User login
DNA Repair Gene Variants in Patients With Prostate Cancer Achieving Durable Clinical Benefit With PARP Inhibitors
BACKGROUND: PARP inhibitors (PARPi’s) were recently approved for the treatment of metastatic prostate cancer among patients harboring mutations in an array of genes responsible for DNA repair. We sought to identify whether a subset of these genes correlates with response to treatment more frequently than others. Consequently, an evaluation of the specific DNA repair genotypes associated with durable clinical benefit (DCB) using real-world patient data was undertaken.
METHODS: The U.S. Department of Veterans Affairs (VA) National Precision Oncology Program’s (NPOP) database and Corporate Data Warehouse (CDW) were reviewed to select patients who (1) carried a diagnosis of prostate cancer, (2) successfully underwent tumor DNA sequencing through NPOP, (3) were prescribed olaparib, rucaparib, nirapib, and/or talazaporib for their prostate cancer between July 2016 and February 2020, and (4) and achieved DCB, defined as no progression in prostate-specific antigen (PSA) for at least 6 months following PARPi initiation without concurrent systemic or non-systemic therapies other than androgen-deprivation. The DNA repair gene variants and orders placed for NPOP consultative support were reviewed.
RESULTS: Of the 44 prostate cancer patients treated with a PARPi, 6 (13.6%) had tumor DNA sequencing through NPOP and had achieved DCB. Five patients were treated with olaparib and 1 with rucaparib. The median PSA progression-free survival was 8.9 (interquartile range = 8.5 – 11.2) months among these selected patients. Regarding gene variants, 5 patients had 7 BRCA2 mutations, including 4 frameshift, 1 nonsense, 1 single nucleotide variant, and 1 splice site. One patient had frameshift and missense ATM mutations. Referrals to the NPOP consult service were ordered for 2 out of the 5 patients with BRCA2 mutations achieving DCB.
CONCLUSIONS: Within the VA’s NPOP, the presence of BRCA2 gene variants was the most common finding from tumor DNA sequencing among patients with prostate cancer achieving DCB with a PARPi. Further analysis of the genotypes of all patients treated with PARPi in NPOP to assess the differential impact of BRCA2 mutations is needed to confirm the clinical implication of this finding.
BACKGROUND: PARP inhibitors (PARPi’s) were recently approved for the treatment of metastatic prostate cancer among patients harboring mutations in an array of genes responsible for DNA repair. We sought to identify whether a subset of these genes correlates with response to treatment more frequently than others. Consequently, an evaluation of the specific DNA repair genotypes associated with durable clinical benefit (DCB) using real-world patient data was undertaken.
METHODS: The U.S. Department of Veterans Affairs (VA) National Precision Oncology Program’s (NPOP) database and Corporate Data Warehouse (CDW) were reviewed to select patients who (1) carried a diagnosis of prostate cancer, (2) successfully underwent tumor DNA sequencing through NPOP, (3) were prescribed olaparib, rucaparib, nirapib, and/or talazaporib for their prostate cancer between July 2016 and February 2020, and (4) and achieved DCB, defined as no progression in prostate-specific antigen (PSA) for at least 6 months following PARPi initiation without concurrent systemic or non-systemic therapies other than androgen-deprivation. The DNA repair gene variants and orders placed for NPOP consultative support were reviewed.
RESULTS: Of the 44 prostate cancer patients treated with a PARPi, 6 (13.6%) had tumor DNA sequencing through NPOP and had achieved DCB. Five patients were treated with olaparib and 1 with rucaparib. The median PSA progression-free survival was 8.9 (interquartile range = 8.5 – 11.2) months among these selected patients. Regarding gene variants, 5 patients had 7 BRCA2 mutations, including 4 frameshift, 1 nonsense, 1 single nucleotide variant, and 1 splice site. One patient had frameshift and missense ATM mutations. Referrals to the NPOP consult service were ordered for 2 out of the 5 patients with BRCA2 mutations achieving DCB.
CONCLUSIONS: Within the VA’s NPOP, the presence of BRCA2 gene variants was the most common finding from tumor DNA sequencing among patients with prostate cancer achieving DCB with a PARPi. Further analysis of the genotypes of all patients treated with PARPi in NPOP to assess the differential impact of BRCA2 mutations is needed to confirm the clinical implication of this finding.
BACKGROUND: PARP inhibitors (PARPi’s) were recently approved for the treatment of metastatic prostate cancer among patients harboring mutations in an array of genes responsible for DNA repair. We sought to identify whether a subset of these genes correlates with response to treatment more frequently than others. Consequently, an evaluation of the specific DNA repair genotypes associated with durable clinical benefit (DCB) using real-world patient data was undertaken.
METHODS: The U.S. Department of Veterans Affairs (VA) National Precision Oncology Program’s (NPOP) database and Corporate Data Warehouse (CDW) were reviewed to select patients who (1) carried a diagnosis of prostate cancer, (2) successfully underwent tumor DNA sequencing through NPOP, (3) were prescribed olaparib, rucaparib, nirapib, and/or talazaporib for their prostate cancer between July 2016 and February 2020, and (4) and achieved DCB, defined as no progression in prostate-specific antigen (PSA) for at least 6 months following PARPi initiation without concurrent systemic or non-systemic therapies other than androgen-deprivation. The DNA repair gene variants and orders placed for NPOP consultative support were reviewed.
RESULTS: Of the 44 prostate cancer patients treated with a PARPi, 6 (13.6%) had tumor DNA sequencing through NPOP and had achieved DCB. Five patients were treated with olaparib and 1 with rucaparib. The median PSA progression-free survival was 8.9 (interquartile range = 8.5 – 11.2) months among these selected patients. Regarding gene variants, 5 patients had 7 BRCA2 mutations, including 4 frameshift, 1 nonsense, 1 single nucleotide variant, and 1 splice site. One patient had frameshift and missense ATM mutations. Referrals to the NPOP consult service were ordered for 2 out of the 5 patients with BRCA2 mutations achieving DCB.
CONCLUSIONS: Within the VA’s NPOP, the presence of BRCA2 gene variants was the most common finding from tumor DNA sequencing among patients with prostate cancer achieving DCB with a PARPi. Further analysis of the genotypes of all patients treated with PARPi in NPOP to assess the differential impact of BRCA2 mutations is needed to confirm the clinical implication of this finding.
Special Considerations for Pediatric Patellar Instability
ABSTRACT
Patellar instability in children and adolescents is a challenging subset to treat. Varied forms of instability, ranging from episodic dislocation to fixed dislocation, have been recognized. It is of utmost importance for the treating physician to recognize these different patterns of instability and their associated risk factors, as more complex patterns of instability would require more extensive surgical procedures. Medial patellofemoral ligament (MPFL) reconstruction, by itself, may not suffice or may not be appropriate for the more complex instability patterns. Appropriate and early treatment of such instability in children would allow for functional progression and possible remodeling of the trochlea. However, early treatment has the associated risk of growth disturbances when surgical procedures are performed around open physis or if adult-type bony procedures are performed in children. Recent knowledge about the relationship between trochlea, MPFL femoral attachment, and distal femoral physis could help to advance safe surgical care for these patients. This article reviews the pathophysiology, risk factors, and the existing classification systems for patellar instability in children and adolescents. It focuses on varied surgical techniques, which are unique to the pediatric population, and summarizes the outcomes of these surgical techniques.
Continue to: EPIDEMIOLOGY
EPIDEMIOLOGY
In a prospective 2-year study of Finnish children, the annual incidence rate of patellar instability was 43/100,000 pediatric population.1 In patients 9 to 15 years of age, the incidence was approximately 1/1000.1 In another study, patients at highest risk for a first-time patellar dislocation were females aged 10 to 17 years.2 In a study in patients with traumatic hemarthrosis, 36% in the younger age group (10-14 years) and 28% in the older age group (15-18 years) had sustained patellar dislocation. In contrast, 22% in the younger age group and 40% in the older age group had sustained an anterior cruciate ligament tear.3
Approximately one-half of patients who dislocate their patella suffer from long-term complications.4,5 These complications include recurrent instability, patellofemoral pain, osteochondral lesions, and eventual arthritis.1,4,5 Young, active individuals are more prone to these issues.6 Also, 39% or more of patellar dislocation patients have an associated osteochondral fracture that might influence the management.1 Thus, patellar instability in young patients is an area of concern.
DEVELOPMENTAL ANATOMY
At 4-week gestation, the patellofemoral joint is an ectodermal sac filled with mesenchyme of the somatic mesoderm.7 Mesenchymal condensations then appear at 4 to 5 weeks gestation, followed by chondrification of both the femur and patella.7 The joint space is present by 6 weeks, and the patellar and distal femoral condyles are present at 7 weeks gestation.7 By 8 weeks gestation, the basic knee anatomy resembles that of an adult with the chondroepiphysis forming the articular surfaces of the femur, tibia, and patella.7 By this time, the extensor mechanism is formed, and active joint motion has begun, facilitating the development of the trochlear sulcus.7 The secondary ossification center in the distal femoral epiphysis forms around 36 weeks gestation.8 Postnatally, both the patella and distal femur grow through endochondral ossification.9,10
The patella is the largest sesamoid bone in the human body.11 The patella begins as a dense consolidation of cells that differentiate as the quadriceps mechanisms develop.12,13 The patellar anlage becomes distinguishable within the quadriceps tendon around 7.5-week gestation.12 The morphology of the patella conforms to the distal femur.12 The patella molds or re-models as the knee begins to move in response to mechanical stresses.7 The patella increases in relative size during the first 6 months of gestation, then enlarges proportionately to the rest of the bones.7 Ossification begins around 3 years of age for females and 4 to 5 years of age for males.8,14 The ossification center may appear irregular as it rapidly expands.14 Ossification proceeds in a proximal to distal direction, thus giving a spurious estimation of patellar height on radiographs in children. The overall morphology of the cartilaginous patella during development is comparable to the final mature shape.14 Abnormal contact stresses on the articular surface of the patella during skeletal immaturity can lead to deformation.7
Ultrasonographic measurements in normal patients show that trochlear groove (TG) morphology is present early and becomes more radiographically apparent as distal femoral ossification is completed.15 Anatomic dissections of aborted fetuses have verified the morphology of the TG as it remains constant during growth and the groove morphology is the same for both fetuses and adults.16 An ultrasound study performed on patients aged 12 to 18 years showed the cartilaginous sulcus angle (CSA) remained constant throughout all age groups (146°).17 The CSA however, differed in patients who suffered a patellar dislocation (average, 164°; range, 154°-195°) compared with normal knees (average CSA, 145°; range, 131°-158°).15,17,18 The osseous sulcus angle, on the other hand, appears flat at birth and the TG deepens with age. This increase in depth is more of a reflection of progressive ossification of a well-formed cartilaginous trochlea, rather than a true deepening of the sulcus.17 Thus, the axial radiographic view of the patella provides misleading information about the sulcus angle in children and should not be used to define trochlear morphology.
Continue to: MEDIAL PATELLOFEMORAL LIGAMENT ANATOMY
MEDIAL PATELLOFEMORAL LIGAMENT ANATOMY
The medial patellofemoral ligament (MPFL) functions to limit the lateral translation of the patella.19 The attachment sites on the femur and patella for the MPFL have been studied in children.20-23 Cadaveric dissections in specimens aged 2 to 11 years have noted the patellar attachment to be an average of 12 mm in length with the midpoint approximately 5 mm superior to the mid-pole of the patella.22 The patellar footprint of the MPFL insertion was a mean 41% of the entire patellar length.22
It is important to be aware of the characteristic anatomy of the MPFL, as fixation points should mimic the anatomic insertion as best as possible while also avoiding violation of the nearby physis. The MPFL originates between the adductor tubercle and the medial femoral epicondyle just distal to the distal femoral physis and attaches to the superomedial aspect of the patella.20-25 In relation to the physis in pediatric patients, the midpoint of MPFL insertion has been measured to be 4 mm to 9 mm distal to the femoral physis.21,24,25 These measurements represent averages as cadaveric studies have reported that some part of MPFL femoral insertion extends proximal to the distal femoral physis.21 A recent report of physeal injury to the posterior distal femoral physis during MPFL reconstruction leading to femoral flexion deformity highlights the importance of physeal-respecting surgery.26
TROCHLEA AND ANTERIOR DISTAL FEMORAL PHYSIS
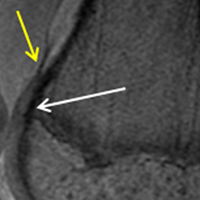
The relationship between the proximal aspect of the trochlea and the anterior distal femoral physis has been recently studied in 175 knees with dysplastic trochlea.27 Based on magnetic resonance imaging evaluation, the lateral aspect of the trochlea extended proximal to the anterior distal femoral physis in 13% of patients and was at the level of the anterior physis in another 13% of patients (Figure 1).27 Hence, a cautious approach is recommended for any surgery to address trochlear dysplasia or trochlear bump in younger patients to prevent iatrogenic injury to anterior distal femoral physis and resultant genu recurvatum. The distance between the trochlea and the physis increased with increasing age.
LIMB ALIGNMENT
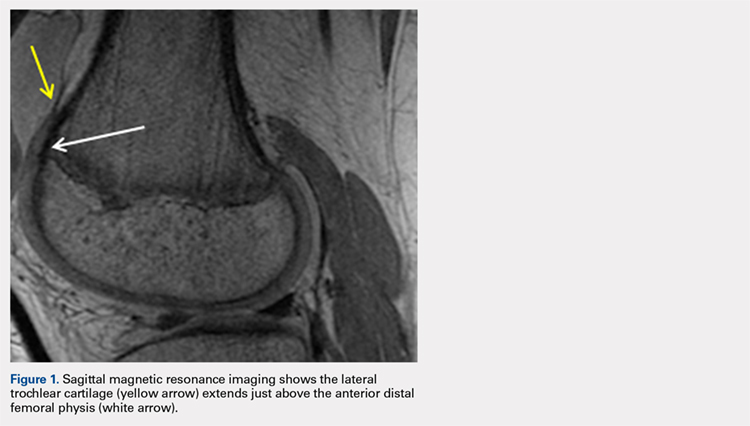
Physiologically, the quadriceps angle (Q angle) changes through the course of growth. As children begin standing and walking, they stand with their feet wider apart and in genu varum.28 Physiologic genu varum can reach 15°.28 This degree lessens during the first 1.5 to 2 years of life, transitioning to physiologic valgus of nearly 12° by 3 years of age.28 Genu valgum, thereafter, gradually decreases to reach the adult value of around 7° to 8° by age 7 years.28 Increased genu valgum is a risk factor for patellar instability. In skeletally immature patients, correction of genu valgum through guided growth may be desirable in patients undergoing patellar stabilization surgery (Figures 2A, 2B).29
PATHOPHYSIOLOGY OF PEDIATRIC PATELLAR DISLOCATION
TROCHLEAR DYSPLASIA
Trochlear dysplasia is an abnormal shape and depth of the TG.30 Up to 96% of patients with patellar dislocation have trochlear dysplasia.30-33 In a study of patellar instability in children, at least 1 of the 3 signs of trochlear dysplasia (the crossing sign, supratrochlear bump, and double contour sign) was present on lateral radiographs.34 In another study on the growth of trochlear dysplasia in children and adolescents, all grades of trochlear dysplasia were present at all ages (ie, the dysplasia was most likely present at birth and did not necessarily worsen with age and growth).35 The linear dimensions of lateral and medial condylar height as well as trochlear bump increased with age but both the sulcus angle and shape of the trochlea did not change significantly.35 Remodeling of a dysplastic trochlea can happen if the patella is stabilized and appropriately located at a younger age, preferably before 10 years of age.36,37
Continue to: PATELLAR HEIGHT
PATELLAR HEIGHT
The role of patellar height in patellar instability has been well established.38 In patients with patella alta, the patella remains proximal to the TG during the greater arc of knee motion, which predisposes it to patellar instability. Calculation of patellar height in children could be challenging due to incomplete ossification, as well as asymmetric ossification of the patella and the tibial tubercle (TT). Since the patella ossifies from proximal to distal, most radiographic methods that measure the patellar height from the distal aspect of the patella provide a spurious elevation of the measurement.
The Caton-Deschamps (CD) method measures the length of the patellar articular surface and the distance from the inferior edge of the articular surface to the anterosuperior corner of the tibial plateau.39 A ratio >1.3 signifies patella alta. The CD ratio has been verified as a simple and reliable index for measuring patellar height in children.40 Two other methods have been described for determining patellar height in children.41,42 Based on anteroposterior (AP) radiographs of the knee in full extension, Micheli and colleagues41 calculated the difference between the distance from the superior pole of the patella to the tibial plateau and the length of the patella. A positive difference signified patella alta. The Koshino method involves the ratio between a reference line from the midpoint of the patella to the midpoint of the proximal tibial physis and a second distance from the midpoint of the distal femoral physis to the midpoint of the proximal tibial physis on lateral knee radiographs.42 Normal values range from 0.99 to 1.20 with the knee in >30° flexion, in children 3 to 18 years of age.
HYPERLAXITY
In contrast to adults, children have increased levels of collagen III compared with collagen I, which is responsible for tissue elasticity.43 Tissue elasticity leads to increased joint mobility, which is more common in children. Joint hypermobility or hyperlaxity has to be differentiated from symptomatic instability. The traditional Beighton score identifies individuals as having joint hypermobility with a score of 5/9 or higher in school-aged children.44-46 Smits-Engelsman and colleagues44 suggested using stricter criteria with scores of 7/9 or higher being indicative of hyperlaxity in school-aged children. A study of 1845 Swedish school children noted that females have a higher degree of joint laxity.45 Maximal laxity was noted in females at 15 years of age.45 Hyperlaxity has been demonstrated to be greater on the left side of the body44 and can be part of generalized syndromes including Down’s syndrome, Marfan’s syndrome, or Ehlers-Danlos syndrome.
LIMB TORSION
Staheli and colleagues47 described the normative values of a lower extremity rotational profile, including femoral anteversion and tibial torsion. Children normally have increased femoral anteversion, which decreases with growth. Miserable malalignment is a term used to denote increased femoral anteversion and increased external tibial torsion.48,49 These rotational abnormalities can increase the Q angle and the lateral forces on the patella. Femoral anteversion or internal rotation of the femur of 30° significantly increases strain in all areas of the MPFL.48 This increased strain may lead to MPFL failure and patellar instability.48 Increased internal rotation of the femur also increases contact pressure on the lateral aspect of the patellofemoral joint.48 Miserable malalignment frequently manifests following a pubertal growth spurt and may require femoral and tibial osteotomy.50
SYNDROMIC ASSOCIATIONS
Several syndromes have patellar instability as a part of their manifestation. The more common syndromes include nail-patella syndrome, Kabuki syndrome, Down’s syndrome, and Rubinstein-Taybi syndrome.51-54 Other syndromes less commonly associated with patellar instability include Turner syndrome, patella aplasia, or absent patella syndrome. Since many patients with syndromic patellar instability are functionally limited, they may not require an aggressive approach to treatment. When treating these patients, it is important to recognize the unique features of a specific syndrome, which may affect the anesthesia risk profile, management decisions, rehabilitation, and prognosis.
Continue to: MPFL TEAR PATTERN
MPFL TEAR PATTERN
The MPFL serves as an important constraint to the patella to prevent lateral dislocation, primarily during the first 20° to 30° of knee flexion.55,56 Injury to the MPFL is noted in over 90% of patients who suffer a patellar dislocation.57 The location of MPFL tears in pediatric patients is variably reported at the patellar attachment (10%-61%), femoral attachment (12%-73%), both (12%-35%) or mid-substance (2.5%-15%).25,57 The most common tear patterns in pediatric patients are tears at the patellar attachment.25,57 This tear pattern may be accompanied by an avulsion fracture of the medial rim of the patella, though this fracture, being extra-articular, seldom needs treatment.
CLASSIFICATION
While several authors have established extensive classification systems of patellar dislocation based on both clinical and radiographic presentation and reviews of the literature, a single classification system has not been recognized as the gold standard. In this section, in addition to presenting our preferred methods of classification, we will review some of the more recent and extensive classification systems for patellar dislocation and patellar instability.
Dejour and colleagues31 initially used both the presence of patellofemoral anatomic abnormalities and pain to define 3 types of patellar instability: major, objective, and potential patellar instability. Major patellar instability indicates that the patient has experienced more than 1 documented dislocation, objective instability involves one dislocation in addition to an associated anatomic abnormality, and potential patellar instability refers to cases in which the patient has radiographic abnormalities and patellar pain.31 Garin and colleagues58 more simplistically divided patellar dislocation patients into 2 groups: major (permanent or habitual) dislocation of the patella and recurrent dislocation. Sillanpaa59 stressed the distinction between first-time dislocation and recurrent dislocation specifically in the context of acute injuries. These classification systems were formulated with adults as the most relevant population; however, classifications targeted specifically to pediatric patients have recently been presented in the literature.
Historically, pediatric patella dislocations were simply categorized as traumatic or congenital.60 In 2014, Chotel and colleagues61 focused on classifying patellar dislocation by extensively reviewing anatomic, biomechanical, pathophysiological, and clinical patterns seen most commonly in children. They included 5 categories: congenital dislocation, permanent dislocation, habitual dislocation during knee flexion, habitual dislocation during knee extension, and recurrent dislocation; however, they did not address traumatic dislocations.61 Congenital dislocation is a rare condition, typically presenting at birth, which produces a pattern of functional genu valgum.62 Permanent dislocation typically presents after the child has started walking, but before the age of 5 years.61 The 2 variations of habitual dislocation typically present between ages 5 and 8 years.61 The final category is the most common and typically occurs during pre-adolescence or adolescence as a result of an atraumatic or trivial traumatic event or sports injury.1 Using more specific terminology, Hiemstra and colleagues63 modeled a classification system based on the traumatic, unilateral, bankart lesion, surgery (TUBS)/atraumatic, multidirectional, bilateral, rehabilitation, inferior shift (AMBRI) for shoulder dislocation classifications. The patellar dislocation system is used to identify 2 distinct subsets of patients in the patellofemoral instability population. One subset is defined by the acronym WARPS (weak, atraumatic, risky anatomy, pain, and subluxation), the other is STAID (strong, traumatic, anatomy normal, instability, and dislocation).64 Patients categorized by the WARPS acronym tend to experience atraumatic onsets of patellofemoral instability and demonstrate anatomic issues that increase this instability. These underlying anatomic issues include valgus alignment, ligamentous laxity, rotational abnormalities, shallow and short TG, and patella alta. On the other hand, STAID patients describe a traumatic dislocation event and do not have underlying anatomic abnormalities that predispose them to instability.64
Taking into account these previous classifications, Frosch and colleagues65 added specific pathologies including “instability,” “maltracking,” and “loss of patellar tracking,” in addition to both clinical and radiographic factors to define 5 types of patellar dislocation and their specific treatment recommendations.65 Type 1 involves simple dislocation with neither maltracking nor instability and a low risk of redislocation.65 Type 2 is defined as primary dislocation followed by subsequent high risk of dislocation and no maltracking.65 Type 3 is divided into 5 subcategories of instability and maltracking issues involving soft tissue contracture, patella alta, pathological tibial tuberosity, and TG distance.65 Type 4 is defined as the highly unstable “floating patella,” and type 5 involves patellar maltracking without instability 65. In terms of treatment, conservative rehabilitation is recommended for type 1 whereas MPFL reconstruction tends to show positive outcomes for both types 2 and 3.66-70
Continue to: Parikh and Lykissas recently published...
Parikh and Lykissas recently published a comprehensive classification system of 4 defined types of patellar dislocation in addition to voluntary patellar instability and syndromic patellar instability (Table).60 The 4 types are Type 1, first-time patellar dislocation; Type 2, recurrent patellar instability; Type 3, dislocatable; and Type 4, dislocated. Type 2 is further subdivided into Type 2A, which presents with positive apprehension signs, and Type 2B, which involves instabilities related to anatomic abnormalities.60 A distinction is also made between Type 3A or passive patellar dislocation and Type 3B habitual patellar dislocation.60
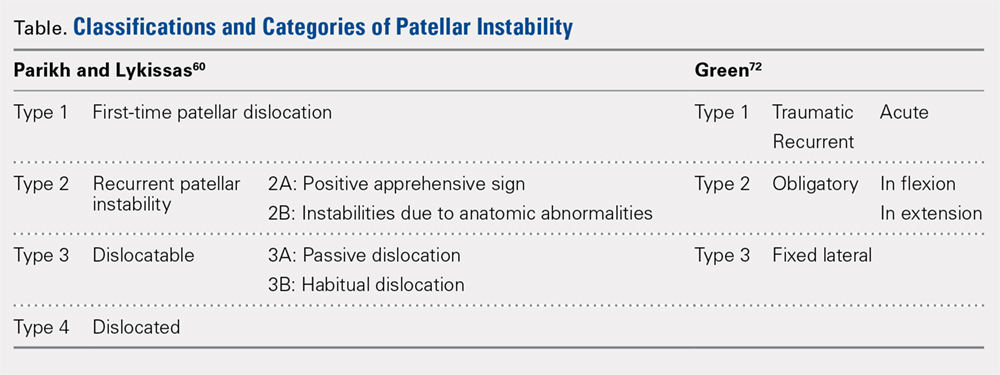
The classification system proposed by Green and colleagues is more simplified with 3 main categories (Table) of pediatric patellar dislocation: traumatic (acute or recurrent), obligatory (either in flexion or extension), and fixed laterally.71,72 The acute traumatic categorization refers to patients who experienced an initial dislocation event due to trauma whereas recurrent traumatic involves repeated patella dislocations following an initial incident. Studies report that between 60% to 70% of these acute traumatic dislocations occur as a result of a sports-related incident.2,33,73 Obligatory dislocations occur with every episode of either knee flexion or extension, depending on the subtype. Obligatory patella dislocation in flexion typically cannot be manipulated or relocated into the trochlea while the knee is fixed but does reduce into the trochlea in full extension. Fixed lateral dislocations are rare, irreducible dislocations in which the patella stays dislocated laterally in flexion and extension. These dislocations often present with other congenital abnormalities. Each of these categories can be further specified as syndromic if the dislocation is associated with genetic or congenital conditions including skeletal dysplasia, Ehlers-Danlos syndrome, cerebral palsy, Marfan disease, nail-patella syndrome, Down syndrome, Rubenstein-Taybi syndrome, and Kabuki syndrome.51-54,61,74-76
SURGICAL TECHNIQUES IN SKELETALLY IMMATURE PATIENTS
While nonsurgical, conservative treatment involving physical therapy and activity modification is recommended for most patients who experience first-time traumatic patellar dislocations, many patients experience complicating factors that indicate them for surgery. These factors include recurrent dislocation, risk factors for patellofemoral instability, underlying malalignment issues, and congenital deformities. When evaluating these factors, particularly patellofemoral instability, the authors recommend assessing osteochondral lesions, age, skeletal maturity, number of previous dislocations, family history, and anatomic risk factors.2,5,77-79 Extra care should be taken when considering surgical treatment for skeletally immature patients at elevated risk for recurrent instability as the risk of cartilage damage in these cases is high.80-82
Recently, there has been a reported increase in surgical treatment for patellar instability in the skeletally immature.83 This finding may be attributed to heightened awareness of factors that indicate patients for surgical treatment and increased familiarity of surgeons with newer techniques.83 Many surgical techniques have been described to address patellar instability involving both soft-tissue procedures and bony corrections.84 In this article, we discuss the various surgical techniques for MPFL reconstruction, quadricepsplasty, and distal realignment. These procedures can be paired with any number of additional procedures including, but not limited to, lateral retinacular release or lengthening, chondroplasty, TT osteotomy (in skeletally mature patients), and removal of loose bodies.83
There is a need for more comprehensive studies, particularly randomized controlled trials, to evaluate the outcomes for both surgical and nonsurgical treatments for first-time dislocations. In the current literature, only very recently have surgical treatments shown outcomes that are more positive. In 2009, Nietosvaara and colleagues85 conducted a randomized controlled trial of nonoperative and operative treatment of primary acute patellar dislocation in both children and adolescents. After a long-term mean follow-up of 14 years, there was not a significant difference between the groups in recurrent dislocation and instability, subjective outcome, or activity scores.85 In a subsequent review of 5 studies including 339 knees, Hing and colleagues86 also found similar results in both the operative and nonoperative cohorts at risk of recurrent dislocations, Kujala scores, and reoperations. However, a recent systematic review comparing redislocation rates and clinical outcomes between surgical and conservative management of acute patellar dislocation reported more positive outcomes for the surgical cohort.87 This review included 627 knees, 470 of which received conservative management, 157 of which received operative treatment. The conservative cohort was followed for an average of 3.9 years and had a 31% rate of recurrent dislocation while the surgical group was followed for a mean 4.7 years and experienced a 22% redislocation rate.87 This study indicates that operative management for acute first-time dislocations may be the preferred treatment option.
Continue to: A potential reason some of these studies...
A potential reason some of these studies did not show any significant difference between the operative and nonoperative cohort could be that the surgical cohorts included a wide range of procedures including lateral releases and MPFL repairs. Recent publications have indicated that these techniques do not produce overall positive outcomes. While each surgical treatment plan is unique depending on the patient; recently, MPFL reconstruction has been shown to have better outcomes than both nonoperative management and simple medial repair and/or lateral
release.67,88-90
MPFL RECONSTRUCTION
INDICATIONS/OVERVIEW
The MPFL is an important stabilizer for the knee that primarily resists lateral translation of the patella. Damage to the MPFL is very common in acute patellar dislocations with up to 90% of first-time dislocations resulting in injury to the MPFL.91,92 Historically, simple medial and/or lateral MPFL repairs have not been shown to improve patellofemoral kinematics significantly and often result in recurrence.90,93 To address this issue, during the past few decades, numerous MPFL reconstruction techniques have been developed to reconstruct a stronger ligament with the same kinematics as the anatomic MPFL.2,19,69,81,94-106 The ultimate goal of MPFL reconstruction is to reestablish the anatomic “checkrein” to guide the patella into the trochlea between 0° and 30° of knee flexion.107,108 An essential secondary surgical goal in skeletally immature patients is to avoid damaging the distal femoral physis.
There are many variations in both the grafts used to replace the MPFL and the means by which to secure them. The ones discussed below include free semitendinosus or gracilis autografts or grafts constructed from a pedicled adductor, patellar, or quadriceps tendon.69,105,109 While not used as frequently, allografts have also been used.110 Methods to secure these grafts in osseous tunnels include suture anchors or tenodesis screws. Incomplete osseous sockets or medial-sided bone tunnels have also been used as a method to decrease patellar fractures as they preserve the lateral patellar cortex.111-114
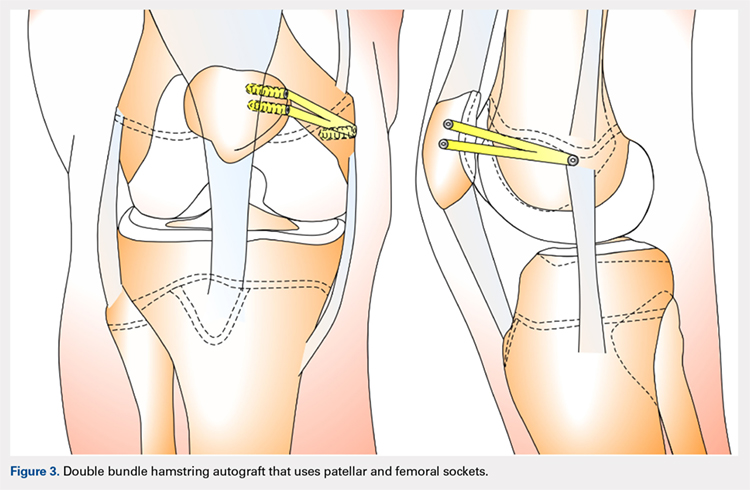
DOUBLE-BUNDLE HAMSTRING AUTOGRAFT
The technique most often used by the author is a double-bundle hamstring autograft harvested from either the semitendinosus or the gracilis secured by short patellar and femoral sockets (Figure 3). After harvesting the hamstring graft from a posteromedial incision, an approximately 90-mm graft is prepared with Krackow stitches to secure 15 mm of the tendon in each socket.115 Lateral radiographs are used intraoperatively to ensure the guidewire for the femoral drill hole falls along the posterior cortex of the diaphysis of the femur while AP radiographs confirm placement distal to the physis. It is important to take both AP and lateral radiographs intraoperatively due to the concave curvature of the distal femoral physis. This unique anatomy can make a point that is located distally to the physis on the AP view appear on or proximal to it on the lateral cross reference view.24,116 For the patellar socket, 2 short sockets are made in the superior half of the patella. Once the sockets have been drilled, the graft is adjusted so that the patella stays seated in the center of the trochlea between 20° and 30° of flexion. This anchoring is accomplished by securing the graft while the knee is kept at 30° of flexion. Proper tension is confirmed by ensuring that the graft does not allow lateral patella movement over one-fourth the width of the patella in extension while crepitation must not appear throughout the ROM.92
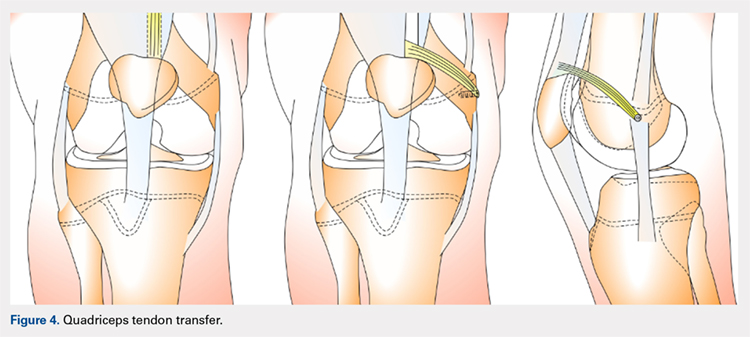
QUADRICPETS TENDON TRANSFER
A combination of techniques by Steensen and colleagues,105 Goyal,109 Noyes and Albright,117 and Pinkowsky and Hennrikus118 describe an MPFL reconstruction in which the proximal end of a small medial portion of the quadriceps tendon is released and then attached to the medial epicondyle through a subcutaneous tunnel (Figure 4). This technique is particularly useful for cases in which the extra strength provided by the bone-quadriceps tendon is necessary to correct more severe dysplasia. Leaving the distal end of the quadriceps tendon intact at its patellar insertion, a graft of about 8 mm x 70 mm thickness is harvested from the tendon. The free distal end of the tendon is then run anatomically through the synovium and retinaculum to be either sutured to the medial intermuscular septum at the medial femoral epicondyle or fixed in femoral tunnel using interference screw.105,109,118 The placement of the femoral fixation point is essential to ensure positive surgical outcomes. If the graft is secured too anteriorly, it may be too loose in extension and too tight in flexion, both of which can lead to postoperative pain, loss of normal kinematics, and overload of the medial patellofemoral cartilage.119-121 Once the ideal placement of the femoral fixation point has been confirmed by intraoperative radiographs, the graft is secured with a small absorbable suture.122,123 While this technique has good clinical results, the longitudinal scar that results from graft harvesting is cosmetically unappealing, and it is technically challenging to harvest a consistent strip of the quadriceps tendon. To address some of these concerns, Fink and colleagues124 described a new harvesting technique that produces more consistent grafts and requires a smaller incision.
Continue to: ADDUCTOR MAGNUS TENDON TRANSFER
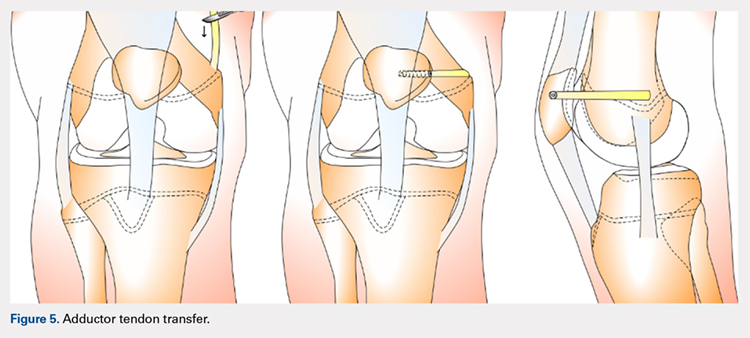
ADDUCTOR MAGNUS TENDON TRANSFER
This technique is a double-bundle MPFL reconstruction that uses a pedicled graft of the distal adductor magnus tendon and suture anchors or incomplete osseous sockets to recreate the MPFL anatomically (Figure 5). Avikainen and colleagues96 and Sillanpää and colleagues125 described this procedure as a progression from the original single-strand adductor magnus transfer technique. First, maintaining the distal insertion, a graft of approximately 14 cm to 18 cm is harvested from the adductor tendon and then passed through a subcutaneous tunnel between the distal vastus medialis obliquus and the superficial joint capsule. The graft is then looped at the medial patella so that the distal bundle runs back to the adductor tubercle.125 With the knee at 30° of flexion to assure proper tension, the graft is secured at both the patella and near the adductor tubercle with suture anchors.125 Hambridge and colleagues126 compared a similar adductor magnus transfer with other pedicled techniques including bone-quadriceps tendon autograft and bone-patellar tendon allograft and found positive results for all 3 methods of reconstruction.
HEMI-PATELLA TENDON TRANSFER
In a similar technique to the adductor tendon transfer, the medial section of the patellar tendon is harvested from the TT and run from its proximal insertion at the medial patella to the medial femoral attachment via a subcutaneous tunnel. The free end of the graft is then secured with suture anchors or incomplete osseous sockets with the knee at 30° of flexion.127
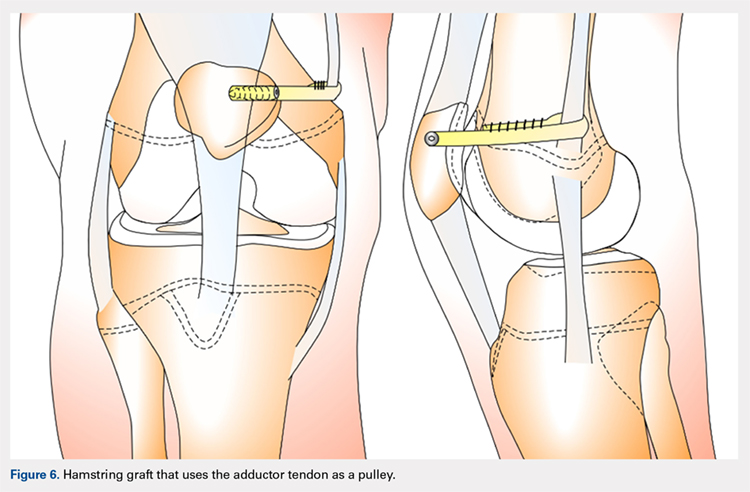
HAMSTRING GRAFT WITH ADDUCTOR TENDON AS A PULLEY
Several techniques opt to use a more dynamic model of MPFL reconstruction in which the adductor tendon or medial collateral ligament (MCL) is used as a pulley for the hamstring graft (Figure 6).128,129 The site of the pulley approximates the normal attachment of the MPFL to the femur and so acts as an effective anatomic replica of the MPFL origin. A semitendinosus graft is harvested and is prepared with continuous sutures, and 2 tunnels to secure the graft are drilled into the patella. The graft is then run subcutaneously from the medial side of the patella to the adductor magnus tubercle into which an osteoperiosteal tunnel is drilled at its distal femoral insertion. The graft is looped through the adductor tunnel and secured with sutures. Proper knee kinematics was ensured by placing the knee at 30° of flexion as the ends of the tendon are secured to the patella.114,130
HAMSTRING GRAFT WITH MCL AS A PULLEY
The MCL can also be used as a pulley rather than the adductor tendon. The semitendinosus graft is harvested and prepared and the patella drilled as it is in the previous technique. The MCL was fashioned into a pulley by making a slit in its posterior one-third. The semitendinosus graft is looped through this slit, and both ends of the graft are held in place with suture anchors on the surface of the patella.129
ADDITIONAL PROCEDURAL COMBINATIONS
Depending on the needs of the individual patient, MPFL reconstruction, and other patellar stabilization techniques can also be combined with additional procedures. Arshi and colleagues83 conducted a review of 6190 adolescents surgically treated for patellar instability and reported the most common additional procedures performed at the time of the stabilization. They found 43.7% of the population underwent lateral retinacular release, which while not effective as an isolated technique to treat patellar instability, has often been used in combination with MPFL reconstruction.131-133 There is currently a lack of consensus regarding the success of adding a lateral release to the reconstruction. Some studies report no difference while others report a decrease in stability after lateral release.90,134-136 While lateral retinacular release has been shown to decrease the force required to displace the patella, it can be surgically indicated in certain patients undergoing MPFL reconstruction.131 The authors advocate that if the lateral retinaculum is tight such that centralized patellar tracking is inhibited following the reconstruction, or if the patella cannot be pushed passively from a laterally tilted position to the neutral horizontal position, lateral retinacular lengthening should be performed to improve kinematics.132
Continue to: Arshi and colleagues...
Arshi and colleagues83 also reported a high rate of cartilage procedures, with chondroplasty performed in 31.1% and chondral fragment/loose body removal in 10.2%. These statistics suggest that a significant level of cartilage damage has occurred by the time of surgery.83
COMPLICATIONS
As MPFL reconstruction techniques have only recently been popularized and developed, there are not many comprehensive studies evaluating the outcomes and complications associated with these procedures. However, in the current literature, there is a general consensus that patients usually experience positive short-term clinical outcomes and relatively low complication rates.68,77 In one of the largest retrospective cohort studies of pediatric patients undergoing MPFL reconstruction, Parikh and colleagues114 reported both the type and rate of complications. They found complications occurred in 16.2% of patients, and the most common complications were recurrent patellar instability, patellar fractures, patellofemoral arthrosis, motion deficits, and stiffness with over half classified as avoidable. Most of these complications were due to technical errors with episodes of recurrent instability only reported in 4.5% of patients.114 In a comprehensive meta-analysis of MPFL reconstruction studies, Shah and colleagues137 reported a complication rate of 26% in both pediatric and adult patients. The cohort was not stratified by age, yet complications were similar to those reported by Parikh and colleagues,114 including pain, loss of knee flexion, wound complications, and patellar fracture.137
As indicated by the frequency of technical complications reported by Parikh and colleagues,114 extra caution should be taken in the operating room to minimize potential errors. In techniques that require drilling of femoral sockets, proper length for and placement of the graft is essential to reestablish proper kinematics. Studies have reported that placing the femoral socket too proximally can result in loss of ROM during flexion and increased compressive forces across the patella.138 A graft that is too short can have similar negative outcomes, and a graft that is too long can result in recurrent instability. Positioning the graft while the knee is in 30° of flexion can help ensure the proper length and tension is achieved. Once the graft is in place, it is important to ensure the ROM and isometry before completing the fixation.72 It is also essential to be vigilant about potential violation of the physes and subsequent growth disturbances. To establish the safest angles for drilling the distal femoral epiphysis for graft placement, Nguyen and colleagues139 conducted a study using high-resolution 3-dimensional images of cadaveric distal femoral epiphyses. By recording which tunnels disrupted the physis before reaching 20 mm of depth, the authors concluded that it is safest to drill distally and anteriorly at an angle between 15° and 20°.139 This technique should minimize damage to the physis, notch, and distal femoral cartilage and decrease potential complications.139
OUTCOMES
In general, the literature reports positive outcomes for MPFL reconstruction—in both studies that address a specific technique and all-encompassing studies. Outcomes are typically reported as Kujala and Tegner scores, results from clinical examinations, and rates of subsequence recurrences. Several recent studies have also evaluated the ability of MPFL reconstruction to restore proper kinematics. Edmonds and colleagues140 evaluated the difference in patellofemoral joint reaction forces and load experienced by 3 groups of adolescents: a cohort treated with MPFL reconstruction, a cohort treated with soft-tissue realignment of the extensor mechanism (the Insall method), and controls. While both surgical techniques were able to restore medial constraints to the patella, the study showed that only the MPFL reconstruction cohort experienced joint reaction forces that were analogous to the control group. In comparison, the cohort that was treated with soft-tissue realignment alone experienced higher patellofemoral joint reaction forces and did not regain normal joint mechanics.140 These results can be used to advocate for the further use of MPFL reconstruction as an effective anatomic replacement of the native ligament. Radiographic studies have similarly reported MPFL reconstruction as an effective means to restore anatomic normality. Fabricant and colleagues141 conducted a radiographic study in which patella alta was corrected to normal childhood ranges in patients who underwent MPFL reconstruction technique using a hamstring autograft. Lykissas and colleagues142 corroborated these results with another radiographic study that reported small but significant decreases in the Blackburne-Peel index and CD index following MPFL reconstruction in 25 adolescents. As correction of patella alta allows the patella to rest in a deeper, more secure position in the TG, these results indicate that effective early MPFL reconstruction can correct for patellar anatomic abnormalities that could be future risk factors.143,144 Several studies have also reported outcomes addressing specific MPFL techniques; these are reported and discussed in this article.
OUTCOMES BY TECHNIQUE
HAMSTRING AUTOGRAFT
Reports on outcomes following MPFL reconstructions using hamstring autografts have been particularly promising. A cohort of 21 skeletally immature patients who underwent MPFL reconstruction was evaluated pre- and postoperatively with an average of a 2.8-year follow-up. The authors of the study reported no redislocation events and significant improvement in the Kujala scores, and patients were able to return to athletic activities safely.145 Previous studies report similar positive increases in Kujala scores, subjective patient reports, and lack of subsequent redislocation for patients who underwent either semitendinosus or gracilis autograft MPFL reconstructions. One such study further documented an average patellar inclination angle decrease from 34.3° to 18.6° following MPFL reconstruction.146 However, while the literature typically reports positive Kujala scores and subjective outcomes for the hamstring autograft procedure, a study arthroscopically evaluating patellar tracking immediately following surgery and then at 6 to 26 months follow-up found that patellar tracking correction was not maintained for all patients who underwent this type of MPFL reconstruction.147
Continue to: QUADRICEPS TENDON TRANSFER OUTCOMES
QUADRICEPS TENDON TRANSFER OUTCOMES
Studies specifically evaluating the quadriceps tendon transfer technique for MPFL reconstruction in children are sparse, but authors have reported positive clinical outcomes and low complication rates in adults. After following 32 young adults who underwent this MPFL reconstruction technique for 3 years, Goyal109 reported a significant increase in mean Kujala scores from 49.31 to 91.25 and no complications or redislocation. He argues this type of quadriceps graft has a high success rate because it is anatomically more similar to the MPFL than other grafts and does not require additional patellar fixation.101,109 Similar positive Kujala scores and minimal complications have been reported in adult patient populations.148 Abouelsoud and colleagues149 conducted one of the few studies in skeletally immature patients and reported similarly positive results with no redislocations and significantly improved Kujala scores at a mean follow-up of 29.25 months in their 16-patient cohorts.
ADDUCTOR MAGNUS TENDON TRANSFER
After initially describing this technique in 14 adult patients, Avikainen and colleagues96 followed this cohort and reported positive subjective results and only 1 redislocation. In a more recent study in which the adductor tendon transfer technique was compared with the quadriceps tendon transfer described above and the bone-patellar tendon allograft, Steiner and colleagues69 reported similarly significant improvement in all cohorts in Lysholm, Kujala, and Tegner scores with no redislocations. Additionally, Malecki and colleagues150 followed a cohort of 33 children with 39 knees diagnosed with recurrent patellar dislocation, who underwent MPFL reconstruction using the adductor magnus tendon. After evaluating this cohort functionally and radiographically, the authors reported improvements in Lysholm and Kujala scores, patellar tilt and congruence angles, and peak torque of the quadriceps muscle and flexor.150 However, this cohort did report postoperative redislocations in 36.4% of patients (4 of 11).150
HEMI-PATELLA TENDON TRANSFER
In 2012, in the first randomized controlled trial, Bitar and colleagues67 compared the outcomes of patients who underwent MPFL reconstruction via the hemi-patellar tendon technique with those who were managed nonoperatively with immobilization and physiotherapy after first-time patellar dislocation. At 2-year follow-up, the surgical cohort presented positive results with a significantly higher mean Kujala score (88.9 to 70.8) and no redislocations or subluxations. In contrast, 35% of nonoperative cases presented with recurrences and subluxations over the 2-year period.67
MCL OR ADDUCTOR TENDON AS A PULLEY
Studies have reported good postoperative results and low complication rates for these dynamic techniques.128,129 In terms of kinematics, while hypermobility and patellar height were not fully corrected, improvements in patellar tilt and lateral shift were reported in a cohort of 6 patients with a minimum 4-year follow-up.129 To further evaluate whether the more dynamic pulley reconstruction technique resulted in better outcomes, Gomes and colleagues128 compared the subjective reports, clinical evaluations, and complication rates of patients who underwent MPFL reconstruction with a rigid adductor magnus fixation vs a semitendinosus tendon dynamic femoral fixation. One case in the rigid cohort experienced a subsequent subluxation, while patients in the semitendinosus group had better subjective reports and a higher rate of return to sport.128 More recently, Kumahashi and colleagues151 specifically studied the outcomes of the MCL tendon as a pulley in 5 patients aged 14 to 15 years. They reported similar successful results as no patients experienced recurrence, and all patients exhibited improvement in radiographic measures of patellar tilt and congruence angle, lateral shift ratio, and both Kujala and Lysholm scores.151
While there has yet to be a randomized controlled trial comparing all of these different techniques, there is a general consensus in the literature that patients tend to perform better following MPFL reconstruction vs MPFL repair.
OTHER STABILIZATION PROCEDURES, INCLUDING DISTAL REALIGNMENT
Patients with additional underlying deficits and malalignment issues such as significant trochlear dysplasia, increased TT-TG distance, patella alta, increased Q angle, and/or positive J sign may require stabilization procedures beyond MPFL reconstruction.152,153 TT osteotomies are often used to correct alignment issues in the adult patient population; however, these procedures are typically contraindicated in skeletally immature patients. Alternative realignment procedures for the pediatric population include both proximal and distal realignment, with proximal realignment performed primarily in children under the age of 12 years.153 Many variations on these procedures exist, some of which are no longer regularly performed due to poor reported outcomes. In this article, we discuss several of the techniques, focusing primarily on those that have demonstrated higher success rates.
Continue to: GALEAZZI TECHNIQUE
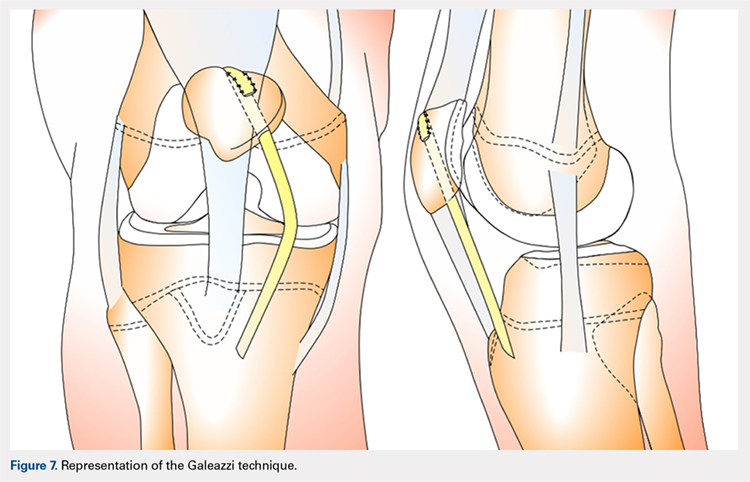
GALEAZZI TECHNIQUE
One of the first and most famous soft-tissue techniques to address patellar instability was the semitendinosus tenodesis, published by Galeazzi154 in 1922 (Figure 7). This technique stabilizes the patella without altering the TT. In the original technique, a portion of the semitendinosus tendon is harvested with its tibial insertion left intact. The free end of the tendon is then secured with sutures at the periosteal groove of the medial patella.154,155 Fiume156 modified this technique by adding a lateral release and medial retinacular reefing. The most recent addition to this procedure was introduced by Baker and colleagues,157 in which a tunnel is drilled from the medial to the lateral border of the patella. Tension placed on the grafted tendon is used to reposition the patella medially and draw it downward. Preliminary literature on this modified procedure reported fair clinical results with success rates of approximately 75%.155,158-160 A recent study evaluating both the clinical and radiographic outcomes of this technique also indicated that while clinical results were excellent in 62.5% of patients, this technique alone was unsuccessful in fully addressing patellar instability in patients with underlying anatomic abnormalities such as patellar alta.161 In light of these less than ideal reports, the authors no longer recommend this technique for patellofemoral instability cases.
