User login
Just a series of fortunate events?
Building a career in hospital medicine
Residents and junior faculty have frequently asked me how they can attain a position similar to mine, focused on quality and leadership in a health care system. When I was first asked to offer advice on this topic, my response was generally something like, “Heck if I know! I just had a series of lucky accidents to get here!”
Back then, I would recount my career history. I established myself as a clinician educator and associate program director soon after Chief Residency. After that, I would explain, a series of fortunate events and health care trends shaped my career. Evidence-based medicine (EBM), the patient safety movement, a shift to incorporate value (as well as volume) into reimbursement models, and the hospital medicine movement all emerged in interesting and often synergistic ways.
A young SHM organization (then known as NAIP) grew rapidly even while the hospitalist programs I led in Phoenix, then at University of California, San Diego, grew in size and influence. Inevitably, it seemed, I was increasingly involved in quality improvement (QI) efforts, and began to publish and speak about them. Collaborative work with SHM and a number of hospital systems broadened my visibility regionally and nationally. Finally, in 2015, I was recruited away from UC San Diego into a new position, as chief quality officer at UC Davis.
On hearing this history, those seeking my sage advice would look a little confused, and then say something like, “So your advice is that I should get lucky??? Gee, thanks a lot! Really helpful!” (Insert sarcasm here).
The honor of being asked to contribute to the “Legacies” series in The Hospitalist gave me an opportunity to think about this a little differently. No one really wanted to know about how past changes in the health care environment led to my career success. They wanted advice on tools and strategies that will allow them to thrive in an environment of ongoing, disruptive change that is likely only going to accelerate. I now present my upgraded points of advice, intertwined with examples of how SHM positively influenced my career (and could assist yours):
Learn how your hospital works. Hospitalists obviously have an inside track on many aspects of hospital operations, but sometimes remain oblivious to the organizational and committee structure, priorities of hospital leadership, and the mechanism for implementing standardized care. Knowing where to go with new ideas, and the process of implementing protocols, will keep you from hitting political land mines and unintentionally encroaching on someone else’s turf, while aligning your efforts with institutional priorities improves the buy-in and resources available to do the work.
Start small, but think big. Don’t bite off more than you can chew, and make sure your ideas for change work on a small scale before trying to sell the world on them. On the other hand, think big! The care you and others provide is dependent on systems that go far beyond your immediate control. Policies, protocols, standardized order sets, checklists, and an array of other tools can be leveraged to influence care across an entire health system, and in the SHM Mentored Implementation programs, can impact hundreds of hospitals.
Broaden your skills. Commit to learning new skills that can increase your impact and career diversity. Procedural skills; information technology; and EMR, EBM, research, public health, QI, business, leadership, public speaking, advocacy, and telehealth, can all open up a whole world of possibilities when combined with a medical degree. These skills can move you into areas that keep you engaged and excited to go to work.
Engage in mentor/mentee relationships. As an associate program director and clinician-educator, I had a lot of opportunity to mentor residents and fellows. It is so rewarding to watch the mentee grow in experience and skills, and to eventually see many of them assume leadership and mentoring roles themselves. You don’t have to be in a teaching position to act as a mentor (my experience mentoring hospitalists and others in leadership and quality improvement now far surpasses my experience with house staff).
The mentor often benefits as much as the mentee from this relationship. I have been inspired by their passion and dedication, educated by their ideas and innovation, and frequently find I am learning more from them, than they are from me. I have had great experiences in the SHM Mentored Implementation program in the role of mentee and mentor.
Participate in a community. When I first joined NAIP, I was amazed that the giants (Wachter, Nelson, Whitcomb, Holman, Williams, Greeno, Howell, Huddleston, Wellikson, and on and on) were not only approachable, they were warm, friendly, interesting, and extraordinarily welcoming. The ever-expanding and evolving community at SHM continues that tradition and offers a forum to share innovative work, discuss common problems and solutions, contact world experts, or just find an empathetic ear. Working on toolkits and collaborative efforts with this community remains a real highlight of my career, and the source of several lasting friendships. So don’t be shy; step right up; and introduce yourself!
Avoid my past mistakes (this might be a long list). Random things you should try to avoid.
- Tribalism – It is natural to be protective of your hospitalist group, and to focus on the injustices heaped upon you from (insert favorite punching bag here, e.g., ED, orthopedists, cardiologists, nursing staff, evil administration penny pinchers, etc). While some of those injustices might be real, tribalism, defensiveness, and circling the wagons generally only makes things worse. Sit down face to face, learn a little bit about the opposing tribe (both about their work, and about them as people), and see how much more fun and productive work can be.
- Storming out of a meeting with the CMO and CEO, slamming the door, etc. – not productive. Administrative leaders are doing their own juggling act and are generally well intentioned and doing the best they can. Respect that, argue your case, but if things don’t pan out, shake their hand, and live to fight another day.
- Using e-mail (evil-mail) to resolve conflict – And if you’re a young whippersnapper, don’t use Twitter, Facebook, Snapchat, or other social media to address conflict either!
- Forgetting to put patients first – Frame decisions for your group around what best serves your patients, not your doctors. Long term, this gives your group credibility and will serve the hospitalists better as well. SHM does this on a large scale with their advocacy efforts, resulting in more credibility and influence on Capitol Hill.
Make time for friends, family, fitness, fun, and reflection. A sense of humor and an occasional laugh when dealing with ill patients, hospital medicine politics, and the EMR all day provides resilience, as does taking the time to foster self-awareness and insight into your own weaknesses, strengths, and how you react to different stressors. A little bit of exercise and time with family and friends can go a long way towards improving your outlook, work, and life in general, while reducing burnout. Oh yeah, it’s also a good idea to choose a great life partner as well. Thanks Michelle!
Dr. Maynard is chief quality officer, University of California Davis Medical Center, Sacramento, Calif.
Building a career in hospital medicine
Building a career in hospital medicine
Residents and junior faculty have frequently asked me how they can attain a position similar to mine, focused on quality and leadership in a health care system. When I was first asked to offer advice on this topic, my response was generally something like, “Heck if I know! I just had a series of lucky accidents to get here!”
Back then, I would recount my career history. I established myself as a clinician educator and associate program director soon after Chief Residency. After that, I would explain, a series of fortunate events and health care trends shaped my career. Evidence-based medicine (EBM), the patient safety movement, a shift to incorporate value (as well as volume) into reimbursement models, and the hospital medicine movement all emerged in interesting and often synergistic ways.
A young SHM organization (then known as NAIP) grew rapidly even while the hospitalist programs I led in Phoenix, then at University of California, San Diego, grew in size and influence. Inevitably, it seemed, I was increasingly involved in quality improvement (QI) efforts, and began to publish and speak about them. Collaborative work with SHM and a number of hospital systems broadened my visibility regionally and nationally. Finally, in 2015, I was recruited away from UC San Diego into a new position, as chief quality officer at UC Davis.
On hearing this history, those seeking my sage advice would look a little confused, and then say something like, “So your advice is that I should get lucky??? Gee, thanks a lot! Really helpful!” (Insert sarcasm here).
The honor of being asked to contribute to the “Legacies” series in The Hospitalist gave me an opportunity to think about this a little differently. No one really wanted to know about how past changes in the health care environment led to my career success. They wanted advice on tools and strategies that will allow them to thrive in an environment of ongoing, disruptive change that is likely only going to accelerate. I now present my upgraded points of advice, intertwined with examples of how SHM positively influenced my career (and could assist yours):
Learn how your hospital works. Hospitalists obviously have an inside track on many aspects of hospital operations, but sometimes remain oblivious to the organizational and committee structure, priorities of hospital leadership, and the mechanism for implementing standardized care. Knowing where to go with new ideas, and the process of implementing protocols, will keep you from hitting political land mines and unintentionally encroaching on someone else’s turf, while aligning your efforts with institutional priorities improves the buy-in and resources available to do the work.
Start small, but think big. Don’t bite off more than you can chew, and make sure your ideas for change work on a small scale before trying to sell the world on them. On the other hand, think big! The care you and others provide is dependent on systems that go far beyond your immediate control. Policies, protocols, standardized order sets, checklists, and an array of other tools can be leveraged to influence care across an entire health system, and in the SHM Mentored Implementation programs, can impact hundreds of hospitals.
Broaden your skills. Commit to learning new skills that can increase your impact and career diversity. Procedural skills; information technology; and EMR, EBM, research, public health, QI, business, leadership, public speaking, advocacy, and telehealth, can all open up a whole world of possibilities when combined with a medical degree. These skills can move you into areas that keep you engaged and excited to go to work.
Engage in mentor/mentee relationships. As an associate program director and clinician-educator, I had a lot of opportunity to mentor residents and fellows. It is so rewarding to watch the mentee grow in experience and skills, and to eventually see many of them assume leadership and mentoring roles themselves. You don’t have to be in a teaching position to act as a mentor (my experience mentoring hospitalists and others in leadership and quality improvement now far surpasses my experience with house staff).
The mentor often benefits as much as the mentee from this relationship. I have been inspired by their passion and dedication, educated by their ideas and innovation, and frequently find I am learning more from them, than they are from me. I have had great experiences in the SHM Mentored Implementation program in the role of mentee and mentor.
Participate in a community. When I first joined NAIP, I was amazed that the giants (Wachter, Nelson, Whitcomb, Holman, Williams, Greeno, Howell, Huddleston, Wellikson, and on and on) were not only approachable, they were warm, friendly, interesting, and extraordinarily welcoming. The ever-expanding and evolving community at SHM continues that tradition and offers a forum to share innovative work, discuss common problems and solutions, contact world experts, or just find an empathetic ear. Working on toolkits and collaborative efforts with this community remains a real highlight of my career, and the source of several lasting friendships. So don’t be shy; step right up; and introduce yourself!
Avoid my past mistakes (this might be a long list). Random things you should try to avoid.
- Tribalism – It is natural to be protective of your hospitalist group, and to focus on the injustices heaped upon you from (insert favorite punching bag here, e.g., ED, orthopedists, cardiologists, nursing staff, evil administration penny pinchers, etc). While some of those injustices might be real, tribalism, defensiveness, and circling the wagons generally only makes things worse. Sit down face to face, learn a little bit about the opposing tribe (both about their work, and about them as people), and see how much more fun and productive work can be.
- Storming out of a meeting with the CMO and CEO, slamming the door, etc. – not productive. Administrative leaders are doing their own juggling act and are generally well intentioned and doing the best they can. Respect that, argue your case, but if things don’t pan out, shake their hand, and live to fight another day.
- Using e-mail (evil-mail) to resolve conflict – And if you’re a young whippersnapper, don’t use Twitter, Facebook, Snapchat, or other social media to address conflict either!
- Forgetting to put patients first – Frame decisions for your group around what best serves your patients, not your doctors. Long term, this gives your group credibility and will serve the hospitalists better as well. SHM does this on a large scale with their advocacy efforts, resulting in more credibility and influence on Capitol Hill.
Make time for friends, family, fitness, fun, and reflection. A sense of humor and an occasional laugh when dealing with ill patients, hospital medicine politics, and the EMR all day provides resilience, as does taking the time to foster self-awareness and insight into your own weaknesses, strengths, and how you react to different stressors. A little bit of exercise and time with family and friends can go a long way towards improving your outlook, work, and life in general, while reducing burnout. Oh yeah, it’s also a good idea to choose a great life partner as well. Thanks Michelle!
Dr. Maynard is chief quality officer, University of California Davis Medical Center, Sacramento, Calif.
Residents and junior faculty have frequently asked me how they can attain a position similar to mine, focused on quality and leadership in a health care system. When I was first asked to offer advice on this topic, my response was generally something like, “Heck if I know! I just had a series of lucky accidents to get here!”
Back then, I would recount my career history. I established myself as a clinician educator and associate program director soon after Chief Residency. After that, I would explain, a series of fortunate events and health care trends shaped my career. Evidence-based medicine (EBM), the patient safety movement, a shift to incorporate value (as well as volume) into reimbursement models, and the hospital medicine movement all emerged in interesting and often synergistic ways.
A young SHM organization (then known as NAIP) grew rapidly even while the hospitalist programs I led in Phoenix, then at University of California, San Diego, grew in size and influence. Inevitably, it seemed, I was increasingly involved in quality improvement (QI) efforts, and began to publish and speak about them. Collaborative work with SHM and a number of hospital systems broadened my visibility regionally and nationally. Finally, in 2015, I was recruited away from UC San Diego into a new position, as chief quality officer at UC Davis.
On hearing this history, those seeking my sage advice would look a little confused, and then say something like, “So your advice is that I should get lucky??? Gee, thanks a lot! Really helpful!” (Insert sarcasm here).
The honor of being asked to contribute to the “Legacies” series in The Hospitalist gave me an opportunity to think about this a little differently. No one really wanted to know about how past changes in the health care environment led to my career success. They wanted advice on tools and strategies that will allow them to thrive in an environment of ongoing, disruptive change that is likely only going to accelerate. I now present my upgraded points of advice, intertwined with examples of how SHM positively influenced my career (and could assist yours):
Learn how your hospital works. Hospitalists obviously have an inside track on many aspects of hospital operations, but sometimes remain oblivious to the organizational and committee structure, priorities of hospital leadership, and the mechanism for implementing standardized care. Knowing where to go with new ideas, and the process of implementing protocols, will keep you from hitting political land mines and unintentionally encroaching on someone else’s turf, while aligning your efforts with institutional priorities improves the buy-in and resources available to do the work.
Start small, but think big. Don’t bite off more than you can chew, and make sure your ideas for change work on a small scale before trying to sell the world on them. On the other hand, think big! The care you and others provide is dependent on systems that go far beyond your immediate control. Policies, protocols, standardized order sets, checklists, and an array of other tools can be leveraged to influence care across an entire health system, and in the SHM Mentored Implementation programs, can impact hundreds of hospitals.
Broaden your skills. Commit to learning new skills that can increase your impact and career diversity. Procedural skills; information technology; and EMR, EBM, research, public health, QI, business, leadership, public speaking, advocacy, and telehealth, can all open up a whole world of possibilities when combined with a medical degree. These skills can move you into areas that keep you engaged and excited to go to work.
Engage in mentor/mentee relationships. As an associate program director and clinician-educator, I had a lot of opportunity to mentor residents and fellows. It is so rewarding to watch the mentee grow in experience and skills, and to eventually see many of them assume leadership and mentoring roles themselves. You don’t have to be in a teaching position to act as a mentor (my experience mentoring hospitalists and others in leadership and quality improvement now far surpasses my experience with house staff).
The mentor often benefits as much as the mentee from this relationship. I have been inspired by their passion and dedication, educated by their ideas and innovation, and frequently find I am learning more from them, than they are from me. I have had great experiences in the SHM Mentored Implementation program in the role of mentee and mentor.
Participate in a community. When I first joined NAIP, I was amazed that the giants (Wachter, Nelson, Whitcomb, Holman, Williams, Greeno, Howell, Huddleston, Wellikson, and on and on) were not only approachable, they were warm, friendly, interesting, and extraordinarily welcoming. The ever-expanding and evolving community at SHM continues that tradition and offers a forum to share innovative work, discuss common problems and solutions, contact world experts, or just find an empathetic ear. Working on toolkits and collaborative efforts with this community remains a real highlight of my career, and the source of several lasting friendships. So don’t be shy; step right up; and introduce yourself!
Avoid my past mistakes (this might be a long list). Random things you should try to avoid.
- Tribalism – It is natural to be protective of your hospitalist group, and to focus on the injustices heaped upon you from (insert favorite punching bag here, e.g., ED, orthopedists, cardiologists, nursing staff, evil administration penny pinchers, etc). While some of those injustices might be real, tribalism, defensiveness, and circling the wagons generally only makes things worse. Sit down face to face, learn a little bit about the opposing tribe (both about their work, and about them as people), and see how much more fun and productive work can be.
- Storming out of a meeting with the CMO and CEO, slamming the door, etc. – not productive. Administrative leaders are doing their own juggling act and are generally well intentioned and doing the best they can. Respect that, argue your case, but if things don’t pan out, shake their hand, and live to fight another day.
- Using e-mail (evil-mail) to resolve conflict – And if you’re a young whippersnapper, don’t use Twitter, Facebook, Snapchat, or other social media to address conflict either!
- Forgetting to put patients first – Frame decisions for your group around what best serves your patients, not your doctors. Long term, this gives your group credibility and will serve the hospitalists better as well. SHM does this on a large scale with their advocacy efforts, resulting in more credibility and influence on Capitol Hill.
Make time for friends, family, fitness, fun, and reflection. A sense of humor and an occasional laugh when dealing with ill patients, hospital medicine politics, and the EMR all day provides resilience, as does taking the time to foster self-awareness and insight into your own weaknesses, strengths, and how you react to different stressors. A little bit of exercise and time with family and friends can go a long way towards improving your outlook, work, and life in general, while reducing burnout. Oh yeah, it’s also a good idea to choose a great life partner as well. Thanks Michelle!
Dr. Maynard is chief quality officer, University of California Davis Medical Center, Sacramento, Calif.
Transitioning From Infusion Insulin
Hyperglycemia due to diabetes or stress is prevalent in the intensive care unit (ICU) and general ward setting. Umpierrez et al.1 reported hyperglycemia in 38% of hospitalized ward patients with 26% having a known history of diabetes. While patients with hyperglycemia admitted to the ICU are primarily treated with infusion insulin, those on the general wards usually receive a subcutaneous regimen of insulin. How best to transition patients from infusion insulin to a subcutaneous regimen remains elusive and under evaluated.
A recent observational pilot study of 24 surgical and 17 cardiac/medical intensive care patients at our university‐based hospital found that glycemic control significantly deteriorated when patients with diabetes transitioned from infusion insulin to subcutaneous insulin. A total of 21 critical care patients with a history of diabetes failed to receive basal insulin prior to discontinuation of the drip and developed uncontrolled hyperglycemia (mean glucose Day 1 of 216 mg/dL and Day 2 of 197 mg/dL). Patients without a history of diabetes did well post transition with a mean glucose of 142 mg/dL Day 1 and 133 mg/dL Day 2. A similar study by Czosnowski et al.2 demonstrated a significant increase in blood glucose from 123 26 mg/dL to 168 50 mg/dL upon discontinuation of infusion insulin.
This failed transition is disappointing, especially in view of the existence of a reliable subcutaneous (SC) insulin order set at our institution, but not surprising, as this is an inherently complex process. The severity of illness, the amount and mode of nutritional intake, geographic location, and provider team may all be in flux at the time of this transition. A few centers have demonstrated that a much improved transition is possible,36 however many of these solutions involve technology or incremental personnel that may not be available or the descriptions may lack sufficient detail to implement theses strategies with confidence elsewhere.
Therefore, we designed and piloted a protocol, coordinated by a multidisciplinary team, to transition patients from infusion insulin to SC insulin. The successful implementation of this protocol could serve as a blueprint to other institutions without the need for additional technology or personnel.
Methods
Patient Population/Setting
This was a prospective study of patients admitted to either the medical/cardiac intensive care unit (MICU/CCU) or surgical intensive care unit (SICU) at an academic medical facility and placed on infusion insulin for >24 hours. The Institutional Review Board (IRB) approved the study for prospective chart review and anonymous results reporting without individual consent.
Patients in the SICU were initiated on infusion insulin after 2 blood glucose readings were above 150 mg/dL, whereas initiation was left to the discretion of the attending physician in the MICU/CCU. A computerized system created in‐house recommends insulin infusion rates based on point‐of‐care (POC) glucose measurements with a target range of 91 mg/dL to 150 mg/dL.
Inclusion/Exclusion Criteria
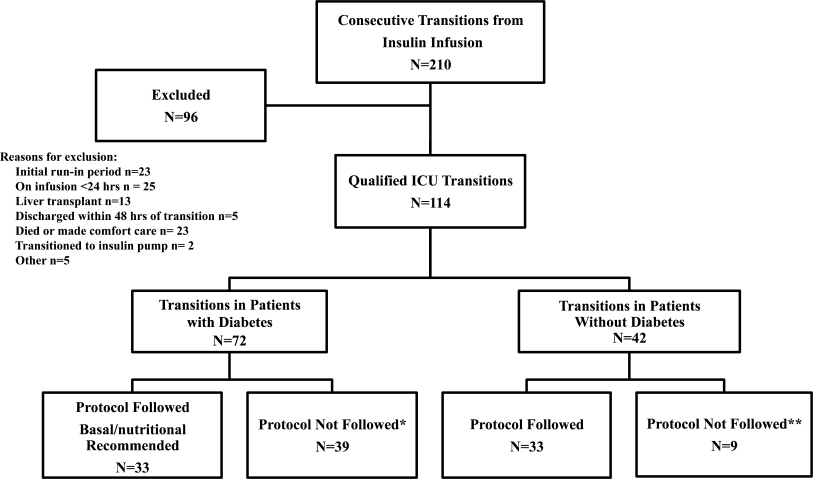
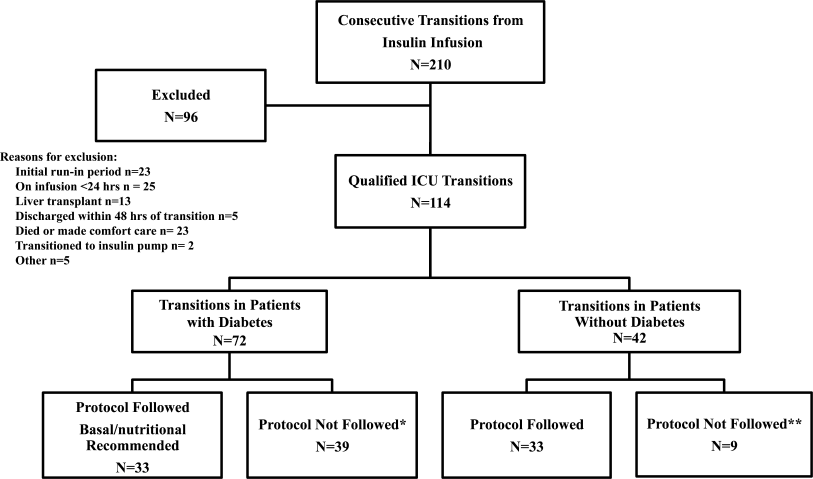
All patients on continuous insulin infusion admitted to the SICU or the MICU/CCU between May 2008 and September 2008 were evaluated for the study (Figure 1). Patients were excluded from analysis if they were on the infusion for less than 24 hours, had a liver transplant, were discharged within 48 hours of transition, were made comfort care or transitioned to an insulin pump. All other patients were included in the final analysis.
Transition Protocol
Step 1: Does the Patient Need Basal SC Insulin?
Patients were recommended to receive basal SC insulin if they either: (1) were on medications for diabetes; (2) had an A1c 6%; or (3) received the equivalent of 60 mg of prednisone; AND had an infusion rate 1 unit/hour (Supporting Information Appendix 1). Patients on infusion insulin due to stress hyperglycemia, regardless of the infusion rate, were not placed on basal SC insulin. Patients on high dose steroids due to spinal injuries were excluded because their duration of steroid use was typically less than 48 hours and usually ended prior to the time of transition. The protocol recommends premeal correctional insulin for those not qualifying for basal insulin.
In order to establish patients in need of basal/nutritional insulin we opted to use A1c as well as past medical history to identify patients with diabetes. The American Diabetes Association (ADA) has recently accepted using an A1c 6.5% to make a new diagnosis of diabetes.7 In a 2‐week trial prior to initiating the protocol we used a cut off A1c of 6.5%. However, we found that patients with an A1c of 6% to 6.5% had poor glucose control post transition; therefore we chose 6% as our identifier. In addition, using a cut off A1c of 6% was reported by Rohlfing et al.8 and Greci et al.9 to be more than 97% sensitive at identifying a new diagnosis of diabetes.
To ensure an A1c was ordered and available at the time of transition, critical care pharmacists were given Pharmacy and Therapeutics Committee authorization to order an A1c at the start of the infusion. Pharmacists would also guide the primary team through the protocol's recommendations as well as alert the project team when a patient was expected to transition.
Step 2: Evaluate the Patient's Nutritional Intake to Calculate the Total Daily Dose (TDD) of Insulin
TDD is the total amount of insulin needed to cover both the nutritional and basal requirements of a patient over the course of 24 hours. TDD was calculated by averaging the hourly drip rate over the prior 6 hours and multiplying by 20 if taking in full nutrition or 40 if taking minimal nutrition while on the drip. A higher multiplier was used for those on minimal nutrition with the expectation that their insulin requirements would double once tolerating a full diet. Full nutrition was defined as eating >50% of meals, on goal tube feeds, or receiving total parenteral nutrition (TPN). Minimal nutrition was defined as taking nothing by mouth (or NPO), tolerating <50% of meals, or on a clear liquid diet.
Step 3: Divide the TDD Into the Appropriate Components of Insulin Treatment (Basal, Nutritional and Correction), Depending on the Nutritional Status
In Step 3, the TDD was evenly divided into basal and nutritional insulin. A total of 50% of the TDD was given as glargine (Lantus) 2 hours prior to stopping the infusion. The remaining 50% was divided into nutritional components as either Regular insulin every 6 hours for patients on tube feeds or lispro (Humalog) before meals if tolerating an oral diet. For patients on minimal nutrition, the 50% nutritional insulin dose was not initiated until the patient was tolerating full nutrition.
The protocol recommended basal insulin administration 2 hours prior to infusion discontinuation as recommended by the American Association of Clinical Endocrinologists (AACE) and ADA consensus statement on inpatient glycemic control as well as pharmacokinetics.10, 11 For these reasons, failure to receive basal insulin prior to transition was viewed as failure to follow the protocol.
Safety features of the protocol included a maximum TDD of 100 units unless the patient was on >100 units/day of insulin prior to admission. A pager was carried by rotating hospitalists or pharmacist study investigators at all hours during the protocol implementation phase to answer any questions regarding a patient's transition.
Data Collection/Monitoring
A multidisciplinary team consisting of hospitalists, ICU pharmacists, critical care physicians and nursing representatives was assembled during the study period. This team was responsible for protocol implementation, data collection, and surveillance of patient response to the protocol. Educational sessions with house staff and nurses in each unit were held prior to the beginning of the study as well as continued monthly educational efforts during the study. In addition, biweekly huddles to review ongoing patient transitions as well as more formal monthly reviews were held.
The primary objective was to improve glycemic control, defined as the mean daily glucose, during the first 48 hours post transition without a significant increase in the percentage of patients with hypoglycemia (41‐70 mg/dL) or severe hypoglycemia (40 mg/dL). Secondary endpoints included the percent of patients with severe hyperglycemia (300 mg/dL), length of stay (LOS) calculated from the day of transition, number of restarts back onto infusion insulin within 72 hours of transition, and day‐weighted glucose mean up to 12 days following transition for patients with diabetes.
Glucose values were collected and averaged over 6‐hour periods for 48 hours post transition. For patients with diabetes, POC glucose values were collected up to 12 days of hospitalization. Day‐weighted means were obtained by calculating the mean glucose for each hospital day, averaged across all hospital days.12
Analysis
Subjects were divided by the presence or absence of diabetes. Those with diabetes were recommended to receive basal SC insulin during the transition period. Within each group, subjects were further divided by adherence to the protocol. Failure to transition per protocol was defined as: not receiving at least 80% of the recommended basal insulin dose, receiving the initial dose of insulin after the drip was discontinued, or receiving basal insulin when none was recommended.
Descriptive statistics within subgroups comparing age, gender, LOS by analysis of variance for continuous data and by chi‐square for nominal data, were compared. Twenty‐four and 48‐hour post transition mean glucose values and the 12 day weighted‐mean glucose were compared using analysis of variance (Stata ver. 10). All data are expressed as mean standard deviation with a significance value established at P < 0.05.
Results
A total of 210 episodes of infusion insulin in ICU patients were evaluated for the study from May of 2008 to September 2008 (Figure 1). Ninety‐six of these episodes were excluded, most commonly due to time on infusion insulin <24 hours or transition to comfort care. The remaining 114 infusions were eligible to use the protocol. Because the protocol recommends insulin therapy based on a diagnosis of diabetes, patients were further divided into these subcategories. Of these 114 transitions, the protocol was followed 66 times (58%).
Patients With Diabetes
(Table 1: Patient Demographics; Table 2: Insulin Use and Glycemic Control; Figure 2: Transition Graph).
| Patients With Diabetes | P Value | Patients Without Diabetes | P Value | |||
|---|---|---|---|---|---|---|
| Protocol Followed, n = 29 Patients* | Protocol NOT Followed, n = 33 Patients | Protocol Followed, n = 30 Patients | Protocol NOT Followed, n = 9 Patients | |||
| ||||||
| Average age, years, mean SD | 57.7 12.1 | 57.8 12.3 | 0.681 | 56.5 18.1 | 62.4 15.5 | 0.532 |
| Male patients | 21 (72%) | 21 (63%) | 0.58 | 20 (66%) | 7 (77%) | 0.691 |
| BMI | 30.7 7.2 | 28.6 6.8 | 0.180 | 27 5.4 | 25.2 3 | 0.081 |
| History of diabetes* | 18 (64%) | 25 (86%) | 0.07 | 0 | 0 | |
| Mean Hgb A1c (%) | 6.61.2 | 7.3 1.8 | 0.136 | 5.6 0.3 | 5.4 0.4 | 0.095 |
| Full nutrition | 26 (79%) | 24 (61%) | 0.131 | 23 (70%) | 9 (100%) | |
| On hemodialysis | 5 (17%) | 9 (27%) | 0.380 | 3 (10%) | 0 | |
| On >60 mg prednisone or equivalent per day | 7 (24%) | 10 (30%) | 0.632 | 0 | 0 | |
| Patients With Diabetes | P Value | Patients Without Diabetes | P Value | |||
|---|---|---|---|---|---|---|
| Protocol Followed, n = 33 transitions | Protocol NOT followed, n = 39 transitions | Protocol Followed, n = 33 transitions | Protocol NOT Followed, n = 9 transitions | |||
| ||||||
| Average infusion rate, hours | 3.96 3.15 | 3.74 3.64 | 0.1597 | 2.34 1.5 | 4.78 1.6 | <0.001 |
| Average BG on infusion insulin (mg/dL) | 122.5 27.5 | 122.5 31.8 | 0.844 | 115.1 22.7 | 127.5 27.2 | 0.006 |
| Average basal dose (units) given | 34.5 14.4 | 14.4 15.3 | <0.001 | 0 | 32.7 | <0.001 |
| Hours before () or after (+) infusion stopped basal insulin given | 1.13 0.9 | 11.6 9.3 | <0.001 | n/a | 0.33 | * |
| Average BG 6 hours post transition (mg/dL) | 143.7 39.4 | 182 62.5 | 0.019 | 150.2 54.9 | 142.1 34.1 | 0.624 |
| Average BG 0 to 24 hours post transition (mg/dL) | 167.98 50.24 | 211.02 81.01 | <0.001 | 150.24 54.9 | 150.12 32.4 | 0.600 |
| Total insulin used from 0 to 24 hours (units) | 65 32.2 | 26.7 25.4 | <0.001 | 3.2 4.1 | 51.3 30.3 | <0.001 |
| Average BG 25 to 48 hours post transition (mg/dL) | 176.1 55.25 | 218.2 88.54 | <0.001 | 153 35.3 | 154.4 46.7 | 0.711 |
| Total insulin used from 25 to 48 hours (units) | 60.5 35.4 | 28.1 24.4 | <0.001 | 2.8 3.8 | 44.9 34 | <0.001 |
| # of patients with severe hypoglycemia (<40 mg/dL) | 1 (3%) | 1 (2.6%) | * | 0 | 1 | * |
| # of patients with hypoglycemia (4170 mg/dL) | 3 (9%) | 2 (5.1%) | * | 1 | 0 | * |
| % of BG values in goal range (80180 mg/dL) (# in range/total #) | 60.2% (153/254) | 38.2% (104/272) | 0.004 | 80.1% (173/216) | 75.4% (49/65) | 0.83 |
| # of patients with severe hyperglycemia (>300 mg/dL) | 5 (15.2%) | 19 (48.7%) | 0.002 | 1 (3%) | 1 (11.1%) | * |
| LOS from transition (days) | 14.6 11.3 | 14 11.4 | 0.836 | 25.3 24.4 | 13.6 7.5 | 0.168 |
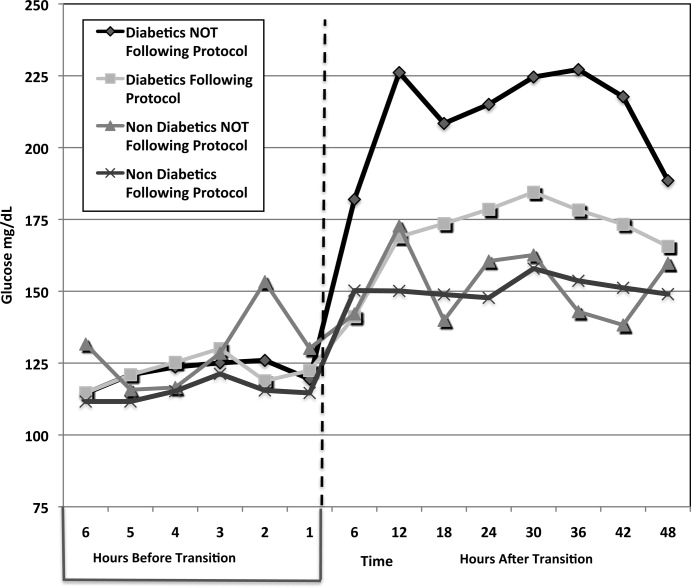
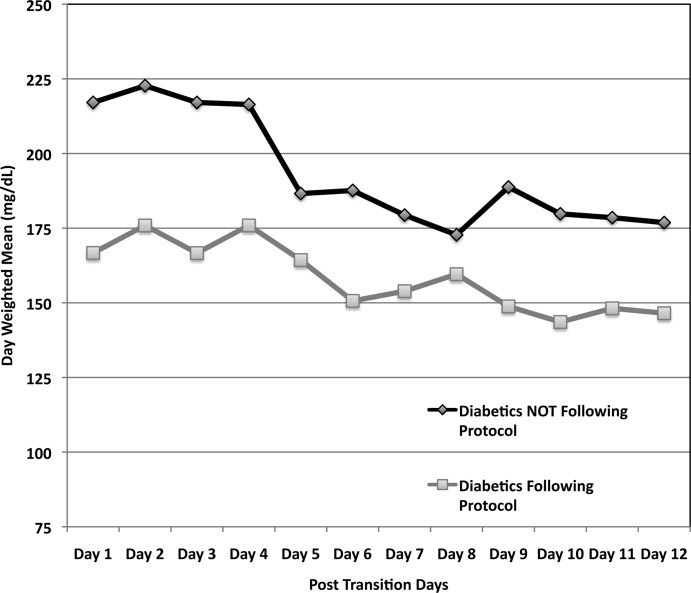
A total of 62 individual patients accounted for 72 separate transitions in patients with diabetes based on past medical history or an A1c 6% (n = 14). Of these 72 transitions, 33 (46%) adhered to the protocol while the remaining 39 (54%) transitions varied from the protocol at the treatment team's discretion. Despite similar insulin infusion rates and mean glucose values pretransition, patients with diabetes following the protocol had better glycemic control at both 24 hours and 48 hours after transition than those patients transitioned without the protocol. Day 1 mean blood glucose was 168 mg/dL vs. 211 mg/dL (P = <0.001) and day 2 mean blood glucose was 176 mg/dL vs. 218 mg/dL (P = <0.001) in protocol vs. nonprotocol patients with diabetes respectively (Figure 2).
There was a severe hypoglycemic event (40 mg/dL) in 1 patient with diabetes following the protocol and 1 patient not following the protocol within 48 hours of transition. Both events were secondary to nutritional‐insulin mismatch with emesis after insulin in one case and tube feeds being held in the second case. These findings were consistent with our prior examination of hypoglycemia cases.13 Severe hyperglycemia (glucose 300mg/dL) occurred in 5 (15 %) patients following the protocol vs. 19 (49%) patients not following protocol (P = 0.002.) Patients with diabetes following the protocol received significantly more insulin in the first 24 hours (mean of 65 units vs. 27 units, P 0.001) and 24 to 48 hours after transition (mean of 61 units vs. 28 units, p0.001) than those not following protocol.
An alternate method used at our institution and others14, 15 to calculate TDD is based on the patient's weight and body habitus. When we compared the projected TDD based on weight with the TDD using the transition protocol, we found that the weight based method was much less aggressive. For patients following the protocol, the weight based method projected a mean TDD of 46.3 16.9 units whereas the protocol projected a mean TDD of 65 33.2 units (P = 0.001).
Patients with diabetes following protocol received basal insulin an average of 1.13 hours prior to discontinuing the insulin infusion versus 11.6 hours after for those not following protocol.
Three patients with diabetes following the protocol and 3 patients with diabetes not following the protocol were restarted on infusion insulin within 72 hours of transition.
LOS from final transition to discharge was similar between protocol vs. nonprotocol patients (14.6 vs. 14 days, P = 0.836).
Figure 3 demonstrates that when used correctly, the protocol provides an extended period of glycemic control up to 12 days post transition. Patients transitioned per protocol had a day‐weighted mean glucose of 155 mg/dL vs. 184 mg/dL (P = 0.043) in patients not following protocol. There was only 1 glucose value less than 40 mg/dL between days 2 to 12 in the protocol group.
Patients Without Diabetes
Of the 39 individual patients without diabetes there were 42 transition events, 33 transitions (78.6%) were per protocol and placed on correctional insulin only. The remaining 9 transitions failed to follow protocol in that basal insulin was prescribed, but these patients maintained comparable glycemic control without an increase in hypoglycemic events. Following transition, patients without diabetes on protocol maintained a mean glucose of 150 mg/dL in the first 24 hours and 153 mg/dL in 24 to 48 hours post transition. They required a mean daily correctional insulin dose of 3.2 units on Day 1 and 2.8 units on Day 2 despite having an average drip rate of 2.3 units/hour at the time of transition (Table 2). There were no severe hypoglycemic events and 80% of blood sugars were within the goal range of 80 mg/dL to 180 mg/dL. Only 1 patient had a single blood glucose of >300mg/dL. No patient was restarted on infusion insulin once transitioned.
Patients without diabetes had a longer LOS after transition off of infusion insulin when compared to their diabetic counterparts (22 vs. 14 days).
Discussion
This study demonstrates the utility of hospitalist‐pharmacist collaboration in the creation and implementation of a safe and effective transition protocol for patients on infusion insulin. The protocol identifies patients appropriate for transition to a basal/nutritional insulin regimen versus those who will do well with premeal correctional insulin alone. Daily mean glucose was improved post transition for diabetic patients following the protocol compared to those not following the protocol without an increase in hypoglycemic events.
We found an equal number of insulin infusion restarts within 72 hours of transition and a similar LOS in protocol vs. nonprotocol patients with diabetes. The LOS was increased for patients without diabetes. This may be due to worse outcomes noted in patients with stress hyperglycemia in other studies.1, 16
The use of the higher multiplier for patients on minimal nutrition led to confusion among many protocol users. The protocol has since been modified to start by averaging the infusion rate over the prior 6 hours and then multiplying by 20 for all patients. This essentially calculates 80% of projected insulin requirements for the next 24 hours based on the patient's current needs. This calculation is then given as 50% basal and 50% nutritional for those on full nutrition vs. 100% basal for those on minimal nutrition. This protocol change has no impact on the amount of insulin received by the patient, but is more intuitive to providers. Instead of calculating the TDD as the projected requirement when full nutrition is obtained, the TDD is now calculated based on current insulin needs, and then doubled when patients who are receiving minimal nutrition advance to full nutrition.
Our study is limited by the lack of a true randomized control group. In lieu of this, we used our patients who did not follow protocol as our control. While not truly randomized, this group is comparable based on their age, gender mix, infusion rate, mean A1c, and projected TDD. This group was also similar to our preprotocol group mentioned in the Introduction.
Additional study limitations include the small number of nondiabetic patients not following the protocol (n = 9). We noted higher infusion rates in nondiabetics not following protocol versus those following protocol, which may have driven the primary team to give basal insulin. It is possible that these 9 patients were not yet ready to transition from infusion insulin or had other stressors not measured in our study. Unfortunately their small population size limits more extensive analysis.
The protocol was followed only 50% of the time for a variety of reasons. Patients who transitioned at night or on weekends were monitored by covering pharmacists and physicians who may not have been familiar with the protocol. Many physicians and nurses remain fearful of hypoglycemia and the outcomes of our study were not yet available for education. Some reported difficulty fully understanding how to use the protocol and why a higher multiplier was used for patients who were on minimal nutrition.
Efforts to improve adherence to the protocol are ongoing with some success, aided by the data demonstrating the safety and efficacy of the transition protocol.
Conclusion
By collaborating with ICU pharmacists we were able to design and implement a protocol that successfully and safely transitioned patients from infusion insulin to subcutaneous insulin. Patients following the protocol had a higher percentage of glucose values within the goal glucose range of 80 mg/dL to 180 mg/dL. In the future, we hope to automate the calculation of TDD and directly recommend a basal/bolus regimen for the clinical provider.
- ,,,,,.Hyperglycemia: an independent marker of in‐hospital mortality in patients with undiagnosed diabetes.J Clin Endocrinol Metab.2002;87:978–982.
- ,,,,,.Evaluation of glycemic control following discontinuation of an intensive insulin protocol.J Hosp Med.2009;4:28–34.
- ,,, et al.Inpatient management of hyperglycemia: the Northwestern experience.Endocr Pract.2006;12:491–505.
- ,,,.Intravenous insulin infusion therapy: indications, methods, and transition to subcutaneous insulin therapy.Endocr Pract.2004;10Suppl 2:71–80.
- ,,, et al.Conversion of intravenous insulin infusions to subcutaneously administered insulin glargine in patients with hyperglycemia.Endocr Pract.2006;12:641–650.
- ,.Effects of outcome on in‐hospital transition from intravenous insulin infusion to subcutaneous therapy.Am J Cardiol.2006;98:557–564.
- International Expert Committee report on the role of the A1C assay in the diagnosis of diabetes.Diabetes Care.2009;32:1327–1334.
- ,,, et al.Use of GHb (HbA1c) in screening for undiagnosed diabetes in the U.S. population.Diabetes Care.2000;23:187–191.
- ,,, et al.Utility of HbA(1c) levels for diabetes case finding in hospitalized patients with hyperglycemia.Diabetes Care.2003;26:1064–1068.
- ,,, et al.American Association of Clinical Endocrinologists and American Diabetes Association consensus statement on inpatient glycemic control.Endocr Pract.2009;15(4):353–369.
- ,,, et al.Pharmacokinetics and pharmacodynamics of subcutaneous injection of long‐acting human insulin analog glargine, NPH insulin, and ultralente human insulin and continuous subcutaneous infusion of insulin lispro.Diabetes.2000;49:2142–2148.
- ,,, et al.“Glucometrics”‐‐assessing the quality of inpatient glucose management.Diabetes Technol Ther.2006;8:560–569.
- ,,.Iatrogenic Inpatient Hypoglycemia: Risk Factors, Treatment, and Prevention: Analysis of Current Practice at an Academic Medical Center With Implications for Improvement Efforts.Diabetes Spectr.2008;21:241–247.
- ,,, et al.Management of diabetes and hyperglycemia in hospitals.Diabetes Care.2004;27:553–591.
- .Insulin management of diabetic patients on general medical and surgical floors.Endocr Pract.2006;12Suppl 3:86–90.
- ,,,.Inadequate blood glucose control is associated with in‐hospital mortality and morbidity in diabetic and nondiabetic patients undergoing cardiac surgery.Circulation.2008;118:113–123.
Hyperglycemia due to diabetes or stress is prevalent in the intensive care unit (ICU) and general ward setting. Umpierrez et al.1 reported hyperglycemia in 38% of hospitalized ward patients with 26% having a known history of diabetes. While patients with hyperglycemia admitted to the ICU are primarily treated with infusion insulin, those on the general wards usually receive a subcutaneous regimen of insulin. How best to transition patients from infusion insulin to a subcutaneous regimen remains elusive and under evaluated.
A recent observational pilot study of 24 surgical and 17 cardiac/medical intensive care patients at our university‐based hospital found that glycemic control significantly deteriorated when patients with diabetes transitioned from infusion insulin to subcutaneous insulin. A total of 21 critical care patients with a history of diabetes failed to receive basal insulin prior to discontinuation of the drip and developed uncontrolled hyperglycemia (mean glucose Day 1 of 216 mg/dL and Day 2 of 197 mg/dL). Patients without a history of diabetes did well post transition with a mean glucose of 142 mg/dL Day 1 and 133 mg/dL Day 2. A similar study by Czosnowski et al.2 demonstrated a significant increase in blood glucose from 123 26 mg/dL to 168 50 mg/dL upon discontinuation of infusion insulin.
This failed transition is disappointing, especially in view of the existence of a reliable subcutaneous (SC) insulin order set at our institution, but not surprising, as this is an inherently complex process. The severity of illness, the amount and mode of nutritional intake, geographic location, and provider team may all be in flux at the time of this transition. A few centers have demonstrated that a much improved transition is possible,36 however many of these solutions involve technology or incremental personnel that may not be available or the descriptions may lack sufficient detail to implement theses strategies with confidence elsewhere.
Therefore, we designed and piloted a protocol, coordinated by a multidisciplinary team, to transition patients from infusion insulin to SC insulin. The successful implementation of this protocol could serve as a blueprint to other institutions without the need for additional technology or personnel.
Methods
Patient Population/Setting
This was a prospective study of patients admitted to either the medical/cardiac intensive care unit (MICU/CCU) or surgical intensive care unit (SICU) at an academic medical facility and placed on infusion insulin for >24 hours. The Institutional Review Board (IRB) approved the study for prospective chart review and anonymous results reporting without individual consent.
Patients in the SICU were initiated on infusion insulin after 2 blood glucose readings were above 150 mg/dL, whereas initiation was left to the discretion of the attending physician in the MICU/CCU. A computerized system created in‐house recommends insulin infusion rates based on point‐of‐care (POC) glucose measurements with a target range of 91 mg/dL to 150 mg/dL.
Inclusion/Exclusion Criteria
All patients on continuous insulin infusion admitted to the SICU or the MICU/CCU between May 2008 and September 2008 were evaluated for the study (Figure 1). Patients were excluded from analysis if they were on the infusion for less than 24 hours, had a liver transplant, were discharged within 48 hours of transition, were made comfort care or transitioned to an insulin pump. All other patients were included in the final analysis.

Transition Protocol
Step 1: Does the Patient Need Basal SC Insulin?
Patients were recommended to receive basal SC insulin if they either: (1) were on medications for diabetes; (2) had an A1c 6%; or (3) received the equivalent of 60 mg of prednisone; AND had an infusion rate 1 unit/hour (Supporting Information Appendix 1). Patients on infusion insulin due to stress hyperglycemia, regardless of the infusion rate, were not placed on basal SC insulin. Patients on high dose steroids due to spinal injuries were excluded because their duration of steroid use was typically less than 48 hours and usually ended prior to the time of transition. The protocol recommends premeal correctional insulin for those not qualifying for basal insulin.
In order to establish patients in need of basal/nutritional insulin we opted to use A1c as well as past medical history to identify patients with diabetes. The American Diabetes Association (ADA) has recently accepted using an A1c 6.5% to make a new diagnosis of diabetes.7 In a 2‐week trial prior to initiating the protocol we used a cut off A1c of 6.5%. However, we found that patients with an A1c of 6% to 6.5% had poor glucose control post transition; therefore we chose 6% as our identifier. In addition, using a cut off A1c of 6% was reported by Rohlfing et al.8 and Greci et al.9 to be more than 97% sensitive at identifying a new diagnosis of diabetes.
To ensure an A1c was ordered and available at the time of transition, critical care pharmacists were given Pharmacy and Therapeutics Committee authorization to order an A1c at the start of the infusion. Pharmacists would also guide the primary team through the protocol's recommendations as well as alert the project team when a patient was expected to transition.
Step 2: Evaluate the Patient's Nutritional Intake to Calculate the Total Daily Dose (TDD) of Insulin
TDD is the total amount of insulin needed to cover both the nutritional and basal requirements of a patient over the course of 24 hours. TDD was calculated by averaging the hourly drip rate over the prior 6 hours and multiplying by 20 if taking in full nutrition or 40 if taking minimal nutrition while on the drip. A higher multiplier was used for those on minimal nutrition with the expectation that their insulin requirements would double once tolerating a full diet. Full nutrition was defined as eating >50% of meals, on goal tube feeds, or receiving total parenteral nutrition (TPN). Minimal nutrition was defined as taking nothing by mouth (or NPO), tolerating <50% of meals, or on a clear liquid diet.
Step 3: Divide the TDD Into the Appropriate Components of Insulin Treatment (Basal, Nutritional and Correction), Depending on the Nutritional Status
In Step 3, the TDD was evenly divided into basal and nutritional insulin. A total of 50% of the TDD was given as glargine (Lantus) 2 hours prior to stopping the infusion. The remaining 50% was divided into nutritional components as either Regular insulin every 6 hours for patients on tube feeds or lispro (Humalog) before meals if tolerating an oral diet. For patients on minimal nutrition, the 50% nutritional insulin dose was not initiated until the patient was tolerating full nutrition.
The protocol recommended basal insulin administration 2 hours prior to infusion discontinuation as recommended by the American Association of Clinical Endocrinologists (AACE) and ADA consensus statement on inpatient glycemic control as well as pharmacokinetics.10, 11 For these reasons, failure to receive basal insulin prior to transition was viewed as failure to follow the protocol.
Safety features of the protocol included a maximum TDD of 100 units unless the patient was on >100 units/day of insulin prior to admission. A pager was carried by rotating hospitalists or pharmacist study investigators at all hours during the protocol implementation phase to answer any questions regarding a patient's transition.
Data Collection/Monitoring
A multidisciplinary team consisting of hospitalists, ICU pharmacists, critical care physicians and nursing representatives was assembled during the study period. This team was responsible for protocol implementation, data collection, and surveillance of patient response to the protocol. Educational sessions with house staff and nurses in each unit were held prior to the beginning of the study as well as continued monthly educational efforts during the study. In addition, biweekly huddles to review ongoing patient transitions as well as more formal monthly reviews were held.
The primary objective was to improve glycemic control, defined as the mean daily glucose, during the first 48 hours post transition without a significant increase in the percentage of patients with hypoglycemia (41‐70 mg/dL) or severe hypoglycemia (40 mg/dL). Secondary endpoints included the percent of patients with severe hyperglycemia (300 mg/dL), length of stay (LOS) calculated from the day of transition, number of restarts back onto infusion insulin within 72 hours of transition, and day‐weighted glucose mean up to 12 days following transition for patients with diabetes.
Glucose values were collected and averaged over 6‐hour periods for 48 hours post transition. For patients with diabetes, POC glucose values were collected up to 12 days of hospitalization. Day‐weighted means were obtained by calculating the mean glucose for each hospital day, averaged across all hospital days.12
Analysis
Subjects were divided by the presence or absence of diabetes. Those with diabetes were recommended to receive basal SC insulin during the transition period. Within each group, subjects were further divided by adherence to the protocol. Failure to transition per protocol was defined as: not receiving at least 80% of the recommended basal insulin dose, receiving the initial dose of insulin after the drip was discontinued, or receiving basal insulin when none was recommended.
Descriptive statistics within subgroups comparing age, gender, LOS by analysis of variance for continuous data and by chi‐square for nominal data, were compared. Twenty‐four and 48‐hour post transition mean glucose values and the 12 day weighted‐mean glucose were compared using analysis of variance (Stata ver. 10). All data are expressed as mean standard deviation with a significance value established at P < 0.05.
Results
A total of 210 episodes of infusion insulin in ICU patients were evaluated for the study from May of 2008 to September 2008 (Figure 1). Ninety‐six of these episodes were excluded, most commonly due to time on infusion insulin <24 hours or transition to comfort care. The remaining 114 infusions were eligible to use the protocol. Because the protocol recommends insulin therapy based on a diagnosis of diabetes, patients were further divided into these subcategories. Of these 114 transitions, the protocol was followed 66 times (58%).
Patients With Diabetes
(Table 1: Patient Demographics; Table 2: Insulin Use and Glycemic Control; Figure 2: Transition Graph).

| Patients With Diabetes | P Value | Patients Without Diabetes | P Value | |||
|---|---|---|---|---|---|---|
| Protocol Followed, n = 29 Patients* | Protocol NOT Followed, n = 33 Patients | Protocol Followed, n = 30 Patients | Protocol NOT Followed, n = 9 Patients | |||
| ||||||
| Average age, years, mean SD | 57.7 12.1 | 57.8 12.3 | 0.681 | 56.5 18.1 | 62.4 15.5 | 0.532 |
| Male patients | 21 (72%) | 21 (63%) | 0.58 | 20 (66%) | 7 (77%) | 0.691 |
| BMI | 30.7 7.2 | 28.6 6.8 | 0.180 | 27 5.4 | 25.2 3 | 0.081 |
| History of diabetes* | 18 (64%) | 25 (86%) | 0.07 | 0 | 0 | |
| Mean Hgb A1c (%) | 6.61.2 | 7.3 1.8 | 0.136 | 5.6 0.3 | 5.4 0.4 | 0.095 |
| Full nutrition | 26 (79%) | 24 (61%) | 0.131 | 23 (70%) | 9 (100%) | |
| On hemodialysis | 5 (17%) | 9 (27%) | 0.380 | 3 (10%) | 0 | |
| On >60 mg prednisone or equivalent per day | 7 (24%) | 10 (30%) | 0.632 | 0 | 0 | |
| Patients With Diabetes | P Value | Patients Without Diabetes | P Value | |||
|---|---|---|---|---|---|---|
| Protocol Followed, n = 33 transitions | Protocol NOT followed, n = 39 transitions | Protocol Followed, n = 33 transitions | Protocol NOT Followed, n = 9 transitions | |||
| ||||||
| Average infusion rate, hours | 3.96 3.15 | 3.74 3.64 | 0.1597 | 2.34 1.5 | 4.78 1.6 | <0.001 |
| Average BG on infusion insulin (mg/dL) | 122.5 27.5 | 122.5 31.8 | 0.844 | 115.1 22.7 | 127.5 27.2 | 0.006 |
| Average basal dose (units) given | 34.5 14.4 | 14.4 15.3 | <0.001 | 0 | 32.7 | <0.001 |
| Hours before () or after (+) infusion stopped basal insulin given | 1.13 0.9 | 11.6 9.3 | <0.001 | n/a | 0.33 | * |
| Average BG 6 hours post transition (mg/dL) | 143.7 39.4 | 182 62.5 | 0.019 | 150.2 54.9 | 142.1 34.1 | 0.624 |
| Average BG 0 to 24 hours post transition (mg/dL) | 167.98 50.24 | 211.02 81.01 | <0.001 | 150.24 54.9 | 150.12 32.4 | 0.600 |
| Total insulin used from 0 to 24 hours (units) | 65 32.2 | 26.7 25.4 | <0.001 | 3.2 4.1 | 51.3 30.3 | <0.001 |
| Average BG 25 to 48 hours post transition (mg/dL) | 176.1 55.25 | 218.2 88.54 | <0.001 | 153 35.3 | 154.4 46.7 | 0.711 |
| Total insulin used from 25 to 48 hours (units) | 60.5 35.4 | 28.1 24.4 | <0.001 | 2.8 3.8 | 44.9 34 | <0.001 |
| # of patients with severe hypoglycemia (<40 mg/dL) | 1 (3%) | 1 (2.6%) | * | 0 | 1 | * |
| # of patients with hypoglycemia (4170 mg/dL) | 3 (9%) | 2 (5.1%) | * | 1 | 0 | * |
| % of BG values in goal range (80180 mg/dL) (# in range/total #) | 60.2% (153/254) | 38.2% (104/272) | 0.004 | 80.1% (173/216) | 75.4% (49/65) | 0.83 |
| # of patients with severe hyperglycemia (>300 mg/dL) | 5 (15.2%) | 19 (48.7%) | 0.002 | 1 (3%) | 1 (11.1%) | * |
| LOS from transition (days) | 14.6 11.3 | 14 11.4 | 0.836 | 25.3 24.4 | 13.6 7.5 | 0.168 |
A total of 62 individual patients accounted for 72 separate transitions in patients with diabetes based on past medical history or an A1c 6% (n = 14). Of these 72 transitions, 33 (46%) adhered to the protocol while the remaining 39 (54%) transitions varied from the protocol at the treatment team's discretion. Despite similar insulin infusion rates and mean glucose values pretransition, patients with diabetes following the protocol had better glycemic control at both 24 hours and 48 hours after transition than those patients transitioned without the protocol. Day 1 mean blood glucose was 168 mg/dL vs. 211 mg/dL (P = <0.001) and day 2 mean blood glucose was 176 mg/dL vs. 218 mg/dL (P = <0.001) in protocol vs. nonprotocol patients with diabetes respectively (Figure 2).
There was a severe hypoglycemic event (40 mg/dL) in 1 patient with diabetes following the protocol and 1 patient not following the protocol within 48 hours of transition. Both events were secondary to nutritional‐insulin mismatch with emesis after insulin in one case and tube feeds being held in the second case. These findings were consistent with our prior examination of hypoglycemia cases.13 Severe hyperglycemia (glucose 300mg/dL) occurred in 5 (15 %) patients following the protocol vs. 19 (49%) patients not following protocol (P = 0.002.) Patients with diabetes following the protocol received significantly more insulin in the first 24 hours (mean of 65 units vs. 27 units, P 0.001) and 24 to 48 hours after transition (mean of 61 units vs. 28 units, p0.001) than those not following protocol.
An alternate method used at our institution and others14, 15 to calculate TDD is based on the patient's weight and body habitus. When we compared the projected TDD based on weight with the TDD using the transition protocol, we found that the weight based method was much less aggressive. For patients following the protocol, the weight based method projected a mean TDD of 46.3 16.9 units whereas the protocol projected a mean TDD of 65 33.2 units (P = 0.001).
Patients with diabetes following protocol received basal insulin an average of 1.13 hours prior to discontinuing the insulin infusion versus 11.6 hours after for those not following protocol.
Three patients with diabetes following the protocol and 3 patients with diabetes not following the protocol were restarted on infusion insulin within 72 hours of transition.
LOS from final transition to discharge was similar between protocol vs. nonprotocol patients (14.6 vs. 14 days, P = 0.836).
Figure 3 demonstrates that when used correctly, the protocol provides an extended period of glycemic control up to 12 days post transition. Patients transitioned per protocol had a day‐weighted mean glucose of 155 mg/dL vs. 184 mg/dL (P = 0.043) in patients not following protocol. There was only 1 glucose value less than 40 mg/dL between days 2 to 12 in the protocol group.

