User login
All together now: Impact of a regionalization and bedside rounding initiative on the efficiency and inclusiveness of clinical rounds
Attending rounds at academic medical centers are often disconnected from patients and non-physician care team members. Time spent bedside is consistently less than one third of total rounding time, with observational studies reporting a range of 9% to 33% over the past several decades.1-8 Rounds are often conducted outside patient rooms, denying patients, families, and nurses the opportunity to participate and offer valuable insights. Lack of bedside rounds thus limits patient and family engagement, patient input into the care plan, teaching of the physical examination, and communication and collaboration with nurses. In one study, physicians and nurses on rounds engaged in interprofessional communication in only 12% of patient cases.1 Studies have found interdisciplinary bedside rounds have several benefits, including subjectively improved communication and teamwork between physicians and nurses; increased patient satisfaction, including feeling more cared for by the medical team; and decreased length of stay and costs of care.2-10
However, there are many barriers to conducting interdisciplinary bedside rounds at large academic medical centers. Patients cared for by a single medical team are often geographically dispersed to several nursing units, and nurses are unable to predict when physicians will round on their patients. This situation limits nursing involvement on rounds and keeps doctors and nurses isolated from each other.2 Regionalization of care teams reduces this fragmentation by facilitating more interaction among doctors, patients, families, and nursing staff.
There are few data on how regionalized patients and interdisciplinary bedside rounds affect rounding time and the nature of rounds. This information is needed to understand how these structural changes mediate their effects, whether other steps are required to optimize outcomes, and how to maximize efficiency. We used time-motion analysis (TMA) to investigate how regionalization of medical teams, encouragement of bedside rounding, and systematic inclusion of nurses on ward rounds affect amount of time spent with patients, nursing presence on rounds, and total rounding time.
METHODS
Setting
This prospective interventional study, approved by the Institutional Review Board of Partners HealthCare, was conducted on the general medical wards at Brigham and Women’s Hospital, an academic 793-bed tertiary-care center in Boston, Massachusetts. Housestaff teams consist of 1 attending, 1 resident, and 2 interns with or without a medical student. Before June 20, 2013, daily rounds on medical inpatients were conducted largely on the patient unit but outside patient rooms. After completing most of a rounding discussion outside a patient’s room, the team might walk in to examine or speak with the patient. A typical medical team had patients dispersed over 7 medical units on average, and over as many as 13. As nurses were unit based, they did not consistently participate in rounds.
Intervention
In June 2013, as part of a general medical service care redesign initiative, the general medical teams were regionalized to specific inpatient units. The goal was to have teams admit patients predominantly to the team’s designated unit and to have all patients on a unit be cared for by the unit’s assigned team as often as possible, with an 85% goal for both. Toward those ends, the admitting structure was changed from a traditional 4-day call cycle to daily admitting for all teams, based on each unit’s bed availability.11
Teams were also expected to conduct rounds with nurses, and a system for facilitating these rounds was established. As physician and nurse care teams were now geographically co-located, it became possible for residents and nurses to check a rounding sheet for the planned patient rounding order, which had been set by the resident and nurse-in-charge before rounds. No more than about 5 minutes was needed to prepare each day’s order. The rounding sheet prioritized sick patients, newly admitted patients, and planned morning discharges, but patients were also always grouped by nurse. For example, the physician team rounded with the first nurse on all 3 of a nurse’s patients, and then proceeded to the next group of 3 patients with the next nurse, until all patients were seen.
Teams were encouraged to conduct patient- and family-centered rounds exclusively at bedside, except when bedside rounding was thought to be detrimental to a patient (eg, one with delirium). After an intern’s bedside presentation, which included a brief summary and details about overnight events and vital signs, the concerns of the patient, family, and nurse were shared, a focused physical examination performed, relevant data (eg, laboratory test results and imaging studies) reviewed, and the day’s plan formulated. The entire team, including the attending, was expected to have read new patients’ admission notes before rounds. Bedside rounds could thus be focused more on patient assessment and patient/family engagement and less on data transfer.
Several actions were taken to facilitate these changes. Residents, attendings, nurses, and other interdisciplinary team members participated in a series of focus groups and conferences to define workflows and share best practices for patient- and family-centered bedside rounds. Tips on bedside rounding were included in a general medicine rotation guidebook made available to residents and attendings. At the beginning of each post-intervention general medicine rotation, attendings and residents attended brief orientation sessions to review the new daily schedule, have interdisciplinary huddles, and share expectations for patient- and family-centered bedside rounds. On the general medicine units, new medical directors were hired to partner with existing nursing directors to support adoption of the workflows. Last, an interdisciplinary leadership team was formed to support the care redesign efforts. This team started meeting every 2 weeks.
Study Design
We used a pre–post analysis to study the effects of care redesign. Analysis was performed at the same time of year for 2 consecutive years to control for the stage of training and experience of the housestaff. TMA was performed by trained medical students using computer tablets linked to a customized Microsoft Access database form (Redmond, Washington). The form and the database were designed with specific buttons that, when pressed, recorded the time of particular events, such as the coming and going of each participant, the location of rounds, and the beginning and the end of rounding encounters with a patient. One research assistant using an Access entry form was able to dynamically track all events in real time, as they occurred. We collected data on 4 teams at baseline and 5 teams after the intervention. Each of the 4 baseline teams was followed for 4 consecutive weekdays—16 rounds total, April-June 2013—to capture the 4-day call cycle. Each of the 5 post-intervention teams was followed for 5 consecutive weekdays—25 rounds total, April–June 2014—to capture the 5-day cycle. (Because of technical difficulties, data from 1 rounding session were not captured.) For inclusion in the statistical analyses, TMA captured 166 on-service patients before the intervention and 304 afterward. Off-service patients, those with an attending other than the team attending, were excluded because their rounds were conducted separately.
We examined 2 primary outcomes, the proportion of time each clinical team member was present on rounds and the proportion of bedside rounding time. Secondary outcomes were round duration, rounding time per patient, and total non-patient time per rounding session (total rounding time minus total patient time).
Statistical Analysis
TMA data were organized in an Access database and analyzed with SAS Version 9.3 (SAS Institute, Cary, North Carolina). We analyzed the data by round session as well as by patient.
Data are presented as means with standard deviations, medians with interquartile ranges, and proportions, as appropriate. For analyses by round session, we used unadjusted linear regression; for patient-level analyses, we used general estimating equations to adjust for clustering of patients within each session; for nurse presence during any part of a round by patient, we used a χ2 test. Total non-patient time per round session was compared with use of patient-clustered general estimating equations using a γ distribution to account for the non-normality of the data.
RESULTS
Patient and Care Team Characteristics
Over the first year of the initiative, 85% of a team’s patients were on their assigned unit, and 87% of a unit’s patients were with the assigned team. Census numbers were 10.4 patients per general medicine team in April-June 2013 and 12.7 patients per team in April-June 2014, a 22% increase after care redesign. There were no statistically significant differences in patient characteristics, including age, sex, race, language, admission source, and comorbidity measure (Elixhauser score), between the pre-intervention and post-intervention study periods, except for a slightly higher proportion of patients admitted from home and fewer patients admitted directly from clinic (Table 1).
Primary Outcomes
Mean proportion of time the nurse was present on rounds per round session increased significantly (P < 0.001), from 24.1% to 67.8% (Figure 1A, Table 2). For individual patient encounters, the increased overall nursing presence was attributable to having more nurses on rounds and having nurses present for a larger proportion of individual rounding encounters (Figure 1B, Table 2). Nurses were present for at least some part of rounds for 53% of patients before the intervention and 93% afterward (P < 0.001). Mean proportion of round time by each of the 2 interns on each team decreased from 59.6% to 49.6% (P = 0.007).
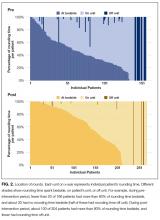
Total bedside rounding time increased significantly ( P < 0.001), from 39.9% before the intervention to 55.8% afterward (Table 2). Meanwhile, percentage of rounding time spent on the unit but outside patient rooms decreased significantly ( P = 0.004), from 55.2% to 42.2%, as did rounding time on a unit completely different from the patient’s (4.9% before intervention, 2.0% afterward; P = 0.03). Again, patient-level results were similar (Figure 2, Table 2), but the decreased time spent on the unit, outside the patient rooms, was not significant.
Secondary Outcomes
Total rounding time decreased significantly, from a mean of 182 minutes (3.0 hours) at baseline to a mean of 146 minutes (2.4 hours) after the intervention, despite the higher post-intervention census. (When adjusted for patient census, the difference increased from 35.5 to 53.8 minutes; Table 2.) Mean rounding time per patient decreased significantly, from 14.7 minutes at baseline to 10.5 minutes after the intervention. For newly admitted patients, mean rounding time per patient decreased from 30.0 minutes before implementation to 16.3 minutes afterward. Mean rounding time also decreased, though much less, for subsequent-day patients (Table 2). For both new and existing patients, the decrease in rounding time largely was a reduction in time spent rounding outside patient rooms, with minimal impact on bedside time (Table 2). Mean time nurses were present during a patient’s rounds increased significantly, from 4.5 to 8.0 minutes (Table 2). Total nurse rounding time increased from 45.1 minutes per session to 98.8 minutes. Rounding time not related to patient discussion or evaluation decreased from 22.7 minutes per session to 13.3 minutes ( P = 0.003).
DISCUSSION
TMA of our care redesign initiative showed that this multipronged intervention, which included team regionalization, encouragement of bedside rounding with nurses, call structure changes, and attendings’ reading of admission notes before rounds, resulted in an increased proportion of rounding time spent with patients and an increased proportion of time nurses were present on rounds. Secondarily, round duration decreased even as patient census increased.
Regionalized teams have been found to improve interdisciplinary communication.1 The present study elaborates on that finding by demonstrating a dramatic increase in nursing presence on rounds, likely resulting from the unit’s use of rounding schedules and nurses’ prioritization of rounding orders, both of which were made possible by geographic co-localization. Other research has noted that one of the most significant barriers to interdisciplinary rounds is difficulty coordinating the start times of physician/nurse bedside rounding encounters. The system we have studied directly addresses this difficulty.9 Of note, nursing presence on rounds is necessary but not sufficient for true physician–nurse collaboration and effective communication,1 as reflected in a separate study of the intervention showing no significant difference in the concordance of the patient care plan between nurses and physicians before and after regionalization.12 Additional interventions may be needed to ensure that communication during bedside rounds is effective.
Our regionalized teams spent a significantly higher proportion of rounding time bedside, likely because of a cultural shift in expectations and the increased convenience of seeing patients on the team’s unit. Nevertheless, bedside time was not 100%. Structural barriers (eg, patients off-unit for dialysis) and cultural barriers likely contributed to the less than full adoption of bedside rounding. As described previously, cultural barriers to bedside rounding include trainees’ anxiety about being questioned in front of patients, the desire to freely exchange academic ideas in a conference room, and attendings’ doubts about their bedside teaching ability.1,9,13 Bedside rounds provide an important opportunity to apply the principles of patient- and family-centered care, including promotion of dignity and respect, information sharing, and collaboration. Thus, overcoming the concerns of housestaff and attendings and helping them feel prepared for bedside rounds can benefit the patient experience. More attention should be given to these practices as these types of interventions are implemented at Brigham and Women’s Hospital and elsewhere.1,13-15
Another primary concern about interdisciplinary bedside rounding is the perception that it takes more time.9 Therefore, it was important for us to measure round duration as a balancing measure to be considered for our intervention. Fortunately, we found round duration decreased with regionalization and encouragement of bedside rounding. This decrease was driven largely by a significant decrease in mean rounding time per new patient, which may be attributable at least in part to setting expectations that attendings and residents will read admission notes before rounds and that interns will summarize rather than recount information from admission notes. However, we also found rounding time decreases for subsequent-day patients, suggesting an underlying time savings. Spending a larger proportion of time bedside may therefore result in more efficient rounds. Bedside presentations can reduce redundancies, such as discussing a patient’s case outside his or her room and subsequently walking in and going over much of the same information with the patient. Our model de-emphasizes data transfer in favor of discussion of care plans. There was also a decrease in non-patient time, likely reflecting reduced transit time for regionalized teams. This decrease aligns with a recent finding that bedside rounding was at least as efficient as rounding outside the room.16
Of note, though a larger percentage of time was spent bedside after implementation of the care redesign, the absolute amount of bedside time did not change significantly. Our data showed that, even with shorter rounds, the same amount of absolute time can be spent bedside, face to face with the patient, by increasing the proportion of bedside rounding time. In other words, teams on average did not spend more time with patients, though the content and the structure of those encounters may have changed. This finding may be attributable to eliminating redundancy, forgoing the outside-the-room discussion, and thus the largest time reductions were realized there. In addition, teams incompletely adopted beside rounds, as reflected in the data. We expect that, with more complete adoption, an even larger proportion of time will be spent bedside, and absolute time bedside might increase as a result.
An unexpected result of the care redesign was that interns’ proportion of rounding time decreased after the intervention. This decrease most likely is attributable to interns’ being less likely to participate in rounds for a co-intern’s patient, and to their staying outside that patient’s room to give themselves more time to advance the care of their own patients. Before the intervention, when more rounding time was spent outside patient rooms, interns were more likely to join rounds for their co-intern’s patients because they could easily break away, as needed, to continue care of their own patients. The resident is now encouraged to use the morning huddle to identify which patients likely have the most educational value, and both interns are expected to join the bedside rounds for these patients.
This study had a few limitations. First, the pre–post design made it difficult to exclude the possibility that other temporal changes may have affected outcomes, though we did account for time-of-year effects by aligning our data-collection phases. In addition, the authors, including the director of the general medical service, are unaware of any co-interventions during the study period. Second, the multipronged intervention included care team regionalization, encouragement of bedside rounding with nurses, call structure changes (from 4 days to daily admitting), and attendings’ reading of admission notes before rounds. Thus, parsing which component(s) contributed to the results was difficult, though all the changes instituted likely were necessary for system redesign. For example, regionalization of clinicians to unit-based teams was made possible by switching to a daily admitting system.
Time that team members spent preparing for rounds was not recorded before or after the intervention. Thus, the decrease in total rounding time could have been accompanied by an increase in time spent preparing for rounds. However, admission notes were available in our electronic medical record before and after the intervention, and most residents and attendings were already reading them pre-intervention. After the intervention, pre-round note reading was more clearly defined as an expectation, and we were able to set the expectation that interns should use their presentations to summarize rather than recount information. In addition, in the post-intervention period, we did not include time spent preparing rounding orders; as already noted, however, preparation took only 5 minutes per day. Also, we did not analyze the content or the quality of the discussion on rounds, but simply recorded who was present where and when. Regarding the effect of the intervention on patient care, results were mixed. As reported in 2016, we saw no difference in frequency of adverse events with this intervention.12 However, a more sensitive measure of adverse events—used in a study on handoffs—showed our regionalization efforts had an additive effect on reducing overnight adverse events.17Researchers should now focus on the effects of care redesign on clinical outcomes, interdisciplinary care team communication, patient engagement and satisfaction, provider opinions of communication, workflow, patient care, and housestaff education. Our methodology can be used as a model to link structure, process, and outcome related to rounds and thereby better understand how best to optimize patient care and efficiency. Additional studies are needed to analyze the content of rounds and their association with patient and educational outcomes. Last, it will be important to conduct a study to see if the effects we have identified can be sustained. Such a study is already under way.
