglycemia (>200 mg/dL), and in nearly 1 in 5 of these patients hyperglycemia persists for 3 days or more.1 Hyperglycemia among inpatients without a previous history of diabetes mellitus is also very common.2 Observational studies have shown that hyperglycemia in hospitalized patients is associated with adverse outcomes including infectious complications, increased length of stay, and increased mortality.27 Recent randomized controlled trials have demonstrated that aggressive treatment of inpatient hyperglycemia improves outcomes in surgical and medical intensive care units.8, 9
Based on the available data, the American Diabetes Association (ADA) now advocates good metabolic control, defined as preprandial glucose levels of 90 to 130 mg/dL and peak postprandial glucose levels <180 mg/dL in hospitalized nonintensive care unit (ICU) patients.10 To reach these targets, the ADA and American College of Endocrinology (ACE) suggest that multidisciplinary teams develop and implement hyperglycemia management guidelines and protocols.11 Protocols should promote the use of continuous intravenous insulin infusions or scheduled basal‐bolus subcutaneous insulin regimens. Subcutaneous insulin protocols should include target glucose levels, basal, nutritional, and supplemental insulin, and daily dose adjustments.6 A recent randomized controlled trial of non‐ICU inpatients demonstrated that such a basal‐bolus insulin regimen results in improved glucose control compared with a sliding scale only regimen.12
To date, few published studies have investigated the best ways to implement such management protocols; those that have are often resource‐intensive, for example involving daily involvement of nurse practitioners or diabetologists.13, 14 It is therefore not known how best to implement an inpatient diabetes management program that is effective, efficient, and self‐perpetuating. At Brigham and Women's Hospital (BWH), we have been refining a subcutaneous insulin protocol, focused provider education, and more recently a computerized order set to overcome barriers related to fear of hypoglycemia, delays in insulin prescribing, and unfamiliarity with inpatient glucose management.15 The aims of this current trial were to evaluate the effects of these interventions on a geographically localized general medical service previously naive to these interventions to evaluate their effects on glycemic control, patient safety, and processes of care. We hypothesized that these interventions would improve glycemic control and increase use of basal‐bolus insulin orders without increasing the rate of hypoglycemia.
METHODS
Setting and Participants
This prospective, before‐after trial was conducted at BWH from July 15, 2005 through June 22, 2006. Eligible subjects were patients scheduled for admission to the BWH Physician Assistant/Clinician Educator (PACE) Service with either a known diagnosis of type 2 diabetes mellitus or inpatient hyperglycemia (at least 1 random laboratory glucose >180 mg/dL). The PACE service is a geographically‐localized general medicine service of up to 15 beds where patients are cared for by a single cadre of nurses, 2 physician's assistants (PAs), and 1 hospitalist attending. A moonlighter covers the service at night. The PACE service does not accept patients transferred from other acute care hospitals or from ICUs, but does not otherwise have triage guidelines related to diagnosis, complexity, or acuity. Patients were excluded if they had type 1 diabetes, presented with hyperosmolar hyperglycemic state (HHS) or diabetic ketoacidosis (DKA), received total parenteral nutrition (TPN), or were receiving palliative care. This study was approved by the BWH Institutional Review Board; patient consent was deemed not to be necessary for this study given the relatively nonsensitive nature of the data, noninvasive means of data collection, and the steps taken by research personnel to minimize any breach in patient confidentiality.
Intervention
The study intervention consisted of three components, initiated in January 2006:
Glycemic management protocol: a multidisciplinary team of a diabetologist (M.L.P.), a hospitalist (J.L.S.), and a pharmacist (Jennifer Trujillo) developed a subcutaneous insulin protocol based on ADA guidelines (Table 1; see the appendix for complete protocol). The protocol was approved by the BWH Pharmacy and Therapeutics Diabetes Subcommittee and refined through 6 months of pilot testing on other general medical services.15 The protocol consisted of a set of specific treatment recommendations, including: (1) bedside glucose monitoring; (2) stopping oral diabetes agents in most patients; (3) estimating total daily insulin requirements; (4) prescribing basal, nutritional, and supplemental insulin based on the patient's total insulin requirements, preadmission medication regimen, and nutritional status; (5) adjusting insulin on a daily basis as needed; (6) managing hypoglycemia; (7) suggestions for discharge orders; and (8) indications for an endocrinology consultation. The protocol was printed as a pocket guide, distributed to all members of the PACE service, and used to guide all other interventions.
Diabetes education: all PAs received 2 one‐hour educational sessions: a lecture by a diabetologist (M.L.P.) reviewing the rationale for tight glycemic control and general principles of management, and a workshop by a hospitalist (J.L.S.) in which specific cases were reviewed to illustrate how the protocol could be used in practice (eg, when oral agents could be safely continued, how to prescribe insulin on admission, and how to make subsequent adjustments in dose). All hospitalist attendings received a 1‐hour lecture summarizing the above material. All nurses on the service received a lecture that focused on issues unique to nursing care, such as insulin administration, glucose testing, managing patients with unpredictable oral (PO) intake, and patient education. (All materials are available from the authors upon request).
Order Set: an order set, built into BWH's proprietary computer provider order entry (CPOE) system, was created to parallel the glycemic management protocol and facilitate insulin orders for patients eating discrete meals, receiving continuous liquid enteral nutrition (tube feeds), or receiving nothing by mouth (NPO). Other components of the order set facilitated glucose monitoring and other laboratory tests and ordering consultation when appropriate.
| Oral Agents | Stop Oral Agents in Most Patients |
|---|---|
| |
| Glucose testing | Check bedside blood glucose before meals and at bedtime if eating, or every 6 hours if NPO |
| Insulin | |
| 1. Estimate total daily insulin dose | 0.5 to 0.7 units/kg/day, depending on patient's age, size, renal function, insulin sensitivity, history of hypoglycemia, and steroid use |
| 2. Start basal insulin | Patient's home dose or 50% of calculated total daily dose; NPH qAM/qHS or insulin glargine qHS; If NPO, use one‐half the home dose unless hyperglycemic |
| 3. Start nutritional insulin if not NPO | Patient's home dose or 50% of calculated total daily dose, less if poor or unknown intake; discrete meals: insulin aspart split over 3 meals, 0 to 15 minutes prior to eating; continuous tube feeds or IV dextrose: regular insulin every 6 hours |
| 4. Start correctional insulin | 1 of 3 scales provided based on total daily dose of insulin; same type as nutritional insulin; regular insulin if NPO |
| 5. Daily adjustment | Calculate total administered dose from prior day, adjust for degree of hyperglycemia or hypoglycemia, renal function, PO intake, steroid use, and degree of illness, and redistribute as 50% basal, 50% nutritional, or 100% basal if NPO |
| Hypoglycemia orders | Juice, IV dextrose, or IM glucagon depending on ability to take oral nutrition and IV access |
| Discharge orders | Based on A1C: either home regimen, titration of home regimen, or new insulin regimen (if latter, simple regimen with aggressive patient education and prompt follow‐up) |
| Indications for endocrine consultation | Labile blood sugars, poor control, prolonged NPO period, question of type 1 or type 2 diabetes |
Study Protocol and Data Collection
A research assistant prospectively identified eligible patients each weekday by screening all patients scheduled for admission to the PACE service using the daily computerized sign‐out system used on all general medical teams. Specifically, laboratory random glucose levels, inpatient medications, and medical histories were reviewed to determine if each patient met eligibility criteria. Eligibility criteria were then confirmed by medical record review and adjudicated by one study author (J.L.S.) if necessary. Further medical record review was performed to identify specific patient populations (eg, diet‐controlled, steroid‐induced, or previously undiagnosed diabetes), determine preadmission diabetes medications, and determine the patient's weight. Hospital computerized clinical and administrative records were abstracted to obtain patient demographics (age, sex, race, insurance status), laboratory data (glucose level on admission, A1C level [taken during or within 6 months prior to admission]), clinical data (length of stay, billing‐based Charlson comorbidity score,16 and diagnosis‐related group [DRG] case mix index), all inpatient insulin and oral diabetes medication orders, frequency of bedside glucose testing, and diet orders. Electronic medication administration record (eMAR) data were used to determine all doses and times of insulin administration.
Outcomes
The primary outcome was the mean percent of glucose readings between 60 and 180 mg/dL per patient (ie, calculated for each patient and averaged across all eligible patients in each study arm). Only bedside glucose readings were used given the lack of additional useful information typically provided by laboratory (venous plasma) glucose readings.17 Readings drawn within 1 hour of a previous reading were excluded to avoid ascertainment bias caused by follow‐up testing of abnormal glucose values. Only readings while on the study service were used. Readings on hospital day 1 were excluded because our intervention was expected to have little impact on the first day's glucose control; for patients with undiagnosed diabetes, data collection began the day following the first elevated glucose reading. Readings beyond hospital day 14 were also excluded to avoid biased data from patients with exceptionally long lengths of stay.
Secondary outcomes included the following:
Glycemic control:
Patient‐day weighted mean glucose (ie, mean glucose for each patient‐day, averaged across all patient days);
Mean glucose per patient for each hospital day (days 17).
Patient safety:
Proportion of patient‐days with any glucose reading <60 mg/dL (hypoglycemia) and <40 mg/dL (severe hypoglycemia).
Processes of care:
Use of any NPH insulin or insulin glargine (basal) insulin during the hospitalization if 2 or more glucose readings were >180 mg/dL.
Adequacy of basal dose on day first prescribed: for patients prescribed a diet, within 20% of preadmission basal dose or 0.20 to 0.42 units/kg if not known or not taken prior to admission. If not eating, half the above calculations.
Use of any scheduled nutritional insulin during the hospitalization if ever prescribed a diet and 2 or more glucose readings were greater than 180 mg/dL.
Adequacy of nutritional dose on day first prescribed: for patients prescribed a diet, within 20% of preadmission nutritional dose or 0.20 to 0.42 units/kg/day if not known or not taken prior to admission. Patients on clear liquid diets, enteral feeds, or receiving glucocorticoids were excluded from this analysis.
Correct type of nutritional insulin: if eating discrete meals, insulin aspart (the rapid‐acting insulin on formulary at BWH); if prescribed tube feeds, regular insulin.
Use of supplemental insulin by itself (without scheduled basal or nutritional insulin), a marker of poor care.
A1C testing within 1 month prior to or during hospitalization.
Clinical inertia: if at least two glucose readings <60 mg/dL or >180 mg/dL on a patient‐day, lack of any change to any insulin order the following day if still on the study service.
Healthcare utilization:
Hospital length of stay in hours, calculated from the exact time of admission until the exact time of discharge, using hospital administrative data.
Analyses
Study results were compared prior to the intervention (July 15 through December 12, 2005) with those during the intervention (January 18 through June 20, 2006). Patient data and clinical outcomes were analyzed descriptively using proportions, means with standard deviations (SDs), or medians with interquartile ranges (IQRs) as appropriate. Comparisons between groups were calculated using Fisher's exact test for dichotomous and categorical variables, and Student t test or Wilcoxon rank sum test for continuous variables as appropriate. The primary outcome was first analyzed using linear regression with study group as the independent variable and percent of glucose readings within range per patient as the dependent variable. We then adjusted for potential confounders by putting each covariate into the model, one at a time. All significant predictors of the outcome at a P value <0.10 were retained in the final model. We used general estimating equations to adjust for clustering of results by each PA. Similar analyses were performed for hospital length of stay per patient using a negative binomial model, so chosen because it fit the data distribution much better than the typically used Poisson model. With a planned sample size of 115 patients and 1250 glucose readings per arm, an intraclass correlation coefficient of 0.10, and an alpha of 0.05, the study had 90% power to detect an increase in percent of glucose readings in range from 67% to 75%. All analyses were based on the intention‐to‐treat principle. Except as above, 2‐sided P values <0.05 were considered significant. SAS version 9.1 (SAS Institute, Cary, NC) was used for all analyses.
RESULTS
We prospectively identified 248 potential patients for the study. We subsequently excluded 79 patients for the following reasons: no glucose readings beyond hospital day 1 while on PACE service (34 patients); never admitted to PACE service (15 patients); no diabetes or inpatient hyperglycemia (9 patients, mostly patients prescribed an insulin sliding scale prophylactically to avoid steroid‐induced hyperglycemia); type 1 diabetes (13 patients); TPN, DKA, or HHS (5 patients); and palliative care (3 patients). The remaining 169 patients included 63 from the preintervention period(out of 489 total admissions to the PACE service; 13%) and 106 patients in the postintervention period (out of 565 admissions; 19%). These patients had 2447 glucose readings, or an average of 3.6 glucose readings per monitored patient‐day in the preintervention period and 3.3 glucose readings per patient‐day in the postintervention period. Even including the 34 patients who were excluded for lack of glucose readings, glucose data were still available for 717 out of a potential 775 patient‐days (93%). Characteristics for all included patients are shown in Table 2. The mean admission glucose was 197 mg/dL, mean A1C was 8.4%, 54% of the patients were prescribed insulin prior to admission, and 7% had no prior diagnosis of diabetes. There were no significant differences in baseline characteristics between the 2 patient groups except for Charlson score, which was higher in the preintervention group (87% versus 74% with score 2 or higher; Table 2). The top diagnosis‐related groups for the entire cohort included: heart failure and shock (12 patients); kidney and urinary tract infections (12 patients); esophagitis, gastroenteritis, and miscellaneous digestive disorders (11 patients); chronic obstructive pulmonary disease (10 patients); renal failure (10 patients); simple pneumonia and pleurisy (7 patients); disorders of the pancreas except malignancy (6 patients); chest pain (5 patients); and cellulitis (5 patients).
| Preintervention (n = 63) | Postintervention (n = 106) | P Value | |
|---|---|---|---|
| |||
| Mean age, year (SD) | 63.0 (15.7) | 64.7 (14.3) | 0.52 |
| Male, n (%) | 25 (40) | 52 (49) | 0.27 |
| Race, n (%) | 0.33 | ||
| White | 29 (46) | 42 (40) | |
| Black | 21 (33) | 28 (26) | |
| Hispanic | 11 (17) | 30 (28) | |
| Unknown | 2 (3) | 6 (6) | |
| Admission glucose, mg/dL (SD) | 188 (90.9) | 203 (96.1) | 0.33 |
| A1C, % (SD) | 8.5 (2.4) | 8.3 (2.4) | 0.85 |
| Insulin use prior to admission, n (%) | 38 (60) | 54 (51) | 0.48 |
| Case mix index, median (IQR) | 0.89 (0.781.11) | 0.91 (0.841.22) | 0.33 |
| Charlson index, n (%) | 0.03 | ||
| 01 | 8 (13) | 28 (26) | |
| 23 | 29 (46) | 27 (26) | |
| 45 | 15 (24) | 29 (27) | |
| >5 | 11 (17) | 22 (21) | |
| Known history of diabetes, n (%) | 62 (98) | 96 (91) | 0.06 |
With respect to insulin ordering practices, there was no significant difference in the use of basal insulin in hyperglycemic patients between the preintervention period and postintervention period (81% versus 91%; P = 0.17), nor in the dose of basal insulin prescribed (results not shown), but there was an increase in the use of scheduled nutritional insulin for those patients with hyperglycemia receiving nutrition: 40% versus 75%, P < 0.001 (Table 3). The percent of patients receiving supplemental (sliding scale) insulin by itself (ie, without ever receiving basal or nutritional insulin) was lower during the postintervention period (29% versus 8%, P < 0.001). Nonsignificant differences were seen in the rates of prescribing an appropriate dose and type of nutritional insulin. Notably, there was no difference at all in the proportion of patient‐days in which insulin adjustments were made when 2 or more episodes of hyperglycemia or hypoglycemia were present during the previous day (56% of patient‐days in both groups; P = 0.90).
| Preintervention (n = 63) | Postintervention (n = 106) | Unadjusted Effect Size (95% CI) | Adjusted Effect Size (95% CI) | |
|---|---|---|---|---|
| ||||
| Mean percent glucose readings 60180 mg/dL per patient (SD) | 59.1 (0.28) | 64.7 (0.27) | +5.6 (3.0 to +14.3) | +9.7 (+0.6 to +18.8)*, |
| Patient‐day weighted mean glucose, mg/dL (SD) | 174.7 (60.0) | 164.6 (54.2) | 10.1 (1.6 to 18.5) | 15.6 (6.4 to 24.9), |
| Percent patient‐days with any glucose <60 mg/dL | 16/293 (5.5%) | 26/424 (6.1%) | 1.1 (0.6 to 2.1) | 1.1 (0.6 to 2.1) |
| Percent patient‐days with any glucose <40 mg/dL | 3/293 (1.0%) | 5/424 (1.2%) | 1.3 (0.3 to 5.9) | 1.1 (0.3 to 5.1) |
| Hospital length of stay, hours, mean (SD) | 112.2 (63.3) | 86.0 (89.6) | 30% (5% to 51%) | 25% (6% to 44%),∥ |
| Basal insulin if inpatient hyperglycemia (2 or more readings >180 mg/dL) | 39/48 (81%) | 67/74 (91%) | 2.2 (0.8 to 6.4) | |
| Nutritional insulin if inpatient hyperglycemia and PO intake | 19/48 (40%) | 53/71 (75%) | 4.5 (2.0 to 9.9), | |
| Adequate initial dose of nutritional insulin (home dose or 0.200.42 units/kg/day)# | 2/9 (22%) | 22/49 (45%) | 2.9 (0.5 to 15.1) | |
| Supplemental insulin alone (without basal or nutritional insulin) | 16/56 (29%) | 7/92 (8%) | 0.2 (0.08 to 0.5), | |
| Insulin changed if previous day's glucose out of range (2 or more values <60 or >180 mg/dL) | 70/126 (56%) | 76/135 (56%) | 1.0 (0.6 to 1.6) | |
| A1C tested during hospitalization if not available within 30 days prior | 38/63 (60%) | 74/106 (70%) | 1.5 (0.8 to 2.9) | |
The primary outcome, the mean percent of glucose readings between 60 and 180 mg/dL per patient, was 59.1% in the preintervention period and 64.7% in the postintervention (P = 0.13 in unadjusted analysis; Table 3). When adjusted for A1C, admission glucose, and insulin use prior to admission, the adjusted absolute difference in the percent of glucose readings within range was 9.7% (95% confidence interval [CI], 0.6%‐18.8%; P = 0.04; Table 3). Regarding other measures of glucose control, the patient‐day weighted mean glucose was 174.7 mg/dL in the preintervention period and 164.6 mg/dL postintervention (P = 0.02), and there was no significant difference in the percent of patient‐days with any hypoglycemia (glucose <60 mg/dL) or severe hypoglycemia (glucose <40 mg/dL; Table 3). There were also no significant differences in the mean number of hypoglycemic events per patient‐day (6.8 versus 6.6 per 100 patient‐days; relative risk, 0.95; 95% CI, 0.541.67; P = 0.87) or severe hypoglycemic events per patient‐day (1.0 versus 1.4 per 100 patient‐days; relative risk, 1.38; 95% CI, 0.355.53; P = 0.65).
We also compared hospital length of stay in hours between the study groups (Table 3). Length of stay (LOS) was shorter in the postintervention arm in unadjusted analyses (112 versus 86 hours; P < 0.001), and this difference persisted when adjusted for patient insurance, race, gender, and Charlson comorbidity score (25% shorter; 95% CI, 6%‐44%). A comparison of LOS among nonstudy patients on the PACE service during these 2 time periods revealed no difference (105 versus 101 hours). When the length of stay analysis was limited to study patients with a known diagnosis of diabetes, the adjusted effect size was a 31% relative decrease in length of stay.
Figure 1A shows the percent glucose readings within range per patient by hospital day. The greatest differences between groups can be seen on hospital days 2 and 3 (11% absolute differences on both days). Similarly, Figure 1B shows the mean glucose per patient by hospital day. Again, the biggest differences are seen on hospital days 2 and 3 (20 and 23 mg/dL difference between groups, respectively). In both cases, only the day 3 comparisons were significantly different between study groups.

DISCUSSION
In this before‐after study, we found that a multifaceted intervention consisting of a subcutaneous insulin protocol, focused education, and an order set built into the hospital's CPOE system was associated with a significantly higher percentage of glucose readings within range per patient in analyses adjusted for patient demographics and severity of diabetes. We also found a significant decrease in patient‐day weighted mean glucose, a marked increase in appropriate use of scheduled nutritional insulin, and a concomitant decrease in sliding scale insulin only regimens during the postintervention period. Moreover, we found a shorter length of stay during the postintervention period that persisted after adjustment for several clinical factors. Importantly, the interventions described in this study require very few resources to continue indefinitely: printing costs for the management protocol, 4 hours of education delivered per year, and routine upkeep of an electronic order set.
Because this was a before‐after study, we cannot exclude the possibility that these improvements in process and outcome were due to cointerventions and/or temporal trends. However, we know of no other interventions aimed at improving diabetes care in this self‐contained service of nurses, PAs, and hospitalists. Moreover, the process improvements, especially the increase in scheduled nutritional insulin, were rather marked, unlikely to be due to temporal trends alone, and likely capable of producing the corresponding improvements in glucose control. That glucose control stopped improving after hospital day 3 may be due to the fact that subsequent adjustment to insulin orders occurred infrequently and no more often than prior to the intervention. That we did not see greater improvements in glycemic control overall may also reflect the fact that 81% of study patients with inpatient hyperglycemia received basal insulin prior to the intervention.
The reduction in patient LOS was somewhat surprising given the relatively small sample size. However, the results are consistent with those of other studies linking hyperglycemia to LOS18, 19 and we found no evidence for a temporal trend toward lower LOS on the PACE service as a whole during the same time period. While a greater proportion of patients on the PACE service were in the study in the post‐intervention period compared with the preintervention period, we found no evidence that the difference in length of stay was due to increased surveillance for nondiabetics, especially because eligibility criteria depended on phlebotomy glucose values, which were uniformly tested in all inpatients. Also, effects on length of stay were actually stronger when limited to patients with known diabetes. Finally, we controlled for several predictors of length of stay, although we still cannot exclude the possibility of unmeasured confounding between groups.
Since ADA and ACE issued guidelines for inpatient management of diabetes and hyperglycemia, many institutions have developed subcutaneous insulin algorithms, educational curricula, and/or order sets to increase compliance with these guidelines and improve glycemic control. Some of these efforts have been studied and some have been successful in their efforts.13, 14, 2023 Unfortunately, most of these programs have not rigorously assessed their impact on process and outcomes, and the most effective studies published to date have involved interventions much more intensive than those described here. For example, Rush University's intervention was associated with a 50 mg/dL decrease in mean blood glucose but involved an endocrinologist rounding twice daily with house officers for 2 weeks at a time.13 At Northwestern University, a diabetes management service run by nurse practitioners was established, and the focus was on the conversion from intravenous to subcutaneous insulin regimens.14 The RABBIT 2 study that demonstrated the benefits of a basal‐bolus insulin regimen used daily rounding with an endocrinologist.12 More modestly, a program in Pitt County Memorial Hospital in Greenville, NC, relied mostly on diabetes nurse case managers, a strategy which reduced hospital‐wide mean glucose levels as well as LOS, although the greatest improvements in glycemic control were seen in the ICU.19 Our findings are much more consistent with those from University of California San Diego, as yet unpublished, which also used an algorithm, computerized order set, education, as well as continuous quality improvement methods to achieve its aims.22
Our study has several limitations, including being conducted on 1 general medicine service at 1 academic medical center. Moreover, this service, using a physician assistant/hospitalist model, a closed geographic unit, and fairly generous staffing ratio, is likely different from those in many settings and may limit the generalizability of our findings. However, this model allowed us to conduct the study in a laboratory relatively untouched by other cointerventions. Furthermore, the use of PAs in this way may become more common as both academic and community hospitals rely more on mid‐level providers. Our study had a relatively low percentage of patients without a known diagnosis of diabetes compared with other studies, again potentially but not necessarily limiting generalizability. This finding has been shown in other studies at our institution24 and may be due to the high rate of screening for diabetes in the community. Another limitation is that this was a nonrandomized, before‐after trial. However, all subjects were prospectively enrolled to improve comparability, and we performed rigorous adjustment for multiple potential confounding factors. Also, this study had limited statistical power to detect differences in hypoglycemia rates. The preintervention arm was smaller than planned due to fewer diabetic patients than expected on the service and a higher number of exclusions; we prolonged the postintervention period to achieve the desired sample size for that arm of the study.
Our study also has several strengths, including electronic capture of many processes of care and a methodology to operationalize them into measures of protocol adherence. Our metrics of glycemic control were rigorously designed and based on a national task force on inpatient glycemic control sponsored by the Society of Hospital Medicine, with representation from the ADA and AACE.25
Potential future improvements to this intervention include modifications to the daily adjustment algorithm to improve its usability and ability to improve glucose control. Another is the use of high‐reliability methods to improve order set use and daily insulin adjustment, including alerts within the CPOE system and nurse empowerment to contact medical teams if glucose levels are out of range (eg, if greater than 180 mg/dL, not just if greater than 350 or 400 mg/dL). Future research directions include multicenter, randomized controlled trials of these types of interventions and an analysis of more distal patient outcomes including total healthcare utilization, infection rates, end‐organ damage, and mortality.
In conclusion, we found a relationship between a relatively low‐cost quality improvement intervention and improved glycemic control in the non‐ICU general medical setting. Such a finding suggests the benefits of the algorithm itself to improve glucose control and of our implementation strategy. Other institutions may find this intervention a useful starting point for their own quality improvement efforts. Both the algorithm and implementation strategy are deserving of further improvements and future study.
Acknowledgements
We thank Paul Szumita, Karen Fiumara, Jennifer Trujillo, and the other members of the BWH Diabetes Pharmacy and Therapeutics Subcommittee for their help designing and implementing the intervention; Aubre McClendon, Nicole Auclair, Emily Dattwyler, Mariya Fiman, and Alison Pietras for valuable research assistance; Deborah Williams for data analysis; Amy Bloom for project support; and Stuart Lipsitz for biostatistical expertise.
APPENDIX
INPATIENT DIABETES MANAGEMENT PROTOCOL
Management of Diabetes and Hyperglycemia in Hospitalized Non‐ICU Patients
Rationale
Increasing data show a strong association between hyperglycemia and adverse inpatient outcomes. The American Diabetes Association and the American College of Clinical Endocrinology recommend all glucose levels be below 180 mg/dL in non‐ICU patients. Because hospitalizations are unstable situations, even patients who are well controlled on oral agents as outpatients are usually best managed with insulin.
Insulin may be safely administered even to patients without previously diagnosed diabetes. As long as the prescribed doses are below what is normally produced by the pancreas, the patient will not become hypoglycemic. If the glucose level drops, endogenous insulin secretion will reduce to compensate.
Total insulin requirements in insulin‐sensitive patients (eg, type 1 diabetes mellitus) is 0.50.7/units/kg/day. Insulin requirements in insulin‐resistant type 2 diabetic patients may vary greatly, and can exceed 12 units/kg/day. A conservative estimate for initial insulin therapy in any patient with diabetes is to start with the type 1 diabetes mellitus dose, 0.50.7 units/kg/day.
Overview
Effective inpatient insulin regimens typically include 3 components:
Basal insulin (eg, scheduled NPH or insulin glargine [Lantus]), which is used to manage fasting and premeal hyperglycemia.
Nutritional or prandial insulin (eg, scheduled regular insulin, insulin lispro [Humalog] or insulin aspart [Novolog]) which controls hyperglycemia from nutritional (eg, discrete meals, TPN, IV dextrose) sources.
Supplemental or correctional insulin (eg, regular insulin, insulin lispro, or insulin aspart), which is used in addition to scheduled insulin to meet unexpected basal hyperglycemia that is not covered by the scheduled insulin.
Sample Orders (Not for Patients with Uncontrolled Type 1 Diabetes, DKA, Hyperglycemic Hyperosmolar State, or Other Absolute Need for IV Insulin)
Check (fingerstick) capillary blood glucose qAC, qHS.
NPH insulin subcutaneously (SC) ___ units qAM, ___ units qHS.
Insulin aspart SC ___ units pre‐breakfast, ___ units pre‐lunch, ___ units pre‐dinner, hold if NPO or premeal BS <60 mg/dL; give 015 minutes before meals.
Insulin aspart SC sliding scale (see Table 6) qAC, in addition to standing nutritional insulin, 015 minutes before meals.
For BS <60 mg/dL:
If patient can take PO
Give 15 g of fast acting carbohydrate (4 oz fruit juice/nondiet soda, 8 oz nonfat milk, or 34 glucose tablets).
Repeat finger capillary glucose every 15 (q15) minutes and repeat above (5.a.i.) if BG <60 mg/dL.
When BG >60 mg/dL, give snack or meal in a half‐hour.
If patient cannot take PO
Give 25 mL of 50% dextrose (D50) as an IV push.;
Repeat finger capillary glucose q15 minutes and repeat above (5.b.i.) if BG <80 mg/dL.
Guidelines
Stop oral diabetes agents in most patients (see Table 7 for list of contraindications and precautions).
Check bedside blood glucose (BBG or fingerstick) qAC and qHS (or at 0600 hours, 1200 hours, 1800 hours, and 2400 hours if no discrete meals).
Estimate total daily insulin requirement:
For most patients, conservative estimate is 0.50.7 units/kg/day, but may be much higher.
Reasons for lower end of the range: renal insufficiency, small size, insulin sensitive (eg, type 1), recent hypoglycemia, decreasing doses of steroids, older age.
Reasons for higher end of the range: obese, initiation or increasing doses of steroids, marked hyperglycemia.
Start basal insulin if any premeal BG >140 mg/dL and no recent glucose <60 mg/dL off insulin (Table 5).
Start nutritional or prandial insulinhold if nutrition is stopped/held or premeal BS <60 (Table 5).
Start supplemental/correctional insulin in addition to nutritional (prandial) insulin (Table 6):
Discrete meals: Insulin aspart qAC (with nutritional insulin). 0
No discrete meals: Regular insulin q6h.
On a daily basis, adjust scheduled insulin based on previous days' blood sugars:
Add up total insulin given the previous day, including scheduled and supplemental insulin, to determine new total daily insulin requirement.
Adjust total daily insulin requirement based on clinical considerations (eg, give more if marked hyperglycemia, eating more, improving renal function, increasing steroids; give less if eating less, worsening renal function, tapering steroids, recovering from severe illness).
Give 50% of requirement as basal and 50% as nutritional, as above (may need proportionately less nutritional insulin if appetite poor or unknown).
Adjust sliding scale if needed based on total scheduled insulin dose (see step 6, above).
For BG <60 mg/dL:
If patient can take PO, give 15 g of fast acting carbohydrate.
(4 oz fruit juice/nondiet soda, 8 oz nonfat milk, or 34 glucose tablets; not juice plus sugar).
Repeat finger capillary glucose q15 minutes and repeat above if BG <60.
When BG >60, give snack or meal in half an hour.
If patient cannot take PO, give 25 mL of D50 as IV push.
Check finger capillary glucose q15 minutes and repeat above if BG <80.
Discharge orders:
Patient should be discharged home on a medication regimen that was similar to the admission regimen (ie, the regimen prescribed by their PCP). Exceptions include
The patient has a contraindication to an admission medication.
There is evidence of severe hyperglycemia (eg, very high A1C) or hypoglycemia on admission regimen.
If a patient is admitted with no insulin, and requires insulin to be continued as an outpatient (eg, newly‐diagnosed type 1 diabetes, A1C very high, and contraindication to or on maximum oral regimen), limit discharge insulin regimen to no more than 1 injection per day (eg, hs NPH; an exception to this is for type 1 diabetic patients, who are optimally treated with 34 injections/day). Make sure the patient has prompt follow‐up with their primary care provider (PCP).
Avoid discharging home on sliding scale.
If a patient is going to require insulin injections and self‐monitoring blood glucose as an outpatient, make sure they are instructed about how to perform these.
Indications for calling an endocrine consult:
Labile blood sugars.
Prolonged periods of NPO, eg, for procedures, especially in patients with type 1 diabetes
Marked hyperglycemia despite following this guideline.
Question of type 1 versus type 2 versus other type of diabetes. 0
Basil Insulin Guidelines Home Insulin Regimen Starting Dose of Basal Insulin Considerations NOTE: Patients with T1DM require basal insulin at all times! Basal never should be held!
Abbreviations: NPO, nothing by mouth.
On basal (eg, NPH or glargine) insulin at home Patient's home dose of NPH or glargine If NPO, consider starting half of NPH or glargine home dose, unless hyperglycemic at home. Not on basal (eg, NPH or glargine) insulin at home NPH 50% of total daily insulin requirement, given qHS or split qAM/qHS (maximum starting dose 20 units/day) Same dose if patient has previously diagnosed or undiagnosed diabetes Nutritional Insulin Guidelines Type of Nutrition Common Nutritional Regimens Sample Starting Doses Abbreviation: qAM, every morning; qHS, at bed time.
If receiving cycled tube feeds at night, give nutritional NPH qHS only.
Discrete meals Aspart given 015 minutes before meals Home dose, if known or 50% of total insulin requirement, split over 3 meals, may need less if poor or unknown appetite Continuous tube feeding,* IV dextrose NPH qHS or qAM/qHS 50% of total insulin requirement (in addition to basal dose), may need less if not at goal caloric intake Glargine given every day (qd), anytime Regular every 6 hours (q6h) Sample Supplemental/Correctional Insulin Scales Blood Glucose Scheduled Insulin < 40 Units/Day Scheduled Insulin of 4080 Units/Day Scheduled Insulin > 80 Units/Day Individualized NOTE: Avoid supplemental insulin qHS unless patient is very hyperglycemic and obese.
150199 1 unit 1 unit 2 units ____ units 200249 2 units 3 units 4 units ____ units 250299 3 units 5 units 7 units ____ units 300349 4 units 7 units 10 units ____ units >349 5 units + call HO 8 units + call HO 12 units + call HO ___ units + call HO Notes on Oral Agents Agents Considerations Metabolism Sulfonylureas/secretagogues: glyburide, glipizide, glimeperide (Amaryl); repaglinide (Prandin); nateglinide (Starlix) Risk for hypoglycemia Metabolized in liver; Glyburide metabolized to active metabolites; 50% renally eliminated Metformin Contraindicated in heart failure and renal dysfunction (creatinine [Cr] >1.5 mg/dL in men and 1.4 mg/dL in women) Eliminated renally Should be held at time of iodinated contrast studies. (May be restarted after normal postcontrast renal function is confirmed) Adverse effects include diarrhea, nausea, and anorexia Thiazolidinediones: pioglitazone (Actos), rosiglitazone (Avandia) Contraindicated in class III and IV heart failure Metabolized in liver Use with caution in patients with edema Adverse effects include increased intravascular volume Slow onset of action Avoid in hepatic dysfunction Glucosidease inhibitors: acarbose (Precose), miglitol (Glycet) Gastrointestinal intolerance Acarbose eliminated in gut and renally
Diabetes mellitus and/or inpatient hyperglycemia are common comorbid conditions in hospitalized patients. Recent surveys show that over 90% of hospitalized diabetic patients experience hyperglycemia (>200 mg/dL), and in nearly 1 in 5 of these patients hyperglycemia persists for 3 days or more.1 Hyperglycemia among inpatients without a previous history of diabetes mellitus is also very common.2 Observational studies have shown that hyperglycemia in hospitalized patients is associated with adverse outcomes including infectious complications, increased length of stay, and increased mortality.27 Recent randomized controlled trials have demonstrated that aggressive treatment of inpatient hyperglycemia improves outcomes in surgical and medical intensive care units.8, 9
Based on the available data, the American Diabetes Association (ADA) now advocates good metabolic control, defined as preprandial glucose levels of 90 to 130 mg/dL and peak postprandial glucose levels <180 mg/dL in hospitalized nonintensive care unit (ICU) patients.10 To reach these targets, the ADA and American College of Endocrinology (ACE) suggest that multidisciplinary teams develop and implement hyperglycemia management guidelines and protocols.11 Protocols should promote the use of continuous intravenous insulin infusions or scheduled basal‐bolus subcutaneous insulin regimens. Subcutaneous insulin protocols should include target glucose levels, basal, nutritional, and supplemental insulin, and daily dose adjustments.6 A recent randomized controlled trial of non‐ICU inpatients demonstrated that such a basal‐bolus insulin regimen results in improved glucose control compared with a sliding scale only regimen.12
To date, few published studies have investigated the best ways to implement such management protocols; those that have are often resource‐intensive, for example involving daily involvement of nurse practitioners or diabetologists.13, 14 It is therefore not known how best to implement an inpatient diabetes management program that is effective, efficient, and self‐perpetuating. At Brigham and Women's Hospital (BWH), we have been refining a subcutaneous insulin protocol, focused provider education, and more recently a computerized order set to overcome barriers related to fear of hypoglycemia, delays in insulin prescribing, and unfamiliarity with inpatient glucose management.15 The aims of this current trial were to evaluate the effects of these interventions on a geographically localized general medical service previously naive to these interventions to evaluate their effects on glycemic control, patient safety, and processes of care. We hypothesized that these interventions would improve glycemic control and increase use of basal‐bolus insulin orders without increasing the rate of hypoglycemia.
METHODS
Setting and Participants
This prospective, before‐after trial was conducted at BWH from July 15, 2005 through June 22, 2006. Eligible subjects were patients scheduled for admission to the BWH Physician Assistant/Clinician Educator (PACE) Service with either a known diagnosis of type 2 diabetes mellitus or inpatient hyperglycemia (at least 1 random laboratory glucose >180 mg/dL). The PACE service is a geographically‐localized general medicine service of up to 15 beds where patients are cared for by a single cadre of nurses, 2 physician's assistants (PAs), and 1 hospitalist attending. A moonlighter covers the service at night. The PACE service does not accept patients transferred from other acute care hospitals or from ICUs, but does not otherwise have triage guidelines related to diagnosis, complexity, or acuity. Patients were excluded if they had type 1 diabetes, presented with hyperosmolar hyperglycemic state (HHS) or diabetic ketoacidosis (DKA), received total parenteral nutrition (TPN), or were receiving palliative care. This study was approved by the BWH Institutional Review Board; patient consent was deemed not to be necessary for this study given the relatively nonsensitive nature of the data, noninvasive means of data collection, and the steps taken by research personnel to minimize any breach in patient confidentiality.
Intervention
The study intervention consisted of three components, initiated in January 2006:
Glycemic management protocol: a multidisciplinary team of a diabetologist (M.L.P.), a hospitalist (J.L.S.), and a pharmacist (Jennifer Trujillo) developed a subcutaneous insulin protocol based on ADA guidelines (Table 1; see the appendix for complete protocol). The protocol was approved by the BWH Pharmacy and Therapeutics Diabetes Subcommittee and refined through 6 months of pilot testing on other general medical services.15 The protocol consisted of a set of specific treatment recommendations, including: (1) bedside glucose monitoring; (2) stopping oral diabetes agents in most patients; (3) estimating total daily insulin requirements; (4) prescribing basal, nutritional, and supplemental insulin based on the patient's total insulin requirements, preadmission medication regimen, and nutritional status; (5) adjusting insulin on a daily basis as needed; (6) managing hypoglycemia; (7) suggestions for discharge orders; and (8) indications for an endocrinology consultation. The protocol was printed as a pocket guide, distributed to all members of the PACE service, and used to guide all other interventions.
Diabetes education: all PAs received 2 one‐hour educational sessions: a lecture by a diabetologist (M.L.P.) reviewing the rationale for tight glycemic control and general principles of management, and a workshop by a hospitalist (J.L.S.) in which specific cases were reviewed to illustrate how the protocol could be used in practice (eg, when oral agents could be safely continued, how to prescribe insulin on admission, and how to make subsequent adjustments in dose). All hospitalist attendings received a 1‐hour lecture summarizing the above material. All nurses on the service received a lecture that focused on issues unique to nursing care, such as insulin administration, glucose testing, managing patients with unpredictable oral (PO) intake, and patient education. (All materials are available from the authors upon request).
Order Set: an order set, built into BWH's proprietary computer provider order entry (CPOE) system, was created to parallel the glycemic management protocol and facilitate insulin orders for patients eating discrete meals, receiving continuous liquid enteral nutrition (tube feeds), or receiving nothing by mouth (NPO). Other components of the order set facilitated glucose monitoring and other laboratory tests and ordering consultation when appropriate.
| Oral Agents | Stop Oral Agents in Most Patients |
|---|---|
| |
| Glucose testing | Check bedside blood glucose before meals and at bedtime if eating, or every 6 hours if NPO |
| Insulin | |
| 1. Estimate total daily insulin dose | 0.5 to 0.7 units/kg/day, depending on patient's age, size, renal function, insulin sensitivity, history of hypoglycemia, and steroid use |
| 2. Start basal insulin | Patient's home dose or 50% of calculated total daily dose; NPH qAM/qHS or insulin glargine qHS; If NPO, use one‐half the home dose unless hyperglycemic |
| 3. Start nutritional insulin if not NPO | Patient's home dose or 50% of calculated total daily dose, less if poor or unknown intake; discrete meals: insulin aspart split over 3 meals, 0 to 15 minutes prior to eating; continuous tube feeds or IV dextrose: regular insulin every 6 hours |
| 4. Start correctional insulin | 1 of 3 scales provided based on total daily dose of insulin; same type as nutritional insulin; regular insulin if NPO |
| 5. Daily adjustment | Calculate total administered dose from prior day, adjust for degree of hyperglycemia or hypoglycemia, renal function, PO intake, steroid use, and degree of illness, and redistribute as 50% basal, 50% nutritional, or 100% basal if NPO |
| Hypoglycemia orders | Juice, IV dextrose, or IM glucagon depending on ability to take oral nutrition and IV access |
| Discharge orders | Based on A1C: either home regimen, titration of home regimen, or new insulin regimen (if latter, simple regimen with aggressive patient education and prompt follow‐up) |
| Indications for endocrine consultation | Labile blood sugars, poor control, prolonged NPO period, question of type 1 or type 2 diabetes |
Study Protocol and Data Collection
A research assistant prospectively identified eligible patients each weekday by screening all patients scheduled for admission to the PACE service using the daily computerized sign‐out system used on all general medical teams. Specifically, laboratory random glucose levels, inpatient medications, and medical histories were reviewed to determine if each patient met eligibility criteria. Eligibility criteria were then confirmed by medical record review and adjudicated by one study author (J.L.S.) if necessary. Further medical record review was performed to identify specific patient populations (eg, diet‐controlled, steroid‐induced, or previously undiagnosed diabetes), determine preadmission diabetes medications, and determine the patient's weight. Hospital computerized clinical and administrative records were abstracted to obtain patient demographics (age, sex, race, insurance status), laboratory data (glucose level on admission, A1C level [taken during or within 6 months prior to admission]), clinical data (length of stay, billing‐based Charlson comorbidity score,16 and diagnosis‐related group [DRG] case mix index), all inpatient insulin and oral diabetes medication orders, frequency of bedside glucose testing, and diet orders. Electronic medication administration record (eMAR) data were used to determine all doses and times of insulin administration.
Outcomes
The primary outcome was the mean percent of glucose readings between 60 and 180 mg/dL per patient (ie, calculated for each patient and averaged across all eligible patients in each study arm). Only bedside glucose readings were used given the lack of additional useful information typically provided by laboratory (venous plasma) glucose readings.17 Readings drawn within 1 hour of a previous reading were excluded to avoid ascertainment bias caused by follow‐up testing of abnormal glucose values. Only readings while on the study service were used. Readings on hospital day 1 were excluded because our intervention was expected to have little impact on the first day's glucose control; for patients with undiagnosed diabetes, data collection began the day following the first elevated glucose reading. Readings beyond hospital day 14 were also excluded to avoid biased data from patients with exceptionally long lengths of stay.
Secondary outcomes included the following:
Glycemic control:
Patient‐day weighted mean glucose (ie, mean glucose for each patient‐day, averaged across all patient days);
Mean glucose per patient for each hospital day (days 17).
Patient safety:
Proportion of patient‐days with any glucose reading <60 mg/dL (hypoglycemia) and <40 mg/dL (severe hypoglycemia).
Processes of care:
Use of any NPH insulin or insulin glargine (basal) insulin during the hospitalization if 2 or more glucose readings were >180 mg/dL.
Adequacy of basal dose on day first prescribed: for patients prescribed a diet, within 20% of preadmission basal dose or 0.20 to 0.42 units/kg if not known or not taken prior to admission. If not eating, half the above calculations.
Use of any scheduled nutritional insulin during the hospitalization if ever prescribed a diet and 2 or more glucose readings were greater than 180 mg/dL.
Adequacy of nutritional dose on day first prescribed: for patients prescribed a diet, within 20% of preadmission nutritional dose or 0.20 to 0.42 units/kg/day if not known or not taken prior to admission. Patients on clear liquid diets, enteral feeds, or receiving glucocorticoids were excluded from this analysis.
Correct type of nutritional insulin: if eating discrete meals, insulin aspart (the rapid‐acting insulin on formulary at BWH); if prescribed tube feeds, regular insulin.
Use of supplemental insulin by itself (without scheduled basal or nutritional insulin), a marker of poor care.
A1C testing within 1 month prior to or during hospitalization.
Clinical inertia: if at least two glucose readings <60 mg/dL or >180 mg/dL on a patient‐day, lack of any change to any insulin order the following day if still on the study service.
Healthcare utilization:
Hospital length of stay in hours, calculated from the exact time of admission until the exact time of discharge, using hospital administrative data.
Analyses
Study results were compared prior to the intervention (July 15 through December 12, 2005) with those during the intervention (January 18 through June 20, 2006). Patient data and clinical outcomes were analyzed descriptively using proportions, means with standard deviations (SDs), or medians with interquartile ranges (IQRs) as appropriate. Comparisons between groups were calculated using Fisher's exact test for dichotomous and categorical variables, and Student t test or Wilcoxon rank sum test for continuous variables as appropriate. The primary outcome was first analyzed using linear regression with study group as the independent variable and percent of glucose readings within range per patient as the dependent variable. We then adjusted for potential confounders by putting each covariate into the model, one at a time. All significant predictors of the outcome at a P value <0.10 were retained in the final model. We used general estimating equations to adjust for clustering of results by each PA. Similar analyses were performed for hospital length of stay per patient using a negative binomial model, so chosen because it fit the data distribution much better than the typically used Poisson model. With a planned sample size of 115 patients and 1250 glucose readings per arm, an intraclass correlation coefficient of 0.10, and an alpha of 0.05, the study had 90% power to detect an increase in percent of glucose readings in range from 67% to 75%. All analyses were based on the intention‐to‐treat principle. Except as above, 2‐sided P values <0.05 were considered significant. SAS version 9.1 (SAS Institute, Cary, NC) was used for all analyses.
RESULTS
We prospectively identified 248 potential patients for the study. We subsequently excluded 79 patients for the following reasons: no glucose readings beyond hospital day 1 while on PACE service (34 patients); never admitted to PACE service (15 patients); no diabetes or inpatient hyperglycemia (9 patients, mostly patients prescribed an insulin sliding scale prophylactically to avoid steroid‐induced hyperglycemia); type 1 diabetes (13 patients); TPN, DKA, or HHS (5 patients); and palliative care (3 patients). The remaining 169 patients included 63 from the preintervention period(out of 489 total admissions to the PACE service; 13%) and 106 patients in the postintervention period (out of 565 admissions; 19%). These patients had 2447 glucose readings, or an average of 3.6 glucose readings per monitored patient‐day in the preintervention period and 3.3 glucose readings per patient‐day in the postintervention period. Even including the 34 patients who were excluded for lack of glucose readings, glucose data were still available for 717 out of a potential 775 patient‐days (93%). Characteristics for all included patients are shown in Table 2. The mean admission glucose was 197 mg/dL, mean A1C was 8.4%, 54% of the patients were prescribed insulin prior to admission, and 7% had no prior diagnosis of diabetes. There were no significant differences in baseline characteristics between the 2 patient groups except for Charlson score, which was higher in the preintervention group (87% versus 74% with score 2 or higher; Table 2). The top diagnosis‐related groups for the entire cohort included: heart failure and shock (12 patients); kidney and urinary tract infections (12 patients); esophagitis, gastroenteritis, and miscellaneous digestive disorders (11 patients); chronic obstructive pulmonary disease (10 patients); renal failure (10 patients); simple pneumonia and pleurisy (7 patients); disorders of the pancreas except malignancy (6 patients); chest pain (5 patients); and cellulitis (5 patients).
| Preintervention (n = 63) | Postintervention (n = 106) | P Value | |
|---|---|---|---|
| |||
| Mean age, year (SD) | 63.0 (15.7) | 64.7 (14.3) | 0.52 |
| Male, n (%) | 25 (40) | 52 (49) | 0.27 |
| Race, n (%) | 0.33 | ||
| White | 29 (46) | 42 (40) | |
| Black | 21 (33) | 28 (26) | |
| Hispanic | 11 (17) | 30 (28) | |
| Unknown | 2 (3) | 6 (6) | |
| Admission glucose, mg/dL (SD) | 188 (90.9) | 203 (96.1) | 0.33 |
| A1C, % (SD) | 8.5 (2.4) | 8.3 (2.4) | 0.85 |
| Insulin use prior to admission, n (%) | 38 (60) | 54 (51) | 0.48 |
| Case mix index, median (IQR) | 0.89 (0.781.11) | 0.91 (0.841.22) | 0.33 |
| Charlson index, n (%) | 0.03 | ||
| 01 | 8 (13) | 28 (26) | |
| 23 | 29 (46) | 27 (26) | |
| 45 | 15 (24) | 29 (27) | |
| >5 | 11 (17) | 22 (21) | |
| Known history of diabetes, n (%) | 62 (98) | 96 (91) | 0.06 |
With respect to insulin ordering practices, there was no significant difference in the use of basal insulin in hyperglycemic patients between the preintervention period and postintervention period (81% versus 91%; P = 0.17), nor in the dose of basal insulin prescribed (results not shown), but there was an increase in the use of scheduled nutritional insulin for those patients with hyperglycemia receiving nutrition: 40% versus 75%, P < 0.001 (Table 3). The percent of patients receiving supplemental (sliding scale) insulin by itself (ie, without ever receiving basal or nutritional insulin) was lower during the postintervention period (29% versus 8%, P < 0.001). Nonsignificant differences were seen in the rates of prescribing an appropriate dose and type of nutritional insulin. Notably, there was no difference at all in the proportion of patient‐days in which insulin adjustments were made when 2 or more episodes of hyperglycemia or hypoglycemia were present during the previous day (56% of patient‐days in both groups; P = 0.90).
| Preintervention (n = 63) | Postintervention (n = 106) | Unadjusted Effect Size (95% CI) | Adjusted Effect Size (95% CI) | |
|---|---|---|---|---|
| ||||
| Mean percent glucose readings 60180 mg/dL per patient (SD) | 59.1 (0.28) | 64.7 (0.27) | +5.6 (3.0 to +14.3) | +9.7 (+0.6 to +18.8)*, |
| Patient‐day weighted mean glucose, mg/dL (SD) | 174.7 (60.0) | 164.6 (54.2) | 10.1 (1.6 to 18.5) | 15.6 (6.4 to 24.9), |
| Percent patient‐days with any glucose <60 mg/dL | 16/293 (5.5%) | 26/424 (6.1%) | 1.1 (0.6 to 2.1) | 1.1 (0.6 to 2.1) |
| Percent patient‐days with any glucose <40 mg/dL | 3/293 (1.0%) | 5/424 (1.2%) | 1.3 (0.3 to 5.9) | 1.1 (0.3 to 5.1) |
| Hospital length of stay, hours, mean (SD) | 112.2 (63.3) | 86.0 (89.6) | 30% (5% to 51%) | 25% (6% to 44%),∥ |
| Basal insulin if inpatient hyperglycemia (2 or more readings >180 mg/dL) | 39/48 (81%) | 67/74 (91%) | 2.2 (0.8 to 6.4) | |
| Nutritional insulin if inpatient hyperglycemia and PO intake | 19/48 (40%) | 53/71 (75%) | 4.5 (2.0 to 9.9), | |
| Adequate initial dose of nutritional insulin (home dose or 0.200.42 units/kg/day)# | 2/9 (22%) | 22/49 (45%) | 2.9 (0.5 to 15.1) | |
| Supplemental insulin alone (without basal or nutritional insulin) | 16/56 (29%) | 7/92 (8%) | 0.2 (0.08 to 0.5), | |
| Insulin changed if previous day's glucose out of range (2 or more values <60 or >180 mg/dL) | 70/126 (56%) | 76/135 (56%) | 1.0 (0.6 to 1.6) | |
| A1C tested during hospitalization if not available within 30 days prior | 38/63 (60%) | 74/106 (70%) | 1.5 (0.8 to 2.9) | |
The primary outcome, the mean percent of glucose readings between 60 and 180 mg/dL per patient, was 59.1% in the preintervention period and 64.7% in the postintervention (P = 0.13 in unadjusted analysis; Table 3). When adjusted for A1C, admission glucose, and insulin use prior to admission, the adjusted absolute difference in the percent of glucose readings within range was 9.7% (95% confidence interval [CI], 0.6%‐18.8%; P = 0.04; Table 3). Regarding other measures of glucose control, the patient‐day weighted mean glucose was 174.7 mg/dL in the preintervention period and 164.6 mg/dL postintervention (P = 0.02), and there was no significant difference in the percent of patient‐days with any hypoglycemia (glucose <60 mg/dL) or severe hypoglycemia (glucose <40 mg/dL; Table 3). There were also no significant differences in the mean number of hypoglycemic events per patient‐day (6.8 versus 6.6 per 100 patient‐days; relative risk, 0.95; 95% CI, 0.541.67; P = 0.87) or severe hypoglycemic events per patient‐day (1.0 versus 1.4 per 100 patient‐days; relative risk, 1.38; 95% CI, 0.355.53; P = 0.65).
We also compared hospital length of stay in hours between the study groups (Table 3). Length of stay (LOS) was shorter in the postintervention arm in unadjusted analyses (112 versus 86 hours; P < 0.001), and this difference persisted when adjusted for patient insurance, race, gender, and Charlson comorbidity score (25% shorter; 95% CI, 6%‐44%). A comparison of LOS among nonstudy patients on the PACE service during these 2 time periods revealed no difference (105 versus 101 hours). When the length of stay analysis was limited to study patients with a known diagnosis of diabetes, the adjusted effect size was a 31% relative decrease in length of stay.
Figure 1A shows the percent glucose readings within range per patient by hospital day. The greatest differences between groups can be seen on hospital days 2 and 3 (11% absolute differences on both days). Similarly, Figure 1B shows the mean glucose per patient by hospital day. Again, the biggest differences are seen on hospital days 2 and 3 (20 and 23 mg/dL difference between groups, respectively). In both cases, only the day 3 comparisons were significantly different between study groups.

DISCUSSION
In this before‐after study, we found that a multifaceted intervention consisting of a subcutaneous insulin protocol, focused education, and an order set built into the hospital's CPOE system was associated with a significantly higher percentage of glucose readings within range per patient in analyses adjusted for patient demographics and severity of diabetes. We also found a significant decrease in patient‐day weighted mean glucose, a marked increase in appropriate use of scheduled nutritional insulin, and a concomitant decrease in sliding scale insulin only regimens during the postintervention period. Moreover, we found a shorter length of stay during the postintervention period that persisted after adjustment for several clinical factors. Importantly, the interventions described in this study require very few resources to continue indefinitely: printing costs for the management protocol, 4 hours of education delivered per year, and routine upkeep of an electronic order set.
Because this was a before‐after study, we cannot exclude the possibility that these improvements in process and outcome were due to cointerventions and/or temporal trends. However, we know of no other interventions aimed at improving diabetes care in this self‐contained service of nurses, PAs, and hospitalists. Moreover, the process improvements, especially the increase in scheduled nutritional insulin, were rather marked, unlikely to be due to temporal trends alone, and likely capable of producing the corresponding improvements in glucose control. That glucose control stopped improving after hospital day 3 may be due to the fact that subsequent adjustment to insulin orders occurred infrequently and no more often than prior to the intervention. That we did not see greater improvements in glycemic control overall may also reflect the fact that 81% of study patients with inpatient hyperglycemia received basal insulin prior to the intervention.
The reduction in patient LOS was somewhat surprising given the relatively small sample size. However, the results are consistent with those of other studies linking hyperglycemia to LOS18, 19 and we found no evidence for a temporal trend toward lower LOS on the PACE service as a whole during the same time period. While a greater proportion of patients on the PACE service were in the study in the post‐intervention period compared with the preintervention period, we found no evidence that the difference in length of stay was due to increased surveillance for nondiabetics, especially because eligibility criteria depended on phlebotomy glucose values, which were uniformly tested in all inpatients. Also, effects on length of stay were actually stronger when limited to patients with known diabetes. Finally, we controlled for several predictors of length of stay, although we still cannot exclude the possibility of unmeasured confounding between groups.
Since ADA and ACE issued guidelines for inpatient management of diabetes and hyperglycemia, many institutions have developed subcutaneous insulin algorithms, educational curricula, and/or order sets to increase compliance with these guidelines and improve glycemic control. Some of these efforts have been studied and some have been successful in their efforts.13, 14, 2023 Unfortunately, most of these programs have not rigorously assessed their impact on process and outcomes, and the most effective studies published to date have involved interventions much more intensive than those described here. For example, Rush University's intervention was associated with a 50 mg/dL decrease in mean blood glucose but involved an endocrinologist rounding twice daily with house officers for 2 weeks at a time.13 At Northwestern University, a diabetes management service run by nurse practitioners was established, and the focus was on the conversion from intravenous to subcutaneous insulin regimens.14 The RABBIT 2 study that demonstrated the benefits of a basal‐bolus insulin regimen used daily rounding with an endocrinologist.12 More modestly, a program in Pitt County Memorial Hospital in Greenville, NC, relied mostly on diabetes nurse case managers, a strategy which reduced hospital‐wide mean glucose levels as well as LOS, although the greatest improvements in glycemic control were seen in the ICU.19 Our findings are much more consistent with those from University of California San Diego, as yet unpublished, which also used an algorithm, computerized order set, education, as well as continuous quality improvement methods to achieve its aims.22
Our study has several limitations, including being conducted on 1 general medicine service at 1 academic medical center. Moreover, this service, using a physician assistant/hospitalist model, a closed geographic unit, and fairly generous staffing ratio, is likely different from those in many settings and may limit the generalizability of our findings. However, this model allowed us to conduct the study in a laboratory relatively untouched by other cointerventions. Furthermore, the use of PAs in this way may become more common as both academic and community hospitals rely more on mid‐level providers. Our study had a relatively low percentage of patients without a known diagnosis of diabetes compared with other studies, again potentially but not necessarily limiting generalizability. This finding has been shown in other studies at our institution24 and may be due to the high rate of screening for diabetes in the community. Another limitation is that this was a nonrandomized, before‐after trial. However, all subjects were prospectively enrolled to improve comparability, and we performed rigorous adjustment for multiple potential confounding factors. Also, this study had limited statistical power to detect differences in hypoglycemia rates. The preintervention arm was smaller than planned due to fewer diabetic patients than expected on the service and a higher number of exclusions; we prolonged the postintervention period to achieve the desired sample size for that arm of the study.
Our study also has several strengths, including electronic capture of many processes of care and a methodology to operationalize them into measures of protocol adherence. Our metrics of glycemic control were rigorously designed and based on a national task force on inpatient glycemic control sponsored by the Society of Hospital Medicine, with representation from the ADA and AACE.25
Potential future improvements to this intervention include modifications to the daily adjustment algorithm to improve its usability and ability to improve glucose control. Another is the use of high‐reliability methods to improve order set use and daily insulin adjustment, including alerts within the CPOE system and nurse empowerment to contact medical teams if glucose levels are out of range (eg, if greater than 180 mg/dL, not just if greater than 350 or 400 mg/dL). Future research directions include multicenter, randomized controlled trials of these types of interventions and an analysis of more distal patient outcomes including total healthcare utilization, infection rates, end‐organ damage, and mortality.
In conclusion, we found a relationship between a relatively low‐cost quality improvement intervention and improved glycemic control in the non‐ICU general medical setting. Such a finding suggests the benefits of the algorithm itself to improve glucose control and of our implementation strategy. Other institutions may find this intervention a useful starting point for their own quality improvement efforts. Both the algorithm and implementation strategy are deserving of further improvements and future study.
Acknowledgements
We thank Paul Szumita, Karen Fiumara, Jennifer Trujillo, and the other members of the BWH Diabetes Pharmacy and Therapeutics Subcommittee for their help designing and implementing the intervention; Aubre McClendon, Nicole Auclair, Emily Dattwyler, Mariya Fiman, and Alison Pietras for valuable research assistance; Deborah Williams for data analysis; Amy Bloom for project support; and Stuart Lipsitz for biostatistical expertise.
APPENDIX
INPATIENT DIABETES MANAGEMENT PROTOCOL
Management of Diabetes and Hyperglycemia in Hospitalized Non‐ICU Patients
Rationale
Increasing data show a strong association between hyperglycemia and adverse inpatient outcomes. The American Diabetes Association and the American College of Clinical Endocrinology recommend all glucose levels be below 180 mg/dL in non‐ICU patients. Because hospitalizations are unstable situations, even patients who are well controlled on oral agents as outpatients are usually best managed with insulin.
Insulin may be safely administered even to patients without previously diagnosed diabetes. As long as the prescribed doses are below what is normally produced by the pancreas, the patient will not become hypoglycemic. If the glucose level drops, endogenous insulin secretion will reduce to compensate.
Total insulin requirements in insulin‐sensitive patients (eg, type 1 diabetes mellitus) is 0.50.7/units/kg/day. Insulin requirements in insulin‐resistant type 2 diabetic patients may vary greatly, and can exceed 12 units/kg/day. A conservative estimate for initial insulin therapy in any patient with diabetes is to start with the type 1 diabetes mellitus dose, 0.50.7 units/kg/day.
Overview
Effective inpatient insulin regimens typically include 3 components:
Basal insulin (eg, scheduled NPH or insulin glargine [Lantus]), which is used to manage fasting and premeal hyperglycemia.
Nutritional or prandial insulin (eg, scheduled regular insulin, insulin lispro [Humalog] or insulin aspart [Novolog]) which controls hyperglycemia from nutritional (eg, discrete meals, TPN, IV dextrose) sources.
Supplemental or correctional insulin (eg, regular insulin, insulin lispro, or insulin aspart), which is used in addition to scheduled insulin to meet unexpected basal hyperglycemia that is not covered by the scheduled insulin.
Sample Orders (Not for Patients with Uncontrolled Type 1 Diabetes, DKA, Hyperglycemic Hyperosmolar State, or Other Absolute Need for IV Insulin)
Check (fingerstick) capillary blood glucose qAC, qHS.
NPH insulin subcutaneously (SC) ___ units qAM, ___ units qHS.
Insulin aspart SC ___ units pre‐breakfast, ___ units pre‐lunch, ___ units pre‐dinner, hold if NPO or premeal BS <60 mg/dL; give 015 minutes before meals.
Insulin aspart SC sliding scale (see Table 6) qAC, in addition to standing nutritional insulin, 015 minutes before meals.
For BS <60 mg/dL:
If patient can take PO
Give 15 g of fast acting carbohydrate (4 oz fruit juice/nondiet soda, 8 oz nonfat milk, or 34 glucose tablets).
Repeat finger capillary glucose every 15 (q15) minutes and repeat above (5.a.i.) if BG <60 mg/dL.
When BG >60 mg/dL, give snack or meal in a half‐hour.
If patient cannot take PO
Give 25 mL of 50% dextrose (D50) as an IV push.;
Repeat finger capillary glucose q15 minutes and repeat above (5.b.i.) if BG <80 mg/dL.
Guidelines
Stop oral diabetes agents in most patients (see Table 7 for list of contraindications and precautions).
Check bedside blood glucose (BBG or fingerstick) qAC and qHS (or at 0600 hours, 1200 hours, 1800 hours, and 2400 hours if no discrete meals).
Estimate total daily insulin requirement:
For most patients, conservative estimate is 0.50.7 units/kg/day, but may be much higher.
Reasons for lower end of the range: renal insufficiency, small size, insulin sensitive (eg, type 1), recent hypoglycemia, decreasing doses of steroids, older age.
Reasons for higher end of the range: obese, initiation or increasing doses of steroids, marked hyperglycemia.
Start basal insulin if any premeal BG >140 mg/dL and no recent glucose <60 mg/dL off insulin (Table 5).
Start nutritional or prandial insulinhold if nutrition is stopped/held or premeal BS <60 (Table 5).
Start supplemental/correctional insulin in addition to nutritional (prandial) insulin (Table 6):
Discrete meals: Insulin aspart qAC (with nutritional insulin). 0
No discrete meals: Regular insulin q6h.
On a daily basis, adjust scheduled insulin based on previous days' blood sugars:
Add up total insulin given the previous day, including scheduled and supplemental insulin, to determine new total daily insulin requirement.
Adjust total daily insulin requirement based on clinical considerations (eg, give more if marked hyperglycemia, eating more, improving renal function, increasing steroids; give less if eating less, worsening renal function, tapering steroids, recovering from severe illness).
Give 50% of requirement as basal and 50% as nutritional, as above (may need proportionately less nutritional insulin if appetite poor or unknown).
Adjust sliding scale if needed based on total scheduled insulin dose (see step 6, above).
For BG <60 mg/dL:
If patient can take PO, give 15 g of fast acting carbohydrate.
(4 oz fruit juice/nondiet soda, 8 oz nonfat milk, or 34 glucose tablets; not juice plus sugar).
Repeat finger capillary glucose q15 minutes and repeat above if BG <60.
When BG >60, give snack or meal in half an hour.
If patient cannot take PO, give 25 mL of D50 as IV push.
Check finger capillary glucose q15 minutes and repeat above if BG <80.
Discharge orders:
Patient should be discharged home on a medication regimen that was similar to the admission regimen (ie, the regimen prescribed by their PCP). Exceptions include
The patient has a contraindication to an admission medication.
There is evidence of severe hyperglycemia (eg, very high A1C) or hypoglycemia on admission regimen.
If a patient is admitted with no insulin, and requires insulin to be continued as an outpatient (eg, newly‐diagnosed type 1 diabetes, A1C very high, and contraindication to or on maximum oral regimen), limit discharge insulin regimen to no more than 1 injection per day (eg, hs NPH; an exception to this is for type 1 diabetic patients, who are optimally treated with 34 injections/day). Make sure the patient has prompt follow‐up with their primary care provider (PCP).
Avoid discharging home on sliding scale.
If a patient is going to require insulin injections and self‐monitoring blood glucose as an outpatient, make sure they are instructed about how to perform these.
Indications for calling an endocrine consult:
Labile blood sugars.
Prolonged periods of NPO, eg, for procedures, especially in patients with type 1 diabetes
Marked hyperglycemia despite following this guideline.
Question of type 1 versus type 2 versus other type of diabetes. 0
Basil Insulin Guidelines Home Insulin Regimen Starting Dose of Basal Insulin Considerations NOTE: Patients with T1DM require basal insulin at all times! Basal never should be held!
Abbreviations: NPO, nothing by mouth.
On basal (eg, NPH or glargine) insulin at home Patient's home dose of NPH or glargine If NPO, consider starting half of NPH or glargine home dose, unless hyperglycemic at home. Not on basal (eg, NPH or glargine) insulin at home NPH 50% of total daily insulin requirement, given qHS or split qAM/qHS (maximum starting dose 20 units/day) Same dose if patient has previously diagnosed or undiagnosed diabetes Nutritional Insulin Guidelines Type of Nutrition Common Nutritional Regimens Sample Starting Doses Abbreviation: qAM, every morning; qHS, at bed time.
If receiving cycled tube feeds at night, give nutritional NPH qHS only.
Discrete meals Aspart given 015 minutes before meals Home dose, if known or 50% of total insulin requirement, split over 3 meals, may need less if poor or unknown appetite Continuous tube feeding,* IV dextrose NPH qHS or qAM/qHS 50% of total insulin requirement (in addition to basal dose), may need less if not at goal caloric intake Glargine given every day (qd), anytime Regular every 6 hours (q6h) Sample Supplemental/Correctional Insulin Scales Blood Glucose Scheduled Insulin < 40 Units/Day Scheduled Insulin of 4080 Units/Day Scheduled Insulin > 80 Units/Day Individualized NOTE: Avoid supplemental insulin qHS unless patient is very hyperglycemic and obese.
150199 1 unit 1 unit 2 units ____ units 200249 2 units 3 units 4 units ____ units 250299 3 units 5 units 7 units ____ units 300349 4 units 7 units 10 units ____ units >349 5 units + call HO 8 units + call HO 12 units + call HO ___ units + call HO Notes on Oral Agents Agents Considerations Metabolism Sulfonylureas/secretagogues: glyburide, glipizide, glimeperide (Amaryl); repaglinide (Prandin); nateglinide (Starlix) Risk for hypoglycemia Metabolized in liver; Glyburide metabolized to active metabolites; 50% renally eliminated Metformin Contraindicated in heart failure and renal dysfunction (creatinine [Cr] >1.5 mg/dL in men and 1.4 mg/dL in women) Eliminated renally Should be held at time of iodinated contrast studies. (May be restarted after normal postcontrast renal function is confirmed) Adverse effects include diarrhea, nausea, and anorexia Thiazolidinediones: pioglitazone (Actos), rosiglitazone (Avandia) Contraindicated in class III and IV heart failure Metabolized in liver Use with caution in patients with edema Adverse effects include increased intravascular volume Slow onset of action Avoid in hepatic dysfunction Glucosidease inhibitors: acarbose (Precose), miglitol (Glycet) Gastrointestinal intolerance Acarbose eliminated in gut and renally
- ,,,,.Prevalence of hyper‐ and hypoglycemia among inpatients with diabetes: a national survey of 44 U.S. hospitals.Diabetes Care.2007;30:367–369.
- ,,,,,.Hyperglycemia: an independent marker of in‐hospital mortality in patients with undiagnosed diabetes.J Clin Endocrinol Metab.2002;87:978–982.
- ,,, et al.Hyperglycaemia is associated with poor outcomes in patients admitted to hospital with acute exacerbations of chronic obstructive pulmonary disease.Thorax.2006;61:284–289.
- ,,,,.Stress hyperglycemia and prognosis of stroke in nondiabetic and diabetic patients: a systematic overview.Stroke.2001;32:2426–2432.
- ,,,.Hyperglycemia is associated with adverse outcomes in patients receiving total parenteral nutrition.Diabetes Care.2005;28:2367–2371.
- ,,, et al.Management of diabetes and hyperglycemia in hospitals.Diabetes Care.2004;27:553–597.
- ,,,,,.The relation between hyperglycemia and outcomes in 2471 patients admitted to the hospital with community‐acquired pneumonia.Diabetes Care.2005;28:810–815.
- ,,, et al.Intensive insulin therapy in the medical ICU.N Engl J Med.2006;354:449–461.
- ,,, et al.Intensive insulin therapy in the critically ill patients.N Engl J Med.2001;345:1359–1367.
- Standards of medical care in diabetes, 2007.Diabetes Care.2007;30(Suppl 1):S4–S41.
- American College of Endocrinology and American Diabetes Association Consensus statement on inpatient diabetes and glycemic control: a call to action.Diabetes Care.2006;29:1955–1962.
- ,,, et al.Randomized study of basal‐bolus insulin therapy in the inpatient management of patients with type 2 diabetes (RABBIT 2 trial).Diabetes Care.2007;30:2181–2186.
- ,,,.Eliminating inpatient sliding‐scale insulin: a reeducation project with medical house staff.Diabetes Care.2005;28:1008–1011.
- ,,, et al.Inpatient management of hyperglycemia: the Northwestern experience.Endocr Pract.2006;12:491–505.
- ,,, et al.Improving glycemic control in medical inpatients: a pilot study.J Hosp Med.2008;3:55–63.
- ,,.Adapting a clinical comorbidity index for use with ICD‐9‐CM administrative databases.J Clin Epidemiol.1992;45:613–619.
- ,,, et al.“Glucometrics”—assessing the quality of inpatient glucose management.Diabetes Technol Ther.2006;8:560–569.
- ,,,.Hyperglycemia and length of stay in patients hospitalized for bone marrow transplantation.Diabetes Care.2007;30:993–994.
- ,.Financial implications of glycemic control: results of an inpatient diabetes management program.Endocr Pract.2006;12(Suppl 3):43–48.
- ,,, et al.In‐hospital treatment of hyperglycemia: effects of intensified subcutaneous insulin treatment.Curr Med Res Opin.2007;23:757–765.
- ,,,.Impact of endocrine and diabetes team consultation on hospital length of stay for patients with diabetes.Am J Med.1995;99:22–28.
- ,,,.Effect of a standardized insulin order set and an insulin management algorithm on inpatient glycemic control and hypoglycemia. Society of Hospital Medicine Annual Meeting, 2007; Dallas, TX;2007.
- ,,, et al.Trends in bed occupancy for inpatients with diabetes before and after the introduction of a diabetes inpatient specialist nurse service.Diabet Med.2006;23:1008–1015.
- ,,,,.Inpatient management of diabetes and hyperglycemia among general medicine patients at a large teaching hospital.J Hosp Med.2006;1:145–150.
- ,,, et al. Improving glycemic control, preventing hypoglycemia, and optimizing care of the inpatient with hyperglycemia and diabetes, 2006. Available at:http://www.hospitalmedicine.org/ResourceRoomRedesign/html/GC_Imp_Guide.cfm. Accessed October 2008.
- ,,,,.Prevalence of hyper‐ and hypoglycemia among inpatients with diabetes: a national survey of 44 U.S. hospitals.Diabetes Care.2007;30:367–369.
- ,,,,,.Hyperglycemia: an independent marker of in‐hospital mortality in patients with undiagnosed diabetes.J Clin Endocrinol Metab.2002;87:978–982.
- ,,, et al.Hyperglycaemia is associated with poor outcomes in patients admitted to hospital with acute exacerbations of chronic obstructive pulmonary disease.Thorax.2006;61:284–289.
- ,,,,.Stress hyperglycemia and prognosis of stroke in nondiabetic and diabetic patients: a systematic overview.Stroke.2001;32:2426–2432.
- ,,,.Hyperglycemia is associated with adverse outcomes in patients receiving total parenteral nutrition.Diabetes Care.2005;28:2367–2371.
- ,,, et al.Management of diabetes and hyperglycemia in hospitals.Diabetes Care.2004;27:553–597.
- ,,,,,.The relation between hyperglycemia and outcomes in 2471 patients admitted to the hospital with community‐acquired pneumonia.Diabetes Care.2005;28:810–815.
- ,,, et al.Intensive insulin therapy in the medical ICU.N Engl J Med.2006;354:449–461.
- ,,, et al.Intensive insulin therapy in the critically ill patients.N Engl J Med.2001;345:1359–1367.
- Standards of medical care in diabetes, 2007.Diabetes Care.2007;30(Suppl 1):S4–S41.
- American College of Endocrinology and American Diabetes Association Consensus statement on inpatient diabetes and glycemic control: a call to action.Diabetes Care.2006;29:1955–1962.
- ,,, et al.Randomized study of basal‐bolus insulin therapy in the inpatient management of patients with type 2 diabetes (RABBIT 2 trial).Diabetes Care.2007;30:2181–2186.
- ,,,.Eliminating inpatient sliding‐scale insulin: a reeducation project with medical house staff.Diabetes Care.2005;28:1008–1011.
- ,,, et al.Inpatient management of hyperglycemia: the Northwestern experience.Endocr Pract.2006;12:491–505.
- ,,, et al.Improving glycemic control in medical inpatients: a pilot study.J Hosp Med.2008;3:55–63.
- ,,.Adapting a clinical comorbidity index for use with ICD‐9‐CM administrative databases.J Clin Epidemiol.1992;45:613–619.
- ,,, et al.“Glucometrics”—assessing the quality of inpatient glucose management.Diabetes Technol Ther.2006;8:560–569.
- ,,,.Hyperglycemia and length of stay in patients hospitalized for bone marrow transplantation.Diabetes Care.2007;30:993–994.
- ,.Financial implications of glycemic control: results of an inpatient diabetes management program.Endocr Pract.2006;12(Suppl 3):43–48.
- ,,, et al.In‐hospital treatment of hyperglycemia: effects of intensified subcutaneous insulin treatment.Curr Med Res Opin.2007;23:757–765.
- ,,,.Impact of endocrine and diabetes team consultation on hospital length of stay for patients with diabetes.Am J Med.1995;99:22–28.
- ,,,.Effect of a standardized insulin order set and an insulin management algorithm on inpatient glycemic control and hypoglycemia. Society of Hospital Medicine Annual Meeting, 2007; Dallas, TX;2007.
- ,,, et al.Trends in bed occupancy for inpatients with diabetes before and after the introduction of a diabetes inpatient specialist nurse service.Diabet Med.2006;23:1008–1015.
- ,,,,.Inpatient management of diabetes and hyperglycemia among general medicine patients at a large teaching hospital.J Hosp Med.2006;1:145–150.
- ,,, et al. Improving glycemic control, preventing hypoglycemia, and optimizing care of the inpatient with hyperglycemia and diabetes, 2006. Available at:http://www.hospitalmedicine.org/ResourceRoomRedesign/html/GC_Imp_Guide.cfm. Accessed October 2008.
Copyright © 2009 Society of Hospital Medicine
Metrics for Inpatient Glycemic Control
Data collection, analysis, and presentation are key to the success of any hospital glycemic control initiative. Such efforts enable the management team to track improvements in processes and outcomes, make necessary changes to their quality improvement efforts, justify the provision of necessary time and resources, and share their results with others. Reliable metrics for assessing glycemic control and frequency of hypoglycemia are essential to accomplish these tasks and to assess whether interventions result in more benefit than harm. Hypoglycemia metrics must be especially convincing because fear of hypoglycemia remains a major source of clinical inertia, impeding efforts to improve glucose control.
