User login
Biomechanical Consequences of Anterior Femoral Notching in Cruciate-Retaining Versus Posterior-Stabilized Total Knee Arthroplasty
Although rare, periprosthetic fractures remain a significant complication after total knee arthroplasty (TKA), occurring in 0.3% to 2.5% of cases.1-4 Hirsh and colleagues5 were among the first to suggest that anterior femoral notching during TKA was a potential risk factor for postoperative periprosthetic femoral fracture because notching may weaken the anterior femoral cortex. Anterior femoral notching, a cortex violation occurring during an anterior bone cut, occurs in up to 30% of cases.6 Using a theoretical biomechanical model, Culp and colleagues1 found that increasing the depth of the notch defect into the cortex led to reduced torsional strength. In more recent, cadaveric biomechanical studies, notching of the anterior femoral cortex decreased torsional strength by up to 39%.7,8 Contrary to these biomechanical studies, a retrospective study evaluating 1089 TKAs using 2 implant designs (Anatomic Graduated Component, Biomet and Legacy, Zimmer) demonstrated no significant effect of anterior femoral notching with respect to incidence of supracondylar femur fractures.6 That study, however, did not address whether implant design is associated with a differential risk for fracture in the presence of anterior notching.
Previous biomechanical studies have primarily investigated cruciate-retaining (CR) femoral components and properties with respect to anterior notching, even though the posterior-stabilized (PS) design is used more often in the United States.1,7 According to a Mayo Clinic survey, TKAs with a PS design increased from <10% in 1990 to almost 75% by 1997.9 Today, there is little to no consensus about which implant is better, and use of one or the other depends largely on the surgeon and varies widely between countries and regions.10 PS designs require more bone resection and demonstrate prosthesis-controlled rollback during flexion, whereas CR designs preserve more bone and achieve posterior stabilization via the posterior cruciate ligament.11 Despite these differences in design and mechanics, a 2013 Cochrane review of TKA design found no clinically significant differences between CR and PS with respect to pain, range of motion, or clinical and radiologic outcomes.10 The reviewers did not specifically address periprosthetic fractures associated with either femoral notching or TKA design, as they could not quantitatively analyze postoperative complications because of the diversity of reports. Given the limited number of reported cases, a review of radiographic findings pertaining to the characteristics of supracondylar fractures in anterior femoral notching was unsuccessful.12 As the previous biomechanical studies of anterior notching used primarily CR models or no prostheses at all, a study of biomechanical differences between CR and PS designs in the presence of anterior notching is warranted.1,7,8 Therefore, we conducted a study to assess the effect of anterior femoral notching on torsional strength and load to failure in CR and PS femoral components.
Materials and Methods
Twelve fourth-generation composite adult left femur synthetic sawbones (Sawbones; Pacific Research Laboratories) were selected for their consistent biomechanical properties, vs those of cadaveric specimens; in addition, low intersample variability made them preferable to cadaveric bones given the small sample used in this study.13,14 All bones were from the same lot. All were visually inspected for defects and found to be acceptable. In each sample, an anterior cortical defect was created by making an anterior cut with an undersized (size 4) posterior referencing guide. In addition, the distance from the proximal end of the notch to the implant fell within 15 mm, as that is the maximum distance from the implant a notch can be placed using a standard femoral cutting jig.15 Six femora were instrumented with CR implants and 6 with PS implants (DePuy Synthes). Implants were placed using standardized cuts. Before testing, each implant was inspected for proper fit and found to be securely fastened to the femur. In addition, precision calipers were used to measure notch depth and distance from notch to implant before loading. A custom polymethylmethacrylate torsion jig was used to fix each instrumented femur proximally and distally on the femoral implant (Figure 1). Care was taken to ensure the distal jig engaged only the implant, thus isolating the notch as a stress riser. Each femur was loaded in external rotation through the proximal femoral jig along the anatomical axis. Use of external rotation was based on study findings implicating external rotation of the tibia as the most likely mechanism for generating a fracture in the event of a fall.12 Furthermore, distal femur fractures are predominantly spiral as opposed to butterfly or bending—an indication that torsion is the most likely mechanism of failure.16 With no axial rotation possible within the prosthesis, increased torsional stress is undoubtedly generated within adjacent bone. Each specimen underwent torsional stiffness testing and then load to failure. Torsional stiffness was measured by slowly loading each femur in external rotation, from 1 to 18 Nm for 3 cycles at a displacement rate of 0.5° per second. Each specimen then underwent torsional load-to-failure testing on an Instron 5800R machine at a rate of 0.5° per second. Failure was defined as the moment of fracture and subsequent decrease in torsional load—determined graphically by the peak torsional load followed immediately by a sharp decrease in load. Stiffness was determined as the slope of torque to the displacement curve for each cycle, and torque to failure was the highest recorded torque before fracture. Fracture pattern was noted after failure. A sample size of 6 specimens per group provided 80% power to detect a between-group difference of 1 Nm per degree in stiffness, using an estimated SD of 0.7 Nm per degree. In our statistical analysis, continuous variables are reported as means and SDs. Data from our torsional stiffness and load-to-failure testing were analyzed with unpaired 2-sample t tests, and P < .05 was considered statistically significant.
Results
We did not detect a statistical difference in notch depth, notch-to-implant distance, or femoral length between the CR and PS groups. Mean (SD) notch depth was 6.0 (1.3) mm for CR and 4.9 (1.0) mm for PS (P = .13); mean (SD) distance from the proximal end of the notch to the implant was 13.8 (1.7) mm for CR and 11.1 (3.2) mm for PS (P = .08); and mean (SD) femoral length was 46.2 (0.1) cm for CR and 46.2 (0.1) cm for PS (P = .60).
Mean (SD) torsional stiffness for the first 3 precycles was 6.2 (1.2), 8.7 (1.5), and 8.8 (1.4) Nm per degree for the CR group and 6.0 (0.7), 8.4 (1.4), and 8.6 (1.4) Nm per degree for the PS group; the differences were not statistically significant (Figure 2A). In addition, there were no statistically significant differences in mean (SD) stiffness at failure between CR, 6.5 (0.7) Nm per degree, and PS, 7.1 (0.9) Nm per degree (P = .24; Figure 2B) or in mean (SD) final torque at failure between CR, 62.4 (9.4) Nm, and PS, 62.7 (12.2) Nm (P = .95; Figure 2C).
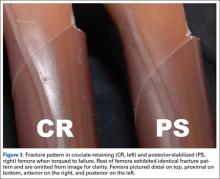
All fractures in both groups were oblique fractures originating at the proximal angle of the notch and extended proximally. None extended distally into the box. Fracture locations and patterns were identical in the CR and PS groups of femurs (Figure 3).
Discussion
Periprosthetic fractures after TKA remain rare. However, these fractures can significantly increase morbidity and complications. Anterior femoral notching occurs inadvertently in 30% to 40% of TKAs.6,17 The impact of femoral notching on supracondylar femur fracture is inconsistent between biomechanical and retrospective clinical studies. Retrospective studies failed to find a significant correlation between anterior femoral notching and supracondylar femur fractures.6,17 However, findings of biomechanical studies have suggested that a notch 3 mm deep will reduce the torsional strength of the femur by 29%.7 Another study, using 3-dimensional finite element analysis, showed a significant increase in local stress with a notch deeper than 3 mm.15
To our knowledge, no clinical studies, including the aforementioned Cochrane review,10 have specifically evaluated the difference in risk for periprosthetic fracture between different TKA models in the presence of notching.11 The biomechanical differences between implant designs could be a confounding factor in the results of past studies. More bone resection is required in PS designs than in CR designs. The position of the PS intercondylar cutout, much lower than the top of the patella flange, should not increase susceptibility to fractures more than in CR designs, but this hypothesis, though accepted, has not been validated biomechanically or addressed specifically in prospective or retrospective clinical analysis. In the present study, we used a biomechanical model to replicate an external rotation failure mechanism and quantify the differences in torsional strength and load to failure between CR TKA and PS TKA models in the presence of anterior femoral notching. Our results showed no significant differences in torsional stiffness, stiffness at failure, or torque at failure between the CR and PS design groups in the presence of anterior femoral notching.
In this study, all femoral fractures were oblique, and they all originated at the site of the cortical defect, not the notch—a situation markedly different from having bending forces applied to the femur. Previous biomechanical data indicated that bending forces applied to a notched femur cause fractures originating at the notch, whereas torsional forces applied to a notched femur cause fractures originating at the anterior aspect of the bone–component interface.7 The difference is attributable to study design. Our femurs were held fixed at their proximal end, which may have exacerbated any bending forces applied during external rotation, but we thought constraining the proximal femur would better replicate a fall involving external rotation.
More important for our study, an oblique fracture pattern was noted for both design groups (CR and PS), indicating the fracture pattern was unrelated to the area from which bone was resected for the PS design. All femur fractures in both design groups occurred proximal to a well-fixed prosthesis, indicating they should be classified as Vancouver C fractures. This is significant because intercondylar fossa resection (PS group) did not convert the fractures into Vancouver B2 fractures, which involve prosthesis loosening caused by pericomponent fracture.18 This simple observation validated our hypothesis that there would be no biomechanical differences between CR and PS designs with respect to the effects of anterior femoral notching. This lack of a significant difference may be attributed to the PS intercondylar cutout being much lower than the top of the anterior flange shielding the resected bone deep to the anterior flange.7 In addition, given the rarity of supracondylar fractures and the lack of sufficient relevant clinical data, it is difficult to speculate on the fracture patterns observed in clinical cases versus biomechanical studies.12
The use of synthetic bone models instead of cadaveric specimens could be seen as a limitation. Although synthetic bones may not reproduce the mechanism of failure in living and cadaveric femurs, the mechanical properties of synthetic bones have previously been found to fall within the range of those of cadaveric bones under axial loading, bending, and torsion testing.13,14 As a uniform testing material, synthetic bones allow removal of the confounding variations in bone size and quality that plague biomechanical studies in cadaveric bones.13,14 Interfemoral variability was 20 to 200 times higher in cadaveric femurs than in synthetic bones, which makes synthetic femurs preferable to cadaveric femurs, especially in studies with a small sample size.13,14 In addition, a uniform specimen provides consistent, reproducible osteotomies, which were crucial for consistent mechanical evaluation of each configuration in this study.