In conclusion, creating regionalized care teams and encouraging focused bedside rounds increased the proportion of bedside time and the presence of nurses on rounds. Rounds were shorter despite higher patient census. TMA revealed that regionalized care teams and bedside rounding at a large academic hospital are feasible, and are useful in establishing the necessary structures for increasing physician–nurse and provider–patient interactions.
Acknowledgments
The authors acknowledge Dr. Stan Ashley, Dr. Jacqueline Somerville, and Sheila Harris for their support of the regionalization initiative.
Disclosures
Dr. Schnipper received funding from Sanofi-aventis to conduct an investigator-initiated study to implement and evaluate a multi-faceted intervention to improve transitions of care in patients discharged home on insulin. The study was also supported by funding from the Marshall A. Wolf Medical Education Fund, Brigham and Women’s Hospital, and Dr. Stan Ashley, Chief Medical Officer, Brigham and Women’s Hospital. Some of the content of this article was orally presented at the annual meeting of the Society of Hospital Medicine; March 29-April 1, 2015; National Harbor, MD.
1. Crumlish CM, Yialamas MA, McMahon GT. Quantification of bedside teaching by an academic hospitalist group. J Hosp Med. 2009;4(5):304-307. PubMed
2. Gonzalo JD, Masters PA, Simons RJ, Chuang CH. Attending rounds and bedside case presentations: medical student and medicine resident experiences and attitudes. Teach Learn Med. 2009;21(2):105-110. PubMed
3. Elliot DL, Hickam DH. Attending rounds on in-patient units: differences between medical and non-medical services. Med Educ. 1993;27(6):503-508. PubMed
4. Payson HE, Barchas JD. A time study of medical teaching rounds. N Engl J Med. 1965;273(27):1468-1471. PubMed
5. Tremonti LP, Biddle WB. Teaching behaviors of residents and faculty members. J Med Educ. 1982;57(11):854-859. PubMed
6. Miller M, Johnson B, Greene HL, Baier M, Nowlin S. An observational study of attending rounds. J Gen Intern Med. 1992;7(6):646-648. PubMed
7. Collins GF, Cassie JM, Daggett CJ. The role of the attending physician in clinical training. J Med Educ. 1978;53(5):429-431. PubMed
8. Ward DR, Ghali WA, Graham A, Lemaire JB. A real-time locating system observes physician time-motion patterns during walk-rounds: a pilot study. BMC Med Educ. 2014;14:37. PubMed
9. Gonzalo JD, Kuperman E, Lehman E, Haidet P. Bedside interprofessional rounds: perceptions of benefits and barriers by internal medicine nursing staff, attending physicians, and housestaff physicians. J Hosp Med. 2014;9(10):646-651. PubMed
10. Stickrath C, Noble M, Prochazka A, et al. Attending rounds in the current era: what is and is not happening. JAMA Intern Med. 2013;173(12):1084-1089. PubMed
11. Boxer R, Vitale M, Gershanik EF, et al. 5th time’s a charm: creation of unit-based care teams in a high occupancy hospital [abstract]. J Hosp Med. 2015;10(suppl 2).
12. Mueller SK, Schnipper JL, Giannelli K, Roy CL, Boxer R. Impact of regionalized care on concordance of plan and preventable adverse events on general medicine services. J Hosp Med. 2016;11(9):620-627. PubMed
13. Chauke HL, Pattinson RC. Ward rounds—bedside or conference room? S Afr Med J. 2006;96(5):398-400. PubMed
14. Wang-Cheng RM, Barnas GP, Sigmann P, Riendl PA, Young MJ. Bedside case presentations: why patients like them but learners don’t. J Gen Intern Med. 1989;4(4):284-287. PubMed
15. Lehmann LS, Brancati FL, Chen MC, Roter D, Dobs AS. The effect of bedside case presentations on patients’ perceptions of their medical care. N Engl J Med. 1997;336(16):1150-1155. PubMed
16. Gonzalo JD, Chuang CH, Huang G, Smith C. The return of bedside rounds: an educational intervention. J Gen Intern Med. 2010;25(8):792-798. PubMed
17. Mueller SK, Yoon C, Schnipper JL. Association of a web-based handoff tool with rates of medical errors. JAMA Intern Med. 2016;176(9):1400-1402. PubMed
Attending rounds at academic medical centers are often disconnected from patients and non-physician care team members. Time spent bedside is consistently less than one third of total rounding time, with observational studies reporting a range of 9% to 33% over the past several decades.1-8 Rounds are often conducted outside patient rooms, denying patients, families, and nurses the opportunity to participate and offer valuable insights. Lack of bedside rounds thus limits patient and family engagement, patient input into the care plan, teaching of the physical examination, and communication and collaboration with nurses. In one study, physicians and nurses on rounds engaged in interprofessional communication in only 12% of patient cases.1 Studies have found interdisciplinary bedside rounds have several benefits, including subjectively improved communication and teamwork between physicians and nurses; increased patient satisfaction, including feeling more cared for by the medical team; and decreased length of stay and costs of care.2-10
However, there are many barriers to conducting interdisciplinary bedside rounds at large academic medical centers. Patients cared for by a single medical team are often geographically dispersed to several nursing units, and nurses are unable to predict when physicians will round on their patients. This situation limits nursing involvement on rounds and keeps doctors and nurses isolated from each other.2 Regionalization of care teams reduces this fragmentation by facilitating more interaction among doctors, patients, families, and nursing staff.
There are few data on how regionalized patients and interdisciplinary bedside rounds affect rounding time and the nature of rounds. This information is needed to understand how these structural changes mediate their effects, whether other steps are required to optimize outcomes, and how to maximize efficiency. We used time-motion analysis (TMA) to investigate how regionalization of medical teams, encouragement of bedside rounding, and systematic inclusion of nurses on ward rounds affect amount of time spent with patients, nursing presence on rounds, and total rounding time.
METHODS
Setting
This prospective interventional study, approved by the Institutional Review Board of Partners HealthCare, was conducted on the general medical wards at Brigham and Women’s Hospital, an academic 793-bed tertiary-care center in Boston, Massachusetts. Housestaff teams consist of 1 attending, 1 resident, and 2 interns with or without a medical student. Before June 20, 2013, daily rounds on medical inpatients were conducted largely on the patient unit but outside patient rooms. After completing most of a rounding discussion outside a patient’s room, the team might walk in to examine or speak with the patient. A typical medical team had patients dispersed over 7 medical units on average, and over as many as 13. As nurses were unit based, they did not consistently participate in rounds.
Intervention
In June 2013, as part of a general medical service care redesign initiative, the general medical teams were regionalized to specific inpatient units. The goal was to have teams admit patients predominantly to the team’s designated unit and to have all patients on a unit be cared for by the unit’s assigned team as often as possible, with an 85% goal for both. Toward those ends, the admitting structure was changed from a traditional 4-day call cycle to daily admitting for all teams, based on each unit’s bed availability.11
Teams were also expected to conduct rounds with nurses, and a system for facilitating these rounds was established. As physician and nurse care teams were now geographically co-located, it became possible for residents and nurses to check a rounding sheet for the planned patient rounding order, which had been set by the resident and nurse-in-charge before rounds. No more than about 5 minutes was needed to prepare each day’s order. The rounding sheet prioritized sick patients, newly admitted patients, and planned morning discharges, but patients were also always grouped by nurse. For example, the physician team rounded with the first nurse on all 3 of a nurse’s patients, and then proceeded to the next group of 3 patients with the next nurse, until all patients were seen.
Teams were encouraged to conduct patient- and family-centered rounds exclusively at bedside, except when bedside rounding was thought to be detrimental to a patient (eg, one with delirium). After an intern’s bedside presentation, which included a brief summary and details about overnight events and vital signs, the concerns of the patient, family, and nurse were shared, a focused physical examination performed, relevant data (eg, laboratory test results and imaging studies) reviewed, and the day’s plan formulated. The entire team, including the attending, was expected to have read new patients’ admission notes before rounds. Bedside rounds could thus be focused more on patient assessment and patient/family engagement and less on data transfer.
Several actions were taken to facilitate these changes. Residents, attendings, nurses, and other interdisciplinary team members participated in a series of focus groups and conferences to define workflows and share best practices for patient- and family-centered bedside rounds. Tips on bedside rounding were included in a general medicine rotation guidebook made available to residents and attendings. At the beginning of each post-intervention general medicine rotation, attendings and residents attended brief orientation sessions to review the new daily schedule, have interdisciplinary huddles, and share expectations for patient- and family-centered bedside rounds. On the general medicine units, new medical directors were hired to partner with existing nursing directors to support adoption of the workflows. Last, an interdisciplinary leadership team was formed to support the care redesign efforts. This team started meeting every 2 weeks.
Study Design
We used a pre–post analysis to study the effects of care redesign. Analysis was performed at the same time of year for 2 consecutive years to control for the stage of training and experience of the housestaff. TMA was performed by trained medical students using computer tablets linked to a customized Microsoft Access database form (Redmond, Washington). The form and the database were designed with specific buttons that, when pressed, recorded the time of particular events, such as the coming and going of each participant, the location of rounds, and the beginning and the end of rounding encounters with a patient. One research assistant using an Access entry form was able to dynamically track all events in real time, as they occurred. We collected data on 4 teams at baseline and 5 teams after the intervention. Each of the 4 baseline teams was followed for 4 consecutive weekdays—16 rounds total, April-June 2013—to capture the 4-day call cycle. Each of the 5 post-intervention teams was followed for 5 consecutive weekdays—25 rounds total, April–June 2014—to capture the 5-day cycle. (Because of technical difficulties, data from 1 rounding session were not captured.) For inclusion in the statistical analyses, TMA captured 166 on-service patients before the intervention and 304 afterward. Off-service patients, those with an attending other than the team attending, were excluded because their rounds were conducted separately.
We examined 2 primary outcomes, the proportion of time each clinical team member was present on rounds and the proportion of bedside rounding time. Secondary outcomes were round duration, rounding time per patient, and total non-patient time per rounding session (total rounding time minus total patient time).
Statistical Analysis
TMA data were organized in an Access database and analyzed with SAS Version 9.3 (SAS Institute, Cary, North Carolina). We analyzed the data by round session as well as by patient.
Data are presented as means with standard deviations, medians with interquartile ranges, and proportions, as appropriate. For analyses by round session, we used unadjusted linear regression; for patient-level analyses, we used general estimating equations to adjust for clustering of patients within each session; for nurse presence during any part of a round by patient, we used a χ2 test. Total non-patient time per round session was compared with use of patient-clustered general estimating equations using a γ distribution to account for the non-normality of the data.
RESULTS
Patient and Care Team Characteristics
Over the first year of the initiative, 85% of a team’s patients were on their assigned unit, and 87% of a unit’s patients were with the assigned team. Census numbers were 10.4 patients per general medicine team in April-June 2013 and 12.7 patients per team in April-June 2014, a 22% increase after care redesign. There were no statistically significant differences in patient characteristics, including age, sex, race, language, admission source, and comorbidity measure (Elixhauser score), between the pre-intervention and post-intervention study periods, except for a slightly higher proportion of patients admitted from home and fewer patients admitted directly from clinic (Table 1).
Primary Outcomes
Mean proportion of time the nurse was present on rounds per round session increased significantly (P < 0.001), from 24.1% to 67.8% (Figure 1A, Table 2). For individual patient encounters, the increased overall nursing presence was attributable to having more nurses on rounds and having nurses present for a larger proportion of individual rounding encounters (Figure 1B, Table 2). Nurses were present for at least some part of rounds for 53% of patients before the intervention and 93% afterward (P < 0.001). Mean proportion of round time by each of the 2 interns on each team decreased from 59.6% to 49.6% (P = 0.007).
Total bedside rounding time increased significantly ( P < 0.001), from 39.9% before the intervention to 55.8% afterward (Table 2). Meanwhile, percentage of rounding time spent on the unit but outside patient rooms decreased significantly ( P = 0.004), from 55.2% to 42.2%, as did rounding time on a unit completely different from the patient’s (4.9% before intervention, 2.0% afterward; P = 0.03). Again, patient-level results were similar (Figure 2, Table 2), but the decreased time spent on the unit, outside the patient rooms, was not significant.
Secondary Outcomes
Total rounding time decreased significantly, from a mean of 182 minutes (3.0 hours) at baseline to a mean of 146 minutes (2.4 hours) after the intervention, despite the higher post-intervention census. (When adjusted for patient census, the difference increased from 35.5 to 53.8 minutes; Table 2.) Mean rounding time per patient decreased significantly, from 14.7 minutes at baseline to 10.5 minutes after the intervention. For newly admitted patients, mean rounding time per patient decreased from 30.0 minutes before implementation to 16.3 minutes afterward. Mean rounding time also decreased, though much less, for subsequent-day patients (Table 2). For both new and existing patients, the decrease in rounding time largely was a reduction in time spent rounding outside patient rooms, with minimal impact on bedside time (Table 2). Mean time nurses were present during a patient’s rounds increased significantly, from 4.5 to 8.0 minutes (Table 2). Total nurse rounding time increased from 45.1 minutes per session to 98.8 minutes. Rounding time not related to patient discussion or evaluation decreased from 22.7 minutes per session to 13.3 minutes ( P = 0.003).
DISCUSSION
TMA of our care redesign initiative showed that this multipronged intervention, which included team regionalization, encouragement of bedside rounding with nurses, call structure changes, and attendings’ reading of admission notes before rounds, resulted in an increased proportion of rounding time spent with patients and an increased proportion of time nurses were present on rounds. Secondarily, round duration decreased even as patient census increased.
Regionalized teams have been found to improve interdisciplinary communication.1 The present study elaborates on that finding by demonstrating a dramatic increase in nursing presence on rounds, likely resulting from the unit’s use of rounding schedules and nurses’ prioritization of rounding orders, both of which were made possible by geographic co-localization. Other research has noted that one of the most significant barriers to interdisciplinary rounds is difficulty coordinating the start times of physician/nurse bedside rounding encounters. The system we have studied directly addresses this difficulty.9 Of note, nursing presence on rounds is necessary but not sufficient for true physician–nurse collaboration and effective communication,1 as reflected in a separate study of the intervention showing no significant difference in the concordance of the patient care plan between nurses and physicians before and after regionalization.12 Additional interventions may be needed to ensure that communication during bedside rounds is effective.