Currently, there are no official standards or guidelines for formulating metrics on the quality of inpatient glycemic control. This creates several problems. First, different metrics vary in their biases and in their responsiveness to change. Thus, use of a poor metric could lead to either a falsely positive or falsely negative impression that a quality improvement intervention is in fact improving glycemic control. Second, the proliferation of different measures and analytical plans in the research and quality improvement literature make it very difficult for hospitals to compare baseline performance, determine need for improvement, and understand which interventions may be most effective.
A related article in this supplement provides the rationale for improved inpatient glycemic control. That article argues that the current state of inpatient glycemic control, with the frequent occurrence of severe hyperglycemia and irrational insulin ordering, cannot be considered acceptable, especially given the large body of data (albeit largely observational) linking hyperglycemia to negative patient outcomes. However, regardless of whether one is an advocate or skeptic of tighter glucose control in the intensive care unit (ICU) and especially the non‐ICU setting, there is no question that standardized, valid, and reliable metrics are needed to compare efforts to improve glycemic control, better understand whether such control actually improves patient care, and closely monitor patient safety.
This article provides a summary of practical suggestions to assess glycemic control, insulin use patterns, and safety (hypoglycemia and severe hyperglycemia). In particular, we discuss the pros and cons of various measurement choices. We conclude with a tiered summary of recommendations for practical metrics that we hope will be useful to individual improvement teams. This article is not a consensus statement but rather a starting place that we hope will begin to standardize measurement across institutions and advance the dialogue on this subject. To more definitely address this problem, we call on the American Association of Clinical Endocrinologists (AACE), American Diabetes Association (ADA), Society of Hospital Medicine (SHM), and others to agree on consensus standards regarding metrics for the quality of inpatient glycemic control.
MEASURING GLYCEMIC CONTROL: GLUCOMETRICS
Glucometrics may be defined as the systematic analysis of blood glucose (BG) dataa phrase initially coined specifically for the inpatient setting. There are numerous ways to do these analyses, depending on which patients and glucose values are considered, the definitions used for hypoglycemia and hyperglycemia, the unit of measurement (eg, patient, patient‐day, individual glucose value), and the measure of control (eg, mean, median, percent of glucose readings within a certain range). We consider each of these dimensions in turn.
Defining the Target Patient Population
The first decision to be made is which patients to include in your analysis. Choices include the following:
-
Patients with a discharge diagnosis of diabetes: this group has face validity and intuitive appeal, is easy to identify retrospectively, and may capture some untested/untreated diabetics, but will miss patients with otherwise undiagnosed diabetes and stress hyperglycemia. It is also subject to the variable accuracy of billing codes.
-
Patients with a certain number of point‐of‐care (POC) glucose measurements: this group is also easy to identify, easy to measure, and will include patients with hyperglycemia without a previous diagnosis of diabetes, but will miss patients with untested/untreated hyperglycemia. Also, if glucose levels are checked on normoglycemic, nondiabetic patients, these values may dilute the overall assessment of glycemic control.
-
Patients treated with insulin in the hospital: this is a good choice if the purpose is mainly drug safety and avoidance of hypoglycemia, but by definition excludes most untreated patients.
-
Patients with 2 or more BG values (laboratory and/or POC) over a certain threshold (eg, >180 mg/dL). This will likely capture more patients with inpatient hyperglycemia, whether or not detected by the medical team, but is subject to wide variations in the frequency and timing of laboratory glucose testing, including whether or not the values are pre‐prandial (note that even preprandial POC glucose measurements are not always in fact fasting values).
Other considerations include the following:
-
Are there natural patient subgroups that should be measured and analyzed separately because of different guidelines? For example, there probably should be separate/emndependent inclusion criteria and analyses for critical care and noncritical care units because their glycemic targets and management considerations differ.
-
Which patients should be excluded? For example, if targeting subcutaneous insulin use in general hospitalized patients, one might eliminate those patients who are admitted specifically as the result of a diabetes emergency (eg, diabetic ketoacidosis [DKA] and hyperglycemic hyperosmolar state [HHS]), as their marked and prolonged hyperglycemia will skew BG data. Pregnant women should generally be excluded from broad‐based analyses or considered as a discrete category because they have very different targets for BG therapy. Patients with short lengths of stay may be less likely to benefit from tight glucose control and may also be considered for post hoc exclusion. One might also exclude patients with very few evaluable glucose readings (eg, fewer than 5) to ensure that measurement is meaningful for a given patient, keeping in mind that this may also exclude patients with undetected hyperglycemia, as mentioned above. Finally, patients receiving palliative care should also be considered for exclusion if feasible.
Recommendation: Do not limit analyses to only those patients with a diagnosis of diabetes or only those on insulin, which will lead to biased results.
-
For noncritical care patients, we recommend a combined approach: adult patients with a diagnosis of diabetes (e. g. using diagnosis‐related group [DRG] codes 294 or 295 or International Classification of Diseases 9th edition [ICD9] codes 250.xx) or with hyperglycemia (eg, 2 or more random laboratory and/or point of care (POC) BG values >180 mg/dL or 2 or more fasting BG values >130 mg/dL), excluding patients with DKA or HHS or who are pregnant.
-
For critical care units, we recommend either all patients, or patients with at least mild hyperglycemia (eg, 2 random glucose levels >140 mg/dL). Critical care patients with DKA, HHS, and pregnancy should be evaluated separately if possible.
Which Glucose Values to Include and Exclude
To answer this question, we first need to decide which method to use for BG measurement. There are several ways to measure BG, including the type of sample collected (capillary [fingerstick], arterial, and venous) and the technique used (central laboratory analyzing plasma, central laboratory analyzing whole blood [eg, from an arterial blood gas sample], glucose meter [usually calibrated to plasma], etc.). The POC (eg, capillary, glucose meter) glucose measurements alone are often preferred in the non‐ICU setting because laboratory plasma values generally provide little additional information and typically lower the mean glucose by including redundant fasting values.1 In critical care units, several different methods are often used together, and each merits inclusion. The inherent differences in calibration between the methods do not generally require separate analyses, especially given the frequency of testing in the ICU setting.
The next question is which values to include in analyses. In some situations, it may be most useful to focus on a certain period of hospitalization, such as the day of a procedure and the next 2 days in assessing the impact of the quality of perioperative care, or the first 14 days of a noncritical care stay to keep outliers for length of stay (LOS) from skewing the data. In the non‐ICU setting, it may be reasonable to exclude the first day of hospitalization, as early BG control is impacted by multiple variables beyond direct control of the clinician (eg, glucose control prior to admission, severity of presenting illness) and may not realistically reflect your interventions. (Keep in mind, however, that it may be useful to adjust for the admission glucose value in multivariable models given its importance to clinical outcomes and its strong relationship to subsequent inpatient glucose control.) However, in critical care units, it is reasonable to include the first day's readings in analyses given the high frequency of glucose measurements in this setting and the expectation that glucose control should be achieved within a few hours of starting an intravenous insulin infusion.
If feasible to do so with your institution's data capture methods, you may wish to select only the regularly scheduled (before each meal [qAC] and at bedtime [qHS], or every 6 hours [q6h]) glucose readings for inclusion in the summary data of glycemic control in the non‐ICU setting, thereby reducing bias caused by repeated measurements around extremes of glycemic excursions. An alternative in the non‐ICU setting is to censor glucose readings within 60 minutes of a previous reading.
Recommendation:
-
In the non‐ICU setting, we recommend first looking at all POC glucose values and if possible repeating the analyses excluding hospital day 1 and hospital day 15 and beyond, and also excluding glucose values measured within 60 minutes of a previous value.
-
In critical care units, we recommend evaluating all glucose readings used to guide care.
Units of Analysis
There are several different units of analysis, each with its own advantages and disadvantages:
-
Glucose value: this is the simplest measure and the one with the most statistical power. All glucose values for all patients of interest comprise the denominator. A report might say, for example, that 1% of the 1000 glucose values were <70 mg/dL during a certain period or that the mean of all glucose values collected for the month from patients in noncritical care areas was 160 mg/dL. The potential disadvantages of this approach are that these analyses are less clinically relevant than patient‐level analyses and that patients with many glucose readings and long hospitalizations may skew the data.
-
Patient (or the Patient Stay, [ie, the entire hospitalization]): all patients who are monitored make up the denominator. The numerator may be the percentage of patients with any hypoglycemia during their hospital stay or the percentage of patients achieving a certain mean glucose during their hospitalization, for example. This is inherently more clinically meaningful than using glucose value as a unit of analysis. A major disadvantage is not controlling for LOS effects. For example, a hospitalized patient with a long LOS is much more likely to be characterized as having at least 1 hypoglycemic value than is a patient with a shorter LOS. Another shortcoming is that this approach does not correct for uneven distribution of testing. A patient's mean glucose might be calculated on the basis of 8 glucose values on the first day of hospitalization, 4 on the second day, and 1 on the third day. Despite all these shortcomings, reporting by patient remains a popular and valid method of presenting glycemic control results, particularly when complemented by other views and refined to control for the number of readings per day.
-
Monitored Patient‐Day: The denominator in this setting is the total number of days a patient glucose level is monitored. The benefits of this method have been described and advocated in the literature.1 As with patient‐level analyses, this measure will be more rigorous and meaningful if the BG measures to be evaluated have been standardized. Typical reports might include percentage of monitored days with any hypoglycemia, or percentage of monitored days with all glucose values in the desired range. This unit of analysis may be considered more difficult to generate and to interpret. On the other hand, it is clinically relevant, less biased by LOS effects, and may be considered the most actionable metric by clinicians. This method provides a good balance when presented with data organized by patient.
The following example uses all 3 units of measurement, in this case to determine the rate of hypoglycemia, demonstrating the different but complementary information that each method provides:
-
In 1 month, 3900 POC glucose measurements were obtained from 286 patients representing 986 monitored patient‐days. With hypoglycemia defined as POC BG 60 mg/dL, the results showed the following:
-
50 of 3900 measurements (1.4%) were hypoglycemic 22 of 286 patients (7.7%) had 1 hypoglycemic episodes
-
40 of 986 monitored days (4.4%) had 1 hypoglycemic episodes.
The metric based on the number of glucose readings could be considered the least clinically relevant because it is unclear how many patients were affected; moreover, it may be based on variable testing patterns among patients, and could be influenced disproportionately by 1 patient with frequent hypoglycemia, many glucose readings, and/or a long LOS. One could argue that the patient‐stay metric is artificially elevated because a single hypoglycemic episode characterizes the entire stay as hypoglycemic. On the other hand, at least it acknowledges the number of patients affected by hypoglycemia. The patient‐day unit of analysis likely provides the most balanced view, one that is clinically relevant and measured over a standard period of time, and less biased by LOS and frequency of testing.
One way to express patient‐day glycemic control that deserves special mention is the patient‐day weighted mean. A mean glucose is calculated for each patient‐day, and then the mean is calculated across all patient‐days. The advantage of this approach is that it corrects for variation in the number of glucose readings each day; all hospital days are weighted equally.
Recommendation:
-
In noncritical care units, we recommend a combination of patient‐day and patient‐stay measures.
-
In critical care units, it is acceptable to also use glucose reading as the unit of measurement given more frequent and uniform data collection, but it should be complemented by more meaningful patient‐day and patient‐stay measures.
Measures of Control
In addition to deciding the unit(s) of analysis, another issue concerns which measures of control to use. These could include rates of hypoglycemia and hyperglycemia, percentage of glucose readings within various ranges (eg, <70, 70180, >180 mg/dL), mean glucose value, percentage of patient‐days during which the mean glucose is within various ranges, or the in control rate (ie, when all glucose values are within a certain range).
As with the various units of analysis, each of these measures of control has various advantages and disadvantages. For example, mean glucose is easy to report and understand, but masks extreme values. Percentage of glucose values within a certain range (eg, per patient, averaged across patients) presents a more complete picture but is a little harder to understand and will vary depending on the frequency of glucose monitoring. As mentioned above, this latter problem can be corrected in part by including only certain glucose values. Percent of glucose values within range may also be less sensitive to change than mean glucose (eg, a glucose that is lowered from 300 mg/dL to 200 mg/dL is still out of range). We recommend choosing a few, but not all, measures of control in order to get a complete picture of glycemic control. Over time one can then refine the measures being used to meet the needs of the glycemic control team and provide data that will drive the performance improvement process.
In critical care and perioperative settings, interest in glycemic control is often more intense around the time of a particular event such as major surgery or after admission to the ICU. Some measures commonly used in performing such analyses are:
-
All values outside a target range within a designated crucial period. For example, the University Healthcare Consortium and other organizations use a simple metric to gauge perioperative glycemic control. They collect the fasting glucose on postoperative days 1 and 2 and then calculate the percentage of postoperative days with any fasting glucose >200 mg/dL. Of course, this is a very liberal target, but it can always be lowered in a stepwise fashion once it is regularly being reached.
-
Three‐day blood glucose average. The Portland group uses the mean glucose of each patient for the period that includes the day of coronary artery bypass graft (CABG) surgery and the following 2 days. The 3‐day BG average (3‐BG) correlates very well with patient outcomes and can serve as a well‐defined target.2 It is likely that use of the 3‐BG would work well in other perioperative/trauma settings and could work in the medical ICU as well, with admission to the ICU as the starting point for calculation of the 3‐BG.
Hyperglycemic Index
Measuring the hyperglycemic index (HGI) is a validated method of summarizing glycemic control of ICU patients.3 It is designed to take into account the sometimes uneven distribution of patient testing. Time is plotted on the x‐axis and glucose values on the y‐axis. The HGI is calculated the area under the curve of glycemic values but above the upper limit of normal (ie, 110 mg/dL). Glucose values in the normal or hypoglycemic range are not included in the AUC. Mortality correlated well with this glycemic index. However, a recent observational study of glucometrics in patients hospitalized with acute myocardial infarction found that the simple mean of each patient's glucose values over the entire hospitalization was as predictive of in‐hospital mortality as the HGI or the time‐averaged glucose (AUC for all glucose values).4 In this study, metrics derived from glucose readings for the entire hospitalization were more predictive than those based on the first 24 or 48 hours or on the admission glucose.
Analyses Describing Change in Glycemic Control Over Time in the Hospital
In the critical care setting, this unit of analysis may be as simple as the mean time to reach the glycemic target on your insulin infusion protocol. On noncritical care wards, it is a bit more challenging to characterize the improvement (or clinical inertia) implied by failure of hyperglycemia to lessen as an inpatient stay progresses. One method is to calculate the mean glucose (or percentage of glucose values in a given range) for each patient on hospital day (HD) 1, and repeat for each HD (up to some reasonable limit, such as 5 or 7 days).
Recommendations:
-
In noncritical units, we recommend a limited set of complementary measures, such as the patient‐day weighted mean glucose, mean percent of glucose readings per patient that are within a certain range, and percentage of patients whose mean glucose is within a certain range on each hospital day.
-
In critical care units, it is often useful to focus measures around a certain critical event such as the 3‐day blood glucose average and to use measures such as the HGI that take advantage of more frequent blood glucose testing.
Definitions of Hyperglycemia and Hypoglycemia
Glucometrics outcomes will obviously depend on the thresholds established for hyperglycemia and hypoglycemia. Many centers define hypoglycemia as 60 mg/dL, whereas the ADA definition, based on physiologic changes that may take place, defines hypoglycemia (at least in the outpatient setting) as 70 mg/dL. Hypoglycemia may be further stratified by severity, with any glucose 40 mg/dL, for instance, defined as severe hypoglycemia.
Similarly, the definition of hyperglycemia (and therefore good control) must also be defined. Based on definitions developed by the ADA and AACE, the state of the medical literature, and current understanding of the pathophysiology of hyperglycemia, thresholds for critical care units include 110 mg/dL, 130 mg/dL, and 140 mg/dL, and options in noncritical care units include 130 mg/dL, 140 mg/dL, and 180 mg/dL. Because these thresholds implicitly assume adverse effects when glucose levels are above them, these levels are subject to revision as data become available confirming the benefits and safety of targeted glycemic control in various settings and patient populations.
Introducing optimal BG targets in a stepped fashion over time should also be considered. Furnary et al.2 have done this in the Portland Project, which tracks glycemic control in cardiac surgery patients receiving intravenous insulin therapy. The initial BG target for this project was <200 mg/dL; it was subsequently lowered stepwise over several years to 150 mg/dL, then to 120 mg/dL, and most recently to 110 mg/dL. This approach allows the safe introduction of targeted glycemic control and promotes acceptance of the concept by physicians and the allied nursing and medical staff.
Recommendations:
-
In noncritical care units, it is reasonable to use 40 mg/dL for severe hypoglycemia, 70 mg/dL for hypoglycemia, 130 mg/dL for fasting hyperglycemia, 180 mg/dL for random or postprandial hyperglycemia, and 300 mg/dL for severe hyperglycemia, keeping in mind that these thresholds are arbitrary. In critical care units, values from 110 mg/dL to 140 mg/dL might be better thresholds for hyperglycemia, but it may take time to safely and effectively move an organization toward these lower targets.
Other Considerations Relative to Glucometrics
Yale Glucometrics Website
The Yale Informatics group has put together a Web‐based resource (
Other Analytic Resources
Commercially available software, such as the RALS system (Medical Automation Systems, Inc., Charlottesville, VA) can gather POC glucose measurements directly from devices and provide real‐time reports of glycemic control, stratified by inpatient unit, using user‐defined targets for hypoglycemia and hyperglycemia. While they are no substitute for a dedicated, on‐site data analyst, such systems can be very useful for smaller hospitals with minimal data or information technology support staff.
APPROACHES TO ANALYSIS: RUN CHARTS
Most conventional clinical trials hold interventions fixed for a period of time and compare results with and without the intervention. For quality improvement studies, this is still a valid way to proceed, especially if studied as a randomized controlled trial. Such methods may be preferred when the clinical question is Does this type of intervention work in general? and the desired output is publication in peer‐reviewed journals so that others can learn about and adopt the intervention to their own institution. A before and after study with a similar analytic approach may also be valid, although concerns about temporal trends and cointerventions potentially compromise the validity of such studies. This approach again assumes that an intervention is held fixed over time such that it is clear what patients received during each time period.
If the desired result is improvement at a given institution (the question is Did we improve care?) then it may be preferable to present results over time using run‐charts. In a run chart, the x‐axis is time and the y‐axis the desired metric, such as patient‐day weighted mean glucose. Points in time when interventions were introduced or modified can be highlighted. Run charts have several advantages over before‐and‐after summaries: they do not require interventions remaining fixed and are more compatible with continuous quality improvement methods, it is easier to see the effect of different aspects of the interventions as they occur, one can get a quicker picture of whether something is working, and it is easier to separate out the impact of the intervention from secular trends. Finally, the use of run charts does not imply the absence of statistical rigor. Run charts with statistical process control (SPC) limits5 can easily convey when the observed time trend is unlikely to be due to chance using prespecified P values. (A full discussion of SPC and other methods to study quality improvement interventions is beyond the scope of this article.)
ASSESSING PATTERNS OF INSULIN USE AND ORDER SET UTILIZATION
Besides measuring the impact of quality improvement interventions on glucose control, it is important to measure processes such as proper insulin use. As mentioned in other articles in this supplement, processes are much more sensitive to change than outcomes. Failure to change processes should lead one to make changes to the intervention.
ICU and Perioperative Settings
For ICU and perioperative settings, the major process measure will likely be use of the insulin infusion order set. Designation of BG levels that trigger insulin infusion in these settings should be agreed upon in advance. The number of patients who meet the predefined glycemic criteria would make up the denominator, and the number of patients on the insulin infusion order set would make up the numerator.
NonCritical Care Units
On noncritical care units, measuring the percentage of subcutaneous insulin regimens that contain a basal insulin is a useful way to monitor the impact of an intervention. A more detailed analysis could examine the percentage of patients on simultaneous basal and nutritional insulin (if applicable). An important measure of clinical inertia is to track the percentage of patients who had changes in their insulin regimens on days after hypoglycemic or hyperglycemic excursions. Another important measure is the frequency with which the standardized order set is being used, analogous to the measure of insulin infusion use in the ICU. A final process measure, indirectly related to insulin use, is the frequency of use of oral diabetes agents, especially by patients for whom their use is contraindicated (eg, patients with congestive heart failure who are on thiazolidinediones and patients with renal insufficiency or receiving intravenous contrast continued on metformin).
OTHER CONSIDERATIONS AND METRICS
Examples of other metrics that can be used to track the success of quality improvement efforts include:
-
Glucose measurement within 8 hours of hospital admission.
-
Glycated hemoglobin (A1C) measurement obtained or available within 30 days of admission to help guide inpatient and especially discharge management.
-
Appropriate glucose testing in patients with diabetes or hyperglycemia (eg, 4 times per day in patients not on insulin infusion protocols, at least until 24 hours of euglycemia is documented).
-
The percentage of patients on insulin with on‐time tray delivery.
-
The timing of subcutaneous insulin administration in relation to glucose testing and nutrition delivery.
-
Documentation of carbohydrate intake among patients who are eating.
-
Satisfaction of physicians and nurses with order sets or protocols, using standard surveys.
-
Physician and nurse knowledge, attitudes, and beliefs about insulin administration, fear of hypoglycemia, treatment of hypoglycemia, and glycemic control in the hospital.
-
Patient satisfaction with their diabetes care in the hospital, including the education they received.
-
Nursing and physician education/certification in insulin prescribing, insulin administration, and other diabetes care issues.
-
Patient outcomes strongly associated with glycemic control, (eg, surgical wound infections, ICU LOS, catheter‐related bloodstream infections).
-
Appropriate treatment and documentation of hypoglycemia (eg, in accordance with hospital policy).
-
Documentation of severe hypoglycemic events through the hospital's adverse events reporting system (these may actually increase as change comes to the organization and as clinical personnel are more attuned to glycemic control).
-
Root causes of hypoglycemic events, which can be used to understand and prevent future events.
-
Appropriate transitions from IV to SC insulin regimens, (eg, starting basal insulin prior to discontinuing infusion in patients who have been on an insulin infusion of at least 2 units/hour or who have a known diagnosis of diabetes or A1C >7).
(Survey instruments and other measurement tools are available from the authors upon request.)
SHM GLYCEMIC CONTROL TASK FORCE SUMMARY RECOMMENDATIONS
The SHM Glycemic Control Task Force is working to develop standardized measures of inpatient glucose control and related indicators to track progress of hospital glycemic control initiatives (see the introduction to this supplement for a description of the charge and membership of this task force). The goals of the Task Force's metrics recommendations (Table 1) are several‐fold: (1) create a set of measurements that are complete but not overly burdensome; (2) create realistic measures that can be applied to institutions with different data management capabilities; and (3) allow for comparison across institutions for benchmarking purposes, evaluation of quality improvement projects, and reporting of results for formal research studies in this field.
| Measurement Issue | NonCritical Care Units | Critical Care Units | ||
|---|---|---|---|---|
| Tier 1 Recommendations | Tier 2 Recommendations | Tier 1 Recommendations | Tier 2 Recommendations | |
| ||||
| Patient inclusion and exclusion criteria | All adult patients with POC glucose testing (sampling acceptable). Exclude patients with DKA or HHS or who are pregnant. | All adult patients with diagnosis of diabetes by ICD‐9 code* or by glucose testing: random glucose (POC or laboratory) >180 mg/dL 2 or fasting glucose >130 mg/dL 2, excluding patients with DKA or HHS or who are pregnant. Additional analysis: exclude patients with <5 evaluable glucose readings, patients with LOS <2 days, or receiving palliative care. | All patients in every critical care unit (sampling acceptable). | Patients with DKA, HHS, or pregnancy in separate analyses. All patients in every critical care unit with random glucose (POC or laboratory) >140 mg/dL 2. |
| Glucose reading inclusion and exclusion criteria | All POC glucose values. | Additional analysis: exclude glucose values on hospital day 1 and on hospital day 15 and after. Additional analysis: exclude glucose values measured within 60 minutes of a previous value. | All POC and other glucose values used to guide care. | |
| Measures of safety | Analysis by patient‐day: Percentage of patient‐days with 1 or more values <40, <70, and >300 mg/dL. | Analysis by patient‐day: Percentage of patient‐days with 1 or more values <40, <70, and >300 mg/dL. | ||
| Measures of glucose control | Analysis by patient‐day: Percentage of patient‐days with mean <140, <180 mg/dL and/or Percentage of patient‐days with all values <180 mg/dL. | Analysis by patient‐day: Patient day‐weighted mean glucose. | Analysis by glucose reading: Percentage of readings <110, <140 mg/dL. | 3‐BG as above for all patients in critical care units.∥ Hyperglycemic index for all patients in critical care units (AUC of glucose values above target). |
| Analysis by patient stay: Percentage of patient stays with mean <140, <180 mg/dL. | Analysis by patient stay: Mean percentage of glucose readings of each patient <180 mg/dL. | Analysis by patient‐day: Percentage of patient‐days with mean <110, <140 mg/dL, and/or Percentage of patient‐days with all values <110, <140 mg/dL. | ||
| Analysis by hospital day: Percentage of patients with mean glucose readings <140, <180 mg/dL by hospital day (days 17). | Analysis by patient stay: 3‐day blood glucose average (3‐BG) for selected perioperative patients: Percentage of patients with 3‐BG <110, <140 mg/dL. Mean time (hours) to reach glycemic target (BG <110 or <140 mg/dL) on insulin infusion. | |||
| Measures of insulin use | Percentage of patients on any subcutaneous insulin that has a scheduled basal insulin component (glargine, NPH, or detemir). | Percentage of patients with at least 2 POC and/or laboratory glucose readings >180 mg/dL who have a scheduled basal insulin component. Percentage of eating patients with hyperglycemia as defined above with scheduled basal insulin and nutritional insulin. Percentage of patients and patient‐days with any changes in insulin orders the day after 2 or more episodes of hypoglycemia or hyperglycemia (ie, <70 or >180 mg/dL). | Percentage of patients with 2 POC or laboratory glucose readings >140 mg/dL placed on insulin infusion protocol. | |
| Other process measures | Glucose measured within 8 hours of hospital admission. | POC glucose testing at least 4 times a day for all patients with diabetes or hyperglycemia as defined above. | Glucose measured within 8 hours of hospital admission. | Appropriateness of hypoglycemia treatment and documentation. |
| A1C measurement obtained or available within 30 days of admission. | Measures of adherence to specific components of management protocol. | Frequency of BG testing (eg, per protocol if on insulin infusion; every 68 hours if not). | Clinical events of severe hypoglycemia reported through the organization's critical events reporting tool. | |
| Appropriateness of hypoglycemia treatment and documentation. | Root causes of hypoglycemia. | |||
| Clinical events of severe hypoglycemia reported through the organization's critical events reporting tool. | Appropriate use of IV‐to‐SC insulin transition protocol. | |||
| Root causes of hypoglycemia. | ||||
For each domain of glycemic management (glycemic control, safety, and insulin use), the task force chose a set of best measures. They are presented as two tiers of measurement standards, depending on the capabilities of the institution and the planned uses of the data. Tier 1 includes measures that, although they do take time and resources to collect, are feasible for most institutions. Tier 2 measures are recommended for hospitals with easy manipulation of electronic sources of data and for reporting quality‐of‐care measures for widespread publication, that is, in the context of a research study. It should be emphasized that these recommendations are only meant as a guide: the actual measures chosen should meet the needs and capabilities of each institution.
We recognize that few data support the recommendations made by this task force, that such data are needed, and that the field of data collection and analysis for hospital glycemic management is rapidly evolving. The hope is to begin the standardization process, promote dialogue in this field, and eventually reach a consensus in collaboration with the ADA, AACE, and other pertinent stakeholders.
CONCLUSIONS
Like the field of inpatient glycemic management itself, the field of devising metrics to measure the quality of inpatient glycemic control is also in its infancy and quickly evolving. One should not be paralyzed by the lack of consensus regarding measurementthe important point is to pick a few complementary metrics and begin the process. The table of recommendations can hopefully serve as a starting point for many institutions, with a focus on efficacy (glycemic control), safety (hypoglycemia), and process (insulin use patterns). As your institution gains experience with measurement and the field evolves, your metrics will likely change. We recommend keeping all process and outcome data in its raw form so that it can be summarized in different ways over time. It is also important not to wait for the perfect data collection tool before beginning to analyze data: sampling and paper processes are acceptable if automated data collection is not yet possible. Eventually, blood glucose meter readings should be downloaded into a central database that interfaces with hospital data repositories so data can be analyzed in conjunction with patient, service, and unit‐level information. Only with a rigorous measurement process can institutions hope to know whether their changes are resulting in improved care for patients.
- ,,, et al.“Glucometrics”—assessing the quality of inpatient glucose management.Diabetes Technol Ther.2006;8:560–569.
- ,,.Effect of hyperglycemia and continuous intravenous insulin infusions on outcomes of cardiac surgical procedures: the Portland Diabetic Project.Endocr Pract.2004;10(suppl 2):21–33.
- ,,.Hyperglycaemic index as a tool to assess glucose control: a retrospective study.Crit Care.2004;8:R122–R127.
- ,,, et al.Glucometrics in patients hospitalized with acute myocardial infarction: defining the optimal outcomes‐based measure of risk.Circulation.2008;117:1018–1027.
- ,,.Statistical process control as a tool for research and healthcare improvement.Qual Saf Health Care.2003;12:458–464.
Data collection, analysis, and presentation are key to the success of any hospital glycemic control initiative. Such efforts enable the management team to track improvements in processes and outcomes, make necessary changes to their quality improvement efforts, justify the provision of necessary time and resources, and share their results with others. Reliable metrics for assessing glycemic control and frequency of hypoglycemia are essential to accomplish these tasks and to assess whether interventions result in more benefit than harm. Hypoglycemia metrics must be especially convincing because fear of hypoglycemia remains a major source of clinical inertia, impeding efforts to improve glucose control.
Currently, there are no official standards or guidelines for formulating metrics on the quality of inpatient glycemic control. This creates several problems. First, different metrics vary in their biases and in their responsiveness to change. Thus, use of a poor metric could lead to either a falsely positive or falsely negative impression that a quality improvement intervention is in fact improving glycemic control. Second, the proliferation of different measures and analytical plans in the research and quality improvement literature make it very difficult for hospitals to compare baseline performance, determine need for improvement, and understand which interventions may be most effective.
A related article in this supplement provides the rationale for improved inpatient glycemic control. That article argues that the current state of inpatient glycemic control, with the frequent occurrence of severe hyperglycemia and irrational insulin ordering, cannot be considered acceptable, especially given the large body of data (albeit largely observational) linking hyperglycemia to negative patient outcomes. However, regardless of whether one is an advocate or skeptic of tighter glucose control in the intensive care unit (ICU) and especially the non‐ICU setting, there is no question that standardized, valid, and reliable metrics are needed to compare efforts to improve glycemic control, better understand whether such control actually improves patient care, and closely monitor patient safety.
This article provides a summary of practical suggestions to assess glycemic control, insulin use patterns, and safety (hypoglycemia and severe hyperglycemia). In particular, we discuss the pros and cons of various measurement choices. We conclude with a tiered summary of recommendations for practical metrics that we hope will be useful to individual improvement teams. This article is not a consensus statement but rather a starting place that we hope will begin to standardize measurement across institutions and advance the dialogue on this subject. To more definitely address this problem, we call on the American Association of Clinical Endocrinologists (AACE), American Diabetes Association (ADA), Society of Hospital Medicine (SHM), and others to agree on consensus standards regarding metrics for the quality of inpatient glycemic control.
MEASURING GLYCEMIC CONTROL: GLUCOMETRICS
Glucometrics may be defined as the systematic analysis of blood glucose (BG) dataa phrase initially coined specifically for the inpatient setting. There are numerous ways to do these analyses, depending on which patients and glucose values are considered, the definitions used for hypoglycemia and hyperglycemia, the unit of measurement (eg, patient, patient‐day, individual glucose value), and the measure of control (eg, mean, median, percent of glucose readings within a certain range). We consider each of these dimensions in turn.
Defining the Target Patient Population
The first decision to be made is which patients to include in your analysis. Choices include the following:
-
Patients with a discharge diagnosis of diabetes: this group has face validity and intuitive appeal, is easy to identify retrospectively, and may capture some untested/untreated diabetics, but will miss patients with otherwise undiagnosed diabetes and stress hyperglycemia. It is also subject to the variable accuracy of billing codes.
-
Patients with a certain number of point‐of‐care (POC) glucose measurements: this group is also easy to identify, easy to measure, and will include patients with hyperglycemia without a previous diagnosis of diabetes, but will miss patients with untested/untreated hyperglycemia. Also, if glucose levels are checked on normoglycemic, nondiabetic patients, these values may dilute the overall assessment of glycemic control.
-
Patients treated with insulin in the hospital: this is a good choice if the purpose is mainly drug safety and avoidance of hypoglycemia, but by definition excludes most untreated patients.
-
Patients with 2 or more BG values (laboratory and/or POC) over a certain threshold (eg, >180 mg/dL). This will likely capture more patients with inpatient hyperglycemia, whether or not detected by the medical team, but is subject to wide variations in the frequency and timing of laboratory glucose testing, including whether or not the values are pre‐prandial (note that even preprandial POC glucose measurements are not always in fact fasting values).
Other considerations include the following:
-
Are there natural patient subgroups that should be measured and analyzed separately because of different guidelines? For example, there probably should be separate/emndependent inclusion criteria and analyses for critical care and noncritical care units because their glycemic targets and management considerations differ.
-
Which patients should be excluded? For example, if targeting subcutaneous insulin use in general hospitalized patients, one might eliminate those patients who are admitted specifically as the result of a diabetes emergency (eg, diabetic ketoacidosis [DKA] and hyperglycemic hyperosmolar state [HHS]), as their marked and prolonged hyperglycemia will skew BG data. Pregnant women should generally be excluded from broad‐based analyses or considered as a discrete category because they have very different targets for BG therapy. Patients with short lengths of stay may be less likely to benefit from tight glucose control and may also be considered for post hoc exclusion. One might also exclude patients with very few evaluable glucose readings (eg, fewer than 5) to ensure that measurement is meaningful for a given patient, keeping in mind that this may also exclude patients with undetected hyperglycemia, as mentioned above. Finally, patients receiving palliative care should also be considered for exclusion if feasible.
Recommendation: Do not limit analyses to only those patients with a diagnosis of diabetes or only those on insulin, which will lead to biased results.
-
For noncritical care patients, we recommend a combined approach: adult patients with a diagnosis of diabetes (e. g. using diagnosis‐related group [DRG] codes 294 or 295 or International Classification of Diseases 9th edition [ICD9] codes 250.xx) or with hyperglycemia (eg, 2 or more random laboratory and/or point of care (POC) BG values >180 mg/dL or 2 or more fasting BG values >130 mg/dL), excluding patients with DKA or HHS or who are pregnant.
-
For critical care units, we recommend either all patients, or patients with at least mild hyperglycemia (eg, 2 random glucose levels >140 mg/dL). Critical care patients with DKA, HHS, and pregnancy should be evaluated separately if possible.
Which Glucose Values to Include and Exclude
To answer this question, we first need to decide which method to use for BG measurement. There are several ways to measure BG, including the type of sample collected (capillary [fingerstick], arterial, and venous) and the technique used (central laboratory analyzing plasma, central laboratory analyzing whole blood [eg, from an arterial blood gas sample], glucose meter [usually calibrated to plasma], etc.). The POC (eg, capillary, glucose meter) glucose measurements alone are often preferred in the non‐ICU setting because laboratory plasma values generally provide little additional information and typically lower the mean glucose by including redundant fasting values.1 In critical care units, several different methods are often used together, and each merits inclusion. The inherent differences in calibration between the methods do not generally require separate analyses, especially given the frequency of testing in the ICU setting.
The next question is which values to include in analyses. In some situations, it may be most useful to focus on a certain period of hospitalization, such as the day of a procedure and the next 2 days in assessing the impact of the quality of perioperative care, or the first 14 days of a noncritical care stay to keep outliers for length of stay (LOS) from skewing the data. In the non‐ICU setting, it may be reasonable to exclude the first day of hospitalization, as early BG control is impacted by multiple variables beyond direct control of the clinician (eg, glucose control prior to admission, severity of presenting illness) and may not realistically reflect your interventions. (Keep in mind, however, that it may be useful to adjust for the admission glucose value in multivariable models given its importance to clinical outcomes and its strong relationship to subsequent inpatient glucose control.) However, in critical care units, it is reasonable to include the first day's readings in analyses given the high frequency of glucose measurements in this setting and the expectation that glucose control should be achieved within a few hours of starting an intravenous insulin infusion.
If feasible to do so with your institution's data capture methods, you may wish to select only the regularly scheduled (before each meal [qAC] and at bedtime [qHS], or every 6 hours [q6h]) glucose readings for inclusion in the summary data of glycemic control in the non‐ICU setting, thereby reducing bias caused by repeated measurements around extremes of glycemic excursions. An alternative in the non‐ICU setting is to censor glucose readings within 60 minutes of a previous reading.
Recommendation:
-
In the non‐ICU setting, we recommend first looking at all POC glucose values and if possible repeating the analyses excluding hospital day 1 and hospital day 15 and beyond, and also excluding glucose values measured within 60 minutes of a previous value.
-
In critical care units, we recommend evaluating all glucose readings used to guide care.
Units of Analysis
There are several different units of analysis, each with its own advantages and disadvantages:
-
Glucose value: this is the simplest measure and the one with the most statistical power. All glucose values for all patients of interest comprise the denominator. A report might say, for example, that 1% of the 1000 glucose values were <70 mg/dL during a certain period or that the mean of all glucose values collected for the month from patients in noncritical care areas was 160 mg/dL. The potential disadvantages of this approach are that these analyses are less clinically relevant than patient‐level analyses and that patients with many glucose readings and long hospitalizations may skew the data.
-
Patient (or the Patient Stay, [ie, the entire hospitalization]): all patients who are monitored make up the denominator. The numerator may be the percentage of patients with any hypoglycemia during their hospital stay or the percentage of patients achieving a certain mean glucose during their hospitalization, for example. This is inherently more clinically meaningful than using glucose value as a unit of analysis. A major disadvantage is not controlling for LOS effects. For example, a hospitalized patient with a long LOS is much more likely to be characterized as having at least 1 hypoglycemic value than is a patient with a shorter LOS. Another shortcoming is that this approach does not correct for uneven distribution of testing. A patient's mean glucose might be calculated on the basis of 8 glucose values on the first day of hospitalization, 4 on the second day, and 1 on the third day. Despite all these shortcomings, reporting by patient remains a popular and valid method of presenting glycemic control results, particularly when complemented by other views and refined to control for the number of readings per day.
-
Monitored Patient‐Day: The denominator in this setting is the total number of days a patient glucose level is monitored. The benefits of this method have been described and advocated in the literature.1 As with patient‐level analyses, this measure will be more rigorous and meaningful if the BG measures to be evaluated have been standardized. Typical reports might include percentage of monitored days with any hypoglycemia, or percentage of monitored days with all glucose values in the desired range. This unit of analysis may be considered more difficult to generate and to interpret. On the other hand, it is clinically relevant, less biased by LOS effects, and may be considered the most actionable metric by clinicians. This method provides a good balance when presented with data organized by patient.
The following example uses all 3 units of measurement, in this case to determine the rate of hypoglycemia, demonstrating the different but complementary information that each method provides:
-
In 1 month, 3900 POC glucose measurements were obtained from 286 patients representing 986 monitored patient‐days. With hypoglycemia defined as POC BG 60 mg/dL, the results showed the following:
-
50 of 3900 measurements (1.4%) were hypoglycemic 22 of 286 patients (7.7%) had 1 hypoglycemic episodes
-
40 of 986 monitored days (4.4%) had 1 hypoglycemic episodes.
The metric based on the number of glucose readings could be considered the least clinically relevant because it is unclear how many patients were affected; moreover, it may be based on variable testing patterns among patients, and could be influenced disproportionately by 1 patient with frequent hypoglycemia, many glucose readings, and/or a long LOS. One could argue that the patient‐stay metric is artificially elevated because a single hypoglycemic episode characterizes the entire stay as hypoglycemic. On the other hand, at least it acknowledges the number of patients affected by hypoglycemia. The patient‐day unit of analysis likely provides the most balanced view, one that is clinically relevant and measured over a standard period of time, and less biased by LOS and frequency of testing.
One way to express patient‐day glycemic control that deserves special mention is the patient‐day weighted mean. A mean glucose is calculated for each patient‐day, and then the mean is calculated across all patient‐days. The advantage of this approach is that it corrects for variation in the number of glucose readings each day; all hospital days are weighted equally.
Recommendation:
-
In noncritical care units, we recommend a combination of patient‐day and patient‐stay measures.
-
In critical care units, it is acceptable to also use glucose reading as the unit of measurement given more frequent and uniform data collection, but it should be complemented by more meaningful patient‐day and patient‐stay measures.
Measures of Control
In addition to deciding the unit(s) of analysis, another issue concerns which measures of control to use. These could include rates of hypoglycemia and hyperglycemia, percentage of glucose readings within various ranges (eg, <70, 70180, >180 mg/dL), mean glucose value, percentage of patient‐days during which the mean glucose is within various ranges, or the in control rate (ie, when all glucose values are within a certain range).
As with the various units of analysis, each of these measures of control has various advantages and disadvantages. For example, mean glucose is easy to report and understand, but masks extreme values. Percentage of glucose values within a certain range (eg, per patient, averaged across patients) presents a more complete picture but is a little harder to understand and will vary depending on the frequency of glucose monitoring. As mentioned above, this latter problem can be corrected in part by including only certain glucose values. Percent of glucose values within range may also be less sensitive to change than mean glucose (eg, a glucose that is lowered from 300 mg/dL to 200 mg/dL is still out of range). We recommend choosing a few, but not all, measures of control in order to get a complete picture of glycemic control. Over time one can then refine the measures being used to meet the needs of the glycemic control team and provide data that will drive the performance improvement process.
In critical care and perioperative settings, interest in glycemic control is often more intense around the time of a particular event such as major surgery or after admission to the ICU. Some measures commonly used in performing such analyses are:
-
All values outside a target range within a designated crucial period. For example, the University Healthcare Consortium and other organizations use a simple metric to gauge perioperative glycemic control. They collect the fasting glucose on postoperative days 1 and 2 and then calculate the percentage of postoperative days with any fasting glucose >200 mg/dL. Of course, this is a very liberal target, but it can always be lowered in a stepwise fashion once it is regularly being reached.
-
Three‐day blood glucose average. The Portland group uses the mean glucose of each patient for the period that includes the day of coronary artery bypass graft (CABG) surgery and the following 2 days. The 3‐day BG average (3‐BG) correlates very well with patient outcomes and can serve as a well‐defined target.2 It is likely that use of the 3‐BG would work well in other perioperative/trauma settings and could work in the medical ICU as well, with admission to the ICU as the starting point for calculation of the 3‐BG.
Hyperglycemic Index
Measuring the hyperglycemic index (HGI) is a validated method of summarizing glycemic control of ICU patients.3 It is designed to take into account the sometimes uneven distribution of patient testing. Time is plotted on the x‐axis and glucose values on the y‐axis. The HGI is calculated the area under the curve of glycemic values but above the upper limit of normal (ie, 110 mg/dL). Glucose values in the normal or hypoglycemic range are not included in the AUC. Mortality correlated well with this glycemic index. However, a recent observational study of glucometrics in patients hospitalized with acute myocardial infarction found that the simple mean of each patient's glucose values over the entire hospitalization was as predictive of in‐hospital mortality as the HGI or the time‐averaged glucose (AUC for all glucose values).4 In this study, metrics derived from glucose readings for the entire hospitalization were more predictive than those based on the first 24 or 48 hours or on the admission glucose.
Analyses Describing Change in Glycemic Control Over Time in the Hospital
In the critical care setting, this unit of analysis may be as simple as the mean time to reach the glycemic target on your insulin infusion protocol. On noncritical care wards, it is a bit more challenging to characterize the improvement (or clinical inertia) implied by failure of hyperglycemia to lessen as an inpatient stay progresses. One method is to calculate the mean glucose (or percentage of glucose values in a given range) for each patient on hospital day (HD) 1, and repeat for each HD (up to some reasonable limit, such as 5 or 7 days).
Recommendations:
-
In noncritical units, we recommend a limited set of complementary measures, such as the patient‐day weighted mean glucose, mean percent of glucose readings per patient that are within a certain range, and percentage of patients whose mean glucose is within a certain range on each hospital day.
-
In critical care units, it is often useful to focus measures around a certain critical event such as the 3‐day blood glucose average and to use measures such as the HGI that take advantage of more frequent blood glucose testing.
Definitions of Hyperglycemia and Hypoglycemia
Glucometrics outcomes will obviously depend on the thresholds established for hyperglycemia and hypoglycemia. Many centers define hypoglycemia as 60 mg/dL, whereas the ADA definition, based on physiologic changes that may take place, defines hypoglycemia (at least in the outpatient setting) as 70 mg/dL. Hypoglycemia may be further stratified by severity, with any glucose 40 mg/dL, for instance, defined as severe hypoglycemia.
Similarly, the definition of hyperglycemia (and therefore good control) must also be defined. Based on definitions developed by the ADA and AACE, the state of the medical literature, and current understanding of the pathophysiology of hyperglycemia, thresholds for critical care units include 110 mg/dL, 130 mg/dL, and 140 mg/dL, and options in noncritical care units include 130 mg/dL, 140 mg/dL, and 180 mg/dL. Because these thresholds implicitly assume adverse effects when glucose levels are above them, these levels are subject to revision as data become available confirming the benefits and safety of targeted glycemic control in various settings and patient populations.
Introducing optimal BG targets in a stepped fashion over time should also be considered. Furnary et al.2 have done this in the Portland Project, which tracks glycemic control in cardiac surgery patients receiving intravenous insulin therapy. The initial BG target for this project was <200 mg/dL; it was subsequently lowered stepwise over several years to 150 mg/dL, then to 120 mg/dL, and most recently to 110 mg/dL. This approach allows the safe introduction of targeted glycemic control and promotes acceptance of the concept by physicians and the allied nursing and medical staff.
Recommendations:
-
In noncritical care units, it is reasonable to use 40 mg/dL for severe hypoglycemia, 70 mg/dL for hypoglycemia, 130 mg/dL for fasting hyperglycemia, 180 mg/dL for random or postprandial hyperglycemia, and 300 mg/dL for severe hyperglycemia, keeping in mind that these thresholds are arbitrary. In critical care units, values from 110 mg/dL to 140 mg/dL might be better thresholds for hyperglycemia, but it may take time to safely and effectively move an organization toward these lower targets.
Other Considerations Relative to Glucometrics
Yale Glucometrics Website
The Yale Informatics group has put together a Web‐based resource (
Other Analytic Resources
Commercially available software, such as the RALS system (Medical Automation Systems, Inc., Charlottesville, VA) can gather POC glucose measurements directly from devices and provide real‐time reports of glycemic control, stratified by inpatient unit, using user‐defined targets for hypoglycemia and hyperglycemia. While they are no substitute for a dedicated, on‐site data analyst, such systems can be very useful for smaller hospitals with minimal data or information technology support staff.
APPROACHES TO ANALYSIS: RUN CHARTS
Most conventional clinical trials hold interventions fixed for a period of time and compare results with and without the intervention. For quality improvement studies, this is still a valid way to proceed, especially if studied as a randomized controlled trial. Such methods may be preferred when the clinical question is Does this type of intervention work in general? and the desired output is publication in peer‐reviewed journals so that others can learn about and adopt the intervention to their own institution. A before and after study with a similar analytic approach may also be valid, although concerns about temporal trends and cointerventions potentially compromise the validity of such studies. This approach again assumes that an intervention is held fixed over time such that it is clear what patients received during each time period.
If the desired result is improvement at a given institution (the question is Did we improve care?) then it may be preferable to present results over time using run‐charts. In a run chart, the x‐axis is time and the y‐axis the desired metric, such as patient‐day weighted mean glucose. Points in time when interventions were introduced or modified can be highlighted. Run charts have several advantages over before‐and‐after summaries: they do not require interventions remaining fixed and are more compatible with continuous quality improvement methods, it is easier to see the effect of different aspects of the interventions as they occur, one can get a quicker picture of whether something is working, and it is easier to separate out the impact of the intervention from secular trends. Finally, the use of run charts does not imply the absence of statistical rigor. Run charts with statistical process control (SPC) limits5 can easily convey when the observed time trend is unlikely to be due to chance using prespecified P values. (A full discussion of SPC and other methods to study quality improvement interventions is beyond the scope of this article.)
ASSESSING PATTERNS OF INSULIN USE AND ORDER SET UTILIZATION
Besides measuring the impact of quality improvement interventions on glucose control, it is important to measure processes such as proper insulin use. As mentioned in other articles in this supplement, processes are much more sensitive to change than outcomes. Failure to change processes should lead one to make changes to the intervention.
ICU and Perioperative Settings
For ICU and perioperative settings, the major process measure will likely be use of the insulin infusion order set. Designation of BG levels that trigger insulin infusion in these settings should be agreed upon in advance. The number of patients who meet the predefined glycemic criteria would make up the denominator, and the number of patients on the insulin infusion order set would make up the numerator.
NonCritical Care Units
On noncritical care units, measuring the percentage of subcutaneous insulin regimens that contain a basal insulin is a useful way to monitor the impact of an intervention. A more detailed analysis could examine the percentage of patients on simultaneous basal and nutritional insulin (if applicable). An important measure of clinical inertia is to track the percentage of patients who had changes in their insulin regimens on days after hypoglycemic or hyperglycemic excursions. Another important measure is the frequency with which the standardized order set is being used, analogous to the measure of insulin infusion use in the ICU. A final process measure, indirectly related to insulin use, is the frequency of use of oral diabetes agents, especially by patients for whom their use is contraindicated (eg, patients with congestive heart failure who are on thiazolidinediones and patients with renal insufficiency or receiving intravenous contrast continued on metformin).
OTHER CONSIDERATIONS AND METRICS
Examples of other metrics that can be used to track the success of quality improvement efforts include:
-
Glucose measurement within 8 hours of hospital admission.
-
Glycated hemoglobin (A1C) measurement obtained or available within 30 days of admission to help guide inpatient and especially discharge management.
-
Appropriate glucose testing in patients with diabetes or hyperglycemia (eg, 4 times per day in patients not on insulin infusion protocols, at least until 24 hours of euglycemia is documented).
-
The percentage of patients on insulin with on‐time tray delivery.
-
The timing of subcutaneous insulin administration in relation to glucose testing and nutrition delivery.
-
Documentation of carbohydrate intake among patients who are eating.
-
Satisfaction of physicians and nurses with order sets or protocols, using standard surveys.
-
Physician and nurse knowledge, attitudes, and beliefs about insulin administration, fear of hypoglycemia, treatment of hypoglycemia, and glycemic control in the hospital.
-
Patient satisfaction with their diabetes care in the hospital, including the education they received.
-
Nursing and physician education/certification in insulin prescribing, insulin administration, and other diabetes care issues.
-
Patient outcomes strongly associated with glycemic control, (eg, surgical wound infections, ICU LOS, catheter‐related bloodstream infections).
-
Appropriate treatment and documentation of hypoglycemia (eg, in accordance with hospital policy).
-
Documentation of severe hypoglycemic events through the hospital's adverse events reporting system (these may actually increase as change comes to the organization and as clinical personnel are more attuned to glycemic control).
-
Root causes of hypoglycemic events, which can be used to understand and prevent future events.
-
Appropriate transitions from IV to SC insulin regimens, (eg, starting basal insulin prior to discontinuing infusion in patients who have been on an insulin infusion of at least 2 units/hour or who have a known diagnosis of diabetes or A1C >7).
(Survey instruments and other measurement tools are available from the authors upon request.)
SHM GLYCEMIC CONTROL TASK FORCE SUMMARY RECOMMENDATIONS
The SHM Glycemic Control Task Force is working to develop standardized measures of inpatient glucose control and related indicators to track progress of hospital glycemic control initiatives (see the introduction to this supplement for a description of the charge and membership of this task force). The goals of the Task Force's metrics recommendations (Table 1) are several‐fold: (1) create a set of measurements that are complete but not overly burdensome; (2) create realistic measures that can be applied to institutions with different data management capabilities; and (3) allow for comparison across institutions for benchmarking purposes, evaluation of quality improvement projects, and reporting of results for formal research studies in this field.
| Measurement Issue | NonCritical Care Units | Critical Care Units | ||
|---|---|---|---|---|
| Tier 1 Recommendations | Tier 2 Recommendations | Tier 1 Recommendations | Tier 2 Recommendations | |
| ||||
| Patient inclusion and exclusion criteria | All adult patients with POC glucose testing (sampling acceptable). Exclude patients with DKA or HHS or who are pregnant. | All adult patients with diagnosis of diabetes by ICD‐9 code* or by glucose testing: random glucose (POC or laboratory) >180 mg/dL 2 or fasting glucose >130 mg/dL 2, excluding patients with DKA or HHS or who are pregnant. Additional analysis: exclude patients with <5 evaluable glucose readings, patients with LOS <2 days, or receiving palliative care. | All patients in every critical care unit (sampling acceptable). | Patients with DKA, HHS, or pregnancy in separate analyses. All patients in every critical care unit with random glucose (POC or laboratory) >140 mg/dL 2. |
| Glucose reading inclusion and exclusion criteria | All POC glucose values. | Additional analysis: exclude glucose values on hospital day 1 and on hospital day 15 and after. Additional analysis: exclude glucose values measured within 60 minutes of a previous value. | All POC and other glucose values used to guide care. | |
| Measures of safety | Analysis by patient‐day: Percentage of patient‐days with 1 or more values <40, <70, and >300 mg/dL. | Analysis by patient‐day: Percentage of patient‐days with 1 or more values <40, <70, and >300 mg/dL. | ||
| Measures of glucose control | Analysis by patient‐day: Percentage of patient‐days with mean <140, <180 mg/dL and/or Percentage of patient‐days with all values <180 mg/dL. | Analysis by patient‐day: Patient day‐weighted mean glucose. | Analysis by glucose reading: Percentage of readings <110, <140 mg/dL. | 3‐BG as above for all patients in critical care units.∥ Hyperglycemic index for all patients in critical care units (AUC of glucose values above target). |
| Analysis by patient stay: Percentage of patient stays with mean <140, <180 mg/dL. | Analysis by patient stay: Mean percentage of glucose readings of each patient <180 mg/dL. | Analysis by patient‐day: Percentage of patient‐days with mean <110, <140 mg/dL, and/or Percentage of patient‐days with all values <110, <140 mg/dL. | ||
| Analysis by hospital day: Percentage of patients with mean glucose readings <140, <180 mg/dL by hospital day (days 17). | Analysis by patient stay: 3‐day blood glucose average (3‐BG) for selected perioperative patients: Percentage of patients with 3‐BG <110, <140 mg/dL. Mean time (hours) to reach glycemic target (BG <110 or <140 mg/dL) on insulin infusion. | |||
| Measures of insulin use | Percentage of patients on any subcutaneous insulin that has a scheduled basal insulin component (glargine, NPH, or detemir). | Percentage of patients with at least 2 POC and/or laboratory glucose readings >180 mg/dL who have a scheduled basal insulin component. Percentage of eating patients with hyperglycemia as defined above with scheduled basal insulin and nutritional insulin. Percentage of patients and patient‐days with any changes in insulin orders the day after 2 or more episodes of hypoglycemia or hyperglycemia (ie, <70 or >180 mg/dL). | Percentage of patients with 2 POC or laboratory glucose readings >140 mg/dL placed on insulin infusion protocol. | |
| Other process measures | Glucose measured within 8 hours of hospital admission. | POC glucose testing at least 4 times a day for all patients with diabetes or hyperglycemia as defined above. | Glucose measured within 8 hours of hospital admission. | Appropriateness of hypoglycemia treatment and documentation. |
| A1C measurement obtained or available within 30 days of admission. | Measures of adherence to specific components of management protocol. | Frequency of BG testing (eg, per protocol if on insulin infusion; every 68 hours if not). | Clinical events of severe hypoglycemia reported through the organization's critical events reporting tool. | |
| Appropriateness of hypoglycemia treatment and documentation. | Root causes of hypoglycemia. | |||
| Clinical events of severe hypoglycemia reported through the organization's critical events reporting tool. | Appropriate use of IV‐to‐SC insulin transition protocol. | |||
| Root causes of hypoglycemia. | ||||
For each domain of glycemic management (glycemic control, safety, and insulin use), the task force chose a set of best measures. They are presented as two tiers of measurement standards, depending on the capabilities of the institution and the planned uses of the data. Tier 1 includes measures that, although they do take time and resources to collect, are feasible for most institutions. Tier 2 measures are recommended for hospitals with easy manipulation of electronic sources of data and for reporting quality‐of‐care measures for widespread publication, that is, in the context of a research study. It should be emphasized that these recommendations are only meant as a guide: the actual measures chosen should meet the needs and capabilities of each institution.
We recognize that few data support the recommendations made by this task force, that such data are needed, and that the field of data collection and analysis for hospital glycemic management is rapidly evolving. The hope is to begin the standardization process, promote dialogue in this field, and eventually reach a consensus in collaboration with the ADA, AACE, and other pertinent stakeholders.
CONCLUSIONS
Like the field of inpatient glycemic management itself, the field of devising metrics to measure the quality of inpatient glycemic control is also in its infancy and quickly evolving. One should not be paralyzed by the lack of consensus regarding measurementthe important point is to pick a few complementary metrics and begin the process. The table of recommendations can hopefully serve as a starting point for many institutions, with a focus on efficacy (glycemic control), safety (hypoglycemia), and process (insulin use patterns). As your institution gains experience with measurement and the field evolves, your metrics will likely change. We recommend keeping all process and outcome data in its raw form so that it can be summarized in different ways over time. It is also important not to wait for the perfect data collection tool before beginning to analyze data: sampling and paper processes are acceptable if automated data collection is not yet possible. Eventually, blood glucose meter readings should be downloaded into a central database that interfaces with hospital data repositories so data can be analyzed in conjunction with patient, service, and unit‐level information. Only with a rigorous measurement process can institutions hope to know whether their changes are resulting in improved care for patients.
Data collection, analysis, and presentation are key to the success of any hospital glycemic control initiative. Such efforts enable the management team to track improvements in processes and outcomes, make necessary changes to their quality improvement efforts, justify the provision of necessary time and resources, and share their results with others. Reliable metrics for assessing glycemic control and frequency of hypoglycemia are essential to accomplish these tasks and to assess whether interventions result in more benefit than harm. Hypoglycemia metrics must be especially convincing because fear of hypoglycemia remains a major source of clinical inertia, impeding efforts to improve glucose control.
Currently, there are no official standards or guidelines for formulating metrics on the quality of inpatient glycemic control. This creates several problems. First, different metrics vary in their biases and in their responsiveness to change. Thus, use of a poor metric could lead to either a falsely positive or falsely negative impression that a quality improvement intervention is in fact improving glycemic control. Second, the proliferation of different measures and analytical plans in the research and quality improvement literature make it very difficult for hospitals to compare baseline performance, determine need for improvement, and understand which interventions may be most effective.
A related article in this supplement provides the rationale for improved inpatient glycemic control. That article argues that the current state of inpatient glycemic control, with the frequent occurrence of severe hyperglycemia and irrational insulin ordering, cannot be considered acceptable, especially given the large body of data (albeit largely observational) linking hyperglycemia to negative patient outcomes. However, regardless of whether one is an advocate or skeptic of tighter glucose control in the intensive care unit (ICU) and especially the non‐ICU setting, there is no question that standardized, valid, and reliable metrics are needed to compare efforts to improve glycemic control, better understand whether such control actually improves patient care, and closely monitor patient safety.
This article provides a summary of practical suggestions to assess glycemic control, insulin use patterns, and safety (hypoglycemia and severe hyperglycemia). In particular, we discuss the pros and cons of various measurement choices. We conclude with a tiered summary of recommendations for practical metrics that we hope will be useful to individual improvement teams. This article is not a consensus statement but rather a starting place that we hope will begin to standardize measurement across institutions and advance the dialogue on this subject. To more definitely address this problem, we call on the American Association of Clinical Endocrinologists (AACE), American Diabetes Association (ADA), Society of Hospital Medicine (SHM), and others to agree on consensus standards regarding metrics for the quality of inpatient glycemic control.
MEASURING GLYCEMIC CONTROL: GLUCOMETRICS
Glucometrics may be defined as the systematic analysis of blood glucose (BG) dataa phrase initially coined specifically for the inpatient setting. There are numerous ways to do these analyses, depending on which patients and glucose values are considered, the definitions used for hypoglycemia and hyperglycemia, the unit of measurement (eg, patient, patient‐day, individual glucose value), and the measure of control (eg, mean, median, percent of glucose readings within a certain range). We consider each of these dimensions in turn.
Defining the Target Patient Population
The first decision to be made is which patients to include in your analysis. Choices include the following:
-
Patients with a discharge diagnosis of diabetes: this group has face validity and intuitive appeal, is easy to identify retrospectively, and may capture some untested/untreated diabetics, but will miss patients with otherwise undiagnosed diabetes and stress hyperglycemia. It is also subject to the variable accuracy of billing codes.
-
Patients with a certain number of point‐of‐care (POC) glucose measurements: this group is also easy to identify, easy to measure, and will include patients with hyperglycemia without a previous diagnosis of diabetes, but will miss patients with untested/untreated hyperglycemia. Also, if glucose levels are checked on normoglycemic, nondiabetic patients, these values may dilute the overall assessment of glycemic control.
-
Patients treated with insulin in the hospital: this is a good choice if the purpose is mainly drug safety and avoidance of hypoglycemia, but by definition excludes most untreated patients.
-
Patients with 2 or more BG values (laboratory and/or POC) over a certain threshold (eg, >180 mg/dL). This will likely capture more patients with inpatient hyperglycemia, whether or not detected by the medical team, but is subject to wide variations in the frequency and timing of laboratory glucose testing, including whether or not the values are pre‐prandial (note that even preprandial POC glucose measurements are not always in fact fasting values).
Other considerations include the following:
-
Are there natural patient subgroups that should be measured and analyzed separately because of different guidelines? For example, there probably should be separate/emndependent inclusion criteria and analyses for critical care and noncritical care units because their glycemic targets and management considerations differ.
-
Which patients should be excluded? For example, if targeting subcutaneous insulin use in general hospitalized patients, one might eliminate those patients who are admitted specifically as the result of a diabetes emergency (eg, diabetic ketoacidosis [DKA] and hyperglycemic hyperosmolar state [HHS]), as their marked and prolonged hyperglycemia will skew BG data. Pregnant women should generally be excluded from broad‐based analyses or considered as a discrete category because they have very different targets for BG therapy. Patients with short lengths of stay may be less likely to benefit from tight glucose control and may also be considered for post hoc exclusion. One might also exclude patients with very few evaluable glucose readings (eg, fewer than 5) to ensure that measurement is meaningful for a given patient, keeping in mind that this may also exclude patients with undetected hyperglycemia, as mentioned above. Finally, patients receiving palliative care should also be considered for exclusion if feasible.
Recommendation: Do not limit analyses to only those patients with a diagnosis of diabetes or only those on insulin, which will lead to biased results.
-
For noncritical care patients, we recommend a combined approach: adult patients with a diagnosis of diabetes (e. g. using diagnosis‐related group [DRG] codes 294 or 295 or International Classification of Diseases 9th edition [ICD9] codes 250.xx) or with hyperglycemia (eg, 2 or more random laboratory and/or point of care (POC) BG values >180 mg/dL or 2 or more fasting BG values >130 mg/dL), excluding patients with DKA or HHS or who are pregnant.
-
For critical care units, we recommend either all patients, or patients with at least mild hyperglycemia (eg, 2 random glucose levels >140 mg/dL). Critical care patients with DKA, HHS, and pregnancy should be evaluated separately if possible.
Which Glucose Values to Include and Exclude
To answer this question, we first need to decide which method to use for BG measurement. There are several ways to measure BG, including the type of sample collected (capillary [fingerstick], arterial, and venous) and the technique used (central laboratory analyzing plasma, central laboratory analyzing whole blood [eg, from an arterial blood gas sample], glucose meter [usually calibrated to plasma], etc.). The POC (eg, capillary, glucose meter) glucose measurements alone are often preferred in the non‐ICU setting because laboratory plasma values generally provide little additional information and typically lower the mean glucose by including redundant fasting values.1 In critical care units, several different methods are often used together, and each merits inclusion. The inherent differences in calibration between the methods do not generally require separate analyses, especially given the frequency of testing in the ICU setting.
The next question is which values to include in analyses. In some situations, it may be most useful to focus on a certain period of hospitalization, such as the day of a procedure and the next 2 days in assessing the impact of the quality of perioperative care, or the first 14 days of a noncritical care stay to keep outliers for length of stay (LOS) from skewing the data. In the non‐ICU setting, it may be reasonable to exclude the first day of hospitalization, as early BG control is impacted by multiple variables beyond direct control of the clinician (eg, glucose control prior to admission, severity of presenting illness) and may not realistically reflect your interventions. (Keep in mind, however, that it may be useful to adjust for the admission glucose value in multivariable models given its importance to clinical outcomes and its strong relationship to subsequent inpatient glucose control.) However, in critical care units, it is reasonable to include the first day's readings in analyses given the high frequency of glucose measurements in this setting and the expectation that glucose control should be achieved within a few hours of starting an intravenous insulin infusion.
If feasible to do so with your institution's data capture methods, you may wish to select only the regularly scheduled (before each meal [qAC] and at bedtime [qHS], or every 6 hours [q6h]) glucose readings for inclusion in the summary data of glycemic control in the non‐ICU setting, thereby reducing bias caused by repeated measurements around extremes of glycemic excursions. An alternative in the non‐ICU setting is to censor glucose readings within 60 minutes of a previous reading.
Recommendation:
-
In the non‐ICU setting, we recommend first looking at all POC glucose values and if possible repeating the analyses excluding hospital day 1 and hospital day 15 and beyond, and also excluding glucose values measured within 60 minutes of a previous value.
-
In critical care units, we recommend evaluating all glucose readings used to guide care.
Units of Analysis
There are several different units of analysis, each with its own advantages and disadvantages:
-
Glucose value: this is the simplest measure and the one with the most statistical power. All glucose values for all patients of interest comprise the denominator. A report might say, for example, that 1% of the 1000 glucose values were <70 mg/dL during a certain period or that the mean of all glucose values collected for the month from patients in noncritical care areas was 160 mg/dL. The potential disadvantages of this approach are that these analyses are less clinically relevant than patient‐level analyses and that patients with many glucose readings and long hospitalizations may skew the data.
-
Patient (or the Patient Stay, [ie, the entire hospitalization]): all patients who are monitored make up the denominator. The numerator may be the percentage of patients with any hypoglycemia during their hospital stay or the percentage of patients achieving a certain mean glucose during their hospitalization, for example. This is inherently more clinically meaningful than using glucose value as a unit of analysis. A major disadvantage is not controlling for LOS effects. For example, a hospitalized patient with a long LOS is much more likely to be characterized as having at least 1 hypoglycemic value than is a patient with a shorter LOS. Another shortcoming is that this approach does not correct for uneven distribution of testing. A patient's mean glucose might be calculated on the basis of 8 glucose values on the first day of hospitalization, 4 on the second day, and 1 on the third day. Despite all these shortcomings, reporting by patient remains a popular and valid method of presenting glycemic control results, particularly when complemented by other views and refined to control for the number of readings per day.
-
Monitored Patient‐Day: The denominator in this setting is the total number of days a patient glucose level is monitored. The benefits of this method have been described and advocated in the literature.1 As with patient‐level analyses, this measure will be more rigorous and meaningful if the BG measures to be evaluated have been standardized. Typical reports might include percentage of monitored days with any hypoglycemia, or percentage of monitored days with all glucose values in the desired range. This unit of analysis may be considered more difficult to generate and to interpret. On the other hand, it is clinically relevant, less biased by LOS effects, and may be considered the most actionable metric by clinicians. This method provides a good balance when presented with data organized by patient.
The following example uses all 3 units of measurement, in this case to determine the rate of hypoglycemia, demonstrating the different but complementary information that each method provides:
-
In 1 month, 3900 POC glucose measurements were obtained from 286 patients representing 986 monitored patient‐days. With hypoglycemia defined as POC BG 60 mg/dL, the results showed the following:
-
50 of 3900 measurements (1.4%) were hypoglycemic 22 of 286 patients (7.7%) had 1 hypoglycemic episodes
-
40 of 986 monitored days (4.4%) had 1 hypoglycemic episodes.
The metric based on the number of glucose readings could be considered the least clinically relevant because it is unclear how many patients were affected; moreover, it may be based on variable testing patterns among patients, and could be influenced disproportionately by 1 patient with frequent hypoglycemia, many glucose readings, and/or a long LOS. One could argue that the patient‐stay metric is artificially elevated because a single hypoglycemic episode characterizes the entire stay as hypoglycemic. On the other hand, at least it acknowledges the number of patients affected by hypoglycemia. The patient‐day unit of analysis likely provides the most balanced view, one that is clinically relevant and measured over a standard period of time, and less biased by LOS and frequency of testing.
One way to express patient‐day glycemic control that deserves special mention is the patient‐day weighted mean. A mean glucose is calculated for each patient‐day, and then the mean is calculated across all patient‐days. The advantage of this approach is that it corrects for variation in the number of glucose readings each day; all hospital days are weighted equally.
Recommendation:
-
In noncritical care units, we recommend a combination of patient‐day and patient‐stay measures.
-
In critical care units, it is acceptable to also use glucose reading as the unit of measurement given more frequent and uniform data collection, but it should be complemented by more meaningful patient‐day and patient‐stay measures.
Measures of Control
In addition to deciding the unit(s) of analysis, another issue concerns which measures of control to use. These could include rates of hypoglycemia and hyperglycemia, percentage of glucose readings within various ranges (eg, <70, 70180, >180 mg/dL), mean glucose value, percentage of patient‐days during which the mean glucose is within various ranges, or the in control rate (ie, when all glucose values are within a certain range).
As with the various units of analysis, each of these measures of control has various advantages and disadvantages. For example, mean glucose is easy to report and understand, but masks extreme values. Percentage of glucose values within a certain range (eg, per patient, averaged across patients) presents a more complete picture but is a little harder to understand and will vary depending on the frequency of glucose monitoring. As mentioned above, this latter problem can be corrected in part by including only certain glucose values. Percent of glucose values within range may also be less sensitive to change than mean glucose (eg, a glucose that is lowered from 300 mg/dL to 200 mg/dL is still out of range). We recommend choosing a few, but not all, measures of control in order to get a complete picture of glycemic control. Over time one can then refine the measures being used to meet the needs of the glycemic control team and provide data that will drive the performance improvement process.
In critical care and perioperative settings, interest in glycemic control is often more intense around the time of a particular event such as major surgery or after admission to the ICU. Some measures commonly used in performing such analyses are:
-
All values outside a target range within a designated crucial period. For example, the University Healthcare Consortium and other organizations use a simple metric to gauge perioperative glycemic control. They collect the fasting glucose on postoperative days 1 and 2 and then calculate the percentage of postoperative days with any fasting glucose >200 mg/dL. Of course, this is a very liberal target, but it can always be lowered in a stepwise fashion once it is regularly being reached.
-
Three‐day blood glucose average. The Portland group uses the mean glucose of each patient for the period that includes the day of coronary artery bypass graft (CABG) surgery and the following 2 days. The 3‐day BG average (3‐BG) correlates very well with patient outcomes and can serve as a well‐defined target.2 It is likely that use of the 3‐BG would work well in other perioperative/trauma settings and could work in the medical ICU as well, with admission to the ICU as the starting point for calculation of the 3‐BG.
Hyperglycemic Index
Measuring the hyperglycemic index (HGI) is a validated method of summarizing glycemic control of ICU patients.3 It is designed to take into account the sometimes uneven distribution of patient testing. Time is plotted on the x‐axis and glucose values on the y‐axis. The HGI is calculated the area under the curve of glycemic values but above the upper limit of normal (ie, 110 mg/dL). Glucose values in the normal or hypoglycemic range are not included in the AUC. Mortality correlated well with this glycemic index. However, a recent observational study of glucometrics in patients hospitalized with acute myocardial infarction found that the simple mean of each patient's glucose values over the entire hospitalization was as predictive of in‐hospital mortality as the HGI or the time‐averaged glucose (AUC for all glucose values).4 In this study, metrics derived from glucose readings for the entire hospitalization were more predictive than those based on the first 24 or 48 hours or on the admission glucose.
Analyses Describing Change in Glycemic Control Over Time in the Hospital
In the critical care setting, this unit of analysis may be as simple as the mean time to reach the glycemic target on your insulin infusion protocol. On noncritical care wards, it is a bit more challenging to characterize the improvement (or clinical inertia) implied by failure of hyperglycemia to lessen as an inpatient stay progresses. One method is to calculate the mean glucose (or percentage of glucose values in a given range) for each patient on hospital day (HD) 1, and repeat for each HD (up to some reasonable limit, such as 5 or 7 days).
Recommendations:
-
In noncritical units, we recommend a limited set of complementary measures, such as the patient‐day weighted mean glucose, mean percent of glucose readings per patient that are within a certain range, and percentage of patients whose mean glucose is within a certain range on each hospital day.
-
In critical care units, it is often useful to focus measures around a certain critical event such as the 3‐day blood glucose average and to use measures such as the HGI that take advantage of more frequent blood glucose testing.
Definitions of Hyperglycemia and Hypoglycemia
Glucometrics outcomes will obviously depend on the thresholds established for hyperglycemia and hypoglycemia. Many centers define hypoglycemia as 60 mg/dL, whereas the ADA definition, based on physiologic changes that may take place, defines hypoglycemia (at least in the outpatient setting) as 70 mg/dL. Hypoglycemia may be further stratified by severity, with any glucose 40 mg/dL, for instance, defined as severe hypoglycemia.
Similarly, the definition of hyperglycemia (and therefore good control) must also be defined. Based on definitions developed by the ADA and AACE, the state of the medical literature, and current understanding of the pathophysiology of hyperglycemia, thresholds for critical care units include 110 mg/dL, 130 mg/dL, and 140 mg/dL, and options in noncritical care units include 130 mg/dL, 140 mg/dL, and 180 mg/dL. Because these thresholds implicitly assume adverse effects when glucose levels are above them, these levels are subject to revision as data become available confirming the benefits and safety of targeted glycemic control in various settings and patient populations.
Introducing optimal BG targets in a stepped fashion over time should also be considered. Furnary et al.2 have done this in the Portland Project, which tracks glycemic control in cardiac surgery patients receiving intravenous insulin therapy. The initial BG target for this project was <200 mg/dL; it was subsequently lowered stepwise over several years to 150 mg/dL, then to 120 mg/dL, and most recently to 110 mg/dL. This approach allows the safe introduction of targeted glycemic control and promotes acceptance of the concept by physicians and the allied nursing and medical staff.
Recommendations:
-
In noncritical care units, it is reasonable to use 40 mg/dL for severe hypoglycemia, 70 mg/dL for hypoglycemia, 130 mg/dL for fasting hyperglycemia, 180 mg/dL for random or postprandial hyperglycemia, and 300 mg/dL for severe hyperglycemia, keeping in mind that these thresholds are arbitrary. In critical care units, values from 110 mg/dL to 140 mg/dL might be better thresholds for hyperglycemia, but it may take time to safely and effectively move an organization toward these lower targets.
Other Considerations Relative to Glucometrics
Yale Glucometrics Website
The Yale Informatics group has put together a Web‐based resource (
Other Analytic Resources
Commercially available software, such as the RALS system (Medical Automation Systems, Inc., Charlottesville, VA) can gather POC glucose measurements directly from devices and provide real‐time reports of glycemic control, stratified by inpatient unit, using user‐defined targets for hypoglycemia and hyperglycemia. While they are no substitute for a dedicated, on‐site data analyst, such systems can be very useful for smaller hospitals with minimal data or information technology support staff.
APPROACHES TO ANALYSIS: RUN CHARTS
Most conventional clinical trials hold interventions fixed for a period of time and compare results with and without the intervention. For quality improvement studies, this is still a valid way to proceed, especially if studied as a randomized controlled trial. Such methods may be preferred when the clinical question is Does this type of intervention work in general? and the desired output is publication in peer‐reviewed journals so that others can learn about and adopt the intervention to their own institution. A before and after study with a similar analytic approach may also be valid, although concerns about temporal trends and cointerventions potentially compromise the validity of such studies. This approach again assumes that an intervention is held fixed over time such that it is clear what patients received during each time period.
If the desired result is improvement at a given institution (the question is Did we improve care?) then it may be preferable to present results over time using run‐charts. In a run chart, the x‐axis is time and the y‐axis the desired metric, such as patient‐day weighted mean glucose. Points in time when interventions were introduced or modified can be highlighted. Run charts have several advantages over before‐and‐after summaries: they do not require interventions remaining fixed and are more compatible with continuous quality improvement methods, it is easier to see the effect of different aspects of the interventions as they occur, one can get a quicker picture of whether something is working, and it is easier to separate out the impact of the intervention from secular trends. Finally, the use of run charts does not imply the absence of statistical rigor. Run charts with statistical process control (SPC) limits5 can easily convey when the observed time trend is unlikely to be due to chance using prespecified P values. (A full discussion of SPC and other methods to study quality improvement interventions is beyond the scope of this article.)
ASSESSING PATTERNS OF INSULIN USE AND ORDER SET UTILIZATION
Besides measuring the impact of quality improvement interventions on glucose control, it is important to measure processes such as proper insulin use. As mentioned in other articles in this supplement, processes are much more sensitive to change than outcomes. Failure to change processes should lead one to make changes to the intervention.
ICU and Perioperative Settings
For ICU and perioperative settings, the major process measure will likely be use of the insulin infusion order set. Designation of BG levels that trigger insulin infusion in these settings should be agreed upon in advance. The number of patients who meet the predefined glycemic criteria would make up the denominator, and the number of patients on the insulin infusion order set would make up the numerator.