The long-term clinical significance of anterior femoral notching in periprosthetic fractures is equivocal, possibly because most studies predominantly use CR implants.6 This may not be an issue if it is shown that CR and PS implants have the same mechanical properties. Despite the differences between clinical studies and our biomechanical study, reevaluation of clinical data is not warranted given the biomechanical data we present here. Results of biomechanical studies like ours still suggest an increased immediate postoperative risk for supracondylar fracture after anterior cortical notching of the femur.5,7 Ultimately, this study found that, compared with a CR design, a PS design did not alter the torsional biomechanical properties or fracture pattern of an anteriorly notched femur.
1. Culp RW, Schmidt RG, Hanks G, Mak A, Esterhai JL Jr, Heppenstall RB. Supracondylar fracture of the femur following prosthetic knee arthroplasty. Clin Orthop Relat Res. 1987;(222):212-222.
2. Delport PH, Van Audekercke R, Martens M, Mulier JC. Conservative treatment of ipsilateral supracondylar femoral fracture after total knee arthroplasty. J Trauma. 1984;24(9):846-849.
3. Figgie MP, Goldberg VM, Figgie HE 3rd, Sobel M. The results of treatment of supracondylar fracture above total knee arthroplasty. J Arthroplasty. 1990;5(3):267-276.
4. Rorabeck CH, Taylor JW. Periprosthetic fractures of the femur complicating total knee arthroplasty. Orthop Clin North Am. 1999;30(2):265-277.
5. Hirsh DM, Bhalla S, Roffman M. Supracondylar fracture of the femur following total knee replacement. Report of four cases. J Bone Joint Surg Am. 1981;63(1):162-163.
6. Ritter MA, Thong AE, Keating EM, et al. The effect of femoral notching during total knee arthroplasty on the prevalence of postoperative femoral fractures and on clinical outcome. J Bone Joint Surg Am. 2005;87(11):2411-2414.
7. Lesh ML, Schneider DJ, Deol G, Davis B, Jacobs CR, Pellegrini VD Jr. The consequences of anterior femoral notching in total knee arthroplasty. A biomechanical study. J Bone Joint Surg Am. 2000;82(8):1096-1101.
8. Shawen SB, Belmont PJ Jr, Klemme WR, Topoleski LD, Xenos JS, Orchowski JR. Osteoporosis and anterior femoral notching in periprosthetic supracondylar femoral fractures: a biomechanical analysis. J Bone Joint Surg Am. 2003;85(1):115-121.
9. Scuderi GR, Pagnano MW. Review article: the rationale for posterior cruciate substituting total knee arthroplasty. J Orthop Surg (Hong Kong). 2001;9(2):81-88.
10. Verra WC, van den Boom LG, Jacobs W, Clement DJ, Wymenga AA, Nelissen RG. Retention versus sacrifice of the posterior cruciate ligament in total knee arthroplasty for treating osteoarthritis. Cochrane Database Syst Rev. 2013;10:CD004803.
11. Kolisek FR, McGrath MS, Marker DR, et al. Posterior-stabilized versus posterior cruciate ligament-retaining total knee arthroplasty. Iowa Orthop J. 2009;29:23-27.
12. Dennis DA. Periprosthetic fractures following total knee arthroplasty. Instr Course Lect. 2001;50:379-389.
13. Cristofolini L, Viceconti M, Cappello A, Toni A. Mechanical validation of whole bone composite femur models. J Biomech. 1996;29(4):525-535.
14. Heiner AD, Brown TD. Structural properties of a new design of composite replicate femurs and tibias. J Biomech. 2001;34(6):773-781.
15. Beals RK, Tower SS. Periprosthetic fractures of the femur. An analysis of 93 fractures. Clin Orthop Relat Res. 1996;(327):238-246.
16. Gujarathi N, Putti AB, Abboud RJ, MacLean JG, Espley AJ, Kellett CF. Risk of periprosthetic fracture after anterior femoral notching. Acta Orthop. 2009;80(5):553-556.
17. Zalzal P, Backstein D, Gross AE, Papini M. Notching of the anterior femoral cortex during total knee arthroplasty: characteristics that increase local stresses. J Arthroplasty. 2006;21(5):737-743.
18. Gaski GE, Scully SP. In brief: classifications in brief: Vancouver classification of postoperative periprosthetic femur fractures. Clin Orthop Relat Res. 2011;469(5):1507-1510.
Although rare, periprosthetic fractures remain a significant complication after total knee arthroplasty (TKA), occurring in 0.3% to 2.5% of cases.1-4 Hirsh and colleagues5 were among the first to suggest that anterior femoral notching during TKA was a potential risk factor for postoperative periprosthetic femoral fracture because notching may weaken the anterior femoral cortex. Anterior femoral notching, a cortex violation occurring during an anterior bone cut, occurs in up to 30% of cases.6 Using a theoretical biomechanical model, Culp and colleagues1 found that increasing the depth of the notch defect into the cortex led to reduced torsional strength. In more recent, cadaveric biomechanical studies, notching of the anterior femoral cortex decreased torsional strength by up to 39%.7,8 Contrary to these biomechanical studies, a retrospective study evaluating 1089 TKAs using 2 implant designs (Anatomic Graduated Component, Biomet and Legacy, Zimmer) demonstrated no significant effect of anterior femoral notching with respect to incidence of supracondylar femur fractures.6 That study, however, did not address whether implant design is associated with a differential risk for fracture in the presence of anterior notching.
Previous biomechanical studies have primarily investigated cruciate-retaining (CR) femoral components and properties with respect to anterior notching, even though the posterior-stabilized (PS) design is used more often in the United States.1,7 According to a Mayo Clinic survey, TKAs with a PS design increased from <10% in 1990 to almost 75% by 1997.9 Today, there is little to no consensus about which implant is better, and use of one or the other depends largely on the surgeon and varies widely between countries and regions.10 PS designs require more bone resection and demonstrate prosthesis-controlled rollback during flexion, whereas CR designs preserve more bone and achieve posterior stabilization via the posterior cruciate ligament.11 Despite these differences in design and mechanics, a 2013 Cochrane review of TKA design found no clinically significant differences between CR and PS with respect to pain, range of motion, or clinical and radiologic outcomes.10 The reviewers did not specifically address periprosthetic fractures associated with either femoral notching or TKA design, as they could not quantitatively analyze postoperative complications because of the diversity of reports. Given the limited number of reported cases, a review of radiographic findings pertaining to the characteristics of supracondylar fractures in anterior femoral notching was unsuccessful.12 As the previous biomechanical studies of anterior notching used primarily CR models or no prostheses at all, a study of biomechanical differences between CR and PS designs in the presence of anterior notching is warranted.1,7,8 Therefore, we conducted a study to assess the effect of anterior femoral notching on torsional strength and load to failure in CR and PS femoral components.
Materials and Methods
Twelve fourth-generation composite adult left femur synthetic sawbones (Sawbones; Pacific Research Laboratories) were selected for their consistent biomechanical properties, vs those of cadaveric specimens; in addition, low intersample variability made them preferable to cadaveric bones given the small sample used in this study.13,14 All bones were from the same lot. All were visually inspected for defects and found to be acceptable. In each sample, an anterior cortical defect was created by making an anterior cut with an undersized (size 4) posterior referencing guide. In addition, the distance from the proximal end of the notch to the implant fell within 15 mm, as that is the maximum distance from the implant a notch can be placed using a standard femoral cutting jig.15 Six femora were instrumented with CR implants and 6 with PS implants (DePuy Synthes). Implants were placed using standardized cuts. Before testing, each implant was inspected for proper fit and found to be securely fastened to the femur. In addition, precision calipers were used to measure notch depth and distance from notch to implant before loading. A custom polymethylmethacrylate torsion jig was used to fix each instrumented femur proximally and distally on the femoral implant (Figure 1). Care was taken to ensure the distal jig engaged only the implant, thus isolating the notch as a stress riser. Each femur was loaded in external rotation through the proximal femoral jig along the anatomical axis. Use of external rotation was based on study findings implicating external rotation of the tibia as the most likely mechanism for generating a fracture in the event of a fall.12 Furthermore, distal femur fractures are predominantly spiral as opposed to butterfly or bending—an indication that torsion is the most likely mechanism of failure.16 With no axial rotation possible within the prosthesis, increased torsional stress is undoubtedly generated within adjacent bone. Each specimen underwent torsional stiffness testing and then load to failure. Torsional stiffness was measured by slowly loading each femur in external rotation, from 1 to 18 Nm for 3 cycles at a displacement rate of 0.5° per second. Each specimen then underwent torsional load-to-failure testing on an Instron 5800R machine at a rate of 0.5° per second. Failure was defined as the moment of fracture and subsequent decrease in torsional load—determined graphically by the peak torsional load followed immediately by a sharp decrease in load. Stiffness was determined as the slope of torque to the displacement curve for each cycle, and torque to failure was the highest recorded torque before fracture. Fracture pattern was noted after failure. A sample size of 6 specimens per group provided 80% power to detect a between-group difference of 1 Nm per degree in stiffness, using an estimated SD of 0.7 Nm per degree. In our statistical analysis, continuous variables are reported as means and SDs. Data from our torsional stiffness and load-to-failure testing were analyzed with unpaired 2-sample t tests, and P < .05 was considered statistically significant.