Our regionalized teams spent a significantly higher proportion of rounding time bedside, likely because of a cultural shift in expectations and the increased convenience of seeing patients on the team’s unit. Nevertheless, bedside time was not 100%. Structural barriers (eg, patients off-unit for dialysis) and cultural barriers likely contributed to the less than full adoption of bedside rounding. As described previously, cultural barriers to bedside rounding include trainees’ anxiety about being questioned in front of patients, the desire to freely exchange academic ideas in a conference room, and attendings’ doubts about their bedside teaching ability.1,9,13 Bedside rounds provide an important opportunity to apply the principles of patient- and family-centered care, including promotion of dignity and respect, information sharing, and collaboration. Thus, overcoming the concerns of housestaff and attendings and helping them feel prepared for bedside rounds can benefit the patient experience. More attention should be given to these practices as these types of interventions are implemented at Brigham and Women’s Hospital and elsewhere.1,13-15
Another primary concern about interdisciplinary bedside rounding is the perception that it takes more time.9 Therefore, it was important for us to measure round duration as a balancing measure to be considered for our intervention. Fortunately, we found round duration decreased with regionalization and encouragement of bedside rounding. This decrease was driven largely by a significant decrease in mean rounding time per new patient, which may be attributable at least in part to setting expectations that attendings and residents will read admission notes before rounds and that interns will summarize rather than recount information from admission notes. However, we also found rounding time decreases for subsequent-day patients, suggesting an underlying time savings. Spending a larger proportion of time bedside may therefore result in more efficient rounds. Bedside presentations can reduce redundancies, such as discussing a patient’s case outside his or her room and subsequently walking in and going over much of the same information with the patient. Our model de-emphasizes data transfer in favor of discussion of care plans. There was also a decrease in non-patient time, likely reflecting reduced transit time for regionalized teams. This decrease aligns with a recent finding that bedside rounding was at least as efficient as rounding outside the room.16
Of note, though a larger percentage of time was spent bedside after implementation of the care redesign, the absolute amount of bedside time did not change significantly. Our data showed that, even with shorter rounds, the same amount of absolute time can be spent bedside, face to face with the patient, by increasing the proportion of bedside rounding time. In other words, teams on average did not spend more time with patients, though the content and the structure of those encounters may have changed. This finding may be attributable to eliminating redundancy, forgoing the outside-the-room discussion, and thus the largest time reductions were realized there. In addition, teams incompletely adopted beside rounds, as reflected in the data. We expect that, with more complete adoption, an even larger proportion of time will be spent bedside, and absolute time bedside might increase as a result.
An unexpected result of the care redesign was that interns’ proportion of rounding time decreased after the intervention. This decrease most likely is attributable to interns’ being less likely to participate in rounds for a co-intern’s patient, and to their staying outside that patient’s room to give themselves more time to advance the care of their own patients. Before the intervention, when more rounding time was spent outside patient rooms, interns were more likely to join rounds for their co-intern’s patients because they could easily break away, as needed, to continue care of their own patients. The resident is now encouraged to use the morning huddle to identify which patients likely have the most educational value, and both interns are expected to join the bedside rounds for these patients.
This study had a few limitations. First, the pre–post design made it difficult to exclude the possibility that other temporal changes may have affected outcomes, though we did account for time-of-year effects by aligning our data-collection phases. In addition, the authors, including the director of the general medical service, are unaware of any co-interventions during the study period. Second, the multipronged intervention included care team regionalization, encouragement of bedside rounding with nurses, call structure changes (from 4 days to daily admitting), and attendings’ reading of admission notes before rounds. Thus, parsing which component(s) contributed to the results was difficult, though all the changes instituted likely were necessary for system redesign. For example, regionalization of clinicians to unit-based teams was made possible by switching to a daily admitting system.
Time that team members spent preparing for rounds was not recorded before or after the intervention. Thus, the decrease in total rounding time could have been accompanied by an increase in time spent preparing for rounds. However, admission notes were available in our electronic medical record before and after the intervention, and most residents and attendings were already reading them pre-intervention. After the intervention, pre-round note reading was more clearly defined as an expectation, and we were able to set the expectation that interns should use their presentations to summarize rather than recount information. In addition, in the post-intervention period, we did not include time spent preparing rounding orders; as already noted, however, preparation took only 5 minutes per day. Also, we did not analyze the content or the quality of the discussion on rounds, but simply recorded who was present where and when. Regarding the effect of the intervention on patient care, results were mixed. As reported in 2016, we saw no difference in frequency of adverse events with this intervention.12 However, a more sensitive measure of adverse events—used in a study on handoffs—showed our regionalization efforts had an additive effect on reducing overnight adverse events.17Researchers should now focus on the effects of care redesign on clinical outcomes, interdisciplinary care team communication, patient engagement and satisfaction, provider opinions of communication, workflow, patient care, and housestaff education. Our methodology can be used as a model to link structure, process, and outcome related to rounds and thereby better understand how best to optimize patient care and efficiency. Additional studies are needed to analyze the content of rounds and their association with patient and educational outcomes. Last, it will be important to conduct a study to see if the effects we have identified can be sustained. Such a study is already under way.
In conclusion, creating regionalized care teams and encouraging focused bedside rounds increased the proportion of bedside time and the presence of nurses on rounds. Rounds were shorter despite higher patient census. TMA revealed that regionalized care teams and bedside rounding at a large academic hospital are feasible, and are useful in establishing the necessary structures for increasing physician–nurse and provider–patient interactions.
Acknowledgments
The authors acknowledge Dr. Stan Ashley, Dr. Jacqueline Somerville, and Sheila Harris for their support of the regionalization initiative.
Disclosures
Dr. Schnipper received funding from Sanofi-aventis to conduct an investigator-initiated study to implement and evaluate a multi-faceted intervention to improve transitions of care in patients discharged home on insulin. The study was also supported by funding from the Marshall A. Wolf Medical Education Fund, Brigham and Women’s Hospital, and Dr. Stan Ashley, Chief Medical Officer, Brigham and Women’s Hospital. Some of the content of this article was orally presented at the annual meeting of the Society of Hospital Medicine; March 29-April 1, 2015; National Harbor, MD.
Attending rounds at academic medical centers are often disconnected from patients and non-physician care team members. Time spent bedside is consistently less than one third of total rounding time, with observational studies reporting a range of 9% to 33% over the past several decades.1-8 Rounds are often conducted outside patient rooms, denying patients, families, and nurses the opportunity to participate and offer valuable insights. Lack of bedside rounds thus limits patient and family engagement, patient input into the care plan, teaching of the physical examination, and communication and collaboration with nurses. In one study, physicians and nurses on rounds engaged in interprofessional communication in only 12% of patient cases.1 Studies have found interdisciplinary bedside rounds have several benefits, including subjectively improved communication and teamwork between physicians and nurses; increased patient satisfaction, including feeling more cared for by the medical team; and decreased length of stay and costs of care.2-10
However, there are many barriers to conducting interdisciplinary bedside rounds at large academic medical centers. Patients cared for by a single medical team are often geographically dispersed to several nursing units, and nurses are unable to predict when physicians will round on their patients. This situation limits nursing involvement on rounds and keeps doctors and nurses isolated from each other.2 Regionalization of care teams reduces this fragmentation by facilitating more interaction among doctors, patients, families, and nursing staff.
There are few data on how regionalized patients and interdisciplinary bedside rounds affect rounding time and the nature of rounds. This information is needed to understand how these structural changes mediate their effects, whether other steps are required to optimize outcomes, and how to maximize efficiency. We used time-motion analysis (TMA) to investigate how regionalization of medical teams, encouragement of bedside rounding, and systematic inclusion of nurses on ward rounds affect amount of time spent with patients, nursing presence on rounds, and total rounding time.
METHODS
Setting
This prospective interventional study, approved by the Institutional Review Board of Partners HealthCare, was conducted on the general medical wards at Brigham and Women’s Hospital, an academic 793-bed tertiary-care center in Boston, Massachusetts. Housestaff teams consist of 1 attending, 1 resident, and 2 interns with or without a medical student. Before June 20, 2013, daily rounds on medical inpatients were conducted largely on the patient unit but outside patient rooms. After completing most of a rounding discussion outside a patient’s room, the team might walk in to examine or speak with the patient. A typical medical team had patients dispersed over 7 medical units on average, and over as many as 13. As nurses were unit based, they did not consistently participate in rounds.
Intervention
In June 2013, as part of a general medical service care redesign initiative, the general medical teams were regionalized to specific inpatient units. The goal was to have teams admit patients predominantly to the team’s designated unit and to have all patients on a unit be cared for by the unit’s assigned team as often as possible, with an 85% goal for both. Toward those ends, the admitting structure was changed from a traditional 4-day call cycle to daily admitting for all teams, based on each unit’s bed availability.11
Teams were also expected to conduct rounds with nurses, and a system for facilitating these rounds was established. As physician and nurse care teams were now geographically co-located, it became possible for residents and nurses to check a rounding sheet for the planned patient rounding order, which had been set by the resident and nurse-in-charge before rounds. No more than about 5 minutes was needed to prepare each day’s order. The rounding sheet prioritized sick patients, newly admitted patients, and planned morning discharges, but patients were also always grouped by nurse. For example, the physician team rounded with the first nurse on all 3 of a nurse’s patients, and then proceeded to the next group of 3 patients with the next nurse, until all patients were seen.
Teams were encouraged to conduct patient- and family-centered rounds exclusively at bedside, except when bedside rounding was thought to be detrimental to a patient (eg, one with delirium). After an intern’s bedside presentation, which included a brief summary and details about overnight events and vital signs, the concerns of the patient, family, and nurse were shared, a focused physical examination performed, relevant data (eg, laboratory test results and imaging studies) reviewed, and the day’s plan formulated. The entire team, including the attending, was expected to have read new patients’ admission notes before rounds. Bedside rounds could thus be focused more on patient assessment and patient/family engagement and less on data transfer.
Several actions were taken to facilitate these changes. Residents, attendings, nurses, and other interdisciplinary team members participated in a series of focus groups and conferences to define workflows and share best practices for patient- and family-centered bedside rounds. Tips on bedside rounding were included in a general medicine rotation guidebook made available to residents and attendings. At the beginning of each post-intervention general medicine rotation, attendings and residents attended brief orientation sessions to review the new daily schedule, have interdisciplinary huddles, and share expectations for patient- and family-centered bedside rounds. On the general medicine units, new medical directors were hired to partner with existing nursing directors to support adoption of the workflows. Last, an interdisciplinary leadership team was formed to support the care redesign efforts. This team started meeting every 2 weeks.
Study Design
We used a pre–post analysis to study the effects of care redesign. Analysis was performed at the same time of year for 2 consecutive years to control for the stage of training and experience of the housestaff. TMA was performed by trained medical students using computer tablets linked to a customized Microsoft Access database form (Redmond, Washington). The form and the database were designed with specific buttons that, when pressed, recorded the time of particular events, such as the coming and going of each participant, the location of rounds, and the beginning and the end of rounding encounters with a patient. One research assistant using an Access entry form was able to dynamically track all events in real time, as they occurred. We collected data on 4 teams at baseline and 5 teams after the intervention. Each of the 4 baseline teams was followed for 4 consecutive weekdays—16 rounds total, April-June 2013—to capture the 4-day call cycle. Each of the 5 post-intervention teams was followed for 5 consecutive weekdays—25 rounds total, April–June 2014—to capture the 5-day cycle. (Because of technical difficulties, data from 1 rounding session were not captured.) For inclusion in the statistical analyses, TMA captured 166 on-service patients before the intervention and 304 afterward. Off-service patients, those with an attending other than the team attending, were excluded because their rounds were conducted separately.
We examined 2 primary outcomes, the proportion of time each clinical team member was present on rounds and the proportion of bedside rounding time. Secondary outcomes were round duration, rounding time per patient, and total non-patient time per rounding session (total rounding time minus total patient time).
Statistical Analysis
TMA data were organized in an Access database and analyzed with SAS Version 9.3 (SAS Institute, Cary, North Carolina). We analyzed the data by round session as well as by patient.
Data are presented as means with standard deviations, medians with interquartile ranges, and proportions, as appropriate. For analyses by round session, we used unadjusted linear regression; for patient-level analyses, we used general estimating equations to adjust for clustering of patients within each session; for nurse presence during any part of a round by patient, we used a χ2 test. Total non-patient time per round session was compared with use of patient-clustered general estimating equations using a γ distribution to account for the non-normality of the data.
RESULTS
Patient and Care Team Characteristics
Over the first year of the initiative, 85% of a team’s patients were on their assigned unit, and 87% of a unit’s patients were with the assigned team. Census numbers were 10.4 patients per general medicine team in April-June 2013 and 12.7 patients per team in April-June 2014, a 22% increase after care redesign. There were no statistically significant differences in patient characteristics, including age, sex, race, language, admission source, and comorbidity measure (Elixhauser score), between the pre-intervention and post-intervention study periods, except for a slightly higher proportion of patients admitted from home and fewer patients admitted directly from clinic (Table 1).
Primary Outcomes
Mean proportion of time the nurse was present on rounds per round session increased significantly (P < 0.001), from 24.1% to 67.8% (Figure 1A, Table 2). For individual patient encounters, the increased overall nursing presence was attributable to having more nurses on rounds and having nurses present for a larger proportion of individual rounding encounters (Figure 1B, Table 2). Nurses were present for at least some part of rounds for 53% of patients before the intervention and 93% afterward (P < 0.001). Mean proportion of round time by each of the 2 interns on each team decreased from 59.6% to 49.6% (P = 0.007).
Total bedside rounding time increased significantly ( P < 0.001), from 39.9% before the intervention to 55.8% afterward (Table 2). Meanwhile, percentage of rounding time spent on the unit but outside patient rooms decreased significantly ( P = 0.004), from 55.2% to 42.2%, as did rounding time on a unit completely different from the patient’s (4.9% before intervention, 2.0% afterward; P = 0.03). Again, patient-level results were similar (Figure 2, Table 2), but the decreased time spent on the unit, outside the patient rooms, was not significant.
Secondary Outcomes
Total rounding time decreased significantly, from a mean of 182 minutes (3.0 hours) at baseline to a mean of 146 minutes (2.4 hours) after the intervention, despite the higher post-intervention census. (When adjusted for patient census, the difference increased from 35.5 to 53.8 minutes; Table 2.) Mean rounding time per patient decreased significantly, from 14.7 minutes at baseline to 10.5 minutes after the intervention. For newly admitted patients, mean rounding time per patient decreased from 30.0 minutes before implementation to 16.3 minutes afterward. Mean rounding time also decreased, though much less, for subsequent-day patients (Table 2). For both new and existing patients, the decrease in rounding time largely was a reduction in time spent rounding outside patient rooms, with minimal impact on bedside time (Table 2). Mean time nurses were present during a patient’s rounds increased significantly, from 4.5 to 8.0 minutes (Table 2). Total nurse rounding time increased from 45.1 minutes per session to 98.8 minutes. Rounding time not related to patient discussion or evaluation decreased from 22.7 minutes per session to 13.3 minutes ( P = 0.003).
DISCUSSION
TMA of our care redesign initiative showed that this multipronged intervention, which included team regionalization, encouragement of bedside rounding with nurses, call structure changes, and attendings’ reading of admission notes before rounds, resulted in an increased proportion of rounding time spent with patients and an increased proportion of time nurses were present on rounds. Secondarily, round duration decreased even as patient census increased.