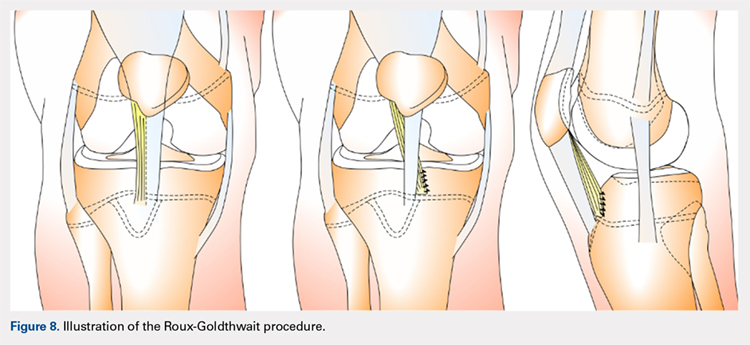
ROUX-GOLDTHWAIT PROCEDURE
The Roux-Goldthwait procedure, first described by both Roux162 and Goldthwait163 in 1888 and 1895 respectively, was later modified in 1985 to involve a lateral release, plication of the medial retinaculum, medial transfer of the lateral patellar tendon without advancement, and advancement of the vastus medialis (Figure 8).164 More recently, Marsh and colleagues152 introduced an addition to aligning the extensor mechanism with the femoral shaft better. In this technique modification, the patellar tendon is split longitudinally, and its lateral half is detached and transferred distally beneath its medial half. The free end is then sutured to the periosteum on the medial side of the tibia.152 With a mean long-term follow-up of 6.2 years, Marsh and colleagues152 reported excellent results in 65%, good in 11%, and fair in 3% of the knees operated on with this modified technique. Of the patients in this cohort whose strength was evaluated, 80% had their strength returned to 90% of preoperative levels in the operated leg.152 While this study and others report improved outcomes, an increasing body of literature has found high rates of recurrence, patella infera, and other complications following the modified Roux-Goldthwait procedure.36,165-171 Also, a study comparing MPFL reconstruction using adductus magnus transfer with the Roux-Goldthwait procedure reported that patients in the MPFL cohort reported less pain postoperatively.150 In addition, whereas the Kujala and Lysholm scores, recurrence rates, patellofemoral angles, and apprehension test results did not demonstrate significant differences between these 2 groups, the MPFL group had significantly fewer abnormal congruence angles, better patellar medialization, and higher peak torque of the hamstring.150
COMBINED MPFL AND MEDIAL PATELLOTIBIAL LIGAMENT RECONSTRUCTION
While the medial patellotibial ligament (MPTL) has not received much attention with regard to patellar stability, recent studies have indicated its role during higher degrees of both flexion and extension.172 The MPTL acts as a secondary restrictor ligament which helps release stress on the MPFL by decreasing the Q angle and further normalizing patellar kinematics.173 Patients who present with hyperlaxity or knee hyperextension combined with extension subluxation and flexion instability could be indicated for this additional stabilizing procedure. Both Nietosvaara and colleagues85 and Brown and Ahmad174 have described a dual MPTL and MPFL reconstruction technique using a semitendinosus hamstring graft. More recently Hinckel and colleagues172 described a combined MPFL and MPTL reconstruction, using a graft from the quadriceps tendon to reconstruct the MPFL and one from the patellar tendon to reconstruct the MPTL. In this technique, once the respective grafts have been harvested, a femoral insertion for the graft recreating the MPFL is fluoroscopically established so that an anchor can be inserted distal to the femoral physeal growth plate. For the MPTL insertion, attachment to the tibia below the joint line and 2 cm medial to the patellar tendon is established fluoroscopically just above the physeal growth plate on the proximal epiphysis.19,175 The MPTL graft is sutured first with the knee at 90° of flexion to establish tension similar to that of the patellar tendon.176 Then, the knee is placed in 30° of flexion to fix the MPFL graft to the medial patella to prevent excessive lateral translation of the patella.
PATELLAR TENDON TRANSFER
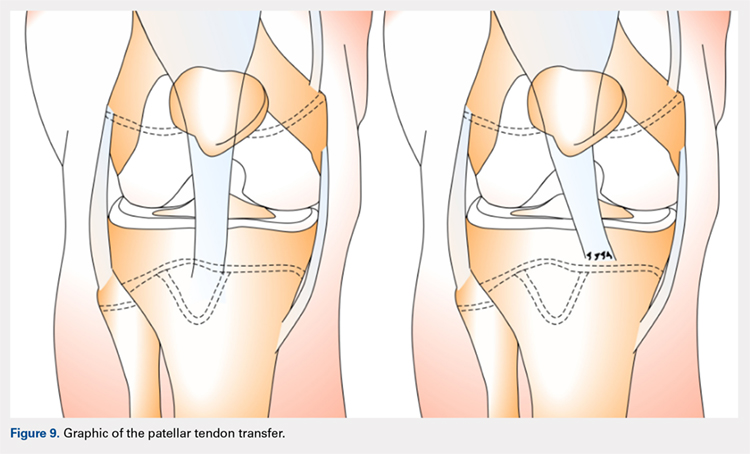
Patellar tendon transfer with proximal realignment is a technique used in particularly young patients to address cases of patellofemoral instability involving concomitant bony or anatomic abnormalities. This procedure is effective for young children with substantial amounts of remaining growth as it better mimics native anatomy than other realignment procedures and does not require bony remodeling.152-154 It is important to familiarize with surgical techniques to address malalignment issues in young patients as neglected alignment issues can lead to worsening of trochlear dysplasia and instability, which are very difficult to treat later on when patients are older.153
The patellar tendon transfer technique (Figure 9), as described by Gordon and Schoenecker,177 starts with an extensive lateral retinacular release. The patellar tendon is then released from its distal insertion at the TT so that it can be moved medially without moving it inferiorly. After confirming patellar tracking and alignment by flexing the knee from 0° to 90° with the graft in place, the patellar tendon graft is secured with multiple nonabsorbable horizontal sutures.177 Of note, in skeletally mature patients, a TT osteotomy is used to accomplish the same goal. This osteotomy has been shown to improve both patellar height and TT-TG distance in skeletally mature patients, but is contraindicated in skeletally immature patients.92,178
Continue to: Initial studies conducted on patellar tendon...
Initial studies conducted on patellar tendon transfer have positive outcomes.179 At a mean follow-up of 5.1 years, patients reported a decrease in pain and increased the ROM and activity, and only 1 reported a postoperative redislocation.179 In more recent studies, both Benoit and colleagues36 and Garin and colleagues58 reviewed cases of patellar instability treated with patellar tendon transfer to address concomitant patellar alignment and anatomic abnormalities. They reported good functional, clinical, and radiographic outcomes with 12.5% and 16% recurrence rates, respectively.36,58 They also noted radiographic improvements in femoral sulcus angle, particularly in younger patients, which indicate this procedure is effective in addressing bony abnormalities that can result from neglected malalignment issues.36,58,154
QUADRICEPSPLASTY
Quadricepsplasty is a lengthening and remodeling technique not frequently used in the pediatric population. The goal of this procedure in patients with significant amounts of growth remaining is to reposition the patella to ameliorate trochlear remodeling and prevent worsening symptoms and anatomic abnormalities.36 A quadricepsplasty accomplishes this by de-rotating and/or lengthening the extensor mechanism and may or may not involve a concomitant MPFL reconstruction. This procedure is particularly effective in young patients who experience obligatory dislocation.60,72 Several quadricepsplasty techniques have been described including Thompson, Curtis and Fisher, Judet, Stanisavljevic, and V-Y technique.180-186 Most techniques initially involve sharp dissection of the vastus medialis and lateralis from the rectus femoral tendon. A tongue is then fashioned out of the rectus femoral tendon. Once the vastus medialis and lateralis are detached from the margins of the patella, the knee is extended, and the distal ends of the vasti are sutured to the tongue of the rectus tendon. Effective extension facilitates flexion to 90°.184 The authors recommend a modification of this technique in which a Z lengthening of the quadriceps tendon is performed after the vastus lateralis is removed distally from the patella and the quadriceps tendon.
Several series and case reports evaluating quadricepsplasty in adult patients report positive outcomes with most patients achieving good or excellent flexion with minimal complications.183,185,187-189 Reports on quadricepsplasty used to treat conditions other than patellofemoral instability in children have reported similar positive outcomes.190-192 As quadricepsplasty for patellar instability is relatively rare in pediatric patients, there is not much relevant literature. However, Kocon and colleagues193 reported results of quadricepsplasty and quadricepsplasty combined with the modified Galeazzi procedure in 8 children (10 knees) with a mean follow-up of 3.25 years. Seventy percent of cases resulted in stabilization and correction of patellar position, and only 2 postoperative redislocations were noted.193 Additionally, in a study evaluating 6 patients suffering from patellar instability, 2 of whom were obligate dislocators, quadricepsplasty resulted in patellar stability, satisfaction, and near normal gait patterns.194
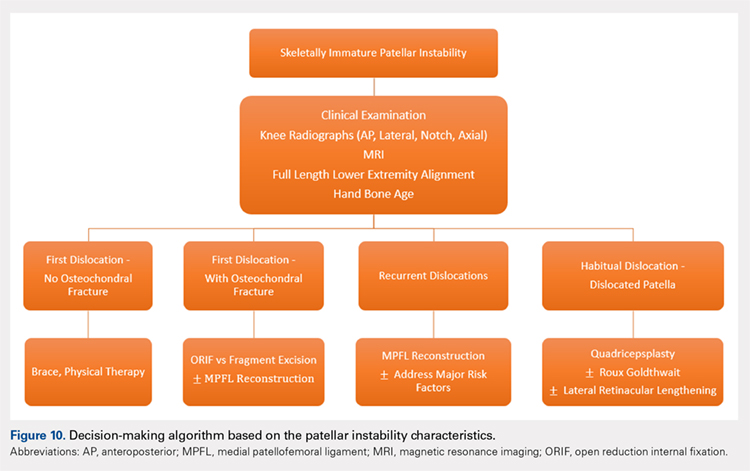
Figure 10 shows the surgical algorithm used for patellar instability characteristics.
CONCLUSION
Patellofemoral joint stability relies on a complex interplay of musculotendinous units, ligaments and the osteocartilaginous morphology of the patellofemoral joint. Patellar instability in pediatric patients is different from adults. Having an in-depth understanding of the remodeling potential, the insertion sites for the MPFL and its relationship to the physis are of utmost importance when planning surgery. Reducing and maintaining the patella within the patellofemoral joint early enough can allow for remodeling of the patella and/or the trochlea to provide for lasting stability. Appropriate surgical principles, such as tensioning, can help both prevent continued pain and minimize future complications.
1. Nietosvaara Y, Aalto K, Kallio PE. Acute patellar dislocation in children: incidence and associated osteochondral fractures. J Pediatr Orthop. 1994;14(4):513-515.
2. Fithian DC, Paxton EW, Stone ML, et al. Epidemiology and natural history of acute patellar dislocation. Am J Sports Med. 2004;32(5):1114-1121. doi:10.1177/0363546503260788.
3. Abbasi D, May MM, Wall EJ, Chan G, Parikh SN. MRI findings in adolescent patients with acute traumatic knee hemarthrosis. J Pediatr Orthop. 2012;32(8):760-764. doi:10.1097/BPO.0b013e3182648d45.
4. Mäenpää H, Lehto MU. Patellar dislocation. The long-term results of nonoperative management in 100 patients. Am J Sports Med. 1997;25(2):213-217. doi:10.1177/036354659702500213.
5. Hawkins RJ, Bell RH, Anisette G. Acute patellar dislocations. The natural history. Am J Sports Med. 1986;14(2):117-120. doi:10.1177/036354658601400204.
6. Cofield RH, Bryan RS. Acute dislocation of the patella: results of conservative treatment. J Trauma. 1977;17(7):526-531.
7. Wasserlauf BL, Paletta GA. Developmental anatomy of the pediatric and adolescent knee. In: Micheli LJ, Kocher MS, eds. The Pediatric and Adolescent Knee. 1st ed. Elsevier; 2006:27-32.
8. Birch JG. Growth and development. In: Herring J, ed. Tachdjian’s Pediatric Orthopedics. 4th ed. Saunders/Elsevier; 2007:3-22.
9. Sarin VK, Carter DR. Mechanobiology and joint conformity regulate endochondral ossification of sesamoids. J Orthop Res. 2000;18(5):706-712. doi:10.1002/jor.1100180505.
10. Maes C, Kronenberg HM. Postnatal bone growth: growth plate biology, bone formation, and remodeling. In: Pediatric Bone. Elsevier; 2012:55-82.
11. Tecklenburg K, Dejour D, Hoser C, Fink C. Bony and cartilaginous anatomy of the patellofemoral joint. Knee Surg Sports Traumatol Arthrosc. 2006;14(3):235-240. doi:10.1007/s00167-005-0683-0.
12. Walmsley R. The development of the patella. J Anat. 1940;74(Pt 3):360-368.3.
13. Fulkerson J, Hungerford D. Disorders of the patellofemoral Joint. In: Normal Anatomy. 2nd ed. Baltimore: Williams & Wilkins; 1990:1-24.
14. Ogden JA. Radiology of postnatal skeletal development. X. Patella and tibial tuberosity. Skeletal Radiol. 1984;11(4):246-257.
15. Nietosvaara Y, Aalto K. The cartilaginous femoral sulcus in children with patellar dislocation: an ultrasonographic study. J Pediatr Orthop. 1997;17(1):50-53.
16. Glard Y, Jouve JL, Garron E, Adalian P, Tardieu C, Bollini G. Anatomic study of femoral patellar groove in fetus. J Pediatr Orthop. 2005;25(3):305-308.
17. Nietosvaara Y. The femoral sulcus in children. An ultrasonographic study. J Bone Joint Surg Br. 1994;76(5):807-809.
18. Nietosvaara AY, Aalto KA. Ultrasonographic evaluation of patellar tracking in children. Clin Orthop Relat Res. 1993;(297):62-64.
19. Desio SM, Burks RT, Bachus KN. Soft tissue restraints to lateral patellar translation in the human knee. Am J Sports Med. 26(1):59-65. doi:10.1177/03635465980260012701.
20. Shea KG, Styhl AC, Jacobs JC, et al. The relationship of the femoral physis and the medial patellofemoral ligament in children: a cadaveric study. Am J Sports Med. 2016;44(11):2833-2837. doi:10.1177/0363546516656366.
21. Shea KG, Polousky JD, Jacobs JC, et al. The relationship of the femoral physis and the medial patellofemoral ligament in children. J Pediatr Orthop. 2014;34(8):808-813. doi:10.1097/BPO.0000000000000165.
22. Shea KG, Polousky JD, Jacobs JC, et al. The patellar insertion of the medial patellofemoral ligament in children. J Pediatr Orthop. 2015;35(4):e31-e35. doi:10.1097/BPO.0000000000000399.
23. Shea KG, Grimm NL, Belzer J, Burks RT, Pfeiffer R. The relation of the femoral physis and the medial patellofemoral ligament. Arthroscopy. 2010;26(8):1083-1087. doi:10.1016/j.arthro.2009.12.020.
24. Nelitz M, Dornacher D, Dreyhaupt J, Reichel H, Lippacher S. The relation of the distal femoral physis and the medial patellofemoral ligament. Knee Surg Sport Traumatol Arthrosc. 2011;19(12):2067-2071. doi:10.1007/s00167-011-1548-3.
25. Kepler CK, Bogner EA, Hammoud S, Malcolmson G, Potter HG, Green DW. Zone of injury of the medial patellofemoral ligament after acute patellar dislocation in children and adolescents. Am J Sports Med. 2011;39(7):1444-1449. doi:10.1177/0363546510397174.
26. Seitlinger G, Moroder P, Fink C, Wierer G. Acquired femoral flexion deformity due to physeal injury during medial patellofemoral ligament reconstruction. Knee. 2017;24(3):680-685. doi:10.1016/j.knee.2017.02.003.
27. Parikh SN, Rajdev N. Relationship between trochlear dysplasia and the anterior distal femoral physis in pediatric and adolescent patients with patellar instability. In: 36th Annual Meething AANA. Denver; 2017.
28. Salenius P, Vankka E. The development of the tibiofemoral angle in children. J Bone Joint Surg Am. 1975;57(2):259-261.
29. Parikh SN. Medial patellofemoral ligament reconstruction and simultaneous guided correction of genu valgum for patellar instability in skeletally immature patients. In: EPOSNA. 2017.
30. Brattstroem H. Shape of the intercondylar groove normally and in recurrent dislocation of patella. A clinical and X-ray-Anatomical investigation. Acta Orthop Scand Suppl. 1964;68(suppl 68):1-148.
31. Dejour H, Walch G, Nove-Josserand L, Guier C. Factors of patellar instability: an anatomic radiographic study. Knee Surg Sports Traumatol Arthrosc. 1994;2(1):19-26.
32. Lewallen LW, McIntosh AL, Dahm DL. Predictors of recurrent instability after acute patellofemoral dislocation in pediatric and adolescent patients. Am J Sports Med. 2013;41(3):575-581. doi:10.1177/0363546512472873.
33. Jaquith BP, Parikh SN. Predictors of recurrent patellar instability in children and adolescents after first-time dislocation. J Pediatr Orthop. 2017;37(7):484-490. doi:10.1097/BPO.0000000000000674.
34. Lippacher S, Reichel H, Nelitz M. Radiological criteria for trochlear dysplasia in children and adolescents. J Pediatr Orthop B. 2011;20(5):341-344. doi:10.1097/BPB.0b013e3283474c8b.
35. Parikh SN, Rajdev N, Sun Q. The growth of trochlear dysplasia during adolescence. J Pediatr Orthop. 2018. doi:10.1097/BPO.0000000000001168. [Epub ahead of print]
36. Benoit B, Laflamme GY, Laflamme GH, Rouleau D, Delisle J, Morin B. Long-term outcome of surgically-treated habitual patellar dislocation in children with coexistent patella alta. Minimum follow-up of 11 years. J Bone Joint Surg Br. 2007;89(9):1172-1177. doi:10.1302/0301-620X.89B9.19065.
37. Sugimoto D, Christino MA, Micheli LJ. Effects of surgical intervention on trochlear remodeling in pediatric patients with recurrent patella dislocation cases. J Pediatr Orthop B. 2016;25(4):349-353. doi:10.1097/BPB.0000000000000341.
38. Insall J, Goldberg V, Salvati E. Recurrent dislocation and the high-riding patella. Clin Orthop Relat Res. 1972;88:67-69.
39. Caton J. [Method of measuring the height of the patella]. Acta Orthop Belg. 1989;55(3):385-386.
40. Thévenin-Lemoine C, Ferrand M, Courvoisier A, Damsin JP, Ducou le Pointe H, Vialle R. Is the Caton-Deschamps index a valuable ratio to investigate patellar height in children? J Bone Joint Surg Am. 2011;93(8):e35. doi:10.2106/JBJS.J.00759.
41. Micheli LJ, Slater JA, Woods E, Gerbino PG. Patella alta and the adolescent growth spurt. Clin Orthop Relat Res. 1986;213:159-162.
42. Koshino T, Sugimoto K. New measurement of patellar height in the knees of children using the epiphyseal line midpoint. J Pediatr Orthop. 1989;9(2):216-218.
43. Mays PK, Bishop JE, Laurent GJ. Age-related changes in the proportion of types I and III collagen. Mech Ageing Dev. 1988;45(3):203-212.
44. Smits-Engelsman B, Klerks M, Kirby A. Beighton score: a valid measure for generalized hypermobility in children. J Pediatr. 2011;158(1):119-123. doi:10.1016/j.jpeds.2010.07.021.
45. Jansson A, Saartok T, Werner S, Renström P. General joint laxity in 1845 Swedish school children of different ages: age- and gender-specific distributions. Acta Paediatr. 2004;93(9):1202-1206.
46. Beighton P, Solomon L, Soskolnet CL. Articular mobility in an African population. Ann rheum Dis. 1973;32(5):413-418.
47. Staheli LT, Corbett M, Wyss C, King H. Lower-extremity rotational problems in children. Normal values to guide management. J Bone Joint Surg Am. 1985;67(1):39-47.
48. Kijowski R, Plagens D, Shaeh S, Teitge R. The effects of rotational deformities of the femur on contact pressure and contact area in the patellofemoral joint and on strain in the medial patellofemoral ligament. Annu Meet Int Patellofemoral Study Group, Napa Val San Fr CA. 1999.
49. Post W, Teitge R, Amis A. Patellofemoral malalignment: looking beyond the viewbox. Clin Sports Med. 2002;21(3):521-546.
50. Bruce WD, Stevens PM. Surgical correction of miserable malalignment syndrome. J Pediatr Orthop. 2004;24(4):392-396.
51. Kurosawa K, Kawame H, Ochiai Y, Nakashima M, Tohma T, Ohashi H. Patellar dislocation in Kabuki syndrome. Am J Med Genet. 2002;108(2):160-163.
52. Mik G, Gholve PA, Scher DM, Widmann RF, Green DW. Down syndrome: orthopedic issues. Curr Opin Pediatr. 2008;20(1):30-36. doi:10.1097/MOP.0b013e3282f35f19.
53. Sweeney E, Fryer A, Mountford R, Green A, McIntosh I. Nail patella syndrome: a review of the phenotype aided by developmental biology. J Med Genet. 2003;40(3):153-162.
54. Stevens CA. Patellar dislocation in Rubenstein-Taybi syndrome. Am J Med Genet. 1997;72(2):188-190.
55. Andrish J. The biomechanics of patellofemoral stability. J Knee Surg. 2004;17(1):35-39.
56. Conlan T, Garth WP, Lemons JE. Evaluation of the medial soft-tissue restraints of the extensor mechanism of the knee. J Bone Joint Surg Am. 1993;75(5):682-693.
57. Balcarek P, Ammon J, Frosch S, et al. Magnetic resonance imaging characteristics of the medial patellofemoral ligament lesion in acute lateral patellar dislocations considering trochlear dysplasia, patella alta, and tibial tuberosity-TG distance. Arthroscopy. 2010;26(7):926-935. doi:10.1016/j.arthro.2009.11.004.
58. Garin C, Chaker M, Dohin B, Kohler R. Permanent, habitual dislocation and recurrent dislocation of the patella in children: surgical management by patellar ligamentous transfer in 50 knees. Rev Chir Orthop Reparatrice Appar Mot. 2007;93(7):690-700.
59. Sillanpaa P. Terminology of patellar dislocation. In: Sillanpaa P, ed. Trauma. Saarbrucken, Germany: Lambert Academic Publishing; 2010:16-18.
60. Parikh SN, Lykissas MG. Classification of lateral patellar instability in children and adolescents. Orthop Clin North Am. 2016;47(1):145-152. doi:10.1016/j.ocl.2015.08.016.
61. Chotel F, Bérard J, Raux S. Patellar instability in children and adolescents. Orthop Traumatol Surg Res. 2014;100(suppl 1):125-137. doi:10.1016/j.otsr.2013.06.014.
62. Wada A, Fujii T, Takamura K, Yanagida H, Surijamorn P. Congenital dislocation of the patella. J Child Orthop. 2008;2(2):119-123. doi:10.1007/s11832-008-0090-4.
63. Südkamp NP, Granrath M, Hoffmann R, Haas NP. Instability of the shoulder joint in the athlete. Chirurg. 1994;65(11):901-909.
64. Hiemstra LA, Kerslake S, Lafave M, Heard SM, Buchko GML. Introduction of a classification system for patients with patellofemoral instability (WARPS and STAID). Knee Surg Sport Traumatol Arthrosc. 2014;22(11):2776-2782. doi:10.1007/s00167-013-2477-0.
65. Frosch KH, Schmeling A. A new classification system of patellar instability and patellar maltracking. Arch Orthop Trauma Surg. 2016;136(4):485-497. doi:10.1007/s00402-015-2381-9.
66. Balcarek P, Oberthür S, Hopfensitz S, et al. Which patellae are likely to redislocate? Knee Surg Sport Traumatol Arthrosc. 2014;22(10):2308-2314. doi:10.1007/s00167-013-2650-5.
67. Bitar AC, Demange MK, D’Elia CO, Camanho GL. Traumatic patellar dislocation: nonoperative treatment compared with mpfl reconstruction using patellar tendon. Am J Sports Med. 2012;40(1):114-122. doi:10.1177/0363546511423742.
68. Smith TO, Walker J, Russell N. Outcomes of medial patellofemoral ligament reconstruction for patellar instability: a systematic review. Knee Surg Sport Traumatol Arthrosc. 2007;15(11):1301-1314. doi:10.1007/s00167-007-0390-0.
69. Steiner TM, Torga-Spak R, Teitge RA. Medial patellofemoral ligament reconstruction in patients with lateral patellar instability and trochlear dysplasia. Am J Sports Med. 2006;34(8):1254-1261. doi:10.1177/0363546505285584.
70. Ma LF, Wang F, Chen BC, Wang CH, Zhou JW, Wang HY. Medial retinaculum plasty versus medial patellofemoral ligament reconstruction for recurrent patellar instability in adults: a randomized controlled trial. Arthrosc J Arthrosc Relat Surg. 2013;29(5):891-897. doi:10.1016/j.arthro.2013.01.030.
71. Weeks KD, Fabricant PD, Ladenhauf HN, Green DW. Surgical options for patellar stabilization in the skeletally immature patient. Sports Med Arthrosc Rev. 2012;20(3):194-202.
72. Green D. Surgical treatment of pediatric patella instability. Die Ther der Instabilen patella. 2016:80-89.
73. Lewallen L, McIntosh A, Dahm D. First-time patellofemoral dislocation: risk factors for recurrent instability. J Knee Surg. 2015;28(4):303-309. doi:10.1055/s-0034-1398373.
74. Ghanem I, Wattincourt L, Seringe R. Congenital dislocation of the patella. Part I: pathologic anatomy. J Pediatr Orthop. 2000;20(6):812-816.
75. Bongers E, Van Kampen A, Van Bokhoven H, Knoers N. Human syndromes with congenital patellar anomalies and the underlying gene defects. Clin Genet. 2005;68(4):302-319. doi:10.1111/j.1399-0004.2005.00508.x.
76. Beighton P, Horan F. Orthopaedic aspects of the Ehlers-Danlos syndrome. J Bone Joint Surg Br. 1969;51(3):444-453.
77. Gausden EB, Fabricant PD, Taylor SA, et al. Medial patellofemoral reconstruction in children and adolescents. JBJS Rev. 2015;3(10):1-11. doi:10.2106/JBJS.RVW.N.00091.
78. Palmu S, Kallio PE, Donell ST, Helenius I, Nietosvaara Y. Acute patellar dislocation in children and adolescents: a randomized clinical trial. J Bone Joint Surg Am. 2008;90(3):463-470. doi:10.2106/JBJS.G.00072.
79. Webb JE, Lewallen LW, Christophersen C, Krych AJ, McIntosh AL. Clinical outcome of internal fixation of unstable juvenile osteochondritis dissecans lesions of the knee. Orthopedics. 2013;36(11):e1444-e1449. doi:10.3928/01477447-20131021-30.
80. Hennrikus W, Pylawka T. Patellofemoral instability in skeletally immature athletes. Instr Course Lect. 2013;62:445-453.
81. Nomura E, Inoue M. Surgical technique and rationale for medial patellofemoral ligament reconstruction for recurrent patellar dislocation. Arthroscopy. 2003;19(5):e47. doi:10.1053/jars.2003.50167.
82. Nomura E, Inoue M, Kobayashi S. Long-term follow-up and knee osteoarthritis change after medial patellofemoral ligament reconstruction for recurrent patellar dislocation. Am J Sports Med. 2007;35(11):1851-1858. doi:10.1177/0363546507306161.
83. Arshi A, Cohen JR, Wang JC, Hame SL, McAllister DR, Jones KJ. Operative management of patellar instability in the United States. Orthop J Sport Med. 2016;4(8):2325967116662873. doi:10.1177/2325967116662873.
84. Servien E, Verdonk PC, Neyret P. Tibial tuberosity transfer for episodic patellar dislocation. Sports Med Arthrosc. 2007;15(2):61-67. doi:10.1097/JSA.0b013e3180479464.
85. Nietosvaara Y, Paukku R, Palmu S, Donell ST. Acute patellar dislocation in children and adolescents. Surgical technique. J Bone Joint Surg Am. 2009;91(suppl 2):139-145. doi:10.2106/JBJS.H.01289.
86. Hing CBC, Smith TO, Donell S, Song F. Surgical versus non-surgical interventions for treating patellar dislocation. In Hing CB, ed. Cochrane Database Syst Rev. 2011;(11):CD008106. doi:10.1002/14651858.CD008106.pub2.
87. Nwachukwu BU, So C, Schairer WW, Green DW, Dodwell ER. Surgical versus conservative management of acute patellar dislocation in children and adolescents: a systematic review. Knee Surgery, Sport Traumatol Arthrosc. 2016;24(3):760-767. doi:10.1007/s00167-015-3948-2.
88. Camp CL, Krych AJ, Dahm DL, Levy BA, Stuart MJ. Medial patellofemoral ligament repair for recurrent patellar dislocation. Am J Sports Med. 2010;38(11):2248-2254. doi:10.1177/0363546510376230.
89. Nikku R, Nietosvaara Y, Aalto K, Kallio PE. Operative treatment of primary patellar dislocation does not improve medium-term outcome: a 7-year follow-up report and risk analysis of 127 randomized patients. Acta Orthop. 2005;76(5):699-704. doi:10.1080/17453670510041790.
90. Ostermeier S, Holst M, Hurschler C, Windhagen H, Stukenborg-Colsman C. Dynamic measurement of patellofemoral kinematics and contact pressure after lateral retinacular release: an in vitro study. Knee Surg Sport Traumatol Arthrosc. 2007;15(5):547-554. doi:10.1007/s00167-006-0261-0.
91. Sallay PI, Poggi J, Speer KP, Garrett WE. Acute dislocation of the patella. A correlative pathoanatomic study. Am J Sports Med. 1996;24(1):52-60. doi: 10.1177/036354659602400110.
92. Arendt EA, Fithian DC, Cohen E. Current concepts of lateral patella dislocation. Clin Sports Med. 2002;21(3):499-519.
93. Arendt EA, Moeller A, Agel J. Clinical outcomes of medial patellofemoral ligament repair in recurrent (chronic) lateral patella dislocations. Knee Surg Sports Traumatol Arthrosc. 2011;19(11):1909-1914. doi:10.1007/s00167-011-1516-y.
94. Ellera Gomes JL. Medial patellofemoral ligament reconstruction for recurrent dislocation of the patella: a preliminary report. Arthroscopy. 1992;8(3):335-340.
95. Amis AA, Firer P, Mountney J, Senavongse W, Thomas NP. Anatomy and biomechanics of the medial patellofemoral ligament. Knee. 2003;10(3):215-220.
96. Avikainen VJ, Nikku RK, Seppänen-Lehmonen TK. Adductor magnus tenodesis for patellar dislocation. Technique and preliminary results. Clin Orthop Relat Res. 1993;(297):12-16.
97. Davis DK, Fithian DC. Techniques of medial retinacular repair and reconstruction. Clin Orthop Relat Res. 2002;(402):38-52.
98. Dhillon MS, Mohan P, Nagi ON. Does harvesting the medial third of the patellar tendon cause lateral shift of the patella after ACL reconstruction? Acta Orthop Belg. 2003;69(4):334-340.
99. Drez D, Edwards TB, Williams CS. Results of medial patellofemoral ligament reconstruction in the treatment of patellar dislocation. Arthrosc J Arthrosc Relat Surg. 2001;17(3):298-306. doi:10.1053/jars.2001.21490.
100. Ellera Gomes JL, Stigler Marczyk LR, César de César P, Jungblut CF. Medial patellofemoral ligament reconstruction with semitendinosus autograft for chronic patellar instability: a follow-up study. Arthrosc J Arthrosc Relat Surg. 2004;20(2):147-151. doi:10.1016/j.arthro.2003.11.006.
101. Feller JA, Amis AA, Andrish JT, Arendt EA, Erasmus PJ, Powers CM. Surgical biomechanics of the patellofemoral joint. Arthrosc J Arthrosc Relat Surg. 2007;23(5):542-553. doi:10.1016/j.arthro.2007.03.006.
102. Nomura E, Horiuchi Y, Kihara M. Medial patellofemoral ligament restraint in lateral patellar translation and reconstruction. Knee. 2000;7(2):121-127.
103. Ostermeier S, Stukenborg-Colsman C, Hurschler C, Wirth CJ. In vitro investigation of the effect of medial patellofemoral ligament reconstruction and medial tibial tuberosity transfer on lateral patellar stability. Arthrosc J Arthrosc Relat Surg. 2006;22(3):308-319. doi:10.1016/j.arthro.2005.09.024.
104. Smirk C, Morris H. The anatomy and reconstruction of the medial patellofemoral ligament. Knee. 2003;10(3):221-227.
105. Steensen RN, Dopirak RM, Maurus PB. A simple technique for reconstruction of the medial patellofemoral ligament using a quadriceps tendon graft. Arthroscopy. 2005;21(3):365-370. doi:10.1016/j.arthro.2004.10.007.
106. Steensen RN, Dopirak RM, McDonald WG. The anatomy and isometry of the medial patellofemoral ligament: implications for reconstruction. Am J Sports Med. 2004;32(6):1509-1513. doi:10.1177/0363546503261505.
107. Farahmand F, Tahmasbi MN, Amis AA. Lateral force–displacement behaviour of the human patella and its variation with knee flexion—a biomechanical study in vitro. J Biomech. 1998;31(12):1147-1152.
108. Heegaard J, Leyvraz PF, Van Kampen A, Rakotomanana L, Rubin PJ, Blankevoort L. Influence of soft structures on patellar 3-dimensional tracking. Clin Orthop Relat Res. 1994;(299):235-243.
109. Goyal D. Medial patellofemoral ligament reconstruction: the superficial quad technique. Am J Sports Med. 2013;41(5):1022-1029. doi:10.1177/0363546513477828.
110. Hohn E, Pandya NK. Does the utilization of allograft tissue in medial patellofemoral ligament reconstruction in pediatric and adolescent patients restore patellar stability? Clin Orthop Relat Res. 2017;475(6):1563-1569. doi:10.1007/s11999-016-5060-4.
111. Csintalan R, Latt L, Fornalski S, Raiszadeh K, Inacio M, Fithian D. Medial patellofemoral ligament (MPFL) reconstruction for the treatment of patellofemoral instability. J Knee Surg. 2013;27(2):139-146. doi:10.1055/s-0033-1360652.
112. Ahmad CS, Brown GD, Stein BS. The docking technique for medial patellofemoral ligament reconstruction: surgical technique and clinical outcome. Am J Sports Med. 2009;37(10):2021-2027. doi:10.1177/0363546509336261.
113. Fernandez E, Sala D, Castejon M. Reconstruction of the medial patellofemoral ligament for patellar instability using a semitendinosus autograft. Acta Orthop Belg. 2005;71(3):303-308.
114. Parikh SN, Nathan ST, Wall EJ, Eismann EA. Complications of medial patellofemoral ligament reconstruction in young patients. Am J Sports Med. 2013;41(5):1030-1038. doi:10.1177/0363546513482085.
115. Green D, Gausden E. Medial patellofemoral ligament reconstruction: hamstring technique. In: Green D, Cordasco F, eds. Pediatr. Adolesc. Knee Surg. New York, NY: Wolters Kluwer; 2015:150-157.
116. Craig JG, Cody DD, van Holsbeeck M. The distal femoral and proximal tibial growth plates: MR imaging, 3-dimensional modeling and estimation of area and volume. Skeletal Radiol. 2004;33(6):337-344.
117. Noyes FR, Albright JC. Reconstruction of the medial patellofemoral ligament with autologous quadriceps tendon. Arthroscopy. 2006;22(8):904.e1-e7. doi:10.1016/j.arthro.2005.12.058.
118. Pinkowsky G, Hennrikus. Technique: quad tendon medial patellofemoral ligament reconstruction. In: Green DW, Cordasco FA, eds. Pediatr. Adolesc. Knee Surg. New York, NY: Wolters Kluwer; 2015:133-139.
119. Bollier M, Fulkerson J, Cosgarea A, Tanaka M. Technical failure of medial patellofemoral ligament reconstruction. Arthroscopy. 2011;27(8):1153-1159. doi:10.1016/j.arthro.2011.02.014.
120. Elias JJ, Cosgarea AJ. Technical errors during medial patellofemoral ligament reconstruction could overload medial patellofemoral cartilage: a computational analysis. Am J Sports Med. 2006;34(9):1478-1485. doi:10.1177/0363546506287486.
121. Thaunat M, Erasmus PJ. Management of overtight medial patellofemoral ligament reconstruction. Knee Surg Sports Traumatol Arthrosc. 2009;17(5):480-483. doi:10.1007/s00167-008-0702-z.
122. Schöttle PB, Schmeling A, Rosenstiel N, Weiler A. Radiographic landmarks for femoral tunnel placement in medial patellofemoral ligament reconstruction. Am J Sports Med. 2007;35(5):801-804. doi:10.1177/0363546506296415.
123. Redfern J, Kamath G, Burks R. Anatomical confirmation of the use of radiographic landmarks in medial patellofemoral ligament reconstruction. Am J Sports Med. 2010;38(2):293-297. doi:10.1177/0363546509347602.
124. Fink C, Veselko M, Herbort M, Hoser C. Minimally invasive reconstruction of the medial patellofemoral ligament using quadriceps tendon. Arthrosc Tech. 2014;3(3):e325-e329. doi:10.1016/j.eats.2014.01.012.
125. Sillanpää PJ, Mäenpää HM, Mattila VM, Visuri T, Pihlajamäki H. A mini-invasive adductor magnus tendon transfer technique for medial patellofemoral ligament reconstruction: a technical note. Knee Surg Sports Traumatol Arthrosc. 2009;17(5):508-512. doi:10.1007/s00167-008-0713-9.
126. Hambidge SJ, Davidson AJ, Gonzales R, Steiner JF. Epidemiology of pediatric injury-related primary care office visits in the United States. Pediatrics. 2002;109(4):559-565.
127. Camanho GL, Bitar AC, Hernandez AJ, Olivi R. Medial patellofemoral ligament reconstruction: a novel technique using the patellar ligament. Arthroscopy. 2007;23(1):108.e1-e4. doi:10.1016/j.arthro.2006.07.008.
128. Gomes JE. Comparison between a static and a dynamic technique for medial patellofemoral ligament reconstruction. Arthroscopy. 2008;24(4):430-435. doi:10.1016/j.arthro.2007.11.005.
129. Deie M, Ochi M, Sumen Y, Yasumoto M, Kobayashi K, Kimura H. Reconstruction of the medial patellofemoral ligament for the treatment of habitual or recurrent dislocation of the patella in children. J Bone Joint Surg Br. 2003;85(6):887-890.
130. Ladenhauf HN, Berkes MB, Green DW. Medial patellofemoral ligament reconstruction using hamstring autograft in children and adolescents. Arthrosc Tech. 2013;2(2):e151-e154. doi:10.1016/j.eats.2013.01.006.
131. Bedi H, Marzo J. The biomechanics of medial patellofemoral ligament repair followed by lateral retinacular release. Am J Sports Med. 2010;38(7):1462-1467. doi:10.1177/0363546510373581.
132. Lattermann C, Toth J, Bach BR. The role of lateral retinacular release in the treatment of patellar instability. Sports Med Arthrosc Rev. 2007;15(2):57-60. doi:10.1097/JSA.0b013e318042af30
133. Ricchetti ET, Mehta S, Sennett BJ, Huffman GR. Comparison of lateral release versus lateral release with medial soft-tissue realignment for the treatment of recurrent patellar instability: a systematic review. Arthroscopy. 2007;23(5):463-468. doi:10.1016/j.arthro.2007.01.007.
134. Dainer RD, Barrack RL, Buckley SL, Alexander AH. Arthroscopic treatment of acute patellar dislocations. Arthroscopy. 1988;4(4):267-271.
135. Miller JR, Adamson GJ, Pink MM, Fraipont MJ, Durand P. Arthroscopically assisted medial reefing without routine lateral release for patellar instability. Am J Sports Med. 2007;35(4):622-629. doi:10.1177/0363546506296041.
136. Vainionpää S, Laasonen E, Silvennoinen T, Vasenius J, Rokkanen P. Acute dislocation of the patella. A prospective review of operative treatment. J Bone Joint Surg Br. 1990;72(3):366-369.
137. Shah JN, Howard JS, Flanigan DC, Brophy RH, Carey JL, Lattermann C. A systematic review of complications and failures associated with medial patellofemoral ligament reconstruction for recurrent patellar dislocation. Am J Sports Med. 2012;40(8):1916-1923. doi:10.1177/0363546512442330.
138. Tanaka MJ, Bollier MJ, Andrish JT, Fulkerson JP, Cosgarea AJ. Complications of medial patellofemoral ligament reconstruction: common technical errors and factors for success: AAOS exhibit selection. J Bone Joint Surg Am. 2012;94(12):e87. doi:10.2106/JBJS.K.01449.
139. Nguyen CV, Farrow LD, Liu RW, Gilmore A. Safe drilling paths in the distal femoral epiphysis for pediatric medial patellofemoral ligament reconstruction. Am J Sports Med. 2017;45(5):1085-1089. doi:10.1177/0363546516677795.
140. Edmonds EW, Glaser DA. Adolescent patella instability extensor mechanics. J Pediatr Orthop. 2016;36(3):262-267. doi:10.1097/BPO.0000000000000430.
141. Fabricant PD, Ladenhauf HN, Salvati EA, Green DW. Medial patellofemoral ligament (MPFL) reconstruction improves radiographic measures of patella alta in children. Knee. 2014;21(6):1180-1184. doi:10.1016/j.knee.2014.07.023.
142. Lykissas MG, Li T, Eismann EA, Parikh SN. Does medial patellofemoral ligament reconstruction decrease patellar height? A preliminary report. J Pediatr Orthop. 2014;34(1):78-85. doi:10.1097/BPO.0b013e3182a12102.
143. Magnussen RA, De Simone V, Lustig S, Neyret P, Flanigan DC. Treatment of patella alta in patients with episodic patellar dislocation: a systematic review. Knee Surgery, Sport Traumatol Arthrosc. 2014;22(10):2545-2550. doi:10.1007/s00167-013-2445-8.
144. Schöttle PB, Fucentese SF, Romero J. Clinical and radiological outcome of medial patellofemoral ligament reconstruction with a semitendinosus autograft for patella instability. Knee Surg Sports Traumatol Arthrosc. 2005;13(7):516-521. doi:10.1007/s00167-005-0659-0.
145. Nelitz M, Dreyhaupt J, Reichel H, Woelfle J, Lippacher S. Anatomic reconstruction of the medial patellofemoral ligament in children and adolescents with open growth plates: surgical technique and clinical outcome. Am J Sports Med. 2013;41(1):58-63. doi:10.1177/0363546512463683.
146. Raghuveer RK, Mishra CB. Reconstruction of medial patellofemoral ligament for chronic patellar instability. Indian J Orthop. 2012;46(4):447-454. doi:10.4103/0019-5413.97259.
147. Kita K, Horibe S, Toritsuka Y, et al. Effects of medial patellofemoral ligament reconstruction on patellar tracking. Knee Surg Sports Traumatol Arthrosc. 2012;20(5):829-837. doi:10.1007/s00167-011-1609-7.
148. Lenschow S, Herbort M, Fink C. Medial patellofemoral ligament reconstruction using quadriceps tendon. Oper Orthop Traumatol. 2015;27(6):474-483. doi:10.1007/s00064-015-0416-6.
149. Abouelsoud MM, Abdelhady A, Elshazly O. Anatomic physeal-sparing technique for medial patellofemoral ligament reconstruction in skeletally immature patients with ligamentous laxity. Eur J Orthop Surg Traumatol. 2015;25(5):921-926. doi:10.1007/s00590-015-1618-1.
150. Malecki K, Fabis J, Flont P, Niedzielski KR. The results of adductor magnus tenodesis in adolescents with recurrent patellar dislocation. Biomed Res Int. 2015;2015:1-7. doi:10.1155/2015/456858.
151. Kumahashi N, Kuwata S, Tadenuma T, Kadowaki M, Uchio Y. A “sandwich” method of reconstruction of the medial patellofemoral ligament using a titanium interference screw for patellar instability in skeletally immature patients. Arch Orthop Trauma Surg. 2012;132(8):1077-1083. doi:10.1007/s00402-012-1516-5.
152. Marsh JS, Daigneault JP, Sethi P, Polzhofer GK. Treatment of recurrent patellar instability with a modification of the Roux-Goldthwait technique. J Pediatr Orthop. 2006;26(4):461-465. doi:10.1097/01.bpo.0000217711.34492.48.
153. Nepple J, Luhmann S. Medial patellar tendon transfer with proximal realignment. In: Green D, Cordasco F, eds. Pediatric and Adolescent Knee Surgery. New York, NY: Wolters Kluwer; 2015:148-153.
154. Galeazzi R. Nuove applicazioni del trapianto musculare e tendineo. Ard Di Orthop Milano. 1922;38:315-323.
155. Grannatt K, Heyworth BE, Ogunwole O, Micheli LJ, Kocher MS. Galeazzi semitendinosus tenodesis for patellofemoral instability in skeletally immature patients. J Pediatr Orthop. 2012;32(6):621-625. doi:10.1097/BPO.0b013e318263a230.
156. Fiume M. La rotulopessi secondi Galeazzi nella lussazione recidivante di rotula [in Italian]. Minerva Ortop. 1954;5:171-174.
157. Baker RH, Carroll N, Dewar FP, Hall JE. The semitendinosus tenodesis for recurrent dislocation of the patella. J Bone Joint Surg Br. 1972;54(1):103-109.
158. Hall JE, Micheli LJ, McManama GB. Semitendinosus tenodesis for recurrent subluxation or dislocation of the patella. Clin Orthop Relat Res. 1979;(144):31-35.
159. Moyad TF, Blakemore L. Modified Galeazzi technique for recurrent patellar dislocation in children. Orthopedics. 2006;29(4):302-304.
160. Letts RM, Davidson D, Beaule P. Semitendinosus tenodesis for repair of recurrent dislocation of the patella in children. J Pediatr Orthop. 1999;19(6):742-747.
161. Aulisa AG, Falciglia F, Giordano M, Savignoni P, Guzzanti V. Galeazzi’s modified technique for recurrent patella dislocation in skeletally immature patients. J Orthop Sci. 2012;17(2):148-155. doi:10.1007/s00776-011-0189-1.
162. Roux C. Recurrent dislocation of the patella: operative treatment. 1888. Clin Orthop Relat Res. 2006;452:17-20.
163. Goldthwait J. Dislocation of the Patella. Trans Am Orthop Assn. 1895.
164. Fondren FB, Goldner JL, Bassett FH. Recurrent dislocation of the patella treated by the modified Roux-Goldthwait procedure. A prospective study of forty-seven knees. J Bone Joint Surg Am. 1985;67(7):993-1005.
165. Aärimaa V, Ranne J, Mattila K, Rahi K, Virolainen P, Hiltunen A. Patellar tendon shortening after treatment of patellar instability with a patellar tendon medialization procedure. Scand J Med Sci Sports. 2008;18(4):442-446. doi:10.1111/j.1600-0838.2007.00730.x.
166. Nelitz M, Reichel H, Dornacher D, Lippacher S. Anatomical reconstruction of the medial patellofemoral ligament in children with open growth-plates. Arch Orthop Trauma Surg. 2012;132(11):1647-1651. doi:10.1007/s00402-012-1593-5.
167. Vähäsarja V, Kinnunen P, Lanning P, Serlo W. Operative realignment of patellar malalignment in children. J Pediatr Orthop. 1995;15(3):281-285.
168. Abraham E, Washington E, Huang TL. Insall proximal realignment for disorders of the patella. Clin Orthop Relat Res. 1989;248:61-65.
169. Insall JN, Aglietti P, Tria AJ. Patellar pain and incongruence. II: Clinical application. Clin Orthop Relat Res. 1983;176:225-232.
170. Chrisman OD, Snook GA, Wilson TC. A long-term prospective study of the Hauser and Roux-Goldthwait procedures for recurrent patellar dislocation. Clin Orthop Relat Res. 1979;144:27-30.
171. Niedzielski KR, Malecki K, Flont P, Fabis J. The results of an extensive soft-tissue procedure in the treatment of obligatory patellar dislocation in children with ligamentous laxity: a post-operative isokinetic study. Bone Joint J. 2015;97-B(1):129-133. doi:10.1302/0301-620X.97B1.33941.
172. Hinckel BB, Gobbi RG, Demange MK, Bonadio MB, Pécora JR, Camanho GL. Combined reconstruction of the medial patellofemoral ligament with quadricipital tendon and the medial patellotibial ligament with patellar tendon. Arthrosc Tech. 2016;5(1):e79-e84. doi:10.1016/j.eats.2015.10.004.
173. Mani S, Kirkpatrick MS, Saranathan A, Smith LG, Cosgarea AJ, Elias JJ. Tibial tuberosity osteotomy for patellofemoral realignment alters tibiofemoral kinematics. Am J Sports Med. 2011;39(5):1024-1031. doi:10.1177/0363546510390188.
174. Brown GD, Ahmad CS. Combined medial patellofemoral ligament and medial patellotibial ligament reconstruction in skeletally immature patients. J Knee Surg. 2008;21(4):328-332.
175. Panagiotopoulos E, Strzelczyk P, Herrmann M, Scuderi G. Cadaveric study on static medial patellar stabilizers: the dynamizing role of the vastus medialis obliquus on medial patellofemoral ligament. Knee Surg Sports Traumatol Arthrosc. 2006;14(1):7-12. doi:10.1007/s00167-005-0631-z.
176. Philippot R, Boyer B, Testa R, Farizon F, Moyen B. The role of the medial ligamentous structures on patellar tracking during knee flexion. Knee Surg Sports Traumatol Arthrosc. 2012;20(2):331-336. doi:10.1007/s00167-011-1598-6.
177. Gordon JE, Schoenecker PL. Surgical treatment of congenital dislocation of the patella. J Pediatr Orthop. 1999;19(2):260-264.
178. Koh JL, Stewart C. Patellar instability. Orthop Clin North Am. 2015;46(1):147-157. doi:10.1016/j.ocl.2014.09.011.
179. Pal S, Besier TF, Draper CE, et al. Patellar tilt correlates with vastus lateralis: vastus medialis activation ratio in maltracking patellofemoral pain patients. J Orthop Res. 2012;30(6):927-933. doi:10.1002/jor.22008.
180. Thompson TC. Quadricepsplasty. Ann Surg. 1945;121(5):751-754.
181. Daoud H, O’Farrell T, Cruess RL. Quadricepsplasty. The Judet technique and results of six cases. J Bone Joint Surg Br. 1982;64(2):194-197.
182. Stanisavljevic S, Zemenick G, Miller D. Congenital, irreducible, permanent lateral dislocation of the patella. Clin Orthop Relat Res. 1976;(116):190-199.
183. Kundu Z, Sangwan S, Guliani G, Siwach R, Kamboj P, Singh R. Thompson’s quadricepsplasty for stiff knee. Indian J Orthop. 2007;41(4):390-394. doi:10.4103/0019-5413.37004.
184. Tercier S, Shah H, Joseph B. Quadricepsplasty for congenital dislocation of the knee and congenital quadriceps contracture. J Child Orthop. 2012;6(5):397-410. doi:10.1007/s11832-012-0437-8.
185. Rose RE. Judet quadricepsplasty for extension contracture of the knee. West Indian Med J. 2005;54(4):238-241.
186. Tsukamoto N, Miura H, Matsuda S, Mawatari T, Kato H, Iwamoto Y. Functional evaluation of four patients treated with V-Y quadricepsplasty in total knee arthroplasty. J Orthop Sci. 2006;11(4):394-400. doi:10.1007/s00776-006-1023-z.
187. Dao Q, Chen DB, Scott RD. Proximal patellar quadricepsplasty realignment during total knee arthroplasty for irreducible congenital dislocation of the patella. J Bone Joint Surg Am. 2010;92(14):2457-2461. doi:10.2106/JBJS.H.00812.
188. Judet R, Judet J, Lord G. Results of treatment of stiffness of the knee caused by arthrolysis and disinsertion of the quadriceps femoris. Mem Acad Chir. 1959;85:645-654.
189. Oliveira VG, D’Elia LF, Tirico LEP, et al. Judet quadricepsplasty in the treatment of posttraumatic knee rigidity: long-term outcomes of 45 cases. J Trauma Acute Care Surg. 2012;72(2):e77-e80.
190. Hahn SB, Choi YR, Kang HJ, Lee SH. Prognostic factors and long-term outcomes following a modified Thompson’s quadricepsplasty for severely stiff knees. J Bone Joint Surg Br. 2010;92(2):217-221. doi:10.1302/0301-620X.92B2.22936.
191. Hosalkar HS, Jones S, Chowdhury M, Hartley J, Hill RA. Quadricepsplasty for knee stiffness after femoral lengthening in congenital short femur. J Bone Joint Surg Br. 2003;85(2):261-264.
192. Massè A, Biasibetti A, Demangos J, Dutto E, Pazzano S, Gallinaro P. The judet quadricepsplasty: long-term outcome of 21 cases. J Trauma. 2006;61(2):358-362. doi:10.1097/01.ta.0000230281.31144.1d.
193. Kocon H, Kabacyj M, Zgoda M. The results of the operative treatment of patellar instability in children with Down’s syndrome. J Pediatr Orthop B. 2012;21(5):407-410. doi:10.1097/BPB.0b013e328354f684.
194. Martin BD, Cherkashin AM, Tulchin K, Samchukov M, Birch JG. Treatment of femoral lengthening-related knee stiffness with a novel quadricepsplasty. J Pediatr Orthop. 2013;33(4):446-452. doi:10.1097/BPO.0b013e3182784e5d.
ABSTRACT
Patellar instability in children and adolescents is a challenging subset to treat. Varied forms of instability, ranging from episodic dislocation to fixed dislocation, have been recognized. It is of utmost importance for the treating physician to recognize these different patterns of instability and their associated risk factors, as more complex patterns of instability would require more extensive surgical procedures. Medial patellofemoral ligament (MPFL) reconstruction, by itself, may not suffice or may not be appropriate for the more complex instability patterns. Appropriate and early treatment of such instability in children would allow for functional progression and possible remodeling of the trochlea. However, early treatment has the associated risk of growth disturbances when surgical procedures are performed around open physis or if adult-type bony procedures are performed in children. Recent knowledge about the relationship between trochlea, MPFL femoral attachment, and distal femoral physis could help to advance safe surgical care for these patients. This article reviews the pathophysiology, risk factors, and the existing classification systems for patellar instability in children and adolescents. It focuses on varied surgical techniques, which are unique to the pediatric population, and summarizes the outcomes of these surgical techniques.
Continue to: EPIDEMIOLOGY
EPIDEMIOLOGY
In a prospective 2-year study of Finnish children, the annual incidence rate of patellar instability was 43/100,000 pediatric population.1 In patients 9 to 15 years of age, the incidence was approximately 1/1000.1 In another study, patients at highest risk for a first-time patellar dislocation were females aged 10 to 17 years.2 In a study in patients with traumatic hemarthrosis, 36% in the younger age group (10-14 years) and 28% in the older age group (15-18 years) had sustained patellar dislocation. In contrast, 22% in the younger age group and 40% in the older age group had sustained an anterior cruciate ligament tear.3
Approximately one-half of patients who dislocate their patella suffer from long-term complications.4,5 These complications include recurrent instability, patellofemoral pain, osteochondral lesions, and eventual arthritis.1,4,5 Young, active individuals are more prone to these issues.6 Also, 39% or more of patellar dislocation patients have an associated osteochondral fracture that might influence the management.1 Thus, patellar instability in young patients is an area of concern.
DEVELOPMENTAL ANATOMY
At 4-week gestation, the patellofemoral joint is an ectodermal sac filled with mesenchyme of the somatic mesoderm.7 Mesenchymal condensations then appear at 4 to 5 weeks gestation, followed by chondrification of both the femur and patella.7 The joint space is present by 6 weeks, and the patellar and distal femoral condyles are present at 7 weeks gestation.7 By 8 weeks gestation, the basic knee anatomy resembles that of an adult with the chondroepiphysis forming the articular surfaces of the femur, tibia, and patella.7 By this time, the extensor mechanism is formed, and active joint motion has begun, facilitating the development of the trochlear sulcus.7 The secondary ossification center in the distal femoral epiphysis forms around 36 weeks gestation.8 Postnatally, both the patella and distal femur grow through endochondral ossification.9,10
The patella is the largest sesamoid bone in the human body.11 The patella begins as a dense consolidation of cells that differentiate as the quadriceps mechanisms develop.12,13 The patellar anlage becomes distinguishable within the quadriceps tendon around 7.5-week gestation.12 The morphology of the patella conforms to the distal femur.12 The patella molds or re-models as the knee begins to move in response to mechanical stresses.7 The patella increases in relative size during the first 6 months of gestation, then enlarges proportionately to the rest of the bones.7 Ossification begins around 3 years of age for females and 4 to 5 years of age for males.8,14 The ossification center may appear irregular as it rapidly expands.14 Ossification proceeds in a proximal to distal direction, thus giving a spurious estimation of patellar height on radiographs in children. The overall morphology of the cartilaginous patella during development is comparable to the final mature shape.14 Abnormal contact stresses on the articular surface of the patella during skeletal immaturity can lead to deformation.7
Ultrasonographic measurements in normal patients show that trochlear groove (TG) morphology is present early and becomes more radiographically apparent as distal femoral ossification is completed.15 Anatomic dissections of aborted fetuses have verified the morphology of the TG as it remains constant during growth and the groove morphology is the same for both fetuses and adults.16 An ultrasound study performed on patients aged 12 to 18 years showed the cartilaginous sulcus angle (CSA) remained constant throughout all age groups (146°).17 The CSA however, differed in patients who suffered a patellar dislocation (average, 164°; range, 154°-195°) compared with normal knees (average CSA, 145°; range, 131°-158°).15,17,18 The osseous sulcus angle, on the other hand, appears flat at birth and the TG deepens with age. This increase in depth is more of a reflection of progressive ossification of a well-formed cartilaginous trochlea, rather than a true deepening of the sulcus.17 Thus, the axial radiographic view of the patella provides misleading information about the sulcus angle in children and should not be used to define trochlear morphology.
Continue to: MEDIAL PATELLOFEMORAL LIGAMENT ANATOMY
MEDIAL PATELLOFEMORAL LIGAMENT ANATOMY
The medial patellofemoral ligament (MPFL) functions to limit the lateral translation of the patella.19 The attachment sites on the femur and patella for the MPFL have been studied in children.20-23 Cadaveric dissections in specimens aged 2 to 11 years have noted the patellar attachment to be an average of 12 mm in length with the midpoint approximately 5 mm superior to the mid-pole of the patella.22 The patellar footprint of the MPFL insertion was a mean 41% of the entire patellar length.22
It is important to be aware of the characteristic anatomy of the MPFL, as fixation points should mimic the anatomic insertion as best as possible while also avoiding violation of the nearby physis. The MPFL originates between the adductor tubercle and the medial femoral epicondyle just distal to the distal femoral physis and attaches to the superomedial aspect of the patella.20-25 In relation to the physis in pediatric patients, the midpoint of MPFL insertion has been measured to be 4 mm to 9 mm distal to the femoral physis.21,24,25 These measurements represent averages as cadaveric studies have reported that some part of MPFL femoral insertion extends proximal to the distal femoral physis.21 A recent report of physeal injury to the posterior distal femoral physis during MPFL reconstruction leading to femoral flexion deformity highlights the importance of physeal-respecting surgery.26
TROCHLEA AND ANTERIOR DISTAL FEMORAL PHYSIS
The relationship between the proximal aspect of the trochlea and the anterior distal femoral physis has been recently studied in 175 knees with dysplastic trochlea.27 Based on magnetic resonance imaging evaluation, the lateral aspect of the trochlea extended proximal to the anterior distal femoral physis in 13% of patients and was at the level of the anterior physis in another 13% of patients (Figure 1).27 Hence, a cautious approach is recommended for any surgery to address trochlear dysplasia or trochlear bump in younger patients to prevent iatrogenic injury to anterior distal femoral physis and resultant genu recurvatum. The distance between the trochlea and the physis increased with increasing age.