Results
We did not detect a statistical difference in notch depth, notch-to-implant distance, or femoral length between the CR and PS groups. Mean (SD) notch depth was 6.0 (1.3) mm for CR and 4.9 (1.0) mm for PS (P = .13); mean (SD) distance from the proximal end of the notch to the implant was 13.8 (1.7) mm for CR and 11.1 (3.2) mm for PS (P = .08); and mean (SD) femoral length was 46.2 (0.1) cm for CR and 46.2 (0.1) cm for PS (P = .60).
Mean (SD) torsional stiffness for the first 3 precycles was 6.2 (1.2), 8.7 (1.5), and 8.8 (1.4) Nm per degree for the CR group and 6.0 (0.7), 8.4 (1.4), and 8.6 (1.4) Nm per degree for the PS group; the differences were not statistically significant (Figure 2A). In addition, there were no statistically significant differences in mean (SD) stiffness at failure between CR, 6.5 (0.7) Nm per degree, and PS, 7.1 (0.9) Nm per degree (P = .24; Figure 2B) or in mean (SD) final torque at failure between CR, 62.4 (9.4) Nm, and PS, 62.7 (12.2) Nm (P = .95; Figure 2C).
All fractures in both groups were oblique fractures originating at the proximal angle of the notch and extended proximally. None extended distally into the box. Fracture locations and patterns were identical in the CR and PS groups of femurs (Figure 3).
Discussion
Periprosthetic fractures after TKA remain rare. However, these fractures can significantly increase morbidity and complications. Anterior femoral notching occurs inadvertently in 30% to 40% of TKAs.6,17 The impact of femoral notching on supracondylar femur fracture is inconsistent between biomechanical and retrospective clinical studies. Retrospective studies failed to find a significant correlation between anterior femoral notching and supracondylar femur fractures.6,17 However, findings of biomechanical studies have suggested that a notch 3 mm deep will reduce the torsional strength of the femur by 29%.7 Another study, using 3-dimensional finite element analysis, showed a significant increase in local stress with a notch deeper than 3 mm.15
To our knowledge, no clinical studies, including the aforementioned Cochrane review,10 have specifically evaluated the difference in risk for periprosthetic fracture between different TKA models in the presence of notching.11 The biomechanical differences between implant designs could be a confounding factor in the results of past studies. More bone resection is required in PS designs than in CR designs. The position of the PS intercondylar cutout, much lower than the top of the patella flange, should not increase susceptibility to fractures more than in CR designs, but this hypothesis, though accepted, has not been validated biomechanically or addressed specifically in prospective or retrospective clinical analysis. In the present study, we used a biomechanical model to replicate an external rotation failure mechanism and quantify the differences in torsional strength and load to failure between CR TKA and PS TKA models in the presence of anterior femoral notching. Our results showed no significant differences in torsional stiffness, stiffness at failure, or torque at failure between the CR and PS design groups in the presence of anterior femoral notching.
In this study, all femoral fractures were oblique, and they all originated at the site of the cortical defect, not the notch—a situation markedly different from having bending forces applied to the femur. Previous biomechanical data indicated that bending forces applied to a notched femur cause fractures originating at the notch, whereas torsional forces applied to a notched femur cause fractures originating at the anterior aspect of the bone–component interface.7 The difference is attributable to study design. Our femurs were held fixed at their proximal end, which may have exacerbated any bending forces applied during external rotation, but we thought constraining the proximal femur would better replicate a fall involving external rotation.
More important for our study, an oblique fracture pattern was noted for both design groups (CR and PS), indicating the fracture pattern was unrelated to the area from which bone was resected for the PS design. All femur fractures in both design groups occurred proximal to a well-fixed prosthesis, indicating they should be classified as Vancouver C fractures. This is significant because intercondylar fossa resection (PS group) did not convert the fractures into Vancouver B2 fractures, which involve prosthesis loosening caused by pericomponent fracture.18 This simple observation validated our hypothesis that there would be no biomechanical differences between CR and PS designs with respect to the effects of anterior femoral notching. This lack of a significant difference may be attributed to the PS intercondylar cutout being much lower than the top of the anterior flange shielding the resected bone deep to the anterior flange.7 In addition, given the rarity of supracondylar fractures and the lack of sufficient relevant clinical data, it is difficult to speculate on the fracture patterns observed in clinical cases versus biomechanical studies.12
The use of synthetic bone models instead of cadaveric specimens could be seen as a limitation. Although synthetic bones may not reproduce the mechanism of failure in living and cadaveric femurs, the mechanical properties of synthetic bones have previously been found to fall within the range of those of cadaveric bones under axial loading, bending, and torsion testing.13,14 As a uniform testing material, synthetic bones allow removal of the confounding variations in bone size and quality that plague biomechanical studies in cadaveric bones.13,14 Interfemoral variability was 20 to 200 times higher in cadaveric femurs than in synthetic bones, which makes synthetic femurs preferable to cadaveric femurs, especially in studies with a small sample size.13,14 In addition, a uniform specimen provides consistent, reproducible osteotomies, which were crucial for consistent mechanical evaluation of each configuration in this study.
The long-term clinical significance of anterior femoral notching in periprosthetic fractures is equivocal, possibly because most studies predominantly use CR implants.6 This may not be an issue if it is shown that CR and PS implants have the same mechanical properties. Despite the differences between clinical studies and our biomechanical study, reevaluation of clinical data is not warranted given the biomechanical data we present here. Results of biomechanical studies like ours still suggest an increased immediate postoperative risk for supracondylar fracture after anterior cortical notching of the femur.5,7 Ultimately, this study found that, compared with a CR design, a PS design did not alter the torsional biomechanical properties or fracture pattern of an anteriorly notched femur.
Although rare, periprosthetic fractures remain a significant complication after total knee arthroplasty (TKA), occurring in 0.3% to 2.5% of cases.1-4 Hirsh and colleagues5 were among the first to suggest that anterior femoral notching during TKA was a potential risk factor for postoperative periprosthetic femoral fracture because notching may weaken the anterior femoral cortex. Anterior femoral notching, a cortex violation occurring during an anterior bone cut, occurs in up to 30% of cases.6 Using a theoretical biomechanical model, Culp and colleagues1 found that increasing the depth of the notch defect into the cortex led to reduced torsional strength. In more recent, cadaveric biomechanical studies, notching of the anterior femoral cortex decreased torsional strength by up to 39%.7,8 Contrary to these biomechanical studies, a retrospective study evaluating 1089 TKAs using 2 implant designs (Anatomic Graduated Component, Biomet and Legacy, Zimmer) demonstrated no significant effect of anterior femoral notching with respect to incidence of supracondylar femur fractures.6 That study, however, did not address whether implant design is associated with a differential risk for fracture in the presence of anterior notching.
Previous biomechanical studies have primarily investigated cruciate-retaining (CR) femoral components and properties with respect to anterior notching, even though the posterior-stabilized (PS) design is used more often in the United States.1,7 According to a Mayo Clinic survey, TKAs with a PS design increased from <10% in 1990 to almost 75% by 1997.9 Today, there is little to no consensus about which implant is better, and use of one or the other depends largely on the surgeon and varies widely between countries and regions.10 PS designs require more bone resection and demonstrate prosthesis-controlled rollback during flexion, whereas CR designs preserve more bone and achieve posterior stabilization via the posterior cruciate ligament.11 Despite these differences in design and mechanics, a 2013 Cochrane review of TKA design found no clinically significant differences between CR and PS with respect to pain, range of motion, or clinical and radiologic outcomes.10 The reviewers did not specifically address periprosthetic fractures associated with either femoral notching or TKA design, as they could not quantitatively analyze postoperative complications because of the diversity of reports. Given the limited number of reported cases, a review of radiographic findings pertaining to the characteristics of supracondylar fractures in anterior femoral notching was unsuccessful.12 As the previous biomechanical studies of anterior notching used primarily CR models or no prostheses at all, a study of biomechanical differences between CR and PS designs in the presence of anterior notching is warranted.1,7,8 Therefore, we conducted a study to assess the effect of anterior femoral notching on torsional strength and load to failure in CR and PS femoral components.
