User login
Glucose Management and Inpatient Mortality
Patients with diabetes currently comprise over 8% of the US population (over 25 million people) and more than 20% of hospitalized patients.[1, 2] Hospitalizations of patients with diabetes account for 23% of total hospital costs in the United States,[2] and patients with diabetes have worse outcomes after hospitalization for a variety of common medical conditions,[3, 4, 5, 6] as well as in intensive care unit (ICU) settings.[7, 8] Individuals with diabetes have historically experienced higher inpatient mortality than individuals without diabetes.[9] However, we recently reported that patients with diabetes at our large academic medical center have experienced a disproportionate reduction in in‐hospital mortality relative to patients without diabetes over the past decade.[10] This surprising trend begs further inquiry.
Improvement in in‐hospital mortality among patients with diabetes may stem from improved inpatient glycemic management. The landmark 2001 study by van den Berghe et al. demonstrating that intensive insulin therapy reduced postsurgical mortality among ICU patients ushered in an era of intensive inpatient glucose control.[11] However, follow‐up multicenter studies have not been able to replicate these results.[12, 13, 14, 15] In non‐ICU and nonsurgical settings, intensive glucose control has not yet been shown to have any mortality benefit, although it may impact other morbidities, such as postoperative infections.[16] Consequently, less stringent glycemic targets are now recommended.[17] Nonetheless, hospitals are being held accountable for certain aspects of inpatient glucose control. For example, the Centers for Medicare & Medicaid Services (CMS) began asking hospitals to report inpatient glucose control in cardiac surgery patients in 2004.[18] This measure is now publicly reported, and as of 2013 is included in the CMS Value‐Based Purchasing Program, which financially penalizes hospitals that do not meet targets.
Outpatient diabetes standards have also evolved in the past decade. The Diabetes Control and Complications Trial in 1993 and the United Kingdom Prospective Diabetes Study in 1997 demonstrated that better glycemic control in type 1 and newly diagnosed type 2 diabetes patients, respectively, improved clinical outcomes, and prompted guidelines for pharmacologic treatment of diabetic patients.[19, 20] However, subsequent randomized clinical trials have failed to establish a clear beneficial effect of intensive glucose control on primary cardiovascular endpoints among higher‐risk patients with longstanding type 2 diabetes,[21, 22, 23] and clinical practice recommendations now accept a more individualized approach to glycemic control.[24] Nonetheless, clinicians are also being held accountable for outpatient glucose control.[25]
To better understand the disproportionate reduction in mortality among hospitalized patients with diabetes that we observed, we first examined whether it was limited to surgical patients or patients in the ICU, the populations that have been demonstrated to benefit from intensive inpatient glucose control. Furthermore, given recent improvements in inpatient and outpatient glycemic control,[26, 27] we examined whether inpatient or outpatient glucose control explained the mortality trends. Results from this study contribute empirical evidence on real‐world effects of efforts to improve inpatient and outpatient glycemic control.
METHODS
Setting
During the study period, YaleNew Haven Hospital (YNHH) was an urban academic medical center in New Haven, Connecticut, with over 950 beds and an average of approximately 32,000 annual adult nonobstetric admissions. YNHH conducted a variety of inpatient glucose control initiatives during the study period. The surgical ICU began an informal medical teamdirected insulin infusion protocol in 2000 to 2001. In 2002, the medical ICU instituted a formal insulin infusion protocol with a target of 100 to 140 mg/dL, which spread to remaining hospital ICUs by the end of 2003. In 2005, YNHH launched a consultative inpatient diabetes management team to assist clinicians in controlling glucose in non‐ICU patients with diabetes. This team covered approximately 10 to 15 patients at a time and consisted of an advanced‐practice nurse practitioner, a supervising endocrinologist and endocrinology fellow, and a nurse educator to provide diabetic teaching. Additionally, in 2005, basal‐boluscorrection insulin order sets became available. The surgical ICU implemented a stringent insulin infusion protocol with target glucose of 80 to 110 mg/dL in 2006, but relaxed it (goal 80150 mg/dL) in 2007. Similarly, in 2006, YNHH made ICU insulin infusion recommendations more stringent in remaining ICUs (goal 90130 mg/dL), but relaxed them in 2010 (goal 120160 mg/dL), based on emerging data from clinical trials and prevailing national guidelines.
Participants and Data Sources
We included all adult, nonobstetric discharges from YNHH between January 1, 2000 and December 31, 2010. Repeat visits by the same patient were linked by medical record number. We obtained data from YNHH administrative billing, laboratory, and point‐of‐care capillary blood glucose databases. The Yale Human Investigation Committee approved our study design and granted a Health Insurance Portability and Accountability Act waiver and a waiver of patient consent.
Variables
Our primary endpoint was in‐hospital mortality. The primary exposure of interest was whether a patient had diabetes mellitus, defined as the presence of International Classification of Diseases, Ninth Revision codes 249.x, 250.x, V4585, V5391, or V6546 in any of the primary or secondary diagnosis codes in the index admission, or in any hospital encounter in the year prior to the index admission.
We assessed 2 effect‐modifying variables: ICU status (as measured by a charge for at least 1 night in the ICU) and service assignment to surgery (including neurosurgery and orthopedics), compared to medicine (including neurology). Independent explanatory variables included time between the start of the study and patient admission (measured as days/365), diabetes status, inpatient glucose control, and long‐term glucose control (as measured by hemoglobin A1c at any time in the 180 days prior to hospital admission in order to have adequate sample size). We assessed inpatient blood glucose control through point‐of‐care blood glucose meters (OneTouch SureStep; LifeScan, Inc., Milipitas, CA) at YNHH. We used 4 validated measures of inpatient glucose control: the proportion of days in each hospitalization in which there was any hypoglycemic episode (blood glucose value <70 mg/dL), the proportion of days in which there was any severely hyperglycemic episode (blood glucose value >299 mg/dL), the proportion of days in which mean blood glucose was considered to be within adequate control (all blood glucose values between 70 and 179 mg/dL), and the standard deviation of mean glucose during hospitalization as a measure of glycemic variability.[28]
Covariates included gender, age at time of admission, length of stay in days, race (defined by hospital registration), payer, Elixhauser comorbidity dummy variables (revised to exclude diabetes and to use only secondary diagnosis codes),[29] and primary discharge diagnosis grouped using Clinical Classifications Software,[30] based on established associations with in‐hospital mortality.
Statistical Analysis
We summarized demographic characteristics numerically and graphically for patients with and without diabetes and compared them using [2] and t tests. We summarized changes in inpatient and outpatient measures of glucose control over time numerically and graphically, and compared across years using the Wilcoxon rank sum test adjusted for multiple hypothesis testing.
We stratified all analyses first by ICU status and then by service assignment (medicine vs surgery). Statistical analyses within each stratum paralleled our previous approach to the full study cohort.[10] Taking each stratum separately (ie, only ICU patients or only medicine patients), we used a difference‐in‐differences approach comparing changes over time in in‐hospital mortality among patients with diabetes compared to those without diabetes. This approach enabled us to determine whether patients with diabetes had a different time trend in risk of in‐hospital mortality than those without diabetes. That is, for each stratum, we constructed multivariate logistic regression models including time in years, diabetes status, and the interaction between time and diabetes status as well as the aforementioned covariates. We calculated odds of death and confidence intervals for each additional year for patients with diabetes by exponentiating the sum of parameter estimates for time and the diabetes‐time interaction term. We evaluated all 2‐way interactions between year or diabetes status and the covariates in a multiple degree of freedom likelihood ratio test. We investigated nonlinearity of the relation between mortality and time by evaluating first and second‐order polynomials.
Because we found a significant decline in mortality risk for patients with versus without diabetes among ICU patients but not among non‐ICU patients, and because service assignment was not found to be an effect modifier, we then limited our sample to ICU patients with diabetes to better understand the role of inpatient and outpatient glucose control in accounting for observed mortality trends. First, we determined the relation between the measures of inpatient glucose control and changes in mortality over time using logistic regression. Then, we repeated this analysis in the subsets of patients who had inpatient glucose data and both inpatient and outpatient glycemic control data, adding inpatient and outpatient measures sequentially. Given the high level of missing outpatient glycemic control data, we compared demographic characteristics for diabetic ICU patients with and without such data using [2] and t tests, and found that patients with data were younger and less likely to be white and had longer mean length of stay, slightly worse performance on several measures of inpatient glucose control, and lower mortality (see Supporting Table 1 in the online version of this article).
| Characteristic | Overall, N=322,939 | Any ICU Stay, N=54,646 | No ICU Stay, N=268,293 | Medical Service, N=196,325 | Surgical Service, N=126,614 |
|---|---|---|---|---|---|
| |||||
| Died during admission, n (%) | 7,587 (2.3) | 5,439 (10.0) | 2,147 (0.8) | 5,705 (2.9) | 1,883 (1.5) |
| Diabetes, n (%) | 76,758 (23.8) | 14,364 (26.3) | 62,394 (23.2) | 55,453 (28.2) | 21,305 (16.8) |
| Age, y, mean (SD) | 55.5 (20.0) | 61.0 (17.0) | 54.4 (21.7) | 60.3 (18.9) | 48.0 (23.8) |
| Age, full range (interquartile range) | 0118 (4273) | 18112 (4975) | 0118 (4072) | 0118 (4776) | 0111 (3266) |
| Female, n (%) | 159,227 (49.3) | 23,208 (42.5) | 134,296 (50.1) | 99,805 (50.8) | 59,422 (46.9) |
| White race, n (%) | 226,586 (70.2) | 41,982 (76.8) | 184,604 (68.8) | 132,749 (67.6) | 93,838 (74.1) |
| Insurance, n (%) | |||||
| Medicaid | 54,590 (16.9) | 7,222 (13.2) | 47,378 (17.7) | 35,229 (17.9) | 19,361 (15.3) |
| Medicare | 141,638 (43.9) | 27,458 (50.2) | 114,180 (42.6) | 100,615 (51.2) | 41,023 (32.4) |
| Commercial | 113,013 (35.0) | 18,248 (33.4) | 94,765 (35.3) | 53,510 (27.2) | 59,503 (47.0) |
| Uninsured | 13,521 (4.2) | 1,688 (3.1) | 11,833 (4.4) | 6,878 (3.5) | 6,643 (5.2) |
| Length of stay, d, mean (SD) | 5.4 (9.5) | 11.8 (17.8) | 4.2 (6.2) | 5.46 (10.52) | 5.42 (9.75) |
| Service, n (%) | |||||
| Medicine | 184,495 (57.1) | 27,190 (49.8) | 157,305 (58.6) | 184,496 (94.0) | |
| Surgery | 126,614 (39.2) | 25,602 (46.9) | 101,012 (37.7) | 126,614 (100%) | |
| Neurology | 11,829 (3.7) | 1,853 (3.4) | 9,976 (3.7) | 11,829 (6.0) | |
To explore the effects of dependence among observations from patients with multiple encounters, we compared parameter estimates derived from a model with all patient encounters (including repeated admissions for the same patient) with those from a model with a randomly sampled single visit per patient, and observed that there was no difference in parameter estimates between the 2 classes of models. For all analyses, we used a type I error of 5% (2 sided) to test for statistical significance using SAS version 9.3 (SAS Institute, Cary, NC) or R software (
RESULTS
We included 322,938 patient admissions. Of this sample, 54,645 (16.9%) had spent at least 1 night in the ICU. Overall, 76,758 patients (23.8%) had diabetes, representing 26.3% of ICU patients, 23.2% of non‐ICU patients, 28.2% of medical patients, and 16.8% of surgical patients (see Table 1 for demographic characteristics).
Mortality Trends Within Strata
Among ICU patients, the overall mortality rate was 9.9%: 10.5% of patients with diabetes and 9.8% of patients without diabetes. Among non‐ICU patients, the overall mortality rate was 0.8%: 0.9% of patients with diabetes and 0.7% of patients without diabetes.
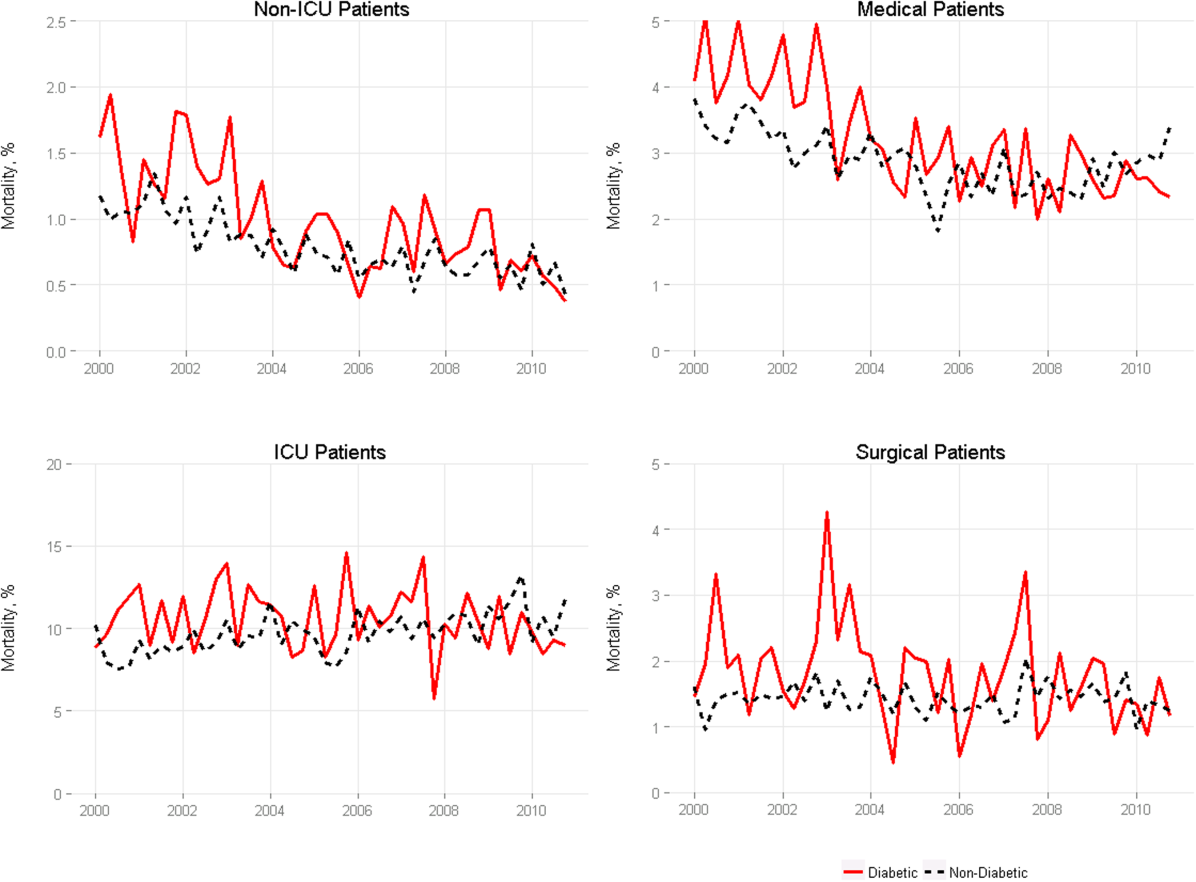
Among medical patients, the overall mortality rate was 2.9%: 3.1% of patients with diabetes and 2.8% of patients without diabetes. Among surgical patients, the overall mortality rate was 1.4%: 1.8% of patients with diabetes and 1.4% of patients without diabetes. Figure 1 shows quarterly in‐hospital mortality for patients with and without diabetes from 2000 to 2010 stratified by ICU status and by service assignment.
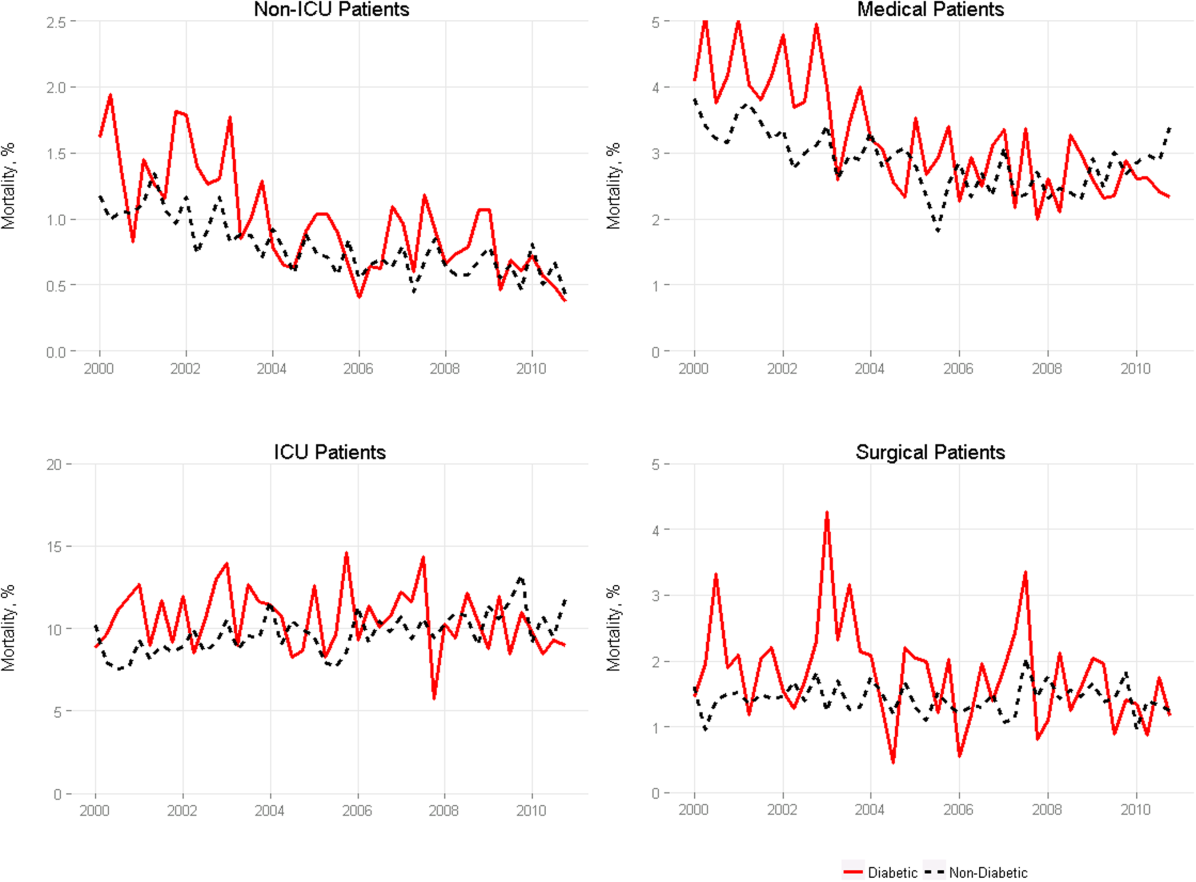
Table 2 describes the difference‐in‐differences regression analyses, stratified by ICU status and service assignment. Among ICU patients (Table 2, model 1), each successive year was associated with a 2.6% relative reduction in the adjusted odds of mortality (odds ratio [OR]: 0.974, 95% confidence interval [CI]: 0.963‐0.985) for patients without diabetes compared to a 7.8% relative reduction for those with diabetes (OR: 0.923, 95% CI: 0.906‐0.940). In other words, patients with diabetes compared to patients without diabetes had a significantly greater decline in odds of adjusted mortality of 5.3% per year (OR: 0.947, 95% CI: 0.927‐0.967). As a result, the adjusted odds of mortality among patients with versus without diabetes decreased from 1.352 in 2000 to 0.772 in 2010.
| Independent Variables | ICU Patients, N=54,646, OR (95% CI) | Non‐ICU Patients, N=268,293, OR (95% CI) | Medical Patients, N=196,325, OR (95% CI) | Surgical Patients, N=126,614, OR (95% CI) |
|---|---|---|---|---|
| Model 1 | Model 2 | Model 3 | Model 4 | |
| ||||
| Year | 0.974 (0.963‐0.985) | 0.925 (0.909‐0.940) | 0.943 (0.933‐0.954) | 0.995 (0.977‐1.103) |
| Diabetes | 1.352 (1.562‐1.171) | 0.958 (0.783‐1.173) | 1.186 (1.037‐1.356) | 1.213 (0.942‐1.563) |
| Diabetes*year | 0.947 (0.927‐0.967) | 0.977 (0.946‐1.008) | 0.961 (0.942‐0.980) | 0.955 (0.918‐0.994) |
| C statistic | 0.812 | 0.907 | 0.880 | 0.919 |
Among non‐ICU patients (Table 2, model 2), each successive year was associated with a 7.5% relative reduction in the adjusted odds of mortality (OR: 0.925, 95% CI: 0.909‐0.940) for patients without diabetes compared to a 9.6% relative reduction for those with diabetes (OR: 0.904, 95% CI: 0.879‐0.929); this greater decline in odds of adjusted mortality of 2.3% per year (OR: 0.977, 95% CI: 0.946‐1.008; P=0.148) was not statistically significant.
We found greater decline in odds of mortality among patients with diabetes than among patients without diabetes over time in both medical patients (3.9% greater decline per year; OR: 0.961, 95% CI: 0.942‐0.980) and surgical patients (4.5% greater decline per year; OR: 0.955, 95% CI: 0.918‐0.994), without a difference between the 2. Detailed results are shown in Table 2, models 3 and 4.
Glycemic Control
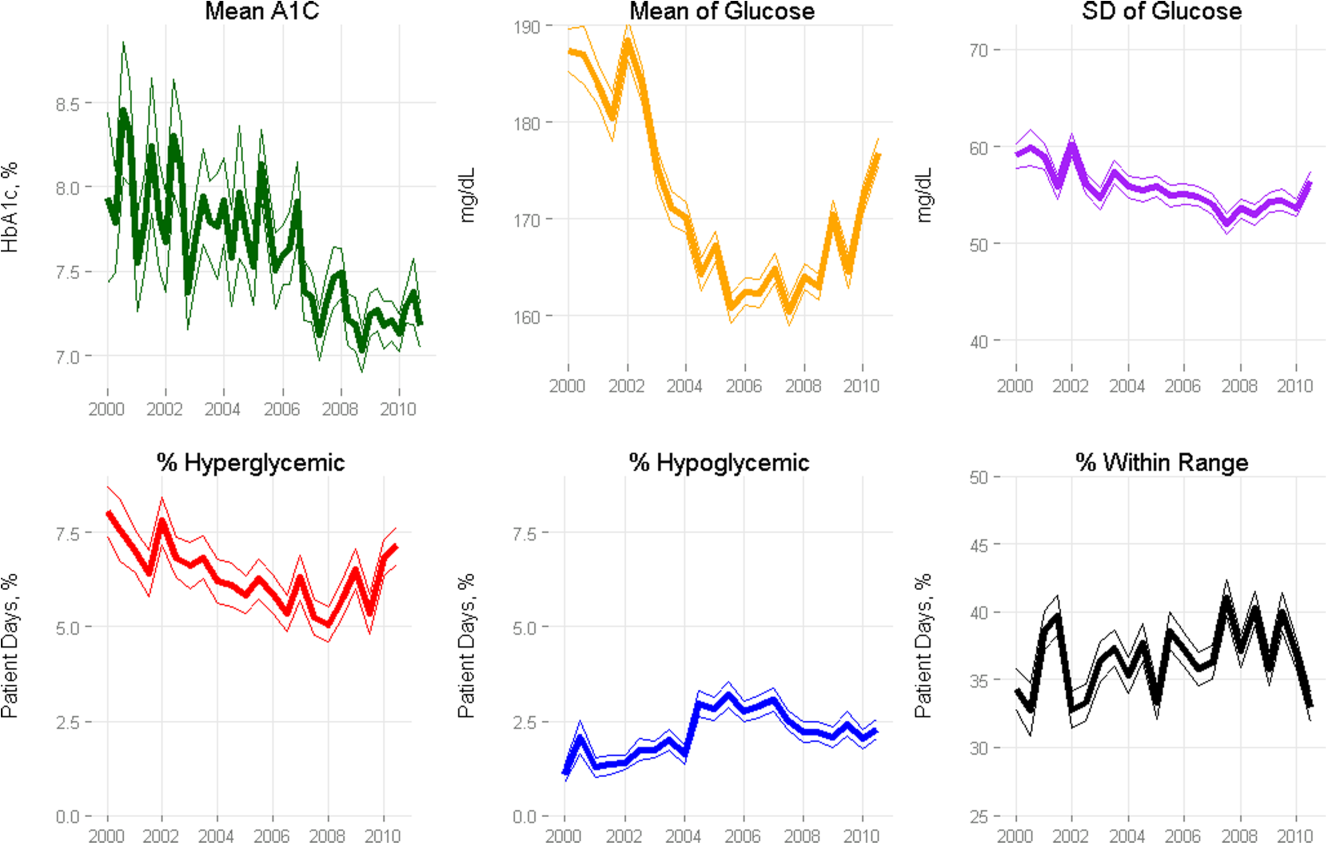
Among ICU patients with diabetes (N=14,364), at least 2 inpatient point‐of‐care glucose readings were available for 13,136 (91.5%), with a mean of 4.67 readings per day, whereas hemoglobin A1c data were available for only 5321 patients (37.0%). Both inpatient glucose data and hemoglobin A1c were available for 4989 patients (34.7%). Figure 2 shows trends in inpatient and outpatient glycemic control measures among ICU patients with diabetes over the study period. Mean hemoglobin A1c decreased from 7.7 in 2000 to 7.3 in 2010. Mean hospitalization glucose began at 187.2, reached a nadir of 162.4 in the third quarter (Q3) of 2007, and rose subsequently to 174.4 with loosened glucose control targets. Standard deviation of mean glucose and percentage of patient‐days with a severe hyperglycemic episode followed a similar pattern, though with nadirs in Q4 2007 and Q2 2008, respectively, whereas percentage of patient‐days with a hypoglycemic episode rose from 1.46% in 2000, peaked at 3.00% in Q3 2005, and returned to 2.15% in 2010. All changes in glucose control are significant with P<0.001.
Mortality Trends and Glycemic Control
To determine whether glucose control explained the excess decline in odds of mortality among patients with diabetes in the ICU, we restricted our sample to ICU patients with diabetes and examined the association of diabetes with mortality after including measures of glucose control.
We first verified that the overall adjusted mortality trend among ICU patients with diabetes for whom we had measures of inpatient glucose control was similar to that of the full sample of ICU patients with diabetes. Similar to the full sample, we found that the adjusted excess odds of death significantly declined by a relative 7.3% each successive year (OR: 0.927, 95% CI: 0.907‐0.947; Table 3, model 1). We then included measures of inpatient glucose control in the model and found, as expected, that a higher percentage of days with severe hyperglycemia and with hypoglycemia was associated with an increased odds of death (P<0.001 for both; Table 3, model 2). Nonetheless, after including measures of inpatient glucose control, we found that the rate of change of excess odds of death for patients with diabetes was unchanged (OR: 0.926, 95% CI: 0.905‐0.947).
| Patients With Inpatient Glucose Control Measures, n=13,136 | Patients With Inpatient and Outpatient Glucose Control Measures, n=4,989 | ||||
|---|---|---|---|---|---|
| Independent Variables | Model 1, OR (95% CI) | Model 2, OR (95% CI) | Model 3, OR (95% CI) | Model 4, OR (95% CI) | Model 5, OR (95% CI) |
| |||||
| Year | 0.927 (0.907‐0.947) | 0.926 (0.905‐0.947) | 0.958 (0.919‐0.998) | 0.956 (0.916‐0.997) | 0.953 (0.914‐0.994) |
| % Severe hyperglycemic days | 1.016 (1.010‐1.021) | 1.009 (0.998‐1.020) | 1.010 (0.999‐1.021) | ||
| % Hypoglycemic days | 1.047 (1.040‐1.055) | 1.051 (1.037‐1.065) | 1.049 (1.036‐1.063) | ||
| % Normoglycemic days | 0.997 (0.994‐1.000) | 0.994 (0.989‐0.999) | 0.993 (0.988‐0.998) | ||
| SD of mean glucose | 0.996 (0.992‐1.000) | 0.993 (0.986‐1.000) | 0.994 (0.987‐1.002) | ||
| Mean HbA1c | 0.892 (0.828‐0.961) | ||||
| C statistic | 0.806 | 0.825 | 0.825 | 0.838 | 0.841 |
We then restricted our sample to patients with diabetes with both inpatient and outpatient glycemic control data and found that, in this subpopulation, the adjusted excess odds of death among patients with diabetes relative to those without significantly declined by a relative 4.2% each progressive year (OR: 0.958, 95% CI: 0.918‐0.998; Table 3, model 3). Including measures of inpatient glucose control in the model did not significantly change the rate of change of excess odds of death (OR: 0.956, 95% CI: 0.916‐0.997; Table 3, model 4), nor did including both measures of inpatient and outpatient glycemic control (OR: 0.953, 95% CI: 0.914‐0.994; Table 3, model 5).
DISCUSSION
We conducted a difference‐in‐difference analysis of in‐hospital mortality rates among adult patients with diabetes compared to patients without diabetes over 10 years, stratifying by ICU status and service assignment. For patients with any ICU stay, we found that the reduction in odds of mortality for patients with diabetes has been 3 times larger than the reduction in odds of mortality for patients without diabetes. For those without an ICU stay, we found no significant difference between patients with and without diabetes in the rate at which in‐hospital mortality declined. We did not find stratification by assignment to a medical or surgical service to be an effect modifier. Finally, despite the fact that our institution achieved better aggregate inpatient glucose control, less severe hyperglycemia, and better long‐term glucose control over the course of the decade, we did not find that either inpatient or outpatient glucose control explained the trend in mortality for patients with diabetes in the ICU. Our study is unique in its inclusion of all hospitalized patients and its ability to simultaneously assess whether both inpatient and outpatient glucose control are explanatory factors in the observed mortality trends.
The fact that improved inpatient glucose control did not explain the trend in mortality for patients with diabetes in the ICU is consistent with the majority of the literature on intensive inpatient glucose control. In randomized trials, intensive glucose control appears to be of greater benefit for patients without diabetes than for patients with diabetes.[31] In fact, in 1 study, patients with diabetes were the only group that did not benefit from intensive glucose control.[32] In our study, it is possible that the rise in hypoglycemia nullified some of the benefits of glucose control. Nationally, hospital admissions for hypoglycemia among Medicare beneficiaries now outnumber admissions for hyperglycemia.[27]
We also do not find that the decline in hemoglobin A1c attenuated the reduction in mortality in the minority of patients for whom these data were available. This is concordant with evidence from 3 randomized clinical trials that have failed to establish a clear beneficial effect of intensive outpatient glucose control on primary cardiovascular endpoints among older, high‐risk patients with type 2 diabetes using glucose‐lowering agents.[21, 22, 23] It is notable, however, that the population for whom we had available hemoglobin A1c results was not representative of the overall population of ICU patients with diabetes. Consequently, there may be an association of outpatient glucose control with inpatient mortality in the overall population of ICU patients with diabetes that we were not able to detect.
The decline in mortality among ICU patients with diabetes in our study may stem from factors other than glycemic control. It is possible that patients were diagnosed earlier in their course of disease in later years of the study period, making the population of patients with diabetes younger or healthier. Of note, however, our risk adjustment models were very robust, with C statistics from 0.82 to 0.92, suggesting that we were able to account for much of the mortality risk attributable to patient clinical and demographic factors. More intensive glucose management may have nonglycemic benefits, such as closer patient observation, which may themselves affect mortality. Alternatively, improved cardiovascular management for patients with diabetes may have decreased the incidence of cardiovascular events. During the study period, evidence from large clinical trials demonstrated the importance of tight blood pressure and lipid management in improving outcomes for patients with diabetes,[33, 34, 35, 36] guidelines for lipid management for patients with diabetes changed,[37] and fewer patients developed cardiovascular complications.[38] Finally, it is possible that our findings can be explained by an improvement in treatment of complications for which patients with diabetes previously have had disproportionately worse outcomes, such as percutaneous coronary intervention.[39]
Our findings may have important implications for both clinicians and policymakers. Changes in inpatient glucose management have required substantial additional resources on the part of hospitals. Our evidence regarding the questionable impact of inpatient glucose control on in‐hospital mortality trends for patients with diabetes is disappointing and highlights the need for multifaceted evaluation of the impact of such quality initiatives. There may, for instance, be benefits from tighter blood glucose control in the hospital beyond mortality, such as reduced infections, costs, or length of stay. On the outpatient side, our more limited data are consistent with recent studies that have not been able to show a mortality benefit in older diabetic patients from more stringent glycemic control. A reassessment of prevailing diabetes‐related quality measures, as recently called for by some,[40, 41] seems reasonable.
Our study must be interpreted in light of its limitations. It is possible that the improvements in glucose management were too small to result in a mortality benefit. The overall reduction of 25 mg dL achieved at our institution is less than the 33 to 50 mg/dL difference between intensive and conventional groups in those randomized clinical trials that have found reductions in mortality.[11, 42] In addition, an increase in mean glucose during the last 1 to 2 years of the observation period (in response to prevailing guidelines) could potentially have attenuated any benefit on mortality. The study does not include other important clinical endpoints, such as infections, complications, length of stay, and hospital costs. Additionally, we did not examine postdischarge mortality, which might have shown a different pattern. The small proportion of patients with hemoglobin A1c results may have hampered our ability to detect an effect of outpatient glucose control. Consequently, our findings regarding outpatient glucose control are only suggestive. Finally, our findings represent the experience of a single, large academic medical center and may not be generalizable to all settings.
Overall, we found that patients with diabetes in the ICU have experienced a disproportionate reduction in in‐hospital mortality over time that does not appear to be explained by improvements in either inpatient or outpatient glucose control. Although improved glycemic control may have other benefits, it does not appear to impact in‐hospital mortality. Our real‐world empirical results contribute to the discourse among clinicians and policymakers with regards to refocusing the approach to managing glucose in‐hospital and readjudication of diabetes‐related quality measures.
Acknowledgments
The authors would like to acknowledge the YaleNew Haven Hospital diabetes management team: Gael Ulisse, APRN, Helen Psarakis, APRN, Anne Kaisen, APRN, and the Yale Endocrine Fellows.
Disclosures: Design and conduct of the study: N. B., J. D., S. I., T. B., L. H. Collection, management, analysis, and interpretation of the data: N. B., B. J., J. D., J. R., J. B., S. I., L. H. Preparation, review, or approval of the manuscript: N. B., B. J., J. D., J. R., S. I., T. B., L. H. Leora Horwitz, MD, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis. Dr. Horwitz is supported by the National Institute on Aging (K08 AG038336) and by the American Federation for Aging Research through the Paul B. Beeson Career Development Award Program. This publication was also made possible by CTSA grant number UL1 RR024139 from the National Center for Research Resources and the National Center for Advancing Translational Science, components of the National Institutes of Health (NIH), and NIH roadmap for Medical Research. Its contents are solely the responsibility of the authors and do not necessarily represent the official view of the NIH. No funding source had any role in design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; or decision to submit the manuscript for publication. Silvio E. Inzucchi, MD, serves on a Data Safety Monitoring Board for Novo Nordisk, a manufacturer of insulin products used in the hospital setting. The remaining authors declare no conflicts of interest.
- National Diabetes Information Clearinghouse. National Diabetes Statistics; 2011. Available at: http://diabetes.niddk.nih.gov/dm/pubs/america/index.aspx. Accessed November 12, 2013.
- Healthcare Cost and Utilization Project. Statistical brief #93; 2010. Available at: http://www.hcup‐us.ahrq.gov/reports/statbriefs/sb93.pdf. Accessed November 12, 2013.
- , , , et al. Association between diabetes mellitus and post‐discharge outcomes in patients hospitalized with heart failure: findings from the EVEREST trial. Eur J Heart Fail. 2013;15(2):194–202.