NonCritical Care Units
On noncritical care units, measuring the percentage of subcutaneous insulin regimens that contain a basal insulin is a useful way to monitor the impact of an intervention. A more detailed analysis could examine the percentage of patients on simultaneous basal and nutritional insulin (if applicable). An important measure of clinical inertia is to track the percentage of patients who had changes in their insulin regimens on days after hypoglycemic or hyperglycemic excursions. Another important measure is the frequency with which the standardized order set is being used, analogous to the measure of insulin infusion use in the ICU. A final process measure, indirectly related to insulin use, is the frequency of use of oral diabetes agents, especially by patients for whom their use is contraindicated (eg, patients with congestive heart failure who are on thiazolidinediones and patients with renal insufficiency or receiving intravenous contrast continued on metformin).
OTHER CONSIDERATIONS AND METRICS
Examples of other metrics that can be used to track the success of quality improvement efforts include:
-
Glucose measurement within 8 hours of hospital admission.
-
Glycated hemoglobin (A1C) measurement obtained or available within 30 days of admission to help guide inpatient and especially discharge management.
-
Appropriate glucose testing in patients with diabetes or hyperglycemia (eg, 4 times per day in patients not on insulin infusion protocols, at least until 24 hours of euglycemia is documented).
-
The percentage of patients on insulin with on‐time tray delivery.
-
The timing of subcutaneous insulin administration in relation to glucose testing and nutrition delivery.
-
Documentation of carbohydrate intake among patients who are eating.
-
Satisfaction of physicians and nurses with order sets or protocols, using standard surveys.
-
Physician and nurse knowledge, attitudes, and beliefs about insulin administration, fear of hypoglycemia, treatment of hypoglycemia, and glycemic control in the hospital.
-
Patient satisfaction with their diabetes care in the hospital, including the education they received.
-
Nursing and physician education/certification in insulin prescribing, insulin administration, and other diabetes care issues.
-
Patient outcomes strongly associated with glycemic control, (eg, surgical wound infections, ICU LOS, catheter‐related bloodstream infections).
-
Appropriate treatment and documentation of hypoglycemia (eg, in accordance with hospital policy).
-
Documentation of severe hypoglycemic events through the hospital's adverse events reporting system (these may actually increase as change comes to the organization and as clinical personnel are more attuned to glycemic control).
-
Root causes of hypoglycemic events, which can be used to understand and prevent future events.
-
Appropriate transitions from IV to SC insulin regimens, (eg, starting basal insulin prior to discontinuing infusion in patients who have been on an insulin infusion of at least 2 units/hour or who have a known diagnosis of diabetes or A1C >7).
(Survey instruments and other measurement tools are available from the authors upon request.)
SHM GLYCEMIC CONTROL TASK FORCE SUMMARY RECOMMENDATIONS
The SHM Glycemic Control Task Force is working to develop standardized measures of inpatient glucose control and related indicators to track progress of hospital glycemic control initiatives (see the introduction to this supplement for a description of the charge and membership of this task force). The goals of the Task Force's metrics recommendations (Table 1) are several‐fold: (1) create a set of measurements that are complete but not overly burdensome; (2) create realistic measures that can be applied to institutions with different data management capabilities; and (3) allow for comparison across institutions for benchmarking purposes, evaluation of quality improvement projects, and reporting of results for formal research studies in this field.
| Measurement Issue | NonCritical Care Units | Critical Care Units | ||
|---|---|---|---|---|
| Tier 1 Recommendations | Tier 2 Recommendations | Tier 1 Recommendations | Tier 2 Recommendations | |
| ||||
| Patient inclusion and exclusion criteria | All adult patients with POC glucose testing (sampling acceptable). Exclude patients with DKA or HHS or who are pregnant. | All adult patients with diagnosis of diabetes by ICD‐9 code* or by glucose testing: random glucose (POC or laboratory) >180 mg/dL 2 or fasting glucose >130 mg/dL 2, excluding patients with DKA or HHS or who are pregnant. Additional analysis: exclude patients with <5 evaluable glucose readings, patients with LOS <2 days, or receiving palliative care. | All patients in every critical care unit (sampling acceptable). | Patients with DKA, HHS, or pregnancy in separate analyses. All patients in every critical care unit with random glucose (POC or laboratory) >140 mg/dL 2. |
| Glucose reading inclusion and exclusion criteria | All POC glucose values. | Additional analysis: exclude glucose values on hospital day 1 and on hospital day 15 and after. Additional analysis: exclude glucose values measured within 60 minutes of a previous value. | All POC and other glucose values used to guide care. | |
| Measures of safety | Analysis by patient‐day: Percentage of patient‐days with 1 or more values <40, <70, and >300 mg/dL. | Analysis by patient‐day: Percentage of patient‐days with 1 or more values <40, <70, and >300 mg/dL. | ||
| Measures of glucose control | Analysis by patient‐day: Percentage of patient‐days with mean <140, <180 mg/dL and/or Percentage of patient‐days with all values <180 mg/dL. | Analysis by patient‐day: Patient day‐weighted mean glucose. | Analysis by glucose reading: Percentage of readings <110, <140 mg/dL. | 3‐BG as above for all patients in critical care units.∥ Hyperglycemic index for all patients in critical care units (AUC of glucose values above target). |
| Analysis by patient stay: Percentage of patient stays with mean <140, <180 mg/dL. | Analysis by patient stay: Mean percentage of glucose readings of each patient <180 mg/dL. | Analysis by patient‐day: Percentage of patient‐days with mean <110, <140 mg/dL, and/or Percentage of patient‐days with all values <110, <140 mg/dL. | ||
| Analysis by hospital day: Percentage of patients with mean glucose readings <140, <180 mg/dL by hospital day (days 17). | Analysis by patient stay: 3‐day blood glucose average (3‐BG) for selected perioperative patients: Percentage of patients with 3‐BG <110, <140 mg/dL. Mean time (hours) to reach glycemic target (BG <110 or <140 mg/dL) on insulin infusion. | |||
| Measures of insulin use | Percentage of patients on any subcutaneous insulin that has a scheduled basal insulin component (glargine, NPH, or detemir). | Percentage of patients with at least 2 POC and/or laboratory glucose readings >180 mg/dL who have a scheduled basal insulin component. Percentage of eating patients with hyperglycemia as defined above with scheduled basal insulin and nutritional insulin. Percentage of patients and patient‐days with any changes in insulin orders the day after 2 or more episodes of hypoglycemia or hyperglycemia (ie, <70 or >180 mg/dL). | Percentage of patients with 2 POC or laboratory glucose readings >140 mg/dL placed on insulin infusion protocol. | |
| Other process measures | Glucose measured within 8 hours of hospital admission. | POC glucose testing at least 4 times a day for all patients with diabetes or hyperglycemia as defined above. | Glucose measured within 8 hours of hospital admission. | Appropriateness of hypoglycemia treatment and documentation. |
| A1C measurement obtained or available within 30 days of admission. | Measures of adherence to specific components of management protocol. | Frequency of BG testing (eg, per protocol if on insulin infusion; every 68 hours if not). | Clinical events of severe hypoglycemia reported through the organization's critical events reporting tool. | |
| Appropriateness of hypoglycemia treatment and documentation. | Root causes of hypoglycemia. | |||
| Clinical events of severe hypoglycemia reported through the organization's critical events reporting tool. | Appropriate use of IV‐to‐SC insulin transition protocol. | |||
| Root causes of hypoglycemia. | ||||
For each domain of glycemic management (glycemic control, safety, and insulin use), the task force chose a set of best measures. They are presented as two tiers of measurement standards, depending on the capabilities of the institution and the planned uses of the data. Tier 1 includes measures that, although they do take time and resources to collect, are feasible for most institutions. Tier 2 measures are recommended for hospitals with easy manipulation of electronic sources of data and for reporting quality‐of‐care measures for widespread publication, that is, in the context of a research study. It should be emphasized that these recommendations are only meant as a guide: the actual measures chosen should meet the needs and capabilities of each institution.
We recognize that few data support the recommendations made by this task force, that such data are needed, and that the field of data collection and analysis for hospital glycemic management is rapidly evolving. The hope is to begin the standardization process, promote dialogue in this field, and eventually reach a consensus in collaboration with the ADA, AACE, and other pertinent stakeholders.
CONCLUSIONS
Like the field of inpatient glycemic management itself, the field of devising metrics to measure the quality of inpatient glycemic control is also in its infancy and quickly evolving. One should not be paralyzed by the lack of consensus regarding measurementthe important point is to pick a few complementary metrics and begin the process. The table of recommendations can hopefully serve as a starting point for many institutions, with a focus on efficacy (glycemic control), safety (hypoglycemia), and process (insulin use patterns). As your institution gains experience with measurement and the field evolves, your metrics will likely change. We recommend keeping all process and outcome data in its raw form so that it can be summarized in different ways over time. It is also important not to wait for the perfect data collection tool before beginning to analyze data: sampling and paper processes are acceptable if automated data collection is not yet possible. Eventually, blood glucose meter readings should be downloaded into a central database that interfaces with hospital data repositories so data can be analyzed in conjunction with patient, service, and unit‐level information. Only with a rigorous measurement process can institutions hope to know whether their changes are resulting in improved care for patients.
- ,,, et al.“Glucometrics”—assessing the quality of inpatient glucose management.Diabetes Technol Ther.2006;8:560–569.
- ,,.Effect of hyperglycemia and continuous intravenous insulin infusions on outcomes of cardiac surgical procedures: the Portland Diabetic Project.Endocr Pract.2004;10(suppl 2):21–33.
- ,,.Hyperglycaemic index as a tool to assess glucose control: a retrospective study.Crit Care.2004;8:R122–R127.
- ,,, et al.Glucometrics in patients hospitalized with acute myocardial infarction: defining the optimal outcomes‐based measure of risk.Circulation.2008;117:1018–1027.
- ,,.Statistical process control as a tool for research and healthcare improvement.Qual Saf Health Care.2003;12:458–464.
- ,,, et al.“Glucometrics”—assessing the quality of inpatient glucose management.Diabetes Technol Ther.2006;8:560–569.
- ,,.Effect of hyperglycemia and continuous intravenous insulin infusions on outcomes of cardiac surgical procedures: the Portland Diabetic Project.Endocr Pract.2004;10(suppl 2):21–33.
- ,,.Hyperglycaemic index as a tool to assess glucose control: a retrospective study.Crit Care.2004;8:R122–R127.
- ,,, et al.Glucometrics in patients hospitalized with acute myocardial infarction: defining the optimal outcomes‐based measure of risk.Circulation.2008;117:1018–1027.
- ,,.Statistical process control as a tool for research and healthcare improvement.Qual Saf Health Care.2003;12:458–464.
Supporting Inpatient Glycemic Control Programs Now
Medical centers are faced with multiple competing priorities when deciding how to focus their improvement efforts and meet the ever expanding menu of publicly reported and regulatory issues. In this article we expand on the rationale for supporting inpatient glycemic control programs as a priority that should be moved near the top of the list. We review the evidence for establishing glycemic range targets, and also review the limitations of this evidence, acknowledging, as does the American Diabetes Association (ADA), that in both the critical care and non‐critical care venue, glycemic goals must take into account the individual patient's situation as well as hospital system support for achieving these goals.1, 2 We emphasize that inpatient glycemic control programs are needed to address a wide variety of quality and safety issues surrounding the care of the inpatient with diabetes and hyperglycemia, and we wish to elevate the dialogue beyond arguments surrounding adoption of one glycemic target versus another. The Society of Hospital Medicine Glycemic Control Task Force members are not in unanimous agreement with the American Association of Clinical Endocrinologists (AACE)/ADA inpatient glycemic targets. However, we do agree on several other important points, which we will expand on in this article:
-
Uncontrolled hyperglycemia and iatrogenic hypoglycemia are common and potentially dangerous situations that are largely preventable with safe and proven methods.
-
The current state of care for our inpatients with hyperglycemia is unacceptably poor on a broad scale, with substandard education, communication, coordination, and treatment issues.
-
Concerted efforts with changes in the design of the process of care are needed to improve this state of affairs.
DIABETES AND HYPERGLYCEMIA ARE VERY COMMON INPATIENT CONDITIONS
Diabetes mellitus (DM) has reached epidemic proportions in the United States. A reported 9.3% of adults over 20 years of age have diabetes, representing over 20 million persons. Despite increasing awareness, diabetes remains undiagnosed in approximately 30% of these persons.3 Concurrent with the increasing prevalence of diabetes in the U.S. population from 1980 through 2003, the number of hospital discharges with diabetes as any listed diagnosis more than doubled, going from 2.2 to 5.1 million discharges.4 Hospital care for patients with diabetes and hyperglycemia poses a significant health economic burden in the United States, representing over 40 billion dollars in annual direct medical expenditures.5
Hyperglycemia in the hospital may be due to known diabetes, to previously unrecognized diabetes, to prediabetes, and/or to the stress of surgery or illness. Deterioration in glycemic control in the hospital setting is most commonly associated with one or more factors, including stress‐induced release of insulin counterregulatory hormones (catecholamines, cortisol, glucagon, and growth hormone), exogenous administration of high dose glucocorticoids, and suboptimal glycemic management strategies.68 In a Belgian medical intensive care unit (MICU) randomized controlled trial (RCT) of strict versus conventional glycemic control, mean blood glucose (BG) on admission to the unit in the intention to treat group was 162 70 mg/dL (n = 1200),9 and in this group's RCT of 1548 surgical intensive care unit (SICU) patients, BG > 110 mg/dL was observed in over 70% of subjects.10 Mean BG of >145 mg/dL has been reported in 39%11 and BG >200 mg/dL in anywhere from 11% to 31% of intensive care unit (ICU) patients.10, 12 For general medicine and surgery, 1 study of 2030 patients admitted to a teaching hospital revealed that 26% of admissions had a known history of DM and 12% had new hyperglycemia, as evidenced by an admission or in‐hospital fasting BG of 126 mg/dL or more or a random BG of 200 mg/dL or more on 2 or more determinations.13 National and regional estimates on hospital use maintained by the Agency for Healthcare Research and Quality include data concerning diabetes diagnoses alone, without hyperglycemia, and may be displayed by querying its Web site.14 In cardiovascular populations almost 70% of patients having a first myocardial infarction have been reported to have either known DM, previously unrecognized diabetes, or impaired glucose tolerance.15
THE EVIDENCE SUPPORTS INPATIENT GLYCEMIC CONTROL
Evidence: Physiology
The pathophysiologic mechanisms through which hyperglycemia is linked to suboptimal outcomes in the hospital are complex and multifactorial. Although it is beyond the scope of this article to discuss these mechanisms in detail, research has broadly focused in the following areas: (1) immune system dysfunction, associated with a proinflammatory state and impaired white blood cell function; (2) metabolic derangements leading to oxidative stress, release of free fatty acids, reduction in endogenous insulin secretion, and fluid and electrolyte imbalance; and (3) a wide variety of vascular system responses (eg, endothelial dysfunction with impairment of tissue perfusion, a prothrombotic state, increased platelet aggregation, and left ventricular dysfunction).8, 1618
Conversely administration of insulin suppresses or reverses many of these abnormalities including generation of reactive oxygen species (ROS) and activation of inflammatory mechanisms,19 and leads to a fall in C‐reactive protein, which accompanied the clinical benefit of intensive insulin therapy (IIT) in the Leuven, Belgium, ICU population,20 and prevents mitochondrial abnormalities in hepatocytes.21 In the same surgical ICU cohort, Langouche et al.22 report suppression of intracellular adhesion molecule‐1 (ICAM‐1) and E‐selectin, markers of inflammation, and reduction in plasma nitric oxide (NO) and innate nitric oxide (iNOS) expression with insulin administration in patients treated with intravenous (IV) IIT.22 These data further support the role of insulin infusion in suppressing inflammation and endothelial dysfunction. The authors suggest that maintaining normoglycemia with IIT during critical illness protects the endothelium, thereby contributing to prevention of organ failure and death.22 Based on accumulating data in the literature such as that cited above, it has been suggested that a new paradigm in which glucose and insulin are related not only through their metabolic action but also through inflammatory mechanisms offers important potential therapeutic opportunities.19
Evidence: Epidemiology/Observational Studies/Non‐RCT Interventional Studies
A strong association between hospital hyperglycemia and negative outcomes has been reported in numerous observational studies in diverse adult medical and surgical settings. In over 1800 hospital admissions, those with new hyperglycemia had an in‐hospital mortality rate of 16% compared with 3% mortality in patients with known diabetes and 1.7% in normoglycemic patients (P < 0.01). These data suggest that hyperglycemia due to previously unrecognized diabetes may be an independent marker of in‐hospital mortality.13
Hyperglycemia has been linked to adverse outcomes in myocardial infarction, stroke,2328 postoperative nosocomial infection risk, pneumonia, renal transplant, cancer chemotherapy, percutaneous coronary interventions, and cardiac surgery.2938 These observational studies have the usual limitations inherent in their design. Demonstrating a strong association of hyperglycemia with adverse outcomes is not a guarantee that the hyperglycemia is the cause for the poor outcome, as hyperglycemia can reflect a patient under more stress who is at a higher risk for adverse outcome. By the same token, the strong association of hyperglycemia with the risk of poor outcomes seen in these studies does not guarantee that euglycemia would mitigate this risk.
Nonetheless, there are several factors that make the body of evidence for glycemic control more compelling. First, the association has a rational physiologic basis as described above. Second, the associations are consistent across a variety of patient populations and disease entities, and demonstrate a dose‐response relationship. Third, in studies that control for comorbidities and severity of illness, hyperglycemia persists as an independent risk factor for adverse outcomes, whether the patient has a preexisting diagnosis of diabetes or not. Last, non‐RCT interventional studies and RCTs largely reinforce these studies.
The Portland Diabetic Project has reported prospective, nonrandomized data over 17 years on the use of an IV insulin therapy protocol in cardiac surgery patients.38 This program has implemented stepped lowering of target BG, with the most recent data report implementing a goal BG <150 mg/dL.35 The current protocol uses a BG target of 70110 mg/dL, but results have not yet been published.39 Mortality and deep sternal wound infection rates for patients with diabetes who remain on the IV insulin protocol for 3 days have been lowered to levels equivalent to those for nondiabetic patients. This group has also reported reductions in length of stay and cost‐effectiveness of targeted glycemic control in the cardiac surgery population.35 Their data have to a large extent driven a nationwide movement to implement targeted BG control in cardiac surgery patients.
Another large ICU study (mixed medical‐surgical, n = 800 patients) also supports a benefit through targeted BG control (130.7 versus 152.3 mg/dL, P < 0.001) when compared with historical controls. This study demonstrated reduction in in‐hospital mortality (relative risk reduction 29.3%, P = 0.002), duration of ICU stay (10.8%, P = 0.04), acute renal failure (75%, P = 0.03), and blood transfusions (18.7%, P = 0.002),40 representing a similar magnitude of effect as was demonstrated by the Belgian group.
Evidence: RCTs
Evidence is accumulating that demonstrates an advantage in terms of morbidity and mortality when targeted glycemic control using intravenous insulin infusion is implemented in the hospital. The most robust data have been reported from ICU and cardiac surgery settings. The largest randomized, controlled study to date enrolled 1548 patients in a surgical ICU in Leuven, Belgium who were randomized to either intensive (IT) or conventional (CT) insulin therapy. Mean glucose attained was 103 19 and 153 33 mg/dL in each arm, respectively. The intensive insulin group demonstrated a reduction in both ICU (4.6% versus 8.0%) and in‐hospital mortality (7.2% versus 10.9%), as well as bloodstream infections, acute renal failure, transfusions, and polyneuropathy, the latter being reflected by duration of mechanical ventilation (P < 0.01 for all). Although a similar study in an MICU did not achieve statistical significance in the overall intention‐to‐treat analysis, it did demonstrate reductions in mortality (from 52.5% to 43.0%) in patients with at least 3 days of ICU treatment. It should also be noted that in this MICU population hypoglycemia rates were higher and level of glycemic control attained not as rigorous as in the same group's SICU cohort, factors which may have had an impact on observed outcomes. A meta‐analysis of these two Leuven, Belgium, studies demonstrated a reduction in mortality (23.6% versus 20.4%, absolute risk reduction [ARR] 3.2%, P = 0.004)) in all patients treated with IIT, with a larger reduction in mortality (37.9% versus 30.1%, ARR 7.8%, P = 0.002) observed in patients with at least 3 days of IIT, as well as substantial reductions in morbidity.9, 10, 41, 42
Several other studies must be mentioned in this context. A small (n = 61), randomized study in another SICU did not show a mortality benefit, perhaps because the number of subjects was not adequate to reach statistical significance, but did result in a significant reduction in nosocomial infections in patients receiving IIT (BG = 125 versus 179 mg/dL, P < 0.001).43 Two international multicenter studies recently stopped enrollment due to excess rates of hypoglycemia. The Volume Substitution and Insulin Therapy in Severe Sepsis (VISEP) study, in a mixed medical and surgical sepsis population, showed no significant reduction in mortality in the intensively‐treated group. Serious adverse events were reported according to standard definitions. Enrollment was stopped before the full number of subjects had been randomized. Among the 537 evaluable cases, hypoglycemia (BG < 40 mg/dL) was reported as 17.0% in the IT group and 4.1% (P < 0.001) in the control group,44 and the rate of serious adverse events was higher in the IT group (10.9% versus 5.2%, P = 0.01). It is notable that the rate of hypoglycemia was comparable to the 18.7% rate seen in the IT group in the Leuven, Belgium, medical ICU study.9 The Glucontrol study enrolled 855 medical and surgical ICU patients and was similarly terminated because of hypoglycemia (BG < 40 mg/dL) at a rate of 8.6% compared to 2.4% in the control group (P < 0.001). Insulin infusion protocols and outcome data have not yet been published.42, 45
These studies with very high hypoglycemia rates each used an algorithm based on the Leuven, Belgium, protocol. The rates of severe hypoglycemia are 34 that reported by a variety of others achieving similar or identical glycemic targets. Hypoglycemia should not be construed as a reason to not use a standardized insulin infusion protocol. In comparing protocols that have been published, it is apparent that rates of hypoglycemia differ substantially and that performance results of some algorithms are not necessarily replicable across sites.46 Dose‐defining designs can be substantively more sophisticated than those used in the trials mentioned, in some cases incorporating principles of control engineering. The variability of hypoglycemia rates under differing insulin infusion protocols is a compelling reason to devote institutional effort to monitoring the efficacy and safety of the infusion protocols that are used.
High‐level evidence from randomized, controlled trials demonstrating outcomes benefit through targeted BG control outside the ICU is lacking at this point in time, but it must be noted that feasibility is suggested by a recent randomized control trial (RABBIT2) that demonstrated the superiority of basal bolus insulin regimens to sliding scale insulin in securing glycemic control, without any increase in hypoglycemia.47
Summing Up the Evidence
It is clear that hyperglycemia is associated with negative clinical outcomes throughout the hospital, and level A evidence is available to support tight glucose control in the SICU setting. However, in view of the imperfect and incomplete nature of the evidence, controversy persists around how stringent glycemic targets should be in the ICU, on whether glycemic targets should differ between SICU and MICU patients, and especially what the targets should be in the non‐ICU setting. There should be hesitancy to extrapolate glycemic targets to be applied beyond the populations that have been studied with RCTs or to assume benefit for medical conditions that have not been examined for the impact of interventions to control hyperglycemia. Institutions might justifiably choose more liberal targets than those promoted in national recommendations/guidelines2, 4850 until safe attainment of more moderate goals is demonstrated. However, even critics agree that uncontrolled hyperglycemia exceeding 180200 mg/dL in any acute care setting is undesirable. Moreover, strong observational data showing the hazards of hyperglycemia in noncritical care units (even after adjustment for severity of illness) combined with the high rate of adverse drug events associated with insulin use, argue strongly for a standardized approach to treating diabetes and hyperglycemia in the hospital. Even though no RCTs exist demonstrating outcomes benefits of achieving glycemic target on wards, the alternatives to control of hyperglycemia using scheduled insulin therapy are unacceptable. Oral agent therapy is potentially dangerous and within the necessary timeframe is likely to be ineffective; sliding scale management is inferior to basal‐bolus insulin therapy, as shown inan RCT,47 and is unsafe; and on the wards improved glycemic control can be achieved simultaneously with a reduction in hypoglycemia.51
INPATIENT GLYCEMIC CONTROL IS INCREASINGLY INCORPORATED INTO PUBLIC REPORTING, GUIDELINES, REGULATORY AGENCY, AND NATIONAL QUALITY INITIATIVE PRIORITIES
National quality initiatives, public reporting, pay‐for‐performance, and guideline‐based care continue to play an increasingly important role in the U.S. healthcare system. Over the years these initiatives have focused on various disease states (venous thromboembolism, congestive heart failure, community‐acquired pneumonia, etc.) in an attempt to standardize care and improve patient safety and quality. Inpatient hyperglycemic control is also increasingly being incorporated into public reporting, regulatory compliance, and national quality initiatives.
Professional organizations such as the ADA2 and AACE50 have published guidelines supporting improved glycemic control, the safe use of insulin, and other measures to improve care for hyperglycemic inpatients. The AACE has a Web site dedicated to hospital hyperglycemia.52 The Society of Hospital Medicine48 has created a resource room on its Web site and a workbook for improvement49 on optimizing the care of inpatients with hyperglycemia and diabetes. The guidelines and Web sites help raise awareness and educate physicians and healthcare workers in inpatient glucose management. The American Heart Association has incorporated specific recommendation regarding inpatient diabetic management in its Get With the Guidelines.53
The Joint Commission54 has developed an advanced disease‐specific certification on inpatient diabetes. Disease management programs are important components of complex healthcare systems that serve to coordinate chronic care, promote early detection and prevention, and reduce overall healthcare costs. Certification is increasingly important to providers, payers, and healthcare institutions because it demonstrates a commitment to quality and patient safety. The Joint Commission disease‐specific care certification is a patient‐centered model focusing on the delivery of clinical care and relationship between the practitioner and the patient. The evaluation and resulting certification by the Joint Commission is based on 3 core components: (1) an assessment of compliance with consensus‐based national standards; (2) the effective use of established clinical practice guidelines to manage and optimize care; and (3) an organized approach to performance measurement and improved activities.55 For inpatient diabetes, the Joint Commission program has 7 major elements following the ADA recommendations, including general recommendations regarding diabetic documentation, BG targets, preventing hypoglycemia, diabetes care providers, diabetes self‐management education, medical nutrition therapy, and BG monitoring.54 This mirrors the Call to Action Consensus Conference essential elements for successful glycemic control programs.1
Other organizations such as the Surgical Care Improvement Partnership (SCIP) and National Surgical Quality Improvement Program (NSQIP) have included perioperative glycemic control measures, as it impacts surgical wound infections. The University HealthSystem Consortium (UHC) has benchmarking data and endorses perioperative glycemic control measures, whereas the Institute for Healthcare Improvement (IHI) has focused on safe use of insulin practices in its 5 Million Lives campaign.
HOSPITALIZATION IS A MOMENT OF OPPORTUNITY TO ASSESS AND INTERVENE
The benefits of outpatient glycemic control and quality preventive care are well established, and the reduction of adverse consequences of uncontrolled diabetes are a high priority in ambulatory medicine.5658 Hospitalization provides an opportunity to identify previously undiagnosed diabetes or prediabetes and, for patients with known diabetes, to assess and impact upon the long term course of diabetes.
As a first step, unless a recent hemoglobin A1C (HbA1c) is known, among hospitalized hyperglycemic patients an HbA1C should be obtained upon admission. Greci et al.59 showed that an HbA1c level >6.0% was 100% specific (14/14) and 57% sensitive (12/21) for the diagnosis of diabetes. Among patients having known diabetes, an HbA1C elevation on admission may justify intensification of preadmission management at the time of discharge. If discharge and postdischarge adjustments of preadmission regimens are planned in response to admission A1C elevations, then the modified long‐term treatment strategy can improve the A1C in the ambulatory setting.60 Moreover, the event of hospitalization is the ideal teachable moment for patients and their caregivers to improve self‐care activities. Yet floor nurses may be overwhelmed by the tasks of patient education. For ideal patient education, both a nutritionist and a diabetes nurse educator are needed to assess compliance with medication, diet, and other aspects of care.6163 There also is need for outpatient follow‐up education. Finally, at the time of discharge, there is a duty and an opportunity for the diabetes provider to communicate with outpatient care providers about the patient's regimen and glycemic control, and also, based on information gathered during the admission, to convey any evidence that might support the need for a change of long‐term strategy.64 Unfortunately, the opportunity that hospitalization presents to assess, educate, and intervene frequently is underused.1, 8, 51, 65
LARGE GAPS EXIST BETWEEN CURRENT AND OPTIMAL CARE
Despite the evidence that inpatient glycemic control is important for patient outcomes, and despite guidelines recommending tighter inpatient glycemic control, clinical practice has been slow to change. In many institutions, inpatient glycemic management has not improved over the past decade, and large gaps remain between current practice and optimal practice.
Studies of individual institutions provide several insights into gaps in care. For example, Schnipper et al.66 examined practices on the general medicine service of an academic medical center in Boston in 2004. Among 107 prospectively identified patients with a known diagnosis of diabetes or at least 1 glucose reading >200 mg/dL (excluding patients with diabetic ketoacidosis, hyperglycemic hyperosmolar state, or pregnancy), they found scheduled long‐acting insulin prescribed in 43% of patients, scheduled short‐acting/rapid‐acting insulin in only 4% of patients, and 80 of 89 patients (90%) on the same sliding scale insulin regimen despite widely varying insulin requirements. Thirty‐one percent of glucose readings were >180 mg/dL compared with 1.2% of readings <60 mg/dL (but 11% of patients had at least 1 episode of hypoglycemia). Of the 75 patients with at least 1 episode of hyperglycemia or hypoglycemia, only 35% had any change to their insulin regimen during the first 5 days of the hospitalization.
Other studies have confirmed this concept of clinical inertia (ie, recognition of the problem but failure to act).67 A study by Cook et al.68 of all hospitalized non‐ICU patients with diabetes or hyperglycemia and length of stay of 3 days between 2001 and 2004 showed that 20% of patients had persistent hyperglycemia during the hospitalization (defined as a mean glucose >200 mg/dL). Forty‐six percent of patients whose average glucose was in the top tertile did not have their insulin regimen intensified to a combination of short‐acting/rapid‐acting and long‐acting insulin, and 35% of these patients either had no change in their total daily insulin dose or actually had a decrease in their dose when comparing the last 24 hours with the first 24 hours of hospitalization, a concept they term negative therapeutic momentum.
Perhaps the most well‐balanced view of the current state of medical practice comes from the UHC benchmarking project.69 UHC is an alliance of 90 academic health centers. For the diabetes project, each institution reviewed the records of 50 randomly selected patients over 18 years of age with at least a 72‐hour length of stay, 1 of 7 prespecified Diagnosis Related Group (DRG) codes, and at least 2 consecutive glucose readings >180 mg/dL or the receipt of insulin any time during the hospitalization. Patients with a history of pancreatic transplant, pregnant at the time of admission, receiving hospice or comfort care, or receiving insulin for a reason other than glucose management were excluded. The study showed widespread gaps in processes and outcomes (Table 1). Moreover, performance varied widely across hospitals. For example, the morning glucose in the ICU on the second measurement day was 110 mg/dL in 18% of patients for the median‐performing hospital, with a range of 0% to 67% across all 37 measured hospitals. In the non‐ICU setting on the second measurement day, 26% of patients had all BG measurements = 180 mg/dL in the median‐performing hospital, with a range of 7% to 48%. Of note, hypoglycemia was relatively uncommon: in the median hospital, 2.4% of patient‐days had 1 or more BG readings <50 mg/dL (range: 0%8.6%). Finally, in the median‐performing hospital, effective insulin therapy (defined as short‐acting/rapid‐acting and long‐acting subcutaneous insulin, continuous insulin infusion, or subcutaneous insulin pump therapy) was prescribed in 45% of patients, with a range of 12% to 77% across measured hospitals.
| Key Performance Measure | Results for Median‐Performing Hospital (%) |
|---|---|
| |
| Documentation of diabetes | 100 |
| Hob A1c assessment within 30 days | 36.1 |
| Glucose measurement within 8 hours of admission | 78.6 |
| Glucose monitoring 4 times a day | 85.4 |
| Median glucose reading > 200 mg/dL | 10.3 |
| Effective insulin therapy* | 44.7 |
| ICU day 2 morning glucose 110 mg/dL | 17.7 |
| Non‐ICU day 2 all glucose readings 180 mg/dL | 26.3 |
| Patient‐days with at least 1 glucose reading < 50 mg/dL | 2.4 |
FREQUENT PROBLEMS WITH COMMUNICATION AND COORDINATION
Those who work closely with frontline practitioners striving to improve inpatient glycemic management have noticed other deficiencies in care.1, 70 These include: a lack of coordination between feeding, BG measurement, and insulin administration, leading to mistimed and incorrectly dosed insulin; frequent use of sliding‐scale only regimens despite evidence that they are useless at best and harmful at worst;6, 47, 60, 71 discharge summaries that often do not mention follow‐up plans for hyperglycemic management; incomplete patient educational programs; breakdowns in care at transition points; nursing and medical staffs that are unevenly educated about the proper use of insulin; and patients who are often angry or confused about their diabetes care in the hospital. Collectively, these gaps in care serve as prime targets for any glycemic control program.
HYPOGLYCEMIA IS A PROMINENT INPATIENT SAFETY CONCERN
Hypoglycemia is common in the inpatient setting and is a legitimate safety concern. In a recently reported series of 2174 hospitalized patients receiving antihyperglycemic agents, it was found that 9.5% of patients experienced a total 484 hypoglycemic episodes (defined as 60 mg/dL).72 Hypoglycemia often occurred in the setting of insulin therapy and frequently resulted from a failure to recognize trends in BG readings or other clues that a patient was at risk for developing hypoglycemia.73 A common thread is the risk created by interruption of carbohydrate intake, noted by Fischer et al.73 and once again in the recent ICU study by Vriesendorp et al.74 Sources of error include: lack of coordination between feeding and medication administration, leading to mistiming of insulin action; lack of sufficient frequency in BG monitoring; lack of clarity or uniformity in the writing of orders; failure to recognize changes in insulin requirements due to advanced age, renal failure, liver disease, or change in clinical status; steroid use with subsequent tapering or interruption; changes in feeding; failure to reconcile medications; inappropriate use of oral antihyperglycemic agents, and communication or handoff failures.
It has been difficult to sort out whether hypoglycemia is a marker of severity of illness or whether it is an independent factor leading to poor outcomes. Observational studies lend credibility to the concept that patients having congestive heart failure or myocardial infarction may be at risk for excessive mortality if their average BG resides in the low end of the normal range.7578 Sympathetic activation occurs as the threshold for hypoglycemia is approached, such as occurs at BG = 70 or 72 mg/dL.79 Patients living with BG levels observed to be in the low end of the normal range might experience more severe but unobserved and undocumented episodes of neuroglycopenia. Arrhythmia and fatality have been directly attributed to strict glycemic control.80, 81 We are confronted with the need to interpret well conducted observational studies, evaluating subgroups at risk, and using multivariate analysis to assess the impact of hypoglycemia upon outcomes.82 In such studies, we will need to examine high‐risk subgroups, including cardiac patients, in particular, for the possibility that there is a J‐shaped curve for mortality as a function of average BG.
Unfortunately, clinical inertia exists in response to hypoglycemia just as it does with hyperglycemia. One recent study examined 52 patients who received intravenous 50% dextrose solution for an episode of hypoglycemia.83 Changes to insulin regimens were subsequently made in only 21 patients (40%), and diabetes specialists agreed with the changes for 11 of these patients. The other 31 patients (60%) received no changes in treatment, and diabetes specialists agreed with that decision for only 10 of these patients.
Although some increase in hypoglycemia might be expected with initiation of tight glycemic control efforts, the solution is not to undertreat hyperglycemia. Hyperglycemia creates an unsafe setting for the treatment of illness and disease. Sliding‐scaleonly regimens are ineffective in securing glycemic control and can result in increases in hypoglycemia as well as hyperglycemic excursions.6, 66 Inappropriate withholding of insulin doses can lead to severe glycemic excursions and even iatrogenic diabetic ketoacidosis (DKA). Systems approaches to avoid the errors outlined above can minimize or even reverse the increased risk of hypoglycemia expected with tighter glycemic targets.51
A SYSTEMS APPROACH IS NEEDED FOR THESE MULTIPLE COMPLEX PROBLEMS
Care is of the hyperglycemic inpatient is inherently complex. Previously established treatments are often inappropriate under conditions of altered insulin resistance, changing patterns of nutrition and carbohydrate exposure, comorbidities, concomitant medications, and rapidly changing medical and surgical status. Patients frequently undergo changes in the route and amount of nutritional exposure, including discrete meals, continuous intravenous dextrose, nil per orem (nothing by mouth status; NPO) status, grazing on nutritional supplements or liquid diets with or without meals, bolus enteral feedings, overnight enteral feedings with daytime grazing, total parenteral nutrition, continuous peritoneal dialysis, and overnight cycling of peritoneal dialysis. Relying on individual expertise and vigilance to negotiate this complex terrain without safeguards, protocols, standardization of orders, and other systems change is impractical and unwise.
Transitions across care providers and locations lead to multiple opportunities for breakdown in the quality, consistency, and safety of care.64, 65 At the time of ward transfer or change of patient status, previous medication and monitoring orders sometimes are purged. At the time of discharge, there may be risk of continuation of anti‐hyperglycemic therapy, initiated to cover medical stress, in doses that will subsequently be unsafe.
In the face of this complexity, educational programs alone will not suffice to improve care. Institutional commitment and systems changes are essential.
MARKED IMPROVEMENT IS POSSIBLE AND TOOLS EXIST: A ROADMAP IS IN PLACE
Fortunately, a roadmap is in place to help us achieve better glycemic control, improve insulin management, and address the long list of current deficiencies in care. This is imperative to develop consistent processes in order to achieve maximum patient quality outcomes that effective glycemic management offers. This roadmap entails 4 components: (1) national awareness, (2) national guidelines, (3) consensus statements, and (4) effective tools. As mentioned above, the first two components of this roadmap are now in place.
As these national guidelines become more widely accepted, the next step will be the incorporation of this into programs like Pay‐for Performance and the Physician Quality Reporting Initiative (PQRI), which will impact reimbursement to both hospitals and providers.
Regarding the third component, a recent multidisciplinary consensus conference1 outlined the essential elements needed for successful implementation of an inpatient glycemic control program which include:
-
An appropriate level of administrative support.
-
Formation of a multidisciplinary steering committee to drive the development of initiatives and empowered to enact change.
-
Assessment of current processes, quality of care, and barriers to practice change.
-
Development and implementation of interventions including standardized order sets, protocols, policies and algorithms with associated educational programs.
-
Metrics for evaluation of glycemic control, hypoglycemia, insulin use patterns, and other aspects of care.
Finally, extensive resources and effective tools are now available to help institutions achieve better inpatient glucose control. The Society of Hospital Medicine (SHM), in conjunction with the ADA, AACE, the American College of Physicians (ACP), the Case Management Society of America (CMSA), the American Society of Consultant Pharmacists, nursing, and diabetic educators have all partnered to produce a comprehensive guide to effective implementation of glycemic control and preventing hypoglycemia.49 This comprehensive workbook is a proven performance improvement framework and is available on the SHM Web site.48 Details and examples of all essential elements are covered in this workbook along with opportunities for marked improvement bolstered by integration of high reliability design features and attention to effective implementation techniques. The remainder of this supplement crystallizes a substantial portion of this material. The AACE has also recently offered a valuable web‐based resource to encourage institutional glycemic control efforts.49
GLYCEMIC CONTROL INITIATIVES CAN BE COST‐EFFECTIVE
Achieving optimal glycemic control safely requires monitoring, education, and other measures, which can be expensive, labor intensive, and require coordination of the services of many hospital divisions. This incremental expense has been shown to be cost‐effective in a variety of settings.1, 84, 85 The costs of glycemic control initiatives have demonstrated a good return on investment via:
-
Improved LOS, readmission rates, morbidity, and mortality.
-
Improved documentation of patient acuity and related payment for acuity.
-
Income generated via incremental physician and allied health professional billing.
CONCLUSION AND SUMMARY
Evidence exists that appropriate management of hyperglycemia improves outcomes, whereas the current state of affairs is that most medical centers currently manage this suboptimally. This is concerning given the magnitude of diabetes and hyperglycemia in our inpatient setting in the United States. To bring awareness to this issue, multiple initiatives (guidelines, certification programs, workbooks, etc.) are available by various organizations including the ADA, AACE, SCIP, NSQIP, IHI, UHC, the Joint Commission, and SHM. However, this is not enough. Change occurs at the local level, and institutional prioritization and support is needed to empower a multidisciplinary steering committee, with appropriate administrative support, to standardize and improve systems in the face of substantial cultural issues and complex barriers. Improved data collection and reporting, incremental monitoring, creation of metrics, and improved documentation are an absolutely necessary necessity to achieve breakthrough levels of improvement.
Now the time is right to make an assertive effort to improve inpatient glycemic control and related issues, and push for appropriate support at your institution to help achieve this in the interest of patient safety and optimal outcomes.
- American College of Endocrinology and American Diabetes Association Consensus Statement on Inpatient Diabetes and Glycemic Control: A call to action.Diabetes Care.2006;29:1955–1962.
- Standards of medical care in diabetes‐‐2008.Diabetes Care.2008;31(Suppl 1):S12–S54.
- ,,, et al.Prevalence of diabetes and impaired fasting glucose in adults in the U.S. population: National Health And Nutrition Examination Survey 1999–2002.Diabetes Care.2006;29:1263–1268.
- Centers for Disease Control and Prevention.National Diabetes Fact Sheet: General Information and National Estimates on Diabetes in the United States, 2005.Atlanta, GA:U.S. Department of Health and Human Services, Centers for Disease Control and Prevention,2005. Available at: http://www.cdc.gov/diabetes/pubs/factsheet05.htm. Accessed September 2007.
- ,,.Economic costs of diabetes in the US in 2002.Diabetes Care.2003;26:917–932.
- ,,.Glycemic control and sliding scale insulin use in medical inpatients with diabetes mellitus.Arch Intern Med.1997;157:545–552.
- ,,.Inpatient management of diabetes mellitus.Am J Med.2002;113:317–323.
- ,,, et al.Management of diabetes and hyperglycemia in hospitals.Diabetes Care.2004;27:553–591.
- ,,, et al.Intensive insulin therapy in the medical ICU.N Engl J Med.2006;354:449–461.
- ,,, et al.Intensive insulin therapy in critically ill patients.N Engl J Med.2001;345:1359–1367.
- .Association between hyperglycemia and increased hospital mortality in a heterogeneous population of critically ill patients.Mayo Clin Proc.2003;78:1471–1478.
- ,,,,.Unrecognized diabetes among hospitalized patients.Diabetes Care.1998;21:246–249.
- ,,,,,.Hyperglycemia: an independent marker of in‐hospital mortality in patients with undiagnosed diabetes.J Clin Endocrinol Metab.2002;87:978–982.
- United States Department of Health and Human Services Agency for Healthcare Research and Quality.2007. Available at: http://hcupnet.ahrq.gov. Accessed December 2007.
- ,,, et al.Glucose metabolism in patients with acute myocardial infarction and no previous diagnosis of diabetes mellitus: a prospective study.Lancet.2002;359:2140–2144.
- .Mechanism by which hyperglycemia plays a role in the setting of acute cardiovascular illness.Rev Cardiovasc Med.2006;7(Suppl 2):S35–S43.
- ,,,,,, et al.Stress hyperglycaemia is an independent predictor of left ventricular remodelling after first anterior myocardial infarction in non‐diabetic patients.Eur Heart J.2007;28:546–552.
- ,.Implications and treatment of acute hyperglycemia in the setting of acute myocardial infarction.Circulation.2007;115:e436–e439.
- ,,,,.Insulin infusion in acute illness.J Clin Invest.2005;115:2069–2072.
- ,,,,.Intensive insulin therapy exerts antiinflammatory effects in critically ill patients and counteracts the adverse effect of low mannose‐gind lectin levels.J Clin Endocrinol Metab.2003;88:1082–1088.
- ,,,,,.Protection of hepatocyte mitochondrial ultrastructure and function by strict blood glucose control with insulin in critically ill patients.Lancet.2005;365:53–59.
- ,,, et al.Intensive insulin therapy protects the endothelium of critically ill patients.J Clin Invest.2005;115:2277–2286.
- ,,,.The association between hyperglycaemia on admission and 180‐day mortality in acute myocardial infarction patients with and without diabetes.Diabet Med.2005;22:1321–1325.
- ,,, et al.Admission glucose and mortality in elderly patients hospitalized with acute myocardial infarction: implications for patients with and without recognized diabetes.Circulation.2005;111:3078–3086.
- ,,,.Glycometabolic state at admission: important risk marker of mortality in conventionally treated patients with diabetes mellitus and acute myocardial infarction: long‐term results from the Diabetes and Insulin‐Glucose Infusion in Acute Myocardial Infarction (DIGAMI) study.Circulation.1999;99:2626–2632.
- ,,,.Stress hyperglycaemia and increased risk of death after myocardial infarction in patients with and without diabetes: a systematic overview.Lancet.2000;355:773–778.
- ,,.How important is hyperglycemia during acute brain infarction?Neurologist.2004;10:195–200.
- ,,,,.Stress hyperglycemia and prognosis of stroke in nondiabetic and diabetic patients: a systematic overview.Stroke.2001;32:2426–2432.
- ,,,.Perioperative glycemic control and the risk of infectious complications in a cohort of adults with diabetes.Diabetes Care.1999;22:1408–1414.
- ,,, et al.Early postoperative glucose control predicts nosocomial infection rate in diabetic patients.JPEN J Parenter Enteral Nutr.1998;22:77–81.
- ,,,,,.The relation between hyperglycemia and outcomes in 2,471 patients admitted to the hospital with community‐acquired pneumonia.Diabetes Care.2005;28:810–815.
- ,,,,.Early peri‐operative glycaemic control and allograft rejection in patients with diabetes mellitus: a pilot study.Transplantation.2001;72:1321–1324.
- ,,, et al.Relation between the duration of remission and hyperglycemia during induction chemotherapy for acute lymphocytic leukemia with a hyperfractionated cyclophosphamide, vincristine, doxorubicin, and dexamethasone/methotrexate‐cytarabine regimen.Cancer.2004;100:1179–1185.
- ,,, et al.Effect of fasting glucose levels on mortality rate in patients with and without diabetes mellitus and coronary artery disease undergoing percutaneous coronary intervention.Am Heart J.2003;146:351–358.
- ,,.Effect of hyperglycemia and continuous intravenous insulin infusions on outcomes of cardiac surgical procedures: the Portland diabetic project.Endocr Pract.2004;10(Suppl 2):21–33.
- ,,, et al.Intraoperative hyperglycemia and perioperative outcomes in cardiac surgery patients.Mayo Clin Proc.2005;80:862–866.
- ,,,,.The association of diabetes and glucose control with surgical‐site infections among cardiothoracic surgery patients.Infect Control Hosp Epidemiol.2001;22:607–612.
- ,,.Glucose control lowers the risk of wound infection in diabetics after open heart operations.Ann Thorac Surg.1997;63:356–361.
- The Portland Protocol. Available at: http://www.providence.org/oregon/grograms_and_services/heart/portlandprotocol/. Accessed September2007.
- .Effect of an intensive glucose management protocol on the mortality of critically ill adult patients.Mayo Clin Proc.2004;79:992–1000.
- ,,, et al.Intensive insulin therapy in mixed medical/surgical intensive care units: benefit versus harm.Diabetes.2006;55:3151–3159.
- ,,.Tight blood glucose control with insulin in the ICU: facts and controversies.Chest.2007;132:268–278.
- ,.Reduction of nosocomial infections in the surgical intensive‐care unit by strict glycemic control.Endocr Pract.2004;10(Suppl 2):46–52.
- ,,,,,, et al.Intensive insulin therapy and pentastarch resuscitation in severe sepsis.N Engl J Med.2008;358:125–139.
- ,.Current controversies around tight glucose control in critically ill patients.Curr Opin Clin Nutr Metab Care.2007;10:206–209.
- ,,,.Designing and implementing insulin infusion protocols and order sets.J Hosp Med.2008;3(5):S42–S54.
- ,,,,,, et al.Randomized study of basal‐bolus insulin therapy in the inpatient management of patients with type 2 diabetes (RABBIT 2 trial).Diabetes Care.2007;30:2181–2186.
- Society of Hospital Medicine. Glycemic control resource room. Available at: http://www.hospitalmedicine.org/ResourceRoomRedesign/GlycemicControl.cfm. Accessed November2007.
- Society of Hospital Medicine. Workbook for improvement: improving glycemic control, preventing hypoglycemia, and optimizing care of the inpatient with hyperglycemia and diabetes. Available at: http://www.hospitalmedicine.org/ResourceRoomRedesign/pdf/GC_Workbook.pdf. Accessed November2007.
- ,,, et al.American College of Endocrinology position statement on inpatient diabetes and metabolic control.Endocr Pract.2004;10:77–82.
- ,,,,.Improved inpatient use of basal insulin, reduced hypoglycemia, and improved glycemic control: effect of structured subcutaneous insulin orders and an insulin management algorithm.J Hosp Med.2008. In press.
- American Association of Clinical Endocrinologists Inpatient Glycemic Control Resource Center.2007. Available at: http://resources.aace.com/index.asp. Accessed December 2007.
- American Heart Association. Get With the Guidelines. Available at: http://www.americanheart.org/getwiththeguidelines. Accessed November2007.
- Joint Commission. Disease Specific‐Care Certification. Available at:http://www.jointcommission.org/CertificationPrograms. Accessed November2007.
- The Joint Commission Disease‐Specific Certification Program. Range JE. Oncology issues. July/August2007:40–41.
- Anonymous.The Diabetes Control and Complications Trial Research Group (DCCT). The effect of intensive treatment of diabetes on the development and progression of long‐term complications in insulin‐dependent diabetes mellitus.N Engl J Med.1993;329:977–986.
- Intensive blood‐glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type, 2 diabetes (UKPDS 33). UK Prospective Diabetes Study (UKPDS) Group.Lancet.1998;352:837–853.
- ,,,.Intensified multifactorial intervention in patients with type 2 diabetes mellitus and microalbuminuria: the Steno type 2 randomised study.Lancet.1999;353:617–622.
- ,,, et al.Utility of HbA1c levels for diabetes case finding in hospitalized patients with hyperglycemia.Diabetes Care.2003;26:1064–1068.
- ,,,.Eliminating inpatient sliding‐scale insulin: a reeducation project with medical house staff.Diabetes Care.2005;28:1008–1011.
- ,.Advanced carbohydrate counting. In:Practical Carbohydrate Counting: A How‐to‐Teach Guide for Health Professionals.Alexandria, VA:American Diabetes Association;2001:26–28.
- ,,,,.The evidence for the effectiveness of medical nutrition therapy in diabetes management.Diabetes Care.2002;25:608–613.
- ,,, et al.Inpatient management of diabetes and hyperglycemia: implications for nutrition practice and the food and nutrition professional.J Am Diet Assoc.2007;107:105–111.
- .The transition from insulin infusions to long‐term diabetes therapy: the argument for insulin analogs.Semin Thorac Cardiovasc Surg.2006;18:366–378.
- .Transitions paper.J Hosp Med.2008.
- ,,,,.Inpatient management of diabetes and hyperglycemia among general medicine patients at a large teaching hospital.J Hosp Med.2006;1:145–150.
- ,,, et al.Clinical inertia.Ann Intern Med.2001;135:825–834.
- ,,, et al.Diabetes care in hospitalized noncritically ill patients: more evidence for clinical inertia and negative therapeutic momentum.J Hosp Med.2007;2:203–211.
- University HealthSystem Consortium.Glycemic control 2005 findings and conclusions. Presented at: Glycemic Control 2005 Knowledge Transfer Meeting; 2005 August 19,2005; Chicago, IL.
- ,.Glycemic chaos (not glycemic control) still the rule for inpatient care: how do we stop the insanity?J Hosp Med.2006;1:141–144.
- ,,,,.Management of diabetes mellitus in hospitalized patients: efficiency and effectiveness of sliding‐scale insulin therapy.Pharmacotherapy.2006;26:1421–1432.
- ,,,,,.Hypoglycemia in hospitalized patients treated with antihyperglycemic agents.J Hosp Med.2007;2:234–240.
- ,,.Hypoglycemia in hospitalized patients.N Engl J Med.1986;315:1245–1250.
- ,,, et al.Predisposing factors for hypoglycemia in the intensive care unit.Crit Care Med.2006;34:96–101.
- ,,,.Association between hyper‐ and hypoglycaemia and 2 year all‐cause mortality risk in diabetic patients with acute coronary events.Eur Heart J.2005;26:1255–1261.
- ,,, et al.U‐shaped relationship of blood glucose with adverse outcomes among patients with ST‐segment elevation myocardial infarction.J Am Coll Cardiol.2005;46:178–180.
- ,,.An unexpected inverse relationship between HbA1c levels and mortality in patients with diabetes and advanced systolic heart failure.Am Heart J.2006;151:91.
- ,,, et al.Glucometrics in patients hospitalized with acute myocardial infarction: defining the optimal outcomes‐based measure of risk.Circulation.2008;117:1018–1027.
- ,,.Hypoglycemia in diabetes.Diabetes Care.2003;26:1902–1912.
- ,,.Hypoglycemia and cardiac arrest in a critically ill patient on strict glycemic control.Anesth Analg.2006;102:549–551.
- ,,,,,.Tight glycemic control in critically injured trauma patients.Ann Surg.2007;246:605–610; discussion 10–12.
- ,.Severe hypoglycemia in critically ill patients: risk factors and outcomes.Crit Care Med.2007;35:2262–2267.
- ,,,.Provider response to insulin‐induced hypoglycemia in hospitalized patients.J Hosp Med.2007;2:258–260.
- ,.Financial implications of glycemic control: results of an inpatient diabetes management program.Endocr Pract.2006;12(Suppl 3):43–48.
- ,,,.Impact of endocrine and diabetes team consultation on hospital length of stay for patients with diabetes.Am J Med.1995;99:22–28.
Medical centers are faced with multiple competing priorities when deciding how to focus their improvement efforts and meet the ever expanding menu of publicly reported and regulatory issues. In this article we expand on the rationale for supporting inpatient glycemic control programs as a priority that should be moved near the top of the list. We review the evidence for establishing glycemic range targets, and also review the limitations of this evidence, acknowledging, as does the American Diabetes Association (ADA), that in both the critical care and non‐critical care venue, glycemic goals must take into account the individual patient's situation as well as hospital system support for achieving these goals.1, 2 We emphasize that inpatient glycemic control programs are needed to address a wide variety of quality and safety issues surrounding the care of the inpatient with diabetes and hyperglycemia, and we wish to elevate the dialogue beyond arguments surrounding adoption of one glycemic target versus another. The Society of Hospital Medicine Glycemic Control Task Force members are not in unanimous agreement with the American Association of Clinical Endocrinologists (AACE)/ADA inpatient glycemic targets. However, we do agree on several other important points, which we will expand on in this article:
-
Uncontrolled hyperglycemia and iatrogenic hypoglycemia are common and potentially dangerous situations that are largely preventable with safe and proven methods.
-
The current state of care for our inpatients with hyperglycemia is unacceptably poor on a broad scale, with substandard education, communication, coordination, and treatment issues.
-
Concerted efforts with changes in the design of the process of care are needed to improve this state of affairs.
DIABETES AND HYPERGLYCEMIA ARE VERY COMMON INPATIENT CONDITIONS
Diabetes mellitus (DM) has reached epidemic proportions in the United States. A reported 9.3% of adults over 20 years of age have diabetes, representing over 20 million persons. Despite increasing awareness, diabetes remains undiagnosed in approximately 30% of these persons.3 Concurrent with the increasing prevalence of diabetes in the U.S. population from 1980 through 2003, the number of hospital discharges with diabetes as any listed diagnosis more than doubled, going from 2.2 to 5.1 million discharges.4 Hospital care for patients with diabetes and hyperglycemia poses a significant health economic burden in the United States, representing over 40 billion dollars in annual direct medical expenditures.5
Hyperglycemia in the hospital may be due to known diabetes, to previously unrecognized diabetes, to prediabetes, and/or to the stress of surgery or illness. Deterioration in glycemic control in the hospital setting is most commonly associated with one or more factors, including stress‐induced release of insulin counterregulatory hormones (catecholamines, cortisol, glucagon, and growth hormone), exogenous administration of high dose glucocorticoids, and suboptimal glycemic management strategies.68 In a Belgian medical intensive care unit (MICU) randomized controlled trial (RCT) of strict versus conventional glycemic control, mean blood glucose (BG) on admission to the unit in the intention to treat group was 162 70 mg/dL (n = 1200),9 and in this group's RCT of 1548 surgical intensive care unit (SICU) patients, BG > 110 mg/dL was observed in over 70% of subjects.10 Mean BG of >145 mg/dL has been reported in 39%11 and BG >200 mg/dL in anywhere from 11% to 31% of intensive care unit (ICU) patients.10, 12 For general medicine and surgery, 1 study of 2030 patients admitted to a teaching hospital revealed that 26% of admissions had a known history of DM and 12% had new hyperglycemia, as evidenced by an admission or in‐hospital fasting BG of 126 mg/dL or more or a random BG of 200 mg/dL or more on 2 or more determinations.13 National and regional estimates on hospital use maintained by the Agency for Healthcare Research and Quality include data concerning diabetes diagnoses alone, without hyperglycemia, and may be displayed by querying its Web site.14 In cardiovascular populations almost 70% of patients having a first myocardial infarction have been reported to have either known DM, previously unrecognized diabetes, or impaired glucose tolerance.15
THE EVIDENCE SUPPORTS INPATIENT GLYCEMIC CONTROL
Evidence: Physiology
The pathophysiologic mechanisms through which hyperglycemia is linked to suboptimal outcomes in the hospital are complex and multifactorial. Although it is beyond the scope of this article to discuss these mechanisms in detail, research has broadly focused in the following areas: (1) immune system dysfunction, associated with a proinflammatory state and impaired white blood cell function; (2) metabolic derangements leading to oxidative stress, release of free fatty acids, reduction in endogenous insulin secretion, and fluid and electrolyte imbalance; and (3) a wide variety of vascular system responses (eg, endothelial dysfunction with impairment of tissue perfusion, a prothrombotic state, increased platelet aggregation, and left ventricular dysfunction).8, 1618
Conversely administration of insulin suppresses or reverses many of these abnormalities including generation of reactive oxygen species (ROS) and activation of inflammatory mechanisms,19 and leads to a fall in C‐reactive protein, which accompanied the clinical benefit of intensive insulin therapy (IIT) in the Leuven, Belgium, ICU population,20 and prevents mitochondrial abnormalities in hepatocytes.21 In the same surgical ICU cohort, Langouche et al.22 report suppression of intracellular adhesion molecule‐1 (ICAM‐1) and E‐selectin, markers of inflammation, and reduction in plasma nitric oxide (NO) and innate nitric oxide (iNOS) expression with insulin administration in patients treated with intravenous (IV) IIT.22 These data further support the role of insulin infusion in suppressing inflammation and endothelial dysfunction. The authors suggest that maintaining normoglycemia with IIT during critical illness protects the endothelium, thereby contributing to prevention of organ failure and death.22 Based on accumulating data in the literature such as that cited above, it has been suggested that a new paradigm in which glucose and insulin are related not only through their metabolic action but also through inflammatory mechanisms offers important potential therapeutic opportunities.19
Evidence: Epidemiology/Observational Studies/Non‐RCT Interventional Studies
A strong association between hospital hyperglycemia and negative outcomes has been reported in numerous observational studies in diverse adult medical and surgical settings. In over 1800 hospital admissions, those with new hyperglycemia had an in‐hospital mortality rate of 16% compared with 3% mortality in patients with known diabetes and 1.7% in normoglycemic patients (P < 0.01). These data suggest that hyperglycemia due to previously unrecognized diabetes may be an independent marker of in‐hospital mortality.13
Hyperglycemia has been linked to adverse outcomes in myocardial infarction, stroke,2328 postoperative nosocomial infection risk, pneumonia, renal transplant, cancer chemotherapy, percutaneous coronary interventions, and cardiac surgery.2938 These observational studies have the usual limitations inherent in their design. Demonstrating a strong association of hyperglycemia with adverse outcomes is not a guarantee that the hyperglycemia is the cause for the poor outcome, as hyperglycemia can reflect a patient under more stress who is at a higher risk for adverse outcome. By the same token, the strong association of hyperglycemia with the risk of poor outcomes seen in these studies does not guarantee that euglycemia would mitigate this risk.
Nonetheless, there are several factors that make the body of evidence for glycemic control more compelling. First, the association has a rational physiologic basis as described above. Second, the associations are consistent across a variety of patient populations and disease entities, and demonstrate a dose‐response relationship. Third, in studies that control for comorbidities and severity of illness, hyperglycemia persists as an independent risk factor for adverse outcomes, whether the patient has a preexisting diagnosis of diabetes or not. Last, non‐RCT interventional studies and RCTs largely reinforce these studies.
The Portland Diabetic Project has reported prospective, nonrandomized data over 17 years on the use of an IV insulin therapy protocol in cardiac surgery patients.38 This program has implemented stepped lowering of target BG, with the most recent data report implementing a goal BG <150 mg/dL.35 The current protocol uses a BG target of 70110 mg/dL, but results have not yet been published.39 Mortality and deep sternal wound infection rates for patients with diabetes who remain on the IV insulin protocol for 3 days have been lowered to levels equivalent to those for nondiabetic patients. This group has also reported reductions in length of stay and cost‐effectiveness of targeted glycemic control in the cardiac surgery population.35 Their data have to a large extent driven a nationwide movement to implement targeted BG control in cardiac surgery patients.
Another large ICU study (mixed medical‐surgical, n = 800 patients) also supports a benefit through targeted BG control (130.7 versus 152.3 mg/dL, P < 0.001) when compared with historical controls. This study demonstrated reduction in in‐hospital mortality (relative risk reduction 29.3%, P = 0.002), duration of ICU stay (10.8%, P = 0.04), acute renal failure (75%, P = 0.03), and blood transfusions (18.7%, P = 0.002),40 representing a similar magnitude of effect as was demonstrated by the Belgian group.
Evidence: RCTs
Evidence is accumulating that demonstrates an advantage in terms of morbidity and mortality when targeted glycemic control using intravenous insulin infusion is implemented in the hospital. The most robust data have been reported from ICU and cardiac surgery settings. The largest randomized, controlled study to date enrolled 1548 patients in a surgical ICU in Leuven, Belgium who were randomized to either intensive (IT) or conventional (CT) insulin therapy. Mean glucose attained was 103 19 and 153 33 mg/dL in each arm, respectively. The intensive insulin group demonstrated a reduction in both ICU (4.6% versus 8.0%) and in‐hospital mortality (7.2% versus 10.9%), as well as bloodstream infections, acute renal failure, transfusions, and polyneuropathy, the latter being reflected by duration of mechanical ventilation (P < 0.01 for all). Although a similar study in an MICU did not achieve statistical significance in the overall intention‐to‐treat analysis, it did demonstrate reductions in mortality (from 52.5% to 43.0%) in patients with at least 3 days of ICU treatment. It should also be noted that in this MICU population hypoglycemia rates were higher and level of glycemic control attained not as rigorous as in the same group's SICU cohort, factors which may have had an impact on observed outcomes. A meta‐analysis of these two Leuven, Belgium, studies demonstrated a reduction in mortality (23.6% versus 20.4%, absolute risk reduction [ARR] 3.2%, P = 0.004)) in all patients treated with IIT, with a larger reduction in mortality (37.9% versus 30.1%, ARR 7.8%, P = 0.002) observed in patients with at least 3 days of IIT, as well as substantial reductions in morbidity.9, 10, 41, 42
Several other studies must be mentioned in this context. A small (n = 61), randomized study in another SICU did not show a mortality benefit, perhaps because the number of subjects was not adequate to reach statistical significance, but did result in a significant reduction in nosocomial infections in patients receiving IIT (BG = 125 versus 179 mg/dL, P < 0.001).43 Two international multicenter studies recently stopped enrollment due to excess rates of hypoglycemia. The Volume Substitution and Insulin Therapy in Severe Sepsis (VISEP) study, in a mixed medical and surgical sepsis population, showed no significant reduction in mortality in the intensively‐treated group. Serious adverse events were reported according to standard definitions. Enrollment was stopped before the full number of subjects had been randomized. Among the 537 evaluable cases, hypoglycemia (BG < 40 mg/dL) was reported as 17.0% in the IT group and 4.1% (P < 0.001) in the control group,44 and the rate of serious adverse events was higher in the IT group (10.9% versus 5.2%, P = 0.01). It is notable that the rate of hypoglycemia was comparable to the 18.7% rate seen in the IT group in the Leuven, Belgium, medical ICU study.9 The Glucontrol study enrolled 855 medical and surgical ICU patients and was similarly terminated because of hypoglycemia (BG < 40 mg/dL) at a rate of 8.6% compared to 2.4% in the control group (P < 0.001). Insulin infusion protocols and outcome data have not yet been published.42, 45
These studies with very high hypoglycemia rates each used an algorithm based on the Leuven, Belgium, protocol. The rates of severe hypoglycemia are 34 that reported by a variety of others achieving similar or identical glycemic targets. Hypoglycemia should not be construed as a reason to not use a standardized insulin infusion protocol. In comparing protocols that have been published, it is apparent that rates of hypoglycemia differ substantially and that performance results of some algorithms are not necessarily replicable across sites.46 Dose‐defining designs can be substantively more sophisticated than those used in the trials mentioned, in some cases incorporating principles of control engineering. The variability of hypoglycemia rates under differing insulin infusion protocols is a compelling reason to devote institutional effort to monitoring the efficacy and safety of the infusion protocols that are used.
High‐level evidence from randomized, controlled trials demonstrating outcomes benefit through targeted BG control outside the ICU is lacking at this point in time, but it must be noted that feasibility is suggested by a recent randomized control trial (RABBIT2) that demonstrated the superiority of basal bolus insulin regimens to sliding scale insulin in securing glycemic control, without any increase in hypoglycemia.47
Summing Up the Evidence
It is clear that hyperglycemia is associated with negative clinical outcomes throughout the hospital, and level A evidence is available to support tight glucose control in the SICU setting. However, in view of the imperfect and incomplete nature of the evidence, controversy persists around how stringent glycemic targets should be in the ICU, on whether glycemic targets should differ between SICU and MICU patients, and especially what the targets should be in the non‐ICU setting. There should be hesitancy to extrapolate glycemic targets to be applied beyond the populations that have been studied with RCTs or to assume benefit for medical conditions that have not been examined for the impact of interventions to control hyperglycemia. Institutions might justifiably choose more liberal targets than those promoted in national recommendations/guidelines2, 4850 until safe attainment of more moderate goals is demonstrated. However, even critics agree that uncontrolled hyperglycemia exceeding 180200 mg/dL in any acute care setting is undesirable. Moreover, strong observational data showing the hazards of hyperglycemia in noncritical care units (even after adjustment for severity of illness) combined with the high rate of adverse drug events associated with insulin use, argue strongly for a standardized approach to treating diabetes and hyperglycemia in the hospital. Even though no RCTs exist demonstrating outcomes benefits of achieving glycemic target on wards, the alternatives to control of hyperglycemia using scheduled insulin therapy are unacceptable. Oral agent therapy is potentially dangerous and within the necessary timeframe is likely to be ineffective; sliding scale management is inferior to basal‐bolus insulin therapy, as shown inan RCT,47 and is unsafe; and on the wards improved glycemic control can be achieved simultaneously with a reduction in hypoglycemia.51
INPATIENT GLYCEMIC CONTROL IS INCREASINGLY INCORPORATED INTO PUBLIC REPORTING, GUIDELINES, REGULATORY AGENCY, AND NATIONAL QUALITY INITIATIVE PRIORITIES
National quality initiatives, public reporting, pay‐for‐performance, and guideline‐based care continue to play an increasingly important role in the U.S. healthcare system. Over the years these initiatives have focused on various disease states (venous thromboembolism, congestive heart failure, community‐acquired pneumonia, etc.) in an attempt to standardize care and improve patient safety and quality. Inpatient hyperglycemic control is also increasingly being incorporated into public reporting, regulatory compliance, and national quality initiatives.
Professional organizations such as the ADA2 and AACE50 have published guidelines supporting improved glycemic control, the safe use of insulin, and other measures to improve care for hyperglycemic inpatients. The AACE has a Web site dedicated to hospital hyperglycemia.52 The Society of Hospital Medicine48 has created a resource room on its Web site and a workbook for improvement49 on optimizing the care of inpatients with hyperglycemia and diabetes. The guidelines and Web sites help raise awareness and educate physicians and healthcare workers in inpatient glucose management. The American Heart Association has incorporated specific recommendation regarding inpatient diabetic management in its Get With the Guidelines.53
The Joint Commission54 has developed an advanced disease‐specific certification on inpatient diabetes. Disease management programs are important components of complex healthcare systems that serve to coordinate chronic care, promote early detection and prevention, and reduce overall healthcare costs. Certification is increasingly important to providers, payers, and healthcare institutions because it demonstrates a commitment to quality and patient safety. The Joint Commission disease‐specific care certification is a patient‐centered model focusing on the delivery of clinical care and relationship between the practitioner and the patient. The evaluation and resulting certification by the Joint Commission is based on 3 core components: (1) an assessment of compliance with consensus‐based national standards; (2) the effective use of established clinical practice guidelines to manage and optimize care; and (3) an organized approach to performance measurement and improved activities.55 For inpatient diabetes, the Joint Commission program has 7 major elements following the ADA recommendations, including general recommendations regarding diabetic documentation, BG targets, preventing hypoglycemia, diabetes care providers, diabetes self‐management education, medical nutrition therapy, and BG monitoring.54 This mirrors the Call to Action Consensus Conference essential elements for successful glycemic control programs.1
Other organizations such as the Surgical Care Improvement Partnership (SCIP) and National Surgical Quality Improvement Program (NSQIP) have included perioperative glycemic control measures, as it impacts surgical wound infections. The University HealthSystem Consortium (UHC) has benchmarking data and endorses perioperative glycemic control measures, whereas the Institute for Healthcare Improvement (IHI) has focused on safe use of insulin practices in its 5 Million Lives campaign.
HOSPITALIZATION IS A MOMENT OF OPPORTUNITY TO ASSESS AND INTERVENE
The benefits of outpatient glycemic control and quality preventive care are well established, and the reduction of adverse consequences of uncontrolled diabetes are a high priority in ambulatory medicine.5658 Hospitalization provides an opportunity to identify previously undiagnosed diabetes or prediabetes and, for patients with known diabetes, to assess and impact upon the long term course of diabetes.
As a first step, unless a recent hemoglobin A1C (HbA1c) is known, among hospitalized hyperglycemic patients an HbA1C should be obtained upon admission. Greci et al.59 showed that an HbA1c level >6.0% was 100% specific (14/14) and 57% sensitive (12/21) for the diagnosis of diabetes. Among patients having known diabetes, an HbA1C elevation on admission may justify intensification of preadmission management at the time of discharge. If discharge and postdischarge adjustments of preadmission regimens are planned in response to admission A1C elevations, then the modified long‐term treatment strategy can improve the A1C in the ambulatory setting.60 Moreover, the event of hospitalization is the ideal teachable moment for patients and their caregivers to improve self‐care activities. Yet floor nurses may be overwhelmed by the tasks of patient education. For ideal patient education, both a nutritionist and a diabetes nurse educator are needed to assess compliance with medication, diet, and other aspects of care.6163 There also is need for outpatient follow‐up education. Finally, at the time of discharge, there is a duty and an opportunity for the diabetes provider to communicate with outpatient care providers about the patient's regimen and glycemic control, and also, based on information gathered during the admission, to convey any evidence that might support the need for a change of long‐term strategy.64 Unfortunately, the opportunity that hospitalization presents to assess, educate, and intervene frequently is underused.1, 8, 51, 65
LARGE GAPS EXIST BETWEEN CURRENT AND OPTIMAL CARE
Despite the evidence that inpatient glycemic control is important for patient outcomes, and despite guidelines recommending tighter inpatient glycemic control, clinical practice has been slow to change. In many institutions, inpatient glycemic management has not improved over the past decade, and large gaps remain between current practice and optimal practice.
Studies of individual institutions provide several insights into gaps in care. For example, Schnipper et al.66 examined practices on the general medicine service of an academic medical center in Boston in 2004. Among 107 prospectively identified patients with a known diagnosis of diabetes or at least 1 glucose reading >200 mg/dL (excluding patients with diabetic ketoacidosis, hyperglycemic hyperosmolar state, or pregnancy), they found scheduled long‐acting insulin prescribed in 43% of patients, scheduled short‐acting/rapid‐acting insulin in only 4% of patients, and 80 of 89 patients (90%) on the same sliding scale insulin regimen despite widely varying insulin requirements. Thirty‐one percent of glucose readings were >180 mg/dL compared with 1.2% of readings <60 mg/dL (but 11% of patients had at least 1 episode of hypoglycemia). Of the 75 patients with at least 1 episode of hyperglycemia or hypoglycemia, only 35% had any change to their insulin regimen during the first 5 days of the hospitalization.
Other studies have confirmed this concept of clinical inertia (ie, recognition of the problem but failure to act).67 A study by Cook et al.68 of all hospitalized non‐ICU patients with diabetes or hyperglycemia and length of stay of 3 days between 2001 and 2004 showed that 20% of patients had persistent hyperglycemia during the hospitalization (defined as a mean glucose >200 mg/dL). Forty‐six percent of patients whose average glucose was in the top tertile did not have their insulin regimen intensified to a combination of short‐acting/rapid‐acting and long‐acting insulin, and 35% of these patients either had no change in their total daily insulin dose or actually had a decrease in their dose when comparing the last 24 hours with the first 24 hours of hospitalization, a concept they term negative therapeutic momentum.
Perhaps the most well‐balanced view of the current state of medical practice comes from the UHC benchmarking project.69 UHC is an alliance of 90 academic health centers. For the diabetes project, each institution reviewed the records of 50 randomly selected patients over 18 years of age with at least a 72‐hour length of stay, 1 of 7 prespecified Diagnosis Related Group (DRG) codes, and at least 2 consecutive glucose readings >180 mg/dL or the receipt of insulin any time during the hospitalization. Patients with a history of pancreatic transplant, pregnant at the time of admission, receiving hospice or comfort care, or receiving insulin for a reason other than glucose management were excluded. The study showed widespread gaps in processes and outcomes (Table 1). Moreover, performance varied widely across hospitals. For example, the morning glucose in the ICU on the second measurement day was 110 mg/dL in 18% of patients for the median‐performing hospital, with a range of 0% to 67% across all 37 measured hospitals. In the non‐ICU setting on the second measurement day, 26% of patients had all BG measurements = 180 mg/dL in the median‐performing hospital, with a range of 7% to 48%. Of note, hypoglycemia was relatively uncommon: in the median hospital, 2.4% of patient‐days had 1 or more BG readings <50 mg/dL (range: 0%8.6%). Finally, in the median‐performing hospital, effective insulin therapy (defined as short‐acting/rapid‐acting and long‐acting subcutaneous insulin, continuous insulin infusion, or subcutaneous insulin pump therapy) was prescribed in 45% of patients, with a range of 12% to 77% across measured hospitals.
| Key Performance Measure | Results for Median‐Performing Hospital (%) |
|---|---|
| |
| Documentation of diabetes | 100 |
| Hob A1c assessment within 30 days | 36.1 |
| Glucose measurement within 8 hours of admission | 78.6 |
| Glucose monitoring 4 times a day | 85.4 |
| Median glucose reading > 200 mg/dL | 10.3 |
| Effective insulin therapy* | 44.7 |
| ICU day 2 morning glucose 110 mg/dL | 17.7 |
| Non‐ICU day 2 all glucose readings 180 mg/dL | 26.3 |
| Patient‐days with at least 1 glucose reading < 50 mg/dL | 2.4 |
FREQUENT PROBLEMS WITH COMMUNICATION AND COORDINATION
Those who work closely with frontline practitioners striving to improve inpatient glycemic management have noticed other deficiencies in care.1, 70 These include: a lack of coordination between feeding, BG measurement, and insulin administration, leading to mistimed and incorrectly dosed insulin; frequent use of sliding‐scale only regimens despite evidence that they are useless at best and harmful at worst;6, 47, 60, 71 discharge summaries that often do not mention follow‐up plans for hyperglycemic management; incomplete patient educational programs; breakdowns in care at transition points; nursing and medical staffs that are unevenly educated about the proper use of insulin; and patients who are often angry or confused about their diabetes care in the hospital. Collectively, these gaps in care serve as prime targets for any glycemic control program.
HYPOGLYCEMIA IS A PROMINENT INPATIENT SAFETY CONCERN
Hypoglycemia is common in the inpatient setting and is a legitimate safety concern. In a recently reported series of 2174 hospitalized patients receiving antihyperglycemic agents, it was found that 9.5% of patients experienced a total 484 hypoglycemic episodes (defined as 60 mg/dL).72 Hypoglycemia often occurred in the setting of insulin therapy and frequently resulted from a failure to recognize trends in BG readings or other clues that a patient was at risk for developing hypoglycemia.73 A common thread is the risk created by interruption of carbohydrate intake, noted by Fischer et al.73 and once again in the recent ICU study by Vriesendorp et al.74 Sources of error include: lack of coordination between feeding and medication administration, leading to mistiming of insulin action; lack of sufficient frequency in BG monitoring; lack of clarity or uniformity in the writing of orders; failure to recognize changes in insulin requirements due to advanced age, renal failure, liver disease, or change in clinical status; steroid use with subsequent tapering or interruption; changes in feeding; failure to reconcile medications; inappropriate use of oral antihyperglycemic agents, and communication or handoff failures.