LIMB ALIGNMENT
Physiologically, the quadriceps angle (Q angle) changes through the course of growth. As children begin standing and walking, they stand with their feet wider apart and in genu varum.28 Physiologic genu varum can reach 15°.28 This degree lessens during the first 1.5 to 2 years of life, transitioning to physiologic valgus of nearly 12° by 3 years of age.28 Genu valgum, thereafter, gradually decreases to reach the adult value of around 7° to 8° by age 7 years.28 Increased genu valgum is a risk factor for patellar instability. In skeletally immature patients, correction of genu valgum through guided growth may be desirable in patients undergoing patellar stabilization surgery (Figures 2A, 2B).29

PATHOPHYSIOLOGY OF PEDIATRIC PATELLAR DISLOCATION
TROCHLEAR DYSPLASIA
Trochlear dysplasia is an abnormal shape and depth of the TG.30 Up to 96% of patients with patellar dislocation have trochlear dysplasia.30-33 In a study of patellar instability in children, at least 1 of the 3 signs of trochlear dysplasia (the crossing sign, supratrochlear bump, and double contour sign) was present on lateral radiographs.34 In another study on the growth of trochlear dysplasia in children and adolescents, all grades of trochlear dysplasia were present at all ages (ie, the dysplasia was most likely present at birth and did not necessarily worsen with age and growth).35 The linear dimensions of lateral and medial condylar height as well as trochlear bump increased with age but both the sulcus angle and shape of the trochlea did not change significantly.35 Remodeling of a dysplastic trochlea can happen if the patella is stabilized and appropriately located at a younger age, preferably before 10 years of age.36,37
Continue to: PATELLAR HEIGHT
PATELLAR HEIGHT
The role of patellar height in patellar instability has been well established.38 In patients with patella alta, the patella remains proximal to the TG during the greater arc of knee motion, which predisposes it to patellar instability. Calculation of patellar height in children could be challenging due to incomplete ossification, as well as asymmetric ossification of the patella and the tibial tubercle (TT). Since the patella ossifies from proximal to distal, most radiographic methods that measure the patellar height from the distal aspect of the patella provide a spurious elevation of the measurement.
The Caton-Deschamps (CD) method measures the length of the patellar articular surface and the distance from the inferior edge of the articular surface to the anterosuperior corner of the tibial plateau.39 A ratio >1.3 signifies patella alta. The CD ratio has been verified as a simple and reliable index for measuring patellar height in children.40 Two other methods have been described for determining patellar height in children.41,42 Based on anteroposterior (AP) radiographs of the knee in full extension, Micheli and colleagues41 calculated the difference between the distance from the superior pole of the patella to the tibial plateau and the length of the patella. A positive difference signified patella alta. The Koshino method involves the ratio between a reference line from the midpoint of the patella to the midpoint of the proximal tibial physis and a second distance from the midpoint of the distal femoral physis to the midpoint of the proximal tibial physis on lateral knee radiographs.42 Normal values range from 0.99 to 1.20 with the knee in >30° flexion, in children 3 to 18 years of age.
HYPERLAXITY
In contrast to adults, children have increased levels of collagen III compared with collagen I, which is responsible for tissue elasticity.43 Tissue elasticity leads to increased joint mobility, which is more common in children. Joint hypermobility or hyperlaxity has to be differentiated from symptomatic instability. The traditional Beighton score identifies individuals as having joint hypermobility with a score of 5/9 or higher in school-aged children.44-46 Smits-Engelsman and colleagues44 suggested using stricter criteria with scores of 7/9 or higher being indicative of hyperlaxity in school-aged children. A study of 1845 Swedish school children noted that females have a higher degree of joint laxity.45 Maximal laxity was noted in females at 15 years of age.45 Hyperlaxity has been demonstrated to be greater on the left side of the body44 and can be part of generalized syndromes including Down’s syndrome, Marfan’s syndrome, or Ehlers-Danlos syndrome.
LIMB TORSION
Staheli and colleagues47 described the normative values of a lower extremity rotational profile, including femoral anteversion and tibial torsion. Children normally have increased femoral anteversion, which decreases with growth. Miserable malalignment is a term used to denote increased femoral anteversion and increased external tibial torsion.48,49 These rotational abnormalities can increase the Q angle and the lateral forces on the patella. Femoral anteversion or internal rotation of the femur of 30° significantly increases strain in all areas of the MPFL.48 This increased strain may lead to MPFL failure and patellar instability.48 Increased internal rotation of the femur also increases contact pressure on the lateral aspect of the patellofemoral joint.48 Miserable malalignment frequently manifests following a pubertal growth spurt and may require femoral and tibial osteotomy.50
SYNDROMIC ASSOCIATIONS
Several syndromes have patellar instability as a part of their manifestation. The more common syndromes include nail-patella syndrome, Kabuki syndrome, Down’s syndrome, and Rubinstein-Taybi syndrome.51-54 Other syndromes less commonly associated with patellar instability include Turner syndrome, patella aplasia, or absent patella syndrome. Since many patients with syndromic patellar instability are functionally limited, they may not require an aggressive approach to treatment. When treating these patients, it is important to recognize the unique features of a specific syndrome, which may affect the anesthesia risk profile, management decisions, rehabilitation, and prognosis.
Continue to: MPFL TEAR PATTERN
MPFL TEAR PATTERN
The MPFL serves as an important constraint to the patella to prevent lateral dislocation, primarily during the first 20° to 30° of knee flexion.55,56 Injury to the MPFL is noted in over 90% of patients who suffer a patellar dislocation.57 The location of MPFL tears in pediatric patients is variably reported at the patellar attachment (10%-61%), femoral attachment (12%-73%), both (12%-35%) or mid-substance (2.5%-15%).25,57 The most common tear patterns in pediatric patients are tears at the patellar attachment.25,57 This tear pattern may be accompanied by an avulsion fracture of the medial rim of the patella, though this fracture, being extra-articular, seldom needs treatment.
CLASSIFICATION
While several authors have established extensive classification systems of patellar dislocation based on both clinical and radiographic presentation and reviews of the literature, a single classification system has not been recognized as the gold standard. In this section, in addition to presenting our preferred methods of classification, we will review some of the more recent and extensive classification systems for patellar dislocation and patellar instability.
Dejour and colleagues31 initially used both the presence of patellofemoral anatomic abnormalities and pain to define 3 types of patellar instability: major, objective, and potential patellar instability. Major patellar instability indicates that the patient has experienced more than 1 documented dislocation, objective instability involves one dislocation in addition to an associated anatomic abnormality, and potential patellar instability refers to cases in which the patient has radiographic abnormalities and patellar pain.31 Garin and colleagues58 more simplistically divided patellar dislocation patients into 2 groups: major (permanent or habitual) dislocation of the patella and recurrent dislocation. Sillanpaa59 stressed the distinction between first-time dislocation and recurrent dislocation specifically in the context of acute injuries. These classification systems were formulated with adults as the most relevant population; however, classifications targeted specifically to pediatric patients have recently been presented in the literature.
Historically, pediatric patella dislocations were simply categorized as traumatic or congenital.60 In 2014, Chotel and colleagues61 focused on classifying patellar dislocation by extensively reviewing anatomic, biomechanical, pathophysiological, and clinical patterns seen most commonly in children. They included 5 categories: congenital dislocation, permanent dislocation, habitual dislocation during knee flexion, habitual dislocation during knee extension, and recurrent dislocation; however, they did not address traumatic dislocations.61 Congenital dislocation is a rare condition, typically presenting at birth, which produces a pattern of functional genu valgum.62 Permanent dislocation typically presents after the child has started walking, but before the age of 5 years.61 The 2 variations of habitual dislocation typically present between ages 5 and 8 years.61 The final category is the most common and typically occurs during pre-adolescence or adolescence as a result of an atraumatic or trivial traumatic event or sports injury.1 Using more specific terminology, Hiemstra and colleagues63 modeled a classification system based on the traumatic, unilateral, bankart lesion, surgery (TUBS)/atraumatic, multidirectional, bilateral, rehabilitation, inferior shift (AMBRI) for shoulder dislocation classifications. The patellar dislocation system is used to identify 2 distinct subsets of patients in the patellofemoral instability population. One subset is defined by the acronym WARPS (weak, atraumatic, risky anatomy, pain, and subluxation), the other is STAID (strong, traumatic, anatomy normal, instability, and dislocation).64 Patients categorized by the WARPS acronym tend to experience atraumatic onsets of patellofemoral instability and demonstrate anatomic issues that increase this instability. These underlying anatomic issues include valgus alignment, ligamentous laxity, rotational abnormalities, shallow and short TG, and patella alta. On the other hand, STAID patients describe a traumatic dislocation event and do not have underlying anatomic abnormalities that predispose them to instability.64
Taking into account these previous classifications, Frosch and colleagues65 added specific pathologies including “instability,” “maltracking,” and “loss of patellar tracking,” in addition to both clinical and radiographic factors to define 5 types of patellar dislocation and their specific treatment recommendations.65 Type 1 involves simple dislocation with neither maltracking nor instability and a low risk of redislocation.65 Type 2 is defined as primary dislocation followed by subsequent high risk of dislocation and no maltracking.65 Type 3 is divided into 5 subcategories of instability and maltracking issues involving soft tissue contracture, patella alta, pathological tibial tuberosity, and TG distance.65 Type 4 is defined as the highly unstable “floating patella,” and type 5 involves patellar maltracking without instability 65. In terms of treatment, conservative rehabilitation is recommended for type 1 whereas MPFL reconstruction tends to show positive outcomes for both types 2 and 3.66-70
Continue to: Parikh and Lykissas recently published...
Parikh and Lykissas recently published a comprehensive classification system of 4 defined types of patellar dislocation in addition to voluntary patellar instability and syndromic patellar instability (Table).60 The 4 types are Type 1, first-time patellar dislocation; Type 2, recurrent patellar instability; Type 3, dislocatable; and Type 4, dislocated. Type 2 is further subdivided into Type 2A, which presents with positive apprehension signs, and Type 2B, which involves instabilities related to anatomic abnormalities.60 A distinction is also made between Type 3A or passive patellar dislocation and Type 3B habitual patellar dislocation.60