Regionalized teams have been found to improve interdisciplinary communication.1 The present study elaborates on that finding by demonstrating a dramatic increase in nursing presence on rounds, likely resulting from the unit’s use of rounding schedules and nurses’ prioritization of rounding orders, both of which were made possible by geographic co-localization. Other research has noted that one of the most significant barriers to interdisciplinary rounds is difficulty coordinating the start times of physician/nurse bedside rounding encounters. The system we have studied directly addresses this difficulty.9 Of note, nursing presence on rounds is necessary but not sufficient for true physician–nurse collaboration and effective communication,1 as reflected in a separate study of the intervention showing no significant difference in the concordance of the patient care plan between nurses and physicians before and after regionalization.12 Additional interventions may be needed to ensure that communication during bedside rounds is effective.
Our regionalized teams spent a significantly higher proportion of rounding time bedside, likely because of a cultural shift in expectations and the increased convenience of seeing patients on the team’s unit. Nevertheless, bedside time was not 100%. Structural barriers (eg, patients off-unit for dialysis) and cultural barriers likely contributed to the less than full adoption of bedside rounding. As described previously, cultural barriers to bedside rounding include trainees’ anxiety about being questioned in front of patients, the desire to freely exchange academic ideas in a conference room, and attendings’ doubts about their bedside teaching ability.1,9,13 Bedside rounds provide an important opportunity to apply the principles of patient- and family-centered care, including promotion of dignity and respect, information sharing, and collaboration. Thus, overcoming the concerns of housestaff and attendings and helping them feel prepared for bedside rounds can benefit the patient experience. More attention should be given to these practices as these types of interventions are implemented at Brigham and Women’s Hospital and elsewhere.1,13-15
Another primary concern about interdisciplinary bedside rounding is the perception that it takes more time.9 Therefore, it was important for us to measure round duration as a balancing measure to be considered for our intervention. Fortunately, we found round duration decreased with regionalization and encouragement of bedside rounding. This decrease was driven largely by a significant decrease in mean rounding time per new patient, which may be attributable at least in part to setting expectations that attendings and residents will read admission notes before rounds and that interns will summarize rather than recount information from admission notes. However, we also found rounding time decreases for subsequent-day patients, suggesting an underlying time savings. Spending a larger proportion of time bedside may therefore result in more efficient rounds. Bedside presentations can reduce redundancies, such as discussing a patient’s case outside his or her room and subsequently walking in and going over much of the same information with the patient. Our model de-emphasizes data transfer in favor of discussion of care plans. There was also a decrease in non-patient time, likely reflecting reduced transit time for regionalized teams. This decrease aligns with a recent finding that bedside rounding was at least as efficient as rounding outside the room.16
Of note, though a larger percentage of time was spent bedside after implementation of the care redesign, the absolute amount of bedside time did not change significantly. Our data showed that, even with shorter rounds, the same amount of absolute time can be spent bedside, face to face with the patient, by increasing the proportion of bedside rounding time. In other words, teams on average did not spend more time with patients, though the content and the structure of those encounters may have changed. This finding may be attributable to eliminating redundancy, forgoing the outside-the-room discussion, and thus the largest time reductions were realized there. In addition, teams incompletely adopted beside rounds, as reflected in the data. We expect that, with more complete adoption, an even larger proportion of time will be spent bedside, and absolute time bedside might increase as a result.
An unexpected result of the care redesign was that interns’ proportion of rounding time decreased after the intervention. This decrease most likely is attributable to interns’ being less likely to participate in rounds for a co-intern’s patient, and to their staying outside that patient’s room to give themselves more time to advance the care of their own patients. Before the intervention, when more rounding time was spent outside patient rooms, interns were more likely to join rounds for their co-intern’s patients because they could easily break away, as needed, to continue care of their own patients. The resident is now encouraged to use the morning huddle to identify which patients likely have the most educational value, and both interns are expected to join the bedside rounds for these patients.
This study had a few limitations. First, the pre–post design made it difficult to exclude the possibility that other temporal changes may have affected outcomes, though we did account for time-of-year effects by aligning our data-collection phases. In addition, the authors, including the director of the general medical service, are unaware of any co-interventions during the study period. Second, the multipronged intervention included care team regionalization, encouragement of bedside rounding with nurses, call structure changes (from 4 days to daily admitting), and attendings’ reading of admission notes before rounds. Thus, parsing which component(s) contributed to the results was difficult, though all the changes instituted likely were necessary for system redesign. For example, regionalization of clinicians to unit-based teams was made possible by switching to a daily admitting system.
Time that team members spent preparing for rounds was not recorded before or after the intervention. Thus, the decrease in total rounding time could have been accompanied by an increase in time spent preparing for rounds. However, admission notes were available in our electronic medical record before and after the intervention, and most residents and attendings were already reading them pre-intervention. After the intervention, pre-round note reading was more clearly defined as an expectation, and we were able to set the expectation that interns should use their presentations to summarize rather than recount information. In addition, in the post-intervention period, we did not include time spent preparing rounding orders; as already noted, however, preparation took only 5 minutes per day. Also, we did not analyze the content or the quality of the discussion on rounds, but simply recorded who was present where and when. Regarding the effect of the intervention on patient care, results were mixed. As reported in 2016, we saw no difference in frequency of adverse events with this intervention.12 However, a more sensitive measure of adverse events—used in a study on handoffs—showed our regionalization efforts had an additive effect on reducing overnight adverse events.17Researchers should now focus on the effects of care redesign on clinical outcomes, interdisciplinary care team communication, patient engagement and satisfaction, provider opinions of communication, workflow, patient care, and housestaff education. Our methodology can be used as a model to link structure, process, and outcome related to rounds and thereby better understand how best to optimize patient care and efficiency. Additional studies are needed to analyze the content of rounds and their association with patient and educational outcomes. Last, it will be important to conduct a study to see if the effects we have identified can be sustained. Such a study is already under way.
In conclusion, creating regionalized care teams and encouraging focused bedside rounds increased the proportion of bedside time and the presence of nurses on rounds. Rounds were shorter despite higher patient census. TMA revealed that regionalized care teams and bedside rounding at a large academic hospital are feasible, and are useful in establishing the necessary structures for increasing physician–nurse and provider–patient interactions.
Acknowledgments
The authors acknowledge Dr. Stan Ashley, Dr. Jacqueline Somerville, and Sheila Harris for their support of the regionalization initiative.
Disclosures
Dr. Schnipper received funding from Sanofi-aventis to conduct an investigator-initiated study to implement and evaluate a multi-faceted intervention to improve transitions of care in patients discharged home on insulin. The study was also supported by funding from the Marshall A. Wolf Medical Education Fund, Brigham and Women’s Hospital, and Dr. Stan Ashley, Chief Medical Officer, Brigham and Women’s Hospital. Some of the content of this article was orally presented at the annual meeting of the Society of Hospital Medicine; March 29-April 1, 2015; National Harbor, MD.
1. Crumlish CM, Yialamas MA, McMahon GT. Quantification of bedside teaching by an academic hospitalist group. J Hosp Med. 2009;4(5):304-307. PubMed
2. Gonzalo JD, Masters PA, Simons RJ, Chuang CH. Attending rounds and bedside case presentations: medical student and medicine resident experiences and attitudes. Teach Learn Med. 2009;21(2):105-110. PubMed
3. Elliot DL, Hickam DH. Attending rounds on in-patient units: differences between medical and non-medical services. Med Educ. 1993;27(6):503-508. PubMed
4. Payson HE, Barchas JD. A time study of medical teaching rounds. N Engl J Med. 1965;273(27):1468-1471. PubMed
5. Tremonti LP, Biddle WB. Teaching behaviors of residents and faculty members. J Med Educ. 1982;57(11):854-859. PubMed
6. Miller M, Johnson B, Greene HL, Baier M, Nowlin S. An observational study of attending rounds. J Gen Intern Med. 1992;7(6):646-648. PubMed
7. Collins GF, Cassie JM, Daggett CJ. The role of the attending physician in clinical training. J Med Educ. 1978;53(5):429-431. PubMed
8. Ward DR, Ghali WA, Graham A, Lemaire JB. A real-time locating system observes physician time-motion patterns during walk-rounds: a pilot study. BMC Med Educ. 2014;14:37. PubMed
9. Gonzalo JD, Kuperman E, Lehman E, Haidet P. Bedside interprofessional rounds: perceptions of benefits and barriers by internal medicine nursing staff, attending physicians, and housestaff physicians. J Hosp Med. 2014;9(10):646-651. PubMed
10. Stickrath C, Noble M, Prochazka A, et al. Attending rounds in the current era: what is and is not happening. JAMA Intern Med. 2013;173(12):1084-1089. PubMed
11. Boxer R, Vitale M, Gershanik EF, et al. 5th time’s a charm: creation of unit-based care teams in a high occupancy hospital [abstract]. J Hosp Med. 2015;10(suppl 2).
12. Mueller SK, Schnipper JL, Giannelli K, Roy CL, Boxer R. Impact of regionalized care on concordance of plan and preventable adverse events on general medicine services. J Hosp Med. 2016;11(9):620-627. PubMed
13. Chauke HL, Pattinson RC. Ward rounds—bedside or conference room? S Afr Med J. 2006;96(5):398-400. PubMed
14. Wang-Cheng RM, Barnas GP, Sigmann P, Riendl PA, Young MJ. Bedside case presentations: why patients like them but learners don’t. J Gen Intern Med. 1989;4(4):284-287. PubMed
15. Lehmann LS, Brancati FL, Chen MC, Roter D, Dobs AS. The effect of bedside case presentations on patients’ perceptions of their medical care. N Engl J Med. 1997;336(16):1150-1155. PubMed
16. Gonzalo JD, Chuang CH, Huang G, Smith C. The return of bedside rounds: an educational intervention. J Gen Intern Med. 2010;25(8):792-798. PubMed
17. Mueller SK, Yoon C, Schnipper JL. Association of a web-based handoff tool with rates of medical errors. JAMA Intern Med. 2016;176(9):1400-1402. PubMed
1. Crumlish CM, Yialamas MA, McMahon GT. Quantification of bedside teaching by an academic hospitalist group. J Hosp Med. 2009;4(5):304-307. PubMed
2. Gonzalo JD, Masters PA, Simons RJ, Chuang CH. Attending rounds and bedside case presentations: medical student and medicine resident experiences and attitudes. Teach Learn Med. 2009;21(2):105-110. PubMed
3. Elliot DL, Hickam DH. Attending rounds on in-patient units: differences between medical and non-medical services. Med Educ. 1993;27(6):503-508. PubMed
4. Payson HE, Barchas JD. A time study of medical teaching rounds. N Engl J Med. 1965;273(27):1468-1471. PubMed
5. Tremonti LP, Biddle WB. Teaching behaviors of residents and faculty members. J Med Educ. 1982;57(11):854-859. PubMed
6. Miller M, Johnson B, Greene HL, Baier M, Nowlin S. An observational study of attending rounds. J Gen Intern Med. 1992;7(6):646-648. PubMed
7. Collins GF, Cassie JM, Daggett CJ. The role of the attending physician in clinical training. J Med Educ. 1978;53(5):429-431. PubMed
8. Ward DR, Ghali WA, Graham A, Lemaire JB. A real-time locating system observes physician time-motion patterns during walk-rounds: a pilot study. BMC Med Educ. 2014;14:37. PubMed
9. Gonzalo JD, Kuperman E, Lehman E, Haidet P. Bedside interprofessional rounds: perceptions of benefits and barriers by internal medicine nursing staff, attending physicians, and housestaff physicians. J Hosp Med. 2014;9(10):646-651. PubMed
10. Stickrath C, Noble M, Prochazka A, et al. Attending rounds in the current era: what is and is not happening. JAMA Intern Med. 2013;173(12):1084-1089. PubMed
11. Boxer R, Vitale M, Gershanik EF, et al. 5th time’s a charm: creation of unit-based care teams in a high occupancy hospital [abstract]. J Hosp Med. 2015;10(suppl 2).
12. Mueller SK, Schnipper JL, Giannelli K, Roy CL, Boxer R. Impact of regionalized care on concordance of plan and preventable adverse events on general medicine services. J Hosp Med. 2016;11(9):620-627. PubMed
13. Chauke HL, Pattinson RC. Ward rounds—bedside or conference room? S Afr Med J. 2006;96(5):398-400. PubMed
14. Wang-Cheng RM, Barnas GP, Sigmann P, Riendl PA, Young MJ. Bedside case presentations: why patients like them but learners don’t. J Gen Intern Med. 1989;4(4):284-287. PubMed
15. Lehmann LS, Brancati FL, Chen MC, Roter D, Dobs AS. The effect of bedside case presentations on patients’ perceptions of their medical care. N Engl J Med. 1997;336(16):1150-1155. PubMed
16. Gonzalo JD, Chuang CH, Huang G, Smith C. The return of bedside rounds: an educational intervention. J Gen Intern Med. 2010;25(8):792-798. PubMed
17. Mueller SK, Yoon C, Schnipper JL. Association of a web-based handoff tool with rates of medical errors. JAMA Intern Med. 2016;176(9):1400-1402. PubMed
© 2017 Society of Hospital Medicine
Continuing Medical Education Program in
If you wish to receive credit for this activity, please refer to the website: www.wileyblackwellcme.com.
Accreditation and Designation Statement
Blackwell Futura Media Services designates this journal‐based CME activity for a maximum of 1 AMA PRA Category 1 Credit.. Physicians should only claim credit commensurate with the extent of their participation in the activity.
Blackwell Futura Media Services is accredited by the Accreditation Council for Continuing Medical Education to provide continuing medical education for physicians.
Educational Objectives
Upon completion of this educational activity, participants will be better able to:
Illustrate the elements of a systematic approach to successful hospital smoking cessation programs.
Describe the efficacy of a coordinated real world” hospital smoking cessation program in a U.S. hospital.
Evaluate the barriers to successful hospital smoking cessation programs.
This manuscript underwent peer review in line with the standards of editorial integrity and publication ethics maintained by Journal of Hospital Medicine. The peer reviewers have no relevant financial relationships. The peer review process for Journal of Hospital Medicine is blinded. As such, the identities of the reviewers are not disclosed in line with the standard accepted practices of medical journal peer review.
Conflicts of interest have been identified and resolved in accordance with Blackwell Futura Media Services's Policy on Activity Disclosure and Conflict of Interest. The primary resolution method used was peer review and review by a non‐conflicted expert.
Instructions on Receiving Credit
For information on applicability and acceptance of CME credit for this activity, please consult your professional licensing board.
This activity is designed to be completed within an hour; physicians should claim only those credits that reflect the time actually spent in the activity. To successfully earn credit, participants must complete the activity during the valid credit period, which is up to two years from initial publication.
Follow these steps to earn credit:
Log on to www.wileyblackwellcme.com
Read the target audience, learning objectives, and author disclosures.
Read the article in print or online format.
Reflect on the article.
Access the CME Exam, and choose the best answer to each question.