It has been difficult to sort out whether hypoglycemia is a marker of severity of illness or whether it is an independent factor leading to poor outcomes. Observational studies lend credibility to the concept that patients having congestive heart failure or myocardial infarction may be at risk for excessive mortality if their average BG resides in the low end of the normal range.7578 Sympathetic activation occurs as the threshold for hypoglycemia is approached, such as occurs at BG = 70 or 72 mg/dL.79 Patients living with BG levels observed to be in the low end of the normal range might experience more severe but unobserved and undocumented episodes of neuroglycopenia. Arrhythmia and fatality have been directly attributed to strict glycemic control.80, 81 We are confronted with the need to interpret well conducted observational studies, evaluating subgroups at risk, and using multivariate analysis to assess the impact of hypoglycemia upon outcomes.82 In such studies, we will need to examine high‐risk subgroups, including cardiac patients, in particular, for the possibility that there is a J‐shaped curve for mortality as a function of average BG.
Unfortunately, clinical inertia exists in response to hypoglycemia just as it does with hyperglycemia. One recent study examined 52 patients who received intravenous 50% dextrose solution for an episode of hypoglycemia.83 Changes to insulin regimens were subsequently made in only 21 patients (40%), and diabetes specialists agreed with the changes for 11 of these patients. The other 31 patients (60%) received no changes in treatment, and diabetes specialists agreed with that decision for only 10 of these patients.
Although some increase in hypoglycemia might be expected with initiation of tight glycemic control efforts, the solution is not to undertreat hyperglycemia. Hyperglycemia creates an unsafe setting for the treatment of illness and disease. Sliding‐scaleonly regimens are ineffective in securing glycemic control and can result in increases in hypoglycemia as well as hyperglycemic excursions.6, 66 Inappropriate withholding of insulin doses can lead to severe glycemic excursions and even iatrogenic diabetic ketoacidosis (DKA). Systems approaches to avoid the errors outlined above can minimize or even reverse the increased risk of hypoglycemia expected with tighter glycemic targets.51
A SYSTEMS APPROACH IS NEEDED FOR THESE MULTIPLE COMPLEX PROBLEMS
Care is of the hyperglycemic inpatient is inherently complex. Previously established treatments are often inappropriate under conditions of altered insulin resistance, changing patterns of nutrition and carbohydrate exposure, comorbidities, concomitant medications, and rapidly changing medical and surgical status. Patients frequently undergo changes in the route and amount of nutritional exposure, including discrete meals, continuous intravenous dextrose, nil per orem (nothing by mouth status; NPO) status, grazing on nutritional supplements or liquid diets with or without meals, bolus enteral feedings, overnight enteral feedings with daytime grazing, total parenteral nutrition, continuous peritoneal dialysis, and overnight cycling of peritoneal dialysis. Relying on individual expertise and vigilance to negotiate this complex terrain without safeguards, protocols, standardization of orders, and other systems change is impractical and unwise.
Transitions across care providers and locations lead to multiple opportunities for breakdown in the quality, consistency, and safety of care.64, 65 At the time of ward transfer or change of patient status, previous medication and monitoring orders sometimes are purged. At the time of discharge, there may be risk of continuation of anti‐hyperglycemic therapy, initiated to cover medical stress, in doses that will subsequently be unsafe.
In the face of this complexity, educational programs alone will not suffice to improve care. Institutional commitment and systems changes are essential.
MARKED IMPROVEMENT IS POSSIBLE AND TOOLS EXIST: A ROADMAP IS IN PLACE
Fortunately, a roadmap is in place to help us achieve better glycemic control, improve insulin management, and address the long list of current deficiencies in care. This is imperative to develop consistent processes in order to achieve maximum patient quality outcomes that effective glycemic management offers. This roadmap entails 4 components: (1) national awareness, (2) national guidelines, (3) consensus statements, and (4) effective tools. As mentioned above, the first two components of this roadmap are now in place.
As these national guidelines become more widely accepted, the next step will be the incorporation of this into programs like Pay‐for Performance and the Physician Quality Reporting Initiative (PQRI), which will impact reimbursement to both hospitals and providers.
Regarding the third component, a recent multidisciplinary consensus conference1 outlined the essential elements needed for successful implementation of an inpatient glycemic control program which include:
-
An appropriate level of administrative support.
-
Formation of a multidisciplinary steering committee to drive the development of initiatives and empowered to enact change.
-
Assessment of current processes, quality of care, and barriers to practice change.
-
Development and implementation of interventions including standardized order sets, protocols, policies and algorithms with associated educational programs.
-
Metrics for evaluation of glycemic control, hypoglycemia, insulin use patterns, and other aspects of care.
Finally, extensive resources and effective tools are now available to help institutions achieve better inpatient glucose control. The Society of Hospital Medicine (SHM), in conjunction with the ADA, AACE, the American College of Physicians (ACP), the Case Management Society of America (CMSA), the American Society of Consultant Pharmacists, nursing, and diabetic educators have all partnered to produce a comprehensive guide to effective implementation of glycemic control and preventing hypoglycemia.49 This comprehensive workbook is a proven performance improvement framework and is available on the SHM Web site.48 Details and examples of all essential elements are covered in this workbook along with opportunities for marked improvement bolstered by integration of high reliability design features and attention to effective implementation techniques. The remainder of this supplement crystallizes a substantial portion of this material. The AACE has also recently offered a valuable web‐based resource to encourage institutional glycemic control efforts.49
GLYCEMIC CONTROL INITIATIVES CAN BE COST‐EFFECTIVE
Achieving optimal glycemic control safely requires monitoring, education, and other measures, which can be expensive, labor intensive, and require coordination of the services of many hospital divisions. This incremental expense has been shown to be cost‐effective in a variety of settings.1, 84, 85 The costs of glycemic control initiatives have demonstrated a good return on investment via:
-
Improved LOS, readmission rates, morbidity, and mortality.
-
Improved documentation of patient acuity and related payment for acuity.
-
Income generated via incremental physician and allied health professional billing.
CONCLUSION AND SUMMARY
Evidence exists that appropriate management of hyperglycemia improves outcomes, whereas the current state of affairs is that most medical centers currently manage this suboptimally. This is concerning given the magnitude of diabetes and hyperglycemia in our inpatient setting in the United States. To bring awareness to this issue, multiple initiatives (guidelines, certification programs, workbooks, etc.) are available by various organizations including the ADA, AACE, SCIP, NSQIP, IHI, UHC, the Joint Commission, and SHM. However, this is not enough. Change occurs at the local level, and institutional prioritization and support is needed to empower a multidisciplinary steering committee, with appropriate administrative support, to standardize and improve systems in the face of substantial cultural issues and complex barriers. Improved data collection and reporting, incremental monitoring, creation of metrics, and improved documentation are an absolutely necessary necessity to achieve breakthrough levels of improvement.
Now the time is right to make an assertive effort to improve inpatient glycemic control and related issues, and push for appropriate support at your institution to help achieve this in the interest of patient safety and optimal outcomes.
Medical centers are faced with multiple competing priorities when deciding how to focus their improvement efforts and meet the ever expanding menu of publicly reported and regulatory issues. In this article we expand on the rationale for supporting inpatient glycemic control programs as a priority that should be moved near the top of the list. We review the evidence for establishing glycemic range targets, and also review the limitations of this evidence, acknowledging, as does the American Diabetes Association (ADA), that in both the critical care and non‐critical care venue, glycemic goals must take into account the individual patient's situation as well as hospital system support for achieving these goals.1, 2 We emphasize that inpatient glycemic control programs are needed to address a wide variety of quality and safety issues surrounding the care of the inpatient with diabetes and hyperglycemia, and we wish to elevate the dialogue beyond arguments surrounding adoption of one glycemic target versus another. The Society of Hospital Medicine Glycemic Control Task Force members are not in unanimous agreement with the American Association of Clinical Endocrinologists (AACE)/ADA inpatient glycemic targets. However, we do agree on several other important points, which we will expand on in this article:
-
Uncontrolled hyperglycemia and iatrogenic hypoglycemia are common and potentially dangerous situations that are largely preventable with safe and proven methods.
-
The current state of care for our inpatients with hyperglycemia is unacceptably poor on a broad scale, with substandard education, communication, coordination, and treatment issues.
-
Concerted efforts with changes in the design of the process of care are needed to improve this state of affairs.
DIABETES AND HYPERGLYCEMIA ARE VERY COMMON INPATIENT CONDITIONS
Diabetes mellitus (DM) has reached epidemic proportions in the United States. A reported 9.3% of adults over 20 years of age have diabetes, representing over 20 million persons. Despite increasing awareness, diabetes remains undiagnosed in approximately 30% of these persons.3 Concurrent with the increasing prevalence of diabetes in the U.S. population from 1980 through 2003, the number of hospital discharges with diabetes as any listed diagnosis more than doubled, going from 2.2 to 5.1 million discharges.4 Hospital care for patients with diabetes and hyperglycemia poses a significant health economic burden in the United States, representing over 40 billion dollars in annual direct medical expenditures.5
Hyperglycemia in the hospital may be due to known diabetes, to previously unrecognized diabetes, to prediabetes, and/or to the stress of surgery or illness. Deterioration in glycemic control in the hospital setting is most commonly associated with one or more factors, including stress‐induced release of insulin counterregulatory hormones (catecholamines, cortisol, glucagon, and growth hormone), exogenous administration of high dose glucocorticoids, and suboptimal glycemic management strategies.68 In a Belgian medical intensive care unit (MICU) randomized controlled trial (RCT) of strict versus conventional glycemic control, mean blood glucose (BG) on admission to the unit in the intention to treat group was 162 70 mg/dL (n = 1200),9 and in this group's RCT of 1548 surgical intensive care unit (SICU) patients, BG > 110 mg/dL was observed in over 70% of subjects.10 Mean BG of >145 mg/dL has been reported in 39%11 and BG >200 mg/dL in anywhere from 11% to 31% of intensive care unit (ICU) patients.10, 12 For general medicine and surgery, 1 study of 2030 patients admitted to a teaching hospital revealed that 26% of admissions had a known history of DM and 12% had new hyperglycemia, as evidenced by an admission or in‐hospital fasting BG of 126 mg/dL or more or a random BG of 200 mg/dL or more on 2 or more determinations.13 National and regional estimates on hospital use maintained by the Agency for Healthcare Research and Quality include data concerning diabetes diagnoses alone, without hyperglycemia, and may be displayed by querying its Web site.14 In cardiovascular populations almost 70% of patients having a first myocardial infarction have been reported to have either known DM, previously unrecognized diabetes, or impaired glucose tolerance.15
THE EVIDENCE SUPPORTS INPATIENT GLYCEMIC CONTROL
Evidence: Physiology
The pathophysiologic mechanisms through which hyperglycemia is linked to suboptimal outcomes in the hospital are complex and multifactorial. Although it is beyond the scope of this article to discuss these mechanisms in detail, research has broadly focused in the following areas: (1) immune system dysfunction, associated with a proinflammatory state and impaired white blood cell function; (2) metabolic derangements leading to oxidative stress, release of free fatty acids, reduction in endogenous insulin secretion, and fluid and electrolyte imbalance; and (3) a wide variety of vascular system responses (eg, endothelial dysfunction with impairment of tissue perfusion, a prothrombotic state, increased platelet aggregation, and left ventricular dysfunction).8, 1618
Conversely administration of insulin suppresses or reverses many of these abnormalities including generation of reactive oxygen species (ROS) and activation of inflammatory mechanisms,19 and leads to a fall in C‐reactive protein, which accompanied the clinical benefit of intensive insulin therapy (IIT) in the Leuven, Belgium, ICU population,20 and prevents mitochondrial abnormalities in hepatocytes.21 In the same surgical ICU cohort, Langouche et al.22 report suppression of intracellular adhesion molecule‐1 (ICAM‐1) and E‐selectin, markers of inflammation, and reduction in plasma nitric oxide (NO) and innate nitric oxide (iNOS) expression with insulin administration in patients treated with intravenous (IV) IIT.22 These data further support the role of insulin infusion in suppressing inflammation and endothelial dysfunction. The authors suggest that maintaining normoglycemia with IIT during critical illness protects the endothelium, thereby contributing to prevention of organ failure and death.22 Based on accumulating data in the literature such as that cited above, it has been suggested that a new paradigm in which glucose and insulin are related not only through their metabolic action but also through inflammatory mechanisms offers important potential therapeutic opportunities.19
Evidence: Epidemiology/Observational Studies/Non‐RCT Interventional Studies
A strong association between hospital hyperglycemia and negative outcomes has been reported in numerous observational studies in diverse adult medical and surgical settings. In over 1800 hospital admissions, those with new hyperglycemia had an in‐hospital mortality rate of 16% compared with 3% mortality in patients with known diabetes and 1.7% in normoglycemic patients (P < 0.01). These data suggest that hyperglycemia due to previously unrecognized diabetes may be an independent marker of in‐hospital mortality.13
Hyperglycemia has been linked to adverse outcomes in myocardial infarction, stroke,2328 postoperative nosocomial infection risk, pneumonia, renal transplant, cancer chemotherapy, percutaneous coronary interventions, and cardiac surgery.2938 These observational studies have the usual limitations inherent in their design. Demonstrating a strong association of hyperglycemia with adverse outcomes is not a guarantee that the hyperglycemia is the cause for the poor outcome, as hyperglycemia can reflect a patient under more stress who is at a higher risk for adverse outcome. By the same token, the strong association of hyperglycemia with the risk of poor outcomes seen in these studies does not guarantee that euglycemia would mitigate this risk.
Nonetheless, there are several factors that make the body of evidence for glycemic control more compelling. First, the association has a rational physiologic basis as described above. Second, the associations are consistent across a variety of patient populations and disease entities, and demonstrate a dose‐response relationship. Third, in studies that control for comorbidities and severity of illness, hyperglycemia persists as an independent risk factor for adverse outcomes, whether the patient has a preexisting diagnosis of diabetes or not. Last, non‐RCT interventional studies and RCTs largely reinforce these studies.
The Portland Diabetic Project has reported prospective, nonrandomized data over 17 years on the use of an IV insulin therapy protocol in cardiac surgery patients.38 This program has implemented stepped lowering of target BG, with the most recent data report implementing a goal BG <150 mg/dL.35 The current protocol uses a BG target of 70110 mg/dL, but results have not yet been published.39 Mortality and deep sternal wound infection rates for patients with diabetes who remain on the IV insulin protocol for 3 days have been lowered to levels equivalent to those for nondiabetic patients. This group has also reported reductions in length of stay and cost‐effectiveness of targeted glycemic control in the cardiac surgery population.35 Their data have to a large extent driven a nationwide movement to implement targeted BG control in cardiac surgery patients.
Another large ICU study (mixed medical‐surgical, n = 800 patients) also supports a benefit through targeted BG control (130.7 versus 152.3 mg/dL, P < 0.001) when compared with historical controls. This study demonstrated reduction in in‐hospital mortality (relative risk reduction 29.3%, P = 0.002), duration of ICU stay (10.8%, P = 0.04), acute renal failure (75%, P = 0.03), and blood transfusions (18.7%, P = 0.002),40 representing a similar magnitude of effect as was demonstrated by the Belgian group.
Evidence: RCTs
Evidence is accumulating that demonstrates an advantage in terms of morbidity and mortality when targeted glycemic control using intravenous insulin infusion is implemented in the hospital. The most robust data have been reported from ICU and cardiac surgery settings. The largest randomized, controlled study to date enrolled 1548 patients in a surgical ICU in Leuven, Belgium who were randomized to either intensive (IT) or conventional (CT) insulin therapy. Mean glucose attained was 103 19 and 153 33 mg/dL in each arm, respectively. The intensive insulin group demonstrated a reduction in both ICU (4.6% versus 8.0%) and in‐hospital mortality (7.2% versus 10.9%), as well as bloodstream infections, acute renal failure, transfusions, and polyneuropathy, the latter being reflected by duration of mechanical ventilation (P < 0.01 for all). Although a similar study in an MICU did not achieve statistical significance in the overall intention‐to‐treat analysis, it did demonstrate reductions in mortality (from 52.5% to 43.0%) in patients with at least 3 days of ICU treatment. It should also be noted that in this MICU population hypoglycemia rates were higher and level of glycemic control attained not as rigorous as in the same group's SICU cohort, factors which may have had an impact on observed outcomes. A meta‐analysis of these two Leuven, Belgium, studies demonstrated a reduction in mortality (23.6% versus 20.4%, absolute risk reduction [ARR] 3.2%, P = 0.004)) in all patients treated with IIT, with a larger reduction in mortality (37.9% versus 30.1%, ARR 7.8%, P = 0.002) observed in patients with at least 3 days of IIT, as well as substantial reductions in morbidity.9, 10, 41, 42
Several other studies must be mentioned in this context. A small (n = 61), randomized study in another SICU did not show a mortality benefit, perhaps because the number of subjects was not adequate to reach statistical significance, but did result in a significant reduction in nosocomial infections in patients receiving IIT (BG = 125 versus 179 mg/dL, P < 0.001).43 Two international multicenter studies recently stopped enrollment due to excess rates of hypoglycemia. The Volume Substitution and Insulin Therapy in Severe Sepsis (VISEP) study, in a mixed medical and surgical sepsis population, showed no significant reduction in mortality in the intensively‐treated group. Serious adverse events were reported according to standard definitions. Enrollment was stopped before the full number of subjects had been randomized. Among the 537 evaluable cases, hypoglycemia (BG < 40 mg/dL) was reported as 17.0% in the IT group and 4.1% (P < 0.001) in the control group,44 and the rate of serious adverse events was higher in the IT group (10.9% versus 5.2%, P = 0.01). It is notable that the rate of hypoglycemia was comparable to the 18.7% rate seen in the IT group in the Leuven, Belgium, medical ICU study.9 The Glucontrol study enrolled 855 medical and surgical ICU patients and was similarly terminated because of hypoglycemia (BG < 40 mg/dL) at a rate of 8.6% compared to 2.4% in the control group (P < 0.001). Insulin infusion protocols and outcome data have not yet been published.42, 45
These studies with very high hypoglycemia rates each used an algorithm based on the Leuven, Belgium, protocol. The rates of severe hypoglycemia are 34 that reported by a variety of others achieving similar or identical glycemic targets. Hypoglycemia should not be construed as a reason to not use a standardized insulin infusion protocol. In comparing protocols that have been published, it is apparent that rates of hypoglycemia differ substantially and that performance results of some algorithms are not necessarily replicable across sites.46 Dose‐defining designs can be substantively more sophisticated than those used in the trials mentioned, in some cases incorporating principles of control engineering. The variability of hypoglycemia rates under differing insulin infusion protocols is a compelling reason to devote institutional effort to monitoring the efficacy and safety of the infusion protocols that are used.
High‐level evidence from randomized, controlled trials demonstrating outcomes benefit through targeted BG control outside the ICU is lacking at this point in time, but it must be noted that feasibility is suggested by a recent randomized control trial (RABBIT2) that demonstrated the superiority of basal bolus insulin regimens to sliding scale insulin in securing glycemic control, without any increase in hypoglycemia.47
Summing Up the Evidence
It is clear that hyperglycemia is associated with negative clinical outcomes throughout the hospital, and level A evidence is available to support tight glucose control in the SICU setting. However, in view of the imperfect and incomplete nature of the evidence, controversy persists around how stringent glycemic targets should be in the ICU, on whether glycemic targets should differ between SICU and MICU patients, and especially what the targets should be in the non‐ICU setting. There should be hesitancy to extrapolate glycemic targets to be applied beyond the populations that have been studied with RCTs or to assume benefit for medical conditions that have not been examined for the impact of interventions to control hyperglycemia. Institutions might justifiably choose more liberal targets than those promoted in national recommendations/guidelines2, 4850 until safe attainment of more moderate goals is demonstrated. However, even critics agree that uncontrolled hyperglycemia exceeding 180200 mg/dL in any acute care setting is undesirable. Moreover, strong observational data showing the hazards of hyperglycemia in noncritical care units (even after adjustment for severity of illness) combined with the high rate of adverse drug events associated with insulin use, argue strongly for a standardized approach to treating diabetes and hyperglycemia in the hospital. Even though no RCTs exist demonstrating outcomes benefits of achieving glycemic target on wards, the alternatives to control of hyperglycemia using scheduled insulin therapy are unacceptable. Oral agent therapy is potentially dangerous and within the necessary timeframe is likely to be ineffective; sliding scale management is inferior to basal‐bolus insulin therapy, as shown inan RCT,47 and is unsafe; and on the wards improved glycemic control can be achieved simultaneously with a reduction in hypoglycemia.51
INPATIENT GLYCEMIC CONTROL IS INCREASINGLY INCORPORATED INTO PUBLIC REPORTING, GUIDELINES, REGULATORY AGENCY, AND NATIONAL QUALITY INITIATIVE PRIORITIES
National quality initiatives, public reporting, pay‐for‐performance, and guideline‐based care continue to play an increasingly important role in the U.S. healthcare system. Over the years these initiatives have focused on various disease states (venous thromboembolism, congestive heart failure, community‐acquired pneumonia, etc.) in an attempt to standardize care and improve patient safety and quality. Inpatient hyperglycemic control is also increasingly being incorporated into public reporting, regulatory compliance, and national quality initiatives.
Professional organizations such as the ADA2 and AACE50 have published guidelines supporting improved glycemic control, the safe use of insulin, and other measures to improve care for hyperglycemic inpatients. The AACE has a Web site dedicated to hospital hyperglycemia.52 The Society of Hospital Medicine48 has created a resource room on its Web site and a workbook for improvement49 on optimizing the care of inpatients with hyperglycemia and diabetes. The guidelines and Web sites help raise awareness and educate physicians and healthcare workers in inpatient glucose management. The American Heart Association has incorporated specific recommendation regarding inpatient diabetic management in its Get With the Guidelines.53
The Joint Commission54 has developed an advanced disease‐specific certification on inpatient diabetes. Disease management programs are important components of complex healthcare systems that serve to coordinate chronic care, promote early detection and prevention, and reduce overall healthcare costs. Certification is increasingly important to providers, payers, and healthcare institutions because it demonstrates a commitment to quality and patient safety. The Joint Commission disease‐specific care certification is a patient‐centered model focusing on the delivery of clinical care and relationship between the practitioner and the patient. The evaluation and resulting certification by the Joint Commission is based on 3 core components: (1) an assessment of compliance with consensus‐based national standards; (2) the effective use of established clinical practice guidelines to manage and optimize care; and (3) an organized approach to performance measurement and improved activities.55 For inpatient diabetes, the Joint Commission program has 7 major elements following the ADA recommendations, including general recommendations regarding diabetic documentation, BG targets, preventing hypoglycemia, diabetes care providers, diabetes self‐management education, medical nutrition therapy, and BG monitoring.54 This mirrors the Call to Action Consensus Conference essential elements for successful glycemic control programs.1
Other organizations such as the Surgical Care Improvement Partnership (SCIP) and National Surgical Quality Improvement Program (NSQIP) have included perioperative glycemic control measures, as it impacts surgical wound infections. The University HealthSystem Consortium (UHC) has benchmarking data and endorses perioperative glycemic control measures, whereas the Institute for Healthcare Improvement (IHI) has focused on safe use of insulin practices in its 5 Million Lives campaign.
HOSPITALIZATION IS A MOMENT OF OPPORTUNITY TO ASSESS AND INTERVENE
The benefits of outpatient glycemic control and quality preventive care are well established, and the reduction of adverse consequences of uncontrolled diabetes are a high priority in ambulatory medicine.5658 Hospitalization provides an opportunity to identify previously undiagnosed diabetes or prediabetes and, for patients with known diabetes, to assess and impact upon the long term course of diabetes.
As a first step, unless a recent hemoglobin A1C (HbA1c) is known, among hospitalized hyperglycemic patients an HbA1C should be obtained upon admission. Greci et al.59 showed that an HbA1c level >6.0% was 100% specific (14/14) and 57% sensitive (12/21) for the diagnosis of diabetes. Among patients having known diabetes, an HbA1C elevation on admission may justify intensification of preadmission management at the time of discharge. If discharge and postdischarge adjustments of preadmission regimens are planned in response to admission A1C elevations, then the modified long‐term treatment strategy can improve the A1C in the ambulatory setting.60 Moreover, the event of hospitalization is the ideal teachable moment for patients and their caregivers to improve self‐care activities. Yet floor nurses may be overwhelmed by the tasks of patient education. For ideal patient education, both a nutritionist and a diabetes nurse educator are needed to assess compliance with medication, diet, and other aspects of care.6163 There also is need for outpatient follow‐up education. Finally, at the time of discharge, there is a duty and an opportunity for the diabetes provider to communicate with outpatient care providers about the patient's regimen and glycemic control, and also, based on information gathered during the admission, to convey any evidence that might support the need for a change of long‐term strategy.64 Unfortunately, the opportunity that hospitalization presents to assess, educate, and intervene frequently is underused.1, 8, 51, 65
LARGE GAPS EXIST BETWEEN CURRENT AND OPTIMAL CARE
Despite the evidence that inpatient glycemic control is important for patient outcomes, and despite guidelines recommending tighter inpatient glycemic control, clinical practice has been slow to change. In many institutions, inpatient glycemic management has not improved over the past decade, and large gaps remain between current practice and optimal practice.
Studies of individual institutions provide several insights into gaps in care. For example, Schnipper et al.66 examined practices on the general medicine service of an academic medical center in Boston in 2004. Among 107 prospectively identified patients with a known diagnosis of diabetes or at least 1 glucose reading >200 mg/dL (excluding patients with diabetic ketoacidosis, hyperglycemic hyperosmolar state, or pregnancy), they found scheduled long‐acting insulin prescribed in 43% of patients, scheduled short‐acting/rapid‐acting insulin in only 4% of patients, and 80 of 89 patients (90%) on the same sliding scale insulin regimen despite widely varying insulin requirements. Thirty‐one percent of glucose readings were >180 mg/dL compared with 1.2% of readings <60 mg/dL (but 11% of patients had at least 1 episode of hypoglycemia). Of the 75 patients with at least 1 episode of hyperglycemia or hypoglycemia, only 35% had any change to their insulin regimen during the first 5 days of the hospitalization.
Other studies have confirmed this concept of clinical inertia (ie, recognition of the problem but failure to act).67 A study by Cook et al.68 of all hospitalized non‐ICU patients with diabetes or hyperglycemia and length of stay of 3 days between 2001 and 2004 showed that 20% of patients had persistent hyperglycemia during the hospitalization (defined as a mean glucose >200 mg/dL). Forty‐six percent of patients whose average glucose was in the top tertile did not have their insulin regimen intensified to a combination of short‐acting/rapid‐acting and long‐acting insulin, and 35% of these patients either had no change in their total daily insulin dose or actually had a decrease in their dose when comparing the last 24 hours with the first 24 hours of hospitalization, a concept they term negative therapeutic momentum.
Perhaps the most well‐balanced view of the current state of medical practice comes from the UHC benchmarking project.69 UHC is an alliance of 90 academic health centers. For the diabetes project, each institution reviewed the records of 50 randomly selected patients over 18 years of age with at least a 72‐hour length of stay, 1 of 7 prespecified Diagnosis Related Group (DRG) codes, and at least 2 consecutive glucose readings >180 mg/dL or the receipt of insulin any time during the hospitalization. Patients with a history of pancreatic transplant, pregnant at the time of admission, receiving hospice or comfort care, or receiving insulin for a reason other than glucose management were excluded. The study showed widespread gaps in processes and outcomes (Table 1). Moreover, performance varied widely across hospitals. For example, the morning glucose in the ICU on the second measurement day was 110 mg/dL in 18% of patients for the median‐performing hospital, with a range of 0% to 67% across all 37 measured hospitals. In the non‐ICU setting on the second measurement day, 26% of patients had all BG measurements = 180 mg/dL in the median‐performing hospital, with a range of 7% to 48%. Of note, hypoglycemia was relatively uncommon: in the median hospital, 2.4% of patient‐days had 1 or more BG readings <50 mg/dL (range: 0%8.6%). Finally, in the median‐performing hospital, effective insulin therapy (defined as short‐acting/rapid‐acting and long‐acting subcutaneous insulin, continuous insulin infusion, or subcutaneous insulin pump therapy) was prescribed in 45% of patients, with a range of 12% to 77% across measured hospitals.
| Key Performance Measure | Results for Median‐Performing Hospital (%) |
|---|---|
| |
| Documentation of diabetes | 100 |
| Hob A1c assessment within 30 days | 36.1 |
| Glucose measurement within 8 hours of admission | 78.6 |
| Glucose monitoring 4 times a day | 85.4 |
| Median glucose reading > 200 mg/dL | 10.3 |
| Effective insulin therapy* | 44.7 |
| ICU day 2 morning glucose 110 mg/dL | 17.7 |
| Non‐ICU day 2 all glucose readings 180 mg/dL | 26.3 |
| Patient‐days with at least 1 glucose reading < 50 mg/dL | 2.4 |
FREQUENT PROBLEMS WITH COMMUNICATION AND COORDINATION
Those who work closely with frontline practitioners striving to improve inpatient glycemic management have noticed other deficiencies in care.1, 70 These include: a lack of coordination between feeding, BG measurement, and insulin administration, leading to mistimed and incorrectly dosed insulin; frequent use of sliding‐scale only regimens despite evidence that they are useless at best and harmful at worst;6, 47, 60, 71 discharge summaries that often do not mention follow‐up plans for hyperglycemic management; incomplete patient educational programs; breakdowns in care at transition points; nursing and medical staffs that are unevenly educated about the proper use of insulin; and patients who are often angry or confused about their diabetes care in the hospital. Collectively, these gaps in care serve as prime targets for any glycemic control program.
HYPOGLYCEMIA IS A PROMINENT INPATIENT SAFETY CONCERN
Hypoglycemia is common in the inpatient setting and is a legitimate safety concern. In a recently reported series of 2174 hospitalized patients receiving antihyperglycemic agents, it was found that 9.5% of patients experienced a total 484 hypoglycemic episodes (defined as 60 mg/dL).72 Hypoglycemia often occurred in the setting of insulin therapy and frequently resulted from a failure to recognize trends in BG readings or other clues that a patient was at risk for developing hypoglycemia.73 A common thread is the risk created by interruption of carbohydrate intake, noted by Fischer et al.73 and once again in the recent ICU study by Vriesendorp et al.74 Sources of error include: lack of coordination between feeding and medication administration, leading to mistiming of insulin action; lack of sufficient frequency in BG monitoring; lack of clarity or uniformity in the writing of orders; failure to recognize changes in insulin requirements due to advanced age, renal failure, liver disease, or change in clinical status; steroid use with subsequent tapering or interruption; changes in feeding; failure to reconcile medications; inappropriate use of oral antihyperglycemic agents, and communication or handoff failures.
It has been difficult to sort out whether hypoglycemia is a marker of severity of illness or whether it is an independent factor leading to poor outcomes. Observational studies lend credibility to the concept that patients having congestive heart failure or myocardial infarction may be at risk for excessive mortality if their average BG resides in the low end of the normal range.7578 Sympathetic activation occurs as the threshold for hypoglycemia is approached, such as occurs at BG = 70 or 72 mg/dL.79 Patients living with BG levels observed to be in the low end of the normal range might experience more severe but unobserved and undocumented episodes of neuroglycopenia. Arrhythmia and fatality have been directly attributed to strict glycemic control.80, 81 We are confronted with the need to interpret well conducted observational studies, evaluating subgroups at risk, and using multivariate analysis to assess the impact of hypoglycemia upon outcomes.82 In such studies, we will need to examine high‐risk subgroups, including cardiac patients, in particular, for the possibility that there is a J‐shaped curve for mortality as a function of average BG.
Unfortunately, clinical inertia exists in response to hypoglycemia just as it does with hyperglycemia. One recent study examined 52 patients who received intravenous 50% dextrose solution for an episode of hypoglycemia.83 Changes to insulin regimens were subsequently made in only 21 patients (40%), and diabetes specialists agreed with the changes for 11 of these patients. The other 31 patients (60%) received no changes in treatment, and diabetes specialists agreed with that decision for only 10 of these patients.
Although some increase in hypoglycemia might be expected with initiation of tight glycemic control efforts, the solution is not to undertreat hyperglycemia. Hyperglycemia creates an unsafe setting for the treatment of illness and disease. Sliding‐scaleonly regimens are ineffective in securing glycemic control and can result in increases in hypoglycemia as well as hyperglycemic excursions.6, 66 Inappropriate withholding of insulin doses can lead to severe glycemic excursions and even iatrogenic diabetic ketoacidosis (DKA). Systems approaches to avoid the errors outlined above can minimize or even reverse the increased risk of hypoglycemia expected with tighter glycemic targets.51
A SYSTEMS APPROACH IS NEEDED FOR THESE MULTIPLE COMPLEX PROBLEMS
Care is of the hyperglycemic inpatient is inherently complex. Previously established treatments are often inappropriate under conditions of altered insulin resistance, changing patterns of nutrition and carbohydrate exposure, comorbidities, concomitant medications, and rapidly changing medical and surgical status. Patients frequently undergo changes in the route and amount of nutritional exposure, including discrete meals, continuous intravenous dextrose, nil per orem (nothing by mouth status; NPO) status, grazing on nutritional supplements or liquid diets with or without meals, bolus enteral feedings, overnight enteral feedings with daytime grazing, total parenteral nutrition, continuous peritoneal dialysis, and overnight cycling of peritoneal dialysis. Relying on individual expertise and vigilance to negotiate this complex terrain without safeguards, protocols, standardization of orders, and other systems change is impractical and unwise.
Transitions across care providers and locations lead to multiple opportunities for breakdown in the quality, consistency, and safety of care.64, 65 At the time of ward transfer or change of patient status, previous medication and monitoring orders sometimes are purged. At the time of discharge, there may be risk of continuation of anti‐hyperglycemic therapy, initiated to cover medical stress, in doses that will subsequently be unsafe.
In the face of this complexity, educational programs alone will not suffice to improve care. Institutional commitment and systems changes are essential.
MARKED IMPROVEMENT IS POSSIBLE AND TOOLS EXIST: A ROADMAP IS IN PLACE
Fortunately, a roadmap is in place to help us achieve better glycemic control, improve insulin management, and address the long list of current deficiencies in care. This is imperative to develop consistent processes in order to achieve maximum patient quality outcomes that effective glycemic management offers. This roadmap entails 4 components: (1) national awareness, (2) national guidelines, (3) consensus statements, and (4) effective tools. As mentioned above, the first two components of this roadmap are now in place.
As these national guidelines become more widely accepted, the next step will be the incorporation of this into programs like Pay‐for Performance and the Physician Quality Reporting Initiative (PQRI), which will impact reimbursement to both hospitals and providers.
Regarding the third component, a recent multidisciplinary consensus conference1 outlined the essential elements needed for successful implementation of an inpatient glycemic control program which include:
-
An appropriate level of administrative support.
-
Formation of a multidisciplinary steering committee to drive the development of initiatives and empowered to enact change.
-
Assessment of current processes, quality of care, and barriers to practice change.
-
Development and implementation of interventions including standardized order sets, protocols, policies and algorithms with associated educational programs.
-
Metrics for evaluation of glycemic control, hypoglycemia, insulin use patterns, and other aspects of care.
Finally, extensive resources and effective tools are now available to help institutions achieve better inpatient glucose control. The Society of Hospital Medicine (SHM), in conjunction with the ADA, AACE, the American College of Physicians (ACP), the Case Management Society of America (CMSA), the American Society of Consultant Pharmacists, nursing, and diabetic educators have all partnered to produce a comprehensive guide to effective implementation of glycemic control and preventing hypoglycemia.49 This comprehensive workbook is a proven performance improvement framework and is available on the SHM Web site.48 Details and examples of all essential elements are covered in this workbook along with opportunities for marked improvement bolstered by integration of high reliability design features and attention to effective implementation techniques. The remainder of this supplement crystallizes a substantial portion of this material. The AACE has also recently offered a valuable web‐based resource to encourage institutional glycemic control efforts.49
GLYCEMIC CONTROL INITIATIVES CAN BE COST‐EFFECTIVE
Achieving optimal glycemic control safely requires monitoring, education, and other measures, which can be expensive, labor intensive, and require coordination of the services of many hospital divisions. This incremental expense has been shown to be cost‐effective in a variety of settings.1, 84, 85 The costs of glycemic control initiatives have demonstrated a good return on investment via:
-
Improved LOS, readmission rates, morbidity, and mortality.
-
Improved documentation of patient acuity and related payment for acuity.
-
Income generated via incremental physician and allied health professional billing.
CONCLUSION AND SUMMARY
Evidence exists that appropriate management of hyperglycemia improves outcomes, whereas the current state of affairs is that most medical centers currently manage this suboptimally. This is concerning given the magnitude of diabetes and hyperglycemia in our inpatient setting in the United States. To bring awareness to this issue, multiple initiatives (guidelines, certification programs, workbooks, etc.) are available by various organizations including the ADA, AACE, SCIP, NSQIP, IHI, UHC, the Joint Commission, and SHM. However, this is not enough. Change occurs at the local level, and institutional prioritization and support is needed to empower a multidisciplinary steering committee, with appropriate administrative support, to standardize and improve systems in the face of substantial cultural issues and complex barriers. Improved data collection and reporting, incremental monitoring, creation of metrics, and improved documentation are an absolutely necessary necessity to achieve breakthrough levels of improvement.
Now the time is right to make an assertive effort to improve inpatient glycemic control and related issues, and push for appropriate support at your institution to help achieve this in the interest of patient safety and optimal outcomes.
- American College of Endocrinology and American Diabetes Association Consensus Statement on Inpatient Diabetes and Glycemic Control: A call to action.Diabetes Care.2006;29:1955–1962.
- Standards of medical care in diabetes‐‐2008.Diabetes Care.2008;31(Suppl 1):S12–S54.
- ,,, et al.Prevalence of diabetes and impaired fasting glucose in adults in the U.S. population: National Health And Nutrition Examination Survey 1999–2002.Diabetes Care.2006;29:1263–1268.
- Centers for Disease Control and Prevention.National Diabetes Fact Sheet: General Information and National Estimates on Diabetes in the United States, 2005.Atlanta, GA:U.S. Department of Health and Human Services, Centers for Disease Control and Prevention,2005. Available at: http://www.cdc.gov/diabetes/pubs/factsheet05.htm. Accessed September 2007.
- ,,.Economic costs of diabetes in the US in 2002.Diabetes Care.2003;26:917–932.
- ,,.Glycemic control and sliding scale insulin use in medical inpatients with diabetes mellitus.Arch Intern Med.1997;157:545–552.
- ,,.Inpatient management of diabetes mellitus.Am J Med.2002;113:317–323.
- ,,, et al.Management of diabetes and hyperglycemia in hospitals.Diabetes Care.2004;27:553–591.
- ,,, et al.Intensive insulin therapy in the medical ICU.N Engl J Med.2006;354:449–461.
- ,,, et al.Intensive insulin therapy in critically ill patients.N Engl J Med.2001;345:1359–1367.
- .Association between hyperglycemia and increased hospital mortality in a heterogeneous population of critically ill patients.Mayo Clin Proc.2003;78:1471–1478.
- ,,,,.Unrecognized diabetes among hospitalized patients.Diabetes Care.1998;21:246–249.
- ,,,,,.Hyperglycemia: an independent marker of in‐hospital mortality in patients with undiagnosed diabetes.J Clin Endocrinol Metab.2002;87:978–982.
- United States Department of Health and Human Services Agency for Healthcare Research and Quality.2007. Available at: http://hcupnet.ahrq.gov. Accessed December 2007.
- ,,, et al.Glucose metabolism in patients with acute myocardial infarction and no previous diagnosis of diabetes mellitus: a prospective study.Lancet.2002;359:2140–2144.
- .Mechanism by which hyperglycemia plays a role in the setting of acute cardiovascular illness.Rev Cardiovasc Med.2006;7(Suppl 2):S35–S43.
- ,,,,,, et al.Stress hyperglycaemia is an independent predictor of left ventricular remodelling after first anterior myocardial infarction in non‐diabetic patients.Eur Heart J.2007;28:546–552.
- ,.Implications and treatment of acute hyperglycemia in the setting of acute myocardial infarction.Circulation.2007;115:e436–e439.
- ,,,,.Insulin infusion in acute illness.J Clin Invest.2005;115:2069–2072.
- ,,,,.Intensive insulin therapy exerts antiinflammatory effects in critically ill patients and counteracts the adverse effect of low mannose‐gind lectin levels.J Clin Endocrinol Metab.2003;88:1082–1088.
- ,,,,,.Protection of hepatocyte mitochondrial ultrastructure and function by strict blood glucose control with insulin in critically ill patients.Lancet.2005;365:53–59.
- ,,, et al.Intensive insulin therapy protects the endothelium of critically ill patients.J Clin Invest.2005;115:2277–2286.
- ,,,.The association between hyperglycaemia on admission and 180‐day mortality in acute myocardial infarction patients with and without diabetes.Diabet Med.2005;22:1321–1325.
- ,,, et al.Admission glucose and mortality in elderly patients hospitalized with acute myocardial infarction: implications for patients with and without recognized diabetes.Circulation.2005;111:3078–3086.
- ,,,.Glycometabolic state at admission: important risk marker of mortality in conventionally treated patients with diabetes mellitus and acute myocardial infarction: long‐term results from the Diabetes and Insulin‐Glucose Infusion in Acute Myocardial Infarction (DIGAMI) study.Circulation.1999;99:2626–2632.
- ,,,.Stress hyperglycaemia and increased risk of death after myocardial infarction in patients with and without diabetes: a systematic overview.Lancet.2000;355:773–778.
- ,,.How important is hyperglycemia during acute brain infarction?Neurologist.2004;10:195–200.
- ,,,,.Stress hyperglycemia and prognosis of stroke in nondiabetic and diabetic patients: a systematic overview.Stroke.2001;32:2426–2432.
- ,,,.Perioperative glycemic control and the risk of infectious complications in a cohort of adults with diabetes.Diabetes Care.1999;22:1408–1414.
- ,,, et al.Early postoperative glucose control predicts nosocomial infection rate in diabetic patients.JPEN J Parenter Enteral Nutr.1998;22:77–81.
- ,,,,,.The relation between hyperglycemia and outcomes in 2,471 patients admitted to the hospital with community‐acquired pneumonia.Diabetes Care.2005;28:810–815.
- ,,,,.Early peri‐operative glycaemic control and allograft rejection in patients with diabetes mellitus: a pilot study.Transplantation.2001;72:1321–1324.
- ,,, et al.Relation between the duration of remission and hyperglycemia during induction chemotherapy for acute lymphocytic leukemia with a hyperfractionated cyclophosphamide, vincristine, doxorubicin, and dexamethasone/methotrexate‐cytarabine regimen.Cancer.2004;100:1179–1185.
- ,,, et al.Effect of fasting glucose levels on mortality rate in patients with and without diabetes mellitus and coronary artery disease undergoing percutaneous coronary intervention.Am Heart J.2003;146:351–358.
- ,,.Effect of hyperglycemia and continuous intravenous insulin infusions on outcomes of cardiac surgical procedures: the Portland diabetic project.Endocr Pract.2004;10(Suppl 2):21–33.
- ,,, et al.Intraoperative hyperglycemia and perioperative outcomes in cardiac surgery patients.Mayo Clin Proc.2005;80:862–866.
- ,,,,.The association of diabetes and glucose control with surgical‐site infections among cardiothoracic surgery patients.Infect Control Hosp Epidemiol.2001;22:607–612.
- ,,.Glucose control lowers the risk of wound infection in diabetics after open heart operations.Ann Thorac Surg.1997;63:356–361.
- The Portland Protocol. Available at: http://www.providence.org/oregon/grograms_and_services/heart/portlandprotocol/. Accessed September2007.
- .Effect of an intensive glucose management protocol on the mortality of critically ill adult patients.Mayo Clin Proc.2004;79:992–1000.
- ,,, et al.Intensive insulin therapy in mixed medical/surgical intensive care units: benefit versus harm.Diabetes.2006;55:3151–3159.
- ,,.Tight blood glucose control with insulin in the ICU: facts and controversies.Chest.2007;132:268–278.
- ,.Reduction of nosocomial infections in the surgical intensive‐care unit by strict glycemic control.Endocr Pract.2004;10(Suppl 2):46–52.
- ,,,,,, et al.Intensive insulin therapy and pentastarch resuscitation in severe sepsis.N Engl J Med.2008;358:125–139.
- ,.Current controversies around tight glucose control in critically ill patients.Curr Opin Clin Nutr Metab Care.2007;10:206–209.
- ,,,.Designing and implementing insulin infusion protocols and order sets.J Hosp Med.2008;3(5):S42–S54.
- ,,,,,, et al.Randomized study of basal‐bolus insulin therapy in the inpatient management of patients with type 2 diabetes (RABBIT 2 trial).Diabetes Care.2007;30:2181–2186.
- Society of Hospital Medicine. Glycemic control resource room. Available at: http://www.hospitalmedicine.org/ResourceRoomRedesign/GlycemicControl.cfm. Accessed November2007.
- Society of Hospital Medicine. Workbook for improvement: improving glycemic control, preventing hypoglycemia, and optimizing care of the inpatient with hyperglycemia and diabetes. Available at: http://www.hospitalmedicine.org/ResourceRoomRedesign/pdf/GC_Workbook.pdf. Accessed November2007.
- ,,, et al.American College of Endocrinology position statement on inpatient diabetes and metabolic control.Endocr Pract.2004;10:77–82.
- ,,,,.Improved inpatient use of basal insulin, reduced hypoglycemia, and improved glycemic control: effect of structured subcutaneous insulin orders and an insulin management algorithm.J Hosp Med.2008. In press.
- American Association of Clinical Endocrinologists Inpatient Glycemic Control Resource Center.2007. Available at: http://resources.aace.com/index.asp. Accessed December 2007.
- American Heart Association. Get With the Guidelines. Available at: http://www.americanheart.org/getwiththeguidelines. Accessed November2007.
- Joint Commission. Disease Specific‐Care Certification. Available at:http://www.jointcommission.org/CertificationPrograms. Accessed November2007.
- The Joint Commission Disease‐Specific Certification Program. Range JE. Oncology issues. July/August2007:40–41.
- Anonymous.The Diabetes Control and Complications Trial Research Group (DCCT). The effect of intensive treatment of diabetes on the development and progression of long‐term complications in insulin‐dependent diabetes mellitus.N Engl J Med.1993;329:977–986.
- Intensive blood‐glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type, 2 diabetes (UKPDS 33). UK Prospective Diabetes Study (UKPDS) Group.Lancet.1998;352:837–853.
- ,,,.Intensified multifactorial intervention in patients with type 2 diabetes mellitus and microalbuminuria: the Steno type 2 randomised study.Lancet.1999;353:617–622.
- ,,, et al.Utility of HbA1c levels for diabetes case finding in hospitalized patients with hyperglycemia.Diabetes Care.2003;26:1064–1068.
- ,,,.Eliminating inpatient sliding‐scale insulin: a reeducation project with medical house staff.Diabetes Care.2005;28:1008–1011.
- ,.Advanced carbohydrate counting. In:Practical Carbohydrate Counting: A How‐to‐Teach Guide for Health Professionals.Alexandria, VA:American Diabetes Association;2001:26–28.
- ,,,,.The evidence for the effectiveness of medical nutrition therapy in diabetes management.Diabetes Care.2002;25:608–613.
- ,,, et al.Inpatient management of diabetes and hyperglycemia: implications for nutrition practice and the food and nutrition professional.J Am Diet Assoc.2007;107:105–111.
- .The transition from insulin infusions to long‐term diabetes therapy: the argument for insulin analogs.Semin Thorac Cardiovasc Surg.2006;18:366–378.
- .Transitions paper.J Hosp Med.2008.
- ,,,,.Inpatient management of diabetes and hyperglycemia among general medicine patients at a large teaching hospital.J Hosp Med.2006;1:145–150.
- ,,, et al.Clinical inertia.Ann Intern Med.2001;135:825–834.
- ,,, et al.Diabetes care in hospitalized noncritically ill patients: more evidence for clinical inertia and negative therapeutic momentum.J Hosp Med.2007;2:203–211.
- University HealthSystem Consortium.Glycemic control 2005 findings and conclusions. Presented at: Glycemic Control 2005 Knowledge Transfer Meeting; 2005 August 19,2005; Chicago, IL.
- ,.Glycemic chaos (not glycemic control) still the rule for inpatient care: how do we stop the insanity?J Hosp Med.2006;1:141–144.
- ,,,,.Management of diabetes mellitus in hospitalized patients: efficiency and effectiveness of sliding‐scale insulin therapy.Pharmacotherapy.2006;26:1421–1432.
- ,,,,,.Hypoglycemia in hospitalized patients treated with antihyperglycemic agents.J Hosp Med.2007;2:234–240.
- ,,.Hypoglycemia in hospitalized patients.N Engl J Med.1986;315:1245–1250.
- ,,, et al.Predisposing factors for hypoglycemia in the intensive care unit.Crit Care Med.2006;34:96–101.
- ,,,.Association between hyper‐ and hypoglycaemia and 2 year all‐cause mortality risk in diabetic patients with acute coronary events.Eur Heart J.2005;26:1255–1261.
- ,,, et al.U‐shaped relationship of blood glucose with adverse outcomes among patients with ST‐segment elevation myocardial infarction.J Am Coll Cardiol.2005;46:178–180.
- ,,.An unexpected inverse relationship between HbA1c levels and mortality in patients with diabetes and advanced systolic heart failure.Am Heart J.2006;151:91.
- ,,, et al.Glucometrics in patients hospitalized with acute myocardial infarction: defining the optimal outcomes‐based measure of risk.Circulation.2008;117:1018–1027.
- ,,.Hypoglycemia in diabetes.Diabetes Care.2003;26:1902–1912.
- ,,.Hypoglycemia and cardiac arrest in a critically ill patient on strict glycemic control.Anesth Analg.2006;102:549–551.
- ,,,,,.Tight glycemic control in critically injured trauma patients.Ann Surg.2007;246:605–610; discussion 10–12.
- ,.Severe hypoglycemia in critically ill patients: risk factors and outcomes.Crit Care Med.2007;35:2262–2267.
- ,,,.Provider response to insulin‐induced hypoglycemia in hospitalized patients.J Hosp Med.2007;2:258–260.
- ,.Financial implications of glycemic control: results of an inpatient diabetes management program.Endocr Pract.2006;12(Suppl 3):43–48.
- ,,,.Impact of endocrine and diabetes team consultation on hospital length of stay for patients with diabetes.Am J Med.1995;99:22–28.
- American College of Endocrinology and American Diabetes Association Consensus Statement on Inpatient Diabetes and Glycemic Control: A call to action.Diabetes Care.2006;29:1955–1962.
- Standards of medical care in diabetes‐‐2008.Diabetes Care.2008;31(Suppl 1):S12–S54.
- ,,, et al.Prevalence of diabetes and impaired fasting glucose in adults in the U.S. population: National Health And Nutrition Examination Survey 1999–2002.Diabetes Care.2006;29:1263–1268.
- Centers for Disease Control and Prevention.National Diabetes Fact Sheet: General Information and National Estimates on Diabetes in the United States, 2005.Atlanta, GA:U.S. Department of Health and Human Services, Centers for Disease Control and Prevention,2005. Available at: http://www.cdc.gov/diabetes/pubs/factsheet05.htm. Accessed September 2007.
- ,,.Economic costs of diabetes in the US in 2002.Diabetes Care.2003;26:917–932.
- ,,.Glycemic control and sliding scale insulin use in medical inpatients with diabetes mellitus.Arch Intern Med.1997;157:545–552.
- ,,.Inpatient management of diabetes mellitus.Am J Med.2002;113:317–323.
- ,,, et al.Management of diabetes and hyperglycemia in hospitals.Diabetes Care.2004;27:553–591.
- ,,, et al.Intensive insulin therapy in the medical ICU.N Engl J Med.2006;354:449–461.
- ,,, et al.Intensive insulin therapy in critically ill patients.N Engl J Med.2001;345:1359–1367.
- .Association between hyperglycemia and increased hospital mortality in a heterogeneous population of critically ill patients.Mayo Clin Proc.2003;78:1471–1478.
- ,,,,.Unrecognized diabetes among hospitalized patients.Diabetes Care.1998;21:246–249.
- ,,,,,.Hyperglycemia: an independent marker of in‐hospital mortality in patients with undiagnosed diabetes.J Clin Endocrinol Metab.2002;87:978–982.
- United States Department of Health and Human Services Agency for Healthcare Research and Quality.2007. Available at: http://hcupnet.ahrq.gov. Accessed December 2007.
- ,,, et al.Glucose metabolism in patients with acute myocardial infarction and no previous diagnosis of diabetes mellitus: a prospective study.Lancet.2002;359:2140–2144.
- .Mechanism by which hyperglycemia plays a role in the setting of acute cardiovascular illness.Rev Cardiovasc Med.2006;7(Suppl 2):S35–S43.
- ,,,,,, et al.Stress hyperglycaemia is an independent predictor of left ventricular remodelling after first anterior myocardial infarction in non‐diabetic patients.Eur Heart J.2007;28:546–552.
- ,.Implications and treatment of acute hyperglycemia in the setting of acute myocardial infarction.Circulation.2007;115:e436–e439.
- ,,,,.Insulin infusion in acute illness.J Clin Invest.2005;115:2069–2072.
- ,,,,.Intensive insulin therapy exerts antiinflammatory effects in critically ill patients and counteracts the adverse effect of low mannose‐gind lectin levels.J Clin Endocrinol Metab.2003;88:1082–1088.
- ,,,,,.Protection of hepatocyte mitochondrial ultrastructure and function by strict blood glucose control with insulin in critically ill patients.Lancet.2005;365:53–59.
- ,,, et al.Intensive insulin therapy protects the endothelium of critically ill patients.J Clin Invest.2005;115:2277–2286.
- ,,,.The association between hyperglycaemia on admission and 180‐day mortality in acute myocardial infarction patients with and without diabetes.Diabet Med.2005;22:1321–1325.
- ,,, et al.Admission glucose and mortality in elderly patients hospitalized with acute myocardial infarction: implications for patients with and without recognized diabetes.Circulation.2005;111:3078–3086.
- ,,,.Glycometabolic state at admission: important risk marker of mortality in conventionally treated patients with diabetes mellitus and acute myocardial infarction: long‐term results from the Diabetes and Insulin‐Glucose Infusion in Acute Myocardial Infarction (DIGAMI) study.Circulation.1999;99:2626–2632.
- ,,,.Stress hyperglycaemia and increased risk of death after myocardial infarction in patients with and without diabetes: a systematic overview.Lancet.2000;355:773–778.
- ,,.How important is hyperglycemia during acute brain infarction?Neurologist.2004;10:195–200.
- ,,,,.Stress hyperglycemia and prognosis of stroke in nondiabetic and diabetic patients: a systematic overview.Stroke.2001;32:2426–2432.
- ,,,.Perioperative glycemic control and the risk of infectious complications in a cohort of adults with diabetes.Diabetes Care.1999;22:1408–1414.
- ,,, et al.Early postoperative glucose control predicts nosocomial infection rate in diabetic patients.JPEN J Parenter Enteral Nutr.1998;22:77–81.
- ,,,,,.The relation between hyperglycemia and outcomes in 2,471 patients admitted to the hospital with community‐acquired pneumonia.Diabetes Care.2005;28:810–815.
- ,,,,.Early peri‐operative glycaemic control and allograft rejection in patients with diabetes mellitus: a pilot study.Transplantation.2001;72:1321–1324.
- ,,, et al.Relation between the duration of remission and hyperglycemia during induction chemotherapy for acute lymphocytic leukemia with a hyperfractionated cyclophosphamide, vincristine, doxorubicin, and dexamethasone/methotrexate‐cytarabine regimen.Cancer.2004;100:1179–1185.
- ,,, et al.Effect of fasting glucose levels on mortality rate in patients with and without diabetes mellitus and coronary artery disease undergoing percutaneous coronary intervention.Am Heart J.2003;146:351–358.
- ,,.Effect of hyperglycemia and continuous intravenous insulin infusions on outcomes of cardiac surgical procedures: the Portland diabetic project.Endocr Pract.2004;10(Suppl 2):21–33.
- ,,, et al.Intraoperative hyperglycemia and perioperative outcomes in cardiac surgery patients.Mayo Clin Proc.2005;80:862–866.
- ,,,,.The association of diabetes and glucose control with surgical‐site infections among cardiothoracic surgery patients.Infect Control Hosp Epidemiol.2001;22:607–612.
- ,,.Glucose control lowers the risk of wound infection in diabetics after open heart operations.Ann Thorac Surg.1997;63:356–361.
- The Portland Protocol. Available at: http://www.providence.org/oregon/grograms_and_services/heart/portlandprotocol/. Accessed September2007.
- .Effect of an intensive glucose management protocol on the mortality of critically ill adult patients.Mayo Clin Proc.2004;79:992–1000.
- ,,, et al.Intensive insulin therapy in mixed medical/surgical intensive care units: benefit versus harm.Diabetes.2006;55:3151–3159.
- ,,.Tight blood glucose control with insulin in the ICU: facts and controversies.Chest.2007;132:268–278.
- ,.Reduction of nosocomial infections in the surgical intensive‐care unit by strict glycemic control.Endocr Pract.2004;10(Suppl 2):46–52.
- ,,,,,, et al.Intensive insulin therapy and pentastarch resuscitation in severe sepsis.N Engl J Med.2008;358:125–139.
- ,.Current controversies around tight glucose control in critically ill patients.Curr Opin Clin Nutr Metab Care.2007;10:206–209.
- ,,,.Designing and implementing insulin infusion protocols and order sets.J Hosp Med.2008;3(5):S42–S54.
- ,,,,,, et al.Randomized study of basal‐bolus insulin therapy in the inpatient management of patients with type 2 diabetes (RABBIT 2 trial).Diabetes Care.2007;30:2181–2186.
- Society of Hospital Medicine. Glycemic control resource room. Available at: http://www.hospitalmedicine.org/ResourceRoomRedesign/GlycemicControl.cfm. Accessed November2007.
- Society of Hospital Medicine. Workbook for improvement: improving glycemic control, preventing hypoglycemia, and optimizing care of the inpatient with hyperglycemia and diabetes. Available at: http://www.hospitalmedicine.org/ResourceRoomRedesign/pdf/GC_Workbook.pdf. Accessed November2007.
- ,,, et al.American College of Endocrinology position statement on inpatient diabetes and metabolic control.Endocr Pract.2004;10:77–82.
- ,,,,.Improved inpatient use of basal insulin, reduced hypoglycemia, and improved glycemic control: effect of structured subcutaneous insulin orders and an insulin management algorithm.J Hosp Med.2008. In press.
- American Association of Clinical Endocrinologists Inpatient Glycemic Control Resource Center.2007. Available at: http://resources.aace.com/index.asp. Accessed December 2007.
- American Heart Association. Get With the Guidelines. Available at: http://www.americanheart.org/getwiththeguidelines. Accessed November2007.
- Joint Commission. Disease Specific‐Care Certification. Available at:http://www.jointcommission.org/CertificationPrograms. Accessed November2007.
- The Joint Commission Disease‐Specific Certification Program. Range JE. Oncology issues. July/August2007:40–41.
- Anonymous.The Diabetes Control and Complications Trial Research Group (DCCT). The effect of intensive treatment of diabetes on the development and progression of long‐term complications in insulin‐dependent diabetes mellitus.N Engl J Med.1993;329:977–986.
- Intensive blood‐glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type, 2 diabetes (UKPDS 33). UK Prospective Diabetes Study (UKPDS) Group.Lancet.1998;352:837–853.
- ,,,.Intensified multifactorial intervention in patients with type 2 diabetes mellitus and microalbuminuria: the Steno type 2 randomised study.Lancet.1999;353:617–622.
- ,,, et al.Utility of HbA1c levels for diabetes case finding in hospitalized patients with hyperglycemia.Diabetes Care.2003;26:1064–1068.
- ,,,.Eliminating inpatient sliding‐scale insulin: a reeducation project with medical house staff.Diabetes Care.2005;28:1008–1011.
- ,.Advanced carbohydrate counting. In:Practical Carbohydrate Counting: A How‐to‐Teach Guide for Health Professionals.Alexandria, VA:American Diabetes Association;2001:26–28.
- ,,,,.The evidence for the effectiveness of medical nutrition therapy in diabetes management.Diabetes Care.2002;25:608–613.
- ,,, et al.Inpatient management of diabetes and hyperglycemia: implications for nutrition practice and the food and nutrition professional.J Am Diet Assoc.2007;107:105–111.
- .The transition from insulin infusions to long‐term diabetes therapy: the argument for insulin analogs.Semin Thorac Cardiovasc Surg.2006;18:366–378.
- .Transitions paper.J Hosp Med.2008.
- ,,,,.Inpatient management of diabetes and hyperglycemia among general medicine patients at a large teaching hospital.J Hosp Med.2006;1:145–150.
- ,,, et al.Clinical inertia.Ann Intern Med.2001;135:825–834.
- ,,, et al.Diabetes care in hospitalized noncritically ill patients: more evidence for clinical inertia and negative therapeutic momentum.J Hosp Med.2007;2:203–211.
- University HealthSystem Consortium.Glycemic control 2005 findings and conclusions. Presented at: Glycemic Control 2005 Knowledge Transfer Meeting; 2005 August 19,2005; Chicago, IL.
- ,.Glycemic chaos (not glycemic control) still the rule for inpatient care: how do we stop the insanity?J Hosp Med.2006;1:141–144.
- ,,,,.Management of diabetes mellitus in hospitalized patients: efficiency and effectiveness of sliding‐scale insulin therapy.Pharmacotherapy.2006;26:1421–1432.
- ,,,,,.Hypoglycemia in hospitalized patients treated with antihyperglycemic agents.J Hosp Med.2007;2:234–240.
- ,,.Hypoglycemia in hospitalized patients.N Engl J Med.1986;315:1245–1250.
- ,,, et al.Predisposing factors for hypoglycemia in the intensive care unit.Crit Care Med.2006;34:96–101.
- ,,,.Association between hyper‐ and hypoglycaemia and 2 year all‐cause mortality risk in diabetic patients with acute coronary events.Eur Heart J.2005;26:1255–1261.
- ,,, et al.U‐shaped relationship of blood glucose with adverse outcomes among patients with ST‐segment elevation myocardial infarction.J Am Coll Cardiol.2005;46:178–180.
- ,,.An unexpected inverse relationship between HbA1c levels and mortality in patients with diabetes and advanced systolic heart failure.Am Heart J.2006;151:91.
- ,,, et al.Glucometrics in patients hospitalized with acute myocardial infarction: defining the optimal outcomes‐based measure of risk.Circulation.2008;117:1018–1027.
- ,,.Hypoglycemia in diabetes.Diabetes Care.2003;26:1902–1912.
- ,,.Hypoglycemia and cardiac arrest in a critically ill patient on strict glycemic control.Anesth Analg.2006;102:549–551.
- ,,,,,.Tight glycemic control in critically injured trauma patients.Ann Surg.2007;246:605–610; discussion 10–12.
- ,.Severe hypoglycemia in critically ill patients: risk factors and outcomes.Crit Care Med.2007;35:2262–2267.
- ,,,.Provider response to insulin‐induced hypoglycemia in hospitalized patients.J Hosp Med.2007;2:258–260.
- ,.Financial implications of glycemic control: results of an inpatient diabetes management program.Endocr Pract.2006;12(Suppl 3):43–48.
- ,,,.Impact of endocrine and diabetes team consultation on hospital length of stay for patients with diabetes.Am J Med.1995;99:22–28.
Physician Assistant/Hospitalist Service
Midlevel providers (physician assistants and nurse practitioners) have long been employed by academic medical centers, predominantly on surgical services, or on medical subspecialty services, where they have typically had a limited scope of practice, focused in a narrowly defined area or set of procedures.17 In contrast, there are relatively few reports of experiences deploying midlevel providers to replace house staff on inpatient general medicine services in academic centers,810 and few studies of the effect of midlevel providers on quality and efficiency of care in the academic setting. Despite this, reductions in house officer duty hours as mandated by the Accreditation Council on Graduate Medical Education (ACGME)11 have resulted in academic centers increasingly using midlevel providers to decrease house staff workload on inpatient services.12, 13 In general, midlevel practitioners on general medicine services have been deployed to: (1) care for a population of patients separate from and in parallel with house staff; this population may be narrowly defined (eg, patients with chest pain) or not; (2) assist with the management of patients cared for by house staff by performing certain tasks (eg, scheduling appointments, discharging patients). Even as midlevel providers become more prevalent on academic general medicine services, the best model of care incorporating them into clinical care remains unclear, and few studies have rigorously examined the care provided on services that use them.
We developed an inpatient general medicine service within a large academic medical center staffed by physician assistants and hospitalists to help our residency program meet ACGME duty hour requirements. We hypothesized that by creating a service that is geographically localized and supervised by full‐time hospitalists, by instituting multidisciplinary rounds, and by investing in the professional development of highly‐skilled physician assistants, we could provide care for medically complex, acutely ill general medicine inpatients with similar quality and efficiency as compared to house staff teams. We report our experience during the first year of implementing the service, and compare quality and efficiency of care on this service with that of our traditional house staff services. We also evaluate the effects of this service on patient satisfaction and self‐reported house staff workload.
PATIENTS AND METHODS
Study Setting
The study was conducted in a 747‐bed urban, academic medical center in the northeastern United States. The hospital's human research committee reviewed and approved the study design. The hospital has accredited residency and fellowship programs in all major specialties. Prior to July 2005, physician assistants were employed only on surgical and medical subspecialty services (ie, bone marrow transplant, interventional cardiology); none were employed on the inpatient general medicine service. There were approximately 44,000 inpatient admissions during the year of the study, with approximately 6500 of these to the general medicine service.
Description of the General Medicine Service
The General Medicine Service consisted of 8 traditional house staff teams, with 1 attending, 1 junior or senior resident, 2 interns, and 1 or 2 medical students. These teams admitted patients on a rotating basis every fourth day. On 4 of these teams, the attending was a hospitalist, with clinical responsibility for the majority of the patients admitted to the team. On the remaining 4 teams, the teaching attending was a primary care physician or medical subspecialist, responsible for the direct care of a small number of the team's patients, with the remainder cared for by private primary care physicians or subspecialists.
Description of the Physician Assistant/Hospitalist Service
The Physician Assistant/Clinician Educator (PACE) service opened in July 2005, and consisted of 15 beds localized to 2 adjacent inpatient pods, staffed by a single cadre of nurses and medically staffed by 1 hospitalist and 2 physician assistants from 7:00 AM to 7:00 PM on weekdays and by 1 hospitalist, 1 physician assistant, and 1 moonlighter (usually a senior medical resident or fellow) from 7:00 AM to 7:00 PM on weekends. A moonlighter, typically a senior resident or medical subspecialty fellow, admitted patients and covered nights on the service from 7:00 PM to 7:00 AM 7 days a week. The daily census goal for the service was 15 patients, limited by the number of available beds on the 2 pods, and the service accepted admissions 24 hours per day, 7 days per week, whenever beds were available. Daily morning rounds occurred at 8:00 AM and included the hospitalist, physician assistants, nurses, a care coordinator, and a pharmacist. The PACE service did not have triage guidelines related to diagnosis, complexity, or acuity, but only accepted patients via the emergency department or via a primary care physician's office, and did not accept patients transferred from outside hospitals or from the intensive care units.
Physician Assistants
All of the physician assistants on the PACE service had prior inpatient medicine experience, ranging from 6 months to 5 years. The physician assistants worked in 3‐day to 6‐day blocks of 12‐hour shifts. Their clinical responsibilities were similar to those of interns at the study hospital, and included taking histories and performing physical examinations, writing notes and orders, reviewing and assimilating data, creating and updating patient signouts, completing discharge summaries, consulting other services as needed, and communicating with nurses and family members.
Many physician assistants also had nonclinical responsibilities, taking on physician‐mentored roles in education, quality improvement, and administration. They were involved in several initiatives: (1) developing a physician assistant curriculum in hospital medicine, (2) presenting at hospital‐wide physician assistant grand rounds, (3) surveying and tracking patient and family satisfaction on the service, (4) reviewing all 72‐hour hospital readmissions, intensive care unit transfers, and deaths on the service, and (5) maintaining the service's compliance with state regulations regarding physician assistant scope of practice and prescribing.
Hospitalists
The 3 hospitalists on the PACE service worked in 7‐day blocks of 12‐hour shifts (7:00 AM to 7:00 PM). They directly supervised the physician assistants and had no competing responsibilities. The hospitalists were all recent graduates of the study hospital's internal medicine residency, with no prior clinical experience beyond residency. All were planning to work on the service for 1 to 2 years before beginning a subspecialty fellowship. In addition to supervising the clinical work of the physician assistants, the hospitalists were responsible for teaching the physician assistants on rounds and in weekly didactic sessions, guided by a curriculum in hospital medicine that focused on the most common general medicine diagnoses seen on the PACE service. The medical director of the PACE service periodically reviewed each physician assistant's clinical experience, skills and knowledge base, and held semiannual feedback sessions.
Study Patients
All general medicine patients admitted to the PACE service from July 1, 2005 to June 30, 2006 comprised the study population. The comparison group consisted of general medicine patients admitted to the 8 house staff general medicine teams; patients transferred from an intensive care unit (ICU) or another facility were excluded in order to match the admission criteria for the PACE service and improve comparability between the 2 study arms.
Data Collection and Study Outcomes
We obtained all patient data from the hospital's administrative databases. We identified patients assigned to the PACE service or to the comparison group based on the admitting service, team, and attending. We obtained patient demographics, insurance, admission source and discharge destination, admission and discharge times, dates, diagnoses, and diagnosis‐related groups (DRGs), as well as dates and times of transfers to other services, including to the intensive care unit. We also obtained the Medicare case‐mix index (CMI, based on DRG weight), and calculated a Charlson score based on billing diagnoses coded in the year prior to the index admission.14 Outcomes included length of stay (LOS) to the nearest hour, in‐hospital mortality, transfers to the intensive care unit, readmissions to the study hospital within 72 hours, 14 days, and 30 days, and total costs as derived from the hospital's cost accounting system (Transition Systems Inc., Boston, MA). Other outcomes included patient satisfaction as measured by responses to the Press‐Ganey survey routinely administered to a randomly selected 70% of recently discharged patients and effect on self‐reported resident work hours.
Statistical Analysis
Patient demographics, clinical characteristics, and study outcomes are presented using proportions, means with standard deviations, and medians with inter‐quartile ranges as appropriate. Unadjusted differences in outcomes between the two services were calculated using univariable regression techniques with service as the independent variable and each outcome as the dependent variable. We used logistic regression for dichotomous outcomes (readmissions, ICU transfers, and inpatient mortality), and linear regression for log‐transformed LOS and log‐transformed total costs of care. To adjust each outcome for potential confounders, we then built multivariable regression models. Each potential confounder was entered into the model one at a time as the independent variable. All variables found to be significant predictors of the outcome at the P < 0.10 level were then retained in the final model along with service as the predictor of interest. We used general estimating equations in all multivariable models to adjust for clustering of patients by attending physician. For logistic regression models, the effect size is presented as an odds ratio (OR); for log‐transformed linear regression models, the effect size is presented as the percent difference between groups. We also performed 2 subgroup analyses, limited to (1) the patients with the 10 most common discharge DRGs, and (2) patients admitted between the hours of 7:00 AM and 7:00 PM to remove the effects of moonlighters performing the initial admission. Except as noted above, 2‐sided P values < 0.05 were considered significant. SAS 9.1 (SAS Institute, Cary, NC) was used for all analyses.
RESULTS
Patient Demographics
Table 1 shows patient demographics and clinical characteristics of the PACE service and the comparison group. Patients in the comparison group were slightly older and tended to have slightly higher CMI and Charlson scores. Patients on the PACE service were more likely to be admitted at night (10:00 PM to 7:00 AM; 43.8% versus 30.3%; P < 0.0001). There were no significant differences in sex, race, insurance, or percentage of patients discharged to home. The 10 most common DRGs in the comparison group accounted for 37.0% of discharges, and these same DRGs accounted for 37.5% of discharges on the PACE service (Table 2).
| Characteristic | PACE Service (n = 992) | House Staff Services (n = 4,202) | P value |
|---|---|---|---|
| |||
| Age (years) | |||
| 1844 | 19.1 | 18.2 | |
| 4564 | 35.5 | 31.9 | 0.04 |
| 65+ | 45.5 | 49.9 | |
| Sex (% female) | 57.7 | 60.0 | NS |
| Race/ethnicity | |||
| White | 57.3 | 59.3 | |
| Black | 24.0 | 23.5 | NS |
| Hispanic | 14.1 | 13.3 | |
| Other | 4.6 | 3.9 | |
| Insurance | |||
| Medicare | 41.9 | 43.8 | |
| Commercial | 34.9 | 35.9 | |
| Medicaid | 14.4 | 11.7 | NS |
| Free care | 4.5 | 3.9 | |
| Self pay | 1.1 | 0.8 | |
| Median income by zip code of residence, USD (IQR) | 45,517 (32,49362,932) | 45,517 (35,88963,275) | NS |
| Case‐mix index, median (IQR) | 1.1 (0.81.5) | 1.2 (0.91.8) | 0.001 |
| Charlson score | |||
| 0 | 27.2 | 24.9 | |
| 1 | 22.6 | 21.1 | 0.02 |
| 2 | 16.2 | 16.5 | |
| 3+ | 34.0 | 37.6 | |
| Admissions between 10:00 PM and 7:00 AM | 43.8 | 30.3 | <0.0001 |
| Discharged to home | 81.1 | 80.5 | NS |
| Diagnosis‐Related Group at Discharge | PACE Service (n = 992)* | House Staff Services (n = 4,202)* |
|---|---|---|
| ||
| Chest pain | 5.4 | 6.4 |
| Esophagitis, gastroenteritis, and miscellaneous digestive disorders | 4.5 | 4.4 |
| Heart failure and shock | 3.4 | 4.6 |
| Simple pneumonia and pleurisy | 2.7 | 4.4 |
| Kidney and urinary tract infections | 4.7 | 3.2 |
| Chronic obstructive pulmonary disease | 4.0 | 3.3 |
| Renal failure | 2.7 | 3.5 |
| Gastrointestinal hemorrhage | 3.7 | 2.7 |
| Nutritional and miscellaneous metabolic disorders | 3.3 | 2.4 |
| Disorders of the pancreas except malignancy | 3.1 | 2.1 |
| Cumulative percent | 37.5 | 37.0 |
Efficiency and Quality of Care
Table 3 compares the performance of the PACE service and the comparison group on several efficiency and quality measures. Unadjusted LOS was not significantly different, and adjusted LOS was slightly but not statistically significantly higher on the study service (adjusted LOS 5.0% higher; 95% confidence interval [CI], 0.4% to +10%). Unadjusted and adjusted total costs of care were marginally lower on the study service (adjusted total cost of care 3.9% lower; 95% CI, 7.5% to 0.3%).
| PACE Service | House Staff Services | Unadjusted % Difference (95%CI) | Adjusted % Difference (95%CI)* | |
|---|---|---|---|---|
| PACE Service | House Staff Services | Unadjusted OR (95% CI) | Adjusted OR (95% CI) | |
| ||||
| Efficiency measure | ||||
| Length of stay, days, median (IQR) | 2.6 (1.6, 4.4) | 2.6 (1.4, 4.6) | +0.1% (5.6% to +6.1%) | +5.0% (0.4% to +10.0%) |
| Total costs, USD, median (IQR) | 4,536 (2,848, 7,201) | 4,749 (3,046, 8,161) | 9.1% (14.0% to 3.8%) | 3.9% (7.5% to 0.3%)‖ |
| Quality measure | ||||
| 72‐hour readmissions/100 discharges | 0.8 | 1.3 | 0.6 (0.31.3) | 0.7 (0.21.8) |
| 14‐day readmissions/100 discharges | 5.4 | 5.4 | 1.0 (0.71.4) | 1.1 (0.81.4) |
| 30‐day readmissions/100 discharges | 8.0 | 8.1 | 1.0 (0.81.3) | 1.1 (0.91.3) |
| ICU transfers/100 discharges | 2.0 | 2.3 | 0.9 (0.51.4) | 1.4 (0.82.4)# |
| Inpatient mortality/100 discharges | 0.7 | 1.2 | 0.6 (0.31.3) | 0.8 (0.31.8)** |
We found no differences between the PACE service and comparison group in unadjusted rates of hospital readmissions within 72 hours, 14 days, and 30 days, transfer to the intensive care units, or inpatient mortality (Table 3). The associated ORs for each outcome were similar after adjusting for patient demographics and clinical characteristics including severity of illness, as well as for clustering by attending physician.
Subgroup Analyses
When the analysis was limited to the subset of patients with the 10 most common discharge DRGs, the difference in adjusted total cost of care was similar but lost statistical significance (4.0% lower on PACE service; 95% CI, 11.0% to +3.3%). In this subgroup, LOS, readmission rates, and ICU transfer rates were not different. ORs for mortality could not be calculated because there were no deaths in this subgroup on the PACE service (data not shown). When analysis was limited to daytime admissions (to remove any potential effect of admitting by a moonlighter), the difference in total cost of care was attenuated and lost statistical significance (0.2% lower on PACE service; 95%CI, 5.9% to +5.5%). No differences were seen in LOS, mortality, and ICU transfers (data not shown). However, 14‐day readmissions (but not 72‐hour or 30‐day readmissions) were lower on the PACE service (OR, 0.49; 95% CI, 0.25‐0.93).