The classification system proposed by Green and colleagues is more simplified with 3 main categories (Table) of pediatric patellar dislocation: traumatic (acute or recurrent), obligatory (either in flexion or extension), and fixed laterally.71,72 The acute traumatic categorization refers to patients who experienced an initial dislocation event due to trauma whereas recurrent traumatic involves repeated patella dislocations following an initial incident. Studies report that between 60% to 70% of these acute traumatic dislocations occur as a result of a sports-related incident.2,33,73 Obligatory dislocations occur with every episode of either knee flexion or extension, depending on the subtype. Obligatory patella dislocation in flexion typically cannot be manipulated or relocated into the trochlea while the knee is fixed but does reduce into the trochlea in full extension. Fixed lateral dislocations are rare, irreducible dislocations in which the patella stays dislocated laterally in flexion and extension. These dislocations often present with other congenital abnormalities. Each of these categories can be further specified as syndromic if the dislocation is associated with genetic or congenital conditions including skeletal dysplasia, Ehlers-Danlos syndrome, cerebral palsy, Marfan disease, nail-patella syndrome, Down syndrome, Rubenstein-Taybi syndrome, and Kabuki syndrome.51-54,61,74-76
SURGICAL TECHNIQUES IN SKELETALLY IMMATURE PATIENTS
While nonsurgical, conservative treatment involving physical therapy and activity modification is recommended for most patients who experience first-time traumatic patellar dislocations, many patients experience complicating factors that indicate them for surgery. These factors include recurrent dislocation, risk factors for patellofemoral instability, underlying malalignment issues, and congenital deformities. When evaluating these factors, particularly patellofemoral instability, the authors recommend assessing osteochondral lesions, age, skeletal maturity, number of previous dislocations, family history, and anatomic risk factors.2,5,77-79 Extra care should be taken when considering surgical treatment for skeletally immature patients at elevated risk for recurrent instability as the risk of cartilage damage in these cases is high.80-82
Recently, there has been a reported increase in surgical treatment for patellar instability in the skeletally immature.83 This finding may be attributed to heightened awareness of factors that indicate patients for surgical treatment and increased familiarity of surgeons with newer techniques.83 Many surgical techniques have been described to address patellar instability involving both soft-tissue procedures and bony corrections.84 In this article, we discuss the various surgical techniques for MPFL reconstruction, quadricepsplasty, and distal realignment. These procedures can be paired with any number of additional procedures including, but not limited to, lateral retinacular release or lengthening, chondroplasty, TT osteotomy (in skeletally mature patients), and removal of loose bodies.83
There is a need for more comprehensive studies, particularly randomized controlled trials, to evaluate the outcomes for both surgical and nonsurgical treatments for first-time dislocations. In the current literature, only very recently have surgical treatments shown outcomes that are more positive. In 2009, Nietosvaara and colleagues85 conducted a randomized controlled trial of nonoperative and operative treatment of primary acute patellar dislocation in both children and adolescents. After a long-term mean follow-up of 14 years, there was not a significant difference between the groups in recurrent dislocation and instability, subjective outcome, or activity scores.85 In a subsequent review of 5 studies including 339 knees, Hing and colleagues86 also found similar results in both the operative and nonoperative cohorts at risk of recurrent dislocations, Kujala scores, and reoperations. However, a recent systematic review comparing redislocation rates and clinical outcomes between surgical and conservative management of acute patellar dislocation reported more positive outcomes for the surgical cohort.87 This review included 627 knees, 470 of which received conservative management, 157 of which received operative treatment. The conservative cohort was followed for an average of 3.9 years and had a 31% rate of recurrent dislocation while the surgical group was followed for a mean 4.7 years and experienced a 22% redislocation rate.87 This study indicates that operative management for acute first-time dislocations may be the preferred treatment option.
Continue to: A potential reason some of these studies...
A potential reason some of these studies did not show any significant difference between the operative and nonoperative cohort could be that the surgical cohorts included a wide range of procedures including lateral releases and MPFL repairs. Recent publications have indicated that these techniques do not produce overall positive outcomes. While each surgical treatment plan is unique depending on the patient; recently, MPFL reconstruction has been shown to have better outcomes than both nonoperative management and simple medial repair and/or lateral
release.67,88-90
MPFL RECONSTRUCTION
INDICATIONS/OVERVIEW
The MPFL is an important stabilizer for the knee that primarily resists lateral translation of the patella. Damage to the MPFL is very common in acute patellar dislocations with up to 90% of first-time dislocations resulting in injury to the MPFL.91,92 Historically, simple medial and/or lateral MPFL repairs have not been shown to improve patellofemoral kinematics significantly and often result in recurrence.90,93 To address this issue, during the past few decades, numerous MPFL reconstruction techniques have been developed to reconstruct a stronger ligament with the same kinematics as the anatomic MPFL.2,19,69,81,94-106 The ultimate goal of MPFL reconstruction is to reestablish the anatomic “checkrein” to guide the patella into the trochlea between 0° and 30° of knee flexion.107,108 An essential secondary surgical goal in skeletally immature patients is to avoid damaging the distal femoral physis.
There are many variations in both the grafts used to replace the MPFL and the means by which to secure them. The ones discussed below include free semitendinosus or gracilis autografts or grafts constructed from a pedicled adductor, patellar, or quadriceps tendon.69,105,109 While not used as frequently, allografts have also been used.110 Methods to secure these grafts in osseous tunnels include suture anchors or tenodesis screws. Incomplete osseous sockets or medial-sided bone tunnels have also been used as a method to decrease patellar fractures as they preserve the lateral patellar cortex.111-114
DOUBLE-BUNDLE HAMSTRING AUTOGRAFT
The technique most often used by the author is a double-bundle hamstring autograft harvested from either the semitendinosus or the gracilis secured by short patellar and femoral sockets (Figure 3). After harvesting the hamstring graft from a posteromedial incision, an approximately 90-mm graft is prepared with Krackow stitches to secure 15 mm of the tendon in each socket.115 Lateral radiographs are used intraoperatively to ensure the guidewire for the femoral drill hole falls along the posterior cortex of the diaphysis of the femur while AP radiographs confirm placement distal to the physis. It is important to take both AP and lateral radiographs intraoperatively due to the concave curvature of the distal femoral physis. This unique anatomy can make a point that is located distally to the physis on the AP view appear on or proximal to it on the lateral cross reference view.24,116 For the patellar socket, 2 short sockets are made in the superior half of the patella. Once the sockets have been drilled, the graft is adjusted so that the patella stays seated in the center of the trochlea between 20° and 30° of flexion. This anchoring is accomplished by securing the graft while the knee is kept at 30° of flexion. Proper tension is confirmed by ensuring that the graft does not allow lateral patella movement over one-fourth the width of the patella in extension while crepitation must not appear throughout the ROM.92

QUADRICPETS TENDON TRANSFER
A combination of techniques by Steensen and colleagues,105 Goyal,109 Noyes and Albright,117 and Pinkowsky and Hennrikus118 describe an MPFL reconstruction in which the proximal end of a small medial portion of the quadriceps tendon is released and then attached to the medial epicondyle through a subcutaneous tunnel (Figure 4). This technique is particularly useful for cases in which the extra strength provided by the bone-quadriceps tendon is necessary to correct more severe dysplasia. Leaving the distal end of the quadriceps tendon intact at its patellar insertion, a graft of about 8 mm x 70 mm thickness is harvested from the tendon. The free distal end of the tendon is then run anatomically through the synovium and retinaculum to be either sutured to the medial intermuscular septum at the medial femoral epicondyle or fixed in femoral tunnel using interference screw.105,109,118 The placement of the femoral fixation point is essential to ensure positive surgical outcomes. If the graft is secured too anteriorly, it may be too loose in extension and too tight in flexion, both of which can lead to postoperative pain, loss of normal kinematics, and overload of the medial patellofemoral cartilage.119-121 Once the ideal placement of the femoral fixation point has been confirmed by intraoperative radiographs, the graft is secured with a small absorbable suture.122,123 While this technique has good clinical results, the longitudinal scar that results from graft harvesting is cosmetically unappealing, and it is technically challenging to harvest a consistent strip of the quadriceps tendon. To address some of these concerns, Fink and colleagues124 described a new harvesting technique that produces more consistent grafts and requires a smaller incision.

Continue to: ADDUCTOR MAGNUS TENDON TRANSFER
ADDUCTOR MAGNUS TENDON TRANSFER
This technique is a double-bundle MPFL reconstruction that uses a pedicled graft of the distal adductor magnus tendon and suture anchors or incomplete osseous sockets to recreate the MPFL anatomically (Figure 5). Avikainen and colleagues96 and Sillanpää and colleagues125 described this procedure as a progression from the original single-strand adductor magnus transfer technique. First, maintaining the distal insertion, a graft of approximately 14 cm to 18 cm is harvested from the adductor tendon and then passed through a subcutaneous tunnel between the distal vastus medialis obliquus and the superficial joint capsule. The graft is then looped at the medial patella so that the distal bundle runs back to the adductor tubercle.125 With the knee at 30° of flexion to assure proper tension, the graft is secured at both the patella and near the adductor tubercle with suture anchors.125 Hambridge and colleagues126 compared a similar adductor magnus transfer with other pedicled techniques including bone-quadriceps tendon autograft and bone-patellar tendon allograft and found positive results for all 3 methods of reconstruction.

HEMI-PATELLA TENDON TRANSFER
In a similar technique to the adductor tendon transfer, the medial section of the patellar tendon is harvested from the TT and run from its proximal insertion at the medial patella to the medial femoral attachment via a subcutaneous tunnel. The free end of the graft is then secured with suture anchors or incomplete osseous sockets with the knee at 30° of flexion.127
HAMSTRING GRAFT WITH ADDUCTOR TENDON AS A PULLEY
Several techniques opt to use a more dynamic model of MPFL reconstruction in which the adductor tendon or medial collateral ligament (MCL) is used as a pulley for the hamstring graft (Figure 6).128,129 The site of the pulley approximates the normal attachment of the MPFL to the femur and so acts as an effective anatomic replica of the MPFL origin. A semitendinosus graft is harvested and is prepared with continuous sutures, and 2 tunnels to secure the graft are drilled into the patella. The graft is then run subcutaneously from the medial side of the patella to the adductor magnus tubercle into which an osteoperiosteal tunnel is drilled at its distal femoral insertion. The graft is looped through the adductor tunnel and secured with sutures. Proper knee kinematics was ensured by placing the knee at 30° of flexion as the ends of the tendon are secured to the patella.114,130

HAMSTRING GRAFT WITH MCL AS A PULLEY
The MCL can also be used as a pulley rather than the adductor tendon. The semitendinosus graft is harvested and prepared and the patella drilled as it is in the previous technique. The MCL was fashioned into a pulley by making a slit in its posterior one-third. The semitendinosus graft is looped through this slit, and both ends of the graft are held in place with suture anchors on the surface of the patella.129
ADDITIONAL PROCEDURAL COMBINATIONS
Depending on the needs of the individual patient, MPFL reconstruction, and other patellar stabilization techniques can also be combined with additional procedures. Arshi and colleagues83 conducted a review of 6190 adolescents surgically treated for patellar instability and reported the most common additional procedures performed at the time of the stabilization. They found 43.7% of the population underwent lateral retinacular release, which while not effective as an isolated technique to treat patellar instability, has often been used in combination with MPFL reconstruction.131-133 There is currently a lack of consensus regarding the success of adding a lateral release to the reconstruction. Some studies report no difference while others report a decrease in stability after lateral release.90,134-136 While lateral retinacular release has been shown to decrease the force required to displace the patella, it can be surgically indicated in certain patients undergoing MPFL reconstruction.131 The authors advocate that if the lateral retinaculum is tight such that centralized patellar tracking is inhibited following the reconstruction, or if the patella cannot be pushed passively from a laterally tilted position to the neutral horizontal position, lateral retinacular lengthening should be performed to improve kinematics.132
Continue to: Arshi and colleagues...
Arshi and colleagues83 also reported a high rate of cartilage procedures, with chondroplasty performed in 31.1% and chondral fragment/loose body removal in 10.2%. These statistics suggest that a significant level of cartilage damage has occurred by the time of surgery.83
COMPLICATIONS
As MPFL reconstruction techniques have only recently been popularized and developed, there are not many comprehensive studies evaluating the outcomes and complications associated with these procedures. However, in the current literature, there is a general consensus that patients usually experience positive short-term clinical outcomes and relatively low complication rates.68,77 In one of the largest retrospective cohort studies of pediatric patients undergoing MPFL reconstruction, Parikh and colleagues114 reported both the type and rate of complications. They found complications occurred in 16.2% of patients, and the most common complications were recurrent patellar instability, patellar fractures, patellofemoral arthrosis, motion deficits, and stiffness with over half classified as avoidable. Most of these complications were due to technical errors with episodes of recurrent instability only reported in 4.5% of patients.114 In a comprehensive meta-analysis of MPFL reconstruction studies, Shah and colleagues137 reported a complication rate of 26% in both pediatric and adult patients. The cohort was not stratified by age, yet complications were similar to those reported by Parikh and colleagues,114 including pain, loss of knee flexion, wound complications, and patellar fracture.137
As indicated by the frequency of technical complications reported by Parikh and colleagues,114 extra caution should be taken in the operating room to minimize potential errors. In techniques that require drilling of femoral sockets, proper length for and placement of the graft is essential to reestablish proper kinematics. Studies have reported that placing the femoral socket too proximally can result in loss of ROM during flexion and increased compressive forces across the patella.138 A graft that is too short can have similar negative outcomes, and a graft that is too long can result in recurrent instability. Positioning the graft while the knee is in 30° of flexion can help ensure the proper length and tension is achieved. Once the graft is in place, it is important to ensure the ROM and isometry before completing the fixation.72 It is also essential to be vigilant about potential violation of the physes and subsequent growth disturbances. To establish the safest angles for drilling the distal femoral epiphysis for graft placement, Nguyen and colleagues139 conducted a study using high-resolution 3-dimensional images of cadaveric distal femoral epiphyses. By recording which tunnels disrupted the physis before reaching 20 mm of depth, the authors concluded that it is safest to drill distally and anteriorly at an angle between 15° and 20°.139 This technique should minimize damage to the physis, notch, and distal femoral cartilage and decrease potential complications.139
OUTCOMES
In general, the literature reports positive outcomes for MPFL reconstruction—in both studies that address a specific technique and all-encompassing studies. Outcomes are typically reported as Kujala and Tegner scores, results from clinical examinations, and rates of subsequence recurrences. Several recent studies have also evaluated the ability of MPFL reconstruction to restore proper kinematics. Edmonds and colleagues140 evaluated the difference in patellofemoral joint reaction forces and load experienced by 3 groups of adolescents: a cohort treated with MPFL reconstruction, a cohort treated with soft-tissue realignment of the extensor mechanism (the Insall method), and controls. While both surgical techniques were able to restore medial constraints to the patella, the study showed that only the MPFL reconstruction cohort experienced joint reaction forces that were analogous to the control group. In comparison, the cohort that was treated with soft-tissue realignment alone experienced higher patellofemoral joint reaction forces and did not regain normal joint mechanics.140 These results can be used to advocate for the further use of MPFL reconstruction as an effective anatomic replacement of the native ligament. Radiographic studies have similarly reported MPFL reconstruction as an effective means to restore anatomic normality. Fabricant and colleagues141 conducted a radiographic study in which patella alta was corrected to normal childhood ranges in patients who underwent MPFL reconstruction technique using a hamstring autograft. Lykissas and colleagues142 corroborated these results with another radiographic study that reported small but significant decreases in the Blackburne-Peel index and CD index following MPFL reconstruction in 25 adolescents. As correction of patella alta allows the patella to rest in a deeper, more secure position in the TG, these results indicate that effective early MPFL reconstruction can correct for patellar anatomic abnormalities that could be future risk factors.143,144 Several studies have also reported outcomes addressing specific MPFL techniques; these are reported and discussed in this article.
OUTCOMES BY TECHNIQUE
HAMSTRING AUTOGRAFT
Reports on outcomes following MPFL reconstructions using hamstring autografts have been particularly promising. A cohort of 21 skeletally immature patients who underwent MPFL reconstruction was evaluated pre- and postoperatively with an average of a 2.8-year follow-up. The authors of the study reported no redislocation events and significant improvement in the Kujala scores, and patients were able to return to athletic activities safely.145 Previous studies report similar positive increases in Kujala scores, subjective patient reports, and lack of subsequent redislocation for patients who underwent either semitendinosus or gracilis autograft MPFL reconstructions. One such study further documented an average patellar inclination angle decrease from 34.3° to 18.6° following MPFL reconstruction.146 However, while the literature typically reports positive Kujala scores and subjective outcomes for the hamstring autograft procedure, a study arthroscopically evaluating patellar tracking immediately following surgery and then at 6 to 26 months follow-up found that patellar tracking correction was not maintained for all patients who underwent this type of MPFL reconstruction.147
Continue to: QUADRICEPS TENDON TRANSFER OUTCOMES
QUADRICEPS TENDON TRANSFER OUTCOMES
Studies specifically evaluating the quadriceps tendon transfer technique for MPFL reconstruction in children are sparse, but authors have reported positive clinical outcomes and low complication rates in adults. After following 32 young adults who underwent this MPFL reconstruction technique for 3 years, Goyal109 reported a significant increase in mean Kujala scores from 49.31 to 91.25 and no complications or redislocation. He argues this type of quadriceps graft has a high success rate because it is anatomically more similar to the MPFL than other grafts and does not require additional patellar fixation.101,109 Similar positive Kujala scores and minimal complications have been reported in adult patient populations.148 Abouelsoud and colleagues149 conducted one of the few studies in skeletally immature patients and reported similarly positive results with no redislocations and significantly improved Kujala scores at a mean follow-up of 29.25 months in their 16-patient cohorts.
ADDUCTOR MAGNUS TENDON TRANSFER
After initially describing this technique in 14 adult patients, Avikainen and colleagues96 followed this cohort and reported positive subjective results and only 1 redislocation. In a more recent study in which the adductor tendon transfer technique was compared with the quadriceps tendon transfer described above and the bone-patellar tendon allograft, Steiner and colleagues69 reported similarly significant improvement in all cohorts in Lysholm, Kujala, and Tegner scores with no redislocations. Additionally, Malecki and colleagues150 followed a cohort of 33 children with 39 knees diagnosed with recurrent patellar dislocation, who underwent MPFL reconstruction using the adductor magnus tendon. After evaluating this cohort functionally and radiographically, the authors reported improvements in Lysholm and Kujala scores, patellar tilt and congruence angles, and peak torque of the quadriceps muscle and flexor.150 However, this cohort did report postoperative redislocations in 36.4% of patients (4 of 11).150
HEMI-PATELLA TENDON TRANSFER
In 2012, in the first randomized controlled trial, Bitar and colleagues67 compared the outcomes of patients who underwent MPFL reconstruction via the hemi-patellar tendon technique with those who were managed nonoperatively with immobilization and physiotherapy after first-time patellar dislocation. At 2-year follow-up, the surgical cohort presented positive results with a significantly higher mean Kujala score (88.9 to 70.8) and no redislocations or subluxations. In contrast, 35% of nonoperative cases presented with recurrences and subluxations over the 2-year period.67
MCL OR ADDUCTOR TENDON AS A PULLEY
Studies have reported good postoperative results and low complication rates for these dynamic techniques.128,129 In terms of kinematics, while hypermobility and patellar height were not fully corrected, improvements in patellar tilt and lateral shift were reported in a cohort of 6 patients with a minimum 4-year follow-up.129 To further evaluate whether the more dynamic pulley reconstruction technique resulted in better outcomes, Gomes and colleagues128 compared the subjective reports, clinical evaluations, and complication rates of patients who underwent MPFL reconstruction with a rigid adductor magnus fixation vs a semitendinosus tendon dynamic femoral fixation. One case in the rigid cohort experienced a subsequent subluxation, while patients in the semitendinosus group had better subjective reports and a higher rate of return to sport.128 More recently, Kumahashi and colleagues151 specifically studied the outcomes of the MCL tendon as a pulley in 5 patients aged 14 to 15 years. They reported similar successful results as no patients experienced recurrence, and all patients exhibited improvement in radiographic measures of patellar tilt and congruence angle, lateral shift ratio, and both Kujala and Lysholm scores.151
While there has yet to be a randomized controlled trial comparing all of these different techniques, there is a general consensus in the literature that patients tend to perform better following MPFL reconstruction vs MPFL repair.
OTHER STABILIZATION PROCEDURES, INCLUDING DISTAL REALIGNMENT
Patients with additional underlying deficits and malalignment issues such as significant trochlear dysplasia, increased TT-TG distance, patella alta, increased Q angle, and/or positive J sign may require stabilization procedures beyond MPFL reconstruction.152,153 TT osteotomies are often used to correct alignment issues in the adult patient population; however, these procedures are typically contraindicated in skeletally immature patients. Alternative realignment procedures for the pediatric population include both proximal and distal realignment, with proximal realignment performed primarily in children under the age of 12 years.153 Many variations on these procedures exist, some of which are no longer regularly performed due to poor reported outcomes. In this article, we discuss several of the techniques, focusing primarily on those that have demonstrated higher success rates.
Continue to: GALEAZZI TECHNIQUE
GALEAZZI TECHNIQUE
One of the first and most famous soft-tissue techniques to address patellar instability was the semitendinosus tenodesis, published by Galeazzi154 in 1922 (Figure 7). This technique stabilizes the patella without altering the TT. In the original technique, a portion of the semitendinosus tendon is harvested with its tibial insertion left intact. The free end of the tendon is then secured with sutures at the periosteal groove of the medial patella.154,155 Fiume156 modified this technique by adding a lateral release and medial retinacular reefing. The most recent addition to this procedure was introduced by Baker and colleagues,157 in which a tunnel is drilled from the medial to the lateral border of the patella. Tension placed on the grafted tendon is used to reposition the patella medially and draw it downward. Preliminary literature on this modified procedure reported fair clinical results with success rates of approximately 75%.155,158-160 A recent study evaluating both the clinical and radiographic outcomes of this technique also indicated that while clinical results were excellent in 62.5% of patients, this technique alone was unsuccessful in fully addressing patellar instability in patients with underlying anatomic abnormalities such as patellar alta.161 In light of these less than ideal reports, the authors no longer recommend this technique for patellofemoral instability cases.