Patients Without Diabetes
Of the 39 individual patients without diabetes there were 42 transition events, 33 transitions (78.6%) were per protocol and placed on correctional insulin only. The remaining 9 transitions failed to follow protocol in that basal insulin was prescribed, but these patients maintained comparable glycemic control without an increase in hypoglycemic events. Following transition, patients without diabetes on protocol maintained a mean glucose of 150 mg/dL in the first 24 hours and 153 mg/dL in 24 to 48 hours post transition. They required a mean daily correctional insulin dose of 3.2 units on Day 1 and 2.8 units on Day 2 despite having an average drip rate of 2.3 units/hour at the time of transition (Table 2). There were no severe hypoglycemic events and 80% of blood sugars were within the goal range of 80 mg/dL to 180 mg/dL. Only 1 patient had a single blood glucose of >300mg/dL. No patient was restarted on infusion insulin once transitioned.
Patients without diabetes had a longer LOS after transition off of infusion insulin when compared to their diabetic counterparts (22 vs. 14 days).
Discussion
This study demonstrates the utility of hospitalist‐pharmacist collaboration in the creation and implementation of a safe and effective transition protocol for patients on infusion insulin. The protocol identifies patients appropriate for transition to a basal/nutritional insulin regimen versus those who will do well with premeal correctional insulin alone. Daily mean glucose was improved post transition for diabetic patients following the protocol compared to those not following the protocol without an increase in hypoglycemic events.
We found an equal number of insulin infusion restarts within 72 hours of transition and a similar LOS in protocol vs. nonprotocol patients with diabetes. The LOS was increased for patients without diabetes. This may be due to worse outcomes noted in patients with stress hyperglycemia in other studies.1, 16
The use of the higher multiplier for patients on minimal nutrition led to confusion among many protocol users. The protocol has since been modified to start by averaging the infusion rate over the prior 6 hours and then multiplying by 20 for all patients. This essentially calculates 80% of projected insulin requirements for the next 24 hours based on the patient's current needs. This calculation is then given as 50% basal and 50% nutritional for those on full nutrition vs. 100% basal for those on minimal nutrition. This protocol change has no impact on the amount of insulin received by the patient, but is more intuitive to providers. Instead of calculating the TDD as the projected requirement when full nutrition is obtained, the TDD is now calculated based on current insulin needs, and then doubled when patients who are receiving minimal nutrition advance to full nutrition.
Our study is limited by the lack of a true randomized control group. In lieu of this, we used our patients who did not follow protocol as our control. While not truly randomized, this group is comparable based on their age, gender mix, infusion rate, mean A1c, and projected TDD. This group was also similar to our preprotocol group mentioned in the Introduction.
Additional study limitations include the small number of nondiabetic patients not following the protocol (n = 9). We noted higher infusion rates in nondiabetics not following protocol versus those following protocol, which may have driven the primary team to give basal insulin. It is possible that these 9 patients were not yet ready to transition from infusion insulin or had other stressors not measured in our study. Unfortunately their small population size limits more extensive analysis.
The protocol was followed only 50% of the time for a variety of reasons. Patients who transitioned at night or on weekends were monitored by covering pharmacists and physicians who may not have been familiar with the protocol. Many physicians and nurses remain fearful of hypoglycemia and the outcomes of our study were not yet available for education. Some reported difficulty fully understanding how to use the protocol and why a higher multiplier was used for patients who were on minimal nutrition.
Efforts to improve adherence to the protocol are ongoing with some success, aided by the data demonstrating the safety and efficacy of the transition protocol.
Conclusion
By collaborating with ICU pharmacists we were able to design and implement a protocol that successfully and safely transitioned patients from infusion insulin to subcutaneous insulin. Patients following the protocol had a higher percentage of glucose values within the goal glucose range of 80 mg/dL to 180 mg/dL. In the future, we hope to automate the calculation of TDD and directly recommend a basal/bolus regimen for the clinical provider.
Hyperglycemia due to diabetes or stress is prevalent in the intensive care unit (ICU) and general ward setting. Umpierrez et al.1 reported hyperglycemia in 38% of hospitalized ward patients with 26% having a known history of diabetes. While patients with hyperglycemia admitted to the ICU are primarily treated with infusion insulin, those on the general wards usually receive a subcutaneous regimen of insulin. How best to transition patients from infusion insulin to a subcutaneous regimen remains elusive and under evaluated.
A recent observational pilot study of 24 surgical and 17 cardiac/medical intensive care patients at our university‐based hospital found that glycemic control significantly deteriorated when patients with diabetes transitioned from infusion insulin to subcutaneous insulin. A total of 21 critical care patients with a history of diabetes failed to receive basal insulin prior to discontinuation of the drip and developed uncontrolled hyperglycemia (mean glucose Day 1 of 216 mg/dL and Day 2 of 197 mg/dL). Patients without a history of diabetes did well post transition with a mean glucose of 142 mg/dL Day 1 and 133 mg/dL Day 2. A similar study by Czosnowski et al.2 demonstrated a significant increase in blood glucose from 123 26 mg/dL to 168 50 mg/dL upon discontinuation of infusion insulin.
This failed transition is disappointing, especially in view of the existence of a reliable subcutaneous (SC) insulin order set at our institution, but not surprising, as this is an inherently complex process. The severity of illness, the amount and mode of nutritional intake, geographic location, and provider team may all be in flux at the time of this transition. A few centers have demonstrated that a much improved transition is possible,36 however many of these solutions involve technology or incremental personnel that may not be available or the descriptions may lack sufficient detail to implement theses strategies with confidence elsewhere.
Therefore, we designed and piloted a protocol, coordinated by a multidisciplinary team, to transition patients from infusion insulin to SC insulin. The successful implementation of this protocol could serve as a blueprint to other institutions without the need for additional technology or personnel.
Methods
Patient Population/Setting
This was a prospective study of patients admitted to either the medical/cardiac intensive care unit (MICU/CCU) or surgical intensive care unit (SICU) at an academic medical facility and placed on infusion insulin for >24 hours. The Institutional Review Board (IRB) approved the study for prospective chart review and anonymous results reporting without individual consent.
Patients in the SICU were initiated on infusion insulin after 2 blood glucose readings were above 150 mg/dL, whereas initiation was left to the discretion of the attending physician in the MICU/CCU. A computerized system created in‐house recommends insulin infusion rates based on point‐of‐care (POC) glucose measurements with a target range of 91 mg/dL to 150 mg/dL.
Inclusion/Exclusion Criteria
All patients on continuous insulin infusion admitted to the SICU or the MICU/CCU between May 2008 and September 2008 were evaluated for the study (Figure 1). Patients were excluded from analysis if they were on the infusion for less than 24 hours, had a liver transplant, were discharged within 48 hours of transition, were made comfort care or transitioned to an insulin pump. All other patients were included in the final analysis.

Transition Protocol
Step 1: Does the Patient Need Basal SC Insulin?
Patients were recommended to receive basal SC insulin if they either: (1) were on medications for diabetes; (2) had an A1c 6%; or (3) received the equivalent of 60 mg of prednisone; AND had an infusion rate 1 unit/hour (Supporting Information Appendix 1). Patients on infusion insulin due to stress hyperglycemia, regardless of the infusion rate, were not placed on basal SC insulin. Patients on high dose steroids due to spinal injuries were excluded because their duration of steroid use was typically less than 48 hours and usually ended prior to the time of transition. The protocol recommends premeal correctional insulin for those not qualifying for basal insulin.
In order to establish patients in need of basal/nutritional insulin we opted to use A1c as well as past medical history to identify patients with diabetes. The American Diabetes Association (ADA) has recently accepted using an A1c 6.5% to make a new diagnosis of diabetes.7 In a 2‐week trial prior to initiating the protocol we used a cut off A1c of 6.5%. However, we found that patients with an A1c of 6% to 6.5% had poor glucose control post transition; therefore we chose 6% as our identifier. In addition, using a cut off A1c of 6% was reported by Rohlfing et al.8 and Greci et al.9 to be more than 97% sensitive at identifying a new diagnosis of diabetes.
To ensure an A1c was ordered and available at the time of transition, critical care pharmacists were given Pharmacy and Therapeutics Committee authorization to order an A1c at the start of the infusion. Pharmacists would also guide the primary team through the protocol's recommendations as well as alert the project team when a patient was expected to transition.
Step 2: Evaluate the Patient's Nutritional Intake to Calculate the Total Daily Dose (TDD) of Insulin
TDD is the total amount of insulin needed to cover both the nutritional and basal requirements of a patient over the course of 24 hours. TDD was calculated by averaging the hourly drip rate over the prior 6 hours and multiplying by 20 if taking in full nutrition or 40 if taking minimal nutrition while on the drip. A higher multiplier was used for those on minimal nutrition with the expectation that their insulin requirements would double once tolerating a full diet. Full nutrition was defined as eating >50% of meals, on goal tube feeds, or receiving total parenteral nutrition (TPN). Minimal nutrition was defined as taking nothing by mouth (or NPO), tolerating <50% of meals, or on a clear liquid diet.
Step 3: Divide the TDD Into the Appropriate Components of Insulin Treatment (Basal, Nutritional and Correction), Depending on the Nutritional Status
In Step 3, the TDD was evenly divided into basal and nutritional insulin. A total of 50% of the TDD was given as glargine (Lantus) 2 hours prior to stopping the infusion. The remaining 50% was divided into nutritional components as either Regular insulin every 6 hours for patients on tube feeds or lispro (Humalog) before meals if tolerating an oral diet. For patients on minimal nutrition, the 50% nutritional insulin dose was not initiated until the patient was tolerating full nutrition.
The protocol recommended basal insulin administration 2 hours prior to infusion discontinuation as recommended by the American Association of Clinical Endocrinologists (AACE) and ADA consensus statement on inpatient glycemic control as well as pharmacokinetics.10, 11 For these reasons, failure to receive basal insulin prior to transition was viewed as failure to follow the protocol.
Safety features of the protocol included a maximum TDD of 100 units unless the patient was on >100 units/day of insulin prior to admission. A pager was carried by rotating hospitalists or pharmacist study investigators at all hours during the protocol implementation phase to answer any questions regarding a patient's transition.
Data Collection/Monitoring
A multidisciplinary team consisting of hospitalists, ICU pharmacists, critical care physicians and nursing representatives was assembled during the study period. This team was responsible for protocol implementation, data collection, and surveillance of patient response to the protocol. Educational sessions with house staff and nurses in each unit were held prior to the beginning of the study as well as continued monthly educational efforts during the study. In addition, biweekly huddles to review ongoing patient transitions as well as more formal monthly reviews were held.
The primary objective was to improve glycemic control, defined as the mean daily glucose, during the first 48 hours post transition without a significant increase in the percentage of patients with hypoglycemia (41‐70 mg/dL) or severe hypoglycemia (40 mg/dL). Secondary endpoints included the percent of patients with severe hyperglycemia (300 mg/dL), length of stay (LOS) calculated from the day of transition, number of restarts back onto infusion insulin within 72 hours of transition, and day‐weighted glucose mean up to 12 days following transition for patients with diabetes.
Glucose values were collected and averaged over 6‐hour periods for 48 hours post transition. For patients with diabetes, POC glucose values were collected up to 12 days of hospitalization. Day‐weighted means were obtained by calculating the mean glucose for each hospital day, averaged across all hospital days.12
Analysis
Subjects were divided by the presence or absence of diabetes. Those with diabetes were recommended to receive basal SC insulin during the transition period. Within each group, subjects were further divided by adherence to the protocol. Failure to transition per protocol was defined as: not receiving at least 80% of the recommended basal insulin dose, receiving the initial dose of insulin after the drip was discontinued, or receiving basal insulin when none was recommended.
Descriptive statistics within subgroups comparing age, gender, LOS by analysis of variance for continuous data and by chi‐square for nominal data, were compared. Twenty‐four and 48‐hour post transition mean glucose values and the 12 day weighted‐mean glucose were compared using analysis of variance (Stata ver. 10). All data are expressed as mean standard deviation with a significance value established at P < 0.05.
Results
A total of 210 episodes of infusion insulin in ICU patients were evaluated for the study from May of 2008 to September 2008 (Figure 1). Ninety‐six of these episodes were excluded, most commonly due to time on infusion insulin <24 hours or transition to comfort care. The remaining 114 infusions were eligible to use the protocol. Because the protocol recommends insulin therapy based on a diagnosis of diabetes, patients were further divided into these subcategories. Of these 114 transitions, the protocol was followed 66 times (58%).
Patients With Diabetes
(Table 1: Patient Demographics; Table 2: Insulin Use and Glycemic Control; Figure 2: Transition Graph).

| Patients With Diabetes | P Value | Patients Without Diabetes | P Value | |||
|---|---|---|---|---|---|---|
| Protocol Followed, n = 29 Patients* | Protocol NOT Followed, n = 33 Patients | Protocol Followed, n = 30 Patients | Protocol NOT Followed, n = 9 Patients | |||
| ||||||
| Average age, years, mean SD | 57.7 12.1 | 57.8 12.3 | 0.681 | 56.5 18.1 | 62.4 15.5 | 0.532 |
| Male patients | 21 (72%) | 21 (63%) | 0.58 | 20 (66%) | 7 (77%) | 0.691 |
| BMI | 30.7 7.2 | 28.6 6.8 | 0.180 | 27 5.4 | 25.2 3 | 0.081 |
| History of diabetes* | 18 (64%) | 25 (86%) | 0.07 | 0 | 0 | |
| Mean Hgb A1c (%) | 6.61.2 | 7.3 1.8 | 0.136 | 5.6 0.3 | 5.4 0.4 | 0.095 |
| Full nutrition | 26 (79%) | 24 (61%) | 0.131 | 23 (70%) | 9 (100%) | |
| On hemodialysis | 5 (17%) | 9 (27%) | 0.380 | 3 (10%) | 0 | |
| On >60 mg prednisone or equivalent per day | 7 (24%) | 10 (30%) | 0.632 | 0 | 0 | |
| Patients With Diabetes | P Value | Patients Without Diabetes | P Value | |||
|---|---|---|---|---|---|---|
| Protocol Followed, n = 33 transitions | Protocol NOT followed, n = 39 transitions | Protocol Followed, n = 33 transitions | Protocol NOT Followed, n = 9 transitions | |||
| ||||||
| Average infusion rate, hours | 3.96 3.15 | 3.74 3.64 | 0.1597 | 2.34 1.5 | 4.78 1.6 | <0.001 |
| Average BG on infusion insulin (mg/dL) | 122.5 27.5 | 122.5 31.8 | 0.844 | 115.1 22.7 | 127.5 27.2 | 0.006 |
| Average basal dose (units) given | 34.5 14.4 | 14.4 15.3 | <0.001 | 0 | 32.7 | <0.001 |
| Hours before () or after (+) infusion stopped basal insulin given | 1.13 0.9 | 11.6 9.3 | <0.001 | n/a | 0.33 | * |
| Average BG 6 hours post transition (mg/dL) | 143.7 39.4 | 182 62.5 | 0.019 | 150.2 54.9 | 142.1 34.1 | 0.624 |
| Average BG 0 to 24 hours post transition (mg/dL) | 167.98 50.24 | 211.02 81.01 | <0.001 | 150.24 54.9 | 150.12 32.4 | 0.600 |
| Total insulin used from 0 to 24 hours (units) | 65 32.2 | 26.7 25.4 | <0.001 | 3.2 4.1 | 51.3 30.3 | <0.001 |
| Average BG 25 to 48 hours post transition (mg/dL) | 176.1 55.25 | 218.2 88.54 | <0.001 | 153 35.3 | 154.4 46.7 | 0.711 |
| Total insulin used from 25 to 48 hours (units) | 60.5 35.4 | 28.1 24.4 | <0.001 | 2.8 3.8 | 44.9 34 | <0.001 |
| # of patients with severe hypoglycemia (<40 mg/dL) | 1 (3%) | 1 (2.6%) | * | 0 | 1 | * |
| # of patients with hypoglycemia (4170 mg/dL) | 3 (9%) | 2 (5.1%) | * | 1 | 0 | * |
| % of BG values in goal range (80180 mg/dL) (# in range/total #) | 60.2% (153/254) | 38.2% (104/272) | 0.004 | 80.1% (173/216) | 75.4% (49/65) | 0.83 |
| # of patients with severe hyperglycemia (>300 mg/dL) | 5 (15.2%) | 19 (48.7%) | 0.002 | 1 (3%) | 1 (11.1%) | * |
| LOS from transition (days) | 14.6 11.3 | 14 11.4 | 0.836 | 25.3 24.4 | 13.6 7.5 | 0.168 |
A total of 62 individual patients accounted for 72 separate transitions in patients with diabetes based on past medical history or an A1c 6% (n = 14). Of these 72 transitions, 33 (46%) adhered to the protocol while the remaining 39 (54%) transitions varied from the protocol at the treatment team's discretion. Despite similar insulin infusion rates and mean glucose values pretransition, patients with diabetes following the protocol had better glycemic control at both 24 hours and 48 hours after transition than those patients transitioned without the protocol. Day 1 mean blood glucose was 168 mg/dL vs. 211 mg/dL (P = <0.001) and day 2 mean blood glucose was 176 mg/dL vs. 218 mg/dL (P = <0.001) in protocol vs. nonprotocol patients with diabetes respectively (Figure 2).
There was a severe hypoglycemic event (40 mg/dL) in 1 patient with diabetes following the protocol and 1 patient not following the protocol within 48 hours of transition. Both events were secondary to nutritional‐insulin mismatch with emesis after insulin in one case and tube feeds being held in the second case. These findings were consistent with our prior examination of hypoglycemia cases.13 Severe hyperglycemia (glucose 300mg/dL) occurred in 5 (15 %) patients following the protocol vs. 19 (49%) patients not following protocol (P = 0.002.) Patients with diabetes following the protocol received significantly more insulin in the first 24 hours (mean of 65 units vs. 27 units, P 0.001) and 24 to 48 hours after transition (mean of 61 units vs. 28 units, p0.001) than those not following protocol.
An alternate method used at our institution and others14, 15 to calculate TDD is based on the patient's weight and body habitus. When we compared the projected TDD based on weight with the TDD using the transition protocol, we found that the weight based method was much less aggressive. For patients following the protocol, the weight based method projected a mean TDD of 46.3 16.9 units whereas the protocol projected a mean TDD of 65 33.2 units (P = 0.001).
Patients with diabetes following protocol received basal insulin an average of 1.13 hours prior to discontinuing the insulin infusion versus 11.6 hours after for those not following protocol.
Three patients with diabetes following the protocol and 3 patients with diabetes not following the protocol were restarted on infusion insulin within 72 hours of transition.
LOS from final transition to discharge was similar between protocol vs. nonprotocol patients (14.6 vs. 14 days, P = 0.836).
Figure 3 demonstrates that when used correctly, the protocol provides an extended period of glycemic control up to 12 days post transition. Patients transitioned per protocol had a day‐weighted mean glucose of 155 mg/dL vs. 184 mg/dL (P = 0.043) in patients not following protocol. There was only 1 glucose value less than 40 mg/dL between days 2 to 12 in the protocol group.

Patients Without Diabetes
Of the 39 individual patients without diabetes there were 42 transition events, 33 transitions (78.6%) were per protocol and placed on correctional insulin only. The remaining 9 transitions failed to follow protocol in that basal insulin was prescribed, but these patients maintained comparable glycemic control without an increase in hypoglycemic events. Following transition, patients without diabetes on protocol maintained a mean glucose of 150 mg/dL in the first 24 hours and 153 mg/dL in 24 to 48 hours post transition. They required a mean daily correctional insulin dose of 3.2 units on Day 1 and 2.8 units on Day 2 despite having an average drip rate of 2.3 units/hour at the time of transition (Table 2). There were no severe hypoglycemic events and 80% of blood sugars were within the goal range of 80 mg/dL to 180 mg/dL. Only 1 patient had a single blood glucose of >300mg/dL. No patient was restarted on infusion insulin once transitioned.
Patients without diabetes had a longer LOS after transition off of infusion insulin when compared to their diabetic counterparts (22 vs. 14 days).
Discussion
This study demonstrates the utility of hospitalist‐pharmacist collaboration in the creation and implementation of a safe and effective transition protocol for patients on infusion insulin. The protocol identifies patients appropriate for transition to a basal/nutritional insulin regimen versus those who will do well with premeal correctional insulin alone. Daily mean glucose was improved post transition for diabetic patients following the protocol compared to those not following the protocol without an increase in hypoglycemic events.
We found an equal number of insulin infusion restarts within 72 hours of transition and a similar LOS in protocol vs. nonprotocol patients with diabetes. The LOS was increased for patients without diabetes. This may be due to worse outcomes noted in patients with stress hyperglycemia in other studies.1, 16
The use of the higher multiplier for patients on minimal nutrition led to confusion among many protocol users. The protocol has since been modified to start by averaging the infusion rate over the prior 6 hours and then multiplying by 20 for all patients. This essentially calculates 80% of projected insulin requirements for the next 24 hours based on the patient's current needs. This calculation is then given as 50% basal and 50% nutritional for those on full nutrition vs. 100% basal for those on minimal nutrition. This protocol change has no impact on the amount of insulin received by the patient, but is more intuitive to providers. Instead of calculating the TDD as the projected requirement when full nutrition is obtained, the TDD is now calculated based on current insulin needs, and then doubled when patients who are receiving minimal nutrition advance to full nutrition.
Our study is limited by the lack of a true randomized control group. In lieu of this, we used our patients who did not follow protocol as our control. While not truly randomized, this group is comparable based on their age, gender mix, infusion rate, mean A1c, and projected TDD. This group was also similar to our preprotocol group mentioned in the Introduction.
Additional study limitations include the small number of nondiabetic patients not following the protocol (n = 9). We noted higher infusion rates in nondiabetics not following protocol versus those following protocol, which may have driven the primary team to give basal insulin. It is possible that these 9 patients were not yet ready to transition from infusion insulin or had other stressors not measured in our study. Unfortunately their small population size limits more extensive analysis.
The protocol was followed only 50% of the time for a variety of reasons. Patients who transitioned at night or on weekends were monitored by covering pharmacists and physicians who may not have been familiar with the protocol. Many physicians and nurses remain fearful of hypoglycemia and the outcomes of our study were not yet available for education. Some reported difficulty fully understanding how to use the protocol and why a higher multiplier was used for patients who were on minimal nutrition.
Efforts to improve adherence to the protocol are ongoing with some success, aided by the data demonstrating the safety and efficacy of the transition protocol.
Conclusion
By collaborating with ICU pharmacists we were able to design and implement a protocol that successfully and safely transitioned patients from infusion insulin to subcutaneous insulin. Patients following the protocol had a higher percentage of glucose values within the goal glucose range of 80 mg/dL to 180 mg/dL. In the future, we hope to automate the calculation of TDD and directly recommend a basal/bolus regimen for the clinical provider.
- ,,,,,.Hyperglycemia: an independent marker of in‐hospital mortality in patients with undiagnosed diabetes.J Clin Endocrinol Metab.2002;87:978–982.
- ,,,,,.Evaluation of glycemic control following discontinuation of an intensive insulin protocol.J Hosp Med.2009;4:28–34.
- ,,, et al.Inpatient management of hyperglycemia: the Northwestern experience.Endocr Pract.2006;12:491–505.
- ,,,.Intravenous insulin infusion therapy: indications, methods, and transition to subcutaneous insulin therapy.Endocr Pract.2004;10Suppl 2:71–80.
- ,,, et al.Conversion of intravenous insulin infusions to subcutaneously administered insulin glargine in patients with hyperglycemia.Endocr Pract.2006;12:641–650.
- ,.Effects of outcome on in‐hospital transition from intravenous insulin infusion to subcutaneous therapy.Am J Cardiol.2006;98:557–564.
- International Expert Committee report on the role of the A1C assay in the diagnosis of diabetes.Diabetes Care.2009;32:1327–1334.
- ,,, et al.Use of GHb (HbA1c) in screening for undiagnosed diabetes in the U.S. population.Diabetes Care.2000;23:187–191.
- ,,, et al.Utility of HbA(1c) levels for diabetes case finding in hospitalized patients with hyperglycemia.Diabetes Care.2003;26:1064–1068.
- ,,, et al.American Association of Clinical Endocrinologists and American Diabetes Association consensus statement on inpatient glycemic control.Endocr Pract.2009;15(4):353–369.
- ,,, et al.Pharmacokinetics and pharmacodynamics of subcutaneous injection of long‐acting human insulin analog glargine, NPH insulin, and ultralente human insulin and continuous subcutaneous infusion of insulin lispro.Diabetes.2000;49:2142–2148.
- ,,, et al.“Glucometrics”‐‐assessing the quality of inpatient glucose management.Diabetes Technol Ther.2006;8:560–569.
- ,,.Iatrogenic Inpatient Hypoglycemia: Risk Factors, Treatment, and Prevention: Analysis of Current Practice at an Academic Medical Center With Implications for Improvement Efforts.Diabetes Spectr.2008;21:241–247.
- ,,, et al.Management of diabetes and hyperglycemia in hospitals.Diabetes Care.2004;27:553–591.
- .Insulin management of diabetic patients on general medical and surgical floors.Endocr Pract.2006;12Suppl 3:86–90.
- ,,,.Inadequate blood glucose control is associated with in‐hospital mortality and morbidity in diabetic and nondiabetic patients undergoing cardiac surgery.Circulation.2008;118:113–123.
- ,,,,,.Hyperglycemia: an independent marker of in‐hospital mortality in patients with undiagnosed diabetes.J Clin Endocrinol Metab.2002;87:978–982.
- ,,,,,.Evaluation of glycemic control following discontinuation of an intensive insulin protocol.J Hosp Med.2009;4:28–34.
- ,,, et al.Inpatient management of hyperglycemia: the Northwestern experience.Endocr Pract.2006;12:491–505.
- ,,,.Intravenous insulin infusion therapy: indications, methods, and transition to subcutaneous insulin therapy.Endocr Pract.2004;10Suppl 2:71–80.
- ,,, et al.Conversion of intravenous insulin infusions to subcutaneously administered insulin glargine in patients with hyperglycemia.Endocr Pract.2006;12:641–650.
- ,.Effects of outcome on in‐hospital transition from intravenous insulin infusion to subcutaneous therapy.Am J Cardiol.2006;98:557–564.
- International Expert Committee report on the role of the A1C assay in the diagnosis of diabetes.Diabetes Care.2009;32:1327–1334.
- ,,, et al.Use of GHb (HbA1c) in screening for undiagnosed diabetes in the U.S. population.Diabetes Care.2000;23:187–191.
- ,,, et al.Utility of HbA(1c) levels for diabetes case finding in hospitalized patients with hyperglycemia.Diabetes Care.2003;26:1064–1068.
- ,,, et al.American Association of Clinical Endocrinologists and American Diabetes Association consensus statement on inpatient glycemic control.Endocr Pract.2009;15(4):353–369.
- ,,, et al.Pharmacokinetics and pharmacodynamics of subcutaneous injection of long‐acting human insulin analog glargine, NPH insulin, and ultralente human insulin and continuous subcutaneous infusion of insulin lispro.Diabetes.2000;49:2142–2148.
- ,,, et al.“Glucometrics”‐‐assessing the quality of inpatient glucose management.Diabetes Technol Ther.2006;8:560–569.
- ,,.Iatrogenic Inpatient Hypoglycemia: Risk Factors, Treatment, and Prevention: Analysis of Current Practice at an Academic Medical Center With Implications for Improvement Efforts.Diabetes Spectr.2008;21:241–247.
- ,,, et al.Management of diabetes and hyperglycemia in hospitals.Diabetes Care.2004;27:553–591.
- .Insulin management of diabetic patients on general medical and surgical floors.Endocr Pract.2006;12Suppl 3:86–90.
- ,,,.Inadequate blood glucose control is associated with in‐hospital mortality and morbidity in diabetic and nondiabetic patients undergoing cardiac surgery.Circulation.2008;118:113–123.
Copyright © 2010 Society of Hospital Medicine
Improved Glycemic Control and Hypoglycemia
Diabetes has reached epidemic proportions in the United States, affecting over 20 million individuals,1 and further rises are expected. A disproportionate increase in diabetes has occurred in the inpatient setting.2 Furthermore, for every 2 patients in the hospital with known diabetes, there may be an additional 1 with newly observed hyperglycemia. Both are common. In 1 report, for example, 24% of inpatients with hyperglycemia had a prior diagnosis of diabetes, whereas another 12% had hyperglycemia without a prior diagnosis of diabetes.3
Although there is a paucity of high quality randomized controlled trials to support tight glycemic control in non‐critical care inpatient settings, poor glycemic control in hospitalized patients is strongly associated with undesirable outcomes for a variety of conditions, including pneumonia,4 cancer chemotherapy,5 renal transplant,6 and postsurgical wound infections.7, 8 Hyperglycemia also induces dehydration, fluid and electrolyte imbalance, gastric motility problems, and venous thromboembolism formation.9
Structured subcutaneous insulin order sets and insulin management protocols have been widely advocated as a method to encourage basal bolus insulin regimens and enhance glycemic control,2, 9, 10 but the effect of these interventions on glycemic control, hypoglycemia, and insulin use patterns in the real world setting has not been well reported. Fear of inducing hypoglycemia is often the main barrier for initiating basal insulin containing regimens and pursuing glycemic targets.2 The evidence would suggest, however, that sliding scale regimens, as opposed to more physiologic basal bolus regimens, may actually increase both hypoglycemic and hyperglycemic excursions.11 A convincing demonstration of the efficacy (improved insulin use patterns and reduced hyperglycemia) and safety (reduced hypoglycemia) of structured insulin order sets and insulin management protocols would foster a more rapid adoption of these strategies.
PATIENTS AND METHODS
In our 400‐bed university hospital, we formed a hospitalist‐led multidisciplinary team in early 2003, with the focus of improving the care delivered to non‐critical care patients with diabetes or hyperglycemia. We used a Plan‐Do‐Study‐Act (PDSA) performance improvement framework, and conducted institutional review board (IRB)‐approved prospective observational research in parallel with the performance improvement efforts, with a waiver for individual informed consent. The study population consisted of all adult inpatients on non‐critical care units with electronically reported point of care (POC) glucose testing from November 2002 through December 2005. We excluded patients who did not have either a discharge diagnosis of Diabetes (ICD 9 codes 250‐251.XX) or demonstrated hyperglycemia (fasting POC glucose >130 mg/dL 2, or a random value of >180 mg/dL) from analysis of glycemic control and hypoglycemia. Women admitted to Obstetrics were excluded. Monthly and quarterly summaries on glycemic control, hypoglycemia, and insulin use patterns (metrics described below) were reported to the improvement team and other groups on a regular basis throughout the intervention period. POC glucose data, demographics, markers of severity of illness, and diagnosis codes were retrieved from the electronic health record.
Interventions
We introduced several interventions and educational efforts throughout the course of our improvement. The 2 key interventions were as follows:
Structured subcutaneous insulin order sets (November, 2003).
An insulin management algorithm, described below (May 2005).
Key Intervention #1: Structured Subcutaneous Insulin Order Set Implementation
In November 2003, we introduced a paper‐based structured subcutaneous insulin order set. This order set encouraged the use of scheduled basal and nutritional insulin, provided guidance for monitoring glucose levels, and for insulin dosing. A hypoglycemia protocol and a standardized correction insulin table were embedded in the order set. This set was similar to examples of structured insulin ordering subsequently presented in the literature.9 In a parallel effort, the University of California, San Diego Medical Center (UCSDMC) was developing a computer physician order entry (CPOE) module for our comprehensive clinical information system, Invision (Siemens Medical Systems, Malvern, PA), that heretofore had primarily focused on result review, patient schedule management, and nursing documentation. In anticipation of CPOE and for the purpose of standardization, we removed outdated sliding scale insulin regimens from a variety preexisting order sets and inserted references to the standardized subcutaneous insulin order set in their stead. The medication administration record (MAR) was changed to reflect the basal/nutritional/correction insulin terminology. It became more difficult to order a stand‐alone insulin sliding scale even before CPOE versions became available. The standardized order set was the only preprinted correction scale insulin order available, and ordering physicians have to specifically opt out of basal and nutritional insulin choices to order sliding scale only regimens. Verbal orders for correction dose scales were deemed unacceptable by medical staff committees. Correctional insulin doses could be ordered as a 1‐time order, but the pharmacy rejected ongoing insulin orders that were not entered on the structured form.
We introduced our first standardized CPOE subcutaneous insulin order set in January 2004 at the smaller of our 2 campuses, and subsequently completed full deployment across both campuses in all adult medical‐surgical care areas by September 2004.
The CPOE version, like the paper version that immediately preceded it, encouraged the use of basal/bolus insulin regimens, promoted the terms basal, nutritional or premeal, and adjustment dose insulin in the order sets and the medication administration record, and was mandatory for providers wishing to order anything but a 1‐time order of insulin. Figure 1 depicts a screen shot of the CPOE version. Similar to the paper version, the ordering physician had to specifically opt out of ordering scheduled premeal and basal insulin to order a sliding scale only regimen. The first screen also ensured that appropriate POC glucose monitoring was ordered and endorsed a standing hypoglycemia protocol order. The CPOE version had only a few additional features not possible on paper. Obvious benefits included elimination of unapproved abbreviations and handwriting errors. Nutritional and correction insulin types were forced to be identical. Fundamentally, however, both the paper and online structured ordering experiences had the same degree of control over provider ordering patterns, and there was no increment in guidance for choosing insulin regimens, hence their combined analysis as structured orders.
Key Intervention #2: Insulin Management Algorithm
The structured insulin order set had many advantages, but also had many limitations. Guidance for preferred insulin regimens for patients in different nutritional situations was not inherent in the order set, and all basal and nutritional insulin options were offered as equally acceptable choices. The order set gave very general guidance for insulin dosing, but did not calculate insulin doses or assist in the apportionment of insulin between basal and nutritional components, and guidance for setting a glycemic target or adjusting insulin was lacking.
Recognizing these limitations, we devised an insulin management algorithm to provide guidance incremental to that offered in the order set. In April 2005, 3 hospitalists piloted a paper‐based insulin management algorithm (Figure 2, front; Figure 3, reverse) on their teaching services. This 1‐page algorithm provided guidance on insulin dosing and monitoring, and provided institutionally preferred insulin regimens for patients in different nutritional situations. As an example, of the several acceptable subcutaneous insulin regimens that an eating patient might use in the inpatient setting, we advocated the use of 1 preferred regimen (a relatively peakless, long‐acting basal insulin once a day, along with a rapid acting analog nutritional insulin with each meal). We introduced the concept of a ward glycemic target, provided prompts for diabetes education, and generally recommended discontinuation of oral hypoglycemic agents in the inpatient setting. The hospitalists were introduced to the concepts and the algorithm via 1 of the authors (G.M.) in a 1‐hour session. The algorithm was introduced on each teaching team during routine teaching rounds with a slide set (approximately 15 slides) that outlined the basic principles of insulin dosing, and gave example cases which modeled the proper use of the algorithm. The principles were reinforced on daily patient work rounds as they were applied on inpatients with hyperglycemia. The pilot results on 25 patients, compared to 250 historical control patients, were very promising, with markedly improved glycemic control and no increase in hypoglycemia. We therefore sought to spread the use of the algorithm. In May 2005 the insulin management algorithm and teaching slide set were promoted on all 7 hospitalist‐run services, and the results of the pilot and concepts of the algorithm were shared with a variety of house staff and service leaders in approximately a dozen sessions: educational grand rounds, assorted noon lectures, and subsequently, at new intern orientations. Easy access to the algorithm was assured by providing a link to the file within the CPOE insulin order set.
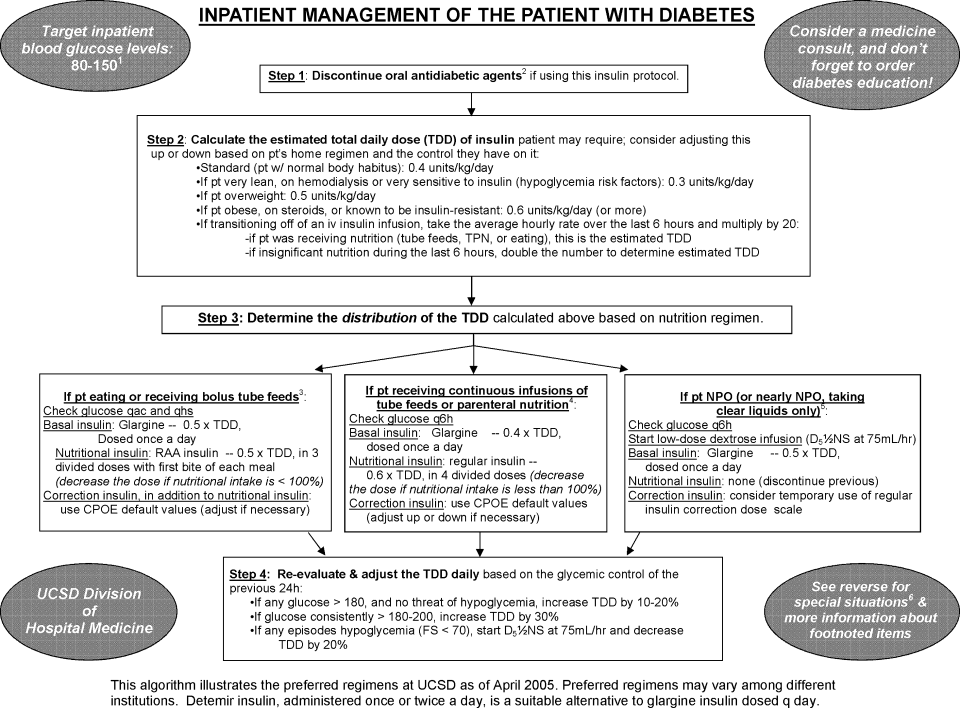