Materials and Methods
Twelve fourth-generation composite adult left femur synthetic sawbones (Sawbones; Pacific Research Laboratories) were selected for their consistent biomechanical properties, vs those of cadaveric specimens; in addition, low intersample variability made them preferable to cadaveric bones given the small sample used in this study.13,14 All bones were from the same lot. All were visually inspected for defects and found to be acceptable. In each sample, an anterior cortical defect was created by making an anterior cut with an undersized (size 4) posterior referencing guide. In addition, the distance from the proximal end of the notch to the implant fell within 15 mm, as that is the maximum distance from the implant a notch can be placed using a standard femoral cutting jig.15 Six femora were instrumented with CR implants and 6 with PS implants (DePuy Synthes). Implants were placed using standardized cuts. Before testing, each implant was inspected for proper fit and found to be securely fastened to the femur. In addition, precision calipers were used to measure notch depth and distance from notch to implant before loading. A custom polymethylmethacrylate torsion jig was used to fix each instrumented femur proximally and distally on the femoral implant (Figure 1). Care was taken to ensure the distal jig engaged only the implant, thus isolating the notch as a stress riser. Each femur was loaded in external rotation through the proximal femoral jig along the anatomical axis. Use of external rotation was based on study findings implicating external rotation of the tibia as the most likely mechanism for generating a fracture in the event of a fall.12 Furthermore, distal femur fractures are predominantly spiral as opposed to butterfly or bending—an indication that torsion is the most likely mechanism of failure.16 With no axial rotation possible within the prosthesis, increased torsional stress is undoubtedly generated within adjacent bone. Each specimen underwent torsional stiffness testing and then load to failure. Torsional stiffness was measured by slowly loading each femur in external rotation, from 1 to 18 Nm for 3 cycles at a displacement rate of 0.5° per second. Each specimen then underwent torsional load-to-failure testing on an Instron 5800R machine at a rate of 0.5° per second. Failure was defined as the moment of fracture and subsequent decrease in torsional load—determined graphically by the peak torsional load followed immediately by a sharp decrease in load. Stiffness was determined as the slope of torque to the displacement curve for each cycle, and torque to failure was the highest recorded torque before fracture. Fracture pattern was noted after failure. A sample size of 6 specimens per group provided 80% power to detect a between-group difference of 1 Nm per degree in stiffness, using an estimated SD of 0.7 Nm per degree. In our statistical analysis, continuous variables are reported as means and SDs. Data from our torsional stiffness and load-to-failure testing were analyzed with unpaired 2-sample t tests, and P < .05 was considered statistically significant.
Results
We did not detect a statistical difference in notch depth, notch-to-implant distance, or femoral length between the CR and PS groups. Mean (SD) notch depth was 6.0 (1.3) mm for CR and 4.9 (1.0) mm for PS (P = .13); mean (SD) distance from the proximal end of the notch to the implant was 13.8 (1.7) mm for CR and 11.1 (3.2) mm for PS (P = .08); and mean (SD) femoral length was 46.2 (0.1) cm for CR and 46.2 (0.1) cm for PS (P = .60).
Mean (SD) torsional stiffness for the first 3 precycles was 6.2 (1.2), 8.7 (1.5), and 8.8 (1.4) Nm per degree for the CR group and 6.0 (0.7), 8.4 (1.4), and 8.6 (1.4) Nm per degree for the PS group; the differences were not statistically significant (Figure 2A). In addition, there were no statistically significant differences in mean (SD) stiffness at failure between CR, 6.5 (0.7) Nm per degree, and PS, 7.1 (0.9) Nm per degree (P = .24; Figure 2B) or in mean (SD) final torque at failure between CR, 62.4 (9.4) Nm, and PS, 62.7 (12.2) Nm (P = .95; Figure 2C).
All fractures in both groups were oblique fractures originating at the proximal angle of the notch and extended proximally. None extended distally into the box. Fracture locations and patterns were identical in the CR and PS groups of femurs (Figure 3).
Discussion
Periprosthetic fractures after TKA remain rare. However, these fractures can significantly increase morbidity and complications. Anterior femoral notching occurs inadvertently in 30% to 40% of TKAs.6,17 The impact of femoral notching on supracondylar femur fracture is inconsistent between biomechanical and retrospective clinical studies. Retrospective studies failed to find a significant correlation between anterior femoral notching and supracondylar femur fractures.6,17 However, findings of biomechanical studies have suggested that a notch 3 mm deep will reduce the torsional strength of the femur by 29%.7 Another study, using 3-dimensional finite element analysis, showed a significant increase in local stress with a notch deeper than 3 mm.15
To our knowledge, no clinical studies, including the aforementioned Cochrane review,10 have specifically evaluated the difference in risk for periprosthetic fracture between different TKA models in the presence of notching.11 The biomechanical differences between implant designs could be a confounding factor in the results of past studies. More bone resection is required in PS designs than in CR designs. The position of the PS intercondylar cutout, much lower than the top of the patella flange, should not increase susceptibility to fractures more than in CR designs, but this hypothesis, though accepted, has not been validated biomechanically or addressed specifically in prospective or retrospective clinical analysis. In the present study, we used a biomechanical model to replicate an external rotation failure mechanism and quantify the differences in torsional strength and load to failure between CR TKA and PS TKA models in the presence of anterior femoral notching. Our results showed no significant differences in torsional stiffness, stiffness at failure, or torque at failure between the CR and PS design groups in the presence of anterior femoral notching.
In this study, all femoral fractures were oblique, and they all originated at the site of the cortical defect, not the notch—a situation markedly different from having bending forces applied to the femur. Previous biomechanical data indicated that bending forces applied to a notched femur cause fractures originating at the notch, whereas torsional forces applied to a notched femur cause fractures originating at the anterior aspect of the bone–component interface.7 The difference is attributable to study design. Our femurs were held fixed at their proximal end, which may have exacerbated any bending forces applied during external rotation, but we thought constraining the proximal femur would better replicate a fall involving external rotation.
More important for our study, an oblique fracture pattern was noted for both design groups (CR and PS), indicating the fracture pattern was unrelated to the area from which bone was resected for the PS design. All femur fractures in both design groups occurred proximal to a well-fixed prosthesis, indicating they should be classified as Vancouver C fractures. This is significant because intercondylar fossa resection (PS group) did not convert the fractures into Vancouver B2 fractures, which involve prosthesis loosening caused by pericomponent fracture.18 This simple observation validated our hypothesis that there would be no biomechanical differences between CR and PS designs with respect to the effects of anterior femoral notching. This lack of a significant difference may be attributed to the PS intercondylar cutout being much lower than the top of the anterior flange shielding the resected bone deep to the anterior flange.7 In addition, given the rarity of supracondylar fractures and the lack of sufficient relevant clinical data, it is difficult to speculate on the fracture patterns observed in clinical cases versus biomechanical studies.12
The use of synthetic bone models instead of cadaveric specimens could be seen as a limitation. Although synthetic bones may not reproduce the mechanism of failure in living and cadaveric femurs, the mechanical properties of synthetic bones have previously been found to fall within the range of those of cadaveric bones under axial loading, bending, and torsion testing.13,14 As a uniform testing material, synthetic bones allow removal of the confounding variations in bone size and quality that plague biomechanical studies in cadaveric bones.13,14 Interfemoral variability was 20 to 200 times higher in cadaveric femurs than in synthetic bones, which makes synthetic femurs preferable to cadaveric femurs, especially in studies with a small sample size.13,14 In addition, a uniform specimen provides consistent, reproducible osteotomies, which were crucial for consistent mechanical evaluation of each configuration in this study.
The long-term clinical significance of anterior femoral notching in periprosthetic fractures is equivocal, possibly because most studies predominantly use CR implants.6 This may not be an issue if it is shown that CR and PS implants have the same mechanical properties. Despite the differences between clinical studies and our biomechanical study, reevaluation of clinical data is not warranted given the biomechanical data we present here. Results of biomechanical studies like ours still suggest an increased immediate postoperative risk for supracondylar fracture after anterior cortical notching of the femur.5,7 Ultimately, this study found that, compared with a CR design, a PS design did not alter the torsional biomechanical properties or fracture pattern of an anteriorly notched femur.
1. Culp RW, Schmidt RG, Hanks G, Mak A, Esterhai JL Jr, Heppenstall RB. Supracondylar fracture of the femur following prosthetic knee arthroplasty. Clin Orthop Relat Res. 1987;(222):212-222.
2. Delport PH, Van Audekercke R, Martens M, Mulier JC. Conservative treatment of ipsilateral supracondylar femoral fracture after total knee arthroplasty. J Trauma. 1984;24(9):846-849.
3. Figgie MP, Goldberg VM, Figgie HE 3rd, Sobel M. The results of treatment of supracondylar fracture above total knee arthroplasty. J Arthroplasty. 1990;5(3):267-276.
4. Rorabeck CH, Taylor JW. Periprosthetic fractures of the femur complicating total knee arthroplasty. Orthop Clin North Am. 1999;30(2):265-277.
5. Hirsh DM, Bhalla S, Roffman M. Supracondylar fracture of the femur following total knee replacement. Report of four cases. J Bone Joint Surg Am. 1981;63(1):162-163.
6. Ritter MA, Thong AE, Keating EM, et al. The effect of femoral notching during total knee arthroplasty on the prevalence of postoperative femoral fractures and on clinical outcome. J Bone Joint Surg Am. 2005;87(11):2411-2414.
7. Lesh ML, Schneider DJ, Deol G, Davis B, Jacobs CR, Pellegrini VD Jr. The consequences of anterior femoral notching in total knee arthroplasty. A biomechanical study. J Bone Joint Surg Am. 2000;82(8):1096-1101.
8. Shawen SB, Belmont PJ Jr, Klemme WR, Topoleski LD, Xenos JS, Orchowski JR. Osteoporosis and anterior femoral notching in periprosthetic supracondylar femoral fractures: a biomechanical analysis. J Bone Joint Surg Am. 2003;85(1):115-121.
9. Scuderi GR, Pagnano MW. Review article: the rationale for posterior cruciate substituting total knee arthroplasty. J Orthop Surg (Hong Kong). 2001;9(2):81-88.
10. Verra WC, van den Boom LG, Jacobs W, Clement DJ, Wymenga AA, Nelissen RG. Retention versus sacrifice of the posterior cruciate ligament in total knee arthroplasty for treating osteoarthritis. Cochrane Database Syst Rev. 2013;10:CD004803.