ROUX-GOLDTHWAIT PROCEDURE
The Roux-Goldthwait procedure, first described by both Roux162 and Goldthwait163 in 1888 and 1895 respectively, was later modified in 1985 to involve a lateral release, plication of the medial retinaculum, medial transfer of the lateral patellar tendon without advancement, and advancement of the vastus medialis (Figure 8).164 More recently, Marsh and colleagues152 introduced an addition to aligning the extensor mechanism with the femoral shaft better. In this technique modification, the patellar tendon is split longitudinally, and its lateral half is detached and transferred distally beneath its medial half. The free end is then sutured to the periosteum on the medial side of the tibia.152 With a mean long-term follow-up of 6.2 years, Marsh and colleagues152 reported excellent results in 65%, good in 11%, and fair in 3% of the knees operated on with this modified technique. Of the patients in this cohort whose strength was evaluated, 80% had their strength returned to 90% of preoperative levels in the operated leg.152 While this study and others report improved outcomes, an increasing body of literature has found high rates of recurrence, patella infera, and other complications following the modified Roux-Goldthwait procedure.36,165-171 Also, a study comparing MPFL reconstruction using adductus magnus transfer with the Roux-Goldthwait procedure reported that patients in the MPFL cohort reported less pain postoperatively.150 In addition, whereas the Kujala and Lysholm scores, recurrence rates, patellofemoral angles, and apprehension test results did not demonstrate significant differences between these 2 groups, the MPFL group had significantly fewer abnormal congruence angles, better patellar medialization, and higher peak torque of the hamstring.150

COMBINED MPFL AND MEDIAL PATELLOTIBIAL LIGAMENT RECONSTRUCTION
While the medial patellotibial ligament (MPTL) has not received much attention with regard to patellar stability, recent studies have indicated its role during higher degrees of both flexion and extension.172 The MPTL acts as a secondary restrictor ligament which helps release stress on the MPFL by decreasing the Q angle and further normalizing patellar kinematics.173 Patients who present with hyperlaxity or knee hyperextension combined with extension subluxation and flexion instability could be indicated for this additional stabilizing procedure. Both Nietosvaara and colleagues85 and Brown and Ahmad174 have described a dual MPTL and MPFL reconstruction technique using a semitendinosus hamstring graft. More recently Hinckel and colleagues172 described a combined MPFL and MPTL reconstruction, using a graft from the quadriceps tendon to reconstruct the MPFL and one from the patellar tendon to reconstruct the MPTL. In this technique, once the respective grafts have been harvested, a femoral insertion for the graft recreating the MPFL is fluoroscopically established so that an anchor can be inserted distal to the femoral physeal growth plate. For the MPTL insertion, attachment to the tibia below the joint line and 2 cm medial to the patellar tendon is established fluoroscopically just above the physeal growth plate on the proximal epiphysis.19,175 The MPTL graft is sutured first with the knee at 90° of flexion to establish tension similar to that of the patellar tendon.176 Then, the knee is placed in 30° of flexion to fix the MPFL graft to the medial patella to prevent excessive lateral translation of the patella.
PATELLAR TENDON TRANSFER
Patellar tendon transfer with proximal realignment is a technique used in particularly young patients to address cases of patellofemoral instability involving concomitant bony or anatomic abnormalities. This procedure is effective for young children with substantial amounts of remaining growth as it better mimics native anatomy than other realignment procedures and does not require bony remodeling.152-154 It is important to familiarize with surgical techniques to address malalignment issues in young patients as neglected alignment issues can lead to worsening of trochlear dysplasia and instability, which are very difficult to treat later on when patients are older.153
The patellar tendon transfer technique (Figure 9), as described by Gordon and Schoenecker,177 starts with an extensive lateral retinacular release. The patellar tendon is then released from its distal insertion at the TT so that it can be moved medially without moving it inferiorly. After confirming patellar tracking and alignment by flexing the knee from 0° to 90° with the graft in place, the patellar tendon graft is secured with multiple nonabsorbable horizontal sutures.177 Of note, in skeletally mature patients, a TT osteotomy is used to accomplish the same goal. This osteotomy has been shown to improve both patellar height and TT-TG distance in skeletally mature patients, but is contraindicated in skeletally immature patients.92,178

Continue to: Initial studies conducted on patellar tendon...
Initial studies conducted on patellar tendon transfer have positive outcomes.179 At a mean follow-up of 5.1 years, patients reported a decrease in pain and increased the ROM and activity, and only 1 reported a postoperative redislocation.179 In more recent studies, both Benoit and colleagues36 and Garin and colleagues58 reviewed cases of patellar instability treated with patellar tendon transfer to address concomitant patellar alignment and anatomic abnormalities. They reported good functional, clinical, and radiographic outcomes with 12.5% and 16% recurrence rates, respectively.36,58 They also noted radiographic improvements in femoral sulcus angle, particularly in younger patients, which indicate this procedure is effective in addressing bony abnormalities that can result from neglected malalignment issues.36,58,154
QUADRICEPSPLASTY
Quadricepsplasty is a lengthening and remodeling technique not frequently used in the pediatric population. The goal of this procedure in patients with significant amounts of growth remaining is to reposition the patella to ameliorate trochlear remodeling and prevent worsening symptoms and anatomic abnormalities.36 A quadricepsplasty accomplishes this by de-rotating and/or lengthening the extensor mechanism and may or may not involve a concomitant MPFL reconstruction. This procedure is particularly effective in young patients who experience obligatory dislocation.60,72 Several quadricepsplasty techniques have been described including Thompson, Curtis and Fisher, Judet, Stanisavljevic, and V-Y technique.180-186 Most techniques initially involve sharp dissection of the vastus medialis and lateralis from the rectus femoral tendon. A tongue is then fashioned out of the rectus femoral tendon. Once the vastus medialis and lateralis are detached from the margins of the patella, the knee is extended, and the distal ends of the vasti are sutured to the tongue of the rectus tendon. Effective extension facilitates flexion to 90°.184 The authors recommend a modification of this technique in which a Z lengthening of the quadriceps tendon is performed after the vastus lateralis is removed distally from the patella and the quadriceps tendon.
Several series and case reports evaluating quadricepsplasty in adult patients report positive outcomes with most patients achieving good or excellent flexion with minimal complications.183,185,187-189 Reports on quadricepsplasty used to treat conditions other than patellofemoral instability in children have reported similar positive outcomes.190-192 As quadricepsplasty for patellar instability is relatively rare in pediatric patients, there is not much relevant literature. However, Kocon and colleagues193 reported results of quadricepsplasty and quadricepsplasty combined with the modified Galeazzi procedure in 8 children (10 knees) with a mean follow-up of 3.25 years. Seventy percent of cases resulted in stabilization and correction of patellar position, and only 2 postoperative redislocations were noted.193 Additionally, in a study evaluating 6 patients suffering from patellar instability, 2 of whom were obligate dislocators, quadricepsplasty resulted in patellar stability, satisfaction, and near normal gait patterns.194
Figure 10 shows the surgical algorithm used for patellar instability characteristics.

CONCLUSION
Patellofemoral joint stability relies on a complex interplay of musculotendinous units, ligaments and the osteocartilaginous morphology of the patellofemoral joint. Patellar instability in pediatric patients is different from adults. Having an in-depth understanding of the remodeling potential, the insertion sites for the MPFL and its relationship to the physis are of utmost importance when planning surgery. Reducing and maintaining the patella within the patellofemoral joint early enough can allow for remodeling of the patella and/or the trochlea to provide for lasting stability. Appropriate surgical principles, such as tensioning, can help both prevent continued pain and minimize future complications.
ABSTRACT
Patellar instability in children and adolescents is a challenging subset to treat. Varied forms of instability, ranging from episodic dislocation to fixed dislocation, have been recognized. It is of utmost importance for the treating physician to recognize these different patterns of instability and their associated risk factors, as more complex patterns of instability would require more extensive surgical procedures. Medial patellofemoral ligament (MPFL) reconstruction, by itself, may not suffice or may not be appropriate for the more complex instability patterns. Appropriate and early treatment of such instability in children would allow for functional progression and possible remodeling of the trochlea. However, early treatment has the associated risk of growth disturbances when surgical procedures are performed around open physis or if adult-type bony procedures are performed in children. Recent knowledge about the relationship between trochlea, MPFL femoral attachment, and distal femoral physis could help to advance safe surgical care for these patients. This article reviews the pathophysiology, risk factors, and the existing classification systems for patellar instability in children and adolescents. It focuses on varied surgical techniques, which are unique to the pediatric population, and summarizes the outcomes of these surgical techniques.
Continue to: EPIDEMIOLOGY
EPIDEMIOLOGY
In a prospective 2-year study of Finnish children, the annual incidence rate of patellar instability was 43/100,000 pediatric population.1 In patients 9 to 15 years of age, the incidence was approximately 1/1000.1 In another study, patients at highest risk for a first-time patellar dislocation were females aged 10 to 17 years.2 In a study in patients with traumatic hemarthrosis, 36% in the younger age group (10-14 years) and 28% in the older age group (15-18 years) had sustained patellar dislocation. In contrast, 22% in the younger age group and 40% in the older age group had sustained an anterior cruciate ligament tear.3
Approximately one-half of patients who dislocate their patella suffer from long-term complications.4,5 These complications include recurrent instability, patellofemoral pain, osteochondral lesions, and eventual arthritis.1,4,5 Young, active individuals are more prone to these issues.6 Also, 39% or more of patellar dislocation patients have an associated osteochondral fracture that might influence the management.1 Thus, patellar instability in young patients is an area of concern.
DEVELOPMENTAL ANATOMY
At 4-week gestation, the patellofemoral joint is an ectodermal sac filled with mesenchyme of the somatic mesoderm.7 Mesenchymal condensations then appear at 4 to 5 weeks gestation, followed by chondrification of both the femur and patella.7 The joint space is present by 6 weeks, and the patellar and distal femoral condyles are present at 7 weeks gestation.7 By 8 weeks gestation, the basic knee anatomy resembles that of an adult with the chondroepiphysis forming the articular surfaces of the femur, tibia, and patella.7 By this time, the extensor mechanism is formed, and active joint motion has begun, facilitating the development of the trochlear sulcus.7 The secondary ossification center in the distal femoral epiphysis forms around 36 weeks gestation.8 Postnatally, both the patella and distal femur grow through endochondral ossification.9,10
The patella is the largest sesamoid bone in the human body.11 The patella begins as a dense consolidation of cells that differentiate as the quadriceps mechanisms develop.12,13 The patellar anlage becomes distinguishable within the quadriceps tendon around 7.5-week gestation.12 The morphology of the patella conforms to the distal femur.12 The patella molds or re-models as the knee begins to move in response to mechanical stresses.7 The patella increases in relative size during the first 6 months of gestation, then enlarges proportionately to the rest of the bones.7 Ossification begins around 3 years of age for females and 4 to 5 years of age for males.8,14 The ossification center may appear irregular as it rapidly expands.14 Ossification proceeds in a proximal to distal direction, thus giving a spurious estimation of patellar height on radiographs in children. The overall morphology of the cartilaginous patella during development is comparable to the final mature shape.14 Abnormal contact stresses on the articular surface of the patella during skeletal immaturity can lead to deformation.7
Ultrasonographic measurements in normal patients show that trochlear groove (TG) morphology is present early and becomes more radiographically apparent as distal femoral ossification is completed.15 Anatomic dissections of aborted fetuses have verified the morphology of the TG as it remains constant during growth and the groove morphology is the same for both fetuses and adults.16 An ultrasound study performed on patients aged 12 to 18 years showed the cartilaginous sulcus angle (CSA) remained constant throughout all age groups (146°).17 The CSA however, differed in patients who suffered a patellar dislocation (average, 164°; range, 154°-195°) compared with normal knees (average CSA, 145°; range, 131°-158°).15,17,18 The osseous sulcus angle, on the other hand, appears flat at birth and the TG deepens with age. This increase in depth is more of a reflection of progressive ossification of a well-formed cartilaginous trochlea, rather than a true deepening of the sulcus.17 Thus, the axial radiographic view of the patella provides misleading information about the sulcus angle in children and should not be used to define trochlear morphology.
Continue to: MEDIAL PATELLOFEMORAL LIGAMENT ANATOMY
MEDIAL PATELLOFEMORAL LIGAMENT ANATOMY
The medial patellofemoral ligament (MPFL) functions to limit the lateral translation of the patella.19 The attachment sites on the femur and patella for the MPFL have been studied in children.20-23 Cadaveric dissections in specimens aged 2 to 11 years have noted the patellar attachment to be an average of 12 mm in length with the midpoint approximately 5 mm superior to the mid-pole of the patella.22 The patellar footprint of the MPFL insertion was a mean 41% of the entire patellar length.22
It is important to be aware of the characteristic anatomy of the MPFL, as fixation points should mimic the anatomic insertion as best as possible while also avoiding violation of the nearby physis. The MPFL originates between the adductor tubercle and the medial femoral epicondyle just distal to the distal femoral physis and attaches to the superomedial aspect of the patella.20-25 In relation to the physis in pediatric patients, the midpoint of MPFL insertion has been measured to be 4 mm to 9 mm distal to the femoral physis.21,24,25 These measurements represent averages as cadaveric studies have reported that some part of MPFL femoral insertion extends proximal to the distal femoral physis.21 A recent report of physeal injury to the posterior distal femoral physis during MPFL reconstruction leading to femoral flexion deformity highlights the importance of physeal-respecting surgery.26
TROCHLEA AND ANTERIOR DISTAL FEMORAL PHYSIS
The relationship between the proximal aspect of the trochlea and the anterior distal femoral physis has been recently studied in 175 knees with dysplastic trochlea.27 Based on magnetic resonance imaging evaluation, the lateral aspect of the trochlea extended proximal to the anterior distal femoral physis in 13% of patients and was at the level of the anterior physis in another 13% of patients (Figure 1).27 Hence, a cautious approach is recommended for any surgery to address trochlear dysplasia or trochlear bump in younger patients to prevent iatrogenic injury to anterior distal femoral physis and resultant genu recurvatum. The distance between the trochlea and the physis increased with increasing age.

LIMB ALIGNMENT
Physiologically, the quadriceps angle (Q angle) changes through the course of growth. As children begin standing and walking, they stand with their feet wider apart and in genu varum.28 Physiologic genu varum can reach 15°.28 This degree lessens during the first 1.5 to 2 years of life, transitioning to physiologic valgus of nearly 12° by 3 years of age.28 Genu valgum, thereafter, gradually decreases to reach the adult value of around 7° to 8° by age 7 years.28 Increased genu valgum is a risk factor for patellar instability. In skeletally immature patients, correction of genu valgum through guided growth may be desirable in patients undergoing patellar stabilization surgery (Figures 2A, 2B).29

PATHOPHYSIOLOGY OF PEDIATRIC PATELLAR DISLOCATION
TROCHLEAR DYSPLASIA
Trochlear dysplasia is an abnormal shape and depth of the TG.30 Up to 96% of patients with patellar dislocation have trochlear dysplasia.30-33 In a study of patellar instability in children, at least 1 of the 3 signs of trochlear dysplasia (the crossing sign, supratrochlear bump, and double contour sign) was present on lateral radiographs.34 In another study on the growth of trochlear dysplasia in children and adolescents, all grades of trochlear dysplasia were present at all ages (ie, the dysplasia was most likely present at birth and did not necessarily worsen with age and growth).35 The linear dimensions of lateral and medial condylar height as well as trochlear bump increased with age but both the sulcus angle and shape of the trochlea did not change significantly.35 Remodeling of a dysplastic trochlea can happen if the patella is stabilized and appropriately located at a younger age, preferably before 10 years of age.36,37
Continue to: PATELLAR HEIGHT
PATELLAR HEIGHT
The role of patellar height in patellar instability has been well established.38 In patients with patella alta, the patella remains proximal to the TG during the greater arc of knee motion, which predisposes it to patellar instability. Calculation of patellar height in children could be challenging due to incomplete ossification, as well as asymmetric ossification of the patella and the tibial tubercle (TT). Since the patella ossifies from proximal to distal, most radiographic methods that measure the patellar height from the distal aspect of the patella provide a spurious elevation of the measurement.
The Caton-Deschamps (CD) method measures the length of the patellar articular surface and the distance from the inferior edge of the articular surface to the anterosuperior corner of the tibial plateau.39 A ratio >1.3 signifies patella alta. The CD ratio has been verified as a simple and reliable index for measuring patellar height in children.40 Two other methods have been described for determining patellar height in children.41,42 Based on anteroposterior (AP) radiographs of the knee in full extension, Micheli and colleagues41 calculated the difference between the distance from the superior pole of the patella to the tibial plateau and the length of the patella. A positive difference signified patella alta. The Koshino method involves the ratio between a reference line from the midpoint of the patella to the midpoint of the proximal tibial physis and a second distance from the midpoint of the distal femoral physis to the midpoint of the proximal tibial physis on lateral knee radiographs.42 Normal values range from 0.99 to 1.20 with the knee in >30° flexion, in children 3 to 18 years of age.
HYPERLAXITY
In contrast to adults, children have increased levels of collagen III compared with collagen I, which is responsible for tissue elasticity.43 Tissue elasticity leads to increased joint mobility, which is more common in children. Joint hypermobility or hyperlaxity has to be differentiated from symptomatic instability. The traditional Beighton score identifies individuals as having joint hypermobility with a score of 5/9 or higher in school-aged children.44-46 Smits-Engelsman and colleagues44 suggested using stricter criteria with scores of 7/9 or higher being indicative of hyperlaxity in school-aged children. A study of 1845 Swedish school children noted that females have a higher degree of joint laxity.45 Maximal laxity was noted in females at 15 years of age.45 Hyperlaxity has been demonstrated to be greater on the left side of the body44 and can be part of generalized syndromes including Down’s syndrome, Marfan’s syndrome, or Ehlers-Danlos syndrome.
LIMB TORSION
Staheli and colleagues47 described the normative values of a lower extremity rotational profile, including femoral anteversion and tibial torsion. Children normally have increased femoral anteversion, which decreases with growth. Miserable malalignment is a term used to denote increased femoral anteversion and increased external tibial torsion.48,49 These rotational abnormalities can increase the Q angle and the lateral forces on the patella. Femoral anteversion or internal rotation of the femur of 30° significantly increases strain in all areas of the MPFL.48 This increased strain may lead to MPFL failure and patellar instability.48 Increased internal rotation of the femur also increases contact pressure on the lateral aspect of the patellofemoral joint.48 Miserable malalignment frequently manifests following a pubertal growth spurt and may require femoral and tibial osteotomy.50
SYNDROMIC ASSOCIATIONS
Several syndromes have patellar instability as a part of their manifestation. The more common syndromes include nail-patella syndrome, Kabuki syndrome, Down’s syndrome, and Rubinstein-Taybi syndrome.51-54 Other syndromes less commonly associated with patellar instability include Turner syndrome, patella aplasia, or absent patella syndrome. Since many patients with syndromic patellar instability are functionally limited, they may not require an aggressive approach to treatment. When treating these patients, it is important to recognize the unique features of a specific syndrome, which may affect the anesthesia risk profile, management decisions, rehabilitation, and prognosis.
Continue to: MPFL TEAR PATTERN
MPFL TEAR PATTERN
The MPFL serves as an important constraint to the patella to prevent lateral dislocation, primarily during the first 20° to 30° of knee flexion.55,56 Injury to the MPFL is noted in over 90% of patients who suffer a patellar dislocation.57 The location of MPFL tears in pediatric patients is variably reported at the patellar attachment (10%-61%), femoral attachment (12%-73%), both (12%-35%) or mid-substance (2.5%-15%).25,57 The most common tear patterns in pediatric patients are tears at the patellar attachment.25,57 This tear pattern may be accompanied by an avulsion fracture of the medial rim of the patella, though this fracture, being extra-articular, seldom needs treatment.
CLASSIFICATION
While several authors have established extensive classification systems of patellar dislocation based on both clinical and radiographic presentation and reviews of the literature, a single classification system has not been recognized as the gold standard. In this section, in addition to presenting our preferred methods of classification, we will review some of the more recent and extensive classification systems for patellar dislocation and patellar instability.
Dejour and colleagues31 initially used both the presence of patellofemoral anatomic abnormalities and pain to define 3 types of patellar instability: major, objective, and potential patellar instability. Major patellar instability indicates that the patient has experienced more than 1 documented dislocation, objective instability involves one dislocation in addition to an associated anatomic abnormality, and potential patellar instability refers to cases in which the patient has radiographic abnormalities and patellar pain.31 Garin and colleagues58 more simplistically divided patellar dislocation patients into 2 groups: major (permanent or habitual) dislocation of the patella and recurrent dislocation. Sillanpaa59 stressed the distinction between first-time dislocation and recurrent dislocation specifically in the context of acute injuries. These classification systems were formulated with adults as the most relevant population; however, classifications targeted specifically to pediatric patients have recently been presented in the literature.
Historically, pediatric patella dislocations were simply categorized as traumatic or congenital.60 In 2014, Chotel and colleagues61 focused on classifying patellar dislocation by extensively reviewing anatomic, biomechanical, pathophysiological, and clinical patterns seen most commonly in children. They included 5 categories: congenital dislocation, permanent dislocation, habitual dislocation during knee flexion, habitual dislocation during knee extension, and recurrent dislocation; however, they did not address traumatic dislocations.61 Congenital dislocation is a rare condition, typically presenting at birth, which produces a pattern of functional genu valgum.62 Permanent dislocation typically presents after the child has started walking, but before the age of 5 years.61 The 2 variations of habitual dislocation typically present between ages 5 and 8 years.61 The final category is the most common and typically occurs during pre-adolescence or adolescence as a result of an atraumatic or trivial traumatic event or sports injury.1 Using more specific terminology, Hiemstra and colleagues63 modeled a classification system based on the traumatic, unilateral, bankart lesion, surgery (TUBS)/atraumatic, multidirectional, bilateral, rehabilitation, inferior shift (AMBRI) for shoulder dislocation classifications. The patellar dislocation system is used to identify 2 distinct subsets of patients in the patellofemoral instability population. One subset is defined by the acronym WARPS (weak, atraumatic, risky anatomy, pain, and subluxation), the other is STAID (strong, traumatic, anatomy normal, instability, and dislocation).64 Patients categorized by the WARPS acronym tend to experience atraumatic onsets of patellofemoral instability and demonstrate anatomic issues that increase this instability. These underlying anatomic issues include valgus alignment, ligamentous laxity, rotational abnormalities, shallow and short TG, and patella alta. On the other hand, STAID patients describe a traumatic dislocation event and do not have underlying anatomic abnormalities that predispose them to instability.64
Taking into account these previous classifications, Frosch and colleagues65 added specific pathologies including “instability,” “maltracking,” and “loss of patellar tracking,” in addition to both clinical and radiographic factors to define 5 types of patellar dislocation and their specific treatment recommendations.65 Type 1 involves simple dislocation with neither maltracking nor instability and a low risk of redislocation.65 Type 2 is defined as primary dislocation followed by subsequent high risk of dislocation and no maltracking.65 Type 3 is divided into 5 subcategories of instability and maltracking issues involving soft tissue contracture, patella alta, pathological tibial tuberosity, and TG distance.65 Type 4 is defined as the highly unstable “floating patella,” and type 5 involves patellar maltracking without instability 65. In terms of treatment, conservative rehabilitation is recommended for type 1 whereas MPFL reconstruction tends to show positive outcomes for both types 2 and 3.66-70
Continue to: Parikh and Lykissas recently published...
Parikh and Lykissas recently published a comprehensive classification system of 4 defined types of patellar dislocation in addition to voluntary patellar instability and syndromic patellar instability (Table).60 The 4 types are Type 1, first-time patellar dislocation; Type 2, recurrent patellar instability; Type 3, dislocatable; and Type 4, dislocated. Type 2 is further subdivided into Type 2A, which presents with positive apprehension signs, and Type 2B, which involves instabilities related to anatomic abnormalities.60 A distinction is also made between Type 3A or passive patellar dislocation and Type 3B habitual patellar dislocation.60

The classification system proposed by Green and colleagues is more simplified with 3 main categories (Table) of pediatric patellar dislocation: traumatic (acute or recurrent), obligatory (either in flexion or extension), and fixed laterally.71,72 The acute traumatic categorization refers to patients who experienced an initial dislocation event due to trauma whereas recurrent traumatic involves repeated patella dislocations following an initial incident. Studies report that between 60% to 70% of these acute traumatic dislocations occur as a result of a sports-related incident.2,33,73 Obligatory dislocations occur with every episode of either knee flexion or extension, depending on the subtype. Obligatory patella dislocation in flexion typically cannot be manipulated or relocated into the trochlea while the knee is fixed but does reduce into the trochlea in full extension. Fixed lateral dislocations are rare, irreducible dislocations in which the patella stays dislocated laterally in flexion and extension. These dislocations often present with other congenital abnormalities. Each of these categories can be further specified as syndromic if the dislocation is associated with genetic or congenital conditions including skeletal dysplasia, Ehlers-Danlos syndrome, cerebral palsy, Marfan disease, nail-patella syndrome, Down syndrome, Rubenstein-Taybi syndrome, and Kabuki syndrome.51-54,61,74-76
SURGICAL TECHNIQUES IN SKELETALLY IMMATURE PATIENTS
While nonsurgical, conservative treatment involving physical therapy and activity modification is recommended for most patients who experience first-time traumatic patellar dislocations, many patients experience complicating factors that indicate them for surgery. These factors include recurrent dislocation, risk factors for patellofemoral instability, underlying malalignment issues, and congenital deformities. When evaluating these factors, particularly patellofemoral instability, the authors recommend assessing osteochondral lesions, age, skeletal maturity, number of previous dislocations, family history, and anatomic risk factors.2,5,77-79 Extra care should be taken when considering surgical treatment for skeletally immature patients at elevated risk for recurrent instability as the risk of cartilage damage in these cases is high.80-82
Recently, there has been a reported increase in surgical treatment for patellar instability in the skeletally immature.83 This finding may be attributed to heightened awareness of factors that indicate patients for surgical treatment and increased familiarity of surgeons with newer techniques.83 Many surgical techniques have been described to address patellar instability involving both soft-tissue procedures and bony corrections.84 In this article, we discuss the various surgical techniques for MPFL reconstruction, quadricepsplasty, and distal realignment. These procedures can be paired with any number of additional procedures including, but not limited to, lateral retinacular release or lengthening, chondroplasty, TT osteotomy (in skeletally mature patients), and removal of loose bodies.83
There is a need for more comprehensive studies, particularly randomized controlled trials, to evaluate the outcomes for both surgical and nonsurgical treatments for first-time dislocations. In the current literature, only very recently have surgical treatments shown outcomes that are more positive. In 2009, Nietosvaara and colleagues85 conducted a randomized controlled trial of nonoperative and operative treatment of primary acute patellar dislocation in both children and adolescents. After a long-term mean follow-up of 14 years, there was not a significant difference between the groups in recurrent dislocation and instability, subjective outcome, or activity scores.85 In a subsequent review of 5 studies including 339 knees, Hing and colleagues86 also found similar results in both the operative and nonoperative cohorts at risk of recurrent dislocations, Kujala scores, and reoperations. However, a recent systematic review comparing redislocation rates and clinical outcomes between surgical and conservative management of acute patellar dislocation reported more positive outcomes for the surgical cohort.87 This review included 627 knees, 470 of which received conservative management, 157 of which received operative treatment. The conservative cohort was followed for an average of 3.9 years and had a 31% rate of recurrent dislocation while the surgical group was followed for a mean 4.7 years and experienced a 22% redislocation rate.87 This study indicates that operative management for acute first-time dislocations may be the preferred treatment option.
Continue to: A potential reason some of these studies...
A potential reason some of these studies did not show any significant difference between the operative and nonoperative cohort could be that the surgical cohorts included a wide range of procedures including lateral releases and MPFL repairs. Recent publications have indicated that these techniques do not produce overall positive outcomes. While each surgical treatment plan is unique depending on the patient; recently, MPFL reconstruction has been shown to have better outcomes than both nonoperative management and simple medial repair and/or lateral
release.67,88-90
MPFL RECONSTRUCTION
INDICATIONS/OVERVIEW
The MPFL is an important stabilizer for the knee that primarily resists lateral translation of the patella. Damage to the MPFL is very common in acute patellar dislocations with up to 90% of first-time dislocations resulting in injury to the MPFL.91,92 Historically, simple medial and/or lateral MPFL repairs have not been shown to improve patellofemoral kinematics significantly and often result in recurrence.90,93 To address this issue, during the past few decades, numerous MPFL reconstruction techniques have been developed to reconstruct a stronger ligament with the same kinematics as the anatomic MPFL.2,19,69,81,94-106 The ultimate goal of MPFL reconstruction is to reestablish the anatomic “checkrein” to guide the patella into the trochlea between 0° and 30° of knee flexion.107,108 An essential secondary surgical goal in skeletally immature patients is to avoid damaging the distal femoral physis.
There are many variations in both the grafts used to replace the MPFL and the means by which to secure them. The ones discussed below include free semitendinosus or gracilis autografts or grafts constructed from a pedicled adductor, patellar, or quadriceps tendon.69,105,109 While not used as frequently, allografts have also been used.110 Methods to secure these grafts in osseous tunnels include suture anchors or tenodesis screws. Incomplete osseous sockets or medial-sided bone tunnels have also been used as a method to decrease patellar fractures as they preserve the lateral patellar cortex.111-114
DOUBLE-BUNDLE HAMSTRING AUTOGRAFT
The technique most often used by the author is a double-bundle hamstring autograft harvested from either the semitendinosus or the gracilis secured by short patellar and femoral sockets (Figure 3). After harvesting the hamstring graft from a posteromedial incision, an approximately 90-mm graft is prepared with Krackow stitches to secure 15 mm of the tendon in each socket.115 Lateral radiographs are used intraoperatively to ensure the guidewire for the femoral drill hole falls along the posterior cortex of the diaphysis of the femur while AP radiographs confirm placement distal to the physis. It is important to take both AP and lateral radiographs intraoperatively due to the concave curvature of the distal femoral physis. This unique anatomy can make a point that is located distally to the physis on the AP view appear on or proximal to it on the lateral cross reference view.24,116 For the patellar socket, 2 short sockets are made in the superior half of the patella. Once the sockets have been drilled, the graft is adjusted so that the patella stays seated in the center of the trochlea between 20° and 30° of flexion. This anchoring is accomplished by securing the graft while the knee is kept at 30° of flexion. Proper tension is confirmed by ensuring that the graft does not allow lateral patella movement over one-fourth the width of the patella in extension while crepitation must not appear throughout the ROM.92

QUADRICPETS TENDON TRANSFER
A combination of techniques by Steensen and colleagues,105 Goyal,109 Noyes and Albright,117 and Pinkowsky and Hennrikus118 describe an MPFL reconstruction in which the proximal end of a small medial portion of the quadriceps tendon is released and then attached to the medial epicondyle through a subcutaneous tunnel (Figure 4). This technique is particularly useful for cases in which the extra strength provided by the bone-quadriceps tendon is necessary to correct more severe dysplasia. Leaving the distal end of the quadriceps tendon intact at its patellar insertion, a graft of about 8 mm x 70 mm thickness is harvested from the tendon. The free distal end of the tendon is then run anatomically through the synovium and retinaculum to be either sutured to the medial intermuscular septum at the medial femoral epicondyle or fixed in femoral tunnel using interference screw.105,109,118 The placement of the femoral fixation point is essential to ensure positive surgical outcomes. If the graft is secured too anteriorly, it may be too loose in extension and too tight in flexion, both of which can lead to postoperative pain, loss of normal kinematics, and overload of the medial patellofemoral cartilage.119-121 Once the ideal placement of the femoral fixation point has been confirmed by intraoperative radiographs, the graft is secured with a small absorbable suture.122,123 While this technique has good clinical results, the longitudinal scar that results from graft harvesting is cosmetically unappealing, and it is technically challenging to harvest a consistent strip of the quadriceps tendon. To address some of these concerns, Fink and colleagues124 described a new harvesting technique that produces more consistent grafts and requires a smaller incision.

Continue to: ADDUCTOR MAGNUS TENDON TRANSFER
ADDUCTOR MAGNUS TENDON TRANSFER
This technique is a double-bundle MPFL reconstruction that uses a pedicled graft of the distal adductor magnus tendon and suture anchors or incomplete osseous sockets to recreate the MPFL anatomically (Figure 5). Avikainen and colleagues96 and Sillanpää and colleagues125 described this procedure as a progression from the original single-strand adductor magnus transfer technique. First, maintaining the distal insertion, a graft of approximately 14 cm to 18 cm is harvested from the adductor tendon and then passed through a subcutaneous tunnel between the distal vastus medialis obliquus and the superficial joint capsule. The graft is then looped at the medial patella so that the distal bundle runs back to the adductor tubercle.125 With the knee at 30° of flexion to assure proper tension, the graft is secured at both the patella and near the adductor tubercle with suture anchors.125 Hambridge and colleagues126 compared a similar adductor magnus transfer with other pedicled techniques including bone-quadriceps tendon autograft and bone-patellar tendon allograft and found positive results for all 3 methods of reconstruction.