Other Attempts to Improve Care
Several other issues were addressed in the context of the larger performance improvement effort by the team. In many cases, hard data were not gathered to assess the effectiveness of the interventions, or the interventions were ongoing and could be considered the background milieu for the key interventions listed above.
During each intervention, education sessions were given throughout the hospital to staff, including physicians, residents, and nurses, using departmental grand rounds, nursing rounds, and in‐services to describe the process and goals. Patient education programs were also redesigned and implemented, using preprinted brochure. Front‐line nursing staff teaching skills were bolstered via Clinical Nurse Specialist educational sessions, and the use of a template for patient teaching. The educational template assessed patient readiness to learn, home environment, current knowledge, and other factors. Approximately 6 conferences directed at various physician staff per year became part of the regular curriculum.
We recognized that there was often poor coordination between glucose monitoring, nutrition delivery, and insulin administration. The traditional nursing practice of the 6:00 AM finger stick and insulin administration was changed to match a formalized nutrition delivery schedule. Nutrition services and nursing were engaged to address timeliness of nutrition delivery, insulin administration, and POC glucose documentation in the electronic health record.
Feedback to individual medicine resident teams on reaching glycemic targets, with movie ticket/coffee coupon rewards to high performing teams, was tried from April 2004 to September 2004.
Measures and Analyses
Assessing Insulin Use Patterns
A convenience sample gathering all subcutaneous insulin orders from 4 to 5 selected days per month yielded 70 to 90 subcutaneous insulin orders for review each month. Sampling was originally performed each month, followed by less frequent sampling once stability in insulin use patterns was reached. Regimens were categorized by pharmacy and hospitalist review as to whether basal insulin was part of the insulin regimen or not. The percentage of insulin regimens incorporating basal insulin was calculated for each sampled month and followed in run charts, and comparisons between preorder set and postorder set time periods were made using Pearson's chi square statistic.
Assessing Glycemic Control
Glycemic control and hypoglycemia parameters were monitored for the entire 38‐month observation period.
Routinely monitored POC glucose values were used to assess glycemic control. During the initial data examination, it was found after 14 days of the hospital stay, there was a notable stabilization and improvement in glucose control and fewer hypoglycemic events, therefore we examined only the first 14 days of hospitalization, thereby eliminating a potential source of bias from length of stay outliers.
A mean glucose value was recorded for each patient‐day with 1 or more recorded values. Glycemic control for each patient‐stay was calculated by averaging the patient‐day mean values, which we will refer to as the day‐weighted mean. Hypoglycemic values (60 mg/dL) were excluded from calculation of the mean glucose, to avoid equating frequent hypoglycemia with optimal glycemic control. An uncontrolled patient‐day was defined as a monitored patient‐day with a mean glucose 180 mg/dL. An uncontrolled patient‐stay is defined as a patient‐stay with a day‐weighted mean glucose value 180 mg/dL.
We theorized that the greatest impact of the interventions would be realized in patients with longer monitoring periods, and that those with only a few POC glucose values could potentially misrepresent the impact of our interventions: therefore we performed a second analysis restricted to patients with 8 POC glucose values.
Assessing Hypoglycemia
Hypoglycemia was defined as a glucose 60 mg/dL, and severe hypoglycemia was defined as a glucose 40 mg/dL. These parameters were characterized by 2 methods. First, we calculated the percentage of monitored patients suffering from 1 or more hypoglycemic events or severe hypoglycemic events over the course of their entire admission. A second method tracked the percentage of monitored patient‐days with hypoglycemia and severe hypoglycemia, thereby correcting for potential misinterpretation from clustered repeated measures or variable length of stay. As with the glycemic control analysis, we repeated the hypoglycemia analysis in the subset of patients with 8 POC glucose values.
Summary Analysis of Glycemic Control and Hypoglycemia
Pearson chi square values, with relative risks (RRs) and 95% confidence intervals (CIs) were calculated to compare glycemic control and hypoglycemia in the 2 key interventions and baseline. The interventions and data reporting were grouped as follows:
Baseline: November 2002 to October 2003) = Time Period 1 (TP1)
Structured Order Set: November 2003 to April 2005) = Time Period 2 (TP2)
Algorithm plus Structured Order Set: May 2005 to December 2005) = Time Period 3 (TP3)
A P value of less than 0.05 was determined as significant and data were analyzed using STATA, Version 8 (STATA Corp., College Station, TX).
We assigned the RR of uncontrolled hyperglycemia and the RR of hypoglycemia during the baseline time (TP1) with values of 1.0, and calculated the RR and CIs for the same parameters during TP2 and TP3.
RESULTS
Just over 11,000 patients were identified for POC glucose testing over the 38 month observation period. Of these, 9314 patients had either a diagnosis of diabetes or documented hyperglycemia. The characteristics of this study population are depicted in Table 1. There were no differences between the groups and the demographics of age, gender, or length of stay (P > 0.05 for all parameters). There was a slight increase in the percent of patients with any intensive care unit days over the 3 time periods and a similar increase in the case mix index.
| Patients Meeting Criteria of Diabetes Mellitus Diagnosis or Hyperglycemia (n = 9,314 patients) | Baseline | TP2 | TP3 |
|---|---|---|---|
| |||
| Time period (TP) | November 2002 to October 2003 | November 2003 to April 2005 | May 2005 to December 2005 |
| Monitored patient days (44,232) | 11,571 | 21,126 | 11,535 |
| Number of patients (9,314) | 2,504 | 4,515 | 2,295 |
| Males (%) | 55 | 54 | 56 |
| Average age standard deviation | 56 17 | 56 17 | 56 16 |
| Length of stay (excluding highest 1% of outliers) | 4.6 5.9 | 4.6 5.7 | 4.8 5.8 |
| % With any intensive care unit days* | 18 | 20 | 22 |
| Case mix index score (mean SD) | 1.8 2.1 | 2.0 2.3 | 2.1 2.1 |
| Case mix index (median score) | 1.1 | 1.3 | 1.3 |
Of the 9314 study patients, 5530 had 8 or more POC glucose values, and were included in a secondary analysis of glycemic control and hypoglycemia.
Insulin Use Patterns
Figure 4 demonstrates the dramatic improvement that took place with the introduction of the structured order set. In the 6 months preceding the introduction of the structured insulin order set (May‐October 2003) 72% of 477 sampled patients with insulin orders were on sliding scale‐only insulin regimens (with no basal insulin), compared to just 26% of 499 patients sampled in the March to August 2004 time period subsequent to order set implementation (P < .0001, chi square statistic). Intermittent monthly checks on insulin use patterns reveal this change has been sustained.
Glycemic Control
A total of 9314 patients with 44,232 monitored patient‐days and over 120,000 POC glucose values were analyzed to assess glycemic control, which was improved with structured insulin orders and improved incrementally with the introduction of the insulin management algorithm.
The percent of patient‐days that were uncontrolled, defined as a monitored day with a mean glucose of 180 mg/dL, was reduced over the 3 time periods (37.8% versus 33.9% versus 30.1%, P < 0.005, Pearson chi square statistic), representing a 21% RR reduction of uncontrolled patient‐days from TP1 versus TP3. Table 2 shows the summary results for glycemic control, including the RR and CIs between the 3 time periods.
| Time Period (TP) | Baseline | TP2 Structured Orders | TP3 Orders Plus Algorithm | Relative Risk TP3:TP2 |
|---|---|---|---|---|
| ||||
| Patient‐day glucose | ||||
| Mean SD | 179 66 | 170 65 | 165 58 | |
| Median | 160 | 155 | 151 | |
| Uncontrolled patient‐days* | 4,372 | 7,162 | 3,465 | |
| Monitored patient‐days | 11,555 | 21,135 | 11,531 | |
| % Uncontrolled patient‐days | 37.8 | 33.9 | 30.1 | |
| RR: uncontrolled patient‐day (95% confidence interval) | 1.0 | 0.89 (0.87‐0.92) | 0.79 (0.77‐0.82) | 0.89 (0.86‐0.92) |
| Glycemic control by patient‐stay | ||||
| Day‐weighted mean SD | 177 57 | 174 54 | 170 50 | |
| Day‐weighted median | 167 | 162 | 158 | |
| Uncontrolled patient‐stay (%) | 1,038 | 1,696 | 784 | |
| Monitored patient‐stay | 2,504 | 4,515 | 2,295 | |
| % Uncontrolled patient‐stays | 41.5 | 37.6 | 34.2 | |
| RR: uncontrolled patient‐stay (95% confidence interval) | 0.91 (0.85‐0.96) | 0.84 (0.77‐0.89) | 0.91 (0.85‐0.97) | |
In a similar fashion, the percent of patients with uncontrolled patient‐stays (day‐weighted mean glucose 180 mg/dL) was also reduced over the 3 time periods (41.5% versus 37.6% versus 34.2%, P < 0.05, Pearson chi square statistic, with an RR reduction of 16% for TP3:TP1). Figure 5 depicts a statistical process control chart of the percent of patients experiencing uncontrolled patient‐stays over time, and is more effective in displaying the temporal relationship of the interventions with the improved results.
Uncontrolled hyperglycemic days and stays were reduced incrementally from TP3 versus TP2, reflecting the added benefit of the insulin management algorithm, compared to the benefit enjoyed with the structured order set alone.
When the analyses were repeated after excluding patients with fewer than 8 POC glucose readings (Table 3), the findings were similar, but as predicted, the effect was slightly more pronounced, with a 23% relative reduction in uncontrolled patient‐days and a 27% reduction in uncontrolled patient‐stays of TP3 versus TP1.
| Time Period (TP) | Baseline | TP2 Structured Orders | TP3 Orders Plus Algorithm | Relative Risk TP3:TP2 |
|---|---|---|---|---|
| ||||
| Patient‐day glucose | ||||
| Mean SD | 172 65 | 169 64 | 163 57 | |
| Median | 159 | 154 | 149 | |
| Uncontrolled patient‐days* | 3,469 | 5,639 | 2,766 | |
| Monitored patient‐days | 9,304 | 17,278 | 9,671 | |
| % Uncontrolled patient‐days | 37.3 | 32.6 | 28.6 | |
| RR: uncontrolled patient‐day (95% confidence interval) | 1.0 | 0.87 (0.85‐0.90) | 0.77 (0.74‐0.80) | 0.88 (0.84‐0.91) |
| Glycemic control by patient‐stay | ||||
| Day‐weighted mean SD | 175 51 | 169 47 | 166 45 | |
| Day‐weighted median | 167 | 158 | 155 | |
| Uncontrolled patient‐stay (%) | 588 | 908 | 425 | |
| Monitored patient‐stay | 1,439 | 2,659 | 1,426 | |
| % Uncontrolled patient‐stays | 40.1 | 34.1 | 29.8 | |
| RR: Uncontrolled patient‐stay (95% confidence interval) | 0.84 (0.77‐0.91) | 0.73 (0.66‐0.81) | 0.87 (0.79‐0.96) | |
Hypoglycemia
Table 4 summarizes the results for hypoglycemia and severe hypoglycemia in the study population, and Table 5 summarizes the secondary analyses of hypoglycemia in the subset with at least 8 POC glucose readings.
| TP (Time Period) | Baseline | TP2 | TP3 | Relative Risk TP3:TP2 |
|---|---|---|---|---|
| ||||
| Monitored patient‐stays | 2504 | 4515 | 2295 | |
| Stays with hypoglycemia (%) | 296 (11.8) | 437 (9.7) | 210 (9.2) | |
| RR hypoglycemic stay (CI) | 1.0 | 0.82 (0.72‐0.94) | 0.77 (0.65‐0.92) | 0.95 (0.81‐1.10) |
| Stays with severe hypoglycemia (%) | 73 (2.9) | 96 (2.1) | 55 (2.4) | |
| RR severe hypoglycemic stay (CI) | 1.0 | 0.73 (0.54‐0.98) | 0.82 (0.58‐1.16) | 1.13 (0.81‐1.56) |
| Monitored patient‐days | 11,584 | 21,158 | 11,548 | |
| Days with hypoglycemia (%) | 441 (3.8) | 623 (2.9) | 300 (2.6) | |
| RR hypoglycemic day (CI) | 1.0 | 0.77 (0.69‐0.87) | 0.68 (0.59‐0.78) | 0.88 (0.77‐1.01) |
| Days with severe hypoglycemia (%) | 86 (0.74) | 109 (0.52) | 66 (0.57) | |
| RR Severe hypoglycemic day (CI) | 1.0 | 0.69 (0.52‐0.92) | 0.77 (0.56‐1.06) | 1.10 (0.82‐1.5) |
| TP (Time Period) | Baseline | TP2 | TP3 | Relative Risk TP3:TP2 |
|---|---|---|---|---|
| ||||
| Monitored patient‐stays | 1440 | 2664 | 1426 | |
| Stays with hypoglycemia (%) | 237 (16.5) | 384 (14.4) | 180 (12.6) | |
| RR hypoglycemic stay (CI) | 1.0 | 0.88 (0.76‐1.02) | 0.77 (0.64‐0.92) | 0.88 (0.75‐1.03) |
| Stays with severe hypoglycemia (%) | 58 (4.0) | 93 (3.5) | 47 (3.3) | |
| RR severe hypoglycemic stay (CI) | 1.0 | 0.87 (0.63‐1.2) | 0.82 (0.56‐1.19) | 0.94 (0.67‐1.33) |
| Monitored patient‐days | 9,317 | 17,310 | 9,684 | |
| Days with hypoglycemia (%) | 379 (4.1) | 569 (3.3) | 269 (2.7) | |
| RR hypoglycemic day (CI) | 1.0 | 0.81 (0.71‐0.92) | 0.68 (0.59‐0.80) | 0.85 (0.73‐0.98) |
| Days with severe hypoglycemia (%) | 71 (0.76) | 106 (0.61) | 58 (0.60) | |
| RR severe hypoglycemic day (CI) | 1.0 | 0.80 (0.60‐1.08) | 0.79 (0.56‐1.11) | 0.98 (0.71‐1.34) |
Analysis by Patient‐Stay
The percent of patients that suffered 1 or more hypoglycemic event over the course of their inpatient stay was 11.8% in TP1, 9.7% in TP2, and 9.2% in TP3. The RR of a patient suffering from a hypoglycemic event was significantly improved in the intervention time periods compared to baseline, with the RR of TP3:TP1 = 0.77 (CI, 0.65‐0.92). There was a strong trend for incremental improvement in hypoglycemic patient‐stays for TP3 versus TP2, but the trend just missed statistical significance (P < 0.07). Similar trends in improvement were found for severe hypoglycemia by patient‐stay, but these trends were only statistically significant for TP2 versus TP1. The findings were similar in the subset of patients with at least 8 POC glucose readings (Table 5).
Analysis by Patient‐Day
Of monitored patient days in the baseline TP1, 3.8% contained a hypoglycemic value of 60 mg/dL. With the introduction of structured insulin orders in TP2, this was reduced to 2.9%, and in TP3 it was 2.6%. The RR of a hypoglycemic patient‐day of TP2 compared to TP1 was 0.77 (CI, 0.69‐0.87), whereas the cumulative impact of the structured order set and algorithm (TP3:TP1) was 0.68 (CI, 0.59‐0.78), representing a 32% reduction of the baseline risk of suffering from a hypoglycemic day. Similar reductions were seen for the risk of a severe hypoglycemic patient‐day.
The secondary analysis of hypoglycemic and severe hypoglycemic patient‐days showed very similar results, except that the TP3:TP2 RR for hypoglycemia of 0.85 (CI, 0.73‐0.98) reached statistical significance, again demonstrating the incrementally beneficial effect of the insulin management algorithm.
DISCUSSION
Our study convincingly demonstrates that significant improvement in glycemic control can be achieved with implementation of structured subcutaneous insulin orders and a simple insulin management protocol. Perhaps more importantly, these gains in glycemic control are not gained at the expense of increased iatrogenic hypoglycemia, and in fact, we observed a 32% decline in the percent of patient‐days with hypoglycemia. This is extremely important because fear of hypoglycemia is the most significant barrier to glycemic control efforts.
Strengths and Limitations
Our study has several strengths. The study is large and incorporates all patients with diabetes or hyperglycemia captured by POC glucose testing, and the observation period is long enough that bias from merely being observed is not a factor. We used metrics for glycemic control, hypoglycemia, and insulin use patterns that are of high quality and are generally in line with the Society of Hospital Medicine (SHM) Glycemic Control Task force recommendations,12, 13 and examined data by both patient‐stay and patient‐day.
The increased use of anticipatory physiologic subcutaneous insulin regimens, and the subsequent decline in the use of sliding scale insulin, is the most likely mechanism for improvement. The improvements seen are fairly dramatic for an institution in absolute terms, because inpatient hyperglycemia and hypoglycemia are so common. For example, on an annualized basis for our 400‐bed medical center, these interventions prevent 124 patients from experiencing 208 hypoglycemic days.
Other institutions should be able to replicate our results. We received administrative support to create a multidisciplinary steering committee, but we did not have incremental resources to create a dedicated team for insulin management, mandated endocrinology comanagement or consultations, or manual data collection. In fact, we had only 1 diabetes educator for 400 adult beds at 2 sites, and were relatively underresourced in this area by community standards. There was some time and expense in creating the glycemic control reports, but all of the glucose data collected were part of normal care, and the data retrieval became automated.
The main limitation of this study lies in the observational study design. There were multiple interventions in addition to structured insulin orders and the insulin management algorithm, and these educational and organizational changes undoubtedly also contributed to the overall success of our program. Since we did not perform a randomized controlled trial, the reader might reasonably question if the structured order sets and insulin management algorithm were actually the cause of the improvement seen, as opposed to these ancillary efforts or secular change. However, there are several factors that make this unlikely. First, the study population was well‐defined, having diabetes or documented hyperglycemia in all 3 time periods. Second, the demographics remained constant or actually worked against improvement trends, since the markers of patient acuity suggest increased patient acuity over the observation period. Third, the temporal relationship of the improvement to the introduction of our key interventions, as viewed on statistical process control charts shown in Figure 5, strongly suggest a causal relationship. This temporal relationship was consistently observed no matter how we chose to define uncontrolled hyperglycemia, and was also seen on hypoglycemia control charts. We view the ancillary interventions (such as educational efforts) as necessary, but not sufficient, in and of themselves, to effect major improvement.
We did not analyze the impact of the improved glycemic control on patient outcomes. In the absence of a randomized controlled trial design, controlling for the various confounders is a challenging task. Also, it is likely that not all hypoglycemic events were attributable to inpatient glycemic control regimens, though the secondary analysis probably eliminated many hypoglycemia admissions.
Lessons Learned: Implications from our study
We agree with the American Association of Clinical Endocrinologists (AACE)/American Diabetes Association (ADA)2 and the SHM Glycemic Control Task Force12 about the essential elements needed for successful implementation of inpatient glycemic control programs:
An appropriate level of administrative support.
Formation of a multidisciplinary steering committee to drive the development of initiatives, empowered to enact changes.
Assessment of current processes, quality of care, and barriers to practice change.
Development and implementation of interventions, including standardized order sets, protocols, policies, and algorithms with associated educational programs.
Metrics for evaluation of glycemic control, hypoglycemia, insulin use patterns, and other aspects of care.
Metrics to follow hypoglycemia are extremely important. The voluntary reporting on insulin‐induced hypoglycemia fluctuated widely over the course of our project. These fluctuations did not correlate well with the more objective and accurate measures we followed, and this objective data was very helpful in reducing the fear of hypoglycemia, and spreading the wider use of basal bolus insulin regimens. We strongly recommend that improvement teams formulate and follow measures of glycemic control, hypoglycemia, and insulin use, similar to those outlined in the SHM Glycemic Control Improvement Guide12 and the SHM Glycemic Control Task Force summary on glucometrics.13
Although we introduced our structured insulin order set first, with a long lag time until we introduced the insulin management algorithm, we advocate a different approach for institutions grappling with these issues. This approach is well‐described by the SHM Glycemic Control Task Force.14 An insulin management algorithm should be crafted first, integrating guidance for insulin dosing, preferred insulin regimens for different nutritional situations, a glycemic target, insulin dosing adjustment, glucose monitoring, and prompts for ordering a glycosylated hemoglobin (A1c) level. Next, the order set and the supporting educational programs should integrate this guidance as much as possible, making the key guidance available at the point of patient care.
This guidance was available in our algorithm but was not inherent in the structured insulin orders described in this report, and all basal and nutritional insulin options were offered as equally acceptable choices. This version did not calculate insulin doses or assist in the apportionment of insulin between basal and nutritional components. Only a single adjustment dose scale was offered, leaving appropriate modifications up to the end user, and from a usability standpoint, our CPOE insulin orders lacked dynamic flexibility (revising a single insulin required discontinuing all prior orders and reentering all orders). These limitations have subsequently been addressed with Version 2 of our CPOE insulin orders, and the details will soon be available in the literature.15
We are now exploring further improvement with concurrent identification and intervention of hyperglycemic patients that are not on physiologic insulin regimens or not meeting glycemic targets, and implementing protocols addressing the transition from infusion insulin.
CONCLUSION
We significantly improved glycemic control and simultaneously reduced hypoglycemia across all major medical and surgical services at our medical center, thereby addressing the number 1 barrier to improved inpatient glycemic control. We achieved this via systems changes with the introduction of structured subcutaneous insulin orders and the insulin management algorithm, along with education, but did not otherwise mandate or monitor adherence to our algorithm.
Implementing an institutional insulin management algorithm and structured insulin orders should now be viewed as a potent safety intervention as well as an intervention to enhance quality, and we have demonstrated that non‐critical care glycemic control efforts can clearly be a win‐win situation.
- Centers for Disease Control and Prevention.National Diabetes Fact Sheet: General Information and National Estimates on Diabetes in the United States, 2002.Atlanta, GA:U.S. Department of Health and Human Services, Centers for Disease Control and Prevention;2003. Available at: www.cdc.gov/diabetes/pubs/factsheet.htm. Accessed January 21, 2006.
- American College of Endocrinology and American Diabetes Association Consensus statement on inpatient diabetes and glycemic control: a call to action.Diabetes Care.2006;29:1955‐1962.
- ,,, et al.Hyperglycemia: an independent marker of in‐hospital mortality in patients with undiagnosed diabetes.J Clin Endocrinol Metab.2002;87:978‐982.
- ,,, et al.The relation between hyperglycemia and outcomes in 2471 patients admitted to the hospital with community‐acquired pneumonia.Diabetes Care.2005;28:810‐815.
- ,,, et al.Cancer.2004;100:1179‐1185.
- ,,, et al.Early perioperative glycaemic control and allograft rejection in patients with diabetes mellitus: a pilot study.Transplantation.2001;72:1321‐1324.
- ,,, et al.Early postoperative glucose control predicts nosocomial infection rate in diabetic patients.J Parenter Enteral Nutr.1998;22:77‐81.
- ,,, et al.Glucose control lowers the risk of wound infection in diabetics after open heart operations.Ann Thorac Surg.1997;63:356‐361.
- ,,, et al.Management of diabetes and hyperglycemia in hospitals.Diabetes Care.2004;27:553‐591.
- ,,, et al.American College of Endocrinology position statement on inpatient diabetes and metabolic control.Endocr Pract.2004;10:77‐82.
- ,.Glycemic chaos (not glycemic control) still the rule for inpatient care: how do we stop the insanity? [Editorial].J Hosp Med.2006;1:141‐144.
- Society of Hospital Medicine Glycemic Control Task Force: Optimizing Glycemic Control and Reducing Hypoglycemia at Your Medical Center. Society of Hospital Medicine, Glycemic Control Quality Improvement Resource Room. Available at: http://www.hospitalmedicine.org/ResourceRoomRedesign/GlycemicControl.cfm. Accessed October2008.
- ,,,,,.SHM Glycemic Control Task Force summary: practical recommendations for assessing the impact of glycemic control efforts.J Hosp Med.2008;3(S5):66–75.
- ,,,;for the SHM Glycemic Control Task Force.Subcutaneous insulin order sets and protocols: effective design and implementation strategies.J Hosp Med.2008;3(S5):29–41.
- ,,,.Indication‐based ordering: a new paradigm for glycemic control in hospitalized inpatients.J Diabetes Sci Tech.2008;2(3):349‐356.
Diabetes has reached epidemic proportions in the United States, affecting over 20 million individuals,1 and further rises are expected. A disproportionate increase in diabetes has occurred in the inpatient setting.2 Furthermore, for every 2 patients in the hospital with known diabetes, there may be an additional 1 with newly observed hyperglycemia. Both are common. In 1 report, for example, 24% of inpatients with hyperglycemia had a prior diagnosis of diabetes, whereas another 12% had hyperglycemia without a prior diagnosis of diabetes.3
Although there is a paucity of high quality randomized controlled trials to support tight glycemic control in non‐critical care inpatient settings, poor glycemic control in hospitalized patients is strongly associated with undesirable outcomes for a variety of conditions, including pneumonia,4 cancer chemotherapy,5 renal transplant,6 and postsurgical wound infections.7, 8 Hyperglycemia also induces dehydration, fluid and electrolyte imbalance, gastric motility problems, and venous thromboembolism formation.9
Structured subcutaneous insulin order sets and insulin management protocols have been widely advocated as a method to encourage basal bolus insulin regimens and enhance glycemic control,2, 9, 10 but the effect of these interventions on glycemic control, hypoglycemia, and insulin use patterns in the real world setting has not been well reported. Fear of inducing hypoglycemia is often the main barrier for initiating basal insulin containing regimens and pursuing glycemic targets.2 The evidence would suggest, however, that sliding scale regimens, as opposed to more physiologic basal bolus regimens, may actually increase both hypoglycemic and hyperglycemic excursions.11 A convincing demonstration of the efficacy (improved insulin use patterns and reduced hyperglycemia) and safety (reduced hypoglycemia) of structured insulin order sets and insulin management protocols would foster a more rapid adoption of these strategies.
PATIENTS AND METHODS
In our 400‐bed university hospital, we formed a hospitalist‐led multidisciplinary team in early 2003, with the focus of improving the care delivered to non‐critical care patients with diabetes or hyperglycemia. We used a Plan‐Do‐Study‐Act (PDSA) performance improvement framework, and conducted institutional review board (IRB)‐approved prospective observational research in parallel with the performance improvement efforts, with a waiver for individual informed consent. The study population consisted of all adult inpatients on non‐critical care units with electronically reported point of care (POC) glucose testing from November 2002 through December 2005. We excluded patients who did not have either a discharge diagnosis of Diabetes (ICD 9 codes 250‐251.XX) or demonstrated hyperglycemia (fasting POC glucose >130 mg/dL 2, or a random value of >180 mg/dL) from analysis of glycemic control and hypoglycemia. Women admitted to Obstetrics were excluded. Monthly and quarterly summaries on glycemic control, hypoglycemia, and insulin use patterns (metrics described below) were reported to the improvement team and other groups on a regular basis throughout the intervention period. POC glucose data, demographics, markers of severity of illness, and diagnosis codes were retrieved from the electronic health record.
Interventions
We introduced several interventions and educational efforts throughout the course of our improvement. The 2 key interventions were as follows:
Structured subcutaneous insulin order sets (November, 2003).
An insulin management algorithm, described below (May 2005).
Key Intervention #1: Structured Subcutaneous Insulin Order Set Implementation
In November 2003, we introduced a paper‐based structured subcutaneous insulin order set. This order set encouraged the use of scheduled basal and nutritional insulin, provided guidance for monitoring glucose levels, and for insulin dosing. A hypoglycemia protocol and a standardized correction insulin table were embedded in the order set. This set was similar to examples of structured insulin ordering subsequently presented in the literature.9 In a parallel effort, the University of California, San Diego Medical Center (UCSDMC) was developing a computer physician order entry (CPOE) module for our comprehensive clinical information system, Invision (Siemens Medical Systems, Malvern, PA), that heretofore had primarily focused on result review, patient schedule management, and nursing documentation. In anticipation of CPOE and for the purpose of standardization, we removed outdated sliding scale insulin regimens from a variety preexisting order sets and inserted references to the standardized subcutaneous insulin order set in their stead. The medication administration record (MAR) was changed to reflect the basal/nutritional/correction insulin terminology. It became more difficult to order a stand‐alone insulin sliding scale even before CPOE versions became available. The standardized order set was the only preprinted correction scale insulin order available, and ordering physicians have to specifically opt out of basal and nutritional insulin choices to order sliding scale only regimens. Verbal orders for correction dose scales were deemed unacceptable by medical staff committees. Correctional insulin doses could be ordered as a 1‐time order, but the pharmacy rejected ongoing insulin orders that were not entered on the structured form.
We introduced our first standardized CPOE subcutaneous insulin order set in January 2004 at the smaller of our 2 campuses, and subsequently completed full deployment across both campuses in all adult medical‐surgical care areas by September 2004.
The CPOE version, like the paper version that immediately preceded it, encouraged the use of basal/bolus insulin regimens, promoted the terms basal, nutritional or premeal, and adjustment dose insulin in the order sets and the medication administration record, and was mandatory for providers wishing to order anything but a 1‐time order of insulin. Figure 1 depicts a screen shot of the CPOE version. Similar to the paper version, the ordering physician had to specifically opt out of ordering scheduled premeal and basal insulin to order a sliding scale only regimen. The first screen also ensured that appropriate POC glucose monitoring was ordered and endorsed a standing hypoglycemia protocol order. The CPOE version had only a few additional features not possible on paper. Obvious benefits included elimination of unapproved abbreviations and handwriting errors. Nutritional and correction insulin types were forced to be identical. Fundamentally, however, both the paper and online structured ordering experiences had the same degree of control over provider ordering patterns, and there was no increment in guidance for choosing insulin regimens, hence their combined analysis as structured orders.

Key Intervention #2: Insulin Management Algorithm
The structured insulin order set had many advantages, but also had many limitations. Guidance for preferred insulin regimens for patients in different nutritional situations was not inherent in the order set, and all basal and nutritional insulin options were offered as equally acceptable choices. The order set gave very general guidance for insulin dosing, but did not calculate insulin doses or assist in the apportionment of insulin between basal and nutritional components, and guidance for setting a glycemic target or adjusting insulin was lacking.
Recognizing these limitations, we devised an insulin management algorithm to provide guidance incremental to that offered in the order set. In April 2005, 3 hospitalists piloted a paper‐based insulin management algorithm (Figure 2, front; Figure 3, reverse) on their teaching services. This 1‐page algorithm provided guidance on insulin dosing and monitoring, and provided institutionally preferred insulin regimens for patients in different nutritional situations. As an example, of the several acceptable subcutaneous insulin regimens that an eating patient might use in the inpatient setting, we advocated the use of 1 preferred regimen (a relatively peakless, long‐acting basal insulin once a day, along with a rapid acting analog nutritional insulin with each meal). We introduced the concept of a ward glycemic target, provided prompts for diabetes education, and generally recommended discontinuation of oral hypoglycemic agents in the inpatient setting. The hospitalists were introduced to the concepts and the algorithm via 1 of the authors (G.M.) in a 1‐hour session. The algorithm was introduced on each teaching team during routine teaching rounds with a slide set (approximately 15 slides) that outlined the basic principles of insulin dosing, and gave example cases which modeled the proper use of the algorithm. The principles were reinforced on daily patient work rounds as they were applied on inpatients with hyperglycemia. The pilot results on 25 patients, compared to 250 historical control patients, were very promising, with markedly improved glycemic control and no increase in hypoglycemia. We therefore sought to spread the use of the algorithm. In May 2005 the insulin management algorithm and teaching slide set were promoted on all 7 hospitalist‐run services, and the results of the pilot and concepts of the algorithm were shared with a variety of house staff and service leaders in approximately a dozen sessions: educational grand rounds, assorted noon lectures, and subsequently, at new intern orientations. Easy access to the algorithm was assured by providing a link to the file within the CPOE insulin order set.


Other Attempts to Improve Care
Several other issues were addressed in the context of the larger performance improvement effort by the team. In many cases, hard data were not gathered to assess the effectiveness of the interventions, or the interventions were ongoing and could be considered the background milieu for the key interventions listed above.
During each intervention, education sessions were given throughout the hospital to staff, including physicians, residents, and nurses, using departmental grand rounds, nursing rounds, and in‐services to describe the process and goals. Patient education programs were also redesigned and implemented, using preprinted brochure. Front‐line nursing staff teaching skills were bolstered via Clinical Nurse Specialist educational sessions, and the use of a template for patient teaching. The educational template assessed patient readiness to learn, home environment, current knowledge, and other factors. Approximately 6 conferences directed at various physician staff per year became part of the regular curriculum.
We recognized that there was often poor coordination between glucose monitoring, nutrition delivery, and insulin administration. The traditional nursing practice of the 6:00 AM finger stick and insulin administration was changed to match a formalized nutrition delivery schedule. Nutrition services and nursing were engaged to address timeliness of nutrition delivery, insulin administration, and POC glucose documentation in the electronic health record.
Feedback to individual medicine resident teams on reaching glycemic targets, with movie ticket/coffee coupon rewards to high performing teams, was tried from April 2004 to September 2004.
Measures and Analyses
Assessing Insulin Use Patterns
A convenience sample gathering all subcutaneous insulin orders from 4 to 5 selected days per month yielded 70 to 90 subcutaneous insulin orders for review each month. Sampling was originally performed each month, followed by less frequent sampling once stability in insulin use patterns was reached. Regimens were categorized by pharmacy and hospitalist review as to whether basal insulin was part of the insulin regimen or not. The percentage of insulin regimens incorporating basal insulin was calculated for each sampled month and followed in run charts, and comparisons between preorder set and postorder set time periods were made using Pearson's chi square statistic.
Assessing Glycemic Control
Glycemic control and hypoglycemia parameters were monitored for the entire 38‐month observation period.
Routinely monitored POC glucose values were used to assess glycemic control. During the initial data examination, it was found after 14 days of the hospital stay, there was a notable stabilization and improvement in glucose control and fewer hypoglycemic events, therefore we examined only the first 14 days of hospitalization, thereby eliminating a potential source of bias from length of stay outliers.
A mean glucose value was recorded for each patient‐day with 1 or more recorded values. Glycemic control for each patient‐stay was calculated by averaging the patient‐day mean values, which we will refer to as the day‐weighted mean. Hypoglycemic values (60 mg/dL) were excluded from calculation of the mean glucose, to avoid equating frequent hypoglycemia with optimal glycemic control. An uncontrolled patient‐day was defined as a monitored patient‐day with a mean glucose 180 mg/dL. An uncontrolled patient‐stay is defined as a patient‐stay with a day‐weighted mean glucose value 180 mg/dL.
We theorized that the greatest impact of the interventions would be realized in patients with longer monitoring periods, and that those with only a few POC glucose values could potentially misrepresent the impact of our interventions: therefore we performed a second analysis restricted to patients with 8 POC glucose values.
Assessing Hypoglycemia
Hypoglycemia was defined as a glucose 60 mg/dL, and severe hypoglycemia was defined as a glucose 40 mg/dL. These parameters were characterized by 2 methods. First, we calculated the percentage of monitored patients suffering from 1 or more hypoglycemic events or severe hypoglycemic events over the course of their entire admission. A second method tracked the percentage of monitored patient‐days with hypoglycemia and severe hypoglycemia, thereby correcting for potential misinterpretation from clustered repeated measures or variable length of stay. As with the glycemic control analysis, we repeated the hypoglycemia analysis in the subset of patients with 8 POC glucose values.
Summary Analysis of Glycemic Control and Hypoglycemia
Pearson chi square values, with relative risks (RRs) and 95% confidence intervals (CIs) were calculated to compare glycemic control and hypoglycemia in the 2 key interventions and baseline. The interventions and data reporting were grouped as follows:
Baseline: November 2002 to October 2003) = Time Period 1 (TP1)
Structured Order Set: November 2003 to April 2005) = Time Period 2 (TP2)
Algorithm plus Structured Order Set: May 2005 to December 2005) = Time Period 3 (TP3)
A P value of less than 0.05 was determined as significant and data were analyzed using STATA, Version 8 (STATA Corp., College Station, TX).
We assigned the RR of uncontrolled hyperglycemia and the RR of hypoglycemia during the baseline time (TP1) with values of 1.0, and calculated the RR and CIs for the same parameters during TP2 and TP3.
RESULTS
Just over 11,000 patients were identified for POC glucose testing over the 38 month observation period. Of these, 9314 patients had either a diagnosis of diabetes or documented hyperglycemia. The characteristics of this study population are depicted in Table 1. There were no differences between the groups and the demographics of age, gender, or length of stay (P > 0.05 for all parameters). There was a slight increase in the percent of patients with any intensive care unit days over the 3 time periods and a similar increase in the case mix index.
| Patients Meeting Criteria of Diabetes Mellitus Diagnosis or Hyperglycemia (n = 9,314 patients) | Baseline | TP2 | TP3 |
|---|---|---|---|
| |||
| Time period (TP) | November 2002 to October 2003 | November 2003 to April 2005 | May 2005 to December 2005 |
| Monitored patient days (44,232) | 11,571 | 21,126 | 11,535 |
| Number of patients (9,314) | 2,504 | 4,515 | 2,295 |
| Males (%) | 55 | 54 | 56 |
| Average age standard deviation | 56 17 | 56 17 | 56 16 |
| Length of stay (excluding highest 1% of outliers) | 4.6 5.9 | 4.6 5.7 | 4.8 5.8 |
| % With any intensive care unit days* | 18 | 20 | 22 |
| Case mix index score (mean SD) | 1.8 2.1 | 2.0 2.3 | 2.1 2.1 |
| Case mix index (median score) | 1.1 | 1.3 | 1.3 |
Of the 9314 study patients, 5530 had 8 or more POC glucose values, and were included in a secondary analysis of glycemic control and hypoglycemia.
Insulin Use Patterns
Figure 4 demonstrates the dramatic improvement that took place with the introduction of the structured order set. In the 6 months preceding the introduction of the structured insulin order set (May‐October 2003) 72% of 477 sampled patients with insulin orders were on sliding scale‐only insulin regimens (with no basal insulin), compared to just 26% of 499 patients sampled in the March to August 2004 time period subsequent to order set implementation (P < .0001, chi square statistic). Intermittent monthly checks on insulin use patterns reveal this change has been sustained.

Glycemic Control
A total of 9314 patients with 44,232 monitored patient‐days and over 120,000 POC glucose values were analyzed to assess glycemic control, which was improved with structured insulin orders and improved incrementally with the introduction of the insulin management algorithm.
The percent of patient‐days that were uncontrolled, defined as a monitored day with a mean glucose of 180 mg/dL, was reduced over the 3 time periods (37.8% versus 33.9% versus 30.1%, P < 0.005, Pearson chi square statistic), representing a 21% RR reduction of uncontrolled patient‐days from TP1 versus TP3. Table 2 shows the summary results for glycemic control, including the RR and CIs between the 3 time periods.
| Time Period (TP) | Baseline | TP2 Structured Orders | TP3 Orders Plus Algorithm | Relative Risk TP3:TP2 |
|---|---|---|---|---|
| ||||
| Patient‐day glucose | ||||
| Mean SD | 179 66 | 170 65 | 165 58 | |
| Median | 160 | 155 | 151 | |
| Uncontrolled patient‐days* | 4,372 | 7,162 | 3,465 | |
| Monitored patient‐days | 11,555 | 21,135 | 11,531 | |
| % Uncontrolled patient‐days | 37.8 | 33.9 | 30.1 | |
| RR: uncontrolled patient‐day (95% confidence interval) | 1.0 | 0.89 (0.87‐0.92) | 0.79 (0.77‐0.82) | 0.89 (0.86‐0.92) |
| Glycemic control by patient‐stay | ||||
| Day‐weighted mean SD | 177 57 | 174 54 | 170 50 | |
| Day‐weighted median | 167 | 162 | 158 | |
| Uncontrolled patient‐stay (%) | 1,038 | 1,696 | 784 | |
| Monitored patient‐stay | 2,504 | 4,515 | 2,295 | |
| % Uncontrolled patient‐stays | 41.5 | 37.6 | 34.2 | |
| RR: uncontrolled patient‐stay (95% confidence interval) | 0.91 (0.85‐0.96) | 0.84 (0.77‐0.89) | 0.91 (0.85‐0.97) | |
In a similar fashion, the percent of patients with uncontrolled patient‐stays (day‐weighted mean glucose 180 mg/dL) was also reduced over the 3 time periods (41.5% versus 37.6% versus 34.2%, P < 0.05, Pearson chi square statistic, with an RR reduction of 16% for TP3:TP1). Figure 5 depicts a statistical process control chart of the percent of patients experiencing uncontrolled patient‐stays over time, and is more effective in displaying the temporal relationship of the interventions with the improved results.

Uncontrolled hyperglycemic days and stays were reduced incrementally from TP3 versus TP2, reflecting the added benefit of the insulin management algorithm, compared to the benefit enjoyed with the structured order set alone.
When the analyses were repeated after excluding patients with fewer than 8 POC glucose readings (Table 3), the findings were similar, but as predicted, the effect was slightly more pronounced, with a 23% relative reduction in uncontrolled patient‐days and a 27% reduction in uncontrolled patient‐stays of TP3 versus TP1.
| Time Period (TP) | Baseline | TP2 Structured Orders | TP3 Orders Plus Algorithm | Relative Risk TP3:TP2 |
|---|---|---|---|---|
| ||||
| Patient‐day glucose | ||||
| Mean SD | 172 65 | 169 64 | 163 57 | |
| Median | 159 | 154 | 149 | |
| Uncontrolled patient‐days* | 3,469 | 5,639 | 2,766 | |
| Monitored patient‐days | 9,304 | 17,278 | 9,671 | |
| % Uncontrolled patient‐days | 37.3 | 32.6 | 28.6 | |
| RR: uncontrolled patient‐day (95% confidence interval) | 1.0 | 0.87 (0.85‐0.90) | 0.77 (0.74‐0.80) | 0.88 (0.84‐0.91) |
| Glycemic control by patient‐stay | ||||
| Day‐weighted mean SD | 175 51 | 169 47 | 166 45 | |
| Day‐weighted median | 167 | 158 | 155 | |
| Uncontrolled patient‐stay (%) | 588 | 908 | 425 | |
| Monitored patient‐stay | 1,439 | 2,659 | 1,426 | |
| % Uncontrolled patient‐stays | 40.1 | 34.1 | 29.8 | |
| RR: Uncontrolled patient‐stay (95% confidence interval) | 0.84 (0.77‐0.91) | 0.73 (0.66‐0.81) | 0.87 (0.79‐0.96) | |
Hypoglycemia
Table 4 summarizes the results for hypoglycemia and severe hypoglycemia in the study population, and Table 5 summarizes the secondary analyses of hypoglycemia in the subset with at least 8 POC glucose readings.
| TP (Time Period) | Baseline | TP2 | TP3 | Relative Risk TP3:TP2 |
|---|---|---|---|---|
| ||||
| Monitored patient‐stays | 2504 | 4515 | 2295 | |
| Stays with hypoglycemia (%) | 296 (11.8) | 437 (9.7) | 210 (9.2) | |
| RR hypoglycemic stay (CI) | 1.0 | 0.82 (0.72‐0.94) | 0.77 (0.65‐0.92) | 0.95 (0.81‐1.10) |
| Stays with severe hypoglycemia (%) | 73 (2.9) | 96 (2.1) | 55 (2.4) | |
| RR severe hypoglycemic stay (CI) | 1.0 | 0.73 (0.54‐0.98) | 0.82 (0.58‐1.16) | 1.13 (0.81‐1.56) |
| Monitored patient‐days | 11,584 | 21,158 | 11,548 | |
| Days with hypoglycemia (%) | 441 (3.8) | 623 (2.9) | 300 (2.6) | |
| RR hypoglycemic day (CI) | 1.0 | 0.77 (0.69‐0.87) | 0.68 (0.59‐0.78) | 0.88 (0.77‐1.01) |
| Days with severe hypoglycemia (%) | 86 (0.74) | 109 (0.52) | 66 (0.57) | |
| RR Severe hypoglycemic day (CI) | 1.0 | 0.69 (0.52‐0.92) | 0.77 (0.56‐1.06) | 1.10 (0.82‐1.5) |
| TP (Time Period) | Baseline | TP2 | TP3 | Relative Risk TP3:TP2 |
|---|---|---|---|---|
| ||||
| Monitored patient‐stays | 1440 | 2664 | 1426 | |
| Stays with hypoglycemia (%) | 237 (16.5) | 384 (14.4) | 180 (12.6) | |
| RR hypoglycemic stay (CI) | 1.0 | 0.88 (0.76‐1.02) | 0.77 (0.64‐0.92) | 0.88 (0.75‐1.03) |
| Stays with severe hypoglycemia (%) | 58 (4.0) | 93 (3.5) | 47 (3.3) | |
| RR severe hypoglycemic stay (CI) | 1.0 | 0.87 (0.63‐1.2) | 0.82 (0.56‐1.19) | 0.94 (0.67‐1.33) |
| Monitored patient‐days | 9,317 | 17,310 | 9,684 | |
| Days with hypoglycemia (%) | 379 (4.1) | 569 (3.3) | 269 (2.7) | |
| RR hypoglycemic day (CI) | 1.0 | 0.81 (0.71‐0.92) | 0.68 (0.59‐0.80) | 0.85 (0.73‐0.98) |
| Days with severe hypoglycemia (%) | 71 (0.76) | 106 (0.61) | 58 (0.60) | |
| RR severe hypoglycemic day (CI) | 1.0 | 0.80 (0.60‐1.08) | 0.79 (0.56‐1.11) | 0.98 (0.71‐1.34) |
Analysis by Patient‐Stay
The percent of patients that suffered 1 or more hypoglycemic event over the course of their inpatient stay was 11.8% in TP1, 9.7% in TP2, and 9.2% in TP3. The RR of a patient suffering from a hypoglycemic event was significantly improved in the intervention time periods compared to baseline, with the RR of TP3:TP1 = 0.77 (CI, 0.65‐0.92). There was a strong trend for incremental improvement in hypoglycemic patient‐stays for TP3 versus TP2, but the trend just missed statistical significance (P < 0.07). Similar trends in improvement were found for severe hypoglycemia by patient‐stay, but these trends were only statistically significant for TP2 versus TP1. The findings were similar in the subset of patients with at least 8 POC glucose readings (Table 5).
Analysis by Patient‐Day
Of monitored patient days in the baseline TP1, 3.8% contained a hypoglycemic value of 60 mg/dL. With the introduction of structured insulin orders in TP2, this was reduced to 2.9%, and in TP3 it was 2.6%. The RR of a hypoglycemic patient‐day of TP2 compared to TP1 was 0.77 (CI, 0.69‐0.87), whereas the cumulative impact of the structured order set and algorithm (TP3:TP1) was 0.68 (CI, 0.59‐0.78), representing a 32% reduction of the baseline risk of suffering from a hypoglycemic day. Similar reductions were seen for the risk of a severe hypoglycemic patient‐day.
The secondary analysis of hypoglycemic and severe hypoglycemic patient‐days showed very similar results, except that the TP3:TP2 RR for hypoglycemia of 0.85 (CI, 0.73‐0.98) reached statistical significance, again demonstrating the incrementally beneficial effect of the insulin management algorithm.
DISCUSSION
Our study convincingly demonstrates that significant improvement in glycemic control can be achieved with implementation of structured subcutaneous insulin orders and a simple insulin management protocol. Perhaps more importantly, these gains in glycemic control are not gained at the expense of increased iatrogenic hypoglycemia, and in fact, we observed a 32% decline in the percent of patient‐days with hypoglycemia. This is extremely important because fear of hypoglycemia is the most significant barrier to glycemic control efforts.
Strengths and Limitations
Our study has several strengths. The study is large and incorporates all patients with diabetes or hyperglycemia captured by POC glucose testing, and the observation period is long enough that bias from merely being observed is not a factor. We used metrics for glycemic control, hypoglycemia, and insulin use patterns that are of high quality and are generally in line with the Society of Hospital Medicine (SHM) Glycemic Control Task force recommendations,12, 13 and examined data by both patient‐stay and patient‐day.
The increased use of anticipatory physiologic subcutaneous insulin regimens, and the subsequent decline in the use of sliding scale insulin, is the most likely mechanism for improvement. The improvements seen are fairly dramatic for an institution in absolute terms, because inpatient hyperglycemia and hypoglycemia are so common. For example, on an annualized basis for our 400‐bed medical center, these interventions prevent 124 patients from experiencing 208 hypoglycemic days.
Other institutions should be able to replicate our results. We received administrative support to create a multidisciplinary steering committee, but we did not have incremental resources to create a dedicated team for insulin management, mandated endocrinology comanagement or consultations, or manual data collection. In fact, we had only 1 diabetes educator for 400 adult beds at 2 sites, and were relatively underresourced in this area by community standards. There was some time and expense in creating the glycemic control reports, but all of the glucose data collected were part of normal care, and the data retrieval became automated.
The main limitation of this study lies in the observational study design. There were multiple interventions in addition to structured insulin orders and the insulin management algorithm, and these educational and organizational changes undoubtedly also contributed to the overall success of our program. Since we did not perform a randomized controlled trial, the reader might reasonably question if the structured order sets and insulin management algorithm were actually the cause of the improvement seen, as opposed to these ancillary efforts or secular change. However, there are several factors that make this unlikely. First, the study population was well‐defined, having diabetes or documented hyperglycemia in all 3 time periods. Second, the demographics remained constant or actually worked against improvement trends, since the markers of patient acuity suggest increased patient acuity over the observation period. Third, the temporal relationship of the improvement to the introduction of our key interventions, as viewed on statistical process control charts shown in Figure 5, strongly suggest a causal relationship. This temporal relationship was consistently observed no matter how we chose to define uncontrolled hyperglycemia, and was also seen on hypoglycemia control charts. We view the ancillary interventions (such as educational efforts) as necessary, but not sufficient, in and of themselves, to effect major improvement.
We did not analyze the impact of the improved glycemic control on patient outcomes. In the absence of a randomized controlled trial design, controlling for the various confounders is a challenging task. Also, it is likely that not all hypoglycemic events were attributable to inpatient glycemic control regimens, though the secondary analysis probably eliminated many hypoglycemia admissions.
Lessons Learned: Implications from our study
We agree with the American Association of Clinical Endocrinologists (AACE)/American Diabetes Association (ADA)2 and the SHM Glycemic Control Task Force12 about the essential elements needed for successful implementation of inpatient glycemic control programs:
An appropriate level of administrative support.
Formation of a multidisciplinary steering committee to drive the development of initiatives, empowered to enact changes.
Assessment of current processes, quality of care, and barriers to practice change.
Development and implementation of interventions, including standardized order sets, protocols, policies, and algorithms with associated educational programs.
Metrics for evaluation of glycemic control, hypoglycemia, insulin use patterns, and other aspects of care.
Metrics to follow hypoglycemia are extremely important. The voluntary reporting on insulin‐induced hypoglycemia fluctuated widely over the course of our project. These fluctuations did not correlate well with the more objective and accurate measures we followed, and this objective data was very helpful in reducing the fear of hypoglycemia, and spreading the wider use of basal bolus insulin regimens. We strongly recommend that improvement teams formulate and follow measures of glycemic control, hypoglycemia, and insulin use, similar to those outlined in the SHM Glycemic Control Improvement Guide12 and the SHM Glycemic Control Task Force summary on glucometrics.13
Although we introduced our structured insulin order set first, with a long lag time until we introduced the insulin management algorithm, we advocate a different approach for institutions grappling with these issues. This approach is well‐described by the SHM Glycemic Control Task Force.14 An insulin management algorithm should be crafted first, integrating guidance for insulin dosing, preferred insulin regimens for different nutritional situations, a glycemic target, insulin dosing adjustment, glucose monitoring, and prompts for ordering a glycosylated hemoglobin (A1c) level. Next, the order set and the supporting educational programs should integrate this guidance as much as possible, making the key guidance available at the point of patient care.
This guidance was available in our algorithm but was not inherent in the structured insulin orders described in this report, and all basal and nutritional insulin options were offered as equally acceptable choices. This version did not calculate insulin doses or assist in the apportionment of insulin between basal and nutritional components. Only a single adjustment dose scale was offered, leaving appropriate modifications up to the end user, and from a usability standpoint, our CPOE insulin orders lacked dynamic flexibility (revising a single insulin required discontinuing all prior orders and reentering all orders). These limitations have subsequently been addressed with Version 2 of our CPOE insulin orders, and the details will soon be available in the literature.15
We are now exploring further improvement with concurrent identification and intervention of hyperglycemic patients that are not on physiologic insulin regimens or not meeting glycemic targets, and implementing protocols addressing the transition from infusion insulin.
CONCLUSION
We significantly improved glycemic control and simultaneously reduced hypoglycemia across all major medical and surgical services at our medical center, thereby addressing the number 1 barrier to improved inpatient glycemic control. We achieved this via systems changes with the introduction of structured subcutaneous insulin orders and the insulin management algorithm, along with education, but did not otherwise mandate or monitor adherence to our algorithm.
Implementing an institutional insulin management algorithm and structured insulin orders should now be viewed as a potent safety intervention as well as an intervention to enhance quality, and we have demonstrated that non‐critical care glycemic control efforts can clearly be a win‐win situation.
Diabetes has reached epidemic proportions in the United States, affecting over 20 million individuals,1 and further rises are expected. A disproportionate increase in diabetes has occurred in the inpatient setting.2 Furthermore, for every 2 patients in the hospital with known diabetes, there may be an additional 1 with newly observed hyperglycemia. Both are common. In 1 report, for example, 24% of inpatients with hyperglycemia had a prior diagnosis of diabetes, whereas another 12% had hyperglycemia without a prior diagnosis of diabetes.3
Although there is a paucity of high quality randomized controlled trials to support tight glycemic control in non‐critical care inpatient settings, poor glycemic control in hospitalized patients is strongly associated with undesirable outcomes for a variety of conditions, including pneumonia,4 cancer chemotherapy,5 renal transplant,6 and postsurgical wound infections.7, 8 Hyperglycemia also induces dehydration, fluid and electrolyte imbalance, gastric motility problems, and venous thromboembolism formation.9
Structured subcutaneous insulin order sets and insulin management protocols have been widely advocated as a method to encourage basal bolus insulin regimens and enhance glycemic control,2, 9, 10 but the effect of these interventions on glycemic control, hypoglycemia, and insulin use patterns in the real world setting has not been well reported. Fear of inducing hypoglycemia is often the main barrier for initiating basal insulin containing regimens and pursuing glycemic targets.2 The evidence would suggest, however, that sliding scale regimens, as opposed to more physiologic basal bolus regimens, may actually increase both hypoglycemic and hyperglycemic excursions.11 A convincing demonstration of the efficacy (improved insulin use patterns and reduced hyperglycemia) and safety (reduced hypoglycemia) of structured insulin order sets and insulin management protocols would foster a more rapid adoption of these strategies.
PATIENTS AND METHODS
In our 400‐bed university hospital, we formed a hospitalist‐led multidisciplinary team in early 2003, with the focus of improving the care delivered to non‐critical care patients with diabetes or hyperglycemia. We used a Plan‐Do‐Study‐Act (PDSA) performance improvement framework, and conducted institutional review board (IRB)‐approved prospective observational research in parallel with the performance improvement efforts, with a waiver for individual informed consent. The study population consisted of all adult inpatients on non‐critical care units with electronically reported point of care (POC) glucose testing from November 2002 through December 2005. We excluded patients who did not have either a discharge diagnosis of Diabetes (ICD 9 codes 250‐251.XX) or demonstrated hyperglycemia (fasting POC glucose >130 mg/dL 2, or a random value of >180 mg/dL) from analysis of glycemic control and hypoglycemia. Women admitted to Obstetrics were excluded. Monthly and quarterly summaries on glycemic control, hypoglycemia, and insulin use patterns (metrics described below) were reported to the improvement team and other groups on a regular basis throughout the intervention period. POC glucose data, demographics, markers of severity of illness, and diagnosis codes were retrieved from the electronic health record.
Interventions
We introduced several interventions and educational efforts throughout the course of our improvement. The 2 key interventions were as follows:
Structured subcutaneous insulin order sets (November, 2003).
An insulin management algorithm, described below (May 2005).
Key Intervention #1: Structured Subcutaneous Insulin Order Set Implementation
In November 2003, we introduced a paper‐based structured subcutaneous insulin order set. This order set encouraged the use of scheduled basal and nutritional insulin, provided guidance for monitoring glucose levels, and for insulin dosing. A hypoglycemia protocol and a standardized correction insulin table were embedded in the order set. This set was similar to examples of structured insulin ordering subsequently presented in the literature.9 In a parallel effort, the University of California, San Diego Medical Center (UCSDMC) was developing a computer physician order entry (CPOE) module for our comprehensive clinical information system, Invision (Siemens Medical Systems, Malvern, PA), that heretofore had primarily focused on result review, patient schedule management, and nursing documentation. In anticipation of CPOE and for the purpose of standardization, we removed outdated sliding scale insulin regimens from a variety preexisting order sets and inserted references to the standardized subcutaneous insulin order set in their stead. The medication administration record (MAR) was changed to reflect the basal/nutritional/correction insulin terminology. It became more difficult to order a stand‐alone insulin sliding scale even before CPOE versions became available. The standardized order set was the only preprinted correction scale insulin order available, and ordering physicians have to specifically opt out of basal and nutritional insulin choices to order sliding scale only regimens. Verbal orders for correction dose scales were deemed unacceptable by medical staff committees. Correctional insulin doses could be ordered as a 1‐time order, but the pharmacy rejected ongoing insulin orders that were not entered on the structured form.
We introduced our first standardized CPOE subcutaneous insulin order set in January 2004 at the smaller of our 2 campuses, and subsequently completed full deployment across both campuses in all adult medical‐surgical care areas by September 2004.
The CPOE version, like the paper version that immediately preceded it, encouraged the use of basal/bolus insulin regimens, promoted the terms basal, nutritional or premeal, and adjustment dose insulin in the order sets and the medication administration record, and was mandatory for providers wishing to order anything but a 1‐time order of insulin. Figure 1 depicts a screen shot of the CPOE version. Similar to the paper version, the ordering physician had to specifically opt out of ordering scheduled premeal and basal insulin to order a sliding scale only regimen. The first screen also ensured that appropriate POC glucose monitoring was ordered and endorsed a standing hypoglycemia protocol order. The CPOE version had only a few additional features not possible on paper. Obvious benefits included elimination of unapproved abbreviations and handwriting errors. Nutritional and correction insulin types were forced to be identical. Fundamentally, however, both the paper and online structured ordering experiences had the same degree of control over provider ordering patterns, and there was no increment in guidance for choosing insulin regimens, hence their combined analysis as structured orders.

Key Intervention #2: Insulin Management Algorithm
The structured insulin order set had many advantages, but also had many limitations. Guidance for preferred insulin regimens for patients in different nutritional situations was not inherent in the order set, and all basal and nutritional insulin options were offered as equally acceptable choices. The order set gave very general guidance for insulin dosing, but did not calculate insulin doses or assist in the apportionment of insulin between basal and nutritional components, and guidance for setting a glycemic target or adjusting insulin was lacking.
Recognizing these limitations, we devised an insulin management algorithm to provide guidance incremental to that offered in the order set. In April 2005, 3 hospitalists piloted a paper‐based insulin management algorithm (Figure 2, front; Figure 3, reverse) on their teaching services. This 1‐page algorithm provided guidance on insulin dosing and monitoring, and provided institutionally preferred insulin regimens for patients in different nutritional situations. As an example, of the several acceptable subcutaneous insulin regimens that an eating patient might use in the inpatient setting, we advocated the use of 1 preferred regimen (a relatively peakless, long‐acting basal insulin once a day, along with a rapid acting analog nutritional insulin with each meal). We introduced the concept of a ward glycemic target, provided prompts for diabetes education, and generally recommended discontinuation of oral hypoglycemic agents in the inpatient setting. The hospitalists were introduced to the concepts and the algorithm via 1 of the authors (G.M.) in a 1‐hour session. The algorithm was introduced on each teaching team during routine teaching rounds with a slide set (approximately 15 slides) that outlined the basic principles of insulin dosing, and gave example cases which modeled the proper use of the algorithm. The principles were reinforced on daily patient work rounds as they were applied on inpatients with hyperglycemia. The pilot results on 25 patients, compared to 250 historical control patients, were very promising, with markedly improved glycemic control and no increase in hypoglycemia. We therefore sought to spread the use of the algorithm. In May 2005 the insulin management algorithm and teaching slide set were promoted on all 7 hospitalist‐run services, and the results of the pilot and concepts of the algorithm were shared with a variety of house staff and service leaders in approximately a dozen sessions: educational grand rounds, assorted noon lectures, and subsequently, at new intern orientations. Easy access to the algorithm was assured by providing a link to the file within the CPOE insulin order set.


Other Attempts to Improve Care
Several other issues were addressed in the context of the larger performance improvement effort by the team. In many cases, hard data were not gathered to assess the effectiveness of the interventions, or the interventions were ongoing and could be considered the background milieu for the key interventions listed above.
During each intervention, education sessions were given throughout the hospital to staff, including physicians, residents, and nurses, using departmental grand rounds, nursing rounds, and in‐services to describe the process and goals. Patient education programs were also redesigned and implemented, using preprinted brochure. Front‐line nursing staff teaching skills were bolstered via Clinical Nurse Specialist educational sessions, and the use of a template for patient teaching. The educational template assessed patient readiness to learn, home environment, current knowledge, and other factors. Approximately 6 conferences directed at various physician staff per year became part of the regular curriculum.
We recognized that there was often poor coordination between glucose monitoring, nutrition delivery, and insulin administration. The traditional nursing practice of the 6:00 AM finger stick and insulin administration was changed to match a formalized nutrition delivery schedule. Nutrition services and nursing were engaged to address timeliness of nutrition delivery, insulin administration, and POC glucose documentation in the electronic health record.
Feedback to individual medicine resident teams on reaching glycemic targets, with movie ticket/coffee coupon rewards to high performing teams, was tried from April 2004 to September 2004.
Measures and Analyses
Assessing Insulin Use Patterns
A convenience sample gathering all subcutaneous insulin orders from 4 to 5 selected days per month yielded 70 to 90 subcutaneous insulin orders for review each month. Sampling was originally performed each month, followed by less frequent sampling once stability in insulin use patterns was reached. Regimens were categorized by pharmacy and hospitalist review as to whether basal insulin was part of the insulin regimen or not. The percentage of insulin regimens incorporating basal insulin was calculated for each sampled month and followed in run charts, and comparisons between preorder set and postorder set time periods were made using Pearson's chi square statistic.
Assessing Glycemic Control
Glycemic control and hypoglycemia parameters were monitored for the entire 38‐month observation period.
Routinely monitored POC glucose values were used to assess glycemic control. During the initial data examination, it was found after 14 days of the hospital stay, there was a notable stabilization and improvement in glucose control and fewer hypoglycemic events, therefore we examined only the first 14 days of hospitalization, thereby eliminating a potential source of bias from length of stay outliers.
A mean glucose value was recorded for each patient‐day with 1 or more recorded values. Glycemic control for each patient‐stay was calculated by averaging the patient‐day mean values, which we will refer to as the day‐weighted mean. Hypoglycemic values (60 mg/dL) were excluded from calculation of the mean glucose, to avoid equating frequent hypoglycemia with optimal glycemic control. An uncontrolled patient‐day was defined as a monitored patient‐day with a mean glucose 180 mg/dL. An uncontrolled patient‐stay is defined as a patient‐stay with a day‐weighted mean glucose value 180 mg/dL.
We theorized that the greatest impact of the interventions would be realized in patients with longer monitoring periods, and that those with only a few POC glucose values could potentially misrepresent the impact of our interventions: therefore we performed a second analysis restricted to patients with 8 POC glucose values.
Assessing Hypoglycemia
Hypoglycemia was defined as a glucose 60 mg/dL, and severe hypoglycemia was defined as a glucose 40 mg/dL. These parameters were characterized by 2 methods. First, we calculated the percentage of monitored patients suffering from 1 or more hypoglycemic events or severe hypoglycemic events over the course of their entire admission. A second method tracked the percentage of monitored patient‐days with hypoglycemia and severe hypoglycemia, thereby correcting for potential misinterpretation from clustered repeated measures or variable length of stay. As with the glycemic control analysis, we repeated the hypoglycemia analysis in the subset of patients with 8 POC glucose values.
Summary Analysis of Glycemic Control and Hypoglycemia
Pearson chi square values, with relative risks (RRs) and 95% confidence intervals (CIs) were calculated to compare glycemic control and hypoglycemia in the 2 key interventions and baseline. The interventions and data reporting were grouped as follows:
Baseline: November 2002 to October 2003) = Time Period 1 (TP1)
Structured Order Set: November 2003 to April 2005) = Time Period 2 (TP2)
Algorithm plus Structured Order Set: May 2005 to December 2005) = Time Period 3 (TP3)
A P value of less than 0.05 was determined as significant and data were analyzed using STATA, Version 8 (STATA Corp., College Station, TX).
We assigned the RR of uncontrolled hyperglycemia and the RR of hypoglycemia during the baseline time (TP1) with values of 1.0, and calculated the RR and CIs for the same parameters during TP2 and TP3.
RESULTS
Just over 11,000 patients were identified for POC glucose testing over the 38 month observation period. Of these, 9314 patients had either a diagnosis of diabetes or documented hyperglycemia. The characteristics of this study population are depicted in Table 1. There were no differences between the groups and the demographics of age, gender, or length of stay (P > 0.05 for all parameters). There was a slight increase in the percent of patients with any intensive care unit days over the 3 time periods and a similar increase in the case mix index.
| Patients Meeting Criteria of Diabetes Mellitus Diagnosis or Hyperglycemia (n = 9,314 patients) | Baseline | TP2 | TP3 |
|---|---|---|---|
| |||
| Time period (TP) | November 2002 to October 2003 | November 2003 to April 2005 | May 2005 to December 2005 |
| Monitored patient days (44,232) | 11,571 | 21,126 | 11,535 |
| Number of patients (9,314) | 2,504 | 4,515 | 2,295 |
| Males (%) | 55 | 54 | 56 |
| Average age standard deviation | 56 17 | 56 17 | 56 16 |
| Length of stay (excluding highest 1% of outliers) | 4.6 5.9 | 4.6 5.7 | 4.8 5.8 |
| % With any intensive care unit days* | 18 | 20 | 22 |
| Case mix index score (mean SD) | 1.8 2.1 | 2.0 2.3 | 2.1 2.1 |
| Case mix index (median score) | 1.1 | 1.3 | 1.3 |
Of the 9314 study patients, 5530 had 8 or more POC glucose values, and were included in a secondary analysis of glycemic control and hypoglycemia.
Insulin Use Patterns
Figure 4 demonstrates the dramatic improvement that took place with the introduction of the structured order set. In the 6 months preceding the introduction of the structured insulin order set (May‐October 2003) 72% of 477 sampled patients with insulin orders were on sliding scale‐only insulin regimens (with no basal insulin), compared to just 26% of 499 patients sampled in the March to August 2004 time period subsequent to order set implementation (P < .0001, chi square statistic). Intermittent monthly checks on insulin use patterns reveal this change has been sustained.

Glycemic Control
A total of 9314 patients with 44,232 monitored patient‐days and over 120,000 POC glucose values were analyzed to assess glycemic control, which was improved with structured insulin orders and improved incrementally with the introduction of the insulin management algorithm.
The percent of patient‐days that were uncontrolled, defined as a monitored day with a mean glucose of 180 mg/dL, was reduced over the 3 time periods (37.8% versus 33.9% versus 30.1%, P < 0.005, Pearson chi square statistic), representing a 21% RR reduction of uncontrolled patient‐days from TP1 versus TP3. Table 2 shows the summary results for glycemic control, including the RR and CIs between the 3 time periods.
| Time Period (TP) | Baseline | TP2 Structured Orders | TP3 Orders Plus Algorithm | Relative Risk TP3:TP2 |
|---|---|---|---|---|
| ||||
| Patient‐day glucose | ||||
| Mean SD | 179 66 | 170 65 | 165 58 | |
| Median | 160 | 155 | 151 | |
| Uncontrolled patient‐days* | 4,372 | 7,162 | 3,465 | |
| Monitored patient‐days | 11,555 | 21,135 | 11,531 | |
| % Uncontrolled patient‐days | 37.8 | 33.9 | 30.1 | |
| RR: uncontrolled patient‐day (95% confidence interval) | 1.0 | 0.89 (0.87‐0.92) | 0.79 (0.77‐0.82) | 0.89 (0.86‐0.92) |
| Glycemic control by patient‐stay | ||||
| Day‐weighted mean SD | 177 57 | 174 54 | 170 50 | |
| Day‐weighted median | 167 | 162 | 158 | |
| Uncontrolled patient‐stay (%) | 1,038 | 1,696 | 784 | |
| Monitored patient‐stay | 2,504 | 4,515 | 2,295 | |
| % Uncontrolled patient‐stays | 41.5 | 37.6 | 34.2 | |
| RR: uncontrolled patient‐stay (95% confidence interval) | 0.91 (0.85‐0.96) | 0.84 (0.77‐0.89) | 0.91 (0.85‐0.97) | |
In a similar fashion, the percent of patients with uncontrolled patient‐stays (day‐weighted mean glucose 180 mg/dL) was also reduced over the 3 time periods (41.5% versus 37.6% versus 34.2%, P < 0.05, Pearson chi square statistic, with an RR reduction of 16% for TP3:TP1). Figure 5 depicts a statistical process control chart of the percent of patients experiencing uncontrolled patient‐stays over time, and is more effective in displaying the temporal relationship of the interventions with the improved results.