- , , , et al. Influence of diabetes mellitus on clinical outcome in the thrombolytic era of acute myocardial infarction. GUSTO‐I Investigators. Global Utilization of Streptokinase and Tissue Plasminogen Activator for Occluded Coronary Arteries. J Am Coll Cardiol. 1997;30(1):171–179.
- , , , , , . Type 2 diabetes and pneumonia outcomes: a population‐based cohort study. Diabetes Care. 2007;30(9):2251–2257.
- , , , . Prevalence and outcomes of diabetes, hypertension and cardiovascular disease in COPD. Eur Respir J. 2008;32(4):962–969.
- , , , , . The role of body mass index and diabetes in the development of acute organ failure and subsequent mortality in an observational cohort. Crit Care. 2006;10(5):R137.
- , , , , , . Type 2 diabetes and 1‐year mortality in intensive care unit patients. Eur J Clin Invest. 2013;43(3):238–247.
- , , . Excess mortality during hospital stays among patients with recorded diabetes compared with those without diabetes. Diabet Med. 2013;30(12):1393–1402.
- , , , et al. Decade‐long trends in mortality among patients with and without diabetes mellitus at a major academic medical center. JAMA Intern Med. 2014;174(7):1187–1188.
- , , , et al. Intensive insulin therapy in critically ill patients. N Engl J Med. 2001;345(19):1359–1367.
- , , , et al. Intensive versus conventional glucose control in critically ill patients. N Engl J Med. 2009;360(13):1283–1297.
- , , , et al. A prospective randomised multi‐centre controlled trial on tight glucose control by intensive insulin therapy in adult intensive care units: the Glucontrol study. Intensive Care Med. 2009;35(10):1738–1748.
- , , , et al. Intensive versus conventional insulin therapy: a randomized controlled trial in medical and surgical critically ill patients. Crit Care Med. 2008;36(12):3190–3197.
- , , , et al. Intensive insulin therapy in the medical ICU. N Engl J Med. 2006;354(5):449–461.
- , , , et al. Glycemic control in non‐critically ill hospitalized patients: a systematic review and meta‐analysis. J Clin Endocrinol Metab. 2012;97(1):49–58.
- , , , et al. American Association of Clinical Endocrinologists and American Diabetes Association consensus statement on inpatient glycemic control. Diabetes Care. 2009;32(6):1119–1131.
- Agency for Healthcare Research and Quality National Quality Measures Clearinghouse. Percent of cardiac surgery patients with controlled 6 A.M. postoperative blood glucose; 2012. Available at: http://www.qualitymeasures.ahrq.gov/content.aspx?id=35532. Accessed November 12, 2013.
- The effect of intensive treatment of diabetes on the development and progression of long‐term complications in insulin‐dependent diabetes mellitus. The Diabetes Control and Complications Trial Research Group. N Engl J Med. 1993;329(14):977–986.
- , , , et al. Intensive blood‐glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33). Lancet. 1998;352(9131):837–853.
- Effects of intensive glucose lowering in type 2 diabetes. N Engl J Med. 2008;358(24):2545–2559.
- , , , et al. Glucose control and vascular complications in veterans with type 2 diabetes. N Engl J Med. 2009;360(2):129–139.
- , , , et al. Intensive blood glucose control and vascular outcomes in patients with type 2 diabetes. N Engl J Med. 2008;358(24):2560–2572.
- . Standards of medical care in diabetes—2014. Diabetes Care. 2014;37(suppl 1):S14–S80.
- National Committee for Quality Assurance. HEDIS 2013. Available at: http://www.ncqa.org/HEDISQualityMeasurement.aspx. Accessed November 12, 2013.
- , , , . Is glycemic control improving in US adults? Diabetes Care. 2008;31(1):81–86.
- , , , et al. National trends in US hospital admissions for hyperglycemia and hypoglycemia among medicare beneficiaries, 1999 to 2011. JAMA Intern Med. 2014;174(7):1116–1124.
- , , , et al. "Glucometrics"—assessing the quality of inpatient glucose management. Diabetes Technol Ther. 2006;8(5):560–569.
- , , , , . A modification of the Elixhauser comorbidity measures into a point system for hospital death using administrative data. Med Care. 2009;47(6):626–633.
- Healthcare Cost and Utilization Project. Clinical Classifications Software (CCS) for ICD‐9‐CM; 2013. Available at: http://www.hcup‐us.ahrq.gov/toolssoftware/ccs/ccs.jsp. Accessed November 12, 2013.
- , , , , . The impact of premorbid diabetic status on the relationship between the three domains of glycemic control and mortality in critically ill patients. Curr Opin Clin Nutr Metab Care. 2012;15(2):151–160.
- , , , et al. Intensive insulin therapy in mixed medical/surgical intensive care units: benefit versus harm. Diabetes. 2006;55(11):3151–3159.
- Tight blood pressure control and risk of macrovascular and microvascular complications in type 2 diabetes: UKPDS 38. UK Prospective Diabetes Study Group. BMJ. 1998;317(7160):703–713.
- , , , et al. Effects of a fixed combination of perindopril and indapamide on macrovascular and microvascular outcomes in patients with type 2 diabetes mellitus (the ADVANCE trial): a randomised controlled trial. Lancet. 2007;370(9590):829–840.
- , , , , . MRC/BHF heart protection study of cholesterol‐lowering with simvastatin in 5963 people with diabetes: a randomised placebo‐controlled trial. Lancet. 2003;361(9374):2005–2016.
- , , , et al. Primary prevention of cardiovascular disease with atorvastatin in type 2 diabetes in the Collaborative Atorvastatin Diabetes Study (CARDS): multicentre randomised placebo‐controlled trial. Lancet. 2004;364(9435):685–696.
- , , , . Expert panel on detection, evaluation and treatment of high blood cholesterol in adults. Executive summary of the third report of the national cholesterol education program (NCEP) adult treatment panel (atp III). JAMA. 2001;285(19):2486–2497.
- , , , et al. Changes in diabetes‐related complications in the United States, 1990–2010. N Engl J Med. 2014;370(16):1514–1523.
- , , . Coronary heart disease in patients with diabetes: part II: recent advances in coronary revascularization. J Am Coll Cardiol. 2007;49(6):643–656.
- , , , et al. Management of hyperglycemia in type 2 diabetes: a patient‐centered approach position statement of the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD). Diabetes Care. 2012;35(6):1364–1379.
- , , , , . Assessing potential glycemic overtreatment in persons at hypoglycemic risk. JAMA Intern Med. 2013;174(2):259–268.
- , , , . Glycometabolic state at admission: important risk marker of mortality in conventionally treated patients with diabetes mellitus and acute myocardial infarction: long‐term results from the Diabetes and Insulin‐Glucose Infusion in Acute Myocardial Infarction (DIGAMI) study. Circulation. 1999;99(20):2626–2632.
Patients with diabetes currently comprise over 8% of the US population (over 25 million people) and more than 20% of hospitalized patients.[1, 2] Hospitalizations of patients with diabetes account for 23% of total hospital costs in the United States,[2] and patients with diabetes have worse outcomes after hospitalization for a variety of common medical conditions,[3, 4, 5, 6] as well as in intensive care unit (ICU) settings.[7, 8] Individuals with diabetes have historically experienced higher inpatient mortality than individuals without diabetes.[9] However, we recently reported that patients with diabetes at our large academic medical center have experienced a disproportionate reduction in in‐hospital mortality relative to patients without diabetes over the past decade.[10] This surprising trend begs further inquiry.
Improvement in in‐hospital mortality among patients with diabetes may stem from improved inpatient glycemic management. The landmark 2001 study by van den Berghe et al. demonstrating that intensive insulin therapy reduced postsurgical mortality among ICU patients ushered in an era of intensive inpatient glucose control.[11] However, follow‐up multicenter studies have not been able to replicate these results.[12, 13, 14, 15] In non‐ICU and nonsurgical settings, intensive glucose control has not yet been shown to have any mortality benefit, although it may impact other morbidities, such as postoperative infections.[16] Consequently, less stringent glycemic targets are now recommended.[17] Nonetheless, hospitals are being held accountable for certain aspects of inpatient glucose control. For example, the Centers for Medicare & Medicaid Services (CMS) began asking hospitals to report inpatient glucose control in cardiac surgery patients in 2004.[18] This measure is now publicly reported, and as of 2013 is included in the CMS Value‐Based Purchasing Program, which financially penalizes hospitals that do not meet targets.
Outpatient diabetes standards have also evolved in the past decade. The Diabetes Control and Complications Trial in 1993 and the United Kingdom Prospective Diabetes Study in 1997 demonstrated that better glycemic control in type 1 and newly diagnosed type 2 diabetes patients, respectively, improved clinical outcomes, and prompted guidelines for pharmacologic treatment of diabetic patients.[19, 20] However, subsequent randomized clinical trials have failed to establish a clear beneficial effect of intensive glucose control on primary cardiovascular endpoints among higher‐risk patients with longstanding type 2 diabetes,[21, 22, 23] and clinical practice recommendations now accept a more individualized approach to glycemic control.[24] Nonetheless, clinicians are also being held accountable for outpatient glucose control.[25]
To better understand the disproportionate reduction in mortality among hospitalized patients with diabetes that we observed, we first examined whether it was limited to surgical patients or patients in the ICU, the populations that have been demonstrated to benefit from intensive inpatient glucose control. Furthermore, given recent improvements in inpatient and outpatient glycemic control,[26, 27] we examined whether inpatient or outpatient glucose control explained the mortality trends. Results from this study contribute empirical evidence on real‐world effects of efforts to improve inpatient and outpatient glycemic control.
METHODS
Setting
During the study period, YaleNew Haven Hospital (YNHH) was an urban academic medical center in New Haven, Connecticut, with over 950 beds and an average of approximately 32,000 annual adult nonobstetric admissions. YNHH conducted a variety of inpatient glucose control initiatives during the study period. The surgical ICU began an informal medical teamdirected insulin infusion protocol in 2000 to 2001. In 2002, the medical ICU instituted a formal insulin infusion protocol with a target of 100 to 140 mg/dL, which spread to remaining hospital ICUs by the end of 2003. In 2005, YNHH launched a consultative inpatient diabetes management team to assist clinicians in controlling glucose in non‐ICU patients with diabetes. This team covered approximately 10 to 15 patients at a time and consisted of an advanced‐practice nurse practitioner, a supervising endocrinologist and endocrinology fellow, and a nurse educator to provide diabetic teaching. Additionally, in 2005, basal‐boluscorrection insulin order sets became available. The surgical ICU implemented a stringent insulin infusion protocol with target glucose of 80 to 110 mg/dL in 2006, but relaxed it (goal 80150 mg/dL) in 2007. Similarly, in 2006, YNHH made ICU insulin infusion recommendations more stringent in remaining ICUs (goal 90130 mg/dL), but relaxed them in 2010 (goal 120160 mg/dL), based on emerging data from clinical trials and prevailing national guidelines.
Participants and Data Sources
We included all adult, nonobstetric discharges from YNHH between January 1, 2000 and December 31, 2010. Repeat visits by the same patient were linked by medical record number. We obtained data from YNHH administrative billing, laboratory, and point‐of‐care capillary blood glucose databases. The Yale Human Investigation Committee approved our study design and granted a Health Insurance Portability and Accountability Act waiver and a waiver of patient consent.
Variables
Our primary endpoint was in‐hospital mortality. The primary exposure of interest was whether a patient had diabetes mellitus, defined as the presence of International Classification of Diseases, Ninth Revision codes 249.x, 250.x, V4585, V5391, or V6546 in any of the primary or secondary diagnosis codes in the index admission, or in any hospital encounter in the year prior to the index admission.
We assessed 2 effect‐modifying variables: ICU status (as measured by a charge for at least 1 night in the ICU) and service assignment to surgery (including neurosurgery and orthopedics), compared to medicine (including neurology). Independent explanatory variables included time between the start of the study and patient admission (measured as days/365), diabetes status, inpatient glucose control, and long‐term glucose control (as measured by hemoglobin A1c at any time in the 180 days prior to hospital admission in order to have adequate sample size). We assessed inpatient blood glucose control through point‐of‐care blood glucose meters (OneTouch SureStep; LifeScan, Inc., Milipitas, CA) at YNHH. We used 4 validated measures of inpatient glucose control: the proportion of days in each hospitalization in which there was any hypoglycemic episode (blood glucose value <70 mg/dL), the proportion of days in which there was any severely hyperglycemic episode (blood glucose value >299 mg/dL), the proportion of days in which mean blood glucose was considered to be within adequate control (all blood glucose values between 70 and 179 mg/dL), and the standard deviation of mean glucose during hospitalization as a measure of glycemic variability.[28]
Covariates included gender, age at time of admission, length of stay in days, race (defined by hospital registration), payer, Elixhauser comorbidity dummy variables (revised to exclude diabetes and to use only secondary diagnosis codes),[29] and primary discharge diagnosis grouped using Clinical Classifications Software,[30] based on established associations with in‐hospital mortality.
Statistical Analysis
We summarized demographic characteristics numerically and graphically for patients with and without diabetes and compared them using [2] and t tests. We summarized changes in inpatient and outpatient measures of glucose control over time numerically and graphically, and compared across years using the Wilcoxon rank sum test adjusted for multiple hypothesis testing.
We stratified all analyses first by ICU status and then by service assignment (medicine vs surgery). Statistical analyses within each stratum paralleled our previous approach to the full study cohort.[10] Taking each stratum separately (ie, only ICU patients or only medicine patients), we used a difference‐in‐differences approach comparing changes over time in in‐hospital mortality among patients with diabetes compared to those without diabetes. This approach enabled us to determine whether patients with diabetes had a different time trend in risk of in‐hospital mortality than those without diabetes. That is, for each stratum, we constructed multivariate logistic regression models including time in years, diabetes status, and the interaction between time and diabetes status as well as the aforementioned covariates. We calculated odds of death and confidence intervals for each additional year for patients with diabetes by exponentiating the sum of parameter estimates for time and the diabetes‐time interaction term. We evaluated all 2‐way interactions between year or diabetes status and the covariates in a multiple degree of freedom likelihood ratio test. We investigated nonlinearity of the relation between mortality and time by evaluating first and second‐order polynomials.
Because we found a significant decline in mortality risk for patients with versus without diabetes among ICU patients but not among non‐ICU patients, and because service assignment was not found to be an effect modifier, we then limited our sample to ICU patients with diabetes to better understand the role of inpatient and outpatient glucose control in accounting for observed mortality trends. First, we determined the relation between the measures of inpatient glucose control and changes in mortality over time using logistic regression. Then, we repeated this analysis in the subsets of patients who had inpatient glucose data and both inpatient and outpatient glycemic control data, adding inpatient and outpatient measures sequentially. Given the high level of missing outpatient glycemic control data, we compared demographic characteristics for diabetic ICU patients with and without such data using [2] and t tests, and found that patients with data were younger and less likely to be white and had longer mean length of stay, slightly worse performance on several measures of inpatient glucose control, and lower mortality (see Supporting Table 1 in the online version of this article).
| Characteristic | Overall, N=322,939 | Any ICU Stay, N=54,646 | No ICU Stay, N=268,293 | Medical Service, N=196,325 | Surgical Service, N=126,614 |
|---|---|---|---|---|---|
| |||||
| Died during admission, n (%) | 7,587 (2.3) | 5,439 (10.0) | 2,147 (0.8) | 5,705 (2.9) | 1,883 (1.5) |
| Diabetes, n (%) | 76,758 (23.8) | 14,364 (26.3) | 62,394 (23.2) | 55,453 (28.2) | 21,305 (16.8) |
| Age, y, mean (SD) | 55.5 (20.0) | 61.0 (17.0) | 54.4 (21.7) | 60.3 (18.9) | 48.0 (23.8) |
| Age, full range (interquartile range) | 0118 (4273) | 18112 (4975) | 0118 (4072) | 0118 (4776) | 0111 (3266) |
| Female, n (%) | 159,227 (49.3) | 23,208 (42.5) | 134,296 (50.1) | 99,805 (50.8) | 59,422 (46.9) |
| White race, n (%) | 226,586 (70.2) | 41,982 (76.8) | 184,604 (68.8) | 132,749 (67.6) | 93,838 (74.1) |
| Insurance, n (%) | |||||
| Medicaid | 54,590 (16.9) | 7,222 (13.2) | 47,378 (17.7) | 35,229 (17.9) | 19,361 (15.3) |
| Medicare | 141,638 (43.9) | 27,458 (50.2) | 114,180 (42.6) | 100,615 (51.2) | 41,023 (32.4) |
| Commercial | 113,013 (35.0) | 18,248 (33.4) | 94,765 (35.3) | 53,510 (27.2) | 59,503 (47.0) |
| Uninsured | 13,521 (4.2) | 1,688 (3.1) | 11,833 (4.4) | 6,878 (3.5) | 6,643 (5.2) |
| Length of stay, d, mean (SD) | 5.4 (9.5) | 11.8 (17.8) | 4.2 (6.2) | 5.46 (10.52) | 5.42 (9.75) |
| Service, n (%) | |||||
| Medicine | 184,495 (57.1) | 27,190 (49.8) | 157,305 (58.6) | 184,496 (94.0) | |
| Surgery | 126,614 (39.2) | 25,602 (46.9) | 101,012 (37.7) | 126,614 (100%) | |
| Neurology | 11,829 (3.7) | 1,853 (3.4) | 9,976 (3.7) | 11,829 (6.0) | |
To explore the effects of dependence among observations from patients with multiple encounters, we compared parameter estimates derived from a model with all patient encounters (including repeated admissions for the same patient) with those from a model with a randomly sampled single visit per patient, and observed that there was no difference in parameter estimates between the 2 classes of models. For all analyses, we used a type I error of 5% (2 sided) to test for statistical significance using SAS version 9.3 (SAS Institute, Cary, NC) or R software (
RESULTS
We included 322,938 patient admissions. Of this sample, 54,645 (16.9%) had spent at least 1 night in the ICU. Overall, 76,758 patients (23.8%) had diabetes, representing 26.3% of ICU patients, 23.2% of non‐ICU patients, 28.2% of medical patients, and 16.8% of surgical patients (see Table 1 for demographic characteristics).
Mortality Trends Within Strata
Among ICU patients, the overall mortality rate was 9.9%: 10.5% of patients with diabetes and 9.8% of patients without diabetes. Among non‐ICU patients, the overall mortality rate was 0.8%: 0.9% of patients with diabetes and 0.7% of patients without diabetes.
Among medical patients, the overall mortality rate was 2.9%: 3.1% of patients with diabetes and 2.8% of patients without diabetes. Among surgical patients, the overall mortality rate was 1.4%: 1.8% of patients with diabetes and 1.4% of patients without diabetes. Figure 1 shows quarterly in‐hospital mortality for patients with and without diabetes from 2000 to 2010 stratified by ICU status and by service assignment.

Table 2 describes the difference‐in‐differences regression analyses, stratified by ICU status and service assignment. Among ICU patients (Table 2, model 1), each successive year was associated with a 2.6% relative reduction in the adjusted odds of mortality (odds ratio [OR]: 0.974, 95% confidence interval [CI]: 0.963‐0.985) for patients without diabetes compared to a 7.8% relative reduction for those with diabetes (OR: 0.923, 95% CI: 0.906‐0.940). In other words, patients with diabetes compared to patients without diabetes had a significantly greater decline in odds of adjusted mortality of 5.3% per year (OR: 0.947, 95% CI: 0.927‐0.967). As a result, the adjusted odds of mortality among patients with versus without diabetes decreased from 1.352 in 2000 to 0.772 in 2010.
| Independent Variables | ICU Patients, N=54,646, OR (95% CI) | Non‐ICU Patients, N=268,293, OR (95% CI) | Medical Patients, N=196,325, OR (95% CI) | Surgical Patients, N=126,614, OR (95% CI) |
|---|---|---|---|---|
| Model 1 | Model 2 | Model 3 | Model 4 | |
| ||||
| Year | 0.974 (0.963‐0.985) | 0.925 (0.909‐0.940) | 0.943 (0.933‐0.954) | 0.995 (0.977‐1.103) |
| Diabetes | 1.352 (1.562‐1.171) | 0.958 (0.783‐1.173) | 1.186 (1.037‐1.356) | 1.213 (0.942‐1.563) |
| Diabetes*year | 0.947 (0.927‐0.967) | 0.977 (0.946‐1.008) | 0.961 (0.942‐0.980) | 0.955 (0.918‐0.994) |
| C statistic | 0.812 | 0.907 | 0.880 | 0.919 |
Among non‐ICU patients (Table 2, model 2), each successive year was associated with a 7.5% relative reduction in the adjusted odds of mortality (OR: 0.925, 95% CI: 0.909‐0.940) for patients without diabetes compared to a 9.6% relative reduction for those with diabetes (OR: 0.904, 95% CI: 0.879‐0.929); this greater decline in odds of adjusted mortality of 2.3% per year (OR: 0.977, 95% CI: 0.946‐1.008; P=0.148) was not statistically significant.
We found greater decline in odds of mortality among patients with diabetes than among patients without diabetes over time in both medical patients (3.9% greater decline per year; OR: 0.961, 95% CI: 0.942‐0.980) and surgical patients (4.5% greater decline per year; OR: 0.955, 95% CI: 0.918‐0.994), without a difference between the 2. Detailed results are shown in Table 2, models 3 and 4.
Glycemic Control
Among ICU patients with diabetes (N=14,364), at least 2 inpatient point‐of‐care glucose readings were available for 13,136 (91.5%), with a mean of 4.67 readings per day, whereas hemoglobin A1c data were available for only 5321 patients (37.0%). Both inpatient glucose data and hemoglobin A1c were available for 4989 patients (34.7%). Figure 2 shows trends in inpatient and outpatient glycemic control measures among ICU patients with diabetes over the study period. Mean hemoglobin A1c decreased from 7.7 in 2000 to 7.3 in 2010. Mean hospitalization glucose began at 187.2, reached a nadir of 162.4 in the third quarter (Q3) of 2007, and rose subsequently to 174.4 with loosened glucose control targets. Standard deviation of mean glucose and percentage of patient‐days with a severe hyperglycemic episode followed a similar pattern, though with nadirs in Q4 2007 and Q2 2008, respectively, whereas percentage of patient‐days with a hypoglycemic episode rose from 1.46% in 2000, peaked at 3.00% in Q3 2005, and returned to 2.15% in 2010. All changes in glucose control are significant with P<0.001.

Mortality Trends and Glycemic Control
To determine whether glucose control explained the excess decline in odds of mortality among patients with diabetes in the ICU, we restricted our sample to ICU patients with diabetes and examined the association of diabetes with mortality after including measures of glucose control.
We first verified that the overall adjusted mortality trend among ICU patients with diabetes for whom we had measures of inpatient glucose control was similar to that of the full sample of ICU patients with diabetes. Similar to the full sample, we found that the adjusted excess odds of death significantly declined by a relative 7.3% each successive year (OR: 0.927, 95% CI: 0.907‐0.947; Table 3, model 1). We then included measures of inpatient glucose control in the model and found, as expected, that a higher percentage of days with severe hyperglycemia and with hypoglycemia was associated with an increased odds of death (P<0.001 for both; Table 3, model 2). Nonetheless, after including measures of inpatient glucose control, we found that the rate of change of excess odds of death for patients with diabetes was unchanged (OR: 0.926, 95% CI: 0.905‐0.947).
| Patients With Inpatient Glucose Control Measures, n=13,136 | Patients With Inpatient and Outpatient Glucose Control Measures, n=4,989 | ||||
|---|---|---|---|---|---|
| Independent Variables | Model 1, OR (95% CI) | Model 2, OR (95% CI) | Model 3, OR (95% CI) | Model 4, OR (95% CI) | Model 5, OR (95% CI) |
| |||||
| Year | 0.927 (0.907‐0.947) | 0.926 (0.905‐0.947) | 0.958 (0.919‐0.998) | 0.956 (0.916‐0.997) | 0.953 (0.914‐0.994) |
| % Severe hyperglycemic days | 1.016 (1.010‐1.021) | 1.009 (0.998‐1.020) | 1.010 (0.999‐1.021) | ||
| % Hypoglycemic days | 1.047 (1.040‐1.055) | 1.051 (1.037‐1.065) | 1.049 (1.036‐1.063) | ||
| % Normoglycemic days | 0.997 (0.994‐1.000) | 0.994 (0.989‐0.999) | 0.993 (0.988‐0.998) | ||
| SD of mean glucose | 0.996 (0.992‐1.000) | 0.993 (0.986‐1.000) | 0.994 (0.987‐1.002) | ||
| Mean HbA1c | 0.892 (0.828‐0.961) | ||||
| C statistic | 0.806 | 0.825 | 0.825 | 0.838 | 0.841 |
We then restricted our sample to patients with diabetes with both inpatient and outpatient glycemic control data and found that, in this subpopulation, the adjusted excess odds of death among patients with diabetes relative to those without significantly declined by a relative 4.2% each progressive year (OR: 0.958, 95% CI: 0.918‐0.998; Table 3, model 3). Including measures of inpatient glucose control in the model did not significantly change the rate of change of excess odds of death (OR: 0.956, 95% CI: 0.916‐0.997; Table 3, model 4), nor did including both measures of inpatient and outpatient glycemic control (OR: 0.953, 95% CI: 0.914‐0.994; Table 3, model 5).
DISCUSSION
We conducted a difference‐in‐difference analysis of in‐hospital mortality rates among adult patients with diabetes compared to patients without diabetes over 10 years, stratifying by ICU status and service assignment. For patients with any ICU stay, we found that the reduction in odds of mortality for patients with diabetes has been 3 times larger than the reduction in odds of mortality for patients without diabetes. For those without an ICU stay, we found no significant difference between patients with and without diabetes in the rate at which in‐hospital mortality declined. We did not find stratification by assignment to a medical or surgical service to be an effect modifier. Finally, despite the fact that our institution achieved better aggregate inpatient glucose control, less severe hyperglycemia, and better long‐term glucose control over the course of the decade, we did not find that either inpatient or outpatient glucose control explained the trend in mortality for patients with diabetes in the ICU. Our study is unique in its inclusion of all hospitalized patients and its ability to simultaneously assess whether both inpatient and outpatient glucose control are explanatory factors in the observed mortality trends.
The fact that improved inpatient glucose control did not explain the trend in mortality for patients with diabetes in the ICU is consistent with the majority of the literature on intensive inpatient glucose control. In randomized trials, intensive glucose control appears to be of greater benefit for patients without diabetes than for patients with diabetes.[31] In fact, in 1 study, patients with diabetes were the only group that did not benefit from intensive glucose control.[32] In our study, it is possible that the rise in hypoglycemia nullified some of the benefits of glucose control. Nationally, hospital admissions for hypoglycemia among Medicare beneficiaries now outnumber admissions for hyperglycemia.[27]
We also do not find that the decline in hemoglobin A1c attenuated the reduction in mortality in the minority of patients for whom these data were available. This is concordant with evidence from 3 randomized clinical trials that have failed to establish a clear beneficial effect of intensive outpatient glucose control on primary cardiovascular endpoints among older, high‐risk patients with type 2 diabetes using glucose‐lowering agents.[21, 22, 23] It is notable, however, that the population for whom we had available hemoglobin A1c results was not representative of the overall population of ICU patients with diabetes. Consequently, there may be an association of outpatient glucose control with inpatient mortality in the overall population of ICU patients with diabetes that we were not able to detect.
The decline in mortality among ICU patients with diabetes in our study may stem from factors other than glycemic control. It is possible that patients were diagnosed earlier in their course of disease in later years of the study period, making the population of patients with diabetes younger or healthier. Of note, however, our risk adjustment models were very robust, with C statistics from 0.82 to 0.92, suggesting that we were able to account for much of the mortality risk attributable to patient clinical and demographic factors. More intensive glucose management may have nonglycemic benefits, such as closer patient observation, which may themselves affect mortality. Alternatively, improved cardiovascular management for patients with diabetes may have decreased the incidence of cardiovascular events. During the study period, evidence from large clinical trials demonstrated the importance of tight blood pressure and lipid management in improving outcomes for patients with diabetes,[33, 34, 35, 36] guidelines for lipid management for patients with diabetes changed,[37] and fewer patients developed cardiovascular complications.[38] Finally, it is possible that our findings can be explained by an improvement in treatment of complications for which patients with diabetes previously have had disproportionately worse outcomes, such as percutaneous coronary intervention.[39]
Our findings may have important implications for both clinicians and policymakers. Changes in inpatient glucose management have required substantial additional resources on the part of hospitals. Our evidence regarding the questionable impact of inpatient glucose control on in‐hospital mortality trends for patients with diabetes is disappointing and highlights the need for multifaceted evaluation of the impact of such quality initiatives. There may, for instance, be benefits from tighter blood glucose control in the hospital beyond mortality, such as reduced infections, costs, or length of stay. On the outpatient side, our more limited data are consistent with recent studies that have not been able to show a mortality benefit in older diabetic patients from more stringent glycemic control. A reassessment of prevailing diabetes‐related quality measures, as recently called for by some,[40, 41] seems reasonable.
Our study must be interpreted in light of its limitations. It is possible that the improvements in glucose management were too small to result in a mortality benefit. The overall reduction of 25 mg dL achieved at our institution is less than the 33 to 50 mg/dL difference between intensive and conventional groups in those randomized clinical trials that have found reductions in mortality.[11, 42] In addition, an increase in mean glucose during the last 1 to 2 years of the observation period (in response to prevailing guidelines) could potentially have attenuated any benefit on mortality. The study does not include other important clinical endpoints, such as infections, complications, length of stay, and hospital costs. Additionally, we did not examine postdischarge mortality, which might have shown a different pattern. The small proportion of patients with hemoglobin A1c results may have hampered our ability to detect an effect of outpatient glucose control. Consequently, our findings regarding outpatient glucose control are only suggestive. Finally, our findings represent the experience of a single, large academic medical center and may not be generalizable to all settings.
Overall, we found that patients with diabetes in the ICU have experienced a disproportionate reduction in in‐hospital mortality over time that does not appear to be explained by improvements in either inpatient or outpatient glucose control. Although improved glycemic control may have other benefits, it does not appear to impact in‐hospital mortality. Our real‐world empirical results contribute to the discourse among clinicians and policymakers with regards to refocusing the approach to managing glucose in‐hospital and readjudication of diabetes‐related quality measures.
Acknowledgments
The authors would like to acknowledge the YaleNew Haven Hospital diabetes management team: Gael Ulisse, APRN, Helen Psarakis, APRN, Anne Kaisen, APRN, and the Yale Endocrine Fellows.
Disclosures: Design and conduct of the study: N. B., J. D., S. I., T. B., L. H. Collection, management, analysis, and interpretation of the data: N. B., B. J., J. D., J. R., J. B., S. I., L. H. Preparation, review, or approval of the manuscript: N. B., B. J., J. D., J. R., S. I., T. B., L. H. Leora Horwitz, MD, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis. Dr. Horwitz is supported by the National Institute on Aging (K08 AG038336) and by the American Federation for Aging Research through the Paul B. Beeson Career Development Award Program. This publication was also made possible by CTSA grant number UL1 RR024139 from the National Center for Research Resources and the National Center for Advancing Translational Science, components of the National Institutes of Health (NIH), and NIH roadmap for Medical Research. Its contents are solely the responsibility of the authors and do not necessarily represent the official view of the NIH. No funding source had any role in design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; or decision to submit the manuscript for publication. Silvio E. Inzucchi, MD, serves on a Data Safety Monitoring Board for Novo Nordisk, a manufacturer of insulin products used in the hospital setting. The remaining authors declare no conflicts of interest.
Patients with diabetes currently comprise over 8% of the US population (over 25 million people) and more than 20% of hospitalized patients.[1, 2] Hospitalizations of patients with diabetes account for 23% of total hospital costs in the United States,[2] and patients with diabetes have worse outcomes after hospitalization for a variety of common medical conditions,[3, 4, 5, 6] as well as in intensive care unit (ICU) settings.[7, 8] Individuals with diabetes have historically experienced higher inpatient mortality than individuals without diabetes.[9] However, we recently reported that patients with diabetes at our large academic medical center have experienced a disproportionate reduction in in‐hospital mortality relative to patients without diabetes over the past decade.[10] This surprising trend begs further inquiry.
Improvement in in‐hospital mortality among patients with diabetes may stem from improved inpatient glycemic management. The landmark 2001 study by van den Berghe et al. demonstrating that intensive insulin therapy reduced postsurgical mortality among ICU patients ushered in an era of intensive inpatient glucose control.[11] However, follow‐up multicenter studies have not been able to replicate these results.[12, 13, 14, 15] In non‐ICU and nonsurgical settings, intensive glucose control has not yet been shown to have any mortality benefit, although it may impact other morbidities, such as postoperative infections.[16] Consequently, less stringent glycemic targets are now recommended.[17] Nonetheless, hospitals are being held accountable for certain aspects of inpatient glucose control. For example, the Centers for Medicare & Medicaid Services (CMS) began asking hospitals to report inpatient glucose control in cardiac surgery patients in 2004.[18] This measure is now publicly reported, and as of 2013 is included in the CMS Value‐Based Purchasing Program, which financially penalizes hospitals that do not meet targets.
Outpatient diabetes standards have also evolved in the past decade. The Diabetes Control and Complications Trial in 1993 and the United Kingdom Prospective Diabetes Study in 1997 demonstrated that better glycemic control in type 1 and newly diagnosed type 2 diabetes patients, respectively, improved clinical outcomes, and prompted guidelines for pharmacologic treatment of diabetic patients.[19, 20] However, subsequent randomized clinical trials have failed to establish a clear beneficial effect of intensive glucose control on primary cardiovascular endpoints among higher‐risk patients with longstanding type 2 diabetes,[21, 22, 23] and clinical practice recommendations now accept a more individualized approach to glycemic control.[24] Nonetheless, clinicians are also being held accountable for outpatient glucose control.[25]
To better understand the disproportionate reduction in mortality among hospitalized patients with diabetes that we observed, we first examined whether it was limited to surgical patients or patients in the ICU, the populations that have been demonstrated to benefit from intensive inpatient glucose control. Furthermore, given recent improvements in inpatient and outpatient glycemic control,[26, 27] we examined whether inpatient or outpatient glucose control explained the mortality trends. Results from this study contribute empirical evidence on real‐world effects of efforts to improve inpatient and outpatient glycemic control.
METHODS
Setting
During the study period, YaleNew Haven Hospital (YNHH) was an urban academic medical center in New Haven, Connecticut, with over 950 beds and an average of approximately 32,000 annual adult nonobstetric admissions. YNHH conducted a variety of inpatient glucose control initiatives during the study period. The surgical ICU began an informal medical teamdirected insulin infusion protocol in 2000 to 2001. In 2002, the medical ICU instituted a formal insulin infusion protocol with a target of 100 to 140 mg/dL, which spread to remaining hospital ICUs by the end of 2003. In 2005, YNHH launched a consultative inpatient diabetes management team to assist clinicians in controlling glucose in non‐ICU patients with diabetes. This team covered approximately 10 to 15 patients at a time and consisted of an advanced‐practice nurse practitioner, a supervising endocrinologist and endocrinology fellow, and a nurse educator to provide diabetic teaching. Additionally, in 2005, basal‐boluscorrection insulin order sets became available. The surgical ICU implemented a stringent insulin infusion protocol with target glucose of 80 to 110 mg/dL in 2006, but relaxed it (goal 80150 mg/dL) in 2007. Similarly, in 2006, YNHH made ICU insulin infusion recommendations more stringent in remaining ICUs (goal 90130 mg/dL), but relaxed them in 2010 (goal 120160 mg/dL), based on emerging data from clinical trials and prevailing national guidelines.
Participants and Data Sources
We included all adult, nonobstetric discharges from YNHH between January 1, 2000 and December 31, 2010. Repeat visits by the same patient were linked by medical record number. We obtained data from YNHH administrative billing, laboratory, and point‐of‐care capillary blood glucose databases. The Yale Human Investigation Committee approved our study design and granted a Health Insurance Portability and Accountability Act waiver and a waiver of patient consent.