Complete the required evaluation component of the activity.
This activity will be available for CME credit for twelve months following its publication date. At that time, it will be reviewed and potentially updated and extended for an additional twelve months.
If you wish to receive credit for this activity, please refer to the website: www.wileyblackwellcme.com.
Accreditation and Designation Statement
Blackwell Futura Media Services designates this journal‐based CME activity for a maximum of 1 AMA PRA Category 1 Credit.. Physicians should only claim credit commensurate with the extent of their participation in the activity.
Blackwell Futura Media Services is accredited by the Accreditation Council for Continuing Medical Education to provide continuing medical education for physicians.
Educational Objectives
Upon completion of this educational activity, participants will be better able to:
Illustrate the elements of a systematic approach to successful hospital smoking cessation programs.
Describe the efficacy of a coordinated real world” hospital smoking cessation program in a U.S. hospital.
Evaluate the barriers to successful hospital smoking cessation programs.
This manuscript underwent peer review in line with the standards of editorial integrity and publication ethics maintained by Journal of Hospital Medicine. The peer reviewers have no relevant financial relationships. The peer review process for Journal of Hospital Medicine is blinded. As such, the identities of the reviewers are not disclosed in line with the standard accepted practices of medical journal peer review.
Conflicts of interest have been identified and resolved in accordance with Blackwell Futura Media Services's Policy on Activity Disclosure and Conflict of Interest. The primary resolution method used was peer review and review by a non‐conflicted expert.
Instructions on Receiving Credit
For information on applicability and acceptance of CME credit for this activity, please consult your professional licensing board.
This activity is designed to be completed within an hour; physicians should claim only those credits that reflect the time actually spent in the activity. To successfully earn credit, participants must complete the activity during the valid credit period, which is up to two years from initial publication.
Follow these steps to earn credit:
Log on to www.wileyblackwellcme.com
Read the target audience, learning objectives, and author disclosures.
Read the article in print or online format.
Reflect on the article.
Access the CME Exam, and choose the best answer to each question.
Complete the required evaluation component of the activity.
This activity will be available for CME credit for twelve months following its publication date. At that time, it will be reviewed and potentially updated and extended for an additional twelve months.
If you wish to receive credit for this activity, please refer to the website: www.wileyblackwellcme.com.
Accreditation and Designation Statement
Blackwell Futura Media Services designates this journal‐based CME activity for a maximum of 1 AMA PRA Category 1 Credit.. Physicians should only claim credit commensurate with the extent of their participation in the activity.
Blackwell Futura Media Services is accredited by the Accreditation Council for Continuing Medical Education to provide continuing medical education for physicians.
Educational Objectives
Upon completion of this educational activity, participants will be better able to:
Illustrate the elements of a systematic approach to successful hospital smoking cessation programs.
Describe the efficacy of a coordinated real world” hospital smoking cessation program in a U.S. hospital.
Evaluate the barriers to successful hospital smoking cessation programs.
This manuscript underwent peer review in line with the standards of editorial integrity and publication ethics maintained by Journal of Hospital Medicine. The peer reviewers have no relevant financial relationships. The peer review process for Journal of Hospital Medicine is blinded. As such, the identities of the reviewers are not disclosed in line with the standard accepted practices of medical journal peer review.
Conflicts of interest have been identified and resolved in accordance with Blackwell Futura Media Services's Policy on Activity Disclosure and Conflict of Interest. The primary resolution method used was peer review and review by a non‐conflicted expert.
Instructions on Receiving Credit
For information on applicability and acceptance of CME credit for this activity, please consult your professional licensing board.
This activity is designed to be completed within an hour; physicians should claim only those credits that reflect the time actually spent in the activity. To successfully earn credit, participants must complete the activity during the valid credit period, which is up to two years from initial publication.
Follow these steps to earn credit:
Log on to www.wileyblackwellcme.com
Read the target audience, learning objectives, and author disclosures.
Read the article in print or online format.
Reflect on the article.
Access the CME Exam, and choose the best answer to each question.
Complete the required evaluation component of the activity.
This activity will be available for CME credit for twelve months following its publication date. At that time, it will be reviewed and potentially updated and extended for an additional twelve months.
Copyright © 2010 Society of Hospital Medicine
Important Postdischarge Culture Results
Many hospitalized patients have microbiology test results pending at the time of discharge.1, 2 Failure to follow up on these results in a timely fashion can lead to delays in diagnosis and adequate treatment of important infections. Prompt communication of the results of these pending tests to the responsible providers is crucial to minimize these delays.36 As hospitalized patients are increasingly cared for by clinicians other than their primary care providers,7 important information may be lost during the discharge process.8 This increasing fragmentation makes reliable communication of pending tests even more crucial.9, 10
Studies to date have primarily investigated tests from general medical services. In that setting, there is clearly room for improvement in test result communication. Discharge summaries often do not reach the outpatient providers at the time of the patients' follow‐up visits after hospitalization.11 When the discharge summaries are present, the majority of pending tests are not mentioned in them,2, 12, 13 and both inpatient and outpatient physicians are unaware of most of these results when they return.1 However, the specific characteristics of postdischarge microbiology results and the extent to which these results represent potential follow‐up errors in specialties other than general medicine have not been adequately studied.
We aimed to describe the issue of microbiology tests pending at the time of discharge from a hospital‐wide perspective. Specifically, we sought to determine: (1) frequency and characteristics of these results across all admitting services; and (2) how often these results potentially require a change in antimicrobial therapy.
Methods
Study Setting
We conducted our study at a 777‐bed, tertiary‐care academic hospital in Boston, MA with 13 medical and 18 surgical admitting specialties. The human research committee reviewed and approved the study design. For inpatient services, the hospital had well‐established computerized order entry and electronic discharge medication list systems, along with paper clinical notes. The affiliated outpatient practices used an internally developed electronic health record that could access the test results obtained during hospitalization.
Data Collection
We analyzed all 111,331 results of blood, urine, cerebrospinal fluid (CSF), and sputum cultures that were finalized by the hospital's microbiology laboratory in calendar year 2007. For each result, we determined the type of culture, the date of collection, the date of final result, and the identity and antibiotic susceptibility of any organisms isolated in the microbiology lab. For blood and CSF cultures, we also collected the date of preliminary susceptibilities. Preliminary susceptibilities are not reported for urine and sputum cultures at our institution. For cultures collected during hospital admission, we determined the dates of hospital admission and discharge, hospital service caring for the patient at the time of discharge, and the list of medications prescribed to the patient at discharge.
Case Selection Criteria
Our goal was to screen for postdischarge microbiology results that were likely to require action from the clinicians. To this end, we identified cases that were: (1) clinically important, which we defined as likely to represent a true infection or require further evaluation; and (2) were untreated at the time of discharge, which we defined as cases with no antibiotic or inadequate antibiotic therapy. We first excluded cultures obtained while patients were in the outpatient setting. We further excluded all cultures for which the preliminary susceptibilities or final results returned on or before the day of discharge from the hospital.
For each of the four culture types, we developed criteria to identify clinically important results. For blood cultures, we used a prediction model developed and validated at our institution that was based on the identity of the organism, time to first growth, and prior matching culture results.14 For the remaining three culture types, we defined clinical importance based on Centers for Disease Control and Prevention (CDC) definitions of nosocomial infections. These criteria were felt to be adequate to screen for both community‐acquired and nosocomial infections. For urine cultures, we required at least 100,000 colony‐forming units and growth of no more than two distinct organisms. For CSF, any growth was considered clinically important. For sputum, we required a positive culture as well as a discharge diagnosis of pneumonia based on International Classification of Diseases, Ninth Revision (ICD‐9) codes. The discharge diagnosis was included to incorporate the clinical interpretation required to separate true infections from contaminated samples or colonization.
To identify the untreated cultures, we compared the antibiotic susceptibility of the clinically important postdischarge results against the list of antibiotics prescribed to the patients at the time of hospital discharge. We considered the infections treated if there was at least one antibiotic on the discharge medication list to which the organism was found to be susceptible.
Manual Review
We manually reviewed a random sample of 94 of the clinically important and untreated postdischarge results to determine if the results potentially required a change in therapy and therefore required follow‐up. For each case, the electronic patient chart was reviewed by two internal medicine‐trained physicians on the study staff. Each reviewer was blinded to events that occurred after the cultures returned, and determined whether the results necessitated a potential change in antibiotic. The reviewer then indicated the level of certainty of that determination on a 6‐point Likert scale that had been previously used in reviews to identify adverse medical events15, 16: 1 = little or no evidence, 2 = slight evidence, 3 = not quite likely (<50:50 but close call), 4 = more likely than not (>50:50 but close call), 5 = strong evidence, and 6 = virtually certain evidence. To standardize the assignment of certainty for potential need for antibiotic change, we used a set of review guidelines developed by our study staff (Figure 1). A microbiology result was defined as potentially necessitating antibiotic change if both reviewers indicated as such and recorded a certainty with a score 4. Differences in assessments were resolved through discussion of the case between the reviewers.
Statistical Analysis
Using the 94 manually reviewed results, we examined how the proportion of clinically important and untreated microbiology results requiring follow‐up varied by type of culture and primary discharging service. We created a multivariable logistic regression model to predict which of the untreated, postdischarge results required follow‐up. The covariates in our model were selected a priori and included type of culture, hospital service at the time of discharge, patient age, sex, and insurance status. Type of culture and hospital service were included to determine how the distribution of untreated results varied across hospital specialties. Patient age, sex, and insurance status were included to account for differences in the prevalence of antibiotic‐resistant organisms and the clinician's choice of which empiric antimicrobial agent, if any, to initiate based on these patient‐level factors. We calculated a kappa statistic to measure the concordance of the assessments of the two reviewers prior to resolution of disagreements. All analyses were performed using SAS (version 9.2, Cary, NC).
Results
Of the 111,331 blood, urine, sputum, and CSF cultures analyzed, 77,349 (69%) were collected from hospitalized patients. The majority (63%) of the inpatient results were for blood cultures and one quarter (24%) were for urine cultures. Table 1 shows the distribution of the microbiology results across primary services responsible for the patients at the time of discharge. Half (49%) of the patients from whom the specimens were collected were female. The mean age of patients was 55 years. Most (68%) were white and most (86%) had either commercial insurance or Medicare (Table 1).
| Variable | Results for Admitted Patients (n = 77,349) | Results Finalized Postdischarge (n = 8,668) |
|---|---|---|
| ||
| Type of culture, n (%) | ||
| Urine | 18,746 (24) | 2,843 (33) |
| Blood | 48,546 (63) | 4,696 (54) |
| Sputum | 8,466 (11) | 1,059 (12) |
| CSF | 1,591 (2) | 70 (1) |
| Hospital service at discharge, n (%) | ||
| General Medicine | 15,997 (21) | 2,548 (29) |
| Oncology | 13,138 (17) | 1,341 (15) |
| Medical subspecialties | 20,846 (27) | 2,025 (23) |
| Surgery | 23,380 (30) | 2,031 (23) |
| Other | 3,988 (5) | 723 (8) |
| Patient characteristics | ||
| Female, n (%) | 38,125 (49) | 4,539 (52) |
| Age, n (SD) | 55 (21) | 56 (19) |
| Race, n (%) | ||
| White | 52,824 (68) | 5,669 (65) |
| Black | 9,319 (12) | 1,241 (14) |
| Asian | 1,565 (2) | 183 (2) |
| Hispanic | 5,116 (7) | 897 (10) |
| Other | 1,330 (2) | 146 (2) |
| Unavailable | 7,195 (9) | 532 (6) |
| Insurance, n (%) | ||
| Commercial | 35,893 (46) | 3,977 (46) |
| Medicare | 30,553 (40) | 3,473 (40) |
| Medicaid | 9,514 (12) | 1,034 (12) |
| Other | 1,389 (2) | 184 (2) |
Of the 77,349 microbiology tests obtained during hospital stays, 8668 (11%) of the inpatient microbiology results were reported after the patients were discharged from the hospital. Most (54%) of these postdischarge results were for blood cultures. The distribution of results across primary hospital service, patient sex, race, insurance, and mean patient age were similar to those for all inpatient results (Table 1). Of the 8668 postdischarge results, 385 (4%) met our screening criteria of being both clinically important and not treated by an antibiotic to which the organism was found susceptible at the time of discharge from the hospital. After manual review of a random subset of 94 of these screen‐positive cases, 50 (53%) required follow‐up (Figure 2). The interrater reliability for the reviewers was found to be kappa = 0.58 (P < 0.001). From our results, we estimated that 2.4% of the postdischarge microbiology results required follow‐up and potential change in therapy.
Potential need for antibiotic change was present in 30 of 45 (67%) urine cultures, 12 of 32 (38%) blood cultures, 8 of 16 (50%) sputum cultures, and 0 of 1 (0%) CSF cultures. By primary service, reviewers identified a potential need for antibiotic change in 19 of 25 (76%) of results from surgical services, 17 of 29 (59%) from general medicine, 6 of 16 (38%) from oncology, and 8 of 23 (35%) from medical subspecialties. Examples of cases that potentially required antibiotic change are shown in Table 2.
| Culture Type | Scenario |
|---|---|
| Urine | 42‐year‐old woman with dysuria after admission for hysterectomy; no empiric antibiotic treatment given; postdischarge urine culture grew Klebsiella pneumoniae |
| Blood | 81‐year‐old man with Crohn's disease on total parenteral nutrition (TPN) who was initially treated for sepsis from suspected line infection, but discharged without antibiotics, given negative cultures during admission; postdischarge blood culture grew Klebsiella pneumoniae |
| Sputum | 46‐year‐old woman prescribed levofloxacin for pneumonia; sputum culture returns postdischarge with Pseudomonas aeruginosa resistant to levofloxacin |
In our logistic regression model, both the type of culture and the primary hospital service were found to be significant predictors of a potential need for antibiotic change in the manually reviewed cases. Urine cultures were more likely than non‐urine cultures to potentially require antibiotic change (P = 0.03; OR 2.8, 95% CI 1.1‐7.2). Results from surgical services were most likely to potentially require antibiotic change, followed by general medicine, oncology, and medical subspecialties (Table 3).
| Variable | Results Potentially Requiring Change in Therapy (n = 50) | Results Not Requiring Change in Therapy (n = 44) | Odds Ratio (95% CI)* | Adjusted P‐value* |
|---|---|---|---|---|
| ||||
| Type of culture, n (%) | ||||
| Urine | 30 (60) | 15 (34) | 2.84 (1.13‐7.17) | 0.03 |
| Non‐urine | 20 (40) | 29 (66) | Ref | |
| Hospital service at discharge, n (%) | ||||
| General Medicine | 17 (34) | 12 (27) | Ref | |
| Oncology | 6 (12) | 10 (23) | 0.41 (0.11‐1.56) | 0.02 |
| Medical subspecialties | 8 (16) | 16 (36) | 0.34 (0.10‐1.16) | |
| Surgery | 19 (38) | 6 (14) | 2.40 (0.65‐8.89) | |
| Age, mean (SD) | 61 (20) | 59 (21) | 1.01 (0.98‐1.04) | 0.62 |
| Female, n (%) | 29 (58) | 21 (42) | 1.15 (0.44‐2.98) | 0.77 |
| Insurance, n (%) | ||||
| Commercial | 17 (34) | 19 (43) | Ref | |
| Medicare | 25 (50) | 19 (43) | 1.60 (0.42‐6.11) | 0.65 |
| Medicaid and other | 8 (16) | 6 (14) | 1.78 (0.43‐7.36) | |
Discussion
We performed a retrospective analysis of all blood, urine, sputum, and CSF cultures finalized at our institution in 2007 and found that many returned after patients were discharged. Overall, we estimated that 2.4% of these postdischarge results potentially required a change in antibiotic. This proportion varied by culture type and by primary hospital service at the time of discharge, with urine cultures and cultures from surgical services being most likely to potentially need change in antibiotic.