Uncontrolled hyperglycemic days and stays were reduced incrementally from TP3 versus TP2, reflecting the added benefit of the insulin management algorithm, compared to the benefit enjoyed with the structured order set alone.
When the analyses were repeated after excluding patients with fewer than 8 POC glucose readings (Table 3), the findings were similar, but as predicted, the effect was slightly more pronounced, with a 23% relative reduction in uncontrolled patient‐days and a 27% reduction in uncontrolled patient‐stays of TP3 versus TP1.
| Time Period (TP) | Baseline | TP2 Structured Orders | TP3 Orders Plus Algorithm | Relative Risk TP3:TP2 |
|---|---|---|---|---|
| ||||
| Patient‐day glucose | ||||
| Mean SD | 172 65 | 169 64 | 163 57 | |
| Median | 159 | 154 | 149 | |
| Uncontrolled patient‐days* | 3,469 | 5,639 | 2,766 | |
| Monitored patient‐days | 9,304 | 17,278 | 9,671 | |
| % Uncontrolled patient‐days | 37.3 | 32.6 | 28.6 | |
| RR: uncontrolled patient‐day (95% confidence interval) | 1.0 | 0.87 (0.85‐0.90) | 0.77 (0.74‐0.80) | 0.88 (0.84‐0.91) |
| Glycemic control by patient‐stay | ||||
| Day‐weighted mean SD | 175 51 | 169 47 | 166 45 | |
| Day‐weighted median | 167 | 158 | 155 | |
| Uncontrolled patient‐stay (%) | 588 | 908 | 425 | |
| Monitored patient‐stay | 1,439 | 2,659 | 1,426 | |
| % Uncontrolled patient‐stays | 40.1 | 34.1 | 29.8 | |
| RR: Uncontrolled patient‐stay (95% confidence interval) | 0.84 (0.77‐0.91) | 0.73 (0.66‐0.81) | 0.87 (0.79‐0.96) | |
Hypoglycemia
Table 4 summarizes the results for hypoglycemia and severe hypoglycemia in the study population, and Table 5 summarizes the secondary analyses of hypoglycemia in the subset with at least 8 POC glucose readings.
| TP (Time Period) | Baseline | TP2 | TP3 | Relative Risk TP3:TP2 |
|---|---|---|---|---|
| ||||
| Monitored patient‐stays | 2504 | 4515 | 2295 | |
| Stays with hypoglycemia (%) | 296 (11.8) | 437 (9.7) | 210 (9.2) | |
| RR hypoglycemic stay (CI) | 1.0 | 0.82 (0.72‐0.94) | 0.77 (0.65‐0.92) | 0.95 (0.81‐1.10) |
| Stays with severe hypoglycemia (%) | 73 (2.9) | 96 (2.1) | 55 (2.4) | |
| RR severe hypoglycemic stay (CI) | 1.0 | 0.73 (0.54‐0.98) | 0.82 (0.58‐1.16) | 1.13 (0.81‐1.56) |
| Monitored patient‐days | 11,584 | 21,158 | 11,548 | |
| Days with hypoglycemia (%) | 441 (3.8) | 623 (2.9) | 300 (2.6) | |
| RR hypoglycemic day (CI) | 1.0 | 0.77 (0.69‐0.87) | 0.68 (0.59‐0.78) | 0.88 (0.77‐1.01) |
| Days with severe hypoglycemia (%) | 86 (0.74) | 109 (0.52) | 66 (0.57) | |
| RR Severe hypoglycemic day (CI) | 1.0 | 0.69 (0.52‐0.92) | 0.77 (0.56‐1.06) | 1.10 (0.82‐1.5) |
| TP (Time Period) | Baseline | TP2 | TP3 | Relative Risk TP3:TP2 |
|---|---|---|---|---|
| ||||
| Monitored patient‐stays | 1440 | 2664 | 1426 | |
| Stays with hypoglycemia (%) | 237 (16.5) | 384 (14.4) | 180 (12.6) | |
| RR hypoglycemic stay (CI) | 1.0 | 0.88 (0.76‐1.02) | 0.77 (0.64‐0.92) | 0.88 (0.75‐1.03) |
| Stays with severe hypoglycemia (%) | 58 (4.0) | 93 (3.5) | 47 (3.3) | |
| RR severe hypoglycemic stay (CI) | 1.0 | 0.87 (0.63‐1.2) | 0.82 (0.56‐1.19) | 0.94 (0.67‐1.33) |
| Monitored patient‐days | 9,317 | 17,310 | 9,684 | |
| Days with hypoglycemia (%) | 379 (4.1) | 569 (3.3) | 269 (2.7) | |
| RR hypoglycemic day (CI) | 1.0 | 0.81 (0.71‐0.92) | 0.68 (0.59‐0.80) | 0.85 (0.73‐0.98) |
| Days with severe hypoglycemia (%) | 71 (0.76) | 106 (0.61) | 58 (0.60) | |
| RR severe hypoglycemic day (CI) | 1.0 | 0.80 (0.60‐1.08) | 0.79 (0.56‐1.11) | 0.98 (0.71‐1.34) |
Analysis by Patient‐Stay
The percent of patients that suffered 1 or more hypoglycemic event over the course of their inpatient stay was 11.8% in TP1, 9.7% in TP2, and 9.2% in TP3. The RR of a patient suffering from a hypoglycemic event was significantly improved in the intervention time periods compared to baseline, with the RR of TP3:TP1 = 0.77 (CI, 0.65‐0.92). There was a strong trend for incremental improvement in hypoglycemic patient‐stays for TP3 versus TP2, but the trend just missed statistical significance (P < 0.07). Similar trends in improvement were found for severe hypoglycemia by patient‐stay, but these trends were only statistically significant for TP2 versus TP1. The findings were similar in the subset of patients with at least 8 POC glucose readings (Table 5).
Analysis by Patient‐Day
Of monitored patient days in the baseline TP1, 3.8% contained a hypoglycemic value of 60 mg/dL. With the introduction of structured insulin orders in TP2, this was reduced to 2.9%, and in TP3 it was 2.6%. The RR of a hypoglycemic patient‐day of TP2 compared to TP1 was 0.77 (CI, 0.69‐0.87), whereas the cumulative impact of the structured order set and algorithm (TP3:TP1) was 0.68 (CI, 0.59‐0.78), representing a 32% reduction of the baseline risk of suffering from a hypoglycemic day. Similar reductions were seen for the risk of a severe hypoglycemic patient‐day.
The secondary analysis of hypoglycemic and severe hypoglycemic patient‐days showed very similar results, except that the TP3:TP2 RR for hypoglycemia of 0.85 (CI, 0.73‐0.98) reached statistical significance, again demonstrating the incrementally beneficial effect of the insulin management algorithm.
DISCUSSION
Our study convincingly demonstrates that significant improvement in glycemic control can be achieved with implementation of structured subcutaneous insulin orders and a simple insulin management protocol. Perhaps more importantly, these gains in glycemic control are not gained at the expense of increased iatrogenic hypoglycemia, and in fact, we observed a 32% decline in the percent of patient‐days with hypoglycemia. This is extremely important because fear of hypoglycemia is the most significant barrier to glycemic control efforts.
Strengths and Limitations
Our study has several strengths. The study is large and incorporates all patients with diabetes or hyperglycemia captured by POC glucose testing, and the observation period is long enough that bias from merely being observed is not a factor. We used metrics for glycemic control, hypoglycemia, and insulin use patterns that are of high quality and are generally in line with the Society of Hospital Medicine (SHM) Glycemic Control Task force recommendations,12, 13 and examined data by both patient‐stay and patient‐day.
The increased use of anticipatory physiologic subcutaneous insulin regimens, and the subsequent decline in the use of sliding scale insulin, is the most likely mechanism for improvement. The improvements seen are fairly dramatic for an institution in absolute terms, because inpatient hyperglycemia and hypoglycemia are so common. For example, on an annualized basis for our 400‐bed medical center, these interventions prevent 124 patients from experiencing 208 hypoglycemic days.
Other institutions should be able to replicate our results. We received administrative support to create a multidisciplinary steering committee, but we did not have incremental resources to create a dedicated team for insulin management, mandated endocrinology comanagement or consultations, or manual data collection. In fact, we had only 1 diabetes educator for 400 adult beds at 2 sites, and were relatively underresourced in this area by community standards. There was some time and expense in creating the glycemic control reports, but all of the glucose data collected were part of normal care, and the data retrieval became automated.
The main limitation of this study lies in the observational study design. There were multiple interventions in addition to structured insulin orders and the insulin management algorithm, and these educational and organizational changes undoubtedly also contributed to the overall success of our program. Since we did not perform a randomized controlled trial, the reader might reasonably question if the structured order sets and insulin management algorithm were actually the cause of the improvement seen, as opposed to these ancillary efforts or secular change. However, there are several factors that make this unlikely. First, the study population was well‐defined, having diabetes or documented hyperglycemia in all 3 time periods. Second, the demographics remained constant or actually worked against improvement trends, since the markers of patient acuity suggest increased patient acuity over the observation period. Third, the temporal relationship of the improvement to the introduction of our key interventions, as viewed on statistical process control charts shown in Figure 5, strongly suggest a causal relationship. This temporal relationship was consistently observed no matter how we chose to define uncontrolled hyperglycemia, and was also seen on hypoglycemia control charts. We view the ancillary interventions (such as educational efforts) as necessary, but not sufficient, in and of themselves, to effect major improvement.
We did not analyze the impact of the improved glycemic control on patient outcomes. In the absence of a randomized controlled trial design, controlling for the various confounders is a challenging task. Also, it is likely that not all hypoglycemic events were attributable to inpatient glycemic control regimens, though the secondary analysis probably eliminated many hypoglycemia admissions.
Lessons Learned: Implications from our study
We agree with the American Association of Clinical Endocrinologists (AACE)/American Diabetes Association (ADA)2 and the SHM Glycemic Control Task Force12 about the essential elements needed for successful implementation of inpatient glycemic control programs:
An appropriate level of administrative support.
Formation of a multidisciplinary steering committee to drive the development of initiatives, empowered to enact changes.
Assessment of current processes, quality of care, and barriers to practice change.
Development and implementation of interventions, including standardized order sets, protocols, policies, and algorithms with associated educational programs.
Metrics for evaluation of glycemic control, hypoglycemia, insulin use patterns, and other aspects of care.
Metrics to follow hypoglycemia are extremely important. The voluntary reporting on insulin‐induced hypoglycemia fluctuated widely over the course of our project. These fluctuations did not correlate well with the more objective and accurate measures we followed, and this objective data was very helpful in reducing the fear of hypoglycemia, and spreading the wider use of basal bolus insulin regimens. We strongly recommend that improvement teams formulate and follow measures of glycemic control, hypoglycemia, and insulin use, similar to those outlined in the SHM Glycemic Control Improvement Guide12 and the SHM Glycemic Control Task Force summary on glucometrics.13
Although we introduced our structured insulin order set first, with a long lag time until we introduced the insulin management algorithm, we advocate a different approach for institutions grappling with these issues. This approach is well‐described by the SHM Glycemic Control Task Force.14 An insulin management algorithm should be crafted first, integrating guidance for insulin dosing, preferred insulin regimens for different nutritional situations, a glycemic target, insulin dosing adjustment, glucose monitoring, and prompts for ordering a glycosylated hemoglobin (A1c) level. Next, the order set and the supporting educational programs should integrate this guidance as much as possible, making the key guidance available at the point of patient care.
This guidance was available in our algorithm but was not inherent in the structured insulin orders described in this report, and all basal and nutritional insulin options were offered as equally acceptable choices. This version did not calculate insulin doses or assist in the apportionment of insulin between basal and nutritional components. Only a single adjustment dose scale was offered, leaving appropriate modifications up to the end user, and from a usability standpoint, our CPOE insulin orders lacked dynamic flexibility (revising a single insulin required discontinuing all prior orders and reentering all orders). These limitations have subsequently been addressed with Version 2 of our CPOE insulin orders, and the details will soon be available in the literature.15
We are now exploring further improvement with concurrent identification and intervention of hyperglycemic patients that are not on physiologic insulin regimens or not meeting glycemic targets, and implementing protocols addressing the transition from infusion insulin.
CONCLUSION
We significantly improved glycemic control and simultaneously reduced hypoglycemia across all major medical and surgical services at our medical center, thereby addressing the number 1 barrier to improved inpatient glycemic control. We achieved this via systems changes with the introduction of structured subcutaneous insulin orders and the insulin management algorithm, along with education, but did not otherwise mandate or monitor adherence to our algorithm.
Implementing an institutional insulin management algorithm and structured insulin orders should now be viewed as a potent safety intervention as well as an intervention to enhance quality, and we have demonstrated that non‐critical care glycemic control efforts can clearly be a win‐win situation.
- Centers for Disease Control and Prevention.National Diabetes Fact Sheet: General Information and National Estimates on Diabetes in the United States, 2002.Atlanta, GA:U.S. Department of Health and Human Services, Centers for Disease Control and Prevention;2003. Available at: www.cdc.gov/diabetes/pubs/factsheet.htm. Accessed January 21, 2006.
- American College of Endocrinology and American Diabetes Association Consensus statement on inpatient diabetes and glycemic control: a call to action.Diabetes Care.2006;29:1955‐1962.
- ,,, et al.Hyperglycemia: an independent marker of in‐hospital mortality in patients with undiagnosed diabetes.J Clin Endocrinol Metab.2002;87:978‐982.
- ,,, et al.The relation between hyperglycemia and outcomes in 2471 patients admitted to the hospital with community‐acquired pneumonia.Diabetes Care.2005;28:810‐815.
- ,,, et al.Cancer.2004;100:1179‐1185.
- ,,, et al.Early perioperative glycaemic control and allograft rejection in patients with diabetes mellitus: a pilot study.Transplantation.2001;72:1321‐1324.
- ,,, et al.Early postoperative glucose control predicts nosocomial infection rate in diabetic patients.J Parenter Enteral Nutr.1998;22:77‐81.
- ,,, et al.Glucose control lowers the risk of wound infection in diabetics after open heart operations.Ann Thorac Surg.1997;63:356‐361.
- ,,, et al.Management of diabetes and hyperglycemia in hospitals.Diabetes Care.2004;27:553‐591.
- ,,, et al.American College of Endocrinology position statement on inpatient diabetes and metabolic control.Endocr Pract.2004;10:77‐82.
- ,.Glycemic chaos (not glycemic control) still the rule for inpatient care: how do we stop the insanity? [Editorial].J Hosp Med.2006;1:141‐144.
- Society of Hospital Medicine Glycemic Control Task Force: Optimizing Glycemic Control and Reducing Hypoglycemia at Your Medical Center. Society of Hospital Medicine, Glycemic Control Quality Improvement Resource Room. Available at: http://www.hospitalmedicine.org/ResourceRoomRedesign/GlycemicControl.cfm. Accessed October2008.
- ,,,,,.SHM Glycemic Control Task Force summary: practical recommendations for assessing the impact of glycemic control efforts.J Hosp Med.2008;3(S5):66–75.
- ,,,;for the SHM Glycemic Control Task Force.Subcutaneous insulin order sets and protocols: effective design and implementation strategies.J Hosp Med.2008;3(S5):29–41.
- ,,,.Indication‐based ordering: a new paradigm for glycemic control in hospitalized inpatients.J Diabetes Sci Tech.2008;2(3):349‐356.
- Centers for Disease Control and Prevention.National Diabetes Fact Sheet: General Information and National Estimates on Diabetes in the United States, 2002.Atlanta, GA:U.S. Department of Health and Human Services, Centers for Disease Control and Prevention;2003. Available at: www.cdc.gov/diabetes/pubs/factsheet.htm. Accessed January 21, 2006.
- American College of Endocrinology and American Diabetes Association Consensus statement on inpatient diabetes and glycemic control: a call to action.Diabetes Care.2006;29:1955‐1962.
- ,,, et al.Hyperglycemia: an independent marker of in‐hospital mortality in patients with undiagnosed diabetes.J Clin Endocrinol Metab.2002;87:978‐982.
- ,,, et al.The relation between hyperglycemia and outcomes in 2471 patients admitted to the hospital with community‐acquired pneumonia.Diabetes Care.2005;28:810‐815.
- ,,, et al.Cancer.2004;100:1179‐1185.
- ,,, et al.Early perioperative glycaemic control and allograft rejection in patients with diabetes mellitus: a pilot study.Transplantation.2001;72:1321‐1324.
- ,,, et al.Early postoperative glucose control predicts nosocomial infection rate in diabetic patients.J Parenter Enteral Nutr.1998;22:77‐81.
- ,,, et al.Glucose control lowers the risk of wound infection in diabetics after open heart operations.Ann Thorac Surg.1997;63:356‐361.
- ,,, et al.Management of diabetes and hyperglycemia in hospitals.Diabetes Care.2004;27:553‐591.
- ,,, et al.American College of Endocrinology position statement on inpatient diabetes and metabolic control.Endocr Pract.2004;10:77‐82.
- ,.Glycemic chaos (not glycemic control) still the rule for inpatient care: how do we stop the insanity? [Editorial].J Hosp Med.2006;1:141‐144.
- Society of Hospital Medicine Glycemic Control Task Force: Optimizing Glycemic Control and Reducing Hypoglycemia at Your Medical Center. Society of Hospital Medicine, Glycemic Control Quality Improvement Resource Room. Available at: http://www.hospitalmedicine.org/ResourceRoomRedesign/GlycemicControl.cfm. Accessed October2008.
- ,,,,,.SHM Glycemic Control Task Force summary: practical recommendations for assessing the impact of glycemic control efforts.J Hosp Med.2008;3(S5):66–75.
- ,,,;for the SHM Glycemic Control Task Force.Subcutaneous insulin order sets and protocols: effective design and implementation strategies.J Hosp Med.2008;3(S5):29–41.
- ,,,.Indication‐based ordering: a new paradigm for glycemic control in hospitalized inpatients.J Diabetes Sci Tech.2008;2(3):349‐356.
Copyright © 2009 Society of Hospital Medicine
Introduction
The articles on these pages represent the culmination of 3 years of effort by the Society of Hospital Medicine (SHM) Glycemic Control Task Force. In this brief introduction, we share a few insights and comments about this multidisciplinary collaborative effort to address the care of inpatients with hyperglycemia.
The SHM Glycemic Control Task Force was assembled in 2005, with the intent of improving the care of inpatients with diabetes. We wished to provide hospitalists and quality improvement teams with an understanding of the best practices to achieve safe glycemic control in the hospital. Additionally, this task force sought to identify tools and strategies to obtain improved communication, medication safety, education, and other aspects of care. A distinguished panel of experts attended their inaugural meeting in Chicago, Illinois, in October 2005, including hospitalists, endocrinologists, nurses, case managers, diabetes educators, and pharmacists. A roster of the individuals and organizations is given in the Appendix.
Many members of the SHM Glycemic Control Task Force also participated in the Call to Action consensus conference1 hosted by the American Association of Clinical Endocrinologists (AACE) and the American Diabetes Association (ADA) in January 2006. Both groups identified several barriers to improvement, and methods to overcome these barriers were summarized in this quote from the Consensus Conference,
Successful implementation of a program to improve glycemic control in the inpatient setting should include the following components:
-
An appropriate level of administrative support.
-
Formation of a multidisciplinary steering committee to drive the development of initiatives.
-
Assessment of current processes, quality of care, and barriers to practice change.
-
Development and implementation of interventions including standardized order sets, protocols, policies, and algorithms with associated educational programs.
-
Metrics for evaluation.
Both groups also called for a web‐based compendium of tested tools and strategies to assist local improvement teams.
After countless hours of development and revision by the SHM Glycemic Task Force and the Resource Room team, such a compendium addressing all of these components was launched on the SHM web site in the form of the SHM Glycemic Control Resource Room.2 A comprehensive implementation guide3 is available for downloading free of charge, and serves as the centerpiece of the Resource Room. Subsequently, this comprehensive but somewhat sprawling implementation guide evolved into these more sophisticated and concise articles.410 The topics include a review of the rationale for improving inpatient glycemic control,4 an important call for standardizing the metrics of glycemic control,9 subcutaneous insulin regimens and order sets,5, 6 insulin infusion protocols,7 transitions of care,8 and the business case for glycemic control.10 It has been a long but rewarding and educational journey, drawing on the collective experience from dozens of institutions in all kinds of inpatient care settings. A few key points and insights seem worth sharing.
THE IMPROVEMENT EFFORT IS NOT JUST ABOUT REACHING A GLYCEMIC TARGET
The term glycemic control team and the label for the SHM Glycemic Control Task Force itself are somewhat misnomers. Task Force members agree that desirable institutional glycemic target ranges should be established, but many among us believe the glycemic targets endorsed by national guidelines (ref ADA and AACE guidelines)11, 12 are too stringent. Furthermore, we believe that achieving glycemic control is just one small part of the needed improvement efforts. Uncontrolled hyperglycemia is common, potentially dangerous, and largely preventable with safe and proven methodsbut so are the iatrogenic hypoglycemia episodes, substandard education, poor communication, lack of care coordination, and inadequate monitoring that typify care of the hyperglycemic inpatient. We address all of these issues and urge the adoption of this broader perspective.
THE EVIDENCE IS INCOMPLETEBUT ACTION IS REQUIRED
We acknowledge gaps and inconsistencies in the literature surrounding inpatient diabetes management and the controversy around tight glycemic control. In many cases, high level evidence is not available to guide the formulation of protocols, order sets, or other improvement tools. We are all struck by how pervasive the lack of evidence is. What is the best metric for inpatient glycemic control or hypoglycemia? What is the best regimen for a patient on continuous tube feedings? Which insulin infusion protocol is superior in reaching and maintaining a glycemic target range?
Rather than make no recommendations or accept negative inertia on the basis of less than perfect evidence, we make recommendations based on the best evidence available. When we make recommendations based on consensus opinion or the collective experience from dozens of medical centers, rather than randomized trials, we have made every effort to make this clear in the text of the articles. In our view, incomplete evidence is not an adequate excuse to persist in the unacceptable status quo, clinging on to methods (such as sliding scale insulin regimens) that have been shown to be ineffective and potentially dangerous.1315
COLLABORATION PAYS DIVIDENDS
It takes a multidisciplinary approach to make substantial improvement in glycemic control of hospitalized patients. By the same token, it is unlikely that any one group can advance the national agenda for improved care as well as a multidisciplinary coordinated effort. Team members, especially the endocrinologists and hospitalists, collaborated skillfully throughout this effort. The hospitalists learned a tremendous amount from the expertise, insight, and mastery of the literature, offered by the endocrinology members, whereas the endocrinologists appreciated the front line expertise and practical quality improvement approach of the hospitalist members. This collaboration serves as a model for making guidelines and best practices become more of a practical reality for a variety of important clinical problems. Hospitalists can partner with and learn from a variety of other disciplines, while they assist these disciplines on effective improvement and implementation efforts. On a more personal note, this work has fostered mutual respect, friendship, and career long collaborative opportunities. The potential for these same opportunities with nursing, pharmacy, and all medical and surgical fields seems compelling and exciting.
THERE'S MORE!
By the time this is published, these articles will be integrated into the third iteration of the SHM Glycemic Control Resource Room. This online resource has already undergone 2 major revisions since its inception just a few years ago, reflecting SHM's dedication to the continuous improvement of the products and services that it offers. The Glycemic Control Implementation Guide and Resource Room will continue to be a work in progress. We highly encourage and welcome constructive criticism and feedback via E‐mail to
NEXT STEPS
More research and demonstration projects are obviously needed in this field. Local collaborative activities have sprung up in several cities and regions, as well as Glycemic Control Champions courses. A longitudinal mentoring program (similar to the SHM Venous Thromboembolism Prevention collaborative) would undoubtedly be beneficial, and may become available within the next year or so. These items and more will be promoted and posted in the resource room whenever possible.
Finally, the next step is up to you and the institutions in which you workyou have to decide, as individuals and institutions, if you believe the status quo is good enough. We believe that if you look, you'll find the care of our inpatients with diabetes and hyperglycemia disturbingly suboptimal, and hope that the work of the SHM Glycemic Control Task Force can help you rapidly improve on this state of affairs.
APPENDIX: GLYCEMIC CONTROL TASK FORCE
The Society of Hospital Medicine thanks all the members of the Glycemic Control Task Force, who encompass a distinguished panel of experts with representation from the AACE, ADA, ACP, and other organizations whose expertise was essential to the construction of the Glycemic Control Resource Room and the Implementation Guide for Glycemic Control and Prevention of Hypoglycemia.
Hospitalists
Representing the Society of Hospital Medicine
-
Gregory Maynard, MD. Lead Author and Editor of Glycemic Control Implementation Guide (web product); Glycemic Control Initiative Project Director; Clinical Professor of Medicine and Chief, Division of Hospital Medicine. University of California, San Diego (UCSD) Medical Center, San Diego, California.
-
David H. Wesorick, MD. Co‐editor of Glycemic Control Implementation Guide (web product); Clinical Assistant Professor of Internal Medicine, University of Michigan, Ann Arbor, Michigan.
-
Cheryl O'Malley, MD. Associate Program Director, Internal Medicine Faculty, Medicine/Pediatrics Banner, Good Samaritan Medical Center; Clinical Assistant Professor of Medicine, University of Arizona College of Medicine, Phoenix, Arizona.
-
Kevin Larsen, MD. Assistant Professor of Internal Medicine, University of Minnesota; Associate Program Director, Internal Medicine Residency, Hennepin County Medical Center, Minneapolis, Minnesota.
-
Jeffrey L. Schnipper, MD, MPH. Associate Physician, Brigham and Women's Hospital, Boston, Massachusetts
-
Alpesh Amin, MD, MBA, FACP. Executive Director and Vice Chair, University of California (UC) Irvine Hospitalist Program, Irvine, California.
-
Lakshmi Halasyamani, MD. Associate Chair, Department of Medicine, St. Joseph Mercy Medical Center, Ann Arbor, Michigan.
-
Mitchell J. Wilson, MD. Associate Professor of Medicine, University of North Carolina, Chapel Hill, North Carolina.
Representing the American College of Physicians
-
Doren Schneider, MD. Associate Program Director, Internal Medicine Residency Director, Ambulatory Service Unit, Abington Adult Medical Associates; Assistant Professor of Medicine, Temple University School of Medicine, Abington, Pennsylvania.
Endocrinologists
Representing the American Diabetes Association
-
Andrew J. Ahmann, MD. Associate Professor of Medicine, Director, Diabetes Center, Oregon Health & Science University, Portland, Oregon.
-
Michelle F. Magee, MD. Associate Professor of Medicine, Georgetown University School of Medicine Medstar Diabetes and Research Institutes, Washington Hospital Center, Washington, DC.
Representing the American Association of Clinical Endocrinologists
-
Richard Hellman, MD, FACP, FACE. Clinical Professor of Medicine, University of MissouriKansas City, North Kansas City, Missouri.
Endocringology Expert Panel
-
Susan Shapiro Braithwaite, MD, FACP, FACE. Clinical Professor of Medicine, University of North Carolina, Chapel Hill, North Carolina.
-
Mary Ann Emanuele, MD, FACP. Professor of Medicine, Endocrinology, Cell Biology, Neurobiology, and Anatomy Biochemistry, Loyola University Medical Center, Maywood, Illinois.
-
Irl B. Hirsch, MD. Professor of Medicine, University of Washington, Seattle, Washington.
-
Robert Rushakoff, MD. Clinical Professor of Medicine, Director, Diabetes Program, University of California, San Francisco (UCSF)/Mt. Zion, San Francisco, California.
-
Silvio E. Inzucchi, MD. Professor of Medicine, Clinical Director, Section of Endocrinology, Yale University School of Medicine, New Haven, Connecticut.
Education
-
Marcia D. Draheim, RN, CDE. Program Supervisor, Diabetes Center, St Luke's Hospital, Cedar Rapids, Iowa.
-
Sharon Mahowald, RN, CDE. Inpatient Diabetes Coordinator, Hennepin County Medical Center, Minneapolis, Minnesota.
Financial
-
Adam Beck, MHS, FABC. MedStar Research Institute, Washington, DC.
Pharmacists
-
Stuart T. Haines, PharmD, FASHP, FCCP, BCPS. Associate Professor/Vice Chair for Education, University of Maryland School of Pharmacy Baltimore, Maryland.
Representing the American Society of Consultant Pharmacists
-
Donald K. Zettervall, RPh, CDE, CDM. Owner/Director, The Diabetes Education Center, Old Saybrook, Connecticut.
Case Management
Representing the Case Management Society of America
-
Cheri Lattimer, RN, BSN. Executive Director, Case Management Society of America, Little Rock, Arkansas.
-
Nancy Skinner, RN, CCM. Director, Case Management Society of America Principle Consultant, Riverside HealthCare Consulting, Whitwell, Tennessee.
Dietetics
-
Carrie Swift, MS, RD, BC‐ADM. Dietetics Coordinator, Veterans Affairs Medical Center, Walla Walla, Washington.
SHM Staff Members
-
Geri Barnes and Joy Wittnebert.
Glycemic Control Resource Room Project Team
-
Greg Maynard, Jason Stein, David Wesorick, Mary Ann Emanuele, Kevin Larsen, Geri Barnes, Joy Wittnebert, and Bruce Hansen.
- Inpatient Diabetes and Glycemic Control: A Call to Action Conference. Position statement. AACE, February 2006. Available at: http://www.aace.com/meetings/consensus/IIDC/IDGC0207.pdf. Accessed October 2006. Garber et al. Endocr Pract.2006;12(suppl 3):3–13.
- Society of Hospital Medicine. Glycemic Control Resource Room. Available at: http://www.hospitalmedicine.org/ResourceRoomRedesign/GlycemicControl.cfm. Accessed May2008.
- Society of Hospital Medicine Glycemic Control Task Force. Implementation guide: improving glycemic control, preventing hypoglycemia, and optimizing care of the inpatient with hyperglycemia and diabetes. Published January 2007 on the Society of Hospital Medicine Website. Available at: http://www.hospitalmedicine.org. Accessed May2008.
- ,,,,,.The case for supporting inpatient glycemic control programs now: the evidence and beyond.J Hosp Med.2008;3(5 suppl 5)( ):S6–S16.
- ,,,,.Management of diabetes and hyperglycemia in the hospital: a practical guide to subcutaneous insulin use in the non‐critically ill adult patient.J Hosp Med.2008;3(5 suppl 5)( ):S17–S28.
- ,,,.Subcutaneous insulin order sets and protocols: effective design and implementation strategies.J Hosp Med.2008;3(5 suppl 5)( ):S29–S41.
- ,.Designing and implementing insulin infusion protocols and order sets.J Hosp Med.2008;3(5 suppl 5)( ):S42–S54.
- ,,,.Bridge over troubled waters: safe and effective transitions of the inpatient with hyperglycemia.J Hosp Med.2008;3(5 suppl 5)( ):S55–S65.
- ,,,,,.Society of Hospital Medicine glycemic control task force summary: practical recommendations for assessing the impact of glycemic control efforts.J Hosp Med.2008;3(suppl 5):S66–S75.
- ,.Practical strategies for developing the business case for hospital glycemic control teams.J Hosp Med.2008;3(suppl 5):S76–S83.
- ,,, et al.American College of Endocrinology position statement on inpatient diabetes and metabolic control.Endocr Pract.2004;10(1):77–82.
- Standards of medical care in diabetes‐‐2008.Diabetes Care.2008;31(suppl 1):S12–S54.
- ,,.Glycemic control and sliding scale insulin use in medical inpatients with diabetes mellitus.Arch Intern Med.1997;157(5):545–552.
- ,,, et al.Randomized study of basal‐bolus insulin therapy in the inpatient management of patients with type 2 diabetes (RABBIT 2 trial).Diabetes Care.2007;30(9):2181–2186.
- ,.Glycemic chaos (not glycemic control) still the rule for inpatient care: how do we stop the insanity?J Hosp Med.2006;1(3):141–144.
The articles on these pages represent the culmination of 3 years of effort by the Society of Hospital Medicine (SHM) Glycemic Control Task Force. In this brief introduction, we share a few insights and comments about this multidisciplinary collaborative effort to address the care of inpatients with hyperglycemia.
The SHM Glycemic Control Task Force was assembled in 2005, with the intent of improving the care of inpatients with diabetes. We wished to provide hospitalists and quality improvement teams with an understanding of the best practices to achieve safe glycemic control in the hospital. Additionally, this task force sought to identify tools and strategies to obtain improved communication, medication safety, education, and other aspects of care. A distinguished panel of experts attended their inaugural meeting in Chicago, Illinois, in October 2005, including hospitalists, endocrinologists, nurses, case managers, diabetes educators, and pharmacists. A roster of the individuals and organizations is given in the Appendix.
Many members of the SHM Glycemic Control Task Force also participated in the Call to Action consensus conference1 hosted by the American Association of Clinical Endocrinologists (AACE) and the American Diabetes Association (ADA) in January 2006. Both groups identified several barriers to improvement, and methods to overcome these barriers were summarized in this quote from the Consensus Conference,
Successful implementation of a program to improve glycemic control in the inpatient setting should include the following components:
-
An appropriate level of administrative support.
-
Formation of a multidisciplinary steering committee to drive the development of initiatives.
-
Assessment of current processes, quality of care, and barriers to practice change.
-
Development and implementation of interventions including standardized order sets, protocols, policies, and algorithms with associated educational programs.
-
Metrics for evaluation.
Both groups also called for a web‐based compendium of tested tools and strategies to assist local improvement teams.
After countless hours of development and revision by the SHM Glycemic Task Force and the Resource Room team, such a compendium addressing all of these components was launched on the SHM web site in the form of the SHM Glycemic Control Resource Room.2 A comprehensive implementation guide3 is available for downloading free of charge, and serves as the centerpiece of the Resource Room. Subsequently, this comprehensive but somewhat sprawling implementation guide evolved into these more sophisticated and concise articles.410 The topics include a review of the rationale for improving inpatient glycemic control,4 an important call for standardizing the metrics of glycemic control,9 subcutaneous insulin regimens and order sets,5, 6 insulin infusion protocols,7 transitions of care,8 and the business case for glycemic control.10 It has been a long but rewarding and educational journey, drawing on the collective experience from dozens of institutions in all kinds of inpatient care settings. A few key points and insights seem worth sharing.
THE IMPROVEMENT EFFORT IS NOT JUST ABOUT REACHING A GLYCEMIC TARGET
The term glycemic control team and the label for the SHM Glycemic Control Task Force itself are somewhat misnomers. Task Force members agree that desirable institutional glycemic target ranges should be established, but many among us believe the glycemic targets endorsed by national guidelines (ref ADA and AACE guidelines)11, 12 are too stringent. Furthermore, we believe that achieving glycemic control is just one small part of the needed improvement efforts. Uncontrolled hyperglycemia is common, potentially dangerous, and largely preventable with safe and proven methodsbut so are the iatrogenic hypoglycemia episodes, substandard education, poor communication, lack of care coordination, and inadequate monitoring that typify care of the hyperglycemic inpatient. We address all of these issues and urge the adoption of this broader perspective.
THE EVIDENCE IS INCOMPLETEBUT ACTION IS REQUIRED
We acknowledge gaps and inconsistencies in the literature surrounding inpatient diabetes management and the controversy around tight glycemic control. In many cases, high level evidence is not available to guide the formulation of protocols, order sets, or other improvement tools. We are all struck by how pervasive the lack of evidence is. What is the best metric for inpatient glycemic control or hypoglycemia? What is the best regimen for a patient on continuous tube feedings? Which insulin infusion protocol is superior in reaching and maintaining a glycemic target range?
Rather than make no recommendations or accept negative inertia on the basis of less than perfect evidence, we make recommendations based on the best evidence available. When we make recommendations based on consensus opinion or the collective experience from dozens of medical centers, rather than randomized trials, we have made every effort to make this clear in the text of the articles. In our view, incomplete evidence is not an adequate excuse to persist in the unacceptable status quo, clinging on to methods (such as sliding scale insulin regimens) that have been shown to be ineffective and potentially dangerous.1315
COLLABORATION PAYS DIVIDENDS
It takes a multidisciplinary approach to make substantial improvement in glycemic control of hospitalized patients. By the same token, it is unlikely that any one group can advance the national agenda for improved care as well as a multidisciplinary coordinated effort. Team members, especially the endocrinologists and hospitalists, collaborated skillfully throughout this effort. The hospitalists learned a tremendous amount from the expertise, insight, and mastery of the literature, offered by the endocrinology members, whereas the endocrinologists appreciated the front line expertise and practical quality improvement approach of the hospitalist members. This collaboration serves as a model for making guidelines and best practices become more of a practical reality for a variety of important clinical problems. Hospitalists can partner with and learn from a variety of other disciplines, while they assist these disciplines on effective improvement and implementation efforts. On a more personal note, this work has fostered mutual respect, friendship, and career long collaborative opportunities. The potential for these same opportunities with nursing, pharmacy, and all medical and surgical fields seems compelling and exciting.
THERE'S MORE!
By the time this is published, these articles will be integrated into the third iteration of the SHM Glycemic Control Resource Room. This online resource has already undergone 2 major revisions since its inception just a few years ago, reflecting SHM's dedication to the continuous improvement of the products and services that it offers. The Glycemic Control Implementation Guide and Resource Room will continue to be a work in progress. We highly encourage and welcome constructive criticism and feedback via E‐mail to
NEXT STEPS
More research and demonstration projects are obviously needed in this field. Local collaborative activities have sprung up in several cities and regions, as well as Glycemic Control Champions courses. A longitudinal mentoring program (similar to the SHM Venous Thromboembolism Prevention collaborative) would undoubtedly be beneficial, and may become available within the next year or so. These items and more will be promoted and posted in the resource room whenever possible.
Finally, the next step is up to you and the institutions in which you workyou have to decide, as individuals and institutions, if you believe the status quo is good enough. We believe that if you look, you'll find the care of our inpatients with diabetes and hyperglycemia disturbingly suboptimal, and hope that the work of the SHM Glycemic Control Task Force can help you rapidly improve on this state of affairs.
APPENDIX: GLYCEMIC CONTROL TASK FORCE
The Society of Hospital Medicine thanks all the members of the Glycemic Control Task Force, who encompass a distinguished panel of experts with representation from the AACE, ADA, ACP, and other organizations whose expertise was essential to the construction of the Glycemic Control Resource Room and the Implementation Guide for Glycemic Control and Prevention of Hypoglycemia.
Hospitalists
Representing the Society of Hospital Medicine
-
Gregory Maynard, MD. Lead Author and Editor of Glycemic Control Implementation Guide (web product); Glycemic Control Initiative Project Director; Clinical Professor of Medicine and Chief, Division of Hospital Medicine. University of California, San Diego (UCSD) Medical Center, San Diego, California.
-
David H. Wesorick, MD. Co‐editor of Glycemic Control Implementation Guide (web product); Clinical Assistant Professor of Internal Medicine, University of Michigan, Ann Arbor, Michigan.
-
Cheryl O'Malley, MD. Associate Program Director, Internal Medicine Faculty, Medicine/Pediatrics Banner, Good Samaritan Medical Center; Clinical Assistant Professor of Medicine, University of Arizona College of Medicine, Phoenix, Arizona.
-
Kevin Larsen, MD. Assistant Professor of Internal Medicine, University of Minnesota; Associate Program Director, Internal Medicine Residency, Hennepin County Medical Center, Minneapolis, Minnesota.
-
Jeffrey L. Schnipper, MD, MPH. Associate Physician, Brigham and Women's Hospital, Boston, Massachusetts
-
Alpesh Amin, MD, MBA, FACP. Executive Director and Vice Chair, University of California (UC) Irvine Hospitalist Program, Irvine, California.
-
Lakshmi Halasyamani, MD. Associate Chair, Department of Medicine, St. Joseph Mercy Medical Center, Ann Arbor, Michigan.
-
Mitchell J. Wilson, MD. Associate Professor of Medicine, University of North Carolina, Chapel Hill, North Carolina.
Representing the American College of Physicians
-
Doren Schneider, MD. Associate Program Director, Internal Medicine Residency Director, Ambulatory Service Unit, Abington Adult Medical Associates; Assistant Professor of Medicine, Temple University School of Medicine, Abington, Pennsylvania.
Endocrinologists
Representing the American Diabetes Association
-
Andrew J. Ahmann, MD. Associate Professor of Medicine, Director, Diabetes Center, Oregon Health & Science University, Portland, Oregon.
-
Michelle F. Magee, MD. Associate Professor of Medicine, Georgetown University School of Medicine Medstar Diabetes and Research Institutes, Washington Hospital Center, Washington, DC.
Representing the American Association of Clinical Endocrinologists
-
Richard Hellman, MD, FACP, FACE. Clinical Professor of Medicine, University of MissouriKansas City, North Kansas City, Missouri.
Endocringology Expert Panel
-
Susan Shapiro Braithwaite, MD, FACP, FACE. Clinical Professor of Medicine, University of North Carolina, Chapel Hill, North Carolina.
-
Mary Ann Emanuele, MD, FACP. Professor of Medicine, Endocrinology, Cell Biology, Neurobiology, and Anatomy Biochemistry, Loyola University Medical Center, Maywood, Illinois.
-
Irl B. Hirsch, MD. Professor of Medicine, University of Washington, Seattle, Washington.
-
Robert Rushakoff, MD. Clinical Professor of Medicine, Director, Diabetes Program, University of California, San Francisco (UCSF)/Mt. Zion, San Francisco, California.
-
Silvio E. Inzucchi, MD. Professor of Medicine, Clinical Director, Section of Endocrinology, Yale University School of Medicine, New Haven, Connecticut.
Education
-
Marcia D. Draheim, RN, CDE. Program Supervisor, Diabetes Center, St Luke's Hospital, Cedar Rapids, Iowa.
-
Sharon Mahowald, RN, CDE. Inpatient Diabetes Coordinator, Hennepin County Medical Center, Minneapolis, Minnesota.
Financial
-
Adam Beck, MHS, FABC. MedStar Research Institute, Washington, DC.
Pharmacists
-
Stuart T. Haines, PharmD, FASHP, FCCP, BCPS. Associate Professor/Vice Chair for Education, University of Maryland School of Pharmacy Baltimore, Maryland.
Representing the American Society of Consultant Pharmacists
-
Donald K. Zettervall, RPh, CDE, CDM. Owner/Director, The Diabetes Education Center, Old Saybrook, Connecticut.
Case Management
Representing the Case Management Society of America
-
Cheri Lattimer, RN, BSN. Executive Director, Case Management Society of America, Little Rock, Arkansas.
-
Nancy Skinner, RN, CCM. Director, Case Management Society of America Principle Consultant, Riverside HealthCare Consulting, Whitwell, Tennessee.
Dietetics
-
Carrie Swift, MS, RD, BC‐ADM. Dietetics Coordinator, Veterans Affairs Medical Center, Walla Walla, Washington.
SHM Staff Members
-
Geri Barnes and Joy Wittnebert.
Glycemic Control Resource Room Project Team
-
Greg Maynard, Jason Stein, David Wesorick, Mary Ann Emanuele, Kevin Larsen, Geri Barnes, Joy Wittnebert, and Bruce Hansen.
The articles on these pages represent the culmination of 3 years of effort by the Society of Hospital Medicine (SHM) Glycemic Control Task Force. In this brief introduction, we share a few insights and comments about this multidisciplinary collaborative effort to address the care of inpatients with hyperglycemia.
The SHM Glycemic Control Task Force was assembled in 2005, with the intent of improving the care of inpatients with diabetes. We wished to provide hospitalists and quality improvement teams with an understanding of the best practices to achieve safe glycemic control in the hospital. Additionally, this task force sought to identify tools and strategies to obtain improved communication, medication safety, education, and other aspects of care. A distinguished panel of experts attended their inaugural meeting in Chicago, Illinois, in October 2005, including hospitalists, endocrinologists, nurses, case managers, diabetes educators, and pharmacists. A roster of the individuals and organizations is given in the Appendix.
Many members of the SHM Glycemic Control Task Force also participated in the Call to Action consensus conference1 hosted by the American Association of Clinical Endocrinologists (AACE) and the American Diabetes Association (ADA) in January 2006. Both groups identified several barriers to improvement, and methods to overcome these barriers were summarized in this quote from the Consensus Conference,
Successful implementation of a program to improve glycemic control in the inpatient setting should include the following components:
-
An appropriate level of administrative support.
-
Formation of a multidisciplinary steering committee to drive the development of initiatives.
-
Assessment of current processes, quality of care, and barriers to practice change.
-
Development and implementation of interventions including standardized order sets, protocols, policies, and algorithms with associated educational programs.
-
Metrics for evaluation.
Both groups also called for a web‐based compendium of tested tools and strategies to assist local improvement teams.
After countless hours of development and revision by the SHM Glycemic Task Force and the Resource Room team, such a compendium addressing all of these components was launched on the SHM web site in the form of the SHM Glycemic Control Resource Room.2 A comprehensive implementation guide3 is available for downloading free of charge, and serves as the centerpiece of the Resource Room. Subsequently, this comprehensive but somewhat sprawling implementation guide evolved into these more sophisticated and concise articles.410 The topics include a review of the rationale for improving inpatient glycemic control,4 an important call for standardizing the metrics of glycemic control,9 subcutaneous insulin regimens and order sets,5, 6 insulin infusion protocols,7 transitions of care,8 and the business case for glycemic control.10 It has been a long but rewarding and educational journey, drawing on the collective experience from dozens of institutions in all kinds of inpatient care settings. A few key points and insights seem worth sharing.
THE IMPROVEMENT EFFORT IS NOT JUST ABOUT REACHING A GLYCEMIC TARGET
The term glycemic control team and the label for the SHM Glycemic Control Task Force itself are somewhat misnomers. Task Force members agree that desirable institutional glycemic target ranges should be established, but many among us believe the glycemic targets endorsed by national guidelines (ref ADA and AACE guidelines)11, 12 are too stringent. Furthermore, we believe that achieving glycemic control is just one small part of the needed improvement efforts. Uncontrolled hyperglycemia is common, potentially dangerous, and largely preventable with safe and proven methodsbut so are the iatrogenic hypoglycemia episodes, substandard education, poor communication, lack of care coordination, and inadequate monitoring that typify care of the hyperglycemic inpatient. We address all of these issues and urge the adoption of this broader perspective.
THE EVIDENCE IS INCOMPLETEBUT ACTION IS REQUIRED
We acknowledge gaps and inconsistencies in the literature surrounding inpatient diabetes management and the controversy around tight glycemic control. In many cases, high level evidence is not available to guide the formulation of protocols, order sets, or other improvement tools. We are all struck by how pervasive the lack of evidence is. What is the best metric for inpatient glycemic control or hypoglycemia? What is the best regimen for a patient on continuous tube feedings? Which insulin infusion protocol is superior in reaching and maintaining a glycemic target range?
Rather than make no recommendations or accept negative inertia on the basis of less than perfect evidence, we make recommendations based on the best evidence available. When we make recommendations based on consensus opinion or the collective experience from dozens of medical centers, rather than randomized trials, we have made every effort to make this clear in the text of the articles. In our view, incomplete evidence is not an adequate excuse to persist in the unacceptable status quo, clinging on to methods (such as sliding scale insulin regimens) that have been shown to be ineffective and potentially dangerous.1315
COLLABORATION PAYS DIVIDENDS
It takes a multidisciplinary approach to make substantial improvement in glycemic control of hospitalized patients. By the same token, it is unlikely that any one group can advance the national agenda for improved care as well as a multidisciplinary coordinated effort. Team members, especially the endocrinologists and hospitalists, collaborated skillfully throughout this effort. The hospitalists learned a tremendous amount from the expertise, insight, and mastery of the literature, offered by the endocrinology members, whereas the endocrinologists appreciated the front line expertise and practical quality improvement approach of the hospitalist members. This collaboration serves as a model for making guidelines and best practices become more of a practical reality for a variety of important clinical problems. Hospitalists can partner with and learn from a variety of other disciplines, while they assist these disciplines on effective improvement and implementation efforts. On a more personal note, this work has fostered mutual respect, friendship, and career long collaborative opportunities. The potential for these same opportunities with nursing, pharmacy, and all medical and surgical fields seems compelling and exciting.
THERE'S MORE!
By the time this is published, these articles will be integrated into the third iteration of the SHM Glycemic Control Resource Room. This online resource has already undergone 2 major revisions since its inception just a few years ago, reflecting SHM's dedication to the continuous improvement of the products and services that it offers. The Glycemic Control Implementation Guide and Resource Room will continue to be a work in progress. We highly encourage and welcome constructive criticism and feedback via E‐mail to
NEXT STEPS
More research and demonstration projects are obviously needed in this field. Local collaborative activities have sprung up in several cities and regions, as well as Glycemic Control Champions courses. A longitudinal mentoring program (similar to the SHM Venous Thromboembolism Prevention collaborative) would undoubtedly be beneficial, and may become available within the next year or so. These items and more will be promoted and posted in the resource room whenever possible.
Finally, the next step is up to you and the institutions in which you workyou have to decide, as individuals and institutions, if you believe the status quo is good enough. We believe that if you look, you'll find the care of our inpatients with diabetes and hyperglycemia disturbingly suboptimal, and hope that the work of the SHM Glycemic Control Task Force can help you rapidly improve on this state of affairs.
APPENDIX: GLYCEMIC CONTROL TASK FORCE
The Society of Hospital Medicine thanks all the members of the Glycemic Control Task Force, who encompass a distinguished panel of experts with representation from the AACE, ADA, ACP, and other organizations whose expertise was essential to the construction of the Glycemic Control Resource Room and the Implementation Guide for Glycemic Control and Prevention of Hypoglycemia.
Hospitalists
Representing the Society of Hospital Medicine
-
Gregory Maynard, MD. Lead Author and Editor of Glycemic Control Implementation Guide (web product); Glycemic Control Initiative Project Director; Clinical Professor of Medicine and Chief, Division of Hospital Medicine. University of California, San Diego (UCSD) Medical Center, San Diego, California.
-
David H. Wesorick, MD. Co‐editor of Glycemic Control Implementation Guide (web product); Clinical Assistant Professor of Internal Medicine, University of Michigan, Ann Arbor, Michigan.
-
Cheryl O'Malley, MD. Associate Program Director, Internal Medicine Faculty, Medicine/Pediatrics Banner, Good Samaritan Medical Center; Clinical Assistant Professor of Medicine, University of Arizona College of Medicine, Phoenix, Arizona.
-
Kevin Larsen, MD. Assistant Professor of Internal Medicine, University of Minnesota; Associate Program Director, Internal Medicine Residency, Hennepin County Medical Center, Minneapolis, Minnesota.
-
Jeffrey L. Schnipper, MD, MPH. Associate Physician, Brigham and Women's Hospital, Boston, Massachusetts
-
Alpesh Amin, MD, MBA, FACP. Executive Director and Vice Chair, University of California (UC) Irvine Hospitalist Program, Irvine, California.
-
Lakshmi Halasyamani, MD. Associate Chair, Department of Medicine, St. Joseph Mercy Medical Center, Ann Arbor, Michigan.
-
Mitchell J. Wilson, MD. Associate Professor of Medicine, University of North Carolina, Chapel Hill, North Carolina.
Representing the American College of Physicians
-
Doren Schneider, MD. Associate Program Director, Internal Medicine Residency Director, Ambulatory Service Unit, Abington Adult Medical Associates; Assistant Professor of Medicine, Temple University School of Medicine, Abington, Pennsylvania.
Endocrinologists
Representing the American Diabetes Association
-
Andrew J. Ahmann, MD. Associate Professor of Medicine, Director, Diabetes Center, Oregon Health & Science University, Portland, Oregon.
-
Michelle F. Magee, MD. Associate Professor of Medicine, Georgetown University School of Medicine Medstar Diabetes and Research Institutes, Washington Hospital Center, Washington, DC.
Representing the American Association of Clinical Endocrinologists
-
Richard Hellman, MD, FACP, FACE. Clinical Professor of Medicine, University of MissouriKansas City, North Kansas City, Missouri.
Endocringology Expert Panel
-
Susan Shapiro Braithwaite, MD, FACP, FACE. Clinical Professor of Medicine, University of North Carolina, Chapel Hill, North Carolina.
-
Mary Ann Emanuele, MD, FACP. Professor of Medicine, Endocrinology, Cell Biology, Neurobiology, and Anatomy Biochemistry, Loyola University Medical Center, Maywood, Illinois.
-
Irl B. Hirsch, MD. Professor of Medicine, University of Washington, Seattle, Washington.
-
Robert Rushakoff, MD. Clinical Professor of Medicine, Director, Diabetes Program, University of California, San Francisco (UCSF)/Mt. Zion, San Francisco, California.
-
Silvio E. Inzucchi, MD. Professor of Medicine, Clinical Director, Section of Endocrinology, Yale University School of Medicine, New Haven, Connecticut.
Education
-
Marcia D. Draheim, RN, CDE. Program Supervisor, Diabetes Center, St Luke's Hospital, Cedar Rapids, Iowa.
-
Sharon Mahowald, RN, CDE. Inpatient Diabetes Coordinator, Hennepin County Medical Center, Minneapolis, Minnesota.
Financial
-
Adam Beck, MHS, FABC. MedStar Research Institute, Washington, DC.
Pharmacists
-
Stuart T. Haines, PharmD, FASHP, FCCP, BCPS. Associate Professor/Vice Chair for Education, University of Maryland School of Pharmacy Baltimore, Maryland.
Representing the American Society of Consultant Pharmacists
-
Donald K. Zettervall, RPh, CDE, CDM. Owner/Director, The Diabetes Education Center, Old Saybrook, Connecticut.
Case Management
Representing the Case Management Society of America
-
Cheri Lattimer, RN, BSN. Executive Director, Case Management Society of America, Little Rock, Arkansas.
-
Nancy Skinner, RN, CCM. Director, Case Management Society of America Principle Consultant, Riverside HealthCare Consulting, Whitwell, Tennessee.
Dietetics
-
Carrie Swift, MS, RD, BC‐ADM. Dietetics Coordinator, Veterans Affairs Medical Center, Walla Walla, Washington.
SHM Staff Members
-
Geri Barnes and Joy Wittnebert.
Glycemic Control Resource Room Project Team
-
Greg Maynard, Jason Stein, David Wesorick, Mary Ann Emanuele, Kevin Larsen, Geri Barnes, Joy Wittnebert, and Bruce Hansen.
- Inpatient Diabetes and Glycemic Control: A Call to Action Conference. Position statement. AACE, February 2006. Available at: http://www.aace.com/meetings/consensus/IIDC/IDGC0207.pdf. Accessed October 2006. Garber et al. Endocr Pract.2006;12(suppl 3):3–13.
- Society of Hospital Medicine. Glycemic Control Resource Room. Available at: http://www.hospitalmedicine.org/ResourceRoomRedesign/GlycemicControl.cfm. Accessed May2008.
- Society of Hospital Medicine Glycemic Control Task Force. Implementation guide: improving glycemic control, preventing hypoglycemia, and optimizing care of the inpatient with hyperglycemia and diabetes. Published January 2007 on the Society of Hospital Medicine Website. Available at: http://www.hospitalmedicine.org. Accessed May2008.
- ,,,,,.The case for supporting inpatient glycemic control programs now: the evidence and beyond.J Hosp Med.2008;3(5 suppl 5)( ):S6–S16.
- ,,,,.Management of diabetes and hyperglycemia in the hospital: a practical guide to subcutaneous insulin use in the non‐critically ill adult patient.J Hosp Med.2008;3(5 suppl 5)( ):S17–S28.
- ,,,.Subcutaneous insulin order sets and protocols: effective design and implementation strategies.J Hosp Med.2008;3(5 suppl 5)( ):S29–S41.
- ,.Designing and implementing insulin infusion protocols and order sets.J Hosp Med.2008;3(5 suppl 5)( ):S42–S54.
- ,,,.Bridge over troubled waters: safe and effective transitions of the inpatient with hyperglycemia.J Hosp Med.2008;3(5 suppl 5)( ):S55–S65.
- ,,,,,.Society of Hospital Medicine glycemic control task force summary: practical recommendations for assessing the impact of glycemic control efforts.J Hosp Med.2008;3(suppl 5):S66–S75.
- ,.Practical strategies for developing the business case for hospital glycemic control teams.J Hosp Med.2008;3(suppl 5):S76–S83.
- ,,, et al.American College of Endocrinology position statement on inpatient diabetes and metabolic control.Endocr Pract.2004;10(1):77–82.
- Standards of medical care in diabetes‐‐2008.Diabetes Care.2008;31(suppl 1):S12–S54.
- ,,.Glycemic control and sliding scale insulin use in medical inpatients with diabetes mellitus.Arch Intern Med.1997;157(5):545–552.
- ,,, et al.Randomized study of basal‐bolus insulin therapy in the inpatient management of patients with type 2 diabetes (RABBIT 2 trial).Diabetes Care.2007;30(9):2181–2186.
- ,.Glycemic chaos (not glycemic control) still the rule for inpatient care: how do we stop the insanity?J Hosp Med.2006;1(3):141–144.
- Inpatient Diabetes and Glycemic Control: A Call to Action Conference. Position statement. AACE, February 2006. Available at: http://www.aace.com/meetings/consensus/IIDC/IDGC0207.pdf. Accessed October 2006. Garber et al. Endocr Pract.2006;12(suppl 3):3–13.
- Society of Hospital Medicine. Glycemic Control Resource Room. Available at: http://www.hospitalmedicine.org/ResourceRoomRedesign/GlycemicControl.cfm. Accessed May2008.
- Society of Hospital Medicine Glycemic Control Task Force. Implementation guide: improving glycemic control, preventing hypoglycemia, and optimizing care of the inpatient with hyperglycemia and diabetes. Published January 2007 on the Society of Hospital Medicine Website. Available at: http://www.hospitalmedicine.org. Accessed May2008.
- ,,,,,.The case for supporting inpatient glycemic control programs now: the evidence and beyond.J Hosp Med.2008;3(5 suppl 5)( ):S6–S16.
- ,,,,.Management of diabetes and hyperglycemia in the hospital: a practical guide to subcutaneous insulin use in the non‐critically ill adult patient.J Hosp Med.2008;3(5 suppl 5)( ):S17–S28.
- ,,,.Subcutaneous insulin order sets and protocols: effective design and implementation strategies.J Hosp Med.2008;3(5 suppl 5)( ):S29–S41.
- ,.Designing and implementing insulin infusion protocols and order sets.J Hosp Med.2008;3(5 suppl 5)( ):S42–S54.
- ,,,.Bridge over troubled waters: safe and effective transitions of the inpatient with hyperglycemia.J Hosp Med.2008;3(5 suppl 5)( ):S55–S65.
- ,,,,,.Society of Hospital Medicine glycemic control task force summary: practical recommendations for assessing the impact of glycemic control efforts.J Hosp Med.2008;3(suppl 5):S66–S75.
- ,.Practical strategies for developing the business case for hospital glycemic control teams.J Hosp Med.2008;3(suppl 5):S76–S83.
- ,,, et al.American College of Endocrinology position statement on inpatient diabetes and metabolic control.Endocr Pract.2004;10(1):77–82.
- Standards of medical care in diabetes‐‐2008.Diabetes Care.2008;31(suppl 1):S12–S54.
- ,,.Glycemic control and sliding scale insulin use in medical inpatients with diabetes mellitus.Arch Intern Med.1997;157(5):545–552.
- ,,, et al.Randomized study of basal‐bolus insulin therapy in the inpatient management of patients with type 2 diabetes (RABBIT 2 trial).Diabetes Care.2007;30(9):2181–2186.
- ,.Glycemic chaos (not glycemic control) still the rule for inpatient care: how do we stop the insanity?J Hosp Med.2006;1(3):141–144.
Implementing Insulin Infusion Protocols
The delayed and variable absorption of subcutaneous (SC) insulin has always provided challenges for the rapid and predictable control of hyperglycemia in the acute care setting. Conversely, intravenous infusion of human regular insulin provides a continuous and essentially immediate delivery mechanism. Once in the circulation, insulin has a very short half‐life of about 5 to 7 minutes and a biological effect of about 20 minutes.1 Intravenous insulin infusions are well‐established in several acute care settings including hyperglycemic emergencies, perioperative glucose management, and glucose control during labor and delivery.2, 3