11. Kolisek FR, McGrath MS, Marker DR, et al. Posterior-stabilized versus posterior cruciate ligament-retaining total knee arthroplasty. Iowa Orthop J. 2009;29:23-27.
12. Dennis DA. Periprosthetic fractures following total knee arthroplasty. Instr Course Lect. 2001;50:379-389.
13. Cristofolini L, Viceconti M, Cappello A, Toni A. Mechanical validation of whole bone composite femur models. J Biomech. 1996;29(4):525-535.
14. Heiner AD, Brown TD. Structural properties of a new design of composite replicate femurs and tibias. J Biomech. 2001;34(6):773-781.
15. Beals RK, Tower SS. Periprosthetic fractures of the femur. An analysis of 93 fractures. Clin Orthop Relat Res. 1996;(327):238-246.
16. Gujarathi N, Putti AB, Abboud RJ, MacLean JG, Espley AJ, Kellett CF. Risk of periprosthetic fracture after anterior femoral notching. Acta Orthop. 2009;80(5):553-556.
17. Zalzal P, Backstein D, Gross AE, Papini M. Notching of the anterior femoral cortex during total knee arthroplasty: characteristics that increase local stresses. J Arthroplasty. 2006;21(5):737-743.
18. Gaski GE, Scully SP. In brief: classifications in brief: Vancouver classification of postoperative periprosthetic femur fractures. Clin Orthop Relat Res. 2011;469(5):1507-1510.
1. Culp RW, Schmidt RG, Hanks G, Mak A, Esterhai JL Jr, Heppenstall RB. Supracondylar fracture of the femur following prosthetic knee arthroplasty. Clin Orthop Relat Res. 1987;(222):212-222.
2. Delport PH, Van Audekercke R, Martens M, Mulier JC. Conservative treatment of ipsilateral supracondylar femoral fracture after total knee arthroplasty. J Trauma. 1984;24(9):846-849.
3. Figgie MP, Goldberg VM, Figgie HE 3rd, Sobel M. The results of treatment of supracondylar fracture above total knee arthroplasty. J Arthroplasty. 1990;5(3):267-276.
4. Rorabeck CH, Taylor JW. Periprosthetic fractures of the femur complicating total knee arthroplasty. Orthop Clin North Am. 1999;30(2):265-277.
5. Hirsh DM, Bhalla S, Roffman M. Supracondylar fracture of the femur following total knee replacement. Report of four cases. J Bone Joint Surg Am. 1981;63(1):162-163.
6. Ritter MA, Thong AE, Keating EM, et al. The effect of femoral notching during total knee arthroplasty on the prevalence of postoperative femoral fractures and on clinical outcome. J Bone Joint Surg Am. 2005;87(11):2411-2414.
7. Lesh ML, Schneider DJ, Deol G, Davis B, Jacobs CR, Pellegrini VD Jr. The consequences of anterior femoral notching in total knee arthroplasty. A biomechanical study. J Bone Joint Surg Am. 2000;82(8):1096-1101.
8. Shawen SB, Belmont PJ Jr, Klemme WR, Topoleski LD, Xenos JS, Orchowski JR. Osteoporosis and anterior femoral notching in periprosthetic supracondylar femoral fractures: a biomechanical analysis. J Bone Joint Surg Am. 2003;85(1):115-121.
9. Scuderi GR, Pagnano MW. Review article: the rationale for posterior cruciate substituting total knee arthroplasty. J Orthop Surg (Hong Kong). 2001;9(2):81-88.
10. Verra WC, van den Boom LG, Jacobs W, Clement DJ, Wymenga AA, Nelissen RG. Retention versus sacrifice of the posterior cruciate ligament in total knee arthroplasty for treating osteoarthritis. Cochrane Database Syst Rev. 2013;10:CD004803.
11. Kolisek FR, McGrath MS, Marker DR, et al. Posterior-stabilized versus posterior cruciate ligament-retaining total knee arthroplasty. Iowa Orthop J. 2009;29:23-27.
12. Dennis DA. Periprosthetic fractures following total knee arthroplasty. Instr Course Lect. 2001;50:379-389.
13. Cristofolini L, Viceconti M, Cappello A, Toni A. Mechanical validation of whole bone composite femur models. J Biomech. 1996;29(4):525-535.
14. Heiner AD, Brown TD. Structural properties of a new design of composite replicate femurs and tibias. J Biomech. 2001;34(6):773-781.
15. Beals RK, Tower SS. Periprosthetic fractures of the femur. An analysis of 93 fractures. Clin Orthop Relat Res. 1996;(327):238-246.
16. Gujarathi N, Putti AB, Abboud RJ, MacLean JG, Espley AJ, Kellett CF. Risk of periprosthetic fracture after anterior femoral notching. Acta Orthop. 2009;80(5):553-556.
17. Zalzal P, Backstein D, Gross AE, Papini M. Notching of the anterior femoral cortex during total knee arthroplasty: characteristics that increase local stresses. J Arthroplasty. 2006;21(5):737-743.
18. Gaski GE, Scully SP. In brief: classifications in brief: Vancouver classification of postoperative periprosthetic femur fractures. Clin Orthop Relat Res. 2011;469(5):1507-1510.
Periprosthetic Supracondylar Femur Fracture Treated With Spanning External Fixation
The incidence of periprosthetic supracondylar fractures of the femur after total knee arthroplasty (TKA) ranges from 0.6% to 2.5%.1 Treatment of periprosthetic fractures is often complicated by advanced patient age and osteoporosis, which frequently accompanies these fractures. Management of a periprosthetic fracture depends on the relation between the fracture site and the prosthesis, displacement of the prosthesis, integrity of the fixation of the prosthesis, extent of the bone loss caused by fracture comminution or preexisting osteolysis, general health of the patient, and surgeon expertise.2,3 The aim is to achieve fracture union around a stable, well-aligned arthroplasty with preserved or restored bone stock and therefore to return the patient to previous level of function. Although nonoperative treatments have been shown to be successful,4,5 in the great majority of cases surgical treatment is advised for these fractures.6-10 In cases in which bone stock is adequate for fixation rather than replacement of the distal femur, 2 modalities are commonly used: retrograde intramedullary nailing and locking plates. Each has its drawbacks and advantages.11,12
Although external fixation has been used in the treatment of distal femoral fractures,13 it is seldom considered in the treatment of periprosthetic fractures. Several authors have described cases that used external fixators, occasionally spanning the knee. The specific types of external fixators discussed in the literature have included ring fixators,14-17 hybrid fixators,18 and uniplanar nonspanning fixators14,19 (Table). Use of a simple anterior spanning external fixator in treating a periprosthetic femoral fracture has received little attention in the literature.
The patient provided written informed consent for print and electronic publication of this case report.
Case Report
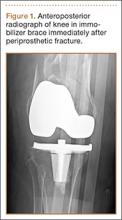
A 54-year-old woman with previous total hip arthroplasty (THA) and ipsilateral TKA tripped on a carpet and sustained a comminuted fracture of the distal femur just above the TKA prosthesis (Figure 1). She was a Jehovah’s Witness and thus refused all blood products. She had an extensive history of osteoporosis, morbid obesity (5 feet tall, 250 pounds; body mass index, 49), diabetes, and rheumatoid arthritis. Evaluation by the internal medicine service revealed severe coronary artery disease on a stress thallium test and anemia with hematocrit of 24%. Given the patient’s medical comorbidities and religious status, and the location of the comminuted distal femur fracture, several treatment options were considered. First was nonoperative treatment with a cast or cast-brace (hinged cast). Because of her body habitus, however, we thought she would very likely experience skin complications, inadequate immobilization of the bone, and significant discomfort. Ultimately, use of a spanning external fixator was chosen as the safest course, given the significant medical risks accompanying a more extensive surgical reconstruction. With the spanning external fixator, the main risks were the inability to fully control fracture alignment and the potential introduction of infection into the functional THA. We thought that, by limiting the amount of time in the fixator and managing the pin site aggressively, we could minimize the risk for infection in this setting.
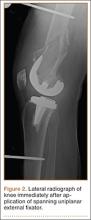
The procedure was performed with the patient under general anesthesia. During surgery, a lateral image of the femur was used to identify the distal end of the THA prosthesis. A level was marked 2 to 3 cm distal to the tip of this prosthesis, and another about 1 cm above the fracture (noted to be above the most proximal extent of the knee joint). These planned pin-entry sites were prepared from an anterior approach with incisions (using a No. 11 blade) of about 1 cm each. Blunt dissection was carried down to the femur. Each planned pin site was predrilled with a 3.5-mm drill; then, a 5-mm Shanz pin was placed. This process was repeated immediately distal to the tibial component and at the junction of the mid and distal thirds of the tibia (Figure 2). The preliminary external fixator frame was then applied. Once the reduction was satisfactory in the anteroposterior and lateral planes, the fixator clamps were tightened. A second row of bars was then incorporated.