HEMI-PATELLA TENDON TRANSFER
In a similar technique to the adductor tendon transfer, the medial section of the patellar tendon is harvested from the TT and run from its proximal insertion at the medial patella to the medial femoral attachment via a subcutaneous tunnel. The free end of the graft is then secured with suture anchors or incomplete osseous sockets with the knee at 30° of flexion.127
HAMSTRING GRAFT WITH ADDUCTOR TENDON AS A PULLEY
Several techniques opt to use a more dynamic model of MPFL reconstruction in which the adductor tendon or medial collateral ligament (MCL) is used as a pulley for the hamstring graft (Figure 6).128,129 The site of the pulley approximates the normal attachment of the MPFL to the femur and so acts as an effective anatomic replica of the MPFL origin. A semitendinosus graft is harvested and is prepared with continuous sutures, and 2 tunnels to secure the graft are drilled into the patella. The graft is then run subcutaneously from the medial side of the patella to the adductor magnus tubercle into which an osteoperiosteal tunnel is drilled at its distal femoral insertion. The graft is looped through the adductor tunnel and secured with sutures. Proper knee kinematics was ensured by placing the knee at 30° of flexion as the ends of the tendon are secured to the patella.114,130

HAMSTRING GRAFT WITH MCL AS A PULLEY
The MCL can also be used as a pulley rather than the adductor tendon. The semitendinosus graft is harvested and prepared and the patella drilled as it is in the previous technique. The MCL was fashioned into a pulley by making a slit in its posterior one-third. The semitendinosus graft is looped through this slit, and both ends of the graft are held in place with suture anchors on the surface of the patella.129
ADDITIONAL PROCEDURAL COMBINATIONS
Depending on the needs of the individual patient, MPFL reconstruction, and other patellar stabilization techniques can also be combined with additional procedures. Arshi and colleagues83 conducted a review of 6190 adolescents surgically treated for patellar instability and reported the most common additional procedures performed at the time of the stabilization. They found 43.7% of the population underwent lateral retinacular release, which while not effective as an isolated technique to treat patellar instability, has often been used in combination with MPFL reconstruction.131-133 There is currently a lack of consensus regarding the success of adding a lateral release to the reconstruction. Some studies report no difference while others report a decrease in stability after lateral release.90,134-136 While lateral retinacular release has been shown to decrease the force required to displace the patella, it can be surgically indicated in certain patients undergoing MPFL reconstruction.131 The authors advocate that if the lateral retinaculum is tight such that centralized patellar tracking is inhibited following the reconstruction, or if the patella cannot be pushed passively from a laterally tilted position to the neutral horizontal position, lateral retinacular lengthening should be performed to improve kinematics.132
Continue to: Arshi and colleagues...
Arshi and colleagues83 also reported a high rate of cartilage procedures, with chondroplasty performed in 31.1% and chondral fragment/loose body removal in 10.2%. These statistics suggest that a significant level of cartilage damage has occurred by the time of surgery.83
COMPLICATIONS
As MPFL reconstruction techniques have only recently been popularized and developed, there are not many comprehensive studies evaluating the outcomes and complications associated with these procedures. However, in the current literature, there is a general consensus that patients usually experience positive short-term clinical outcomes and relatively low complication rates.68,77 In one of the largest retrospective cohort studies of pediatric patients undergoing MPFL reconstruction, Parikh and colleagues114 reported both the type and rate of complications. They found complications occurred in 16.2% of patients, and the most common complications were recurrent patellar instability, patellar fractures, patellofemoral arthrosis, motion deficits, and stiffness with over half classified as avoidable. Most of these complications were due to technical errors with episodes of recurrent instability only reported in 4.5% of patients.114 In a comprehensive meta-analysis of MPFL reconstruction studies, Shah and colleagues137 reported a complication rate of 26% in both pediatric and adult patients. The cohort was not stratified by age, yet complications were similar to those reported by Parikh and colleagues,114 including pain, loss of knee flexion, wound complications, and patellar fracture.137
As indicated by the frequency of technical complications reported by Parikh and colleagues,114 extra caution should be taken in the operating room to minimize potential errors. In techniques that require drilling of femoral sockets, proper length for and placement of the graft is essential to reestablish proper kinematics. Studies have reported that placing the femoral socket too proximally can result in loss of ROM during flexion and increased compressive forces across the patella.138 A graft that is too short can have similar negative outcomes, and a graft that is too long can result in recurrent instability. Positioning the graft while the knee is in 30° of flexion can help ensure the proper length and tension is achieved. Once the graft is in place, it is important to ensure the ROM and isometry before completing the fixation.72 It is also essential to be vigilant about potential violation of the physes and subsequent growth disturbances. To establish the safest angles for drilling the distal femoral epiphysis for graft placement, Nguyen and colleagues139 conducted a study using high-resolution 3-dimensional images of cadaveric distal femoral epiphyses. By recording which tunnels disrupted the physis before reaching 20 mm of depth, the authors concluded that it is safest to drill distally and anteriorly at an angle between 15° and 20°.139 This technique should minimize damage to the physis, notch, and distal femoral cartilage and decrease potential complications.139
OUTCOMES
In general, the literature reports positive outcomes for MPFL reconstruction—in both studies that address a specific technique and all-encompassing studies. Outcomes are typically reported as Kujala and Tegner scores, results from clinical examinations, and rates of subsequence recurrences. Several recent studies have also evaluated the ability of MPFL reconstruction to restore proper kinematics. Edmonds and colleagues140 evaluated the difference in patellofemoral joint reaction forces and load experienced by 3 groups of adolescents: a cohort treated with MPFL reconstruction, a cohort treated with soft-tissue realignment of the extensor mechanism (the Insall method), and controls. While both surgical techniques were able to restore medial constraints to the patella, the study showed that only the MPFL reconstruction cohort experienced joint reaction forces that were analogous to the control group. In comparison, the cohort that was treated with soft-tissue realignment alone experienced higher patellofemoral joint reaction forces and did not regain normal joint mechanics.140 These results can be used to advocate for the further use of MPFL reconstruction as an effective anatomic replacement of the native ligament. Radiographic studies have similarly reported MPFL reconstruction as an effective means to restore anatomic normality. Fabricant and colleagues141 conducted a radiographic study in which patella alta was corrected to normal childhood ranges in patients who underwent MPFL reconstruction technique using a hamstring autograft. Lykissas and colleagues142 corroborated these results with another radiographic study that reported small but significant decreases in the Blackburne-Peel index and CD index following MPFL reconstruction in 25 adolescents. As correction of patella alta allows the patella to rest in a deeper, more secure position in the TG, these results indicate that effective early MPFL reconstruction can correct for patellar anatomic abnormalities that could be future risk factors.143,144 Several studies have also reported outcomes addressing specific MPFL techniques; these are reported and discussed in this article.
OUTCOMES BY TECHNIQUE
HAMSTRING AUTOGRAFT
Reports on outcomes following MPFL reconstructions using hamstring autografts have been particularly promising. A cohort of 21 skeletally immature patients who underwent MPFL reconstruction was evaluated pre- and postoperatively with an average of a 2.8-year follow-up. The authors of the study reported no redislocation events and significant improvement in the Kujala scores, and patients were able to return to athletic activities safely.145 Previous studies report similar positive increases in Kujala scores, subjective patient reports, and lack of subsequent redislocation for patients who underwent either semitendinosus or gracilis autograft MPFL reconstructions. One such study further documented an average patellar inclination angle decrease from 34.3° to 18.6° following MPFL reconstruction.146 However, while the literature typically reports positive Kujala scores and subjective outcomes for the hamstring autograft procedure, a study arthroscopically evaluating patellar tracking immediately following surgery and then at 6 to 26 months follow-up found that patellar tracking correction was not maintained for all patients who underwent this type of MPFL reconstruction.147
Continue to: QUADRICEPS TENDON TRANSFER OUTCOMES
QUADRICEPS TENDON TRANSFER OUTCOMES
Studies specifically evaluating the quadriceps tendon transfer technique for MPFL reconstruction in children are sparse, but authors have reported positive clinical outcomes and low complication rates in adults. After following 32 young adults who underwent this MPFL reconstruction technique for 3 years, Goyal109 reported a significant increase in mean Kujala scores from 49.31 to 91.25 and no complications or redislocation. He argues this type of quadriceps graft has a high success rate because it is anatomically more similar to the MPFL than other grafts and does not require additional patellar fixation.101,109 Similar positive Kujala scores and minimal complications have been reported in adult patient populations.148 Abouelsoud and colleagues149 conducted one of the few studies in skeletally immature patients and reported similarly positive results with no redislocations and significantly improved Kujala scores at a mean follow-up of 29.25 months in their 16-patient cohorts.
ADDUCTOR MAGNUS TENDON TRANSFER
After initially describing this technique in 14 adult patients, Avikainen and colleagues96 followed this cohort and reported positive subjective results and only 1 redislocation. In a more recent study in which the adductor tendon transfer technique was compared with the quadriceps tendon transfer described above and the bone-patellar tendon allograft, Steiner and colleagues69 reported similarly significant improvement in all cohorts in Lysholm, Kujala, and Tegner scores with no redislocations. Additionally, Malecki and colleagues150 followed a cohort of 33 children with 39 knees diagnosed with recurrent patellar dislocation, who underwent MPFL reconstruction using the adductor magnus tendon. After evaluating this cohort functionally and radiographically, the authors reported improvements in Lysholm and Kujala scores, patellar tilt and congruence angles, and peak torque of the quadriceps muscle and flexor.150 However, this cohort did report postoperative redislocations in 36.4% of patients (4 of 11).150
HEMI-PATELLA TENDON TRANSFER
In 2012, in the first randomized controlled trial, Bitar and colleagues67 compared the outcomes of patients who underwent MPFL reconstruction via the hemi-patellar tendon technique with those who were managed nonoperatively with immobilization and physiotherapy after first-time patellar dislocation. At 2-year follow-up, the surgical cohort presented positive results with a significantly higher mean Kujala score (88.9 to 70.8) and no redislocations or subluxations. In contrast, 35% of nonoperative cases presented with recurrences and subluxations over the 2-year period.67
MCL OR ADDUCTOR TENDON AS A PULLEY
Studies have reported good postoperative results and low complication rates for these dynamic techniques.128,129 In terms of kinematics, while hypermobility and patellar height were not fully corrected, improvements in patellar tilt and lateral shift were reported in a cohort of 6 patients with a minimum 4-year follow-up.129 To further evaluate whether the more dynamic pulley reconstruction technique resulted in better outcomes, Gomes and colleagues128 compared the subjective reports, clinical evaluations, and complication rates of patients who underwent MPFL reconstruction with a rigid adductor magnus fixation vs a semitendinosus tendon dynamic femoral fixation. One case in the rigid cohort experienced a subsequent subluxation, while patients in the semitendinosus group had better subjective reports and a higher rate of return to sport.128 More recently, Kumahashi and colleagues151 specifically studied the outcomes of the MCL tendon as a pulley in 5 patients aged 14 to 15 years. They reported similar successful results as no patients experienced recurrence, and all patients exhibited improvement in radiographic measures of patellar tilt and congruence angle, lateral shift ratio, and both Kujala and Lysholm scores.151
While there has yet to be a randomized controlled trial comparing all of these different techniques, there is a general consensus in the literature that patients tend to perform better following MPFL reconstruction vs MPFL repair.
OTHER STABILIZATION PROCEDURES, INCLUDING DISTAL REALIGNMENT
Patients with additional underlying deficits and malalignment issues such as significant trochlear dysplasia, increased TT-TG distance, patella alta, increased Q angle, and/or positive J sign may require stabilization procedures beyond MPFL reconstruction.152,153 TT osteotomies are often used to correct alignment issues in the adult patient population; however, these procedures are typically contraindicated in skeletally immature patients. Alternative realignment procedures for the pediatric population include both proximal and distal realignment, with proximal realignment performed primarily in children under the age of 12 years.153 Many variations on these procedures exist, some of which are no longer regularly performed due to poor reported outcomes. In this article, we discuss several of the techniques, focusing primarily on those that have demonstrated higher success rates.
Continue to: GALEAZZI TECHNIQUE
GALEAZZI TECHNIQUE
One of the first and most famous soft-tissue techniques to address patellar instability was the semitendinosus tenodesis, published by Galeazzi154 in 1922 (Figure 7). This technique stabilizes the patella without altering the TT. In the original technique, a portion of the semitendinosus tendon is harvested with its tibial insertion left intact. The free end of the tendon is then secured with sutures at the periosteal groove of the medial patella.154,155 Fiume156 modified this technique by adding a lateral release and medial retinacular reefing. The most recent addition to this procedure was introduced by Baker and colleagues,157 in which a tunnel is drilled from the medial to the lateral border of the patella. Tension placed on the grafted tendon is used to reposition the patella medially and draw it downward. Preliminary literature on this modified procedure reported fair clinical results with success rates of approximately 75%.155,158-160 A recent study evaluating both the clinical and radiographic outcomes of this technique also indicated that while clinical results were excellent in 62.5% of patients, this technique alone was unsuccessful in fully addressing patellar instability in patients with underlying anatomic abnormalities such as patellar alta.161 In light of these less than ideal reports, the authors no longer recommend this technique for patellofemoral instability cases.

ROUX-GOLDTHWAIT PROCEDURE
The Roux-Goldthwait procedure, first described by both Roux162 and Goldthwait163 in 1888 and 1895 respectively, was later modified in 1985 to involve a lateral release, plication of the medial retinaculum, medial transfer of the lateral patellar tendon without advancement, and advancement of the vastus medialis (Figure 8).164 More recently, Marsh and colleagues152 introduced an addition to aligning the extensor mechanism with the femoral shaft better. In this technique modification, the patellar tendon is split longitudinally, and its lateral half is detached and transferred distally beneath its medial half. The free end is then sutured to the periosteum on the medial side of the tibia.152 With a mean long-term follow-up of 6.2 years, Marsh and colleagues152 reported excellent results in 65%, good in 11%, and fair in 3% of the knees operated on with this modified technique. Of the patients in this cohort whose strength was evaluated, 80% had their strength returned to 90% of preoperative levels in the operated leg.152 While this study and others report improved outcomes, an increasing body of literature has found high rates of recurrence, patella infera, and other complications following the modified Roux-Goldthwait procedure.36,165-171 Also, a study comparing MPFL reconstruction using adductus magnus transfer with the Roux-Goldthwait procedure reported that patients in the MPFL cohort reported less pain postoperatively.150 In addition, whereas the Kujala and Lysholm scores, recurrence rates, patellofemoral angles, and apprehension test results did not demonstrate significant differences between these 2 groups, the MPFL group had significantly fewer abnormal congruence angles, better patellar medialization, and higher peak torque of the hamstring.150

COMBINED MPFL AND MEDIAL PATELLOTIBIAL LIGAMENT RECONSTRUCTION
While the medial patellotibial ligament (MPTL) has not received much attention with regard to patellar stability, recent studies have indicated its role during higher degrees of both flexion and extension.172 The MPTL acts as a secondary restrictor ligament which helps release stress on the MPFL by decreasing the Q angle and further normalizing patellar kinematics.173 Patients who present with hyperlaxity or knee hyperextension combined with extension subluxation and flexion instability could be indicated for this additional stabilizing procedure. Both Nietosvaara and colleagues85 and Brown and Ahmad174 have described a dual MPTL and MPFL reconstruction technique using a semitendinosus hamstring graft. More recently Hinckel and colleagues172 described a combined MPFL and MPTL reconstruction, using a graft from the quadriceps tendon to reconstruct the MPFL and one from the patellar tendon to reconstruct the MPTL. In this technique, once the respective grafts have been harvested, a femoral insertion for the graft recreating the MPFL is fluoroscopically established so that an anchor can be inserted distal to the femoral physeal growth plate. For the MPTL insertion, attachment to the tibia below the joint line and 2 cm medial to the patellar tendon is established fluoroscopically just above the physeal growth plate on the proximal epiphysis.19,175 The MPTL graft is sutured first with the knee at 90° of flexion to establish tension similar to that of the patellar tendon.176 Then, the knee is placed in 30° of flexion to fix the MPFL graft to the medial patella to prevent excessive lateral translation of the patella.
PATELLAR TENDON TRANSFER
Patellar tendon transfer with proximal realignment is a technique used in particularly young patients to address cases of patellofemoral instability involving concomitant bony or anatomic abnormalities. This procedure is effective for young children with substantial amounts of remaining growth as it better mimics native anatomy than other realignment procedures and does not require bony remodeling.152-154 It is important to familiarize with surgical techniques to address malalignment issues in young patients as neglected alignment issues can lead to worsening of trochlear dysplasia and instability, which are very difficult to treat later on when patients are older.153
The patellar tendon transfer technique (Figure 9), as described by Gordon and Schoenecker,177 starts with an extensive lateral retinacular release. The patellar tendon is then released from its distal insertion at the TT so that it can be moved medially without moving it inferiorly. After confirming patellar tracking and alignment by flexing the knee from 0° to 90° with the graft in place, the patellar tendon graft is secured with multiple nonabsorbable horizontal sutures.177 Of note, in skeletally mature patients, a TT osteotomy is used to accomplish the same goal. This osteotomy has been shown to improve both patellar height and TT-TG distance in skeletally mature patients, but is contraindicated in skeletally immature patients.92,178

Continue to: Initial studies conducted on patellar tendon...
Initial studies conducted on patellar tendon transfer have positive outcomes.179 At a mean follow-up of 5.1 years, patients reported a decrease in pain and increased the ROM and activity, and only 1 reported a postoperative redislocation.179 In more recent studies, both Benoit and colleagues36 and Garin and colleagues58 reviewed cases of patellar instability treated with patellar tendon transfer to address concomitant patellar alignment and anatomic abnormalities. They reported good functional, clinical, and radiographic outcomes with 12.5% and 16% recurrence rates, respectively.36,58 They also noted radiographic improvements in femoral sulcus angle, particularly in younger patients, which indicate this procedure is effective in addressing bony abnormalities that can result from neglected malalignment issues.36,58,154
QUADRICEPSPLASTY
Quadricepsplasty is a lengthening and remodeling technique not frequently used in the pediatric population. The goal of this procedure in patients with significant amounts of growth remaining is to reposition the patella to ameliorate trochlear remodeling and prevent worsening symptoms and anatomic abnormalities.36 A quadricepsplasty accomplishes this by de-rotating and/or lengthening the extensor mechanism and may or may not involve a concomitant MPFL reconstruction. This procedure is particularly effective in young patients who experience obligatory dislocation.60,72 Several quadricepsplasty techniques have been described including Thompson, Curtis and Fisher, Judet, Stanisavljevic, and V-Y technique.180-186 Most techniques initially involve sharp dissection of the vastus medialis and lateralis from the rectus femoral tendon. A tongue is then fashioned out of the rectus femoral tendon. Once the vastus medialis and lateralis are detached from the margins of the patella, the knee is extended, and the distal ends of the vasti are sutured to the tongue of the rectus tendon. Effective extension facilitates flexion to 90°.184 The authors recommend a modification of this technique in which a Z lengthening of the quadriceps tendon is performed after the vastus lateralis is removed distally from the patella and the quadriceps tendon.
Several series and case reports evaluating quadricepsplasty in adult patients report positive outcomes with most patients achieving good or excellent flexion with minimal complications.183,185,187-189 Reports on quadricepsplasty used to treat conditions other than patellofemoral instability in children have reported similar positive outcomes.190-192 As quadricepsplasty for patellar instability is relatively rare in pediatric patients, there is not much relevant literature. However, Kocon and colleagues193 reported results of quadricepsplasty and quadricepsplasty combined with the modified Galeazzi procedure in 8 children (10 knees) with a mean follow-up of 3.25 years. Seventy percent of cases resulted in stabilization and correction of patellar position, and only 2 postoperative redislocations were noted.193 Additionally, in a study evaluating 6 patients suffering from patellar instability, 2 of whom were obligate dislocators, quadricepsplasty resulted in patellar stability, satisfaction, and near normal gait patterns.194
Figure 10 shows the surgical algorithm used for patellar instability characteristics.