The beneficial effects seen in early trials of strict glycemic control involving intensive care unit (ICU) patients47 (particularly cardiac surgery patients) and guidelines for inpatient glycemic control8 stimulated widespread interest in adopting insulin infusion protocols (IIPs) focused on achieving strict glycemic targets. The initial enthusiasm has been tempered by uneven results in trials of tight glycemic control, concerns about the effects of excessive hypoglycemia, and the resources needed to implement and maintain an IIP.9 Although considerable controversy about the ideal glycemic target for different patient populations exists, and will likely continue for some time, this article is not a review of the evidence for supporting 1 glycemic target versus another. Regardless of the glycemic target chosen, a standardized algorithm and accompanying program of monitoring are widely endorsed for both safety and quality reasons.1014 Most medical centers are at least attempting to implement nurse‐driven protocols that have demonstrated better perfomance than SC regimens15 and physician‐driven insulin infusions.16, 17 In this article, we outline several variables in IIP design and implementation and endorse several aspects of design and implementation that will likely result in improved staff acceptance and, ultimately, a more safe and effective IIP.
PREPARING TO IMPLEMENT AN IIP
Building The Team
Implementing a medical centerwide standardized insulin infusion order set with supporting policies, protocols, monitoring standards, and the requisite educational programs is a major task for any hospital. This is not a simple maneuver involving only 1 or 2 interested individuals and requires much more than selecting a published protocol and disseminating it to various patient care units. Instead, to manage the full spectrum of diabetes programs and protocols, an institution must convene a multidisciplinary steering committee or task force. This should include representation from nursing, nursing administration, pharmacy, nutrition services, and the quality improvement department. Physicians should include hospitalists, intensivists, and endocrinologists but may also involve anesthesiologists and surgeons, as applicable. At times, additional members may need to be recruited according to project needs.
Identifying the Stakeholders and Current Practices
In developing or improving currently utilized IIPs, the multidisciplinary committee would benefit from careful background work before moving forward. First, administrative and institutional support must be secured to endorse uniform standards for insulin infusions, and to provide the important infrastructure needed to facilitate the work involved. Clinical and administrative stakeholders from the key departments then need to be identified.
All insulin infusion orders and policies/procedures presently used in the institution should be identified and examined. The developers and/or users of these order sets should be engaged in a dialogue and encouraged to share their experiences regarding their current practice and the attendant work flow, glucose monitoring, and data collection and reporting. Immediate concerns should be clearly addressed. Measurement systems for glycemic control, hypoglycemia, and insulin use patterns should assess current practice and the impact of subsequent modifications of the protocol or initiation of new protocols. We recommend using glucometrics consistent with those endorsed by the Society of Hospital Medicine (SHM) Glycemic Control Task Force elsewhere in this supplement.18
Addressing the Burden of Change
Through this process, the committee will uncover barriers, dysfunctional and inconsistent practices, and individuals who will pose challenges. Identifying these issues should not discourage the team, but rather it should guide the interventional strategies, and help build consensus that change may be required. There must be caution not to exclude significant individuals simply because they resist changes. Indeed, if they are included and have the opportunity to contribute to the process, success is much more likely.
It is important for process leaders to understand the implications of what is proposed, particularly for nursing services.19 For example, it has been shown that IIPs require about 5 minutes per patient per hour for glucose monitoring and dose adjustments.20 Acknowledging and attempting to address this burden proactively (often well over 2 hours per day) can gain staff acceptance more effectively than a laissez faire approach. In this regard, some effort should be invested in nursing education of the benefits of tight glycemic management on critical care outcomes. The difficult‐to‐quantify work involved when patients' blood glucose is not well controlled (eg, paging physicians for stat insulin orders) should also be part of this discussion.
Identifying and Addressing Barriers to IIP implementation
There are numerous potential barriers to implementation of IIPs. Table 1 identifies some of the most frequent ones along with potential strategies or solutions. Very common barriers include skepticism surrounding the benefits of tight glycemic control, fear of hypoglycemia, and difficulty agreeing on glycemic targets.
| Barrier | Strategy/Solution |
|---|---|
| Insufficient glucose meters to accommodate the increased testing needs | Purchase additional glucose meters. |
| Ask the vendor to provide extra on‐site replacement meters at no charge until they are activated. | |
| Nursing time requirements involved in monitoring and adjustments. | Get ancillary help to check glucose values |
| ○eg, nurse assistants | |
| Make extra efforts to make protocols clear with few required calculations | |
| Avoid duplicate recording | |
| Consider meters requiring shorter time and a smaller sample (to avoid need for re‐sampling) | |
| Requirement for uncomfortable frequent sticks | Utilize central lines or arterial lines. |
| ○These tend to vary by < 10% from POC readings | |
| ○May not be available in noncritical care settings | |
| Staff fear of hypoglycemia | Educate on the benefit of glucose control and the true definition of hypoglycemia |
| ○Measure staff fasting glucose levels to demonstrate normal range. | |
| Establish metrics and publicly report hypoglycemia event rates. | |
| Pilot IIP on small scale. | |
| Protocol and education for prevention of hypoglycemia. | |
| Difficulty gaining consensus on glycemic target | Compromise if needed on the glucose target |
| ○eg, start with a higher goal such as 90‐140 mg/dL. | |
| ○Others will be willing to lower the goal when feasibility is seen. | |
| Allow for different targets in different units if indicated | |
| ○maintain consistency in other respects. | |
| Focal points of resistance | Identify a local nurse or physician champion within resistant site. |
| Pilot the protocol in an area with least resistance | |
| ○Will gain momentum with initial success and adjustments | |
| Lack of integrated information and reporting systems | Incorporate information systems personnel onto team |
| Advocate for improved reporting capability with administrative leaders | |
| Use sampling methods to collect data until automated systems are available. | |
| Multiple providers, hand offs, and opportunities for error and communication breakdown, diffusion of responsibility for glycemic control | Involvement of varied front line providers |
| Check lists for important items to communicate on transfer/transport | |
| Common protocols/education for similar units |
Whereas the national guidelines call for a glycemic target in critical care areas with an upper limit of 110 mg/dL, there is room for debate, and tailoring of the glycemic target to fit individual patient circumstances is often advisable. Starting with a less aggressive glycemic target can be good politics (and perhaps good medicine as well). Once the higher glycemic targets are achieved safely, it can pave the way for a more aggressive approach.
Fear of hypoglycemia is one of the most potent barriers to intensive insulin infusion implementation. Because hyperglycemia is such a common condition in critical care units, nursing and physician staff may have developed a skewed view of the definition of hypoglycemia, at times fearing for their patient when the glucose values reach a level of 100 mg/dL or so. Polling the nurses on what they think their own fasting glucose levels are, and then actually measuring them can be an effective strategy (the nurses may be surprised that the patient's scary 90 mg/dL reading is higher than their own). It should also be emphasized that properly designed and implemented protocols may actually decrease the incidence of hypoglycemia when compared to standard care which may involve individually and sometimes improperly adjusted intravenous (IV) insulin (discussed further below).
CHOOSING AN IIP FOR YOUR HOSPITAL
See Table 2 for a comparison of several features identified in selected published protocols.7, 2127 This is not a comprehensive list of all protocols found in the literature. Rather, the authors consider it representative of the various types of IIPs or best recognized IIPs in the literature. Note that there is variability in study population, the glucose targets, hypoglycemia, the time to reach the target, and the time spent in the target range. Other practical factors to consider in reviewing the literature and selecting an IIP design are the complexity of the protocol, the required process steps or calculations, the evidence for staff acceptance, and the level of resources supporting the published protocol.
| Author (reference) | IIP Description | Patient population | Glucose Target (mg/dL) | Mean Glucose (mg/dL) | Time to Reach Target | Hypoglycemia |
|---|---|---|---|---|---|---|
| Van den Berghe7 | Initial and subsequent rates based on BG | Surgical ICU | 80‐110 | 6 AM glucose 103 19 | Not reported | 5.1% of patient < 41 mg/dL |
| Nurses had latitude | ||||||
| Van den Berghe21 | TPN standard with IIP | Medical ICU | 80‐110 | 6 AM glucose 105 | Not reported | 19% of patients < 41 mg/dL |
| Furnary22 (2001‐2003 version) | Initial dose determined by BG and type of DM | CABG | 100‐150 (there is a newer protocol with lower targets) | Not mentioned. Appears to be < 150 | 94% within 3 hours | 0.5% < 60 (% of readings?) Not reported with any specificity |
| Changes based on present BG and last change | ||||||
| Relatively complex | ||||||
| Goldberg23 | Dosing based on: | MICU | 100‐139 | Not specified | 10.1 hours | 0.3% of readings <60 BG <60 in 5.4% of patient days |
| ○Current BG | ||||||
| ○Velocity of glucose change | ||||||
| Goldberg24 | ○Infusion rate | CT Surgery | 100‐139 | 122 17 Once target attained. | 5 hours | 0.2% of all BGs < 60 mg/Dl BG < 60 in 2.9% of patient days |
| Uses 3 tables | ||||||
| Relatively complex | ||||||
| DeSantis25 | Initial dose based on BG | Mostly surgical ICU and CVICU (75% surgical) | 80‐110 | 135 49 (higher in SICU and CVICU alone) | 10.6 hours | 1.5% of readings |
| < 60 mg/dL (lower for CVICU & SICU alone) | ||||||
| Changes based on present glucose and rate of change. | ||||||
| IV bolus used with changes. | ||||||
| Braithwaite26 | 6‐column method | Trauma ICU | <110 mg/dL | 129 25 | 16.7 hours | 2.4% of readings < 70 mg/dL |
| Davidson27 | Computer‐directed algorithm (similar to column method) | Full spectrum | Varied by year and situation | Not reported (approximately 125 mg/dL when stable) | 90% < 150 mg/dL in 3 hours | 0.6% of readings <50 mg/dL 2.6% of patients |
Structural Differences in Protocols
Protocols that vary by level of insulin sensitivity generally use column methods with the individual columns representing different categories of insulin sensitivity, placing the most sensitive category on the left with the highest level of insulin resistance on the far right. These methods use a multiplier to adjust for sensitivity. They are constructed according to the rule of 1500, 1700, or 1800, thereby adjusting for changes in insulin sensitivity that follow surgery or other changes in physiologic stress in the acute setting. Rate of change is addressed by shifting to the right if correction of hyperglycemia is too slow and to the left if the glucose is dropping too rapidly.
Most institutions using the column/emnsulin sensitivity method, implement paper orders as first published by Markovitz.28 The same concepts have been used to develop computer‐assisted insulin infusion protocols. One published method is the Glucommander,27 but a number of institutions are using similar computer‐assisted methods developed locally.
Other methods use the present glucose and change from last glucose to constantly adjust to any situation.7, 2125 They usually involve 2 or 3 steps and often require more calculations by the nurse. These methods are purported to be more agile or flexible but there have been no direct comparisons with the column methods looking at effectiveness, nursing errors, or hypoglycemic risk. The Yale Protocol23, 24 and the Portland Protocol22 are 2 prominent examples of this type of protocol. Adjustments are defined as units, percent change, or a combination of both.
Limitations of the IIP Literature
There had been few published insulin protocols aimed at reaching specific glucose goals when the Leuven surgical ICU experience7 was published in 2001. These early publications featured algorithms that adjusted insulin infusions solely on the basis of glucose level, and did not take the velocity of change, direction of change, proximity to glycemic target, or different insulin sensitivities into account. Also, the targeted glucose goals in those reports were not consistent with the present standards.2931 Other published protocols featured glucose‐insulin‐potassium infusion (GIK) protocols that focused on the amount of insulin administered and failed to attain appropriately defined glucose targets.32, 33 These publications offer no real guidance in crafting a modern glycemic targetoriented protocol.
Whereas more than 20 modern IIPs directed at glycemic targets have been published,7, 1517, 2127, 3450 many of the published protocols represent local modifications of ones previously published elsewhere, and no published, prospective, head‐to‐head comparisons of the best‐known IIPs are available.
Several reviews of previous published reports include comparisons of IIPs with varied areas of emphasis.5154 The reliability of such comparisons is limited by the inconsistency in methodology between studies and the different populations studied. For example, medical populations are generally harder to control and are more prone to hypoglycemia than surgical populations,7, 21, 23, 24, 50 and some studies include only patients with diabetes.4, 5, 38 In addition, definitions of hypoglycemia and methods of analyzing glycemic control are highly variable, making comparisons of IIPs challenging.39 As a result, attempts to compare published IIP results without consideration of the population studied could lead to erroneous conclusions.
In spite of the aforementioned limitations, there are several lessons we can learn from the IIP literature and accumulated clinical experience at a variety of centers.
Glycemic Targets and Other IIP Features Can Evolve over Time
Revisions of protocol details, including glycemic targets, often evolve over time. This deliberate approach can facilitate staff acceptance by demonstrating safe achievement of higher glycemic targets. The Yale team initially selected a conservative glycemic target of 100‐139 mg/dL, as depicted in Table 1.23, 24 They subsequently lowered the target range to 90‐119 mg/dL and increased the initial insulin bolus amount by 40%.50 The modified protocol displayed improved performance and yet retained safety, with only 1 glucose reading < 40 mg/dL in 101 patients over 117 runs of insulin infusion.
The Portland Protocol, used primarily in cardiac surgery patients, has also evolved over time. This group has altered the IIP in a number of ways at least 4 times. These protocols, including the most recent protocol targeting glucose levels of 70‐110 mg/dL, is found at
Medical and Surgical Patients Are Different
The most convincing evidence for stringent glycemic control evolved from studies of surgical patients.4, 5, 7 Acknowledging this fact, along with the greater degree of difficulty in achieving glycemic targets safely in critically ill medical patients, have led many to endorse higher glycemic targets for certain populations than others.57 Although we generally favor a uniform glycemic target for a unit serving a particular patient population, adopting a less stringent glycemic target in medical ICU settings compared to surgical ICU settings is reasonable and prudent in many institutional settings. However, the accompanying challenges regarding boarding patients and floating nurses between units would also need to be considered.
Local Factors and Implementation Methods Matter
The success or failure of a protocol likely depends on local factors and implementation methods as much as it does on the structure of the protocol itself. IIP development and implementation is a process that must be approached systematically and with attention to detail.19 Errors in approach can delay or abort the implementation, or potentially lead to an ineffective or unsafe protocol for the institution.
The Yale experience again provides us with a salient example.58 Initial efforts to implement an IIP failed due to a number of factors: a complicated protocol, insufficient nursing involvement, and inadequate training and education led to incomplete buy‐in, and nursing concerns over hypoglycemia that actually was within the goal range of the protocol. Successful implementation was not achieved until the leaders learned from their mistakes. Nurses and other clinical allies were involved and educated. Important stakeholders, who were not included earlier, were now involved, and front‐line nursing staff were engaged in proactive troubleshooting.
In another example, multisite studies like VISEP59 and Glucontrol9 have tried to adapt the Leuven protocol, only to struggle with excessive hypoglycemia and an inability to replicate the tight glycemic control enjoyed by van den Berghe and colleagues.7, 21 The Leuven care team augmented the performance of the IIP by their experience with the protocol, physician involvement, and adjustments made by the nursing staff.
Some IIPs Have Lower Hypoglycemia Rates than Others
Recent articles have highlighted the association of intensive insulin treatment, hypoglycemia, and mortality in the medical ICU population.60 Concerns that excessive hypoglycemia could reduce or reverse the overall benefits of intensive insulin therapy has led some to call for moderation of critical care glycemic targets.61 Comparisons of IIPs5254, 61 that include information about the propensity of the IIP to produce hypoglycemia are therefore important.
The Leuven IIP has been reported to yield high rates of hypoglycemia with almost 20% of patients suffering from severe hypoglycemia (glucose < 40 mg/dL) in some studies.9, 21, 59 By way of contrast, several different IIP regimens have frequently achieved identical or very similar glycemic control with less than 5% of the patients experiencing severe hypoglycemia.27, 50, 62 Although implementation factors, severity of illness, and other population factors play some role, some protocols have an inherent structural propensity to produce more hypoglycemia than others. In the case of the Leuven protocol, there is more of a role for clinician adjustment, and the written protocol itself does not call for much active adjustment as the patient enters into the hypoglycemic range.52 In contrast, more sophisticated and complicated regimens adjust more aggressively in this range and consistently induce less hypoglycemia.
Successful Methods to Manage the Complexity of an IIP
Many modern IIPs use bolus insulin to expedite control and adjust the infusion rate based on the velocity and direction of glucose change, not just the glucose value.52 Insulin resistance is taken into account in some models, and multiple calculation steps are required in several reported protocols.52 The improved automation and control refinements come at a cost of increased complexity.
Intensive implementation efforts and strong leadership can overcome some issues, as demonstrated by the Yale experience, but 2 other strategies have commonly demonstrated success. First, focused expert glycemic management teams that directly oversee many aspects of the IIP, or even assume direct management roles, can be quite effective,25 especially during early implementation.
Automation of calculations and computerization is another method to consider, and many recent reports involve applications of web‐based or other computerized models.27, 40, 42, 43, 46, 48, 63 Comparisons of computerized versus manual methods, computerized versus column methods, and a computerized nomogram‐versus‐chart method are now available.42, 45, 46, 48, 62 Computerized protocols show significant promise and would be expected to reduce dosing errors. These instruments generally present the nurse with a specific infusion rate after each monitored glucose and then recommend an interval for the next glucose determination. In direct comparisons, they tend to perform as well or often better than conventional methods. For example, in the most recent randomized trial comparing the Glucommander to a paper‐based column method in an ICU, Newton and colleagues found the computerized method reached the goal more rapidly and reached a lower mean glucose without increasing the rate of severe hypoglycemia (< 40 mg/dL).62 Computerized methods also facilitate data collection for analytical purposes.
Computerized systems (both commercial and home‐grown versions) are becoming more common and appear to hold significant benefits as long as they are backed by a validated algorithm. Whereas many institutions are not yet in a position to integrate such protocols into their standard or electronic record systems, we expect the trend for increased implementation to continue.
ENHANCING THE DESIGN OF YOUR IIP
Once the improvement team has identified and examined current order sets and protocols in the context of the literature, we encourage consolidation of institutional insulin infusion orders into a common basic structure. This basic structure should be enhanced by incorporating a variety of elements designed to enhance the reliability of use and safety of the IIP. These design features are outlined in Table 3. Randomized trial evidence of the effectiveness of these design features are largely lacking, but they are well grounded in reliability principles and common experience, and have also been recommended by others.1013, 58
| □Identifies the glycemic target range |
| □Includes clear dosing instructions with acceptable calculation requirements for nurses |
| □Incorporates glucose monitoring expectations |
| □Easy physician ordering, check box simplicity |
| □Criteria for calling the physician |
| □Includes guidance on steps to follow for interruption of nutrition |
| □States guidelines on when to initiate the infusion and when to stop |
| □Defines the insulin concentration clearly and consistently |
| □Considers changing insulin sensitivity as well as the current glucose value and rate of change in attempting to reach goal and avoid hypoglycemia |
| □Includes or refers to a standardized hypoglycemia treatment protocol and prevention protocol. |
| □Incorporates guidelines and cautions for transition to subcutaneous insulin |
| □Ideally adaptable outside of critical care unitclear definition of locations where order set is to be used. |
The orders should require a single physician signature and limited physician choices as the vehicle initiating the nurse‐driven protocol. The glycemic target range should be explicitly identified, and guidance for calling the physician and how to handle interruptions in nutrition should be embedded in the order set. Frequent monitoring of glucose levels is necessary for the safe infusion of insulin. Guidance for how often the monitoring is required must be explicit and included in the infusion order set, and standardization of documentation of the infusion rates and glucose values is highly desirable. IIPs that adjust based on the velocity of glucose change and insulin sensitivity are desirable. Certain elements may be more appropriate for some institutions than others, partly based on previous protocols and methods of practice. For example, the hypoglycemia protocol may be embedded or referred to as a separate standard of care with clear presence in the chart. The intended timing of conversion from IV to SC insulin should be included, but the actual method may be the subject of a separate order set. At other times, the conversion formula will be part of the initial intravenous order set.
IMPLEMENTATION: ADDRESSING SAFETY ISSUES
The use of insulin infusions comes with several potential hazards. Many of these potential complications can be proactively addressed, thereby minimizing accidental injuries to the patient on an insulin infusion.
Standardizing Insulin Infusion Preparations and Priming New Tubing
Varied concentrations or types of insulin for insulin infusions can lead to serious errors. Insulin infusions should generally be centrally prepared with a standard concentration of regular insulin in the pharmacy (usually 1 unit/cc), and the infusion concentration should be included in your infusion order set. Insulin binding to IV tubing can lead to false elevation of insulin requirements, potentially followed by serious hypoglycemia. When nurses change IV tubing or initially set up an insulin drip, education/emnstructions on priming new tubing with a small amount of insulin infusion to saturate the binding to the polyvinyl chloride tubing should be incorporated into their routine. Although 50 mL has often been recommended for priming, a recent study64 found that 20 mL of insulin infusion is enough to reach the saturation point.
Avoiding Over‐Reliance on the Insulin Protocol
Nurse‐driven insulin infusion protocols automate frequent insulin adjustment and reduce unnecessary calls to the physician. Although this is generally a decided advantage, the care team can be lulled into a sense of false security by the presence of orders that allow for such adjustment. Increasing the rate of an insulin infusion without thoughtful attention to factors that may be playing a role in this increased requirement (such as developing sepsis, other medical decompensation, steroid boluses, or an increase in carbohydrate intake) can have serious consequences. By the same token, an unanticipated rapid decrease in insulin requirement should lead to a reassessment of the infusion, and an inquiry about cessation of glucocorticoid therapy or nutrition. Rarely, a pharmacy or nursing error may induce a pseudo‐change in insulin requirements. The protocol should lead the nurse to seek advice and alert the physician to review potential causes of dramatic changes in insulin requirements, rather than simply adjusting insulin or nutrition to correct the present abnormal value.
Interruption of the Insulin Infusion
Interruption of insulin infusions may occur for many reasons, either intended or unintended. At times, the doctors or nurses may temporarily stop the protocol to allow for delivery of blood products or medications when IV access is limited. Infusions may mistakenly not be restarted, or deliberate discontinuation may not be adequately communicated, potentially leading to worsening hyperglycemia or even the development of ketoacidosis, and other adverse clinical outcomes. Therefore, the algorithm should have clear orders for the nurses to contact the ordering physician if the infusion is stopped for any reason, other than protocol‐driven cessation due to falling blood glucose concentrations.
Interruption of Nutrition, Field Trips, and Communication
Insulin infusion commonly provides both basal and nutritional insulin requirements. Interruptions in nutritional intake are extremely common in the inpatient setting, with a potential to cause serious hypoglycemia. Feeding tubes are often pulled out without warning; enteral nutrition may also need be halted if high gastric residuals are noted or during certain diagnostic tests. At times, IV carbohydrate sources (dextrose, partial parenteral nutrition, total parenteral nutrition) may be interrupted as well. In some cases, field trips out of the critical care units to the operating room, imaging studies, or other hospital locations add another layer of challenges to managing the IIP. Staff in these various areas may not be familiar with the IIP or monitoring standards and techniques, and potentially may not even be aware that the patient is on an insulin infusion. It is therefore crucial to anticipate these pitfalls and develop effective institutional procedures for addressing them. For example, many institutions use D10 solution to replace the carbohydrate calories that are lost when tube feedings have to be interrupted in a gram‐per‐gram fashion. Patients should be clearly identified as being on an insulin infusion. The requirement for consistent glucose monitoring, hypoglycemia recognition and treatment, and insulin infusion adjustment requires either critical care nurse care of the patient on the field trip, or training in the same skills in areas such as endoscopy, interventional radiology, and operating rooms including the preoperative and postoperative care units. In any case, all services should be involved in crafting solutions that will ensure a consistent approach to glycemic control as the patient travels off‐unit. Monitoring and treatment equipment needs to be readily available in all sites, and hypoglycemia protocols need to be distributed and supported in all areas.
Preventing and Treating Hypoglycemia
Some hypoglycemia will occur with infusion protocols, no matter how carefully a protocol is crafted and how well it is administered. Hypoglycemia protocols should therefore be incorporated directly into an infusion order set. Treatment of hypoglycemic events with a full 50 mL of D50 solution is equivalent to 25 g of carbohydrate, which will raise glucose levels in the average patient by 125 mg/dL. Many institutions discourage the overcorrection of hypoglycemic events by encouraging giving lesser aliquots of D50 based on the degree of hypoglycemia.
Preventing hypoglycemia by recognizing hypoglycemia risk factors, proper monitoring, and anticipating reductions in insulin requirements from decreasing severity of illness, nutritional intake, or steroid dosing can also reduce the frequency of hypoglycemic events.
IMPLEMENTATION: EDUCATING AND ENGAGING NURSING AND PHYSICIAN STAFF
Nursing staff generally bear the brunt of the burden on the front line of implementing intensive IIPs. Educational efforts for nurses should include the rationale for intensive insulin therapy and use of an IIP. Additionally, detailed, case‐based instruction on utilization of the IIP is required. Properly educated, nursing staff often become the strongest advocates of the IIP. In addition, they can frequently provide important input when situations arise that require troubleshooting. Regular feedback sessions early in implementation that address ease of use, clarity of orders, and difficulties encountered by nurses can be invaluable. Improvement teams need to provide frequent in‐service training and updates on the IIP selected after implementation. This is imperative to promote nursing acceptance and adherence to the IIP chosen, particularly with consideration for traveling nurses. The importance of nursing champions to design and carry out this work cannot be overstated. Educational programs focusing on the physician staff can also be very useful, particularly when focused on high‐volume physicians and influential thought leaders.
IMPLEMENTATION: ADDRESSING COMMON CLINICAL SITUATIONS
Steroids
Steroid boluses are commonly an integral part of regimens targeting a variety of conditions, such as transplant rejection, reactive airways disease, certain infections, cancer, and a variety of autoimmune disorders. This can lead to glycemic excursions and rapidly varying insulin requirements. Educational efforts and treatment regimens should address the disproportionate impact that steroids have on postprandial glycemic excursions. To minimize the glycemic impact of glucocorticoid therapy, a team should investigate promoting the use of steroid infusions in situations when a bolus is not absolutely necessary.
Dealing with the Eating Patient and Other Sources of Carbohydrate‐Induced Glycemic Excursions
Glucose levels can be difficult to control in patients who are eating while on insulin infusion, because the infusion chases the glycemic excursions through frequent adjustments, often with a late overshoot and inappropriate reduction in dose. We instead recommend providing bolus nutritional insulin to cover the expected glycemic excursion caused by carbohydrate ingestion. Carbohydrate counting and using a unit of insulin for each 10‐15 g of carbohydrate consumed can smooth out the rapid fluctuations in glucose. Guidance for this should be incorporated into the order set.
Transition Off of Insulin Infusion
Rational strategies for dealing with this transition are covered in detail elsewhere in this supplement.65 Guidance for managing this transition should be integrated into your insulin infusion and SC order sets. The transition to SC insulin may represent a separate order set but is sometimes best integrated into the IV insulin infusion order set itself.
IIPs Outside of the Critical Care Setting
IIPs are most commonly used in the critical care setting. In some institutions, IV insulin protocols are safely and effectively employed outside the ICU. Obviously, the number of nurses and other personnel who must be familiar with such protocols is much higher outside the ICU, and protocol errors are therefore likely to be somewhat higher. In addition the nurse‐per‐patient ratio is usually lower outside of the critical care setting. As a result, suggestions for safe implementation of insulin infusion regimens outside of the critical care setting include:
-
Choose an infusion protocol with a higher glycemic target.
-
Limit the medical and surgical units where this expertise will be developed.
-
Consider simplified infusion protocols but stay consistent with format.
-
Automated or computerized assistance of calculations may reduce human error and nursing burden.
ASSESSING THE IMPACT OF YOUR EFFORTS: FOLLOW‐UP AND FOLLOW‐THROUGH
Monitoring, Recording, and Analyzing Glycemic Control Data
Once the IIP is implemented, it is critical that the impact on glycemic control, hypoglycemia, insulin use, and other factors be analyzed and used for improving the IIP and care delivery. Frequent monitoring of glucose levels is necessary for the safe infusion of insulin. Guidance for how often the monitoring is required must be explicit and included in the infusion order set. Intermittent auditing for compliance with the frequency of glucose testing and appropriate dose selection is good practice. Attention should be paid to how the glucose level is obtained, recorded, and made available to the health care team in your institution. All glucose readings should be recorded electronically for ongoing analyses and retrieval, and ideally, this could be done in an automated or single‐step method. Try to eliminate duplication of effort, such as asking the nurse to record the glucose level and their reaction to it on paper and again in an electronic format. Your team should also provide guidance about the potential problems of using point‐of‐care glucose testing in settings with hypotension, sepsis, pressor use, and other conditions that may impair the accuracy of capillary glucose readings.
Reports on the time to reach the glycemic target, glycemic control while on infusion, and the incidence of hypoglycemia should be reviewed by the multidisciplinary steering committee. The Society of Hospital Medicine Glycemic Control Task Force recommends analysis by patient day and by patient stay (or insulin infusion run) as preferred methodologies for analysis of glycemic control and hypoglycemia rates over the method of using each individual glucose reading as the unit of analysis. (The latter tends to under‐value the frequency of hypoglycemia.) Detailed practical recommendations for analyzing and summarizing glycemic control data are available elsewhere in this supplement.18 These data should drive decisions on modification of glycemic targets and the protocol structure. Patients meeting prespecified criteria should be referred to the improvement team for review. For example, patients who experience any glucose readings of 40 mg/dL, or who take more than 12 hours to reach the upper limit glycemic target should be referred to the team for a case review.
Assessing Adherence to the Protocol and Ease‐of‐Use Issues
Focused audits in the pilot and early implementation phases should look for nonadherence to the protocol. Deviations should be evaluated according to the patterns identified. For example, variation in application in some cases is specific for an individual and in others is characteristic of a specific group or the whole. Accordingly, this may point to gaps in education or attitudes about the importance of this endeavor. Front‐line staff may deviate from the protocol because they find it ineffective, unsafe, or impractical for certain situations or specific patients. Many IIPs are the subject of nursing errors related to the knowledge and acceptance of the nurse but also the complexity of the protocol. Appropriate modifications to the protocol based on these cases can frequently improve the ease of use and effectiveness of the protocol. The ongoing review process should identify issues that must be addressed with permanent solutions rather than accepting frequent individual alterations to meet goals. Revisions require supplementary education and rapid and wide dissemination. Although educational efforts and monitoring are often most intense in the early implementation phase, periodic retraining should continue to achieve optimal results and safety. Educational tools must consider nursing time commitments and will often include an interactive web‐based module that gives more flexibility for trainers and clinical nurses alike.
CONCLUSIONS
Insulin infusions are a powerful clinical tool in the inpatient setting to maintain glycemic control. Many IIPs have been developed and used successfully. The institutional challenge is to select, modify, and implement the IIP to reduce hyperglycemia and improve outcomes without excess hypoglycemia. In order to accomplish this goal safely and efficiently, standardized processes and collaboration between physicians, nurses, and pharmacists are needed. The keys to minimizing errors include developing a culture of safety and cooperation, back‐up checks, standardization, automation, and robust training for all those who are involved in the care of a patient on an insulin infusion. Although we encourage standardization and the use of protocols, providers always need to consider the unique clinical circumstances and potential problems presented by each individual patient. It is important to recognize the many barriers to successful implementation of an IIP, but strategies exist to overcome these. Finally, remember that the process does not end with the development phase. Continued review is paramount to success. Note variations in use, analyze them, and learn from them, in order to continually improve the process of care.
- ,,,,,.A comparison of the activity and disposal of semi‐synthetic human insulin and porcine insulin in normal man by the glucose clamp technique.Diabetologia.1982;22(1):41–45.
- .Perioperative management of the diabetic patient.Endocrinol Metab Clin North Am.1992;21(2):457–475.
- ,.Pregnancy in the diabetic woman. Guidelines for a successful outcome.Endocrinol Metab Clin North Am.1992;21(2):433–456.
- ,,,.Continuous intravenous insulin infusion reduces the incidence of deep sternal wound infection in diabetic patients after cardiac surgical procedures.Ann Thorac Surg.1999;67(2):352–360; discussion 360–362.
- ,,, et al.Continuous insulin infusion reduces mortality in patients with diabetes undergoing coronary artery bypass grafting.J Thorac Cardiovasc Surg.2003;125(5):1007–1021.
- .Effect of an intensive glucose management protocol on the mortality of critically ill adult patients.Mayo Clin Proc.2004;79(8):992–1000.
- ,,, et al.Intensive insulin therapy in the critically ill patients.N Engl J Med.2001;345(19):1359–1367.
- American College of Endocrinology Position Statement on Inpatient Diabetes and Metobolic Control.Endocr Pract.2004;10(suppl 2):5–9.
- ,.Current controversies around tight glucose control in critically ill patients.Curr Opin Clin Nutr Metab Care.2007;10(2):206–209.
- American Society of Health‐System Pharmacists and the Hospital and Health‐System Association of Pennsylvania. Recommendations for Safe Use of Insulin in Hospitals. http://www.ashp.org/s_ashp/docs/files/Safe_Use_of_Insulin.pdf. Accessed September 5,2008.
- ,,, et al.Management of diabetes and hyperglycemia in hospitals.Diabetes Care.2004;27(2):553–591.
- Joint Commission. Disease Specific‐Care Certification. http://www.jointcommission.org/CertificationPrograms. Accessed September 5,2008.
- American College of Endocrinology and American Diabetes Association consensus statement on inpatient diabetes and glycemic control: a call to action.Diabetes Care2006;29(8):1955–1962.
- Standards of medical care in diabetes–2008.Diabetes Care2008;31(Suppl 1):S12–S54.
- ,,.Continuous insulin infusion improves postoperative glucose control in patients with diabetes mellitus undergoing coronary artery bypass surgery.Tex Heart Inst J.2006;33(4):445–451.
- ,,,,.A practical approach to hyperglycemia management in the intensive care unit: evaluation of an intensive insulin infusion protocol.Pharmacotherapy.2006;26(10):1410–1420.
- ,,,,,.Standardization of intravenous insulin therapy improves the efficiency and safety of blood glucose control in critically ill adults.Intensive Care Med.2004;30(5):804–810.
- ,,,,,.SHM Glycemic Control Task Force summary: practical recommendations for assessing the impact of glycemic control efforts.J Hosp Med supplement. In press.
- ,,,.Introducing intensive insulin therapy: the nursing perspective.Nurs Crit Care.2006;11(2):75–79.
- .Evaluation of nursing work effort and perceptions about blood glucose testing in tight glycemic control.Am J Crit Care. Jul2006;15(4):370–377.
- ,,, et al.Intensive insulin therapy in the medical ICU.N Engl J Med.2006;354(5):449–461.
- ,,.Effect of hyperglycemia and continuous intravenous insulin infusions on outcomes of cardiac surgical procedures: the Portland Diabetic Project.Endocr Pract.2004;10(Suppl 2):21–33.
- ,,, et al.Implementation of a safe and effective insulin infusion protocol in a medical intensive care unit.Diabetes Care.2004;27(2):461–467.
- ,,, et al.Improving glycemic control in the cardiothoracic intensive care unit: clinical experience in two hospital settings.J Cardiothorac Vasc Anesth.2004;18(6):690–697.
- ,,, et al.Inpatient management of hyperglycemia: the Northwestern experience.Endocr Pract.2006;12(5):491–505.
- ,,, et al.Performance of a dose‐defining insulin infusion protocol among trauma service intensive care unit admissions.Diabetes Technol Ther.2006;8(4):476–488.
- ,,.Glucommander: a computer‐directed intravenous insulin system shown to be safe, simple, and effective in 120,618 h of operation.Diabetes Care.2005;28(10):2418–2423.
- ,,, et al.Description and evaluation of a glycemic management protocol for patients with diabetes undergoing heart surgery.Endocr Pract.2002;8(1):10–18.
- ,,,,,.Tight glycemic control in diabetic coronary artery bypass graft patients improves perioperative outcomes and decreases recurrent ischemic events.Circulation.2004;109(12):1497–1502.
- ,,,.Practical management of diabetes in critically ill patients.Am J Respir Crit Care Med.2001;164(10 Pt 1):1763–1767.
- ,,,.Postoperative management of diabetes mellitus: steady‐state glucose control with bedside algorithm for insulin adjustment.Diabetes Care.1987;10(6):722–728.
- ,,,,.Glucose‐insulin‐potassium solutions improve outcomes in diabetics who have coronary artery operations.Ann Thorac Surg.2000;70(1):145–150.
- ,,, et al.Randomized trial of insulin‐glucose infusion followed by subcutaneous insulin treatment in diabetic patients with acute myocardial infarction (DIGAMI study): effects on mortality at 1 year.J Am Coll Cardiol.1995;26(1):57–65.
- ,.Eliminating the diabetic disadvantage: the Portland Diabetic Project.Semin Thorac Cardiovasc Surg.2006;18(4):302–308.
- ,,.Validation of an insulin infusion nomogram for intensive glucose control in critically ill patients.Pharmacotherapy.2005;25(3):352–359.
- ,,,,.Achieving tight glycemic control in the operating room: lessons learned from 12 years in the trenches of a paradigm shift in anesthetic care.Semin Thorac Cardiovasc Surg.2006;18(4):339–345.
- ,,.The rationale and management of hyperglycemia for in‐patients with cardiovascular disease: time for change.J Clin Endocrinol Metab.2003;88(6):2430–2437.
- ,,,,.An insulin infusion protocol in critically ill cardiothoracic surgery patients.Ann Pharmacother.2004;38(7–8):1123–1129.
- ,,, et al.Efficacy and safety of an insulin infusion protocol in a surgical ICU.J Am Coll Surg.2006;202(1):1–9.
- ,,,.Implementation of a tight glycaemic control protocol using a web‐based insulin dose calculator.Anaesthesia.2005;60(11):1093–1100.
- ,,,.Intensive insulin therapy to non‐cardiac ICU patients: a prospective study.Eur J Anaesthesiol.2006;23(8):705–709.
- ,,, et al.Blood glucose control by a model predictive control algorithm with variable sampling rate versus a routine glucose management protocol in cardiac surgery patients: a randomized controlled trial.J Clin Endocrinol Metab.2007;92(8):2960–2964.
- ,,, et al.Outcomes of a cardiothoracic intensive care web‐based online intravenous insulin infusion calculator study at a medical university hospital.Diabetes Technol Ther.2007;9(6):523–534.
- ,,,.Rush University guidelines and protocols for the management of hyperglycemia in hospitalized patients: elimination of the sliding scale and improvement of glycemic control throughout the hospital.Diabetes Educ.2006;32(6):954–962.
- ,,,.Collaborative development of an insulin nomogram for intensive insulin therapy.Crit Care Nurs Q.2006;29(1):96–105.
- ,,, et al.Computer‐based insulin infusion protocol improves glycemia control over manual protocol.J Am Med Inform Assoc.2007;14(3):278–287.
- ,,, et al.Implementing an intravenous insulin infusion protocol in the intensive care unit.Am J Health Syst Pharm.2007;64(4):385–395.
- ,,,,.Use of a computerized guideline for glucose regulation in the intensive care unit improved both guideline adherence and glucose regulation.J Am Med Inform Assoc.2005;12(2):172–180.
- ,,, et al.Improving hyperglycemia management in the intensive care unit: preliminary report of a nurse‐driven quality improvement project using a redesigned insulin infusion algorithm.Diabetes Educ.2006;32(3):394–403.
- ,,.Clinical results of an updated insulin infusion protocol in critically ill patients.Diabetes Spectrum.2005;18(1):28–33.
- ,,.Insulin therapy for critically ill hospitalized patients: a meta‐analysis of randomized controlled trials.Arch Intern Med.2004;164(18):2005–2011.
- ,,.Intensive insulin therapy in critical care: a review of 12 protocols.Diabetes Care.2007;30(4):1005–1011.
- ,,.Hypoglycemia in the intensive care unit.Curr Opin Clin Nutr Metab Care.2007;10(2):193–196.
- ,,.Insulin infusion protocols for critically ill patients: a highlight of differences and similarities.Endocr Pract.2007;13(2):137–146.
- ,,.Effect of hyperglycemia and continuous intravenous insulin infusions on outcomes of cardiac surgical procedures: the Portland Diabetic Project.Endocr Pract.2004;10(Suppl 2):21–33.
- Providence Health 10(Suppl 2):71–80.
- .Memoirs of a root canal salesman: the successful implementation of a hospital‐wide intravenous insulin infusion protocol.Endocr Pract.2006;12(Suppl 3):79–85.
- ,,, et al.Intensive insulin therapy and pentastarch resuscitation in severe sepsis.N Engl J Med.2008;358(2):125–139.
- ,.Severe hypoglycemia in critically ill patients: risk factors and outcomes.Crit Care Med.2007;35(10):2262–2267.
- ,,.Evidence for strict inpatient blood glucose control: time to revise glycemic goals in hospitalized patients.Metabolism.2008;57(1):116–120.
- ,,, et al.Comparison of insulin infusion protocols in the ICU: computer‐guided versus standard column‐based insulin regimens [Abstract]. American Diabetes Association abstract,2008.
- ,,.Design and implementation of GRIP: a computerized glucose control system at a surgical intensive care unit.BMC Med Inform Decis Mak.2005;5:38.
- ,,, et al.“Waste not, want not”: determining the optimal priming volume for intravenous infusions.Diabetes Technol Ther.2006;8(5):598–601.
- ,,,.Bridge over troubled waters: safe and effective transitions of the inpatient with hyperglycemia.J Hosp Med supplement. In press.
The delayed and variable absorption of subcutaneous (SC) insulin has always provided challenges for the rapid and predictable control of hyperglycemia in the acute care setting. Conversely, intravenous infusion of human regular insulin provides a continuous and essentially immediate delivery mechanism. Once in the circulation, insulin has a very short half‐life of about 5 to 7 minutes and a biological effect of about 20 minutes.1 Intravenous insulin infusions are well‐established in several acute care settings including hyperglycemic emergencies, perioperative glucose management, and glucose control during labor and delivery.2, 3
The beneficial effects seen in early trials of strict glycemic control involving intensive care unit (ICU) patients47 (particularly cardiac surgery patients) and guidelines for inpatient glycemic control8 stimulated widespread interest in adopting insulin infusion protocols (IIPs) focused on achieving strict glycemic targets. The initial enthusiasm has been tempered by uneven results in trials of tight glycemic control, concerns about the effects of excessive hypoglycemia, and the resources needed to implement and maintain an IIP.9 Although considerable controversy about the ideal glycemic target for different patient populations exists, and will likely continue for some time, this article is not a review of the evidence for supporting 1 glycemic target versus another. Regardless of the glycemic target chosen, a standardized algorithm and accompanying program of monitoring are widely endorsed for both safety and quality reasons.1014 Most medical centers are at least attempting to implement nurse‐driven protocols that have demonstrated better perfomance than SC regimens15 and physician‐driven insulin infusions.16, 17 In this article, we outline several variables in IIP design and implementation and endorse several aspects of design and implementation that will likely result in improved staff acceptance and, ultimately, a more safe and effective IIP.
PREPARING TO IMPLEMENT AN IIP
Building The Team
Implementing a medical centerwide standardized insulin infusion order set with supporting policies, protocols, monitoring standards, and the requisite educational programs is a major task for any hospital. This is not a simple maneuver involving only 1 or 2 interested individuals and requires much more than selecting a published protocol and disseminating it to various patient care units. Instead, to manage the full spectrum of diabetes programs and protocols, an institution must convene a multidisciplinary steering committee or task force. This should include representation from nursing, nursing administration, pharmacy, nutrition services, and the quality improvement department. Physicians should include hospitalists, intensivists, and endocrinologists but may also involve anesthesiologists and surgeons, as applicable. At times, additional members may need to be recruited according to project needs.
Identifying the Stakeholders and Current Practices
In developing or improving currently utilized IIPs, the multidisciplinary committee would benefit from careful background work before moving forward. First, administrative and institutional support must be secured to endorse uniform standards for insulin infusions, and to provide the important infrastructure needed to facilitate the work involved. Clinical and administrative stakeholders from the key departments then need to be identified.
All insulin infusion orders and policies/procedures presently used in the institution should be identified and examined. The developers and/or users of these order sets should be engaged in a dialogue and encouraged to share their experiences regarding their current practice and the attendant work flow, glucose monitoring, and data collection and reporting. Immediate concerns should be clearly addressed. Measurement systems for glycemic control, hypoglycemia, and insulin use patterns should assess current practice and the impact of subsequent modifications of the protocol or initiation of new protocols. We recommend using glucometrics consistent with those endorsed by the Society of Hospital Medicine (SHM) Glycemic Control Task Force elsewhere in this supplement.18
Addressing the Burden of Change
Through this process, the committee will uncover barriers, dysfunctional and inconsistent practices, and individuals who will pose challenges. Identifying these issues should not discourage the team, but rather it should guide the interventional strategies, and help build consensus that change may be required. There must be caution not to exclude significant individuals simply because they resist changes. Indeed, if they are included and have the opportunity to contribute to the process, success is much more likely.
It is important for process leaders to understand the implications of what is proposed, particularly for nursing services.19 For example, it has been shown that IIPs require about 5 minutes per patient per hour for glucose monitoring and dose adjustments.20 Acknowledging and attempting to address this burden proactively (often well over 2 hours per day) can gain staff acceptance more effectively than a laissez faire approach. In this regard, some effort should be invested in nursing education of the benefits of tight glycemic management on critical care outcomes. The difficult‐to‐quantify work involved when patients' blood glucose is not well controlled (eg, paging physicians for stat insulin orders) should also be part of this discussion.
Identifying and Addressing Barriers to IIP implementation
There are numerous potential barriers to implementation of IIPs. Table 1 identifies some of the most frequent ones along with potential strategies or solutions. Very common barriers include skepticism surrounding the benefits of tight glycemic control, fear of hypoglycemia, and difficulty agreeing on glycemic targets.
| Barrier | Strategy/Solution |
|---|---|
| Insufficient glucose meters to accommodate the increased testing needs | Purchase additional glucose meters. |
| Ask the vendor to provide extra on‐site replacement meters at no charge until they are activated. | |
| Nursing time requirements involved in monitoring and adjustments. | Get ancillary help to check glucose values |
| ○eg, nurse assistants | |
| Make extra efforts to make protocols clear with few required calculations | |
| Avoid duplicate recording | |
| Consider meters requiring shorter time and a smaller sample (to avoid need for re‐sampling) | |
| Requirement for uncomfortable frequent sticks | Utilize central lines or arterial lines. |
| ○These tend to vary by < 10% from POC readings | |
| ○May not be available in noncritical care settings | |
| Staff fear of hypoglycemia | Educate on the benefit of glucose control and the true definition of hypoglycemia |
| ○Measure staff fasting glucose levels to demonstrate normal range. | |
| Establish metrics and publicly report hypoglycemia event rates. | |
| Pilot IIP on small scale. | |
| Protocol and education for prevention of hypoglycemia. | |
| Difficulty gaining consensus on glycemic target | Compromise if needed on the glucose target |
| ○eg, start with a higher goal such as 90‐140 mg/dL. | |
| ○Others will be willing to lower the goal when feasibility is seen. | |
| Allow for different targets in different units if indicated | |
| ○maintain consistency in other respects. | |
| Focal points of resistance | Identify a local nurse or physician champion within resistant site. |
| Pilot the protocol in an area with least resistance | |
| ○Will gain momentum with initial success and adjustments | |
| Lack of integrated information and reporting systems | Incorporate information systems personnel onto team |
| Advocate for improved reporting capability with administrative leaders | |
| Use sampling methods to collect data until automated systems are available. | |
| Multiple providers, hand offs, and opportunities for error and communication breakdown, diffusion of responsibility for glycemic control | Involvement of varied front line providers |
| Check lists for important items to communicate on transfer/transport | |
| Common protocols/education for similar units |
Whereas the national guidelines call for a glycemic target in critical care areas with an upper limit of 110 mg/dL, there is room for debate, and tailoring of the glycemic target to fit individual patient circumstances is often advisable. Starting with a less aggressive glycemic target can be good politics (and perhaps good medicine as well). Once the higher glycemic targets are achieved safely, it can pave the way for a more aggressive approach.
Fear of hypoglycemia is one of the most potent barriers to intensive insulin infusion implementation. Because hyperglycemia is such a common condition in critical care units, nursing and physician staff may have developed a skewed view of the definition of hypoglycemia, at times fearing for their patient when the glucose values reach a level of 100 mg/dL or so. Polling the nurses on what they think their own fasting glucose levels are, and then actually measuring them can be an effective strategy (the nurses may be surprised that the patient's scary 90 mg/dL reading is higher than their own). It should also be emphasized that properly designed and implemented protocols may actually decrease the incidence of hypoglycemia when compared to standard care which may involve individually and sometimes improperly adjusted intravenous (IV) insulin (discussed further below).
CHOOSING AN IIP FOR YOUR HOSPITAL
See Table 2 for a comparison of several features identified in selected published protocols.7, 2127 This is not a comprehensive list of all protocols found in the literature. Rather, the authors consider it representative of the various types of IIPs or best recognized IIPs in the literature. Note that there is variability in study population, the glucose targets, hypoglycemia, the time to reach the target, and the time spent in the target range. Other practical factors to consider in reviewing the literature and selecting an IIP design are the complexity of the protocol, the required process steps or calculations, the evidence for staff acceptance, and the level of resources supporting the published protocol.
| Author (reference) | IIP Description | Patient population | Glucose Target (mg/dL) | Mean Glucose (mg/dL) | Time to Reach Target | Hypoglycemia |
|---|---|---|---|---|---|---|
| Van den Berghe7 | Initial and subsequent rates based on BG | Surgical ICU | 80‐110 | 6 AM glucose 103 19 | Not reported | 5.1% of patient < 41 mg/dL |
| Nurses had latitude | ||||||
| Van den Berghe21 | TPN standard with IIP | Medical ICU | 80‐110 | 6 AM glucose 105 | Not reported | 19% of patients < 41 mg/dL |
| Furnary22 (2001‐2003 version) | Initial dose determined by BG and type of DM | CABG | 100‐150 (there is a newer protocol with lower targets) | Not mentioned. Appears to be < 150 | 94% within 3 hours | 0.5% < 60 (% of readings?) Not reported with any specificity |
| Changes based on present BG and last change | ||||||
| Relatively complex | ||||||
| Goldberg23 | Dosing based on: | MICU | 100‐139 | Not specified | 10.1 hours | 0.3% of readings <60 BG <60 in 5.4% of patient days |
| ○Current BG | ||||||
| ○Velocity of glucose change | ||||||
| Goldberg24 | ○Infusion rate | CT Surgery | 100‐139 | 122 17 Once target attained. | 5 hours | 0.2% of all BGs < 60 mg/Dl BG < 60 in 2.9% of patient days |
| Uses 3 tables | ||||||
| Relatively complex | ||||||
| DeSantis25 | Initial dose based on BG | Mostly surgical ICU and CVICU (75% surgical) | 80‐110 | 135 49 (higher in SICU and CVICU alone) | 10.6 hours | 1.5% of readings |
| < 60 mg/dL (lower for CVICU & SICU alone) | ||||||
| Changes based on present glucose and rate of change. | ||||||
| IV bolus used with changes. | ||||||
| Braithwaite26 | 6‐column method | Trauma ICU | <110 mg/dL | 129 25 | 16.7 hours | 2.4% of readings < 70 mg/dL |
| Davidson27 | Computer‐directed algorithm (similar to column method) | Full spectrum | Varied by year and situation | Not reported (approximately 125 mg/dL when stable) | 90% < 150 mg/dL in 3 hours | 0.6% of readings <50 mg/dL 2.6% of patients |
Structural Differences in Protocols
Protocols that vary by level of insulin sensitivity generally use column methods with the individual columns representing different categories of insulin sensitivity, placing the most sensitive category on the left with the highest level of insulin resistance on the far right. These methods use a multiplier to adjust for sensitivity. They are constructed according to the rule of 1500, 1700, or 1800, thereby adjusting for changes in insulin sensitivity that follow surgery or other changes in physiologic stress in the acute setting. Rate of change is addressed by shifting to the right if correction of hyperglycemia is too slow and to the left if the glucose is dropping too rapidly.
Most institutions using the column/emnsulin sensitivity method, implement paper orders as first published by Markovitz.28 The same concepts have been used to develop computer‐assisted insulin infusion protocols. One published method is the Glucommander,27 but a number of institutions are using similar computer‐assisted methods developed locally.
Other methods use the present glucose and change from last glucose to constantly adjust to any situation.7, 2125 They usually involve 2 or 3 steps and often require more calculations by the nurse. These methods are purported to be more agile or flexible but there have been no direct comparisons with the column methods looking at effectiveness, nursing errors, or hypoglycemic risk. The Yale Protocol23, 24 and the Portland Protocol22 are 2 prominent examples of this type of protocol. Adjustments are defined as units, percent change, or a combination of both.
Limitations of the IIP Literature
There had been few published insulin protocols aimed at reaching specific glucose goals when the Leuven surgical ICU experience7 was published in 2001. These early publications featured algorithms that adjusted insulin infusions solely on the basis of glucose level, and did not take the velocity of change, direction of change, proximity to glycemic target, or different insulin sensitivities into account. Also, the targeted glucose goals in those reports were not consistent with the present standards.2931 Other published protocols featured glucose‐insulin‐potassium infusion (GIK) protocols that focused on the amount of insulin administered and failed to attain appropriately defined glucose targets.32, 33 These publications offer no real guidance in crafting a modern glycemic targetoriented protocol.
Whereas more than 20 modern IIPs directed at glycemic targets have been published,7, 1517, 2127, 3450 many of the published protocols represent local modifications of ones previously published elsewhere, and no published, prospective, head‐to‐head comparisons of the best‐known IIPs are available.
Several reviews of previous published reports include comparisons of IIPs with varied areas of emphasis.5154 The reliability of such comparisons is limited by the inconsistency in methodology between studies and the different populations studied. For example, medical populations are generally harder to control and are more prone to hypoglycemia than surgical populations,7, 21, 23, 24, 50 and some studies include only patients with diabetes.4, 5, 38 In addition, definitions of hypoglycemia and methods of analyzing glycemic control are highly variable, making comparisons of IIPs challenging.39 As a result, attempts to compare published IIP results without consideration of the population studied could lead to erroneous conclusions.
In spite of the aforementioned limitations, there are several lessons we can learn from the IIP literature and accumulated clinical experience at a variety of centers.
Glycemic Targets and Other IIP Features Can Evolve over Time
Revisions of protocol details, including glycemic targets, often evolve over time. This deliberate approach can facilitate staff acceptance by demonstrating safe achievement of higher glycemic targets. The Yale team initially selected a conservative glycemic target of 100‐139 mg/dL, as depicted in Table 1.23, 24 They subsequently lowered the target range to 90‐119 mg/dL and increased the initial insulin bolus amount by 40%.50 The modified protocol displayed improved performance and yet retained safety, with only 1 glucose reading < 40 mg/dL in 101 patients over 117 runs of insulin infusion.