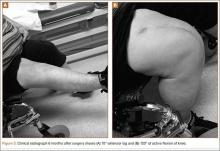
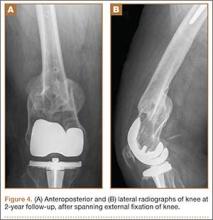
Six weeks after surgery, radiographs showed early callus formation. Removing the external fixator and examining the knee under anesthesia confirmed there was no significant motion through the fracture site. A cast-brace (fiberglass thigh segment, fiberglass lower leg cast with hinged knee segment) was then applied. We remained concerned about skin complications but were encouraged by the early healing achieved with the fixator. The patient was started on a physical therapy program of gait training with a walker and toe-touch weight-bearing on the injured extremity. She also started a limited lower-extremity strengthening program. Three months after surgery, she was tolerating weight-bearing on the injured extremity with no pain. At 6 months, knee radiographs showed fracture consolidation with active range of motion of 10° to 120° and no pain (Figures 3A, 3B). Distal sensation, motor function, and vascular examination were normal. Two years after surgery, radiographs of the right knee showed minor malalignment in the coronal and sagittal planes (Figures 4A, 4B) and complete consolidation of the fracture.
Discussion
Periprosthetic fractures of the femur after TKA often occur in the setting of osteopenia, and some are associated with concurrent implant loosening. In most cases, these fractures require surgical stabilization. Nevertheless, the goals of treatment are to obtain and maintain anatomical alignment and stability to allow early range of motion. Nonoperative options include skeletal traction, cast, pins and plaster, and cast-brace.3-5,20 Operative options include intramedullary fixation,12,21 stabilization with various plates,21-23 revision knee arthroplasty, and arthrodesis.1 Treatment selection should be based on patient health, fracture displacement, comminution, osteopenia severity, and status of the prosthetic components.
The present case exemplifies some of the highest degrees of medical and surgical risk factors in people with a periprosthetic femoral fracture after TKA. Patients with rheumatoid arthritis, patients having corticosteroid treatment, patients of advanced age, and female patients are all at higher risk for supracondylar femoral fracture.9 Our patient had these risk factors on a background of anemia and extensive coronary artery disease. Given her past medical history and refusal of blood products out of religious belief, we thought she was too high risk for extensive surgical treatment for her fracture. In addition, she was not an ideal candidate for nonoperative treatment, as a periprosthetic fracture typically is treated with surgical revision or open reduction and internal fixation. Therefore, we selected an unconventional treatment modality, typically used as a temporizing measure in severe fractures around the knee—a spanning external fixator worn for 6 weeks and a cast-brace for an additional 6 weeks. This led to successful clinical and radiographic outcomes. We consider spanning external fixation a viable option for periprosthetic fractures after TKA in morbidly obese patients with relatively well-aligned fractures and extremely high risk for medical complications associated with traditional open surgery.
1. Figgie MP, Goldberg VM, Figgie HE 3rd, Sobel M. The results of treatment of supracondylar fracture above total knee arthroplasty. J Arthroplasty. 1990;5(3):267-276.
2. Su ET, Kubiak EN, Dewal H, Hiebert R, Di Cesare PE. A proposed classification of supracondylar femur fractures above total knee arthroplasties. J Arthroplasty. 2006;21(3):405-408.
3. Kim KI, Egol KA, Hozack WJ, Parvizi J. Periprosthetic fractures after total knee arthroplasties. Clin Orthop. 2006;(446):167-175.
4. Sochart DH, Hardinge K. Nonsurgical management of supracondylar fracture above total knee arthroplasty. Still the nineties option. J Arthroplasty. 1997;12(7):830-834.
5. Delport PH, Van Audekercke R, Martens M, Mulier JC. Conservative treatment of ipsilateral supracondylar femoral fracture after total knee arthroplasty. J Trauma. 1984;24(9):846-849.
6. Frigg R, Appenzeller A, Christensen R, Frenk A, Gilbert S, Schavan R. The development of the distal femur less invasive stabilization system (LISS). Injury. 2001;32(suppl 3):SC24-SC31.
7. Goesling T, Frenk A, Appenzeller A, Garapati R, Marti A, Krettek C. LISS PLT: design, mechanical and biomechanical characteristics. Injury. 2003;34(suppl 1):A11-A15.
8. Huang HT, Huang PJ, Su JY, Lin SY. Indirect reduction and bridge plating of supracondylar fractures of the femur. Injury. 2003;34(2):135-140.
9. Dennis DA. Periprosthetic fractures following total knee arthroplasty. Instr Course Lect. 2001;50:379-389.
10. Jamali AA, Lee MA, Donthineni R, Meehan JP. Minimally invasive management of a floating prosthesis injury with locking plates. J Arthroplasty. 2007;22(6):928-933.
11. Bong MR, Egol KA, Koval KJ, et al. Comparison of the LISS and a retrograde-inserted supracondylar intramedullary nail for fixation of a periprosthetic distal femur fracture proximal to a total knee arthroplasty. J Arthroplasty. 2002;17(7):876-881.
12. Firoozbakhsh K, Behzadi K, DeCoster TA, Moneim MS, Naraghi FF. Mechanics of retrograde nail versus plate fixation for supracondylar femur fractures. J Orthop Trauma. 1995;9(2):152-157.
13. Arazi M, Memik R, Ogun TC, Yel M. Ilizarov external fixation for severely comminuted supracondylar and intercondylar fractures of the distal femur. J Bone Joint Surg Br. 2001;83(5):663-667.
14. Pleva L, Sir M, Madeja R. Our experiences with the treatment of periprosthetic fractures of femur. Biomed Pap Med Fac Univ Palacky Olomouc Czech Repub. 2004;148(1):75-79.
15. Simon RG, Brinker MR. Use of Ilizarov external fixation for a periprosthetic supracondylar femur fracture. J Arthroplasty. 1999;14(1):118-121.
16. Hurson C, Synnott K, McCormack D. Above-knee Ilizarov external fixation for early periprosthetic supracondylar femoral fracture—a case report. Knee. 2005;12(2):145-147.
17. Beris AE, Lykissas MG, Sioros V, Mavrodontidis AN, Korompilias AV. Femoral periprosthetic fracture in osteoporotic bone after a total knee replacement: treatment with Ilizarov external fixation. J Arthroplasty. 2010;25(7):1168.e9-e12.
18. Pafilas D, Kourtzis N. Hybrid external fixation as a new treatment method for periprosthetic femoral fracture. A case report. J Bone Joint Surg Am. 2006;88(1):188-192.
19. Merkel KD, Johnson EW Jr. Supracondylar fracture of the femur after total knee arthroplasty. J Bone Joint Surg Am. 1986;68(1):29-43.
20. Cordeiro EN, Costa RC, Carazzato JG, Silva Jdos S. Periprosthetic fractures in patients with total knee arthroplasties. Clin Orthop. 1990;(252):182-189.
21. Riemer BL, Butterfield SL, Burke CJ 3rd, Mathews D. Immediate plate fixation of highly comminuted femoral diaphyseal fractures in blunt polytrauma patients. Orthopedics. 1992;15(8):907-916.
22. Kregor PJ, Hughes JL, Cole PA. Fixation of distal femoral fractures above total knee arthroplasty utilizing the less invasive stabilization system (L.I.S.S.). Injury. 2001;32(suppl 3):SC64-SC75.
23. Althausen PL, Lee MA, Finkemeier CG, Meehan JP, Rodrigo JJ. Operative stabilization of supracondylar femur fractures above total knee arthroplasty: a comparison of four treatment methods. J Arthroplasty. 2003;18(7):834-839.
The incidence of periprosthetic supracondylar fractures of the femur after total knee arthroplasty (TKA) ranges from 0.6% to 2.5%.1 Treatment of periprosthetic fractures is often complicated by advanced patient age and osteoporosis, which frequently accompanies these fractures. Management of a periprosthetic fracture depends on the relation between the fracture site and the prosthesis, displacement of the prosthesis, integrity of the fixation of the prosthesis, extent of the bone loss caused by fracture comminution or preexisting osteolysis, general health of the patient, and surgeon expertise.2,3 The aim is to achieve fracture union around a stable, well-aligned arthroplasty with preserved or restored bone stock and therefore to return the patient to previous level of function. Although nonoperative treatments have been shown to be successful,4,5 in the great majority of cases surgical treatment is advised for these fractures.6-10 In cases in which bone stock is adequate for fixation rather than replacement of the distal femur, 2 modalities are commonly used: retrograde intramedullary nailing and locking plates. Each has its drawbacks and advantages.11,12
Although external fixation has been used in the treatment of distal femoral fractures,13 it is seldom considered in the treatment of periprosthetic fractures. Several authors have described cases that used external fixators, occasionally spanning the knee. The specific types of external fixators discussed in the literature have included ring fixators,14-17 hybrid fixators,18 and uniplanar nonspanning fixators14,19 (Table). Use of a simple anterior spanning external fixator in treating a periprosthetic femoral fracture has received little attention in the literature.
The patient provided written informed consent for print and electronic publication of this case report.
Case Report
A 54-year-old woman with previous total hip arthroplasty (THA) and ipsilateral TKA tripped on a carpet and sustained a comminuted fracture of the distal femur just above the TKA prosthesis (Figure 1). She was a Jehovah’s Witness and thus refused all blood products. She had an extensive history of osteoporosis, morbid obesity (5 feet tall, 250 pounds; body mass index, 49), diabetes, and rheumatoid arthritis. Evaluation by the internal medicine service revealed severe coronary artery disease on a stress thallium test and anemia with hematocrit of 24%. Given the patient’s medical comorbidities and religious status, and the location of the comminuted distal femur fracture, several treatment options were considered. First was nonoperative treatment with a cast or cast-brace (hinged cast). Because of her body habitus, however, we thought she would very likely experience skin complications, inadequate immobilization of the bone, and significant discomfort. Ultimately, use of a spanning external fixator was chosen as the safest course, given the significant medical risks accompanying a more extensive surgical reconstruction. With the spanning external fixator, the main risks were the inability to fully control fracture alignment and the potential introduction of infection into the functional THA. We thought that, by limiting the amount of time in the fixator and managing the pin site aggressively, we could minimize the risk for infection in this setting.