We speculate that postdischarge urine cultures may have been more likely to require antibiotic change in part due to different urgency that clinicians assign to different culture types. Urinary tract infections may present with more vague, transient, or minor complaints compared with bacteremia, pneumonia, and cerebrospinal fluid infections. For that reason, clinicians may be more likely to forego empiric antibiotics for pending urine cultures in favor of watchful waiting. Therefore, the postdischarge urine cultures with growth may include a higher proportion of untreated true infections compared with other culture types.
A similar difference in prescription of empiric antibiotics may help explain the differences seen across primary hospital specialties. For example, if patients on surgical services were less likely to receive empiric antibiotics, then the pool of postdischarge results would be more likely to include true infections that require antibiotic change. Furthermore, it is possible that surgical services may tend to order cultures for patients only if they already have convincing evidence of infections. It may be that selecting a group with higher likelihood of infection led to a higher proportion of true infections in surgical patients with cultures with growth.
Prior studies led by Roy and Were illustrated that pending microbiology results from general medicine services were often not communicated and followed up adequately.1, 2 For patients discharged with pending test results, between 47% and 89% of discharge summaries did not mention the pending tests.2, 12, 13, 17 These deficiencies in discharge summaries likely have a substantial impact on the proportion of tests followed up by outpatient clinicians. By extending the analysis hospital‐wide, our study suggests that pending microbiology results occur for a wide range of hospital services. While our study was not designed to determine whether these results were followed up appropriately, opportunities for miscommunication and missed follow‐up likely exist for all specialties.
The potential harms associated with inadequate test follow‐up have gained the attention of the patient safety community. In 2005, the Joint Commission underscored the importance of proper communication of critical lab results.3, 5, 18 Their recommendations included the development of systems to ensure adequate follow‐up of critical results in high‐risk scenarios including the postdischarge period.5 While many of the microbiology results do not fall into the criticalcategory, we feel that these results should be considered for inclusion in hospital efforts to track postdischarge results. These efforts should also address issues specific to microbiology results, such as preliminary status before antibiotic sensitivities are known.
Developing a comprehensive strategy for test result communication is challenging, and more so for results that return after transitions of care. Even defining the proper target of communication interventions can involve complex organizational and cultural issues. As these results span the inpatient and outpatient domains, there may be some ambiguity as to which provider is responsible when the results return. The inpatient clinicians ordering the microbiology cultures are in the best position to put the results into the patient's clinical context. However, these clinicians may no longer be on clinical duty when the results return, or they may not have a system to ensure that they are notified about these results. While the outpatient providers may be available, they have often not seen the patient in follow‐up at the time the results return and would need to repeat a clinical assessment to determine whether a change in antibiotics is required. While many feel that the ordering provider is a logical choice to perform the follow‐up of the result, not all agree and few institutions have developed clear policies on this issue. To avoid this ambiguity, future work will require institutions to clearly outline which party is responsible for test result follow‐up during transitions of care.
Potential solutions to improve communication of these results must be tailored to the local infrastructure of the institution. In hospitals that do not have extensive electronic systems, a solution might involve a registered nurse, nurse practitioner, or lab technician whose responsibilities include identifying postdischarge results and communicating them to the ordering clinician, primary care provider, and patient. In settings with more advanced electronic infrastructure, solutions could be designed to automatically notify the responsible providers electronically, as well as post the results to a patient portal. Regardless of the level of technical sophistication, it is vital to create a system that has is highly reliable to prevent these important results from falling through the cracks.
Our study did have some limitations. First, we evaluated results from only one institution. It is unclear how substantially differences in practice patterns or patient populations would affect the number of postdischarge microbiology results in other settings. Second, we did not assess whether these results were actually followed up or whether treatment regimens were altered. As this study was retrospective in nature, we could not expect clinicians to recall the clinical scenarios surrounding each result and decided that documentation in clinical notes would be an unreliable indicator of whether any follow‐up action had been taken. Even without this information, however, we would submit that our findings represent a substantial near‐miss rate and threat to patient safety (approximately one potentially actionable, postdischarge microbiology result every other day for our hospital), and call for a fail‐safe system to ensure appropriate actions are taken.
In conclusion, microbiology results are often pending at the time patients are discharged from the hospital and roughly 2.4% of these results potentially require a change in therapy. This proportion was highest for urine cultures and cultures drawn from surgical patients. Our results suggest that a hospital‐wide system is warranted to ensure adequate communication of postdischarge microbiology results. Further research is required to evaluate the impact of such a system on the follow‐up rates of pending microbiology tests.
Acknowledgements
The authors thank Deborah Williams from the Brigham and Women's Division of General Medicine for her programming assistance.
- ,,, et al.Patient safety concerns arising from test results that return after hospital discharge.Ann Intern Med.2005;143(2):121–128.
- ,,, et al.Adequacy of hospital discharge summaries in documenting tests with pending results and outpatient follow‐up providers.J Gen Intern Med.2009;24(9):1002–1006.
- ,.Doing better with critical test results.Jt Comm J Qual Patient Saf.2005;31(2):61,66–67.
- .Fumbled handoffs: one dropped ball after another.Ann Intern Med.2005;142(5):352–358.
- ,,,.Communicating critical test results: safe practice recommendations.Jt Comm J Qual Patient Saf.2005;31(2):68–80.
- .Introduction: Communicating critical test results.Jt Comm J Qual Patient Saf.2005;31(2):61,63–65.
- ,,,.Growth in the care of older patients by hospitalists in the United States.N Engl J Med.2009;360(11):1102–1112.
- ,,.Gaps in the continuity of care and progress on patient safety.BMJ.2000;320(7237):791–794.
- .Key legal principles for hospitalists.Am J Med.2001;111(9B):5S–9S.
- ,,.Passing the clinical baton: 6 principles to guide the hospitalist.Am J Med.2001;111(9B):36S–39S.
- ,,.Dissemination of discharge summaries. Not reaching follow‐up physicians.Can Fam Physician.2002;48:737–742.
- ,,, et al.Communication and information deficits in patients discharged to rehabilitation facilities: an evaluation of five acute care hospitals.J Hosp Med.2009;4(8):E28–33.
- , , , , .Pending laboratory tests and the hospital discharge summary in patients discharged to sub‐acute care.J Gen Intern Med.2010;26(4):393–398.
- ,,,,,.Using electronic data to predict the probability of true bacteremia from positive blood cultures.Proc AMIA Symp.2000:893–897.
- ,,, et al.The nature of adverse events in hospitalized patients. Results of the Harvard Medical Practice Study II.N Engl J Med.1991;324(6):377–384.
- ,,, et al.Incidence and types of adverse events and negligent care in Utah and Colorado.Med Care.2000;38(3):261–271.
- ,,,.General practitioner‐hospital communications: a review of discharge summaries.J Qual Clin Pract.2001;21(4):104–108.
- ,,,,,.Failure to recognize and act on abnormal test results: the case of screening bone densitometry.Jt Comm J Qual Patient Saf.2005;31(2):90–97.
Many hospitalized patients have microbiology test results pending at the time of discharge.1, 2 Failure to follow up on these results in a timely fashion can lead to delays in diagnosis and adequate treatment of important infections. Prompt communication of the results of these pending tests to the responsible providers is crucial to minimize these delays.36 As hospitalized patients are increasingly cared for by clinicians other than their primary care providers,7 important information may be lost during the discharge process.8 This increasing fragmentation makes reliable communication of pending tests even more crucial.9, 10
Studies to date have primarily investigated tests from general medical services. In that setting, there is clearly room for improvement in test result communication. Discharge summaries often do not reach the outpatient providers at the time of the patients' follow‐up visits after hospitalization.11 When the discharge summaries are present, the majority of pending tests are not mentioned in them,2, 12, 13 and both inpatient and outpatient physicians are unaware of most of these results when they return.1 However, the specific characteristics of postdischarge microbiology results and the extent to which these results represent potential follow‐up errors in specialties other than general medicine have not been adequately studied.
We aimed to describe the issue of microbiology tests pending at the time of discharge from a hospital‐wide perspective. Specifically, we sought to determine: (1) frequency and characteristics of these results across all admitting services; and (2) how often these results potentially require a change in antimicrobial therapy.
Methods
Study Setting
We conducted our study at a 777‐bed, tertiary‐care academic hospital in Boston, MA with 13 medical and 18 surgical admitting specialties. The human research committee reviewed and approved the study design. For inpatient services, the hospital had well‐established computerized order entry and electronic discharge medication list systems, along with paper clinical notes. The affiliated outpatient practices used an internally developed electronic health record that could access the test results obtained during hospitalization.
Data Collection
We analyzed all 111,331 results of blood, urine, cerebrospinal fluid (CSF), and sputum cultures that were finalized by the hospital's microbiology laboratory in calendar year 2007. For each result, we determined the type of culture, the date of collection, the date of final result, and the identity and antibiotic susceptibility of any organisms isolated in the microbiology lab. For blood and CSF cultures, we also collected the date of preliminary susceptibilities. Preliminary susceptibilities are not reported for urine and sputum cultures at our institution. For cultures collected during hospital admission, we determined the dates of hospital admission and discharge, hospital service caring for the patient at the time of discharge, and the list of medications prescribed to the patient at discharge.
Case Selection Criteria
Our goal was to screen for postdischarge microbiology results that were likely to require action from the clinicians. To this end, we identified cases that were: (1) clinically important, which we defined as likely to represent a true infection or require further evaluation; and (2) were untreated at the time of discharge, which we defined as cases with no antibiotic or inadequate antibiotic therapy. We first excluded cultures obtained while patients were in the outpatient setting. We further excluded all cultures for which the preliminary susceptibilities or final results returned on or before the day of discharge from the hospital.
For each of the four culture types, we developed criteria to identify clinically important results. For blood cultures, we used a prediction model developed and validated at our institution that was based on the identity of the organism, time to first growth, and prior matching culture results.14 For the remaining three culture types, we defined clinical importance based on Centers for Disease Control and Prevention (CDC) definitions of nosocomial infections. These criteria were felt to be adequate to screen for both community‐acquired and nosocomial infections. For urine cultures, we required at least 100,000 colony‐forming units and growth of no more than two distinct organisms. For CSF, any growth was considered clinically important. For sputum, we required a positive culture as well as a discharge diagnosis of pneumonia based on International Classification of Diseases, Ninth Revision (ICD‐9) codes. The discharge diagnosis was included to incorporate the clinical interpretation required to separate true infections from contaminated samples or colonization.
To identify the untreated cultures, we compared the antibiotic susceptibility of the clinically important postdischarge results against the list of antibiotics prescribed to the patients at the time of hospital discharge. We considered the infections treated if there was at least one antibiotic on the discharge medication list to which the organism was found to be susceptible.
Manual Review
We manually reviewed a random sample of 94 of the clinically important and untreated postdischarge results to determine if the results potentially required a change in therapy and therefore required follow‐up. For each case, the electronic patient chart was reviewed by two internal medicine‐trained physicians on the study staff. Each reviewer was blinded to events that occurred after the cultures returned, and determined whether the results necessitated a potential change in antibiotic. The reviewer then indicated the level of certainty of that determination on a 6‐point Likert scale that had been previously used in reviews to identify adverse medical events15, 16: 1 = little or no evidence, 2 = slight evidence, 3 = not quite likely (<50:50 but close call), 4 = more likely than not (>50:50 but close call), 5 = strong evidence, and 6 = virtually certain evidence. To standardize the assignment of certainty for potential need for antibiotic change, we used a set of review guidelines developed by our study staff (Figure 1). A microbiology result was defined as potentially necessitating antibiotic change if both reviewers indicated as such and recorded a certainty with a score 4. Differences in assessments were resolved through discussion of the case between the reviewers.

Statistical Analysis
Using the 94 manually reviewed results, we examined how the proportion of clinically important and untreated microbiology results requiring follow‐up varied by type of culture and primary discharging service. We created a multivariable logistic regression model to predict which of the untreated, postdischarge results required follow‐up. The covariates in our model were selected a priori and included type of culture, hospital service at the time of discharge, patient age, sex, and insurance status. Type of culture and hospital service were included to determine how the distribution of untreated results varied across hospital specialties. Patient age, sex, and insurance status were included to account for differences in the prevalence of antibiotic‐resistant organisms and the clinician's choice of which empiric antimicrobial agent, if any, to initiate based on these patient‐level factors. We calculated a kappa statistic to measure the concordance of the assessments of the two reviewers prior to resolution of disagreements. All analyses were performed using SAS (version 9.2, Cary, NC).
Results
Of the 111,331 blood, urine, sputum, and CSF cultures analyzed, 77,349 (69%) were collected from hospitalized patients. The majority (63%) of the inpatient results were for blood cultures and one quarter (24%) were for urine cultures. Table 1 shows the distribution of the microbiology results across primary services responsible for the patients at the time of discharge. Half (49%) of the patients from whom the specimens were collected were female. The mean age of patients was 55 years. Most (68%) were white and most (86%) had either commercial insurance or Medicare (Table 1).
| Variable | Results for Admitted Patients (n = 77,349) | Results Finalized Postdischarge (n = 8,668) |
|---|---|---|
| ||
| Type of culture, n (%) | ||
| Urine | 18,746 (24) | 2,843 (33) |
| Blood | 48,546 (63) | 4,696 (54) |
| Sputum | 8,466 (11) | 1,059 (12) |
| CSF | 1,591 (2) | 70 (1) |
| Hospital service at discharge, n (%) | ||
| General Medicine | 15,997 (21) | 2,548 (29) |
| Oncology | 13,138 (17) | 1,341 (15) |
| Medical subspecialties | 20,846 (27) | 2,025 (23) |
| Surgery | 23,380 (30) | 2,031 (23) |
| Other | 3,988 (5) | 723 (8) |
| Patient characteristics | ||
| Female, n (%) | 38,125 (49) | 4,539 (52) |
| Age, n (SD) | 55 (21) | 56 (19) |
| Race, n (%) | ||
| White | 52,824 (68) | 5,669 (65) |
| Black | 9,319 (12) | 1,241 (14) |
| Asian | 1,565 (2) | 183 (2) |
| Hispanic | 5,116 (7) | 897 (10) |
| Other | 1,330 (2) | 146 (2) |
| Unavailable | 7,195 (9) | 532 (6) |
| Insurance, n (%) | ||
| Commercial | 35,893 (46) | 3,977 (46) |
| Medicare | 30,553 (40) | 3,473 (40) |
| Medicaid | 9,514 (12) | 1,034 (12) |
| Other | 1,389 (2) | 184 (2) |
Of the 77,349 microbiology tests obtained during hospital stays, 8668 (11%) of the inpatient microbiology results were reported after the patients were discharged from the hospital. Most (54%) of these postdischarge results were for blood cultures. The distribution of results across primary hospital service, patient sex, race, insurance, and mean patient age were similar to those for all inpatient results (Table 1). Of the 8668 postdischarge results, 385 (4%) met our screening criteria of being both clinically important and not treated by an antibiotic to which the organism was found susceptible at the time of discharge from the hospital. After manual review of a random subset of 94 of these screen‐positive cases, 50 (53%) required follow‐up (Figure 2). The interrater reliability for the reviewers was found to be kappa = 0.58 (P < 0.001). From our results, we estimated that 2.4% of the postdischarge microbiology results required follow‐up and potential change in therapy.