CONCLUSION
Patellofemoral joint stability relies on a complex interplay of musculotendinous units, ligaments and the osteocartilaginous morphology of the patellofemoral joint. Patellar instability in pediatric patients is different from adults. Having an in-depth understanding of the remodeling potential, the insertion sites for the MPFL and its relationship to the physis are of utmost importance when planning surgery. Reducing and maintaining the patella within the patellofemoral joint early enough can allow for remodeling of the patella and/or the trochlea to provide for lasting stability. Appropriate surgical principles, such as tensioning, can help both prevent continued pain and minimize future complications.
1. Nietosvaara Y, Aalto K, Kallio PE. Acute patellar dislocation in children: incidence and associated osteochondral fractures. J Pediatr Orthop. 1994;14(4):513-515.
2. Fithian DC, Paxton EW, Stone ML, et al. Epidemiology and natural history of acute patellar dislocation. Am J Sports Med. 2004;32(5):1114-1121. doi:10.1177/0363546503260788.
3. Abbasi D, May MM, Wall EJ, Chan G, Parikh SN. MRI findings in adolescent patients with acute traumatic knee hemarthrosis. J Pediatr Orthop. 2012;32(8):760-764. doi:10.1097/BPO.0b013e3182648d45.
4. Mäenpää H, Lehto MU. Patellar dislocation. The long-term results of nonoperative management in 100 patients. Am J Sports Med. 1997;25(2):213-217. doi:10.1177/036354659702500213.
5. Hawkins RJ, Bell RH, Anisette G. Acute patellar dislocations. The natural history. Am J Sports Med. 1986;14(2):117-120. doi:10.1177/036354658601400204.
6. Cofield RH, Bryan RS. Acute dislocation of the patella: results of conservative treatment. J Trauma. 1977;17(7):526-531.
7. Wasserlauf BL, Paletta GA. Developmental anatomy of the pediatric and adolescent knee. In: Micheli LJ, Kocher MS, eds. The Pediatric and Adolescent Knee. 1st ed. Elsevier; 2006:27-32.
8. Birch JG. Growth and development. In: Herring J, ed. Tachdjian’s Pediatric Orthopedics. 4th ed. Saunders/Elsevier; 2007:3-22.
9. Sarin VK, Carter DR. Mechanobiology and joint conformity regulate endochondral ossification of sesamoids. J Orthop Res. 2000;18(5):706-712. doi:10.1002/jor.1100180505.
10. Maes C, Kronenberg HM. Postnatal bone growth: growth plate biology, bone formation, and remodeling. In: Pediatric Bone. Elsevier; 2012:55-82.
11. Tecklenburg K, Dejour D, Hoser C, Fink C. Bony and cartilaginous anatomy of the patellofemoral joint. Knee Surg Sports Traumatol Arthrosc. 2006;14(3):235-240. doi:10.1007/s00167-005-0683-0.
12. Walmsley R. The development of the patella. J Anat. 1940;74(Pt 3):360-368.3.
13. Fulkerson J, Hungerford D. Disorders of the patellofemoral Joint. In: Normal Anatomy. 2nd ed. Baltimore: Williams & Wilkins; 1990:1-24.
14. Ogden JA. Radiology of postnatal skeletal development. X. Patella and tibial tuberosity. Skeletal Radiol. 1984;11(4):246-257.
15. Nietosvaara Y, Aalto K. The cartilaginous femoral sulcus in children with patellar dislocation: an ultrasonographic study. J Pediatr Orthop. 1997;17(1):50-53.
16. Glard Y, Jouve JL, Garron E, Adalian P, Tardieu C, Bollini G. Anatomic study of femoral patellar groove in fetus. J Pediatr Orthop. 2005;25(3):305-308.
17. Nietosvaara Y. The femoral sulcus in children. An ultrasonographic study. J Bone Joint Surg Br. 1994;76(5):807-809.
18. Nietosvaara AY, Aalto KA. Ultrasonographic evaluation of patellar tracking in children. Clin Orthop Relat Res. 1993;(297):62-64.
19. Desio SM, Burks RT, Bachus KN. Soft tissue restraints to lateral patellar translation in the human knee. Am J Sports Med. 26(1):59-65. doi:10.1177/03635465980260012701.
20. Shea KG, Styhl AC, Jacobs JC, et al. The relationship of the femoral physis and the medial patellofemoral ligament in children: a cadaveric study. Am J Sports Med. 2016;44(11):2833-2837. doi:10.1177/0363546516656366.
21. Shea KG, Polousky JD, Jacobs JC, et al. The relationship of the femoral physis and the medial patellofemoral ligament in children. J Pediatr Orthop. 2014;34(8):808-813. doi:10.1097/BPO.0000000000000165.
22. Shea KG, Polousky JD, Jacobs JC, et al. The patellar insertion of the medial patellofemoral ligament in children. J Pediatr Orthop. 2015;35(4):e31-e35. doi:10.1097/BPO.0000000000000399.
23. Shea KG, Grimm NL, Belzer J, Burks RT, Pfeiffer R. The relation of the femoral physis and the medial patellofemoral ligament. Arthroscopy. 2010;26(8):1083-1087. doi:10.1016/j.arthro.2009.12.020.
24. Nelitz M, Dornacher D, Dreyhaupt J, Reichel H, Lippacher S. The relation of the distal femoral physis and the medial patellofemoral ligament. Knee Surg Sport Traumatol Arthrosc. 2011;19(12):2067-2071. doi:10.1007/s00167-011-1548-3.
25. Kepler CK, Bogner EA, Hammoud S, Malcolmson G, Potter HG, Green DW. Zone of injury of the medial patellofemoral ligament after acute patellar dislocation in children and adolescents. Am J Sports Med. 2011;39(7):1444-1449. doi:10.1177/0363546510397174.
26. Seitlinger G, Moroder P, Fink C, Wierer G. Acquired femoral flexion deformity due to physeal injury during medial patellofemoral ligament reconstruction. Knee. 2017;24(3):680-685. doi:10.1016/j.knee.2017.02.003.
27. Parikh SN, Rajdev N. Relationship between trochlear dysplasia and the anterior distal femoral physis in pediatric and adolescent patients with patellar instability. In: 36th Annual Meething AANA. Denver; 2017.
28. Salenius P, Vankka E. The development of the tibiofemoral angle in children. J Bone Joint Surg Am. 1975;57(2):259-261.
29. Parikh SN. Medial patellofemoral ligament reconstruction and simultaneous guided correction of genu valgum for patellar instability in skeletally immature patients. In: EPOSNA. 2017.
30. Brattstroem H. Shape of the intercondylar groove normally and in recurrent dislocation of patella. A clinical and X-ray-Anatomical investigation. Acta Orthop Scand Suppl. 1964;68(suppl 68):1-148.
31. Dejour H, Walch G, Nove-Josserand L, Guier C. Factors of patellar instability: an anatomic radiographic study. Knee Surg Sports Traumatol Arthrosc. 1994;2(1):19-26.
32. Lewallen LW, McIntosh AL, Dahm DL. Predictors of recurrent instability after acute patellofemoral dislocation in pediatric and adolescent patients. Am J Sports Med. 2013;41(3):575-581. doi:10.1177/0363546512472873.
33. Jaquith BP, Parikh SN. Predictors of recurrent patellar instability in children and adolescents after first-time dislocation. J Pediatr Orthop. 2017;37(7):484-490. doi:10.1097/BPO.0000000000000674.
34. Lippacher S, Reichel H, Nelitz M. Radiological criteria for trochlear dysplasia in children and adolescents. J Pediatr Orthop B. 2011;20(5):341-344. doi:10.1097/BPB.0b013e3283474c8b.
35. Parikh SN, Rajdev N, Sun Q. The growth of trochlear dysplasia during adolescence. J Pediatr Orthop. 2018. doi:10.1097/BPO.0000000000001168. [Epub ahead of print]
36. Benoit B, Laflamme GY, Laflamme GH, Rouleau D, Delisle J, Morin B. Long-term outcome of surgically-treated habitual patellar dislocation in children with coexistent patella alta. Minimum follow-up of 11 years. J Bone Joint Surg Br. 2007;89(9):1172-1177. doi:10.1302/0301-620X.89B9.19065.
37. Sugimoto D, Christino MA, Micheli LJ. Effects of surgical intervention on trochlear remodeling in pediatric patients with recurrent patella dislocation cases. J Pediatr Orthop B. 2016;25(4):349-353. doi:10.1097/BPB.0000000000000341.
38. Insall J, Goldberg V, Salvati E. Recurrent dislocation and the high-riding patella. Clin Orthop Relat Res. 1972;88:67-69.
39. Caton J. [Method of measuring the height of the patella]. Acta Orthop Belg. 1989;55(3):385-386.
40. Thévenin-Lemoine C, Ferrand M, Courvoisier A, Damsin JP, Ducou le Pointe H, Vialle R. Is the Caton-Deschamps index a valuable ratio to investigate patellar height in children? J Bone Joint Surg Am. 2011;93(8):e35. doi:10.2106/JBJS.J.00759.
41. Micheli LJ, Slater JA, Woods E, Gerbino PG. Patella alta and the adolescent growth spurt. Clin Orthop Relat Res. 1986;213:159-162.
42. Koshino T, Sugimoto K. New measurement of patellar height in the knees of children using the epiphyseal line midpoint. J Pediatr Orthop. 1989;9(2):216-218.
43. Mays PK, Bishop JE, Laurent GJ. Age-related changes in the proportion of types I and III collagen. Mech Ageing Dev. 1988;45(3):203-212.
44. Smits-Engelsman B, Klerks M, Kirby A. Beighton score: a valid measure for generalized hypermobility in children. J Pediatr. 2011;158(1):119-123. doi:10.1016/j.jpeds.2010.07.021.
45. Jansson A, Saartok T, Werner S, Renström P. General joint laxity in 1845 Swedish school children of different ages: age- and gender-specific distributions. Acta Paediatr. 2004;93(9):1202-1206.
46. Beighton P, Solomon L, Soskolnet CL. Articular mobility in an African population. Ann rheum Dis. 1973;32(5):413-418.
47. Staheli LT, Corbett M, Wyss C, King H. Lower-extremity rotational problems in children. Normal values to guide management. J Bone Joint Surg Am. 1985;67(1):39-47.
48. Kijowski R, Plagens D, Shaeh S, Teitge R. The effects of rotational deformities of the femur on contact pressure and contact area in the patellofemoral joint and on strain in the medial patellofemoral ligament. Annu Meet Int Patellofemoral Study Group, Napa Val San Fr CA. 1999.
49. Post W, Teitge R, Amis A. Patellofemoral malalignment: looking beyond the viewbox. Clin Sports Med. 2002;21(3):521-546.
50. Bruce WD, Stevens PM. Surgical correction of miserable malalignment syndrome. J Pediatr Orthop. 2004;24(4):392-396.
51. Kurosawa K, Kawame H, Ochiai Y, Nakashima M, Tohma T, Ohashi H. Patellar dislocation in Kabuki syndrome. Am J Med Genet. 2002;108(2):160-163.
52. Mik G, Gholve PA, Scher DM, Widmann RF, Green DW. Down syndrome: orthopedic issues. Curr Opin Pediatr. 2008;20(1):30-36. doi:10.1097/MOP.0b013e3282f35f19.
53. Sweeney E, Fryer A, Mountford R, Green A, McIntosh I. Nail patella syndrome: a review of the phenotype aided by developmental biology. J Med Genet. 2003;40(3):153-162.
54. Stevens CA. Patellar dislocation in Rubenstein-Taybi syndrome. Am J Med Genet. 1997;72(2):188-190.
55. Andrish J. The biomechanics of patellofemoral stability. J Knee Surg. 2004;17(1):35-39.
56. Conlan T, Garth WP, Lemons JE. Evaluation of the medial soft-tissue restraints of the extensor mechanism of the knee. J Bone Joint Surg Am. 1993;75(5):682-693.
57. Balcarek P, Ammon J, Frosch S, et al. Magnetic resonance imaging characteristics of the medial patellofemoral ligament lesion in acute lateral patellar dislocations considering trochlear dysplasia, patella alta, and tibial tuberosity-TG distance. Arthroscopy. 2010;26(7):926-935. doi:10.1016/j.arthro.2009.11.004.
58. Garin C, Chaker M, Dohin B, Kohler R. Permanent, habitual dislocation and recurrent dislocation of the patella in children: surgical management by patellar ligamentous transfer in 50 knees. Rev Chir Orthop Reparatrice Appar Mot. 2007;93(7):690-700.
59. Sillanpaa P. Terminology of patellar dislocation. In: Sillanpaa P, ed. Trauma. Saarbrucken, Germany: Lambert Academic Publishing; 2010:16-18.
60. Parikh SN, Lykissas MG. Classification of lateral patellar instability in children and adolescents. Orthop Clin North Am. 2016;47(1):145-152. doi:10.1016/j.ocl.2015.08.016.
61. Chotel F, Bérard J, Raux S. Patellar instability in children and adolescents. Orthop Traumatol Surg Res. 2014;100(suppl 1):125-137. doi:10.1016/j.otsr.2013.06.014.
62. Wada A, Fujii T, Takamura K, Yanagida H, Surijamorn P. Congenital dislocation of the patella. J Child Orthop. 2008;2(2):119-123. doi:10.1007/s11832-008-0090-4.
63. Südkamp NP, Granrath M, Hoffmann R, Haas NP. Instability of the shoulder joint in the athlete. Chirurg. 1994;65(11):901-909.
64. Hiemstra LA, Kerslake S, Lafave M, Heard SM, Buchko GML. Introduction of a classification system for patients with patellofemoral instability (WARPS and STAID). Knee Surg Sport Traumatol Arthrosc. 2014;22(11):2776-2782. doi:10.1007/s00167-013-2477-0.
65. Frosch KH, Schmeling A. A new classification system of patellar instability and patellar maltracking. Arch Orthop Trauma Surg. 2016;136(4):485-497. doi:10.1007/s00402-015-2381-9.
66. Balcarek P, Oberthür S, Hopfensitz S, et al. Which patellae are likely to redislocate? Knee Surg Sport Traumatol Arthrosc. 2014;22(10):2308-2314. doi:10.1007/s00167-013-2650-5.
67. Bitar AC, Demange MK, D’Elia CO, Camanho GL. Traumatic patellar dislocation: nonoperative treatment compared with mpfl reconstruction using patellar tendon. Am J Sports Med. 2012;40(1):114-122. doi:10.1177/0363546511423742.
68. Smith TO, Walker J, Russell N. Outcomes of medial patellofemoral ligament reconstruction for patellar instability: a systematic review. Knee Surg Sport Traumatol Arthrosc. 2007;15(11):1301-1314. doi:10.1007/s00167-007-0390-0.
69. Steiner TM, Torga-Spak R, Teitge RA. Medial patellofemoral ligament reconstruction in patients with lateral patellar instability and trochlear dysplasia. Am J Sports Med. 2006;34(8):1254-1261. doi:10.1177/0363546505285584.
70. Ma LF, Wang F, Chen BC, Wang CH, Zhou JW, Wang HY. Medial retinaculum plasty versus medial patellofemoral ligament reconstruction for recurrent patellar instability in adults: a randomized controlled trial. Arthrosc J Arthrosc Relat Surg. 2013;29(5):891-897. doi:10.1016/j.arthro.2013.01.030.
71. Weeks KD, Fabricant PD, Ladenhauf HN, Green DW. Surgical options for patellar stabilization in the skeletally immature patient. Sports Med Arthrosc Rev. 2012;20(3):194-202.
72. Green D. Surgical treatment of pediatric patella instability. Die Ther der Instabilen patella. 2016:80-89.
73. Lewallen L, McIntosh A, Dahm D. First-time patellofemoral dislocation: risk factors for recurrent instability. J Knee Surg. 2015;28(4):303-309. doi:10.1055/s-0034-1398373.
74. Ghanem I, Wattincourt L, Seringe R. Congenital dislocation of the patella. Part I: pathologic anatomy. J Pediatr Orthop. 2000;20(6):812-816.
75. Bongers E, Van Kampen A, Van Bokhoven H, Knoers N. Human syndromes with congenital patellar anomalies and the underlying gene defects. Clin Genet. 2005;68(4):302-319. doi:10.1111/j.1399-0004.2005.00508.x.
76. Beighton P, Horan F. Orthopaedic aspects of the Ehlers-Danlos syndrome. J Bone Joint Surg Br. 1969;51(3):444-453.
77. Gausden EB, Fabricant PD, Taylor SA, et al. Medial patellofemoral reconstruction in children and adolescents. JBJS Rev. 2015;3(10):1-11. doi:10.2106/JBJS.RVW.N.00091.
78. Palmu S, Kallio PE, Donell ST, Helenius I, Nietosvaara Y. Acute patellar dislocation in children and adolescents: a randomized clinical trial. J Bone Joint Surg Am. 2008;90(3):463-470. doi:10.2106/JBJS.G.00072.
79. Webb JE, Lewallen LW, Christophersen C, Krych AJ, McIntosh AL. Clinical outcome of internal fixation of unstable juvenile osteochondritis dissecans lesions of the knee. Orthopedics. 2013;36(11):e1444-e1449. doi:10.3928/01477447-20131021-30.
80. Hennrikus W, Pylawka T. Patellofemoral instability in skeletally immature athletes. Instr Course Lect. 2013;62:445-453.
81. Nomura E, Inoue M. Surgical technique and rationale for medial patellofemoral ligament reconstruction for recurrent patellar dislocation. Arthroscopy. 2003;19(5):e47. doi:10.1053/jars.2003.50167.
82. Nomura E, Inoue M, Kobayashi S. Long-term follow-up and knee osteoarthritis change after medial patellofemoral ligament reconstruction for recurrent patellar dislocation. Am J Sports Med. 2007;35(11):1851-1858. doi:10.1177/0363546507306161.
83. Arshi A, Cohen JR, Wang JC, Hame SL, McAllister DR, Jones KJ. Operative management of patellar instability in the United States. Orthop J Sport Med. 2016;4(8):2325967116662873. doi:10.1177/2325967116662873.
84. Servien E, Verdonk PC, Neyret P. Tibial tuberosity transfer for episodic patellar dislocation. Sports Med Arthrosc. 2007;15(2):61-67. doi:10.1097/JSA.0b013e3180479464.
85. Nietosvaara Y, Paukku R, Palmu S, Donell ST. Acute patellar dislocation in children and adolescents. Surgical technique. J Bone Joint Surg Am. 2009;91(suppl 2):139-145. doi:10.2106/JBJS.H.01289.
86. Hing CBC, Smith TO, Donell S, Song F. Surgical versus non-surgical interventions for treating patellar dislocation. In Hing CB, ed. Cochrane Database Syst Rev. 2011;(11):CD008106. doi:10.1002/14651858.CD008106.pub2.
87. Nwachukwu BU, So C, Schairer WW, Green DW, Dodwell ER. Surgical versus conservative management of acute patellar dislocation in children and adolescents: a systematic review. Knee Surgery, Sport Traumatol Arthrosc. 2016;24(3):760-767. doi:10.1007/s00167-015-3948-2.
88. Camp CL, Krych AJ, Dahm DL, Levy BA, Stuart MJ. Medial patellofemoral ligament repair for recurrent patellar dislocation. Am J Sports Med. 2010;38(11):2248-2254. doi:10.1177/0363546510376230.
89. Nikku R, Nietosvaara Y, Aalto K, Kallio PE. Operative treatment of primary patellar dislocation does not improve medium-term outcome: a 7-year follow-up report and risk analysis of 127 randomized patients. Acta Orthop. 2005;76(5):699-704. doi:10.1080/17453670510041790.
90. Ostermeier S, Holst M, Hurschler C, Windhagen H, Stukenborg-Colsman C. Dynamic measurement of patellofemoral kinematics and contact pressure after lateral retinacular release: an in vitro study. Knee Surg Sport Traumatol Arthrosc. 2007;15(5):547-554. doi:10.1007/s00167-006-0261-0.
91. Sallay PI, Poggi J, Speer KP, Garrett WE. Acute dislocation of the patella. A correlative pathoanatomic study. Am J Sports Med. 1996;24(1):52-60. doi: 10.1177/036354659602400110.
92. Arendt EA, Fithian DC, Cohen E. Current concepts of lateral patella dislocation. Clin Sports Med. 2002;21(3):499-519.
93. Arendt EA, Moeller A, Agel J. Clinical outcomes of medial patellofemoral ligament repair in recurrent (chronic) lateral patella dislocations. Knee Surg Sports Traumatol Arthrosc. 2011;19(11):1909-1914. doi:10.1007/s00167-011-1516-y.
94. Ellera Gomes JL. Medial patellofemoral ligament reconstruction for recurrent dislocation of the patella: a preliminary report. Arthroscopy. 1992;8(3):335-340.
95. Amis AA, Firer P, Mountney J, Senavongse W, Thomas NP. Anatomy and biomechanics of the medial patellofemoral ligament. Knee. 2003;10(3):215-220.
96. Avikainen VJ, Nikku RK, Seppänen-Lehmonen TK. Adductor magnus tenodesis for patellar dislocation. Technique and preliminary results. Clin Orthop Relat Res. 1993;(297):12-16.
97. Davis DK, Fithian DC. Techniques of medial retinacular repair and reconstruction. Clin Orthop Relat Res. 2002;(402):38-52.
98. Dhillon MS, Mohan P, Nagi ON. Does harvesting the medial third of the patellar tendon cause lateral shift of the patella after ACL reconstruction? Acta Orthop Belg. 2003;69(4):334-340.
99. Drez D, Edwards TB, Williams CS. Results of medial patellofemoral ligament reconstruction in the treatment of patellar dislocation. Arthrosc J Arthrosc Relat Surg. 2001;17(3):298-306. doi:10.1053/jars.2001.21490.
100. Ellera Gomes JL, Stigler Marczyk LR, César de César P, Jungblut CF. Medial patellofemoral ligament reconstruction with semitendinosus autograft for chronic patellar instability: a follow-up study. Arthrosc J Arthrosc Relat Surg. 2004;20(2):147-151. doi:10.1016/j.arthro.2003.11.006.
101. Feller JA, Amis AA, Andrish JT, Arendt EA, Erasmus PJ, Powers CM. Surgical biomechanics of the patellofemoral joint. Arthrosc J Arthrosc Relat Surg. 2007;23(5):542-553. doi:10.1016/j.arthro.2007.03.006.
102. Nomura E, Horiuchi Y, Kihara M. Medial patellofemoral ligament restraint in lateral patellar translation and reconstruction. Knee. 2000;7(2):121-127.
103. Ostermeier S, Stukenborg-Colsman C, Hurschler C, Wirth CJ. In vitro investigation of the effect of medial patellofemoral ligament reconstruction and medial tibial tuberosity transfer on lateral patellar stability. Arthrosc J Arthrosc Relat Surg. 2006;22(3):308-319. doi:10.1016/j.arthro.2005.09.024.
104. Smirk C, Morris H. The anatomy and reconstruction of the medial patellofemoral ligament. Knee. 2003;10(3):221-227.
105. Steensen RN, Dopirak RM, Maurus PB. A simple technique for reconstruction of the medial patellofemoral ligament using a quadriceps tendon graft. Arthroscopy. 2005;21(3):365-370. doi:10.1016/j.arthro.2004.10.007.
106. Steensen RN, Dopirak RM, McDonald WG. The anatomy and isometry of the medial patellofemoral ligament: implications for reconstruction. Am J Sports Med. 2004;32(6):1509-1513. doi:10.1177/0363546503261505.
107. Farahmand F, Tahmasbi MN, Amis AA. Lateral force–displacement behaviour of the human patella and its variation with knee flexion—a biomechanical study in vitro. J Biomech. 1998;31(12):1147-1152.
108. Heegaard J, Leyvraz PF, Van Kampen A, Rakotomanana L, Rubin PJ, Blankevoort L. Influence of soft structures on patellar 3-dimensional tracking. Clin Orthop Relat Res. 1994;(299):235-243.
109. Goyal D. Medial patellofemoral ligament reconstruction: the superficial quad technique. Am J Sports Med. 2013;41(5):1022-1029. doi:10.1177/0363546513477828.
110. Hohn E, Pandya NK. Does the utilization of allograft tissue in medial patellofemoral ligament reconstruction in pediatric and adolescent patients restore patellar stability? Clin Orthop Relat Res. 2017;475(6):1563-1569. doi:10.1007/s11999-016-5060-4.
111. Csintalan R, Latt L, Fornalski S, Raiszadeh K, Inacio M, Fithian D. Medial patellofemoral ligament (MPFL) reconstruction for the treatment of patellofemoral instability. J Knee Surg. 2013;27(2):139-146. doi:10.1055/s-0033-1360652.
112. Ahmad CS, Brown GD, Stein BS. The docking technique for medial patellofemoral ligament reconstruction: surgical technique and clinical outcome. Am J Sports Med. 2009;37(10):2021-2027. doi:10.1177/0363546509336261.
113. Fernandez E, Sala D, Castejon M. Reconstruction of the medial patellofemoral ligament for patellar instability using a semitendinosus autograft. Acta Orthop Belg. 2005;71(3):303-308.
114. Parikh SN, Nathan ST, Wall EJ, Eismann EA. Complications of medial patellofemoral ligament reconstruction in young patients. Am J Sports Med. 2013;41(5):1030-1038. doi:10.1177/0363546513482085.
115. Green D, Gausden E. Medial patellofemoral ligament reconstruction: hamstring technique. In: Green D, Cordasco F, eds. Pediatr. Adolesc. Knee Surg. New York, NY: Wolters Kluwer; 2015:150-157.
116. Craig JG, Cody DD, van Holsbeeck M. The distal femoral and proximal tibial growth plates: MR imaging, 3-dimensional modeling and estimation of area and volume. Skeletal Radiol. 2004;33(6):337-344.
117. Noyes FR, Albright JC. Reconstruction of the medial patellofemoral ligament with autologous quadriceps tendon. Arthroscopy. 2006;22(8):904.e1-e7. doi:10.1016/j.arthro.2005.12.058.
118. Pinkowsky G, Hennrikus. Technique: quad tendon medial patellofemoral ligament reconstruction. In: Green DW, Cordasco FA, eds. Pediatr. Adolesc. Knee Surg. New York, NY: Wolters Kluwer; 2015:133-139.
119. Bollier M, Fulkerson J, Cosgarea A, Tanaka M. Technical failure of medial patellofemoral ligament reconstruction. Arthroscopy. 2011;27(8):1153-1159. doi:10.1016/j.arthro.2011.02.014.
120. Elias JJ, Cosgarea AJ. Technical errors during medial patellofemoral ligament reconstruction could overload medial patellofemoral cartilage: a computational analysis. Am J Sports Med. 2006;34(9):1478-1485. doi:10.1177/0363546506287486.
121. Thaunat M, Erasmus PJ. Management of overtight medial patellofemoral ligament reconstruction. Knee Surg Sports Traumatol Arthrosc. 2009;17(5):480-483. doi:10.1007/s00167-008-0702-z.
122. Schöttle PB, Schmeling A, Rosenstiel N, Weiler A. Radiographic landmarks for femoral tunnel placement in medial patellofemoral ligament reconstruction. Am J Sports Med. 2007;35(5):801-804. doi:10.1177/0363546506296415.
123. Redfern J, Kamath G, Burks R. Anatomical confirmation of the use of radiographic landmarks in medial patellofemoral ligament reconstruction. Am J Sports Med. 2010;38(2):293-297. doi:10.1177/0363546509347602.
124. Fink C, Veselko M, Herbort M, Hoser C. Minimally invasive reconstruction of the medial patellofemoral ligament using quadriceps tendon. Arthrosc Tech. 2014;3(3):e325-e329. doi:10.1016/j.eats.2014.01.012.
125. Sillanpää PJ, Mäenpää HM, Mattila VM, Visuri T, Pihlajamäki H. A mini-invasive adductor magnus tendon transfer technique for medial patellofemoral ligament reconstruction: a technical note. Knee Surg Sports Traumatol Arthrosc. 2009;17(5):508-512. doi:10.1007/s00167-008-0713-9.
126. Hambidge SJ, Davidson AJ, Gonzales R, Steiner JF. Epidemiology of pediatric injury-related primary care office visits in the United States. Pediatrics. 2002;109(4):559-565.
127. Camanho GL, Bitar AC, Hernandez AJ, Olivi R. Medial patellofemoral ligament reconstruction: a novel technique using the patellar ligament. Arthroscopy. 2007;23(1):108.e1-e4. doi:10.1016/j.arthro.2006.07.008.
128. Gomes JE. Comparison between a static and a dynamic technique for medial patellofemoral ligament reconstruction. Arthroscopy. 2008;24(4):430-435. doi:10.1016/j.arthro.2007.11.005.
129. Deie M, Ochi M, Sumen Y, Yasumoto M, Kobayashi K, Kimura H. Reconstruction of the medial patellofemoral ligament for the treatment of habitual or recurrent dislocation of the patella in children. J Bone Joint Surg Br. 2003;85(6):887-890.
130. Ladenhauf HN, Berkes MB, Green DW. Medial patellofemoral ligament reconstruction using hamstring autograft in children and adolescents. Arthrosc Tech. 2013;2(2):e151-e154. doi:10.1016/j.eats.2013.01.006.
131. Bedi H, Marzo J. The biomechanics of medial patellofemoral ligament repair followed by lateral retinacular release. Am J Sports Med. 2010;38(7):1462-1467. doi:10.1177/0363546510373581.
132. Lattermann C, Toth J, Bach BR. The role of lateral retinacular release in the treatment of patellar instability. Sports Med Arthrosc Rev. 2007;15(2):57-60. doi:10.1097/JSA.0b013e318042af30
133. Ricchetti ET, Mehta S, Sennett BJ, Huffman GR. Comparison of lateral release versus lateral release with medial soft-tissue realignment for the treatment of recurrent patellar instability: a systematic review. Arthroscopy. 2007;23(5):463-468. doi:10.1016/j.arthro.2007.01.007.
134. Dainer RD, Barrack RL, Buckley SL, Alexander AH. Arthroscopic treatment of acute patellar dislocations. Arthroscopy. 1988;4(4):267-271.
135. Miller JR, Adamson GJ, Pink MM, Fraipont MJ, Durand P. Arthroscopically assisted medial reefing without routine lateral release for patellar instability. Am J Sports Med. 2007;35(4):622-629. doi:10.1177/0363546506296041.
136. Vainionpää S, Laasonen E, Silvennoinen T, Vasenius J, Rokkanen P. Acute dislocation of the patella. A prospective review of operative treatment. J Bone Joint Surg Br. 1990;72(3):366-369.
137. Shah JN, Howard JS, Flanigan DC, Brophy RH, Carey JL, Lattermann C. A systematic review of complications and failures associated with medial patellofemoral ligament reconstruction for recurrent patellar dislocation. Am J Sports Med. 2012;40(8):1916-1923. doi:10.1177/0363546512442330.
138. Tanaka MJ, Bollier MJ, Andrish JT, Fulkerson JP, Cosgarea AJ. Complications of medial patellofemoral ligament reconstruction: common technical errors and factors for success: AAOS exhibit selection. J Bone Joint Surg Am. 2012;94(12):e87. doi:10.2106/JBJS.K.01449.
139. Nguyen CV, Farrow LD, Liu RW, Gilmore A. Safe drilling paths in the distal femoral epiphysis for pediatric medial patellofemoral ligament reconstruction. Am J Sports Med. 2017;45(5):1085-1089. doi:10.1177/0363546516677795.
140. Edmonds EW, Glaser DA. Adolescent patella instability extensor mechanics. J Pediatr Orthop. 2016;36(3):262-267. doi:10.1097/BPO.0000000000000430.
141. Fabricant PD, Ladenhauf HN, Salvati EA, Green DW. Medial patellofemoral ligament (MPFL) reconstruction improves radiographic measures of patella alta in children. Knee. 2014;21(6):1180-1184. doi:10.1016/j.knee.2014.07.023.
142. Lykissas MG, Li T, Eismann EA, Parikh SN. Does medial patellofemoral ligament reconstruction decrease patellar height? A preliminary report. J Pediatr Orthop. 2014;34(1):78-85. doi:10.1097/BPO.0b013e3182a12102.
143. Magnussen RA, De Simone V, Lustig S, Neyret P, Flanigan DC. Treatment of patella alta in patients with episodic patellar dislocation: a systematic review. Knee Surgery, Sport Traumatol Arthrosc. 2014;22(10):2545-2550. doi:10.1007/s00167-013-2445-8.
144. Schöttle PB, Fucentese SF, Romero J. Clinical and radiological outcome of medial patellofemoral ligament reconstruction with a semitendinosus autograft for patella instability. Knee Surg Sports Traumatol Arthrosc. 2005;13(7):516-521. doi:10.1007/s00167-005-0659-0.
145. Nelitz M, Dreyhaupt J, Reichel H, Woelfle J, Lippacher S. Anatomic reconstruction of the medial patellofemoral ligament in children and adolescents with open growth plates: surgical technique and clinical outcome. Am J Sports Med. 2013;41(1):58-63. doi:10.1177/0363546512463683.
146. Raghuveer RK, Mishra CB. Reconstruction of medial patellofemoral ligament for chronic patellar instability. Indian J Orthop. 2012;46(4):447-454. doi:10.4103/0019-5413.97259.
147. Kita K, Horibe S, Toritsuka Y, et al. Effects of medial patellofemoral ligament reconstruction on patellar tracking. Knee Surg Sports Traumatol Arthrosc. 2012;20(5):829-837. doi:10.1007/s00167-011-1609-7.
148. Lenschow S, Herbort M, Fink C. Medial patellofemoral ligament reconstruction using quadriceps tendon. Oper Orthop Traumatol. 2015;27(6):474-483. doi:10.1007/s00064-015-0416-6.
149. Abouelsoud MM, Abdelhady A, Elshazly O. Anatomic physeal-sparing technique for medial patellofemoral ligament reconstruction in skeletally immature patients with ligamentous laxity. Eur J Orthop Surg Traumatol. 2015;25(5):921-926. doi:10.1007/s00590-015-1618-1.
150. Malecki K, Fabis J, Flont P, Niedzielski KR. The results of adductor magnus tenodesis in adolescents with recurrent patellar dislocation. Biomed Res Int. 2015;2015:1-7. doi:10.1155/2015/456858.
151. Kumahashi N, Kuwata S, Tadenuma T, Kadowaki M, Uchio Y. A “sandwich” method of reconstruction of the medial patellofemoral ligament using a titanium interference screw for patellar instability in skeletally immature patients. Arch Orthop Trauma Surg. 2012;132(8):1077-1083. doi:10.1007/s00402-012-1516-5.
152. Marsh JS, Daigneault JP, Sethi P, Polzhofer GK. Treatment of recurrent patellar instability with a modification of the Roux-Goldthwait technique. J Pediatr Orthop. 2006;26(4):461-465. doi:10.1097/01.bpo.0000217711.34492.48.
153. Nepple J, Luhmann S. Medial patellar tendon transfer with proximal realignment. In: Green D, Cordasco F, eds. Pediatric and Adolescent Knee Surgery. New York, NY: Wolters Kluwer; 2015:148-153.
154. Galeazzi R. Nuove applicazioni del trapianto musculare e tendineo. Ard Di Orthop Milano. 1922;38:315-323.
155. Grannatt K, Heyworth BE, Ogunwole O, Micheli LJ, Kocher MS. Galeazzi semitendinosus tenodesis for patellofemoral instability in skeletally immature patients. J Pediatr Orthop. 2012;32(6):621-625. doi:10.1097/BPO.0b013e318263a230.
156. Fiume M. La rotulopessi secondi Galeazzi nella lussazione recidivante di rotula [in Italian]. Minerva Ortop. 1954;5:171-174.
157. Baker RH, Carroll N, Dewar FP, Hall JE. The semitendinosus tenodesis for recurrent dislocation of the patella. J Bone Joint Surg Br. 1972;54(1):103-109.
158. Hall JE, Micheli LJ, McManama GB. Semitendinosus tenodesis for recurrent subluxation or dislocation of the patella. Clin Orthop Relat Res. 1979;(144):31-35.
159. Moyad TF, Blakemore L. Modified Galeazzi technique for recurrent patellar dislocation in children. Orthopedics. 2006;29(4):302-304.
160. Letts RM, Davidson D, Beaule P. Semitendinosus tenodesis for repair of recurrent dislocation of the patella in children. J Pediatr Orthop. 1999;19(6):742-747.
161. Aulisa AG, Falciglia F, Giordano M, Savignoni P, Guzzanti V. Galeazzi’s modified technique for recurrent patella dislocation in skeletally immature patients. J Orthop Sci. 2012;17(2):148-155. doi:10.1007/s00776-011-0189-1.
162. Roux C. Recurrent dislocation of the patella: operative treatment. 1888. Clin Orthop Relat Res. 2006;452:17-20.
163. Goldthwait J. Dislocation of the Patella. Trans Am Orthop Assn. 1895.
164. Fondren FB, Goldner JL, Bassett FH. Recurrent dislocation of the patella treated by the modified Roux-Goldthwait procedure. A prospective study of forty-seven knees. J Bone Joint Surg Am. 1985;67(7):993-1005.
165. Aärimaa V, Ranne J, Mattila K, Rahi K, Virolainen P, Hiltunen A. Patellar tendon shortening after treatment of patellar instability with a patellar tendon medialization procedure. Scand J Med Sci Sports. 2008;18(4):442-446. doi:10.1111/j.1600-0838.2007.00730.x.
166. Nelitz M, Reichel H, Dornacher D, Lippacher S. Anatomical reconstruction of the medial patellofemoral ligament in children with open growth-plates. Arch Orthop Trauma Surg. 2012;132(11):1647-1651. doi:10.1007/s00402-012-1593-5.
167. Vähäsarja V, Kinnunen P, Lanning P, Serlo W. Operative realignment of patellar malalignment in children. J Pediatr Orthop. 1995;15(3):281-285.
168. Abraham E, Washington E, Huang TL. Insall proximal realignment for disorders of the patella. Clin Orthop Relat Res. 1989;248:61-65.
169. Insall JN, Aglietti P, Tria AJ. Patellar pain and incongruence. II: Clinical application. Clin Orthop Relat Res. 1983;176:225-232.
170. Chrisman OD, Snook GA, Wilson TC. A long-term prospective study of the Hauser and Roux-Goldthwait procedures for recurrent patellar dislocation. Clin Orthop Relat Res. 1979;144:27-30.
171. Niedzielski KR, Malecki K, Flont P, Fabis J. The results of an extensive soft-tissue procedure in the treatment of obligatory patellar dislocation in children with ligamentous laxity: a post-operative isokinetic study. Bone Joint J. 2015;97-B(1):129-133. doi:10.1302/0301-620X.97B1.33941.
172. Hinckel BB, Gobbi RG, Demange MK, Bonadio MB, Pécora JR, Camanho GL. Combined reconstruction of the medial patellofemoral ligament with quadricipital tendon and the medial patellotibial ligament with patellar tendon. Arthrosc Tech. 2016;5(1):e79-e84. doi:10.1016/j.eats.2015.10.004.
173. Mani S, Kirkpatrick MS, Saranathan A, Smith LG, Cosgarea AJ, Elias JJ. Tibial tuberosity osteotomy for patellofemoral realignment alters tibiofemoral kinematics. Am J Sports Med. 2011;39(5):1024-1031. doi:10.1177/0363546510390188.
174. Brown GD, Ahmad CS. Combined medial patellofemoral ligament and medial patellotibial ligament reconstruction in skeletally immature patients. J Knee Surg. 2008;21(4):328-332.
175. Panagiotopoulos E, Strzelczyk P, Herrmann M, Scuderi G. Cadaveric study on static medial patellar stabilizers: the dynamizing role of the vastus medialis obliquus on medial patellofemoral ligament. Knee Surg Sports Traumatol Arthrosc. 2006;14(1):7-12. doi:10.1007/s00167-005-0631-z.
176. Philippot R, Boyer B, Testa R, Farizon F, Moyen B. The role of the medial ligamentous structures on patellar tracking during knee flexion. Knee Surg Sports Traumatol Arthrosc. 2012;20(2):331-336. doi:10.1007/s00167-011-1598-6.
177. Gordon JE, Schoenecker PL. Surgical treatment of congenital dislocation of the patella. J Pediatr Orthop. 1999;19(2):260-264.
178. Koh JL, Stewart C. Patellar instability. Orthop Clin North Am. 2015;46(1):147-157. doi:10.1016/j.ocl.2014.09.011.
179. Pal S, Besier TF, Draper CE, et al. Patellar tilt correlates with vastus lateralis: vastus medialis activation ratio in maltracking patellofemoral pain patients. J Orthop Res. 2012;30(6):927-933. doi:10.1002/jor.22008.
180. Thompson TC. Quadricepsplasty. Ann Surg. 1945;121(5):751-754.
181. Daoud H, O’Farrell T, Cruess RL. Quadricepsplasty. The Judet technique and results of six cases. J Bone Joint Surg Br. 1982;64(2):194-197.
182. Stanisavljevic S, Zemenick G, Miller D. Congenital, irreducible, permanent lateral dislocation of the patella. Clin Orthop Relat Res. 1976;(116):190-199.
183. Kundu Z, Sangwan S, Guliani G, Siwach R, Kamboj P, Singh R. Thompson’s quadricepsplasty for stiff knee. Indian J Orthop. 2007;41(4):390-394. doi:10.4103/0019-5413.37004.
184. Tercier S, Shah H, Joseph B. Quadricepsplasty for congenital dislocation of the knee and congenital quadriceps contracture. J Child Orthop. 2012;6(5):397-410. doi:10.1007/s11832-012-0437-8.
185. Rose RE. Judet quadricepsplasty for extension contracture of the knee. West Indian Med J. 2005;54(4):238-241.
186. Tsukamoto N, Miura H, Matsuda S, Mawatari T, Kato H, Iwamoto Y. Functional evaluation of four patients treated with V-Y quadricepsplasty in total knee arthroplasty. J Orthop Sci. 2006;11(4):394-400. doi:10.1007/s00776-006-1023-z.
187. Dao Q, Chen DB, Scott RD. Proximal patellar quadricepsplasty realignment during total knee arthroplasty for irreducible congenital dislocation of the patella. J Bone Joint Surg Am. 2010;92(14):2457-2461. doi:10.2106/JBJS.H.00812.
188. Judet R, Judet J, Lord G. Results of treatment of stiffness of the knee caused by arthrolysis and disinsertion of the quadriceps femoris. Mem Acad Chir. 1959;85:645-654.
189. Oliveira VG, D’Elia LF, Tirico LEP, et al. Judet quadricepsplasty in the treatment of posttraumatic knee rigidity: long-term outcomes of 45 cases. J Trauma Acute Care Surg. 2012;72(2):e77-e80.
190. Hahn SB, Choi YR, Kang HJ, Lee SH. Prognostic factors and long-term outcomes following a modified Thompson’s quadricepsplasty for severely stiff knees. J Bone Joint Surg Br. 2010;92(2):217-221. doi:10.1302/0301-620X.92B2.22936.
191. Hosalkar HS, Jones S, Chowdhury M, Hartley J, Hill RA. Quadricepsplasty for knee stiffness after femoral lengthening in congenital short femur. J Bone Joint Surg Br. 2003;85(2):261-264.
192. Massè A, Biasibetti A, Demangos J, Dutto E, Pazzano S, Gallinaro P. The judet quadricepsplasty: long-term outcome of 21 cases. J Trauma. 2006;61(2):358-362. doi:10.1097/01.ta.0000230281.31144.1d.
193. Kocon H, Kabacyj M, Zgoda M. The results of the operative treatment of patellar instability in children with Down’s syndrome. J Pediatr Orthop B. 2012;21(5):407-410. doi:10.1097/BPB.0b013e328354f684.
194. Martin BD, Cherkashin AM, Tulchin K, Samchukov M, Birch JG. Treatment of femoral lengthening-related knee stiffness with a novel quadricepsplasty. J Pediatr Orthop. 2013;33(4):446-452. doi:10.1097/BPO.0b013e3182784e5d.