The Portland Protocol, used primarily in cardiac surgery patients, has also evolved over time. This group has altered the IIP in a number of ways at least 4 times. These protocols, including the most recent protocol targeting glucose levels of 70‐110 mg/dL, is found at
Medical and Surgical Patients Are Different
The most convincing evidence for stringent glycemic control evolved from studies of surgical patients.4, 5, 7 Acknowledging this fact, along with the greater degree of difficulty in achieving glycemic targets safely in critically ill medical patients, have led many to endorse higher glycemic targets for certain populations than others.57 Although we generally favor a uniform glycemic target for a unit serving a particular patient population, adopting a less stringent glycemic target in medical ICU settings compared to surgical ICU settings is reasonable and prudent in many institutional settings. However, the accompanying challenges regarding boarding patients and floating nurses between units would also need to be considered.
Local Factors and Implementation Methods Matter
The success or failure of a protocol likely depends on local factors and implementation methods as much as it does on the structure of the protocol itself. IIP development and implementation is a process that must be approached systematically and with attention to detail.19 Errors in approach can delay or abort the implementation, or potentially lead to an ineffective or unsafe protocol for the institution.
The Yale experience again provides us with a salient example.58 Initial efforts to implement an IIP failed due to a number of factors: a complicated protocol, insufficient nursing involvement, and inadequate training and education led to incomplete buy‐in, and nursing concerns over hypoglycemia that actually was within the goal range of the protocol. Successful implementation was not achieved until the leaders learned from their mistakes. Nurses and other clinical allies were involved and educated. Important stakeholders, who were not included earlier, were now involved, and front‐line nursing staff were engaged in proactive troubleshooting.
In another example, multisite studies like VISEP59 and Glucontrol9 have tried to adapt the Leuven protocol, only to struggle with excessive hypoglycemia and an inability to replicate the tight glycemic control enjoyed by van den Berghe and colleagues.7, 21 The Leuven care team augmented the performance of the IIP by their experience with the protocol, physician involvement, and adjustments made by the nursing staff.
Some IIPs Have Lower Hypoglycemia Rates than Others
Recent articles have highlighted the association of intensive insulin treatment, hypoglycemia, and mortality in the medical ICU population.60 Concerns that excessive hypoglycemia could reduce or reverse the overall benefits of intensive insulin therapy has led some to call for moderation of critical care glycemic targets.61 Comparisons of IIPs5254, 61 that include information about the propensity of the IIP to produce hypoglycemia are therefore important.
The Leuven IIP has been reported to yield high rates of hypoglycemia with almost 20% of patients suffering from severe hypoglycemia (glucose < 40 mg/dL) in some studies.9, 21, 59 By way of contrast, several different IIP regimens have frequently achieved identical or very similar glycemic control with less than 5% of the patients experiencing severe hypoglycemia.27, 50, 62 Although implementation factors, severity of illness, and other population factors play some role, some protocols have an inherent structural propensity to produce more hypoglycemia than others. In the case of the Leuven protocol, there is more of a role for clinician adjustment, and the written protocol itself does not call for much active adjustment as the patient enters into the hypoglycemic range.52 In contrast, more sophisticated and complicated regimens adjust more aggressively in this range and consistently induce less hypoglycemia.
Successful Methods to Manage the Complexity of an IIP
Many modern IIPs use bolus insulin to expedite control and adjust the infusion rate based on the velocity and direction of glucose change, not just the glucose value.52 Insulin resistance is taken into account in some models, and multiple calculation steps are required in several reported protocols.52 The improved automation and control refinements come at a cost of increased complexity.
Intensive implementation efforts and strong leadership can overcome some issues, as demonstrated by the Yale experience, but 2 other strategies have commonly demonstrated success. First, focused expert glycemic management teams that directly oversee many aspects of the IIP, or even assume direct management roles, can be quite effective,25 especially during early implementation.
Automation of calculations and computerization is another method to consider, and many recent reports involve applications of web‐based or other computerized models.27, 40, 42, 43, 46, 48, 63 Comparisons of computerized versus manual methods, computerized versus column methods, and a computerized nomogram‐versus‐chart method are now available.42, 45, 46, 48, 62 Computerized protocols show significant promise and would be expected to reduce dosing errors. These instruments generally present the nurse with a specific infusion rate after each monitored glucose and then recommend an interval for the next glucose determination. In direct comparisons, they tend to perform as well or often better than conventional methods. For example, in the most recent randomized trial comparing the Glucommander to a paper‐based column method in an ICU, Newton and colleagues found the computerized method reached the goal more rapidly and reached a lower mean glucose without increasing the rate of severe hypoglycemia (< 40 mg/dL).62 Computerized methods also facilitate data collection for analytical purposes.
Computerized systems (both commercial and home‐grown versions) are becoming more common and appear to hold significant benefits as long as they are backed by a validated algorithm. Whereas many institutions are not yet in a position to integrate such protocols into their standard or electronic record systems, we expect the trend for increased implementation to continue.
ENHANCING THE DESIGN OF YOUR IIP
Once the improvement team has identified and examined current order sets and protocols in the context of the literature, we encourage consolidation of institutional insulin infusion orders into a common basic structure. This basic structure should be enhanced by incorporating a variety of elements designed to enhance the reliability of use and safety of the IIP. These design features are outlined in Table 3. Randomized trial evidence of the effectiveness of these design features are largely lacking, but they are well grounded in reliability principles and common experience, and have also been recommended by others.1013, 58
| □Identifies the glycemic target range |
| □Includes clear dosing instructions with acceptable calculation requirements for nurses |
| □Incorporates glucose monitoring expectations |
| □Easy physician ordering, check box simplicity |
| □Criteria for calling the physician |
| □Includes guidance on steps to follow for interruption of nutrition |
| □States guidelines on when to initiate the infusion and when to stop |
| □Defines the insulin concentration clearly and consistently |
| □Considers changing insulin sensitivity as well as the current glucose value and rate of change in attempting to reach goal and avoid hypoglycemia |
| □Includes or refers to a standardized hypoglycemia treatment protocol and prevention protocol. |
| □Incorporates guidelines and cautions for transition to subcutaneous insulin |
| □Ideally adaptable outside of critical care unitclear definition of locations where order set is to be used. |
The orders should require a single physician signature and limited physician choices as the vehicle initiating the nurse‐driven protocol. The glycemic target range should be explicitly identified, and guidance for calling the physician and how to handle interruptions in nutrition should be embedded in the order set. Frequent monitoring of glucose levels is necessary for the safe infusion of insulin. Guidance for how often the monitoring is required must be explicit and included in the infusion order set, and standardization of documentation of the infusion rates and glucose values is highly desirable. IIPs that adjust based on the velocity of glucose change and insulin sensitivity are desirable. Certain elements may be more appropriate for some institutions than others, partly based on previous protocols and methods of practice. For example, the hypoglycemia protocol may be embedded or referred to as a separate standard of care with clear presence in the chart. The intended timing of conversion from IV to SC insulin should be included, but the actual method may be the subject of a separate order set. At other times, the conversion formula will be part of the initial intravenous order set.
IMPLEMENTATION: ADDRESSING SAFETY ISSUES
The use of insulin infusions comes with several potential hazards. Many of these potential complications can be proactively addressed, thereby minimizing accidental injuries to the patient on an insulin infusion.
Standardizing Insulin Infusion Preparations and Priming New Tubing
Varied concentrations or types of insulin for insulin infusions can lead to serious errors. Insulin infusions should generally be centrally prepared with a standard concentration of regular insulin in the pharmacy (usually 1 unit/cc), and the infusion concentration should be included in your infusion order set. Insulin binding to IV tubing can lead to false elevation of insulin requirements, potentially followed by serious hypoglycemia. When nurses change IV tubing or initially set up an insulin drip, education/emnstructions on priming new tubing with a small amount of insulin infusion to saturate the binding to the polyvinyl chloride tubing should be incorporated into their routine. Although 50 mL has often been recommended for priming, a recent study64 found that 20 mL of insulin infusion is enough to reach the saturation point.
Avoiding Over‐Reliance on the Insulin Protocol
Nurse‐driven insulin infusion protocols automate frequent insulin adjustment and reduce unnecessary calls to the physician. Although this is generally a decided advantage, the care team can be lulled into a sense of false security by the presence of orders that allow for such adjustment. Increasing the rate of an insulin infusion without thoughtful attention to factors that may be playing a role in this increased requirement (such as developing sepsis, other medical decompensation, steroid boluses, or an increase in carbohydrate intake) can have serious consequences. By the same token, an unanticipated rapid decrease in insulin requirement should lead to a reassessment of the infusion, and an inquiry about cessation of glucocorticoid therapy or nutrition. Rarely, a pharmacy or nursing error may induce a pseudo‐change in insulin requirements. The protocol should lead the nurse to seek advice and alert the physician to review potential causes of dramatic changes in insulin requirements, rather than simply adjusting insulin or nutrition to correct the present abnormal value.
Interruption of the Insulin Infusion
Interruption of insulin infusions may occur for many reasons, either intended or unintended. At times, the doctors or nurses may temporarily stop the protocol to allow for delivery of blood products or medications when IV access is limited. Infusions may mistakenly not be restarted, or deliberate discontinuation may not be adequately communicated, potentially leading to worsening hyperglycemia or even the development of ketoacidosis, and other adverse clinical outcomes. Therefore, the algorithm should have clear orders for the nurses to contact the ordering physician if the infusion is stopped for any reason, other than protocol‐driven cessation due to falling blood glucose concentrations.
Interruption of Nutrition, Field Trips, and Communication
Insulin infusion commonly provides both basal and nutritional insulin requirements. Interruptions in nutritional intake are extremely common in the inpatient setting, with a potential to cause serious hypoglycemia. Feeding tubes are often pulled out without warning; enteral nutrition may also need be halted if high gastric residuals are noted or during certain diagnostic tests. At times, IV carbohydrate sources (dextrose, partial parenteral nutrition, total parenteral nutrition) may be interrupted as well. In some cases, field trips out of the critical care units to the operating room, imaging studies, or other hospital locations add another layer of challenges to managing the IIP. Staff in these various areas may not be familiar with the IIP or monitoring standards and techniques, and potentially may not even be aware that the patient is on an insulin infusion. It is therefore crucial to anticipate these pitfalls and develop effective institutional procedures for addressing them. For example, many institutions use D10 solution to replace the carbohydrate calories that are lost when tube feedings have to be interrupted in a gram‐per‐gram fashion. Patients should be clearly identified as being on an insulin infusion. The requirement for consistent glucose monitoring, hypoglycemia recognition and treatment, and insulin infusion adjustment requires either critical care nurse care of the patient on the field trip, or training in the same skills in areas such as endoscopy, interventional radiology, and operating rooms including the preoperative and postoperative care units. In any case, all services should be involved in crafting solutions that will ensure a consistent approach to glycemic control as the patient travels off‐unit. Monitoring and treatment equipment needs to be readily available in all sites, and hypoglycemia protocols need to be distributed and supported in all areas.
Preventing and Treating Hypoglycemia
Some hypoglycemia will occur with infusion protocols, no matter how carefully a protocol is crafted and how well it is administered. Hypoglycemia protocols should therefore be incorporated directly into an infusion order set. Treatment of hypoglycemic events with a full 50 mL of D50 solution is equivalent to 25 g of carbohydrate, which will raise glucose levels in the average patient by 125 mg/dL. Many institutions discourage the overcorrection of hypoglycemic events by encouraging giving lesser aliquots of D50 based on the degree of hypoglycemia.
Preventing hypoglycemia by recognizing hypoglycemia risk factors, proper monitoring, and anticipating reductions in insulin requirements from decreasing severity of illness, nutritional intake, or steroid dosing can also reduce the frequency of hypoglycemic events.
IMPLEMENTATION: EDUCATING AND ENGAGING NURSING AND PHYSICIAN STAFF
Nursing staff generally bear the brunt of the burden on the front line of implementing intensive IIPs. Educational efforts for nurses should include the rationale for intensive insulin therapy and use of an IIP. Additionally, detailed, case‐based instruction on utilization of the IIP is required. Properly educated, nursing staff often become the strongest advocates of the IIP. In addition, they can frequently provide important input when situations arise that require troubleshooting. Regular feedback sessions early in implementation that address ease of use, clarity of orders, and difficulties encountered by nurses can be invaluable. Improvement teams need to provide frequent in‐service training and updates on the IIP selected after implementation. This is imperative to promote nursing acceptance and adherence to the IIP chosen, particularly with consideration for traveling nurses. The importance of nursing champions to design and carry out this work cannot be overstated. Educational programs focusing on the physician staff can also be very useful, particularly when focused on high‐volume physicians and influential thought leaders.
IMPLEMENTATION: ADDRESSING COMMON CLINICAL SITUATIONS
Steroids
Steroid boluses are commonly an integral part of regimens targeting a variety of conditions, such as transplant rejection, reactive airways disease, certain infections, cancer, and a variety of autoimmune disorders. This can lead to glycemic excursions and rapidly varying insulin requirements. Educational efforts and treatment regimens should address the disproportionate impact that steroids have on postprandial glycemic excursions. To minimize the glycemic impact of glucocorticoid therapy, a team should investigate promoting the use of steroid infusions in situations when a bolus is not absolutely necessary.
Dealing with the Eating Patient and Other Sources of Carbohydrate‐Induced Glycemic Excursions
Glucose levels can be difficult to control in patients who are eating while on insulin infusion, because the infusion chases the glycemic excursions through frequent adjustments, often with a late overshoot and inappropriate reduction in dose. We instead recommend providing bolus nutritional insulin to cover the expected glycemic excursion caused by carbohydrate ingestion. Carbohydrate counting and using a unit of insulin for each 10‐15 g of carbohydrate consumed can smooth out the rapid fluctuations in glucose. Guidance for this should be incorporated into the order set.
Transition Off of Insulin Infusion
Rational strategies for dealing with this transition are covered in detail elsewhere in this supplement.65 Guidance for managing this transition should be integrated into your insulin infusion and SC order sets. The transition to SC insulin may represent a separate order set but is sometimes best integrated into the IV insulin infusion order set itself.
IIPs Outside of the Critical Care Setting
IIPs are most commonly used in the critical care setting. In some institutions, IV insulin protocols are safely and effectively employed outside the ICU. Obviously, the number of nurses and other personnel who must be familiar with such protocols is much higher outside the ICU, and protocol errors are therefore likely to be somewhat higher. In addition the nurse‐per‐patient ratio is usually lower outside of the critical care setting. As a result, suggestions for safe implementation of insulin infusion regimens outside of the critical care setting include:
-
Choose an infusion protocol with a higher glycemic target.
-
Limit the medical and surgical units where this expertise will be developed.
-
Consider simplified infusion protocols but stay consistent with format.
-
Automated or computerized assistance of calculations may reduce human error and nursing burden.
ASSESSING THE IMPACT OF YOUR EFFORTS: FOLLOW‐UP AND FOLLOW‐THROUGH
Monitoring, Recording, and Analyzing Glycemic Control Data
Once the IIP is implemented, it is critical that the impact on glycemic control, hypoglycemia, insulin use, and other factors be analyzed and used for improving the IIP and care delivery. Frequent monitoring of glucose levels is necessary for the safe infusion of insulin. Guidance for how often the monitoring is required must be explicit and included in the infusion order set. Intermittent auditing for compliance with the frequency of glucose testing and appropriate dose selection is good practice. Attention should be paid to how the glucose level is obtained, recorded, and made available to the health care team in your institution. All glucose readings should be recorded electronically for ongoing analyses and retrieval, and ideally, this could be done in an automated or single‐step method. Try to eliminate duplication of effort, such as asking the nurse to record the glucose level and their reaction to it on paper and again in an electronic format. Your team should also provide guidance about the potential problems of using point‐of‐care glucose testing in settings with hypotension, sepsis, pressor use, and other conditions that may impair the accuracy of capillary glucose readings.
Reports on the time to reach the glycemic target, glycemic control while on infusion, and the incidence of hypoglycemia should be reviewed by the multidisciplinary steering committee. The Society of Hospital Medicine Glycemic Control Task Force recommends analysis by patient day and by patient stay (or insulin infusion run) as preferred methodologies for analysis of glycemic control and hypoglycemia rates over the method of using each individual glucose reading as the unit of analysis. (The latter tends to under‐value the frequency of hypoglycemia.) Detailed practical recommendations for analyzing and summarizing glycemic control data are available elsewhere in this supplement.18 These data should drive decisions on modification of glycemic targets and the protocol structure. Patients meeting prespecified criteria should be referred to the improvement team for review. For example, patients who experience any glucose readings of 40 mg/dL, or who take more than 12 hours to reach the upper limit glycemic target should be referred to the team for a case review.
Assessing Adherence to the Protocol and Ease‐of‐Use Issues
Focused audits in the pilot and early implementation phases should look for nonadherence to the protocol. Deviations should be evaluated according to the patterns identified. For example, variation in application in some cases is specific for an individual and in others is characteristic of a specific group or the whole. Accordingly, this may point to gaps in education or attitudes about the importance of this endeavor. Front‐line staff may deviate from the protocol because they find it ineffective, unsafe, or impractical for certain situations or specific patients. Many IIPs are the subject of nursing errors related to the knowledge and acceptance of the nurse but also the complexity of the protocol. Appropriate modifications to the protocol based on these cases can frequently improve the ease of use and effectiveness of the protocol. The ongoing review process should identify issues that must be addressed with permanent solutions rather than accepting frequent individual alterations to meet goals. Revisions require supplementary education and rapid and wide dissemination. Although educational efforts and monitoring are often most intense in the early implementation phase, periodic retraining should continue to achieve optimal results and safety. Educational tools must consider nursing time commitments and will often include an interactive web‐based module that gives more flexibility for trainers and clinical nurses alike.
CONCLUSIONS
Insulin infusions are a powerful clinical tool in the inpatient setting to maintain glycemic control. Many IIPs have been developed and used successfully. The institutional challenge is to select, modify, and implement the IIP to reduce hyperglycemia and improve outcomes without excess hypoglycemia. In order to accomplish this goal safely and efficiently, standardized processes and collaboration between physicians, nurses, and pharmacists are needed. The keys to minimizing errors include developing a culture of safety and cooperation, back‐up checks, standardization, automation, and robust training for all those who are involved in the care of a patient on an insulin infusion. Although we encourage standardization and the use of protocols, providers always need to consider the unique clinical circumstances and potential problems presented by each individual patient. It is important to recognize the many barriers to successful implementation of an IIP, but strategies exist to overcome these. Finally, remember that the process does not end with the development phase. Continued review is paramount to success. Note variations in use, analyze them, and learn from them, in order to continually improve the process of care.
The delayed and variable absorption of subcutaneous (SC) insulin has always provided challenges for the rapid and predictable control of hyperglycemia in the acute care setting. Conversely, intravenous infusion of human regular insulin provides a continuous and essentially immediate delivery mechanism. Once in the circulation, insulin has a very short half‐life of about 5 to 7 minutes and a biological effect of about 20 minutes.1 Intravenous insulin infusions are well‐established in several acute care settings including hyperglycemic emergencies, perioperative glucose management, and glucose control during labor and delivery.2, 3
The beneficial effects seen in early trials of strict glycemic control involving intensive care unit (ICU) patients47 (particularly cardiac surgery patients) and guidelines for inpatient glycemic control8 stimulated widespread interest in adopting insulin infusion protocols (IIPs) focused on achieving strict glycemic targets. The initial enthusiasm has been tempered by uneven results in trials of tight glycemic control, concerns about the effects of excessive hypoglycemia, and the resources needed to implement and maintain an IIP.9 Although considerable controversy about the ideal glycemic target for different patient populations exists, and will likely continue for some time, this article is not a review of the evidence for supporting 1 glycemic target versus another. Regardless of the glycemic target chosen, a standardized algorithm and accompanying program of monitoring are widely endorsed for both safety and quality reasons.1014 Most medical centers are at least attempting to implement nurse‐driven protocols that have demonstrated better perfomance than SC regimens15 and physician‐driven insulin infusions.16, 17 In this article, we outline several variables in IIP design and implementation and endorse several aspects of design and implementation that will likely result in improved staff acceptance and, ultimately, a more safe and effective IIP.
PREPARING TO IMPLEMENT AN IIP
Building The Team
Implementing a medical centerwide standardized insulin infusion order set with supporting policies, protocols, monitoring standards, and the requisite educational programs is a major task for any hospital. This is not a simple maneuver involving only 1 or 2 interested individuals and requires much more than selecting a published protocol and disseminating it to various patient care units. Instead, to manage the full spectrum of diabetes programs and protocols, an institution must convene a multidisciplinary steering committee or task force. This should include representation from nursing, nursing administration, pharmacy, nutrition services, and the quality improvement department. Physicians should include hospitalists, intensivists, and endocrinologists but may also involve anesthesiologists and surgeons, as applicable. At times, additional members may need to be recruited according to project needs.
Identifying the Stakeholders and Current Practices
In developing or improving currently utilized IIPs, the multidisciplinary committee would benefit from careful background work before moving forward. First, administrative and institutional support must be secured to endorse uniform standards for insulin infusions, and to provide the important infrastructure needed to facilitate the work involved. Clinical and administrative stakeholders from the key departments then need to be identified.
All insulin infusion orders and policies/procedures presently used in the institution should be identified and examined. The developers and/or users of these order sets should be engaged in a dialogue and encouraged to share their experiences regarding their current practice and the attendant work flow, glucose monitoring, and data collection and reporting. Immediate concerns should be clearly addressed. Measurement systems for glycemic control, hypoglycemia, and insulin use patterns should assess current practice and the impact of subsequent modifications of the protocol or initiation of new protocols. We recommend using glucometrics consistent with those endorsed by the Society of Hospital Medicine (SHM) Glycemic Control Task Force elsewhere in this supplement.18
Addressing the Burden of Change
Through this process, the committee will uncover barriers, dysfunctional and inconsistent practices, and individuals who will pose challenges. Identifying these issues should not discourage the team, but rather it should guide the interventional strategies, and help build consensus that change may be required. There must be caution not to exclude significant individuals simply because they resist changes. Indeed, if they are included and have the opportunity to contribute to the process, success is much more likely.
It is important for process leaders to understand the implications of what is proposed, particularly for nursing services.19 For example, it has been shown that IIPs require about 5 minutes per patient per hour for glucose monitoring and dose adjustments.20 Acknowledging and attempting to address this burden proactively (often well over 2 hours per day) can gain staff acceptance more effectively than a laissez faire approach. In this regard, some effort should be invested in nursing education of the benefits of tight glycemic management on critical care outcomes. The difficult‐to‐quantify work involved when patients' blood glucose is not well controlled (eg, paging physicians for stat insulin orders) should also be part of this discussion.
Identifying and Addressing Barriers to IIP implementation
There are numerous potential barriers to implementation of IIPs. Table 1 identifies some of the most frequent ones along with potential strategies or solutions. Very common barriers include skepticism surrounding the benefits of tight glycemic control, fear of hypoglycemia, and difficulty agreeing on glycemic targets.
| Barrier | Strategy/Solution |
|---|---|
| Insufficient glucose meters to accommodate the increased testing needs | Purchase additional glucose meters. |
| Ask the vendor to provide extra on‐site replacement meters at no charge until they are activated. | |
| Nursing time requirements involved in monitoring and adjustments. | Get ancillary help to check glucose values |
| ○eg, nurse assistants | |
| Make extra efforts to make protocols clear with few required calculations | |
| Avoid duplicate recording | |
| Consider meters requiring shorter time and a smaller sample (to avoid need for re‐sampling) | |
| Requirement for uncomfortable frequent sticks | Utilize central lines or arterial lines. |
| ○These tend to vary by < 10% from POC readings | |
| ○May not be available in noncritical care settings | |
| Staff fear of hypoglycemia | Educate on the benefit of glucose control and the true definition of hypoglycemia |
| ○Measure staff fasting glucose levels to demonstrate normal range. | |
| Establish metrics and publicly report hypoglycemia event rates. | |
| Pilot IIP on small scale. | |
| Protocol and education for prevention of hypoglycemia. | |
| Difficulty gaining consensus on glycemic target | Compromise if needed on the glucose target |
| ○eg, start with a higher goal such as 90‐140 mg/dL. | |
| ○Others will be willing to lower the goal when feasibility is seen. | |
| Allow for different targets in different units if indicated | |
| ○maintain consistency in other respects. | |
| Focal points of resistance | Identify a local nurse or physician champion within resistant site. |
| Pilot the protocol in an area with least resistance | |
| ○Will gain momentum with initial success and adjustments | |
| Lack of integrated information and reporting systems | Incorporate information systems personnel onto team |
| Advocate for improved reporting capability with administrative leaders | |
| Use sampling methods to collect data until automated systems are available. | |
| Multiple providers, hand offs, and opportunities for error and communication breakdown, diffusion of responsibility for glycemic control | Involvement of varied front line providers |
| Check lists for important items to communicate on transfer/transport | |
| Common protocols/education for similar units |
Whereas the national guidelines call for a glycemic target in critical care areas with an upper limit of 110 mg/dL, there is room for debate, and tailoring of the glycemic target to fit individual patient circumstances is often advisable. Starting with a less aggressive glycemic target can be good politics (and perhaps good medicine as well). Once the higher glycemic targets are achieved safely, it can pave the way for a more aggressive approach.
Fear of hypoglycemia is one of the most potent barriers to intensive insulin infusion implementation. Because hyperglycemia is such a common condition in critical care units, nursing and physician staff may have developed a skewed view of the definition of hypoglycemia, at times fearing for their patient when the glucose values reach a level of 100 mg/dL or so. Polling the nurses on what they think their own fasting glucose levels are, and then actually measuring them can be an effective strategy (the nurses may be surprised that the patient's scary 90 mg/dL reading is higher than their own). It should also be emphasized that properly designed and implemented protocols may actually decrease the incidence of hypoglycemia when compared to standard care which may involve individually and sometimes improperly adjusted intravenous (IV) insulin (discussed further below).
CHOOSING AN IIP FOR YOUR HOSPITAL
See Table 2 for a comparison of several features identified in selected published protocols.7, 2127 This is not a comprehensive list of all protocols found in the literature. Rather, the authors consider it representative of the various types of IIPs or best recognized IIPs in the literature. Note that there is variability in study population, the glucose targets, hypoglycemia, the time to reach the target, and the time spent in the target range. Other practical factors to consider in reviewing the literature and selecting an IIP design are the complexity of the protocol, the required process steps or calculations, the evidence for staff acceptance, and the level of resources supporting the published protocol.
| Author (reference) | IIP Description | Patient population | Glucose Target (mg/dL) | Mean Glucose (mg/dL) | Time to Reach Target | Hypoglycemia |
|---|---|---|---|---|---|---|
| Van den Berghe7 | Initial and subsequent rates based on BG | Surgical ICU | 80‐110 | 6 AM glucose 103 19 | Not reported | 5.1% of patient < 41 mg/dL |
| Nurses had latitude | ||||||
| Van den Berghe21 | TPN standard with IIP | Medical ICU | 80‐110 | 6 AM glucose 105 | Not reported | 19% of patients < 41 mg/dL |
| Furnary22 (2001‐2003 version) | Initial dose determined by BG and type of DM | CABG | 100‐150 (there is a newer protocol with lower targets) | Not mentioned. Appears to be < 150 | 94% within 3 hours | 0.5% < 60 (% of readings?) Not reported with any specificity |
| Changes based on present BG and last change | ||||||
| Relatively complex | ||||||
| Goldberg23 | Dosing based on: | MICU | 100‐139 | Not specified | 10.1 hours | 0.3% of readings <60 BG <60 in 5.4% of patient days |
| ○Current BG | ||||||
| ○Velocity of glucose change | ||||||
| Goldberg24 | ○Infusion rate | CT Surgery | 100‐139 | 122 17 Once target attained. | 5 hours | 0.2% of all BGs < 60 mg/Dl BG < 60 in 2.9% of patient days |
| Uses 3 tables | ||||||
| Relatively complex | ||||||
| DeSantis25 | Initial dose based on BG | Mostly surgical ICU and CVICU (75% surgical) | 80‐110 | 135 49 (higher in SICU and CVICU alone) | 10.6 hours | 1.5% of readings |
| < 60 mg/dL (lower for CVICU & SICU alone) | ||||||
| Changes based on present glucose and rate of change. | ||||||
| IV bolus used with changes. | ||||||
| Braithwaite26 | 6‐column method | Trauma ICU | <110 mg/dL | 129 25 | 16.7 hours | 2.4% of readings < 70 mg/dL |
| Davidson27 | Computer‐directed algorithm (similar to column method) | Full spectrum | Varied by year and situation | Not reported (approximately 125 mg/dL when stable) | 90% < 150 mg/dL in 3 hours | 0.6% of readings <50 mg/dL 2.6% of patients |
Structural Differences in Protocols
Protocols that vary by level of insulin sensitivity generally use column methods with the individual columns representing different categories of insulin sensitivity, placing the most sensitive category on the left with the highest level of insulin resistance on the far right. These methods use a multiplier to adjust for sensitivity. They are constructed according to the rule of 1500, 1700, or 1800, thereby adjusting for changes in insulin sensitivity that follow surgery or other changes in physiologic stress in the acute setting. Rate of change is addressed by shifting to the right if correction of hyperglycemia is too slow and to the left if the glucose is dropping too rapidly.
Most institutions using the column/emnsulin sensitivity method, implement paper orders as first published by Markovitz.28 The same concepts have been used to develop computer‐assisted insulin infusion protocols. One published method is the Glucommander,27 but a number of institutions are using similar computer‐assisted methods developed locally.
Other methods use the present glucose and change from last glucose to constantly adjust to any situation.7, 2125 They usually involve 2 or 3 steps and often require more calculations by the nurse. These methods are purported to be more agile or flexible but there have been no direct comparisons with the column methods looking at effectiveness, nursing errors, or hypoglycemic risk. The Yale Protocol23, 24 and the Portland Protocol22 are 2 prominent examples of this type of protocol. Adjustments are defined as units, percent change, or a combination of both.
Limitations of the IIP Literature
There had been few published insulin protocols aimed at reaching specific glucose goals when the Leuven surgical ICU experience7 was published in 2001. These early publications featured algorithms that adjusted insulin infusions solely on the basis of glucose level, and did not take the velocity of change, direction of change, proximity to glycemic target, or different insulin sensitivities into account. Also, the targeted glucose goals in those reports were not consistent with the present standards.2931 Other published protocols featured glucose‐insulin‐potassium infusion (GIK) protocols that focused on the amount of insulin administered and failed to attain appropriately defined glucose targets.32, 33 These publications offer no real guidance in crafting a modern glycemic targetoriented protocol.
Whereas more than 20 modern IIPs directed at glycemic targets have been published,7, 1517, 2127, 3450 many of the published protocols represent local modifications of ones previously published elsewhere, and no published, prospective, head‐to‐head comparisons of the best‐known IIPs are available.
Several reviews of previous published reports include comparisons of IIPs with varied areas of emphasis.5154 The reliability of such comparisons is limited by the inconsistency in methodology between studies and the different populations studied. For example, medical populations are generally harder to control and are more prone to hypoglycemia than surgical populations,7, 21, 23, 24, 50 and some studies include only patients with diabetes.4, 5, 38 In addition, definitions of hypoglycemia and methods of analyzing glycemic control are highly variable, making comparisons of IIPs challenging.39 As a result, attempts to compare published IIP results without consideration of the population studied could lead to erroneous conclusions.
In spite of the aforementioned limitations, there are several lessons we can learn from the IIP literature and accumulated clinical experience at a variety of centers.
Glycemic Targets and Other IIP Features Can Evolve over Time
Revisions of protocol details, including glycemic targets, often evolve over time. This deliberate approach can facilitate staff acceptance by demonstrating safe achievement of higher glycemic targets. The Yale team initially selected a conservative glycemic target of 100‐139 mg/dL, as depicted in Table 1.23, 24 They subsequently lowered the target range to 90‐119 mg/dL and increased the initial insulin bolus amount by 40%.50 The modified protocol displayed improved performance and yet retained safety, with only 1 glucose reading < 40 mg/dL in 101 patients over 117 runs of insulin infusion.
The Portland Protocol, used primarily in cardiac surgery patients, has also evolved over time. This group has altered the IIP in a number of ways at least 4 times. These protocols, including the most recent protocol targeting glucose levels of 70‐110 mg/dL, is found at
Medical and Surgical Patients Are Different
The most convincing evidence for stringent glycemic control evolved from studies of surgical patients.4, 5, 7 Acknowledging this fact, along with the greater degree of difficulty in achieving glycemic targets safely in critically ill medical patients, have led many to endorse higher glycemic targets for certain populations than others.57 Although we generally favor a uniform glycemic target for a unit serving a particular patient population, adopting a less stringent glycemic target in medical ICU settings compared to surgical ICU settings is reasonable and prudent in many institutional settings. However, the accompanying challenges regarding boarding patients and floating nurses between units would also need to be considered.
Local Factors and Implementation Methods Matter
The success or failure of a protocol likely depends on local factors and implementation methods as much as it does on the structure of the protocol itself. IIP development and implementation is a process that must be approached systematically and with attention to detail.19 Errors in approach can delay or abort the implementation, or potentially lead to an ineffective or unsafe protocol for the institution.
The Yale experience again provides us with a salient example.58 Initial efforts to implement an IIP failed due to a number of factors: a complicated protocol, insufficient nursing involvement, and inadequate training and education led to incomplete buy‐in, and nursing concerns over hypoglycemia that actually was within the goal range of the protocol. Successful implementation was not achieved until the leaders learned from their mistakes. Nurses and other clinical allies were involved and educated. Important stakeholders, who were not included earlier, were now involved, and front‐line nursing staff were engaged in proactive troubleshooting.
In another example, multisite studies like VISEP59 and Glucontrol9 have tried to adapt the Leuven protocol, only to struggle with excessive hypoglycemia and an inability to replicate the tight glycemic control enjoyed by van den Berghe and colleagues.7, 21 The Leuven care team augmented the performance of the IIP by their experience with the protocol, physician involvement, and adjustments made by the nursing staff.
Some IIPs Have Lower Hypoglycemia Rates than Others
Recent articles have highlighted the association of intensive insulin treatment, hypoglycemia, and mortality in the medical ICU population.60 Concerns that excessive hypoglycemia could reduce or reverse the overall benefits of intensive insulin therapy has led some to call for moderation of critical care glycemic targets.61 Comparisons of IIPs5254, 61 that include information about the propensity of the IIP to produce hypoglycemia are therefore important.
The Leuven IIP has been reported to yield high rates of hypoglycemia with almost 20% of patients suffering from severe hypoglycemia (glucose < 40 mg/dL) in some studies.9, 21, 59 By way of contrast, several different IIP regimens have frequently achieved identical or very similar glycemic control with less than 5% of the patients experiencing severe hypoglycemia.27, 50, 62 Although implementation factors, severity of illness, and other population factors play some role, some protocols have an inherent structural propensity to produce more hypoglycemia than others. In the case of the Leuven protocol, there is more of a role for clinician adjustment, and the written protocol itself does not call for much active adjustment as the patient enters into the hypoglycemic range.52 In contrast, more sophisticated and complicated regimens adjust more aggressively in this range and consistently induce less hypoglycemia.
Successful Methods to Manage the Complexity of an IIP
Many modern IIPs use bolus insulin to expedite control and adjust the infusion rate based on the velocity and direction of glucose change, not just the glucose value.52 Insulin resistance is taken into account in some models, and multiple calculation steps are required in several reported protocols.52 The improved automation and control refinements come at a cost of increased complexity.
Intensive implementation efforts and strong leadership can overcome some issues, as demonstrated by the Yale experience, but 2 other strategies have commonly demonstrated success. First, focused expert glycemic management teams that directly oversee many aspects of the IIP, or even assume direct management roles, can be quite effective,25 especially during early implementation.
Automation of calculations and computerization is another method to consider, and many recent reports involve applications of web‐based or other computerized models.27, 40, 42, 43, 46, 48, 63 Comparisons of computerized versus manual methods, computerized versus column methods, and a computerized nomogram‐versus‐chart method are now available.42, 45, 46, 48, 62 Computerized protocols show significant promise and would be expected to reduce dosing errors. These instruments generally present the nurse with a specific infusion rate after each monitored glucose and then recommend an interval for the next glucose determination. In direct comparisons, they tend to perform as well or often better than conventional methods. For example, in the most recent randomized trial comparing the Glucommander to a paper‐based column method in an ICU, Newton and colleagues found the computerized method reached the goal more rapidly and reached a lower mean glucose without increasing the rate of severe hypoglycemia (< 40 mg/dL).62 Computerized methods also facilitate data collection for analytical purposes.
Computerized systems (both commercial and home‐grown versions) are becoming more common and appear to hold significant benefits as long as they are backed by a validated algorithm. Whereas many institutions are not yet in a position to integrate such protocols into their standard or electronic record systems, we expect the trend for increased implementation to continue.
ENHANCING THE DESIGN OF YOUR IIP
Once the improvement team has identified and examined current order sets and protocols in the context of the literature, we encourage consolidation of institutional insulin infusion orders into a common basic structure. This basic structure should be enhanced by incorporating a variety of elements designed to enhance the reliability of use and safety of the IIP. These design features are outlined in Table 3. Randomized trial evidence of the effectiveness of these design features are largely lacking, but they are well grounded in reliability principles and common experience, and have also been recommended by others.1013, 58
| □Identifies the glycemic target range |
| □Includes clear dosing instructions with acceptable calculation requirements for nurses |
| □Incorporates glucose monitoring expectations |
| □Easy physician ordering, check box simplicity |
| □Criteria for calling the physician |
| □Includes guidance on steps to follow for interruption of nutrition |
| □States guidelines on when to initiate the infusion and when to stop |
| □Defines the insulin concentration clearly and consistently |
| □Considers changing insulin sensitivity as well as the current glucose value and rate of change in attempting to reach goal and avoid hypoglycemia |
| □Includes or refers to a standardized hypoglycemia treatment protocol and prevention protocol. |
| □Incorporates guidelines and cautions for transition to subcutaneous insulin |
| □Ideally adaptable outside of critical care unitclear definition of locations where order set is to be used. |
The orders should require a single physician signature and limited physician choices as the vehicle initiating the nurse‐driven protocol. The glycemic target range should be explicitly identified, and guidance for calling the physician and how to handle interruptions in nutrition should be embedded in the order set. Frequent monitoring of glucose levels is necessary for the safe infusion of insulin. Guidance for how often the monitoring is required must be explicit and included in the infusion order set, and standardization of documentation of the infusion rates and glucose values is highly desirable. IIPs that adjust based on the velocity of glucose change and insulin sensitivity are desirable. Certain elements may be more appropriate for some institutions than others, partly based on previous protocols and methods of practice. For example, the hypoglycemia protocol may be embedded or referred to as a separate standard of care with clear presence in the chart. The intended timing of conversion from IV to SC insulin should be included, but the actual method may be the subject of a separate order set. At other times, the conversion formula will be part of the initial intravenous order set.
IMPLEMENTATION: ADDRESSING SAFETY ISSUES
The use of insulin infusions comes with several potential hazards. Many of these potential complications can be proactively addressed, thereby minimizing accidental injuries to the patient on an insulin infusion.
Standardizing Insulin Infusion Preparations and Priming New Tubing
Varied concentrations or types of insulin for insulin infusions can lead to serious errors. Insulin infusions should generally be centrally prepared with a standard concentration of regular insulin in the pharmacy (usually 1 unit/cc), and the infusion concentration should be included in your infusion order set. Insulin binding to IV tubing can lead to false elevation of insulin requirements, potentially followed by serious hypoglycemia. When nurses change IV tubing or initially set up an insulin drip, education/emnstructions on priming new tubing with a small amount of insulin infusion to saturate the binding to the polyvinyl chloride tubing should be incorporated into their routine. Although 50 mL has often been recommended for priming, a recent study64 found that 20 mL of insulin infusion is enough to reach the saturation point.
Avoiding Over‐Reliance on the Insulin Protocol
Nurse‐driven insulin infusion protocols automate frequent insulin adjustment and reduce unnecessary calls to the physician. Although this is generally a decided advantage, the care team can be lulled into a sense of false security by the presence of orders that allow for such adjustment. Increasing the rate of an insulin infusion without thoughtful attention to factors that may be playing a role in this increased requirement (such as developing sepsis, other medical decompensation, steroid boluses, or an increase in carbohydrate intake) can have serious consequences. By the same token, an unanticipated rapid decrease in insulin requirement should lead to a reassessment of the infusion, and an inquiry about cessation of glucocorticoid therapy or nutrition. Rarely, a pharmacy or nursing error may induce a pseudo‐change in insulin requirements. The protocol should lead the nurse to seek advice and alert the physician to review potential causes of dramatic changes in insulin requirements, rather than simply adjusting insulin or nutrition to correct the present abnormal value.
Interruption of the Insulin Infusion
Interruption of insulin infusions may occur for many reasons, either intended or unintended. At times, the doctors or nurses may temporarily stop the protocol to allow for delivery of blood products or medications when IV access is limited. Infusions may mistakenly not be restarted, or deliberate discontinuation may not be adequately communicated, potentially leading to worsening hyperglycemia or even the development of ketoacidosis, and other adverse clinical outcomes. Therefore, the algorithm should have clear orders for the nurses to contact the ordering physician if the infusion is stopped for any reason, other than protocol‐driven cessation due to falling blood glucose concentrations.
Interruption of Nutrition, Field Trips, and Communication
Insulin infusion commonly provides both basal and nutritional insulin requirements. Interruptions in nutritional intake are extremely common in the inpatient setting, with a potential to cause serious hypoglycemia. Feeding tubes are often pulled out without warning; enteral nutrition may also need be halted if high gastric residuals are noted or during certain diagnostic tests. At times, IV carbohydrate sources (dextrose, partial parenteral nutrition, total parenteral nutrition) may be interrupted as well. In some cases, field trips out of the critical care units to the operating room, imaging studies, or other hospital locations add another layer of challenges to managing the IIP. Staff in these various areas may not be familiar with the IIP or monitoring standards and techniques, and potentially may not even be aware that the patient is on an insulin infusion. It is therefore crucial to anticipate these pitfalls and develop effective institutional procedures for addressing them. For example, many institutions use D10 solution to replace the carbohydrate calories that are lost when tube feedings have to be interrupted in a gram‐per‐gram fashion. Patients should be clearly identified as being on an insulin infusion. The requirement for consistent glucose monitoring, hypoglycemia recognition and treatment, and insulin infusion adjustment requires either critical care nurse care of the patient on the field trip, or training in the same skills in areas such as endoscopy, interventional radiology, and operating rooms including the preoperative and postoperative care units. In any case, all services should be involved in crafting solutions that will ensure a consistent approach to glycemic control as the patient travels off‐unit. Monitoring and treatment equipment needs to be readily available in all sites, and hypoglycemia protocols need to be distributed and supported in all areas.
Preventing and Treating Hypoglycemia
Some hypoglycemia will occur with infusion protocols, no matter how carefully a protocol is crafted and how well it is administered. Hypoglycemia protocols should therefore be incorporated directly into an infusion order set. Treatment of hypoglycemic events with a full 50 mL of D50 solution is equivalent to 25 g of carbohydrate, which will raise glucose levels in the average patient by 125 mg/dL. Many institutions discourage the overcorrection of hypoglycemic events by encouraging giving lesser aliquots of D50 based on the degree of hypoglycemia.
Preventing hypoglycemia by recognizing hypoglycemia risk factors, proper monitoring, and anticipating reductions in insulin requirements from decreasing severity of illness, nutritional intake, or steroid dosing can also reduce the frequency of hypoglycemic events.
IMPLEMENTATION: EDUCATING AND ENGAGING NURSING AND PHYSICIAN STAFF
Nursing staff generally bear the brunt of the burden on the front line of implementing intensive IIPs. Educational efforts for nurses should include the rationale for intensive insulin therapy and use of an IIP. Additionally, detailed, case‐based instruction on utilization of the IIP is required. Properly educated, nursing staff often become the strongest advocates of the IIP. In addition, they can frequently provide important input when situations arise that require troubleshooting. Regular feedback sessions early in implementation that address ease of use, clarity of orders, and difficulties encountered by nurses can be invaluable. Improvement teams need to provide frequent in‐service training and updates on the IIP selected after implementation. This is imperative to promote nursing acceptance and adherence to the IIP chosen, particularly with consideration for traveling nurses. The importance of nursing champions to design and carry out this work cannot be overstated. Educational programs focusing on the physician staff can also be very useful, particularly when focused on high‐volume physicians and influential thought leaders.
IMPLEMENTATION: ADDRESSING COMMON CLINICAL SITUATIONS
Steroids
Steroid boluses are commonly an integral part of regimens targeting a variety of conditions, such as transplant rejection, reactive airways disease, certain infections, cancer, and a variety of autoimmune disorders. This can lead to glycemic excursions and rapidly varying insulin requirements. Educational efforts and treatment regimens should address the disproportionate impact that steroids have on postprandial glycemic excursions. To minimize the glycemic impact of glucocorticoid therapy, a team should investigate promoting the use of steroid infusions in situations when a bolus is not absolutely necessary.
Dealing with the Eating Patient and Other Sources of Carbohydrate‐Induced Glycemic Excursions
Glucose levels can be difficult to control in patients who are eating while on insulin infusion, because the infusion chases the glycemic excursions through frequent adjustments, often with a late overshoot and inappropriate reduction in dose. We instead recommend providing bolus nutritional insulin to cover the expected glycemic excursion caused by carbohydrate ingestion. Carbohydrate counting and using a unit of insulin for each 10‐15 g of carbohydrate consumed can smooth out the rapid fluctuations in glucose. Guidance for this should be incorporated into the order set.
Transition Off of Insulin Infusion
Rational strategies for dealing with this transition are covered in detail elsewhere in this supplement.65 Guidance for managing this transition should be integrated into your insulin infusion and SC order sets. The transition to SC insulin may represent a separate order set but is sometimes best integrated into the IV insulin infusion order set itself.
IIPs Outside of the Critical Care Setting
IIPs are most commonly used in the critical care setting. In some institutions, IV insulin protocols are safely and effectively employed outside the ICU. Obviously, the number of nurses and other personnel who must be familiar with such protocols is much higher outside the ICU, and protocol errors are therefore likely to be somewhat higher. In addition the nurse‐per‐patient ratio is usually lower outside of the critical care setting. As a result, suggestions for safe implementation of insulin infusion regimens outside of the critical care setting include:
-
Choose an infusion protocol with a higher glycemic target.
-
Limit the medical and surgical units where this expertise will be developed.
-
Consider simplified infusion protocols but stay consistent with format.
-
Automated or computerized assistance of calculations may reduce human error and nursing burden.
ASSESSING THE IMPACT OF YOUR EFFORTS: FOLLOW‐UP AND FOLLOW‐THROUGH
Monitoring, Recording, and Analyzing Glycemic Control Data
Once the IIP is implemented, it is critical that the impact on glycemic control, hypoglycemia, insulin use, and other factors be analyzed and used for improving the IIP and care delivery. Frequent monitoring of glucose levels is necessary for the safe infusion of insulin. Guidance for how often the monitoring is required must be explicit and included in the infusion order set. Intermittent auditing for compliance with the frequency of glucose testing and appropriate dose selection is good practice. Attention should be paid to how the glucose level is obtained, recorded, and made available to the health care team in your institution. All glucose readings should be recorded electronically for ongoing analyses and retrieval, and ideally, this could be done in an automated or single‐step method. Try to eliminate duplication of effort, such as asking the nurse to record the glucose level and their reaction to it on paper and again in an electronic format. Your team should also provide guidance about the potential problems of using point‐of‐care glucose testing in settings with hypotension, sepsis, pressor use, and other conditions that may impair the accuracy of capillary glucose readings.
Reports on the time to reach the glycemic target, glycemic control while on infusion, and the incidence of hypoglycemia should be reviewed by the multidisciplinary steering committee. The Society of Hospital Medicine Glycemic Control Task Force recommends analysis by patient day and by patient stay (or insulin infusion run) as preferred methodologies for analysis of glycemic control and hypoglycemia rates over the method of using each individual glucose reading as the unit of analysis. (The latter tends to under‐value the frequency of hypoglycemia.) Detailed practical recommendations for analyzing and summarizing glycemic control data are available elsewhere in this supplement.18 These data should drive decisions on modification of glycemic targets and the protocol structure. Patients meeting prespecified criteria should be referred to the improvement team for review. For example, patients who experience any glucose readings of 40 mg/dL, or who take more than 12 hours to reach the upper limit glycemic target should be referred to the team for a case review.
Assessing Adherence to the Protocol and Ease‐of‐Use Issues
Focused audits in the pilot and early implementation phases should look for nonadherence to the protocol. Deviations should be evaluated according to the patterns identified. For example, variation in application in some cases is specific for an individual and in others is characteristic of a specific group or the whole. Accordingly, this may point to gaps in education or attitudes about the importance of this endeavor. Front‐line staff may deviate from the protocol because they find it ineffective, unsafe, or impractical for certain situations or specific patients. Many IIPs are the subject of nursing errors related to the knowledge and acceptance of the nurse but also the complexity of the protocol. Appropriate modifications to the protocol based on these cases can frequently improve the ease of use and effectiveness of the protocol. The ongoing review process should identify issues that must be addressed with permanent solutions rather than accepting frequent individual alterations to meet goals. Revisions require supplementary education and rapid and wide dissemination. Although educational efforts and monitoring are often most intense in the early implementation phase, periodic retraining should continue to achieve optimal results and safety. Educational tools must consider nursing time commitments and will often include an interactive web‐based module that gives more flexibility for trainers and clinical nurses alike.
CONCLUSIONS
Insulin infusions are a powerful clinical tool in the inpatient setting to maintain glycemic control. Many IIPs have been developed and used successfully. The institutional challenge is to select, modify, and implement the IIP to reduce hyperglycemia and improve outcomes without excess hypoglycemia. In order to accomplish this goal safely and efficiently, standardized processes and collaboration between physicians, nurses, and pharmacists are needed. The keys to minimizing errors include developing a culture of safety and cooperation, back‐up checks, standardization, automation, and robust training for all those who are involved in the care of a patient on an insulin infusion. Although we encourage standardization and the use of protocols, providers always need to consider the unique clinical circumstances and potential problems presented by each individual patient. It is important to recognize the many barriers to successful implementation of an IIP, but strategies exist to overcome these. Finally, remember that the process does not end with the development phase. Continued review is paramount to success. Note variations in use, analyze them, and learn from them, in order to continually improve the process of care.
- ,,,,,.A comparison of the activity and disposal of semi‐synthetic human insulin and porcine insulin in normal man by the glucose clamp technique.Diabetologia.1982;22(1):41–45.
- .Perioperative management of the diabetic patient.Endocrinol Metab Clin North Am.1992;21(2):457–475.
- ,.Pregnancy in the diabetic woman. Guidelines for a successful outcome.Endocrinol Metab Clin North Am.1992;21(2):433–456.
- ,,,.Continuous intravenous insulin infusion reduces the incidence of deep sternal wound infection in diabetic patients after cardiac surgical procedures.Ann Thorac Surg.1999;67(2):352–360; discussion 360–362.
- ,,, et al.Continuous insulin infusion reduces mortality in patients with diabetes undergoing coronary artery bypass grafting.J Thorac Cardiovasc Surg.2003;125(5):1007–1021.
- .Effect of an intensive glucose management protocol on the mortality of critically ill adult patients.Mayo Clin Proc.2004;79(8):992–1000.
- ,,, et al.Intensive insulin therapy in the critically ill patients.N Engl J Med.2001;345(19):1359–1367.
- American College of Endocrinology Position Statement on Inpatient Diabetes and Metobolic Control.Endocr Pract.2004;10(suppl 2):5–9.