Patient Satisfaction
Patients were similarly satisfied with their care on the PACE service and on the house staff services. In specific areas and globally, percentages of patients satisfied with their physicians and with the discharge process were not different, as measured by the Press‐Ganey survey (Press‐Ganey Associates, South Bend, IN; Figures 1 and 2). The survey distinguishes between attendings and residents, but not physician assistants; therefore, Figure 1 only includes responses to the attending questions. Given the sampling procedure of the Press‐Ganey survey, exact response rates cannot be calculated, but Press‐Ganey reports a response rate of about 40% for the English survey and about 20% for the Spanish survey.
Resident Duty Hours
Comparing the same month 1 year prior to implementation of the PACE service, mean self‐reported resident duty hours on the general medicine service were unchanged; however, self‐reported data were incomplete, and multiple changes took place in the residency program during the study period. For example, implementation of the PACE service allowed for the dissolution of one full house staff general medicine team and redistribution of these house staff to night float positions and an expanded medical intensive care unit.
Costs of Implementation
The costs associated with implementing the PACE service included physician and physician assistant salaries (2.5 full‐time physicians, 5 full‐time physician assistants, plus fringe) and night coverage by resident and fellow moonlighters (without fringe, and estimated at 50% effort given other moonlighter coverage responsibilities on subspecialty services). We estimated these costs at $257.50/patient‐day ($115/patient‐day for attending physician compensation, $110/patient‐day for physician assistant compensation, and $32.50/patient‐day for moonlighting coverage).
DISCUSSION
As academic centers struggle with developing a workforce to provide patient care no longer provided by residents, questions about the ideal structure of nonhouse staff inpatient services abound. Although solutions to this problem will be determined to some extent by local factors such as institutional culture and resources, some lessons learned in developing such services will be more widely applicable. We found that by implementing a geographically localized, physician assistant‐staffed hospitalist service, we were able to provide care of similar quality and efficiency to that of traditional house staff services, despite inexperienced hospitalists staffing the service and a medical residency program commonly recognized as one of the best in the country. Adjusted total costs were slightly lower on the PACE service, but this difference was small and of borderline statistical significance. Likewise, no significant differences were seen in any of several quality measures or in patient satisfaction.
Our findings add to the available evidence supporting the use of physician assistants on academic general medicine services, and are germane to academic centers facing reductions in house staff availability and seeking alternative models of care for inpatients. Several specific characteristics of the PACE service and the implications of these should be considered:
The service accepted all patients, regardless of diagnosis, acuity, or complexity of illness. This was unlike many previously described nonhouse staff services which were more limited in scope, and allowed more flexibility with patient flow. However, in the end, patients on the PACE service did have a modestly lower case mix index and Charlson score, suggesting that, despite a lack of triage guidelines, there was some bias in the triage of admissions, possibly due to a perception that physician assistants should take care of lower complexity patients. If it is desirable to have a similar distribution of higher complexity patients across house staff and nonhouse staff services, extra efforts may be necessary to overcome this perception.
The service was geographically regionalized. Geographic regionalization offered many important advantages, especially with regards to communication among staff, nursing, and consultants, and allowed for multidisciplinary rounds. However, it is possible that the modest, but not statistically significant, trend toward an increased LOS seen on the PACE service might be a reflection of geographic admitting (less incentive to discharge since discharging a patient means taking a new admission).
The education and professional development of the physician assistants was a priority. Physician assistants had considerable autonomy and responsibility, and rather than being assigned only lower level administrative tasks, performed all aspects of patient care. They also received regular teaching from the hospitalists, attended house staff teaching conferences, and developed nonclinical roles in education and quality improvement. The higher standards expected of the physician assistants were quite possibly a factor in the quality of care delivered, and almost certainly contributed to physician assistant satisfaction and retention.
Our findings contrast with those of Myers et al.,9 who found that a nonteaching service staffed by hospitalists and nurse practitioners had a significantly lower median LOS and hospital charges compared to similar patients on resident‐based services. However, unlike ours, their service cared for a select patient population, and only accepted patients with chest pain at low risk for acute coronary syndrome. Van Rhee et al.10 found that physician assistants on a general medicine service used fewer resources for patients with pneumonia, stroke, and congestive heart failure than resident physicians, and did not exceed the resources used by residents in other diagnoses. The authors did not find a difference in LOS, but did find a significantly higher mortality among patients with pneumonia cared for by physician assistants.
Several limitations should be noted. First, the study was a retrospective analysis of administrative data rather than a randomized trial, and although we employed a standard approach to adjust for a wide range of patient characteristics including severity of illness, there may have been undetected differences in the patient populations studied that may have confounded our results. Second, resident moonlighters admitted patients to the PACE service and, at other times, to the house staff services, and this may have diluted any differences between the groups. However, when we limited our analysis to the subgroup of patients admitted during the day, similar results were obtained, with the exception that the PACE service had a lower rate of 14‐day readmissions, an unexpected finding deserving of further study. Third, the study was conducted in a single academic institution and our findings may not be generalizable to others with different needs and resources; indeed, the costs associated with implementing such a service may be prohibitive for some institutions. Fourth, because of simultaneous changes that were taking place in our residency program, we are unable to accurately assess the impact of the PACE service on resident duty hours. However, resident duty hours did not increase over this time period on the general medicine service, and implementation of the service allowed for redistribution of house staff to other services and positions. Fifth, patient satisfaction data were obtained from responses to the mailed Press‐Ganey survey, to which there is a relatively low response rate. Also, we did not survey providers regarding their satisfaction with the service during the study period. Sixth, the study had limited power to detect clinically important differences in mortality and ICU transfers. Finally, this study is unable to compare this particular model of incorporating midlevel providers into general medical services with other models, only with traditional house staff services.
Future research should focus on determining the most effective and efficient ways to incorporate midlevel providers on academic general medicine services. One important question from the standpoint of house staff training is whether such services should be separate but equal, or should house staff gain experience during residency working with midlevel providers, since they are likely to encounter them in the future whether they stay in academics or not. Different models of care will likely have large implications for the quality and efficiency of patient care, house staff education and satisfaction, and physician assistant job satisfaction and turnover.
In summary, our study demonstrates that a geographically regionalized, multidisciplinary service staffed by hospitalists and physician assistants can be a safe alternative to house staff‐based services for the care of general medicine inpatients in an academic medical center.
- ,,,,,.The physician's assistant as resident on surgical service. An example of creative problem solving in surgical manpower.Arch Surg.1980;115:310–314.
- ,,,.Coronary arteriography performed by a physician assistant.Am J Cardiol.1987;60:784–787.
- .The specialized physician assistant: an alternative to the clinical cardiology trainee.Am J Cardiol.1987;60:901–902.
- ,,.One hospital's successful 20‐year experience with physician assistants in graduate medical education.Acad Med.1999;74:641–645.
- ,.Physicians assistants in cardiothoracic surgery: a 30‐year experience in a university center.Ann Thorac Surg.2006;81:195–199; discussion 199–200.
- ,,.Comparative review of use of physician assistants in a level I trauma center.Am Surg.2004;70:272–279.
- ,,,,.Integrating midlevel practitioners into a teaching service.Am J Surg.2006;192:119–124.
- ,.Acute care nurse practitioners: creating and implementing a model of care for an inpatient general medical service.Am J Crit Care.2002;11:448–458.
- ,,,,.Improving resource utilization in a teaching hospital: development of a nonteaching service for chest pain admissions.Acad Med.2006;81:432–435.
- ,,.Resource use by physician assistant services versus teaching services.JAAPA.2002;15:33–38.
- ,,, for the ACGME Work Group on Resident Duty Hours, Accreditation Council for Graduate Medical Education.New requirements for resident duty hours.JAMA.2002;288:1112–1114.
- ,,.The substitution of physician assistants and nurse practitioners for physician residents in teaching hospitals.Health Aff.1995;14:181–191.
- ,,,,.Challenges of the 80‐hour resident work rules: collaboration between surgeons and nonphysician practitioners.Surg Clin North Am.2004;84:1573–1586.
- ,,.Adapting a clinical comorbidity index for use with ICD‐9‐CM administrative databases.J Clin Epidemiol.1992;45:613–619.
Midlevel providers (physician assistants and nurse practitioners) have long been employed by academic medical centers, predominantly on surgical services, or on medical subspecialty services, where they have typically had a limited scope of practice, focused in a narrowly defined area or set of procedures.17 In contrast, there are relatively few reports of experiences deploying midlevel providers to replace house staff on inpatient general medicine services in academic centers,810 and few studies of the effect of midlevel providers on quality and efficiency of care in the academic setting. Despite this, reductions in house officer duty hours as mandated by the Accreditation Council on Graduate Medical Education (ACGME)11 have resulted in academic centers increasingly using midlevel providers to decrease house staff workload on inpatient services.12, 13 In general, midlevel practitioners on general medicine services have been deployed to: (1) care for a population of patients separate from and in parallel with house staff; this population may be narrowly defined (eg, patients with chest pain) or not; (2) assist with the management of patients cared for by house staff by performing certain tasks (eg, scheduling appointments, discharging patients). Even as midlevel providers become more prevalent on academic general medicine services, the best model of care incorporating them into clinical care remains unclear, and few studies have rigorously examined the care provided on services that use them.
We developed an inpatient general medicine service within a large academic medical center staffed by physician assistants and hospitalists to help our residency program meet ACGME duty hour requirements. We hypothesized that by creating a service that is geographically localized and supervised by full‐time hospitalists, by instituting multidisciplinary rounds, and by investing in the professional development of highly‐skilled physician assistants, we could provide care for medically complex, acutely ill general medicine inpatients with similar quality and efficiency as compared to house staff teams. We report our experience during the first year of implementing the service, and compare quality and efficiency of care on this service with that of our traditional house staff services. We also evaluate the effects of this service on patient satisfaction and self‐reported house staff workload.
PATIENTS AND METHODS
Study Setting
The study was conducted in a 747‐bed urban, academic medical center in the northeastern United States. The hospital's human research committee reviewed and approved the study design. The hospital has accredited residency and fellowship programs in all major specialties. Prior to July 2005, physician assistants were employed only on surgical and medical subspecialty services (ie, bone marrow transplant, interventional cardiology); none were employed on the inpatient general medicine service. There were approximately 44,000 inpatient admissions during the year of the study, with approximately 6500 of these to the general medicine service.
Description of the General Medicine Service
The General Medicine Service consisted of 8 traditional house staff teams, with 1 attending, 1 junior or senior resident, 2 interns, and 1 or 2 medical students. These teams admitted patients on a rotating basis every fourth day. On 4 of these teams, the attending was a hospitalist, with clinical responsibility for the majority of the patients admitted to the team. On the remaining 4 teams, the teaching attending was a primary care physician or medical subspecialist, responsible for the direct care of a small number of the team's patients, with the remainder cared for by private primary care physicians or subspecialists.
Description of the Physician Assistant/Hospitalist Service
The Physician Assistant/Clinician Educator (PACE) service opened in July 2005, and consisted of 15 beds localized to 2 adjacent inpatient pods, staffed by a single cadre of nurses and medically staffed by 1 hospitalist and 2 physician assistants from 7:00 AM to 7:00 PM on weekdays and by 1 hospitalist, 1 physician assistant, and 1 moonlighter (usually a senior medical resident or fellow) from 7:00 AM to 7:00 PM on weekends. A moonlighter, typically a senior resident or medical subspecialty fellow, admitted patients and covered nights on the service from 7:00 PM to 7:00 AM 7 days a week. The daily census goal for the service was 15 patients, limited by the number of available beds on the 2 pods, and the service accepted admissions 24 hours per day, 7 days per week, whenever beds were available. Daily morning rounds occurred at 8:00 AM and included the hospitalist, physician assistants, nurses, a care coordinator, and a pharmacist. The PACE service did not have triage guidelines related to diagnosis, complexity, or acuity, but only accepted patients via the emergency department or via a primary care physician's office, and did not accept patients transferred from outside hospitals or from the intensive care units.
Physician Assistants
All of the physician assistants on the PACE service had prior inpatient medicine experience, ranging from 6 months to 5 years. The physician assistants worked in 3‐day to 6‐day blocks of 12‐hour shifts. Their clinical responsibilities were similar to those of interns at the study hospital, and included taking histories and performing physical examinations, writing notes and orders, reviewing and assimilating data, creating and updating patient signouts, completing discharge summaries, consulting other services as needed, and communicating with nurses and family members.
Many physician assistants also had nonclinical responsibilities, taking on physician‐mentored roles in education, quality improvement, and administration. They were involved in several initiatives: (1) developing a physician assistant curriculum in hospital medicine, (2) presenting at hospital‐wide physician assistant grand rounds, (3) surveying and tracking patient and family satisfaction on the service, (4) reviewing all 72‐hour hospital readmissions, intensive care unit transfers, and deaths on the service, and (5) maintaining the service's compliance with state regulations regarding physician assistant scope of practice and prescribing.
Hospitalists
The 3 hospitalists on the PACE service worked in 7‐day blocks of 12‐hour shifts (7:00 AM to 7:00 PM). They directly supervised the physician assistants and had no competing responsibilities. The hospitalists were all recent graduates of the study hospital's internal medicine residency, with no prior clinical experience beyond residency. All were planning to work on the service for 1 to 2 years before beginning a subspecialty fellowship. In addition to supervising the clinical work of the physician assistants, the hospitalists were responsible for teaching the physician assistants on rounds and in weekly didactic sessions, guided by a curriculum in hospital medicine that focused on the most common general medicine diagnoses seen on the PACE service. The medical director of the PACE service periodically reviewed each physician assistant's clinical experience, skills and knowledge base, and held semiannual feedback sessions.
Study Patients
All general medicine patients admitted to the PACE service from July 1, 2005 to June 30, 2006 comprised the study population. The comparison group consisted of general medicine patients admitted to the 8 house staff general medicine teams; patients transferred from an intensive care unit (ICU) or another facility were excluded in order to match the admission criteria for the PACE service and improve comparability between the 2 study arms.
Data Collection and Study Outcomes
We obtained all patient data from the hospital's administrative databases. We identified patients assigned to the PACE service or to the comparison group based on the admitting service, team, and attending. We obtained patient demographics, insurance, admission source and discharge destination, admission and discharge times, dates, diagnoses, and diagnosis‐related groups (DRGs), as well as dates and times of transfers to other services, including to the intensive care unit. We also obtained the Medicare case‐mix index (CMI, based on DRG weight), and calculated a Charlson score based on billing diagnoses coded in the year prior to the index admission.14 Outcomes included length of stay (LOS) to the nearest hour, in‐hospital mortality, transfers to the intensive care unit, readmissions to the study hospital within 72 hours, 14 days, and 30 days, and total costs as derived from the hospital's cost accounting system (Transition Systems Inc., Boston, MA). Other outcomes included patient satisfaction as measured by responses to the Press‐Ganey survey routinely administered to a randomly selected 70% of recently discharged patients and effect on self‐reported resident work hours.
Statistical Analysis
Patient demographics, clinical characteristics, and study outcomes are presented using proportions, means with standard deviations, and medians with inter‐quartile ranges as appropriate. Unadjusted differences in outcomes between the two services were calculated using univariable regression techniques with service as the independent variable and each outcome as the dependent variable. We used logistic regression for dichotomous outcomes (readmissions, ICU transfers, and inpatient mortality), and linear regression for log‐transformed LOS and log‐transformed total costs of care. To adjust each outcome for potential confounders, we then built multivariable regression models. Each potential confounder was entered into the model one at a time as the independent variable. All variables found to be significant predictors of the outcome at the P < 0.10 level were then retained in the final model along with service as the predictor of interest. We used general estimating equations in all multivariable models to adjust for clustering of patients by attending physician. For logistic regression models, the effect size is presented as an odds ratio (OR); for log‐transformed linear regression models, the effect size is presented as the percent difference between groups. We also performed 2 subgroup analyses, limited to (1) the patients with the 10 most common discharge DRGs, and (2) patients admitted between the hours of 7:00 AM and 7:00 PM to remove the effects of moonlighters performing the initial admission. Except as noted above, 2‐sided P values < 0.05 were considered significant. SAS 9.1 (SAS Institute, Cary, NC) was used for all analyses.
RESULTS
Patient Demographics
Table 1 shows patient demographics and clinical characteristics of the PACE service and the comparison group. Patients in the comparison group were slightly older and tended to have slightly higher CMI and Charlson scores. Patients on the PACE service were more likely to be admitted at night (10:00 PM to 7:00 AM; 43.8% versus 30.3%; P < 0.0001). There were no significant differences in sex, race, insurance, or percentage of patients discharged to home. The 10 most common DRGs in the comparison group accounted for 37.0% of discharges, and these same DRGs accounted for 37.5% of discharges on the PACE service (Table 2).
| Characteristic | PACE Service (n = 992) | House Staff Services (n = 4,202) | P value |
|---|---|---|---|
| |||
| Age (years) | |||
| 1844 | 19.1 | 18.2 | |
| 4564 | 35.5 | 31.9 | 0.04 |
| 65+ | 45.5 | 49.9 | |
| Sex (% female) | 57.7 | 60.0 | NS |
| Race/ethnicity | |||
| White | 57.3 | 59.3 | |
| Black | 24.0 | 23.5 | NS |
| Hispanic | 14.1 | 13.3 | |
| Other | 4.6 | 3.9 | |
| Insurance | |||
| Medicare | 41.9 | 43.8 | |
| Commercial | 34.9 | 35.9 | |
| Medicaid | 14.4 | 11.7 | NS |
| Free care | 4.5 | 3.9 | |
| Self pay | 1.1 | 0.8 | |
| Median income by zip code of residence, USD (IQR) | 45,517 (32,49362,932) | 45,517 (35,88963,275) | NS |
| Case‐mix index, median (IQR) | 1.1 (0.81.5) | 1.2 (0.91.8) | 0.001 |
| Charlson score | |||
| 0 | 27.2 | 24.9 | |
| 1 | 22.6 | 21.1 | 0.02 |
| 2 | 16.2 | 16.5 | |
| 3+ | 34.0 | 37.6 | |
| Admissions between 10:00 PM and 7:00 AM | 43.8 | 30.3 | <0.0001 |
| Discharged to home | 81.1 | 80.5 | NS |
| Diagnosis‐Related Group at Discharge | PACE Service (n = 992)* | House Staff Services (n = 4,202)* |
|---|---|---|
| ||
| Chest pain | 5.4 | 6.4 |
| Esophagitis, gastroenteritis, and miscellaneous digestive disorders | 4.5 | 4.4 |
| Heart failure and shock | 3.4 | 4.6 |
| Simple pneumonia and pleurisy | 2.7 | 4.4 |
| Kidney and urinary tract infections | 4.7 | 3.2 |
| Chronic obstructive pulmonary disease | 4.0 | 3.3 |
| Renal failure | 2.7 | 3.5 |
| Gastrointestinal hemorrhage | 3.7 | 2.7 |
| Nutritional and miscellaneous metabolic disorders | 3.3 | 2.4 |
| Disorders of the pancreas except malignancy | 3.1 | 2.1 |
| Cumulative percent | 37.5 | 37.0 |
Efficiency and Quality of Care
Table 3 compares the performance of the PACE service and the comparison group on several efficiency and quality measures. Unadjusted LOS was not significantly different, and adjusted LOS was slightly but not statistically significantly higher on the study service (adjusted LOS 5.0% higher; 95% confidence interval [CI], 0.4% to +10%). Unadjusted and adjusted total costs of care were marginally lower on the study service (adjusted total cost of care 3.9% lower; 95% CI, 7.5% to 0.3%).
| PACE Service | House Staff Services | Unadjusted % Difference (95%CI) | Adjusted % Difference (95%CI)* | |
|---|---|---|---|---|
| PACE Service | House Staff Services | Unadjusted OR (95% CI) | Adjusted OR (95% CI) | |
| ||||
| Efficiency measure | ||||
| Length of stay, days, median (IQR) | 2.6 (1.6, 4.4) | 2.6 (1.4, 4.6) | +0.1% (5.6% to +6.1%) | +5.0% (0.4% to +10.0%) |
| Total costs, USD, median (IQR) | 4,536 (2,848, 7,201) | 4,749 (3,046, 8,161) | 9.1% (14.0% to 3.8%) | 3.9% (7.5% to 0.3%)‖ |
| Quality measure | ||||
| 72‐hour readmissions/100 discharges | 0.8 | 1.3 | 0.6 (0.31.3) | 0.7 (0.21.8) |
| 14‐day readmissions/100 discharges | 5.4 | 5.4 | 1.0 (0.71.4) | 1.1 (0.81.4) |
| 30‐day readmissions/100 discharges | 8.0 | 8.1 | 1.0 (0.81.3) | 1.1 (0.91.3) |
| ICU transfers/100 discharges | 2.0 | 2.3 | 0.9 (0.51.4) | 1.4 (0.82.4)# |
| Inpatient mortality/100 discharges | 0.7 | 1.2 | 0.6 (0.31.3) | 0.8 (0.31.8)** |
We found no differences between the PACE service and comparison group in unadjusted rates of hospital readmissions within 72 hours, 14 days, and 30 days, transfer to the intensive care units, or inpatient mortality (Table 3). The associated ORs for each outcome were similar after adjusting for patient demographics and clinical characteristics including severity of illness, as well as for clustering by attending physician.
Subgroup Analyses
When the analysis was limited to the subset of patients with the 10 most common discharge DRGs, the difference in adjusted total cost of care was similar but lost statistical significance (4.0% lower on PACE service; 95% CI, 11.0% to +3.3%). In this subgroup, LOS, readmission rates, and ICU transfer rates were not different. ORs for mortality could not be calculated because there were no deaths in this subgroup on the PACE service (data not shown). When analysis was limited to daytime admissions (to remove any potential effect of admitting by a moonlighter), the difference in total cost of care was attenuated and lost statistical significance (0.2% lower on PACE service; 95%CI, 5.9% to +5.5%). No differences were seen in LOS, mortality, and ICU transfers (data not shown). However, 14‐day readmissions (but not 72‐hour or 30‐day readmissions) were lower on the PACE service (OR, 0.49; 95% CI, 0.25‐0.93).
Patient Satisfaction
Patients were similarly satisfied with their care on the PACE service and on the house staff services. In specific areas and globally, percentages of patients satisfied with their physicians and with the discharge process were not different, as measured by the Press‐Ganey survey (Press‐Ganey Associates, South Bend, IN; Figures 1 and 2). The survey distinguishes between attendings and residents, but not physician assistants; therefore, Figure 1 only includes responses to the attending questions. Given the sampling procedure of the Press‐Ganey survey, exact response rates cannot be calculated, but Press‐Ganey reports a response rate of about 40% for the English survey and about 20% for the Spanish survey.


Resident Duty Hours
Comparing the same month 1 year prior to implementation of the PACE service, mean self‐reported resident duty hours on the general medicine service were unchanged; however, self‐reported data were incomplete, and multiple changes took place in the residency program during the study period. For example, implementation of the PACE service allowed for the dissolution of one full house staff general medicine team and redistribution of these house staff to night float positions and an expanded medical intensive care unit.
Costs of Implementation
The costs associated with implementing the PACE service included physician and physician assistant salaries (2.5 full‐time physicians, 5 full‐time physician assistants, plus fringe) and night coverage by resident and fellow moonlighters (without fringe, and estimated at 50% effort given other moonlighter coverage responsibilities on subspecialty services). We estimated these costs at $257.50/patient‐day ($115/patient‐day for attending physician compensation, $110/patient‐day for physician assistant compensation, and $32.50/patient‐day for moonlighting coverage).
DISCUSSION
As academic centers struggle with developing a workforce to provide patient care no longer provided by residents, questions about the ideal structure of nonhouse staff inpatient services abound. Although solutions to this problem will be determined to some extent by local factors such as institutional culture and resources, some lessons learned in developing such services will be more widely applicable. We found that by implementing a geographically localized, physician assistant‐staffed hospitalist service, we were able to provide care of similar quality and efficiency to that of traditional house staff services, despite inexperienced hospitalists staffing the service and a medical residency program commonly recognized as one of the best in the country. Adjusted total costs were slightly lower on the PACE service, but this difference was small and of borderline statistical significance. Likewise, no significant differences were seen in any of several quality measures or in patient satisfaction.
Our findings add to the available evidence supporting the use of physician assistants on academic general medicine services, and are germane to academic centers facing reductions in house staff availability and seeking alternative models of care for inpatients. Several specific characteristics of the PACE service and the implications of these should be considered:
The service accepted all patients, regardless of diagnosis, acuity, or complexity of illness. This was unlike many previously described nonhouse staff services which were more limited in scope, and allowed more flexibility with patient flow. However, in the end, patients on the PACE service did have a modestly lower case mix index and Charlson score, suggesting that, despite a lack of triage guidelines, there was some bias in the triage of admissions, possibly due to a perception that physician assistants should take care of lower complexity patients. If it is desirable to have a similar distribution of higher complexity patients across house staff and nonhouse staff services, extra efforts may be necessary to overcome this perception.
The service was geographically regionalized. Geographic regionalization offered many important advantages, especially with regards to communication among staff, nursing, and consultants, and allowed for multidisciplinary rounds. However, it is possible that the modest, but not statistically significant, trend toward an increased LOS seen on the PACE service might be a reflection of geographic admitting (less incentive to discharge since discharging a patient means taking a new admission).
The education and professional development of the physician assistants was a priority. Physician assistants had considerable autonomy and responsibility, and rather than being assigned only lower level administrative tasks, performed all aspects of patient care. They also received regular teaching from the hospitalists, attended house staff teaching conferences, and developed nonclinical roles in education and quality improvement. The higher standards expected of the physician assistants were quite possibly a factor in the quality of care delivered, and almost certainly contributed to physician assistant satisfaction and retention.
Our findings contrast with those of Myers et al.,9 who found that a nonteaching service staffed by hospitalists and nurse practitioners had a significantly lower median LOS and hospital charges compared to similar patients on resident‐based services. However, unlike ours, their service cared for a select patient population, and only accepted patients with chest pain at low risk for acute coronary syndrome. Van Rhee et al.10 found that physician assistants on a general medicine service used fewer resources for patients with pneumonia, stroke, and congestive heart failure than resident physicians, and did not exceed the resources used by residents in other diagnoses. The authors did not find a difference in LOS, but did find a significantly higher mortality among patients with pneumonia cared for by physician assistants.
Several limitations should be noted. First, the study was a retrospective analysis of administrative data rather than a randomized trial, and although we employed a standard approach to adjust for a wide range of patient characteristics including severity of illness, there may have been undetected differences in the patient populations studied that may have confounded our results. Second, resident moonlighters admitted patients to the PACE service and, at other times, to the house staff services, and this may have diluted any differences between the groups. However, when we limited our analysis to the subgroup of patients admitted during the day, similar results were obtained, with the exception that the PACE service had a lower rate of 14‐day readmissions, an unexpected finding deserving of further study. Third, the study was conducted in a single academic institution and our findings may not be generalizable to others with different needs and resources; indeed, the costs associated with implementing such a service may be prohibitive for some institutions. Fourth, because of simultaneous changes that were taking place in our residency program, we are unable to accurately assess the impact of the PACE service on resident duty hours. However, resident duty hours did not increase over this time period on the general medicine service, and implementation of the service allowed for redistribution of house staff to other services and positions. Fifth, patient satisfaction data were obtained from responses to the mailed Press‐Ganey survey, to which there is a relatively low response rate. Also, we did not survey providers regarding their satisfaction with the service during the study period. Sixth, the study had limited power to detect clinically important differences in mortality and ICU transfers. Finally, this study is unable to compare this particular model of incorporating midlevel providers into general medical services with other models, only with traditional house staff services.
Future research should focus on determining the most effective and efficient ways to incorporate midlevel providers on academic general medicine services. One important question from the standpoint of house staff training is whether such services should be separate but equal, or should house staff gain experience during residency working with midlevel providers, since they are likely to encounter them in the future whether they stay in academics or not. Different models of care will likely have large implications for the quality and efficiency of patient care, house staff education and satisfaction, and physician assistant job satisfaction and turnover.
In summary, our study demonstrates that a geographically regionalized, multidisciplinary service staffed by hospitalists and physician assistants can be a safe alternative to house staff‐based services for the care of general medicine inpatients in an academic medical center.
Midlevel providers (physician assistants and nurse practitioners) have long been employed by academic medical centers, predominantly on surgical services, or on medical subspecialty services, where they have typically had a limited scope of practice, focused in a narrowly defined area or set of procedures.17 In contrast, there are relatively few reports of experiences deploying midlevel providers to replace house staff on inpatient general medicine services in academic centers,810 and few studies of the effect of midlevel providers on quality and efficiency of care in the academic setting. Despite this, reductions in house officer duty hours as mandated by the Accreditation Council on Graduate Medical Education (ACGME)11 have resulted in academic centers increasingly using midlevel providers to decrease house staff workload on inpatient services.12, 13 In general, midlevel practitioners on general medicine services have been deployed to: (1) care for a population of patients separate from and in parallel with house staff; this population may be narrowly defined (eg, patients with chest pain) or not; (2) assist with the management of patients cared for by house staff by performing certain tasks (eg, scheduling appointments, discharging patients). Even as midlevel providers become more prevalent on academic general medicine services, the best model of care incorporating them into clinical care remains unclear, and few studies have rigorously examined the care provided on services that use them.
We developed an inpatient general medicine service within a large academic medical center staffed by physician assistants and hospitalists to help our residency program meet ACGME duty hour requirements. We hypothesized that by creating a service that is geographically localized and supervised by full‐time hospitalists, by instituting multidisciplinary rounds, and by investing in the professional development of highly‐skilled physician assistants, we could provide care for medically complex, acutely ill general medicine inpatients with similar quality and efficiency as compared to house staff teams. We report our experience during the first year of implementing the service, and compare quality and efficiency of care on this service with that of our traditional house staff services. We also evaluate the effects of this service on patient satisfaction and self‐reported house staff workload.
PATIENTS AND METHODS
Study Setting
The study was conducted in a 747‐bed urban, academic medical center in the northeastern United States. The hospital's human research committee reviewed and approved the study design. The hospital has accredited residency and fellowship programs in all major specialties. Prior to July 2005, physician assistants were employed only on surgical and medical subspecialty services (ie, bone marrow transplant, interventional cardiology); none were employed on the inpatient general medicine service. There were approximately 44,000 inpatient admissions during the year of the study, with approximately 6500 of these to the general medicine service.
Description of the General Medicine Service
The General Medicine Service consisted of 8 traditional house staff teams, with 1 attending, 1 junior or senior resident, 2 interns, and 1 or 2 medical students. These teams admitted patients on a rotating basis every fourth day. On 4 of these teams, the attending was a hospitalist, with clinical responsibility for the majority of the patients admitted to the team. On the remaining 4 teams, the teaching attending was a primary care physician or medical subspecialist, responsible for the direct care of a small number of the team's patients, with the remainder cared for by private primary care physicians or subspecialists.
Description of the Physician Assistant/Hospitalist Service
The Physician Assistant/Clinician Educator (PACE) service opened in July 2005, and consisted of 15 beds localized to 2 adjacent inpatient pods, staffed by a single cadre of nurses and medically staffed by 1 hospitalist and 2 physician assistants from 7:00 AM to 7:00 PM on weekdays and by 1 hospitalist, 1 physician assistant, and 1 moonlighter (usually a senior medical resident or fellow) from 7:00 AM to 7:00 PM on weekends. A moonlighter, typically a senior resident or medical subspecialty fellow, admitted patients and covered nights on the service from 7:00 PM to 7:00 AM 7 days a week. The daily census goal for the service was 15 patients, limited by the number of available beds on the 2 pods, and the service accepted admissions 24 hours per day, 7 days per week, whenever beds were available. Daily morning rounds occurred at 8:00 AM and included the hospitalist, physician assistants, nurses, a care coordinator, and a pharmacist. The PACE service did not have triage guidelines related to diagnosis, complexity, or acuity, but only accepted patients via the emergency department or via a primary care physician's office, and did not accept patients transferred from outside hospitals or from the intensive care units.
Physician Assistants
All of the physician assistants on the PACE service had prior inpatient medicine experience, ranging from 6 months to 5 years. The physician assistants worked in 3‐day to 6‐day blocks of 12‐hour shifts. Their clinical responsibilities were similar to those of interns at the study hospital, and included taking histories and performing physical examinations, writing notes and orders, reviewing and assimilating data, creating and updating patient signouts, completing discharge summaries, consulting other services as needed, and communicating with nurses and family members.
Many physician assistants also had nonclinical responsibilities, taking on physician‐mentored roles in education, quality improvement, and administration. They were involved in several initiatives: (1) developing a physician assistant curriculum in hospital medicine, (2) presenting at hospital‐wide physician assistant grand rounds, (3) surveying and tracking patient and family satisfaction on the service, (4) reviewing all 72‐hour hospital readmissions, intensive care unit transfers, and deaths on the service, and (5) maintaining the service's compliance with state regulations regarding physician assistant scope of practice and prescribing.
Hospitalists
The 3 hospitalists on the PACE service worked in 7‐day blocks of 12‐hour shifts (7:00 AM to 7:00 PM). They directly supervised the physician assistants and had no competing responsibilities. The hospitalists were all recent graduates of the study hospital's internal medicine residency, with no prior clinical experience beyond residency. All were planning to work on the service for 1 to 2 years before beginning a subspecialty fellowship. In addition to supervising the clinical work of the physician assistants, the hospitalists were responsible for teaching the physician assistants on rounds and in weekly didactic sessions, guided by a curriculum in hospital medicine that focused on the most common general medicine diagnoses seen on the PACE service. The medical director of the PACE service periodically reviewed each physician assistant's clinical experience, skills and knowledge base, and held semiannual feedback sessions.
Study Patients
All general medicine patients admitted to the PACE service from July 1, 2005 to June 30, 2006 comprised the study population. The comparison group consisted of general medicine patients admitted to the 8 house staff general medicine teams; patients transferred from an intensive care unit (ICU) or another facility were excluded in order to match the admission criteria for the PACE service and improve comparability between the 2 study arms.
Data Collection and Study Outcomes
We obtained all patient data from the hospital's administrative databases. We identified patients assigned to the PACE service or to the comparison group based on the admitting service, team, and attending. We obtained patient demographics, insurance, admission source and discharge destination, admission and discharge times, dates, diagnoses, and diagnosis‐related groups (DRGs), as well as dates and times of transfers to other services, including to the intensive care unit. We also obtained the Medicare case‐mix index (CMI, based on DRG weight), and calculated a Charlson score based on billing diagnoses coded in the year prior to the index admission.14 Outcomes included length of stay (LOS) to the nearest hour, in‐hospital mortality, transfers to the intensive care unit, readmissions to the study hospital within 72 hours, 14 days, and 30 days, and total costs as derived from the hospital's cost accounting system (Transition Systems Inc., Boston, MA). Other outcomes included patient satisfaction as measured by responses to the Press‐Ganey survey routinely administered to a randomly selected 70% of recently discharged patients and effect on self‐reported resident work hours.
Statistical Analysis
Patient demographics, clinical characteristics, and study outcomes are presented using proportions, means with standard deviations, and medians with inter‐quartile ranges as appropriate. Unadjusted differences in outcomes between the two services were calculated using univariable regression techniques with service as the independent variable and each outcome as the dependent variable. We used logistic regression for dichotomous outcomes (readmissions, ICU transfers, and inpatient mortality), and linear regression for log‐transformed LOS and log‐transformed total costs of care. To adjust each outcome for potential confounders, we then built multivariable regression models. Each potential confounder was entered into the model one at a time as the independent variable. All variables found to be significant predictors of the outcome at the P < 0.10 level were then retained in the final model along with service as the predictor of interest. We used general estimating equations in all multivariable models to adjust for clustering of patients by attending physician. For logistic regression models, the effect size is presented as an odds ratio (OR); for log‐transformed linear regression models, the effect size is presented as the percent difference between groups. We also performed 2 subgroup analyses, limited to (1) the patients with the 10 most common discharge DRGs, and (2) patients admitted between the hours of 7:00 AM and 7:00 PM to remove the effects of moonlighters performing the initial admission. Except as noted above, 2‐sided P values < 0.05 were considered significant. SAS 9.1 (SAS Institute, Cary, NC) was used for all analyses.
RESULTS
Patient Demographics
Table 1 shows patient demographics and clinical characteristics of the PACE service and the comparison group. Patients in the comparison group were slightly older and tended to have slightly higher CMI and Charlson scores. Patients on the PACE service were more likely to be admitted at night (10:00 PM to 7:00 AM; 43.8% versus 30.3%; P < 0.0001). There were no significant differences in sex, race, insurance, or percentage of patients discharged to home. The 10 most common DRGs in the comparison group accounted for 37.0% of discharges, and these same DRGs accounted for 37.5% of discharges on the PACE service (Table 2).
| Characteristic | PACE Service (n = 992) | House Staff Services (n = 4,202) | P value |
|---|---|---|---|
| |||
| Age (years) | |||
| 1844 | 19.1 | 18.2 | |
| 4564 | 35.5 | 31.9 | 0.04 |
| 65+ | 45.5 | 49.9 | |
| Sex (% female) | 57.7 | 60.0 | NS |
| Race/ethnicity | |||
| White | 57.3 | 59.3 | |
| Black | 24.0 | 23.5 | NS |
| Hispanic | 14.1 | 13.3 | |
| Other | 4.6 | 3.9 | |
| Insurance | |||
| Medicare | 41.9 | 43.8 | |
| Commercial | 34.9 | 35.9 | |
| Medicaid | 14.4 | 11.7 | NS |
| Free care | 4.5 | 3.9 | |
| Self pay | 1.1 | 0.8 | |
| Median income by zip code of residence, USD (IQR) | 45,517 (32,49362,932) | 45,517 (35,88963,275) | NS |
| Case‐mix index, median (IQR) | 1.1 (0.81.5) | 1.2 (0.91.8) | 0.001 |
| Charlson score | |||
| 0 | 27.2 | 24.9 | |
| 1 | 22.6 | 21.1 | 0.02 |
| 2 | 16.2 | 16.5 | |
| 3+ | 34.0 | 37.6 | |
| Admissions between 10:00 PM and 7:00 AM | 43.8 | 30.3 | <0.0001 |
| Discharged to home | 81.1 | 80.5 | NS |
| Diagnosis‐Related Group at Discharge | PACE Service (n = 992)* | House Staff Services (n = 4,202)* |
|---|---|---|
| ||
| Chest pain | 5.4 | 6.4 |
| Esophagitis, gastroenteritis, and miscellaneous digestive disorders | 4.5 | 4.4 |
| Heart failure and shock | 3.4 | 4.6 |
| Simple pneumonia and pleurisy | 2.7 | 4.4 |
| Kidney and urinary tract infections | 4.7 | 3.2 |
| Chronic obstructive pulmonary disease | 4.0 | 3.3 |
| Renal failure | 2.7 | 3.5 |
| Gastrointestinal hemorrhage | 3.7 | 2.7 |
| Nutritional and miscellaneous metabolic disorders | 3.3 | 2.4 |
| Disorders of the pancreas except malignancy | 3.1 | 2.1 |
| Cumulative percent | 37.5 | 37.0 |
Efficiency and Quality of Care
Table 3 compares the performance of the PACE service and the comparison group on several efficiency and quality measures. Unadjusted LOS was not significantly different, and adjusted LOS was slightly but not statistically significantly higher on the study service (adjusted LOS 5.0% higher; 95% confidence interval [CI], 0.4% to +10%). Unadjusted and adjusted total costs of care were marginally lower on the study service (adjusted total cost of care 3.9% lower; 95% CI, 7.5% to 0.3%).
| PACE Service | House Staff Services | Unadjusted % Difference (95%CI) | Adjusted % Difference (95%CI)* | |
|---|---|---|---|---|
| PACE Service | House Staff Services | Unadjusted OR (95% CI) | Adjusted OR (95% CI) | |
| ||||
| Efficiency measure | ||||
| Length of stay, days, median (IQR) | 2.6 (1.6, 4.4) | 2.6 (1.4, 4.6) | +0.1% (5.6% to +6.1%) | +5.0% (0.4% to +10.0%) |
| Total costs, USD, median (IQR) | 4,536 (2,848, 7,201) | 4,749 (3,046, 8,161) | 9.1% (14.0% to 3.8%) | 3.9% (7.5% to 0.3%)‖ |
| Quality measure | ||||
| 72‐hour readmissions/100 discharges | 0.8 | 1.3 | 0.6 (0.31.3) | 0.7 (0.21.8) |
| 14‐day readmissions/100 discharges | 5.4 | 5.4 | 1.0 (0.71.4) | 1.1 (0.81.4) |
| 30‐day readmissions/100 discharges | 8.0 | 8.1 | 1.0 (0.81.3) | 1.1 (0.91.3) |
| ICU transfers/100 discharges | 2.0 | 2.3 | 0.9 (0.51.4) | 1.4 (0.82.4)# |
| Inpatient mortality/100 discharges | 0.7 | 1.2 | 0.6 (0.31.3) | 0.8 (0.31.8)** |
We found no differences between the PACE service and comparison group in unadjusted rates of hospital readmissions within 72 hours, 14 days, and 30 days, transfer to the intensive care units, or inpatient mortality (Table 3). The associated ORs for each outcome were similar after adjusting for patient demographics and clinical characteristics including severity of illness, as well as for clustering by attending physician.
Subgroup Analyses
When the analysis was limited to the subset of patients with the 10 most common discharge DRGs, the difference in adjusted total cost of care was similar but lost statistical significance (4.0% lower on PACE service; 95% CI, 11.0% to +3.3%). In this subgroup, LOS, readmission rates, and ICU transfer rates were not different. ORs for mortality could not be calculated because there were no deaths in this subgroup on the PACE service (data not shown). When analysis was limited to daytime admissions (to remove any potential effect of admitting by a moonlighter), the difference in total cost of care was attenuated and lost statistical significance (0.2% lower on PACE service; 95%CI, 5.9% to +5.5%). No differences were seen in LOS, mortality, and ICU transfers (data not shown). However, 14‐day readmissions (but not 72‐hour or 30‐day readmissions) were lower on the PACE service (OR, 0.49; 95% CI, 0.25‐0.93).
Patient Satisfaction
Patients were similarly satisfied with their care on the PACE service and on the house staff services. In specific areas and globally, percentages of patients satisfied with their physicians and with the discharge process were not different, as measured by the Press‐Ganey survey (Press‐Ganey Associates, South Bend, IN; Figures 1 and 2). The survey distinguishes between attendings and residents, but not physician assistants; therefore, Figure 1 only includes responses to the attending questions. Given the sampling procedure of the Press‐Ganey survey, exact response rates cannot be calculated, but Press‐Ganey reports a response rate of about 40% for the English survey and about 20% for the Spanish survey.


Resident Duty Hours
Comparing the same month 1 year prior to implementation of the PACE service, mean self‐reported resident duty hours on the general medicine service were unchanged; however, self‐reported data were incomplete, and multiple changes took place in the residency program during the study period. For example, implementation of the PACE service allowed for the dissolution of one full house staff general medicine team and redistribution of these house staff to night float positions and an expanded medical intensive care unit.
Costs of Implementation
The costs associated with implementing the PACE service included physician and physician assistant salaries (2.5 full‐time physicians, 5 full‐time physician assistants, plus fringe) and night coverage by resident and fellow moonlighters (without fringe, and estimated at 50% effort given other moonlighter coverage responsibilities on subspecialty services). We estimated these costs at $257.50/patient‐day ($115/patient‐day for attending physician compensation, $110/patient‐day for physician assistant compensation, and $32.50/patient‐day for moonlighting coverage).
DISCUSSION
As academic centers struggle with developing a workforce to provide patient care no longer provided by residents, questions about the ideal structure of nonhouse staff inpatient services abound. Although solutions to this problem will be determined to some extent by local factors such as institutional culture and resources, some lessons learned in developing such services will be more widely applicable. We found that by implementing a geographically localized, physician assistant‐staffed hospitalist service, we were able to provide care of similar quality and efficiency to that of traditional house staff services, despite inexperienced hospitalists staffing the service and a medical residency program commonly recognized as one of the best in the country. Adjusted total costs were slightly lower on the PACE service, but this difference was small and of borderline statistical significance. Likewise, no significant differences were seen in any of several quality measures or in patient satisfaction.
Our findings add to the available evidence supporting the use of physician assistants on academic general medicine services, and are germane to academic centers facing reductions in house staff availability and seeking alternative models of care for inpatients. Several specific characteristics of the PACE service and the implications of these should be considered:
The service accepted all patients, regardless of diagnosis, acuity, or complexity of illness. This was unlike many previously described nonhouse staff services which were more limited in scope, and allowed more flexibility with patient flow. However, in the end, patients on the PACE service did have a modestly lower case mix index and Charlson score, suggesting that, despite a lack of triage guidelines, there was some bias in the triage of admissions, possibly due to a perception that physician assistants should take care of lower complexity patients. If it is desirable to have a similar distribution of higher complexity patients across house staff and nonhouse staff services, extra efforts may be necessary to overcome this perception.
The service was geographically regionalized. Geographic regionalization offered many important advantages, especially with regards to communication among staff, nursing, and consultants, and allowed for multidisciplinary rounds. However, it is possible that the modest, but not statistically significant, trend toward an increased LOS seen on the PACE service might be a reflection of geographic admitting (less incentive to discharge since discharging a patient means taking a new admission).
The education and professional development of the physician assistants was a priority. Physician assistants had considerable autonomy and responsibility, and rather than being assigned only lower level administrative tasks, performed all aspects of patient care. They also received regular teaching from the hospitalists, attended house staff teaching conferences, and developed nonclinical roles in education and quality improvement. The higher standards expected of the physician assistants were quite possibly a factor in the quality of care delivered, and almost certainly contributed to physician assistant satisfaction and retention.
Our findings contrast with those of Myers et al.,9 who found that a nonteaching service staffed by hospitalists and nurse practitioners had a significantly lower median LOS and hospital charges compared to similar patients on resident‐based services. However, unlike ours, their service cared for a select patient population, and only accepted patients with chest pain at low risk for acute coronary syndrome. Van Rhee et al.10 found that physician assistants on a general medicine service used fewer resources for patients with pneumonia, stroke, and congestive heart failure than resident physicians, and did not exceed the resources used by residents in other diagnoses. The authors did not find a difference in LOS, but did find a significantly higher mortality among patients with pneumonia cared for by physician assistants.
Several limitations should be noted. First, the study was a retrospective analysis of administrative data rather than a randomized trial, and although we employed a standard approach to adjust for a wide range of patient characteristics including severity of illness, there may have been undetected differences in the patient populations studied that may have confounded our results. Second, resident moonlighters admitted patients to the PACE service and, at other times, to the house staff services, and this may have diluted any differences between the groups. However, when we limited our analysis to the subgroup of patients admitted during the day, similar results were obtained, with the exception that the PACE service had a lower rate of 14‐day readmissions, an unexpected finding deserving of further study. Third, the study was conducted in a single academic institution and our findings may not be generalizable to others with different needs and resources; indeed, the costs associated with implementing such a service may be prohibitive for some institutions. Fourth, because of simultaneous changes that were taking place in our residency program, we are unable to accurately assess the impact of the PACE service on resident duty hours. However, resident duty hours did not increase over this time period on the general medicine service, and implementation of the service allowed for redistribution of house staff to other services and positions. Fifth, patient satisfaction data were obtained from responses to the mailed Press‐Ganey survey, to which there is a relatively low response rate. Also, we did not survey providers regarding their satisfaction with the service during the study period. Sixth, the study had limited power to detect clinically important differences in mortality and ICU transfers. Finally, this study is unable to compare this particular model of incorporating midlevel providers into general medical services with other models, only with traditional house staff services.
Future research should focus on determining the most effective and efficient ways to incorporate midlevel providers on academic general medicine services. One important question from the standpoint of house staff training is whether such services should be separate but equal, or should house staff gain experience during residency working with midlevel providers, since they are likely to encounter them in the future whether they stay in academics or not. Different models of care will likely have large implications for the quality and efficiency of patient care, house staff education and satisfaction, and physician assistant job satisfaction and turnover.
In summary, our study demonstrates that a geographically regionalized, multidisciplinary service staffed by hospitalists and physician assistants can be a safe alternative to house staff‐based services for the care of general medicine inpatients in an academic medical center.
- ,,,,,.The physician's assistant as resident on surgical service. An example of creative problem solving in surgical manpower.Arch Surg.1980;115:310–314.
- ,,,.Coronary arteriography performed by a physician assistant.Am J Cardiol.1987;60:784–787.
- .The specialized physician assistant: an alternative to the clinical cardiology trainee.Am J Cardiol.1987;60:901–902.
- ,,.One hospital's successful 20‐year experience with physician assistants in graduate medical education.Acad Med.1999;74:641–645.
- ,.Physicians assistants in cardiothoracic surgery: a 30‐year experience in a university center.Ann Thorac Surg.2006;81:195–199; discussion 199–200.
- ,,.Comparative review of use of physician assistants in a level I trauma center.Am Surg.2004;70:272–279.
- ,,,,.Integrating midlevel practitioners into a teaching service.Am J Surg.2006;192:119–124.
- ,.Acute care nurse practitioners: creating and implementing a model of care for an inpatient general medical service.Am J Crit Care.2002;11:448–458.
- ,,,,.Improving resource utilization in a teaching hospital: development of a nonteaching service for chest pain admissions.Acad Med.2006;81:432–435.
- ,,.Resource use by physician assistant services versus teaching services.JAAPA.2002;15:33–38.
- ,,, for the ACGME Work Group on Resident Duty Hours, Accreditation Council for Graduate Medical Education.New requirements for resident duty hours.JAMA.2002;288:1112–1114.
- ,,.The substitution of physician assistants and nurse practitioners for physician residents in teaching hospitals.Health Aff.1995;14:181–191.
- ,,,,.Challenges of the 80‐hour resident work rules: collaboration between surgeons and nonphysician practitioners.Surg Clin North Am.2004;84:1573–1586.
- ,,.Adapting a clinical comorbidity index for use with ICD‐9‐CM administrative databases.J Clin Epidemiol.1992;45:613–619.
- ,,,,,.The physician's assistant as resident on surgical service. An example of creative problem solving in surgical manpower.Arch Surg.1980;115:310–314.
- ,,,.Coronary arteriography performed by a physician assistant.Am J Cardiol.1987;60:784–787.
- .The specialized physician assistant: an alternative to the clinical cardiology trainee.Am J Cardiol.1987;60:901–902.
- ,,.One hospital's successful 20‐year experience with physician assistants in graduate medical education.Acad Med.1999;74:641–645.
- ,.Physicians assistants in cardiothoracic surgery: a 30‐year experience in a university center.Ann Thorac Surg.2006;81:195–199; discussion 199–200.
- ,,.Comparative review of use of physician assistants in a level I trauma center.Am Surg.2004;70:272–279.
- ,,,,.Integrating midlevel practitioners into a teaching service.Am J Surg.2006;192:119–124.
- ,.Acute care nurse practitioners: creating and implementing a model of care for an inpatient general medical service.Am J Crit Care.2002;11:448–458.
- ,,,,.Improving resource utilization in a teaching hospital: development of a nonteaching service for chest pain admissions.Acad Med.2006;81:432–435.
- ,,.Resource use by physician assistant services versus teaching services.JAAPA.2002;15:33–38.
- ,,, for the ACGME Work Group on Resident Duty Hours, Accreditation Council for Graduate Medical Education.New requirements for resident duty hours.JAMA.2002;288:1112–1114.
- ,,.The substitution of physician assistants and nurse practitioners for physician residents in teaching hospitals.Health Aff.1995;14:181–191.
- ,,,,.Challenges of the 80‐hour resident work rules: collaboration between surgeons and nonphysician practitioners.Surg Clin North Am.2004;84:1573–1586.
- ,,.Adapting a clinical comorbidity index for use with ICD‐9‐CM administrative databases.J Clin Epidemiol.1992;45:613–619.
Copyright © 2008 Society of Hospital Medicine
Glycemic Control in Medical Inpatients
Diabetes mellitus is a common comorbid condition in hospitalized patients. In 2003, diabetes was listed as a diagnosis in 17.2% of hospital discharges in the United States.1 Because these diagnosis codes do not account for undiagnosed diabetes or hospital‐related hyperglycemia, the true prevalence of diabetes or hyperglycemia in hospitalized patients is likely higher and has been estimated to be as great as 38%.2 Hyperglycemia has been associated with adverse outcomes among hospitalized patients, including infectious complications, increased length of stay, and increased mortality.27 However, because hyperglycemia is not usually the primary reason patients with diabetes are hospitalized, its management is often not a focus in the inpatient setting. Sliding‐scale insulin alone continues to be commonly prescribed despite clinical evidence showing it to be ineffective in achieving glycemic control.8, 9
Recent randomized controlled trials have demonstrated that aggressive treatment of inpatient hyperglycemia improves outcomes in surgical and medical intensive care units10, 11 and in patients admitted for myocardial infarction.12, 13 Based on this clinical evidence and strong observational data linking hyperglycemia to poor patient outcomes in the non‐ICU setting,27 the American Diabetes Association (ADA) now advocates good metabolic control, defined as preprandial glucose levels of 90‐130 mg/dL and peak postprandial glucose levels < 180 mg/dL in hospitalized non‐ICU patients with hyperglycemia14 (note that these targets are less aggressive than those for ICU patients, for whom randomized controlled trials showed the benefits of reduced mortality provided by tight glucose control).11 To reach these targets, the ADA and American College of Endocrinology suggest that multidisciplinary teams develop and implement hyperglycemia management guidelines and protocols.15 Protocols should promote the use of continuous intravenous insulin or scheduled subcutaneous insulin as opposed to the use of sliding‐scale insulin alone. Subcutaneous insulin protocols should include target glucose levels; basal, nutritional, and supplemental insulin; and daily adjustments based on previous glucose levels, insulin sensitivity, nutritional intake, illness, and medications.6, 15 To date, few published protocols or algorithms for inpatient subcutaneous insulin have been shown to be effective.16, 17 It is therefore not known how best to design and implement an inpatient diabetes management protocol that is effective, efficient, and self‐perpetuating. The aims of our pilot study were to develop and implement a subcutaneous insulin protocol on a general medicine service, to identify barriers to implementation, and to determine the effect of this protocol on glycemic control.
METHODS
Setting and Participants
This prospective quality‐improvement pilot study was conducted at Brigham and Women's Hospital (BWH) from January 10, 2005, through June 23, 2005. Patients were eligible to participate if they were admitted to either of 2 General Medicine Service (GMS) teams with either a known diagnosis of type 2 diabetes or inpatient hyperglycemia (random laboratory glucose level > 180 mg/dL) and at least 1 fasting point‐of‐care glucose reading > 140 mg/dL. Patients were excluded if they had diabetic ketoacidosis, hyperosmolar hyperglycemic state, another absolute indication for intravenous insulin, or fasting glucose < 60 mg/dL on no insulin or if they were pregnant. Each GMS team consisted of a teaching attending, a junior or senior resident, 2 interns, and a clinical pharmacist. Twenty‐six physicians attended on these 2 teams during the study period, 13 of whom were hospitalists. This study was approved by the BWH Institutional Review Board; patient consent to participate in this study was deemed not necessary because of the relatively nonsensitive nature of the data (eg, glucose control, insulin orders), the noninvasive means of data collection (eg, chart review), and the steps taken by research personnel to minimize any breach in patient confidentiality.
Intervention
A multidisciplinary team composed of a diabetologist (M.L.P.), a hospitalist (J.L.S.), and a pharmacist (J.M.T.) developed a subcutaneous insulin protocol that was approved by the BWH Pharmacy and Therapeutics Diabetes Subcommittee. The protocol consisted of a set of treatment recommendations made by a pharmacist to be carried out by the medical team. The primary components are shown in Table 1 (a full description can be found in the Appendix). The main emphasis of the protocol was on discontinuing oral antihyperglycemic agents during hospitalization, initiating basal insulin in most patients, and adjusting basal insulin daily as needed.
|
| Oral agents |
| 1. Stop oral agents in most patients |
| Glucose testing |
| 2. Check bedside blood glucose before meals and at bedtime if eating, or every 6 hours if not eating |
| Insulin |
| 3. Start basal insulin Patient's home dose or NPH 0.1 units/kg before breakfast and at bedtime or insulin glargine 0.2 units/kg at bedtime (max dose 20 units) If NPO, consider half dose unless hyperglycemic |
| 4. Start nutritional insulin Discrete meals: insulin aspart 0.05‐0.1 units/kg per meal or home dose 0‐15 minutes prior to eating Continuous tube feeds: regular insulin every 6 hours or NPH every morning and at bedtime (0.1‐0.2 units/kg per day in addition to basal insulin) Hold if NPO |
| 5. Start correctional insulin Scale provided based on blood glucose and daily scheduled insulin requirements |
| Daily Adjustments |
6. Adjust scheduled insulin daily
|
| Other Considerations |
| 7. Hypoglycemia management (protocols for fruit juice, glucagons, IV dextrose, and when to call physician) |
| 8. Discharge orders (recommendations to discharge most patients on admission medication regimen, avoid sliding scale insulin, simplify dosing for patients requiring new insulin regimens, ensure adequate patient education and prompt outpatient follow‐up) |
All medical residents received general instructions regarding inpatient diabetes control by the research team's diabetologist (M.L.P.) through a 1‐hour department‐wide didactic lecture. The standards of care taught were identical to those in the protocol. In addition, the research team's hospitalist (J.L.S.) contacted each medical resident assigned to the 2 GMS teams electronically to introduce the protocol and describe the purpose and logistics of the pilot study.
A research assistant prospectively identified eligible patients each weekday by screening all patients admitted to the 2 GMS teams using the daily computerized sign‐out system used by all medical residents. Specifically, laboratory random glucose levels, inpatient medications, and medical history were reviewed to determine if each patient met eligibility criteria. Eligibility criteria were confirmed by medical record review. The pharmacist recommended to the primary team that the protocol be initiated for eligible patients. In addition, the pharmacist recommended daily adjustment of the insulin dose according to the protocol as appropriate. A chronologically organized summary of clinical data relevant to glycemic management for each patient, including bedside blood glucose measurements, general dietary intake, use of intravenous dextrose solutions, and administration of systemic steroids, oral diabetes medications, and all insulins, was provided to the team each day by the research assistant.
Measurements
The resident's acceptance of the protocol or reasons for declining it were recorded by the pharmacist on the day the protocol was recommended. Protocol acceptance was categorized as yes, no, or partial. Partial acceptance was defined as resident agreement to use the protocol, but with stated caveats or modifications. Clinical data were collected on each eligible patient for up to 7 days on GMS. Several data sources were used, including physician admission notes, the hospital's computerized clinical data system, vital‐sign sheets, medication administration records, and personal communication with nurses regarding any missing or discrepant data.
All insulin use (prescribed drug, dose, route, schedule and actual administered drug, dose, route, and time) was recorded each day by the research assistant. Use of basal and nutritional insulin and daily dose adjustments if previous hypo‐ or hyperglycemia (categorized as yes, no, or not applicable for each patient each day) were determined by the study pharmacist (J.M.T.) through retrospective review of all orders.
Up to 4 routine bedside blood glucose measurements were recorded each day: for patients eating discrete meals, these were the measurements taken before meals and at bedtime; for patients not eating or receiving continuous nutrition, these were the measurements taken closest to 6 AM, noon, 6 PM, and midnight. Additional measurements were not recorded to avoid ascertainment bias caused by follow‐up testing of abnormal glucose values. Glucose readings on the day of admission were excluded from analysis because these values are not amenable to inpatient ordering practices.
Study outcomes included overall protocol acceptance rate, insulin prescribing practices including use of basal insulin (ie, long‐acting agents such as NPH and insulin glargine), nutritional insulin (ie, scheduled regular, lispro, or aspart insulin given before each meal), daily dose adjustments under the protocol, and mean percentage of glucose readings per person greater than 180 mg/dL (hyperglycemia) and below 60 mg/dL (hypoglycemia). Comparable data from a previous cohort study of 91 GMS patients were used as baseline data for comparisons with the results of the present study.9
Other patient data collected included age, sex, weight, baseline A1C (taken at or within 6 months of admission), diabetic medications used prior to admission (none, oral agents only, or any insulin use); daily inpatient use of oral or intravenous steroids, oral diabetic medications, dextrose‐containing intravenous fluids, tube feeds, total parenteral nutrition, and general nutritional intake (nothing by mouth, clear diet, low carbohydrate diet, house diet).
Statistical Analysis
Characteristics of the study subjects and process and outcome measures were analyzed descriptively using rates, means, and standard deviations or medians with interquartile ranges as appropriate. Comparisons between the pilot study and baseline cohorts were performed using Fisher's exact test for dichotomous outcomes (eg, use of basal insulin). For rates of hyperglycemia (ie, fraction of readings > 180 mg/dL), we used binomial logistic regression, accounting for potential correlation among repeated events by individual patients with a dispersion parameter18 (note that we did not use the same analysis for rates of hypoglycemia because it was such a rare event; for analysis of hypoglycemia, the variables were dichotomized). We also analyzed outcomes by hospital day (through hospital day 5, the limit used in the baseline study) to determine daily trends during the course of hospitalization; for these analyses we used the Mantel‐Haenszel chi‐square test for dichotomous variables and binomial logistic regression with hospital day as the independent variable for rates of hyperglycemia. Two‐sided P values < .05 were considered significant. SAS version 9.1 (Cary, NC) was used for all analyses.
RESULTS
After screening all 785 admissions to the 2 medical teams during the study period, we prospectively identified 109 patients (14%) for the pilot study. Twenty patients were subsequently excluded: 7 patients who were discharged the same day they were identified, 4 who did not have a fasting blood glucose value greater than 140 mg/dL, 4 patients who had type 1 diabetes, 2 patients who were admitted with diabetic ketoacidosis, and 3 patients whose data could not be accessed because of repeated unavailability of the medical record. Characteristics of the remaining 89 study subjects are shown in Table 2 and are compared to 91 baseline subjects. The mean age of the study subjects was 68.7 years; 45% were men. Five patients (6%) did not have a previous diagnosis of diabetes, and 51% were taking insulin prior to admission; the median A1C was 6.8%.
| Characteristic | Baseline (n = 91) | Pilot (n = 89) |
|---|---|---|
| ||
| Age (years), mean (SD) | 66.0 (14.5) | 68.7 (14.7) |
| Male | 53/91 (58%) | 40/89 (45%) |
| No diagnosis of diabetes at admission | 7/91 (8%) | 5/89 (6%) |
| Preadmission diabetes regimen | ||
| None | 15/91 (16%) | 14/78 (18%) |
| Oral medications only | 32/91 (35%) | 24/78 (31%) |
| Insulin | 44/91 (48%) | 40/78 (51%) |
| A1C (IQR) | 7.0 (6.0, 8.0) | 6.8 (6.3, 7.8) |
| Hospital length of stay (days), median (IQR) | 5 (3, 7) | 5 (3, 7) |
The medical residents agreed, at least in theory, to follow the subcutaneous insulin protocol for 50 patients (56%), partially accepted it for 8 (9%), and declined for 31 (35%). Reasons for declining the protocol included fear of hypoglycemia, severity of patient's other disease states or overall poor health of patient, concern for the effects of renal insufficiency on insulin clearance, concern for the effect of steroid tapers on glucose levels, desire to titrate oral medications, and anticipation of patient's imminent discharge. Other reasons such as the glucose levels are not that bad and let's watch the glucose levels for one more day suggest that some residents did not view hyperglycemia as an acute problem requiring immediate attention.
Regarding insulin‐ordering practices (Table 3), basal insulin was prescribed for 57 patients (64%) in the pilot group compared to 45 patients (49%) in the baseline group (P = .05). Nutritional insulin was prescribed to 12 patients (13%) in the pilot group compared to no patients in the baseline group (P < .001). Oral hypoglycemic agents were prescribed less often in the pilot study than at baseline (20% vs. 38%, P = .01). The use of a standard default sliding scale from the hospital computer order set was high and was not significantly different in the pilot study compared with that at baseline (93% vs. 90%, P = .78). Twenty‐four of the 83 patients in the pilot group (29%) received sliding‐scale insulin without ever receiving basal or nutritional insulin during hospitalization compared to 45 of 91 patients in the baseline group (49%; P = .01 for comparison). Among patients started on basal insulin, 42% (24 of 57) were started after the first full hospital day. The initial basal insulin dose was appropriate according to the protocol (within 20%) in 38 of 57 patients (67%). Only 20 of 61 patients (33%) who had any hypo‐ or hyperglycemia had any change to their insulin regimen made during days 2 through 7 of their hospitalization on GMS, similar to the rate noted at baseline (36%).
| Measure | Baseline | Pilot | P value |
|---|---|---|---|
| |||
| Process | |||
| Any basal insulin during hospitalization | 45/91 (49%) | 57/89 (64%) | 0.05 |
| Any nutritional insulin during hospitalization | 0/91 (0%) | 12/89 (13%) | < 0.001 |
| Change in dose to any insulin order during hospitalization | 24/66 (36%) | 20/61 (33%) | 0.71 |
| Standard sliding scale from hospital computer order set | 75/83 (90%) | 76/82 (93%) | 0.78 |
| Any oral antihyperglycemic agents during hospitalization | 35/91 (38%) | 18/89 (20%) | 0.01 |
| Outcome | |||
| Mean percentage of glucose readings > 180 mg/dL (SD) | 33.3% (33.3%) | 31.6% (29.6%) | 0.85 |
| Any hyperglycemia (glucose > 180 mg/dL) | 66/89 (74%) | 59/78 (76%) | 0.86 |
| 1%‐20% of readings | 17/89 (19%) | 15/78 (19%) | 0.85 for trend |
| 20%‐40% | 15/89 (17%) | 15/78 (19%) | |
| 40%‐60% | 15/89 (17%) | 15/78 (19%) | |
| 60%‐80% | 7/89 (8%) | 6/78 (8%) | |
| >80% | 12/89 (13%) | 8/78 (10%) | |
| Any hypoglycemia (glucose < 60 mg/dL) | 6/89 (7%) | 10/78 (13%) | 0.20 |
Regarding glucose control (Table 3), the mean percentage of glucose readings per patient greater than 180 mg/dL was not significantly different in the pilot study compared to baseline (31.6% vs. 33.3%, P = .85). Despite implementation of the protocol and increased use of basal and nutritional insulin, 76% of patients had at least 1 routine glucose reading greater than 180 mg/dL, and 37% of patients had at least 40% of their routine glucose readings greater than 180 mg/dL, comparable to baseline (74% and 38%, respectively, P = NS for both comparisons). At least 1 hypoglycemic event (glucose reading below 60 mg/dL) occurred in 7% of patients at baseline and 13% during the pilot study (P = .20). Eleven hypoglycemic events in the pilot study were between 50 and 59 mg/dL (55%), 6 were between 40 and 49 mg/dL (30%), 3 were between 30 and 39 mg/dL (15%), and none were less than 30 mg/dL. Nine occurred before breakfast (45%), 5 before dinner (25%), 3 before lunch (15%), and 3 at bedtime (15%).
During the pilot study, the use of basal insulin did improve over the first 5 days of hospitalization (Fig. 1), in both the percentage of patients prescribed any basal insulin and the percentage of each patient's total insulin dose (basal, nutritional, and supplemental) given as basal (both P < .001 for trend). Hyperglycemia rates also improved during hospitalization (Fig. 1), decreasing from 48% on hospital day 1 to 34% on hospital day 5 (P = .004 for trend). These trends were not observed in the baseline group, with hyperglycemia rates of 37% on hospital day 1 and 34% on hospital day 5 (P = .16 for trend).
Patients for whom the resident accepted or partially accepted the protocol had higher use of basal insulin (91% vs. 13%, P < .0001), higher use of nutritional insulin (21% vs. 0%, P = .01), and more frequent dose adjustments (47% vs. 7%, P = .01) compared with patients for whom the resident declined the protocol. However, the rate of hyperglycemia was higher in patients for whom the protocol was accepted or partially accepted than in patients for whom the protocol was declined (37% vs. 20%, P = .02).
DISCUSSION
Our subcutaneous insulin protocol focused on increasing the use of basal and nutritional insulin, avoiding the use of sliding‐scale insulin by itself, and performing daily insulin adjustments in response to the hypo‐ or hyperglycemia of general medical inpatients with diabetes or hyperglycemia.
The most notable finding of our pilot study was that residents were resistant to using the protocol, both in general and in its specific recommendations. Despite receiving education about inpatient diabetes control and protocol recommendations from the team pharmacist, and despite being on a hospitalist‐run medical service, the residents accepted use of the protocol for only half the eligible patients. Patients who were started on basal insulin were often underdosed or started after the first day of hospitalization, and daily dose adjustments were not consistently made despite persistent hypo‐ or hyperglycemia. Although the use of nutritional insulin was greater compared with that in the baseline group, it was still only prescribed for 13% of patients. Use of a standard sliding scale from the hospital computer order set was common in the pilot study and similar to that in the baseline group. These results suggest significant resistance to changing the current standard of practice.
Despite this lack of adherence to the protocol, some modest improvements in processes of care were seen. Basal insulin was ordered more often during the pilot study than at baseline, especially over the course of a hospital stay. Nutritional insulin was also ordered more often during the pilot study than at baseline, but was still infrequent. Oral antihyperglycemic agents were ordered less often during the pilot study than at baseline. This demonstrates that use of the protocol may be able to improve process outcomes. However, the modest improvements in process outcomes could have simply been a result of increased awareness and education, not the protocol itself.
Regarding patient outcomes, the overall hyperglycemia rate did not improve in the pilot study relative to that at baseline. Importantly, hypoglycemia rates did not increase significantly compared with those at baseline. However, because of the small number of hypoglycemia events, the sample size may not have been sufficient to detect a true difference between groups.
The most likely reason that the protocol did not show an effect on glycemic control was that its recommendations were not adhered to. In turn, this may have been a result of incomplete education, training, and implementation measures and/or inherent problems with the protocol that made its recommendations difficult to follow. Another possibility is that the protocol itself may not have been capable of improving glucose control, even when properly used. However, we do know that resident agreement to use the protocol did lead to higher rates of recommended best practices being carried out, such as basal insulin use and daily insulin dose adjustments, and that use of the protocol was associated with improvements in glucose control over the hospital stay. A larger study with a higher degree of protocol adherence would be better able to evaluate the merits of the protocol itself, as would a randomized controlled trial using instrumental variables to measure treatment efficacy. Another possibility explanation for the lack of effect is that glucose control on admission happened to be worse in the pilot group than in the control group: rates of hyperglycemia on day 1 were 48% in the pilot group compared with 37% in the baseline group (Fig. 1). Also, the decreased use of oral agents in the pilot group, a purposeful change to decrease the risk of hypoglycemia, may have counteracted the beneficial effects of more appropriate insulin use. Finally, there were few patients with poorly controlled diabetes at baseline (18 patients with A1C 8.0 in the baseline group and 12 such patients in the pilot group), arguably those most likely to benefit.
There is a pressing need to identify protocols that can improve glucose control in the non‐ICU inpatient setting and successfully implement these protocols with a minimum of resources and effort. To date, most studies that have improved glucose control in the non‐ICU setting have relied on frequent input from diabetologists or nurse‐practitioners.14, 15
The results of this study should be viewed in light of its limitations, including its relatively small sample size (thus limiting our ability to detect possible significant differences between groups) and that it was conducted at a single institution (thus limiting its generalizability). Patients were enrolled on weekdays, so patients admitted and discharged over a weekend or on a holiday may have been missed. Also, because of the nonrandomized design of the study, we cannot exclude the possibility that the improvements noted in the pilot study were a result of the increased education provided or of increased awareness and general improvement in diabetes management over the course of the study. Finally, implementation of the protocol was somewhat labor intensive and required staff support that could be difficult to replicate in other institutions. However, most of the study staff's effort was necessary either to implement the protocol in the absence of an order set or to evaluate barriers to implementation. Widespread implementation of a protocol with an order set, education, and the use of highly reliable tools should be possible with much less effort and resources. The strengths of this study include its prospective data collection methods, which included rigorous inclusion criteria and collection of detailed clinical data.
Our study findings suggest several approaches to improve care in the future. To combat resistance to change, the American Association of Clinical Endocrinologists strongly recommends that each institution ensure that all its clinicians involved agree about general philosophies of diabetes management.19 A more expansive, hospital‐wide educational and promotional plan may increase the initial acceptance of the protocol. Interviews with residents also indicated there was unfamiliarity with diabetes management and significant concerns about the harmful affects of tight glucose control (ie, risk of hypoglycemia), especially in certain patient subgroups. These results confirmed the need for more practical individualized training and sparked the implementation of small‐group, case‐based educational sessions on inpatient diabetes management for all house officers, with a particular focus on patients with multiple comorbidities, on steroid tapers, and/or with renal failure.
The lack of nutritional insulin orders, delays in ordering basal insulin, and use of inadequate doses of insulin may be counteracted by the use of an order set, in our case built into our computer physician order entry (CPOE) system. The use of CPOE also allows reminders to be automatically sent to clinicians if eligible patients are not started on these orders. Clinical inertia (eg, failure to adjust the insulin doses of specific patients despite hyperglycemia) is more difficult to combat but may be addressed through better organization of clinical data, individualized, case‐based education, and CPOE reminders and eventually through culture change.
As a result of our pilot study, additional revisions were made to the protocol in hopes of increasing protocol adherence. For example, for patients eating discrete meals who are not taking insulin at home, the pilot protocol had suggested a starting insulin dose range for basal and nutritional insulin that required 2 separate calculations. The revised protocol was simplified to recommend a total daily insulin dose to be split evenly between basal and nutritional insulin. The daily adjustment instructions were also simplified. The pilot protocol had included a complicated table of adjustment recommendations based on bedside glucose trends. The revised protocol recommends adjusting the new daily dose by adding the total units of insulin given the previous day (including supplemental doses), making minor adjustments for hyper‐ or hypoglycemia and other clinical factors (like renal failure), and splitting this dose evenly between scheduled basal and nutritional insulin. In addition, 3 order sets were built into our computerized physician order entry system to facilitate early and appropriate insulin orders for patients with different diets (discrete meals, continuous tube feeds, and nothing by mouth); 3 different insulin sliding scales were created for patients with different degrees of insulin resistance; a diabetes management page for our electronic medication administration record is being developed to better organize clinical data; and hospital‐wide education and individualized training are ongoing.
In conclusion, the adherence to an inpatient glycemic management protocol that focused on increasing use of basal insulin and performing daily insulin adjustments was only fair. Barriers to successful implementation included clinical inertia regarding individual patients, unfamiliarity with inpatient diabetes management strategies, fear of hypoglycemia, and resistance to changing the current standard of practice. Targeted education, standard order sets, better organization of clinical data, protocol simplification, and institutional culture changes may be necessary for successful protocol implementation and improved inpatient glucose control.
- Agency for Healthcare Research and Quality. HCUPnet, Healthcare Cost and Utilization Project. 8/17/05; http://www.ahrq.gove/HCUPnet/. Accessed 7/17/06,2006.
- ,,,,,.Hyperglycemia: an independent marker of in‐hospital mortality in patients with undiagnosed diabetes.J Clin Endocrinol Metab.2002;87:978–982.
- ,,, et al.Hyperglycaemia is associated with poor outcomes in patients admitted to hospital with acute exacerbations of chronic obstructive pulmonary disease.Thorax2006;61:284–289.
- ,,,,.Stress hyperglycemia and prognosis of stroke in nondiabetic and diabetic patients: a systematic overview.Stroke.2001;32:2426–2432.
- ,,,.Hyperglycemia is associated with adverse outcomes in patients receiving total parenteral nutrition.Diabetes Care.2005;28:2367–2371.
- ,,, et al.Management of diabetes and hyperglycemia in hospitals.Diabetes Care.2004;27:553–597.
- ,,,,,.The relation between hyperglycemia and outcomes in 2,471 patients admitted to the hospital with community‐acquired pneumonia.Diabetes Care.2005;28:810–815.
- ,,.Glycemic control and sliding scale insulin use in medical inpatients with diabetes mellitus.Arch Intern Med.1997;157:545–552.
- ,,,,.Inpatient management of diabetes and hyperglycemia among general medicine patients at a large teaching hospital.J Hosp Med.2006;1:145–150.
- ,,, et al.Intensive insulin therapy in the medical ICU.N Engl J Med.2006;354:449–461.
- ,,, et al.Intensive insulin therapy in the critically ill patients.N Engl J Med.2001;345:1359–1367.
- .Prospective randomised study of intensive insulin treatment on long term survival after acute myocardial infarction in patients with diabetes mellitus.DIGAMI (Diabetes Mellitus, Insulin Glucose Infusion in Acute Myocardial Infarction) Study Group.BMJ.1997;314:1512–1515.
- ,,, et al.Intense metabolic control by means of insulin in patients with diabetes mellitus and acute myocardial infarction (DIGAMI 2): effects on mortality and morbidity.Eur Heart J.2005;26:650–661.
- American Diabetes Association.Standards of Medical Care in Diabetes ‐ 2006.Diabetes Care.2006;29:S4–S42.
- ACE/ADA Task Force on Inpatient Diabetes.American College of Endocrinology and American Diabetes Association consensus statement on inpatient diabetes and glycemic control: A call to action.Diabetes Care.2006;29:1955–1962.
- ,,,.Eliminating inpatient sliding‐scale insulin: a reeducation project with medical house staff.Diabetes Care.2005;28:1008–11.
- ,,, et al.Randomized study of basal‐bolus insulin therapy in the inpatient management of patients with type 2 diabetes (RABBIT 2 Trial).Diabetes Care.2007;30:2181–2186.
- .Extra‐binomial variation in logistic linear models.Appl Stat.1982;31:144–148.
- ,.Hospital management of diabetes.Endocrinol Metab Clin North Am.2005;34:99–116.