Variables
Our primary endpoint was in‐hospital mortality. The primary exposure of interest was whether a patient had diabetes mellitus, defined as the presence of International Classification of Diseases, Ninth Revision codes 249.x, 250.x, V4585, V5391, or V6546 in any of the primary or secondary diagnosis codes in the index admission, or in any hospital encounter in the year prior to the index admission.
We assessed 2 effect‐modifying variables: ICU status (as measured by a charge for at least 1 night in the ICU) and service assignment to surgery (including neurosurgery and orthopedics), compared to medicine (including neurology). Independent explanatory variables included time between the start of the study and patient admission (measured as days/365), diabetes status, inpatient glucose control, and long‐term glucose control (as measured by hemoglobin A1c at any time in the 180 days prior to hospital admission in order to have adequate sample size). We assessed inpatient blood glucose control through point‐of‐care blood glucose meters (OneTouch SureStep; LifeScan, Inc., Milipitas, CA) at YNHH. We used 4 validated measures of inpatient glucose control: the proportion of days in each hospitalization in which there was any hypoglycemic episode (blood glucose value <70 mg/dL), the proportion of days in which there was any severely hyperglycemic episode (blood glucose value >299 mg/dL), the proportion of days in which mean blood glucose was considered to be within adequate control (all blood glucose values between 70 and 179 mg/dL), and the standard deviation of mean glucose during hospitalization as a measure of glycemic variability.[28]
Covariates included gender, age at time of admission, length of stay in days, race (defined by hospital registration), payer, Elixhauser comorbidity dummy variables (revised to exclude diabetes and to use only secondary diagnosis codes),[29] and primary discharge diagnosis grouped using Clinical Classifications Software,[30] based on established associations with in‐hospital mortality.
Statistical Analysis
We summarized demographic characteristics numerically and graphically for patients with and without diabetes and compared them using [2] and t tests. We summarized changes in inpatient and outpatient measures of glucose control over time numerically and graphically, and compared across years using the Wilcoxon rank sum test adjusted for multiple hypothesis testing.
We stratified all analyses first by ICU status and then by service assignment (medicine vs surgery). Statistical analyses within each stratum paralleled our previous approach to the full study cohort.[10] Taking each stratum separately (ie, only ICU patients or only medicine patients), we used a difference‐in‐differences approach comparing changes over time in in‐hospital mortality among patients with diabetes compared to those without diabetes. This approach enabled us to determine whether patients with diabetes had a different time trend in risk of in‐hospital mortality than those without diabetes. That is, for each stratum, we constructed multivariate logistic regression models including time in years, diabetes status, and the interaction between time and diabetes status as well as the aforementioned covariates. We calculated odds of death and confidence intervals for each additional year for patients with diabetes by exponentiating the sum of parameter estimates for time and the diabetes‐time interaction term. We evaluated all 2‐way interactions between year or diabetes status and the covariates in a multiple degree of freedom likelihood ratio test. We investigated nonlinearity of the relation between mortality and time by evaluating first and second‐order polynomials.
Because we found a significant decline in mortality risk for patients with versus without diabetes among ICU patients but not among non‐ICU patients, and because service assignment was not found to be an effect modifier, we then limited our sample to ICU patients with diabetes to better understand the role of inpatient and outpatient glucose control in accounting for observed mortality trends. First, we determined the relation between the measures of inpatient glucose control and changes in mortality over time using logistic regression. Then, we repeated this analysis in the subsets of patients who had inpatient glucose data and both inpatient and outpatient glycemic control data, adding inpatient and outpatient measures sequentially. Given the high level of missing outpatient glycemic control data, we compared demographic characteristics for diabetic ICU patients with and without such data using [2] and t tests, and found that patients with data were younger and less likely to be white and had longer mean length of stay, slightly worse performance on several measures of inpatient glucose control, and lower mortality (see Supporting Table 1 in the online version of this article).
| Characteristic | Overall, N=322,939 | Any ICU Stay, N=54,646 | No ICU Stay, N=268,293 | Medical Service, N=196,325 | Surgical Service, N=126,614 |
|---|---|---|---|---|---|
| |||||
| Died during admission, n (%) | 7,587 (2.3) | 5,439 (10.0) | 2,147 (0.8) | 5,705 (2.9) | 1,883 (1.5) |
| Diabetes, n (%) | 76,758 (23.8) | 14,364 (26.3) | 62,394 (23.2) | 55,453 (28.2) | 21,305 (16.8) |
| Age, y, mean (SD) | 55.5 (20.0) | 61.0 (17.0) | 54.4 (21.7) | 60.3 (18.9) | 48.0 (23.8) |
| Age, full range (interquartile range) | 0118 (4273) | 18112 (4975) | 0118 (4072) | 0118 (4776) | 0111 (3266) |
| Female, n (%) | 159,227 (49.3) | 23,208 (42.5) | 134,296 (50.1) | 99,805 (50.8) | 59,422 (46.9) |
| White race, n (%) | 226,586 (70.2) | 41,982 (76.8) | 184,604 (68.8) | 132,749 (67.6) | 93,838 (74.1) |
| Insurance, n (%) | |||||
| Medicaid | 54,590 (16.9) | 7,222 (13.2) | 47,378 (17.7) | 35,229 (17.9) | 19,361 (15.3) |
| Medicare | 141,638 (43.9) | 27,458 (50.2) | 114,180 (42.6) | 100,615 (51.2) | 41,023 (32.4) |
| Commercial | 113,013 (35.0) | 18,248 (33.4) | 94,765 (35.3) | 53,510 (27.2) | 59,503 (47.0) |
| Uninsured | 13,521 (4.2) | 1,688 (3.1) | 11,833 (4.4) | 6,878 (3.5) | 6,643 (5.2) |
| Length of stay, d, mean (SD) | 5.4 (9.5) | 11.8 (17.8) | 4.2 (6.2) | 5.46 (10.52) | 5.42 (9.75) |
| Service, n (%) | |||||
| Medicine | 184,495 (57.1) | 27,190 (49.8) | 157,305 (58.6) | 184,496 (94.0) | |
| Surgery | 126,614 (39.2) | 25,602 (46.9) | 101,012 (37.7) | 126,614 (100%) | |
| Neurology | 11,829 (3.7) | 1,853 (3.4) | 9,976 (3.7) | 11,829 (6.0) | |
To explore the effects of dependence among observations from patients with multiple encounters, we compared parameter estimates derived from a model with all patient encounters (including repeated admissions for the same patient) with those from a model with a randomly sampled single visit per patient, and observed that there was no difference in parameter estimates between the 2 classes of models. For all analyses, we used a type I error of 5% (2 sided) to test for statistical significance using SAS version 9.3 (SAS Institute, Cary, NC) or R software (
RESULTS
We included 322,938 patient admissions. Of this sample, 54,645 (16.9%) had spent at least 1 night in the ICU. Overall, 76,758 patients (23.8%) had diabetes, representing 26.3% of ICU patients, 23.2% of non‐ICU patients, 28.2% of medical patients, and 16.8% of surgical patients (see Table 1 for demographic characteristics).
Mortality Trends Within Strata
Among ICU patients, the overall mortality rate was 9.9%: 10.5% of patients with diabetes and 9.8% of patients without diabetes. Among non‐ICU patients, the overall mortality rate was 0.8%: 0.9% of patients with diabetes and 0.7% of patients without diabetes.
Among medical patients, the overall mortality rate was 2.9%: 3.1% of patients with diabetes and 2.8% of patients without diabetes. Among surgical patients, the overall mortality rate was 1.4%: 1.8% of patients with diabetes and 1.4% of patients without diabetes. Figure 1 shows quarterly in‐hospital mortality for patients with and without diabetes from 2000 to 2010 stratified by ICU status and by service assignment.

Table 2 describes the difference‐in‐differences regression analyses, stratified by ICU status and service assignment. Among ICU patients (Table 2, model 1), each successive year was associated with a 2.6% relative reduction in the adjusted odds of mortality (odds ratio [OR]: 0.974, 95% confidence interval [CI]: 0.963‐0.985) for patients without diabetes compared to a 7.8% relative reduction for those with diabetes (OR: 0.923, 95% CI: 0.906‐0.940). In other words, patients with diabetes compared to patients without diabetes had a significantly greater decline in odds of adjusted mortality of 5.3% per year (OR: 0.947, 95% CI: 0.927‐0.967). As a result, the adjusted odds of mortality among patients with versus without diabetes decreased from 1.352 in 2000 to 0.772 in 2010.
| Independent Variables | ICU Patients, N=54,646, OR (95% CI) | Non‐ICU Patients, N=268,293, OR (95% CI) | Medical Patients, N=196,325, OR (95% CI) | Surgical Patients, N=126,614, OR (95% CI) |
|---|---|---|---|---|
| Model 1 | Model 2 | Model 3 | Model 4 | |
| ||||
| Year | 0.974 (0.963‐0.985) | 0.925 (0.909‐0.940) | 0.943 (0.933‐0.954) | 0.995 (0.977‐1.103) |
| Diabetes | 1.352 (1.562‐1.171) | 0.958 (0.783‐1.173) | 1.186 (1.037‐1.356) | 1.213 (0.942‐1.563) |
| Diabetes*year | 0.947 (0.927‐0.967) | 0.977 (0.946‐1.008) | 0.961 (0.942‐0.980) | 0.955 (0.918‐0.994) |
| C statistic | 0.812 | 0.907 | 0.880 | 0.919 |
Among non‐ICU patients (Table 2, model 2), each successive year was associated with a 7.5% relative reduction in the adjusted odds of mortality (OR: 0.925, 95% CI: 0.909‐0.940) for patients without diabetes compared to a 9.6% relative reduction for those with diabetes (OR: 0.904, 95% CI: 0.879‐0.929); this greater decline in odds of adjusted mortality of 2.3% per year (OR: 0.977, 95% CI: 0.946‐1.008; P=0.148) was not statistically significant.
We found greater decline in odds of mortality among patients with diabetes than among patients without diabetes over time in both medical patients (3.9% greater decline per year; OR: 0.961, 95% CI: 0.942‐0.980) and surgical patients (4.5% greater decline per year; OR: 0.955, 95% CI: 0.918‐0.994), without a difference between the 2. Detailed results are shown in Table 2, models 3 and 4.
Glycemic Control
Among ICU patients with diabetes (N=14,364), at least 2 inpatient point‐of‐care glucose readings were available for 13,136 (91.5%), with a mean of 4.67 readings per day, whereas hemoglobin A1c data were available for only 5321 patients (37.0%). Both inpatient glucose data and hemoglobin A1c were available for 4989 patients (34.7%). Figure 2 shows trends in inpatient and outpatient glycemic control measures among ICU patients with diabetes over the study period. Mean hemoglobin A1c decreased from 7.7 in 2000 to 7.3 in 2010. Mean hospitalization glucose began at 187.2, reached a nadir of 162.4 in the third quarter (Q3) of 2007, and rose subsequently to 174.4 with loosened glucose control targets. Standard deviation of mean glucose and percentage of patient‐days with a severe hyperglycemic episode followed a similar pattern, though with nadirs in Q4 2007 and Q2 2008, respectively, whereas percentage of patient‐days with a hypoglycemic episode rose from 1.46% in 2000, peaked at 3.00% in Q3 2005, and returned to 2.15% in 2010. All changes in glucose control are significant with P<0.001.

Mortality Trends and Glycemic Control
To determine whether glucose control explained the excess decline in odds of mortality among patients with diabetes in the ICU, we restricted our sample to ICU patients with diabetes and examined the association of diabetes with mortality after including measures of glucose control.
We first verified that the overall adjusted mortality trend among ICU patients with diabetes for whom we had measures of inpatient glucose control was similar to that of the full sample of ICU patients with diabetes. Similar to the full sample, we found that the adjusted excess odds of death significantly declined by a relative 7.3% each successive year (OR: 0.927, 95% CI: 0.907‐0.947; Table 3, model 1). We then included measures of inpatient glucose control in the model and found, as expected, that a higher percentage of days with severe hyperglycemia and with hypoglycemia was associated with an increased odds of death (P<0.001 for both; Table 3, model 2). Nonetheless, after including measures of inpatient glucose control, we found that the rate of change of excess odds of death for patients with diabetes was unchanged (OR: 0.926, 95% CI: 0.905‐0.947).
| Patients With Inpatient Glucose Control Measures, n=13,136 | Patients With Inpatient and Outpatient Glucose Control Measures, n=4,989 | ||||
|---|---|---|---|---|---|
| Independent Variables | Model 1, OR (95% CI) | Model 2, OR (95% CI) | Model 3, OR (95% CI) | Model 4, OR (95% CI) | Model 5, OR (95% CI) |
| |||||
| Year | 0.927 (0.907‐0.947) | 0.926 (0.905‐0.947) | 0.958 (0.919‐0.998) | 0.956 (0.916‐0.997) | 0.953 (0.914‐0.994) |
| % Severe hyperglycemic days | 1.016 (1.010‐1.021) | 1.009 (0.998‐1.020) | 1.010 (0.999‐1.021) | ||
| % Hypoglycemic days | 1.047 (1.040‐1.055) | 1.051 (1.037‐1.065) | 1.049 (1.036‐1.063) | ||
| % Normoglycemic days | 0.997 (0.994‐1.000) | 0.994 (0.989‐0.999) | 0.993 (0.988‐0.998) | ||
| SD of mean glucose | 0.996 (0.992‐1.000) | 0.993 (0.986‐1.000) | 0.994 (0.987‐1.002) | ||
| Mean HbA1c | 0.892 (0.828‐0.961) | ||||
| C statistic | 0.806 | 0.825 | 0.825 | 0.838 | 0.841 |
We then restricted our sample to patients with diabetes with both inpatient and outpatient glycemic control data and found that, in this subpopulation, the adjusted excess odds of death among patients with diabetes relative to those without significantly declined by a relative 4.2% each progressive year (OR: 0.958, 95% CI: 0.918‐0.998; Table 3, model 3). Including measures of inpatient glucose control in the model did not significantly change the rate of change of excess odds of death (OR: 0.956, 95% CI: 0.916‐0.997; Table 3, model 4), nor did including both measures of inpatient and outpatient glycemic control (OR: 0.953, 95% CI: 0.914‐0.994; Table 3, model 5).
DISCUSSION
We conducted a difference‐in‐difference analysis of in‐hospital mortality rates among adult patients with diabetes compared to patients without diabetes over 10 years, stratifying by ICU status and service assignment. For patients with any ICU stay, we found that the reduction in odds of mortality for patients with diabetes has been 3 times larger than the reduction in odds of mortality for patients without diabetes. For those without an ICU stay, we found no significant difference between patients with and without diabetes in the rate at which in‐hospital mortality declined. We did not find stratification by assignment to a medical or surgical service to be an effect modifier. Finally, despite the fact that our institution achieved better aggregate inpatient glucose control, less severe hyperglycemia, and better long‐term glucose control over the course of the decade, we did not find that either inpatient or outpatient glucose control explained the trend in mortality for patients with diabetes in the ICU. Our study is unique in its inclusion of all hospitalized patients and its ability to simultaneously assess whether both inpatient and outpatient glucose control are explanatory factors in the observed mortality trends.
The fact that improved inpatient glucose control did not explain the trend in mortality for patients with diabetes in the ICU is consistent with the majority of the literature on intensive inpatient glucose control. In randomized trials, intensive glucose control appears to be of greater benefit for patients without diabetes than for patients with diabetes.[31] In fact, in 1 study, patients with diabetes were the only group that did not benefit from intensive glucose control.[32] In our study, it is possible that the rise in hypoglycemia nullified some of the benefits of glucose control. Nationally, hospital admissions for hypoglycemia among Medicare beneficiaries now outnumber admissions for hyperglycemia.[27]
We also do not find that the decline in hemoglobin A1c attenuated the reduction in mortality in the minority of patients for whom these data were available. This is concordant with evidence from 3 randomized clinical trials that have failed to establish a clear beneficial effect of intensive outpatient glucose control on primary cardiovascular endpoints among older, high‐risk patients with type 2 diabetes using glucose‐lowering agents.[21, 22, 23] It is notable, however, that the population for whom we had available hemoglobin A1c results was not representative of the overall population of ICU patients with diabetes. Consequently, there may be an association of outpatient glucose control with inpatient mortality in the overall population of ICU patients with diabetes that we were not able to detect.
The decline in mortality among ICU patients with diabetes in our study may stem from factors other than glycemic control. It is possible that patients were diagnosed earlier in their course of disease in later years of the study period, making the population of patients with diabetes younger or healthier. Of note, however, our risk adjustment models were very robust, with C statistics from 0.82 to 0.92, suggesting that we were able to account for much of the mortality risk attributable to patient clinical and demographic factors. More intensive glucose management may have nonglycemic benefits, such as closer patient observation, which may themselves affect mortality. Alternatively, improved cardiovascular management for patients with diabetes may have decreased the incidence of cardiovascular events. During the study period, evidence from large clinical trials demonstrated the importance of tight blood pressure and lipid management in improving outcomes for patients with diabetes,[33, 34, 35, 36] guidelines for lipid management for patients with diabetes changed,[37] and fewer patients developed cardiovascular complications.[38] Finally, it is possible that our findings can be explained by an improvement in treatment of complications for which patients with diabetes previously have had disproportionately worse outcomes, such as percutaneous coronary intervention.[39]
Our findings may have important implications for both clinicians and policymakers. Changes in inpatient glucose management have required substantial additional resources on the part of hospitals. Our evidence regarding the questionable impact of inpatient glucose control on in‐hospital mortality trends for patients with diabetes is disappointing and highlights the need for multifaceted evaluation of the impact of such quality initiatives. There may, for instance, be benefits from tighter blood glucose control in the hospital beyond mortality, such as reduced infections, costs, or length of stay. On the outpatient side, our more limited data are consistent with recent studies that have not been able to show a mortality benefit in older diabetic patients from more stringent glycemic control. A reassessment of prevailing diabetes‐related quality measures, as recently called for by some,[40, 41] seems reasonable.
Our study must be interpreted in light of its limitations. It is possible that the improvements in glucose management were too small to result in a mortality benefit. The overall reduction of 25 mg dL achieved at our institution is less than the 33 to 50 mg/dL difference between intensive and conventional groups in those randomized clinical trials that have found reductions in mortality.[11, 42] In addition, an increase in mean glucose during the last 1 to 2 years of the observation period (in response to prevailing guidelines) could potentially have attenuated any benefit on mortality. The study does not include other important clinical endpoints, such as infections, complications, length of stay, and hospital costs. Additionally, we did not examine postdischarge mortality, which might have shown a different pattern. The small proportion of patients with hemoglobin A1c results may have hampered our ability to detect an effect of outpatient glucose control. Consequently, our findings regarding outpatient glucose control are only suggestive. Finally, our findings represent the experience of a single, large academic medical center and may not be generalizable to all settings.
Overall, we found that patients with diabetes in the ICU have experienced a disproportionate reduction in in‐hospital mortality over time that does not appear to be explained by improvements in either inpatient or outpatient glucose control. Although improved glycemic control may have other benefits, it does not appear to impact in‐hospital mortality. Our real‐world empirical results contribute to the discourse among clinicians and policymakers with regards to refocusing the approach to managing glucose in‐hospital and readjudication of diabetes‐related quality measures.
Acknowledgments
The authors would like to acknowledge the YaleNew Haven Hospital diabetes management team: Gael Ulisse, APRN, Helen Psarakis, APRN, Anne Kaisen, APRN, and the Yale Endocrine Fellows.
Disclosures: Design and conduct of the study: N. B., J. D., S. I., T. B., L. H. Collection, management, analysis, and interpretation of the data: N. B., B. J., J. D., J. R., J. B., S. I., L. H. Preparation, review, or approval of the manuscript: N. B., B. J., J. D., J. R., S. I., T. B., L. H. Leora Horwitz, MD, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis. Dr. Horwitz is supported by the National Institute on Aging (K08 AG038336) and by the American Federation for Aging Research through the Paul B. Beeson Career Development Award Program. This publication was also made possible by CTSA grant number UL1 RR024139 from the National Center for Research Resources and the National Center for Advancing Translational Science, components of the National Institutes of Health (NIH), and NIH roadmap for Medical Research. Its contents are solely the responsibility of the authors and do not necessarily represent the official view of the NIH. No funding source had any role in design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; or decision to submit the manuscript for publication. Silvio E. Inzucchi, MD, serves on a Data Safety Monitoring Board for Novo Nordisk, a manufacturer of insulin products used in the hospital setting. The remaining authors declare no conflicts of interest.
- National Diabetes Information Clearinghouse. National Diabetes Statistics; 2011. Available at: http://diabetes.niddk.nih.gov/dm/pubs/america/index.aspx. Accessed November 12, 2013.
- Healthcare Cost and Utilization Project. Statistical brief #93; 2010. Available at: http://www.hcup‐us.ahrq.gov/reports/statbriefs/sb93.pdf. Accessed November 12, 2013.
- , , , et al. Association between diabetes mellitus and post‐discharge outcomes in patients hospitalized with heart failure: findings from the EVEREST trial. Eur J Heart Fail. 2013;15(2):194–202.
- , , , et al. Influence of diabetes mellitus on clinical outcome in the thrombolytic era of acute myocardial infarction. GUSTO‐I Investigators. Global Utilization of Streptokinase and Tissue Plasminogen Activator for Occluded Coronary Arteries. J Am Coll Cardiol. 1997;30(1):171–179.
- , , , , , . Type 2 diabetes and pneumonia outcomes: a population‐based cohort study. Diabetes Care. 2007;30(9):2251–2257.
- , , , . Prevalence and outcomes of diabetes, hypertension and cardiovascular disease in COPD. Eur Respir J. 2008;32(4):962–969.
- , , , , . The role of body mass index and diabetes in the development of acute organ failure and subsequent mortality in an observational cohort. Crit Care. 2006;10(5):R137.
- , , , , , . Type 2 diabetes and 1‐year mortality in intensive care unit patients. Eur J Clin Invest. 2013;43(3):238–247.
- , , . Excess mortality during hospital stays among patients with recorded diabetes compared with those without diabetes. Diabet Med. 2013;30(12):1393–1402.
- , , , et al. Decade‐long trends in mortality among patients with and without diabetes mellitus at a major academic medical center. JAMA Intern Med. 2014;174(7):1187–1188.
- , , , et al. Intensive insulin therapy in critically ill patients. N Engl J Med. 2001;345(19):1359–1367.
- , , , et al. Intensive versus conventional glucose control in critically ill patients. N Engl J Med. 2009;360(13):1283–1297.
- , , , et al. A prospective randomised multi‐centre controlled trial on tight glucose control by intensive insulin therapy in adult intensive care units: the Glucontrol study. Intensive Care Med. 2009;35(10):1738–1748.
- , , , et al. Intensive versus conventional insulin therapy: a randomized controlled trial in medical and surgical critically ill patients. Crit Care Med. 2008;36(12):3190–3197.
- , , , et al. Intensive insulin therapy in the medical ICU. N Engl J Med. 2006;354(5):449–461.
- , , , et al. Glycemic control in non‐critically ill hospitalized patients: a systematic review and meta‐analysis. J Clin Endocrinol Metab. 2012;97(1):49–58.
- , , , et al. American Association of Clinical Endocrinologists and American Diabetes Association consensus statement on inpatient glycemic control. Diabetes Care. 2009;32(6):1119–1131.
- Agency for Healthcare Research and Quality National Quality Measures Clearinghouse. Percent of cardiac surgery patients with controlled 6 A.M. postoperative blood glucose; 2012. Available at: http://www.qualitymeasures.ahrq.gov/content.aspx?id=35532. Accessed November 12, 2013.
- The effect of intensive treatment of diabetes on the development and progression of long‐term complications in insulin‐dependent diabetes mellitus. The Diabetes Control and Complications Trial Research Group. N Engl J Med. 1993;329(14):977–986.
- , , , et al. Intensive blood‐glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33). Lancet. 1998;352(9131):837–853.
- Effects of intensive glucose lowering in type 2 diabetes. N Engl J Med. 2008;358(24):2545–2559.
- , , , et al. Glucose control and vascular complications in veterans with type 2 diabetes. N Engl J Med. 2009;360(2):129–139.
- , , , et al. Intensive blood glucose control and vascular outcomes in patients with type 2 diabetes. N Engl J Med. 2008;358(24):2560–2572.
- . Standards of medical care in diabetes—2014. Diabetes Care. 2014;37(suppl 1):S14–S80.
- National Committee for Quality Assurance. HEDIS 2013. Available at: http://www.ncqa.org/HEDISQualityMeasurement.aspx. Accessed November 12, 2013.
- , , , . Is glycemic control improving in US adults? Diabetes Care. 2008;31(1):81–86.
- , , , et al. National trends in US hospital admissions for hyperglycemia and hypoglycemia among medicare beneficiaries, 1999 to 2011. JAMA Intern Med. 2014;174(7):1116–1124.
- , , , et al. "Glucometrics"—assessing the quality of inpatient glucose management. Diabetes Technol Ther. 2006;8(5):560–569.
- , , , , . A modification of the Elixhauser comorbidity measures into a point system for hospital death using administrative data. Med Care. 2009;47(6):626–633.
- Healthcare Cost and Utilization Project. Clinical Classifications Software (CCS) for ICD‐9‐CM; 2013. Available at: http://www.hcup‐us.ahrq.gov/toolssoftware/ccs/ccs.jsp. Accessed November 12, 2013.
- , , , , . The impact of premorbid diabetic status on the relationship between the three domains of glycemic control and mortality in critically ill patients. Curr Opin Clin Nutr Metab Care. 2012;15(2):151–160.
- , , , et al. Intensive insulin therapy in mixed medical/surgical intensive care units: benefit versus harm. Diabetes. 2006;55(11):3151–3159.
- Tight blood pressure control and risk of macrovascular and microvascular complications in type 2 diabetes: UKPDS 38. UK Prospective Diabetes Study Group. BMJ. 1998;317(7160):703–713.
- , , , et al. Effects of a fixed combination of perindopril and indapamide on macrovascular and microvascular outcomes in patients with type 2 diabetes mellitus (the ADVANCE trial): a randomised controlled trial. Lancet. 2007;370(9590):829–840.
- , , , , . MRC/BHF heart protection study of cholesterol‐lowering with simvastatin in 5963 people with diabetes: a randomised placebo‐controlled trial. Lancet. 2003;361(9374):2005–2016.
- , , , et al. Primary prevention of cardiovascular disease with atorvastatin in type 2 diabetes in the Collaborative Atorvastatin Diabetes Study (CARDS): multicentre randomised placebo‐controlled trial. Lancet. 2004;364(9435):685–696.
- , , , . Expert panel on detection, evaluation and treatment of high blood cholesterol in adults. Executive summary of the third report of the national cholesterol education program (NCEP) adult treatment panel (atp III). JAMA. 2001;285(19):2486–2497.
- , , , et al. Changes in diabetes‐related complications in the United States, 1990–2010. N Engl J Med. 2014;370(16):1514–1523.
- , , . Coronary heart disease in patients with diabetes: part II: recent advances in coronary revascularization. J Am Coll Cardiol. 2007;49(6):643–656.
- , , , et al. Management of hyperglycemia in type 2 diabetes: a patient‐centered approach position statement of the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD). Diabetes Care. 2012;35(6):1364–1379.
- , , , , . Assessing potential glycemic overtreatment in persons at hypoglycemic risk. JAMA Intern Med. 2013;174(2):259–268.
- , , , . Glycometabolic state at admission: important risk marker of mortality in conventionally treated patients with diabetes mellitus and acute myocardial infarction: long‐term results from the Diabetes and Insulin‐Glucose Infusion in Acute Myocardial Infarction (DIGAMI) study. Circulation. 1999;99(20):2626–2632.
- National Diabetes Information Clearinghouse. National Diabetes Statistics; 2011. Available at: http://diabetes.niddk.nih.gov/dm/pubs/america/index.aspx. Accessed November 12, 2013.
- Healthcare Cost and Utilization Project. Statistical brief #93; 2010. Available at: http://www.hcup‐us.ahrq.gov/reports/statbriefs/sb93.pdf. Accessed November 12, 2013.
- , , , et al. Association between diabetes mellitus and post‐discharge outcomes in patients hospitalized with heart failure: findings from the EVEREST trial. Eur J Heart Fail. 2013;15(2):194–202.
- , , , et al. Influence of diabetes mellitus on clinical outcome in the thrombolytic era of acute myocardial infarction. GUSTO‐I Investigators. Global Utilization of Streptokinase and Tissue Plasminogen Activator for Occluded Coronary Arteries. J Am Coll Cardiol. 1997;30(1):171–179.
- , , , , , . Type 2 diabetes and pneumonia outcomes: a population‐based cohort study. Diabetes Care. 2007;30(9):2251–2257.
- , , , . Prevalence and outcomes of diabetes, hypertension and cardiovascular disease in COPD. Eur Respir J. 2008;32(4):962–969.
- , , , , . The role of body mass index and diabetes in the development of acute organ failure and subsequent mortality in an observational cohort. Crit Care. 2006;10(5):R137.
- , , , , , . Type 2 diabetes and 1‐year mortality in intensive care unit patients. Eur J Clin Invest. 2013;43(3):238–247.
- , , . Excess mortality during hospital stays among patients with recorded diabetes compared with those without diabetes. Diabet Med. 2013;30(12):1393–1402.
- , , , et al. Decade‐long trends in mortality among patients with and without diabetes mellitus at a major academic medical center. JAMA Intern Med. 2014;174(7):1187–1188.
- , , , et al. Intensive insulin therapy in critically ill patients. N Engl J Med. 2001;345(19):1359–1367.
- , , , et al. Intensive versus conventional glucose control in critically ill patients. N Engl J Med. 2009;360(13):1283–1297.
- , , , et al. A prospective randomised multi‐centre controlled trial on tight glucose control by intensive insulin therapy in adult intensive care units: the Glucontrol study. Intensive Care Med. 2009;35(10):1738–1748.
- , , , et al. Intensive versus conventional insulin therapy: a randomized controlled trial in medical and surgical critically ill patients. Crit Care Med. 2008;36(12):3190–3197.
- , , , et al. Intensive insulin therapy in the medical ICU. N Engl J Med. 2006;354(5):449–461.
- , , , et al. Glycemic control in non‐critically ill hospitalized patients: a systematic review and meta‐analysis. J Clin Endocrinol Metab. 2012;97(1):49–58.
- , , , et al. American Association of Clinical Endocrinologists and American Diabetes Association consensus statement on inpatient glycemic control. Diabetes Care. 2009;32(6):1119–1131.
- Agency for Healthcare Research and Quality National Quality Measures Clearinghouse. Percent of cardiac surgery patients with controlled 6 A.M. postoperative blood glucose; 2012. Available at: http://www.qualitymeasures.ahrq.gov/content.aspx?id=35532. Accessed November 12, 2013.
- The effect of intensive treatment of diabetes on the development and progression of long‐term complications in insulin‐dependent diabetes mellitus. The Diabetes Control and Complications Trial Research Group. N Engl J Med. 1993;329(14):977–986.
- , , , et al. Intensive blood‐glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33). Lancet. 1998;352(9131):837–853.
- Effects of intensive glucose lowering in type 2 diabetes. N Engl J Med. 2008;358(24):2545–2559.
- , , , et al. Glucose control and vascular complications in veterans with type 2 diabetes. N Engl J Med. 2009;360(2):129–139.
- , , , et al. Intensive blood glucose control and vascular outcomes in patients with type 2 diabetes. N Engl J Med. 2008;358(24):2560–2572.
- . Standards of medical care in diabetes—2014. Diabetes Care. 2014;37(suppl 1):S14–S80.
- National Committee for Quality Assurance. HEDIS 2013. Available at: http://www.ncqa.org/HEDISQualityMeasurement.aspx. Accessed November 12, 2013.
- , , , . Is glycemic control improving in US adults? Diabetes Care. 2008;31(1):81–86.
- , , , et al. National trends in US hospital admissions for hyperglycemia and hypoglycemia among medicare beneficiaries, 1999 to 2011. JAMA Intern Med. 2014;174(7):1116–1124.
- , , , et al. "Glucometrics"—assessing the quality of inpatient glucose management. Diabetes Technol Ther. 2006;8(5):560–569.
- , , , , . A modification of the Elixhauser comorbidity measures into a point system for hospital death using administrative data. Med Care. 2009;47(6):626–633.
- Healthcare Cost and Utilization Project. Clinical Classifications Software (CCS) for ICD‐9‐CM; 2013. Available at: http://www.hcup‐us.ahrq.gov/toolssoftware/ccs/ccs.jsp. Accessed November 12, 2013.
- , , , , . The impact of premorbid diabetic status on the relationship between the three domains of glycemic control and mortality in critically ill patients. Curr Opin Clin Nutr Metab Care. 2012;15(2):151–160.
- , , , et al. Intensive insulin therapy in mixed medical/surgical intensive care units: benefit versus harm. Diabetes. 2006;55(11):3151–3159.
- Tight blood pressure control and risk of macrovascular and microvascular complications in type 2 diabetes: UKPDS 38. UK Prospective Diabetes Study Group. BMJ. 1998;317(7160):703–713.
- , , , et al. Effects of a fixed combination of perindopril and indapamide on macrovascular and microvascular outcomes in patients with type 2 diabetes mellitus (the ADVANCE trial): a randomised controlled trial. Lancet. 2007;370(9590):829–840.
- , , , , . MRC/BHF heart protection study of cholesterol‐lowering with simvastatin in 5963 people with diabetes: a randomised placebo‐controlled trial. Lancet. 2003;361(9374):2005–2016.
- , , , et al. Primary prevention of cardiovascular disease with atorvastatin in type 2 diabetes in the Collaborative Atorvastatin Diabetes Study (CARDS): multicentre randomised placebo‐controlled trial. Lancet. 2004;364(9435):685–696.
- , , , . Expert panel on detection, evaluation and treatment of high blood cholesterol in adults. Executive summary of the third report of the national cholesterol education program (NCEP) adult treatment panel (atp III). JAMA. 2001;285(19):2486–2497.
- , , , et al. Changes in diabetes‐related complications in the United States, 1990–2010. N Engl J Med. 2014;370(16):1514–1523.
- , , . Coronary heart disease in patients with diabetes: part II: recent advances in coronary revascularization. J Am Coll Cardiol. 2007;49(6):643–656.
- , , , et al. Management of hyperglycemia in type 2 diabetes: a patient‐centered approach position statement of the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD). Diabetes Care. 2012;35(6):1364–1379.
- , , , , . Assessing potential glycemic overtreatment in persons at hypoglycemic risk. JAMA Intern Med. 2013;174(2):259–268.
- , , , . Glycometabolic state at admission: important risk marker of mortality in conventionally treated patients with diabetes mellitus and acute myocardial infarction: long‐term results from the Diabetes and Insulin‐Glucose Infusion in Acute Myocardial Infarction (DIGAMI) study. Circulation. 1999;99(20):2626–2632.
© 2015 Society of Hospital Medicine
Metrics for Inpatient Glycemic Control
Data collection, analysis, and presentation are key to the success of any hospital glycemic control initiative. Such efforts enable the management team to track improvements in processes and outcomes, make necessary changes to their quality improvement efforts, justify the provision of necessary time and resources, and share their results with others. Reliable metrics for assessing glycemic control and frequency of hypoglycemia are essential to accomplish these tasks and to assess whether interventions result in more benefit than harm. Hypoglycemia metrics must be especially convincing because fear of hypoglycemia remains a major source of clinical inertia, impeding efforts to improve glucose control.
Currently, there are no official standards or guidelines for formulating metrics on the quality of inpatient glycemic control. This creates several problems. First, different metrics vary in their biases and in their responsiveness to change. Thus, use of a poor metric could lead to either a falsely positive or falsely negative impression that a quality improvement intervention is in fact improving glycemic control. Second, the proliferation of different measures and analytical plans in the research and quality improvement literature make it very difficult for hospitals to compare baseline performance, determine need for improvement, and understand which interventions may be most effective.