Potential need for antibiotic change was present in 30 of 45 (67%) urine cultures, 12 of 32 (38%) blood cultures, 8 of 16 (50%) sputum cultures, and 0 of 1 (0%) CSF cultures. By primary service, reviewers identified a potential need for antibiotic change in 19 of 25 (76%) of results from surgical services, 17 of 29 (59%) from general medicine, 6 of 16 (38%) from oncology, and 8 of 23 (35%) from medical subspecialties. Examples of cases that potentially required antibiotic change are shown in Table 2.
| Culture Type | Scenario |
|---|---|
| Urine | 42‐year‐old woman with dysuria after admission for hysterectomy; no empiric antibiotic treatment given; postdischarge urine culture grew Klebsiella pneumoniae |
| Blood | 81‐year‐old man with Crohn's disease on total parenteral nutrition (TPN) who was initially treated for sepsis from suspected line infection, but discharged without antibiotics, given negative cultures during admission; postdischarge blood culture grew Klebsiella pneumoniae |
| Sputum | 46‐year‐old woman prescribed levofloxacin for pneumonia; sputum culture returns postdischarge with Pseudomonas aeruginosa resistant to levofloxacin |
In our logistic regression model, both the type of culture and the primary hospital service were found to be significant predictors of a potential need for antibiotic change in the manually reviewed cases. Urine cultures were more likely than non‐urine cultures to potentially require antibiotic change (P = 0.03; OR 2.8, 95% CI 1.1‐7.2). Results from surgical services were most likely to potentially require antibiotic change, followed by general medicine, oncology, and medical subspecialties (Table 3).
| Variable | Results Potentially Requiring Change in Therapy (n = 50) | Results Not Requiring Change in Therapy (n = 44) | Odds Ratio (95% CI)* | Adjusted P‐value* |
|---|---|---|---|---|
| ||||
| Type of culture, n (%) | ||||
| Urine | 30 (60) | 15 (34) | 2.84 (1.13‐7.17) | 0.03 |
| Non‐urine | 20 (40) | 29 (66) | Ref | |
| Hospital service at discharge, n (%) | ||||
| General Medicine | 17 (34) | 12 (27) | Ref | |
| Oncology | 6 (12) | 10 (23) | 0.41 (0.11‐1.56) | 0.02 |
| Medical subspecialties | 8 (16) | 16 (36) | 0.34 (0.10‐1.16) | |
| Surgery | 19 (38) | 6 (14) | 2.40 (0.65‐8.89) | |
| Age, mean (SD) | 61 (20) | 59 (21) | 1.01 (0.98‐1.04) | 0.62 |
| Female, n (%) | 29 (58) | 21 (42) | 1.15 (0.44‐2.98) | 0.77 |
| Insurance, n (%) | ||||
| Commercial | 17 (34) | 19 (43) | Ref | |
| Medicare | 25 (50) | 19 (43) | 1.60 (0.42‐6.11) | 0.65 |
| Medicaid and other | 8 (16) | 6 (14) | 1.78 (0.43‐7.36) | |
Discussion
We performed a retrospective analysis of all blood, urine, sputum, and CSF cultures finalized at our institution in 2007 and found that many returned after patients were discharged. Overall, we estimated that 2.4% of these postdischarge results potentially required a change in antibiotic. This proportion varied by culture type and by primary hospital service at the time of discharge, with urine cultures and cultures from surgical services being most likely to potentially need change in antibiotic.
We speculate that postdischarge urine cultures may have been more likely to require antibiotic change in part due to different urgency that clinicians assign to different culture types. Urinary tract infections may present with more vague, transient, or minor complaints compared with bacteremia, pneumonia, and cerebrospinal fluid infections. For that reason, clinicians may be more likely to forego empiric antibiotics for pending urine cultures in favor of watchful waiting. Therefore, the postdischarge urine cultures with growth may include a higher proportion of untreated true infections compared with other culture types.
A similar difference in prescription of empiric antibiotics may help explain the differences seen across primary hospital specialties. For example, if patients on surgical services were less likely to receive empiric antibiotics, then the pool of postdischarge results would be more likely to include true infections that require antibiotic change. Furthermore, it is possible that surgical services may tend to order cultures for patients only if they already have convincing evidence of infections. It may be that selecting a group with higher likelihood of infection led to a higher proportion of true infections in surgical patients with cultures with growth.
Prior studies led by Roy and Were illustrated that pending microbiology results from general medicine services were often not communicated and followed up adequately.1, 2 For patients discharged with pending test results, between 47% and 89% of discharge summaries did not mention the pending tests.2, 12, 13, 17 These deficiencies in discharge summaries likely have a substantial impact on the proportion of tests followed up by outpatient clinicians. By extending the analysis hospital‐wide, our study suggests that pending microbiology results occur for a wide range of hospital services. While our study was not designed to determine whether these results were followed up appropriately, opportunities for miscommunication and missed follow‐up likely exist for all specialties.
The potential harms associated with inadequate test follow‐up have gained the attention of the patient safety community. In 2005, the Joint Commission underscored the importance of proper communication of critical lab results.3, 5, 18 Their recommendations included the development of systems to ensure adequate follow‐up of critical results in high‐risk scenarios including the postdischarge period.5 While many of the microbiology results do not fall into the criticalcategory, we feel that these results should be considered for inclusion in hospital efforts to track postdischarge results. These efforts should also address issues specific to microbiology results, such as preliminary status before antibiotic sensitivities are known.
Developing a comprehensive strategy for test result communication is challenging, and more so for results that return after transitions of care. Even defining the proper target of communication interventions can involve complex organizational and cultural issues. As these results span the inpatient and outpatient domains, there may be some ambiguity as to which provider is responsible when the results return. The inpatient clinicians ordering the microbiology cultures are in the best position to put the results into the patient's clinical context. However, these clinicians may no longer be on clinical duty when the results return, or they may not have a system to ensure that they are notified about these results. While the outpatient providers may be available, they have often not seen the patient in follow‐up at the time the results return and would need to repeat a clinical assessment to determine whether a change in antibiotics is required. While many feel that the ordering provider is a logical choice to perform the follow‐up of the result, not all agree and few institutions have developed clear policies on this issue. To avoid this ambiguity, future work will require institutions to clearly outline which party is responsible for test result follow‐up during transitions of care.
Potential solutions to improve communication of these results must be tailored to the local infrastructure of the institution. In hospitals that do not have extensive electronic systems, a solution might involve a registered nurse, nurse practitioner, or lab technician whose responsibilities include identifying postdischarge results and communicating them to the ordering clinician, primary care provider, and patient. In settings with more advanced electronic infrastructure, solutions could be designed to automatically notify the responsible providers electronically, as well as post the results to a patient portal. Regardless of the level of technical sophistication, it is vital to create a system that has is highly reliable to prevent these important results from falling through the cracks.
Our study did have some limitations. First, we evaluated results from only one institution. It is unclear how substantially differences in practice patterns or patient populations would affect the number of postdischarge microbiology results in other settings. Second, we did not assess whether these results were actually followed up or whether treatment regimens were altered. As this study was retrospective in nature, we could not expect clinicians to recall the clinical scenarios surrounding each result and decided that documentation in clinical notes would be an unreliable indicator of whether any follow‐up action had been taken. Even without this information, however, we would submit that our findings represent a substantial near‐miss rate and threat to patient safety (approximately one potentially actionable, postdischarge microbiology result every other day for our hospital), and call for a fail‐safe system to ensure appropriate actions are taken.
In conclusion, microbiology results are often pending at the time patients are discharged from the hospital and roughly 2.4% of these results potentially require a change in therapy. This proportion was highest for urine cultures and cultures drawn from surgical patients. Our results suggest that a hospital‐wide system is warranted to ensure adequate communication of postdischarge microbiology results. Further research is required to evaluate the impact of such a system on the follow‐up rates of pending microbiology tests.
Acknowledgements
The authors thank Deborah Williams from the Brigham and Women's Division of General Medicine for her programming assistance.
Many hospitalized patients have microbiology test results pending at the time of discharge.1, 2 Failure to follow up on these results in a timely fashion can lead to delays in diagnosis and adequate treatment of important infections. Prompt communication of the results of these pending tests to the responsible providers is crucial to minimize these delays.36 As hospitalized patients are increasingly cared for by clinicians other than their primary care providers,7 important information may be lost during the discharge process.8 This increasing fragmentation makes reliable communication of pending tests even more crucial.9, 10
Studies to date have primarily investigated tests from general medical services. In that setting, there is clearly room for improvement in test result communication. Discharge summaries often do not reach the outpatient providers at the time of the patients' follow‐up visits after hospitalization.11 When the discharge summaries are present, the majority of pending tests are not mentioned in them,2, 12, 13 and both inpatient and outpatient physicians are unaware of most of these results when they return.1 However, the specific characteristics of postdischarge microbiology results and the extent to which these results represent potential follow‐up errors in specialties other than general medicine have not been adequately studied.
We aimed to describe the issue of microbiology tests pending at the time of discharge from a hospital‐wide perspective. Specifically, we sought to determine: (1) frequency and characteristics of these results across all admitting services; and (2) how often these results potentially require a change in antimicrobial therapy.
Methods
Study Setting
We conducted our study at a 777‐bed, tertiary‐care academic hospital in Boston, MA with 13 medical and 18 surgical admitting specialties. The human research committee reviewed and approved the study design. For inpatient services, the hospital had well‐established computerized order entry and electronic discharge medication list systems, along with paper clinical notes. The affiliated outpatient practices used an internally developed electronic health record that could access the test results obtained during hospitalization.
Data Collection
We analyzed all 111,331 results of blood, urine, cerebrospinal fluid (CSF), and sputum cultures that were finalized by the hospital's microbiology laboratory in calendar year 2007. For each result, we determined the type of culture, the date of collection, the date of final result, and the identity and antibiotic susceptibility of any organisms isolated in the microbiology lab. For blood and CSF cultures, we also collected the date of preliminary susceptibilities. Preliminary susceptibilities are not reported for urine and sputum cultures at our institution. For cultures collected during hospital admission, we determined the dates of hospital admission and discharge, hospital service caring for the patient at the time of discharge, and the list of medications prescribed to the patient at discharge.
Case Selection Criteria
Our goal was to screen for postdischarge microbiology results that were likely to require action from the clinicians. To this end, we identified cases that were: (1) clinically important, which we defined as likely to represent a true infection or require further evaluation; and (2) were untreated at the time of discharge, which we defined as cases with no antibiotic or inadequate antibiotic therapy. We first excluded cultures obtained while patients were in the outpatient setting. We further excluded all cultures for which the preliminary susceptibilities or final results returned on or before the day of discharge from the hospital.
For each of the four culture types, we developed criteria to identify clinically important results. For blood cultures, we used a prediction model developed and validated at our institution that was based on the identity of the organism, time to first growth, and prior matching culture results.14 For the remaining three culture types, we defined clinical importance based on Centers for Disease Control and Prevention (CDC) definitions of nosocomial infections. These criteria were felt to be adequate to screen for both community‐acquired and nosocomial infections. For urine cultures, we required at least 100,000 colony‐forming units and growth of no more than two distinct organisms. For CSF, any growth was considered clinically important. For sputum, we required a positive culture as well as a discharge diagnosis of pneumonia based on International Classification of Diseases, Ninth Revision (ICD‐9) codes. The discharge diagnosis was included to incorporate the clinical interpretation required to separate true infections from contaminated samples or colonization.
To identify the untreated cultures, we compared the antibiotic susceptibility of the clinically important postdischarge results against the list of antibiotics prescribed to the patients at the time of hospital discharge. We considered the infections treated if there was at least one antibiotic on the discharge medication list to which the organism was found to be susceptible.
Manual Review
We manually reviewed a random sample of 94 of the clinically important and untreated postdischarge results to determine if the results potentially required a change in therapy and therefore required follow‐up. For each case, the electronic patient chart was reviewed by two internal medicine‐trained physicians on the study staff. Each reviewer was blinded to events that occurred after the cultures returned, and determined whether the results necessitated a potential change in antibiotic. The reviewer then indicated the level of certainty of that determination on a 6‐point Likert scale that had been previously used in reviews to identify adverse medical events15, 16: 1 = little or no evidence, 2 = slight evidence, 3 = not quite likely (<50:50 but close call), 4 = more likely than not (>50:50 but close call), 5 = strong evidence, and 6 = virtually certain evidence. To standardize the assignment of certainty for potential need for antibiotic change, we used a set of review guidelines developed by our study staff (Figure 1). A microbiology result was defined as potentially necessitating antibiotic change if both reviewers indicated as such and recorded a certainty with a score 4. Differences in assessments were resolved through discussion of the case between the reviewers.

Statistical Analysis
Using the 94 manually reviewed results, we examined how the proportion of clinically important and untreated microbiology results requiring follow‐up varied by type of culture and primary discharging service. We created a multivariable logistic regression model to predict which of the untreated, postdischarge results required follow‐up. The covariates in our model were selected a priori and included type of culture, hospital service at the time of discharge, patient age, sex, and insurance status. Type of culture and hospital service were included to determine how the distribution of untreated results varied across hospital specialties. Patient age, sex, and insurance status were included to account for differences in the prevalence of antibiotic‐resistant organisms and the clinician's choice of which empiric antimicrobial agent, if any, to initiate based on these patient‐level factors. We calculated a kappa statistic to measure the concordance of the assessments of the two reviewers prior to resolution of disagreements. All analyses were performed using SAS (version 9.2, Cary, NC).