Diabetes mellitus is a common comorbid condition in hospitalized patients. In 2003, diabetes was listed as a diagnosis in 17.2% of hospital discharges in the United States.1 Because these diagnosis codes do not account for undiagnosed diabetes or hospital‐related hyperglycemia, the true prevalence of diabetes or hyperglycemia in hospitalized patients is likely higher and has been estimated to be as great as 38%.2 Hyperglycemia has been associated with adverse outcomes among hospitalized patients, including infectious complications, increased length of stay, and increased mortality.27 However, because hyperglycemia is not usually the primary reason patients with diabetes are hospitalized, its management is often not a focus in the inpatient setting. Sliding‐scale insulin alone continues to be commonly prescribed despite clinical evidence showing it to be ineffective in achieving glycemic control.8, 9
Recent randomized controlled trials have demonstrated that aggressive treatment of inpatient hyperglycemia improves outcomes in surgical and medical intensive care units10, 11 and in patients admitted for myocardial infarction.12, 13 Based on this clinical evidence and strong observational data linking hyperglycemia to poor patient outcomes in the non‐ICU setting,27 the American Diabetes Association (ADA) now advocates good metabolic control, defined as preprandial glucose levels of 90‐130 mg/dL and peak postprandial glucose levels < 180 mg/dL in hospitalized non‐ICU patients with hyperglycemia14 (note that these targets are less aggressive than those for ICU patients, for whom randomized controlled trials showed the benefits of reduced mortality provided by tight glucose control).11 To reach these targets, the ADA and American College of Endocrinology suggest that multidisciplinary teams develop and implement hyperglycemia management guidelines and protocols.15 Protocols should promote the use of continuous intravenous insulin or scheduled subcutaneous insulin as opposed to the use of sliding‐scale insulin alone. Subcutaneous insulin protocols should include target glucose levels; basal, nutritional, and supplemental insulin; and daily adjustments based on previous glucose levels, insulin sensitivity, nutritional intake, illness, and medications.6, 15 To date, few published protocols or algorithms for inpatient subcutaneous insulin have been shown to be effective.16, 17 It is therefore not known how best to design and implement an inpatient diabetes management protocol that is effective, efficient, and self‐perpetuating. The aims of our pilot study were to develop and implement a subcutaneous insulin protocol on a general medicine service, to identify barriers to implementation, and to determine the effect of this protocol on glycemic control.
METHODS
Setting and Participants
This prospective quality‐improvement pilot study was conducted at Brigham and Women's Hospital (BWH) from January 10, 2005, through June 23, 2005. Patients were eligible to participate if they were admitted to either of 2 General Medicine Service (GMS) teams with either a known diagnosis of type 2 diabetes or inpatient hyperglycemia (random laboratory glucose level > 180 mg/dL) and at least 1 fasting point‐of‐care glucose reading > 140 mg/dL. Patients were excluded if they had diabetic ketoacidosis, hyperosmolar hyperglycemic state, another absolute indication for intravenous insulin, or fasting glucose < 60 mg/dL on no insulin or if they were pregnant. Each GMS team consisted of a teaching attending, a junior or senior resident, 2 interns, and a clinical pharmacist. Twenty‐six physicians attended on these 2 teams during the study period, 13 of whom were hospitalists. This study was approved by the BWH Institutional Review Board; patient consent to participate in this study was deemed not necessary because of the relatively nonsensitive nature of the data (eg, glucose control, insulin orders), the noninvasive means of data collection (eg, chart review), and the steps taken by research personnel to minimize any breach in patient confidentiality.
Intervention
A multidisciplinary team composed of a diabetologist (M.L.P.), a hospitalist (J.L.S.), and a pharmacist (J.M.T.) developed a subcutaneous insulin protocol that was approved by the BWH Pharmacy and Therapeutics Diabetes Subcommittee. The protocol consisted of a set of treatment recommendations made by a pharmacist to be carried out by the medical team. The primary components are shown in Table 1 (a full description can be found in the Appendix). The main emphasis of the protocol was on discontinuing oral antihyperglycemic agents during hospitalization, initiating basal insulin in most patients, and adjusting basal insulin daily as needed.
|
| Oral agents |
| 1. Stop oral agents in most patients |
| Glucose testing |
| 2. Check bedside blood glucose before meals and at bedtime if eating, or every 6 hours if not eating |
| Insulin |
| 3. Start basal insulin Patient's home dose or NPH 0.1 units/kg before breakfast and at bedtime or insulin glargine 0.2 units/kg at bedtime (max dose 20 units) If NPO, consider half dose unless hyperglycemic |
| 4. Start nutritional insulin Discrete meals: insulin aspart 0.05‐0.1 units/kg per meal or home dose 0‐15 minutes prior to eating Continuous tube feeds: regular insulin every 6 hours or NPH every morning and at bedtime (0.1‐0.2 units/kg per day in addition to basal insulin) Hold if NPO |
| 5. Start correctional insulin Scale provided based on blood glucose and daily scheduled insulin requirements |
| Daily Adjustments |
6. Adjust scheduled insulin daily
|
| Other Considerations |
| 7. Hypoglycemia management (protocols for fruit juice, glucagons, IV dextrose, and when to call physician) |
| 8. Discharge orders (recommendations to discharge most patients on admission medication regimen, avoid sliding scale insulin, simplify dosing for patients requiring new insulin regimens, ensure adequate patient education and prompt outpatient follow‐up) |
All medical residents received general instructions regarding inpatient diabetes control by the research team's diabetologist (M.L.P.) through a 1‐hour department‐wide didactic lecture. The standards of care taught were identical to those in the protocol. In addition, the research team's hospitalist (J.L.S.) contacted each medical resident assigned to the 2 GMS teams electronically to introduce the protocol and describe the purpose and logistics of the pilot study.
A research assistant prospectively identified eligible patients each weekday by screening all patients admitted to the 2 GMS teams using the daily computerized sign‐out system used by all medical residents. Specifically, laboratory random glucose levels, inpatient medications, and medical history were reviewed to determine if each patient met eligibility criteria. Eligibility criteria were confirmed by medical record review. The pharmacist recommended to the primary team that the protocol be initiated for eligible patients. In addition, the pharmacist recommended daily adjustment of the insulin dose according to the protocol as appropriate. A chronologically organized summary of clinical data relevant to glycemic management for each patient, including bedside blood glucose measurements, general dietary intake, use of intravenous dextrose solutions, and administration of systemic steroids, oral diabetes medications, and all insulins, was provided to the team each day by the research assistant.
Measurements
The resident's acceptance of the protocol or reasons for declining it were recorded by the pharmacist on the day the protocol was recommended. Protocol acceptance was categorized as yes, no, or partial. Partial acceptance was defined as resident agreement to use the protocol, but with stated caveats or modifications. Clinical data were collected on each eligible patient for up to 7 days on GMS. Several data sources were used, including physician admission notes, the hospital's computerized clinical data system, vital‐sign sheets, medication administration records, and personal communication with nurses regarding any missing or discrepant data.
All insulin use (prescribed drug, dose, route, schedule and actual administered drug, dose, route, and time) was recorded each day by the research assistant. Use of basal and nutritional insulin and daily dose adjustments if previous hypo‐ or hyperglycemia (categorized as yes, no, or not applicable for each patient each day) were determined by the study pharmacist (J.M.T.) through retrospective review of all orders.
Up to 4 routine bedside blood glucose measurements were recorded each day: for patients eating discrete meals, these were the measurements taken before meals and at bedtime; for patients not eating or receiving continuous nutrition, these were the measurements taken closest to 6 AM, noon, 6 PM, and midnight. Additional measurements were not recorded to avoid ascertainment bias caused by follow‐up testing of abnormal glucose values. Glucose readings on the day of admission were excluded from analysis because these values are not amenable to inpatient ordering practices.
Study outcomes included overall protocol acceptance rate, insulin prescribing practices including use of basal insulin (ie, long‐acting agents such as NPH and insulin glargine), nutritional insulin (ie, scheduled regular, lispro, or aspart insulin given before each meal), daily dose adjustments under the protocol, and mean percentage of glucose readings per person greater than 180 mg/dL (hyperglycemia) and below 60 mg/dL (hypoglycemia). Comparable data from a previous cohort study of 91 GMS patients were used as baseline data for comparisons with the results of the present study.9
Other patient data collected included age, sex, weight, baseline A1C (taken at or within 6 months of admission), diabetic medications used prior to admission (none, oral agents only, or any insulin use); daily inpatient use of oral or intravenous steroids, oral diabetic medications, dextrose‐containing intravenous fluids, tube feeds, total parenteral nutrition, and general nutritional intake (nothing by mouth, clear diet, low carbohydrate diet, house diet).
Statistical Analysis
Characteristics of the study subjects and process and outcome measures were analyzed descriptively using rates, means, and standard deviations or medians with interquartile ranges as appropriate. Comparisons between the pilot study and baseline cohorts were performed using Fisher's exact test for dichotomous outcomes (eg, use of basal insulin). For rates of hyperglycemia (ie, fraction of readings > 180 mg/dL), we used binomial logistic regression, accounting for potential correlation among repeated events by individual patients with a dispersion parameter18 (note that we did not use the same analysis for rates of hypoglycemia because it was such a rare event; for analysis of hypoglycemia, the variables were dichotomized). We also analyzed outcomes by hospital day (through hospital day 5, the limit used in the baseline study) to determine daily trends during the course of hospitalization; for these analyses we used the Mantel‐Haenszel chi‐square test for dichotomous variables and binomial logistic regression with hospital day as the independent variable for rates of hyperglycemia. Two‐sided P values < .05 were considered significant. SAS version 9.1 (Cary, NC) was used for all analyses.
RESULTS
After screening all 785 admissions to the 2 medical teams during the study period, we prospectively identified 109 patients (14%) for the pilot study. Twenty patients were subsequently excluded: 7 patients who were discharged the same day they were identified, 4 who did not have a fasting blood glucose value greater than 140 mg/dL, 4 patients who had type 1 diabetes, 2 patients who were admitted with diabetic ketoacidosis, and 3 patients whose data could not be accessed because of repeated unavailability of the medical record. Characteristics of the remaining 89 study subjects are shown in Table 2 and are compared to 91 baseline subjects. The mean age of the study subjects was 68.7 years; 45% were men. Five patients (6%) did not have a previous diagnosis of diabetes, and 51% were taking insulin prior to admission; the median A1C was 6.8%.
| Characteristic | Baseline (n = 91) | Pilot (n = 89) |
|---|---|---|
| ||
| Age (years), mean (SD) | 66.0 (14.5) | 68.7 (14.7) |
| Male | 53/91 (58%) | 40/89 (45%) |
| No diagnosis of diabetes at admission | 7/91 (8%) | 5/89 (6%) |
| Preadmission diabetes regimen | ||
| None | 15/91 (16%) | 14/78 (18%) |
| Oral medications only | 32/91 (35%) | 24/78 (31%) |
| Insulin | 44/91 (48%) | 40/78 (51%) |
| A1C (IQR) | 7.0 (6.0, 8.0) | 6.8 (6.3, 7.8) |
| Hospital length of stay (days), median (IQR) | 5 (3, 7) | 5 (3, 7) |
The medical residents agreed, at least in theory, to follow the subcutaneous insulin protocol for 50 patients (56%), partially accepted it for 8 (9%), and declined for 31 (35%). Reasons for declining the protocol included fear of hypoglycemia, severity of patient's other disease states or overall poor health of patient, concern for the effects of renal insufficiency on insulin clearance, concern for the effect of steroid tapers on glucose levels, desire to titrate oral medications, and anticipation of patient's imminent discharge. Other reasons such as the glucose levels are not that bad and let's watch the glucose levels for one more day suggest that some residents did not view hyperglycemia as an acute problem requiring immediate attention.
Regarding insulin‐ordering practices (Table 3), basal insulin was prescribed for 57 patients (64%) in the pilot group compared to 45 patients (49%) in the baseline group (P = .05). Nutritional insulin was prescribed to 12 patients (13%) in the pilot group compared to no patients in the baseline group (P < .001). Oral hypoglycemic agents were prescribed less often in the pilot study than at baseline (20% vs. 38%, P = .01). The use of a standard default sliding scale from the hospital computer order set was high and was not significantly different in the pilot study compared with that at baseline (93% vs. 90%, P = .78). Twenty‐four of the 83 patients in the pilot group (29%) received sliding‐scale insulin without ever receiving basal or nutritional insulin during hospitalization compared to 45 of 91 patients in the baseline group (49%; P = .01 for comparison). Among patients started on basal insulin, 42% (24 of 57) were started after the first full hospital day. The initial basal insulin dose was appropriate according to the protocol (within 20%) in 38 of 57 patients (67%). Only 20 of 61 patients (33%) who had any hypo‐ or hyperglycemia had any change to their insulin regimen made during days 2 through 7 of their hospitalization on GMS, similar to the rate noted at baseline (36%).
| Measure | Baseline | Pilot | P value |
|---|---|---|---|
| |||
| Process | |||
| Any basal insulin during hospitalization | 45/91 (49%) | 57/89 (64%) | 0.05 |
| Any nutritional insulin during hospitalization | 0/91 (0%) | 12/89 (13%) | < 0.001 |
| Change in dose to any insulin order during hospitalization | 24/66 (36%) | 20/61 (33%) | 0.71 |
| Standard sliding scale from hospital computer order set | 75/83 (90%) | 76/82 (93%) | 0.78 |
| Any oral antihyperglycemic agents during hospitalization | 35/91 (38%) | 18/89 (20%) | 0.01 |
| Outcome | |||
| Mean percentage of glucose readings > 180 mg/dL (SD) | 33.3% (33.3%) | 31.6% (29.6%) | 0.85 |
| Any hyperglycemia (glucose > 180 mg/dL) | 66/89 (74%) | 59/78 (76%) | 0.86 |
| 1%‐20% of readings | 17/89 (19%) | 15/78 (19%) | 0.85 for trend |
| 20%‐40% | 15/89 (17%) | 15/78 (19%) | |
| 40%‐60% | 15/89 (17%) | 15/78 (19%) | |
| 60%‐80% | 7/89 (8%) | 6/78 (8%) | |
| >80% | 12/89 (13%) | 8/78 (10%) | |
| Any hypoglycemia (glucose < 60 mg/dL) | 6/89 (7%) | 10/78 (13%) | 0.20 |
Regarding glucose control (Table 3), the mean percentage of glucose readings per patient greater than 180 mg/dL was not significantly different in the pilot study compared to baseline (31.6% vs. 33.3%, P = .85). Despite implementation of the protocol and increased use of basal and nutritional insulin, 76% of patients had at least 1 routine glucose reading greater than 180 mg/dL, and 37% of patients had at least 40% of their routine glucose readings greater than 180 mg/dL, comparable to baseline (74% and 38%, respectively, P = NS for both comparisons). At least 1 hypoglycemic event (glucose reading below 60 mg/dL) occurred in 7% of patients at baseline and 13% during the pilot study (P = .20). Eleven hypoglycemic events in the pilot study were between 50 and 59 mg/dL (55%), 6 were between 40 and 49 mg/dL (30%), 3 were between 30 and 39 mg/dL (15%), and none were less than 30 mg/dL. Nine occurred before breakfast (45%), 5 before dinner (25%), 3 before lunch (15%), and 3 at bedtime (15%).
During the pilot study, the use of basal insulin did improve over the first 5 days of hospitalization (Fig. 1), in both the percentage of patients prescribed any basal insulin and the percentage of each patient's total insulin dose (basal, nutritional, and supplemental) given as basal (both P < .001 for trend). Hyperglycemia rates also improved during hospitalization (Fig. 1), decreasing from 48% on hospital day 1 to 34% on hospital day 5 (P = .004 for trend). These trends were not observed in the baseline group, with hyperglycemia rates of 37% on hospital day 1 and 34% on hospital day 5 (P = .16 for trend).

Patients for whom the resident accepted or partially accepted the protocol had higher use of basal insulin (91% vs. 13%, P < .0001), higher use of nutritional insulin (21% vs. 0%, P = .01), and more frequent dose adjustments (47% vs. 7%, P = .01) compared with patients for whom the resident declined the protocol. However, the rate of hyperglycemia was higher in patients for whom the protocol was accepted or partially accepted than in patients for whom the protocol was declined (37% vs. 20%, P = .02).
DISCUSSION
Our subcutaneous insulin protocol focused on increasing the use of basal and nutritional insulin, avoiding the use of sliding‐scale insulin by itself, and performing daily insulin adjustments in response to the hypo‐ or hyperglycemia of general medical inpatients with diabetes or hyperglycemia.
The most notable finding of our pilot study was that residents were resistant to using the protocol, both in general and in its specific recommendations. Despite receiving education about inpatient diabetes control and protocol recommendations from the team pharmacist, and despite being on a hospitalist‐run medical service, the residents accepted use of the protocol for only half the eligible patients. Patients who were started on basal insulin were often underdosed or started after the first day of hospitalization, and daily dose adjustments were not consistently made despite persistent hypo‐ or hyperglycemia. Although the use of nutritional insulin was greater compared with that in the baseline group, it was still only prescribed for 13% of patients. Use of a standard sliding scale from the hospital computer order set was common in the pilot study and similar to that in the baseline group. These results suggest significant resistance to changing the current standard of practice.
Despite this lack of adherence to the protocol, some modest improvements in processes of care were seen. Basal insulin was ordered more often during the pilot study than at baseline, especially over the course of a hospital stay. Nutritional insulin was also ordered more often during the pilot study than at baseline, but was still infrequent. Oral antihyperglycemic agents were ordered less often during the pilot study than at baseline. This demonstrates that use of the protocol may be able to improve process outcomes. However, the modest improvements in process outcomes could have simply been a result of increased awareness and education, not the protocol itself.
Regarding patient outcomes, the overall hyperglycemia rate did not improve in the pilot study relative to that at baseline. Importantly, hypoglycemia rates did not increase significantly compared with those at baseline. However, because of the small number of hypoglycemia events, the sample size may not have been sufficient to detect a true difference between groups.
The most likely reason that the protocol did not show an effect on glycemic control was that its recommendations were not adhered to. In turn, this may have been a result of incomplete education, training, and implementation measures and/or inherent problems with the protocol that made its recommendations difficult to follow. Another possibility is that the protocol itself may not have been capable of improving glucose control, even when properly used. However, we do know that resident agreement to use the protocol did lead to higher rates of recommended best practices being carried out, such as basal insulin use and daily insulin dose adjustments, and that use of the protocol was associated with improvements in glucose control over the hospital stay. A larger study with a higher degree of protocol adherence would be better able to evaluate the merits of the protocol itself, as would a randomized controlled trial using instrumental variables to measure treatment efficacy. Another possibility explanation for the lack of effect is that glucose control on admission happened to be worse in the pilot group than in the control group: rates of hyperglycemia on day 1 were 48% in the pilot group compared with 37% in the baseline group (Fig. 1). Also, the decreased use of oral agents in the pilot group, a purposeful change to decrease the risk of hypoglycemia, may have counteracted the beneficial effects of more appropriate insulin use. Finally, there were few patients with poorly controlled diabetes at baseline (18 patients with A1C 8.0 in the baseline group and 12 such patients in the pilot group), arguably those most likely to benefit.
There is a pressing need to identify protocols that can improve glucose control in the non‐ICU inpatient setting and successfully implement these protocols with a minimum of resources and effort. To date, most studies that have improved glucose control in the non‐ICU setting have relied on frequent input from diabetologists or nurse‐practitioners.14, 15
The results of this study should be viewed in light of its limitations, including its relatively small sample size (thus limiting our ability to detect possible significant differences between groups) and that it was conducted at a single institution (thus limiting its generalizability). Patients were enrolled on weekdays, so patients admitted and discharged over a weekend or on a holiday may have been missed. Also, because of the nonrandomized design of the study, we cannot exclude the possibility that the improvements noted in the pilot study were a result of the increased education provided or of increased awareness and general improvement in diabetes management over the course of the study. Finally, implementation of the protocol was somewhat labor intensive and required staff support that could be difficult to replicate in other institutions. However, most of the study staff's effort was necessary either to implement the protocol in the absence of an order set or to evaluate barriers to implementation. Widespread implementation of a protocol with an order set, education, and the use of highly reliable tools should be possible with much less effort and resources. The strengths of this study include its prospective data collection methods, which included rigorous inclusion criteria and collection of detailed clinical data.
Our study findings suggest several approaches to improve care in the future. To combat resistance to change, the American Association of Clinical Endocrinologists strongly recommends that each institution ensure that all its clinicians involved agree about general philosophies of diabetes management.19 A more expansive, hospital‐wide educational and promotional plan may increase the initial acceptance of the protocol. Interviews with residents also indicated there was unfamiliarity with diabetes management and significant concerns about the harmful affects of tight glucose control (ie, risk of hypoglycemia), especially in certain patient subgroups. These results confirmed the need for more practical individualized training and sparked the implementation of small‐group, case‐based educational sessions on inpatient diabetes management for all house officers, with a particular focus on patients with multiple comorbidities, on steroid tapers, and/or with renal failure.
The lack of nutritional insulin orders, delays in ordering basal insulin, and use of inadequate doses of insulin may be counteracted by the use of an order set, in our case built into our computer physician order entry (CPOE) system. The use of CPOE also allows reminders to be automatically sent to clinicians if eligible patients are not started on these orders. Clinical inertia (eg, failure to adjust the insulin doses of specific patients despite hyperglycemia) is more difficult to combat but may be addressed through better organization of clinical data, individualized, case‐based education, and CPOE reminders and eventually through culture change.
As a result of our pilot study, additional revisions were made to the protocol in hopes of increasing protocol adherence. For example, for patients eating discrete meals who are not taking insulin at home, the pilot protocol had suggested a starting insulin dose range for basal and nutritional insulin that required 2 separate calculations. The revised protocol was simplified to recommend a total daily insulin dose to be split evenly between basal and nutritional insulin. The daily adjustment instructions were also simplified. The pilot protocol had included a complicated table of adjustment recommendations based on bedside glucose trends. The revised protocol recommends adjusting the new daily dose by adding the total units of insulin given the previous day (including supplemental doses), making minor adjustments for hyper‐ or hypoglycemia and other clinical factors (like renal failure), and splitting this dose evenly between scheduled basal and nutritional insulin. In addition, 3 order sets were built into our computerized physician order entry system to facilitate early and appropriate insulin orders for patients with different diets (discrete meals, continuous tube feeds, and nothing by mouth); 3 different insulin sliding scales were created for patients with different degrees of insulin resistance; a diabetes management page for our electronic medication administration record is being developed to better organize clinical data; and hospital‐wide education and individualized training are ongoing.
In conclusion, the adherence to an inpatient glycemic management protocol that focused on increasing use of basal insulin and performing daily insulin adjustments was only fair. Barriers to successful implementation included clinical inertia regarding individual patients, unfamiliarity with inpatient diabetes management strategies, fear of hypoglycemia, and resistance to changing the current standard of practice. Targeted education, standard order sets, better organization of clinical data, protocol simplification, and institutional culture changes may be necessary for successful protocol implementation and improved inpatient glucose control.
Diabetes mellitus is a common comorbid condition in hospitalized patients. In 2003, diabetes was listed as a diagnosis in 17.2% of hospital discharges in the United States.1 Because these diagnosis codes do not account for undiagnosed diabetes or hospital‐related hyperglycemia, the true prevalence of diabetes or hyperglycemia in hospitalized patients is likely higher and has been estimated to be as great as 38%.2 Hyperglycemia has been associated with adverse outcomes among hospitalized patients, including infectious complications, increased length of stay, and increased mortality.27 However, because hyperglycemia is not usually the primary reason patients with diabetes are hospitalized, its management is often not a focus in the inpatient setting. Sliding‐scale insulin alone continues to be commonly prescribed despite clinical evidence showing it to be ineffective in achieving glycemic control.8, 9
Recent randomized controlled trials have demonstrated that aggressive treatment of inpatient hyperglycemia improves outcomes in surgical and medical intensive care units10, 11 and in patients admitted for myocardial infarction.12, 13 Based on this clinical evidence and strong observational data linking hyperglycemia to poor patient outcomes in the non‐ICU setting,27 the American Diabetes Association (ADA) now advocates good metabolic control, defined as preprandial glucose levels of 90‐130 mg/dL and peak postprandial glucose levels < 180 mg/dL in hospitalized non‐ICU patients with hyperglycemia14 (note that these targets are less aggressive than those for ICU patients, for whom randomized controlled trials showed the benefits of reduced mortality provided by tight glucose control).11 To reach these targets, the ADA and American College of Endocrinology suggest that multidisciplinary teams develop and implement hyperglycemia management guidelines and protocols.15 Protocols should promote the use of continuous intravenous insulin or scheduled subcutaneous insulin as opposed to the use of sliding‐scale insulin alone. Subcutaneous insulin protocols should include target glucose levels; basal, nutritional, and supplemental insulin; and daily adjustments based on previous glucose levels, insulin sensitivity, nutritional intake, illness, and medications.6, 15 To date, few published protocols or algorithms for inpatient subcutaneous insulin have been shown to be effective.16, 17 It is therefore not known how best to design and implement an inpatient diabetes management protocol that is effective, efficient, and self‐perpetuating. The aims of our pilot study were to develop and implement a subcutaneous insulin protocol on a general medicine service, to identify barriers to implementation, and to determine the effect of this protocol on glycemic control.
METHODS
Setting and Participants
This prospective quality‐improvement pilot study was conducted at Brigham and Women's Hospital (BWH) from January 10, 2005, through June 23, 2005. Patients were eligible to participate if they were admitted to either of 2 General Medicine Service (GMS) teams with either a known diagnosis of type 2 diabetes or inpatient hyperglycemia (random laboratory glucose level > 180 mg/dL) and at least 1 fasting point‐of‐care glucose reading > 140 mg/dL. Patients were excluded if they had diabetic ketoacidosis, hyperosmolar hyperglycemic state, another absolute indication for intravenous insulin, or fasting glucose < 60 mg/dL on no insulin or if they were pregnant. Each GMS team consisted of a teaching attending, a junior or senior resident, 2 interns, and a clinical pharmacist. Twenty‐six physicians attended on these 2 teams during the study period, 13 of whom were hospitalists. This study was approved by the BWH Institutional Review Board; patient consent to participate in this study was deemed not necessary because of the relatively nonsensitive nature of the data (eg, glucose control, insulin orders), the noninvasive means of data collection (eg, chart review), and the steps taken by research personnel to minimize any breach in patient confidentiality.
Intervention
A multidisciplinary team composed of a diabetologist (M.L.P.), a hospitalist (J.L.S.), and a pharmacist (J.M.T.) developed a subcutaneous insulin protocol that was approved by the BWH Pharmacy and Therapeutics Diabetes Subcommittee. The protocol consisted of a set of treatment recommendations made by a pharmacist to be carried out by the medical team. The primary components are shown in Table 1 (a full description can be found in the Appendix). The main emphasis of the protocol was on discontinuing oral antihyperglycemic agents during hospitalization, initiating basal insulin in most patients, and adjusting basal insulin daily as needed.
|
| Oral agents |
| 1. Stop oral agents in most patients |
| Glucose testing |
| 2. Check bedside blood glucose before meals and at bedtime if eating, or every 6 hours if not eating |
| Insulin |
| 3. Start basal insulin Patient's home dose or NPH 0.1 units/kg before breakfast and at bedtime or insulin glargine 0.2 units/kg at bedtime (max dose 20 units) If NPO, consider half dose unless hyperglycemic |
| 4. Start nutritional insulin Discrete meals: insulin aspart 0.05‐0.1 units/kg per meal or home dose 0‐15 minutes prior to eating Continuous tube feeds: regular insulin every 6 hours or NPH every morning and at bedtime (0.1‐0.2 units/kg per day in addition to basal insulin) Hold if NPO |
| 5. Start correctional insulin Scale provided based on blood glucose and daily scheduled insulin requirements |
| Daily Adjustments |
6. Adjust scheduled insulin daily
|
| Other Considerations |
| 7. Hypoglycemia management (protocols for fruit juice, glucagons, IV dextrose, and when to call physician) |
| 8. Discharge orders (recommendations to discharge most patients on admission medication regimen, avoid sliding scale insulin, simplify dosing for patients requiring new insulin regimens, ensure adequate patient education and prompt outpatient follow‐up) |
All medical residents received general instructions regarding inpatient diabetes control by the research team's diabetologist (M.L.P.) through a 1‐hour department‐wide didactic lecture. The standards of care taught were identical to those in the protocol. In addition, the research team's hospitalist (J.L.S.) contacted each medical resident assigned to the 2 GMS teams electronically to introduce the protocol and describe the purpose and logistics of the pilot study.
A research assistant prospectively identified eligible patients each weekday by screening all patients admitted to the 2 GMS teams using the daily computerized sign‐out system used by all medical residents. Specifically, laboratory random glucose levels, inpatient medications, and medical history were reviewed to determine if each patient met eligibility criteria. Eligibility criteria were confirmed by medical record review. The pharmacist recommended to the primary team that the protocol be initiated for eligible patients. In addition, the pharmacist recommended daily adjustment of the insulin dose according to the protocol as appropriate. A chronologically organized summary of clinical data relevant to glycemic management for each patient, including bedside blood glucose measurements, general dietary intake, use of intravenous dextrose solutions, and administration of systemic steroids, oral diabetes medications, and all insulins, was provided to the team each day by the research assistant.
Measurements
The resident's acceptance of the protocol or reasons for declining it were recorded by the pharmacist on the day the protocol was recommended. Protocol acceptance was categorized as yes, no, or partial. Partial acceptance was defined as resident agreement to use the protocol, but with stated caveats or modifications. Clinical data were collected on each eligible patient for up to 7 days on GMS. Several data sources were used, including physician admission notes, the hospital's computerized clinical data system, vital‐sign sheets, medication administration records, and personal communication with nurses regarding any missing or discrepant data.
All insulin use (prescribed drug, dose, route, schedule and actual administered drug, dose, route, and time) was recorded each day by the research assistant. Use of basal and nutritional insulin and daily dose adjustments if previous hypo‐ or hyperglycemia (categorized as yes, no, or not applicable for each patient each day) were determined by the study pharmacist (J.M.T.) through retrospective review of all orders.
Up to 4 routine bedside blood glucose measurements were recorded each day: for patients eating discrete meals, these were the measurements taken before meals and at bedtime; for patients not eating or receiving continuous nutrition, these were the measurements taken closest to 6 AM, noon, 6 PM, and midnight. Additional measurements were not recorded to avoid ascertainment bias caused by follow‐up testing of abnormal glucose values. Glucose readings on the day of admission were excluded from analysis because these values are not amenable to inpatient ordering practices.
Study outcomes included overall protocol acceptance rate, insulin prescribing practices including use of basal insulin (ie, long‐acting agents such as NPH and insulin glargine), nutritional insulin (ie, scheduled regular, lispro, or aspart insulin given before each meal), daily dose adjustments under the protocol, and mean percentage of glucose readings per person greater than 180 mg/dL (hyperglycemia) and below 60 mg/dL (hypoglycemia). Comparable data from a previous cohort study of 91 GMS patients were used as baseline data for comparisons with the results of the present study.9
Other patient data collected included age, sex, weight, baseline A1C (taken at or within 6 months of admission), diabetic medications used prior to admission (none, oral agents only, or any insulin use); daily inpatient use of oral or intravenous steroids, oral diabetic medications, dextrose‐containing intravenous fluids, tube feeds, total parenteral nutrition, and general nutritional intake (nothing by mouth, clear diet, low carbohydrate diet, house diet).
Statistical Analysis
Characteristics of the study subjects and process and outcome measures were analyzed descriptively using rates, means, and standard deviations or medians with interquartile ranges as appropriate. Comparisons between the pilot study and baseline cohorts were performed using Fisher's exact test for dichotomous outcomes (eg, use of basal insulin). For rates of hyperglycemia (ie, fraction of readings > 180 mg/dL), we used binomial logistic regression, accounting for potential correlation among repeated events by individual patients with a dispersion parameter18 (note that we did not use the same analysis for rates of hypoglycemia because it was such a rare event; for analysis of hypoglycemia, the variables were dichotomized). We also analyzed outcomes by hospital day (through hospital day 5, the limit used in the baseline study) to determine daily trends during the course of hospitalization; for these analyses we used the Mantel‐Haenszel chi‐square test for dichotomous variables and binomial logistic regression with hospital day as the independent variable for rates of hyperglycemia. Two‐sided P values < .05 were considered significant. SAS version 9.1 (Cary, NC) was used for all analyses.
RESULTS
After screening all 785 admissions to the 2 medical teams during the study period, we prospectively identified 109 patients (14%) for the pilot study. Twenty patients were subsequently excluded: 7 patients who were discharged the same day they were identified, 4 who did not have a fasting blood glucose value greater than 140 mg/dL, 4 patients who had type 1 diabetes, 2 patients who were admitted with diabetic ketoacidosis, and 3 patients whose data could not be accessed because of repeated unavailability of the medical record. Characteristics of the remaining 89 study subjects are shown in Table 2 and are compared to 91 baseline subjects. The mean age of the study subjects was 68.7 years; 45% were men. Five patients (6%) did not have a previous diagnosis of diabetes, and 51% were taking insulin prior to admission; the median A1C was 6.8%.
| Characteristic | Baseline (n = 91) | Pilot (n = 89) |
|---|---|---|
| ||
| Age (years), mean (SD) | 66.0 (14.5) | 68.7 (14.7) |
| Male | 53/91 (58%) | 40/89 (45%) |
| No diagnosis of diabetes at admission | 7/91 (8%) | 5/89 (6%) |
| Preadmission diabetes regimen | ||
| None | 15/91 (16%) | 14/78 (18%) |
| Oral medications only | 32/91 (35%) | 24/78 (31%) |
| Insulin | 44/91 (48%) | 40/78 (51%) |
| A1C (IQR) | 7.0 (6.0, 8.0) | 6.8 (6.3, 7.8) |
| Hospital length of stay (days), median (IQR) | 5 (3, 7) | 5 (3, 7) |
The medical residents agreed, at least in theory, to follow the subcutaneous insulin protocol for 50 patients (56%), partially accepted it for 8 (9%), and declined for 31 (35%). Reasons for declining the protocol included fear of hypoglycemia, severity of patient's other disease states or overall poor health of patient, concern for the effects of renal insufficiency on insulin clearance, concern for the effect of steroid tapers on glucose levels, desire to titrate oral medications, and anticipation of patient's imminent discharge. Other reasons such as the glucose levels are not that bad and let's watch the glucose levels for one more day suggest that some residents did not view hyperglycemia as an acute problem requiring immediate attention.
Regarding insulin‐ordering practices (Table 3), basal insulin was prescribed for 57 patients (64%) in the pilot group compared to 45 patients (49%) in the baseline group (P = .05). Nutritional insulin was prescribed to 12 patients (13%) in the pilot group compared to no patients in the baseline group (P < .001). Oral hypoglycemic agents were prescribed less often in the pilot study than at baseline (20% vs. 38%, P = .01). The use of a standard default sliding scale from the hospital computer order set was high and was not significantly different in the pilot study compared with that at baseline (93% vs. 90%, P = .78). Twenty‐four of the 83 patients in the pilot group (29%) received sliding‐scale insulin without ever receiving basal or nutritional insulin during hospitalization compared to 45 of 91 patients in the baseline group (49%; P = .01 for comparison). Among patients started on basal insulin, 42% (24 of 57) were started after the first full hospital day. The initial basal insulin dose was appropriate according to the protocol (within 20%) in 38 of 57 patients (67%). Only 20 of 61 patients (33%) who had any hypo‐ or hyperglycemia had any change to their insulin regimen made during days 2 through 7 of their hospitalization on GMS, similar to the rate noted at baseline (36%).
| Measure | Baseline | Pilot | P value |
|---|---|---|---|
| |||
| Process | |||
| Any basal insulin during hospitalization | 45/91 (49%) | 57/89 (64%) | 0.05 |
| Any nutritional insulin during hospitalization | 0/91 (0%) | 12/89 (13%) | < 0.001 |
| Change in dose to any insulin order during hospitalization | 24/66 (36%) | 20/61 (33%) | 0.71 |
| Standard sliding scale from hospital computer order set | 75/83 (90%) | 76/82 (93%) | 0.78 |
| Any oral antihyperglycemic agents during hospitalization | 35/91 (38%) | 18/89 (20%) | 0.01 |
| Outcome | |||
| Mean percentage of glucose readings > 180 mg/dL (SD) | 33.3% (33.3%) | 31.6% (29.6%) | 0.85 |
| Any hyperglycemia (glucose > 180 mg/dL) | 66/89 (74%) | 59/78 (76%) | 0.86 |
| 1%‐20% of readings | 17/89 (19%) | 15/78 (19%) | 0.85 for trend |
| 20%‐40% | 15/89 (17%) | 15/78 (19%) | |
| 40%‐60% | 15/89 (17%) | 15/78 (19%) | |
| 60%‐80% | 7/89 (8%) | 6/78 (8%) | |
| >80% | 12/89 (13%) | 8/78 (10%) | |
| Any hypoglycemia (glucose < 60 mg/dL) | 6/89 (7%) | 10/78 (13%) | 0.20 |
Regarding glucose control (Table 3), the mean percentage of glucose readings per patient greater than 180 mg/dL was not significantly different in the pilot study compared to baseline (31.6% vs. 33.3%, P = .85). Despite implementation of the protocol and increased use of basal and nutritional insulin, 76% of patients had at least 1 routine glucose reading greater than 180 mg/dL, and 37% of patients had at least 40% of their routine glucose readings greater than 180 mg/dL, comparable to baseline (74% and 38%, respectively, P = NS for both comparisons). At least 1 hypoglycemic event (glucose reading below 60 mg/dL) occurred in 7% of patients at baseline and 13% during the pilot study (P = .20). Eleven hypoglycemic events in the pilot study were between 50 and 59 mg/dL (55%), 6 were between 40 and 49 mg/dL (30%), 3 were between 30 and 39 mg/dL (15%), and none were less than 30 mg/dL. Nine occurred before breakfast (45%), 5 before dinner (25%), 3 before lunch (15%), and 3 at bedtime (15%).
During the pilot study, the use of basal insulin did improve over the first 5 days of hospitalization (Fig. 1), in both the percentage of patients prescribed any basal insulin and the percentage of each patient's total insulin dose (basal, nutritional, and supplemental) given as basal (both P < .001 for trend). Hyperglycemia rates also improved during hospitalization (Fig. 1), decreasing from 48% on hospital day 1 to 34% on hospital day 5 (P = .004 for trend). These trends were not observed in the baseline group, with hyperglycemia rates of 37% on hospital day 1 and 34% on hospital day 5 (P = .16 for trend).

Patients for whom the resident accepted or partially accepted the protocol had higher use of basal insulin (91% vs. 13%, P < .0001), higher use of nutritional insulin (21% vs. 0%, P = .01), and more frequent dose adjustments (47% vs. 7%, P = .01) compared with patients for whom the resident declined the protocol. However, the rate of hyperglycemia was higher in patients for whom the protocol was accepted or partially accepted than in patients for whom the protocol was declined (37% vs. 20%, P = .02).
DISCUSSION
Our subcutaneous insulin protocol focused on increasing the use of basal and nutritional insulin, avoiding the use of sliding‐scale insulin by itself, and performing daily insulin adjustments in response to the hypo‐ or hyperglycemia of general medical inpatients with diabetes or hyperglycemia.
The most notable finding of our pilot study was that residents were resistant to using the protocol, both in general and in its specific recommendations. Despite receiving education about inpatient diabetes control and protocol recommendations from the team pharmacist, and despite being on a hospitalist‐run medical service, the residents accepted use of the protocol for only half the eligible patients. Patients who were started on basal insulin were often underdosed or started after the first day of hospitalization, and daily dose adjustments were not consistently made despite persistent hypo‐ or hyperglycemia. Although the use of nutritional insulin was greater compared with that in the baseline group, it was still only prescribed for 13% of patients. Use of a standard sliding scale from the hospital computer order set was common in the pilot study and similar to that in the baseline group. These results suggest significant resistance to changing the current standard of practice.
Despite this lack of adherence to the protocol, some modest improvements in processes of care were seen. Basal insulin was ordered more often during the pilot study than at baseline, especially over the course of a hospital stay. Nutritional insulin was also ordered more often during the pilot study than at baseline, but was still infrequent. Oral antihyperglycemic agents were ordered less often during the pilot study than at baseline. This demonstrates that use of the protocol may be able to improve process outcomes. However, the modest improvements in process outcomes could have simply been a result of increased awareness and education, not the protocol itself.
Regarding patient outcomes, the overall hyperglycemia rate did not improve in the pilot study relative to that at baseline. Importantly, hypoglycemia rates did not increase significantly compared with those at baseline. However, because of the small number of hypoglycemia events, the sample size may not have been sufficient to detect a true difference between groups.
The most likely reason that the protocol did not show an effect on glycemic control was that its recommendations were not adhered to. In turn, this may have been a result of incomplete education, training, and implementation measures and/or inherent problems with the protocol that made its recommendations difficult to follow. Another possibility is that the protocol itself may not have been capable of improving glucose control, even when properly used. However, we do know that resident agreement to use the protocol did lead to higher rates of recommended best practices being carried out, such as basal insulin use and daily insulin dose adjustments, and that use of the protocol was associated with improvements in glucose control over the hospital stay. A larger study with a higher degree of protocol adherence would be better able to evaluate the merits of the protocol itself, as would a randomized controlled trial using instrumental variables to measure treatment efficacy. Another possibility explanation for the lack of effect is that glucose control on admission happened to be worse in the pilot group than in the control group: rates of hyperglycemia on day 1 were 48% in the pilot group compared with 37% in the baseline group (Fig. 1). Also, the decreased use of oral agents in the pilot group, a purposeful change to decrease the risk of hypoglycemia, may have counteracted the beneficial effects of more appropriate insulin use. Finally, there were few patients with poorly controlled diabetes at baseline (18 patients with A1C 8.0 in the baseline group and 12 such patients in the pilot group), arguably those most likely to benefit.
There is a pressing need to identify protocols that can improve glucose control in the non‐ICU inpatient setting and successfully implement these protocols with a minimum of resources and effort. To date, most studies that have improved glucose control in the non‐ICU setting have relied on frequent input from diabetologists or nurse‐practitioners.14, 15
The results of this study should be viewed in light of its limitations, including its relatively small sample size (thus limiting our ability to detect possible significant differences between groups) and that it was conducted at a single institution (thus limiting its generalizability). Patients were enrolled on weekdays, so patients admitted and discharged over a weekend or on a holiday may have been missed. Also, because of the nonrandomized design of the study, we cannot exclude the possibility that the improvements noted in the pilot study were a result of the increased education provided or of increased awareness and general improvement in diabetes management over the course of the study. Finally, implementation of the protocol was somewhat labor intensive and required staff support that could be difficult to replicate in other institutions. However, most of the study staff's effort was necessary either to implement the protocol in the absence of an order set or to evaluate barriers to implementation. Widespread implementation of a protocol with an order set, education, and the use of highly reliable tools should be possible with much less effort and resources. The strengths of this study include its prospective data collection methods, which included rigorous inclusion criteria and collection of detailed clinical data.
Our study findings suggest several approaches to improve care in the future. To combat resistance to change, the American Association of Clinical Endocrinologists strongly recommends that each institution ensure that all its clinicians involved agree about general philosophies of diabetes management.19 A more expansive, hospital‐wide educational and promotional plan may increase the initial acceptance of the protocol. Interviews with residents also indicated there was unfamiliarity with diabetes management and significant concerns about the harmful affects of tight glucose control (ie, risk of hypoglycemia), especially in certain patient subgroups. These results confirmed the need for more practical individualized training and sparked the implementation of small‐group, case‐based educational sessions on inpatient diabetes management for all house officers, with a particular focus on patients with multiple comorbidities, on steroid tapers, and/or with renal failure.
The lack of nutritional insulin orders, delays in ordering basal insulin, and use of inadequate doses of insulin may be counteracted by the use of an order set, in our case built into our computer physician order entry (CPOE) system. The use of CPOE also allows reminders to be automatically sent to clinicians if eligible patients are not started on these orders. Clinical inertia (eg, failure to adjust the insulin doses of specific patients despite hyperglycemia) is more difficult to combat but may be addressed through better organization of clinical data, individualized, case‐based education, and CPOE reminders and eventually through culture change.
As a result of our pilot study, additional revisions were made to the protocol in hopes of increasing protocol adherence. For example, for patients eating discrete meals who are not taking insulin at home, the pilot protocol had suggested a starting insulin dose range for basal and nutritional insulin that required 2 separate calculations. The revised protocol was simplified to recommend a total daily insulin dose to be split evenly between basal and nutritional insulin. The daily adjustment instructions were also simplified. The pilot protocol had included a complicated table of adjustment recommendations based on bedside glucose trends. The revised protocol recommends adjusting the new daily dose by adding the total units of insulin given the previous day (including supplemental doses), making minor adjustments for hyper‐ or hypoglycemia and other clinical factors (like renal failure), and splitting this dose evenly between scheduled basal and nutritional insulin. In addition, 3 order sets were built into our computerized physician order entry system to facilitate early and appropriate insulin orders for patients with different diets (discrete meals, continuous tube feeds, and nothing by mouth); 3 different insulin sliding scales were created for patients with different degrees of insulin resistance; a diabetes management page for our electronic medication administration record is being developed to better organize clinical data; and hospital‐wide education and individualized training are ongoing.
In conclusion, the adherence to an inpatient glycemic management protocol that focused on increasing use of basal insulin and performing daily insulin adjustments was only fair. Barriers to successful implementation included clinical inertia regarding individual patients, unfamiliarity with inpatient diabetes management strategies, fear of hypoglycemia, and resistance to changing the current standard of practice. Targeted education, standard order sets, better organization of clinical data, protocol simplification, and institutional culture changes may be necessary for successful protocol implementation and improved inpatient glucose control.
- Agency for Healthcare Research and Quality. HCUPnet, Healthcare Cost and Utilization Project. 8/17/05; http://www.ahrq.gove/HCUPnet/. Accessed 7/17/06,2006.
- ,,,,,.Hyperglycemia: an independent marker of in‐hospital mortality in patients with undiagnosed diabetes.J Clin Endocrinol Metab.2002;87:978–982.
- ,,, et al.Hyperglycaemia is associated with poor outcomes in patients admitted to hospital with acute exacerbations of chronic obstructive pulmonary disease.Thorax2006;61:284–289.
- ,,,,.Stress hyperglycemia and prognosis of stroke in nondiabetic and diabetic patients: a systematic overview.Stroke.2001;32:2426–2432.
- ,,,.Hyperglycemia is associated with adverse outcomes in patients receiving total parenteral nutrition.Diabetes Care.2005;28:2367–2371.
- ,,, et al.Management of diabetes and hyperglycemia in hospitals.Diabetes Care.2004;27:553–597.
- ,,,,,.The relation between hyperglycemia and outcomes in 2,471 patients admitted to the hospital with community‐acquired pneumonia.Diabetes Care.2005;28:810–815.
- ,,.Glycemic control and sliding scale insulin use in medical inpatients with diabetes mellitus.Arch Intern Med.1997;157:545–552.
- ,,,,.Inpatient management of diabetes and hyperglycemia among general medicine patients at a large teaching hospital.J Hosp Med.2006;1:145–150.
- ,,, et al.Intensive insulin therapy in the medical ICU.N Engl J Med.2006;354:449–461.
- ,,, et al.Intensive insulin therapy in the critically ill patients.N Engl J Med.2001;345:1359–1367.
- .Prospective randomised study of intensive insulin treatment on long term survival after acute myocardial infarction in patients with diabetes mellitus.DIGAMI (Diabetes Mellitus, Insulin Glucose Infusion in Acute Myocardial Infarction) Study Group.BMJ.1997;314:1512–1515.
- ,,, et al.Intense metabolic control by means of insulin in patients with diabetes mellitus and acute myocardial infarction (DIGAMI 2): effects on mortality and morbidity.Eur Heart J.2005;26:650–661.
- American Diabetes Association.Standards of Medical Care in Diabetes ‐ 2006.Diabetes Care.2006;29:S4–S42.
- ACE/ADA Task Force on Inpatient Diabetes.American College of Endocrinology and American Diabetes Association consensus statement on inpatient diabetes and glycemic control: A call to action.Diabetes Care.2006;29:1955–1962.
- ,,,.Eliminating inpatient sliding‐scale insulin: a reeducation project with medical house staff.Diabetes Care.2005;28:1008–11.
- ,,, et al.Randomized study of basal‐bolus insulin therapy in the inpatient management of patients with type 2 diabetes (RABBIT 2 Trial).Diabetes Care.2007;30:2181–2186.
- .Extra‐binomial variation in logistic linear models.Appl Stat.1982;31:144–148.
- ,.Hospital management of diabetes.Endocrinol Metab Clin North Am.2005;34:99–116.
- Agency for Healthcare Research and Quality. HCUPnet, Healthcare Cost and Utilization Project. 8/17/05; http://www.ahrq.gove/HCUPnet/. Accessed 7/17/06,2006.
- ,,,,,.Hyperglycemia: an independent marker of in‐hospital mortality in patients with undiagnosed diabetes.J Clin Endocrinol Metab.2002;87:978–982.
- ,,, et al.Hyperglycaemia is associated with poor outcomes in patients admitted to hospital with acute exacerbations of chronic obstructive pulmonary disease.Thorax2006;61:284–289.
- ,,,,.Stress hyperglycemia and prognosis of stroke in nondiabetic and diabetic patients: a systematic overview.Stroke.2001;32:2426–2432.
- ,,,.Hyperglycemia is associated with adverse outcomes in patients receiving total parenteral nutrition.Diabetes Care.2005;28:2367–2371.
- ,,, et al.Management of diabetes and hyperglycemia in hospitals.Diabetes Care.2004;27:553–597.
- ,,,,,.The relation between hyperglycemia and outcomes in 2,471 patients admitted to the hospital with community‐acquired pneumonia.Diabetes Care.2005;28:810–815.
- ,,.Glycemic control and sliding scale insulin use in medical inpatients with diabetes mellitus.Arch Intern Med.1997;157:545–552.
- ,,,,.Inpatient management of diabetes and hyperglycemia among general medicine patients at a large teaching hospital.J Hosp Med.2006;1:145–150.
- ,,, et al.Intensive insulin therapy in the medical ICU.N Engl J Med.2006;354:449–461.
- ,,, et al.Intensive insulin therapy in the critically ill patients.N Engl J Med.2001;345:1359–1367.
- .Prospective randomised study of intensive insulin treatment on long term survival after acute myocardial infarction in patients with diabetes mellitus.DIGAMI (Diabetes Mellitus, Insulin Glucose Infusion in Acute Myocardial Infarction) Study Group.BMJ.1997;314:1512–1515.
- ,,, et al.Intense metabolic control by means of insulin in patients with diabetes mellitus and acute myocardial infarction (DIGAMI 2): effects on mortality and morbidity.Eur Heart J.2005;26:650–661.
- American Diabetes Association.Standards of Medical Care in Diabetes ‐ 2006.Diabetes Care.2006;29:S4–S42.
- ACE/ADA Task Force on Inpatient Diabetes.American College of Endocrinology and American Diabetes Association consensus statement on inpatient diabetes and glycemic control: A call to action.Diabetes Care.2006;29:1955–1962.
- ,,,.Eliminating inpatient sliding‐scale insulin: a reeducation project with medical house staff.Diabetes Care.2005;28:1008–11.
- ,,, et al.Randomized study of basal‐bolus insulin therapy in the inpatient management of patients with type 2 diabetes (RABBIT 2 Trial).Diabetes Care.2007;30:2181–2186.
- .Extra‐binomial variation in logistic linear models.Appl Stat.1982;31:144–148.
- ,.Hospital management of diabetes.Endocrinol Metab Clin North Am.2005;34:99–116.
Transitions of Care at Hospital Discharge
As the counterpart to hospital admission, hospital discharge is a necessary process experienced by each living patient. For all patients except those being transferred to a continuing care facility, discharge is a period of transition from hospital to home that involves a transfer in responsibility from the inpatient provider or hospitalist to the patient and primary care physician (PCP).1 Prescription medications are commonly altered at this transition point, with patients asked to discontinue some medications, switch to a new dosage schedule of others, or begin new treatments.2, 3 Self‐care responsibilities also increase in number and importance, presenting new challenges for patients and their families as they return home.4 Under these circumstances, ineffective planning and coordination of care can undermine patient satisfaction, facilitate adverse events, and contribute to more frequent hospital readmissions.58
Following hospital discharge nearly half (49%) of hospitalized patients experience at least 1 medical error in medication continuity, diagnostic workup, or test follow‐up.7 It has been reported that 19%23% of patients suffer an adverse event, most frequently an adverse drug event (ADE).911 Half of ADEs are considered preventable or ameliorable (ie, their severity or duration could have been decreased). Most errors and adverse events in this setting result from a breakdown in communication between the hospital team and the patient or primary care physician.10
To promote more effective care transitions, The Joint Commission now requires accredited facilities to accurately and completely reconcile medications across the continuum of care.12 The Society of Hospital Medicine recently published recommendations for the discharge of elderly patients.13 The joint Society of Hospital MedicineSociety of General Internal Medicine Continuity of Care Task Force also recently published a systematic review with recommendations for improving the handoff of patient information at discharge.14 Apart from these reports, however, it is uncommon to find evidence‐based recommendations for hospital discharge applicable to a broad range of patients.15 This review highlights several important challenges for physicians who seek to provide high‐quality care during hospital discharge and the subsequent period of transition. Based on the best available evidence, recommendations are also provided for how to improve communication and facilitate the care transition for adult inpatients returning home.
INPATIENTOUTPATIENT PHYSICIAN DISCONTINUITY
Traditionally, primary care physicians have admitted their own patients, provided hospital care (in addition to seeing outpatients during the day), and followed patients after discharge. Under this model, continuity of care has been preserved; however, this method of care has faltered under the weight of inpatients and outpatients with more severe illnesses, rapid technological advancements, managed care pressuring outpatient physicians to see more patients, and a thrust toward reduced hospital costs and length of stay.16 Increases in the efficiency and quality of hospital care have accompanied a new reliance on the field of hospital medicine, while allowing PCPs to focus on outpatient care.1719 With more than 14,000 hospitalists currently practicing in the United States and 25,000 anticipated to be practicing by 2010, transfer of care from hospital‐based providers to PCPs has become increasingly common at discharge.20
Patient discharge summaries are the most common means of communication between inpatient and outpatient providers. However, numerous studies have shown that discharge summaries often fail to provide important administrative and medical information, such as the primary diagnosis, results of abnormal diagnostics, details about the hospital course, follow‐up plans, whether laboratory test results are pending, and patient or family counseling.14 Summaries also may not arrive in a timely manner and sometimes may not reach the PCP at all.2123
At the time patients first follow up with their PCPs after hospitalization, discharge summaries have not yet arrived about 75% of the time,22, 24, 25 restricting the PCPs' ability to provide adequate follow‐up care in 24% of hospital follow‐up visits, according to one study.26 In another investigation, PCPs reported being unaware of 62% of the pending test results that returned after discharge, of which 37% were considered actionable.27
Improving Physician Information Transfer and Continuity
To improve information transfer from hospitalist to PCP, attention must be paid to the content, format, and timely delivery of discharge information (Table 1).14 Surveys of primary care physicians suggest the following information should be included in discharge summaries: diagnoses, abnormal physical findings, important test results, discharge medications, follow‐up arrangements made and appointments that still need to be made, counseling provided to the patient and family, and tests still pending at discharge.24, 2833 These domains are consistent with Joint Commission guidelines for discharge summaries,34 and the inclusion of a detailed medication list and pending test results also has implications for patient safety.911, 27
| Challenge | Recommended approaches |
|---|---|
| Inpatientoutpatient physician discontinuity | When possible, involve the primary care physician (PCP) in discharge planning and work together to develop a follow‐up plan |
| At minimum, communicate the following to the PCP on the day of discharge: diagnoses, medications, results of procedures, pending tests, follow‐up arrangements, and suggested next steps | |
| Provide the PCP with a detailed discharge summary within 1 week | |
| In discharge summaries include: diagnoses, abnormal physical findings, important test results, discharge medications with rationale for new or changed medications, follow‐up arrangements made, counseling provided to the patient and family, and tasks to be completed (eg, appointments that still need to be made and tests that require follow‐up) | |
| Follow a structured template with subheadings in discharge communications | |
| When possible, use health information technology to create and disseminate discharge summaries | |
| Changes and discrepancies in medication regimen | Obtain a complete medication history by asking patients about: medications taken at different times of day; medications prescribed by different physicians; nonoral medications; over‐the‐counter products; dosage, indication, length of therapy, and timing of last dose of all drugs; allergies; and adherence |
| Compare and reconcile medication information obtained from patient and caregiver reports, patient lists, prescription bottles, medical records, and pharmacy records | |
| Display preadmission medication list prominently in the chart | |
| Reconcile medications at all care transitions, including admission, intrahospital transfer, and discharge | |
| Communicate complete and accurate medication information to the next provider at discharge, including indications for new medications and reasons for any changes | |
| When possible, partner with clinical pharmacists to manage medication information and reconciliation, especially for high‐risk patients | |
| Self‐care responsibilities and social support | Use multidisciplinary discharge planning teams to assess the needs of patients and their families |
| Arrange a specific follow‐up appointment prior to discharge | |
| Contact patients by telephone a few days after discharge to assess questions, symptoms, and medication‐related issues | |
| Order home health services when indicated | |
| Consider home visits for frail elderly patients | |
| Ineffective physicianpatient communication | Focus discharge counseling on informing patients of major diagnoses, medication changes, dates of follow‐up appointments, self‐care instructions, and who to contact if problems develop |
| Ensure that staff members communicate consistent instructions | |
| For high‐volume conditions, consider using audiovisual recordings for discharge education, combined with an opportunity for additional counseling and questions | |
| Use trained interpreters when a language gap exists | |
| Provide simply written materials that include illustrations when possible to reinforce verbal instructions | |
| Ensure patients and family members comprehend key points by asking them to teach back the information in their own words and demonstrate any self‐care behaviors | |
| Encourage patients and family members to ask questions through an open‐ended invitation like, What questions do you have? instead of Do you have any questions? |
Because many patients follow up with their PCPs within a few days of discharge, it becomes important to provide the PCPs with some information about the hospitalization on the day of discharge. This can be accomplished via a quick telephone call, fax, or e‐mail update to the PCP.24, 35 Important things to include in this communiqu are the discharge diagnosis, medications, results of procedures, pending test results, follow‐up arrangements, and suggested next steps. Within 1 week, a detailed discharge summary should have been received.26, 33, 36 As electronic medical records become more widely available, computer‐generated summaries offer a way to more quickly and completely highlight the key elements of the hospitalization, and they are ready for delivery sooner than traditional dictated summaries.37 Additionally, all forms of discharge summariescomputer‐generated, handwritten, and dictatedshould include subheadings to better organize and present the information instead of unstructured narrative summaries.38
There is increasing interest in moving away from the traditional 1‐way transfer of information about a hospitalization toward a 2‐way dialogue between hospitalist and primary care physician.39 Preferences about how to do this will vary among physicians. One strategy might be to provide the PCP with the hospitalist's contact information and encouraging questions about the hospitalization. Another approach would involve contacting the PCP during the discharge planning process to exchange information about the patient, provide an opportunity for the PCP to ask questions about the hospitalization, and formulate a cohesive plan for follow‐up, particularly about contingency planning (ie, what is most likely to go wrong and what should be done about it) and specific follow‐up needs (ie, what tasks should be accomplished at the first postdischarge visit).
CHANGES AND DISCREPANCIES IN THE MEDICATION REGIMEN
Medication errors make up a large portion of the adverse events patients may experience in the period following hospital discharge.7 In fact, errors during the ordering of admission or discharge medications make up almost half of all hospital medication errors.4043 At transition points such as admission and discharge, errors are often associated with changes in the medication regimen, including discrepancies between the new set of medication orders and what the patient was taking previously. In 2 recent studies, 54% of patients experienced at least 1 unintended medication discrepancy on admission to the hospital, and 39%‐45% of these discrepancies were considered a potential threat to the patient.44, 45
At discharge, differences between the prescribed medication regimen and the prehospital regimen may exist for several reasons. First, physicians may not obtain a comprehensive and accurate medication history at the time of admission.46 The medication history elicited from the patient at hospital admission is often affected by health literacy, language barriers, current health status, medication‐history interviewing skills, and time constraints.47 Physicians may not consult other important sources of medication information, including family members, prescription lists or bottles, and community pharmacy records. The most common error in the admission medication history is omitting a medication taken at home.46 Additionally, several providers, including a physician, a nurse, and an inpatient pharmacist, may independently take medication histories for the same patient. These multiple accounts lead to discrepancies that are rarely recognized or corrected.
Second, a patient's medication regimen can be significantly altered several times during a hospitalization. Acute illness may cause physicians to hold certain medications, discontinue others, or change prescribed doses during hospitalization.48 In addition, at most hospitals closed drug formularies necessitate automatic substitution of 1 medication for another drug in the same class during the patient's hospital stay.49 Changes from long‐acting to short‐acting medications are also routinely made in the name of tighter control (eg, of blood pressure). One study of hospitalized elders found that 40% of all admission medications had been discontinued by discharge and that 45% of all discharge medications were newly started during the hospitalization.3
Finally, at discharge, the current medication regimen needs to be reconciled with the preadmission medication regimen in a thoughtful manner.2 This includes resuming medications held or modified at admission for clinical reasons, resuming medications that were substituted in the hospital for formulary or pharmacokinetic reasons, and stopping newly started medications that were only required during the hospitalization (eg, for prevention of venous thromboembolism or stress ulcers).50 It is difficult, even in hospitals with advanced electronic health information systems, to prompt physicians to make these necessary changes. In a recent study, unexplained discrepancies between the preadmission medication list and discharge medication orders were noted in 49% of hospital discharges.51 Errors in discharge medication reconciliation may subsequently increase the risk of postdischarge ADEs.51
Medication Reconciliation and Education
An optimal strategy for obtaining a complete medication history may include asking patients about the following: a typical day and what medications are taken at different times of day; whether prescriptions come from more than 1 doctor; medications not taken orally (eg, inhalers, patches); dosages and indications for all medications; length of therapy and timing of last dose; over‐the‐counter products, herbals, vitamins, and supplements used and vaccinations received; allergies; and number of doses missed in the last week (Table 1).5254 Forms are also available to help patients maintain a list of current medications.5557
Ideally, the process of obtaining a medication history involves integration of information from several sources, including patient and caregiver recollections, patient‐provided lists of medications, prescription bottles, outpatient medical records, and prescription refill information from community pharmacies.58, 59 Any discrepancies in the information obtained should be explicitly resolved with the patient and/or caregiver. Assistance from a pharmacist or the patient's PCP may also be required.
Once the preadmission medication regimen is confirmed, it should be entered on a standardized form and placed in a prominent place in the chart. This list should then be compared against the patient's medication orders at admission, throughout the hospital stay, and at discharge.12 The planned action for each of these medications (eg, continue at same dose/route/frequency, substitute) should be made explicit. At discharge, this preadmission list also needs to be compared with the current hospital medications in order to create a coherent set of discharge orders.
Staff responsibilities for obtaining and documenting an accurate list of preadmission medications and reconciling medications at admission, transfer, and discharge should be well defined and based on the resources available at each institution. Redundant work (eg, multiple personnel independently taking a medication history) should be replaced by interdisciplinary communication (ie, a member of the team confirming the accuracy of a list obtained by another member of the team). When discrepancies are found (eg, between preadmission and discharge medications), reconciliation requires correction of unintentional discrepancies and appropriate documentation of intentional changes.60
Because a patient's medications change frequently during the transitions of admission, intrahospital transfer, and discharge, reconciliation is an active and ongoing process that aims to ensure the patient is receiving the correct medication regimen at all times. Reconciliation also allows for a review of the safety and appropriateness of the regimen and discontinuation of any unsuitable or needless medications.61, 62
Finally, a comprehensive list of a patient's medications should be reported to the next service provider when the patient is referred or transferred to another setting, service, practitioner, or level of care within or outside the organization. Avoiding overarching orders such as continue home medications and resume all medications becomes crucial to patient safety during transitions in care. At discharge, physicians should provide patients with a complete list of medications to be taken at home with indications and instructions for administration written in everyday language. Physicians should also highlight the results of medication reconciliation by pointing out any changes from the preadmission regimen, especially medications that are at home but should no longer be taken.
Ultimately, physicians have the duty to ensure that correct and complete medication information is provided. However, to achieve optimal results, physicians should partner with clinical pharmacists when possible. Pharmacists have been formally educated about and are experienced at taking medication histories, which may make them the ideal individuals to interview newly admitted patients about their medication histories.63 Unfortunately, according to a recent survey, pharmacists perform admission drug histories in only 5% of U.S. hospitals and provide drug therapy counseling in just 49% of U.S. hospitals.64 Patients who are elderly, have limited literacy skills, take more than 5 medications daily, or take high‐risk medications such as insulin, warfarin, cardiovascular drugs (including antiarrhythmics), inhalers, antiseizure medications, eye medications, analgesics, oral hypoglycemics, oral methotrexate, and immunosuppressants may require additional counseling or pharmacist involvement for effective reconciliation.10, 65, 66
Although the evidence supporting medication reconciliation is limited, it is convincing enough to support carrying out such reconciliations. In 1 investigation, when the nursing staff obtained and pharmacists verified orders for home medications, the accuracy of admission medication orders increased from 40% to 95%.67 In another work, in which there was pharmacist‐led medication reconciliation, significant discrepancies were found in approximately 25% of patients' medication histories and admission orders.45 In the absence of pharmacist intervention, the authors predicted that 22% of the discrepancies could have caused some form of patient harm during hospitalization and that 59% of the discrepancies might have contributed to an adverse event if the error continued after discharge.45 Others report that orders were changed as a result of reconciliation for 94% of patients being transferred out of the intensive care unit.2 Finally, in a randomized controlled trial of a pharmacist intervention at discharge in which medication reconciliation was the most common action performed, after 30 days preventable ADEs were detected in 11% of control patients and 1% of intervention patients. Medication discrepancy was the cause of half the preventable ADEs in the control group.51
SELF‐CARE RESPONSIBILITIES AND SOCIAL SUPPORT
Compounding the difficulties at discharge are the economic pressures on our health care system, causing patients to be released from the hospital quicker and sicker than ever before.68 The scope of care provided to patients also undergoes a major shift at discharge. Multidisciplinary providers no longer continually review the health status and needs of patients; instead, patients must follow up with their outpatient physician over a period of days to weeks. In the interim, the patients themselves are responsible for administering new medications, participating in physical therapy, and tracking their own symptoms to see if they are worsening. For many patients, sufficient social and family support is not available to help perform these activities effectively. Unfortunately, hospital personnel often inaccurately assess patients' functional status and overestimate patients' knowledge of required self‐care activities.69
Providing Adequate Medical and Social Support
A multidisciplinary discharge planning team can facilitate proper assessments of the social needs of patients and their families (Table 1).7072 This team is often composed of a nurse case manager and a social worker but may also include a physical therapist, an occupational therapist, a pharmacist, and other health care providers. Following discussion with a patient and the patient's family, the team may suggest home health services during the transition home to supplement available medical support,73 or they may decide that discharge to a rehabilitation or skilled nursing facility is more appropriate.
In addition, follow‐up should be arranged prior to discharge. Patients who are given a set appointment are more likely to show up for their follow‐up visits than are those who are simply asked to call and arrange their own visits.74 Typically, follow‐up with the PCP should be conducted within 2 weeks of hospital discharge. However, depending on a patient's functional status, pending test results, and need for medication monitoring or follow‐up testing, this may need to take place sooner. Interestingly, research indicates that follow‐up appointments with the inpatient provider can result in a lower combined rate of readmission and 30‐day mortality.75 Thus, hospitalists may consider operating a hospitalist‐staffed follow‐up clinic, especially for patients without a regular PCP.
Telephone follow‐up conducted a few days after discharge can also be an effective means of bridging the inpatientoutpatient transition.35 Such follow‐up provides a chance to attend to any patient questions, new or concerning symptoms, and medication‐related issues (eg, not filling the discharge medications or difficulty comprehending the new medication regimen).76 A physician, physician assistant, advanced practice nurse, registered nurse, pharmacist, or care manager can effectively carry out this telephone follow‐up. No matter who telephones, the caller must be aware of the patient's recent course of events as well as the care plan decided at discharge. Published evidence indicates that telephone follow‐up fosters patient satisfaction, increases medication adherence, decreases preventable ADEs, and decreases the number of subsequent emergency room visits and hospital readmissions,51, 77, 78 although not all evaluations have demonstrated benefit.79 As with medication histories performed by pharmacists, limited resources may mean that such follow‐up be restricted to those patients at highest risk for readmission.
Home visits may be appropriate for certain patient populations, such as the frail elderly.80 Home visits enable a patient's daily needs and safety (eg, fall risk) to be assessed. They can also be a means of assessing medication safety and adherence by reviewing all prescription and over‐the‐counter products in the household.81 Close follow‐up of at‐risk or elderly patients after discharge can help to minimize hospital readmission and total health care costs.4, 8285
INEFFECTIVE PHYSICIANPATIENT COMMUNICATION
Physicianpatient communication is fundamental to the practice of medicine and is crucially important at discharge. However, several studies have demonstrated a disconnect between physician information giving and patient understanding.76, 9690 When providing instructions, physicians commonly use medical jargon and attempt to cover a wealth of information in a limited amount of time.69, 87 They also tend to rely on verbal instructions and fail to provide supplementary audiovisual materials (eg, educational handouts or videos) that could aid patient comprehension. Physicians may not point out important self‐care tasks that patients should carry out at home. The entire interaction may be rushed or seem rushed. Moreover, when physicians solicit questions from patients, they may only allow for yes/no responses by using statements like Any questions? or Do you have any questions? that make it easy for patients to simply respond, No. The encounter usually comes to an end without true confirmation of a patient's level of understanding or assessment of a patient's ability to perform the self‐care activities and medication management required on returning home.81
Adding to the challenges of effective physicianpatient communication is the large number of adult Americans (more than 90 million) who have limited functional literacy skills.91, 92 Such patients typically have difficulty reading and understanding medical instructions, medication labels, and appointment slips.9396 Not surprisingly, patients with limited literacy skills know less about their chronic illnesses and how to manage their diseases.97 Having low literacy is also linked to increased use of emergency department services, a higher risk of hospitalization, and higher health care costs.9799 Patients with limited English proficiency have similar or even greater challenges and also have longer stays in the hospital.100
More Effective PhysicianPatient Communication
Discharge counseling should concentrate on the few key points that are of the greatest interest and the most importance to patients: major diagnoses, medication changes, dates of follow‐up appointments, self‐care instructions, and who to contact if problems develop (Table 1).101 Furthermore, these key instructions should be reinforced by other hospital staff, including nurses and pharmacists. For common conditions (eg, high‐volume cardiac procedures), offering standardized audiovisual instructions can be both efficient and worthwhile if used in conjunction with questionanswer sessions.102 In the event that physicians and hospital staff cannot fluently communicate in a patient's language, it is essential to engage trained interpreters, not rely on rudimentary language skills, the patient's family, or other ad hoc ways to communicate.103
Because patients are unlikely to fully remember verbal instructions at discharge, it is helpful to provide patients and family members with written materials to take home in order to reinforce important self‐care instructions.76, 87 These materials, written at a 5th‐ to 8th‐grade reading level, should outline key information in a simple format with little or no medical jargon. Illustrated materials are often better comprehended and subsequently remembered by patients.104, 105 If preprinted illustrated materials are not on hand, then physicians can convey key points by drawing simple pictures.
Confirming patient comprehension with the teach‐back method is perhaps the most important step in effectively communicating discharge instructions.106 With this method, patients are asked to repeat back what they understand from the discharge instructions. Application of this simple technique is advocated as one of the most effective means of improving patient safety.107, 108 Patients should also be asked to demonstrate any new self‐care tasks that they will be required to carry out at home, such as using an inhaler or administering a subcutaneous injection.
Last, The Joint Commission recently created a National Patent Safety Goal to encourage the active involvement of patients and their families in the patient's own care.12 This charge requires that physicians offer ample time for patients and their family members to ask questions. Physicians should avoid questions with yes/no responses and instead invite patient and family member questions in a more open‐ended manner (eg, What questions do you have?) to help ensure comprehension and comfort with the care plan.
CONCLUSIONS
The transition from hospital to home is a vulnerable period of discontinuity and potential adverse events. Hospitalists and other inpatient providers should not view discharge as an end to their obligation to patients but rather should attempt to promote a safe and efficient transition of care. Hospitalists can play an important role in bridging the gap between inpatient and outpatient care through appropriate discharge planning and effective communication with patients, their family members, and outpatient physicians.
Acknowledgements
The authors thank Marra Katz for her editorial assistance in the preparation of this manuscript
- .Whither continuity of care?N Engl J Med.1999;340:1362–1363.
- ,,, et al.Medication reconciliation: a practical tool to reduce the risk of medication errors.J Crit Care.2003;18(4):201–205.
- ,,,.Influence of hospitalization on drug therapy in the elderly.J Am Geriatr Soc.1989;37:679–683.
- ,,,,,.Preparing patients and caregivers to participate in care delivered across settings: the Care Transitions Intervention.J Am Geriatr Soc.2004;52:1817–1825.
- ,,.Predictors of elder and family caregiver satisfaction with discharge planning.J Cardiovasc Nurs.2000;14(3):76–87.
- ,.Discharge planning and continuity of care for aged people: indicators of satisfaction and implications for practice.Aust J Adv Nurs.1998;16(1):7–13.
- ,,,.Medical errors related to discontinuity of care from an inpatient to an outpatient setting.J Gen Intern Med.2003;18:646–651.
- ,,.Assessing the quality of preparation for posthospital care from the patient's perspective: the care transitions measure.Med Care.2005;43(3):246–255.
- ,,, et al.Adverse events among medical patients after discharge from hospital.CMAJ.2004;170:345–349.
- ,,,,.The incidence and severity of adverse events affecting patients after discharge from the hospital.Ann Intern Med.2003;138:161–167.
- ,,,,.Adverse drug events occurring following hospital discharge.J Gen Intern Med.2005;20:317–323.
- The Joint Commission. Joint Commission National Patient Safety Goals. Available at: http://www.jointcommission.org/PatientSafety/NationalPatientSafetyGoals/. Accessed July 17,2006.
- ,,, et al.Transition of care for hospitalized elderly patients ‐ Development of a discharge checklist for hospitalists.J Hosp Med.2006;1:354–360.
- ,,,,,.Deficits in communication and information transfer between hospital‐based and primary care physicians: implications for patient safety and continuity of care.JAMA.2007;297:831–841.
- Agency for Healthcare Research and Quality. National Guideline Clearinghouse. Available at: http://www.guideline.gov. Accessed January 3,2007.
- ,.The emerging role of “hospitalists” in the American health care system.N Engl J Med.1996;335:514–517.
- .Hospitalists in the United States—mission accomplished or work in progress?N Engl J Med.2004;350:1935–1936.
- .The evolution of the hospitalist model in the United States.Med Clin North Am2002;86(4):687–706.
- ,.The hospitalist movement 5 years later.JAMA.2002;287:487–494.
- Society of Hospital Medicine. Growth of hospital medicine nationwide. Available at: http://www.hospitalmedicine.org/Content/NavigationMenu/Media/GrowthofHospitalMedicineNationwide/Growth_of_Hospital_M.htm. Accessed July 17,2006.
- ,,,,.Quality assessment of discharge letters in a French university hospital.Int J Health Care Qual Assur Inc Leadersh Health Serv.1998;11(2‐3):90–95.
- ,,.Dissemination of discharge summaries. Not reaching follow‐up physicians.Can Fam Physician.2002;48:737–742.
- ,.Quality assessment of a discharge summary system.CMAJ.1995;152:1437–1442.
- ,,,.Primary care physician attitudes regarding communication with hospitalists.Am J Med.2001;111(9B):15S–20S.
- ,,,.Effect of discharge summary availability during post‐discharge visits on hospital readmission.J Gen Intern Med.2002;17(3):186–192.
- .Study of discharge communications from hospital doctors to an inner London general practice.J R Coll Gen Pract.1987;37:494–495.
- ,,, et al.Patient safety concerns arising from test results that return after hospital discharge.Ann Intern Med.2005;143(2):121–128.
- ,,.Communication between general practitioners and consultants: what should their letters contain?BMJ1992;304(6830):821–824.
- ,,,.Written communication from specialists to general practitioners in cancer care. What are the expectations and how are they met?Scand J Prim Health Care.1998;16(3):154–159.
- ,.Improving the continuity of care between general practitioners and public hospitals.Med J Aust.1994;161:656–659.
- ,.Towards better discharge summaries: brevity and structure.West Engl Med J.1991;106(2):40–41,55.
- ,,.Content of a discharge summary from a medical ward: views of general practitioners and hospital doctors.J R Coll Physicians Lond.1995;29:307–310.
- ,.What is necessary for high‐quality discharge summaries?Am J Med Qual.1999;14(4):160–169.
- The Joint Commission.Standard IM.6.10. Hospital Accreditation Standards.Oakbrook Terrace, IL:The Joint Commission;2006:338–340.
- .The importance of postdischarge telephone follow‐up for hospitalists: a view from the trenches.Am J Med.2001;111(9B):43S–44S.
- ,,,.The quality of communication between hospitals and general practitioners: an assessment.J Qual Clin Pract.1998;18:241–247.
- ,,,.Dictated versus database‐generated discharge summaries: a randomized clinical trial.CMAJ.1999;160:319–326.
- ,,,.Standardized or narrative discharge summaries. Which do family physicians prefer?Can Fam Physician.1998;44:62–69.
- ,.Moving from information transfer to information exchange in health and health care.Soc Sci Med.2003;56:449–464.
- ,,, et al.The costs of adverse drug events in hospitalized patients.Adverse Drug Events Prevention Study Group.JAMA.1997;277:307–311.
- ,,, et al.Systems analysis of adverse drug events.ADE Prevention Study Group.JAMA.1995;274(1):35–43.