- ,.Current controversies around tight glucose control in critically ill patients.Curr Opin Clin Nutr Metab Care.2007;10(2):206–209.
- American Society of Health‐System Pharmacists and the Hospital and Health‐System Association of Pennsylvania. Recommendations for Safe Use of Insulin in Hospitals. http://www.ashp.org/s_ashp/docs/files/Safe_Use_of_Insulin.pdf. Accessed September 5,2008.
- ,,, et al.Management of diabetes and hyperglycemia in hospitals.Diabetes Care.2004;27(2):553–591.
- Joint Commission. Disease Specific‐Care Certification. http://www.jointcommission.org/CertificationPrograms. Accessed September 5,2008.
- American College of Endocrinology and American Diabetes Association consensus statement on inpatient diabetes and glycemic control: a call to action.Diabetes Care2006;29(8):1955–1962.
- Standards of medical care in diabetes–2008.Diabetes Care2008;31(Suppl 1):S12–S54.
- ,,.Continuous insulin infusion improves postoperative glucose control in patients with diabetes mellitus undergoing coronary artery bypass surgery.Tex Heart Inst J.2006;33(4):445–451.
- ,,,,.A practical approach to hyperglycemia management in the intensive care unit: evaluation of an intensive insulin infusion protocol.Pharmacotherapy.2006;26(10):1410–1420.
- ,,,,,.Standardization of intravenous insulin therapy improves the efficiency and safety of blood glucose control in critically ill adults.Intensive Care Med.2004;30(5):804–810.
- ,,,,,.SHM Glycemic Control Task Force summary: practical recommendations for assessing the impact of glycemic control efforts.J Hosp Med supplement. In press.
- ,,,.Introducing intensive insulin therapy: the nursing perspective.Nurs Crit Care.2006;11(2):75–79.
- .Evaluation of nursing work effort and perceptions about blood glucose testing in tight glycemic control.Am J Crit Care. Jul2006;15(4):370–377.
- ,,, et al.Intensive insulin therapy in the medical ICU.N Engl J Med.2006;354(5):449–461.
- ,,.Effect of hyperglycemia and continuous intravenous insulin infusions on outcomes of cardiac surgical procedures: the Portland Diabetic Project.Endocr Pract.2004;10(Suppl 2):21–33.
- ,,, et al.Implementation of a safe and effective insulin infusion protocol in a medical intensive care unit.Diabetes Care.2004;27(2):461–467.
- ,,, et al.Improving glycemic control in the cardiothoracic intensive care unit: clinical experience in two hospital settings.J Cardiothorac Vasc Anesth.2004;18(6):690–697.
- ,,, et al.Inpatient management of hyperglycemia: the Northwestern experience.Endocr Pract.2006;12(5):491–505.
- ,,, et al.Performance of a dose‐defining insulin infusion protocol among trauma service intensive care unit admissions.Diabetes Technol Ther.2006;8(4):476–488.
- ,,.Glucommander: a computer‐directed intravenous insulin system shown to be safe, simple, and effective in 120,618 h of operation.Diabetes Care.2005;28(10):2418–2423.
- ,,, et al.Description and evaluation of a glycemic management protocol for patients with diabetes undergoing heart surgery.Endocr Pract.2002;8(1):10–18.
- ,,,,,.Tight glycemic control in diabetic coronary artery bypass graft patients improves perioperative outcomes and decreases recurrent ischemic events.Circulation.2004;109(12):1497–1502.
- ,,,.Practical management of diabetes in critically ill patients.Am J Respir Crit Care Med.2001;164(10 Pt 1):1763–1767.
- ,,,.Postoperative management of diabetes mellitus: steady‐state glucose control with bedside algorithm for insulin adjustment.Diabetes Care.1987;10(6):722–728.
- ,,,,.Glucose‐insulin‐potassium solutions improve outcomes in diabetics who have coronary artery operations.Ann Thorac Surg.2000;70(1):145–150.
- ,,, et al.Randomized trial of insulin‐glucose infusion followed by subcutaneous insulin treatment in diabetic patients with acute myocardial infarction (DIGAMI study): effects on mortality at 1 year.J Am Coll Cardiol.1995;26(1):57–65.
- ,.Eliminating the diabetic disadvantage: the Portland Diabetic Project.Semin Thorac Cardiovasc Surg.2006;18(4):302–308.
- ,,.Validation of an insulin infusion nomogram for intensive glucose control in critically ill patients.Pharmacotherapy.2005;25(3):352–359.
- ,,,,.Achieving tight glycemic control in the operating room: lessons learned from 12 years in the trenches of a paradigm shift in anesthetic care.Semin Thorac Cardiovasc Surg.2006;18(4):339–345.
- ,,.The rationale and management of hyperglycemia for in‐patients with cardiovascular disease: time for change.J Clin Endocrinol Metab.2003;88(6):2430–2437.
- ,,,,.An insulin infusion protocol in critically ill cardiothoracic surgery patients.Ann Pharmacother.2004;38(7–8):1123–1129.
- ,,, et al.Efficacy and safety of an insulin infusion protocol in a surgical ICU.J Am Coll Surg.2006;202(1):1–9.
- ,,,.Implementation of a tight glycaemic control protocol using a web‐based insulin dose calculator.Anaesthesia.2005;60(11):1093–1100.
- ,,,.Intensive insulin therapy to non‐cardiac ICU patients: a prospective study.Eur J Anaesthesiol.2006;23(8):705–709.
- ,,, et al.Blood glucose control by a model predictive control algorithm with variable sampling rate versus a routine glucose management protocol in cardiac surgery patients: a randomized controlled trial.J Clin Endocrinol Metab.2007;92(8):2960–2964.
- ,,, et al.Outcomes of a cardiothoracic intensive care web‐based online intravenous insulin infusion calculator study at a medical university hospital.Diabetes Technol Ther.2007;9(6):523–534.
- ,,,.Rush University guidelines and protocols for the management of hyperglycemia in hospitalized patients: elimination of the sliding scale and improvement of glycemic control throughout the hospital.Diabetes Educ.2006;32(6):954–962.
- ,,,.Collaborative development of an insulin nomogram for intensive insulin therapy.Crit Care Nurs Q.2006;29(1):96–105.
- ,,, et al.Computer‐based insulin infusion protocol improves glycemia control over manual protocol.J Am Med Inform Assoc.2007;14(3):278–287.
- ,,, et al.Implementing an intravenous insulin infusion protocol in the intensive care unit.Am J Health Syst Pharm.2007;64(4):385–395.
- ,,,,.Use of a computerized guideline for glucose regulation in the intensive care unit improved both guideline adherence and glucose regulation.J Am Med Inform Assoc.2005;12(2):172–180.
- ,,, et al.Improving hyperglycemia management in the intensive care unit: preliminary report of a nurse‐driven quality improvement project using a redesigned insulin infusion algorithm.Diabetes Educ.2006;32(3):394–403.
- ,,.Clinical results of an updated insulin infusion protocol in critically ill patients.Diabetes Spectrum.2005;18(1):28–33.
- ,,.Insulin therapy for critically ill hospitalized patients: a meta‐analysis of randomized controlled trials.Arch Intern Med.2004;164(18):2005–2011.
- ,,.Intensive insulin therapy in critical care: a review of 12 protocols.Diabetes Care.2007;30(4):1005–1011.
- ,,.Hypoglycemia in the intensive care unit.Curr Opin Clin Nutr Metab Care.2007;10(2):193–196.
- ,,.Insulin infusion protocols for critically ill patients: a highlight of differences and similarities.Endocr Pract.2007;13(2):137–146.
- ,,.Effect of hyperglycemia and continuous intravenous insulin infusions on outcomes of cardiac surgical procedures: the Portland Diabetic Project.Endocr Pract.2004;10(Suppl 2):21–33.
- Providence Health 10(Suppl 2):71–80.
- .Memoirs of a root canal salesman: the successful implementation of a hospital‐wide intravenous insulin infusion protocol.Endocr Pract.2006;12(Suppl 3):79–85.
- ,,, et al.Intensive insulin therapy and pentastarch resuscitation in severe sepsis.N Engl J Med.2008;358(2):125–139.
- ,.Severe hypoglycemia in critically ill patients: risk factors and outcomes.Crit Care Med.2007;35(10):2262–2267.
- ,,.Evidence for strict inpatient blood glucose control: time to revise glycemic goals in hospitalized patients.Metabolism.2008;57(1):116–120.
- ,,, et al.Comparison of insulin infusion protocols in the ICU: computer‐guided versus standard column‐based insulin regimens [Abstract]. American Diabetes Association abstract,2008.
- ,,.Design and implementation of GRIP: a computerized glucose control system at a surgical intensive care unit.BMC Med Inform Decis Mak.2005;5:38.
- ,,, et al.“Waste not, want not”: determining the optimal priming volume for intravenous infusions.Diabetes Technol Ther.2006;8(5):598–601.
- ,,,.Bridge over troubled waters: safe and effective transitions of the inpatient with hyperglycemia.J Hosp Med supplement. In press.
- ,,,,,.A comparison of the activity and disposal of semi‐synthetic human insulin and porcine insulin in normal man by the glucose clamp technique.Diabetologia.1982;22(1):41–45.
- .Perioperative management of the diabetic patient.Endocrinol Metab Clin North Am.1992;21(2):457–475.
- ,.Pregnancy in the diabetic woman. Guidelines for a successful outcome.Endocrinol Metab Clin North Am.1992;21(2):433–456.
- ,,,.Continuous intravenous insulin infusion reduces the incidence of deep sternal wound infection in diabetic patients after cardiac surgical procedures.Ann Thorac Surg.1999;67(2):352–360; discussion 360–362.
- ,,, et al.Continuous insulin infusion reduces mortality in patients with diabetes undergoing coronary artery bypass grafting.J Thorac Cardiovasc Surg.2003;125(5):1007–1021.
- .Effect of an intensive glucose management protocol on the mortality of critically ill adult patients.Mayo Clin Proc.2004;79(8):992–1000.
- ,,, et al.Intensive insulin therapy in the critically ill patients.N Engl J Med.2001;345(19):1359–1367.
- American College of Endocrinology Position Statement on Inpatient Diabetes and Metobolic Control.Endocr Pract.2004;10(suppl 2):5–9.
- ,.Current controversies around tight glucose control in critically ill patients.Curr Opin Clin Nutr Metab Care.2007;10(2):206–209.
- American Society of Health‐System Pharmacists and the Hospital and Health‐System Association of Pennsylvania. Recommendations for Safe Use of Insulin in Hospitals. http://www.ashp.org/s_ashp/docs/files/Safe_Use_of_Insulin.pdf. Accessed September 5,2008.
- ,,, et al.Management of diabetes and hyperglycemia in hospitals.Diabetes Care.2004;27(2):553–591.
- Joint Commission. Disease Specific‐Care Certification. http://www.jointcommission.org/CertificationPrograms. Accessed September 5,2008.
- American College of Endocrinology and American Diabetes Association consensus statement on inpatient diabetes and glycemic control: a call to action.Diabetes Care2006;29(8):1955–1962.
- Standards of medical care in diabetes–2008.Diabetes Care2008;31(Suppl 1):S12–S54.
- ,,.Continuous insulin infusion improves postoperative glucose control in patients with diabetes mellitus undergoing coronary artery bypass surgery.Tex Heart Inst J.2006;33(4):445–451.
- ,,,,.A practical approach to hyperglycemia management in the intensive care unit: evaluation of an intensive insulin infusion protocol.Pharmacotherapy.2006;26(10):1410–1420.
- ,,,,,.Standardization of intravenous insulin therapy improves the efficiency and safety of blood glucose control in critically ill adults.Intensive Care Med.2004;30(5):804–810.
- ,,,,,.SHM Glycemic Control Task Force summary: practical recommendations for assessing the impact of glycemic control efforts.J Hosp Med supplement. In press.
- ,,,.Introducing intensive insulin therapy: the nursing perspective.Nurs Crit Care.2006;11(2):75–79.
- .Evaluation of nursing work effort and perceptions about blood glucose testing in tight glycemic control.Am J Crit Care. Jul2006;15(4):370–377.
- ,,, et al.Intensive insulin therapy in the medical ICU.N Engl J Med.2006;354(5):449–461.
- ,,.Effect of hyperglycemia and continuous intravenous insulin infusions on outcomes of cardiac surgical procedures: the Portland Diabetic Project.Endocr Pract.2004;10(Suppl 2):21–33.
- ,,, et al.Implementation of a safe and effective insulin infusion protocol in a medical intensive care unit.Diabetes Care.2004;27(2):461–467.
- ,,, et al.Improving glycemic control in the cardiothoracic intensive care unit: clinical experience in two hospital settings.J Cardiothorac Vasc Anesth.2004;18(6):690–697.
- ,,, et al.Inpatient management of hyperglycemia: the Northwestern experience.Endocr Pract.2006;12(5):491–505.
- ,,, et al.Performance of a dose‐defining insulin infusion protocol among trauma service intensive care unit admissions.Diabetes Technol Ther.2006;8(4):476–488.
- ,,.Glucommander: a computer‐directed intravenous insulin system shown to be safe, simple, and effective in 120,618 h of operation.Diabetes Care.2005;28(10):2418–2423.
- ,,, et al.Description and evaluation of a glycemic management protocol for patients with diabetes undergoing heart surgery.Endocr Pract.2002;8(1):10–18.
- ,,,,,.Tight glycemic control in diabetic coronary artery bypass graft patients improves perioperative outcomes and decreases recurrent ischemic events.Circulation.2004;109(12):1497–1502.
- ,,,.Practical management of diabetes in critically ill patients.Am J Respir Crit Care Med.2001;164(10 Pt 1):1763–1767.
- ,,,.Postoperative management of diabetes mellitus: steady‐state glucose control with bedside algorithm for insulin adjustment.Diabetes Care.1987;10(6):722–728.
- ,,,,.Glucose‐insulin‐potassium solutions improve outcomes in diabetics who have coronary artery operations.Ann Thorac Surg.2000;70(1):145–150.
- ,,, et al.Randomized trial of insulin‐glucose infusion followed by subcutaneous insulin treatment in diabetic patients with acute myocardial infarction (DIGAMI study): effects on mortality at 1 year.J Am Coll Cardiol.1995;26(1):57–65.
- ,.Eliminating the diabetic disadvantage: the Portland Diabetic Project.Semin Thorac Cardiovasc Surg.2006;18(4):302–308.
- ,,.Validation of an insulin infusion nomogram for intensive glucose control in critically ill patients.Pharmacotherapy.2005;25(3):352–359.
- ,,,,.Achieving tight glycemic control in the operating room: lessons learned from 12 years in the trenches of a paradigm shift in anesthetic care.Semin Thorac Cardiovasc Surg.2006;18(4):339–345.
- ,,.The rationale and management of hyperglycemia for in‐patients with cardiovascular disease: time for change.J Clin Endocrinol Metab.2003;88(6):2430–2437.
- ,,,,.An insulin infusion protocol in critically ill cardiothoracic surgery patients.Ann Pharmacother.2004;38(7–8):1123–1129.
- ,,, et al.Efficacy and safety of an insulin infusion protocol in a surgical ICU.J Am Coll Surg.2006;202(1):1–9.
- ,,,.Implementation of a tight glycaemic control protocol using a web‐based insulin dose calculator.Anaesthesia.2005;60(11):1093–1100.
- ,,,.Intensive insulin therapy to non‐cardiac ICU patients: a prospective study.Eur J Anaesthesiol.2006;23(8):705–709.
- ,,, et al.Blood glucose control by a model predictive control algorithm with variable sampling rate versus a routine glucose management protocol in cardiac surgery patients: a randomized controlled trial.J Clin Endocrinol Metab.2007;92(8):2960–2964.
- ,,, et al.Outcomes of a cardiothoracic intensive care web‐based online intravenous insulin infusion calculator study at a medical university hospital.Diabetes Technol Ther.2007;9(6):523–534.
- ,,,.Rush University guidelines and protocols for the management of hyperglycemia in hospitalized patients: elimination of the sliding scale and improvement of glycemic control throughout the hospital.Diabetes Educ.2006;32(6):954–962.
- ,,,.Collaborative development of an insulin nomogram for intensive insulin therapy.Crit Care Nurs Q.2006;29(1):96–105.
- ,,, et al.Computer‐based insulin infusion protocol improves glycemia control over manual protocol.J Am Med Inform Assoc.2007;14(3):278–287.
- ,,, et al.Implementing an intravenous insulin infusion protocol in the intensive care unit.Am J Health Syst Pharm.2007;64(4):385–395.
- ,,,,.Use of a computerized guideline for glucose regulation in the intensive care unit improved both guideline adherence and glucose regulation.J Am Med Inform Assoc.2005;12(2):172–180.
- ,,, et al.Improving hyperglycemia management in the intensive care unit: preliminary report of a nurse‐driven quality improvement project using a redesigned insulin infusion algorithm.Diabetes Educ.2006;32(3):394–403.
- ,,.Clinical results of an updated insulin infusion protocol in critically ill patients.Diabetes Spectrum.2005;18(1):28–33.
- ,,.Insulin therapy for critically ill hospitalized patients: a meta‐analysis of randomized controlled trials.Arch Intern Med.2004;164(18):2005–2011.
- ,,.Intensive insulin therapy in critical care: a review of 12 protocols.Diabetes Care.2007;30(4):1005–1011.
- ,,.Hypoglycemia in the intensive care unit.Curr Opin Clin Nutr Metab Care.2007;10(2):193–196.
- ,,.Insulin infusion protocols for critically ill patients: a highlight of differences and similarities.Endocr Pract.2007;13(2):137–146.
- ,,.Effect of hyperglycemia and continuous intravenous insulin infusions on outcomes of cardiac surgical procedures: the Portland Diabetic Project.Endocr Pract.2004;10(Suppl 2):21–33.
- Providence Health 10(Suppl 2):71–80.
- .Memoirs of a root canal salesman: the successful implementation of a hospital‐wide intravenous insulin infusion protocol.Endocr Pract.2006;12(Suppl 3):79–85.
- ,,, et al.Intensive insulin therapy and pentastarch resuscitation in severe sepsis.N Engl J Med.2008;358(2):125–139.
- ,.Severe hypoglycemia in critically ill patients: risk factors and outcomes.Crit Care Med.2007;35(10):2262–2267.
- ,,.Evidence for strict inpatient blood glucose control: time to revise glycemic goals in hospitalized patients.Metabolism.2008;57(1):116–120.
- ,,, et al.Comparison of insulin infusion protocols in the ICU: computer‐guided versus standard column‐based insulin regimens [Abstract]. American Diabetes Association abstract,2008.
- ,,.Design and implementation of GRIP: a computerized glucose control system at a surgical intensive care unit.BMC Med Inform Decis Mak.2005;5:38.
- ,,, et al.“Waste not, want not”: determining the optimal priming volume for intravenous infusions.Diabetes Technol Ther.2006;8(5):598–601.
- ,,,.Bridge over troubled waters: safe and effective transitions of the inpatient with hyperglycemia.J Hosp Med supplement. In press.
SC Insulin Order Sets and Protocols
Inpatient glycemic control and hypoglycemia are issues with well deserved increased attention in recent years. Prominent guidelines and technical reviews have been published,13 and a recent, randomized controlled trial demonstrated the superiority of basal bolus insulin regimens compared to sliding‐scale regimens.4 Effective glycemic control for inpatients has remained elusive in most medical centers. Recent reports57 detail clinical inertia and the continued widespread use of sliding‐scale subcutaneous insulin regimens, as opposed to the anticipatory, physiologic basal‐nutrition‐correction dose insulin regimens endorsed by these reviews.
Inpatient glycemic control faces a number of barriers, including fears of inducing hypoglycemia, uneven knowledge and training among staff, and competing institutional and patient priorities. These barriers occur in the background of an inherently complex inpatient environment that poses unique challenges in maintaining safe glycemic control. Patients frequently move across a variety of care teams and geographic locations during a single inpatient stay, giving rise to multiple opportunities for failed communication, incomplete handoffs, and inconsistent treatment. In addition, insulin requirements may change dramatically due to variations in the stress of illness, exposure to medications that effect glucose levels, and varied forms of nutritional intake with frequent interruption. Although insulin is recognized as one of the medications most likely to be associated with adverse events in the hospital, many hospitals do not have protocols or order sets in place to standardize its use.
A Call to Action consensus conference,8, 9 hosted by the American Association of Clinical Endocrinologists (AACE) and the American Diabetes Association (ADA), brought together many thought leaders and organizations, including representation from the Society of Hospital Medicine (SHM), to address these barriers and to outline components necessary for successful implementation of a program to improve inpatient glycemic control in the face of these difficulties. Institutional insulin management protocols and standardized insulin order sets (supported by appropriate educational efforts) were identified as key interventions. It may be tempting to quickly deploy a generic insulin order set in an effort to improve care. This often results in mediocre results, due to inadequate incorporation of standardization and guidance into the order set and other documentation tools, and uneven use of the order set.
The SHM Glycemic Control Task Force (GCTF) recommends the following steps for developing and implementing successful protocols and order sets addressing the needs of the noncritical care inpatient with diabetes/hyperglycemia.
-
Form a steering committee for this work, and assess the current processes of care.
-
Identify best practices and preferred regimens to manage diabetes and hyperglycemia in the hospital.
-
Integrate best practices and preferred institutional choices into an inpatient glycemic control protocol. Crystallize your protocol into a one page summary.
-
Place guidance from your protocol into the flow of work, by integrating it into standardized subcutaneous insulin order sets and other documentation and treatment tools.
-
Monitor the use of your order sets and protocol. Intervene actively on nonadherents to your protocol and those with poor glycemic control, and revise your protocol/order sets as needed.
IDENTIFYING AND INCORPORATING KEY CONCEPTS AND BEST PRACTICES
A protocol is a document that endorses specific monitoring and treatment strategies in a given institution. This potentially extensive document should provide guidance for transitions, special situations (like steroids and total parenteral nutrition [TPN]) and should outline preferred insulin regimens for all of the most common nutritional situations. One of the most difficult parts of creating a protocol is the assimilation of all of the important information on which to base decisions. Your protocol and order set will be promoting a set of clinical practices. Fortunately, the current best practice for noncritical care hyperglycemic patients has been summarized by several authoritative sources,13, 811 including references from the SHM Glycemic Task Force published in this supplement.4, 12
Table 1 summarizes the key concepts that should be emphasized in a protocol for subcutaneous insulin management in the hospital. We recommend embedding guidance from your protocol into order sets, the medication administration record, and educational materials. Although the details contained in a protocol and order set might vary from one institution to another, the key concepts should not. The remainder of this article provides practical information about how these concepts and guidance for how preferred insulin regimens should be included in these tools. Appendices 1 and 2 give examples of an institutional one‐page summary protocol and subcutaneous insulin order set, respectively.
| 1. Establish a target range for blood glucose levels. |
| 2. Standardize monitoring of glucose levels and assessment of long‐term control (HbA1c). |
| 3. Incorporate nutritional management. |
| 4. Prompt clinicians to consider discontinuing oral antihyperglycemic medications. |
| 5. Prescribe physiologic (basal‐nutrition‐correction) insulin regimens. |
| a. Choose a total daily dose (TDD). |
| b. Divide the TDD into physiologic components of insulin therapy and provide basal and nutritional/correction separately. |
| c. Choose and dose a basal insulin. |
| d. Choose and dose a nutritional (prandial) insulin |
|
i. Match exactly to nutritional intake (see Table 2). |
| ii. Include standing orders to allow nurses to hold nutritional insulin for nutritional interruptions and to modify nutritional insulin depending on the actual nutritional intake. |
| e. Add correction insulin |
| i. Match to an estimate of the patients insulin sensitivity using prefabricated scales. |
| ii. Use the same insulin as nutritional insulin. |
| 6. Miscellaneous |
| a. Manage hypoglycemia in a standardized fashion and adjust regimen to prevent recurrences. |
| b. Provide diabetes education and appropriate consultation. |
| c. Coordinate glucose testing, nutrition delivery, and insulin administration. |
| d. Tailor discharge treatment regimens to the patient's individual circumstances and arrange for proper follow‐up. |
Standardize the Monitoring of Blood Glucose Values and Glucosylated Hemoglobin
Guidance for the coordination of glucose testing, nutrition delivery, and insulin administration, should be integrated into your protocols, and order sets. For noncritical care areas, the minimal frequency for blood glucose monitoring for patients who are eating is before meals and at bedtime. For the patient designated nothing by mouth (NPO) or the patient on continuous tube feeding, the type of nutritional/correction insulin used should drive the minimum frequency (every 4‐6 hours if rapid acting analog insulins [RAA‐I] are used, and every 6 hours if regular insulin is used). Directions for administering scheduled RAA‐I immediately before or immediately after nutrition delivery should be incorporated into protocols, order sets, and medication administration records. Unfortunately, having this guidance in the order sets and protocols does not automatically translate into its being carried out in the real world. Wide variability in the coordination of glucose monitoring, nutritional delivery, and insulin administration is common, so monitoring the process to make sure the protocol is followed is important.
Obtaining a glucosylated hemoglobin (HbA1c) level is important in gauging how well the patient's outpatient regimen is maintaining glycemic control, distinguishing stress hyperglycemia from established diabetes, and guiding the inpatient approach to glycemic control. ADA guidelines2, 3 endorse obtaining HbA1c levels of inpatients if these levels are not already available from the month prior to admission.
Establish a Target Range for Blood Glucose in NonCritical Care Areas
It is important to adopt a glycemic target that is institution‐wide, for critical care areas and noncritical care areas alike. Your glycemic target need not be identical to the ADA/AACE glycemic targets, but should be similar to them.
Examples of institutional glycemic targets for noncritical care areas:
-
Preprandial target 90‐130 mg/dL, maximum random glucose <180 mg/dL (ADA/AACE consensus target)
-
90‐150 mg/dL (a target used in some hospitals)
-
Preprandial target 90‐130 mg/dL for most patients, 100‐150 mg/dL if there are hypoglycemia risk factors, and <180 mg/dL if comfort‐care or end‐of‐life care (a more refined target, allowing for customization based on patient characteristics).
Your multidisciplinary glycemic control steering committee should pick the glycemic target it can most successfully implement and disseminate. It is fine to start with a conservative target and then ratchet down the goals as the environment becomes more accepting of the concept of tighter control of blood glucose in the hospital.
Although the choice of glycemic target is somewhat arbitrary, establishing an institutional glycemic target is critical to motivate clinical action. Your committee should design interventions, for instances when a patient's glycemic target is consistently not being met, including an assignment of responsibility.
Prompt Clinicians to Consider Discontinuing Oral Agents
Oral antihyperglycemic agents, in general, are difficult to quickly titrate to effect, and have side effects that limit their use in the hospital. In contrast, insulin acts rapidly and can be used in virtually all patients and clinical situations, making it the treatment of choice for treatment of hyperglycemia in the hospital.3, 11, 12 In certain circumstances, it may be entirely appropriate to continue a well‐controlled patient on his or her prior outpatient oral regimen. It is often also reasonable to resume oral agents in some patients when preparing for hospital discharge.
Incorporate Nutritional Management
Because diet is so integral to the management of diabetes and hyperglycemia, diet orders should be embedded in all diabetes or insulin‐related order sets. Diets with the same amount of carbohydrate with each meal should be the default rule for patients with diabetes. Nutritionist consultation should be considered and easy to access for patients with malnutrition, obesity, and other common conditions of the inpatient with diabetes.
Access Diabetes Education and Appropriate Consultation
Diabetes education should be offered to all hyperglycemic patients with normal mental status, complete with written materials, a listing of community resources, and survival skills. Consultation with physicians in internal medicine or endocrinology for difficult‐to‐control cases, or for cases in which the primary physician of record is not familiar with (or not adherent to) principles of inpatient glycemic management, should be very easy to obtain, or perhaps mandated, depending on your institution‐specific environment.
Prescribe Physiologic (Basal‐Nutritional‐Correction Dose) Insulin Regimens
Physiologic insulin use is the backbone of the recommended best practice for diabetes and hyperglycemia management in the hospital. The principles of such regimens are summarized elsewhere in this supplement.12 These principles will not be reiterated in detail here, but the major concepts that should be integrated into the protocols and order sets will be highlighted.
Choose a Total Daily Dose
Clinicians need guidance on how much subcutaneous insulin they should give a patient. These doses are well known from clinical experience and the published literature. The fear of hypoglycemia usually results in substantial underdosing of insulin, or total avoidance of scheduled insulin on admission. Your team should provide guidance for how much insulin to start a patient on when it is unclear from past experience how much insulin the patient needs. Waiting a few days to see how much insulin is required via sliding‐scale‐only regimens is a bad practice that should be discouraged for patients whose glucose values are substantially above the glycemic target. The total daily dose (TDD) can be estimated in several different ways (as demonstrated in Appendix 1 and 2), and protocols should make this step very clear for clinicians. Providing a specific location on the order set to declare the TDD may help ensure this step gets done more reliably. Some institutions with computer physician order entry (CPOE) provide assistance with calculating the TDD and the allocation of basal and nutritional components, based on data the ordering physician inputs into the system.
Select and Dose a Basal Insulin
Your protocol should describe how the TDD should be divided between basal and nutritional insulin. We generally recommend 50% of the TDD be given as basal insulin, with the other 50% administered on a scheduled basis to cover glycemic excursions from nutritional intake. The 50/50 rule is simple and generally works well, and should be widely promoted. However, there are exceptions to this rule that should be incorporated into your full protocol and educational programs. The order set should have separate steps for ordering basal insulin, nutritional insulin, and correction insulin. The advantage to providing these insulin components separately is that it allows them to be independently manipulated (eg, if a patient is unable to tolerate a meal, nutritional insulin is held, but basal insulin and correction insulin are continued).
The SHM GCTF specifically endorses long acting insulin (glargine and detemir) as the preferred basal insulin in the hospital setting, thus discouraging the use of neutral protamine Hagedorn (NPH) insulin and fixed combination insulin formulations (Table 2). In the absence of randomized controlled trials demonstrating superiority of the glargine or detemir to NPH insulin in the hospital, this endorsement deserves some further explanation. Although we believe that correctly dosed NPH containing insulin regimens can attain effective and safe glycemic control in the hospital setting, it is more difficult to standardize their use and adjust for fluctuations in nutritional intake. Glargine and detemir have much less pronounced spikes in their effect than NPH, rendering them relatively peakless in comparison. This pharmacokinetic profile allows for continued dosing with minimal or no correction when nutrition intake is variable, and allow for consistent reinforcement of the basal‐nutritional‐correction insulin concept.
| Nutritional situation | Necessary insulin components | Preferred regimen* |
|---|---|---|
| ||
| NPO (or clear liquids) | Basal insulin: 50% of TDD. Nutritional insulin: None. | Basal insulin: glargine given once daily or detemir given twice daily. Nutritional insulin: None. Correctional insulin: Regular insulin q 6 hours or RAA insulin q 4 hours. Other comments: Dextrose infusion (e.g., D5 containing solution at 75‐150 cc/hour) recommended when nutrition is held. An IV insulin infusion is preferred for management of prolonged fasts or fasting type 1 diabetes patients. |
| Eating meals | Basal insulin: 50% of TDD. Nutritional insulin: 50% of TDD, divided equally before each meal. | Basal insulin: glargine given once daily or detemir given twice daily. Nutritional insulin: RAA insulin with meals. Correctional insulin: RAA insulin q AC and HS (reduced dose at HS). |
| Bolus tube feeds | Basal insulin: 40% of TDD. Nutritional insulin: 60% of the TDD, divided equally before each bolus feed. | Basal insulin: glargine given once daily or detemir given twice daily. Nutritional insulin: RAA insulin with each bolus. Correctional insulin: RAA insulin with each bolus. |
| Continuous tube feeds | Basal insulin: 40% (conservative) of TDD. Nutritional insulin: 60% of the TDD in divided doses. | Basal insulin: glargine given once daily or detemir given twice daily. Nutritional insulin: RAA insulin q 4 hours or regular insulin q 6 hours. Correctional insulin: Should match nutritional insulin choice. |
| Parenteral nutrition | Insulin is usually given parenterally, with the nutrition | Initially, a separate insulin drip allows for accurate dose‐finding. Then, 80% of amount determined as TDD using drip is added to subsequent TPN bags as regular insulin. Use correctional subcutaneous insulin doses cautiously, in addition. |
There are some caveats to this general recommendation. First, patients who are well controlled on home regimens with NPH basal insulin can (and sometimes should) stay on the regimen that has worked well for them. However, extra vigilance in reducing the dose for reductions in nutrition is required, because NPH is generally used to cover both nutritional and basal requirements. Second, extensive experience with glargine and detemir are not available in obstetric populations. They are not U.S. Food and Drug Administration (FDA) approved for use in pregnant patients and formally carry a Class C rating, whereas NPH insulin has been used safely in obstetric populations for decades. Third, the insulin regimen used as an inpatient is not necessarily the preferred regimen to prescribe at discharge: cost, patient preferences, HbA1c level, and other factors should be considered in making this choice.
Select and Dose a Nutritional (Prandial) Insulin
The step for ordering nutritional insulin should assist the clinician in matching the insulin to the type of nutrition that the patient is receiving. For example, rapid‐acting insulin analogs are preferred over regular insulin in the eating patient, in view of their more physiologic profile, which averts the insulin stacking that can occur with regular insulin. If regular insulin is used as the preferred institutional choice for eating patients, the lunchtime dose should be reduced or eliminated altogether, to eliminate insulin stacking.
Table 2 outlines the SHM GCTF preferred regimens for different nutritional situations.
There should be a standing order for nutritional insulin to be held when nutrition is interrupted, whether intentional or unintentional. Patients with interrupted tube feedings could have standing orders for a dextrose infusion to replace the tube feeding carbohydrate load and prevent hypoglycemia. Ideally, there should also be a standing order allowing for real‐time management of the patient with uncertain nutritional intake. For example, when a patient's premeal assessment reveals that she may not tolerate the meal, the patient should be allowed to attempt to eat, and then the nutritional insulin should be given after the meal, in proportion to the amount of food that was eaten. This type of order will require significant nursing education and process redesign in many hospitals, but is essential for matching nutritional insulin to actual intake.
Add Correction Insulin
There is no convincing evidence for the benefit of correction (sliding‐scale) insulin in the inpatient setting, although a randomized trial demonstrating the superiority of basal/nutritional insulin regimens to sliding‐scale only regimens did incorporate a correction insulin scale as an adjunct to the superior basal/nutritional regimen.4 The SHM GCTF again emphasizes that control of hyperglycemia should be proactive and anticipatory of insulin needs, rather than reactive to hyperglycemia. Nonetheless, unexpected hyperglycemic excursions are common, and the use of correction insulin remains a pervasive and arguably logical practice. If correction insulin is used, it should be ordered as a separate step after considering basal and nutrition insulin needs. The doses of scheduled insulin should be adjusted regularly if correction insulin is consistently being required. Ideally, the prescriber should choose a preformatted corrective insulin scale, based on the patient's insulin sensitivity (Appendix 2). There should be a prompt to use the same type of insulin that is being used for nutritional insulin, and there should be instructions that this insulin is given in addition to the basal and nutritional insulin to correct for hyperglycemia. Nocturnal correction‐dose scales should be reduced in the eating patient.
Even after limiting insulin regimens to those in Table 2, multidisciplinary glycemic control teams are still left with several options within these SHM‐preferred regimens. We recommend that your team choose a single, institutionally‐preferred basal‐nutritional‐correction insulin combination for each situation.
Choosing one preferred option for these situations is advantageous because:
-
You can communicate preferred regimens more simply and succinctly to all staff.
-
You eliminate all inappropriate choices for insulin regimens for that situation, as well as some other less preferred, but acceptable choices.
-
You can encourage regimens that are most economical (by promoting the insulin regimens that reflect your hospital formulary choices).
-
Staff members can become very familiar with a few regimens, instead of being confused by a multitude of them. They can identify variations from your preferred choices and target these patients for extra scrutiny and actions should they fail to meet glycemic targets.
Although virtually every institution can provide specific guidance on insulin management in a protocol, there are tradeoffs inherent in how restrictive you can be in pushing these preferred choices in your order sets. Should you eliminate alternate basal or nutritional insulin choices from your order sets? As you integrate more and more of your preferred algorithm and regimens into your order set, you will gain incremental improvement in the standardization of inpatient insulin management. However, you reduce not only variability in ordering, but also the choices available to your prescribers and patients, and in effect you are pushing the providers to use an insulin regimen that often differs from the patient's outpatient regimen. If your institution is not yet ready to go with a single preferred insulin, simply listing your preferred insulin first with the annotation preferred can be enough to increase the use of the preferred insulin.
We endorse building the most protocol‐driven, proscriptive, insulin order set that the Glycemic Control Steering Committee believes their medical staff will accept. There are some caveats to this endorsement. First, there must be extra efforts on the backend of the admission, to ensure that the antihyperglycemic regimen is tailored to the unique needs of the patient (this is discussed further below). Second, a protocol‐driven approach is not a substitute for a good educational program for health care providers or well‐informed clinical judgment. Education should reinforce major concepts driving the protocol and should also highlight exceptions to the rule. Variance from the protocol endorsed choices should be allowed (and even encouraged) when the variance is driven by patient factors (as opposed to provider whim). Learning from this variance is a key concept in refining protocols. Education ideally should not be limited to only protocol‐endorsed choices, as staff should be familiar with the full range of antihyperglycemia regimens seen in inpatient and outpatient settings.
Special Situations
Most of the preferred regimens for different situations are outlined in Table 2 in a straightforward manner, and can be depicted in your protocols and order sets in the same way. Some conditions have enough complexity, however, that you will have difficulty placing all of the details into your one‐page protocol and order set. Details should be placed on your more detailed protocol, and educational programs should include the topics outlined below. Although insulin infusion is often the option that would provide the most reliable and expedient control of hyperglycemia in these special situations, it is an option not available in many noncritical care settings. Therefore, the discussion is limited to subcutaneous insulin control regimens.
Patient on Continuous Tube Feeding
The SHM GCTF endorses glargine or detemir as the basal insulin of choice for this setting. The nutritional and correction insulin of choice is either an RAA‐I every 4 hours (q4h), or regular insulin every 6 hours (q6h). We endorse this choice because it retains the basal‐nutritional‐correction dose concept, generally allows for continued basal insulin use if the tube feedings become interrupted, and is amenable to building a consistent institutional protocol.
There are some important caveats to this recommendation. First, realize that almost any regimen that provides a stable insulin supply would be acceptable, and many institutions will use glargine or detemir to cover both basal and nutritional needs. The downside to using large boluses of long‐acting insulin in this clinical situation is that any unexpected interruption of the feedings will necessitate prolonged infusions of dextrate 10% solution (D10) to avoid hypoglycemia
Second, because of the glycemic load inherent in tube feedings, maintenance of glycemic control in the setting of enteral feeding may be best managed by providing a higher percentage of the TDD as nutritional insulin. In these cases, ratios of basal to nutritional insulin of 40:60, or even less basal insulin, may be appropriate.
Glucocorticoid Therapy
High‐dose glucocorticoids are strongly associated with increased insulin requirements. The degree of hyperglycemia induced by steroids varies significantly from patient to patient, and the pattern of hyperglycemia will vary depending on the pattern of steroid administration. The general principle to keep in mind is that the hyperglycemia induced by a steroid dose will peak 8‐12 hours after it is given, so insulin regimens to address this should take this effect into account. For example, giving a long‐acting basal insulin like glargine to accommodate the hyperglycemic effect of a steroid bolus given in the morning would be inappropriate because the steroid effect would wane and then disappear overnight, leading to insulin‐induced hypoglycemia. NPH insulin can be ideal in this setting, either by itself, or by layering it on top of an existing regimen.
Another caveat: glucocorticoids exert their predominate effect on insulin sensitivity in muscle (as opposed to the liver), and as a result, have their most notable effect on postprandial glucose. For this reason, the best insulin regimens for this situation may use proportionally less basal insulin and more nutritional insulin. One common regimen calls for keeping the basal insulin dose the same as the preglucocorticoid dose, while escalating the RAA insulin dose at lunch and dinner.
Given the complexities of covering steroid‐induced hyperglycemia and its high prevalence in certain populations (such as transplantation patients and patients undergoing chemotherapy), this would be an excellent area on which to focus expertise. Examples include routine endocrinology consultation, intervention by a special glycemic control team, or incorporating routine glucose monitoring and triggers for initiating insulin infusion into the protocols for chemotherapy and transplantation patients.
Regiment the Management of Hypoglycemia
Hypoglycemia is defined by the ADA as a blood glucose of 70 mg/dL or less, based on the physiologic changes that can occur at this glucose level, even in subjectively asymptomatic patients.3 Protocols for management of hypoglycemia should be linked to your diabetes/hyperglycemia protocols. There are many hypoglycemia protocols available for review in the SHM Glycemic Control Resource Room and Glycemic Control Implementation Guide.10 Some common themes for effective implementation stand out. First, the protocols need to walk the balance between simplicity of use, and the need to provide instructions that will provide guidance in a variety of patient situations. Second, the protocols need to be nurse driven, so that nurses can initiate treatment without waiting for a physician order. Third, education and instruction regarding recognition of risk factors, and avoidance of hypoglycemia are needed to support a successful protocol. Importantly, any hypoglycemic event should lead to a reconsideration of the current anti‐hyperglycemic regimen so that future events can be prevented.
Plan for Discharge and Provide Guidance for the Transition
Your institution should have policies and procedures outlining all the steps needed to complete the important transition out of the hospital. At a minimum, this planning should include adequate education (including a learner assessment), appropriate follow‐up, referral to community resources, and a discharge glycemic control regimen that is tailored to the educational, financial, and motivational profile of a patient. The more your inpatient insulin management is driven by protocol, the more likely it is the patient will be on an inpatient treatment plan that differs from their outpatient regimen; therefore, it is even more important to plan this transition carefully and reliably.
Communicating the accurate hyperglycemia related diagnosis and related problems to the primary care provider is important for good care, perhaps even more so for patients who had hyperglycemia while hospitalized without a prior diagnosis of diabetes. Some centers place a prompt for hyperglycemia related diagnosis in the order set and/or discharge paperwork, to remind the clinician to convey the diagnosis to the primary provider, and to encourage more complete documentation. Improved documentation can also improve the business case for glycemic control, along with other strategies outlined elsewhere in this supplement.13
Transitions in care (including transitions out of the hospital and off of infusion insulin) are discussed in more detail14, 15 elsewhere in this supplement. The principles outlined in these references should be incorporated into your institutional protocol. Briefly, not all patients require or are capable of intensive basal‐bolus regimens upon discharge. The HbA1c can be very valuable in arriving at the optimal outpatient regimen.14 The capacities and preferences of the patient and the context of his or her outpatient care environment (including the preferences of the primary care provider) must be taken into consideration as an outpatient management program is planned.
PULLING IT ALL TOGETHER: MAKE SURE YOUR PROTOCOL/ORDER SET IS EASY TO USE AND WIDELY UTILIZED
When standardizing hospital management of diabetes and hyperglycemia, we recommend building the full protocol first, then crystallizing the protocol into a one‐page summary that can be widely disseminated. The protocol guidance is then incorporated into the order set and nursing medical administration record (MAR). Again, we recommend the most proscriptive and protocol‐driven order set feasible within the constraints of medical staff support. The example order set in Appendix 2 illustrates this approach along with other desirable features:
-
Check‐box simplicity on when to order appropriate glucose monitoring.
-
Prompt for the proper hyperglycemia‐related diagnosis.
-
Prompts to document diagnosis and to order HbA1c level.
-
Use of encouraged insulin terminology: basal, prandial (or nutritional), and correction. Language is a powerful thing, and just getting staff to use these terms goes a long way toward the more physiologic prescribing of insulin.
-
Statement/reminder of a glycemic goal.
-
Prompts and contact information for appropriate consultation.
-
Elimination of unapproved abbreviations (such as U for units).
-
Stating both generic and brand names of insulin preparations.
-
Important timing cues for administration of insulin.
-
Several correction‐dose scales suitable for different insulin sensitivities. One size does NOT fit all.
-
Incorporation of a simple hypoglycemia protocol into the order set.
-
Insulin dosing guidelines available at the point of care (in this case, on the back of the order set).
Additional nursing‐specific cues (such as an admonition to never mix glargine insulin with other types of insulin) can also be included in the MAR whenever glargine is ordered.
Once you have protocols and order sets to guide providers, you need to assure that they are used for the majority of hyperglycemic patients. Educational programs should introduce your interventions and the rationale for them. In order to make your method the default method of care, your team should survey all preprinted or CPOE insulin order sets of your institution. A review of postoperative, transfer, and admission order sets that all services use may reveal a half‐dozen or more embedded sliding‐scale insulin order sets that should be removed, with prompts to use the standardized insulin order set being placed in their stead.
Computerized order sets present both challenges and opportunities. Wording limitations and the scrolling nature can make concepts less clear, yet there is a capability for incorporating a hierarchical structure that allows for guiding the user through a more algorithmic approach. There is also a capacity to provide assistance with dosing calculations that do not exist in the paper world. Education remains of key importance for both methods.
MONITOR THE USE AND EFFECTIVENESS OF YOUR PROTOCOLS AND ORDER SETS
Creating and implementing protocols, order sets, and other tools is not the end of the journey to improve care. It is important to monitor order set utilization, insulin use patterns, and parameters measuring glycemic control and hypoglycemia, as outlined in more detail in another article in this supplement.16 In addition to summary data every month or so, we recommend daily reports that spur action in near real time. Triggers such as uncontrolled hyperglycemia, markedly elevated HbA1c levels, and nonphysiologic insulin regimens should initiate consultation, extra diabetes education, or referral to a glucose control team. If appropriate consultation is not readily available, the glycemic control steering group should lobby the administration to bolster this capability. Qualitative feedback from the frontline caregivers, as well as this quantitative data, can assist the local glycemic control champions in designing even more effective protocols, order sets, focused educational efforts, and concurrent mitigation of suboptimal care.
CONCLUSION
Diabetes, hyperglycemia, and iatrogenic hypoglycemia are common and important conditions affecting the noncritically ill inpatient. Interventional trials to validate the recommended noncritical care unit glycemic targets are needed. Although there is a growing consensus on best practices to care for these patients, numerous barriers and the complexity of caring for inpatients hamper the reliability of best practice delivery. Institutional protocols and protocol driven subcutaneous insulin orders, when implemented with the strategies outlined here, can be the key to delivering these best practices more reliably.
Appendix
- American College of Endocrinology Task Force on Inpatient Diabetes and Metabolic Control.American College of Endocrinology Position Statement on Inpatient Diabetes and Metabolic Control.Endocr Pract.2004;10:77–82.
- ,,,,,,.Management of diabetes and hyperglycemia in hospitals.Diabetes Care.2004;27:553–591.
- American Diabetes Association.Standards of Medical Carein Diabetes‐2006.Diabetes Care.2006;29(suppl 1):s4–s42.
- ,,, et al.Randomized study of basal‐bolus insulin therapy in the inpatient management of patients with type 2 diabetes (RABBIT 2 Trial).Diabetes Care.2007;30:2181–2186.
- ,,,,.Inpatient management of diabetes and hyperglycemia among general medicine patients at a large teaching hospital.J Hosp Med.2006;1:145–150.
- ,,, et al.Diabetes care in the hospital: is there clinical inertia?J Hosp Med.2006;1:151–160.
- ,,, et al.Diabetes care in hospitalized non‐critically ill patients: more evidence for clinical inertia and negative therapeutic momentum.JHosp Med.2007;2:203–211.
- Inpatient Diabetes and Glycemic Control: A Call to Action Conference. Position Statement. AACE, February2006. Available at: http://www.aace.com/meetings/consensus/IIDC/IDGC0207.pdf. Accessed October, 2006.
- Proceedings of the American College of Endocrinology and American Diabetes Association Consensus Conference, Washington, DC, January 30–31, 2006. Endocr Pract.2006; 12(suppl 3):3–13.
- Society of Hospital Medicine Glycemic Control Task Force. Implementation Guide: Improving Glycemic Control, Preventing Hypoglycemia, and Optimizing Care of the Inpatient with Hyperglycemia and Diabetes. Published January 2007 on the Society of Hospital Medicine website. Available at: http://www.hospitalmedicine.org. Accessed August,2007.
- .Management of hyperglycemia in the hospital setting.N Engl J Med.2006;355:1903–1911.
- ,,,,.Management of diabetes and hyperglycemia in the hospital: a practical guide to subcutaneous insulin use in the non‐critically ill adult patient.J Hosp Med.2008;3(5):S17–S28.
- ,.Practical strategies for developing the business case for hospital glycemic control teams.J Hosp Med2008;3(5):S76–S83.
- ,,,.Bridge over troubled waters: safe and effective transitions of the inpatient with hyperglycemia.J Hosp Med.2008;3(5):S55–S65.
- ,,,.Designing and implementing insulin infusion protocols and order sets.J Hosp Med.2008;3(5):S42–S54.
- ,,,,,.SHM Glycemic Control Task Force summary: practical recommendations for assessing the impact of glycemic control efforts.J Hosp Med.2008;3(5):S66–S75.
Inpatient glycemic control and hypoglycemia are issues with well deserved increased attention in recent years. Prominent guidelines and technical reviews have been published,13 and a recent, randomized controlled trial demonstrated the superiority of basal bolus insulin regimens compared to sliding‐scale regimens.4 Effective glycemic control for inpatients has remained elusive in most medical centers. Recent reports57 detail clinical inertia and the continued widespread use of sliding‐scale subcutaneous insulin regimens, as opposed to the anticipatory, physiologic basal‐nutrition‐correction dose insulin regimens endorsed by these reviews.
Inpatient glycemic control faces a number of barriers, including fears of inducing hypoglycemia, uneven knowledge and training among staff, and competing institutional and patient priorities. These barriers occur in the background of an inherently complex inpatient environment that poses unique challenges in maintaining safe glycemic control. Patients frequently move across a variety of care teams and geographic locations during a single inpatient stay, giving rise to multiple opportunities for failed communication, incomplete handoffs, and inconsistent treatment. In addition, insulin requirements may change dramatically due to variations in the stress of illness, exposure to medications that effect glucose levels, and varied forms of nutritional intake with frequent interruption. Although insulin is recognized as one of the medications most likely to be associated with adverse events in the hospital, many hospitals do not have protocols or order sets in place to standardize its use.
A Call to Action consensus conference,8, 9 hosted by the American Association of Clinical Endocrinologists (AACE) and the American Diabetes Association (ADA), brought together many thought leaders and organizations, including representation from the Society of Hospital Medicine (SHM), to address these barriers and to outline components necessary for successful implementation of a program to improve inpatient glycemic control in the face of these difficulties. Institutional insulin management protocols and standardized insulin order sets (supported by appropriate educational efforts) were identified as key interventions. It may be tempting to quickly deploy a generic insulin order set in an effort to improve care. This often results in mediocre results, due to inadequate incorporation of standardization and guidance into the order set and other documentation tools, and uneven use of the order set.
The SHM Glycemic Control Task Force (GCTF) recommends the following steps for developing and implementing successful protocols and order sets addressing the needs of the noncritical care inpatient with diabetes/hyperglycemia.
-
Form a steering committee for this work, and assess the current processes of care.
-
Identify best practices and preferred regimens to manage diabetes and hyperglycemia in the hospital.
-
Integrate best practices and preferred institutional choices into an inpatient glycemic control protocol. Crystallize your protocol into a one page summary.
-
Place guidance from your protocol into the flow of work, by integrating it into standardized subcutaneous insulin order sets and other documentation and treatment tools.
-
Monitor the use of your order sets and protocol. Intervene actively on nonadherents to your protocol and those with poor glycemic control, and revise your protocol/order sets as needed.
IDENTIFYING AND INCORPORATING KEY CONCEPTS AND BEST PRACTICES
A protocol is a document that endorses specific monitoring and treatment strategies in a given institution. This potentially extensive document should provide guidance for transitions, special situations (like steroids and total parenteral nutrition [TPN]) and should outline preferred insulin regimens for all of the most common nutritional situations. One of the most difficult parts of creating a protocol is the assimilation of all of the important information on which to base decisions. Your protocol and order set will be promoting a set of clinical practices. Fortunately, the current best practice for noncritical care hyperglycemic patients has been summarized by several authoritative sources,13, 811 including references from the SHM Glycemic Task Force published in this supplement.4, 12
Table 1 summarizes the key concepts that should be emphasized in a protocol for subcutaneous insulin management in the hospital. We recommend embedding guidance from your protocol into order sets, the medication administration record, and educational materials. Although the details contained in a protocol and order set might vary from one institution to another, the key concepts should not. The remainder of this article provides practical information about how these concepts and guidance for how preferred insulin regimens should be included in these tools. Appendices 1 and 2 give examples of an institutional one‐page summary protocol and subcutaneous insulin order set, respectively.
| 1. Establish a target range for blood glucose levels. |
| 2. Standardize monitoring of glucose levels and assessment of long‐term control (HbA1c). |
| 3. Incorporate nutritional management. |
| 4. Prompt clinicians to consider discontinuing oral antihyperglycemic medications. |
| 5. Prescribe physiologic (basal‐nutrition‐correction) insulin regimens. |
| a. Choose a total daily dose (TDD). |
| b. Divide the TDD into physiologic components of insulin therapy and provide basal and nutritional/correction separately. |
| c. Choose and dose a basal insulin. |
| d. Choose and dose a nutritional (prandial) insulin |
|
i. Match exactly to nutritional intake (see Table 2). |
| ii. Include standing orders to allow nurses to hold nutritional insulin for nutritional interruptions and to modify nutritional insulin depending on the actual nutritional intake. |
| e. Add correction insulin |
| i. Match to an estimate of the patients insulin sensitivity using prefabricated scales. |
| ii. Use the same insulin as nutritional insulin. |
| 6. Miscellaneous |
| a. Manage hypoglycemia in a standardized fashion and adjust regimen to prevent recurrences. |
| b. Provide diabetes education and appropriate consultation. |
| c. Coordinate glucose testing, nutrition delivery, and insulin administration. |
| d. Tailor discharge treatment regimens to the patient's individual circumstances and arrange for proper follow‐up. |
Standardize the Monitoring of Blood Glucose Values and Glucosylated Hemoglobin
Guidance for the coordination of glucose testing, nutrition delivery, and insulin administration, should be integrated into your protocols, and order sets. For noncritical care areas, the minimal frequency for blood glucose monitoring for patients who are eating is before meals and at bedtime. For the patient designated nothing by mouth (NPO) or the patient on continuous tube feeding, the type of nutritional/correction insulin used should drive the minimum frequency (every 4‐6 hours if rapid acting analog insulins [RAA‐I] are used, and every 6 hours if regular insulin is used). Directions for administering scheduled RAA‐I immediately before or immediately after nutrition delivery should be incorporated into protocols, order sets, and medication administration records. Unfortunately, having this guidance in the order sets and protocols does not automatically translate into its being carried out in the real world. Wide variability in the coordination of glucose monitoring, nutritional delivery, and insulin administration is common, so monitoring the process to make sure the protocol is followed is important.
Obtaining a glucosylated hemoglobin (HbA1c) level is important in gauging how well the patient's outpatient regimen is maintaining glycemic control, distinguishing stress hyperglycemia from established diabetes, and guiding the inpatient approach to glycemic control. ADA guidelines2, 3 endorse obtaining HbA1c levels of inpatients if these levels are not already available from the month prior to admission.
Establish a Target Range for Blood Glucose in NonCritical Care Areas
It is important to adopt a glycemic target that is institution‐wide, for critical care areas and noncritical care areas alike. Your glycemic target need not be identical to the ADA/AACE glycemic targets, but should be similar to them.
Examples of institutional glycemic targets for noncritical care areas:
-
Preprandial target 90‐130 mg/dL, maximum random glucose <180 mg/dL (ADA/AACE consensus target)
-
90‐150 mg/dL (a target used in some hospitals)
-
Preprandial target 90‐130 mg/dL for most patients, 100‐150 mg/dL if there are hypoglycemia risk factors, and <180 mg/dL if comfort‐care or end‐of‐life care (a more refined target, allowing for customization based on patient characteristics).
Your multidisciplinary glycemic control steering committee should pick the glycemic target it can most successfully implement and disseminate. It is fine to start with a conservative target and then ratchet down the goals as the environment becomes more accepting of the concept of tighter control of blood glucose in the hospital.
Although the choice of glycemic target is somewhat arbitrary, establishing an institutional glycemic target is critical to motivate clinical action. Your committee should design interventions, for instances when a patient's glycemic target is consistently not being met, including an assignment of responsibility.
Prompt Clinicians to Consider Discontinuing Oral Agents