A related article in this supplement provides the rationale for improved inpatient glycemic control. That article argues that the current state of inpatient glycemic control, with the frequent occurrence of severe hyperglycemia and irrational insulin ordering, cannot be considered acceptable, especially given the large body of data (albeit largely observational) linking hyperglycemia to negative patient outcomes. However, regardless of whether one is an advocate or skeptic of tighter glucose control in the intensive care unit (ICU) and especially the non‐ICU setting, there is no question that standardized, valid, and reliable metrics are needed to compare efforts to improve glycemic control, better understand whether such control actually improves patient care, and closely monitor patient safety.
This article provides a summary of practical suggestions to assess glycemic control, insulin use patterns, and safety (hypoglycemia and severe hyperglycemia). In particular, we discuss the pros and cons of various measurement choices. We conclude with a tiered summary of recommendations for practical metrics that we hope will be useful to individual improvement teams. This article is not a consensus statement but rather a starting place that we hope will begin to standardize measurement across institutions and advance the dialogue on this subject. To more definitely address this problem, we call on the American Association of Clinical Endocrinologists (AACE), American Diabetes Association (ADA), Society of Hospital Medicine (SHM), and others to agree on consensus standards regarding metrics for the quality of inpatient glycemic control.
MEASURING GLYCEMIC CONTROL: GLUCOMETRICS
Glucometrics may be defined as the systematic analysis of blood glucose (BG) dataa phrase initially coined specifically for the inpatient setting. There are numerous ways to do these analyses, depending on which patients and glucose values are considered, the definitions used for hypoglycemia and hyperglycemia, the unit of measurement (eg, patient, patient‐day, individual glucose value), and the measure of control (eg, mean, median, percent of glucose readings within a certain range). We consider each of these dimensions in turn.
Defining the Target Patient Population
The first decision to be made is which patients to include in your analysis. Choices include the following:
-
Patients with a discharge diagnosis of diabetes: this group has face validity and intuitive appeal, is easy to identify retrospectively, and may capture some untested/untreated diabetics, but will miss patients with otherwise undiagnosed diabetes and stress hyperglycemia. It is also subject to the variable accuracy of billing codes.
-
Patients with a certain number of point‐of‐care (POC) glucose measurements: this group is also easy to identify, easy to measure, and will include patients with hyperglycemia without a previous diagnosis of diabetes, but will miss patients with untested/untreated hyperglycemia. Also, if glucose levels are checked on normoglycemic, nondiabetic patients, these values may dilute the overall assessment of glycemic control.
-
Patients treated with insulin in the hospital: this is a good choice if the purpose is mainly drug safety and avoidance of hypoglycemia, but by definition excludes most untreated patients.
-
Patients with 2 or more BG values (laboratory and/or POC) over a certain threshold (eg, >180 mg/dL). This will likely capture more patients with inpatient hyperglycemia, whether or not detected by the medical team, but is subject to wide variations in the frequency and timing of laboratory glucose testing, including whether or not the values are pre‐prandial (note that even preprandial POC glucose measurements are not always in fact fasting values).
Other considerations include the following:
-
Are there natural patient subgroups that should be measured and analyzed separately because of different guidelines? For example, there probably should be separate/emndependent inclusion criteria and analyses for critical care and noncritical care units because their glycemic targets and management considerations differ.
-
Which patients should be excluded? For example, if targeting subcutaneous insulin use in general hospitalized patients, one might eliminate those patients who are admitted specifically as the result of a diabetes emergency (eg, diabetic ketoacidosis [DKA] and hyperglycemic hyperosmolar state [HHS]), as their marked and prolonged hyperglycemia will skew BG data. Pregnant women should generally be excluded from broad‐based analyses or considered as a discrete category because they have very different targets for BG therapy. Patients with short lengths of stay may be less likely to benefit from tight glucose control and may also be considered for post hoc exclusion. One might also exclude patients with very few evaluable glucose readings (eg, fewer than 5) to ensure that measurement is meaningful for a given patient, keeping in mind that this may also exclude patients with undetected hyperglycemia, as mentioned above. Finally, patients receiving palliative care should also be considered for exclusion if feasible.
Recommendation: Do not limit analyses to only those patients with a diagnosis of diabetes or only those on insulin, which will lead to biased results.
-
For noncritical care patients, we recommend a combined approach: adult patients with a diagnosis of diabetes (e. g. using diagnosis‐related group [DRG] codes 294 or 295 or International Classification of Diseases 9th edition [ICD9] codes 250.xx) or with hyperglycemia (eg, 2 or more random laboratory and/or point of care (POC) BG values >180 mg/dL or 2 or more fasting BG values >130 mg/dL), excluding patients with DKA or HHS or who are pregnant.
-
For critical care units, we recommend either all patients, or patients with at least mild hyperglycemia (eg, 2 random glucose levels >140 mg/dL). Critical care patients with DKA, HHS, and pregnancy should be evaluated separately if possible.
Which Glucose Values to Include and Exclude
To answer this question, we first need to decide which method to use for BG measurement. There are several ways to measure BG, including the type of sample collected (capillary [fingerstick], arterial, and venous) and the technique used (central laboratory analyzing plasma, central laboratory analyzing whole blood [eg, from an arterial blood gas sample], glucose meter [usually calibrated to plasma], etc.). The POC (eg, capillary, glucose meter) glucose measurements alone are often preferred in the non‐ICU setting because laboratory plasma values generally provide little additional information and typically lower the mean glucose by including redundant fasting values.1 In critical care units, several different methods are often used together, and each merits inclusion. The inherent differences in calibration between the methods do not generally require separate analyses, especially given the frequency of testing in the ICU setting.
The next question is which values to include in analyses. In some situations, it may be most useful to focus on a certain period of hospitalization, such as the day of a procedure and the next 2 days in assessing the impact of the quality of perioperative care, or the first 14 days of a noncritical care stay to keep outliers for length of stay (LOS) from skewing the data. In the non‐ICU setting, it may be reasonable to exclude the first day of hospitalization, as early BG control is impacted by multiple variables beyond direct control of the clinician (eg, glucose control prior to admission, severity of presenting illness) and may not realistically reflect your interventions. (Keep in mind, however, that it may be useful to adjust for the admission glucose value in multivariable models given its importance to clinical outcomes and its strong relationship to subsequent inpatient glucose control.) However, in critical care units, it is reasonable to include the first day's readings in analyses given the high frequency of glucose measurements in this setting and the expectation that glucose control should be achieved within a few hours of starting an intravenous insulin infusion.
If feasible to do so with your institution's data capture methods, you may wish to select only the regularly scheduled (before each meal [qAC] and at bedtime [qHS], or every 6 hours [q6h]) glucose readings for inclusion in the summary data of glycemic control in the non‐ICU setting, thereby reducing bias caused by repeated measurements around extremes of glycemic excursions. An alternative in the non‐ICU setting is to censor glucose readings within 60 minutes of a previous reading.
Recommendation:
-
In the non‐ICU setting, we recommend first looking at all POC glucose values and if possible repeating the analyses excluding hospital day 1 and hospital day 15 and beyond, and also excluding glucose values measured within 60 minutes of a previous value.
-
In critical care units, we recommend evaluating all glucose readings used to guide care.
Units of Analysis
There are several different units of analysis, each with its own advantages and disadvantages:
-
Glucose value: this is the simplest measure and the one with the most statistical power. All glucose values for all patients of interest comprise the denominator. A report might say, for example, that 1% of the 1000 glucose values were <70 mg/dL during a certain period or that the mean of all glucose values collected for the month from patients in noncritical care areas was 160 mg/dL. The potential disadvantages of this approach are that these analyses are less clinically relevant than patient‐level analyses and that patients with many glucose readings and long hospitalizations may skew the data.
-
Patient (or the Patient Stay, [ie, the entire hospitalization]): all patients who are monitored make up the denominator. The numerator may be the percentage of patients with any hypoglycemia during their hospital stay or the percentage of patients achieving a certain mean glucose during their hospitalization, for example. This is inherently more clinically meaningful than using glucose value as a unit of analysis. A major disadvantage is not controlling for LOS effects. For example, a hospitalized patient with a long LOS is much more likely to be characterized as having at least 1 hypoglycemic value than is a patient with a shorter LOS. Another shortcoming is that this approach does not correct for uneven distribution of testing. A patient's mean glucose might be calculated on the basis of 8 glucose values on the first day of hospitalization, 4 on the second day, and 1 on the third day. Despite all these shortcomings, reporting by patient remains a popular and valid method of presenting glycemic control results, particularly when complemented by other views and refined to control for the number of readings per day.
-
Monitored Patient‐Day: The denominator in this setting is the total number of days a patient glucose level is monitored. The benefits of this method have been described and advocated in the literature.1 As with patient‐level analyses, this measure will be more rigorous and meaningful if the BG measures to be evaluated have been standardized. Typical reports might include percentage of monitored days with any hypoglycemia, or percentage of monitored days with all glucose values in the desired range. This unit of analysis may be considered more difficult to generate and to interpret. On the other hand, it is clinically relevant, less biased by LOS effects, and may be considered the most actionable metric by clinicians. This method provides a good balance when presented with data organized by patient.
The following example uses all 3 units of measurement, in this case to determine the rate of hypoglycemia, demonstrating the different but complementary information that each method provides:
-
In 1 month, 3900 POC glucose measurements were obtained from 286 patients representing 986 monitored patient‐days. With hypoglycemia defined as POC BG 60 mg/dL, the results showed the following:
-
50 of 3900 measurements (1.4%) were hypoglycemic 22 of 286 patients (7.7%) had 1 hypoglycemic episodes
-
40 of 986 monitored days (4.4%) had 1 hypoglycemic episodes.
The metric based on the number of glucose readings could be considered the least clinically relevant because it is unclear how many patients were affected; moreover, it may be based on variable testing patterns among patients, and could be influenced disproportionately by 1 patient with frequent hypoglycemia, many glucose readings, and/or a long LOS. One could argue that the patient‐stay metric is artificially elevated because a single hypoglycemic episode characterizes the entire stay as hypoglycemic. On the other hand, at least it acknowledges the number of patients affected by hypoglycemia. The patient‐day unit of analysis likely provides the most balanced view, one that is clinically relevant and measured over a standard period of time, and less biased by LOS and frequency of testing.
One way to express patient‐day glycemic control that deserves special mention is the patient‐day weighted mean. A mean glucose is calculated for each patient‐day, and then the mean is calculated across all patient‐days. The advantage of this approach is that it corrects for variation in the number of glucose readings each day; all hospital days are weighted equally.
Recommendation:
-
In noncritical care units, we recommend a combination of patient‐day and patient‐stay measures.
-
In critical care units, it is acceptable to also use glucose reading as the unit of measurement given more frequent and uniform data collection, but it should be complemented by more meaningful patient‐day and patient‐stay measures.
Measures of Control
In addition to deciding the unit(s) of analysis, another issue concerns which measures of control to use. These could include rates of hypoglycemia and hyperglycemia, percentage of glucose readings within various ranges (eg, <70, 70180, >180 mg/dL), mean glucose value, percentage of patient‐days during which the mean glucose is within various ranges, or the in control rate (ie, when all glucose values are within a certain range).
As with the various units of analysis, each of these measures of control has various advantages and disadvantages. For example, mean glucose is easy to report and understand, but masks extreme values. Percentage of glucose values within a certain range (eg, per patient, averaged across patients) presents a more complete picture but is a little harder to understand and will vary depending on the frequency of glucose monitoring. As mentioned above, this latter problem can be corrected in part by including only certain glucose values. Percent of glucose values within range may also be less sensitive to change than mean glucose (eg, a glucose that is lowered from 300 mg/dL to 200 mg/dL is still out of range). We recommend choosing a few, but not all, measures of control in order to get a complete picture of glycemic control. Over time one can then refine the measures being used to meet the needs of the glycemic control team and provide data that will drive the performance improvement process.
In critical care and perioperative settings, interest in glycemic control is often more intense around the time of a particular event such as major surgery or after admission to the ICU. Some measures commonly used in performing such analyses are:
-
All values outside a target range within a designated crucial period. For example, the University Healthcare Consortium and other organizations use a simple metric to gauge perioperative glycemic control. They collect the fasting glucose on postoperative days 1 and 2 and then calculate the percentage of postoperative days with any fasting glucose >200 mg/dL. Of course, this is a very liberal target, but it can always be lowered in a stepwise fashion once it is regularly being reached.
-
Three‐day blood glucose average. The Portland group uses the mean glucose of each patient for the period that includes the day of coronary artery bypass graft (CABG) surgery and the following 2 days. The 3‐day BG average (3‐BG) correlates very well with patient outcomes and can serve as a well‐defined target.2 It is likely that use of the 3‐BG would work well in other perioperative/trauma settings and could work in the medical ICU as well, with admission to the ICU as the starting point for calculation of the 3‐BG.
Hyperglycemic Index
Measuring the hyperglycemic index (HGI) is a validated method of summarizing glycemic control of ICU patients.3 It is designed to take into account the sometimes uneven distribution of patient testing. Time is plotted on the x‐axis and glucose values on the y‐axis. The HGI is calculated the area under the curve of glycemic values but above the upper limit of normal (ie, 110 mg/dL). Glucose values in the normal or hypoglycemic range are not included in the AUC. Mortality correlated well with this glycemic index. However, a recent observational study of glucometrics in patients hospitalized with acute myocardial infarction found that the simple mean of each patient's glucose values over the entire hospitalization was as predictive of in‐hospital mortality as the HGI or the time‐averaged glucose (AUC for all glucose values).4 In this study, metrics derived from glucose readings for the entire hospitalization were more predictive than those based on the first 24 or 48 hours or on the admission glucose.
Analyses Describing Change in Glycemic Control Over Time in the Hospital
In the critical care setting, this unit of analysis may be as simple as the mean time to reach the glycemic target on your insulin infusion protocol. On noncritical care wards, it is a bit more challenging to characterize the improvement (or clinical inertia) implied by failure of hyperglycemia to lessen as an inpatient stay progresses. One method is to calculate the mean glucose (or percentage of glucose values in a given range) for each patient on hospital day (HD) 1, and repeat for each HD (up to some reasonable limit, such as 5 or 7 days).
Recommendations:
-
In noncritical units, we recommend a limited set of complementary measures, such as the patient‐day weighted mean glucose, mean percent of glucose readings per patient that are within a certain range, and percentage of patients whose mean glucose is within a certain range on each hospital day.
-
In critical care units, it is often useful to focus measures around a certain critical event such as the 3‐day blood glucose average and to use measures such as the HGI that take advantage of more frequent blood glucose testing.
Definitions of Hyperglycemia and Hypoglycemia
Glucometrics outcomes will obviously depend on the thresholds established for hyperglycemia and hypoglycemia. Many centers define hypoglycemia as 60 mg/dL, whereas the ADA definition, based on physiologic changes that may take place, defines hypoglycemia (at least in the outpatient setting) as 70 mg/dL. Hypoglycemia may be further stratified by severity, with any glucose 40 mg/dL, for instance, defined as severe hypoglycemia.
Similarly, the definition of hyperglycemia (and therefore good control) must also be defined. Based on definitions developed by the ADA and AACE, the state of the medical literature, and current understanding of the pathophysiology of hyperglycemia, thresholds for critical care units include 110 mg/dL, 130 mg/dL, and 140 mg/dL, and options in noncritical care units include 130 mg/dL, 140 mg/dL, and 180 mg/dL. Because these thresholds implicitly assume adverse effects when glucose levels are above them, these levels are subject to revision as data become available confirming the benefits and safety of targeted glycemic control in various settings and patient populations.
Introducing optimal BG targets in a stepped fashion over time should also be considered. Furnary et al.2 have done this in the Portland Project, which tracks glycemic control in cardiac surgery patients receiving intravenous insulin therapy. The initial BG target for this project was <200 mg/dL; it was subsequently lowered stepwise over several years to 150 mg/dL, then to 120 mg/dL, and most recently to 110 mg/dL. This approach allows the safe introduction of targeted glycemic control and promotes acceptance of the concept by physicians and the allied nursing and medical staff.
Recommendations:
-
In noncritical care units, it is reasonable to use 40 mg/dL for severe hypoglycemia, 70 mg/dL for hypoglycemia, 130 mg/dL for fasting hyperglycemia, 180 mg/dL for random or postprandial hyperglycemia, and 300 mg/dL for severe hyperglycemia, keeping in mind that these thresholds are arbitrary. In critical care units, values from 110 mg/dL to 140 mg/dL might be better thresholds for hyperglycemia, but it may take time to safely and effectively move an organization toward these lower targets.
Other Considerations Relative to Glucometrics
Yale Glucometrics Website
The Yale Informatics group has put together a Web‐based resource (
Other Analytic Resources
Commercially available software, such as the RALS system (Medical Automation Systems, Inc., Charlottesville, VA) can gather POC glucose measurements directly from devices and provide real‐time reports of glycemic control, stratified by inpatient unit, using user‐defined targets for hypoglycemia and hyperglycemia. While they are no substitute for a dedicated, on‐site data analyst, such systems can be very useful for smaller hospitals with minimal data or information technology support staff.
APPROACHES TO ANALYSIS: RUN CHARTS
Most conventional clinical trials hold interventions fixed for a period of time and compare results with and without the intervention. For quality improvement studies, this is still a valid way to proceed, especially if studied as a randomized controlled trial. Such methods may be preferred when the clinical question is Does this type of intervention work in general? and the desired output is publication in peer‐reviewed journals so that others can learn about and adopt the intervention to their own institution. A before and after study with a similar analytic approach may also be valid, although concerns about temporal trends and cointerventions potentially compromise the validity of such studies. This approach again assumes that an intervention is held fixed over time such that it is clear what patients received during each time period.
If the desired result is improvement at a given institution (the question is Did we improve care?) then it may be preferable to present results over time using run‐charts. In a run chart, the x‐axis is time and the y‐axis the desired metric, such as patient‐day weighted mean glucose. Points in time when interventions were introduced or modified can be highlighted. Run charts have several advantages over before‐and‐after summaries: they do not require interventions remaining fixed and are more compatible with continuous quality improvement methods, it is easier to see the effect of different aspects of the interventions as they occur, one can get a quicker picture of whether something is working, and it is easier to separate out the impact of the intervention from secular trends. Finally, the use of run charts does not imply the absence of statistical rigor. Run charts with statistical process control (SPC) limits5 can easily convey when the observed time trend is unlikely to be due to chance using prespecified P values. (A full discussion of SPC and other methods to study quality improvement interventions is beyond the scope of this article.)
ASSESSING PATTERNS OF INSULIN USE AND ORDER SET UTILIZATION
Besides measuring the impact of quality improvement interventions on glucose control, it is important to measure processes such as proper insulin use. As mentioned in other articles in this supplement, processes are much more sensitive to change than outcomes. Failure to change processes should lead one to make changes to the intervention.
ICU and Perioperative Settings
For ICU and perioperative settings, the major process measure will likely be use of the insulin infusion order set. Designation of BG levels that trigger insulin infusion in these settings should be agreed upon in advance. The number of patients who meet the predefined glycemic criteria would make up the denominator, and the number of patients on the insulin infusion order set would make up the numerator.
NonCritical Care Units
On noncritical care units, measuring the percentage of subcutaneous insulin regimens that contain a basal insulin is a useful way to monitor the impact of an intervention. A more detailed analysis could examine the percentage of patients on simultaneous basal and nutritional insulin (if applicable). An important measure of clinical inertia is to track the percentage of patients who had changes in their insulin regimens on days after hypoglycemic or hyperglycemic excursions. Another important measure is the frequency with which the standardized order set is being used, analogous to the measure of insulin infusion use in the ICU. A final process measure, indirectly related to insulin use, is the frequency of use of oral diabetes agents, especially by patients for whom their use is contraindicated (eg, patients with congestive heart failure who are on thiazolidinediones and patients with renal insufficiency or receiving intravenous contrast continued on metformin).
OTHER CONSIDERATIONS AND METRICS
Examples of other metrics that can be used to track the success of quality improvement efforts include:
-
Glucose measurement within 8 hours of hospital admission.
-
Glycated hemoglobin (A1C) measurement obtained or available within 30 days of admission to help guide inpatient and especially discharge management.
-
Appropriate glucose testing in patients with diabetes or hyperglycemia (eg, 4 times per day in patients not on insulin infusion protocols, at least until 24 hours of euglycemia is documented).
-
The percentage of patients on insulin with on‐time tray delivery.
-
The timing of subcutaneous insulin administration in relation to glucose testing and nutrition delivery.
-
Documentation of carbohydrate intake among patients who are eating.
-
Satisfaction of physicians and nurses with order sets or protocols, using standard surveys.
-
Physician and nurse knowledge, attitudes, and beliefs about insulin administration, fear of hypoglycemia, treatment of hypoglycemia, and glycemic control in the hospital.
-
Patient satisfaction with their diabetes care in the hospital, including the education they received.
-
Nursing and physician education/certification in insulin prescribing, insulin administration, and other diabetes care issues.
-
Patient outcomes strongly associated with glycemic control, (eg, surgical wound infections, ICU LOS, catheter‐related bloodstream infections).
-
Appropriate treatment and documentation of hypoglycemia (eg, in accordance with hospital policy).
-
Documentation of severe hypoglycemic events through the hospital's adverse events reporting system (these may actually increase as change comes to the organization and as clinical personnel are more attuned to glycemic control).
-
Root causes of hypoglycemic events, which can be used to understand and prevent future events.
-
Appropriate transitions from IV to SC insulin regimens, (eg, starting basal insulin prior to discontinuing infusion in patients who have been on an insulin infusion of at least 2 units/hour or who have a known diagnosis of diabetes or A1C >7).
(Survey instruments and other measurement tools are available from the authors upon request.)
SHM GLYCEMIC CONTROL TASK FORCE SUMMARY RECOMMENDATIONS
The SHM Glycemic Control Task Force is working to develop standardized measures of inpatient glucose control and related indicators to track progress of hospital glycemic control initiatives (see the introduction to this supplement for a description of the charge and membership of this task force). The goals of the Task Force's metrics recommendations (Table 1) are several‐fold: (1) create a set of measurements that are complete but not overly burdensome; (2) create realistic measures that can be applied to institutions with different data management capabilities; and (3) allow for comparison across institutions for benchmarking purposes, evaluation of quality improvement projects, and reporting of results for formal research studies in this field.
| Measurement Issue | NonCritical Care Units | Critical Care Units | ||
|---|---|---|---|---|
| Tier 1 Recommendations | Tier 2 Recommendations | Tier 1 Recommendations | Tier 2 Recommendations | |
| ||||
| Patient inclusion and exclusion criteria | All adult patients with POC glucose testing (sampling acceptable). Exclude patients with DKA or HHS or who are pregnant. | All adult patients with diagnosis of diabetes by ICD‐9 code* or by glucose testing: random glucose (POC or laboratory) >180 mg/dL 2 or fasting glucose >130 mg/dL 2, excluding patients with DKA or HHS or who are pregnant. Additional analysis: exclude patients with <5 evaluable glucose readings, patients with LOS <2 days, or receiving palliative care. | All patients in every critical care unit (sampling acceptable). | Patients with DKA, HHS, or pregnancy in separate analyses. All patients in every critical care unit with random glucose (POC or laboratory) >140 mg/dL 2. |
| Glucose reading inclusion and exclusion criteria | All POC glucose values. | Additional analysis: exclude glucose values on hospital day 1 and on hospital day 15 and after. Additional analysis: exclude glucose values measured within 60 minutes of a previous value. | All POC and other glucose values used to guide care. | |
| Measures of safety | Analysis by patient‐day: Percentage of patient‐days with 1 or more values <40, <70, and >300 mg/dL. | Analysis by patient‐day: Percentage of patient‐days with 1 or more values <40, <70, and >300 mg/dL. | ||
| Measures of glucose control | Analysis by patient‐day: Percentage of patient‐days with mean <140, <180 mg/dL and/or Percentage of patient‐days with all values <180 mg/dL. | Analysis by patient‐day: Patient day‐weighted mean glucose. | Analysis by glucose reading: Percentage of readings <110, <140 mg/dL. | 3‐BG as above for all patients in critical care units.∥ Hyperglycemic index for all patients in critical care units (AUC of glucose values above target). |
| Analysis by patient stay: Percentage of patient stays with mean <140, <180 mg/dL. | Analysis by patient stay: Mean percentage of glucose readings of each patient <180 mg/dL. | Analysis by patient‐day: Percentage of patient‐days with mean <110, <140 mg/dL, and/or Percentage of patient‐days with all values <110, <140 mg/dL. | ||
| Analysis by hospital day: Percentage of patients with mean glucose readings <140, <180 mg/dL by hospital day (days 17). | Analysis by patient stay: 3‐day blood glucose average (3‐BG) for selected perioperative patients: Percentage of patients with 3‐BG <110, <140 mg/dL. Mean time (hours) to reach glycemic target (BG <110 or <140 mg/dL) on insulin infusion. | |||
| Measures of insulin use | Percentage of patients on any subcutaneous insulin that has a scheduled basal insulin component (glargine, NPH, or detemir). | Percentage of patients with at least 2 POC and/or laboratory glucose readings >180 mg/dL who have a scheduled basal insulin component. Percentage of eating patients with hyperglycemia as defined above with scheduled basal insulin and nutritional insulin. Percentage of patients and patient‐days with any changes in insulin orders the day after 2 or more episodes of hypoglycemia or hyperglycemia (ie, <70 or >180 mg/dL). | Percentage of patients with 2 POC or laboratory glucose readings >140 mg/dL placed on insulin infusion protocol. | |
| Other process measures | Glucose measured within 8 hours of hospital admission. | POC glucose testing at least 4 times a day for all patients with diabetes or hyperglycemia as defined above. | Glucose measured within 8 hours of hospital admission. | Appropriateness of hypoglycemia treatment and documentation. |
| A1C measurement obtained or available within 30 days of admission. | Measures of adherence to specific components of management protocol. | Frequency of BG testing (eg, per protocol if on insulin infusion; every 68 hours if not). | Clinical events of severe hypoglycemia reported through the organization's critical events reporting tool. | |
| Appropriateness of hypoglycemia treatment and documentation. | Root causes of hypoglycemia. | |||
| Clinical events of severe hypoglycemia reported through the organization's critical events reporting tool. | Appropriate use of IV‐to‐SC insulin transition protocol. | |||
| Root causes of hypoglycemia. | ||||
For each domain of glycemic management (glycemic control, safety, and insulin use), the task force chose a set of best measures. They are presented as two tiers of measurement standards, depending on the capabilities of the institution and the planned uses of the data. Tier 1 includes measures that, although they do take time and resources to collect, are feasible for most institutions. Tier 2 measures are recommended for hospitals with easy manipulation of electronic sources of data and for reporting quality‐of‐care measures for widespread publication, that is, in the context of a research study. It should be emphasized that these recommendations are only meant as a guide: the actual measures chosen should meet the needs and capabilities of each institution.
We recognize that few data support the recommendations made by this task force, that such data are needed, and that the field of data collection and analysis for hospital glycemic management is rapidly evolving. The hope is to begin the standardization process, promote dialogue in this field, and eventually reach a consensus in collaboration with the ADA, AACE, and other pertinent stakeholders.
CONCLUSIONS
Like the field of inpatient glycemic management itself, the field of devising metrics to measure the quality of inpatient glycemic control is also in its infancy and quickly evolving. One should not be paralyzed by the lack of consensus regarding measurementthe important point is to pick a few complementary metrics and begin the process. The table of recommendations can hopefully serve as a starting point for many institutions, with a focus on efficacy (glycemic control), safety (hypoglycemia), and process (insulin use patterns). As your institution gains experience with measurement and the field evolves, your metrics will likely change. We recommend keeping all process and outcome data in its raw form so that it can be summarized in different ways over time. It is also important not to wait for the perfect data collection tool before beginning to analyze data: sampling and paper processes are acceptable if automated data collection is not yet possible. Eventually, blood glucose meter readings should be downloaded into a central database that interfaces with hospital data repositories so data can be analyzed in conjunction with patient, service, and unit‐level information. Only with a rigorous measurement process can institutions hope to know whether their changes are resulting in improved care for patients.
- ,,, et al.“Glucometrics”—assessing the quality of inpatient glucose management.Diabetes Technol Ther.2006;8:560–569.
- ,,.Effect of hyperglycemia and continuous intravenous insulin infusions on outcomes of cardiac surgical procedures: the Portland Diabetic Project.Endocr Pract.2004;10(suppl 2):21–33.
- ,,.Hyperglycaemic index as a tool to assess glucose control: a retrospective study.Crit Care.2004;8:R122–R127.
- ,,, et al.Glucometrics in patients hospitalized with acute myocardial infarction: defining the optimal outcomes‐based measure of risk.Circulation.2008;117:1018–1027.
- ,,.Statistical process control as a tool for research and healthcare improvement.Qual Saf Health Care.2003;12:458–464.
Data collection, analysis, and presentation are key to the success of any hospital glycemic control initiative. Such efforts enable the management team to track improvements in processes and outcomes, make necessary changes to their quality improvement efforts, justify the provision of necessary time and resources, and share their results with others. Reliable metrics for assessing glycemic control and frequency of hypoglycemia are essential to accomplish these tasks and to assess whether interventions result in more benefit than harm. Hypoglycemia metrics must be especially convincing because fear of hypoglycemia remains a major source of clinical inertia, impeding efforts to improve glucose control.
Currently, there are no official standards or guidelines for formulating metrics on the quality of inpatient glycemic control. This creates several problems. First, different metrics vary in their biases and in their responsiveness to change. Thus, use of a poor metric could lead to either a falsely positive or falsely negative impression that a quality improvement intervention is in fact improving glycemic control. Second, the proliferation of different measures and analytical plans in the research and quality improvement literature make it very difficult for hospitals to compare baseline performance, determine need for improvement, and understand which interventions may be most effective.
A related article in this supplement provides the rationale for improved inpatient glycemic control. That article argues that the current state of inpatient glycemic control, with the frequent occurrence of severe hyperglycemia and irrational insulin ordering, cannot be considered acceptable, especially given the large body of data (albeit largely observational) linking hyperglycemia to negative patient outcomes. However, regardless of whether one is an advocate or skeptic of tighter glucose control in the intensive care unit (ICU) and especially the non‐ICU setting, there is no question that standardized, valid, and reliable metrics are needed to compare efforts to improve glycemic control, better understand whether such control actually improves patient care, and closely monitor patient safety.
This article provides a summary of practical suggestions to assess glycemic control, insulin use patterns, and safety (hypoglycemia and severe hyperglycemia). In particular, we discuss the pros and cons of various measurement choices. We conclude with a tiered summary of recommendations for practical metrics that we hope will be useful to individual improvement teams. This article is not a consensus statement but rather a starting place that we hope will begin to standardize measurement across institutions and advance the dialogue on this subject. To more definitely address this problem, we call on the American Association of Clinical Endocrinologists (AACE), American Diabetes Association (ADA), Society of Hospital Medicine (SHM), and others to agree on consensus standards regarding metrics for the quality of inpatient glycemic control.
MEASURING GLYCEMIC CONTROL: GLUCOMETRICS
Glucometrics may be defined as the systematic analysis of blood glucose (BG) dataa phrase initially coined specifically for the inpatient setting. There are numerous ways to do these analyses, depending on which patients and glucose values are considered, the definitions used for hypoglycemia and hyperglycemia, the unit of measurement (eg, patient, patient‐day, individual glucose value), and the measure of control (eg, mean, median, percent of glucose readings within a certain range). We consider each of these dimensions in turn.
Defining the Target Patient Population
The first decision to be made is which patients to include in your analysis. Choices include the following:
-
Patients with a discharge diagnosis of diabetes: this group has face validity and intuitive appeal, is easy to identify retrospectively, and may capture some untested/untreated diabetics, but will miss patients with otherwise undiagnosed diabetes and stress hyperglycemia. It is also subject to the variable accuracy of billing codes.
-
Patients with a certain number of point‐of‐care (POC) glucose measurements: this group is also easy to identify, easy to measure, and will include patients with hyperglycemia without a previous diagnosis of diabetes, but will miss patients with untested/untreated hyperglycemia. Also, if glucose levels are checked on normoglycemic, nondiabetic patients, these values may dilute the overall assessment of glycemic control.
-
Patients treated with insulin in the hospital: this is a good choice if the purpose is mainly drug safety and avoidance of hypoglycemia, but by definition excludes most untreated patients.
-
Patients with 2 or more BG values (laboratory and/or POC) over a certain threshold (eg, >180 mg/dL). This will likely capture more patients with inpatient hyperglycemia, whether or not detected by the medical team, but is subject to wide variations in the frequency and timing of laboratory glucose testing, including whether or not the values are pre‐prandial (note that even preprandial POC glucose measurements are not always in fact fasting values).
Other considerations include the following:
-
Are there natural patient subgroups that should be measured and analyzed separately because of different guidelines? For example, there probably should be separate/emndependent inclusion criteria and analyses for critical care and noncritical care units because their glycemic targets and management considerations differ.
-
Which patients should be excluded? For example, if targeting subcutaneous insulin use in general hospitalized patients, one might eliminate those patients who are admitted specifically as the result of a diabetes emergency (eg, diabetic ketoacidosis [DKA] and hyperglycemic hyperosmolar state [HHS]), as their marked and prolonged hyperglycemia will skew BG data. Pregnant women should generally be excluded from broad‐based analyses or considered as a discrete category because they have very different targets for BG therapy. Patients with short lengths of stay may be less likely to benefit from tight glucose control and may also be considered for post hoc exclusion. One might also exclude patients with very few evaluable glucose readings (eg, fewer than 5) to ensure that measurement is meaningful for a given patient, keeping in mind that this may also exclude patients with undetected hyperglycemia, as mentioned above. Finally, patients receiving palliative care should also be considered for exclusion if feasible.
Recommendation: Do not limit analyses to only those patients with a diagnosis of diabetes or only those on insulin, which will lead to biased results.
-
For noncritical care patients, we recommend a combined approach: adult patients with a diagnosis of diabetes (e. g. using diagnosis‐related group [DRG] codes 294 or 295 or International Classification of Diseases 9th edition [ICD9] codes 250.xx) or with hyperglycemia (eg, 2 or more random laboratory and/or point of care (POC) BG values >180 mg/dL or 2 or more fasting BG values >130 mg/dL), excluding patients with DKA or HHS or who are pregnant.
-
For critical care units, we recommend either all patients, or patients with at least mild hyperglycemia (eg, 2 random glucose levels >140 mg/dL). Critical care patients with DKA, HHS, and pregnancy should be evaluated separately if possible.
Which Glucose Values to Include and Exclude
To answer this question, we first need to decide which method to use for BG measurement. There are several ways to measure BG, including the type of sample collected (capillary [fingerstick], arterial, and venous) and the technique used (central laboratory analyzing plasma, central laboratory analyzing whole blood [eg, from an arterial blood gas sample], glucose meter [usually calibrated to plasma], etc.). The POC (eg, capillary, glucose meter) glucose measurements alone are often preferred in the non‐ICU setting because laboratory plasma values generally provide little additional information and typically lower the mean glucose by including redundant fasting values.1 In critical care units, several different methods are often used together, and each merits inclusion. The inherent differences in calibration between the methods do not generally require separate analyses, especially given the frequency of testing in the ICU setting.