The procedure was performed with the patient under general anesthesia. During surgery, a lateral image of the femur was used to identify the distal end of the THA prosthesis. A level was marked 2 to 3 cm distal to the tip of this prosthesis, and another about 1 cm above the fracture (noted to be above the most proximal extent of the knee joint). These planned pin-entry sites were prepared from an anterior approach with incisions (using a No. 11 blade) of about 1 cm each. Blunt dissection was carried down to the femur. Each planned pin site was predrilled with a 3.5-mm drill; then, a 5-mm Shanz pin was placed. This process was repeated immediately distal to the tibial component and at the junction of the mid and distal thirds of the tibia (Figure 2). The preliminary external fixator frame was then applied. Once the reduction was satisfactory in the anteroposterior and lateral planes, the fixator clamps were tightened. A second row of bars was then incorporated.
Six weeks after surgery, radiographs showed early callus formation. Removing the external fixator and examining the knee under anesthesia confirmed there was no significant motion through the fracture site. A cast-brace (fiberglass thigh segment, fiberglass lower leg cast with hinged knee segment) was then applied. We remained concerned about skin complications but were encouraged by the early healing achieved with the fixator. The patient was started on a physical therapy program of gait training with a walker and toe-touch weight-bearing on the injured extremity. She also started a limited lower-extremity strengthening program. Three months after surgery, she was tolerating weight-bearing on the injured extremity with no pain. At 6 months, knee radiographs showed fracture consolidation with active range of motion of 10° to 120° and no pain (Figures 3A, 3B). Distal sensation, motor function, and vascular examination were normal. Two years after surgery, radiographs of the right knee showed minor malalignment in the coronal and sagittal planes (Figures 4A, 4B) and complete consolidation of the fracture.
Discussion
Periprosthetic fractures of the femur after TKA often occur in the setting of osteopenia, and some are associated with concurrent implant loosening. In most cases, these fractures require surgical stabilization. Nevertheless, the goals of treatment are to obtain and maintain anatomical alignment and stability to allow early range of motion. Nonoperative options include skeletal traction, cast, pins and plaster, and cast-brace.3-5,20 Operative options include intramedullary fixation,12,21 stabilization with various plates,21-23 revision knee arthroplasty, and arthrodesis.1 Treatment selection should be based on patient health, fracture displacement, comminution, osteopenia severity, and status of the prosthetic components.
The present case exemplifies some of the highest degrees of medical and surgical risk factors in people with a periprosthetic femoral fracture after TKA. Patients with rheumatoid arthritis, patients having corticosteroid treatment, patients of advanced age, and female patients are all at higher risk for supracondylar femoral fracture.9 Our patient had these risk factors on a background of anemia and extensive coronary artery disease. Given her past medical history and refusal of blood products out of religious belief, we thought she was too high risk for extensive surgical treatment for her fracture. In addition, she was not an ideal candidate for nonoperative treatment, as a periprosthetic fracture typically is treated with surgical revision or open reduction and internal fixation. Therefore, we selected an unconventional treatment modality, typically used as a temporizing measure in severe fractures around the knee—a spanning external fixator worn for 6 weeks and a cast-brace for an additional 6 weeks. This led to successful clinical and radiographic outcomes. We consider spanning external fixation a viable option for periprosthetic fractures after TKA in morbidly obese patients with relatively well-aligned fractures and extremely high risk for medical complications associated with traditional open surgery.
The incidence of periprosthetic supracondylar fractures of the femur after total knee arthroplasty (TKA) ranges from 0.6% to 2.5%.1 Treatment of periprosthetic fractures is often complicated by advanced patient age and osteoporosis, which frequently accompanies these fractures. Management of a periprosthetic fracture depends on the relation between the fracture site and the prosthesis, displacement of the prosthesis, integrity of the fixation of the prosthesis, extent of the bone loss caused by fracture comminution or preexisting osteolysis, general health of the patient, and surgeon expertise.2,3 The aim is to achieve fracture union around a stable, well-aligned arthroplasty with preserved or restored bone stock and therefore to return the patient to previous level of function. Although nonoperative treatments have been shown to be successful,4,5 in the great majority of cases surgical treatment is advised for these fractures.6-10 In cases in which bone stock is adequate for fixation rather than replacement of the distal femur, 2 modalities are commonly used: retrograde intramedullary nailing and locking plates. Each has its drawbacks and advantages.11,12
Although external fixation has been used in the treatment of distal femoral fractures,13 it is seldom considered in the treatment of periprosthetic fractures. Several authors have described cases that used external fixators, occasionally spanning the knee. The specific types of external fixators discussed in the literature have included ring fixators,14-17 hybrid fixators,18 and uniplanar nonspanning fixators14,19 (Table). Use of a simple anterior spanning external fixator in treating a periprosthetic femoral fracture has received little attention in the literature.
The patient provided written informed consent for print and electronic publication of this case report.
Case Report
A 54-year-old woman with previous total hip arthroplasty (THA) and ipsilateral TKA tripped on a carpet and sustained a comminuted fracture of the distal femur just above the TKA prosthesis (Figure 1). She was a Jehovah’s Witness and thus refused all blood products. She had an extensive history of osteoporosis, morbid obesity (5 feet tall, 250 pounds; body mass index, 49), diabetes, and rheumatoid arthritis. Evaluation by the internal medicine service revealed severe coronary artery disease on a stress thallium test and anemia with hematocrit of 24%. Given the patient’s medical comorbidities and religious status, and the location of the comminuted distal femur fracture, several treatment options were considered. First was nonoperative treatment with a cast or cast-brace (hinged cast). Because of her body habitus, however, we thought she would very likely experience skin complications, inadequate immobilization of the bone, and significant discomfort. Ultimately, use of a spanning external fixator was chosen as the safest course, given the significant medical risks accompanying a more extensive surgical reconstruction. With the spanning external fixator, the main risks were the inability to fully control fracture alignment and the potential introduction of infection into the functional THA. We thought that, by limiting the amount of time in the fixator and managing the pin site aggressively, we could minimize the risk for infection in this setting.
The procedure was performed with the patient under general anesthesia. During surgery, a lateral image of the femur was used to identify the distal end of the THA prosthesis. A level was marked 2 to 3 cm distal to the tip of this prosthesis, and another about 1 cm above the fracture (noted to be above the most proximal extent of the knee joint). These planned pin-entry sites were prepared from an anterior approach with incisions (using a No. 11 blade) of about 1 cm each. Blunt dissection was carried down to the femur. Each planned pin site was predrilled with a 3.5-mm drill; then, a 5-mm Shanz pin was placed. This process was repeated immediately distal to the tibial component and at the junction of the mid and distal thirds of the tibia (Figure 2). The preliminary external fixator frame was then applied. Once the reduction was satisfactory in the anteroposterior and lateral planes, the fixator clamps were tightened. A second row of bars was then incorporated.
Six weeks after surgery, radiographs showed early callus formation. Removing the external fixator and examining the knee under anesthesia confirmed there was no significant motion through the fracture site. A cast-brace (fiberglass thigh segment, fiberglass lower leg cast with hinged knee segment) was then applied. We remained concerned about skin complications but were encouraged by the early healing achieved with the fixator. The patient was started on a physical therapy program of gait training with a walker and toe-touch weight-bearing on the injured extremity. She also started a limited lower-extremity strengthening program. Three months after surgery, she was tolerating weight-bearing on the injured extremity with no pain. At 6 months, knee radiographs showed fracture consolidation with active range of motion of 10° to 120° and no pain (Figures 3A, 3B). Distal sensation, motor function, and vascular examination were normal. Two years after surgery, radiographs of the right knee showed minor malalignment in the coronal and sagittal planes (Figures 4A, 4B) and complete consolidation of the fracture.
Discussion
Periprosthetic fractures of the femur after TKA often occur in the setting of osteopenia, and some are associated with concurrent implant loosening. In most cases, these fractures require surgical stabilization. Nevertheless, the goals of treatment are to obtain and maintain anatomical alignment and stability to allow early range of motion. Nonoperative options include skeletal traction, cast, pins and plaster, and cast-brace.3-5,20 Operative options include intramedullary fixation,12,21 stabilization with various plates,21-23 revision knee arthroplasty, and arthrodesis.1 Treatment selection should be based on patient health, fracture displacement, comminution, osteopenia severity, and status of the prosthetic components.
The present case exemplifies some of the highest degrees of medical and surgical risk factors in people with a periprosthetic femoral fracture after TKA. Patients with rheumatoid arthritis, patients having corticosteroid treatment, patients of advanced age, and female patients are all at higher risk for supracondylar femoral fracture.9 Our patient had these risk factors on a background of anemia and extensive coronary artery disease. Given her past medical history and refusal of blood products out of religious belief, we thought she was too high risk for extensive surgical treatment for her fracture. In addition, she was not an ideal candidate for nonoperative treatment, as a periprosthetic fracture typically is treated with surgical revision or open reduction and internal fixation. Therefore, we selected an unconventional treatment modality, typically used as a temporizing measure in severe fractures around the knee—a spanning external fixator worn for 6 weeks and a cast-brace for an additional 6 weeks. This led to successful clinical and radiographic outcomes. We consider spanning external fixation a viable option for periprosthetic fractures after TKA in morbidly obese patients with relatively well-aligned fractures and extremely high risk for medical complications associated with traditional open surgery.