Results
Of the 111,331 blood, urine, sputum, and CSF cultures analyzed, 77,349 (69%) were collected from hospitalized patients. The majority (63%) of the inpatient results were for blood cultures and one quarter (24%) were for urine cultures. Table 1 shows the distribution of the microbiology results across primary services responsible for the patients at the time of discharge. Half (49%) of the patients from whom the specimens were collected were female. The mean age of patients was 55 years. Most (68%) were white and most (86%) had either commercial insurance or Medicare (Table 1).
| Variable | Results for Admitted Patients (n = 77,349) | Results Finalized Postdischarge (n = 8,668) |
|---|---|---|
| ||
| Type of culture, n (%) | ||
| Urine | 18,746 (24) | 2,843 (33) |
| Blood | 48,546 (63) | 4,696 (54) |
| Sputum | 8,466 (11) | 1,059 (12) |
| CSF | 1,591 (2) | 70 (1) |
| Hospital service at discharge, n (%) | ||
| General Medicine | 15,997 (21) | 2,548 (29) |
| Oncology | 13,138 (17) | 1,341 (15) |
| Medical subspecialties | 20,846 (27) | 2,025 (23) |
| Surgery | 23,380 (30) | 2,031 (23) |
| Other | 3,988 (5) | 723 (8) |
| Patient characteristics | ||
| Female, n (%) | 38,125 (49) | 4,539 (52) |
| Age, n (SD) | 55 (21) | 56 (19) |
| Race, n (%) | ||
| White | 52,824 (68) | 5,669 (65) |
| Black | 9,319 (12) | 1,241 (14) |
| Asian | 1,565 (2) | 183 (2) |
| Hispanic | 5,116 (7) | 897 (10) |
| Other | 1,330 (2) | 146 (2) |
| Unavailable | 7,195 (9) | 532 (6) |
| Insurance, n (%) | ||
| Commercial | 35,893 (46) | 3,977 (46) |
| Medicare | 30,553 (40) | 3,473 (40) |
| Medicaid | 9,514 (12) | 1,034 (12) |
| Other | 1,389 (2) | 184 (2) |
Of the 77,349 microbiology tests obtained during hospital stays, 8668 (11%) of the inpatient microbiology results were reported after the patients were discharged from the hospital. Most (54%) of these postdischarge results were for blood cultures. The distribution of results across primary hospital service, patient sex, race, insurance, and mean patient age were similar to those for all inpatient results (Table 1). Of the 8668 postdischarge results, 385 (4%) met our screening criteria of being both clinically important and not treated by an antibiotic to which the organism was found susceptible at the time of discharge from the hospital. After manual review of a random subset of 94 of these screen‐positive cases, 50 (53%) required follow‐up (Figure 2). The interrater reliability for the reviewers was found to be kappa = 0.58 (P < 0.001). From our results, we estimated that 2.4% of the postdischarge microbiology results required follow‐up and potential change in therapy.

Potential need for antibiotic change was present in 30 of 45 (67%) urine cultures, 12 of 32 (38%) blood cultures, 8 of 16 (50%) sputum cultures, and 0 of 1 (0%) CSF cultures. By primary service, reviewers identified a potential need for antibiotic change in 19 of 25 (76%) of results from surgical services, 17 of 29 (59%) from general medicine, 6 of 16 (38%) from oncology, and 8 of 23 (35%) from medical subspecialties. Examples of cases that potentially required antibiotic change are shown in Table 2.
| Culture Type | Scenario |
|---|---|
| Urine | 42‐year‐old woman with dysuria after admission for hysterectomy; no empiric antibiotic treatment given; postdischarge urine culture grew Klebsiella pneumoniae |
| Blood | 81‐year‐old man with Crohn's disease on total parenteral nutrition (TPN) who was initially treated for sepsis from suspected line infection, but discharged without antibiotics, given negative cultures during admission; postdischarge blood culture grew Klebsiella pneumoniae |
| Sputum | 46‐year‐old woman prescribed levofloxacin for pneumonia; sputum culture returns postdischarge with Pseudomonas aeruginosa resistant to levofloxacin |
In our logistic regression model, both the type of culture and the primary hospital service were found to be significant predictors of a potential need for antibiotic change in the manually reviewed cases. Urine cultures were more likely than non‐urine cultures to potentially require antibiotic change (P = 0.03; OR 2.8, 95% CI 1.1‐7.2). Results from surgical services were most likely to potentially require antibiotic change, followed by general medicine, oncology, and medical subspecialties (Table 3).
| Variable | Results Potentially Requiring Change in Therapy (n = 50) | Results Not Requiring Change in Therapy (n = 44) | Odds Ratio (95% CI)* | Adjusted P‐value* |
|---|---|---|---|---|
| ||||
| Type of culture, n (%) | ||||
| Urine | 30 (60) | 15 (34) | 2.84 (1.13‐7.17) | 0.03 |
| Non‐urine | 20 (40) | 29 (66) | Ref | |
| Hospital service at discharge, n (%) | ||||
| General Medicine | 17 (34) | 12 (27) | Ref | |
| Oncology | 6 (12) | 10 (23) | 0.41 (0.11‐1.56) | 0.02 |
| Medical subspecialties | 8 (16) | 16 (36) | 0.34 (0.10‐1.16) | |
| Surgery | 19 (38) | 6 (14) | 2.40 (0.65‐8.89) | |
| Age, mean (SD) | 61 (20) | 59 (21) | 1.01 (0.98‐1.04) | 0.62 |
| Female, n (%) | 29 (58) | 21 (42) | 1.15 (0.44‐2.98) | 0.77 |
| Insurance, n (%) | ||||
| Commercial | 17 (34) | 19 (43) | Ref | |
| Medicare | 25 (50) | 19 (43) | 1.60 (0.42‐6.11) | 0.65 |
| Medicaid and other | 8 (16) | 6 (14) | 1.78 (0.43‐7.36) | |
Discussion
We performed a retrospective analysis of all blood, urine, sputum, and CSF cultures finalized at our institution in 2007 and found that many returned after patients were discharged. Overall, we estimated that 2.4% of these postdischarge results potentially required a change in antibiotic. This proportion varied by culture type and by primary hospital service at the time of discharge, with urine cultures and cultures from surgical services being most likely to potentially need change in antibiotic.
We speculate that postdischarge urine cultures may have been more likely to require antibiotic change in part due to different urgency that clinicians assign to different culture types. Urinary tract infections may present with more vague, transient, or minor complaints compared with bacteremia, pneumonia, and cerebrospinal fluid infections. For that reason, clinicians may be more likely to forego empiric antibiotics for pending urine cultures in favor of watchful waiting. Therefore, the postdischarge urine cultures with growth may include a higher proportion of untreated true infections compared with other culture types.
A similar difference in prescription of empiric antibiotics may help explain the differences seen across primary hospital specialties. For example, if patients on surgical services were less likely to receive empiric antibiotics, then the pool of postdischarge results would be more likely to include true infections that require antibiotic change. Furthermore, it is possible that surgical services may tend to order cultures for patients only if they already have convincing evidence of infections. It may be that selecting a group with higher likelihood of infection led to a higher proportion of true infections in surgical patients with cultures with growth.
Prior studies led by Roy and Were illustrated that pending microbiology results from general medicine services were often not communicated and followed up adequately.1, 2 For patients discharged with pending test results, between 47% and 89% of discharge summaries did not mention the pending tests.2, 12, 13, 17 These deficiencies in discharge summaries likely have a substantial impact on the proportion of tests followed up by outpatient clinicians. By extending the analysis hospital‐wide, our study suggests that pending microbiology results occur for a wide range of hospital services. While our study was not designed to determine whether these results were followed up appropriately, opportunities for miscommunication and missed follow‐up likely exist for all specialties.
The potential harms associated with inadequate test follow‐up have gained the attention of the patient safety community. In 2005, the Joint Commission underscored the importance of proper communication of critical lab results.3, 5, 18 Their recommendations included the development of systems to ensure adequate follow‐up of critical results in high‐risk scenarios including the postdischarge period.5 While many of the microbiology results do not fall into the criticalcategory, we feel that these results should be considered for inclusion in hospital efforts to track postdischarge results. These efforts should also address issues specific to microbiology results, such as preliminary status before antibiotic sensitivities are known.
Developing a comprehensive strategy for test result communication is challenging, and more so for results that return after transitions of care. Even defining the proper target of communication interventions can involve complex organizational and cultural issues. As these results span the inpatient and outpatient domains, there may be some ambiguity as to which provider is responsible when the results return. The inpatient clinicians ordering the microbiology cultures are in the best position to put the results into the patient's clinical context. However, these clinicians may no longer be on clinical duty when the results return, or they may not have a system to ensure that they are notified about these results. While the outpatient providers may be available, they have often not seen the patient in follow‐up at the time the results return and would need to repeat a clinical assessment to determine whether a change in antibiotics is required. While many feel that the ordering provider is a logical choice to perform the follow‐up of the result, not all agree and few institutions have developed clear policies on this issue. To avoid this ambiguity, future work will require institutions to clearly outline which party is responsible for test result follow‐up during transitions of care.
Potential solutions to improve communication of these results must be tailored to the local infrastructure of the institution. In hospitals that do not have extensive electronic systems, a solution might involve a registered nurse, nurse practitioner, or lab technician whose responsibilities include identifying postdischarge results and communicating them to the ordering clinician, primary care provider, and patient. In settings with more advanced electronic infrastructure, solutions could be designed to automatically notify the responsible providers electronically, as well as post the results to a patient portal. Regardless of the level of technical sophistication, it is vital to create a system that has is highly reliable to prevent these important results from falling through the cracks.
Our study did have some limitations. First, we evaluated results from only one institution. It is unclear how substantially differences in practice patterns or patient populations would affect the number of postdischarge microbiology results in other settings. Second, we did not assess whether these results were actually followed up or whether treatment regimens were altered. As this study was retrospective in nature, we could not expect clinicians to recall the clinical scenarios surrounding each result and decided that documentation in clinical notes would be an unreliable indicator of whether any follow‐up action had been taken. Even without this information, however, we would submit that our findings represent a substantial near‐miss rate and threat to patient safety (approximately one potentially actionable, postdischarge microbiology result every other day for our hospital), and call for a fail‐safe system to ensure appropriate actions are taken.
In conclusion, microbiology results are often pending at the time patients are discharged from the hospital and roughly 2.4% of these results potentially require a change in therapy. This proportion was highest for urine cultures and cultures drawn from surgical patients. Our results suggest that a hospital‐wide system is warranted to ensure adequate communication of postdischarge microbiology results. Further research is required to evaluate the impact of such a system on the follow‐up rates of pending microbiology tests.
Acknowledgements
The authors thank Deborah Williams from the Brigham and Women's Division of General Medicine for her programming assistance.
- ,,, et al.Patient safety concerns arising from test results that return after hospital discharge.Ann Intern Med.2005;143(2):121–128.
- ,,, et al.Adequacy of hospital discharge summaries in documenting tests with pending results and outpatient follow‐up providers.J Gen Intern Med.2009;24(9):1002–1006.
- ,.Doing better with critical test results.Jt Comm J Qual Patient Saf.2005;31(2):61,66–67.
- .Fumbled handoffs: one dropped ball after another.Ann Intern Med.2005;142(5):352–358.
- ,,,.Communicating critical test results: safe practice recommendations.Jt Comm J Qual Patient Saf.2005;31(2):68–80.
- .Introduction: Communicating critical test results.Jt Comm J Qual Patient Saf.2005;31(2):61,63–65.
- ,,,.Growth in the care of older patients by hospitalists in the United States.N Engl J Med.2009;360(11):1102–1112.
- ,,.Gaps in the continuity of care and progress on patient safety.BMJ.2000;320(7237):791–794.
- .Key legal principles for hospitalists.Am J Med.2001;111(9B):5S–9S.
- ,,.Passing the clinical baton: 6 principles to guide the hospitalist.Am J Med.2001;111(9B):36S–39S.
- ,,.Dissemination of discharge summaries. Not reaching follow‐up physicians.Can Fam Physician.2002;48:737–742.
- ,,, et al.Communication and information deficits in patients discharged to rehabilitation facilities: an evaluation of five acute care hospitals.J Hosp Med.2009;4(8):E28–33.
- , , , , .Pending laboratory tests and the hospital discharge summary in patients discharged to sub‐acute care.J Gen Intern Med.2010;26(4):393–398.
- ,,,,,.Using electronic data to predict the probability of true bacteremia from positive blood cultures.Proc AMIA Symp.2000:893–897.
- ,,, et al.The nature of adverse events in hospitalized patients. Results of the Harvard Medical Practice Study II.N Engl J Med.1991;324(6):377–384.
- ,,, et al.Incidence and types of adverse events and negligent care in Utah and Colorado.Med Care.2000;38(3):261–271.
- ,,,.General practitioner‐hospital communications: a review of discharge summaries.J Qual Clin Pract.2001;21(4):104–108.
- ,,,,,.Failure to recognize and act on abnormal test results: the case of screening bone densitometry.Jt Comm J Qual Patient Saf.2005;31(2):90–97.
- ,,, et al.Patient safety concerns arising from test results that return after hospital discharge.Ann Intern Med.2005;143(2):121–128.
- ,,, et al.Adequacy of hospital discharge summaries in documenting tests with pending results and outpatient follow‐up providers.J Gen Intern Med.2009;24(9):1002–1006.
- ,.Doing better with critical test results.Jt Comm J Qual Patient Saf.2005;31(2):61,66–67.
- .Fumbled handoffs: one dropped ball after another.Ann Intern Med.2005;142(5):352–358.
- ,,,.Communicating critical test results: safe practice recommendations.Jt Comm J Qual Patient Saf.2005;31(2):68–80.
- .Introduction: Communicating critical test results.Jt Comm J Qual Patient Saf.2005;31(2):61,63–65.
- ,,,.Growth in the care of older patients by hospitalists in the United States.N Engl J Med.2009;360(11):1102–1112.
- ,,.Gaps in the continuity of care and progress on patient safety.BMJ.2000;320(7237):791–794.
- .Key legal principles for hospitalists.Am J Med.2001;111(9B):5S–9S.
- ,,.Passing the clinical baton: 6 principles to guide the hospitalist.Am J Med.2001;111(9B):36S–39S.
- ,,.Dissemination of discharge summaries. Not reaching follow‐up physicians.Can Fam Physician.2002;48:737–742.
- ,,, et al.Communication and information deficits in patients discharged to rehabilitation facilities: an evaluation of five acute care hospitals.J Hosp Med.2009;4(8):E28–33.
- , , , , .Pending laboratory tests and the hospital discharge summary in patients discharged to sub‐acute care.J Gen Intern Med.2010;26(4):393–398.
- ,,,,,.Using electronic data to predict the probability of true bacteremia from positive blood cultures.Proc AMIA Symp.2000:893–897.
- ,,, et al.The nature of adverse events in hospitalized patients. Results of the Harvard Medical Practice Study II.N Engl J Med.1991;324(6):377–384.
- ,,, et al.Incidence and types of adverse events and negligent care in Utah and Colorado.Med Care.2000;38(3):261–271.
- ,,,.General practitioner‐hospital communications: a review of discharge summaries.J Qual Clin Pract.2001;21(4):104–108.
- ,,,,,.Failure to recognize and act on abnormal test results: the case of screening bone densitometry.Jt Comm J Qual Patient Saf.2005;31(2):90–97.
Copyright © 2010 Society of Hospital Medicine