The next question is which values to include in analyses. In some situations, it may be most useful to focus on a certain period of hospitalization, such as the day of a procedure and the next 2 days in assessing the impact of the quality of perioperative care, or the first 14 days of a noncritical care stay to keep outliers for length of stay (LOS) from skewing the data. In the non‐ICU setting, it may be reasonable to exclude the first day of hospitalization, as early BG control is impacted by multiple variables beyond direct control of the clinician (eg, glucose control prior to admission, severity of presenting illness) and may not realistically reflect your interventions. (Keep in mind, however, that it may be useful to adjust for the admission glucose value in multivariable models given its importance to clinical outcomes and its strong relationship to subsequent inpatient glucose control.) However, in critical care units, it is reasonable to include the first day's readings in analyses given the high frequency of glucose measurements in this setting and the expectation that glucose control should be achieved within a few hours of starting an intravenous insulin infusion.
If feasible to do so with your institution's data capture methods, you may wish to select only the regularly scheduled (before each meal [qAC] and at bedtime [qHS], or every 6 hours [q6h]) glucose readings for inclusion in the summary data of glycemic control in the non‐ICU setting, thereby reducing bias caused by repeated measurements around extremes of glycemic excursions. An alternative in the non‐ICU setting is to censor glucose readings within 60 minutes of a previous reading.
Recommendation:
-
In the non‐ICU setting, we recommend first looking at all POC glucose values and if possible repeating the analyses excluding hospital day 1 and hospital day 15 and beyond, and also excluding glucose values measured within 60 minutes of a previous value.
-
In critical care units, we recommend evaluating all glucose readings used to guide care.
Units of Analysis
There are several different units of analysis, each with its own advantages and disadvantages:
-
Glucose value: this is the simplest measure and the one with the most statistical power. All glucose values for all patients of interest comprise the denominator. A report might say, for example, that 1% of the 1000 glucose values were <70 mg/dL during a certain period or that the mean of all glucose values collected for the month from patients in noncritical care areas was 160 mg/dL. The potential disadvantages of this approach are that these analyses are less clinically relevant than patient‐level analyses and that patients with many glucose readings and long hospitalizations may skew the data.
-
Patient (or the Patient Stay, [ie, the entire hospitalization]): all patients who are monitored make up the denominator. The numerator may be the percentage of patients with any hypoglycemia during their hospital stay or the percentage of patients achieving a certain mean glucose during their hospitalization, for example. This is inherently more clinically meaningful than using glucose value as a unit of analysis. A major disadvantage is not controlling for LOS effects. For example, a hospitalized patient with a long LOS is much more likely to be characterized as having at least 1 hypoglycemic value than is a patient with a shorter LOS. Another shortcoming is that this approach does not correct for uneven distribution of testing. A patient's mean glucose might be calculated on the basis of 8 glucose values on the first day of hospitalization, 4 on the second day, and 1 on the third day. Despite all these shortcomings, reporting by patient remains a popular and valid method of presenting glycemic control results, particularly when complemented by other views and refined to control for the number of readings per day.
-
Monitored Patient‐Day: The denominator in this setting is the total number of days a patient glucose level is monitored. The benefits of this method have been described and advocated in the literature.1 As with patient‐level analyses, this measure will be more rigorous and meaningful if the BG measures to be evaluated have been standardized. Typical reports might include percentage of monitored days with any hypoglycemia, or percentage of monitored days with all glucose values in the desired range. This unit of analysis may be considered more difficult to generate and to interpret. On the other hand, it is clinically relevant, less biased by LOS effects, and may be considered the most actionable metric by clinicians. This method provides a good balance when presented with data organized by patient.
The following example uses all 3 units of measurement, in this case to determine the rate of hypoglycemia, demonstrating the different but complementary information that each method provides:
-
In 1 month, 3900 POC glucose measurements were obtained from 286 patients representing 986 monitored patient‐days. With hypoglycemia defined as POC BG 60 mg/dL, the results showed the following:
-
50 of 3900 measurements (1.4%) were hypoglycemic 22 of 286 patients (7.7%) had 1 hypoglycemic episodes
-
40 of 986 monitored days (4.4%) had 1 hypoglycemic episodes.
The metric based on the number of glucose readings could be considered the least clinically relevant because it is unclear how many patients were affected; moreover, it may be based on variable testing patterns among patients, and could be influenced disproportionately by 1 patient with frequent hypoglycemia, many glucose readings, and/or a long LOS. One could argue that the patient‐stay metric is artificially elevated because a single hypoglycemic episode characterizes the entire stay as hypoglycemic. On the other hand, at least it acknowledges the number of patients affected by hypoglycemia. The patient‐day unit of analysis likely provides the most balanced view, one that is clinically relevant and measured over a standard period of time, and less biased by LOS and frequency of testing.
One way to express patient‐day glycemic control that deserves special mention is the patient‐day weighted mean. A mean glucose is calculated for each patient‐day, and then the mean is calculated across all patient‐days. The advantage of this approach is that it corrects for variation in the number of glucose readings each day; all hospital days are weighted equally.
Recommendation:
-
In noncritical care units, we recommend a combination of patient‐day and patient‐stay measures.
-
In critical care units, it is acceptable to also use glucose reading as the unit of measurement given more frequent and uniform data collection, but it should be complemented by more meaningful patient‐day and patient‐stay measures.
Measures of Control
In addition to deciding the unit(s) of analysis, another issue concerns which measures of control to use. These could include rates of hypoglycemia and hyperglycemia, percentage of glucose readings within various ranges (eg, <70, 70180, >180 mg/dL), mean glucose value, percentage of patient‐days during which the mean glucose is within various ranges, or the in control rate (ie, when all glucose values are within a certain range).
As with the various units of analysis, each of these measures of control has various advantages and disadvantages. For example, mean glucose is easy to report and understand, but masks extreme values. Percentage of glucose values within a certain range (eg, per patient, averaged across patients) presents a more complete picture but is a little harder to understand and will vary depending on the frequency of glucose monitoring. As mentioned above, this latter problem can be corrected in part by including only certain glucose values. Percent of glucose values within range may also be less sensitive to change than mean glucose (eg, a glucose that is lowered from 300 mg/dL to 200 mg/dL is still out of range). We recommend choosing a few, but not all, measures of control in order to get a complete picture of glycemic control. Over time one can then refine the measures being used to meet the needs of the glycemic control team and provide data that will drive the performance improvement process.
In critical care and perioperative settings, interest in glycemic control is often more intense around the time of a particular event such as major surgery or after admission to the ICU. Some measures commonly used in performing such analyses are:
-
All values outside a target range within a designated crucial period. For example, the University Healthcare Consortium and other organizations use a simple metric to gauge perioperative glycemic control. They collect the fasting glucose on postoperative days 1 and 2 and then calculate the percentage of postoperative days with any fasting glucose >200 mg/dL. Of course, this is a very liberal target, but it can always be lowered in a stepwise fashion once it is regularly being reached.
-
Three‐day blood glucose average. The Portland group uses the mean glucose of each patient for the period that includes the day of coronary artery bypass graft (CABG) surgery and the following 2 days. The 3‐day BG average (3‐BG) correlates very well with patient outcomes and can serve as a well‐defined target.2 It is likely that use of the 3‐BG would work well in other perioperative/trauma settings and could work in the medical ICU as well, with admission to the ICU as the starting point for calculation of the 3‐BG.
Hyperglycemic Index
Measuring the hyperglycemic index (HGI) is a validated method of summarizing glycemic control of ICU patients.3 It is designed to take into account the sometimes uneven distribution of patient testing. Time is plotted on the x‐axis and glucose values on the y‐axis. The HGI is calculated the area under the curve of glycemic values but above the upper limit of normal (ie, 110 mg/dL). Glucose values in the normal or hypoglycemic range are not included in the AUC. Mortality correlated well with this glycemic index. However, a recent observational study of glucometrics in patients hospitalized with acute myocardial infarction found that the simple mean of each patient's glucose values over the entire hospitalization was as predictive of in‐hospital mortality as the HGI or the time‐averaged glucose (AUC for all glucose values).4 In this study, metrics derived from glucose readings for the entire hospitalization were more predictive than those based on the first 24 or 48 hours or on the admission glucose.
Analyses Describing Change in Glycemic Control Over Time in the Hospital
In the critical care setting, this unit of analysis may be as simple as the mean time to reach the glycemic target on your insulin infusion protocol. On noncritical care wards, it is a bit more challenging to characterize the improvement (or clinical inertia) implied by failure of hyperglycemia to lessen as an inpatient stay progresses. One method is to calculate the mean glucose (or percentage of glucose values in a given range) for each patient on hospital day (HD) 1, and repeat for each HD (up to some reasonable limit, such as 5 or 7 days).
Recommendations:
-
In noncritical units, we recommend a limited set of complementary measures, such as the patient‐day weighted mean glucose, mean percent of glucose readings per patient that are within a certain range, and percentage of patients whose mean glucose is within a certain range on each hospital day.
-
In critical care units, it is often useful to focus measures around a certain critical event such as the 3‐day blood glucose average and to use measures such as the HGI that take advantage of more frequent blood glucose testing.
Definitions of Hyperglycemia and Hypoglycemia
Glucometrics outcomes will obviously depend on the thresholds established for hyperglycemia and hypoglycemia. Many centers define hypoglycemia as 60 mg/dL, whereas the ADA definition, based on physiologic changes that may take place, defines hypoglycemia (at least in the outpatient setting) as 70 mg/dL. Hypoglycemia may be further stratified by severity, with any glucose 40 mg/dL, for instance, defined as severe hypoglycemia.
Similarly, the definition of hyperglycemia (and therefore good control) must also be defined. Based on definitions developed by the ADA and AACE, the state of the medical literature, and current understanding of the pathophysiology of hyperglycemia, thresholds for critical care units include 110 mg/dL, 130 mg/dL, and 140 mg/dL, and options in noncritical care units include 130 mg/dL, 140 mg/dL, and 180 mg/dL. Because these thresholds implicitly assume adverse effects when glucose levels are above them, these levels are subject to revision as data become available confirming the benefits and safety of targeted glycemic control in various settings and patient populations.
Introducing optimal BG targets in a stepped fashion over time should also be considered. Furnary et al.2 have done this in the Portland Project, which tracks glycemic control in cardiac surgery patients receiving intravenous insulin therapy. The initial BG target for this project was <200 mg/dL; it was subsequently lowered stepwise over several years to 150 mg/dL, then to 120 mg/dL, and most recently to 110 mg/dL. This approach allows the safe introduction of targeted glycemic control and promotes acceptance of the concept by physicians and the allied nursing and medical staff.
Recommendations:
-
In noncritical care units, it is reasonable to use 40 mg/dL for severe hypoglycemia, 70 mg/dL for hypoglycemia, 130 mg/dL for fasting hyperglycemia, 180 mg/dL for random or postprandial hyperglycemia, and 300 mg/dL for severe hyperglycemia, keeping in mind that these thresholds are arbitrary. In critical care units, values from 110 mg/dL to 140 mg/dL might be better thresholds for hyperglycemia, but it may take time to safely and effectively move an organization toward these lower targets.
Other Considerations Relative to Glucometrics
Yale Glucometrics Website
The Yale Informatics group has put together a Web‐based resource (
Other Analytic Resources
Commercially available software, such as the RALS system (Medical Automation Systems, Inc., Charlottesville, VA) can gather POC glucose measurements directly from devices and provide real‐time reports of glycemic control, stratified by inpatient unit, using user‐defined targets for hypoglycemia and hyperglycemia. While they are no substitute for a dedicated, on‐site data analyst, such systems can be very useful for smaller hospitals with minimal data or information technology support staff.
APPROACHES TO ANALYSIS: RUN CHARTS
Most conventional clinical trials hold interventions fixed for a period of time and compare results with and without the intervention. For quality improvement studies, this is still a valid way to proceed, especially if studied as a randomized controlled trial. Such methods may be preferred when the clinical question is Does this type of intervention work in general? and the desired output is publication in peer‐reviewed journals so that others can learn about and adopt the intervention to their own institution. A before and after study with a similar analytic approach may also be valid, although concerns about temporal trends and cointerventions potentially compromise the validity of such studies. This approach again assumes that an intervention is held fixed over time such that it is clear what patients received during each time period.
If the desired result is improvement at a given institution (the question is Did we improve care?) then it may be preferable to present results over time using run‐charts. In a run chart, the x‐axis is time and the y‐axis the desired metric, such as patient‐day weighted mean glucose. Points in time when interventions were introduced or modified can be highlighted. Run charts have several advantages over before‐and‐after summaries: they do not require interventions remaining fixed and are more compatible with continuous quality improvement methods, it is easier to see the effect of different aspects of the interventions as they occur, one can get a quicker picture of whether something is working, and it is easier to separate out the impact of the intervention from secular trends. Finally, the use of run charts does not imply the absence of statistical rigor. Run charts with statistical process control (SPC) limits5 can easily convey when the observed time trend is unlikely to be due to chance using prespecified P values. (A full discussion of SPC and other methods to study quality improvement interventions is beyond the scope of this article.)
ASSESSING PATTERNS OF INSULIN USE AND ORDER SET UTILIZATION
Besides measuring the impact of quality improvement interventions on glucose control, it is important to measure processes such as proper insulin use. As mentioned in other articles in this supplement, processes are much more sensitive to change than outcomes. Failure to change processes should lead one to make changes to the intervention.
ICU and Perioperative Settings
For ICU and perioperative settings, the major process measure will likely be use of the insulin infusion order set. Designation of BG levels that trigger insulin infusion in these settings should be agreed upon in advance. The number of patients who meet the predefined glycemic criteria would make up the denominator, and the number of patients on the insulin infusion order set would make up the numerator.
NonCritical Care Units
On noncritical care units, measuring the percentage of subcutaneous insulin regimens that contain a basal insulin is a useful way to monitor the impact of an intervention. A more detailed analysis could examine the percentage of patients on simultaneous basal and nutritional insulin (if applicable). An important measure of clinical inertia is to track the percentage of patients who had changes in their insulin regimens on days after hypoglycemic or hyperglycemic excursions. Another important measure is the frequency with which the standardized order set is being used, analogous to the measure of insulin infusion use in the ICU. A final process measure, indirectly related to insulin use, is the frequency of use of oral diabetes agents, especially by patients for whom their use is contraindicated (eg, patients with congestive heart failure who are on thiazolidinediones and patients with renal insufficiency or receiving intravenous contrast continued on metformin).
OTHER CONSIDERATIONS AND METRICS
Examples of other metrics that can be used to track the success of quality improvement efforts include:
-
Glucose measurement within 8 hours of hospital admission.
-
Glycated hemoglobin (A1C) measurement obtained or available within 30 days of admission to help guide inpatient and especially discharge management.
-
Appropriate glucose testing in patients with diabetes or hyperglycemia (eg, 4 times per day in patients not on insulin infusion protocols, at least until 24 hours of euglycemia is documented).
-
The percentage of patients on insulin with on‐time tray delivery.
-
The timing of subcutaneous insulin administration in relation to glucose testing and nutrition delivery.
-
Documentation of carbohydrate intake among patients who are eating.
-
Satisfaction of physicians and nurses with order sets or protocols, using standard surveys.
-
Physician and nurse knowledge, attitudes, and beliefs about insulin administration, fear of hypoglycemia, treatment of hypoglycemia, and glycemic control in the hospital.
-
Patient satisfaction with their diabetes care in the hospital, including the education they received.
-
Nursing and physician education/certification in insulin prescribing, insulin administration, and other diabetes care issues.
-
Patient outcomes strongly associated with glycemic control, (eg, surgical wound infections, ICU LOS, catheter‐related bloodstream infections).
-
Appropriate treatment and documentation of hypoglycemia (eg, in accordance with hospital policy).
-
Documentation of severe hypoglycemic events through the hospital's adverse events reporting system (these may actually increase as change comes to the organization and as clinical personnel are more attuned to glycemic control).
-
Root causes of hypoglycemic events, which can be used to understand and prevent future events.
-
Appropriate transitions from IV to SC insulin regimens, (eg, starting basal insulin prior to discontinuing infusion in patients who have been on an insulin infusion of at least 2 units/hour or who have a known diagnosis of diabetes or A1C >7).
(Survey instruments and other measurement tools are available from the authors upon request.)
SHM GLYCEMIC CONTROL TASK FORCE SUMMARY RECOMMENDATIONS
The SHM Glycemic Control Task Force is working to develop standardized measures of inpatient glucose control and related indicators to track progress of hospital glycemic control initiatives (see the introduction to this supplement for a description of the charge and membership of this task force). The goals of the Task Force's metrics recommendations (Table 1) are several‐fold: (1) create a set of measurements that are complete but not overly burdensome; (2) create realistic measures that can be applied to institutions with different data management capabilities; and (3) allow for comparison across institutions for benchmarking purposes, evaluation of quality improvement projects, and reporting of results for formal research studies in this field.
| Measurement Issue | NonCritical Care Units | Critical Care Units | ||
|---|---|---|---|---|
| Tier 1 Recommendations | Tier 2 Recommendations | Tier 1 Recommendations | Tier 2 Recommendations | |
| ||||
| Patient inclusion and exclusion criteria | All adult patients with POC glucose testing (sampling acceptable). Exclude patients with DKA or HHS or who are pregnant. | All adult patients with diagnosis of diabetes by ICD‐9 code* or by glucose testing: random glucose (POC or laboratory) >180 mg/dL 2 or fasting glucose >130 mg/dL 2, excluding patients with DKA or HHS or who are pregnant. Additional analysis: exclude patients with <5 evaluable glucose readings, patients with LOS <2 days, or receiving palliative care. | All patients in every critical care unit (sampling acceptable). | Patients with DKA, HHS, or pregnancy in separate analyses. All patients in every critical care unit with random glucose (POC or laboratory) >140 mg/dL 2. |
| Glucose reading inclusion and exclusion criteria | All POC glucose values. | Additional analysis: exclude glucose values on hospital day 1 and on hospital day 15 and after. Additional analysis: exclude glucose values measured within 60 minutes of a previous value. | All POC and other glucose values used to guide care. | |
| Measures of safety | Analysis by patient‐day: Percentage of patient‐days with 1 or more values <40, <70, and >300 mg/dL. | Analysis by patient‐day: Percentage of patient‐days with 1 or more values <40, <70, and >300 mg/dL. | ||
| Measures of glucose control | Analysis by patient‐day: Percentage of patient‐days with mean <140, <180 mg/dL and/or Percentage of patient‐days with all values <180 mg/dL. | Analysis by patient‐day: Patient day‐weighted mean glucose. | Analysis by glucose reading: Percentage of readings <110, <140 mg/dL. | 3‐BG as above for all patients in critical care units.∥ Hyperglycemic index for all patients in critical care units (AUC of glucose values above target). |
| Analysis by patient stay: Percentage of patient stays with mean <140, <180 mg/dL. | Analysis by patient stay: Mean percentage of glucose readings of each patient <180 mg/dL. | Analysis by patient‐day: Percentage of patient‐days with mean <110, <140 mg/dL, and/or Percentage of patient‐days with all values <110, <140 mg/dL. | ||
| Analysis by hospital day: Percentage of patients with mean glucose readings <140, <180 mg/dL by hospital day (days 17). | Analysis by patient stay: 3‐day blood glucose average (3‐BG) for selected perioperative patients: Percentage of patients with 3‐BG <110, <140 mg/dL. Mean time (hours) to reach glycemic target (BG <110 or <140 mg/dL) on insulin infusion. | |||
| Measures of insulin use | Percentage of patients on any subcutaneous insulin that has a scheduled basal insulin component (glargine, NPH, or detemir). | Percentage of patients with at least 2 POC and/or laboratory glucose readings >180 mg/dL who have a scheduled basal insulin component. Percentage of eating patients with hyperglycemia as defined above with scheduled basal insulin and nutritional insulin. Percentage of patients and patient‐days with any changes in insulin orders the day after 2 or more episodes of hypoglycemia or hyperglycemia (ie, <70 or >180 mg/dL). | Percentage of patients with 2 POC or laboratory glucose readings >140 mg/dL placed on insulin infusion protocol. | |
| Other process measures | Glucose measured within 8 hours of hospital admission. | POC glucose testing at least 4 times a day for all patients with diabetes or hyperglycemia as defined above. | Glucose measured within 8 hours of hospital admission. | Appropriateness of hypoglycemia treatment and documentation. |
| A1C measurement obtained or available within 30 days of admission. | Measures of adherence to specific components of management protocol. | Frequency of BG testing (eg, per protocol if on insulin infusion; every 68 hours if not). | Clinical events of severe hypoglycemia reported through the organization's critical events reporting tool. | |
| Appropriateness of hypoglycemia treatment and documentation. | Root causes of hypoglycemia. | |||
| Clinical events of severe hypoglycemia reported through the organization's critical events reporting tool. | Appropriate use of IV‐to‐SC insulin transition protocol. | |||
| Root causes of hypoglycemia. | ||||
For each domain of glycemic management (glycemic control, safety, and insulin use), the task force chose a set of best measures. They are presented as two tiers of measurement standards, depending on the capabilities of the institution and the planned uses of the data. Tier 1 includes measures that, although they do take time and resources to collect, are feasible for most institutions. Tier 2 measures are recommended for hospitals with easy manipulation of electronic sources of data and for reporting quality‐of‐care measures for widespread publication, that is, in the context of a research study. It should be emphasized that these recommendations are only meant as a guide: the actual measures chosen should meet the needs and capabilities of each institution.
We recognize that few data support the recommendations made by this task force, that such data are needed, and that the field of data collection and analysis for hospital glycemic management is rapidly evolving. The hope is to begin the standardization process, promote dialogue in this field, and eventually reach a consensus in collaboration with the ADA, AACE, and other pertinent stakeholders.
CONCLUSIONS
Like the field of inpatient glycemic management itself, the field of devising metrics to measure the quality of inpatient glycemic control is also in its infancy and quickly evolving. One should not be paralyzed by the lack of consensus regarding measurementthe important point is to pick a few complementary metrics and begin the process. The table of recommendations can hopefully serve as a starting point for many institutions, with a focus on efficacy (glycemic control), safety (hypoglycemia), and process (insulin use patterns). As your institution gains experience with measurement and the field evolves, your metrics will likely change. We recommend keeping all process and outcome data in its raw form so that it can be summarized in different ways over time. It is also important not to wait for the perfect data collection tool before beginning to analyze data: sampling and paper processes are acceptable if automated data collection is not yet possible. Eventually, blood glucose meter readings should be downloaded into a central database that interfaces with hospital data repositories so data can be analyzed in conjunction with patient, service, and unit‐level information. Only with a rigorous measurement process can institutions hope to know whether their changes are resulting in improved care for patients.
Data collection, analysis, and presentation are key to the success of any hospital glycemic control initiative. Such efforts enable the management team to track improvements in processes and outcomes, make necessary changes to their quality improvement efforts, justify the provision of necessary time and resources, and share their results with others. Reliable metrics for assessing glycemic control and frequency of hypoglycemia are essential to accomplish these tasks and to assess whether interventions result in more benefit than harm. Hypoglycemia metrics must be especially convincing because fear of hypoglycemia remains a major source of clinical inertia, impeding efforts to improve glucose control.
Currently, there are no official standards or guidelines for formulating metrics on the quality of inpatient glycemic control. This creates several problems. First, different metrics vary in their biases and in their responsiveness to change. Thus, use of a poor metric could lead to either a falsely positive or falsely negative impression that a quality improvement intervention is in fact improving glycemic control. Second, the proliferation of different measures and analytical plans in the research and quality improvement literature make it very difficult for hospitals to compare baseline performance, determine need for improvement, and understand which interventions may be most effective.
A related article in this supplement provides the rationale for improved inpatient glycemic control. That article argues that the current state of inpatient glycemic control, with the frequent occurrence of severe hyperglycemia and irrational insulin ordering, cannot be considered acceptable, especially given the large body of data (albeit largely observational) linking hyperglycemia to negative patient outcomes. However, regardless of whether one is an advocate or skeptic of tighter glucose control in the intensive care unit (ICU) and especially the non‐ICU setting, there is no question that standardized, valid, and reliable metrics are needed to compare efforts to improve glycemic control, better understand whether such control actually improves patient care, and closely monitor patient safety.
This article provides a summary of practical suggestions to assess glycemic control, insulin use patterns, and safety (hypoglycemia and severe hyperglycemia). In particular, we discuss the pros and cons of various measurement choices. We conclude with a tiered summary of recommendations for practical metrics that we hope will be useful to individual improvement teams. This article is not a consensus statement but rather a starting place that we hope will begin to standardize measurement across institutions and advance the dialogue on this subject. To more definitely address this problem, we call on the American Association of Clinical Endocrinologists (AACE), American Diabetes Association (ADA), Society of Hospital Medicine (SHM), and others to agree on consensus standards regarding metrics for the quality of inpatient glycemic control.
MEASURING GLYCEMIC CONTROL: GLUCOMETRICS
Glucometrics may be defined as the systematic analysis of blood glucose (BG) dataa phrase initially coined specifically for the inpatient setting. There are numerous ways to do these analyses, depending on which patients and glucose values are considered, the definitions used for hypoglycemia and hyperglycemia, the unit of measurement (eg, patient, patient‐day, individual glucose value), and the measure of control (eg, mean, median, percent of glucose readings within a certain range). We consider each of these dimensions in turn.
Defining the Target Patient Population
The first decision to be made is which patients to include in your analysis. Choices include the following:
-
Patients with a discharge diagnosis of diabetes: this group has face validity and intuitive appeal, is easy to identify retrospectively, and may capture some untested/untreated diabetics, but will miss patients with otherwise undiagnosed diabetes and stress hyperglycemia. It is also subject to the variable accuracy of billing codes.
-
Patients with a certain number of point‐of‐care (POC) glucose measurements: this group is also easy to identify, easy to measure, and will include patients with hyperglycemia without a previous diagnosis of diabetes, but will miss patients with untested/untreated hyperglycemia. Also, if glucose levels are checked on normoglycemic, nondiabetic patients, these values may dilute the overall assessment of glycemic control.
-
Patients treated with insulin in the hospital: this is a good choice if the purpose is mainly drug safety and avoidance of hypoglycemia, but by definition excludes most untreated patients.
-
Patients with 2 or more BG values (laboratory and/or POC) over a certain threshold (eg, >180 mg/dL). This will likely capture more patients with inpatient hyperglycemia, whether or not detected by the medical team, but is subject to wide variations in the frequency and timing of laboratory glucose testing, including whether or not the values are pre‐prandial (note that even preprandial POC glucose measurements are not always in fact fasting values).
Other considerations include the following:
-
Are there natural patient subgroups that should be measured and analyzed separately because of different guidelines? For example, there probably should be separate/emndependent inclusion criteria and analyses for critical care and noncritical care units because their glycemic targets and management considerations differ.
-
Which patients should be excluded? For example, if targeting subcutaneous insulin use in general hospitalized patients, one might eliminate those patients who are admitted specifically as the result of a diabetes emergency (eg, diabetic ketoacidosis [DKA] and hyperglycemic hyperosmolar state [HHS]), as their marked and prolonged hyperglycemia will skew BG data. Pregnant women should generally be excluded from broad‐based analyses or considered as a discrete category because they have very different targets for BG therapy. Patients with short lengths of stay may be less likely to benefit from tight glucose control and may also be considered for post hoc exclusion. One might also exclude patients with very few evaluable glucose readings (eg, fewer than 5) to ensure that measurement is meaningful for a given patient, keeping in mind that this may also exclude patients with undetected hyperglycemia, as mentioned above. Finally, patients receiving palliative care should also be considered for exclusion if feasible.
Recommendation: Do not limit analyses to only those patients with a diagnosis of diabetes or only those on insulin, which will lead to biased results.
-
For noncritical care patients, we recommend a combined approach: adult patients with a diagnosis of diabetes (e. g. using diagnosis‐related group [DRG] codes 294 or 295 or International Classification of Diseases 9th edition [ICD9] codes 250.xx) or with hyperglycemia (eg, 2 or more random laboratory and/or point of care (POC) BG values >180 mg/dL or 2 or more fasting BG values >130 mg/dL), excluding patients with DKA or HHS or who are pregnant.
-
For critical care units, we recommend either all patients, or patients with at least mild hyperglycemia (eg, 2 random glucose levels >140 mg/dL). Critical care patients with DKA, HHS, and pregnancy should be evaluated separately if possible.
Which Glucose Values to Include and Exclude
To answer this question, we first need to decide which method to use for BG measurement. There are several ways to measure BG, including the type of sample collected (capillary [fingerstick], arterial, and venous) and the technique used (central laboratory analyzing plasma, central laboratory analyzing whole blood [eg, from an arterial blood gas sample], glucose meter [usually calibrated to plasma], etc.). The POC (eg, capillary, glucose meter) glucose measurements alone are often preferred in the non‐ICU setting because laboratory plasma values generally provide little additional information and typically lower the mean glucose by including redundant fasting values.1 In critical care units, several different methods are often used together, and each merits inclusion. The inherent differences in calibration between the methods do not generally require separate analyses, especially given the frequency of testing in the ICU setting.
The next question is which values to include in analyses. In some situations, it may be most useful to focus on a certain period of hospitalization, such as the day of a procedure and the next 2 days in assessing the impact of the quality of perioperative care, or the first 14 days of a noncritical care stay to keep outliers for length of stay (LOS) from skewing the data. In the non‐ICU setting, it may be reasonable to exclude the first day of hospitalization, as early BG control is impacted by multiple variables beyond direct control of the clinician (eg, glucose control prior to admission, severity of presenting illness) and may not realistically reflect your interventions. (Keep in mind, however, that it may be useful to adjust for the admission glucose value in multivariable models given its importance to clinical outcomes and its strong relationship to subsequent inpatient glucose control.) However, in critical care units, it is reasonable to include the first day's readings in analyses given the high frequency of glucose measurements in this setting and the expectation that glucose control should be achieved within a few hours of starting an intravenous insulin infusion.
If feasible to do so with your institution's data capture methods, you may wish to select only the regularly scheduled (before each meal [qAC] and at bedtime [qHS], or every 6 hours [q6h]) glucose readings for inclusion in the summary data of glycemic control in the non‐ICU setting, thereby reducing bias caused by repeated measurements around extremes of glycemic excursions. An alternative in the non‐ICU setting is to censor glucose readings within 60 minutes of a previous reading.
Recommendation:
-
In the non‐ICU setting, we recommend first looking at all POC glucose values and if possible repeating the analyses excluding hospital day 1 and hospital day 15 and beyond, and also excluding glucose values measured within 60 minutes of a previous value.
-
In critical care units, we recommend evaluating all glucose readings used to guide care.
Units of Analysis
There are several different units of analysis, each with its own advantages and disadvantages:
-
Glucose value: this is the simplest measure and the one with the most statistical power. All glucose values for all patients of interest comprise the denominator. A report might say, for example, that 1% of the 1000 glucose values were <70 mg/dL during a certain period or that the mean of all glucose values collected for the month from patients in noncritical care areas was 160 mg/dL. The potential disadvantages of this approach are that these analyses are less clinically relevant than patient‐level analyses and that patients with many glucose readings and long hospitalizations may skew the data.
-
Patient (or the Patient Stay, [ie, the entire hospitalization]): all patients who are monitored make up the denominator. The numerator may be the percentage of patients with any hypoglycemia during their hospital stay or the percentage of patients achieving a certain mean glucose during their hospitalization, for example. This is inherently more clinically meaningful than using glucose value as a unit of analysis. A major disadvantage is not controlling for LOS effects. For example, a hospitalized patient with a long LOS is much more likely to be characterized as having at least 1 hypoglycemic value than is a patient with a shorter LOS. Another shortcoming is that this approach does not correct for uneven distribution of testing. A patient's mean glucose might be calculated on the basis of 8 glucose values on the first day of hospitalization, 4 on the second day, and 1 on the third day. Despite all these shortcomings, reporting by patient remains a popular and valid method of presenting glycemic control results, particularly when complemented by other views and refined to control for the number of readings per day.
-
Monitored Patient‐Day: The denominator in this setting is the total number of days a patient glucose level is monitored. The benefits of this method have been described and advocated in the literature.1 As with patient‐level analyses, this measure will be more rigorous and meaningful if the BG measures to be evaluated have been standardized. Typical reports might include percentage of monitored days with any hypoglycemia, or percentage of monitored days with all glucose values in the desired range. This unit of analysis may be considered more difficult to generate and to interpret. On the other hand, it is clinically relevant, less biased by LOS effects, and may be considered the most actionable metric by clinicians. This method provides a good balance when presented with data organized by patient.
The following example uses all 3 units of measurement, in this case to determine the rate of hypoglycemia, demonstrating the different but complementary information that each method provides:
-
In 1 month, 3900 POC glucose measurements were obtained from 286 patients representing 986 monitored patient‐days. With hypoglycemia defined as POC BG 60 mg/dL, the results showed the following:
-
50 of 3900 measurements (1.4%) were hypoglycemic 22 of 286 patients (7.7%) had 1 hypoglycemic episodes
-
40 of 986 monitored days (4.4%) had 1 hypoglycemic episodes.
The metric based on the number of glucose readings could be considered the least clinically relevant because it is unclear how many patients were affected; moreover, it may be based on variable testing patterns among patients, and could be influenced disproportionately by 1 patient with frequent hypoglycemia, many glucose readings, and/or a long LOS. One could argue that the patient‐stay metric is artificially elevated because a single hypoglycemic episode characterizes the entire stay as hypoglycemic. On the other hand, at least it acknowledges the number of patients affected by hypoglycemia. The patient‐day unit of analysis likely provides the most balanced view, one that is clinically relevant and measured over a standard period of time, and less biased by LOS and frequency of testing.
One way to express patient‐day glycemic control that deserves special mention is the patient‐day weighted mean. A mean glucose is calculated for each patient‐day, and then the mean is calculated across all patient‐days. The advantage of this approach is that it corrects for variation in the number of glucose readings each day; all hospital days are weighted equally.
Recommendation:
-
In noncritical care units, we recommend a combination of patient‐day and patient‐stay measures.
-
In critical care units, it is acceptable to also use glucose reading as the unit of measurement given more frequent and uniform data collection, but it should be complemented by more meaningful patient‐day and patient‐stay measures.
Measures of Control
In addition to deciding the unit(s) of analysis, another issue concerns which measures of control to use. These could include rates of hypoglycemia and hyperglycemia, percentage of glucose readings within various ranges (eg, <70, 70180, >180 mg/dL), mean glucose value, percentage of patient‐days during which the mean glucose is within various ranges, or the in control rate (ie, when all glucose values are within a certain range).
As with the various units of analysis, each of these measures of control has various advantages and disadvantages. For example, mean glucose is easy to report and understand, but masks extreme values. Percentage of glucose values within a certain range (eg, per patient, averaged across patients) presents a more complete picture but is a little harder to understand and will vary depending on the frequency of glucose monitoring. As mentioned above, this latter problem can be corrected in part by including only certain glucose values. Percent of glucose values within range may also be less sensitive to change than mean glucose (eg, a glucose that is lowered from 300 mg/dL to 200 mg/dL is still out of range). We recommend choosing a few, but not all, measures of control in order to get a complete picture of glycemic control. Over time one can then refine the measures being used to meet the needs of the glycemic control team and provide data that will drive the performance improvement process.
In critical care and perioperative settings, interest in glycemic control is often more intense around the time of a particular event such as major surgery or after admission to the ICU. Some measures commonly used in performing such analyses are:
-
All values outside a target range within a designated crucial period. For example, the University Healthcare Consortium and other organizations use a simple metric to gauge perioperative glycemic control. They collect the fasting glucose on postoperative days 1 and 2 and then calculate the percentage of postoperative days with any fasting glucose >200 mg/dL. Of course, this is a very liberal target, but it can always be lowered in a stepwise fashion once it is regularly being reached.
-
Three‐day blood glucose average. The Portland group uses the mean glucose of each patient for the period that includes the day of coronary artery bypass graft (CABG) surgery and the following 2 days. The 3‐day BG average (3‐BG) correlates very well with patient outcomes and can serve as a well‐defined target.2 It is likely that use of the 3‐BG would work well in other perioperative/trauma settings and could work in the medical ICU as well, with admission to the ICU as the starting point for calculation of the 3‐BG.