1. Nietosvaara Y, Aalto K, Kallio PE. Acute patellar dislocation in children: incidence and associated osteochondral fractures. J Pediatr Orthop. 1994;14(4):513-515.
2. Fithian DC, Paxton EW, Stone ML, et al. Epidemiology and natural history of acute patellar dislocation. Am J Sports Med. 2004;32(5):1114-1121. doi:10.1177/0363546503260788.
3. Abbasi D, May MM, Wall EJ, Chan G, Parikh SN. MRI findings in adolescent patients with acute traumatic knee hemarthrosis. J Pediatr Orthop. 2012;32(8):760-764. doi:10.1097/BPO.0b013e3182648d45.
4. Mäenpää H, Lehto MU. Patellar dislocation. The long-term results of nonoperative management in 100 patients. Am J Sports Med. 1997;25(2):213-217. doi:10.1177/036354659702500213.
5. Hawkins RJ, Bell RH, Anisette G. Acute patellar dislocations. The natural history. Am J Sports Med. 1986;14(2):117-120. doi:10.1177/036354658601400204.
6. Cofield RH, Bryan RS. Acute dislocation of the patella: results of conservative treatment. J Trauma. 1977;17(7):526-531.
7. Wasserlauf BL, Paletta GA. Developmental anatomy of the pediatric and adolescent knee. In: Micheli LJ, Kocher MS, eds. The Pediatric and Adolescent Knee. 1st ed. Elsevier; 2006:27-32.
8. Birch JG. Growth and development. In: Herring J, ed. Tachdjian’s Pediatric Orthopedics. 4th ed. Saunders/Elsevier; 2007:3-22.
9. Sarin VK, Carter DR. Mechanobiology and joint conformity regulate endochondral ossification of sesamoids. J Orthop Res. 2000;18(5):706-712. doi:10.1002/jor.1100180505.
10. Maes C, Kronenberg HM. Postnatal bone growth: growth plate biology, bone formation, and remodeling. In: Pediatric Bone. Elsevier; 2012:55-82.
11. Tecklenburg K, Dejour D, Hoser C, Fink C. Bony and cartilaginous anatomy of the patellofemoral joint. Knee Surg Sports Traumatol Arthrosc. 2006;14(3):235-240. doi:10.1007/s00167-005-0683-0.
12. Walmsley R. The development of the patella. J Anat. 1940;74(Pt 3):360-368.3.
13. Fulkerson J, Hungerford D. Disorders of the patellofemoral Joint. In: Normal Anatomy. 2nd ed. Baltimore: Williams & Wilkins; 1990:1-24.
14. Ogden JA. Radiology of postnatal skeletal development. X. Patella and tibial tuberosity. Skeletal Radiol. 1984;11(4):246-257.
15. Nietosvaara Y, Aalto K. The cartilaginous femoral sulcus in children with patellar dislocation: an ultrasonographic study. J Pediatr Orthop. 1997;17(1):50-53.
16. Glard Y, Jouve JL, Garron E, Adalian P, Tardieu C, Bollini G. Anatomic study of femoral patellar groove in fetus. J Pediatr Orthop. 2005;25(3):305-308.
17. Nietosvaara Y. The femoral sulcus in children. An ultrasonographic study. J Bone Joint Surg Br. 1994;76(5):807-809.
18. Nietosvaara AY, Aalto KA. Ultrasonographic evaluation of patellar tracking in children. Clin Orthop Relat Res. 1993;(297):62-64.
19. Desio SM, Burks RT, Bachus KN. Soft tissue restraints to lateral patellar translation in the human knee. Am J Sports Med. 26(1):59-65. doi:10.1177/03635465980260012701.
20. Shea KG, Styhl AC, Jacobs JC, et al. The relationship of the femoral physis and the medial patellofemoral ligament in children: a cadaveric study. Am J Sports Med. 2016;44(11):2833-2837. doi:10.1177/0363546516656366.
21. Shea KG, Polousky JD, Jacobs JC, et al. The relationship of the femoral physis and the medial patellofemoral ligament in children. J Pediatr Orthop. 2014;34(8):808-813. doi:10.1097/BPO.0000000000000165.
22. Shea KG, Polousky JD, Jacobs JC, et al. The patellar insertion of the medial patellofemoral ligament in children. J Pediatr Orthop. 2015;35(4):e31-e35. doi:10.1097/BPO.0000000000000399.
23. Shea KG, Grimm NL, Belzer J, Burks RT, Pfeiffer R. The relation of the femoral physis and the medial patellofemoral ligament. Arthroscopy. 2010;26(8):1083-1087. doi:10.1016/j.arthro.2009.12.020.
24. Nelitz M, Dornacher D, Dreyhaupt J, Reichel H, Lippacher S. The relation of the distal femoral physis and the medial patellofemoral ligament. Knee Surg Sport Traumatol Arthrosc. 2011;19(12):2067-2071. doi:10.1007/s00167-011-1548-3.
25. Kepler CK, Bogner EA, Hammoud S, Malcolmson G, Potter HG, Green DW. Zone of injury of the medial patellofemoral ligament after acute patellar dislocation in children and adolescents. Am J Sports Med. 2011;39(7):1444-1449. doi:10.1177/0363546510397174.
26. Seitlinger G, Moroder P, Fink C, Wierer G. Acquired femoral flexion deformity due to physeal injury during medial patellofemoral ligament reconstruction. Knee. 2017;24(3):680-685. doi:10.1016/j.knee.2017.02.003.
27. Parikh SN, Rajdev N. Relationship between trochlear dysplasia and the anterior distal femoral physis in pediatric and adolescent patients with patellar instability. In: 36th Annual Meething AANA. Denver; 2017.
28. Salenius P, Vankka E. The development of the tibiofemoral angle in children. J Bone Joint Surg Am. 1975;57(2):259-261.
29. Parikh SN. Medial patellofemoral ligament reconstruction and simultaneous guided correction of genu valgum for patellar instability in skeletally immature patients. In: EPOSNA. 2017.
30. Brattstroem H. Shape of the intercondylar groove normally and in recurrent dislocation of patella. A clinical and X-ray-Anatomical investigation. Acta Orthop Scand Suppl. 1964;68(suppl 68):1-148.
31. Dejour H, Walch G, Nove-Josserand L, Guier C. Factors of patellar instability: an anatomic radiographic study. Knee Surg Sports Traumatol Arthrosc. 1994;2(1):19-26.
32. Lewallen LW, McIntosh AL, Dahm DL. Predictors of recurrent instability after acute patellofemoral dislocation in pediatric and adolescent patients. Am J Sports Med. 2013;41(3):575-581. doi:10.1177/0363546512472873.
33. Jaquith BP, Parikh SN. Predictors of recurrent patellar instability in children and adolescents after first-time dislocation. J Pediatr Orthop. 2017;37(7):484-490. doi:10.1097/BPO.0000000000000674.
34. Lippacher S, Reichel H, Nelitz M. Radiological criteria for trochlear dysplasia in children and adolescents. J Pediatr Orthop B. 2011;20(5):341-344. doi:10.1097/BPB.0b013e3283474c8b.
35. Parikh SN, Rajdev N, Sun Q. The growth of trochlear dysplasia during adolescence. J Pediatr Orthop. 2018. doi:10.1097/BPO.0000000000001168. [Epub ahead of print]
36. Benoit B, Laflamme GY, Laflamme GH, Rouleau D, Delisle J, Morin B. Long-term outcome of surgically-treated habitual patellar dislocation in children with coexistent patella alta. Minimum follow-up of 11 years. J Bone Joint Surg Br. 2007;89(9):1172-1177. doi:10.1302/0301-620X.89B9.19065.
37. Sugimoto D, Christino MA, Micheli LJ. Effects of surgical intervention on trochlear remodeling in pediatric patients with recurrent patella dislocation cases. J Pediatr Orthop B. 2016;25(4):349-353. doi:10.1097/BPB.0000000000000341.
38. Insall J, Goldberg V, Salvati E. Recurrent dislocation and the high-riding patella. Clin Orthop Relat Res. 1972;88:67-69.
39. Caton J. [Method of measuring the height of the patella]. Acta Orthop Belg. 1989;55(3):385-386.
40. Thévenin-Lemoine C, Ferrand M, Courvoisier A, Damsin JP, Ducou le Pointe H, Vialle R. Is the Caton-Deschamps index a valuable ratio to investigate patellar height in children? J Bone Joint Surg Am. 2011;93(8):e35. doi:10.2106/JBJS.J.00759.
41. Micheli LJ, Slater JA, Woods E, Gerbino PG. Patella alta and the adolescent growth spurt. Clin Orthop Relat Res. 1986;213:159-162.
42. Koshino T, Sugimoto K. New measurement of patellar height in the knees of children using the epiphyseal line midpoint. J Pediatr Orthop. 1989;9(2):216-218.
43. Mays PK, Bishop JE, Laurent GJ. Age-related changes in the proportion of types I and III collagen. Mech Ageing Dev. 1988;45(3):203-212.
44. Smits-Engelsman B, Klerks M, Kirby A. Beighton score: a valid measure for generalized hypermobility in children. J Pediatr. 2011;158(1):119-123. doi:10.1016/j.jpeds.2010.07.021.
45. Jansson A, Saartok T, Werner S, Renström P. General joint laxity in 1845 Swedish school children of different ages: age- and gender-specific distributions. Acta Paediatr. 2004;93(9):1202-1206.
46. Beighton P, Solomon L, Soskolnet CL. Articular mobility in an African population. Ann rheum Dis. 1973;32(5):413-418.
47. Staheli LT, Corbett M, Wyss C, King H. Lower-extremity rotational problems in children. Normal values to guide management. J Bone Joint Surg Am. 1985;67(1):39-47.
48. Kijowski R, Plagens D, Shaeh S, Teitge R. The effects of rotational deformities of the femur on contact pressure and contact area in the patellofemoral joint and on strain in the medial patellofemoral ligament. Annu Meet Int Patellofemoral Study Group, Napa Val San Fr CA. 1999.
49. Post W, Teitge R, Amis A. Patellofemoral malalignment: looking beyond the viewbox. Clin Sports Med. 2002;21(3):521-546.
50. Bruce WD, Stevens PM. Surgical correction of miserable malalignment syndrome. J Pediatr Orthop. 2004;24(4):392-396.
51. Kurosawa K, Kawame H, Ochiai Y, Nakashima M, Tohma T, Ohashi H. Patellar dislocation in Kabuki syndrome. Am J Med Genet. 2002;108(2):160-163.
52. Mik G, Gholve PA, Scher DM, Widmann RF, Green DW. Down syndrome: orthopedic issues. Curr Opin Pediatr. 2008;20(1):30-36. doi:10.1097/MOP.0b013e3282f35f19.
53. Sweeney E, Fryer A, Mountford R, Green A, McIntosh I. Nail patella syndrome: a review of the phenotype aided by developmental biology. J Med Genet. 2003;40(3):153-162.
54. Stevens CA. Patellar dislocation in Rubenstein-Taybi syndrome. Am J Med Genet. 1997;72(2):188-190.
55. Andrish J. The biomechanics of patellofemoral stability. J Knee Surg. 2004;17(1):35-39.
56. Conlan T, Garth WP, Lemons JE. Evaluation of the medial soft-tissue restraints of the extensor mechanism of the knee. J Bone Joint Surg Am. 1993;75(5):682-693.
57. Balcarek P, Ammon J, Frosch S, et al. Magnetic resonance imaging characteristics of the medial patellofemoral ligament lesion in acute lateral patellar dislocations considering trochlear dysplasia, patella alta, and tibial tuberosity-TG distance. Arthroscopy. 2010;26(7):926-935. doi:10.1016/j.arthro.2009.11.004.
58. Garin C, Chaker M, Dohin B, Kohler R. Permanent, habitual dislocation and recurrent dislocation of the patella in children: surgical management by patellar ligamentous transfer in 50 knees. Rev Chir Orthop Reparatrice Appar Mot. 2007;93(7):690-700.
59. Sillanpaa P. Terminology of patellar dislocation. In: Sillanpaa P, ed. Trauma. Saarbrucken, Germany: Lambert Academic Publishing; 2010:16-18.
60. Parikh SN, Lykissas MG. Classification of lateral patellar instability in children and adolescents. Orthop Clin North Am. 2016;47(1):145-152. doi:10.1016/j.ocl.2015.08.016.
61. Chotel F, Bérard J, Raux S. Patellar instability in children and adolescents. Orthop Traumatol Surg Res. 2014;100(suppl 1):125-137. doi:10.1016/j.otsr.2013.06.014.
62. Wada A, Fujii T, Takamura K, Yanagida H, Surijamorn P. Congenital dislocation of the patella. J Child Orthop. 2008;2(2):119-123. doi:10.1007/s11832-008-0090-4.
63. Südkamp NP, Granrath M, Hoffmann R, Haas NP. Instability of the shoulder joint in the athlete. Chirurg. 1994;65(11):901-909.
64. Hiemstra LA, Kerslake S, Lafave M, Heard SM, Buchko GML. Introduction of a classification system for patients with patellofemoral instability (WARPS and STAID). Knee Surg Sport Traumatol Arthrosc. 2014;22(11):2776-2782. doi:10.1007/s00167-013-2477-0.
65. Frosch KH, Schmeling A. A new classification system of patellar instability and patellar maltracking. Arch Orthop Trauma Surg. 2016;136(4):485-497. doi:10.1007/s00402-015-2381-9.
66. Balcarek P, Oberthür S, Hopfensitz S, et al. Which patellae are likely to redislocate? Knee Surg Sport Traumatol Arthrosc. 2014;22(10):2308-2314. doi:10.1007/s00167-013-2650-5.
67. Bitar AC, Demange MK, D’Elia CO, Camanho GL. Traumatic patellar dislocation: nonoperative treatment compared with mpfl reconstruction using patellar tendon. Am J Sports Med. 2012;40(1):114-122. doi:10.1177/0363546511423742.
68. Smith TO, Walker J, Russell N. Outcomes of medial patellofemoral ligament reconstruction for patellar instability: a systematic review. Knee Surg Sport Traumatol Arthrosc. 2007;15(11):1301-1314. doi:10.1007/s00167-007-0390-0.
69. Steiner TM, Torga-Spak R, Teitge RA. Medial patellofemoral ligament reconstruction in patients with lateral patellar instability and trochlear dysplasia. Am J Sports Med. 2006;34(8):1254-1261. doi:10.1177/0363546505285584.
70. Ma LF, Wang F, Chen BC, Wang CH, Zhou JW, Wang HY. Medial retinaculum plasty versus medial patellofemoral ligament reconstruction for recurrent patellar instability in adults: a randomized controlled trial. Arthrosc J Arthrosc Relat Surg. 2013;29(5):891-897. doi:10.1016/j.arthro.2013.01.030.
71. Weeks KD, Fabricant PD, Ladenhauf HN, Green DW. Surgical options for patellar stabilization in the skeletally immature patient. Sports Med Arthrosc Rev. 2012;20(3):194-202.
72. Green D. Surgical treatment of pediatric patella instability. Die Ther der Instabilen patella. 2016:80-89.
73. Lewallen L, McIntosh A, Dahm D. First-time patellofemoral dislocation: risk factors for recurrent instability. J Knee Surg. 2015;28(4):303-309. doi:10.1055/s-0034-1398373.
74. Ghanem I, Wattincourt L, Seringe R. Congenital dislocation of the patella. Part I: pathologic anatomy. J Pediatr Orthop. 2000;20(6):812-816.
75. Bongers E, Van Kampen A, Van Bokhoven H, Knoers N. Human syndromes with congenital patellar anomalies and the underlying gene defects. Clin Genet. 2005;68(4):302-319. doi:10.1111/j.1399-0004.2005.00508.x.
76. Beighton P, Horan F. Orthopaedic aspects of the Ehlers-Danlos syndrome. J Bone Joint Surg Br. 1969;51(3):444-453.
77. Gausden EB, Fabricant PD, Taylor SA, et al. Medial patellofemoral reconstruction in children and adolescents. JBJS Rev. 2015;3(10):1-11. doi:10.2106/JBJS.RVW.N.00091.
78. Palmu S, Kallio PE, Donell ST, Helenius I, Nietosvaara Y. Acute patellar dislocation in children and adolescents: a randomized clinical trial. J Bone Joint Surg Am. 2008;90(3):463-470. doi:10.2106/JBJS.G.00072.
79. Webb JE, Lewallen LW, Christophersen C, Krych AJ, McIntosh AL. Clinical outcome of internal fixation of unstable juvenile osteochondritis dissecans lesions of the knee. Orthopedics. 2013;36(11):e1444-e1449. doi:10.3928/01477447-20131021-30.
80. Hennrikus W, Pylawka T. Patellofemoral instability in skeletally immature athletes. Instr Course Lect. 2013;62:445-453.
81. Nomura E, Inoue M. Surgical technique and rationale for medial patellofemoral ligament reconstruction for recurrent patellar dislocation. Arthroscopy. 2003;19(5):e47. doi:10.1053/jars.2003.50167.
82. Nomura E, Inoue M, Kobayashi S. Long-term follow-up and knee osteoarthritis change after medial patellofemoral ligament reconstruction for recurrent patellar dislocation. Am J Sports Med. 2007;35(11):1851-1858. doi:10.1177/0363546507306161.
83. Arshi A, Cohen JR, Wang JC, Hame SL, McAllister DR, Jones KJ. Operative management of patellar instability in the United States. Orthop J Sport Med. 2016;4(8):2325967116662873. doi:10.1177/2325967116662873.
84. Servien E, Verdonk PC, Neyret P. Tibial tuberosity transfer for episodic patellar dislocation. Sports Med Arthrosc. 2007;15(2):61-67. doi:10.1097/JSA.0b013e3180479464.
85. Nietosvaara Y, Paukku R, Palmu S, Donell ST. Acute patellar dislocation in children and adolescents. Surgical technique. J Bone Joint Surg Am. 2009;91(suppl 2):139-145. doi:10.2106/JBJS.H.01289.
86. Hing CBC, Smith TO, Donell S, Song F. Surgical versus non-surgical interventions for treating patellar dislocation. In Hing CB, ed. Cochrane Database Syst Rev. 2011;(11):CD008106. doi:10.1002/14651858.CD008106.pub2.
87. Nwachukwu BU, So C, Schairer WW, Green DW, Dodwell ER. Surgical versus conservative management of acute patellar dislocation in children and adolescents: a systematic review. Knee Surgery, Sport Traumatol Arthrosc. 2016;24(3):760-767. doi:10.1007/s00167-015-3948-2.
88. Camp CL, Krych AJ, Dahm DL, Levy BA, Stuart MJ. Medial patellofemoral ligament repair for recurrent patellar dislocation. Am J Sports Med. 2010;38(11):2248-2254. doi:10.1177/0363546510376230.
89. Nikku R, Nietosvaara Y, Aalto K, Kallio PE. Operative treatment of primary patellar dislocation does not improve medium-term outcome: a 7-year follow-up report and risk analysis of 127 randomized patients. Acta Orthop. 2005;76(5):699-704. doi:10.1080/17453670510041790.
90. Ostermeier S, Holst M, Hurschler C, Windhagen H, Stukenborg-Colsman C. Dynamic measurement of patellofemoral kinematics and contact pressure after lateral retinacular release: an in vitro study. Knee Surg Sport Traumatol Arthrosc. 2007;15(5):547-554. doi:10.1007/s00167-006-0261-0.
91. Sallay PI, Poggi J, Speer KP, Garrett WE. Acute dislocation of the patella. A correlative pathoanatomic study. Am J Sports Med. 1996;24(1):52-60. doi: 10.1177/036354659602400110.
92. Arendt EA, Fithian DC, Cohen E. Current concepts of lateral patella dislocation. Clin Sports Med. 2002;21(3):499-519.
93. Arendt EA, Moeller A, Agel J. Clinical outcomes of medial patellofemoral ligament repair in recurrent (chronic) lateral patella dislocations. Knee Surg Sports Traumatol Arthrosc. 2011;19(11):1909-1914. doi:10.1007/s00167-011-1516-y.
94. Ellera Gomes JL. Medial patellofemoral ligament reconstruction for recurrent dislocation of the patella: a preliminary report. Arthroscopy. 1992;8(3):335-340.
95. Amis AA, Firer P, Mountney J, Senavongse W, Thomas NP. Anatomy and biomechanics of the medial patellofemoral ligament. Knee. 2003;10(3):215-220.
96. Avikainen VJ, Nikku RK, Seppänen-Lehmonen TK. Adductor magnus tenodesis for patellar dislocation. Technique and preliminary results. Clin Orthop Relat Res. 1993;(297):12-16.
97. Davis DK, Fithian DC. Techniques of medial retinacular repair and reconstruction. Clin Orthop Relat Res. 2002;(402):38-52.
98. Dhillon MS, Mohan P, Nagi ON. Does harvesting the medial third of the patellar tendon cause lateral shift of the patella after ACL reconstruction? Acta Orthop Belg. 2003;69(4):334-340.
99. Drez D, Edwards TB, Williams CS. Results of medial patellofemoral ligament reconstruction in the treatment of patellar dislocation. Arthrosc J Arthrosc Relat Surg. 2001;17(3):298-306. doi:10.1053/jars.2001.21490.
100. Ellera Gomes JL, Stigler Marczyk LR, César de César P, Jungblut CF. Medial patellofemoral ligament reconstruction with semitendinosus autograft for chronic patellar instability: a follow-up study. Arthrosc J Arthrosc Relat Surg. 2004;20(2):147-151. doi:10.1016/j.arthro.2003.11.006.
101. Feller JA, Amis AA, Andrish JT, Arendt EA, Erasmus PJ, Powers CM. Surgical biomechanics of the patellofemoral joint. Arthrosc J Arthrosc Relat Surg. 2007;23(5):542-553. doi:10.1016/j.arthro.2007.03.006.
102. Nomura E, Horiuchi Y, Kihara M. Medial patellofemoral ligament restraint in lateral patellar translation and reconstruction. Knee. 2000;7(2):121-127.
103. Ostermeier S, Stukenborg-Colsman C, Hurschler C, Wirth CJ. In vitro investigation of the effect of medial patellofemoral ligament reconstruction and medial tibial tuberosity transfer on lateral patellar stability. Arthrosc J Arthrosc Relat Surg. 2006;22(3):308-319. doi:10.1016/j.arthro.2005.09.024.
104. Smirk C, Morris H. The anatomy and reconstruction of the medial patellofemoral ligament. Knee. 2003;10(3):221-227.
105. Steensen RN, Dopirak RM, Maurus PB. A simple technique for reconstruction of the medial patellofemoral ligament using a quadriceps tendon graft. Arthroscopy. 2005;21(3):365-370. doi:10.1016/j.arthro.2004.10.007.
106. Steensen RN, Dopirak RM, McDonald WG. The anatomy and isometry of the medial patellofemoral ligament: implications for reconstruction. Am J Sports Med. 2004;32(6):1509-1513. doi:10.1177/0363546503261505.
107. Farahmand F, Tahmasbi MN, Amis AA. Lateral force–displacement behaviour of the human patella and its variation with knee flexion—a biomechanical study in vitro. J Biomech. 1998;31(12):1147-1152.
108. Heegaard J, Leyvraz PF, Van Kampen A, Rakotomanana L, Rubin PJ, Blankevoort L. Influence of soft structures on patellar 3-dimensional tracking. Clin Orthop Relat Res. 1994;(299):235-243.
109. Goyal D. Medial patellofemoral ligament reconstruction: the superficial quad technique. Am J Sports Med. 2013;41(5):1022-1029. doi:10.1177/0363546513477828.
110. Hohn E, Pandya NK. Does the utilization of allograft tissue in medial patellofemoral ligament reconstruction in pediatric and adolescent patients restore patellar stability? Clin Orthop Relat Res. 2017;475(6):1563-1569. doi:10.1007/s11999-016-5060-4.
111. Csintalan R, Latt L, Fornalski S, Raiszadeh K, Inacio M, Fithian D. Medial patellofemoral ligament (MPFL) reconstruction for the treatment of patellofemoral instability. J Knee Surg. 2013;27(2):139-146. doi:10.1055/s-0033-1360652.
112. Ahmad CS, Brown GD, Stein BS. The docking technique for medial patellofemoral ligament reconstruction: surgical technique and clinical outcome. Am J Sports Med. 2009;37(10):2021-2027. doi:10.1177/0363546509336261.
113. Fernandez E, Sala D, Castejon M. Reconstruction of the medial patellofemoral ligament for patellar instability using a semitendinosus autograft. Acta Orthop Belg. 2005;71(3):303-308.
114. Parikh SN, Nathan ST, Wall EJ, Eismann EA. Complications of medial patellofemoral ligament reconstruction in young patients. Am J Sports Med. 2013;41(5):1030-1038. doi:10.1177/0363546513482085.
115. Green D, Gausden E. Medial patellofemoral ligament reconstruction: hamstring technique. In: Green D, Cordasco F, eds. Pediatr. Adolesc. Knee Surg. New York, NY: Wolters Kluwer; 2015:150-157.
116. Craig JG, Cody DD, van Holsbeeck M. The distal femoral and proximal tibial growth plates: MR imaging, 3-dimensional modeling and estimation of area and volume. Skeletal Radiol. 2004;33(6):337-344.
117. Noyes FR, Albright JC. Reconstruction of the medial patellofemoral ligament with autologous quadriceps tendon. Arthroscopy. 2006;22(8):904.e1-e7. doi:10.1016/j.arthro.2005.12.058.
118. Pinkowsky G, Hennrikus. Technique: quad tendon medial patellofemoral ligament reconstruction. In: Green DW, Cordasco FA, eds. Pediatr. Adolesc. Knee Surg. New York, NY: Wolters Kluwer; 2015:133-139.
119. Bollier M, Fulkerson J, Cosgarea A, Tanaka M. Technical failure of medial patellofemoral ligament reconstruction. Arthroscopy. 2011;27(8):1153-1159. doi:10.1016/j.arthro.2011.02.014.
120. Elias JJ, Cosgarea AJ. Technical errors during medial patellofemoral ligament reconstruction could overload medial patellofemoral cartilage: a computational analysis. Am J Sports Med. 2006;34(9):1478-1485. doi:10.1177/0363546506287486.
121. Thaunat M, Erasmus PJ. Management of overtight medial patellofemoral ligament reconstruction. Knee Surg Sports Traumatol Arthrosc. 2009;17(5):480-483. doi:10.1007/s00167-008-0702-z.
122. Schöttle PB, Schmeling A, Rosenstiel N, Weiler A. Radiographic landmarks for femoral tunnel placement in medial patellofemoral ligament reconstruction. Am J Sports Med. 2007;35(5):801-804. doi:10.1177/0363546506296415.
123. Redfern J, Kamath G, Burks R. Anatomical confirmation of the use of radiographic landmarks in medial patellofemoral ligament reconstruction. Am J Sports Med. 2010;38(2):293-297. doi:10.1177/0363546509347602.
124. Fink C, Veselko M, Herbort M, Hoser C. Minimally invasive reconstruction of the medial patellofemoral ligament using quadriceps tendon. Arthrosc Tech. 2014;3(3):e325-e329. doi:10.1016/j.eats.2014.01.012.
125. Sillanpää PJ, Mäenpää HM, Mattila VM, Visuri T, Pihlajamäki H. A mini-invasive adductor magnus tendon transfer technique for medial patellofemoral ligament reconstruction: a technical note. Knee Surg Sports Traumatol Arthrosc. 2009;17(5):508-512. doi:10.1007/s00167-008-0713-9.
126. Hambidge SJ, Davidson AJ, Gonzales R, Steiner JF. Epidemiology of pediatric injury-related primary care office visits in the United States. Pediatrics. 2002;109(4):559-565.
127. Camanho GL, Bitar AC, Hernandez AJ, Olivi R. Medial patellofemoral ligament reconstruction: a novel technique using the patellar ligament. Arthroscopy. 2007;23(1):108.e1-e4. doi:10.1016/j.arthro.2006.07.008.
128. Gomes JE. Comparison between a static and a dynamic technique for medial patellofemoral ligament reconstruction. Arthroscopy. 2008;24(4):430-435. doi:10.1016/j.arthro.2007.11.005.
129. Deie M, Ochi M, Sumen Y, Yasumoto M, Kobayashi K, Kimura H. Reconstruction of the medial patellofemoral ligament for the treatment of habitual or recurrent dislocation of the patella in children. J Bone Joint Surg Br. 2003;85(6):887-890.
130. Ladenhauf HN, Berkes MB, Green DW. Medial patellofemoral ligament reconstruction using hamstring autograft in children and adolescents. Arthrosc Tech. 2013;2(2):e151-e154. doi:10.1016/j.eats.2013.01.006.
131. Bedi H, Marzo J. The biomechanics of medial patellofemoral ligament repair followed by lateral retinacular release. Am J Sports Med. 2010;38(7):1462-1467. doi:10.1177/0363546510373581.
132. Lattermann C, Toth J, Bach BR. The role of lateral retinacular release in the treatment of patellar instability. Sports Med Arthrosc Rev. 2007;15(2):57-60. doi:10.1097/JSA.0b013e318042af30
133. Ricchetti ET, Mehta S, Sennett BJ, Huffman GR. Comparison of lateral release versus lateral release with medial soft-tissue realignment for the treatment of recurrent patellar instability: a systematic review. Arthroscopy. 2007;23(5):463-468. doi:10.1016/j.arthro.2007.01.007.
134. Dainer RD, Barrack RL, Buckley SL, Alexander AH. Arthroscopic treatment of acute patellar dislocations. Arthroscopy. 1988;4(4):267-271.
135. Miller JR, Adamson GJ, Pink MM, Fraipont MJ, Durand P. Arthroscopically assisted medial reefing without routine lateral release for patellar instability. Am J Sports Med. 2007;35(4):622-629. doi:10.1177/0363546506296041.
136. Vainionpää S, Laasonen E, Silvennoinen T, Vasenius J, Rokkanen P. Acute dislocation of the patella. A prospective review of operative treatment. J Bone Joint Surg Br. 1990;72(3):366-369.
137. Shah JN, Howard JS, Flanigan DC, Brophy RH, Carey JL, Lattermann C. A systematic review of complications and failures associated with medial patellofemoral ligament reconstruction for recurrent patellar dislocation. Am J Sports Med. 2012;40(8):1916-1923. doi:10.1177/0363546512442330.
138. Tanaka MJ, Bollier MJ, Andrish JT, Fulkerson JP, Cosgarea AJ. Complications of medial patellofemoral ligament reconstruction: common technical errors and factors for success: AAOS exhibit selection. J Bone Joint Surg Am. 2012;94(12):e87. doi:10.2106/JBJS.K.01449.
139. Nguyen CV, Farrow LD, Liu RW, Gilmore A. Safe drilling paths in the distal femoral epiphysis for pediatric medial patellofemoral ligament reconstruction. Am J Sports Med. 2017;45(5):1085-1089. doi:10.1177/0363546516677795.
140. Edmonds EW, Glaser DA. Adolescent patella instability extensor mechanics. J Pediatr Orthop. 2016;36(3):262-267. doi:10.1097/BPO.0000000000000430.
141. Fabricant PD, Ladenhauf HN, Salvati EA, Green DW. Medial patellofemoral ligament (MPFL) reconstruction improves radiographic measures of patella alta in children. Knee. 2014;21(6):1180-1184. doi:10.1016/j.knee.2014.07.023.
142. Lykissas MG, Li T, Eismann EA, Parikh SN. Does medial patellofemoral ligament reconstruction decrease patellar height? A preliminary report. J Pediatr Orthop. 2014;34(1):78-85. doi:10.1097/BPO.0b013e3182a12102.
143. Magnussen RA, De Simone V, Lustig S, Neyret P, Flanigan DC. Treatment of patella alta in patients with episodic patellar dislocation: a systematic review. Knee Surgery, Sport Traumatol Arthrosc. 2014;22(10):2545-2550. doi:10.1007/s00167-013-2445-8.
144. Schöttle PB, Fucentese SF, Romero J. Clinical and radiological outcome of medial patellofemoral ligament reconstruction with a semitendinosus autograft for patella instability. Knee Surg Sports Traumatol Arthrosc. 2005;13(7):516-521. doi:10.1007/s00167-005-0659-0.
145. Nelitz M, Dreyhaupt J, Reichel H, Woelfle J, Lippacher S. Anatomic reconstruction of the medial patellofemoral ligament in children and adolescents with open growth plates: surgical technique and clinical outcome. Am J Sports Med. 2013;41(1):58-63. doi:10.1177/0363546512463683.
146. Raghuveer RK, Mishra CB. Reconstruction of medial patellofemoral ligament for chronic patellar instability. Indian J Orthop. 2012;46(4):447-454. doi:10.4103/0019-5413.97259.
147. Kita K, Horibe S, Toritsuka Y, et al. Effects of medial patellofemoral ligament reconstruction on patellar tracking. Knee Surg Sports Traumatol Arthrosc. 2012;20(5):829-837. doi:10.1007/s00167-011-1609-7.
148. Lenschow S, Herbort M, Fink C. Medial patellofemoral ligament reconstruction using quadriceps tendon. Oper Orthop Traumatol. 2015;27(6):474-483. doi:10.1007/s00064-015-0416-6.
149. Abouelsoud MM, Abdelhady A, Elshazly O. Anatomic physeal-sparing technique for medial patellofemoral ligament reconstruction in skeletally immature patients with ligamentous laxity. Eur J Orthop Surg Traumatol. 2015;25(5):921-926. doi:10.1007/s00590-015-1618-1.
150. Malecki K, Fabis J, Flont P, Niedzielski KR. The results of adductor magnus tenodesis in adolescents with recurrent patellar dislocation. Biomed Res Int. 2015;2015:1-7. doi:10.1155/2015/456858.
151. Kumahashi N, Kuwata S, Tadenuma T, Kadowaki M, Uchio Y. A “sandwich” method of reconstruction of the medial patellofemoral ligament using a titanium interference screw for patellar instability in skeletally immature patients. Arch Orthop Trauma Surg. 2012;132(8):1077-1083. doi:10.1007/s00402-012-1516-5.
152. Marsh JS, Daigneault JP, Sethi P, Polzhofer GK. Treatment of recurrent patellar instability with a modification of the Roux-Goldthwait technique. J Pediatr Orthop. 2006;26(4):461-465. doi:10.1097/01.bpo.0000217711.34492.48.
153. Nepple J, Luhmann S. Medial patellar tendon transfer with proximal realignment. In: Green D, Cordasco F, eds. Pediatric and Adolescent Knee Surgery. New York, NY: Wolters Kluwer; 2015:148-153.
154. Galeazzi R. Nuove applicazioni del trapianto musculare e tendineo. Ard Di Orthop Milano. 1922;38:315-323.
155. Grannatt K, Heyworth BE, Ogunwole O, Micheli LJ, Kocher MS. Galeazzi semitendinosus tenodesis for patellofemoral instability in skeletally immature patients. J Pediatr Orthop. 2012;32(6):621-625. doi:10.1097/BPO.0b013e318263a230.
156. Fiume M. La rotulopessi secondi Galeazzi nella lussazione recidivante di rotula [in Italian]. Minerva Ortop. 1954;5:171-174.
157. Baker RH, Carroll N, Dewar FP, Hall JE. The semitendinosus tenodesis for recurrent dislocation of the patella. J Bone Joint Surg Br. 1972;54(1):103-109.
158. Hall JE, Micheli LJ, McManama GB. Semitendinosus tenodesis for recurrent subluxation or dislocation of the patella. Clin Orthop Relat Res. 1979;(144):31-35.
159. Moyad TF, Blakemore L. Modified Galeazzi technique for recurrent patellar dislocation in children. Orthopedics. 2006;29(4):302-304.
160. Letts RM, Davidson D, Beaule P. Semitendinosus tenodesis for repair of recurrent dislocation of the patella in children. J Pediatr Orthop. 1999;19(6):742-747.
161. Aulisa AG, Falciglia F, Giordano M, Savignoni P, Guzzanti V. Galeazzi’s modified technique for recurrent patella dislocation in skeletally immature patients. J Orthop Sci. 2012;17(2):148-155. doi:10.1007/s00776-011-0189-1.
162. Roux C. Recurrent dislocation of the patella: operative treatment. 1888. Clin Orthop Relat Res. 2006;452:17-20.
163. Goldthwait J. Dislocation of the Patella. Trans Am Orthop Assn. 1895.
164. Fondren FB, Goldner JL, Bassett FH. Recurrent dislocation of the patella treated by the modified Roux-Goldthwait procedure. A prospective study of forty-seven knees. J Bone Joint Surg Am. 1985;67(7):993-1005.
165. Aärimaa V, Ranne J, Mattila K, Rahi K, Virolainen P, Hiltunen A. Patellar tendon shortening after treatment of patellar instability with a patellar tendon medialization procedure. Scand J Med Sci Sports. 2008;18(4):442-446. doi:10.1111/j.1600-0838.2007.00730.x.
166. Nelitz M, Reichel H, Dornacher D, Lippacher S. Anatomical reconstruction of the medial patellofemoral ligament in children with open growth-plates. Arch Orthop Trauma Surg. 2012;132(11):1647-1651. doi:10.1007/s00402-012-1593-5.
167. Vähäsarja V, Kinnunen P, Lanning P, Serlo W. Operative realignment of patellar malalignment in children. J Pediatr Orthop. 1995;15(3):281-285.
168. Abraham E, Washington E, Huang TL. Insall proximal realignment for disorders of the patella. Clin Orthop Relat Res. 1989;248:61-65.
169. Insall JN, Aglietti P, Tria AJ. Patellar pain and incongruence. II: Clinical application. Clin Orthop Relat Res. 1983;176:225-232.
170. Chrisman OD, Snook GA, Wilson TC. A long-term prospective study of the Hauser and Roux-Goldthwait procedures for recurrent patellar dislocation. Clin Orthop Relat Res. 1979;144:27-30.
171. Niedzielski KR, Malecki K, Flont P, Fabis J. The results of an extensive soft-tissue procedure in the treatment of obligatory patellar dislocation in children with ligamentous laxity: a post-operative isokinetic study. Bone Joint J. 2015;97-B(1):129-133. doi:10.1302/0301-620X.97B1.33941.
172. Hinckel BB, Gobbi RG, Demange MK, Bonadio MB, Pécora JR, Camanho GL. Combined reconstruction of the medial patellofemoral ligament with quadricipital tendon and the medial patellotibial ligament with patellar tendon. Arthrosc Tech. 2016;5(1):e79-e84. doi:10.1016/j.eats.2015.10.004.
173. Mani S, Kirkpatrick MS, Saranathan A, Smith LG, Cosgarea AJ, Elias JJ. Tibial tuberosity osteotomy for patellofemoral realignment alters tibiofemoral kinematics. Am J Sports Med. 2011;39(5):1024-1031. doi:10.1177/0363546510390188.
174. Brown GD, Ahmad CS. Combined medial patellofemoral ligament and medial patellotibial ligament reconstruction in skeletally immature patients. J Knee Surg. 2008;21(4):328-332.
175. Panagiotopoulos E, Strzelczyk P, Herrmann M, Scuderi G. Cadaveric study on static medial patellar stabilizers: the dynamizing role of the vastus medialis obliquus on medial patellofemoral ligament. Knee Surg Sports Traumatol Arthrosc. 2006;14(1):7-12. doi:10.1007/s00167-005-0631-z.
176. Philippot R, Boyer B, Testa R, Farizon F, Moyen B. The role of the medial ligamentous structures on patellar tracking during knee flexion. Knee Surg Sports Traumatol Arthrosc. 2012;20(2):331-336. doi:10.1007/s00167-011-1598-6.
177. Gordon JE, Schoenecker PL. Surgical treatment of congenital dislocation of the patella. J Pediatr Orthop. 1999;19(2):260-264.
178. Koh JL, Stewart C. Patellar instability. Orthop Clin North Am. 2015;46(1):147-157. doi:10.1016/j.ocl.2014.09.011.
179. Pal S, Besier TF, Draper CE, et al. Patellar tilt correlates with vastus lateralis: vastus medialis activation ratio in maltracking patellofemoral pain patients. J Orthop Res. 2012;30(6):927-933. doi:10.1002/jor.22008.
180. Thompson TC. Quadricepsplasty. Ann Surg. 1945;121(5):751-754.
181. Daoud H, O’Farrell T, Cruess RL. Quadricepsplasty. The Judet technique and results of six cases. J Bone Joint Surg Br. 1982;64(2):194-197.
182. Stanisavljevic S, Zemenick G, Miller D. Congenital, irreducible, permanent lateral dislocation of the patella. Clin Orthop Relat Res. 1976;(116):190-199.
183. Kundu Z, Sangwan S, Guliani G, Siwach R, Kamboj P, Singh R. Thompson’s quadricepsplasty for stiff knee. Indian J Orthop. 2007;41(4):390-394. doi:10.4103/0019-5413.37004.
184. Tercier S, Shah H, Joseph B. Quadricepsplasty for congenital dislocation of the knee and congenital quadriceps contracture. J Child Orthop. 2012;6(5):397-410. doi:10.1007/s11832-012-0437-8.
185. Rose RE. Judet quadricepsplasty for extension contracture of the knee. West Indian Med J. 2005;54(4):238-241.
186. Tsukamoto N, Miura H, Matsuda S, Mawatari T, Kato H, Iwamoto Y. Functional evaluation of four patients treated with V-Y quadricepsplasty in total knee arthroplasty. J Orthop Sci. 2006;11(4):394-400. doi:10.1007/s00776-006-1023-z.
187. Dao Q, Chen DB, Scott RD. Proximal patellar quadricepsplasty realignment during total knee arthroplasty for irreducible congenital dislocation of the patella. J Bone Joint Surg Am. 2010;92(14):2457-2461. doi:10.2106/JBJS.H.00812.
188. Judet R, Judet J, Lord G. Results of treatment of stiffness of the knee caused by arthrolysis and disinsertion of the quadriceps femoris. Mem Acad Chir. 1959;85:645-654.
189. Oliveira VG, D’Elia LF, Tirico LEP, et al. Judet quadricepsplasty in the treatment of posttraumatic knee rigidity: long-term outcomes of 45 cases. J Trauma Acute Care Surg. 2012;72(2):e77-e80.
190. Hahn SB, Choi YR, Kang HJ, Lee SH. Prognostic factors and long-term outcomes following a modified Thompson’s quadricepsplasty for severely stiff knees. J Bone Joint Surg Br. 2010;92(2):217-221. doi:10.1302/0301-620X.92B2.22936.
191. Hosalkar HS, Jones S, Chowdhury M, Hartley J, Hill RA. Quadricepsplasty for knee stiffness after femoral lengthening in congenital short femur. J Bone Joint Surg Br. 2003;85(2):261-264.
192. Massè A, Biasibetti A, Demangos J, Dutto E, Pazzano S, Gallinaro P. The judet quadricepsplasty: long-term outcome of 21 cases. J Trauma. 2006;61(2):358-362. doi:10.1097/01.ta.0000230281.31144.1d.
193. Kocon H, Kabacyj M, Zgoda M. The results of the operative treatment of patellar instability in children with Down’s syndrome. J Pediatr Orthop B. 2012;21(5):407-410. doi:10.1097/BPB.0b013e328354f684.
194. Martin BD, Cherkashin AM, Tulchin K, Samchukov M, Birch JG. Treatment of femoral lengthening-related knee stiffness with a novel quadricepsplasty. J Pediatr Orthop. 2013;33(4):446-452. doi:10.1097/BPO.0b013e3182784e5d.
TAKE-HOME POINTS
- Patellofemoral joint stability is dependent on a complex interplay of musculotendinous units, ligaments, and the osteocartilaginous morphology of the patellofemoral joint.
- Varied patterns of patellar instability in the pediatric population should be recognized. Habitual dislocation in flexion and permanent dislocation are the more severe types.
- Assessment of major risk factors and, if required, their correction would influence management decisions and would have prognostic value related to outcomes.
- Physeal-sparing MPFL reconstruction can suffice for most children and adolescents with recurrent patellar dislocation.
- Distal stabilization techniques and quadricepsplasty are an important part of surgical armamentarium, especially for the more complex patellar instability patterns.