- ,,, et al.Incidence of adverse drug events and potential adverse drug events. Implications for prevention.ADE Prevention Study Group.JAMA.1995;274(1):29–34.
- ,.Medication errors in hospitalized cardiovascular patients.Arch Intern Med.2003;163:1461–1466.
- ,,, et al.Unintended medication discrepancies at the time of hospital admission.Arch Intern Med.2005;165:424–429.
- ,,,,,.Reconciliation of discrepancies in medication histories and admission orders of newly hospitalized patients.Am J Health Syst Pharm.2004;61:1689–1695.
- ,,,,,.Frequency, type and clinical importance of medication history errors at admission to hospital: a systematic review.CMAJ.2005;173:510–515.
- ,,,,.Medication reconciliation in the acute care setting: opportunity and challenge for nursing.J Nurs Care Qual.2005;20(2):95–98.
- ,,.The adverse effects of hospitalization on drug regimens.Arch Intern Med.1991;151:1562–1564.
- ,,,,.Prevalence and cost savings of therapeutic interchange among U.S. hospitals.Am J Health Syst Pharm.2002;59:529–533.
- ,,, et al.Medication reconciliation: are we meeting the requirements?JCOM.2006;13:441–444.
- ,,, et al.Role of pharmacist counseling in preventing adverse drug events after hospitalization.Arch Intern Med.2006;166:565–571.
- ,.Effectiveness of a pharmacist‐acquired medication history in promoting patient safety.Am J Health Syst Pharm.2002;59:2221–2225.
- .Ensuring continuity of care and accuracy of patients' medication history on hospital admission.Am J Health Syst Pharm.2002;59:1054–1055.
- ,,,.Current methods used to teach the medication history interview to doctor of pharmacy students.Am J Pharm Educ.2002;66(Summer):103–107.
- AARP. My Personal Medication Record. Available at: http://assets.aarp.org/www.aarp.org_/articles/learntech/wellbeing/medication‐record.pdf. Accessed October 20,2006.
- AARP. Mi registro de medicacion. Available at: http://assets.aarp.org/www.aarp.org_/articles/health/docs/PersonalMedRecordSP.pdf. Accessed October 20,2006.
- Institute for Safe Medication Practices. Available at: http://www.ismp.org. Accessed September 8,2006.
- ,,.Parents as partners in obtaining the medication history.J Am Med Inform Assoc.2005;12:299–305.
- ,,.Medication history on internal medicine wards: assessment of extra information collected from second drug interviews and GP lists.Pharmacoepidemiology Drug Saf.2003;12:491–498.
- Medication Discrepancy Tool. Available at: http://www.caretransitions.org. Accessed July 28,2005.
- ,,,,,.Updating the Beers criteria for potentially inappropriate medication use in older adults: results of a US consensus panel of experts.Arch Intern Med.2003;163:2716–2724.
- .Using the NO TEARS tool for medication review.BMJ2004;329(7463):434.
- ,,,.Clinical pharmacists and inpatient medical care: a systematic review.Arch Intern Med.2006;166:955–964.
- ,.Clinical pharmacy services, pharmacy staffing, and adverse drug reactions in United States hospitals.Pharmacotherapy.2006;26:735–747.
- ,,, et al.Adverse drug events in ambulatory care.N Engl J Med.2003;348:1556–1564.
- MA Coalition for the Prevention of Medical Errors. Reconciling medications. Recommended practices. Available at: http://www.macoalition.org/documents/RecMedPractices.pdf. Accessed July 27,2005.
- ,.OSF healthcare's journey in patient safety.Qual Manag Health Care.2004;13(1):53–59.
- ,,, et al.Prospective payment system and impairment at discharge. The ‘quicker‐and‐sicker’ story revisited.JAMA.1990;264:1980–1983.
- ,,,,,.Discharge planning: comparison of patients and nurses' perceptions of patients following hospital discharge.Image J Nurs Sch.1996;28(2):143–147.
- ,,,.The Care Transitions Intervention: results of a randomized controlled trial.Arch Intern Med.2006;166:1822–1828.
- ,,,,,.Comprehensive discharge planning with postdischarge support for older patients with congestive heart failure: a meta‐analysis.JAMA.2004;291:1358–1367.
- ,,,.Discharge planning from hospital to home.Cochrane Database Syst Rev.2006;4.
- .Falling through the cracks: challenges and opportunities for improving transitional care for persons with continuous complex care needs.J Am Geriatr Soc.2003;51:549–555.
- .The best way to improve emergency department follow‐up is actually to give the patient a specific appointment.J Gen Intern Med.2006;21:398; author reply398.
- ,,,.Continuity of care and patient outcomes after hospital discharge.J Gen Intern Med.2004;19:624–631.
- ,.Patients' understanding of their treatment plans and diagnosis at discharge.Mayo Clin Proc.2005;80:991–994.
- ,,,.The impact of follow‐up telephone calls to patients after hospitalization.Am J Med.2001;111(9B):26S–30S.
- ,.The impact of clinical pharmacists' consultations on geriatric patients' compliance and medical care use: a randomized controlled trial.Gerontologist.1994;34:307–315.
- ,.Telephone follow‐up, initiated by a hospital‐based health professional, for postdischarge problems in patients discharged from hospital to home.Cochrane Database Syst Rev.2007;1.
- ,,, et al.Geriatric home assessment after hospital discharge.J Am Geriatr Soc.1994;42:1229–1234.
- ,.Uncovering a multitude of sins: medication management in the home post acute hospitalisation among the chronically ill.Aust N Z J Med.1999;29(2):220–227.
- ,,, et al.Comprehensive discharge planning and home follow‐up of hospitalized elders: a randomized clinical trial.JAMA.1999;281:613–620.
- ,,,.The cost‐effectiveness of intensive postdischarge care. A randomized trial.Med Care.1988;26:1092–1102.
- ,,,,.The effects of aftercare on chronic patients and frail elderly patients when discharged from hospital: a systematic review.J Adv Nurs.1998;27:1076–1086.
- ,,,,,.A multidisciplinary intervention to prevent the readmission of elderly patients with congestive heart failure.N Engl J Med.1995;333:1190–1195.
- ,,.Medication education of acutely hospitalized older patients.J Gen Intern Med.1999;14:610–616.
- ,,, et al.Patient‐physician communication at hospital discharge and patients' understanding of the postdischarge treatment plan.Arch Intern Med.1997;157:1026–1030.
- ,,.Patient understanding of commonly used medical vocabulary.J Fam Pract.1987;25:176–178.
- .The understanding of medical terminology used in printed health education materials.Heal Educ J.1979;38:111–121.
- ,,,,.The gap between patient reading comprehension and the readability of patient education materials.J Fam Pract.1990;31:533–538.
- ,,. National Assessment of Adult Literacy (NAAL). A first look at the literacy of America's adults in the 21st century. Available at: http://nces.ed.gov/naal. Accessed May 2,2006.
- ,,,.Adult Literacy in America: A First Look at the Findings of the National Adult Literacy Survey.Washington, DC:National Center for Education Statistics, U.S. Department of Education;1993.
- ,,,.The role of health literacy in patient‐physician communication.Fam Med.2002;34:383–389.
- American Medical Association Council on Scientific Affairs.Health literacy.JAMA.1999;281:552–557.
- National Work Group on Literacy and Health.Communicating with patients who have limited literacy skills.J Fam Pract.1998;46:168–176.
- ,.Communicating with patients who cannot read.N Engl J Med.1997;337:272–274.
- ,,,,.Literacy and health outcomes: a systematic review of the literature.J Gen Intern Med.2004;19:1129–1139.
- ,,.The impact of low health literacy on the medical costs of Medicare managed care enrollees.Am J Med.2005;118:371–377.
- ,,,.Health literacy and the risk of hospital admission.J Gen Intern Med.1998;13:791–798.
- ,,, et al.The effect of English language proficiency on length of stay and in‐hospital mortality.J Gen Intern Med.2004;19(3):221–228.
- ,,, et al.Effects of a structured patient‐centered discharge interview on patients' knowledge about their medications.Am J Med.2004;117:563–568.
- ,,.Effects of a videotape information intervention at discharge on diet and exercise compliance after coronary artery bypass surgery.J Cardiopulm Rehab.1999;19(3):170–177.
- .The impact of medical interpreter services on the quality of health care: a systematic review.Med Care Res Rev.2005;62(3):255–299.
- ,,.Use of pictorial aids in medication instructions: a review of the literature.Am J Health Syst Pharm.2006;63:2391–2397.
- ,,,.The role of pictures in improving health communication: A review of research on attention, comprehension, recall, and adherence.Patient Educ Couns.2006;61(2):173–190.
- ,,, et al.Closing the loop. Physician communication with diabetic patients who have low health literacy.Arch Intern Med.2003;163:83–90.
- National Quality Forum.Safe Practices for Better Healthcare,2003; Washington, DC.
- Shojania KG, Duncan BW, McDonald KM, Wachter RM, eds.Making Healthcare Safer: A Critical Analysis of Patient Safety Practices. Evidence Report No. 43 from the Agency for Healthcare Research and Quality. AHRQ Publication No. 01‐E058;2001.
As the counterpart to hospital admission, hospital discharge is a necessary process experienced by each living patient. For all patients except those being transferred to a continuing care facility, discharge is a period of transition from hospital to home that involves a transfer in responsibility from the inpatient provider or hospitalist to the patient and primary care physician (PCP).1 Prescription medications are commonly altered at this transition point, with patients asked to discontinue some medications, switch to a new dosage schedule of others, or begin new treatments.2, 3 Self‐care responsibilities also increase in number and importance, presenting new challenges for patients and their families as they return home.4 Under these circumstances, ineffective planning and coordination of care can undermine patient satisfaction, facilitate adverse events, and contribute to more frequent hospital readmissions.58
Following hospital discharge nearly half (49%) of hospitalized patients experience at least 1 medical error in medication continuity, diagnostic workup, or test follow‐up.7 It has been reported that 19%23% of patients suffer an adverse event, most frequently an adverse drug event (ADE).911 Half of ADEs are considered preventable or ameliorable (ie, their severity or duration could have been decreased). Most errors and adverse events in this setting result from a breakdown in communication between the hospital team and the patient or primary care physician.10
To promote more effective care transitions, The Joint Commission now requires accredited facilities to accurately and completely reconcile medications across the continuum of care.12 The Society of Hospital Medicine recently published recommendations for the discharge of elderly patients.13 The joint Society of Hospital MedicineSociety of General Internal Medicine Continuity of Care Task Force also recently published a systematic review with recommendations for improving the handoff of patient information at discharge.14 Apart from these reports, however, it is uncommon to find evidence‐based recommendations for hospital discharge applicable to a broad range of patients.15 This review highlights several important challenges for physicians who seek to provide high‐quality care during hospital discharge and the subsequent period of transition. Based on the best available evidence, recommendations are also provided for how to improve communication and facilitate the care transition for adult inpatients returning home.
INPATIENTOUTPATIENT PHYSICIAN DISCONTINUITY
Traditionally, primary care physicians have admitted their own patients, provided hospital care (in addition to seeing outpatients during the day), and followed patients after discharge. Under this model, continuity of care has been preserved; however, this method of care has faltered under the weight of inpatients and outpatients with more severe illnesses, rapid technological advancements, managed care pressuring outpatient physicians to see more patients, and a thrust toward reduced hospital costs and length of stay.16 Increases in the efficiency and quality of hospital care have accompanied a new reliance on the field of hospital medicine, while allowing PCPs to focus on outpatient care.1719 With more than 14,000 hospitalists currently practicing in the United States and 25,000 anticipated to be practicing by 2010, transfer of care from hospital‐based providers to PCPs has become increasingly common at discharge.20
Patient discharge summaries are the most common means of communication between inpatient and outpatient providers. However, numerous studies have shown that discharge summaries often fail to provide important administrative and medical information, such as the primary diagnosis, results of abnormal diagnostics, details about the hospital course, follow‐up plans, whether laboratory test results are pending, and patient or family counseling.14 Summaries also may not arrive in a timely manner and sometimes may not reach the PCP at all.2123
At the time patients first follow up with their PCPs after hospitalization, discharge summaries have not yet arrived about 75% of the time,22, 24, 25 restricting the PCPs' ability to provide adequate follow‐up care in 24% of hospital follow‐up visits, according to one study.26 In another investigation, PCPs reported being unaware of 62% of the pending test results that returned after discharge, of which 37% were considered actionable.27
Improving Physician Information Transfer and Continuity
To improve information transfer from hospitalist to PCP, attention must be paid to the content, format, and timely delivery of discharge information (Table 1).14 Surveys of primary care physicians suggest the following information should be included in discharge summaries: diagnoses, abnormal physical findings, important test results, discharge medications, follow‐up arrangements made and appointments that still need to be made, counseling provided to the patient and family, and tests still pending at discharge.24, 2833 These domains are consistent with Joint Commission guidelines for discharge summaries,34 and the inclusion of a detailed medication list and pending test results also has implications for patient safety.911, 27
| Challenge | Recommended approaches |
|---|---|
| Inpatientoutpatient physician discontinuity | When possible, involve the primary care physician (PCP) in discharge planning and work together to develop a follow‐up plan |
| At minimum, communicate the following to the PCP on the day of discharge: diagnoses, medications, results of procedures, pending tests, follow‐up arrangements, and suggested next steps | |
| Provide the PCP with a detailed discharge summary within 1 week | |
| In discharge summaries include: diagnoses, abnormal physical findings, important test results, discharge medications with rationale for new or changed medications, follow‐up arrangements made, counseling provided to the patient and family, and tasks to be completed (eg, appointments that still need to be made and tests that require follow‐up) | |
| Follow a structured template with subheadings in discharge communications | |
| When possible, use health information technology to create and disseminate discharge summaries | |
| Changes and discrepancies in medication regimen | Obtain a complete medication history by asking patients about: medications taken at different times of day; medications prescribed by different physicians; nonoral medications; over‐the‐counter products; dosage, indication, length of therapy, and timing of last dose of all drugs; allergies; and adherence |
| Compare and reconcile medication information obtained from patient and caregiver reports, patient lists, prescription bottles, medical records, and pharmacy records | |
| Display preadmission medication list prominently in the chart | |
| Reconcile medications at all care transitions, including admission, intrahospital transfer, and discharge | |
| Communicate complete and accurate medication information to the next provider at discharge, including indications for new medications and reasons for any changes | |
| When possible, partner with clinical pharmacists to manage medication information and reconciliation, especially for high‐risk patients | |
| Self‐care responsibilities and social support | Use multidisciplinary discharge planning teams to assess the needs of patients and their families |
| Arrange a specific follow‐up appointment prior to discharge | |
| Contact patients by telephone a few days after discharge to assess questions, symptoms, and medication‐related issues | |
| Order home health services when indicated | |
| Consider home visits for frail elderly patients | |
| Ineffective physicianpatient communication | Focus discharge counseling on informing patients of major diagnoses, medication changes, dates of follow‐up appointments, self‐care instructions, and who to contact if problems develop |
| Ensure that staff members communicate consistent instructions | |
| For high‐volume conditions, consider using audiovisual recordings for discharge education, combined with an opportunity for additional counseling and questions | |
| Use trained interpreters when a language gap exists | |
| Provide simply written materials that include illustrations when possible to reinforce verbal instructions | |
| Ensure patients and family members comprehend key points by asking them to teach back the information in their own words and demonstrate any self‐care behaviors | |
| Encourage patients and family members to ask questions through an open‐ended invitation like, What questions do you have? instead of Do you have any questions? |
Because many patients follow up with their PCPs within a few days of discharge, it becomes important to provide the PCPs with some information about the hospitalization on the day of discharge. This can be accomplished via a quick telephone call, fax, or e‐mail update to the PCP.24, 35 Important things to include in this communiqu are the discharge diagnosis, medications, results of procedures, pending test results, follow‐up arrangements, and suggested next steps. Within 1 week, a detailed discharge summary should have been received.26, 33, 36 As electronic medical records become more widely available, computer‐generated summaries offer a way to more quickly and completely highlight the key elements of the hospitalization, and they are ready for delivery sooner than traditional dictated summaries.37 Additionally, all forms of discharge summariescomputer‐generated, handwritten, and dictatedshould include subheadings to better organize and present the information instead of unstructured narrative summaries.38
There is increasing interest in moving away from the traditional 1‐way transfer of information about a hospitalization toward a 2‐way dialogue between hospitalist and primary care physician.39 Preferences about how to do this will vary among physicians. One strategy might be to provide the PCP with the hospitalist's contact information and encouraging questions about the hospitalization. Another approach would involve contacting the PCP during the discharge planning process to exchange information about the patient, provide an opportunity for the PCP to ask questions about the hospitalization, and formulate a cohesive plan for follow‐up, particularly about contingency planning (ie, what is most likely to go wrong and what should be done about it) and specific follow‐up needs (ie, what tasks should be accomplished at the first postdischarge visit).
CHANGES AND DISCREPANCIES IN THE MEDICATION REGIMEN
Medication errors make up a large portion of the adverse events patients may experience in the period following hospital discharge.7 In fact, errors during the ordering of admission or discharge medications make up almost half of all hospital medication errors.4043 At transition points such as admission and discharge, errors are often associated with changes in the medication regimen, including discrepancies between the new set of medication orders and what the patient was taking previously. In 2 recent studies, 54% of patients experienced at least 1 unintended medication discrepancy on admission to the hospital, and 39%‐45% of these discrepancies were considered a potential threat to the patient.44, 45
At discharge, differences between the prescribed medication regimen and the prehospital regimen may exist for several reasons. First, physicians may not obtain a comprehensive and accurate medication history at the time of admission.46 The medication history elicited from the patient at hospital admission is often affected by health literacy, language barriers, current health status, medication‐history interviewing skills, and time constraints.47 Physicians may not consult other important sources of medication information, including family members, prescription lists or bottles, and community pharmacy records. The most common error in the admission medication history is omitting a medication taken at home.46 Additionally, several providers, including a physician, a nurse, and an inpatient pharmacist, may independently take medication histories for the same patient. These multiple accounts lead to discrepancies that are rarely recognized or corrected.
Second, a patient's medication regimen can be significantly altered several times during a hospitalization. Acute illness may cause physicians to hold certain medications, discontinue others, or change prescribed doses during hospitalization.48 In addition, at most hospitals closed drug formularies necessitate automatic substitution of 1 medication for another drug in the same class during the patient's hospital stay.49 Changes from long‐acting to short‐acting medications are also routinely made in the name of tighter control (eg, of blood pressure). One study of hospitalized elders found that 40% of all admission medications had been discontinued by discharge and that 45% of all discharge medications were newly started during the hospitalization.3
Finally, at discharge, the current medication regimen needs to be reconciled with the preadmission medication regimen in a thoughtful manner.2 This includes resuming medications held or modified at admission for clinical reasons, resuming medications that were substituted in the hospital for formulary or pharmacokinetic reasons, and stopping newly started medications that were only required during the hospitalization (eg, for prevention of venous thromboembolism or stress ulcers).50 It is difficult, even in hospitals with advanced electronic health information systems, to prompt physicians to make these necessary changes. In a recent study, unexplained discrepancies between the preadmission medication list and discharge medication orders were noted in 49% of hospital discharges.51 Errors in discharge medication reconciliation may subsequently increase the risk of postdischarge ADEs.51
Medication Reconciliation and Education
An optimal strategy for obtaining a complete medication history may include asking patients about the following: a typical day and what medications are taken at different times of day; whether prescriptions come from more than 1 doctor; medications not taken orally (eg, inhalers, patches); dosages and indications for all medications; length of therapy and timing of last dose; over‐the‐counter products, herbals, vitamins, and supplements used and vaccinations received; allergies; and number of doses missed in the last week (Table 1).5254 Forms are also available to help patients maintain a list of current medications.5557
Ideally, the process of obtaining a medication history involves integration of information from several sources, including patient and caregiver recollections, patient‐provided lists of medications, prescription bottles, outpatient medical records, and prescription refill information from community pharmacies.58, 59 Any discrepancies in the information obtained should be explicitly resolved with the patient and/or caregiver. Assistance from a pharmacist or the patient's PCP may also be required.
Once the preadmission medication regimen is confirmed, it should be entered on a standardized form and placed in a prominent place in the chart. This list should then be compared against the patient's medication orders at admission, throughout the hospital stay, and at discharge.12 The planned action for each of these medications (eg, continue at same dose/route/frequency, substitute) should be made explicit. At discharge, this preadmission list also needs to be compared with the current hospital medications in order to create a coherent set of discharge orders.
Staff responsibilities for obtaining and documenting an accurate list of preadmission medications and reconciling medications at admission, transfer, and discharge should be well defined and based on the resources available at each institution. Redundant work (eg, multiple personnel independently taking a medication history) should be replaced by interdisciplinary communication (ie, a member of the team confirming the accuracy of a list obtained by another member of the team). When discrepancies are found (eg, between preadmission and discharge medications), reconciliation requires correction of unintentional discrepancies and appropriate documentation of intentional changes.60
Because a patient's medications change frequently during the transitions of admission, intrahospital transfer, and discharge, reconciliation is an active and ongoing process that aims to ensure the patient is receiving the correct medication regimen at all times. Reconciliation also allows for a review of the safety and appropriateness of the regimen and discontinuation of any unsuitable or needless medications.61, 62
Finally, a comprehensive list of a patient's medications should be reported to the next service provider when the patient is referred or transferred to another setting, service, practitioner, or level of care within or outside the organization. Avoiding overarching orders such as continue home medications and resume all medications becomes crucial to patient safety during transitions in care. At discharge, physicians should provide patients with a complete list of medications to be taken at home with indications and instructions for administration written in everyday language. Physicians should also highlight the results of medication reconciliation by pointing out any changes from the preadmission regimen, especially medications that are at home but should no longer be taken.
Ultimately, physicians have the duty to ensure that correct and complete medication information is provided. However, to achieve optimal results, physicians should partner with clinical pharmacists when possible. Pharmacists have been formally educated about and are experienced at taking medication histories, which may make them the ideal individuals to interview newly admitted patients about their medication histories.63 Unfortunately, according to a recent survey, pharmacists perform admission drug histories in only 5% of U.S. hospitals and provide drug therapy counseling in just 49% of U.S. hospitals.64 Patients who are elderly, have limited literacy skills, take more than 5 medications daily, or take high‐risk medications such as insulin, warfarin, cardiovascular drugs (including antiarrhythmics), inhalers, antiseizure medications, eye medications, analgesics, oral hypoglycemics, oral methotrexate, and immunosuppressants may require additional counseling or pharmacist involvement for effective reconciliation.10, 65, 66
Although the evidence supporting medication reconciliation is limited, it is convincing enough to support carrying out such reconciliations. In 1 investigation, when the nursing staff obtained and pharmacists verified orders for home medications, the accuracy of admission medication orders increased from 40% to 95%.67 In another work, in which there was pharmacist‐led medication reconciliation, significant discrepancies were found in approximately 25% of patients' medication histories and admission orders.45 In the absence of pharmacist intervention, the authors predicted that 22% of the discrepancies could have caused some form of patient harm during hospitalization and that 59% of the discrepancies might have contributed to an adverse event if the error continued after discharge.45 Others report that orders were changed as a result of reconciliation for 94% of patients being transferred out of the intensive care unit.2 Finally, in a randomized controlled trial of a pharmacist intervention at discharge in which medication reconciliation was the most common action performed, after 30 days preventable ADEs were detected in 11% of control patients and 1% of intervention patients. Medication discrepancy was the cause of half the preventable ADEs in the control group.51
SELF‐CARE RESPONSIBILITIES AND SOCIAL SUPPORT
Compounding the difficulties at discharge are the economic pressures on our health care system, causing patients to be released from the hospital quicker and sicker than ever before.68 The scope of care provided to patients also undergoes a major shift at discharge. Multidisciplinary providers no longer continually review the health status and needs of patients; instead, patients must follow up with their outpatient physician over a period of days to weeks. In the interim, the patients themselves are responsible for administering new medications, participating in physical therapy, and tracking their own symptoms to see if they are worsening. For many patients, sufficient social and family support is not available to help perform these activities effectively. Unfortunately, hospital personnel often inaccurately assess patients' functional status and overestimate patients' knowledge of required self‐care activities.69
Providing Adequate Medical and Social Support
A multidisciplinary discharge planning team can facilitate proper assessments of the social needs of patients and their families (Table 1).7072 This team is often composed of a nurse case manager and a social worker but may also include a physical therapist, an occupational therapist, a pharmacist, and other health care providers. Following discussion with a patient and the patient's family, the team may suggest home health services during the transition home to supplement available medical support,73 or they may decide that discharge to a rehabilitation or skilled nursing facility is more appropriate.
In addition, follow‐up should be arranged prior to discharge. Patients who are given a set appointment are more likely to show up for their follow‐up visits than are those who are simply asked to call and arrange their own visits.74 Typically, follow‐up with the PCP should be conducted within 2 weeks of hospital discharge. However, depending on a patient's functional status, pending test results, and need for medication monitoring or follow‐up testing, this may need to take place sooner. Interestingly, research indicates that follow‐up appointments with the inpatient provider can result in a lower combined rate of readmission and 30‐day mortality.75 Thus, hospitalists may consider operating a hospitalist‐staffed follow‐up clinic, especially for patients without a regular PCP.
Telephone follow‐up conducted a few days after discharge can also be an effective means of bridging the inpatientoutpatient transition.35 Such follow‐up provides a chance to attend to any patient questions, new or concerning symptoms, and medication‐related issues (eg, not filling the discharge medications or difficulty comprehending the new medication regimen).76 A physician, physician assistant, advanced practice nurse, registered nurse, pharmacist, or care manager can effectively carry out this telephone follow‐up. No matter who telephones, the caller must be aware of the patient's recent course of events as well as the care plan decided at discharge. Published evidence indicates that telephone follow‐up fosters patient satisfaction, increases medication adherence, decreases preventable ADEs, and decreases the number of subsequent emergency room visits and hospital readmissions,51, 77, 78 although not all evaluations have demonstrated benefit.79 As with medication histories performed by pharmacists, limited resources may mean that such follow‐up be restricted to those patients at highest risk for readmission.
Home visits may be appropriate for certain patient populations, such as the frail elderly.80 Home visits enable a patient's daily needs and safety (eg, fall risk) to be assessed. They can also be a means of assessing medication safety and adherence by reviewing all prescription and over‐the‐counter products in the household.81 Close follow‐up of at‐risk or elderly patients after discharge can help to minimize hospital readmission and total health care costs.4, 8285
INEFFECTIVE PHYSICIANPATIENT COMMUNICATION
Physicianpatient communication is fundamental to the practice of medicine and is crucially important at discharge. However, several studies have demonstrated a disconnect between physician information giving and patient understanding.76, 9690 When providing instructions, physicians commonly use medical jargon and attempt to cover a wealth of information in a limited amount of time.69, 87 They also tend to rely on verbal instructions and fail to provide supplementary audiovisual materials (eg, educational handouts or videos) that could aid patient comprehension. Physicians may not point out important self‐care tasks that patients should carry out at home. The entire interaction may be rushed or seem rushed. Moreover, when physicians solicit questions from patients, they may only allow for yes/no responses by using statements like Any questions? or Do you have any questions? that make it easy for patients to simply respond, No. The encounter usually comes to an end without true confirmation of a patient's level of understanding or assessment of a patient's ability to perform the self‐care activities and medication management required on returning home.81
Adding to the challenges of effective physicianpatient communication is the large number of adult Americans (more than 90 million) who have limited functional literacy skills.91, 92 Such patients typically have difficulty reading and understanding medical instructions, medication labels, and appointment slips.9396 Not surprisingly, patients with limited literacy skills know less about their chronic illnesses and how to manage their diseases.97 Having low literacy is also linked to increased use of emergency department services, a higher risk of hospitalization, and higher health care costs.9799 Patients with limited English proficiency have similar or even greater challenges and also have longer stays in the hospital.100
More Effective PhysicianPatient Communication
Discharge counseling should concentrate on the few key points that are of the greatest interest and the most importance to patients: major diagnoses, medication changes, dates of follow‐up appointments, self‐care instructions, and who to contact if problems develop (Table 1).101 Furthermore, these key instructions should be reinforced by other hospital staff, including nurses and pharmacists. For common conditions (eg, high‐volume cardiac procedures), offering standardized audiovisual instructions can be both efficient and worthwhile if used in conjunction with questionanswer sessions.102 In the event that physicians and hospital staff cannot fluently communicate in a patient's language, it is essential to engage trained interpreters, not rely on rudimentary language skills, the patient's family, or other ad hoc ways to communicate.103
Because patients are unlikely to fully remember verbal instructions at discharge, it is helpful to provide patients and family members with written materials to take home in order to reinforce important self‐care instructions.76, 87 These materials, written at a 5th‐ to 8th‐grade reading level, should outline key information in a simple format with little or no medical jargon. Illustrated materials are often better comprehended and subsequently remembered by patients.104, 105 If preprinted illustrated materials are not on hand, then physicians can convey key points by drawing simple pictures.
Confirming patient comprehension with the teach‐back method is perhaps the most important step in effectively communicating discharge instructions.106 With this method, patients are asked to repeat back what they understand from the discharge instructions. Application of this simple technique is advocated as one of the most effective means of improving patient safety.107, 108 Patients should also be asked to demonstrate any new self‐care tasks that they will be required to carry out at home, such as using an inhaler or administering a subcutaneous injection.
Last, The Joint Commission recently created a National Patent Safety Goal to encourage the active involvement of patients and their families in the patient's own care.12 This charge requires that physicians offer ample time for patients and their family members to ask questions. Physicians should avoid questions with yes/no responses and instead invite patient and family member questions in a more open‐ended manner (eg, What questions do you have?) to help ensure comprehension and comfort with the care plan.
CONCLUSIONS
The transition from hospital to home is a vulnerable period of discontinuity and potential adverse events. Hospitalists and other inpatient providers should not view discharge as an end to their obligation to patients but rather should attempt to promote a safe and efficient transition of care. Hospitalists can play an important role in bridging the gap between inpatient and outpatient care through appropriate discharge planning and effective communication with patients, their family members, and outpatient physicians.
Acknowledgements
The authors thank Marra Katz for her editorial assistance in the preparation of this manuscript
As the counterpart to hospital admission, hospital discharge is a necessary process experienced by each living patient. For all patients except those being transferred to a continuing care facility, discharge is a period of transition from hospital to home that involves a transfer in responsibility from the inpatient provider or hospitalist to the patient and primary care physician (PCP).1 Prescription medications are commonly altered at this transition point, with patients asked to discontinue some medications, switch to a new dosage schedule of others, or begin new treatments.2, 3 Self‐care responsibilities also increase in number and importance, presenting new challenges for patients and their families as they return home.4 Under these circumstances, ineffective planning and coordination of care can undermine patient satisfaction, facilitate adverse events, and contribute to more frequent hospital readmissions.58
Following hospital discharge nearly half (49%) of hospitalized patients experience at least 1 medical error in medication continuity, diagnostic workup, or test follow‐up.7 It has been reported that 19%23% of patients suffer an adverse event, most frequently an adverse drug event (ADE).911 Half of ADEs are considered preventable or ameliorable (ie, their severity or duration could have been decreased). Most errors and adverse events in this setting result from a breakdown in communication between the hospital team and the patient or primary care physician.10
To promote more effective care transitions, The Joint Commission now requires accredited facilities to accurately and completely reconcile medications across the continuum of care.12 The Society of Hospital Medicine recently published recommendations for the discharge of elderly patients.13 The joint Society of Hospital MedicineSociety of General Internal Medicine Continuity of Care Task Force also recently published a systematic review with recommendations for improving the handoff of patient information at discharge.14 Apart from these reports, however, it is uncommon to find evidence‐based recommendations for hospital discharge applicable to a broad range of patients.15 This review highlights several important challenges for physicians who seek to provide high‐quality care during hospital discharge and the subsequent period of transition. Based on the best available evidence, recommendations are also provided for how to improve communication and facilitate the care transition for adult inpatients returning home.
INPATIENTOUTPATIENT PHYSICIAN DISCONTINUITY
Traditionally, primary care physicians have admitted their own patients, provided hospital care (in addition to seeing outpatients during the day), and followed patients after discharge. Under this model, continuity of care has been preserved; however, this method of care has faltered under the weight of inpatients and outpatients with more severe illnesses, rapid technological advancements, managed care pressuring outpatient physicians to see more patients, and a thrust toward reduced hospital costs and length of stay.16 Increases in the efficiency and quality of hospital care have accompanied a new reliance on the field of hospital medicine, while allowing PCPs to focus on outpatient care.1719 With more than 14,000 hospitalists currently practicing in the United States and 25,000 anticipated to be practicing by 2010, transfer of care from hospital‐based providers to PCPs has become increasingly common at discharge.20
Patient discharge summaries are the most common means of communication between inpatient and outpatient providers. However, numerous studies have shown that discharge summaries often fail to provide important administrative and medical information, such as the primary diagnosis, results of abnormal diagnostics, details about the hospital course, follow‐up plans, whether laboratory test results are pending, and patient or family counseling.14 Summaries also may not arrive in a timely manner and sometimes may not reach the PCP at all.2123
At the time patients first follow up with their PCPs after hospitalization, discharge summaries have not yet arrived about 75% of the time,22, 24, 25 restricting the PCPs' ability to provide adequate follow‐up care in 24% of hospital follow‐up visits, according to one study.26 In another investigation, PCPs reported being unaware of 62% of the pending test results that returned after discharge, of which 37% were considered actionable.27
Improving Physician Information Transfer and Continuity
To improve information transfer from hospitalist to PCP, attention must be paid to the content, format, and timely delivery of discharge information (Table 1).14 Surveys of primary care physicians suggest the following information should be included in discharge summaries: diagnoses, abnormal physical findings, important test results, discharge medications, follow‐up arrangements made and appointments that still need to be made, counseling provided to the patient and family, and tests still pending at discharge.24, 2833 These domains are consistent with Joint Commission guidelines for discharge summaries,34 and the inclusion of a detailed medication list and pending test results also has implications for patient safety.911, 27
| Challenge | Recommended approaches |
|---|---|
| Inpatientoutpatient physician discontinuity | When possible, involve the primary care physician (PCP) in discharge planning and work together to develop a follow‐up plan |
| At minimum, communicate the following to the PCP on the day of discharge: diagnoses, medications, results of procedures, pending tests, follow‐up arrangements, and suggested next steps | |
| Provide the PCP with a detailed discharge summary within 1 week | |
| In discharge summaries include: diagnoses, abnormal physical findings, important test results, discharge medications with rationale for new or changed medications, follow‐up arrangements made, counseling provided to the patient and family, and tasks to be completed (eg, appointments that still need to be made and tests that require follow‐up) | |
| Follow a structured template with subheadings in discharge communications | |
| When possible, use health information technology to create and disseminate discharge summaries | |
| Changes and discrepancies in medication regimen | Obtain a complete medication history by asking patients about: medications taken at different times of day; medications prescribed by different physicians; nonoral medications; over‐the‐counter products; dosage, indication, length of therapy, and timing of last dose of all drugs; allergies; and adherence |
| Compare and reconcile medication information obtained from patient and caregiver reports, patient lists, prescription bottles, medical records, and pharmacy records | |
| Display preadmission medication list prominently in the chart | |
| Reconcile medications at all care transitions, including admission, intrahospital transfer, and discharge | |
| Communicate complete and accurate medication information to the next provider at discharge, including indications for new medications and reasons for any changes | |
| When possible, partner with clinical pharmacists to manage medication information and reconciliation, especially for high‐risk patients | |
| Self‐care responsibilities and social support | Use multidisciplinary discharge planning teams to assess the needs of patients and their families |
| Arrange a specific follow‐up appointment prior to discharge | |
| Contact patients by telephone a few days after discharge to assess questions, symptoms, and medication‐related issues | |
| Order home health services when indicated | |
| Consider home visits for frail elderly patients | |
| Ineffective physicianpatient communication | Focus discharge counseling on informing patients of major diagnoses, medication changes, dates of follow‐up appointments, self‐care instructions, and who to contact if problems develop |
| Ensure that staff members communicate consistent instructions | |
| For high‐volume conditions, consider using audiovisual recordings for discharge education, combined with an opportunity for additional counseling and questions | |
| Use trained interpreters when a language gap exists | |
| Provide simply written materials that include illustrations when possible to reinforce verbal instructions | |
| Ensure patients and family members comprehend key points by asking them to teach back the information in their own words and demonstrate any self‐care behaviors | |
| Encourage patients and family members to ask questions through an open‐ended invitation like, What questions do you have? instead of Do you have any questions? |
Because many patients follow up with their PCPs within a few days of discharge, it becomes important to provide the PCPs with some information about the hospitalization on the day of discharge. This can be accomplished via a quick telephone call, fax, or e‐mail update to the PCP.24, 35 Important things to include in this communiqu are the discharge diagnosis, medications, results of procedures, pending test results, follow‐up arrangements, and suggested next steps. Within 1 week, a detailed discharge summary should have been received.26, 33, 36 As electronic medical records become more widely available, computer‐generated summaries offer a way to more quickly and completely highlight the key elements of the hospitalization, and they are ready for delivery sooner than traditional dictated summaries.37 Additionally, all forms of discharge summariescomputer‐generated, handwritten, and dictatedshould include subheadings to better organize and present the information instead of unstructured narrative summaries.38
There is increasing interest in moving away from the traditional 1‐way transfer of information about a hospitalization toward a 2‐way dialogue between hospitalist and primary care physician.39 Preferences about how to do this will vary among physicians. One strategy might be to provide the PCP with the hospitalist's contact information and encouraging questions about the hospitalization. Another approach would involve contacting the PCP during the discharge planning process to exchange information about the patient, provide an opportunity for the PCP to ask questions about the hospitalization, and formulate a cohesive plan for follow‐up, particularly about contingency planning (ie, what is most likely to go wrong and what should be done about it) and specific follow‐up needs (ie, what tasks should be accomplished at the first postdischarge visit).
CHANGES AND DISCREPANCIES IN THE MEDICATION REGIMEN
Medication errors make up a large portion of the adverse events patients may experience in the period following hospital discharge.7 In fact, errors during the ordering of admission or discharge medications make up almost half of all hospital medication errors.4043 At transition points such as admission and discharge, errors are often associated with changes in the medication regimen, including discrepancies between the new set of medication orders and what the patient was taking previously. In 2 recent studies, 54% of patients experienced at least 1 unintended medication discrepancy on admission to the hospital, and 39%‐45% of these discrepancies were considered a potential threat to the patient.44, 45
At discharge, differences between the prescribed medication regimen and the prehospital regimen may exist for several reasons. First, physicians may not obtain a comprehensive and accurate medication history at the time of admission.46 The medication history elicited from the patient at hospital admission is often affected by health literacy, language barriers, current health status, medication‐history interviewing skills, and time constraints.47 Physicians may not consult other important sources of medication information, including family members, prescription lists or bottles, and community pharmacy records. The most common error in the admission medication history is omitting a medication taken at home.46 Additionally, several providers, including a physician, a nurse, and an inpatient pharmacist, may independently take medication histories for the same patient. These multiple accounts lead to discrepancies that are rarely recognized or corrected.
Second, a patient's medication regimen can be significantly altered several times during a hospitalization. Acute illness may cause physicians to hold certain medications, discontinue others, or change prescribed doses during hospitalization.48 In addition, at most hospitals closed drug formularies necessitate automatic substitution of 1 medication for another drug in the same class during the patient's hospital stay.49 Changes from long‐acting to short‐acting medications are also routinely made in the name of tighter control (eg, of blood pressure). One study of hospitalized elders found that 40% of all admission medications had been discontinued by discharge and that 45% of all discharge medications were newly started during the hospitalization.3
Finally, at discharge, the current medication regimen needs to be reconciled with the preadmission medication regimen in a thoughtful manner.2 This includes resuming medications held or modified at admission for clinical reasons, resuming medications that were substituted in the hospital for formulary or pharmacokinetic reasons, and stopping newly started medications that were only required during the hospitalization (eg, for prevention of venous thromboembolism or stress ulcers).50 It is difficult, even in hospitals with advanced electronic health information systems, to prompt physicians to make these necessary changes. In a recent study, unexplained discrepancies between the preadmission medication list and discharge medication orders were noted in 49% of hospital discharges.51 Errors in discharge medication reconciliation may subsequently increase the risk of postdischarge ADEs.51
Medication Reconciliation and Education
An optimal strategy for obtaining a complete medication history may include asking patients about the following: a typical day and what medications are taken at different times of day; whether prescriptions come from more than 1 doctor; medications not taken orally (eg, inhalers, patches); dosages and indications for all medications; length of therapy and timing of last dose; over‐the‐counter products, herbals, vitamins, and supplements used and vaccinations received; allergies; and number of doses missed in the last week (Table 1).5254 Forms are also available to help patients maintain a list of current medications.5557
Ideally, the process of obtaining a medication history involves integration of information from several sources, including patient and caregiver recollections, patient‐provided lists of medications, prescription bottles, outpatient medical records, and prescription refill information from community pharmacies.58, 59 Any discrepancies in the information obtained should be explicitly resolved with the patient and/or caregiver. Assistance from a pharmacist or the patient's PCP may also be required.
Once the preadmission medication regimen is confirmed, it should be entered on a standardized form and placed in a prominent place in the chart. This list should then be compared against the patient's medication orders at admission, throughout the hospital stay, and at discharge.12 The planned action for each of these medications (eg, continue at same dose/route/frequency, substitute) should be made explicit. At discharge, this preadmission list also needs to be compared with the current hospital medications in order to create a coherent set of discharge orders.
Staff responsibilities for obtaining and documenting an accurate list of preadmission medications and reconciling medications at admission, transfer, and discharge should be well defined and based on the resources available at each institution. Redundant work (eg, multiple personnel independently taking a medication history) should be replaced by interdisciplinary communication (ie, a member of the team confirming the accuracy of a list obtained by another member of the team). When discrepancies are found (eg, between preadmission and discharge medications), reconciliation requires correction of unintentional discrepancies and appropriate documentation of intentional changes.60
Because a patient's medications change frequently during the transitions of admission, intrahospital transfer, and discharge, reconciliation is an active and ongoing process that aims to ensure the patient is receiving the correct medication regimen at all times. Reconciliation also allows for a review of the safety and appropriateness of the regimen and discontinuation of any unsuitable or needless medications.61, 62
Finally, a comprehensive list of a patient's medications should be reported to the next service provider when the patient is referred or transferred to another setting, service, practitioner, or level of care within or outside the organization. Avoiding overarching orders such as continue home medications and resume all medications becomes crucial to patient safety during transitions in care. At discharge, physicians should provide patients with a complete list of medications to be taken at home with indications and instructions for administration written in everyday language. Physicians should also highlight the results of medication reconciliation by pointing out any changes from the preadmission regimen, especially medications that are at home but should no longer be taken.
Ultimately, physicians have the duty to ensure that correct and complete medication information is provided. However, to achieve optimal results, physicians should partner with clinical pharmacists when possible. Pharmacists have been formally educated about and are experienced at taking medication histories, which may make them the ideal individuals to interview newly admitted patients about their medication histories.63 Unfortunately, according to a recent survey, pharmacists perform admission drug histories in only 5% of U.S. hospitals and provide drug therapy counseling in just 49% of U.S. hospitals.64 Patients who are elderly, have limited literacy skills, take more than 5 medications daily, or take high‐risk medications such as insulin, warfarin, cardiovascular drugs (including antiarrhythmics), inhalers, antiseizure medications, eye medications, analgesics, oral hypoglycemics, oral methotrexate, and immunosuppressants may require additional counseling or pharmacist involvement for effective reconciliation.10, 65, 66
Although the evidence supporting medication reconciliation is limited, it is convincing enough to support carrying out such reconciliations. In 1 investigation, when the nursing staff obtained and pharmacists verified orders for home medications, the accuracy of admission medication orders increased from 40% to 95%.67 In another work, in which there was pharmacist‐led medication reconciliation, significant discrepancies were found in approximately 25% of patients' medication histories and admission orders.45 In the absence of pharmacist intervention, the authors predicted that 22% of the discrepancies could have caused some form of patient harm during hospitalization and that 59% of the discrepancies might have contributed to an adverse event if the error continued after discharge.45 Others report that orders were changed as a result of reconciliation for 94% of patients being transferred out of the intensive care unit.2 Finally, in a randomized controlled trial of a pharmacist intervention at discharge in which medication reconciliation was the most common action performed, after 30 days preventable ADEs were detected in 11% of control patients and 1% of intervention patients. Medication discrepancy was the cause of half the preventable ADEs in the control group.51
SELF‐CARE RESPONSIBILITIES AND SOCIAL SUPPORT
Compounding the difficulties at discharge are the economic pressures on our health care system, causing patients to be released from the hospital quicker and sicker than ever before.68 The scope of care provided to patients also undergoes a major shift at discharge. Multidisciplinary providers no longer continually review the health status and needs of patients; instead, patients must follow up with their outpatient physician over a period of days to weeks. In the interim, the patients themselves are responsible for administering new medications, participating in physical therapy, and tracking their own symptoms to see if they are worsening. For many patients, sufficient social and family support is not available to help perform these activities effectively. Unfortunately, hospital personnel often inaccurately assess patients' functional status and overestimate patients' knowledge of required self‐care activities.69
Providing Adequate Medical and Social Support
A multidisciplinary discharge planning team can facilitate proper assessments of the social needs of patients and their families (Table 1).7072 This team is often composed of a nurse case manager and a social worker but may also include a physical therapist, an occupational therapist, a pharmacist, and other health care providers. Following discussion with a patient and the patient's family, the team may suggest home health services during the transition home to supplement available medical support,73 or they may decide that discharge to a rehabilitation or skilled nursing facility is more appropriate.
In addition, follow‐up should be arranged prior to discharge. Patients who are given a set appointment are more likely to show up for their follow‐up visits than are those who are simply asked to call and arrange their own visits.74 Typically, follow‐up with the PCP should be conducted within 2 weeks of hospital discharge. However, depending on a patient's functional status, pending test results, and need for medication monitoring or follow‐up testing, this may need to take place sooner. Interestingly, research indicates that follow‐up appointments with the inpatient provider can result in a lower combined rate of readmission and 30‐day mortality.75 Thus, hospitalists may consider operating a hospitalist‐staffed follow‐up clinic, especially for patients without a regular PCP.
Telephone follow‐up conducted a few days after discharge can also be an effective means of bridging the inpatientoutpatient transition.35 Such follow‐up provides a chance to attend to any patient questions, new or concerning symptoms, and medication‐related issues (eg, not filling the discharge medications or difficulty comprehending the new medication regimen).76 A physician, physician assistant, advanced practice nurse, registered nurse, pharmacist, or care manager can effectively carry out this telephone follow‐up. No matter who telephones, the caller must be aware of the patient's recent course of events as well as the care plan decided at discharge. Published evidence indicates that telephone follow‐up fosters patient satisfaction, increases medication adherence, decreases preventable ADEs, and decreases the number of subsequent emergency room visits and hospital readmissions,51, 77, 78 although not all evaluations have demonstrated benefit.79 As with medication histories performed by pharmacists, limited resources may mean that such follow‐up be restricted to those patients at highest risk for readmission.
Home visits may be appropriate for certain patient populations, such as the frail elderly.80 Home visits enable a patient's daily needs and safety (eg, fall risk) to be assessed. They can also be a means of assessing medication safety and adherence by reviewing all prescription and over‐the‐counter products in the household.81 Close follow‐up of at‐risk or elderly patients after discharge can help to minimize hospital readmission and total health care costs.4, 8285
INEFFECTIVE PHYSICIANPATIENT COMMUNICATION
Physicianpatient communication is fundamental to the practice of medicine and is crucially important at discharge. However, several studies have demonstrated a disconnect between physician information giving and patient understanding.76, 9690 When providing instructions, physicians commonly use medical jargon and attempt to cover a wealth of information in a limited amount of time.69, 87 They also tend to rely on verbal instructions and fail to provide supplementary audiovisual materials (eg, educational handouts or videos) that could aid patient comprehension. Physicians may not point out important self‐care tasks that patients should carry out at home. The entire interaction may be rushed or seem rushed. Moreover, when physicians solicit questions from patients, they may only allow for yes/no responses by using statements like Any questions? or Do you have any questions? that make it easy for patients to simply respond, No. The encounter usually comes to an end without true confirmation of a patient's level of understanding or assessment of a patient's ability to perform the self‐care activities and medication management required on returning home.81
Adding to the challenges of effective physicianpatient communication is the large number of adult Americans (more than 90 million) who have limited functional literacy skills.91, 92 Such patients typically have difficulty reading and understanding medical instructions, medication labels, and appointment slips.9396 Not surprisingly, patients with limited literacy skills know less about their chronic illnesses and how to manage their diseases.97 Having low literacy is also linked to increased use of emergency department services, a higher risk of hospitalization, and higher health care costs.9799 Patients with limited English proficiency have similar or even greater challenges and also have longer stays in the hospital.100
More Effective PhysicianPatient Communication
Discharge counseling should concentrate on the few key points that are of the greatest interest and the most importance to patients: major diagnoses, medication changes, dates of follow‐up appointments, self‐care instructions, and who to contact if problems develop (Table 1).101 Furthermore, these key instructions should be reinforced by other hospital staff, including nurses and pharmacists. For common conditions (eg, high‐volume cardiac procedures), offering standardized audiovisual instructions can be both efficient and worthwhile if used in conjunction with questionanswer sessions.102 In the event that physicians and hospital staff cannot fluently communicate in a patient's language, it is essential to engage trained interpreters, not rely on rudimentary language skills, the patient's family, or other ad hoc ways to communicate.103
Because patients are unlikely to fully remember verbal instructions at discharge, it is helpful to provide patients and family members with written materials to take home in order to reinforce important self‐care instructions.76, 87 These materials, written at a 5th‐ to 8th‐grade reading level, should outline key information in a simple format with little or no medical jargon. Illustrated materials are often better comprehended and subsequently remembered by patients.104, 105 If preprinted illustrated materials are not on hand, then physicians can convey key points by drawing simple pictures.
Confirming patient comprehension with the teach‐back method is perhaps the most important step in effectively communicating discharge instructions.106 With this method, patients are asked to repeat back what they understand from the discharge instructions. Application of this simple technique is advocated as one of the most effective means of improving patient safety.107, 108 Patients should also be asked to demonstrate any new self‐care tasks that they will be required to carry out at home, such as using an inhaler or administering a subcutaneous injection.
Last, The Joint Commission recently created a National Patent Safety Goal to encourage the active involvement of patients and their families in the patient's own care.12 This charge requires that physicians offer ample time for patients and their family members to ask questions. Physicians should avoid questions with yes/no responses and instead invite patient and family member questions in a more open‐ended manner (eg, What questions do you have?) to help ensure comprehension and comfort with the care plan.
CONCLUSIONS
The transition from hospital to home is a vulnerable period of discontinuity and potential adverse events. Hospitalists and other inpatient providers should not view discharge as an end to their obligation to patients but rather should attempt to promote a safe and efficient transition of care. Hospitalists can play an important role in bridging the gap between inpatient and outpatient care through appropriate discharge planning and effective communication with patients, their family members, and outpatient physicians.
Acknowledgements
The authors thank Marra Katz for her editorial assistance in the preparation of this manuscript
- .Whither continuity of care?N Engl J Med.1999;340:1362–1363.
- ,,, et al.Medication reconciliation: a practical tool to reduce the risk of medication errors.J Crit Care.2003;18(4):201–205.
- ,,,.Influence of hospitalization on drug therapy in the elderly.J Am Geriatr Soc.1989;37:679–683.
- ,,,,,.Preparing patients and caregivers to participate in care delivered across settings: the Care Transitions Intervention.J Am Geriatr Soc.2004;52:1817–1825.
- ,,.Predictors of elder and family caregiver satisfaction with discharge planning.J Cardiovasc Nurs.2000;14(3):76–87.
- ,.Discharge planning and continuity of care for aged people: indicators of satisfaction and implications for practice.Aust J Adv Nurs.1998;16(1):7–13.
- ,,,.Medical errors related to discontinuity of care from an inpatient to an outpatient setting.J Gen Intern Med.2003;18:646–651.
- ,,.Assessing the quality of preparation for posthospital care from the patient's perspective: the care transitions measure.Med Care.2005;43(3):246–255.
- ,,, et al.Adverse events among medical patients after discharge from hospital.CMAJ.2004;170:345–349.
- ,,,,.The incidence and severity of adverse events affecting patients after discharge from the hospital.Ann Intern Med.2003;138:161–167.
- ,,,,.Adverse drug events occurring following hospital discharge.J Gen Intern Med.2005;20:317–323.
- The Joint Commission. Joint Commission National Patient Safety Goals. Available at: http://www.jointcommission.org/PatientSafety/NationalPatientSafetyGoals/. Accessed July 17,2006.
- ,,, et al.Transition of care for hospitalized elderly patients ‐ Development of a discharge checklist for hospitalists.J Hosp Med.2006;1:354–360.
- ,,,,,.Deficits in communication and information transfer between hospital‐based and primary care physicians: implications for patient safety and continuity of care.JAMA.2007;297:831–841.
- Agency for Healthcare Research and Quality. National Guideline Clearinghouse. Available at: http://www.guideline.gov. Accessed January 3,2007.
- ,.The emerging role of “hospitalists” in the American health care system.N Engl J Med.1996;335:514–517.
- .Hospitalists in the United States—mission accomplished or work in progress?N Engl J Med.2004;350:1935–1936.
- .The evolution of the hospitalist model in the United States.Med Clin North Am2002;86(4):687–706.
- ,.The hospitalist movement 5 years later.JAMA.2002;287:487–494.
- Society of Hospital Medicine. Growth of hospital medicine nationwide. Available at: http://www.hospitalmedicine.org/Content/NavigationMenu/Media/GrowthofHospitalMedicineNationwide/Growth_of_Hospital_M.htm. Accessed July 17,2006.
- ,,,,.Quality assessment of discharge letters in a French university hospital.Int J Health Care Qual Assur Inc Leadersh Health Serv.1998;11(2‐3):90–95.
- ,,.Dissemination of discharge summaries. Not reaching follow‐up physicians.Can Fam Physician.2002;48:737–742.
- ,.Quality assessment of a discharge summary system.CMAJ.1995;152:1437–1442.
- ,,,.Primary care physician attitudes regarding communication with hospitalists.Am J Med.2001;111(9B):15S–20S.
- ,,,.Effect of discharge summary availability during post‐discharge visits on hospital readmission.J Gen Intern Med.2002;17(3):186–192.
- .Study of discharge communications from hospital doctors to an inner London general practice.J R Coll Gen Pract.1987;37:494–495.
- ,,, et al.Patient safety concerns arising from test results that return after hospital discharge.Ann Intern Med.2005;143(2):121–128.
- ,,.Communication between general practitioners and consultants: what should their letters contain?BMJ1992;304(6830):821–824.
- ,,,.Written communication from specialists to general practitioners in cancer care. What are the expectations and how are they met?Scand J Prim Health Care.1998;16(3):154–159.
- ,.Improving the continuity of care between general practitioners and public hospitals.Med J Aust.1994;161:656–659.
- ,.Towards better discharge summaries: brevity and structure.West Engl Med J.1991;106(2):40–41,55.
- ,,.Content of a discharge summary from a medical ward: views of general practitioners and hospital doctors.J R Coll Physicians Lond.1995;29:307–310.
- ,.What is necessary for high‐quality discharge summaries?Am J Med Qual.1999;14(4):160–169.
- The Joint Commission.Standard IM.6.10. Hospital Accreditation Standards.Oakbrook Terrace, IL:The Joint Commission;2006:338–340.
- .The importance of postdischarge telephone follow‐up for hospitalists: a view from the trenches.Am J Med.2001;111(9B):43S–44S.
- ,,,.The quality of communication between hospitals and general practitioners: an assessment.J Qual Clin Pract.1998;18:241–247.
- ,,,.Dictated versus database‐generated discharge summaries: a randomized clinical trial.CMAJ.1999;160:319–326.
- ,,,.Standardized or narrative discharge summaries. Which do family physicians prefer?Can Fam Physician.1998;44:62–69.
- ,.Moving from information transfer to information exchange in health and health care.Soc Sci Med.2003;56:449–464.
- ,,, et al.The costs of adverse drug events in hospitalized patients.Adverse Drug Events Prevention Study Group.JAMA.1997;277:307–311.
- ,,, et al.Systems analysis of adverse drug events.ADE Prevention Study Group.JAMA.1995;274(1):35–43.
- ,,, et al.Incidence of adverse drug events and potential adverse drug events. Implications for prevention.ADE Prevention Study Group.JAMA.1995;274(1):29–34.
- ,.Medication errors in hospitalized cardiovascular patients.Arch Intern Med.2003;163:1461–1466.
- ,,, et al.Unintended medication discrepancies at the time of hospital admission.Arch Intern Med.2005;165:424–429.
- ,,,,,.Reconciliation of discrepancies in medication histories and admission orders of newly hospitalized patients.Am J Health Syst Pharm.2004;61:1689–1695.
- ,,,,,.Frequency, type and clinical importance of medication history errors at admission to hospital: a systematic review.CMAJ.2005;173:510–515.
- ,,,,.Medication reconciliation in the acute care setting: opportunity and challenge for nursing.J Nurs Care Qual.2005;20(2):95–98.
- ,,.The adverse effects of hospitalization on drug regimens.Arch Intern Med.1991;151:1562–1564.
- ,,,,.Prevalence and cost savings of therapeutic interchange among U.S. hospitals.Am J Health Syst Pharm.2002;59:529–533.
- ,,, et al.Medication reconciliation: are we meeting the requirements?JCOM.2006;13:441–444.
- ,,, et al.Role of pharmacist counseling in preventing adverse drug events after hospitalization.Arch Intern Med.2006;166:565–571.
- ,.Effectiveness of a pharmacist‐acquired medication history in promoting patient safety.Am J Health Syst Pharm.2002;59:2221–2225.
- .Ensuring continuity of care and accuracy of patients' medication history on hospital admission.Am J Health Syst Pharm.2002;59:1054–1055.
- ,,,.Current methods used to teach the medication history interview to doctor of pharmacy students.Am J Pharm Educ.2002;66(Summer):103–107.
- AARP. My Personal Medication Record. Available at: http://assets.aarp.org/www.aarp.org_/articles/learntech/wellbeing/medication‐record.pdf. Accessed October 20,2006.
- AARP. Mi registro de medicacion. Available at: http://assets.aarp.org/www.aarp.org_/articles/health/docs/PersonalMedRecordSP.pdf. Accessed October 20,2006.
- Institute for Safe Medication Practices. Available at: http://www.ismp.org. Accessed September 8,2006.
- ,,.Parents as partners in obtaining the medication history.J Am Med Inform Assoc.2005;12:299–305.
- ,,.Medication history on internal medicine wards: assessment of extra information collected from second drug interviews and GP lists.Pharmacoepidemiology Drug Saf.2003;12:491–498.
- Medication Discrepancy Tool. Available at: http://www.caretransitions.org. Accessed July 28,2005.
- ,,,,,.Updating the Beers criteria for potentially inappropriate medication use in older adults: results of a US consensus panel of experts.Arch Intern Med.2003;163:2716–2724.
- .Using the NO TEARS tool for medication review.BMJ2004;329(7463):434.
- ,,,.Clinical pharmacists and inpatient medical care: a systematic review.Arch Intern Med.2006;166:955–964.
- ,.Clinical pharmacy services, pharmacy staffing, and adverse drug reactions in United States hospitals.Pharmacotherapy.2006;26:735–747.
- ,,, et al.Adverse drug events in ambulatory care.N Engl J Med.2003;348:1556–1564.
- MA Coalition for the Prevention of Medical Errors. Reconciling medications. Recommended practices. Available at: http://www.macoalition.org/documents/RecMedPractices.pdf. Accessed July 27,2005.
- ,.OSF healthcare's journey in patient safety.Qual Manag Health Care.2004;13(1):53–59.
- ,,, et al.Prospective payment system and impairment at discharge. The ‘quicker‐and‐sicker’ story revisited.JAMA.1990;264:1980–1983.
- ,,,,,.Discharge planning: comparison of patients and nurses' perceptions of patients following hospital discharge.Image J Nurs Sch.1996;28(2):143–147.
- ,,,.The Care Transitions Intervention: results of a randomized controlled trial.Arch Intern Med.2006;166:1822–1828.
- ,,,,,.Comprehensive discharge planning with postdischarge support for older patients with congestive heart failure: a meta‐analysis.JAMA.2004;291:1358–1367.
- ,,,.Discharge planning from hospital to home.Cochrane Database Syst Rev.2006;4.
- .Falling through the cracks: challenges and opportunities for improving transitional care for persons with continuous complex care needs.J Am Geriatr Soc.2003;51:549–555.
- .The best way to improve emergency department follow‐up is actually to give the patient a specific appointment.J Gen Intern Med.2006;21:398; author reply398.
- ,,,.Continuity of care and patient outcomes after hospital discharge.J Gen Intern Med.2004;19:624–631.
- ,.Patients' understanding of their treatment plans and diagnosis at discharge.Mayo Clin Proc.2005;80:991–994.
- ,,,.The impact of follow‐up telephone calls to patients after hospitalization.Am J Med.2001;111(9B):26S–30S.
- ,.The impact of clinical pharmacists' consultations on geriatric patients' compliance and medical care use: a randomized controlled trial.Gerontologist.1994;34:307–315.
- ,.Telephone follow‐up, initiated by a hospital‐based health professional, for postdischarge problems in patients discharged from hospital to home.Cochrane Database Syst Rev.2007;1.
- ,,, et al.Geriatric home assessment after hospital discharge.J Am Geriatr Soc.1994;42:1229–1234.
- ,.Uncovering a multitude of sins: medication management in the home post acute hospitalisation among the chronically ill.Aust N Z J Med.1999;29(2):220–227.
- ,,, et al.Comprehensive discharge planning and home follow‐up of hospitalized elders: a randomized clinical trial.JAMA.1999;281:613–620.
- ,,,.The cost‐effectiveness of intensive postdischarge care. A randomized trial.Med Care.1988;26:1092–1102.
- ,,,,.The effects of aftercare on chronic patients and frail elderly patients when discharged from hospital: a systematic review.J Adv Nurs.1998;27:1076–1086.
- ,,,,,.A multidisciplinary intervention to prevent the readmission of elderly patients with congestive heart failure.N Engl J Med.1995;333:1190–1195.
- ,,.Medication education of acutely hospitalized older patients.J Gen Intern Med.1999;14:610–616.
- ,,, et al.Patient‐physician communication at hospital discharge and patients' understanding of the postdischarge treatment plan.Arch Intern Med.1997;157:1026–1030.
- ,,.Patient understanding of commonly used medical vocabulary.J Fam Pract.1987;25:176–178.
- .The understanding of medical terminology used in printed health education materials.Heal Educ J.1979;38:111–121.
- ,,,,.The gap between patient reading comprehension and the readability of patient education materials.J Fam Pract.1990;31:533–538.
- ,,. National Assessment of Adult Literacy (NAAL). A first look at the literacy of America's adults in the 21st century. Available at: http://nces.ed.gov/naal. Accessed May 2,2006.
- ,,,.Adult Literacy in America: A First Look at the Findings of the National Adult Literacy Survey.Washington, DC:National Center for Education Statistics, U.S. Department of Education;1993.
- ,,,.The role of health literacy in patient‐physician communication.Fam Med.2002;34:383–389.
- American Medical Association Council on Scientific Affairs.Health literacy.JAMA.1999;281:552–557.
- National Work Group on Literacy and Health.Communicating with patients who have limited literacy skills.J Fam Pract.1998;46:168–176.
- ,.Communicating with patients who cannot read.N Engl J Med.1997;337:272–274.
- ,,,,.Literacy and health outcomes: a systematic review of the literature.J Gen Intern Med.2004;19:1129–1139.
- ,,.The impact of low health literacy on the medical costs of Medicare managed care enrollees.Am J Med.2005;118:371–377.
- ,,,.Health literacy and the risk of hospital admission.J Gen Intern Med.1998;13:791–798.
- ,,, et al.The effect of English language proficiency on length of stay and in‐hospital mortality.J Gen Intern Med.2004;19(3):221–228.
- ,,, et al.Effects of a structured patient‐centered discharge interview on patients' knowledge about their medications.Am J Med.2004;117:563–568.
- ,,.Effects of a videotape information intervention at discharge on diet and exercise compliance after coronary artery bypass surgery.J Cardiopulm Rehab.1999;19(3):170–177.
- .The impact of medical interpreter services on the quality of health care: a systematic review.Med Care Res Rev.2005;62(3):255–299.
- ,,.Use of pictorial aids in medication instructions: a review of the literature.Am J Health Syst Pharm.2006;63:2391–2397.
- ,,,.The role of pictures in improving health communication: A review of research on attention, comprehension, recall, and adherence.Patient Educ Couns.2006;61(2):173–190.
- ,,, et al.Closing the loop. Physician communication with diabetic patients who have low health literacy.Arch Intern Med.2003;163:83–90.
- National Quality Forum.Safe Practices for Better Healthcare,2003; Washington, DC.
- Shojania KG, Duncan BW, McDonald KM, Wachter RM, eds.Making Healthcare Safer: A Critical Analysis of Patient Safety Practices. Evidence Report No. 43 from the Agency for Healthcare Research and Quality. AHRQ Publication No. 01‐E058;2001.
- .Whither continuity of care?N Engl J Med.1999;340:1362–1363.
- ,,, et al.Medication reconciliation: a practical tool to reduce the risk of medication errors.J Crit Care.2003;18(4):201–205.
- ,,,.Influence of hospitalization on drug therapy in the elderly.J Am Geriatr Soc.1989;37:679–683.
- ,,,,,.Preparing patients and caregivers to participate in care delivered across settings: the Care Transitions Intervention.J Am Geriatr Soc.2004;52:1817–1825.
- ,,.Predictors of elder and family caregiver satisfaction with discharge planning.J Cardiovasc Nurs.2000;14(3):76–87.
- ,.Discharge planning and continuity of care for aged people: indicators of satisfaction and implications for practice.Aust J Adv Nurs.1998;16(1):7–13.
- ,,,.Medical errors related to discontinuity of care from an inpatient to an outpatient setting.J Gen Intern Med.2003;18:646–651.
- ,,.Assessing the quality of preparation for posthospital care from the patient's perspective: the care transitions measure.Med Care.2005;43(3):246–255.
- ,,, et al.Adverse events among medical patients after discharge from hospital.CMAJ.2004;170:345–349.
- ,,,,.The incidence and severity of adverse events affecting patients after discharge from the hospital.Ann Intern Med.2003;138:161–167.
- ,,,,.Adverse drug events occurring following hospital discharge.J Gen Intern Med.2005;20:317–323.
- The Joint Commission. Joint Commission National Patient Safety Goals. Available at: http://www.jointcommission.org/PatientSafety/NationalPatientSafetyGoals/. Accessed July 17,2006.
- ,,, et al.Transition of care for hospitalized elderly patients ‐ Development of a discharge checklist for hospitalists.J Hosp Med.2006;1:354–360.
- ,,,,,.Deficits in communication and information transfer between hospital‐based and primary care physicians: implications for patient safety and continuity of care.JAMA.2007;297:831–841.
- Agency for Healthcare Research and Quality. National Guideline Clearinghouse. Available at: http://www.guideline.gov. Accessed January 3,2007.
- ,.The emerging role of “hospitalists” in the American health care system.N Engl J Med.1996;335:514–517.
- .Hospitalists in the United States—mission accomplished or work in progress?N Engl J Med.2004;350:1935–1936.
- .The evolution of the hospitalist model in the United States.Med Clin North Am2002;86(4):687–706.
- ,.The hospitalist movement 5 years later.JAMA.2002;287:487–494.
- Society of Hospital Medicine. Growth of hospital medicine nationwide. Available at: http://www.hospitalmedicine.org/Content/NavigationMenu/Media/GrowthofHospitalMedicineNationwide/Growth_of_Hospital_M.htm. Accessed July 17,2006.
- ,,,,.Quality assessment of discharge letters in a French university hospital.Int J Health Care Qual Assur Inc Leadersh Health Serv.1998;11(2‐3):90–95.
- ,,.Dissemination of discharge summaries. Not reaching follow‐up physicians.Can Fam Physician.2002;48:737–742.
- ,.Quality assessment of a discharge summary system.CMAJ.1995;152:1437–1442.
- ,,,.Primary care physician attitudes regarding communication with hospitalists.Am J Med.2001;111(9B):15S–20S.
- ,,,.Effect of discharge summary availability during post‐discharge visits on hospital readmission.J Gen Intern Med.2002;17(3):186–192.
- .Study of discharge communications from hospital doctors to an inner London general practice.J R Coll Gen Pract.1987;37:494–495.
- ,,, et al.Patient safety concerns arising from test results that return after hospital discharge.Ann Intern Med.2005;143(2):121–128.
- ,,.Communication between general practitioners and consultants: what should their letters contain?BMJ1992;304(6830):821–824.
- ,,,.Written communication from specialists to general practitioners in cancer care. What are the expectations and how are they met?Scand J Prim Health Care.1998;16(3):154–159.
- ,.Improving the continuity of care between general practitioners and public hospitals.Med J Aust.1994;161:656–659.
- ,.Towards better discharge summaries: brevity and structure.West Engl Med J.1991;106(2):40–41,55.
- ,,.Content of a discharge summary from a medical ward: views of general practitioners and hospital doctors.J R Coll Physicians Lond.1995;29:307–310.
- ,.What is necessary for high‐quality discharge summaries?Am J Med Qual.1999;14(4):160–169.
- The Joint Commission.Standard IM.6.10. Hospital Accreditation Standards.Oakbrook Terrace, IL:The Joint Commission;2006:338–340.
- .The importance of postdischarge telephone follow‐up for hospitalists: a view from the trenches.Am J Med.2001;111(9B):43S–44S.
- ,,,.The quality of communication between hospitals and general practitioners: an assessment.J Qual Clin Pract.1998;18:241–247.
- ,,,.Dictated versus database‐generated discharge summaries: a randomized clinical trial.CMAJ.1999;160:319–326.
- ,,,.Standardized or narrative discharge summaries. Which do family physicians prefer?Can Fam Physician.1998;44:62–69.
- ,.Moving from information transfer to information exchange in health and health care.Soc Sci Med.2003;56:449–464.
- ,,, et al.The costs of adverse drug events in hospitalized patients.Adverse Drug Events Prevention Study Group.JAMA.1997;277:307–311.
- ,,, et al.Systems analysis of adverse drug events.ADE Prevention Study Group.JAMA.1995;274(1):35–43.
- ,,, et al.Incidence of adverse drug events and potential adverse drug events. Implications for prevention.ADE Prevention Study Group.JAMA.1995;274(1):29–34.
- ,.Medication errors in hospitalized cardiovascular patients.Arch Intern Med.2003;163:1461–1466.
- ,,, et al.Unintended medication discrepancies at the time of hospital admission.Arch Intern Med.2005;165:424–429.
- ,,,,,.Reconciliation of discrepancies in medication histories and admission orders of newly hospitalized patients.Am J Health Syst Pharm.2004;61:1689–1695.
- ,,,,,.Frequency, type and clinical importance of medication history errors at admission to hospital: a systematic review.CMAJ.2005;173:510–515.
- ,,,,.Medication reconciliation in the acute care setting: opportunity and challenge for nursing.J Nurs Care Qual.2005;20(2):95–98.
- ,,.The adverse effects of hospitalization on drug regimens.Arch Intern Med.1991;151:1562–1564.
- ,,,,.Prevalence and cost savings of therapeutic interchange among U.S. hospitals.Am J Health Syst Pharm.2002;59:529–533.
- ,,, et al.Medication reconciliation: are we meeting the requirements?JCOM.2006;13:441–444.
- ,,, et al.Role of pharmacist counseling in preventing adverse drug events after hospitalization.Arch Intern Med.2006;166:565–571.
- ,.Effectiveness of a pharmacist‐acquired medication history in promoting patient safety.Am J Health Syst Pharm.2002;59:2221–2225.
- .Ensuring continuity of care and accuracy of patients' medication history on hospital admission.Am J Health Syst Pharm.2002;59:1054–1055.
- ,,,.Current methods used to teach the medication history interview to doctor of pharmacy students.Am J Pharm Educ.2002;66(Summer):103–107.
- AARP. My Personal Medication Record. Available at: http://assets.aarp.org/www.aarp.org_/articles/learntech/wellbeing/medication‐record.pdf. Accessed October 20,2006.
- AARP. Mi registro de medicacion. Available at: http://assets.aarp.org/www.aarp.org_/articles/health/docs/PersonalMedRecordSP.pdf. Accessed October 20,2006.
- Institute for Safe Medication Practices. Available at: http://www.ismp.org. Accessed September 8,2006.
- ,,.Parents as partners in obtaining the medication history.J Am Med Inform Assoc.2005;12:299–305.
- ,,.Medication history on internal medicine wards: assessment of extra information collected from second drug interviews and GP lists.Pharmacoepidemiology Drug Saf.2003;12:491–498.
- Medication Discrepancy Tool. Available at: http://www.caretransitions.org. Accessed July 28,2005.
- ,,,,,.Updating the Beers criteria for potentially inappropriate medication use in older adults: results of a US consensus panel of experts.Arch Intern Med.2003;163:2716–2724.
- .Using the NO TEARS tool for medication review.BMJ2004;329(7463):434.
- ,,,.Clinical pharmacists and inpatient medical care: a systematic review.Arch Intern Med.2006;166:955–964.
- ,.Clinical pharmacy services, pharmacy staffing, and adverse drug reactions in United States hospitals.Pharmacotherapy.2006;26:735–747.
- ,,, et al.Adverse drug events in ambulatory care.N Engl J Med.2003;348:1556–1564.
- MA Coalition for the Prevention of Medical Errors. Reconciling medications. Recommended practices. Available at: http://www.macoalition.org/documents/RecMedPractices.pdf. Accessed July 27,2005.
- ,.OSF healthcare's journey in patient safety.Qual Manag Health Care.2004;13(1):53–59.
- ,,, et al.Prospective payment system and impairment at discharge. The ‘quicker‐and‐sicker’ story revisited.JAMA.1990;264:1980–1983.
- ,,,,,.Discharge planning: comparison of patients and nurses' perceptions of patients following hospital discharge.Image J Nurs Sch.1996;28(2):143–147.
- ,,,.The Care Transitions Intervention: results of a randomized controlled trial.Arch Intern Med.2006;166:1822–1828.
- ,,,,,.Comprehensive discharge planning with postdischarge support for older patients with congestive heart failure: a meta‐analysis.JAMA.2004;291:1358–1367.
- ,,,.Discharge planning from hospital to home.Cochrane Database Syst Rev.2006;4.
- .Falling through the cracks: challenges and opportunities for improving transitional care for persons with continuous complex care needs.J Am Geriatr Soc.2003;51:549–555.
- .The best way to improve emergency department follow‐up is actually to give the patient a specific appointment.J Gen Intern Med.2006;21:398; author reply398.
- ,,,.Continuity of care and patient outcomes after hospital discharge.J Gen Intern Med.2004;19:624–631.
- ,.Patients' understanding of their treatment plans and diagnosis at discharge.Mayo Clin Proc.2005;80:991–994.
- ,,,.The impact of follow‐up telephone calls to patients after hospitalization.Am J Med.2001;111(9B):26S–30S.
- ,.The impact of clinical pharmacists' consultations on geriatric patients' compliance and medical care use: a randomized controlled trial.Gerontologist.1994;34:307–315.
- ,.Telephone follow‐up, initiated by a hospital‐based health professional, for postdischarge problems in patients discharged from hospital to home.Cochrane Database Syst Rev.2007;1.
- ,,, et al.Geriatric home assessment after hospital discharge.J Am Geriatr Soc.1994;42:1229–1234.
- ,.Uncovering a multitude of sins: medication management in the home post acute hospitalisation among the chronically ill.Aust N Z J Med.1999;29(2):220–227.
- ,,, et al.Comprehensive discharge planning and home follow‐up of hospitalized elders: a randomized clinical trial.JAMA.1999;281:613–620.
- ,,,.The cost‐effectiveness of intensive postdischarge care. A randomized trial.Med Care.1988;26:1092–1102.
- ,,,,.The effects of aftercare on chronic patients and frail elderly patients when discharged from hospital: a systematic review.J Adv Nurs.1998;27:1076–1086.
- ,,,,,.A multidisciplinary intervention to prevent the readmission of elderly patients with congestive heart failure.N Engl J Med.1995;333:1190–1195.
- ,,.Medication education of acutely hospitalized older patients.J Gen Intern Med.1999;14:610–616.
- ,,, et al.Patient‐physician communication at hospital discharge and patients' understanding of the postdischarge treatment plan.Arch Intern Med.1997;157:1026–1030.
- ,,.Patient understanding of commonly used medical vocabulary.J Fam Pract.1987;25:176–178.
- .The understanding of medical terminology used in printed health education materials.Heal Educ J.1979;38:111–121.
- ,,,,.The gap between patient reading comprehension and the readability of patient education materials.J Fam Pract.1990;31:533–538.
- ,,. National Assessment of Adult Literacy (NAAL). A first look at the literacy of America's adults in the 21st century. Available at: http://nces.ed.gov/naal. Accessed May 2,2006.
- ,,,.Adult Literacy in America: A First Look at the Findings of the National Adult Literacy Survey.Washington, DC:National Center for Education Statistics, U.S. Department of Education;1993.
- ,,,.The role of health literacy in patient‐physician communication.Fam Med.2002;34:383–389.
- American Medical Association Council on Scientific Affairs.Health literacy.JAMA.1999;281:552–557.
- National Work Group on Literacy and Health.Communicating with patients who have limited literacy skills.J Fam Pract.1998;46:168–176.
- ,.Communicating with patients who cannot read.N Engl J Med.1997;337:272–274.
- ,,,,.Literacy and health outcomes: a systematic review of the literature.J Gen Intern Med.2004;19:1129–1139.