Hyperglycemic Index
Measuring the hyperglycemic index (HGI) is a validated method of summarizing glycemic control of ICU patients.3 It is designed to take into account the sometimes uneven distribution of patient testing. Time is plotted on the x‐axis and glucose values on the y‐axis. The HGI is calculated the area under the curve of glycemic values but above the upper limit of normal (ie, 110 mg/dL). Glucose values in the normal or hypoglycemic range are not included in the AUC. Mortality correlated well with this glycemic index. However, a recent observational study of glucometrics in patients hospitalized with acute myocardial infarction found that the simple mean of each patient's glucose values over the entire hospitalization was as predictive of in‐hospital mortality as the HGI or the time‐averaged glucose (AUC for all glucose values).4 In this study, metrics derived from glucose readings for the entire hospitalization were more predictive than those based on the first 24 or 48 hours or on the admission glucose.
Analyses Describing Change in Glycemic Control Over Time in the Hospital
In the critical care setting, this unit of analysis may be as simple as the mean time to reach the glycemic target on your insulin infusion protocol. On noncritical care wards, it is a bit more challenging to characterize the improvement (or clinical inertia) implied by failure of hyperglycemia to lessen as an inpatient stay progresses. One method is to calculate the mean glucose (or percentage of glucose values in a given range) for each patient on hospital day (HD) 1, and repeat for each HD (up to some reasonable limit, such as 5 or 7 days).
Recommendations:
-
In noncritical units, we recommend a limited set of complementary measures, such as the patient‐day weighted mean glucose, mean percent of glucose readings per patient that are within a certain range, and percentage of patients whose mean glucose is within a certain range on each hospital day.
-
In critical care units, it is often useful to focus measures around a certain critical event such as the 3‐day blood glucose average and to use measures such as the HGI that take advantage of more frequent blood glucose testing.
Definitions of Hyperglycemia and Hypoglycemia
Glucometrics outcomes will obviously depend on the thresholds established for hyperglycemia and hypoglycemia. Many centers define hypoglycemia as 60 mg/dL, whereas the ADA definition, based on physiologic changes that may take place, defines hypoglycemia (at least in the outpatient setting) as 70 mg/dL. Hypoglycemia may be further stratified by severity, with any glucose 40 mg/dL, for instance, defined as severe hypoglycemia.
Similarly, the definition of hyperglycemia (and therefore good control) must also be defined. Based on definitions developed by the ADA and AACE, the state of the medical literature, and current understanding of the pathophysiology of hyperglycemia, thresholds for critical care units include 110 mg/dL, 130 mg/dL, and 140 mg/dL, and options in noncritical care units include 130 mg/dL, 140 mg/dL, and 180 mg/dL. Because these thresholds implicitly assume adverse effects when glucose levels are above them, these levels are subject to revision as data become available confirming the benefits and safety of targeted glycemic control in various settings and patient populations.
Introducing optimal BG targets in a stepped fashion over time should also be considered. Furnary et al.2 have done this in the Portland Project, which tracks glycemic control in cardiac surgery patients receiving intravenous insulin therapy. The initial BG target for this project was <200 mg/dL; it was subsequently lowered stepwise over several years to 150 mg/dL, then to 120 mg/dL, and most recently to 110 mg/dL. This approach allows the safe introduction of targeted glycemic control and promotes acceptance of the concept by physicians and the allied nursing and medical staff.
Recommendations:
-
In noncritical care units, it is reasonable to use 40 mg/dL for severe hypoglycemia, 70 mg/dL for hypoglycemia, 130 mg/dL for fasting hyperglycemia, 180 mg/dL for random or postprandial hyperglycemia, and 300 mg/dL for severe hyperglycemia, keeping in mind that these thresholds are arbitrary. In critical care units, values from 110 mg/dL to 140 mg/dL might be better thresholds for hyperglycemia, but it may take time to safely and effectively move an organization toward these lower targets.
Other Considerations Relative to Glucometrics
Yale Glucometrics Website
The Yale Informatics group has put together a Web‐based resource (
Other Analytic Resources
Commercially available software, such as the RALS system (Medical Automation Systems, Inc., Charlottesville, VA) can gather POC glucose measurements directly from devices and provide real‐time reports of glycemic control, stratified by inpatient unit, using user‐defined targets for hypoglycemia and hyperglycemia. While they are no substitute for a dedicated, on‐site data analyst, such systems can be very useful for smaller hospitals with minimal data or information technology support staff.
APPROACHES TO ANALYSIS: RUN CHARTS
Most conventional clinical trials hold interventions fixed for a period of time and compare results with and without the intervention. For quality improvement studies, this is still a valid way to proceed, especially if studied as a randomized controlled trial. Such methods may be preferred when the clinical question is Does this type of intervention work in general? and the desired output is publication in peer‐reviewed journals so that others can learn about and adopt the intervention to their own institution. A before and after study with a similar analytic approach may also be valid, although concerns about temporal trends and cointerventions potentially compromise the validity of such studies. This approach again assumes that an intervention is held fixed over time such that it is clear what patients received during each time period.
If the desired result is improvement at a given institution (the question is Did we improve care?) then it may be preferable to present results over time using run‐charts. In a run chart, the x‐axis is time and the y‐axis the desired metric, such as patient‐day weighted mean glucose. Points in time when interventions were introduced or modified can be highlighted. Run charts have several advantages over before‐and‐after summaries: they do not require interventions remaining fixed and are more compatible with continuous quality improvement methods, it is easier to see the effect of different aspects of the interventions as they occur, one can get a quicker picture of whether something is working, and it is easier to separate out the impact of the intervention from secular trends. Finally, the use of run charts does not imply the absence of statistical rigor. Run charts with statistical process control (SPC) limits5 can easily convey when the observed time trend is unlikely to be due to chance using prespecified P values. (A full discussion of SPC and other methods to study quality improvement interventions is beyond the scope of this article.)
ASSESSING PATTERNS OF INSULIN USE AND ORDER SET UTILIZATION
Besides measuring the impact of quality improvement interventions on glucose control, it is important to measure processes such as proper insulin use. As mentioned in other articles in this supplement, processes are much more sensitive to change than outcomes. Failure to change processes should lead one to make changes to the intervention.
ICU and Perioperative Settings
For ICU and perioperative settings, the major process measure will likely be use of the insulin infusion order set. Designation of BG levels that trigger insulin infusion in these settings should be agreed upon in advance. The number of patients who meet the predefined glycemic criteria would make up the denominator, and the number of patients on the insulin infusion order set would make up the numerator.
NonCritical Care Units
On noncritical care units, measuring the percentage of subcutaneous insulin regimens that contain a basal insulin is a useful way to monitor the impact of an intervention. A more detailed analysis could examine the percentage of patients on simultaneous basal and nutritional insulin (if applicable). An important measure of clinical inertia is to track the percentage of patients who had changes in their insulin regimens on days after hypoglycemic or hyperglycemic excursions. Another important measure is the frequency with which the standardized order set is being used, analogous to the measure of insulin infusion use in the ICU. A final process measure, indirectly related to insulin use, is the frequency of use of oral diabetes agents, especially by patients for whom their use is contraindicated (eg, patients with congestive heart failure who are on thiazolidinediones and patients with renal insufficiency or receiving intravenous contrast continued on metformin).
OTHER CONSIDERATIONS AND METRICS
Examples of other metrics that can be used to track the success of quality improvement efforts include:
-
Glucose measurement within 8 hours of hospital admission.
-
Glycated hemoglobin (A1C) measurement obtained or available within 30 days of admission to help guide inpatient and especially discharge management.
-
Appropriate glucose testing in patients with diabetes or hyperglycemia (eg, 4 times per day in patients not on insulin infusion protocols, at least until 24 hours of euglycemia is documented).
-
The percentage of patients on insulin with on‐time tray delivery.
-
The timing of subcutaneous insulin administration in relation to glucose testing and nutrition delivery.
-
Documentation of carbohydrate intake among patients who are eating.
-
Satisfaction of physicians and nurses with order sets or protocols, using standard surveys.
-
Physician and nurse knowledge, attitudes, and beliefs about insulin administration, fear of hypoglycemia, treatment of hypoglycemia, and glycemic control in the hospital.
-
Patient satisfaction with their diabetes care in the hospital, including the education they received.
-
Nursing and physician education/certification in insulin prescribing, insulin administration, and other diabetes care issues.
-
Patient outcomes strongly associated with glycemic control, (eg, surgical wound infections, ICU LOS, catheter‐related bloodstream infections).
-
Appropriate treatment and documentation of hypoglycemia (eg, in accordance with hospital policy).
-
Documentation of severe hypoglycemic events through the hospital's adverse events reporting system (these may actually increase as change comes to the organization and as clinical personnel are more attuned to glycemic control).
-
Root causes of hypoglycemic events, which can be used to understand and prevent future events.
-
Appropriate transitions from IV to SC insulin regimens, (eg, starting basal insulin prior to discontinuing infusion in patients who have been on an insulin infusion of at least 2 units/hour or who have a known diagnosis of diabetes or A1C >7).
(Survey instruments and other measurement tools are available from the authors upon request.)
SHM GLYCEMIC CONTROL TASK FORCE SUMMARY RECOMMENDATIONS
The SHM Glycemic Control Task Force is working to develop standardized measures of inpatient glucose control and related indicators to track progress of hospital glycemic control initiatives (see the introduction to this supplement for a description of the charge and membership of this task force). The goals of the Task Force's metrics recommendations (Table 1) are several‐fold: (1) create a set of measurements that are complete but not overly burdensome; (2) create realistic measures that can be applied to institutions with different data management capabilities; and (3) allow for comparison across institutions for benchmarking purposes, evaluation of quality improvement projects, and reporting of results for formal research studies in this field.
| Measurement Issue | NonCritical Care Units | Critical Care Units | ||
|---|---|---|---|---|
| Tier 1 Recommendations | Tier 2 Recommendations | Tier 1 Recommendations | Tier 2 Recommendations | |
| ||||
| Patient inclusion and exclusion criteria | All adult patients with POC glucose testing (sampling acceptable). Exclude patients with DKA or HHS or who are pregnant. | All adult patients with diagnosis of diabetes by ICD‐9 code* or by glucose testing: random glucose (POC or laboratory) >180 mg/dL 2 or fasting glucose >130 mg/dL 2, excluding patients with DKA or HHS or who are pregnant. Additional analysis: exclude patients with <5 evaluable glucose readings, patients with LOS <2 days, or receiving palliative care. | All patients in every critical care unit (sampling acceptable). | Patients with DKA, HHS, or pregnancy in separate analyses. All patients in every critical care unit with random glucose (POC or laboratory) >140 mg/dL 2. |
| Glucose reading inclusion and exclusion criteria | All POC glucose values. | Additional analysis: exclude glucose values on hospital day 1 and on hospital day 15 and after. Additional analysis: exclude glucose values measured within 60 minutes of a previous value. | All POC and other glucose values used to guide care. | |
| Measures of safety | Analysis by patient‐day: Percentage of patient‐days with 1 or more values <40, <70, and >300 mg/dL. | Analysis by patient‐day: Percentage of patient‐days with 1 or more values <40, <70, and >300 mg/dL. | ||
| Measures of glucose control | Analysis by patient‐day: Percentage of patient‐days with mean <140, <180 mg/dL and/or Percentage of patient‐days with all values <180 mg/dL. | Analysis by patient‐day: Patient day‐weighted mean glucose. | Analysis by glucose reading: Percentage of readings <110, <140 mg/dL. | 3‐BG as above for all patients in critical care units.∥ Hyperglycemic index for all patients in critical care units (AUC of glucose values above target). |
| Analysis by patient stay: Percentage of patient stays with mean <140, <180 mg/dL. | Analysis by patient stay: Mean percentage of glucose readings of each patient <180 mg/dL. | Analysis by patient‐day: Percentage of patient‐days with mean <110, <140 mg/dL, and/or Percentage of patient‐days with all values <110, <140 mg/dL. | ||
| Analysis by hospital day: Percentage of patients with mean glucose readings <140, <180 mg/dL by hospital day (days 17). | Analysis by patient stay: 3‐day blood glucose average (3‐BG) for selected perioperative patients: Percentage of patients with 3‐BG <110, <140 mg/dL. Mean time (hours) to reach glycemic target (BG <110 or <140 mg/dL) on insulin infusion. | |||
| Measures of insulin use | Percentage of patients on any subcutaneous insulin that has a scheduled basal insulin component (glargine, NPH, or detemir). | Percentage of patients with at least 2 POC and/or laboratory glucose readings >180 mg/dL who have a scheduled basal insulin component. Percentage of eating patients with hyperglycemia as defined above with scheduled basal insulin and nutritional insulin. Percentage of patients and patient‐days with any changes in insulin orders the day after 2 or more episodes of hypoglycemia or hyperglycemia (ie, <70 or >180 mg/dL). | Percentage of patients with 2 POC or laboratory glucose readings >140 mg/dL placed on insulin infusion protocol. | |
| Other process measures | Glucose measured within 8 hours of hospital admission. | POC glucose testing at least 4 times a day for all patients with diabetes or hyperglycemia as defined above. | Glucose measured within 8 hours of hospital admission. | Appropriateness of hypoglycemia treatment and documentation. |
| A1C measurement obtained or available within 30 days of admission. | Measures of adherence to specific components of management protocol. | Frequency of BG testing (eg, per protocol if on insulin infusion; every 68 hours if not). | Clinical events of severe hypoglycemia reported through the organization's critical events reporting tool. | |
| Appropriateness of hypoglycemia treatment and documentation. | Root causes of hypoglycemia. | |||
| Clinical events of severe hypoglycemia reported through the organization's critical events reporting tool. | Appropriate use of IV‐to‐SC insulin transition protocol. | |||
| Root causes of hypoglycemia. | ||||
For each domain of glycemic management (glycemic control, safety, and insulin use), the task force chose a set of best measures. They are presented as two tiers of measurement standards, depending on the capabilities of the institution and the planned uses of the data. Tier 1 includes measures that, although they do take time and resources to collect, are feasible for most institutions. Tier 2 measures are recommended for hospitals with easy manipulation of electronic sources of data and for reporting quality‐of‐care measures for widespread publication, that is, in the context of a research study. It should be emphasized that these recommendations are only meant as a guide: the actual measures chosen should meet the needs and capabilities of each institution.
We recognize that few data support the recommendations made by this task force, that such data are needed, and that the field of data collection and analysis for hospital glycemic management is rapidly evolving. The hope is to begin the standardization process, promote dialogue in this field, and eventually reach a consensus in collaboration with the ADA, AACE, and other pertinent stakeholders.
CONCLUSIONS
Like the field of inpatient glycemic management itself, the field of devising metrics to measure the quality of inpatient glycemic control is also in its infancy and quickly evolving. One should not be paralyzed by the lack of consensus regarding measurementthe important point is to pick a few complementary metrics and begin the process. The table of recommendations can hopefully serve as a starting point for many institutions, with a focus on efficacy (glycemic control), safety (hypoglycemia), and process (insulin use patterns). As your institution gains experience with measurement and the field evolves, your metrics will likely change. We recommend keeping all process and outcome data in its raw form so that it can be summarized in different ways over time. It is also important not to wait for the perfect data collection tool before beginning to analyze data: sampling and paper processes are acceptable if automated data collection is not yet possible. Eventually, blood glucose meter readings should be downloaded into a central database that interfaces with hospital data repositories so data can be analyzed in conjunction with patient, service, and unit‐level information. Only with a rigorous measurement process can institutions hope to know whether their changes are resulting in improved care for patients.
- ,,, et al.“Glucometrics”—assessing the quality of inpatient glucose management.Diabetes Technol Ther.2006;8:560–569.
- ,,.Effect of hyperglycemia and continuous intravenous insulin infusions on outcomes of cardiac surgical procedures: the Portland Diabetic Project.Endocr Pract.2004;10(suppl 2):21–33.
- ,,.Hyperglycaemic index as a tool to assess glucose control: a retrospective study.Crit Care.2004;8:R122–R127.
- ,,, et al.Glucometrics in patients hospitalized with acute myocardial infarction: defining the optimal outcomes‐based measure of risk.Circulation.2008;117:1018–1027.
- ,,.Statistical process control as a tool for research and healthcare improvement.Qual Saf Health Care.2003;12:458–464.
- ,,, et al.“Glucometrics”—assessing the quality of inpatient glucose management.Diabetes Technol Ther.2006;8:560–569.
- ,,.Effect of hyperglycemia and continuous intravenous insulin infusions on outcomes of cardiac surgical procedures: the Portland Diabetic Project.Endocr Pract.2004;10(suppl 2):21–33.
- ,,.Hyperglycaemic index as a tool to assess glucose control: a retrospective study.Crit Care.2004;8:R122–R127.
- ,,, et al.Glucometrics in patients hospitalized with acute myocardial infarction: defining the optimal outcomes‐based measure of risk.Circulation.2008;117:1018–1027.
- ,,.Statistical process control as a tool for research and healthcare improvement.Qual Saf Health Care.2003;12:458–464.
SC Insulin Order Sets and Protocols
Inpatient glycemic control and hypoglycemia are issues with well deserved increased attention in recent years. Prominent guidelines and technical reviews have been published,13 and a recent, randomized controlled trial demonstrated the superiority of basal bolus insulin regimens compared to sliding‐scale regimens.4 Effective glycemic control for inpatients has remained elusive in most medical centers. Recent reports57 detail clinical inertia and the continued widespread use of sliding‐scale subcutaneous insulin regimens, as opposed to the anticipatory, physiologic basal‐nutrition‐correction dose insulin regimens endorsed by these reviews.
Inpatient glycemic control faces a number of barriers, including fears of inducing hypoglycemia, uneven knowledge and training among staff, and competing institutional and patient priorities. These barriers occur in the background of an inherently complex inpatient environment that poses unique challenges in maintaining safe glycemic control. Patients frequently move across a variety of care teams and geographic locations during a single inpatient stay, giving rise to multiple opportunities for failed communication, incomplete handoffs, and inconsistent treatment. In addition, insulin requirements may change dramatically due to variations in the stress of illness, exposure to medications that effect glucose levels, and varied forms of nutritional intake with frequent interruption. Although insulin is recognized as one of the medications most likely to be associated with adverse events in the hospital, many hospitals do not have protocols or order sets in place to standardize its use.
A Call to Action consensus conference,8, 9 hosted by the American Association of Clinical Endocrinologists (AACE) and the American Diabetes Association (ADA), brought together many thought leaders and organizations, including representation from the Society of Hospital Medicine (SHM), to address these barriers and to outline components necessary for successful implementation of a program to improve inpatient glycemic control in the face of these difficulties. Institutional insulin management protocols and standardized insulin order sets (supported by appropriate educational efforts) were identified as key interventions. It may be tempting to quickly deploy a generic insulin order set in an effort to improve care. This often results in mediocre results, due to inadequate incorporation of standardization and guidance into the order set and other documentation tools, and uneven use of the order set.
The SHM Glycemic Control Task Force (GCTF) recommends the following steps for developing and implementing successful protocols and order sets addressing the needs of the noncritical care inpatient with diabetes/hyperglycemia.
-
Form a steering committee for this work, and assess the current processes of care.
-
Identify best practices and preferred regimens to manage diabetes and hyperglycemia in the hospital.
-
Integrate best practices and preferred institutional choices into an inpatient glycemic control protocol. Crystallize your protocol into a one page summary.
-
Place guidance from your protocol into the flow of work, by integrating it into standardized subcutaneous insulin order sets and other documentation and treatment tools.
-
Monitor the use of your order sets and protocol. Intervene actively on nonadherents to your protocol and those with poor glycemic control, and revise your protocol/order sets as needed.
IDENTIFYING AND INCORPORATING KEY CONCEPTS AND BEST PRACTICES
A protocol is a document that endorses specific monitoring and treatment strategies in a given institution. This potentially extensive document should provide guidance for transitions, special situations (like steroids and total parenteral nutrition [TPN]) and should outline preferred insulin regimens for all of the most common nutritional situations. One of the most difficult parts of creating a protocol is the assimilation of all of the important information on which to base decisions. Your protocol and order set will be promoting a set of clinical practices. Fortunately, the current best practice for noncritical care hyperglycemic patients has been summarized by several authoritative sources,13, 811 including references from the SHM Glycemic Task Force published in this supplement.4, 12
Table 1 summarizes the key concepts that should be emphasized in a protocol for subcutaneous insulin management in the hospital. We recommend embedding guidance from your protocol into order sets, the medication administration record, and educational materials. Although the details contained in a protocol and order set might vary from one institution to another, the key concepts should not. The remainder of this article provides practical information about how these concepts and guidance for how preferred insulin regimens should be included in these tools. Appendices 1 and 2 give examples of an institutional one‐page summary protocol and subcutaneous insulin order set, respectively.
| 1. Establish a target range for blood glucose levels. |
| 2. Standardize monitoring of glucose levels and assessment of long‐term control (HbA1c). |
| 3. Incorporate nutritional management. |
| 4. Prompt clinicians to consider discontinuing oral antihyperglycemic medications. |
| 5. Prescribe physiologic (basal‐nutrition‐correction) insulin regimens. |
| a. Choose a total daily dose (TDD). |
| b. Divide the TDD into physiologic components of insulin therapy and provide basal and nutritional/correction separately. |
| c. Choose and dose a basal insulin. |
| d. Choose and dose a nutritional (prandial) insulin |
|
i. Match exactly to nutritional intake (see Table 2). |
| ii. Include standing orders to allow nurses to hold nutritional insulin for nutritional interruptions and to modify nutritional insulin depending on the actual nutritional intake. |
| e. Add correction insulin |
| i. Match to an estimate of the patients insulin sensitivity using prefabricated scales. |
| ii. Use the same insulin as nutritional insulin. |
| 6. Miscellaneous |
| a. Manage hypoglycemia in a standardized fashion and adjust regimen to prevent recurrences. |
| b. Provide diabetes education and appropriate consultation. |
| c. Coordinate glucose testing, nutrition delivery, and insulin administration. |
| d. Tailor discharge treatment regimens to the patient's individual circumstances and arrange for proper follow‐up. |
Standardize the Monitoring of Blood Glucose Values and Glucosylated Hemoglobin
Guidance for the coordination of glucose testing, nutrition delivery, and insulin administration, should be integrated into your protocols, and order sets. For noncritical care areas, the minimal frequency for blood glucose monitoring for patients who are eating is before meals and at bedtime. For the patient designated nothing by mouth (NPO) or the patient on continuous tube feeding, the type of nutritional/correction insulin used should drive the minimum frequency (every 4‐6 hours if rapid acting analog insulins [RAA‐I] are used, and every 6 hours if regular insulin is used). Directions for administering scheduled RAA‐I immediately before or immediately after nutrition delivery should be incorporated into protocols, order sets, and medication administration records. Unfortunately, having this guidance in the order sets and protocols does not automatically translate into its being carried out in the real world. Wide variability in the coordination of glucose monitoring, nutritional delivery, and insulin administration is common, so monitoring the process to make sure the protocol is followed is important.
Obtaining a glucosylated hemoglobin (HbA1c) level is important in gauging how well the patient's outpatient regimen is maintaining glycemic control, distinguishing stress hyperglycemia from established diabetes, and guiding the inpatient approach to glycemic control. ADA guidelines2, 3 endorse obtaining HbA1c levels of inpatients if these levels are not already available from the month prior to admission.
Establish a Target Range for Blood Glucose in NonCritical Care Areas
It is important to adopt a glycemic target that is institution‐wide, for critical care areas and noncritical care areas alike. Your glycemic target need not be identical to the ADA/AACE glycemic targets, but should be similar to them.
Examples of institutional glycemic targets for noncritical care areas:
-
Preprandial target 90‐130 mg/dL, maximum random glucose <180 mg/dL (ADA/AACE consensus target)
-
90‐150 mg/dL (a target used in some hospitals)
-
Preprandial target 90‐130 mg/dL for most patients, 100‐150 mg/dL if there are hypoglycemia risk factors, and <180 mg/dL if comfort‐care or end‐of‐life care (a more refined target, allowing for customization based on patient characteristics).
Your multidisciplinary glycemic control steering committee should pick the glycemic target it can most successfully implement and disseminate. It is fine to start with a conservative target and then ratchet down the goals as the environment becomes more accepting of the concept of tighter control of blood glucose in the hospital.
Although the choice of glycemic target is somewhat arbitrary, establishing an institutional glycemic target is critical to motivate clinical action. Your committee should design interventions, for instances when a patient's glycemic target is consistently not being met, including an assignment of responsibility.
Prompt Clinicians to Consider Discontinuing Oral Agents
Oral antihyperglycemic agents, in general, are difficult to quickly titrate to effect, and have side effects that limit their use in the hospital. In contrast, insulin acts rapidly and can be used in virtually all patients and clinical situations, making it the treatment of choice for treatment of hyperglycemia in the hospital.3, 11, 12 In certain circumstances, it may be entirely appropriate to continue a well‐controlled patient on his or her prior outpatient oral regimen. It is often also reasonable to resume oral agents in some patients when preparing for hospital discharge.
Incorporate Nutritional Management
Because diet is so integral to the management of diabetes and hyperglycemia, diet orders should be embedded in all diabetes or insulin‐related order sets. Diets with the same amount of carbohydrate with each meal should be the default rule for patients with diabetes. Nutritionist consultation should be considered and easy to access for patients with malnutrition, obesity, and other common conditions of the inpatient with diabetes.
Access Diabetes Education and Appropriate Consultation
Diabetes education should be offered to all hyperglycemic patients with normal mental status, complete with written materials, a listing of community resources, and survival skills. Consultation with physicians in internal medicine or endocrinology for difficult‐to‐control cases, or for cases in which the primary physician of record is not familiar with (or not adherent to) principles of inpatient glycemic management, should be very easy to obtain, or perhaps mandated, depending on your institution‐specific environment.
Prescribe Physiologic (Basal‐Nutritional‐Correction Dose) Insulin Regimens
Physiologic insulin use is the backbone of the recommended best practice for diabetes and hyperglycemia management in the hospital. The principles of such regimens are summarized elsewhere in this supplement.12 These principles will not be reiterated in detail here, but the major concepts that should be integrated into the protocols and order sets will be highlighted.
Choose a Total Daily Dose
Clinicians need guidance on how much subcutaneous insulin they should give a patient. These doses are well known from clinical experience and the published literature. The fear of hypoglycemia usually results in substantial underdosing of insulin, or total avoidance of scheduled insulin on admission. Your team should provide guidance for how much insulin to start a patient on when it is unclear from past experience how much insulin the patient needs. Waiting a few days to see how much insulin is required via sliding‐scale‐only regimens is a bad practice that should be discouraged for patients whose glucose values are substantially above the glycemic target. The total daily dose (TDD) can be estimated in several different ways (as demonstrated in Appendix 1 and 2), and protocols should make this step very clear for clinicians. Providing a specific location on the order set to declare the TDD may help ensure this step gets done more reliably. Some institutions with computer physician order entry (CPOE) provide assistance with calculating the TDD and the allocation of basal and nutritional components, based on data the ordering physician inputs into the system.
Select and Dose a Basal Insulin
Your protocol should describe how the TDD should be divided between basal and nutritional insulin. We generally recommend 50% of the TDD be given as basal insulin, with the other 50% administered on a scheduled basis to cover glycemic excursions from nutritional intake. The 50/50 rule is simple and generally works well, and should be widely promoted. However, there are exceptions to this rule that should be incorporated into your full protocol and educational programs. The order set should have separate steps for ordering basal insulin, nutritional insulin, and correction insulin. The advantage to providing these insulin components separately is that it allows them to be independently manipulated (eg, if a patient is unable to tolerate a meal, nutritional insulin is held, but basal insulin and correction insulin are continued).
The SHM GCTF specifically endorses long acting insulin (glargine and detemir) as the preferred basal insulin in the hospital setting, thus discouraging the use of neutral protamine Hagedorn (NPH) insulin and fixed combination insulin formulations (Table 2). In the absence of randomized controlled trials demonstrating superiority of the glargine or detemir to NPH insulin in the hospital, this endorsement deserves some further explanation. Although we believe that correctly dosed NPH containing insulin regimens can attain effective and safe glycemic control in the hospital setting, it is more difficult to standardize their use and adjust for fluctuations in nutritional intake. Glargine and detemir have much less pronounced spikes in their effect than NPH, rendering them relatively peakless in comparison. This pharmacokinetic profile allows for continued dosing with minimal or no correction when nutrition intake is variable, and allow for consistent reinforcement of the basal‐nutritional‐correction insulin concept.
| Nutritional situation | Necessary insulin components | Preferred regimen* |
|---|---|---|
| ||
| NPO (or clear liquids) | Basal insulin: 50% of TDD. Nutritional insulin: None. | Basal insulin: glargine given once daily or detemir given twice daily. Nutritional insulin: None. Correctional insulin: Regular insulin q 6 hours or RAA insulin q 4 hours. Other comments: Dextrose infusion (e.g., D5 containing solution at 75‐150 cc/hour) recommended when nutrition is held. An IV insulin infusion is preferred for management of prolonged fasts or fasting type 1 diabetes patients. |
| Eating meals | Basal insulin: 50% of TDD. Nutritional insulin: 50% of TDD, divided equally before each meal. | Basal insulin: glargine given once daily or detemir given twice daily. Nutritional insulin: RAA insulin with meals. Correctional insulin: RAA insulin q AC and HS (reduced dose at HS). |
| Bolus tube feeds | Basal insulin: 40% of TDD. Nutritional insulin: 60% of the TDD, divided equally before each bolus feed. | Basal insulin: glargine given once daily or detemir given twice daily. Nutritional insulin: RAA insulin with each bolus. Correctional insulin: RAA insulin with each bolus. |
| Continuous tube feeds | Basal insulin: 40% (conservative) of TDD. Nutritional insulin: 60% of the TDD in divided doses. | Basal insulin: glargine given once daily or detemir given twice daily. Nutritional insulin: RAA insulin q 4 hours or regular insulin q 6 hours. Correctional insulin: Should match nutritional insulin choice. |
| Parenteral nutrition | Insulin is usually given parenterally, with the nutrition | Initially, a separate insulin drip allows for accurate dose‐finding. Then, 80% of amount determined as TDD using drip is added to subsequent TPN bags as regular insulin. Use correctional subcutaneous insulin doses cautiously, in addition. |
There are some caveats to this general recommendation. First, patients who are well controlled on home regimens with NPH basal insulin can (and sometimes should) stay on the regimen that has worked well for them. However, extra vigilance in reducing the dose for reductions in nutrition is required, because NPH is generally used to cover both nutritional and basal requirements. Second, extensive experience with glargine and detemir are not available in obstetric populations. They are not U.S. Food and Drug Administration (FDA) approved for use in pregnant patients and formally carry a Class C rating, whereas NPH insulin has been used safely in obstetric populations for decades. Third, the insulin regimen used as an inpatient is not necessarily the preferred regimen to prescribe at discharge: cost, patient preferences, HbA1c level, and other factors should be considered in making this choice.
Select and Dose a Nutritional (Prandial) Insulin
The step for ordering nutritional insulin should assist the clinician in matching the insulin to the type of nutrition that the patient is receiving. For example, rapid‐acting insulin analogs are preferred over regular insulin in the eating patient, in view of their more physiologic profile, which averts the insulin stacking that can occur with regular insulin. If regular insulin is used as the preferred institutional choice for eating patients, the lunchtime dose should be reduced or eliminated altogether, to eliminate insulin stacking.
Table 2 outlines the SHM GCTF preferred regimens for different nutritional situations.
There should be a standing order for nutritional insulin to be held when nutrition is interrupted, whether intentional or unintentional. Patients with interrupted tube feedings could have standing orders for a dextrose infusion to replace the tube feeding carbohydrate load and prevent hypoglycemia. Ideally, there should also be a standing order allowing for real‐time management of the patient with uncertain nutritional intake. For example, when a patient's premeal assessment reveals that she may not tolerate the meal, the patient should be allowed to attempt to eat, and then the nutritional insulin should be given after the meal, in proportion to the amount of food that was eaten. This type of order will require significant nursing education and process redesign in many hospitals, but is essential for matching nutritional insulin to actual intake.
Add Correction Insulin
There is no convincing evidence for the benefit of correction (sliding‐scale) insulin in the inpatient setting, although a randomized trial demonstrating the superiority of basal/nutritional insulin regimens to sliding‐scale only regimens did incorporate a correction insulin scale as an adjunct to the superior basal/nutritional regimen.4 The SHM GCTF again emphasizes that control of hyperglycemia should be proactive and anticipatory of insulin needs, rather than reactive to hyperglycemia. Nonetheless, unexpected hyperglycemic excursions are common, and the use of correction insulin remains a pervasive and arguably logical practice. If correction insulin is used, it should be ordered as a separate step after considering basal and nutrition insulin needs. The doses of scheduled insulin should be adjusted regularly if correction insulin is consistently being required. Ideally, the prescriber should choose a preformatted corrective insulin scale, based on the patient's insulin sensitivity (Appendix 2). There should be a prompt to use the same type of insulin that is being used for nutritional insulin, and there should be instructions that this insulin is given in addition to the basal and nutritional insulin to correct for hyperglycemia. Nocturnal correction‐dose scales should be reduced in the eating patient.
Even after limiting insulin regimens to those in Table 2, multidisciplinary glycemic control teams are still left with several options within these SHM‐preferred regimens. We recommend that your team choose a single, institutionally‐preferred basal‐nutritional‐correction insulin combination for each situation.
Choosing one preferred option for these situations is advantageous because:
-
You can communicate preferred regimens more simply and succinctly to all staff.
-
You eliminate all inappropriate choices for insulin regimens for that situation, as well as some other less preferred, but acceptable choices.
-
You can encourage regimens that are most economical (by promoting the insulin regimens that reflect your hospital formulary choices).
-
Staff members can become very familiar with a few regimens, instead of being confused by a multitude of them. They can identify variations from your preferred choices and target these patients for extra scrutiny and actions should they fail to meet glycemic targets.
Although virtually every institution can provide specific guidance on insulin management in a protocol, there are tradeoffs inherent in how restrictive you can be in pushing these preferred choices in your order sets. Should you eliminate alternate basal or nutritional insulin choices from your order sets? As you integrate more and more of your preferred algorithm and regimens into your order set, you will gain incremental improvement in the standardization of inpatient insulin management. However, you reduce not only variability in ordering, but also the choices available to your prescribers and patients, and in effect you are pushing the providers to use an insulin regimen that often differs from the patient's outpatient regimen. If your institution is not yet ready to go with a single preferred insulin, simply listing your preferred insulin first with the annotation preferred can be enough to increase the use of the preferred insulin.
We endorse building the most protocol‐driven, proscriptive, insulin order set that the Glycemic Control Steering Committee believes their medical staff will accept. There are some caveats to this endorsement. First, there must be extra efforts on the backend of the admission, to ensure that the antihyperglycemic regimen is tailored to the unique needs of the patient (this is discussed further below). Second, a protocol‐driven approach is not a substitute for a good educational program for health care providers or well‐informed clinical judgment. Education should reinforce major concepts driving the protocol and should also highlight exceptions to the rule. Variance from the protocol endorsed choices should be allowed (and even encouraged) when the variance is driven by patient factors (as opposed to provider whim). Learning from this variance is a key concept in refining protocols. Education ideally should not be limited to only protocol‐endorsed choices, as staff should be familiar with the full range of antihyperglycemia regimens seen in inpatient and outpatient settings.
Special Situations
Most of the preferred regimens for different situations are outlined in Table 2 in a straightforward manner, and can be depicted in your protocols and order sets in the same way. Some conditions have enough complexity, however, that you will have difficulty placing all of the details into your one‐page protocol and order set. Details should be placed on your more detailed protocol, and educational programs should include the topics outlined below. Although insulin infusion is often the option that would provide the most reliable and expedient control of hyperglycemia in these special situations, it is an option not available in many noncritical care settings. Therefore, the discussion is limited to subcutaneous insulin control regimens.