Oral antihyperglycemic agents, in general, are difficult to quickly titrate to effect, and have side effects that limit their use in the hospital. In contrast, insulin acts rapidly and can be used in virtually all patients and clinical situations, making it the treatment of choice for treatment of hyperglycemia in the hospital.3, 11, 12 In certain circumstances, it may be entirely appropriate to continue a well‐controlled patient on his or her prior outpatient oral regimen. It is often also reasonable to resume oral agents in some patients when preparing for hospital discharge.
Incorporate Nutritional Management
Because diet is so integral to the management of diabetes and hyperglycemia, diet orders should be embedded in all diabetes or insulin‐related order sets. Diets with the same amount of carbohydrate with each meal should be the default rule for patients with diabetes. Nutritionist consultation should be considered and easy to access for patients with malnutrition, obesity, and other common conditions of the inpatient with diabetes.
Access Diabetes Education and Appropriate Consultation
Diabetes education should be offered to all hyperglycemic patients with normal mental status, complete with written materials, a listing of community resources, and survival skills. Consultation with physicians in internal medicine or endocrinology for difficult‐to‐control cases, or for cases in which the primary physician of record is not familiar with (or not adherent to) principles of inpatient glycemic management, should be very easy to obtain, or perhaps mandated, depending on your institution‐specific environment.
Prescribe Physiologic (Basal‐Nutritional‐Correction Dose) Insulin Regimens
Physiologic insulin use is the backbone of the recommended best practice for diabetes and hyperglycemia management in the hospital. The principles of such regimens are summarized elsewhere in this supplement.12 These principles will not be reiterated in detail here, but the major concepts that should be integrated into the protocols and order sets will be highlighted.
Choose a Total Daily Dose
Clinicians need guidance on how much subcutaneous insulin they should give a patient. These doses are well known from clinical experience and the published literature. The fear of hypoglycemia usually results in substantial underdosing of insulin, or total avoidance of scheduled insulin on admission. Your team should provide guidance for how much insulin to start a patient on when it is unclear from past experience how much insulin the patient needs. Waiting a few days to see how much insulin is required via sliding‐scale‐only regimens is a bad practice that should be discouraged for patients whose glucose values are substantially above the glycemic target. The total daily dose (TDD) can be estimated in several different ways (as demonstrated in Appendix 1 and 2), and protocols should make this step very clear for clinicians. Providing a specific location on the order set to declare the TDD may help ensure this step gets done more reliably. Some institutions with computer physician order entry (CPOE) provide assistance with calculating the TDD and the allocation of basal and nutritional components, based on data the ordering physician inputs into the system.
Select and Dose a Basal Insulin
Your protocol should describe how the TDD should be divided between basal and nutritional insulin. We generally recommend 50% of the TDD be given as basal insulin, with the other 50% administered on a scheduled basis to cover glycemic excursions from nutritional intake. The 50/50 rule is simple and generally works well, and should be widely promoted. However, there are exceptions to this rule that should be incorporated into your full protocol and educational programs. The order set should have separate steps for ordering basal insulin, nutritional insulin, and correction insulin. The advantage to providing these insulin components separately is that it allows them to be independently manipulated (eg, if a patient is unable to tolerate a meal, nutritional insulin is held, but basal insulin and correction insulin are continued).
The SHM GCTF specifically endorses long acting insulin (glargine and detemir) as the preferred basal insulin in the hospital setting, thus discouraging the use of neutral protamine Hagedorn (NPH) insulin and fixed combination insulin formulations (Table 2). In the absence of randomized controlled trials demonstrating superiority of the glargine or detemir to NPH insulin in the hospital, this endorsement deserves some further explanation. Although we believe that correctly dosed NPH containing insulin regimens can attain effective and safe glycemic control in the hospital setting, it is more difficult to standardize their use and adjust for fluctuations in nutritional intake. Glargine and detemir have much less pronounced spikes in their effect than NPH, rendering them relatively peakless in comparison. This pharmacokinetic profile allows for continued dosing with minimal or no correction when nutrition intake is variable, and allow for consistent reinforcement of the basal‐nutritional‐correction insulin concept.
| Nutritional situation | Necessary insulin components | Preferred regimen* |
|---|---|---|
| ||
| NPO (or clear liquids) | Basal insulin: 50% of TDD. Nutritional insulin: None. | Basal insulin: glargine given once daily or detemir given twice daily. Nutritional insulin: None. Correctional insulin: Regular insulin q 6 hours or RAA insulin q 4 hours. Other comments: Dextrose infusion (e.g., D5 containing solution at 75‐150 cc/hour) recommended when nutrition is held. An IV insulin infusion is preferred for management of prolonged fasts or fasting type 1 diabetes patients. |
| Eating meals | Basal insulin: 50% of TDD. Nutritional insulin: 50% of TDD, divided equally before each meal. | Basal insulin: glargine given once daily or detemir given twice daily. Nutritional insulin: RAA insulin with meals. Correctional insulin: RAA insulin q AC and HS (reduced dose at HS). |
| Bolus tube feeds | Basal insulin: 40% of TDD. Nutritional insulin: 60% of the TDD, divided equally before each bolus feed. | Basal insulin: glargine given once daily or detemir given twice daily. Nutritional insulin: RAA insulin with each bolus. Correctional insulin: RAA insulin with each bolus. |
| Continuous tube feeds | Basal insulin: 40% (conservative) of TDD. Nutritional insulin: 60% of the TDD in divided doses. | Basal insulin: glargine given once daily or detemir given twice daily. Nutritional insulin: RAA insulin q 4 hours or regular insulin q 6 hours. Correctional insulin: Should match nutritional insulin choice. |
| Parenteral nutrition | Insulin is usually given parenterally, with the nutrition | Initially, a separate insulin drip allows for accurate dose‐finding. Then, 80% of amount determined as TDD using drip is added to subsequent TPN bags as regular insulin. Use correctional subcutaneous insulin doses cautiously, in addition. |
There are some caveats to this general recommendation. First, patients who are well controlled on home regimens with NPH basal insulin can (and sometimes should) stay on the regimen that has worked well for them. However, extra vigilance in reducing the dose for reductions in nutrition is required, because NPH is generally used to cover both nutritional and basal requirements. Second, extensive experience with glargine and detemir are not available in obstetric populations. They are not U.S. Food and Drug Administration (FDA) approved for use in pregnant patients and formally carry a Class C rating, whereas NPH insulin has been used safely in obstetric populations for decades. Third, the insulin regimen used as an inpatient is not necessarily the preferred regimen to prescribe at discharge: cost, patient preferences, HbA1c level, and other factors should be considered in making this choice.
Select and Dose a Nutritional (Prandial) Insulin
The step for ordering nutritional insulin should assist the clinician in matching the insulin to the type of nutrition that the patient is receiving. For example, rapid‐acting insulin analogs are preferred over regular insulin in the eating patient, in view of their more physiologic profile, which averts the insulin stacking that can occur with regular insulin. If regular insulin is used as the preferred institutional choice for eating patients, the lunchtime dose should be reduced or eliminated altogether, to eliminate insulin stacking.
Table 2 outlines the SHM GCTF preferred regimens for different nutritional situations.
There should be a standing order for nutritional insulin to be held when nutrition is interrupted, whether intentional or unintentional. Patients with interrupted tube feedings could have standing orders for a dextrose infusion to replace the tube feeding carbohydrate load and prevent hypoglycemia. Ideally, there should also be a standing order allowing for real‐time management of the patient with uncertain nutritional intake. For example, when a patient's premeal assessment reveals that she may not tolerate the meal, the patient should be allowed to attempt to eat, and then the nutritional insulin should be given after the meal, in proportion to the amount of food that was eaten. This type of order will require significant nursing education and process redesign in many hospitals, but is essential for matching nutritional insulin to actual intake.
Add Correction Insulin
There is no convincing evidence for the benefit of correction (sliding‐scale) insulin in the inpatient setting, although a randomized trial demonstrating the superiority of basal/nutritional insulin regimens to sliding‐scale only regimens did incorporate a correction insulin scale as an adjunct to the superior basal/nutritional regimen.4 The SHM GCTF again emphasizes that control of hyperglycemia should be proactive and anticipatory of insulin needs, rather than reactive to hyperglycemia. Nonetheless, unexpected hyperglycemic excursions are common, and the use of correction insulin remains a pervasive and arguably logical practice. If correction insulin is used, it should be ordered as a separate step after considering basal and nutrition insulin needs. The doses of scheduled insulin should be adjusted regularly if correction insulin is consistently being required. Ideally, the prescriber should choose a preformatted corrective insulin scale, based on the patient's insulin sensitivity (Appendix 2). There should be a prompt to use the same type of insulin that is being used for nutritional insulin, and there should be instructions that this insulin is given in addition to the basal and nutritional insulin to correct for hyperglycemia. Nocturnal correction‐dose scales should be reduced in the eating patient.
Even after limiting insulin regimens to those in Table 2, multidisciplinary glycemic control teams are still left with several options within these SHM‐preferred regimens. We recommend that your team choose a single, institutionally‐preferred basal‐nutritional‐correction insulin combination for each situation.
Choosing one preferred option for these situations is advantageous because:
-
You can communicate preferred regimens more simply and succinctly to all staff.
-
You eliminate all inappropriate choices for insulin regimens for that situation, as well as some other less preferred, but acceptable choices.
-
You can encourage regimens that are most economical (by promoting the insulin regimens that reflect your hospital formulary choices).
-
Staff members can become very familiar with a few regimens, instead of being confused by a multitude of them. They can identify variations from your preferred choices and target these patients for extra scrutiny and actions should they fail to meet glycemic targets.
Although virtually every institution can provide specific guidance on insulin management in a protocol, there are tradeoffs inherent in how restrictive you can be in pushing these preferred choices in your order sets. Should you eliminate alternate basal or nutritional insulin choices from your order sets? As you integrate more and more of your preferred algorithm and regimens into your order set, you will gain incremental improvement in the standardization of inpatient insulin management. However, you reduce not only variability in ordering, but also the choices available to your prescribers and patients, and in effect you are pushing the providers to use an insulin regimen that often differs from the patient's outpatient regimen. If your institution is not yet ready to go with a single preferred insulin, simply listing your preferred insulin first with the annotation preferred can be enough to increase the use of the preferred insulin.
We endorse building the most protocol‐driven, proscriptive, insulin order set that the Glycemic Control Steering Committee believes their medical staff will accept. There are some caveats to this endorsement. First, there must be extra efforts on the backend of the admission, to ensure that the antihyperglycemic regimen is tailored to the unique needs of the patient (this is discussed further below). Second, a protocol‐driven approach is not a substitute for a good educational program for health care providers or well‐informed clinical judgment. Education should reinforce major concepts driving the protocol and should also highlight exceptions to the rule. Variance from the protocol endorsed choices should be allowed (and even encouraged) when the variance is driven by patient factors (as opposed to provider whim). Learning from this variance is a key concept in refining protocols. Education ideally should not be limited to only protocol‐endorsed choices, as staff should be familiar with the full range of antihyperglycemia regimens seen in inpatient and outpatient settings.
Special Situations
Most of the preferred regimens for different situations are outlined in Table 2 in a straightforward manner, and can be depicted in your protocols and order sets in the same way. Some conditions have enough complexity, however, that you will have difficulty placing all of the details into your one‐page protocol and order set. Details should be placed on your more detailed protocol, and educational programs should include the topics outlined below. Although insulin infusion is often the option that would provide the most reliable and expedient control of hyperglycemia in these special situations, it is an option not available in many noncritical care settings. Therefore, the discussion is limited to subcutaneous insulin control regimens.
Patient on Continuous Tube Feeding
The SHM GCTF endorses glargine or detemir as the basal insulin of choice for this setting. The nutritional and correction insulin of choice is either an RAA‐I every 4 hours (q4h), or regular insulin every 6 hours (q6h). We endorse this choice because it retains the basal‐nutritional‐correction dose concept, generally allows for continued basal insulin use if the tube feedings become interrupted, and is amenable to building a consistent institutional protocol.
There are some important caveats to this recommendation. First, realize that almost any regimen that provides a stable insulin supply would be acceptable, and many institutions will use glargine or detemir to cover both basal and nutritional needs. The downside to using large boluses of long‐acting insulin in this clinical situation is that any unexpected interruption of the feedings will necessitate prolonged infusions of dextrate 10% solution (D10) to avoid hypoglycemia
Second, because of the glycemic load inherent in tube feedings, maintenance of glycemic control in the setting of enteral feeding may be best managed by providing a higher percentage of the TDD as nutritional insulin. In these cases, ratios of basal to nutritional insulin of 40:60, or even less basal insulin, may be appropriate.
Glucocorticoid Therapy
High‐dose glucocorticoids are strongly associated with increased insulin requirements. The degree of hyperglycemia induced by steroids varies significantly from patient to patient, and the pattern of hyperglycemia will vary depending on the pattern of steroid administration. The general principle to keep in mind is that the hyperglycemia induced by a steroid dose will peak 8‐12 hours after it is given, so insulin regimens to address this should take this effect into account. For example, giving a long‐acting basal insulin like glargine to accommodate the hyperglycemic effect of a steroid bolus given in the morning would be inappropriate because the steroid effect would wane and then disappear overnight, leading to insulin‐induced hypoglycemia. NPH insulin can be ideal in this setting, either by itself, or by layering it on top of an existing regimen.
Another caveat: glucocorticoids exert their predominate effect on insulin sensitivity in muscle (as opposed to the liver), and as a result, have their most notable effect on postprandial glucose. For this reason, the best insulin regimens for this situation may use proportionally less basal insulin and more nutritional insulin. One common regimen calls for keeping the basal insulin dose the same as the preglucocorticoid dose, while escalating the RAA insulin dose at lunch and dinner.
Given the complexities of covering steroid‐induced hyperglycemia and its high prevalence in certain populations (such as transplantation patients and patients undergoing chemotherapy), this would be an excellent area on which to focus expertise. Examples include routine endocrinology consultation, intervention by a special glycemic control team, or incorporating routine glucose monitoring and triggers for initiating insulin infusion into the protocols for chemotherapy and transplantation patients.
Regiment the Management of Hypoglycemia
Hypoglycemia is defined by the ADA as a blood glucose of 70 mg/dL or less, based on the physiologic changes that can occur at this glucose level, even in subjectively asymptomatic patients.3 Protocols for management of hypoglycemia should be linked to your diabetes/hyperglycemia protocols. There are many hypoglycemia protocols available for review in the SHM Glycemic Control Resource Room and Glycemic Control Implementation Guide.10 Some common themes for effective implementation stand out. First, the protocols need to walk the balance between simplicity of use, and the need to provide instructions that will provide guidance in a variety of patient situations. Second, the protocols need to be nurse driven, so that nurses can initiate treatment without waiting for a physician order. Third, education and instruction regarding recognition of risk factors, and avoidance of hypoglycemia are needed to support a successful protocol. Importantly, any hypoglycemic event should lead to a reconsideration of the current anti‐hyperglycemic regimen so that future events can be prevented.
Plan for Discharge and Provide Guidance for the Transition
Your institution should have policies and procedures outlining all the steps needed to complete the important transition out of the hospital. At a minimum, this planning should include adequate education (including a learner assessment), appropriate follow‐up, referral to community resources, and a discharge glycemic control regimen that is tailored to the educational, financial, and motivational profile of a patient. The more your inpatient insulin management is driven by protocol, the more likely it is the patient will be on an inpatient treatment plan that differs from their outpatient regimen; therefore, it is even more important to plan this transition carefully and reliably.
Communicating the accurate hyperglycemia related diagnosis and related problems to the primary care provider is important for good care, perhaps even more so for patients who had hyperglycemia while hospitalized without a prior diagnosis of diabetes. Some centers place a prompt for hyperglycemia related diagnosis in the order set and/or discharge paperwork, to remind the clinician to convey the diagnosis to the primary provider, and to encourage more complete documentation. Improved documentation can also improve the business case for glycemic control, along with other strategies outlined elsewhere in this supplement.13
Transitions in care (including transitions out of the hospital and off of infusion insulin) are discussed in more detail14, 15 elsewhere in this supplement. The principles outlined in these references should be incorporated into your institutional protocol. Briefly, not all patients require or are capable of intensive basal‐bolus regimens upon discharge. The HbA1c can be very valuable in arriving at the optimal outpatient regimen.14 The capacities and preferences of the patient and the context of his or her outpatient care environment (including the preferences of the primary care provider) must be taken into consideration as an outpatient management program is planned.
PULLING IT ALL TOGETHER: MAKE SURE YOUR PROTOCOL/ORDER SET IS EASY TO USE AND WIDELY UTILIZED
When standardizing hospital management of diabetes and hyperglycemia, we recommend building the full protocol first, then crystallizing the protocol into a one‐page summary that can be widely disseminated. The protocol guidance is then incorporated into the order set and nursing medical administration record (MAR). Again, we recommend the most proscriptive and protocol‐driven order set feasible within the constraints of medical staff support. The example order set in Appendix 2 illustrates this approach along with other desirable features:
-
Check‐box simplicity on when to order appropriate glucose monitoring.
-
Prompt for the proper hyperglycemia‐related diagnosis.
-
Prompts to document diagnosis and to order HbA1c level.
-
Use of encouraged insulin terminology: basal, prandial (or nutritional), and correction. Language is a powerful thing, and just getting staff to use these terms goes a long way toward the more physiologic prescribing of insulin.
-
Statement/reminder of a glycemic goal.
-
Prompts and contact information for appropriate consultation.
-
Elimination of unapproved abbreviations (such as U for units).
-
Stating both generic and brand names of insulin preparations.
-
Important timing cues for administration of insulin.
-
Several correction‐dose scales suitable for different insulin sensitivities. One size does NOT fit all.
-
Incorporation of a simple hypoglycemia protocol into the order set.
-
Insulin dosing guidelines available at the point of care (in this case, on the back of the order set).
Additional nursing‐specific cues (such as an admonition to never mix glargine insulin with other types of insulin) can also be included in the MAR whenever glargine is ordered.
Once you have protocols and order sets to guide providers, you need to assure that they are used for the majority of hyperglycemic patients. Educational programs should introduce your interventions and the rationale for them. In order to make your method the default method of care, your team should survey all preprinted or CPOE insulin order sets of your institution. A review of postoperative, transfer, and admission order sets that all services use may reveal a half‐dozen or more embedded sliding‐scale insulin order sets that should be removed, with prompts to use the standardized insulin order set being placed in their stead.
Computerized order sets present both challenges and opportunities. Wording limitations and the scrolling nature can make concepts less clear, yet there is a capability for incorporating a hierarchical structure that allows for guiding the user through a more algorithmic approach. There is also a capacity to provide assistance with dosing calculations that do not exist in the paper world. Education remains of key importance for both methods.
MONITOR THE USE AND EFFECTIVENESS OF YOUR PROTOCOLS AND ORDER SETS
Creating and implementing protocols, order sets, and other tools is not the end of the journey to improve care. It is important to monitor order set utilization, insulin use patterns, and parameters measuring glycemic control and hypoglycemia, as outlined in more detail in another article in this supplement.16 In addition to summary data every month or so, we recommend daily reports that spur action in near real time. Triggers such as uncontrolled hyperglycemia, markedly elevated HbA1c levels, and nonphysiologic insulin regimens should initiate consultation, extra diabetes education, or referral to a glucose control team. If appropriate consultation is not readily available, the glycemic control steering group should lobby the administration to bolster this capability. Qualitative feedback from the frontline caregivers, as well as this quantitative data, can assist the local glycemic control champions in designing even more effective protocols, order sets, focused educational efforts, and concurrent mitigation of suboptimal care.
CONCLUSION
Diabetes, hyperglycemia, and iatrogenic hypoglycemia are common and important conditions affecting the noncritically ill inpatient. Interventional trials to validate the recommended noncritical care unit glycemic targets are needed. Although there is a growing consensus on best practices to care for these patients, numerous barriers and the complexity of caring for inpatients hamper the reliability of best practice delivery. Institutional protocols and protocol driven subcutaneous insulin orders, when implemented with the strategies outlined here, can be the key to delivering these best practices more reliably.
Appendix
Inpatient glycemic control and hypoglycemia are issues with well deserved increased attention in recent years. Prominent guidelines and technical reviews have been published,13 and a recent, randomized controlled trial demonstrated the superiority of basal bolus insulin regimens compared to sliding‐scale regimens.4 Effective glycemic control for inpatients has remained elusive in most medical centers. Recent reports57 detail clinical inertia and the continued widespread use of sliding‐scale subcutaneous insulin regimens, as opposed to the anticipatory, physiologic basal‐nutrition‐correction dose insulin regimens endorsed by these reviews.
Inpatient glycemic control faces a number of barriers, including fears of inducing hypoglycemia, uneven knowledge and training among staff, and competing institutional and patient priorities. These barriers occur in the background of an inherently complex inpatient environment that poses unique challenges in maintaining safe glycemic control. Patients frequently move across a variety of care teams and geographic locations during a single inpatient stay, giving rise to multiple opportunities for failed communication, incomplete handoffs, and inconsistent treatment. In addition, insulin requirements may change dramatically due to variations in the stress of illness, exposure to medications that effect glucose levels, and varied forms of nutritional intake with frequent interruption. Although insulin is recognized as one of the medications most likely to be associated with adverse events in the hospital, many hospitals do not have protocols or order sets in place to standardize its use.
A Call to Action consensus conference,8, 9 hosted by the American Association of Clinical Endocrinologists (AACE) and the American Diabetes Association (ADA), brought together many thought leaders and organizations, including representation from the Society of Hospital Medicine (SHM), to address these barriers and to outline components necessary for successful implementation of a program to improve inpatient glycemic control in the face of these difficulties. Institutional insulin management protocols and standardized insulin order sets (supported by appropriate educational efforts) were identified as key interventions. It may be tempting to quickly deploy a generic insulin order set in an effort to improve care. This often results in mediocre results, due to inadequate incorporation of standardization and guidance into the order set and other documentation tools, and uneven use of the order set.
The SHM Glycemic Control Task Force (GCTF) recommends the following steps for developing and implementing successful protocols and order sets addressing the needs of the noncritical care inpatient with diabetes/hyperglycemia.
-
Form a steering committee for this work, and assess the current processes of care.
-
Identify best practices and preferred regimens to manage diabetes and hyperglycemia in the hospital.
-
Integrate best practices and preferred institutional choices into an inpatient glycemic control protocol. Crystallize your protocol into a one page summary.
-
Place guidance from your protocol into the flow of work, by integrating it into standardized subcutaneous insulin order sets and other documentation and treatment tools.
-
Monitor the use of your order sets and protocol. Intervene actively on nonadherents to your protocol and those with poor glycemic control, and revise your protocol/order sets as needed.
IDENTIFYING AND INCORPORATING KEY CONCEPTS AND BEST PRACTICES
A protocol is a document that endorses specific monitoring and treatment strategies in a given institution. This potentially extensive document should provide guidance for transitions, special situations (like steroids and total parenteral nutrition [TPN]) and should outline preferred insulin regimens for all of the most common nutritional situations. One of the most difficult parts of creating a protocol is the assimilation of all of the important information on which to base decisions. Your protocol and order set will be promoting a set of clinical practices. Fortunately, the current best practice for noncritical care hyperglycemic patients has been summarized by several authoritative sources,13, 811 including references from the SHM Glycemic Task Force published in this supplement.4, 12
Table 1 summarizes the key concepts that should be emphasized in a protocol for subcutaneous insulin management in the hospital. We recommend embedding guidance from your protocol into order sets, the medication administration record, and educational materials. Although the details contained in a protocol and order set might vary from one institution to another, the key concepts should not. The remainder of this article provides practical information about how these concepts and guidance for how preferred insulin regimens should be included in these tools. Appendices 1 and 2 give examples of an institutional one‐page summary protocol and subcutaneous insulin order set, respectively.
| 1. Establish a target range for blood glucose levels. |
| 2. Standardize monitoring of glucose levels and assessment of long‐term control (HbA1c). |
| 3. Incorporate nutritional management. |
| 4. Prompt clinicians to consider discontinuing oral antihyperglycemic medications. |
| 5. Prescribe physiologic (basal‐nutrition‐correction) insulin regimens. |
| a. Choose a total daily dose (TDD). |
| b. Divide the TDD into physiologic components of insulin therapy and provide basal and nutritional/correction separately. |
| c. Choose and dose a basal insulin. |
| d. Choose and dose a nutritional (prandial) insulin |
|
i. Match exactly to nutritional intake (see Table 2). |
| ii. Include standing orders to allow nurses to hold nutritional insulin for nutritional interruptions and to modify nutritional insulin depending on the actual nutritional intake. |
| e. Add correction insulin |
| i. Match to an estimate of the patients insulin sensitivity using prefabricated scales. |
| ii. Use the same insulin as nutritional insulin. |
| 6. Miscellaneous |
| a. Manage hypoglycemia in a standardized fashion and adjust regimen to prevent recurrences. |
| b. Provide diabetes education and appropriate consultation. |
| c. Coordinate glucose testing, nutrition delivery, and insulin administration. |
| d. Tailor discharge treatment regimens to the patient's individual circumstances and arrange for proper follow‐up. |
Standardize the Monitoring of Blood Glucose Values and Glucosylated Hemoglobin
Guidance for the coordination of glucose testing, nutrition delivery, and insulin administration, should be integrated into your protocols, and order sets. For noncritical care areas, the minimal frequency for blood glucose monitoring for patients who are eating is before meals and at bedtime. For the patient designated nothing by mouth (NPO) or the patient on continuous tube feeding, the type of nutritional/correction insulin used should drive the minimum frequency (every 4‐6 hours if rapid acting analog insulins [RAA‐I] are used, and every 6 hours if regular insulin is used). Directions for administering scheduled RAA‐I immediately before or immediately after nutrition delivery should be incorporated into protocols, order sets, and medication administration records. Unfortunately, having this guidance in the order sets and protocols does not automatically translate into its being carried out in the real world. Wide variability in the coordination of glucose monitoring, nutritional delivery, and insulin administration is common, so monitoring the process to make sure the protocol is followed is important.
Obtaining a glucosylated hemoglobin (HbA1c) level is important in gauging how well the patient's outpatient regimen is maintaining glycemic control, distinguishing stress hyperglycemia from established diabetes, and guiding the inpatient approach to glycemic control. ADA guidelines2, 3 endorse obtaining HbA1c levels of inpatients if these levels are not already available from the month prior to admission.
Establish a Target Range for Blood Glucose in NonCritical Care Areas
It is important to adopt a glycemic target that is institution‐wide, for critical care areas and noncritical care areas alike. Your glycemic target need not be identical to the ADA/AACE glycemic targets, but should be similar to them.
Examples of institutional glycemic targets for noncritical care areas:
-
Preprandial target 90‐130 mg/dL, maximum random glucose <180 mg/dL (ADA/AACE consensus target)
-
90‐150 mg/dL (a target used in some hospitals)
-
Preprandial target 90‐130 mg/dL for most patients, 100‐150 mg/dL if there are hypoglycemia risk factors, and <180 mg/dL if comfort‐care or end‐of‐life care (a more refined target, allowing for customization based on patient characteristics).
Your multidisciplinary glycemic control steering committee should pick the glycemic target it can most successfully implement and disseminate. It is fine to start with a conservative target and then ratchet down the goals as the environment becomes more accepting of the concept of tighter control of blood glucose in the hospital.
Although the choice of glycemic target is somewhat arbitrary, establishing an institutional glycemic target is critical to motivate clinical action. Your committee should design interventions, for instances when a patient's glycemic target is consistently not being met, including an assignment of responsibility.
Prompt Clinicians to Consider Discontinuing Oral Agents
Oral antihyperglycemic agents, in general, are difficult to quickly titrate to effect, and have side effects that limit their use in the hospital. In contrast, insulin acts rapidly and can be used in virtually all patients and clinical situations, making it the treatment of choice for treatment of hyperglycemia in the hospital.3, 11, 12 In certain circumstances, it may be entirely appropriate to continue a well‐controlled patient on his or her prior outpatient oral regimen. It is often also reasonable to resume oral agents in some patients when preparing for hospital discharge.
Incorporate Nutritional Management
Because diet is so integral to the management of diabetes and hyperglycemia, diet orders should be embedded in all diabetes or insulin‐related order sets. Diets with the same amount of carbohydrate with each meal should be the default rule for patients with diabetes. Nutritionist consultation should be considered and easy to access for patients with malnutrition, obesity, and other common conditions of the inpatient with diabetes.
Access Diabetes Education and Appropriate Consultation
Diabetes education should be offered to all hyperglycemic patients with normal mental status, complete with written materials, a listing of community resources, and survival skills. Consultation with physicians in internal medicine or endocrinology for difficult‐to‐control cases, or for cases in which the primary physician of record is not familiar with (or not adherent to) principles of inpatient glycemic management, should be very easy to obtain, or perhaps mandated, depending on your institution‐specific environment.
Prescribe Physiologic (Basal‐Nutritional‐Correction Dose) Insulin Regimens
Physiologic insulin use is the backbone of the recommended best practice for diabetes and hyperglycemia management in the hospital. The principles of such regimens are summarized elsewhere in this supplement.12 These principles will not be reiterated in detail here, but the major concepts that should be integrated into the protocols and order sets will be highlighted.
Choose a Total Daily Dose
Clinicians need guidance on how much subcutaneous insulin they should give a patient. These doses are well known from clinical experience and the published literature. The fear of hypoglycemia usually results in substantial underdosing of insulin, or total avoidance of scheduled insulin on admission. Your team should provide guidance for how much insulin to start a patient on when it is unclear from past experience how much insulin the patient needs. Waiting a few days to see how much insulin is required via sliding‐scale‐only regimens is a bad practice that should be discouraged for patients whose glucose values are substantially above the glycemic target. The total daily dose (TDD) can be estimated in several different ways (as demonstrated in Appendix 1 and 2), and protocols should make this step very clear for clinicians. Providing a specific location on the order set to declare the TDD may help ensure this step gets done more reliably. Some institutions with computer physician order entry (CPOE) provide assistance with calculating the TDD and the allocation of basal and nutritional components, based on data the ordering physician inputs into the system.
Select and Dose a Basal Insulin
Your protocol should describe how the TDD should be divided between basal and nutritional insulin. We generally recommend 50% of the TDD be given as basal insulin, with the other 50% administered on a scheduled basis to cover glycemic excursions from nutritional intake. The 50/50 rule is simple and generally works well, and should be widely promoted. However, there are exceptions to this rule that should be incorporated into your full protocol and educational programs. The order set should have separate steps for ordering basal insulin, nutritional insulin, and correction insulin. The advantage to providing these insulin components separately is that it allows them to be independently manipulated (eg, if a patient is unable to tolerate a meal, nutritional insulin is held, but basal insulin and correction insulin are continued).
The SHM GCTF specifically endorses long acting insulin (glargine and detemir) as the preferred basal insulin in the hospital setting, thus discouraging the use of neutral protamine Hagedorn (NPH) insulin and fixed combination insulin formulations (Table 2). In the absence of randomized controlled trials demonstrating superiority of the glargine or detemir to NPH insulin in the hospital, this endorsement deserves some further explanation. Although we believe that correctly dosed NPH containing insulin regimens can attain effective and safe glycemic control in the hospital setting, it is more difficult to standardize their use and adjust for fluctuations in nutritional intake. Glargine and detemir have much less pronounced spikes in their effect than NPH, rendering them relatively peakless in comparison. This pharmacokinetic profile allows for continued dosing with minimal or no correction when nutrition intake is variable, and allow for consistent reinforcement of the basal‐nutritional‐correction insulin concept.
| Nutritional situation | Necessary insulin components | Preferred regimen* |
|---|---|---|
| ||
| NPO (or clear liquids) | Basal insulin: 50% of TDD. Nutritional insulin: None. | Basal insulin: glargine given once daily or detemir given twice daily. Nutritional insulin: None. Correctional insulin: Regular insulin q 6 hours or RAA insulin q 4 hours. Other comments: Dextrose infusion (e.g., D5 containing solution at 75‐150 cc/hour) recommended when nutrition is held. An IV insulin infusion is preferred for management of prolonged fasts or fasting type 1 diabetes patients. |
| Eating meals | Basal insulin: 50% of TDD. Nutritional insulin: 50% of TDD, divided equally before each meal. | Basal insulin: glargine given once daily or detemir given twice daily. Nutritional insulin: RAA insulin with meals. Correctional insulin: RAA insulin q AC and HS (reduced dose at HS). |
| Bolus tube feeds | Basal insulin: 40% of TDD. Nutritional insulin: 60% of the TDD, divided equally before each bolus feed. | Basal insulin: glargine given once daily or detemir given twice daily. Nutritional insulin: RAA insulin with each bolus. Correctional insulin: RAA insulin with each bolus. |
| Continuous tube feeds | Basal insulin: 40% (conservative) of TDD. Nutritional insulin: 60% of the TDD in divided doses. | Basal insulin: glargine given once daily or detemir given twice daily. Nutritional insulin: RAA insulin q 4 hours or regular insulin q 6 hours. Correctional insulin: Should match nutritional insulin choice. |
| Parenteral nutrition | Insulin is usually given parenterally, with the nutrition | Initially, a separate insulin drip allows for accurate dose‐finding. Then, 80% of amount determined as TDD using drip is added to subsequent TPN bags as regular insulin. Use correctional subcutaneous insulin doses cautiously, in addition. |
There are some caveats to this general recommendation. First, patients who are well controlled on home regimens with NPH basal insulin can (and sometimes should) stay on the regimen that has worked well for them. However, extra vigilance in reducing the dose for reductions in nutrition is required, because NPH is generally used to cover both nutritional and basal requirements. Second, extensive experience with glargine and detemir are not available in obstetric populations. They are not U.S. Food and Drug Administration (FDA) approved for use in pregnant patients and formally carry a Class C rating, whereas NPH insulin has been used safely in obstetric populations for decades. Third, the insulin regimen used as an inpatient is not necessarily the preferred regimen to prescribe at discharge: cost, patient preferences, HbA1c level, and other factors should be considered in making this choice.
Select and Dose a Nutritional (Prandial) Insulin
The step for ordering nutritional insulin should assist the clinician in matching the insulin to the type of nutrition that the patient is receiving. For example, rapid‐acting insulin analogs are preferred over regular insulin in the eating patient, in view of their more physiologic profile, which averts the insulin stacking that can occur with regular insulin. If regular insulin is used as the preferred institutional choice for eating patients, the lunchtime dose should be reduced or eliminated altogether, to eliminate insulin stacking.
Table 2 outlines the SHM GCTF preferred regimens for different nutritional situations.
There should be a standing order for nutritional insulin to be held when nutrition is interrupted, whether intentional or unintentional. Patients with interrupted tube feedings could have standing orders for a dextrose infusion to replace the tube feeding carbohydrate load and prevent hypoglycemia. Ideally, there should also be a standing order allowing for real‐time management of the patient with uncertain nutritional intake. For example, when a patient's premeal assessment reveals that she may not tolerate the meal, the patient should be allowed to attempt to eat, and then the nutritional insulin should be given after the meal, in proportion to the amount of food that was eaten. This type of order will require significant nursing education and process redesign in many hospitals, but is essential for matching nutritional insulin to actual intake.
Add Correction Insulin
There is no convincing evidence for the benefit of correction (sliding‐scale) insulin in the inpatient setting, although a randomized trial demonstrating the superiority of basal/nutritional insulin regimens to sliding‐scale only regimens did incorporate a correction insulin scale as an adjunct to the superior basal/nutritional regimen.4 The SHM GCTF again emphasizes that control of hyperglycemia should be proactive and anticipatory of insulin needs, rather than reactive to hyperglycemia. Nonetheless, unexpected hyperglycemic excursions are common, and the use of correction insulin remains a pervasive and arguably logical practice. If correction insulin is used, it should be ordered as a separate step after considering basal and nutrition insulin needs. The doses of scheduled insulin should be adjusted regularly if correction insulin is consistently being required. Ideally, the prescriber should choose a preformatted corrective insulin scale, based on the patient's insulin sensitivity (Appendix 2). There should be a prompt to use the same type of insulin that is being used for nutritional insulin, and there should be instructions that this insulin is given in addition to the basal and nutritional insulin to correct for hyperglycemia. Nocturnal correction‐dose scales should be reduced in the eating patient.
Even after limiting insulin regimens to those in Table 2, multidisciplinary glycemic control teams are still left with several options within these SHM‐preferred regimens. We recommend that your team choose a single, institutionally‐preferred basal‐nutritional‐correction insulin combination for each situation.
Choosing one preferred option for these situations is advantageous because:
-
You can communicate preferred regimens more simply and succinctly to all staff.
-
You eliminate all inappropriate choices for insulin regimens for that situation, as well as some other less preferred, but acceptable choices.
-
You can encourage regimens that are most economical (by promoting the insulin regimens that reflect your hospital formulary choices).
-
Staff members can become very familiar with a few regimens, instead of being confused by a multitude of them. They can identify variations from your preferred choices and target these patients for extra scrutiny and actions should they fail to meet glycemic targets.
Although virtually every institution can provide specific guidance on insulin management in a protocol, there are tradeoffs inherent in how restrictive you can be in pushing these preferred choices in your order sets. Should you eliminate alternate basal or nutritional insulin choices from your order sets? As you integrate more and more of your preferred algorithm and regimens into your order set, you will gain incremental improvement in the standardization of inpatient insulin management. However, you reduce not only variability in ordering, but also the choices available to your prescribers and patients, and in effect you are pushing the providers to use an insulin regimen that often differs from the patient's outpatient regimen. If your institution is not yet ready to go with a single preferred insulin, simply listing your preferred insulin first with the annotation preferred can be enough to increase the use of the preferred insulin.
We endorse building the most protocol‐driven, proscriptive, insulin order set that the Glycemic Control Steering Committee believes their medical staff will accept. There are some caveats to this endorsement. First, there must be extra efforts on the backend of the admission, to ensure that the antihyperglycemic regimen is tailored to the unique needs of the patient (this is discussed further below). Second, a protocol‐driven approach is not a substitute for a good educational program for health care providers or well‐informed clinical judgment. Education should reinforce major concepts driving the protocol and should also highlight exceptions to the rule. Variance from the protocol endorsed choices should be allowed (and even encouraged) when the variance is driven by patient factors (as opposed to provider whim). Learning from this variance is a key concept in refining protocols. Education ideally should not be limited to only protocol‐endorsed choices, as staff should be familiar with the full range of antihyperglycemia regimens seen in inpatient and outpatient settings.
Special Situations
Most of the preferred regimens for different situations are outlined in Table 2 in a straightforward manner, and can be depicted in your protocols and order sets in the same way. Some conditions have enough complexity, however, that you will have difficulty placing all of the details into your one‐page protocol and order set. Details should be placed on your more detailed protocol, and educational programs should include the topics outlined below. Although insulin infusion is often the option that would provide the most reliable and expedient control of hyperglycemia in these special situations, it is an option not available in many noncritical care settings. Therefore, the discussion is limited to subcutaneous insulin control regimens.
Patient on Continuous Tube Feeding
The SHM GCTF endorses glargine or detemir as the basal insulin of choice for this setting. The nutritional and correction insulin of choice is either an RAA‐I every 4 hours (q4h), or regular insulin every 6 hours (q6h). We endorse this choice because it retains the basal‐nutritional‐correction dose concept, generally allows for continued basal insulin use if the tube feedings become interrupted, and is amenable to building a consistent institutional protocol.
There are some important caveats to this recommendation. First, realize that almost any regimen that provides a stable insulin supply would be acceptable, and many institutions will use glargine or detemir to cover both basal and nutritional needs. The downside to using large boluses of long‐acting insulin in this clinical situation is that any unexpected interruption of the feedings will necessitate prolonged infusions of dextrate 10% solution (D10) to avoid hypoglycemia
Second, because of the glycemic load inherent in tube feedings, maintenance of glycemic control in the setting of enteral feeding may be best managed by providing a higher percentage of the TDD as nutritional insulin. In these cases, ratios of basal to nutritional insulin of 40:60, or even less basal insulin, may be appropriate.
Glucocorticoid Therapy
High‐dose glucocorticoids are strongly associated with increased insulin requirements. The degree of hyperglycemia induced by steroids varies significantly from patient to patient, and the pattern of hyperglycemia will vary depending on the pattern of steroid administration. The general principle to keep in mind is that the hyperglycemia induced by a steroid dose will peak 8‐12 hours after it is given, so insulin regimens to address this should take this effect into account. For example, giving a long‐acting basal insulin like glargine to accommodate the hyperglycemic effect of a steroid bolus given in the morning would be inappropriate because the steroid effect would wane and then disappear overnight, leading to insulin‐induced hypoglycemia. NPH insulin can be ideal in this setting, either by itself, or by layering it on top of an existing regimen.
Another caveat: glucocorticoids exert their predominate effect on insulin sensitivity in muscle (as opposed to the liver), and as a result, have their most notable effect on postprandial glucose. For this reason, the best insulin regimens for this situation may use proportionally less basal insulin and more nutritional insulin. One common regimen calls for keeping the basal insulin dose the same as the preglucocorticoid dose, while escalating the RAA insulin dose at lunch and dinner.
Given the complexities of covering steroid‐induced hyperglycemia and its high prevalence in certain populations (such as transplantation patients and patients undergoing chemotherapy), this would be an excellent area on which to focus expertise. Examples include routine endocrinology consultation, intervention by a special glycemic control team, or incorporating routine glucose monitoring and triggers for initiating insulin infusion into the protocols for chemotherapy and transplantation patients.
Regiment the Management of Hypoglycemia
Hypoglycemia is defined by the ADA as a blood glucose of 70 mg/dL or less, based on the physiologic changes that can occur at this glucose level, even in subjectively asymptomatic patients.3 Protocols for management of hypoglycemia should be linked to your diabetes/hyperglycemia protocols. There are many hypoglycemia protocols available for review in the SHM Glycemic Control Resource Room and Glycemic Control Implementation Guide.10 Some common themes for effective implementation stand out. First, the protocols need to walk the balance between simplicity of use, and the need to provide instructions that will provide guidance in a variety of patient situations. Second, the protocols need to be nurse driven, so that nurses can initiate treatment without waiting for a physician order. Third, education and instruction regarding recognition of risk factors, and avoidance of hypoglycemia are needed to support a successful protocol. Importantly, any hypoglycemic event should lead to a reconsideration of the current anti‐hyperglycemic regimen so that future events can be prevented.
Plan for Discharge and Provide Guidance for the Transition
Your institution should have policies and procedures outlining all the steps needed to complete the important transition out of the hospital. At a minimum, this planning should include adequate education (including a learner assessment), appropriate follow‐up, referral to community resources, and a discharge glycemic control regimen that is tailored to the educational, financial, and motivational profile of a patient. The more your inpatient insulin management is driven by protocol, the more likely it is the patient will be on an inpatient treatment plan that differs from their outpatient regimen; therefore, it is even more important to plan this transition carefully and reliably.
Communicating the accurate hyperglycemia related diagnosis and related problems to the primary care provider is important for good care, perhaps even more so for patients who had hyperglycemia while hospitalized without a prior diagnosis of diabetes. Some centers place a prompt for hyperglycemia related diagnosis in the order set and/or discharge paperwork, to remind the clinician to convey the diagnosis to the primary provider, and to encourage more complete documentation. Improved documentation can also improve the business case for glycemic control, along with other strategies outlined elsewhere in this supplement.13
Transitions in care (including transitions out of the hospital and off of infusion insulin) are discussed in more detail14, 15 elsewhere in this supplement. The principles outlined in these references should be incorporated into your institutional protocol. Briefly, not all patients require or are capable of intensive basal‐bolus regimens upon discharge. The HbA1c can be very valuable in arriving at the optimal outpatient regimen.14 The capacities and preferences of the patient and the context of his or her outpatient care environment (including the preferences of the primary care provider) must be taken into consideration as an outpatient management program is planned.
PULLING IT ALL TOGETHER: MAKE SURE YOUR PROTOCOL/ORDER SET IS EASY TO USE AND WIDELY UTILIZED
When standardizing hospital management of diabetes and hyperglycemia, we recommend building the full protocol first, then crystallizing the protocol into a one‐page summary that can be widely disseminated. The protocol guidance is then incorporated into the order set and nursing medical administration record (MAR). Again, we recommend the most proscriptive and protocol‐driven order set feasible within the constraints of medical staff support. The example order set in Appendix 2 illustrates this approach along with other desirable features:
-
Check‐box simplicity on when to order appropriate glucose monitoring.
-
Prompt for the proper hyperglycemia‐related diagnosis.
-
Prompts to document diagnosis and to order HbA1c level.
-
Use of encouraged insulin terminology: basal, prandial (or nutritional), and correction. Language is a powerful thing, and just getting staff to use these terms goes a long way toward the more physiologic prescribing of insulin.
-
Statement/reminder of a glycemic goal.
-
Prompts and contact information for appropriate consultation.
-
Elimination of unapproved abbreviations (such as U for units).
-
Stating both generic and brand names of insulin preparations.
-
Important timing cues for administration of insulin.
-
Several correction‐dose scales suitable for different insulin sensitivities. One size does NOT fit all.
-
Incorporation of a simple hypoglycemia protocol into the order set.
-
Insulin dosing guidelines available at the point of care (in this case, on the back of the order set).
Additional nursing‐specific cues (such as an admonition to never mix glargine insulin with other types of insulin) can also be included in the MAR whenever glargine is ordered.
Once you have protocols and order sets to guide providers, you need to assure that they are used for the majority of hyperglycemic patients. Educational programs should introduce your interventions and the rationale for them. In order to make your method the default method of care, your team should survey all preprinted or CPOE insulin order sets of your institution. A review of postoperative, transfer, and admission order sets that all services use may reveal a half‐dozen or more embedded sliding‐scale insulin order sets that should be removed, with prompts to use the standardized insulin order set being placed in their stead.
Computerized order sets present both challenges and opportunities. Wording limitations and the scrolling nature can make concepts less clear, yet there is a capability for incorporating a hierarchical structure that allows for guiding the user through a more algorithmic approach. There is also a capacity to provide assistance with dosing calculations that do not exist in the paper world. Education remains of key importance for both methods.
MONITOR THE USE AND EFFECTIVENESS OF YOUR PROTOCOLS AND ORDER SETS
Creating and implementing protocols, order sets, and other tools is not the end of the journey to improve care. It is important to monitor order set utilization, insulin use patterns, and parameters measuring glycemic control and hypoglycemia, as outlined in more detail in another article in this supplement.16 In addition to summary data every month or so, we recommend daily reports that spur action in near real time. Triggers such as uncontrolled hyperglycemia, markedly elevated HbA1c levels, and nonphysiologic insulin regimens should initiate consultation, extra diabetes education, or referral to a glucose control team. If appropriate consultation is not readily available, the glycemic control steering group should lobby the administration to bolster this capability. Qualitative feedback from the frontline caregivers, as well as this quantitative data, can assist the local glycemic control champions in designing even more effective protocols, order sets, focused educational efforts, and concurrent mitigation of suboptimal care.
CONCLUSION
Diabetes, hyperglycemia, and iatrogenic hypoglycemia are common and important conditions affecting the noncritically ill inpatient. Interventional trials to validate the recommended noncritical care unit glycemic targets are needed. Although there is a growing consensus on best practices to care for these patients, numerous barriers and the complexity of caring for inpatients hamper the reliability of best practice delivery. Institutional protocols and protocol driven subcutaneous insulin orders, when implemented with the strategies outlined here, can be the key to delivering these best practices more reliably.
Appendix
- American College of Endocrinology Task Force on Inpatient Diabetes and Metabolic Control.American College of Endocrinology Position Statement on Inpatient Diabetes and Metabolic Control.Endocr Pract.2004;10:77–82.
- ,,,,,,.Management of diabetes and hyperglycemia in hospitals.Diabetes Care.2004;27:553–591.
- American Diabetes Association.Standards of Medical Carein Diabetes‐2006.Diabetes Care.2006;29(suppl 1):s4–s42.
- ,,, et al.Randomized study of basal‐bolus insulin therapy in the inpatient management of patients with type 2 diabetes (RABBIT 2 Trial).Diabetes Care.2007;30:2181–2186.
- ,,,,.Inpatient management of diabetes and hyperglycemia among general medicine patients at a large teaching hospital.J Hosp Med.2006;1:145–150.
- ,,, et al.Diabetes care in the hospital: is there clinical inertia?J Hosp Med.2006;1:151–160.
- ,,, et al.Diabetes care in hospitalized non‐critically ill patients: more evidence for clinical inertia and negative therapeutic momentum.JHosp Med.2007;2:203–211.
- Inpatient Diabetes and Glycemic Control: A Call to Action Conference. Position Statement. AACE, February2006. Available at: http://www.aace.com/meetings/consensus/IIDC/IDGC0207.pdf. Accessed October, 2006.
- Proceedings of the American College of Endocrinology and American Diabetes Association Consensus Conference, Washington, DC, January 30–31, 2006. Endocr Pract.2006; 12(suppl 3):3–13.
- Society of Hospital Medicine Glycemic Control Task Force. Implementation Guide: Improving Glycemic Control, Preventing Hypoglycemia, and Optimizing Care of the Inpatient with Hyperglycemia and Diabetes. Published January 2007 on the Society of Hospital Medicine website. Available at: http://www.hospitalmedicine.org. Accessed August,2007.
- .Management of hyperglycemia in the hospital setting.N Engl J Med.2006;355:1903–1911.
- ,,,,.Management of diabetes and hyperglycemia in the hospital: a practical guide to subcutaneous insulin use in the non‐critically ill adult patient.J Hosp Med.2008;3(5):S17–S28.
- ,.Practical strategies for developing the business case for hospital glycemic control teams.J Hosp Med2008;3(5):S76–S83.
- ,,,.Bridge over troubled waters: safe and effective transitions of the inpatient with hyperglycemia.J Hosp Med.2008;3(5):S55–S65.
- ,,,.Designing and implementing insulin infusion protocols and order sets.J Hosp Med.2008;3(5):S42–S54.
- ,,,,,.SHM Glycemic Control Task Force summary: practical recommendations for assessing the impact of glycemic control efforts.J Hosp Med.2008;3(5):S66–S75.
- American College of Endocrinology Task Force on Inpatient Diabetes and Metabolic Control.American College of Endocrinology Position Statement on Inpatient Diabetes and Metabolic Control.Endocr Pract.2004;10:77–82.
- ,,,,,,.Management of diabetes and hyperglycemia in hospitals.Diabetes Care.2004;27:553–591.
- American Diabetes Association.Standards of Medical Carein Diabetes‐2006.Diabetes Care.2006;29(suppl 1):s4–s42.
- ,,, et al.Randomized study of basal‐bolus insulin therapy in the inpatient management of patients with type 2 diabetes (RABBIT 2 Trial).Diabetes Care.2007;30:2181–2186.
- ,,,,.Inpatient management of diabetes and hyperglycemia among general medicine patients at a large teaching hospital.J Hosp Med.2006;1:145–150.
- ,,, et al.Diabetes care in the hospital: is there clinical inertia?J Hosp Med.2006;1:151–160.
- ,,, et al.Diabetes care in hospitalized non‐critically ill patients: more evidence for clinical inertia and negative therapeutic momentum.JHosp Med.2007;2:203–211.
- Inpatient Diabetes and Glycemic Control: A Call to Action Conference. Position Statement. AACE, February2006. Available at: http://www.aace.com/meetings/consensus/IIDC/IDGC0207.pdf. Accessed October, 2006.
- Proceedings of the American College of Endocrinology and American Diabetes Association Consensus Conference, Washington, DC, January 30–31, 2006. Endocr Pract.2006; 12(suppl 3):3–13.
- Society of Hospital Medicine Glycemic Control Task Force. Implementation Guide: Improving Glycemic Control, Preventing Hypoglycemia, and Optimizing Care of the Inpatient with Hyperglycemia and Diabetes. Published January 2007 on the Society of Hospital Medicine website. Available at: http://www.hospitalmedicine.org. Accessed August,2007.
- .Management of hyperglycemia in the hospital setting.N Engl J Med.2006;355:1903–1911.
- ,,,,.Management of diabetes and hyperglycemia in the hospital: a practical guide to subcutaneous insulin use in the non‐critically ill adult patient.J Hosp Med.2008;3(5):S17–S28.
- ,.Practical strategies for developing the business case for hospital glycemic control teams.J Hosp Med2008;3(5):S76–S83.
- ,,,.Bridge over troubled waters: safe and effective transitions of the inpatient with hyperglycemia.J Hosp Med.2008;3(5):S55–S65.
- ,,,.Designing and implementing insulin infusion protocols and order sets.J Hosp Med.2008;3(5):S42–S54.
- ,,,,,.SHM Glycemic Control Task Force summary: practical recommendations for assessing the impact of glycemic control efforts.J Hosp Med.2008;3(5):S66–S75.