- ,,.The impact of low health literacy on the medical costs of Medicare managed care enrollees.Am J Med.2005;118:371–377.
- ,,,.Health literacy and the risk of hospital admission.J Gen Intern Med.1998;13:791–798.
- ,,, et al.The effect of English language proficiency on length of stay and in‐hospital mortality.J Gen Intern Med.2004;19(3):221–228.
- ,,, et al.Effects of a structured patient‐centered discharge interview on patients' knowledge about their medications.Am J Med.2004;117:563–568.
- ,,.Effects of a videotape information intervention at discharge on diet and exercise compliance after coronary artery bypass surgery.J Cardiopulm Rehab.1999;19(3):170–177.
- .The impact of medical interpreter services on the quality of health care: a systematic review.Med Care Res Rev.2005;62(3):255–299.
- ,,.Use of pictorial aids in medication instructions: a review of the literature.Am J Health Syst Pharm.2006;63:2391–2397.
- ,,,.The role of pictures in improving health communication: A review of research on attention, comprehension, recall, and adherence.Patient Educ Couns.2006;61(2):173–190.
- ,,, et al.Closing the loop. Physician communication with diabetic patients who have low health literacy.Arch Intern Med.2003;163:83–90.
- National Quality Forum.Safe Practices for Better Healthcare,2003; Washington, DC.
- Shojania KG, Duncan BW, McDonald KM, Wachter RM, eds.Making Healthcare Safer: A Critical Analysis of Patient Safety Practices. Evidence Report No. 43 from the Agency for Healthcare Research and Quality. AHRQ Publication No. 01‐E058;2001.
Strategies for a Safe and Effective Resident Sign‐Out
Modern‐day continuity of patient care in teaching hospitals, once remarkably high because of a cadre of sleep‐deprived residents, is now peppered with breaks, each accompanied by the transfer of patient care responsibility from one resident to another; a process often referred to as a handoff. Such transitions have long been a part of medical practice but have recently received increased attention because of restrictions in the duty hours of house staff. In July 2003 the Accreditation Council for Graduate Medical Education (ACGME) mandated reduced duty hours for all trainees in hopes of improving resident education and well‐being and patient safety.1 In fact, some studies have shown improved resident well‐being2 and fewer medical errors with reductions in duty hours,3, 4 but the growing consensus about the negative consequences of resident fatigue on patient safety has been accompanied by parallel concerns about the potential for information loss with each break in the continuity of care.5, 6
Although the tradeoff of increased discontinuity of care for fewer hours worked is sometimes characterized as an unintended consequence of duty hour regulations, it is in fact predictable and essential. As individuals work fewer hours, discontinuity must necessarily increase (assuming 24‐hour coverage).7 The extent to which this occurs may vary, but the link is consistent. At the University of California, San Francisco (UCSF), for example, we found that compliance with new duty hour requirements for internal medicine resulted in an average of 15 handoffs per patient during a 5‐day hospitalization. Each individual intern was involved in more than 300 handoffs in an average month‐long rotation, an increase of 40% since system changes were introduced to decrease duty hours. We found similar increases at Brigham and Women's Hospital (BWH) and the University of Chicago. Because U.S. teaching hospitals care for more than 6 million patients each year,8 the impact of these handoffs on the quality and efficiency of care is tremendous.
Discontinuity of care is currently managed by sign‐out, or the transfer of patient information from one physician to another. Recognizing the importance of information transfer at these vulnerable transition times for patients, the Joint Commission on Accreditation of Hospital Organizations (JCAHO) issued the 2006 National Patient Safety Goal 2E: Implement a standardized approach to hand off communications, including an opportunity to ask and respond to questions.9 Hospitals have little data to draw on to determine how to comply with this mandate and even less data to guide them in how to achieve its intended goals of improving communication and thus patient safety.
In an effort to better understand sign‐outs and ways to improve this process for house staff on in‐patient services, we reviewed data from the fields of aviation, communications, systems engineering, and human factors research, and we also searched the medical literature using key words pass‐off, handoff, sign‐out, duty hours, work hours, and discontinuity of care and MeSH headings Continuity of Patient Care Internship and Residency/*organization & administration, Personnel Staffing and Scheduling/*organization & administration, and Quality of Health Care. We also searched the websites of the Agency of Healthcare Quality and Research and the National Patient Safety Foundation. On the basis of these reviews, our experiences as hospitalist medical educators organizing resident sign‐out efforts at the University of California, San Francisco, the University of Chicago, and Brigham and Women's Hospital, and our efforts leading national training sessions on sign‐outs at the Society of General Internal Medicine (2004 and 2005), the Society of Hospital Medicine (2004), and the Association of Program Directors in Internal Medicine (2005, 2006), we propose a set of best practices regarding the content and process of sign‐out in an effort to improve communication between residents caring for hospitalized patients, assist programs in building safe and effective sign‐out systems, and improve the quality of patient care.
Effects of Discontinuity on Patient Safety
Research on the effects of discontinuity of care, although limited, suggests it has a negative impact on patient safety. In a study that investigated the institution of code 405 (the regulation that reduced duty hours in New York State), researchers found that the presumed increase in discontinuity with decreased duty hours resulted in delayed test ordering and an increased number of hospital complications.10 Another study found that the number of potentially preventable adverse events doubled when patients were under the care of a physician from a nonprimary team (eg, the cross‐covering intern).11 Studies have also linked resident discontinuity with longer length of stay, increased laboratory testing, and increased medication errors.12, 13
Managing Discontinuity: Sign‐Out as the Means of Information Transfer
In theory, more effective sign‐out systems should mitigate the potential for patient harm, but there is little in the literature describing current effective sign‐out practices or the best ways to design and implement such systems in the health care field. Examining information transfer mechanisms used in fields outside health care can assist in developing these systems.
Information Transfer in Other Industries
Although there is a paucity of data on sign‐out in the medical literature, information transfer has been the subject of substantial research in other industries in which safety depends on effective communication.
Aviation, for example, created systems and processes to improve handoff communication in response to accidents linked to failures in information transfer. One example, the 1977 collision of 2 747s on an airport runway in Tenerife, the Canary Islands, occurred after a garbled transmission from an air traffic controller to the cockpit of one of the aircraft. It was determined that a culture of adherence to a steep hierarchy prevented subordinates from questioning the captain's mistaken certainty that a runway was clear,14 an erroneous belief that was the basis for his decision to continue the aircraft on its course, resulting in its collision with the other airplane.
Subsequently, commercial aviation designed systems that standardized and formalized the process of information transfer and improved teamwork and coordination. These interventions were developed on the basis of detailed observations of cockpit interactions, reviews of communication errors, and focus groups.15 Because of these efforts, today's pilots use standardized checklists to transfer information content, communicate at designated times in specific undistracted environments, and use standard language and read‐backs to enhance understanding.16 The result has been a remarkable decrease in the risk of aviation crashes, one that most experts attribute in large part to these efforts to improve communication.17
Observation of how communication occurs in other high‐risk industries has informed the arena of effective information transfer. For example, direct observation of information transfer at NASA, in nuclear power plants, and in the railway industry identified specific strategies for effective handoffs/sign‐outs such as standardizing the information transferred, ensuring information is up to date, limiting interruptions, and having a structured face‐to‐face verbal interchange.18
Other strategies noted to be effective in diminishing errors are the use of a standardized phonetic alphabet to ensure that information is correctly heard and understood4 and having interactive verbal communication occur at a whiteboard.19
Information Transfer in Health Care
Those in the discipline of nursing have vast experience in the transfer of patient care information. The sign‐out process employed by nurses includes face‐to‐face discussions, typed information, and, most commonly, taped verbal communication.20 Interestingly, this process has not been subject to detailed scrutiny, and there is little information in the literature about best practices in sign‐out. Most articles in the literature on nursing handoffs are ethnographic descriptions of patient care responsibilities,21 on the basis of which, the authors advocate standardization of the information to be transferred, formalization of the channel used to communicate, and attention to increasing a culture of professionalism during sign‐out in order to improve efficiency.20, 22
There is little in the literature on transfer of care among physicians. Improvements in sign‐out have been suggested as part of broad strategies, such as increased training and information technology support,4, 7, 23, 24 and specific strategies have been offered such as managing barriers to communication, including specific types of data when transferring care,25 and involving nurses and senior physicians in sign‐outs.26 Specific outcomes data in this area have focused primarily on the use of computerized systems to improve information transfer. For example, the use of a computerized sign‐out system at Brigham and Women's Hospital (BWH), linked to the hospital's information system to ensure up‐to‐date information on patient demographics, medications, and laboratory values, has resulted in fewer errors,27 as have other similar systems.28 At the University of Washington, use of a similarly linked computerized sign‐out system resulted in fewer patients being missed on rounds and improvement in the quality of sign‐out and continuity of care according to resident self‐reports.29 Unfortunately, fewer than 10% of hospitals have such integrated hospitalwide information systems to support the sign‐out function.30
It has been noted that verbal communication, in concert with advances in technological communication, is important in information transfer in health care,18, 31 especially in emergent or urgent conditions.32 For example, eliminating the phoned‐in report from the lab to the ER and replacing it with delivery by an electronic reporting system lacking verbal communicationresulted in 45% of emergent lab results going unchecked.32 Structured verbal communication tools have been efficacious in improving information transfer outside the formal sign‐outfor example, read‐backs, which reduced errors in the reporting of critical laboratory values,33 and the SBAR (situation, background, assessment, recommendation) tool (designed to frame the transfer of critical information), which improved physician and nurse patient care information transfer in the in‐patient setting of the Kaiser Permanente health system.34
In focus groups and in response to formal and informal surveys, residents at our 3 sites suggested inclusion of the following information, provided in writing and orally, to improve sign‐outs: up‐to‐date administrative information (eg, room number, primary care physician); patient's recent cognitive or cardiopulmonary status; problems the patient had already experienced and treatments previously tried, both successfully and unsuccessfully; patient's code status and discussions on level of care; test results or consultation recommendations that were likely to come back while covering the patient and what to do with the results; and relevant psychosocial information (eg, complex family dynamics).35
The Current Practice of Sign‐Out
In examining sign‐outs at our 3 institutions, we found them to be unstructured and unstandardized. From discussion with faculty participating in national workshops on sign‐out, we found that most sign‐outs are conducted by interns, usually with little or no formal training. Templates, checklists, or other methods to standardize the content of the information transferred were rarely used.
We also noted that the vehicle for written sign‐out is highly variable. At UCSF, different residency training programs used a variety of modalities for written sign‐outs, including index cards, Excel spreadsheets, Word documents, and loose sheets of paper. Recently, the UCSF Department of Medicine designed a simple database (on Filemaker Pro) that allows members of the house staff to update their sign‐out information, share it with other house staff and nurses, and access it at locations throughout the hospital (Fig. 1). Although this database is not yet linked to the hospital information system (planned for 2006), anecdotally resident satisfaction with sign‐out has vastly improved since its implementation. The cost of design and implementation was approximately $10,000. At the University of Chicago, interns used Microsoft Word to create sign‐out sheets containing patient summaries to transfer information. However, during structured interviews, 95% of the interns reported that these sheets were frequently lost or misplaced.7 Although medicine residents at BWH use a computerized system to produce sign‐out sheets, this system did not guarantee complete and structured information. For example, a survey at BWH found that 56% of cross‐covering residents said that when paged about a patient overnight, the relevant information needed to care for that patient was present less than half the time; and 27% of residents reported being paged more than 3 times in the previous 2 weeks about a test result or consultant recommendation that they did not know was pending.36
The process of sign‐out also varied across disciplines and institutions. From our experiences at our sites and at the sites of faculty nationally, we found limited standardization about whether sign‐out was verbal, the data transmitted, and the setting in which it was transmitted. In fact, at UCSF most residents signed out verbally on the fly, wherever and whenever they could find the cross‐coverage intern. At BWH, only 37% of residents said that sign‐out occurred in a quiet place most of the time, and only 52% signed out on every patient both orally and in writing.36 At the University of Chicago, the sign‐out process was characterized by outright failures in communication because of omission of needed information (ie, medications, active or anticipated medical problems, etc.) or by failure‐prone communication (ie, lack of face‐to‐face communication, illegible writing). These failures often led to uncertainty in making patient care decisions, potentially resulting in inefficient or suboptimal care.35
Strategies for Safe and Effective Sign‐Out
Given the current landscape of variability in sign‐outs, the recognition that information lost during sign‐out may result in harm to patients, and evidence of improvements in information transfer in areas outside health care, we aimed to develop mechanisms to improve the sign‐out process for residents working in a hospital setting. These strategies are based on our review of the existing literature supplemented by our experiences at our 3 institutions.
Content of Sign‐Out
The elements of content necessary for safe and effective sign‐out can be divided into 5 broad categories (Table 1), contained in the mnemonic ANTICipate: Administrative information, New clinical information, specific Tasks to be performed, assessment of severity of Illness, and Contingency plans or anticipated problems (Table 1, Fig. 2).
| ✓ Administrative data |
| □ Patient name, age, sex |
| □ Medical record number |
| □ Room number |
| □ Admission date |
| □ Primary inpatient medical team, primary care physician |
| □ Family contact information |
| ✓ New information (clinical update) |
| □ Chief complaint, brief HPI, and diagnosis (or differential diagnosis) |
| □ Updated list of medications with doses, updated allergies |
| □ Updated, brief assessment by system/problem, with dates |
| □ Current baseline status (eg, mental status, cardiopulmonary, vital signs, especially if abnormal but stable) |
| □ Recent procedures and significant events |
| ✓ Tasks (what needs to be done) |
| □ Specific, using if‐then statements |
| □ Prepare cross‐coverage (eg, patient consent for blood transfusion) |
| □ Alert to incoming information (eg, study results, consultant recommendations), and what action, if any, needs to be taken during the cross‐coverage |
| ✓ Illness |
| □ Is the patient sick? |
| ✓ Contingency planning/Code status |
| □ What may go wrong and what to do about it |
| □ What has or has not worked before (eg, responds to 40 mg IV furosemide) |
| □ Difficult family or psychosocial situations |
| □ Code status, especially recent changes or family discussions |

Several general points about this list should be noted. First, the sign‐out content is not meant to replace the chart. The information included reflects the goal of a sign‐out, namely, to provide enough information to allow for a safe transition in patient care. Information we believe is not essential to the sign‐out includes: a complete history and physical exam from the day of admission, a list of tasks already completed, and data necessary only to complete a discharge summary.
Sign‐out must be also be a closed loopthe process of signing in is as important as the process of signing out. This usually entails members of the primary team obtaining information from the cross‐covering physician when they resume care of the patient. The information conveyed in this case is different and includes details on events during cross‐coverage such as: 1) time called to assess patient; 2) reason for call; 3) a brief assessment of the patient, including vital signs; 4) actions taken, for example, medications given and tests ordered; and 5) rationale for those actions. Some of this information may also be included in the chart as an event note (see Fig. 3).
The Vehicle for Sign‐Out
We recommend a computer‐assisted vehicle for patient information transfer. Ideally, this would be linked to the hospital information system to ensure accurate and up‐to‐date information Easy access to the computerized sign‐out is essential (eg, using a hospitalwide computer system, shared hard drive service, intranet, or PDA linked to the computer system), and it should be customizable for the varied needs of different services and departments. The system should have templates to standardize the content of sign‐out, contain robust backup systems, and be HIPAA compliant (ie, restrict access to required health care personnel). However, the perfect should not be the enemy of the good: systems that do not meet these criteria may still help to protect patients by providing legible, predictable, and accessible information.
Sign‐Out Processes
Verbal communication.
Although electronic solutions can facilitate the standardization of written content, face‐to‐face verbal communication adds additional value.19 We recommend that each patient be reviewed separately. Identification of each patient verbally ensures that those engaged in the sign‐out are discussing the same patient. Reiterating the major medical problems gives a snapshot of the patient and frames the sign‐out. The to‐do list, the list of tasks that the cross‐cover resident needs to complete during cross coverage, should be specific and articulated as if, then statements (eg, if the urine output is less than 1 L, then give 40 mg of IV furosemide). The receiver of sign‐out should read back to the person giving the sign‐out each item on the to‐do list (eg, So, I should check the ins and outs at about 10:00 pm, and give 40 of furosemide if the patient is not 1 L negative, right?).
Anticipated problems should also be verbally communicated to promote a dialogue. Points that cannot be adequately transferred in the written sign‐out are particularly important to transmit verbally. Examples include previous code discussions, unusual responses to treatment, and psychosocial and family issues. When delivering verbal sign‐out, it is important to consider the a priori knowledge of the recipient. How much knowledge about a patient is already shared between the outgoing and incoming physicians and the level of experience of the physicians may affect the extent to which information needs be transmitted.37 For instance, 2 experienced physicians who already have been working to cover the same patient will likely have an abbreviated discussion, in contrast to the lengthier sign‐out necessary if the outgoing and incoming physicians are interns, and the incoming intern has no prior knowledge of the patient. Similarly, it is likely the level of detail transmitted will need to be greater during a permanent transfer of patient care (ie, at the end of a resident's rotation) than during a brief, temporary transition (eg, overnight coverage).
The challenges of a busy inpatient service may preclude a complete verbal sign‐out for all patients; we contend, though, it is best to use these practices to the extent possible, especially for patients with treatment plans in flux, those whose status is tenuous, and those who have anticipated changes in status during cross‐coverage. One tool that may be effectively used in signing out such patients is the SBAR tool, according to which a brief description of the situation is given, followed by the background and the physician's specific assessment and complete recommendation.38 For example, a resident signing out might begin by stating, I have 18 patients to sign‐out to you. I'm going to describe 6 active patients in detail. Twelve others are fairly stable, and I will give you basic information about them, and the details are in the written sign‐out. One patient has a plan in flux. The situation is Mr. S. is having trouble breathing, the background is that he has both CHF and COPD, my assessment is that this is more cardiac than pulmonary, and I recommend that you see him first and discuss with the cardiology consultant. Using the tools described here (Table 2), a sign‐out of 15 patients of variable acuity could be verbally signed out in less than 10 minutes.
| ✓ WHO should participate in the sign‐out process? |
| □ Outgoing clinician primarily responsible for patient's care |
| □ Oncoming clinician who will be primarily responsible for patient's care (avoid passing this task to someone else, even if busy) |
| □ Consider supervision by experienced clinicians if early in training |
| ✓ WHAT content needs to be verbally communicated? |
| Use situation briefing model, or SBAR, technique: |
| □ SituationIdentify each patient (name, age, sex, chief complaint) and briefly state any major problems (active and those that may become active during cross‐coverage). |
| □ Backgroundpertinent information relevant to current care (eg, recent vitals and/or baseline exam, labs, test results, etc). |
| □ Assessmentworking diagnosis, response to treatment, anticipated problems during cross‐coverage including anything not adequately described using written form (eg, complex family discussions). |
| □ Recommendationto‐do lists and if/then recommendations. |
| ✓ WHERE should sign‐out occur? |
| □ Designated room or place for sign‐out (eg, avoid patient areas because of HIPPA requirements) |
| □ Proper lighting |
| □ Avoid excessive noise (eg, high‐traffic areas) |
| □ Minimize disruptions (eg, hand over pagers) |
| □ Ensure systems support for sign‐out (eg, computers, printer, paper, etc.) |
| ✓ WHEN is the optimal time for sign‐out? |
| □ Designated time when both parties can be present and pay attention (eg, beware of clinic, other obligations) |
| □ Have enough time for interactive questions at the end (eg, avoid rush at the end of the shift) |
| ✓ HOW should verbal communication be performed? |
| □ Face to face, allowing for questions |
| □ Verbalize data in the same order for each patient at each sign‐out |
| □ Read back all to‐do items |
| □ Adjust length and depth of review according to baseline knowledge of parties involved and type of transition in care |
The Environment and setting.
To improve the setting of sign‐out, we recommend: a designated space that is well lit, quiet, and respects patient confidentiality and a designated time when sign‐out will occur. To limit known distractions and interruptions39, 40 in the hospital, we also recommend the outgoing physician hand off his or her pager to someone else during sign‐out. Also key to an environment conducive to information transfer is ensuring adequate computer support for electronic sign‐out and access to updated clinical information.
Organizational culture and institutional leadership.
The way residents transfer patient care information reflects the culture of the institution. Changing the culture to one in which interactive questioning is valued regardless of position in the hierarchy has been shown to reduce errors in aviation.41 Educating residents on the impact of sign‐outs on patient care is a first step toward improving the culture of sign‐out. Resident commitment to the new sign‐out can be gained by engaging residents in development of the process itself. To cement these changes into the culture, practitioners at all levels should be aware of and support the new system. The role of an institution's leaders in achieving these changes cannot be overlooked. Leaders will need to be creative in order to support sign‐out as described within the obvious constraints of money, time, personnel, and space. Gaining institutional buy‐in can start with heightening the awareness of leaders of the issues surrounding sign‐out, including patient safety, resident efficiency, and the financial impact of discontinuity. Ongoing evaluation of efforts to improve sign‐out is also crucial and can be accomplished with surveys, focus groups, and direct observation. Feeding back the positive impact of the changes to all involved stakeholders will promote confidence in the new systems and pride in their efforts.
CONCLUSIONS
Sign‐outs are a part of the current landscape of academic medical centers as well as hospitals at large. Interns, residents, and consulting fellows, not to mention nurses, physical therapists, and nutritionists, transfer patient care information at each transition point. There are few resources that can assist these caregivers in identifying and implementing the most effective ways to transfer patient care information. Hospitals and other care facilities are now mandated to develop standards and systems to improve sign‐out. On the basis of the limited literature to date and our own experiences, we have proposed standards and best practices to assist hospitals, training programs, and institutional leaders in designing safe and usable sign‐out systems. Effective implementation of the standards must include appropriate allocation of resources, individualization to meet specific needs of each program or institution, intensive training, and ongoing evaluation. Future research should focus on developing valid surrogate measures of continuity of care, conducting rigorous trials to determine the elements of sign‐out that lead to the best patient outcomes, and studying the most effective ways of implementing these improvements. By improving the content and process of sign‐out, we can meet the challenges of the new health care landscape while putting patient safety at the forefront.
- ,,.New requirements for resident duty hours.JAMA.2002;288:1112–1114.
- ,,,,.Effects of limited work hours on surgical training.J Am Coll Surg.2002;195:531–538.
- ,,, et al.Effect of reducing interns' weekly work hours on sleep and attentional failures.N Engl J Med.2004;351:1829–1837.
- ,,, et al.Effect of reducing interns' work hours on serious medical errors in intensive care units.N Engl J Med.2004;351:1838–1848.
- .A precarious exchange.N Engl J Med.2004;351:1822–1824.
- .Awake and informed.N Engl J Med.2004;351:1884.
- . Fumbled handoff: missed communication between teams. Cases and Commentary: Hospital Medicine, Morbidity 269:374–378.
- ,,,,.Does housestaff discontinuity of care increase the risk for preventable adverse events?Ann Intern Med.1994;121:866–872.
- ,,,.Post‐call transfer of resident responsibility: its effect on patient care.J Gen Intern Med.1990;5:501–505.
- ,,,.Effect of a change in house staff work schedule on resource utilization and patient care.Arch Intern Med.1991;151:2065–2070.
- ,.Internal Bleeding: the Truth behind America's Terrifying Epidemic of Medical Mistakes.New York City:Rugged Land, LLC;2004:448.
- ,,.Crew resource management and its applications in medicine. In:Making Health Care Safer: A Critical Analysis of Patient Safety Practices. Evidence Report/Technology Assessment Number 43, AHRQ Publication 01‐E058.Rockville, MD:Agency for Healthcare Research and Quality;2001.
- ,,.System safety and threat and error management: the line operations safety audit (LOSA). In:Jensen RS, ed. Proceedings of the Eleventh International Symposium on Aviation Psychology.Columbus, OH:Ohio State University;2001:1–6.
- ,,.Translating teamwork behaviours from aviation to healthcare: development of behavioural markers for neonatal resuscitation.Qual Saf Health Care.2004;13(Suppl 1):i57–i64.
- ,,,.Handoff strategies in settings with high consequences for failure: lessons for healthcare operations.Intl J Qual Health Care.2004;16:125–132.
- . Available at: http://www.agilemodeling.com/essays/communication.htm. Accessed December 15,2005.
- .Ensuring continuing care: styles and efficiency of the handover process.Aust J Adv Nurs.1998;16:23–27.
- ,.The handover: uncovering the hidden practices of nurses.Intensive Crit Care Nurs.2000;16:373–383.
- .The patient handover: a study of its form, function and efficiency.Nurs Stand.1995;9(52):33–36.
- ,.Residents' suggestions for reducing errors in teaching hospitals.N Engl J Med.2003;348:851–855.
- ,.Is 80 the cost of saving lives? Reduced duty hours, errors, and cost.J Gen Intern Med.2005;20:969–970.
- ,,,.Lost in translation: challenges and opportunities in physician‐to‐physician communication during patient handoffs.Acad Med.2005;80:1094–1099.
- British Medical Association.Safe Handover: Safe Patients: Guidance on Clinical Handover for Clinicians and Managers.London:British Medical Association, Junior Doctors Committee;2004.
- ,,,,.Using a computerized sign‐out program to improve continuity of inpatient care and prevent adverse events.Jt Comm J Qual Improv.1998;24(2):77–87.
- ,,,.Organizing the transfer of patient care information: the development of a computerized resident sign‐out system.Surgery.2004;136:5–13.
- ,,,,.A randomized, controlled trial evaluating the impact of a computerized rounding and sign‐out system on continuity of care and resident work hours.J Am Coll Surg.2005;200:538–545.
- ,,,.Computerized physician order entry in U.S. hospitals: results of a 2002 survey.J Am Med Inform Assoc.2004;11:95–99.
- ,,,,,.The impact of verbal communication on physician prescribing patterns in hospitalized patients with diabetes.Diabetes Educ.2003;29:827–836.
- ,.Use of computer terminals on wards to access emergency test results: a retrospective audit.Br Med J.2001;322:1101–1103.
- ,,,,,.Improving patient safety by repeating (read‐back) telephone reports of critical information.Am J Clin Pathol.2004;121:801–803.
- ,.The human factor: the critical importance of effective teamwork and communication in providing safe care.Qual Saf Health Care.2004;13(Suppl 1):i85–i90.
- ,,,,.Communication failures in patient sign‐out and suggestions for improvement: a critical incident analysis.Qual Saf Health Care.2005;14:401–407.
- ,,.Intern curriculum: the impact of a focused training program on the process and content of signout out patients. Harvard Medical School Education Day, Boston, MA;2004.
- .When conversation is better than computation.J Am Med Inform Assoc.2000;7:277–286.
- SBAR technique for communication: a situational briefing model. Available at: http://www.ihi.org/IHI/Topics/PatientSafety/SafetyGeneral/Tools/SBARTechniqueforCommunicationASituationalBriefingModel.htm. Accessed December2005.
- ,,,,.Impact of reduced duty hours on residents' educational satisfaction at the University of California, San Francisco.Acad Med.2006;81:76–81.
- ,.Communication behaviours in a hospital setting: an observational study.Br Med J.1998;316:673–676.
- ,,.Communication failures: an insidious contributor to medical mishapsAcad Med.2004;79(2):186–194.
Modern‐day continuity of patient care in teaching hospitals, once remarkably high because of a cadre of sleep‐deprived residents, is now peppered with breaks, each accompanied by the transfer of patient care responsibility from one resident to another; a process often referred to as a handoff. Such transitions have long been a part of medical practice but have recently received increased attention because of restrictions in the duty hours of house staff. In July 2003 the Accreditation Council for Graduate Medical Education (ACGME) mandated reduced duty hours for all trainees in hopes of improving resident education and well‐being and patient safety.1 In fact, some studies have shown improved resident well‐being2 and fewer medical errors with reductions in duty hours,3, 4 but the growing consensus about the negative consequences of resident fatigue on patient safety has been accompanied by parallel concerns about the potential for information loss with each break in the continuity of care.5, 6
Although the tradeoff of increased discontinuity of care for fewer hours worked is sometimes characterized as an unintended consequence of duty hour regulations, it is in fact predictable and essential. As individuals work fewer hours, discontinuity must necessarily increase (assuming 24‐hour coverage).7 The extent to which this occurs may vary, but the link is consistent. At the University of California, San Francisco (UCSF), for example, we found that compliance with new duty hour requirements for internal medicine resulted in an average of 15 handoffs per patient during a 5‐day hospitalization. Each individual intern was involved in more than 300 handoffs in an average month‐long rotation, an increase of 40% since system changes were introduced to decrease duty hours. We found similar increases at Brigham and Women's Hospital (BWH) and the University of Chicago. Because U.S. teaching hospitals care for more than 6 million patients each year,8 the impact of these handoffs on the quality and efficiency of care is tremendous.
Discontinuity of care is currently managed by sign‐out, or the transfer of patient information from one physician to another. Recognizing the importance of information transfer at these vulnerable transition times for patients, the Joint Commission on Accreditation of Hospital Organizations (JCAHO) issued the 2006 National Patient Safety Goal 2E: Implement a standardized approach to hand off communications, including an opportunity to ask and respond to questions.9 Hospitals have little data to draw on to determine how to comply with this mandate and even less data to guide them in how to achieve its intended goals of improving communication and thus patient safety.
In an effort to better understand sign‐outs and ways to improve this process for house staff on in‐patient services, we reviewed data from the fields of aviation, communications, systems engineering, and human factors research, and we also searched the medical literature using key words pass‐off, handoff, sign‐out, duty hours, work hours, and discontinuity of care and MeSH headings Continuity of Patient Care Internship and Residency/*organization & administration, Personnel Staffing and Scheduling/*organization & administration, and Quality of Health Care. We also searched the websites of the Agency of Healthcare Quality and Research and the National Patient Safety Foundation. On the basis of these reviews, our experiences as hospitalist medical educators organizing resident sign‐out efforts at the University of California, San Francisco, the University of Chicago, and Brigham and Women's Hospital, and our efforts leading national training sessions on sign‐outs at the Society of General Internal Medicine (2004 and 2005), the Society of Hospital Medicine (2004), and the Association of Program Directors in Internal Medicine (2005, 2006), we propose a set of best practices regarding the content and process of sign‐out in an effort to improve communication between residents caring for hospitalized patients, assist programs in building safe and effective sign‐out systems, and improve the quality of patient care.
Effects of Discontinuity on Patient Safety
Research on the effects of discontinuity of care, although limited, suggests it has a negative impact on patient safety. In a study that investigated the institution of code 405 (the regulation that reduced duty hours in New York State), researchers found that the presumed increase in discontinuity with decreased duty hours resulted in delayed test ordering and an increased number of hospital complications.10 Another study found that the number of potentially preventable adverse events doubled when patients were under the care of a physician from a nonprimary team (eg, the cross‐covering intern).11 Studies have also linked resident discontinuity with longer length of stay, increased laboratory testing, and increased medication errors.12, 13
Managing Discontinuity: Sign‐Out as the Means of Information Transfer
In theory, more effective sign‐out systems should mitigate the potential for patient harm, but there is little in the literature describing current effective sign‐out practices or the best ways to design and implement such systems in the health care field. Examining information transfer mechanisms used in fields outside health care can assist in developing these systems.
Information Transfer in Other Industries
Although there is a paucity of data on sign‐out in the medical literature, information transfer has been the subject of substantial research in other industries in which safety depends on effective communication.
Aviation, for example, created systems and processes to improve handoff communication in response to accidents linked to failures in information transfer. One example, the 1977 collision of 2 747s on an airport runway in Tenerife, the Canary Islands, occurred after a garbled transmission from an air traffic controller to the cockpit of one of the aircraft. It was determined that a culture of adherence to a steep hierarchy prevented subordinates from questioning the captain's mistaken certainty that a runway was clear,14 an erroneous belief that was the basis for his decision to continue the aircraft on its course, resulting in its collision with the other airplane.
Subsequently, commercial aviation designed systems that standardized and formalized the process of information transfer and improved teamwork and coordination. These interventions were developed on the basis of detailed observations of cockpit interactions, reviews of communication errors, and focus groups.15 Because of these efforts, today's pilots use standardized checklists to transfer information content, communicate at designated times in specific undistracted environments, and use standard language and read‐backs to enhance understanding.16 The result has been a remarkable decrease in the risk of aviation crashes, one that most experts attribute in large part to these efforts to improve communication.17
Observation of how communication occurs in other high‐risk industries has informed the arena of effective information transfer. For example, direct observation of information transfer at NASA, in nuclear power plants, and in the railway industry identified specific strategies for effective handoffs/sign‐outs such as standardizing the information transferred, ensuring information is up to date, limiting interruptions, and having a structured face‐to‐face verbal interchange.18
Other strategies noted to be effective in diminishing errors are the use of a standardized phonetic alphabet to ensure that information is correctly heard and understood4 and having interactive verbal communication occur at a whiteboard.19
Information Transfer in Health Care
Those in the discipline of nursing have vast experience in the transfer of patient care information. The sign‐out process employed by nurses includes face‐to‐face discussions, typed information, and, most commonly, taped verbal communication.20 Interestingly, this process has not been subject to detailed scrutiny, and there is little information in the literature about best practices in sign‐out. Most articles in the literature on nursing handoffs are ethnographic descriptions of patient care responsibilities,21 on the basis of which, the authors advocate standardization of the information to be transferred, formalization of the channel used to communicate, and attention to increasing a culture of professionalism during sign‐out in order to improve efficiency.20, 22
There is little in the literature on transfer of care among physicians. Improvements in sign‐out have been suggested as part of broad strategies, such as increased training and information technology support,4, 7, 23, 24 and specific strategies have been offered such as managing barriers to communication, including specific types of data when transferring care,25 and involving nurses and senior physicians in sign‐outs.26 Specific outcomes data in this area have focused primarily on the use of computerized systems to improve information transfer. For example, the use of a computerized sign‐out system at Brigham and Women's Hospital (BWH), linked to the hospital's information system to ensure up‐to‐date information on patient demographics, medications, and laboratory values, has resulted in fewer errors,27 as have other similar systems.28 At the University of Washington, use of a similarly linked computerized sign‐out system resulted in fewer patients being missed on rounds and improvement in the quality of sign‐out and continuity of care according to resident self‐reports.29 Unfortunately, fewer than 10% of hospitals have such integrated hospitalwide information systems to support the sign‐out function.30
It has been noted that verbal communication, in concert with advances in technological communication, is important in information transfer in health care,18, 31 especially in emergent or urgent conditions.32 For example, eliminating the phoned‐in report from the lab to the ER and replacing it with delivery by an electronic reporting system lacking verbal communicationresulted in 45% of emergent lab results going unchecked.32 Structured verbal communication tools have been efficacious in improving information transfer outside the formal sign‐outfor example, read‐backs, which reduced errors in the reporting of critical laboratory values,33 and the SBAR (situation, background, assessment, recommendation) tool (designed to frame the transfer of critical information), which improved physician and nurse patient care information transfer in the in‐patient setting of the Kaiser Permanente health system.34
In focus groups and in response to formal and informal surveys, residents at our 3 sites suggested inclusion of the following information, provided in writing and orally, to improve sign‐outs: up‐to‐date administrative information (eg, room number, primary care physician); patient's recent cognitive or cardiopulmonary status; problems the patient had already experienced and treatments previously tried, both successfully and unsuccessfully; patient's code status and discussions on level of care; test results or consultation recommendations that were likely to come back while covering the patient and what to do with the results; and relevant psychosocial information (eg, complex family dynamics).35
The Current Practice of Sign‐Out
In examining sign‐outs at our 3 institutions, we found them to be unstructured and unstandardized. From discussion with faculty participating in national workshops on sign‐out, we found that most sign‐outs are conducted by interns, usually with little or no formal training. Templates, checklists, or other methods to standardize the content of the information transferred were rarely used.
We also noted that the vehicle for written sign‐out is highly variable. At UCSF, different residency training programs used a variety of modalities for written sign‐outs, including index cards, Excel spreadsheets, Word documents, and loose sheets of paper. Recently, the UCSF Department of Medicine designed a simple database (on Filemaker Pro) that allows members of the house staff to update their sign‐out information, share it with other house staff and nurses, and access it at locations throughout the hospital (Fig. 1). Although this database is not yet linked to the hospital information system (planned for 2006), anecdotally resident satisfaction with sign‐out has vastly improved since its implementation. The cost of design and implementation was approximately $10,000. At the University of Chicago, interns used Microsoft Word to create sign‐out sheets containing patient summaries to transfer information. However, during structured interviews, 95% of the interns reported that these sheets were frequently lost or misplaced.7 Although medicine residents at BWH use a computerized system to produce sign‐out sheets, this system did not guarantee complete and structured information. For example, a survey at BWH found that 56% of cross‐covering residents said that when paged about a patient overnight, the relevant information needed to care for that patient was present less than half the time; and 27% of residents reported being paged more than 3 times in the previous 2 weeks about a test result or consultant recommendation that they did not know was pending.36

The process of sign‐out also varied across disciplines and institutions. From our experiences at our sites and at the sites of faculty nationally, we found limited standardization about whether sign‐out was verbal, the data transmitted, and the setting in which it was transmitted. In fact, at UCSF most residents signed out verbally on the fly, wherever and whenever they could find the cross‐coverage intern. At BWH, only 37% of residents said that sign‐out occurred in a quiet place most of the time, and only 52% signed out on every patient both orally and in writing.36 At the University of Chicago, the sign‐out process was characterized by outright failures in communication because of omission of needed information (ie, medications, active or anticipated medical problems, etc.) or by failure‐prone communication (ie, lack of face‐to‐face communication, illegible writing). These failures often led to uncertainty in making patient care decisions, potentially resulting in inefficient or suboptimal care.35
Strategies for Safe and Effective Sign‐Out
Given the current landscape of variability in sign‐outs, the recognition that information lost during sign‐out may result in harm to patients, and evidence of improvements in information transfer in areas outside health care, we aimed to develop mechanisms to improve the sign‐out process for residents working in a hospital setting. These strategies are based on our review of the existing literature supplemented by our experiences at our 3 institutions.
Content of Sign‐Out
The elements of content necessary for safe and effective sign‐out can be divided into 5 broad categories (Table 1), contained in the mnemonic ANTICipate: Administrative information, New clinical information, specific Tasks to be performed, assessment of severity of Illness, and Contingency plans or anticipated problems (Table 1, Fig. 2).
| ✓ Administrative data |
| □ Patient name, age, sex |
| □ Medical record number |
| □ Room number |
| □ Admission date |
| □ Primary inpatient medical team, primary care physician |
| □ Family contact information |
| ✓ New information (clinical update) |
| □ Chief complaint, brief HPI, and diagnosis (or differential diagnosis) |
| □ Updated list of medications with doses, updated allergies |
| □ Updated, brief assessment by system/problem, with dates |
| □ Current baseline status (eg, mental status, cardiopulmonary, vital signs, especially if abnormal but stable) |
| □ Recent procedures and significant events |
| ✓ Tasks (what needs to be done) |
| □ Specific, using if‐then statements |
| □ Prepare cross‐coverage (eg, patient consent for blood transfusion) |
| □ Alert to incoming information (eg, study results, consultant recommendations), and what action, if any, needs to be taken during the cross‐coverage |
| ✓ Illness |
| □ Is the patient sick? |
| ✓ Contingency planning/Code status |
| □ What may go wrong and what to do about it |
| □ What has or has not worked before (eg, responds to 40 mg IV furosemide) |
| □ Difficult family or psychosocial situations |
| □ Code status, especially recent changes or family discussions |

Several general points about this list should be noted. First, the sign‐out content is not meant to replace the chart. The information included reflects the goal of a sign‐out, namely, to provide enough information to allow for a safe transition in patient care. Information we believe is not essential to the sign‐out includes: a complete history and physical exam from the day of admission, a list of tasks already completed, and data necessary only to complete a discharge summary.
Sign‐out must be also be a closed loopthe process of signing in is as important as the process of signing out. This usually entails members of the primary team obtaining information from the cross‐covering physician when they resume care of the patient. The information conveyed in this case is different and includes details on events during cross‐coverage such as: 1) time called to assess patient; 2) reason for call; 3) a brief assessment of the patient, including vital signs; 4) actions taken, for example, medications given and tests ordered; and 5) rationale for those actions. Some of this information may also be included in the chart as an event note (see Fig. 3).

The Vehicle for Sign‐Out
We recommend a computer‐assisted vehicle for patient information transfer. Ideally, this would be linked to the hospital information system to ensure accurate and up‐to‐date information Easy access to the computerized sign‐out is essential (eg, using a hospitalwide computer system, shared hard drive service, intranet, or PDA linked to the computer system), and it should be customizable for the varied needs of different services and departments. The system should have templates to standardize the content of sign‐out, contain robust backup systems, and be HIPAA compliant (ie, restrict access to required health care personnel). However, the perfect should not be the enemy of the good: systems that do not meet these criteria may still help to protect patients by providing legible, predictable, and accessible information.
Sign‐Out Processes
Verbal communication.
Although electronic solutions can facilitate the standardization of written content, face‐to‐face verbal communication adds additional value.19 We recommend that each patient be reviewed separately. Identification of each patient verbally ensures that those engaged in the sign‐out are discussing the same patient. Reiterating the major medical problems gives a snapshot of the patient and frames the sign‐out. The to‐do list, the list of tasks that the cross‐cover resident needs to complete during cross coverage, should be specific and articulated as if, then statements (eg, if the urine output is less than 1 L, then give 40 mg of IV furosemide). The receiver of sign‐out should read back to the person giving the sign‐out each item on the to‐do list (eg, So, I should check the ins and outs at about 10:00 pm, and give 40 of furosemide if the patient is not 1 L negative, right?).
Anticipated problems should also be verbally communicated to promote a dialogue. Points that cannot be adequately transferred in the written sign‐out are particularly important to transmit verbally. Examples include previous code discussions, unusual responses to treatment, and psychosocial and family issues. When delivering verbal sign‐out, it is important to consider the a priori knowledge of the recipient. How much knowledge about a patient is already shared between the outgoing and incoming physicians and the level of experience of the physicians may affect the extent to which information needs be transmitted.37 For instance, 2 experienced physicians who already have been working to cover the same patient will likely have an abbreviated discussion, in contrast to the lengthier sign‐out necessary if the outgoing and incoming physicians are interns, and the incoming intern has no prior knowledge of the patient. Similarly, it is likely the level of detail transmitted will need to be greater during a permanent transfer of patient care (ie, at the end of a resident's rotation) than during a brief, temporary transition (eg, overnight coverage).
The challenges of a busy inpatient service may preclude a complete verbal sign‐out for all patients; we contend, though, it is best to use these practices to the extent possible, especially for patients with treatment plans in flux, those whose status is tenuous, and those who have anticipated changes in status during cross‐coverage. One tool that may be effectively used in signing out such patients is the SBAR tool, according to which a brief description of the situation is given, followed by the background and the physician's specific assessment and complete recommendation.38 For example, a resident signing out might begin by stating, I have 18 patients to sign‐out to you. I'm going to describe 6 active patients in detail. Twelve others are fairly stable, and I will give you basic information about them, and the details are in the written sign‐out. One patient has a plan in flux. The situation is Mr. S. is having trouble breathing, the background is that he has both CHF and COPD, my assessment is that this is more cardiac than pulmonary, and I recommend that you see him first and discuss with the cardiology consultant. Using the tools described here (Table 2), a sign‐out of 15 patients of variable acuity could be verbally signed out in less than 10 minutes.
| ✓ WHO should participate in the sign‐out process? |
| □ Outgoing clinician primarily responsible for patient's care |
| □ Oncoming clinician who will be primarily responsible for patient's care (avoid passing this task to someone else, even if busy) |
| □ Consider supervision by experienced clinicians if early in training |
| ✓ WHAT content needs to be verbally communicated? |
| Use situation briefing model, or SBAR, technique: |
| □ SituationIdentify each patient (name, age, sex, chief complaint) and briefly state any major problems (active and those that may become active during cross‐coverage). |
| □ Backgroundpertinent information relevant to current care (eg, recent vitals and/or baseline exam, labs, test results, etc). |
| □ Assessmentworking diagnosis, response to treatment, anticipated problems during cross‐coverage including anything not adequately described using written form (eg, complex family discussions). |
| □ Recommendationto‐do lists and if/then recommendations. |
| ✓ WHERE should sign‐out occur? |
| □ Designated room or place for sign‐out (eg, avoid patient areas because of HIPPA requirements) |
| □ Proper lighting |
| □ Avoid excessive noise (eg, high‐traffic areas) |
| □ Minimize disruptions (eg, hand over pagers) |
| □ Ensure systems support for sign‐out (eg, computers, printer, paper, etc.) |
| ✓ WHEN is the optimal time for sign‐out? |
| □ Designated time when both parties can be present and pay attention (eg, beware of clinic, other obligations) |
| □ Have enough time for interactive questions at the end (eg, avoid rush at the end of the shift) |
| ✓ HOW should verbal communication be performed? |
| □ Face to face, allowing for questions |
| □ Verbalize data in the same order for each patient at each sign‐out |
| □ Read back all to‐do items |
| □ Adjust length and depth of review according to baseline knowledge of parties involved and type of transition in care |
The Environment and setting.
To improve the setting of sign‐out, we recommend: a designated space that is well lit, quiet, and respects patient confidentiality and a designated time when sign‐out will occur. To limit known distractions and interruptions39, 40 in the hospital, we also recommend the outgoing physician hand off his or her pager to someone else during sign‐out. Also key to an environment conducive to information transfer is ensuring adequate computer support for electronic sign‐out and access to updated clinical information.
Organizational culture and institutional leadership.
The way residents transfer patient care information reflects the culture of the institution. Changing the culture to one in which interactive questioning is valued regardless of position in the hierarchy has been shown to reduce errors in aviation.41 Educating residents on the impact of sign‐outs on patient care is a first step toward improving the culture of sign‐out. Resident commitment to the new sign‐out can be gained by engaging residents in development of the process itself. To cement these changes into the culture, practitioners at all levels should be aware of and support the new system. The role of an institution's leaders in achieving these changes cannot be overlooked. Leaders will need to be creative in order to support sign‐out as described within the obvious constraints of money, time, personnel, and space. Gaining institutional buy‐in can start with heightening the awareness of leaders of the issues surrounding sign‐out, including patient safety, resident efficiency, and the financial impact of discontinuity. Ongoing evaluation of efforts to improve sign‐out is also crucial and can be accomplished with surveys, focus groups, and direct observation. Feeding back the positive impact of the changes to all involved stakeholders will promote confidence in the new systems and pride in their efforts.
CONCLUSIONS
Sign‐outs are a part of the current landscape of academic medical centers as well as hospitals at large. Interns, residents, and consulting fellows, not to mention nurses, physical therapists, and nutritionists, transfer patient care information at each transition point. There are few resources that can assist these caregivers in identifying and implementing the most effective ways to transfer patient care information. Hospitals and other care facilities are now mandated to develop standards and systems to improve sign‐out. On the basis of the limited literature to date and our own experiences, we have proposed standards and best practices to assist hospitals, training programs, and institutional leaders in designing safe and usable sign‐out systems. Effective implementation of the standards must include appropriate allocation of resources, individualization to meet specific needs of each program or institution, intensive training, and ongoing evaluation. Future research should focus on developing valid surrogate measures of continuity of care, conducting rigorous trials to determine the elements of sign‐out that lead to the best patient outcomes, and studying the most effective ways of implementing these improvements. By improving the content and process of sign‐out, we can meet the challenges of the new health care landscape while putting patient safety at the forefront.
Modern‐day continuity of patient care in teaching hospitals, once remarkably high because of a cadre of sleep‐deprived residents, is now peppered with breaks, each accompanied by the transfer of patient care responsibility from one resident to another; a process often referred to as a handoff. Such transitions have long been a part of medical practice but have recently received increased attention because of restrictions in the duty hours of house staff. In July 2003 the Accreditation Council for Graduate Medical Education (ACGME) mandated reduced duty hours for all trainees in hopes of improving resident education and well‐being and patient safety.1 In fact, some studies have shown improved resident well‐being2 and fewer medical errors with reductions in duty hours,3, 4 but the growing consensus about the negative consequences of resident fatigue on patient safety has been accompanied by parallel concerns about the potential for information loss with each break in the continuity of care.5, 6
Although the tradeoff of increased discontinuity of care for fewer hours worked is sometimes characterized as an unintended consequence of duty hour regulations, it is in fact predictable and essential. As individuals work fewer hours, discontinuity must necessarily increase (assuming 24‐hour coverage).7 The extent to which this occurs may vary, but the link is consistent. At the University of California, San Francisco (UCSF), for example, we found that compliance with new duty hour requirements for internal medicine resulted in an average of 15 handoffs per patient during a 5‐day hospitalization. Each individual intern was involved in more than 300 handoffs in an average month‐long rotation, an increase of 40% since system changes were introduced to decrease duty hours. We found similar increases at Brigham and Women's Hospital (BWH) and the University of Chicago. Because U.S. teaching hospitals care for more than 6 million patients each year,8 the impact of these handoffs on the quality and efficiency of care is tremendous.
Discontinuity of care is currently managed by sign‐out, or the transfer of patient information from one physician to another. Recognizing the importance of information transfer at these vulnerable transition times for patients, the Joint Commission on Accreditation of Hospital Organizations (JCAHO) issued the 2006 National Patient Safety Goal 2E: Implement a standardized approach to hand off communications, including an opportunity to ask and respond to questions.9 Hospitals have little data to draw on to determine how to comply with this mandate and even less data to guide them in how to achieve its intended goals of improving communication and thus patient safety.
In an effort to better understand sign‐outs and ways to improve this process for house staff on in‐patient services, we reviewed data from the fields of aviation, communications, systems engineering, and human factors research, and we also searched the medical literature using key words pass‐off, handoff, sign‐out, duty hours, work hours, and discontinuity of care and MeSH headings Continuity of Patient Care Internship and Residency/*organization & administration, Personnel Staffing and Scheduling/*organization & administration, and Quality of Health Care. We also searched the websites of the Agency of Healthcare Quality and Research and the National Patient Safety Foundation. On the basis of these reviews, our experiences as hospitalist medical educators organizing resident sign‐out efforts at the University of California, San Francisco, the University of Chicago, and Brigham and Women's Hospital, and our efforts leading national training sessions on sign‐outs at the Society of General Internal Medicine (2004 and 2005), the Society of Hospital Medicine (2004), and the Association of Program Directors in Internal Medicine (2005, 2006), we propose a set of best practices regarding the content and process of sign‐out in an effort to improve communication between residents caring for hospitalized patients, assist programs in building safe and effective sign‐out systems, and improve the quality of patient care.
Effects of Discontinuity on Patient Safety
Research on the effects of discontinuity of care, although limited, suggests it has a negative impact on patient safety. In a study that investigated the institution of code 405 (the regulation that reduced duty hours in New York State), researchers found that the presumed increase in discontinuity with decreased duty hours resulted in delayed test ordering and an increased number of hospital complications.10 Another study found that the number of potentially preventable adverse events doubled when patients were under the care of a physician from a nonprimary team (eg, the cross‐covering intern).11 Studies have also linked resident discontinuity with longer length of stay, increased laboratory testing, and increased medication errors.12, 13
Managing Discontinuity: Sign‐Out as the Means of Information Transfer
In theory, more effective sign‐out systems should mitigate the potential for patient harm, but there is little in the literature describing current effective sign‐out practices or the best ways to design and implement such systems in the health care field. Examining information transfer mechanisms used in fields outside health care can assist in developing these systems.
Information Transfer in Other Industries
Although there is a paucity of data on sign‐out in the medical literature, information transfer has been the subject of substantial research in other industries in which safety depends on effective communication.
Aviation, for example, created systems and processes to improve handoff communication in response to accidents linked to failures in information transfer. One example, the 1977 collision of 2 747s on an airport runway in Tenerife, the Canary Islands, occurred after a garbled transmission from an air traffic controller to the cockpit of one of the aircraft. It was determined that a culture of adherence to a steep hierarchy prevented subordinates from questioning the captain's mistaken certainty that a runway was clear,14 an erroneous belief that was the basis for his decision to continue the aircraft on its course, resulting in its collision with the other airplane.
Subsequently, commercial aviation designed systems that standardized and formalized the process of information transfer and improved teamwork and coordination. These interventions were developed on the basis of detailed observations of cockpit interactions, reviews of communication errors, and focus groups.15 Because of these efforts, today's pilots use standardized checklists to transfer information content, communicate at designated times in specific undistracted environments, and use standard language and read‐backs to enhance understanding.16 The result has been a remarkable decrease in the risk of aviation crashes, one that most experts attribute in large part to these efforts to improve communication.17
Observation of how communication occurs in other high‐risk industries has informed the arena of effective information transfer. For example, direct observation of information transfer at NASA, in nuclear power plants, and in the railway industry identified specific strategies for effective handoffs/sign‐outs such as standardizing the information transferred, ensuring information is up to date, limiting interruptions, and having a structured face‐to‐face verbal interchange.18
Other strategies noted to be effective in diminishing errors are the use of a standardized phonetic alphabet to ensure that information is correctly heard and understood4 and having interactive verbal communication occur at a whiteboard.19
Information Transfer in Health Care
Those in the discipline of nursing have vast experience in the transfer of patient care information. The sign‐out process employed by nurses includes face‐to‐face discussions, typed information, and, most commonly, taped verbal communication.20 Interestingly, this process has not been subject to detailed scrutiny, and there is little information in the literature about best practices in sign‐out. Most articles in the literature on nursing handoffs are ethnographic descriptions of patient care responsibilities,21 on the basis of which, the authors advocate standardization of the information to be transferred, formalization of the channel used to communicate, and attention to increasing a culture of professionalism during sign‐out in order to improve efficiency.20, 22
There is little in the literature on transfer of care among physicians. Improvements in sign‐out have been suggested as part of broad strategies, such as increased training and information technology support,4, 7, 23, 24 and specific strategies have been offered such as managing barriers to communication, including specific types of data when transferring care,25 and involving nurses and senior physicians in sign‐outs.26 Specific outcomes data in this area have focused primarily on the use of computerized systems to improve information transfer. For example, the use of a computerized sign‐out system at Brigham and Women's Hospital (BWH), linked to the hospital's information system to ensure up‐to‐date information on patient demographics, medications, and laboratory values, has resulted in fewer errors,27 as have other similar systems.28 At the University of Washington, use of a similarly linked computerized sign‐out system resulted in fewer patients being missed on rounds and improvement in the quality of sign‐out and continuity of care according to resident self‐reports.29 Unfortunately, fewer than 10% of hospitals have such integrated hospitalwide information systems to support the sign‐out function.30
It has been noted that verbal communication, in concert with advances in technological communication, is important in information transfer in health care,18, 31 especially in emergent or urgent conditions.32 For example, eliminating the phoned‐in report from the lab to the ER and replacing it with delivery by an electronic reporting system lacking verbal communicationresulted in 45% of emergent lab results going unchecked.32 Structured verbal communication tools have been efficacious in improving information transfer outside the formal sign‐outfor example, read‐backs, which reduced errors in the reporting of critical laboratory values,33 and the SBAR (situation, background, assessment, recommendation) tool (designed to frame the transfer of critical information), which improved physician and nurse patient care information transfer in the in‐patient setting of the Kaiser Permanente health system.34
In focus groups and in response to formal and informal surveys, residents at our 3 sites suggested inclusion of the following information, provided in writing and orally, to improve sign‐outs: up‐to‐date administrative information (eg, room number, primary care physician); patient's recent cognitive or cardiopulmonary status; problems the patient had already experienced and treatments previously tried, both successfully and unsuccessfully; patient's code status and discussions on level of care; test results or consultation recommendations that were likely to come back while covering the patient and what to do with the results; and relevant psychosocial information (eg, complex family dynamics).35
The Current Practice of Sign‐Out
In examining sign‐outs at our 3 institutions, we found them to be unstructured and unstandardized. From discussion with faculty participating in national workshops on sign‐out, we found that most sign‐outs are conducted by interns, usually with little or no formal training. Templates, checklists, or other methods to standardize the content of the information transferred were rarely used.
We also noted that the vehicle for written sign‐out is highly variable. At UCSF, different residency training programs used a variety of modalities for written sign‐outs, including index cards, Excel spreadsheets, Word documents, and loose sheets of paper. Recently, the UCSF Department of Medicine designed a simple database (on Filemaker Pro) that allows members of the house staff to update their sign‐out information, share it with other house staff and nurses, and access it at locations throughout the hospital (Fig. 1). Although this database is not yet linked to the hospital information system (planned for 2006), anecdotally resident satisfaction with sign‐out has vastly improved since its implementation. The cost of design and implementation was approximately $10,000. At the University of Chicago, interns used Microsoft Word to create sign‐out sheets containing patient summaries to transfer information. However, during structured interviews, 95% of the interns reported that these sheets were frequently lost or misplaced.7 Although medicine residents at BWH use a computerized system to produce sign‐out sheets, this system did not guarantee complete and structured information. For example, a survey at BWH found that 56% of cross‐covering residents said that when paged about a patient overnight, the relevant information needed to care for that patient was present less than half the time; and 27% of residents reported being paged more than 3 times in the previous 2 weeks about a test result or consultant recommendation that they did not know was pending.36

The process of sign‐out also varied across disciplines and institutions. From our experiences at our sites and at the sites of faculty nationally, we found limited standardization about whether sign‐out was verbal, the data transmitted, and the setting in which it was transmitted. In fact, at UCSF most residents signed out verbally on the fly, wherever and whenever they could find the cross‐coverage intern. At BWH, only 37% of residents said that sign‐out occurred in a quiet place most of the time, and only 52% signed out on every patient both orally and in writing.36 At the University of Chicago, the sign‐out process was characterized by outright failures in communication because of omission of needed information (ie, medications, active or anticipated medical problems, etc.) or by failure‐prone communication (ie, lack of face‐to‐face communication, illegible writing). These failures often led to uncertainty in making patient care decisions, potentially resulting in inefficient or suboptimal care.35
Strategies for Safe and Effective Sign‐Out
Given the current landscape of variability in sign‐outs, the recognition that information lost during sign‐out may result in harm to patients, and evidence of improvements in information transfer in areas outside health care, we aimed to develop mechanisms to improve the sign‐out process for residents working in a hospital setting. These strategies are based on our review of the existing literature supplemented by our experiences at our 3 institutions.
Content of Sign‐Out
The elements of content necessary for safe and effective sign‐out can be divided into 5 broad categories (Table 1), contained in the mnemonic ANTICipate: Administrative information, New clinical information, specific Tasks to be performed, assessment of severity of Illness, and Contingency plans or anticipated problems (Table 1, Fig. 2).
| ✓ Administrative data |
| □ Patient name, age, sex |
| □ Medical record number |
| □ Room number |
| □ Admission date |
| □ Primary inpatient medical team, primary care physician |
| □ Family contact information |
| ✓ New information (clinical update) |
| □ Chief complaint, brief HPI, and diagnosis (or differential diagnosis) |
| □ Updated list of medications with doses, updated allergies |
| □ Updated, brief assessment by system/problem, with dates |
| □ Current baseline status (eg, mental status, cardiopulmonary, vital signs, especially if abnormal but stable) |
| □ Recent procedures and significant events |
| ✓ Tasks (what needs to be done) |
| □ Specific, using if‐then statements |
| □ Prepare cross‐coverage (eg, patient consent for blood transfusion) |
| □ Alert to incoming information (eg, study results, consultant recommendations), and what action, if any, needs to be taken during the cross‐coverage |
| ✓ Illness |
| □ Is the patient sick? |
| ✓ Contingency planning/Code status |
| □ What may go wrong and what to do about it |
| □ What has or has not worked before (eg, responds to 40 mg IV furosemide) |
| □ Difficult family or psychosocial situations |
| □ Code status, especially recent changes or family discussions |

Several general points about this list should be noted. First, the sign‐out content is not meant to replace the chart. The information included reflects the goal of a sign‐out, namely, to provide enough information to allow for a safe transition in patient care. Information we believe is not essential to the sign‐out includes: a complete history and physical exam from the day of admission, a list of tasks already completed, and data necessary only to complete a discharge summary.
Sign‐out must be also be a closed loopthe process of signing in is as important as the process of signing out. This usually entails members of the primary team obtaining information from the cross‐covering physician when they resume care of the patient. The information conveyed in this case is different and includes details on events during cross‐coverage such as: 1) time called to assess patient; 2) reason for call; 3) a brief assessment of the patient, including vital signs; 4) actions taken, for example, medications given and tests ordered; and 5) rationale for those actions. Some of this information may also be included in the chart as an event note (see Fig. 3).

The Vehicle for Sign‐Out
We recommend a computer‐assisted vehicle for patient information transfer. Ideally, this would be linked to the hospital information system to ensure accurate and up‐to‐date information Easy access to the computerized sign‐out is essential (eg, using a hospitalwide computer system, shared hard drive service, intranet, or PDA linked to the computer system), and it should be customizable for the varied needs of different services and departments. The system should have templates to standardize the content of sign‐out, contain robust backup systems, and be HIPAA compliant (ie, restrict access to required health care personnel). However, the perfect should not be the enemy of the good: systems that do not meet these criteria may still help to protect patients by providing legible, predictable, and accessible information.
Sign‐Out Processes
Verbal communication.
Although electronic solutions can facilitate the standardization of written content, face‐to‐face verbal communication adds additional value.19 We recommend that each patient be reviewed separately. Identification of each patient verbally ensures that those engaged in the sign‐out are discussing the same patient. Reiterating the major medical problems gives a snapshot of the patient and frames the sign‐out. The to‐do list, the list of tasks that the cross‐cover resident needs to complete during cross coverage, should be specific and articulated as if, then statements (eg, if the urine output is less than 1 L, then give 40 mg of IV furosemide). The receiver of sign‐out should read back to the person giving the sign‐out each item on the to‐do list (eg, So, I should check the ins and outs at about 10:00 pm, and give 40 of furosemide if the patient is not 1 L negative, right?).
Anticipated problems should also be verbally communicated to promote a dialogue. Points that cannot be adequately transferred in the written sign‐out are particularly important to transmit verbally. Examples include previous code discussions, unusual responses to treatment, and psychosocial and family issues. When delivering verbal sign‐out, it is important to consider the a priori knowledge of the recipient. How much knowledge about a patient is already shared between the outgoing and incoming physicians and the level of experience of the physicians may affect the extent to which information needs be transmitted.37 For instance, 2 experienced physicians who already have been working to cover the same patient will likely have an abbreviated discussion, in contrast to the lengthier sign‐out necessary if the outgoing and incoming physicians are interns, and the incoming intern has no prior knowledge of the patient. Similarly, it is likely the level of detail transmitted will need to be greater during a permanent transfer of patient care (ie, at the end of a resident's rotation) than during a brief, temporary transition (eg, overnight coverage).
The challenges of a busy inpatient service may preclude a complete verbal sign‐out for all patients; we contend, though, it is best to use these practices to the extent possible, especially for patients with treatment plans in flux, those whose status is tenuous, and those who have anticipated changes in status during cross‐coverage. One tool that may be effectively used in signing out such patients is the SBAR tool, according to which a brief description of the situation is given, followed by the background and the physician's specific assessment and complete recommendation.38 For example, a resident signing out might begin by stating, I have 18 patients to sign‐out to you. I'm going to describe 6 active patients in detail. Twelve others are fairly stable, and I will give you basic information about them, and the details are in the written sign‐out. One patient has a plan in flux. The situation is Mr. S. is having trouble breathing, the background is that he has both CHF and COPD, my assessment is that this is more cardiac than pulmonary, and I recommend that you see him first and discuss with the cardiology consultant. Using the tools described here (Table 2), a sign‐out of 15 patients of variable acuity could be verbally signed out in less than 10 minutes.
| ✓ WHO should participate in the sign‐out process? |
| □ Outgoing clinician primarily responsible for patient's care |
| □ Oncoming clinician who will be primarily responsible for patient's care (avoid passing this task to someone else, even if busy) |
| □ Consider supervision by experienced clinicians if early in training |
| ✓ WHAT content needs to be verbally communicated? |
| Use situation briefing model, or SBAR, technique: |
| □ SituationIdentify each patient (name, age, sex, chief complaint) and briefly state any major problems (active and those that may become active during cross‐coverage). |
| □ Backgroundpertinent information relevant to current care (eg, recent vitals and/or baseline exam, labs, test results, etc). |
| □ Assessmentworking diagnosis, response to treatment, anticipated problems during cross‐coverage including anything not adequately described using written form (eg, complex family discussions). |
| □ Recommendationto‐do lists and if/then recommendations. |
| ✓ WHERE should sign‐out occur? |
| □ Designated room or place for sign‐out (eg, avoid patient areas because of HIPPA requirements) |
| □ Proper lighting |
| □ Avoid excessive noise (eg, high‐traffic areas) |
| □ Minimize disruptions (eg, hand over pagers) |
| □ Ensure systems support for sign‐out (eg, computers, printer, paper, etc.) |
| ✓ WHEN is the optimal time for sign‐out? |
| □ Designated time when both parties can be present and pay attention (eg, beware of clinic, other obligations) |
| □ Have enough time for interactive questions at the end (eg, avoid rush at the end of the shift) |
| ✓ HOW should verbal communication be performed? |
| □ Face to face, allowing for questions |
| □ Verbalize data in the same order for each patient at each sign‐out |
| □ Read back all to‐do items |
| □ Adjust length and depth of review according to baseline knowledge of parties involved and type of transition in care |
The Environment and setting.
To improve the setting of sign‐out, we recommend: a designated space that is well lit, quiet, and respects patient confidentiality and a designated time when sign‐out will occur. To limit known distractions and interruptions39, 40 in the hospital, we also recommend the outgoing physician hand off his or her pager to someone else during sign‐out. Also key to an environment conducive to information transfer is ensuring adequate computer support for electronic sign‐out and access to updated clinical information.
Organizational culture and institutional leadership.
The way residents transfer patient care information reflects the culture of the institution. Changing the culture to one in which interactive questioning is valued regardless of position in the hierarchy has been shown to reduce errors in aviation.41 Educating residents on the impact of sign‐outs on patient care is a first step toward improving the culture of sign‐out. Resident commitment to the new sign‐out can be gained by engaging residents in development of the process itself. To cement these changes into the culture, practitioners at all levels should be aware of and support the new system. The role of an institution's leaders in achieving these changes cannot be overlooked. Leaders will need to be creative in order to support sign‐out as described within the obvious constraints of money, time, personnel, and space. Gaining institutional buy‐in can start with heightening the awareness of leaders of the issues surrounding sign‐out, including patient safety, resident efficiency, and the financial impact of discontinuity. Ongoing evaluation of efforts to improve sign‐out is also crucial and can be accomplished with surveys, focus groups, and direct observation. Feeding back the positive impact of the changes to all involved stakeholders will promote confidence in the new systems and pride in their efforts.
CONCLUSIONS
Sign‐outs are a part of the current landscape of academic medical centers as well as hospitals at large. Interns, residents, and consulting fellows, not to mention nurses, physical therapists, and nutritionists, transfer patient care information at each transition point. There are few resources that can assist these caregivers in identifying and implementing the most effective ways to transfer patient care information. Hospitals and other care facilities are now mandated to develop standards and systems to improve sign‐out. On the basis of the limited literature to date and our own experiences, we have proposed standards and best practices to assist hospitals, training programs, and institutional leaders in designing safe and usable sign‐out systems. Effective implementation of the standards must include appropriate allocation of resources, individualization to meet specific needs of each program or institution, intensive training, and ongoing evaluation. Future research should focus on developing valid surrogate measures of continuity of care, conducting rigorous trials to determine the elements of sign‐out that lead to the best patient outcomes, and studying the most effective ways of implementing these improvements. By improving the content and process of sign‐out, we can meet the challenges of the new health care landscape while putting patient safety at the forefront.
- ,,.New requirements for resident duty hours.JAMA.2002;288:1112–1114.
- ,,,,.Effects of limited work hours on surgical training.J Am Coll Surg.2002;195:531–538.
- ,,, et al.Effect of reducing interns' weekly work hours on sleep and attentional failures.N Engl J Med.2004;351:1829–1837.
- ,,, et al.Effect of reducing interns' work hours on serious medical errors in intensive care units.N Engl J Med.2004;351:1838–1848.
- .A precarious exchange.N Engl J Med.2004;351:1822–1824.
- .Awake and informed.N Engl J Med.2004;351:1884.
- . Fumbled handoff: missed communication between teams. Cases and Commentary: Hospital Medicine, Morbidity 269:374–378.
- ,,,,.Does housestaff discontinuity of care increase the risk for preventable adverse events?Ann Intern Med.1994;121:866–872.
- ,,,.Post‐call transfer of resident responsibility: its effect on patient care.J Gen Intern Med.1990;5:501–505.
- ,,,.Effect of a change in house staff work schedule on resource utilization and patient care.Arch Intern Med.1991;151:2065–2070.
- ,.Internal Bleeding: the Truth behind America's Terrifying Epidemic of Medical Mistakes.New York City:Rugged Land, LLC;2004:448.
- ,,.Crew resource management and its applications in medicine. In:Making Health Care Safer: A Critical Analysis of Patient Safety Practices. Evidence Report/Technology Assessment Number 43, AHRQ Publication 01‐E058.Rockville, MD:Agency for Healthcare Research and Quality;2001.
- ,,.System safety and threat and error management: the line operations safety audit (LOSA). In:Jensen RS, ed. Proceedings of the Eleventh International Symposium on Aviation Psychology.Columbus, OH:Ohio State University;2001:1–6.
- ,,.Translating teamwork behaviours from aviation to healthcare: development of behavioural markers for neonatal resuscitation.Qual Saf Health Care.2004;13(Suppl 1):i57–i64.
- ,,,.Handoff strategies in settings with high consequences for failure: lessons for healthcare operations.Intl J Qual Health Care.2004;16:125–132.
- . Available at: http://www.agilemodeling.com/essays/communication.htm. Accessed December 15,2005.
- .Ensuring continuing care: styles and efficiency of the handover process.Aust J Adv Nurs.1998;16:23–27.
- ,.The handover: uncovering the hidden practices of nurses.Intensive Crit Care Nurs.2000;16:373–383.
- .The patient handover: a study of its form, function and efficiency.Nurs Stand.1995;9(52):33–36.
- ,.Residents' suggestions for reducing errors in teaching hospitals.N Engl J Med.2003;348:851–855.
- ,.Is 80 the cost of saving lives? Reduced duty hours, errors, and cost.J Gen Intern Med.2005;20:969–970.
- ,,,.Lost in translation: challenges and opportunities in physician‐to‐physician communication during patient handoffs.Acad Med.2005;80:1094–1099.
- British Medical Association.Safe Handover: Safe Patients: Guidance on Clinical Handover for Clinicians and Managers.London:British Medical Association, Junior Doctors Committee;2004.
- ,,,,.Using a computerized sign‐out program to improve continuity of inpatient care and prevent adverse events.Jt Comm J Qual Improv.1998;24(2):77–87.
- ,,,.Organizing the transfer of patient care information: the development of a computerized resident sign‐out system.Surgery.2004;136:5–13.
- ,,,,.A randomized, controlled trial evaluating the impact of a computerized rounding and sign‐out system on continuity of care and resident work hours.J Am Coll Surg.2005;200:538–545.
- ,,,.Computerized physician order entry in U.S. hospitals: results of a 2002 survey.J Am Med Inform Assoc.2004;11:95–99.
- ,,,,,.The impact of verbal communication on physician prescribing patterns in hospitalized patients with diabetes.Diabetes Educ.2003;29:827–836.
- ,.Use of computer terminals on wards to access emergency test results: a retrospective audit.Br Med J.2001;322:1101–1103.
- ,,,,,.Improving patient safety by repeating (read‐back) telephone reports of critical information.Am J Clin Pathol.2004;121:801–803.
- ,.The human factor: the critical importance of effective teamwork and communication in providing safe care.Qual Saf Health Care.2004;13(Suppl 1):i85–i90.
- ,,,,.Communication failures in patient sign‐out and suggestions for improvement: a critical incident analysis.Qual Saf Health Care.2005;14:401–407.
- ,,.Intern curriculum: the impact of a focused training program on the process and content of signout out patients. Harvard Medical School Education Day, Boston, MA;2004.
- .When conversation is better than computation.J Am Med Inform Assoc.2000;7:277–286.
- SBAR technique for communication: a situational briefing model. Available at: http://www.ihi.org/IHI/Topics/PatientSafety/SafetyGeneral/Tools/SBARTechniqueforCommunicationASituationalBriefingModel.htm. Accessed December2005.
- ,,,,.Impact of reduced duty hours on residents' educational satisfaction at the University of California, San Francisco.Acad Med.2006;81:76–81.
- ,.Communication behaviours in a hospital setting: an observational study.Br Med J.1998;316:673–676.
- ,,.Communication failures: an insidious contributor to medical mishapsAcad Med.2004;79(2):186–194.
- ,,.New requirements for resident duty hours.JAMA.2002;288:1112–1114.
- ,,,,.Effects of limited work hours on surgical training.J Am Coll Surg.2002;195:531–538.
- ,,, et al.Effect of reducing interns' weekly work hours on sleep and attentional failures.N Engl J Med.2004;351:1829–1837.
- ,,, et al.Effect of reducing interns' work hours on serious medical errors in intensive care units.N Engl J Med.2004;351:1838–1848.
- .A precarious exchange.N Engl J Med.2004;351:1822–1824.
- .Awake and informed.N Engl J Med.2004;351:1884.
- . Fumbled handoff: missed communication between teams. Cases and Commentary: Hospital Medicine, Morbidity 269:374–378.
- ,,,,.Does housestaff discontinuity of care increase the risk for preventable adverse events?Ann Intern Med.1994;121:866–872.
- ,,,.Post‐call transfer of resident responsibility: its effect on patient care.J Gen Intern Med.1990;5:501–505.
- ,,,.Effect of a change in house staff work schedule on resource utilization and patient care.Arch Intern Med.1991;151:2065–2070.
- ,.Internal Bleeding: the Truth behind America's Terrifying Epidemic of Medical Mistakes.New York City:Rugged Land, LLC;2004:448.
- ,,.Crew resource management and its applications in medicine. In:Making Health Care Safer: A Critical Analysis of Patient Safety Practices. Evidence Report/Technology Assessment Number 43, AHRQ Publication 01‐E058.Rockville, MD:Agency for Healthcare Research and Quality;2001.
- ,,.System safety and threat and error management: the line operations safety audit (LOSA). In:Jensen RS, ed. Proceedings of the Eleventh International Symposium on Aviation Psychology.Columbus, OH:Ohio State University;2001:1–6.
- ,,.Translating teamwork behaviours from aviation to healthcare: development of behavioural markers for neonatal resuscitation.Qual Saf Health Care.2004;13(Suppl 1):i57–i64.
- ,,,.Handoff strategies in settings with high consequences for failure: lessons for healthcare operations.Intl J Qual Health Care.2004;16:125–132.
- . Available at: http://www.agilemodeling.com/essays/communication.htm. Accessed December 15,2005.
- .Ensuring continuing care: styles and efficiency of the handover process.Aust J Adv Nurs.1998;16:23–27.
- ,.The handover: uncovering the hidden practices of nurses.Intensive Crit Care Nurs.2000;16:373–383.
- .The patient handover: a study of its form, function and efficiency.Nurs Stand.1995;9(52):33–36.
- ,.Residents' suggestions for reducing errors in teaching hospitals.N Engl J Med.2003;348:851–855.
- ,.Is 80 the cost of saving lives? Reduced duty hours, errors, and cost.J Gen Intern Med.2005;20:969–970.
- ,,,.Lost in translation: challenges and opportunities in physician‐to‐physician communication during patient handoffs.Acad Med.2005;80:1094–1099.
- British Medical Association.Safe Handover: Safe Patients: Guidance on Clinical Handover for Clinicians and Managers.London:British Medical Association, Junior Doctors Committee;2004.
- ,,,,.Using a computerized sign‐out program to improve continuity of inpatient care and prevent adverse events.Jt Comm J Qual Improv.1998;24(2):77–87.
- ,,,.Organizing the transfer of patient care information: the development of a computerized resident sign‐out system.Surgery.2004;136:5–13.
- ,,,,.A randomized, controlled trial evaluating the impact of a computerized rounding and sign‐out system on continuity of care and resident work hours.J Am Coll Surg.2005;200:538–545.
- ,,,.Computerized physician order entry in U.S. hospitals: results of a 2002 survey.J Am Med Inform Assoc.2004;11:95–99.
- ,,,,,.The impact of verbal communication on physician prescribing patterns in hospitalized patients with diabetes.Diabetes Educ.2003;29:827–836.
- ,.Use of computer terminals on wards to access emergency test results: a retrospective audit.Br Med J.2001;322:1101–1103.
- ,,,,,.Improving patient safety by repeating (read‐back) telephone reports of critical information.Am J Clin Pathol.2004;121:801–803.
- ,.The human factor: the critical importance of effective teamwork and communication in providing safe care.Qual Saf Health Care.2004;13(Suppl 1):i85–i90.
- ,,,,.Communication failures in patient sign‐out and suggestions for improvement: a critical incident analysis.Qual Saf Health Care.2005;14:401–407.
- ,,.Intern curriculum: the impact of a focused training program on the process and content of signout out patients. Harvard Medical School Education Day, Boston, MA;2004.
- .When conversation is better than computation.J Am Med Inform Assoc.2000;7:277–286.
- SBAR technique for communication: a situational briefing model. Available at: http://www.ihi.org/IHI/Topics/PatientSafety/SafetyGeneral/Tools/SBARTechniqueforCommunicationASituationalBriefingModel.htm. Accessed December2005.
- ,,,,.Impact of reduced duty hours on residents' educational satisfaction at the University of California, San Francisco.Acad Med.2006;81:76–81.
- ,.Communication behaviours in a hospital setting: an observational study.Br Med J.1998;316:673–676.
- ,,.Communication failures: an insidious contributor to medical mishapsAcad Med.2004;79(2):186–194.