Patient on Continuous Tube Feeding
The SHM GCTF endorses glargine or detemir as the basal insulin of choice for this setting. The nutritional and correction insulin of choice is either an RAA‐I every 4 hours (q4h), or regular insulin every 6 hours (q6h). We endorse this choice because it retains the basal‐nutritional‐correction dose concept, generally allows for continued basal insulin use if the tube feedings become interrupted, and is amenable to building a consistent institutional protocol.
There are some important caveats to this recommendation. First, realize that almost any regimen that provides a stable insulin supply would be acceptable, and many institutions will use glargine or detemir to cover both basal and nutritional needs. The downside to using large boluses of long‐acting insulin in this clinical situation is that any unexpected interruption of the feedings will necessitate prolonged infusions of dextrate 10% solution (D10) to avoid hypoglycemia
Second, because of the glycemic load inherent in tube feedings, maintenance of glycemic control in the setting of enteral feeding may be best managed by providing a higher percentage of the TDD as nutritional insulin. In these cases, ratios of basal to nutritional insulin of 40:60, or even less basal insulin, may be appropriate.
Glucocorticoid Therapy
High‐dose glucocorticoids are strongly associated with increased insulin requirements. The degree of hyperglycemia induced by steroids varies significantly from patient to patient, and the pattern of hyperglycemia will vary depending on the pattern of steroid administration. The general principle to keep in mind is that the hyperglycemia induced by a steroid dose will peak 8‐12 hours after it is given, so insulin regimens to address this should take this effect into account. For example, giving a long‐acting basal insulin like glargine to accommodate the hyperglycemic effect of a steroid bolus given in the morning would be inappropriate because the steroid effect would wane and then disappear overnight, leading to insulin‐induced hypoglycemia. NPH insulin can be ideal in this setting, either by itself, or by layering it on top of an existing regimen.
Another caveat: glucocorticoids exert their predominate effect on insulin sensitivity in muscle (as opposed to the liver), and as a result, have their most notable effect on postprandial glucose. For this reason, the best insulin regimens for this situation may use proportionally less basal insulin and more nutritional insulin. One common regimen calls for keeping the basal insulin dose the same as the preglucocorticoid dose, while escalating the RAA insulin dose at lunch and dinner.
Given the complexities of covering steroid‐induced hyperglycemia and its high prevalence in certain populations (such as transplantation patients and patients undergoing chemotherapy), this would be an excellent area on which to focus expertise. Examples include routine endocrinology consultation, intervention by a special glycemic control team, or incorporating routine glucose monitoring and triggers for initiating insulin infusion into the protocols for chemotherapy and transplantation patients.
Regiment the Management of Hypoglycemia
Hypoglycemia is defined by the ADA as a blood glucose of 70 mg/dL or less, based on the physiologic changes that can occur at this glucose level, even in subjectively asymptomatic patients.3 Protocols for management of hypoglycemia should be linked to your diabetes/hyperglycemia protocols. There are many hypoglycemia protocols available for review in the SHM Glycemic Control Resource Room and Glycemic Control Implementation Guide.10 Some common themes for effective implementation stand out. First, the protocols need to walk the balance between simplicity of use, and the need to provide instructions that will provide guidance in a variety of patient situations. Second, the protocols need to be nurse driven, so that nurses can initiate treatment without waiting for a physician order. Third, education and instruction regarding recognition of risk factors, and avoidance of hypoglycemia are needed to support a successful protocol. Importantly, any hypoglycemic event should lead to a reconsideration of the current anti‐hyperglycemic regimen so that future events can be prevented.
Plan for Discharge and Provide Guidance for the Transition
Your institution should have policies and procedures outlining all the steps needed to complete the important transition out of the hospital. At a minimum, this planning should include adequate education (including a learner assessment), appropriate follow‐up, referral to community resources, and a discharge glycemic control regimen that is tailored to the educational, financial, and motivational profile of a patient. The more your inpatient insulin management is driven by protocol, the more likely it is the patient will be on an inpatient treatment plan that differs from their outpatient regimen; therefore, it is even more important to plan this transition carefully and reliably.
Communicating the accurate hyperglycemia related diagnosis and related problems to the primary care provider is important for good care, perhaps even more so for patients who had hyperglycemia while hospitalized without a prior diagnosis of diabetes. Some centers place a prompt for hyperglycemia related diagnosis in the order set and/or discharge paperwork, to remind the clinician to convey the diagnosis to the primary provider, and to encourage more complete documentation. Improved documentation can also improve the business case for glycemic control, along with other strategies outlined elsewhere in this supplement.13
Transitions in care (including transitions out of the hospital and off of infusion insulin) are discussed in more detail14, 15 elsewhere in this supplement. The principles outlined in these references should be incorporated into your institutional protocol. Briefly, not all patients require or are capable of intensive basal‐bolus regimens upon discharge. The HbA1c can be very valuable in arriving at the optimal outpatient regimen.14 The capacities and preferences of the patient and the context of his or her outpatient care environment (including the preferences of the primary care provider) must be taken into consideration as an outpatient management program is planned.
PULLING IT ALL TOGETHER: MAKE SURE YOUR PROTOCOL/ORDER SET IS EASY TO USE AND WIDELY UTILIZED
When standardizing hospital management of diabetes and hyperglycemia, we recommend building the full protocol first, then crystallizing the protocol into a one‐page summary that can be widely disseminated. The protocol guidance is then incorporated into the order set and nursing medical administration record (MAR). Again, we recommend the most proscriptive and protocol‐driven order set feasible within the constraints of medical staff support. The example order set in Appendix 2 illustrates this approach along with other desirable features:
-
Check‐box simplicity on when to order appropriate glucose monitoring.
-
Prompt for the proper hyperglycemia‐related diagnosis.
-
Prompts to document diagnosis and to order HbA1c level.
-
Use of encouraged insulin terminology: basal, prandial (or nutritional), and correction. Language is a powerful thing, and just getting staff to use these terms goes a long way toward the more physiologic prescribing of insulin.
-
Statement/reminder of a glycemic goal.
-
Prompts and contact information for appropriate consultation.
-
Elimination of unapproved abbreviations (such as U for units).
-
Stating both generic and brand names of insulin preparations.
-
Important timing cues for administration of insulin.
-
Several correction‐dose scales suitable for different insulin sensitivities. One size does NOT fit all.
-
Incorporation of a simple hypoglycemia protocol into the order set.
-
Insulin dosing guidelines available at the point of care (in this case, on the back of the order set).
Additional nursing‐specific cues (such as an admonition to never mix glargine insulin with other types of insulin) can also be included in the MAR whenever glargine is ordered.
Once you have protocols and order sets to guide providers, you need to assure that they are used for the majority of hyperglycemic patients. Educational programs should introduce your interventions and the rationale for them. In order to make your method the default method of care, your team should survey all preprinted or CPOE insulin order sets of your institution. A review of postoperative, transfer, and admission order sets that all services use may reveal a half‐dozen or more embedded sliding‐scale insulin order sets that should be removed, with prompts to use the standardized insulin order set being placed in their stead.
Computerized order sets present both challenges and opportunities. Wording limitations and the scrolling nature can make concepts less clear, yet there is a capability for incorporating a hierarchical structure that allows for guiding the user through a more algorithmic approach. There is also a capacity to provide assistance with dosing calculations that do not exist in the paper world. Education remains of key importance for both methods.
MONITOR THE USE AND EFFECTIVENESS OF YOUR PROTOCOLS AND ORDER SETS
Creating and implementing protocols, order sets, and other tools is not the end of the journey to improve care. It is important to monitor order set utilization, insulin use patterns, and parameters measuring glycemic control and hypoglycemia, as outlined in more detail in another article in this supplement.16 In addition to summary data every month or so, we recommend daily reports that spur action in near real time. Triggers such as uncontrolled hyperglycemia, markedly elevated HbA1c levels, and nonphysiologic insulin regimens should initiate consultation, extra diabetes education, or referral to a glucose control team. If appropriate consultation is not readily available, the glycemic control steering group should lobby the administration to bolster this capability. Qualitative feedback from the frontline caregivers, as well as this quantitative data, can assist the local glycemic control champions in designing even more effective protocols, order sets, focused educational efforts, and concurrent mitigation of suboptimal care.
CONCLUSION
Diabetes, hyperglycemia, and iatrogenic hypoglycemia are common and important conditions affecting the noncritically ill inpatient. Interventional trials to validate the recommended noncritical care unit glycemic targets are needed. Although there is a growing consensus on best practices to care for these patients, numerous barriers and the complexity of caring for inpatients hamper the reliability of best practice delivery. Institutional protocols and protocol driven subcutaneous insulin orders, when implemented with the strategies outlined here, can be the key to delivering these best practices more reliably.
Appendix
- American College of Endocrinology Task Force on Inpatient Diabetes and Metabolic Control.American College of Endocrinology Position Statement on Inpatient Diabetes and Metabolic Control.Endocr Pract.2004;10:77–82.
- ,,,,,,.Management of diabetes and hyperglycemia in hospitals.Diabetes Care.2004;27:553–591.
- American Diabetes Association.Standards of Medical Carein Diabetes‐2006.Diabetes Care.2006;29(suppl 1):s4–s42.
- ,,, et al.Randomized study of basal‐bolus insulin therapy in the inpatient management of patients with type 2 diabetes (RABBIT 2 Trial).Diabetes Care.2007;30:2181–2186.
- ,,,,.Inpatient management of diabetes and hyperglycemia among general medicine patients at a large teaching hospital.J Hosp Med.2006;1:145–150.
- ,,, et al.Diabetes care in the hospital: is there clinical inertia?J Hosp Med.2006;1:151–160.
- ,,, et al.Diabetes care in hospitalized non‐critically ill patients: more evidence for clinical inertia and negative therapeutic momentum.JHosp Med.2007;2:203–211.
- Inpatient Diabetes and Glycemic Control: A Call to Action Conference. Position Statement. AACE, February2006. Available at: http://www.aace.com/meetings/consensus/IIDC/IDGC0207.pdf. Accessed October, 2006.
- Proceedings of the American College of Endocrinology and American Diabetes Association Consensus Conference, Washington, DC, January 30–31, 2006. Endocr Pract.2006; 12(suppl 3):3–13.
- Society of Hospital Medicine Glycemic Control Task Force. Implementation Guide: Improving Glycemic Control, Preventing Hypoglycemia, and Optimizing Care of the Inpatient with Hyperglycemia and Diabetes. Published January 2007 on the Society of Hospital Medicine website. Available at: http://www.hospitalmedicine.org. Accessed August,2007.
- .Management of hyperglycemia in the hospital setting.N Engl J Med.2006;355:1903–1911.
- ,,,,.Management of diabetes and hyperglycemia in the hospital: a practical guide to subcutaneous insulin use in the non‐critically ill adult patient.J Hosp Med.2008;3(5):S17–S28.
- ,.Practical strategies for developing the business case for hospital glycemic control teams.J Hosp Med2008;3(5):S76–S83.
- ,,,.Bridge over troubled waters: safe and effective transitions of the inpatient with hyperglycemia.J Hosp Med.2008;3(5):S55–S65.
- ,,,.Designing and implementing insulin infusion protocols and order sets.J Hosp Med.2008;3(5):S42–S54.
- ,,,,,.SHM Glycemic Control Task Force summary: practical recommendations for assessing the impact of glycemic control efforts.J Hosp Med.2008;3(5):S66–S75.
Inpatient glycemic control and hypoglycemia are issues with well deserved increased attention in recent years. Prominent guidelines and technical reviews have been published,13 and a recent, randomized controlled trial demonstrated the superiority of basal bolus insulin regimens compared to sliding‐scale regimens.4 Effective glycemic control for inpatients has remained elusive in most medical centers. Recent reports57 detail clinical inertia and the continued widespread use of sliding‐scale subcutaneous insulin regimens, as opposed to the anticipatory, physiologic basal‐nutrition‐correction dose insulin regimens endorsed by these reviews.
Inpatient glycemic control faces a number of barriers, including fears of inducing hypoglycemia, uneven knowledge and training among staff, and competing institutional and patient priorities. These barriers occur in the background of an inherently complex inpatient environment that poses unique challenges in maintaining safe glycemic control. Patients frequently move across a variety of care teams and geographic locations during a single inpatient stay, giving rise to multiple opportunities for failed communication, incomplete handoffs, and inconsistent treatment. In addition, insulin requirements may change dramatically due to variations in the stress of illness, exposure to medications that effect glucose levels, and varied forms of nutritional intake with frequent interruption. Although insulin is recognized as one of the medications most likely to be associated with adverse events in the hospital, many hospitals do not have protocols or order sets in place to standardize its use.
A Call to Action consensus conference,8, 9 hosted by the American Association of Clinical Endocrinologists (AACE) and the American Diabetes Association (ADA), brought together many thought leaders and organizations, including representation from the Society of Hospital Medicine (SHM), to address these barriers and to outline components necessary for successful implementation of a program to improve inpatient glycemic control in the face of these difficulties. Institutional insulin management protocols and standardized insulin order sets (supported by appropriate educational efforts) were identified as key interventions. It may be tempting to quickly deploy a generic insulin order set in an effort to improve care. This often results in mediocre results, due to inadequate incorporation of standardization and guidance into the order set and other documentation tools, and uneven use of the order set.
The SHM Glycemic Control Task Force (GCTF) recommends the following steps for developing and implementing successful protocols and order sets addressing the needs of the noncritical care inpatient with diabetes/hyperglycemia.
-
Form a steering committee for this work, and assess the current processes of care.
-
Identify best practices and preferred regimens to manage diabetes and hyperglycemia in the hospital.
-
Integrate best practices and preferred institutional choices into an inpatient glycemic control protocol. Crystallize your protocol into a one page summary.
-
Place guidance from your protocol into the flow of work, by integrating it into standardized subcutaneous insulin order sets and other documentation and treatment tools.
-
Monitor the use of your order sets and protocol. Intervene actively on nonadherents to your protocol and those with poor glycemic control, and revise your protocol/order sets as needed.
IDENTIFYING AND INCORPORATING KEY CONCEPTS AND BEST PRACTICES
A protocol is a document that endorses specific monitoring and treatment strategies in a given institution. This potentially extensive document should provide guidance for transitions, special situations (like steroids and total parenteral nutrition [TPN]) and should outline preferred insulin regimens for all of the most common nutritional situations. One of the most difficult parts of creating a protocol is the assimilation of all of the important information on which to base decisions. Your protocol and order set will be promoting a set of clinical practices. Fortunately, the current best practice for noncritical care hyperglycemic patients has been summarized by several authoritative sources,13, 811 including references from the SHM Glycemic Task Force published in this supplement.4, 12
Table 1 summarizes the key concepts that should be emphasized in a protocol for subcutaneous insulin management in the hospital. We recommend embedding guidance from your protocol into order sets, the medication administration record, and educational materials. Although the details contained in a protocol and order set might vary from one institution to another, the key concepts should not. The remainder of this article provides practical information about how these concepts and guidance for how preferred insulin regimens should be included in these tools. Appendices 1 and 2 give examples of an institutional one‐page summary protocol and subcutaneous insulin order set, respectively.
| 1. Establish a target range for blood glucose levels. |
| 2. Standardize monitoring of glucose levels and assessment of long‐term control (HbA1c). |
| 3. Incorporate nutritional management. |
| 4. Prompt clinicians to consider discontinuing oral antihyperglycemic medications. |
| 5. Prescribe physiologic (basal‐nutrition‐correction) insulin regimens. |
| a. Choose a total daily dose (TDD). |
| b. Divide the TDD into physiologic components of insulin therapy and provide basal and nutritional/correction separately. |
| c. Choose and dose a basal insulin. |
| d. Choose and dose a nutritional (prandial) insulin |
|
i. Match exactly to nutritional intake (see Table 2). |
| ii. Include standing orders to allow nurses to hold nutritional insulin for nutritional interruptions and to modify nutritional insulin depending on the actual nutritional intake. |
| e. Add correction insulin |
| i. Match to an estimate of the patients insulin sensitivity using prefabricated scales. |
| ii. Use the same insulin as nutritional insulin. |
| 6. Miscellaneous |
| a. Manage hypoglycemia in a standardized fashion and adjust regimen to prevent recurrences. |
| b. Provide diabetes education and appropriate consultation. |
| c. Coordinate glucose testing, nutrition delivery, and insulin administration. |
| d. Tailor discharge treatment regimens to the patient's individual circumstances and arrange for proper follow‐up. |
Standardize the Monitoring of Blood Glucose Values and Glucosylated Hemoglobin
Guidance for the coordination of glucose testing, nutrition delivery, and insulin administration, should be integrated into your protocols, and order sets. For noncritical care areas, the minimal frequency for blood glucose monitoring for patients who are eating is before meals and at bedtime. For the patient designated nothing by mouth (NPO) or the patient on continuous tube feeding, the type of nutritional/correction insulin used should drive the minimum frequency (every 4‐6 hours if rapid acting analog insulins [RAA‐I] are used, and every 6 hours if regular insulin is used). Directions for administering scheduled RAA‐I immediately before or immediately after nutrition delivery should be incorporated into protocols, order sets, and medication administration records. Unfortunately, having this guidance in the order sets and protocols does not automatically translate into its being carried out in the real world. Wide variability in the coordination of glucose monitoring, nutritional delivery, and insulin administration is common, so monitoring the process to make sure the protocol is followed is important.
Obtaining a glucosylated hemoglobin (HbA1c) level is important in gauging how well the patient's outpatient regimen is maintaining glycemic control, distinguishing stress hyperglycemia from established diabetes, and guiding the inpatient approach to glycemic control. ADA guidelines2, 3 endorse obtaining HbA1c levels of inpatients if these levels are not already available from the month prior to admission.
Establish a Target Range for Blood Glucose in NonCritical Care Areas
It is important to adopt a glycemic target that is institution‐wide, for critical care areas and noncritical care areas alike. Your glycemic target need not be identical to the ADA/AACE glycemic targets, but should be similar to them.
Examples of institutional glycemic targets for noncritical care areas:
-
Preprandial target 90‐130 mg/dL, maximum random glucose <180 mg/dL (ADA/AACE consensus target)
-
90‐150 mg/dL (a target used in some hospitals)
-
Preprandial target 90‐130 mg/dL for most patients, 100‐150 mg/dL if there are hypoglycemia risk factors, and <180 mg/dL if comfort‐care or end‐of‐life care (a more refined target, allowing for customization based on patient characteristics).
Your multidisciplinary glycemic control steering committee should pick the glycemic target it can most successfully implement and disseminate. It is fine to start with a conservative target and then ratchet down the goals as the environment becomes more accepting of the concept of tighter control of blood glucose in the hospital.
Although the choice of glycemic target is somewhat arbitrary, establishing an institutional glycemic target is critical to motivate clinical action. Your committee should design interventions, for instances when a patient's glycemic target is consistently not being met, including an assignment of responsibility.
Prompt Clinicians to Consider Discontinuing Oral Agents
Oral antihyperglycemic agents, in general, are difficult to quickly titrate to effect, and have side effects that limit their use in the hospital. In contrast, insulin acts rapidly and can be used in virtually all patients and clinical situations, making it the treatment of choice for treatment of hyperglycemia in the hospital.3, 11, 12 In certain circumstances, it may be entirely appropriate to continue a well‐controlled patient on his or her prior outpatient oral regimen. It is often also reasonable to resume oral agents in some patients when preparing for hospital discharge.
Incorporate Nutritional Management
Because diet is so integral to the management of diabetes and hyperglycemia, diet orders should be embedded in all diabetes or insulin‐related order sets. Diets with the same amount of carbohydrate with each meal should be the default rule for patients with diabetes. Nutritionist consultation should be considered and easy to access for patients with malnutrition, obesity, and other common conditions of the inpatient with diabetes.
Access Diabetes Education and Appropriate Consultation
Diabetes education should be offered to all hyperglycemic patients with normal mental status, complete with written materials, a listing of community resources, and survival skills. Consultation with physicians in internal medicine or endocrinology for difficult‐to‐control cases, or for cases in which the primary physician of record is not familiar with (or not adherent to) principles of inpatient glycemic management, should be very easy to obtain, or perhaps mandated, depending on your institution‐specific environment.
Prescribe Physiologic (Basal‐Nutritional‐Correction Dose) Insulin Regimens
Physiologic insulin use is the backbone of the recommended best practice for diabetes and hyperglycemia management in the hospital. The principles of such regimens are summarized elsewhere in this supplement.12 These principles will not be reiterated in detail here, but the major concepts that should be integrated into the protocols and order sets will be highlighted.
Choose a Total Daily Dose
Clinicians need guidance on how much subcutaneous insulin they should give a patient. These doses are well known from clinical experience and the published literature. The fear of hypoglycemia usually results in substantial underdosing of insulin, or total avoidance of scheduled insulin on admission. Your team should provide guidance for how much insulin to start a patient on when it is unclear from past experience how much insulin the patient needs. Waiting a few days to see how much insulin is required via sliding‐scale‐only regimens is a bad practice that should be discouraged for patients whose glucose values are substantially above the glycemic target. The total daily dose (TDD) can be estimated in several different ways (as demonstrated in Appendix 1 and 2), and protocols should make this step very clear for clinicians. Providing a specific location on the order set to declare the TDD may help ensure this step gets done more reliably. Some institutions with computer physician order entry (CPOE) provide assistance with calculating the TDD and the allocation of basal and nutritional components, based on data the ordering physician inputs into the system.
Select and Dose a Basal Insulin
Your protocol should describe how the TDD should be divided between basal and nutritional insulin. We generally recommend 50% of the TDD be given as basal insulin, with the other 50% administered on a scheduled basis to cover glycemic excursions from nutritional intake. The 50/50 rule is simple and generally works well, and should be widely promoted. However, there are exceptions to this rule that should be incorporated into your full protocol and educational programs. The order set should have separate steps for ordering basal insulin, nutritional insulin, and correction insulin. The advantage to providing these insulin components separately is that it allows them to be independently manipulated (eg, if a patient is unable to tolerate a meal, nutritional insulin is held, but basal insulin and correction insulin are continued).
The SHM GCTF specifically endorses long acting insulin (glargine and detemir) as the preferred basal insulin in the hospital setting, thus discouraging the use of neutral protamine Hagedorn (NPH) insulin and fixed combination insulin formulations (Table 2). In the absence of randomized controlled trials demonstrating superiority of the glargine or detemir to NPH insulin in the hospital, this endorsement deserves some further explanation. Although we believe that correctly dosed NPH containing insulin regimens can attain effective and safe glycemic control in the hospital setting, it is more difficult to standardize their use and adjust for fluctuations in nutritional intake. Glargine and detemir have much less pronounced spikes in their effect than NPH, rendering them relatively peakless in comparison. This pharmacokinetic profile allows for continued dosing with minimal or no correction when nutrition intake is variable, and allow for consistent reinforcement of the basal‐nutritional‐correction insulin concept.
| Nutritional situation | Necessary insulin components | Preferred regimen* |
|---|---|---|
| ||
| NPO (or clear liquids) | Basal insulin: 50% of TDD. Nutritional insulin: None. | Basal insulin: glargine given once daily or detemir given twice daily. Nutritional insulin: None. Correctional insulin: Regular insulin q 6 hours or RAA insulin q 4 hours. Other comments: Dextrose infusion (e.g., D5 containing solution at 75‐150 cc/hour) recommended when nutrition is held. An IV insulin infusion is preferred for management of prolonged fasts or fasting type 1 diabetes patients. |
| Eating meals | Basal insulin: 50% of TDD. Nutritional insulin: 50% of TDD, divided equally before each meal. | Basal insulin: glargine given once daily or detemir given twice daily. Nutritional insulin: RAA insulin with meals. Correctional insulin: RAA insulin q AC and HS (reduced dose at HS). |
| Bolus tube feeds | Basal insulin: 40% of TDD. Nutritional insulin: 60% of the TDD, divided equally before each bolus feed. | Basal insulin: glargine given once daily or detemir given twice daily. Nutritional insulin: RAA insulin with each bolus. Correctional insulin: RAA insulin with each bolus. |
| Continuous tube feeds | Basal insulin: 40% (conservative) of TDD. Nutritional insulin: 60% of the TDD in divided doses. | Basal insulin: glargine given once daily or detemir given twice daily. Nutritional insulin: RAA insulin q 4 hours or regular insulin q 6 hours. Correctional insulin: Should match nutritional insulin choice. |
| Parenteral nutrition | Insulin is usually given parenterally, with the nutrition | Initially, a separate insulin drip allows for accurate dose‐finding. Then, 80% of amount determined as TDD using drip is added to subsequent TPN bags as regular insulin. Use correctional subcutaneous insulin doses cautiously, in addition. |
There are some caveats to this general recommendation. First, patients who are well controlled on home regimens with NPH basal insulin can (and sometimes should) stay on the regimen that has worked well for them. However, extra vigilance in reducing the dose for reductions in nutrition is required, because NPH is generally used to cover both nutritional and basal requirements. Second, extensive experience with glargine and detemir are not available in obstetric populations. They are not U.S. Food and Drug Administration (FDA) approved for use in pregnant patients and formally carry a Class C rating, whereas NPH insulin has been used safely in obstetric populations for decades. Third, the insulin regimen used as an inpatient is not necessarily the preferred regimen to prescribe at discharge: cost, patient preferences, HbA1c level, and other factors should be considered in making this choice.
Select and Dose a Nutritional (Prandial) Insulin
The step for ordering nutritional insulin should assist the clinician in matching the insulin to the type of nutrition that the patient is receiving. For example, rapid‐acting insulin analogs are preferred over regular insulin in the eating patient, in view of their more physiologic profile, which averts the insulin stacking that can occur with regular insulin. If regular insulin is used as the preferred institutional choice for eating patients, the lunchtime dose should be reduced or eliminated altogether, to eliminate insulin stacking.
Table 2 outlines the SHM GCTF preferred regimens for different nutritional situations.
There should be a standing order for nutritional insulin to be held when nutrition is interrupted, whether intentional or unintentional. Patients with interrupted tube feedings could have standing orders for a dextrose infusion to replace the tube feeding carbohydrate load and prevent hypoglycemia. Ideally, there should also be a standing order allowing for real‐time management of the patient with uncertain nutritional intake. For example, when a patient's premeal assessment reveals that she may not tolerate the meal, the patient should be allowed to attempt to eat, and then the nutritional insulin should be given after the meal, in proportion to the amount of food that was eaten. This type of order will require significant nursing education and process redesign in many hospitals, but is essential for matching nutritional insulin to actual intake.
Add Correction Insulin
There is no convincing evidence for the benefit of correction (sliding‐scale) insulin in the inpatient setting, although a randomized trial demonstrating the superiority of basal/nutritional insulin regimens to sliding‐scale only regimens did incorporate a correction insulin scale as an adjunct to the superior basal/nutritional regimen.4 The SHM GCTF again emphasizes that control of hyperglycemia should be proactive and anticipatory of insulin needs, rather than reactive to hyperglycemia. Nonetheless, unexpected hyperglycemic excursions are common, and the use of correction insulin remains a pervasive and arguably logical practice. If correction insulin is used, it should be ordered as a separate step after considering basal and nutrition insulin needs. The doses of scheduled insulin should be adjusted regularly if correction insulin is consistently being required. Ideally, the prescriber should choose a preformatted corrective insulin scale, based on the patient's insulin sensitivity (Appendix 2). There should be a prompt to use the same type of insulin that is being used for nutritional insulin, and there should be instructions that this insulin is given in addition to the basal and nutritional insulin to correct for hyperglycemia. Nocturnal correction‐dose scales should be reduced in the eating patient.
Even after limiting insulin regimens to those in Table 2, multidisciplinary glycemic control teams are still left with several options within these SHM‐preferred regimens. We recommend that your team choose a single, institutionally‐preferred basal‐nutritional‐correction insulin combination for each situation.
Choosing one preferred option for these situations is advantageous because:
-
You can communicate preferred regimens more simply and succinctly to all staff.
-
You eliminate all inappropriate choices for insulin regimens for that situation, as well as some other less preferred, but acceptable choices.
-
You can encourage regimens that are most economical (by promoting the insulin regimens that reflect your hospital formulary choices).
-
Staff members can become very familiar with a few regimens, instead of being confused by a multitude of them. They can identify variations from your preferred choices and target these patients for extra scrutiny and actions should they fail to meet glycemic targets.
Although virtually every institution can provide specific guidance on insulin management in a protocol, there are tradeoffs inherent in how restrictive you can be in pushing these preferred choices in your order sets. Should you eliminate alternate basal or nutritional insulin choices from your order sets? As you integrate more and more of your preferred algorithm and regimens into your order set, you will gain incremental improvement in the standardization of inpatient insulin management. However, you reduce not only variability in ordering, but also the choices available to your prescribers and patients, and in effect you are pushing the providers to use an insulin regimen that often differs from the patient's outpatient regimen. If your institution is not yet ready to go with a single preferred insulin, simply listing your preferred insulin first with the annotation preferred can be enough to increase the use of the preferred insulin.
We endorse building the most protocol‐driven, proscriptive, insulin order set that the Glycemic Control Steering Committee believes their medical staff will accept. There are some caveats to this endorsement. First, there must be extra efforts on the backend of the admission, to ensure that the antihyperglycemic regimen is tailored to the unique needs of the patient (this is discussed further below). Second, a protocol‐driven approach is not a substitute for a good educational program for health care providers or well‐informed clinical judgment. Education should reinforce major concepts driving the protocol and should also highlight exceptions to the rule. Variance from the protocol endorsed choices should be allowed (and even encouraged) when the variance is driven by patient factors (as opposed to provider whim). Learning from this variance is a key concept in refining protocols. Education ideally should not be limited to only protocol‐endorsed choices, as staff should be familiar with the full range of antihyperglycemia regimens seen in inpatient and outpatient settings.
Special Situations
Most of the preferred regimens for different situations are outlined in Table 2 in a straightforward manner, and can be depicted in your protocols and order sets in the same way. Some conditions have enough complexity, however, that you will have difficulty placing all of the details into your one‐page protocol and order set. Details should be placed on your more detailed protocol, and educational programs should include the topics outlined below. Although insulin infusion is often the option that would provide the most reliable and expedient control of hyperglycemia in these special situations, it is an option not available in many noncritical care settings. Therefore, the discussion is limited to subcutaneous insulin control regimens.
Patient on Continuous Tube Feeding
The SHM GCTF endorses glargine or detemir as the basal insulin of choice for this setting. The nutritional and correction insulin of choice is either an RAA‐I every 4 hours (q4h), or regular insulin every 6 hours (q6h). We endorse this choice because it retains the basal‐nutritional‐correction dose concept, generally allows for continued basal insulin use if the tube feedings become interrupted, and is amenable to building a consistent institutional protocol.
There are some important caveats to this recommendation. First, realize that almost any regimen that provides a stable insulin supply would be acceptable, and many institutions will use glargine or detemir to cover both basal and nutritional needs. The downside to using large boluses of long‐acting insulin in this clinical situation is that any unexpected interruption of the feedings will necessitate prolonged infusions of dextrate 10% solution (D10) to avoid hypoglycemia
Second, because of the glycemic load inherent in tube feedings, maintenance of glycemic control in the setting of enteral feeding may be best managed by providing a higher percentage of the TDD as nutritional insulin. In these cases, ratios of basal to nutritional insulin of 40:60, or even less basal insulin, may be appropriate.
Glucocorticoid Therapy
High‐dose glucocorticoids are strongly associated with increased insulin requirements. The degree of hyperglycemia induced by steroids varies significantly from patient to patient, and the pattern of hyperglycemia will vary depending on the pattern of steroid administration. The general principle to keep in mind is that the hyperglycemia induced by a steroid dose will peak 8‐12 hours after it is given, so insulin regimens to address this should take this effect into account. For example, giving a long‐acting basal insulin like glargine to accommodate the hyperglycemic effect of a steroid bolus given in the morning would be inappropriate because the steroid effect would wane and then disappear overnight, leading to insulin‐induced hypoglycemia. NPH insulin can be ideal in this setting, either by itself, or by layering it on top of an existing regimen.
Another caveat: glucocorticoids exert their predominate effect on insulin sensitivity in muscle (as opposed to the liver), and as a result, have their most notable effect on postprandial glucose. For this reason, the best insulin regimens for this situation may use proportionally less basal insulin and more nutritional insulin. One common regimen calls for keeping the basal insulin dose the same as the preglucocorticoid dose, while escalating the RAA insulin dose at lunch and dinner.
Given the complexities of covering steroid‐induced hyperglycemia and its high prevalence in certain populations (such as transplantation patients and patients undergoing chemotherapy), this would be an excellent area on which to focus expertise. Examples include routine endocrinology consultation, intervention by a special glycemic control team, or incorporating routine glucose monitoring and triggers for initiating insulin infusion into the protocols for chemotherapy and transplantation patients.
Regiment the Management of Hypoglycemia
Hypoglycemia is defined by the ADA as a blood glucose of 70 mg/dL or less, based on the physiologic changes that can occur at this glucose level, even in subjectively asymptomatic patients.3 Protocols for management of hypoglycemia should be linked to your diabetes/hyperglycemia protocols. There are many hypoglycemia protocols available for review in the SHM Glycemic Control Resource Room and Glycemic Control Implementation Guide.10 Some common themes for effective implementation stand out. First, the protocols need to walk the balance between simplicity of use, and the need to provide instructions that will provide guidance in a variety of patient situations. Second, the protocols need to be nurse driven, so that nurses can initiate treatment without waiting for a physician order. Third, education and instruction regarding recognition of risk factors, and avoidance of hypoglycemia are needed to support a successful protocol. Importantly, any hypoglycemic event should lead to a reconsideration of the current anti‐hyperglycemic regimen so that future events can be prevented.
Plan for Discharge and Provide Guidance for the Transition
Your institution should have policies and procedures outlining all the steps needed to complete the important transition out of the hospital. At a minimum, this planning should include adequate education (including a learner assessment), appropriate follow‐up, referral to community resources, and a discharge glycemic control regimen that is tailored to the educational, financial, and motivational profile of a patient. The more your inpatient insulin management is driven by protocol, the more likely it is the patient will be on an inpatient treatment plan that differs from their outpatient regimen; therefore, it is even more important to plan this transition carefully and reliably.
Communicating the accurate hyperglycemia related diagnosis and related problems to the primary care provider is important for good care, perhaps even more so for patients who had hyperglycemia while hospitalized without a prior diagnosis of diabetes. Some centers place a prompt for hyperglycemia related diagnosis in the order set and/or discharge paperwork, to remind the clinician to convey the diagnosis to the primary provider, and to encourage more complete documentation. Improved documentation can also improve the business case for glycemic control, along with other strategies outlined elsewhere in this supplement.13
Transitions in care (including transitions out of the hospital and off of infusion insulin) are discussed in more detail14, 15 elsewhere in this supplement. The principles outlined in these references should be incorporated into your institutional protocol. Briefly, not all patients require or are capable of intensive basal‐bolus regimens upon discharge. The HbA1c can be very valuable in arriving at the optimal outpatient regimen.14 The capacities and preferences of the patient and the context of his or her outpatient care environment (including the preferences of the primary care provider) must be taken into consideration as an outpatient management program is planned.
PULLING IT ALL TOGETHER: MAKE SURE YOUR PROTOCOL/ORDER SET IS EASY TO USE AND WIDELY UTILIZED
When standardizing hospital management of diabetes and hyperglycemia, we recommend building the full protocol first, then crystallizing the protocol into a one‐page summary that can be widely disseminated. The protocol guidance is then incorporated into the order set and nursing medical administration record (MAR). Again, we recommend the most proscriptive and protocol‐driven order set feasible within the constraints of medical staff support. The example order set in Appendix 2 illustrates this approach along with other desirable features:
-
Check‐box simplicity on when to order appropriate glucose monitoring.
-
Prompt for the proper hyperglycemia‐related diagnosis.