1. Figgie MP, Goldberg VM, Figgie HE 3rd, Sobel M. The results of treatment of supracondylar fracture above total knee arthroplasty. J Arthroplasty. 1990;5(3):267-276.
2. Su ET, Kubiak EN, Dewal H, Hiebert R, Di Cesare PE. A proposed classification of supracondylar femur fractures above total knee arthroplasties. J Arthroplasty. 2006;21(3):405-408.
3. Kim KI, Egol KA, Hozack WJ, Parvizi J. Periprosthetic fractures after total knee arthroplasties. Clin Orthop. 2006;(446):167-175.
4. Sochart DH, Hardinge K. Nonsurgical management of supracondylar fracture above total knee arthroplasty. Still the nineties option. J Arthroplasty. 1997;12(7):830-834.
5. Delport PH, Van Audekercke R, Martens M, Mulier JC. Conservative treatment of ipsilateral supracondylar femoral fracture after total knee arthroplasty. J Trauma. 1984;24(9):846-849.
6. Frigg R, Appenzeller A, Christensen R, Frenk A, Gilbert S, Schavan R. The development of the distal femur less invasive stabilization system (LISS). Injury. 2001;32(suppl 3):SC24-SC31.
7. Goesling T, Frenk A, Appenzeller A, Garapati R, Marti A, Krettek C. LISS PLT: design, mechanical and biomechanical characteristics. Injury. 2003;34(suppl 1):A11-A15.
8. Huang HT, Huang PJ, Su JY, Lin SY. Indirect reduction and bridge plating of supracondylar fractures of the femur. Injury. 2003;34(2):135-140.
9. Dennis DA. Periprosthetic fractures following total knee arthroplasty. Instr Course Lect. 2001;50:379-389.
10. Jamali AA, Lee MA, Donthineni R, Meehan JP. Minimally invasive management of a floating prosthesis injury with locking plates. J Arthroplasty. 2007;22(6):928-933.
11. Bong MR, Egol KA, Koval KJ, et al. Comparison of the LISS and a retrograde-inserted supracondylar intramedullary nail for fixation of a periprosthetic distal femur fracture proximal to a total knee arthroplasty. J Arthroplasty. 2002;17(7):876-881.
12. Firoozbakhsh K, Behzadi K, DeCoster TA, Moneim MS, Naraghi FF. Mechanics of retrograde nail versus plate fixation for supracondylar femur fractures. J Orthop Trauma. 1995;9(2):152-157.
13. Arazi M, Memik R, Ogun TC, Yel M. Ilizarov external fixation for severely comminuted supracondylar and intercondylar fractures of the distal femur. J Bone Joint Surg Br. 2001;83(5):663-667.
14. Pleva L, Sir M, Madeja R. Our experiences with the treatment of periprosthetic fractures of femur. Biomed Pap Med Fac Univ Palacky Olomouc Czech Repub. 2004;148(1):75-79.
15. Simon RG, Brinker MR. Use of Ilizarov external fixation for a periprosthetic supracondylar femur fracture. J Arthroplasty. 1999;14(1):118-121.
16. Hurson C, Synnott K, McCormack D. Above-knee Ilizarov external fixation for early periprosthetic supracondylar femoral fracture—a case report. Knee. 2005;12(2):145-147.
17. Beris AE, Lykissas MG, Sioros V, Mavrodontidis AN, Korompilias AV. Femoral periprosthetic fracture in osteoporotic bone after a total knee replacement: treatment with Ilizarov external fixation. J Arthroplasty. 2010;25(7):1168.e9-e12.
18. Pafilas D, Kourtzis N. Hybrid external fixation as a new treatment method for periprosthetic femoral fracture. A case report. J Bone Joint Surg Am. 2006;88(1):188-192.
19. Merkel KD, Johnson EW Jr. Supracondylar fracture of the femur after total knee arthroplasty. J Bone Joint Surg Am. 1986;68(1):29-43.
20. Cordeiro EN, Costa RC, Carazzato JG, Silva Jdos S. Periprosthetic fractures in patients with total knee arthroplasties. Clin Orthop. 1990;(252):182-189.
21. Riemer BL, Butterfield SL, Burke CJ 3rd, Mathews D. Immediate plate fixation of highly comminuted femoral diaphyseal fractures in blunt polytrauma patients. Orthopedics. 1992;15(8):907-916.
22. Kregor PJ, Hughes JL, Cole PA. Fixation of distal femoral fractures above total knee arthroplasty utilizing the less invasive stabilization system (L.I.S.S.). Injury. 2001;32(suppl 3):SC64-SC75.
23. Althausen PL, Lee MA, Finkemeier CG, Meehan JP, Rodrigo JJ. Operative stabilization of supracondylar femur fractures above total knee arthroplasty: a comparison of four treatment methods. J Arthroplasty. 2003;18(7):834-839.
1. Figgie MP, Goldberg VM, Figgie HE 3rd, Sobel M. The results of treatment of supracondylar fracture above total knee arthroplasty. J Arthroplasty. 1990;5(3):267-276.
2. Su ET, Kubiak EN, Dewal H, Hiebert R, Di Cesare PE. A proposed classification of supracondylar femur fractures above total knee arthroplasties. J Arthroplasty. 2006;21(3):405-408.
3. Kim KI, Egol KA, Hozack WJ, Parvizi J. Periprosthetic fractures after total knee arthroplasties. Clin Orthop. 2006;(446):167-175.
4. Sochart DH, Hardinge K. Nonsurgical management of supracondylar fracture above total knee arthroplasty. Still the nineties option. J Arthroplasty. 1997;12(7):830-834.
5. Delport PH, Van Audekercke R, Martens M, Mulier JC. Conservative treatment of ipsilateral supracondylar femoral fracture after total knee arthroplasty. J Trauma. 1984;24(9):846-849.
6. Frigg R, Appenzeller A, Christensen R, Frenk A, Gilbert S, Schavan R. The development of the distal femur less invasive stabilization system (LISS). Injury. 2001;32(suppl 3):SC24-SC31.
7. Goesling T, Frenk A, Appenzeller A, Garapati R, Marti A, Krettek C. LISS PLT: design, mechanical and biomechanical characteristics. Injury. 2003;34(suppl 1):A11-A15.
8. Huang HT, Huang PJ, Su JY, Lin SY. Indirect reduction and bridge plating of supracondylar fractures of the femur. Injury. 2003;34(2):135-140.
9. Dennis DA. Periprosthetic fractures following total knee arthroplasty. Instr Course Lect. 2001;50:379-389.
10. Jamali AA, Lee MA, Donthineni R, Meehan JP. Minimally invasive management of a floating prosthesis injury with locking plates. J Arthroplasty. 2007;22(6):928-933.
11. Bong MR, Egol KA, Koval KJ, et al. Comparison of the LISS and a retrograde-inserted supracondylar intramedullary nail for fixation of a periprosthetic distal femur fracture proximal to a total knee arthroplasty. J Arthroplasty. 2002;17(7):876-881.
12. Firoozbakhsh K, Behzadi K, DeCoster TA, Moneim MS, Naraghi FF. Mechanics of retrograde nail versus plate fixation for supracondylar femur fractures. J Orthop Trauma. 1995;9(2):152-157.
13. Arazi M, Memik R, Ogun TC, Yel M. Ilizarov external fixation for severely comminuted supracondylar and intercondylar fractures of the distal femur. J Bone Joint Surg Br. 2001;83(5):663-667.
14. Pleva L, Sir M, Madeja R. Our experiences with the treatment of periprosthetic fractures of femur. Biomed Pap Med Fac Univ Palacky Olomouc Czech Repub. 2004;148(1):75-79.
15. Simon RG, Brinker MR. Use of Ilizarov external fixation for a periprosthetic supracondylar femur fracture. J Arthroplasty. 1999;14(1):118-121.
16. Hurson C, Synnott K, McCormack D. Above-knee Ilizarov external fixation for early periprosthetic supracondylar femoral fracture—a case report. Knee. 2005;12(2):145-147.
17. Beris AE, Lykissas MG, Sioros V, Mavrodontidis AN, Korompilias AV. Femoral periprosthetic fracture in osteoporotic bone after a total knee replacement: treatment with Ilizarov external fixation. J Arthroplasty. 2010;25(7):1168.e9-e12.
18. Pafilas D, Kourtzis N. Hybrid external fixation as a new treatment method for periprosthetic femoral fracture. A case report. J Bone Joint Surg Am. 2006;88(1):188-192.
19. Merkel KD, Johnson EW Jr. Supracondylar fracture of the femur after total knee arthroplasty. J Bone Joint Surg Am. 1986;68(1):29-43.
20. Cordeiro EN, Costa RC, Carazzato JG, Silva Jdos S. Periprosthetic fractures in patients with total knee arthroplasties. Clin Orthop. 1990;(252):182-189.
21. Riemer BL, Butterfield SL, Burke CJ 3rd, Mathews D. Immediate plate fixation of highly comminuted femoral diaphyseal fractures in blunt polytrauma patients. Orthopedics. 1992;15(8):907-916.
22. Kregor PJ, Hughes JL, Cole PA. Fixation of distal femoral fractures above total knee arthroplasty utilizing the less invasive stabilization system (L.I.S.S.). Injury. 2001;32(suppl 3):SC64-SC75.
23. Althausen PL, Lee MA, Finkemeier CG, Meehan JP, Rodrigo JJ. Operative stabilization of supracondylar femur fractures above total knee arthroplasty: a comparison of four treatment methods. J Arthroplasty. 2003;18(7):834-839.