-
Prompts to document diagnosis and to order HbA1c level.
-
Use of encouraged insulin terminology: basal, prandial (or nutritional), and correction. Language is a powerful thing, and just getting staff to use these terms goes a long way toward the more physiologic prescribing of insulin.
-
Statement/reminder of a glycemic goal.
-
Prompts and contact information for appropriate consultation.
-
Elimination of unapproved abbreviations (such as U for units).
-
Stating both generic and brand names of insulin preparations.
-
Important timing cues for administration of insulin.
-
Several correction‐dose scales suitable for different insulin sensitivities. One size does NOT fit all.
-
Incorporation of a simple hypoglycemia protocol into the order set.
-
Insulin dosing guidelines available at the point of care (in this case, on the back of the order set).
Additional nursing‐specific cues (such as an admonition to never mix glargine insulin with other types of insulin) can also be included in the MAR whenever glargine is ordered.
Once you have protocols and order sets to guide providers, you need to assure that they are used for the majority of hyperglycemic patients. Educational programs should introduce your interventions and the rationale for them. In order to make your method the default method of care, your team should survey all preprinted or CPOE insulin order sets of your institution. A review of postoperative, transfer, and admission order sets that all services use may reveal a half‐dozen or more embedded sliding‐scale insulin order sets that should be removed, with prompts to use the standardized insulin order set being placed in their stead.
Computerized order sets present both challenges and opportunities. Wording limitations and the scrolling nature can make concepts less clear, yet there is a capability for incorporating a hierarchical structure that allows for guiding the user through a more algorithmic approach. There is also a capacity to provide assistance with dosing calculations that do not exist in the paper world. Education remains of key importance for both methods.
MONITOR THE USE AND EFFECTIVENESS OF YOUR PROTOCOLS AND ORDER SETS
Creating and implementing protocols, order sets, and other tools is not the end of the journey to improve care. It is important to monitor order set utilization, insulin use patterns, and parameters measuring glycemic control and hypoglycemia, as outlined in more detail in another article in this supplement.16 In addition to summary data every month or so, we recommend daily reports that spur action in near real time. Triggers such as uncontrolled hyperglycemia, markedly elevated HbA1c levels, and nonphysiologic insulin regimens should initiate consultation, extra diabetes education, or referral to a glucose control team. If appropriate consultation is not readily available, the glycemic control steering group should lobby the administration to bolster this capability. Qualitative feedback from the frontline caregivers, as well as this quantitative data, can assist the local glycemic control champions in designing even more effective protocols, order sets, focused educational efforts, and concurrent mitigation of suboptimal care.
CONCLUSION
Diabetes, hyperglycemia, and iatrogenic hypoglycemia are common and important conditions affecting the noncritically ill inpatient. Interventional trials to validate the recommended noncritical care unit glycemic targets are needed. Although there is a growing consensus on best practices to care for these patients, numerous barriers and the complexity of caring for inpatients hamper the reliability of best practice delivery. Institutional protocols and protocol driven subcutaneous insulin orders, when implemented with the strategies outlined here, can be the key to delivering these best practices more reliably.
Appendix
Inpatient glycemic control and hypoglycemia are issues with well deserved increased attention in recent years. Prominent guidelines and technical reviews have been published,13 and a recent, randomized controlled trial demonstrated the superiority of basal bolus insulin regimens compared to sliding‐scale regimens.4 Effective glycemic control for inpatients has remained elusive in most medical centers. Recent reports57 detail clinical inertia and the continued widespread use of sliding‐scale subcutaneous insulin regimens, as opposed to the anticipatory, physiologic basal‐nutrition‐correction dose insulin regimens endorsed by these reviews.
Inpatient glycemic control faces a number of barriers, including fears of inducing hypoglycemia, uneven knowledge and training among staff, and competing institutional and patient priorities. These barriers occur in the background of an inherently complex inpatient environment that poses unique challenges in maintaining safe glycemic control. Patients frequently move across a variety of care teams and geographic locations during a single inpatient stay, giving rise to multiple opportunities for failed communication, incomplete handoffs, and inconsistent treatment. In addition, insulin requirements may change dramatically due to variations in the stress of illness, exposure to medications that effect glucose levels, and varied forms of nutritional intake with frequent interruption. Although insulin is recognized as one of the medications most likely to be associated with adverse events in the hospital, many hospitals do not have protocols or order sets in place to standardize its use.
A Call to Action consensus conference,8, 9 hosted by the American Association of Clinical Endocrinologists (AACE) and the American Diabetes Association (ADA), brought together many thought leaders and organizations, including representation from the Society of Hospital Medicine (SHM), to address these barriers and to outline components necessary for successful implementation of a program to improve inpatient glycemic control in the face of these difficulties. Institutional insulin management protocols and standardized insulin order sets (supported by appropriate educational efforts) were identified as key interventions. It may be tempting to quickly deploy a generic insulin order set in an effort to improve care. This often results in mediocre results, due to inadequate incorporation of standardization and guidance into the order set and other documentation tools, and uneven use of the order set.
The SHM Glycemic Control Task Force (GCTF) recommends the following steps for developing and implementing successful protocols and order sets addressing the needs of the noncritical care inpatient with diabetes/hyperglycemia.
-
Form a steering committee for this work, and assess the current processes of care.
-
Identify best practices and preferred regimens to manage diabetes and hyperglycemia in the hospital.
-
Integrate best practices and preferred institutional choices into an inpatient glycemic control protocol. Crystallize your protocol into a one page summary.
-
Place guidance from your protocol into the flow of work, by integrating it into standardized subcutaneous insulin order sets and other documentation and treatment tools.
-
Monitor the use of your order sets and protocol. Intervene actively on nonadherents to your protocol and those with poor glycemic control, and revise your protocol/order sets as needed.
IDENTIFYING AND INCORPORATING KEY CONCEPTS AND BEST PRACTICES
A protocol is a document that endorses specific monitoring and treatment strategies in a given institution. This potentially extensive document should provide guidance for transitions, special situations (like steroids and total parenteral nutrition [TPN]) and should outline preferred insulin regimens for all of the most common nutritional situations. One of the most difficult parts of creating a protocol is the assimilation of all of the important information on which to base decisions. Your protocol and order set will be promoting a set of clinical practices. Fortunately, the current best practice for noncritical care hyperglycemic patients has been summarized by several authoritative sources,13, 811 including references from the SHM Glycemic Task Force published in this supplement.4, 12
Table 1 summarizes the key concepts that should be emphasized in a protocol for subcutaneous insulin management in the hospital. We recommend embedding guidance from your protocol into order sets, the medication administration record, and educational materials. Although the details contained in a protocol and order set might vary from one institution to another, the key concepts should not. The remainder of this article provides practical information about how these concepts and guidance for how preferred insulin regimens should be included in these tools. Appendices 1 and 2 give examples of an institutional one‐page summary protocol and subcutaneous insulin order set, respectively.
| 1. Establish a target range for blood glucose levels. |
| 2. Standardize monitoring of glucose levels and assessment of long‐term control (HbA1c). |
| 3. Incorporate nutritional management. |
| 4. Prompt clinicians to consider discontinuing oral antihyperglycemic medications. |
| 5. Prescribe physiologic (basal‐nutrition‐correction) insulin regimens. |
| a. Choose a total daily dose (TDD). |
| b. Divide the TDD into physiologic components of insulin therapy and provide basal and nutritional/correction separately. |
| c. Choose and dose a basal insulin. |
| d. Choose and dose a nutritional (prandial) insulin |
|
i. Match exactly to nutritional intake (see Table 2). |
| ii. Include standing orders to allow nurses to hold nutritional insulin for nutritional interruptions and to modify nutritional insulin depending on the actual nutritional intake. |
| e. Add correction insulin |
| i. Match to an estimate of the patients insulin sensitivity using prefabricated scales. |
| ii. Use the same insulin as nutritional insulin. |
| 6. Miscellaneous |
| a. Manage hypoglycemia in a standardized fashion and adjust regimen to prevent recurrences. |
| b. Provide diabetes education and appropriate consultation. |
| c. Coordinate glucose testing, nutrition delivery, and insulin administration. |
| d. Tailor discharge treatment regimens to the patient's individual circumstances and arrange for proper follow‐up. |
Standardize the Monitoring of Blood Glucose Values and Glucosylated Hemoglobin
Guidance for the coordination of glucose testing, nutrition delivery, and insulin administration, should be integrated into your protocols, and order sets. For noncritical care areas, the minimal frequency for blood glucose monitoring for patients who are eating is before meals and at bedtime. For the patient designated nothing by mouth (NPO) or the patient on continuous tube feeding, the type of nutritional/correction insulin used should drive the minimum frequency (every 4‐6 hours if rapid acting analog insulins [RAA‐I] are used, and every 6 hours if regular insulin is used). Directions for administering scheduled RAA‐I immediately before or immediately after nutrition delivery should be incorporated into protocols, order sets, and medication administration records. Unfortunately, having this guidance in the order sets and protocols does not automatically translate into its being carried out in the real world. Wide variability in the coordination of glucose monitoring, nutritional delivery, and insulin administration is common, so monitoring the process to make sure the protocol is followed is important.
Obtaining a glucosylated hemoglobin (HbA1c) level is important in gauging how well the patient's outpatient regimen is maintaining glycemic control, distinguishing stress hyperglycemia from established diabetes, and guiding the inpatient approach to glycemic control. ADA guidelines2, 3 endorse obtaining HbA1c levels of inpatients if these levels are not already available from the month prior to admission.
Establish a Target Range for Blood Glucose in NonCritical Care Areas
It is important to adopt a glycemic target that is institution‐wide, for critical care areas and noncritical care areas alike. Your glycemic target need not be identical to the ADA/AACE glycemic targets, but should be similar to them.
Examples of institutional glycemic targets for noncritical care areas:
-
Preprandial target 90‐130 mg/dL, maximum random glucose <180 mg/dL (ADA/AACE consensus target)
-
90‐150 mg/dL (a target used in some hospitals)
-
Preprandial target 90‐130 mg/dL for most patients, 100‐150 mg/dL if there are hypoglycemia risk factors, and <180 mg/dL if comfort‐care or end‐of‐life care (a more refined target, allowing for customization based on patient characteristics).
Your multidisciplinary glycemic control steering committee should pick the glycemic target it can most successfully implement and disseminate. It is fine to start with a conservative target and then ratchet down the goals as the environment becomes more accepting of the concept of tighter control of blood glucose in the hospital.
Although the choice of glycemic target is somewhat arbitrary, establishing an institutional glycemic target is critical to motivate clinical action. Your committee should design interventions, for instances when a patient's glycemic target is consistently not being met, including an assignment of responsibility.
Prompt Clinicians to Consider Discontinuing Oral Agents
Oral antihyperglycemic agents, in general, are difficult to quickly titrate to effect, and have side effects that limit their use in the hospital. In contrast, insulin acts rapidly and can be used in virtually all patients and clinical situations, making it the treatment of choice for treatment of hyperglycemia in the hospital.3, 11, 12 In certain circumstances, it may be entirely appropriate to continue a well‐controlled patient on his or her prior outpatient oral regimen. It is often also reasonable to resume oral agents in some patients when preparing for hospital discharge.
Incorporate Nutritional Management
Because diet is so integral to the management of diabetes and hyperglycemia, diet orders should be embedded in all diabetes or insulin‐related order sets. Diets with the same amount of carbohydrate with each meal should be the default rule for patients with diabetes. Nutritionist consultation should be considered and easy to access for patients with malnutrition, obesity, and other common conditions of the inpatient with diabetes.
Access Diabetes Education and Appropriate Consultation
Diabetes education should be offered to all hyperglycemic patients with normal mental status, complete with written materials, a listing of community resources, and survival skills. Consultation with physicians in internal medicine or endocrinology for difficult‐to‐control cases, or for cases in which the primary physician of record is not familiar with (or not adherent to) principles of inpatient glycemic management, should be very easy to obtain, or perhaps mandated, depending on your institution‐specific environment.
Prescribe Physiologic (Basal‐Nutritional‐Correction Dose) Insulin Regimens
Physiologic insulin use is the backbone of the recommended best practice for diabetes and hyperglycemia management in the hospital. The principles of such regimens are summarized elsewhere in this supplement.12 These principles will not be reiterated in detail here, but the major concepts that should be integrated into the protocols and order sets will be highlighted.
Choose a Total Daily Dose
Clinicians need guidance on how much subcutaneous insulin they should give a patient. These doses are well known from clinical experience and the published literature. The fear of hypoglycemia usually results in substantial underdosing of insulin, or total avoidance of scheduled insulin on admission. Your team should provide guidance for how much insulin to start a patient on when it is unclear from past experience how much insulin the patient needs. Waiting a few days to see how much insulin is required via sliding‐scale‐only regimens is a bad practice that should be discouraged for patients whose glucose values are substantially above the glycemic target. The total daily dose (TDD) can be estimated in several different ways (as demonstrated in Appendix 1 and 2), and protocols should make this step very clear for clinicians. Providing a specific location on the order set to declare the TDD may help ensure this step gets done more reliably. Some institutions with computer physician order entry (CPOE) provide assistance with calculating the TDD and the allocation of basal and nutritional components, based on data the ordering physician inputs into the system.
Select and Dose a Basal Insulin
Your protocol should describe how the TDD should be divided between basal and nutritional insulin. We generally recommend 50% of the TDD be given as basal insulin, with the other 50% administered on a scheduled basis to cover glycemic excursions from nutritional intake. The 50/50 rule is simple and generally works well, and should be widely promoted. However, there are exceptions to this rule that should be incorporated into your full protocol and educational programs. The order set should have separate steps for ordering basal insulin, nutritional insulin, and correction insulin. The advantage to providing these insulin components separately is that it allows them to be independently manipulated (eg, if a patient is unable to tolerate a meal, nutritional insulin is held, but basal insulin and correction insulin are continued).
The SHM GCTF specifically endorses long acting insulin (glargine and detemir) as the preferred basal insulin in the hospital setting, thus discouraging the use of neutral protamine Hagedorn (NPH) insulin and fixed combination insulin formulations (Table 2). In the absence of randomized controlled trials demonstrating superiority of the glargine or detemir to NPH insulin in the hospital, this endorsement deserves some further explanation. Although we believe that correctly dosed NPH containing insulin regimens can attain effective and safe glycemic control in the hospital setting, it is more difficult to standardize their use and adjust for fluctuations in nutritional intake. Glargine and detemir have much less pronounced spikes in their effect than NPH, rendering them relatively peakless in comparison. This pharmacokinetic profile allows for continued dosing with minimal or no correction when nutrition intake is variable, and allow for consistent reinforcement of the basal‐nutritional‐correction insulin concept.
| Nutritional situation | Necessary insulin components | Preferred regimen* |
|---|---|---|
| ||
| NPO (or clear liquids) | Basal insulin: 50% of TDD. Nutritional insulin: None. | Basal insulin: glargine given once daily or detemir given twice daily. Nutritional insulin: None. Correctional insulin: Regular insulin q 6 hours or RAA insulin q 4 hours. Other comments: Dextrose infusion (e.g., D5 containing solution at 75‐150 cc/hour) recommended when nutrition is held. An IV insulin infusion is preferred for management of prolonged fasts or fasting type 1 diabetes patients. |
| Eating meals | Basal insulin: 50% of TDD. Nutritional insulin: 50% of TDD, divided equally before each meal. | Basal insulin: glargine given once daily or detemir given twice daily. Nutritional insulin: RAA insulin with meals. Correctional insulin: RAA insulin q AC and HS (reduced dose at HS). |
| Bolus tube feeds | Basal insulin: 40% of TDD. Nutritional insulin: 60% of the TDD, divided equally before each bolus feed. | Basal insulin: glargine given once daily or detemir given twice daily. Nutritional insulin: RAA insulin with each bolus. Correctional insulin: RAA insulin with each bolus. |
| Continuous tube feeds | Basal insulin: 40% (conservative) of TDD. Nutritional insulin: 60% of the TDD in divided doses. | Basal insulin: glargine given once daily or detemir given twice daily. Nutritional insulin: RAA insulin q 4 hours or regular insulin q 6 hours. Correctional insulin: Should match nutritional insulin choice. |
| Parenteral nutrition | Insulin is usually given parenterally, with the nutrition | Initially, a separate insulin drip allows for accurate dose‐finding. Then, 80% of amount determined as TDD using drip is added to subsequent TPN bags as regular insulin. Use correctional subcutaneous insulin doses cautiously, in addition. |
There are some caveats to this general recommendation. First, patients who are well controlled on home regimens with NPH basal insulin can (and sometimes should) stay on the regimen that has worked well for them. However, extra vigilance in reducing the dose for reductions in nutrition is required, because NPH is generally used to cover both nutritional and basal requirements. Second, extensive experience with glargine and detemir are not available in obstetric populations. They are not U.S. Food and Drug Administration (FDA) approved for use in pregnant patients and formally carry a Class C rating, whereas NPH insulin has been used safely in obstetric populations for decades. Third, the insulin regimen used as an inpatient is not necessarily the preferred regimen to prescribe at discharge: cost, patient preferences, HbA1c level, and other factors should be considered in making this choice.
Select and Dose a Nutritional (Prandial) Insulin
The step for ordering nutritional insulin should assist the clinician in matching the insulin to the type of nutrition that the patient is receiving. For example, rapid‐acting insulin analogs are preferred over regular insulin in the eating patient, in view of their more physiologic profile, which averts the insulin stacking that can occur with regular insulin. If regular insulin is used as the preferred institutional choice for eating patients, the lunchtime dose should be reduced or eliminated altogether, to eliminate insulin stacking.
Table 2 outlines the SHM GCTF preferred regimens for different nutritional situations.
There should be a standing order for nutritional insulin to be held when nutrition is interrupted, whether intentional or unintentional. Patients with interrupted tube feedings could have standing orders for a dextrose infusion to replace the tube feeding carbohydrate load and prevent hypoglycemia. Ideally, there should also be a standing order allowing for real‐time management of the patient with uncertain nutritional intake. For example, when a patient's premeal assessment reveals that she may not tolerate the meal, the patient should be allowed to attempt to eat, and then the nutritional insulin should be given after the meal, in proportion to the amount of food that was eaten. This type of order will require significant nursing education and process redesign in many hospitals, but is essential for matching nutritional insulin to actual intake.
Add Correction Insulin
There is no convincing evidence for the benefit of correction (sliding‐scale) insulin in the inpatient setting, although a randomized trial demonstrating the superiority of basal/nutritional insulin regimens to sliding‐scale only regimens did incorporate a correction insulin scale as an adjunct to the superior basal/nutritional regimen.4 The SHM GCTF again emphasizes that control of hyperglycemia should be proactive and anticipatory of insulin needs, rather than reactive to hyperglycemia. Nonetheless, unexpected hyperglycemic excursions are common, and the use of correction insulin remains a pervasive and arguably logical practice. If correction insulin is used, it should be ordered as a separate step after considering basal and nutrition insulin needs. The doses of scheduled insulin should be adjusted regularly if correction insulin is consistently being required. Ideally, the prescriber should choose a preformatted corrective insulin scale, based on the patient's insulin sensitivity (Appendix 2). There should be a prompt to use the same type of insulin that is being used for nutritional insulin, and there should be instructions that this insulin is given in addition to the basal and nutritional insulin to correct for hyperglycemia. Nocturnal correction‐dose scales should be reduced in the eating patient.
Even after limiting insulin regimens to those in Table 2, multidisciplinary glycemic control teams are still left with several options within these SHM‐preferred regimens. We recommend that your team choose a single, institutionally‐preferred basal‐nutritional‐correction insulin combination for each situation.
Choosing one preferred option for these situations is advantageous because:
-
You can communicate preferred regimens more simply and succinctly to all staff.
-
You eliminate all inappropriate choices for insulin regimens for that situation, as well as some other less preferred, but acceptable choices.
-
You can encourage regimens that are most economical (by promoting the insulin regimens that reflect your hospital formulary choices).
-
Staff members can become very familiar with a few regimens, instead of being confused by a multitude of them. They can identify variations from your preferred choices and target these patients for extra scrutiny and actions should they fail to meet glycemic targets.
Although virtually every institution can provide specific guidance on insulin management in a protocol, there are tradeoffs inherent in how restrictive you can be in pushing these preferred choices in your order sets. Should you eliminate alternate basal or nutritional insulin choices from your order sets? As you integrate more and more of your preferred algorithm and regimens into your order set, you will gain incremental improvement in the standardization of inpatient insulin management. However, you reduce not only variability in ordering, but also the choices available to your prescribers and patients, and in effect you are pushing the providers to use an insulin regimen that often differs from the patient's outpatient regimen. If your institution is not yet ready to go with a single preferred insulin, simply listing your preferred insulin first with the annotation preferred can be enough to increase the use of the preferred insulin.
We endorse building the most protocol‐driven, proscriptive, insulin order set that the Glycemic Control Steering Committee believes their medical staff will accept. There are some caveats to this endorsement. First, there must be extra efforts on the backend of the admission, to ensure that the antihyperglycemic regimen is tailored to the unique needs of the patient (this is discussed further below). Second, a protocol‐driven approach is not a substitute for a good educational program for health care providers or well‐informed clinical judgment. Education should reinforce major concepts driving the protocol and should also highlight exceptions to the rule. Variance from the protocol endorsed choices should be allowed (and even encouraged) when the variance is driven by patient factors (as opposed to provider whim). Learning from this variance is a key concept in refining protocols. Education ideally should not be limited to only protocol‐endorsed choices, as staff should be familiar with the full range of antihyperglycemia regimens seen in inpatient and outpatient settings.
Special Situations
Most of the preferred regimens for different situations are outlined in Table 2 in a straightforward manner, and can be depicted in your protocols and order sets in the same way. Some conditions have enough complexity, however, that you will have difficulty placing all of the details into your one‐page protocol and order set. Details should be placed on your more detailed protocol, and educational programs should include the topics outlined below. Although insulin infusion is often the option that would provide the most reliable and expedient control of hyperglycemia in these special situations, it is an option not available in many noncritical care settings. Therefore, the discussion is limited to subcutaneous insulin control regimens.
Patient on Continuous Tube Feeding
The SHM GCTF endorses glargine or detemir as the basal insulin of choice for this setting. The nutritional and correction insulin of choice is either an RAA‐I every 4 hours (q4h), or regular insulin every 6 hours (q6h). We endorse this choice because it retains the basal‐nutritional‐correction dose concept, generally allows for continued basal insulin use if the tube feedings become interrupted, and is amenable to building a consistent institutional protocol.
There are some important caveats to this recommendation. First, realize that almost any regimen that provides a stable insulin supply would be acceptable, and many institutions will use glargine or detemir to cover both basal and nutritional needs. The downside to using large boluses of long‐acting insulin in this clinical situation is that any unexpected interruption of the feedings will necessitate prolonged infusions of dextrate 10% solution (D10) to avoid hypoglycemia
Second, because of the glycemic load inherent in tube feedings, maintenance of glycemic control in the setting of enteral feeding may be best managed by providing a higher percentage of the TDD as nutritional insulin. In these cases, ratios of basal to nutritional insulin of 40:60, or even less basal insulin, may be appropriate.
Glucocorticoid Therapy
High‐dose glucocorticoids are strongly associated with increased insulin requirements. The degree of hyperglycemia induced by steroids varies significantly from patient to patient, and the pattern of hyperglycemia will vary depending on the pattern of steroid administration. The general principle to keep in mind is that the hyperglycemia induced by a steroid dose will peak 8‐12 hours after it is given, so insulin regimens to address this should take this effect into account. For example, giving a long‐acting basal insulin like glargine to accommodate the hyperglycemic effect of a steroid bolus given in the morning would be inappropriate because the steroid effect would wane and then disappear overnight, leading to insulin‐induced hypoglycemia. NPH insulin can be ideal in this setting, either by itself, or by layering it on top of an existing regimen.
Another caveat: glucocorticoids exert their predominate effect on insulin sensitivity in muscle (as opposed to the liver), and as a result, have their most notable effect on postprandial glucose. For this reason, the best insulin regimens for this situation may use proportionally less basal insulin and more nutritional insulin. One common regimen calls for keeping the basal insulin dose the same as the preglucocorticoid dose, while escalating the RAA insulin dose at lunch and dinner.
Given the complexities of covering steroid‐induced hyperglycemia and its high prevalence in certain populations (such as transplantation patients and patients undergoing chemotherapy), this would be an excellent area on which to focus expertise. Examples include routine endocrinology consultation, intervention by a special glycemic control team, or incorporating routine glucose monitoring and triggers for initiating insulin infusion into the protocols for chemotherapy and transplantation patients.
Regiment the Management of Hypoglycemia
Hypoglycemia is defined by the ADA as a blood glucose of 70 mg/dL or less, based on the physiologic changes that can occur at this glucose level, even in subjectively asymptomatic patients.3 Protocols for management of hypoglycemia should be linked to your diabetes/hyperglycemia protocols. There are many hypoglycemia protocols available for review in the SHM Glycemic Control Resource Room and Glycemic Control Implementation Guide.10 Some common themes for effective implementation stand out. First, the protocols need to walk the balance between simplicity of use, and the need to provide instructions that will provide guidance in a variety of patient situations. Second, the protocols need to be nurse driven, so that nurses can initiate treatment without waiting for a physician order. Third, education and instruction regarding recognition of risk factors, and avoidance of hypoglycemia are needed to support a successful protocol. Importantly, any hypoglycemic event should lead to a reconsideration of the current anti‐hyperglycemic regimen so that future events can be prevented.
Plan for Discharge and Provide Guidance for the Transition
Your institution should have policies and procedures outlining all the steps needed to complete the important transition out of the hospital. At a minimum, this planning should include adequate education (including a learner assessment), appropriate follow‐up, referral to community resources, and a discharge glycemic control regimen that is tailored to the educational, financial, and motivational profile of a patient. The more your inpatient insulin management is driven by protocol, the more likely it is the patient will be on an inpatient treatment plan that differs from their outpatient regimen; therefore, it is even more important to plan this transition carefully and reliably.
Communicating the accurate hyperglycemia related diagnosis and related problems to the primary care provider is important for good care, perhaps even more so for patients who had hyperglycemia while hospitalized without a prior diagnosis of diabetes. Some centers place a prompt for hyperglycemia related diagnosis in the order set and/or discharge paperwork, to remind the clinician to convey the diagnosis to the primary provider, and to encourage more complete documentation. Improved documentation can also improve the business case for glycemic control, along with other strategies outlined elsewhere in this supplement.13
Transitions in care (including transitions out of the hospital and off of infusion insulin) are discussed in more detail14, 15 elsewhere in this supplement. The principles outlined in these references should be incorporated into your institutional protocol. Briefly, not all patients require or are capable of intensive basal‐bolus regimens upon discharge. The HbA1c can be very valuable in arriving at the optimal outpatient regimen.14 The capacities and preferences of the patient and the context of his or her outpatient care environment (including the preferences of the primary care provider) must be taken into consideration as an outpatient management program is planned.
PULLING IT ALL TOGETHER: MAKE SURE YOUR PROTOCOL/ORDER SET IS EASY TO USE AND WIDELY UTILIZED
When standardizing hospital management of diabetes and hyperglycemia, we recommend building the full protocol first, then crystallizing the protocol into a one‐page summary that can be widely disseminated. The protocol guidance is then incorporated into the order set and nursing medical administration record (MAR). Again, we recommend the most proscriptive and protocol‐driven order set feasible within the constraints of medical staff support. The example order set in Appendix 2 illustrates this approach along with other desirable features:
-
Check‐box simplicity on when to order appropriate glucose monitoring.
-
Prompt for the proper hyperglycemia‐related diagnosis.
-
Prompts to document diagnosis and to order HbA1c level.
-
Use of encouraged insulin terminology: basal, prandial (or nutritional), and correction. Language is a powerful thing, and just getting staff to use these terms goes a long way toward the more physiologic prescribing of insulin.
-
Statement/reminder of a glycemic goal.
-
Prompts and contact information for appropriate consultation.
-
Elimination of unapproved abbreviations (such as U for units).
-
Stating both generic and brand names of insulin preparations.
-
Important timing cues for administration of insulin.
-
Several correction‐dose scales suitable for different insulin sensitivities. One size does NOT fit all.
-
Incorporation of a simple hypoglycemia protocol into the order set.
-
Insulin dosing guidelines available at the point of care (in this case, on the back of the order set).
Additional nursing‐specific cues (such as an admonition to never mix glargine insulin with other types of insulin) can also be included in the MAR whenever glargine is ordered.
Once you have protocols and order sets to guide providers, you need to assure that they are used for the majority of hyperglycemic patients. Educational programs should introduce your interventions and the rationale for them. In order to make your method the default method of care, your team should survey all preprinted or CPOE insulin order sets of your institution. A review of postoperative, transfer, and admission order sets that all services use may reveal a half‐dozen or more embedded sliding‐scale insulin order sets that should be removed, with prompts to use the standardized insulin order set being placed in their stead.
Computerized order sets present both challenges and opportunities. Wording limitations and the scrolling nature can make concepts less clear, yet there is a capability for incorporating a hierarchical structure that allows for guiding the user through a more algorithmic approach. There is also a capacity to provide assistance with dosing calculations that do not exist in the paper world. Education remains of key importance for both methods.
MONITOR THE USE AND EFFECTIVENESS OF YOUR PROTOCOLS AND ORDER SETS
Creating and implementing protocols, order sets, and other tools is not the end of the journey to improve care. It is important to monitor order set utilization, insulin use patterns, and parameters measuring glycemic control and hypoglycemia, as outlined in more detail in another article in this supplement.16 In addition to summary data every month or so, we recommend daily reports that spur action in near real time. Triggers such as uncontrolled hyperglycemia, markedly elevated HbA1c levels, and nonphysiologic insulin regimens should initiate consultation, extra diabetes education, or referral to a glucose control team. If appropriate consultation is not readily available, the glycemic control steering group should lobby the administration to bolster this capability. Qualitative feedback from the frontline caregivers, as well as this quantitative data, can assist the local glycemic control champions in designing even more effective protocols, order sets, focused educational efforts, and concurrent mitigation of suboptimal care.
CONCLUSION
Diabetes, hyperglycemia, and iatrogenic hypoglycemia are common and important conditions affecting the noncritically ill inpatient. Interventional trials to validate the recommended noncritical care unit glycemic targets are needed. Although there is a growing consensus on best practices to care for these patients, numerous barriers and the complexity of caring for inpatients hamper the reliability of best practice delivery. Institutional protocols and protocol driven subcutaneous insulin orders, when implemented with the strategies outlined here, can be the key to delivering these best practices more reliably.
Appendix
- American College of Endocrinology Task Force on Inpatient Diabetes and Metabolic Control.American College of Endocrinology Position Statement on Inpatient Diabetes and Metabolic Control.Endocr Pract.2004;10:77–82.
- ,,,,,,.Management of diabetes and hyperglycemia in hospitals.Diabetes Care.2004;27:553–591.
- American Diabetes Association.Standards of Medical Carein Diabetes‐2006.Diabetes Care.2006;29(suppl 1):s4–s42.
- ,,, et al.Randomized study of basal‐bolus insulin therapy in the inpatient management of patients with type 2 diabetes (RABBIT 2 Trial).Diabetes Care.2007;30:2181–2186.
- ,,,,.Inpatient management of diabetes and hyperglycemia among general medicine patients at a large teaching hospital.J Hosp Med.2006;1:145–150.
- ,,, et al.Diabetes care in the hospital: is there clinical inertia?J Hosp Med.2006;1:151–160.
- ,,, et al.Diabetes care in hospitalized non‐critically ill patients: more evidence for clinical inertia and negative therapeutic momentum.JHosp Med.2007;2:203–211.
- Inpatient Diabetes and Glycemic Control: A Call to Action Conference. Position Statement. AACE, February2006. Available at: http://www.aace.com/meetings/consensus/IIDC/IDGC0207.pdf. Accessed October, 2006.
- Proceedings of the American College of Endocrinology and American Diabetes Association Consensus Conference, Washington, DC, January 30–31, 2006. Endocr Pract.2006; 12(suppl 3):3–13.
- Society of Hospital Medicine Glycemic Control Task Force. Implementation Guide: Improving Glycemic Control, Preventing Hypoglycemia, and Optimizing Care of the Inpatient with Hyperglycemia and Diabetes. Published January 2007 on the Society of Hospital Medicine website. Available at: http://www.hospitalmedicine.org. Accessed August,2007.
- .Management of hyperglycemia in the hospital setting.N Engl J Med.2006;355:1903–1911.
- ,,,,.Management of diabetes and hyperglycemia in the hospital: a practical guide to subcutaneous insulin use in the non‐critically ill adult patient.J Hosp Med.2008;3(5):S17–S28.
- ,.Practical strategies for developing the business case for hospital glycemic control teams.J Hosp Med2008;3(5):S76–S83.
- ,,,.Bridge over troubled waters: safe and effective transitions of the inpatient with hyperglycemia.J Hosp Med.2008;3(5):S55–S65.
- ,,,.Designing and implementing insulin infusion protocols and order sets.J Hosp Med.2008;3(5):S42–S54.
- ,,,,,.SHM Glycemic Control Task Force summary: practical recommendations for assessing the impact of glycemic control efforts.J Hosp Med.2008;3(5):S66–S75.
- American College of Endocrinology Task Force on Inpatient Diabetes and Metabolic Control.American College of Endocrinology Position Statement on Inpatient Diabetes and Metabolic Control.Endocr Pract.2004;10:77–82.
- ,,,,,,.Management of diabetes and hyperglycemia in hospitals.Diabetes Care.2004;27:553–591.
- American Diabetes Association.Standards of Medical Carein Diabetes‐2006.Diabetes Care.2006;29(suppl 1):s4–s42.
- ,,, et al.Randomized study of basal‐bolus insulin therapy in the inpatient management of patients with type 2 diabetes (RABBIT 2 Trial).Diabetes Care.2007;30:2181–2186.
- ,,,,.Inpatient management of diabetes and hyperglycemia among general medicine patients at a large teaching hospital.J Hosp Med.2006;1:145–150.
- ,,, et al.Diabetes care in the hospital: is there clinical inertia?J Hosp Med.2006;1:151–160.
- ,,, et al.Diabetes care in hospitalized non‐critically ill patients: more evidence for clinical inertia and negative therapeutic momentum.JHosp Med.2007;2:203–211.
- Inpatient Diabetes and Glycemic Control: A Call to Action Conference. Position Statement. AACE, February2006. Available at: http://www.aace.com/meetings/consensus/IIDC/IDGC0207.pdf. Accessed October, 2006.
- Proceedings of the American College of Endocrinology and American Diabetes Association Consensus Conference, Washington, DC, January 30–31, 2006. Endocr Pract.2006; 12(suppl 3):3–13.
- Society of Hospital Medicine Glycemic Control Task Force. Implementation Guide: Improving Glycemic Control, Preventing Hypoglycemia, and Optimizing Care of the Inpatient with Hyperglycemia and Diabetes. Published January 2007 on the Society of Hospital Medicine website. Available at: http://www.hospitalmedicine.org. Accessed August,2007.
- .Management of hyperglycemia in the hospital setting.N Engl J Med.2006;355:1903–1911.
- ,,,,.Management of diabetes and hyperglycemia in the hospital: a practical guide to subcutaneous insulin use in the non‐critically ill adult patient.J Hosp Med.2008;3(5):S17–S28.
- ,.Practical strategies for developing the business case for hospital glycemic control teams.J Hosp Med2008;3(5):S76–S83.
- ,,,.Bridge over troubled waters: safe and effective transitions of the inpatient with hyperglycemia.J Hosp Med.2008;3(5):S55–S65.
- ,,,.Designing and implementing insulin infusion protocols and order sets.J Hosp Med.2008;3(5):S42–S54.
- ,,,,,.SHM Glycemic Control Task Force summary: practical recommendations for assessing the impact of glycemic control efforts.J Hosp Med.2008;3(5):S66–S75.